User login
Patients with viral hepatitis are living longer, increasing risk of extrahepatic mortality
Patients with viral hepatitis may live longer after treatment with direct-acting antiviral agents (DAAs), but their risk of extrahepatic causes of death may rise as a result, according to investigators.
Importantly, this increasing rate of extrahepatic mortality shouldn’t be seen as a causal link with DAA use, cautioned lead author Donghee Kim, MD, PhD, of Stanford (Calif.) University, and colleagues. Instead, the upward trend is more likely because of successful treatment with DAAs, which can increase lifespan, and with it, time for susceptibility to extrahepatic conditions.
This was just one finding from a retrospective study that used U.S. Census and National Center for Health Statistics mortality records to evaluate almost 28 million deaths that occurred between 2007 and 2017. The investigators looked for mortality trends among patients with common chronic liver diseases, including viral hepatitis, alcoholic liver disease (ALD), and nonalcoholic fatty liver disease (NAFLD), noting that each of these conditions is associated with extrahepatic complications. The study included deaths due to extrahepatic cancer, cardiovascular disease, and diabetes.
While the efficacy of therapy for viral hepatitis has improved markedly since 2014, treatments for ALD and NAFLD have remained static, the investigators noted.
“Unfortunately, there have been no significant breakthroughs in the treatment of [ALD] over the last 2 decades, resulting in an increase in estimated global mortality to 3.8%,” the investigators wrote in Gastroenterology.
“[NAFLD] is the most common chronic liver disease in the world,” they added. “The leading cause of death in individuals with NAFLD is cardiovascular disease, followed by extrahepatic malignancies, and then liver-related mortality. However, recent trends in ALD and NAFLD-related extrahepatic complications in comparison to viral hepatitis have not been studied.”
The results of the current study supported the positive impact of DAAs, which began to see widespread use in 2014. Age-standardized mortality among patients with hepatitis C virus rose until 2014 (2.2% per year) and dropped thereafter (–6.5% per year). Mortality among those with hepatitis B virus steadily decreased over the study period (–1.2% per year).
Of note, while deaths because of HCV-related liver disease dropped from 2014 to 2017, extrahepatic causes of death didn’t follow suit. Age-standardized mortality for cardiovascular disease and diabetes increased at average annual rates of 1.9% and 3.3%, respectively, while the rate of extrahepatic cancer-related deaths held steady.
“The widespread use, higher efficacy and durable response to DAA agents in individuals with HCV infection may have resulted in a paradigm shift in the clinical progression of coexisting disease entities following response to DAA agents in the virus-free environment,” the investigators wrote. “These findings suggest assessment and identification of risk and risk factors for extrahepatic cancer, cardiovascular disease, and diabetes in individuals who have been successfully treated and cured of HCV infection.”
In sharp contrast with the viral hepatitis findings, mortality rates among patients with ALD and NAFLD increased at an accelerating rate over the 11-year study period.
Among patients with ALD, all-cause mortality increased by an average of 3.4% per year, at a higher rate in the second half of the study than the first (4.6% vs 2.1%). Liver disease–related mortality rose at a similar, accelerating rate. In the same group, deaths due to cardiovascular disease increased at an average annual rate of 2.1%, which was accelerating, while extrahepatic cancer-related deaths increased at a more constant rate of 3.6%.
For patients with NAFLD, all-cause mortality increased by 8.1% per year, accelerating from 6.1% in the first half of the study to 11.2% in the second. Deaths from liver disease increased at an average rate of 12.6% per year, while extrahepatic deaths increased significantly for all three included types: cardiovascular disease (2.0%), extrahepatic cancer (15.1%), and diabetes (9.7%).
Concerning the worsening rates of mortality among patients with ALD and NAFLD, the investigators cited a lack of progress in treatments, and suggested that “the quest for newer therapies must remain the cornerstone in our efforts.”
The investigators reported no external funding or conflicts of interest.
SOURCE: Kim D et al. Gastroenterology. 2019 Jun 25. doi: 10.1053/j.gastro.2019.06.026.
Chronic liver disease is one of the leading causes of death in the United States. Whereas mortality from other causes (e.g., heart disease and cancer) has declined, age-adjusted mortality from chronic liver disease has continued to increase. There have been a few major advances in the treatment of several chronic liver diseases in recent years. These include nucleos(t)ide analogues for hepatitis B virus (HBV) and direct-acting antiviral agents for the treatment of hepatitis C virus infection (HCV). Many studies show that these treatments are highly effective in improving patient outcomes, including patient survival. However, whether these individual-level benefits have translated into population-level improvements remains unclear.
Overall, the results were mixed; they were encouraging for viral hepatitis but concerning for alcoholic and nonalcoholic liver disease. Specifically, all-cause mortality from HCV was on an upward trajectory in the first 7 years (from 2007 to 2014) but the trend shifted from 2014 onward. Importantly, this inflection point coincided with the timing of the new HCV treatments. Most of this positive shift post 2014 was related to a strong downward trend in liver-related mortality. In contrast, upward trends in mortality related to extrahepatic causes (such as cardiovascular mortality) continued unabated. The authors found similar results for HBV. The story, however, was different for alcohol and nonalcohol-related liver disease – both conditions lacking effective treatments; liver-related mortality for both continued to increase during the study period.
Although we cannot make causal inferences from this study, overall, the results are good news. They suggest that HBV and HCV treatments have reached enough infected people to result in tangible improvements in the burden of chronic liver disease. We may now need to shift the focus of secondary prevention efforts from liver to nonliver (extrahepatic) morbidity in the newer cohorts of patients with treated HCV and HBV.
Fasiha Kanwal, MD, MSHS, is an investigator in the clinical epidemiology and comparative effectiveness program for the Center for Innovations in Quality, Effectiveness, and Safety in collaboration with the Michael E. DeBakey VA Medical Center, as well as an associate professor of medicine in gastroenterology and hepatology at Baylor College of Medicine in Houston. She has no conflicts of interest.
Chronic liver disease is one of the leading causes of death in the United States. Whereas mortality from other causes (e.g., heart disease and cancer) has declined, age-adjusted mortality from chronic liver disease has continued to increase. There have been a few major advances in the treatment of several chronic liver diseases in recent years. These include nucleos(t)ide analogues for hepatitis B virus (HBV) and direct-acting antiviral agents for the treatment of hepatitis C virus infection (HCV). Many studies show that these treatments are highly effective in improving patient outcomes, including patient survival. However, whether these individual-level benefits have translated into population-level improvements remains unclear.
Overall, the results were mixed; they were encouraging for viral hepatitis but concerning for alcoholic and nonalcoholic liver disease. Specifically, all-cause mortality from HCV was on an upward trajectory in the first 7 years (from 2007 to 2014) but the trend shifted from 2014 onward. Importantly, this inflection point coincided with the timing of the new HCV treatments. Most of this positive shift post 2014 was related to a strong downward trend in liver-related mortality. In contrast, upward trends in mortality related to extrahepatic causes (such as cardiovascular mortality) continued unabated. The authors found similar results for HBV. The story, however, was different for alcohol and nonalcohol-related liver disease – both conditions lacking effective treatments; liver-related mortality for both continued to increase during the study period.
Although we cannot make causal inferences from this study, overall, the results are good news. They suggest that HBV and HCV treatments have reached enough infected people to result in tangible improvements in the burden of chronic liver disease. We may now need to shift the focus of secondary prevention efforts from liver to nonliver (extrahepatic) morbidity in the newer cohorts of patients with treated HCV and HBV.
Fasiha Kanwal, MD, MSHS, is an investigator in the clinical epidemiology and comparative effectiveness program for the Center for Innovations in Quality, Effectiveness, and Safety in collaboration with the Michael E. DeBakey VA Medical Center, as well as an associate professor of medicine in gastroenterology and hepatology at Baylor College of Medicine in Houston. She has no conflicts of interest.
Chronic liver disease is one of the leading causes of death in the United States. Whereas mortality from other causes (e.g., heart disease and cancer) has declined, age-adjusted mortality from chronic liver disease has continued to increase. There have been a few major advances in the treatment of several chronic liver diseases in recent years. These include nucleos(t)ide analogues for hepatitis B virus (HBV) and direct-acting antiviral agents for the treatment of hepatitis C virus infection (HCV). Many studies show that these treatments are highly effective in improving patient outcomes, including patient survival. However, whether these individual-level benefits have translated into population-level improvements remains unclear.
Overall, the results were mixed; they were encouraging for viral hepatitis but concerning for alcoholic and nonalcoholic liver disease. Specifically, all-cause mortality from HCV was on an upward trajectory in the first 7 years (from 2007 to 2014) but the trend shifted from 2014 onward. Importantly, this inflection point coincided with the timing of the new HCV treatments. Most of this positive shift post 2014 was related to a strong downward trend in liver-related mortality. In contrast, upward trends in mortality related to extrahepatic causes (such as cardiovascular mortality) continued unabated. The authors found similar results for HBV. The story, however, was different for alcohol and nonalcohol-related liver disease – both conditions lacking effective treatments; liver-related mortality for both continued to increase during the study period.
Although we cannot make causal inferences from this study, overall, the results are good news. They suggest that HBV and HCV treatments have reached enough infected people to result in tangible improvements in the burden of chronic liver disease. We may now need to shift the focus of secondary prevention efforts from liver to nonliver (extrahepatic) morbidity in the newer cohorts of patients with treated HCV and HBV.
Fasiha Kanwal, MD, MSHS, is an investigator in the clinical epidemiology and comparative effectiveness program for the Center for Innovations in Quality, Effectiveness, and Safety in collaboration with the Michael E. DeBakey VA Medical Center, as well as an associate professor of medicine in gastroenterology and hepatology at Baylor College of Medicine in Houston. She has no conflicts of interest.
Patients with viral hepatitis may live longer after treatment with direct-acting antiviral agents (DAAs), but their risk of extrahepatic causes of death may rise as a result, according to investigators.
Importantly, this increasing rate of extrahepatic mortality shouldn’t be seen as a causal link with DAA use, cautioned lead author Donghee Kim, MD, PhD, of Stanford (Calif.) University, and colleagues. Instead, the upward trend is more likely because of successful treatment with DAAs, which can increase lifespan, and with it, time for susceptibility to extrahepatic conditions.
This was just one finding from a retrospective study that used U.S. Census and National Center for Health Statistics mortality records to evaluate almost 28 million deaths that occurred between 2007 and 2017. The investigators looked for mortality trends among patients with common chronic liver diseases, including viral hepatitis, alcoholic liver disease (ALD), and nonalcoholic fatty liver disease (NAFLD), noting that each of these conditions is associated with extrahepatic complications. The study included deaths due to extrahepatic cancer, cardiovascular disease, and diabetes.
While the efficacy of therapy for viral hepatitis has improved markedly since 2014, treatments for ALD and NAFLD have remained static, the investigators noted.
“Unfortunately, there have been no significant breakthroughs in the treatment of [ALD] over the last 2 decades, resulting in an increase in estimated global mortality to 3.8%,” the investigators wrote in Gastroenterology.
“[NAFLD] is the most common chronic liver disease in the world,” they added. “The leading cause of death in individuals with NAFLD is cardiovascular disease, followed by extrahepatic malignancies, and then liver-related mortality. However, recent trends in ALD and NAFLD-related extrahepatic complications in comparison to viral hepatitis have not been studied.”
The results of the current study supported the positive impact of DAAs, which began to see widespread use in 2014. Age-standardized mortality among patients with hepatitis C virus rose until 2014 (2.2% per year) and dropped thereafter (–6.5% per year). Mortality among those with hepatitis B virus steadily decreased over the study period (–1.2% per year).
Of note, while deaths because of HCV-related liver disease dropped from 2014 to 2017, extrahepatic causes of death didn’t follow suit. Age-standardized mortality for cardiovascular disease and diabetes increased at average annual rates of 1.9% and 3.3%, respectively, while the rate of extrahepatic cancer-related deaths held steady.
“The widespread use, higher efficacy and durable response to DAA agents in individuals with HCV infection may have resulted in a paradigm shift in the clinical progression of coexisting disease entities following response to DAA agents in the virus-free environment,” the investigators wrote. “These findings suggest assessment and identification of risk and risk factors for extrahepatic cancer, cardiovascular disease, and diabetes in individuals who have been successfully treated and cured of HCV infection.”
In sharp contrast with the viral hepatitis findings, mortality rates among patients with ALD and NAFLD increased at an accelerating rate over the 11-year study period.
Among patients with ALD, all-cause mortality increased by an average of 3.4% per year, at a higher rate in the second half of the study than the first (4.6% vs 2.1%). Liver disease–related mortality rose at a similar, accelerating rate. In the same group, deaths due to cardiovascular disease increased at an average annual rate of 2.1%, which was accelerating, while extrahepatic cancer-related deaths increased at a more constant rate of 3.6%.
For patients with NAFLD, all-cause mortality increased by 8.1% per year, accelerating from 6.1% in the first half of the study to 11.2% in the second. Deaths from liver disease increased at an average rate of 12.6% per year, while extrahepatic deaths increased significantly for all three included types: cardiovascular disease (2.0%), extrahepatic cancer (15.1%), and diabetes (9.7%).
Concerning the worsening rates of mortality among patients with ALD and NAFLD, the investigators cited a lack of progress in treatments, and suggested that “the quest for newer therapies must remain the cornerstone in our efforts.”
The investigators reported no external funding or conflicts of interest.
SOURCE: Kim D et al. Gastroenterology. 2019 Jun 25. doi: 10.1053/j.gastro.2019.06.026.
Patients with viral hepatitis may live longer after treatment with direct-acting antiviral agents (DAAs), but their risk of extrahepatic causes of death may rise as a result, according to investigators.
Importantly, this increasing rate of extrahepatic mortality shouldn’t be seen as a causal link with DAA use, cautioned lead author Donghee Kim, MD, PhD, of Stanford (Calif.) University, and colleagues. Instead, the upward trend is more likely because of successful treatment with DAAs, which can increase lifespan, and with it, time for susceptibility to extrahepatic conditions.
This was just one finding from a retrospective study that used U.S. Census and National Center for Health Statistics mortality records to evaluate almost 28 million deaths that occurred between 2007 and 2017. The investigators looked for mortality trends among patients with common chronic liver diseases, including viral hepatitis, alcoholic liver disease (ALD), and nonalcoholic fatty liver disease (NAFLD), noting that each of these conditions is associated with extrahepatic complications. The study included deaths due to extrahepatic cancer, cardiovascular disease, and diabetes.
While the efficacy of therapy for viral hepatitis has improved markedly since 2014, treatments for ALD and NAFLD have remained static, the investigators noted.
“Unfortunately, there have been no significant breakthroughs in the treatment of [ALD] over the last 2 decades, resulting in an increase in estimated global mortality to 3.8%,” the investigators wrote in Gastroenterology.
“[NAFLD] is the most common chronic liver disease in the world,” they added. “The leading cause of death in individuals with NAFLD is cardiovascular disease, followed by extrahepatic malignancies, and then liver-related mortality. However, recent trends in ALD and NAFLD-related extrahepatic complications in comparison to viral hepatitis have not been studied.”
