User login
Does vitamin D supplementation reduce asthma exacerbations?
EVIDENCE SUMMARY
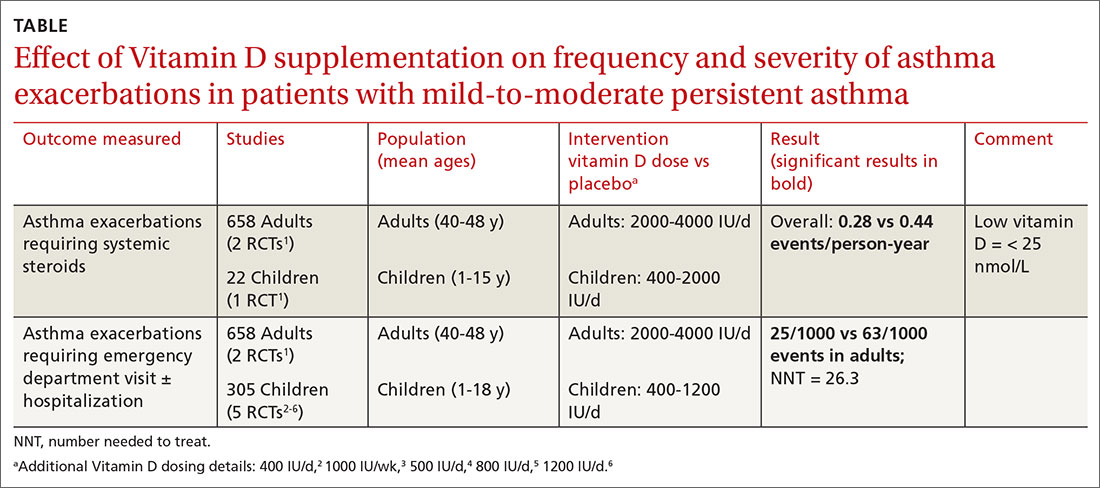
A Cochrane systematic review of vitamin D for managing asthma performed meta-analyses on RCTs that evaluated several outcomes.1 The review found improvement in the primary outcome of asthma exacerbations requiring systemic steroids, mainly in adult patients, and in the secondary outcomes of emergency department visits or hospitalization, in a mix of adults and children (TABLE1-6).
Most participants had mild-to-moderate asthma; trials lasted 4 to 12 months. Vitamin D dosage regimens varied, with a median daily dose of 900 IU/d (range, 400-4000 IU/d). Six RCTs were rated high-quality, and 1 had unclear risk of bias.
Supplementation reduced exacerbations in patients with low vitamin D levels
A subsequent (2017) systematic review and meta-analysis evaluating the primary outcome of exacerbations requiring steroids7 included another study8 (in addition to the 6 RCTs in the Cochrane review).
When researchers reanalyzed individual participant data from the trials in the Cochrane review, plus the additional RCT, to include baseline vitamin D levels, they found that vitamin D supplementation reduced exacerbations overall (NNT = 7.7) and in patients with low baseline vitamin D levels (25[OH] vitamin D < 25 nmol/L; 92 participants in 3 RCTs; NNT = 4.3) but not in patients with higher baseline levels (764 participants in 6 RCTs). Vitamin D supplementation reduced the asthma exacerbation rate in patients with low baseline vitamin D levels (0.19 vs 0.42 events per participant-year; P = .046).
Smaller benefit found on ED visits and hospitalizations
The Cochrane review, with 2 RCTs with adults (n = 658)1 and 5 RCTs with children (n = 305),2-6 evaluated whether Vitamin D reduced the need for emergency department visits and hospitalization with asthma exacerbations; they found a smaller benefit (NNT = 26.3).
Effects on FEV1, daily asthma symptoms, and serious adverse effects
Several RCTs included in the 2017 meta-analysis found no effect of vitamin D supplementation on FEV1, daily asthma symptoms (evaluated with the standardized Asthma Control Test Score), or reported serious adverse events.2-6,9,10 No deaths occurred in any trial.
Additional findings in children from lower-quality studies
A 2015 systematic review and meta-analysis of RCTs evaluating vitamin D supplementation for children with asthma found11:
- moderate-quality evidence for decreased emergency department visits (1 RCT from India, 100 children ages 3 to 14 years, decrease not specified; P = .015);
- low-quality evidence for reduced exacerbations (6 RCTs [3 RCTs also in Cochrane review], 507 children ages 3 to 17 years; risk ratio = 0.41; 95% confidence interval, 0.27-0.63); and
- low-quality evidence for reduced standardized asthma symptom scores (6 RCTs [2 RCTs also in Cochrane review], 231 children ages 3 to 17 years; amount of reduction not listed; P = .01).
Continue to: RECOMMENDATIONS
RECOMMENDATIONS
No published guidelines discuss using vitamin D in managing asthma. An American Academy of Family Physicians (AAFP) summary of the Cochrane systematic review recommends that family physicians await further studies and updated guidelines before recommending vitamin D for patients with asthma.12 The AAFP also points out that the Endocrine Society has recommended vitamin D supplementation for adults (1500-2000 IU/d) and children (at least 1000 IU/d) at risk for deficiency.
Editor's takeaway
In the meta-analyses highlighted here, researchers evaluated asthma patients with a wide range of ages, baseline vitamin D levels, and vitamin D supplementation protocols. Although vitamin D reduced asthma exacerbations requiring steroids overall, the effect was driven by 3 studies of patients with low baseline vitamin D levels. As a result, disentangling who might benefit the most remains a challenge. The conservative course for now is to manage asthma according to current guidelines and supplement vitamin D in patients at risk for, or with known, deficiency.
, , , . Vitamin D for the management of asthma. Cochrane Database Syst Rev. 2016;9:CD011511.
2. Jensen M, Mailhot G, Alos N, et al. Vitamin D intervention in preschoolers with viral-induced asthma (DIVA): a pilot randomised controlled trial. Trials. 2016;26:17:353.
, , , et al. Correlation of vitamin D with Foxp3 induction and steroid-sparing effect of immunotherapy in asthmatic children. Ann Allergy Asthma Immunol. 2012;109:329-335.
, , , et al. Vitamin D supplementation in children may prevent asthma exacerbation triggered by acute respiratory infection. J Allergy Clin Immunol. 2011;127:1294-1296.
, , , et al. Improved control of childhood asthma with low-dose, short-term vitamin D supplementation: a randomized, double-blind, placebo-controlled trial. Allergy. 2016;71:1001-1009.
, , , et al. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in school children. Am J Clin Nutr. 2010;91:1255-1260.
7. Joliffe DA, Greenberg L, Hooper RL, et al. Vitamin D supplementation to prevent asthma exacerbations: a systematic review and meta-analysis of individual participant data. Lancet 2017;5:881-890.
8. Kerley CP, Hutchinson K, Cormical L, et al. Vitamin D3 for uncontrolled childhood asthma: a pilot study. Pediatr Allergy Immunol. 2016;27:404-412.
, , , et al. Effect of vitamin D3 on asthma treatment failures in adults with symptomatic asthma and lower vitamin D levels: the VIDA randomized clinical trial. JAMA. 2014;311:2083-2091.
, , , et al. Double-blind multi-centre randomised controlled trial of vitamin D3 supplementation in adults with inhaled corticosteroid-treated asthma (ViDiAs). Thorax. 2015:70:451-457.
11. Riverin B, Maguire J, Li P. Vitamin D supplementation for childhood asthma: a systematic review and meta-analysis. PLOS One. 2015;10:e0136841.
EVIDENCE SUMMARY
A Cochrane systematic review of vitamin D for managing asthma performed meta-analyses on RCTs that evaluated several outcomes.1 The review found improvement in the primary outcome of asthma exacerbations requiring systemic steroids, mainly in adult patients, and in the secondary outcomes of emergency department visits or hospitalization, in a mix of adults and children (TABLE1-6).
Most participants had mild-to-moderate asthma; trials lasted 4 to 12 months. Vitamin D dosage regimens varied, with a median daily dose of 900 IU/d (range, 400-4000 IU/d). Six RCTs were rated high-quality, and 1 had unclear risk of bias.
Supplementation reduced exacerbations in patients with low vitamin D levels
A subsequent (2017) systematic review and meta-analysis evaluating the primary outcome of exacerbations requiring steroids7 included another study8 (in addition to the 6 RCTs in the Cochrane review).
When researchers reanalyzed individual participant data from the trials in the Cochrane review, plus the additional RCT, to include baseline vitamin D levels, they found that vitamin D supplementation reduced exacerbations overall (NNT = 7.7) and in patients with low baseline vitamin D levels (25[OH] vitamin D < 25 nmol/L; 92 participants in 3 RCTs; NNT = 4.3) but not in patients with higher baseline levels (764 participants in 6 RCTs). Vitamin D supplementation reduced the asthma exacerbation rate in patients with low baseline vitamin D levels (0.19 vs 0.42 events per participant-year; P = .046).
Smaller benefit found on ED visits and hospitalizations
The Cochrane review, with 2 RCTs with adults (n = 658)1 and 5 RCTs with children (n = 305),2-6 evaluated whether Vitamin D reduced the need for emergency department visits and hospitalization with asthma exacerbations; they found a smaller benefit (NNT = 26.3).
Effects on FEV1, daily asthma symptoms, and serious adverse effects
Several RCTs included in the 2017 meta-analysis found no effect of vitamin D supplementation on FEV1, daily asthma symptoms (evaluated with the standardized Asthma Control Test Score), or reported serious adverse events.2-6,9,10 No deaths occurred in any trial.
Additional findings in children from lower-quality studies
A 2015 systematic review and meta-analysis of RCTs evaluating vitamin D supplementation for children with asthma found11:
- moderate-quality evidence for decreased emergency department visits (1 RCT from India, 100 children ages 3 to 14 years, decrease not specified; P = .015);
- low-quality evidence for reduced exacerbations (6 RCTs [3 RCTs also in Cochrane review], 507 children ages 3 to 17 years; risk ratio = 0.41; 95% confidence interval, 0.27-0.63); and
- low-quality evidence for reduced standardized asthma symptom scores (6 RCTs [2 RCTs also in Cochrane review], 231 children ages 3 to 17 years; amount of reduction not listed; P = .01).
Continue to: RECOMMENDATIONS
RECOMMENDATIONS
No published guidelines discuss using vitamin D in managing asthma. An American Academy of Family Physicians (AAFP) summary of the Cochrane systematic review recommends that family physicians await further studies and updated guidelines before recommending vitamin D for patients with asthma.12 The AAFP also points out that the Endocrine Society has recommended vitamin D supplementation for adults (1500-2000 IU/d) and children (at least 1000 IU/d) at risk for deficiency.
Editor's takeaway
In the meta-analyses highlighted here, researchers evaluated asthma patients with a wide range of ages, baseline vitamin D levels, and vitamin D supplementation protocols. Although vitamin D reduced asthma exacerbations requiring steroids overall, the effect was driven by 3 studies of patients with low baseline vitamin D levels. As a result, disentangling who might benefit the most remains a challenge. The conservative course for now is to manage asthma according to current guidelines and supplement vitamin D in patients at risk for, or with known, deficiency.
EVIDENCE SUMMARY
A Cochrane systematic review of vitamin D for managing asthma performed meta-analyses on RCTs that evaluated several outcomes.1 The review found improvement in the primary outcome of asthma exacerbations requiring systemic steroids, mainly in adult patients, and in the secondary outcomes of emergency department visits or hospitalization, in a mix of adults and children (TABLE1-6).
Most participants had mild-to-moderate asthma; trials lasted 4 to 12 months. Vitamin D dosage regimens varied, with a median daily dose of 900 IU/d (range, 400-4000 IU/d). Six RCTs were rated high-quality, and 1 had unclear risk of bias.
Supplementation reduced exacerbations in patients with low vitamin D levels
A subsequent (2017) systematic review and meta-analysis evaluating the primary outcome of exacerbations requiring steroids7 included another study8 (in addition to the 6 RCTs in the Cochrane review).
When researchers reanalyzed individual participant data from the trials in the Cochrane review, plus the additional RCT, to include baseline vitamin D levels, they found that vitamin D supplementation reduced exacerbations overall (NNT = 7.7) and in patients with low baseline vitamin D levels (25[OH] vitamin D < 25 nmol/L; 92 participants in 3 RCTs; NNT = 4.3) but not in patients with higher baseline levels (764 participants in 6 RCTs). Vitamin D supplementation reduced the asthma exacerbation rate in patients with low baseline vitamin D levels (0.19 vs 0.42 events per participant-year; P = .046).
Smaller benefit found on ED visits and hospitalizations
The Cochrane review, with 2 RCTs with adults (n = 658)1 and 5 RCTs with children (n = 305),2-6 evaluated whether Vitamin D reduced the need for emergency department visits and hospitalization with asthma exacerbations; they found a smaller benefit (NNT = 26.3).
Effects on FEV1, daily asthma symptoms, and serious adverse effects
Several RCTs included in the 2017 meta-analysis found no effect of vitamin D supplementation on FEV1, daily asthma symptoms (evaluated with the standardized Asthma Control Test Score), or reported serious adverse events.2-6,9,10 No deaths occurred in any trial.
Additional findings in children from lower-quality studies
A 2015 systematic review and meta-analysis of RCTs evaluating vitamin D supplementation for children with asthma found11:
- moderate-quality evidence for decreased emergency department visits (1 RCT from India, 100 children ages 3 to 14 years, decrease not specified; P = .015);
- low-quality evidence for reduced exacerbations (6 RCTs [3 RCTs also in Cochrane review], 507 children ages 3 to 17 years; risk ratio = 0.41; 95% confidence interval, 0.27-0.63); and
- low-quality evidence for reduced standardized asthma symptom scores (6 RCTs [2 RCTs also in Cochrane review], 231 children ages 3 to 17 years; amount of reduction not listed; P = .01).
Continue to: RECOMMENDATIONS
RECOMMENDATIONS
No published guidelines discuss using vitamin D in managing asthma. An American Academy of Family Physicians (AAFP) summary of the Cochrane systematic review recommends that family physicians await further studies and updated guidelines before recommending vitamin D for patients with asthma.12 The AAFP also points out that the Endocrine Society has recommended vitamin D supplementation for adults (1500-2000 IU/d) and children (at least 1000 IU/d) at risk for deficiency.
Editor's takeaway
In the meta-analyses highlighted here, researchers evaluated asthma patients with a wide range of ages, baseline vitamin D levels, and vitamin D supplementation protocols. Although vitamin D reduced asthma exacerbations requiring steroids overall, the effect was driven by 3 studies of patients with low baseline vitamin D levels. As a result, disentangling who might benefit the most remains a challenge. The conservative course for now is to manage asthma according to current guidelines and supplement vitamin D in patients at risk for, or with known, deficiency.
, , , . Vitamin D for the management of asthma. Cochrane Database Syst Rev. 2016;9:CD011511.
2. Jensen M, Mailhot G, Alos N, et al. Vitamin D intervention in preschoolers with viral-induced asthma (DIVA): a pilot randomised controlled trial. Trials. 2016;26:17:353.
, , , et al. Correlation of vitamin D with Foxp3 induction and steroid-sparing effect of immunotherapy in asthmatic children. Ann Allergy Asthma Immunol. 2012;109:329-335.
, , , et al. Vitamin D supplementation in children may prevent asthma exacerbation triggered by acute respiratory infection. J Allergy Clin Immunol. 2011;127:1294-1296.
, , , et al. Improved control of childhood asthma with low-dose, short-term vitamin D supplementation: a randomized, double-blind, placebo-controlled trial. Allergy. 2016;71:1001-1009.
, , , et al. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in school children. Am J Clin Nutr. 2010;91:1255-1260.
7. Joliffe DA, Greenberg L, Hooper RL, et al. Vitamin D supplementation to prevent asthma exacerbations: a systematic review and meta-analysis of individual participant data. Lancet 2017;5:881-890.
8. Kerley CP, Hutchinson K, Cormical L, et al. Vitamin D3 for uncontrolled childhood asthma: a pilot study. Pediatr Allergy Immunol. 2016;27:404-412.
, , , et al. Effect of vitamin D3 on asthma treatment failures in adults with symptomatic asthma and lower vitamin D levels: the VIDA randomized clinical trial. JAMA. 2014;311:2083-2091.
, , , et al. Double-blind multi-centre randomised controlled trial of vitamin D3 supplementation in adults with inhaled corticosteroid-treated asthma (ViDiAs). Thorax. 2015:70:451-457.
11. Riverin B, Maguire J, Li P. Vitamin D supplementation for childhood asthma: a systematic review and meta-analysis. PLOS One. 2015;10:e0136841.
, , , . Vitamin D for the management of asthma. Cochrane Database Syst Rev. 2016;9:CD011511.
2. Jensen M, Mailhot G, Alos N, et al. Vitamin D intervention in preschoolers with viral-induced asthma (DIVA): a pilot randomised controlled trial. Trials. 2016;26:17:353.
, , , et al. Correlation of vitamin D with Foxp3 induction and steroid-sparing effect of immunotherapy in asthmatic children. Ann Allergy Asthma Immunol. 2012;109:329-335.
, , , et al. Vitamin D supplementation in children may prevent asthma exacerbation triggered by acute respiratory infection. J Allergy Clin Immunol. 2011;127:1294-1296.
, , , et al. Improved control of childhood asthma with low-dose, short-term vitamin D supplementation: a randomized, double-blind, placebo-controlled trial. Allergy. 2016;71:1001-1009.
, , , et al. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in school children. Am J Clin Nutr. 2010;91:1255-1260.
7. Joliffe DA, Greenberg L, Hooper RL, et al. Vitamin D supplementation to prevent asthma exacerbations: a systematic review and meta-analysis of individual participant data. Lancet 2017;5:881-890.
8. Kerley CP, Hutchinson K, Cormical L, et al. Vitamin D3 for uncontrolled childhood asthma: a pilot study. Pediatr Allergy Immunol. 2016;27:404-412.
, , , et al. Effect of vitamin D3 on asthma treatment failures in adults with symptomatic asthma and lower vitamin D levels: the VIDA randomized clinical trial. JAMA. 2014;311:2083-2091.
, , , et al. Double-blind multi-centre randomised controlled trial of vitamin D3 supplementation in adults with inhaled corticosteroid-treated asthma (ViDiAs). Thorax. 2015:70:451-457.
11. Riverin B, Maguire J, Li P. Vitamin D supplementation for childhood asthma: a systematic review and meta-analysis. PLOS One. 2015;10:e0136841.
EVIDENCE-BASED ANSWER:
Yes, to some extent it does, and primarily in patients with low vitamin D levels. Supplementation reduces asthma exacerbations requiring systemic steroids by 30% overall in adults and children with mild-to-moderate asthma (number needed to treat [NNT] = 7.7). The outcome is driven by the effect in patients with vitamin D levels < 25 nmol/L (NNT = 4.3), however; supplementation doesn’t decrease exacerbations in patients with higher levels. Supplementation also reduces, by a smaller amount (NNT = 26.3), the odds of exacerbations requiring emergency department care or hospitalization (strength of recommendation [SOR]: A, meta-analysis of randomized controlled trials [RCTs]).
In children, vitamin D supplementation may also reduce exacerbations and improve symptom scores (SOR: C, low-quality RCTs).
Vitamin D doesn’t improve forced expiratory volume in 1 second (FEV1) or standardized asthma control test scores. Also, it isn’t associated with serious adverse effects (SOR: A, meta-analysis of RCTs).
FDA approves olaparib/bevacizumab maintenance
The Food and Drug Administration has announced a new approved indication for olaparib (Lynparza) in adults with advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer.
Olaparib is now FDA-approved for use in combination with bevacizumab as maintenance therapy in patients who responded to first-line platinum-based chemotherapy and whose cancer is homologous recombination deficiency positive, as defined by a deleterious or suspected deleterious BRCA mutation and/or genomic instability.
The FDA also approved the Myriad myChoice CDx test as a companion diagnostic for olaparib.
Trial results
The efficacy of olaparib and the myChoice CDx test were assessed in patients in the phase 3 PAOLA-1 trial (NCT02477644). The study enrolled patients with advanced high-grade epithelial ovarian, fallopian tube, or primary peritoneal cancer who had received first-line platinum-based chemotherapy and bevacizumab.
Patients were stratified by first-line treatment outcome and BRCA mutation status, as determined by prospective local testing. All available clinical samples were retrospectively tested with the Myriad myChoice CDx test.
The patients were randomized to receive olaparib at 300 mg orally twice daily in combination with bevacizumab at 15 mg/kg every 3 weeks (n = 537) or placebo plus bevacizumab (n = 269). Patients continued bevacizumab in the maintenance setting and started olaparib 3-9 weeks after their last chemotherapy dose. Olaparib could be continued for up to 2 years or until disease progression or unacceptable toxicity.
The median progression-free survival among the 387 patients with homologous recombination deficiency-positive tumors was 37.2 months in the olaparib arm and 17.7 months in the placebo arm (hazard ratio, 0.33), according to the prescribing information for olaparib.
Serious adverse events occurred in 31% of patients in the olaparib arm. The most common were hypertension (19%) and anemia (17%).
Dose interruptions from adverse events occurred in 54% of patients in the olaparib arm, and dose reductions from adverse events occurred in 41%.
The Food and Drug Administration has announced a new approved indication for olaparib (Lynparza) in adults with advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer.
Olaparib is now FDA-approved for use in combination with bevacizumab as maintenance therapy in patients who responded to first-line platinum-based chemotherapy and whose cancer is homologous recombination deficiency positive, as defined by a deleterious or suspected deleterious BRCA mutation and/or genomic instability.
The FDA also approved the Myriad myChoice CDx test as a companion diagnostic for olaparib.
Trial results
The efficacy of olaparib and the myChoice CDx test were assessed in patients in the phase 3 PAOLA-1 trial (NCT02477644). The study enrolled patients with advanced high-grade epithelial ovarian, fallopian tube, or primary peritoneal cancer who had received first-line platinum-based chemotherapy and bevacizumab.
Patients were stratified by first-line treatment outcome and BRCA mutation status, as determined by prospective local testing. All available clinical samples were retrospectively tested with the Myriad myChoice CDx test.
The patients were randomized to receive olaparib at 300 mg orally twice daily in combination with bevacizumab at 15 mg/kg every 3 weeks (n = 537) or placebo plus bevacizumab (n = 269). Patients continued bevacizumab in the maintenance setting and started olaparib 3-9 weeks after their last chemotherapy dose. Olaparib could be continued for up to 2 years or until disease progression or unacceptable toxicity.
The median progression-free survival among the 387 patients with homologous recombination deficiency-positive tumors was 37.2 months in the olaparib arm and 17.7 months in the placebo arm (hazard ratio, 0.33), according to the prescribing information for olaparib.
Serious adverse events occurred in 31% of patients in the olaparib arm. The most common were hypertension (19%) and anemia (17%).
Dose interruptions from adverse events occurred in 54% of patients in the olaparib arm, and dose reductions from adverse events occurred in 41%.
The Food and Drug Administration has announced a new approved indication for olaparib (Lynparza) in adults with advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer.
Olaparib is now FDA-approved for use in combination with bevacizumab as maintenance therapy in patients who responded to first-line platinum-based chemotherapy and whose cancer is homologous recombination deficiency positive, as defined by a deleterious or suspected deleterious BRCA mutation and/or genomic instability.
The FDA also approved the Myriad myChoice CDx test as a companion diagnostic for olaparib.
Trial results
The efficacy of olaparib and the myChoice CDx test were assessed in patients in the phase 3 PAOLA-1 trial (NCT02477644). The study enrolled patients with advanced high-grade epithelial ovarian, fallopian tube, or primary peritoneal cancer who had received first-line platinum-based chemotherapy and bevacizumab.
Patients were stratified by first-line treatment outcome and BRCA mutation status, as determined by prospective local testing. All available clinical samples were retrospectively tested with the Myriad myChoice CDx test.
The patients were randomized to receive olaparib at 300 mg orally twice daily in combination with bevacizumab at 15 mg/kg every 3 weeks (n = 537) or placebo plus bevacizumab (n = 269). Patients continued bevacizumab in the maintenance setting and started olaparib 3-9 weeks after their last chemotherapy dose. Olaparib could be continued for up to 2 years or until disease progression or unacceptable toxicity.
The median progression-free survival among the 387 patients with homologous recombination deficiency-positive tumors was 37.2 months in the olaparib arm and 17.7 months in the placebo arm (hazard ratio, 0.33), according to the prescribing information for olaparib.
Serious adverse events occurred in 31% of patients in the olaparib arm. The most common were hypertension (19%) and anemia (17%).
Dose interruptions from adverse events occurred in 54% of patients in the olaparib arm, and dose reductions from adverse events occurred in 41%.
Justices appear split over birth control mandate case
U.S. Supreme Court justices appear divided over whether the Trump administration acted properly when it expanded exemptions under the Affordable Care Act’s contraception mandate.
During oral arguments on May 6, the court expressed differing perspectives about the administration’s authority to allow for more exemptions under the health law’s birth control mandate and whether the expansions were reasonable. Justices heard the consolidated cases – Little Sisters of the Poor v. Pennsylvania and Trump v. Pennsylvania – by teleconference because of the COVID-19 pandemic. They are expected to make a decision by the summer.
Associate justice Ruth Bader Ginsburg, who participated in the telephone conference call from a hospital where she was recovering from a gallbladder condition, said the exemptions ignored the intent of Congress to provide women with comprehensive coverage through the ACA.
“The glaring feature of what the government has done in expanding this exemption is to toss to the winds entirely Congress’s instruction that women need and shall have seamless, no-cost, comprehensive coverage,” she said during oral arguments. “This leaves the women to hunt for other government programs that might cover them, and for those who are not covered by Medicaid or one of the other government programs, they can get contraceptive coverage only from paying out of their own pocket, which is exactly what Congress didn’t want to happen.”
Associate Justice Samuel Alito Jr., meanwhile, indicated that a lower court opinion that had blocked the exemptions from going forward conflicts with the Supreme Court’s ruling in a related case, Burwell v. Hobby Lobby.
“Explain to me why the Third Circuit’s analysis of the question of substantial burden is not squarely inconsistent with our reasoning in Hobby Lobby,” Associate Justice Alito said during oral arguments. “Hobby Lobby held that, if a person sincerely believes that it is immoral to perform an act that has the effect of enabling another person to commit an immoral act, a federal court does not have the right to say that this person is wrong on the question of moral complicity. That’s precisely the situation here. Reading the Third Circuit’s discussion of the substantial burden question, I wondered whether they had read that part of the Hobby Lobby decision.”
The dispute surrounding the ACA’s birth control mandate and the extent of exemptions afforded has gone on for a decade and has led to numerous legal challenges. The ACA initially required all employers to cover birth control for employees with no copayments, but exempted group health plans of religious employers. Those religious employers were primarily churches and other houses of worship. After a number of complaints and lawsuits, the Obama administration created a workaround for nonprofit religious employers not included in that exemption to opt out of the mandate. However, critics argued the process itself was a violation of their religious freedom.
The issue led to the case of Zubik v. Burwell, a legal challenge over the mandate exemption that went before the U.S. Supreme Court in March 2016. The issue was never resolved however, and in May 2016, the Supreme Court vacated the lower court rulings related to Zubik v. Burwell and remanded the case back to the four appeals courts that had originally ruled on the issue.
In 2018, the Trump administration announced new rules aimed at broadening exemptions to the ACA’s contraceptive mandate to entities that object to services covered by the mandate on the basis of “sincerely held religious beliefs.” A second rule allowed nonprofit organizations and small businesses that had nonreligious moral convictions against the mandate to opt out.
Thirteen states and the District of Columbia then sued the Trump administration over the rules, as well as Pennsylvania and New Jersey in a separate case. Little Sisters of the Poor, a religious nonprofit operating a home in Pittsburgh, intervened in the case as an aggrieved party. An appeal court temporarily barred the regulations from moving forward.
During oral arguments, Solicitor General for the Department of Justice Noel J. Francisco said the exemptions are lawful because they are authorized under a provision of the ACA as well as the Religious Freedom Restoration Act (RFRA).
“RFRA at the very least authorizes the religious exemption,” Mr. Francisco said during oral arguments.
Chief Deputy Attorney General for Pennsylvania Michael J. Fischer argued that the Trump administration’s moral and religious exemption rules rest on overly broad assertions of agency authority.
