User login
8-year-old boy • palpable purpura on the legs with arthralgia • absence of coagulopathy • upper respiratory infection • Dx?
THE CASE
An 8-year-old boy presented to his family physician (FP) with pharyngitis, nasal drainage, and a dry cough of 3 days’ duration. He denied any fever, chills, vomiting, or diarrhea. He had no sick contacts or prior history of streptococcal pharyngitis, but a rapid strep test was positive. No throat culture was performed at this time. The patient was started on amoxicillin 250 mg 3 times daily for 10 days.
On Day 7 of symptoms, the patient presented to the emergency department with elbow and knee pain, as well as mild swelling and purpura of his legs of 3 days’ duration. He was normotensive and reported no abdominal pain. A laboratory workup, including a complete blood cell count and differential, prothrombin time, partial thromboplastin time, comprehensive metabolic panel, creatinine kinase test, urinalysis, and chest radiograph, was normal, but his erythrocyte sedimentation rate (ESR) was mildly elevated at 22 mm/h (reference range, 0–20 mm/h). The patient was discharged on acetaminophen 15 mg/kg every 4 hours as needed for pain.
THE DIAGNOSIS
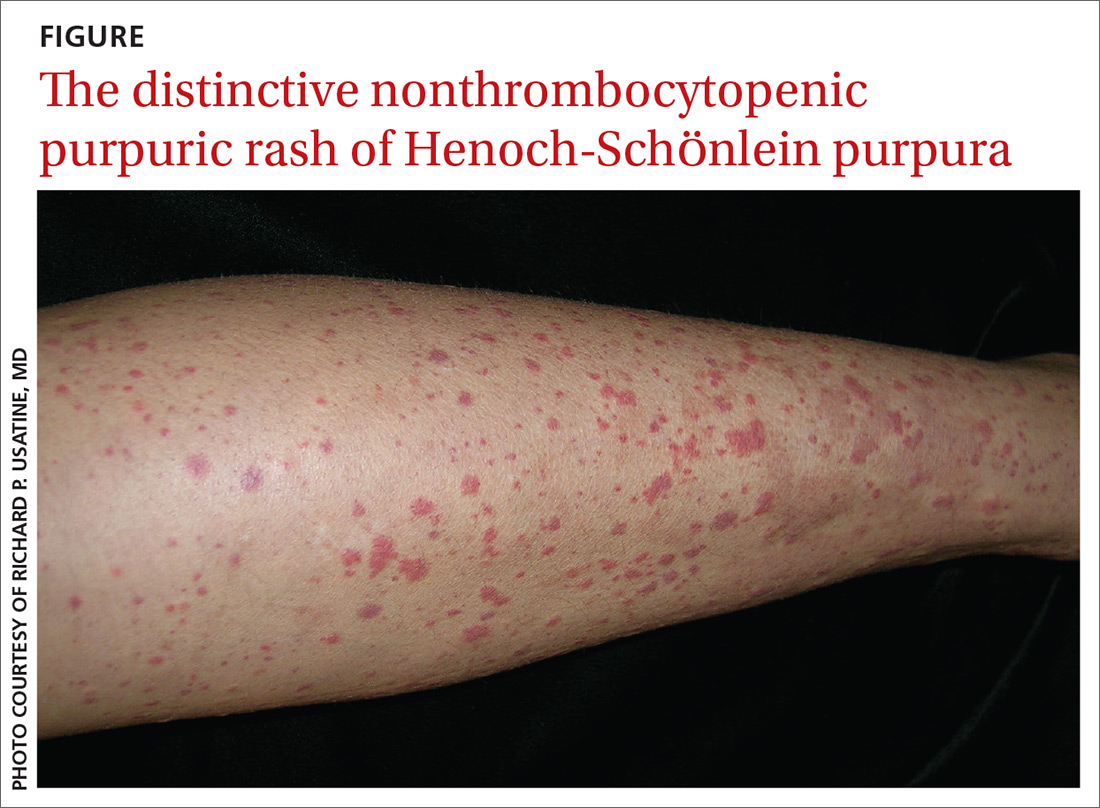
Based on the distinctive palpable purpura on the legs, arthralgia, upper respiratory infection, and lack of thrombocytopenia and coagulopathy, a presumptive diagnosis of Henoch-Schönlein purpura (HSP) was made.
On Day 9 of symptoms, the patient returned to his FP’s office because the arthralgia persisted in his ankles, knees, and hips. He had developed lower back pain, but the pharyngitis and upper respiratory symptoms had resolved. On physical examination, he was normotensive with a normal abdominal exam. The patient reported that it hurt to move his wrists, hands, elbows, shoulders, knees, and ankles. He also had mild swelling in his left wrist, hand, and ankle. The paraspinal muscles in the lower thoracic and lumbar back were mildly tender to palpation. A complete metabolic panel and urinalysis were normal. Dermatologic examination revealed discrete purpuric lesions ranging from 1 to 8 mm in diameter on the child’s shins, thighs, and buttocks. Urinalysis, blood urea nitrogen, and creatinine kinase were normal. His ESR remained mildly elevated at 24 mm/h. Since there was no evidence of glomerulonephritis, ibuprofen 10 mg/kg every 8 hours as needed was added for pain management.
The child was brought back to his FP on Day 18 for a scheduled follow-up visit. The parents reported that his arthralgia was improved during the day, but by the evening, his knees and ankles hurt so much that they had to carry him to the bathroom. On physical examination, he still had palpable purpura of the legs. There was no swelling, but his joints were still tender to palpation. His parents were reminded to give him ibuprofen after school to control evening pain. Over the next 2 weeks, the patient showed gradual improvement, and by Day 33 the rash and all of the associated symptoms had resolved.
DISCUSSION
Clinical presentation. HSP is an IgA immune complex vasculitis in which abnormal glycosylation of IgA creates large immune complexes that are deposited in the walls of the skin capillaries and arterioles. The primary clinical finding in HSP is a distinctive nonthrombocytopenic purpuric rash that is not associated with coagulopathy and is characterized by reddish purple macules that progress to palpable purpura with petechiae (
A preceding upper respiratory infection has been found in 37% of patients,1 and in patients with renal complications, 20% to 50% have been found to have a group A Streptococcus infection.2 Other associations include food allergies, cold exposure, insect bites, and drug allergies.
Continue to: HSP vasculitis causes...
HSP vasculitis causes abdominal pain in 50% to 75% of patients due to proximal small-bowel submucosal hemorrhage and bowel wall edema.3 In children with HSP, 20% to 55% have been shown to develop renal disease,4 which can range in severity from microscopic hematuria to nephrotic syndrome.3 To ensure prompt treatment of renal manifestations, renal function should be monitored regularly via blood pressure and urinalysis during the course of HSP and after resolution. Renal disease associated with HSP can be acute or chronic.
This case was different because our patient did not exhibit all elements of the classic tetrad of HSP, which includes the characteristic rash, abdominal pain, renal involvement, and arthralgia.
Incidence. HSP is more common in children than adults, with average annual incidence rates of 20/100,000 and 70/100,000 in children in the United States and Asia, respectively.5 While 90% of HSP cases occur in children < 10 years, the peak incidence is at 6 years of age.6 Complications from HSP are more common in adults than in children.7 Caucasian and Asian populations have a 3- to 4-times higher prevalence of HSP than black populations. The male-to-female ratio is 2 to 1.6
The diagnosis of HSP is usually made clinically, based on the distinctive rash, which typically is symmetrical, involving the buttocks, lower legs, elbows, and/or knees. HSP also can be confirmed via skin biopsy and/or direct immunofluorescence, which can identify the presence of IgA in the vessel walls.
The presence of 3 or more of the following criteria also suggests HSP: palpable purpura, bowel angina, gastrointestinal (GI) bleeding, hematuria, ≤ 20 years of age at onset, and no medications prior to presentation of symptoms (87% of cases correctly classified). Fewer than 3 of these factors favor hypersensitivity vasculitis (74% of cases correctly classified).8
Continue to: The differential diagnosis
The differential diagnosis for HSP includes polyarteritis nodosa, a vasculitis with a different characteristic rash; acute abdomen, distinguished by the absence of purpura or arthralgia; meningococcemia, in which fever and meningeal signs may occur; hypersensitivity vasculitis, which arises due to prior exposure to medications or food allergens; and thrombocytopenic purpura, which is characterized by low platelet count.9
Treatment focuses on pain management
In the absence of renal disease, HSP commonly is treated with naproxen for pain management (dosage for children < 2 years of age: 5-7 mg/kg orally every 8-12 hours; dosage for children ≥ 2 years of age, adolescents, and adults: 10-20 mg/kg/d divided into 2 doses; maximum adolescent and adult dose is 1500 mg/d for 3 days followed by a maximum of 1000 mg/d thereafter).
For patients of all ages with severe pain and those with GI effects limiting oral intake of medication, use oral prednisone (1-2 mg/kg/d [maximum dose, 60-80 mg/d]) or intravenous methylprednisolone (0.8-1.6 mg/kg/d [maximum dose, 64 mg/d). Glucocorticoids may then be tapered slowly over 4 to 8 weeks to avoid rebound since they help with inflammation but do not shorten the course of disease. Steroids can ease GI and joint symptoms in HSP but will not improve the rash.
THE TAKEAWAY
The classic tetrad of HSP includes the characteristic rash, abdominal pain, renal involvement, and arthralgia. Diagnosis usually is made clinically, but skin biopsy and direct immunofluorescence can confirm small vessel vasculitis with IgA deposits. More severe manifestations of HSP such as renal disease, hemorrhage, severe anemia, signs of intestinal obstruction, or peritonitis require rapid subspecialty referral.
CORRESPONDENCE
Rachel Bramson, MD, Department of Primary Care, Baylor Scott and White Health, University Clinic, 1700 University Drive, College Station, TX 77840; Rachel.Bramson@BSWHealth.org
1. Rigante D, Castellazzi L, Bosco A, et al. Is there a crossroad between infections, genetics, and Henoch-Schönlein purpura? Autoimmun Rev. 2013;12:1016-1021.
2. LaConti JJ, Donet JA, Cho-Vega JH, et al. Henoch-Schönlein Purpura with adalimumab therapy for ulcerative colitis: a case report and review of the literature [published online July 27, 2016]. Case Rep Rheumatol. 2016;2016:2812980.
3. Trnka P. Henoch-Schönlein purpura in children. J Paediatr Child Health. 2013;49:995-1003.
4. Audemard-Verger A, Pillebout E, Guillevin L, et al. IgA vasculitis (Henoch-Shönlein purpura) in adults: diagnostic and therapeutic aspects. Autoimmun Rev. 2015;14:579-585.
5. Chen J, Mao J. Henoch-Schönlein purpura nephritis in children: incidence, pathogenesis and management. World J Pediatr. 2015;11:29-34.
6. Michel B, Hunder G, Bloch D, et al. Hypersensitivity vasculitis and Henoch-Schönlein purpura: a comparison between the 2 disorders. J Rheumatol. 1992;19:721-728.
7. Reamy BV, Williams PM, Lindsay TJ. Henoch-Schönlein purpura. Am Fam Physician. 2009;80:697-704.
8. Yang YH, Yu HH, Chiang BL. The diagnosis and classification of Henoch-Schönlein purpura: an updated review. Autoimmun Rev. 2014;13:355-358.
9. Floege J, Feehally J. Treatment of IgA nephropathy and Henoch-Schönlein nephritis. Nat Rev Nephrol. 2013;9:320-327.
THE CASE
An 8-year-old boy presented to his family physician (FP) with pharyngitis, nasal drainage, and a dry cough of 3 days’ duration. He denied any fever, chills, vomiting, or diarrhea. He had no sick contacts or prior history of streptococcal pharyngitis, but a rapid strep test was positive. No throat culture was performed at this time. The patient was started on amoxicillin 250 mg 3 times daily for 10 days.
On Day 7 of symptoms, the patient presented to the emergency department with elbow and knee pain, as well as mild swelling and purpura of his legs of 3 days’ duration. He was normotensive and reported no abdominal pain. A laboratory workup, including a complete blood cell count and differential, prothrombin time, partial thromboplastin time, comprehensive metabolic panel, creatinine kinase test, urinalysis, and chest radiograph, was normal, but his erythrocyte sedimentation rate (ESR) was mildly elevated at 22 mm/h (reference range, 0–20 mm/h). The patient was discharged on acetaminophen 15 mg/kg every 4 hours as needed for pain.
THE DIAGNOSIS
Based on the distinctive palpable purpura on the legs, arthralgia, upper respiratory infection, and lack of thrombocytopenia and coagulopathy, a presumptive diagnosis of Henoch-Schönlein purpura (HSP) was made.
On Day 9 of symptoms, the patient returned to his FP’s office because the arthralgia persisted in his ankles, knees, and hips. He had developed lower back pain, but the pharyngitis and upper respiratory symptoms had resolved. On physical examination, he was normotensive with a normal abdominal exam. The patient reported that it hurt to move his wrists, hands, elbows, shoulders, knees, and ankles. He also had mild swelling in his left wrist, hand, and ankle. The paraspinal muscles in the lower thoracic and lumbar back were mildly tender to palpation. A complete metabolic panel and urinalysis were normal. Dermatologic examination revealed discrete purpuric lesions ranging from 1 to 8 mm in diameter on the child’s shins, thighs, and buttocks. Urinalysis, blood urea nitrogen, and creatinine kinase were normal. His ESR remained mildly elevated at 24 mm/h. Since there was no evidence of glomerulonephritis, ibuprofen 10 mg/kg every 8 hours as needed was added for pain management.
The child was brought back to his FP on Day 18 for a scheduled follow-up visit. The parents reported that his arthralgia was improved during the day, but by the evening, his knees and ankles hurt so much that they had to carry him to the bathroom. On physical examination, he still had palpable purpura of the legs. There was no swelling, but his joints were still tender to palpation. His parents were reminded to give him ibuprofen after school to control evening pain. Over the next 2 weeks, the patient showed gradual improvement, and by Day 33 the rash and all of the associated symptoms had resolved.
DISCUSSION
Clinical presentation. HSP is an IgA immune complex vasculitis in which abnormal glycosylation of IgA creates large immune complexes that are deposited in the walls of the skin capillaries and arterioles. The primary clinical finding in HSP is a distinctive nonthrombocytopenic purpuric rash that is not associated with coagulopathy and is characterized by reddish purple macules that progress to palpable purpura with petechiae (
A preceding upper respiratory infection has been found in 37% of patients,1 and in patients with renal complications, 20% to 50% have been found to have a group A Streptococcus infection.2 Other associations include food allergies, cold exposure, insect bites, and drug allergies.
Continue to: HSP vasculitis causes...
HSP vasculitis causes abdominal pain in 50% to 75% of patients due to proximal small-bowel submucosal hemorrhage and bowel wall edema.3 In children with HSP, 20% to 55% have been shown to develop renal disease,4 which can range in severity from microscopic hematuria to nephrotic syndrome.3 To ensure prompt treatment of renal manifestations, renal function should be monitored regularly via blood pressure and urinalysis during the course of HSP and after resolution. Renal disease associated with HSP can be acute or chronic.
This case was different because our patient did not exhibit all elements of the classic tetrad of HSP, which includes the characteristic rash, abdominal pain, renal involvement, and arthralgia.
Incidence. HSP is more common in children than adults, with average annual incidence rates of 20/100,000 and 70/100,000 in children in the United States and Asia, respectively.5 While 90% of HSP cases occur in children < 10 years, the peak incidence is at 6 years of age.6 Complications from HSP are more common in adults than in children.7 Caucasian and Asian populations have a 3- to 4-times higher prevalence of HSP than black populations. The male-to-female ratio is 2 to 1.6
The diagnosis of HSP is usually made clinically, based on the distinctive rash, which typically is symmetrical, involving the buttocks, lower legs, elbows, and/or knees. HSP also can be confirmed via skin biopsy and/or direct immunofluorescence, which can identify the presence of IgA in the vessel walls.
The presence of 3 or more of the following criteria also suggests HSP: palpable purpura, bowel angina, gastrointestinal (GI) bleeding, hematuria, ≤ 20 years of age at onset, and no medications prior to presentation of symptoms (87% of cases correctly classified). Fewer than 3 of these factors favor hypersensitivity vasculitis (74% of cases correctly classified).8
Continue to: The differential diagnosis
The differential diagnosis for HSP includes polyarteritis nodosa, a vasculitis with a different characteristic rash; acute abdomen, distinguished by the absence of purpura or arthralgia; meningococcemia, in which fever and meningeal signs may occur; hypersensitivity vasculitis, which arises due to prior exposure to medications or food allergens; and thrombocytopenic purpura, which is characterized by low platelet count.9
Treatment focuses on pain management
In the absence of renal disease, HSP commonly is treated with naproxen for pain management (dosage for children < 2 years of age: 5-7 mg/kg orally every 8-12 hours; dosage for children ≥ 2 years of age, adolescents, and adults: 10-20 mg/kg/d divided into 2 doses; maximum adolescent and adult dose is 1500 mg/d for 3 days followed by a maximum of 1000 mg/d thereafter).
For patients of all ages with severe pain and those with GI effects limiting oral intake of medication, use oral prednisone (1-2 mg/kg/d [maximum dose, 60-80 mg/d]) or intravenous methylprednisolone (0.8-1.6 mg/kg/d [maximum dose, 64 mg/d). Glucocorticoids may then be tapered slowly over 4 to 8 weeks to avoid rebound since they help with inflammation but do not shorten the course of disease. Steroids can ease GI and joint symptoms in HSP but will not improve the rash.
THE TAKEAWAY
The classic tetrad of HSP includes the characteristic rash, abdominal pain, renal involvement, and arthralgia. Diagnosis usually is made clinically, but skin biopsy and direct immunofluorescence can confirm small vessel vasculitis with IgA deposits. More severe manifestations of HSP such as renal disease, hemorrhage, severe anemia, signs of intestinal obstruction, or peritonitis require rapid subspecialty referral.
CORRESPONDENCE
Rachel Bramson, MD, Department of Primary Care, Baylor Scott and White Health, University Clinic, 1700 University Drive, College Station, TX 77840; Rachel.Bramson@BSWHealth.org
THE CASE
An 8-year-old boy presented to his family physician (FP) with pharyngitis, nasal drainage, and a dry cough of 3 days’ duration. He denied any fever, chills, vomiting, or diarrhea. He had no sick contacts or prior history of streptococcal pharyngitis, but a rapid strep test was positive. No throat culture was performed at this time. The patient was started on amoxicillin 250 mg 3 times daily for 10 days.
On Day 7 of symptoms, the patient presented to the emergency department with elbow and knee pain, as well as mild swelling and purpura of his legs of 3 days’ duration. He was normotensive and reported no abdominal pain. A laboratory workup, including a complete blood cell count and differential, prothrombin time, partial thromboplastin time, comprehensive metabolic panel, creatinine kinase test, urinalysis, and chest radiograph, was normal, but his erythrocyte sedimentation rate (ESR) was mildly elevated at 22 mm/h (reference range, 0–20 mm/h). The patient was discharged on acetaminophen 15 mg/kg every 4 hours as needed for pain.
THE DIAGNOSIS
Based on the distinctive palpable purpura on the legs, arthralgia, upper respiratory infection, and lack of thrombocytopenia and coagulopathy, a presumptive diagnosis of Henoch-Schönlein purpura (HSP) was made.
On Day 9 of symptoms, the patient returned to his FP’s office because the arthralgia persisted in his ankles, knees, and hips. He had developed lower back pain, but the pharyngitis and upper respiratory symptoms had resolved. On physical examination, he was normotensive with a normal abdominal exam. The patient reported that it hurt to move his wrists, hands, elbows, shoulders, knees, and ankles. He also had mild swelling in his left wrist, hand, and ankle. The paraspinal muscles in the lower thoracic and lumbar back were mildly tender to palpation. A complete metabolic panel and urinalysis were normal. Dermatologic examination revealed discrete purpuric lesions ranging from 1 to 8 mm in diameter on the child’s shins, thighs, and buttocks. Urinalysis, blood urea nitrogen, and creatinine kinase were normal. His ESR remained mildly elevated at 24 mm/h. Since there was no evidence of glomerulonephritis, ibuprofen 10 mg/kg every 8 hours as needed was added for pain management.
The child was brought back to his FP on Day 18 for a scheduled follow-up visit. The parents reported that his arthralgia was improved during the day, but by the evening, his knees and ankles hurt so much that they had to carry him to the bathroom. On physical examination, he still had palpable purpura of the legs. There was no swelling, but his joints were still tender to palpation. His parents were reminded to give him ibuprofen after school to control evening pain. Over the next 2 weeks, the patient showed gradual improvement, and by Day 33 the rash and all of the associated symptoms had resolved.
DISCUSSION
Clinical presentation. HSP is an IgA immune complex vasculitis in which abnormal glycosylation of IgA creates large immune complexes that are deposited in the walls of the skin capillaries and arterioles. The primary clinical finding in HSP is a distinctive nonthrombocytopenic purpuric rash that is not associated with coagulopathy and is characterized by reddish purple macules that progress to palpable purpura with petechiae (
A preceding upper respiratory infection has been found in 37% of patients,1 and in patients with renal complications, 20% to 50% have been found to have a group A Streptococcus infection.2 Other associations include food allergies, cold exposure, insect bites, and drug allergies.
Continue to: HSP vasculitis causes...
HSP vasculitis causes abdominal pain in 50% to 75% of patients due to proximal small-bowel submucosal hemorrhage and bowel wall edema.3 In children with HSP, 20% to 55% have been shown to develop renal disease,4 which can range in severity from microscopic hematuria to nephrotic syndrome.3 To ensure prompt treatment of renal manifestations, renal function should be monitored regularly via blood pressure and urinalysis during the course of HSP and after resolution. Renal disease associated with HSP can be acute or chronic.
This case was different because our patient did not exhibit all elements of the classic tetrad of HSP, which includes the characteristic rash, abdominal pain, renal involvement, and arthralgia.
Incidence. HSP is more common in children than adults, with average annual incidence rates of 20/100,000 and 70/100,000 in children in the United States and Asia, respectively.5 While 90% of HSP cases occur in children < 10 years, the peak incidence is at 6 years of age.6 Complications from HSP are more common in adults than in children.7 Caucasian and Asian populations have a 3- to 4-times higher prevalence of HSP than black populations. The male-to-female ratio is 2 to 1.6
The diagnosis of HSP is usually made clinically, based on the distinctive rash, which typically is symmetrical, involving the buttocks, lower legs, elbows, and/or knees. HSP also can be confirmed via skin biopsy and/or direct immunofluorescence, which can identify the presence of IgA in the vessel walls.
The presence of 3 or more of the following criteria also suggests HSP: palpable purpura, bowel angina, gastrointestinal (GI) bleeding, hematuria, ≤ 20 years of age at onset, and no medications prior to presentation of symptoms (87% of cases correctly classified). Fewer than 3 of these factors favor hypersensitivity vasculitis (74% of cases correctly classified).8
Continue to: The differential diagnosis
The differential diagnosis for HSP includes polyarteritis nodosa, a vasculitis with a different characteristic rash; acute abdomen, distinguished by the absence of purpura or arthralgia; meningococcemia, in which fever and meningeal signs may occur; hypersensitivity vasculitis, which arises due to prior exposure to medications or food allergens; and thrombocytopenic purpura, which is characterized by low platelet count.9
Treatment focuses on pain management
In the absence of renal disease, HSP commonly is treated with naproxen for pain management (dosage for children < 2 years of age: 5-7 mg/kg orally every 8-12 hours; dosage for children ≥ 2 years of age, adolescents, and adults: 10-20 mg/kg/d divided into 2 doses; maximum adolescent and adult dose is 1500 mg/d for 3 days followed by a maximum of 1000 mg/d thereafter).
For patients of all ages with severe pain and those with GI effects limiting oral intake of medication, use oral prednisone (1-2 mg/kg/d [maximum dose, 60-80 mg/d]) or intravenous methylprednisolone (0.8-1.6 mg/kg/d [maximum dose, 64 mg/d). Glucocorticoids may then be tapered slowly over 4 to 8 weeks to avoid rebound since they help with inflammation but do not shorten the course of disease. Steroids can ease GI and joint symptoms in HSP but will not improve the rash.
THE TAKEAWAY
The classic tetrad of HSP includes the characteristic rash, abdominal pain, renal involvement, and arthralgia. Diagnosis usually is made clinically, but skin biopsy and direct immunofluorescence can confirm small vessel vasculitis with IgA deposits. More severe manifestations of HSP such as renal disease, hemorrhage, severe anemia, signs of intestinal obstruction, or peritonitis require rapid subspecialty referral.
CORRESPONDENCE
Rachel Bramson, MD, Department of Primary Care, Baylor Scott and White Health, University Clinic, 1700 University Drive, College Station, TX 77840; Rachel.Bramson@BSWHealth.org
1. Rigante D, Castellazzi L, Bosco A, et al. Is there a crossroad between infections, genetics, and Henoch-Schönlein purpura? Autoimmun Rev. 2013;12:1016-1021.
2. LaConti JJ, Donet JA, Cho-Vega JH, et al. Henoch-Schönlein Purpura with adalimumab therapy for ulcerative colitis: a case report and review of the literature [published online July 27, 2016]. Case Rep Rheumatol. 2016;2016:2812980.
3. Trnka P. Henoch-Schönlein purpura in children. J Paediatr Child Health. 2013;49:995-1003.
4. Audemard-Verger A, Pillebout E, Guillevin L, et al. IgA vasculitis (Henoch-Shönlein purpura) in adults: diagnostic and therapeutic aspects. Autoimmun Rev. 2015;14:579-585.
5. Chen J, Mao J. Henoch-Schönlein purpura nephritis in children: incidence, pathogenesis and management. World J Pediatr. 2015;11:29-34.
6. Michel B, Hunder G, Bloch D, et al. Hypersensitivity vasculitis and Henoch-Schönlein purpura: a comparison between the 2 disorders. J Rheumatol. 1992;19:721-728.
7. Reamy BV, Williams PM, Lindsay TJ. Henoch-Schönlein purpura. Am Fam Physician. 2009;80:697-704.
8. Yang YH, Yu HH, Chiang BL. The diagnosis and classification of Henoch-Schönlein purpura: an updated review. Autoimmun Rev. 2014;13:355-358.
9. Floege J, Feehally J. Treatment of IgA nephropathy and Henoch-Schönlein nephritis. Nat Rev Nephrol. 2013;9:320-327.
1. Rigante D, Castellazzi L, Bosco A, et al. Is there a crossroad between infections, genetics, and Henoch-Schönlein purpura? Autoimmun Rev. 2013;12:1016-1021.
2. LaConti JJ, Donet JA, Cho-Vega JH, et al. Henoch-Schönlein Purpura with adalimumab therapy for ulcerative colitis: a case report and review of the literature [published online July 27, 2016]. Case Rep Rheumatol. 2016;2016:2812980.
3. Trnka P. Henoch-Schönlein purpura in children. J Paediatr Child Health. 2013;49:995-1003.
4. Audemard-Verger A, Pillebout E, Guillevin L, et al. IgA vasculitis (Henoch-Shönlein purpura) in adults: diagnostic and therapeutic aspects. Autoimmun Rev. 2015;14:579-585.
5. Chen J, Mao J. Henoch-Schönlein purpura nephritis in children: incidence, pathogenesis and management. World J Pediatr. 2015;11:29-34.
6. Michel B, Hunder G, Bloch D, et al. Hypersensitivity vasculitis and Henoch-Schönlein purpura: a comparison between the 2 disorders. J Rheumatol. 1992;19:721-728.
7. Reamy BV, Williams PM, Lindsay TJ. Henoch-Schönlein purpura. Am Fam Physician. 2009;80:697-704.
8. Yang YH, Yu HH, Chiang BL. The diagnosis and classification of Henoch-Schönlein purpura: an updated review. Autoimmun Rev. 2014;13:355-358.
9. Floege J, Feehally J. Treatment of IgA nephropathy and Henoch-Schönlein nephritis. Nat Rev Nephrol. 2013;9:320-327.
Antidepressant Tx for anxiety disorders: How long?
ILLUSTRATIVE CASE
A 42-year-old woman with generalized anxiety disorder and panic attacks has been treated with sertraline 100 mg/d for the past 8 months. She has also engaged in cognitive behavioral therapy (CBT) for 6 months. Her Generalized Anxiety Disorder-7 score has decreased from 19 prior to treatment to 5 at present. Now she would like to stop her antidepressant medication because she feels better. Would you recommend that she discontinue her medication at this point?
Anxiety disorders are common, often chronic, and can cause significant morbidity and impairment.2,3 First-line treatments for anxiety disorders include CBT and antidepressants, particularly selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors.4-6
There is limited evidence regarding duration of antidepressant therapy for anxiety disorders. Previous studies have shown a high risk of relapse after discontinuation of antidepressants.6 A review of current practice patterns regarding pharmacologic treatment of depression and anxiety indicates an uptick in longer term antidepressant use for up to 2 years.7 However, long-term studies to guide treatment decisions are lacking.
STUDY SUMMARY
Clear benefit of continuing treatment up to 1 year
This systematic review and meta-analysis evaluated studies that looked at relapse rates and time to relapse in patients treated for anxiety disorders.1 The authors used PubMed, Cochrane, and Embase to identify studies involving patients treated for a variety of disorders, including generalized anxiety disorder (GAD), posttraumatic stress disorder (PTSD), panic disorder (PD), obsessive-compulsive disorder (OCD), and social phobia. Eligible studies enrolled patients with anxiety disorders who had a positive response to an antidepressant and then randomized them in a double-blind fashion to either discontinuation of antidepressants and starting placebo (stopping group) or continuation of antidepressants (continuation group) for a duration of 8 to 52 weeks. The primary outcomes were relapse rate and time to relapse.
Twenty-eight studies met the inclusion criteria for the meta-analysis, with a total of 5233 patients (2625 patients in the antidepressant group and 2608 patients in the placebo group). A breakdown of the trials by indiication included OCD (7), PD (6), GAD (6), social phobia (5), and PTSD (4). The authors graded the overall risk of bias to be low but noted that attrition bias was present in most studies.
Results. Relapse was more likely in the stopping group (odds ratio [OR] = 3.11; 95% confidence interval [CI], 2.48-3.89; n = 28 studies). Heterogeneity for relapse rate was low (I2 = 8.07%). Subgroup analyses by type of antidepressant, mode of discontinuation, and exclusion of patient comorbidities yielded similar results. Relapse prevalence was 16.4% in the antidepressant group and 36.4% in the stopping group. Additionally, time to relapse was shorter when antidepressants were discontinued (hazard ratio [HR] = 3.63; 95% CI, 2.58-5.10; n = 11 studies). Again, the heterogeneity for relapse rate was low (I2 = 0%). The original publications did not consistently report medication tolerability or withdrawal symptoms, preventing analysis of these. Dropout rates were higher in the stopping group (OR = 1.31; 95% CI, 1.06-1.63; n = 27 studies).
WHAT’S NEW
No more guessing about how long to treat
Previously, there was limited evidence to guide decisions about the duration of antidepressant treatment for anxiety disorders. This study provides evidence that stopping antidepressant treatment before 1 year increases the risk of relapse.
Continue to: CAVEATS
CAVEATS
Potential bias … bias … and more bias
While the authors used standard and appropriate methodologies for this type of study, some significant threats to validity remained. All but 2 studies in the analysis were industry funded. Publication bias is another potential issue, even though the authors identified and included 6 unpublished studies, 4 of which had negative results.
Additionally, the authors graded 11 of 28 trials as having a high likelihood of selective reporting bias, meaning that important portions of the original studies’ results may not have been published. Most studies were at high risk for attrition bias, resulting in loss of information when patients dropped out of the study. While this happened more often in the stopping groups, it is still possible that there are unidentified harms or unexpected outcomes in the medication groups.
While PTSD and OCD are no longer considered anxiety disorders, subgroup analyses found no difference in relapse rates between these diagnoses and the others included in the studies. Finally, treatment duration longer than 52 weeks has not been studied, so the optimal treatment duration is unknown.
CHALLENGES TO IMPLEMENTATION
Patients may resist continuing treatment once symptoms abate
Some patients may want to discontinue antidepressant treatment if their anxiety symptoms improve prior to 1 year. It may be difficult to convince them that continuing treatment will prevent relapse of their condition. Providing patients with information about the increased relapse rate with stopping medication early (with an estimated number needed to treat of 5) may help patients make a more informed decision.
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Batelaan NM, Bosman RC, Muntingh A, et al. Risk of relapse after antidepressant discontinuation in anxiety disorders, obsessive-compulsive disorder, and post-traumatic stress disorder: systematic review and meta-analysis of relapse prevention trials. BMJ. 2017;358:j3927. Erratum in: BMJ. 2017;358:j4461.
2. National Institute of Mental Health. Prevalence of any anxiety disorder among adults. https://www.nimh.nih.gov/health/statistics/any-anxiety-disorder.shtml#part_155094. Updated November 2017. Accessed July 11, 2019.
3. Kessler RC, Petukhova M, Sampson NA, et al. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res. 2012;21:169-184.
4. Bandelow B, Sher L, Bunevicius R, et al. Guidelines for the pharmacological treatment of anxiety disorders, obsessive-compulsive disorder and posttraumatic stress disorder in primary care. Int J Psychiatry Clin Pract. 2012;16:77-84.
5. Kaczkurkin AN, Foa EB. Cognitive-behavioral therapy for anxiety disorders: an update on the empirical evidence. Dialogues Clin Neurosci. 2015;17:337-346.
6. Donovan MR, Glue P, Kolluri S, et al. Comparative efficacy of antidepressants in preventing relapse in anxiety disorders—a meta-analysis. J Affect Disord. 2010;123:9-16.
7. Mojtabai R, Olfson M. National trends in long-term use of antidepressant medications: results from the U.S. National Health and Nutrition Examination Survey. J Clin Psychiatry. 2014;75:169-177.
ILLUSTRATIVE CASE
A 42-year-old woman with generalized anxiety disorder and panic attacks has been treated with sertraline 100 mg/d for the past 8 months. She has also engaged in cognitive behavioral therapy (CBT) for 6 months. Her Generalized Anxiety Disorder-7 score has decreased from 19 prior to treatment to 5 at present. Now she would like to stop her antidepressant medication because she feels better. Would you recommend that she discontinue her medication at this point?
Anxiety disorders are common, often chronic, and can cause significant morbidity and impairment.2,3 First-line treatments for anxiety disorders include CBT and antidepressants, particularly selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors.4-6
There is limited evidence regarding duration of antidepressant therapy for anxiety disorders. Previous studies have shown a high risk of relapse after discontinuation of antidepressants.6 A review of current practice patterns regarding pharmacologic treatment of depression and anxiety indicates an uptick in longer term antidepressant use for up to 2 years.7 However, long-term studies to guide treatment decisions are lacking.
STUDY SUMMARY
Clear benefit of continuing treatment up to 1 year
This systematic review and meta-analysis evaluated studies that looked at relapse rates and time to relapse in patients treated for anxiety disorders.1 The authors used PubMed, Cochrane, and Embase to identify studies involving patients treated for a variety of disorders, including generalized anxiety disorder (GAD), posttraumatic stress disorder (PTSD), panic disorder (PD), obsessive-compulsive disorder (OCD), and social phobia. Eligible studies enrolled patients with anxiety disorders who had a positive response to an antidepressant and then randomized them in a double-blind fashion to either discontinuation of antidepressants and starting placebo (stopping group) or continuation of antidepressants (continuation group) for a duration of 8 to 52 weeks. The primary outcomes were relapse rate and time to relapse.
Twenty-eight studies met the inclusion criteria for the meta-analysis, with a total of 5233 patients (2625 patients in the antidepressant group and 2608 patients in the placebo group). A breakdown of the trials by indiication included OCD (7), PD (6), GAD (6), social phobia (5), and PTSD (4). The authors graded the overall risk of bias to be low but noted that attrition bias was present in most studies.
Results. Relapse was more likely in the stopping group (odds ratio [OR] = 3.11; 95% confidence interval [CI], 2.48-3.89; n = 28 studies). Heterogeneity for relapse rate was low (I2 = 8.07%). Subgroup analyses by type of antidepressant, mode of discontinuation, and exclusion of patient comorbidities yielded similar results. Relapse prevalence was 16.4% in the antidepressant group and 36.4% in the stopping group. Additionally, time to relapse was shorter when antidepressants were discontinued (hazard ratio [HR] = 3.63; 95% CI, 2.58-5.10; n = 11 studies). Again, the heterogeneity for relapse rate was low (I2 = 0%). The original publications did not consistently report medication tolerability or withdrawal symptoms, preventing analysis of these. Dropout rates were higher in the stopping group (OR = 1.31; 95% CI, 1.06-1.63; n = 27 studies).
WHAT’S NEW
No more guessing about how long to treat
Previously, there was limited evidence to guide decisions about the duration of antidepressant treatment for anxiety disorders. This study provides evidence that stopping antidepressant treatment before 1 year increases the risk of relapse.
Continue to: CAVEATS
CAVEATS
Potential bias … bias … and more bias
While the authors used standard and appropriate methodologies for this type of study, some significant threats to validity remained. All but 2 studies in the analysis were industry funded. Publication bias is another potential issue, even though the authors identified and included 6 unpublished studies, 4 of which had negative results.
Additionally, the authors graded 11 of 28 trials as having a high likelihood of selective reporting bias, meaning that important portions of the original studies’ results may not have been published. Most studies were at high risk for attrition bias, resulting in loss of information when patients dropped out of the study. While this happened more often in the stopping groups, it is still possible that there are unidentified harms or unexpected outcomes in the medication groups.
While PTSD and OCD are no longer considered anxiety disorders, subgroup analyses found no difference in relapse rates between these diagnoses and the others included in the studies. Finally, treatment duration longer than 52 weeks has not been studied, so the optimal treatment duration is unknown.
CHALLENGES TO IMPLEMENTATION
Patients may resist continuing treatment once symptoms abate
Some patients may want to discontinue antidepressant treatment if their anxiety symptoms improve prior to 1 year. It may be difficult to convince them that continuing treatment will prevent relapse of their condition. Providing patients with information about the increased relapse rate with stopping medication early (with an estimated number needed to treat of 5) may help patients make a more informed decision.
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 42-year-old woman with generalized anxiety disorder and panic attacks has been treated with sertraline 100 mg/d for the past 8 months. She has also engaged in cognitive behavioral therapy (CBT) for 6 months. Her Generalized Anxiety Disorder-7 score has decreased from 19 prior to treatment to 5 at present. Now she would like to stop her antidepressant medication because she feels better. Would you recommend that she discontinue her medication at this point?
Anxiety disorders are common, often chronic, and can cause significant morbidity and impairment.2,3 First-line treatments for anxiety disorders include CBT and antidepressants, particularly selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors.4-6
There is limited evidence regarding duration of antidepressant therapy for anxiety disorders. Previous studies have shown a high risk of relapse after discontinuation of antidepressants.6 A review of current practice patterns regarding pharmacologic treatment of depression and anxiety indicates an uptick in longer term antidepressant use for up to 2 years.7 However, long-term studies to guide treatment decisions are lacking.
STUDY SUMMARY
Clear benefit of continuing treatment up to 1 year
This systematic review and meta-analysis evaluated studies that looked at relapse rates and time to relapse in patients treated for anxiety disorders.1 The authors used PubMed, Cochrane, and Embase to identify studies involving patients treated for a variety of disorders, including generalized anxiety disorder (GAD), posttraumatic stress disorder (PTSD), panic disorder (PD), obsessive-compulsive disorder (OCD), and social phobia. Eligible studies enrolled patients with anxiety disorders who had a positive response to an antidepressant and then randomized them in a double-blind fashion to either discontinuation of antidepressants and starting placebo (stopping group) or continuation of antidepressants (continuation group) for a duration of 8 to 52 weeks. The primary outcomes were relapse rate and time to relapse.
Twenty-eight studies met the inclusion criteria for the meta-analysis, with a total of 5233 patients (2625 patients in the antidepressant group and 2608 patients in the placebo group). A breakdown of the trials by indiication included OCD (7), PD (6), GAD (6), social phobia (5), and PTSD (4). The authors graded the overall risk of bias to be low but noted that attrition bias was present in most studies.
Results. Relapse was more likely in the stopping group (odds ratio [OR] = 3.11; 95% confidence interval [CI], 2.48-3.89; n = 28 studies). Heterogeneity for relapse rate was low (I2 = 8.07%). Subgroup analyses by type of antidepressant, mode of discontinuation, and exclusion of patient comorbidities yielded similar results. Relapse prevalence was 16.4% in the antidepressant group and 36.4% in the stopping group. Additionally, time to relapse was shorter when antidepressants were discontinued (hazard ratio [HR] = 3.63; 95% CI, 2.58-5.10; n = 11 studies). Again, the heterogeneity for relapse rate was low (I2 = 0%). The original publications did not consistently report medication tolerability or withdrawal symptoms, preventing analysis of these. Dropout rates were higher in the stopping group (OR = 1.31; 95% CI, 1.06-1.63; n = 27 studies).
WHAT’S NEW
No more guessing about how long to treat
Previously, there was limited evidence to guide decisions about the duration of antidepressant treatment for anxiety disorders. This study provides evidence that stopping antidepressant treatment before 1 year increases the risk of relapse.
Continue to: CAVEATS
CAVEATS
Potential bias … bias … and more bias
While the authors used standard and appropriate methodologies for this type of study, some significant threats to validity remained. All but 2 studies in the analysis were industry funded. Publication bias is another potential issue, even though the authors identified and included 6 unpublished studies, 4 of which had negative results.
Additionally, the authors graded 11 of 28 trials as having a high likelihood of selective reporting bias, meaning that important portions of the original studies’ results may not have been published. Most studies were at high risk for attrition bias, resulting in loss of information when patients dropped out of the study. While this happened more often in the stopping groups, it is still possible that there are unidentified harms or unexpected outcomes in the medication groups.
While PTSD and OCD are no longer considered anxiety disorders, subgroup analyses found no difference in relapse rates between these diagnoses and the others included in the studies. Finally, treatment duration longer than 52 weeks has not been studied, so the optimal treatment duration is unknown.
CHALLENGES TO IMPLEMENTATION
Patients may resist continuing treatment once symptoms abate
Some patients may want to discontinue antidepressant treatment if their anxiety symptoms improve prior to 1 year. It may be difficult to convince them that continuing treatment will prevent relapse of their condition. Providing patients with information about the increased relapse rate with stopping medication early (with an estimated number needed to treat of 5) may help patients make a more informed decision.
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Batelaan NM, Bosman RC, Muntingh A, et al. Risk of relapse after antidepressant discontinuation in anxiety disorders, obsessive-compulsive disorder, and post-traumatic stress disorder: systematic review and meta-analysis of relapse prevention trials. BMJ. 2017;358:j3927. Erratum in: BMJ. 2017;358:j4461.
2. National Institute of Mental Health. Prevalence of any anxiety disorder among adults. https://www.nimh.nih.gov/health/statistics/any-anxiety-disorder.shtml#part_155094. Updated November 2017. Accessed July 11, 2019.
3. Kessler RC, Petukhova M, Sampson NA, et al. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res. 2012;21:169-184.
4. Bandelow B, Sher L, Bunevicius R, et al. Guidelines for the pharmacological treatment of anxiety disorders, obsessive-compulsive disorder and posttraumatic stress disorder in primary care. Int J Psychiatry Clin Pract. 2012;16:77-84.
5. Kaczkurkin AN, Foa EB. Cognitive-behavioral therapy for anxiety disorders: an update on the empirical evidence. Dialogues Clin Neurosci. 2015;17:337-346.
6. Donovan MR, Glue P, Kolluri S, et al. Comparative efficacy of antidepressants in preventing relapse in anxiety disorders—a meta-analysis. J Affect Disord. 2010;123:9-16.
7. Mojtabai R, Olfson M. National trends in long-term use of antidepressant medications: results from the U.S. National Health and Nutrition Examination Survey. J Clin Psychiatry. 2014;75:169-177.
1. Batelaan NM, Bosman RC, Muntingh A, et al. Risk of relapse after antidepressant discontinuation in anxiety disorders, obsessive-compulsive disorder, and post-traumatic stress disorder: systematic review and meta-analysis of relapse prevention trials. BMJ. 2017;358:j3927. Erratum in: BMJ. 2017;358:j4461.
2. National Institute of Mental Health. Prevalence of any anxiety disorder among adults. https://www.nimh.nih.gov/health/statistics/any-anxiety-disorder.shtml#part_155094. Updated November 2017. Accessed July 11, 2019.
3. Kessler RC, Petukhova M, Sampson NA, et al. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res. 2012;21:169-184.
4. Bandelow B, Sher L, Bunevicius R, et al. Guidelines for the pharmacological treatment of anxiety disorders, obsessive-compulsive disorder and posttraumatic stress disorder in primary care. Int J Psychiatry Clin Pract. 2012;16:77-84.
5. Kaczkurkin AN, Foa EB. Cognitive-behavioral therapy for anxiety disorders: an update on the empirical evidence. Dialogues Clin Neurosci. 2015;17:337-346.
6. Donovan MR, Glue P, Kolluri S, et al. Comparative efficacy of antidepressants in preventing relapse in anxiety disorders—a meta-analysis. J Affect Disord. 2010;123:9-16.
7. Mojtabai R, Olfson M. National trends in long-term use of antidepressant medications: results from the U.S. National Health and Nutrition Examination Survey. J Clin Psychiatry. 2014;75:169-177.
PRACTICE CHANGER
Keep patients on antidepressant therapy for anxiety disorders for a year or longer before considering a taper.
STRENGTH OF RECOMMENDATION
A: Based on a systematic review/meta-analysis of several good quality randomized controlled trials.1
Batelaan NM, Bosman RC, Muntingh A, et al. Risk of relapse after antidepressant discontinuation in anxiety disorders, obsessive-compulsive disorder, and post-traumatic stress disorder: systematic review and meta-analysis of relapse prevention trials. BMJ. 2017;358:j3927. Erratum in: BMJ. 2017;358:j4461.
Caring for patients with co-occurring mental health & substance use disorders
THE CASE
Janice J* visits her family physician with complaints of chest pain, shortness of breath, and heart palpitations that are usually worse at night. Her medical history is significant for deep vein thrombosis secondary to an underlying hypercoagulability condition (rheumatoid arthritis) diagnosed 2 months earlier. She also has a history of opioid use disorder and has been on buprenorphine/naloxone therapy for 3 years. Her family medical history is unremarkable. She works full-time and lives with her 8-year-old son. On physical exam, she appears anxious; her cardiac and pulmonary exams are normal. A completed workup rules out cardiac or pulmonary problems.
- What is your diagnosis?
- How would you treat this patient?
* The patient’s name has been changed to protect her identity.
CO-OCCURRING DISORDERS: SCOPE OF THE PROBLEM
Co-occurring disorders, previously called “dual diagnosis,” refers to the coexistence of a mental health disorder and a substance use disorder. The obsolete term, dual diagnosis, specified the presence of 2 co-occurring Axis I diagnoses or the presence of an Axis I diagnosis and an Axis II diagnosis (such as mental disability). The change in nomenclature more precisely describes the co-existing mental health and substance use disorders.
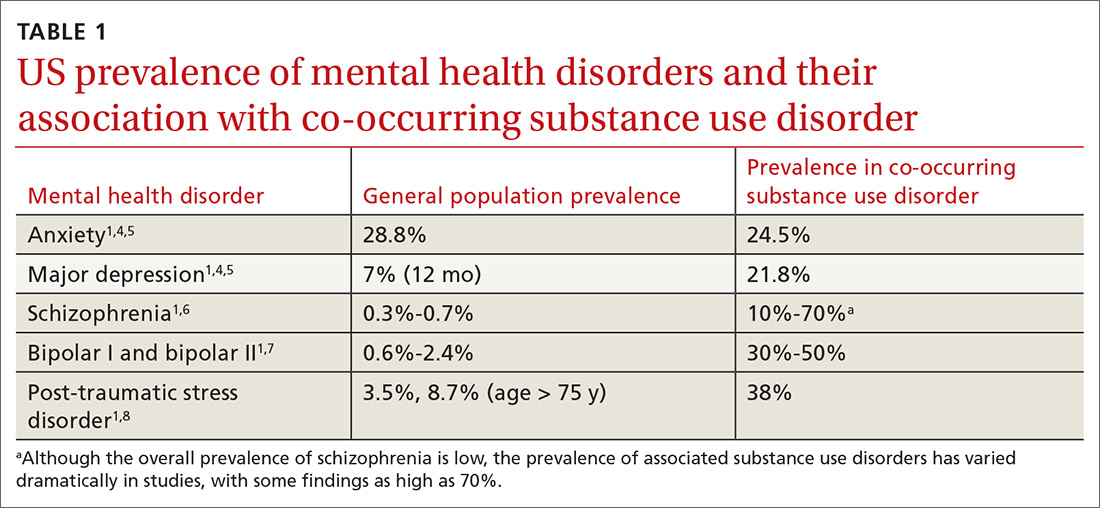
Currently the Diagnostic and Statistical Manual of Mental Disorders, 5th edition, (DSM-5) includes no diagnostic criteria for this dual condition.1 The criteria for mental health disorders and for substance use disorders comprise separate lists. Criteria for substance use disorder fall broadly into categories of “impaired [self] control, social impairment, risky behaviors, increased tolerance, and withdrawal symptoms.”1 It is estimated that 8.5 million US adults have co-occurring disorders, per the 2017 National Survey on Drug Use and Health conducted by the Substance Abuse and Mental Health Services Administration.2 Distinguishing which of the 2 conditions occurred first can be challenging. It has been suggested that the lifetime prevalence of a mental health disorder with a coexisting substance use disorder is greater than 40%3,4 (TABLE 11,4-8). For patients with schizophrenia and bipolar disorder, these numbers may be higher.
The consequences of undiagnosed and untreated co-occurring disorders include poor medication adherence, physical comorbidities (and decreased overall health), diminished self-care, increased suicide risk or aggression, increased risky sexual behavior, and possible incarceration.9
WHEN SHOULD YOU SUSPECT CO-OCCURRING DISORDERS?
Diagnosing a second condition can also be difficult when a patient’s symptoms are actually adverse effects of substances or prescribed medications. For example, a patient with worsening anxiety may also exhibit increasing blood pressure resistant to treatment. The cause of the patient’s fluctuating blood pressures may actually be the result of his or her use of alcohol to self-treat the anxiety. In addition to self-medication, other underlying factors may be at play, including genetic vulnerability, environment, and lifestyle.14 In the case we present, the patient’s conditions arose independently.
Anxiety disorders, with a lifetime risk of 28.8% in the US population,4 may be the primary mental health issue in many patients with co-occurring disorders, but this cannot be assumed in lieu of a complete workup.2,8,9,15 Substance use disorders in the general population have a past-year and lifetime prevalence of 14.6%.1,4,16,17 Because the causal and temporal association between anxiety and substance abuse is not always clear, it’s important to separate the diagnoses of the mental health and substance use disorders.
Continue to: MAKING THE DIAGNOSIS
MAKING THE DIAGNOSIS
To make an accurate diagnosis of co-occurring disorder, it is essential to take a complete history focusing on the timeline of symptoms, previous diagnoses and treatments, if any, and substance-free periods. Details gathered from these inquiries will help to separate symptoms of a primary mental health disorder from adverse effects of medication, withdrawal symptoms, or symptoms related to an underlying chronic medical condition.
Optimally, the diagnosis of a mental health disorder should be considered following a substance-free period. If this is not possible, a chart review may reveal a time when the patient did not have a substance use disorder.18
A diagnosis of substance use disorder requires that the patient manifest at least 2 of 11 behaviors listed in the DSM-5 over a 12-month period.1 The criteria focus on the amount of substance used, the time spent securing the substance, risky behaviors associated with the substance, and tolerance to the substance.
DON'T DEFER MENTAL HEALTH Tx
It is necessary to treat co-occurring disorders simultaneously. The old idea of deferring treatment of a mental health issue until the substance use disorder is resolved no longer applies.19,20 Treating substance use problems without addressing comorbid mental health issues can negatively impact treatment progress and increase risk for relapse. In a similar way, leaving substance use problems untreated is associated with nonadherence in mental health treatment, poor engagement, and dropout.21,22
Integrated services. Due to this condition’s level of clinical complexity, the optimal treatment approach is an interdisciplinary one in which integrated services are offered at a single location by a team of medical, mental health, and substance use providers (see “The case for behavioral health integration into primary care” in the June issue). An evidence-based example of such an approach is the Integrated Dual Disorder Treatment (IDDT) model—a comprehensive, integrated method of treating severe mental health disorders, including substance use disorders.21,22 IDDT combines coordinated services such as pharmacologic, psychological, educational, and social interventions to address the needs of patients and their family members. The IDDT model conceptualizes and treats co-occurring disorders within a biopsychosocial framework. Specific services may include medical detoxification, pharmacotherapy, patient and family education, behavioral and cognitive therapies, contingency management, self-help support groups, supported employment, residential/housing assistance, and case management services.23,24
Continue to: Medications for the mental health component
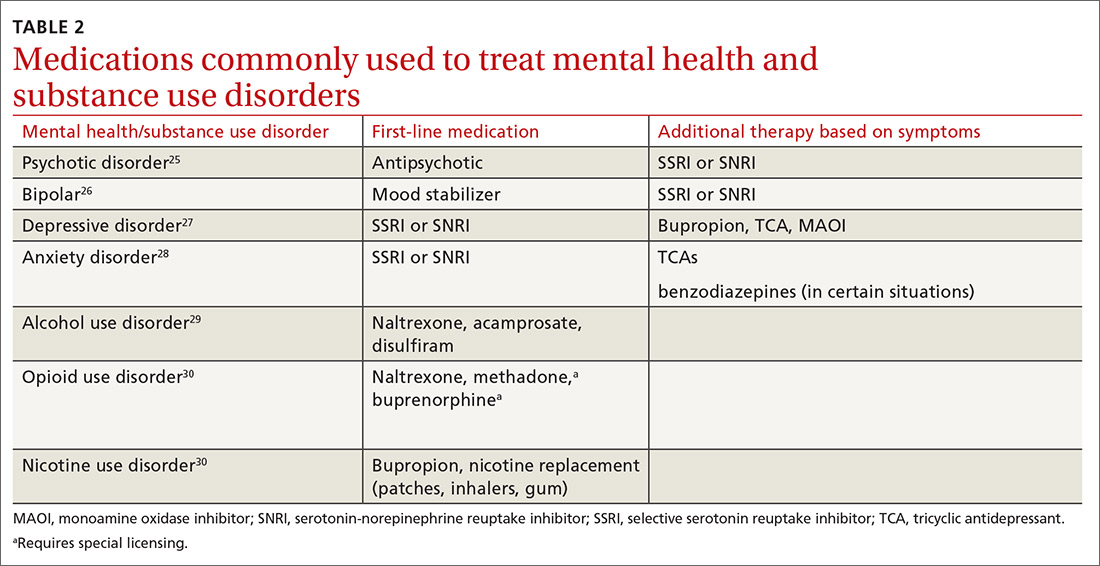
Medications for the mental health component. For patients who prefer medication treatment to cognitive behavioral therapy (CBT), or for whom CBT is unavailable, treat the mental health disorder per customary practice for the diagnosis (TABLE 225-30). For psychotic disorders, use an antipsychotic, adding a selective serotonin reuptake inhibitor (SSRI) or serotonin-norepinephrine reuptake inhibitor (SNRI) as needed depending on the presence of negative symptoms.25,31 For bipolar spectrum disorder, start a mood stabilizer32; for depressive disorders initiate an SSRI or SNRI.27 Anxiety disorders respond optimally when treated with SSRIs or SNRIs. Buspirone may be prescribed alone or as an adjunct for anxiety, and it does not cause mood-altering or withdrawal effects. Benzodiazepines in a controlled and monitored setting are an option in some antianxiety treatment plans. Consultation with a psychiatrist will help to determine the best treatment in these situations.
In all cases, treat the substance use disorder concurrently. Treatment options vary depending on the substance of choice. Although often overlooked, there can be simultaneous nicotine abuse. Oral or inhaled medications for nicotine abuse treatment are limited. The range of pharmacologic options for alcohol use disorder includes naltrexone, acamprosate, and disulfiram.29,33 Pharmacologic treatment options for opioid use disorder include naltrexone, methadone, and a combination of naloxone and buprenorphine.34
Physicians who wish to prescribe buprenorphine must qualify for and complete a certified 8 hour waiver-training course, which is then approved by the Drug Enforcement Agency (under the DATA 2000 – Drug and Alcohol Act 2000). The physician obtains the designation of a data-waived physician and is assigned a special identification number to prescribe these medications.35,36 Methadone may be provided only in a licensed methadone maintenance program. Regular and random drug urine screen requirements apply to all treatment programs.
Psychosocial and behavioral interventions are essential to the successful treatment of co-occurring disorders. Evidence-based behavioral and cognitive therapies are recommended for promoting adaptive coping skills and healthy lifestyle behaviors in co-occurring disorder populations.23,24,37-40 Motivational interviewing enhances motivation and adherence when patients demonstrate resistance or ambivalence.41,42 Mindfulness-based interventions have been shown to be effective and may be particularly beneficial for treating cravings/urges and promoting relapse prevention.37,39,40,43-46
Psychotropic medications, as with other treatment components, are most effective when used in combination with services that simultaneously address the patient’s biological, psychological, and social needs.
Continue to: The grassroots organization...
The grassroots organization National Alliance on Mental Illness (www.nami.org) recommends self-help and support groups, which include 12-step, faith-based and non-faith–based programs.20
For any treatment method to be successful, there needs to be a level of customization and individualization. Some patients may respond to medication or nonmedication treatments only, and others may need a combination of treatments.
CASE
The physician recalls a past diagnosis of anxiety and asks Ms. J if there are any new stressors or changes causing concern. The patient expresses concern about an opioid use relapse secondary to her recent diagnosis of rheumatoid arthritis, which may be life altering or limiting.
Even though she has been doing well and has been adherent to her daily buprenorphine treatment, she worries for the well-being of her family and what would happen if she cannot work, becomes incapacitated, or dies at a young age. She has never considered herself an anxious person and is surprised that anxiety could cause such pronounced physical symptoms.
The physician discusses different modalities of treatment, including counseling with an onsite psychologist, a trial of an anti-anxiety medication such as sertraline, or return office visits with the physician. They decide first to schedule an appointment with the psychologist, and Ms. J promises to find more time for self-wellness activities, such as exercise.
After 3 months of therapy, the patient decides to space out treatment to every 2 to 3 months and does not report any more episodes of chest pain or shortness of breath.
CORRESPONDENCE
Kristen Rundell, MD, Northwood-High Building, 2231 N. High Street, Suite 211, Columbus, OH 43201; kristen.rundell@osumc.edu.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: APA; 2013.
2. SAMHSA. Key substance use and mental health indicators in the United States: results from the 2017 National Survey on Drug Use and Health. 2017. https://www.samhsa.gov/data/sites/default/files/cbhsq-reports/NSDUHFFR2017/NSDUHFFR2017.htm#cooccur2. Accessed August 16, 2019.
3. Conway KP, Compton W, Stinson FS, et al. Lifetime comorbidity of DSM-IV mood and anxiety disorders and specific drug use disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2006;67:247-257.
4. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593-602.
5. Grant BF, Stinson FS, Dawson DA, et al. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2004;61:807-816.
6. Dixon L. Dual diagnosis of substance abuse in schizophrenia: prevalence and impact on outcomes. Schizophr Res. 1999;35(suppl):S93-S100.
7. Merikangas KR, Jin R, He JP, et al. Prevalence and correlates of bipolar spectrum disorder in the World Mental Health Survey Initiative. Arch Gen Psychiatry. 2011;68:241-251.
8. Cottler LB, Compton WM 3rd, Mager D, et al. Posttraumatic stress disorder among substance users from the general population. Am J Psychiatry. 1992;149:664-670.
9. Kessler RC, Angermeyer M, Anthony JC, et al. Lifetime prevalence and age-of-onset distributions of mental disorders in the World Health Organization’s World Mental Health Survey Initiative. World Psychiatry. 2007;6:168-176.
10. Burns L, Teesson M, O’Neill K. The impact of comorbid anxiety and depression on alcohol treatment outcomes. Addiction. 2005;100:787-796.
11. Magidson JF, Liu SM, Lejuez CW, et al. Comparison of the course of substance use disorders among individuals with and without generalized anxiety disorder in a nationally representative sample. J Psychiatr Res. 2012;46:659666.
12. Boschloo L, Vogelzangs N, van den Brink W, et al. Alcohol use disorders and the course of depressive and anxiety disorders. Br J Psychiatry. 2012;200:476-484.
13. Schuckit MA. Comorbidity between substance use disorders and psychiatric conditions. Addiction. 2006;101(suppl 1):76-88.
14. Buckley PF. Prevalence and consequences of the dual diagnosis of substance abuse and severe mental illness. J Clin Psychiatry. 2006;67(suppl 7):5-9.
15. Salo R, Flower K, Kielstein A, et al. Psychiatric comorbidity in methamphetamine dependence. Psychiatry Res. 2011;186:356-361.
16. Torrens M, Gilchrist G, Domingo-Salvany A. Psychiatric comorbidity in illicit drug users: substance-induced versus independent disorders. Drug Alcohol Depend. 2011;113:147-156.
17. Buckner JD, Timpano KR, Zvolensky MJ, et al. Implications of comorbid alcohol dependence among individuals with social anxiety disorder. Depress Anxiety. 2008;25:1028-1037.
18. Kushner MG, Abrams K, Borchardt C. The relationship between anxiety disorders and alcohol use disorders: a review of major perspectives and findings. Clin Psychol Rev. 2000;20:149-171.
19. McHugh RK. Treatment of co-occurring anxiety disorders and substance use disorders. Harv Rev Psychiatry. 2015;23:99-111.
20. National Alliance on Mental Illness. Dual diagnosis. NAMI Web site. www.nami.org/Learn-More/Mental-Health-Conditions/related-conditions/dual-diagnosis. Reviewed August 2017. Accessed July 23, 2019.
21. SAMSHA. Substance Abuse Treatment for Persons with Co-Occurring Disorders. Treatment Improvement Protocol (TIP) series No. 42. HHS Publication No. (SMA) 13-3992. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2013.
22. SAMHSA. Treatment of co-occurring disorders. In: Medication-Assisted Treatment for Opioid Addiction in Opioid Treatment Programs. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2005.
23. Drake RE, Mueser KT, Brunette MF, et al. A review of treatments for people with severe mental illnesses and co-occurring substance use disorders. Psychiatr Rehabil J. 2004;27:360-374.
24. Kola LA, Kruszynski R. Adapting the integrated dual-disorder treatment model for addiction services. Alcohol Treat Q. 2010;28:437-450.
25. American Psychiatric Association. Practice guideline for the treatment of patients with schizophrenia, 2nd ed. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/schizophrenia.pdf. Published 2010. Accessed August 2, 2019.
26. American Psychiatric Association. Practice guideline for the treatment of patients with bipolar disorder, 2nd ed. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/bipolar.pdf. Published 2010. Accessed August 2, 2019.
27. American Psychiatric Association. Practice Guideline for the Treatment of Patients with Major Depressive Disorder. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf. Published October 2010. Accessed July 23, 2019.
28. American Psychiatric Association. Practice guideline for the treatment of patients with panic disorder, 2nd ed. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/panicdisorder.pdf. Published January 2009. Accessed August 2, 2019.
29. American Psychiatric Association. Practice guideline for the pharmacological treatment of patients with alcohol use disorder. https://psychiatryonline.org/doi/pdf/10.1176/appi.books.9781615371969. Accessed August 2, 2019.
30. American Psychiatric Association. Practice guideline for the treatment of patients with substance use disorders, 2nd ed. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/substanceuse.pdf. Published 2010. Accessed August 2, 2019.
31. Petrakis IL, Nich C, Ralevski E. Psychotic spectrum disorders and alcohol abuse: a review of pharmacotherapeutic strategies and a report on the effectiveness of naltrexone and disulfiram. Schizophr Bull. 2006;32:644-654.
32. McIntyre RS, Yoon J. Efficacy of antimanic treatments in mixed states. Bipolar Disord. 2012;14(suppl 2):22-36.
33. Volpicelli JR, Alterman AI, Hayashida M, et al. Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry. 1992;49:876-880.
34. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomized controlled trial. Lancet. 2018;391:309-318.
35. US Department of Justice. DEA requirements for DATA waived physicians (DWPs). Drug Enforcement Administration, Diversion Control Division Web site. www.deadiversion.usdoj.gov/pubs/docs/dwp_buprenorphine.htm. Accessed August 2, 2019.
36. SAMHSA. Buprenorphine waiver management. https://www.samhsa.gov/medication-assisted-treatment/buprenorphine-waiver-management. SAMHSA Web site. Updated May 7, 2019. Accessed August 2, 2019.
37. Bowen S, Chawla N, Witkiewitz K. Mindfulness-based relapse prevention for addictive behaviors. In: Baer RA, ed. Mindfulness-Based Treatment Approaches: A Clinician’s Guide to Evidence Base and Applications. London, UK: Elsevier; 2014.
38. Dixon L, McFarlane W, Lefley H, et al. Evidence-based practices for services to families of people with psychiatric disabilities. Psychiatr Serv. 2001;52:903-910.
39. Hayes SC, Levin M, Plumb-Vilardaga J, et al. Acceptance and commitment therapy and contextual behavioral science: examining the progress of a distinctive model of behavioral and cognitive therapy. Behav Ther. 2013;44:180-198.
40. Osilla KC, Hepner KA, Muñoz RF, et al. Developing an integrated treatment for substance use and depression using cognitive behavioral therapy. J Subst Abuse Treat. 2009;37:412-420.
41. Martino S, Carroll K, Kostas D, et al. Dual diagnosis motivational interviewing: a modification of motivational interviewing for substance-abusing patients with psychotic disorders. J Subst Abuse Treat. 2002;23:297-308.
42. Rollnick S, Miller WR. What is motivational interviewing? Behav Cogn Psychother. 1995;23:325-334.
43. Garland EL. Disrupting the downward spiral of chronic pain and opioid addiction with mindfulness-oriented recovery enhancement: a review of clinical outcomes and neurocognitive targets. J Pain Palliat Care Pharmacother. 2014;28:122-129.
44. Garland EL, Manusov EG, Froeliger B, et al. Mindfulness-oriented recovery enhancement for chronic pain and prescription opioid misuse: results from an early-stage randomized controlled trial. J Consult Clin Psychol. 2014;82:448-459.
45. Marlatt GA, Donovan DM. Relapse Prevention: Maintenance Strategies in the Treatment of Addictive Behaviors, 2nd ed. New York, NY: Guilford Press; 2007.
46. Zgierska A, Rabago D, Chawla N, et al. Mindfulness meditation for substance use disorders: a systematic review. Subst Abus. 2009;30:266-294.
THE CASE
Janice J* visits her family physician with complaints of chest pain, shortness of breath, and heart palpitations that are usually worse at night. Her medical history is significant for deep vein thrombosis secondary to an underlying hypercoagulability condition (rheumatoid arthritis) diagnosed 2 months earlier. She also has a history of opioid use disorder and has been on buprenorphine/naloxone therapy for 3 years. Her family medical history is unremarkable. She works full-time and lives with her 8-year-old son. On physical exam, she appears anxious; her cardiac and pulmonary exams are normal. A completed workup rules out cardiac or pulmonary problems.
- What is your diagnosis?
- How would you treat this patient?
* The patient’s name has been changed to protect her identity.
CO-OCCURRING DISORDERS: SCOPE OF THE PROBLEM
Co-occurring disorders, previously called “dual diagnosis,” refers to the coexistence of a mental health disorder and a substance use disorder. The obsolete term, dual diagnosis, specified the presence of 2 co-occurring Axis I diagnoses or the presence of an Axis I diagnosis and an Axis II diagnosis (such as mental disability). The change in nomenclature more precisely describes the co-existing mental health and substance use disorders.
Currently the Diagnostic and Statistical Manual of Mental Disorders, 5th edition, (DSM-5) includes no diagnostic criteria for this dual condition.1 The criteria for mental health disorders and for substance use disorders comprise separate lists. Criteria for substance use disorder fall broadly into categories of “impaired [self] control, social impairment, risky behaviors, increased tolerance, and withdrawal symptoms.”1 It is estimated that 8.5 million US adults have co-occurring disorders, per the 2017 National Survey on Drug Use and Health conducted by the Substance Abuse and Mental Health Services Administration.2 Distinguishing which of the 2 conditions occurred first can be challenging. It has been suggested that the lifetime prevalence of a mental health disorder with a coexisting substance use disorder is greater than 40%3,4 (TABLE 11,4-8). For patients with schizophrenia and bipolar disorder, these numbers may be higher.
The consequences of undiagnosed and untreated co-occurring disorders include poor medication adherence, physical comorbidities (and decreased overall health), diminished self-care, increased suicide risk or aggression, increased risky sexual behavior, and possible incarceration.9
WHEN SHOULD YOU SUSPECT CO-OCCURRING DISORDERS?
Diagnosing a second condition can also be difficult when a patient’s symptoms are actually adverse effects of substances or prescribed medications. For example, a patient with worsening anxiety may also exhibit increasing blood pressure resistant to treatment. The cause of the patient’s fluctuating blood pressures may actually be the result of his or her use of alcohol to self-treat the anxiety. In addition to self-medication, other underlying factors may be at play, including genetic vulnerability, environment, and lifestyle.14 In the case we present, the patient’s conditions arose independently.
Anxiety disorders, with a lifetime risk of 28.8% in the US population,4 may be the primary mental health issue in many patients with co-occurring disorders, but this cannot be assumed in lieu of a complete workup.2,8,9,15 Substance use disorders in the general population have a past-year and lifetime prevalence of 14.6%.1,4,16,17 Because the causal and temporal association between anxiety and substance abuse is not always clear, it’s important to separate the diagnoses of the mental health and substance use disorders.
Continue to: MAKING THE DIAGNOSIS
MAKING THE DIAGNOSIS
To make an accurate diagnosis of co-occurring disorder, it is essential to take a complete history focusing on the timeline of symptoms, previous diagnoses and treatments, if any, and substance-free periods. Details gathered from these inquiries will help to separate symptoms of a primary mental health disorder from adverse effects of medication, withdrawal symptoms, or symptoms related to an underlying chronic medical condition.
Optimally, the diagnosis of a mental health disorder should be considered following a substance-free period. If this is not possible, a chart review may reveal a time when the patient did not have a substance use disorder.18
A diagnosis of substance use disorder requires that the patient manifest at least 2 of 11 behaviors listed in the DSM-5 over a 12-month period.1 The criteria focus on the amount of substance used, the time spent securing the substance, risky behaviors associated with the substance, and tolerance to the substance.
DON'T DEFER MENTAL HEALTH Tx
It is necessary to treat co-occurring disorders simultaneously. The old idea of deferring treatment of a mental health issue until the substance use disorder is resolved no longer applies.19,20 Treating substance use problems without addressing comorbid mental health issues can negatively impact treatment progress and increase risk for relapse. In a similar way, leaving substance use problems untreated is associated with nonadherence in mental health treatment, poor engagement, and dropout.21,22
Integrated services. Due to this condition’s level of clinical complexity, the optimal treatment approach is an interdisciplinary one in which integrated services are offered at a single location by a team of medical, mental health, and substance use providers (see “The case for behavioral health integration into primary care” in the June issue). An evidence-based example of such an approach is the Integrated Dual Disorder Treatment (IDDT) model—a comprehensive, integrated method of treating severe mental health disorders, including substance use disorders.21,22 IDDT combines coordinated services such as pharmacologic, psychological, educational, and social interventions to address the needs of patients and their family members. The IDDT model conceptualizes and treats co-occurring disorders within a biopsychosocial framework. Specific services may include medical detoxification, pharmacotherapy, patient and family education, behavioral and cognitive therapies, contingency management, self-help support groups, supported employment, residential/housing assistance, and case management services.23,24
Continue to: Medications for the mental health component
Medications for the mental health component. For patients who prefer medication treatment to cognitive behavioral therapy (CBT), or for whom CBT is unavailable, treat the mental health disorder per customary practice for the diagnosis (TABLE 225-30). For psychotic disorders, use an antipsychotic, adding a selective serotonin reuptake inhibitor (SSRI) or serotonin-norepinephrine reuptake inhibitor (SNRI) as needed depending on the presence of negative symptoms.25,31 For bipolar spectrum disorder, start a mood stabilizer32; for depressive disorders initiate an SSRI or SNRI.27 Anxiety disorders respond optimally when treated with SSRIs or SNRIs. Buspirone may be prescribed alone or as an adjunct for anxiety, and it does not cause mood-altering or withdrawal effects. Benzodiazepines in a controlled and monitored setting are an option in some antianxiety treatment plans. Consultation with a psychiatrist will help to determine the best treatment in these situations.
In all cases, treat the substance use disorder concurrently. Treatment options vary depending on the substance of choice. Although often overlooked, there can be simultaneous nicotine abuse. Oral or inhaled medications for nicotine abuse treatment are limited. The range of pharmacologic options for alcohol use disorder includes naltrexone, acamprosate, and disulfiram.29,33 Pharmacologic treatment options for opioid use disorder include naltrexone, methadone, and a combination of naloxone and buprenorphine.34
Physicians who wish to prescribe buprenorphine must qualify for and complete a certified 8 hour waiver-training course, which is then approved by the Drug Enforcement Agency (under the DATA 2000 – Drug and Alcohol Act 2000). The physician obtains the designation of a data-waived physician and is assigned a special identification number to prescribe these medications.35,36 Methadone may be provided only in a licensed methadone maintenance program. Regular and random drug urine screen requirements apply to all treatment programs.
Psychosocial and behavioral interventions are essential to the successful treatment of co-occurring disorders. Evidence-based behavioral and cognitive therapies are recommended for promoting adaptive coping skills and healthy lifestyle behaviors in co-occurring disorder populations.23,24,37-40 Motivational interviewing enhances motivation and adherence when patients demonstrate resistance or ambivalence.41,42 Mindfulness-based interventions have been shown to be effective and may be particularly beneficial for treating cravings/urges and promoting relapse prevention.37,39,40,43-46
Psychotropic medications, as with other treatment components, are most effective when used in combination with services that simultaneously address the patient’s biological, psychological, and social needs.
Continue to: The grassroots organization...
The grassroots organization National Alliance on Mental Illness (www.nami.org) recommends self-help and support groups, which include 12-step, faith-based and non-faith–based programs.20
For any treatment method to be successful, there needs to be a level of customization and individualization. Some patients may respond to medication or nonmedication treatments only, and others may need a combination of treatments.
CASE
The physician recalls a past diagnosis of anxiety and asks Ms. J if there are any new stressors or changes causing concern. The patient expresses concern about an opioid use relapse secondary to her recent diagnosis of rheumatoid arthritis, which may be life altering or limiting.
Even though she has been doing well and has been adherent to her daily buprenorphine treatment, she worries for the well-being of her family and what would happen if she cannot work, becomes incapacitated, or dies at a young age. She has never considered herself an anxious person and is surprised that anxiety could cause such pronounced physical symptoms.
The physician discusses different modalities of treatment, including counseling with an onsite psychologist, a trial of an anti-anxiety medication such as sertraline, or return office visits with the physician. They decide first to schedule an appointment with the psychologist, and Ms. J promises to find more time for self-wellness activities, such as exercise.
After 3 months of therapy, the patient decides to space out treatment to every 2 to 3 months and does not report any more episodes of chest pain or shortness of breath.
CORRESPONDENCE
Kristen Rundell, MD, Northwood-High Building, 2231 N. High Street, Suite 211, Columbus, OH 43201; kristen.rundell@osumc.edu.
THE CASE
Janice J* visits her family physician with complaints of chest pain, shortness of breath, and heart palpitations that are usually worse at night. Her medical history is significant for deep vein thrombosis secondary to an underlying hypercoagulability condition (rheumatoid arthritis) diagnosed 2 months earlier. She also has a history of opioid use disorder and has been on buprenorphine/naloxone therapy for 3 years. Her family medical history is unremarkable. She works full-time and lives with her 8-year-old son. On physical exam, she appears anxious; her cardiac and pulmonary exams are normal. A completed workup rules out cardiac or pulmonary problems.
- What is your diagnosis?
- How would you treat this patient?
* The patient’s name has been changed to protect her identity.
CO-OCCURRING DISORDERS: SCOPE OF THE PROBLEM
Co-occurring disorders, previously called “dual diagnosis,” refers to the coexistence of a mental health disorder and a substance use disorder. The obsolete term, dual diagnosis, specified the presence of 2 co-occurring Axis I diagnoses or the presence of an Axis I diagnosis and an Axis II diagnosis (such as mental disability). The change in nomenclature more precisely describes the co-existing mental health and substance use disorders.
Currently the Diagnostic and Statistical Manual of Mental Disorders, 5th edition, (DSM-5) includes no diagnostic criteria for this dual condition.1 The criteria for mental health disorders and for substance use disorders comprise separate lists. Criteria for substance use disorder fall broadly into categories of “impaired [self] control, social impairment, risky behaviors, increased tolerance, and withdrawal symptoms.”1 It is estimated that 8.5 million US adults have co-occurring disorders, per the 2017 National Survey on Drug Use and Health conducted by the Substance Abuse and Mental Health Services Administration.2 Distinguishing which of the 2 conditions occurred first can be challenging. It has been suggested that the lifetime prevalence of a mental health disorder with a coexisting substance use disorder is greater than 40%3,4 (TABLE 11,4-8). For patients with schizophrenia and bipolar disorder, these numbers may be higher.
The consequences of undiagnosed and untreated co-occurring disorders include poor medication adherence, physical comorbidities (and decreased overall health), diminished self-care, increased suicide risk or aggression, increased risky sexual behavior, and possible incarceration.9
WHEN SHOULD YOU SUSPECT CO-OCCURRING DISORDERS?
Diagnosing a second condition can also be difficult when a patient’s symptoms are actually adverse effects of substances or prescribed medications. For example, a patient with worsening anxiety may also exhibit increasing blood pressure resistant to treatment. The cause of the patient’s fluctuating blood pressures may actually be the result of his or her use of alcohol to self-treat the anxiety. In addition to self-medication, other underlying factors may be at play, including genetic vulnerability, environment, and lifestyle.14 In the case we present, the patient’s conditions arose independently.
Anxiety disorders, with a lifetime risk of 28.8% in the US population,4 may be the primary mental health issue in many patients with co-occurring disorders, but this cannot be assumed in lieu of a complete workup.2,8,9,15 Substance use disorders in the general population have a past-year and lifetime prevalence of 14.6%.1,4,16,17 Because the causal and temporal association between anxiety and substance abuse is not always clear, it’s important to separate the diagnoses of the mental health and substance use disorders.
Continue to: MAKING THE DIAGNOSIS
MAKING THE DIAGNOSIS
To make an accurate diagnosis of co-occurring disorder, it is essential to take a complete history focusing on the timeline of symptoms, previous diagnoses and treatments, if any, and substance-free periods. Details gathered from these inquiries will help to separate symptoms of a primary mental health disorder from adverse effects of medication, withdrawal symptoms, or symptoms related to an underlying chronic medical condition.
Optimally, the diagnosis of a mental health disorder should be considered following a substance-free period. If this is not possible, a chart review may reveal a time when the patient did not have a substance use disorder.18
A diagnosis of substance use disorder requires that the patient manifest at least 2 of 11 behaviors listed in the DSM-5 over a 12-month period.1 The criteria focus on the amount of substance used, the time spent securing the substance, risky behaviors associated with the substance, and tolerance to the substance.
DON'T DEFER MENTAL HEALTH Tx
It is necessary to treat co-occurring disorders simultaneously. The old idea of deferring treatment of a mental health issue until the substance use disorder is resolved no longer applies.19,20 Treating substance use problems without addressing comorbid mental health issues can negatively impact treatment progress and increase risk for relapse. In a similar way, leaving substance use problems untreated is associated with nonadherence in mental health treatment, poor engagement, and dropout.21,22
Integrated services. Due to this condition’s level of clinical complexity, the optimal treatment approach is an interdisciplinary one in which integrated services are offered at a single location by a team of medical, mental health, and substance use providers (see “The case for behavioral health integration into primary care” in the June issue). An evidence-based example of such an approach is the Integrated Dual Disorder Treatment (IDDT) model—a comprehensive, integrated method of treating severe mental health disorders, including substance use disorders.21,22 IDDT combines coordinated services such as pharmacologic, psychological, educational, and social interventions to address the needs of patients and their family members. The IDDT model conceptualizes and treats co-occurring disorders within a biopsychosocial framework. Specific services may include medical detoxification, pharmacotherapy, patient and family education, behavioral and cognitive therapies, contingency management, self-help support groups, supported employment, residential/housing assistance, and case management services.23,24
Continue to: Medications for the mental health component
Medications for the mental health component. For patients who prefer medication treatment to cognitive behavioral therapy (CBT), or for whom CBT is unavailable, treat the mental health disorder per customary practice for the diagnosis (TABLE 225-30). For psychotic disorders, use an antipsychotic, adding a selective serotonin reuptake inhibitor (SSRI) or serotonin-norepinephrine reuptake inhibitor (SNRI) as needed depending on the presence of negative symptoms.25,31 For bipolar spectrum disorder, start a mood stabilizer32; for depressive disorders initiate an SSRI or SNRI.27 Anxiety disorders respond optimally when treated with SSRIs or SNRIs. Buspirone may be prescribed alone or as an adjunct for anxiety, and it does not cause mood-altering or withdrawal effects. Benzodiazepines in a controlled and monitored setting are an option in some antianxiety treatment plans. Consultation with a psychiatrist will help to determine the best treatment in these situations.
In all cases, treat the substance use disorder concurrently. Treatment options vary depending on the substance of choice. Although often overlooked, there can be simultaneous nicotine abuse. Oral or inhaled medications for nicotine abuse treatment are limited. The range of pharmacologic options for alcohol use disorder includes naltrexone, acamprosate, and disulfiram.29,33 Pharmacologic treatment options for opioid use disorder include naltrexone, methadone, and a combination of naloxone and buprenorphine.34
Physicians who wish to prescribe buprenorphine must qualify for and complete a certified 8 hour waiver-training course, which is then approved by the Drug Enforcement Agency (under the DATA 2000 – Drug and Alcohol Act 2000). The physician obtains the designation of a data-waived physician and is assigned a special identification number to prescribe these medications.35,36 Methadone may be provided only in a licensed methadone maintenance program. Regular and random drug urine screen requirements apply to all treatment programs.
Psychosocial and behavioral interventions are essential to the successful treatment of co-occurring disorders. Evidence-based behavioral and cognitive therapies are recommended for promoting adaptive coping skills and healthy lifestyle behaviors in co-occurring disorder populations.23,24,37-40 Motivational interviewing enhances motivation and adherence when patients demonstrate resistance or ambivalence.41,42 Mindfulness-based interventions have been shown to be effective and may be particularly beneficial for treating cravings/urges and promoting relapse prevention.37,39,40,43-46
Psychotropic medications, as with other treatment components, are most effective when used in combination with services that simultaneously address the patient’s biological, psychological, and social needs.