The results of the current study supported the positive impact of DAAs, which began to see widespread use in 2014. Age-standardized mortality among patients with hepatitis C virus rose until 2014 (2.2% per year) and dropped thereafter (–6.5% per year). Mortality among those with hepatitis B virus steadily decreased over the study period (–1.2% per year).
Of note, while deaths because of HCV-related liver disease dropped from 2014 to 2017, extrahepatic causes of death didn’t follow suit. Age-standardized mortality for cardiovascular disease and diabetes increased at average annual rates of 1.9% and 3.3%, respectively, while the rate of extrahepatic cancer-related deaths held steady.
“The widespread use, higher efficacy and durable response to DAA agents in individuals with HCV infection may have resulted in a paradigm shift in the clinical progression of coexisting disease entities following response to DAA agents in the virus-free environment,” the investigators wrote. “These findings suggest assessment and identification of risk and risk factors for extrahepatic cancer, cardiovascular disease, and diabetes in individuals who have been successfully treated and cured of HCV infection.”
In sharp contrast with the viral hepatitis findings, mortality rates among patients with ALD and NAFLD increased at an accelerating rate over the 11-year study period.
Among patients with ALD, all-cause mortality increased by an average of 3.4% per year, at a higher rate in the second half of the study than the first (4.6% vs 2.1%). Liver disease–related mortality rose at a similar, accelerating rate. In the same group, deaths due to cardiovascular disease increased at an average annual rate of 2.1%, which was accelerating, while extrahepatic cancer-related deaths increased at a more constant rate of 3.6%.
For patients with NAFLD, all-cause mortality increased by 8.1% per year, accelerating from 6.1% in the first half of the study to 11.2% in the second. Deaths from liver disease increased at an average rate of 12.6% per year, while extrahepatic deaths increased significantly for all three included types: cardiovascular disease (2.0%), extrahepatic cancer (15.1%), and diabetes (9.7%).
Concerning the worsening rates of mortality among patients with ALD and NAFLD, the investigators cited a lack of progress in treatments, and suggested that “the quest for newer therapies must remain the cornerstone in our efforts.”
The investigators reported no external funding or conflicts of interest.
SOURCE: Kim D et al. Gastroenterology. 2019 Jun 25. doi: 10.1053/j.gastro.2019.06.026.
FROM GASTROENTEROLOGY
Michigan becomes first state to ban flavored e-cigarettes
The state health agency is expected to issue rules outlining the ban within the next 30 days. The emergency ban will be in effect for 6 months, with the possibility of a 6-month extension while state health regulators craft rules to set in place a permanent ban.
The ban will also prohibit “misleading marketing of vaping products, including the use of terms like ‘clean,’ ‘safe,’ and ‘healthy,’ that perpetuate beliefs that these products are harmless,” according to a statement issued by Gov. Whitmer.
Companies selling vaping products “are using candy flavors to hook children on nicotine and misleading claims to promote the belief that these products are safe,” she said in a statement. “That ends today. Our kids deserve leaders who are going to fight to protect them. These bold steps will finally put an end to these irresponsible and deceptive practices and protect Michiganders’ public health.”
The ban also will cover mint- and menthol-flavors in addition to sweet flavors but will not ban tobacco-flavored e-cigarette products.
The American Academy of Pediatrics, American Heart Association, American Lung Association, American Cancer Society Cancer Action Network and other organizations praised the action taken by the state, calling the steps “necessary and appropriate.”
“The need for action is even more urgent in light of the recent outbreak of severe lung illness associated with e-cigarette use and the failure of the U.S. Food and Drug Administration to take strong regulatory action such as prohibiting the sale of the flavored products nationwide that have attracted shocking numbers of our nation’s youth,” the organizations said in a statement.
The groups noted that “health authorities are investigating reports of severe respiratory illness associated with e-cigarette use in at least 215 people ... in 25 states,” adding that many are youth and young adults.
The U.S. Department of Health & Human Services Secretary Alex Azar said in an Aug. 30 statement that the federal government is “using every tool we have to get to the bottom of this deeply concerning outbreak of illness in Americans who use e-cigarettes. More broadly, we will continue using every regulatory and enforcement power we have to stop the epidemic of youth e-cigarette use.”
HHS noted that no single substance or e-cigarette product has been consistently associated with the reports of illness. The agency called upon clinicians to report any new cases as appropriate to their state and local health departments.
Gov. Whitmer earlier this year signed bills that clarify that it is illegal to sell nontraditional nicotine products to minors, but the governor’s statement notes her criticism that the bills did not go far enough to protect the state’s youth, necessitating this further action.
The state health agency is expected to issue rules outlining the ban within the next 30 days. The emergency ban will be in effect for 6 months, with the possibility of a 6-month extension while state health regulators craft rules to set in place a permanent ban.
The ban will also prohibit “misleading marketing of vaping products, including the use of terms like ‘clean,’ ‘safe,’ and ‘healthy,’ that perpetuate beliefs that these products are harmless,” according to a statement issued by Gov. Whitmer.
Companies selling vaping products “are using candy flavors to hook children on nicotine and misleading claims to promote the belief that these products are safe,” she said in a statement. “That ends today. Our kids deserve leaders who are going to fight to protect them. These bold steps will finally put an end to these irresponsible and deceptive practices and protect Michiganders’ public health.”
The ban also will cover mint- and menthol-flavors in addition to sweet flavors but will not ban tobacco-flavored e-cigarette products.
The American Academy of Pediatrics, American Heart Association, American Lung Association, American Cancer Society Cancer Action Network and other organizations praised the action taken by the state, calling the steps “necessary and appropriate.”
“The need for action is even more urgent in light of the recent outbreak of severe lung illness associated with e-cigarette use and the failure of the U.S. Food and Drug Administration to take strong regulatory action such as prohibiting the sale of the flavored products nationwide that have attracted shocking numbers of our nation’s youth,” the organizations said in a statement.
The groups noted that “health authorities are investigating reports of severe respiratory illness associated with e-cigarette use in at least 215 people ... in 25 states,” adding that many are youth and young adults.
The U.S. Department of Health & Human Services Secretary Alex Azar said in an Aug. 30 statement that the federal government is “using every tool we have to get to the bottom of this deeply concerning outbreak of illness in Americans who use e-cigarettes. More broadly, we will continue using every regulatory and enforcement power we have to stop the epidemic of youth e-cigarette use.”
HHS noted that no single substance or e-cigarette product has been consistently associated with the reports of illness. The agency called upon clinicians to report any new cases as appropriate to their state and local health departments.
Gov. Whitmer earlier this year signed bills that clarify that it is illegal to sell nontraditional nicotine products to minors, but the governor’s statement notes her criticism that the bills did not go far enough to protect the state’s youth, necessitating this further action.
The state health agency is expected to issue rules outlining the ban within the next 30 days. The emergency ban will be in effect for 6 months, with the possibility of a 6-month extension while state health regulators craft rules to set in place a permanent ban.
The ban will also prohibit “misleading marketing of vaping products, including the use of terms like ‘clean,’ ‘safe,’ and ‘healthy,’ that perpetuate beliefs that these products are harmless,” according to a statement issued by Gov. Whitmer.
Companies selling vaping products “are using candy flavors to hook children on nicotine and misleading claims to promote the belief that these products are safe,” she said in a statement. “That ends today. Our kids deserve leaders who are going to fight to protect them. These bold steps will finally put an end to these irresponsible and deceptive practices and protect Michiganders’ public health.”
The ban also will cover mint- and menthol-flavors in addition to sweet flavors but will not ban tobacco-flavored e-cigarette products.
The American Academy of Pediatrics, American Heart Association, American Lung Association, American Cancer Society Cancer Action Network and other organizations praised the action taken by the state, calling the steps “necessary and appropriate.”
“The need for action is even more urgent in light of the recent outbreak of severe lung illness associated with e-cigarette use and the failure of the U.S. Food and Drug Administration to take strong regulatory action such as prohibiting the sale of the flavored products nationwide that have attracted shocking numbers of our nation’s youth,” the organizations said in a statement.
The groups noted that “health authorities are investigating reports of severe respiratory illness associated with e-cigarette use in at least 215 people ... in 25 states,” adding that many are youth and young adults.
The U.S. Department of Health & Human Services Secretary Alex Azar said in an Aug. 30 statement that the federal government is “using every tool we have to get to the bottom of this deeply concerning outbreak of illness in Americans who use e-cigarettes. More broadly, we will continue using every regulatory and enforcement power we have to stop the epidemic of youth e-cigarette use.”
HHS noted that no single substance or e-cigarette product has been consistently associated with the reports of illness. The agency called upon clinicians to report any new cases as appropriate to their state and local health departments.
Gov. Whitmer earlier this year signed bills that clarify that it is illegal to sell nontraditional nicotine products to minors, but the governor’s statement notes her criticism that the bills did not go far enough to protect the state’s youth, necessitating this further action.
Werewolf babies, blinding fries, and the gut library
Someone needs carrots, stat
Eat your veggies or you’ll … go blind? A U.K. teen took picky eating to a whole new level, literally losing his vision after a steady decade-long diet of strictly fries, Pringles, white bread, and ham.
Looks like Pringles needs to change their tagline a little bit: “Once you pop, the fun don’t stop – until you start losing hearing and vision!” We think it’s really catchy.
The teen first visited a doctor several years ago complaining of tiredness and was given B12 injections and sent on his merry way. Unfortunately, he quickly developed hearing and vision loss, and by age 17 years was diagnosed with nutritional optic neuropathy.
Somehow through all of this, he maintained a normal weight, proving once and for all the metabolism of teenage boys can withstand just about anything.
The chip-loving teen now joins the (very small) Nutritional Optic Neuropathy Hall of Fame of Developed Countries, previously only occupied by a man who pretty much drank vodka every day for breakfast, lunch, and dinner. Cheers!
Teen wolf? Try baby wolf
Every parent just wants their child to be happy, healthy, and covered in a thick layer of hair, right?
No?
Well, that’s too bad for dozens of parents in Spain, whose children developed hypertrichosis, aka “werewolf syndrome,” and suddenly sprouted full-body hair growth that Tom Selleck would be jealous of. After a brief investigation, they discovered the fast-paced hair growth was caused by an unfortunate medicine mix-up at the lab. Instead of receiving omeprazole for their gastric reflux, the children had been given minoxidil, a drug that treats alopecia.
Luckily for the children who don’t want to impersonate Michael J. Fox anymore, the hair will go away when they stop taking the drug.
No official statement yet on the mysterious sightings of wolf children roaming the Spanish countryside terrorizing locals and howling at the full moon.
Mouthwash in my veins
Let’s say you’re a person with hypertension. After years of your doctor badgering you to do more cardio exercise, you’ve finally committed to the morning jog. It’s a pain getting up that early in the morning, but the benefits will be worth it, right? You head back to your physician, eager to show off the new you. The doctor weighs you (down a few pounds, not bad), then takes a blood pressure reading, and ... it’s exactly the same.
What happened? Was all that work wasted?
Well, according to a study published in Free Radical Biology and Medicine, you may have an excuse: mouthwash usage.
It all has to do with nitric acid. Normally during cardio exercise, bacteria in the mouth convert nitrates into nitrites, and when these nitrites are swallowed, they are converted into nitric acid after being absorbed by the circulatory system. That widens the blood vessels and reduces blood pressure. Mouthwash changes all that. It inhibits those oral bacteria, and the whole process is stopped before it can begin.
The investigators found that, after 1 hour of exercise on a treadmill, study participants who received mouthwash beforehand saw their systolic blood pressure reduced by 2 mm Hg. And those who received the placebo (mint-flavored water)? They saw a 5.2-mm Hg reduction.
Bottom line to those with hypertension: You may have to start flossing. We know it’s annoying, but it’ll make your doctor happy, and it’ll make your dentist especially happy.
No books at this library
The human microbiome is a pretty hot scientific topic right now, but we here at LOTME are not scientists, or doctors, or experts of any kind, so we have a simple question: What’s in a gut?
Happily (yes, this is the sort of thing that makes us happy), researchers at MIT and the Broad Institute, both in Cambridge, Mass., have taken a very detailed look at the guts of about 90 people, with a dozen or so providing samples for up to 2 years, and can now tell us what’s in a gut: bacteria. Lots of bacteria … 7,758 different strains of bacteria.
According to a statement from MIT, the samples were obtained “through the OpenBiome organization, which collects stool samples for research and therapeutic purposes.” It also sounds like a fun place to work.
Those samples presented “a unique opportunity, and we thought that would be a great set of individuals to really try to dig down and characterize the microbial populations more thoroughly,” said Eric Alm, PhD, one of the investigators.
All of their data, along with samples of the bacteria strains they isolated, are available online at the Broad Institute–OpenBiome Microbiome Library. Which, if you think about it (and that is what we do here), makes it kind of like an Amazon for bacteria.
Hmmm … Alexa, order Turicibacter sanguinis. Uncle Leo’s birthday is coming up.
Someone needs carrots, stat
Eat your veggies or you’ll … go blind? A U.K. teen took picky eating to a whole new level, literally losing his vision after a steady decade-long diet of strictly fries, Pringles, white bread, and ham.
Looks like Pringles needs to change their tagline a little bit: “Once you pop, the fun don’t stop – until you start losing hearing and vision!” We think it’s really catchy.
The teen first visited a doctor several years ago complaining of tiredness and was given B12 injections and sent on his merry way. Unfortunately, he quickly developed hearing and vision loss, and by age 17 years was diagnosed with nutritional optic neuropathy.
Somehow through all of this, he maintained a normal weight, proving once and for all the metabolism of teenage boys can withstand just about anything.
The chip-loving teen now joins the (very small) Nutritional Optic Neuropathy Hall of Fame of Developed Countries, previously only occupied by a man who pretty much drank vodka every day for breakfast, lunch, and dinner. Cheers!
Teen wolf? Try baby wolf
Every parent just wants their child to be happy, healthy, and covered in a thick layer of hair, right?
No?
Well, that’s too bad for dozens of parents in Spain, whose children developed hypertrichosis, aka “werewolf syndrome,” and suddenly sprouted full-body hair growth that Tom Selleck would be jealous of. After a brief investigation, they discovered the fast-paced hair growth was caused by an unfortunate medicine mix-up at the lab. Instead of receiving omeprazole for their gastric reflux, the children had been given minoxidil, a drug that treats alopecia.
Luckily for the children who don’t want to impersonate Michael J. Fox anymore, the hair will go away when they stop taking the drug.
No official statement yet on the mysterious sightings of wolf children roaming the Spanish countryside terrorizing locals and howling at the full moon.
Mouthwash in my veins
Let’s say you’re a person with hypertension. After years of your doctor badgering you to do more cardio exercise, you’ve finally committed to the morning jog. It’s a pain getting up that early in the morning, but the benefits will be worth it, right? You head back to your physician, eager to show off the new you. The doctor weighs you (down a few pounds, not bad), then takes a blood pressure reading, and ... it’s exactly the same.
What happened? Was all that work wasted?
Well, according to a study published in Free Radical Biology and Medicine, you may have an excuse: mouthwash usage.