“First, the agencies twist a narrow delegation that allows the Health Resources and Services Administration to decide which preventive services insurers must cover under the Women’s Health Amendment into a grant of authority so broad it allows them to permit virtually any employer or college to opt out of providing contraceptive coverage entirely, including for reasons as amorphous as vaguely defined moral beliefs,” he said during oral arguments. “Second, the agencies claim that RFRA, a statute that limits government action, affirmatively authorizes them to permit employers to deny women their rights to contraceptive coverage even in the absence of a RFRA violation in the first place.”
U.S. Supreme Court justices appear divided over whether the Trump administration acted properly when it expanded exemptions under the Affordable Care Act’s contraception mandate.
During oral arguments on May 6, the court expressed differing perspectives about the administration’s authority to allow for more exemptions under the health law’s birth control mandate and whether the expansions were reasonable. Justices heard the consolidated cases – Little Sisters of the Poor v. Pennsylvania and Trump v. Pennsylvania – by teleconference because of the COVID-19 pandemic. They are expected to make a decision by the summer.
Associate justice Ruth Bader Ginsburg, who participated in the telephone conference call from a hospital where she was recovering from a gallbladder condition, said the exemptions ignored the intent of Congress to provide women with comprehensive coverage through the ACA.
“The glaring feature of what the government has done in expanding this exemption is to toss to the winds entirely Congress’s instruction that women need and shall have seamless, no-cost, comprehensive coverage,” she said during oral arguments. “This leaves the women to hunt for other government programs that might cover them, and for those who are not covered by Medicaid or one of the other government programs, they can get contraceptive coverage only from paying out of their own pocket, which is exactly what Congress didn’t want to happen.”
Associate Justice Samuel Alito Jr., meanwhile, indicated that a lower court opinion that had blocked the exemptions from going forward conflicts with the Supreme Court’s ruling in a related case, Burwell v. Hobby Lobby.
“Explain to me why the Third Circuit’s analysis of the question of substantial burden is not squarely inconsistent with our reasoning in Hobby Lobby,” Associate Justice Alito said during oral arguments. “Hobby Lobby held that, if a person sincerely believes that it is immoral to perform an act that has the effect of enabling another person to commit an immoral act, a federal court does not have the right to say that this person is wrong on the question of moral complicity. That’s precisely the situation here. Reading the Third Circuit’s discussion of the substantial burden question, I wondered whether they had read that part of the Hobby Lobby decision.”
The dispute surrounding the ACA’s birth control mandate and the extent of exemptions afforded has gone on for a decade and has led to numerous legal challenges. The ACA initially required all employers to cover birth control for employees with no copayments, but exempted group health plans of religious employers. Those religious employers were primarily churches and other houses of worship. After a number of complaints and lawsuits, the Obama administration created a workaround for nonprofit religious employers not included in that exemption to opt out of the mandate. However, critics argued the process itself was a violation of their religious freedom.
The issue led to the case of Zubik v. Burwell, a legal challenge over the mandate exemption that went before the U.S. Supreme Court in March 2016. The issue was never resolved however, and in May 2016, the Supreme Court vacated the lower court rulings related to Zubik v. Burwell and remanded the case back to the four appeals courts that had originally ruled on the issue.
In 2018, the Trump administration announced new rules aimed at broadening exemptions to the ACA’s contraceptive mandate to entities that object to services covered by the mandate on the basis of “sincerely held religious beliefs.” A second rule allowed nonprofit organizations and small businesses that had nonreligious moral convictions against the mandate to opt out.
Thirteen states and the District of Columbia then sued the Trump administration over the rules, as well as Pennsylvania and New Jersey in a separate case. Little Sisters of the Poor, a religious nonprofit operating a home in Pittsburgh, intervened in the case as an aggrieved party. An appeal court temporarily barred the regulations from moving forward.
During oral arguments, Solicitor General for the Department of Justice Noel J. Francisco said the exemptions are lawful because they are authorized under a provision of the ACA as well as the Religious Freedom Restoration Act (RFRA).
“RFRA at the very least authorizes the religious exemption,” Mr. Francisco said during oral arguments.
Chief Deputy Attorney General for Pennsylvania Michael J. Fischer argued that the Trump administration’s moral and religious exemption rules rest on overly broad assertions of agency authority.
“First, the agencies twist a narrow delegation that allows the Health Resources and Services Administration to decide which preventive services insurers must cover under the Women’s Health Amendment into a grant of authority so broad it allows them to permit virtually any employer or college to opt out of providing contraceptive coverage entirely, including for reasons as amorphous as vaguely defined moral beliefs,” he said during oral arguments. “Second, the agencies claim that RFRA, a statute that limits government action, affirmatively authorizes them to permit employers to deny women their rights to contraceptive coverage even in the absence of a RFRA violation in the first place.”
U.S. Supreme Court justices appear divided over whether the Trump administration acted properly when it expanded exemptions under the Affordable Care Act’s contraception mandate.
During oral arguments on May 6, the court expressed differing perspectives about the administration’s authority to allow for more exemptions under the health law’s birth control mandate and whether the expansions were reasonable. Justices heard the consolidated cases – Little Sisters of the Poor v. Pennsylvania and Trump v. Pennsylvania – by teleconference because of the COVID-19 pandemic. They are expected to make a decision by the summer.
Associate justice Ruth Bader Ginsburg, who participated in the telephone conference call from a hospital where she was recovering from a gallbladder condition, said the exemptions ignored the intent of Congress to provide women with comprehensive coverage through the ACA.
“The glaring feature of what the government has done in expanding this exemption is to toss to the winds entirely Congress’s instruction that women need and shall have seamless, no-cost, comprehensive coverage,” she said during oral arguments. “This leaves the women to hunt for other government programs that might cover them, and for those who are not covered by Medicaid or one of the other government programs, they can get contraceptive coverage only from paying out of their own pocket, which is exactly what Congress didn’t want to happen.”
Associate Justice Samuel Alito Jr., meanwhile, indicated that a lower court opinion that had blocked the exemptions from going forward conflicts with the Supreme Court’s ruling in a related case, Burwell v. Hobby Lobby.
“Explain to me why the Third Circuit’s analysis of the question of substantial burden is not squarely inconsistent with our reasoning in Hobby Lobby,” Associate Justice Alito said during oral arguments. “Hobby Lobby held that, if a person sincerely believes that it is immoral to perform an act that has the effect of enabling another person to commit an immoral act, a federal court does not have the right to say that this person is wrong on the question of moral complicity. That’s precisely the situation here. Reading the Third Circuit’s discussion of the substantial burden question, I wondered whether they had read that part of the Hobby Lobby decision.”
The dispute surrounding the ACA’s birth control mandate and the extent of exemptions afforded has gone on for a decade and has led to numerous legal challenges. The ACA initially required all employers to cover birth control for employees with no copayments, but exempted group health plans of religious employers. Those religious employers were primarily churches and other houses of worship. After a number of complaints and lawsuits, the Obama administration created a workaround for nonprofit religious employers not included in that exemption to opt out of the mandate. However, critics argued the process itself was a violation of their religious freedom.
The issue led to the case of Zubik v. Burwell, a legal challenge over the mandate exemption that went before the U.S. Supreme Court in March 2016. The issue was never resolved however, and in May 2016, the Supreme Court vacated the lower court rulings related to Zubik v. Burwell and remanded the case back to the four appeals courts that had originally ruled on the issue.
In 2018, the Trump administration announced new rules aimed at broadening exemptions to the ACA’s contraceptive mandate to entities that object to services covered by the mandate on the basis of “sincerely held religious beliefs.” A second rule allowed nonprofit organizations and small businesses that had nonreligious moral convictions against the mandate to opt out.
Thirteen states and the District of Columbia then sued the Trump administration over the rules, as well as Pennsylvania and New Jersey in a separate case. Little Sisters of the Poor, a religious nonprofit operating a home in Pittsburgh, intervened in the case as an aggrieved party. An appeal court temporarily barred the regulations from moving forward.
During oral arguments, Solicitor General for the Department of Justice Noel J. Francisco said the exemptions are lawful because they are authorized under a provision of the ACA as well as the Religious Freedom Restoration Act (RFRA).
“RFRA at the very least authorizes the religious exemption,” Mr. Francisco said during oral arguments.
Chief Deputy Attorney General for Pennsylvania Michael J. Fischer argued that the Trump administration’s moral and religious exemption rules rest on overly broad assertions of agency authority.
“First, the agencies twist a narrow delegation that allows the Health Resources and Services Administration to decide which preventive services insurers must cover under the Women’s Health Amendment into a grant of authority so broad it allows them to permit virtually any employer or college to opt out of providing contraceptive coverage entirely, including for reasons as amorphous as vaguely defined moral beliefs,” he said during oral arguments. “Second, the agencies claim that RFRA, a statute that limits government action, affirmatively authorizes them to permit employers to deny women their rights to contraceptive coverage even in the absence of a RFRA violation in the first place.”
Triple-antiviral combo speeds COVID-19 recovery
A triple-antiviral therapy regimen of interferon-beta1, lopinavir/ritonavir, and ribavirin shortened median time to COVID-19 viral negativity by 5 days in a small trial from Hong Kong.
In an open-label, randomized phase 2 trial in patients with mild or moderate COVID-19 infections, the median time to viral negativity by nasopharyngeal swab was 7 days for 86 patients assigned to receive a 14-day course of lopinavir 400 mg and ritonavir 100 mg every 12 hours, ribavirin 400 mg every 12 hours, and three doses of 8 million international units of interferon beta-1b on alternate days, compared with a median time to negativity of 12 days for patients treated with lopinavir/ritonavir alone (P = .0010), wrote Ivan Fan-Ngai Hung, MD, from Gleaneagles Hospital in Hong Kong, and colleagues.
“Triple-antiviral therapy with interferon beta-1b, lopinavir/ritonavir, and ribavirin were safe and superior to lopinavir/ritonavir alone in shortening virus shedding, alleviating symptoms, and facilitating discharge of patients with mild to moderate COVID-19,” they wrote in a study published online in The Lancet.
Patients who received the combination also had significantly shorter time to complete alleviation of symptoms as assessed by a National Early Warning Score 2 (NEWS2, a system for detecting clinical deterioration in patients with acute illnesses) score of 0 (4 vs. 8 days, respectively; hazard ratio 3.92, P < .0001), and to a Sequential Organ Failure Assessment (SOFA) score of 0 (3 vs. 8 days, HR 1.89, P = .041).
The median hospital stay was 9 days for patients treated with the combination, compared with 14.5 days for controls (HR 2.72, P = .016).
In most patients treated with the combination, SARS-CoV-2 viral load was effectively suppressed in all clinical specimens, including nasopharyngeal swabs, throat and posterior oropharyngeal saliva, and stool.
In addition, serum levels of interleukin 6 (IL-6) – an inflammatory cytokine implicated in the cytokine storm frequently seen in patients with severe COVID-19 infections – were significantly lower on treatment days 2, 6, and 8 in patients treated with the combination, compared with those treated with lopinavir/ritonavir alone.
“Our trial demonstrates that early treatment of mild to moderate COVID-19 with a triple combination of antiviral drugs may rapidly suppress the amount of virus in a patient’s body, relieve symptoms, and reduce the risk to health care workers by reducing the duration and quantity of viral shedding (when the virus is detectable and potentially transmissible). Furthermore, the treatment combination appeared safe and well tolerated by patients,” said lead investigator Professor Kwok-Yung Yuen from the University of Hong Kong, in a statement.
“Despite these encouraging findings,” he continued, “we must confirm in larger phase 3 trials that interferon beta-1b alone or in combination with other drugs is effective in patients with more severe illness (in whom the virus has had more time to replicate).”
Plausible rationale
Benjamin Medoff, MD, chief of the division of pulmonary and critical care medicine at Massachusetts General Hospital in Boston, who was not involved in the study, said in an interview that the biologic rationale for the combination is plausible.
“I think this is a promising study that suggests that a regimen of interferon beta-1b, lopinavir/ritonavir, and ribavirin can shorten the duration of infection and improve symptoms in COVID-19 patients especially if started early in disease, in less than 7 days of symptom onset,” he said in reply to a request for expert analysis.
“The open-label nature and small size of the study limits the broad use of the regimen as noted by the authors, and it’s important to emphasize that the subjects enrolled did not have very severe disease (not in the ICU). However, the study does suggest that a larger truly randomized study is warranted,” he said.
AIDS drugs repurposed
Lopinavir/ritonavir is commonly used to treat HIV/AIDS throughout the world, and the investigators had previously reported that the antiviral agents combined with ribavirin reduced deaths and the need for intensive ventilator support among patients with SARS-CoV, the betacoronavirus that causes severe acute respiratory syndrome (SARS), and antivirals have shown in vitro activity against both SARS-CoV and MERS-CoV, the closely related pathogen that causes Middle East respiratory syndrome.
“ However the viral load of SARS and MERS peaks at around day 7-10 after symptom onset, whereas the viral load of COVID-19 peaks at the time of presentation, similar to influenza. Experience from the treatment of patients with influenza who are admitted to hospital suggested that a combination of multiple antiviral drugs is more effective than single-drug treatments in this setting of patients with a high viral load at presentation,” the investigators wrote.
To test this, they enrolled adults patients admitted to one of six Hong Kong Hospitals for virologically confirmed COVID-19 infections from Feb. 10 through March 20, 2020.
A total of 86 patients were randomly assigned to the combination and 41 to lopinavir/ritonavir alone as controls, at doses described above.
Patients who entered the trial within less than 7 days of symptom onset received the triple combination, with interferon dosing adjusted according to the day that treatment started. Patients recruited 1 or 2 days after symptom onset received three doses of interferon, patients started on day 3 or 4 received two doses, and those started on days 5 or 6 received one interferon dose. Patients recruited 7 days or later from symptom onset did not receive interferon beta-1b because of its proinflammatory effects.
In post-hoc analysis by day of treatment initiation, clinical and virological outcomes (except stool samples) were superior in patients admitted less than 7 days after symptom onset for the 52 patients who received a least one interferon dose plus lopinavir/ritonavir and ribavirin, compared with 24 patients randomized to the control arm (lopinavir/ritonavir only). In contrast, among patients admitted and started on treatment at day 7 or later after symptom onset, there were no differences between those who received lopinavir/ritonavir alone or combined with ribavirin.
Adverse events were reported in 41 of 86 patients in the combination group and 20 of 41 patients in the control arm. The most common adverse events were diarrhea, occurring in 52 of all 127 patients, fever in 48, nausea in 43, and elevated alanine transaminase level in 18. The side effects generally resolved within 3 days of the start of treatments.
There were no serious adverse events reported in the combination group. One patient in the control group had impaired hepatic enzymes requiring discontinuation of treatment. No patients died during the study.
The study was funded by the Shaw Foundation, Richard and Carol Yu, May Tam Mak Mei Yin, and Sanming Project of Medicine. The authors and Dr. Medoff declared no competing interests.
SOURCE: Hung IFN et al. Lancet. 2020 May 8. doi: 10.1016/S0140-6736(20)31101-6.
A triple-antiviral therapy regimen of interferon-beta1, lopinavir/ritonavir, and ribavirin shortened median time to COVID-19 viral negativity by 5 days in a small trial from Hong Kong.
In an open-label, randomized phase 2 trial in patients with mild or moderate COVID-19 infections, the median time to viral negativity by nasopharyngeal swab was 7 days for 86 patients assigned to receive a 14-day course of lopinavir 400 mg and ritonavir 100 mg every 12 hours, ribavirin 400 mg every 12 hours, and three doses of 8 million international units of interferon beta-1b on alternate days, compared with a median time to negativity of 12 days for patients treated with lopinavir/ritonavir alone (P = .0010), wrote Ivan Fan-Ngai Hung, MD, from Gleaneagles Hospital in Hong Kong, and colleagues.
“Triple-antiviral therapy with interferon beta-1b, lopinavir/ritonavir, and ribavirin were safe and superior to lopinavir/ritonavir alone in shortening virus shedding, alleviating symptoms, and facilitating discharge of patients with mild to moderate COVID-19,” they wrote in a study published online in The Lancet.
Patients who received the combination also had significantly shorter time to complete alleviation of symptoms as assessed by a National Early Warning Score 2 (NEWS2, a system for detecting clinical deterioration in patients with acute illnesses) score of 0 (4 vs. 8 days, respectively; hazard ratio 3.92, P < .0001), and to a Sequential Organ Failure Assessment (SOFA) score of 0 (3 vs. 8 days, HR 1.89, P = .041).
The median hospital stay was 9 days for patients treated with the combination, compared with 14.5 days for controls (HR 2.72, P = .016).
In most patients treated with the combination, SARS-CoV-2 viral load was effectively suppressed in all clinical specimens, including nasopharyngeal swabs, throat and posterior oropharyngeal saliva, and stool.
In addition, serum levels of interleukin 6 (IL-6) – an inflammatory cytokine implicated in the cytokine storm frequently seen in patients with severe COVID-19 infections – were significantly lower on treatment days 2, 6, and 8 in patients treated with the combination, compared with those treated with lopinavir/ritonavir alone.
“Our trial demonstrates that early treatment of mild to moderate COVID-19 with a triple combination of antiviral drugs may rapidly suppress the amount of virus in a patient’s body, relieve symptoms, and reduce the risk to health care workers by reducing the duration and quantity of viral shedding (when the virus is detectable and potentially transmissible). Furthermore, the treatment combination appeared safe and well tolerated by patients,” said lead investigator Professor Kwok-Yung Yuen from the University of Hong Kong, in a statement.
“Despite these encouraging findings,” he continued, “we must confirm in larger phase 3 trials that interferon beta-1b alone or in combination with other drugs is effective in patients with more severe illness (in whom the virus has had more time to replicate).”
Plausible rationale
Benjamin Medoff, MD, chief of the division of pulmonary and critical care medicine at Massachusetts General Hospital in Boston, who was not involved in the study, said in an interview that the biologic rationale for the combination is plausible.
“I think this is a promising study that suggests that a regimen of interferon beta-1b, lopinavir/ritonavir, and ribavirin can shorten the duration of infection and improve symptoms in COVID-19 patients especially if started early in disease, in less than 7 days of symptom onset,” he said in reply to a request for expert analysis.
“The open-label nature and small size of the study limits the broad use of the regimen as noted by the authors, and it’s important to emphasize that the subjects enrolled did not have very severe disease (not in the ICU). However, the study does suggest that a larger truly randomized study is warranted,” he said.
AIDS drugs repurposed
Lopinavir/ritonavir is commonly used to treat HIV/AIDS throughout the world, and the investigators had previously reported that the antiviral agents combined with ribavirin reduced deaths and the need for intensive ventilator support among patients with SARS-CoV, the betacoronavirus that causes severe acute respiratory syndrome (SARS), and antivirals have shown in vitro activity against both SARS-CoV and MERS-CoV, the closely related pathogen that causes Middle East respiratory syndrome.
“ However the viral load of SARS and MERS peaks at around day 7-10 after symptom onset, whereas the viral load of COVID-19 peaks at the time of presentation, similar to influenza. Experience from the treatment of patients with influenza who are admitted to hospital suggested that a combination of multiple antiviral drugs is more effective than single-drug treatments in this setting of patients with a high viral load at presentation,” the investigators wrote.
To test this, they enrolled adults patients admitted to one of six Hong Kong Hospitals for virologically confirmed COVID-19 infections from Feb. 10 through March 20, 2020.
A total of 86 patients were randomly assigned to the combination and 41 to lopinavir/ritonavir alone as controls, at doses described above.
Patients who entered the trial within less than 7 days of symptom onset received the triple combination, with interferon dosing adjusted according to the day that treatment started. Patients recruited 1 or 2 days after symptom onset received three doses of interferon, patients started on day 3 or 4 received two doses, and those started on days 5 or 6 received one interferon dose. Patients recruited 7 days or later from symptom onset did not receive interferon beta-1b because of its proinflammatory effects.
In post-hoc analysis by day of treatment initiation, clinical and virological outcomes (except stool samples) were superior in patients admitted less than 7 days after symptom onset for the 52 patients who received a least one interferon dose plus lopinavir/ritonavir and ribavirin, compared with 24 patients randomized to the control arm (lopinavir/ritonavir only). In contrast, among patients admitted and started on treatment at day 7 or later after symptom onset, there were no differences between those who received lopinavir/ritonavir alone or combined with ribavirin.
Adverse events were reported in 41 of 86 patients in the combination group and 20 of 41 patients in the control arm. The most common adverse events were diarrhea, occurring in 52 of all 127 patients, fever in 48, nausea in 43, and elevated alanine transaminase level in 18. The side effects generally resolved within 3 days of the start of treatments.
There were no serious adverse events reported in the combination group. One patient in the control group had impaired hepatic enzymes requiring discontinuation of treatment. No patients died during the study.
The study was funded by the Shaw Foundation, Richard and Carol Yu, May Tam Mak Mei Yin, and Sanming Project of Medicine. The authors and Dr. Medoff declared no competing interests.
SOURCE: Hung IFN et al. Lancet. 2020 May 8. doi: 10.1016/S0140-6736(20)31101-6.
A triple-antiviral therapy regimen of interferon-beta1, lopinavir/ritonavir, and ribavirin shortened median time to COVID-19 viral negativity by 5 days in a small trial from Hong Kong.
In an open-label, randomized phase 2 trial in patients with mild or moderate COVID-19 infections, the median time to viral negativity by nasopharyngeal swab was 7 days for 86 patients assigned to receive a 14-day course of lopinavir 400 mg and ritonavir 100 mg every 12 hours, ribavirin 400 mg every 12 hours, and three doses of 8 million international units of interferon beta-1b on alternate days, compared with a median time to negativity of 12 days for patients treated with lopinavir/ritonavir alone (P = .0010), wrote Ivan Fan-Ngai Hung, MD, from Gleaneagles Hospital in Hong Kong, and colleagues.
“Triple-antiviral therapy with interferon beta-1b, lopinavir/ritonavir, and ribavirin were safe and superior to lopinavir/ritonavir alone in shortening virus shedding, alleviating symptoms, and facilitating discharge of patients with mild to moderate COVID-19,” they wrote in a study published online in The Lancet.
Patients who received the combination also had significantly shorter time to complete alleviation of symptoms as assessed by a National Early Warning Score 2 (NEWS2, a system for detecting clinical deterioration in patients with acute illnesses) score of 0 (4 vs. 8 days, respectively; hazard ratio 3.92, P < .0001), and to a Sequential Organ Failure Assessment (SOFA) score of 0 (3 vs. 8 days, HR 1.89, P = .041).
The median hospital stay was 9 days for patients treated with the combination, compared with 14.5 days for controls (HR 2.72, P = .016).
In most patients treated with the combination, SARS-CoV-2 viral load was effectively suppressed in all clinical specimens, including nasopharyngeal swabs, throat and posterior oropharyngeal saliva, and stool.
In addition, serum levels of interleukin 6 (IL-6) – an inflammatory cytokine implicated in the cytokine storm frequently seen in patients with severe COVID-19 infections – were significantly lower on treatment days 2, 6, and 8 in patients treated with the combination, compared with those treated with lopinavir/ritonavir alone.
“Our trial demonstrates that early treatment of mild to moderate COVID-19 with a triple combination of antiviral drugs may rapidly suppress the amount of virus in a patient’s body, relieve symptoms, and reduce the risk to health care workers by reducing the duration and quantity of viral shedding (when the virus is detectable and potentially transmissible). Furthermore, the treatment combination appeared safe and well tolerated by patients,” said lead investigator Professor Kwok-Yung Yuen from the University of Hong Kong, in a statement.
“Despite these encouraging findings,” he continued, “we must confirm in larger phase 3 trials that interferon beta-1b alone or in combination with other drugs is effective in patients with more severe illness (in whom the virus has had more time to replicate).”
Plausible rationale
Benjamin Medoff, MD, chief of the division of pulmonary and critical care medicine at Massachusetts General Hospital in Boston, who was not involved in the study, said in an interview that the biologic rationale for the combination is plausible.
“I think this is a promising study that suggests that a regimen of interferon beta-1b, lopinavir/ritonavir, and ribavirin can shorten the duration of infection and improve symptoms in COVID-19 patients especially if started early in disease, in less than 7 days of symptom onset,” he said in reply to a request for expert analysis.
“The open-label nature and small size of the study limits the broad use of the regimen as noted by the authors, and it’s important to emphasize that the subjects enrolled did not have very severe disease (not in the ICU). However, the study does suggest that a larger truly randomized study is warranted,” he said.
AIDS drugs repurposed
Lopinavir/ritonavir is commonly used to treat HIV/AIDS throughout the world, and the investigators had previously reported that the antiviral agents combined with ribavirin reduced deaths and the need for intensive ventilator support among patients with SARS-CoV, the betacoronavirus that causes severe acute respiratory syndrome (SARS), and antivirals have shown in vitro activity against both SARS-CoV and MERS-CoV, the closely related pathogen that causes Middle East respiratory syndrome.
“ However the viral load of SARS and MERS peaks at around day 7-10 after symptom onset, whereas the viral load of COVID-19 peaks at the time of presentation, similar to influenza. Experience from the treatment of patients with influenza who are admitted to hospital suggested that a combination of multiple antiviral drugs is more effective than single-drug treatments in this setting of patients with a high viral load at presentation,” the investigators wrote.
To test this, they enrolled adults patients admitted to one of six Hong Kong Hospitals for virologically confirmed COVID-19 infections from Feb. 10 through March 20, 2020.
A total of 86 patients were randomly assigned to the combination and 41 to lopinavir/ritonavir alone as controls, at doses described above.
Patients who entered the trial within less than 7 days of symptom onset received the triple combination, with interferon dosing adjusted according to the day that treatment started. Patients recruited 1 or 2 days after symptom onset received three doses of interferon, patients started on day 3 or 4 received two doses, and those started on days 5 or 6 received one interferon dose. Patients recruited 7 days or later from symptom onset did not receive interferon beta-1b because of its proinflammatory effects.
In post-hoc analysis by day of treatment initiation, clinical and virological outcomes (except stool samples) were superior in patients admitted less than 7 days after symptom onset for the 52 patients who received a least one interferon dose plus lopinavir/ritonavir and ribavirin, compared with 24 patients randomized to the control arm (lopinavir/ritonavir only). In contrast, among patients admitted and started on treatment at day 7 or later after symptom onset, there were no differences between those who received lopinavir/ritonavir alone or combined with ribavirin.
Adverse events were reported in 41 of 86 patients in the combination group and 20 of 41 patients in the control arm. The most common adverse events were diarrhea, occurring in 52 of all 127 patients, fever in 48, nausea in 43, and elevated alanine transaminase level in 18. The side effects generally resolved within 3 days of the start of treatments.
There were no serious adverse events reported in the combination group. One patient in the control group had impaired hepatic enzymes requiring discontinuation of treatment. No patients died during the study.
The study was funded by the Shaw Foundation, Richard and Carol Yu, May Tam Mak Mei Yin, and Sanming Project of Medicine. The authors and Dr. Medoff declared no competing interests.
SOURCE: Hung IFN et al. Lancet. 2020 May 8. doi: 10.1016/S0140-6736(20)31101-6.
FROM THE LANCET
Sun-damage selfies give kids motivation to protect skin
Photo-manipulated selfies can provide adolescents an influential window into the wrinkled, sun-damaged future that may be theirs if they’re not careful, a new study suggests.
In the study, researchers found that Brazilian teenagers, especially girls, were more likely to protect themselves from the sun if they got glimpses of how sun exposure could damage their faces. “The intervention used in this study was effective in convincing a substantial part of the students to take up regular sunscreen use and to examine their own skin regularly,” they wrote. “Moreover, these effects were maintained for at least half a year.”
The study, led by Titus J. Brinker, MD, of the department of dermatology, in the National Center for Tumor Diseases, German Cancer Research Center in Heidelberg, Germany, appeared online on May 6 in JAMA Dermatology (2020 May 6. doi: 10.1001/jamadermatol.2020.0511.
Dr. Brinker and colleagues launched the study in 2018 at eight public schools that serve grades 9-12 in Itaúna, a city in southeast Brazil, randomly assigning 1,573 students (52% girls, 48% boys; mean age, 16 years) to the intervention or control group.
Those in the intervention group attended seminars in which medical students showed them selfies of their classmates altered with a mobile phone app called Sunface, developed by Dr. Brinker.
The app, which takes the skin types of the subjects into account, was described by the Vice news site as “terrifying” in a 2018 article. It “could very well scare you into using sunscreen and wearing hats,” the author of that article wrote.