Continue to: The grassroots organization...
The grassroots organization National Alliance on Mental Illness (www.nami.org) recommends self-help and support groups, which include 12-step, faith-based and non-faith–based programs.20
For any treatment method to be successful, there needs to be a level of customization and individualization. Some patients may respond to medication or nonmedication treatments only, and others may need a combination of treatments.
CASE
The physician recalls a past diagnosis of anxiety and asks Ms. J if there are any new stressors or changes causing concern. The patient expresses concern about an opioid use relapse secondary to her recent diagnosis of rheumatoid arthritis, which may be life altering or limiting.
Even though she has been doing well and has been adherent to her daily buprenorphine treatment, she worries for the well-being of her family and what would happen if she cannot work, becomes incapacitated, or dies at a young age. She has never considered herself an anxious person and is surprised that anxiety could cause such pronounced physical symptoms.
The physician discusses different modalities of treatment, including counseling with an onsite psychologist, a trial of an anti-anxiety medication such as sertraline, or return office visits with the physician. They decide first to schedule an appointment with the psychologist, and Ms. J promises to find more time for self-wellness activities, such as exercise.
After 3 months of therapy, the patient decides to space out treatment to every 2 to 3 months and does not report any more episodes of chest pain or shortness of breath.
CORRESPONDENCE
Kristen Rundell, MD, Northwood-High Building, 2231 N. High Street, Suite 211, Columbus, OH 43201; kristen.rundell@osumc.edu.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: APA; 2013.
2. SAMHSA. Key substance use and mental health indicators in the United States: results from the 2017 National Survey on Drug Use and Health. 2017. https://www.samhsa.gov/data/sites/default/files/cbhsq-reports/NSDUHFFR2017/NSDUHFFR2017.htm#cooccur2. Accessed August 16, 2019.
3. Conway KP, Compton W, Stinson FS, et al. Lifetime comorbidity of DSM-IV mood and anxiety disorders and specific drug use disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2006;67:247-257.
4. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593-602.
5. Grant BF, Stinson FS, Dawson DA, et al. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2004;61:807-816.
6. Dixon L. Dual diagnosis of substance abuse in schizophrenia: prevalence and impact on outcomes. Schizophr Res. 1999;35(suppl):S93-S100.
7. Merikangas KR, Jin R, He JP, et al. Prevalence and correlates of bipolar spectrum disorder in the World Mental Health Survey Initiative. Arch Gen Psychiatry. 2011;68:241-251.
8. Cottler LB, Compton WM 3rd, Mager D, et al. Posttraumatic stress disorder among substance users from the general population. Am J Psychiatry. 1992;149:664-670.
9. Kessler RC, Angermeyer M, Anthony JC, et al. Lifetime prevalence and age-of-onset distributions of mental disorders in the World Health Organization’s World Mental Health Survey Initiative. World Psychiatry. 2007;6:168-176.
10. Burns L, Teesson M, O’Neill K. The impact of comorbid anxiety and depression on alcohol treatment outcomes. Addiction. 2005;100:787-796.
11. Magidson JF, Liu SM, Lejuez CW, et al. Comparison of the course of substance use disorders among individuals with and without generalized anxiety disorder in a nationally representative sample. J Psychiatr Res. 2012;46:659666.
12. Boschloo L, Vogelzangs N, van den Brink W, et al. Alcohol use disorders and the course of depressive and anxiety disorders. Br J Psychiatry. 2012;200:476-484.
13. Schuckit MA. Comorbidity between substance use disorders and psychiatric conditions. Addiction. 2006;101(suppl 1):76-88.
14. Buckley PF. Prevalence and consequences of the dual diagnosis of substance abuse and severe mental illness. J Clin Psychiatry. 2006;67(suppl 7):5-9.
15. Salo R, Flower K, Kielstein A, et al. Psychiatric comorbidity in methamphetamine dependence. Psychiatry Res. 2011;186:356-361.
16. Torrens M, Gilchrist G, Domingo-Salvany A. Psychiatric comorbidity in illicit drug users: substance-induced versus independent disorders. Drug Alcohol Depend. 2011;113:147-156.
17. Buckner JD, Timpano KR, Zvolensky MJ, et al. Implications of comorbid alcohol dependence among individuals with social anxiety disorder. Depress Anxiety. 2008;25:1028-1037.
18. Kushner MG, Abrams K, Borchardt C. The relationship between anxiety disorders and alcohol use disorders: a review of major perspectives and findings. Clin Psychol Rev. 2000;20:149-171.
19. McHugh RK. Treatment of co-occurring anxiety disorders and substance use disorders. Harv Rev Psychiatry. 2015;23:99-111.
20. National Alliance on Mental Illness. Dual diagnosis. NAMI Web site. www.nami.org/Learn-More/Mental-Health-Conditions/related-conditions/dual-diagnosis. Reviewed August 2017. Accessed July 23, 2019.
21. SAMSHA. Substance Abuse Treatment for Persons with Co-Occurring Disorders. Treatment Improvement Protocol (TIP) series No. 42. HHS Publication No. (SMA) 13-3992. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2013.
22. SAMHSA. Treatment of co-occurring disorders. In: Medication-Assisted Treatment for Opioid Addiction in Opioid Treatment Programs. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2005.
23. Drake RE, Mueser KT, Brunette MF, et al. A review of treatments for people with severe mental illnesses and co-occurring substance use disorders. Psychiatr Rehabil J. 2004;27:360-374.
24. Kola LA, Kruszynski R. Adapting the integrated dual-disorder treatment model for addiction services. Alcohol Treat Q. 2010;28:437-450.
25. American Psychiatric Association. Practice guideline for the treatment of patients with schizophrenia, 2nd ed. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/schizophrenia.pdf. Published 2010. Accessed August 2, 2019.
26. American Psychiatric Association. Practice guideline for the treatment of patients with bipolar disorder, 2nd ed. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/bipolar.pdf. Published 2010. Accessed August 2, 2019.
27. American Psychiatric Association. Practice Guideline for the Treatment of Patients with Major Depressive Disorder. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf. Published October 2010. Accessed July 23, 2019.
28. American Psychiatric Association. Practice guideline for the treatment of patients with panic disorder, 2nd ed. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/panicdisorder.pdf. Published January 2009. Accessed August 2, 2019.
29. American Psychiatric Association. Practice guideline for the pharmacological treatment of patients with alcohol use disorder. https://psychiatryonline.org/doi/pdf/10.1176/appi.books.9781615371969. Accessed August 2, 2019.
30. American Psychiatric Association. Practice guideline for the treatment of patients with substance use disorders, 2nd ed. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/substanceuse.pdf. Published 2010. Accessed August 2, 2019.
31. Petrakis IL, Nich C, Ralevski E. Psychotic spectrum disorders and alcohol abuse: a review of pharmacotherapeutic strategies and a report on the effectiveness of naltrexone and disulfiram. Schizophr Bull. 2006;32:644-654.
32. McIntyre RS, Yoon J. Efficacy of antimanic treatments in mixed states. Bipolar Disord. 2012;14(suppl 2):22-36.
33. Volpicelli JR, Alterman AI, Hayashida M, et al. Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry. 1992;49:876-880.
34. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomized controlled trial. Lancet. 2018;391:309-318.
35. US Department of Justice. DEA requirements for DATA waived physicians (DWPs). Drug Enforcement Administration, Diversion Control Division Web site. www.deadiversion.usdoj.gov/pubs/docs/dwp_buprenorphine.htm. Accessed August 2, 2019.
36. SAMHSA. Buprenorphine waiver management. https://www.samhsa.gov/medication-assisted-treatment/buprenorphine-waiver-management. SAMHSA Web site. Updated May 7, 2019. Accessed August 2, 2019.
37. Bowen S, Chawla N, Witkiewitz K. Mindfulness-based relapse prevention for addictive behaviors. In: Baer RA, ed. Mindfulness-Based Treatment Approaches: A Clinician’s Guide to Evidence Base and Applications. London, UK: Elsevier; 2014.
38. Dixon L, McFarlane W, Lefley H, et al. Evidence-based practices for services to families of people with psychiatric disabilities. Psychiatr Serv. 2001;52:903-910.
39. Hayes SC, Levin M, Plumb-Vilardaga J, et al. Acceptance and commitment therapy and contextual behavioral science: examining the progress of a distinctive model of behavioral and cognitive therapy. Behav Ther. 2013;44:180-198.
40. Osilla KC, Hepner KA, Muñoz RF, et al. Developing an integrated treatment for substance use and depression using cognitive behavioral therapy. J Subst Abuse Treat. 2009;37:412-420.
41. Martino S, Carroll K, Kostas D, et al. Dual diagnosis motivational interviewing: a modification of motivational interviewing for substance-abusing patients with psychotic disorders. J Subst Abuse Treat. 2002;23:297-308.
42. Rollnick S, Miller WR. What is motivational interviewing? Behav Cogn Psychother. 1995;23:325-334.
43. Garland EL. Disrupting the downward spiral of chronic pain and opioid addiction with mindfulness-oriented recovery enhancement: a review of clinical outcomes and neurocognitive targets. J Pain Palliat Care Pharmacother. 2014;28:122-129.
44. Garland EL, Manusov EG, Froeliger B, et al. Mindfulness-oriented recovery enhancement for chronic pain and prescription opioid misuse: results from an early-stage randomized controlled trial. J Consult Clin Psychol. 2014;82:448-459.
45. Marlatt GA, Donovan DM. Relapse Prevention: Maintenance Strategies in the Treatment of Addictive Behaviors, 2nd ed. New York, NY: Guilford Press; 2007.
46. Zgierska A, Rabago D, Chawla N, et al. Mindfulness meditation for substance use disorders: a systematic review. Subst Abus. 2009;30:266-294.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: APA; 2013.
2. SAMHSA. Key substance use and mental health indicators in the United States: results from the 2017 National Survey on Drug Use and Health. 2017. https://www.samhsa.gov/data/sites/default/files/cbhsq-reports/NSDUHFFR2017/NSDUHFFR2017.htm#cooccur2. Accessed August 16, 2019.
3. Conway KP, Compton W, Stinson FS, et al. Lifetime comorbidity of DSM-IV mood and anxiety disorders and specific drug use disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2006;67:247-257.
4. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593-602.
5. Grant BF, Stinson FS, Dawson DA, et al. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2004;61:807-816.
6. Dixon L. Dual diagnosis of substance abuse in schizophrenia: prevalence and impact on outcomes. Schizophr Res. 1999;35(suppl):S93-S100.
7. Merikangas KR, Jin R, He JP, et al. Prevalence and correlates of bipolar spectrum disorder in the World Mental Health Survey Initiative. Arch Gen Psychiatry. 2011;68:241-251.
8. Cottler LB, Compton WM 3rd, Mager D, et al. Posttraumatic stress disorder among substance users from the general population. Am J Psychiatry. 1992;149:664-670.
9. Kessler RC, Angermeyer M, Anthony JC, et al. Lifetime prevalence and age-of-onset distributions of mental disorders in the World Health Organization’s World Mental Health Survey Initiative. World Psychiatry. 2007;6:168-176.
10. Burns L, Teesson M, O’Neill K. The impact of comorbid anxiety and depression on alcohol treatment outcomes. Addiction. 2005;100:787-796.
11. Magidson JF, Liu SM, Lejuez CW, et al. Comparison of the course of substance use disorders among individuals with and without generalized anxiety disorder in a nationally representative sample. J Psychiatr Res. 2012;46:659666.
12. Boschloo L, Vogelzangs N, van den Brink W, et al. Alcohol use disorders and the course of depressive and anxiety disorders. Br J Psychiatry. 2012;200:476-484.
13. Schuckit MA. Comorbidity between substance use disorders and psychiatric conditions. Addiction. 2006;101(suppl 1):76-88.
14. Buckley PF. Prevalence and consequences of the dual diagnosis of substance abuse and severe mental illness. J Clin Psychiatry. 2006;67(suppl 7):5-9.
15. Salo R, Flower K, Kielstein A, et al. Psychiatric comorbidity in methamphetamine dependence. Psychiatry Res. 2011;186:356-361.
16. Torrens M, Gilchrist G, Domingo-Salvany A. Psychiatric comorbidity in illicit drug users: substance-induced versus independent disorders. Drug Alcohol Depend. 2011;113:147-156.
17. Buckner JD, Timpano KR, Zvolensky MJ, et al. Implications of comorbid alcohol dependence among individuals with social anxiety disorder. Depress Anxiety. 2008;25:1028-1037.
18. Kushner MG, Abrams K, Borchardt C. The relationship between anxiety disorders and alcohol use disorders: a review of major perspectives and findings. Clin Psychol Rev. 2000;20:149-171.
19. McHugh RK. Treatment of co-occurring anxiety disorders and substance use disorders. Harv Rev Psychiatry. 2015;23:99-111.
20. National Alliance on Mental Illness. Dual diagnosis. NAMI Web site. www.nami.org/Learn-More/Mental-Health-Conditions/related-conditions/dual-diagnosis. Reviewed August 2017. Accessed July 23, 2019.
21. SAMSHA. Substance Abuse Treatment for Persons with Co-Occurring Disorders. Treatment Improvement Protocol (TIP) series No. 42. HHS Publication No. (SMA) 13-3992. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2013.
22. SAMHSA. Treatment of co-occurring disorders. In: Medication-Assisted Treatment for Opioid Addiction in Opioid Treatment Programs. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2005.
23. Drake RE, Mueser KT, Brunette MF, et al. A review of treatments for people with severe mental illnesses and co-occurring substance use disorders. Psychiatr Rehabil J. 2004;27:360-374.
24. Kola LA, Kruszynski R. Adapting the integrated dual-disorder treatment model for addiction services. Alcohol Treat Q. 2010;28:437-450.
25. American Psychiatric Association. Practice guideline for the treatment of patients with schizophrenia, 2nd ed. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/schizophrenia.pdf. Published 2010. Accessed August 2, 2019.
26. American Psychiatric Association. Practice guideline for the treatment of patients with bipolar disorder, 2nd ed. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/bipolar.pdf. Published 2010. Accessed August 2, 2019.
27. American Psychiatric Association. Practice Guideline for the Treatment of Patients with Major Depressive Disorder. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf. Published October 2010. Accessed July 23, 2019.
28. American Psychiatric Association. Practice guideline for the treatment of patients with panic disorder, 2nd ed. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/panicdisorder.pdf. Published January 2009. Accessed August 2, 2019.
29. American Psychiatric Association. Practice guideline for the pharmacological treatment of patients with alcohol use disorder. https://psychiatryonline.org/doi/pdf/10.1176/appi.books.9781615371969. Accessed August 2, 2019.
30. American Psychiatric Association. Practice guideline for the treatment of patients with substance use disorders, 2nd ed. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/substanceuse.pdf. Published 2010. Accessed August 2, 2019.
31. Petrakis IL, Nich C, Ralevski E. Psychotic spectrum disorders and alcohol abuse: a review of pharmacotherapeutic strategies and a report on the effectiveness of naltrexone and disulfiram. Schizophr Bull. 2006;32:644-654.
32. McIntyre RS, Yoon J. Efficacy of antimanic treatments in mixed states. Bipolar Disord. 2012;14(suppl 2):22-36.
33. Volpicelli JR, Alterman AI, Hayashida M, et al. Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry. 1992;49:876-880.
34. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomized controlled trial. Lancet. 2018;391:309-318.
35. US Department of Justice. DEA requirements for DATA waived physicians (DWPs). Drug Enforcement Administration, Diversion Control Division Web site. www.deadiversion.usdoj.gov/pubs/docs/dwp_buprenorphine.htm. Accessed August 2, 2019.
36. SAMHSA. Buprenorphine waiver management. https://www.samhsa.gov/medication-assisted-treatment/buprenorphine-waiver-management. SAMHSA Web site. Updated May 7, 2019. Accessed August 2, 2019.
37. Bowen S, Chawla N, Witkiewitz K. Mindfulness-based relapse prevention for addictive behaviors. In: Baer RA, ed. Mindfulness-Based Treatment Approaches: A Clinician’s Guide to Evidence Base and Applications. London, UK: Elsevier; 2014.
38. Dixon L, McFarlane W, Lefley H, et al. Evidence-based practices for services to families of people with psychiatric disabilities. Psychiatr Serv. 2001;52:903-910.
39. Hayes SC, Levin M, Plumb-Vilardaga J, et al. Acceptance and commitment therapy and contextual behavioral science: examining the progress of a distinctive model of behavioral and cognitive therapy. Behav Ther. 2013;44:180-198.
40. Osilla KC, Hepner KA, Muñoz RF, et al. Developing an integrated treatment for substance use and depression using cognitive behavioral therapy. J Subst Abuse Treat. 2009;37:412-420.
41. Martino S, Carroll K, Kostas D, et al. Dual diagnosis motivational interviewing: a modification of motivational interviewing for substance-abusing patients with psychotic disorders. J Subst Abuse Treat. 2002;23:297-308.
42. Rollnick S, Miller WR. What is motivational interviewing? Behav Cogn Psychother. 1995;23:325-334.
43. Garland EL. Disrupting the downward spiral of chronic pain and opioid addiction with mindfulness-oriented recovery enhancement: a review of clinical outcomes and neurocognitive targets. J Pain Palliat Care Pharmacother. 2014;28:122-129.
44. Garland EL, Manusov EG, Froeliger B, et al. Mindfulness-oriented recovery enhancement for chronic pain and prescription opioid misuse: results from an early-stage randomized controlled trial. J Consult Clin Psychol. 2014;82:448-459.
45. Marlatt GA, Donovan DM. Relapse Prevention: Maintenance Strategies in the Treatment of Addictive Behaviors, 2nd ed. New York, NY: Guilford Press; 2007.
46. Zgierska A, Rabago D, Chawla N, et al. Mindfulness meditation for substance use disorders: a systematic review. Subst Abus. 2009;30:266-294.
How best to address breast pain in nonbreastfeeding women
CASE 1
Robin S is a 40-year-old woman who has never had children or been pregnant. She is in a relationship with a woman so does not use contraception. She has no family history of cancer. She presents with worsening bilateral breast pain that starts 10 days before the onset of her period. The pain has been present for about 4 years, but it has worsened over the last 6 months such that she is unable to wear a bra during these 10 days, finds lying in bed on her side too painful for sleep, and is unable to exercise. She has tried to eliminate caffeine from her diet and takes ibuprofen, but neither of these interventions has controlled her pain. Her breast exam is normal except for diffuse tenderness over both breasts.
CASE 2
Meg R is a 50-year-old healthy woman. She is a G2P2 who breastfed each of her children for 1 year. She does not smoke. She has no family history of breast cancer or other malignancies. She presents with 2 months of deep, left-sided breast pain. She describes the pain as constant, progressive, dull, and achy. She points to a spot in the upper outer quadrant of her left breast and describes the pain as being close to her ribs. She had a screening mammogram 3 weeks earlier that was normal, with findings of dense breasts. She did not tell the technician that she was having pain. Clinical breast examination of both breasts reveals tenderness to deep palpation of the left breast. She has dense breasts but a focal mass is not palpated.
Mastalgia, or breast pain, is one of the most common breast symptoms seen in primary care and a common reason for referrals to breast surgeons. Up to 70% of women will experience breast pain during their lifetime—most in their premenopausal years.1,2
The most common type of breast pain is cyclic (ie, relating to the menstrual cycle); it accounts for up to 70% of all cases of breast pain in women.1,3 The other 2 types of breast pain are noncyclic and extramammary. The cause of cyclic breast pain is unclear, but it is likely hormonally mediated and multifactorial. In the vast majority of women with breast pain, no distinct etiology is found, and there is a very low incidence of breast cancer.2,4
In this review, we describe how to proceed when a woman who is not breastfeeding presents with cyclic or noncyclic breast pain.
Evaluation: Focus on the pain, medications, and history
Evaluation of breast pain should begin with the patient describing the pain, including its quality, location, radiation, and relationship to the menstrual cycle. It’s important to inquire about recent trauma or aggravating activities and to order a pregnancy test for women of childbearing age.1
Cyclic mastalgia is typically described as diffuse, either unilateral or bilateral, with an aching or heavy quality. The pain is often felt in the upper outer quadrant of the breast with radiation to the axilla. It most commonly occurs during the luteal phase of the menstrual cycle, improves with the onset of menses, and is thought to be related to the increased water content in breast stroma caused by increasing hormone levels during the luteal phase.5-7
Continue to: Noncyclic mastalgia
Noncyclic mastalgia is typically unilateral and localized within 1 quadrant of the breast; however, women may report diffuse pain with radiation to the axilla. The pain is often described as burning, achy, or as soreness.5,6 There can be considerable overlap in the presentations of cyclic and noncyclic pain and differentiating between the 2 is often not necessary as management is similar.8
A thorough review of medications is important as several drugs have been associated with breast pain. These include oral contraceptives, hormone therapy, antidepressants (selective serotonin reuptake inhibitors [SSRIs], venlafaxine, mirtazapine), antipsychotics (haloperidol), and some cardiovascular agents (spironolactone, digoxin).5
Inquiring about stress, caffeine intake, smoking status, and bra usage may also yield useful information. Increased stress and caffeine intake have been associated with mastalgia,7 and women who are heavy smokers are more likely to have noncyclic hypersensitive breast pain.9 In addition, women with large breasts often have noncyclic breast pain, particularly if they don’t wear a sufficiently supportive bra.3
Medical, surgical, family history. Relevant aspects of a woman’s past medical, surgical, and family history include prior breast mass or biopsy, breast surgery, and risk factors associated with breast cancer (menarche age < 12 years, menopause age > 55 years, nulliparity, exposure to ionizing radiation, and family history of breast or ovarian cancer).1 A thorough history should include questions to evaluate for extra-mammary etiologies of breast pain such as those that are musculoskeletal or dermatologic in nature (TABLE 11,5,8,10).
Using an objective measure of pain is not only helpful for evaluating the pain itself, but also for determining the effectiveness of treatment strategies. When using the Cardiff Breast Pain Chart, for example, menstrual cycle and level of pain are recorded on a calendar (see www.breastcancercare.org.uk/sites/default/files/files/breast_pain_chart.pdf).11 If the pain is determined to be cyclic, the concern for malignancy is significantly lower.2
Continue to: Ensure that the physical exam is thorough
Ensure that the physical exam is thorough
Women presenting with breast pain should undergo a clinical breast exam in both the upright and supine positions. Inspect for asymmetry, erythema, rashes, skin dimpling, nipple discharge, and retraction/inversion. Palpate the breasts for any suspicious masses, asymmetry, or tenderness, as well as for axillary and/or supraclavicular lymphadenopathy and chest wall tenderness. This is facilitated by having the patient lie in the lateral decubitus position, allowing the breast to fall away from the chest wall.5,12,13
Imaging: Preferred method depends on the age of the patient
Women with a palpable mass should be referred for diagnostic imaging (FIGURE 11,14). Ultrasonography is the recommended modality for women < 30 years of age (TABLE 215). For women between the ages of 30 and 39 years, appropriate initial imaging includes ultrasound, diagnostic mammography, or digital breast tomosynthesis (DBT). For women ≥ 40 years of age, diagnostic mammography or DBT is recommended.15
Cyclic breast pain. Women with cyclic breast pain do not require further evaluation with imaging. Reassurance and symptomatic treatment is appropriate in most cases, as the risk of malignancy is very low in the absence of other concerning signs or symptoms. A screening mammogram may be appropriate for women > 40 years of age who have not had one in the preceding 12 months.1-3,10,12,15
Noncyclic breast pain. In contrast, imaging may be appropriate in women who present with noncyclic breast pain depending on the woman’s age and whether the pain is focal (≤ 25% of the breast and axillary tissue) or diffuse (> 25% of the breast and axillary tissue). Although evidence suggests that the risk of malignancy in women with noncyclic breast pain is low, the American College of Radiology advises that imaging may be useful in some patients to provide reassurance and to exclude a treatable cause of breast pain.3,14 In women with focal pain, ultrasound alone is the preferred modality for women < 30 years of age and ultrasound plus diagnostic mammography is recommended for women ≥ 30 years of age.3,14
In one small study, the use of ultrasonography in women ages < 30 years with focal breast pain had a sensitivity of 100% and a negative predictive value of 100%.16 Similarly, another small retrospective study in older women (average age 56 years) with focal breast pain and no palpable mass showed that ultrasound plus diagnostic mammography had a negative predictive value of 100%.4 DBT may be used in place of mammography to rule out malignancy in this setting.
Continue to: In general...
In general, routine imaging is not indicated for women with noncyclic diffuse breast pain, although diagnostic mammography or DBT may be considered in women ≥ 40 years of age 14 (see “Less common diagnoses with breast pain”4,5,17-21).
SIDEBAR
Less common diagnoses with breast pain
Many women presenting with breast pain are concerned about malignancy. Breast cancer is an uncommon cause of breast pain; only 0.5% of patients presenting with mastalgia without other clinical findings have a malignancy.4 Mastalgia is not a risk factor for breast cancer.
When mastalgia is associated with breast cancer, it is more likely to be unilateral, intense, noncyclic, and progressive.5 Concerning features that warrant further evaluation include new onset focal pain with or without an abnormal exam. If symptoms cannot be explained by an obvious cause (such as trauma, costochondritis, radicular back or intercostal pain, herpes zoster, or superficial thrombophlebitis that does not resolve), diagnostic breast imaging is indicated.
Inflammatory breast cancer (IBC) is an aggressive form of breast cancer that initially presents with breast pain and rapidly enlarging diffuse erythema of the breast in the absence of a discrete breast lump. The initial presentation is similar to that seen with benign inflammatory etiologies of the breast tissue like cellulitis or abscess, duct ectasia, mastitis, phlebitis of the thoracoepigastric vein (Mondor’s disease), or fat necrosis.17 Benign breast conditions due to these causes will generally resolve with appropriate treatment for those conditions within 7 days and will generally not present with the warning signs of IBC, which include a personal history of breast cancer, nonlactational status, and palpable axillary adenopathy. Although uncommon (accounting for 1%-6% of all breast cancer diagnoses), IBC spreads rapidly over a few weeks; thus, urgent imaging is warranted.17
Mastitis is inflammation of the breast tissue that may or may not be associated with a bacterial infection and uncommonly occurs in nonbreastfeeding women. Periductal mastitis is characterized by inflammation of the subareolar ducts and can present with pain, periareolar inflammation, and purulent nipple discharge.18 The condition is typically chronic, and the inflamed ducts may become secondarily infected leading to duct damage and abscess formation. Treatment generally includes antibiotics along with incision and drainage of any associated abscesses or duct excision.18,19
Idiopathic granulomatous mastitis (IGM) is a rare inflammatory breast disease that typically affects young parous women. The presentation can vary from a single peripheral breast mass to multiple areas of infection with abscesses and skin ulceration. The etiology is unknown. Diagnosis requires a core needle biopsy to rule out malignancy or other causes of granulomatous disease. IGM is a benign condition and typically resolves without treatment over the course of several months, although antibiotics and/or drainage may be required for secondary infections.20,21
Continue to: Treatment...
Treatment: When reassurance isn’t enough
Nonrandomized studies suggest that reassurance that mastalgia is benign is enough to treat up to 70% of women.8,22,23 Cyclic breast pain is usually treated symptomatically since the likelihood of breast cancer is extremely low in absence of clinical breast examination abnormalities.2 Because treatment for cyclic and noncyclic mastalgia overlaps, available treatments are discussed together on the following pages.
Lifestyle factors associated with breast pain include stress, caffeine consumption, smoking, and having breastfed 3 or more children (P < .05).9 Although restriction of caffeine, fat, and salt intake may be attempted to address breast pain, no randomized control trials (RCTs) of these interventions have demonstrated effectiveness in reducing mastalgia.8,10
Although not supported by RCTs, first-line treatment of mastalgia includes a recommendation that women, particularly those with large, heavy breasts, wear a well-fitted and supportive bra.8,10
Complementary and alternative medicine treatments for mastalgia
A number of complementary and alternative medicine treatments have demonstrated benefit in treating mastalgia and are often tried before pharmacologic agents (TABLE 324-28). Keep in mind, though, that these therapies are not regulated by the US Food and Drug Administration (FDA). So it’s wise to review particular products with your patient before she buys them (or ask her to bring in any bottles of product for you to review).
Flaxseed, omega-3 fatty acids, and soy milk. Flaxseed, a source of phytoestrogens and omega-3 fatty acids, has been shown to reduce cyclic breast pain in 2 small RCTs.24,25 Breast pain scores were significantly lower for patients ingesting 25 g/d of flaxseed powder compared with placebo.24,25 Omega-3 fatty acids were also more effective than placebo for relief of cyclic breast pain in 2 small RCTs.25,26 Another small RCT demonstrated that women who drank soy milk had a nonsignificant improvement in breast pain compared with those who drank cow’s milk.27
Continue to: Chasteberry
Chasteberry. One RCT demonstrated that Vitex agnus-castus, a chasteberry fruit extract, produced significant and clinically meaningful improvement in visual analogue pain scores for mastalgia, with few adverse effects.29 Another RCT assessing breast fullness as part of the premenstrual syndrome showed significant improvement in breast discomfort for women treated with Vitex agnus-castus.30
Evening primrose oil (EPO). In at least one small study, EPO was effective in controlling breast pain.28 A more recent meta-analysis of all of the EPO trials including gamolenic acid (the active ingredient of EPO) showed no significant difference in mastalgia compared with placebo.31
Pharmacologic Tx options: Start with NSAIDs
Oral nonsteroidal anti-inflammatory drugs (NSAIDs) are often recommended as a first-line treatment for mastalgia and are likely effective for some women; however, there is currently insufficient evidence that oral NSAIDs (or acetaminophen) improve pain (TABLE 432-37; FIGURE 25,13,17). Nevertheless, the potential benefits are thought to outweigh the risk of adverse effects in most patients. A small RCT did demonstrate that topical diclofenac was effective in patients with cyclic and noncyclic mastalgia.38
SSRIs. A meta-analysis of 10 double-blind RCTs of SSRIs used in women with premenstrual symptoms, including 4 studies that specifically included physical symptoms such as breast pain, showed SSRIs to be more effective than placebo at relieving breast pain.35
Progesterones. Several studies have found topical, oral, and injected progesterone ineffective at reducing breast pain.8,36,39 However, one RCT did show topical vaginal micronized progesterone used in the luteal phase to be effective in reducing breast pain by at least 50%.36
Continue to: Oral contraceptives
Oral contraceptives. For women who use oral contraceptive pills and experience cyclic breast pain, continuous dosing (skipping the pill-free week) or using a lower dose of estrogen may improve symptoms. Postmenopausal women with mastalgia that developed with initiation of hormone therapy may benefit from discontinuing hormone therapy or decreasing the estrogen dose; however, there are no RCTs to offer conclusive evidence of the effectiveness of these interventions.10
Danazol. Women with severe mastalgia that does not respond to more benign therapies may require hormone therapy. As with all symptom management, it is imperative to engage the patient in a shared decision-making conversation about the risks and benefits of this treatment strategy. Women must be able to balance the potential adverse effects of agents such as danazol and tamoxifen with the need to alleviate pain and improve quality of life.
Danazol is the only medication FDA-approved for the treatment of mastalgia. Danazol is an androgen that blocks the release of other gonadotropins to limit hormonal stimulation of breast tissue. One RCT demonstrated that danazol (100 mg bid) reduces breast pain in 60% to 90% of women, although adverse effects often limit utility.40 Adverse effects of danazol include weight gain, hot flashes, deepening of the voice, hirsutism, menorrhagia or amenorrhea, muscle cramps, and androgenic effects on a fetus.8,31,40 Danazol may be best used cyclically during the luteal phase of the menstrual cycle to limit these adverse effects with reduction of the dose to 100 mg/d after relief of symptoms.31,40
Tamoxifen, a selective estrogen receptor modulator, has been shown to reduce breast pain in 80% to 90% of women, although it is not indicated for mastalgia.40 Tamoxifen may cause endometrial thickening, hot flashes, menstrual irregularity, venous thromboembolism, and teratogenicity. The 10 mg/d dose appears to be as effective at improving symptoms as the 20 mg/d dose with fewer adverse effects.8,31,40
In a head-to-head randomized trial, tamoxifen was superior to danazol for relief of breast pain with fewer adverse effects.34 Experts recommend limiting use of tamoxifen and danazol to 3 to 6 months. Neither of these drugs is considered safe in pregnancy.
Continue to: Bromocriptine
Bromocriptine, a prolactin inhibitor, has been shown to be more effective than placebo in reducing breast pain, although nausea and dizziness contribute to high discontinuation rates. Bromocriptine is less effective than danazol.40
Goserelin, which is not available in the United States, is a gonadorelin analog (luteinizing hormone-releasing hormone analog) that produces reversible ovarian suppression. One RCT showed that goserelin injection may be more effective than placebo in reducing breast pain.37 Adverse effects include vaginal dryness, hot flashes, decreased libido, oily skin or hair, decreased breast size, and irritability. It is recommended as treatment only for severe refractory mastalgia and that it be used no longer than 6 months.31,37
CASE 1
You reassure Ms. S that her history and physical exam are consistent with cyclic breast pain and not malignancy. You review the current US Preventive Services Task Force recommendations for breast cancer screening in women ages 40 to 49 years (Grade C; women who place a higher value on the potential benefit than the potential harms may choose screening).41 Based on shared decision-making,you offer her a screening mammogram, which returns normal. After confirming that she is using an appropriately-sized supportive bra, you recommend adding 25 g/d of ground flaxseed to her diet.
After 2 months she reports a 30% improvement in her pain. You then recommend chasteberry extract 4.2 mg/d, which provides additional relief to the point where she can now sleep better and walk for exercise.
CASE 2
You order a diagnostic mammogram of the left breast, which is normal, and an ultrasound that demonstrates a 6-cm deep mass. A biopsy determines that Ms. R has invasive lobular breast cancer—an extremely unlikely outcome of breast pain. She elects to have a double mastectomy and reconstruction and is doing well 4 years later.
CORRESPONDENCE
Sarina Schrager, MD, MS, University of Wisconsin Department of Family Medicine and Community Health, 1100 Delaplaine Ct., Madison, WI, 53715; sbschrag@wisc.edu.
1. Salzman B, Fleegle S, Tully AS. Common breast problems. Am Fam Physician. 2012;86:343-349.
2. Chetlen AL, Kapoor MM, Watts MR. Mastalgia: imaging work-up appropriateness. Acad Radiol. 2017;24:345-349.
3. Expert Panel on Breast Imaging: Jokich PM, Bailey L, D’Orsi C, et al. ACR Appropriateness Criteria Breast Pain. J Am Coll Radiol. 2017;14:S25-S33.
4. Arslan M, Küçükerdem HS, Can H, et al. Retrospective analysis of women with only mastalgia. J Breast Health. 2016;12:151-154.
5. Smith RL, Pruthi S, Fitzpatrick LA. Evaluation and management of breast pain. Mayo Clin Proc. 2004;79:353-372.
6. Mansel RE. ABC of breast diseases. Breast pain. BMJ. 1994;309:866-868.
7. Ader DN, South-Paul J, Adera T, et al. Cyclical mastalgia: prevalence and associated health and behavioral factors. J Psychosom Obstet Gynaecol. 2001;22:71-76.
8. Iddon J, Dixon JM. Mastalgia. BMJ. 2013;347:f3288.
9. Eren T, Aslan A, Ozemir IA, et al. Factors effecting mastalgia. Breast Care (Basel). 2016;11:188-193.
10. Pearlman MD, Griffin JL. Benign breast disease. Obstet Gynecol. 2010;116:747-758.
11. Gateley CA, Mansel RE. The Cardiff Breast Score. Br J Hosp Med. 1991;45:16.
12. Michigan Medicine. University of Michigan. Common breast problems: guidelines for clinical care. https://www.med.umich.edu/1info/FHP/practiceguides/breast/breast.pdf. Updated June 2013. Accessed September 3, 2019.
13. Millet AV, Dirbas FM. Clinical management of breast pain: a review. Obstet Gynecol Surv. 2002;57:451-461.
14. American College of Radiology. ACR Appropriateness Criteria: Breast Pain. https://acsearch.acr.org/docs/3091546/Narrative/. Revised 2018. Accessed July 2, 2019.
15. American College of Radiology. ACR Appropriateness Criteria: Palpable Breast Masses. https://acsearch.acr.org/docs/69495/Narrative/. Revised 2016. Accessed September 3, 2019.
16. Loving VA, DeMartini WB, Eby PR, et al. Targeted ultrasound in women younger than 30 years with focal breast signs or symptoms: outcomes analyses and management implications. AJR Am J Roentgenol. 2010;195:1472-1477.
17. Molckovsky A, Fitzgerald B, Freedman O, et al. Approach to inflammatory breast cancer. Can Fam Physician. 2009;55:25-31.
18. Ammari FF, Yaghan RJ, Omari AK. Periductal mastitis: clinical characteristics and outcome. Saudi Med J. 2002;23:819-822.
19. Lannin DR. Twenty-two year experience with recurring subareolar abscess and lactiferous duct fistula treated by a single breast surgeon. Am J Surg. 2004;188:407-410.
20. Wilson JP, Massoll N, Marshall J, et al. Idiopathic granulomatous mastitis: in search of a therapeutic paradigm. Am Surg. 2007;73:798-802.
21. Bouton ME, Jayaram L, O’Neill PJ, et al. Management of idiopathic granulomatous mastitis with observation. Am J Surg. 2015;210:258-262.
22. Olawaiye A, Withiam-Leitch M, Danakas G, et al. Mastalgia: a review of management. J Reprod Med. 2005;50:933-939.
23. American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins-Gynecology. Practice Bulletin No. 164: Diagnosis and management of benign breast disorders. Obstet Gynecol. 2016;127:e141-e156.
24. Mirghafourvand M, Mohammad-Alizadeh-Charandabi S, Ahmadpour P, et al. Effects of Vitex agnus and flaxseed on cyclic mastalgia: a randomized controlled trial. Complement Ther Med. 2016;24:90-95.
25. Vaziri F, Zamani Lari M, Sansami Dehaghani A, et al. Comparing the effects of dietary flaxseed and omega-3 fatty acids supplement on cyclical mastalgia in Iranian women: a randomized clinical trial. Int J Fam Med. 2014;2014:174532.
26. Sohrabi N, Kashanian M, Ghafoori SS, et al. Evaluation of the effect of omega-3 fatty acids in the treatment of premenstrual syndrome: “a pilot trial”. Complement Ther Med. 2013;21:141-146.
27. McFayden IJ, Chetty U, Setchell KD, et al. A randomized double blind-cross over trial of soya protein for the treatment of cyclical breast pain. Breast. 2000;9:271-276.
28. Pruthi S, Wahner-Roedler DL, Torkelson CJ, et al. Vitamin E and evening primrose oil for management of cyclical mastalgia: a randomized pilot study. Altern Med Rev. 2010;15:59-67.
29. Halaska M, Raus K, Beles P, et al. Treatment of cyclical mastodynia using an extract of Vitex agnus castus: results of a double-blind comparison with a placebo. Ceska Gynekol. 1998;63:388-392.
30. Schellenberg R. Treatment for the premenstrual syndrome with agnus castus fruit extract: prospective randomised placebo controlled study. BMJ. 2001;322:134-137.
31. Goyal A. Breast pain. BMJ Clin Evid. 2011;2011:0812.
32. Maddox PR, Harrison BJ, Mansel RE. Low-dose danazol for mastalgia. Br J Clin Pract Suppl. 1989;68:43-47.
33. Ahmadinejad M, Delfan B, Haghdani S, et al. Comparing the effect of diclofenac gel and piroxicam gel on mastalgia. Breast J. 2010;16:213-214.
34. Kontostolis E, Stefanidis K, Navrozoglou I, et al. Comparison of tamoxifen with danazol for treatment of cyclical mastalgia. Gynecol Endocrinol. 1997;11:393-397.
35. Marjoribanks J, Brown J, O’Brien PM, et al. Selective serotonin reuptake inhibitors for premenstrual syndrome. Cochrane Database Syst Rev. 2013;(6):CD001396. doi: 10.1002/14651858.CD001396.pub3.
36. Nappi C, Affinito P, Di Carlo C, et al. Double-blind controlled trial of progesterone vaginal cream treatment for cyclical mastodynia in women with benign breast disease. J Endocrinol Invest. 1992;15:801-806.
37. Mansel RE, Goyal A, Preece P, et al. European randomized, multicenter study of goserelin (Zoladex) in the management of mastalgia. Am J Obstet Gynecol. 2004;191:1942-1949.
38. Colak T, Ipek T, Kanik A, et al. Efficacy of topical nonsteroidal antiinflammatory drugs in mastalgia treatment. J Am Coll Surg. 2003;196:525-530.
39. Goyal A. Breast pain. Am Fam Physician. 2016;93:872-873.
40. Srivastava A, Mansel RE, Arvind N, et al. Evidence-based management of mastalgia: a meta-analysis of randomised trials. Breast. 2007;16:503-512.
41. US Preventive Services Task Force. Breast cancer: Screening. Release date: January 2016. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/breast-cancer-screening1. Accessed August 13, 2019.
CASE 1
Robin S is a 40-year-old woman who has never had children or been pregnant. She is in a relationship with a woman so does not use contraception. She has no family history of cancer. She presents with worsening bilateral breast pain that starts 10 days before the onset of her period. The pain has been present for about 4 years, but it has worsened over the last 6 months such that she is unable to wear a bra during these 10 days, finds lying in bed on her side too painful for sleep, and is unable to exercise. She has tried to eliminate caffeine from her diet and takes ibuprofen, but neither of these interventions has controlled her pain. Her breast exam is normal except for diffuse tenderness over both breasts.
CASE 2
Meg R is a 50-year-old healthy woman. She is a G2P2 who breastfed each of her children for 1 year. She does not smoke. She has no family history of breast cancer or other malignancies. She presents with 2 months of deep, left-sided breast pain. She describes the pain as constant, progressive, dull, and achy. She points to a spot in the upper outer quadrant of her left breast and describes the pain as being close to her ribs. She had a screening mammogram 3 weeks earlier that was normal, with findings of dense breasts. She did not tell the technician that she was having pain. Clinical breast examination of both breasts reveals tenderness to deep palpation of the left breast. She has dense breasts but a focal mass is not palpated.
Mastalgia, or breast pain, is one of the most common breast symptoms seen in primary care and a common reason for referrals to breast surgeons. Up to 70% of women will experience breast pain during their lifetime—most in their premenopausal years.1,2
The most common type of breast pain is cyclic (ie, relating to the menstrual cycle); it accounts for up to 70% of all cases of breast pain in women.1,3 The other 2 types of breast pain are noncyclic and extramammary. The cause of cyclic breast pain is unclear, but it is likely hormonally mediated and multifactorial. In the vast majority of women with breast pain, no distinct etiology is found, and there is a very low incidence of breast cancer.2,4
In this review, we describe how to proceed when a woman who is not breastfeeding presents with cyclic or noncyclic breast pain.
Evaluation: Focus on the pain, medications, and history
Evaluation of breast pain should begin with the patient describing the pain, including its quality, location, radiation, and relationship to the menstrual cycle. It’s important to inquire about recent trauma or aggravating activities and to order a pregnancy test for women of childbearing age.1
Cyclic mastalgia is typically described as diffuse, either unilateral or bilateral, with an aching or heavy quality. The pain is often felt in the upper outer quadrant of the breast with radiation to the axilla. It most commonly occurs during the luteal phase of the menstrual cycle, improves with the onset of menses, and is thought to be related to the increased water content in breast stroma caused by increasing hormone levels during the luteal phase.5-7
Continue to: Noncyclic mastalgia
Noncyclic mastalgia is typically unilateral and localized within 1 quadrant of the breast; however, women may report diffuse pain with radiation to the axilla. The pain is often described as burning, achy, or as soreness.5,6 There can be considerable overlap in the presentations of cyclic and noncyclic pain and differentiating between the 2 is often not necessary as management is similar.8
A thorough review of medications is important as several drugs have been associated with breast pain. These include oral contraceptives, hormone therapy, antidepressants (selective serotonin reuptake inhibitors [SSRIs], venlafaxine, mirtazapine), antipsychotics (haloperidol), and some cardiovascular agents (spironolactone, digoxin).5
Inquiring about stress, caffeine intake, smoking status, and bra usage may also yield useful information. Increased stress and caffeine intake have been associated with mastalgia,7 and women who are heavy smokers are more likely to have noncyclic hypersensitive breast pain.9 In addition, women with large breasts often have noncyclic breast pain, particularly if they don’t wear a sufficiently supportive bra.3
Medical, surgical, family history. Relevant aspects of a woman’s past medical, surgical, and family history include prior breast mass or biopsy, breast surgery, and risk factors associated with breast cancer (menarche age < 12 years, menopause age > 55 years, nulliparity, exposure to ionizing radiation, and family history of breast or ovarian cancer).1 A thorough history should include questions to evaluate for extra-mammary etiologies of breast pain such as those that are musculoskeletal or dermatologic in nature (TABLE 11,5,8,10).
Using an objective measure of pain is not only helpful for evaluating the pain itself, but also for determining the effectiveness of treatment strategies. When using the Cardiff Breast Pain Chart, for example, menstrual cycle and level of pain are recorded on a calendar (see www.breastcancercare.org.uk/sites/default/files/files/breast_pain_chart.pdf).11 If the pain is determined to be cyclic, the concern for malignancy is significantly lower.2
Continue to: Ensure that the physical exam is thorough
Ensure that the physical exam is thorough
Women presenting with breast pain should undergo a clinical breast exam in both the upright and supine positions. Inspect for asymmetry, erythema, rashes, skin dimpling, nipple discharge, and retraction/inversion. Palpate the breasts for any suspicious masses, asymmetry, or tenderness, as well as for axillary and/or supraclavicular lymphadenopathy and chest wall tenderness. This is facilitated by having the patient lie in the lateral decubitus position, allowing the breast to fall away from the chest wall.5,12,13
Imaging: Preferred method depends on the age of the patient
Women with a palpable mass should be referred for diagnostic imaging (FIGURE 11,14). Ultrasonography is the recommended modality for women < 30 years of age (TABLE 215). For women between the ages of 30 and 39 years, appropriate initial imaging includes ultrasound, diagnostic mammography, or digital breast tomosynthesis (DBT). For women ≥ 40 years of age, diagnostic mammography or DBT is recommended.15
Cyclic breast pain. Women with cyclic breast pain do not require further evaluation with imaging. Reassurance and symptomatic treatment is appropriate in most cases, as the risk of malignancy is very low in the absence of other concerning signs or symptoms. A screening mammogram may be appropriate for women > 40 years of age who have not had one in the preceding 12 months.1-3,10,12,15
Noncyclic breast pain. In contrast, imaging may be appropriate in women who present with noncyclic breast pain depending on the woman’s age and whether the pain is focal (≤ 25% of the breast and axillary tissue) or diffuse (> 25% of the breast and axillary tissue). Although evidence suggests that the risk of malignancy in women with noncyclic breast pain is low, the American College of Radiology advises that imaging may be useful in some patients to provide reassurance and to exclude a treatable cause of breast pain.3,14 In women with focal pain, ultrasound alone is the preferred modality for women < 30 years of age and ultrasound plus diagnostic mammography is recommended for women ≥ 30 years of age.3,14
In one small study, the use of ultrasonography in women ages < 30 years with focal breast pain had a sensitivity of 100% and a negative predictive value of 100%.16 Similarly, another small retrospective study in older women (average age 56 years) with focal breast pain and no palpable mass showed that ultrasound plus diagnostic mammography had a negative predictive value of 100%.4 DBT may be used in place of mammography to rule out malignancy in this setting.
Continue to: In general...
In general, routine imaging is not indicated for women with noncyclic diffuse breast pain, although diagnostic mammography or DBT may be considered in women ≥ 40 years of age 14 (see “Less common diagnoses with breast pain”4,5,17-21).
SIDEBAR
Less common diagnoses with breast pain
Many women presenting with breast pain are concerned about malignancy. Breast cancer is an uncommon cause of breast pain; only 0.5% of patients presenting with mastalgia without other clinical findings have a malignancy.4 Mastalgia is not a risk factor for breast cancer.
When mastalgia is associated with breast cancer, it is more likely to be unilateral, intense, noncyclic, and progressive.5 Concerning features that warrant further evaluation include new onset focal pain with or without an abnormal exam. If symptoms cannot be explained by an obvious cause (such as trauma, costochondritis, radicular back or intercostal pain, herpes zoster, or superficial thrombophlebitis that does not resolve), diagnostic breast imaging is indicated.
Inflammatory breast cancer (IBC) is an aggressive form of breast cancer that initially presents with breast pain and rapidly enlarging diffuse erythema of the breast in the absence of a discrete breast lump. The initial presentation is similar to that seen with benign inflammatory etiologies of the breast tissue like cellulitis or abscess, duct ectasia, mastitis, phlebitis of the thoracoepigastric vein (Mondor’s disease), or fat necrosis.17 Benign breast conditions due to these causes will generally resolve with appropriate treatment for those conditions within 7 days and will generally not present with the warning signs of IBC, which include a personal history of breast cancer, nonlactational status, and palpable axillary adenopathy. Although uncommon (accounting for 1%-6% of all breast cancer diagnoses), IBC spreads rapidly over a few weeks; thus, urgent imaging is warranted.17
Mastitis is inflammation of the breast tissue that may or may not be associated with a bacterial infection and uncommonly occurs in nonbreastfeeding women. Periductal mastitis is characterized by inflammation of the subareolar ducts and can present with pain, periareolar inflammation, and purulent nipple discharge.18 The condition is typically chronic, and the inflamed ducts may become secondarily infected leading to duct damage and abscess formation. Treatment generally includes antibiotics along with incision and drainage of any associated abscesses or duct excision.18,19
Idiopathic granulomatous mastitis (IGM) is a rare inflammatory breast disease that typically affects young parous women. The presentation can vary from a single peripheral breast mass to multiple areas of infection with abscesses and skin ulceration. The etiology is unknown. Diagnosis requires a core needle biopsy to rule out malignancy or other causes of granulomatous disease. IGM is a benign condition and typically resolves without treatment over the course of several months, although antibiotics and/or drainage may be required for secondary infections.20,21
Continue to: Treatment...
Treatment: When reassurance isn’t enough
Nonrandomized studies suggest that reassurance that mastalgia is benign is enough to treat up to 70% of women.8,22,23 Cyclic breast pain is usually treated symptomatically since the likelihood of breast cancer is extremely low in absence of clinical breast examination abnormalities.2 Because treatment for cyclic and noncyclic mastalgia overlaps, available treatments are discussed together on the following pages.
Lifestyle factors associated with breast pain include stress, caffeine consumption, smoking, and having breastfed 3 or more children (P < .05).9 Although restriction of caffeine, fat, and salt intake may be attempted to address breast pain, no randomized control trials (RCTs) of these interventions have demonstrated effectiveness in reducing mastalgia.8,10
Although not supported by RCTs, first-line treatment of mastalgia includes a recommendation that women, particularly those with large, heavy breasts, wear a well-fitted and supportive bra.8,10
Complementary and alternative medicine treatments for mastalgia
A number of complementary and alternative medicine treatments have demonstrated benefit in treating mastalgia and are often tried before pharmacologic agents (TABLE 324-28). Keep in mind, though, that these therapies are not regulated by the US Food and Drug Administration (FDA). So it’s wise to review particular products with your patient before she buys them (or ask her to bring in any bottles of product for you to review).
Flaxseed, omega-3 fatty acids, and soy milk. Flaxseed, a source of phytoestrogens and omega-3 fatty acids, has been shown to reduce cyclic breast pain in 2 small RCTs.24,25 Breast pain scores were significantly lower for patients ingesting 25 g/d of flaxseed powder compared with placebo.24,25 Omega-3 fatty acids were also more effective than placebo for relief of cyclic breast pain in 2 small RCTs.25,26 Another small RCT demonstrated that women who drank soy milk had a nonsignificant improvement in breast pain compared with those who drank cow’s milk.27
Continue to: Chasteberry
Chasteberry. One RCT demonstrated that Vitex agnus-castus, a chasteberry fruit extract, produced significant and clinically meaningful improvement in visual analogue pain scores for mastalgia, with few adverse effects.29 Another RCT assessing breast fullness as part of the premenstrual syndrome showed significant improvement in breast discomfort for women treated with Vitex agnus-castus.30
Evening primrose oil (EPO). In at least one small study, EPO was effective in controlling breast pain.28 A more recent meta-analysis of all of the EPO trials including gamolenic acid (the active ingredient of EPO) showed no significant difference in mastalgia compared with placebo.31
Pharmacologic Tx options: Start with NSAIDs
Oral nonsteroidal anti-inflammatory drugs (NSAIDs) are often recommended as a first-line treatment for mastalgia and are likely effective for some women; however, there is currently insufficient evidence that oral NSAIDs (or acetaminophen) improve pain (TABLE 432-37; FIGURE 25,13,17). Nevertheless, the potential benefits are thought to outweigh the risk of adverse effects in most patients. A small RCT did demonstrate that topical diclofenac was effective in patients with cyclic and noncyclic mastalgia.38
SSRIs. A meta-analysis of 10 double-blind RCTs of SSRIs used in women with premenstrual symptoms, including 4 studies that specifically included physical symptoms such as breast pain, showed SSRIs to be more effective than placebo at relieving breast pain.35
Progesterones. Several studies have found topical, oral, and injected progesterone ineffective at reducing breast pain.8,36,39 However, one RCT did show topical vaginal micronized progesterone used in the luteal phase to be effective in reducing breast pain by at least 50%.36
Continue to: Oral contraceptives
Oral contraceptives. For women who use oral contraceptive pills and experience cyclic breast pain, continuous dosing (skipping the pill-free week) or using a lower dose of estrogen may improve symptoms. Postmenopausal women with mastalgia that developed with initiation of hormone therapy may benefit from discontinuing hormone therapy or decreasing the estrogen dose; however, there are no RCTs to offer conclusive evidence of the effectiveness of these interventions.10
Danazol. Women with severe mastalgia that does not respond to more benign therapies may require hormone therapy. As with all symptom management, it is imperative to engage the patient in a shared decision-making conversation about the risks and benefits of this treatment strategy. Women must be able to balance the potential adverse effects of agents such as danazol and tamoxifen with the need to alleviate pain and improve quality of life.
Danazol is the only medication FDA-approved for the treatment of mastalgia. Danazol is an androgen that blocks the release of other gonadotropins to limit hormonal stimulation of breast tissue. One RCT demonstrated that danazol (100 mg bid) reduces breast pain in 60% to 90% of women, although adverse effects often limit utility.40 Adverse effects of danazol include weight gain, hot flashes, deepening of the voice, hirsutism, menorrhagia or amenorrhea, muscle cramps, and androgenic effects on a fetus.8,31,40 Danazol may be best used cyclically during the luteal phase of the menstrual cycle to limit these adverse effects with reduction of the dose to 100 mg/d after relief of symptoms.31,40
Tamoxifen, a selective estrogen receptor modulator, has been shown to reduce breast pain in 80% to 90% of women, although it is not indicated for mastalgia.40 Tamoxifen may cause endometrial thickening, hot flashes, menstrual irregularity, venous thromboembolism, and teratogenicity. The 10 mg/d dose appears to be as effective at improving symptoms as the 20 mg/d dose with fewer adverse effects.8,31,40
In a head-to-head randomized trial, tamoxifen was superior to danazol for relief of breast pain with fewer adverse effects.34 Experts recommend limiting use of tamoxifen and danazol to 3 to 6 months. Neither of these drugs is considered safe in pregnancy.
Continue to: Bromocriptine
Bromocriptine, a prolactin inhibitor, has been shown to be more effective than placebo in reducing breast pain, although nausea and dizziness contribute to high discontinuation rates. Bromocriptine is less effective than danazol.40
Goserelin, which is not available in the United States, is a gonadorelin analog (luteinizing hormone-releasing hormone analog) that produces reversible ovarian suppression. One RCT showed that goserelin injection may be more effective than placebo in reducing breast pain.37 Adverse effects include vaginal dryness, hot flashes, decreased libido, oily skin or hair, decreased breast size, and irritability. It is recommended as treatment only for severe refractory mastalgia and that it be used no longer than 6 months.31,37
CASE 1
You reassure Ms. S that her history and physical exam are consistent with cyclic breast pain and not malignancy. You review the current US Preventive Services Task Force recommendations for breast cancer screening in women ages 40 to 49 years (Grade C; women who place a higher value on the potential benefit than the potential harms may choose screening).41 Based on shared decision-making,you offer her a screening mammogram, which returns normal. After confirming that she is using an appropriately-sized supportive bra, you recommend adding 25 g/d of ground flaxseed to her diet.
After 2 months she reports a 30% improvement in her pain. You then recommend chasteberry extract 4.2 mg/d, which provides additional relief to the point where she can now sleep better and walk for exercise.
CASE 2
You order a diagnostic mammogram of the left breast, which is normal, and an ultrasound that demonstrates a 6-cm deep mass. A biopsy determines that Ms. R has invasive lobular breast cancer—an extremely unlikely outcome of breast pain. She elects to have a double mastectomy and reconstruction and is doing well 4 years later.
CORRESPONDENCE
Sarina Schrager, MD, MS, University of Wisconsin Department of Family Medicine and Community Health, 1100 Delaplaine Ct., Madison, WI, 53715; sbschrag@wisc.edu.
CASE 1
Robin S is a 40-year-old woman who has never had children or been pregnant. She is in a relationship with a woman so does not use contraception. She has no family history of cancer. She presents with worsening bilateral breast pain that starts 10 days before the onset of her period. The pain has been present for about 4 years, but it has worsened over the last 6 months such that she is unable to wear a bra during these 10 days, finds lying in bed on her side too painful for sleep, and is unable to exercise. She has tried to eliminate caffeine from her diet and takes ibuprofen, but neither of these interventions has controlled her pain. Her breast exam is normal except for diffuse tenderness over both breasts.
CASE 2
Meg R is a 50-year-old healthy woman. She is a G2P2 who breastfed each of her children for 1 year. She does not smoke. She has no family history of breast cancer or other malignancies. She presents with 2 months of deep, left-sided breast pain. She describes the pain as constant, progressive, dull, and achy. She points to a spot in the upper outer quadrant of her left breast and describes the pain as being close to her ribs. She had a screening mammogram 3 weeks earlier that was normal, with findings of dense breasts. She did not tell the technician that she was having pain. Clinical breast examination of both breasts reveals tenderness to deep palpation of the left breast. She has dense breasts but a focal mass is not palpated.
Mastalgia, or breast pain, is one of the most common breast symptoms seen in primary care and a common reason for referrals to breast surgeons. Up to 70% of women will experience breast pain during their lifetime—most in their premenopausal years.1,2
The most common type of breast pain is cyclic (ie, relating to the menstrual cycle); it accounts for up to 70% of all cases of breast pain in women.1,3 The other 2 types of breast pain are noncyclic and extramammary. The cause of cyclic breast pain is unclear, but it is likely hormonally mediated and multifactorial. In the vast majority of women with breast pain, no distinct etiology is found, and there is a very low incidence of breast cancer.2,4
In this review, we describe how to proceed when a woman who is not breastfeeding presents with cyclic or noncyclic breast pain.
Evaluation: Focus on the pain, medications, and history
Evaluation of breast pain should begin with the patient describing the pain, including its quality, location, radiation, and relationship to the menstrual cycle. It’s important to inquire about recent trauma or aggravating activities and to order a pregnancy test for women of childbearing age.1
Cyclic mastalgia is typically described as diffuse, either unilateral or bilateral, with an aching or heavy quality. The pain is often felt in the upper outer quadrant of the breast with radiation to the axilla. It most commonly occurs during the luteal phase of the menstrual cycle, improves with the onset of menses, and is thought to be related to the increased water content in breast stroma caused by increasing hormone levels during the luteal phase.5-7
Continue to: Noncyclic mastalgia
Noncyclic mastalgia is typically unilateral and localized within 1 quadrant of the breast; however, women may report diffuse pain with radiation to the axilla. The pain is often described as burning, achy, or as soreness.5,6 There can be considerable overlap in the presentations of cyclic and noncyclic pain and differentiating between the 2 is often not necessary as management is similar.8
A thorough review of medications is important as several drugs have been associated with breast pain. These include oral contraceptives, hormone therapy, antidepressants (selective serotonin reuptake inhibitors [SSRIs], venlafaxine, mirtazapine), antipsychotics (haloperidol), and some cardiovascular agents (spironolactone, digoxin).5
Inquiring about stress, caffeine intake, smoking status, and bra usage may also yield useful information. Increased stress and caffeine intake have been associated with mastalgia,7 and women who are heavy smokers are more likely to have noncyclic hypersensitive breast pain.9 In addition, women with large breasts often have noncyclic breast pain, particularly if they don’t wear a sufficiently supportive bra.3
Medical, surgical, family history. Relevant aspects of a woman’s past medical, surgical, and family history include prior breast mass or biopsy, breast surgery, and risk factors associated with breast cancer (menarche age < 12 years, menopause age > 55 years, nulliparity, exposure to ionizing radiation, and family history of breast or ovarian cancer).1 A thorough history should include questions to evaluate for extra-mammary etiologies of breast pain such as those that are musculoskeletal or dermatologic in nature (TABLE 11,5,8,10).
Using an objective measure of pain is not only helpful for evaluating the pain itself, but also for determining the effectiveness of treatment strategies. When using the Cardiff Breast Pain Chart, for example, menstrual cycle and level of pain are recorded on a calendar (see www.breastcancercare.org.uk/sites/default/files/files/breast_pain_chart.pdf).11 If the pain is determined to be cyclic, the concern for malignancy is significantly lower.2
Continue to: Ensure that the physical exam is thorough
Ensure that the physical exam is thorough
Women presenting with breast pain should undergo a clinical breast exam in both the upright and supine positions. Inspect for asymmetry, erythema, rashes, skin dimpling, nipple discharge, and retraction/inversion. Palpate the breasts for any suspicious masses, asymmetry, or tenderness, as well as for axillary and/or supraclavicular lymphadenopathy and chest wall tenderness. This is facilitated by having the patient lie in the lateral decubitus position, allowing the breast to fall away from the chest wall.5,12,13
Imaging: Preferred method depends on the age of the patient
Women with a palpable mass should be referred for diagnostic imaging (FIGURE 11,14). Ultrasonography is the recommended modality for women < 30 years of age (TABLE 215). For women between the ages of 30 and 39 years, appropriate initial imaging includes ultrasound, diagnostic mammography, or digital breast tomosynthesis (DBT). For women ≥ 40 years of age, diagnostic mammography or DBT is recommended.15
Cyclic breast pain. Women with cyclic breast pain do not require further evaluation with imaging. Reassurance and symptomatic treatment is appropriate in most cases, as the risk of malignancy is very low in the absence of other concerning signs or symptoms. A screening mammogram may be appropriate for women > 40 years of age who have not had one in the preceding 12 months.1-3,10,12,15
Noncyclic breast pain. In contrast, imaging may be appropriate in women who present with noncyclic breast pain depending on the woman’s age and whether the pain is focal (≤ 25% of the breast and axillary tissue) or diffuse (> 25% of the breast and axillary tissue). Although evidence suggests that the risk of malignancy in women with noncyclic breast pain is low, the American College of Radiology advises that imaging may be useful in some patients to provide reassurance and to exclude a treatable cause of breast pain.3,14 In women with focal pain, ultrasound alone is the preferred modality for women < 30 years of age and ultrasound plus diagnostic mammography is recommended for women ≥ 30 years of age.3,14
In one small study, the use of ultrasonography in women ages < 30 years with focal breast pain had a sensitivity of 100% and a negative predictive value of 100%.16 Similarly, another small retrospective study in older women (average age 56 years) with focal breast pain and no palpable mass showed that ultrasound plus diagnostic mammography had a negative predictive value of 100%.4 DBT may be used in place of mammography to rule out malignancy in this setting.
Continue to: In general...
In general, routine imaging is not indicated for women with noncyclic diffuse breast pain, although diagnostic mammography or DBT may be considered in women ≥ 40 years of age 14 (see “Less common diagnoses with breast pain”4,5,17-21).
SIDEBAR
Less common diagnoses with breast pain
Many women presenting with breast pain are concerned about malignancy. Breast cancer is an uncommon cause of breast pain; only 0.5% of patients presenting with mastalgia without other clinical findings have a malignancy.4 Mastalgia is not a risk factor for breast cancer.
When mastalgia is associated with breast cancer, it is more likely to be unilateral, intense, noncyclic, and progressive.5 Concerning features that warrant further evaluation include new onset focal pain with or without an abnormal exam. If symptoms cannot be explained by an obvious cause (such as trauma, costochondritis, radicular back or intercostal pain, herpes zoster, or superficial thrombophlebitis that does not resolve), diagnostic breast imaging is indicated.
Inflammatory breast cancer (IBC) is an aggressive form of breast cancer that initially presents with breast pain and rapidly enlarging diffuse erythema of the breast in the absence of a discrete breast lump. The initial presentation is similar to that seen with benign inflammatory etiologies of the breast tissue like cellulitis or abscess, duct ectasia, mastitis, phlebitis of the thoracoepigastric vein (Mondor’s disease), or fat necrosis.17 Benign breast conditions due to these causes will generally resolve with appropriate treatment for those conditions within 7 days and will generally not present with the warning signs of IBC, which include a personal history of breast cancer, nonlactational status, and palpable axillary adenopathy. Although uncommon (accounting for 1%-6% of all breast cancer diagnoses), IBC spreads rapidly over a few weeks; thus, urgent imaging is warranted.17
Mastitis is inflammation of the breast tissue that may or may not be associated with a bacterial infection and uncommonly occurs in nonbreastfeeding women. Periductal mastitis is characterized by inflammation of the subareolar ducts and can present with pain, periareolar inflammation, and purulent nipple discharge.18 The condition is typically chronic, and the inflamed ducts may become secondarily infected leading to duct damage and abscess formation. Treatment generally includes antibiotics along with incision and drainage of any associated abscesses or duct excision.18,19
Idiopathic granulomatous mastitis (IGM) is a rare inflammatory breast disease that typically affects young parous women. The presentation can vary from a single peripheral breast mass to multiple areas of infection with abscesses and skin ulceration. The etiology is unknown. Diagnosis requires a core needle biopsy to rule out malignancy or other causes of granulomatous disease. IGM is a benign condition and typically resolves without treatment over the course of several months, although antibiotics and/or drainage may be required for secondary infections.20,21
Continue to: Treatment...
Treatment: When reassurance isn’t enough
Nonrandomized studies suggest that reassurance that mastalgia is benign is enough to treat up to 70% of women.8,22,23 Cyclic breast pain is usually treated symptomatically since the likelihood of breast cancer is extremely low in absence of clinical breast examination abnormalities.2 Because treatment for cyclic and noncyclic mastalgia overlaps, available treatments are discussed together on the following pages.
Lifestyle factors associated with breast pain include stress, caffeine consumption, smoking, and having breastfed 3 or more children (P < .05).9 Although restriction of caffeine, fat, and salt intake may be attempted to address breast pain, no randomized control trials (RCTs) of these interventions have demonstrated effectiveness in reducing mastalgia.8,10
Although not supported by RCTs, first-line treatment of mastalgia includes a recommendation that women, particularly those with large, heavy breasts, wear a well-fitted and supportive bra.8,10
Complementary and alternative medicine treatments for mastalgia
A number of complementary and alternative medicine treatments have demonstrated benefit in treating mastalgia and are often tried before pharmacologic agents (TABLE 324-28). Keep in mind, though, that these therapies are not regulated by the US Food and Drug Administration (FDA). So it’s wise to review particular products with your patient before she buys them (or ask her to bring in any bottles of product for you to review).
Flaxseed, omega-3 fatty acids, and soy milk. Flaxseed, a source of phytoestrogens and omega-3 fatty acids, has been shown to reduce cyclic breast pain in 2 small RCTs.24,25 Breast pain scores were significantly lower for patients ingesting 25 g/d of flaxseed powder compared with placebo.24,25 Omega-3 fatty acids were also more effective than placebo for relief of cyclic breast pain in 2 small RCTs.25,26 Another small RCT demonstrated that women who drank soy milk had a nonsignificant improvement in breast pain compared with those who drank cow’s milk.27
Continue to: Chasteberry
Chasteberry. One RCT demonstrated that Vitex agnus-castus, a chasteberry fruit extract, produced significant and clinically meaningful improvement in visual analogue pain scores for mastalgia, with few adverse effects.29 Another RCT assessing breast fullness as part of the premenstrual syndrome showed significant improvement in breast discomfort for women treated with Vitex agnus-castus.30
Evening primrose oil (EPO). In at least one small study, EPO was effective in controlling breast pain.28 A more recent meta-analysis of all of the EPO trials including gamolenic acid (the active ingredient of EPO) showed no significant difference in mastalgia compared with placebo.31
Pharmacologic Tx options: Start with NSAIDs
Oral nonsteroidal anti-inflammatory drugs (NSAIDs) are often recommended as a first-line treatment for mastalgia and are likely effective for some women; however, there is currently insufficient evidence that oral NSAIDs (or acetaminophen) improve pain (TABLE 432-37; FIGURE 25,13,17). Nevertheless, the potential benefits are thought to outweigh the risk of adverse effects in most patients. A small RCT did demonstrate that topical diclofenac was effective in patients with cyclic and noncyclic mastalgia.38
SSRIs. A meta-analysis of 10 double-blind RCTs of SSRIs used in women with premenstrual symptoms, including 4 studies that specifically included physical symptoms such as breast pain, showed SSRIs to be more effective than placebo at relieving breast pain.35
Progesterones. Several studies have found topical, oral, and injected progesterone ineffective at reducing breast pain.8,36,39 However, one RCT did show topical vaginal micronized progesterone used in the luteal phase to be effective in reducing breast pain by at least 50%.36
Continue to: Oral contraceptives
Oral contraceptives. For women who use oral contraceptive pills and experience cyclic breast pain, continuous dosing (skipping the pill-free week) or using a lower dose of estrogen may improve symptoms. Postmenopausal women with mastalgia that developed with initiation of hormone therapy may benefit from discontinuing hormone therapy or decreasing the estrogen dose; however, there are no RCTs to offer conclusive evidence of the effectiveness of these interventions.10
Danazol. Women with severe mastalgia that does not respond to more benign therapies may require hormone therapy. As with all symptom management, it is imperative to engage the patient in a shared decision-making conversation about the risks and benefits of this treatment strategy. Women must be able to balance the potential adverse effects of agents such as danazol and tamoxifen with the need to alleviate pain and improve quality of life.
Danazol is the only medication FDA-approved for the treatment of mastalgia. Danazol is an androgen that blocks the release of other gonadotropins to limit hormonal stimulation of breast tissue. One RCT demonstrated that danazol (100 mg bid) reduces breast pain in 60% to 90% of women, although adverse effects often limit utility.40 Adverse effects of danazol include weight gain, hot flashes, deepening of the voice, hirsutism, menorrhagia or amenorrhea, muscle cramps, and androgenic effects on a fetus.8,31,40 Danazol may be best used cyclically during the luteal phase of the menstrual cycle to limit these adverse effects with reduction of the dose to 100 mg/d after relief of symptoms.31,40
Tamoxifen, a selective estrogen receptor modulator, has been shown to reduce breast pain in 80% to 90% of women, although it is not indicated for mastalgia.40 Tamoxifen may cause endometrial thickening, hot flashes, menstrual irregularity, venous thromboembolism, and teratogenicity. The 10 mg/d dose appears to be as effective at improving symptoms as the 20 mg/d dose with fewer adverse effects.8,31,40
In a head-to-head randomized trial, tamoxifen was superior to danazol for relief of breast pain with fewer adverse effects.34 Experts recommend limiting use of tamoxifen and danazol to 3 to 6 months. Neither of these drugs is considered safe in pregnancy.
Continue to: Bromocriptine
Bromocriptine, a prolactin inhibitor, has been shown to be more effective than placebo in reducing breast pain, although nausea and dizziness contribute to high discontinuation rates. Bromocriptine is less effective than danazol.40
Goserelin, which is not available in the United States, is a gonadorelin analog (luteinizing hormone-releasing hormone analog) that produces reversible ovarian suppression. One RCT showed that goserelin injection may be more effective than placebo in reducing breast pain.37 Adverse effects include vaginal dryness, hot flashes, decreased libido, oily skin or hair, decreased breast size, and irritability. It is recommended as treatment only for severe refractory mastalgia and that it be used no longer than 6 months.31,37
CASE 1
You reassure Ms. S that her history and physical exam are consistent with cyclic breast pain and not malignancy. You review the current US Preventive Services Task Force recommendations for breast cancer screening in women ages 40 to 49 years (Grade C; women who place a higher value on the potential benefit than the potential harms may choose screening).41 Based on shared decision-making,you offer her a screening mammogram, which returns normal. After confirming that she is using an appropriately-sized supportive bra, you recommend adding 25 g/d of ground flaxseed to her diet.
After 2 months she reports a 30% improvement in her pain. You then recommend chasteberry extract 4.2 mg/d, which provides additional relief to the point where she can now sleep better and walk for exercise.
CASE 2
You order a diagnostic mammogram of the left breast, which is normal, and an ultrasound that demonstrates a 6-cm deep mass. A biopsy determines that Ms. R has invasive lobular breast cancer—an extremely unlikely outcome of breast pain. She elects to have a double mastectomy and reconstruction and is doing well 4 years later.
CORRESPONDENCE
Sarina Schrager, MD, MS, University of Wisconsin Department of Family Medicine and Community Health, 1100 Delaplaine Ct., Madison, WI, 53715; sbschrag@wisc.edu.
1. Salzman B, Fleegle S, Tully AS. Common breast problems. Am Fam Physician. 2012;86:343-349.
2. Chetlen AL, Kapoor MM, Watts MR. Mastalgia: imaging work-up appropriateness. Acad Radiol. 2017;24:345-349.
3. Expert Panel on Breast Imaging: Jokich PM, Bailey L, D’Orsi C, et al. ACR Appropriateness Criteria Breast Pain. J Am Coll Radiol. 2017;14:S25-S33.
4. Arslan M, Küçükerdem HS, Can H, et al. Retrospective analysis of women with only mastalgia. J Breast Health. 2016;12:151-154.
5. Smith RL, Pruthi S, Fitzpatrick LA. Evaluation and management of breast pain. Mayo Clin Proc. 2004;79:353-372.
6. Mansel RE. ABC of breast diseases. Breast pain. BMJ. 1994;309:866-868.
7. Ader DN, South-Paul J, Adera T, et al. Cyclical mastalgia: prevalence and associated health and behavioral factors. J Psychosom Obstet Gynaecol. 2001;22:71-76.
8. Iddon J, Dixon JM. Mastalgia. BMJ. 2013;347:f3288.
9. Eren T, Aslan A, Ozemir IA, et al. Factors effecting mastalgia. Breast Care (Basel). 2016;11:188-193.
10. Pearlman MD, Griffin JL. Benign breast disease. Obstet Gynecol. 2010;116:747-758.
11. Gateley CA, Mansel RE. The Cardiff Breast Score. Br J Hosp Med. 1991;45:16.
12. Michigan Medicine. University of Michigan. Common breast problems: guidelines for clinical care. https://www.med.umich.edu/1info/FHP/practiceguides/breast/breast.pdf. Updated June 2013. Accessed September 3, 2019.
13. Millet AV, Dirbas FM. Clinical management of breast pain: a review. Obstet Gynecol Surv. 2002;57:451-461.
14. American College of Radiology. ACR Appropriateness Criteria: Breast Pain. https://acsearch.acr.org/docs/3091546/Narrative/. Revised 2018. Accessed July 2, 2019.
15. American College of Radiology. ACR Appropriateness Criteria: Palpable Breast Masses. https://acsearch.acr.org/docs/69495/Narrative/. Revised 2016. Accessed September 3, 2019.
16. Loving VA, DeMartini WB, Eby PR, et al. Targeted ultrasound in women younger than 30 years with focal breast signs or symptoms: outcomes analyses and management implications. AJR Am J Roentgenol. 2010;195:1472-1477.
17. Molckovsky A, Fitzgerald B, Freedman O, et al. Approach to inflammatory breast cancer. Can Fam Physician. 2009;55:25-31.
18. Ammari FF, Yaghan RJ, Omari AK. Periductal mastitis: clinical characteristics and outcome. Saudi Med J. 2002;23:819-822.
19. Lannin DR. Twenty-two year experience with recurring subareolar abscess and lactiferous duct fistula treated by a single breast surgeon. Am J Surg. 2004;188:407-410.
20. Wilson JP, Massoll N, Marshall J, et al. Idiopathic granulomatous mastitis: in search of a therapeutic paradigm. Am Surg. 2007;73:798-802.
21. Bouton ME, Jayaram L, O’Neill PJ, et al. Management of idiopathic granulomatous mastitis with observation. Am J Surg. 2015;210:258-262.
22. Olawaiye A, Withiam-Leitch M, Danakas G, et al. Mastalgia: a review of management. J Reprod Med. 2005;50:933-939.
23. American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins-Gynecology. Practice Bulletin No. 164: Diagnosis and management of benign breast disorders. Obstet Gynecol. 2016;127:e141-e156.
24. Mirghafourvand M, Mohammad-Alizadeh-Charandabi S, Ahmadpour P, et al. Effects of Vitex agnus and flaxseed on cyclic mastalgia: a randomized controlled trial. Complement Ther Med. 2016;24:90-95.
25. Vaziri F, Zamani Lari M, Sansami Dehaghani A, et al. Comparing the effects of dietary flaxseed and omega-3 fatty acids supplement on cyclical mastalgia in Iranian women: a randomized clinical trial. Int J Fam Med. 2014;2014:174532.
26. Sohrabi N, Kashanian M, Ghafoori SS, et al. Evaluation of the effect of omega-3 fatty acids in the treatment of premenstrual syndrome: “a pilot trial”. Complement Ther Med. 2013;21:141-146.
27. McFayden IJ, Chetty U, Setchell KD, et al. A randomized double blind-cross over trial of soya protein for the treatment of cyclical breast pain. Breast. 2000;9:271-276.
28. Pruthi S, Wahner-Roedler DL, Torkelson CJ, et al. Vitamin E and evening primrose oil for management of cyclical mastalgia: a randomized pilot study. Altern Med Rev. 2010;15:59-67.
29. Halaska M, Raus K, Beles P, et al. Treatment of cyclical mastodynia using an extract of Vitex agnus castus: results of a double-blind comparison with a placebo. Ceska Gynekol. 1998;63:388-392.
30. Schellenberg R. Treatment for the premenstrual syndrome with agnus castus fruit extract: prospective randomised placebo controlled study. BMJ. 2001;322:134-137.
31. Goyal A. Breast pain. BMJ Clin Evid. 2011;2011:0812.
32. Maddox PR, Harrison BJ, Mansel RE. Low-dose danazol for mastalgia. Br J Clin Pract Suppl. 1989;68:43-47.
33. Ahmadinejad M, Delfan B, Haghdani S, et al. Comparing the effect of diclofenac gel and piroxicam gel on mastalgia. Breast J. 2010;16:213-214.
34. Kontostolis E, Stefanidis K, Navrozoglou I, et al. Comparison of tamoxifen with danazol for treatment of cyclical mastalgia. Gynecol Endocrinol. 1997;11:393-397.
35. Marjoribanks J, Brown J, O’Brien PM, et al. Selective serotonin reuptake inhibitors for premenstrual syndrome. Cochrane Database Syst Rev. 2013;(6):CD001396. doi: 10.1002/14651858.CD001396.pub3.
36. Nappi C, Affinito P, Di Carlo C, et al. Double-blind controlled trial of progesterone vaginal cream treatment for cyclical mastodynia in women with benign breast disease. J Endocrinol Invest. 1992;15:801-806.
37. Mansel RE, Goyal A, Preece P, et al. European randomized, multicenter study of goserelin (Zoladex) in the management of mastalgia. Am J Obstet Gynecol. 2004;191:1942-1949.
38. Colak T, Ipek T, Kanik A, et al. Efficacy of topical nonsteroidal antiinflammatory drugs in mastalgia treatment. J Am Coll Surg. 2003;196:525-530.
39. Goyal A. Breast pain. Am Fam Physician. 2016;93:872-873.
40. Srivastava A, Mansel RE, Arvind N, et al. Evidence-based management of mastalgia: a meta-analysis of randomised trials. Breast. 2007;16:503-512.
41. US Preventive Services Task Force. Breast cancer: Screening. Release date: January 2016. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/breast-cancer-screening1. Accessed August 13, 2019.
1. Salzman B, Fleegle S, Tully AS. Common breast problems. Am Fam Physician. 2012;86:343-349.
2. Chetlen AL, Kapoor MM, Watts MR. Mastalgia: imaging work-up appropriateness. Acad Radiol. 2017;24:345-349.
3. Expert Panel on Breast Imaging: Jokich PM, Bailey L, D’Orsi C, et al. ACR Appropriateness Criteria Breast Pain. J Am Coll Radiol. 2017;14:S25-S33.
4. Arslan M, Küçükerdem HS, Can H, et al. Retrospective analysis of women with only mastalgia. J Breast Health. 2016;12:151-154.
5. Smith RL, Pruthi S, Fitzpatrick LA. Evaluation and management of breast pain. Mayo Clin Proc. 2004;79:353-372.
6. Mansel RE. ABC of breast diseases. Breast pain. BMJ. 1994;309:866-868.
7. Ader DN, South-Paul J, Adera T, et al. Cyclical mastalgia: prevalence and associated health and behavioral factors. J Psychosom Obstet Gynaecol. 2001;22:71-76.
8. Iddon J, Dixon JM. Mastalgia. BMJ. 2013;347:f3288.
9. Eren T, Aslan A, Ozemir IA, et al. Factors effecting mastalgia. Breast Care (Basel). 2016;11:188-193.
10. Pearlman MD, Griffin JL. Benign breast disease. Obstet Gynecol. 2010;116:747-758.
11. Gateley CA, Mansel RE. The Cardiff Breast Score. Br J Hosp Med. 1991;45:16.
12. Michigan Medicine. University of Michigan. Common breast problems: guidelines for clinical care. https://www.med.umich.edu/1info/FHP/practiceguides/breast/breast.pdf. Updated June 2013. Accessed September 3, 2019.
13. Millet AV, Dirbas FM. Clinical management of breast pain: a review. Obstet Gynecol Surv. 2002;57:451-461.
14. American College of Radiology. ACR Appropriateness Criteria: Breast Pain. https://acsearch.acr.org/docs/3091546/Narrative/. Revised 2018. Accessed July 2, 2019.
15. American College of Radiology. ACR Appropriateness Criteria: Palpable Breast Masses. https://acsearch.acr.org/docs/69495/Narrative/. Revised 2016. Accessed September 3, 2019.
