User login
Proteogenomics provides molecular insights into endometrial carcinoma
according to a proteogenomic study.
Further insights into the regulatory mechanisms of the disease were also identified, based on findings from genome-wide phosphoproteome and acetylome surveys.
“This study provides a comprehensive overview of the molecular systems of endometrial carcinoma at the genomic, transcriptomic, and proteomic levels,” wrote Yongchao Dou, PhD, of Baylor College of Medicine, Houston, and colleagues. The findings were published in Cell.
The researchers prospectively analyzed proteogenomic data from 95 endometrial carcinoma tumors, including 83 endometrioid and 12 serous samples, and 49 nonmalignant tissue samples. Whole genome and exome, DNA methylation, and total and microRNA sequencing analyses were performed for each sample.
An integrated evaluation of proteins, posttranslational modifications (phosphorylation and acetylation), DNA, and RNA were used to detect novel regulatory mechanisms and potential therapeutic targets.
The researchers confirmed previous data on the impact of gain-of-function TP53 mutations on the Aurora kinase pathway, notably the relationship between AURKA expression and negative outcomes in endometrial carcinoma.
“[These findings] provide a theoretical basis for the use of AURKA inhibitors in these tumors,” the researchers wrote.
In addition, the team found evidence of a new regulatory pathway involving ESRP2, circular RNA (circRNA), and QKI, each of which plays a key role in regulatory function.
“Through its known function in isoform regulation, ESRP2 could also directly regulate circRNA levels, and, if so, it could compete with QKI in circRNA-mediated gene regulation,” the researchers wrote.
Furthermore, they identified several gene products that could play a role in optimizing selection of patients for checkpoint blockade immunotherapy. One product in particular, CDK12, may better clinical response rates to immune checkpoint blockade.
The researchers also found evidence to suggest that measuring tumor antigen presentation defects could be more effective than measuring tumor mutation burden when selecting immunotherapy for patients with endometrial carcinoma.
“Although the results presented herein are predominantly observational, they provide the basis for multiple hypotheses of clinical relevance that can and should be further explored,” the researchers concluded.
The study was funded by the National Cancer Institute, the Cancer Prevention & Research Institutes of Texas, and the Robert and Janice McNair Foundation. The authors reported having no conflicts of interest.
SOURCE: Dou Y et al. Cell. 2020 Feb 13. doi: 10.1016/j.cell.2020.01.026.
according to a proteogenomic study.
Further insights into the regulatory mechanisms of the disease were also identified, based on findings from genome-wide phosphoproteome and acetylome surveys.
“This study provides a comprehensive overview of the molecular systems of endometrial carcinoma at the genomic, transcriptomic, and proteomic levels,” wrote Yongchao Dou, PhD, of Baylor College of Medicine, Houston, and colleagues. The findings were published in Cell.
The researchers prospectively analyzed proteogenomic data from 95 endometrial carcinoma tumors, including 83 endometrioid and 12 serous samples, and 49 nonmalignant tissue samples. Whole genome and exome, DNA methylation, and total and microRNA sequencing analyses were performed for each sample.
An integrated evaluation of proteins, posttranslational modifications (phosphorylation and acetylation), DNA, and RNA were used to detect novel regulatory mechanisms and potential therapeutic targets.
The researchers confirmed previous data on the impact of gain-of-function TP53 mutations on the Aurora kinase pathway, notably the relationship between AURKA expression and negative outcomes in endometrial carcinoma.
“[These findings] provide a theoretical basis for the use of AURKA inhibitors in these tumors,” the researchers wrote.
In addition, the team found evidence of a new regulatory pathway involving ESRP2, circular RNA (circRNA), and QKI, each of which plays a key role in regulatory function.
“Through its known function in isoform regulation, ESRP2 could also directly regulate circRNA levels, and, if so, it could compete with QKI in circRNA-mediated gene regulation,” the researchers wrote.
Furthermore, they identified several gene products that could play a role in optimizing selection of patients for checkpoint blockade immunotherapy. One product in particular, CDK12, may better clinical response rates to immune checkpoint blockade.
The researchers also found evidence to suggest that measuring tumor antigen presentation defects could be more effective than measuring tumor mutation burden when selecting immunotherapy for patients with endometrial carcinoma.
“Although the results presented herein are predominantly observational, they provide the basis for multiple hypotheses of clinical relevance that can and should be further explored,” the researchers concluded.
The study was funded by the National Cancer Institute, the Cancer Prevention & Research Institutes of Texas, and the Robert and Janice McNair Foundation. The authors reported having no conflicts of interest.
SOURCE: Dou Y et al. Cell. 2020 Feb 13. doi: 10.1016/j.cell.2020.01.026.
according to a proteogenomic study.
Further insights into the regulatory mechanisms of the disease were also identified, based on findings from genome-wide phosphoproteome and acetylome surveys.
“This study provides a comprehensive overview of the molecular systems of endometrial carcinoma at the genomic, transcriptomic, and proteomic levels,” wrote Yongchao Dou, PhD, of Baylor College of Medicine, Houston, and colleagues. The findings were published in Cell.
The researchers prospectively analyzed proteogenomic data from 95 endometrial carcinoma tumors, including 83 endometrioid and 12 serous samples, and 49 nonmalignant tissue samples. Whole genome and exome, DNA methylation, and total and microRNA sequencing analyses were performed for each sample.
An integrated evaluation of proteins, posttranslational modifications (phosphorylation and acetylation), DNA, and RNA were used to detect novel regulatory mechanisms and potential therapeutic targets.
The researchers confirmed previous data on the impact of gain-of-function TP53 mutations on the Aurora kinase pathway, notably the relationship between AURKA expression and negative outcomes in endometrial carcinoma.
“[These findings] provide a theoretical basis for the use of AURKA inhibitors in these tumors,” the researchers wrote.
In addition, the team found evidence of a new regulatory pathway involving ESRP2, circular RNA (circRNA), and QKI, each of which plays a key role in regulatory function.
“Through its known function in isoform regulation, ESRP2 could also directly regulate circRNA levels, and, if so, it could compete with QKI in circRNA-mediated gene regulation,” the researchers wrote.
Furthermore, they identified several gene products that could play a role in optimizing selection of patients for checkpoint blockade immunotherapy. One product in particular, CDK12, may better clinical response rates to immune checkpoint blockade.
The researchers also found evidence to suggest that measuring tumor antigen presentation defects could be more effective than measuring tumor mutation burden when selecting immunotherapy for patients with endometrial carcinoma.
“Although the results presented herein are predominantly observational, they provide the basis for multiple hypotheses of clinical relevance that can and should be further explored,” the researchers concluded.
The study was funded by the National Cancer Institute, the Cancer Prevention & Research Institutes of Texas, and the Robert and Janice McNair Foundation. The authors reported having no conflicts of interest.
SOURCE: Dou Y et al. Cell. 2020 Feb 13. doi: 10.1016/j.cell.2020.01.026.
FROM CELL
‘A glimmer of hope’ for stroke/mortality benefit with AFib catheter ablation
SNOWMASS, COLO. – stroke, major bleeding, or cardiac arrest, compared with rhythm and/or rate control drugs in a propensity score–weighted, retrospective, observational study.
Findings of the investigation, which included more than 183,000 real-world patients in routine clinical practice, were reported by Peter S. Noseworthy, MD, during the annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
The results breathe new life into the controversy created by the previously reported CABANA trial (Catheter Ablation vs. Antiarrhythmic Drug Therapy for Atrial Fibrillation), a 10-country study in which 2,204 patients with atrial fibrillation (AFib) were randomized to catheter ablation or antiarrhythmic and/or rhythm control medications and followed for a mean of about 4 years. CABANA yielded a negative result (JAMA. 2019 Apr 2;321[13]:1261-74), with the prespecified intent-to-treat analysis indicating no significant between-group difference in the primary composite endpoint – the very same one that was positive in the large observational study.
However, CABANA was marred by major problems arising from protocol deviations: Nearly 28% of patients assigned to medical therapy crossed over to catheter ablation, typically because their antiarrhythmic drugs failed, and 10% of patients randomized to catheter ablation never got it. This muddies the waters when trying to identify a true stroke/mortality benefit for catheter ablation, if indeed any such benefit was actually present.
Here’s where the controversy arose: While CABANA must be called a negative trial based upon the disappointing results of the intent-to-treat analysis, a prespecified post hoc analysis of patients as actually treated showed a statistically significant 27% relative risk reduction for the primary composite endpoint in the catheter ablation group. That’s strikingly similar to the 30% relative risk reduction for catheter ablation seen in the huge observational study, where the CABANA-type primary outcome occurred in 22.5% of the medically managed patients and 16.8% of those who underwent catheter ablation, noted Dr. Noseworthy, professor of medicine and director of heart rhythm and physiology at the Mayo Clinic in Rochester, Minn.
He ought to know: He was both an investigator in CABANA and first author of the published observational study (Eur Heart J. 2019 Apr 21;40[16]:1257-64).
In the observational study, Dr. Noseworthy and coinvestigators utilized a huge U.S. administrative health claims database in order to identify a nationally representative group of 183,760 AFib patients, 12,032 of whom were treated with catheter ablation and the rest with antiarrhythmic and/or rhythm control drugs during the same years the CABANA trial was enrolling patients. The two groups were balanced using propensity score weighting to adjust for baseline differences in 90 variables.
The investigators sought to learn if the CABANA study population was representative of real-world AFib patients, and whether the observational experience could help resolve the CABANA controversy. It turned out that most AFib patients seen in daily clinical practice were CABANA like; that is, 74% of them would have been eligible for the clinical trial because they were symptomatic, over age 65, or younger than 65 with at least one CHADS2 stroke risk factor. About 22% of the large real-world sample would have been excluded from CABANA because they’d failed on amiodarone and other antiarrhythmic agents or had previously undergone ablation. About 4% of patients failed to meet the CABANA inclusion criteria.
The risk reduction for the composite endpoint associated with catheter ablation in the large retrospective study was greatest in the CABANA-like patients, at 30%. It was less robust but still statistically significant at 15% in patients who met at least one of the exclusion criteria for the trial.
The sheer size of this study provides greater statistical power than in CABANA. Of course, a nonrandomized, propensity score–based comparison such as this is always susceptible to confounding, even after adjustment for 90 variables. But the observational study does offer “a glimmer of hope” that catheter ablation, done in the right patients, might confer a stroke risk reduction and mortality benefit, he said.
The 33% relative risk reduction in the small group of real-world patients who failed to meet the CABANA inclusion criteria, while numerically impressive, wasn’t close to statistical significance, probably because event rates in that population were so low.
“Even if you could reduce stroke risk with ablation in that low-risk group, it would be a very inefficient way to reduce the population burden of stroke,” Dr. Noseworthy observed.
Putting together the results of CABANA and the large observational study to sum up his view of where catheter ablation for AF[ib] stands today, Dr. Noseworthy commented, “Ablation is reasonable for symptom control in many patients, basically anyone who is either breaking through on drugs or doesn’t want to take the drugs and is highly symptomatic. And there may be a small stroke and/or mortality benefit for people who are in the sweet spot – and those are people who look a lot like the patients enrolled in CABANA.”
Patients who met the exclusion criteria for CABANA are too advanced in their AFib to be likely to derive a stroke or mortality benefit from catheter ablation. “It’s very hard to move the needle in these patients with either a drug or catheter ablation approach. I wouldn’t try to reduce the risk of stroke here with an expensive and invasive procedure,” the electrophysiologist concluded.
He reported having no financial conflicts regarding his presentation.
SNOWMASS, COLO. – stroke, major bleeding, or cardiac arrest, compared with rhythm and/or rate control drugs in a propensity score–weighted, retrospective, observational study.
Findings of the investigation, which included more than 183,000 real-world patients in routine clinical practice, were reported by Peter S. Noseworthy, MD, during the annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
The results breathe new life into the controversy created by the previously reported CABANA trial (Catheter Ablation vs. Antiarrhythmic Drug Therapy for Atrial Fibrillation), a 10-country study in which 2,204 patients with atrial fibrillation (AFib) were randomized to catheter ablation or antiarrhythmic and/or rhythm control medications and followed for a mean of about 4 years. CABANA yielded a negative result (JAMA. 2019 Apr 2;321[13]:1261-74), with the prespecified intent-to-treat analysis indicating no significant between-group difference in the primary composite endpoint – the very same one that was positive in the large observational study.
However, CABANA was marred by major problems arising from protocol deviations: Nearly 28% of patients assigned to medical therapy crossed over to catheter ablation, typically because their antiarrhythmic drugs failed, and 10% of patients randomized to catheter ablation never got it. This muddies the waters when trying to identify a true stroke/mortality benefit for catheter ablation, if indeed any such benefit was actually present.
Here’s where the controversy arose: While CABANA must be called a negative trial based upon the disappointing results of the intent-to-treat analysis, a prespecified post hoc analysis of patients as actually treated showed a statistically significant 27% relative risk reduction for the primary composite endpoint in the catheter ablation group. That’s strikingly similar to the 30% relative risk reduction for catheter ablation seen in the huge observational study, where the CABANA-type primary outcome occurred in 22.5% of the medically managed patients and 16.8% of those who underwent catheter ablation, noted Dr. Noseworthy, professor of medicine and director of heart rhythm and physiology at the Mayo Clinic in Rochester, Minn.
He ought to know: He was both an investigator in CABANA and first author of the published observational study (Eur Heart J. 2019 Apr 21;40[16]:1257-64).
In the observational study, Dr. Noseworthy and coinvestigators utilized a huge U.S. administrative health claims database in order to identify a nationally representative group of 183,760 AFib patients, 12,032 of whom were treated with catheter ablation and the rest with antiarrhythmic and/or rhythm control drugs during the same years the CABANA trial was enrolling patients. The two groups were balanced using propensity score weighting to adjust for baseline differences in 90 variables.
The investigators sought to learn if the CABANA study population was representative of real-world AFib patients, and whether the observational experience could help resolve the CABANA controversy. It turned out that most AFib patients seen in daily clinical practice were CABANA like; that is, 74% of them would have been eligible for the clinical trial because they were symptomatic, over age 65, or younger than 65 with at least one CHADS2 stroke risk factor. About 22% of the large real-world sample would have been excluded from CABANA because they’d failed on amiodarone and other antiarrhythmic agents or had previously undergone ablation. About 4% of patients failed to meet the CABANA inclusion criteria.
The risk reduction for the composite endpoint associated with catheter ablation in the large retrospective study was greatest in the CABANA-like patients, at 30%. It was less robust but still statistically significant at 15% in patients who met at least one of the exclusion criteria for the trial.
The sheer size of this study provides greater statistical power than in CABANA. Of course, a nonrandomized, propensity score–based comparison such as this is always susceptible to confounding, even after adjustment for 90 variables. But the observational study does offer “a glimmer of hope” that catheter ablation, done in the right patients, might confer a stroke risk reduction and mortality benefit, he said.
The 33% relative risk reduction in the small group of real-world patients who failed to meet the CABANA inclusion criteria, while numerically impressive, wasn’t close to statistical significance, probably because event rates in that population were so low.
“Even if you could reduce stroke risk with ablation in that low-risk group, it would be a very inefficient way to reduce the population burden of stroke,” Dr. Noseworthy observed.
Putting together the results of CABANA and the large observational study to sum up his view of where catheter ablation for AF[ib] stands today, Dr. Noseworthy commented, “Ablation is reasonable for symptom control in many patients, basically anyone who is either breaking through on drugs or doesn’t want to take the drugs and is highly symptomatic. And there may be a small stroke and/or mortality benefit for people who are in the sweet spot – and those are people who look a lot like the patients enrolled in CABANA.”
Patients who met the exclusion criteria for CABANA are too advanced in their AFib to be likely to derive a stroke or mortality benefit from catheter ablation. “It’s very hard to move the needle in these patients with either a drug or catheter ablation approach. I wouldn’t try to reduce the risk of stroke here with an expensive and invasive procedure,” the electrophysiologist concluded.
He reported having no financial conflicts regarding his presentation.
SNOWMASS, COLO. – stroke, major bleeding, or cardiac arrest, compared with rhythm and/or rate control drugs in a propensity score–weighted, retrospective, observational study.
Findings of the investigation, which included more than 183,000 real-world patients in routine clinical practice, were reported by Peter S. Noseworthy, MD, during the annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
The results breathe new life into the controversy created by the previously reported CABANA trial (Catheter Ablation vs. Antiarrhythmic Drug Therapy for Atrial Fibrillation), a 10-country study in which 2,204 patients with atrial fibrillation (AFib) were randomized to catheter ablation or antiarrhythmic and/or rhythm control medications and followed for a mean of about 4 years. CABANA yielded a negative result (JAMA. 2019 Apr 2;321[13]:1261-74), with the prespecified intent-to-treat analysis indicating no significant between-group difference in the primary composite endpoint – the very same one that was positive in the large observational study.
However, CABANA was marred by major problems arising from protocol deviations: Nearly 28% of patients assigned to medical therapy crossed over to catheter ablation, typically because their antiarrhythmic drugs failed, and 10% of patients randomized to catheter ablation never got it. This muddies the waters when trying to identify a true stroke/mortality benefit for catheter ablation, if indeed any such benefit was actually present.
Here’s where the controversy arose: While CABANA must be called a negative trial based upon the disappointing results of the intent-to-treat analysis, a prespecified post hoc analysis of patients as actually treated showed a statistically significant 27% relative risk reduction for the primary composite endpoint in the catheter ablation group. That’s strikingly similar to the 30% relative risk reduction for catheter ablation seen in the huge observational study, where the CABANA-type primary outcome occurred in 22.5% of the medically managed patients and 16.8% of those who underwent catheter ablation, noted Dr. Noseworthy, professor of medicine and director of heart rhythm and physiology at the Mayo Clinic in Rochester, Minn.
He ought to know: He was both an investigator in CABANA and first author of the published observational study (Eur Heart J. 2019 Apr 21;40[16]:1257-64).
In the observational study, Dr. Noseworthy and coinvestigators utilized a huge U.S. administrative health claims database in order to identify a nationally representative group of 183,760 AFib patients, 12,032 of whom were treated with catheter ablation and the rest with antiarrhythmic and/or rhythm control drugs during the same years the CABANA trial was enrolling patients. The two groups were balanced using propensity score weighting to adjust for baseline differences in 90 variables.
The investigators sought to learn if the CABANA study population was representative of real-world AFib patients, and whether the observational experience could help resolve the CABANA controversy. It turned out that most AFib patients seen in daily clinical practice were CABANA like; that is, 74% of them would have been eligible for the clinical trial because they were symptomatic, over age 65, or younger than 65 with at least one CHADS2 stroke risk factor. About 22% of the large real-world sample would have been excluded from CABANA because they’d failed on amiodarone and other antiarrhythmic agents or had previously undergone ablation. About 4% of patients failed to meet the CABANA inclusion criteria.
The risk reduction for the composite endpoint associated with catheter ablation in the large retrospective study was greatest in the CABANA-like patients, at 30%. It was less robust but still statistically significant at 15% in patients who met at least one of the exclusion criteria for the trial.
The sheer size of this study provides greater statistical power than in CABANA. Of course, a nonrandomized, propensity score–based comparison such as this is always susceptible to confounding, even after adjustment for 90 variables. But the observational study does offer “a glimmer of hope” that catheter ablation, done in the right patients, might confer a stroke risk reduction and mortality benefit, he said.
The 33% relative risk reduction in the small group of real-world patients who failed to meet the CABANA inclusion criteria, while numerically impressive, wasn’t close to statistical significance, probably because event rates in that population were so low.
“Even if you could reduce stroke risk with ablation in that low-risk group, it would be a very inefficient way to reduce the population burden of stroke,” Dr. Noseworthy observed.
Putting together the results of CABANA and the large observational study to sum up his view of where catheter ablation for AF[ib] stands today, Dr. Noseworthy commented, “Ablation is reasonable for symptom control in many patients, basically anyone who is either breaking through on drugs or doesn’t want to take the drugs and is highly symptomatic. And there may be a small stroke and/or mortality benefit for people who are in the sweet spot – and those are people who look a lot like the patients enrolled in CABANA.”
Patients who met the exclusion criteria for CABANA are too advanced in their AFib to be likely to derive a stroke or mortality benefit from catheter ablation. “It’s very hard to move the needle in these patients with either a drug or catheter ablation approach. I wouldn’t try to reduce the risk of stroke here with an expensive and invasive procedure,” the electrophysiologist concluded.
He reported having no financial conflicts regarding his presentation.
REPORTING FROM ACC SNOWMASS 2020
Zilucoplan improved efficacy outcomes in myasthenia gravis
The clinical effect of the self-administered macrocyclic peptide inhibitor was “similar,” the investigators wrote, to what was seen in studies of the intravenously administered complement inhibitor eculizumab, which is approved by the Food and Drug Administration for treatment of gMG.
While eculizumab studies were restricted to patients with refractory gMG, the investigators wrote that their study of zilucoplan included a broader population, including patients who had not failed prior therapies, who were earlier in their disease course, and who had a history of thymoma.
“This observation is important because in gMG, disease severity frequently peaks within the first few years after diagnosis, before all treatment options have been exhausted, and before patients may be formally declared treatment refractory,” wrote James F. Howard Jr, MD, of the University of North Carolina in Chapel Hill, and coauthors.
Complement inhibition is a “targeted approach” that addresses the primary mechanism of tissue damage in gMG, the investigators wrote.
That stands in contrast to conventional gMG treatments including pyridostigmine, corticosteroids, and other immunosuppressants. “These treatments lack strong evidence from clinical trials to support their efficacy, are often poorly tolerated, and can be associated with considerable long-term toxicities,” Dr. Howard and colleagues wrote in their report, which was published in JAMA Neurology.
A total of 44 adult patients with gMG were randomized to receive daily zilucoplan 0.1 mg/kg, 0.3 mg/kg, or placebo for 12 weeks in this 25-center North American study. All patients had acetylcholine receptor autoantibody–positive disease and a Quantitative Myasthenia Gravis (QMG) score of 12 or higher. The QMG score ranges from 0, indicating no muscle weakness, to 39, or severe weakness.
Per the study protocol, patients had to keep taking their current gMG medication without changing the dose.
Change in QMG score from baseline to 12 weeks, the primary efficacy endpoint of the study, showed a significant and clinically meaningful difference favoring zilucoplan 0.3 mg/kg over placebo, according to the investigators.
The mean change was –6.0 points for zilucoplan 0.3 mg/kg and –3.2 for placebo (P = .05), according to their report, which indicated a rapid onset of action apparent 1 week after starting treatment.
Zilucoplan 0.1 mg/kg also yielded a significant and clinically meaningful improvement versus placebo, but its magnitude was smaller and took 4 weeks to become apparent.
Treatment with zilucoplan also significantly improved MG Activities of Daily Living scores versus placebo, a key secondary endpoint of the trial, according to the researchers.
Treatment-emergent adverse events, which included local injection-site reactions, were mild and judged to be unrelated to the study treatment, according to the report.
