User login
Out-of-pocket cost of oral TKIs linked to poor lung cancer survival
ALEXANDRIA, VA. – Higher out-of-pocket costs for oral tyrosine kinase inhibitors (TKIs) were linked to inferior survival in patients with advanced, biomarker-positive lung cancers in an analysis of state-level registry data, an investigator reported at a press conference ahead of the Quality Care Symposium, sponsored by the American Society of Clinical Oncology.
Higher out-of-pocket cost burden was also linked to lower numbers of TKI prescriptions and shorter duration of TKI therapy in the study, which included patients diagnosed with EGFR- and ALK-positive non–small cell lung cancer (NSCLC) between 2010 and 2016.
The findings would suggest a need for a review of coverage for these effective medications, according to Bernardo H. L. Goulart, MD, a thoracic oncologist and health services researcher at the University of Washington and Fred Hutchinson Cancer Research Center, both in Seattle.
“Making sure that the cost to the patients is affordable could mitigate this financial toxicity, and hopefully help patients stay on therapy and derive the survival benefit that these medications are supposed to offer,” Dr. Goulart said in an interview.
The study included data on 106 patients with EGFR- and ALK-positive stage IV NSCLC in the Washington Surveillance, Epidemiology, and End Results registry who had at least one oral TKI prescription. Investigators linked that registry data with commercial and Medicare claims, then divided this patient cohort into quartiles based on out-of-pocket costs.
In the top quartile, the median monthly out-of-pocket cost for TKI treatment was $2,888, compared with just $1,431 in the other three quartiles – essentially half the cost, Dr. Goulart said.
Median survival in the patients in the top out-of-pocket cost quartile was just 9 months, compared with 22 months in the lower three quartiles, he added.
“That difference is remarkable,” Dr. Goulart said, adding that the survival in the top-cost quartile reflects a survival that might be expected with conventional, nontargeted chemotherapy, while survival in the remaining patients mirrored what might be expected based on clinical trials of TKIs in this setting.
Patients in the high-cost quartile were 2.31 times as likely to die as patients in the lower quartiles, according to results of a multivariable analysis that adjusted for patient, disease-specific, and financial characteristics including qualification for low-income subsidies.
The mean medication possession ratio, a measure of medication adherence, was lower in the high-cost quartile (1.06 vs. 1.20 for the lower three quartiles; P = .02), and median duration of therapy was likewise lower in the high-cost quartile (4 vs. 8 months; P less than .01), according to data reported in the study abstract.
While multiple previous studies have linked out-of-pocket costs to decreased adherence and duration of therapy, the present study is one of the few to evaluate the link between cost of oral cancer medications and survival, according to Dr. Goulart.
In one other recent study showing a relationship between financial toxicity and survival, researchers at Fred Hutch showing that Washington cancer patients who filed for bankruptcy were more likely to die, compared with cancer patients not filing for bankruptcy, even after adjustment for a variety of patient characteristics.
The present study results raise a “serious concern” that some patients are unable to afford their medications, which is having a detrimental effect on survival, Dr. Goulart said. Alternatively, the out-of-pocket costs may not have an effect on survival; rather, they may be a “marker of very poor insurance coverage” that reflects higher costs for multiple other aspects of their care.
“The out-of-pocket cost for these drugs can be pretty astronomical, and we have at least a plausible hypothesis that they are taking a toll on patient’s survival,” he added.
If the findings of this study are confirmed in more and larger studies, there could be important implications for health policy and oncology practice, according to Dr. Goulart.
“The biggest action would be to involve patient advocates and physician groups such as ASCO, and advocate for changes in policy for coverage and out-of-pocket costs for these oral TKIs, at least for the patients that have the mutations,” he explained.
Another action, according to Dr. Goulart, would be to try to equip oncology clinics everywhere with patient financial-assistance programs to link patients to entities that can help them afford the cost of TKIs.
“Patients who attend small, remote cancer clinics might not have access to a financial specialist who can help them navigate these costs,” he said.
Funding for the study came from the National Institutes of Health. Dr. Goulart reported disclosures related to Flatiron Health (travel, accommodations, and expenses).
SOURCE: Goulart BHL et al. SCS 2019, Abstract 3.
ALEXANDRIA, VA. – Higher out-of-pocket costs for oral tyrosine kinase inhibitors (TKIs) were linked to inferior survival in patients with advanced, biomarker-positive lung cancers in an analysis of state-level registry data, an investigator reported at a press conference ahead of the Quality Care Symposium, sponsored by the American Society of Clinical Oncology.
Higher out-of-pocket cost burden was also linked to lower numbers of TKI prescriptions and shorter duration of TKI therapy in the study, which included patients diagnosed with EGFR- and ALK-positive non–small cell lung cancer (NSCLC) between 2010 and 2016.
The findings would suggest a need for a review of coverage for these effective medications, according to Bernardo H. L. Goulart, MD, a thoracic oncologist and health services researcher at the University of Washington and Fred Hutchinson Cancer Research Center, both in Seattle.
“Making sure that the cost to the patients is affordable could mitigate this financial toxicity, and hopefully help patients stay on therapy and derive the survival benefit that these medications are supposed to offer,” Dr. Goulart said in an interview.
The study included data on 106 patients with EGFR- and ALK-positive stage IV NSCLC in the Washington Surveillance, Epidemiology, and End Results registry who had at least one oral TKI prescription. Investigators linked that registry data with commercial and Medicare claims, then divided this patient cohort into quartiles based on out-of-pocket costs.
In the top quartile, the median monthly out-of-pocket cost for TKI treatment was $2,888, compared with just $1,431 in the other three quartiles – essentially half the cost, Dr. Goulart said.
Median survival in the patients in the top out-of-pocket cost quartile was just 9 months, compared with 22 months in the lower three quartiles, he added.
“That difference is remarkable,” Dr. Goulart said, adding that the survival in the top-cost quartile reflects a survival that might be expected with conventional, nontargeted chemotherapy, while survival in the remaining patients mirrored what might be expected based on clinical trials of TKIs in this setting.
Patients in the high-cost quartile were 2.31 times as likely to die as patients in the lower quartiles, according to results of a multivariable analysis that adjusted for patient, disease-specific, and financial characteristics including qualification for low-income subsidies.
The mean medication possession ratio, a measure of medication adherence, was lower in the high-cost quartile (1.06 vs. 1.20 for the lower three quartiles; P = .02), and median duration of therapy was likewise lower in the high-cost quartile (4 vs. 8 months; P less than .01), according to data reported in the study abstract.
While multiple previous studies have linked out-of-pocket costs to decreased adherence and duration of therapy, the present study is one of the few to evaluate the link between cost of oral cancer medications and survival, according to Dr. Goulart.
In one other recent study showing a relationship between financial toxicity and survival, researchers at Fred Hutch showing that Washington cancer patients who filed for bankruptcy were more likely to die, compared with cancer patients not filing for bankruptcy, even after adjustment for a variety of patient characteristics.
The present study results raise a “serious concern” that some patients are unable to afford their medications, which is having a detrimental effect on survival, Dr. Goulart said. Alternatively, the out-of-pocket costs may not have an effect on survival; rather, they may be a “marker of very poor insurance coverage” that reflects higher costs for multiple other aspects of their care.
“The out-of-pocket cost for these drugs can be pretty astronomical, and we have at least a plausible hypothesis that they are taking a toll on patient’s survival,” he added.
If the findings of this study are confirmed in more and larger studies, there could be important implications for health policy and oncology practice, according to Dr. Goulart.
“The biggest action would be to involve patient advocates and physician groups such as ASCO, and advocate for changes in policy for coverage and out-of-pocket costs for these oral TKIs, at least for the patients that have the mutations,” he explained.
Another action, according to Dr. Goulart, would be to try to equip oncology clinics everywhere with patient financial-assistance programs to link patients to entities that can help them afford the cost of TKIs.
“Patients who attend small, remote cancer clinics might not have access to a financial specialist who can help them navigate these costs,” he said.
Funding for the study came from the National Institutes of Health. Dr. Goulart reported disclosures related to Flatiron Health (travel, accommodations, and expenses).
SOURCE: Goulart BHL et al. SCS 2019, Abstract 3.
ALEXANDRIA, VA. – Higher out-of-pocket costs for oral tyrosine kinase inhibitors (TKIs) were linked to inferior survival in patients with advanced, biomarker-positive lung cancers in an analysis of state-level registry data, an investigator reported at a press conference ahead of the Quality Care Symposium, sponsored by the American Society of Clinical Oncology.
Higher out-of-pocket cost burden was also linked to lower numbers of TKI prescriptions and shorter duration of TKI therapy in the study, which included patients diagnosed with EGFR- and ALK-positive non–small cell lung cancer (NSCLC) between 2010 and 2016.
The findings would suggest a need for a review of coverage for these effective medications, according to Bernardo H. L. Goulart, MD, a thoracic oncologist and health services researcher at the University of Washington and Fred Hutchinson Cancer Research Center, both in Seattle.
“Making sure that the cost to the patients is affordable could mitigate this financial toxicity, and hopefully help patients stay on therapy and derive the survival benefit that these medications are supposed to offer,” Dr. Goulart said in an interview.
The study included data on 106 patients with EGFR- and ALK-positive stage IV NSCLC in the Washington Surveillance, Epidemiology, and End Results registry who had at least one oral TKI prescription. Investigators linked that registry data with commercial and Medicare claims, then divided this patient cohort into quartiles based on out-of-pocket costs.
In the top quartile, the median monthly out-of-pocket cost for TKI treatment was $2,888, compared with just $1,431 in the other three quartiles – essentially half the cost, Dr. Goulart said.
Median survival in the patients in the top out-of-pocket cost quartile was just 9 months, compared with 22 months in the lower three quartiles, he added.
“That difference is remarkable,” Dr. Goulart said, adding that the survival in the top-cost quartile reflects a survival that might be expected with conventional, nontargeted chemotherapy, while survival in the remaining patients mirrored what might be expected based on clinical trials of TKIs in this setting.
Patients in the high-cost quartile were 2.31 times as likely to die as patients in the lower quartiles, according to results of a multivariable analysis that adjusted for patient, disease-specific, and financial characteristics including qualification for low-income subsidies.
The mean medication possession ratio, a measure of medication adherence, was lower in the high-cost quartile (1.06 vs. 1.20 for the lower three quartiles; P = .02), and median duration of therapy was likewise lower in the high-cost quartile (4 vs. 8 months; P less than .01), according to data reported in the study abstract.
While multiple previous studies have linked out-of-pocket costs to decreased adherence and duration of therapy, the present study is one of the few to evaluate the link between cost of oral cancer medications and survival, according to Dr. Goulart.
In one other recent study showing a relationship between financial toxicity and survival, researchers at Fred Hutch showing that Washington cancer patients who filed for bankruptcy were more likely to die, compared with cancer patients not filing for bankruptcy, even after adjustment for a variety of patient characteristics.
The present study results raise a “serious concern” that some patients are unable to afford their medications, which is having a detrimental effect on survival, Dr. Goulart said. Alternatively, the out-of-pocket costs may not have an effect on survival; rather, they may be a “marker of very poor insurance coverage” that reflects higher costs for multiple other aspects of their care.
“The out-of-pocket cost for these drugs can be pretty astronomical, and we have at least a plausible hypothesis that they are taking a toll on patient’s survival,” he added.
If the findings of this study are confirmed in more and larger studies, there could be important implications for health policy and oncology practice, according to Dr. Goulart.
“The biggest action would be to involve patient advocates and physician groups such as ASCO, and advocate for changes in policy for coverage and out-of-pocket costs for these oral TKIs, at least for the patients that have the mutations,” he explained.
Another action, according to Dr. Goulart, would be to try to equip oncology clinics everywhere with patient financial-assistance programs to link patients to entities that can help them afford the cost of TKIs.
“Patients who attend small, remote cancer clinics might not have access to a financial specialist who can help them navigate these costs,” he said.
Funding for the study came from the National Institutes of Health. Dr. Goulart reported disclosures related to Flatiron Health (travel, accommodations, and expenses).
SOURCE: Goulart BHL et al. SCS 2019, Abstract 3.
REPORTING FROM QCS 2019
siRNA drug safely halved LDL cholesterol in phase 3 ORION-11
PARIS – A small interfering RNA drug, inclisiran, safely halved LDL cholesterol levels in more than 800 patients in a phase 3, multicenter study, in a big step toward this drug coming onto the market and offering an alternative way to harness the potent cholesterol-lowering power of PCSK9 inhibition.
In the reported study – which enrolled patients with established cardiovascular disease, familial hypercholesterolemia, type 2 diabetes, or a high Framingham Risk Score – participants received inclisiran as a semiannual subcutaneous injection. The safe efficacy this produced showed the viability of a new way to deliver lipid-lowering therapy that guarantees compliance and is convenient for patients.
The prospect of lowering cholesterol with about the same potency as the monoclonal antibodies that block PCSK9 (proprotein convertase subtilisin/kexin type 9) activity but administered as a biannual injection “enables provider control over medication adherence, and may offer patients a meaningful new choice that is safe and convenient and has assured results,” Kausik K. Ray, MD, said at the annual congress of the European Society of Cardiology. The durable effect of the small interfering RNA (siRNA) agent “offers a huge advantage,” and “opens the field,” said Dr. Ray, a cardiologist and professor of public health at Imperial College, London.
He also highlighted the “excellent” safety profile seen in the 811 patients treated with inclisiran, compared with 804 patients in the study who received placebo. After four total injections of inclisiran spaced out over 450 days (about 15 months), the rate of treatment-emergent adverse events and serious events was virtually the same in the two treatment arms, and with no signal of inclisiran causing liver effects, renal or muscle injury, damage to blood components, or malignancy. The serial treatment with inclisiran that patients received – at baseline, 90, 270, and 450 days – produced no severe injection-site reactions, and transient mild or moderate injection-site reactions in just under 5% of patients.
Safety issues, such as more-severe injection-site reactions, thrombocytopenia, hepatotoxicity, and flu-like symptoms, plagued siRNA drugs during their earlier days of development, but more recently next-generation siRNA drugs with modified structures have produced much better safety performance, noted Richard C. Becker, MD, professor of medicine and director of the Heart, Lung, & Vascular Institute at the University of Cincinnati. The new-generation siRNAs such as inclisiran are “very well tolerated,” he said in an interview.
The Food and Drug Administration approved the first siRNA drug in August 2018, and in the year since then a few others have also come onto the U.S. market, Dr. Becker said.