The app appeared to do just that – but not universally, according to the new study.
At 6 months, there was no change in sun protection habits in the control group. But among those remaining in the intervention group, the use of daily sunscreen significantly increased from 15% (110 of 734 students) during the 30 days prior to the survey, to 23% (139 of 607 students) at the 6-month follow-up (P less than .001), as did the percentage of those who performed at least one skin self-examination within the 6 months (25% to 49%; P less than .001). The students were slightly less likely to use tanning beds within the previous month (19% to 15%; P = .04); the researchers speculate that it’s easier to gain a new healthy habit than get rid of an old unhealthy one.
Girls were much more likely to change their habits than boys. The number needed to treat to reach the primary endpoint, daily sunscreen use, was 8 for girls and 31 for boys.
The researchers noted that the dropout rate was higher in the intervention group (17%) vs. the control group (6%). “The intervention may have led to strong adverse reactions in some students, leading to the observed higher dropout rate in the intervention group,” they wrote. Changes to the way the app is used could improve the dropout rate, but potentially hurt the intervention’s impact, they added.
In an accompanying editorial in JAMA Dermatology, two health intervention researchers wrote that “this work represents a needed shift toward scalable interventions that bring messaging to target populations using their preferred technology” (2020 May 6. doi: 10.1001/jamadermatol.2020.0510).
Referring to the finding that sunscreen use did not change much among the boys in the study, the authors, Sherry L. Pagoto, PhD, of the Institute for Collaborations on Health, Interventions, and Policy at the University of Connecticut, Storrs, and Alan C. Geller, MPH, RN, of the Harvard TH Chan School of Public Health, Boston, also noted that “teen boys have been largely resistant to traditional and nontraditional forms of sun safety education.”
“Teasing out sex differences is important,” they added, “because sun protection interventions woven into existing programs at pools, beaches, and sporting events might be more appealing and enduring for boys, particularly if the technology they regularly use is leveraged.”
Dr. Brinker disclosed receiving an award from La Fondation la Roche-Posay, which also provided support for the study which partially funded the study, for his research on the Sunface app. The University of Itaúna provided other study funding. Several other study authors had various disclosures. Dr. Pagoto disclosed consulting work and personal fees from Johnson & Johnson, unrelated to the topic of the commentary; Dr. Geller had no disclosures.
SOURCES: Brinker TJ et al. JAMA Dermatol. 2020 May 6. doi: 10.1001/jamadermatol.2020.0511; Pagoto SL and Geller AC. JAMA Dermatol. 2020 May 6. doi: 10.1001/jamadermatol.2020.0510.
Photo-manipulated selfies can provide adolescents an influential window into the wrinkled, sun-damaged future that may be theirs if they’re not careful, a new study suggests.
In the study, researchers found that Brazilian teenagers, especially girls, were more likely to protect themselves from the sun if they got glimpses of how sun exposure could damage their faces. “The intervention used in this study was effective in convincing a substantial part of the students to take up regular sunscreen use and to examine their own skin regularly,” they wrote. “Moreover, these effects were maintained for at least half a year.”
The study, led by Titus J. Brinker, MD, of the department of dermatology, in the National Center for Tumor Diseases, German Cancer Research Center in Heidelberg, Germany, appeared online on May 6 in JAMA Dermatology (2020 May 6. doi: 10.1001/jamadermatol.2020.0511.
Dr. Brinker and colleagues launched the study in 2018 at eight public schools that serve grades 9-12 in Itaúna, a city in southeast Brazil, randomly assigning 1,573 students (52% girls, 48% boys; mean age, 16 years) to the intervention or control group.
Those in the intervention group attended seminars in which medical students showed them selfies of their classmates altered with a mobile phone app called Sunface, developed by Dr. Brinker.
The app, which takes the skin types of the subjects into account, was described by the Vice news site as “terrifying” in a 2018 article. It “could very well scare you into using sunscreen and wearing hats,” the author of that article wrote.
The app appeared to do just that – but not universally, according to the new study.
At 6 months, there was no change in sun protection habits in the control group. But among those remaining in the intervention group, the use of daily sunscreen significantly increased from 15% (110 of 734 students) during the 30 days prior to the survey, to 23% (139 of 607 students) at the 6-month follow-up (P less than .001), as did the percentage of those who performed at least one skin self-examination within the 6 months (25% to 49%; P less than .001). The students were slightly less likely to use tanning beds within the previous month (19% to 15%; P = .04); the researchers speculate that it’s easier to gain a new healthy habit than get rid of an old unhealthy one.
Girls were much more likely to change their habits than boys. The number needed to treat to reach the primary endpoint, daily sunscreen use, was 8 for girls and 31 for boys.
The researchers noted that the dropout rate was higher in the intervention group (17%) vs. the control group (6%). “The intervention may have led to strong adverse reactions in some students, leading to the observed higher dropout rate in the intervention group,” they wrote. Changes to the way the app is used could improve the dropout rate, but potentially hurt the intervention’s impact, they added.
In an accompanying editorial in JAMA Dermatology, two health intervention researchers wrote that “this work represents a needed shift toward scalable interventions that bring messaging to target populations using their preferred technology” (2020 May 6. doi: 10.1001/jamadermatol.2020.0510).
Referring to the finding that sunscreen use did not change much among the boys in the study, the authors, Sherry L. Pagoto, PhD, of the Institute for Collaborations on Health, Interventions, and Policy at the University of Connecticut, Storrs, and Alan C. Geller, MPH, RN, of the Harvard TH Chan School of Public Health, Boston, also noted that “teen boys have been largely resistant to traditional and nontraditional forms of sun safety education.”
“Teasing out sex differences is important,” they added, “because sun protection interventions woven into existing programs at pools, beaches, and sporting events might be more appealing and enduring for boys, particularly if the technology they regularly use is leveraged.”
Dr. Brinker disclosed receiving an award from La Fondation la Roche-Posay, which also provided support for the study which partially funded the study, for his research on the Sunface app. The University of Itaúna provided other study funding. Several other study authors had various disclosures. Dr. Pagoto disclosed consulting work and personal fees from Johnson & Johnson, unrelated to the topic of the commentary; Dr. Geller had no disclosures.
SOURCES: Brinker TJ et al. JAMA Dermatol. 2020 May 6. doi: 10.1001/jamadermatol.2020.0511; Pagoto SL and Geller AC. JAMA Dermatol. 2020 May 6. doi: 10.1001/jamadermatol.2020.0510.
Photo-manipulated selfies can provide adolescents an influential window into the wrinkled, sun-damaged future that may be theirs if they’re not careful, a new study suggests.
In the study, researchers found that Brazilian teenagers, especially girls, were more likely to protect themselves from the sun if they got glimpses of how sun exposure could damage their faces. “The intervention used in this study was effective in convincing a substantial part of the students to take up regular sunscreen use and to examine their own skin regularly,” they wrote. “Moreover, these effects were maintained for at least half a year.”
The study, led by Titus J. Brinker, MD, of the department of dermatology, in the National Center for Tumor Diseases, German Cancer Research Center in Heidelberg, Germany, appeared online on May 6 in JAMA Dermatology (2020 May 6. doi: 10.1001/jamadermatol.2020.0511.
Dr. Brinker and colleagues launched the study in 2018 at eight public schools that serve grades 9-12 in Itaúna, a city in southeast Brazil, randomly assigning 1,573 students (52% girls, 48% boys; mean age, 16 years) to the intervention or control group.
Those in the intervention group attended seminars in which medical students showed them selfies of their classmates altered with a mobile phone app called Sunface, developed by Dr. Brinker.
The app, which takes the skin types of the subjects into account, was described by the Vice news site as “terrifying” in a 2018 article. It “could very well scare you into using sunscreen and wearing hats,” the author of that article wrote.
The app appeared to do just that – but not universally, according to the new study.
At 6 months, there was no change in sun protection habits in the control group. But among those remaining in the intervention group, the use of daily sunscreen significantly increased from 15% (110 of 734 students) during the 30 days prior to the survey, to 23% (139 of 607 students) at the 6-month follow-up (P less than .001), as did the percentage of those who performed at least one skin self-examination within the 6 months (25% to 49%; P less than .001). The students were slightly less likely to use tanning beds within the previous month (19% to 15%; P = .04); the researchers speculate that it’s easier to gain a new healthy habit than get rid of an old unhealthy one.
Girls were much more likely to change their habits than boys. The number needed to treat to reach the primary endpoint, daily sunscreen use, was 8 for girls and 31 for boys.
The researchers noted that the dropout rate was higher in the intervention group (17%) vs. the control group (6%). “The intervention may have led to strong adverse reactions in some students, leading to the observed higher dropout rate in the intervention group,” they wrote. Changes to the way the app is used could improve the dropout rate, but potentially hurt the intervention’s impact, they added.
In an accompanying editorial in JAMA Dermatology, two health intervention researchers wrote that “this work represents a needed shift toward scalable interventions that bring messaging to target populations using their preferred technology” (2020 May 6. doi: 10.1001/jamadermatol.2020.0510).
Referring to the finding that sunscreen use did not change much among the boys in the study, the authors, Sherry L. Pagoto, PhD, of the Institute for Collaborations on Health, Interventions, and Policy at the University of Connecticut, Storrs, and Alan C. Geller, MPH, RN, of the Harvard TH Chan School of Public Health, Boston, also noted that “teen boys have been largely resistant to traditional and nontraditional forms of sun safety education.”
“Teasing out sex differences is important,” they added, “because sun protection interventions woven into existing programs at pools, beaches, and sporting events might be more appealing and enduring for boys, particularly if the technology they regularly use is leveraged.”
Dr. Brinker disclosed receiving an award from La Fondation la Roche-Posay, which also provided support for the study which partially funded the study, for his research on the Sunface app. The University of Itaúna provided other study funding. Several other study authors had various disclosures. Dr. Pagoto disclosed consulting work and personal fees from Johnson & Johnson, unrelated to the topic of the commentary; Dr. Geller had no disclosures.
SOURCES: Brinker TJ et al. JAMA Dermatol. 2020 May 6. doi: 10.1001/jamadermatol.2020.0511; Pagoto SL and Geller AC. JAMA Dermatol. 2020 May 6. doi: 10.1001/jamadermatol.2020.0510.
FROM JAMA DERMATOLOGY
ASCO goes ahead online, as conference center is used as hospital
Traditionally at this time of year, everyone working in cancer turns their attention toward Chicago, and 40,000 or so travel to the city for the annual meeting of the American Society of Clinical Oncology (ASCO).
Not this year.
The McCormick Place convention center has been converted to a field hospital to cope with the ongoing COVID-19 pandemic. The cavernous meeting halls have been filled with makeshift wards with 750 acute care beds, as shown in a tweet from Toni Choueiri, MD, chief of genitourinary oncology at the Dana Farber Cancer Center in Boston.
But the annual meeting is still going ahead, having been transferred online.
“We have to remember that even though there’s a pandemic going on and people are dying every day from coronavirus, people are still dying every day from cancer,” Richard Schilsky, MD, PhD, chief medical officer at ASCO, told Medscape Medical News.
“This pandemic will end, but cancer will continue, and we need to be able to continue to get the most cutting edge scientific results out there to our members and our constituents so they can act on those results on behalf of their patients,” he said.
The ASCO Virtual Scientific Program will take place over the weekend of May 30-31.
“We’re certainly hoping that we’re going to deliver a program that features all of the most important science that would have been presented in person in Chicago,” Schilsky commented in an interview.
Most of the presentations will be prerecorded and then streamed, which “we hope will mitigate any of the technical glitches that could come from trying to do a live broadcast of the meeting,” he said.
There will be 250 oral and 2500 poster presentations in 24 disease-based and specialty tracks.
The majority of the abstracts will be released online on May 13. The majority of the on-demand content will be released on May 29. Some of the abstracts will be highlighted at ASCO press briefings and released on those two dates.
But some of the material will be made available only on the weekend of the meeting. The opening session, plenaries featuring late-breaking abstracts, special highlights sessions, and other clinical science symposia will be broadcast on Saturday, May 30, and Sunday, May 31 (the schedule for the weekend program is available on the ASCO meeting website).
Among the plenary presentations are some clinical results that are likely to change practice immediately, Schilsky predicted. These include data to be presented in the following abstracts:
- Abstract LBA4 on the KEYNOTE-177 study comparing immunotherapy using pembrolizumab (Keytruda, Merck & Co) with chemotherapy in patients with metastatic colorectal cancer whose tumors show microsatellite instability or mismatch repair deficiency;
- Abstract LBA5 on the ADAURA study exploring osimertinib (Tagrisso, AstraZeneca) as adjuvant therapy after complete tumor reseaction in patients with early-stage non–small cell lung cancer whose tumors are EGFR mutation positive;
- Abstract LBA1 on the JAVELIN Bladder 100 study exploring maintenance avelumab (Bavencio, Merck and Pfizer) with best supportive care after platinum-based first-line chemotherapy in patients with advanced urothelial carcinoma.
However, some of the material that would have been part of the annual meeting, which includes mostly educational sessions and invited talks, has been moved to another event, the ASCO Educational Program, to be held in August 2020.
“So I suppose, in the grand scheme of things, the meeting is going to be compressed a little bit,” Schilsky commented. “Obviously, we can’t deliver all the interactions that happen in the hallways and everywhere else at the meeting that really gives so much energy to the meeting, but, at this moment in our history, probably getting the science out there is what’s most important.”
Virtual exhibition hall
There will also be a virtual exhibition hall, which will open on May 29.
“Just as there is a typical exhibit hall in the convention center,” Schilsky commented, most of the companies that were planning to be in Chicago have “now transitioned to creating a virtual booth that people who are participating in the virtual meeting can visit.
“I don’t know exactly how each company is going to use their time and their virtual space, and that’s part of the whole learning process here to see how this whole experiment is going to work out,” he added.
Unlike some of the other conferences that have gone virtual, in which access has been made available to everyone for free, registration is still required for the ASCO meeting. But the society notes that the registration fee has been discounted for nonmembers and has been waived for ASCO members. Also, the fee covers both the Virtual Scientific Program in May and the ASCO Educational Program in August.
Registrants will have access to video and slide presentations, as well as discussant commentaries, for 180 days.
The article first appeared on Medscape.com.
Traditionally at this time of year, everyone working in cancer turns their attention toward Chicago, and 40,000 or so travel to the city for the annual meeting of the American Society of Clinical Oncology (ASCO).
Not this year.
The McCormick Place convention center has been converted to a field hospital to cope with the ongoing COVID-19 pandemic. The cavernous meeting halls have been filled with makeshift wards with 750 acute care beds, as shown in a tweet from Toni Choueiri, MD, chief of genitourinary oncology at the Dana Farber Cancer Center in Boston.
But the annual meeting is still going ahead, having been transferred online.
“We have to remember that even though there’s a pandemic going on and people are dying every day from coronavirus, people are still dying every day from cancer,” Richard Schilsky, MD, PhD, chief medical officer at ASCO, told Medscape Medical News.
“This pandemic will end, but cancer will continue, and we need to be able to continue to get the most cutting edge scientific results out there to our members and our constituents so they can act on those results on behalf of their patients,” he said.
The ASCO Virtual Scientific Program will take place over the weekend of May 30-31.
“We’re certainly hoping that we’re going to deliver a program that features all of the most important science that would have been presented in person in Chicago,” Schilsky commented in an interview.
Most of the presentations will be prerecorded and then streamed, which “we hope will mitigate any of the technical glitches that could come from trying to do a live broadcast of the meeting,” he said.
There will be 250 oral and 2500 poster presentations in 24 disease-based and specialty tracks.
The majority of the abstracts will be released online on May 13. The majority of the on-demand content will be released on May 29. Some of the abstracts will be highlighted at ASCO press briefings and released on those two dates.
But some of the material will be made available only on the weekend of the meeting. The opening session, plenaries featuring late-breaking abstracts, special highlights sessions, and other clinical science symposia will be broadcast on Saturday, May 30, and Sunday, May 31 (the schedule for the weekend program is available on the ASCO meeting website).
Among the plenary presentations are some clinical results that are likely to change practice immediately, Schilsky predicted. These include data to be presented in the following abstracts:
- Abstract LBA4 on the KEYNOTE-177 study comparing immunotherapy using pembrolizumab (Keytruda, Merck & Co) with chemotherapy in patients with metastatic colorectal cancer whose tumors show microsatellite instability or mismatch repair deficiency;
- Abstract LBA5 on the ADAURA study exploring osimertinib (Tagrisso, AstraZeneca) as adjuvant therapy after complete tumor reseaction in patients with early-stage non–small cell lung cancer whose tumors are EGFR mutation positive;
- Abstract LBA1 on the JAVELIN Bladder 100 study exploring maintenance avelumab (Bavencio, Merck and Pfizer) with best supportive care after platinum-based first-line chemotherapy in patients with advanced urothelial carcinoma.
However, some of the material that would have been part of the annual meeting, which includes mostly educational sessions and invited talks, has been moved to another event, the ASCO Educational Program, to be held in August 2020.
“So I suppose, in the grand scheme of things, the meeting is going to be compressed a little bit,” Schilsky commented. “Obviously, we can’t deliver all the interactions that happen in the hallways and everywhere else at the meeting that really gives so much energy to the meeting, but, at this moment in our history, probably getting the science out there is what’s most important.”
Virtual exhibition hall
There will also be a virtual exhibition hall, which will open on May 29.
“Just as there is a typical exhibit hall in the convention center,” Schilsky commented, most of the companies that were planning to be in Chicago have “now transitioned to creating a virtual booth that people who are participating in the virtual meeting can visit.
“I don’t know exactly how each company is going to use their time and their virtual space, and that’s part of the whole learning process here to see how this whole experiment is going to work out,” he added.
Unlike some of the other conferences that have gone virtual, in which access has been made available to everyone for free, registration is still required for the ASCO meeting. But the society notes that the registration fee has been discounted for nonmembers and has been waived for ASCO members. Also, the fee covers both the Virtual Scientific Program in May and the ASCO Educational Program in August.
Registrants will have access to video and slide presentations, as well as discussant commentaries, for 180 days.
The article first appeared on Medscape.com.
Traditionally at this time of year, everyone working in cancer turns their attention toward Chicago, and 40,000 or so travel to the city for the annual meeting of the American Society of Clinical Oncology (ASCO).
Not this year.
The McCormick Place convention center has been converted to a field hospital to cope with the ongoing COVID-19 pandemic. The cavernous meeting halls have been filled with makeshift wards with 750 acute care beds, as shown in a tweet from Toni Choueiri, MD, chief of genitourinary oncology at the Dana Farber Cancer Center in Boston.
But the annual meeting is still going ahead, having been transferred online.
“We have to remember that even though there’s a pandemic going on and people are dying every day from coronavirus, people are still dying every day from cancer,” Richard Schilsky, MD, PhD, chief medical officer at ASCO, told Medscape Medical News.
“This pandemic will end, but cancer will continue, and we need to be able to continue to get the most cutting edge scientific results out there to our members and our constituents so they can act on those results on behalf of their patients,” he said.
The ASCO Virtual Scientific Program will take place over the weekend of May 30-31.
“We’re certainly hoping that we’re going to deliver a program that features all of the most important science that would have been presented in person in Chicago,” Schilsky commented in an interview.
Most of the presentations will be prerecorded and then streamed, which “we hope will mitigate any of the technical glitches that could come from trying to do a live broadcast of the meeting,” he said.
There will be 250 oral and 2500 poster presentations in 24 disease-based and specialty tracks.
The majority of the abstracts will be released online on May 13. The majority of the on-demand content will be released on May 29. Some of the abstracts will be highlighted at ASCO press briefings and released on those two dates.
But some of the material will be made available only on the weekend of the meeting. The opening session, plenaries featuring late-breaking abstracts, special highlights sessions, and other clinical science symposia will be broadcast on Saturday, May 30, and Sunday, May 31 (the schedule for the weekend program is available on the ASCO meeting website).
Among the plenary presentations are some clinical results that are likely to change practice immediately, Schilsky predicted. These include data to be presented in the following abstracts:
- Abstract LBA4 on the KEYNOTE-177 study comparing immunotherapy using pembrolizumab (Keytruda, Merck & Co) with chemotherapy in patients with metastatic colorectal cancer whose tumors show microsatellite instability or mismatch repair deficiency;
- Abstract LBA5 on the ADAURA study exploring osimertinib (Tagrisso, AstraZeneca) as adjuvant therapy after complete tumor reseaction in patients with early-stage non–small cell lung cancer whose tumors are EGFR mutation positive;
- Abstract LBA1 on the JAVELIN Bladder 100 study exploring maintenance avelumab (Bavencio, Merck and Pfizer) with best supportive care after platinum-based first-line chemotherapy in patients with advanced urothelial carcinoma.
However, some of the material that would have been part of the annual meeting, which includes mostly educational sessions and invited talks, has been moved to another event, the ASCO Educational Program, to be held in August 2020.
“So I suppose, in the grand scheme of things, the meeting is going to be compressed a little bit,” Schilsky commented. “Obviously, we can’t deliver all the interactions that happen in the hallways and everywhere else at the meeting that really gives so much energy to the meeting, but, at this moment in our history, probably getting the science out there is what’s most important.”
Virtual exhibition hall
There will also be a virtual exhibition hall, which will open on May 29.
“Just as there is a typical exhibit hall in the convention center,” Schilsky commented, most of the companies that were planning to be in Chicago have “now transitioned to creating a virtual booth that people who are participating in the virtual meeting can visit.
“I don’t know exactly how each company is going to use their time and their virtual space, and that’s part of the whole learning process here to see how this whole experiment is going to work out,” he added.
Unlike some of the other conferences that have gone virtual, in which access has been made available to everyone for free, registration is still required for the ASCO meeting. But the society notes that the registration fee has been discounted for nonmembers and has been waived for ASCO members. Also, the fee covers both the Virtual Scientific Program in May and the ASCO Educational Program in August.
Registrants will have access to video and slide presentations, as well as discussant commentaries, for 180 days.
The article first appeared on Medscape.com.
How to minimize the pain of local anesthetic administration
In-office procedures are increasingly emphasized as a way to reduce referrals, avoid treatment delay, and increase practice revenue. Local analgesia is administered before many in-office procedures such as biopsies, toenail removal, and laceration repair. Skin procedures are performed most commonly; nearly three-quarters (74%) of family physicians (FPs) provided these services in 2018.1 Administration of local anesthetic is often the most feared and uncomfortable step in the entire process.2
Knowledge of strategies to reduce pain associated with anesthetic administration can make a huge difference in the patient experience. This article explores evidence-based techniques for administering a local anesthetic with minimal patient discomfort.
4 factors influence the painof local anesthetic administration
Pain is perceived during the administration of local anesthetic because of the insertion of the needle and the increased pressure from the injection of fluid. The needle causes sharp, pricking “first pain” via large diameter, myelinated A-delta fibers, and the fluid induces unmyelinated C-fiber activation via tissue distention resulting in dull, diffuse “second pain.”
Four factors influence the experience of pain during administration of local anesthetic: the pharmacologic properties of the anesthetic itself, the equipment used, the environment, and the injection technique. Optimizing all 4 factors limits patient discomfort.
Pharmacologic agents: Lidocaine is often the agent of choice
Local anesthetics differ in maximal dosing, onset of action, and duration of effect (TABLE3). Given its ubiquity in clinics and hospitals, 1% lidocaine is often the agent of choice. Onset of effect occurs within minutes and lasts up to 2 hours. Alternative agents, such as bupivacaine or ropivacaine, may be considered to prolong the anesthetic effect; however, limited evidence exists to support their use in office-based procedures. Additionally, bupivacaine and ropivacaine may be associated with greater pain on injection and parasthesias lasting longer than the duration of pain control.4-6 In practice, maximal dosing is most important in the pediatric population, given the smaller size of the patients and their increased susceptibility to toxicity.
Calculating the maximum recommended dose. To calculate the maximum recommended dose of local anesthetic, you need to know the concentration of the anesthetic, the maximum allowable dose (mg/kg), and the weight of the patient.7,8 The concentration of the local anesthetic is converted from percentage to weight per unit volume (eg, 1% = 10 mg/mL; 0.5% = 5 mg/mL). Multiply the patient's weight (kg) by the maximum dose of local anesthetic (mg/kg) and divide by the concentration of the local anesthetic (mg/mL) to get the maximum recommended dose in milliliters. Walsh et al9 described a simplified formula to calculate the maximum allowable volume of local anesthetics in milliliters:
(maximum allowable dose in mg/kg) × (weight in kg) × (1 divided by the concentration of anesthetic).
For delivery of lidocaine with epinephrine in a 50-lb (22.7-kg) child, the calculation would be (7 mg/kg) × (22.7 kg) × (1 divided by 10 mg/mL) = 15.9 mL.
Continue to: The advantages (and misconceptions) of epinephrine
The advantages (and misconceptions) of epinephrine
The advantage of adding epinephrine is that it prolongs the effect of the anesthesia and it decreases bleeding. Epinephrine is commonly available as a premixed solution with lidocaine or bupivacaine at a concentration of 1:100,000 and is generally differentiated from “plain” local anesthetic by a red label and cap. Although maximum vasoconstriction may occur as long as 30 minutes after injection,10 adequate vasoconstriction is achieved in 7 to 10 minutes for excision of skin lesions.11
Traditional teaching recommends against using epinephrine in the “fingers, toes, penis, ears, or nose” because of potential arterial spasm, ischemia, and gangrene distal to the injection site.12 These concerns were based on experiences with procaine and cocaine mixed with epinephrine. Studies suffered from multiple confounders, including tourniquets and nonstandardized epinephrine concentrations.13-15
No association of distal ischemia with epinephrine use was identified in a recent Cochrane Review or in another multicenter prospective study.16,17 Phentolamine, a non-selective alpha-adrenergic receptor antagonist and vasodilator, can be administered to reverse vasoconstriction following inadvertent administration of high-dose epinephrine (1:1000) via anaphylaxis autoinjector kits.
Dosing of phentolamine is 1 mL of 1 mg/mL solution delivered subcutaneously to the affected area; reversal decreases the duration of vasoconstriction from 320 minutes to approximately 85 minutes.18 As always, when applying literature to clinical practice, one must keep in mind the risks and benefits of any intervention. As such, in patients with pre-existing vascular disease, vaso-occlusive or vasospastic disease, or compromised perfusion due to trauma, one must weigh the benefits of the hemostatic effect against potential ischemia of already susceptible tissues. In such instances, omitting epinephrine from the solution is reasonable.
The benefits of sodium bicarbonate
The acidity of the solution contributes to the level of pain associated with administration of local anesthesia. Previously opened containers become more acidic.19 Addition of 8.4% sodium bicarbonate, at a ratio of 1 mL per 10 mL of 1% lidocaine with 1:100,000 epinephrine, neutralizes the pH to 7.4.19 A Cochrane Review showed that correction of pH to physiologic levels results in a significant reduction in pain.20
Continue to: This solution can be...
This solution can be easily prepared, as standard syringes hold an additional milliliter (ie, 10-mL syringes hold 11 mL) and, thus, can accommodate the additional volume of bicarbonate.21
Warming the solution helps, too
Warming the solution to body temperature prior to injection decreases pain on injection.22 This may be done in a variety of ways depending on available in-office equipment. Water baths, incubators, fluid warmers, heating pads, or specific syringe warmers may be used. Multiple studies have shown improvement in patient satisfaction with warming.23 Moreover, warming and buffering solution provide a synergistic effect on pain reduction.23
Equipment: Size matters
Smaller diameter needles. Reducing the outer diameter of the needle used for injection improves pain by reducing activation of nociceptors.24-26 Reduced inner diameter restricts injection speed, which further reduces pain.25 We recommend 27- to 30-gauge needles for subcutaneous injection and 25- to 27-gauge needles for intra-articular or tendon sheath injections.
Appropriate syringe size. Filling a syringe to capacity results in maximal deployment of the plunger. This requires greater handspan, which can lead to fatigue and loss of control during injection.26,27 Using a syringe filled to approximately half its capacity results in improved dexterity. We recommend 10-mL syringes with 5 mL to 6 mL of local anesthetic for small procedures and 20-mL syringes filled with 10 mL to 12 mL for larger procedures.