Ra Pharmaceuticals funded the study. Dr. Howard reported disclosures related to Ra Pharmaceuticals, Alexion Pharmaceuticals, argenx, Viela Bio, and others.
SOURCE: Howard Jr JF et al. JAMA Neurol. 2020 Feb 17. doi: 10.1001/jamaneurol.2019.5125.
The clinical effect of the self-administered macrocyclic peptide inhibitor was “similar,” the investigators wrote, to what was seen in studies of the intravenously administered complement inhibitor eculizumab, which is approved by the Food and Drug Administration for treatment of gMG.
While eculizumab studies were restricted to patients with refractory gMG, the investigators wrote that their study of zilucoplan included a broader population, including patients who had not failed prior therapies, who were earlier in their disease course, and who had a history of thymoma.
“This observation is important because in gMG, disease severity frequently peaks within the first few years after diagnosis, before all treatment options have been exhausted, and before patients may be formally declared treatment refractory,” wrote James F. Howard Jr, MD, of the University of North Carolina in Chapel Hill, and coauthors.
Complement inhibition is a “targeted approach” that addresses the primary mechanism of tissue damage in gMG, the investigators wrote.
That stands in contrast to conventional gMG treatments including pyridostigmine, corticosteroids, and other immunosuppressants. “These treatments lack strong evidence from clinical trials to support their efficacy, are often poorly tolerated, and can be associated with considerable long-term toxicities,” Dr. Howard and colleagues wrote in their report, which was published in JAMA Neurology.
A total of 44 adult patients with gMG were randomized to receive daily zilucoplan 0.1 mg/kg, 0.3 mg/kg, or placebo for 12 weeks in this 25-center North American study. All patients had acetylcholine receptor autoantibody–positive disease and a Quantitative Myasthenia Gravis (QMG) score of 12 or higher. The QMG score ranges from 0, indicating no muscle weakness, to 39, or severe weakness.
Per the study protocol, patients had to keep taking their current gMG medication without changing the dose.
Change in QMG score from baseline to 12 weeks, the primary efficacy endpoint of the study, showed a significant and clinically meaningful difference favoring zilucoplan 0.3 mg/kg over placebo, according to the investigators.
The mean change was –6.0 points for zilucoplan 0.3 mg/kg and –3.2 for placebo (P = .05), according to their report, which indicated a rapid onset of action apparent 1 week after starting treatment.
Zilucoplan 0.1 mg/kg also yielded a significant and clinically meaningful improvement versus placebo, but its magnitude was smaller and took 4 weeks to become apparent.
Treatment with zilucoplan also significantly improved MG Activities of Daily Living scores versus placebo, a key secondary endpoint of the trial, according to the researchers.
Treatment-emergent adverse events, which included local injection-site reactions, were mild and judged to be unrelated to the study treatment, according to the report.
Ra Pharmaceuticals funded the study. Dr. Howard reported disclosures related to Ra Pharmaceuticals, Alexion Pharmaceuticals, argenx, Viela Bio, and others.
SOURCE: Howard Jr JF et al. JAMA Neurol. 2020 Feb 17. doi: 10.1001/jamaneurol.2019.5125.
The clinical effect of the self-administered macrocyclic peptide inhibitor was “similar,” the investigators wrote, to what was seen in studies of the intravenously administered complement inhibitor eculizumab, which is approved by the Food and Drug Administration for treatment of gMG.
While eculizumab studies were restricted to patients with refractory gMG, the investigators wrote that their study of zilucoplan included a broader population, including patients who had not failed prior therapies, who were earlier in their disease course, and who had a history of thymoma.
“This observation is important because in gMG, disease severity frequently peaks within the first few years after diagnosis, before all treatment options have been exhausted, and before patients may be formally declared treatment refractory,” wrote James F. Howard Jr, MD, of the University of North Carolina in Chapel Hill, and coauthors.
Complement inhibition is a “targeted approach” that addresses the primary mechanism of tissue damage in gMG, the investigators wrote.
That stands in contrast to conventional gMG treatments including pyridostigmine, corticosteroids, and other immunosuppressants. “These treatments lack strong evidence from clinical trials to support their efficacy, are often poorly tolerated, and can be associated with considerable long-term toxicities,” Dr. Howard and colleagues wrote in their report, which was published in JAMA Neurology.
A total of 44 adult patients with gMG were randomized to receive daily zilucoplan 0.1 mg/kg, 0.3 mg/kg, or placebo for 12 weeks in this 25-center North American study. All patients had acetylcholine receptor autoantibody–positive disease and a Quantitative Myasthenia Gravis (QMG) score of 12 or higher. The QMG score ranges from 0, indicating no muscle weakness, to 39, or severe weakness.
Per the study protocol, patients had to keep taking their current gMG medication without changing the dose.
Change in QMG score from baseline to 12 weeks, the primary efficacy endpoint of the study, showed a significant and clinically meaningful difference favoring zilucoplan 0.3 mg/kg over placebo, according to the investigators.
The mean change was –6.0 points for zilucoplan 0.3 mg/kg and –3.2 for placebo (P = .05), according to their report, which indicated a rapid onset of action apparent 1 week after starting treatment.
Zilucoplan 0.1 mg/kg also yielded a significant and clinically meaningful improvement versus placebo, but its magnitude was smaller and took 4 weeks to become apparent.
Treatment with zilucoplan also significantly improved MG Activities of Daily Living scores versus placebo, a key secondary endpoint of the trial, according to the researchers.
Treatment-emergent adverse events, which included local injection-site reactions, were mild and judged to be unrelated to the study treatment, according to the report.
Ra Pharmaceuticals funded the study. Dr. Howard reported disclosures related to Ra Pharmaceuticals, Alexion Pharmaceuticals, argenx, Viela Bio, and others.
SOURCE: Howard Jr JF et al. JAMA Neurol. 2020 Feb 17. doi: 10.1001/jamaneurol.2019.5125.
FROM JAMA NEUROLOGY
Critical care admissions up for pediatric opioid poisonings
ORLANDO – The proportion of children and adolescents admitted to critical care for serious poisonings has increased in recent years, according to authors of a study of more than 750,000 reported opioid exposures.
Critical care units were involved in 10% of pediatric opioid poisoning cases registered in 2015-2018, up from 7% in 2005-2009, reported Megan E. Land, MD, of Emory University, Atlanta, and coinvestigators.
Attempted suicide has represented an increasingly large proportion of pediatric opioid poisonings from 2005 to 2018, according to the researchers, based on retrospective analysis of cases reported to U.S. poison centers.
Mortality related to these pediatric poisonings increased over time, and among children and adolescents admitted to a pediatric ICU, CPR and naloxone use also increased over time, Dr. Land and associates noted.
These said Dr. Land, who presented the findings at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
“I think that this really requires a two-pronged approach,” she explained. “One is that we need to increase mental health resources for kids to address adolescent suicidality, and secondly, we need to decrease access to opioids in the hands of pediatric patients by decreasing prescribing and then also getting those that are unused out of the homes.”
Jeffrey Zimmerman, MD, past president of SCCM, said these findings on pediatric opioid poisonings represent the “iceberg tip” of a much larger societal issue that has impacts well beyond critical care.
“I think acutely, we’re well equipped to deal with the situation in terms of interventions,” Dr. Zimmerman said in an interview. “The bigger issue is dealing with what happens afterward, when the patient leaves the ICU in the hospital.”
When the issue is chronic opioid use among adolescents or children, critical care specialists can help by initiating opioid tapering in the hospital setting, rather than allowing the complete weaning process to play out at home, he said.
All clinicians can help prevent future injury by asking questions of the child and family to ensure that any opiates and other prescription medications at home are locked up, he added.
“These aren’t very glamorous things, but they’re common sense, and there’s more need for this common sense now than there ever has been,” Dr. Zimmerman concluded.
The study by Dr. Land and colleagues included data on primary opioid ingestions registered at 55 poison control centers in the United States. They assessed trends over three time periods: 2005-2009, 2010-2014, and 2015-2018.
They found that children under 19 years of age accounted for 28% of the 753,592 opioid poisonings reported over that time period.
The overall number of reported opioid poisonings among children declined somewhat since about 2010. However, the proportion admitted to a critical care unit increased from 7% in the 2005-2009 period to 10% in the 2015-2018 period, said Dr. Land, who added that the probability of a moderate or major effect increased by 0.55% and 0.11% per year, respectively, over the 14 years studied.
Mortality – 0.21% overall – increased from 0.18% in the earliest era to 0.28% in the most recent era, according to the investigators.
Suicidal intent increased from 14% in the earliest era to 21% in the most recent era, and was linked to near tenfold odds of undergoing a pediatric ICU procedure, Dr. Land and colleagues reported.
Among those children admitted to a pediatric ICU, use of CPR increased from 1% to 3% in the earliest and latest time periods, respectively; likewise, naloxone administration increased from 42% to 51% over those two time periods. By contrast, there was no change in use of mechanical ventilation (12%) or vasopressors (3%) over time, they added.
The opioids most commonly linked to pediatric ICU procedures were fentanyl (odds ratio, 12), heroin (OR, 11), and methadone (OR, 15).
Some funding for the study came from the Georgia Poison Center. Dr. Land had no disclosures relevant to the research.
SOURCE: Land M et al. Crit Care Med. 2020 doi: 10.1097/01.ccm.0000618708.38414.ea.
ORLANDO – The proportion of children and adolescents admitted to critical care for serious poisonings has increased in recent years, according to authors of a study of more than 750,000 reported opioid exposures.
Critical care units were involved in 10% of pediatric opioid poisoning cases registered in 2015-2018, up from 7% in 2005-2009, reported Megan E. Land, MD, of Emory University, Atlanta, and coinvestigators.
Attempted suicide has represented an increasingly large proportion of pediatric opioid poisonings from 2005 to 2018, according to the researchers, based on retrospective analysis of cases reported to U.S. poison centers.
Mortality related to these pediatric poisonings increased over time, and among children and adolescents admitted to a pediatric ICU, CPR and naloxone use also increased over time, Dr. Land and associates noted.
These said Dr. Land, who presented the findings at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
“I think that this really requires a two-pronged approach,” she explained. “One is that we need to increase mental health resources for kids to address adolescent suicidality, and secondly, we need to decrease access to opioids in the hands of pediatric patients by decreasing prescribing and then also getting those that are unused out of the homes.”
Jeffrey Zimmerman, MD, past president of SCCM, said these findings on pediatric opioid poisonings represent the “iceberg tip” of a much larger societal issue that has impacts well beyond critical care.
“I think acutely, we’re well equipped to deal with the situation in terms of interventions,” Dr. Zimmerman said in an interview. “The bigger issue is dealing with what happens afterward, when the patient leaves the ICU in the hospital.”
When the issue is chronic opioid use among adolescents or children, critical care specialists can help by initiating opioid tapering in the hospital setting, rather than allowing the complete weaning process to play out at home, he said.
All clinicians can help prevent future injury by asking questions of the child and family to ensure that any opiates and other prescription medications at home are locked up, he added.
“These aren’t very glamorous things, but they’re common sense, and there’s more need for this common sense now than there ever has been,” Dr. Zimmerman concluded.
The study by Dr. Land and colleagues included data on primary opioid ingestions registered at 55 poison control centers in the United States. They assessed trends over three time periods: 2005-2009, 2010-2014, and 2015-2018.
They found that children under 19 years of age accounted for 28% of the 753,592 opioid poisonings reported over that time period.
The overall number of reported opioid poisonings among children declined somewhat since about 2010. However, the proportion admitted to a critical care unit increased from 7% in the 2005-2009 period to 10% in the 2015-2018 period, said Dr. Land, who added that the probability of a moderate or major effect increased by 0.55% and 0.11% per year, respectively, over the 14 years studied.
Mortality – 0.21% overall – increased from 0.18% in the earliest era to 0.28% in the most recent era, according to the investigators.
Suicidal intent increased from 14% in the earliest era to 21% in the most recent era, and was linked to near tenfold odds of undergoing a pediatric ICU procedure, Dr. Land and colleagues reported.
Among those children admitted to a pediatric ICU, use of CPR increased from 1% to 3% in the earliest and latest time periods, respectively; likewise, naloxone administration increased from 42% to 51% over those two time periods. By contrast, there was no change in use of mechanical ventilation (12%) or vasopressors (3%) over time, they added.
The opioids most commonly linked to pediatric ICU procedures were fentanyl (odds ratio, 12), heroin (OR, 11), and methadone (OR, 15).
Some funding for the study came from the Georgia Poison Center. Dr. Land had no disclosures relevant to the research.
SOURCE: Land M et al. Crit Care Med. 2020 doi: 10.1097/01.ccm.0000618708.38414.ea.
ORLANDO – The proportion of children and adolescents admitted to critical care for serious poisonings has increased in recent years, according to authors of a study of more than 750,000 reported opioid exposures.
Critical care units were involved in 10% of pediatric opioid poisoning cases registered in 2015-2018, up from 7% in 2005-2009, reported Megan E. Land, MD, of Emory University, Atlanta, and coinvestigators.
Attempted suicide has represented an increasingly large proportion of pediatric opioid poisonings from 2005 to 2018, according to the researchers, based on retrospective analysis of cases reported to U.S. poison centers.
Mortality related to these pediatric poisonings increased over time, and among children and adolescents admitted to a pediatric ICU, CPR and naloxone use also increased over time, Dr. Land and associates noted.
These said Dr. Land, who presented the findings at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
“I think that this really requires a two-pronged approach,” she explained. “One is that we need to increase mental health resources for kids to address adolescent suicidality, and secondly, we need to decrease access to opioids in the hands of pediatric patients by decreasing prescribing and then also getting those that are unused out of the homes.”
Jeffrey Zimmerman, MD, past president of SCCM, said these findings on pediatric opioid poisonings represent the “iceberg tip” of a much larger societal issue that has impacts well beyond critical care.
“I think acutely, we’re well equipped to deal with the situation in terms of interventions,” Dr. Zimmerman said in an interview. “The bigger issue is dealing with what happens afterward, when the patient leaves the ICU in the hospital.”
When the issue is chronic opioid use among adolescents or children, critical care specialists can help by initiating opioid tapering in the hospital setting, rather than allowing the complete weaning process to play out at home, he said.
All clinicians can help prevent future injury by asking questions of the child and family to ensure that any opiates and other prescription medications at home are locked up, he added.
“These aren’t very glamorous things, but they’re common sense, and there’s more need for this common sense now than there ever has been,” Dr. Zimmerman concluded.
The study by Dr. Land and colleagues included data on primary opioid ingestions registered at 55 poison control centers in the United States. They assessed trends over three time periods: 2005-2009, 2010-2014, and 2015-2018.
They found that children under 19 years of age accounted for 28% of the 753,592 opioid poisonings reported over that time period.
The overall number of reported opioid poisonings among children declined somewhat since about 2010. However, the proportion admitted to a critical care unit increased from 7% in the 2005-2009 period to 10% in the 2015-2018 period, said Dr. Land, who added that the probability of a moderate or major effect increased by 0.55% and 0.11% per year, respectively, over the 14 years studied.
Mortality – 0.21% overall – increased from 0.18% in the earliest era to 0.28% in the most recent era, according to the investigators.
Suicidal intent increased from 14% in the earliest era to 21% in the most recent era, and was linked to near tenfold odds of undergoing a pediatric ICU procedure, Dr. Land and colleagues reported.
Among those children admitted to a pediatric ICU, use of CPR increased from 1% to 3% in the earliest and latest time periods, respectively; likewise, naloxone administration increased from 42% to 51% over those two time periods. By contrast, there was no change in use of mechanical ventilation (12%) or vasopressors (3%) over time, they added.
The opioids most commonly linked to pediatric ICU procedures were fentanyl (odds ratio, 12), heroin (OR, 11), and methadone (OR, 15).
Some funding for the study came from the Georgia Poison Center. Dr. Land had no disclosures relevant to the research.
SOURCE: Land M et al. Crit Care Med. 2020 doi: 10.1097/01.ccm.0000618708.38414.ea.
REPORTING FROM CCC49
Outcomes Comparison of the Veterans’ Choice Program With the Veterans Affairs Healthcare System for Hepatitis C Treatment
Population studies show high prevalence of chronic hepatitis C virus (HCV) infection among veterans, especially Vietnam War era veterans.1,2 The development of safe and efficacious direct-acting antiviral (DAA) medications to treat HCV infection made the majority of those infected eligible for treatment. However, the large number of veterans needing DAA treatment stressed the resources of the US Department of Veterans Affairs (VA) health care system. This occurred while Congress was focused on reducing wait times for veterans receiving care at the VA.
Congress passed the Veterans Access, Choice, and Accountability Act (Choice) on August 7, 2014, leading to the creation of the Veterans Choice Program. Legislators felt there were inappropriate delays in care at the VA, and the Choice program was meant to address this problem and provide an “apples-to-apples comparison [of the VA] with non-VA hospitals.”3
Congress acknowledged the importance of curing HCV in the veteran population and allocated $1.5 billion for fiscal year (FY) 2016 for DAAs. The VA Central Office (VACO) carefully monitored these resources. The first policy memorandum from VACO for HCV care, issued on May 21, 2015, recommended that the sickest patients who will benefit from the treatment “receive priority over those who are less ill.”4,5 Those who met criteria for advanced liver disease were prioritized for treatment at the VA, while those who did not meet criteria were given the option of receiving treatment through Choice, or waiting for a change in policy.6 Over time, revisions to the guidelines relaxed the criteria for VA treatment eligibility, and on February 24, 2016, all restrictions on HCV treatment at the VA were lifted.7,8
The aim of this study was to provide a comparison of VA and non-VA care, specifically to determine whether care provided through Choice was timelier and more cost effective than care provided by the VA, and whether there was a quality difference. The high prevalence among veterans, well-established standards of care, and finite treatment course with clear indicators of success and failure makes HCV treatment an ideal disease with which to make this comparison.
Methods
We retrospectively analyzed the VA electronic health records of all veterans seen in the VA Loma Linda Healthcare System (VALLHCS) Hepatology clinic for chronic HCV infection during FY 2016 who were referred to Choice for HCV treatment. One hundred veterans met these criteria, encompassing the Choice population; 71 were seen at least once by a non-VA (Choice) health care provider (HCP) and 61 completed a treatment regimen through Choice. Treatment completion was defined as cessation of medication after the planned duration of therapy, or early termination of medication without resumption by that HCP. The Choice population was matched to an equal number of veterans who received HCV treatment from VALLHCS HCPs.
Data collected included age, gender, HCV genotype, determinants of liver fibrosis, and treatment success (defined as sustained virologic response at 12 weeks after the last dose of medication [SVR12]). Determinants of liver fibrosis included documented cirrhosis or complications of cirrhosis, Fibrosis-4 score (Fib-4), and platelet count.
Treatment failures were categorized as nonresponse (defined as detectable HCV viral load at the end of treatment), relapse (defined as an undetectable HCV viral load at the end of treatment with a subsequent positive test), and early termination (defined as a failure to complete the planned treatment regimen). Documented patient nonadherence, medical comorbidities that affected the treatment protocol, mental health diagnoses, and active social issues (defined as active or history of heavy alcohol use, active or history of illicit drug use, lack of social support, and homelessness) were noted.
Timeliness of delivery of care was measured in days. For the VA group, the wait time was defined as the date the consult for HCV treatment was placed to the date of the initial appointment with the HCV treatment provider. For the Choice group, the wait time was defined as the date the referral to the Choice program was made to the date of the initial appointment with the Choice HCP. Treatment regimens were evaluated for appropriateness based on guidelines from VACO and the American Association for the Study of Liver Diseases.9-11
Tests performed by Choice providers were evaluated for whether they were relevant to HCV care and whether similar data already were available from VALLHCS. Tests that were not indicated were identified as unnecessary costs incurred by the Choice program, as were tests that had to be repeated at the VA because of a lack of documentation from the Choice provider. All medications given inappropriately were considered added costs. Medicare reimbursement rates for the most applicable Current Procedural Terminology (CPT) code and VA national contract pricing for medications were used for calculations. This study was approved by the VALLHCS institutional review board.
Statistical Analysis
IBM (Armonk, NY) Statistical Package for Social Sciences software was used to evaluate for differences in Fib-4, platelet count, prevalence of cirrhosis, prevalence of medical comorbidities, prevalence of mental health comorbidities, prevalence of the social issues defined in the Methods section, time from referral to time of appointment date, and SVR12 rate between the VA and Choice groups.
Exclusions
There were 15 veterans in the VA group who had a wait time of > 100 days. Of these, 5 (33%) were initially Choice referrals, but due to negative interactions with the Choice provider, the veterans returned to VALLHCS for care. Two of the 15 (13%) did not keep appointments and were lost to follow up. Six of the 15 (40%) had medical comorbidities that required more immediate attention, so HCV treatment initiation was deliberately moved back. The final 2 veterans scheduled their appointments unusually far apart, artificially increasing their wait time. Given that these were unique situations and some of the veterans received care from both Choice and VA providers, a decision was made to exclude these individuals from the study.
It has been shown that platelet count correlates with degree of liver fibrosis, a concept that is the basis for the Fib-4 scoring system.12 Studies have shown that platelet count is a survival predictor in those with cirrhosis, and thrombocytopenia is a negative predictor of HCV treatment success using peginterferon and ribavirin.13,14 Therefore, the VA memorandum automatically assigned the sickest individuals to the VA for HCV treatment. The goal of this study was to compare the impact of factors other than stage of fibrosis on HCV treatment success, which is why the 12 veterans with platelet count < 100,000 in the VA group were excluded. There were no veterans with platelet count < 100,000 in the Choice group.
When comparing SVR12 rates between the VA and Choice groups, every veteran treated at VALLHCS in FY 2016 was included, increasing the number in the VA group from 100 to 320 and therefore the power of this comparison.
Results
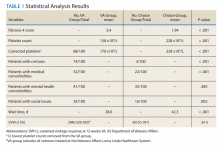
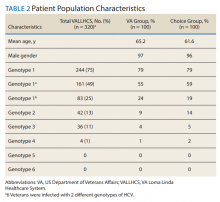
A summary of the statistical analysis can be found in Table 1. The genotype distribution was consistent with epidemiological studies, including those specific to veterans.15,16 There were statistically significant differences (P < .001) in mean Fib-4 and mean platelet count. The VA group had a higher Fib-4 and lower platelet count. Seventy-four percent of the VA population was defined as cirrhotic, while only 3% of the Choice population met similar criteria (P < .001). The VA and Choice groups were similar in terms of age, gender, and genotype distribution (Table 2).
The VA group was found to have a higher prevalence of comorbidities that affected HCV treatment. These conditions included but were not limited to: chronic kidney disease that precluded the use of certain medications, any condition that required medication with a known interaction with DAAs (ie, proton pump inhibitors, statins, and amiodarone), and cirrhosis if it impacted the treatment regimen. The difference in the prevalence of mental health comorbidities was not significant (P = .39), but there was a higher prevalence of social issues among the VA group (P = .002).