It all has to do with nitric acid. Normally during cardio exercise, bacteria in the mouth convert nitrates into nitrites, and when these nitrites are swallowed, they are converted into nitric acid after being absorbed by the circulatory system. That widens the blood vessels and reduces blood pressure. Mouthwash changes all that. It inhibits those oral bacteria, and the whole process is stopped before it can begin.
The investigators found that, after 1 hour of exercise on a treadmill, study participants who received mouthwash beforehand saw their systolic blood pressure reduced by 2 mm Hg. And those who received the placebo (mint-flavored water)? They saw a 5.2-mm Hg reduction.
Bottom line to those with hypertension: You may have to start flossing. We know it’s annoying, but it’ll make your doctor happy, and it’ll make your dentist especially happy.
No books at this library
The human microbiome is a pretty hot scientific topic right now, but we here at LOTME are not scientists, or doctors, or experts of any kind, so we have a simple question: What’s in a gut?
Happily (yes, this is the sort of thing that makes us happy), researchers at MIT and the Broad Institute, both in Cambridge, Mass., have taken a very detailed look at the guts of about 90 people, with a dozen or so providing samples for up to 2 years, and can now tell us what’s in a gut: bacteria. Lots of bacteria … 7,758 different strains of bacteria.
According to a statement from MIT, the samples were obtained “through the OpenBiome organization, which collects stool samples for research and therapeutic purposes.” It also sounds like a fun place to work.
Those samples presented “a unique opportunity, and we thought that would be a great set of individuals to really try to dig down and characterize the microbial populations more thoroughly,” said Eric Alm, PhD, one of the investigators.
All of their data, along with samples of the bacteria strains they isolated, are available online at the Broad Institute–OpenBiome Microbiome Library. Which, if you think about it (and that is what we do here), makes it kind of like an Amazon for bacteria.
Hmmm … Alexa, order Turicibacter sanguinis. Uncle Leo’s birthday is coming up.

Someone needs carrots, stat
Eat your veggies or you’ll … go blind? A U.K. teen took picky eating to a whole new level, literally losing his vision after a steady decade-long diet of strictly fries, Pringles, white bread, and ham.
Looks like Pringles needs to change their tagline a little bit: “Once you pop, the fun don’t stop – until you start losing hearing and vision!” We think it’s really catchy.
The teen first visited a doctor several years ago complaining of tiredness and was given B12 injections and sent on his merry way. Unfortunately, he quickly developed hearing and vision loss, and by age 17 years was diagnosed with nutritional optic neuropathy.
Somehow through all of this, he maintained a normal weight, proving once and for all the metabolism of teenage boys can withstand just about anything.
The chip-loving teen now joins the (very small) Nutritional Optic Neuropathy Hall of Fame of Developed Countries, previously only occupied by a man who pretty much drank vodka every day for breakfast, lunch, and dinner. Cheers!
Teen wolf? Try baby wolf
Every parent just wants their child to be happy, healthy, and covered in a thick layer of hair, right?
No?
Well, that’s too bad for dozens of parents in Spain, whose children developed hypertrichosis, aka “werewolf syndrome,” and suddenly sprouted full-body hair growth that Tom Selleck would be jealous of. After a brief investigation, they discovered the fast-paced hair growth was caused by an unfortunate medicine mix-up at the lab. Instead of receiving omeprazole for their gastric reflux, the children had been given minoxidil, a drug that treats alopecia.
Luckily for the children who don’t want to impersonate Michael J. Fox anymore, the hair will go away when they stop taking the drug.
No official statement yet on the mysterious sightings of wolf children roaming the Spanish countryside terrorizing locals and howling at the full moon.
Mouthwash in my veins
Let’s say you’re a person with hypertension. After years of your doctor badgering you to do more cardio exercise, you’ve finally committed to the morning jog. It’s a pain getting up that early in the morning, but the benefits will be worth it, right? You head back to your physician, eager to show off the new you. The doctor weighs you (down a few pounds, not bad), then takes a blood pressure reading, and ... it’s exactly the same.
What happened? Was all that work wasted?
Well, according to a study published in Free Radical Biology and Medicine, you may have an excuse: mouthwash usage.
It all has to do with nitric acid. Normally during cardio exercise, bacteria in the mouth convert nitrates into nitrites, and when these nitrites are swallowed, they are converted into nitric acid after being absorbed by the circulatory system. That widens the blood vessels and reduces blood pressure. Mouthwash changes all that. It inhibits those oral bacteria, and the whole process is stopped before it can begin.
The investigators found that, after 1 hour of exercise on a treadmill, study participants who received mouthwash beforehand saw their systolic blood pressure reduced by 2 mm Hg. And those who received the placebo (mint-flavored water)? They saw a 5.2-mm Hg reduction.
Bottom line to those with hypertension: You may have to start flossing. We know it’s annoying, but it’ll make your doctor happy, and it’ll make your dentist especially happy.
No books at this library
The human microbiome is a pretty hot scientific topic right now, but we here at LOTME are not scientists, or doctors, or experts of any kind, so we have a simple question: What’s in a gut?
Happily (yes, this is the sort of thing that makes us happy), researchers at MIT and the Broad Institute, both in Cambridge, Mass., have taken a very detailed look at the guts of about 90 people, with a dozen or so providing samples for up to 2 years, and can now tell us what’s in a gut: bacteria. Lots of bacteria … 7,758 different strains of bacteria.
According to a statement from MIT, the samples were obtained “through the OpenBiome organization, which collects stool samples for research and therapeutic purposes.” It also sounds like a fun place to work.
Those samples presented “a unique opportunity, and we thought that would be a great set of individuals to really try to dig down and characterize the microbial populations more thoroughly,” said Eric Alm, PhD, one of the investigators.
All of their data, along with samples of the bacteria strains they isolated, are available online at the Broad Institute–OpenBiome Microbiome Library. Which, if you think about it (and that is what we do here), makes it kind of like an Amazon for bacteria.
Hmmm … Alexa, order Turicibacter sanguinis. Uncle Leo’s birthday is coming up.

Hyperphosphorylated tau visible in TBI survivors decades after brain injury
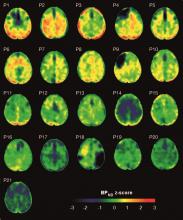
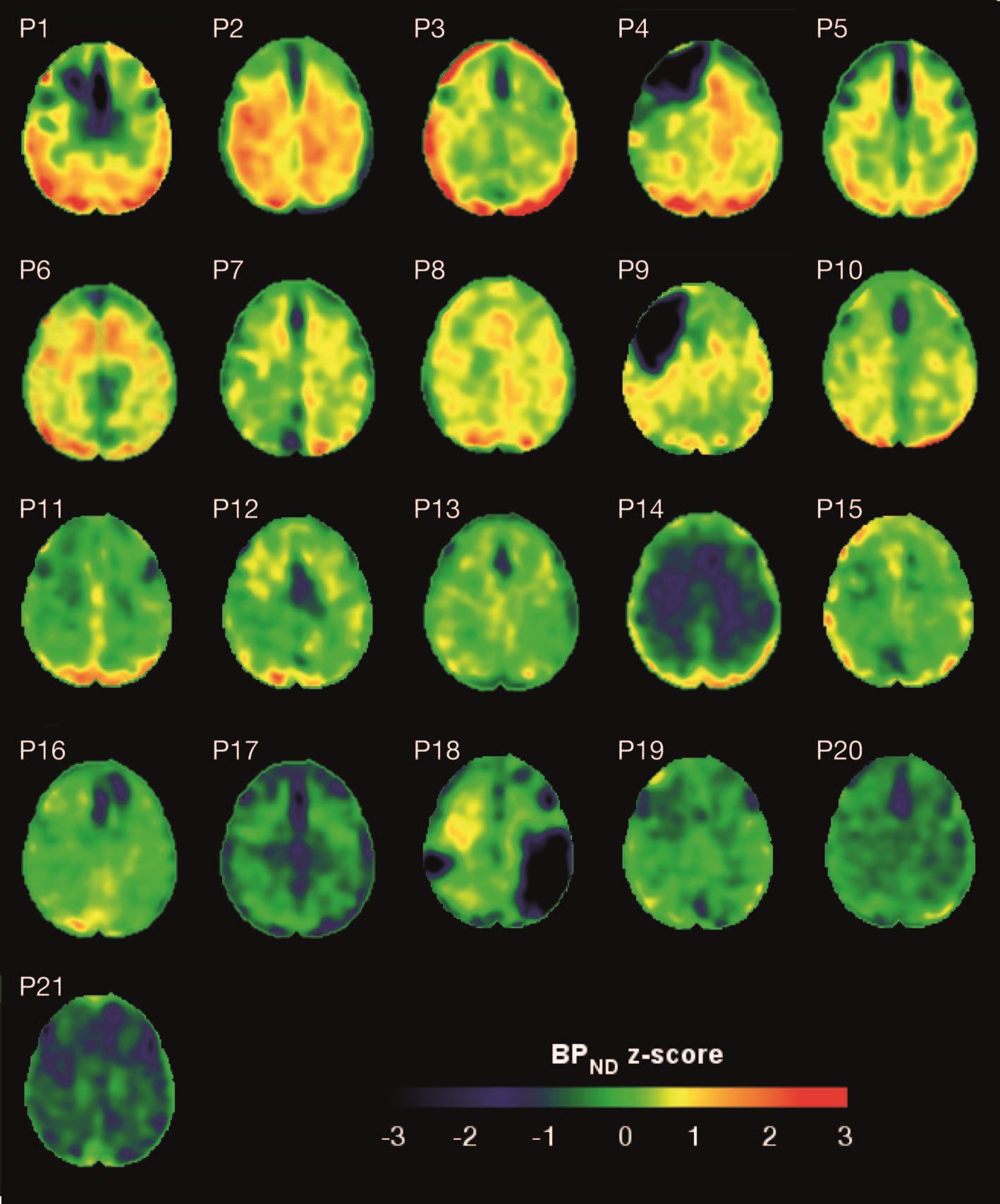
Brain deposits of hyperphosphorylated tau are detectable in traumatic brain injury (TBI) patients 18-51 years after a single moderate to severe incident occurred, researchers reported Sept. 4 in Science Translational Medicine.
Imaging with the tau-specific PET radioligand flortaucipir showed that the protein was most apparent in the right occipital cortex, and was associated with changes in cognitive scores, tau and beta amyloid in cerebrospinal fluid (CSF), and white matter density, Nikos Gorgoraptis, PhD, of Imperial College London and his colleagues wrote.
“The ability to detect tau pathology in vivo after TBI has major potential implications for diagnosis and prognostication of clinical outcomes after TBI,” the researchers explained. “It is also likely to assist in patient selection and stratification for future treatment trials targeting tau.”
The cohort study comprised 21 subjects (median age, 49 years) who had experienced a single moderate to severe TBI a median of 32 years (range, 18-51 years) before enrollment. A control group comprised 11 noninjured adults who were matched for age and other demographic factors. Everyone underwent a PET scan with flortaucipir, brain MRI, CSF sampling, apolipoprotein E genotyping, and neuropsychological testing.
TBI subjects were grouped according to recovery status: good and disabled. Overall, they showed impairments on multiple cognitive domains (processing speed, executive function, motivation, inhibition, and verbal and visual memory), compared with controls. These findings were largely driven by the disabled group.
Eight TBI subjects had elevated tau binding greater than 2,000 voxels above the threshold of detection (equivalent to 16 cm3 of brain volume), and seven had an increase of 249-1,999 voxels above threshold. Tau binding in the remainder was similar to that in controls. Recovery status didn’t correlate with the tau-binding strength.
Overall, the tau-binding signal appeared most strongly in the right lateral occipital cortex, regardless of functional recovery status.
In TBI subjects, CSF total tau correlated significantly with flortaucipir uptake in cortical gray matter, but not white matter. CSF phosphorylated tau correlated with uptake in white matter, but not gray matter.
The investigators also examined fractional anisotropy, a measure of fiber density, axonal diameter, and myelination in white matter. In TBI subjects, there was more flortaucipir uptake in areas of decreased fractional anisotropy, including association, commissural, and projection tracts.
“Correlations were observed in the genu and body of the corpus callosum, as well as in several association tracts within the ipsilateral (right) hemisphere, including the cingulum bundle, inferior longitudinal fasciculus, uncinate fasciculus, and anterior thalamic radiation, but not in the contralateral hemisphere. Higher cortical flortaucipir [signal] was associated with reduced tissue density in remote white matter regions including the corpus callosum and right prefrontal white matter. The same analysis for gray matter density did not show an association.”
The increased tau signal in TBI subjects “is in keeping with a causative role for traumatic axonal injury in the pathophysiology of posttraumatic tau pathology,” the authors said. “Mechanical forces exerted at the time of head injury are thought to disrupt axonal organization, producing damage to microtubule structure and associated axonal tau. This damage may lead to hyperphosphorylation of tau, misfolding, and neurofibrillary tangle formation, which eventually causes neurodegeneration. Mechanical forces are maximal in points of geometric inflection such as the base of cortical sulci, where tau pathology is seen in chronic traumatic encephalopathy.”
These patterns suggest that tau imaging could provide valuable diagnostic information about the type of posttraumatic neurodegeneration, they said.
The work was supported by the Medical Research Council and UK Dementia Research Institute. None of the authors declared having any competing interests related to the current study. Some authors reported financial ties to pharmaceutical companies.
SOURCE: Gorgoraptis N et al. Sci Transl Med. 2019;11:eaaw1993. doi: 10.1126/scitranslmed.aaw1993.
Brain deposits of hyperphosphorylated tau are detectable in traumatic brain injury (TBI) patients 18-51 years after a single moderate to severe incident occurred, researchers reported Sept. 4 in Science Translational Medicine.
Imaging with the tau-specific PET radioligand flortaucipir showed that the protein was most apparent in the right occipital cortex, and was associated with changes in cognitive scores, tau and beta amyloid in cerebrospinal fluid (CSF), and white matter density, Nikos Gorgoraptis, PhD, of Imperial College London and his colleagues wrote.
“The ability to detect tau pathology in vivo after TBI has major potential implications for diagnosis and prognostication of clinical outcomes after TBI,” the researchers explained. “It is also likely to assist in patient selection and stratification for future treatment trials targeting tau.”
The cohort study comprised 21 subjects (median age, 49 years) who had experienced a single moderate to severe TBI a median of 32 years (range, 18-51 years) before enrollment. A control group comprised 11 noninjured adults who were matched for age and other demographic factors. Everyone underwent a PET scan with flortaucipir, brain MRI, CSF sampling, apolipoprotein E genotyping, and neuropsychological testing.
TBI subjects were grouped according to recovery status: good and disabled. Overall, they showed impairments on multiple cognitive domains (processing speed, executive function, motivation, inhibition, and verbal and visual memory), compared with controls. These findings were largely driven by the disabled group.
Eight TBI subjects had elevated tau binding greater than 2,000 voxels above the threshold of detection (equivalent to 16 cm3 of brain volume), and seven had an increase of 249-1,999 voxels above threshold. Tau binding in the remainder was similar to that in controls. Recovery status didn’t correlate with the tau-binding strength.