Like other siRNA drugs, the activity of inclisiran comes from a short RNA segment that is antisense to a particular messenger RNA (mRNA) target. In the case of inclisiran, the target is the mRNA for the PCSK9 enzyme produced in hepatocytes, and that decreases the number of LDL cholesterol receptors on the cell’s surface. When the antisense RNA molecule encounters a PCSK9 mRNA, the two bind and the mRNA is then degraded by a normal cell process. This cuts the cell’s production of the PCSK9 protein, resulting in more LDL cholesterol receptors on the cell’s surface that pull more LDL cholesterol from the blood. The blocking of PCSK9 activity by inclisiran is roughly equivalent to the action of the PCSK9 monoclonal antibodies that have now been on the U.S. market for a few years. Also like other siRNA drugs, the RNA of inclisiran is packaged so that, once injected into a patient, the RNA molecules travel to the liver and enter hepatocytes, where they exert their activity.
“No one has concerns about inclisiran being able to lower LDL [cholesterol], and there have been no safety signals. The data we have seen so far look very reassuring, and in particular has been very safe for the liver,” commented Marc S. Sabatine, MD, professor of medicine at Harvard Medical School, Boston, who has led several studies involving PCSK9-targeted drugs and is helping to run ORION-4, a 15,000-patient study of inclisiran designed to assess the drug’s effect on clinical events. Results from ORION-4 are not expected until about 2024.
“The PCSK9 inhibitors in general have been a huge advance for patients, and the more kinds of drugs we have to target PCSK9, the better,” he said in an interview.
The study reported by Dr. Ray, ORION-11, enrolled 1,617 patients with high atherosclerotic disease risk at 70 sites in six European countries and South Africa. The study’s primary efficacy endpoint was reduction from baseline in LDL cholesterol both at 510 days (about 17 months) after the first dose and also throughout the 15-month period that started 3 months after the first dose. The average reduction seen after 510 days was 54%, compared with baseline, and the time-averaged reduction during the 15-month window examined was 50%, Dr. Ray said. The results also showed a consistent reduction in LDL cholesterol in virtually every patient treated with inclisiran.
Two other phase 3 studies of inclisiran with similar design have been completed, and the results will come out before the end of 2019, according to a statement from the Medicines Company, which is developing the drug. The statement also said that the company plans to file their data with the FDA for marketing approval for inclisiran before the end of 2019. In the recent past, the FDA has approved drugs for the indication of lowering LDL cholesterol before evidence is available to prove that the agent has benefits for reducing clinical events.
Future studies of inclisiran will explore the efficacy of a single annual injection of the drug as an approach to primary prevention of cardiovascular disease, Dr. Ray said.
ORION-11 was sponsored by the Medicines Company. Dr. Ray is a consultant to it and to several other companies. Dr. Becker had no relevant disclosures. Dr. Sabatine has received research support from the Medicines Company and several other companies, and has received personal fees from Anthos Therapeutics, Bristol-Myers Squibb, CVS Caremark, Daiichi Sankyo, DalCor Pharmaceuticals, Dyrnamix, and Ionis.
PARIS – A small interfering RNA drug, inclisiran, safely halved LDL cholesterol levels in more than 800 patients in a phase 3, multicenter study, in a big step toward this drug coming onto the market and offering an alternative way to harness the potent cholesterol-lowering power of PCSK9 inhibition.
In the reported study – which enrolled patients with established cardiovascular disease, familial hypercholesterolemia, type 2 diabetes, or a high Framingham Risk Score – participants received inclisiran as a semiannual subcutaneous injection. The safe efficacy this produced showed the viability of a new way to deliver lipid-lowering therapy that guarantees compliance and is convenient for patients.
The prospect of lowering cholesterol with about the same potency as the monoclonal antibodies that block PCSK9 (proprotein convertase subtilisin/kexin type 9) activity but administered as a biannual injection “enables provider control over medication adherence, and may offer patients a meaningful new choice that is safe and convenient and has assured results,” Kausik K. Ray, MD, said at the annual congress of the European Society of Cardiology. The durable effect of the small interfering RNA (siRNA) agent “offers a huge advantage,” and “opens the field,” said Dr. Ray, a cardiologist and professor of public health at Imperial College, London.
He also highlighted the “excellent” safety profile seen in the 811 patients treated with inclisiran, compared with 804 patients in the study who received placebo. After four total injections of inclisiran spaced out over 450 days (about 15 months), the rate of treatment-emergent adverse events and serious events was virtually the same in the two treatment arms, and with no signal of inclisiran causing liver effects, renal or muscle injury, damage to blood components, or malignancy. The serial treatment with inclisiran that patients received – at baseline, 90, 270, and 450 days – produced no severe injection-site reactions, and transient mild or moderate injection-site reactions in just under 5% of patients.
Safety issues, such as more-severe injection-site reactions, thrombocytopenia, hepatotoxicity, and flu-like symptoms, plagued siRNA drugs during their earlier days of development, but more recently next-generation siRNA drugs with modified structures have produced much better safety performance, noted Richard C. Becker, MD, professor of medicine and director of the Heart, Lung, & Vascular Institute at the University of Cincinnati. The new-generation siRNAs such as inclisiran are “very well tolerated,” he said in an interview.
The Food and Drug Administration approved the first siRNA drug in August 2018, and in the year since then a few others have also come onto the U.S. market, Dr. Becker said.
Like other siRNA drugs, the activity of inclisiran comes from a short RNA segment that is antisense to a particular messenger RNA (mRNA) target. In the case of inclisiran, the target is the mRNA for the PCSK9 enzyme produced in hepatocytes, and that decreases the number of LDL cholesterol receptors on the cell’s surface. When the antisense RNA molecule encounters a PCSK9 mRNA, the two bind and the mRNA is then degraded by a normal cell process. This cuts the cell’s production of the PCSK9 protein, resulting in more LDL cholesterol receptors on the cell’s surface that pull more LDL cholesterol from the blood. The blocking of PCSK9 activity by inclisiran is roughly equivalent to the action of the PCSK9 monoclonal antibodies that have now been on the U.S. market for a few years. Also like other siRNA drugs, the RNA of inclisiran is packaged so that, once injected into a patient, the RNA molecules travel to the liver and enter hepatocytes, where they exert their activity.
“No one has concerns about inclisiran being able to lower LDL [cholesterol], and there have been no safety signals. The data we have seen so far look very reassuring, and in particular has been very safe for the liver,” commented Marc S. Sabatine, MD, professor of medicine at Harvard Medical School, Boston, who has led several studies involving PCSK9-targeted drugs and is helping to run ORION-4, a 15,000-patient study of inclisiran designed to assess the drug’s effect on clinical events. Results from ORION-4 are not expected until about 2024.
“The PCSK9 inhibitors in general have been a huge advance for patients, and the more kinds of drugs we have to target PCSK9, the better,” he said in an interview.
The study reported by Dr. Ray, ORION-11, enrolled 1,617 patients with high atherosclerotic disease risk at 70 sites in six European countries and South Africa. The study’s primary efficacy endpoint was reduction from baseline in LDL cholesterol both at 510 days (about 17 months) after the first dose and also throughout the 15-month period that started 3 months after the first dose. The average reduction seen after 510 days was 54%, compared with baseline, and the time-averaged reduction during the 15-month window examined was 50%, Dr. Ray said. The results also showed a consistent reduction in LDL cholesterol in virtually every patient treated with inclisiran.
Two other phase 3 studies of inclisiran with similar design have been completed, and the results will come out before the end of 2019, according to a statement from the Medicines Company, which is developing the drug. The statement also said that the company plans to file their data with the FDA for marketing approval for inclisiran before the end of 2019. In the recent past, the FDA has approved drugs for the indication of lowering LDL cholesterol before evidence is available to prove that the agent has benefits for reducing clinical events.
Future studies of inclisiran will explore the efficacy of a single annual injection of the drug as an approach to primary prevention of cardiovascular disease, Dr. Ray said.
ORION-11 was sponsored by the Medicines Company. Dr. Ray is a consultant to it and to several other companies. Dr. Becker had no relevant disclosures. Dr. Sabatine has received research support from the Medicines Company and several other companies, and has received personal fees from Anthos Therapeutics, Bristol-Myers Squibb, CVS Caremark, Daiichi Sankyo, DalCor Pharmaceuticals, Dyrnamix, and Ionis.
PARIS – A small interfering RNA drug, inclisiran, safely halved LDL cholesterol levels in more than 800 patients in a phase 3, multicenter study, in a big step toward this drug coming onto the market and offering an alternative way to harness the potent cholesterol-lowering power of PCSK9 inhibition.
In the reported study – which enrolled patients with established cardiovascular disease, familial hypercholesterolemia, type 2 diabetes, or a high Framingham Risk Score – participants received inclisiran as a semiannual subcutaneous injection. The safe efficacy this produced showed the viability of a new way to deliver lipid-lowering therapy that guarantees compliance and is convenient for patients.
The prospect of lowering cholesterol with about the same potency as the monoclonal antibodies that block PCSK9 (proprotein convertase subtilisin/kexin type 9) activity but administered as a biannual injection “enables provider control over medication adherence, and may offer patients a meaningful new choice that is safe and convenient and has assured results,” Kausik K. Ray, MD, said at the annual congress of the European Society of Cardiology. The durable effect of the small interfering RNA (siRNA) agent “offers a huge advantage,” and “opens the field,” said Dr. Ray, a cardiologist and professor of public health at Imperial College, London.
He also highlighted the “excellent” safety profile seen in the 811 patients treated with inclisiran, compared with 804 patients in the study who received placebo. After four total injections of inclisiran spaced out over 450 days (about 15 months), the rate of treatment-emergent adverse events and serious events was virtually the same in the two treatment arms, and with no signal of inclisiran causing liver effects, renal or muscle injury, damage to blood components, or malignancy. The serial treatment with inclisiran that patients received – at baseline, 90, 270, and 450 days – produced no severe injection-site reactions, and transient mild or moderate injection-site reactions in just under 5% of patients.
Safety issues, such as more-severe injection-site reactions, thrombocytopenia, hepatotoxicity, and flu-like symptoms, plagued siRNA drugs during their earlier days of development, but more recently next-generation siRNA drugs with modified structures have produced much better safety performance, noted Richard C. Becker, MD, professor of medicine and director of the Heart, Lung, & Vascular Institute at the University of Cincinnati. The new-generation siRNAs such as inclisiran are “very well tolerated,” he said in an interview.
The Food and Drug Administration approved the first siRNA drug in August 2018, and in the year since then a few others have also come onto the U.S. market, Dr. Becker said.
Like other siRNA drugs, the activity of inclisiran comes from a short RNA segment that is antisense to a particular messenger RNA (mRNA) target. In the case of inclisiran, the target is the mRNA for the PCSK9 enzyme produced in hepatocytes, and that decreases the number of LDL cholesterol receptors on the cell’s surface. When the antisense RNA molecule encounters a PCSK9 mRNA, the two bind and the mRNA is then degraded by a normal cell process. This cuts the cell’s production of the PCSK9 protein, resulting in more LDL cholesterol receptors on the cell’s surface that pull more LDL cholesterol from the blood. The blocking of PCSK9 activity by inclisiran is roughly equivalent to the action of the PCSK9 monoclonal antibodies that have now been on the U.S. market for a few years. Also like other siRNA drugs, the RNA of inclisiran is packaged so that, once injected into a patient, the RNA molecules travel to the liver and enter hepatocytes, where they exert their activity.
“No one has concerns about inclisiran being able to lower LDL [cholesterol], and there have been no safety signals. The data we have seen so far look very reassuring, and in particular has been very safe for the liver,” commented Marc S. Sabatine, MD, professor of medicine at Harvard Medical School, Boston, who has led several studies involving PCSK9-targeted drugs and is helping to run ORION-4, a 15,000-patient study of inclisiran designed to assess the drug’s effect on clinical events. Results from ORION-4 are not expected until about 2024.
“The PCSK9 inhibitors in general have been a huge advance for patients, and the more kinds of drugs we have to target PCSK9, the better,” he said in an interview.
The study reported by Dr. Ray, ORION-11, enrolled 1,617 patients with high atherosclerotic disease risk at 70 sites in six European countries and South Africa. The study’s primary efficacy endpoint was reduction from baseline in LDL cholesterol both at 510 days (about 17 months) after the first dose and also throughout the 15-month period that started 3 months after the first dose. The average reduction seen after 510 days was 54%, compared with baseline, and the time-averaged reduction during the 15-month window examined was 50%, Dr. Ray said. The results also showed a consistent reduction in LDL cholesterol in virtually every patient treated with inclisiran.
Two other phase 3 studies of inclisiran with similar design have been completed, and the results will come out before the end of 2019, according to a statement from the Medicines Company, which is developing the drug. The statement also said that the company plans to file their data with the FDA for marketing approval for inclisiran before the end of 2019. In the recent past, the FDA has approved drugs for the indication of lowering LDL cholesterol before evidence is available to prove that the agent has benefits for reducing clinical events.
Future studies of inclisiran will explore the efficacy of a single annual injection of the drug as an approach to primary prevention of cardiovascular disease, Dr. Ray said.
ORION-11 was sponsored by the Medicines Company. Dr. Ray is a consultant to it and to several other companies. Dr. Becker had no relevant disclosures. Dr. Sabatine has received research support from the Medicines Company and several other companies, and has received personal fees from Anthos Therapeutics, Bristol-Myers Squibb, CVS Caremark, Daiichi Sankyo, DalCor Pharmaceuticals, Dyrnamix, and Ionis.
REPORTING FROM THE ESC CONGRESS 2019
Ocular Complications of Atopic Dermatitis
Atopic dermatitis (AD) is a chronic inflammatory skin condition with a lifetime prevalence of 15% to 20% in industrialized countries.1 It affects both children and adults and is predominantly characterized by a waxing and waning course of eczematous skin lesions and pruritus. In recent years, there is increasing recognition that AD can present with extracutaneous findings. Large-scale epidemiologic studies have reported a notably higher prevalence of ophthalmic complications in the AD population compared to the general population, in a severity-dependent manner.2,3 Potential complications include blepharitis, keratoconjunctivitis, keratoconus, glaucoma, cataracts, retinal detachment, ophthalmic herpes simplex virus infections, and dupilumab-associated ocular complications.
The etiology of each ocular complication in the context of AD is complex and likely multifactorial. Intrinsic immune dysregulation, physical trauma from eye rubbing, AD medication side effects, and genetics all have been speculated to play a role.2 Some of these ocular complications have a chronic course, while others present with sudden onset of symptoms; many of them can result in visual impairment if undiagnosed or left untreated. This article reviews several of the most common ocular comorbidities associated with AD. We discuss the clinical presentation, pathophysiology, and management strategies for each condition.