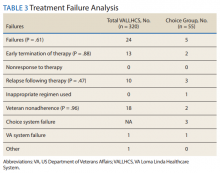
The mean wait time from referral to appointment was 28.6 days for the VA group and 42.3 days for the Choice group (P < .001), indicating that a Choice referral took longer to complete than a referral within the VA for HCV treatment. Thirty of the 71 (42%) veterans seen by a Choice provider accrued extraneous cost, with a mean additional cost of $8,561.40 per veteran. In the Choice group, 61 veterans completed a treatment regimen with the Choice HCP. Fifty-five veterans completed treatment and had available SVR12 data (6 were lost to follow up without SVR12 testing) and 50 (91%) had confirmed SVR12. The charts of the 5 treatment failures were reviewed to discern the cause for failure. Two cases involved early termination of therapy, 3 involved relapse and 2 failed to comply with medication instructions. There was 1 case of the Choice HCP not addressing simultaneous use of ledipasvir and a proton pump inhibitor, potentially causing an interaction, and 1 case where both the VA and Choice providers failed to recognize indicators of cirrhosis, which impacted the regimen used.
In the VALLHCS group, records of 320 veterans who completed treatment and had SVR12 testing were reviewed. While the Choice memorandum was active, veterans selected to be treated at VALLHCS had advanced liver fibrosis or cirrhosis, medical and mental health comorbidities that increased the risk of treatment complications or were considered to have difficulty adhering to the medication regimen. For this group, 296 (93%) had confirmed SVR12. Eighteen of the 24 (75%) treatment failures were complicated by nonadherence, including all 13 cases of early termination. One patient died from complications of decompensated cirrhosis before completing treatment, and 1 did not receive HCV medications during a hospital admission due to poor coordination of care between the VA inpatient and outpatient pharmacy services, leading to multiple missed doses.
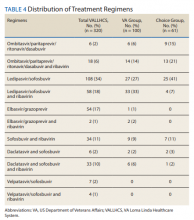
The difference in SVR12 rates (ie, treatment failure rates), between the VA and Choice groups was not statistically significant (P = .61). None of the specific reasons for treatment failure had a statistically significant difference between groups. A treatment failure analysis is shown in Table 3, and Table 4 indicates the breakdown of treatment regimens.
Discussion
The Veterans Health Administration (VHA) is the largest integrated health care system in the US, consisting of 152 medical centers and > 1,700 sites of care. The VA has the potential to meet the health care needs of 21.6 million veterans. About 9 million veterans are enrolled in the VA system and 5.9 million received health care through VHA.17 However, every medical service cannot realistically be made available at every facility, and some veterans have difficulty gaining access to VHA care; distance and wait times have been well-publicized issues that need further exploration.18,19 The Choice program is an attempt to meet gaps in VA coverage using non-VA HCPs.
HCV infection is a specific diagnosis with national treatment guidelines and well-studied treatments; it can be cured, with an evidence-based definition of cure. The VACO policy memorandum to refer less sick veterans to Choice while treating sicker veterans at the VA provided the opportunity to directly compare the quality of the 2 programs. The SVR12 rates of VALLHCS and Choice providers were comparable to the national average at the time, and while the difference in SVR12 rate was not significant, VALLHCS treated a significantly higher number of patients with cirrhosis because of the referral criteria.20
The significant difference in medical comorbidities between the VA and Choice groups was not surprising, partly because of the referral criteria. Cirrhosis can impact the treatment regimen, especially in regard to use of ribavirin. Since the presence of mental health comorbidities did not affect selection into the Choice group, it makes sense that there was no significant difference in prevalence between the groups.
VACO allowed veterans with HCV treatment plans that VA HCPs felt were too complicated for the Choice program to be treated by VHA HCPs.9 VALLHCS exercised this right for veterans at risk for nonadherence, because in HCV treatment, nonadherence leads to treatment failure and development of drug resistant virus strains. Therefore, veterans who would have difficulty traveling to VALLHCS to pick up medications, those who lacked means of communication (such as those who were homeless), and those who had active substance abuse were treated at the VA, where closer monitoring and immediate access to a wide range of services was possible. Studies have confirmed the impact of these types of issues on HCV treatment adherence and success.21 This explains the higher prevalence of social issues in the VA group.
For an internal referral for HCV treatment at VALLHCS, the hepatology provider submits a consult request to the HCV treatment provider, who works in the same office, making direct communication simple. The main administrative limiting factor to minimizing wait times is the number of HCPs, which is dependent on hiring allowances.
When a veteran is referred to Choice, the VA provider places a consult for non-VA care, which the VA Office of Community Care processes by compiling relevant documents and sending the package to Triwest Healthcare Alliance, a private insurance processor contracted with the VA. Triwest selects the Choice provider, often without any input from the VA, and arranges the veteran’s initial appointment.22 Geographic distance to the veteran’s address is the main selection criteria for Triwest. Documents sent between the Choice and VA HCPs go through the Office of Community Care and Triwest. This significantly increases the potential for delays and failed communication. Triwest had a comprehensive list of providers deemed to be qualified to treat HCV within the geographic catchment of VALLHCS. This list was reviewed, and all veterans referred to Choice had HCPs near their home address; therefore, availability of Choice HCPs was not an issue.
The VA can provide guidance on management of the veteran in the form of bundle packages containing a list of services for which the Choice provider is authorized to provide, and ones the Choice provider is not authorized to provide. Some Choice HCPs ordered tests that were not authorized for HCV treatment such as esophagogastroduodenoscopy, colonoscopy, and liver biopsy. In all, 17 of 71 (24%) veterans seen by Choice HCPs had tests performed or ordered that VA HCPs would not have obtained for the purpose of HCV treatment (Table 5).
In order to prevent veterans from receiving unnecessary tests, a VALLHCS hepatologist asked to be notified by VA administrators overseeing Choice referrals whenever a secondary authorization request (SAR) was submitted by a Choice HCP. This strategy is not standard VA practice, therefore at many VA sites these requested tests would have been performed by the Choice HCP, which is why SARs were factored into cost analysis.
SVR12 test results that were drawn too early and had to be repeated at VALLHCS were a cost unique to the Choice program. Duplicate tests, particularly imaging studies and blood work, were extraneous costs. The largest extraneous costs were treatment regimens prescribed by Choice HCPs that did not follow standard of care and required VA provider intervention. Thirty of the 71 (42%) veterans seen by a Choice provider accrued a mean $8,561.40 in extra costs. As a result, the Choice program cost VHA $250,000 more to provide care for 30 veterans (enough to pay for a physician’s annual salary).
Some inappropriate treatment regimens were the result of Choice HCP error, such as 1 case in which a veteran was inadvertently switched from ledipasvir/sofosbuvir to ombitasvir/paritaprevir/ritonavir/dasabuvir after week 4. The veteran had to start therapy over but still achieved SVR12. Other cases saw veterans receive regimens for which they had clear contraindications, such as creatinine clearance < 30 mL/min/1.73m2 for sofosbuvir or a positive resistance panel for specific medications. Eleven of 62 (18%) veterans who were started on HCV treatment by a Choice HCP received a regimen not consistent with VA guidelines—an alarming result.
Follow up for veterans referred to Choice was extremely labor intensive, and assessment of personnel requirements in a Choice-based VA system must take this into consideration. The Choice HCP has no obligation to communicate with the VA HCP. At the time of chart review, 57 of 71 (80%) Choice veterans had inadequate documentation to make a confident assessment of the treatment outcome. Multiple calls to the offices of the Choice HCP were needed to acquire records, and veterans had to be tracked down for additional tests. Veterans often would complete treatment and stop following up with the Choice provider before SVR12 confirmation. The VA hepatology provider reviewing Choice referrals served as clinician, case manager, and clerk in order to get veterans to an appropriate end point in their hepatitis C treatment, with mostly unmeasured hours of work.
Limitations
The study population size was limited by the number of veterans able to complete treatment through Choice. The parameters in the VACO policy memos automatically selected the VA and Choice groups but made them clinically distinct populations. New treatment medications were released during the study period, which impacted management strategy. Occasionally, VA and non-VA HCPs preferred different treatment regimens, leading to variation in the distribution of regimens used despite similar genotype distribution (Tables 2 and 4). In addition, a retrospective study is at risk for recall bias. A prospective study randomizing veterans to the Choice and VA groups is an important future endeavor. Comparing veteran satisfaction for Choice and VA services is also crucial.
Conclusions
This study demonstrates that the VA was able to provide more cost-effective and more timely care for HCV treatment to a relatively sicker population with no reduction in treatment success when compared with non-VA HCPs through the Choice program. While the Choice program can help veterans receive services they may otherwise not have access to and reduce travel time, the current system introduces inefficiencies that delay care and decrease cost-effectiveness. The Choice HCP selection process is based on proximity rather than quality, which may place the veteran at risk for receiving substandard care. Large-scale quality of care studies that compare efficiency measures, clinical outcomes, patient demographics, travel distance, cost efficacy and patient satisfaction for veterans receiving similar services at a VA facility and through Choice should be performed to ensure that veterans receive the best care available.
1. Denniston MM, Jiles RB, Drobeniuc J, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160(5):293-300.
2. Dominitz JA, Boyko EJ, Koepsell TD, et al. Elevated prevalence of hepatitis C infection in users of United States veterans medical centers. Hepatology. 2005;41(1):88-96.
3. Veterans Access, Choice, and Accountability Act of 2014. 42 USC §1395 (2014).
4. Tuchschmidt J. Attachment C: Provision of hepatitis C treatment. US Department of Veterans Affairs Central Office Memorandum from the Principal Deputy Under Secretary for Health. http://vaww.hepatitis.va.gov/education/choice-provision-hcv-treatment.asp. Published May 21, 2015. [Nonpublic site.]
5. Tuchschmidt J. Attachment A: Provision of hepatitis C (HCV) treatment through the Choice program. US Department of Veterans Affairs Central Office Memorandum from the Principal Deputy Under Secretary for Health. http://vaww.hepatitis.va.gov/pdf/choice-attachment-a-FY16.pdf. Published May 21, 2015. [Nonpublic site.]
6. Tuchschmidt J. Attachment B: Initiation of hepatitis C virus (HCV) treatment: protocol for prioritization. US Department of Veterans Affairs Central Office Memorandum from the Principal Deputy Under Secretary for Health. http://vaww.hepatitis.va.gov/pdf/provision-HCV-treatment-attachment-b.pdf. Published May 21, 2015. [Nonpublic site.]
7. Murphy, JP. Hepatitis C virus funding and prioritization status. US Department of Veterans Affairs Central Office Memorandum from the Assistant Deputy Under Secretary for Health for Clinical Operations. http://vaww.hepatitis.va.gov/education/choice-memo-hcv-funding-and-prioritization-status-01272016.asp. Published January 27, 2016. [Nonpublic site.]
8. Lynch TJ, McCarthy MF. Hepatitis C virus funding and prioritization status update. US Department of Veterans Affairs Central Office Memorandum from the Assistant Deputy Under Secretary for Health for Clinical Operations and Acting Assistant Deputy Under Secretary for Health for Patient Care Services. http://vaww.hepatitis.va.gov/education/choice-funding-update-feb-2016.asp. Published February 24, 2016. [Nonpublic site.]
9. Morgan TR, Yee H; US Department of Veterans Affairs National Hepatitis C Resource Center Program and the National Viral Hepatitis Program in the Office of Patient Care Services. Chronic hepatitis C virus (HCV) infection: treatment considerations. http://vaww.hepatitis.va.gov/pdf/treatment-considerations-2016-03-28.pdf. Published March 28, 2016. [Nonpublic site.]
10. American Association for the Study of Liver Diseases; Infectious Diseases Society of America. Initial treatment box. http://hcvguidelines.org/full-report/initial-treatment-box-summary-recommendations-patients-who-are-initiating-therapy-hcv. Updated November 6, 2019. Accessed September 27, 2016
11. AASLD/IDSA HCV Guidance Panel. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 2015;62(3): 932-954.
12. Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006; 43(6):1317-1325.
13. Realdi G, Fattovich G, Hadziyannis S, et al. Survival and prognostic factors in 366 patients with compensated cirrhosis type B: a multicenter study. The Investigators of the European Concerted Action on Viral Hepatitis (EUROHEP). J Hepatol. 1994;21(4):656-666.
14. Kanda T, Kato K, Tsubota A, et al. Platelet count and sustained virological response in hepatitis C treatment. World J Hepatol. 2013;5(4):182-188.
15. Manos MM, Shvachko VA, Murphy RC, Arduino JM, Shire NJ. Distribution of hepatitis C virus genotypes in a diverse US integrated health care population. J Med Virol. 2012;84(11):1744-1750.
16. Cheung RC. Epidemiology of hepatitis C virus infection in American veterans. Am J Gastroenterol. 2000;95(3):740-747.
17. Bagalman E. The number of Veterans that use VA health care services: a fact sheet. Congressional Research Service Report R43579. https://fas.org/sgp/crs/misc/R43579.pdf. Published June 3, 2014. Accessed November 25, 2019.
18. US General Accounting Office. Report to the Ranking Minority Member, Subcommittee on Compensation, Pension, Insurance, and Memorial Affairs, Committee on Veterans’ Affairs, US House of Representatives. How distance from VA facilities affects veterans’ use of VA services. https://www.gao.gov/assets/230/221992.pdf. Published December 1995. Accessed November 25, 2019.
19. Bronstein S, Griffin D. A fatal wait: Veterans languish and die on a VA hospital’s secret list. http://www.cnn.com/2014/04/23/health/veterans-dying-health-care-delays/index.html. Published April 23, 2014. Accessed November 25, 2019.
20. Ioannou GN, Beste LA, Chang MF, et al. Effectiveness of sofosbuvir, ledipasvir/sofosbuvir, or paritaprevir/ritonavir/ombitasvir and dasabuvir regimens for treatment of patients with hepatitis C in the Veterans Affairs national health care system. Gastroenterology. 2016;151(3):457-471.
21. Malespin MH, Harris C, Kanar O, et al. Barriers to treatment of chronic hepatitis C with direct acting antivirals in an urban clinic. Ann Hepatol. 2019;18(2):304–309.
22. Tuchschmidt J. Attachment D: Hepatitis C virus (HCV) fact sheet for Veterans Choice Program for both VA and Choice providers. US Department of Veterans Affairs Central Office Memorandum from the Deputy Under Secretary for Health for Policy and Services and the Acting Deputy Undersecretary for Health for Operations and Management. http://vaww.hepatitis.va.gov/educatiochoice-provision-HCV-treatment-additional.asp. [Nonpublic site.]
Population studies show high prevalence of chronic hepatitis C virus (HCV) infection among veterans, especially Vietnam War era veterans.1,2 The development of safe and efficacious direct-acting antiviral (DAA) medications to treat HCV infection made the majority of those infected eligible for treatment. However, the large number of veterans needing DAA treatment stressed the resources of the US Department of Veterans Affairs (VA) health care system. This occurred while Congress was focused on reducing wait times for veterans receiving care at the VA.
Congress passed the Veterans Access, Choice, and Accountability Act (Choice) on August 7, 2014, leading to the creation of the Veterans Choice Program. Legislators felt there were inappropriate delays in care at the VA, and the Choice program was meant to address this problem and provide an “apples-to-apples comparison [of the VA] with non-VA hospitals.”3
Congress acknowledged the importance of curing HCV in the veteran population and allocated $1.5 billion for fiscal year (FY) 2016 for DAAs. The VA Central Office (VACO) carefully monitored these resources. The first policy memorandum from VACO for HCV care, issued on May 21, 2015, recommended that the sickest patients who will benefit from the treatment “receive priority over those who are less ill.”4,5 Those who met criteria for advanced liver disease were prioritized for treatment at the VA, while those who did not meet criteria were given the option of receiving treatment through Choice, or waiting for a change in policy.6 Over time, revisions to the guidelines relaxed the criteria for VA treatment eligibility, and on February 24, 2016, all restrictions on HCV treatment at the VA were lifted.7,8
The aim of this study was to provide a comparison of VA and non-VA care, specifically to determine whether care provided through Choice was timelier and more cost effective than care provided by the VA, and whether there was a quality difference. The high prevalence among veterans, well-established standards of care, and finite treatment course with clear indicators of success and failure makes HCV treatment an ideal disease with which to make this comparison.
Methods
We retrospectively analyzed the VA electronic health records of all veterans seen in the VA Loma Linda Healthcare System (VALLHCS) Hepatology clinic for chronic HCV infection during FY 2016 who were referred to Choice for HCV treatment. One hundred veterans met these criteria, encompassing the Choice population; 71 were seen at least once by a non-VA (Choice) health care provider (HCP) and 61 completed a treatment regimen through Choice. Treatment completion was defined as cessation of medication after the planned duration of therapy, or early termination of medication without resumption by that HCP. The Choice population was matched to an equal number of veterans who received HCV treatment from VALLHCS HCPs.
Data collected included age, gender, HCV genotype, determinants of liver fibrosis, and treatment success (defined as sustained virologic response at 12 weeks after the last dose of medication [SVR12]). Determinants of liver fibrosis included documented cirrhosis or complications of cirrhosis, Fibrosis-4 score (Fib-4), and platelet count.
Treatment failures were categorized as nonresponse (defined as detectable HCV viral load at the end of treatment), relapse (defined as an undetectable HCV viral load at the end of treatment with a subsequent positive test), and early termination (defined as a failure to complete the planned treatment regimen). Documented patient nonadherence, medical comorbidities that affected the treatment protocol, mental health diagnoses, and active social issues (defined as active or history of heavy alcohol use, active or history of illicit drug use, lack of social support, and homelessness) were noted.
Timeliness of delivery of care was measured in days. For the VA group, the wait time was defined as the date the consult for HCV treatment was placed to the date of the initial appointment with the HCV treatment provider. For the Choice group, the wait time was defined as the date the referral to the Choice program was made to the date of the initial appointment with the Choice HCP. Treatment regimens were evaluated for appropriateness based on guidelines from VACO and the American Association for the Study of Liver Diseases.9-11
Tests performed by Choice providers were evaluated for whether they were relevant to HCV care and whether similar data already were available from VALLHCS. Tests that were not indicated were identified as unnecessary costs incurred by the Choice program, as were tests that had to be repeated at the VA because of a lack of documentation from the Choice provider. All medications given inappropriately were considered added costs. Medicare reimbursement rates for the most applicable Current Procedural Terminology (CPT) code and VA national contract pricing for medications were used for calculations. This study was approved by the VALLHCS institutional review board.
Statistical Analysis
IBM (Armonk, NY) Statistical Package for Social Sciences software was used to evaluate for differences in Fib-4, platelet count, prevalence of cirrhosis, prevalence of medical comorbidities, prevalence of mental health comorbidities, prevalence of the social issues defined in the Methods section, time from referral to time of appointment date, and SVR12 rate between the VA and Choice groups.
Exclusions
There were 15 veterans in the VA group who had a wait time of > 100 days. Of these, 5 (33%) were initially Choice referrals, but due to negative interactions with the Choice provider, the veterans returned to VALLHCS for care. Two of the 15 (13%) did not keep appointments and were lost to follow up. Six of the 15 (40%) had medical comorbidities that required more immediate attention, so HCV treatment initiation was deliberately moved back. The final 2 veterans scheduled their appointments unusually far apart, artificially increasing their wait time. Given that these were unique situations and some of the veterans received care from both Choice and VA providers, a decision was made to exclude these individuals from the study.
It has been shown that platelet count correlates with degree of liver fibrosis, a concept that is the basis for the Fib-4 scoring system.12 Studies have shown that platelet count is a survival predictor in those with cirrhosis, and thrombocytopenia is a negative predictor of HCV treatment success using peginterferon and ribavirin.13,14 Therefore, the VA memorandum automatically assigned the sickest individuals to the VA for HCV treatment. The goal of this study was to compare the impact of factors other than stage of fibrosis on HCV treatment success, which is why the 12 veterans with platelet count < 100,000 in the VA group were excluded. There were no veterans with platelet count < 100,000 in the Choice group.
When comparing SVR12 rates between the VA and Choice groups, every veteran treated at VALLHCS in FY 2016 was included, increasing the number in the VA group from 100 to 320 and therefore the power of this comparison.
Results
A summary of the statistical analysis can be found in Table 1. The genotype distribution was consistent with epidemiological studies, including those specific to veterans.15,16 There were statistically significant differences (P < .001) in mean Fib-4 and mean platelet count. The VA group had a higher Fib-4 and lower platelet count. Seventy-four percent of the VA population was defined as cirrhotic, while only 3% of the Choice population met similar criteria (P < .001). The VA and Choice groups were similar in terms of age, gender, and genotype distribution (Table 2).
The VA group was found to have a higher prevalence of comorbidities that affected HCV treatment. These conditions included but were not limited to: chronic kidney disease that precluded the use of certain medications, any condition that required medication with a known interaction with DAAs (ie, proton pump inhibitors, statins, and amiodarone), and cirrhosis if it impacted the treatment regimen. The difference in the prevalence of mental health comorbidities was not significant (P = .39), but there was a higher prevalence of social issues among the VA group (P = .002).
The mean wait time from referral to appointment was 28.6 days for the VA group and 42.3 days for the Choice group (P < .001), indicating that a Choice referral took longer to complete than a referral within the VA for HCV treatment. Thirty of the 71 (42%) veterans seen by a Choice provider accrued extraneous cost, with a mean additional cost of $8,561.40 per veteran. In the Choice group, 61 veterans completed a treatment regimen with the Choice HCP. Fifty-five veterans completed treatment and had available SVR12 data (6 were lost to follow up without SVR12 testing) and 50 (91%) had confirmed SVR12. The charts of the 5 treatment failures were reviewed to discern the cause for failure. Two cases involved early termination of therapy, 3 involved relapse and 2 failed to comply with medication instructions. There was 1 case of the Choice HCP not addressing simultaneous use of ledipasvir and a proton pump inhibitor, potentially causing an interaction, and 1 case where both the VA and Choice providers failed to recognize indicators of cirrhosis, which impacted the regimen used.
In the VALLHCS group, records of 320 veterans who completed treatment and had SVR12 testing were reviewed. While the Choice memorandum was active, veterans selected to be treated at VALLHCS had advanced liver fibrosis or cirrhosis, medical and mental health comorbidities that increased the risk of treatment complications or were considered to have difficulty adhering to the medication regimen. For this group, 296 (93%) had confirmed SVR12. Eighteen of the 24 (75%) treatment failures were complicated by nonadherence, including all 13 cases of early termination. One patient died from complications of decompensated cirrhosis before completing treatment, and 1 did not receive HCV medications during a hospital admission due to poor coordination of care between the VA inpatient and outpatient pharmacy services, leading to multiple missed doses.
The difference in SVR12 rates (ie, treatment failure rates), between the VA and Choice groups was not statistically significant (P = .61). None of the specific reasons for treatment failure had a statistically significant difference between groups. A treatment failure analysis is shown in Table 3, and Table 4 indicates the breakdown of treatment regimens.