16. Loving VA, DeMartini WB, Eby PR, et al. Targeted ultrasound in women younger than 30 years with focal breast signs or symptoms: outcomes analyses and management implications. AJR Am J Roentgenol. 2010;195:1472-1477.
17. Molckovsky A, Fitzgerald B, Freedman O, et al. Approach to inflammatory breast cancer. Can Fam Physician. 2009;55:25-31.
18. Ammari FF, Yaghan RJ, Omari AK. Periductal mastitis: clinical characteristics and outcome. Saudi Med J. 2002;23:819-822.
19. Lannin DR. Twenty-two year experience with recurring subareolar abscess and lactiferous duct fistula treated by a single breast surgeon. Am J Surg. 2004;188:407-410.
20. Wilson JP, Massoll N, Marshall J, et al. Idiopathic granulomatous mastitis: in search of a therapeutic paradigm. Am Surg. 2007;73:798-802.
21. Bouton ME, Jayaram L, O’Neill PJ, et al. Management of idiopathic granulomatous mastitis with observation. Am J Surg. 2015;210:258-262.
22. Olawaiye A, Withiam-Leitch M, Danakas G, et al. Mastalgia: a review of management. J Reprod Med. 2005;50:933-939.
23. American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins-Gynecology. Practice Bulletin No. 164: Diagnosis and management of benign breast disorders. Obstet Gynecol. 2016;127:e141-e156.
24. Mirghafourvand M, Mohammad-Alizadeh-Charandabi S, Ahmadpour P, et al. Effects of Vitex agnus and flaxseed on cyclic mastalgia: a randomized controlled trial. Complement Ther Med. 2016;24:90-95.
25. Vaziri F, Zamani Lari M, Sansami Dehaghani A, et al. Comparing the effects of dietary flaxseed and omega-3 fatty acids supplement on cyclical mastalgia in Iranian women: a randomized clinical trial. Int J Fam Med. 2014;2014:174532.
26. Sohrabi N, Kashanian M, Ghafoori SS, et al. Evaluation of the effect of omega-3 fatty acids in the treatment of premenstrual syndrome: “a pilot trial”. Complement Ther Med. 2013;21:141-146.
27. McFayden IJ, Chetty U, Setchell KD, et al. A randomized double blind-cross over trial of soya protein for the treatment of cyclical breast pain. Breast. 2000;9:271-276.
28. Pruthi S, Wahner-Roedler DL, Torkelson CJ, et al. Vitamin E and evening primrose oil for management of cyclical mastalgia: a randomized pilot study. Altern Med Rev. 2010;15:59-67.
29. Halaska M, Raus K, Beles P, et al. Treatment of cyclical mastodynia using an extract of Vitex agnus castus: results of a double-blind comparison with a placebo. Ceska Gynekol. 1998;63:388-392.
30. Schellenberg R. Treatment for the premenstrual syndrome with agnus castus fruit extract: prospective randomised placebo controlled study. BMJ. 2001;322:134-137.
31. Goyal A. Breast pain. BMJ Clin Evid. 2011;2011:0812.
32. Maddox PR, Harrison BJ, Mansel RE. Low-dose danazol for mastalgia. Br J Clin Pract Suppl. 1989;68:43-47.
33. Ahmadinejad M, Delfan B, Haghdani S, et al. Comparing the effect of diclofenac gel and piroxicam gel on mastalgia. Breast J. 2010;16:213-214.
34. Kontostolis E, Stefanidis K, Navrozoglou I, et al. Comparison of tamoxifen with danazol for treatment of cyclical mastalgia. Gynecol Endocrinol. 1997;11:393-397.
35. Marjoribanks J, Brown J, O’Brien PM, et al. Selective serotonin reuptake inhibitors for premenstrual syndrome. Cochrane Database Syst Rev. 2013;(6):CD001396. doi: 10.1002/14651858.CD001396.pub3.
36. Nappi C, Affinito P, Di Carlo C, et al. Double-blind controlled trial of progesterone vaginal cream treatment for cyclical mastodynia in women with benign breast disease. J Endocrinol Invest. 1992;15:801-806.
37. Mansel RE, Goyal A, Preece P, et al. European randomized, multicenter study of goserelin (Zoladex) in the management of mastalgia. Am J Obstet Gynecol. 2004;191:1942-1949.
38. Colak T, Ipek T, Kanik A, et al. Efficacy of topical nonsteroidal antiinflammatory drugs in mastalgia treatment. J Am Coll Surg. 2003;196:525-530.
39. Goyal A. Breast pain. Am Fam Physician. 2016;93:872-873.
40. Srivastava A, Mansel RE, Arvind N, et al. Evidence-based management of mastalgia: a meta-analysis of randomised trials. Breast. 2007;16:503-512.
41. US Preventive Services Task Force. Breast cancer: Screening. Release date: January 2016. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/breast-cancer-screening1. Accessed August 13, 2019.
PRACTICE RECOMMENDATIONS
› Instruct patients to maintain a pain diary, which, along with a careful history and physical examination, helps to determine the cause of breast pain and the type of evaluation needed. C
› Treat cyclic, bilateral breast pain with chasteberry and flaxseed. B
› Consider short-term treatment with danazol or tamoxifen for women with severe pain. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Medical Cannabis: A guide to the clinical and legal landscapes
CASE
Barry S, a 45-year-old man with a new diagnosis of non-Hodgkin’s lymphoma, recently started induction chemotherapy. He has struggled with nausea, profound gustatory changes, and poor appetite; various antiemetics have provided only minimal relief. He tells you that he is hesitant to try “yet another pill” but has heard and read that marijuana (genus Cannabis) is used to alleviate disruptive chemotherapy-induced adverse effects. He asks if this is a treatment you’d recommend for him.
As Mr. S’s physician, how do you respond?
Understandably, some family physicians are hesitant to recommend an unregulated, federally illegal substance characterized by conflicting or absent evidence of safety and effectiveness.1 Nevertheless, throughout history and in the current court of public opinion, medical Cannabis has overwhelming support,2 leading to legalization in most of the United States.
As with many traditionally accepted therapies (whether they are or are not supported by substantial evidence), physicians are expected to provide individualized guidance regarding minimizing risk and maximizing benefit of the therapeutic use of Cannabis. The rapidly growing scientific and commercial fields of medical Cannabis guarantee that information on this topic will constantly be changing—and will often be contradictory. In this article, we review the most common concerns about medical Cannabis and provide up-to-date evidence on its use.
The pharmacology of cannabis
Cannabis sativa was among the earliest plants cultivated by man, with the first evidence of its use in China, approximately 4000 BC, to make twine and rope from its fibers.3 Records of medicinal Cannabis date back to the world’s oldest pharmacopoeia, a written summary of what was known about herbal medicine through the late 16th century.4
The 2 principal species of Cannabis are sativa and indica. There is no good medical evidence to separate the impacts of either strain; however, a staggering amount of lay information exists about the reported differing effects of each strain.5
Chemical constituents. Phytocannabinoids derived from C sativa are the plant’s best-known proteins, constituting a complex lipid-signaling network involved in numerous physiological processes. There are more than 100 known phytocannabinoids, the most well-recognized being Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD). Additional sources of cannabinoids include endogenous cannabinoids, or endocannabinoids, and synthetic cannabinoids.
The endocannabinoid system, comprising cannabinoid receptors, endocannabinoids, and their specific enzymes, is a potential therapeutic target for a variety of pathologic processes.6,7 The 2 most well-studied targets for cannabinoids in the human body are the cannabinoid receptors CB1 and CB2, found throughout the body: CB1, predominantly in the central and peripheral nervous system, and CB2 in a more limited distribution in the immune and hematopoietic systems. Other pathways activated or antagonized by THC and CBD exist, but are less well-mapped than CB1 and CB2.
[polldaddy:10402702]
Continue to: Botanical or synthetic?
Botanical or synthetic? It is important to distinguish between synthetic and plant-based cannabinoids, for you and your patients' benefit. Pharmaceutical (synthetic) THC is just that: THC alone. Whole-plant Cannabis, on the other hand, has hundreds of additional chemicals—most notably, phytocannabinoids and terpenoids. Data on the mechanisms of action and interactions of these additional chemicals are limited.
Although clinical trials have been undertaken with synthetic cannabinoids, there is increasing understanding and interest in the medical community of whole-plant Cannabis as a distinct entity. For example, nabiximols is a novel development in plant-based Cannabis products. Available as an oromucosal spray, a dose provides THC and CBD at 2.7 mg/100 mcL. Nabiximols is not approved by the US Food and Drug Administration (FDA) but is widely used
A third class of Cannabis comprises nonregulated synthetic cannabinoids that have no medically recognized benefit. They are solely a drug of abuse; common names include “K2” and “Spice.” These cannabinoids are outside of the scope of our discussion, but patients and providers should be aware of these cannabinoids because they are street-available. Unsuspecting patients might not know the difference between abusive and therapeutic formulations.8
Delivery and strength. Common forms of plant-based Cannabis include leaf that is smoked or vaporized, oral tincture, pill, and oil concentrate that can be vaporized. All forms come in a range of THC:CBD ratios—from as high as 90% THC content to 0% THC and all CBD-based content. Patients who are naïve to Cannabis might be concerned about formulations with a high THC concentration because of the psychoactive effects of this substance. Given the minimal CNS activity of CBD, a tolerable therapeutic starting point often is a THC:CBD ratio of 1:1, which contains a lower percentage of THC.4
Physiologic effects. THC is a partial agonist of CB1 and CB2 receptors; CBD functions as an antagonist at both receptors. The primary effects of THC result from activation of CB1 receptors, which exist in various areas of the cerebrum and cerebellum, as well as in the spinal cord.7 THC exerts its psychotropic effects at CB1 sites in the central nervous system; CBD can antagonize these THC effects at CB1 receptors. CBD also has anti-inflammatory and other effects that are mediated through peripherally distributed CB2 receptors.9
Continue to: THC has tremendously...
THC has tremendously complex capacity for activation and inhibition within various neuronal circuits, resulting in effects on mood, appetite, and movement.1,7 Adverse effects associated with Cannabis are wide-ranging: Most commonly, nausea, drowsiness, fatigue, dry mouth, and dizziness are reported alongside cognitive effects. Rarely, tachycardia, hypotension, hyperemesis, and depression can be seen.
Clinical implications and indications
Clinical indications for legal medical Cannabis vary by state; typically, indications include human immunodeficiency virus (HIV) infection and acquired immune deficiency syndrome (AIDS), cachexia, cancer, glaucoma, epilepsy and other seizure disorders, severe and chronic pain, spasticity from neurodegenerative disorders, and irritable bowel syndrome and Crohn’s disease, as well as a wide range of less-universal diagnoses. A patient may have a so-called qualifying diagnosis (ie, having the potential to allow the patient to be certified to purchase and use Cannabis) in one state but not have the same standing in a neighboring state, posing a complex legal issue. Given the significant complexities of performing medical research with plant-based Cannabis in the United States, little research has been done. The result? Policymakers are grappling with questions that only scientific research can answer:
- For which conditions does Cannabis provide medicinal benefit equal to or superior to alternatives?
- What are the appropriate dosages (or CBD:THC ratios), formulations (plant-derived or synthetic), and routes of administration (smoked, ingested, or topical) for various conditions?
Bird’s-eye view of clinical research. A meta-analysis of isolated synthetic and plant-based cannabinoids for medical use was published in 2015.10 The analysis included more than 6000 patients in 79 trials, most of which assessed whether dronabinol or nabilone (both synthetic isolates) were effective compared to placebo or alternative non-Cannabis-based therapy. The studies examined chemotherapy-induced nausea and vomiting, appetite stimulation in HIV and AIDS, chronic pain, spasticity, depression and anxiety, sleep disorders, and psychosis.
Twenty-eight studies assessed chemotherapy-induced nausea and vomiting. All of these studies indicated a greater benefit from cannabinoids than from alternative antiemetic regimens and placebo; however, that finding did not reach statistical significance across all studies.
There was moderate evidence to suggest the use of Cannabis for neuropathic and nonneuropathic cancer-related pain. However, there is an increased short-term risk of adverse events with synthetic isolates dronabinol (when used for pain) and nabilone (when used for nausea and vomiting).
Continue to: The primary conclusion...
The primary conclusion of the meta-analysis is that further study is required because little evidence exists on the effects and the adverse events of plant-based Cannabis.
HIV infection. Data on Cannabis for the treatment of refractory neuropathy and appetite stimulation in HIV infection is mixed.10,11 Smoked Cannabis for medically refractory neuropathy was examined in several trials:
- In a randomized crossover trial, researchers found statistically significant subjective improvement in neuropathic pain, with minimal intolerable adverse effects, in the 28 HIV-infected participants who completed the trial.11
- In another study,Cannabis ingested in various forms resulted in appetite stimulation in late-stage HIV infection but did not produce statistically significant weight gain.10
Pediatric epilepsy. Research on pediatric patients who have epilepsy characterized by refractory seizures has shown that the impact of Cannabis on their disease is promising. Specifically, CBD has shown tremendous potential impact: Patients experienced a statistically significant reduction in the number of seizures.9 In 2018, the FDA approved the first plant-based derivative of Cannabis: an oral cannabidiol (marketed as Epidiolex [Greenwich Biosciences, Inc.]) for the treatment of intractable seizures associated with Lennox-Gastaut syndrome and Dravet syndrome, rare and severe forms of epilepsy. Epidiolex is the first FDA-approved drug that contains a purified drug substance derived from marijuana.
CASE
Mr. S’s diagnosis of cancer is broadly included in the list of Cannabis-qualifying illnesses in all 34 states that certify patients for medical Cannabis. He qualifies both because (1) he is a cancer patient and (2) he has not found relief from chemotherapy-induced nausea and vomiting with several targeted therapies, including 5-hydroxytryptamine-receptor antagonists, steroids, and antipsychotics. Evidence supports CB1 and CB2 as potential targets for antiemetic treatment.
Given Mr. S’s consequent anorexia, his frustration with taking an increasing number of medications, and possible adverse effects of additional therapy, Cannabis is a reasonable course of action to treat nausea and vomiting. He would be able to use oral tincture or vaporization of oil to further limit his pill burden—likely, with a THC:CBD ratio of 1:1 or similar.
Continue to: Based on recent observational data...
Based on recent observational data from New York Cannabis dispensaries, cancer patients pursing Cannabis to treat chemotherapy-induced symptoms report that (1) either products with a high concentration of THC or products that contain THC and CBD in a 1:1 ratio are most effective and (2) products in 1:1 ratio of THC and CBD are most tolerable.
A legal system at oddsover the status of medical Cannabis
The core legal issue underlying medical Cannabis is a contradiction between federal and state laws.
At the federal level. The federal government regulates the lawful production, possession, and distribution of controlled substances through the Controlled Substances Act (CSA).12 The CSA is the basis for categorizing certain plants, drugs, and chemicals into 5 schedules, based on the substance’s medical use, potential for abuse, and safety or dependence liability.13 Under the CSA, marijuana (along with substances such as heroin and methamphetamine) is categorized as Schedule I14; ie, the substance
- has high potential for abuse,
- has no accepted therapeutic medical use in the United States, and
- lacks acceptable safety for use under medical supervision.
Despite waxing and waning efforts to protect states from federal prosecution, any use of a Schedule-1 substance violates federal law.15
In June 2018, a bipartisan group of federal lawmakers introduced a bill designed to amend the CSA and guarantee the rights of states and territories to self-determine marijuana regulation. The bill established a so-called STATES (Strengthening the Tenth Amendment Through Entrusting States) Act that “amends the Controlled Substances Act (21 U.S.C. § 801 et seq.) so that—as states and tribes comply with a few basic protections—its provisions no longer apply to any person acting in compliance with state or tribal laws relating to the manufacture, production, possession, distribution, dispensation, administration, or delivery of marijuana.”15
Continue to: The bill was referred to the Senate...
The bill was referred to the Senate and House Judiciary Committees but, ultimately, the STATES Act was blocked from debate in 2018.
On April 4, 2019, the Act was reintroduced in the House (H.R. 2093) and Senate (S. 1028) of the 116th Congress. Although there is bipartisan support for this bill, the timeline for moving it forward is unclear.16,17
At the state level. Thirty-four states have comprehensive public medical marijuana and Cannabis programs. The National Conference of State Legislatures18 (www.ncsl.org) designates a program “comprehensive” if it
- includes protection from criminal penalties for using marijuana for a medical purpose,
- allows access to marijuana through home cultivation, dispensaries, or other system,
- permits a variety of strains, including those more potent than what is labeled “low-THC,” and
- allows smoking or vaporization of marijuana products, plant-based material, or extract.
An additional 14 states allow for “low-THC, high-CBD” products for medical reasons, in limited situations, or as a legal defense. Regulation in these states varies widely, however: Some states allow industrialized hemp products only; others do not provide for any in-state production.18
Last, many states have some form of so-called “affirmative-defense” statutes that allow people charged with marijuana possession to mention use of marijuana for medical purposes as a possible defense.
Continue to: Physician shield
Physician shield. Despite inconsistent and evolving state and federal laws, physicians are protected, based on the Conant v Walters decision, from prosecution or revocation of their prescriptive authority for the professional “recommendation” of the use of medical marijuana.19 In 2002, the US Ninth Circuit Court of Appeals upheld the permanent injunction, based on a physician’s First Amendment right to discuss medical marijuana with patients.
CASE
Mr. S is amenable to trial of Cannabis to relieve nausea and anorexia. He asks you if he is allowed to use Cannabis at work, were he to return to an office-based desk job—even part-time—during treatment for cancer.
How would you answer Mr. S? Patients are legally protected from workplace penalties and dismissal for using and consuming Cannabis in states with a medical Cannabis law (including the state in which Mr. S resides). However, all employers have some variability in corporate policy, especially if a person works in a federally supported or regulated occupation. It’s always helpful to advise patients who will be using medical Cannabis to be proactive and speak with a human resources or employee health department staff member before beginning a course of medical Cannabis. Additionally, Cannabis with any amount of THC has the ability to alter focus, concentration, and perceptions of time. Thus, if a patient using medical Cannabis with THC asks about driving to work, he should be given the same advice one would offer about driving after consuming alcohol or ingesting opioids.
Common concerns
Ignorance of legal status. Theoretically, the Conant v Walters decision protects physicians from investigation for recommending medical Cannabis even in states where it is illegal. However, you should adhere closely to procedures set out by your state. The National Council of State Legislatures provides up-to-date information on each state’s procedures and programs,18 and the American Society of Addiction Medicine (www.asam.org) has established standards of professionalism for physicians who discuss medical Cannabis with patients (TABLE).20
Exposure to smoke. Cannabis smoke carries many of the same carcinogens found in tobacco smoke; furthermore, use of Cannabis and tobacco are highly correlated, confounding many population-based studies. The manner of inhalation of Cannabis can result in significantly higher levels of tar and carbon dioxide than with tobacco smoking. Because the effects of Cannabis last longer, however, people who smoke Cannabis may smoke it less often than tobacco smokers smoke tobacco.21
Continue to: Large cross-sectional...
Large cross-sectional and longitudinal studies have not found a link between Cannabis smoking and long-term pulmonary consequences, such as chronic obstructive pulmonary disease and lung cancer.22,23 The technology of Cannabis delivery systems has progressed far more rapidly than the clinical evidence for or against such technology.
“Vaping” is an informal term for inhalation of aerosolized Cannabis components and water vapor. Vaporizers do not heat Cannabis to the point of combustion; therefore, they provide less exposure to smoke-related toxicants while providing similar time of onset.
Neuropsychiatric adverse effects. Data regarding the relationship between Cannabis use and psychiatric disorders are incompletely understood, in conflict, and related to cannabinoid type. Consider Pennsylvania’s addition of anxiety disorder as a “serious medical condition” covered under the Pennsylvania Medical Marijuana Act.24 Although patients often report the use of medical Cannabis to treat anxiety,25 panic attacks are often associated with Cannabis use.26
While there is a clear association between Cannabis use and psychotic disorder, a causal link has yet to be unequivocally established. However, the rate of psychiatric hospitalization is increased in bipolar disorder and schizophrenia patients who use Cannabis heavily.27
We recommend, therefore, that physicians screen patients for serious mental health concerns before recommending or certifying them to use medical Cannabis.
Continue to: Overconsumption of edibles
Overconsumption of edibles. Cannabis edibles (ie, food products infused with Cannabis extract) are distinct from inhaled Cannabis in regard to onset, duration, and potential for adverse effects. Cannabis edibles might be more popular than inhaled products among older medical Cannabis users.28
Edible Cannabis has a reported onset of 1 to 3 hours (compared to 5-10 minutes with inhaled Cannabis) and a duration of effect of 6 to 8 hours (compared with 2-4 hours for inhaled products).29 These qualities might render Cannabis edibles preferable to inhaled formulations for controlling chronic symptoms and conditions. However, delayed onset of edible products and wide variation in the concentration of THC also increase the risk of overconsumption, which can lead to overdose and self-limited Cannabis-induced psychosis. We recommend providing patient education about the effects of the physiologically active therapeutic compounds tetrahydrocannabinol and cannabidiol, to prevent overconsumption of high-THC products.30
CASE
Mr. S returns to your office after a trial of Cannabis as vaporized oil and reports some relief of nausea and a mild increase in appetite, but no weight gain. He is concerned about overconsumption or overdose, and asks you what the risks of these problems are.
How should you counsel Mr. S? Explain that ingestion of Cannabis has a prolonged onset of action; vaporization has a more rapid onset of action; therefore, he could more easily self-regulate ingestion with the vehicle he has chosen. In states where edible Cannabis products are legal, education is necessary so that patients know how much of the edible to consume and how long they will wait to feel the full impact of the effects of THC.30
Cannabis use disorder in the context of medical marijuana
Cannabis use disorder (CUD) incorporates general diagnostic features of a substance use disorder, including behavioral, cognitive, and physiologic symptoms such as cravings, tolerance, and withdrawal, in the setting of persistent use despite significant substance-related problems.31 Features of Cannabis withdrawal syndrome include irritability, anger or aggression, anxiety, depressed mood, restlessness, sleep difficulty, and decreased appetite or weight loss.31 Cannabis use disorder can develop in people who use medical Cannabis; however, physiologic symptoms of tolerance and withdrawal can also develop in the setting of appropriate medical use and do not, in isolation, represent CUD.
Continue to: A recent study...
A recent study considered nationwide cross-sectional survey data from the US National Survey of Drug Use and Health to examine the relationship between medical marijuana laws and CUD.32 Study findings did not show an increase in the prevalence of CUD or marijuana use among adults in states with a legalized medical marijuana program. Importantly, when researchers looked at marijuana use among adolescents and young adults, they found no increase in measured outcomes (eg, active [ie, past-month] marijuana use, heavy [> 300 d/yr] use, and a diagnosis of CUD) after medical marijuana laws were passed.32
A paucity of pediatric data
The adolescent brain might be more vulnerable to the adverse long-term effects of Cannabis; there is potential significant harm associated with Cannabis in children and adolescence. However, accurate data concerning risk and benefit are limited.
The most recent policy statement of the American Academy of Pediatrics (AAP) reflects this paucity of data.33 The AAP opposes the use of medical Cannabis outside regulation by the FDA, although the organization allows for consideration of compassionate use of medical Cannabis for children who have life-threatening or severely disabling conditions. The AAP does support (1) additional research into pharmaceutical cannabinoids and (2) changing Cannabis from Schedule I to Schedule II to facilitate this process. Since the publication of the policy statement, Pediatrics, the official journal of the AAP, has published a review of medical cannabinoids and found (1) strong evidence for benefit in chemotherapy-induced nausea and vomiting and (2) accumulating evidence of benefit in epilepsy.34
Recognized risk: Not supporting medical Cannabis
As with all medical decisions, the risks and benefits of certifying patients for medical Cannabis must be balanced against the risks and benefits of not doing so. The risks that accompany failure to certify a patient for medical marijuana fall into 3 categories:
Blocking access to a substance that has potential therapeutic benefit. More data regarding the potential benefits and risks of medical Cannabis will, undoubtedly, dispel some of the uncertainty regarding the decision to certify a patient for medical Cannabis. When you recommend medical Cannabis and certify patients for its use, you do so with the certainty that the Cannabis safety index (ie, risk of overdose or serious adverse effects) is exceedingly low.35
Continue to: Limiting patients to other medications
Limiting patients to other medications that, potentially, carry a risk of more or greater harmful effects. An example is the decision to prescribe an opioid for chronic pain instead of certifying a patient for medical Cannabis. For certain other conditions, including chemotherapy-induced nausea and vomiting, FDA-approved pharmaceuticals might have more reported serious adverse events and interactions than medical Cannabis.36
Resigning patients to obtain Cannabis from an illegal source. This speaks to harm reduction and social justice, because obtaining Cannabis from an illegal source carries health and legal risks:
- Increased health risks result from lacing or cutting botanical or synthetic Cannabis products with potentially toxic substances. Cocaine, the rodenticide brodifacoum, methamphetamine, and phencyclidine are all known, or have been reported, to be added to botanical and synthetic Cannabis.37
- Legal repercussions of Cannabis possession are disproportionately racially based, with a significantly higher arrest rate among people of color, even in states where medical Cannabis has been legalized.38
CORRESPONDENCE
Lara Carson Weinstein, MD, MPH, DrPH, Department of Family and Community Medicine, Sidney Kimmel Medical College at Thomas Jefferson University, 1015 Walnut Street, Suite 401, Philadelphia, PA 19107; Lara.weinstein@jefferson.edu.
1. College of Family Physicians of Canada. Authorizing Dried Cannabis for Chronic Pain or Anxiety: Preliminary Guidance from the College of Family Physicians of Canada. Mississauga, Ontario: College of Family Physicians of Canada; 2014. www.cfpc.ca/uploadedFiles/Resources/_PDFs/Authorizing%20Dried%20Cannabis%20for%20Chronic%20Pain%20or%20Anxiety.pdf. Accessed July 10, 2019.
2. Hartig H, Geiger AW. About six-in-ten Americans support marijuana legalization. Pew Research Center Web site. www.pewresearch.org/fact-tank/2018/10/08/americans-support-marijuana-legalization/. Published October 8, 2018. Accessed July 10, 2019.
3. Li H-L. An archaeological and historical account of cannabis in China. Econ Bot. 1974:28:437-448.
4. Zuardi AW. History of cannabis as a medicine: a review. Braz J Psychiatry. 2006;28:153-157.
5. Marijuana strains and infused products. Leafly Web site. www.leafly.com/start-exploring. Accessed July 10, 2019.
6. Fraguas-Sánchez AI, Torres-Suárez AI. Medical use of cannabinoids. Drugs. 2018;78:1665-1703.
7. Maurya N, Velmurugan BK. Therapeutic applications of cannabinoids. Chem Biol Interact. 2018;293:77-88.
8. Kelkar AH, Smith NA, Martial A, et al. An outbreak of synthetic cannabinoid-associated coagulopathy in Illinois. N Engl J Med. 2018;379:1216-1223.
9. Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Δ9-tetrahydrocannabinol, cannabidiol and Δ9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153:199-215.
10. Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313:2456-2473.
11. Ellis RJ, Toperoff W, Vaida F, et al. Smoked medicinal cannabis for neuropathic pain in HIV: a randomized, crossover clinical trial. Neuropsychopharmacology. 2008;34:672-680.
12. US Department of Justice, Drug Enforcement Administration, Diversion Control Division. Title 21 United States Code (USC) Controlled Substances Act. Subchapter I—Control and Enforcement. Part A—Introductory Provisions. §801. Congressional findings and declarations: controlled substances. www.deadiversion.usdoj.gov/21cfr/21usc/801.htm. Accessed July 10, 2019.
13. Yeh BT. The Controlled Substances Act: regulatory requirements. Congressional Research Service 7-5700. https://fas.org/sgp/crs/misc/RL34635.pdf. Published December 13, 2012. Accessed July 10, 2019.
14. US Department of Justice, Drug Enforcement Administration, Diversion Control Division. Title 21 United States Code (USC) Controlled Substances Act. Subchapter I—Control and Enforcement. Part B—Authority to Control; Standards and Schedules. §812. Schedules of controlled substances. www.deadiversion.usdoj.gov/21cfr/21usc/812.htm. Accessed July 10, 2019.
15. United States Senate. The STATES Act. Senator Elizabeth Warren and Senator Cory Gardner. 2018. www.warren.senate.gov/imo/media/doc/STATES%20Act%20One%20Pager.pdf. Accessed July 10, 2019.
16. Strengthening the Tenth Amendment Through Entrusting States (STATES) Act of 2019, HR 2093. 116th Cong, 1st Session (2019). www.congress.gov/bill/116th-congress/house-bill/2093/text. Accessed July 20, 2019.
17. Strengthening the Tenth Amendment Through Entrusting States (STATES) Act of 2019, S 1028. 116th Cong, 1st Session (2019). www.congress.gov/bill/116th-congress/senate-bill/1028/all-info?r=3&s=6. Accessed August 8, 2019.
18. State medical marijuana laws. National Conference of State Legislatures Web site. www.ncsl.org/research/health/state-medical-marijuana-laws.aspx#3. Published July 2, 2019. Accessed July 10, 2019.
19. Conant v Walters. 309 F.3d 629 (9th cir. 2002).
20. American Society of Addiction Medicine. The role of the physician in “medical” marijuana. www.asam.org/docs/publicy-policy-statements/1role_of_phys_in_med_mj_9-10.pdf?sfvrsn=0. Published September 2010. Accessed July 12, 2019.
21. What are marijuana’s effects on lung health? National Institute on Drug Abuse Web site. www.drugabuse.gov/publications/research-reports/marijuana/what-are-marijuanas-effects-lung-health. Updated July 2019. Accessed July 10, 2019.
22. Tashkin DP. Effects of marijuana smoking on the lung. Ann Am Thorac Soc. 2013;10:239-247.
23. Zhang LR, Morgenstern H, Greenland S, et al. Cannabis smoking and lung cancer risk: pooled analysis in the International Lung Cancer Consortium. Int J Cancer. 2015;136:894-903.
24. Getting medical marijuana. Commonwealth of Pennsylvania Web site. www.pa.gov/guides/pennsylvania-medical-marijuana-program/. Accessed July 20, 2019.
25. Kosiba JD, Maisto SA, Ditre JW. Patient-reported use of medical cannabis for pain, anxiety, and depression symptoms: systematic review and meta-analysis. Soc Sci Med. 2019;233:181-192.
26. Crippa JA, Zuardi AW, Martín-Santos R, et al. Cannabis and anxiety: a critical review of the evidence. Hum Psychopharmacol. 2009;24:515-523.
27. Moore TH, Zammit S, Lingford-Hughes A, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370:319-328.
28. Barrus DG, Capogrossi KL, Cates S, et al. Tasty THC: Promises and Challenges of Cannabis Edibles. Publication No. OP-0035-1611. Research Triangle Park, NC: RTI Press; 2016. www.rti.org/sites/default/files/resources/rti-publication-file-6ff047d7-3fa4-41ad-90ed-9fb11663bc89.pdf. Accessed July 10, 2019.
29. MacCallum CA, Russo EB. Practical considerations in medical cannabis administration and dosing. Eur J Intern Med. 2018;49:12-19.
30. MacCoun RJ, Mello MM. Half-baked—the retail promotion of marijuana edibles. N Engl J Med. 2015;372:989-991.
31. Cannabis use disorder [305.20, 304.30]. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). Washington, DC: American Psychiatric Association; 2013:509-516.
32. Williams AR, Santaella-Tenorio J, Mauro CM, et al. Loose regulation of medical marijuana programs associated with higher rates of adult marijuana use but not cannabis use disorder. Addiction. 2017;112:1985-1991.
33. American Academy of Pediatrics Committee on Substance Abuse, American Academy of Pediatrics Committee on Adolescents. The impact of marijuana policies on youth: clinical, research, and legal update. Pediatrics. 2015;135:584-587.
34. Wong SS, Wilens TE. Medical cannabinoids in children and adolescents: a systematic review. Pediatrics. 2017;140. pii: e20171818.
35. Drug Enforcement Administration. Drugs of abuse: a DEA resource guide. www.dea.gov/sites/default/files/drug_of_abuse.pdf. Published 2017. Accessed July 10, 2019.
36. National Academies of Science, Engineering, and Medicine. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. Washington, DC: The National Academies Press; 2017. www.nap.edu/read/24625/chapter/12017:2017-2019. Published 2017. Accessed July 10, 2019.
37. Emerging trend and alerts. National Institute on Drug Abuse Web site. www.drugabuse.gov/drugs-abuse/emerging-trends-alerts. Accessed July 10, 2019.
38. Drug Policy Alliance. From prohibition to progress: a status report on marijuana legalization. www.drugpolicy.org/sites/default/files/dpa_marijuana_legalization_report_feb14_2018_0.pdf. Published January 2018. Accessed July 10, 2019.
CASE
Barry S, a 45-year-old man with a new diagnosis of non-Hodgkin’s lymphoma, recently started induction chemotherapy. He has struggled with nausea, profound gustatory changes, and poor appetite; various antiemetics have provided only minimal relief. He tells you that he is hesitant to try “yet another pill” but has heard and read that marijuana (genus Cannabis) is used to alleviate disruptive chemotherapy-induced adverse effects. He asks if this is a treatment you’d recommend for him.
As Mr. S’s physician, how do you respond?
Understandably, some family physicians are hesitant to recommend an unregulated, federally illegal substance characterized by conflicting or absent evidence of safety and effectiveness.1 Nevertheless, throughout history and in the current court of public opinion, medical Cannabis has overwhelming support,2 leading to legalization in most of the United States.
As with many traditionally accepted therapies (whether they are or are not supported by substantial evidence), physicians are expected to provide individualized guidance regarding minimizing risk and maximizing benefit of the therapeutic use of Cannabis. The rapidly growing scientific and commercial fields of medical Cannabis guarantee that information on this topic will constantly be changing—and will often be contradictory. In this article, we review the most common concerns about medical Cannabis and provide up-to-date evidence on its use.
The pharmacology of cannabis
Cannabis sativa was among the earliest plants cultivated by man, with the first evidence of its use in China, approximately 4000 BC, to make twine and rope from its fibers.3 Records of medicinal Cannabis date back to the world’s oldest pharmacopoeia, a written summary of what was known about herbal medicine through the late 16th century.4
The 2 principal species of Cannabis are sativa and indica. There is no good medical evidence to separate the impacts of either strain; however, a staggering amount of lay information exists about the reported differing effects of each strain.5
Chemical constituents. Phytocannabinoids derived from C sativa are the plant’s best-known proteins, constituting a complex lipid-signaling network involved in numerous physiological processes. There are more than 100 known phytocannabinoids, the most well-recognized being Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD). Additional sources of cannabinoids include endogenous cannabinoids, or endocannabinoids, and synthetic cannabinoids.
The endocannabinoid system, comprising cannabinoid receptors, endocannabinoids, and their specific enzymes, is a potential therapeutic target for a variety of pathologic processes.6,7 The 2 most well-studied targets for cannabinoids in the human body are the cannabinoid receptors CB1 and CB2, found throughout the body: CB1, predominantly in the central and peripheral nervous system, and CB2 in a more limited distribution in the immune and hematopoietic systems. Other pathways activated or antagonized by THC and CBD exist, but are less well-mapped than CB1 and CB2.
[polldaddy:10402702]
Continue to: Botanical or synthetic?
Botanical or synthetic? It is important to distinguish between synthetic and plant-based cannabinoids, for you and your patients' benefit. Pharmaceutical (synthetic) THC is just that: THC alone. Whole-plant Cannabis, on the other hand, has hundreds of additional chemicals—most notably, phytocannabinoids and terpenoids. Data on the mechanisms of action and interactions of these additional chemicals are limited.
Although clinical trials have been undertaken with synthetic cannabinoids, there is increasing understanding and interest in the medical community of whole-plant Cannabis as a distinct entity. For example, nabiximols is a novel development in plant-based Cannabis products. Available as an oromucosal spray, a dose provides THC and CBD at 2.7 mg/100 mcL. Nabiximols is not approved by the US Food and Drug Administration (FDA) but is widely used
A third class of Cannabis comprises nonregulated synthetic cannabinoids that have no medically recognized benefit. They are solely a drug of abuse; common names include “K2” and “Spice.” These cannabinoids are outside of the scope of our discussion, but patients and providers should be aware of these cannabinoids because they are street-available. Unsuspecting patients might not know the difference between abusive and therapeutic formulations.8
Delivery and strength. Common forms of plant-based Cannabis include leaf that is smoked or vaporized, oral tincture, pill, and oil concentrate that can be vaporized. All forms come in a range of THC:CBD ratios—from as high as 90% THC content to 0% THC and all CBD-based content. Patients who are naïve to Cannabis might be concerned about formulations with a high THC concentration because of the psychoactive effects of this substance. Given the minimal CNS activity of CBD, a tolerable therapeutic starting point often is a THC:CBD ratio of 1:1, which contains a lower percentage of THC.4
Physiologic effects. THC is a partial agonist of CB1 and CB2 receptors; CBD functions as an antagonist at both receptors. The primary effects of THC result from activation of CB1 receptors, which exist in various areas of the cerebrum and cerebellum, as well as in the spinal cord.7 THC exerts its psychotropic effects at CB1 sites in the central nervous system; CBD can antagonize these THC effects at CB1 receptors. CBD also has anti-inflammatory and other effects that are mediated through peripherally distributed CB2 receptors.9
Continue to: THC has tremendously...
THC has tremendously complex capacity for activation and inhibition within various neuronal circuits, resulting in effects on mood, appetite, and movement.1,7 Adverse effects associated with Cannabis are wide-ranging: Most commonly, nausea, drowsiness, fatigue, dry mouth, and dizziness are reported alongside cognitive effects. Rarely, tachycardia, hypotension, hyperemesis, and depression can be seen.
Clinical implications and indications
Clinical indications for legal medical Cannabis vary by state; typically, indications include human immunodeficiency virus (HIV) infection and acquired immune deficiency syndrome (AIDS), cachexia, cancer, glaucoma, epilepsy and other seizure disorders, severe and chronic pain, spasticity from neurodegenerative disorders, and irritable bowel syndrome and Crohn’s disease, as well as a wide range of less-universal diagnoses. A patient may have a so-called qualifying diagnosis (ie, having the potential to allow the patient to be certified to purchase and use Cannabis) in one state but not have the same standing in a neighboring state, posing a complex legal issue. Given the significant complexities of performing medical research with plant-based Cannabis in the United States, little research has been done. The result? Policymakers are grappling with questions that only scientific research can answer:
- For which conditions does Cannabis provide medicinal benefit equal to or superior to alternatives?
- What are the appropriate dosages (or CBD:THC ratios), formulations (plant-derived or synthetic), and routes of administration (smoked, ingested, or topical) for various conditions?
Bird’s-eye view of clinical research. A meta-analysis of isolated synthetic and plant-based cannabinoids for medical use was published in 2015.10 The analysis included more than 6000 patients in 79 trials, most of which assessed whether dronabinol or nabilone (both synthetic isolates) were effective compared to placebo or alternative non-Cannabis-based therapy. The studies examined chemotherapy-induced nausea and vomiting, appetite stimulation in HIV and AIDS, chronic pain, spasticity, depression and anxiety, sleep disorders, and psychosis.
Twenty-eight studies assessed chemotherapy-induced nausea and vomiting. All of these studies indicated a greater benefit from cannabinoids than from alternative antiemetic regimens and placebo; however, that finding did not reach statistical significance across all studies.
There was moderate evidence to suggest the use of Cannabis for neuropathic and nonneuropathic cancer-related pain. However, there is an increased short-term risk of adverse events with synthetic isolates dronabinol (when used for pain) and nabilone (when used for nausea and vomiting).
Continue to: The primary conclusion...
The primary conclusion of the meta-analysis is that further study is required because little evidence exists on the effects and the adverse events of plant-based Cannabis.
HIV infection. Data on Cannabis for the treatment of refractory neuropathy and appetite stimulation in HIV infection is mixed.10,11 Smoked Cannabis for medically refractory neuropathy was examined in several trials:
- In a randomized crossover trial, researchers found statistically significant subjective improvement in neuropathic pain, with minimal intolerable adverse effects, in the 28 HIV-infected participants who completed the trial.11
- In another study,Cannabis ingested in various forms resulted in appetite stimulation in late-stage HIV infection but did not produce statistically significant weight gain.10
Pediatric epilepsy. Research on pediatric patients who have epilepsy characterized by refractory seizures has shown that the impact of Cannabis on their disease is promising. Specifically, CBD has shown tremendous potential impact: Patients experienced a statistically significant reduction in the number of seizures.9 In 2018, the FDA approved the first plant-based derivative of Cannabis: an oral cannabidiol (marketed as Epidiolex [Greenwich Biosciences, Inc.]) for the treatment of intractable seizures associated with Lennox-Gastaut syndrome and Dravet syndrome, rare and severe forms of epilepsy. Epidiolex is the first FDA-approved drug that contains a purified drug substance derived from marijuana.
CASE
Mr. S’s diagnosis of cancer is broadly included in the list of Cannabis-qualifying illnesses in all 34 states that certify patients for medical Cannabis. He qualifies both because (1) he is a cancer patient and (2) he has not found relief from chemotherapy-induced nausea and vomiting with several targeted therapies, including 5-hydroxytryptamine-receptor antagonists, steroids, and antipsychotics. Evidence supports CB1 and CB2 as potential targets for antiemetic treatment.
Given Mr. S’s consequent anorexia, his frustration with taking an increasing number of medications, and possible adverse effects of additional therapy, Cannabis is a reasonable course of action to treat nausea and vomiting. He would be able to use oral tincture or vaporization of oil to further limit his pill burden—likely, with a THC:CBD ratio of 1:1 or similar.
Continue to: Based on recent observational data...
Based on recent observational data from New York Cannabis dispensaries, cancer patients pursing Cannabis to treat chemotherapy-induced symptoms report that (1) either products with a high concentration of THC or products that contain THC and CBD in a 1:1 ratio are most effective and (2) products in 1:1 ratio of THC and CBD are most tolerable.
A legal system at oddsover the status of medical Cannabis
The core legal issue underlying medical Cannabis is a contradiction between federal and state laws.
At the federal level. The federal government regulates the lawful production, possession, and distribution of controlled substances through the Controlled Substances Act (CSA).12 The CSA is the basis for categorizing certain plants, drugs, and chemicals into 5 schedules, based on the substance’s medical use, potential for abuse, and safety or dependence liability.13 Under the CSA, marijuana (along with substances such as heroin and methamphetamine) is categorized as Schedule I14; ie, the substance
- has high potential for abuse,
- has no accepted therapeutic medical use in the United States, and
- lacks acceptable safety for use under medical supervision.
Despite waxing and waning efforts to protect states from federal prosecution, any use of a Schedule-1 substance violates federal law.15
In June 2018, a bipartisan group of federal lawmakers introduced a bill designed to amend the CSA and guarantee the rights of states and territories to self-determine marijuana regulation. The bill established a so-called STATES (Strengthening the Tenth Amendment Through Entrusting States) Act that “amends the Controlled Substances Act (21 U.S.C. § 801 et seq.) so that—as states and tribes comply with a few basic protections—its provisions no longer apply to any person acting in compliance with state or tribal laws relating to the manufacture, production, possession, distribution, dispensation, administration, or delivery of marijuana.”15
Continue to: The bill was referred to the Senate...
The bill was referred to the Senate and House Judiciary Committees but, ultimately, the STATES Act was blocked from debate in 2018.
On April 4, 2019, the Act was reintroduced in the House (H.R. 2093) and Senate (S. 1028) of the 116th Congress. Although there is bipartisan support for this bill, the timeline for moving it forward is unclear.16,17
At the state level. Thirty-four states have comprehensive public medical marijuana and Cannabis programs. The National Conference of State Legislatures18 (www.ncsl.org) designates a program “comprehensive” if it
- includes protection from criminal penalties for using marijuana for a medical purpose,
- allows access to marijuana through home cultivation, dispensaries, or other system,
- permits a variety of strains, including those more potent than what is labeled “low-THC,” and
- allows smoking or vaporization of marijuana products, plant-based material, or extract.
An additional 14 states allow for “low-THC, high-CBD” products for medical reasons, in limited situations, or as a legal defense. Regulation in these states varies widely, however: Some states allow industrialized hemp products only; others do not provide for any in-state production.18
Last, many states have some form of so-called “affirmative-defense” statutes that allow people charged with marijuana possession to mention use of marijuana for medical purposes as a possible defense.
Continue to: Physician shield
Physician shield. Despite inconsistent and evolving state and federal laws, physicians are protected, based on the Conant v Walters decision, from prosecution or revocation of their prescriptive authority for the professional “recommendation” of the use of medical marijuana.19 In 2002, the US Ninth Circuit Court of Appeals upheld the permanent injunction, based on a physician’s First Amendment right to discuss medical marijuana with patients.
CASE
Mr. S is amenable to trial of Cannabis to relieve nausea and anorexia. He asks you if he is allowed to use Cannabis at work, were he to return to an office-based desk job—even part-time—during treatment for cancer.
How would you answer Mr. S? Patients are legally protected from workplace penalties and dismissal for using and consuming Cannabis in states with a medical Cannabis law (including the state in which Mr. S resides). However, all employers have some variability in corporate policy, especially if a person works in a federally supported or regulated occupation. It’s always helpful to advise patients who will be using medical Cannabis to be proactive and speak with a human resources or employee health department staff member before beginning a course of medical Cannabis. Additionally, Cannabis with any amount of THC has the ability to alter focus, concentration, and perceptions of time. Thus, if a patient using medical Cannabis with THC asks about driving to work, he should be given the same advice one would offer about driving after consuming alcohol or ingesting opioids.
Common concerns
Ignorance of legal status. Theoretically, the Conant v Walters decision protects physicians from investigation for recommending medical Cannabis even in states where it is illegal. However, you should adhere closely to procedures set out by your state. The National Council of State Legislatures provides up-to-date information on each state’s procedures and programs,18 and the American Society of Addiction Medicine (www.asam.org) has established standards of professionalism for physicians who discuss medical Cannabis with patients (TABLE).20
Exposure to smoke. Cannabis smoke carries many of the same carcinogens found in tobacco smoke; furthermore, use of Cannabis and tobacco are highly correlated, confounding many population-based studies. The manner of inhalation of Cannabis can result in significantly higher levels of tar and carbon dioxide than with tobacco smoking. Because the effects of Cannabis last longer, however, people who smoke Cannabis may smoke it less often than tobacco smokers smoke tobacco.21
Continue to: Large cross-sectional...
Large cross-sectional and longitudinal studies have not found a link between Cannabis smoking and long-term pulmonary consequences, such as chronic obstructive pulmonary disease and lung cancer.22,23 The technology of Cannabis delivery systems has progressed far more rapidly than the clinical evidence for or against such technology.
“Vaping” is an informal term for inhalation of aerosolized Cannabis components and water vapor. Vaporizers do not heat Cannabis to the point of combustion; therefore, they provide less exposure to smoke-related toxicants while providing similar time of onset.
Neuropsychiatric adverse effects. Data regarding the relationship between Cannabis use and psychiatric disorders are incompletely understood, in conflict, and related to cannabinoid type. Consider Pennsylvania’s addition of anxiety disorder as a “serious medical condition” covered under the Pennsylvania Medical Marijuana Act.24 Although patients often report the use of medical Cannabis to treat anxiety,25 panic attacks are often associated with Cannabis use.26
While there is a clear association between Cannabis use and psychotic disorder, a causal link has yet to be unequivocally established. However, the rate of psychiatric hospitalization is increased in bipolar disorder and schizophrenia patients who use Cannabis heavily.27
We recommend, therefore, that physicians screen patients for serious mental health concerns before recommending or certifying them to use medical Cannabis.
Continue to: Overconsumption of edibles
Overconsumption of edibles. Cannabis edibles (ie, food products infused with Cannabis extract) are distinct from inhaled Cannabis in regard to onset, duration, and potential for adverse effects. Cannabis edibles might be more popular than inhaled products among older medical Cannabis users.28
Edible Cannabis has a reported onset of 1 to 3 hours (compared to 5-10 minutes with inhaled Cannabis) and a duration of effect of 6 to 8 hours (compared with 2-4 hours for inhaled products).29 These qualities might render Cannabis edibles preferable to inhaled formulations for controlling chronic symptoms and conditions. However, delayed onset of edible products and wide variation in the concentration of THC also increase the risk of overconsumption, which can lead to overdose and self-limited Cannabis-induced psychosis. We recommend providing patient education about the effects of the physiologically active therapeutic compounds tetrahydrocannabinol and cannabidiol, to prevent overconsumption of high-THC products.30
CASE
Mr. S returns to your office after a trial of Cannabis as vaporized oil and reports some relief of nausea and a mild increase in appetite, but no weight gain. He is concerned about overconsumption or overdose, and asks you what the risks of these problems are.
How should you counsel Mr. S? Explain that ingestion of Cannabis has a prolonged onset of action; vaporization has a more rapid onset of action; therefore, he could more easily self-regulate ingestion with the vehicle he has chosen. In states where edible Cannabis products are legal, education is necessary so that patients know how much of the edible to consume and how long they will wait to feel the full impact of the effects of THC.30
Cannabis use disorder in the context of medical marijuana
Cannabis use disorder (CUD) incorporates general diagnostic features of a substance use disorder, including behavioral, cognitive, and physiologic symptoms such as cravings, tolerance, and withdrawal, in the setting of persistent use despite significant substance-related problems.31 Features of Cannabis withdrawal syndrome include irritability, anger or aggression, anxiety, depressed mood, restlessness, sleep difficulty, and decreased appetite or weight loss.31 Cannabis use disorder can develop in people who use medical Cannabis; however, physiologic symptoms of tolerance and withdrawal can also develop in the setting of appropriate medical use and do not, in isolation, represent CUD.
Continue to: A recent study...
A recent study considered nationwide cross-sectional survey data from the US National Survey of Drug Use and Health to examine the relationship between medical marijuana laws and CUD.32 Study findings did not show an increase in the prevalence of CUD or marijuana use among adults in states with a legalized medical marijuana program. Importantly, when researchers looked at marijuana use among adolescents and young adults, they found no increase in measured outcomes (eg, active [ie, past-month] marijuana use, heavy [> 300 d/yr] use, and a diagnosis of CUD) after medical marijuana laws were passed.32
A paucity of pediatric data
The adolescent brain might be more vulnerable to the adverse long-term effects of Cannabis; there is potential significant harm associated with Cannabis in children and adolescence. However, accurate data concerning risk and benefit are limited.
The most recent policy statement of the American Academy of Pediatrics (AAP) reflects this paucity of data.33 The AAP opposes the use of medical Cannabis outside regulation by the FDA, although the organization allows for consideration of compassionate use of medical Cannabis for children who have life-threatening or severely disabling conditions. The AAP does support (1) additional research into pharmaceutical cannabinoids and (2) changing Cannabis from Schedule I to Schedule II to facilitate this process. Since the publication of the policy statement, Pediatrics, the official journal of the AAP, has published a review of medical cannabinoids and found (1) strong evidence for benefit in chemotherapy-induced nausea and vomiting and (2) accumulating evidence of benefit in epilepsy.34
Recognized risk: Not supporting medical Cannabis
As with all medical decisions, the risks and benefits of certifying patients for medical Cannabis must be balanced against the risks and benefits of not doing so. The risks that accompany failure to certify a patient for medical marijuana fall into 3 categories:
Blocking access to a substance that has potential therapeutic benefit. More data regarding the potential benefits and risks of medical Cannabis will, undoubtedly, dispel some of the uncertainty regarding the decision to certify a patient for medical Cannabis. When you recommend medical Cannabis and certify patients for its use, you do so with the certainty that the Cannabis safety index (ie, risk of overdose or serious adverse effects) is exceedingly low.35
Continue to: Limiting patients to other medications
Limiting patients to other medications that, potentially, carry a risk of more or greater harmful effects. An example is the decision to prescribe an opioid for chronic pain instead of certifying a patient for medical Cannabis. For certain other conditions, including chemotherapy-induced nausea and vomiting, FDA-approved pharmaceuticals might have more reported serious adverse events and interactions than medical Cannabis.36
Resigning patients to obtain Cannabis from an illegal source. This speaks to harm reduction and social justice, because obtaining Cannabis from an illegal source carries health and legal risks:
- Increased health risks result from lacing or cutting botanical or synthetic Cannabis products with potentially toxic substances. Cocaine, the rodenticide brodifacoum, methamphetamine, and phencyclidine are all known, or have been reported, to be added to botanical and synthetic Cannabis.37
- Legal repercussions of Cannabis possession are disproportionately racially based, with a significantly higher arrest rate among people of color, even in states where medical Cannabis has been legalized.38
CORRESPONDENCE
Lara Carson Weinstein, MD, MPH, DrPH, Department of Family and Community Medicine, Sidney Kimmel Medical College at Thomas Jefferson University, 1015 Walnut Street, Suite 401, Philadelphia, PA 19107; Lara.weinstein@jefferson.edu.
CASE
Barry S, a 45-year-old man with a new diagnosis of non-Hodgkin’s lymphoma, recently started induction chemotherapy. He has struggled with nausea, profound gustatory changes, and poor appetite; various antiemetics have provided only minimal relief. He tells you that he is hesitant to try “yet another pill” but has heard and read that marijuana (genus Cannabis) is used to alleviate disruptive chemotherapy-induced adverse effects. He asks if this is a treatment you’d recommend for him.
As Mr. S’s physician, how do you respond?
Understandably, some family physicians are hesitant to recommend an unregulated, federally illegal substance characterized by conflicting or absent evidence of safety and effectiveness.1 Nevertheless, throughout history and in the current court of public opinion, medical Cannabis has overwhelming support,2 leading to legalization in most of the United States.
As with many traditionally accepted therapies (whether they are or are not supported by substantial evidence), physicians are expected to provide individualized guidance regarding minimizing risk and maximizing benefit of the therapeutic use of Cannabis. The rapidly growing scientific and commercial fields of medical Cannabis guarantee that information on this topic will constantly be changing—and will often be contradictory. In this article, we review the most common concerns about medical Cannabis and provide up-to-date evidence on its use.
The pharmacology of cannabis
Cannabis sativa was among the earliest plants cultivated by man, with the first evidence of its use in China, approximately 4000 BC, to make twine and rope from its fibers.3 Records of medicinal Cannabis date back to the world’s oldest pharmacopoeia, a written summary of what was known about herbal medicine through the late 16th century.4
The 2 principal species of Cannabis are sativa and indica. There is no good medical evidence to separate the impacts of either strain; however, a staggering amount of lay information exists about the reported differing effects of each strain.5
Chemical constituents. Phytocannabinoids derived from C sativa are the plant’s best-known proteins, constituting a complex lipid-signaling network involved in numerous physiological processes. There are more than 100 known phytocannabinoids, the most well-recognized being Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD). Additional sources of cannabinoids include endogenous cannabinoids, or endocannabinoids, and synthetic cannabinoids.
The endocannabinoid system, comprising cannabinoid receptors, endocannabinoids, and their specific enzymes, is a potential therapeutic target for a variety of pathologic processes.6,7 The 2 most well-studied targets for cannabinoids in the human body are the cannabinoid receptors CB1 and CB2, found throughout the body: CB1, predominantly in the central and peripheral nervous system, and CB2 in a more limited distribution in the immune and hematopoietic systems. Other pathways activated or antagonized by THC and CBD exist, but are less well-mapped than CB1 and CB2.
[polldaddy:10402702]
Continue to: Botanical or synthetic?
Botanical or synthetic? It is important to distinguish between synthetic and plant-based cannabinoids, for you and your patients' benefit. Pharmaceutical (synthetic) THC is just that: THC alone. Whole-plant Cannabis, on the other hand, has hundreds of additional chemicals—most notably, phytocannabinoids and terpenoids. Data on the mechanisms of action and interactions of these additional chemicals are limited.
Although clinical trials have been undertaken with synthetic cannabinoids, there is increasing understanding and interest in the medical community of whole-plant Cannabis as a distinct entity. For example, nabiximols is a novel development in plant-based Cannabis products. Available as an oromucosal spray, a dose provides THC and CBD at 2.7 mg/100 mcL. Nabiximols is not approved by the US Food and Drug Administration (FDA) but is widely used
A third class of Cannabis comprises nonregulated synthetic cannabinoids that have no medically recognized benefit. They are solely a drug of abuse; common names include “K2” and “Spice.” These cannabinoids are outside of the scope of our discussion, but patients and providers should be aware of these cannabinoids because they are street-available. Unsuspecting patients might not know the difference between abusive and therapeutic formulations.8
Delivery and strength. Common forms of plant-based Cannabis include leaf that is smoked or vaporized, oral tincture, pill, and oil concentrate that can be vaporized. All forms come in a range of THC:CBD ratios—from as high as 90% THC content to 0% THC and all CBD-based content. Patients who are naïve to Cannabis might be concerned about formulations with a high THC concentration because of the psychoactive effects of this substance. Given the minimal CNS activity of CBD, a tolerable therapeutic starting point often is a THC:CBD ratio of 1:1, which contains a lower percentage of THC.4
Physiologic effects. THC is a partial agonist of CB1 and CB2 receptors; CBD functions as an antagonist at both receptors. The primary effects of THC result from activation of CB1 receptors, which exist in various areas of the cerebrum and cerebellum, as well as in the spinal cord.7 THC exerts its psychotropic effects at CB1 sites in the central nervous system; CBD can antagonize these THC effects at CB1 receptors. CBD also has anti-inflammatory and other effects that are mediated through peripherally distributed CB2 receptors.9
Continue to: THC has tremendously...
THC has tremendously complex capacity for activation and inhibition within various neuronal circuits, resulting in effects on mood, appetite, and movement.1,7 Adverse effects associated with Cannabis are wide-ranging: Most commonly, nausea, drowsiness, fatigue, dry mouth, and dizziness are reported alongside cognitive effects. Rarely, tachycardia, hypotension, hyperemesis, and depression can be seen.
Clinical implications and indications
Clinical indications for legal medical Cannabis vary by state; typically, indications include human immunodeficiency virus (HIV) infection and acquired immune deficiency syndrome (AIDS), cachexia, cancer, glaucoma, epilepsy and other seizure disorders, severe and chronic pain, spasticity from neurodegenerative disorders, and irritable bowel syndrome and Crohn’s disease, as well as a wide range of less-universal diagnoses. A patient may have a so-called qualifying diagnosis (ie, having the potential to allow the patient to be certified to purchase and use Cannabis) in one state but not have the same standing in a neighboring state, posing a complex legal issue. Given the significant complexities of performing medical research with plant-based Cannabis in the United States, little research has been done. The result? Policymakers are grappling with questions that only scientific research can answer:
- For which conditions does Cannabis provide medicinal benefit equal to or superior to alternatives?
- What are the appropriate dosages (or CBD:THC ratios), formulations (plant-derived or synthetic), and routes of administration (smoked, ingested, or topical) for various conditions?
Bird’s-eye view of clinical research. A meta-analysis of isolated synthetic and plant-based cannabinoids for medical use was published in 2015.10 The analysis included more than 6000 patients in 79 trials, most of which assessed whether dronabinol or nabilone (both synthetic isolates) were effective compared to placebo or alternative non-Cannabis-based therapy. The studies examined chemotherapy-induced nausea and vomiting, appetite stimulation in HIV and AIDS, chronic pain, spasticity, depression and anxiety, sleep disorders, and psychosis.
Twenty-eight studies assessed chemotherapy-induced nausea and vomiting. All of these studies indicated a greater benefit from cannabinoids than from alternative antiemetic regimens and placebo; however, that finding did not reach statistical significance across all studies.
There was moderate evidence to suggest the use of Cannabis for neuropathic and nonneuropathic cancer-related pain. However, there is an increased short-term risk of adverse events with synthetic isolates dronabinol (when used for pain) and nabilone (when used for nausea and vomiting).
Continue to: The primary conclusion...
The primary conclusion of the meta-analysis is that further study is required because little evidence exists on the effects and the adverse events of plant-based Cannabis.
HIV infection. Data on Cannabis for the treatment of refractory neuropathy and appetite stimulation in HIV infection is mixed.10,11 Smoked Cannabis for medically refractory neuropathy was examined in several trials:
- In a randomized crossover trial, researchers found statistically significant subjective improvement in neuropathic pain, with minimal intolerable adverse effects, in the 28 HIV-infected participants who completed the trial.11
- In another study,Cannabis ingested in various forms resulted in appetite stimulation in late-stage HIV infection but did not produce statistically significant weight gain.10
Pediatric epilepsy. Research on pediatric patients who have epilepsy characterized by refractory seizures has shown that the impact of Cannabis on their disease is promising. Specifically, CBD has shown tremendous potential impact: Patients experienced a statistically significant reduction in the number of seizures.9 In 2018, the FDA approved the first plant-based derivative of Cannabis: an oral cannabidiol (marketed as Epidiolex [Greenwich Biosciences, Inc.]) for the treatment of intractable seizures associated with Lennox-Gastaut syndrome and Dravet syndrome, rare and severe forms of epilepsy. Epidiolex is the first FDA-approved drug that contains a purified drug substance derived from marijuana.
CASE
Mr. S’s diagnosis of cancer is broadly included in the list of Cannabis-qualifying illnesses in all 34 states that certify patients for medical Cannabis. He qualifies both because (1) he is a cancer patient and (2) he has not found relief from chemotherapy-induced nausea and vomiting with several targeted therapies, including 5-hydroxytryptamine-receptor antagonists, steroids, and antipsychotics. Evidence supports CB1 and CB2 as potential targets for antiemetic treatment.
Given Mr. S’s consequent anorexia, his frustration with taking an increasing number of medications, and possible adverse effects of additional therapy, Cannabis is a reasonable course of action to treat nausea and vomiting. He would be able to use oral tincture or vaporization of oil to further limit his pill burden—likely, with a THC:CBD ratio of 1:1 or similar.
Continue to: Based on recent observational data...
Based on recent observational data from New York Cannabis dispensaries, cancer patients pursing Cannabis to treat chemotherapy-induced symptoms report that (1) either products with a high concentration of THC or products that contain THC and CBD in a 1:1 ratio are most effective and (2) products in 1:1 ratio of THC and CBD are most tolerable.
A legal system at oddsover the status of medical Cannabis
The core legal issue underlying medical Cannabis is a contradiction between federal and state laws.
At the federal level. The federal government regulates the lawful production, possession, and distribution of controlled substances through the Controlled Substances Act (CSA).12 The CSA is the basis for categorizing certain plants, drugs, and chemicals into 5 schedules, based on the substance’s medical use, potential for abuse, and safety or dependence liability.13 Under the CSA, marijuana (along with substances such as heroin and methamphetamine) is categorized as Schedule I14; ie, the substance
- has high potential for abuse,
- has no accepted therapeutic medical use in the United States, and
- lacks acceptable safety for use under medical supervision.
Despite waxing and waning efforts to protect states from federal prosecution, any use of a Schedule-1 substance violates federal law.15
In June 2018, a bipartisan group of federal lawmakers introduced a bill designed to amend the CSA and guarantee the rights of states and territories to self-determine marijuana regulation. The bill established a so-called STATES (Strengthening the Tenth Amendment Through Entrusting States) Act that “amends the Controlled Substances Act (21 U.S.C. § 801 et seq.) so that—as states and tribes comply with a few basic protections—its provisions no longer apply to any person acting in compliance with state or tribal laws relating to the manufacture, production, possession, distribution, dispensation, administration, or delivery of marijuana.”15
Continue to: The bill was referred to the Senate...
The bill was referred to the Senate and House Judiciary Committees but, ultimately, the STATES Act was blocked from debate in 2018.
On April 4, 2019, the Act was reintroduced in the House (H.R. 2093) and Senate (S. 1028) of the 116th Congress. Although there is bipartisan support for this bill, the timeline for moving it forward is unclear.16,17
At the state level. Thirty-four states have comprehensive public medical marijuana and Cannabis programs. The National Conference of State Legislatures18 (www.ncsl.org) designates a program “comprehensive” if it
- includes protection from criminal penalties for using marijuana for a medical purpose,
- allows access to marijuana through home cultivation, dispensaries, or other system,
- permits a variety of strains, including those more potent than what is labeled “low-THC,” and
- allows smoking or vaporization of marijuana products, plant-based material, or extract.
An additional 14 states allow for “low-THC, high-CBD” products for medical reasons, in limited situations, or as a legal defense. Regulation in these states varies widely, however: Some states allow industrialized hemp products only; others do not provide for any in-state production.18
Last, many states have some form of so-called “affirmative-defense” statutes that allow people charged with marijuana possession to mention use of marijuana for medical purposes as a possible defense.
Continue to: Physician shield
Physician shield. Despite inconsistent and evolving state and federal laws, physicians are protected, based on the Conant v Walters decision, from prosecution or revocation of their prescriptive authority for the professional “recommendation” of the use of medical marijuana.19 In 2002, the US Ninth Circuit Court of Appeals upheld the permanent injunction, based on a physician’s First Amendment right to discuss medical marijuana with patients.
CASE
Mr. S is amenable to trial of Cannabis to relieve nausea and anorexia. He asks you if he is allowed to use Cannabis at work, were he to return to an office-based desk job—even part-time—during treatment for cancer.
How would you answer Mr. S? Patients are legally protected from workplace penalties and dismissal for using and consuming Cannabis in states with a medical Cannabis law (including the state in which Mr. S resides). However, all employers have some variability in corporate policy, especially if a person works in a federally supported or regulated occupation. It’s always helpful to advise patients who will be using medical Cannabis to be proactive and speak with a human resources or employee health department staff member before beginning a course of medical Cannabis. Additionally, Cannabis with any amount of THC has the ability to alter focus, concentration, and perceptions of time. Thus, if a patient using medical Cannabis with THC asks about driving to work, he should be given the same advice one would offer about driving after consuming alcohol or ingesting opioids.
Common concerns
Ignorance of legal status. Theoretically, the Conant v Walters decision protects physicians from investigation for recommending medical Cannabis even in states where it is illegal. However, you should adhere closely to procedures set out by your state. The National Council of State Legislatures provides up-to-date information on each state’s procedures and programs,18 and the American Society of Addiction Medicine (www.asam.org) has established standards of professionalism for physicians who discuss medical Cannabis with patients (TABLE).20
Exposure to smoke. Cannabis smoke carries many of the same carcinogens found in tobacco smoke; furthermore, use of Cannabis and tobacco are highly correlated, confounding many population-based studies. The manner of inhalation of Cannabis can result in significantly higher levels of tar and carbon dioxide than with tobacco smoking. Because the effects of Cannabis last longer, however, people who smoke Cannabis may smoke it less often than tobacco smokers smoke tobacco.21
Continue to: Large cross-sectional...
Large cross-sectional and longitudinal studies have not found a link between Cannabis smoking and long-term pulmonary consequences, such as chronic obstructive pulmonary disease and lung cancer.22,23 The technology of Cannabis delivery systems has progressed far more rapidly than the clinical evidence for or against such technology.
“Vaping” is an informal term for inhalation of aerosolized Cannabis components and water vapor. Vaporizers do not heat Cannabis to the point of combustion; therefore, they provide less exposure to smoke-related toxicants while providing similar time of onset.
Neuropsychiatric adverse effects. Data regarding the relationship between Cannabis use and psychiatric disorders are incompletely understood, in conflict, and related to cannabinoid type. Consider Pennsylvania’s addition of anxiety disorder as a “serious medical condition” covered under the Pennsylvania Medical Marijuana Act.24 Although patients often report the use of medical Cannabis to treat anxiety,25 panic attacks are often associated with Cannabis use.26
While there is a clear association between Cannabis use and psychotic disorder, a causal link has yet to be unequivocally established. However, the rate of psychiatric hospitalization is increased in bipolar disorder and schizophrenia patients who use Cannabis heavily.27
We recommend, therefore, that physicians screen patients for serious mental health concerns before recommending or certifying them to use medical Cannabis.
Continue to: Overconsumption of edibles
Overconsumption of edibles. Cannabis edibles (ie, food products infused with Cannabis extract) are distinct from inhaled Cannabis in regard to onset, duration, and potential for adverse effects. Cannabis edibles might be more popular than inhaled products among older medical Cannabis users.28
Edible Cannabis has a reported onset of 1 to 3 hours (compared to 5-10 minutes with inhaled Cannabis) and a duration of effect of 6 to 8 hours (compared with 2-4 hours for inhaled products).29 These qualities might render Cannabis edibles preferable to inhaled formulations for controlling chronic symptoms and conditions. However, delayed onset of edible products and wide variation in the concentration of THC also increase the risk of overconsumption, which can lead to overdose and self-limited Cannabis-induced psychosis. We recommend providing patient education about the effects of the physiologically active therapeutic compounds tetrahydrocannabinol and cannabidiol, to prevent overconsumption of high-THC products.30
CASE
Mr. S returns to your office after a trial of Cannabis as vaporized oil and reports some relief of nausea and a mild increase in appetite, but no weight gain. He is concerned about overconsumption or overdose, and asks you what the risks of these problems are.
How should you counsel Mr. S? Explain that ingestion of Cannabis has a prolonged onset of action; vaporization has a more rapid onset of action; therefore, he could more easily self-regulate ingestion with the vehicle he has chosen. In states where edible Cannabis products are legal, education is necessary so that patients know how much of the edible to consume and how long they will wait to feel the full impact of the effects of THC.30
Cannabis use disorder in the context of medical marijuana
Cannabis use disorder (CUD) incorporates general diagnostic features of a substance use disorder, including behavioral, cognitive, and physiologic symptoms such as cravings, tolerance, and withdrawal, in the setting of persistent use despite significant substance-related problems.31 Features of Cannabis withdrawal syndrome include irritability, anger or aggression, anxiety, depressed mood, restlessness, sleep difficulty, and decreased appetite or weight loss.31 Cannabis use disorder can develop in people who use medical Cannabis; however, physiologic symptoms of tolerance and withdrawal can also develop in the setting of appropriate medical use and do not, in isolation, represent CUD.
Continue to: A recent study...
A recent study considered nationwide cross-sectional survey data from the US National Survey of Drug Use and Health to examine the relationship between medical marijuana laws and CUD.32 Study findings did not show an increase in the prevalence of CUD or marijuana use among adults in states with a legalized medical marijuana program. Importantly, when researchers looked at marijuana use among adolescents and young adults, they found no increase in measured outcomes (eg, active [ie, past-month] marijuana use, heavy [> 300 d/yr] use, and a diagnosis of CUD) after medical marijuana laws were passed.32
A paucity of pediatric data
The adolescent brain might be more vulnerable to the adverse long-term effects of Cannabis; there is potential significant harm associated with Cannabis in children and adolescence. However, accurate data concerning risk and benefit are limited.
The most recent policy statement of the American Academy of Pediatrics (AAP) reflects this paucity of data.33 The AAP opposes the use of medical Cannabis outside regulation by the FDA, although the organization allows for consideration of compassionate use of medical Cannabis for children who have life-threatening or severely disabling conditions. The AAP does support (1) additional research into pharmaceutical cannabinoids and (2) changing Cannabis from Schedule I to Schedule II to facilitate this process. Since the publication of the policy statement, Pediatrics, the official journal of the AAP, has published a review of medical cannabinoids and found (1) strong evidence for benefit in chemotherapy-induced nausea and vomiting and (2) accumulating evidence of benefit in epilepsy.34
Recognized risk: Not supporting medical Cannabis
As with all medical decisions, the risks and benefits of certifying patients for medical Cannabis must be balanced against the risks and benefits of not doing so. The risks that accompany failure to certify a patient for medical marijuana fall into 3 categories:
Blocking access to a substance that has potential therapeutic benefit. More data regarding the potential benefits and risks of medical Cannabis will, undoubtedly, dispel some of the uncertainty regarding the decision to certify a patient for medical Cannabis. When you recommend medical Cannabis and certify patients for its use, you do so with the certainty that the Cannabis safety index (ie, risk of overdose or serious adverse effects) is exceedingly low.35
Continue to: Limiting patients to other medications
Limiting patients to other medications that, potentially, carry a risk of more or greater harmful effects. An example is the decision to prescribe an opioid for chronic pain instead of certifying a patient for medical Cannabis. For certain other conditions, including chemotherapy-induced nausea and vomiting, FDA-approved pharmaceuticals might have more reported serious adverse events and interactions than medical Cannabis.36
Resigning patients to obtain Cannabis from an illegal source. This speaks to harm reduction and social justice, because obtaining Cannabis from an illegal source carries health and legal risks:
- Increased health risks result from lacing or cutting botanical or synthetic Cannabis products with potentially toxic substances. Cocaine, the rodenticide brodifacoum, methamphetamine, and phencyclidine are all known, or have been reported, to be added to botanical and synthetic Cannabis.37
- Legal repercussions of Cannabis possession are disproportionately racially based, with a significantly higher arrest rate among people of color, even in states where medical Cannabis has been legalized.38
CORRESPONDENCE
Lara Carson Weinstein, MD, MPH, DrPH, Department of Family and Community Medicine, Sidney Kimmel Medical College at Thomas Jefferson University, 1015 Walnut Street, Suite 401, Philadelphia, PA 19107; Lara.weinstein@jefferson.edu.
1. College of Family Physicians of Canada. Authorizing Dried Cannabis for Chronic Pain or Anxiety: Preliminary Guidance from the College of Family Physicians of Canada. Mississauga, Ontario: College of Family Physicians of Canada; 2014. www.cfpc.ca/uploadedFiles/Resources/_PDFs/Authorizing%20Dried%20Cannabis%20for%20Chronic%20Pain%20or%20Anxiety.pdf. Accessed July 10, 2019.
2. Hartig H, Geiger AW. About six-in-ten Americans support marijuana legalization. Pew Research Center Web site. www.pewresearch.org/fact-tank/2018/10/08/americans-support-marijuana-legalization/. Published October 8, 2018. Accessed July 10, 2019.
3. Li H-L. An archaeological and historical account of cannabis in China. Econ Bot. 1974:28:437-448.
4. Zuardi AW. History of cannabis as a medicine: a review. Braz J Psychiatry. 2006;28:153-157.
5. Marijuana strains and infused products. Leafly Web site. www.leafly.com/start-exploring. Accessed July 10, 2019.
6. Fraguas-Sánchez AI, Torres-Suárez AI. Medical use of cannabinoids. Drugs. 2018;78:1665-1703.
7. Maurya N, Velmurugan BK. Therapeutic applications of cannabinoids. Chem Biol Interact. 2018;293:77-88.
8. Kelkar AH, Smith NA, Martial A, et al. An outbreak of synthetic cannabinoid-associated coagulopathy in Illinois. N Engl J Med. 2018;379:1216-1223.
9. Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Δ9-tetrahydrocannabinol, cannabidiol and Δ9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153:199-215.
10. Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313:2456-2473.
11. Ellis RJ, Toperoff W, Vaida F, et al. Smoked medicinal cannabis for neuropathic pain in HIV: a randomized, crossover clinical trial. Neuropsychopharmacology. 2008;34:672-680.
12. US Department of Justice, Drug Enforcement Administration, Diversion Control Division. Title 21 United States Code (USC) Controlled Substances Act. Subchapter I—Control and Enforcement. Part A—Introductory Provisions. §801. Congressional findings and declarations: controlled substances. www.deadiversion.usdoj.gov/21cfr/21usc/801.htm. Accessed July 10, 2019.
13. Yeh BT. The Controlled Substances Act: regulatory requirements. Congressional Research Service 7-5700. https://fas.org/sgp/crs/misc/RL34635.pdf. Published December 13, 2012. Accessed July 10, 2019.
14. US Department of Justice, Drug Enforcement Administration, Diversion Control Division. Title 21 United States Code (USC) Controlled Substances Act. Subchapter I—Control and Enforcement. Part B—Authority to Control; Standards and Schedules. §812. Schedules of controlled substances. www.deadiversion.usdoj.gov/21cfr/21usc/812.htm. Accessed July 10, 2019.
15. United States Senate. The STATES Act. Senator Elizabeth Warren and Senator Cory Gardner. 2018. www.warren.senate.gov/imo/media/doc/STATES%20Act%20One%20Pager.pdf. Accessed July 10, 2019.
16. Strengthening the Tenth Amendment Through Entrusting States (STATES) Act of 2019, HR 2093. 116th Cong, 1st Session (2019). www.congress.gov/bill/116th-congress/house-bill/2093/text. Accessed July 20, 2019.
17. Strengthening the Tenth Amendment Through Entrusting States (STATES) Act of 2019, S 1028. 116th Cong, 1st Session (2019). www.congress.gov/bill/116th-congress/senate-bill/1028/all-info?r=3&s=6. Accessed August 8, 2019.
18. State medical marijuana laws. National Conference of State Legislatures Web site. www.ncsl.org/research/health/state-medical-marijuana-laws.aspx#3. Published July 2, 2019. Accessed July 10, 2019.
19. Conant v Walters. 309 F.3d 629 (9th cir. 2002).
20. American Society of Addiction Medicine. The role of the physician in “medical” marijuana. www.asam.org/docs/publicy-policy-statements/1role_of_phys_in_med_mj_9-10.pdf?sfvrsn=0. Published September 2010. Accessed July 12, 2019.
21. What are marijuana’s effects on lung health? National Institute on Drug Abuse Web site. www.drugabuse.gov/publications/research-reports/marijuana/what-are-marijuanas-effects-lung-health. Updated July 2019. Accessed July 10, 2019.
22. Tashkin DP. Effects of marijuana smoking on the lung. Ann Am Thorac Soc. 2013;10:239-247.
23. Zhang LR, Morgenstern H, Greenland S, et al. Cannabis smoking and lung cancer risk: pooled analysis in the International Lung Cancer Consortium. Int J Cancer. 2015;136:894-903.
24. Getting medical marijuana. Commonwealth of Pennsylvania Web site. www.pa.gov/guides/pennsylvania-medical-marijuana-program/. Accessed July 20, 2019.
25. Kosiba JD, Maisto SA, Ditre JW. Patient-reported use of medical cannabis for pain, anxiety, and depression symptoms: systematic review and meta-analysis. Soc Sci Med. 2019;233:181-192.
26. Crippa JA, Zuardi AW, Martín-Santos R, et al. Cannabis and anxiety: a critical review of the evidence. Hum Psychopharmacol. 2009;24:515-523.
27. Moore TH, Zammit S, Lingford-Hughes A, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370:319-328.
28. Barrus DG, Capogrossi KL, Cates S, et al. Tasty THC: Promises and Challenges of Cannabis Edibles. Publication No. OP-0035-1611. Research Triangle Park, NC: RTI Press; 2016. www.rti.org/sites/default/files/resources/rti-publication-file-6ff047d7-3fa4-41ad-90ed-9fb11663bc89.pdf. Accessed July 10, 2019.
29. MacCallum CA, Russo EB. Practical considerations in medical cannabis administration and dosing. Eur J Intern Med. 2018;49:12-19.
30. MacCoun RJ, Mello MM. Half-baked—the retail promotion of marijuana edibles. N Engl J Med. 2015;372:989-991.
31. Cannabis use disorder [305.20, 304.30]. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). Washington, DC: American Psychiatric Association; 2013:509-516.
32. Williams AR, Santaella-Tenorio J, Mauro CM, et al. Loose regulation of medical marijuana programs associated with higher rates of adult marijuana use but not cannabis use disorder. Addiction. 2017;112:1985-1991.
33. American Academy of Pediatrics Committee on Substance Abuse, American Academy of Pediatrics Committee on Adolescents. The impact of marijuana policies on youth: clinical, research, and legal update. Pediatrics. 2015;135:584-587.
34. Wong SS, Wilens TE. Medical cannabinoids in children and adolescents: a systematic review. Pediatrics. 2017;140. pii: e20171818.
35. Drug Enforcement Administration. Drugs of abuse: a DEA resource guide. www.dea.gov/sites/default/files/drug_of_abuse.pdf. Published 2017. Accessed July 10, 2019.
36. National Academies of Science, Engineering, and Medicine. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. Washington, DC: The National Academies Press; 2017. www.nap.edu/read/24625/chapter/12017:2017-2019. Published 2017. Accessed July 10, 2019.
37. Emerging trend and alerts. National Institute on Drug Abuse Web site. www.drugabuse.gov/drugs-abuse/emerging-trends-alerts. Accessed July 10, 2019.
38. Drug Policy Alliance. From prohibition to progress: a status report on marijuana legalization. www.drugpolicy.org/sites/default/files/dpa_marijuana_legalization_report_feb14_2018_0.pdf. Published January 2018. Accessed July 10, 2019.
1. College of Family Physicians of Canada. Authorizing Dried Cannabis for Chronic Pain or Anxiety: Preliminary Guidance from the College of Family Physicians of Canada. Mississauga, Ontario: College of Family Physicians of Canada; 2014. www.cfpc.ca/uploadedFiles/Resources/_PDFs/Authorizing%20Dried%20Cannabis%20for%20Chronic%20Pain%20or%20Anxiety.pdf. Accessed July 10, 2019.
2. Hartig H, Geiger AW. About six-in-ten Americans support marijuana legalization. Pew Research Center Web site. www.pewresearch.org/fact-tank/2018/10/08/americans-support-marijuana-legalization/. Published October 8, 2018. Accessed July 10, 2019.
3. Li H-L. An archaeological and historical account of cannabis in China. Econ Bot. 1974:28:437-448.
4. Zuardi AW. History of cannabis as a medicine: a review. Braz J Psychiatry. 2006;28:153-157.
5. Marijuana strains and infused products. Leafly Web site. www.leafly.com/start-exploring. Accessed July 10, 2019.
6. Fraguas-Sánchez AI, Torres-Suárez AI. Medical use of cannabinoids. Drugs. 2018;78:1665-1703.
7. Maurya N, Velmurugan BK. Therapeutic applications of cannabinoids. Chem Biol Interact. 2018;293:77-88.
8. Kelkar AH, Smith NA, Martial A, et al. An outbreak of synthetic cannabinoid-associated coagulopathy in Illinois. N Engl J Med. 2018;379:1216-1223.
9. Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Δ9-tetrahydrocannabinol, cannabidiol and Δ9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153:199-215.
10. Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313:2456-2473.
11. Ellis RJ, Toperoff W, Vaida F, et al. Smoked medicinal cannabis for neuropathic pain in HIV: a randomized, crossover clinical trial. Neuropsychopharmacology. 2008;34:672-680.
12. US Department of Justice, Drug Enforcement Administration, Diversion Control Division. Title 21 United States Code (USC) Controlled Substances Act. Subchapter I—Control and Enforcement. Part A—Introductory Provisions. §801. Congressional findings and declarations: controlled substances. www.deadiversion.usdoj.gov/21cfr/21usc/801.htm. Accessed July 10, 2019.
13. Yeh BT. The Controlled Substances Act: regulatory requirements. Congressional Research Service 7-5700. https://fas.org/sgp/crs/misc/RL34635.pdf. Published December 13, 2012. Accessed July 10, 2019.