Overall, the tau-binding signal appeared most strongly in the right lateral occipital cortex, regardless of functional recovery status.
In TBI subjects, CSF total tau correlated significantly with flortaucipir uptake in cortical gray matter, but not white matter. CSF phosphorylated tau correlated with uptake in white matter, but not gray matter.
The investigators also examined fractional anisotropy, a measure of fiber density, axonal diameter, and myelination in white matter. In TBI subjects, there was more flortaucipir uptake in areas of decreased fractional anisotropy, including association, commissural, and projection tracts.
“Correlations were observed in the genu and body of the corpus callosum, as well as in several association tracts within the ipsilateral (right) hemisphere, including the cingulum bundle, inferior longitudinal fasciculus, uncinate fasciculus, and anterior thalamic radiation, but not in the contralateral hemisphere. Higher cortical flortaucipir [signal] was associated with reduced tissue density in remote white matter regions including the corpus callosum and right prefrontal white matter. The same analysis for gray matter density did not show an association.”
The increased tau signal in TBI subjects “is in keeping with a causative role for traumatic axonal injury in the pathophysiology of posttraumatic tau pathology,” the authors said. “Mechanical forces exerted at the time of head injury are thought to disrupt axonal organization, producing damage to microtubule structure and associated axonal tau. This damage may lead to hyperphosphorylation of tau, misfolding, and neurofibrillary tangle formation, which eventually causes neurodegeneration. Mechanical forces are maximal in points of geometric inflection such as the base of cortical sulci, where tau pathology is seen in chronic traumatic encephalopathy.”
These patterns suggest that tau imaging could provide valuable diagnostic information about the type of posttraumatic neurodegeneration, they said.
The work was supported by the Medical Research Council and UK Dementia Research Institute. None of the authors declared having any competing interests related to the current study. Some authors reported financial ties to pharmaceutical companies.
SOURCE: Gorgoraptis N et al. Sci Transl Med. 2019;11:eaaw1993. doi: 10.1126/scitranslmed.aaw1993.
Brain deposits of hyperphosphorylated tau are detectable in traumatic brain injury (TBI) patients 18-51 years after a single moderate to severe incident occurred, researchers reported Sept. 4 in Science Translational Medicine.
Imaging with the tau-specific PET radioligand flortaucipir showed that the protein was most apparent in the right occipital cortex, and was associated with changes in cognitive scores, tau and beta amyloid in cerebrospinal fluid (CSF), and white matter density, Nikos Gorgoraptis, PhD, of Imperial College London and his colleagues wrote.
“The ability to detect tau pathology in vivo after TBI has major potential implications for diagnosis and prognostication of clinical outcomes after TBI,” the researchers explained. “It is also likely to assist in patient selection and stratification for future treatment trials targeting tau.”
The cohort study comprised 21 subjects (median age, 49 years) who had experienced a single moderate to severe TBI a median of 32 years (range, 18-51 years) before enrollment. A control group comprised 11 noninjured adults who were matched for age and other demographic factors. Everyone underwent a PET scan with flortaucipir, brain MRI, CSF sampling, apolipoprotein E genotyping, and neuropsychological testing.
TBI subjects were grouped according to recovery status: good and disabled. Overall, they showed impairments on multiple cognitive domains (processing speed, executive function, motivation, inhibition, and verbal and visual memory), compared with controls. These findings were largely driven by the disabled group.
Eight TBI subjects had elevated tau binding greater than 2,000 voxels above the threshold of detection (equivalent to 16 cm3 of brain volume), and seven had an increase of 249-1,999 voxels above threshold. Tau binding in the remainder was similar to that in controls. Recovery status didn’t correlate with the tau-binding strength.
Overall, the tau-binding signal appeared most strongly in the right lateral occipital cortex, regardless of functional recovery status.
In TBI subjects, CSF total tau correlated significantly with flortaucipir uptake in cortical gray matter, but not white matter. CSF phosphorylated tau correlated with uptake in white matter, but not gray matter.
The investigators also examined fractional anisotropy, a measure of fiber density, axonal diameter, and myelination in white matter. In TBI subjects, there was more flortaucipir uptake in areas of decreased fractional anisotropy, including association, commissural, and projection tracts.
“Correlations were observed in the genu and body of the corpus callosum, as well as in several association tracts within the ipsilateral (right) hemisphere, including the cingulum bundle, inferior longitudinal fasciculus, uncinate fasciculus, and anterior thalamic radiation, but not in the contralateral hemisphere. Higher cortical flortaucipir [signal] was associated with reduced tissue density in remote white matter regions including the corpus callosum and right prefrontal white matter. The same analysis for gray matter density did not show an association.”
The increased tau signal in TBI subjects “is in keeping with a causative role for traumatic axonal injury in the pathophysiology of posttraumatic tau pathology,” the authors said. “Mechanical forces exerted at the time of head injury are thought to disrupt axonal organization, producing damage to microtubule structure and associated axonal tau. This damage may lead to hyperphosphorylation of tau, misfolding, and neurofibrillary tangle formation, which eventually causes neurodegeneration. Mechanical forces are maximal in points of geometric inflection such as the base of cortical sulci, where tau pathology is seen in chronic traumatic encephalopathy.”
These patterns suggest that tau imaging could provide valuable diagnostic information about the type of posttraumatic neurodegeneration, they said.
The work was supported by the Medical Research Council and UK Dementia Research Institute. None of the authors declared having any competing interests related to the current study. Some authors reported financial ties to pharmaceutical companies.
SOURCE: Gorgoraptis N et al. Sci Transl Med. 2019;11:eaaw1993. doi: 10.1126/scitranslmed.aaw1993.
FROM SCIENCE TRANSLATIONAL MEDICINE
Type of renal dysfunction affects liver cirrhosis mortality risk
For non–status 1 patients with cirrhosis who are awaiting liver transplantation, type of renal dysfunction may be a key determinant of mortality risk, based on a retrospective analysis of more than 22,000 patients.
Risk of death was greatest for patients with acute on chronic kidney disease (AKI on CKD), followed by AKI alone, then CKD alone, reported lead author Giuseppe Cullaro, MD, of the University of California, San Francisco, and colleagues.
Although it is well known that renal dysfunction worsens outcomes among patients with liver cirrhosis, the impact of different types of kidney pathology on mortality risk has been minimally researched, the investigators wrote in Clinical Gastroenterology and Hepatology. “To date, studies evaluating the impact of renal dysfunction on prognosis in patients with cirrhosis have mostly focused on AKI.”
To learn more, the investigators performed a retrospective study involving acute, chronic, and acute on chronic kidney disease among patients with cirrhosis. They included data from 22,680 non–status 1 adults who were awaiting liver transplantation between 2007 and 2014, with at least 90 days on the wait list. Information was gathered from the Organ Procurement and Transplantation Network registry.
AKI was defined by fewer than 72 days of hemodialysis, or an increase in creatinine of at least 0.3 mg/dL or at least 50% in the last 7 days. CKD was identified by more than 72 days of hemodialysis, or an estimated glomerular filtration rate less than 60 mL/min/1.73 m2 for 90 days with a final rate of at least 30 mL/min/1.73 m2. Using these criteria, the researchers put patients into four possible categories: AKI on CKD, AKI, CKD, or normal renal function. The primary outcome was wait list mortality, which was defined as death, or removal from the wait list for illness. Follow-up started at the time of addition to the wait list and continued until transplant, removal from the wait list, or death.
Multivariate analysis, which accounted for final MELD-Na score and other confounders, showed that patients with AKI on CKD fared worst, with a 2.86-fold higher mortality risk (subhazard [SHR] ratio, 2.86) than that of patients with normal renal function. The mortality risk for acute on chronic kidney disease was followed closely by patients with AKI alone (SHR, 2.42), and more distantly by patients with CKD alone (SHR, 1.56). Further analysis showed that the disparity between mortality risks of each subgroup became more pronounced with increased MELD-Na score. In addition, evaluation of receiver operating characteristic curves for 6-month wait list mortality showed that the addition of renal function to MELD-Na score increased the accuracy of prognosis from an area under the curve of 0.71 to 0.80 (P less than .001).
“This suggests that incorporating the pattern of renal function could provide an opportunity to better prognosticate risk of mortality in the patients with cirrhosis who are the sickest,” the investigators concluded.
They also speculated about why outcomes may vary by type of kidney dysfunction.
“We suspect that those patients who experience AKI and AKI on CKD in our cohort likely had a triggering event – infection, bleeding, hypovolemia – that put these patients at greater risk for waitlist mortality,” the investigators wrote. “These events inherently carry more risk than stable nonliver-related elevations in serum creatinine that are seen in patients with CKD. Because of this heterogeneity of etiology in renal dysfunction in patients with cirrhosis, it is perhaps not surprising that unique renal function patterns variably impact mortality.”
The investigators noted that the findings from the study have “important implications for clinical practice,” and suggested that including type of renal dysfunction would have the most significant affect on accuracy of prognoses among patients at greatest risk of mortality.
The study was funded by a Paul B. Beeson Career Development Award and the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Verna disclosed relationships with Salix, Merck, and Gilead.
SOURCE: Cullaro et al. Clin Gastroenterol Hepatol. 2019 Feb 1. doi: 10.1016/j.cgh.2019.01.043.
Cirrhotic patients with renal failure have a sevenfold increase in mortality compared with those without renal failure. Acute kidney injury (AKI) is common in cirrhosis; increasingly, cirrhotic patients awaiting liver transplantation have or are also at risk for CKD. They are sicker, older, and have more comorbidities such as obesity and diabetes. In this study, the cumulative incidence of death on the wait list was much more pronounced for any form of AKI, with those with AKI on CKD having the highest cumulative incidence of wait list mortality compared with those with normal renal function. The study notably raises several important issues. First, AKI exerts a greater influence in risk of mortality on CKD than it does on those with normal renal function. This is relevant given the increasing prevalence of CKD in this population. Second, it emphasizes the need to effectively measure renal function. All serum creatinine-based equations overestimate glomerular filtration rate in the presence of renal dysfunction. Finally, the study highlights the importance of extrahepatic factors in determining mortality on the wait list. While in all comers, a mathematical model such as the MELDNa score may be able to predict mortality, for a specific patient the presence of comorbid conditions, malnutrition and sarcopenia, infections, critical illness, and now pattern of renal dysfunction, may all play a role.
Sumeet K. Asrani, MD, MSc, is a hepatologist affiliated with Baylor University Medical Center, Dallas. He has no conflicts of interest.
Cirrhotic patients with renal failure have a sevenfold increase in mortality compared with those without renal failure. Acute kidney injury (AKI) is common in cirrhosis; increasingly, cirrhotic patients awaiting liver transplantation have or are also at risk for CKD. They are sicker, older, and have more comorbidities such as obesity and diabetes. In this study, the cumulative incidence of death on the wait list was much more pronounced for any form of AKI, with those with AKI on CKD having the highest cumulative incidence of wait list mortality compared with those with normal renal function. The study notably raises several important issues. First, AKI exerts a greater influence in risk of mortality on CKD than it does on those with normal renal function. This is relevant given the increasing prevalence of CKD in this population. Second, it emphasizes the need to effectively measure renal function. All serum creatinine-based equations overestimate glomerular filtration rate in the presence of renal dysfunction. Finally, the study highlights the importance of extrahepatic factors in determining mortality on the wait list. While in all comers, a mathematical model such as the MELDNa score may be able to predict mortality, for a specific patient the presence of comorbid conditions, malnutrition and sarcopenia, infections, critical illness, and now pattern of renal dysfunction, may all play a role.
Sumeet K. Asrani, MD, MSc, is a hepatologist affiliated with Baylor University Medical Center, Dallas. He has no conflicts of interest.
Cirrhotic patients with renal failure have a sevenfold increase in mortality compared with those without renal failure. Acute kidney injury (AKI) is common in cirrhosis; increasingly, cirrhotic patients awaiting liver transplantation have or are also at risk for CKD. They are sicker, older, and have more comorbidities such as obesity and diabetes. In this study, the cumulative incidence of death on the wait list was much more pronounced for any form of AKI, with those with AKI on CKD having the highest cumulative incidence of wait list mortality compared with those with normal renal function. The study notably raises several important issues. First, AKI exerts a greater influence in risk of mortality on CKD than it does on those with normal renal function. This is relevant given the increasing prevalence of CKD in this population. Second, it emphasizes the need to effectively measure renal function. All serum creatinine-based equations overestimate glomerular filtration rate in the presence of renal dysfunction. Finally, the study highlights the importance of extrahepatic factors in determining mortality on the wait list. While in all comers, a mathematical model such as the MELDNa score may be able to predict mortality, for a specific patient the presence of comorbid conditions, malnutrition and sarcopenia, infections, critical illness, and now pattern of renal dysfunction, may all play a role.
Sumeet K. Asrani, MD, MSc, is a hepatologist affiliated with Baylor University Medical Center, Dallas. He has no conflicts of interest.
For non–status 1 patients with cirrhosis who are awaiting liver transplantation, type of renal dysfunction may be a key determinant of mortality risk, based on a retrospective analysis of more than 22,000 patients.
Risk of death was greatest for patients with acute on chronic kidney disease (AKI on CKD), followed by AKI alone, then CKD alone, reported lead author Giuseppe Cullaro, MD, of the University of California, San Francisco, and colleagues.
Although it is well known that renal dysfunction worsens outcomes among patients with liver cirrhosis, the impact of different types of kidney pathology on mortality risk has been minimally researched, the investigators wrote in Clinical Gastroenterology and Hepatology. “To date, studies evaluating the impact of renal dysfunction on prognosis in patients with cirrhosis have mostly focused on AKI.”
To learn more, the investigators performed a retrospective study involving acute, chronic, and acute on chronic kidney disease among patients with cirrhosis. They included data from 22,680 non–status 1 adults who were awaiting liver transplantation between 2007 and 2014, with at least 90 days on the wait list. Information was gathered from the Organ Procurement and Transplantation Network registry.
AKI was defined by fewer than 72 days of hemodialysis, or an increase in creatinine of at least 0.3 mg/dL or at least 50% in the last 7 days. CKD was identified by more than 72 days of hemodialysis, or an estimated glomerular filtration rate less than 60 mL/min/1.73 m2 for 90 days with a final rate of at least 30 mL/min/1.73 m2. Using these criteria, the researchers put patients into four possible categories: AKI on CKD, AKI, CKD, or normal renal function. The primary outcome was wait list mortality, which was defined as death, or removal from the wait list for illness. Follow-up started at the time of addition to the wait list and continued until transplant, removal from the wait list, or death.
Multivariate analysis, which accounted for final MELD-Na score and other confounders, showed that patients with AKI on CKD fared worst, with a 2.86-fold higher mortality risk (subhazard [SHR] ratio, 2.86) than that of patients with normal renal function. The mortality risk for acute on chronic kidney disease was followed closely by patients with AKI alone (SHR, 2.42), and more distantly by patients with CKD alone (SHR, 1.56). Further analysis showed that the disparity between mortality risks of each subgroup became more pronounced with increased MELD-Na score. In addition, evaluation of receiver operating characteristic curves for 6-month wait list mortality showed that the addition of renal function to MELD-Na score increased the accuracy of prognosis from an area under the curve of 0.71 to 0.80 (P less than .001).