Discussion
The Veterans Health Administration (VHA) is the largest integrated health care system in the US, consisting of 152 medical centers and > 1,700 sites of care. The VA has the potential to meet the health care needs of 21.6 million veterans. About 9 million veterans are enrolled in the VA system and 5.9 million received health care through VHA.17 However, every medical service cannot realistically be made available at every facility, and some veterans have difficulty gaining access to VHA care; distance and wait times have been well-publicized issues that need further exploration.18,19 The Choice program is an attempt to meet gaps in VA coverage using non-VA HCPs.
HCV infection is a specific diagnosis with national treatment guidelines and well-studied treatments; it can be cured, with an evidence-based definition of cure. The VACO policy memorandum to refer less sick veterans to Choice while treating sicker veterans at the VA provided the opportunity to directly compare the quality of the 2 programs. The SVR12 rates of VALLHCS and Choice providers were comparable to the national average at the time, and while the difference in SVR12 rate was not significant, VALLHCS treated a significantly higher number of patients with cirrhosis because of the referral criteria.20
The significant difference in medical comorbidities between the VA and Choice groups was not surprising, partly because of the referral criteria. Cirrhosis can impact the treatment regimen, especially in regard to use of ribavirin. Since the presence of mental health comorbidities did not affect selection into the Choice group, it makes sense that there was no significant difference in prevalence between the groups.
VACO allowed veterans with HCV treatment plans that VA HCPs felt were too complicated for the Choice program to be treated by VHA HCPs.9 VALLHCS exercised this right for veterans at risk for nonadherence, because in HCV treatment, nonadherence leads to treatment failure and development of drug resistant virus strains. Therefore, veterans who would have difficulty traveling to VALLHCS to pick up medications, those who lacked means of communication (such as those who were homeless), and those who had active substance abuse were treated at the VA, where closer monitoring and immediate access to a wide range of services was possible. Studies have confirmed the impact of these types of issues on HCV treatment adherence and success.21 This explains the higher prevalence of social issues in the VA group.
For an internal referral for HCV treatment at VALLHCS, the hepatology provider submits a consult request to the HCV treatment provider, who works in the same office, making direct communication simple. The main administrative limiting factor to minimizing wait times is the number of HCPs, which is dependent on hiring allowances.
When a veteran is referred to Choice, the VA provider places a consult for non-VA care, which the VA Office of Community Care processes by compiling relevant documents and sending the package to Triwest Healthcare Alliance, a private insurance processor contracted with the VA. Triwest selects the Choice provider, often without any input from the VA, and arranges the veteran’s initial appointment.22 Geographic distance to the veteran’s address is the main selection criteria for Triwest. Documents sent between the Choice and VA HCPs go through the Office of Community Care and Triwest. This significantly increases the potential for delays and failed communication. Triwest had a comprehensive list of providers deemed to be qualified to treat HCV within the geographic catchment of VALLHCS. This list was reviewed, and all veterans referred to Choice had HCPs near their home address; therefore, availability of Choice HCPs was not an issue.
The VA can provide guidance on management of the veteran in the form of bundle packages containing a list of services for which the Choice provider is authorized to provide, and ones the Choice provider is not authorized to provide. Some Choice HCPs ordered tests that were not authorized for HCV treatment such as esophagogastroduodenoscopy, colonoscopy, and liver biopsy. In all, 17 of 71 (24%) veterans seen by Choice HCPs had tests performed or ordered that VA HCPs would not have obtained for the purpose of HCV treatment (Table 5).
In order to prevent veterans from receiving unnecessary tests, a VALLHCS hepatologist asked to be notified by VA administrators overseeing Choice referrals whenever a secondary authorization request (SAR) was submitted by a Choice HCP. This strategy is not standard VA practice, therefore at many VA sites these requested tests would have been performed by the Choice HCP, which is why SARs were factored into cost analysis.
SVR12 test results that were drawn too early and had to be repeated at VALLHCS were a cost unique to the Choice program. Duplicate tests, particularly imaging studies and blood work, were extraneous costs. The largest extraneous costs were treatment regimens prescribed by Choice HCPs that did not follow standard of care and required VA provider intervention. Thirty of the 71 (42%) veterans seen by a Choice provider accrued a mean $8,561.40 in extra costs. As a result, the Choice program cost VHA $250,000 more to provide care for 30 veterans (enough to pay for a physician’s annual salary).
Some inappropriate treatment regimens were the result of Choice HCP error, such as 1 case in which a veteran was inadvertently switched from ledipasvir/sofosbuvir to ombitasvir/paritaprevir/ritonavir/dasabuvir after week 4. The veteran had to start therapy over but still achieved SVR12. Other cases saw veterans receive regimens for which they had clear contraindications, such as creatinine clearance < 30 mL/min/1.73m2 for sofosbuvir or a positive resistance panel for specific medications. Eleven of 62 (18%) veterans who were started on HCV treatment by a Choice HCP received a regimen not consistent with VA guidelines—an alarming result.
Follow up for veterans referred to Choice was extremely labor intensive, and assessment of personnel requirements in a Choice-based VA system must take this into consideration. The Choice HCP has no obligation to communicate with the VA HCP. At the time of chart review, 57 of 71 (80%) Choice veterans had inadequate documentation to make a confident assessment of the treatment outcome. Multiple calls to the offices of the Choice HCP were needed to acquire records, and veterans had to be tracked down for additional tests. Veterans often would complete treatment and stop following up with the Choice provider before SVR12 confirmation. The VA hepatology provider reviewing Choice referrals served as clinician, case manager, and clerk in order to get veterans to an appropriate end point in their hepatitis C treatment, with mostly unmeasured hours of work.
Limitations
The study population size was limited by the number of veterans able to complete treatment through Choice. The parameters in the VACO policy memos automatically selected the VA and Choice groups but made them clinically distinct populations. New treatment medications were released during the study period, which impacted management strategy. Occasionally, VA and non-VA HCPs preferred different treatment regimens, leading to variation in the distribution of regimens used despite similar genotype distribution (Tables 2 and 4). In addition, a retrospective study is at risk for recall bias. A prospective study randomizing veterans to the Choice and VA groups is an important future endeavor. Comparing veteran satisfaction for Choice and VA services is also crucial.
Conclusions
This study demonstrates that the VA was able to provide more cost-effective and more timely care for HCV treatment to a relatively sicker population with no reduction in treatment success when compared with non-VA HCPs through the Choice program. While the Choice program can help veterans receive services they may otherwise not have access to and reduce travel time, the current system introduces inefficiencies that delay care and decrease cost-effectiveness. The Choice HCP selection process is based on proximity rather than quality, which may place the veteran at risk for receiving substandard care. Large-scale quality of care studies that compare efficiency measures, clinical outcomes, patient demographics, travel distance, cost efficacy and patient satisfaction for veterans receiving similar services at a VA facility and through Choice should be performed to ensure that veterans receive the best care available.
Population studies show high prevalence of chronic hepatitis C virus (HCV) infection among veterans, especially Vietnam War era veterans.1,2 The development of safe and efficacious direct-acting antiviral (DAA) medications to treat HCV infection made the majority of those infected eligible for treatment. However, the large number of veterans needing DAA treatment stressed the resources of the US Department of Veterans Affairs (VA) health care system. This occurred while Congress was focused on reducing wait times for veterans receiving care at the VA.
Congress passed the Veterans Access, Choice, and Accountability Act (Choice) on August 7, 2014, leading to the creation of the Veterans Choice Program. Legislators felt there were inappropriate delays in care at the VA, and the Choice program was meant to address this problem and provide an “apples-to-apples comparison [of the VA] with non-VA hospitals.”3
Congress acknowledged the importance of curing HCV in the veteran population and allocated $1.5 billion for fiscal year (FY) 2016 for DAAs. The VA Central Office (VACO) carefully monitored these resources. The first policy memorandum from VACO for HCV care, issued on May 21, 2015, recommended that the sickest patients who will benefit from the treatment “receive priority over those who are less ill.”4,5 Those who met criteria for advanced liver disease were prioritized for treatment at the VA, while those who did not meet criteria were given the option of receiving treatment through Choice, or waiting for a change in policy.6 Over time, revisions to the guidelines relaxed the criteria for VA treatment eligibility, and on February 24, 2016, all restrictions on HCV treatment at the VA were lifted.7,8
The aim of this study was to provide a comparison of VA and non-VA care, specifically to determine whether care provided through Choice was timelier and more cost effective than care provided by the VA, and whether there was a quality difference. The high prevalence among veterans, well-established standards of care, and finite treatment course with clear indicators of success and failure makes HCV treatment an ideal disease with which to make this comparison.
Methods
We retrospectively analyzed the VA electronic health records of all veterans seen in the VA Loma Linda Healthcare System (VALLHCS) Hepatology clinic for chronic HCV infection during FY 2016 who were referred to Choice for HCV treatment. One hundred veterans met these criteria, encompassing the Choice population; 71 were seen at least once by a non-VA (Choice) health care provider (HCP) and 61 completed a treatment regimen through Choice. Treatment completion was defined as cessation of medication after the planned duration of therapy, or early termination of medication without resumption by that HCP. The Choice population was matched to an equal number of veterans who received HCV treatment from VALLHCS HCPs.
Data collected included age, gender, HCV genotype, determinants of liver fibrosis, and treatment success (defined as sustained virologic response at 12 weeks after the last dose of medication [SVR12]). Determinants of liver fibrosis included documented cirrhosis or complications of cirrhosis, Fibrosis-4 score (Fib-4), and platelet count.
Treatment failures were categorized as nonresponse (defined as detectable HCV viral load at the end of treatment), relapse (defined as an undetectable HCV viral load at the end of treatment with a subsequent positive test), and early termination (defined as a failure to complete the planned treatment regimen). Documented patient nonadherence, medical comorbidities that affected the treatment protocol, mental health diagnoses, and active social issues (defined as active or history of heavy alcohol use, active or history of illicit drug use, lack of social support, and homelessness) were noted.
Timeliness of delivery of care was measured in days. For the VA group, the wait time was defined as the date the consult for HCV treatment was placed to the date of the initial appointment with the HCV treatment provider. For the Choice group, the wait time was defined as the date the referral to the Choice program was made to the date of the initial appointment with the Choice HCP. Treatment regimens were evaluated for appropriateness based on guidelines from VACO and the American Association for the Study of Liver Diseases.9-11
Tests performed by Choice providers were evaluated for whether they were relevant to HCV care and whether similar data already were available from VALLHCS. Tests that were not indicated were identified as unnecessary costs incurred by the Choice program, as were tests that had to be repeated at the VA because of a lack of documentation from the Choice provider. All medications given inappropriately were considered added costs. Medicare reimbursement rates for the most applicable Current Procedural Terminology (CPT) code and VA national contract pricing for medications were used for calculations. This study was approved by the VALLHCS institutional review board.
Statistical Analysis
IBM (Armonk, NY) Statistical Package for Social Sciences software was used to evaluate for differences in Fib-4, platelet count, prevalence of cirrhosis, prevalence of medical comorbidities, prevalence of mental health comorbidities, prevalence of the social issues defined in the Methods section, time from referral to time of appointment date, and SVR12 rate between the VA and Choice groups.
Exclusions
There were 15 veterans in the VA group who had a wait time of > 100 days. Of these, 5 (33%) were initially Choice referrals, but due to negative interactions with the Choice provider, the veterans returned to VALLHCS for care. Two of the 15 (13%) did not keep appointments and were lost to follow up. Six of the 15 (40%) had medical comorbidities that required more immediate attention, so HCV treatment initiation was deliberately moved back. The final 2 veterans scheduled their appointments unusually far apart, artificially increasing their wait time. Given that these were unique situations and some of the veterans received care from both Choice and VA providers, a decision was made to exclude these individuals from the study.
It has been shown that platelet count correlates with degree of liver fibrosis, a concept that is the basis for the Fib-4 scoring system.12 Studies have shown that platelet count is a survival predictor in those with cirrhosis, and thrombocytopenia is a negative predictor of HCV treatment success using peginterferon and ribavirin.13,14 Therefore, the VA memorandum automatically assigned the sickest individuals to the VA for HCV treatment. The goal of this study was to compare the impact of factors other than stage of fibrosis on HCV treatment success, which is why the 12 veterans with platelet count < 100,000 in the VA group were excluded. There were no veterans with platelet count < 100,000 in the Choice group.
When comparing SVR12 rates between the VA and Choice groups, every veteran treated at VALLHCS in FY 2016 was included, increasing the number in the VA group from 100 to 320 and therefore the power of this comparison.
Results
A summary of the statistical analysis can be found in Table 1. The genotype distribution was consistent with epidemiological studies, including those specific to veterans.15,16 There were statistically significant differences (P < .001) in mean Fib-4 and mean platelet count. The VA group had a higher Fib-4 and lower platelet count. Seventy-four percent of the VA population was defined as cirrhotic, while only 3% of the Choice population met similar criteria (P < .001). The VA and Choice groups were similar in terms of age, gender, and genotype distribution (Table 2).
The VA group was found to have a higher prevalence of comorbidities that affected HCV treatment. These conditions included but were not limited to: chronic kidney disease that precluded the use of certain medications, any condition that required medication with a known interaction with DAAs (ie, proton pump inhibitors, statins, and amiodarone), and cirrhosis if it impacted the treatment regimen. The difference in the prevalence of mental health comorbidities was not significant (P = .39), but there was a higher prevalence of social issues among the VA group (P = .002).
The mean wait time from referral to appointment was 28.6 days for the VA group and 42.3 days for the Choice group (P < .001), indicating that a Choice referral took longer to complete than a referral within the VA for HCV treatment. Thirty of the 71 (42%) veterans seen by a Choice provider accrued extraneous cost, with a mean additional cost of $8,561.40 per veteran. In the Choice group, 61 veterans completed a treatment regimen with the Choice HCP. Fifty-five veterans completed treatment and had available SVR12 data (6 were lost to follow up without SVR12 testing) and 50 (91%) had confirmed SVR12. The charts of the 5 treatment failures were reviewed to discern the cause for failure. Two cases involved early termination of therapy, 3 involved relapse and 2 failed to comply with medication instructions. There was 1 case of the Choice HCP not addressing simultaneous use of ledipasvir and a proton pump inhibitor, potentially causing an interaction, and 1 case where both the VA and Choice providers failed to recognize indicators of cirrhosis, which impacted the regimen used.
In the VALLHCS group, records of 320 veterans who completed treatment and had SVR12 testing were reviewed. While the Choice memorandum was active, veterans selected to be treated at VALLHCS had advanced liver fibrosis or cirrhosis, medical and mental health comorbidities that increased the risk of treatment complications or were considered to have difficulty adhering to the medication regimen. For this group, 296 (93%) had confirmed SVR12. Eighteen of the 24 (75%) treatment failures were complicated by nonadherence, including all 13 cases of early termination. One patient died from complications of decompensated cirrhosis before completing treatment, and 1 did not receive HCV medications during a hospital admission due to poor coordination of care between the VA inpatient and outpatient pharmacy services, leading to multiple missed doses.
The difference in SVR12 rates (ie, treatment failure rates), between the VA and Choice groups was not statistically significant (P = .61). None of the specific reasons for treatment failure had a statistically significant difference between groups. A treatment failure analysis is shown in Table 3, and Table 4 indicates the breakdown of treatment regimens.
Discussion
The Veterans Health Administration (VHA) is the largest integrated health care system in the US, consisting of 152 medical centers and > 1,700 sites of care. The VA has the potential to meet the health care needs of 21.6 million veterans. About 9 million veterans are enrolled in the VA system and 5.9 million received health care through VHA.17 However, every medical service cannot realistically be made available at every facility, and some veterans have difficulty gaining access to VHA care; distance and wait times have been well-publicized issues that need further exploration.18,19 The Choice program is an attempt to meet gaps in VA coverage using non-VA HCPs.
HCV infection is a specific diagnosis with national treatment guidelines and well-studied treatments; it can be cured, with an evidence-based definition of cure. The VACO policy memorandum to refer less sick veterans to Choice while treating sicker veterans at the VA provided the opportunity to directly compare the quality of the 2 programs. The SVR12 rates of VALLHCS and Choice providers were comparable to the national average at the time, and while the difference in SVR12 rate was not significant, VALLHCS treated a significantly higher number of patients with cirrhosis because of the referral criteria.20
The significant difference in medical comorbidities between the VA and Choice groups was not surprising, partly because of the referral criteria. Cirrhosis can impact the treatment regimen, especially in regard to use of ribavirin. Since the presence of mental health comorbidities did not affect selection into the Choice group, it makes sense that there was no significant difference in prevalence between the groups.
VACO allowed veterans with HCV treatment plans that VA HCPs felt were too complicated for the Choice program to be treated by VHA HCPs.9 VALLHCS exercised this right for veterans at risk for nonadherence, because in HCV treatment, nonadherence leads to treatment failure and development of drug resistant virus strains. Therefore, veterans who would have difficulty traveling to VALLHCS to pick up medications, those who lacked means of communication (such as those who were homeless), and those who had active substance abuse were treated at the VA, where closer monitoring and immediate access to a wide range of services was possible. Studies have confirmed the impact of these types of issues on HCV treatment adherence and success.21 This explains the higher prevalence of social issues in the VA group.
For an internal referral for HCV treatment at VALLHCS, the hepatology provider submits a consult request to the HCV treatment provider, who works in the same office, making direct communication simple. The main administrative limiting factor to minimizing wait times is the number of HCPs, which is dependent on hiring allowances.
When a veteran is referred to Choice, the VA provider places a consult for non-VA care, which the VA Office of Community Care processes by compiling relevant documents and sending the package to Triwest Healthcare Alliance, a private insurance processor contracted with the VA. Triwest selects the Choice provider, often without any input from the VA, and arranges the veteran’s initial appointment.22 Geographic distance to the veteran’s address is the main selection criteria for Triwest. Documents sent between the Choice and VA HCPs go through the Office of Community Care and Triwest. This significantly increases the potential for delays and failed communication. Triwest had a comprehensive list of providers deemed to be qualified to treat HCV within the geographic catchment of VALLHCS. This list was reviewed, and all veterans referred to Choice had HCPs near their home address; therefore, availability of Choice HCPs was not an issue.
The VA can provide guidance on management of the veteran in the form of bundle packages containing a list of services for which the Choice provider is authorized to provide, and ones the Choice provider is not authorized to provide. Some Choice HCPs ordered tests that were not authorized for HCV treatment such as esophagogastroduodenoscopy, colonoscopy, and liver biopsy. In all, 17 of 71 (24%) veterans seen by Choice HCPs had tests performed or ordered that VA HCPs would not have obtained for the purpose of HCV treatment (Table 5).
In order to prevent veterans from receiving unnecessary tests, a VALLHCS hepatologist asked to be notified by VA administrators overseeing Choice referrals whenever a secondary authorization request (SAR) was submitted by a Choice HCP. This strategy is not standard VA practice, therefore at many VA sites these requested tests would have been performed by the Choice HCP, which is why SARs were factored into cost analysis.
SVR12 test results that were drawn too early and had to be repeated at VALLHCS were a cost unique to the Choice program. Duplicate tests, particularly imaging studies and blood work, were extraneous costs. The largest extraneous costs were treatment regimens prescribed by Choice HCPs that did not follow standard of care and required VA provider intervention. Thirty of the 71 (42%) veterans seen by a Choice provider accrued a mean $8,561.40 in extra costs. As a result, the Choice program cost VHA $250,000 more to provide care for 30 veterans (enough to pay for a physician’s annual salary).
Some inappropriate treatment regimens were the result of Choice HCP error, such as 1 case in which a veteran was inadvertently switched from ledipasvir/sofosbuvir to ombitasvir/paritaprevir/ritonavir/dasabuvir after week 4. The veteran had to start therapy over but still achieved SVR12. Other cases saw veterans receive regimens for which they had clear contraindications, such as creatinine clearance < 30 mL/min/1.73m2 for sofosbuvir or a positive resistance panel for specific medications. Eleven of 62 (18%) veterans who were started on HCV treatment by a Choice HCP received a regimen not consistent with VA guidelines—an alarming result.
Follow up for veterans referred to Choice was extremely labor intensive, and assessment of personnel requirements in a Choice-based VA system must take this into consideration. The Choice HCP has no obligation to communicate with the VA HCP. At the time of chart review, 57 of 71 (80%) Choice veterans had inadequate documentation to make a confident assessment of the treatment outcome. Multiple calls to the offices of the Choice HCP were needed to acquire records, and veterans had to be tracked down for additional tests. Veterans often would complete treatment and stop following up with the Choice provider before SVR12 confirmation. The VA hepatology provider reviewing Choice referrals served as clinician, case manager, and clerk in order to get veterans to an appropriate end point in their hepatitis C treatment, with mostly unmeasured hours of work.
Limitations
The study population size was limited by the number of veterans able to complete treatment through Choice. The parameters in the VACO policy memos automatically selected the VA and Choice groups but made them clinically distinct populations. New treatment medications were released during the study period, which impacted management strategy. Occasionally, VA and non-VA HCPs preferred different treatment regimens, leading to variation in the distribution of regimens used despite similar genotype distribution (Tables 2 and 4). In addition, a retrospective study is at risk for recall bias. A prospective study randomizing veterans to the Choice and VA groups is an important future endeavor. Comparing veteran satisfaction for Choice and VA services is also crucial.
Conclusions
This study demonstrates that the VA was able to provide more cost-effective and more timely care for HCV treatment to a relatively sicker population with no reduction in treatment success when compared with non-VA HCPs through the Choice program. While the Choice program can help veterans receive services they may otherwise not have access to and reduce travel time, the current system introduces inefficiencies that delay care and decrease cost-effectiveness. The Choice HCP selection process is based on proximity rather than quality, which may place the veteran at risk for receiving substandard care. Large-scale quality of care studies that compare efficiency measures, clinical outcomes, patient demographics, travel distance, cost efficacy and patient satisfaction for veterans receiving similar services at a VA facility and through Choice should be performed to ensure that veterans receive the best care available.
1. Denniston MM, Jiles RB, Drobeniuc J, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160(5):293-300.
2. Dominitz JA, Boyko EJ, Koepsell TD, et al. Elevated prevalence of hepatitis C infection in users of United States veterans medical centers. Hepatology. 2005;41(1):88-96.
3. Veterans Access, Choice, and Accountability Act of 2014. 42 USC §1395 (2014).
4. Tuchschmidt J. Attachment C: Provision of hepatitis C treatment. US Department of Veterans Affairs Central Office Memorandum from the Principal Deputy Under Secretary for Health. http://vaww.hepatitis.va.gov/education/choice-provision-hcv-treatment.asp. Published May 21, 2015. [Nonpublic site.]
5. Tuchschmidt J. Attachment A: Provision of hepatitis C (HCV) treatment through the Choice program. US Department of Veterans Affairs Central Office Memorandum from the Principal Deputy Under Secretary for Health. http://vaww.hepatitis.va.gov/pdf/choice-attachment-a-FY16.pdf. Published May 21, 2015. [Nonpublic site.]