14. US Department of Justice, Drug Enforcement Administration, Diversion Control Division. Title 21 United States Code (USC) Controlled Substances Act. Subchapter I—Control and Enforcement. Part B—Authority to Control; Standards and Schedules. §812. Schedules of controlled substances. www.deadiversion.usdoj.gov/21cfr/21usc/812.htm. Accessed July 10, 2019.
15. United States Senate. The STATES Act. Senator Elizabeth Warren and Senator Cory Gardner. 2018. www.warren.senate.gov/imo/media/doc/STATES%20Act%20One%20Pager.pdf. Accessed July 10, 2019.
16. Strengthening the Tenth Amendment Through Entrusting States (STATES) Act of 2019, HR 2093. 116th Cong, 1st Session (2019). www.congress.gov/bill/116th-congress/house-bill/2093/text. Accessed July 20, 2019.
17. Strengthening the Tenth Amendment Through Entrusting States (STATES) Act of 2019, S 1028. 116th Cong, 1st Session (2019). www.congress.gov/bill/116th-congress/senate-bill/1028/all-info?r=3&s=6. Accessed August 8, 2019.
18. State medical marijuana laws. National Conference of State Legislatures Web site. www.ncsl.org/research/health/state-medical-marijuana-laws.aspx#3. Published July 2, 2019. Accessed July 10, 2019.
19. Conant v Walters. 309 F.3d 629 (9th cir. 2002).
20. American Society of Addiction Medicine. The role of the physician in “medical” marijuana. www.asam.org/docs/publicy-policy-statements/1role_of_phys_in_med_mj_9-10.pdf?sfvrsn=0. Published September 2010. Accessed July 12, 2019.
21. What are marijuana’s effects on lung health? National Institute on Drug Abuse Web site. www.drugabuse.gov/publications/research-reports/marijuana/what-are-marijuanas-effects-lung-health. Updated July 2019. Accessed July 10, 2019.
22. Tashkin DP. Effects of marijuana smoking on the lung. Ann Am Thorac Soc. 2013;10:239-247.
23. Zhang LR, Morgenstern H, Greenland S, et al. Cannabis smoking and lung cancer risk: pooled analysis in the International Lung Cancer Consortium. Int J Cancer. 2015;136:894-903.
24. Getting medical marijuana. Commonwealth of Pennsylvania Web site. www.pa.gov/guides/pennsylvania-medical-marijuana-program/. Accessed July 20, 2019.
25. Kosiba JD, Maisto SA, Ditre JW. Patient-reported use of medical cannabis for pain, anxiety, and depression symptoms: systematic review and meta-analysis. Soc Sci Med. 2019;233:181-192.
26. Crippa JA, Zuardi AW, Martín-Santos R, et al. Cannabis and anxiety: a critical review of the evidence. Hum Psychopharmacol. 2009;24:515-523.
27. Moore TH, Zammit S, Lingford-Hughes A, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370:319-328.
28. Barrus DG, Capogrossi KL, Cates S, et al. Tasty THC: Promises and Challenges of Cannabis Edibles. Publication No. OP-0035-1611. Research Triangle Park, NC: RTI Press; 2016. www.rti.org/sites/default/files/resources/rti-publication-file-6ff047d7-3fa4-41ad-90ed-9fb11663bc89.pdf. Accessed July 10, 2019.
29. MacCallum CA, Russo EB. Practical considerations in medical cannabis administration and dosing. Eur J Intern Med. 2018;49:12-19.
30. MacCoun RJ, Mello MM. Half-baked—the retail promotion of marijuana edibles. N Engl J Med. 2015;372:989-991.
31. Cannabis use disorder [305.20, 304.30]. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). Washington, DC: American Psychiatric Association; 2013:509-516.
32. Williams AR, Santaella-Tenorio J, Mauro CM, et al. Loose regulation of medical marijuana programs associated with higher rates of adult marijuana use but not cannabis use disorder. Addiction. 2017;112:1985-1991.
33. American Academy of Pediatrics Committee on Substance Abuse, American Academy of Pediatrics Committee on Adolescents. The impact of marijuana policies on youth: clinical, research, and legal update. Pediatrics. 2015;135:584-587.
34. Wong SS, Wilens TE. Medical cannabinoids in children and adolescents: a systematic review. Pediatrics. 2017;140. pii: e20171818.
35. Drug Enforcement Administration. Drugs of abuse: a DEA resource guide. www.dea.gov/sites/default/files/drug_of_abuse.pdf. Published 2017. Accessed July 10, 2019.
36. National Academies of Science, Engineering, and Medicine. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. Washington, DC: The National Academies Press; 2017. www.nap.edu/read/24625/chapter/12017:2017-2019. Published 2017. Accessed July 10, 2019.
37. Emerging trend and alerts. National Institute on Drug Abuse Web site. www.drugabuse.gov/drugs-abuse/emerging-trends-alerts. Accessed July 10, 2019.
38. Drug Policy Alliance. From prohibition to progress: a status report on marijuana legalization. www.drugpolicy.org/sites/default/files/dpa_marijuana_legalization_report_feb14_2018_0.pdf. Published January 2018. Accessed July 10, 2019.
PRACTICE RECOMMENDATIONS
› Educate patients about the effects of the physiologically active therapeutic compounds in Cannabis; this is critical to prevent overconsumption of products with high levels of tetrahydrocannabinol. B
› Screen patients for serious mental health concerns before recommending or certifying medical Cannabis; this is essential because the rate of psychiatric hospitalization is increased in bipolar disorder and schizophrenia patients who use Cannabis heavily. B
› You can recommend medical Cannabis and certify patients for its use with the certainty that the risk of overdose or serious adverse effects is exceedingly low. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Recommendations on the Use of Ultrasound Guidance for Central and Peripheral Vascular Access in Adults: A Position Statement of the Society of Hospital Medicine
Approximately five million central venous catheters (CVCs) are inserted in the United States annually, with over 15 million catheter days documented in intensive care units alone.1 Traditional CVC insertion techniques using landmarks are associated with a high risk of mechanical complications, particularly pneumothorax and arterial puncture, which occur in 5%-19% patients.2,3
Since the 1990s, several randomized controlled studies and meta-analyses have demonstrated that the use of real-time ultrasound guidance for CVC insertion increases procedure success rates and decreases mechanical complications.4,5 Use of real-time ultrasound guidance was recommended by the Agency for Healthcare Research and Quality, the Institute of Medicine, the National Institute for Health and Care Excellence, the Centers for Disease Control and Prevention, and several medical specialty societies in the early 2000s.6-14 Despite these recommendations, ultrasound guidance has not been universally adopted. Currently, an estimated 20%-55% of CVC insertions in the internal jugular vein are performed without ultrasound guidance.15-17
Following the emergence of literature supporting the use of ultrasound guidance for CVC insertion, observational and randomized controlled studies demonstrated improved procedural success rates with the use of ultrasound guidance for the insertion of peripheral intravenous lines (PIVs), arterial catheters, and peripherally inserted central catheters (PICCs).18-23
The purpose of this position statement is to present evidence-based recommendations on the use of ultrasound guidance for the insertion of central and peripheral vascular access catheters in adult patients. This document presents consensus-based recommendations with supporting evidence for clinical outcomes, techniques, and training for the use of ultrasound guidance for vascular access. We have subdivided the recommendations on techniques for central venous access, peripheral venous access, and arterial access individually, as some providers may not perform all types of vascular access procedures.
These recommendations are intended for hospitalists and other healthcare providers that routinely place central and peripheral vascular access catheters in acutely ill patients. However, this position statement does not mandate that all hospitalists should place central or peripheral vascular access catheters given the diverse array of hospitalist practice settings. For training and competency assessments, we recognize that some of these recommendations may not be feasible in resource-limited settings, such as rural hospitals, where equipment and staffing for assessments are not available. Recommendations and frameworks for initial and ongoing credentialing of hospitalists in ultrasound-guided bedside procedures have been previously published in an Society of Hospital Medicine (SHM) position statement titled, “Credentialing of Hospitalists in Ultrasound-Guided Bedside Procedures.”24
METHODS
Detailed methods are described in Appendix 1. The SHM Point-of-care Ultrasound (POCUS) Task Force was assembled to carry out this guideline development project under the direction of the SHM Board of Directors, Director of Education, and Education Committee. All expert panel members were physicians or advanced practice providers with expertise in POCUS. Expert panel members were divided into working group members, external peer reviewers, and a methodologist. All Task Force members were required to disclose any potential conflicts of interest (Appendix 2). The literature search was conducted in two independent phases. The first phase included literature searches conducted by the vascular access working group members themselves. Key clinical questions and draft recommendations were then prepared. A systematic literature search was conducted by a medical librarian based on the findings of the initial literature search and draft recommendations. The Medline, Embase, CINAHL, and Cochrane medical databases were searched from 1975 to December 2015 initially. Google Scholar was also searched without limiters. An updated search was conducted in November 2017. The literature search strings are included in Appendix 3. All article abstracts were initially screened for relevance by at least two members of the vascular access working group. Full-text versions of screened articles were reviewed, and articles on the use of ultrasound to guide vascular access were selected. The following article types were excluded: non-English language, nonhuman, age <18 years, meeting abstracts, meeting posters, narrative reviews, case reports, letters, and editorials. All relevant systematic reviews, meta-analyses, randomized controlled studies, and observational studies of ultrasound-guided vascular access were screened and selected (Appendix 3, Figure 1). All full-text articles were shared electronically among the working group members, and final article selection was based on working group consensus. Selected articles were incorporated into the draft recommendations.
These recommendations were developed using the Research and Development (RAND) Appropriateness Method that required panel judgment and consensus.14 The 28 voting members of the SHM POCUS Task Force reviewed and voted on the draft recommendations considering five transforming factors: (1) Problem priority and importance, (2) Level of quality of evidence, (3) Benefit/harm balance, (4) Benefit/burden balance, and (5) Certainty/concerns about PEAF (Preferences/Equity/Acceptability/Feasibility). Using an internet-based electronic data collection tool (REDCap™), panel members participated in two rounds of electronic voting, one in August 2018 and the other in October 2018 (Appendix 4). Voting on appropriateness was conducted using a nine-point Likert scale. The three zones of the nine-point Likert scale were inappropriate (1-3 points), uncertain (4-6 points), and appropriate (7-9 points). The degree of consensus was assessed using the RAND algorithm (Appendix 1, Figure 1 and Table 1). Establishing a recommendation required at least 70% agreement that a recommendation was “appropriate.” Disagreement was defined as >30% of panelists voting outside of the zone of the median. A strong recommendation required at least 80% of the votes within one integer of the median per the RAND rules.
Recommendations were classified as strong or weak/conditional based on preset rules defining the panel’s level of consensus, which determined the wording for each recommendation (Table 2). The final version of the consensus-based recommendations underwent internal and external review by members of the SHM POCUS Task Force, the SHM Education Committee, and the SHM Executive Committee. The SHM Executive Committee reviewed and approved this position statement prior to its publication in the Journal of Hospital Medicine.
RESULTS
Literature Search
A total of 5,563 references were pooled from an initial search performed by a certified medical librarian in December 2015 (4,668 citations) which was updated in November 2017 (791 citations), and from the personal bibliographies and searches (104 citations) performed by working group members. A total of 514 full-text articles were reviewed. The final selection included 192 articles that were abstracted into a data table and incorporated into the draft recommendations. See Appendix 3 for details of the literature search strategy.
Recommendations
Four domains (technique, clinical outcomes, training, and knowledge gaps) with 31 draft recommendations were generated based on a review of the literature. Selected references were abstracted and assigned to each draft recommendation. Rationales for each recommendation cite supporting evidence. After two rounds of panel voting, 31 recommendations achieved agreement based on the RAND rules. During the peer review process, two of the recommendations were merged with other recommendations. Thus, a total of 29 recommendations received final approval. The degree of consensus based on the median score and the dispersion of voting around the median are shown in Appendix 5. Twenty-seven statements were approved as strong recommendations, and two were approved as weak/conditional recommendations. The strength of each recommendation and degree of consensus are summarized in Table 3.
Terminology
Central Venous Catheterization
Central venous catheterization refers to insertion of tunneled or nontunneled large bore vascular catheters that are most commonly inserted into the internal jugular, subclavian, or femoral veins with the catheter tip located in a central vein. These vascular access catheters are synonymously referred to as central lines or central venous catheters (CVCs). Nontunneled catheters are designed for short-term use and should be removed promptly when no longer clinically indicated or after a maximum of 14 days.25
Peripherally Inserted Central Catheter (PICC)
Peripherally inserted central catheters, or PICC lines, are inserted most commonly in the basilic or brachial veins in adult patients, and the catheter tip terminates in the distal superior vena cava or cavo-atrial junction. These catheters are designed to remain in place for a duration of several weeks, as long as it is clinically indicated.
Midline Catheterization
Midline catheters are a type of peripheral venous catheter that are an intermediary between a peripheral intravenous catheter and PICC line. Midline catheters are most commonly inserted in the brachial or basilic veins, but unlike PICC lines, the tips of these catheters terminate in the axillary or subclavian vein. Midline catheters are typically 8 cm to 20 cm in length and inserted for a duration <30 days.
Peripheral Intravenous Catheterization
Peripheral intravenous lines (PIV) refer to small bore venous catheters that are most commonly 14G to 24G and inserted into patients for short-term peripheral venous access. Common sites of ultrasound-guided PIV insertion include the superficial and deep veins of the hand, forearm, and arm.
Arterial Catheterization
Arterial catheters are commonly used for reliable blood pressure monitoring, frequent arterial blood
RECOMMENDATIONS
Preprocedure
1. We recommend that providers should be familiar with the operation of their specific ultrasound machine prior to initiation of a vascular access procedure.
Rationale: There is strong consensus that providers must be familiar with the knobs and functions of the specific make and model of ultrasound machine that will be utilized for a vascular access procedure. Minimizing adjustments to the ultrasound machine during the procedure may reduce the risk of contaminating the sterile field.
2. We recommend that providers should use a high-frequency linear transducer with a sterile sheath and sterile gel to perform vascular access procedures.
Rationale: High-frequency linear-array transducers are recommended for the vast majority of vascular access procedures due to their superior resolution compared to other transducer types. Both central and peripheral vascular access procedures, including PIV, PICC, and arterial line placement, should be performed using sterile technique. A sterile transducer cover and sterile gel must be utilized, and providers must be trained in sterile preparation of the ultrasound transducer.13,26,27
The depth of femoral vessels correlates with body mass index (BMI). When accessing these vessels in a morbidly obese patient with a thigh circumference >60 cm and vessel depth >8 cm, a curvilinear transducer may be preferred for its deeper penetration.28 For patients who are poor candidates for bedside insertion of vascular access catheters, such as uncooperative patients, patients with atypical vascular anatomy or poorly visualized target vessels, we recommend consultation with a vascular access specialist prior to attempting the procedure.
3. We recommend that providers should use two-dimensional ultrasound to evaluate for anatomical variations and absence of vascular thrombosis during preprocedural site selection.
Rationale: A thorough ultrasound examination of the target vessel is warranted prior to catheter placement. Anatomical variations that may affect procedural decision-making are easily detected with ultrasound. A focused vascular ultrasound examination is particularly important in patients who have had temporary or tunneled venous catheters, which can cause stenosis or thrombosis of the target vein.
For internal jugular vein (IJV) CVCs, ultrasound is useful for visualizing the relationship between the IJV and common carotid artery (CCA), particularly in terms of vessel overlap. Furthermore, ultrasound allows for immediate revisualization upon changes in head position.29-32 Troianos et al. found >75% overlap of the IJV and CCA in 54% of all patients and in 64% of older patients (age >60 years) whose heads were rotated to the contralateral side.30 In one study of IJV CVC insertion, inadvertent carotid artery punctures were reduced (3% vs 10%) with the use of ultrasound guidance vs landmarks alone.33 In a cohort of 64 high-risk neurosurgical patients, cannulation success was 100% with the use of ultrasound guidance, and there were no injuries to the carotid artery, even though the procedure was performed with a 30-degree head elevation and anomalous IJV anatomy in 39% of patients.34 In a prospective, randomized controlled study of 1,332 patients, ultrasound-guided cannulation in a neutral position was demonstrated to be as safe as the 45-degree rotated position.35
Ultrasound allows for the recognition of anatomical variations which may influence the selection of the vascular access site or technique. Benter et al. found that 36% of patients showed anatomical variations in the IJV and surrounding tissue.36 Similarly Caridi showed the anatomy of the right IJV to be atypical in 29% of patients,37 and Brusasco found that 37% of bariatric patients had anatomical variations of the IJV.38 In a study of 58 patients, there was significant variability in the IJV position and IJV diameter, ranging from 0.5 cm to >2 cm.39 In a study of hemodialysis patients, 75% of patients had sonographic venous abnormalities that led to a change in venous access approach.40
To detect acute or chronic upper extremity deep venous thrombosis or stenosis, two-dimensional visualization with compression should be part of the ultrasound examination prior to central venous catheterization. In a study of patients that had undergone CVC insertion 9-19 weeks earlier, 50% of patients had an IJV thrombosis or stenosis leading to selection of an alternative site. In this study, use of ultrasound for a preprocedural site evaluation reduced unnecessary attempts at catheterizing an occluded vein.41 At least two other studies demonstrated an appreciable likelihood of thrombosis. In a study of bariatric patients, 8% of patients had asymptomatic thrombosis38 and in another study, 9% of patients being evaluated for hemodialysis catheter placement had asymptomatic IJV thrombosis.37
4. We recommend that providers should evaluate the target blood vessel size and depth during a preprocedural ultrasound evaluation.
Rationale: The size, depth, and anatomic location of central veins can vary considerably. These features are easily discernable using ultrasound. Contrary to traditional teaching, the IJV is located 1 cm anterolateral to the CCA in only about two-thirds of patients.37,39,42,43 Furthermore, the diameter of the IJV can vary significantly, ranging from 0.5 cm to >2 cm.39 The laterality of blood vessels may vary considerably as well. A preprocedural ultrasound evaluation of contralateral subclavian and axillary veins showed a significant absolute difference in cross-sectional area of 26.7 mm2 (P < .001).42
Blood vessels can also shift considerably when a patient is in the Trendelenburg position. In one study, the IJV diameter changed from 11.2 (± 1.5) mm to 15.4 (± 1.5) mm in the supine versus the Trendelenburg position at 15 degrees.33 An observational study demonstrated a frog-legged position with reverse Trendelenburg increased the femoral vein size and reduced the common surface area with the common femoral artery compared to a neutral position. Thus, a frog-legged position with reverse Trendelenburg position may be preferred, since overall catheterization success rates are higher in this position.44
Techniques
General Techniques
5. We recommend that providers should avoid using static ultrasound alone to mark the needle insertion site for vascular access procedures.
Rationale: The use of static ultrasound guidance to mark a needle insertion site is not recommended because normal anatomical relationships of vessels vary, and site marking can be inaccurate with minimal changes in patient position, especially of the neck.43,45,46 Benefits of using ultrasound guidance for vascular access are attained when ultrasound is used to track the needle tip in real-time as it is advanced toward the target vessel.
Although continuous-wave Doppler ultrasound without two-dimensional visualization was used in the past, it is no longer recommended for IJV CVC insertion.47 In a study that randomized patients to IJV CVC insertion with continuous-wave Doppler alone vs two-dimensional ultrasound guidance, the use of two-dimensional ultrasound guidance showed significant improvement in first-pass success rates (97% vs 91%, P = .045), particularly in patients with BMI >30 (97% vs 77%, P = .011).48
A randomized study comparing real-time ultrasound-guided, landmark-based, and ultrasound-marked techniques found higher success rates in the real-time ultrasound-guided group than the other two groups (100% vs 74% vs 73%, respectively; P = .01). The total number of mechanical complications was higher in the landmark-based and ultrasound-marked groups than in the real-time ultrasound-guided group (24% and 36% versus 0%, respectively; P = .01).49 Another randomized controlled study found higher success rates with real-time ultrasound guidance (98%) versus an ultrasound-marked (82%) or landmark-based (64%) approach for central line placement.50
6. We recommend that providers should use real-time (dynamic), two-dimensional ultrasound guidance with a high-frequency linear transducer for CVC insertion, regardless of the provider’s level of experience.
7. We suggest using either a transverse (short-axis) or longitudinal (long-axis) approach when performing real-time ultrasound-guided vascular access procedures.
Rationale: In clinical practice, the phrases transverse, short-axis, or out-of-plane approach are synonymous, as are longitudinal, long-axis, and in-plane approach. The short-axis approach involves tracking the needle tip as it approximates the target vessel with the ultrasound beam oriented in a transverse plane perpendicular to the target vessel. The target vessel is seen as a circular structure on the ultrasound screen as the needle tip approaches the target vessel from above. This approach is also called the out-of-plane technique since the needle passes through the ultrasound plane. The advantages of the short-axis approach include better visualization of adjacent vessels or nerves and the relative ease of skill acquisition for novice operators.9 When using the short-axis approach, extra care must be taken to track the needle tip from the point of insertion on the skin to the target vessel. A disadvantage of the short-axis approach is unintended posterior wall puncture of the target vessel.55
In contrast to a short-axis approach, a long-axis approach is performed with the ultrasound beam aligned parallel to the vessel. The vessel appears as a long tubular structure and the entire needle is visualized as it traverses across the ultrasound screen to approach the target vessel. The long-axis approach is also called an in-plane technique because the needle is maintained within the plane of the ultrasound beam. The advantage of a long-axis approach is the ability to visualize the entire needle as it is inserted into the vessel.14 A randomized crossover study with simulation models compared a long-axis versus short-axis approach for both IJV and subclavian vein catheterization. This study showed decreased number of needle redirections (relative risk (RR) 0.5, 95% confidence interval (CI) 0.3 to 0.7), and posterior wall penetrations (OR 0.3, 95% CI 0.1 to 0.9) using a long-axis versus short-axis approach for subclavian vein catheterization.56
A randomized controlled study comparing a long-axis or short-axis approach with ultrasound versus a landmark-based approach for IJV CVC insertion showed higher success rates (100% vs 90%; P < .001), lower insertion time (53 vs 116 seconds; P < .001), and fewer attempts to obtain access (2.5 vs 1.2 attempts, P < .001) with either the long- or short-axis ultrasound approach. The average time to obtain access and number of attempts were comparable between the short-axis and long-axis approaches with ultrasound. The incidence of carotid puncture and hematoma was significantly higher with the landmark-based approach versus either the long- or short-axis ultrasound approach (carotid puncture 17% vs 3%, P = .024; hematoma 23% vs 3%, P = .003).57
High success rates have been reported using a short-axis approach for insertion of PIV lines.58 A prospective, randomized trial compared the short-axis and long-axis approach in patients who had had ≥2 failed PIV insertion attempts. Success rate was 95% (95% CI, 0.85 to 1.00) in the short-axis group compared with 85% (95% CI, 0.69 to 1.00) in the long-axis group. All three subjects with failed PIV placement in the long-axis group had successful rescue placement using a short-axis approach. Furthermore, the short-axis approach was faster than the long-axis approach.59
For radial artery cannulation, limited data exist comparing the short- and long-axis approaches. A randomized controlled study compared a long-axis vs short-axis ultrasound approach for radial artery cannulation. Although the overall procedure success rate was 100% in both groups, the long-axis approach had higher first-pass success rates (1.27 ± 0.4 vs 1.5 ± 0.5, P < .05), shorter cannulation times (24 ± 17 vs 47 ± 34 seconds, P < .05), fewer hematomas (4% vs 43%, P < .05) and fewer posterior wall penetrations (20% vs 56%, P < .05).60
Another technique that has been described for IJV CVC insertion is an oblique-axis approach, a hybrid between the long- and short-axis approaches. In this approach, the transducer is aligned obliquely over the IJV and the needle is inserted using a long-axis or in-plane approach. A prospective randomized trial compared the short-axis, long-axis, and oblique-axis approaches during IJV cannulation. First-pass success rates were 70%, 52%, and 74% with the short-axis, long-axis, and oblique-axis approaches, respectively, and a statistically significant difference was found between the long- and oblique-axis approaches (P = .002). A higher rate of posterior wall puncture was observed with a short-axis approach (15%) compared with the oblique-axis (7%) and long-axis (4%) approaches (P = .047).61
8. We recommend that providers should visualize the needle tip and guidewire in the target vein prior to vessel dilatation.
Rationale: When real-time ultrasound guidance is used, visualization of the needle tip within the vein is the first step to confirm cannulation of the vein and not the artery. After the guidewire is advanced, the provider can use transverse and longitudinal views to reconfirm cannulation of the vein. In a longitudinal view, the guidewire is readily seen positioned within the vein, entering the anterior wall and lying along the posterior wall of the vein. Unintentional perforation of the posterior wall of the vein with entry into the underlying artery can be detected by ultrasound, allowing prompt removal of the needle and guidewire before proceeding with dilation of the vessel. In a prospective observational study that reviewed ultrasound-guided IJV CVC insertions, physicians were able to more readily visualize the guidewire than the needle in the vein.62 A prospective observational study determined that novice operators can visualize intravascular guidewires in simulation models with an overall accuracy of 97%.63
In a retrospective review of CVC insertions where the guidewire position was routinely confirmed in the target vessel prior to dilation, there were no cases of arterial dilation, suggesting confirmation of guidewire position can potentially eliminate the morbidity and mortality associated with arterial dilation during CVC insertion.64
9. To increase the success rate of ultrasound-guided vascular access procedures, we recommend that providers should utilize echogenic needles, plastic needle guides, and/or ultrasound beam steering when available.
Rationale: Echogenic needles have ridged tips that appear brighter on the screen, allowing for better visualization of the needle tip. Plastic needle guides help stabilize the needle alongside the transducer when using either a transverse or longitudinal approach. Although evidence is limited, some studies have reported higher procedural success rates when using echogenic needles, plastic needle guides, and ultrasound beam steering software. In a prospective observational study, Augustides et al. showed significantly higher IJV cannulation rates with versus without use of a needle guide after first (81% vs 69%, P = .0054) and second (93% vs 80%. P = .0001) needle passes.65 A randomized study by Maecken et al. compared subclavian vein CVC insertion with or without use of a needle guide, and found higher procedure success rates within the first and second attempts, reduced time to obtain access (16 seconds vs 30 seconds; P = .0001) and increased needle visibility (86% vs 32%; P < .0001) with the use of a needle guide.66 Another study comparing a short-axis versus long-axis approach with a needle guide showed improved needle visualization using a long-axis approach with a needle guide.67 A randomized study comparing use of a novel, sled-mounted needle guide to a free-hand approach for venous cannulation in simulation models showed the novel, sled-mounted needle guide improved overall success rates and efficiency of cannulation.68
Central Venous Access Techniques
10. We recommend that providers should use a standardized procedure checklist that includes use of real-time ultrasound guidance to reduce the risk of central line-associated bloodstream infection (CLABSI) from CVC insertion.
Rationale: A standardized checklist or protocol should be developed to ensure compliance with all recommendations for insertion of CVCs. Evidence-based protocols address periprocedural issues, such as indications for CVC, and procedural techniques, such as use of maximal sterile barrier precautions to reduce the risk of infection. Protocols and checklists that follow established guidelines for CVC insertion have been shown to decrease CLABSI rates.69,70 Similarly, development of checklists and protocols for maintenance of central venous catheters have been effective in reducing CLABSIs.71 Although no externally-validated checklist has been universally accepted or endorsed by national safety organizations, a few internally-validated checklists are available through peer-reviewed publications.72,73 An observational educational cohort of internal medicine residents who received training using simulation of the entire CVC insertion process was able to demonstrate fewer CLABSIs after the simulator-trained residents rotated in the intensive care unit (ICU) (0.50 vs 3.2 infections per 1,000 catheter days, P = .001).74
11. We recommend that providers should use real-time ultrasound guidance, combined with aseptic technique and maximal sterile barrier precautions, to reduce the incidence of infectious complications from CVC insertion.
Rationale: The use of real-time ultrasound guidance for CVC placement has demonstrated a statistically significant reduction in CLABSIs compared to landmark-based techniques.75 The Centers for Disease Control and Prevention (CDC) guidelines for the prevention of intravascular catheter-related infections recommend the use of ultrasound guidance to reduce the number of cannulation attempts and risk of mechanical complications.69 A prospective, three-arm study comparing ultrasound-guided long-axis, short-axis, and landmark-based approaches showed a CLABSI rate of 20% in the landmark-based group versus 10% in each of the ultrasound groups.57 Another randomized study comparing use of ultrasound guidance to a landmark-based technique for IJV CVC insertion demonstrated significantly lower CLABSI rates with the use of ultrasound (2% vs 10%; P < .05).72
Studies have shown that a systems-based intervention featuring a standardized catheter kit or catheter bundle significantly reduced CLABSI rates.76-78 A complete review of all preventive measures to reduce the risk of CLABSI is beyond the scope of this review, but a few key points will be mentioned. First, aseptic technique includes proper hand hygiene and skin sterilization, which are essential measures to reduce cutaneous colonization of the insertion site and reduce the risk of CLABSIs.79 In a systematic review and meta-analysis of eight studies including over 4,000 catheter insertions, skin antisepsis with chlorhexidine was associated with a 50% reduction in CLABSIs compared with povidone iodine.11 Therefore, a chlorhexidine-containing solution is recommended for skin preparation prior to CVC insertion per guidelines by Healthcare Infection Control Practices Advisory Committee/CDC, Society for Healthcare Epidemiology of America/Infectious Diseases Society of America, and American Society of Anesthesiologists.11,69,80,81 Second, maximal sterile barrier precautions refer to the use of sterile gowns, sterile gloves, caps, masks covering both the mouth and nose, and sterile full-body patient drapes. Use of maximal sterile barrier precautions during CVC insertion has been shown to reduce the incidence of CLABSIs compared to standard precautions.26,79,82-84 Third, catheters containing antimicrobial agents may be considered for hospital units with higher CLABSI rates than institutional goals, despite a comprehensive preventive strategy, and may be considered in specific patient populations at high risk of severe complications from a CLABSI.11,69,80 Finally, providers should use a standardized procedure set-up when inserting CVCs to reduce the risk of CLABSIs. The operator should confirm availability and proper functioning of ultrasound equipment prior to commencing a vascular access procedure. Use of all-inclusive procedure carts or kits with sterile ultrasound probe covers, sterile gel, catheter kits, and other necessary supplies is recommended to minimize interruptions during the procedure, and can ultimately reduce the risk of CLABSIs by ensuring maintenance of a sterile field during the procedure.13
12. We recommend that providers should use real-time ultrasound guidance for internal jugular vein catheterization, which reduces the risk of mechanical and infectious complications, the number of needle passes, and time to cannulation and increases overall procedure success rates.
Rationale: The use of real-time ultrasound guidance for CVC insertion has repeatedly demonstrated better outcomes compared to a landmark-based approach in adults.13 Several randomized controlled studies have demonstrated that real-time ultrasound guidance for IJV cannulation reduces the risk of procedure-related mechanical and infectious complications, and improves first-pass and overall success rates in diverse care settings.27,29,45,50,53,65,75,85-90 Mechanical complications that are reduced with ultrasound guidance include pneumothorax and carotid artery puncture.4,5,45,46,53,62,75,86-93 Currently, several medical societies strongly recommend the use of ultrasound guidance during insertion of IJV CVCs.10-12,14,94-96
A meta-analysis by Hind et al. that included 18 randomized controlled studies demonstrated use of real-time ultrasound guidance reduced failure rates (RR 0.14, 95% CI 0.06 to 0.33; P < .0001), increased first-attempt success rates (RR 0.59, 95% CI 0.39 to 0.88; P = .009), reduced complication rates (RR 0.43, 95% CI 0.22 to 0.87; P = .02) and reduced procedure time (P < .0001), compared to a traditional landmark-based approach when inserting IJV CVCs.5
A Cochrane systematic review compared ultrasound-guided versus landmark-based approaches for IJV CVC insertion and found use of real-time ultrasound guidance reduced total complication rates by 71% (RR 0.29, 95% CI 0.17 to 0.52; P < .0001), arterial puncture rates by 72% (RR 0.28, 95% CI 0.18 to 0.44; P < .00001), and rates of hematoma formation by 73% (RR 0.27, 95% CI 0.13 to 0.55; P = .0004). Furthermore, the number of attempts for successful cannulation was reduced (mean difference -1.19 attempts, 95% CI -1.45 to -0.92; P < .00001), the chance of successful insertion on the first attempt was increased by 57% (RR 1.57, 95% CI 1.36 to 1.82; P < .00001), and overall procedure success rates were modestly increased in all groups by 12% (RR 1.12, 95% CI 1.08 to 1.17; P < .00001).46
An important consideration in performing ultrasound guidance is provider experience. A prospective observational study of patients undergoing elective CVC insertion demonstrated higher complication rates for operators that were inexperienced (25.2%) versus experienced (13.6%).54 A randomized controlled study comparing experts and novices with or without the use of ultrasound guidance for IJV CVC insertion demonstrated higher success rates among expert operators and with the use of ultrasound guidance. Among novice operators, the complication rates were lower with the use of ultrasound guidance.97 One study evaluated the procedural success and complication rates of a two-physician technique with one physician manipulating the transducer and another inserting the needle for IJV CVC insertion. This study concluded that procedural success rates and frequency of complications were directly affected by the experience of the physician manipulating the transducer and not by the experience of the physician inserting the needle.98
The impact of ultrasound guidance on improving procedural success rates and reducing complication rates is greatest in patients that are obese, short necked, hypovolemic, or uncooperative.93 Several studies have demonstrated fewer needle passes and decreased time to cannulation compared to the landmark technique in these populations.46,49,53,86-88,92,93
Ultrasound-guided placement of IJV catheters can safely be performed in patients with disorders of hemostasis and those with multiple previous catheter insertions in the same vein.9 Ultrasound-guided placement of CVCs in patients with disorders of hemostasis is safe with high success and low complication rates. In a case series of liver patients with coagulopathy (mean INR 2.17 ± 1.16, median platelet count 150K), the use of ultrasound guidance for CVC insertion was highly successful with no major bleeding complications.99
A study of renal failure patients found high success rates and low complication rates in the patients with a history of multiple previous catheterizations, poor compliance, skeletal deformities, previous failed cannulations, morbid obesity, and disorders of hemostasis.100 A prospective observational study of 200 ultrasound-guided CVC insertions for apheresis showed a 100% success rate with a 92% first-pass success rate.101
The use of real-time ultrasound guidance for IJV CVC insertion has been shown to be cost effective by reducing procedure-related mechanical complications and improving procedural success rates. A companion cost-effectiveness analysis estimated that for every 1,000 patients, 90 complications would be avoided, with a net cost savings of approximately $3,200 using 2002 prices.102
13. We recommend that providers who routinely insert subclavian vein CVCs should use real-time ultrasound guidance, which has been shown to reduce the risk of mechanical complications and number of needle passes and increase overall procedure success rates compared with landmark-based techniques.
Rationale: In clinical practice, the term ultrasound-guided subclavian vein CVC insertion is commonly used. However, the needle insertion site is often lateral to the first rib and providers are technically inserting the CVC in the axillary vein. The subclavian vein becomes the axillary vein at the lateral border of the first rib where the cephalic vein branches from the subclavian vein. To be consistent with common medical parlance, we use the phrase ultrasound-guided subclavian vein CVC insertion in this document.
Advantages of inserting CVCs in the subclavian vein include reliable surface anatomical landmarks for vein location, patient comfort, and lower risk of infection.103 Several observational studies have demonstrated the technique for ultrasound-guided subclavian vein CVC insertion is feasible and safe.104-107 In a large retrospective observational study of ultrasound-guided central venous access among a complex patient group, the majority of patients were cannulated successfully and safely. The subset of patients undergoing axillary vein CVC insertion (n = 1,923) demonstrated a low rate of complications (0.7%), proving it is a safe and effective alternative to the IJV CVC insertion.107
A Cochrane review of ultrasound-guided subclavian vein cannulation (nine studies, 2,030 participants, 2,049 procedures), demonstrated that real-time two-dimensional ultrasound guidance reduced the risk of inadvertent arterial punctures (three studies, 498 participants, RR 0.21, 95% CI 0.06 to 0.82; P = .02) and hematoma formation (three studies, 498 participants, RR 0.26, 95% CI 0.09 to 0.76; P = .01).46 A systematic review and meta-analysis of 10 randomized controlled studies comparing ultrasound-guided versus landmark-based subclavian vein CVC insertion demonstrated a reduction in the risk of arterial punctures, hematoma formation, pneumothorax, and failed catheterization with the use of ultrasound guidance.105
A randomized controlled study comparing ultrasound-guided vs landmark-based approaches to subclavian vein cannulation found that use of ultrasound guidance had a higher success rate (92% vs 44%, P = .0003), fewer minor complications (1 vs 11, P = .002), fewer attempts (1.4 vs 2.5, P = .007) and fewer catheter kits used (1.0 vs 1.4, P = .0003) per cannulation.108
Fragou et al. randomized patients undergoing subclavian vein CVC insertion to a long-axis approach versus a landmark-based approach and found a significantly higher success rate (100% vs 87.5%, P < .05) and lower rates of mechanical complications: artery puncture (0.5% vs 5.4%), hematoma (1.5% vs 5.4%), hemothorax (0% vs 4.4%), pneumothorax (0% vs 4.9%), brachial plexus injury (0% vs 2.9%), phrenic nerve injury (0% vs 1.5%), and cardiac tamponade (0% vs 0.5%).109 The average time to obtain access and the average number of insertion attempts (1.1 ± 0.3 vs 1.9 ± 0.7, P < .05) were significantly reduced in the ultrasound group compared to the landmark-based group.95
A retrospective review of subclavian vein CVC insertions using a supraclavicular approach found no reported complications with the use of ultrasound guidance vs 23 mechanical complications (8 pneumothorax, 15 arterial punctures) with a landmark-based approach.106 However, it is important to note that a supraclavicular approach is not commonly used in clinical practice.
14. We recommend that providers should use real-time ultrasound guidance for femoral venous access, which has been shown to reduce the risk of arterial punctures and total procedure time and increase overall procedure success rates.
Rationale: Anatomy of the femoral region varies, and close proximity or overlap of the femoral vein and artery is common.51 Early studies showed that ultrasound guidance for femoral vein CVC insertion reduced arterial punctures compared with a landmark-based approach (7% vs 16%), reduced total procedure time (55 ± 19 vs 79 ± 62 seconds), and increased procedure success rates (100% vs 90%).52 A Cochrane review that pooled data from four randomized studies comparing ultrasound-guided vs landmark-based femoral vein CVC insertion found higher first-attempt success rates with the use of ultrasound guidance (RR 1.73, 95% CI 1.34 to 2.22; P < .0001) and a small increase in the overall procedure success rates (RR 1.11, 95% CI 1.00 to 1.23; P = .06). There was no difference in inadvertent arterial punctures or other complications.110
Peripheral Venous Access Techniques
15. We recommend that providers should use real-time ultrasound guidance for the insertion of peripherally inserted central catheters (PICCs), which is associated with higher procedure success rates and may be more cost effective compared with landmark-based techniques.
Rationale: Several studies have demonstrated that providers who use ultrasound guidance vs landmarks for PICC insertion have higher procedural success rates, lower complication rates, and lower total placement costs. A prospective observational report of 350 PICC insertions using ultrasound guidance reported a 99% success rate with an average of 1.2 punctures per insertion and lower total costs.20 A retrospective observational study of 500 PICC insertions by designated specialty nurses revealed an overall success rate of 95%, no evidence of phlebitis, and only one CLABSI among the catheters removed.21 A retrospective observational study comparing several PICC variables found higher success rates (99% vs 77%) and lower thrombosis rates (2% vs 9%) using ultrasound guidance vs landmarks alone.22 A study by Robinson et al. demonstrated that having a dedicated PICC team equipped with ultrasound increased their institutional insertion success rates from 73% to 94%.111
A randomized controlled study comparing ultrasound-guided versus landmark-based PICC insertion found high success rates with both techniques (100% vs 96%). However, there was a reduction in the rate of unplanned catheter removals (4.0% vs 18.7%; P = .02), mechanical phlebitis (0% vs 22.9%; P < .001), and venous thrombosis (0% vs 8.3%; P = .037), but a higher rate of catheter migration (32% vs 2.1%; P < .001). Compared with the landmark-based group, the ultrasound-guided group had significantly lower incidence of severe contact dermatitis (P = .038), and improved comfort and costs up to 3 months after PICC placement (P < .05).112
Routine postprocedure chest x-ray (CXR) is generally considered unnecessary if the PICC is inserted with real-time ultrasound guidance along with use of a newer tracking devices, like the magnetic navigation system with intracardiac electrodes.9 Ultrasound can also be used to detect malpositioning of a PICC immediately after completing the procedure. A randomized controlled study comparing ultrasound versus postprocedure CXR detected one malpositioned PICC in the ultrasound group versus 11 in the control group. This study suggested that ultrasound can detect malpositioning immediately postprocedure and reduce the need for a CXR and the possibility of an additional procedure to reposition a catheter.113
16. We recommend that providers should use real-time ultrasound guidance for the placement of peripheral intravenous lines (PIV) in patients with difficult peripheral venous access to reduce the total procedure time, needle insertion attempts, and needle redirections. Ultrasound-guided PIV insertion is also an effective alternative to CVC insertion in patients with difficult venous access.
Rationale: Difficult venous access refers to patients that have had two unsuccessful attempts at PIV insertion using landmarks or a history of difficult access (i.e. edema, obesity, intravenous drug use, chemotherapy, diabetes, hypovolemia, chronic illness, vasculopathy, multiple prior hospitalizations). A meta-analysis of seven randomized controlled studies concluded that ultrasound guidance increases the likelihood of successful PIV insertion (pooled OR 2.42, 95% CI 1.26 to 4.68; P < .008).18 A second meta-analysis that pooled data from seven studies (six randomized controlled studies) confirmed that ultrasound guidance improves success rates of PIV insertion (OR 3.96, 95% CI 1.75 to 8.94).19 Approximately half of these studies had physician operators while the other half had nurse operators.
In one prospective observational study of emergency department patients with two failed attempts of landmark-based PIV insertion, ultrasound guidance with a modified-Seldinger technique showed a relatively high success rate (96%), fewer needle sticks (mean 1.32 sticks, 95% CI 1.12 to 1.52), and shorter time to obtain access (median time 68 seconds).114 Other prospective observational studies have demonstrated that ultrasound guidance for PIV insertion has a high success rate (87%),115 particularly with brachial or basilic veins PIV insertion, among patients with difficult PIV access, defined as having had ≥2 failed attempts.58
Since insertion of PIVs with ultrasound guidance has a high success rate, there is potential to reduce the reliance on CVC insertion for venous access only. In a study of patients that had had two failed attempts at PIV insertion based on landmarks, a PIV was successfully inserted with ultrasound guidance in 84% of patients, obviating the need for CVC placement for venous access.116 A prospective observational study showed ultrasound-guided PIV insertion was an effective alternative to CVC placement in ED patients with difficult venous access with only 1% of patients requiring a CVC.117 Use of ultrasound by nurses for PIV placement has also been shown to reduce the time to obtain venous access, improve patient satisfaction, and reduce the need for physician intervention.118 In a prospective observational study of patients with difficult access, the majority of patients reported a better experience with ultrasound-guided PIV insertion compared to previous landmark-based attempts with an average satisfaction score of 9.2/10 with 76% of patients rating the experience a 10.119 A strong recommendation has been made for use of ultrasound guidance in patients with difficult PIV placement by la Société Française d’Anesthésie et de Réanimation (The French Society of Anesthesia and Resuscitation).95
17. We suggest using real-time ultrasound guidance to reduce the risk of vascular, infectious, and neurological complications during PIV insertion, particularly in patients with difficult venous access.
Rationale: The incidence of complications from PIV insertion is often underestimated. Vascular complications include arterial puncture, hematoma formation, local infiltration or extravasation of fluid, and superficial or deep venous thrombosis. The most common infectious complications with PIV insertion are phlebitis and cellulitis.120 One observational study reported PIV complications occurring in approximately half of all patients with the most common complications being phlebitis, hematoma formation, and fluid/blood leakage.121
A retrospective review of ICU patients who underwent ultrasound-guided PIV insertion by a single physician showed high success rates (99%) with low rates of phlebitis/cellulitis (0.7%).There was an assumed benefit of risk reduction due to the patients no longer requiring a CVC after successful PIV placement.122 Another study found very low rates of infection with both landmark-based and ultrasound-guided PIV placement performed by emergency department nurses, suggesting that there is no increased risk of infection with the use of ultrasound.123 To reduce the risk of infection from PIV insertion, we recommend the use of sterile gel and sterile transducer cover (See Recommendation 2).
Arterial Access Techniques
18. We recommend that providers should use real-time ultrasound guidance for arterial access, which has been shown to increase first-pass success rates, reduce the time to cannulation, and reduce the risk of hematoma development compared with landmark-based techniques.
Rationale: Several randomized controlled studies have assessed the value of ultrasound in arterial catheter insertion. Shiver et al. randomized 60 patients admitted to a tertiary center emergency department to either palpation or ultrasound-guided arterial cannulation. They demonstrated a first-pass success rate of 87% in the ultrasound group compared with 50% in the landmark technique group. In the same study, the use of ultrasound was also associated with reduced time needed to establish arterial access and a 43% reduction in the development of hematoma at the insertion site.124 Levin et al. demonstrated a first-pass success rate of 62% using ultrasound versus 34% by palpation alone in 69 patients requiring intraoperative invasive hemodynamic monitoring.125 Additional randomized controlled studies have demonstrated that ultrasound guidance increases first-attempt success rates compared to traditional palpation.23,126,127
19. We recommend that providers should use real-time ultrasound guidance for femoral arterial access, which has been shown to increase first-pass success rates and reduce the risk of vascular complications.
Rationale: Although it is a less frequently used site, the femoral artery may be accessed for arterial blood sampling or invasive hemodynamic monitoring, and use of ultrasound guidance has been shown to improve the first-pass success rates of femoral artery cannulation. It is important to note that most of these studies comparing ultrasound-guided vs landmark-based femoral artery cannulation were performed in patients undergoing diagnostic or interventional vascular procedures.
A meta-analysis of randomized controlled studies comparing ultrasound-guided vs landmark-based femoral artery catheterization found use of ultrasound guidance was associated with a 49% reduction in overall complications (RR 0.51, 95% CI 0.28 to 0.91; P > .05) and 42% improvement in first-pass success rates.128 In another study, precise site selection with ultrasound was associated with fewer pseudoaneurysms in patients undergoing femoral artery cannulation by ultrasound guidance vs palpation for cardiac catheterization (3% vs 5%, P < .05).129
A multicenter randomized controlled study comparing ultrasound vs fluoroscopic guidance for femoral artery catheterization demonstrated ultrasound guidance improved rates of common femoral artery (CFA) cannulation in patients with high CFA bifurcations (83% vs 70%, P < .01).130 Furthermore, ultrasound guidance improved first-pass success rates (83% vs 46%, P < .0001), reduced number of attempts (1.3 vs 3.0, P < .0001), reduced risk of venipuncture (2.4% vs 15.8%, P < .0001), and reduced median time to obtain access (136 seconds vs148 seconds, P = .003). Vascular complications occurred in fewer patients in the ultrasound vs fluoroscopy groups (1.4% vs 3.4% P = .04). Reduced risk of hematoma formation with routine use of ultrasound guidance was demonstrated in one retrospective observational study (RR 0.62, 95% CI 0.46 to 0.84; P < .01).131
20. We recommend that providers should use real-time ultrasound guidance for radial arterial access, which has been shown to increase first-pass success rates, reduce the time to successful cannulation, and reduce the risk of complications compared with landmark-based techniques.
Rationale: Ultrasound guidance is particularly useful for radial artery cannulation in patients with altered anatomy, obesity, nonpulsatile blood flow, low perfusion, and previously unsuccessful cannulation attempts using a landmark-guided approach.132
A multicenter randomized controlled study that was not included in the abovementioned meta-analyses showed similar benefits of using ultrasound guidance vs landmarks for radial artery catheterization: a reduction in the number of attempts with ultrasound guidance (1.65 ± 1.2 vs 3.05 ± 3.4, P < .0001) and time to obtain access (88 ± 78 vs 108 ± 112 seconds, P = .006), and increased first-pass success rates (65% vs 44%, P < .0001). The use of ultrasound guidance was found to be particularly useful in patients with difficult access by palpation alone.135
Regarding the level of expertise required to use ultrasound guidance, a prospective observational study demonstrated that physicians with little previous ultrasound experience were able to improve their first-attempt success rates and procedure time for radial artery cannulation compared to historical data of landmark-based insertions.136
Postprocedure
21. We recommend that post-procedure pneumothorax should be ruled out by the detection of bilateral lung sliding using a high-frequency linear transducer before and after insertion of internal jugular and subclavian vein CVCs.
Rationale: Detection of lung sliding with two-dimensional ultrasound rules out pneumothorax, and disappearance of lung sliding in an area where it was previously seen is a strong predictor of postprocedure pneumothorax. In a study of critically ill patients, the disappearance of lung sliding was observed in 100% of patients with pneumothorax vs 8.8% of patients without pneumothorax. For detection of pneumothorax, lung sliding showed a sensitivity of 95%, specificity of 91%, and negative predictive value of 100% (P < .001).137 Another study by the same author showed that the combination of horizontal artifacts (absence of comet-tail artifact) and absence of lung sliding had a sensitivity of 100%, specificity of 96.5%, and negative predictive value of 100% for the detection of pneumothorax.138 A meta-analysis of 10 studies on the diagnostic accuracy of CVC confirmation with bedside ultrasound vs chest radiography reported detection of all 12 pneumothoraces with ultrasound, whereas chest radiography missed two pneumothoraces. The pooled sensitivity and specificity of ultrasound for the detection of pneumothorax was 100%, although an imperfect gold standard bias likely affected the results. An important advantage of bedside ultrasound is the ability to rule out pneumothorax immediately after the procedure while at the bedside. The mean time for confirmation of CVC placement with bedside ultrasound was 6 minutes versus 64 minutes and 143 minutes for completion and interpretation of a chest radiograph, respectively.139
22. We recommend that providers should use ultrasound with rapid infusion of agitated saline to visualize a right atrial swirl sign (RASS) for detecting catheter tip misplacement during CVC insertion. The use of RASS to detect the catheter tip may be considered an advanced skill that requires specific training and expertise.
Rationale: Bedside echocardiography is a reliable tool to detect catheter tip misplacement during CVC insertion. In one study, catheter misplacement was detected by bedside echocardiography with a sensitivity of 96% and specificity of 83% (positive predictive value 98%, negative predictive value 55%) and prevented distal positioning of the catheter tip.140 A prospective observational study assessed for RASS, which is turbulent flow in the right atrium after a rapid saline flush of the distal CVC port, to exclude catheter malposition. In this study with 135 CVC placements, visualization of RASS with ultrasound was able to identify all correct CVC placements and three of four catheter misplacements. Median times to complete the ultrasound exam vs CXR were 1 vs 20 minutes, respectively, with a median difference of 24 minutes (95% CI 19.6 to 29.3, P < .0001) between the two techniques.141
A prospective observational study assessed the ability of bedside transthoracic echocardiography to detect the guidewire, microbubbles, or both, in the right atrium compared to transesophageal echocardiography as the gold standard. Bedside transthoracic echocardiography allowed visualization of the right atrium in 94% of patients, and both microbubbles plus guidewire in 91% of patients.142 Hence, bedside transthoracic echocardiography allows adequate visualization of the right atrium. Another prospective observational study combining ultrasonography and contrast enhanced RASS resulted in 96% sensitivity and 93% specificity for the detection of a misplaced catheter, and the concordance with chest radiography was 96%.143
Training
23. To reduce the risk of mechanical and infectious complications, we recommend that novice providers should complete a systematic training program that includes a combination of simulation-based practice, supervised insertion on patients, and evaluation by an expert operator before attempting ultrasound-guided CVC insertion independently on patients.
Rationale: Cumulative experience has been recognized to not be a proxy for mastery of a clinical skill.144 The National Institute for Clinical Excellence (NICE) has recommended that providers performing ultrasound-guided CVC insertion should receive appropriate training to achieve competence before performing the procedure independently.7 Surveys have demonstrated that lack of training is a commonly reported barrier for not using ultrasound.145,146
Structured training programs on CVC insertion have been shown to reduce the occurrence of infectious and mechanical complications.74,143,147-149 The use of ultrasound and checklists, bundling of supplies, and practice with simulation models, as a part of a structured training program, can improve patient safety related to CVC insertion.9,140,150-154
Simulation-based practice has been used in medical education to provide deliberate practice and foster skill development in a controlled learning environment.155-158 Studies have shown transfer of skills demonstrated in a simulated environment to clinical practice, which can improve CVC insertion practices.159,160 Simulation accelerates learning of all trainees, especially novice trainees, and mitigates risks to patients by allowing trainees to achieve a minimal level of competence before attempting the procedure on real patients.152,161,162 Residents that have been trained using simulation preferentially select the IJV site,147 and more reliably use ultrasound to guide their CVC insertions.160,163
Additionally, simulation-based practice allows exposure to procedures and scenarios that may occur infrequently in clinical practice.
Although there is evidence on efficacy of simulation-based CVC training programs, there is no broadly accepted consensus on timing, duration, and content of CVC training programs for trainees or physicians in practice. The minimum recommended technical skills a trainee must master include the ability to (1) manipulate the ultrasound machine to produce a high-quality image to identify the target vessel, (2) advance the needle under direct visualization to the desired target site and depth, (3) deploy the catheter into the target vessel and confirm catheter placement in the target vessel using ultrasound, and (4) ensure the catheter has not been inadvertently placed in an unintended vessel or structure.153
A variety of simulation models are currently used to practice CVC insertion at the most common sites: the internal jugular, subclavian, basilic, and brachial veins.164,165 Effective simulation models should contain vessels that mimic normal anatomy with muscles, soft tissues, and bones. Animal tissue models, such as turkey or chicken breasts, may be effective for simulated practice of ultrasound-guided CVC insertion.166,167 Ultrasound-guided CVC training using human cadavers has also been shown to be effective.168
24. We recommend that cognitive training in ultrasound-guided CVC insertion should include basic anatomy, ultrasound physics, ultrasound machine knobology, fundamentals of image acquisition and interpretation, detection and management of procedural complications, infection prevention strategies, and pathways to attain competency.
Rationale: After receiving training in ultrasound-guided CVC insertion, physicians report significantly higher comfort with the use of ultrasound compared to those who have not received such training.145 Learners find training sessions worthwhile to increase skill levels,167 and skills learned from simulation-based mastery learning programs have been retained up to one year.158
Several commonalities have been noted across training curricula. Anatomy and physiology didactics should include vessel anatomy (location, size, and course);9 vessel differentiation by ultrasound;9,69 blood flow dynamics;69 Virchow’s triad;69 skin integrity and colonization;150 peripheral nerve identification and distribution;9 respiratory anatomy;9,69 upper and lower extremity, axillary, neck, and chest anatomy.9,69 Vascular anatomy is an essential curricular component that may help avoid preventable CVC insertion complications, such as inadvertent nerve, artery, or lung puncture.150,169 Training curricula should also include ultrasound physics (piezoelectric effect, frequency, resolution, attenuation, echogenicity, Doppler ultrasound, arterial and venous flow characteristics), image acquisition and optimization (imaging mode, focus, dynamic range, probe types), and artifacts (reverberation, mirror, shadowing, enhancement).
CVC-related infections are an important cause of morbidity and mortality in the acute and long-term care environment.69 Infection and thrombosis can both be impacted by the insertion site selection, skin integrity, and catheter–vein ratio.2,3,84 Inexperience generally leads to more insertion attempts that can increase trauma during CVC insertion and potentially increase the risk of infections.170 To reduce the risk of infectious complications, training should include important factors to consider in site selection and maintenance of a sterile environment during CVC insertion, including use of maximal sterile barrier precautions, hand hygiene, and appropriate use of skin antiseptic solutions.
Professional society guidelines have been published with recommendations of appropriate techniques for ultrasound-guided vascular access that include training recommendations.9,154 Training should deconstruct the insertion procedure into readily understood individual steps, and can be aided by demonstration of CVC insertion techniques using video clips. An alternative to face-to-face training is internet-based training that has been shown to be as effective as traditional teaching methods in some medical centers.171 Additional methods to deliver cognitive instruction include textbooks, continuing medical education courses, and digital videos.164,172
25. We recommend that trainees should demonstrate minimal competence before placing ultrasound-guided CVCs independently. A minimum number of CVC insertions may inform this determination, but a proctored assessment of competence is most important.
Rationale: CVC catheter placement carries the risk of serious complications including arterial injury or dissection, pneumothorax, or damage to other local structures; arrhythmias; catheter malposition; infection; and thrombosis. Although there is a lack of consensus and high-quality evidence for the certification of skills to perform ultrasound-guided CVC insertion, recommendations have been published advocating for formal and comprehensive training programs in ultrasound-guided CVC insertion with an emphasis on expert supervision prior to independent practice.9,153,154 Two groups of expert operators have recommended that training should include at least 8-10 supervised ultrasound-guided CVC insertions.154,173,174 A consensus task force from the World Congress of Vascular Access has recommended a minimum of six to eight hours of didactic education, four hours of hands-on training on simulation models, and six hours of hands-on ultrasound training on human volunteers to assess normal anatomy.175 This training should be followed by supervised ultrasound-guided CVC insertions until the learner has demonstrated minimal competence with a low rate of complications.35 There is general consensus that arbitrary numbers should not be the sole determinant of competence, and that the most important determinant of competence should be an evaluation by an expert operator.176
26. We recommend that didactic and hands-on training for trainees should coincide with anticipated times of increased performance of vascular access procedures. Refresher training sessions should be offered periodically.
Rationale: Simulation-based CVC training courses have shown a rapid improvement in skills, but lack of practice leads to deterioration of technical skills.161,162,177,178 Thus, a single immersive training session is insufficient to achieve and maintain mastery of skills, and an important factor to acquire technical expertise is sustained, deliberate practice with feedback.179 Furthermore, an insidious decay in skills may go unrecognized as a learner’s comfort and self-confidence does not always correlate with actual performance, leading to increased risk of errors and potential for procedural complications.147,158,180-183 Given the decay in technical skills over time, simulation-based training sessions are most effective when they occur in close temporal proximity to times when those skills are most likely to be used; for example, a simulation-based training session for trainees may be most effective just before the start of a critical care rotation.152 Regularly scheduled training sessions with monitoring and feedback by expert operators can reinforce procedural skills and prevent decay. Some experts have recommended that a minimum of 10 ultrasound-guided CVC insertions should be performed annually to maintain proficiency.153
27. We recommend that competency assessments should include formal evaluation of knowledge and technical skills using standardized assessment tools.
Rationale: Hospitalists and other healthcare providers that place vascular access catheters should undergo competency assessments proctored by an expert operator to verify that they have the required knowledge and skills.184,185 Knowledge competence can be partially evaluated using a written assessment, such as a multiple-choice test, assessing the provider’s cognitive understanding of the procedure.175 For ultrasound-guided CVC insertion, a written examination should be administered in conjunction with an ultrasound image assessment to test the learner’s recognition of normal vs abnormal vascular anatomy. Minimum passing standards should be established a priori according to local or institutional standards.
The final skills assessment should be objective, and the learner should be required to pass all critical steps of the procedure. Failure of the final skills assessment should lead to continued practice with supervision until the learner can consistently demonstrate correct performance of all critical steps. Checklists are commonly used to rate the technical performance of learners because they provide objective criteria for evaluation, can identify specific skill deficiencies, and can determine a learner’s readiness to perform procedures independently.186,187 The administration of skills assessments and feedback methods should be standardized across faculty. Although passing scores on both knowledge and skills assessments do not guarantee safe performance of a procedure independently, they provide a metric to ensure that a minimum level of competence has been achieved before allowing learners to perform procedures on patients without supervision.188
Competency assessments are a recommended component of intramural and extramural certification of skills in ultrasound-guided procedures. Intramural certification pathways differ by institution and often require additional resources including ultrasound machine(s), simulation equipment, and staff time, particularly when simulation-based assessments are incorporated into certification pathways. We recognize that some of these recommendations may not be feasible in resource-limited settings, such as rural hospitals. However, initial and ongoing competency assessments can be performed during routine performance of procedures on patients. For an in-depth review of credentialing pathways for ultrasound-guided bedside procedures, we recommend reviewing the SHM Position Statement on Credentialing of Hospitalists in Ultrasound-Guided Bedside Procedures.24
28. We recommend that competency assessments should evaluate for proficiency in the following knowledge and skills of CVC insertion:
a. Knowledge of the target vein anatomy, proper vessel identification, and recognition of anatomical variants
b. Demonstration of CVC insertion with no technical errors based on a procedural checklist
c. Recognition and management of acute complications, including emergency management of life-threatening complications
d. Real-time needle tip tracking with ultrasound and cannulation on the first attempt in at least five consecutive simulations.
Rationale: Recommendations have been published with the minimal knowledge and skills learners must demonstrate to perform ultrasound-guided vascular access procedures. These include operation of an ultrasound machine to produce high-quality images of the target vessel, tracking of the needle tip with real-time ultrasound guidance, and recognition and understanding of the management of procedural complications.154,175
First, learners must be able to perform a preprocedural assessment of the target vein, including size and patency of the vein; recognition of adjacent critical structures; and recognition of normal anatomical variants.175,189 Second, learners must be able to demonstrate proficiency in tracking the needle tip penetrating the target vessel, inserting the catheter into the target vessel, and confirming catheter placement in the target vessel with ultrasound.154,175 Third, learners must be able to demonstrate recognition of acute complications, including arterial puncture, hematoma formation, and development of pneumothorax.154,175 Trainees should be familiar with recommended evaluation and management algorithms, including indications for emergent consultation.190
29. We recommend a periodic proficiency assessments of all operators should be conducted to ensure maintenance of competency.
Rationale: Competency extends to periodic assessment and not merely an initial evaluation at the time of training.191 Periodic competency assessments should include assessment of proficiency of all providers that perform a procedure, including instructors and supervisors. Supervising providers should maintain their competency in CVC insertion through routine use of their skills in clinical practice.175 An observational study of emergency medicine residents revealed that lack of faculty comfort with ultrasound hindered the residents’ use of ultrasound.192 Thus, there is a need to examine best practices for procedural supervision of trainees because providers are often supervising procedures that they are not comfortable performing on their own.193
KNOWLEDGE GAPS
The process of producing this position statement revealed areas of uncertainty and important gaps in the literature regarding the use of ultrasound guidance for central and peripheral venous access and arterial access.
This position statement recommends a preprocedural ultrasound evaluation of blood vessels based on evidence that providers may detect anatomic anomalies, thrombosis, or vessel stenosis. Ultrasound can also reveal unsuspected high-risk structures in near proximity to the procedure site. Although previous studies have shown that providers can accurately assess vessels with ultrasound for these features, further study is needed to evaluate the effect of a standardized preprocedural ultrasound exam on clinical and procedural decision-making, as well as procedural outcomes.
Second, two ultrasound applications that are being increasingly used but have not been widely implemented are the use of ultrasound to evaluate lung sliding postprocedure to exclude pneumothorax and the verification of central line placement using a rapid infusion of agitated saline to visualize the RASS.139-141 Both of these applications have the potential to expedite postprocedure clearance of central lines for usage and decrease patient radiation exposure by obviating the need for postprocedure CXRs. Despite the supporting evidence, both of these applications are not yet widely used, as few providers have been trained in these techniques which may be considered advanced skills.
Third, despite advances in our knowledge of effective training for vascular access procedures, there is limited agreement on how to define procedural competence. Notable advancements in training include improved understanding of systematic training programs, development of techniques for proctoring procedures, definition of elements for hands-on assessments, and definition of minimum experience needed to perform vascular access procedures independently. However, application of these concepts to move learners toward independent practice remains variably interpreted at different institutions, likely due to limited resources, engrained cultures about procedures, and a lack of national standards. The development of hospitalist-based procedure services at major academic medical centers with high training standards, close monitoring for quality assurance, and the use of databases to track clinical outcomes may advance our understanding and delivery of optimal procedural training.
Finally, ultrasound technology is rapidly evolving which will affect training, techniques, and clinical outcomes in coming years. Development of advanced imaging software with artificial intelligence can improve needle visualization and tracking. These technologies have the potential to facilitate provider training in real-time ultrasound-guided procedures and improve the overall safety of procedures. Emergence of affordable, handheld ultrasound devices is improving access to ultrasound technology, but their role in vascular access procedures is yet to be defined. Furthermore, availability of wireless handheld ultrasound technology and multifrequency transducers will create new possibilities for use of ultrasound in vascular access procedures.
CONCLUSION
We have presented several evidence-based recommendations on the use of ultrasound guidance for placement of central and peripheral vascular access catheters that are intended for hospitalists and other healthcare providers who routinely perform vascular access procedures. By allowing direct visualization of the needle tip and target vessel, the use of ultrasound guidance has been shown in randomized studies to reduce needle insertion attempts, reduce needle redirections, and increase overall procedure success rates. The accuracy of ultrasound to identify the target vessel, assess for thrombosis, and detect anatomical anomalies is superior to that of physical examination. Hospitalists can attain competence in performing ultrasound-guided vascular access procedures through systematic training programs that combine didactic and hands-on training, which optimally include patient-based competency assessments.
Acknowledgments
The authors thank all the members of the Society of Hospital Medicine Point-of-care Ultrasound Task Force and the Education Committee members for their time and dedication to develop these guidelines.
Collaborators of Society of Hospital Medicine Point-of-care Ultrasound Task Force: Robert Arntfield, Jeffrey Bates, Anjali Bhagra, Michael Blaivas, Daniel Brotman, Richard Hoppmann, Susan Hunt, Trevor P. Jensen, Venkat Kalidindi, Ketino Kobaidze, Joshua Lenchus, Paul Mayo, Satyen Nichani, Vicki Noble, Nitin Puri, Aliaksei Pustavoitau, Kreegan Reierson, Gerard Salame, Kirk Spencer, Vivek Tayal, David Tierney
SHM Point-of-care Ultrasound Task Force: CHAIRS: Nilam J. Soni, Ricardo Franco-Sadud, Jeff Bates. WORKING GROUPS: Thoracentesis Working Group: Ria Dancel (chair), Daniel Schnobrich, Nitin Puri. Vascular Access Working Group: Ricardo Franco (chair), Benji Mathews, Saaid Abdel-Ghani, Sophia Rodgers, Martin Perez, Daniel Schnobrich. Paracentesis Working Group: Joel Cho (chair), Benji Mathews, Kreegan Reierson, Anjali Bhagra, Trevor P. Jensen Lumbar Puncture Working Group: Nilam J. Soni (chair), Ricardo Franco, Gerard Salame, Josh Lenchus, Venkat Kalidindi, Ketino Kobaidze. Credentialing Working Group: Brian P Lucas (chair), David Tierney, Trevor P. Jensen PEER REVIEWERS: Robert Arntfield, Michael Blaivas, Richard Hoppmann, Paul Mayo, Vicki Noble, Aliaksei Pustavoitau, Kirk Spencer, Vivek Tayal. METHODOLOGIST: Mahmoud El-Barbary. LIBRARIAN: Loretta Grikis. SOCIETY OF HOSPITAL MEDICINE EDUCATION COMMITTEE: Daniel Brotman (past chair), Satyen Nichani (current chair), Susan Hunt. SOCIETY OF HOSPITAL MEDICINE STAFF: Nick Marzano.
Disclaimer
The contents of this publication do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
1. Raad I. Intravascular-catheter-related infections. Lancet. 1998;351(9106):893-898. https://doi.org/10.1016/S0140-6736(97)10006-X.
2. Merrer J, De Jonghe B, Golliot F, et al. Complications of femoral and subclavian venous catheterization in critically ill patients: a randomized controlled trial. JAMA. 2001;286(6):700-707. https://doi.org/10.1001/jama.286.6.700.
3. Sznajder JI, Zveibil FR, Bitterman H, Weiner P, Bursztein S. Central vein catheterization. Failure and complication rates by three percutaneous approaches. Arch Intern Med. 1986;146(2):259-261. https://doi.org/10.1001/archinte.146.2.259.
4. Randolph AG, Cook DJ, Gonzales CA, Pribble CG. Ultrasound guidance for placement of central venous catheters: a meta-analysis of the literature. Crit Care Med. 1996;24(12):2053-2058. https://doi.org/10.1097/00003246-199612000-00020.
5. Hind D, Calvert N, McWilliams R, et al. Ultrasonic locating devices for central venous cannulation: meta-analysis. BMJ. 2003;327(7411):361. https://doi.org/10.1136/bmj.327.7411.361.
6. O’Grady NP, Alexander M, Burns LA, et al. Guidelines for the prevention of intravascular catheter-related infections. Am J Infect Control. 2011;39(4):S1-S34. https://doi.org/10.1016/j.ajic.2011.01.003.
7. National Institute for Health and Care Excellence (NICE). Guidance on the Use of Ultrasound Locating Devices for Placing Central Venous Catheters; 2002.
8. Shojania KG, Duncan BW, McDonald KM, et al. Making health care safer: A critical analysis of patient safety practices. Evid Rep Technol Assess. Rockville, MD: Agency for Healthcare Research and Quality. 2001;43(43):i–x, 1.
9. Lamperti M, Bodenham AR, Pittiruti M, et al. International evidence-based recommendations on ultrasound-guided vascular access. Intensive Care Med. 2012;38(7):1105-1117. https://doi.org/10.1007/s00134-012-2597-x.
10. Frykholm P, Pikwer A, Hammarskjöld F, et al. Clinical guidelines on central venous catheterisation. Swedish Society of Anaesthesiology and Intensive Care Medicine. Acta Anaesthesiol Scand. 2014;58(5):508-524. https://doi.org/10.1111/aas.12295.
11. American Society of Anesthesiologists Task Force on Central Venous Access, Rupp SM, Apfelbaum JL, et al. Practice guidelines for central venous access: a report by the American Society of Anesthesiologists Task Force on Central Venous Access. Anesthesiology. 2012;116(3):539-573. https://doi.org/10.1097/ALN.0b013e31823c9569.
12. Frankel HL, Kirkpatrick AW, Elbarbary M, et al. Guidelines for the appropriate use of bedside general and cardiac ultrasonography in the evaluation of critically ill patients-part I: General ultrasonography. Crit Care Med. 2015;43(11):2479-2502. https://doi.org/10.1097/CCM.0000000000001216.
13. Shekelle PG, Wachter RM, Pronovost PJ, et al. Making health care safer II: An updated critical analysis of the evidence for patient safety practices. Evid Rep Technol Assess. 2013;211:1-945.
14. Troianos CA, Hartman GS, Glas KE, et al. Guidelines for performing ultrasound guided vascular cannulation: recommendations of the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. J Am Soc Echocardiogr. 2011;24(12):1291-1318. https://doi.org/10.1016/j.echo.2011.09.021.
15. Soni NJ, Reyes LF, Keyt H, et al. Use of ultrasound guidance for central venous catheterization: a national survey of intensivists and hospitalists. J Crit Care. 2016;36:277-283. https://doi.org/10.1016/j.jcrc.2016.07.014.
16. Parienti JJ, Mongardon N, Mégarbane B, et al. Intravascular complications of central venous catheterization by insertion site. N Engl J Med. 2015;373(13):1220-1229. https://doi.org/10.1056/NEJMoa1500964.
17. Maizel J, Bastide MA, Richecoeur J, et al. Practice of ultrasound-guided central venous catheter technique by the French intensivists: a survey from the BoReal study group. Ann Intensive Care. 2016;6(1):76. https://doi.org/10.1186/s13613-016-0177-x.
18. Egan G, Healy D, O’Neill H, et al. Ultrasound guidance for difficult peripheral venous access: systematic review and meta-analysis. Emerg Med J. 2013;30(7):521-526. https://doi.org/10.1136/emermed-2012-201652.
19. Stolz LA, Stolz U, Howe C, Farrell IJ, Adhikari S. Ultrasound-guided peripheral venous access: a meta-analysis and systematic review. J Vasc Access. 2015;16(4):321-326. https://doi.org/10.5301/jva.5000346.
20. Sofocleous CT, Schur I, Cooper SG, et al. Sonographically guided placement of peripherally inserted central venous catheters: review of 355 procedures. AJR Am J Roentgenol. 1998;170(6):1613-1616. https://doi.org/10.2214/ajr.170.6.9609183.
21. Nichols I, Humphrey JP. The efficacy of upper arm placement of peripherally inserted central catheters using bedside ultrasound and microintroducer technique. J Infus Nurs Off Publ Infus Nurs Soc. 2008;31(3):165-176. https://doi.org/10.1097/01.NAN.0000317703.66395.b8.
22. Stokowski G, Steele D, Wilson D. The use of ultrasound to improve practice and reduce complication rates in peripherally inserted central catheter insertions. J Infus Nurs Off Publ Infus Nurs Soc. 2009;32(3):145-155. https://doi.org/10.1097/NAN.0b013e3181a1a98f.
23. Shiloh AL, Savel RH, Paulin LM, Eisen LA. Ultrasound-guided catheterization of the radial artery: a systematic review and meta-analysis of randomized controlled trials. Chest. 2011;139(3):524-529. https://doi.org/10.1378/chest.10-0919.
24. Lucas BP, Tierney DM, Jensen TP, et al. Credentialing of hospitalists in ultrasound-guided bedside procedures: A position statement of the Society of Hospital Medicine. J Hosp Med. 2018;13(2):117-125. https://doi.org/10.12788/jhm.2917.
25. Chopra V, Flanders SA, Saint S, et al. The Michigan appropriateness guide for intravenous catheters (Magic): results From a Multispecialty Panel Using the RAND/UCLA Appropriateness Method. Ann Intern Med. 2015;163(6):S1-S40. https://doi.org/10.7326/M15-0744.
26. Raad II, Hohn DC, Gilbreath BJ, et al. Prevention of central venous catheter-related infections by using maximal sterile barrier precautions during insertion. Infect Control Hosp Epidemiol. 1994;15(4):231-238.
27. Slama M, Novara A, Safavian A, et al. Improvement of internal jugular vein cannulation using an ultrasound-guided technique. Intensive Care Med. 1997;23(8):916-919. https://doi.org/10.1007/s001340050432.
28. Seyahi N, Kahveci A, Altiparmak MR, Serdengecti K, Erek E. Ultrasound imaging findings of femoral veins in patients with renal failure and its impact on vascular access. Nephrol Dial Transplant. 2005;20(9):1864-1867. https://doi.org/10.1093/ndt/gfh942.
29. Lin BS, Kong CW, Tarng DC, Huang TP, Tang GJ. Anatomical variation of the internal jugular vein and its impact on temporary haemodialysis vascular access: an ultrasonographic survey in uraemic patients. Nephrol Dial Transplant. 1998;13(1):134-138. https://doi.org/10.1093/ndt/13.1.134.
30. Troianos CA, Kuwik RJ, Pasqual JR, Lim AJ, Odasso DP. Internal jugular vein and carotid artery anatomic relation as determined by ultrasonography. Anesthesiology. 1996;85(1):43-48. https://doi.org/10.1097/00000542-199607000-00007.
31. Gordon AC, Wright I, Pugh ND. Duplication of the superficial femoral vein: recognition with duplex ultrasonography. Clin Rad. 1996;51(9):622-624. https://doi.org/10.1016/S0009-9260(96)80055-9.
32. Sulek CA, Gravenstein N, Blackshear RH, Weiss L. Head rotation during internal jugular vein cannulation and the risk of carotid artery puncture. Anesth Analg. 1996;82(1):125-128. https://doi.org/10.1097/00000539-199601000-00022.
33. Shrestha BR, Gautam B. Ultrasound versus the landmark technique: a prospective randomized comparative study of internal jugular vein cannulation in an intensive care unit. JNMA J Nepal Med Assoc. 2011;51(182):56-61. https://doi.org/10.31729/jnma.148.
34. Brederlau J, Greim C, Schwemmer U, et al. Ultrasound-guided cannulation of the internal jugular vein in critically ill patients positioned in 30 degrees dorsal elevation. Eur J Anaesthesiol. 2004;21(9):684-687. https://doi.org/10.1097/00003643-200409000-00003.
35. Lamperti M, Subert M, Cortellazzi P, et al. Is a neutral head position safer than 45-degree neck rotation during ultrasound-guided internal jugular vein cannulation? Results of a randomized controlled clinical trial. Anesth Analg. 2012;114(4):777-784. https://doi.org/10.1213/ANE.0b013e3182459917.
36. Benter T, Teichgräber UK, Klühs L, et al. Anatomical variations in the internal jugular veins of cancer patients affecting central venous access. Anatomical variation of the internal jugular vein. Ultraschall Med. 2001;22(1):23-26. https://doi.org/10.1055/s-2001-11243.
37. Caridi JG, Hawkins IF, Jr, Wiechmann BN, Pevarski DJ, Tonkin JC. Sonographic guidance when using the right internal jugular vein for central vein access. AJR Am J Roentgenol. 1998;171(5):1259-1263. https://doi.org/10.2214/ajr.171.5.9798857.
38. Brusasco C, Corradi F, Zattoni PL, et al. Ultrasound-guided central venous cannulation in bariatric patients. Obes Surg. 2009;19(10):1365-1370. https://doi.org/10.1007/s11695-009-9902-y.
39. Armstrong PJ, Cullen M, Scott DH. The ‘SiteRite’ ultrasound machine--an aid to internal jugular vein cannulation. Anaesthesia. 1993;48(4):319-323. https://doi.org/10.1111/j.1365-2044.1993.tb06953.x.
40. Forauer AR, Glockner JF. Importance of US findings in access planning during jugular vein hemodialysis catheter placements. J Vasc Interv Rad. 2000;11(2 Pt 1):233-238. https://doi.org/10.1016/S1051-0443(07)61471-7.
41. Hassan C, Girishkumar HT, Thatigotla B, et al. Value of ultrasound guidance in placement of hemodialysis access catheters in patients with end-stage renal disease. Am Surg. 2008;74(11):1111-1113.
42. Tan CO, Weinberg L, Peyton P, Story D, McNicol L. Size variation between contralateral infraclavicular axillary veins within individual patients-implications for subclavian venous central line insertion. Crit Care Med. 2013;41(2):457-463. https://doi.org/10.1097/CCM.0b013e31826ab1dd.
43. Wu SY, Ling Q, Cao LH, et al. Real-time two-dimensional ultrasound guidance for central venous cannulation: a meta-analysis. Anesthesiology. 2013;118(2):361-375. https://doi.org/10.1097/ALN.0b013e31827bd172.
44. Kim W, Chung RK, Lee GY, Han JI. The effects of hip abduction with external rotation and reverse Trendelenburg position on the size of the femoral vein; ultrasonographic investigation. Korean J Anesthesiol. 2011;61(3):205-209. https://doi.org/10.4097/kjae.2011.61.3.205.
45. Denys BG, Uretsky BF. Anatomical variations of internal jugular vein location: impact on central venous access. Crit Care Med. 1991;19(12):1516-1519. https://doi.org/10.1097/00003246-199112000-00013.
46. Brass P, Hellmich M, Kolodziej L, Schick G, Smith AF. Ultrasound guidance versus anatomical landmarks for internal jugular vein catheterization. Cochrane Database Syst Rev. 2015;1:CD006962. https://doi.org/10.1002/14651858.CD006962.pub2.
47. Gilbert TB, Seneff MG, Becker RB. Facilitation of internal jugular venous cannulation using an audio-guided Doppler ultrasound vascular access device: results from a prospective, dual-center, randomized, crossover clinical study. Crit Care Med. 1995;23(1):60-65. https://doi.org/10.1097/00003246-199501000-00012.
48. Schummer W, Schummer C, Tuppatsch H, et al. Ultrasound-guided central venous cannulation: is there a difference between Doppler and B-mode ultrasound? J Clin Anesth. 2006;18(3):167-172. https://doi.org/10.1016/j.jclinane.2005.12.010.
49. Airapetian N, Maizel J, Langelle F, et al. Ultrasound-guided central venous cannulation is superior to quick-look ultrasound and landmark methods among inexperienced operators: a prospective randomized study. Intensive Care Med. 2013;39(11):1938-1944. https://doi.org/10.1007/s00134-013-3072-z.
50. Milling TJ, Jr, Rose J, Briggs WM, et al. Randomized, controlled clinical trial of point-of-care limited ultrasonography assistance of central venous cannulation: the Third Sonography Outcomes Assessment Program (SOAP-3) Trial. Crit Care Med. 2005;33(8):1764-1769. https://doi.org/10.1097/01.ccm.0000171533.92856.e5.
51. Beaudoin FL, Merchant RC, Lincoln J, et al. Bedside ultrasonography detects significant femoral vessel overlap: implications for central venous cannulation. CJEM. 2011;13(4):245-250. https://doi.org/10.2310/8000.2011.110482.
52. Kwon TH, Kim YL, Cho DK. Ultrasound-guided cannulation of the femoral vein for acute haemodialysis access. Nephrol Dial Transplant. 1997;12(5):1009-1012. https://doi.org/10.1093/ndt/12.5.1009.
53. Rothschild JM. Ultrasound guidance of central vein catheterization. Evid Rep Technol Assess. 2001;43. Chapter 21.: http://archive.ahrq.gov/clinic/ptsafety/chap21.htm.
54. Lennon M, Zaw NN, Pöpping DM, Wenk M. Procedural complications of central venous catheter insertion. Minerva Anestesiol. 2012;78(11):1234-1240.
55. Blaivas M, Adhikari S. An unseen danger: frequency of posterior vessel wall penetration by needles during attempts to place internal jugular vein central catheters using ultrasound guidance. Crit Care Med. 2009;37(8):2345-2349; quiz 2359. https://doi.org/10.1097/CCM.0b013e3181a067d4.
56. Vogel JA, Haukoos JS, Erickson CL, et al. Is long-axis view superior to short-axis view in ultrasound-guided central venous catheterization? Crit Care Med. 2015;43(4):832-839. https://doi.org/10.1097/CCM.0000000000000823.
57. Tammam TF, El-Shafey EM, Tammam HF. Ultrasound-guided internal jugular vein access: comparison between short axis and long axis techniques. Saudi J Kidney Dis Transpl. 2013;24(4):707-713. https://doi.org/10.4103/1319-2442.113861.
58. Keyes LE, Frazee BW, Snoey ER, Simon BC, Christy D. Ultrasound-guided brachial and basilic vein cannulation in emergency department patients with difficult intravenous access. Ann Emerg Med. 1999;34(6):711-714. https://doi.org/10.1016/S0196-0644(99)70095-8.
59. Mahler SA, Wang H, Lester C, et al. Short- vs long-axis approach to ultrasound-guided peripheral intravenous access: a prospective randomized study. Am J Emerg Med. 2011;29(9):1194-1197. https://doi.org/10.1016/j.ajem.2010.07.015.
60. Berk D, Gurkan Y, Kus A, et al. Ultrasound-guided radial arterial cannulation: long axis/in-plane versus short axis/out-of-plane approaches? J Clin Monit Comput. 2013;27(3):319-324. https://doi.org/10.1007/s10877-013-9437-6.