Topical local anesthetics may be used either as an adjunct to decrease pain during injection or as the primary anesthetic.28 A variety of agents are available for clinical use, including eutectic mixture of local anesthetics (EMLA), lidocaine-epinephrine-tetracaine (LET), lidocaine, benzocaine, and tetracaine. FPs should be familiar with their different pharmacokinetic profiles.
Continue to: EMLA is a mixture of...
EMLA is a mixture of 25 mg/mL of lidocaine and 25 mg/mL of prilocaine. It is indicated for topical anesthesia on intact, nonmucosal, uninjured skin (maximal dose 20 g/200 cm2 of surface area). It is applied in a thick layer and covered with an occlusive dressing (eg, Tegaderm) to enhance dermal penetration. The depth of penetration increases with application time and may reach a maximum depth of 3 mm and 5 mm following 60-minute and 120-minute application times, respectively.28 Duration of effect is 60 to 120 minutes.
LET, which is a mixture of 4% lidocaine, 0.1% epinephrine, and 0.5% tetracaine, may be used on nonintact, nonmucosal surfaces. Typically, 1 mL to 5 mL of gel is applied directly to the target area and is followed by application of direct pressure for 15 to 30 minutes. LET is not effective on intact skin and is contraindicated in children < 2 years of age.28
Cooling sprays or ice. Topical skin refrigerants, or vapocoolants (eg, ethyl chloride spray), offer an option for short-term local anesthesia that is noninvasive and quick acting. Ethyl chloride is a gaseous substance that extracts heat as it evaporates from the skin, resulting in a transient local conduction block. Skin refrigerants are an option to consider for short procedures such as intra-articular injections, venipuncture, or skin tag excision, or as an adjunct prior to local anesthetic delivery.29-32 Research has shown that topical ethyl chloride spray also possesses antiseptic properties.29,33
Environment: Make a few simple changes
Direct observation of needle penetration is associated with increased pain; advising patients to avert their gaze will mitigate the perception of pain.34 Additionally, research has shown that creating a low-anxiety environment improves patient-reported outcomes in both children and adults.35 Music or audiovisual or multimedia aids, for example, decrease pain and anxiety, particularly among children, and can be readily accessed with smart devices.36-39
We also recommend avoiding terms such as “pinch,” “bee sting,” or “stick” in order to reduce patient anxiety. Instead, we use language such as, “This is the medicine that will numb the area so you will be comfortable during the procedure.”40
Continue to: Injection technique
Injection technique: Consider these helpful tips
Site of needle entry. Prior to injecting local anesthesia, assess the area where the procedure is planned (FIGURE 1). The initial injection site should be proximal along the path of innervation. If regional nerves are anesthetized proximally and infiltration of local anesthesia proceeds distally, the initial puncture will be painful; however, further injections will be through anesthetized skin. Additionally, consider and avoid regional vascular anatomy.41,42
Counter-stimulation. Applying firm pressure, massaging, or stroking the site prior to or during the injection decreases pain.43,44 This technique may be performed by firmly pinching the area of planned injection between the thumb and index fingers, inserting the needle into the pinched skin, and maintaining pressure on the area until the anesthetic effect is achieved.
Angle of needle insertion. Perpendicular entry of the needle into the skin appears to reduce injection site pain (FIGURE 1). Anecdotal reports are supported by a randomized, controlled crossover trial that demonstrated significantly reduced pain with perpendicular injection compared to delivery at 45°.45
Depth of injection. Subcutaneous needle placement is associated with significantly less pain than injection into superficial dermis.2,46 Dermal wheals cause distention of the dermis, increased intradermal pressure, and greater activation of pain afferents in comparison to injection in the subcutaneous space.46 One important exception is the shave biopsy in which dermal distention is, in fact, desirable to ensure adequate specimen collection.
Other methods of pain reduction should still be employed. In the setting of traumatic wounds when a laceration is present, injection into the subcutaneous fat through the wound is easy and associated with less pain than injection through intact skin.47
Continue to: Speed of injection
Speed of injection. Rapid injection of anesthesia is associated with worse injection site pain and decreased patient satisfaction.48-50 Slowing the rate of injection causes less rapid distention of the dermis and subcutaneous space, resulting in decreased pain afferent activation and increased time for nerve blockade. Its importance is underscored by a prospective, randomized trial that compared rate of administration with buffering of local anesthetics and demonstrated that slow administration impacted patient-perceived pain more than buffering solution.51
Needle stabilization. Following perpendicular entry of the needle into the area of planned infiltration, deliver 0.5 mL of local anesthetic into the subcutaneous space without movement of the needle tip.52 With a stabilized needle tip, pain associated with initial needle entry is no longer perceived within 15 to 30 seconds.
It is paramount to stabilize both the syringe and the area of infiltration to prevent patient movement from causing iatrogenic injury or the need for multiple needlesticks. This can be accomplished by maintaining the dominant hand in a position to inject (ie, thumb on the plunger).
Needle reinsertion. Once subcutaneous swelling of local anesthesia is obtained, the needle may be slowly advanced, maintaining a palpable subcutaneous wavefront of local anesthesia ahead of the needle tip as it moves proximally to distally.2,52 Any reinsertion of the needle should be through previously anesthetized skin; this blockade is assessed by the presence of palpable tumescence and blanching (from the epinephrine effect).53
An example of the application of these injection pearls is demonstrated in the administration of a digital nerve block in FIGURE 2.54,55 With the use of the techniques outlined here, the patient ideally experiences only the initial needle entry and is comfortable for the remainder of the procedure.
CORRESPONDENCE
Katharine C. DeGeorge, MD, MS, Department of Family Medicine, University of Virginia, 1215 Lee Street, Charlottesville, VA, 22903; kd6fp@viginia.edu.
1. American Academy of Family Physicians. Family Medicine Facts. 2018. www.aafp.org/about/the-aafp/family-medicine-specialty/facts/table-12(rev).html. Accessed April 27, 2020.
2. Strazar AR, Leynes PG, Lalonde DH. Minimizing the pain of local anesthesia injection. Plast Reconstr Surg. 2013;132:675-684.
3. Kouba DJ, LoPiccolo MC, Alam M, et al. Guidelines for the use of local anesthesia in office-based dermatologic surgery. J Am Acad Dermatol. 2016;74:1201-1219.
4. Vinycomb TI, Sahhar LJ. Comparison of local anesthetics for digital nerve blocks: a systematic review. J Hand Surg Am. 2014;39:744-751.e5.
5. Valvano MN, Leffler S. Comparison of bupivacaine and lidocaine/bupivacaine for local anesthesia/digital nerve block. Ann Emerg Med. 1996;27:490-492.
6. Spivey WH, McNamara RM, MacKenzie RS, et al. A clinical comparison of lidocaine and bupivacaine. Ann Emerg Med. 1987;16:752-757.
7. Neal JM, Mulroy MF, Weinberg GL, American Society of Regional Anesthesia and Pain Medicine. American Society of Regional Anesthesia and Pain Medicine checklist for managing local anesthetic systemic toxicity. Reg Anesth Pain Med. 2012;37:16-18.
8. Neal JM, Bernards CM, Butterworth JF, et al. ASRA practice advisory on local anesthetic systemic toxicity. Reg Anesth Pain Med. 2010;35:152-161.
9. Walsh K, Arya R. A simple formula for quick and accurate calculation of maximum allowable volume of local anaesthetic agents. Br J Dermatol. 2015;172:825-826.
10. McKee DE, Lalonde DH, Thoma A, et al. Optimal time delay between epinephrine injection and incision to minimize bleeding. Plast Reconstr Surg. 2013;131:811-814.
11. Hult J, Sheikh R, Nguyen CD, et al. A waiting time of 7 min is sufficient to reduce bleeding in oculoplastic surgery following the administration of epinephrine together with local anaesthesia. Acta Ophthalmol. 2018;96:499-502.
12. McKee DE, Lalonde DH, Thoma A, et al. Achieving the optimal epinephrine effect in wide awake hand surgery using local anesthesia without a tourniquet. Hand (NY). 2015;10:613-615.
13. Krunic AL, Wang LC, Soltani K, et al. Digital anesthesia with epinephrine: an old myth revisited. J Am Acad Dermatol. 2004;51:755-759.
14. Thomson CJ, Lalonde DH, Denkler KA, et al. A critical look at the evidence for and against elective epinephrine use in the finger. Plast Reconstr Surg. 2007;119:260-266.
15. Lalonde DH, Lalonde JF. Discussion. Do not use epinephrine in digital blocks: myth or truth? Part II. A retrospective review of 1111 cases. Plast Reconstr Surg. 2010;126:2035-2036.
16. Prabhakar H, Rath S, Kalaivani M, et al. Adrenaline with lidocaine for digital nerve blocks. Cochrane Database Syst Rev. 2015;(3):CD010645.
17. Lalonde D, Bell M, Benoit P, et al. A multicenter prospective study of 3,110 consecutive cases of elective epinephrine use in the fingers and hand: the Dalhousie Project clinical phase. J Hand Surg Am. 2005;30:1061-1067.
18. Nodwell T, Lalonde D. How long does it take phentolamine to reverse adrenaline-induced vasoconstriction in the finger and hand? A prospective, randomized, blinded study: the Dalhousie Project experimental phase. Can J Plast Surg. 2003;11:187-190.
19. Frank SG, Lalonde DH. How acidic is the lidocaine we are injecting, and how much bicarbonate should we add? Can J Plast Surg. 2012;20:71-73.
20. Cepeda MS, Tzortzopoulou A, Thackrey M, et al. Cochrane Review: adjusting the pH of lidocaine for reducing pain on injection. Evidence-Based Child Heal. 2012;7:149-215.
21. Barros MFFH, da Rocha Luz Júnior A, Roncaglio B, et al. Evaluation of surgical treatment of carpal tunnel syndrome using local anesthesia. Rev Bras Ortop. 2016;51:36-39.
22. Hogan M-E, vanderVaart S, Perampaladas K, et al. Systematic review and meta-analysis of the effect of warming local anesthetics on injection pain. Ann Emerg Med. 2011;58:86-98.e1.
23. Colaric KB, Overton DT, Moore K. Pain reduction in lidocaine administration through buffering and warming. Am J Emerg Med. 1998;16:353-356.
24. Arendt-Nielsen L, Egekvist H, Bjerring P. Pain following controlled cutaneous insertion of needles with different diameters. Somatosens Mot Res. 2006;23:37-43.
25. Edlich RF, Smith JF, Mayer NE, et al. Performance of disposable needle syringe systems for local anesthesia. J Emerg Med. 1987;5:83-90.
26. Reed KL, Malamed SF, Fonner AM. Local anesthesia Part 2: technical considerations. Anesth Prog. 2012;59:127-137.
27. Elliott TG. Tips for a better local anaesthetic. Australas J Dermatol. 1998;39:50-51.
28. Kumar M, Chawla R, Goyal M. Topical anesthesia. J Anaesthesiol Clin Pharmacol. 2015;31:450.
29. Polishchuk D, Gehrmann R, Tan V. Skin sterility after application of ethyl chloride spray. J Bone Joint Surg Am. 2012;94:118-120.
30. Franko OI, Stern PJ. Use and effectiveness of ethyl chloride for hand injections. J Hand Surg Am. 2017;42:175-181.e1.
31. Fossum K, Love SL, April MD. Topical ethyl chloride to reduce pain associated with venous catheterization: a randomized crossover trial. Am J Emerg Med. 2016;34:845-850.
32. Görgülü T, Torun M, Güler R, et al. Fast and painless skin tag excision with ethyl chloride. Aesthetic Plast Surg. 2015;39:644-645.
33. Azar FM, Lake JE, Grace SP, et al. Ethyl chloride improves antiseptic effect of betadine skin preparation for office procedures. J Surg Orthop Adv. 2012;21:84-87.
34. Oliveira NCAC, Santos JLF, Linhares MBM. Audiovisual distraction for pain relief in paediatric inpatients: a crossover study. Eur J Pain. 2017;21:178-187.
35. Pillai Riddell RR, Racine NM, Gennis HG, et al. Non-pharmacological management of infant and young child procedural pain. Cochrane Database Syst Rev. 2015;(12):CD006275.
36. Attar RH, Baghdadi ZD. Comparative efficacy of active and passive distraction during restorative treatment in children using an iPad versus audiovisual eyeglasses: a randomised controlled trial. Eur Arch Paediatr Dent. 2015;16:1-8.
37. Uman LS, Birnie KA, Noel M, et al. Psychological interventions for needle-related procedural pain and distress in children and adolescents. Cochrane Database Syst Rev. 2013;(10):CD005179.
38. Ahmad Z, Chawla R, Jaffe W. A novel distraction technique to facilitate daycase paediatric surgery under local anaesthesia. J Plast Reconstr Aesthetic Surg. 2012;65:e21-e22.
39. Hartling L, Newton AS, Liang Y, et al. Music to reduce pain and distress in the pediatric emergency department. JAMA Pediatr. 2013;167:826.
40. Varelmann D, Pancaro C, Cappiello EC, et al. Nocebo-induced hyperalgesia during local anesthetic injection. Anesth Analg. 2010;110:868-870.
41. Nelson TW. Accidental intravascular injection of local anesthetic? Anesthesiology. 2008;109:1143-1144.
42. Taghavi Zenouz A, Ebrahimi H, Mahdipour M, et al. The incidence of intravascular needle entrance during inferior alveolar nerve block injection. J Dent Res Dent Clin Dent Prospects. 2008;2:38-41.
43. Taddio A, Ilersich AL, Ipp M, et al; HELPinKIDS Team. Physical interventions and injection techniques for reducing injection pain during routine childhood immunizations: systematic review of randomized controlled trials and quasi-randomized controlled trials. Clin Ther. 2009;31:S48-S76.
44. Aminabadi NA, Farahani RMZ, Balayi Gajan E. The efficacy of distraction and counterstimulation in the reduction of pain reaction to intraoral injection by pediatric patients. J Contemp Dent Pract. 2008;9:33-40.
45. Martires KJ, Malbasa CL, Bordeaux JS. A randomized controlled crossover trial: lidocaine injected at a 90-degree angle causes less pain than lidocaine injected at a 45-degree angle. J Am Acad Dermatol. 2011;65:1231-1233.
46. Zilinsky I, Bar-Meir E, Zaslansky R, et al. Ten commandments for minimal pain during administration of local anesthetics. J Drugs Dermatol. 2005;4:212-216.
47. Bartfield JM, Sokaris SJ, Raccio-Robak N. Local anesthesia for lacerations: pain of infiltration inside vs outside the wound. Acad Emerg Med. 1998;5:100-104.
48. Scarfone RJ, Jasani M, Gracely EJ. Pain of local anesthetics: rate of administration and buffering. Ann Emerg Med. 1998;31:36-40.
49. Kattan AE, Al-Shomer F, Al-Jerian A, et al. Pain on administration of non-alkalinised lidocaine for carpal tunnel decompression: a comparison between the Gale and the “advancing wheal” techniques. J Plast Surg Hand Surg. 2016;50:10-14.
50. Tangen LF, Lundbom JS, Skarsvåg TI, et al. The influence of injection speed on pain during injection of local anaesthetic. J Plast Surg Hand Surg. 2016;50:7-9.
51. McGlone R, Bodenham A. Reducing the pain of intradermal lignocaine injection by pH buffering. Arch Emerg Med. 1990;7:65-68.
52. Lalonde D, Wong A. Local anesthetics. Plast Reconstr Surg. 2014;134(4 Suppl 2):40S-49S.
53. Klein JA. Tumescent technique for regional anesthesia permits lidocaine doses of 35 mg/kg for liposuction. J Dermatol Surg Oncol. 1990;16:248-263.
54. Williams JG, Lalonde DH. Randomized comparison of the single-injection volar subcutaneous block and the two-injection dorsal block for digital anesthesia. Plast Reconstr Surg. 2006;118:1195-1200.
55. Thomson CJ, Lalonde DH. Randomized double-blind comparison of duration of anesthesia among three commonly used agents in digital nerve block. Plast Reconstr Surg. 2006;118:429-432.
In-office procedures are increasingly emphasized as a way to reduce referrals, avoid treatment delay, and increase practice revenue. Local analgesia is administered before many in-office procedures such as biopsies, toenail removal, and laceration repair. Skin procedures are performed most commonly; nearly three-quarters (74%) of family physicians (FPs) provided these services in 2018.1 Administration of local anesthetic is often the most feared and uncomfortable step in the entire process.2
Knowledge of strategies to reduce pain associated with anesthetic administration can make a huge difference in the patient experience. This article explores evidence-based techniques for administering a local anesthetic with minimal patient discomfort.
4 factors influence the painof local anesthetic administration
Pain is perceived during the administration of local anesthetic because of the insertion of the needle and the increased pressure from the injection of fluid. The needle causes sharp, pricking “first pain” via large diameter, myelinated A-delta fibers, and the fluid induces unmyelinated C-fiber activation via tissue distention resulting in dull, diffuse “second pain.”
Four factors influence the experience of pain during administration of local anesthetic: the pharmacologic properties of the anesthetic itself, the equipment used, the environment, and the injection technique. Optimizing all 4 factors limits patient discomfort.
Pharmacologic agents: Lidocaine is often the agent of choice
Local anesthetics differ in maximal dosing, onset of action, and duration of effect (TABLE3). Given its ubiquity in clinics and hospitals, 1% lidocaine is often the agent of choice. Onset of effect occurs within minutes and lasts up to 2 hours. Alternative agents, such as bupivacaine or ropivacaine, may be considered to prolong the anesthetic effect; however, limited evidence exists to support their use in office-based procedures. Additionally, bupivacaine and ropivacaine may be associated with greater pain on injection and parasthesias lasting longer than the duration of pain control.4-6 In practice, maximal dosing is most important in the pediatric population, given the smaller size of the patients and their increased susceptibility to toxicity.
Calculating the maximum recommended dose. To calculate the maximum recommended dose of local anesthetic, you need to know the concentration of the anesthetic, the maximum allowable dose (mg/kg), and the weight of the patient.7,8 The concentration of the local anesthetic is converted from percentage to weight per unit volume (eg, 1% = 10 mg/mL; 0.5% = 5 mg/mL). Multiply the patient's weight (kg) by the maximum dose of local anesthetic (mg/kg) and divide by the concentration of the local anesthetic (mg/mL) to get the maximum recommended dose in milliliters. Walsh et al9 described a simplified formula to calculate the maximum allowable volume of local anesthetics in milliliters:
(maximum allowable dose in mg/kg) × (weight in kg) × (1 divided by the concentration of anesthetic).
For delivery of lidocaine with epinephrine in a 50-lb (22.7-kg) child, the calculation would be (7 mg/kg) × (22.7 kg) × (1 divided by 10 mg/mL) = 15.9 mL.
Continue to: The advantages (and misconceptions) of epinephrine
The advantages (and misconceptions) of epinephrine
The advantage of adding epinephrine is that it prolongs the effect of the anesthesia and it decreases bleeding. Epinephrine is commonly available as a premixed solution with lidocaine or bupivacaine at a concentration of 1:100,000 and is generally differentiated from “plain” local anesthetic by a red label and cap. Although maximum vasoconstriction may occur as long as 30 minutes after injection,10 adequate vasoconstriction is achieved in 7 to 10 minutes for excision of skin lesions.11
Traditional teaching recommends against using epinephrine in the “fingers, toes, penis, ears, or nose” because of potential arterial spasm, ischemia, and gangrene distal to the injection site.12 These concerns were based on experiences with procaine and cocaine mixed with epinephrine. Studies suffered from multiple confounders, including tourniquets and nonstandardized epinephrine concentrations.13-15
No association of distal ischemia with epinephrine use was identified in a recent Cochrane Review or in another multicenter prospective study.16,17 Phentolamine, a non-selective alpha-adrenergic receptor antagonist and vasodilator, can be administered to reverse vasoconstriction following inadvertent administration of high-dose epinephrine (1:1000) via anaphylaxis autoinjector kits.
Dosing of phentolamine is 1 mL of 1 mg/mL solution delivered subcutaneously to the affected area; reversal decreases the duration of vasoconstriction from 320 minutes to approximately 85 minutes.18 As always, when applying literature to clinical practice, one must keep in mind the risks and benefits of any intervention. As such, in patients with pre-existing vascular disease, vaso-occlusive or vasospastic disease, or compromised perfusion due to trauma, one must weigh the benefits of the hemostatic effect against potential ischemia of already susceptible tissues. In such instances, omitting epinephrine from the solution is reasonable.
The benefits of sodium bicarbonate
The acidity of the solution contributes to the level of pain associated with administration of local anesthesia. Previously opened containers become more acidic.19 Addition of 8.4% sodium bicarbonate, at a ratio of 1 mL per 10 mL of 1% lidocaine with 1:100,000 epinephrine, neutralizes the pH to 7.4.19 A Cochrane Review showed that correction of pH to physiologic levels results in a significant reduction in pain.20
Continue to: This solution can be...
This solution can be easily prepared, as standard syringes hold an additional milliliter (ie, 10-mL syringes hold 11 mL) and, thus, can accommodate the additional volume of bicarbonate.21
Warming the solution helps, too
Warming the solution to body temperature prior to injection decreases pain on injection.22 This may be done in a variety of ways depending on available in-office equipment. Water baths, incubators, fluid warmers, heating pads, or specific syringe warmers may be used. Multiple studies have shown improvement in patient satisfaction with warming.23 Moreover, warming and buffering solution provide a synergistic effect on pain reduction.23
Equipment: Size matters
Smaller diameter needles. Reducing the outer diameter of the needle used for injection improves pain by reducing activation of nociceptors.24-26 Reduced inner diameter restricts injection speed, which further reduces pain.25 We recommend 27- to 30-gauge needles for subcutaneous injection and 25- to 27-gauge needles for intra-articular or tendon sheath injections.
Appropriate syringe size. Filling a syringe to capacity results in maximal deployment of the plunger. This requires greater handspan, which can lead to fatigue and loss of control during injection.26,27 Using a syringe filled to approximately half its capacity results in improved dexterity. We recommend 10-mL syringes with 5 mL to 6 mL of local anesthetic for small procedures and 20-mL syringes filled with 10 mL to 12 mL for larger procedures.
Topical local anesthetics may be used either as an adjunct to decrease pain during injection or as the primary anesthetic.28 A variety of agents are available for clinical use, including eutectic mixture of local anesthetics (EMLA), lidocaine-epinephrine-tetracaine (LET), lidocaine, benzocaine, and tetracaine. FPs should be familiar with their different pharmacokinetic profiles.
Continue to: EMLA is a mixture of...
EMLA is a mixture of 25 mg/mL of lidocaine and 25 mg/mL of prilocaine. It is indicated for topical anesthesia on intact, nonmucosal, uninjured skin (maximal dose 20 g/200 cm2 of surface area). It is applied in a thick layer and covered with an occlusive dressing (eg, Tegaderm) to enhance dermal penetration. The depth of penetration increases with application time and may reach a maximum depth of 3 mm and 5 mm following 60-minute and 120-minute application times, respectively.28 Duration of effect is 60 to 120 minutes.
LET, which is a mixture of 4% lidocaine, 0.1% epinephrine, and 0.5% tetracaine, may be used on nonintact, nonmucosal surfaces. Typically, 1 mL to 5 mL of gel is applied directly to the target area and is followed by application of direct pressure for 15 to 30 minutes. LET is not effective on intact skin and is contraindicated in children < 2 years of age.28
Cooling sprays or ice. Topical skin refrigerants, or vapocoolants (eg, ethyl chloride spray), offer an option for short-term local anesthesia that is noninvasive and quick acting. Ethyl chloride is a gaseous substance that extracts heat as it evaporates from the skin, resulting in a transient local conduction block. Skin refrigerants are an option to consider for short procedures such as intra-articular injections, venipuncture, or skin tag excision, or as an adjunct prior to local anesthetic delivery.29-32 Research has shown that topical ethyl chloride spray also possesses antiseptic properties.29,33
Environment: Make a few simple changes
Direct observation of needle penetration is associated with increased pain; advising patients to avert their gaze will mitigate the perception of pain.34 Additionally, research has shown that creating a low-anxiety environment improves patient-reported outcomes in both children and adults.35 Music or audiovisual or multimedia aids, for example, decrease pain and anxiety, particularly among children, and can be readily accessed with smart devices.36-39
We also recommend avoiding terms such as “pinch,” “bee sting,” or “stick” in order to reduce patient anxiety. Instead, we use language such as, “This is the medicine that will numb the area so you will be comfortable during the procedure.”40
Continue to: Injection technique
Injection technique: Consider these helpful tips
Site of needle entry. Prior to injecting local anesthesia, assess the area where the procedure is planned (FIGURE 1). The initial injection site should be proximal along the path of innervation. If regional nerves are anesthetized proximally and infiltration of local anesthesia proceeds distally, the initial puncture will be painful; however, further injections will be through anesthetized skin. Additionally, consider and avoid regional vascular anatomy.41,42
Counter-stimulation. Applying firm pressure, massaging, or stroking the site prior to or during the injection decreases pain.43,44 This technique may be performed by firmly pinching the area of planned injection between the thumb and index fingers, inserting the needle into the pinched skin, and maintaining pressure on the area until the anesthetic effect is achieved.
Angle of needle insertion. Perpendicular entry of the needle into the skin appears to reduce injection site pain (FIGURE 1). Anecdotal reports are supported by a randomized, controlled crossover trial that demonstrated significantly reduced pain with perpendicular injection compared to delivery at 45°.45
Depth of injection. Subcutaneous needle placement is associated with significantly less pain than injection into superficial dermis.2,46 Dermal wheals cause distention of the dermis, increased intradermal pressure, and greater activation of pain afferents in comparison to injection in the subcutaneous space.46 One important exception is the shave biopsy in which dermal distention is, in fact, desirable to ensure adequate specimen collection.
Other methods of pain reduction should still be employed. In the setting of traumatic wounds when a laceration is present, injection into the subcutaneous fat through the wound is easy and associated with less pain than injection through intact skin.47
Continue to: Speed of injection
Speed of injection. Rapid injection of anesthesia is associated with worse injection site pain and decreased patient satisfaction.48-50 Slowing the rate of injection causes less rapid distention of the dermis and subcutaneous space, resulting in decreased pain afferent activation and increased time for nerve blockade. Its importance is underscored by a prospective, randomized trial that compared rate of administration with buffering of local anesthetics and demonstrated that slow administration impacted patient-perceived pain more than buffering solution.51
Needle stabilization. Following perpendicular entry of the needle into the area of planned infiltration, deliver 0.5 mL of local anesthetic into the subcutaneous space without movement of the needle tip.52 With a stabilized needle tip, pain associated with initial needle entry is no longer perceived within 15 to 30 seconds.
It is paramount to stabilize both the syringe and the area of infiltration to prevent patient movement from causing iatrogenic injury or the need for multiple needlesticks. This can be accomplished by maintaining the dominant hand in a position to inject (ie, thumb on the plunger).
Needle reinsertion. Once subcutaneous swelling of local anesthesia is obtained, the needle may be slowly advanced, maintaining a palpable subcutaneous wavefront of local anesthesia ahead of the needle tip as it moves proximally to distally.2,52 Any reinsertion of the needle should be through previously anesthetized skin; this blockade is assessed by the presence of palpable tumescence and blanching (from the epinephrine effect).53
An example of the application of these injection pearls is demonstrated in the administration of a digital nerve block in FIGURE 2.54,55 With the use of the techniques outlined here, the patient ideally experiences only the initial needle entry and is comfortable for the remainder of the procedure.
CORRESPONDENCE
Katharine C. DeGeorge, MD, MS, Department of Family Medicine, University of Virginia, 1215 Lee Street, Charlottesville, VA, 22903; kd6fp@viginia.edu.
In-office procedures are increasingly emphasized as a way to reduce referrals, avoid treatment delay, and increase practice revenue. Local analgesia is administered before many in-office procedures such as biopsies, toenail removal, and laceration repair. Skin procedures are performed most commonly; nearly three-quarters (74%) of family physicians (FPs) provided these services in 2018.1 Administration of local anesthetic is often the most feared and uncomfortable step in the entire process.2
Knowledge of strategies to reduce pain associated with anesthetic administration can make a huge difference in the patient experience. This article explores evidence-based techniques for administering a local anesthetic with minimal patient discomfort.
4 factors influence the painof local anesthetic administration
Pain is perceived during the administration of local anesthetic because of the insertion of the needle and the increased pressure from the injection of fluid. The needle causes sharp, pricking “first pain” via large diameter, myelinated A-delta fibers, and the fluid induces unmyelinated C-fiber activation via tissue distention resulting in dull, diffuse “second pain.”