Blepharitis
Blepharitis, an inflammatory condition of the eyelids, is estimated to affect more than 6% of patients with AD compared to less than 1% of the general population.2 Blepharitis can be classified as anterior or posterior, based on the anatomic location of the affected region relative to the lash margin. Affected individuals may experience pruritus and irritation of the eyelids, tearing, a foreign body or burning sensation, crusting of the eyelids, and photophobia.4 Anterior blepharitis commonly is due to staphylococcal disease, and posterior blepharitis is secondary to structural changes and obstruction of meibomian gland orifices.
Although the pathophysiology is not well defined, xerosis in atopic patients is accompanied by barrier disruption and transepidermal water loss, which promote eyelid skin inflammation.
The mainstay of therapy for atopic blepharitis consists of conventional lid hygiene regimens, such as warm compresses and gentle scrubbing of the lid margins to remove crust and debris, which can be done with nonprescription cleansers, pads, and baby shampoos. Acute exacerbations may require topical antibiotics (ie, erythromycin or bacitracin applied to the lid margins once daily), topical calcineurin inhibitors (ie, cyclosporine ophthalmic emulsion 0.05%), or low-potency topical corticosteroids (ie, fluorometholone 0.1% or loteprednol etabonate 0.5% ophthalmic suspensions).5 Due to potential side effects of medications, especially topical corticosteroids, patients should be referred to ophthalmologists for definitive diagnosis and treatment.
Keratoconjunctivitis
Atopic keratoconjunctivitis (AKC) is a noninfectious inflammatory condition of the cornea and conjunctiva that occurs in an estimated 25% to 42% of patients with AD.6,7 It frequently presents in late adolescence and has a peak incidence between 30 and 50 years of age.8 The symptoms of AKC include ocular pruritus, redness, ropy mucoid discharge, burning discomfort, photophobia, and blurring of vision. Corneal involvement can progress to corneal neovascularization and punctate or macroepithelial erosions and ulcerations, which increase the risk for corneal scarring and visual impairment.7
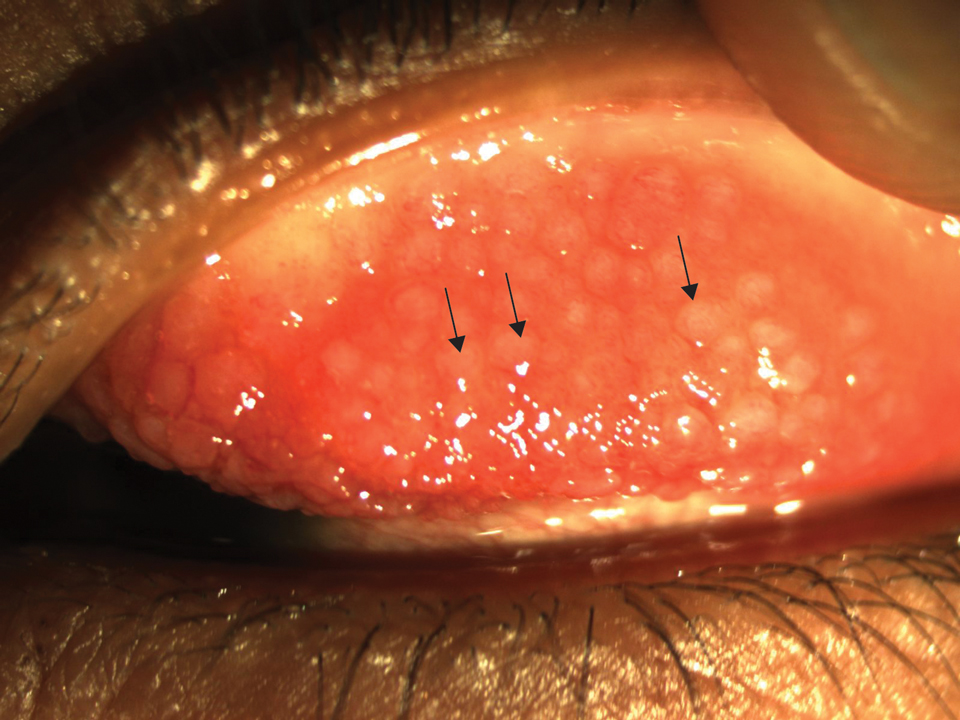
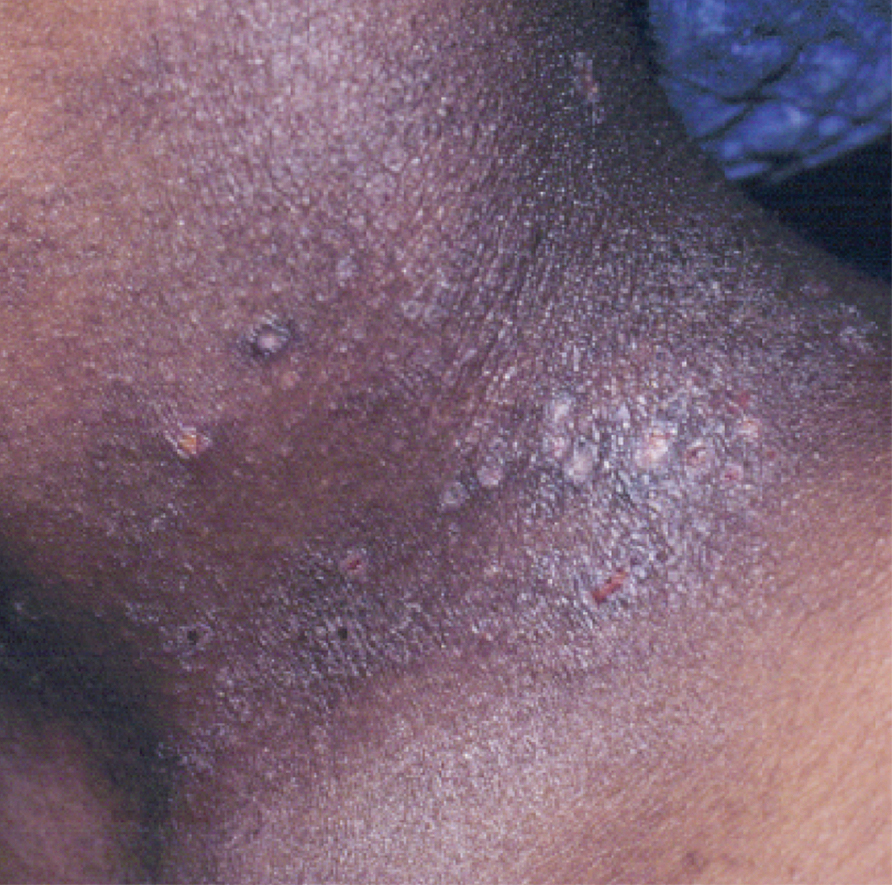
Keratoconjunctivitis is a complex inflammatory disease characterized by infiltration of the conjunctival epithelium by eosinophils, mast cells, and lymphocytes. On examination, patients frequently are found to have concurrent AD of the periorbital skin as well as papillary hypertrophy of the tarsal conjunctiva with accompanying fibrosis, which can lead to entropion (turning inward of the lid margins and lashes) in severe cases.7 Ophthalmic evaluation is strongly recommended for patients with AKC to control symptoms, to limit exacerbations, and to prevent sight-threatening inflammation leading to vision loss. Treatment can be challenging given the chronicity of the condition and may require multiple treatment arms. Conservative measures include cool compresses and treatment with ophthalmic eye drops containing antihistamines (ie, ketotifen 0.025% [available over-the-counter]) and mast cell stabilizers (ie, olopatadine ophthalmic solution 0.1%).8 Atopic keratoconjunctivitis exacerbations may require short-term use of topical steroids or calcineurin inhibitors, or systemic equivalents for refractory cases.6 Long-term maintenance therapy typically consists of proper eye hygiene and steroid-sparing agents that reduce ocular inflammation, such as topical cyclosporine and tacrolimus, neither of which are associated with increased intraocular pressure (IOP)(Figure 1).8 Cornea disease resulting from chronic conjunctival/lid microtrauma can be managed with soft or scleral contact lenses.
Keratoconus
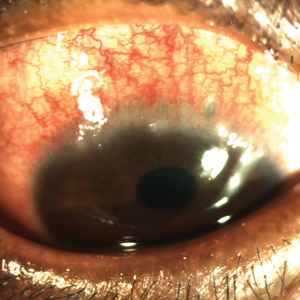
Keratoconus is a noninflammatory ocular disorder characterized by progressive thinning and conelike protrusion of the cornea. The corneal topographic changes result in high irregular astigmatism and reduced visual acuity, which can manifest as image blurring or distortion (Figure 2).2,9 Multiple case series and controlled studies have reported a positive association between keratoconus and a history of atopic disease.10,11
The precise etiology of keratoconus in the context of AD is unclear and likely is multifactorial. Habitual eye rubbing from periocular pruritus and discomfort has been reported to be a notable contributor to keratoconus.12 In addition, intrinsic inflammation and imbalance of cytokines and proteases also may contribute to development of keratoconus.13
Keratoconus is a progressive condition that can severely impact vision, making it critical to diagnose patients before irreversible vision loss occurs. Individuals with risk factors, such as AD of the eyelids, history of eye rubbing, or family history of keratoconus, should be advised to receive routine vision screening for worsening astigmatism, especially during the first few decades of life when keratoconus progresses rapidly.
The conservative management for early keratoconus includes glasses and gas-permeable contact lenses for correction of visual acuity and astigmatism. For advanced keratoconus, scleral lenses often are prescribed. These large-diameter, gas-permeable lenses are designed to rest on the sclera and arch over the entire cornea.9 Alternatively, corneal collagen cross-linking is a newer technique that utilizes riboflavin and UVA irradiation to strengthen the corneal tissue. It has proven to be safe and effective in slowing or stopping the progression of keratoconus, particularly when treated at the early stage, and received US Food and Drug Administration approval in 2016.9
Glaucoma
Glaucoma is a well-known complication of AD and can lead to irreversible ocular hypertension and optic nerve damage. Corticosteroid use is a major risk factor for glaucoma, and the rise in IOP is thought to be due to increased aqueous outflow resistance.14
Multiple case reports have linked glaucoma to long-term use of potent topical corticosteroids in the facial and palpebral regions, which has been attributed to direct steroid contact and absorption by ocular tissues, as glaucoma rarely occurs with topical steroid application elsewhere on the body.15-17 Systemic steroids (ie, prednisolone) taken for more than 8 weeks also have been associated with a marked rise in IOP.18
Certain risk factors may predispose a steroid user to increased IOP, including existing open-angle glaucoma, diabetes mellitus, collagen disease, and high myopia.15,19 Steroid responders and younger individuals also demonstrate increased sensitivity to steroids.20
Given that glaucoma often is asymptomatic until advanced stages, early detection is the key for proper intervention. Periodic glaucoma screening by an ophthalmologist would be appropriate for known steroid responders, as well as patients with a prolonged history of topical steroid application in the palpebral region and systemic steroid use, family history of glaucoma, or known ocular pathology.21 Furthermore, patients with concurrent glaucoma and AD should be jointly managed by dermatology and ophthalmology, and systemic and topical corticosteroid use should be minimized in favor of alterative agents such as calcineurin inhibitors.22
In addition to steroid-induced glaucoma, intrinsic atopic glaucoma recently has been proposed as a clinical entity and is characterized by increased inflammatory cytokines—IL-8 and CCL2—in the aqueous humor and abnormal accumulation of fibers in corneoscleral meshwork.23
Cataracts
Cataracts are estimated to affect 8% to 25% of patients with AD.21,24 Unlike age-related cataracts, cataracts associated with AD are observed in adolescents and young adults in addition to the older population. The progression of lenticular opacity can rapidly occur and has been reported to coincide with AD flares.25,26
Patients with AD typically present with anterior or posterior subcapsular cataracts instead of nuclear and cortical cataracts, which are more common in the general population.27,28 Anterior subcapsular cataracts are more specific to AD, whereas posterior subcapsular cataracts are associated with both prolonged corticosteroid use and AD.26 Children generally are more sensitive to steroids than adults and may develop cataracts more rapidly and at lower concentrations.29
The pathophysiology of cataract formation and progression in the context of AD is multifactorial. Cataract patients with AD have compromised blood-retinal barrier integrity as well as increased oxidative damage in the lens.30,31 Genetics and blunt trauma from eye rubbing are thought to play a role, and the latter has been associated with faster progression of cataracts.28 In contrast, corticosteroid-induced cataracts likely are caused by transcriptional changes and disrupted osmotic balance in the lens fibers, which can lead to fiber rupture and lens opacification.26,32 Systemic corticosteroids show the strongest association with cataract development, but inhaled and topical steroids also have been implicated.26
Although cataracts can be surgically corrected, prevention is critical. Patients with early-onset periorbital AD, prolonged use of topical or systemic corticosteroids, and family history of cataracts should be routinely screened. Anterior and posterior subcapsular cataracts are diagnosed with red reflex examinations that can be readily performed by the primary care physician or ophthalmologist.33 Atopic dermatitis patients with cataracts should be advised to use calcineurin inhibitors and alternative treatments in place of corticosteroids.
Retinal Detachment
Retinal detachment (RD) is a serious complication of AD that can present in individuals younger than 35 years. The incidence of RD in patients with AD has been estimated to be 4% to 8%.34 Retinal detachment manifests with visual disturbances such as flashing lights, shadows, visual field defect, and blurring of vision, but also may occur in the absence of vision changes.35,36
Across multiple case series, patients who developed RD were consistently found to have AD in the facial or periorbital region and a history of chronic eye rubbing. Multiple patients also presented with concurrent proliferative vitreoretinopathy, lens subluxation, and/or cataracts.35,37 The mechanism for RD has been attributed to ocular contusion from vigorous eye rubbing, as fundus findings between traumatic and AD-associated RD are similarly characterized by tractional breaks in the retina at vitreous base borders.37
Avoidance of eye rubbing and optimized treatment of facial AD may help prevent RD in patients with AD. Furthermore, all patients with symptoms of RD should be immediately referred to ophthalmology for surgical repair.
Herpetic Ocular Disease
Ocular herpes simplex virus infections cause ocular pain and are associated with notable visual morbidity, as recurrences can result in irreversible corneal scarring and neovascularization. Two retrospective case-control studies independently reported that individuals with a history of AD are at greater risk for herpetic ocular disease compared to age-matched controls.38,39 Furthermore, atopic disease is associated with higher recurrence rates and slower regeneration of the corneal epithelium.40
These findings suggest that AD patients with a history of recurrent herpetic ocular diseases should be closely monitored and treated with antiviral prophylaxis and/or topical corticosteroids, depending on the type of keratitis (epithelial or stromal).40 Furthermore, active ocular herpetic infections warrant urgent referral to an ophthalmologist.