6. Tuchschmidt J. Attachment B: Initiation of hepatitis C virus (HCV) treatment: protocol for prioritization. US Department of Veterans Affairs Central Office Memorandum from the Principal Deputy Under Secretary for Health. http://vaww.hepatitis.va.gov/pdf/provision-HCV-treatment-attachment-b.pdf. Published May 21, 2015. [Nonpublic site.]
7. Murphy, JP. Hepatitis C virus funding and prioritization status. US Department of Veterans Affairs Central Office Memorandum from the Assistant Deputy Under Secretary for Health for Clinical Operations. http://vaww.hepatitis.va.gov/education/choice-memo-hcv-funding-and-prioritization-status-01272016.asp. Published January 27, 2016. [Nonpublic site.]
8. Lynch TJ, McCarthy MF. Hepatitis C virus funding and prioritization status update. US Department of Veterans Affairs Central Office Memorandum from the Assistant Deputy Under Secretary for Health for Clinical Operations and Acting Assistant Deputy Under Secretary for Health for Patient Care Services. http://vaww.hepatitis.va.gov/education/choice-funding-update-feb-2016.asp. Published February 24, 2016. [Nonpublic site.]
9. Morgan TR, Yee H; US Department of Veterans Affairs National Hepatitis C Resource Center Program and the National Viral Hepatitis Program in the Office of Patient Care Services. Chronic hepatitis C virus (HCV) infection: treatment considerations. http://vaww.hepatitis.va.gov/pdf/treatment-considerations-2016-03-28.pdf. Published March 28, 2016. [Nonpublic site.]
10. American Association for the Study of Liver Diseases; Infectious Diseases Society of America. Initial treatment box. http://hcvguidelines.org/full-report/initial-treatment-box-summary-recommendations-patients-who-are-initiating-therapy-hcv. Updated November 6, 2019. Accessed September 27, 2016
11. AASLD/IDSA HCV Guidance Panel. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 2015;62(3): 932-954.
12. Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006; 43(6):1317-1325.
13. Realdi G, Fattovich G, Hadziyannis S, et al. Survival and prognostic factors in 366 patients with compensated cirrhosis type B: a multicenter study. The Investigators of the European Concerted Action on Viral Hepatitis (EUROHEP). J Hepatol. 1994;21(4):656-666.
14. Kanda T, Kato K, Tsubota A, et al. Platelet count and sustained virological response in hepatitis C treatment. World J Hepatol. 2013;5(4):182-188.
15. Manos MM, Shvachko VA, Murphy RC, Arduino JM, Shire NJ. Distribution of hepatitis C virus genotypes in a diverse US integrated health care population. J Med Virol. 2012;84(11):1744-1750.
16. Cheung RC. Epidemiology of hepatitis C virus infection in American veterans. Am J Gastroenterol. 2000;95(3):740-747.
17. Bagalman E. The number of Veterans that use VA health care services: a fact sheet. Congressional Research Service Report R43579. https://fas.org/sgp/crs/misc/R43579.pdf. Published June 3, 2014. Accessed November 25, 2019.
18. US General Accounting Office. Report to the Ranking Minority Member, Subcommittee on Compensation, Pension, Insurance, and Memorial Affairs, Committee on Veterans’ Affairs, US House of Representatives. How distance from VA facilities affects veterans’ use of VA services. https://www.gao.gov/assets/230/221992.pdf. Published December 1995. Accessed November 25, 2019.
19. Bronstein S, Griffin D. A fatal wait: Veterans languish and die on a VA hospital’s secret list. http://www.cnn.com/2014/04/23/health/veterans-dying-health-care-delays/index.html. Published April 23, 2014. Accessed November 25, 2019.
20. Ioannou GN, Beste LA, Chang MF, et al. Effectiveness of sofosbuvir, ledipasvir/sofosbuvir, or paritaprevir/ritonavir/ombitasvir and dasabuvir regimens for treatment of patients with hepatitis C in the Veterans Affairs national health care system. Gastroenterology. 2016;151(3):457-471.
21. Malespin MH, Harris C, Kanar O, et al. Barriers to treatment of chronic hepatitis C with direct acting antivirals in an urban clinic. Ann Hepatol. 2019;18(2):304–309.
22. Tuchschmidt J. Attachment D: Hepatitis C virus (HCV) fact sheet for Veterans Choice Program for both VA and Choice providers. US Department of Veterans Affairs Central Office Memorandum from the Deputy Under Secretary for Health for Policy and Services and the Acting Deputy Undersecretary for Health for Operations and Management. http://vaww.hepatitis.va.gov/educatiochoice-provision-HCV-treatment-additional.asp. [Nonpublic site.]
1. Denniston MM, Jiles RB, Drobeniuc J, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160(5):293-300.
2. Dominitz JA, Boyko EJ, Koepsell TD, et al. Elevated prevalence of hepatitis C infection in users of United States veterans medical centers. Hepatology. 2005;41(1):88-96.
3. Veterans Access, Choice, and Accountability Act of 2014. 42 USC §1395 (2014).
4. Tuchschmidt J. Attachment C: Provision of hepatitis C treatment. US Department of Veterans Affairs Central Office Memorandum from the Principal Deputy Under Secretary for Health. http://vaww.hepatitis.va.gov/education/choice-provision-hcv-treatment.asp. Published May 21, 2015. [Nonpublic site.]
5. Tuchschmidt J. Attachment A: Provision of hepatitis C (HCV) treatment through the Choice program. US Department of Veterans Affairs Central Office Memorandum from the Principal Deputy Under Secretary for Health. http://vaww.hepatitis.va.gov/pdf/choice-attachment-a-FY16.pdf. Published May 21, 2015. [Nonpublic site.]
6. Tuchschmidt J. Attachment B: Initiation of hepatitis C virus (HCV) treatment: protocol for prioritization. US Department of Veterans Affairs Central Office Memorandum from the Principal Deputy Under Secretary for Health. http://vaww.hepatitis.va.gov/pdf/provision-HCV-treatment-attachment-b.pdf. Published May 21, 2015. [Nonpublic site.]
7. Murphy, JP. Hepatitis C virus funding and prioritization status. US Department of Veterans Affairs Central Office Memorandum from the Assistant Deputy Under Secretary for Health for Clinical Operations. http://vaww.hepatitis.va.gov/education/choice-memo-hcv-funding-and-prioritization-status-01272016.asp. Published January 27, 2016. [Nonpublic site.]
8. Lynch TJ, McCarthy MF. Hepatitis C virus funding and prioritization status update. US Department of Veterans Affairs Central Office Memorandum from the Assistant Deputy Under Secretary for Health for Clinical Operations and Acting Assistant Deputy Under Secretary for Health for Patient Care Services. http://vaww.hepatitis.va.gov/education/choice-funding-update-feb-2016.asp. Published February 24, 2016. [Nonpublic site.]
9. Morgan TR, Yee H; US Department of Veterans Affairs National Hepatitis C Resource Center Program and the National Viral Hepatitis Program in the Office of Patient Care Services. Chronic hepatitis C virus (HCV) infection: treatment considerations. http://vaww.hepatitis.va.gov/pdf/treatment-considerations-2016-03-28.pdf. Published March 28, 2016. [Nonpublic site.]
10. American Association for the Study of Liver Diseases; Infectious Diseases Society of America. Initial treatment box. http://hcvguidelines.org/full-report/initial-treatment-box-summary-recommendations-patients-who-are-initiating-therapy-hcv. Updated November 6, 2019. Accessed September 27, 2016
11. AASLD/IDSA HCV Guidance Panel. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 2015;62(3): 932-954.
12. Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006; 43(6):1317-1325.
13. Realdi G, Fattovich G, Hadziyannis S, et al. Survival and prognostic factors in 366 patients with compensated cirrhosis type B: a multicenter study. The Investigators of the European Concerted Action on Viral Hepatitis (EUROHEP). J Hepatol. 1994;21(4):656-666.
14. Kanda T, Kato K, Tsubota A, et al. Platelet count and sustained virological response in hepatitis C treatment. World J Hepatol. 2013;5(4):182-188.
15. Manos MM, Shvachko VA, Murphy RC, Arduino JM, Shire NJ. Distribution of hepatitis C virus genotypes in a diverse US integrated health care population. J Med Virol. 2012;84(11):1744-1750.
16. Cheung RC. Epidemiology of hepatitis C virus infection in American veterans. Am J Gastroenterol. 2000;95(3):740-747.
17. Bagalman E. The number of Veterans that use VA health care services: a fact sheet. Congressional Research Service Report R43579. https://fas.org/sgp/crs/misc/R43579.pdf. Published June 3, 2014. Accessed November 25, 2019.
18. US General Accounting Office. Report to the Ranking Minority Member, Subcommittee on Compensation, Pension, Insurance, and Memorial Affairs, Committee on Veterans’ Affairs, US House of Representatives. How distance from VA facilities affects veterans’ use of VA services. https://www.gao.gov/assets/230/221992.pdf. Published December 1995. Accessed November 25, 2019.
19. Bronstein S, Griffin D. A fatal wait: Veterans languish and die on a VA hospital’s secret list. http://www.cnn.com/2014/04/23/health/veterans-dying-health-care-delays/index.html. Published April 23, 2014. Accessed November 25, 2019.
20. Ioannou GN, Beste LA, Chang MF, et al. Effectiveness of sofosbuvir, ledipasvir/sofosbuvir, or paritaprevir/ritonavir/ombitasvir and dasabuvir regimens for treatment of patients with hepatitis C in the Veterans Affairs national health care system. Gastroenterology. 2016;151(3):457-471.
21. Malespin MH, Harris C, Kanar O, et al. Barriers to treatment of chronic hepatitis C with direct acting antivirals in an urban clinic. Ann Hepatol. 2019;18(2):304–309.
22. Tuchschmidt J. Attachment D: Hepatitis C virus (HCV) fact sheet for Veterans Choice Program for both VA and Choice providers. US Department of Veterans Affairs Central Office Memorandum from the Deputy Under Secretary for Health for Policy and Services and the Acting Deputy Undersecretary for Health for Operations and Management. http://vaww.hepatitis.va.gov/educatiochoice-provision-HCV-treatment-additional.asp. [Nonpublic site.]
Safer CAR uses modified NK cells for advanced CLL, NHL
A chimeric antigen receptor (CAR) construct using transduced natural killer cells instead of T cells was associated with a high complete remission rate without the cytokine release syndrome frequently seen with CAR T cell therapy, early clinical trial results show.
The construct, consisting of natural killer (NK) cells derived from umbilical cord blood that have been transduced to target CD19-expressing cells combined with interleukin 15 and equipped with an “off” switch, offers the prospect of an off-the-shelf CAR product, reported Enli Liu, MD, and colleagues at the University of Texas MD Anderson Cancer Center in Houston.
“We found that allogeneic CAR-NK cells can be delivered in adoptive transfer without the serious cytokine release syndrome and neurologic toxic effects that have been associated with CAR T-cell therapy,” they wrote in The New England Journal of Medicine.
The modified NK cells were delivered to 9 of 11 patients with only partial human leukocyte antigen (HLA) matching, and in 2 patients with no matching, yet there were no cases of graft-versus host disease (GvHD), and no patients had symptoms of cytokine release syndrome (CRS), neurotoxicity, or hemophagocytic lymphohistiocytosis.
CAR T cell production “is a cumbersome process that requires coordination and collection of the cells and there’s several weeks of manufacturing, during which time patients often can have their lymphoma worsen, and so at times it’s a little bit of a race against the clock to get those cells manufactured,” Brian Hill, MD, PhD, director of the lymphoid malignancies program at Taussig Cancer Institute at Cleveland Clinic, said in an interview.
Dr. Hill, who was not involved in the study, said that the proof-of-principle study shows promising early results and offers the prospect of an effective and safe off-the-shelf therapeutic option for patients with lymphoid malignancies.
Advanced B-cell cancers
The investigators conducted a phase 1/2 trial in patients with B-cell lymphoid malignancies, including five patients with chronic lymphocytic leukemia (CLL), one patient with Richter’s transformation and one with accelerated CLL, three with transformed follicular lymphoma, two with diffuse large B-cell lymphoma (DLBCL), and one with follicular lymphoma (focally grade 3B).
The patients were all heavily pretreated, with 3 to as many as 11 prior lines of therapy.
The patients received cord blood-derived NK cells that had been transduced with a retroviral vector expressing genes that encode anti-CD19 CAR, interleukin-15, and inducible caspase 9 as a safety switch.
The cells were expanded in the lab and after the patients underwent lymphodepleting chemotherapy, they received the cells in a single infusion at one of three doses, either 1×105, 1×106, or 1×107 CAR-NK cells per kilogram of body weight.
As noted before, there were no cases of CRS, neurotoxicity, or GvHD and no increase over baseline in inflammatory cytokines, including interleukin-6, a key factor in the development and severity of CRS. The maximum tolerated dose was not reached.
Early efficacy
Of the 11 patients, 8 had a clinical response, and 7 had a complete remission, including 4 patients with lymphomas and 3 with CLL.
The patient with CLL with Richter’s transformation had a remission of the Richter’s component, but not of the CLL itself.
“This is particularly remarkable, because these patients are notoriously very difficult to treat, and the efficacy of autologous CAR T cell therapy in CLL and Richter’s patients has been hampered by lack of fitness of the patient’s own T cells when manufacturing the CAR T cell product, so this approach may obviate the need for autologous T cells in these patients,” Dr. Hill said.
The responses were rapid and occurred within 30 days of infusion at all dose levels. In addition, there was evidence of expansion and persistence of the modified NK cells at low levels for at least 1 year, despite the HLA mismatches between the NK cells and the recipients. The investigators speculated that the inclusion of interleukin-15 in the NL construct may at least partially account for the persistence of the cells and their antitumor activity.
Of the eight patients with a response, five had postremission therapy, including two patients with CLL who had minimal residual disease (MRD), one patient with follicular lymphoma and one with transformed follicular lymphoma who underwent hematopoietic stem-cell transplantation while in complete remission without evidence of MRD, and the patient with CLLL with Richter’s transformation with remission of the lymphoma component, who received a course of venetoclax.
The authors acknowledged that it may be difficult to assess the durability of response after CAR NK therapy in this study because of the allowed consolidation therapy for patients in remission.
They noted that although the patients in the current study each had a fresh CAR NK product manufactured for them, “we have shown that it is possible to produce more than 100 doses of CAR-NK cells from a single cord-blood unit. This capability, together with the apparently minimal HLA-matching requirements between the donor of CAR-NK cells and the patient, may pave the way for a truly off-the-shelf product that could increase treatment accessibility for many more patients.”
The National Institutes of Health supported the study. Dr. Liu disclosed a pending patent for methods of production of CAR-NK cells, and a patent held by MD Anderson for methods of treatment with NK cells. Dr. Hill is a member of the Hematology News editorial advisory board.
SOURCE: Liu E et al. N Engl J Med. 2020 Feb 6;382:545-53.
A chimeric antigen receptor (CAR) construct using transduced natural killer cells instead of T cells was associated with a high complete remission rate without the cytokine release syndrome frequently seen with CAR T cell therapy, early clinical trial results show.
The construct, consisting of natural killer (NK) cells derived from umbilical cord blood that have been transduced to target CD19-expressing cells combined with interleukin 15 and equipped with an “off” switch, offers the prospect of an off-the-shelf CAR product, reported Enli Liu, MD, and colleagues at the University of Texas MD Anderson Cancer Center in Houston.
“We found that allogeneic CAR-NK cells can be delivered in adoptive transfer without the serious cytokine release syndrome and neurologic toxic effects that have been associated with CAR T-cell therapy,” they wrote in The New England Journal of Medicine.
The modified NK cells were delivered to 9 of 11 patients with only partial human leukocyte antigen (HLA) matching, and in 2 patients with no matching, yet there were no cases of graft-versus host disease (GvHD), and no patients had symptoms of cytokine release syndrome (CRS), neurotoxicity, or hemophagocytic lymphohistiocytosis.
CAR T cell production “is a cumbersome process that requires coordination and collection of the cells and there’s several weeks of manufacturing, during which time patients often can have their lymphoma worsen, and so at times it’s a little bit of a race against the clock to get those cells manufactured,” Brian Hill, MD, PhD, director of the lymphoid malignancies program at Taussig Cancer Institute at Cleveland Clinic, said in an interview.
Dr. Hill, who was not involved in the study, said that the proof-of-principle study shows promising early results and offers the prospect of an effective and safe off-the-shelf therapeutic option for patients with lymphoid malignancies.
Advanced B-cell cancers
The investigators conducted a phase 1/2 trial in patients with B-cell lymphoid malignancies, including five patients with chronic lymphocytic leukemia (CLL), one patient with Richter’s transformation and one with accelerated CLL, three with transformed follicular lymphoma, two with diffuse large B-cell lymphoma (DLBCL), and one with follicular lymphoma (focally grade 3B).
The patients were all heavily pretreated, with 3 to as many as 11 prior lines of therapy.
The patients received cord blood-derived NK cells that had been transduced with a retroviral vector expressing genes that encode anti-CD19 CAR, interleukin-15, and inducible caspase 9 as a safety switch.
The cells were expanded in the lab and after the patients underwent lymphodepleting chemotherapy, they received the cells in a single infusion at one of three doses, either 1×105, 1×106, or 1×107 CAR-NK cells per kilogram of body weight.
As noted before, there were no cases of CRS, neurotoxicity, or GvHD and no increase over baseline in inflammatory cytokines, including interleukin-6, a key factor in the development and severity of CRS. The maximum tolerated dose was not reached.
Early efficacy
Of the 11 patients, 8 had a clinical response, and 7 had a complete remission, including 4 patients with lymphomas and 3 with CLL.
The patient with CLL with Richter’s transformation had a remission of the Richter’s component, but not of the CLL itself.
“This is particularly remarkable, because these patients are notoriously very difficult to treat, and the efficacy of autologous CAR T cell therapy in CLL and Richter’s patients has been hampered by lack of fitness of the patient’s own T cells when manufacturing the CAR T cell product, so this approach may obviate the need for autologous T cells in these patients,” Dr. Hill said.
The responses were rapid and occurred within 30 days of infusion at all dose levels. In addition, there was evidence of expansion and persistence of the modified NK cells at low levels for at least 1 year, despite the HLA mismatches between the NK cells and the recipients. The investigators speculated that the inclusion of interleukin-15 in the NL construct may at least partially account for the persistence of the cells and their antitumor activity.
Of the eight patients with a response, five had postremission therapy, including two patients with CLL who had minimal residual disease (MRD), one patient with follicular lymphoma and one with transformed follicular lymphoma who underwent hematopoietic stem-cell transplantation while in complete remission without evidence of MRD, and the patient with CLLL with Richter’s transformation with remission of the lymphoma component, who received a course of venetoclax.
The authors acknowledged that it may be difficult to assess the durability of response after CAR NK therapy in this study because of the allowed consolidation therapy for patients in remission.
They noted that although the patients in the current study each had a fresh CAR NK product manufactured for them, “we have shown that it is possible to produce more than 100 doses of CAR-NK cells from a single cord-blood unit. This capability, together with the apparently minimal HLA-matching requirements between the donor of CAR-NK cells and the patient, may pave the way for a truly off-the-shelf product that could increase treatment accessibility for many more patients.”
The National Institutes of Health supported the study. Dr. Liu disclosed a pending patent for methods of production of CAR-NK cells, and a patent held by MD Anderson for methods of treatment with NK cells. Dr. Hill is a member of the Hematology News editorial advisory board.
SOURCE: Liu E et al. N Engl J Med. 2020 Feb 6;382:545-53.
A chimeric antigen receptor (CAR) construct using transduced natural killer cells instead of T cells was associated with a high complete remission rate without the cytokine release syndrome frequently seen with CAR T cell therapy, early clinical trial results show.
The construct, consisting of natural killer (NK) cells derived from umbilical cord blood that have been transduced to target CD19-expressing cells combined with interleukin 15 and equipped with an “off” switch, offers the prospect of an off-the-shelf CAR product, reported Enli Liu, MD, and colleagues at the University of Texas MD Anderson Cancer Center in Houston.
“We found that allogeneic CAR-NK cells can be delivered in adoptive transfer without the serious cytokine release syndrome and neurologic toxic effects that have been associated with CAR T-cell therapy,” they wrote in The New England Journal of Medicine.
The modified NK cells were delivered to 9 of 11 patients with only partial human leukocyte antigen (HLA) matching, and in 2 patients with no matching, yet there were no cases of graft-versus host disease (GvHD), and no patients had symptoms of cytokine release syndrome (CRS), neurotoxicity, or hemophagocytic lymphohistiocytosis.
CAR T cell production “is a cumbersome process that requires coordination and collection of the cells and there’s several weeks of manufacturing, during which time patients often can have their lymphoma worsen, and so at times it’s a little bit of a race against the clock to get those cells manufactured,” Brian Hill, MD, PhD, director of the lymphoid malignancies program at Taussig Cancer Institute at Cleveland Clinic, said in an interview.
Dr. Hill, who was not involved in the study, said that the proof-of-principle study shows promising early results and offers the prospect of an effective and safe off-the-shelf therapeutic option for patients with lymphoid malignancies.
Advanced B-cell cancers
The investigators conducted a phase 1/2 trial in patients with B-cell lymphoid malignancies, including five patients with chronic lymphocytic leukemia (CLL), one patient with Richter’s transformation and one with accelerated CLL, three with transformed follicular lymphoma, two with diffuse large B-cell lymphoma (DLBCL), and one with follicular lymphoma (focally grade 3B).
The patients were all heavily pretreated, with 3 to as many as 11 prior lines of therapy.
The patients received cord blood-derived NK cells that had been transduced with a retroviral vector expressing genes that encode anti-CD19 CAR, interleukin-15, and inducible caspase 9 as a safety switch.
The cells were expanded in the lab and after the patients underwent lymphodepleting chemotherapy, they received the cells in a single infusion at one of three doses, either 1×105, 1×106, or 1×107 CAR-NK cells per kilogram of body weight.
As noted before, there were no cases of CRS, neurotoxicity, or GvHD and no increase over baseline in inflammatory cytokines, including interleukin-6, a key factor in the development and severity of CRS. The maximum tolerated dose was not reached.
Early efficacy
Of the 11 patients, 8 had a clinical response, and 7 had a complete remission, including 4 patients with lymphomas and 3 with CLL.
The patient with CLL with Richter’s transformation had a remission of the Richter’s component, but not of the CLL itself.
“This is particularly remarkable, because these patients are notoriously very difficult to treat, and the efficacy of autologous CAR T cell therapy in CLL and Richter’s patients has been hampered by lack of fitness of the patient’s own T cells when manufacturing the CAR T cell product, so this approach may obviate the need for autologous T cells in these patients,” Dr. Hill said.
The responses were rapid and occurred within 30 days of infusion at all dose levels. In addition, there was evidence of expansion and persistence of the modified NK cells at low levels for at least 1 year, despite the HLA mismatches between the NK cells and the recipients. The investigators speculated that the inclusion of interleukin-15 in the NL construct may at least partially account for the persistence of the cells and their antitumor activity.