61. Batllori M, Urra M, Uriarte E, et al. Randomized comparison of three transducer orientation approaches for ultrasound guided internal jugular venous cannulation. Br J Anaesth. 2016;116(3):370-376. https://doi.org/10.1093/bja/aev399.
62. Moak JH, Lyons MS, Wright SW, Lindsell CJ. Needle and guidewire visualization in ultrasound-guided internal jugular vein cannulation. Am J Emerg Med. 2011;29(4):432-436. https://doi.org/10.1016/j.ajem.2010.01.004.
63. Moak JH, Rajkumar JS, Woods WA. The wire is really easy to see (WIRES): sonographic visualization of the guidewire by novices. CJEM. 2013;15(1):18-23. https://doi.org/10.2310/8000.2012.120800.
64. Gillman LM, Blaivas M, Lord J, Al-Kadi A, Kirkpatrick AW. Ultrasound confirmation of guidewire position may eliminate accidental arterial dilatation during central venous cannulation. Scand J Trauma Resusc Emerg Med. 2010;18:39. https://doi.org/10.1186/1757-7241-18-39.
65. Augoustides JG, Horak J, Ochroch AE, et al. A randomized controlled clinical trial of real-time needle-guided ultrasound for internal jugular venous cannulation in a large university anesthesia department. J Cardiothorac Vasc Anesth. 2005;19(3):310-315. https://doi.org/10.1053/j.jvca.2005.03.007.
66. Maecken T, Heite L, Wolf B, Zahn PK, Litz RJ. Ultrasound-guided catheterisation of the subclavian vein: freehand vs needle-guided technique. Anaesthesia. 2015;70(11):1242-1249. https://doi.org/10.1111/anae.13187.
67. Stone MB, Nagdev A, Murphy MC, Sisson CA. Ultrasound detection of guidewire position during central venous catheterization. Am J Emerg Med. 2010;28(1):82-84. https://doi.org/10.1016/j.ajem.2008.09.019.
68. Luyet C, Hartwich V, Urwyler N, et al. Evaluation of a novel needle guide for ultrasound-guided phantom vessel cannulation. Anaesthesia. 2011;66(8):715-720. https://doi.org/10.1111/j.1365-2044.2011.06781.x.
69. O’Grady NP, Alexander M, Burns LA, et al. Summary of recommendations: Guidelines for the Prevention of Intravascular Catheter-related Infections. Clin Infect Dis. 2011;52(9):1087-1099. https://doi.org/10.1093/cid/cir138.
70. Southworth SL, Henman LJ, Kinder LA, Sell JL. The journey to zero central catheter-associated bloodstream infections: culture change in an intensive care unit. Crit Care Nurse. 2012;32(2):49-54. https://doi.org/10.4037/ccn2012915.
71. Guerin K, Wagner J, Rains K, Bessesen M. Reduction in central line-associated bloodstream infections by implementation of a postinsertion care bundle. Am J Infect Control. 2010;38(6):430-433. https://doi.org/10.1016/j.ajic.2010.03.007.
72. Hartman N, Wittler M, Askew K, Manthey D. Delphi method validation of a procedural performance checklist for insertion of an ultrasound-guided internal jugular central line. Am J Med Qual Off J Am Coll Med Qual. 2016;31(1):81-85. https://doi.org/10.1177/1062860614549762.
73. Lenchus JD, Carvalho CM, Ferreri K, et al. Filling the void: defining invasive bedside procedural competency for internal medicine residents. J Grad Med Educ. 2013;5(4):605-612. https://doi.org/10.4300/JGME-D-13-00030.1.
74. Barsuk JH, Cohen ER, Feinglass J, McGaghie WC, Wayne DB. Use of simulation-based education to reduce catheter-related bloodstream infections. Arch Intern Med. 2009;169(15):1420-1423. https://doi.org/10.1001/archinternmed.2009.215.
75. Karakitsos D, Labropoulos N, De Groot E, et al. Real-time ultrasound-guided catheterisation of the internal jugular vein: a prospective comparison with the landmark technique in critical care patients. Crit Care. 2006;10(6):R162. https://doi.org/10.1186/cc5101.
76. Berenholtz SM, Pronovost PJ, Lipsett PA, et al. Eliminating catheter-related bloodstream infections in the intensive care unit. Crit Care Med. 2004;32(10):2014-2020. https://doi.org/10.1097/01.ccm.0000142399.70913.2f.
77. Young EM, Commiskey ML, Wilson SJ. Translating evidence into practice to prevent central venous catheter-associated bloodstream infections: a systems-based intervention. Am J Infect Control. 2006;34(8):503-506. https://doi.org/10.1016/j.ajic.2006.03.011.
78. Galpern D, Guerrero A, Tu A, Fahoum B, Wise L. Effectiveness of a central line bundle campaign on line-associated infections in the intensive care unit. Surgery. 2008;144(4):492-495; discussion 495. https://doi.org/10.1016/j.surg.2008.06.004.
79. Mermel LA, McCormick RD, Springman SR, Maki DG. The pathogenesis and epidemiology of catheter-related infection with pulmonary artery swan-Ganz catheters: a prospective study utilizing molecular subtyping. Am J Med. 1991;91(3b):197S–205S. https://doi.org/10.1016/0002-9343(91)90369-9.
80. Marschall J, Mermel LA, Classen D, et al. Strategies to prevent central line-associated bloodstream infections in acute care hospitals. Infect Control Hosp Epidemiol. 2008;29(1):S22-S30. https://doi.org/10.1086/591059.
81. Marschall J, Mermel LA, Fakih M, et al. Strategies to prevent central line-associated bloodstream infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(7):753-771. https://doi.org/10.1086/676533.
82. Garcia-Rodriguez JF, Álvarez-Díaz H, Vilariño-Maneiro L, et al. Epidemiology and impact of a multifaceted approach in controlling central venous catheter associated blood stream infections outside the intensive care unit. BMC Infect Dis. 2013;13:445. https://doi.org/10.1186/1471-2334-13-445.
83. Lee DH, Jung KY, Choi YH. Use of maximal sterile barrier precautions and/or antimicrobial-coated catheters to reduce the risk of central venous catheter-related bloodstream infection. Infect Control Hosp Epidemiol. 2008;29(10):947-950. https://doi.org/10.1086/590356.
84. Mermel LA. Prevention of intravascular catheter-related infections. Ann Intern Med. 2000;132(5):391-402. https://doi.org/10.7326/0003-4819-132-5-200003070-00009.
85. Troianos CA, Jobes DR, Ellison N. Ultrasound-guided cannulation of the internal jugular vein. A prospective, randomized study. Anesth Analg. 1991;72(6):823-826. https://doi.org/10.1213/00000539-199106000-00020.
86. Troianos CA, Savino JS. Internal jugular vein cannulation guided by echocardiography. Anesthesiology. 1991;74(4):787-789. https://doi.org/10.1097/00000542-199104000-00026.
87. Denys BG, Uretsky BF, Reddy PS, et al. An ultrasound method for safe and rapid central venous access. N Engl J Med. 1991;324(8):566. https://doi.org/10.1056/NEJM199102213240816.
88. Riaz A, Shan Khan RA, Salim F. Ultrasound guided internal jugular venous cannulation: comparison with land-mark technique. J Coll Phys Surg Pak. 2015;25(5):315-319. https://doi.org/05.2015/JCPSP.315319.
89. Gok F, Kilicaslan A, Sarkilar G, Kandemir B, Yosunkaya A. The effect of ultrasound guidance on central venous catheter-associated bloodstream infection in critical care patients. Acta Med Mediterr. 2013;29:677-682.
90. Lamperti M, Cortellazzi P, D’Onofrio G, et al. An outcome study on complications using routine ultrasound assistance for internal jugular vein cannulation. Acta Anaesthesiol Scand. 2007;51(10):1327-1330. https://doi.org/10.1111/j.1399-6576.2007.01442.x.
91. Vezzani A, Manca T, Vercelli A, Braghieri A, Magnacavallo A. Ultrasonography as a guide during vascular access procedures and in the diagnosis of complications. J Ultrasound. 2013;16(4):161-170. https://doi.org/10.1007/s40477-013-0046-5.
92. Palepu GB, Deven J, Subrahmanyam M, Mohan S. Impact of ultrasonography on central venous catheter insertion in intensive care. Indian J Rad Imaging. 2009;19(3):191-198. https://doi.org/10.4103/0971-3026.54877.
93. Agarwal A, Singh DK, Singh AP. Ultrasonography: a novel approach to central venous cannulation. Indian J Crit Care Med. 2009;13(4):213-216. https://doi.org/10.4103/0972-5229.60174.
94. Wong SW, Niazi AU, Chin KJ, Chan VW. Real-time ultrasound-guided spinal anesthesia using the SonixGPS® needle tracking system: a case report. Can J Anaesth. 2013;60(1):50-53. https://doi.org/10.1007/s12630-012-9809-2.
95. Bouaziz H, Zetlaoui PJ, Pierre S, et al. Guidelines on the use of ultrasound guidance for vascular access. Anaesth, Crit Care Pain Med. 2015;34(1):65-69. https://doi.org/10.1016/j.accpm.2015.01.004.
96. Jenssen C, Brkljacic B, Hocke M, et al. EFSUMB guidelines on interventional ultrasound (INVUS), Part VI - Ultrasound-guided vascular interventions. Ultraschall Med. 2016;37(5):473-476. https://doi.org/10.1055/s-0035-1553450.
97. Rando K, Castelli J, Pratt JP, et al. Ultrasound-guided internal jugular vein catheterization: a randomized controlled trial. Heart Lung Vessels. 2014;6(1):13-23.
98. Mey U, Glasmacher A, Hahn C, et al. Evaluation of an ultrasound-guided technique for central venous access via the internal jugular vein in 493 patients. Support Care Cancer Off J Multinat Assoc Support Care Cancer. 2003;11(3):148-155. https://doi.org/10.1007/s00520-002-0399-3.
99. Singh SA, Sharma S, Singh A, et al. The safety of ultrasound guided central venous cannulation in patients with liver disease. Saudi J Anaesth. 2015;9(2):155-160. https://doi.org/10.4103/1658-354X.152842.
100. Akoglu H, Piskinpasa S, Yenigun EC, et al. Real-time ultrasound guided placement of temporary internal jugular vein catheters: assessment of technical success and complication rates in nephrology practice. Nephrol (Carlton). 2012;17(7):603-606. https://doi.org/10.1111/j.1440-1797.2012.01637.x.
101. Sadler DJ, Gordon AC, Klassen J, et al. Image-guided central venous catheters for apheresis. Bone Marrow Transplant. 1999;23(2):179-182. https://doi.org/10.1038/sj.bmt.1701545.
102. Calvert N, Hind D, McWilliams R, et al. Ultrasound for central venous cannulation: economic evaluation of cost-effectiveness. Anaesthesia. 2004;59(11):1116-1120. https://doi.org/10.1111/j.1365-2044.2004.03906.x.
103. Goetz AM, Wagener MM, Miller JM, Muder RR. Risk of infection due to central venous catheters: effect of site of placement and catheter type. Infect Control Hosp Epidemiol. 1998;19(11):842-845.
104. Bertini P, Frediani M. Ultrasound guided supraclavicular central vein cannulation in adults: a technical report. J Vasc Access. 2013;14(1):89-93. https://doi.org/10.5301/jva.5000088.
105. Lalu MM, Fayad A, Ahmed O, et al. Ultrasound-guided subclavian vein catheterization: A systematic review and meta-analysis. Crit Care Med. 2015;43(7):1498-1507. https://doi.org/10.1097/CCM.0000000000000973.
106. Milone M, Di Minno G, Di Minno MN, et al. The real effectiveness of ultrasound guidance in subclavian venous access. Ann ital chir. 2010;81(5):331-334.
107. O’Leary R, Ahmed SM, McLure H, et al. Ultrasound-guided infraclavicular axillary vein cannulation: a useful alternative to the internal jugular vein. Br J Anaesth. 2012;109(5):762-768. https://doi.org/10.1093/bja/aes262.
108. Gualtieri E, Deppe SA, Sipperly ME, Thompson DR. Subclavian venous catheterization: greater success rate for less experienced operators using ultrasound guidance. Crit Care Med. 1995;23(4):692-697. https://doi.org/10.1097/00003246-199504000-00018.
109. Fragou M, Gravvanis A, Dimitriou V, et al. Real-time ultrasound-guided subclavian vein cannulation versus the landmark method in critical care patients: a prospective randomized study. Crit Care Med. 2011;39(7):1607-1612. https://doi.org/10.1097/CCM.0b013e318218a1ae.
110. Brass P, Hellmich M, Kolodziej L, Schick G, Smith AF. Ultrasound guidance versus anatomical landmarks for subclavian or femoral vein catheterization. Cochrane Database Syst Rev. 2015;1:CD011447. https://doi.org/10.1002/14651858.CD011447.
111. Robinson MK, Mogensen KM, Grudinskas GF, Kohler S, Jacobs DO. Improved care and reduced costs for patients requiring peripherally inserted central catheters: the role of bedside ultrasound and a dedicated team. JPEN J Parenter Enter Nutr. 2005;29(5):374-379. https://doi.org/10.1177/0148607105029005374.
112. Li J, Fan YY, Xin MZ, et al. A randomised, controlled trial comparing the long-term effects of peripherally inserted central catheter placement in chemotherapy patients using B-mode ultrasound with modified Seldinger technique versus blind puncture. Eur J Oncol Nurs. 2014;18(1):94-103. https://doi.org/10.1016/j.ejon.2013.08.003.
113. Schweickert WD, Herlitz J, Pohlman AS, et al. A randomized, controlled trial evaluating postinsertion neck ultrasound in peripherally inserted central catheter procedures. Crit Care Med. 2009;37(4):1217-1221. https://doi.org/10.1097/CCM.0b013e31819cee7f.
114. Mahler SA, Wang H, Lester C, Conrad SA. Ultrasound-guided peripheral intravenous access in the emergency department using a modified Seldinger technique. J Emerg Med. 2010;39(3):325-329. https://doi.org/10.1016/j.jemermed.2009.02.013.
115. Brannam L, Blaivas M, Lyon M, Flake M. Emergency nurses’ utilization of ultrasound guidance for placement of peripheral intravenous lines in difficult-access patients. Acad Emerg Med. 2004;11(12):1361-1363. https://doi.org/10.1197/j.aem.2004.08.027.
116. Au A, Rotte M, Gryzbowski R, Ku B, Fields J. 157 Decrease in central venous catheter placement and complications due to utilization of ultrasound-guided peripheral intravenous catheters. Ann Emerg Med. 2011;58(4):S230. https://doi.org/10.1016/j.annemergmed.2011.06.185.
117. Dargin JM, Rebholz CM, Lowenstein RA, Mitchell PM, Feldman JA. Ultrasonography-guided peripheral intravenous catheter survival in ED patients with difficult access. Am J Emerg Med. 2010;28(1):1-7. https://doi.org/10.1016/j.ajem.2008.09.001.
118. Bauman M, Braude D, Crandall C. Ultrasound-guidance vs standard technique in difficult vascular access patients by ED technicians. Am J Emerg Med. 2009;27(2):135-140. https://doi.org/10.1016/j.ajem.2008.02.005.
119. Schoenfeld E, Shokoohi H, Boniface K. Ultrasound-guided peripheral intravenous access in the emergency department: patient-centered survey. West J Emerg Med. 2011;12(4):475-477. https://doi.org/10.5811/westjem.2011.3.1920.
120. Maki DG, Ringer M. Risk factors for infusion-related phlebitis with small peripheral venous catheters. A randomized controlled trial. Ann Intern Med. 1991;114(10):845-854. https://doi.org/10.7326/0003-4819-114-10-845.
121. Miliani K, Taravella R, Thillard D, et al. Peripheral venous catheter-related adverse events: evaluation from a multicentre epidemiological study in France (the CATHEVAL Project). PLOS ONE. 2017;12(1):e0168637. https://doi.org/10.1371/journal.pone.0168637.
122. Gregg SC, Murthi SB, Sisley AC, Stein DM, Scalea TM. Ultrasound-guided peripheral intravenous access in the intensive care unit. J Crit Care. 2010;25(3):514-519. https://doi.org/10.1016/j.jcrc.2009.09.003.
123. Adhikari S, Blaivas M, Morrison D, Lander L. Comparison of infection rates among ultrasound-guided versus traditionally placed peripheral intravenous lines. J Ultrasound Med. 2010;29(5):741-747. https://doi.org/10.7863/jum.2010.29.5.741.
124. Shiver S, Blaivas M, Lyon M. A prospective comparison of ultrasound-guided and blindly placed radial arterial catheters. Acad Emerg Med. 2006;13(12):1275-1279. https://doi.org/10.1197/j.aem.2006.07.015.
125. Levin PD, Sheinin O, Gozal Y. Use of ultrasound guidance in the insertion of radial artery catheters. Crit Care Med. 2003;31(2):481-484. https://doi.org/10.1097/01.CCM.0000050452.17304.2F.
126. Gu WJ, Tie HT, Liu JC, Zeng XT. Efficacy of ultrasound-guided radial artery catheterization: a systematic review and meta-analysis of randomized controlled trials. Crit Care. 2014;18(3):R93. https://doi.org/10.1186/cc13862.
127. Tang L, Wang F, Li Y, et al. Ultrasound guidance for radial artery catheterization: an updated meta-analysis of randomized controlled trials. PLOS ONE. 2014;9(11):e111527. https://doi.org/10.1371/journal.pone.0111527.
128. Sobolev M, Slovut DP, Lee Chang A, Shiloh AL, Eisen LA. Ultrasound-guided catheterization of the femoral artery: A systematic review and meta-analysis of randomized controlled trials. J Invas Cardiol. 2015;27(7):318-323. https://doi.org/10.1378/chest.1991181.
129. Gabriel M, Pawlaczyk K, Waliszewski K, Krasiński Z, Majewski W. Location of femoral artery puncture site and the risk of postcatheterization pseudoaneurysm formation. Int J Cardiol. 2007;120(2):167-171. https://doi.org/10.1016/j.ijcard.2006.09.018.
130. Seto AH, Abu-Fadel MS, Sparling JM, et al. Real-time ultrasound guidance facilitates femoral arterial access and reduces vascular complications: FAUST (Femoral Arterial Access With ultrasound Trial). JACC Cardiovasc Interv. 2010;3(7):751-758. https://doi.org/10.1016/j.jcin.2010.04.015.
131. Kalish J, Eslami M, Gillespie D, et al. Routine use of ultrasound guidance in femoral arterial access for peripheral vascular intervention decreases groin hematoma rates. J Vasc Surg. 2015;61(5):1231-1238. https://doi.org/10.1016/j.jvs2014.12.003.
132. Sandhu NS, Patel B. Use of ultrasonography as a rescue technique for failed radial artery cannulation. J Clin Anesth. 2006;18(2):138-141. https://doi.org/10.1016/j.jclinane.2005.06.011.
133. White L, Halpin A, Turner M, Wallace L. Ultrasound-guided radial artery cannulation in adult and paediatric populations: a systematic review and meta-analysis. Br J Anaesth. 2016;116(5):610-617. https://doi.org/10.1093/bja/aew097.
134. Gao YB, Yan JH, Gao FQ, et al. Effects of ultrasound-guided radial artery catheterization: an updated meta-analysis. Am J Emerg Med. 2015;33(1):50-55. https://doi.org/10.1016/j.ajem.2014.10.008.
135. Seto AH, Roberts JS, Abu-Fadel MS, et al. Real-time ultrasound guidance facilitates transradial access: RAUST (Radial Artery Access with Ultrasound Trial). JACC Cardiovasc Interv. 2015;8(2):283-291. https://doi.org/10.1016/j.jcin.2014.05.036.
136. Roberts J, Manur R. Ultrasound-guided radial artery access by a non-ultrasound trained interventional cardiologist improved first-attempt success rates and shortened time for successful radial artery cannulation. J Invas Cardiol. 2013;25(12):676-679.
137. Lichtenstein DA, Menu Y. A bedside ultrasound sign ruling out pneumothorax in the critically ill. Lung sliding. Chest. 1995;108(5):1345-1348. https://doi.org/10.1378/chest.108.5.1345.
138. Lichtenstein D, Mezière G, Biderman P, Gepner A. The comet-tail artifact: an ultrasound sign ruling out pneumothorax. Intensive Care Med. 1999;25(4):383-388. https://doi.org/10.1007/s001340050862.
139. Ablordeppey EA, Drewry AM, Beyer AB, et al. Diagnostic accuracy of central venous catheter confirmation by bedside ultrasound Versus chest radiography in critically ill patients: A systematic review and meta-analysis. Crit Care Med. 2017;45(4):715-724. https://doi.org/10.1097/CCM.0000000000002188.
140. Bedel J, Vallée F, Mari A, et al. Guidewire localization by transthoracic echocardiography during central venous catheter insertion: a periprocedural method to evaluate catheter placement. Intensive Care Med. 2013;39(11):1932-1937. https://doi.org/10.1007/s00134-013-3097-3.
141. Weekes AJ, Keller SM, Efune B, Ghali S, Runyon M. Prospective comparison of ultrasound and CXR for confirmation of central vascular catheter placement. Emerg Med J EMJ. 2016;33(3):176-180. https://doi.org/10.1136/emermed-2015-205000.
142. Arellano R, Nurmohamed A, Rumman A, et al. The utility of transthoracic echocardiography to confirm central line placement: an observational study. Can J Anaesth. 2014;61(4):340-346. https://doi.org/10.1007/s12630-014-0111-3.
143. Vezzani A, Brusasco C, Palermo S, et al. Ultrasound localization of central vein catheter and detection of postprocedural pneumothorax: an alternative to chest radiography. Crit Care Med. 2010;38(2):533-538. https://doi.org/10.1097/CCM.0b013e3181c0328f.
144. Choudhry NK, Fletcher RH, Soumerai SB. Systematic review: the relationship between clinical experience and quality of health care. Ann Intern Med. 2005;142(4):260-273. https://doi.org/10.7326/0003-4819-142-4-200502150-00008.
145. Backlund BH, Hopkins E, Kendall JL. Ultrasound guidance for central venous access by emergency physicians in Colorado. West J Emerg Med. 2012;13(4):320-325. https://doi.org/10.5811/westjem.2011.11.6821.
146. Buchanan MS, Backlund B, Liao MM, et al. Use of ultrasound guidance for central venous catheter placement: survey from the American Board of Emergency Medicine Longitudinal Study of Emergency Physicians. Acad Emerg Med. 2014;21(4):416-421. https://doi.org/10.1111/acem.12350.
147. Barsuk JH, McGaghie WC, Cohen ER, O’Leary KJ, Wayne DB. Simulation-based mastery learning reduces complications during central venous catheter insertion in a medical intensive care unit. Crit Care Med. 2009;37(10):2697-2701. https://doi.org/10.1097/00003246-200910000-00003.
148. Coopersmith CM, Rebmann TL, Zack JE, et al. Effect of an education program on decreasing catheter-related bloodstream infections in the surgical intensive care unit. Crit Care Med. 2002;30(1):59-64. https://doi.org/10.1097/00003246-200201000-00009.
149. Woo MY, Frank J, Lee AC, et al. Effectiveness of a novel training program for emergency medicine residents in ultrasound-guided insertion of central venous catheters. CJEM. 2009;11(4):343-348. https://doi.org/10.1017/S1481803500011398.
150. McGee DC, Gould MK. Preventing complications of central venous catheterization. N Engl J Med. 2003;348(12):1123-1133. https://doi.org/10.1056/NEJMra011883.
151. Barsuk JH, McGaghie WC, Cohen ER, Balachandran JS, Wayne DB. Use of simulation-based mastery learning to improve the quality of central venous catheter placement in a medical intensive care unit. J Hosp Med. 2009;4(7):397-403. https://doi.org/10.1002/jhm.468.
152. Sekiguchi H, Tokita JE, Minami T, et al. A prerotational, simulation-based workshop improves the safety of central venous catheter insertion: results of a successful internal medicine house staff training program. Chest. 2011;140(3):652-658. https://doi.org/10.1378/chest.10-3319.
153. Feller-Kopman D. Ultrasound-guided internal jugular access: a proposed standardized approach and implications for training and practice. Chest. 2007;132(1):302-309. https://doi.org/10.1378/chest.06-2711.
154. Troianos CA, Hartman GS, Glas KE, et al. Special articles: guidelines for performing ultrasound guided vascular cannulation: recommendations of the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. Anesth Analg. 2012;114(1):46-72. https://doi.org/10.1213/ANE.0b013e3182407cd8.
155. Issenberg SB, McGaghie WC, Hart IR, et al. Simulation technology for health care professional skills training and assessment. JAMA. 1999;282(9):861-866. https://doi.org/10.1001/jama.282.9.861.
156. Millington SJ, Wong RY, Kassen BO, Roberts JM, Ma IW. Improving internal medicine residents’ performance, knowledge, and confidence in central venous catheterization using simulators. J Hosp Med. 2009;4(7):410-416. https://doi.org/10.1002/jhm.570.
157. Beaulieu Y, Laprise R, Drolet P, et al. Bedside ultrasound training using web-based e-learning and simulation early in the curriculum of residents. Crit Ultrasound J. 2015;7:1. https://doi.org/10.1186/s13089-014-0018-9.
158. Barsuk JH, Cohen ER, McGaghie WC, Wayne DB. Long-term retention of central venous catheter insertion skills after simulation-based mastery learning. Acad Med. 2010;85(10 Suppl):S9-S12. https://doi.org/10.1097/ACM.0b013e3181ed436c.
159. Wayne DB, Didwania A, Feinglass J, et al. Simulation-based education improves quality of care during cardiac arrest team responses at an academic teaching hospital: a case-control study. Chest. 2008;133(1):56-61. https://doi.org/10.1378/chest.07-0131.
160. Evans LV, Dodge KL, Shah TD, et al. Simulation training in central venous catheter insertion: improved performance in clinical practice. Acad Med. 2010;85(9):1462-1469. https://doi.org/10.1097/ACM.0b013e3181eac9a3.
161. Smith CC, Huang GC, Newman LR, et al. Simulation training and its effect on long-term resident performance in central venous catheterization. Simul Healthc J Soc Simul Healthc. 2010;5(3):146-151. https://doi.org/10.1097/SIH.0b013e3181dd9672.
162. Laack TA, Dong Y, Goyal DG, et al. Short-term and long-term impact of the central line workshop on resident clinical performance during simulated central line placement. Simul Healthc J Soc Simul Healthc. 2014;9(4):228-233. https://doi.org/10.1097/SIH.0000000000000015.
163. Dodge KL, Lynch CA, Moore CL, Biroscak BJ, Evans LV. Use of ultrasound guidance improves central venous catheter insertion success rates among junior residents. J Ultrasound Med. 2012;31(10):1519-1526. https://doi.org/10.7863/jum.2012.31.10.1519.
164. Bayci AW, Mangla J, Jenkins CS, Ivascu FA, Robbins JM. Novel educational module for subclavian central venous catheter insertion using real-time ultrasound guidance. J Surg Educ. 2015;72(6):1217-1223. https://doi.org/10.1016/j.jsurg.2015.07.010.
165. Andreatta P, Chen Y, Marsh M, Cho K. Simulation-based training improves applied clinical placement of ultrasound-guided PICCs. Support Care Cancer Off J Multinat Assoc Support Care Cancer. 2011;19(4):539-543. https://doi.org/10.1007/s00520-010-0849-2.
166. Rosen BT, Uddin PQ, Harrington AR, Ault BW, Ault MJ. Does personalized vascular access training on a nonhuman tissue model allow for learning and retention of central line placement skills? Phase II of the procedural patient safety initiative (PPSI-II). J Hosp Med. 2009;4(7):423-429. https://doi.org/10.1002/jhm.571.
167. Ault MJ, Rosen BT, Ault B. The use of tissue models for vascular access training. Phase I of the procedural patient safety initiative. J Gen Intern Med. 2006;21(5):514-517. https://doi.org/10.1111/j.1525-1497.2006.00440.x.
168. Varga S, Smith J, Minneti M, et al. Central venous catheterization using a perfused human cadaveric model: application to surgical education. J Surg Educ. 2015;72(1):28-32. https://doi.org/10.1016/j.jsurg.2014.07.005.
169. Sansivero GE. Venous anatomy and physiology. Considerations for vascular access device placement and function. J Intraven Nurs Off Publ Intraven Nurs Soc. 1998;21(5 Suppl):S107-S114.
170. Eisen LA, Narasimhan M, Berger JS, et al. Mechanical complications of central venous catheters. Journal of intensive care medicine. 2006;21(1):40-46. https://doi.org/10.1177/0885066605280884.
171. Chenkin J, Lee S, Huynh T, Bandiera G. Procedures can be learned on the Web: a randomized study of ultrasound-guided vascular access training. Acad Emerg Med. 2008;15(10):949-954. https://doi.org/10.1111/j.1553-2712.2008.00231.x.
172. Abualenain J, Calabrese K, Tansek R, Ranniger C. 319 Comparing standard versus video-based teaching for ultrasound-guided internal jugular central venous catheter access for fourth-year medical students. Ann Emerg Med. 2014;64(4):S113. https://doi.org/10.1016/j.annemergmed.2014.07.347.
173. Pustavoitau A, Blaivas M, Brown SM, et al. Recommendations for achieving and maintaining competence and credentialing in critical care ultrasound with focused cardiac ultrasound and advanced critical care echocardiography. Crit Care Med. 2016.
174. Jensen TP, Soni NJ, Tierney DM, Lucas BP. Hospital privileging practices for bedside procedures: A survey of hospitalist experts. J Hosp Med. 2017;12(10):836-839. https://doi.org/10.12788/jhm.2837.
175. Moureau N, Lamperti M, Kelly LJ, et al. Evidence-based consensus on the insertion of central venous access devices: definition of minimal requirements for training. Br J Anaesth. 2013;110(3):347-356. https://doi.org/10.1093/bja/aes499.
176. Ernst A, Silvestri GA, Johnstone D, American College of Chest Physicians. Interventional pulmonary procedures: guidelines from the American College of Chest Physicians. Chest. 2003;123(5):1693-1717. https://doi.org/10.1378/chest.123.5.1693.
177. Thomas SM, Burch W, Kuehnle SE, et al. Simulation training for pediatric residents on central venous catheter placement: a pilot study. Pediatr Crit Care Med J Soc Crit Care Med.. 2013;14(9):e416-e423. https://doi.org/10.1097/PCC.0b013e31829f5eda.
178. Smith KK, Gilcreast D, Pierce K. Evaluation of staff’s retention of ACLS and BLS skills. Resuscitation. 2008;78(1):59-65. https://doi.org/10.1016/j.resuscitation.2008.02.007.
179. Ericsson KA. Deliberate practice and the acquisition and maintenance of expert performance in medicine and related domains. Acad Med. 2004;79(10 Suppl):S70-S81. https://doi.org/10.1097/00001888-200410001-00022.
180. Gerard JM, Thomas SM, Germino KW, et al. The effect of simulation training on PALS skills among family medicine residents. Fam Med. 2011;43(6):392-399.
181. Brydges R, Nair P, Ma I, Shanks D, Hatala R. Directed self-regulated learning versus instructor-regulated learning in simulation training. Med Educ. 2012;46(7):648-656. https://doi.org/10.1111/j.1365-2923.2012.04268.x.
182. Wayne DB, Butter J, Siddall VJ, et al. Simulation-based training of internal medicine residents in advanced cardiac life support protocols: a randomized trial. Teach Learn Med. 2005;17(3):210-216. https://doi.org/10.1207/s15328015tlm1703_3.
183. Arthur Jr. W, Bennett Jr. W, Stanush PL, McNelly TL. Factors that influence skill decay and retention: A quantitative review and analysis. Hum Perform. 1998;11(1):57-101. https://doi.org/10.1207/s15327043hup1101_3.
184. Rusche JD, Besuner P, Partusch SK, Berning PA. Competency program development across a merged healthcare network. J Nurs Staff Dev. 2001;17(5):234-240; quiz 241-232. https://doi.org/10.1097/00124645-200109000-00004.
185. O’Hearne Rebholz M. A review of methods to assess competency. J Nurs Staff Dev. 2006;22(5):241-245. https://doi.org/10.1097/00124645-200609000-00007.
186. Reznick RK. Teaching and testing technical skills. Am J Surg. 1993;165(3):358-361. https://doi.org/10.1016/s0002-9610(05)80843-8.
187. Reznick RK, MacRae H. Teaching surgical skills--changes in the wind. N Engl J Med. 2006;355(25):2664-2669. https://doi.org/10.1056/NEJMra054785.
188. Murin S, Stollenwerk NS. Simulation in procedural training: at the tipping point. Chest. 2010;137(5):1009-1011. https://doi.org/10.1378/chest.10-0199.
189. American College of Emergency Physicians. Emergency ultrasound guidelines. Ann Emerg Med. 2009;53(4):550-570. https://doi.org/10.1016/j.annemergmed.2008.12.013.
190. Guilbert MC, Elkouri S, Bracco D, et al. Arterial trauma during central venous catheter insertion: case series, review and proposed algorithm. J Vasc Surg. 2008;48(4):918-925; discussion 925. https://doi.org/10.1016/j.jvs2008.04.046.
191. Dong Y, Suri HS, Cook DA, et al. Simulation-based objective assessment discerns clinical proficiency in central line placement: a construct validation. Chest. 2010;137(5):1050-1056. https://doi.org/10.1378/chest.09-1451.
192. Adhikari S, Theodoro D, Raio C, et al. Central venous catheterization: are we using ultrasound guidance? J Ultrasound Med. 2015;34(11):2065-2070. https://doi.org/10.7863/ultra.15.01027.
193. Mourad M, Kohlwes J, Maselli J, MERN Group, Auerbach AD. Supervising the supervisors--procedural training and supervision in internal medicine residency. J Gen Intern Med. 2010;25(4):351-356. https://doi.org/10.1007/s11606-009-1226-z.
Approximately five million central venous catheters (CVCs) are inserted in the United States annually, with over 15 million catheter days documented in intensive care units alone.1 Traditional CVC insertion techniques using landmarks are associated with a high risk of mechanical complications, particularly pneumothorax and arterial puncture, which occur in 5%-19% patients.2,3
Since the 1990s, several randomized controlled studies and meta-analyses have demonstrated that the use of real-time ultrasound guidance for CVC insertion increases procedure success rates and decreases mechanical complications.4,5 Use of real-time ultrasound guidance was recommended by the Agency for Healthcare Research and Quality, the Institute of Medicine, the National Institute for Health and Care Excellence, the Centers for Disease Control and Prevention, and several medical specialty societies in the early 2000s.6-14 Despite these recommendations, ultrasound guidance has not been universally adopted. Currently, an estimated 20%-55% of CVC insertions in the internal jugular vein are performed without ultrasound guidance.15-17
Following the emergence of literature supporting the use of ultrasound guidance for CVC insertion, observational and randomized controlled studies demonstrated improved procedural success rates with the use of ultrasound guidance for the insertion of peripheral intravenous lines (PIVs), arterial catheters, and peripherally inserted central catheters (PICCs).18-23
The purpose of this position statement is to present evidence-based recommendations on the use of ultrasound guidance for the insertion of central and peripheral vascular access catheters in adult patients. This document presents consensus-based recommendations with supporting evidence for clinical outcomes, techniques, and training for the use of ultrasound guidance for vascular access. We have subdivided the recommendations on techniques for central venous access, peripheral venous access, and arterial access individually, as some providers may not perform all types of vascular access procedures.
These recommendations are intended for hospitalists and other healthcare providers that routinely place central and peripheral vascular access catheters in acutely ill patients. However, this position statement does not mandate that all hospitalists should place central or peripheral vascular access catheters given the diverse array of hospitalist practice settings. For training and competency assessments, we recognize that some of these recommendations may not be feasible in resource-limited settings, such as rural hospitals, where equipment and staffing for assessments are not available. Recommendations and frameworks for initial and ongoing credentialing of hospitalists in ultrasound-guided bedside procedures have been previously published in an Society of Hospital Medicine (SHM) position statement titled, “Credentialing of Hospitalists in Ultrasound-Guided Bedside Procedures.”24
METHODS
Detailed methods are described in Appendix 1. The SHM Point-of-care Ultrasound (POCUS) Task Force was assembled to carry out this guideline development project under the direction of the SHM Board of Directors, Director of Education, and Education Committee. All expert panel members were physicians or advanced practice providers with expertise in POCUS. Expert panel members were divided into working group members, external peer reviewers, and a methodologist. All Task Force members were required to disclose any potential conflicts of interest (Appendix 2). The literature search was conducted in two independent phases. The first phase included literature searches conducted by the vascular access working group members themselves. Key clinical questions and draft recommendations were then prepared. A systematic literature search was conducted by a medical librarian based on the findings of the initial literature search and draft recommendations. The Medline, Embase, CINAHL, and Cochrane medical databases were searched from 1975 to December 2015 initially. Google Scholar was also searched without limiters. An updated search was conducted in November 2017. The literature search strings are included in Appendix 3. All article abstracts were initially screened for relevance by at least two members of the vascular access working group. Full-text versions of screened articles were reviewed, and articles on the use of ultrasound to guide vascular access were selected. The following article types were excluded: non-English language, nonhuman, age <18 years, meeting abstracts, meeting posters, narrative reviews, case reports, letters, and editorials. All relevant systematic reviews, meta-analyses, randomized controlled studies, and observational studies of ultrasound-guided vascular access were screened and selected (Appendix 3, Figure 1). All full-text articles were shared electronically among the working group members, and final article selection was based on working group consensus. Selected articles were incorporated into the draft recommendations.
These recommendations were developed using the Research and Development (RAND) Appropriateness Method that required panel judgment and consensus.14 The 28 voting members of the SHM POCUS Task Force reviewed and voted on the draft recommendations considering five transforming factors: (1) Problem priority and importance, (2) Level of quality of evidence, (3) Benefit/harm balance, (4) Benefit/burden balance, and (5) Certainty/concerns about PEAF (Preferences/Equity/Acceptability/Feasibility). Using an internet-based electronic data collection tool (REDCap™), panel members participated in two rounds of electronic voting, one in August 2018 and the other in October 2018 (Appendix 4). Voting on appropriateness was conducted using a nine-point Likert scale. The three zones of the nine-point Likert scale were inappropriate (1-3 points), uncertain (4-6 points), and appropriate (7-9 points). The degree of consensus was assessed using the RAND algorithm (Appendix 1, Figure 1 and Table 1). Establishing a recommendation required at least 70% agreement that a recommendation was “appropriate.” Disagreement was defined as >30% of panelists voting outside of the zone of the median. A strong recommendation required at least 80% of the votes within one integer of the median per the RAND rules.
Recommendations were classified as strong or weak/conditional based on preset rules defining the panel’s level of consensus, which determined the wording for each recommendation (Table 2). The final version of the consensus-based recommendations underwent internal and external review by members of the SHM POCUS Task Force, the SHM Education Committee, and the SHM Executive Committee. The SHM Executive Committee reviewed and approved this position statement prior to its publication in the Journal of Hospital Medicine.
RESULTS
Literature Search
A total of 5,563 references were pooled from an initial search performed by a certified medical librarian in December 2015 (4,668 citations) which was updated in November 2017 (791 citations), and from the personal bibliographies and searches (104 citations) performed by working group members. A total of 514 full-text articles were reviewed. The final selection included 192 articles that were abstracted into a data table and incorporated into the draft recommendations. See Appendix 3 for details of the literature search strategy.
Recommendations
Four domains (technique, clinical outcomes, training, and knowledge gaps) with 31 draft recommendations were generated based on a review of the literature. Selected references were abstracted and assigned to each draft recommendation. Rationales for each recommendation cite supporting evidence. After two rounds of panel voting, 31 recommendations achieved agreement based on the RAND rules. During the peer review process, two of the recommendations were merged with other recommendations. Thus, a total of 29 recommendations received final approval. The degree of consensus based on the median score and the dispersion of voting around the median are shown in Appendix 5. Twenty-seven statements were approved as strong recommendations, and two were approved as weak/conditional recommendations. The strength of each recommendation and degree of consensus are summarized in Table 3.
Terminology
Central Venous Catheterization
Central venous catheterization refers to insertion of tunneled or nontunneled large bore vascular catheters that are most commonly inserted into the internal jugular, subclavian, or femoral veins with the catheter tip located in a central vein. These vascular access catheters are synonymously referred to as central lines or central venous catheters (CVCs). Nontunneled catheters are designed for short-term use and should be removed promptly when no longer clinically indicated or after a maximum of 14 days.25
Peripherally Inserted Central Catheter (PICC)
Peripherally inserted central catheters, or PICC lines, are inserted most commonly in the basilic or brachial veins in adult patients, and the catheter tip terminates in the distal superior vena cava or cavo-atrial junction. These catheters are designed to remain in place for a duration of several weeks, as long as it is clinically indicated.
Midline Catheterization
Midline catheters are a type of peripheral venous catheter that are an intermediary between a peripheral intravenous catheter and PICC line. Midline catheters are most commonly inserted in the brachial or basilic veins, but unlike PICC lines, the tips of these catheters terminate in the axillary or subclavian vein. Midline catheters are typically 8 cm to 20 cm in length and inserted for a duration <30 days.
Peripheral Intravenous Catheterization
Peripheral intravenous lines (PIV) refer to small bore venous catheters that are most commonly 14G to 24G and inserted into patients for short-term peripheral venous access. Common sites of ultrasound-guided PIV insertion include the superficial and deep veins of the hand, forearm, and arm.
Arterial Catheterization
Arterial catheters are commonly used for reliable blood pressure monitoring, frequent arterial blood
RECOMMENDATIONS
Preprocedure
1. We recommend that providers should be familiar with the operation of their specific ultrasound machine prior to initiation of a vascular access procedure.
Rationale: There is strong consensus that providers must be familiar with the knobs and functions of the specific make and model of ultrasound machine that will be utilized for a vascular access procedure. Minimizing adjustments to the ultrasound machine during the procedure may reduce the risk of contaminating the sterile field.
2. We recommend that providers should use a high-frequency linear transducer with a sterile sheath and sterile gel to perform vascular access procedures.
Rationale: High-frequency linear-array transducers are recommended for the vast majority of vascular access procedures due to their superior resolution compared to other transducer types. Both central and peripheral vascular access procedures, including PIV, PICC, and arterial line placement, should be performed using sterile technique. A sterile transducer cover and sterile gel must be utilized, and providers must be trained in sterile preparation of the ultrasound transducer.13,26,27
The depth of femoral vessels correlates with body mass index (BMI). When accessing these vessels in a morbidly obese patient with a thigh circumference >60 cm and vessel depth >8 cm, a curvilinear transducer may be preferred for its deeper penetration.28 For patients who are poor candidates for bedside insertion of vascular access catheters, such as uncooperative patients, patients with atypical vascular anatomy or poorly visualized target vessels, we recommend consultation with a vascular access specialist prior to attempting the procedure.
3. We recommend that providers should use two-dimensional ultrasound to evaluate for anatomical variations and absence of vascular thrombosis during preprocedural site selection.
Rationale: A thorough ultrasound examination of the target vessel is warranted prior to catheter placement. Anatomical variations that may affect procedural decision-making are easily detected with ultrasound. A focused vascular ultrasound examination is particularly important in patients who have had temporary or tunneled venous catheters, which can cause stenosis or thrombosis of the target vein.
For internal jugular vein (IJV) CVCs, ultrasound is useful for visualizing the relationship between the IJV and common carotid artery (CCA), particularly in terms of vessel overlap. Furthermore, ultrasound allows for immediate revisualization upon changes in head position.29-32 Troianos et al. found >75% overlap of the IJV and CCA in 54% of all patients and in 64% of older patients (age >60 years) whose heads were rotated to the contralateral side.30 In one study of IJV CVC insertion, inadvertent carotid artery punctures were reduced (3% vs 10%) with the use of ultrasound guidance vs landmarks alone.33 In a cohort of 64 high-risk neurosurgical patients, cannulation success was 100% with the use of ultrasound guidance, and there were no injuries to the carotid artery, even though the procedure was performed with a 30-degree head elevation and anomalous IJV anatomy in 39% of patients.34 In a prospective, randomized controlled study of 1,332 patients, ultrasound-guided cannulation in a neutral position was demonstrated to be as safe as the 45-degree rotated position.35
Ultrasound allows for the recognition of anatomical variations which may influence the selection of the vascular access site or technique. Benter et al. found that 36% of patients showed anatomical variations in the IJV and surrounding tissue.36 Similarly Caridi showed the anatomy of the right IJV to be atypical in 29% of patients,37 and Brusasco found that 37% of bariatric patients had anatomical variations of the IJV.38 In a study of 58 patients, there was significant variability in the IJV position and IJV diameter, ranging from 0.5 cm to >2 cm.39 In a study of hemodialysis patients, 75% of patients had sonographic venous abnormalities that led to a change in venous access approach.40
To detect acute or chronic upper extremity deep venous thrombosis or stenosis, two-dimensional visualization with compression should be part of the ultrasound examination prior to central venous catheterization. In a study of patients that had undergone CVC insertion 9-19 weeks earlier, 50% of patients had an IJV thrombosis or stenosis leading to selection of an alternative site. In this study, use of ultrasound for a preprocedural site evaluation reduced unnecessary attempts at catheterizing an occluded vein.41 At least two other studies demonstrated an appreciable likelihood of thrombosis. In a study of bariatric patients, 8% of patients had asymptomatic thrombosis38 and in another study, 9% of patients being evaluated for hemodialysis catheter placement had asymptomatic IJV thrombosis.37
4. We recommend that providers should evaluate the target blood vessel size and depth during a preprocedural ultrasound evaluation.
Rationale: The size, depth, and anatomic location of central veins can vary considerably. These features are easily discernable using ultrasound. Contrary to traditional teaching, the IJV is located 1 cm anterolateral to the CCA in only about two-thirds of patients.37,39,42,43 Furthermore, the diameter of the IJV can vary significantly, ranging from 0.5 cm to >2 cm.39 The laterality of blood vessels may vary considerably as well. A preprocedural ultrasound evaluation of contralateral subclavian and axillary veins showed a significant absolute difference in cross-sectional area of 26.7 mm2 (P < .001).42
Blood vessels can also shift considerably when a patient is in the Trendelenburg position. In one study, the IJV diameter changed from 11.2 (± 1.5) mm to 15.4 (± 1.5) mm in the supine versus the Trendelenburg position at 15 degrees.33 An observational study demonstrated a frog-legged position with reverse Trendelenburg increased the femoral vein size and reduced the common surface area with the common femoral artery compared to a neutral position. Thus, a frog-legged position with reverse Trendelenburg position may be preferred, since overall catheterization success rates are higher in this position.44
Techniques
General Techniques
5. We recommend that providers should avoid using static ultrasound alone to mark the needle insertion site for vascular access procedures.
Rationale: The use of static ultrasound guidance to mark a needle insertion site is not recommended because normal anatomical relationships of vessels vary, and site marking can be inaccurate with minimal changes in patient position, especially of the neck.43,45,46 Benefits of using ultrasound guidance for vascular access are attained when ultrasound is used to track the needle tip in real-time as it is advanced toward the target vessel.
Although continuous-wave Doppler ultrasound without two-dimensional visualization was used in the past, it is no longer recommended for IJV CVC insertion.47 In a study that randomized patients to IJV CVC insertion with continuous-wave Doppler alone vs two-dimensional ultrasound guidance, the use of two-dimensional ultrasound guidance showed significant improvement in first-pass success rates (97% vs 91%, P = .045), particularly in patients with BMI >30 (97% vs 77%, P = .011).48
A randomized study comparing real-time ultrasound-guided, landmark-based, and ultrasound-marked techniques found higher success rates in the real-time ultrasound-guided group than the other two groups (100% vs 74% vs 73%, respectively; P = .01). The total number of mechanical complications was higher in the landmark-based and ultrasound-marked groups than in the real-time ultrasound-guided group (24% and 36% versus 0%, respectively; P = .01).49 Another randomized controlled study found higher success rates with real-time ultrasound guidance (98%) versus an ultrasound-marked (82%) or landmark-based (64%) approach for central line placement.50
6. We recommend that providers should use real-time (dynamic), two-dimensional ultrasound guidance with a high-frequency linear transducer for CVC insertion, regardless of the provider’s level of experience.
7. We suggest using either a transverse (short-axis) or longitudinal (long-axis) approach when performing real-time ultrasound-guided vascular access procedures.
Rationale: In clinical practice, the phrases transverse, short-axis, or out-of-plane approach are synonymous, as are longitudinal, long-axis, and in-plane approach. The short-axis approach involves tracking the needle tip as it approximates the target vessel with the ultrasound beam oriented in a transverse plane perpendicular to the target vessel. The target vessel is seen as a circular structure on the ultrasound screen as the needle tip approaches the target vessel from above. This approach is also called the out-of-plane technique since the needle passes through the ultrasound plane. The advantages of the short-axis approach include better visualization of adjacent vessels or nerves and the relative ease of skill acquisition for novice operators.9 When using the short-axis approach, extra care must be taken to track the needle tip from the point of insertion on the skin to the target vessel. A disadvantage of the short-axis approach is unintended posterior wall puncture of the target vessel.55
In contrast to a short-axis approach, a long-axis approach is performed with the ultrasound beam aligned parallel to the vessel. The vessel appears as a long tubular structure and the entire needle is visualized as it traverses across the ultrasound screen to approach the target vessel. The long-axis approach is also called an in-plane technique because the needle is maintained within the plane of the ultrasound beam. The advantage of a long-axis approach is the ability to visualize the entire needle as it is inserted into the vessel.14 A randomized crossover study with simulation models compared a long-axis versus short-axis approach for both IJV and subclavian vein catheterization. This study showed decreased number of needle redirections (relative risk (RR) 0.5, 95% confidence interval (CI) 0.3 to 0.7), and posterior wall penetrations (OR 0.3, 95% CI 0.1 to 0.9) using a long-axis versus short-axis approach for subclavian vein catheterization.56
A randomized controlled study comparing a long-axis or short-axis approach with ultrasound versus a landmark-based approach for IJV CVC insertion showed higher success rates (100% vs 90%; P < .001), lower insertion time (53 vs 116 seconds; P < .001), and fewer attempts to obtain access (2.5 vs 1.2 attempts, P < .001) with either the long- or short-axis ultrasound approach. The average time to obtain access and number of attempts were comparable between the short-axis and long-axis approaches with ultrasound. The incidence of carotid puncture and hematoma was significantly higher with the landmark-based approach versus either the long- or short-axis ultrasound approach (carotid puncture 17% vs 3%, P = .024; hematoma 23% vs 3%, P = .003).57
High success rates have been reported using a short-axis approach for insertion of PIV lines.58 A prospective, randomized trial compared the short-axis and long-axis approach in patients who had had ≥2 failed PIV insertion attempts. Success rate was 95% (95% CI, 0.85 to 1.00) in the short-axis group compared with 85% (95% CI, 0.69 to 1.00) in the long-axis group. All three subjects with failed PIV placement in the long-axis group had successful rescue placement using a short-axis approach. Furthermore, the short-axis approach was faster than the long-axis approach.59
For radial artery cannulation, limited data exist comparing the short- and long-axis approaches. A randomized controlled study compared a long-axis vs short-axis ultrasound approach for radial artery cannulation. Although the overall procedure success rate was 100% in both groups, the long-axis approach had higher first-pass success rates (1.27 ± 0.4 vs 1.5 ± 0.5, P < .05), shorter cannulation times (24 ± 17 vs 47 ± 34 seconds, P < .05), fewer hematomas (4% vs 43%, P < .05) and fewer posterior wall penetrations (20% vs 56%, P < .05).60
Another technique that has been described for IJV CVC insertion is an oblique-axis approach, a hybrid between the long- and short-axis approaches. In this approach, the transducer is aligned obliquely over the IJV and the needle is inserted using a long-axis or in-plane approach. A prospective randomized trial compared the short-axis, long-axis, and oblique-axis approaches during IJV cannulation. First-pass success rates were 70%, 52%, and 74% with the short-axis, long-axis, and oblique-axis approaches, respectively, and a statistically significant difference was found between the long- and oblique-axis approaches (P = .002). A higher rate of posterior wall puncture was observed with a short-axis approach (15%) compared with the oblique-axis (7%) and long-axis (4%) approaches (P = .047).61
8. We recommend that providers should visualize the needle tip and guidewire in the target vein prior to vessel dilatation.
Rationale: When real-time ultrasound guidance is used, visualization of the needle tip within the vein is the first step to confirm cannulation of the vein and not the artery. After the guidewire is advanced, the provider can use transverse and longitudinal views to reconfirm cannulation of the vein. In a longitudinal view, the guidewire is readily seen positioned within the vein, entering the anterior wall and lying along the posterior wall of the vein. Unintentional perforation of the posterior wall of the vein with entry into the underlying artery can be detected by ultrasound, allowing prompt removal of the needle and guidewire before proceeding with dilation of the vessel. In a prospective observational study that reviewed ultrasound-guided IJV CVC insertions, physicians were able to more readily visualize the guidewire than the needle in the vein.62 A prospective observational study determined that novice operators can visualize intravascular guidewires in simulation models with an overall accuracy of 97%.63
In a retrospective review of CVC insertions where the guidewire position was routinely confirmed in the target vessel prior to dilation, there were no cases of arterial dilation, suggesting confirmation of guidewire position can potentially eliminate the morbidity and mortality associated with arterial dilation during CVC insertion.64
9. To increase the success rate of ultrasound-guided vascular access procedures, we recommend that providers should utilize echogenic needles, plastic needle guides, and/or ultrasound beam steering when available.
Rationale: Echogenic needles have ridged tips that appear brighter on the screen, allowing for better visualization of the needle tip. Plastic needle guides help stabilize the needle alongside the transducer when using either a transverse or longitudinal approach. Although evidence is limited, some studies have reported higher procedural success rates when using echogenic needles, plastic needle guides, and ultrasound beam steering software. In a prospective observational study, Augustides et al. showed significantly higher IJV cannulation rates with versus without use of a needle guide after first (81% vs 69%, P = .0054) and second (93% vs 80%. P = .0001) needle passes.65 A randomized study by Maecken et al. compared subclavian vein CVC insertion with or without use of a needle guide, and found higher procedure success rates within the first and second attempts, reduced time to obtain access (16 seconds vs 30 seconds; P = .0001) and increased needle visibility (86% vs 32%; P < .0001) with the use of a needle guide.66 Another study comparing a short-axis versus long-axis approach with a needle guide showed improved needle visualization using a long-axis approach with a needle guide.67 A randomized study comparing use of a novel, sled-mounted needle guide to a free-hand approach for venous cannulation in simulation models showed the novel, sled-mounted needle guide improved overall success rates and efficiency of cannulation.68
Central Venous Access Techniques
10. We recommend that providers should use a standardized procedure checklist that includes use of real-time ultrasound guidance to reduce the risk of central line-associated bloodstream infection (CLABSI) from CVC insertion.
Rationale: A standardized checklist or protocol should be developed to ensure compliance with all recommendations for insertion of CVCs. Evidence-based protocols address periprocedural issues, such as indications for CVC, and procedural techniques, such as use of maximal sterile barrier precautions to reduce the risk of infection. Protocols and checklists that follow established guidelines for CVC insertion have been shown to decrease CLABSI rates.69,70 Similarly, development of checklists and protocols for maintenance of central venous catheters have been effective in reducing CLABSIs.71 Although no externally-validated checklist has been universally accepted or endorsed by national safety organizations, a few internally-validated checklists are available through peer-reviewed publications.72,73 An observational educational cohort of internal medicine residents who received training using simulation of the entire CVC insertion process was able to demonstrate fewer CLABSIs after the simulator-trained residents rotated in the intensive care unit (ICU) (0.50 vs 3.2 infections per 1,000 catheter days, P = .001).74
11. We recommend that providers should use real-time ultrasound guidance, combined with aseptic technique and maximal sterile barrier precautions, to reduce the incidence of infectious complications from CVC insertion.
Rationale: The use of real-time ultrasound guidance for CVC placement has demonstrated a statistically significant reduction in CLABSIs compared to landmark-based techniques.75 The Centers for Disease Control and Prevention (CDC) guidelines for the prevention of intravascular catheter-related infections recommend the use of ultrasound guidance to reduce the number of cannulation attempts and risk of mechanical complications.69 A prospective, three-arm study comparing ultrasound-guided long-axis, short-axis, and landmark-based approaches showed a CLABSI rate of 20% in the landmark-based group versus 10% in each of the ultrasound groups.57 Another randomized study comparing use of ultrasound guidance to a landmark-based technique for IJV CVC insertion demonstrated significantly lower CLABSI rates with the use of ultrasound (2% vs 10%; P < .05).72
Studies have shown that a systems-based intervention featuring a standardized catheter kit or catheter bundle significantly reduced CLABSI rates.76-78 A complete review of all preventive measures to reduce the risk of CLABSI is beyond the scope of this review, but a few key points will be mentioned. First, aseptic technique includes proper hand hygiene and skin sterilization, which are essential measures to reduce cutaneous colonization of the insertion site and reduce the risk of CLABSIs.79 In a systematic review and meta-analysis of eight studies including over 4,000 catheter insertions, skin antisepsis with chlorhexidine was associated with a 50% reduction in CLABSIs compared with povidone iodine.11 Therefore, a chlorhexidine-containing solution is recommended for skin preparation prior to CVC insertion per guidelines by Healthcare Infection Control Practices Advisory Committee/CDC, Society for Healthcare Epidemiology of America/Infectious Diseases Society of America, and American Society of Anesthesiologists.11,69,80,81 Second, maximal sterile barrier precautions refer to the use of sterile gowns, sterile gloves, caps, masks covering both the mouth and nose, and sterile full-body patient drapes. Use of maximal sterile barrier precautions during CVC insertion has been shown to reduce the incidence of CLABSIs compared to standard precautions.26,79,82-84 Third, catheters containing antimicrobial agents may be considered for hospital units with higher CLABSI rates than institutional goals, despite a comprehensive preventive strategy, and may be considered in specific patient populations at high risk of severe complications from a CLABSI.11,69,80 Finally, providers should use a standardized procedure set-up when inserting CVCs to reduce the risk of CLABSIs. The operator should confirm availability and proper functioning of ultrasound equipment prior to commencing a vascular access procedure. Use of all-inclusive procedure carts or kits with sterile ultrasound probe covers, sterile gel, catheter kits, and other necessary supplies is recommended to minimize interruptions during the procedure, and can ultimately reduce the risk of CLABSIs by ensuring maintenance of a sterile field during the procedure.13
12. We recommend that providers should use real-time ultrasound guidance for internal jugular vein catheterization, which reduces the risk of mechanical and infectious complications, the number of needle passes, and time to cannulation and increases overall procedure success rates.
Rationale: The use of real-time ultrasound guidance for CVC insertion has repeatedly demonstrated better outcomes compared to a landmark-based approach in adults.13 Several randomized controlled studies have demonstrated that real-time ultrasound guidance for IJV cannulation reduces the risk of procedure-related mechanical and infectious complications, and improves first-pass and overall success rates in diverse care settings.27,29,45,50,53,65,75,85-90 Mechanical complications that are reduced with ultrasound guidance include pneumothorax and carotid artery puncture.4,5,45,46,53,62,75,86-93 Currently, several medical societies strongly recommend the use of ultrasound guidance during insertion of IJV CVCs.10-12,14,94-96
A meta-analysis by Hind et al. that included 18 randomized controlled studies demonstrated use of real-time ultrasound guidance reduced failure rates (RR 0.14, 95% CI 0.06 to 0.33; P < .0001), increased first-attempt success rates (RR 0.59, 95% CI 0.39 to 0.88; P = .009), reduced complication rates (RR 0.43, 95% CI 0.22 to 0.87; P = .02) and reduced procedure time (P < .0001), compared to a traditional landmark-based approach when inserting IJV CVCs.5
A Cochrane systematic review compared ultrasound-guided versus landmark-based approaches for IJV CVC insertion and found use of real-time ultrasound guidance reduced total complication rates by 71% (RR 0.29, 95% CI 0.17 to 0.52; P < .0001), arterial puncture rates by 72% (RR 0.28, 95% CI 0.18 to 0.44; P < .00001), and rates of hematoma formation by 73% (RR 0.27, 95% CI 0.13 to 0.55; P = .0004). Furthermore, the number of attempts for successful cannulation was reduced (mean difference -1.19 attempts, 95% CI -1.45 to -0.92; P < .00001), the chance of successful insertion on the first attempt was increased by 57% (RR 1.57, 95% CI 1.36 to 1.82; P < .00001), and overall procedure success rates were modestly increased in all groups by 12% (RR 1.12, 95% CI 1.08 to 1.17; P < .00001).46
An important consideration in performing ultrasound guidance is provider experience. A prospective observational study of patients undergoing elective CVC insertion demonstrated higher complication rates for operators that were inexperienced (25.2%) versus experienced (13.6%).54 A randomized controlled study comparing experts and novices with or without the use of ultrasound guidance for IJV CVC insertion demonstrated higher success rates among expert operators and with the use of ultrasound guidance. Among novice operators, the complication rates were lower with the use of ultrasound guidance.97 One study evaluated the procedural success and complication rates of a two-physician technique with one physician manipulating the transducer and another inserting the needle for IJV CVC insertion. This study concluded that procedural success rates and frequency of complications were directly affected by the experience of the physician manipulating the transducer and not by the experience of the physician inserting the needle.98
The impact of ultrasound guidance on improving procedural success rates and reducing complication rates is greatest in patients that are obese, short necked, hypovolemic, or uncooperative.93 Several studies have demonstrated fewer needle passes and decreased time to cannulation compared to the landmark technique in these populations.46,49,53,86-88,92,93
Ultrasound-guided placement of IJV catheters can safely be performed in patients with disorders of hemostasis and those with multiple previous catheter insertions in the same vein.9 Ultrasound-guided placement of CVCs in patients with disorders of hemostasis is safe with high success and low complication rates. In a case series of liver patients with coagulopathy (mean INR 2.17 ± 1.16, median platelet count 150K), the use of ultrasound guidance for CVC insertion was highly successful with no major bleeding complications.99
A study of renal failure patients found high success rates and low complication rates in the patients with a history of multiple previous catheterizations, poor compliance, skeletal deformities, previous failed cannulations, morbid obesity, and disorders of hemostasis.100 A prospective observational study of 200 ultrasound-guided CVC insertions for apheresis showed a 100% success rate with a 92% first-pass success rate.101
The use of real-time ultrasound guidance for IJV CVC insertion has been shown to be cost effective by reducing procedure-related mechanical complications and improving procedural success rates. A companion cost-effectiveness analysis estimated that for every 1,000 patients, 90 complications would be avoided, with a net cost savings of approximately $3,200 using 2002 prices.102
13. We recommend that providers who routinely insert subclavian vein CVCs should use real-time ultrasound guidance, which has been shown to reduce the risk of mechanical complications and number of needle passes and increase overall procedure success rates compared with landmark-based techniques.
Rationale: In clinical practice, the term ultrasound-guided subclavian vein CVC insertion is commonly used. However, the needle insertion site is often lateral to the first rib and providers are technically inserting the CVC in the axillary vein. The subclavian vein becomes the axillary vein at the lateral border of the first rib where the cephalic vein branches from the subclavian vein. To be consistent with common medical parlance, we use the phrase ultrasound-guided subclavian vein CVC insertion in this document.
Advantages of inserting CVCs in the subclavian vein include reliable surface anatomical landmarks for vein location, patient comfort, and lower risk of infection.103 Several observational studies have demonstrated the technique for ultrasound-guided subclavian vein CVC insertion is feasible and safe.104-107 In a large retrospective observational study of ultrasound-guided central venous access among a complex patient group, the majority of patients were cannulated successfully and safely. The subset of patients undergoing axillary vein CVC insertion (n = 1,923) demonstrated a low rate of complications (0.7%), proving it is a safe and effective alternative to the IJV CVC insertion.107
A Cochrane review of ultrasound-guided subclavian vein cannulation (nine studies, 2,030 participants, 2,049 procedures), demonstrated that real-time two-dimensional ultrasound guidance reduced the risk of inadvertent arterial punctures (three studies, 498 participants, RR 0.21, 95% CI 0.06 to 0.82; P = .02) and hematoma formation (three studies, 498 participants, RR 0.26, 95% CI 0.09 to 0.76; P = .01).46 A systematic review and meta-analysis of 10 randomized controlled studies comparing ultrasound-guided versus landmark-based subclavian vein CVC insertion demonstrated a reduction in the risk of arterial punctures, hematoma formation, pneumothorax, and failed catheterization with the use of ultrasound guidance.105
A randomized controlled study comparing ultrasound-guided vs landmark-based approaches to subclavian vein cannulation found that use of ultrasound guidance had a higher success rate (92% vs 44%, P = .0003), fewer minor complications (1 vs 11, P = .002), fewer attempts (1.4 vs 2.5, P = .007) and fewer catheter kits used (1.0 vs 1.4, P = .0003) per cannulation.108
Fragou et al. randomized patients undergoing subclavian vein CVC insertion to a long-axis approach versus a landmark-based approach and found a significantly higher success rate (100% vs 87.5%, P < .05) and lower rates of mechanical complications: artery puncture (0.5% vs 5.4%), hematoma (1.5% vs 5.4%), hemothorax (0% vs 4.4%), pneumothorax (0% vs 4.9%), brachial plexus injury (0% vs 2.9%), phrenic nerve injury (0% vs 1.5%), and cardiac tamponade (0% vs 0.5%).109 The average time to obtain access and the average number of insertion attempts (1.1 ± 0.3 vs 1.9 ± 0.7, P < .05) were significantly reduced in the ultrasound group compared to the landmark-based group.95
A retrospective review of subclavian vein CVC insertions using a supraclavicular approach found no reported complications with the use of ultrasound guidance vs 23 mechanical complications (8 pneumothorax, 15 arterial punctures) with a landmark-based approach.106 However, it is important to note that a supraclavicular approach is not commonly used in clinical practice.
14. We recommend that providers should use real-time ultrasound guidance for femoral venous access, which has been shown to reduce the risk of arterial punctures and total procedure time and increase overall procedure success rates.
Rationale: Anatomy of the femoral region varies, and close proximity or overlap of the femoral vein and artery is common.51 Early studies showed that ultrasound guidance for femoral vein CVC insertion reduced arterial punctures compared with a landmark-based approach (7% vs 16%), reduced total procedure time (55 ± 19 vs 79 ± 62 seconds), and increased procedure success rates (100% vs 90%).52 A Cochrane review that pooled data from four randomized studies comparing ultrasound-guided vs landmark-based femoral vein CVC insertion found higher first-attempt success rates with the use of ultrasound guidance (RR 1.73, 95% CI 1.34 to 2.22; P < .0001) and a small increase in the overall procedure success rates (RR 1.11, 95% CI 1.00 to 1.23; P = .06). There was no difference in inadvertent arterial punctures or other complications.110
Peripheral Venous Access Techniques
15. We recommend that providers should use real-time ultrasound guidance for the insertion of peripherally inserted central catheters (PICCs), which is associated with higher procedure success rates and may be more cost effective compared with landmark-based techniques.
Rationale: Several studies have demonstrated that providers who use ultrasound guidance vs landmarks for PICC insertion have higher procedural success rates, lower complication rates, and lower total placement costs. A prospective observational report of 350 PICC insertions using ultrasound guidance reported a 99% success rate with an average of 1.2 punctures per insertion and lower total costs.20 A retrospective observational study of 500 PICC insertions by designated specialty nurses revealed an overall success rate of 95%, no evidence of phlebitis, and only one CLABSI among the catheters removed.21 A retrospective observational study comparing several PICC variables found higher success rates (99% vs 77%) and lower thrombosis rates (2% vs 9%) using ultrasound guidance vs landmarks alone.22 A study by Robinson et al. demonstrated that having a dedicated PICC team equipped with ultrasound increased their institutional insertion success rates from 73% to 94%.111
A randomized controlled study comparing ultrasound-guided versus landmark-based PICC insertion found high success rates with both techniques (100% vs 96%). However, there was a reduction in the rate of unplanned catheter removals (4.0% vs 18.7%; P = .02), mechanical phlebitis (0% vs 22.9%; P < .001), and venous thrombosis (0% vs 8.3%; P = .037), but a higher rate of catheter migration (32% vs 2.1%; P < .001). Compared with the landmark-based group, the ultrasound-guided group had significantly lower incidence of severe contact dermatitis (P = .038), and improved comfort and costs up to 3 months after PICC placement (P < .05).112
Routine postprocedure chest x-ray (CXR) is generally considered unnecessary if the PICC is inserted with real-time ultrasound guidance along with use of a newer tracking devices, like the magnetic navigation system with intracardiac electrodes.9 Ultrasound can also be used to detect malpositioning of a PICC immediately after completing the procedure. A randomized controlled study comparing ultrasound versus postprocedure CXR detected one malpositioned PICC in the ultrasound group versus 11 in the control group. This study suggested that ultrasound can detect malpositioning immediately postprocedure and reduce the need for a CXR and the possibility of an additional procedure to reposition a catheter.113
16. We recommend that providers should use real-time ultrasound guidance for the placement of peripheral intravenous lines (PIV) in patients with difficult peripheral venous access to reduce the total procedure time, needle insertion attempts, and needle redirections. Ultrasound-guided PIV insertion is also an effective alternative to CVC insertion in patients with difficult venous access.
Rationale: Difficult venous access refers to patients that have had two unsuccessful attempts at PIV insertion using landmarks or a history of difficult access (i.e. edema, obesity, intravenous drug use, chemotherapy, diabetes, hypovolemia, chronic illness, vasculopathy, multiple prior hospitalizations). A meta-analysis of seven randomized controlled studies concluded that ultrasound guidance increases the likelihood of successful PIV insertion (pooled OR 2.42, 95% CI 1.26 to 4.68; P < .008).18 A second meta-analysis that pooled data from seven studies (six randomized controlled studies) confirmed that ultrasound guidance improves success rates of PIV insertion (OR 3.96, 95% CI 1.75 to 8.94).19 Approximately half of these studies had physician operators while the other half had nurse operators.
In one prospective observational study of emergency department patients with two failed attempts of landmark-based PIV insertion, ultrasound guidance with a modified-Seldinger technique showed a relatively high success rate (96%), fewer needle sticks (mean 1.32 sticks, 95% CI 1.12 to 1.52), and shorter time to obtain access (median time 68 seconds).114 Other prospective observational studies have demonstrated that ultrasound guidance for PIV insertion has a high success rate (87%),115 particularly with brachial or basilic veins PIV insertion, among patients with difficult PIV access, defined as having had ≥2 failed attempts.58
Since insertion of PIVs with ultrasound guidance has a high success rate, there is potential to reduce the reliance on CVC insertion for venous access only. In a study of patients that had had two failed attempts at PIV insertion based on landmarks, a PIV was successfully inserted with ultrasound guidance in 84% of patients, obviating the need for CVC placement for venous access.116 A prospective observational study showed ultrasound-guided PIV insertion was an effective alternative to CVC placement in ED patients with difficult venous access with only 1% of patients requiring a CVC.117 Use of ultrasound by nurses for PIV placement has also been shown to reduce the time to obtain venous access, improve patient satisfaction, and reduce the need for physician intervention.118 In a prospective observational study of patients with difficult access, the majority of patients reported a better experience with ultrasound-guided PIV insertion compared to previous landmark-based attempts with an average satisfaction score of 9.2/10 with 76% of patients rating the experience a 10.119 A strong recommendation has been made for use of ultrasound guidance in patients with difficult PIV placement by la Société Française d’Anesthésie et de Réanimation (The French Society of Anesthesia and Resuscitation).95
17. We suggest using real-time ultrasound guidance to reduce the risk of vascular, infectious, and neurological complications during PIV insertion, particularly in patients with difficult venous access.
Rationale: The incidence of complications from PIV insertion is often underestimated. Vascular complications include arterial puncture, hematoma formation, local infiltration or extravasation of fluid, and superficial or deep venous thrombosis. The most common infectious complications with PIV insertion are phlebitis and cellulitis.120 One observational study reported PIV complications occurring in approximately half of all patients with the most common complications being phlebitis, hematoma formation, and fluid/blood leakage.121
A retrospective review of ICU patients who underwent ultrasound-guided PIV insertion by a single physician showed high success rates (99%) with low rates of phlebitis/cellulitis (0.7%).There was an assumed benefit of risk reduction due to the patients no longer requiring a CVC after successful PIV placement.122 Another study found very low rates of infection with both landmark-based and ultrasound-guided PIV placement performed by emergency department nurses, suggesting that there is no increased risk of infection with the use of ultrasound.123 To reduce the risk of infection from PIV insertion, we recommend the use of sterile gel and sterile transducer cover (See Recommendation 2).
Arterial Access Techniques
18. We recommend that providers should use real-time ultrasound guidance for arterial access, which has been shown to increase first-pass success rates, reduce the time to cannulation, and reduce the risk of hematoma development compared with landmark-based techniques.
Rationale: Several randomized controlled studies have assessed the value of ultrasound in arterial catheter insertion. Shiver et al. randomized 60 patients admitted to a tertiary center emergency department to either palpation or ultrasound-guided arterial cannulation. They demonstrated a first-pass success rate of 87% in the ultrasound group compared with 50% in the landmark technique group. In the same study, the use of ultrasound was also associated with reduced time needed to establish arterial access and a 43% reduction in the development of hematoma at the insertion site.124 Levin et al. demonstrated a first-pass success rate of 62% using ultrasound versus 34% by palpation alone in 69 patients requiring intraoperative invasive hemodynamic monitoring.125 Additional randomized controlled studies have demonstrated that ultrasound guidance increases first-attempt success rates compared to traditional palpation.23,126,127
19. We recommend that providers should use real-time ultrasound guidance for femoral arterial access, which has been shown to increase first-pass success rates and reduce the risk of vascular complications.
Rationale: Although it is a less frequently used site, the femoral artery may be accessed for arterial blood sampling or invasive hemodynamic monitoring, and use of ultrasound guidance has been shown to improve the first-pass success rates of femoral artery cannulation. It is important to note that most of these studies comparing ultrasound-guided vs landmark-based femoral artery cannulation were performed in patients undergoing diagnostic or interventional vascular procedures.
A meta-analysis of randomized controlled studies comparing ultrasound-guided vs landmark-based femoral artery catheterization found use of ultrasound guidance was associated with a 49% reduction in overall complications (RR 0.51, 95% CI 0.28 to 0.91; P > .05) and 42% improvement in first-pass success rates.128 In another study, precise site selection with ultrasound was associated with fewer pseudoaneurysms in patients undergoing femoral artery cannulation by ultrasound guidance vs palpation for cardiac catheterization (3% vs 5%, P < .05).129
A multicenter randomized controlled study comparing ultrasound vs fluoroscopic guidance for femoral artery catheterization demonstrated ultrasound guidance improved rates of common femoral artery (CFA) cannulation in patients with high CFA bifurcations (83% vs 70%, P < .01).130 Furthermore, ultrasound guidance improved first-pass success rates (83% vs 46%, P < .0001), reduced number of attempts (1.3 vs 3.0, P < .0001), reduced risk of venipuncture (2.4% vs 15.8%, P < .0001), and reduced median time to obtain access (136 seconds vs148 seconds, P = .003). Vascular complications occurred in fewer patients in the ultrasound vs fluoroscopy groups (1.4% vs 3.4% P = .04). Reduced risk of hematoma formation with routine use of ultrasound guidance was demonstrated in one retrospective observational study (RR 0.62, 95% CI 0.46 to 0.84; P < .01).131
20. We recommend that providers should use real-time ultrasound guidance for radial arterial access, which has been shown to increase first-pass success rates, reduce the time to successful cannulation, and reduce the risk of complications compared with landmark-based techniques.
Rationale: Ultrasound guidance is particularly useful for radial artery cannulation in patients with altered anatomy, obesity, nonpulsatile blood flow, low perfusion, and previously unsuccessful cannulation attempts using a landmark-guided approach.132
A multicenter randomized controlled study that was not included in the abovementioned meta-analyses showed similar benefits of using ultrasound guidance vs landmarks for radial artery catheterization: a reduction in the number of attempts with ultrasound guidance (1.65 ± 1.2 vs 3.05 ± 3.4, P < .0001) and time to obtain access (88 ± 78 vs 108 ± 112 seconds, P = .006), and increased first-pass success rates (65% vs 44%, P < .0001). The use of ultrasound guidance was found to be particularly useful in patients with difficult access by palpation alone.135
Regarding the level of expertise required to use ultrasound guidance, a prospective observational study demonstrated that physicians with little previous ultrasound experience were able to improve their first-attempt success rates and procedure time for radial artery cannulation compared to historical data of landmark-based insertions.136
Postprocedure
21. We recommend that post-procedure pneumothorax should be ruled out by the detection of bilateral lung sliding using a high-frequency linear transducer before and after insertion of internal jugular and subclavian vein CVCs.
Rationale: Detection of lung sliding with two-dimensional ultrasound rules out pneumothorax, and disappearance of lung sliding in an area where it was previously seen is a strong predictor of postprocedure pneumothorax. In a study of critically ill patients, the disappearance of lung sliding was observed in 100% of patients with pneumothorax vs 8.8% of patients without pneumothorax. For detection of pneumothorax, lung sliding showed a sensitivity of 95%, specificity of 91%, and negative predictive value of 100% (P < .001).137 Another study by the same author showed that the combination of horizontal artifacts (absence of comet-tail artifact) and absence of lung sliding had a sensitivity of 100%, specificity of 96.5%, and negative predictive value of 100% for the detection of pneumothorax.138 A meta-analysis of 10 studies on the diagnostic accuracy of CVC confirmation with bedside ultrasound vs chest radiography reported detection of all 12 pneumothoraces with ultrasound, whereas chest radiography missed two pneumothoraces. The pooled sensitivity and specificity of ultrasound for the detection of pneumothorax was 100%, although an imperfect gold standard bias likely affected the results. An important advantage of bedside ultrasound is the ability to rule out pneumothorax immediately after the procedure while at the bedside. The mean time for confirmation of CVC placement with bedside ultrasound was 6 minutes versus 64 minutes and 143 minutes for completion and interpretation of a chest radiograph, respectively.139
22. We recommend that providers should use ultrasound with rapid infusion of agitated saline to visualize a right atrial swirl sign (RASS) for detecting catheter tip misplacement during CVC insertion. The use of RASS to detect the catheter tip may be considered an advanced skill that requires specific training and expertise.
Rationale: Bedside echocardiography is a reliable tool to detect catheter tip misplacement during CVC insertion. In one study, catheter misplacement was detected by bedside echocardiography with a sensitivity of 96% and specificity of 83% (positive predictive value 98%, negative predictive value 55%) and prevented distal positioning of the catheter tip.140 A prospective observational study assessed for RASS, which is turbulent flow in the right atrium after a rapid saline flush of the distal CVC port, to exclude catheter malposition. In this study with 135 CVC placements, visualization of RASS with ultrasound was able to identify all correct CVC placements and three of four catheter misplacements. Median times to complete the ultrasound exam vs CXR were 1 vs 20 minutes, respectively, with a median difference of 24 minutes (95% CI 19.6 to 29.3, P < .0001) between the two techniques.141
A prospective observational study assessed the ability of bedside transthoracic echocardiography to detect the guidewire, microbubbles, or both, in the right atrium compared to transesophageal echocardiography as the gold standard. Bedside transthoracic echocardiography allowed visualization of the right atrium in 94% of patients, and both microbubbles plus guidewire in 91% of patients.142 Hence, bedside transthoracic echocardiography allows adequate visualization of the right atrium. Another prospective observational study combining ultrasonography and contrast enhanced RASS resulted in 96% sensitivity and 93% specificity for the detection of a misplaced catheter, and the concordance with chest radiography was 96%.143
Training
23. To reduce the risk of mechanical and infectious complications, we recommend that novice providers should complete a systematic training program that includes a combination of simulation-based practice, supervised insertion on patients, and evaluation by an expert operator before attempting ultrasound-guided CVC insertion independently on patients.
Rationale: Cumulative experience has been recognized to not be a proxy for mastery of a clinical skill.144 The National Institute for Clinical Excellence (NICE) has recommended that providers performing ultrasound-guided CVC insertion should receive appropriate training to achieve competence before performing the procedure independently.7 Surveys have demonstrated that lack of training is a commonly reported barrier for not using ultrasound.145,146
Structured training programs on CVC insertion have been shown to reduce the occurrence of infectious and mechanical complications.74,143,147-149 The use of ultrasound and checklists, bundling of supplies, and practice with simulation models, as a part of a structured training program, can improve patient safety related to CVC insertion.9,140,150-154
Simulation-based practice has been used in medical education to provide deliberate practice and foster skill development in a controlled learning environment.155-158 Studies have shown transfer of skills demonstrated in a simulated environment to clinical practice, which can improve CVC insertion practices.159,160 Simulation accelerates learning of all trainees, especially novice trainees, and mitigates risks to patients by allowing trainees to achieve a minimal level of competence before attempting the procedure on real patients.152,161,162 Residents that have been trained using simulation preferentially select the IJV site,147 and more reliably use ultrasound to guide their CVC insertions.160,163
Additionally, simulation-based practice allows exposure to procedures and scenarios that may occur infrequently in clinical practice.
Although there is evidence on efficacy of simulation-based CVC training programs, there is no broadly accepted consensus on timing, duration, and content of CVC training programs for trainees or physicians in practice. The minimum recommended technical skills a trainee must master include the ability to (1) manipulate the ultrasound machine to produce a high-quality image to identify the target vessel, (2) advance the needle under direct visualization to the desired target site and depth, (3) deploy the catheter into the target vessel and confirm catheter placement in the target vessel using ultrasound, and (4) ensure the catheter has not been inadvertently placed in an unintended vessel or structure.153
A variety of simulation models are currently used to practice CVC insertion at the most common sites: the internal jugular, subclavian, basilic, and brachial veins.164,165 Effective simulation models should contain vessels that mimic normal anatomy with muscles, soft tissues, and bones. Animal tissue models, such as turkey or chicken breasts, may be effective for simulated practice of ultrasound-guided CVC insertion.166,167 Ultrasound-guided CVC training using human cadavers has also been shown to be effective.168
24. We recommend that cognitive training in ultrasound-guided CVC insertion should include basic anatomy, ultrasound physics, ultrasound machine knobology, fundamentals of image acquisition and interpretation, detection and management of procedural complications, infection prevention strategies, and pathways to attain competency.