Dupilumab-Associated Ocular Complications
Dupilumab, a monoclonal antibody that blocks IL-4 and IL-13 signaling, is the first biologic therapy to be approved for treatment of moderate to severe AD. Prior clinical trials have described a higher incidence of anterior conjunctivitis in dupilumab-treated AD patients (5%–28%) compared to placebo (2%–11%).41 Of note, the incidence may be as high as 70%, as reported in a recent case series.42 Interestingly, independent trials assessing dupilumab treatment in asthma, nasal polyposis, and eosinophilic esophagitis patients did not observe a higher incidence of conjunctivitis in dupilumab-treated patients compared to placebo, suggesting an AD-specific mechanism.43
Prominent features of dupilumab-associated conjunctivitis include hyperemia of the conjunctiva and limbus, in addition to ocular symptoms such as tearing, burning, and bilateral decrease in visual acuity. Marked reduction of conjunctival goblet cells has been reported.44 In addition to conjunctivitis, blepharitis also has been reported during dupilumab treatment.45
Standardized treatment guidelines for dupilumab-associated ocular complications have not yet been established. Surprisingly, antihistamine eye drops appear to be inefficacious in the treatment of dupilumab-associated conjunctivitis.41 However, the condition has been successfully managed with topical steroids (fluorometholone ophthalmic suspension 0.1%) and tacrolimus ointment 0.03%.41 Lifitegrast, an anti-inflammatory agent approved for chronic dry eye, also has been suggested as a treatment option for patients refractory to topical steroids.45 Alternatively, cessation of dupilumab could be considered in AD patients who experience severe ocular complications. Atopic dermatitis patients taking dupilumab who have any concerning signs for ocular complications should be referred to an ophthalmologist for further diagnosis and management.
Conclusion
Practicing dermatologists likely will encounter patients with concurrent AD and ocular complications. Although eye examinations are not routinely performed in the care of AD patients, dermatologists can proactively inquire about ocular symptoms and monitor patients longitudinally. Early diagnosis and treatment of these ocular conditions can prevent vision loss in these patients. Furthermore, symptomatic control of AD and careful consideration of the side-effect profiles of medications can potentially reduce the incidence of ocular complications in individuals with AD.
Patients with visual concerns or risk factors, such as a history of vigorous eye rubbing or chronic corticosteroid use, should be jointly managed with an ophthalmologist for optimized care. Moreover, acute exacerbations of ocular symptoms and visual deterioration warrant urgent referral to ophthalmology.
- Weidinger S, Beck LA, Bieber T, et al. Atopic dermatitis. Nat Rev Dis Primers. 2018;4:1.
- Govind K, Whang K, Khanna R, et al. Atopic dermatitis is associated with increased prevalence of multiple ocular comorbidities. J Allergy Clin Immunol Pract. 2019;7:298-299.
- Thyssen JP, Toft PB, Halling-Overgaard AS, et al. Incidence, prevalence, and risk of selected ocular disease in adults with atopic dermatitis. J Am Acad Dermatol. 2017;77:280-286.e281.
- Putnam CM. Diagnosis and management of blepharitis: an optometrist’s perspective. Clin Optom (Auckl). 2016;8:71-78.
- Amescua G, Akpek EK, Farid M, et al. Blepharitis Preferred Practice Pattern®. Ophthalmology. 2019;126:P56-P93.
- Bielory B, Bielory L. Atopic dermatitis and keratoconjunctivitis. Immunol Allergy Clin North Am. 2010;30:323-336.
- Guglielmetti S, Dart JK, Calder V. Atopic keratoconjunctivitis and atopic dermatitis. Curr Opin Allergy Clin Immunol. 2010;10:478-485.
- Chen JJ, Applebaum DS, Sun GS, et al. Atopic keratoconjunctivitis: a review. J Am Acad Dermatol. 2014;70:569-575.
- Andreanos KD, Hashemi K, Petrelli M, et al. Keratoconus treatment algorithm. Ophthalmol Ther. 2017;6:245-262.
- Rahi A, Davies P, Ruben M, et al. Keratoconus and coexisting atopic disease. Br J Ophthalmol. 1977;61:761-764.
- Gasset AR, Hinson WA, Frias JL. Keratoconus and atopic diseases. Ann Ophthalmol. 1978;10:991-994.
- Bawazeer AM, Hodge WG, Lorimer B. Atopy and keratoconus: a multivariate analysis. Br J Ophthalmol. 2000;84:834-836.
- Galvis V, Sherwin T, Tello A, et al. Keratoconus: an inflammatory disorder? Eye (Lond). 2015;29:843-859.
- Clark AF, Wordinger RJ. The role of steroids in outflow resistance. Exp Eye Res. 2009;88:752-759.
- Daniel BS, Orchard D. Ocular side-effects of topical corticosteroids: what a dermatologist needs to know. Australas J Dermatol. 2015;56:164-169.
- Garrott HM, Walland MJ. Glaucoma from topical corticosteroids to the eyelids. Clin Exp Ophthalmol. 2004;32:224-226.
- Aggarwal RK, Potamitis T, Chong NH, et al. Extensive visual loss with topical facial steroids. Eye (Lond). 1993;7(pt 5):664-666.
- Mandapati JS, Metta AK. Intraocular pressure variation in patients on long-term corticosteroids. Indian Dermatol Online J. 2011;2:67-69.
- Jones R 3rd, Rhee DJ. Corticosteroid-induced ocular hypertension and glaucoma: a brief review and update of the literature. Curr Opin Ophthalmol. 2006;17:163-167.
- Tamagawa-Mineoka R, Yasuoka N, Ueta M, et al. Influence of topical steroids on intraocular pressure in patients with atopic dermatitis. Allergol Int. 2018;67:388-391.
- Bercovitch L. Screening for ocular complications in atopic dermatitis. Arch Dermatol. 2011;147:588-589.
- Abramovits W, Hung P, Tong KB. Efficacy and economics of topical calcineurin inhibitors for the treatment of atopic dermatitis. Am J Clin Dermatol. 2006;7:213-222.
- Takakuwa K, Hamanaka T, Mori K, et al. Atopic glaucoma: clinical and pathophysiological analysis. J Glaucoma. 2015;24:662-668.
- Haeck IM, Rouwen TJ, Timmer-de Mik L, et al. Topical corticosteroids in atopic dermatitis and the risk of glaucoma and cataracts. J Am Acad Dermatol. 2011;64:275-281.
- Amemiya T, Matsuda H, Uehara M. Ocular findings in atopic dermatitis with special reference to the clinical features of atopic cataract. Ophthalmologica. 1980;180:129-132.
- Tatham A. Atopic dermatitis, cutaneous steroids and cataracts in children: two case reports. J Med Case Rep. 2008;2:124.
- Chew M, Chiang PP, Zheng Y, et al. The impact of cataract, cataract types, and cataract grades on vision-specific functioning using Rasch analysis. Am J Ophthalmol. 2012;154:29-38.
- Nagaki Y, Hayasaka S, Kadoi C. Cataract progression in patients with atopic dermatitis. J Cataract Refract Surg. 1999;25:96-99.
- Kaye LD, Kalenak JW, Price RL, et al. Ocular implications of long-term prednisone therapy in children. J Pediatr Ophthalmol Strabismus. 1993;30:142-144.
- Matsuo T, Saito H, Matsuo N. Cataract and aqueous flare levels in patients with atopic dermatitis. Am J Ophthalmol. 1997;124:36-39.
- Namazi MR, Handjani F, Amirahmadi M. Increased oxidative activity from hydrogen peroxide may be the cause of the predisposition to cataracts among patients with atopic dermatitis. Med Hypotheses. 2006;66:863-864.
- James ER. The etiology of steroid cataract. J Ocul Pharmacol Ther. 2007;23:403-420.
- Lambert SR, Teng JMC. Assessing whether the cataracts associated with atopic dermatitis are associated with steroids or inflammatory factors. JAMA Ophthalmol. 2018;136:918-919.
- Sasoh M, Mizutani H, Matsubara H, et al. Incidence of retinal detachment associated with atopic dermatitis in Japan: review of cases from 1992 to 2011. Clin Ophthalmol. 2015;9:1129-1134.
- Yoneda K, Okamoto H, Wada Y, et al. Atopic retinal detachment. report of four cases and a review of the literature. Br J Dermatol. 1995;133:586-591.
- Gnana Jothi V, McGimpsey S, Sharkey JA, et al. Retinal detachment repair and cataract surgery in patients with atopic dermatitis. Eye (Lond). 2017;31:1296-1301.
- Oka C, Ideta H, Nagasaki H, et al. Retinal detachment with atopic dermatitis similar to traumatic retinal detachment. Ophthalmology. 1994;101:1050-1054.
- Prabriputaloong T, Margolis TP, Lietman TM, et al. Atopic disease and herpes simplex eye disease: a population-based case-control study. Am J Ophthalmol. 2006;142:745-749.
- Borkar DS, Gonzales JA, Tham VM, et al. Association between atopy and herpetic eye disease: results from the pacific ocular inflammation study. JAMA Ophthalmol. 2014;132:326-331.
- Rezende RA, Hammersmith K, Bisol T, et al. Comparative study of ocular herpes simplex virus in patients with and without self-reported atopy. Am J Ophthalmol. 2006;141:1120-1125.
- Wollenberg A, Ariens L, Thurau S, et al. Conjunctivitis occurring in atopic dermatitis patients treated with dupilumab-clinical characteristics and treatment. J Allergy Clin Immunol Pract. 2018;6:1778-1780.e1.
- Ivert LU, Wahlgren CF, Ivert L, et al. Eye complications during dupilumab treatment for severe atopic dermatitis. Acta Derm Venereol. 2019;99:375-378.
- Akinlade B, Guttman-Yassky E, de Bruin-Weller M, et al. Conjunctivitis in dupilumab clinical trials [published online March 9, 2019]. Br J Dermatol. doi:10.1111/bjd.17869.
- Bakker DS, Ariens LFM, van Luijk C, et al. Goblet cell scarcity and conjunctival inflammation during treatment with dupilumab in patients with atopic dermatitis. Br J Dermatol. 2019;180:1248-1249.
- Zirwas MJ, Wulff K, Beckman K. Lifitegrast add-on treatment for dupilumab-induced ocular surface disease (DIOSD): a novel case report. JAAD Case Rep. 2019;5:34-36.
Atopic dermatitis (AD) is a chronic inflammatory skin condition with a lifetime prevalence of 15% to 20% in industrialized countries.1 It affects both children and adults and is predominantly characterized by a waxing and waning course of eczematous skin lesions and pruritus. In recent years, there is increasing recognition that AD can present with extracutaneous findings. Large-scale epidemiologic studies have reported a notably higher prevalence of ophthalmic complications in the AD population compared to the general population, in a severity-dependent manner.2,3 Potential complications include blepharitis, keratoconjunctivitis, keratoconus, glaucoma, cataracts, retinal detachment, ophthalmic herpes simplex virus infections, and dupilumab-associated ocular complications.
The etiology of each ocular complication in the context of AD is complex and likely multifactorial. Intrinsic immune dysregulation, physical trauma from eye rubbing, AD medication side effects, and genetics all have been speculated to play a role.2 Some of these ocular complications have a chronic course, while others present with sudden onset of symptoms; many of them can result in visual impairment if undiagnosed or left untreated. This article reviews several of the most common ocular comorbidities associated with AD. We discuss the clinical presentation, pathophysiology, and management strategies for each condition.
Blepharitis
Blepharitis, an inflammatory condition of the eyelids, is estimated to affect more than 6% of patients with AD compared to less than 1% of the general population.2 Blepharitis can be classified as anterior or posterior, based on the anatomic location of the affected region relative to the lash margin. Affected individuals may experience pruritus and irritation of the eyelids, tearing, a foreign body or burning sensation, crusting of the eyelids, and photophobia.4 Anterior blepharitis commonly is due to staphylococcal disease, and posterior blepharitis is secondary to structural changes and obstruction of meibomian gland orifices.
Although the pathophysiology is not well defined, xerosis in atopic patients is accompanied by barrier disruption and transepidermal water loss, which promote eyelid skin inflammation.
The mainstay of therapy for atopic blepharitis consists of conventional lid hygiene regimens, such as warm compresses and gentle scrubbing of the lid margins to remove crust and debris, which can be done with nonprescription cleansers, pads, and baby shampoos. Acute exacerbations may require topical antibiotics (ie, erythromycin or bacitracin applied to the lid margins once daily), topical calcineurin inhibitors (ie, cyclosporine ophthalmic emulsion 0.05%), or low-potency topical corticosteroids (ie, fluorometholone 0.1% or loteprednol etabonate 0.5% ophthalmic suspensions).5 Due to potential side effects of medications, especially topical corticosteroids, patients should be referred to ophthalmologists for definitive diagnosis and treatment.
Keratoconjunctivitis
Atopic keratoconjunctivitis (AKC) is a noninfectious inflammatory condition of the cornea and conjunctiva that occurs in an estimated 25% to 42% of patients with AD.6,7 It frequently presents in late adolescence and has a peak incidence between 30 and 50 years of age.8 The symptoms of AKC include ocular pruritus, redness, ropy mucoid discharge, burning discomfort, photophobia, and blurring of vision. Corneal involvement can progress to corneal neovascularization and punctate or macroepithelial erosions and ulcerations, which increase the risk for corneal scarring and visual impairment.7
Keratoconjunctivitis is a complex inflammatory disease characterized by infiltration of the conjunctival epithelium by eosinophils, mast cells, and lymphocytes. On examination, patients frequently are found to have concurrent AD of the periorbital skin as well as papillary hypertrophy of the tarsal conjunctiva with accompanying fibrosis, which can lead to entropion (turning inward of the lid margins and lashes) in severe cases.7 Ophthalmic evaluation is strongly recommended for patients with AKC to control symptoms, to limit exacerbations, and to prevent sight-threatening inflammation leading to vision loss. Treatment can be challenging given the chronicity of the condition and may require multiple treatment arms. Conservative measures include cool compresses and treatment with ophthalmic eye drops containing antihistamines (ie, ketotifen 0.025% [available over-the-counter]) and mast cell stabilizers (ie, olopatadine ophthalmic solution 0.1%).8 Atopic keratoconjunctivitis exacerbations may require short-term use of topical steroids or calcineurin inhibitors, or systemic equivalents for refractory cases.6 Long-term maintenance therapy typically consists of proper eye hygiene and steroid-sparing agents that reduce ocular inflammation, such as topical cyclosporine and tacrolimus, neither of which are associated with increased intraocular pressure (IOP)(Figure 1).8 Cornea disease resulting from chronic conjunctival/lid microtrauma can be managed with soft or scleral contact lenses.