Four factors influence the experience of pain during administration of local anesthetic: the pharmacologic properties of the anesthetic itself, the equipment used, the environment, and the injection technique. Optimizing all 4 factors limits patient discomfort.
Pharmacologic agents: Lidocaine is often the agent of choice
Local anesthetics differ in maximal dosing, onset of action, and duration of effect (TABLE3). Given its ubiquity in clinics and hospitals, 1% lidocaine is often the agent of choice. Onset of effect occurs within minutes and lasts up to 2 hours. Alternative agents, such as bupivacaine or ropivacaine, may be considered to prolong the anesthetic effect; however, limited evidence exists to support their use in office-based procedures. Additionally, bupivacaine and ropivacaine may be associated with greater pain on injection and parasthesias lasting longer than the duration of pain control.4-6 In practice, maximal dosing is most important in the pediatric population, given the smaller size of the patients and their increased susceptibility to toxicity.
Calculating the maximum recommended dose. To calculate the maximum recommended dose of local anesthetic, you need to know the concentration of the anesthetic, the maximum allowable dose (mg/kg), and the weight of the patient.7,8 The concentration of the local anesthetic is converted from percentage to weight per unit volume (eg, 1% = 10 mg/mL; 0.5% = 5 mg/mL). Multiply the patient's weight (kg) by the maximum dose of local anesthetic (mg/kg) and divide by the concentration of the local anesthetic (mg/mL) to get the maximum recommended dose in milliliters. Walsh et al9 described a simplified formula to calculate the maximum allowable volume of local anesthetics in milliliters:
(maximum allowable dose in mg/kg) × (weight in kg) × (1 divided by the concentration of anesthetic).
For delivery of lidocaine with epinephrine in a 50-lb (22.7-kg) child, the calculation would be (7 mg/kg) × (22.7 kg) × (1 divided by 10 mg/mL) = 15.9 mL.
Continue to: The advantages (and misconceptions) of epinephrine
The advantages (and misconceptions) of epinephrine
The advantage of adding epinephrine is that it prolongs the effect of the anesthesia and it decreases bleeding. Epinephrine is commonly available as a premixed solution with lidocaine or bupivacaine at a concentration of 1:100,000 and is generally differentiated from “plain” local anesthetic by a red label and cap. Although maximum vasoconstriction may occur as long as 30 minutes after injection,10 adequate vasoconstriction is achieved in 7 to 10 minutes for excision of skin lesions.11
Traditional teaching recommends against using epinephrine in the “fingers, toes, penis, ears, or nose” because of potential arterial spasm, ischemia, and gangrene distal to the injection site.12 These concerns were based on experiences with procaine and cocaine mixed with epinephrine. Studies suffered from multiple confounders, including tourniquets and nonstandardized epinephrine concentrations.13-15
No association of distal ischemia with epinephrine use was identified in a recent Cochrane Review or in another multicenter prospective study.16,17 Phentolamine, a non-selective alpha-adrenergic receptor antagonist and vasodilator, can be administered to reverse vasoconstriction following inadvertent administration of high-dose epinephrine (1:1000) via anaphylaxis autoinjector kits.
Dosing of phentolamine is 1 mL of 1 mg/mL solution delivered subcutaneously to the affected area; reversal decreases the duration of vasoconstriction from 320 minutes to approximately 85 minutes.18 As always, when applying literature to clinical practice, one must keep in mind the risks and benefits of any intervention. As such, in patients with pre-existing vascular disease, vaso-occlusive or vasospastic disease, or compromised perfusion due to trauma, one must weigh the benefits of the hemostatic effect against potential ischemia of already susceptible tissues. In such instances, omitting epinephrine from the solution is reasonable.
The benefits of sodium bicarbonate
The acidity of the solution contributes to the level of pain associated with administration of local anesthesia. Previously opened containers become more acidic.19 Addition of 8.4% sodium bicarbonate, at a ratio of 1 mL per 10 mL of 1% lidocaine with 1:100,000 epinephrine, neutralizes the pH to 7.4.19 A Cochrane Review showed that correction of pH to physiologic levels results in a significant reduction in pain.20
Continue to: This solution can be...
This solution can be easily prepared, as standard syringes hold an additional milliliter (ie, 10-mL syringes hold 11 mL) and, thus, can accommodate the additional volume of bicarbonate.21
Warming the solution helps, too
Warming the solution to body temperature prior to injection decreases pain on injection.22 This may be done in a variety of ways depending on available in-office equipment. Water baths, incubators, fluid warmers, heating pads, or specific syringe warmers may be used. Multiple studies have shown improvement in patient satisfaction with warming.23 Moreover, warming and buffering solution provide a synergistic effect on pain reduction.23
Equipment: Size matters
Smaller diameter needles. Reducing the outer diameter of the needle used for injection improves pain by reducing activation of nociceptors.24-26 Reduced inner diameter restricts injection speed, which further reduces pain.25 We recommend 27- to 30-gauge needles for subcutaneous injection and 25- to 27-gauge needles for intra-articular or tendon sheath injections.
Appropriate syringe size. Filling a syringe to capacity results in maximal deployment of the plunger. This requires greater handspan, which can lead to fatigue and loss of control during injection.26,27 Using a syringe filled to approximately half its capacity results in improved dexterity. We recommend 10-mL syringes with 5 mL to 6 mL of local anesthetic for small procedures and 20-mL syringes filled with 10 mL to 12 mL for larger procedures.
Topical local anesthetics may be used either as an adjunct to decrease pain during injection or as the primary anesthetic.28 A variety of agents are available for clinical use, including eutectic mixture of local anesthetics (EMLA), lidocaine-epinephrine-tetracaine (LET), lidocaine, benzocaine, and tetracaine. FPs should be familiar with their different pharmacokinetic profiles.
Continue to: EMLA is a mixture of...
EMLA is a mixture of 25 mg/mL of lidocaine and 25 mg/mL of prilocaine. It is indicated for topical anesthesia on intact, nonmucosal, uninjured skin (maximal dose 20 g/200 cm2 of surface area). It is applied in a thick layer and covered with an occlusive dressing (eg, Tegaderm) to enhance dermal penetration. The depth of penetration increases with application time and may reach a maximum depth of 3 mm and 5 mm following 60-minute and 120-minute application times, respectively.28 Duration of effect is 60 to 120 minutes.
LET, which is a mixture of 4% lidocaine, 0.1% epinephrine, and 0.5% tetracaine, may be used on nonintact, nonmucosal surfaces. Typically, 1 mL to 5 mL of gel is applied directly to the target area and is followed by application of direct pressure for 15 to 30 minutes. LET is not effective on intact skin and is contraindicated in children < 2 years of age.28
Cooling sprays or ice. Topical skin refrigerants, or vapocoolants (eg, ethyl chloride spray), offer an option for short-term local anesthesia that is noninvasive and quick acting. Ethyl chloride is a gaseous substance that extracts heat as it evaporates from the skin, resulting in a transient local conduction block. Skin refrigerants are an option to consider for short procedures such as intra-articular injections, venipuncture, or skin tag excision, or as an adjunct prior to local anesthetic delivery.29-32 Research has shown that topical ethyl chloride spray also possesses antiseptic properties.29,33
Environment: Make a few simple changes
Direct observation of needle penetration is associated with increased pain; advising patients to avert their gaze will mitigate the perception of pain.34 Additionally, research has shown that creating a low-anxiety environment improves patient-reported outcomes in both children and adults.35 Music or audiovisual or multimedia aids, for example, decrease pain and anxiety, particularly among children, and can be readily accessed with smart devices.36-39
We also recommend avoiding terms such as “pinch,” “bee sting,” or “stick” in order to reduce patient anxiety. Instead, we use language such as, “This is the medicine that will numb the area so you will be comfortable during the procedure.”40
Continue to: Injection technique
Injection technique: Consider these helpful tips
Site of needle entry. Prior to injecting local anesthesia, assess the area where the procedure is planned (FIGURE 1). The initial injection site should be proximal along the path of innervation. If regional nerves are anesthetized proximally and infiltration of local anesthesia proceeds distally, the initial puncture will be painful; however, further injections will be through anesthetized skin. Additionally, consider and avoid regional vascular anatomy.41,42
Counter-stimulation. Applying firm pressure, massaging, or stroking the site prior to or during the injection decreases pain.43,44 This technique may be performed by firmly pinching the area of planned injection between the thumb and index fingers, inserting the needle into the pinched skin, and maintaining pressure on the area until the anesthetic effect is achieved.
Angle of needle insertion. Perpendicular entry of the needle into the skin appears to reduce injection site pain (FIGURE 1). Anecdotal reports are supported by a randomized, controlled crossover trial that demonstrated significantly reduced pain with perpendicular injection compared to delivery at 45°.45
Depth of injection. Subcutaneous needle placement is associated with significantly less pain than injection into superficial dermis.2,46 Dermal wheals cause distention of the dermis, increased intradermal pressure, and greater activation of pain afferents in comparison to injection in the subcutaneous space.46 One important exception is the shave biopsy in which dermal distention is, in fact, desirable to ensure adequate specimen collection.
Other methods of pain reduction should still be employed. In the setting of traumatic wounds when a laceration is present, injection into the subcutaneous fat through the wound is easy and associated with less pain than injection through intact skin.47
Continue to: Speed of injection
Speed of injection. Rapid injection of anesthesia is associated with worse injection site pain and decreased patient satisfaction.48-50 Slowing the rate of injection causes less rapid distention of the dermis and subcutaneous space, resulting in decreased pain afferent activation and increased time for nerve blockade. Its importance is underscored by a prospective, randomized trial that compared rate of administration with buffering of local anesthetics and demonstrated that slow administration impacted patient-perceived pain more than buffering solution.51
Needle stabilization. Following perpendicular entry of the needle into the area of planned infiltration, deliver 0.5 mL of local anesthetic into the subcutaneous space without movement of the needle tip.52 With a stabilized needle tip, pain associated with initial needle entry is no longer perceived within 15 to 30 seconds.
It is paramount to stabilize both the syringe and the area of infiltration to prevent patient movement from causing iatrogenic injury or the need for multiple needlesticks. This can be accomplished by maintaining the dominant hand in a position to inject (ie, thumb on the plunger).
Needle reinsertion. Once subcutaneous swelling of local anesthesia is obtained, the needle may be slowly advanced, maintaining a palpable subcutaneous wavefront of local anesthesia ahead of the needle tip as it moves proximally to distally.2,52 Any reinsertion of the needle should be through previously anesthetized skin; this blockade is assessed by the presence of palpable tumescence and blanching (from the epinephrine effect).53
An example of the application of these injection pearls is demonstrated in the administration of a digital nerve block in FIGURE 2.54,55 With the use of the techniques outlined here, the patient ideally experiences only the initial needle entry and is comfortable for the remainder of the procedure.
CORRESPONDENCE
Katharine C. DeGeorge, MD, MS, Department of Family Medicine, University of Virginia, 1215 Lee Street, Charlottesville, VA, 22903; kd6fp@viginia.edu.
1. American Academy of Family Physicians. Family Medicine Facts. 2018. www.aafp.org/about/the-aafp/family-medicine-specialty/facts/table-12(rev).html. Accessed April 27, 2020.
2. Strazar AR, Leynes PG, Lalonde DH. Minimizing the pain of local anesthesia injection. Plast Reconstr Surg. 2013;132:675-684.
3. Kouba DJ, LoPiccolo MC, Alam M, et al. Guidelines for the use of local anesthesia in office-based dermatologic surgery. J Am Acad Dermatol. 2016;74:1201-1219.
4. Vinycomb TI, Sahhar LJ. Comparison of local anesthetics for digital nerve blocks: a systematic review. J Hand Surg Am. 2014;39:744-751.e5.
5. Valvano MN, Leffler S. Comparison of bupivacaine and lidocaine/bupivacaine for local anesthesia/digital nerve block. Ann Emerg Med. 1996;27:490-492.
6. Spivey WH, McNamara RM, MacKenzie RS, et al. A clinical comparison of lidocaine and bupivacaine. Ann Emerg Med. 1987;16:752-757.
7. Neal JM, Mulroy MF, Weinberg GL, American Society of Regional Anesthesia and Pain Medicine. American Society of Regional Anesthesia and Pain Medicine checklist for managing local anesthetic systemic toxicity. Reg Anesth Pain Med. 2012;37:16-18.
8. Neal JM, Bernards CM, Butterworth JF, et al. ASRA practice advisory on local anesthetic systemic toxicity. Reg Anesth Pain Med. 2010;35:152-161.
9. Walsh K, Arya R. A simple formula for quick and accurate calculation of maximum allowable volume of local anaesthetic agents. Br J Dermatol. 2015;172:825-826.
10. McKee DE, Lalonde DH, Thoma A, et al. Optimal time delay between epinephrine injection and incision to minimize bleeding. Plast Reconstr Surg. 2013;131:811-814.
11. Hult J, Sheikh R, Nguyen CD, et al. A waiting time of 7 min is sufficient to reduce bleeding in oculoplastic surgery following the administration of epinephrine together with local anaesthesia. Acta Ophthalmol. 2018;96:499-502.
12. McKee DE, Lalonde DH, Thoma A, et al. Achieving the optimal epinephrine effect in wide awake hand surgery using local anesthesia without a tourniquet. Hand (NY). 2015;10:613-615.
13. Krunic AL, Wang LC, Soltani K, et al. Digital anesthesia with epinephrine: an old myth revisited. J Am Acad Dermatol. 2004;51:755-759.
14. Thomson CJ, Lalonde DH, Denkler KA, et al. A critical look at the evidence for and against elective epinephrine use in the finger. Plast Reconstr Surg. 2007;119:260-266.
15. Lalonde DH, Lalonde JF. Discussion. Do not use epinephrine in digital blocks: myth or truth? Part II. A retrospective review of 1111 cases. Plast Reconstr Surg. 2010;126:2035-2036.
16. Prabhakar H, Rath S, Kalaivani M, et al. Adrenaline with lidocaine for digital nerve blocks. Cochrane Database Syst Rev. 2015;(3):CD010645.
17. Lalonde D, Bell M, Benoit P, et al. A multicenter prospective study of 3,110 consecutive cases of elective epinephrine use in the fingers and hand: the Dalhousie Project clinical phase. J Hand Surg Am. 2005;30:1061-1067.
18. Nodwell T, Lalonde D. How long does it take phentolamine to reverse adrenaline-induced vasoconstriction in the finger and hand? A prospective, randomized, blinded study: the Dalhousie Project experimental phase. Can J Plast Surg. 2003;11:187-190.
19. Frank SG, Lalonde DH. How acidic is the lidocaine we are injecting, and how much bicarbonate should we add? Can J Plast Surg. 2012;20:71-73.
20. Cepeda MS, Tzortzopoulou A, Thackrey M, et al. Cochrane Review: adjusting the pH of lidocaine for reducing pain on injection. Evidence-Based Child Heal. 2012;7:149-215.
21. Barros MFFH, da Rocha Luz Júnior A, Roncaglio B, et al. Evaluation of surgical treatment of carpal tunnel syndrome using local anesthesia. Rev Bras Ortop. 2016;51:36-39.
22. Hogan M-E, vanderVaart S, Perampaladas K, et al. Systematic review and meta-analysis of the effect of warming local anesthetics on injection pain. Ann Emerg Med. 2011;58:86-98.e1.
23. Colaric KB, Overton DT, Moore K. Pain reduction in lidocaine administration through buffering and warming. Am J Emerg Med. 1998;16:353-356.
24. Arendt-Nielsen L, Egekvist H, Bjerring P. Pain following controlled cutaneous insertion of needles with different diameters. Somatosens Mot Res. 2006;23:37-43.
25. Edlich RF, Smith JF, Mayer NE, et al. Performance of disposable needle syringe systems for local anesthesia. J Emerg Med. 1987;5:83-90.
26. Reed KL, Malamed SF, Fonner AM. Local anesthesia Part 2: technical considerations. Anesth Prog. 2012;59:127-137.
27. Elliott TG. Tips for a better local anaesthetic. Australas J Dermatol. 1998;39:50-51.
28. Kumar M, Chawla R, Goyal M. Topical anesthesia. J Anaesthesiol Clin Pharmacol. 2015;31:450.
29. Polishchuk D, Gehrmann R, Tan V. Skin sterility after application of ethyl chloride spray. J Bone Joint Surg Am. 2012;94:118-120.
30. Franko OI, Stern PJ. Use and effectiveness of ethyl chloride for hand injections. J Hand Surg Am. 2017;42:175-181.e1.
31. Fossum K, Love SL, April MD. Topical ethyl chloride to reduce pain associated with venous catheterization: a randomized crossover trial. Am J Emerg Med. 2016;34:845-850.
32. Görgülü T, Torun M, Güler R, et al. Fast and painless skin tag excision with ethyl chloride. Aesthetic Plast Surg. 2015;39:644-645.
33. Azar FM, Lake JE, Grace SP, et al. Ethyl chloride improves antiseptic effect of betadine skin preparation for office procedures. J Surg Orthop Adv. 2012;21:84-87.
34. Oliveira NCAC, Santos JLF, Linhares MBM. Audiovisual distraction for pain relief in paediatric inpatients: a crossover study. Eur J Pain. 2017;21:178-187.
35. Pillai Riddell RR, Racine NM, Gennis HG, et al. Non-pharmacological management of infant and young child procedural pain. Cochrane Database Syst Rev. 2015;(12):CD006275.
36. Attar RH, Baghdadi ZD. Comparative efficacy of active and passive distraction during restorative treatment in children using an iPad versus audiovisual eyeglasses: a randomised controlled trial. Eur Arch Paediatr Dent. 2015;16:1-8.
37. Uman LS, Birnie KA, Noel M, et al. Psychological interventions for needle-related procedural pain and distress in children and adolescents. Cochrane Database Syst Rev. 2013;(10):CD005179.
38. Ahmad Z, Chawla R, Jaffe W. A novel distraction technique to facilitate daycase paediatric surgery under local anaesthesia. J Plast Reconstr Aesthetic Surg. 2012;65:e21-e22.
39. Hartling L, Newton AS, Liang Y, et al. Music to reduce pain and distress in the pediatric emergency department. JAMA Pediatr. 2013;167:826.
40. Varelmann D, Pancaro C, Cappiello EC, et al. Nocebo-induced hyperalgesia during local anesthetic injection. Anesth Analg. 2010;110:868-870.
41. Nelson TW. Accidental intravascular injection of local anesthetic? Anesthesiology. 2008;109:1143-1144.
42. Taghavi Zenouz A, Ebrahimi H, Mahdipour M, et al. The incidence of intravascular needle entrance during inferior alveolar nerve block injection. J Dent Res Dent Clin Dent Prospects. 2008;2:38-41.
43. Taddio A, Ilersich AL, Ipp M, et al; HELPinKIDS Team. Physical interventions and injection techniques for reducing injection pain during routine childhood immunizations: systematic review of randomized controlled trials and quasi-randomized controlled trials. Clin Ther. 2009;31:S48-S76.
44. Aminabadi NA, Farahani RMZ, Balayi Gajan E. The efficacy of distraction and counterstimulation in the reduction of pain reaction to intraoral injection by pediatric patients. J Contemp Dent Pract. 2008;9:33-40.
45. Martires KJ, Malbasa CL, Bordeaux JS. A randomized controlled crossover trial: lidocaine injected at a 90-degree angle causes less pain than lidocaine injected at a 45-degree angle. J Am Acad Dermatol. 2011;65:1231-1233.
46. Zilinsky I, Bar-Meir E, Zaslansky R, et al. Ten commandments for minimal pain during administration of local anesthetics. J Drugs Dermatol. 2005;4:212-216.
47. Bartfield JM, Sokaris SJ, Raccio-Robak N. Local anesthesia for lacerations: pain of infiltration inside vs outside the wound. Acad Emerg Med. 1998;5:100-104.
48. Scarfone RJ, Jasani M, Gracely EJ. Pain of local anesthetics: rate of administration and buffering. Ann Emerg Med. 1998;31:36-40.
49. Kattan AE, Al-Shomer F, Al-Jerian A, et al. Pain on administration of non-alkalinised lidocaine for carpal tunnel decompression: a comparison between the Gale and the “advancing wheal” techniques. J Plast Surg Hand Surg. 2016;50:10-14.
50. Tangen LF, Lundbom JS, Skarsvåg TI, et al. The influence of injection speed on pain during injection of local anaesthetic. J Plast Surg Hand Surg. 2016;50:7-9.
51. McGlone R, Bodenham A. Reducing the pain of intradermal lignocaine injection by pH buffering. Arch Emerg Med. 1990;7:65-68.
52. Lalonde D, Wong A. Local anesthetics. Plast Reconstr Surg. 2014;134(4 Suppl 2):40S-49S.
53. Klein JA. Tumescent technique for regional anesthesia permits lidocaine doses of 35 mg/kg for liposuction. J Dermatol Surg Oncol. 1990;16:248-263.
54. Williams JG, Lalonde DH. Randomized comparison of the single-injection volar subcutaneous block and the two-injection dorsal block for digital anesthesia. Plast Reconstr Surg. 2006;118:1195-1200.
55. Thomson CJ, Lalonde DH. Randomized double-blind comparison of duration of anesthesia among three commonly used agents in digital nerve block. Plast Reconstr Surg. 2006;118:429-432.
1. American Academy of Family Physicians. Family Medicine Facts. 2018. www.aafp.org/about/the-aafp/family-medicine-specialty/facts/table-12(rev).html. Accessed April 27, 2020.
2. Strazar AR, Leynes PG, Lalonde DH. Minimizing the pain of local anesthesia injection. Plast Reconstr Surg. 2013;132:675-684.
3. Kouba DJ, LoPiccolo MC, Alam M, et al. Guidelines for the use of local anesthesia in office-based dermatologic surgery. J Am Acad Dermatol. 2016;74:1201-1219.
4. Vinycomb TI, Sahhar LJ. Comparison of local anesthetics for digital nerve blocks: a systematic review. J Hand Surg Am. 2014;39:744-751.e5.
5. Valvano MN, Leffler S. Comparison of bupivacaine and lidocaine/bupivacaine for local anesthesia/digital nerve block. Ann Emerg Med. 1996;27:490-492.
6. Spivey WH, McNamara RM, MacKenzie RS, et al. A clinical comparison of lidocaine and bupivacaine. Ann Emerg Med. 1987;16:752-757.
7. Neal JM, Mulroy MF, Weinberg GL, American Society of Regional Anesthesia and Pain Medicine. American Society of Regional Anesthesia and Pain Medicine checklist for managing local anesthetic systemic toxicity. Reg Anesth Pain Med. 2012;37:16-18.
8. Neal JM, Bernards CM, Butterworth JF, et al. ASRA practice advisory on local anesthetic systemic toxicity. Reg Anesth Pain Med. 2010;35:152-161.
9. Walsh K, Arya R. A simple formula for quick and accurate calculation of maximum allowable volume of local anaesthetic agents. Br J Dermatol. 2015;172:825-826.
10. McKee DE, Lalonde DH, Thoma A, et al. Optimal time delay between epinephrine injection and incision to minimize bleeding. Plast Reconstr Surg. 2013;131:811-814.
11. Hult J, Sheikh R, Nguyen CD, et al. A waiting time of 7 min is sufficient to reduce bleeding in oculoplastic surgery following the administration of epinephrine together with local anaesthesia. Acta Ophthalmol. 2018;96:499-502.
12. McKee DE, Lalonde DH, Thoma A, et al. Achieving the optimal epinephrine effect in wide awake hand surgery using local anesthesia without a tourniquet. Hand (NY). 2015;10:613-615.
13. Krunic AL, Wang LC, Soltani K, et al. Digital anesthesia with epinephrine: an old myth revisited. J Am Acad Dermatol. 2004;51:755-759.
14. Thomson CJ, Lalonde DH, Denkler KA, et al. A critical look at the evidence for and against elective epinephrine use in the finger. Plast Reconstr Surg. 2007;119:260-266.
15. Lalonde DH, Lalonde JF. Discussion. Do not use epinephrine in digital blocks: myth or truth? Part II. A retrospective review of 1111 cases. Plast Reconstr Surg. 2010;126:2035-2036.
16. Prabhakar H, Rath S, Kalaivani M, et al. Adrenaline with lidocaine for digital nerve blocks. Cochrane Database Syst Rev. 2015;(3):CD010645.
17. Lalonde D, Bell M, Benoit P, et al. A multicenter prospective study of 3,110 consecutive cases of elective epinephrine use in the fingers and hand: the Dalhousie Project clinical phase. J Hand Surg Am. 2005;30:1061-1067.
18. Nodwell T, Lalonde D. How long does it take phentolamine to reverse adrenaline-induced vasoconstriction in the finger and hand? A prospective, randomized, blinded study: the Dalhousie Project experimental phase. Can J Plast Surg. 2003;11:187-190.
19. Frank SG, Lalonde DH. How acidic is the lidocaine we are injecting, and how much bicarbonate should we add? Can J Plast Surg. 2012;20:71-73.
20. Cepeda MS, Tzortzopoulou A, Thackrey M, et al. Cochrane Review: adjusting the pH of lidocaine for reducing pain on injection. Evidence-Based Child Heal. 2012;7:149-215.
21. Barros MFFH, da Rocha Luz Júnior A, Roncaglio B, et al. Evaluation of surgical treatment of carpal tunnel syndrome using local anesthesia. Rev Bras Ortop. 2016;51:36-39.
22. Hogan M-E, vanderVaart S, Perampaladas K, et al. Systematic review and meta-analysis of the effect of warming local anesthetics on injection pain. Ann Emerg Med. 2011;58:86-98.e1.
23. Colaric KB, Overton DT, Moore K. Pain reduction in lidocaine administration through buffering and warming. Am J Emerg Med. 1998;16:353-356.
24. Arendt-Nielsen L, Egekvist H, Bjerring P. Pain following controlled cutaneous insertion of needles with different diameters. Somatosens Mot Res. 2006;23:37-43.
25. Edlich RF, Smith JF, Mayer NE, et al. Performance of disposable needle syringe systems for local anesthesia. J Emerg Med. 1987;5:83-90.
26. Reed KL, Malamed SF, Fonner AM. Local anesthesia Part 2: technical considerations. Anesth Prog. 2012;59:127-137.
27. Elliott TG. Tips for a better local anaesthetic. Australas J Dermatol. 1998;39:50-51.
28. Kumar M, Chawla R, Goyal M. Topical anesthesia. J Anaesthesiol Clin Pharmacol. 2015;31:450.
29. Polishchuk D, Gehrmann R, Tan V. Skin sterility after application of ethyl chloride spray. J Bone Joint Surg Am. 2012;94:118-120.
30. Franko OI, Stern PJ. Use and effectiveness of ethyl chloride for hand injections. J Hand Surg Am. 2017;42:175-181.e1.
31. Fossum K, Love SL, April MD. Topical ethyl chloride to reduce pain associated with venous catheterization: a randomized crossover trial. Am J Emerg Med. 2016;34:845-850.
32. Görgülü T, Torun M, Güler R, et al. Fast and painless skin tag excision with ethyl chloride. Aesthetic Plast Surg. 2015;39:644-645.
33. Azar FM, Lake JE, Grace SP, et al. Ethyl chloride improves antiseptic effect of betadine skin preparation for office procedures. J Surg Orthop Adv. 2012;21:84-87.
34. Oliveira NCAC, Santos JLF, Linhares MBM. Audiovisual distraction for pain relief in paediatric inpatients: a crossover study. Eur J Pain. 2017;21:178-187.
35. Pillai Riddell RR, Racine NM, Gennis HG, et al. Non-pharmacological management of infant and young child procedural pain. Cochrane Database Syst Rev. 2015;(12):CD006275.
36. Attar RH, Baghdadi ZD. Comparative efficacy of active and passive distraction during restorative treatment in children using an iPad versus audiovisual eyeglasses: a randomised controlled trial. Eur Arch Paediatr Dent. 2015;16:1-8.
37. Uman LS, Birnie KA, Noel M, et al. Psychological interventions for needle-related procedural pain and distress in children and adolescents. Cochrane Database Syst Rev. 2013;(10):CD005179.
38. Ahmad Z, Chawla R, Jaffe W. A novel distraction technique to facilitate daycase paediatric surgery under local anaesthesia. J Plast Reconstr Aesthetic Surg. 2012;65:e21-e22.