Rationale: After receiving training in ultrasound-guided CVC insertion, physicians report significantly higher comfort with the use of ultrasound compared to those who have not received such training.145 Learners find training sessions worthwhile to increase skill levels,167 and skills learned from simulation-based mastery learning programs have been retained up to one year.158
Several commonalities have been noted across training curricula. Anatomy and physiology didactics should include vessel anatomy (location, size, and course);9 vessel differentiation by ultrasound;9,69 blood flow dynamics;69 Virchow’s triad;69 skin integrity and colonization;150 peripheral nerve identification and distribution;9 respiratory anatomy;9,69 upper and lower extremity, axillary, neck, and chest anatomy.9,69 Vascular anatomy is an essential curricular component that may help avoid preventable CVC insertion complications, such as inadvertent nerve, artery, or lung puncture.150,169 Training curricula should also include ultrasound physics (piezoelectric effect, frequency, resolution, attenuation, echogenicity, Doppler ultrasound, arterial and venous flow characteristics), image acquisition and optimization (imaging mode, focus, dynamic range, probe types), and artifacts (reverberation, mirror, shadowing, enhancement).
CVC-related infections are an important cause of morbidity and mortality in the acute and long-term care environment.69 Infection and thrombosis can both be impacted by the insertion site selection, skin integrity, and catheter–vein ratio.2,3,84 Inexperience generally leads to more insertion attempts that can increase trauma during CVC insertion and potentially increase the risk of infections.170 To reduce the risk of infectious complications, training should include important factors to consider in site selection and maintenance of a sterile environment during CVC insertion, including use of maximal sterile barrier precautions, hand hygiene, and appropriate use of skin antiseptic solutions.
Professional society guidelines have been published with recommendations of appropriate techniques for ultrasound-guided vascular access that include training recommendations.9,154 Training should deconstruct the insertion procedure into readily understood individual steps, and can be aided by demonstration of CVC insertion techniques using video clips. An alternative to face-to-face training is internet-based training that has been shown to be as effective as traditional teaching methods in some medical centers.171 Additional methods to deliver cognitive instruction include textbooks, continuing medical education courses, and digital videos.164,172
25. We recommend that trainees should demonstrate minimal competence before placing ultrasound-guided CVCs independently. A minimum number of CVC insertions may inform this determination, but a proctored assessment of competence is most important.
Rationale: CVC catheter placement carries the risk of serious complications including arterial injury or dissection, pneumothorax, or damage to other local structures; arrhythmias; catheter malposition; infection; and thrombosis. Although there is a lack of consensus and high-quality evidence for the certification of skills to perform ultrasound-guided CVC insertion, recommendations have been published advocating for formal and comprehensive training programs in ultrasound-guided CVC insertion with an emphasis on expert supervision prior to independent practice.9,153,154 Two groups of expert operators have recommended that training should include at least 8-10 supervised ultrasound-guided CVC insertions.154,173,174 A consensus task force from the World Congress of Vascular Access has recommended a minimum of six to eight hours of didactic education, four hours of hands-on training on simulation models, and six hours of hands-on ultrasound training on human volunteers to assess normal anatomy.175 This training should be followed by supervised ultrasound-guided CVC insertions until the learner has demonstrated minimal competence with a low rate of complications.35 There is general consensus that arbitrary numbers should not be the sole determinant of competence, and that the most important determinant of competence should be an evaluation by an expert operator.176
26. We recommend that didactic and hands-on training for trainees should coincide with anticipated times of increased performance of vascular access procedures. Refresher training sessions should be offered periodically.
Rationale: Simulation-based CVC training courses have shown a rapid improvement in skills, but lack of practice leads to deterioration of technical skills.161,162,177,178 Thus, a single immersive training session is insufficient to achieve and maintain mastery of skills, and an important factor to acquire technical expertise is sustained, deliberate practice with feedback.179 Furthermore, an insidious decay in skills may go unrecognized as a learner’s comfort and self-confidence does not always correlate with actual performance, leading to increased risk of errors and potential for procedural complications.147,158,180-183 Given the decay in technical skills over time, simulation-based training sessions are most effective when they occur in close temporal proximity to times when those skills are most likely to be used; for example, a simulation-based training session for trainees may be most effective just before the start of a critical care rotation.152 Regularly scheduled training sessions with monitoring and feedback by expert operators can reinforce procedural skills and prevent decay. Some experts have recommended that a minimum of 10 ultrasound-guided CVC insertions should be performed annually to maintain proficiency.153
27. We recommend that competency assessments should include formal evaluation of knowledge and technical skills using standardized assessment tools.
Rationale: Hospitalists and other healthcare providers that place vascular access catheters should undergo competency assessments proctored by an expert operator to verify that they have the required knowledge and skills.184,185 Knowledge competence can be partially evaluated using a written assessment, such as a multiple-choice test, assessing the provider’s cognitive understanding of the procedure.175 For ultrasound-guided CVC insertion, a written examination should be administered in conjunction with an ultrasound image assessment to test the learner’s recognition of normal vs abnormal vascular anatomy. Minimum passing standards should be established a priori according to local or institutional standards.
The final skills assessment should be objective, and the learner should be required to pass all critical steps of the procedure. Failure of the final skills assessment should lead to continued practice with supervision until the learner can consistently demonstrate correct performance of all critical steps. Checklists are commonly used to rate the technical performance of learners because they provide objective criteria for evaluation, can identify specific skill deficiencies, and can determine a learner’s readiness to perform procedures independently.186,187 The administration of skills assessments and feedback methods should be standardized across faculty. Although passing scores on both knowledge and skills assessments do not guarantee safe performance of a procedure independently, they provide a metric to ensure that a minimum level of competence has been achieved before allowing learners to perform procedures on patients without supervision.188
Competency assessments are a recommended component of intramural and extramural certification of skills in ultrasound-guided procedures. Intramural certification pathways differ by institution and often require additional resources including ultrasound machine(s), simulation equipment, and staff time, particularly when simulation-based assessments are incorporated into certification pathways. We recognize that some of these recommendations may not be feasible in resource-limited settings, such as rural hospitals. However, initial and ongoing competency assessments can be performed during routine performance of procedures on patients. For an in-depth review of credentialing pathways for ultrasound-guided bedside procedures, we recommend reviewing the SHM Position Statement on Credentialing of Hospitalists in Ultrasound-Guided Bedside Procedures.24
28. We recommend that competency assessments should evaluate for proficiency in the following knowledge and skills of CVC insertion:
a. Knowledge of the target vein anatomy, proper vessel identification, and recognition of anatomical variants
b. Demonstration of CVC insertion with no technical errors based on a procedural checklist
c. Recognition and management of acute complications, including emergency management of life-threatening complications
d. Real-time needle tip tracking with ultrasound and cannulation on the first attempt in at least five consecutive simulations.
Rationale: Recommendations have been published with the minimal knowledge and skills learners must demonstrate to perform ultrasound-guided vascular access procedures. These include operation of an ultrasound machine to produce high-quality images of the target vessel, tracking of the needle tip with real-time ultrasound guidance, and recognition and understanding of the management of procedural complications.154,175
First, learners must be able to perform a preprocedural assessment of the target vein, including size and patency of the vein; recognition of adjacent critical structures; and recognition of normal anatomical variants.175,189 Second, learners must be able to demonstrate proficiency in tracking the needle tip penetrating the target vessel, inserting the catheter into the target vessel, and confirming catheter placement in the target vessel with ultrasound.154,175 Third, learners must be able to demonstrate recognition of acute complications, including arterial puncture, hematoma formation, and development of pneumothorax.154,175 Trainees should be familiar with recommended evaluation and management algorithms, including indications for emergent consultation.190
29. We recommend a periodic proficiency assessments of all operators should be conducted to ensure maintenance of competency.
Rationale: Competency extends to periodic assessment and not merely an initial evaluation at the time of training.191 Periodic competency assessments should include assessment of proficiency of all providers that perform a procedure, including instructors and supervisors. Supervising providers should maintain their competency in CVC insertion through routine use of their skills in clinical practice.175 An observational study of emergency medicine residents revealed that lack of faculty comfort with ultrasound hindered the residents’ use of ultrasound.192 Thus, there is a need to examine best practices for procedural supervision of trainees because providers are often supervising procedures that they are not comfortable performing on their own.193
KNOWLEDGE GAPS
The process of producing this position statement revealed areas of uncertainty and important gaps in the literature regarding the use of ultrasound guidance for central and peripheral venous access and arterial access.
This position statement recommends a preprocedural ultrasound evaluation of blood vessels based on evidence that providers may detect anatomic anomalies, thrombosis, or vessel stenosis. Ultrasound can also reveal unsuspected high-risk structures in near proximity to the procedure site. Although previous studies have shown that providers can accurately assess vessels with ultrasound for these features, further study is needed to evaluate the effect of a standardized preprocedural ultrasound exam on clinical and procedural decision-making, as well as procedural outcomes.
Second, two ultrasound applications that are being increasingly used but have not been widely implemented are the use of ultrasound to evaluate lung sliding postprocedure to exclude pneumothorax and the verification of central line placement using a rapid infusion of agitated saline to visualize the RASS.139-141 Both of these applications have the potential to expedite postprocedure clearance of central lines for usage and decrease patient radiation exposure by obviating the need for postprocedure CXRs. Despite the supporting evidence, both of these applications are not yet widely used, as few providers have been trained in these techniques which may be considered advanced skills.
Third, despite advances in our knowledge of effective training for vascular access procedures, there is limited agreement on how to define procedural competence. Notable advancements in training include improved understanding of systematic training programs, development of techniques for proctoring procedures, definition of elements for hands-on assessments, and definition of minimum experience needed to perform vascular access procedures independently. However, application of these concepts to move learners toward independent practice remains variably interpreted at different institutions, likely due to limited resources, engrained cultures about procedures, and a lack of national standards. The development of hospitalist-based procedure services at major academic medical centers with high training standards, close monitoring for quality assurance, and the use of databases to track clinical outcomes may advance our understanding and delivery of optimal procedural training.
Finally, ultrasound technology is rapidly evolving which will affect training, techniques, and clinical outcomes in coming years. Development of advanced imaging software with artificial intelligence can improve needle visualization and tracking. These technologies have the potential to facilitate provider training in real-time ultrasound-guided procedures and improve the overall safety of procedures. Emergence of affordable, handheld ultrasound devices is improving access to ultrasound technology, but their role in vascular access procedures is yet to be defined. Furthermore, availability of wireless handheld ultrasound technology and multifrequency transducers will create new possibilities for use of ultrasound in vascular access procedures.
CONCLUSION
We have presented several evidence-based recommendations on the use of ultrasound guidance for placement of central and peripheral vascular access catheters that are intended for hospitalists and other healthcare providers who routinely perform vascular access procedures. By allowing direct visualization of the needle tip and target vessel, the use of ultrasound guidance has been shown in randomized studies to reduce needle insertion attempts, reduce needle redirections, and increase overall procedure success rates. The accuracy of ultrasound to identify the target vessel, assess for thrombosis, and detect anatomical anomalies is superior to that of physical examination. Hospitalists can attain competence in performing ultrasound-guided vascular access procedures through systematic training programs that combine didactic and hands-on training, which optimally include patient-based competency assessments.
Acknowledgments
The authors thank all the members of the Society of Hospital Medicine Point-of-care Ultrasound Task Force and the Education Committee members for their time and dedication to develop these guidelines.
Collaborators of Society of Hospital Medicine Point-of-care Ultrasound Task Force: Robert Arntfield, Jeffrey Bates, Anjali Bhagra, Michael Blaivas, Daniel Brotman, Richard Hoppmann, Susan Hunt, Trevor P. Jensen, Venkat Kalidindi, Ketino Kobaidze, Joshua Lenchus, Paul Mayo, Satyen Nichani, Vicki Noble, Nitin Puri, Aliaksei Pustavoitau, Kreegan Reierson, Gerard Salame, Kirk Spencer, Vivek Tayal, David Tierney
SHM Point-of-care Ultrasound Task Force: CHAIRS: Nilam J. Soni, Ricardo Franco-Sadud, Jeff Bates. WORKING GROUPS: Thoracentesis Working Group: Ria Dancel (chair), Daniel Schnobrich, Nitin Puri. Vascular Access Working Group: Ricardo Franco (chair), Benji Mathews, Saaid Abdel-Ghani, Sophia Rodgers, Martin Perez, Daniel Schnobrich. Paracentesis Working Group: Joel Cho (chair), Benji Mathews, Kreegan Reierson, Anjali Bhagra, Trevor P. Jensen Lumbar Puncture Working Group: Nilam J. Soni (chair), Ricardo Franco, Gerard Salame, Josh Lenchus, Venkat Kalidindi, Ketino Kobaidze. Credentialing Working Group: Brian P Lucas (chair), David Tierney, Trevor P. Jensen PEER REVIEWERS: Robert Arntfield, Michael Blaivas, Richard Hoppmann, Paul Mayo, Vicki Noble, Aliaksei Pustavoitau, Kirk Spencer, Vivek Tayal. METHODOLOGIST: Mahmoud El-Barbary. LIBRARIAN: Loretta Grikis. SOCIETY OF HOSPITAL MEDICINE EDUCATION COMMITTEE: Daniel Brotman (past chair), Satyen Nichani (current chair), Susan Hunt. SOCIETY OF HOSPITAL MEDICINE STAFF: Nick Marzano.
Disclaimer
The contents of this publication do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Approximately five million central venous catheters (CVCs) are inserted in the United States annually, with over 15 million catheter days documented in intensive care units alone.1 Traditional CVC insertion techniques using landmarks are associated with a high risk of mechanical complications, particularly pneumothorax and arterial puncture, which occur in 5%-19% patients.2,3
Since the 1990s, several randomized controlled studies and meta-analyses have demonstrated that the use of real-time ultrasound guidance for CVC insertion increases procedure success rates and decreases mechanical complications.4,5 Use of real-time ultrasound guidance was recommended by the Agency for Healthcare Research and Quality, the Institute of Medicine, the National Institute for Health and Care Excellence, the Centers for Disease Control and Prevention, and several medical specialty societies in the early 2000s.6-14 Despite these recommendations, ultrasound guidance has not been universally adopted. Currently, an estimated 20%-55% of CVC insertions in the internal jugular vein are performed without ultrasound guidance.15-17
Following the emergence of literature supporting the use of ultrasound guidance for CVC insertion, observational and randomized controlled studies demonstrated improved procedural success rates with the use of ultrasound guidance for the insertion of peripheral intravenous lines (PIVs), arterial catheters, and peripherally inserted central catheters (PICCs).18-23
The purpose of this position statement is to present evidence-based recommendations on the use of ultrasound guidance for the insertion of central and peripheral vascular access catheters in adult patients. This document presents consensus-based recommendations with supporting evidence for clinical outcomes, techniques, and training for the use of ultrasound guidance for vascular access. We have subdivided the recommendations on techniques for central venous access, peripheral venous access, and arterial access individually, as some providers may not perform all types of vascular access procedures.
These recommendations are intended for hospitalists and other healthcare providers that routinely place central and peripheral vascular access catheters in acutely ill patients. However, this position statement does not mandate that all hospitalists should place central or peripheral vascular access catheters given the diverse array of hospitalist practice settings. For training and competency assessments, we recognize that some of these recommendations may not be feasible in resource-limited settings, such as rural hospitals, where equipment and staffing for assessments are not available. Recommendations and frameworks for initial and ongoing credentialing of hospitalists in ultrasound-guided bedside procedures have been previously published in an Society of Hospital Medicine (SHM) position statement titled, “Credentialing of Hospitalists in Ultrasound-Guided Bedside Procedures.”24
METHODS
Detailed methods are described in Appendix 1. The SHM Point-of-care Ultrasound (POCUS) Task Force was assembled to carry out this guideline development project under the direction of the SHM Board of Directors, Director of Education, and Education Committee. All expert panel members were physicians or advanced practice providers with expertise in POCUS. Expert panel members were divided into working group members, external peer reviewers, and a methodologist. All Task Force members were required to disclose any potential conflicts of interest (Appendix 2). The literature search was conducted in two independent phases. The first phase included literature searches conducted by the vascular access working group members themselves. Key clinical questions and draft recommendations were then prepared. A systematic literature search was conducted by a medical librarian based on the findings of the initial literature search and draft recommendations. The Medline, Embase, CINAHL, and Cochrane medical databases were searched from 1975 to December 2015 initially. Google Scholar was also searched without limiters. An updated search was conducted in November 2017. The literature search strings are included in Appendix 3. All article abstracts were initially screened for relevance by at least two members of the vascular access working group. Full-text versions of screened articles were reviewed, and articles on the use of ultrasound to guide vascular access were selected. The following article types were excluded: non-English language, nonhuman, age <18 years, meeting abstracts, meeting posters, narrative reviews, case reports, letters, and editorials. All relevant systematic reviews, meta-analyses, randomized controlled studies, and observational studies of ultrasound-guided vascular access were screened and selected (Appendix 3, Figure 1). All full-text articles were shared electronically among the working group members, and final article selection was based on working group consensus. Selected articles were incorporated into the draft recommendations.
These recommendations were developed using the Research and Development (RAND) Appropriateness Method that required panel judgment and consensus.14 The 28 voting members of the SHM POCUS Task Force reviewed and voted on the draft recommendations considering five transforming factors: (1) Problem priority and importance, (2) Level of quality of evidence, (3) Benefit/harm balance, (4) Benefit/burden balance, and (5) Certainty/concerns about PEAF (Preferences/Equity/Acceptability/Feasibility). Using an internet-based electronic data collection tool (REDCap™), panel members participated in two rounds of electronic voting, one in August 2018 and the other in October 2018 (Appendix 4). Voting on appropriateness was conducted using a nine-point Likert scale. The three zones of the nine-point Likert scale were inappropriate (1-3 points), uncertain (4-6 points), and appropriate (7-9 points). The degree of consensus was assessed using the RAND algorithm (Appendix 1, Figure 1 and Table 1). Establishing a recommendation required at least 70% agreement that a recommendation was “appropriate.” Disagreement was defined as >30% of panelists voting outside of the zone of the median. A strong recommendation required at least 80% of the votes within one integer of the median per the RAND rules.
Recommendations were classified as strong or weak/conditional based on preset rules defining the panel’s level of consensus, which determined the wording for each recommendation (Table 2). The final version of the consensus-based recommendations underwent internal and external review by members of the SHM POCUS Task Force, the SHM Education Committee, and the SHM Executive Committee. The SHM Executive Committee reviewed and approved this position statement prior to its publication in the Journal of Hospital Medicine.
RESULTS
Literature Search
A total of 5,563 references were pooled from an initial search performed by a certified medical librarian in December 2015 (4,668 citations) which was updated in November 2017 (791 citations), and from the personal bibliographies and searches (104 citations) performed by working group members. A total of 514 full-text articles were reviewed. The final selection included 192 articles that were abstracted into a data table and incorporated into the draft recommendations. See Appendix 3 for details of the literature search strategy.
Recommendations
Four domains (technique, clinical outcomes, training, and knowledge gaps) with 31 draft recommendations were generated based on a review of the literature. Selected references were abstracted and assigned to each draft recommendation. Rationales for each recommendation cite supporting evidence. After two rounds of panel voting, 31 recommendations achieved agreement based on the RAND rules. During the peer review process, two of the recommendations were merged with other recommendations. Thus, a total of 29 recommendations received final approval. The degree of consensus based on the median score and the dispersion of voting around the median are shown in Appendix 5. Twenty-seven statements were approved as strong recommendations, and two were approved as weak/conditional recommendations. The strength of each recommendation and degree of consensus are summarized in Table 3.
Terminology
Central Venous Catheterization
Central venous catheterization refers to insertion of tunneled or nontunneled large bore vascular catheters that are most commonly inserted into the internal jugular, subclavian, or femoral veins with the catheter tip located in a central vein. These vascular access catheters are synonymously referred to as central lines or central venous catheters (CVCs). Nontunneled catheters are designed for short-term use and should be removed promptly when no longer clinically indicated or after a maximum of 14 days.25
Peripherally Inserted Central Catheter (PICC)
Peripherally inserted central catheters, or PICC lines, are inserted most commonly in the basilic or brachial veins in adult patients, and the catheter tip terminates in the distal superior vena cava or cavo-atrial junction. These catheters are designed to remain in place for a duration of several weeks, as long as it is clinically indicated.
Midline Catheterization
Midline catheters are a type of peripheral venous catheter that are an intermediary between a peripheral intravenous catheter and PICC line. Midline catheters are most commonly inserted in the brachial or basilic veins, but unlike PICC lines, the tips of these catheters terminate in the axillary or subclavian vein. Midline catheters are typically 8 cm to 20 cm in length and inserted for a duration <30 days.
Peripheral Intravenous Catheterization
Peripheral intravenous lines (PIV) refer to small bore venous catheters that are most commonly 14G to 24G and inserted into patients for short-term peripheral venous access. Common sites of ultrasound-guided PIV insertion include the superficial and deep veins of the hand, forearm, and arm.
Arterial Catheterization
Arterial catheters are commonly used for reliable blood pressure monitoring, frequent arterial blood
RECOMMENDATIONS
Preprocedure
1. We recommend that providers should be familiar with the operation of their specific ultrasound machine prior to initiation of a vascular access procedure.
Rationale: There is strong consensus that providers must be familiar with the knobs and functions of the specific make and model of ultrasound machine that will be utilized for a vascular access procedure. Minimizing adjustments to the ultrasound machine during the procedure may reduce the risk of contaminating the sterile field.
2. We recommend that providers should use a high-frequency linear transducer with a sterile sheath and sterile gel to perform vascular access procedures.
Rationale: High-frequency linear-array transducers are recommended for the vast majority of vascular access procedures due to their superior resolution compared to other transducer types. Both central and peripheral vascular access procedures, including PIV, PICC, and arterial line placement, should be performed using sterile technique. A sterile transducer cover and sterile gel must be utilized, and providers must be trained in sterile preparation of the ultrasound transducer.13,26,27
The depth of femoral vessels correlates with body mass index (BMI). When accessing these vessels in a morbidly obese patient with a thigh circumference >60 cm and vessel depth >8 cm, a curvilinear transducer may be preferred for its deeper penetration.28 For patients who are poor candidates for bedside insertion of vascular access catheters, such as uncooperative patients, patients with atypical vascular anatomy or poorly visualized target vessels, we recommend consultation with a vascular access specialist prior to attempting the procedure.
3. We recommend that providers should use two-dimensional ultrasound to evaluate for anatomical variations and absence of vascular thrombosis during preprocedural site selection.
Rationale: A thorough ultrasound examination of the target vessel is warranted prior to catheter placement. Anatomical variations that may affect procedural decision-making are easily detected with ultrasound. A focused vascular ultrasound examination is particularly important in patients who have had temporary or tunneled venous catheters, which can cause stenosis or thrombosis of the target vein.
For internal jugular vein (IJV) CVCs, ultrasound is useful for visualizing the relationship between the IJV and common carotid artery (CCA), particularly in terms of vessel overlap. Furthermore, ultrasound allows for immediate revisualization upon changes in head position.29-32 Troianos et al. found >75% overlap of the IJV and CCA in 54% of all patients and in 64% of older patients (age >60 years) whose heads were rotated to the contralateral side.30 In one study of IJV CVC insertion, inadvertent carotid artery punctures were reduced (3% vs 10%) with the use of ultrasound guidance vs landmarks alone.33 In a cohort of 64 high-risk neurosurgical patients, cannulation success was 100% with the use of ultrasound guidance, and there were no injuries to the carotid artery, even though the procedure was performed with a 30-degree head elevation and anomalous IJV anatomy in 39% of patients.34 In a prospective, randomized controlled study of 1,332 patients, ultrasound-guided cannulation in a neutral position was demonstrated to be as safe as the 45-degree rotated position.35
Ultrasound allows for the recognition of anatomical variations which may influence the selection of the vascular access site or technique. Benter et al. found that 36% of patients showed anatomical variations in the IJV and surrounding tissue.36 Similarly Caridi showed the anatomy of the right IJV to be atypical in 29% of patients,37 and Brusasco found that 37% of bariatric patients had anatomical variations of the IJV.38 In a study of 58 patients, there was significant variability in the IJV position and IJV diameter, ranging from 0.5 cm to >2 cm.39 In a study of hemodialysis patients, 75% of patients had sonographic venous abnormalities that led to a change in venous access approach.40
To detect acute or chronic upper extremity deep venous thrombosis or stenosis, two-dimensional visualization with compression should be part of the ultrasound examination prior to central venous catheterization. In a study of patients that had undergone CVC insertion 9-19 weeks earlier, 50% of patients had an IJV thrombosis or stenosis leading to selection of an alternative site. In this study, use of ultrasound for a preprocedural site evaluation reduced unnecessary attempts at catheterizing an occluded vein.41 At least two other studies demonstrated an appreciable likelihood of thrombosis. In a study of bariatric patients, 8% of patients had asymptomatic thrombosis38 and in another study, 9% of patients being evaluated for hemodialysis catheter placement had asymptomatic IJV thrombosis.37
4. We recommend that providers should evaluate the target blood vessel size and depth during a preprocedural ultrasound evaluation.
Rationale: The size, depth, and anatomic location of central veins can vary considerably. These features are easily discernable using ultrasound. Contrary to traditional teaching, the IJV is located 1 cm anterolateral to the CCA in only about two-thirds of patients.37,39,42,43 Furthermore, the diameter of the IJV can vary significantly, ranging from 0.5 cm to >2 cm.39 The laterality of blood vessels may vary considerably as well. A preprocedural ultrasound evaluation of contralateral subclavian and axillary veins showed a significant absolute difference in cross-sectional area of 26.7 mm2 (P < .001).42
Blood vessels can also shift considerably when a patient is in the Trendelenburg position. In one study, the IJV diameter changed from 11.2 (± 1.5) mm to 15.4 (± 1.5) mm in the supine versus the Trendelenburg position at 15 degrees.33 An observational study demonstrated a frog-legged position with reverse Trendelenburg increased the femoral vein size and reduced the common surface area with the common femoral artery compared to a neutral position. Thus, a frog-legged position with reverse Trendelenburg position may be preferred, since overall catheterization success rates are higher in this position.44
Techniques
General Techniques
5. We recommend that providers should avoid using static ultrasound alone to mark the needle insertion site for vascular access procedures.
Rationale: The use of static ultrasound guidance to mark a needle insertion site is not recommended because normal anatomical relationships of vessels vary, and site marking can be inaccurate with minimal changes in patient position, especially of the neck.43,45,46 Benefits of using ultrasound guidance for vascular access are attained when ultrasound is used to track the needle tip in real-time as it is advanced toward the target vessel.
Although continuous-wave Doppler ultrasound without two-dimensional visualization was used in the past, it is no longer recommended for IJV CVC insertion.47 In a study that randomized patients to IJV CVC insertion with continuous-wave Doppler alone vs two-dimensional ultrasound guidance, the use of two-dimensional ultrasound guidance showed significant improvement in first-pass success rates (97% vs 91%, P = .045), particularly in patients with BMI >30 (97% vs 77%, P = .011).48
A randomized study comparing real-time ultrasound-guided, landmark-based, and ultrasound-marked techniques found higher success rates in the real-time ultrasound-guided group than the other two groups (100% vs 74% vs 73%, respectively; P = .01). The total number of mechanical complications was higher in the landmark-based and ultrasound-marked groups than in the real-time ultrasound-guided group (24% and 36% versus 0%, respectively; P = .01).49 Another randomized controlled study found higher success rates with real-time ultrasound guidance (98%) versus an ultrasound-marked (82%) or landmark-based (64%) approach for central line placement.50
6. We recommend that providers should use real-time (dynamic), two-dimensional ultrasound guidance with a high-frequency linear transducer for CVC insertion, regardless of the provider’s level of experience.
7. We suggest using either a transverse (short-axis) or longitudinal (long-axis) approach when performing real-time ultrasound-guided vascular access procedures.
Rationale: In clinical practice, the phrases transverse, short-axis, or out-of-plane approach are synonymous, as are longitudinal, long-axis, and in-plane approach. The short-axis approach involves tracking the needle tip as it approximates the target vessel with the ultrasound beam oriented in a transverse plane perpendicular to the target vessel. The target vessel is seen as a circular structure on the ultrasound screen as the needle tip approaches the target vessel from above. This approach is also called the out-of-plane technique since the needle passes through the ultrasound plane. The advantages of the short-axis approach include better visualization of adjacent vessels or nerves and the relative ease of skill acquisition for novice operators.9 When using the short-axis approach, extra care must be taken to track the needle tip from the point of insertion on the skin to the target vessel. A disadvantage of the short-axis approach is unintended posterior wall puncture of the target vessel.55
In contrast to a short-axis approach, a long-axis approach is performed with the ultrasound beam aligned parallel to the vessel. The vessel appears as a long tubular structure and the entire needle is visualized as it traverses across the ultrasound screen to approach the target vessel. The long-axis approach is also called an in-plane technique because the needle is maintained within the plane of the ultrasound beam. The advantage of a long-axis approach is the ability to visualize the entire needle as it is inserted into the vessel.14 A randomized crossover study with simulation models compared a long-axis versus short-axis approach for both IJV and subclavian vein catheterization. This study showed decreased number of needle redirections (relative risk (RR) 0.5, 95% confidence interval (CI) 0.3 to 0.7), and posterior wall penetrations (OR 0.3, 95% CI 0.1 to 0.9) using a long-axis versus short-axis approach for subclavian vein catheterization.56
A randomized controlled study comparing a long-axis or short-axis approach with ultrasound versus a landmark-based approach for IJV CVC insertion showed higher success rates (100% vs 90%; P < .001), lower insertion time (53 vs 116 seconds; P < .001), and fewer attempts to obtain access (2.5 vs 1.2 attempts, P < .001) with either the long- or short-axis ultrasound approach. The average time to obtain access and number of attempts were comparable between the short-axis and long-axis approaches with ultrasound. The incidence of carotid puncture and hematoma was significantly higher with the landmark-based approach versus either the long- or short-axis ultrasound approach (carotid puncture 17% vs 3%, P = .024; hematoma 23% vs 3%, P = .003).57
High success rates have been reported using a short-axis approach for insertion of PIV lines.58 A prospective, randomized trial compared the short-axis and long-axis approach in patients who had had ≥2 failed PIV insertion attempts. Success rate was 95% (95% CI, 0.85 to 1.00) in the short-axis group compared with 85% (95% CI, 0.69 to 1.00) in the long-axis group. All three subjects with failed PIV placement in the long-axis group had successful rescue placement using a short-axis approach. Furthermore, the short-axis approach was faster than the long-axis approach.59
For radial artery cannulation, limited data exist comparing the short- and long-axis approaches. A randomized controlled study compared a long-axis vs short-axis ultrasound approach for radial artery cannulation. Although the overall procedure success rate was 100% in both groups, the long-axis approach had higher first-pass success rates (1.27 ± 0.4 vs 1.5 ± 0.5, P < .05), shorter cannulation times (24 ± 17 vs 47 ± 34 seconds, P < .05), fewer hematomas (4% vs 43%, P < .05) and fewer posterior wall penetrations (20% vs 56%, P < .05).60
Another technique that has been described for IJV CVC insertion is an oblique-axis approach, a hybrid between the long- and short-axis approaches. In this approach, the transducer is aligned obliquely over the IJV and the needle is inserted using a long-axis or in-plane approach. A prospective randomized trial compared the short-axis, long-axis, and oblique-axis approaches during IJV cannulation. First-pass success rates were 70%, 52%, and 74% with the short-axis, long-axis, and oblique-axis approaches, respectively, and a statistically significant difference was found between the long- and oblique-axis approaches (P = .002). A higher rate of posterior wall puncture was observed with a short-axis approach (15%) compared with the oblique-axis (7%) and long-axis (4%) approaches (P = .047).61
8. We recommend that providers should visualize the needle tip and guidewire in the target vein prior to vessel dilatation.
Rationale: When real-time ultrasound guidance is used, visualization of the needle tip within the vein is the first step to confirm cannulation of the vein and not the artery. After the guidewire is advanced, the provider can use transverse and longitudinal views to reconfirm cannulation of the vein. In a longitudinal view, the guidewire is readily seen positioned within the vein, entering the anterior wall and lying along the posterior wall of the vein. Unintentional perforation of the posterior wall of the vein with entry into the underlying artery can be detected by ultrasound, allowing prompt removal of the needle and guidewire before proceeding with dilation of the vessel. In a prospective observational study that reviewed ultrasound-guided IJV CVC insertions, physicians were able to more readily visualize the guidewire than the needle in the vein.62 A prospective observational study determined that novice operators can visualize intravascular guidewires in simulation models with an overall accuracy of 97%.63
In a retrospective review of CVC insertions where the guidewire position was routinely confirmed in the target vessel prior to dilation, there were no cases of arterial dilation, suggesting confirmation of guidewire position can potentially eliminate the morbidity and mortality associated with arterial dilation during CVC insertion.64
9. To increase the success rate of ultrasound-guided vascular access procedures, we recommend that providers should utilize echogenic needles, plastic needle guides, and/or ultrasound beam steering when available.
Rationale: Echogenic needles have ridged tips that appear brighter on the screen, allowing for better visualization of the needle tip. Plastic needle guides help stabilize the needle alongside the transducer when using either a transverse or longitudinal approach. Although evidence is limited, some studies have reported higher procedural success rates when using echogenic needles, plastic needle guides, and ultrasound beam steering software. In a prospective observational study, Augustides et al. showed significantly higher IJV cannulation rates with versus without use of a needle guide after first (81% vs 69%, P = .0054) and second (93% vs 80%. P = .0001) needle passes.65 A randomized study by Maecken et al. compared subclavian vein CVC insertion with or without use of a needle guide, and found higher procedure success rates within the first and second attempts, reduced time to obtain access (16 seconds vs 30 seconds; P = .0001) and increased needle visibility (86% vs 32%; P < .0001) with the use of a needle guide.66 Another study comparing a short-axis versus long-axis approach with a needle guide showed improved needle visualization using a long-axis approach with a needle guide.67 A randomized study comparing use of a novel, sled-mounted needle guide to a free-hand approach for venous cannulation in simulation models showed the novel, sled-mounted needle guide improved overall success rates and efficiency of cannulation.68
Central Venous Access Techniques
10. We recommend that providers should use a standardized procedure checklist that includes use of real-time ultrasound guidance to reduce the risk of central line-associated bloodstream infection (CLABSI) from CVC insertion.
Rationale: A standardized checklist or protocol should be developed to ensure compliance with all recommendations for insertion of CVCs. Evidence-based protocols address periprocedural issues, such as indications for CVC, and procedural techniques, such as use of maximal sterile barrier precautions to reduce the risk of infection. Protocols and checklists that follow established guidelines for CVC insertion have been shown to decrease CLABSI rates.69,70 Similarly, development of checklists and protocols for maintenance of central venous catheters have been effective in reducing CLABSIs.71 Although no externally-validated checklist has been universally accepted or endorsed by national safety organizations, a few internally-validated checklists are available through peer-reviewed publications.72,73 An observational educational cohort of internal medicine residents who received training using simulation of the entire CVC insertion process was able to demonstrate fewer CLABSIs after the simulator-trained residents rotated in the intensive care unit (ICU) (0.50 vs 3.2 infections per 1,000 catheter days, P = .001).74
11. We recommend that providers should use real-time ultrasound guidance, combined with aseptic technique and maximal sterile barrier precautions, to reduce the incidence of infectious complications from CVC insertion.
Rationale: The use of real-time ultrasound guidance for CVC placement has demonstrated a statistically significant reduction in CLABSIs compared to landmark-based techniques.75 The Centers for Disease Control and Prevention (CDC) guidelines for the prevention of intravascular catheter-related infections recommend the use of ultrasound guidance to reduce the number of cannulation attempts and risk of mechanical complications.69 A prospective, three-arm study comparing ultrasound-guided long-axis, short-axis, and landmark-based approaches showed a CLABSI rate of 20% in the landmark-based group versus 10% in each of the ultrasound groups.57 Another randomized study comparing use of ultrasound guidance to a landmark-based technique for IJV CVC insertion demonstrated significantly lower CLABSI rates with the use of ultrasound (2% vs 10%; P < .05).72
Studies have shown that a systems-based intervention featuring a standardized catheter kit or catheter bundle significantly reduced CLABSI rates.76-78 A complete review of all preventive measures to reduce the risk of CLABSI is beyond the scope of this review, but a few key points will be mentioned. First, aseptic technique includes proper hand hygiene and skin sterilization, which are essential measures to reduce cutaneous colonization of the insertion site and reduce the risk of CLABSIs.79 In a systematic review and meta-analysis of eight studies including over 4,000 catheter insertions, skin antisepsis with chlorhexidine was associated with a 50% reduction in CLABSIs compared with povidone iodine.11 Therefore, a chlorhexidine-containing solution is recommended for skin preparation prior to CVC insertion per guidelines by Healthcare Infection Control Practices Advisory Committee/CDC, Society for Healthcare Epidemiology of America/Infectious Diseases Society of America, and American Society of Anesthesiologists.11,69,80,81 Second, maximal sterile barrier precautions refer to the use of sterile gowns, sterile gloves, caps, masks covering both the mouth and nose, and sterile full-body patient drapes. Use of maximal sterile barrier precautions during CVC insertion has been shown to reduce the incidence of CLABSIs compared to standard precautions.26,79,82-84 Third, catheters containing antimicrobial agents may be considered for hospital units with higher CLABSI rates than institutional goals, despite a comprehensive preventive strategy, and may be considered in specific patient populations at high risk of severe complications from a CLABSI.11,69,80 Finally, providers should use a standardized procedure set-up when inserting CVCs to reduce the risk of CLABSIs. The operator should confirm availability and proper functioning of ultrasound equipment prior to commencing a vascular access procedure. Use of all-inclusive procedure carts or kits with sterile ultrasound probe covers, sterile gel, catheter kits, and other necessary supplies is recommended to minimize interruptions during the procedure, and can ultimately reduce the risk of CLABSIs by ensuring maintenance of a sterile field during the procedure.13
12. We recommend that providers should use real-time ultrasound guidance for internal jugular vein catheterization, which reduces the risk of mechanical and infectious complications, the number of needle passes, and time to cannulation and increases overall procedure success rates.
Rationale: The use of real-time ultrasound guidance for CVC insertion has repeatedly demonstrated better outcomes compared to a landmark-based approach in adults.13 Several randomized controlled studies have demonstrated that real-time ultrasound guidance for IJV cannulation reduces the risk of procedure-related mechanical and infectious complications, and improves first-pass and overall success rates in diverse care settings.27,29,45,50,53,65,75,85-90 Mechanical complications that are reduced with ultrasound guidance include pneumothorax and carotid artery puncture.4,5,45,46,53,62,75,86-93 Currently, several medical societies strongly recommend the use of ultrasound guidance during insertion of IJV CVCs.10-12,14,94-96
A meta-analysis by Hind et al. that included 18 randomized controlled studies demonstrated use of real-time ultrasound guidance reduced failure rates (RR 0.14, 95% CI 0.06 to 0.33; P < .0001), increased first-attempt success rates (RR 0.59, 95% CI 0.39 to 0.88; P = .009), reduced complication rates (RR 0.43, 95% CI 0.22 to 0.87; P = .02) and reduced procedure time (P < .0001), compared to a traditional landmark-based approach when inserting IJV CVCs.5
A Cochrane systematic review compared ultrasound-guided versus landmark-based approaches for IJV CVC insertion and found use of real-time ultrasound guidance reduced total complication rates by 71% (RR 0.29, 95% CI 0.17 to 0.52; P < .0001), arterial puncture rates by 72% (RR 0.28, 95% CI 0.18 to 0.44; P < .00001), and rates of hematoma formation by 73% (RR 0.27, 95% CI 0.13 to 0.55; P = .0004). Furthermore, the number of attempts for successful cannulation was reduced (mean difference -1.19 attempts, 95% CI -1.45 to -0.92; P < .00001), the chance of successful insertion on the first attempt was increased by 57% (RR 1.57, 95% CI 1.36 to 1.82; P < .00001), and overall procedure success rates were modestly increased in all groups by 12% (RR 1.12, 95% CI 1.08 to 1.17; P < .00001).46
An important consideration in performing ultrasound guidance is provider experience. A prospective observational study of patients undergoing elective CVC insertion demonstrated higher complication rates for operators that were inexperienced (25.2%) versus experienced (13.6%).54 A randomized controlled study comparing experts and novices with or without the use of ultrasound guidance for IJV CVC insertion demonstrated higher success rates among expert operators and with the use of ultrasound guidance. Among novice operators, the complication rates were lower with the use of ultrasound guidance.97 One study evaluated the procedural success and complication rates of a two-physician technique with one physician manipulating the transducer and another inserting the needle for IJV CVC insertion. This study concluded that procedural success rates and frequency of complications were directly affected by the experience of the physician manipulating the transducer and not by the experience of the physician inserting the needle.98
The impact of ultrasound guidance on improving procedural success rates and reducing complication rates is greatest in patients that are obese, short necked, hypovolemic, or uncooperative.93 Several studies have demonstrated fewer needle passes and decreased time to cannulation compared to the landmark technique in these populations.46,49,53,86-88,92,93
Ultrasound-guided placement of IJV catheters can safely be performed in patients with disorders of hemostasis and those with multiple previous catheter insertions in the same vein.9 Ultrasound-guided placement of CVCs in patients with disorders of hemostasis is safe with high success and low complication rates. In a case series of liver patients with coagulopathy (mean INR 2.17 ± 1.16, median platelet count 150K), the use of ultrasound guidance for CVC insertion was highly successful with no major bleeding complications.99
A study of renal failure patients found high success rates and low complication rates in the patients with a history of multiple previous catheterizations, poor compliance, skeletal deformities, previous failed cannulations, morbid obesity, and disorders of hemostasis.100 A prospective observational study of 200 ultrasound-guided CVC insertions for apheresis showed a 100% success rate with a 92% first-pass success rate.101
The use of real-time ultrasound guidance for IJV CVC insertion has been shown to be cost effective by reducing procedure-related mechanical complications and improving procedural success rates. A companion cost-effectiveness analysis estimated that for every 1,000 patients, 90 complications would be avoided, with a net cost savings of approximately $3,200 using 2002 prices.102
13. We recommend that providers who routinely insert subclavian vein CVCs should use real-time ultrasound guidance, which has been shown to reduce the risk of mechanical complications and number of needle passes and increase overall procedure success rates compared with landmark-based techniques.
Rationale: In clinical practice, the term ultrasound-guided subclavian vein CVC insertion is commonly used. However, the needle insertion site is often lateral to the first rib and providers are technically inserting the CVC in the axillary vein. The subclavian vein becomes the axillary vein at the lateral border of the first rib where the cephalic vein branches from the subclavian vein. To be consistent with common medical parlance, we use the phrase ultrasound-guided subclavian vein CVC insertion in this document.
Advantages of inserting CVCs in the subclavian vein include reliable surface anatomical landmarks for vein location, patient comfort, and lower risk of infection.103 Several observational studies have demonstrated the technique for ultrasound-guided subclavian vein CVC insertion is feasible and safe.104-107 In a large retrospective observational study of ultrasound-guided central venous access among a complex patient group, the majority of patients were cannulated successfully and safely. The subset of patients undergoing axillary vein CVC insertion (n = 1,923) demonstrated a low rate of complications (0.7%), proving it is a safe and effective alternative to the IJV CVC insertion.107
A Cochrane review of ultrasound-guided subclavian vein cannulation (nine studies, 2,030 participants, 2,049 procedures), demonstrated that real-time two-dimensional ultrasound guidance reduced the risk of inadvertent arterial punctures (three studies, 498 participants, RR 0.21, 95% CI 0.06 to 0.82; P = .02) and hematoma formation (three studies, 498 participants, RR 0.26, 95% CI 0.09 to 0.76; P = .01).46 A systematic review and meta-analysis of 10 randomized controlled studies comparing ultrasound-guided versus landmark-based subclavian vein CVC insertion demonstrated a reduction in the risk of arterial punctures, hematoma formation, pneumothorax, and failed catheterization with the use of ultrasound guidance.105
A randomized controlled study comparing ultrasound-guided vs landmark-based approaches to subclavian vein cannulation found that use of ultrasound guidance had a higher success rate (92% vs 44%, P = .0003), fewer minor complications (1 vs 11, P = .002), fewer attempts (1.4 vs 2.5, P = .007) and fewer catheter kits used (1.0 vs 1.4, P = .0003) per cannulation.108
Fragou et al. randomized patients undergoing subclavian vein CVC insertion to a long-axis approach versus a landmark-based approach and found a significantly higher success rate (100% vs 87.5%, P < .05) and lower rates of mechanical complications: artery puncture (0.5% vs 5.4%), hematoma (1.5% vs 5.4%), hemothorax (0% vs 4.4%), pneumothorax (0% vs 4.9%), brachial plexus injury (0% vs 2.9%), phrenic nerve injury (0% vs 1.5%), and cardiac tamponade (0% vs 0.5%).109 The average time to obtain access and the average number of insertion attempts (1.1 ± 0.3 vs 1.9 ± 0.7, P < .05) were significantly reduced in the ultrasound group compared to the landmark-based group.95
A retrospective review of subclavian vein CVC insertions using a supraclavicular approach found no reported complications with the use of ultrasound guidance vs 23 mechanical complications (8 pneumothorax, 15 arterial punctures) with a landmark-based approach.106 However, it is important to note that a supraclavicular approach is not commonly used in clinical practice.
14. We recommend that providers should use real-time ultrasound guidance for femoral venous access, which has been shown to reduce the risk of arterial punctures and total procedure time and increase overall procedure success rates.
Rationale: Anatomy of the femoral region varies, and close proximity or overlap of the femoral vein and artery is common.51 Early studies showed that ultrasound guidance for femoral vein CVC insertion reduced arterial punctures compared with a landmark-based approach (7% vs 16%), reduced total procedure time (55 ± 19 vs 79 ± 62 seconds), and increased procedure success rates (100% vs 90%).52 A Cochrane review that pooled data from four randomized studies comparing ultrasound-guided vs landmark-based femoral vein CVC insertion found higher first-attempt success rates with the use of ultrasound guidance (RR 1.73, 95% CI 1.34 to 2.22; P < .0001) and a small increase in the overall procedure success rates (RR 1.11, 95% CI 1.00 to 1.23; P = .06). There was no difference in inadvertent arterial punctures or other complications.110
Peripheral Venous Access Techniques
15. We recommend that providers should use real-time ultrasound guidance for the insertion of peripherally inserted central catheters (PICCs), which is associated with higher procedure success rates and may be more cost effective compared with landmark-based techniques.
Rationale: Several studies have demonstrated that providers who use ultrasound guidance vs landmarks for PICC insertion have higher procedural success rates, lower complication rates, and lower total placement costs. A prospective observational report of 350 PICC insertions using ultrasound guidance reported a 99% success rate with an average of 1.2 punctures per insertion and lower total costs.20 A retrospective observational study of 500 PICC insertions by designated specialty nurses revealed an overall success rate of 95%, no evidence of phlebitis, and only one CLABSI among the catheters removed.21 A retrospective observational study comparing several PICC variables found higher success rates (99% vs 77%) and lower thrombosis rates (2% vs 9%) using ultrasound guidance vs landmarks alone.22 A study by Robinson et al. demonstrated that having a dedicated PICC team equipped with ultrasound increased their institutional insertion success rates from 73% to 94%.111
A randomized controlled study comparing ultrasound-guided versus landmark-based PICC insertion found high success rates with both techniques (100% vs 96%). However, there was a reduction in the rate of unplanned catheter removals (4.0% vs 18.7%; P = .02), mechanical phlebitis (0% vs 22.9%; P < .001), and venous thrombosis (0% vs 8.3%; P = .037), but a higher rate of catheter migration (32% vs 2.1%; P < .001). Compared with the landmark-based group, the ultrasound-guided group had significantly lower incidence of severe contact dermatitis (P = .038), and improved comfort and costs up to 3 months after PICC placement (P < .05).112
Routine postprocedure chest x-ray (CXR) is generally considered unnecessary if the PICC is inserted with real-time ultrasound guidance along with use of a newer tracking devices, like the magnetic navigation system with intracardiac electrodes.9 Ultrasound can also be used to detect malpositioning of a PICC immediately after completing the procedure. A randomized controlled study comparing ultrasound versus postprocedure CXR detected one malpositioned PICC in the ultrasound group versus 11 in the control group. This study suggested that ultrasound can detect malpositioning immediately postprocedure and reduce the need for a CXR and the possibility of an additional procedure to reposition a catheter.113
16. We recommend that providers should use real-time ultrasound guidance for the placement of peripheral intravenous lines (PIV) in patients with difficult peripheral venous access to reduce the total procedure time, needle insertion attempts, and needle redirections. Ultrasound-guided PIV insertion is also an effective alternative to CVC insertion in patients with difficult venous access.
Rationale: Difficult venous access refers to patients that have had two unsuccessful attempts at PIV insertion using landmarks or a history of difficult access (i.e. edema, obesity, intravenous drug use, chemotherapy, diabetes, hypovolemia, chronic illness, vasculopathy, multiple prior hospitalizations). A meta-analysis of seven randomized controlled studies concluded that ultrasound guidance increases the likelihood of successful PIV insertion (pooled OR 2.42, 95% CI 1.26 to 4.68; P < .008).18 A second meta-analysis that pooled data from seven studies (six randomized controlled studies) confirmed that ultrasound guidance improves success rates of PIV insertion (OR 3.96, 95% CI 1.75 to 8.94).19 Approximately half of these studies had physician operators while the other half had nurse operators.
In one prospective observational study of emergency department patients with two failed attempts of landmark-based PIV insertion, ultrasound guidance with a modified-Seldinger technique showed a relatively high success rate (96%), fewer needle sticks (mean 1.32 sticks, 95% CI 1.12 to 1.52), and shorter time to obtain access (median time 68 seconds).114 Other prospective observational studies have demonstrated that ultrasound guidance for PIV insertion has a high success rate (87%),115 particularly with brachial or basilic veins PIV insertion, among patients with difficult PIV access, defined as having had ≥2 failed attempts.58
Since insertion of PIVs with ultrasound guidance has a high success rate, there is potential to reduce the reliance on CVC insertion for venous access only. In a study of patients that had had two failed attempts at PIV insertion based on landmarks, a PIV was successfully inserted with ultrasound guidance in 84% of patients, obviating the need for CVC placement for venous access.116 A prospective observational study showed ultrasound-guided PIV insertion was an effective alternative to CVC placement in ED patients with difficult venous access with only 1% of patients requiring a CVC.117 Use of ultrasound by nurses for PIV placement has also been shown to reduce the time to obtain venous access, improve patient satisfaction, and reduce the need for physician intervention.118 In a prospective observational study of patients with difficult access, the majority of patients reported a better experience with ultrasound-guided PIV insertion compared to previous landmark-based attempts with an average satisfaction score of 9.2/10 with 76% of patients rating the experience a 10.119 A strong recommendation has been made for use of ultrasound guidance in patients with difficult PIV placement by la Société Française d’Anesthésie et de Réanimation (The French Society of Anesthesia and Resuscitation).95
17. We suggest using real-time ultrasound guidance to reduce the risk of vascular, infectious, and neurological complications during PIV insertion, particularly in patients with difficult venous access.
Rationale: The incidence of complications from PIV insertion is often underestimated. Vascular complications include arterial puncture, hematoma formation, local infiltration or extravasation of fluid, and superficial or deep venous thrombosis. The most common infectious complications with PIV insertion are phlebitis and cellulitis.120 One observational study reported PIV complications occurring in approximately half of all patients with the most common complications being phlebitis, hematoma formation, and fluid/blood leakage.121
A retrospective review of ICU patients who underwent ultrasound-guided PIV insertion by a single physician showed high success rates (99%) with low rates of phlebitis/cellulitis (0.7%).There was an assumed benefit of risk reduction due to the patients no longer requiring a CVC after successful PIV placement.122 Another study found very low rates of infection with both landmark-based and ultrasound-guided PIV placement performed by emergency department nurses, suggesting that there is no increased risk of infection with the use of ultrasound.123 To reduce the risk of infection from PIV insertion, we recommend the use of sterile gel and sterile transducer cover (See Recommendation 2).
Arterial Access Techniques
18. We recommend that providers should use real-time ultrasound guidance for arterial access, which has been shown to increase first-pass success rates, reduce the time to cannulation, and reduce the risk of hematoma development compared with landmark-based techniques.
Rationale: Several randomized controlled studies have assessed the value of ultrasound in arterial catheter insertion. Shiver et al. randomized 60 patients admitted to a tertiary center emergency department to either palpation or ultrasound-guided arterial cannulation. They demonstrated a first-pass success rate of 87% in the ultrasound group compared with 50% in the landmark technique group. In the same study, the use of ultrasound was also associated with reduced time needed to establish arterial access and a 43% reduction in the development of hematoma at the insertion site.124 Levin et al. demonstrated a first-pass success rate of 62% using ultrasound versus 34% by palpation alone in 69 patients requiring intraoperative invasive hemodynamic monitoring.125 Additional randomized controlled studies have demonstrated that ultrasound guidance increases first-attempt success rates compared to traditional palpation.23,126,127
19. We recommend that providers should use real-time ultrasound guidance for femoral arterial access, which has been shown to increase first-pass success rates and reduce the risk of vascular complications.
Rationale: Although it is a less frequently used site, the femoral artery may be accessed for arterial blood sampling or invasive hemodynamic monitoring, and use of ultrasound guidance has been shown to improve the first-pass success rates of femoral artery cannulation. It is important to note that most of these studies comparing ultrasound-guided vs landmark-based femoral artery cannulation were performed in patients undergoing diagnostic or interventional vascular procedures.
A meta-analysis of randomized controlled studies comparing ultrasound-guided vs landmark-based femoral artery catheterization found use of ultrasound guidance was associated with a 49% reduction in overall complications (RR 0.51, 95% CI 0.28 to 0.91; P > .05) and 42% improvement in first-pass success rates.128 In another study, precise site selection with ultrasound was associated with fewer pseudoaneurysms in patients undergoing femoral artery cannulation by ultrasound guidance vs palpation for cardiac catheterization (3% vs 5%, P < .05).129
A multicenter randomized controlled study comparing ultrasound vs fluoroscopic guidance for femoral artery catheterization demonstrated ultrasound guidance improved rates of common femoral artery (CFA) cannulation in patients with high CFA bifurcations (83% vs 70%, P < .01).130 Furthermore, ultrasound guidance improved first-pass success rates (83% vs 46%, P < .0001), reduced number of attempts (1.3 vs 3.0, P < .0001), reduced risk of venipuncture (2.4% vs 15.8%, P < .0001), and reduced median time to obtain access (136 seconds vs148 seconds, P = .003). Vascular complications occurred in fewer patients in the ultrasound vs fluoroscopy groups (1.4% vs 3.4% P = .04). Reduced risk of hematoma formation with routine use of ultrasound guidance was demonstrated in one retrospective observational study (RR 0.62, 95% CI 0.46 to 0.84; P < .01).131
20. We recommend that providers should use real-time ultrasound guidance for radial arterial access, which has been shown to increase first-pass success rates, reduce the time to successful cannulation, and reduce the risk of complications compared with landmark-based techniques.
Rationale: Ultrasound guidance is particularly useful for radial artery cannulation in patients with altered anatomy, obesity, nonpulsatile blood flow, low perfusion, and previously unsuccessful cannulation attempts using a landmark-guided approach.132
A multicenter randomized controlled study that was not included in the abovementioned meta-analyses showed similar benefits of using ultrasound guidance vs landmarks for radial artery catheterization: a reduction in the number of attempts with ultrasound guidance (1.65 ± 1.2 vs 3.05 ± 3.4, P < .0001) and time to obtain access (88 ± 78 vs 108 ± 112 seconds, P = .006), and increased first-pass success rates (65% vs 44%, P < .0001). The use of ultrasound guidance was found to be particularly useful in patients with difficult access by palpation alone.135
Regarding the level of expertise required to use ultrasound guidance, a prospective observational study demonstrated that physicians with little previous ultrasound experience were able to improve their first-attempt success rates and procedure time for radial artery cannulation compared to historical data of landmark-based insertions.136
Postprocedure
21. We recommend that post-procedure pneumothorax should be ruled out by the detection of bilateral lung sliding using a high-frequency linear transducer before and after insertion of internal jugular and subclavian vein CVCs.
Rationale: Detection of lung sliding with two-dimensional ultrasound rules out pneumothorax, and disappearance of lung sliding in an area where it was previously seen is a strong predictor of postprocedure pneumothorax. In a study of critically ill patients, the disappearance of lung sliding was observed in 100% of patients with pneumothorax vs 8.8% of patients without pneumothorax. For detection of pneumothorax, lung sliding showed a sensitivity of 95%, specificity of 91%, and negative predictive value of 100% (P < .001).137 Another study by the same author showed that the combination of horizontal artifacts (absence of comet-tail artifact) and absence of lung sliding had a sensitivity of 100%, specificity of 96.5%, and negative predictive value of 100% for the detection of pneumothorax.138 A meta-analysis of 10 studies on the diagnostic accuracy of CVC confirmation with bedside ultrasound vs chest radiography reported detection of all 12 pneumothoraces with ultrasound, whereas chest radiography missed two pneumothoraces. The pooled sensitivity and specificity of ultrasound for the detection of pneumothorax was 100%, although an imperfect gold standard bias likely affected the results. An important advantage of bedside ultrasound is the ability to rule out pneumothorax immediately after the procedure while at the bedside. The mean time for confirmation of CVC placement with bedside ultrasound was 6 minutes versus 64 minutes and 143 minutes for completion and interpretation of a chest radiograph, respectively.139
22. We recommend that providers should use ultrasound with rapid infusion of agitated saline to visualize a right atrial swirl sign (RASS) for detecting catheter tip misplacement during CVC insertion. The use of RASS to detect the catheter tip may be considered an advanced skill that requires specific training and expertise.
Rationale: Bedside echocardiography is a reliable tool to detect catheter tip misplacement during CVC insertion. In one study, catheter misplacement was detected by bedside echocardiography with a sensitivity of 96% and specificity of 83% (positive predictive value 98%, negative predictive value 55%) and prevented distal positioning of the catheter tip.140 A prospective observational study assessed for RASS, which is turbulent flow in the right atrium after a rapid saline flush of the distal CVC port, to exclude catheter malposition. In this study with 135 CVC placements, visualization of RASS with ultrasound was able to identify all correct CVC placements and three of four catheter misplacements. Median times to complete the ultrasound exam vs CXR were 1 vs 20 minutes, respectively, with a median difference of 24 minutes (95% CI 19.6 to 29.3, P < .0001) between the two techniques.141
A prospective observational study assessed the ability of bedside transthoracic echocardiography to detect the guidewire, microbubbles, or both, in the right atrium compared to transesophageal echocardiography as the gold standard. Bedside transthoracic echocardiography allowed visualization of the right atrium in 94% of patients, and both microbubbles plus guidewire in 91% of patients.142 Hence, bedside transthoracic echocardiography allows adequate visualization of the right atrium. Another prospective observational study combining ultrasonography and contrast enhanced RASS resulted in 96% sensitivity and 93% specificity for the detection of a misplaced catheter, and the concordance with chest radiography was 96%.143
Training
23. To reduce the risk of mechanical and infectious complications, we recommend that novice providers should complete a systematic training program that includes a combination of simulation-based practice, supervised insertion on patients, and evaluation by an expert operator before attempting ultrasound-guided CVC insertion independently on patients.
Rationale: Cumulative experience has been recognized to not be a proxy for mastery of a clinical skill.144 The National Institute for Clinical Excellence (NICE) has recommended that providers performing ultrasound-guided CVC insertion should receive appropriate training to achieve competence before performing the procedure independently.7 Surveys have demonstrated that lack of training is a commonly reported barrier for not using ultrasound.145,146
Structured training programs on CVC insertion have been shown to reduce the occurrence of infectious and mechanical complications.74,143,147-149 The use of ultrasound and checklists, bundling of supplies, and practice with simulation models, as a part of a structured training program, can improve patient safety related to CVC insertion.9,140,150-154
Simulation-based practice has been used in medical education to provide deliberate practice and foster skill development in a controlled learning environment.155-158 Studies have shown transfer of skills demonstrated in a simulated environment to clinical practice, which can improve CVC insertion practices.159,160 Simulation accelerates learning of all trainees, especially novice trainees, and mitigates risks to patients by allowing trainees to achieve a minimal level of competence before attempting the procedure on real patients.152,161,162 Residents that have been trained using simulation preferentially select the IJV site,147 and more reliably use ultrasound to guide their CVC insertions.160,163
Additionally, simulation-based practice allows exposure to procedures and scenarios that may occur infrequently in clinical practice.
Although there is evidence on efficacy of simulation-based CVC training programs, there is no broadly accepted consensus on timing, duration, and content of CVC training programs for trainees or physicians in practice. The minimum recommended technical skills a trainee must master include the ability to (1) manipulate the ultrasound machine to produce a high-quality image to identify the target vessel, (2) advance the needle under direct visualization to the desired target site and depth, (3) deploy the catheter into the target vessel and confirm catheter placement in the target vessel using ultrasound, and (4) ensure the catheter has not been inadvertently placed in an unintended vessel or structure.153
A variety of simulation models are currently used to practice CVC insertion at the most common sites: the internal jugular, subclavian, basilic, and brachial veins.164,165 Effective simulation models should contain vessels that mimic normal anatomy with muscles, soft tissues, and bones. Animal tissue models, such as turkey or chicken breasts, may be effective for simulated practice of ultrasound-guided CVC insertion.166,167 Ultrasound-guided CVC training using human cadavers has also been shown to be effective.168
24. We recommend that cognitive training in ultrasound-guided CVC insertion should include basic anatomy, ultrasound physics, ultrasound machine knobology, fundamentals of image acquisition and interpretation, detection and management of procedural complications, infection prevention strategies, and pathways to attain competency.
Rationale: After receiving training in ultrasound-guided CVC insertion, physicians report significantly higher comfort with the use of ultrasound compared to those who have not received such training.145 Learners find training sessions worthwhile to increase skill levels,167 and skills learned from simulation-based mastery learning programs have been retained up to one year.158
Several commonalities have been noted across training curricula. Anatomy and physiology didactics should include vessel anatomy (location, size, and course);9 vessel differentiation by ultrasound;9,69 blood flow dynamics;69 Virchow’s triad;69 skin integrity and colonization;150 peripheral nerve identification and distribution;9 respiratory anatomy;9,69 upper and lower extremity, axillary, neck, and chest anatomy.9,69 Vascular anatomy is an essential curricular component that may help avoid preventable CVC insertion complications, such as inadvertent nerve, artery, or lung puncture.150,169 Training curricula should also include ultrasound physics (piezoelectric effect, frequency, resolution, attenuation, echogenicity, Doppler ultrasound, arterial and venous flow characteristics), image acquisition and optimization (imaging mode, focus, dynamic range, probe types), and artifacts (reverberation, mirror, shadowing, enhancement).
CVC-related infections are an important cause of morbidity and mortality in the acute and long-term care environment.69 Infection and thrombosis can both be impacted by the insertion site selection, skin integrity, and catheter–vein ratio.2,3,84 Inexperience generally leads to more insertion attempts that can increase trauma during CVC insertion and potentially increase the risk of infections.170 To reduce the risk of infectious complications, training should include important factors to consider in site selection and maintenance of a sterile environment during CVC insertion, including use of maximal sterile barrier precautions, hand hygiene, and appropriate use of skin antiseptic solutions.
Professional society guidelines have been published with recommendations of appropriate techniques for ultrasound-guided vascular access that include training recommendations.9,154 Training should deconstruct the insertion procedure into readily understood individual steps, and can be aided by demonstration of CVC insertion techniques using video clips. An alternative to face-to-face training is internet-based training that has been shown to be as effective as traditional teaching methods in some medical centers.171 Additional methods to deliver cognitive instruction include textbooks, continuing medical education courses, and digital videos.164,172
25. We recommend that trainees should demonstrate minimal competence before placing ultrasound-guided CVCs independently. A minimum number of CVC insertions may inform this determination, but a proctored assessment of competence is most important.
Rationale: CVC catheter placement carries the risk of serious complications including arterial injury or dissection, pneumothorax, or damage to other local structures; arrhythmias; catheter malposition; infection; and thrombosis. Although there is a lack of consensus and high-quality evidence for the certification of skills to perform ultrasound-guided CVC insertion, recommendations have been published advocating for formal and comprehensive training programs in ultrasound-guided CVC insertion with an emphasis on expert supervision prior to independent practice.9,153,154 Two groups of expert operators have recommended that training should include at least 8-10 supervised ultrasound-guided CVC insertions.154,173,174 A consensus task force from the World Congress of Vascular Access has recommended a minimum of six to eight hours of didactic education, four hours of hands-on training on simulation models, and six hours of hands-on ultrasound training on human volunteers to assess normal anatomy.175 This training should be followed by supervised ultrasound-guided CVC insertions until the learner has demonstrated minimal competence with a low rate of complications.35 There is general consensus that arbitrary numbers should not be the sole determinant of competence, and that the most important determinant of competence should be an evaluation by an expert operator.176
26. We recommend that didactic and hands-on training for trainees should coincide with anticipated times of increased performance of vascular access procedures. Refresher training sessions should be offered periodically.
Rationale: Simulation-based CVC training courses have shown a rapid improvement in skills, but lack of practice leads to deterioration of technical skills.161,162,177,178 Thus, a single immersive training session is insufficient to achieve and maintain mastery of skills, and an important factor to acquire technical expertise is sustained, deliberate practice with feedback.179 Furthermore, an insidious decay in skills may go unrecognized as a learner’s comfort and self-confidence does not always correlate with actual performance, leading to increased risk of errors and potential for procedural complications.147,158,180-183 Given the decay in technical skills over time, simulation-based training sessions are most effective when they occur in close temporal proximity to times when those skills are most likely to be used; for example, a simulation-based training session for trainees may be most effective just before the start of a critical care rotation.152 Regularly scheduled training sessions with monitoring and feedback by expert operators can reinforce procedural skills and prevent decay. Some experts have recommended that a minimum of 10 ultrasound-guided CVC insertions should be performed annually to maintain proficiency.153
27. We recommend that competency assessments should include formal evaluation of knowledge and technical skills using standardized assessment tools.
Rationale: Hospitalists and other healthcare providers that place vascular access catheters should undergo competency assessments proctored by an expert operator to verify that they have the required knowledge and skills.184,185 Knowledge competence can be partially evaluated using a written assessment, such as a multiple-choice test, assessing the provider’s cognitive understanding of the procedure.175 For ultrasound-guided CVC insertion, a written examination should be administered in conjunction with an ultrasound image assessment to test the learner’s recognition of normal vs abnormal vascular anatomy. Minimum passing standards should be established a priori according to local or institutional standards.
The final skills assessment should be objective, and the learner should be required to pass all critical steps of the procedure. Failure of the final skills assessment should lead to continued practice with supervision until the learner can consistently demonstrate correct performance of all critical steps. Checklists are commonly used to rate the technical performance of learners because they provide objective criteria for evaluation, can identify specific skill deficiencies, and can determine a learner’s readiness to perform procedures independently.186,187 The administration of skills assessments and feedback methods should be standardized across faculty. Although passing scores on both knowledge and skills assessments do not guarantee safe performance of a procedure independently, they provide a metric to ensure that a minimum level of competence has been achieved before allowing learners to perform procedures on patients without supervision.188
Competency assessments are a recommended component of intramural and extramural certification of skills in ultrasound-guided procedures. Intramural certification pathways differ by institution and often require additional resources including ultrasound machine(s), simulation equipment, and staff time, particularly when simulation-based assessments are incorporated into certification pathways. We recognize that some of these recommendations may not be feasible in resource-limited settings, such as rural hospitals. However, initial and ongoing competency assessments can be performed during routine performance of procedures on patients. For an in-depth review of credentialing pathways for ultrasound-guided bedside procedures, we recommend reviewing the SHM Position Statement on Credentialing of Hospitalists in Ultrasound-Guided Bedside Procedures.24
28. We recommend that competency assessments should evaluate for proficiency in the following knowledge and skills of CVC insertion:
a. Knowledge of the target vein anatomy, proper vessel identification, and recognition of anatomical variants
b. Demonstration of CVC insertion with no technical errors based on a procedural checklist
c. Recognition and management of acute complications, including emergency management of life-threatening complications
d. Real-time needle tip tracking with ultrasound and cannulation on the first attempt in at least five consecutive simulations.
Rationale: Recommendations have been published with the minimal knowledge and skills learners must demonstrate to perform ultrasound-guided vascular access procedures. These include operation of an ultrasound machine to produce high-quality images of the target vessel, tracking of the needle tip with real-time ultrasound guidance, and recognition and understanding of the management of procedural complications.154,175
First, learners must be able to perform a preprocedural assessment of the target vein, including size and patency of the vein; recognition of adjacent critical structures; and recognition of normal anatomical variants.175,189 Second, learners must be able to demonstrate proficiency in tracking the needle tip penetrating the target vessel, inserting the catheter into the target vessel, and confirming catheter placement in the target vessel with ultrasound.154,175 Third, learners must be able to demonstrate recognition of acute complications, including arterial puncture, hematoma formation, and development of pneumothorax.154,175 Trainees should be familiar with recommended evaluation and management algorithms, including indications for emergent consultation.190
29. We recommend a periodic proficiency assessments of all operators should be conducted to ensure maintenance of competency.
Rationale: Competency extends to periodic assessment and not merely an initial evaluation at the time of training.191 Periodic competency assessments should include assessment of proficiency of all providers that perform a procedure, including instructors and supervisors. Supervising providers should maintain their competency in CVC insertion through routine use of their skills in clinical practice.175 An observational study of emergency medicine residents revealed that lack of faculty comfort with ultrasound hindered the residents’ use of ultrasound.192 Thus, there is a need to examine best practices for procedural supervision of trainees because providers are often supervising procedures that they are not comfortable performing on their own.193
KNOWLEDGE GAPS
The process of producing this position statement revealed areas of uncertainty and important gaps in the literature regarding the use of ultrasound guidance for central and peripheral venous access and arterial access.
This position statement recommends a preprocedural ultrasound evaluation of blood vessels based on evidence that providers may detect anatomic anomalies, thrombosis, or vessel stenosis. Ultrasound can also reveal unsuspected high-risk structures in near proximity to the procedure site. Although previous studies have shown that providers can accurately assess vessels with ultrasound for these features, further study is needed to evaluate the effect of a standardized preprocedural ultrasound exam on clinical and procedural decision-making, as well as procedural outcomes.
Second, two ultrasound applications that are being increasingly used but have not been widely implemented are the use of ultrasound to evaluate lung sliding postprocedure to exclude pneumothorax and the verification of central line placement using a rapid infusion of agitated saline to visualize the RASS.139-141 Both of these applications have the potential to expedite postprocedure clearance of central lines for usage and decrease patient radiation exposure by obviating the need for postprocedure CXRs. Despite the supporting evidence, both of these applications are not yet widely used, as few providers have been trained in these techniques which may be considered advanced skills.
Third, despite advances in our knowledge of effective training for vascular access procedures, there is limited agreement on how to define procedural competence. Notable advancements in training include improved understanding of systematic training programs, development of techniques for proctoring procedures, definition of elements for hands-on assessments, and definition of minimum experience needed to perform vascular access procedures independently. However, application of these concepts to move learners toward independent practice remains variably interpreted at different institutions, likely due to limited resources, engrained cultures about procedures, and a lack of national standards. The development of hospitalist-based procedure services at major academic medical centers with high training standards, close monitoring for quality assurance, and the use of databases to track clinical outcomes may advance our understanding and delivery of optimal procedural training.
Finally, ultrasound technology is rapidly evolving which will affect training, techniques, and clinical outcomes in coming years. Development of advanced imaging software with artificial intelligence can improve needle visualization and tracking. These technologies have the potential to facilitate provider training in real-time ultrasound-guided procedures and improve the overall safety of procedures. Emergence of affordable, handheld ultrasound devices is improving access to ultrasound technology, but their role in vascular access procedures is yet to be defined. Furthermore, availability of wireless handheld ultrasound technology and multifrequency transducers will create new possibilities for use of ultrasound in vascular access procedures.
CONCLUSION
We have presented several evidence-based recommendations on the use of ultrasound guidance for placement of central and peripheral vascular access catheters that are intended for hospitalists and other healthcare providers who routinely perform vascular access procedures. By allowing direct visualization of the needle tip and target vessel, the use of ultrasound guidance has been shown in randomized studies to reduce needle insertion attempts, reduce needle redirections, and increase overall procedure success rates. The accuracy of ultrasound to identify the target vessel, assess for thrombosis, and detect anatomical anomalies is superior to that of physical examination. Hospitalists can attain competence in performing ultrasound-guided vascular access procedures through systematic training programs that combine didactic and hands-on training, which optimally include patient-based competency assessments.
Acknowledgments
The authors thank all the members of the Society of Hospital Medicine Point-of-care Ultrasound Task Force and the Education Committee members for their time and dedication to develop these guidelines.
Collaborators of Society of Hospital Medicine Point-of-care Ultrasound Task Force: Robert Arntfield, Jeffrey Bates, Anjali Bhagra, Michael Blaivas, Daniel Brotman, Richard Hoppmann, Susan Hunt, Trevor P. Jensen, Venkat Kalidindi, Ketino Kobaidze, Joshua Lenchus, Paul Mayo, Satyen Nichani, Vicki Noble, Nitin Puri, Aliaksei Pustavoitau, Kreegan Reierson, Gerard Salame, Kirk Spencer, Vivek Tayal, David Tierney
SHM Point-of-care Ultrasound Task Force: CHAIRS: Nilam J. Soni, Ricardo Franco-Sadud, Jeff Bates. WORKING GROUPS: Thoracentesis Working Group: Ria Dancel (chair), Daniel Schnobrich, Nitin Puri. Vascular Access Working Group: Ricardo Franco (chair), Benji Mathews, Saaid Abdel-Ghani, Sophia Rodgers, Martin Perez, Daniel Schnobrich. Paracentesis Working Group: Joel Cho (chair), Benji Mathews, Kreegan Reierson, Anjali Bhagra, Trevor P. Jensen Lumbar Puncture Working Group: Nilam J. Soni (chair), Ricardo Franco, Gerard Salame, Josh Lenchus, Venkat Kalidindi, Ketino Kobaidze. Credentialing Working Group: Brian P Lucas (chair), David Tierney, Trevor P. Jensen PEER REVIEWERS: Robert Arntfield, Michael Blaivas, Richard Hoppmann, Paul Mayo, Vicki Noble, Aliaksei Pustavoitau, Kirk Spencer, Vivek Tayal. METHODOLOGIST: Mahmoud El-Barbary. LIBRARIAN: Loretta Grikis. SOCIETY OF HOSPITAL MEDICINE EDUCATION COMMITTEE: Daniel Brotman (past chair), Satyen Nichani (current chair), Susan Hunt. SOCIETY OF HOSPITAL MEDICINE STAFF: Nick Marzano.
Disclaimer
The contents of this publication do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
1. Raad I. Intravascular-catheter-related infections. Lancet. 1998;351(9106):893-898. https://doi.org/10.1016/S0140-6736(97)10006-X.
2. Merrer J, De Jonghe B, Golliot F, et al. Complications of femoral and subclavian venous catheterization in critically ill patients: a randomized controlled trial. JAMA. 2001;286(6):700-707. https://doi.org/10.1001/jama.286.6.700.
3. Sznajder JI, Zveibil FR, Bitterman H, Weiner P, Bursztein S. Central vein catheterization. Failure and complication rates by three percutaneous approaches. Arch Intern Med. 1986;146(2):259-261. https://doi.org/10.1001/archinte.146.2.259.
4. Randolph AG, Cook DJ, Gonzales CA, Pribble CG. Ultrasound guidance for placement of central venous catheters: a meta-analysis of the literature. Crit Care Med. 1996;24(12):2053-2058. https://doi.org/10.1097/00003246-199612000-00020.
5. Hind D, Calvert N, McWilliams R, et al. Ultrasonic locating devices for central venous cannulation: meta-analysis. BMJ. 2003;327(7411):361. https://doi.org/10.1136/bmj.327.7411.361.
6. O’Grady NP, Alexander M, Burns LA, et al. Guidelines for the prevention of intravascular catheter-related infections. Am J Infect Control. 2011;39(4):S1-S34. https://doi.org/10.1016/j.ajic.2011.01.003.
7. National Institute for Health and Care Excellence (NICE). Guidance on the Use of Ultrasound Locating Devices for Placing Central Venous Catheters; 2002.
8. Shojania KG, Duncan BW, McDonald KM, et al. Making health care safer: A critical analysis of patient safety practices. Evid Rep Technol Assess. Rockville, MD: Agency for Healthcare Research and Quality. 2001;43(43):i–x, 1.
9. Lamperti M, Bodenham AR, Pittiruti M, et al. International evidence-based recommendations on ultrasound-guided vascular access. Intensive Care Med. 2012;38(7):1105-1117. https://doi.org/10.1007/s00134-012-2597-x.
10. Frykholm P, Pikwer A, Hammarskjöld F, et al. Clinical guidelines on central venous catheterisation. Swedish Society of Anaesthesiology and Intensive Care Medicine. Acta Anaesthesiol Scand. 2014;58(5):508-524. https://doi.org/10.1111/aas.12295.
11. American Society of Anesthesiologists Task Force on Central Venous Access, Rupp SM, Apfelbaum JL, et al. Practice guidelines for central venous access: a report by the American Society of Anesthesiologists Task Force on Central Venous Access. Anesthesiology. 2012;116(3):539-573. https://doi.org/10.1097/ALN.0b013e31823c9569.
12. Frankel HL, Kirkpatrick AW, Elbarbary M, et al. Guidelines for the appropriate use of bedside general and cardiac ultrasonography in the evaluation of critically ill patients-part I: General ultrasonography. Crit Care Med. 2015;43(11):2479-2502. https://doi.org/10.1097/CCM.0000000000001216.
13. Shekelle PG, Wachter RM, Pronovost PJ, et al. Making health care safer II: An updated critical analysis of the evidence for patient safety practices. Evid Rep Technol Assess. 2013;211:1-945.
14. Troianos CA, Hartman GS, Glas KE, et al. Guidelines for performing ultrasound guided vascular cannulation: recommendations of the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. J Am Soc Echocardiogr. 2011;24(12):1291-1318. https://doi.org/10.1016/j.echo.2011.09.021.
15. Soni NJ, Reyes LF, Keyt H, et al. Use of ultrasound guidance for central venous catheterization: a national survey of intensivists and hospitalists. J Crit Care. 2016;36:277-283. https://doi.org/10.1016/j.jcrc.2016.07.014.
16. Parienti JJ, Mongardon N, Mégarbane B, et al. Intravascular complications of central venous catheterization by insertion site. N Engl J Med. 2015;373(13):1220-1229. https://doi.org/10.1056/NEJMoa1500964.
17. Maizel J, Bastide MA, Richecoeur J, et al. Practice of ultrasound-guided central venous catheter technique by the French intensivists: a survey from the BoReal study group. Ann Intensive Care. 2016;6(1):76. https://doi.org/10.1186/s13613-016-0177-x.
18. Egan G, Healy D, O’Neill H, et al. Ultrasound guidance for difficult peripheral venous access: systematic review and meta-analysis. Emerg Med J. 2013;30(7):521-526. https://doi.org/10.1136/emermed-2012-201652.
19. Stolz LA, Stolz U, Howe C, Farrell IJ, Adhikari S. Ultrasound-guided peripheral venous access: a meta-analysis and systematic review. J Vasc Access. 2015;16(4):321-326. https://doi.org/10.5301/jva.5000346.
20. Sofocleous CT, Schur I, Cooper SG, et al. Sonographically guided placement of peripherally inserted central venous catheters: review of 355 procedures. AJR Am J Roentgenol. 1998;170(6):1613-1616. https://doi.org/10.2214/ajr.170.6.9609183.
21. Nichols I, Humphrey JP. The efficacy of upper arm placement of peripherally inserted central catheters using bedside ultrasound and microintroducer technique. J Infus Nurs Off Publ Infus Nurs Soc. 2008;31(3):165-176. https://doi.org/10.1097/01.NAN.0000317703.66395.b8.
22. Stokowski G, Steele D, Wilson D. The use of ultrasound to improve practice and reduce complication rates in peripherally inserted central catheter insertions. J Infus Nurs Off Publ Infus Nurs Soc. 2009;32(3):145-155. https://doi.org/10.1097/NAN.0b013e3181a1a98f.
23. Shiloh AL, Savel RH, Paulin LM, Eisen LA. Ultrasound-guided catheterization of the radial artery: a systematic review and meta-analysis of randomized controlled trials. Chest. 2011;139(3):524-529. https://doi.org/10.1378/chest.10-0919.
24. Lucas BP, Tierney DM, Jensen TP, et al. Credentialing of hospitalists in ultrasound-guided bedside procedures: A position statement of the Society of Hospital Medicine. J Hosp Med. 2018;13(2):117-125. https://doi.org/10.12788/jhm.2917.
25. Chopra V, Flanders SA, Saint S, et al. The Michigan appropriateness guide for intravenous catheters (Magic): results From a Multispecialty Panel Using the RAND/UCLA Appropriateness Method. Ann Intern Med. 2015;163(6):S1-S40. https://doi.org/10.7326/M15-0744.
26. Raad II, Hohn DC, Gilbreath BJ, et al. Prevention of central venous catheter-related infections by using maximal sterile barrier precautions during insertion. Infect Control Hosp Epidemiol. 1994;15(4):231-238.
27. Slama M, Novara A, Safavian A, et al. Improvement of internal jugular vein cannulation using an ultrasound-guided technique. Intensive Care Med. 1997;23(8):916-919. https://doi.org/10.1007/s001340050432.
28. Seyahi N, Kahveci A, Altiparmak MR, Serdengecti K, Erek E. Ultrasound imaging findings of femoral veins in patients with renal failure and its impact on vascular access. Nephrol Dial Transplant. 2005;20(9):1864-1867. https://doi.org/10.1093/ndt/gfh942.
29. Lin BS, Kong CW, Tarng DC, Huang TP, Tang GJ. Anatomical variation of the internal jugular vein and its impact on temporary haemodialysis vascular access: an ultrasonographic survey in uraemic patients. Nephrol Dial Transplant. 1998;13(1):134-138. https://doi.org/10.1093/ndt/13.1.134.
30. Troianos CA, Kuwik RJ, Pasqual JR, Lim AJ, Odasso DP. Internal jugular vein and carotid artery anatomic relation as determined by ultrasonography. Anesthesiology. 1996;85(1):43-48. https://doi.org/10.1097/00000542-199607000-00007.
31. Gordon AC, Wright I, Pugh ND. Duplication of the superficial femoral vein: recognition with duplex ultrasonography. Clin Rad. 1996;51(9):622-624. https://doi.org/10.1016/S0009-9260(96)80055-9.
32. Sulek CA, Gravenstein N, Blackshear RH, Weiss L. Head rotation during internal jugular vein cannulation and the risk of carotid artery puncture. Anesth Analg. 1996;82(1):125-128. https://doi.org/10.1097/00000539-199601000-00022.
33. Shrestha BR, Gautam B. Ultrasound versus the landmark technique: a prospective randomized comparative study of internal jugular vein cannulation in an intensive care unit. JNMA J Nepal Med Assoc. 2011;51(182):56-61. https://doi.org/10.31729/jnma.148.