Keratoconus
Keratoconus is a noninflammatory ocular disorder characterized by progressive thinning and conelike protrusion of the cornea. The corneal topographic changes result in high irregular astigmatism and reduced visual acuity, which can manifest as image blurring or distortion (Figure 2).2,9 Multiple case series and controlled studies have reported a positive association between keratoconus and a history of atopic disease.10,11
The precise etiology of keratoconus in the context of AD is unclear and likely is multifactorial. Habitual eye rubbing from periocular pruritus and discomfort has been reported to be a notable contributor to keratoconus.12 In addition, intrinsic inflammation and imbalance of cytokines and proteases also may contribute to development of keratoconus.13
Keratoconus is a progressive condition that can severely impact vision, making it critical to diagnose patients before irreversible vision loss occurs. Individuals with risk factors, such as AD of the eyelids, history of eye rubbing, or family history of keratoconus, should be advised to receive routine vision screening for worsening astigmatism, especially during the first few decades of life when keratoconus progresses rapidly.
The conservative management for early keratoconus includes glasses and gas-permeable contact lenses for correction of visual acuity and astigmatism. For advanced keratoconus, scleral lenses often are prescribed. These large-diameter, gas-permeable lenses are designed to rest on the sclera and arch over the entire cornea.9 Alternatively, corneal collagen cross-linking is a newer technique that utilizes riboflavin and UVA irradiation to strengthen the corneal tissue. It has proven to be safe and effective in slowing or stopping the progression of keratoconus, particularly when treated at the early stage, and received US Food and Drug Administration approval in 2016.9
Glaucoma
Glaucoma is a well-known complication of AD and can lead to irreversible ocular hypertension and optic nerve damage. Corticosteroid use is a major risk factor for glaucoma, and the rise in IOP is thought to be due to increased aqueous outflow resistance.14
Multiple case reports have linked glaucoma to long-term use of potent topical corticosteroids in the facial and palpebral regions, which has been attributed to direct steroid contact and absorption by ocular tissues, as glaucoma rarely occurs with topical steroid application elsewhere on the body.15-17 Systemic steroids (ie, prednisolone) taken for more than 8 weeks also have been associated with a marked rise in IOP.18
Certain risk factors may predispose a steroid user to increased IOP, including existing open-angle glaucoma, diabetes mellitus, collagen disease, and high myopia.15,19 Steroid responders and younger individuals also demonstrate increased sensitivity to steroids.20
Given that glaucoma often is asymptomatic until advanced stages, early detection is the key for proper intervention. Periodic glaucoma screening by an ophthalmologist would be appropriate for known steroid responders, as well as patients with a prolonged history of topical steroid application in the palpebral region and systemic steroid use, family history of glaucoma, or known ocular pathology.21 Furthermore, patients with concurrent glaucoma and AD should be jointly managed by dermatology and ophthalmology, and systemic and topical corticosteroid use should be minimized in favor of alterative agents such as calcineurin inhibitors.22
In addition to steroid-induced glaucoma, intrinsic atopic glaucoma recently has been proposed as a clinical entity and is characterized by increased inflammatory cytokines—IL-8 and CCL2—in the aqueous humor and abnormal accumulation of fibers in corneoscleral meshwork.23
Cataracts
Cataracts are estimated to affect 8% to 25% of patients with AD.21,24 Unlike age-related cataracts, cataracts associated with AD are observed in adolescents and young adults in addition to the older population. The progression of lenticular opacity can rapidly occur and has been reported to coincide with AD flares.25,26
Patients with AD typically present with anterior or posterior subcapsular cataracts instead of nuclear and cortical cataracts, which are more common in the general population.27,28 Anterior subcapsular cataracts are more specific to AD, whereas posterior subcapsular cataracts are associated with both prolonged corticosteroid use and AD.26 Children generally are more sensitive to steroids than adults and may develop cataracts more rapidly and at lower concentrations.29
The pathophysiology of cataract formation and progression in the context of AD is multifactorial. Cataract patients with AD have compromised blood-retinal barrier integrity as well as increased oxidative damage in the lens.30,31 Genetics and blunt trauma from eye rubbing are thought to play a role, and the latter has been associated with faster progression of cataracts.28 In contrast, corticosteroid-induced cataracts likely are caused by transcriptional changes and disrupted osmotic balance in the lens fibers, which can lead to fiber rupture and lens opacification.26,32 Systemic corticosteroids show the strongest association with cataract development, but inhaled and topical steroids also have been implicated.26
Although cataracts can be surgically corrected, prevention is critical. Patients with early-onset periorbital AD, prolonged use of topical or systemic corticosteroids, and family history of cataracts should be routinely screened. Anterior and posterior subcapsular cataracts are diagnosed with red reflex examinations that can be readily performed by the primary care physician or ophthalmologist.33 Atopic dermatitis patients with cataracts should be advised to use calcineurin inhibitors and alternative treatments in place of corticosteroids.
Retinal Detachment
Retinal detachment (RD) is a serious complication of AD that can present in individuals younger than 35 years. The incidence of RD in patients with AD has been estimated to be 4% to 8%.34 Retinal detachment manifests with visual disturbances such as flashing lights, shadows, visual field defect, and blurring of vision, but also may occur in the absence of vision changes.35,36
Across multiple case series, patients who developed RD were consistently found to have AD in the facial or periorbital region and a history of chronic eye rubbing. Multiple patients also presented with concurrent proliferative vitreoretinopathy, lens subluxation, and/or cataracts.35,37 The mechanism for RD has been attributed to ocular contusion from vigorous eye rubbing, as fundus findings between traumatic and AD-associated RD are similarly characterized by tractional breaks in the retina at vitreous base borders.37
Avoidance of eye rubbing and optimized treatment of facial AD may help prevent RD in patients with AD. Furthermore, all patients with symptoms of RD should be immediately referred to ophthalmology for surgical repair.
Herpetic Ocular Disease
Ocular herpes simplex virus infections cause ocular pain and are associated with notable visual morbidity, as recurrences can result in irreversible corneal scarring and neovascularization. Two retrospective case-control studies independently reported that individuals with a history of AD are at greater risk for herpetic ocular disease compared to age-matched controls.38,39 Furthermore, atopic disease is associated with higher recurrence rates and slower regeneration of the corneal epithelium.40
These findings suggest that AD patients with a history of recurrent herpetic ocular diseases should be closely monitored and treated with antiviral prophylaxis and/or topical corticosteroids, depending on the type of keratitis (epithelial or stromal).40 Furthermore, active ocular herpetic infections warrant urgent referral to an ophthalmologist.
Dupilumab-Associated Ocular Complications
Dupilumab, a monoclonal antibody that blocks IL-4 and IL-13 signaling, is the first biologic therapy to be approved for treatment of moderate to severe AD. Prior clinical trials have described a higher incidence of anterior conjunctivitis in dupilumab-treated AD patients (5%–28%) compared to placebo (2%–11%).41 Of note, the incidence may be as high as 70%, as reported in a recent case series.42 Interestingly, independent trials assessing dupilumab treatment in asthma, nasal polyposis, and eosinophilic esophagitis patients did not observe a higher incidence of conjunctivitis in dupilumab-treated patients compared to placebo, suggesting an AD-specific mechanism.43
Prominent features of dupilumab-associated conjunctivitis include hyperemia of the conjunctiva and limbus, in addition to ocular symptoms such as tearing, burning, and bilateral decrease in visual acuity. Marked reduction of conjunctival goblet cells has been reported.44 In addition to conjunctivitis, blepharitis also has been reported during dupilumab treatment.45
Standardized treatment guidelines for dupilumab-associated ocular complications have not yet been established. Surprisingly, antihistamine eye drops appear to be inefficacious in the treatment of dupilumab-associated conjunctivitis.41 However, the condition has been successfully managed with topical steroids (fluorometholone ophthalmic suspension 0.1%) and tacrolimus ointment 0.03%.41 Lifitegrast, an anti-inflammatory agent approved for chronic dry eye, also has been suggested as a treatment option for patients refractory to topical steroids.45 Alternatively, cessation of dupilumab could be considered in AD patients who experience severe ocular complications. Atopic dermatitis patients taking dupilumab who have any concerning signs for ocular complications should be referred to an ophthalmologist for further diagnosis and management.
Conclusion
Practicing dermatologists likely will encounter patients with concurrent AD and ocular complications. Although eye examinations are not routinely performed in the care of AD patients, dermatologists can proactively inquire about ocular symptoms and monitor patients longitudinally. Early diagnosis and treatment of these ocular conditions can prevent vision loss in these patients. Furthermore, symptomatic control of AD and careful consideration of the side-effect profiles of medications can potentially reduce the incidence of ocular complications in individuals with AD.
Patients with visual concerns or risk factors, such as a history of vigorous eye rubbing or chronic corticosteroid use, should be jointly managed with an ophthalmologist for optimized care. Moreover, acute exacerbations of ocular symptoms and visual deterioration warrant urgent referral to ophthalmology.
Atopic dermatitis (AD) is a chronic inflammatory skin condition with a lifetime prevalence of 15% to 20% in industrialized countries.1 It affects both children and adults and is predominantly characterized by a waxing and waning course of eczematous skin lesions and pruritus. In recent years, there is increasing recognition that AD can present with extracutaneous findings. Large-scale epidemiologic studies have reported a notably higher prevalence of ophthalmic complications in the AD population compared to the general population, in a severity-dependent manner.2,3 Potential complications include blepharitis, keratoconjunctivitis, keratoconus, glaucoma, cataracts, retinal detachment, ophthalmic herpes simplex virus infections, and dupilumab-associated ocular complications.
The etiology of each ocular complication in the context of AD is complex and likely multifactorial. Intrinsic immune dysregulation, physical trauma from eye rubbing, AD medication side effects, and genetics all have been speculated to play a role.2 Some of these ocular complications have a chronic course, while others present with sudden onset of symptoms; many of them can result in visual impairment if undiagnosed or left untreated. This article reviews several of the most common ocular comorbidities associated with AD. We discuss the clinical presentation, pathophysiology, and management strategies for each condition.
Blepharitis
Blepharitis, an inflammatory condition of the eyelids, is estimated to affect more than 6% of patients with AD compared to less than 1% of the general population.2 Blepharitis can be classified as anterior or posterior, based on the anatomic location of the affected region relative to the lash margin. Affected individuals may experience pruritus and irritation of the eyelids, tearing, a foreign body or burning sensation, crusting of the eyelids, and photophobia.4 Anterior blepharitis commonly is due to staphylococcal disease, and posterior blepharitis is secondary to structural changes and obstruction of meibomian gland orifices.
Although the pathophysiology is not well defined, xerosis in atopic patients is accompanied by barrier disruption and transepidermal water loss, which promote eyelid skin inflammation.
The mainstay of therapy for atopic blepharitis consists of conventional lid hygiene regimens, such as warm compresses and gentle scrubbing of the lid margins to remove crust and debris, which can be done with nonprescription cleansers, pads, and baby shampoos. Acute exacerbations may require topical antibiotics (ie, erythromycin or bacitracin applied to the lid margins once daily), topical calcineurin inhibitors (ie, cyclosporine ophthalmic emulsion 0.05%), or low-potency topical corticosteroids (ie, fluorometholone 0.1% or loteprednol etabonate 0.5% ophthalmic suspensions).5 Due to potential side effects of medications, especially topical corticosteroids, patients should be referred to ophthalmologists for definitive diagnosis and treatment.
Keratoconjunctivitis
Atopic keratoconjunctivitis (AKC) is a noninfectious inflammatory condition of the cornea and conjunctiva that occurs in an estimated 25% to 42% of patients with AD.6,7 It frequently presents in late adolescence and has a peak incidence between 30 and 50 years of age.8 The symptoms of AKC include ocular pruritus, redness, ropy mucoid discharge, burning discomfort, photophobia, and blurring of vision. Corneal involvement can progress to corneal neovascularization and punctate or macroepithelial erosions and ulcerations, which increase the risk for corneal scarring and visual impairment.7
Keratoconjunctivitis is a complex inflammatory disease characterized by infiltration of the conjunctival epithelium by eosinophils, mast cells, and lymphocytes. On examination, patients frequently are found to have concurrent AD of the periorbital skin as well as papillary hypertrophy of the tarsal conjunctiva with accompanying fibrosis, which can lead to entropion (turning inward of the lid margins and lashes) in severe cases.7 Ophthalmic evaluation is strongly recommended for patients with AKC to control symptoms, to limit exacerbations, and to prevent sight-threatening inflammation leading to vision loss. Treatment can be challenging given the chronicity of the condition and may require multiple treatment arms. Conservative measures include cool compresses and treatment with ophthalmic eye drops containing antihistamines (ie, ketotifen 0.025% [available over-the-counter]) and mast cell stabilizers (ie, olopatadine ophthalmic solution 0.1%).8 Atopic keratoconjunctivitis exacerbations may require short-term use of topical steroids or calcineurin inhibitors, or systemic equivalents for refractory cases.6 Long-term maintenance therapy typically consists of proper eye hygiene and steroid-sparing agents that reduce ocular inflammation, such as topical cyclosporine and tacrolimus, neither of which are associated with increased intraocular pressure (IOP)(Figure 1).8 Cornea disease resulting from chronic conjunctival/lid microtrauma can be managed with soft or scleral contact lenses.
Keratoconus
Keratoconus is a noninflammatory ocular disorder characterized by progressive thinning and conelike protrusion of the cornea. The corneal topographic changes result in high irregular astigmatism and reduced visual acuity, which can manifest as image blurring or distortion (Figure 2).2,9 Multiple case series and controlled studies have reported a positive association between keratoconus and a history of atopic disease.10,11
The precise etiology of keratoconus in the context of AD is unclear and likely is multifactorial. Habitual eye rubbing from periocular pruritus and discomfort has been reported to be a notable contributor to keratoconus.12 In addition, intrinsic inflammation and imbalance of cytokines and proteases also may contribute to development of keratoconus.13
Keratoconus is a progressive condition that can severely impact vision, making it critical to diagnose patients before irreversible vision loss occurs. Individuals with risk factors, such as AD of the eyelids, history of eye rubbing, or family history of keratoconus, should be advised to receive routine vision screening for worsening astigmatism, especially during the first few decades of life when keratoconus progresses rapidly.