Of the eight patients with a response, five had postremission therapy, including two patients with CLL who had minimal residual disease (MRD), one patient with follicular lymphoma and one with transformed follicular lymphoma who underwent hematopoietic stem-cell transplantation while in complete remission without evidence of MRD, and the patient with CLLL with Richter’s transformation with remission of the lymphoma component, who received a course of venetoclax.
The authors acknowledged that it may be difficult to assess the durability of response after CAR NK therapy in this study because of the allowed consolidation therapy for patients in remission.
They noted that although the patients in the current study each had a fresh CAR NK product manufactured for them, “we have shown that it is possible to produce more than 100 doses of CAR-NK cells from a single cord-blood unit. This capability, together with the apparently minimal HLA-matching requirements between the donor of CAR-NK cells and the patient, may pave the way for a truly off-the-shelf product that could increase treatment accessibility for many more patients.”
The National Institutes of Health supported the study. Dr. Liu disclosed a pending patent for methods of production of CAR-NK cells, and a patent held by MD Anderson for methods of treatment with NK cells. Dr. Hill is a member of the Hematology News editorial advisory board.
SOURCE: Liu E et al. N Engl J Med. 2020 Feb 6;382:545-53.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Vitiligo tied to lower risk of internal malignancies
LAHAINA, HAWAII – from South Korea, Iltefat Hamzavi, MD, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
Previous studies by Dr. Hamzavi and others have established that vitiligo patients have a reduced risk of melanoma and perhaps nonmelanoma skin cancers as well. But the South Korean national study of 101,078 vitiligo patients matched by age and sex to twice as many vitiligo-free controls was the first large examination of the association between vitiligo and internal malignancies. The findings suggest that immunosurveillance in patients with the disease is not merely a skin-deep phenomenon, noted Dr. Hamzavi, of the MultiCultural Dermatology Center at Henry Ford Hospital in Detroit.
“Vitiligo is probably a systemic disease in which there may be increased immunosurveillance. The point here is that as we suppress the disease, we have to be careful that we’re not going to increase cancer rates,” the dermatologist explained in an interview. “This is big data, and something to be aware of, but don’t act on it yet in clinical practice. I just want people to be aware that all of these autoimmune diseases are there for a reason. There are lower rates of melanoma and internal cancers in patients who have vitiligo, but what that means for our new therapies that are coming up we don’t know yet.”
He predicted that the study will open up an active new research domain, but it will take time to find definitive answers as to whether emerging immunomodulatory therapies for patients with vitiligo might, in some instances, increase their current favorably lower risk of internal malignancies. In the meantime, physicians interested in treating vitiligo off label with, for example, Janus kinase (JAK) inhibitors will want to be particularly cautious in patients with a strong history of skin cancer or internal malignancies.
The retrospective, population-based study utilized data from the Korean National Health Insurance claims database. The investigators found that the incidence rate of internal malignancies was 612.9 per 100,000 person-years in the vitiligo group and 708.9 per 100,000 person-years in controls, for a statistically significant and clinically meaningful 14% relative risk reduction after adjustment for age, sex, and comorbid conditions.
Among the most striking organ-specific findings: the vitiligo group had a 38% relative risk reduction in colorectal cancer, a 25% reduction in the risk of lung cancer, and a 38% decrease in ovarian cancer. In contrast, they had a 20% increase in the risk of thyroid cancer (J Clin Oncol. 2019 Apr 10;37[11]:903-11).
Despite the fact that vitiligo is a common disease that affects 0.5%-1% of the population worldwide, for decades it has been something of a pharmacotherapeutic backwater. That’s changed recently and in dramatic fashion as a result of new understanding of the disease pathogenesis. The JAK inhibitors are now under active investigation for the treatment of vitiligo. Indeed, ruxolitinib cream, a potent JAK-1 and -2 inhibitor, is now in phase 3 investigation following a highly successful phase 2 trial. Interleukin-15 blockade is another promising avenue.
Dr. Hamzavi reported serving as a consultant to AbbVie, Aclaris, Novartis, and Pfizer, and receiving research funding from Estee Lauder, Clinuvel Pharmaceuticals, Incyte, and Pfizer. SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
LAHAINA, HAWAII – from South Korea, Iltefat Hamzavi, MD, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
Previous studies by Dr. Hamzavi and others have established that vitiligo patients have a reduced risk of melanoma and perhaps nonmelanoma skin cancers as well. But the South Korean national study of 101,078 vitiligo patients matched by age and sex to twice as many vitiligo-free controls was the first large examination of the association between vitiligo and internal malignancies. The findings suggest that immunosurveillance in patients with the disease is not merely a skin-deep phenomenon, noted Dr. Hamzavi, of the MultiCultural Dermatology Center at Henry Ford Hospital in Detroit.
“Vitiligo is probably a systemic disease in which there may be increased immunosurveillance. The point here is that as we suppress the disease, we have to be careful that we’re not going to increase cancer rates,” the dermatologist explained in an interview. “This is big data, and something to be aware of, but don’t act on it yet in clinical practice. I just want people to be aware that all of these autoimmune diseases are there for a reason. There are lower rates of melanoma and internal cancers in patients who have vitiligo, but what that means for our new therapies that are coming up we don’t know yet.”
He predicted that the study will open up an active new research domain, but it will take time to find definitive answers as to whether emerging immunomodulatory therapies for patients with vitiligo might, in some instances, increase their current favorably lower risk of internal malignancies. In the meantime, physicians interested in treating vitiligo off label with, for example, Janus kinase (JAK) inhibitors will want to be particularly cautious in patients with a strong history of skin cancer or internal malignancies.
The retrospective, population-based study utilized data from the Korean National Health Insurance claims database. The investigators found that the incidence rate of internal malignancies was 612.9 per 100,000 person-years in the vitiligo group and 708.9 per 100,000 person-years in controls, for a statistically significant and clinically meaningful 14% relative risk reduction after adjustment for age, sex, and comorbid conditions.
Among the most striking organ-specific findings: the vitiligo group had a 38% relative risk reduction in colorectal cancer, a 25% reduction in the risk of lung cancer, and a 38% decrease in ovarian cancer. In contrast, they had a 20% increase in the risk of thyroid cancer (J Clin Oncol. 2019 Apr 10;37[11]:903-11).
Despite the fact that vitiligo is a common disease that affects 0.5%-1% of the population worldwide, for decades it has been something of a pharmacotherapeutic backwater. That’s changed recently and in dramatic fashion as a result of new understanding of the disease pathogenesis. The JAK inhibitors are now under active investigation for the treatment of vitiligo. Indeed, ruxolitinib cream, a potent JAK-1 and -2 inhibitor, is now in phase 3 investigation following a highly successful phase 2 trial. Interleukin-15 blockade is another promising avenue.
Dr. Hamzavi reported serving as a consultant to AbbVie, Aclaris, Novartis, and Pfizer, and receiving research funding from Estee Lauder, Clinuvel Pharmaceuticals, Incyte, and Pfizer. SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
LAHAINA, HAWAII – from South Korea, Iltefat Hamzavi, MD, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
Previous studies by Dr. Hamzavi and others have established that vitiligo patients have a reduced risk of melanoma and perhaps nonmelanoma skin cancers as well. But the South Korean national study of 101,078 vitiligo patients matched by age and sex to twice as many vitiligo-free controls was the first large examination of the association between vitiligo and internal malignancies. The findings suggest that immunosurveillance in patients with the disease is not merely a skin-deep phenomenon, noted Dr. Hamzavi, of the MultiCultural Dermatology Center at Henry Ford Hospital in Detroit.
“Vitiligo is probably a systemic disease in which there may be increased immunosurveillance. The point here is that as we suppress the disease, we have to be careful that we’re not going to increase cancer rates,” the dermatologist explained in an interview. “This is big data, and something to be aware of, but don’t act on it yet in clinical practice. I just want people to be aware that all of these autoimmune diseases are there for a reason. There are lower rates of melanoma and internal cancers in patients who have vitiligo, but what that means for our new therapies that are coming up we don’t know yet.”
He predicted that the study will open up an active new research domain, but it will take time to find definitive answers as to whether emerging immunomodulatory therapies for patients with vitiligo might, in some instances, increase their current favorably lower risk of internal malignancies. In the meantime, physicians interested in treating vitiligo off label with, for example, Janus kinase (JAK) inhibitors will want to be particularly cautious in patients with a strong history of skin cancer or internal malignancies.
The retrospective, population-based study utilized data from the Korean National Health Insurance claims database. The investigators found that the incidence rate of internal malignancies was 612.9 per 100,000 person-years in the vitiligo group and 708.9 per 100,000 person-years in controls, for a statistically significant and clinically meaningful 14% relative risk reduction after adjustment for age, sex, and comorbid conditions.
Among the most striking organ-specific findings: the vitiligo group had a 38% relative risk reduction in colorectal cancer, a 25% reduction in the risk of lung cancer, and a 38% decrease in ovarian cancer. In contrast, they had a 20% increase in the risk of thyroid cancer (J Clin Oncol. 2019 Apr 10;37[11]:903-11).
Despite the fact that vitiligo is a common disease that affects 0.5%-1% of the population worldwide, for decades it has been something of a pharmacotherapeutic backwater. That’s changed recently and in dramatic fashion as a result of new understanding of the disease pathogenesis. The JAK inhibitors are now under active investigation for the treatment of vitiligo. Indeed, ruxolitinib cream, a potent JAK-1 and -2 inhibitor, is now in phase 3 investigation following a highly successful phase 2 trial. Interleukin-15 blockade is another promising avenue.
Dr. Hamzavi reported serving as a consultant to AbbVie, Aclaris, Novartis, and Pfizer, and receiving research funding from Estee Lauder, Clinuvel Pharmaceuticals, Incyte, and Pfizer. SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
REPORTING FROM SDEF HAWAII DERMATOLOGY SEMINAR
Anti–TNF-alpha nonresponse in axSpA predicted by socioeconomic, patient-reported factors
A new study has determined modifiable and nonmodifiable factors that can help identify patients with axial spondyloarthritis who are both likely and unlikely to respond to anti–tumor necrosis factor (TNF)–alpha therapy.
“[This study] emphasizes that examination of predictors of nonresponse to pharmacologic therapy in inflammatory arthritis must consider the importance of socioeconomic factors,” wrote Gary J. Macfarlane, MBChB, PhD, of the University of Aberdeen (Scotland) and coauthors. The study was published in Rheumatology.
To identify common factors related to anti–TNF-alpha therapy response, the researchers launched a prospective cohort study of 335 patients with axial spondyloarthritis from the British Society for Rheumatology Biologics Register in Axial Spondyloarthritis (BSRBR-AS) who were naive to biologic therapy. Responses to therapy were assessed through various means, including meeting Assessment of Spondyloarthritis International Society (ASAS) improvement criteria, exhibiting clinically important improvement (1.1 points or more) in Ankylosing Spondylitis Disease Activity Score (ASDAS), or going from a high or very high ASDAS disease state (score of 2.1 or higher) to a moderate or inactive state (score less than 2.1).
All patients also filled out questionnaires at each follow-up on socioeconomic factors, lifestyle factors, and quality of life. Of the 335 participants, 69% were male. They had a median age of 47 years, and about half were employed full time.
At first follow-up – which occurred at a median of 14 weeks – 175 participants (52%) met ASAS20 response criteria and 111 (33%) met ASAS40 response criteria. Of the 261 participants eligible for ASDAS evaluation, 122 (47%) met the criteria for a clinically important ASDAS reduction. Of the 249 participants who had a high or very high disease state at baseline, 87 (35%) were classified as having moderate or inactive disease at follow-up.
Factors that predicted a lack of response across measures included adverse socioeconomic factors, fewer years of education, and not working full time. Clinical and patient-reported factors also associated with a lack of response included comorbidities and poor mental health. The ASDAS models proved best at predicting those unlikely to meet response criteria, with a negative predictive value of 77%.
The study was supported by the British Society for Rheumatology, which receives funding for the BSRBR-AS from Pfizer, AbbVie, and UCB. The authors reported having no conflicts of interest.
SOURCE: Macfarlane GJ et al. Rheumatology. 2020 Jan 28. doi: 10.1093/rheumatology/kez657.
A new study has determined modifiable and nonmodifiable factors that can help identify patients with axial spondyloarthritis who are both likely and unlikely to respond to anti–tumor necrosis factor (TNF)–alpha therapy.
“[This study] emphasizes that examination of predictors of nonresponse to pharmacologic therapy in inflammatory arthritis must consider the importance of socioeconomic factors,” wrote Gary J. Macfarlane, MBChB, PhD, of the University of Aberdeen (Scotland) and coauthors. The study was published in Rheumatology.
To identify common factors related to anti–TNF-alpha therapy response, the researchers launched a prospective cohort study of 335 patients with axial spondyloarthritis from the British Society for Rheumatology Biologics Register in Axial Spondyloarthritis (BSRBR-AS) who were naive to biologic therapy. Responses to therapy were assessed through various means, including meeting Assessment of Spondyloarthritis International Society (ASAS) improvement criteria, exhibiting clinically important improvement (1.1 points or more) in Ankylosing Spondylitis Disease Activity Score (ASDAS), or going from a high or very high ASDAS disease state (score of 2.1 or higher) to a moderate or inactive state (score less than 2.1).
All patients also filled out questionnaires at each follow-up on socioeconomic factors, lifestyle factors, and quality of life. Of the 335 participants, 69% were male. They had a median age of 47 years, and about half were employed full time.
At first follow-up – which occurred at a median of 14 weeks – 175 participants (52%) met ASAS20 response criteria and 111 (33%) met ASAS40 response criteria. Of the 261 participants eligible for ASDAS evaluation, 122 (47%) met the criteria for a clinically important ASDAS reduction. Of the 249 participants who had a high or very high disease state at baseline, 87 (35%) were classified as having moderate or inactive disease at follow-up.
Factors that predicted a lack of response across measures included adverse socioeconomic factors, fewer years of education, and not working full time. Clinical and patient-reported factors also associated with a lack of response included comorbidities and poor mental health. The ASDAS models proved best at predicting those unlikely to meet response criteria, with a negative predictive value of 77%.
The study was supported by the British Society for Rheumatology, which receives funding for the BSRBR-AS from Pfizer, AbbVie, and UCB. The authors reported having no conflicts of interest.
SOURCE: Macfarlane GJ et al. Rheumatology. 2020 Jan 28. doi: 10.1093/rheumatology/kez657.
A new study has determined modifiable and nonmodifiable factors that can help identify patients with axial spondyloarthritis who are both likely and unlikely to respond to anti–tumor necrosis factor (TNF)–alpha therapy.
“[This study] emphasizes that examination of predictors of nonresponse to pharmacologic therapy in inflammatory arthritis must consider the importance of socioeconomic factors,” wrote Gary J. Macfarlane, MBChB, PhD, of the University of Aberdeen (Scotland) and coauthors. The study was published in Rheumatology.
To identify common factors related to anti–TNF-alpha therapy response, the researchers launched a prospective cohort study of 335 patients with axial spondyloarthritis from the British Society for Rheumatology Biologics Register in Axial Spondyloarthritis (BSRBR-AS) who were naive to biologic therapy. Responses to therapy were assessed through various means, including meeting Assessment of Spondyloarthritis International Society (ASAS) improvement criteria, exhibiting clinically important improvement (1.1 points or more) in Ankylosing Spondylitis Disease Activity Score (ASDAS), or going from a high or very high ASDAS disease state (score of 2.1 or higher) to a moderate or inactive state (score less than 2.1).
All patients also filled out questionnaires at each follow-up on socioeconomic factors, lifestyle factors, and quality of life. Of the 335 participants, 69% were male. They had a median age of 47 years, and about half were employed full time.
At first follow-up – which occurred at a median of 14 weeks – 175 participants (52%) met ASAS20 response criteria and 111 (33%) met ASAS40 response criteria. Of the 261 participants eligible for ASDAS evaluation, 122 (47%) met the criteria for a clinically important ASDAS reduction. Of the 249 participants who had a high or very high disease state at baseline, 87 (35%) were classified as having moderate or inactive disease at follow-up.
Factors that predicted a lack of response across measures included adverse socioeconomic factors, fewer years of education, and not working full time. Clinical and patient-reported factors also associated with a lack of response included comorbidities and poor mental health. The ASDAS models proved best at predicting those unlikely to meet response criteria, with a negative predictive value of 77%.
The study was supported by the British Society for Rheumatology, which receives funding for the BSRBR-AS from Pfizer, AbbVie, and UCB. The authors reported having no conflicts of interest.
SOURCE: Macfarlane GJ et al. Rheumatology. 2020 Jan 28. doi: 10.1093/rheumatology/kez657.
FROM RHEUMATOLOGY
Stimulation to titration: An update on hypoglossal nerve stimulation for OSA
Clinical significance
Continuous positive airway pressure remains the gold standard and first-line treatment for moderate to severe OSA. When CPAP and other medical therapies fail or are poorly adopted, surgical solutions - either standalone or in unison - can be directed to target precision therapy.
The newest of these techniques is neuromodulation of the lingual musculature, particularly by way of selective stimulation of the hypoglossal nerve, which first demonstrated success in human clinical trials in 1996.1 Upper airway stimulation (UAS) was formally FDA-approved in 2014 (Inspire Medical Systems, Inc). UAS is designed to eliminate clinically significant OSA through stimulation of the anteriorly directed branches of the hypoglossal nerve, increasing the posterior airway space in a multilevel fashion.2 Since this time, over 7,500 patients have been treated with Inspire in nine countries (United States, Germany, The Netherlands, Switzerland, Belgium, Spain, France, Italy, and Finland). Prospective, international multicenter trials have demonstrated 68% to 96% clinical efficacy in well selected individuals. This is defined as a ≥ 50% reduction in the apnea hypopnea index (AHI) to an overall AHI of ≤ 20/hour.3,4 Additionally, post-UAS analysis demonstrates subjective reduction in daytime sleepiness as reported by Epworth sleepiness scores, with improvements in sleep-related quality of life. Further, UAS reduces socially disruptive snoring with 85% of bedpartners reporting soft to no snoring at 48-month follow-up.5 The procedure has also demonstrated long-term cost benefit in the US health-care system.6
Background and pathophysiology
Oliven and colleagues7 first observed the critical finding that selective intra-muscular stimulation of the genioglossus muscle lowered airway critical closing pressure (PCrit), thereby stabilizing the pharyngeal airway. Conversely, activation of the “retrusor” musculature, namely the hyoglossus and styloglossus muscles, increased Pcrit, increasing collapsibility of the pharyngeal airway.
Therapeutic implantation requires three incisions directed to the neck, chest, and right rib space (between the 4th to 6th intercostal spaces), with an operative time of 90 minutes or less in experienced hands. The majority of patients are discharged on the day of the procedure. Morbidity remains low with minimal pain reported during recovery. The most common complication is that of temporary tongue weakness, which typically resolves within 2 to 3 weeks. While very infrequent, patients should be counseled on the risk of postoperative hematoma, which can precipitate infection and subsequent explant of the device. Average recovery time spans between 3 and 7 days with activation of the device 4 weeks after surgical implantation to allow for appropriate tissue healing and reduce the risk of dislodgement of the implanted components. In contrast to other surgical treatment options, UAS is also reversible with no underlying alteration to existing pharyngeal anatomy apart from external incisions created during the procedure.
Stimulation to titration
As the need for a multidisciplinary approach to salvage of patients failing first-line therapy for OSA continues to grow, UAS with its multilevel impact continues to be of key interest. In similar fashion to established medical therapies such as PAP and oral appliance therapy (OAT), close observation between sleep medicine specialists and the implanting surgeon during the adaptation period with attention paid to titration parameters such as stimulation duration, pulse width, amplitude, and polarity, allow optimization of response outcome.
The stimulation electrode, which is designed in the form of a cuff to envelope the anterior (protrusor) branches of the hypoglossal nerve receives electrical stimulation from the implanted pulse generator, implanted above the pectoralis muscle of the chest wall. This design allows for collaborative awake and overnight titration of the device as directed by a sleep medicine physician. Attention is paid not only to the voltage “strength” administered with each pulse but also the degree of synchronization between respiration and stimulation, as well as pattern of pulse administration. Our experience remains that true success and adaptation to therapy requires not just meticulous surgical technique but a diligent approach to postoperative therapeutic titration to achieve a comfortable, yet effective, voltage for maintaining airway patency. Thus, akin to initiation of CPAP, UAS requires regular follow-up and device fine-tuning with patient comfort taken into consideration to achieve optimal results, and patient expectation should be aligned with this process.
Current indications
Success in UAS relies heavily on appropriate presurgical evaluation and clinical phenotyping. The following surgical indications have been demonstrated in the Stimulation Therapy for Apnea Reduction (STAR) trial and subsequent 3-year clinical follow-up: AHI between 15 and 80 events/hour (with ≤ 25% central apneas) and a BMI ≤ 32.8
As OSA often results from multi-level airway collapse, UAS targets an increase not only in the diameter of the retropalatal/oropharyngeal airway space but also the antero-posterior hypopharyngeal airway. Original criteria for implantation excluded patients with a pattern of complete circumferential collapse (CCC) noted on dynamic airway evaluation during pre-implant drug-induced sleep endoscopy (DISE). DISE aims to precisely target dynamic airway collapse patterns during simulated (propofol or midazolom induced) sleep.
Future directions
The effects of UAS are dependent on upper-airway cross-sectional area, particularly diameter. In patients who demonstrate CCC, the anteroposterior direction of activation derived from the UAS stimulus is unable to overcome CCC. In a recent prospective study, our group demonstrated that CCC can be converted to an airway collapse pattern compatible with UAS implantation, using a modified palatopharyngoplasty prior to UAS implantation. By stabilizing the lateral walls of the oropharyngeal airway with pre-implant palatal surgery, UAS is able to successfully direct widening of the airway cross-sectional area in an antero-posterior fashion. This exciting finding potentially allows for expansion of current indications, thus opening treatment to a wider patient population.9 Still, UAS remains highly studied in a relatively uniform patient population with data in more diverse subsets requiring further directed attention to expand and better define optimal patient populations for treatment.
From the perspective of improving patient adaptation and tolerance in UAS, a well-established concept in the CPAP literature can be applied, as explained by the Starling resistor model. The starling resistor is comprised of two rigid tubes connected by a collapsible segment in between. In parallel, the pharynx is a collapsible muscular tube connected on either end by the nose/nasal cavity and the trachea – both of which are bony/cartilaginous, noncollapsible structures. As has been shown in the use of CPAP, the same pressure required to maintain stability of the collapsible muscular pharynx via nasal breathing may lead to pharyngeal collapse when applied orally.10 This concept has also been directed towards UAS with our clinical experience demonstrating that oro or oronasal breathers tend to require a higher amplitude to maintain airway patency versus nasal breathers. This is an important area for future-directed study as medically/surgically improving nasal breathing in UAS subjects may subsequently lower amplitude requirements and improve patient tolerance.