“This suggests that incorporating the pattern of renal function could provide an opportunity to better prognosticate risk of mortality in the patients with cirrhosis who are the sickest,” the investigators concluded.
They also speculated about why outcomes may vary by type of kidney dysfunction.
“We suspect that those patients who experience AKI and AKI on CKD in our cohort likely had a triggering event – infection, bleeding, hypovolemia – that put these patients at greater risk for waitlist mortality,” the investigators wrote. “These events inherently carry more risk than stable nonliver-related elevations in serum creatinine that are seen in patients with CKD. Because of this heterogeneity of etiology in renal dysfunction in patients with cirrhosis, it is perhaps not surprising that unique renal function patterns variably impact mortality.”
The investigators noted that the findings from the study have “important implications for clinical practice,” and suggested that including type of renal dysfunction would have the most significant affect on accuracy of prognoses among patients at greatest risk of mortality.
The study was funded by a Paul B. Beeson Career Development Award and the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Verna disclosed relationships with Salix, Merck, and Gilead.
SOURCE: Cullaro et al. Clin Gastroenterol Hepatol. 2019 Feb 1. doi: 10.1016/j.cgh.2019.01.043.
For non–status 1 patients with cirrhosis who are awaiting liver transplantation, type of renal dysfunction may be a key determinant of mortality risk, based on a retrospective analysis of more than 22,000 patients.
Risk of death was greatest for patients with acute on chronic kidney disease (AKI on CKD), followed by AKI alone, then CKD alone, reported lead author Giuseppe Cullaro, MD, of the University of California, San Francisco, and colleagues.
Although it is well known that renal dysfunction worsens outcomes among patients with liver cirrhosis, the impact of different types of kidney pathology on mortality risk has been minimally researched, the investigators wrote in Clinical Gastroenterology and Hepatology. “To date, studies evaluating the impact of renal dysfunction on prognosis in patients with cirrhosis have mostly focused on AKI.”
To learn more, the investigators performed a retrospective study involving acute, chronic, and acute on chronic kidney disease among patients with cirrhosis. They included data from 22,680 non–status 1 adults who were awaiting liver transplantation between 2007 and 2014, with at least 90 days on the wait list. Information was gathered from the Organ Procurement and Transplantation Network registry.
AKI was defined by fewer than 72 days of hemodialysis, or an increase in creatinine of at least 0.3 mg/dL or at least 50% in the last 7 days. CKD was identified by more than 72 days of hemodialysis, or an estimated glomerular filtration rate less than 60 mL/min/1.73 m2 for 90 days with a final rate of at least 30 mL/min/1.73 m2. Using these criteria, the researchers put patients into four possible categories: AKI on CKD, AKI, CKD, or normal renal function. The primary outcome was wait list mortality, which was defined as death, or removal from the wait list for illness. Follow-up started at the time of addition to the wait list and continued until transplant, removal from the wait list, or death.
Multivariate analysis, which accounted for final MELD-Na score and other confounders, showed that patients with AKI on CKD fared worst, with a 2.86-fold higher mortality risk (subhazard [SHR] ratio, 2.86) than that of patients with normal renal function. The mortality risk for acute on chronic kidney disease was followed closely by patients with AKI alone (SHR, 2.42), and more distantly by patients with CKD alone (SHR, 1.56). Further analysis showed that the disparity between mortality risks of each subgroup became more pronounced with increased MELD-Na score. In addition, evaluation of receiver operating characteristic curves for 6-month wait list mortality showed that the addition of renal function to MELD-Na score increased the accuracy of prognosis from an area under the curve of 0.71 to 0.80 (P less than .001).
“This suggests that incorporating the pattern of renal function could provide an opportunity to better prognosticate risk of mortality in the patients with cirrhosis who are the sickest,” the investigators concluded.
They also speculated about why outcomes may vary by type of kidney dysfunction.
“We suspect that those patients who experience AKI and AKI on CKD in our cohort likely had a triggering event – infection, bleeding, hypovolemia – that put these patients at greater risk for waitlist mortality,” the investigators wrote. “These events inherently carry more risk than stable nonliver-related elevations in serum creatinine that are seen in patients with CKD. Because of this heterogeneity of etiology in renal dysfunction in patients with cirrhosis, it is perhaps not surprising that unique renal function patterns variably impact mortality.”
The investigators noted that the findings from the study have “important implications for clinical practice,” and suggested that including type of renal dysfunction would have the most significant affect on accuracy of prognoses among patients at greatest risk of mortality.
The study was funded by a Paul B. Beeson Career Development Award and the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Verna disclosed relationships with Salix, Merck, and Gilead.
SOURCE: Cullaro et al. Clin Gastroenterol Hepatol. 2019 Feb 1. doi: 10.1016/j.cgh.2019.01.043.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Part 1: Finding Your Inner Leader
Is it my imagination, or has there been a lot of discussion on leadership lately? In the past 3 years, all the meetings I have attended had at least 1 presentation on leadership or the traits of leaders. Sometimes—even in the oddest places—I have come across an article with “leadership” in the title. In fact, a serendipitous discovery of 2 publications is what inspired me to write this.
I spotted the first one in a reading basket when I was on vacation: It was an interview with Benjamin Zander, the conductor of the Boston Philharmonic Orchestra and the Boston Philharmonic Youth Orchestra.1 I was especially interested to read this because when I was in graduate school, Benjamin (the father of one of my classmates) visited our campus to give a presentation on talent and self-confidence. I can still hear him conducting us to emphatically believe in ourselves and others. This was echoed in his interview: “Never doubt the capacity of the people you lead to accomplish whatever you dream for them.” What a powerful concept: Believe in the possibilities of someone!
The second was an American Legion Auxiliary column, which emphasized that “leadership is not a title, but a responsibility.”2 This struck a chord with me because some prominent leaders don’t appear to ascribe to that assessment (although they should!).
After digesting these articles, I started thinking: What, exactly, is leadership? What do we even mean by leadership? How do we measure it? Is it measurable? How do we know when we see or experience good leadership? Can one learn to become a leader by simply reading a “how-to” article?
I think I can answer these questions with 2 principles that have guided me through my career as an NP: (1) Make use of another leader’s expertise to guide you, and (2) Continue to grow amid any setbacks.
For example, my transition from a full-time clinician to a Health Policy Coordinator or “policy wonk” did not have a distinct trajectory. Although my core set of clinical skills were essential, I knew early on that I had to expand by adapting specific organizational skills that would enable me to grow in my new role. But how was I to prioritize which skills to improve? More than a simple trial-and-error approach was required; I needed guidance. Fortunately, my new boss was willing to share her experience and the lessons she learned on the job. Key among them was to recognize the skills I already had—communicating and coordinating—and to develop those skills to be more effective in my new position.
Later in my career, I worked with colleagues to pursue legislation for NP prescriptive authority in Massachusetts. The political arena of Commonwealth’s health care laws was especially pivotal in changing how I saw setbacks. These weren’t to be accepted as a failure but as a challenge to figure out how to better succeed the next time. For several years, I was told “No” before we finally got a bill passed. But each round of my testimony was an opportunity to educate lawmakers and the public on the valuable role of NPs and the quality of care we provide. I try to share this story with new NPs as a good example of why they should persist through adversity.
Continue to: Over the next 3 weeks...
Over the next 3 weeks, join us on Thursdays as we continue to discuss what it means to be a leader—from the pitfalls to the victories. For those who are leaders or who work for one, please share your thoughts, experiences, and lessons learned. Maybe you can give a shoutout to someone who was a positive influence!
1. Labarre P. Leadership—Ben Zander. Fast Company website. www.fastcompany.com/35825/leadership-ben-zander. Published November 30, 1998. Accessed August 27, 2019.
2. Volunteer beyond the ALA: serve on boards, committees. American Legion Auxiliary. November 2018:29.
Is it my imagination, or has there been a lot of discussion on leadership lately? In the past 3 years, all the meetings I have attended had at least 1 presentation on leadership or the traits of leaders. Sometimes—even in the oddest places—I have come across an article with “leadership” in the title. In fact, a serendipitous discovery of 2 publications is what inspired me to write this.
I spotted the first one in a reading basket when I was on vacation: It was an interview with Benjamin Zander, the conductor of the Boston Philharmonic Orchestra and the Boston Philharmonic Youth Orchestra.1 I was especially interested to read this because when I was in graduate school, Benjamin (the father of one of my classmates) visited our campus to give a presentation on talent and self-confidence. I can still hear him conducting us to emphatically believe in ourselves and others. This was echoed in his interview: “Never doubt the capacity of the people you lead to accomplish whatever you dream for them.” What a powerful concept: Believe in the possibilities of someone!
The second was an American Legion Auxiliary column, which emphasized that “leadership is not a title, but a responsibility.”2 This struck a chord with me because some prominent leaders don’t appear to ascribe to that assessment (although they should!).
After digesting these articles, I started thinking: What, exactly, is leadership? What do we even mean by leadership? How do we measure it? Is it measurable? How do we know when we see or experience good leadership? Can one learn to become a leader by simply reading a “how-to” article?
I think I can answer these questions with 2 principles that have guided me through my career as an NP: (1) Make use of another leader’s expertise to guide you, and (2) Continue to grow amid any setbacks.
For example, my transition from a full-time clinician to a Health Policy Coordinator or “policy wonk” did not have a distinct trajectory. Although my core set of clinical skills were essential, I knew early on that I had to expand by adapting specific organizational skills that would enable me to grow in my new role. But how was I to prioritize which skills to improve? More than a simple trial-and-error approach was required; I needed guidance. Fortunately, my new boss was willing to share her experience and the lessons she learned on the job. Key among them was to recognize the skills I already had—communicating and coordinating—and to develop those skills to be more effective in my new position.
Later in my career, I worked with colleagues to pursue legislation for NP prescriptive authority in Massachusetts. The political arena of Commonwealth’s health care laws was especially pivotal in changing how I saw setbacks. These weren’t to be accepted as a failure but as a challenge to figure out how to better succeed the next time. For several years, I was told “No” before we finally got a bill passed. But each round of my testimony was an opportunity to educate lawmakers and the public on the valuable role of NPs and the quality of care we provide. I try to share this story with new NPs as a good example of why they should persist through adversity.
Continue to: Over the next 3 weeks...
Over the next 3 weeks, join us on Thursdays as we continue to discuss what it means to be a leader—from the pitfalls to the victories. For those who are leaders or who work for one, please share your thoughts, experiences, and lessons learned. Maybe you can give a shoutout to someone who was a positive influence!
Is it my imagination, or has there been a lot of discussion on leadership lately? In the past 3 years, all the meetings I have attended had at least 1 presentation on leadership or the traits of leaders. Sometimes—even in the oddest places—I have come across an article with “leadership” in the title. In fact, a serendipitous discovery of 2 publications is what inspired me to write this.
I spotted the first one in a reading basket when I was on vacation: It was an interview with Benjamin Zander, the conductor of the Boston Philharmonic Orchestra and the Boston Philharmonic Youth Orchestra.1 I was especially interested to read this because when I was in graduate school, Benjamin (the father of one of my classmates) visited our campus to give a presentation on talent and self-confidence. I can still hear him conducting us to emphatically believe in ourselves and others. This was echoed in his interview: “Never doubt the capacity of the people you lead to accomplish whatever you dream for them.” What a powerful concept: Believe in the possibilities of someone!
The second was an American Legion Auxiliary column, which emphasized that “leadership is not a title, but a responsibility.”2 This struck a chord with me because some prominent leaders don’t appear to ascribe to that assessment (although they should!).
After digesting these articles, I started thinking: What, exactly, is leadership? What do we even mean by leadership? How do we measure it? Is it measurable? How do we know when we see or experience good leadership? Can one learn to become a leader by simply reading a “how-to” article?
I think I can answer these questions with 2 principles that have guided me through my career as an NP: (1) Make use of another leader’s expertise to guide you, and (2) Continue to grow amid any setbacks.
For example, my transition from a full-time clinician to a Health Policy Coordinator or “policy wonk” did not have a distinct trajectory. Although my core set of clinical skills were essential, I knew early on that I had to expand by adapting specific organizational skills that would enable me to grow in my new role. But how was I to prioritize which skills to improve? More than a simple trial-and-error approach was required; I needed guidance. Fortunately, my new boss was willing to share her experience and the lessons she learned on the job. Key among them was to recognize the skills I already had—communicating and coordinating—and to develop those skills to be more effective in my new position.
Later in my career, I worked with colleagues to pursue legislation for NP prescriptive authority in Massachusetts. The political arena of Commonwealth’s health care laws was especially pivotal in changing how I saw setbacks. These weren’t to be accepted as a failure but as a challenge to figure out how to better succeed the next time. For several years, I was told “No” before we finally got a bill passed. But each round of my testimony was an opportunity to educate lawmakers and the public on the valuable role of NPs and the quality of care we provide. I try to share this story with new NPs as a good example of why they should persist through adversity.
Continue to: Over the next 3 weeks...
Over the next 3 weeks, join us on Thursdays as we continue to discuss what it means to be a leader—from the pitfalls to the victories. For those who are leaders or who work for one, please share your thoughts, experiences, and lessons learned. Maybe you can give a shoutout to someone who was a positive influence!
1. Labarre P. Leadership—Ben Zander. Fast Company website. www.fastcompany.com/35825/leadership-ben-zander. Published November 30, 1998. Accessed August 27, 2019.
2. Volunteer beyond the ALA: serve on boards, committees. American Legion Auxiliary. November 2018:29.
1. Labarre P. Leadership—Ben Zander. Fast Company website. www.fastcompany.com/35825/leadership-ben-zander. Published November 30, 1998. Accessed August 27, 2019.
2. Volunteer beyond the ALA: serve on boards, committees. American Legion Auxiliary. November 2018:29.
VA Pathologist Indicted for Patient Deaths Due to Misdiagnoses
Levy was chief pathologist at Veterans Health Care System of the Ozarks in Fayetteville, Arkansas. During his 12-year tenure at the US Department of Veterans Affairs (VA), he read almost 34,000 pathology slides. However, at the same time, he was working under the influence of alcohol and 2-methyl-2-butanol (2M2B)—a substance that intoxicates but cannot be detected in routine tests.
The VA fired Levy last year, and the VA Office of the Inspector General (OIG) began an investigation of his actions and of agency lapses in overseeing him. The 18-month review found that 8.9% of Levy’s diagnoses involved clinical errors—the normal misdiagnosis rate for pathologists is 0.7%. Hundreds of Levy’s misdiagnoses were not serious, but ≥ 15 may have led to deaths and harmful illness in 15 other patients. Some patients were not diagnosed when they should have been. Some were told they were sick when they were not and suffered unnecessary invasive treatment.
Levy knowingly falsified diagnoses for 3 veterans. One patient was diagnosed with diffuse large B-cell lymphoma—a type of cancer he did not have. He received the wrong treatment and died. Levy diagnosed another patient, also wrongly, with small cell carcinoma; that patient died of squamous cell carcinoma that spread. The third patient was given a benign test result for prostate cancer. Untreated, he died after the cancer spread.