The conservative management for early keratoconus includes glasses and gas-permeable contact lenses for correction of visual acuity and astigmatism. For advanced keratoconus, scleral lenses often are prescribed. These large-diameter, gas-permeable lenses are designed to rest on the sclera and arch over the entire cornea.9 Alternatively, corneal collagen cross-linking is a newer technique that utilizes riboflavin and UVA irradiation to strengthen the corneal tissue. It has proven to be safe and effective in slowing or stopping the progression of keratoconus, particularly when treated at the early stage, and received US Food and Drug Administration approval in 2016.9
Glaucoma
Glaucoma is a well-known complication of AD and can lead to irreversible ocular hypertension and optic nerve damage. Corticosteroid use is a major risk factor for glaucoma, and the rise in IOP is thought to be due to increased aqueous outflow resistance.14
Multiple case reports have linked glaucoma to long-term use of potent topical corticosteroids in the facial and palpebral regions, which has been attributed to direct steroid contact and absorption by ocular tissues, as glaucoma rarely occurs with topical steroid application elsewhere on the body.15-17 Systemic steroids (ie, prednisolone) taken for more than 8 weeks also have been associated with a marked rise in IOP.18
Certain risk factors may predispose a steroid user to increased IOP, including existing open-angle glaucoma, diabetes mellitus, collagen disease, and high myopia.15,19 Steroid responders and younger individuals also demonstrate increased sensitivity to steroids.20
Given that glaucoma often is asymptomatic until advanced stages, early detection is the key for proper intervention. Periodic glaucoma screening by an ophthalmologist would be appropriate for known steroid responders, as well as patients with a prolonged history of topical steroid application in the palpebral region and systemic steroid use, family history of glaucoma, or known ocular pathology.21 Furthermore, patients with concurrent glaucoma and AD should be jointly managed by dermatology and ophthalmology, and systemic and topical corticosteroid use should be minimized in favor of alterative agents such as calcineurin inhibitors.22
In addition to steroid-induced glaucoma, intrinsic atopic glaucoma recently has been proposed as a clinical entity and is characterized by increased inflammatory cytokines—IL-8 and CCL2—in the aqueous humor and abnormal accumulation of fibers in corneoscleral meshwork.23
Cataracts
Cataracts are estimated to affect 8% to 25% of patients with AD.21,24 Unlike age-related cataracts, cataracts associated with AD are observed in adolescents and young adults in addition to the older population. The progression of lenticular opacity can rapidly occur and has been reported to coincide with AD flares.25,26
Patients with AD typically present with anterior or posterior subcapsular cataracts instead of nuclear and cortical cataracts, which are more common in the general population.27,28 Anterior subcapsular cataracts are more specific to AD, whereas posterior subcapsular cataracts are associated with both prolonged corticosteroid use and AD.26 Children generally are more sensitive to steroids than adults and may develop cataracts more rapidly and at lower concentrations.29
The pathophysiology of cataract formation and progression in the context of AD is multifactorial. Cataract patients with AD have compromised blood-retinal barrier integrity as well as increased oxidative damage in the lens.30,31 Genetics and blunt trauma from eye rubbing are thought to play a role, and the latter has been associated with faster progression of cataracts.28 In contrast, corticosteroid-induced cataracts likely are caused by transcriptional changes and disrupted osmotic balance in the lens fibers, which can lead to fiber rupture and lens opacification.26,32 Systemic corticosteroids show the strongest association with cataract development, but inhaled and topical steroids also have been implicated.26
Although cataracts can be surgically corrected, prevention is critical. Patients with early-onset periorbital AD, prolonged use of topical or systemic corticosteroids, and family history of cataracts should be routinely screened. Anterior and posterior subcapsular cataracts are diagnosed with red reflex examinations that can be readily performed by the primary care physician or ophthalmologist.33 Atopic dermatitis patients with cataracts should be advised to use calcineurin inhibitors and alternative treatments in place of corticosteroids.
Retinal Detachment
Retinal detachment (RD) is a serious complication of AD that can present in individuals younger than 35 years. The incidence of RD in patients with AD has been estimated to be 4% to 8%.34 Retinal detachment manifests with visual disturbances such as flashing lights, shadows, visual field defect, and blurring of vision, but also may occur in the absence of vision changes.35,36
Across multiple case series, patients who developed RD were consistently found to have AD in the facial or periorbital region and a history of chronic eye rubbing. Multiple patients also presented with concurrent proliferative vitreoretinopathy, lens subluxation, and/or cataracts.35,37 The mechanism for RD has been attributed to ocular contusion from vigorous eye rubbing, as fundus findings between traumatic and AD-associated RD are similarly characterized by tractional breaks in the retina at vitreous base borders.37
Avoidance of eye rubbing and optimized treatment of facial AD may help prevent RD in patients with AD. Furthermore, all patients with symptoms of RD should be immediately referred to ophthalmology for surgical repair.
Herpetic Ocular Disease
Ocular herpes simplex virus infections cause ocular pain and are associated with notable visual morbidity, as recurrences can result in irreversible corneal scarring and neovascularization. Two retrospective case-control studies independently reported that individuals with a history of AD are at greater risk for herpetic ocular disease compared to age-matched controls.38,39 Furthermore, atopic disease is associated with higher recurrence rates and slower regeneration of the corneal epithelium.40
These findings suggest that AD patients with a history of recurrent herpetic ocular diseases should be closely monitored and treated with antiviral prophylaxis and/or topical corticosteroids, depending on the type of keratitis (epithelial or stromal).40 Furthermore, active ocular herpetic infections warrant urgent referral to an ophthalmologist.
Dupilumab-Associated Ocular Complications
Dupilumab, a monoclonal antibody that blocks IL-4 and IL-13 signaling, is the first biologic therapy to be approved for treatment of moderate to severe AD. Prior clinical trials have described a higher incidence of anterior conjunctivitis in dupilumab-treated AD patients (5%–28%) compared to placebo (2%–11%).41 Of note, the incidence may be as high as 70%, as reported in a recent case series.42 Interestingly, independent trials assessing dupilumab treatment in asthma, nasal polyposis, and eosinophilic esophagitis patients did not observe a higher incidence of conjunctivitis in dupilumab-treated patients compared to placebo, suggesting an AD-specific mechanism.43
Prominent features of dupilumab-associated conjunctivitis include hyperemia of the conjunctiva and limbus, in addition to ocular symptoms such as tearing, burning, and bilateral decrease in visual acuity. Marked reduction of conjunctival goblet cells has been reported.44 In addition to conjunctivitis, blepharitis also has been reported during dupilumab treatment.45
Standardized treatment guidelines for dupilumab-associated ocular complications have not yet been established. Surprisingly, antihistamine eye drops appear to be inefficacious in the treatment of dupilumab-associated conjunctivitis.41 However, the condition has been successfully managed with topical steroids (fluorometholone ophthalmic suspension 0.1%) and tacrolimus ointment 0.03%.41 Lifitegrast, an anti-inflammatory agent approved for chronic dry eye, also has been suggested as a treatment option for patients refractory to topical steroids.45 Alternatively, cessation of dupilumab could be considered in AD patients who experience severe ocular complications. Atopic dermatitis patients taking dupilumab who have any concerning signs for ocular complications should be referred to an ophthalmologist for further diagnosis and management.
Conclusion
Practicing dermatologists likely will encounter patients with concurrent AD and ocular complications. Although eye examinations are not routinely performed in the care of AD patients, dermatologists can proactively inquire about ocular symptoms and monitor patients longitudinally. Early diagnosis and treatment of these ocular conditions can prevent vision loss in these patients. Furthermore, symptomatic control of AD and careful consideration of the side-effect profiles of medications can potentially reduce the incidence of ocular complications in individuals with AD.
Patients with visual concerns or risk factors, such as a history of vigorous eye rubbing or chronic corticosteroid use, should be jointly managed with an ophthalmologist for optimized care. Moreover, acute exacerbations of ocular symptoms and visual deterioration warrant urgent referral to ophthalmology.
- Weidinger S, Beck LA, Bieber T, et al. Atopic dermatitis. Nat Rev Dis Primers. 2018;4:1.
- Govind K, Whang K, Khanna R, et al. Atopic dermatitis is associated with increased prevalence of multiple ocular comorbidities. J Allergy Clin Immunol Pract. 2019;7:298-299.
- Thyssen JP, Toft PB, Halling-Overgaard AS, et al. Incidence, prevalence, and risk of selected ocular disease in adults with atopic dermatitis. J Am Acad Dermatol. 2017;77:280-286.e281.
- Putnam CM. Diagnosis and management of blepharitis: an optometrist’s perspective. Clin Optom (Auckl). 2016;8:71-78.
- Amescua G, Akpek EK, Farid M, et al. Blepharitis Preferred Practice Pattern®. Ophthalmology. 2019;126:P56-P93.
- Bielory B, Bielory L. Atopic dermatitis and keratoconjunctivitis. Immunol Allergy Clin North Am. 2010;30:323-336.
- Guglielmetti S, Dart JK, Calder V. Atopic keratoconjunctivitis and atopic dermatitis. Curr Opin Allergy Clin Immunol. 2010;10:478-485.
- Chen JJ, Applebaum DS, Sun GS, et al. Atopic keratoconjunctivitis: a review. J Am Acad Dermatol. 2014;70:569-575.
- Andreanos KD, Hashemi K, Petrelli M, et al. Keratoconus treatment algorithm. Ophthalmol Ther. 2017;6:245-262.
- Rahi A, Davies P, Ruben M, et al. Keratoconus and coexisting atopic disease. Br J Ophthalmol. 1977;61:761-764.
- Gasset AR, Hinson WA, Frias JL. Keratoconus and atopic diseases. Ann Ophthalmol. 1978;10:991-994.
- Bawazeer AM, Hodge WG, Lorimer B. Atopy and keratoconus: a multivariate analysis. Br J Ophthalmol. 2000;84:834-836.
- Galvis V, Sherwin T, Tello A, et al. Keratoconus: an inflammatory disorder? Eye (Lond). 2015;29:843-859.
- Clark AF, Wordinger RJ. The role of steroids in outflow resistance. Exp Eye Res. 2009;88:752-759.
- Daniel BS, Orchard D. Ocular side-effects of topical corticosteroids: what a dermatologist needs to know. Australas J Dermatol. 2015;56:164-169.
- Garrott HM, Walland MJ. Glaucoma from topical corticosteroids to the eyelids. Clin Exp Ophthalmol. 2004;32:224-226.
- Aggarwal RK, Potamitis T, Chong NH, et al. Extensive visual loss with topical facial steroids. Eye (Lond). 1993;7(pt 5):664-666.
- Mandapati JS, Metta AK. Intraocular pressure variation in patients on long-term corticosteroids. Indian Dermatol Online J. 2011;2:67-69.
- Jones R 3rd, Rhee DJ. Corticosteroid-induced ocular hypertension and glaucoma: a brief review and update of the literature. Curr Opin Ophthalmol. 2006;17:163-167.
- Tamagawa-Mineoka R, Yasuoka N, Ueta M, et al. Influence of topical steroids on intraocular pressure in patients with atopic dermatitis. Allergol Int. 2018;67:388-391.
- Bercovitch L. Screening for ocular complications in atopic dermatitis. Arch Dermatol. 2011;147:588-589.
- Abramovits W, Hung P, Tong KB. Efficacy and economics of topical calcineurin inhibitors for the treatment of atopic dermatitis. Am J Clin Dermatol. 2006;7:213-222.
- Takakuwa K, Hamanaka T, Mori K, et al. Atopic glaucoma: clinical and pathophysiological analysis. J Glaucoma. 2015;24:662-668.
- Haeck IM, Rouwen TJ, Timmer-de Mik L, et al. Topical corticosteroids in atopic dermatitis and the risk of glaucoma and cataracts. J Am Acad Dermatol. 2011;64:275-281.
- Amemiya T, Matsuda H, Uehara M. Ocular findings in atopic dermatitis with special reference to the clinical features of atopic cataract. Ophthalmologica. 1980;180:129-132.
- Tatham A. Atopic dermatitis, cutaneous steroids and cataracts in children: two case reports. J Med Case Rep. 2008;2:124.
- Chew M, Chiang PP, Zheng Y, et al. The impact of cataract, cataract types, and cataract grades on vision-specific functioning using Rasch analysis. Am J Ophthalmol. 2012;154:29-38.
- Nagaki Y, Hayasaka S, Kadoi C. Cataract progression in patients with atopic dermatitis. J Cataract Refract Surg. 1999;25:96-99.
- Kaye LD, Kalenak JW, Price RL, et al. Ocular implications of long-term prednisone therapy in children. J Pediatr Ophthalmol Strabismus. 1993;30:142-144.
- Matsuo T, Saito H, Matsuo N. Cataract and aqueous flare levels in patients with atopic dermatitis. Am J Ophthalmol. 1997;124:36-39.
- Namazi MR, Handjani F, Amirahmadi M. Increased oxidative activity from hydrogen peroxide may be the cause of the predisposition to cataracts among patients with atopic dermatitis. Med Hypotheses. 2006;66:863-864.
- James ER. The etiology of steroid cataract. J Ocul Pharmacol Ther. 2007;23:403-420.
- Lambert SR, Teng JMC. Assessing whether the cataracts associated with atopic dermatitis are associated with steroids or inflammatory factors. JAMA Ophthalmol. 2018;136:918-919.
- Sasoh M, Mizutani H, Matsubara H, et al. Incidence of retinal detachment associated with atopic dermatitis in Japan: review of cases from 1992 to 2011. Clin Ophthalmol. 2015;9:1129-1134.
- Yoneda K, Okamoto H, Wada Y, et al. Atopic retinal detachment. report of four cases and a review of the literature. Br J Dermatol. 1995;133:586-591.
- Gnana Jothi V, McGimpsey S, Sharkey JA, et al. Retinal detachment repair and cataract surgery in patients with atopic dermatitis. Eye (Lond). 2017;31:1296-1301.
- Oka C, Ideta H, Nagasaki H, et al. Retinal detachment with atopic dermatitis similar to traumatic retinal detachment. Ophthalmology. 1994;101:1050-1054.
- Prabriputaloong T, Margolis TP, Lietman TM, et al. Atopic disease and herpes simplex eye disease: a population-based case-control study. Am J Ophthalmol. 2006;142:745-749.
- Borkar DS, Gonzales JA, Tham VM, et al. Association between atopy and herpetic eye disease: results from the pacific ocular inflammation study. JAMA Ophthalmol. 2014;132:326-331.
- Rezende RA, Hammersmith K, Bisol T, et al. Comparative study of ocular herpes simplex virus in patients with and without self-reported atopy. Am J Ophthalmol. 2006;141:1120-1125.
- Wollenberg A, Ariens L, Thurau S, et al. Conjunctivitis occurring in atopic dermatitis patients treated with dupilumab-clinical characteristics and treatment. J Allergy Clin Immunol Pract. 2018;6:1778-1780.e1.