39. Hartling L, Newton AS, Liang Y, et al. Music to reduce pain and distress in the pediatric emergency department. JAMA Pediatr. 2013;167:826.
40. Varelmann D, Pancaro C, Cappiello EC, et al. Nocebo-induced hyperalgesia during local anesthetic injection. Anesth Analg. 2010;110:868-870.
41. Nelson TW. Accidental intravascular injection of local anesthetic? Anesthesiology. 2008;109:1143-1144.
42. Taghavi Zenouz A, Ebrahimi H, Mahdipour M, et al. The incidence of intravascular needle entrance during inferior alveolar nerve block injection. J Dent Res Dent Clin Dent Prospects. 2008;2:38-41.
43. Taddio A, Ilersich AL, Ipp M, et al; HELPinKIDS Team. Physical interventions and injection techniques for reducing injection pain during routine childhood immunizations: systematic review of randomized controlled trials and quasi-randomized controlled trials. Clin Ther. 2009;31:S48-S76.
44. Aminabadi NA, Farahani RMZ, Balayi Gajan E. The efficacy of distraction and counterstimulation in the reduction of pain reaction to intraoral injection by pediatric patients. J Contemp Dent Pract. 2008;9:33-40.
45. Martires KJ, Malbasa CL, Bordeaux JS. A randomized controlled crossover trial: lidocaine injected at a 90-degree angle causes less pain than lidocaine injected at a 45-degree angle. J Am Acad Dermatol. 2011;65:1231-1233.
46. Zilinsky I, Bar-Meir E, Zaslansky R, et al. Ten commandments for minimal pain during administration of local anesthetics. J Drugs Dermatol. 2005;4:212-216.
47. Bartfield JM, Sokaris SJ, Raccio-Robak N. Local anesthesia for lacerations: pain of infiltration inside vs outside the wound. Acad Emerg Med. 1998;5:100-104.
48. Scarfone RJ, Jasani M, Gracely EJ. Pain of local anesthetics: rate of administration and buffering. Ann Emerg Med. 1998;31:36-40.
49. Kattan AE, Al-Shomer F, Al-Jerian A, et al. Pain on administration of non-alkalinised lidocaine for carpal tunnel decompression: a comparison between the Gale and the “advancing wheal” techniques. J Plast Surg Hand Surg. 2016;50:10-14.
50. Tangen LF, Lundbom JS, Skarsvåg TI, et al. The influence of injection speed on pain during injection of local anaesthetic. J Plast Surg Hand Surg. 2016;50:7-9.
51. McGlone R, Bodenham A. Reducing the pain of intradermal lignocaine injection by pH buffering. Arch Emerg Med. 1990;7:65-68.
52. Lalonde D, Wong A. Local anesthetics. Plast Reconstr Surg. 2014;134(4 Suppl 2):40S-49S.
53. Klein JA. Tumescent technique for regional anesthesia permits lidocaine doses of 35 mg/kg for liposuction. J Dermatol Surg Oncol. 1990;16:248-263.
54. Williams JG, Lalonde DH. Randomized comparison of the single-injection volar subcutaneous block and the two-injection dorsal block for digital anesthesia. Plast Reconstr Surg. 2006;118:1195-1200.
55. Thomson CJ, Lalonde DH. Randomized double-blind comparison of duration of anesthesia among three commonly used agents in digital nerve block. Plast Reconstr Surg. 2006;118:429-432.
PRACTICE RECOMMENDATIONS
› Add epinephrine and sodium bicarbonate buffer to local anesthetic solution to reduce pain and procedural blood loss. A
› Use such techniques as counter-stimulation, a perpendicular angle of injection, a subcutaneous depth of injection, and a slow rate of injection to minimize patient discomfort. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Evolocumab safe, well-tolerated in HIV+ patients
Evolocumab proved effective, well tolerated, and safe for the treatment of refractory dyslipidemia in persons living with HIV in the phase 3, randomized, double-blind BEIJERINCK study.
At 24 weeks, nearly three-quarters of patients randomized to evolocumab (Repatha) achieved at least a 50% reduction in LDL cholesterol while on maximally tolerated background lipid lowering with a statin and/or other drugs. This was accompanied by significant reductions in other atherogenic lipids, Franck Boccara, MD, PhD, reported at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
Evolocumab thus shows the potential to help fill a major unmet need for more effective treatment of dyslipidemia in HIV-positive patients, who number an estimated 38 million worldwide, including 1.1 million in the United States. Access to highly active antiretroviral therapies has transformed HIV infection into a chronic manageable disease, but this major advance has been accompanied by a rate of premature atherosclerotic cardiovascular disease that’s nearly twice that of the general population, observed Dr. Boccara, a cardiologist at Sorbonne University, Paris.
The BEIJERINCK study included 464 HIV-infected patients in the United States and 14 other countries on five continents. Participants had a mean baseline LDL cholesterol of 133 mg/dL and triglycerides of about 190 mg/dL while on maximally tolerated lipid-lowering therapy. They had been diagnosed with HIV an average of 18 years earlier. One-third of them had known atherosclerotic cardiovascular disease. More than one-quarter of participants were cigarette smokers. Patients were randomized 2:1 to 24 weeks of double-blind subcutaneous evolocumab at 420 mg once monthly or placebo, then an additional 24 weeks of open-label evolocumab for all.
The primary endpoint was change in LDL from baseline to week 24: a 56.2% reduction in the evolocumab group and a 0.7% increase with placebo. About 73% of patients on evolocumab achieved at least a 50% reduction in LDL cholesterol, as did less than 1% of controls. Likewise, 73% of the evolocumab group got their LDL cholesterol below 70 mg/dL, compared with 7.9% with placebo.
The evolocumab group also experienced favorable placebo-subtracted differences from baseline of 23% in triglycerides, 27% in lipoprotein(a), and 22% in very-low-density lipoprotein cholesterol.
As was the case in the earlier, much larger landmark clinical trials, evolocumab was well tolerated in BEIJERINCK, with a side effect profile similar to placebo. Notably, there was no increase in liver abnormalities in evolocumab-treated patients on highly active antiretroviral therapy, and no one developed evolocumab neutralizing antibodies.
Dr. Boccara reported receiving a research grant from Amgen, the study sponsor, as well as lecture fees from several other pharmaceutical companies.
Simultaneous with the presentation at ACC 2020, the primary results of the BEIJERINCK study were published online (J Am Coll Cardiol. 2020 Mar 19. doi: 10.1016/j.jacc.2020.03.025).
Evolocumab proved effective, well tolerated, and safe for the treatment of refractory dyslipidemia in persons living with HIV in the phase 3, randomized, double-blind BEIJERINCK study.
At 24 weeks, nearly three-quarters of patients randomized to evolocumab (Repatha) achieved at least a 50% reduction in LDL cholesterol while on maximally tolerated background lipid lowering with a statin and/or other drugs. This was accompanied by significant reductions in other atherogenic lipids, Franck Boccara, MD, PhD, reported at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
Evolocumab thus shows the potential to help fill a major unmet need for more effective treatment of dyslipidemia in HIV-positive patients, who number an estimated 38 million worldwide, including 1.1 million in the United States. Access to highly active antiretroviral therapies has transformed HIV infection into a chronic manageable disease, but this major advance has been accompanied by a rate of premature atherosclerotic cardiovascular disease that’s nearly twice that of the general population, observed Dr. Boccara, a cardiologist at Sorbonne University, Paris.
The BEIJERINCK study included 464 HIV-infected patients in the United States and 14 other countries on five continents. Participants had a mean baseline LDL cholesterol of 133 mg/dL and triglycerides of about 190 mg/dL while on maximally tolerated lipid-lowering therapy. They had been diagnosed with HIV an average of 18 years earlier. One-third of them had known atherosclerotic cardiovascular disease. More than one-quarter of participants were cigarette smokers. Patients were randomized 2:1 to 24 weeks of double-blind subcutaneous evolocumab at 420 mg once monthly or placebo, then an additional 24 weeks of open-label evolocumab for all.
The primary endpoint was change in LDL from baseline to week 24: a 56.2% reduction in the evolocumab group and a 0.7% increase with placebo. About 73% of patients on evolocumab achieved at least a 50% reduction in LDL cholesterol, as did less than 1% of controls. Likewise, 73% of the evolocumab group got their LDL cholesterol below 70 mg/dL, compared with 7.9% with placebo.
The evolocumab group also experienced favorable placebo-subtracted differences from baseline of 23% in triglycerides, 27% in lipoprotein(a), and 22% in very-low-density lipoprotein cholesterol.
As was the case in the earlier, much larger landmark clinical trials, evolocumab was well tolerated in BEIJERINCK, with a side effect profile similar to placebo. Notably, there was no increase in liver abnormalities in evolocumab-treated patients on highly active antiretroviral therapy, and no one developed evolocumab neutralizing antibodies.
Dr. Boccara reported receiving a research grant from Amgen, the study sponsor, as well as lecture fees from several other pharmaceutical companies.
Simultaneous with the presentation at ACC 2020, the primary results of the BEIJERINCK study were published online (J Am Coll Cardiol. 2020 Mar 19. doi: 10.1016/j.jacc.2020.03.025).
Evolocumab proved effective, well tolerated, and safe for the treatment of refractory dyslipidemia in persons living with HIV in the phase 3, randomized, double-blind BEIJERINCK study.
At 24 weeks, nearly three-quarters of patients randomized to evolocumab (Repatha) achieved at least a 50% reduction in LDL cholesterol while on maximally tolerated background lipid lowering with a statin and/or other drugs. This was accompanied by significant reductions in other atherogenic lipids, Franck Boccara, MD, PhD, reported at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
Evolocumab thus shows the potential to help fill a major unmet need for more effective treatment of dyslipidemia in HIV-positive patients, who number an estimated 38 million worldwide, including 1.1 million in the United States. Access to highly active antiretroviral therapies has transformed HIV infection into a chronic manageable disease, but this major advance has been accompanied by a rate of premature atherosclerotic cardiovascular disease that’s nearly twice that of the general population, observed Dr. Boccara, a cardiologist at Sorbonne University, Paris.
The BEIJERINCK study included 464 HIV-infected patients in the United States and 14 other countries on five continents. Participants had a mean baseline LDL cholesterol of 133 mg/dL and triglycerides of about 190 mg/dL while on maximally tolerated lipid-lowering therapy. They had been diagnosed with HIV an average of 18 years earlier. One-third of them had known atherosclerotic cardiovascular disease. More than one-quarter of participants were cigarette smokers. Patients were randomized 2:1 to 24 weeks of double-blind subcutaneous evolocumab at 420 mg once monthly or placebo, then an additional 24 weeks of open-label evolocumab for all.
The primary endpoint was change in LDL from baseline to week 24: a 56.2% reduction in the evolocumab group and a 0.7% increase with placebo. About 73% of patients on evolocumab achieved at least a 50% reduction in LDL cholesterol, as did less than 1% of controls. Likewise, 73% of the evolocumab group got their LDL cholesterol below 70 mg/dL, compared with 7.9% with placebo.
The evolocumab group also experienced favorable placebo-subtracted differences from baseline of 23% in triglycerides, 27% in lipoprotein(a), and 22% in very-low-density lipoprotein cholesterol.
As was the case in the earlier, much larger landmark clinical trials, evolocumab was well tolerated in BEIJERINCK, with a side effect profile similar to placebo. Notably, there was no increase in liver abnormalities in evolocumab-treated patients on highly active antiretroviral therapy, and no one developed evolocumab neutralizing antibodies.
Dr. Boccara reported receiving a research grant from Amgen, the study sponsor, as well as lecture fees from several other pharmaceutical companies.
Simultaneous with the presentation at ACC 2020, the primary results of the BEIJERINCK study were published online (J Am Coll Cardiol. 2020 Mar 19. doi: 10.1016/j.jacc.2020.03.025).
FROM ACC 2020
Bacteroides Fragilis Vertebral Osteomyelitis and Discitis: “Back” to Susceptibility Testing
Acute pyogenic vertebral osteomyelitis is often due to hematogenous spread of aerobic bacteria.1-4 Conversely, only 0.5% of anaerobic bacteremias lead to osteomyelitis.5 Anaerobic osteomyelitis typically results from the contiguous spread of polymicrobial infections through breaks in the gut mucosal barrier and involves the vertebral bodies in only 2% to 5% of cases.5,6 Although Bacteroides fragilis (B fragilis) is the most common anaerobic pathogen cultivated from blood, accounting for about half of all anaerobic blood isolates, it seldom leads to osteomyelitis.1,2,7-11 We report an uncommon case of B fragilis bacteremia and vertebral osteomyelitis confounded by uncertainties in anaerobic identification and susceptibilities.
Case Presentation
A healthy-appearing male aged 55 years presented to the Naval Medical Center Portsmouth (NMCP) with subacute low back pain and fevers of 103 °F for > 3 weeks. While traveling 4 weeks prior, he completed a course of oseltamivir for influenza B infection; afterward, he was diagnosed with community-acquired pneumonia and treated with a dose of ceftriaxone and a 7-day course of doxycycline. The patient presented to the same facility a week later for low back pain and nonresolving respiratory symptoms, and his therapy was changed to azithromycin, cefuroxime, prednisone, and inhalers. Additionally, after being treated for influenza, he developed constipation and hematochezia for which he did not seek care. The hematochezia was similar to a previous episode from an anal fissure 1 year prior that resolved with stool softeners. When he was finally seen at NMCP after 3 weeks of worsening back pain and fevers, lumbosacral magnetic resonance imaging (MRI) demonstrated vertebral osteomyelitis and discitis at L4-L5 and admitted to the hospital (Figure 1).
After a fluoroscopy-guided biopsy of the L4 vertebral body on hospital day 1, the patient was started on cefepime and vancomycin. The biopsy sample was inoculated onto solid media (blood agar, chocolate agar, and MacConkey agar) and incubated at 36 °C for 24 hours in a 5% CO2 atmosphere, as well as onto Shaedler agar with vitamin K and chopped meat glucose broth and incubated at 36 °C for 48 hours under anaerobic conditions. Metronidazole was added and vancomycin discontinued after 2 anaerobic blood culture vials obtained on hospital day 1, incubated in a Becton Dickinson BACTEC FX automated system, which demonstrated Gram-negative bacilli after 48 hours. The blood culture isolates demonstrated a > 99% probability of being identified as ß-lactamase positive Prevotella loescheii using Thermo Fischer Scientific RapID ANA II biochemical testing. Nitrocefinase discs were used to detect ß-lactamase activity.
The biopsy demonstrated nongranulomatous focal areas of necrotic bone and neutrophilia in a hematopoietic background consistent with acute osteomyelitis (Figure 2); on hospital day 4, ß-lactamase positive B fragilis grew from the bone culture. Additionally, 1 anaerobic vial from a surveillance blood culture set that was obtained on hospital day 3 grew ß-lactamasepositive B fragilis using the same identification methods. With these results he was thought to have a polymicrobial infection (B fragilis and Prevotella loescheii [P loescheii]) from a suspected bowel source based on his hematochezia and history of anal fissure. No aerobic, Gram-negative enterobacteriaceae were isolated, but he had previously been on cefuroxime, which has potential activity against these organisms, for ≥ 2 weeks prior to hospitalization and cultures. He was discharged on moxifloxacin and metronidazole pending final culture results, including requested anaerobic susceptibility testing.
At 1-week follow-up, both aerobic and anaerobic vials from surveillance blood cultures remained negative for any microbes, so antibiotics were deescalated to moxifloxacin monotherapy. However, after 3 days the patient was readmitted for increasing C-reactive protein (CRP) levels and intractable back pain with worsening bilateral radiculopathy. A repeat MRI demonstrated interval disease progression with near obliteration of the L4-L5 disc space and hyperenhancement of the prevertebral soft tissues and adjacent psoas musculature without focal rim-enhancing fluid collection (Figure 3). After repeat L4 biopsy, metronidazole was restarted and ertapenem added for enterobacteriaceae coverage, given the known B fragilis and potential suppression from previous cephalosporin therapy; moxifloxacin was discontinued. L4 biopsy cultures showed no growth, and CRP levels trended down from 154.2 mg/L (start of first admission) to 42.4 mg/L (start of second admission) to 14.9 mg/L (day of discharge) (reference range, 5-9.9 mg/L). He was discharged on ertapenem and metronidazole. He completed a 6-week course without further complication.
During antibiotic therapy he had an unremarkable colonoscopy, CRP normalized to 2.6 mg/L (reference range, 0-4.9 mg/L), and he underwent successful L4-L5 transforaminal lumbar interbody fusion 2 weeks after finishing antibiotics.
We retroactively sent both P loescheii isolates and the 1 B fragilis isolate that grew from the surveillance blood culture to the Multidrug-resistant Organism Repository and Surveillance Network (MRSN) at the Walter Reed Army Institute of Research for identification confirmation and susceptibility analysis. Whole genome sequencing with single nucleotide polymorphism (SNP)-based analysis revealed all isolates were 100% identical and consistent with B fragilis and not P loescheii, based on clustering around other B fragilis sequences found in the National Center for Biotechnology Information (NCBI) Genbank database (Figure 4). All isolates carried the antibiotic resistance genes— cepA, sul(2), tetQ— encoding for possible resistance to cephalosporins, sulphonamides, and tetracyclines, respectively; as well as a point mutation in the gyrA gene (Ser82Phe). None of the isolates carried the nim gene, and screening for the 3 subtypes of B fragilis enterotoxin gene (bft-1, bft-2, bft-3) was negative. Eventual susceptibility testing at the Mayo Clinic several months after the conclusion of the case indicated that the B fragilis isolate was sensitive to piperacillin-tazobactam, ertapenem, clindamycin, and metronidazole; however, testing was not performed against moxifloxacin.
Discussion
In the era of growing antibiotic resistance patterns, antimicrobial stewardship programs recommend interventions to improve antimicrobial use through targeted narrow- spectrum antibiotics.12 The Clinical and Laboratory Standards Institute (CLSI) maintains guidelines on the major indications for anaerobic antimicrobial susceptibility testing (AST) to help direct narrow-targeted antimicrobial therapy. However, in a 2008 practice survey Goldstein and colleagues reported that less than half of US hospitals performed anaerobic AST, and only 21% of these facilities did it in-house, while the remainder sent out their isolates for testing.11-14 The CLSI major indications for AST include situations in which the selection of agents is important because of the (1) known resistance of a particular species; (2) confirmation of appropriate therapy for severe infections or for those that may require long-term therapy; (3) persistence of infection despite adequate treatment with an appropriate therapeutic regimen; and (4) difficulty in making empirical decisions based on precedent.14 Additionally, isolates from brain abscess, endocarditis, osteomyelitis, joint infection, infection of prosthetic devices or vascular grafts, bacteremia, and normally sterile body sites (unless contamination suspected) should be tested.14
Because of the lack of anaerobic AST, health care providers must base empiric treatment on reported sensitivities from the medical literature. Empiric selection of antimicrobials for anaerobic infections is made even more challenging by the increased rates of resistance reported in the literature, leading to recommendations to increase susceptibility testing to guide therapy.13,15,16 Empiric therapy of deep-seated anaerobic infections may lead to use of inactive agents or overly broad-spectrum antibiotics. Current antimicrobial stewardship initiatives recognize the importance of narrow-spectrum antibiotics to minimize risk of adverse events and selective pressure for antimicrobial resistance.
Although we attempted to confirm the identification of the anaerobic isolates via commercially available methods, it was not until we performed genetic testing that we were able verify the isolates as B fragilis. Furthermore, earlier susceptibility testing would have allowed for more narrow-targeted antimicrobial therapy and could have potentially prevented our patient’s readmission and use of ertapenem, despite its > 98% susceptibility rates against B fragilis.13,17
All of the B fragilis isolates carried the cepA gene, which is a cephalosporinase that encodes for resistance to cephalosporins and aminopenicillins but not to ß-lactam ß-lactamase inhibitor combinations.13 Although not a substitution for susceptibility analysis, genetic testing showed that all of the isolates carried a nonsynonymous mutation from serine to a phenylalanine at amino acid position 82 (S82F) in the gyrA gene. The S82F mutation has been implicated in fluoroquinolone resistance, via inhibition of substrate–target recognition and binding between fluoroquinolones and the target topoisomerase protein,18 and may potentially explain why our patient clinically worsened while on moxifloxacin monotherapy. Although moxifloxacin susceptibility was not performed, susceptibility rates remain highly variable, ranging from 50% to 70% for B fragilis.13,15,16
It is important to note that the metronidazole the patient received during his first hospital admission could have sterilized the vertebral body without completely eradicating the microbe; thus could explain his clinical worsening while on moxifloxacin monotherapy despite no growth from the repeat biopsy culture. Our rationale for initially continuing moxifloxacin was based on its excellent bioavailability and bone penetration properties. Additionally, of the fluoroquinolones it has the most reliable anaerobic activity and is the only one recommended as monotherapy for complicated intraabdominal infections.19 However, guidelines recommend avoiding its use in patients who have received a fluoroquinolone in the past 90 days or at institutions with high rates of resistance. At our institution Escherichia coli has a > 90% susceptibility rate to fluoroquinolones. Given this rate and our concern that the patient had a polymicrobial infection, we felt that moxifloxacin would provide appropriate anaerobic and aerobic coverage, especially since he had no previous fluoroquinolone exposure.
Additionally, none of the isolates carried the nim or bft toxin genes. Although the nim gene is associated with metronidazole resistance,its presence does not invariably result in resistant strains of B fragilis; in fact, metronidazole resistance is relatively uncommon, with the majority of B fragilis showing < 1% resistance, based on CLSI breakpoints (≥ 32 mg/L).13,20,21 However, one recent epidemiologic study on anaerobic wound isolates from Iraq and Afghanistan casualties found that 12% (2/17) of B fragilis isolates were resistant to metronidazole.15 Given the improvement of the patient’s symptoms while on metronidazole, it is likely that the B fragilis was susceptible. Nevertheless, susceptibility testing with minimum inhibitory concentrations is necessary to verify this result. Also, although enterotoxigenic strains of B fragilis have been associated with bloodstream infections, our patient’s isolates lacked the 3 subtypes of B fragilis enterotoxin gene.22
Conclusions
We report a case of B fragilis bacteremia and vertebral osteomyelitis complicated by challenges in anaerobic identification and sensitivities that led to brief use of a possibly inactive antimicrobial and the subsequent use of carbapenem therapy, which may have been avoided if susceptibility testing were more readily available. This case led to changes in our hospital’s processing of anaerobic isolates to include susceptibility testing on request.
Acknowledgments
We thank Keith Thompson, MD (staff pathologist, Naval Medical Center Portsmouth Virginia), for providing the pathology images from the initial vertebral biopsy, and Dr. Kate Hinkle (director, Multidrug-Resistant Organism Repository and Surveillance Network, Silver Spring, Maryland ) for providing the whole genome sequencing results from the B fragilis isolates.
1. Zimmerli W. Vertebral osteomyelitis. N Eng J Med. 2010;362(11):1022-1029.
2. Chazan B, Strahilevitz J, Millgram MA, Kaufmann S, Raz R. Bacteroides fragilis vertebral osteomyelitis secondary to anal dilatation. Spine (Phila PA 1976). 2001;26(16):E377-E378.
3. Kierzkowska M, Pedzisz P, Babiak I, et al. Orthopedic infections caused by obligatory anaerobic Gram-negative rods: report of two cases. Med Microbiol Immunol. 2017;206(5):363-366.
4. McHenry M, Easley K, Locker G. Vertebral osteomyelitis: long-term outcome for 253 patients from 7 Cleveland-area hospitals. Clin Infect Dis. 2002;34(10):1342-1350.
5. Raff MJ, Melo JC. Anaerobic osteomyelitis. Medicine (Baltimore).1978;57(1):83-103.
6. Lewis R, Sutter V, Finegold S. Bone infections involving anaerobic bacteria. Medicine (Baltimore). 1978;57(1):279-305.
7. Brook I. The role of anaerobic bacteria in bacteremia. Anaerobe. 2010;16(3):183-189.
8. Lassmann B, Gustafson DR, Wood CM, Rosenblatt JE. Reemergence of anaerobic bacteremia. Clin Infect Dis. 2007;44(7):895-900.
9. Lazarovitch T, Freimann S, Shapira G, Blank H. Decrease in anaerobe-related bacteraemias and increase in Bacteroides species isolation rate from 1998 to 2007: a retrospective study. Anaerobe. 2010;16(3):201-205.
10. Keukeleire S, Wybo I, Naessens A, et al. Anaerobic bacteraemia: a 10-year retrospective epidemiological survey. Anaerobe. 2016;39:54-59.
11. Goldstein EJC, Citron DM, Goldman PJ, Goldman RJ. National hospital survey of anaerobic culture and susceptibility methods: III. Anaerobe. 2008;14(2):68-72.
12. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62(10):e51-e77.
13. Schuetz AN. Antimicrobial resistance and susceptibility testing of anaerobic bacteria. Antimicr Resist. 2014;59(5):698-705.
14. Clinical and Laboratory Standards Institute. M11-A8: Methods for Antimicrobial Susceptibility Testing of Anaerobic Bacteria; Approved Standard. 8th ed. Wayne, PA: Clinical and Laboratory Standards Institute; 2012.
15. White B, Mende K, Weintrob A, et al; Infectious Disease Clinical Research Program Trauma Infectious Disease Outcome Study Group. Epidemiology and antimicrobial susceptibilities of wound isolates of obligate anaerobes from combat casualties. Diagn Mircrobiol Infect Dis. 2016;84(2):144-150.
16. Hastey CJ, Boyd H, Schuetz AN, et al; Ad Hoc Working Group on Antimicrobial Susceptibility Testing of Anaerobic Bacteria of CLSI. Changes in the antibiotic susceptibility of anaerobic bacteria from 2007-2009 to 2010-2012 based on the CLSI methodology. Anaerobe. 2016;42:27-30.
17. Brook I, Wexler HM, Goldstein EJC. Antianaerobic antimicrobials: spectrum and susceptibility testing. Clin Microbiol Rev. 2013;26(3):526-546.
18. Pumbwe L, Wareham D, Aduse-Opoku J, Brazier JS, Wexler HM. Genetic analysis of mechanisms of multidrug resistance in a clinical isolate of Bacteroides fragilis. Clin Microbiol Infect. 2007;13(2):183-189.
19. Solomkin J, Mazuski J, Bradley J, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(2):133-164.
20. Breuil J, Dublanchet A, Truffaut N, Sebald M. Transferable 5-nitroimidazole resistance Bacteroides fragilis group. Plasmid. 1989;21(2):151-154.
21. Nagy E, Urbán E, Nord CE; ESCMID Study Group on Antimicrobial Resistance in Anaerobic Bacteria. Antimicrobial susceptibility of Bacteroides fragilis group isolates in Europe: 20 years of experience. Clin Microbiol Infect. 2011;17(3):371-379.
22. Avila-Campos M, Liu C, Song Y, Rowlinson M-C, Finegold SM. Determination of bft gene subtypes in Bacteroides fragilis clinical isolates. J Clin Microbiol. 2007;45(4):1336-1338.
Acute pyogenic vertebral osteomyelitis is often due to hematogenous spread of aerobic bacteria.1-4 Conversely, only 0.5% of anaerobic bacteremias lead to osteomyelitis.5 Anaerobic osteomyelitis typically results from the contiguous spread of polymicrobial infections through breaks in the gut mucosal barrier and involves the vertebral bodies in only 2% to 5% of cases.5,6 Although Bacteroides fragilis (B fragilis) is the most common anaerobic pathogen cultivated from blood, accounting for about half of all anaerobic blood isolates, it seldom leads to osteomyelitis.1,2,7-11 We report an uncommon case of B fragilis bacteremia and vertebral osteomyelitis confounded by uncertainties in anaerobic identification and susceptibilities.
Case Presentation
A healthy-appearing male aged 55 years presented to the Naval Medical Center Portsmouth (NMCP) with subacute low back pain and fevers of 103 °F for > 3 weeks. While traveling 4 weeks prior, he completed a course of oseltamivir for influenza B infection; afterward, he was diagnosed with community-acquired pneumonia and treated with a dose of ceftriaxone and a 7-day course of doxycycline. The patient presented to the same facility a week later for low back pain and nonresolving respiratory symptoms, and his therapy was changed to azithromycin, cefuroxime, prednisone, and inhalers. Additionally, after being treated for influenza, he developed constipation and hematochezia for which he did not seek care. The hematochezia was similar to a previous episode from an anal fissure 1 year prior that resolved with stool softeners. When he was finally seen at NMCP after 3 weeks of worsening back pain and fevers, lumbosacral magnetic resonance imaging (MRI) demonstrated vertebral osteomyelitis and discitis at L4-L5 and admitted to the hospital (Figure 1).