Future direction to allow for improvement in the technology for application in a broader populational segment, external or alternative device powering mechanisms, along with MRI Compatibility and reducing the number of required external incisions will continue to broaden the patient selection criteria. As we move from a “stimulation” to a precision-tailored “stimulation and titration” approach, the mid to long term data supporting UAS remains very promising with 5-year follow up demonstrating sustained polysomnographic and subjective reported outcomes in well selected patients.
Dr. Awad is Assistant Professor – Department of Otolaryngology/Head & Neck Surgery, and Chief – Division of Sleep Surgery; Northwestern University, Chicago, Illinois. Dr. Capasso is Associate Professor – Department of Otolaryngology/Head & Neck Surgery, and Chief – Division of Sleep Surgery; Stanford Hospital and Clinics, Stanford, California.
References
1. Schwartz AR et al. Electrical stimulation of the lingual musculature in obstructive sleep apnea. J Appl Physiol. 1996;81(2):643-52. doi: 10.1152/jappl.1996.81.2.643.
2. Ong AA et al. Efficacy of upper airway stimulation on collapse patterns observed during drug-induced sedation endoscopy. Otolaryngol Head Neck Surg. 2016;154(5):970-7. doi: 10.1177/0194599816636835.
3. Woodson BT et al. Three-year outcomes of cranial nerve stimulation for obstructive sleep apnea: The STAR trial. Otolaryngol Head Neck Surg. 2016;154(1):181-8. doi: 10.1177/0194599815616618.
4. Heiser C et al. Outcomes of upper airway stimulation for obstructive sleep apnea in a multicenter german postmarket study. Otolaryngol Head Neck Surg. 2017;156(2):378-84. doi: 10.1177/0194599816683378.
5. Gillespie MB et al. Upper airway stimulation for obstructive sleep apnea: Patient-reported outcomes after 48 months of follow-up. Otolaryngol Head Neck Surg. 2017;156(4):765-71. doi: 10.1177/0194599817691491.
6. Pietzsch JB et al. Long-term cost-effectiveness of upper airway stimulation for the treatment of obstructive sleep apnea: A model-based projection based on the star trial. Sleep. 2015;38(5):735-44. doi: 10.5665/sleep.4666.
7. Oliven A et al. Improved upper airway patency elicited by electrical stimulation of the hypoglossus nerves. Respiration. 1996;63(4):213-16. doi: 10.1159/000196547.
8. Strollo PJ et al. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med. 2014;370(2):139-49. doi: 10.1056/NEJMoa1308659.
9. Liu YC et al. Palatopharyngoplasty resolves concentric collapse in patients ineligible for upper airway stimulation. Laryngoscope. Forthcoming.
10. De Andrade RGS et al. Impact of the type of mask on the effectiveness of and adherence to continuous positive airway pressure treatment for obstructive sleep apnea. J Bras Pneumol. 2014;40(6):658-68. doi: 10.1590/S1806-37132014000600010
Clinical significance
Continuous positive airway pressure remains the gold standard and first-line treatment for moderate to severe OSA. When CPAP and other medical therapies fail or are poorly adopted, surgical solutions - either standalone or in unison - can be directed to target precision therapy.
The newest of these techniques is neuromodulation of the lingual musculature, particularly by way of selective stimulation of the hypoglossal nerve, which first demonstrated success in human clinical trials in 1996.1 Upper airway stimulation (UAS) was formally FDA-approved in 2014 (Inspire Medical Systems, Inc). UAS is designed to eliminate clinically significant OSA through stimulation of the anteriorly directed branches of the hypoglossal nerve, increasing the posterior airway space in a multilevel fashion.2 Since this time, over 7,500 patients have been treated with Inspire in nine countries (United States, Germany, The Netherlands, Switzerland, Belgium, Spain, France, Italy, and Finland). Prospective, international multicenter trials have demonstrated 68% to 96% clinical efficacy in well selected individuals. This is defined as a ≥ 50% reduction in the apnea hypopnea index (AHI) to an overall AHI of ≤ 20/hour.3,4 Additionally, post-UAS analysis demonstrates subjective reduction in daytime sleepiness as reported by Epworth sleepiness scores, with improvements in sleep-related quality of life. Further, UAS reduces socially disruptive snoring with 85% of bedpartners reporting soft to no snoring at 48-month follow-up.5 The procedure has also demonstrated long-term cost benefit in the US health-care system.6
Background and pathophysiology
Oliven and colleagues7 first observed the critical finding that selective intra-muscular stimulation of the genioglossus muscle lowered airway critical closing pressure (PCrit), thereby stabilizing the pharyngeal airway. Conversely, activation of the “retrusor” musculature, namely the hyoglossus and styloglossus muscles, increased Pcrit, increasing collapsibility of the pharyngeal airway.
Therapeutic implantation requires three incisions directed to the neck, chest, and right rib space (between the 4th to 6th intercostal spaces), with an operative time of 90 minutes or less in experienced hands. The majority of patients are discharged on the day of the procedure. Morbidity remains low with minimal pain reported during recovery. The most common complication is that of temporary tongue weakness, which typically resolves within 2 to 3 weeks. While very infrequent, patients should be counseled on the risk of postoperative hematoma, which can precipitate infection and subsequent explant of the device. Average recovery time spans between 3 and 7 days with activation of the device 4 weeks after surgical implantation to allow for appropriate tissue healing and reduce the risk of dislodgement of the implanted components. In contrast to other surgical treatment options, UAS is also reversible with no underlying alteration to existing pharyngeal anatomy apart from external incisions created during the procedure.
Stimulation to titration
As the need for a multidisciplinary approach to salvage of patients failing first-line therapy for OSA continues to grow, UAS with its multilevel impact continues to be of key interest. In similar fashion to established medical therapies such as PAP and oral appliance therapy (OAT), close observation between sleep medicine specialists and the implanting surgeon during the adaptation period with attention paid to titration parameters such as stimulation duration, pulse width, amplitude, and polarity, allow optimization of response outcome.
The stimulation electrode, which is designed in the form of a cuff to envelope the anterior (protrusor) branches of the hypoglossal nerve receives electrical stimulation from the implanted pulse generator, implanted above the pectoralis muscle of the chest wall. This design allows for collaborative awake and overnight titration of the device as directed by a sleep medicine physician. Attention is paid not only to the voltage “strength” administered with each pulse but also the degree of synchronization between respiration and stimulation, as well as pattern of pulse administration. Our experience remains that true success and adaptation to therapy requires not just meticulous surgical technique but a diligent approach to postoperative therapeutic titration to achieve a comfortable, yet effective, voltage for maintaining airway patency. Thus, akin to initiation of CPAP, UAS requires regular follow-up and device fine-tuning with patient comfort taken into consideration to achieve optimal results, and patient expectation should be aligned with this process.
Current indications
Success in UAS relies heavily on appropriate presurgical evaluation and clinical phenotyping. The following surgical indications have been demonstrated in the Stimulation Therapy for Apnea Reduction (STAR) trial and subsequent 3-year clinical follow-up: AHI between 15 and 80 events/hour (with ≤ 25% central apneas) and a BMI ≤ 32.8
As OSA often results from multi-level airway collapse, UAS targets an increase not only in the diameter of the retropalatal/oropharyngeal airway space but also the antero-posterior hypopharyngeal airway. Original criteria for implantation excluded patients with a pattern of complete circumferential collapse (CCC) noted on dynamic airway evaluation during pre-implant drug-induced sleep endoscopy (DISE). DISE aims to precisely target dynamic airway collapse patterns during simulated (propofol or midazolom induced) sleep.
Future directions
The effects of UAS are dependent on upper-airway cross-sectional area, particularly diameter. In patients who demonstrate CCC, the anteroposterior direction of activation derived from the UAS stimulus is unable to overcome CCC. In a recent prospective study, our group demonstrated that CCC can be converted to an airway collapse pattern compatible with UAS implantation, using a modified palatopharyngoplasty prior to UAS implantation. By stabilizing the lateral walls of the oropharyngeal airway with pre-implant palatal surgery, UAS is able to successfully direct widening of the airway cross-sectional area in an antero-posterior fashion. This exciting finding potentially allows for expansion of current indications, thus opening treatment to a wider patient population.9 Still, UAS remains highly studied in a relatively uniform patient population with data in more diverse subsets requiring further directed attention to expand and better define optimal patient populations for treatment.
From the perspective of improving patient adaptation and tolerance in UAS, a well-established concept in the CPAP literature can be applied, as explained by the Starling resistor model. The starling resistor is comprised of two rigid tubes connected by a collapsible segment in between. In parallel, the pharynx is a collapsible muscular tube connected on either end by the nose/nasal cavity and the trachea – both of which are bony/cartilaginous, noncollapsible structures. As has been shown in the use of CPAP, the same pressure required to maintain stability of the collapsible muscular pharynx via nasal breathing may lead to pharyngeal collapse when applied orally.10 This concept has also been directed towards UAS with our clinical experience demonstrating that oro or oronasal breathers tend to require a higher amplitude to maintain airway patency versus nasal breathers. This is an important area for future-directed study as medically/surgically improving nasal breathing in UAS subjects may subsequently lower amplitude requirements and improve patient tolerance.
Future direction to allow for improvement in the technology for application in a broader populational segment, external or alternative device powering mechanisms, along with MRI Compatibility and reducing the number of required external incisions will continue to broaden the patient selection criteria. As we move from a “stimulation” to a precision-tailored “stimulation and titration” approach, the mid to long term data supporting UAS remains very promising with 5-year follow up demonstrating sustained polysomnographic and subjective reported outcomes in well selected patients.
Dr. Awad is Assistant Professor – Department of Otolaryngology/Head & Neck Surgery, and Chief – Division of Sleep Surgery; Northwestern University, Chicago, Illinois. Dr. Capasso is Associate Professor – Department of Otolaryngology/Head & Neck Surgery, and Chief – Division of Sleep Surgery; Stanford Hospital and Clinics, Stanford, California.
References
1. Schwartz AR et al. Electrical stimulation of the lingual musculature in obstructive sleep apnea. J Appl Physiol. 1996;81(2):643-52. doi: 10.1152/jappl.1996.81.2.643.
2. Ong AA et al. Efficacy of upper airway stimulation on collapse patterns observed during drug-induced sedation endoscopy. Otolaryngol Head Neck Surg. 2016;154(5):970-7. doi: 10.1177/0194599816636835.
3. Woodson BT et al. Three-year outcomes of cranial nerve stimulation for obstructive sleep apnea: The STAR trial. Otolaryngol Head Neck Surg. 2016;154(1):181-8. doi: 10.1177/0194599815616618.
4. Heiser C et al. Outcomes of upper airway stimulation for obstructive sleep apnea in a multicenter german postmarket study. Otolaryngol Head Neck Surg. 2017;156(2):378-84. doi: 10.1177/0194599816683378.
5. Gillespie MB et al. Upper airway stimulation for obstructive sleep apnea: Patient-reported outcomes after 48 months of follow-up. Otolaryngol Head Neck Surg. 2017;156(4):765-71. doi: 10.1177/0194599817691491.
6. Pietzsch JB et al. Long-term cost-effectiveness of upper airway stimulation for the treatment of obstructive sleep apnea: A model-based projection based on the star trial. Sleep. 2015;38(5):735-44. doi: 10.5665/sleep.4666.
7. Oliven A et al. Improved upper airway patency elicited by electrical stimulation of the hypoglossus nerves. Respiration. 1996;63(4):213-16. doi: 10.1159/000196547.
8. Strollo PJ et al. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med. 2014;370(2):139-49. doi: 10.1056/NEJMoa1308659.
9. Liu YC et al. Palatopharyngoplasty resolves concentric collapse in patients ineligible for upper airway stimulation. Laryngoscope. Forthcoming.
10. De Andrade RGS et al. Impact of the type of mask on the effectiveness of and adherence to continuous positive airway pressure treatment for obstructive sleep apnea. J Bras Pneumol. 2014;40(6):658-68. doi: 10.1590/S1806-37132014000600010
Clinical significance
Continuous positive airway pressure remains the gold standard and first-line treatment for moderate to severe OSA. When CPAP and other medical therapies fail or are poorly adopted, surgical solutions - either standalone or in unison - can be directed to target precision therapy.
The newest of these techniques is neuromodulation of the lingual musculature, particularly by way of selective stimulation of the hypoglossal nerve, which first demonstrated success in human clinical trials in 1996.1 Upper airway stimulation (UAS) was formally FDA-approved in 2014 (Inspire Medical Systems, Inc). UAS is designed to eliminate clinically significant OSA through stimulation of the anteriorly directed branches of the hypoglossal nerve, increasing the posterior airway space in a multilevel fashion.2 Since this time, over 7,500 patients have been treated with Inspire in nine countries (United States, Germany, The Netherlands, Switzerland, Belgium, Spain, France, Italy, and Finland). Prospective, international multicenter trials have demonstrated 68% to 96% clinical efficacy in well selected individuals. This is defined as a ≥ 50% reduction in the apnea hypopnea index (AHI) to an overall AHI of ≤ 20/hour.3,4 Additionally, post-UAS analysis demonstrates subjective reduction in daytime sleepiness as reported by Epworth sleepiness scores, with improvements in sleep-related quality of life. Further, UAS reduces socially disruptive snoring with 85% of bedpartners reporting soft to no snoring at 48-month follow-up.5 The procedure has also demonstrated long-term cost benefit in the US health-care system.6
Background and pathophysiology
Oliven and colleagues7 first observed the critical finding that selective intra-muscular stimulation of the genioglossus muscle lowered airway critical closing pressure (PCrit), thereby stabilizing the pharyngeal airway. Conversely, activation of the “retrusor” musculature, namely the hyoglossus and styloglossus muscles, increased Pcrit, increasing collapsibility of the pharyngeal airway.
Therapeutic implantation requires three incisions directed to the neck, chest, and right rib space (between the 4th to 6th intercostal spaces), with an operative time of 90 minutes or less in experienced hands. The majority of patients are discharged on the day of the procedure. Morbidity remains low with minimal pain reported during recovery. The most common complication is that of temporary tongue weakness, which typically resolves within 2 to 3 weeks. While very infrequent, patients should be counseled on the risk of postoperative hematoma, which can precipitate infection and subsequent explant of the device. Average recovery time spans between 3 and 7 days with activation of the device 4 weeks after surgical implantation to allow for appropriate tissue healing and reduce the risk of dislodgement of the implanted components. In contrast to other surgical treatment options, UAS is also reversible with no underlying alteration to existing pharyngeal anatomy apart from external incisions created during the procedure.
Stimulation to titration
As the need for a multidisciplinary approach to salvage of patients failing first-line therapy for OSA continues to grow, UAS with its multilevel impact continues to be of key interest. In similar fashion to established medical therapies such as PAP and oral appliance therapy (OAT), close observation between sleep medicine specialists and the implanting surgeon during the adaptation period with attention paid to titration parameters such as stimulation duration, pulse width, amplitude, and polarity, allow optimization of response outcome.
The stimulation electrode, which is designed in the form of a cuff to envelope the anterior (protrusor) branches of the hypoglossal nerve receives electrical stimulation from the implanted pulse generator, implanted above the pectoralis muscle of the chest wall. This design allows for collaborative awake and overnight titration of the device as directed by a sleep medicine physician. Attention is paid not only to the voltage “strength” administered with each pulse but also the degree of synchronization between respiration and stimulation, as well as pattern of pulse administration. Our experience remains that true success and adaptation to therapy requires not just meticulous surgical technique but a diligent approach to postoperative therapeutic titration to achieve a comfortable, yet effective, voltage for maintaining airway patency. Thus, akin to initiation of CPAP, UAS requires regular follow-up and device fine-tuning with patient comfort taken into consideration to achieve optimal results, and patient expectation should be aligned with this process.
Current indications
Success in UAS relies heavily on appropriate presurgical evaluation and clinical phenotyping. The following surgical indications have been demonstrated in the Stimulation Therapy for Apnea Reduction (STAR) trial and subsequent 3-year clinical follow-up: AHI between 15 and 80 events/hour (with ≤ 25% central apneas) and a BMI ≤ 32.8
As OSA often results from multi-level airway collapse, UAS targets an increase not only in the diameter of the retropalatal/oropharyngeal airway space but also the antero-posterior hypopharyngeal airway. Original criteria for implantation excluded patients with a pattern of complete circumferential collapse (CCC) noted on dynamic airway evaluation during pre-implant drug-induced sleep endoscopy (DISE). DISE aims to precisely target dynamic airway collapse patterns during simulated (propofol or midazolom induced) sleep.
Future directions
The effects of UAS are dependent on upper-airway cross-sectional area, particularly diameter. In patients who demonstrate CCC, the anteroposterior direction of activation derived from the UAS stimulus is unable to overcome CCC. In a recent prospective study, our group demonstrated that CCC can be converted to an airway collapse pattern compatible with UAS implantation, using a modified palatopharyngoplasty prior to UAS implantation. By stabilizing the lateral walls of the oropharyngeal airway with pre-implant palatal surgery, UAS is able to successfully direct widening of the airway cross-sectional area in an antero-posterior fashion. This exciting finding potentially allows for expansion of current indications, thus opening treatment to a wider patient population.9 Still, UAS remains highly studied in a relatively uniform patient population with data in more diverse subsets requiring further directed attention to expand and better define optimal patient populations for treatment.
From the perspective of improving patient adaptation and tolerance in UAS, a well-established concept in the CPAP literature can be applied, as explained by the Starling resistor model. The starling resistor is comprised of two rigid tubes connected by a collapsible segment in between. In parallel, the pharynx is a collapsible muscular tube connected on either end by the nose/nasal cavity and the trachea – both of which are bony/cartilaginous, noncollapsible structures. As has been shown in the use of CPAP, the same pressure required to maintain stability of the collapsible muscular pharynx via nasal breathing may lead to pharyngeal collapse when applied orally.10 This concept has also been directed towards UAS with our clinical experience demonstrating that oro or oronasal breathers tend to require a higher amplitude to maintain airway patency versus nasal breathers. This is an important area for future-directed study as medically/surgically improving nasal breathing in UAS subjects may subsequently lower amplitude requirements and improve patient tolerance.
Future direction to allow for improvement in the technology for application in a broader populational segment, external or alternative device powering mechanisms, along with MRI Compatibility and reducing the number of required external incisions will continue to broaden the patient selection criteria. As we move from a “stimulation” to a precision-tailored “stimulation and titration” approach, the mid to long term data supporting UAS remains very promising with 5-year follow up demonstrating sustained polysomnographic and subjective reported outcomes in well selected patients.
Dr. Awad is Assistant Professor – Department of Otolaryngology/Head & Neck Surgery, and Chief – Division of Sleep Surgery; Northwestern University, Chicago, Illinois. Dr. Capasso is Associate Professor – Department of Otolaryngology/Head & Neck Surgery, and Chief – Division of Sleep Surgery; Stanford Hospital and Clinics, Stanford, California.
References
1. Schwartz AR et al. Electrical stimulation of the lingual musculature in obstructive sleep apnea. J Appl Physiol. 1996;81(2):643-52. doi: 10.1152/jappl.1996.81.2.643.
2. Ong AA et al. Efficacy of upper airway stimulation on collapse patterns observed during drug-induced sedation endoscopy. Otolaryngol Head Neck Surg. 2016;154(5):970-7. doi: 10.1177/0194599816636835.
3. Woodson BT et al. Three-year outcomes of cranial nerve stimulation for obstructive sleep apnea: The STAR trial. Otolaryngol Head Neck Surg. 2016;154(1):181-8. doi: 10.1177/0194599815616618.
4. Heiser C et al. Outcomes of upper airway stimulation for obstructive sleep apnea in a multicenter german postmarket study. Otolaryngol Head Neck Surg. 2017;156(2):378-84. doi: 10.1177/0194599816683378.
5. Gillespie MB et al. Upper airway stimulation for obstructive sleep apnea: Patient-reported outcomes after 48 months of follow-up. Otolaryngol Head Neck Surg. 2017;156(4):765-71. doi: 10.1177/0194599817691491.
6. Pietzsch JB et al. Long-term cost-effectiveness of upper airway stimulation for the treatment of obstructive sleep apnea: A model-based projection based on the star trial. Sleep. 2015;38(5):735-44. doi: 10.5665/sleep.4666.
7. Oliven A et al. Improved upper airway patency elicited by electrical stimulation of the hypoglossus nerves. Respiration. 1996;63(4):213-16. doi: 10.1159/000196547.
8. Strollo PJ et al. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med. 2014;370(2):139-49. doi: 10.1056/NEJMoa1308659.
9. Liu YC et al. Palatopharyngoplasty resolves concentric collapse in patients ineligible for upper airway stimulation. Laryngoscope. Forthcoming.
10. De Andrade RGS et al. Impact of the type of mask on the effectiveness of and adherence to continuous positive airway pressure treatment for obstructive sleep apnea. J Bras Pneumol. 2014;40(6):658-68. doi: 10.1590/S1806-37132014000600010
Clinical Guideline Highlights for the Hospitalist: The GOLD and NICE Guidelines for the Management of COPD
Chronic obstructive pulmonary disease (COPD), projected to be the third leading cause of death by 2020, accounts for 6% of deaths globally.3 Hospitalization for COPD exacerbations is common and impacts patients’ disease trajectory, and mortality, with fewer than half of patients hospitalized for exacerbation surviving 5 years.4 Hospitalization provides an opportunity to optimize care. Due to recent practice-changing evidence, the National Institute for Health and Care Excellence (NICE) and the Global Initiative for Chronic Obstructive Lung Disease (GOLD) published updated guidelines.
KEY RECOMMENDATIONS
These are selected recommendations relevant to adult hospitalists. The GOLD guidelines grade recommendations by evidence strength from category A (randomized control trial data) to category D (expert consensus). The NICE guidelines relay strength of evidence through terminology referring to the presence or absence of a strong recommendation. Recommendations without evidence level specified are NS.
Diagnosis and Classification of COPD Severity
Recommendation 1. In patients with risk factors for and symptoms of COPD, spirometry is required to confirm the diagnosis, defined as a postbronchodilator FEV1/FVC ratio of <0.7 (NS, NICE, GOLD). The Global Lung Function Initiative (GLI) 2012 reference ranges5 are recommended (NS, NICE). Recommendation 2. Severity of airflow obstruction should be assessed according to reduction in the postbronchodilator FEV1 as: Stage I, Mild: FEV1 ≥80%; Stage II, Moderate: FEV1 = 50-79%; Stage III, Severe FEV1 = 30%-49%; Stage IV, FEV1<30% (NS, NICE, GOLD). Recommendation 3. Reversibility testing (aka bronchodilator response) does not indicate long-term response to therapy (NS, NICE, GOLD). Recommendation 4. The combined COPD assessment to classify patient symptoms and disease severity in one of four groups (A, B, C, or D) based on exacerbation history and daily symptom control (NS, GOLD). Use the Medical Research Council dyspnea scale to classify symptoms (strong, NICE).