- Ivert LU, Wahlgren CF, Ivert L, et al. Eye complications during dupilumab treatment for severe atopic dermatitis. Acta Derm Venereol. 2019;99:375-378.
- Akinlade B, Guttman-Yassky E, de Bruin-Weller M, et al. Conjunctivitis in dupilumab clinical trials [published online March 9, 2019]. Br J Dermatol. doi:10.1111/bjd.17869.
- Bakker DS, Ariens LFM, van Luijk C, et al. Goblet cell scarcity and conjunctival inflammation during treatment with dupilumab in patients with atopic dermatitis. Br J Dermatol. 2019;180:1248-1249.
- Zirwas MJ, Wulff K, Beckman K. Lifitegrast add-on treatment for dupilumab-induced ocular surface disease (DIOSD): a novel case report. JAAD Case Rep. 2019;5:34-36.
- Weidinger S, Beck LA, Bieber T, et al. Atopic dermatitis. Nat Rev Dis Primers. 2018;4:1.
- Govind K, Whang K, Khanna R, et al. Atopic dermatitis is associated with increased prevalence of multiple ocular comorbidities. J Allergy Clin Immunol Pract. 2019;7:298-299.
- Thyssen JP, Toft PB, Halling-Overgaard AS, et al. Incidence, prevalence, and risk of selected ocular disease in adults with atopic dermatitis. J Am Acad Dermatol. 2017;77:280-286.e281.
- Putnam CM. Diagnosis and management of blepharitis: an optometrist’s perspective. Clin Optom (Auckl). 2016;8:71-78.
- Amescua G, Akpek EK, Farid M, et al. Blepharitis Preferred Practice Pattern®. Ophthalmology. 2019;126:P56-P93.
- Bielory B, Bielory L. Atopic dermatitis and keratoconjunctivitis. Immunol Allergy Clin North Am. 2010;30:323-336.
- Guglielmetti S, Dart JK, Calder V. Atopic keratoconjunctivitis and atopic dermatitis. Curr Opin Allergy Clin Immunol. 2010;10:478-485.
- Chen JJ, Applebaum DS, Sun GS, et al. Atopic keratoconjunctivitis: a review. J Am Acad Dermatol. 2014;70:569-575.
- Andreanos KD, Hashemi K, Petrelli M, et al. Keratoconus treatment algorithm. Ophthalmol Ther. 2017;6:245-262.
- Rahi A, Davies P, Ruben M, et al. Keratoconus and coexisting atopic disease. Br J Ophthalmol. 1977;61:761-764.
- Gasset AR, Hinson WA, Frias JL. Keratoconus and atopic diseases. Ann Ophthalmol. 1978;10:991-994.
- Bawazeer AM, Hodge WG, Lorimer B. Atopy and keratoconus: a multivariate analysis. Br J Ophthalmol. 2000;84:834-836.
- Galvis V, Sherwin T, Tello A, et al. Keratoconus: an inflammatory disorder? Eye (Lond). 2015;29:843-859.
- Clark AF, Wordinger RJ. The role of steroids in outflow resistance. Exp Eye Res. 2009;88:752-759.
- Daniel BS, Orchard D. Ocular side-effects of topical corticosteroids: what a dermatologist needs to know. Australas J Dermatol. 2015;56:164-169.
- Garrott HM, Walland MJ. Glaucoma from topical corticosteroids to the eyelids. Clin Exp Ophthalmol. 2004;32:224-226.
- Aggarwal RK, Potamitis T, Chong NH, et al. Extensive visual loss with topical facial steroids. Eye (Lond). 1993;7(pt 5):664-666.
- Mandapati JS, Metta AK. Intraocular pressure variation in patients on long-term corticosteroids. Indian Dermatol Online J. 2011;2:67-69.
- Jones R 3rd, Rhee DJ. Corticosteroid-induced ocular hypertension and glaucoma: a brief review and update of the literature. Curr Opin Ophthalmol. 2006;17:163-167.
- Tamagawa-Mineoka R, Yasuoka N, Ueta M, et al. Influence of topical steroids on intraocular pressure in patients with atopic dermatitis. Allergol Int. 2018;67:388-391.
- Bercovitch L. Screening for ocular complications in atopic dermatitis. Arch Dermatol. 2011;147:588-589.
- Abramovits W, Hung P, Tong KB. Efficacy and economics of topical calcineurin inhibitors for the treatment of atopic dermatitis. Am J Clin Dermatol. 2006;7:213-222.
- Takakuwa K, Hamanaka T, Mori K, et al. Atopic glaucoma: clinical and pathophysiological analysis. J Glaucoma. 2015;24:662-668.
- Haeck IM, Rouwen TJ, Timmer-de Mik L, et al. Topical corticosteroids in atopic dermatitis and the risk of glaucoma and cataracts. J Am Acad Dermatol. 2011;64:275-281.
- Amemiya T, Matsuda H, Uehara M. Ocular findings in atopic dermatitis with special reference to the clinical features of atopic cataract. Ophthalmologica. 1980;180:129-132.
- Tatham A. Atopic dermatitis, cutaneous steroids and cataracts in children: two case reports. J Med Case Rep. 2008;2:124.
- Chew M, Chiang PP, Zheng Y, et al. The impact of cataract, cataract types, and cataract grades on vision-specific functioning using Rasch analysis. Am J Ophthalmol. 2012;154:29-38.
- Nagaki Y, Hayasaka S, Kadoi C. Cataract progression in patients with atopic dermatitis. J Cataract Refract Surg. 1999;25:96-99.
- Kaye LD, Kalenak JW, Price RL, et al. Ocular implications of long-term prednisone therapy in children. J Pediatr Ophthalmol Strabismus. 1993;30:142-144.
- Matsuo T, Saito H, Matsuo N. Cataract and aqueous flare levels in patients with atopic dermatitis. Am J Ophthalmol. 1997;124:36-39.
- Namazi MR, Handjani F, Amirahmadi M. Increased oxidative activity from hydrogen peroxide may be the cause of the predisposition to cataracts among patients with atopic dermatitis. Med Hypotheses. 2006;66:863-864.
- James ER. The etiology of steroid cataract. J Ocul Pharmacol Ther. 2007;23:403-420.
- Lambert SR, Teng JMC. Assessing whether the cataracts associated with atopic dermatitis are associated with steroids or inflammatory factors. JAMA Ophthalmol. 2018;136:918-919.
- Sasoh M, Mizutani H, Matsubara H, et al. Incidence of retinal detachment associated with atopic dermatitis in Japan: review of cases from 1992 to 2011. Clin Ophthalmol. 2015;9:1129-1134.
- Yoneda K, Okamoto H, Wada Y, et al. Atopic retinal detachment. report of four cases and a review of the literature. Br J Dermatol. 1995;133:586-591.
- Gnana Jothi V, McGimpsey S, Sharkey JA, et al. Retinal detachment repair and cataract surgery in patients with atopic dermatitis. Eye (Lond). 2017;31:1296-1301.
- Oka C, Ideta H, Nagasaki H, et al. Retinal detachment with atopic dermatitis similar to traumatic retinal detachment. Ophthalmology. 1994;101:1050-1054.
- Prabriputaloong T, Margolis TP, Lietman TM, et al. Atopic disease and herpes simplex eye disease: a population-based case-control study. Am J Ophthalmol. 2006;142:745-749.
- Borkar DS, Gonzales JA, Tham VM, et al. Association between atopy and herpetic eye disease: results from the pacific ocular inflammation study. JAMA Ophthalmol. 2014;132:326-331.
- Rezende RA, Hammersmith K, Bisol T, et al. Comparative study of ocular herpes simplex virus in patients with and without self-reported atopy. Am J Ophthalmol. 2006;141:1120-1125.
- Wollenberg A, Ariens L, Thurau S, et al. Conjunctivitis occurring in atopic dermatitis patients treated with dupilumab-clinical characteristics and treatment. J Allergy Clin Immunol Pract. 2018;6:1778-1780.e1.
- Ivert LU, Wahlgren CF, Ivert L, et al. Eye complications during dupilumab treatment for severe atopic dermatitis. Acta Derm Venereol. 2019;99:375-378.
- Akinlade B, Guttman-Yassky E, de Bruin-Weller M, et al. Conjunctivitis in dupilumab clinical trials [published online March 9, 2019]. Br J Dermatol. doi:10.1111/bjd.17869.
- Bakker DS, Ariens LFM, van Luijk C, et al. Goblet cell scarcity and conjunctival inflammation during treatment with dupilumab in patients with atopic dermatitis. Br J Dermatol. 2019;180:1248-1249.
- Zirwas MJ, Wulff K, Beckman K. Lifitegrast add-on treatment for dupilumab-induced ocular surface disease (DIOSD): a novel case report. JAAD Case Rep. 2019;5:34-36.
Practice Points
- Atopic dermatitis (AD) is associated with various ocular comorbidities that can result in permanent vision loss if untreated.
- Timely recognition of ocular complications in AD patients is critical, and dermatologists should proactively inquire about ocular symptoms in the review of systems.
- Patients with ocular symptoms should be jointly managed with ophthalmology.
Allergic Contact Dermatitis From Sorbitans in Beer and Bread
Sorbitan sesquioleate (SSO), sorbitan monooleate (SMO), and related compounds are increasingly recognized contact allergens. Sorbitan sesquioleate and SMO are nonionic emulsifying agents derived from sorbitol.1
Sorbitan sesquioleate, SMO, and other sorbitol derivatives are used as emulsifiers and dispersing agents in cosmetics, topical medications, topical emollients, produce, and other commercial products. Related compounds also are found in foods such as apples, berries, cherries, and sucrose-free cakes and cookies.1 We present a case of allergic contact dermatitis (ACD) with positive patch testing to sorbitans and clinical correlation with beer and bread exposure.
Case Report
A 62-year-old man presented with a persistent pruritic rash of 6 months’ duration. Erythematous eczematous papules and plaques were observed on the face, neck, chest, abdomen, back, and upper and lower extremities, affecting approximately 60% of the body surface area. His current list of medications was reviewed and included a multivitamin, fish oil, and vitamin C. A punch biopsy revealed spongiotic dermatitis with eosinophils. Patch testing using the North American Contact Dermatitis Group Standard Series with supplemental allergens found in toiletries revealed a positive reaction to SSO and SMO that was persistent at 48 and 96 hours. Notably, patch testing for sodium benzoate, nickel, potassium dichromate, and balsam of Peru were negative. Investigation into the personal care products the patient used identified the presence of sorbitol solution in Vanicream bar soap and Vanicream moisturizing cream (Pharmaceutical Specialties Inc). These products were started after the development of the rash and were discontinued after positive patch testing, but the patient continued to experience the eruption with no improvement.
Retrospectively, the patient was able to correlate exacerbations with drinking beer and eating sandwiches. He habitually ate a sandwich on the same type of bread every single day and enjoyed the same brand of beer 2 to 4 times per week without much variation. To limit allergens, the patient gave up the daily sandwich and avoided bread altogether, noting remarkable clinical improvement over a few weeks. Later, he described even more improvement while on a trip where he did not have access to his usual beer. The eruption recurred when he returned home and excessively indulged in his favorite beer. He also noted recurrence with exposure to certain breads. No new lesions developed with avoidance of beer and bread, and he had less than 1% body surface area involvement at 2-month follow-up and 0% involvement at 1 year. For educational purposes, follow-up patch testing was performed using Vanicream sorbitol solution and the specific beer and bread the patient consumed. The Vanicream solution was obtained from the manufacturer. The beer was placed directly onto a test disc. The bread was moistened with a drop of saline and then placed directly onto a test disc. All were negative at 48 and 96 hours.
Comment
Sorbitol Ingredients
We report a case of systemic ACD with a positive patch test to sorbitans that was exacerbated with consumption of beer and bread and resolved with avoidance of these products. Although it was determined that the patient used personal care products containing a sorbitol solution, discontinuation did not result in clinical improvement. Sorbitol, sorbitans, and sorbitol derivatives are not commonly reported in the ingredient lists of foods such as beer and bread. Both beer and bread are created with the addition of yeast cultures, for fermentation in beer and for leavening in bread. Sorbitol is used as an osmotic stabilizer in the preparation of yeast strains2 and also is a by-product of fermentation by certain bacteria3 found in beer. Additionally, review of commercially available preparations of baker’s and brewer’s yeasts, such as Fleischmann’s and Red Star, list sorbitan monostearate in the ingredients.4-7 We propose that trace amounts are present in the yeast preparations for brewing and baking.
In this case, the offending beer and bread were locally made products (Abita Beer, Covington, Louisiana; Leidenheimer Bread, New Orleans, Louisiana). Both companies were unable to share their yeast sources, limiting our ability to confirm the use of sorbitol in their preparation. We hypothesize that if sorbitol is commonly used in yeast culture preparation and can be a by-product of fermentation, then it is present in trace amounts in many beers and breads and is not specific to these two products.
Contact Allergy
There are few prior reports of ACD due to beer. A case series in 1969 described 4 patients with positive patch testing to ethanol and alcohol by-products and clinical resolution with avoidance of alcohol.8 Another case from 1985 described ACD to beer where patch testing was positive to the beer itself.9 Other published cases of cutaneous reactions to beer demonstrated immediate-type hypersensitivity resulting from both ingestion and skin contact, which is thought to be caused by IgE antibodies to malt and barley proteins.10,11
It is important to distinguish between systemic ACD and oral allergy syndrome (OAS). Although the defining features and criteria for diagnosing OAS have not been officially established, OAS is an IgE-mediated immune reaction commonly described as itching, tingling, or swelling, usually confined to the oral cavity after recent consumption of foods such as raw fruits, vegetables, and nuts.12 Oral allergy syndrome is treated with antihistamines and avoidance of known food allergens. In comparison, ACD is a type IV hypersensitivity, delayed cell-mediated reaction, commonly presenting with widespread rash.