One patient was given antibiotics instead of treatment for what was later diagnosed as late-stage neck and throat cancer. In an interview with the Washington Post he said, “I went from ‘Your earache isn’t anything’ to stage 4.”
How was Levy able to wreak such havoc? One reason was that despite concerns and complaints from colleagues, he looked good on paper. He falsified records to indicate that his deputy concurred with his diagnoses in mandated peer reviews. He also appeared “clean” in inspections through using 2M2B.
Levy was fired not for his work performance but for being arrested for driving while intoxicated. He had been a “star hire” with an medical degree from the University of Chicago, who had completed a pathology residency at the University of California at San Francisco and a fellowship at Duke University focusing on disease of the blood. But he also had a 1996 arrest for a driving under the influence (DUI) on his record when he joined the VA in 2005.
In 2015, a fact-finding panel interviewed Levy about reports that he was under the influence while on duty. He denied the allegations. In 2016, Levy arrived at the radiology department to assist with a biopsy with a blood alcohol level of nearly 0.4. He was suspended, his alcohol impairment was reported to the state medical boards, and his medical privileges were revoked. He entered a VA treatment program in 2016, then returned to work. Levy, who also sat on oversight boards and medical committees, seemed drowsy and was speaking “nonsense” at an October 2017 meeting of the hospital’s tumor board, according to meeting minutes provided to The Post.
He was suspended again in 2017 for being under the influence but allowed to continue with nonclinical work until he was again arrested for DUI in 2018, when the police toxicology test detected 2M2B. He was finally dismissed in April 2018. Nonetheless, even after he had arrived impaired at the laboratory twice, the VA had awarded him 2 performance bonuses, based on the supposedly low clinical error rate and 42 urine and blood samples that turned up negative for alcohol and drugs.
In addition to 3 counts of involuntary manslaughter, the indictment charges that Levy devised a scheme to defraud the VA and to obtain money and property from the VA in the form of salary, benefits, and performance awards. He is charged with 12 counts of wire fraud, 12 counts of mail fraud, and 4 counts of making false statements related to 12 occasions between 2017 and 2018, when Levy was reportedly buying 2M2B over the Internet while he was contractually obligated to submit to random drug and alcohol screens.
After being fired, Levy moved to a small island in the Dutch Caribbean and found a position teaching pathology at a local medical school. At the time of his VA hiring, Levy held a medical license issued by Mississippi. His active medical licenses in California and Florida were revoked only this spring. The VA did not notify the3 states where Levy was licensed that he could no longer practice until June 2018.
The Office of Inspector General (OIG) has identified other VA physicians who continued to practice even after they were found to have compromised patient care, and the Government Accountability Office found “weak systems” for ensuring that problems are addressed in a timely fashion. A VA spokesperson, however, quoted in The Washington Post, said the Levy case was “an isolated incident,” and that the agency has “strengthened internal controls” to ensure that errors are more quickly identified and addressed. The Fayetteville Medical Center also has increased monitoring of its clinical laboratory, according to a Washington Post report. VA officials also said they have added oversight of small specialty staffs across the system to ensure “independent and objective oversight.”
The VA has contacted the families in the 30 most serious cases to advise them of their legal and treatment options, according to the Washington Post.
“The arrest of Dr. Levy was accomplished as a result of the strong leadership of the US Attorney’s Office and the extensive work of special agents of the VA OIG, supported by the medical expertise of the OIG’s health care inspection professionals,” said Michael Missal, the VA’s inspector general, in a press release issued by the US Attorney’s Office in the Western District of Arkansas. “These charges send a clear signal that anyone entrusted with the care of veterans will be held accountable for placing them at risk by working while impaired or through other misconduct.”
Levy is in jail in Fayetteville. The trial date for his case is set for October 7.
Levy was chief pathologist at Veterans Health Care System of the Ozarks in Fayetteville, Arkansas. During his 12-year tenure at the US Department of Veterans Affairs (VA), he read almost 34,000 pathology slides. However, at the same time, he was working under the influence of alcohol and 2-methyl-2-butanol (2M2B)—a substance that intoxicates but cannot be detected in routine tests.
The VA fired Levy last year, and the VA Office of the Inspector General (OIG) began an investigation of his actions and of agency lapses in overseeing him. The 18-month review found that 8.9% of Levy’s diagnoses involved clinical errors—the normal misdiagnosis rate for pathologists is 0.7%. Hundreds of Levy’s misdiagnoses were not serious, but ≥ 15 may have led to deaths and harmful illness in 15 other patients. Some patients were not diagnosed when they should have been. Some were told they were sick when they were not and suffered unnecessary invasive treatment.
Levy knowingly falsified diagnoses for 3 veterans. One patient was diagnosed with diffuse large B-cell lymphoma—a type of cancer he did not have. He received the wrong treatment and died. Levy diagnosed another patient, also wrongly, with small cell carcinoma; that patient died of squamous cell carcinoma that spread. The third patient was given a benign test result for prostate cancer. Untreated, he died after the cancer spread.
One patient was given antibiotics instead of treatment for what was later diagnosed as late-stage neck and throat cancer. In an interview with the Washington Post he said, “I went from ‘Your earache isn’t anything’ to stage 4.”
How was Levy able to wreak such havoc? One reason was that despite concerns and complaints from colleagues, he looked good on paper. He falsified records to indicate that his deputy concurred with his diagnoses in mandated peer reviews. He also appeared “clean” in inspections through using 2M2B.
Levy was fired not for his work performance but for being arrested for driving while intoxicated. He had been a “star hire” with an medical degree from the University of Chicago, who had completed a pathology residency at the University of California at San Francisco and a fellowship at Duke University focusing on disease of the blood. But he also had a 1996 arrest for a driving under the influence (DUI) on his record when he joined the VA in 2005.
In 2015, a fact-finding panel interviewed Levy about reports that he was under the influence while on duty. He denied the allegations. In 2016, Levy arrived at the radiology department to assist with a biopsy with a blood alcohol level of nearly 0.4. He was suspended, his alcohol impairment was reported to the state medical boards, and his medical privileges were revoked. He entered a VA treatment program in 2016, then returned to work. Levy, who also sat on oversight boards and medical committees, seemed drowsy and was speaking “nonsense” at an October 2017 meeting of the hospital’s tumor board, according to meeting minutes provided to The Post.
He was suspended again in 2017 for being under the influence but allowed to continue with nonclinical work until he was again arrested for DUI in 2018, when the police toxicology test detected 2M2B. He was finally dismissed in April 2018. Nonetheless, even after he had arrived impaired at the laboratory twice, the VA had awarded him 2 performance bonuses, based on the supposedly low clinical error rate and 42 urine and blood samples that turned up negative for alcohol and drugs.
In addition to 3 counts of involuntary manslaughter, the indictment charges that Levy devised a scheme to defraud the VA and to obtain money and property from the VA in the form of salary, benefits, and performance awards. He is charged with 12 counts of wire fraud, 12 counts of mail fraud, and 4 counts of making false statements related to 12 occasions between 2017 and 2018, when Levy was reportedly buying 2M2B over the Internet while he was contractually obligated to submit to random drug and alcohol screens.
After being fired, Levy moved to a small island in the Dutch Caribbean and found a position teaching pathology at a local medical school. At the time of his VA hiring, Levy held a medical license issued by Mississippi. His active medical licenses in California and Florida were revoked only this spring. The VA did not notify the3 states where Levy was licensed that he could no longer practice until June 2018.
The Office of Inspector General (OIG) has identified other VA physicians who continued to practice even after they were found to have compromised patient care, and the Government Accountability Office found “weak systems” for ensuring that problems are addressed in a timely fashion. A VA spokesperson, however, quoted in The Washington Post, said the Levy case was “an isolated incident,” and that the agency has “strengthened internal controls” to ensure that errors are more quickly identified and addressed. The Fayetteville Medical Center also has increased monitoring of its clinical laboratory, according to a Washington Post report. VA officials also said they have added oversight of small specialty staffs across the system to ensure “independent and objective oversight.”
The VA has contacted the families in the 30 most serious cases to advise them of their legal and treatment options, according to the Washington Post.
“The arrest of Dr. Levy was accomplished as a result of the strong leadership of the US Attorney’s Office and the extensive work of special agents of the VA OIG, supported by the medical expertise of the OIG’s health care inspection professionals,” said Michael Missal, the VA’s inspector general, in a press release issued by the US Attorney’s Office in the Western District of Arkansas. “These charges send a clear signal that anyone entrusted with the care of veterans will be held accountable for placing them at risk by working while impaired or through other misconduct.”
Levy is in jail in Fayetteville. The trial date for his case is set for October 7.
Levy was chief pathologist at Veterans Health Care System of the Ozarks in Fayetteville, Arkansas. During his 12-year tenure at the US Department of Veterans Affairs (VA), he read almost 34,000 pathology slides. However, at the same time, he was working under the influence of alcohol and 2-methyl-2-butanol (2M2B)—a substance that intoxicates but cannot be detected in routine tests.
The VA fired Levy last year, and the VA Office of the Inspector General (OIG) began an investigation of his actions and of agency lapses in overseeing him. The 18-month review found that 8.9% of Levy’s diagnoses involved clinical errors—the normal misdiagnosis rate for pathologists is 0.7%. Hundreds of Levy’s misdiagnoses were not serious, but ≥ 15 may have led to deaths and harmful illness in 15 other patients. Some patients were not diagnosed when they should have been. Some were told they were sick when they were not and suffered unnecessary invasive treatment.
Levy knowingly falsified diagnoses for 3 veterans. One patient was diagnosed with diffuse large B-cell lymphoma—a type of cancer he did not have. He received the wrong treatment and died. Levy diagnosed another patient, also wrongly, with small cell carcinoma; that patient died of squamous cell carcinoma that spread. The third patient was given a benign test result for prostate cancer. Untreated, he died after the cancer spread.
One patient was given antibiotics instead of treatment for what was later diagnosed as late-stage neck and throat cancer. In an interview with the Washington Post he said, “I went from ‘Your earache isn’t anything’ to stage 4.”
How was Levy able to wreak such havoc? One reason was that despite concerns and complaints from colleagues, he looked good on paper. He falsified records to indicate that his deputy concurred with his diagnoses in mandated peer reviews. He also appeared “clean” in inspections through using 2M2B.
Levy was fired not for his work performance but for being arrested for driving while intoxicated. He had been a “star hire” with an medical degree from the University of Chicago, who had completed a pathology residency at the University of California at San Francisco and a fellowship at Duke University focusing on disease of the blood. But he also had a 1996 arrest for a driving under the influence (DUI) on his record when he joined the VA in 2005.
In 2015, a fact-finding panel interviewed Levy about reports that he was under the influence while on duty. He denied the allegations. In 2016, Levy arrived at the radiology department to assist with a biopsy with a blood alcohol level of nearly 0.4. He was suspended, his alcohol impairment was reported to the state medical boards, and his medical privileges were revoked. He entered a VA treatment program in 2016, then returned to work. Levy, who also sat on oversight boards and medical committees, seemed drowsy and was speaking “nonsense” at an October 2017 meeting of the hospital’s tumor board, according to meeting minutes provided to The Post.
He was suspended again in 2017 for being under the influence but allowed to continue with nonclinical work until he was again arrested for DUI in 2018, when the police toxicology test detected 2M2B. He was finally dismissed in April 2018. Nonetheless, even after he had arrived impaired at the laboratory twice, the VA had awarded him 2 performance bonuses, based on the supposedly low clinical error rate and 42 urine and blood samples that turned up negative for alcohol and drugs.
In addition to 3 counts of involuntary manslaughter, the indictment charges that Levy devised a scheme to defraud the VA and to obtain money and property from the VA in the form of salary, benefits, and performance awards. He is charged with 12 counts of wire fraud, 12 counts of mail fraud, and 4 counts of making false statements related to 12 occasions between 2017 and 2018, when Levy was reportedly buying 2M2B over the Internet while he was contractually obligated to submit to random drug and alcohol screens.
After being fired, Levy moved to a small island in the Dutch Caribbean and found a position teaching pathology at a local medical school. At the time of his VA hiring, Levy held a medical license issued by Mississippi. His active medical licenses in California and Florida were revoked only this spring. The VA did not notify the3 states where Levy was licensed that he could no longer practice until June 2018.
The Office of Inspector General (OIG) has identified other VA physicians who continued to practice even after they were found to have compromised patient care, and the Government Accountability Office found “weak systems” for ensuring that problems are addressed in a timely fashion. A VA spokesperson, however, quoted in The Washington Post, said the Levy case was “an isolated incident,” and that the agency has “strengthened internal controls” to ensure that errors are more quickly identified and addressed. The Fayetteville Medical Center also has increased monitoring of its clinical laboratory, according to a Washington Post report. VA officials also said they have added oversight of small specialty staffs across the system to ensure “independent and objective oversight.”
The VA has contacted the families in the 30 most serious cases to advise them of their legal and treatment options, according to the Washington Post.
“The arrest of Dr. Levy was accomplished as a result of the strong leadership of the US Attorney’s Office and the extensive work of special agents of the VA OIG, supported by the medical expertise of the OIG’s health care inspection professionals,” said Michael Missal, the VA’s inspector general, in a press release issued by the US Attorney’s Office in the Western District of Arkansas. “These charges send a clear signal that anyone entrusted with the care of veterans will be held accountable for placing them at risk by working while impaired or through other misconduct.”
Levy is in jail in Fayetteville. The trial date for his case is set for October 7.
Clinical trial enrollment linked to lower death rate in lung cancer patients
ALEXANDRIA, VA. – Patients with metastatic non–small cell lung cancer (NSCLC) who enrolled in clinical trials had a near 50% lower risk of death versus patients who received treatment outside a clinical trial, according to results of a single-center, retrospective medical record review.
Median survival was almost doubled for those patients with NSCLC enrolled in therapeutic drug trials, according to lead author Christina Merkhofer, MD, a hematology-oncology fellow at the University of Washington and Fred Hutchinson Cancer Center, both in Seattle.
The findings suggest that clinical trials, beyond supporting drug development, provide a direct benefit to NSCLC patients through provision of promising agents, enhanced supportive care, or both, according to Dr. Merkhofer and coauthors, who will present their findings in a poster at the Quality Care Symposium, sponsored by the American Society of Clinical Oncology.
The retrospective study included a total of 371 patients diagnosed with metastatic NSCLC between 2001 and 2015, of whom 118 (32%) enrolled in at least one clinical trial, Dr. Merkhofer reported at a press briefing ahead of the symposium.
Median survival was 838 days for the clinical trial enrollees versus 454 days for nonenrollees, according to the investigators. The risk of death was 47% lower (hazard ratio, 0.53; 95% confidence interval, 0.13-0.92; P = .002) for enrollees relative to nonenrollees after adjustment for variables including sex, performance status, smoking history, histology, presence of brain metastases, and EGFR/ALK status.