34. Brederlau J, Greim C, Schwemmer U, et al. Ultrasound-guided cannulation of the internal jugular vein in critically ill patients positioned in 30 degrees dorsal elevation. Eur J Anaesthesiol. 2004;21(9):684-687. https://doi.org/10.1097/00003643-200409000-00003.
35. Lamperti M, Subert M, Cortellazzi P, et al. Is a neutral head position safer than 45-degree neck rotation during ultrasound-guided internal jugular vein cannulation? Results of a randomized controlled clinical trial. Anesth Analg. 2012;114(4):777-784. https://doi.org/10.1213/ANE.0b013e3182459917.
36. Benter T, Teichgräber UK, Klühs L, et al. Anatomical variations in the internal jugular veins of cancer patients affecting central venous access. Anatomical variation of the internal jugular vein. Ultraschall Med. 2001;22(1):23-26. https://doi.org/10.1055/s-2001-11243.
37. Caridi JG, Hawkins IF, Jr, Wiechmann BN, Pevarski DJ, Tonkin JC. Sonographic guidance when using the right internal jugular vein for central vein access. AJR Am J Roentgenol. 1998;171(5):1259-1263. https://doi.org/10.2214/ajr.171.5.9798857.
38. Brusasco C, Corradi F, Zattoni PL, et al. Ultrasound-guided central venous cannulation in bariatric patients. Obes Surg. 2009;19(10):1365-1370. https://doi.org/10.1007/s11695-009-9902-y.
39. Armstrong PJ, Cullen M, Scott DH. The ‘SiteRite’ ultrasound machine--an aid to internal jugular vein cannulation. Anaesthesia. 1993;48(4):319-323. https://doi.org/10.1111/j.1365-2044.1993.tb06953.x.
40. Forauer AR, Glockner JF. Importance of US findings in access planning during jugular vein hemodialysis catheter placements. J Vasc Interv Rad. 2000;11(2 Pt 1):233-238. https://doi.org/10.1016/S1051-0443(07)61471-7.
41. Hassan C, Girishkumar HT, Thatigotla B, et al. Value of ultrasound guidance in placement of hemodialysis access catheters in patients with end-stage renal disease. Am Surg. 2008;74(11):1111-1113.
42. Tan CO, Weinberg L, Peyton P, Story D, McNicol L. Size variation between contralateral infraclavicular axillary veins within individual patients-implications for subclavian venous central line insertion. Crit Care Med. 2013;41(2):457-463. https://doi.org/10.1097/CCM.0b013e31826ab1dd.
43. Wu SY, Ling Q, Cao LH, et al. Real-time two-dimensional ultrasound guidance for central venous cannulation: a meta-analysis. Anesthesiology. 2013;118(2):361-375. https://doi.org/10.1097/ALN.0b013e31827bd172.
44. Kim W, Chung RK, Lee GY, Han JI. The effects of hip abduction with external rotation and reverse Trendelenburg position on the size of the femoral vein; ultrasonographic investigation. Korean J Anesthesiol. 2011;61(3):205-209. https://doi.org/10.4097/kjae.2011.61.3.205.
45. Denys BG, Uretsky BF. Anatomical variations of internal jugular vein location: impact on central venous access. Crit Care Med. 1991;19(12):1516-1519. https://doi.org/10.1097/00003246-199112000-00013.
46. Brass P, Hellmich M, Kolodziej L, Schick G, Smith AF. Ultrasound guidance versus anatomical landmarks for internal jugular vein catheterization. Cochrane Database Syst Rev. 2015;1:CD006962. https://doi.org/10.1002/14651858.CD006962.pub2.
47. Gilbert TB, Seneff MG, Becker RB. Facilitation of internal jugular venous cannulation using an audio-guided Doppler ultrasound vascular access device: results from a prospective, dual-center, randomized, crossover clinical study. Crit Care Med. 1995;23(1):60-65. https://doi.org/10.1097/00003246-199501000-00012.
48. Schummer W, Schummer C, Tuppatsch H, et al. Ultrasound-guided central venous cannulation: is there a difference between Doppler and B-mode ultrasound? J Clin Anesth. 2006;18(3):167-172. https://doi.org/10.1016/j.jclinane.2005.12.010.
49. Airapetian N, Maizel J, Langelle F, et al. Ultrasound-guided central venous cannulation is superior to quick-look ultrasound and landmark methods among inexperienced operators: a prospective randomized study. Intensive Care Med. 2013;39(11):1938-1944. https://doi.org/10.1007/s00134-013-3072-z.
50. Milling TJ, Jr, Rose J, Briggs WM, et al. Randomized, controlled clinical trial of point-of-care limited ultrasonography assistance of central venous cannulation: the Third Sonography Outcomes Assessment Program (SOAP-3) Trial. Crit Care Med. 2005;33(8):1764-1769. https://doi.org/10.1097/01.ccm.0000171533.92856.e5.
51. Beaudoin FL, Merchant RC, Lincoln J, et al. Bedside ultrasonography detects significant femoral vessel overlap: implications for central venous cannulation. CJEM. 2011;13(4):245-250. https://doi.org/10.2310/8000.2011.110482.
52. Kwon TH, Kim YL, Cho DK. Ultrasound-guided cannulation of the femoral vein for acute haemodialysis access. Nephrol Dial Transplant. 1997;12(5):1009-1012. https://doi.org/10.1093/ndt/12.5.1009.
53. Rothschild JM. Ultrasound guidance of central vein catheterization. Evid Rep Technol Assess. 2001;43. Chapter 21.: http://archive.ahrq.gov/clinic/ptsafety/chap21.htm.
54. Lennon M, Zaw NN, Pöpping DM, Wenk M. Procedural complications of central venous catheter insertion. Minerva Anestesiol. 2012;78(11):1234-1240.
55. Blaivas M, Adhikari S. An unseen danger: frequency of posterior vessel wall penetration by needles during attempts to place internal jugular vein central catheters using ultrasound guidance. Crit Care Med. 2009;37(8):2345-2349; quiz 2359. https://doi.org/10.1097/CCM.0b013e3181a067d4.
56. Vogel JA, Haukoos JS, Erickson CL, et al. Is long-axis view superior to short-axis view in ultrasound-guided central venous catheterization? Crit Care Med. 2015;43(4):832-839. https://doi.org/10.1097/CCM.0000000000000823.
57. Tammam TF, El-Shafey EM, Tammam HF. Ultrasound-guided internal jugular vein access: comparison between short axis and long axis techniques. Saudi J Kidney Dis Transpl. 2013;24(4):707-713. https://doi.org/10.4103/1319-2442.113861.
58. Keyes LE, Frazee BW, Snoey ER, Simon BC, Christy D. Ultrasound-guided brachial and basilic vein cannulation in emergency department patients with difficult intravenous access. Ann Emerg Med. 1999;34(6):711-714. https://doi.org/10.1016/S0196-0644(99)70095-8.
59. Mahler SA, Wang H, Lester C, et al. Short- vs long-axis approach to ultrasound-guided peripheral intravenous access: a prospective randomized study. Am J Emerg Med. 2011;29(9):1194-1197. https://doi.org/10.1016/j.ajem.2010.07.015.
60. Berk D, Gurkan Y, Kus A, et al. Ultrasound-guided radial arterial cannulation: long axis/in-plane versus short axis/out-of-plane approaches? J Clin Monit Comput. 2013;27(3):319-324. https://doi.org/10.1007/s10877-013-9437-6.
61. Batllori M, Urra M, Uriarte E, et al. Randomized comparison of three transducer orientation approaches for ultrasound guided internal jugular venous cannulation. Br J Anaesth. 2016;116(3):370-376. https://doi.org/10.1093/bja/aev399.
62. Moak JH, Lyons MS, Wright SW, Lindsell CJ. Needle and guidewire visualization in ultrasound-guided internal jugular vein cannulation. Am J Emerg Med. 2011;29(4):432-436. https://doi.org/10.1016/j.ajem.2010.01.004.
63. Moak JH, Rajkumar JS, Woods WA. The wire is really easy to see (WIRES): sonographic visualization of the guidewire by novices. CJEM. 2013;15(1):18-23. https://doi.org/10.2310/8000.2012.120800.
64. Gillman LM, Blaivas M, Lord J, Al-Kadi A, Kirkpatrick AW. Ultrasound confirmation of guidewire position may eliminate accidental arterial dilatation during central venous cannulation. Scand J Trauma Resusc Emerg Med. 2010;18:39. https://doi.org/10.1186/1757-7241-18-39.
65. Augoustides JG, Horak J, Ochroch AE, et al. A randomized controlled clinical trial of real-time needle-guided ultrasound for internal jugular venous cannulation in a large university anesthesia department. J Cardiothorac Vasc Anesth. 2005;19(3):310-315. https://doi.org/10.1053/j.jvca.2005.03.007.
66. Maecken T, Heite L, Wolf B, Zahn PK, Litz RJ. Ultrasound-guided catheterisation of the subclavian vein: freehand vs needle-guided technique. Anaesthesia. 2015;70(11):1242-1249. https://doi.org/10.1111/anae.13187.
67. Stone MB, Nagdev A, Murphy MC, Sisson CA. Ultrasound detection of guidewire position during central venous catheterization. Am J Emerg Med. 2010;28(1):82-84. https://doi.org/10.1016/j.ajem.2008.09.019.
68. Luyet C, Hartwich V, Urwyler N, et al. Evaluation of a novel needle guide for ultrasound-guided phantom vessel cannulation. Anaesthesia. 2011;66(8):715-720. https://doi.org/10.1111/j.1365-2044.2011.06781.x.
69. O’Grady NP, Alexander M, Burns LA, et al. Summary of recommendations: Guidelines for the Prevention of Intravascular Catheter-related Infections. Clin Infect Dis. 2011;52(9):1087-1099. https://doi.org/10.1093/cid/cir138.
70. Southworth SL, Henman LJ, Kinder LA, Sell JL. The journey to zero central catheter-associated bloodstream infections: culture change in an intensive care unit. Crit Care Nurse. 2012;32(2):49-54. https://doi.org/10.4037/ccn2012915.
71. Guerin K, Wagner J, Rains K, Bessesen M. Reduction in central line-associated bloodstream infections by implementation of a postinsertion care bundle. Am J Infect Control. 2010;38(6):430-433. https://doi.org/10.1016/j.ajic.2010.03.007.
72. Hartman N, Wittler M, Askew K, Manthey D. Delphi method validation of a procedural performance checklist for insertion of an ultrasound-guided internal jugular central line. Am J Med Qual Off J Am Coll Med Qual. 2016;31(1):81-85. https://doi.org/10.1177/1062860614549762.
73. Lenchus JD, Carvalho CM, Ferreri K, et al. Filling the void: defining invasive bedside procedural competency for internal medicine residents. J Grad Med Educ. 2013;5(4):605-612. https://doi.org/10.4300/JGME-D-13-00030.1.
74. Barsuk JH, Cohen ER, Feinglass J, McGaghie WC, Wayne DB. Use of simulation-based education to reduce catheter-related bloodstream infections. Arch Intern Med. 2009;169(15):1420-1423. https://doi.org/10.1001/archinternmed.2009.215.
75. Karakitsos D, Labropoulos N, De Groot E, et al. Real-time ultrasound-guided catheterisation of the internal jugular vein: a prospective comparison with the landmark technique in critical care patients. Crit Care. 2006;10(6):R162. https://doi.org/10.1186/cc5101.
76. Berenholtz SM, Pronovost PJ, Lipsett PA, et al. Eliminating catheter-related bloodstream infections in the intensive care unit. Crit Care Med. 2004;32(10):2014-2020. https://doi.org/10.1097/01.ccm.0000142399.70913.2f.
77. Young EM, Commiskey ML, Wilson SJ. Translating evidence into practice to prevent central venous catheter-associated bloodstream infections: a systems-based intervention. Am J Infect Control. 2006;34(8):503-506. https://doi.org/10.1016/j.ajic.2006.03.011.
78. Galpern D, Guerrero A, Tu A, Fahoum B, Wise L. Effectiveness of a central line bundle campaign on line-associated infections in the intensive care unit. Surgery. 2008;144(4):492-495; discussion 495. https://doi.org/10.1016/j.surg.2008.06.004.
79. Mermel LA, McCormick RD, Springman SR, Maki DG. The pathogenesis and epidemiology of catheter-related infection with pulmonary artery swan-Ganz catheters: a prospective study utilizing molecular subtyping. Am J Med. 1991;91(3b):197S–205S. https://doi.org/10.1016/0002-9343(91)90369-9.
80. Marschall J, Mermel LA, Classen D, et al. Strategies to prevent central line-associated bloodstream infections in acute care hospitals. Infect Control Hosp Epidemiol. 2008;29(1):S22-S30. https://doi.org/10.1086/591059.
81. Marschall J, Mermel LA, Fakih M, et al. Strategies to prevent central line-associated bloodstream infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(7):753-771. https://doi.org/10.1086/676533.
82. Garcia-Rodriguez JF, Álvarez-Díaz H, Vilariño-Maneiro L, et al. Epidemiology and impact of a multifaceted approach in controlling central venous catheter associated blood stream infections outside the intensive care unit. BMC Infect Dis. 2013;13:445. https://doi.org/10.1186/1471-2334-13-445.
83. Lee DH, Jung KY, Choi YH. Use of maximal sterile barrier precautions and/or antimicrobial-coated catheters to reduce the risk of central venous catheter-related bloodstream infection. Infect Control Hosp Epidemiol. 2008;29(10):947-950. https://doi.org/10.1086/590356.
84. Mermel LA. Prevention of intravascular catheter-related infections. Ann Intern Med. 2000;132(5):391-402. https://doi.org/10.7326/0003-4819-132-5-200003070-00009.
85. Troianos CA, Jobes DR, Ellison N. Ultrasound-guided cannulation of the internal jugular vein. A prospective, randomized study. Anesth Analg. 1991;72(6):823-826. https://doi.org/10.1213/00000539-199106000-00020.
86. Troianos CA, Savino JS. Internal jugular vein cannulation guided by echocardiography. Anesthesiology. 1991;74(4):787-789. https://doi.org/10.1097/00000542-199104000-00026.
87. Denys BG, Uretsky BF, Reddy PS, et al. An ultrasound method for safe and rapid central venous access. N Engl J Med. 1991;324(8):566. https://doi.org/10.1056/NEJM199102213240816.
88. Riaz A, Shan Khan RA, Salim F. Ultrasound guided internal jugular venous cannulation: comparison with land-mark technique. J Coll Phys Surg Pak. 2015;25(5):315-319. https://doi.org/05.2015/JCPSP.315319.
89. Gok F, Kilicaslan A, Sarkilar G, Kandemir B, Yosunkaya A. The effect of ultrasound guidance on central venous catheter-associated bloodstream infection in critical care patients. Acta Med Mediterr. 2013;29:677-682.
90. Lamperti M, Cortellazzi P, D’Onofrio G, et al. An outcome study on complications using routine ultrasound assistance for internal jugular vein cannulation. Acta Anaesthesiol Scand. 2007;51(10):1327-1330. https://doi.org/10.1111/j.1399-6576.2007.01442.x.
91. Vezzani A, Manca T, Vercelli A, Braghieri A, Magnacavallo A. Ultrasonography as a guide during vascular access procedures and in the diagnosis of complications. J Ultrasound. 2013;16(4):161-170. https://doi.org/10.1007/s40477-013-0046-5.
92. Palepu GB, Deven J, Subrahmanyam M, Mohan S. Impact of ultrasonography on central venous catheter insertion in intensive care. Indian J Rad Imaging. 2009;19(3):191-198. https://doi.org/10.4103/0971-3026.54877.
93. Agarwal A, Singh DK, Singh AP. Ultrasonography: a novel approach to central venous cannulation. Indian J Crit Care Med. 2009;13(4):213-216. https://doi.org/10.4103/0972-5229.60174.
94. Wong SW, Niazi AU, Chin KJ, Chan VW. Real-time ultrasound-guided spinal anesthesia using the SonixGPS® needle tracking system: a case report. Can J Anaesth. 2013;60(1):50-53. https://doi.org/10.1007/s12630-012-9809-2.
95. Bouaziz H, Zetlaoui PJ, Pierre S, et al. Guidelines on the use of ultrasound guidance for vascular access. Anaesth, Crit Care Pain Med. 2015;34(1):65-69. https://doi.org/10.1016/j.accpm.2015.01.004.
96. Jenssen C, Brkljacic B, Hocke M, et al. EFSUMB guidelines on interventional ultrasound (INVUS), Part VI - Ultrasound-guided vascular interventions. Ultraschall Med. 2016;37(5):473-476. https://doi.org/10.1055/s-0035-1553450.
97. Rando K, Castelli J, Pratt JP, et al. Ultrasound-guided internal jugular vein catheterization: a randomized controlled trial. Heart Lung Vessels. 2014;6(1):13-23.
98. Mey U, Glasmacher A, Hahn C, et al. Evaluation of an ultrasound-guided technique for central venous access via the internal jugular vein in 493 patients. Support Care Cancer Off J Multinat Assoc Support Care Cancer. 2003;11(3):148-155. https://doi.org/10.1007/s00520-002-0399-3.
99. Singh SA, Sharma S, Singh A, et al. The safety of ultrasound guided central venous cannulation in patients with liver disease. Saudi J Anaesth. 2015;9(2):155-160. https://doi.org/10.4103/1658-354X.152842.
100. Akoglu H, Piskinpasa S, Yenigun EC, et al. Real-time ultrasound guided placement of temporary internal jugular vein catheters: assessment of technical success and complication rates in nephrology practice. Nephrol (Carlton). 2012;17(7):603-606. https://doi.org/10.1111/j.1440-1797.2012.01637.x.
101. Sadler DJ, Gordon AC, Klassen J, et al. Image-guided central venous catheters for apheresis. Bone Marrow Transplant. 1999;23(2):179-182. https://doi.org/10.1038/sj.bmt.1701545.
102. Calvert N, Hind D, McWilliams R, et al. Ultrasound for central venous cannulation: economic evaluation of cost-effectiveness. Anaesthesia. 2004;59(11):1116-1120. https://doi.org/10.1111/j.1365-2044.2004.03906.x.
103. Goetz AM, Wagener MM, Miller JM, Muder RR. Risk of infection due to central venous catheters: effect of site of placement and catheter type. Infect Control Hosp Epidemiol. 1998;19(11):842-845.
104. Bertini P, Frediani M. Ultrasound guided supraclavicular central vein cannulation in adults: a technical report. J Vasc Access. 2013;14(1):89-93. https://doi.org/10.5301/jva.5000088.
105. Lalu MM, Fayad A, Ahmed O, et al. Ultrasound-guided subclavian vein catheterization: A systematic review and meta-analysis. Crit Care Med. 2015;43(7):1498-1507. https://doi.org/10.1097/CCM.0000000000000973.
106. Milone M, Di Minno G, Di Minno MN, et al. The real effectiveness of ultrasound guidance in subclavian venous access. Ann ital chir. 2010;81(5):331-334.
107. O’Leary R, Ahmed SM, McLure H, et al. Ultrasound-guided infraclavicular axillary vein cannulation: a useful alternative to the internal jugular vein. Br J Anaesth. 2012;109(5):762-768. https://doi.org/10.1093/bja/aes262.
108. Gualtieri E, Deppe SA, Sipperly ME, Thompson DR. Subclavian venous catheterization: greater success rate for less experienced operators using ultrasound guidance. Crit Care Med. 1995;23(4):692-697. https://doi.org/10.1097/00003246-199504000-00018.
109. Fragou M, Gravvanis A, Dimitriou V, et al. Real-time ultrasound-guided subclavian vein cannulation versus the landmark method in critical care patients: a prospective randomized study. Crit Care Med. 2011;39(7):1607-1612. https://doi.org/10.1097/CCM.0b013e318218a1ae.
110. Brass P, Hellmich M, Kolodziej L, Schick G, Smith AF. Ultrasound guidance versus anatomical landmarks for subclavian or femoral vein catheterization. Cochrane Database Syst Rev. 2015;1:CD011447. https://doi.org/10.1002/14651858.CD011447.
111. Robinson MK, Mogensen KM, Grudinskas GF, Kohler S, Jacobs DO. Improved care and reduced costs for patients requiring peripherally inserted central catheters: the role of bedside ultrasound and a dedicated team. JPEN J Parenter Enter Nutr. 2005;29(5):374-379. https://doi.org/10.1177/0148607105029005374.
112. Li J, Fan YY, Xin MZ, et al. A randomised, controlled trial comparing the long-term effects of peripherally inserted central catheter placement in chemotherapy patients using B-mode ultrasound with modified Seldinger technique versus blind puncture. Eur J Oncol Nurs. 2014;18(1):94-103. https://doi.org/10.1016/j.ejon.2013.08.003.
113. Schweickert WD, Herlitz J, Pohlman AS, et al. A randomized, controlled trial evaluating postinsertion neck ultrasound in peripherally inserted central catheter procedures. Crit Care Med. 2009;37(4):1217-1221. https://doi.org/10.1097/CCM.0b013e31819cee7f.
114. Mahler SA, Wang H, Lester C, Conrad SA. Ultrasound-guided peripheral intravenous access in the emergency department using a modified Seldinger technique. J Emerg Med. 2010;39(3):325-329. https://doi.org/10.1016/j.jemermed.2009.02.013.
115. Brannam L, Blaivas M, Lyon M, Flake M. Emergency nurses’ utilization of ultrasound guidance for placement of peripheral intravenous lines in difficult-access patients. Acad Emerg Med. 2004;11(12):1361-1363. https://doi.org/10.1197/j.aem.2004.08.027.
116. Au A, Rotte M, Gryzbowski R, Ku B, Fields J. 157 Decrease in central venous catheter placement and complications due to utilization of ultrasound-guided peripheral intravenous catheters. Ann Emerg Med. 2011;58(4):S230. https://doi.org/10.1016/j.annemergmed.2011.06.185.
117. Dargin JM, Rebholz CM, Lowenstein RA, Mitchell PM, Feldman JA. Ultrasonography-guided peripheral intravenous catheter survival in ED patients with difficult access. Am J Emerg Med. 2010;28(1):1-7. https://doi.org/10.1016/j.ajem.2008.09.001.
118. Bauman M, Braude D, Crandall C. Ultrasound-guidance vs standard technique in difficult vascular access patients by ED technicians. Am J Emerg Med. 2009;27(2):135-140. https://doi.org/10.1016/j.ajem.2008.02.005.
119. Schoenfeld E, Shokoohi H, Boniface K. Ultrasound-guided peripheral intravenous access in the emergency department: patient-centered survey. West J Emerg Med. 2011;12(4):475-477. https://doi.org/10.5811/westjem.2011.3.1920.
120. Maki DG, Ringer M. Risk factors for infusion-related phlebitis with small peripheral venous catheters. A randomized controlled trial. Ann Intern Med. 1991;114(10):845-854. https://doi.org/10.7326/0003-4819-114-10-845.
121. Miliani K, Taravella R, Thillard D, et al. Peripheral venous catheter-related adverse events: evaluation from a multicentre epidemiological study in France (the CATHEVAL Project). PLOS ONE. 2017;12(1):e0168637. https://doi.org/10.1371/journal.pone.0168637.
122. Gregg SC, Murthi SB, Sisley AC, Stein DM, Scalea TM. Ultrasound-guided peripheral intravenous access in the intensive care unit. J Crit Care. 2010;25(3):514-519. https://doi.org/10.1016/j.jcrc.2009.09.003.
123. Adhikari S, Blaivas M, Morrison D, Lander L. Comparison of infection rates among ultrasound-guided versus traditionally placed peripheral intravenous lines. J Ultrasound Med. 2010;29(5):741-747. https://doi.org/10.7863/jum.2010.29.5.741.
124. Shiver S, Blaivas M, Lyon M. A prospective comparison of ultrasound-guided and blindly placed radial arterial catheters. Acad Emerg Med. 2006;13(12):1275-1279. https://doi.org/10.1197/j.aem.2006.07.015.
125. Levin PD, Sheinin O, Gozal Y. Use of ultrasound guidance in the insertion of radial artery catheters. Crit Care Med. 2003;31(2):481-484. https://doi.org/10.1097/01.CCM.0000050452.17304.2F.
126. Gu WJ, Tie HT, Liu JC, Zeng XT. Efficacy of ultrasound-guided radial artery catheterization: a systematic review and meta-analysis of randomized controlled trials. Crit Care. 2014;18(3):R93. https://doi.org/10.1186/cc13862.
127. Tang L, Wang F, Li Y, et al. Ultrasound guidance for radial artery catheterization: an updated meta-analysis of randomized controlled trials. PLOS ONE. 2014;9(11):e111527. https://doi.org/10.1371/journal.pone.0111527.
128. Sobolev M, Slovut DP, Lee Chang A, Shiloh AL, Eisen LA. Ultrasound-guided catheterization of the femoral artery: A systematic review and meta-analysis of randomized controlled trials. J Invas Cardiol. 2015;27(7):318-323. https://doi.org/10.1378/chest.1991181.
129. Gabriel M, Pawlaczyk K, Waliszewski K, Krasiński Z, Majewski W. Location of femoral artery puncture site and the risk of postcatheterization pseudoaneurysm formation. Int J Cardiol. 2007;120(2):167-171. https://doi.org/10.1016/j.ijcard.2006.09.018.
130. Seto AH, Abu-Fadel MS, Sparling JM, et al. Real-time ultrasound guidance facilitates femoral arterial access and reduces vascular complications: FAUST (Femoral Arterial Access With ultrasound Trial). JACC Cardiovasc Interv. 2010;3(7):751-758. https://doi.org/10.1016/j.jcin.2010.04.015.
131. Kalish J, Eslami M, Gillespie D, et al. Routine use of ultrasound guidance in femoral arterial access for peripheral vascular intervention decreases groin hematoma rates. J Vasc Surg. 2015;61(5):1231-1238. https://doi.org/10.1016/j.jvs2014.12.003.
132. Sandhu NS, Patel B. Use of ultrasonography as a rescue technique for failed radial artery cannulation. J Clin Anesth. 2006;18(2):138-141. https://doi.org/10.1016/j.jclinane.2005.06.011.
133. White L, Halpin A, Turner M, Wallace L. Ultrasound-guided radial artery cannulation in adult and paediatric populations: a systematic review and meta-analysis. Br J Anaesth. 2016;116(5):610-617. https://doi.org/10.1093/bja/aew097.
134. Gao YB, Yan JH, Gao FQ, et al. Effects of ultrasound-guided radial artery catheterization: an updated meta-analysis. Am J Emerg Med. 2015;33(1):50-55. https://doi.org/10.1016/j.ajem.2014.10.008.
135. Seto AH, Roberts JS, Abu-Fadel MS, et al. Real-time ultrasound guidance facilitates transradial access: RAUST (Radial Artery Access with Ultrasound Trial). JACC Cardiovasc Interv. 2015;8(2):283-291. https://doi.org/10.1016/j.jcin.2014.05.036.
136. Roberts J, Manur R. Ultrasound-guided radial artery access by a non-ultrasound trained interventional cardiologist improved first-attempt success rates and shortened time for successful radial artery cannulation. J Invas Cardiol. 2013;25(12):676-679.
137. Lichtenstein DA, Menu Y. A bedside ultrasound sign ruling out pneumothorax in the critically ill. Lung sliding. Chest. 1995;108(5):1345-1348. https://doi.org/10.1378/chest.108.5.1345.
138. Lichtenstein D, Mezière G, Biderman P, Gepner A. The comet-tail artifact: an ultrasound sign ruling out pneumothorax. Intensive Care Med. 1999;25(4):383-388. https://doi.org/10.1007/s001340050862.
139. Ablordeppey EA, Drewry AM, Beyer AB, et al. Diagnostic accuracy of central venous catheter confirmation by bedside ultrasound Versus chest radiography in critically ill patients: A systematic review and meta-analysis. Crit Care Med. 2017;45(4):715-724. https://doi.org/10.1097/CCM.0000000000002188.
140. Bedel J, Vallée F, Mari A, et al. Guidewire localization by transthoracic echocardiography during central venous catheter insertion: a periprocedural method to evaluate catheter placement. Intensive Care Med. 2013;39(11):1932-1937. https://doi.org/10.1007/s00134-013-3097-3.
141. Weekes AJ, Keller SM, Efune B, Ghali S, Runyon M. Prospective comparison of ultrasound and CXR for confirmation of central vascular catheter placement. Emerg Med J EMJ. 2016;33(3):176-180. https://doi.org/10.1136/emermed-2015-205000.
142. Arellano R, Nurmohamed A, Rumman A, et al. The utility of transthoracic echocardiography to confirm central line placement: an observational study. Can J Anaesth. 2014;61(4):340-346. https://doi.org/10.1007/s12630-014-0111-3.
143. Vezzani A, Brusasco C, Palermo S, et al. Ultrasound localization of central vein catheter and detection of postprocedural pneumothorax: an alternative to chest radiography. Crit Care Med. 2010;38(2):533-538. https://doi.org/10.1097/CCM.0b013e3181c0328f.
144. Choudhry NK, Fletcher RH, Soumerai SB. Systematic review: the relationship between clinical experience and quality of health care. Ann Intern Med. 2005;142(4):260-273. https://doi.org/10.7326/0003-4819-142-4-200502150-00008.
145. Backlund BH, Hopkins E, Kendall JL. Ultrasound guidance for central venous access by emergency physicians in Colorado. West J Emerg Med. 2012;13(4):320-325. https://doi.org/10.5811/westjem.2011.11.6821.
146. Buchanan MS, Backlund B, Liao MM, et al. Use of ultrasound guidance for central venous catheter placement: survey from the American Board of Emergency Medicine Longitudinal Study of Emergency Physicians. Acad Emerg Med. 2014;21(4):416-421. https://doi.org/10.1111/acem.12350.
147. Barsuk JH, McGaghie WC, Cohen ER, O’Leary KJ, Wayne DB. Simulation-based mastery learning reduces complications during central venous catheter insertion in a medical intensive care unit. Crit Care Med. 2009;37(10):2697-2701. https://doi.org/10.1097/00003246-200910000-00003.
148. Coopersmith CM, Rebmann TL, Zack JE, et al. Effect of an education program on decreasing catheter-related bloodstream infections in the surgical intensive care unit. Crit Care Med. 2002;30(1):59-64. https://doi.org/10.1097/00003246-200201000-00009.
149. Woo MY, Frank J, Lee AC, et al. Effectiveness of a novel training program for emergency medicine residents in ultrasound-guided insertion of central venous catheters. CJEM. 2009;11(4):343-348. https://doi.org/10.1017/S1481803500011398.
150. McGee DC, Gould MK. Preventing complications of central venous catheterization. N Engl J Med. 2003;348(12):1123-1133. https://doi.org/10.1056/NEJMra011883.
151. Barsuk JH, McGaghie WC, Cohen ER, Balachandran JS, Wayne DB. Use of simulation-based mastery learning to improve the quality of central venous catheter placement in a medical intensive care unit. J Hosp Med. 2009;4(7):397-403. https://doi.org/10.1002/jhm.468.
152. Sekiguchi H, Tokita JE, Minami T, et al. A prerotational, simulation-based workshop improves the safety of central venous catheter insertion: results of a successful internal medicine house staff training program. Chest. 2011;140(3):652-658. https://doi.org/10.1378/chest.10-3319.
153. Feller-Kopman D. Ultrasound-guided internal jugular access: a proposed standardized approach and implications for training and practice. Chest. 2007;132(1):302-309. https://doi.org/10.1378/chest.06-2711.
154. Troianos CA, Hartman GS, Glas KE, et al. Special articles: guidelines for performing ultrasound guided vascular cannulation: recommendations of the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. Anesth Analg. 2012;114(1):46-72. https://doi.org/10.1213/ANE.0b013e3182407cd8.
155. Issenberg SB, McGaghie WC, Hart IR, et al. Simulation technology for health care professional skills training and assessment. JAMA. 1999;282(9):861-866. https://doi.org/10.1001/jama.282.9.861.
156. Millington SJ, Wong RY, Kassen BO, Roberts JM, Ma IW. Improving internal medicine residents’ performance, knowledge, and confidence in central venous catheterization using simulators. J Hosp Med. 2009;4(7):410-416. https://doi.org/10.1002/jhm.570.
157. Beaulieu Y, Laprise R, Drolet P, et al. Bedside ultrasound training using web-based e-learning and simulation early in the curriculum of residents. Crit Ultrasound J. 2015;7:1. https://doi.org/10.1186/s13089-014-0018-9.
158. Barsuk JH, Cohen ER, McGaghie WC, Wayne DB. Long-term retention of central venous catheter insertion skills after simulation-based mastery learning. Acad Med. 2010;85(10 Suppl):S9-S12. https://doi.org/10.1097/ACM.0b013e3181ed436c.
159. Wayne DB, Didwania A, Feinglass J, et al. Simulation-based education improves quality of care during cardiac arrest team responses at an academic teaching hospital: a case-control study. Chest. 2008;133(1):56-61. https://doi.org/10.1378/chest.07-0131.
160. Evans LV, Dodge KL, Shah TD, et al. Simulation training in central venous catheter insertion: improved performance in clinical practice. Acad Med. 2010;85(9):1462-1469. https://doi.org/10.1097/ACM.0b013e3181eac9a3.
161. Smith CC, Huang GC, Newman LR, et al. Simulation training and its effect on long-term resident performance in central venous catheterization. Simul Healthc J Soc Simul Healthc. 2010;5(3):146-151. https://doi.org/10.1097/SIH.0b013e3181dd9672.
162. Laack TA, Dong Y, Goyal DG, et al. Short-term and long-term impact of the central line workshop on resident clinical performance during simulated central line placement. Simul Healthc J Soc Simul Healthc. 2014;9(4):228-233. https://doi.org/10.1097/SIH.0000000000000015.
163. Dodge KL, Lynch CA, Moore CL, Biroscak BJ, Evans LV. Use of ultrasound guidance improves central venous catheter insertion success rates among junior residents. J Ultrasound Med. 2012;31(10):1519-1526. https://doi.org/10.7863/jum.2012.31.10.1519.
164. Bayci AW, Mangla J, Jenkins CS, Ivascu FA, Robbins JM. Novel educational module for subclavian central venous catheter insertion using real-time ultrasound guidance. J Surg Educ. 2015;72(6):1217-1223. https://doi.org/10.1016/j.jsurg.2015.07.010.
165. Andreatta P, Chen Y, Marsh M, Cho K. Simulation-based training improves applied clinical placement of ultrasound-guided PICCs. Support Care Cancer Off J Multinat Assoc Support Care Cancer. 2011;19(4):539-543. https://doi.org/10.1007/s00520-010-0849-2.
166. Rosen BT, Uddin PQ, Harrington AR, Ault BW, Ault MJ. Does personalized vascular access training on a nonhuman tissue model allow for learning and retention of central line placement skills? Phase II of the procedural patient safety initiative (PPSI-II). J Hosp Med. 2009;4(7):423-429. https://doi.org/10.1002/jhm.571.
167. Ault MJ, Rosen BT, Ault B. The use of tissue models for vascular access training. Phase I of the procedural patient safety initiative. J Gen Intern Med. 2006;21(5):514-517. https://doi.org/10.1111/j.1525-1497.2006.00440.x.
168. Varga S, Smith J, Minneti M, et al. Central venous catheterization using a perfused human cadaveric model: application to surgical education. J Surg Educ. 2015;72(1):28-32. https://doi.org/10.1016/j.jsurg.2014.07.005.
169. Sansivero GE. Venous anatomy and physiology. Considerations for vascular access device placement and function. J Intraven Nurs Off Publ Intraven Nurs Soc. 1998;21(5 Suppl):S107-S114.
170. Eisen LA, Narasimhan M, Berger JS, et al. Mechanical complications of central venous catheters. Journal of intensive care medicine. 2006;21(1):40-46. https://doi.org/10.1177/0885066605280884.
171. Chenkin J, Lee S, Huynh T, Bandiera G. Procedures can be learned on the Web: a randomized study of ultrasound-guided vascular access training. Acad Emerg Med. 2008;15(10):949-954. https://doi.org/10.1111/j.1553-2712.2008.00231.x.
172. Abualenain J, Calabrese K, Tansek R, Ranniger C. 319 Comparing standard versus video-based teaching for ultrasound-guided internal jugular central venous catheter access for fourth-year medical students. Ann Emerg Med. 2014;64(4):S113. https://doi.org/10.1016/j.annemergmed.2014.07.347.
173. Pustavoitau A, Blaivas M, Brown SM, et al. Recommendations for achieving and maintaining competence and credentialing in critical care ultrasound with focused cardiac ultrasound and advanced critical care echocardiography. Crit Care Med. 2016.
174. Jensen TP, Soni NJ, Tierney DM, Lucas BP. Hospital privileging practices for bedside procedures: A survey of hospitalist experts. J Hosp Med. 2017;12(10):836-839. https://doi.org/10.12788/jhm.2837.
175. Moureau N, Lamperti M, Kelly LJ, et al. Evidence-based consensus on the insertion of central venous access devices: definition of minimal requirements for training. Br J Anaesth. 2013;110(3):347-356. https://doi.org/10.1093/bja/aes499.
176. Ernst A, Silvestri GA, Johnstone D, American College of Chest Physicians. Interventional pulmonary procedures: guidelines from the American College of Chest Physicians. Chest. 2003;123(5):1693-1717. https://doi.org/10.1378/chest.123.5.1693.
177. Thomas SM, Burch W, Kuehnle SE, et al. Simulation training for pediatric residents on central venous catheter placement: a pilot study. Pediatr Crit Care Med J Soc Crit Care Med.. 2013;14(9):e416-e423. https://doi.org/10.1097/PCC.0b013e31829f5eda.
178. Smith KK, Gilcreast D, Pierce K. Evaluation of staff’s retention of ACLS and BLS skills. Resuscitation. 2008;78(1):59-65. https://doi.org/10.1016/j.resuscitation.2008.02.007.
179. Ericsson KA. Deliberate practice and the acquisition and maintenance of expert performance in medicine and related domains. Acad Med. 2004;79(10 Suppl):S70-S81. https://doi.org/10.1097/00001888-200410001-00022.
180. Gerard JM, Thomas SM, Germino KW, et al. The effect of simulation training on PALS skills among family medicine residents. Fam Med. 2011;43(6):392-399.
181. Brydges R, Nair P, Ma I, Shanks D, Hatala R. Directed self-regulated learning versus instructor-regulated learning in simulation training. Med Educ. 2012;46(7):648-656. https://doi.org/10.1111/j.1365-2923.2012.04268.x.
182. Wayne DB, Butter J, Siddall VJ, et al. Simulation-based training of internal medicine residents in advanced cardiac life support protocols: a randomized trial. Teach Learn Med. 2005;17(3):210-216. https://doi.org/10.1207/s15328015tlm1703_3.
183. Arthur Jr. W, Bennett Jr. W, Stanush PL, McNelly TL. Factors that influence skill decay and retention: A quantitative review and analysis. Hum Perform. 1998;11(1):57-101. https://doi.org/10.1207/s15327043hup1101_3.
184. Rusche JD, Besuner P, Partusch SK, Berning PA. Competency program development across a merged healthcare network. J Nurs Staff Dev. 2001;17(5):234-240; quiz 241-232. https://doi.org/10.1097/00124645-200109000-00004.
185. O’Hearne Rebholz M. A review of methods to assess competency. J Nurs Staff Dev. 2006;22(5):241-245. https://doi.org/10.1097/00124645-200609000-00007.
186. Reznick RK. Teaching and testing technical skills. Am J Surg. 1993;165(3):358-361. https://doi.org/10.1016/s0002-9610(05)80843-8.
187. Reznick RK, MacRae H. Teaching surgical skills--changes in the wind. N Engl J Med. 2006;355(25):2664-2669. https://doi.org/10.1056/NEJMra054785.
188. Murin S, Stollenwerk NS. Simulation in procedural training: at the tipping point. Chest. 2010;137(5):1009-1011. https://doi.org/10.1378/chest.10-0199.
189. American College of Emergency Physicians. Emergency ultrasound guidelines. Ann Emerg Med. 2009;53(4):550-570. https://doi.org/10.1016/j.annemergmed.2008.12.013.
190. Guilbert MC, Elkouri S, Bracco D, et al. Arterial trauma during central venous catheter insertion: case series, review and proposed algorithm. J Vasc Surg. 2008;48(4):918-925; discussion 925. https://doi.org/10.1016/j.jvs2008.04.046.
191. Dong Y, Suri HS, Cook DA, et al. Simulation-based objective assessment discerns clinical proficiency in central line placement: a construct validation. Chest. 2010;137(5):1050-1056. https://doi.org/10.1378/chest.09-1451.
192. Adhikari S, Theodoro D, Raio C, et al. Central venous catheterization: are we using ultrasound guidance? J Ultrasound Med. 2015;34(11):2065-2070. https://doi.org/10.7863/ultra.15.01027.
193. Mourad M, Kohlwes J, Maselli J, MERN Group, Auerbach AD. Supervising the supervisors--procedural training and supervision in internal medicine residency. J Gen Intern Med. 2010;25(4):351-356. https://doi.org/10.1007/s11606-009-1226-z.
1. Raad I. Intravascular-catheter-related infections. Lancet. 1998;351(9106):893-898. https://doi.org/10.1016/S0140-6736(97)10006-X.
2. Merrer J, De Jonghe B, Golliot F, et al. Complications of femoral and subclavian venous catheterization in critically ill patients: a randomized controlled trial. JAMA. 2001;286(6):700-707. https://doi.org/10.1001/jama.286.6.700.
3. Sznajder JI, Zveibil FR, Bitterman H, Weiner P, Bursztein S. Central vein catheterization. Failure and complication rates by three percutaneous approaches. Arch Intern Med. 1986;146(2):259-261. https://doi.org/10.1001/archinte.146.2.259.
4. Randolph AG, Cook DJ, Gonzales CA, Pribble CG. Ultrasound guidance for placement of central venous catheters: a meta-analysis of the literature. Crit Care Med. 1996;24(12):2053-2058. https://doi.org/10.1097/00003246-199612000-00020.
5. Hind D, Calvert N, McWilliams R, et al. Ultrasonic locating devices for central venous cannulation: meta-analysis. BMJ. 2003;327(7411):361. https://doi.org/10.1136/bmj.327.7411.361.
6. O’Grady NP, Alexander M, Burns LA, et al. Guidelines for the prevention of intravascular catheter-related infections. Am J Infect Control. 2011;39(4):S1-S34. https://doi.org/10.1016/j.ajic.2011.01.003.
7. National Institute for Health and Care Excellence (NICE). Guidance on the Use of Ultrasound Locating Devices for Placing Central Venous Catheters; 2002.
8. Shojania KG, Duncan BW, McDonald KM, et al. Making health care safer: A critical analysis of patient safety practices. Evid Rep Technol Assess. Rockville, MD: Agency for Healthcare Research and Quality. 2001;43(43):i–x, 1.
9. Lamperti M, Bodenham AR, Pittiruti M, et al. International evidence-based recommendations on ultrasound-guided vascular access. Intensive Care Med. 2012;38(7):1105-1117. https://doi.org/10.1007/s00134-012-2597-x.
10. Frykholm P, Pikwer A, Hammarskjöld F, et al. Clinical guidelines on central venous catheterisation. Swedish Society of Anaesthesiology and Intensive Care Medicine. Acta Anaesthesiol Scand. 2014;58(5):508-524. https://doi.org/10.1111/aas.12295.
11. American Society of Anesthesiologists Task Force on Central Venous Access, Rupp SM, Apfelbaum JL, et al. Practice guidelines for central venous access: a report by the American Society of Anesthesiologists Task Force on Central Venous Access. Anesthesiology. 2012;116(3):539-573. https://doi.org/10.1097/ALN.0b013e31823c9569.
12. Frankel HL, Kirkpatrick AW, Elbarbary M, et al. Guidelines for the appropriate use of bedside general and cardiac ultrasonography in the evaluation of critically ill patients-part I: General ultrasonography. Crit Care Med. 2015;43(11):2479-2502. https://doi.org/10.1097/CCM.0000000000001216.
13. Shekelle PG, Wachter RM, Pronovost PJ, et al. Making health care safer II: An updated critical analysis of the evidence for patient safety practices. Evid Rep Technol Assess. 2013;211:1-945.
14. Troianos CA, Hartman GS, Glas KE, et al. Guidelines for performing ultrasound guided vascular cannulation: recommendations of the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. J Am Soc Echocardiogr. 2011;24(12):1291-1318. https://doi.org/10.1016/j.echo.2011.09.021.
15. Soni NJ, Reyes LF, Keyt H, et al. Use of ultrasound guidance for central venous catheterization: a national survey of intensivists and hospitalists. J Crit Care. 2016;36:277-283. https://doi.org/10.1016/j.jcrc.2016.07.014.
16. Parienti JJ, Mongardon N, Mégarbane B, et al. Intravascular complications of central venous catheterization by insertion site. N Engl J Med. 2015;373(13):1220-1229. https://doi.org/10.1056/NEJMoa1500964.
17. Maizel J, Bastide MA, Richecoeur J, et al. Practice of ultrasound-guided central venous catheter technique by the French intensivists: a survey from the BoReal study group. Ann Intensive Care. 2016;6(1):76. https://doi.org/10.1186/s13613-016-0177-x.
18. Egan G, Healy D, O’Neill H, et al. Ultrasound guidance for difficult peripheral venous access: systematic review and meta-analysis. Emerg Med J. 2013;30(7):521-526. https://doi.org/10.1136/emermed-2012-201652.
19. Stolz LA, Stolz U, Howe C, Farrell IJ, Adhikari S. Ultrasound-guided peripheral venous access: a meta-analysis and systematic review. J Vasc Access. 2015;16(4):321-326. https://doi.org/10.5301/jva.5000346.
20. Sofocleous CT, Schur I, Cooper SG, et al. Sonographically guided placement of peripherally inserted central venous catheters: review of 355 procedures. AJR Am J Roentgenol. 1998;170(6):1613-1616. https://doi.org/10.2214/ajr.170.6.9609183.
21. Nichols I, Humphrey JP. The efficacy of upper arm placement of peripherally inserted central catheters using bedside ultrasound and microintroducer technique. J Infus Nurs Off Publ Infus Nurs Soc. 2008;31(3):165-176. https://doi.org/10.1097/01.NAN.0000317703.66395.b8.
22. Stokowski G, Steele D, Wilson D. The use of ultrasound to improve practice and reduce complication rates in peripherally inserted central catheter insertions. J Infus Nurs Off Publ Infus Nurs Soc. 2009;32(3):145-155. https://doi.org/10.1097/NAN.0b013e3181a1a98f.
23. Shiloh AL, Savel RH, Paulin LM, Eisen LA. Ultrasound-guided catheterization of the radial artery: a systematic review and meta-analysis of randomized controlled trials. Chest. 2011;139(3):524-529. https://doi.org/10.1378/chest.10-0919.
24. Lucas BP, Tierney DM, Jensen TP, et al. Credentialing of hospitalists in ultrasound-guided bedside procedures: A position statement of the Society of Hospital Medicine. J Hosp Med. 2018;13(2):117-125. https://doi.org/10.12788/jhm.2917.
25. Chopra V, Flanders SA, Saint S, et al. The Michigan appropriateness guide for intravenous catheters (Magic): results From a Multispecialty Panel Using the RAND/UCLA Appropriateness Method. Ann Intern Med. 2015;163(6):S1-S40. https://doi.org/10.7326/M15-0744.
26. Raad II, Hohn DC, Gilbreath BJ, et al. Prevention of central venous catheter-related infections by using maximal sterile barrier precautions during insertion. Infect Control Hosp Epidemiol. 1994;15(4):231-238.
27. Slama M, Novara A, Safavian A, et al. Improvement of internal jugular vein cannulation using an ultrasound-guided technique. Intensive Care Med. 1997;23(8):916-919. https://doi.org/10.1007/s001340050432.
28. Seyahi N, Kahveci A, Altiparmak MR, Serdengecti K, Erek E. Ultrasound imaging findings of femoral veins in patients with renal failure and its impact on vascular access. Nephrol Dial Transplant. 2005;20(9):1864-1867. https://doi.org/10.1093/ndt/gfh942.
29. Lin BS, Kong CW, Tarng DC, Huang TP, Tang GJ. Anatomical variation of the internal jugular vein and its impact on temporary haemodialysis vascular access: an ultrasonographic survey in uraemic patients. Nephrol Dial Transplant. 1998;13(1):134-138. https://doi.org/10.1093/ndt/13.1.134.
30. Troianos CA, Kuwik RJ, Pasqual JR, Lim AJ, Odasso DP. Internal jugular vein and carotid artery anatomic relation as determined by ultrasonography. Anesthesiology. 1996;85(1):43-48. https://doi.org/10.1097/00000542-199607000-00007.
31. Gordon AC, Wright I, Pugh ND. Duplication of the superficial femoral vein: recognition with duplex ultrasonography. Clin Rad. 1996;51(9):622-624. https://doi.org/10.1016/S0009-9260(96)80055-9.
32. Sulek CA, Gravenstein N, Blackshear RH, Weiss L. Head rotation during internal jugular vein cannulation and the risk of carotid artery puncture. Anesth Analg. 1996;82(1):125-128. https://doi.org/10.1097/00000539-199601000-00022.
33. Shrestha BR, Gautam B. Ultrasound versus the landmark technique: a prospective randomized comparative study of internal jugular vein cannulation in an intensive care unit. JNMA J Nepal Med Assoc. 2011;51(182):56-61. https://doi.org/10.31729/jnma.148.
34. Brederlau J, Greim C, Schwemmer U, et al. Ultrasound-guided cannulation of the internal jugular vein in critically ill patients positioned in 30 degrees dorsal elevation. Eur J Anaesthesiol. 2004;21(9):684-687. https://doi.org/10.1097/00003643-200409000-00003.
35. Lamperti M, Subert M, Cortellazzi P, et al. Is a neutral head position safer than 45-degree neck rotation during ultrasound-guided internal jugular vein cannulation? Results of a randomized controlled clinical trial. Anesth Analg. 2012;114(4):777-784. https://doi.org/10.1213/ANE.0b013e3182459917.
36. Benter T, Teichgräber UK, Klühs L, et al. Anatomical variations in the internal jugular veins of cancer patients affecting central venous access. Anatomical variation of the internal jugular vein. Ultraschall Med. 2001;22(1):23-26. https://doi.org/10.1055/s-2001-11243.
37. Caridi JG, Hawkins IF, Jr, Wiechmann BN, Pevarski DJ, Tonkin JC. Sonographic guidance when using the right internal jugular vein for central vein access. AJR Am J Roentgenol. 1998;171(5):1259-1263. https://doi.org/10.2214/ajr.171.5.9798857.
38. Brusasco C, Corradi F, Zattoni PL, et al. Ultrasound-guided central venous cannulation in bariatric patients. Obes Surg. 2009;19(10):1365-1370. https://doi.org/10.1007/s11695-009-9902-y.
39. Armstrong PJ, Cullen M, Scott DH. The ‘SiteRite’ ultrasound machine--an aid to internal jugular vein cannulation. Anaesthesia. 1993;48(4):319-323. https://doi.org/10.1111/j.1365-2044.1993.tb06953.x.
40. Forauer AR, Glockner JF. Importance of US findings in access planning during jugular vein hemodialysis catheter placements. J Vasc Interv Rad. 2000;11(2 Pt 1):233-238. https://doi.org/10.1016/S1051-0443(07)61471-7.
41. Hassan C, Girishkumar HT, Thatigotla B, et al. Value of ultrasound guidance in placement of hemodialysis access catheters in patients with end-stage renal disease. Am Surg. 2008;74(11):1111-1113.
42. Tan CO, Weinberg L, Peyton P, Story D, McNicol L. Size variation between contralateral infraclavicular axillary veins within individual patients-implications for subclavian venous central line insertion. Crit Care Med. 2013;41(2):457-463. https://doi.org/10.1097/CCM.0b013e31826ab1dd.
43. Wu SY, Ling Q, Cao LH, et al. Real-time two-dimensional ultrasound guidance for central venous cannulation: a meta-analysis. Anesthesiology. 2013;118(2):361-375. https://doi.org/10.1097/ALN.0b013e31827bd172.
44. Kim W, Chung RK, Lee GY, Han JI. The effects of hip abduction with external rotation and reverse Trendelenburg position on the size of the femoral vein; ultrasonographic investigation. Korean J Anesthesiol. 2011;61(3):205-209. https://doi.org/10.4097/kjae.2011.61.3.205.
45. Denys BG, Uretsky BF. Anatomical variations of internal jugular vein location: impact on central venous access. Crit Care Med. 1991;19(12):1516-1519. https://doi.org/10.1097/00003246-199112000-00013.
46. Brass P, Hellmich M, Kolodziej L, Schick G, Smith AF. Ultrasound guidance versus anatomical landmarks for internal jugular vein catheterization. Cochrane Database Syst Rev. 2015;1:CD006962. https://doi.org/10.1002/14651858.CD006962.pub2.
47. Gilbert TB, Seneff MG, Becker RB. Facilitation of internal jugular venous cannulation using an audio-guided Doppler ultrasound vascular access device: results from a prospective, dual-center, randomized, crossover clinical study. Crit Care Med. 1995;23(1):60-65. https://doi.org/10.1097/00003246-199501000-00012.
48. Schummer W, Schummer C, Tuppatsch H, et al. Ultrasound-guided central venous cannulation: is there a difference between Doppler and B-mode ultrasound? J Clin Anesth. 2006;18(3):167-172. https://doi.org/10.1016/j.jclinane.2005.12.010.
49. Airapetian N, Maizel J, Langelle F, et al. Ultrasound-guided central venous cannulation is superior to quick-look ultrasound and landmark methods among inexperienced operators: a prospective randomized study. Intensive Care Med. 2013;39(11):1938-1944. https://doi.org/10.1007/s00134-013-3072-z.
50. Milling TJ, Jr, Rose J, Briggs WM, et al. Randomized, controlled clinical trial of point-of-care limited ultrasonography assistance of central venous cannulation: the Third Sonography Outcomes Assessment Program (SOAP-3) Trial. Crit Care Med. 2005;33(8):1764-1769. https://doi.org/10.1097/01.ccm.0000171533.92856.e5.
51. Beaudoin FL, Merchant RC, Lincoln J, et al. Bedside ultrasonography detects significant femoral vessel overlap: implications for central venous cannulation. CJEM. 2011;13(4):245-250. https://doi.org/10.2310/8000.2011.110482.
52. Kwon TH, Kim YL, Cho DK. Ultrasound-guided cannulation of the femoral vein for acute haemodialysis access. Nephrol Dial Transplant. 1997;12(5):1009-1012. https://doi.org/10.1093/ndt/12.5.1009.
53. Rothschild JM. Ultrasound guidance of central vein catheterization. Evid Rep Technol Assess. 2001;43. Chapter 21.: http://archive.ahrq.gov/clinic/ptsafety/chap21.htm.
54. Lennon M, Zaw NN, Pöpping DM, Wenk M. Procedural complications of central venous catheter insertion. Minerva Anestesiol. 2012;78(11):1234-1240.
55. Blaivas M, Adhikari S. An unseen danger: frequency of posterior vessel wall penetration by needles during attempts to place internal jugular vein central catheters using ultrasound guidance. Crit Care Med. 2009;37(8):2345-2349; quiz 2359. https://doi.org/10.1097/CCM.0b013e3181a067d4.
56. Vogel JA, Haukoos JS, Erickson CL, et al. Is long-axis view superior to short-axis view in ultrasound-guided central venous catheterization? Crit Care Med. 2015;43(4):832-839. https://doi.org/10.1097/CCM.0000000000000823.
57. Tammam TF, El-Shafey EM, Tammam HF. Ultrasound-guided internal jugular vein access: comparison between short axis and long axis techniques. Saudi J Kidney Dis Transpl. 2013;24(4):707-713. https://doi.org/10.4103/1319-2442.113861.
58. Keyes LE, Frazee BW, Snoey ER, Simon BC, Christy D. Ultrasound-guided brachial and basilic vein cannulation in emergency department patients with difficult intravenous access. Ann Emerg Med. 1999;34(6):711-714. https://doi.org/10.1016/S0196-0644(99)70095-8.
59. Mahler SA, Wang H, Lester C, et al. Short- vs long-axis approach to ultrasound-guided peripheral intravenous access: a prospective randomized study. Am J Emerg Med. 2011;29(9):1194-1197. https://doi.org/10.1016/j.ajem.2010.07.015.
60. Berk D, Gurkan Y, Kus A, et al. Ultrasound-guided radial arterial cannulation: long axis/in-plane versus short axis/out-of-plane approaches? J Clin Monit Comput. 2013;27(3):319-324. https://doi.org/10.1007/s10877-013-9437-6.
61. Batllori M, Urra M, Uriarte E, et al. Randomized comparison of three transducer orientation approaches for ultrasound guided internal jugular venous cannulation. Br J Anaesth. 2016;116(3):370-376. https://doi.org/10.1093/bja/aev399.
62. Moak JH, Lyons MS, Wright SW, Lindsell CJ. Needle and guidewire visualization in ultrasound-guided internal jugular vein cannulation. Am J Emerg Med. 2011;29(4):432-436. https://doi.org/10.1016/j.ajem.2010.01.004.
63. Moak JH, Rajkumar JS, Woods WA. The wire is really easy to see (WIRES): sonographic visualization of the guidewire by novices. CJEM. 2013;15(1):18-23. https://doi.org/10.2310/8000.2012.120800.
64. Gillman LM, Blaivas M, Lord J, Al-Kadi A, Kirkpatrick AW. Ultrasound confirmation of guidewire position may eliminate accidental arterial dilatation during central venous cannulation. Scand J Trauma Resusc Emerg Med. 2010;18:39. https://doi.org/10.1186/1757-7241-18-39.
65. Augoustides JG, Horak J, Ochroch AE, et al. A randomized controlled clinical trial of real-time needle-guided ultrasound for internal jugular venous cannulation in a large university anesthesia department. J Cardiothorac Vasc Anesth. 2005;19(3):310-315. https://doi.org/10.1053/j.jvca.2005.03.007.
66. Maecken T, Heite L, Wolf B, Zahn PK, Litz RJ. Ultrasound-guided catheterisation of the subclavian vein: freehand vs needle-guided technique. Anaesthesia. 2015;70(11):1242-1249. https://doi.org/10.1111/anae.13187.
67. Stone MB, Nagdev A, Murphy MC, Sisson CA. Ultrasound detection of guidewire position during central venous catheterization. Am J Emerg Med. 2010;28(1):82-84. https://doi.org/10.1016/j.ajem.2008.09.019.
68. Luyet C, Hartwich V, Urwyler N, et al. Evaluation of a novel needle guide for ultrasound-guided phantom vessel cannulation. Anaesthesia. 2011;66(8):715-720. https://doi.org/10.1111/j.1365-2044.2011.06781.x.
69. O’Grady NP, Alexander M, Burns LA, et al. Summary of recommendations: Guidelines for the Prevention of Intravascular Catheter-related Infections. Clin Infect Dis. 2011;52(9):1087-1099. https://doi.org/10.1093/cid/cir138.
70. Southworth SL, Henman LJ, Kinder LA, Sell JL. The journey to zero central catheter-associated bloodstream infections: culture change in an intensive care unit. Crit Care Nurse. 2012;32(2):49-54. https://doi.org/10.4037/ccn2012915.
71. Guerin K, Wagner J, Rains K, Bessesen M. Reduction in central line-associated bloodstream infections by implementation of a postinsertion care bundle. Am J Infect Control. 2010;38(6):430-433. https://doi.org/10.1016/j.ajic.2010.03.007.
72. Hartman N, Wittler M, Askew K, Manthey D. Delphi method validation of a procedural performance checklist for insertion of an ultrasound-guided internal jugular central line. Am J Med Qual Off J Am Coll Med Qual. 2016;31(1):81-85. https://doi.org/10.1177/1062860614549762.
73. Lenchus JD, Carvalho CM, Ferreri K, et al. Filling the void: defining invasive bedside procedural competency for internal medicine residents. J Grad Med Educ. 2013;5(4):605-612. https://doi.org/10.4300/JGME-D-13-00030.1.
74. Barsuk JH, Cohen ER, Feinglass J, McGaghie WC, Wayne DB. Use of simulation-based education to reduce catheter-related bloodstream infections. Arch Intern Med. 2009;169(15):1420-1423. https://doi.org/10.1001/archinternmed.2009.215.
75. Karakitsos D, Labropoulos N, De Groot E, et al. Real-time ultrasound-guided catheterisation of the internal jugular vein: a prospective comparison with the landmark technique in critical care patients. Crit Care. 2006;10(6):R162. https://doi.org/10.1186/cc5101.
76. Berenholtz SM, Pronovost PJ, Lipsett PA, et al. Eliminating catheter-related bloodstream infections in the intensive care unit. Crit Care Med. 2004;32(10):2014-2020. https://doi.org/10.1097/01.ccm.0000142399.70913.2f.
77. Young EM, Commiskey ML, Wilson SJ. Translating evidence into practice to prevent central venous catheter-associated bloodstream infections: a systems-based intervention. Am J Infect Control. 2006;34(8):503-506. https://doi.org/10.1016/j.ajic.2006.03.011.
78. Galpern D, Guerrero A, Tu A, Fahoum B, Wise L. Effectiveness of a central line bundle campaign on line-associated infections in the intensive care unit. Surgery. 2008;144(4):492-495; discussion 495. https://doi.org/10.1016/j.surg.2008.06.004.
79. Mermel LA, McCormick RD, Springman SR, Maki DG. The pathogenesis and epidemiology of catheter-related infection with pulmonary artery swan-Ganz catheters: a prospective study utilizing molecular subtyping. Am J Med. 1991;91(3b):197S–205S. https://doi.org/10.1016/0002-9343(91)90369-9.
80. Marschall J, Mermel LA, Classen D, et al. Strategies to prevent central line-associated bloodstream infections in acute care hospitals. Infect Control Hosp Epidemiol. 2008;29(1):S22-S30. https://doi.org/10.1086/591059.
81. Marschall J, Mermel LA, Fakih M, et al. Strategies to prevent central line-associated bloodstream infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(7):753-771. https://doi.org/10.1086/676533.
82. Garcia-Rodriguez JF, Álvarez-Díaz H, Vilariño-Maneiro L, et al. Epidemiology and impact of a multifaceted approach in controlling central venous catheter associated blood stream infections outside the intensive care unit. BMC Infect Dis. 2013;13:445. https://doi.org/10.1186/1471-2334-13-445.
83. Lee DH, Jung KY, Choi YH. Use of maximal sterile barrier precautions and/or antimicrobial-coated catheters to reduce the risk of central venous catheter-related bloodstream infection. Infect Control Hosp Epidemiol. 2008;29(10):947-950. https://doi.org/10.1086/590356.
84. Mermel LA. Prevention of intravascular catheter-related infections. Ann Intern Med. 2000;132(5):391-402. https://doi.org/10.7326/0003-4819-132-5-200003070-00009.
85. Troianos CA, Jobes DR, Ellison N. Ultrasound-guided cannulation of the internal jugular vein. A prospective, randomized study. Anesth Analg. 1991;72(6):823-826. https://doi.org/10.1213/00000539-199106000-00020.
86. Troianos CA, Savino JS. Internal jugular vein cannulation guided by echocardiography. Anesthesiology. 1991;74(4):787-789. https://doi.org/10.1097/00000542-199104000-00026.
87. Denys BG, Uretsky BF, Reddy PS, et al. An ultrasound method for safe and rapid central venous access. N Engl J Med. 1991;324(8):566. https://doi.org/10.1056/NEJM199102213240816.
88. Riaz A, Shan Khan RA, Salim F. Ultrasound guided internal jugular venous cannulation: comparison with land-mark technique. J Coll Phys Surg Pak. 2015;25(5):315-319. https://doi.org/05.2015/JCPSP.315319.
89. Gok F, Kilicaslan A, Sarkilar G, Kandemir B, Yosunkaya A. The effect of ultrasound guidance on central venous catheter-associated bloodstream infection in critical care patients. Acta Med Mediterr. 2013;29:677-682.
90. Lamperti M, Cortellazzi P, D’Onofrio G, et al. An outcome study on complications using routine ultrasound assistance for internal jugular vein cannulation. Acta Anaesthesiol Scand. 2007;51(10):1327-1330. https://doi.org/10.1111/j.1399-6576.2007.01442.x.
91. Vezzani A, Manca T, Vercelli A, Braghieri A, Magnacavallo A. Ultrasonography as a guide during vascular access procedures and in the diagnosis of complications. J Ultrasound. 2013;16(4):161-170. https://doi.org/10.1007/s40477-013-0046-5.
92. Palepu GB, Deven J, Subrahmanyam M, Mohan S. Impact of ultrasonography on central venous catheter insertion in intensive care. Indian J Rad Imaging. 2009;19(3):191-198. https://doi.org/10.4103/0971-3026.54877.
93. Agarwal A, Singh DK, Singh AP. Ultrasonography: a novel approach to central venous cannulation. Indian J Crit Care Med. 2009;13(4):213-216. https://doi.org/10.4103/0972-5229.60174.
94. Wong SW, Niazi AU, Chin KJ, Chan VW. Real-time ultrasound-guided spinal anesthesia using the SonixGPS® needle tracking system: a case report. Can J Anaesth. 2013;60(1):50-53. https://doi.org/10.1007/s12630-012-9809-2.
95. Bouaziz H, Zetlaoui PJ, Pierre S, et al. Guidelines on the use of ultrasound guidance for vascular access. Anaesth, Crit Care Pain Med. 2015;34(1):65-69. https://doi.org/10.1016/j.accpm.2015.01.004.
96. Jenssen C, Brkljacic B, Hocke M, et al. EFSUMB guidelines on interventional ultrasound (INVUS), Part VI - Ultrasound-guided vascular interventions. Ultraschall Med. 2016;37(5):473-476. https://doi.org/10.1055/s-0035-1553450.
97. Rando K, Castelli J, Pratt JP, et al. Ultrasound-guided internal jugular vein catheterization: a randomized controlled trial. Heart Lung Vessels. 2014;6(1):13-23.
98. Mey U, Glasmacher A, Hahn C, et al. Evaluation of an ultrasound-guided technique for central venous access via the internal jugular vein in 493 patients. Support Care Cancer Off J Multinat Assoc Support Care Cancer. 2003;11(3):148-155. https://doi.org/10.1007/s00520-002-0399-3.
99. Singh SA, Sharma S, Singh A, et al. The safety of ultrasound guided central venous cannulation in patients with liver disease. Saudi J Anaesth. 2015;9(2):155-160. https://doi.org/10.4103/1658-354X.152842.
100. Akoglu H, Piskinpasa S, Yenigun EC, et al. Real-time ultrasound guided placement of temporary internal jugular vein catheters: assessment of technical success and complication rates in nephrology practice. Nephrol (Carlton). 2012;17(7):603-606. https://doi.org/10.1111/j.1440-1797.2012.01637.x.
101. Sadler DJ, Gordon AC, Klassen J, et al. Image-guided central venous catheters for apheresis. Bone Marrow Transplant. 1999;23(2):179-182. https://doi.org/10.1038/sj.bmt.1701545.
102. Calvert N, Hind D, McWilliams R, et al. Ultrasound for central venous cannulation: economic evaluation of cost-effectiveness. Anaesthesia. 2004;59(11):1116-1120. https://doi.org/10.1111/j.1365-2044.2004.03906.x.
103. Goetz AM, Wagener MM, Miller JM, Muder RR. Risk of infection due to central venous catheters: effect of site of placement and catheter type. Infect Control Hosp Epidemiol. 1998;19(11):842-845.
104. Bertini P, Frediani M. Ultrasound guided supraclavicular central vein cannulation in adults: a technical report. J Vasc Access. 2013;14(1):89-93. https://doi.org/10.5301/jva.5000088.
105. Lalu MM, Fayad A, Ahmed O, et al. Ultrasound-guided subclavian vein catheterization: A systematic review and meta-analysis. Crit Care Med. 2015;43(7):1498-1507. https://doi.org/10.1097/CCM.0000000000000973.
106. Milone M, Di Minno G, Di Minno MN, et al. The real effectiveness of ultrasound guidance in subclavian venous access. Ann ital chir. 2010;81(5):331-334.
107. O’Leary R, Ahmed SM, McLure H, et al. Ultrasound-guided infraclavicular axillary vein cannulation: a useful alternative to the internal jugular vein. Br J Anaesth. 2012;109(5):762-768. https://doi.org/10.1093/bja/aes262.
108. Gualtieri E, Deppe SA, Sipperly ME, Thompson DR. Subclavian venous catheterization: greater success rate for less experienced operators using ultrasound guidance. Crit Care Med. 1995;23(4):692-697. https://doi.org/10.1097/00003246-199504000-00018.
109. Fragou M, Gravvanis A, Dimitriou V, et al. Real-time ultrasound-guided subclavian vein cannulation versus the landmark method in critical care patients: a prospective randomized study. Crit Care Med. 2011;39(7):1607-1612. https://doi.org/10.1097/CCM.0b013e318218a1ae.
110. Brass P, Hellmich M, Kolodziej L, Schick G, Smith AF. Ultrasound guidance versus anatomical landmarks for subclavian or femoral vein catheterization. Cochrane Database Syst Rev. 2015;1:CD011447. https://doi.org/10.1002/14651858.CD011447.
111. Robinson MK, Mogensen KM, Grudinskas GF, Kohler S, Jacobs DO. Improved care and reduced costs for patients requiring peripherally inserted central catheters: the role of bedside ultrasound and a dedicated team. JPEN J Parenter Enter Nutr. 2005;29(5):374-379. https://doi.org/10.1177/0148607105029005374.
112. Li J, Fan YY, Xin MZ, et al. A randomised, controlled trial comparing the long-term effects of peripherally inserted central catheter placement in chemotherapy patients using B-mode ultrasound with modified Seldinger technique versus blind puncture. Eur J Oncol Nurs. 2014;18(1):94-103. https://doi.org/10.1016/j.ejon.2013.08.003.
113. Schweickert WD, Herlitz J, Pohlman AS, et al. A randomized, controlled trial evaluating postinsertion neck ultrasound in peripherally inserted central catheter procedures. Crit Care Med. 2009;37(4):1217-1221. https://doi.org/10.1097/CCM.0b013e31819cee7f.
114. Mahler SA, Wang H, Lester C, Conrad SA. Ultrasound-guided peripheral intravenous access in the emergency department using a modified Seldinger technique. J Emerg Med. 2010;39(3):325-329. https://doi.org/10.1016/j.jemermed.2009.02.013.
115. Brannam L, Blaivas M, Lyon M, Flake M. Emergency nurses’ utilization of ultrasound guidance for placement of peripheral intravenous lines in difficult-access patients. Acad Emerg Med. 2004;11(12):1361-1363. https://doi.org/10.1197/j.aem.2004.08.027.
116. Au A, Rotte M, Gryzbowski R, Ku B, Fields J. 157 Decrease in central venous catheter placement and complications due to utilization of ultrasound-guided peripheral intravenous catheters. Ann Emerg Med. 2011;58(4):S230. https://doi.org/10.1016/j.annemergmed.2011.06.185.
117. Dargin JM, Rebholz CM, Lowenstein RA, Mitchell PM, Feldman JA. Ultrasonography-guided peripheral intravenous catheter survival in ED patients with difficult access. Am J Emerg Med. 2010;28(1):1-7. https://doi.org/10.1016/j.ajem.2008.09.001.
118. Bauman M, Braude D, Crandall C. Ultrasound-guidance vs standard technique in difficult vascular access patients by ED technicians. Am J Emerg Med. 2009;27(2):135-140. https://doi.org/10.1016/j.ajem.2008.02.005.
119. Schoenfeld E, Shokoohi H, Boniface K. Ultrasound-guided peripheral intravenous access in the emergency department: patient-centered survey. West J Emerg Med. 2011;12(4):475-477. https://doi.org/10.5811/westjem.2011.3.1920.
120. Maki DG, Ringer M. Risk factors for infusion-related phlebitis with small peripheral venous catheters. A randomized controlled trial. Ann Intern Med. 1991;114(10):845-854. https://doi.org/10.7326/0003-4819-114-10-845.
121. Miliani K, Taravella R, Thillard D, et al. Peripheral venous catheter-related adverse events: evaluation from a multicentre epidemiological study in France (the CATHEVAL Project). PLOS ONE. 2017;12(1):e0168637. https://doi.org/10.1371/journal.pone.0168637.
122. Gregg SC, Murthi SB, Sisley AC, Stein DM, Scalea TM. Ultrasound-guided peripheral intravenous access in the intensive care unit. J Crit Care. 2010;25(3):514-519. https://doi.org/10.1016/j.jcrc.2009.09.003.
123. Adhikari S, Blaivas M, Morrison D, Lander L. Comparison of infection rates among ultrasound-guided versus traditionally placed peripheral intravenous lines. J Ultrasound Med. 2010;29(5):741-747. https://doi.org/10.7863/jum.2010.29.5.741.
124. Shiver S, Blaivas M, Lyon M. A prospective comparison of ultrasound-guided and blindly placed radial arterial catheters. Acad Emerg Med. 2006;13(12):1275-1279. https://doi.org/10.1197/j.aem.2006.07.015.
125. Levin PD, Sheinin O, Gozal Y. Use of ultrasound guidance in the insertion of radial artery catheters. Crit Care Med. 2003;31(2):481-484. https://doi.org/10.1097/01.CCM.0000050452.17304.2F.
126. Gu WJ, Tie HT, Liu JC, Zeng XT. Efficacy of ultrasound-guided radial artery catheterization: a systematic review and meta-analysis of randomized controlled trials. Crit Care. 2014;18(3):R93. https://doi.org/10.1186/cc13862.
127. Tang L, Wang F, Li Y, et al. Ultrasound guidance for radial artery catheterization: an updated meta-analysis of randomized controlled trials. PLOS ONE. 2014;9(11):e111527. https://doi.org/10.1371/journal.pone.0111527.
128. Sobolev M, Slovut DP, Lee Chang A, Shiloh AL, Eisen LA. Ultrasound-guided catheterization of the femoral artery: A systematic review and meta-analysis of randomized controlled trials. J Invas Cardiol. 2015;27(7):318-323. https://doi.org/10.1378/chest.1991181.
129. Gabriel M, Pawlaczyk K, Waliszewski K, Krasiński Z, Majewski W. Location of femoral artery puncture site and the risk of postcatheterization pseudoaneurysm formation. Int J Cardiol. 2007;120(2):167-171. https://doi.org/10.1016/j.ijcard.2006.09.018.
130. Seto AH, Abu-Fadel MS, Sparling JM, et al. Real-time ultrasound guidance facilitates femoral arterial access and reduces vascular complications: FAUST (Femoral Arterial Access With ultrasound Trial). JACC Cardiovasc Interv. 2010;3(7):751-758. https://doi.org/10.1016/j.jcin.2010.04.015.
131. Kalish J, Eslami M, Gillespie D, et al. Routine use of ultrasound guidance in femoral arterial access for peripheral vascular intervention decreases groin hematoma rates. J Vasc Surg. 2015;61(5):1231-1238. https://doi.org/10.1016/j.jvs2014.12.003.
132. Sandhu NS, Patel B. Use of ultrasonography as a rescue technique for failed radial artery cannulation. J Clin Anesth. 2006;18(2):138-141. https://doi.org/10.1016/j.jclinane.2005.06.011.
133. White L, Halpin A, Turner M, Wallace L. Ultrasound-guided radial artery cannulation in adult and paediatric populations: a systematic review and meta-analysis. Br J Anaesth. 2016;116(5):610-617. https://doi.org/10.1093/bja/aew097.
134. Gao YB, Yan JH, Gao FQ, et al. Effects of ultrasound-guided radial artery catheterization: an updated meta-analysis. Am J Emerg Med. 2015;33(1):50-55. https://doi.org/10.1016/j.ajem.2014.10.008.
135. Seto AH, Roberts JS, Abu-Fadel MS, et al. Real-time ultrasound guidance facilitates transradial access: RAUST (Radial Artery Access with Ultrasound Trial). JACC Cardiovasc Interv. 2015;8(2):283-291. https://doi.org/10.1016/j.jcin.2014.05.036.
136. Roberts J, Manur R. Ultrasound-guided radial artery access by a non-ultrasound trained interventional cardiologist improved first-attempt success rates and shortened time for successful radial artery cannulation. J Invas Cardiol. 2013;25(12):676-679.
137. Lichtenstein DA, Menu Y. A bedside ultrasound sign ruling out pneumothorax in the critically ill. Lung sliding. Chest. 1995;108(5):1345-1348. https://doi.org/10.1378/chest.108.5.1345.
138. Lichtenstein D, Mezière G, Biderman P, Gepner A. The comet-tail artifact: an ultrasound sign ruling out pneumothorax. Intensive Care Med. 1999;25(4):383-388. https://doi.org/10.1007/s001340050862.
139. Ablordeppey EA, Drewry AM, Beyer AB, et al. Diagnostic accuracy of central venous catheter confirmation by bedside ultrasound Versus chest radiography in critically ill patients: A systematic review and meta-analysis. Crit Care Med. 2017;45(4):715-724. https://doi.org/10.1097/CCM.0000000000002188.
140. Bedel J, Vallée F, Mari A, et al. Guidewire localization by transthoracic echocardiography during central venous catheter insertion: a periprocedural method to evaluate catheter placement. Intensive Care Med. 2013;39(11):1932-1937. https://doi.org/10.1007/s00134-013-3097-3.
141. Weekes AJ, Keller SM, Efune B, Ghali S, Runyon M. Prospective comparison of ultrasound and CXR for confirmation of central vascular catheter placement. Emerg Med J EMJ. 2016;33(3):176-180. https://doi.org/10.1136/emermed-2015-205000.
142. Arellano R, Nurmohamed A, Rumman A, et al. The utility of transthoracic echocardiography to confirm central line placement: an observational study. Can J Anaesth. 2014;61(4):340-346. https://doi.org/10.1007/s12630-014-0111-3.
143. Vezzani A, Brusasco C, Palermo S, et al. Ultrasound localization of central vein catheter and detection of postprocedural pneumothorax: an alternative to chest radiography. Crit Care Med. 2010;38(2):533-538. https://doi.org/10.1097/CCM.0b013e3181c0328f.
144. Choudhry NK, Fletcher RH, Soumerai SB. Systematic review: the relationship between clinical experience and quality of health care. Ann Intern Med. 2005;142(4):260-273. https://doi.org/10.7326/0003-4819-142-4-200502150-00008.
145. Backlund BH, Hopkins E, Kendall JL. Ultrasound guidance for central venous access by emergency physicians in Colorado. West J Emerg Med. 2012;13(4):320-325. https://doi.org/10.5811/westjem.2011.11.6821.
146. Buchanan MS, Backlund B, Liao MM, et al. Use of ultrasound guidance for central venous catheter placement: survey from the American Board of Emergency Medicine Longitudinal Study of Emergency Physicians. Acad Emerg Med. 2014;21(4):416-421. https://doi.org/10.1111/acem.12350.
147. Barsuk JH, McGaghie WC, Cohen ER, O’Leary KJ, Wayne DB. Simulation-based mastery learning reduces complications during central venous catheter insertion in a medical intensive care unit. Crit Care Med. 2009;37(10):2697-2701. https://doi.org/10.1097/00003246-200910000-00003.
148. Coopersmith CM, Rebmann TL, Zack JE, et al. Effect of an education program on decreasing catheter-related bloodstream infections in the surgical intensive care unit. Crit Care Med. 2002;30(1):59-64. https://doi.org/10.1097/00003246-200201000-00009.
149. Woo MY, Frank J, Lee AC, et al. Effectiveness of a novel training program for emergency medicine residents in ultrasound-guided insertion of central venous catheters. CJEM. 2009;11(4):343-348. https://doi.org/10.1017/S1481803500011398.
150. McGee DC, Gould MK. Preventing complications of central venous catheterization. N Engl J Med. 2003;348(12):1123-1133. https://doi.org/10.1056/NEJMra011883.
151. Barsuk JH, McGaghie WC, Cohen ER, Balachandran JS, Wayne DB. Use of simulation-based mastery learning to improve the quality of central venous catheter placement in a medical intensive care unit. J Hosp Med. 2009;4(7):397-403. https://doi.org/10.1002/jhm.468.
152. Sekiguchi H, Tokita JE, Minami T, et al. A prerotational, simulation-based workshop improves the safety of central venous catheter insertion: results of a successful internal medicine house staff training program. Chest. 2011;140(3):652-658. https://doi.org/10.1378/chest.10-3319.
153. Feller-Kopman D. Ultrasound-guided internal jugular access: a proposed standardized approach and implications for training and practice. Chest. 2007;132(1):302-309. https://doi.org/10.1378/chest.06-2711.
154. Troianos CA, Hartman GS, Glas KE, et al. Special articles: guidelines for performing ultrasound guided vascular cannulation: recommendations of the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. Anesth Analg. 2012;114(1):46-72. https://doi.org/10.1213/ANE.0b013e3182407cd8.
155. Issenberg SB, McGaghie WC, Hart IR, et al. Simulation technology for health care professional skills training and assessment. JAMA. 1999;282(9):861-866. https://doi.org/10.1001/jama.282.9.861.
156. Millington SJ, Wong RY, Kassen BO, Roberts JM, Ma IW. Improving internal medicine residents’ performance, knowledge, and confidence in central venous catheterization using simulators. J Hosp Med. 2009;4(7):410-416. https://doi.org/10.1002/jhm.570.
157. Beaulieu Y, Laprise R, Drolet P, et al. Bedside ultrasound training using web-based e-learning and simulation early in the curriculum of residents. Crit Ultrasound J. 2015;7:1. https://doi.org/10.1186/s13089-014-0018-9.
158. Barsuk JH, Cohen ER, McGaghie WC, Wayne DB. Long-term retention of central venous catheter insertion skills after simulation-based mastery learning. Acad Med. 2010;85(10 Suppl):S9-S12. https://doi.org/10.1097/ACM.0b013e3181ed436c.
159. Wayne DB, Didwania A, Feinglass J, et al. Simulation-based education improves quality of care during cardiac arrest team responses at an academic teaching hospital: a case-control study. Chest. 2008;133(1):56-61. https://doi.org/10.1378/chest.07-0131.
160. Evans LV, Dodge KL, Shah TD, et al. Simulation training in central venous catheter insertion: improved performance in clinical practice. Acad Med. 2010;85(9):1462-1469. https://doi.org/10.1097/ACM.0b013e3181eac9a3.
161. Smith CC, Huang GC, Newman LR, et al. Simulation training and its effect on long-term resident performance in central venous catheterization. Simul Healthc J Soc Simul Healthc. 2010;5(3):146-151. https://doi.org/10.1097/SIH.0b013e3181dd9672.