After a fluoroscopy-guided biopsy of the L4 vertebral body on hospital day 1, the patient was started on cefepime and vancomycin. The biopsy sample was inoculated onto solid media (blood agar, chocolate agar, and MacConkey agar) and incubated at 36 °C for 24 hours in a 5% CO2 atmosphere, as well as onto Shaedler agar with vitamin K and chopped meat glucose broth and incubated at 36 °C for 48 hours under anaerobic conditions. Metronidazole was added and vancomycin discontinued after 2 anaerobic blood culture vials obtained on hospital day 1, incubated in a Becton Dickinson BACTEC FX automated system, which demonstrated Gram-negative bacilli after 48 hours. The blood culture isolates demonstrated a > 99% probability of being identified as ß-lactamase positive Prevotella loescheii using Thermo Fischer Scientific RapID ANA II biochemical testing. Nitrocefinase discs were used to detect ß-lactamase activity.
The biopsy demonstrated nongranulomatous focal areas of necrotic bone and neutrophilia in a hematopoietic background consistent with acute osteomyelitis (Figure 2); on hospital day 4, ß-lactamase positive B fragilis grew from the bone culture. Additionally, 1 anaerobic vial from a surveillance blood culture set that was obtained on hospital day 3 grew ß-lactamasepositive B fragilis using the same identification methods. With these results he was thought to have a polymicrobial infection (B fragilis and Prevotella loescheii [P loescheii]) from a suspected bowel source based on his hematochezia and history of anal fissure. No aerobic, Gram-negative enterobacteriaceae were isolated, but he had previously been on cefuroxime, which has potential activity against these organisms, for ≥ 2 weeks prior to hospitalization and cultures. He was discharged on moxifloxacin and metronidazole pending final culture results, including requested anaerobic susceptibility testing.
At 1-week follow-up, both aerobic and anaerobic vials from surveillance blood cultures remained negative for any microbes, so antibiotics were deescalated to moxifloxacin monotherapy. However, after 3 days the patient was readmitted for increasing C-reactive protein (CRP) levels and intractable back pain with worsening bilateral radiculopathy. A repeat MRI demonstrated interval disease progression with near obliteration of the L4-L5 disc space and hyperenhancement of the prevertebral soft tissues and adjacent psoas musculature without focal rim-enhancing fluid collection (Figure 3). After repeat L4 biopsy, metronidazole was restarted and ertapenem added for enterobacteriaceae coverage, given the known B fragilis and potential suppression from previous cephalosporin therapy; moxifloxacin was discontinued. L4 biopsy cultures showed no growth, and CRP levels trended down from 154.2 mg/L (start of first admission) to 42.4 mg/L (start of second admission) to 14.9 mg/L (day of discharge) (reference range, 5-9.9 mg/L). He was discharged on ertapenem and metronidazole. He completed a 6-week course without further complication.
During antibiotic therapy he had an unremarkable colonoscopy, CRP normalized to 2.6 mg/L (reference range, 0-4.9 mg/L), and he underwent successful L4-L5 transforaminal lumbar interbody fusion 2 weeks after finishing antibiotics.
We retroactively sent both P loescheii isolates and the 1 B fragilis isolate that grew from the surveillance blood culture to the Multidrug-resistant Organism Repository and Surveillance Network (MRSN) at the Walter Reed Army Institute of Research for identification confirmation and susceptibility analysis. Whole genome sequencing with single nucleotide polymorphism (SNP)-based analysis revealed all isolates were 100% identical and consistent with B fragilis and not P loescheii, based on clustering around other B fragilis sequences found in the National Center for Biotechnology Information (NCBI) Genbank database (Figure 4). All isolates carried the antibiotic resistance genes— cepA, sul(2), tetQ— encoding for possible resistance to cephalosporins, sulphonamides, and tetracyclines, respectively; as well as a point mutation in the gyrA gene (Ser82Phe). None of the isolates carried the nim gene, and screening for the 3 subtypes of B fragilis enterotoxin gene (bft-1, bft-2, bft-3) was negative. Eventual susceptibility testing at the Mayo Clinic several months after the conclusion of the case indicated that the B fragilis isolate was sensitive to piperacillin-tazobactam, ertapenem, clindamycin, and metronidazole; however, testing was not performed against moxifloxacin.
Discussion
In the era of growing antibiotic resistance patterns, antimicrobial stewardship programs recommend interventions to improve antimicrobial use through targeted narrow- spectrum antibiotics.12 The Clinical and Laboratory Standards Institute (CLSI) maintains guidelines on the major indications for anaerobic antimicrobial susceptibility testing (AST) to help direct narrow-targeted antimicrobial therapy. However, in a 2008 practice survey Goldstein and colleagues reported that less than half of US hospitals performed anaerobic AST, and only 21% of these facilities did it in-house, while the remainder sent out their isolates for testing.11-14 The CLSI major indications for AST include situations in which the selection of agents is important because of the (1) known resistance of a particular species; (2) confirmation of appropriate therapy for severe infections or for those that may require long-term therapy; (3) persistence of infection despite adequate treatment with an appropriate therapeutic regimen; and (4) difficulty in making empirical decisions based on precedent.14 Additionally, isolates from brain abscess, endocarditis, osteomyelitis, joint infection, infection of prosthetic devices or vascular grafts, bacteremia, and normally sterile body sites (unless contamination suspected) should be tested.14
Because of the lack of anaerobic AST, health care providers must base empiric treatment on reported sensitivities from the medical literature. Empiric selection of antimicrobials for anaerobic infections is made even more challenging by the increased rates of resistance reported in the literature, leading to recommendations to increase susceptibility testing to guide therapy.13,15,16 Empiric therapy of deep-seated anaerobic infections may lead to use of inactive agents or overly broad-spectrum antibiotics. Current antimicrobial stewardship initiatives recognize the importance of narrow-spectrum antibiotics to minimize risk of adverse events and selective pressure for antimicrobial resistance.
Although we attempted to confirm the identification of the anaerobic isolates via commercially available methods, it was not until we performed genetic testing that we were able verify the isolates as B fragilis. Furthermore, earlier susceptibility testing would have allowed for more narrow-targeted antimicrobial therapy and could have potentially prevented our patient’s readmission and use of ertapenem, despite its > 98% susceptibility rates against B fragilis.13,17
All of the B fragilis isolates carried the cepA gene, which is a cephalosporinase that encodes for resistance to cephalosporins and aminopenicillins but not to ß-lactam ß-lactamase inhibitor combinations.13 Although not a substitution for susceptibility analysis, genetic testing showed that all of the isolates carried a nonsynonymous mutation from serine to a phenylalanine at amino acid position 82 (S82F) in the gyrA gene. The S82F mutation has been implicated in fluoroquinolone resistance, via inhibition of substrate–target recognition and binding between fluoroquinolones and the target topoisomerase protein,18 and may potentially explain why our patient clinically worsened while on moxifloxacin monotherapy. Although moxifloxacin susceptibility was not performed, susceptibility rates remain highly variable, ranging from 50% to 70% for B fragilis.13,15,16
It is important to note that the metronidazole the patient received during his first hospital admission could have sterilized the vertebral body without completely eradicating the microbe; thus could explain his clinical worsening while on moxifloxacin monotherapy despite no growth from the repeat biopsy culture. Our rationale for initially continuing moxifloxacin was based on its excellent bioavailability and bone penetration properties. Additionally, of the fluoroquinolones it has the most reliable anaerobic activity and is the only one recommended as monotherapy for complicated intraabdominal infections.19 However, guidelines recommend avoiding its use in patients who have received a fluoroquinolone in the past 90 days or at institutions with high rates of resistance. At our institution Escherichia coli has a > 90% susceptibility rate to fluoroquinolones. Given this rate and our concern that the patient had a polymicrobial infection, we felt that moxifloxacin would provide appropriate anaerobic and aerobic coverage, especially since he had no previous fluoroquinolone exposure.
Additionally, none of the isolates carried the nim or bft toxin genes. Although the nim gene is associated with metronidazole resistance,its presence does not invariably result in resistant strains of B fragilis; in fact, metronidazole resistance is relatively uncommon, with the majority of B fragilis showing < 1% resistance, based on CLSI breakpoints (≥ 32 mg/L).13,20,21 However, one recent epidemiologic study on anaerobic wound isolates from Iraq and Afghanistan casualties found that 12% (2/17) of B fragilis isolates were resistant to metronidazole.15 Given the improvement of the patient’s symptoms while on metronidazole, it is likely that the B fragilis was susceptible. Nevertheless, susceptibility testing with minimum inhibitory concentrations is necessary to verify this result. Also, although enterotoxigenic strains of B fragilis have been associated with bloodstream infections, our patient’s isolates lacked the 3 subtypes of B fragilis enterotoxin gene.22
Conclusions
We report a case of B fragilis bacteremia and vertebral osteomyelitis complicated by challenges in anaerobic identification and sensitivities that led to brief use of a possibly inactive antimicrobial and the subsequent use of carbapenem therapy, which may have been avoided if susceptibility testing were more readily available. This case led to changes in our hospital’s processing of anaerobic isolates to include susceptibility testing on request.
Acknowledgments
We thank Keith Thompson, MD (staff pathologist, Naval Medical Center Portsmouth Virginia), for providing the pathology images from the initial vertebral biopsy, and Dr. Kate Hinkle (director, Multidrug-Resistant Organism Repository and Surveillance Network, Silver Spring, Maryland ) for providing the whole genome sequencing results from the B fragilis isolates.
Acute pyogenic vertebral osteomyelitis is often due to hematogenous spread of aerobic bacteria.1-4 Conversely, only 0.5% of anaerobic bacteremias lead to osteomyelitis.5 Anaerobic osteomyelitis typically results from the contiguous spread of polymicrobial infections through breaks in the gut mucosal barrier and involves the vertebral bodies in only 2% to 5% of cases.5,6 Although Bacteroides fragilis (B fragilis) is the most common anaerobic pathogen cultivated from blood, accounting for about half of all anaerobic blood isolates, it seldom leads to osteomyelitis.1,2,7-11 We report an uncommon case of B fragilis bacteremia and vertebral osteomyelitis confounded by uncertainties in anaerobic identification and susceptibilities.
Case Presentation
A healthy-appearing male aged 55 years presented to the Naval Medical Center Portsmouth (NMCP) with subacute low back pain and fevers of 103 °F for > 3 weeks. While traveling 4 weeks prior, he completed a course of oseltamivir for influenza B infection; afterward, he was diagnosed with community-acquired pneumonia and treated with a dose of ceftriaxone and a 7-day course of doxycycline. The patient presented to the same facility a week later for low back pain and nonresolving respiratory symptoms, and his therapy was changed to azithromycin, cefuroxime, prednisone, and inhalers. Additionally, after being treated for influenza, he developed constipation and hematochezia for which he did not seek care. The hematochezia was similar to a previous episode from an anal fissure 1 year prior that resolved with stool softeners. When he was finally seen at NMCP after 3 weeks of worsening back pain and fevers, lumbosacral magnetic resonance imaging (MRI) demonstrated vertebral osteomyelitis and discitis at L4-L5 and admitted to the hospital (Figure 1).
After a fluoroscopy-guided biopsy of the L4 vertebral body on hospital day 1, the patient was started on cefepime and vancomycin. The biopsy sample was inoculated onto solid media (blood agar, chocolate agar, and MacConkey agar) and incubated at 36 °C for 24 hours in a 5% CO2 atmosphere, as well as onto Shaedler agar with vitamin K and chopped meat glucose broth and incubated at 36 °C for 48 hours under anaerobic conditions. Metronidazole was added and vancomycin discontinued after 2 anaerobic blood culture vials obtained on hospital day 1, incubated in a Becton Dickinson BACTEC FX automated system, which demonstrated Gram-negative bacilli after 48 hours. The blood culture isolates demonstrated a > 99% probability of being identified as ß-lactamase positive Prevotella loescheii using Thermo Fischer Scientific RapID ANA II biochemical testing. Nitrocefinase discs were used to detect ß-lactamase activity.
The biopsy demonstrated nongranulomatous focal areas of necrotic bone and neutrophilia in a hematopoietic background consistent with acute osteomyelitis (Figure 2); on hospital day 4, ß-lactamase positive B fragilis grew from the bone culture. Additionally, 1 anaerobic vial from a surveillance blood culture set that was obtained on hospital day 3 grew ß-lactamasepositive B fragilis using the same identification methods. With these results he was thought to have a polymicrobial infection (B fragilis and Prevotella loescheii [P loescheii]) from a suspected bowel source based on his hematochezia and history of anal fissure. No aerobic, Gram-negative enterobacteriaceae were isolated, but he had previously been on cefuroxime, which has potential activity against these organisms, for ≥ 2 weeks prior to hospitalization and cultures. He was discharged on moxifloxacin and metronidazole pending final culture results, including requested anaerobic susceptibility testing.
At 1-week follow-up, both aerobic and anaerobic vials from surveillance blood cultures remained negative for any microbes, so antibiotics were deescalated to moxifloxacin monotherapy. However, after 3 days the patient was readmitted for increasing C-reactive protein (CRP) levels and intractable back pain with worsening bilateral radiculopathy. A repeat MRI demonstrated interval disease progression with near obliteration of the L4-L5 disc space and hyperenhancement of the prevertebral soft tissues and adjacent psoas musculature without focal rim-enhancing fluid collection (Figure 3). After repeat L4 biopsy, metronidazole was restarted and ertapenem added for enterobacteriaceae coverage, given the known B fragilis and potential suppression from previous cephalosporin therapy; moxifloxacin was discontinued. L4 biopsy cultures showed no growth, and CRP levels trended down from 154.2 mg/L (start of first admission) to 42.4 mg/L (start of second admission) to 14.9 mg/L (day of discharge) (reference range, 5-9.9 mg/L). He was discharged on ertapenem and metronidazole. He completed a 6-week course without further complication.
During antibiotic therapy he had an unremarkable colonoscopy, CRP normalized to 2.6 mg/L (reference range, 0-4.9 mg/L), and he underwent successful L4-L5 transforaminal lumbar interbody fusion 2 weeks after finishing antibiotics.
We retroactively sent both P loescheii isolates and the 1 B fragilis isolate that grew from the surveillance blood culture to the Multidrug-resistant Organism Repository and Surveillance Network (MRSN) at the Walter Reed Army Institute of Research for identification confirmation and susceptibility analysis. Whole genome sequencing with single nucleotide polymorphism (SNP)-based analysis revealed all isolates were 100% identical and consistent with B fragilis and not P loescheii, based on clustering around other B fragilis sequences found in the National Center for Biotechnology Information (NCBI) Genbank database (Figure 4). All isolates carried the antibiotic resistance genes— cepA, sul(2), tetQ— encoding for possible resistance to cephalosporins, sulphonamides, and tetracyclines, respectively; as well as a point mutation in the gyrA gene (Ser82Phe). None of the isolates carried the nim gene, and screening for the 3 subtypes of B fragilis enterotoxin gene (bft-1, bft-2, bft-3) was negative. Eventual susceptibility testing at the Mayo Clinic several months after the conclusion of the case indicated that the B fragilis isolate was sensitive to piperacillin-tazobactam, ertapenem, clindamycin, and metronidazole; however, testing was not performed against moxifloxacin.
Discussion
In the era of growing antibiotic resistance patterns, antimicrobial stewardship programs recommend interventions to improve antimicrobial use through targeted narrow- spectrum antibiotics.12 The Clinical and Laboratory Standards Institute (CLSI) maintains guidelines on the major indications for anaerobic antimicrobial susceptibility testing (AST) to help direct narrow-targeted antimicrobial therapy. However, in a 2008 practice survey Goldstein and colleagues reported that less than half of US hospitals performed anaerobic AST, and only 21% of these facilities did it in-house, while the remainder sent out their isolates for testing.11-14 The CLSI major indications for AST include situations in which the selection of agents is important because of the (1) known resistance of a particular species; (2) confirmation of appropriate therapy for severe infections or for those that may require long-term therapy; (3) persistence of infection despite adequate treatment with an appropriate therapeutic regimen; and (4) difficulty in making empirical decisions based on precedent.14 Additionally, isolates from brain abscess, endocarditis, osteomyelitis, joint infection, infection of prosthetic devices or vascular grafts, bacteremia, and normally sterile body sites (unless contamination suspected) should be tested.14
Because of the lack of anaerobic AST, health care providers must base empiric treatment on reported sensitivities from the medical literature. Empiric selection of antimicrobials for anaerobic infections is made even more challenging by the increased rates of resistance reported in the literature, leading to recommendations to increase susceptibility testing to guide therapy.13,15,16 Empiric therapy of deep-seated anaerobic infections may lead to use of inactive agents or overly broad-spectrum antibiotics. Current antimicrobial stewardship initiatives recognize the importance of narrow-spectrum antibiotics to minimize risk of adverse events and selective pressure for antimicrobial resistance.
Although we attempted to confirm the identification of the anaerobic isolates via commercially available methods, it was not until we performed genetic testing that we were able verify the isolates as B fragilis. Furthermore, earlier susceptibility testing would have allowed for more narrow-targeted antimicrobial therapy and could have potentially prevented our patient’s readmission and use of ertapenem, despite its > 98% susceptibility rates against B fragilis.13,17
All of the B fragilis isolates carried the cepA gene, which is a cephalosporinase that encodes for resistance to cephalosporins and aminopenicillins but not to ß-lactam ß-lactamase inhibitor combinations.13 Although not a substitution for susceptibility analysis, genetic testing showed that all of the isolates carried a nonsynonymous mutation from serine to a phenylalanine at amino acid position 82 (S82F) in the gyrA gene. The S82F mutation has been implicated in fluoroquinolone resistance, via inhibition of substrate–target recognition and binding between fluoroquinolones and the target topoisomerase protein,18 and may potentially explain why our patient clinically worsened while on moxifloxacin monotherapy. Although moxifloxacin susceptibility was not performed, susceptibility rates remain highly variable, ranging from 50% to 70% for B fragilis.13,15,16
It is important to note that the metronidazole the patient received during his first hospital admission could have sterilized the vertebral body without completely eradicating the microbe; thus could explain his clinical worsening while on moxifloxacin monotherapy despite no growth from the repeat biopsy culture. Our rationale for initially continuing moxifloxacin was based on its excellent bioavailability and bone penetration properties. Additionally, of the fluoroquinolones it has the most reliable anaerobic activity and is the only one recommended as monotherapy for complicated intraabdominal infections.19 However, guidelines recommend avoiding its use in patients who have received a fluoroquinolone in the past 90 days or at institutions with high rates of resistance. At our institution Escherichia coli has a > 90% susceptibility rate to fluoroquinolones. Given this rate and our concern that the patient had a polymicrobial infection, we felt that moxifloxacin would provide appropriate anaerobic and aerobic coverage, especially since he had no previous fluoroquinolone exposure.
Additionally, none of the isolates carried the nim or bft toxin genes. Although the nim gene is associated with metronidazole resistance,its presence does not invariably result in resistant strains of B fragilis; in fact, metronidazole resistance is relatively uncommon, with the majority of B fragilis showing < 1% resistance, based on CLSI breakpoints (≥ 32 mg/L).13,20,21 However, one recent epidemiologic study on anaerobic wound isolates from Iraq and Afghanistan casualties found that 12% (2/17) of B fragilis isolates were resistant to metronidazole.15 Given the improvement of the patient’s symptoms while on metronidazole, it is likely that the B fragilis was susceptible. Nevertheless, susceptibility testing with minimum inhibitory concentrations is necessary to verify this result. Also, although enterotoxigenic strains of B fragilis have been associated with bloodstream infections, our patient’s isolates lacked the 3 subtypes of B fragilis enterotoxin gene.22
Conclusions
We report a case of B fragilis bacteremia and vertebral osteomyelitis complicated by challenges in anaerobic identification and sensitivities that led to brief use of a possibly inactive antimicrobial and the subsequent use of carbapenem therapy, which may have been avoided if susceptibility testing were more readily available. This case led to changes in our hospital’s processing of anaerobic isolates to include susceptibility testing on request.
Acknowledgments
We thank Keith Thompson, MD (staff pathologist, Naval Medical Center Portsmouth Virginia), for providing the pathology images from the initial vertebral biopsy, and Dr. Kate Hinkle (director, Multidrug-Resistant Organism Repository and Surveillance Network, Silver Spring, Maryland ) for providing the whole genome sequencing results from the B fragilis isolates.
1. Zimmerli W. Vertebral osteomyelitis. N Eng J Med. 2010;362(11):1022-1029.
2. Chazan B, Strahilevitz J, Millgram MA, Kaufmann S, Raz R. Bacteroides fragilis vertebral osteomyelitis secondary to anal dilatation. Spine (Phila PA 1976). 2001;26(16):E377-E378.
3. Kierzkowska M, Pedzisz P, Babiak I, et al. Orthopedic infections caused by obligatory anaerobic Gram-negative rods: report of two cases. Med Microbiol Immunol. 2017;206(5):363-366.
4. McHenry M, Easley K, Locker G. Vertebral osteomyelitis: long-term outcome for 253 patients from 7 Cleveland-area hospitals. Clin Infect Dis. 2002;34(10):1342-1350.
5. Raff MJ, Melo JC. Anaerobic osteomyelitis. Medicine (Baltimore).1978;57(1):83-103.
6. Lewis R, Sutter V, Finegold S. Bone infections involving anaerobic bacteria. Medicine (Baltimore). 1978;57(1):279-305.
7. Brook I. The role of anaerobic bacteria in bacteremia. Anaerobe. 2010;16(3):183-189.
8. Lassmann B, Gustafson DR, Wood CM, Rosenblatt JE. Reemergence of anaerobic bacteremia. Clin Infect Dis. 2007;44(7):895-900.
9. Lazarovitch T, Freimann S, Shapira G, Blank H. Decrease in anaerobe-related bacteraemias and increase in Bacteroides species isolation rate from 1998 to 2007: a retrospective study. Anaerobe. 2010;16(3):201-205.
10. Keukeleire S, Wybo I, Naessens A, et al. Anaerobic bacteraemia: a 10-year retrospective epidemiological survey. Anaerobe. 2016;39:54-59.
11. Goldstein EJC, Citron DM, Goldman PJ, Goldman RJ. National hospital survey of anaerobic culture and susceptibility methods: III. Anaerobe. 2008;14(2):68-72.
12. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62(10):e51-e77.
13. Schuetz AN. Antimicrobial resistance and susceptibility testing of anaerobic bacteria. Antimicr Resist. 2014;59(5):698-705.
14. Clinical and Laboratory Standards Institute. M11-A8: Methods for Antimicrobial Susceptibility Testing of Anaerobic Bacteria; Approved Standard. 8th ed. Wayne, PA: Clinical and Laboratory Standards Institute; 2012.
15. White B, Mende K, Weintrob A, et al; Infectious Disease Clinical Research Program Trauma Infectious Disease Outcome Study Group. Epidemiology and antimicrobial susceptibilities of wound isolates of obligate anaerobes from combat casualties. Diagn Mircrobiol Infect Dis. 2016;84(2):144-150.
16. Hastey CJ, Boyd H, Schuetz AN, et al; Ad Hoc Working Group on Antimicrobial Susceptibility Testing of Anaerobic Bacteria of CLSI. Changes in the antibiotic susceptibility of anaerobic bacteria from 2007-2009 to 2010-2012 based on the CLSI methodology. Anaerobe. 2016;42:27-30.
17. Brook I, Wexler HM, Goldstein EJC. Antianaerobic antimicrobials: spectrum and susceptibility testing. Clin Microbiol Rev. 2013;26(3):526-546.
18. Pumbwe L, Wareham D, Aduse-Opoku J, Brazier JS, Wexler HM. Genetic analysis of mechanisms of multidrug resistance in a clinical isolate of Bacteroides fragilis. Clin Microbiol Infect. 2007;13(2):183-189.
19. Solomkin J, Mazuski J, Bradley J, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(2):133-164.
20. Breuil J, Dublanchet A, Truffaut N, Sebald M. Transferable 5-nitroimidazole resistance Bacteroides fragilis group. Plasmid. 1989;21(2):151-154.
21. Nagy E, Urbán E, Nord CE; ESCMID Study Group on Antimicrobial Resistance in Anaerobic Bacteria. Antimicrobial susceptibility of Bacteroides fragilis group isolates in Europe: 20 years of experience. Clin Microbiol Infect. 2011;17(3):371-379.
22. Avila-Campos M, Liu C, Song Y, Rowlinson M-C, Finegold SM. Determination of bft gene subtypes in Bacteroides fragilis clinical isolates. J Clin Microbiol. 2007;45(4):1336-1338.
1. Zimmerli W. Vertebral osteomyelitis. N Eng J Med. 2010;362(11):1022-1029.
2. Chazan B, Strahilevitz J, Millgram MA, Kaufmann S, Raz R. Bacteroides fragilis vertebral osteomyelitis secondary to anal dilatation. Spine (Phila PA 1976). 2001;26(16):E377-E378.
3. Kierzkowska M, Pedzisz P, Babiak I, et al. Orthopedic infections caused by obligatory anaerobic Gram-negative rods: report of two cases. Med Microbiol Immunol. 2017;206(5):363-366.
4. McHenry M, Easley K, Locker G. Vertebral osteomyelitis: long-term outcome for 253 patients from 7 Cleveland-area hospitals. Clin Infect Dis. 2002;34(10):1342-1350.
5. Raff MJ, Melo JC. Anaerobic osteomyelitis. Medicine (Baltimore).1978;57(1):83-103.
6. Lewis R, Sutter V, Finegold S. Bone infections involving anaerobic bacteria. Medicine (Baltimore). 1978;57(1):279-305.
7. Brook I. The role of anaerobic bacteria in bacteremia. Anaerobe. 2010;16(3):183-189.
8. Lassmann B, Gustafson DR, Wood CM, Rosenblatt JE. Reemergence of anaerobic bacteremia. Clin Infect Dis. 2007;44(7):895-900.
9. Lazarovitch T, Freimann S, Shapira G, Blank H. Decrease in anaerobe-related bacteraemias and increase in Bacteroides species isolation rate from 1998 to 2007: a retrospective study. Anaerobe. 2010;16(3):201-205.
10. Keukeleire S, Wybo I, Naessens A, et al. Anaerobic bacteraemia: a 10-year retrospective epidemiological survey. Anaerobe. 2016;39:54-59.
11. Goldstein EJC, Citron DM, Goldman PJ, Goldman RJ. National hospital survey of anaerobic culture and susceptibility methods: III. Anaerobe. 2008;14(2):68-72.
12. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62(10):e51-e77.
13. Schuetz AN. Antimicrobial resistance and susceptibility testing of anaerobic bacteria. Antimicr Resist. 2014;59(5):698-705.
14. Clinical and Laboratory Standards Institute. M11-A8: Methods for Antimicrobial Susceptibility Testing of Anaerobic Bacteria; Approved Standard. 8th ed. Wayne, PA: Clinical and Laboratory Standards Institute; 2012.
15. White B, Mende K, Weintrob A, et al; Infectious Disease Clinical Research Program Trauma Infectious Disease Outcome Study Group. Epidemiology and antimicrobial susceptibilities of wound isolates of obligate anaerobes from combat casualties. Diagn Mircrobiol Infect Dis. 2016;84(2):144-150.
16. Hastey CJ, Boyd H, Schuetz AN, et al; Ad Hoc Working Group on Antimicrobial Susceptibility Testing of Anaerobic Bacteria of CLSI. Changes in the antibiotic susceptibility of anaerobic bacteria from 2007-2009 to 2010-2012 based on the CLSI methodology. Anaerobe. 2016;42:27-30.
17. Brook I, Wexler HM, Goldstein EJC. Antianaerobic antimicrobials: spectrum and susceptibility testing. Clin Microbiol Rev. 2013;26(3):526-546.
18. Pumbwe L, Wareham D, Aduse-Opoku J, Brazier JS, Wexler HM. Genetic analysis of mechanisms of multidrug resistance in a clinical isolate of Bacteroides fragilis. Clin Microbiol Infect. 2007;13(2):183-189.