Based on this study by Dr. Merkhofer and colleagues, participating in therapeutic drug trials appears to improve survival in patients with advanced lung cancer, ASCO expert Merry-Jennifer Markham, MD, said in a statement.
Oncologists have a “duty” to enroll patients in clinical trials as appropriate, said Dr. Markham, chair of the Quality Care Symposium’s news planning team.
“We must work to better understand factors associated with enrollment so that the prospective benefits can be made accessible to all who are eligible,” she said.
The research is part of a larger investigation looking at “areas of uncertainty” in clinical trial participation, such as whether specific trial design characteristics are linked to survival benefit, according to Dr. Merkhofer.
“The study can support research evaluating health care policies or research that looks at incentives for patient participation in trials, such as financing transportation or lodging, [and] can help with research that addresses some of these important barriers to trial participation,” Dr. Merkhofer said in an ASCO press release.
Dr. Merkhofer had no disclosures related to the study. Her coauthors reported disclosures related to numerous pharmaceutical companies.
SOURCE: Merkhofer C et al. QCS2019, Abstract 137.
ALEXANDRIA, VA. – Patients with metastatic non–small cell lung cancer (NSCLC) who enrolled in clinical trials had a near 50% lower risk of death versus patients who received treatment outside a clinical trial, according to results of a single-center, retrospective medical record review.
Median survival was almost doubled for those patients with NSCLC enrolled in therapeutic drug trials, according to lead author Christina Merkhofer, MD, a hematology-oncology fellow at the University of Washington and Fred Hutchinson Cancer Center, both in Seattle.
The findings suggest that clinical trials, beyond supporting drug development, provide a direct benefit to NSCLC patients through provision of promising agents, enhanced supportive care, or both, according to Dr. Merkhofer and coauthors, who will present their findings in a poster at the Quality Care Symposium, sponsored by the American Society of Clinical Oncology.
The retrospective study included a total of 371 patients diagnosed with metastatic NSCLC between 2001 and 2015, of whom 118 (32%) enrolled in at least one clinical trial, Dr. Merkhofer reported at a press briefing ahead of the symposium.
Median survival was 838 days for the clinical trial enrollees versus 454 days for nonenrollees, according to the investigators. The risk of death was 47% lower (hazard ratio, 0.53; 95% confidence interval, 0.13-0.92; P = .002) for enrollees relative to nonenrollees after adjustment for variables including sex, performance status, smoking history, histology, presence of brain metastases, and EGFR/ALK status.
Based on this study by Dr. Merkhofer and colleagues, participating in therapeutic drug trials appears to improve survival in patients with advanced lung cancer, ASCO expert Merry-Jennifer Markham, MD, said in a statement.
Oncologists have a “duty” to enroll patients in clinical trials as appropriate, said Dr. Markham, chair of the Quality Care Symposium’s news planning team.
“We must work to better understand factors associated with enrollment so that the prospective benefits can be made accessible to all who are eligible,” she said.
The research is part of a larger investigation looking at “areas of uncertainty” in clinical trial participation, such as whether specific trial design characteristics are linked to survival benefit, according to Dr. Merkhofer.
“The study can support research evaluating health care policies or research that looks at incentives for patient participation in trials, such as financing transportation or lodging, [and] can help with research that addresses some of these important barriers to trial participation,” Dr. Merkhofer said in an ASCO press release.
Dr. Merkhofer had no disclosures related to the study. Her coauthors reported disclosures related to numerous pharmaceutical companies.
SOURCE: Merkhofer C et al. QCS2019, Abstract 137.
ALEXANDRIA, VA. – Patients with metastatic non–small cell lung cancer (NSCLC) who enrolled in clinical trials had a near 50% lower risk of death versus patients who received treatment outside a clinical trial, according to results of a single-center, retrospective medical record review.
Median survival was almost doubled for those patients with NSCLC enrolled in therapeutic drug trials, according to lead author Christina Merkhofer, MD, a hematology-oncology fellow at the University of Washington and Fred Hutchinson Cancer Center, both in Seattle.
The findings suggest that clinical trials, beyond supporting drug development, provide a direct benefit to NSCLC patients through provision of promising agents, enhanced supportive care, or both, according to Dr. Merkhofer and coauthors, who will present their findings in a poster at the Quality Care Symposium, sponsored by the American Society of Clinical Oncology.
The retrospective study included a total of 371 patients diagnosed with metastatic NSCLC between 2001 and 2015, of whom 118 (32%) enrolled in at least one clinical trial, Dr. Merkhofer reported at a press briefing ahead of the symposium.
Median survival was 838 days for the clinical trial enrollees versus 454 days for nonenrollees, according to the investigators. The risk of death was 47% lower (hazard ratio, 0.53; 95% confidence interval, 0.13-0.92; P = .002) for enrollees relative to nonenrollees after adjustment for variables including sex, performance status, smoking history, histology, presence of brain metastases, and EGFR/ALK status.
Based on this study by Dr. Merkhofer and colleagues, participating in therapeutic drug trials appears to improve survival in patients with advanced lung cancer, ASCO expert Merry-Jennifer Markham, MD, said in a statement.
Oncologists have a “duty” to enroll patients in clinical trials as appropriate, said Dr. Markham, chair of the Quality Care Symposium’s news planning team.
“We must work to better understand factors associated with enrollment so that the prospective benefits can be made accessible to all who are eligible,” she said.
The research is part of a larger investigation looking at “areas of uncertainty” in clinical trial participation, such as whether specific trial design characteristics are linked to survival benefit, according to Dr. Merkhofer.
“The study can support research evaluating health care policies or research that looks at incentives for patient participation in trials, such as financing transportation or lodging, [and] can help with research that addresses some of these important barriers to trial participation,” Dr. Merkhofer said in an ASCO press release.
Dr. Merkhofer had no disclosures related to the study. Her coauthors reported disclosures related to numerous pharmaceutical companies.
SOURCE: Merkhofer C et al. QCS2019, Abstract 137.
REPORTING FROM QCS 2019
Predicted risk of cardiac complications varies among risk calculators
Background: A critical juncture in the American Heart Association/American College of Cardiology 2014 perioperative guidelines relies on clinicians categorizing patients undergoing noncardiac surgery as either low risk (less than 1%) or elevated risk (greater than or equal to 1%) for a MACE. The purpose of this study is to determine whether there is variability between the three risk calculators endorsed by the ACC/AHA guidelines as prediction tools to make this risk stratification.
Study design: Retrospective observational study.
Setting: National Surgical Quality Improvement Program database.
Synopsis: The NSQIP database was used to identify 10,000 patients who had undergone noncardiac surgery. The risk of MACE for each patient was then calculated using the Revised Cardiac Risk Index, the American College of Surgeons National Surgical Quality Improvement Program Surgical Risk Calculator, and the National Surgical Quality Improvement Program Myocardial Infarction or Cardiac Arrest calculator. Data were analyzed using the intraclass correlation coefficient and kappa analysis. Results demonstrated that 29% of the time the three calculators disagreed on which patients were classified as low risk. This suggests that, when following the ACC/AHA perioperative guidelines, a recommendation for further preoperative cardiac testing may depend on which risk prediction tool is used to calculate the risk of MACE.
Bottom line: Nearly one-third of the time, the three risk calculators recommended in the ACC/AHA 2014 perioperative guidelines do not agree on which patients are classified as low risk; this may affect clinical decision making for some patients.
Citation: Glance LG et al. Impact of the choice of risk model for identifying low-risk patients using the 2014 American College of Cardiology/American Heart Association perioperative guidelines. Anesthesiology. 2018;129(5):889-900.
Dr. Bordin-Wosk is an assistant clinical professor in the division of hospital medicine at the University of California, San Diego.
Background: A critical juncture in the American Heart Association/American College of Cardiology 2014 perioperative guidelines relies on clinicians categorizing patients undergoing noncardiac surgery as either low risk (less than 1%) or elevated risk (greater than or equal to 1%) for a MACE. The purpose of this study is to determine whether there is variability between the three risk calculators endorsed by the ACC/AHA guidelines as prediction tools to make this risk stratification.
Study design: Retrospective observational study.
Setting: National Surgical Quality Improvement Program database.
Synopsis: The NSQIP database was used to identify 10,000 patients who had undergone noncardiac surgery. The risk of MACE for each patient was then calculated using the Revised Cardiac Risk Index, the American College of Surgeons National Surgical Quality Improvement Program Surgical Risk Calculator, and the National Surgical Quality Improvement Program Myocardial Infarction or Cardiac Arrest calculator. Data were analyzed using the intraclass correlation coefficient and kappa analysis. Results demonstrated that 29% of the time the three calculators disagreed on which patients were classified as low risk. This suggests that, when following the ACC/AHA perioperative guidelines, a recommendation for further preoperative cardiac testing may depend on which risk prediction tool is used to calculate the risk of MACE.
Bottom line: Nearly one-third of the time, the three risk calculators recommended in the ACC/AHA 2014 perioperative guidelines do not agree on which patients are classified as low risk; this may affect clinical decision making for some patients.
Citation: Glance LG et al. Impact of the choice of risk model for identifying low-risk patients using the 2014 American College of Cardiology/American Heart Association perioperative guidelines. Anesthesiology. 2018;129(5):889-900.
Dr. Bordin-Wosk is an assistant clinical professor in the division of hospital medicine at the University of California, San Diego.
Background: A critical juncture in the American Heart Association/American College of Cardiology 2014 perioperative guidelines relies on clinicians categorizing patients undergoing noncardiac surgery as either low risk (less than 1%) or elevated risk (greater than or equal to 1%) for a MACE. The purpose of this study is to determine whether there is variability between the three risk calculators endorsed by the ACC/AHA guidelines as prediction tools to make this risk stratification.
Study design: Retrospective observational study.
Setting: National Surgical Quality Improvement Program database.
Synopsis: The NSQIP database was used to identify 10,000 patients who had undergone noncardiac surgery. The risk of MACE for each patient was then calculated using the Revised Cardiac Risk Index, the American College of Surgeons National Surgical Quality Improvement Program Surgical Risk Calculator, and the National Surgical Quality Improvement Program Myocardial Infarction or Cardiac Arrest calculator. Data were analyzed using the intraclass correlation coefficient and kappa analysis. Results demonstrated that 29% of the time the three calculators disagreed on which patients were classified as low risk. This suggests that, when following the ACC/AHA perioperative guidelines, a recommendation for further preoperative cardiac testing may depend on which risk prediction tool is used to calculate the risk of MACE.
Bottom line: Nearly one-third of the time, the three risk calculators recommended in the ACC/AHA 2014 perioperative guidelines do not agree on which patients are classified as low risk; this may affect clinical decision making for some patients.
Citation: Glance LG et al. Impact of the choice of risk model for identifying low-risk patients using the 2014 American College of Cardiology/American Heart Association perioperative guidelines. Anesthesiology. 2018;129(5):889-900.
Dr. Bordin-Wosk is an assistant clinical professor in the division of hospital medicine at the University of California, San Diego.
Diagnosis and management of gastroparesis and functional dyspepsia pose challenges
CHICAGO – Because gastroparesis and functional dyspepsia share several symptoms (e.g., upper abdominal pain, fullness, and bloating) and pathophysiological abnormalities (e.g., delayed gastric emptying, impaired gastric accommodation, and visceral hypersensitivity), it can be hard to distinguish the two conditions, according to a lecture presented at Freston Conference 2019, sponsored by the American Gastroenterological Association. Additional research into the role of diet in these conditions will improve the treatment of these patients, said Linda Nguyen, MD, director of neurogastroenterology and motility at Stanford (Calif.) University.
Distinguishing the disorders
The accepted definition of gastroparesis is abnormal gastric emptying in the absence of a mechanical obstruction. The condition’s symptoms include nausea, vomiting, bloating, early satiety, abdominal pain, and weight loss. A previous consensus held that if a patient had abdominal pain, he or she did not have gastroparesis. Yet studies indicate that up to 80% of patients with gastroparesis have pain.
Functional dyspepsia is defined as bothersome postprandial fullness, early satiety, and epigastric pain or burning in the absence of structural abnormality. The disorder can be subdivided into postprandial distress (i.e., meal-related symptomatology) and epigastric pain syndrome (i.e., pain or burning that may or may not be related to meals). Either of these alternatives may entail nausea and vomiting.
Comparing the pathophysiologies of gastroparesis and functional dyspepsia helps to distinguish these disorders from each other. A 2019 review described rapid gastric emptying and duodenal eosinophilia in patients with functional dyspepsia, but not in patients with gastroparesis. Patients with epigastric pain syndrome had sensitivity to acid, bile, and fats. Patients with idiopathic gastroparesis, which is the most common type, had a weak antral pump and abnormal duodenal feedback, but patients with functional dyspepsia did not have these characteristics (J Neurogastroenterol Motil. 2019;25[1]:27-35).
Examining symptoms and severity
One examination of patients with gastroparesis found that approximately 46% of them had a body mass index of 25 or greater. About 26% of patients had a BMI greater than 30. Yet these patients were eating less than 60% of their recommended daily allowances, based on their age, height, weight, and sex (Clin Gastroenterol Hepatol. 2011;9[12]:1056-64).
Accelerating gastric emptying may not relieve symptoms completely in a patient with gastroparesis, said Dr. Nguyen. A 2007 study of patients with gastroparesis found that 43% had impaired accommodation, and 29% had visceral hypersensitivity (Gut. 2007;56[1]:29-36). The same data indicated that gastric emptying time was not correlated with symptom severity. Impaired accommodation, however, was associated with early satiety and weight loss. Visceral hypersensitivity was associated with pain, early satiety, and weight loss. These data suggest that accommodation and visceral hypersensitivity may influence symptom severity in gastroparesis, said Dr. Nguyen.
Other researchers compared mild, moderate, and severe symptoms of early satiety in patients with gastroparesis. They found that patients with severe symptoms of early satiety have more delayed gastric emptying than do patients with mild or moderate symptoms of early satiety (Neurogastroenterol Motil. 2017;29[4].).
Dr. Nguyen and colleagues examined normal gastric emptying, compared with severely delayed gastric emptying, which they defined as greater than 35% retention at 4 hours. They found that severely delayed gastric emptying was associated with more severe symptoms, particularly nausea and vomiting, as measured by Gastroparesis Cardinal Symptom Index (GCSI). Extreme symptoms may help differentiate between gastroparesis and functional dyspepsia, said Dr. Nguyen.
Dietary and pharmacologic treatment
Although clinicians might consider recommending dietary modifications to treat gastroparesis or functional dyspepsia, the literature contains little evidence about their efficacy in these indications, said Dr. Nguyen. Based on a study by Tack and colleagues, some clinicians recommend small, frequent meals that are low in fat and low in fiber to patients with gastroparesis. Such a diet could be harmful, however, to patients with comorbid diabetes, irritable bowel syndrome, or renal failure.
Common dietary recommendations for functional dyspepsia include small, frequent meals; decreased fat consumption; and avoidance of citrus and spicy foods. These recommendations are based on small studies in which patients reported which foods tended to cause their symptoms. Trials of dietary modifications in functional dyspepsia, however, are lacking.