162. Laack TA, Dong Y, Goyal DG, et al. Short-term and long-term impact of the central line workshop on resident clinical performance during simulated central line placement. Simul Healthc J Soc Simul Healthc. 2014;9(4):228-233. https://doi.org/10.1097/SIH.0000000000000015.
163. Dodge KL, Lynch CA, Moore CL, Biroscak BJ, Evans LV. Use of ultrasound guidance improves central venous catheter insertion success rates among junior residents. J Ultrasound Med. 2012;31(10):1519-1526. https://doi.org/10.7863/jum.2012.31.10.1519.
164. Bayci AW, Mangla J, Jenkins CS, Ivascu FA, Robbins JM. Novel educational module for subclavian central venous catheter insertion using real-time ultrasound guidance. J Surg Educ. 2015;72(6):1217-1223. https://doi.org/10.1016/j.jsurg.2015.07.010.
165. Andreatta P, Chen Y, Marsh M, Cho K. Simulation-based training improves applied clinical placement of ultrasound-guided PICCs. Support Care Cancer Off J Multinat Assoc Support Care Cancer. 2011;19(4):539-543. https://doi.org/10.1007/s00520-010-0849-2.
166. Rosen BT, Uddin PQ, Harrington AR, Ault BW, Ault MJ. Does personalized vascular access training on a nonhuman tissue model allow for learning and retention of central line placement skills? Phase II of the procedural patient safety initiative (PPSI-II). J Hosp Med. 2009;4(7):423-429. https://doi.org/10.1002/jhm.571.
167. Ault MJ, Rosen BT, Ault B. The use of tissue models for vascular access training. Phase I of the procedural patient safety initiative. J Gen Intern Med. 2006;21(5):514-517. https://doi.org/10.1111/j.1525-1497.2006.00440.x.
168. Varga S, Smith J, Minneti M, et al. Central venous catheterization using a perfused human cadaveric model: application to surgical education. J Surg Educ. 2015;72(1):28-32. https://doi.org/10.1016/j.jsurg.2014.07.005.
169. Sansivero GE. Venous anatomy and physiology. Considerations for vascular access device placement and function. J Intraven Nurs Off Publ Intraven Nurs Soc. 1998;21(5 Suppl):S107-S114.
170. Eisen LA, Narasimhan M, Berger JS, et al. Mechanical complications of central venous catheters. Journal of intensive care medicine. 2006;21(1):40-46. https://doi.org/10.1177/0885066605280884.
171. Chenkin J, Lee S, Huynh T, Bandiera G. Procedures can be learned on the Web: a randomized study of ultrasound-guided vascular access training. Acad Emerg Med. 2008;15(10):949-954. https://doi.org/10.1111/j.1553-2712.2008.00231.x.
172. Abualenain J, Calabrese K, Tansek R, Ranniger C. 319 Comparing standard versus video-based teaching for ultrasound-guided internal jugular central venous catheter access for fourth-year medical students. Ann Emerg Med. 2014;64(4):S113. https://doi.org/10.1016/j.annemergmed.2014.07.347.
173. Pustavoitau A, Blaivas M, Brown SM, et al. Recommendations for achieving and maintaining competence and credentialing in critical care ultrasound with focused cardiac ultrasound and advanced critical care echocardiography. Crit Care Med. 2016.
174. Jensen TP, Soni NJ, Tierney DM, Lucas BP. Hospital privileging practices for bedside procedures: A survey of hospitalist experts. J Hosp Med. 2017;12(10):836-839. https://doi.org/10.12788/jhm.2837.
175. Moureau N, Lamperti M, Kelly LJ, et al. Evidence-based consensus on the insertion of central venous access devices: definition of minimal requirements for training. Br J Anaesth. 2013;110(3):347-356. https://doi.org/10.1093/bja/aes499.
176. Ernst A, Silvestri GA, Johnstone D, American College of Chest Physicians. Interventional pulmonary procedures: guidelines from the American College of Chest Physicians. Chest. 2003;123(5):1693-1717. https://doi.org/10.1378/chest.123.5.1693.
177. Thomas SM, Burch W, Kuehnle SE, et al. Simulation training for pediatric residents on central venous catheter placement: a pilot study. Pediatr Crit Care Med J Soc Crit Care Med.. 2013;14(9):e416-e423. https://doi.org/10.1097/PCC.0b013e31829f5eda.
178. Smith KK, Gilcreast D, Pierce K. Evaluation of staff’s retention of ACLS and BLS skills. Resuscitation. 2008;78(1):59-65. https://doi.org/10.1016/j.resuscitation.2008.02.007.
179. Ericsson KA. Deliberate practice and the acquisition and maintenance of expert performance in medicine and related domains. Acad Med. 2004;79(10 Suppl):S70-S81. https://doi.org/10.1097/00001888-200410001-00022.
180. Gerard JM, Thomas SM, Germino KW, et al. The effect of simulation training on PALS skills among family medicine residents. Fam Med. 2011;43(6):392-399.
181. Brydges R, Nair P, Ma I, Shanks D, Hatala R. Directed self-regulated learning versus instructor-regulated learning in simulation training. Med Educ. 2012;46(7):648-656. https://doi.org/10.1111/j.1365-2923.2012.04268.x.
182. Wayne DB, Butter J, Siddall VJ, et al. Simulation-based training of internal medicine residents in advanced cardiac life support protocols: a randomized trial. Teach Learn Med. 2005;17(3):210-216. https://doi.org/10.1207/s15328015tlm1703_3.
183. Arthur Jr. W, Bennett Jr. W, Stanush PL, McNelly TL. Factors that influence skill decay and retention: A quantitative review and analysis. Hum Perform. 1998;11(1):57-101. https://doi.org/10.1207/s15327043hup1101_3.
184. Rusche JD, Besuner P, Partusch SK, Berning PA. Competency program development across a merged healthcare network. J Nurs Staff Dev. 2001;17(5):234-240; quiz 241-232. https://doi.org/10.1097/00124645-200109000-00004.
185. O’Hearne Rebholz M. A review of methods to assess competency. J Nurs Staff Dev. 2006;22(5):241-245. https://doi.org/10.1097/00124645-200609000-00007.
186. Reznick RK. Teaching and testing technical skills. Am J Surg. 1993;165(3):358-361. https://doi.org/10.1016/s0002-9610(05)80843-8.
187. Reznick RK, MacRae H. Teaching surgical skills--changes in the wind. N Engl J Med. 2006;355(25):2664-2669. https://doi.org/10.1056/NEJMra054785.
188. Murin S, Stollenwerk NS. Simulation in procedural training: at the tipping point. Chest. 2010;137(5):1009-1011. https://doi.org/10.1378/chest.10-0199.
189. American College of Emergency Physicians. Emergency ultrasound guidelines. Ann Emerg Med. 2009;53(4):550-570. https://doi.org/10.1016/j.annemergmed.2008.12.013.
190. Guilbert MC, Elkouri S, Bracco D, et al. Arterial trauma during central venous catheter insertion: case series, review and proposed algorithm. J Vasc Surg. 2008;48(4):918-925; discussion 925. https://doi.org/10.1016/j.jvs2008.04.046.
191. Dong Y, Suri HS, Cook DA, et al. Simulation-based objective assessment discerns clinical proficiency in central line placement: a construct validation. Chest. 2010;137(5):1050-1056. https://doi.org/10.1378/chest.09-1451.
192. Adhikari S, Theodoro D, Raio C, et al. Central venous catheterization: are we using ultrasound guidance? J Ultrasound Med. 2015;34(11):2065-2070. https://doi.org/10.7863/ultra.15.01027.
193. Mourad M, Kohlwes J, Maselli J, MERN Group, Auerbach AD. Supervising the supervisors--procedural training and supervision in internal medicine residency. J Gen Intern Med. 2010;25(4):351-356. https://doi.org/10.1007/s11606-009-1226-z.
© 2019 Society of Hospital Medicine
The American Board of Pediatrics Response to the Pediatric Hospital Medicine Petition
In August of 2014, the Pediatric Hospital Medicine (PHM) community petitioned the American Board of Pediatrics (ABP) for a subspecialty certificate in PHM. A lengthy vetting process ensued during which the ABP consulted with a wide array of stakeholders. The ABP Board of Directors approved the request from the PHM community for a subspecialty certificate in December 2015 and published the results of the vetting process.1
The ABP received a second petition posted on PHM listserv, which opened with the following statement:
“We submit this petition letter to register a formal complaint, demand immediate action, and request a formal response from the ABP regarding the practice pathway criteria and the application of these criteria for the Pediatric Hospital Medicine specialty exam. Recently there has been considerable discussion on the Pediatric Hospital Medicine ListServ suggesting that the ABP’s implementation of the career pathway criteria has failed to respect and fairly assess the diverse career paths of numerous experienced pediatric hospitalists, which may impede their opportunities for professional advancement. Anecdotal reports on the ListServ also suggest that the use of the current practice pathway criteria to evaluate exam applicants disadvantages women, though sufficient data is not available at this time to evaluate this assertion objectively.”
The ABP response to the PHM community’s concerns regarding the practice pathway for the first certifying exam in PHM is as follows.
THE ABP RESPONSE
ABP thanks the PHM community for the opportunity to respond to the attached petition. Our approach and response are grounded in our mission:
“Advancing child health by certifying pediatricians who meet standards of excellence and are committed to continuous learning and improvement.”
Transparency is one of the ABP’s core values, which underpins this response. The ABP acknowledges that the petitioners did not find the guidance on the ABP website sufficiently transparent. We regret the distress this may have caused, will do our best to answer the questions forthrightly, and have revised the website language for greater clarity.
ALLEGATION OF GENDER BIAS
Some posts on the PHM listserv alleged gender (sex) bias against women in the ABP application process and outcomes. This allegation is not supported by the facts. A peer group of pediatric hospitalists constitutes the ABP PHM subboard which determined the eligibility criteria. The subboard thoughtfully developed these criteria and the American Board of Medical Specialties (ABMS) approved the broad eligibility criteria. The PHM subboard is composed of practicing pediatric hospitalists with a diversity of practice location, age, gender, and race. The majority of ABP PHM subboard members and medical editors are women.
Making unbiased decisions is also a core value of the ABP. Among the 1,627 applicants for the exam, the ABP has approved 1,515 (93%) as of August 15, 2019. Seventy percent of applications were from women, which mirrors the demographics of the pediatric workforce. There was no significant difference between the percentage of women (4.0%) and men (3.7%) who were denied admission to the exam (Table 1). As of August 15, 2019, the credentials committee of the PHM subboard is still reviewing 48 applications, including 35 appeals, of which 60% (N = 21) were from women and 40% (N = 14) were from men. Thirteen (N = 13) remaining applications are under review but not in the appeals process.
PRACTICE PATHWAY CRITERIA USED IN THE APPLICATION PROCESS
PHM is the 15th pediatric subspecialty to begin the certification process with a practice pathway. In none of the prior cases was it possible to do a detailed implementation study to understand the myriad of ways in which individual pediatricians arrange their professional and personal time. This reality has led to the publication of only general, rather than specific practice pathway criteria at the start of the application process for PHM and every other pediatric subspecialty. Rather, in each case, a well-informed and diverse peer group of subspecialists (the subboard) has reviewed the applications to get a sense of the variations of practice and then decided on the criteria that a subspecialist must meet to be considered eligible to sit for the certifying exam. Clear-cut criteria were used consistently in adjudicating all applications. Although the ABP has not done this for other subspecialties, we agree that publishing the specific criteria once they had been decided upon would have improved the process. We commit to doing so in the future.
The eligibility criteria were designed to be true to the mission of the ABP and seek parity with the requirements used by other subspecialties and by the PHM training pathway. The assumption is that competent PHM practice of sufficient duration and breadth, attested to by a supervisor, would allow the ABP to represent to the public that the candidate is qualified to sit for the exam. The eligibility criteria focused on seven practice characteristics (Table 2):
(2) The July 2015 start date follows from the four-year look-back window for the November 2019 exam date.
(3) The minimum percentage full-time equivalent (%FTE) for all PHM professional activities (ie, clinical care, research, education, and PHM administration) was set at 50% FTE. Recognizing that an FTE may be defined differently at different institutions, the ABP defined the workweek as 40 hours and the 50% FTE as 900-1,000 hours per year.
(4) The minimum percentage FTE for PHM direct patient care (as described below) was set at 25% FTE and defined as 450-500 hours per year. Every candidate must satisfy both the minimum hours for all PHM professional activities and the minimum hours for the direct care of hospitalized children. Applicants must meet or exceed these minima if the ABP is to represent to the public that an applicant has the necessary experience to be called a subspecialist. Similarly, all other ABP subspecialties required at least 50% FTE commitment for the candidate to be considered a subspecialist.
(5) The scope of practice seeks to maintain parity with the training pathway by requiring care of the full spectrum of hospitalized children. This full spectrum is defined as children on general pediatric wards, ages birth to 21 years, and specifically includes children with complex chronic disease, surgical care and comanagement, sedation, palliative care, and common procedures. Care devoted exclusively to a narrow patient population (“niched care”), such as newborns in the nursery, does not meet the eligibility requirements.
(6) The location for patient care must have occurred in the United States or Canada.
(7) The possibility of practice interruption was included among the eligibility criteria. Attempting to strike a balance between an applicant demonstrating sufficient recent experience to be called a subspecialist versus the reality of some individuals needing to interrupt professional and clinical practice, the subboard stipulated that interruptions of PHM professional activities should not exceed three months during the preceding four years and six months during the preceding five years.
CLARIFICATION AND SIMPLIFICATION OF ELIGIBILITY CRITERIA
The ABP recognizes that the use of %FTE, work hours, and leave exceptions led to unintended confusion among applicants. The intent had been to acknowledge the many valid reasons for interruption of practice, including parental leave. This response to the petition clarifies that the critical question from the public’s perspective is whether the candidate has accumulated enough hours of sustained practice to be considered competent in the field of PHM and specifically caring for hospitalized children (as defined above). Upon review, the ABP believes the workhours criteria (items 3 and 4) accomplish this critical goal and make the %FTE and practice interruption criteria largely redundant. Table 3 reflects the clarified and streamlined requirements. Re-examination of all the denied applications showed that using the criteria in Table 3 did not have a significant impact on the outcomes. One additional applicant’s appeal was granted, and this applicant has been so notified.
APPEALS PROCESS
The right to appeal and the Appellate Review Procedure are included in a denial letter. The applicant is given a deadline of 14 days to notify the ABP of the intent to appeal. There is no appellate fee. Within one to three days, the ABP acknowledges receipt of the applicant’s intent to appeal and sends the applicant a date by which additional supporting information should be provided.
The appeal material is shared with the subboard credentials committee and each member individually reviews and votes on the appeal. The application is approved if a majority votes in favor of the applicant’s appeal. If there is no majority, the credentials committee discusses the case to reach a decision. The results of the appeal are final according to the ABP Appellate Review Procedure. We remain in the appeal process for several PHM applicants as of the date of this response.
Thank you for the opportunity to respond to the petition. The ABP is committed to dialogue, transparency, and continuously improving its processes.
Acknowledgment
The authors thank the ABP board of directors and the ABP PHM subboard for their review and thoughtful contributions.
Disclosures
Dr. Nichols reports other from The American Board of Pediatrics, during the conduct of the work. Dr. Woods has nothing to disclose.
1. Barrett DJ, McGuinness GA, Cunha CA, et al. Pediatric hospital medicine: A proposed new subspecialty. Pediatrics. 2017;139(3). https://doi.org/10.1542/peds.2016-1823.
In August of 2014, the Pediatric Hospital Medicine (PHM) community petitioned the American Board of Pediatrics (ABP) for a subspecialty certificate in PHM. A lengthy vetting process ensued during which the ABP consulted with a wide array of stakeholders. The ABP Board of Directors approved the request from the PHM community for a subspecialty certificate in December 2015 and published the results of the vetting process.1
The ABP received a second petition posted on PHM listserv, which opened with the following statement:
“We submit this petition letter to register a formal complaint, demand immediate action, and request a formal response from the ABP regarding the practice pathway criteria and the application of these criteria for the Pediatric Hospital Medicine specialty exam. Recently there has been considerable discussion on the Pediatric Hospital Medicine ListServ suggesting that the ABP’s implementation of the career pathway criteria has failed to respect and fairly assess the diverse career paths of numerous experienced pediatric hospitalists, which may impede their opportunities for professional advancement. Anecdotal reports on the ListServ also suggest that the use of the current practice pathway criteria to evaluate exam applicants disadvantages women, though sufficient data is not available at this time to evaluate this assertion objectively.”
The ABP response to the PHM community’s concerns regarding the practice pathway for the first certifying exam in PHM is as follows.
THE ABP RESPONSE
ABP thanks the PHM community for the opportunity to respond to the attached petition. Our approach and response are grounded in our mission:
“Advancing child health by certifying pediatricians who meet standards of excellence and are committed to continuous learning and improvement.”
Transparency is one of the ABP’s core values, which underpins this response. The ABP acknowledges that the petitioners did not find the guidance on the ABP website sufficiently transparent. We regret the distress this may have caused, will do our best to answer the questions forthrightly, and have revised the website language for greater clarity.
ALLEGATION OF GENDER BIAS
Some posts on the PHM listserv alleged gender (sex) bias against women in the ABP application process and outcomes. This allegation is not supported by the facts. A peer group of pediatric hospitalists constitutes the ABP PHM subboard which determined the eligibility criteria. The subboard thoughtfully developed these criteria and the American Board of Medical Specialties (ABMS) approved the broad eligibility criteria. The PHM subboard is composed of practicing pediatric hospitalists with a diversity of practice location, age, gender, and race. The majority of ABP PHM subboard members and medical editors are women.
Making unbiased decisions is also a core value of the ABP. Among the 1,627 applicants for the exam, the ABP has approved 1,515 (93%) as of August 15, 2019. Seventy percent of applications were from women, which mirrors the demographics of the pediatric workforce. There was no significant difference between the percentage of women (4.0%) and men (3.7%) who were denied admission to the exam (Table 1). As of August 15, 2019, the credentials committee of the PHM subboard is still reviewing 48 applications, including 35 appeals, of which 60% (N = 21) were from women and 40% (N = 14) were from men. Thirteen (N = 13) remaining applications are under review but not in the appeals process.
PRACTICE PATHWAY CRITERIA USED IN THE APPLICATION PROCESS
PHM is the 15th pediatric subspecialty to begin the certification process with a practice pathway. In none of the prior cases was it possible to do a detailed implementation study to understand the myriad of ways in which individual pediatricians arrange their professional and personal time. This reality has led to the publication of only general, rather than specific practice pathway criteria at the start of the application process for PHM and every other pediatric subspecialty. Rather, in each case, a well-informed and diverse peer group of subspecialists (the subboard) has reviewed the applications to get a sense of the variations of practice and then decided on the criteria that a subspecialist must meet to be considered eligible to sit for the certifying exam. Clear-cut criteria were used consistently in adjudicating all applications. Although the ABP has not done this for other subspecialties, we agree that publishing the specific criteria once they had been decided upon would have improved the process. We commit to doing so in the future.
The eligibility criteria were designed to be true to the mission of the ABP and seek parity with the requirements used by other subspecialties and by the PHM training pathway. The assumption is that competent PHM practice of sufficient duration and breadth, attested to by a supervisor, would allow the ABP to represent to the public that the candidate is qualified to sit for the exam. The eligibility criteria focused on seven practice characteristics (Table 2):
(2) The July 2015 start date follows from the four-year look-back window for the November 2019 exam date.
(3) The minimum percentage full-time equivalent (%FTE) for all PHM professional activities (ie, clinical care, research, education, and PHM administration) was set at 50% FTE. Recognizing that an FTE may be defined differently at different institutions, the ABP defined the workweek as 40 hours and the 50% FTE as 900-1,000 hours per year.
(4) The minimum percentage FTE for PHM direct patient care (as described below) was set at 25% FTE and defined as 450-500 hours per year. Every candidate must satisfy both the minimum hours for all PHM professional activities and the minimum hours for the direct care of hospitalized children. Applicants must meet or exceed these minima if the ABP is to represent to the public that an applicant has the necessary experience to be called a subspecialist. Similarly, all other ABP subspecialties required at least 50% FTE commitment for the candidate to be considered a subspecialist.
(5) The scope of practice seeks to maintain parity with the training pathway by requiring care of the full spectrum of hospitalized children. This full spectrum is defined as children on general pediatric wards, ages birth to 21 years, and specifically includes children with complex chronic disease, surgical care and comanagement, sedation, palliative care, and common procedures. Care devoted exclusively to a narrow patient population (“niched care”), such as newborns in the nursery, does not meet the eligibility requirements.
(6) The location for patient care must have occurred in the United States or Canada.
(7) The possibility of practice interruption was included among the eligibility criteria. Attempting to strike a balance between an applicant demonstrating sufficient recent experience to be called a subspecialist versus the reality of some individuals needing to interrupt professional and clinical practice, the subboard stipulated that interruptions of PHM professional activities should not exceed three months during the preceding four years and six months during the preceding five years.
CLARIFICATION AND SIMPLIFICATION OF ELIGIBILITY CRITERIA
The ABP recognizes that the use of %FTE, work hours, and leave exceptions led to unintended confusion among applicants. The intent had been to acknowledge the many valid reasons for interruption of practice, including parental leave. This response to the petition clarifies that the critical question from the public’s perspective is whether the candidate has accumulated enough hours of sustained practice to be considered competent in the field of PHM and specifically caring for hospitalized children (as defined above). Upon review, the ABP believes the workhours criteria (items 3 and 4) accomplish this critical goal and make the %FTE and practice interruption criteria largely redundant. Table 3 reflects the clarified and streamlined requirements. Re-examination of all the denied applications showed that using the criteria in Table 3 did not have a significant impact on the outcomes. One additional applicant’s appeal was granted, and this applicant has been so notified.
APPEALS PROCESS
The right to appeal and the Appellate Review Procedure are included in a denial letter. The applicant is given a deadline of 14 days to notify the ABP of the intent to appeal. There is no appellate fee. Within one to three days, the ABP acknowledges receipt of the applicant’s intent to appeal and sends the applicant a date by which additional supporting information should be provided.
The appeal material is shared with the subboard credentials committee and each member individually reviews and votes on the appeal. The application is approved if a majority votes in favor of the applicant’s appeal. If there is no majority, the credentials committee discusses the case to reach a decision. The results of the appeal are final according to the ABP Appellate Review Procedure. We remain in the appeal process for several PHM applicants as of the date of this response.
Thank you for the opportunity to respond to the petition. The ABP is committed to dialogue, transparency, and continuously improving its processes.
Acknowledgment
The authors thank the ABP board of directors and the ABP PHM subboard for their review and thoughtful contributions.
Disclosures
Dr. Nichols reports other from The American Board of Pediatrics, during the conduct of the work. Dr. Woods has nothing to disclose.
In August of 2014, the Pediatric Hospital Medicine (PHM) community petitioned the American Board of Pediatrics (ABP) for a subspecialty certificate in PHM. A lengthy vetting process ensued during which the ABP consulted with a wide array of stakeholders. The ABP Board of Directors approved the request from the PHM community for a subspecialty certificate in December 2015 and published the results of the vetting process.1
The ABP received a second petition posted on PHM listserv, which opened with the following statement:
“We submit this petition letter to register a formal complaint, demand immediate action, and request a formal response from the ABP regarding the practice pathway criteria and the application of these criteria for the Pediatric Hospital Medicine specialty exam. Recently there has been considerable discussion on the Pediatric Hospital Medicine ListServ suggesting that the ABP’s implementation of the career pathway criteria has failed to respect and fairly assess the diverse career paths of numerous experienced pediatric hospitalists, which may impede their opportunities for professional advancement. Anecdotal reports on the ListServ also suggest that the use of the current practice pathway criteria to evaluate exam applicants disadvantages women, though sufficient data is not available at this time to evaluate this assertion objectively.”
The ABP response to the PHM community’s concerns regarding the practice pathway for the first certifying exam in PHM is as follows.
THE ABP RESPONSE
ABP thanks the PHM community for the opportunity to respond to the attached petition. Our approach and response are grounded in our mission:
“Advancing child health by certifying pediatricians who meet standards of excellence and are committed to continuous learning and improvement.”
Transparency is one of the ABP’s core values, which underpins this response. The ABP acknowledges that the petitioners did not find the guidance on the ABP website sufficiently transparent. We regret the distress this may have caused, will do our best to answer the questions forthrightly, and have revised the website language for greater clarity.
ALLEGATION OF GENDER BIAS
Some posts on the PHM listserv alleged gender (sex) bias against women in the ABP application process and outcomes. This allegation is not supported by the facts. A peer group of pediatric hospitalists constitutes the ABP PHM subboard which determined the eligibility criteria. The subboard thoughtfully developed these criteria and the American Board of Medical Specialties (ABMS) approved the broad eligibility criteria. The PHM subboard is composed of practicing pediatric hospitalists with a diversity of practice location, age, gender, and race. The majority of ABP PHM subboard members and medical editors are women.
Making unbiased decisions is also a core value of the ABP. Among the 1,627 applicants for the exam, the ABP has approved 1,515 (93%) as of August 15, 2019. Seventy percent of applications were from women, which mirrors the demographics of the pediatric workforce. There was no significant difference between the percentage of women (4.0%) and men (3.7%) who were denied admission to the exam (Table 1). As of August 15, 2019, the credentials committee of the PHM subboard is still reviewing 48 applications, including 35 appeals, of which 60% (N = 21) were from women and 40% (N = 14) were from men. Thirteen (N = 13) remaining applications are under review but not in the appeals process.
PRACTICE PATHWAY CRITERIA USED IN THE APPLICATION PROCESS
PHM is the 15th pediatric subspecialty to begin the certification process with a practice pathway. In none of the prior cases was it possible to do a detailed implementation study to understand the myriad of ways in which individual pediatricians arrange their professional and personal time. This reality has led to the publication of only general, rather than specific practice pathway criteria at the start of the application process for PHM and every other pediatric subspecialty. Rather, in each case, a well-informed and diverse peer group of subspecialists (the subboard) has reviewed the applications to get a sense of the variations of practice and then decided on the criteria that a subspecialist must meet to be considered eligible to sit for the certifying exam. Clear-cut criteria were used consistently in adjudicating all applications. Although the ABP has not done this for other subspecialties, we agree that publishing the specific criteria once they had been decided upon would have improved the process. We commit to doing so in the future.
The eligibility criteria were designed to be true to the mission of the ABP and seek parity with the requirements used by other subspecialties and by the PHM training pathway. The assumption is that competent PHM practice of sufficient duration and breadth, attested to by a supervisor, would allow the ABP to represent to the public that the candidate is qualified to sit for the exam. The eligibility criteria focused on seven practice characteristics (Table 2):
(2) The July 2015 start date follows from the four-year look-back window for the November 2019 exam date.
(3) The minimum percentage full-time equivalent (%FTE) for all PHM professional activities (ie, clinical care, research, education, and PHM administration) was set at 50% FTE. Recognizing that an FTE may be defined differently at different institutions, the ABP defined the workweek as 40 hours and the 50% FTE as 900-1,000 hours per year.
(4) The minimum percentage FTE for PHM direct patient care (as described below) was set at 25% FTE and defined as 450-500 hours per year. Every candidate must satisfy both the minimum hours for all PHM professional activities and the minimum hours for the direct care of hospitalized children. Applicants must meet or exceed these minima if the ABP is to represent to the public that an applicant has the necessary experience to be called a subspecialist. Similarly, all other ABP subspecialties required at least 50% FTE commitment for the candidate to be considered a subspecialist.
(5) The scope of practice seeks to maintain parity with the training pathway by requiring care of the full spectrum of hospitalized children. This full spectrum is defined as children on general pediatric wards, ages birth to 21 years, and specifically includes children with complex chronic disease, surgical care and comanagement, sedation, palliative care, and common procedures. Care devoted exclusively to a narrow patient population (“niched care”), such as newborns in the nursery, does not meet the eligibility requirements.
(6) The location for patient care must have occurred in the United States or Canada.
(7) The possibility of practice interruption was included among the eligibility criteria. Attempting to strike a balance between an applicant demonstrating sufficient recent experience to be called a subspecialist versus the reality of some individuals needing to interrupt professional and clinical practice, the subboard stipulated that interruptions of PHM professional activities should not exceed three months during the preceding four years and six months during the preceding five years.
CLARIFICATION AND SIMPLIFICATION OF ELIGIBILITY CRITERIA
The ABP recognizes that the use of %FTE, work hours, and leave exceptions led to unintended confusion among applicants. The intent had been to acknowledge the many valid reasons for interruption of practice, including parental leave. This response to the petition clarifies that the critical question from the public’s perspective is whether the candidate has accumulated enough hours of sustained practice to be considered competent in the field of PHM and specifically caring for hospitalized children (as defined above). Upon review, the ABP believes the workhours criteria (items 3 and 4) accomplish this critical goal and make the %FTE and practice interruption criteria largely redundant. Table 3 reflects the clarified and streamlined requirements. Re-examination of all the denied applications showed that using the criteria in Table 3 did not have a significant impact on the outcomes. One additional applicant’s appeal was granted, and this applicant has been so notified.
APPEALS PROCESS
The right to appeal and the Appellate Review Procedure are included in a denial letter. The applicant is given a deadline of 14 days to notify the ABP of the intent to appeal. There is no appellate fee. Within one to three days, the ABP acknowledges receipt of the applicant’s intent to appeal and sends the applicant a date by which additional supporting information should be provided.
The appeal material is shared with the subboard credentials committee and each member individually reviews and votes on the appeal. The application is approved if a majority votes in favor of the applicant’s appeal. If there is no majority, the credentials committee discusses the case to reach a decision. The results of the appeal are final according to the ABP Appellate Review Procedure. We remain in the appeal process for several PHM applicants as of the date of this response.
Thank you for the opportunity to respond to the petition. The ABP is committed to dialogue, transparency, and continuously improving its processes.
Acknowledgment
The authors thank the ABP board of directors and the ABP PHM subboard for their review and thoughtful contributions.
Disclosures
Dr. Nichols reports other from The American Board of Pediatrics, during the conduct of the work. Dr. Woods has nothing to disclose.
1. Barrett DJ, McGuinness GA, Cunha CA, et al. Pediatric hospital medicine: A proposed new subspecialty. Pediatrics. 2017;139(3). https://doi.org/10.1542/peds.2016-1823.
1. Barrett DJ, McGuinness GA, Cunha CA, et al. Pediatric hospital medicine: A proposed new subspecialty. Pediatrics. 2017;139(3). https://doi.org/10.1542/peds.2016-1823.
© 2019 Society of Hospital Medicine
Expanding the View: Implications of the SHM Position Statement on Ultrasound Use in Vascular Access
Is there a single intervention more important to hospitalized patients than vascular access? Since their advent in the 1950s, small plastic tubes have revolutionized medication administration and become a mainstay of modern medicine. Yet, for much of the last 60 years, nurses and doctors have used the same landmark-guided approaches to acquire peripheral and, more specifically, central access.1 Minor improvements to the Seldinger technique and sterile preparation have been reported.2 However, for such a vital and common procedure, the complication rates of landmark-based approaches to central venous access remain unacceptably high.3
In the position statement released by the Society of Hospital Medicine (SHM), Franco–Sadud et al. outline the transformative effects ultrasound can have in obtaining adult vascular access.4 The authors cite comprehensive evidence, leaving little doubt of the technique’s benefits compared with landmark-based approaches. However, several questions remain: Is vascular access the domain of the hospitalist? If so, how can hospitalists pursue and afford ultrasound training? Finally, how will this shift toward ultrasound-guided vascular access affect patients in resource-limited settings?
Through an expert-driven literature review, the authors present 29 succinct recommendations for ultrasound use in vascular access. Supporting data consistently illustrate the association of ultrasound with increased successful vessel cannulation rates and decreased complication rates for all types of vascular access; including central venous access (internal jugular, subclavian, femoral), arterial line placement, peripherally inserted central catheters, and difficult peripheral venous access. Despite this compelling evidence, however, 20%-55% of all central venous catheters are still placed without ultrasound.5 How, then, can hospitalists expand ultrasound use for vascular access or perform these procedures in general?
Hospitalists likely fall into one of three categories in terms of vascular access: (1) they are proficient in ultrasound use for vascular access, (2) they still routinely use traditional landmark-based approaches, or (3) they have little to no involvement in vascular access and defer to intensivists, interventional radiologists, or nurse specialists. Franco-Sadud et al.’s position statement acknowledges the wide range of hospitalist practices and only asserts that, if providers perform vascular access, they should be trained and use ultrasound to do them. We would advocate further that, regardless of their practice, hospitalists have a role in expanding ultrasound use for vascular access given its direct impact on the patients they care for. Hospitalists who do not directly practice vascular access can still leverage the skills that have established hospital medicine’s reputation as leaders in patient safety and quality improvement. Hospitalists can partner with proceduralists in their institutions to ensure that they are supported and trained in the most evidence-based approaches to vascular access and that their patients have access to the highest quality of care.
For the individual hospitalist, the investment of time and resources to incorporate ultrasound into routine practice can seem daunting. In previous position statements, the SHM has advocated for the robust use of simulation and directly observed assessment in credentialing for all bedside procedures.6 However, the Society also acknowledges that this degree of training and monitoring can constitute significant barriers and has argued that the onus for change lies not only with providers but with healthcare institutions at large. How, then, can hospitalists approach their institutions to successfully solicit support? While the evidence is not yet conclusive, Cohen et al. have shown promising data for potential long-term cost savings through ultrasound-guided vascular access.7 Due to decreased complication rates, downstream benefits of lower resource use, higher patient satisfaction, and, theoretically, even lower clinician burnout rates have been attained. These effects, combined with hospitalists acquiring ultrasound skills translatable to other bedside procedures and fundamentals of diagnostic point of care ultrasound, form a compelling argument for institutional support. Many academic medical centers, typically with increased resources and training programs, have been early adopters; but, how will the shift from landmark-based to ultrasound-guided vascular access affect those in resource-limited settings?
While incredible strides have been made in care quality and patient safety over the last 15 years, improvements clearly do not always benefit patients, clinicians, or institutions equally.8 In fact, those in resource-limited settings often experience disproportionately reduced benefits. While focus on the “quality gap” has transformed the culture of the quality improvement and patient safety fields, an “equity gap” has long undermined and limited the impact of those very improvements. Unfortunately, changes in care driven by costly technological advances such as ultrasound are particularly likely to widen this “equity gap.” While ultrasound technology is rapidly becoming more affordable, a lack of access to machines and appropriate training remain significant barriers in the resource-limited settings that hospitalists are most likely to be performing these procedures. Without a focus on equity, the benefits offered by ultrasound will continue to be limited in their reach.
The SHM position statement by Franco-Sadud et al. is an important step in expanding evidence-based ultrasound use for vascular access and improving patient care. While the recommendations are, at times, aspirational and the barriers are real, hospitalists have shown time and again their ability to overcome these challenges and advance the standard of care for all.
1. Beheshti MV. A concise history of central venous access. Tech Vasc Interv Radiol. 2011;14(4):184-5. https://doi.org/10.1053/j.tvir.2011.05.002.
2. Higgs ZC, Macafee DA, Braithwaite BD, Maxwell-Armstrong CA. The Seldinger technique: 50 years on. Lancet. 2005;366(9494):1407-1409. https://doi.org/10.1016/S0140-6736(05)66878-X.
3. Parienti JJ, Mongardon N, Mégarbane B, et al. Intravascular complications of central venous catheterization by insertion site. N Engl J Med. 2015;373(13):1220-1229. https://doi.org/10.1056/NEJMoa1500964.
4. Franco-Sadud R, D Schnobrich, Mathews BK et al. SHM Point-of-care Ultrasound Task Force. Recommendations on the use of ultrasound guidance for central and peripheral vascular access in adults: a position statement of the Society of Hospital Medicine. J Hosp Med. 2019;14:E1-E22. https://doi.org/10.12788/jhm.3287.
5. Soni NJ, Reyes LF, Keyt H, et al. Use of ultrasound guidance for central venous catheterization: a national survey of intensivists and hospitalists. J Crit Care. 2016;36:277-283. https://doi.org/10.1016/j.jcrc.2016.07.014.
6. Lucas BP, Tierney DM, Jensen TP, et al. Credentialing of hospitalists in ultrasound-guided bedside procedures: a position statement of the Society of Hospital Medicine. J Hosp Med. 2018;13(2);117-125. https://doi.org/10.12788/jhm.2917.
7. Cohen ER, Feinglass J, Barsuk JH, et al. Cost savings from reduced catheter-related bloodstream infection after simulation-based education for residents in a medical intensive care unit. Simul Healthc. 2010;5(2):98-102. https://doi.org/10.1097/SIH.0b013e3181bc8304.
8. 2017 National Healthcare Quality and Disparities Report.
Is there a single intervention more important to hospitalized patients than vascular access? Since their advent in the 1950s, small plastic tubes have revolutionized medication administration and become a mainstay of modern medicine. Yet, for much of the last 60 years, nurses and doctors have used the same landmark-guided approaches to acquire peripheral and, more specifically, central access.1 Minor improvements to the Seldinger technique and sterile preparation have been reported.2 However, for such a vital and common procedure, the complication rates of landmark-based approaches to central venous access remain unacceptably high.3
In the position statement released by the Society of Hospital Medicine (SHM), Franco–Sadud et al. outline the transformative effects ultrasound can have in obtaining adult vascular access.4 The authors cite comprehensive evidence, leaving little doubt of the technique’s benefits compared with landmark-based approaches. However, several questions remain: Is vascular access the domain of the hospitalist? If so, how can hospitalists pursue and afford ultrasound training? Finally, how will this shift toward ultrasound-guided vascular access affect patients in resource-limited settings?
Through an expert-driven literature review, the authors present 29 succinct recommendations for ultrasound use in vascular access. Supporting data consistently illustrate the association of ultrasound with increased successful vessel cannulation rates and decreased complication rates for all types of vascular access; including central venous access (internal jugular, subclavian, femoral), arterial line placement, peripherally inserted central catheters, and difficult peripheral venous access. Despite this compelling evidence, however, 20%-55% of all central venous catheters are still placed without ultrasound.5 How, then, can hospitalists expand ultrasound use for vascular access or perform these procedures in general?
Hospitalists likely fall into one of three categories in terms of vascular access: (1) they are proficient in ultrasound use for vascular access, (2) they still routinely use traditional landmark-based approaches, or (3) they have little to no involvement in vascular access and defer to intensivists, interventional radiologists, or nurse specialists. Franco-Sadud et al.’s position statement acknowledges the wide range of hospitalist practices and only asserts that, if providers perform vascular access, they should be trained and use ultrasound to do them. We would advocate further that, regardless of their practice, hospitalists have a role in expanding ultrasound use for vascular access given its direct impact on the patients they care for. Hospitalists who do not directly practice vascular access can still leverage the skills that have established hospital medicine’s reputation as leaders in patient safety and quality improvement. Hospitalists can partner with proceduralists in their institutions to ensure that they are supported and trained in the most evidence-based approaches to vascular access and that their patients have access to the highest quality of care.
For the individual hospitalist, the investment of time and resources to incorporate ultrasound into routine practice can seem daunting. In previous position statements, the SHM has advocated for the robust use of simulation and directly observed assessment in credentialing for all bedside procedures.6 However, the Society also acknowledges that this degree of training and monitoring can constitute significant barriers and has argued that the onus for change lies not only with providers but with healthcare institutions at large. How, then, can hospitalists approach their institutions to successfully solicit support? While the evidence is not yet conclusive, Cohen et al. have shown promising data for potential long-term cost savings through ultrasound-guided vascular access.7 Due to decreased complication rates, downstream benefits of lower resource use, higher patient satisfaction, and, theoretically, even lower clinician burnout rates have been attained. These effects, combined with hospitalists acquiring ultrasound skills translatable to other bedside procedures and fundamentals of diagnostic point of care ultrasound, form a compelling argument for institutional support. Many academic medical centers, typically with increased resources and training programs, have been early adopters; but, how will the shift from landmark-based to ultrasound-guided vascular access affect those in resource-limited settings?
While incredible strides have been made in care quality and patient safety over the last 15 years, improvements clearly do not always benefit patients, clinicians, or institutions equally.8 In fact, those in resource-limited settings often experience disproportionately reduced benefits. While focus on the “quality gap” has transformed the culture of the quality improvement and patient safety fields, an “equity gap” has long undermined and limited the impact of those very improvements. Unfortunately, changes in care driven by costly technological advances such as ultrasound are particularly likely to widen this “equity gap.” While ultrasound technology is rapidly becoming more affordable, a lack of access to machines and appropriate training remain significant barriers in the resource-limited settings that hospitalists are most likely to be performing these procedures. Without a focus on equity, the benefits offered by ultrasound will continue to be limited in their reach.
The SHM position statement by Franco-Sadud et al. is an important step in expanding evidence-based ultrasound use for vascular access and improving patient care. While the recommendations are, at times, aspirational and the barriers are real, hospitalists have shown time and again their ability to overcome these challenges and advance the standard of care for all.
Is there a single intervention more important to hospitalized patients than vascular access? Since their advent in the 1950s, small plastic tubes have revolutionized medication administration and become a mainstay of modern medicine. Yet, for much of the last 60 years, nurses and doctors have used the same landmark-guided approaches to acquire peripheral and, more specifically, central access.1 Minor improvements to the Seldinger technique and sterile preparation have been reported.2 However, for such a vital and common procedure, the complication rates of landmark-based approaches to central venous access remain unacceptably high.3
In the position statement released by the Society of Hospital Medicine (SHM), Franco–Sadud et al. outline the transformative effects ultrasound can have in obtaining adult vascular access.4 The authors cite comprehensive evidence, leaving little doubt of the technique’s benefits compared with landmark-based approaches. However, several questions remain: Is vascular access the domain of the hospitalist? If so, how can hospitalists pursue and afford ultrasound training? Finally, how will this shift toward ultrasound-guided vascular access affect patients in resource-limited settings?
Through an expert-driven literature review, the authors present 29 succinct recommendations for ultrasound use in vascular access. Supporting data consistently illustrate the association of ultrasound with increased successful vessel cannulation rates and decreased complication rates for all types of vascular access; including central venous access (internal jugular, subclavian, femoral), arterial line placement, peripherally inserted central catheters, and difficult peripheral venous access. Despite this compelling evidence, however, 20%-55% of all central venous catheters are still placed without ultrasound.5 How, then, can hospitalists expand ultrasound use for vascular access or perform these procedures in general?
Hospitalists likely fall into one of three categories in terms of vascular access: (1) they are proficient in ultrasound use for vascular access, (2) they still routinely use traditional landmark-based approaches, or (3) they have little to no involvement in vascular access and defer to intensivists, interventional radiologists, or nurse specialists. Franco-Sadud et al.’s position statement acknowledges the wide range of hospitalist practices and only asserts that, if providers perform vascular access, they should be trained and use ultrasound to do them. We would advocate further that, regardless of their practice, hospitalists have a role in expanding ultrasound use for vascular access given its direct impact on the patients they care for. Hospitalists who do not directly practice vascular access can still leverage the skills that have established hospital medicine’s reputation as leaders in patient safety and quality improvement. Hospitalists can partner with proceduralists in their institutions to ensure that they are supported and trained in the most evidence-based approaches to vascular access and that their patients have access to the highest quality of care.
For the individual hospitalist, the investment of time and resources to incorporate ultrasound into routine practice can seem daunting. In previous position statements, the SHM has advocated for the robust use of simulation and directly observed assessment in credentialing for all bedside procedures.6 However, the Society also acknowledges that this degree of training and monitoring can constitute significant barriers and has argued that the onus for change lies not only with providers but with healthcare institutions at large. How, then, can hospitalists approach their institutions to successfully solicit support? While the evidence is not yet conclusive, Cohen et al. have shown promising data for potential long-term cost savings through ultrasound-guided vascular access.7 Due to decreased complication rates, downstream benefits of lower resource use, higher patient satisfaction, and, theoretically, even lower clinician burnout rates have been attained. These effects, combined with hospitalists acquiring ultrasound skills translatable to other bedside procedures and fundamentals of diagnostic point of care ultrasound, form a compelling argument for institutional support. Many academic medical centers, typically with increased resources and training programs, have been early adopters; but, how will the shift from landmark-based to ultrasound-guided vascular access affect those in resource-limited settings?
While incredible strides have been made in care quality and patient safety over the last 15 years, improvements clearly do not always benefit patients, clinicians, or institutions equally.8 In fact, those in resource-limited settings often experience disproportionately reduced benefits. While focus on the “quality gap” has transformed the culture of the quality improvement and patient safety fields, an “equity gap” has long undermined and limited the impact of those very improvements. Unfortunately, changes in care driven by costly technological advances such as ultrasound are particularly likely to widen this “equity gap.” While ultrasound technology is rapidly becoming more affordable, a lack of access to machines and appropriate training remain significant barriers in the resource-limited settings that hospitalists are most likely to be performing these procedures. Without a focus on equity, the benefits offered by ultrasound will continue to be limited in their reach.
The SHM position statement by Franco-Sadud et al. is an important step in expanding evidence-based ultrasound use for vascular access and improving patient care. While the recommendations are, at times, aspirational and the barriers are real, hospitalists have shown time and again their ability to overcome these challenges and advance the standard of care for all.
1. Beheshti MV. A concise history of central venous access. Tech Vasc Interv Radiol. 2011;14(4):184-5. https://doi.org/10.1053/j.tvir.2011.05.002.
2. Higgs ZC, Macafee DA, Braithwaite BD, Maxwell-Armstrong CA. The Seldinger technique: 50 years on. Lancet. 2005;366(9494):1407-1409. https://doi.org/10.1016/S0140-6736(05)66878-X.
3. Parienti JJ, Mongardon N, Mégarbane B, et al. Intravascular complications of central venous catheterization by insertion site. N Engl J Med. 2015;373(13):1220-1229. https://doi.org/10.1056/NEJMoa1500964.
4. Franco-Sadud R, D Schnobrich, Mathews BK et al. SHM Point-of-care Ultrasound Task Force. Recommendations on the use of ultrasound guidance for central and peripheral vascular access in adults: a position statement of the Society of Hospital Medicine. J Hosp Med. 2019;14:E1-E22. https://doi.org/10.12788/jhm.3287.
5. Soni NJ, Reyes LF, Keyt H, et al. Use of ultrasound guidance for central venous catheterization: a national survey of intensivists and hospitalists. J Crit Care. 2016;36:277-283. https://doi.org/10.1016/j.jcrc.2016.07.014.
6. Lucas BP, Tierney DM, Jensen TP, et al. Credentialing of hospitalists in ultrasound-guided bedside procedures: a position statement of the Society of Hospital Medicine. J Hosp Med. 2018;13(2);117-125. https://doi.org/10.12788/jhm.2917.
7. Cohen ER, Feinglass J, Barsuk JH, et al. Cost savings from reduced catheter-related bloodstream infection after simulation-based education for residents in a medical intensive care unit. Simul Healthc. 2010;5(2):98-102. https://doi.org/10.1097/SIH.0b013e3181bc8304.
8. 2017 National Healthcare Quality and Disparities Report.
1. Beheshti MV. A concise history of central venous access. Tech Vasc Interv Radiol. 2011;14(4):184-5. https://doi.org/10.1053/j.tvir.2011.05.002.
2. Higgs ZC, Macafee DA, Braithwaite BD, Maxwell-Armstrong CA. The Seldinger technique: 50 years on. Lancet. 2005;366(9494):1407-1409. https://doi.org/10.1016/S0140-6736(05)66878-X.
3. Parienti JJ, Mongardon N, Mégarbane B, et al. Intravascular complications of central venous catheterization by insertion site. N Engl J Med. 2015;373(13):1220-1229. https://doi.org/10.1056/NEJMoa1500964.
4. Franco-Sadud R, D Schnobrich, Mathews BK et al. SHM Point-of-care Ultrasound Task Force. Recommendations on the use of ultrasound guidance for central and peripheral vascular access in adults: a position statement of the Society of Hospital Medicine. J Hosp Med. 2019;14:E1-E22. https://doi.org/10.12788/jhm.3287.
5. Soni NJ, Reyes LF, Keyt H, et al. Use of ultrasound guidance for central venous catheterization: a national survey of intensivists and hospitalists. J Crit Care. 2016;36:277-283. https://doi.org/10.1016/j.jcrc.2016.07.014.
6. Lucas BP, Tierney DM, Jensen TP, et al. Credentialing of hospitalists in ultrasound-guided bedside procedures: a position statement of the Society of Hospital Medicine. J Hosp Med. 2018;13(2);117-125. https://doi.org/10.12788/jhm.2917.
7. Cohen ER, Feinglass J, Barsuk JH, et al. Cost savings from reduced catheter-related bloodstream infection after simulation-based education for residents in a medical intensive care unit. Simul Healthc. 2010;5(2):98-102. https://doi.org/10.1097/SIH.0b013e3181bc8304.
8. 2017 National Healthcare Quality and Disparities Report.
© 2019 Society of Hospital Medicine
Environmental scan: Drivers of change in technology
Emerging technology has long been a driver of change in health care, and the pace of technological change has accelerated dramatically in the past decade. Physicians are being challenged to incorporate blockchain technology, virtual health care, artificial intelligence, gamification of learning, and the rapidly developing Internet of Things into their work and into their interactions with patients.
Blockchain in health care
Blockchain is a log of activity that is time stamped, tamper proof, and shared across a network of computers. Each transaction that goes into the log of activity is enclosed in a block and linked together in chronological order to form a chain, now called blockchain.
The potential applications of this emerging technology in health-care delivery are almost limitless.1 Shared, secure, and linked data that can be accessed by all can give rise to the automation of complex problems, community-generated solutions to problems that empower patients, and an increase in trust, transparency, and incentive alignment. Currently, insurance claims, prescriptions, and payments mostly reside in sequestered computer systems, but a blockchain of the transactions among them would open up a wealth of learning and efficiency possibilities.2 Hospitals, medical centers, insurance companies, clinical trials, and large practices can collaborate to create a blockchain of transactions in which all members can view access, share, and analyze the data.
Burton Lesnick, MD, FCCP, has given the topic of technology change and the practice of medicine some thought. He is a member of the CHEST Board of Regents and the former founding medical director of The Children’s Care Network, a pediatric accountable care organization of 1,800 providers in metro Atlanta area. Dr. Lesnick notes that blockchain is still in its early phases, partly because it is expensive in terms of computing power and electricity to adequately maintain a distributed ledger.
“I would see it being used in the next decade for high-value public registries, especially where the authenticity of data is critical. For instance, in Europe, we are already seeing a lot of effort to prevent counterfeit drugs from entering the pharmacy chain. We may soon see blockchain being used to track expensive drugs in our health-care system, thus ensuring chain of possession and preventing fraud,” he said.
Virtual care
Some traditional face-to-face encounters between doctor and patient will be replaced by virtual care of different types. Telemedicine is growing, thanks in part to advocacy from Medicare and Medicaid, although the lack of federal guidance on coverage and reimbursement could be a barrier.3 mHealth, the delivery of care via mobile devices, is being utilized for preventive services, appointment confirmation, and follow-up information, but the future of this technology will probably expand into transmission of data from patients and health devices, as well as health alerts.
According to a report by the World Health Organization, an increasing proportion of the population is accessing health information and services through mobile phones.4 According to the Physicians Practice 2018 Mobile Health Survey, a majority of practices that participated in the study stated they use mobile health in their practice on a weekly basis.5 Those still not using mHealth cite concerns over HIPAA compliance. Dr. Lesnick offers some cautionary perspectives.
“Many of us can already download data from medical devices such as CPAP machines and home ventilators. A prominent pharmaceutical company has recently gained FDA approval for an inhaler that date and time stamps when and how the inhaler has been used. Wearable health devices, such as fitness monitors and watches that can alert users about life-threatening arrhythmias are wonderful. But the potential for physicians being overwhelmed by the incoming data flow is concerning. This is especially true when physicians are already reporting high levels of burnout associated with frustration using electronic medical record systems. We can only hope that algorithms will be developed to sift the precious stones from the digital effluent.”
Despite the security concerns, health-care providers, along with the Centers for Medicare & Medicaid Services and the insurance industry, are planning to address the projected shortages in the health-care workforce with virtual care.3
Dr. Lesnick added, “Doctors need to be engaged at the level of their health-care systems and national organizations. Providers are needed to provide context and balance to ensure that new technology utilizes appropriate scope of practice, optimizes care, and reduces costs, while reducing burdens on caregivers.”
Artificial intelligence and the Internet of Things
Artificial intelligence (AI) in health care is the use of complex algorithms and software to approximate human analysis of complicated medical data. The applications in medicine are potentially limitless given the rapid accumulation of data related to health care.
According to Forbes, AI for health-care IT application will cross $1.7 billion by 2019.2 By operationalizing AI platforms across select health-care workflows, organizations could see significant productivity gains during the next few years. Forbes also predicts more AI solutions will be used in imaging diagnostics, drug discovery, and risk-analytics applications.2
At the Icahn School of Medicine at Mount Sinai, New York, researchers use an in-house AI system known as Deep Patient, to predict risk factors for 78 different diseases. Doctors use the system to aid in diagnoses.9 AI is being used to diagnose patient wounds via smartphones, remotely monitor the elderly, and help health systems to digitally verify a patient’s insurance information.
Dr. Lesnick observed that chess computers started beating grand masters more than 20 years ago. However, the best chess players, in combination with a computer, can still reliably beat a computer alone. We need organizations like CHEST to help us become more adept at using technology. AI is a powerful tool but just another instrument to be employed in care of patients.
Big data and AI will combine to create a new ways of practicing medicine in the coming years, but what this trend will mean to individual clinicians remains to seen.
An area of rapid development is the Internet of Things, the extension of internet connectivity into everyday objects and devices designed to monitor and send information. Health-care devices now incorporate AI, real-time analytics, machine learning, physiologic sensors, and embedded systems.10 Physicians will increasingly have access to real-time data on individual patients. For physicians, managing, storing, and analyzing data from the personalized health-care devices of their patients will be a major challenge as the Internet of Things continues to expand into health care.
Dr. Lesnick noted, “In my collaboration with Georgia Tech [in Atlanta], one area I’m really excited about is process mining. Instead of sorting individual data points for statistical correlation, process mining looks at groups of actions and decisions. We’ve applied this to our local emergency room. I’m hoping we can find the most efficient processes and hardwire them in order sets. If we can eventually apply process mining to the health-care system as a whole, we might start to see gains in efficiencies.”
Gamification
Gamification is the term used to describe any tool or platform that applies game mechanics to nongame initiatives in order to encourage and increase engagement. Elements of gamification often include the use of badges, reward points, prizes, social interaction, and leaderboards. Gamification is frequently used by sales teams, marketers, employee training and performance management, onboarding, learning management, and health and wellness.11
The rise in smartphone ownership and wearable technology will likely increase the adoption of gamification technologies to manage health-related concerns and issues. Patient education via gamification is a potentially powerful tool to enhance engagement around disease management. Maintenance of certification and CME are also growth areas for gamification.
Cybersecurity and data breaches
The rapid development of mobile devices and the Internet of Things, in addition to the transmission of health data on a massive scale, will mean more health data will be stolen for a variety of illegal purposes. Hacking and unauthorized access are now common occurrences. Privacy breaches, potential HIPAA violations, and financial damage to patients and institutions are all areas of concern that accompany technological changes.12
Dr. Lesnick stressed that all health-care professionals must be accountable for safeguarding patient information and using the latest security software. “Physicians can be advocates for their patients by cautioning them about the risks of placing their private medical information into public spaces, such as social media. Patients should also know that they may be waiving their privacy rights when they utilize commercial entities that collect and store DNA analyses for purposes of ancestry tracking or medical screening,” he concluded.
References
1. Dhillon V et al. “Blockchain in healthcare: Innovations that empower patients, connect professionals and improve care.” (New York: CRC Press, 2019).
2. Das R. Top 8 healthcare predictions for 2019. Forbes. 2018 Nov 13.
3. 2019 Predictions. Teladoc Health. 2019. http://go.teladochealth.com/predictions/3/.
4. Director-General. “mHealth: Use of appropriate digital technologies for public health.” World Health Organization. 2018 Mar 26.
5. Physicians Practice Staff. 2018 Mobile Health Survey Results. Physicians Practice. 2018 Feb 20.
6. Trend 1: Citizen AI. Accenture. 2018 May 24.
7. Siwicki B. Zocdoc appointment booking app now verifies insurance with AI. Healthcare IT News. 2017 Oct 25
8. Schepke J. What’s your healthcare gamification strategy? Becker’s Healthcare. 2018 May 31.
9. November 2018 healthcare data breach report. HIPAA Journal. 2018 Dec 20.
10. Siwicki, B. Zocdoc appointment booking app now verifies insurance with AI. HeathcareITNews. 2017 Oct 25.
11. Schepke, J. What’s your healthcare gamification strategy? Becker’s Health IT & CIO Report. 2018. May 31.
12. November 2018 healthcare data breach report. HIPAA Journal. 2018 Dec 20.
Note: Background research performed by Avenue M Group.
Emerging technology has long been a driver of change in health care, and the pace of technological change has accelerated dramatically in the past decade. Physicians are being challenged to incorporate blockchain technology, virtual health care, artificial intelligence, gamification of learning, and the rapidly developing Internet of Things into their work and into their interactions with patients.
Blockchain in health care
Blockchain is a log of activity that is time stamped, tamper proof, and shared across a network of computers. Each transaction that goes into the log of activity is enclosed in a block and linked together in chronological order to form a chain, now called blockchain.
The potential applications of this emerging technology in health-care delivery are almost limitless.1 Shared, secure, and linked data that can be accessed by all can give rise to the automation of complex problems, community-generated solutions to problems that empower patients, and an increase in trust, transparency, and incentive alignment. Currently, insurance claims, prescriptions, and payments mostly reside in sequestered computer systems, but a blockchain of the transactions among them would open up a wealth of learning and efficiency possibilities.2 Hospitals, medical centers, insurance companies, clinical trials, and large practices can collaborate to create a blockchain of transactions in which all members can view access, share, and analyze the data.
Burton Lesnick, MD, FCCP, has given the topic of technology change and the practice of medicine some thought. He is a member of the CHEST Board of Regents and the former founding medical director of The Children’s Care Network, a pediatric accountable care organization of 1,800 providers in metro Atlanta area. Dr. Lesnick notes that blockchain is still in its early phases, partly because it is expensive in terms of computing power and electricity to adequately maintain a distributed ledger.
“I would see it being used in the next decade for high-value public registries, especially where the authenticity of data is critical. For instance, in Europe, we are already seeing a lot of effort to prevent counterfeit drugs from entering the pharmacy chain. We may soon see blockchain being used to track expensive drugs in our health-care system, thus ensuring chain of possession and preventing fraud,” he said.
Virtual care
Some traditional face-to-face encounters between doctor and patient will be replaced by virtual care of different types. Telemedicine is growing, thanks in part to advocacy from Medicare and Medicaid, although the lack of federal guidance on coverage and reimbursement could be a barrier.3 mHealth, the delivery of care via mobile devices, is being utilized for preventive services, appointment confirmation, and follow-up information, but the future of this technology will probably expand into transmission of data from patients and health devices, as well as health alerts.
According to a report by the World Health Organization, an increasing proportion of the population is accessing health information and services through mobile phones.4 According to the Physicians Practice 2018 Mobile Health Survey, a majority of practices that participated in the study stated they use mobile health in their practice on a weekly basis.5 Those still not using mHealth cite concerns over HIPAA compliance. Dr. Lesnick offers some cautionary perspectives.
“Many of us can already download data from medical devices such as CPAP machines and home ventilators. A prominent pharmaceutical company has recently gained FDA approval for an inhaler that date and time stamps when and how the inhaler has been used. Wearable health devices, such as fitness monitors and watches that can alert users about life-threatening arrhythmias are wonderful. But the potential for physicians being overwhelmed by the incoming data flow is concerning. This is especially true when physicians are already reporting high levels of burnout associated with frustration using electronic medical record systems. We can only hope that algorithms will be developed to sift the precious stones from the digital effluent.”
Despite the security concerns, health-care providers, along with the Centers for Medicare & Medicaid Services and the insurance industry, are planning to address the projected shortages in the health-care workforce with virtual care.3
Dr. Lesnick added, “Doctors need to be engaged at the level of their health-care systems and national organizations. Providers are needed to provide context and balance to ensure that new technology utilizes appropriate scope of practice, optimizes care, and reduces costs, while reducing burdens on caregivers.”
Artificial intelligence and the Internet of Things
Artificial intelligence (AI) in health care is the use of complex algorithms and software to approximate human analysis of complicated medical data. The applications in medicine are potentially limitless given the rapid accumulation of data related to health care.
According to Forbes, AI for health-care IT application will cross $1.7 billion by 2019.2 By operationalizing AI platforms across select health-care workflows, organizations could see significant productivity gains during the next few years. Forbes also predicts more AI solutions will be used in imaging diagnostics, drug discovery, and risk-analytics applications.2
At the Icahn School of Medicine at Mount Sinai, New York, researchers use an in-house AI system known as Deep Patient, to predict risk factors for 78 different diseases. Doctors use the system to aid in diagnoses.9 AI is being used to diagnose patient wounds via smartphones, remotely monitor the elderly, and help health systems to digitally verify a patient’s insurance information.
Dr. Lesnick observed that chess computers started beating grand masters more than 20 years ago. However, the best chess players, in combination with a computer, can still reliably beat a computer alone. We need organizations like CHEST to help us become more adept at using technology. AI is a powerful tool but just another instrument to be employed in care of patients.
Big data and AI will combine to create a new ways of practicing medicine in the coming years, but what this trend will mean to individual clinicians remains to seen.
An area of rapid development is the Internet of Things, the extension of internet connectivity into everyday objects and devices designed to monitor and send information. Health-care devices now incorporate AI, real-time analytics, machine learning, physiologic sensors, and embedded systems.10 Physicians will increasingly have access to real-time data on individual patients. For physicians, managing, storing, and analyzing data from the personalized health-care devices of their patients will be a major challenge as the Internet of Things continues to expand into health care.
Dr. Lesnick noted, “In my collaboration with Georgia Tech [in Atlanta], one area I’m really excited about is process mining. Instead of sorting individual data points for statistical correlation, process mining looks at groups of actions and decisions. We’ve applied this to our local emergency room. I’m hoping we can find the most efficient processes and hardwire them in order sets. If we can eventually apply process mining to the health-care system as a whole, we might start to see gains in efficiencies.”
Gamification
Gamification is the term used to describe any tool or platform that applies game mechanics to nongame initiatives in order to encourage and increase engagement. Elements of gamification often include the use of badges, reward points, prizes, social interaction, and leaderboards. Gamification is frequently used by sales teams, marketers, employee training and performance management, onboarding, learning management, and health and wellness.11
The rise in smartphone ownership and wearable technology will likely increase the adoption of gamification technologies to manage health-related concerns and issues. Patient education via gamification is a potentially powerful tool to enhance engagement around disease management. Maintenance of certification and CME are also growth areas for gamification.
Cybersecurity and data breaches
The rapid development of mobile devices and the Internet of Things, in addition to the transmission of health data on a massive scale, will mean more health data will be stolen for a variety of illegal purposes. Hacking and unauthorized access are now common occurrences. Privacy breaches, potential HIPAA violations, and financial damage to patients and institutions are all areas of concern that accompany technological changes.12
Dr. Lesnick stressed that all health-care professionals must be accountable for safeguarding patient information and using the latest security software. “Physicians can be advocates for their patients by cautioning them about the risks of placing their private medical information into public spaces, such as social media. Patients should also know that they may be waiving their privacy rights when they utilize commercial entities that collect and store DNA analyses for purposes of ancestry tracking or medical screening,” he concluded.
References
1. Dhillon V et al. “Blockchain in healthcare: Innovations that empower patients, connect professionals and improve care.” (New York: CRC Press, 2019).
2. Das R. Top 8 healthcare predictions for 2019. Forbes. 2018 Nov 13.
3. 2019 Predictions. Teladoc Health. 2019. http://go.teladochealth.com/predictions/3/.
4. Director-General. “mHealth: Use of appropriate digital technologies for public health.” World Health Organization. 2018 Mar 26.
5. Physicians Practice Staff. 2018 Mobile Health Survey Results. Physicians Practice. 2018 Feb 20.
6. Trend 1: Citizen AI. Accenture. 2018 May 24.
7. Siwicki B. Zocdoc appointment booking app now verifies insurance with AI. Healthcare IT News. 2017 Oct 25
8. Schepke J. What’s your healthcare gamification strategy? Becker’s Healthcare. 2018 May 31.
9. November 2018 healthcare data breach report. HIPAA Journal. 2018 Dec 20.
10. Siwicki, B. Zocdoc appointment booking app now verifies insurance with AI. HeathcareITNews. 2017 Oct 25.
11. Schepke, J. What’s your healthcare gamification strategy? Becker’s Health IT & CIO Report. 2018. May 31.
12. November 2018 healthcare data breach report. HIPAA Journal. 2018 Dec 20.
Note: Background research performed by Avenue M Group.
Emerging technology has long been a driver of change in health care, and the pace of technological change has accelerated dramatically in the past decade. Physicians are being challenged to incorporate blockchain technology, virtual health care, artificial intelligence, gamification of learning, and the rapidly developing Internet of Things into their work and into their interactions with patients.
Blockchain in health care
Blockchain is a log of activity that is time stamped, tamper proof, and shared across a network of computers. Each transaction that goes into the log of activity is enclosed in a block and linked together in chronological order to form a chain, now called blockchain.
The potential applications of this emerging technology in health-care delivery are almost limitless.1 Shared, secure, and linked data that can be accessed by all can give rise to the automation of complex problems, community-generated solutions to problems that empower patients, and an increase in trust, transparency, and incentive alignment. Currently, insurance claims, prescriptions, and payments mostly reside in sequestered computer systems, but a blockchain of the transactions among them would open up a wealth of learning and efficiency possibilities.2 Hospitals, medical centers, insurance companies, clinical trials, and large practices can collaborate to create a blockchain of transactions in which all members can view access, share, and analyze the data.
Burton Lesnick, MD, FCCP, has given the topic of technology change and the practice of medicine some thought. He is a member of the CHEST Board of Regents and the former founding medical director of The Children’s Care Network, a pediatric accountable care organization of 1,800 providers in metro Atlanta area. Dr. Lesnick notes that blockchain is still in its early phases, partly because it is expensive in terms of computing power and electricity to adequately maintain a distributed ledger.
“I would see it being used in the next decade for high-value public registries, especially where the authenticity of data is critical. For instance, in Europe, we are already seeing a lot of effort to prevent counterfeit drugs from entering the pharmacy chain. We may soon see blockchain being used to track expensive drugs in our health-care system, thus ensuring chain of possession and preventing fraud,” he said.
Virtual care
Some traditional face-to-face encounters between doctor and patient will be replaced by virtual care of different types. Telemedicine is growing, thanks in part to advocacy from Medicare and Medicaid, although the lack of federal guidance on coverage and reimbursement could be a barrier.3 mHealth, the delivery of care via mobile devices, is being utilized for preventive services, appointment confirmation, and follow-up information, but the future of this technology will probably expand into transmission of data from patients and health devices, as well as health alerts.
According to a report by the World Health Organization, an increasing proportion of the population is accessing health information and services through mobile phones.4 According to the Physicians Practice 2018 Mobile Health Survey, a majority of practices that participated in the study stated they use mobile health in their practice on a weekly basis.5 Those still not using mHealth cite concerns over HIPAA compliance. Dr. Lesnick offers some cautionary perspectives.
“Many of us can already download data from medical devices such as CPAP machines and home ventilators. A prominent pharmaceutical company has recently gained FDA approval for an inhaler that date and time stamps when and how the inhaler has been used. Wearable health devices, such as fitness monitors and watches that can alert users about life-threatening arrhythmias are wonderful. But the potential for physicians being overwhelmed by the incoming data flow is concerning. This is especially true when physicians are already reporting high levels of burnout associated with frustration using electronic medical record systems. We can only hope that algorithms will be developed to sift the precious stones from the digital effluent.”
Despite the security concerns, health-care providers, along with the Centers for Medicare & Medicaid Services and the insurance industry, are planning to address the projected shortages in the health-care workforce with virtual care.3
Dr. Lesnick added, “Doctors need to be engaged at the level of their health-care systems and national organizations. Providers are needed to provide context and balance to ensure that new technology utilizes appropriate scope of practice, optimizes care, and reduces costs, while reducing burdens on caregivers.”
Artificial intelligence and the Internet of Things
Artificial intelligence (AI) in health care is the use of complex algorithms and software to approximate human analysis of complicated medical data. The applications in medicine are potentially limitless given the rapid accumulation of data related to health care.
According to Forbes, AI for health-care IT application will cross $1.7 billion by 2019.2 By operationalizing AI platforms across select health-care workflows, organizations could see significant productivity gains during the next few years. Forbes also predicts more AI solutions will be used in imaging diagnostics, drug discovery, and risk-analytics applications.2
At the Icahn School of Medicine at Mount Sinai, New York, researchers use an in-house AI system known as Deep Patient, to predict risk factors for 78 different diseases. Doctors use the system to aid in diagnoses.9 AI is being used to diagnose patient wounds via smartphones, remotely monitor the elderly, and help health systems to digitally verify a patient’s insurance information.
Dr. Lesnick observed that chess computers started beating grand masters more than 20 years ago. However, the best chess players, in combination with a computer, can still reliably beat a computer alone. We need organizations like CHEST to help us become more adept at using technology. AI is a powerful tool but just another instrument to be employed in care of patients.
Big data and AI will combine to create a new ways of practicing medicine in the coming years, but what this trend will mean to individual clinicians remains to seen.
An area of rapid development is the Internet of Things, the extension of internet connectivity into everyday objects and devices designed to monitor and send information. Health-care devices now incorporate AI, real-time analytics, machine learning, physiologic sensors, and embedded systems.10 Physicians will increasingly have access to real-time data on individual patients. For physicians, managing, storing, and analyzing data from the personalized health-care devices of their patients will be a major challenge as the Internet of Things continues to expand into health care.
Dr. Lesnick noted, “In my collaboration with Georgia Tech [in Atlanta], one area I’m really excited about is process mining. Instead of sorting individual data points for statistical correlation, process mining looks at groups of actions and decisions. We’ve applied this to our local emergency room. I’m hoping we can find the most efficient processes and hardwire them in order sets. If we can eventually apply process mining to the health-care system as a whole, we might start to see gains in efficiencies.”
Gamification
Gamification is the term used to describe any tool or platform that applies game mechanics to nongame initiatives in order to encourage and increase engagement. Elements of gamification often include the use of badges, reward points, prizes, social interaction, and leaderboards. Gamification is frequently used by sales teams, marketers, employee training and performance management, onboarding, learning management, and health and wellness.11
The rise in smartphone ownership and wearable technology will likely increase the adoption of gamification technologies to manage health-related concerns and issues. Patient education via gamification is a potentially powerful tool to enhance engagement around disease management. Maintenance of certification and CME are also growth areas for gamification.
Cybersecurity and data breaches
The rapid development of mobile devices and the Internet of Things, in addition to the transmission of health data on a massive scale, will mean more health data will be stolen for a variety of illegal purposes. Hacking and unauthorized access are now common occurrences. Privacy breaches, potential HIPAA violations, and financial damage to patients and institutions are all areas of concern that accompany technological changes.12
Dr. Lesnick stressed that all health-care professionals must be accountable for safeguarding patient information and using the latest security software. “Physicians can be advocates for their patients by cautioning them about the risks of placing their private medical information into public spaces, such as social media. Patients should also know that they may be waiving their privacy rights when they utilize commercial entities that collect and store DNA analyses for purposes of ancestry tracking or medical screening,” he concluded.
References
1. Dhillon V et al. “Blockchain in healthcare: Innovations that empower patients, connect professionals and improve care.” (New York: CRC Press, 2019).
2. Das R. Top 8 healthcare predictions for 2019. Forbes. 2018 Nov 13.
3. 2019 Predictions. Teladoc Health. 2019. http://go.teladochealth.com/predictions/3/.
4. Director-General. “mHealth: Use of appropriate digital technologies for public health.” World Health Organization. 2018 Mar 26.
5. Physicians Practice Staff. 2018 Mobile Health Survey Results. Physicians Practice. 2018 Feb 20.
6. Trend 1: Citizen AI. Accenture. 2018 May 24.
7. Siwicki B. Zocdoc appointment booking app now verifies insurance with AI. Healthcare IT News. 2017 Oct 25
8. Schepke J. What’s your healthcare gamification strategy? Becker’s Healthcare. 2018 May 31.
9. November 2018 healthcare data breach report. HIPAA Journal. 2018 Dec 20.
10. Siwicki, B. Zocdoc appointment booking app now verifies insurance with AI. HeathcareITNews. 2017 Oct 25.
11. Schepke, J. What’s your healthcare gamification strategy? Becker’s Health IT & CIO Report. 2018. May 31.
12. November 2018 healthcare data breach report. HIPAA Journal. 2018 Dec 20.
Note: Background research performed by Avenue M Group.
Dr. Mark Rosen - My mentor, my friend
By now, most of you know that the CHEST family lost one of our dearest members and leaders in early July, Past President Mark Rosen. This loss has been felt deeply by many, not only because he was taken so suddenly, but because of who Mark was and what he meant to us. We did not get the chance to say goodbye. We shared Mark’s official obituary last month in CHEST Physician. This month, we thought it important to share something more personal.
When I think of Mark, so many words come to mind: master educator, astute and caring clinician, researcher, mentor, leader. So many qualities come to mind: generous, kind, honest, brilliant, and funny. Mark loved CHEST. He gave so much to the organization and was happy to do so. He was one of the Past Presidents who contributed even more after his presidency than during or before. Mark left an enormous footprint on our educational programs, including the annual meeting, Pulmonary Board Review, and SEEK. He was instrumental in building our international educational programs and a key player in assisting our Chinese colleagues in establishing pulmonary fellowships in their country.
When I think of my own journey with Mark, I think back to the first time I saw him. I was a senior fellow taking the Pulmonary Board Review course in Chicago. I don’t remember much from that course – except for Mark’s presentations. They included everything you needed to know, in a very logical outline. More importantly, he had a presence on stage that was larger than life. He made you laugh throughout the entire talk! Mark’s humor was self-deprecating, and he made you feel like you had been best friends forever---even if he’d never met you. From that first encounter, he became a giant in chest medicine to me. It wasn’t too many years later that, as a junior volunteer leader in the organization, I was able to finally meet Mark. He could not have been more welcoming or humble, and he instantly took on the role of mentor. I was so lucky; not only did that mentorship grow, but so did our friendship. I quickly got to the point that I looked forward to the times I would travel for CHEST events, because I knew I would see Mark. I did establish one rule, however, when we started teaching together. I refused to follow Mark in the agenda, as there was no way I could ever live up to his presentation style and humor. I didn’t want to be a let down to the crowd!
Much of what I and others have accomplished with CHEST and in pulmonary medicine is directly related to the wonderful mentors we have had in the organization, and Mark was certainly one of the most prominent. He introduced me to so many additional friends and mentors. And, Mark did this for hundreds of trainees and junior faculty throughout his career. If I were to guess, I would say that this is the thing that made him most proud. Yes, he was an established international expert in several areas of pulmonary medicine; he held several prominent positions in academic medicine and at CHEST. But, what made him most happy was seeing his trainees and mentees succeed – you would have thought we were one of his kids (whom he was also very proud of and loved dearly). Mark was THE example of an outstanding mentor.
The memory I will carry forever of Mark, however, is when he got on stage and was the Master of Ceremonies for the CHEST Challenge Championship. He was in his element as an educator, interacting with the next generation of chest medicine physicians. He spent the entire time making the contestants, and the audience, laugh. People came to the final round to see Mark, even if they had no dog in the fight. I will always fondly recall that way he would look over at me and the other judges if he wasn’t sure about a team’s answer and then have an immediate witty comeback. Many of my CHEST friends have said that Mark was the Jerry Seinfeld of CHEST. I’ve never watched a single episode of Seinfeld, but if this description is true, I plan to!
Mark kept his sense of humor until the very end, telling me in his final days that he chose to focus on “humor markers,” rather than “tumor markers” – he said that always worked out better for him! Mark, we all miss you friend. We can’t wait to share a Chopin Martini with a twist of lemon when we see you on the other side. Thank you for all you did for your family, your patients, your trainees, your colleagues, and CHEST.
By now, most of you know that the CHEST family lost one of our dearest members and leaders in early July, Past President Mark Rosen. This loss has been felt deeply by many, not only because he was taken so suddenly, but because of who Mark was and what he meant to us. We did not get the chance to say goodbye. We shared Mark’s official obituary last month in CHEST Physician. This month, we thought it important to share something more personal.
When I think of Mark, so many words come to mind: master educator, astute and caring clinician, researcher, mentor, leader. So many qualities come to mind: generous, kind, honest, brilliant, and funny. Mark loved CHEST. He gave so much to the organization and was happy to do so. He was one of the Past Presidents who contributed even more after his presidency than during or before. Mark left an enormous footprint on our educational programs, including the annual meeting, Pulmonary Board Review, and SEEK. He was instrumental in building our international educational programs and a key player in assisting our Chinese colleagues in establishing pulmonary fellowships in their country.
When I think of my own journey with Mark, I think back to the first time I saw him. I was a senior fellow taking the Pulmonary Board Review course in Chicago. I don’t remember much from that course – except for Mark’s presentations. They included everything you needed to know, in a very logical outline. More importantly, he had a presence on stage that was larger than life. He made you laugh throughout the entire talk! Mark’s humor was self-deprecating, and he made you feel like you had been best friends forever---even if he’d never met you. From that first encounter, he became a giant in chest medicine to me. It wasn’t too many years later that, as a junior volunteer leader in the organization, I was able to finally meet Mark. He could not have been more welcoming or humble, and he instantly took on the role of mentor. I was so lucky; not only did that mentorship grow, but so did our friendship. I quickly got to the point that I looked forward to the times I would travel for CHEST events, because I knew I would see Mark. I did establish one rule, however, when we started teaching together. I refused to follow Mark in the agenda, as there was no way I could ever live up to his presentation style and humor. I didn’t want to be a let down to the crowd!
Much of what I and others have accomplished with CHEST and in pulmonary medicine is directly related to the wonderful mentors we have had in the organization, and Mark was certainly one of the most prominent. He introduced me to so many additional friends and mentors. And, Mark did this for hundreds of trainees and junior faculty throughout his career. If I were to guess, I would say that this is the thing that made him most proud. Yes, he was an established international expert in several areas of pulmonary medicine; he held several prominent positions in academic medicine and at CHEST. But, what made him most happy was seeing his trainees and mentees succeed – you would have thought we were one of his kids (whom he was also very proud of and loved dearly). Mark was THE example of an outstanding mentor.
The memory I will carry forever of Mark, however, is when he got on stage and was the Master of Ceremonies for the CHEST Challenge Championship. He was in his element as an educator, interacting with the next generation of chest medicine physicians. He spent the entire time making the contestants, and the audience, laugh. People came to the final round to see Mark, even if they had no dog in the fight. I will always fondly recall that way he would look over at me and the other judges if he wasn’t sure about a team’s answer and then have an immediate witty comeback. Many of my CHEST friends have said that Mark was the Jerry Seinfeld of CHEST. I’ve never watched a single episode of Seinfeld, but if this description is true, I plan to!
Mark kept his sense of humor until the very end, telling me in his final days that he chose to focus on “humor markers,” rather than “tumor markers” – he said that always worked out better for him! Mark, we all miss you friend. We can’t wait to share a Chopin Martini with a twist of lemon when we see you on the other side. Thank you for all you did for your family, your patients, your trainees, your colleagues, and CHEST.
By now, most of you know that the CHEST family lost one of our dearest members and leaders in early July, Past President Mark Rosen. This loss has been felt deeply by many, not only because he was taken so suddenly, but because of who Mark was and what he meant to us. We did not get the chance to say goodbye. We shared Mark’s official obituary last month in CHEST Physician. This month, we thought it important to share something more personal.
When I think of Mark, so many words come to mind: master educator, astute and caring clinician, researcher, mentor, leader. So many qualities come to mind: generous, kind, honest, brilliant, and funny. Mark loved CHEST. He gave so much to the organization and was happy to do so. He was one of the Past Presidents who contributed even more after his presidency than during or before. Mark left an enormous footprint on our educational programs, including the annual meeting, Pulmonary Board Review, and SEEK. He was instrumental in building our international educational programs and a key player in assisting our Chinese colleagues in establishing pulmonary fellowships in their country.
When I think of my own journey with Mark, I think back to the first time I saw him. I was a senior fellow taking the Pulmonary Board Review course in Chicago. I don’t remember much from that course – except for Mark’s presentations. They included everything you needed to know, in a very logical outline. More importantly, he had a presence on stage that was larger than life. He made you laugh throughout the entire talk! Mark’s humor was self-deprecating, and he made you feel like you had been best friends forever---even if he’d never met you. From that first encounter, he became a giant in chest medicine to me. It wasn’t too many years later that, as a junior volunteer leader in the organization, I was able to finally meet Mark. He could not have been more welcoming or humble, and he instantly took on the role of mentor. I was so lucky; not only did that mentorship grow, but so did our friendship. I quickly got to the point that I looked forward to the times I would travel for CHEST events, because I knew I would see Mark. I did establish one rule, however, when we started teaching together. I refused to follow Mark in the agenda, as there was no way I could ever live up to his presentation style and humor. I didn’t want to be a let down to the crowd!
Much of what I and others have accomplished with CHEST and in pulmonary medicine is directly related to the wonderful mentors we have had in the organization, and Mark was certainly one of the most prominent. He introduced me to so many additional friends and mentors. And, Mark did this for hundreds of trainees and junior faculty throughout his career. If I were to guess, I would say that this is the thing that made him most proud. Yes, he was an established international expert in several areas of pulmonary medicine; he held several prominent positions in academic medicine and at CHEST. But, what made him most happy was seeing his trainees and mentees succeed – you would have thought we were one of his kids (whom he was also very proud of and loved dearly). Mark was THE example of an outstanding mentor.
The memory I will carry forever of Mark, however, is when he got on stage and was the Master of Ceremonies for the CHEST Challenge Championship. He was in his element as an educator, interacting with the next generation of chest medicine physicians. He spent the entire time making the contestants, and the audience, laugh. People came to the final round to see Mark, even if they had no dog in the fight. I will always fondly recall that way he would look over at me and the other judges if he wasn’t sure about a team’s answer and then have an immediate witty comeback. Many of my CHEST friends have said that Mark was the Jerry Seinfeld of CHEST. I’ve never watched a single episode of Seinfeld, but if this description is true, I plan to!
Mark kept his sense of humor until the very end, telling me in his final days that he chose to focus on “humor markers,” rather than “tumor markers” – he said that always worked out better for him! Mark, we all miss you friend. We can’t wait to share a Chopin Martini with a twist of lemon when we see you on the other side. Thank you for all you did for your family, your patients, your trainees, your colleagues, and CHEST.