19. Solomkin J, Mazuski J, Bradley J, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(2):133-164.
20. Breuil J, Dublanchet A, Truffaut N, Sebald M. Transferable 5-nitroimidazole resistance Bacteroides fragilis group. Plasmid. 1989;21(2):151-154.
21. Nagy E, Urbán E, Nord CE; ESCMID Study Group on Antimicrobial Resistance in Anaerobic Bacteria. Antimicrobial susceptibility of Bacteroides fragilis group isolates in Europe: 20 years of experience. Clin Microbiol Infect. 2011;17(3):371-379.
22. Avila-Campos M, Liu C, Song Y, Rowlinson M-C, Finegold SM. Determination of bft gene subtypes in Bacteroides fragilis clinical isolates. J Clin Microbiol. 2007;45(4):1336-1338.
How to expand the APP role in a crisis
An opportunity to better appreciate the value of PAs, NPs
Advanced practice providers – physician assistants and nurse practitioners – at the 733-bed Emory University Hospital in Atlanta are playing an expanded role in the admission of patients into the hospital, particularly those suspected of having COVID-19.
Before the pandemic crisis, evaluation visits by the APP would have been reviewed on the same day by the supervising physician through an in-person encounter with the patient. The new protocol is not outside of scope-of-practice regulations for APPs in Georgia or of the hospital’s bylaws. But it offers a way to help limit the overall exposure of hospital staff to patients suspected of COVID-19 infection, and the total amount of time providers spend in such patients’ room. Just one provider now needs to meet the patient during the admissions process, while the attending physician can fulfill a requirement for seeing the patient within 24 hours during rounds the following day. Emergency encounters would still be done as needed.
These protocols point toward future conversations about the limits to APPs’ scope of practice, and whether more expansive approaches could be widely adopted once the current crisis is over, say advocates for the APPs’ role.
“Our APPs are primarily doing the admissions to the hospital of COVID patients and of non-COVID patients, as we’ve always done. But with COVID-infected or -suspected patients, we’re trying to minimize exposure for our providers,” explained Susan Ortiz, a certified PA, lead APP at Emory University Hospital. “In this way, we can also see more patients more efficiently.” Ms. Ortiz said she finds in talking to other APP leads in the Emory system that “each facility has its own culture and way of doing things. But for the most part, they’re all trying to do something to limit providers’ time in patients’ rooms.”
In response to the rapidly moving crisis, tactics to limit personnel in COVID patients’ rooms to the “absolutely essential” include gathering much of the needed history and other information requested from the patient by telephone, Ms. Ortiz said. This can be done either over the patient’s own cell phone or a phone placed in the room by hospital staff. Family members may be called to supplement this information, with the patient’s consent.
Once vital sign monitoring equipment is hooked up, it is possible to monitor the patient’s vital signs remotely without making frequent trips into the room. That way, in-person vital sign monitoring doesn’t need to happen routinely – at least not as often. One observation by clinicians on Ms. Ortiz’s team: listening for lung sounds with a stethoscope has not been shown to alter treatment for these patients. Once a chest X-ray shows structural changes in a patient’s lung, all lung exams are going to sound bad.
The admitting provider still needs to meet the patient in person for part of the admission visit and physical exam, but the amount of time spent in close personal contact with the patient can be much shorter, Ms. Ortiz said. For patients who are admitted, if there is a question about difficulty swallowing, they will see a speech pathologist, and if evidence of malnutrition, a nutritionist. “But we have to be extremely thoughtful about when people go into the room. So we are not ordering these ancillary services as routinely as we do during non-COVID times,” she said.
Appropriate levels of fear
Emory’s hospitalists are communicating daily about a rapidly changing situation. “We get a note by email every day, and we have a Dropbox account for downloading more information,” Ms. Ortiz said. A joint on-call system is used to provide backup coverage of APPs at the seven Emory hospitals. When replacement shifts need filling in a hurry, practitioners are able to obtain emergency credentials at any of the other hospitals. “It’s a voluntary process to sign up to be on-call,” Ms. Ortiz said. So far, that has been sufficient.
All staff have their own level of “appropriate fear” of this infection, Ms. Ortiz noted. “We have an extremely supportive group here to back up those of us who, for good reason, don’t want to be admitting the COVID patients.” Ms. Ortiz opted out of doing COVID admissions because her husband’s health places him at particular risk. “But with the cross-coverage we have, sometimes I’ll provide assistance when needed if a patient is suspected of being infected.” APPs are critical to Emory’s hospital medicine group – not ancillaries. “Everyone here feels that way. So we want to give them a lot of support. We’re all pitching in, doing it together,” she said.
“We said when we started with this, a couple of weeks before the surge started, that you could volunteer to see COVID patients,” said Emory hospitalist Jessica Nave, MD. “As we came to realize that the demand would be greater, we said you would need to opt out of seeing these patients, rather than opt in, and have a reason for doing so.” An example is pregnant staff, of which there seems to be a lot at Emory right now, Dr. Nave said, or those who are immunocompromised for other reasons. Those who don’t opt out are seeing the majority of the COVID patients, depending on actual need.
Dr. Nave is married to another hospitalist at Emory. “We can’t isolate from each other or our children. He and I have a regimented protocol for how we handle the risk, which includes taking off our shoes and clothes in the garage, showering and wiping down every place we might have touched. But those steps are not guarantees.” Other staff at Emory are isolating from their families for weeks at a time. Emory has a conference hotel offering discounted rates to staff. Nine physicians at Emory have been tested for the infection based on presenting symptoms, but at press time none had tested positive.
Streamlining code blue
Another area in which Emory has revised its policies in response to COVID-19 is for in-hospital cardiac arrest code response. Codes are inherently unpredictable, and crowd control has always been an issue for them, Dr. Nave said. “Historically, you could have 15 or more people show up when a code was called. Now, more than ever, we need to limit the number of people involved, for the same reason, avoiding unnecessary patient contact.”
The hospital’s Resuscitation Committee took the lead on developing a new policy, approved by the its Critical Care Committee and COVID Task Force, to limit the number of professionals in the room when running a code to an essential six: two doing chest compression, two managing airways, a code leader, and a critical care nurse. Outside the patient’s door, wearing the same personal protective equipment (PPE), are a pharmacist, recorder, and runner. “If you’re not one of those nine, you don’t need to be involved and should leave the area,” Dr. Nave said.
Staff have been instructed that they need to don appropriate PPE, including gown, mask, and eye wear, before entering the room for a code – even if that delays the start of intervention. “We’ve also made a code kit for each unit with quickly accessible gowns and masks. It should be used only for code blues.”
Increasing flexibility for the team
PAs and NPs in other locations are also exploring opportunities for gearing up to play larger roles in hospital care in the current crisis situation. The American Association of Physician Assistants has urged all U.S. governors to issue executive orders to waive state-specific licensing requirements for physician supervision or collaboration during the crisis, in order to increase flexibility of health care teams to deploy APPs.
AAPA believes the supervisory requirement is the biggest current barrier to mobilizing PAs and NPs. That includes those who have been furloughed from outpatient or other settings but are limited in their ability to contribute to the COVID crisis by the need to sign a supervision agreement with a physician at a new hospital.
The crisis is creating an opportunity to better appreciate the value PAs and NPs bring to health care, said Tracy Cardin, ACNP-BC, SFHM, vice president for advanced practice providers at Sound Physicians, a national hospitalist company based in Tacoma, Wash. The company recently sent a memo to the leadership of hospital sites at which it has contracts, requesting suspension of the hospitals’ requirements for a daily physician supervisory visit for APPs – which can be a hurdle when trying to leverage all hands on deck in the crisis.
NPs and PAs are stepping up and volunteering for COVID patients, Ms. Cardin said. Some have even taken leaves from their jobs to go to New York to help out at the epicenter of the U.S. crisis. “They want to make a difference. We’ve been deploying nonhospital medicine APPs from surgery, primary care, and elsewhere, embedding them on the hospital medicine team.”
Before the crisis, APPs at Sound Physicians weren’t always able to practice at the top of their licenses, depending on the hospital setting, added Alicia Scheffer, CNP, the company’s Great Lakes regional director for APPs. “Then COVID-19 showed up and really expedited conversations about how to maximize caseloads using APPs and about the fear of failing patients due to lack of capacity.”
In several locales, Sound Physicians is using quarantined providers to do telephone triage, or staffing ICUs with APPs backed up by telemedicine. “In APP-led ICUs, where the nurses are leading, they are intubating patients, placing central lines, things we weren’t allowed to do before,” Ms. Scheffer said.
A spirit of improvisation
There is a lot of tension at Emory University Hospital these days, reflecting the fears and uncertainties about the crisis, Dr. Nave said. “But there’s also a strangely powerful camaraderie like I’ve never seen before. When you walk onto the COVID units, you feel immediately bonded to the nurses, the techs, the phlebotomists. And you feel like you could talk about anything.”
Changes such as those made at Emory, have been talked about for a while, for example when hospitalists are having a busy night, she said. “But because this is a big cultural change, some physicians resisted it. We trust our APPs. But if the doctor’s name is on a patient chart, they want to see the patient – just for their own comfort level.”
Ms. Ortiz thinks the experience with the COVID crisis could help to advance the conversation about the appropriate role for APPs and their scope of practice in hospital medicine, once the current crisis has passed. “People were used to always doing things a certain way. This experience, hopefully, will get us to the point where attending physicians have more comfort with the APP’s ability to act autonomously,” she said.
“We’ve also talked about piloting telemedicine examinations using Zoom,” Dr. Nave added. “It’s making us think a lot of remote cross-coverage could be done that way. We’ve talked about using the hospital’s iPads with patients. This crisis really makes you think you want to innovate, in a spirit of improvisation,” she said. “Now is the time to try some of these things.”
Editors note: During the COVID-19 pandemic, many hospitals are seeing unprecedented volumes of patients requiring hospital medicine groups to stretch their current resources and recruit providers from outside their groups to bolster their inpatient services. The Society of Hospital Medicine has put together the following stepwise guide for onboarding traditional outpatient and subspecialty-based providers to work on general medicine wards: COVID-19 nonhospitalist onboarding resources.
An opportunity to better appreciate the value of PAs, NPs
An opportunity to better appreciate the value of PAs, NPs
Advanced practice providers – physician assistants and nurse practitioners – at the 733-bed Emory University Hospital in Atlanta are playing an expanded role in the admission of patients into the hospital, particularly those suspected of having COVID-19.
Before the pandemic crisis, evaluation visits by the APP would have been reviewed on the same day by the supervising physician through an in-person encounter with the patient. The new protocol is not outside of scope-of-practice regulations for APPs in Georgia or of the hospital’s bylaws. But it offers a way to help limit the overall exposure of hospital staff to patients suspected of COVID-19 infection, and the total amount of time providers spend in such patients’ room. Just one provider now needs to meet the patient during the admissions process, while the attending physician can fulfill a requirement for seeing the patient within 24 hours during rounds the following day. Emergency encounters would still be done as needed.
These protocols point toward future conversations about the limits to APPs’ scope of practice, and whether more expansive approaches could be widely adopted once the current crisis is over, say advocates for the APPs’ role.
“Our APPs are primarily doing the admissions to the hospital of COVID patients and of non-COVID patients, as we’ve always done. But with COVID-infected or -suspected patients, we’re trying to minimize exposure for our providers,” explained Susan Ortiz, a certified PA, lead APP at Emory University Hospital. “In this way, we can also see more patients more efficiently.” Ms. Ortiz said she finds in talking to other APP leads in the Emory system that “each facility has its own culture and way of doing things. But for the most part, they’re all trying to do something to limit providers’ time in patients’ rooms.”
In response to the rapidly moving crisis, tactics to limit personnel in COVID patients’ rooms to the “absolutely essential” include gathering much of the needed history and other information requested from the patient by telephone, Ms. Ortiz said. This can be done either over the patient’s own cell phone or a phone placed in the room by hospital staff. Family members may be called to supplement this information, with the patient’s consent.
Once vital sign monitoring equipment is hooked up, it is possible to monitor the patient’s vital signs remotely without making frequent trips into the room. That way, in-person vital sign monitoring doesn’t need to happen routinely – at least not as often. One observation by clinicians on Ms. Ortiz’s team: listening for lung sounds with a stethoscope has not been shown to alter treatment for these patients. Once a chest X-ray shows structural changes in a patient’s lung, all lung exams are going to sound bad.
The admitting provider still needs to meet the patient in person for part of the admission visit and physical exam, but the amount of time spent in close personal contact with the patient can be much shorter, Ms. Ortiz said. For patients who are admitted, if there is a question about difficulty swallowing, they will see a speech pathologist, and if evidence of malnutrition, a nutritionist. “But we have to be extremely thoughtful about when people go into the room. So we are not ordering these ancillary services as routinely as we do during non-COVID times,” she said.
Appropriate levels of fear
Emory’s hospitalists are communicating daily about a rapidly changing situation. “We get a note by email every day, and we have a Dropbox account for downloading more information,” Ms. Ortiz said. A joint on-call system is used to provide backup coverage of APPs at the seven Emory hospitals. When replacement shifts need filling in a hurry, practitioners are able to obtain emergency credentials at any of the other hospitals. “It’s a voluntary process to sign up to be on-call,” Ms. Ortiz said. So far, that has been sufficient.
All staff have their own level of “appropriate fear” of this infection, Ms. Ortiz noted. “We have an extremely supportive group here to back up those of us who, for good reason, don’t want to be admitting the COVID patients.” Ms. Ortiz opted out of doing COVID admissions because her husband’s health places him at particular risk. “But with the cross-coverage we have, sometimes I’ll provide assistance when needed if a patient is suspected of being infected.” APPs are critical to Emory’s hospital medicine group – not ancillaries. “Everyone here feels that way. So we want to give them a lot of support. We’re all pitching in, doing it together,” she said.
“We said when we started with this, a couple of weeks before the surge started, that you could volunteer to see COVID patients,” said Emory hospitalist Jessica Nave, MD. “As we came to realize that the demand would be greater, we said you would need to opt out of seeing these patients, rather than opt in, and have a reason for doing so.” An example is pregnant staff, of which there seems to be a lot at Emory right now, Dr. Nave said, or those who are immunocompromised for other reasons. Those who don’t opt out are seeing the majority of the COVID patients, depending on actual need.
Dr. Nave is married to another hospitalist at Emory. “We can’t isolate from each other or our children. He and I have a regimented protocol for how we handle the risk, which includes taking off our shoes and clothes in the garage, showering and wiping down every place we might have touched. But those steps are not guarantees.” Other staff at Emory are isolating from their families for weeks at a time. Emory has a conference hotel offering discounted rates to staff. Nine physicians at Emory have been tested for the infection based on presenting symptoms, but at press time none had tested positive.
Streamlining code blue
Another area in which Emory has revised its policies in response to COVID-19 is for in-hospital cardiac arrest code response. Codes are inherently unpredictable, and crowd control has always been an issue for them, Dr. Nave said. “Historically, you could have 15 or more people show up when a code was called. Now, more than ever, we need to limit the number of people involved, for the same reason, avoiding unnecessary patient contact.”
The hospital’s Resuscitation Committee took the lead on developing a new policy, approved by the its Critical Care Committee and COVID Task Force, to limit the number of professionals in the room when running a code to an essential six: two doing chest compression, two managing airways, a code leader, and a critical care nurse. Outside the patient’s door, wearing the same personal protective equipment (PPE), are a pharmacist, recorder, and runner. “If you’re not one of those nine, you don’t need to be involved and should leave the area,” Dr. Nave said.
Staff have been instructed that they need to don appropriate PPE, including gown, mask, and eye wear, before entering the room for a code – even if that delays the start of intervention. “We’ve also made a code kit for each unit with quickly accessible gowns and masks. It should be used only for code blues.”
Increasing flexibility for the team
PAs and NPs in other locations are also exploring opportunities for gearing up to play larger roles in hospital care in the current crisis situation. The American Association of Physician Assistants has urged all U.S. governors to issue executive orders to waive state-specific licensing requirements for physician supervision or collaboration during the crisis, in order to increase flexibility of health care teams to deploy APPs.
AAPA believes the supervisory requirement is the biggest current barrier to mobilizing PAs and NPs. That includes those who have been furloughed from outpatient or other settings but are limited in their ability to contribute to the COVID crisis by the need to sign a supervision agreement with a physician at a new hospital.
The crisis is creating an opportunity to better appreciate the value PAs and NPs bring to health care, said Tracy Cardin, ACNP-BC, SFHM, vice president for advanced practice providers at Sound Physicians, a national hospitalist company based in Tacoma, Wash. The company recently sent a memo to the leadership of hospital sites at which it has contracts, requesting suspension of the hospitals’ requirements for a daily physician supervisory visit for APPs – which can be a hurdle when trying to leverage all hands on deck in the crisis.
NPs and PAs are stepping up and volunteering for COVID patients, Ms. Cardin said. Some have even taken leaves from their jobs to go to New York to help out at the epicenter of the U.S. crisis. “They want to make a difference. We’ve been deploying nonhospital medicine APPs from surgery, primary care, and elsewhere, embedding them on the hospital medicine team.”
Before the crisis, APPs at Sound Physicians weren’t always able to practice at the top of their licenses, depending on the hospital setting, added Alicia Scheffer, CNP, the company’s Great Lakes regional director for APPs. “Then COVID-19 showed up and really expedited conversations about how to maximize caseloads using APPs and about the fear of failing patients due to lack of capacity.”
In several locales, Sound Physicians is using quarantined providers to do telephone triage, or staffing ICUs with APPs backed up by telemedicine. “In APP-led ICUs, where the nurses are leading, they are intubating patients, placing central lines, things we weren’t allowed to do before,” Ms. Scheffer said.
A spirit of improvisation
There is a lot of tension at Emory University Hospital these days, reflecting the fears and uncertainties about the crisis, Dr. Nave said. “But there’s also a strangely powerful camaraderie like I’ve never seen before. When you walk onto the COVID units, you feel immediately bonded to the nurses, the techs, the phlebotomists. And you feel like you could talk about anything.”
Changes such as those made at Emory, have been talked about for a while, for example when hospitalists are having a busy night, she said. “But because this is a big cultural change, some physicians resisted it. We trust our APPs. But if the doctor’s name is on a patient chart, they want to see the patient – just for their own comfort level.”
Ms. Ortiz thinks the experience with the COVID crisis could help to advance the conversation about the appropriate role for APPs and their scope of practice in hospital medicine, once the current crisis has passed. “People were used to always doing things a certain way. This experience, hopefully, will get us to the point where attending physicians have more comfort with the APP’s ability to act autonomously,” she said.
“We’ve also talked about piloting telemedicine examinations using Zoom,” Dr. Nave added. “It’s making us think a lot of remote cross-coverage could be done that way. We’ve talked about using the hospital’s iPads with patients. This crisis really makes you think you want to innovate, in a spirit of improvisation,” she said. “Now is the time to try some of these things.”
Editors note: During the COVID-19 pandemic, many hospitals are seeing unprecedented volumes of patients requiring hospital medicine groups to stretch their current resources and recruit providers from outside their groups to bolster their inpatient services. The Society of Hospital Medicine has put together the following stepwise guide for onboarding traditional outpatient and subspecialty-based providers to work on general medicine wards: COVID-19 nonhospitalist onboarding resources.
Advanced practice providers – physician assistants and nurse practitioners – at the 733-bed Emory University Hospital in Atlanta are playing an expanded role in the admission of patients into the hospital, particularly those suspected of having COVID-19.
Before the pandemic crisis, evaluation visits by the APP would have been reviewed on the same day by the supervising physician through an in-person encounter with the patient. The new protocol is not outside of scope-of-practice regulations for APPs in Georgia or of the hospital’s bylaws. But it offers a way to help limit the overall exposure of hospital staff to patients suspected of COVID-19 infection, and the total amount of time providers spend in such patients’ room. Just one provider now needs to meet the patient during the admissions process, while the attending physician can fulfill a requirement for seeing the patient within 24 hours during rounds the following day. Emergency encounters would still be done as needed.
These protocols point toward future conversations about the limits to APPs’ scope of practice, and whether more expansive approaches could be widely adopted once the current crisis is over, say advocates for the APPs’ role.
“Our APPs are primarily doing the admissions to the hospital of COVID patients and of non-COVID patients, as we’ve always done. But with COVID-infected or -suspected patients, we’re trying to minimize exposure for our providers,” explained Susan Ortiz, a certified PA, lead APP at Emory University Hospital. “In this way, we can also see more patients more efficiently.” Ms. Ortiz said she finds in talking to other APP leads in the Emory system that “each facility has its own culture and way of doing things. But for the most part, they’re all trying to do something to limit providers’ time in patients’ rooms.”
In response to the rapidly moving crisis, tactics to limit personnel in COVID patients’ rooms to the “absolutely essential” include gathering much of the needed history and other information requested from the patient by telephone, Ms. Ortiz said. This can be done either over the patient’s own cell phone or a phone placed in the room by hospital staff. Family members may be called to supplement this information, with the patient’s consent.
Once vital sign monitoring equipment is hooked up, it is possible to monitor the patient’s vital signs remotely without making frequent trips into the room. That way, in-person vital sign monitoring doesn’t need to happen routinely – at least not as often. One observation by clinicians on Ms. Ortiz’s team: listening for lung sounds with a stethoscope has not been shown to alter treatment for these patients. Once a chest X-ray shows structural changes in a patient’s lung, all lung exams are going to sound bad.
The admitting provider still needs to meet the patient in person for part of the admission visit and physical exam, but the amount of time spent in close personal contact with the patient can be much shorter, Ms. Ortiz said. For patients who are admitted, if there is a question about difficulty swallowing, they will see a speech pathologist, and if evidence of malnutrition, a nutritionist. “But we have to be extremely thoughtful about when people go into the room. So we are not ordering these ancillary services as routinely as we do during non-COVID times,” she said.
Appropriate levels of fear
Emory’s hospitalists are communicating daily about a rapidly changing situation. “We get a note by email every day, and we have a Dropbox account for downloading more information,” Ms. Ortiz said. A joint on-call system is used to provide backup coverage of APPs at the seven Emory hospitals. When replacement shifts need filling in a hurry, practitioners are able to obtain emergency credentials at any of the other hospitals. “It’s a voluntary process to sign up to be on-call,” Ms. Ortiz said. So far, that has been sufficient.
All staff have their own level of “appropriate fear” of this infection, Ms. Ortiz noted. “We have an extremely supportive group here to back up those of us who, for good reason, don’t want to be admitting the COVID patients.” Ms. Ortiz opted out of doing COVID admissions because her husband’s health places him at particular risk. “But with the cross-coverage we have, sometimes I’ll provide assistance when needed if a patient is suspected of being infected.” APPs are critical to Emory’s hospital medicine group – not ancillaries. “Everyone here feels that way. So we want to give them a lot of support. We’re all pitching in, doing it together,” she said.
“We said when we started with this, a couple of weeks before the surge started, that you could volunteer to see COVID patients,” said Emory hospitalist Jessica Nave, MD. “As we came to realize that the demand would be greater, we said you would need to opt out of seeing these patients, rather than opt in, and have a reason for doing so.” An example is pregnant staff, of which there seems to be a lot at Emory right now, Dr. Nave said, or those who are immunocompromised for other reasons. Those who don’t opt out are seeing the majority of the COVID patients, depending on actual need.
Dr. Nave is married to another hospitalist at Emory. “We can’t isolate from each other or our children. He and I have a regimented protocol for how we handle the risk, which includes taking off our shoes and clothes in the garage, showering and wiping down every place we might have touched. But those steps are not guarantees.” Other staff at Emory are isolating from their families for weeks at a time. Emory has a conference hotel offering discounted rates to staff. Nine physicians at Emory have been tested for the infection based on presenting symptoms, but at press time none had tested positive.
Streamlining code blue
Another area in which Emory has revised its policies in response to COVID-19 is for in-hospital cardiac arrest code response. Codes are inherently unpredictable, and crowd control has always been an issue for them, Dr. Nave said. “Historically, you could have 15 or more people show up when a code was called. Now, more than ever, we need to limit the number of people involved, for the same reason, avoiding unnecessary patient contact.”
The hospital’s Resuscitation Committee took the lead on developing a new policy, approved by the its Critical Care Committee and COVID Task Force, to limit the number of professionals in the room when running a code to an essential six: two doing chest compression, two managing airways, a code leader, and a critical care nurse. Outside the patient’s door, wearing the same personal protective equipment (PPE), are a pharmacist, recorder, and runner. “If you’re not one of those nine, you don’t need to be involved and should leave the area,” Dr. Nave said.
Staff have been instructed that they need to don appropriate PPE, including gown, mask, and eye wear, before entering the room for a code – even if that delays the start of intervention. “We’ve also made a code kit for each unit with quickly accessible gowns and masks. It should be used only for code blues.”
Increasing flexibility for the team
PAs and NPs in other locations are also exploring opportunities for gearing up to play larger roles in hospital care in the current crisis situation. The American Association of Physician Assistants has urged all U.S. governors to issue executive orders to waive state-specific licensing requirements for physician supervision or collaboration during the crisis, in order to increase flexibility of health care teams to deploy APPs.
AAPA believes the supervisory requirement is the biggest current barrier to mobilizing PAs and NPs. That includes those who have been furloughed from outpatient or other settings but are limited in their ability to contribute to the COVID crisis by the need to sign a supervision agreement with a physician at a new hospital.
The crisis is creating an opportunity to better appreciate the value PAs and NPs bring to health care, said Tracy Cardin, ACNP-BC, SFHM, vice president for advanced practice providers at Sound Physicians, a national hospitalist company based in Tacoma, Wash. The company recently sent a memo to the leadership of hospital sites at which it has contracts, requesting suspension of the hospitals’ requirements for a daily physician supervisory visit for APPs – which can be a hurdle when trying to leverage all hands on deck in the crisis.
NPs and PAs are stepping up and volunteering for COVID patients, Ms. Cardin said. Some have even taken leaves from their jobs to go to New York to help out at the epicenter of the U.S. crisis. “They want to make a difference. We’ve been deploying nonhospital medicine APPs from surgery, primary care, and elsewhere, embedding them on the hospital medicine team.”
Before the crisis, APPs at Sound Physicians weren’t always able to practice at the top of their licenses, depending on the hospital setting, added Alicia Scheffer, CNP, the company’s Great Lakes regional director for APPs. “Then COVID-19 showed up and really expedited conversations about how to maximize caseloads using APPs and about the fear of failing patients due to lack of capacity.”
In several locales, Sound Physicians is using quarantined providers to do telephone triage, or staffing ICUs with APPs backed up by telemedicine. “In APP-led ICUs, where the nurses are leading, they are intubating patients, placing central lines, things we weren’t allowed to do before,” Ms. Scheffer said.
A spirit of improvisation
There is a lot of tension at Emory University Hospital these days, reflecting the fears and uncertainties about the crisis, Dr. Nave said. “But there’s also a strangely powerful camaraderie like I’ve never seen before. When you walk onto the COVID units, you feel immediately bonded to the nurses, the techs, the phlebotomists. And you feel like you could talk about anything.”
Changes such as those made at Emory, have been talked about for a while, for example when hospitalists are having a busy night, she said. “But because this is a big cultural change, some physicians resisted it. We trust our APPs. But if the doctor’s name is on a patient chart, they want to see the patient – just for their own comfort level.”
Ms. Ortiz thinks the experience with the COVID crisis could help to advance the conversation about the appropriate role for APPs and their scope of practice in hospital medicine, once the current crisis has passed. “People were used to always doing things a certain way. This experience, hopefully, will get us to the point where attending physicians have more comfort with the APP’s ability to act autonomously,” she said.
“We’ve also talked about piloting telemedicine examinations using Zoom,” Dr. Nave added. “It’s making us think a lot of remote cross-coverage could be done that way. We’ve talked about using the hospital’s iPads with patients. This crisis really makes you think you want to innovate, in a spirit of improvisation,” she said. “Now is the time to try some of these things.”
Editors note: During the COVID-19 pandemic, many hospitals are seeing unprecedented volumes of patients requiring hospital medicine groups to stretch their current resources and recruit providers from outside their groups to bolster their inpatient services. The Society of Hospital Medicine has put together the following stepwise guide for onboarding traditional outpatient and subspecialty-based providers to work on general medicine wards: COVID-19 nonhospitalist onboarding resources.