Pharmacologic COPD Management
Recommendation 5. Short-acting inhaled bronchodilators such as short-acting beta2 agonists (SABAs) or short-acting muscarinic antagonists (SAMAs) improve FEV1 and symptoms. Combining SABA/SAMA is superior to monotherapy (A, GOLD). Recommendation 6. Long-acting bronchodilators, such as long-acting antimuscarinics (LAMAs) or long-acting beta2 agonists (LABAs), improve lung function and dyspnea and reduce exacerbations. Combination therapy (LABA/LAMA) is superior to using a single agent (LABA or LAMA) for improving FEV1 and reducing exacerbations (A, GOLD). Recommendation 7. Triple therapy of inhaled corticosteroid ICS/LAMA/LABA is more effective than the individual components in reducing exacerbations in the case of moderate to severe COPD (A, GOLD). Recommendation 8. Treatment with an ICS increases pneumonia risk (A, GOLD). Discuss these side effects (Strong, NICE). Recommendation 9. Use SABAs and SAMAs as initial treatment for patients with COPD (Strong, NICE). LABAs and LAMAs are preferred over short-acting agents except for patients with mild symptoms (A, GOLD). Recommendation 10. For symptomatic patients on long-acting monotherapy, escalate to combination LABA/LAMA, or if asthmatic features or elevated eosinophils (≥300 cells/µL) are present, combination LABA/ICS (A, GOLD; Strong, NICE). Recommendation 11. Assess and correct patient inhaler technique (NS, GOLD; Strong, NICE).
Nonpharmacologic COPD Management
Oxygen. Recommendation 12. Long-term oxygen supplementation increases survival in patients with resting arterial hypoxemia (PaO2<55 mm Hg) or hypoxemia (PaO2<60 mm Hg) with cor pulmonale (A, GOLD). Recommendation 13. In patients with moderate resting (89%-93%) or exercise-induced arterial desaturation (80%-90%), long-term oxygen does not improve outcomes (A, GOLD).6Recommendation 14. Consider long-term oxygan after a risk assessment of fall and burn risk. Do not offer oxygen to those who continue to smoke (Strong, NICE).
Tobacco
Pulmonary Rehabilitation. Recommendation 17. Provide rehabilitation to patients with high exacerbation risk and relevant symptoms (A, GOLD). Offer pulmonary rehabilitation to patients with recent hospitalizations and/or severe dyspnea (Strong, NICE).
Immunizations. Recommendation 18. Influenza and pneumococcal vaccinations (PPSV23 as well as PCV13 when age ≥ 65 years) are recommended for patients with COPD (NS, GOLD; Strong, NICE).
Palliative Care. Recommendation 19. For patients with end-stage COPD or poorly controlled symptoms, provide access to palliative care (NS, GOLD; Strong, NICE).
Management of COPD Exacerbations and Patients at high risk for Exacerbations
Recommendation 20. Use SABAs with or without SAMAs as initial bronchodilators to treat acute exacerbations (C, GOLD). Recommendation 21. Systemic corticosteroids for exacerbations improve lung function, oxygenation, and recovery time. Recommend 5 to 7 days of therapy (A, GOLD; Strong, NICE). Recommendation 22. Antibiotics shorten recovery time and reduce treatment failure and rehospitalization. Treatment should be 5 to 7 days (B, GOLD). Consider antibiotics while balancing the severity of symptoms and hospitalization need (Conditional, NICE). Recommendation 23. Noninvasive mechanical ventilation is the preferred mode of ventilation for COPD patients with acute respiratory failure without acute contraindications (A, GOLD). Recommendation 24. Avoid long-term oral corticosteroids therapy (A, GOLD). Recommendation 25. Consider roflumilast for patients with exacerbations despite LABA/ICS or LABA/LAMA/ICS, and seek respiratory medicine consultation (B, GOLD; Strong, NICE). For former smokers with exacerbations despite appropriate therapy, consider azithromycin (B, GOLD; Strong, NICE).
CRITIQUE
GOLD is an International committee of experts who compile the report based on scientific literature review. NICE is an independent organization funded by Department of Health and Social Care in the United Kingdom responsible for evidence-based guidance on healthcare determined by an expert committee through scientific review and a transparent process that details committee formation and framework (GRADE) used and stakeholder input. While both guidelines review current publications, practice-influencing clinical trials of recent publication may be missed.
On the GOLD Science committee, 17/20 members have pharmaceutical relationships, with no mitigation plan provided. The NICE guidelines detail a panel with few industry ties and a mitigation plan for potential conflicts of interest.
These recommendations comprehensively cover outpatient and inpatient COPD management. The GOLD and NICE guidelines are similar with the exception of recommendations surrounding use of oxygen. The NICE guidelines, based on the adverse events documented in the recent Long-Term Oxygen Treatment Trial,6 recommend against oxygen use by patients who smoke because of the risk of fire-related injuries;7 GOLD guidelines do not differentiate oxygen recommendation by patient population.
Differences in the strength of NICE and GOLD recommendations highlight areas for further study. Investigations determining distinct COPD phenotypes will likely influence future guidelines. More discriminative multidimensional prognostication tools are needed to improve precision surrounding prognosis.
1. NICE. Overview. Chronic obstructive pulmonary disease in over 16s: Diagnosis and management, Guidance. https://www.nice.org.uk/guidance/ng115. Accessed November 21, 2019
2. GOLD Reports for Personal Use. Global Initiative for Chronic Obstructive Lung Disease - GOLD. https://goldcopd.org/gold-reports/. Accessed September 17, 2019.
3. Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095-128. https://doi.org/10.1016/S0140-6736(12)61728-0.
4. Suissa S, Dell’Aniello S, Ernst P. Long-term natural history of chronic obstructive pulmonary disease: Severe exacerbations and mortality. Thorax. 2012;67(11):957-63. https://doi.org/10.1136/thoraxjnl-2011-201518.
5. Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: The global lung function 2012 equations. Eur Respir J. 2012;40(6):1324-43. https://doi.org/10.1183/09031936.00080312.
6. Albert RK, Au DH, Blackford AL, et al. Long-term oxygen treatment trial research group. A randomized trial of long-term oxygen for COPD with moderate desaturation. N Engl J Med. 2016;375(17):1617-27. https://doi.org/10.1056/NEJMoa1604344.
7. National Institute for Health and Care Excellence. Chronic obstructive pulmonary disease in over 16s: diagnosis and management [B} Oxygen therapy in people with stable COPD. https://www.nice.org.uk/guidance/ng115/evidence/b-oxygen-therapy-in-people-with-stable-copd-pdf-6602768751. Accessed November 21, 2019.
Chronic obstructive pulmonary disease (COPD), projected to be the third leading cause of death by 2020, accounts for 6% of deaths globally.3 Hospitalization for COPD exacerbations is common and impacts patients’ disease trajectory, and mortality, with fewer than half of patients hospitalized for exacerbation surviving 5 years.4 Hospitalization provides an opportunity to optimize care. Due to recent practice-changing evidence, the National Institute for Health and Care Excellence (NICE) and the Global Initiative for Chronic Obstructive Lung Disease (GOLD) published updated guidelines.
KEY RECOMMENDATIONS
These are selected recommendations relevant to adult hospitalists. The GOLD guidelines grade recommendations by evidence strength from category A (randomized control trial data) to category D (expert consensus). The NICE guidelines relay strength of evidence through terminology referring to the presence or absence of a strong recommendation. Recommendations without evidence level specified are NS.
Diagnosis and Classification of COPD Severity
Recommendation 1. In patients with risk factors for and symptoms of COPD, spirometry is required to confirm the diagnosis, defined as a postbronchodilator FEV1/FVC ratio of <0.7 (NS, NICE, GOLD). The Global Lung Function Initiative (GLI) 2012 reference ranges5 are recommended (NS, NICE). Recommendation 2. Severity of airflow obstruction should be assessed according to reduction in the postbronchodilator FEV1 as: Stage I, Mild: FEV1 ≥80%; Stage II, Moderate: FEV1 = 50-79%; Stage III, Severe FEV1 = 30%-49%; Stage IV, FEV1<30% (NS, NICE, GOLD). Recommendation 3. Reversibility testing (aka bronchodilator response) does not indicate long-term response to therapy (NS, NICE, GOLD). Recommendation 4. The combined COPD assessment to classify patient symptoms and disease severity in one of four groups (A, B, C, or D) based on exacerbation history and daily symptom control (NS, GOLD). Use the Medical Research Council dyspnea scale to classify symptoms (strong, NICE).
Pharmacologic COPD Management
Recommendation 5. Short-acting inhaled bronchodilators such as short-acting beta2 agonists (SABAs) or short-acting muscarinic antagonists (SAMAs) improve FEV1 and symptoms. Combining SABA/SAMA is superior to monotherapy (A, GOLD). Recommendation 6. Long-acting bronchodilators, such as long-acting antimuscarinics (LAMAs) or long-acting beta2 agonists (LABAs), improve lung function and dyspnea and reduce exacerbations. Combination therapy (LABA/LAMA) is superior to using a single agent (LABA or LAMA) for improving FEV1 and reducing exacerbations (A, GOLD). Recommendation 7. Triple therapy of inhaled corticosteroid ICS/LAMA/LABA is more effective than the individual components in reducing exacerbations in the case of moderate to severe COPD (A, GOLD). Recommendation 8. Treatment with an ICS increases pneumonia risk (A, GOLD). Discuss these side effects (Strong, NICE). Recommendation 9. Use SABAs and SAMAs as initial treatment for patients with COPD (Strong, NICE). LABAs and LAMAs are preferred over short-acting agents except for patients with mild symptoms (A, GOLD). Recommendation 10. For symptomatic patients on long-acting monotherapy, escalate to combination LABA/LAMA, or if asthmatic features or elevated eosinophils (≥300 cells/µL) are present, combination LABA/ICS (A, GOLD; Strong, NICE). Recommendation 11. Assess and correct patient inhaler technique (NS, GOLD; Strong, NICE).
Nonpharmacologic COPD Management
Oxygen. Recommendation 12. Long-term oxygen supplementation increases survival in patients with resting arterial hypoxemia (PaO2<55 mm Hg) or hypoxemia (PaO2<60 mm Hg) with cor pulmonale (A, GOLD). Recommendation 13. In patients with moderate resting (89%-93%) or exercise-induced arterial desaturation (80%-90%), long-term oxygen does not improve outcomes (A, GOLD).6Recommendation 14. Consider long-term oxygan after a risk assessment of fall and burn risk. Do not offer oxygen to those who continue to smoke (Strong, NICE).
Tobacco
Pulmonary Rehabilitation. Recommendation 17. Provide rehabilitation to patients with high exacerbation risk and relevant symptoms (A, GOLD). Offer pulmonary rehabilitation to patients with recent hospitalizations and/or severe dyspnea (Strong, NICE).
Immunizations. Recommendation 18. Influenza and pneumococcal vaccinations (PPSV23 as well as PCV13 when age ≥ 65 years) are recommended for patients with COPD (NS, GOLD; Strong, NICE).
Palliative Care. Recommendation 19. For patients with end-stage COPD or poorly controlled symptoms, provide access to palliative care (NS, GOLD; Strong, NICE).
Management of COPD Exacerbations and Patients at high risk for Exacerbations
Recommendation 20. Use SABAs with or without SAMAs as initial bronchodilators to treat acute exacerbations (C, GOLD). Recommendation 21. Systemic corticosteroids for exacerbations improve lung function, oxygenation, and recovery time. Recommend 5 to 7 days of therapy (A, GOLD; Strong, NICE). Recommendation 22. Antibiotics shorten recovery time and reduce treatment failure and rehospitalization. Treatment should be 5 to 7 days (B, GOLD). Consider antibiotics while balancing the severity of symptoms and hospitalization need (Conditional, NICE). Recommendation 23. Noninvasive mechanical ventilation is the preferred mode of ventilation for COPD patients with acute respiratory failure without acute contraindications (A, GOLD). Recommendation 24. Avoid long-term oral corticosteroids therapy (A, GOLD). Recommendation 25. Consider roflumilast for patients with exacerbations despite LABA/ICS or LABA/LAMA/ICS, and seek respiratory medicine consultation (B, GOLD; Strong, NICE). For former smokers with exacerbations despite appropriate therapy, consider azithromycin (B, GOLD; Strong, NICE).
CRITIQUE
GOLD is an International committee of experts who compile the report based on scientific literature review. NICE is an independent organization funded by Department of Health and Social Care in the United Kingdom responsible for evidence-based guidance on healthcare determined by an expert committee through scientific review and a transparent process that details committee formation and framework (GRADE) used and stakeholder input. While both guidelines review current publications, practice-influencing clinical trials of recent publication may be missed.
On the GOLD Science committee, 17/20 members have pharmaceutical relationships, with no mitigation plan provided. The NICE guidelines detail a panel with few industry ties and a mitigation plan for potential conflicts of interest.
These recommendations comprehensively cover outpatient and inpatient COPD management. The GOLD and NICE guidelines are similar with the exception of recommendations surrounding use of oxygen. The NICE guidelines, based on the adverse events documented in the recent Long-Term Oxygen Treatment Trial,6 recommend against oxygen use by patients who smoke because of the risk of fire-related injuries;7 GOLD guidelines do not differentiate oxygen recommendation by patient population.
Differences in the strength of NICE and GOLD recommendations highlight areas for further study. Investigations determining distinct COPD phenotypes will likely influence future guidelines. More discriminative multidimensional prognostication tools are needed to improve precision surrounding prognosis.
Chronic obstructive pulmonary disease (COPD), projected to be the third leading cause of death by 2020, accounts for 6% of deaths globally.3 Hospitalization for COPD exacerbations is common and impacts patients’ disease trajectory, and mortality, with fewer than half of patients hospitalized for exacerbation surviving 5 years.4 Hospitalization provides an opportunity to optimize care. Due to recent practice-changing evidence, the National Institute for Health and Care Excellence (NICE) and the Global Initiative for Chronic Obstructive Lung Disease (GOLD) published updated guidelines.
KEY RECOMMENDATIONS
These are selected recommendations relevant to adult hospitalists. The GOLD guidelines grade recommendations by evidence strength from category A (randomized control trial data) to category D (expert consensus). The NICE guidelines relay strength of evidence through terminology referring to the presence or absence of a strong recommendation. Recommendations without evidence level specified are NS.
Diagnosis and Classification of COPD Severity
Recommendation 1. In patients with risk factors for and symptoms of COPD, spirometry is required to confirm the diagnosis, defined as a postbronchodilator FEV1/FVC ratio of <0.7 (NS, NICE, GOLD). The Global Lung Function Initiative (GLI) 2012 reference ranges5 are recommended (NS, NICE). Recommendation 2. Severity of airflow obstruction should be assessed according to reduction in the postbronchodilator FEV1 as: Stage I, Mild: FEV1 ≥80%; Stage II, Moderate: FEV1 = 50-79%; Stage III, Severe FEV1 = 30%-49%; Stage IV, FEV1<30% (NS, NICE, GOLD). Recommendation 3. Reversibility testing (aka bronchodilator response) does not indicate long-term response to therapy (NS, NICE, GOLD). Recommendation 4. The combined COPD assessment to classify patient symptoms and disease severity in one of four groups (A, B, C, or D) based on exacerbation history and daily symptom control (NS, GOLD). Use the Medical Research Council dyspnea scale to classify symptoms (strong, NICE).
Pharmacologic COPD Management
Recommendation 5. Short-acting inhaled bronchodilators such as short-acting beta2 agonists (SABAs) or short-acting muscarinic antagonists (SAMAs) improve FEV1 and symptoms. Combining SABA/SAMA is superior to monotherapy (A, GOLD). Recommendation 6. Long-acting bronchodilators, such as long-acting antimuscarinics (LAMAs) or long-acting beta2 agonists (LABAs), improve lung function and dyspnea and reduce exacerbations. Combination therapy (LABA/LAMA) is superior to using a single agent (LABA or LAMA) for improving FEV1 and reducing exacerbations (A, GOLD). Recommendation 7. Triple therapy of inhaled corticosteroid ICS/LAMA/LABA is more effective than the individual components in reducing exacerbations in the case of moderate to severe COPD (A, GOLD). Recommendation 8. Treatment with an ICS increases pneumonia risk (A, GOLD). Discuss these side effects (Strong, NICE). Recommendation 9. Use SABAs and SAMAs as initial treatment for patients with COPD (Strong, NICE). LABAs and LAMAs are preferred over short-acting agents except for patients with mild symptoms (A, GOLD). Recommendation 10. For symptomatic patients on long-acting monotherapy, escalate to combination LABA/LAMA, or if asthmatic features or elevated eosinophils (≥300 cells/µL) are present, combination LABA/ICS (A, GOLD; Strong, NICE). Recommendation 11. Assess and correct patient inhaler technique (NS, GOLD; Strong, NICE).
Nonpharmacologic COPD Management
Oxygen. Recommendation 12. Long-term oxygen supplementation increases survival in patients with resting arterial hypoxemia (PaO2<55 mm Hg) or hypoxemia (PaO2<60 mm Hg) with cor pulmonale (A, GOLD). Recommendation 13. In patients with moderate resting (89%-93%) or exercise-induced arterial desaturation (80%-90%), long-term oxygen does not improve outcomes (A, GOLD).6Recommendation 14. Consider long-term oxygan after a risk assessment of fall and burn risk. Do not offer oxygen to those who continue to smoke (Strong, NICE).
Tobacco
Pulmonary Rehabilitation. Recommendation 17. Provide rehabilitation to patients with high exacerbation risk and relevant symptoms (A, GOLD). Offer pulmonary rehabilitation to patients with recent hospitalizations and/or severe dyspnea (Strong, NICE).
Immunizations. Recommendation 18. Influenza and pneumococcal vaccinations (PPSV23 as well as PCV13 when age ≥ 65 years) are recommended for patients with COPD (NS, GOLD; Strong, NICE).
Palliative Care. Recommendation 19. For patients with end-stage COPD or poorly controlled symptoms, provide access to palliative care (NS, GOLD; Strong, NICE).
Management of COPD Exacerbations and Patients at high risk for Exacerbations
Recommendation 20. Use SABAs with or without SAMAs as initial bronchodilators to treat acute exacerbations (C, GOLD). Recommendation 21. Systemic corticosteroids for exacerbations improve lung function, oxygenation, and recovery time. Recommend 5 to 7 days of therapy (A, GOLD; Strong, NICE). Recommendation 22. Antibiotics shorten recovery time and reduce treatment failure and rehospitalization. Treatment should be 5 to 7 days (B, GOLD). Consider antibiotics while balancing the severity of symptoms and hospitalization need (Conditional, NICE). Recommendation 23. Noninvasive mechanical ventilation is the preferred mode of ventilation for COPD patients with acute respiratory failure without acute contraindications (A, GOLD). Recommendation 24. Avoid long-term oral corticosteroids therapy (A, GOLD). Recommendation 25. Consider roflumilast for patients with exacerbations despite LABA/ICS or LABA/LAMA/ICS, and seek respiratory medicine consultation (B, GOLD; Strong, NICE). For former smokers with exacerbations despite appropriate therapy, consider azithromycin (B, GOLD; Strong, NICE).
CRITIQUE
GOLD is an International committee of experts who compile the report based on scientific literature review. NICE is an independent organization funded by Department of Health and Social Care in the United Kingdom responsible for evidence-based guidance on healthcare determined by an expert committee through scientific review and a transparent process that details committee formation and framework (GRADE) used and stakeholder input. While both guidelines review current publications, practice-influencing clinical trials of recent publication may be missed.
On the GOLD Science committee, 17/20 members have pharmaceutical relationships, with no mitigation plan provided. The NICE guidelines detail a panel with few industry ties and a mitigation plan for potential conflicts of interest.
These recommendations comprehensively cover outpatient and inpatient COPD management. The GOLD and NICE guidelines are similar with the exception of recommendations surrounding use of oxygen. The NICE guidelines, based on the adverse events documented in the recent Long-Term Oxygen Treatment Trial,6 recommend against oxygen use by patients who smoke because of the risk of fire-related injuries;7 GOLD guidelines do not differentiate oxygen recommendation by patient population.
Differences in the strength of NICE and GOLD recommendations highlight areas for further study. Investigations determining distinct COPD phenotypes will likely influence future guidelines. More discriminative multidimensional prognostication tools are needed to improve precision surrounding prognosis.
1. NICE. Overview. Chronic obstructive pulmonary disease in over 16s: Diagnosis and management, Guidance. https://www.nice.org.uk/guidance/ng115. Accessed November 21, 2019
2. GOLD Reports for Personal Use. Global Initiative for Chronic Obstructive Lung Disease - GOLD. https://goldcopd.org/gold-reports/. Accessed September 17, 2019.
3. Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095-128. https://doi.org/10.1016/S0140-6736(12)61728-0.
4. Suissa S, Dell’Aniello S, Ernst P. Long-term natural history of chronic obstructive pulmonary disease: Severe exacerbations and mortality. Thorax. 2012;67(11):957-63. https://doi.org/10.1136/thoraxjnl-2011-201518.
5. Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: The global lung function 2012 equations. Eur Respir J. 2012;40(6):1324-43. https://doi.org/10.1183/09031936.00080312.
6. Albert RK, Au DH, Blackford AL, et al. Long-term oxygen treatment trial research group. A randomized trial of long-term oxygen for COPD with moderate desaturation. N Engl J Med. 2016;375(17):1617-27. https://doi.org/10.1056/NEJMoa1604344.
7. National Institute for Health and Care Excellence. Chronic obstructive pulmonary disease in over 16s: diagnosis and management [B} Oxygen therapy in people with stable COPD. https://www.nice.org.uk/guidance/ng115/evidence/b-oxygen-therapy-in-people-with-stable-copd-pdf-6602768751. Accessed November 21, 2019.
1. NICE. Overview. Chronic obstructive pulmonary disease in over 16s: Diagnosis and management, Guidance. https://www.nice.org.uk/guidance/ng115. Accessed November 21, 2019
2. GOLD Reports for Personal Use. Global Initiative for Chronic Obstructive Lung Disease - GOLD. https://goldcopd.org/gold-reports/. Accessed September 17, 2019.
3. Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095-128. https://doi.org/10.1016/S0140-6736(12)61728-0.
4. Suissa S, Dell’Aniello S, Ernst P. Long-term natural history of chronic obstructive pulmonary disease: Severe exacerbations and mortality. Thorax. 2012;67(11):957-63. https://doi.org/10.1136/thoraxjnl-2011-201518.
5. Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: The global lung function 2012 equations. Eur Respir J. 2012;40(6):1324-43. https://doi.org/10.1183/09031936.00080312.
6. Albert RK, Au DH, Blackford AL, et al. Long-term oxygen treatment trial research group. A randomized trial of long-term oxygen for COPD with moderate desaturation. N Engl J Med. 2016;375(17):1617-27. https://doi.org/10.1056/NEJMoa1604344.
7. National Institute for Health and Care Excellence. Chronic obstructive pulmonary disease in over 16s: diagnosis and management [B} Oxygen therapy in people with stable COPD. https://www.nice.org.uk/guidance/ng115/evidence/b-oxygen-therapy-in-people-with-stable-copd-pdf-6602768751. Accessed November 21, 2019.
© 2020 Society of Hospital Medicine