Nevertheless, the literature can guide the selection of pharmacotherapy for these disorders. Talley et al. examined the effects of neuromodulators such as amitriptyline, a tricyclic antidepressant, and escitalopram in functional dyspepsia. About 70% of the sample had postprandial distress syndrome, and 20% met criteria for idiopathic gastroparesis. Amitriptyline provided greater symptomatic relief to these patients than did placebo, but escitalopram did not. Patients who met criteria for idiopathic gastroparesis did not respond well to tricyclic antidepressants, but patients with epigastric pain syndrome did. Furthermore, compared with patients with normal gastric emptying, those with delayed emptying did not respond to tricyclic antidepressants. A separate study found that the tricyclic antidepressant nortriptyline did not improve symptoms of gastroparesis (JAMA. 2013;310[24]:2640-9).
Promotility agents may be beneficial for certain patients. A study published this year suggests that, compared with placebo, prucalopride is effective for nausea, vomiting, fullness, bloating, and gastric emptying in patients with idiopathic gastroparesis (Am J Gastroenterol. 2019;114[8]:1265-74.). A 2017 meta-analysis, however, found that proton pump inhibitors were more effective than promotility agents in patients with functional dyspepsia (Am J Gastroenterol. 2017;112[7]:988-1013.).
Pyloric dysfunction may accompany gastroparesis in some patients. Increased severity of gastric emptying delay is associated with increased pylorospasm. Endoscopists have gained experience in performing pyloric myotomy, and this treatment has become more popular. Uncontrolled studies indicate that the proportion of patients with decreased symptom severity after this procedure is higher than 70% and can be as high as 86% (Gastrointest Endosc. 2017;85[1]:123-8). The predictors of a good response include idiopathic etiology, male sex, moderate symptom severity, and greater delay in gastric emptying.
Functional dyspepsia should perhaps be understood as normal gastric emptying and symptoms of epigastric pain syndrome, said Dr. Nguyen. Those patients may respond to neuromodulators, she added. Idiopathic gastroparesis appears to be characterized by severe delay in gastric emptying, postprandial symptoms, nausea, and vomiting. “In the middle is the gray zone, where you have these patients with postprandial distress with or without delayed gastric emptying,” said Dr. Nguyen. Functional dyspepsia and gastroparesis could be two ends of a spectrum, and the best management for patients with symptoms that occur in both disorders is unclear.
CHICAGO – Because gastroparesis and functional dyspepsia share several symptoms (e.g., upper abdominal pain, fullness, and bloating) and pathophysiological abnormalities (e.g., delayed gastric emptying, impaired gastric accommodation, and visceral hypersensitivity), it can be hard to distinguish the two conditions, according to a lecture presented at Freston Conference 2019, sponsored by the American Gastroenterological Association. Additional research into the role of diet in these conditions will improve the treatment of these patients, said Linda Nguyen, MD, director of neurogastroenterology and motility at Stanford (Calif.) University.
Distinguishing the disorders
The accepted definition of gastroparesis is abnormal gastric emptying in the absence of a mechanical obstruction. The condition’s symptoms include nausea, vomiting, bloating, early satiety, abdominal pain, and weight loss. A previous consensus held that if a patient had abdominal pain, he or she did not have gastroparesis. Yet studies indicate that up to 80% of patients with gastroparesis have pain.
Functional dyspepsia is defined as bothersome postprandial fullness, early satiety, and epigastric pain or burning in the absence of structural abnormality. The disorder can be subdivided into postprandial distress (i.e., meal-related symptomatology) and epigastric pain syndrome (i.e., pain or burning that may or may not be related to meals). Either of these alternatives may entail nausea and vomiting.
Comparing the pathophysiologies of gastroparesis and functional dyspepsia helps to distinguish these disorders from each other. A 2019 review described rapid gastric emptying and duodenal eosinophilia in patients with functional dyspepsia, but not in patients with gastroparesis. Patients with epigastric pain syndrome had sensitivity to acid, bile, and fats. Patients with idiopathic gastroparesis, which is the most common type, had a weak antral pump and abnormal duodenal feedback, but patients with functional dyspepsia did not have these characteristics (J Neurogastroenterol Motil. 2019;25[1]:27-35).
Examining symptoms and severity
One examination of patients with gastroparesis found that approximately 46% of them had a body mass index of 25 or greater. About 26% of patients had a BMI greater than 30. Yet these patients were eating less than 60% of their recommended daily allowances, based on their age, height, weight, and sex (Clin Gastroenterol Hepatol. 2011;9[12]:1056-64).
Accelerating gastric emptying may not relieve symptoms completely in a patient with gastroparesis, said Dr. Nguyen. A 2007 study of patients with gastroparesis found that 43% had impaired accommodation, and 29% had visceral hypersensitivity (Gut. 2007;56[1]:29-36). The same data indicated that gastric emptying time was not correlated with symptom severity. Impaired accommodation, however, was associated with early satiety and weight loss. Visceral hypersensitivity was associated with pain, early satiety, and weight loss. These data suggest that accommodation and visceral hypersensitivity may influence symptom severity in gastroparesis, said Dr. Nguyen.
Other researchers compared mild, moderate, and severe symptoms of early satiety in patients with gastroparesis. They found that patients with severe symptoms of early satiety have more delayed gastric emptying than do patients with mild or moderate symptoms of early satiety (Neurogastroenterol Motil. 2017;29[4].).
Dr. Nguyen and colleagues examined normal gastric emptying, compared with severely delayed gastric emptying, which they defined as greater than 35% retention at 4 hours. They found that severely delayed gastric emptying was associated with more severe symptoms, particularly nausea and vomiting, as measured by Gastroparesis Cardinal Symptom Index (GCSI). Extreme symptoms may help differentiate between gastroparesis and functional dyspepsia, said Dr. Nguyen.
Dietary and pharmacologic treatment
Although clinicians might consider recommending dietary modifications to treat gastroparesis or functional dyspepsia, the literature contains little evidence about their efficacy in these indications, said Dr. Nguyen. Based on a study by Tack and colleagues, some clinicians recommend small, frequent meals that are low in fat and low in fiber to patients with gastroparesis. Such a diet could be harmful, however, to patients with comorbid diabetes, irritable bowel syndrome, or renal failure.
Common dietary recommendations for functional dyspepsia include small, frequent meals; decreased fat consumption; and avoidance of citrus and spicy foods. These recommendations are based on small studies in which patients reported which foods tended to cause their symptoms. Trials of dietary modifications in functional dyspepsia, however, are lacking.
Nevertheless, the literature can guide the selection of pharmacotherapy for these disorders. Talley et al. examined the effects of neuromodulators such as amitriptyline, a tricyclic antidepressant, and escitalopram in functional dyspepsia. About 70% of the sample had postprandial distress syndrome, and 20% met criteria for idiopathic gastroparesis. Amitriptyline provided greater symptomatic relief to these patients than did placebo, but escitalopram did not. Patients who met criteria for idiopathic gastroparesis did not respond well to tricyclic antidepressants, but patients with epigastric pain syndrome did. Furthermore, compared with patients with normal gastric emptying, those with delayed emptying did not respond to tricyclic antidepressants. A separate study found that the tricyclic antidepressant nortriptyline did not improve symptoms of gastroparesis (JAMA. 2013;310[24]:2640-9).
Promotility agents may be beneficial for certain patients. A study published this year suggests that, compared with placebo, prucalopride is effective for nausea, vomiting, fullness, bloating, and gastric emptying in patients with idiopathic gastroparesis (Am J Gastroenterol. 2019;114[8]:1265-74.). A 2017 meta-analysis, however, found that proton pump inhibitors were more effective than promotility agents in patients with functional dyspepsia (Am J Gastroenterol. 2017;112[7]:988-1013.).
Pyloric dysfunction may accompany gastroparesis in some patients. Increased severity of gastric emptying delay is associated with increased pylorospasm. Endoscopists have gained experience in performing pyloric myotomy, and this treatment has become more popular. Uncontrolled studies indicate that the proportion of patients with decreased symptom severity after this procedure is higher than 70% and can be as high as 86% (Gastrointest Endosc. 2017;85[1]:123-8). The predictors of a good response include idiopathic etiology, male sex, moderate symptom severity, and greater delay in gastric emptying.
Functional dyspepsia should perhaps be understood as normal gastric emptying and symptoms of epigastric pain syndrome, said Dr. Nguyen. Those patients may respond to neuromodulators, she added. Idiopathic gastroparesis appears to be characterized by severe delay in gastric emptying, postprandial symptoms, nausea, and vomiting. “In the middle is the gray zone, where you have these patients with postprandial distress with or without delayed gastric emptying,” said Dr. Nguyen. Functional dyspepsia and gastroparesis could be two ends of a spectrum, and the best management for patients with symptoms that occur in both disorders is unclear.
CHICAGO – Because gastroparesis and functional dyspepsia share several symptoms (e.g., upper abdominal pain, fullness, and bloating) and pathophysiological abnormalities (e.g., delayed gastric emptying, impaired gastric accommodation, and visceral hypersensitivity), it can be hard to distinguish the two conditions, according to a lecture presented at Freston Conference 2019, sponsored by the American Gastroenterological Association. Additional research into the role of diet in these conditions will improve the treatment of these patients, said Linda Nguyen, MD, director of neurogastroenterology and motility at Stanford (Calif.) University.
Distinguishing the disorders
The accepted definition of gastroparesis is abnormal gastric emptying in the absence of a mechanical obstruction. The condition’s symptoms include nausea, vomiting, bloating, early satiety, abdominal pain, and weight loss. A previous consensus held that if a patient had abdominal pain, he or she did not have gastroparesis. Yet studies indicate that up to 80% of patients with gastroparesis have pain.
Functional dyspepsia is defined as bothersome postprandial fullness, early satiety, and epigastric pain or burning in the absence of structural abnormality. The disorder can be subdivided into postprandial distress (i.e., meal-related symptomatology) and epigastric pain syndrome (i.e., pain or burning that may or may not be related to meals). Either of these alternatives may entail nausea and vomiting.
Comparing the pathophysiologies of gastroparesis and functional dyspepsia helps to distinguish these disorders from each other. A 2019 review described rapid gastric emptying and duodenal eosinophilia in patients with functional dyspepsia, but not in patients with gastroparesis. Patients with epigastric pain syndrome had sensitivity to acid, bile, and fats. Patients with idiopathic gastroparesis, which is the most common type, had a weak antral pump and abnormal duodenal feedback, but patients with functional dyspepsia did not have these characteristics (J Neurogastroenterol Motil. 2019;25[1]:27-35).
Examining symptoms and severity
One examination of patients with gastroparesis found that approximately 46% of them had a body mass index of 25 or greater. About 26% of patients had a BMI greater than 30. Yet these patients were eating less than 60% of their recommended daily allowances, based on their age, height, weight, and sex (Clin Gastroenterol Hepatol. 2011;9[12]:1056-64).
Accelerating gastric emptying may not relieve symptoms completely in a patient with gastroparesis, said Dr. Nguyen. A 2007 study of patients with gastroparesis found that 43% had impaired accommodation, and 29% had visceral hypersensitivity (Gut. 2007;56[1]:29-36). The same data indicated that gastric emptying time was not correlated with symptom severity. Impaired accommodation, however, was associated with early satiety and weight loss. Visceral hypersensitivity was associated with pain, early satiety, and weight loss. These data suggest that accommodation and visceral hypersensitivity may influence symptom severity in gastroparesis, said Dr. Nguyen.
Other researchers compared mild, moderate, and severe symptoms of early satiety in patients with gastroparesis. They found that patients with severe symptoms of early satiety have more delayed gastric emptying than do patients with mild or moderate symptoms of early satiety (Neurogastroenterol Motil. 2017;29[4].).
Dr. Nguyen and colleagues examined normal gastric emptying, compared with severely delayed gastric emptying, which they defined as greater than 35% retention at 4 hours. They found that severely delayed gastric emptying was associated with more severe symptoms, particularly nausea and vomiting, as measured by Gastroparesis Cardinal Symptom Index (GCSI). Extreme symptoms may help differentiate between gastroparesis and functional dyspepsia, said Dr. Nguyen.
Dietary and pharmacologic treatment
Although clinicians might consider recommending dietary modifications to treat gastroparesis or functional dyspepsia, the literature contains little evidence about their efficacy in these indications, said Dr. Nguyen. Based on a study by Tack and colleagues, some clinicians recommend small, frequent meals that are low in fat and low in fiber to patients with gastroparesis. Such a diet could be harmful, however, to patients with comorbid diabetes, irritable bowel syndrome, or renal failure.
Common dietary recommendations for functional dyspepsia include small, frequent meals; decreased fat consumption; and avoidance of citrus and spicy foods. These recommendations are based on small studies in which patients reported which foods tended to cause their symptoms. Trials of dietary modifications in functional dyspepsia, however, are lacking.
Nevertheless, the literature can guide the selection of pharmacotherapy for these disorders. Talley et al. examined the effects of neuromodulators such as amitriptyline, a tricyclic antidepressant, and escitalopram in functional dyspepsia. About 70% of the sample had postprandial distress syndrome, and 20% met criteria for idiopathic gastroparesis. Amitriptyline provided greater symptomatic relief to these patients than did placebo, but escitalopram did not. Patients who met criteria for idiopathic gastroparesis did not respond well to tricyclic antidepressants, but patients with epigastric pain syndrome did. Furthermore, compared with patients with normal gastric emptying, those with delayed emptying did not respond to tricyclic antidepressants. A separate study found that the tricyclic antidepressant nortriptyline did not improve symptoms of gastroparesis (JAMA. 2013;310[24]:2640-9).
Promotility agents may be beneficial for certain patients. A study published this year suggests that, compared with placebo, prucalopride is effective for nausea, vomiting, fullness, bloating, and gastric emptying in patients with idiopathic gastroparesis (Am J Gastroenterol. 2019;114[8]:1265-74.). A 2017 meta-analysis, however, found that proton pump inhibitors were more effective than promotility agents in patients with functional dyspepsia (Am J Gastroenterol. 2017;112[7]:988-1013.).
Pyloric dysfunction may accompany gastroparesis in some patients. Increased severity of gastric emptying delay is associated with increased pylorospasm. Endoscopists have gained experience in performing pyloric myotomy, and this treatment has become more popular. Uncontrolled studies indicate that the proportion of patients with decreased symptom severity after this procedure is higher than 70% and can be as high as 86% (Gastrointest Endosc. 2017;85[1]:123-8). The predictors of a good response include idiopathic etiology, male sex, moderate symptom severity, and greater delay in gastric emptying.
Functional dyspepsia should perhaps be understood as normal gastric emptying and symptoms of epigastric pain syndrome, said Dr. Nguyen. Those patients may respond to neuromodulators, she added. Idiopathic gastroparesis appears to be characterized by severe delay in gastric emptying, postprandial symptoms, nausea, and vomiting. “In the middle is the gray zone, where you have these patients with postprandial distress with or without delayed gastric emptying,” said Dr. Nguyen. Functional dyspepsia and gastroparesis could be two ends of a spectrum, and the best management for patients with symptoms that occur in both disorders is unclear.
EXPERT ANALYSIS FROM FRESTON CONFERENCE 2019