Occupational contact dermatitis is common in bakers and food handlers and is more often irritant than allergic. Several relevant allergens have been identified in these groups13,14 and do not include sorbitans; our patient tested positive to both SSO and SMO. Sorbitan sesquioleate and SMO have been increasingly recognized as contact allergens over the last several years, both as standalone allergens and as potential cross-reactors.1 Sorbitan sesquioleate, SMO, and other sorbitol derivatives are found in cosmetics, topical and oral medications, topical emollients, produce, and other commercial products, including but not limited to topical clindamycin, topical metronidazole, topical ketoconazole, tazarotene cream 0.05% and 0.1%, toothpastes, acetaminophen maximum strength liquid, apples, berries, and sucrose-free cakes and cookies.1,15,16
In 2014, a study evaluated 12 oral antihistamines as potential sources for systemic contact allergens; 55% of these 12 oral antihistamine preparations included at least 1 of 10 allergen groups specifically identified. The sorbitans and sorbitol derivatives group ranked highest among the group of allergens found listed in these oral medications.17
Most patients found to have a contact allergy to the products containing SSO, SMO, or sorbitol derivatives reported notable improvement with discontinuation and change to sorbitol-free product use.1,18 It should be noted that SSO is added as an emulsifier to many of the fragrances used for patch testing. A positive patch test to fragrance mix without concomitant sorbitan testing may incorrectly diagnose the allergen.19
Patients with atopic dermatitis, particularly those with a filaggrin mutation, are at increased risk for ACD to sorbitans due to a compromised skin barrier and frequent use of topical steroids. In one study, 75% of patients (n=12) with a positive patch test to SSO were using a topical steroid emulsified with sorbitol or sorbitan derivatives.19
Conclusion
Sorbitan sesquioleate and SMO are increasingly relevant contact allergens. Sorbitol and related substances have been identified in numerous products and may be present in yeast-fermented and leavened goods. When patch testing is positive to SSO and SMO, the dermatologist should inquire about dietary habits with specific attention to beer and bread, in addition to inventorying other dietary preferences, prescription and over-the-counter medications, and personal care products. We suggest dietary considerations only if topical exposures have been eliminated and the rash has not improved.
- Asarch A, Scheinman PL. Sorbitan sesquioleate: an emerging contact allergen. Dermatitis. 2008;19:339-341.
- Lundblad V, Struhl K. Yeast. In: Adelman K, Ausubel F, Brent R, et al. Current Protocols in Molecular Biology, Supplement 64. New York, NY: John Wiley & Sons, Inc; 2008:13.0.1-13.0.4. https://onlinelibrary.wiley.com. Accessed August 19, 2019.
- Spitaels F, Wieme A, Balzarini T, et al. Gluconobacter cerevisiae sp. nov., isolated from the brewery environment. Int J Sys Evol Microbiol. 2014;64(pt 4):1134-1141.
- Fleischmann’s, n.d. Product Label for Rapid Rise Instant Yeast. Memphis, TN. 2017.
- Fleischmann’s, n.d. Product Label for Active Dry Yeast. Memphis, TN. 2017.
- Red Star, n.d. Product Label for Quick-Rise. Milwaukee, WI. 2017.
- Red Star, n.d. Product Label for Platinum Superior Baking Yeast. Milwaukee, WI. 2017.
- Fregert S, Groth O, Hjorth N, et al. Alcohol dermatitis. Acta Derm Venereol. 1969;49:493-497.
- Clarke P. Contact dermatitis due to beer. Med J Aust. 1985;143:92.
- Koelemij I, Van Zuuren EJ. Contact urticaria from beer. Clin Exp Dermatol. 2014;39:395-407.
- Santucci B, Cristaudo A, Cannistraci C, et al. Urticaria from beer in 3 patients. Contact Dermatitis. 1996;34:368.
- Kohn JB. What is oral allergy syndrome? J Acad Nutr Diet. 2017;117:988.
- Vincenzi C, Stinchi C, Ricci C, et al. Contact dermatitis due to an emulsifying agent in a baker. Contact Dermatitis. 1995;32:57.
- Nethercott JR, Holness DL. Occupational dermatitis in food handlers and bakers. J Am Acad Dermatol. 1989;21:485-490.
- Pereira F, Cunha H, Dias M. Contact dermatitis due to emulsifiers. Contact Dermatitis. 1997;36:114.
- Gao Z, Maurousset L, Lemoine R, et al. Cloning, expression, and characterization of sorbitol transporters from developing sour cherry fruit and leaf sink tissues. Plant Physiol. 2003;131:1566-1575.
- McEnery-Stonelake M, Silvestri DL. Contact allergens in oral antihistamines. Dermatitis. 2014;25:83-88.
- Asarch A, Scheinman PL. Sorbitan sesquioleate, a common emulsifier in topical steroids, is an important contact allergen. Dermatitis. 2008;19:323-327.
- Hald M, Menné T, Johansen JD, et al. Allergic contact dermatitis caused by sorbitan sesquioleate imitating severe glove dermatitis in a patient with filaggrin mutation. Contact Dermatitis. 2013;69:311-322.
Sorbitan sesquioleate (SSO), sorbitan monooleate (SMO), and related compounds are increasingly recognized contact allergens. Sorbitan sesquioleate and SMO are nonionic emulsifying agents derived from sorbitol.1
Sorbitan sesquioleate, SMO, and other sorbitol derivatives are used as emulsifiers and dispersing agents in cosmetics, topical medications, topical emollients, produce, and other commercial products. Related compounds also are found in foods such as apples, berries, cherries, and sucrose-free cakes and cookies.1 We present a case of allergic contact dermatitis (ACD) with positive patch testing to sorbitans and clinical correlation with beer and bread exposure.
Case Report
A 62-year-old man presented with a persistent pruritic rash of 6 months’ duration. Erythematous eczematous papules and plaques were observed on the face, neck, chest, abdomen, back, and upper and lower extremities, affecting approximately 60% of the body surface area. His current list of medications was reviewed and included a multivitamin, fish oil, and vitamin C. A punch biopsy revealed spongiotic dermatitis with eosinophils. Patch testing using the North American Contact Dermatitis Group Standard Series with supplemental allergens found in toiletries revealed a positive reaction to SSO and SMO that was persistent at 48 and 96 hours. Notably, patch testing for sodium benzoate, nickel, potassium dichromate, and balsam of Peru were negative. Investigation into the personal care products the patient used identified the presence of sorbitol solution in Vanicream bar soap and Vanicream moisturizing cream (Pharmaceutical Specialties Inc). These products were started after the development of the rash and were discontinued after positive patch testing, but the patient continued to experience the eruption with no improvement.
Retrospectively, the patient was able to correlate exacerbations with drinking beer and eating sandwiches. He habitually ate a sandwich on the same type of bread every single day and enjoyed the same brand of beer 2 to 4 times per week without much variation. To limit allergens, the patient gave up the daily sandwich and avoided bread altogether, noting remarkable clinical improvement over a few weeks. Later, he described even more improvement while on a trip where he did not have access to his usual beer. The eruption recurred when he returned home and excessively indulged in his favorite beer. He also noted recurrence with exposure to certain breads. No new lesions developed with avoidance of beer and bread, and he had less than 1% body surface area involvement at 2-month follow-up and 0% involvement at 1 year. For educational purposes, follow-up patch testing was performed using Vanicream sorbitol solution and the specific beer and bread the patient consumed. The Vanicream solution was obtained from the manufacturer. The beer was placed directly onto a test disc. The bread was moistened with a drop of saline and then placed directly onto a test disc. All were negative at 48 and 96 hours.
Comment
Sorbitol Ingredients
We report a case of systemic ACD with a positive patch test to sorbitans that was exacerbated with consumption of beer and bread and resolved with avoidance of these products. Although it was determined that the patient used personal care products containing a sorbitol solution, discontinuation did not result in clinical improvement. Sorbitol, sorbitans, and sorbitol derivatives are not commonly reported in the ingredient lists of foods such as beer and bread. Both beer and bread are created with the addition of yeast cultures, for fermentation in beer and for leavening in bread. Sorbitol is used as an osmotic stabilizer in the preparation of yeast strains2 and also is a by-product of fermentation by certain bacteria3 found in beer. Additionally, review of commercially available preparations of baker’s and brewer’s yeasts, such as Fleischmann’s and Red Star, list sorbitan monostearate in the ingredients.4-7 We propose that trace amounts are present in the yeast preparations for brewing and baking.
In this case, the offending beer and bread were locally made products (Abita Beer, Covington, Louisiana; Leidenheimer Bread, New Orleans, Louisiana). Both companies were unable to share their yeast sources, limiting our ability to confirm the use of sorbitol in their preparation. We hypothesize that if sorbitol is commonly used in yeast culture preparation and can be a by-product of fermentation, then it is present in trace amounts in many beers and breads and is not specific to these two products.
Contact Allergy
There are few prior reports of ACD due to beer. A case series in 1969 described 4 patients with positive patch testing to ethanol and alcohol by-products and clinical resolution with avoidance of alcohol.8 Another case from 1985 described ACD to beer where patch testing was positive to the beer itself.9 Other published cases of cutaneous reactions to beer demonstrated immediate-type hypersensitivity resulting from both ingestion and skin contact, which is thought to be caused by IgE antibodies to malt and barley proteins.10,11
It is important to distinguish between systemic ACD and oral allergy syndrome (OAS). Although the defining features and criteria for diagnosing OAS have not been officially established, OAS is an IgE-mediated immune reaction commonly described as itching, tingling, or swelling, usually confined to the oral cavity after recent consumption of foods such as raw fruits, vegetables, and nuts.12 Oral allergy syndrome is treated with antihistamines and avoidance of known food allergens. In comparison, ACD is a type IV hypersensitivity, delayed cell-mediated reaction, commonly presenting with widespread rash.
Occupational contact dermatitis is common in bakers and food handlers and is more often irritant than allergic. Several relevant allergens have been identified in these groups13,14 and do not include sorbitans; our patient tested positive to both SSO and SMO. Sorbitan sesquioleate and SMO have been increasingly recognized as contact allergens over the last several years, both as standalone allergens and as potential cross-reactors.1 Sorbitan sesquioleate, SMO, and other sorbitol derivatives are found in cosmetics, topical and oral medications, topical emollients, produce, and other commercial products, including but not limited to topical clindamycin, topical metronidazole, topical ketoconazole, tazarotene cream 0.05% and 0.1%, toothpastes, acetaminophen maximum strength liquid, apples, berries, and sucrose-free cakes and cookies.1,15,16
In 2014, a study evaluated 12 oral antihistamines as potential sources for systemic contact allergens; 55% of these 12 oral antihistamine preparations included at least 1 of 10 allergen groups specifically identified. The sorbitans and sorbitol derivatives group ranked highest among the group of allergens found listed in these oral medications.17
Most patients found to have a contact allergy to the products containing SSO, SMO, or sorbitol derivatives reported notable improvement with discontinuation and change to sorbitol-free product use.1,18 It should be noted that SSO is added as an emulsifier to many of the fragrances used for patch testing. A positive patch test to fragrance mix without concomitant sorbitan testing may incorrectly diagnose the allergen.19
Patients with atopic dermatitis, particularly those with a filaggrin mutation, are at increased risk for ACD to sorbitans due to a compromised skin barrier and frequent use of topical steroids. In one study, 75% of patients (n=12) with a positive patch test to SSO were using a topical steroid emulsified with sorbitol or sorbitan derivatives.19
Conclusion
Sorbitan sesquioleate and SMO are increasingly relevant contact allergens. Sorbitol and related substances have been identified in numerous products and may be present in yeast-fermented and leavened goods. When patch testing is positive to SSO and SMO, the dermatologist should inquire about dietary habits with specific attention to beer and bread, in addition to inventorying other dietary preferences, prescription and over-the-counter medications, and personal care products. We suggest dietary considerations only if topical exposures have been eliminated and the rash has not improved.
Sorbitan sesquioleate (SSO), sorbitan monooleate (SMO), and related compounds are increasingly recognized contact allergens. Sorbitan sesquioleate and SMO are nonionic emulsifying agents derived from sorbitol.1
Sorbitan sesquioleate, SMO, and other sorbitol derivatives are used as emulsifiers and dispersing agents in cosmetics, topical medications, topical emollients, produce, and other commercial products. Related compounds also are found in foods such as apples, berries, cherries, and sucrose-free cakes and cookies.1 We present a case of allergic contact dermatitis (ACD) with positive patch testing to sorbitans and clinical correlation with beer and bread exposure.
Case Report
A 62-year-old man presented with a persistent pruritic rash of 6 months’ duration. Erythematous eczematous papules and plaques were observed on the face, neck, chest, abdomen, back, and upper and lower extremities, affecting approximately 60% of the body surface area. His current list of medications was reviewed and included a multivitamin, fish oil, and vitamin C. A punch biopsy revealed spongiotic dermatitis with eosinophils. Patch testing using the North American Contact Dermatitis Group Standard Series with supplemental allergens found in toiletries revealed a positive reaction to SSO and SMO that was persistent at 48 and 96 hours. Notably, patch testing for sodium benzoate, nickel, potassium dichromate, and balsam of Peru were negative. Investigation into the personal care products the patient used identified the presence of sorbitol solution in Vanicream bar soap and Vanicream moisturizing cream (Pharmaceutical Specialties Inc). These products were started after the development of the rash and were discontinued after positive patch testing, but the patient continued to experience the eruption with no improvement.
Retrospectively, the patient was able to correlate exacerbations with drinking beer and eating sandwiches. He habitually ate a sandwich on the same type of bread every single day and enjoyed the same brand of beer 2 to 4 times per week without much variation. To limit allergens, the patient gave up the daily sandwich and avoided bread altogether, noting remarkable clinical improvement over a few weeks. Later, he described even more improvement while on a trip where he did not have access to his usual beer. The eruption recurred when he returned home and excessively indulged in his favorite beer. He also noted recurrence with exposure to certain breads. No new lesions developed with avoidance of beer and bread, and he had less than 1% body surface area involvement at 2-month follow-up and 0% involvement at 1 year. For educational purposes, follow-up patch testing was performed using Vanicream sorbitol solution and the specific beer and bread the patient consumed. The Vanicream solution was obtained from the manufacturer. The beer was placed directly onto a test disc. The bread was moistened with a drop of saline and then placed directly onto a test disc. All were negative at 48 and 96 hours.
Comment
Sorbitol Ingredients
We report a case of systemic ACD with a positive patch test to sorbitans that was exacerbated with consumption of beer and bread and resolved with avoidance of these products. Although it was determined that the patient used personal care products containing a sorbitol solution, discontinuation did not result in clinical improvement. Sorbitol, sorbitans, and sorbitol derivatives are not commonly reported in the ingredient lists of foods such as beer and bread. Both beer and bread are created with the addition of yeast cultures, for fermentation in beer and for leavening in bread. Sorbitol is used as an osmotic stabilizer in the preparation of yeast strains2 and also is a by-product of fermentation by certain bacteria3 found in beer. Additionally, review of commercially available preparations of baker’s and brewer’s yeasts, such as Fleischmann’s and Red Star, list sorbitan monostearate in the ingredients.4-7 We propose that trace amounts are present in the yeast preparations for brewing and baking.
In this case, the offending beer and bread were locally made products (Abita Beer, Covington, Louisiana; Leidenheimer Bread, New Orleans, Louisiana). Both companies were unable to share their yeast sources, limiting our ability to confirm the use of sorbitol in their preparation. We hypothesize that if sorbitol is commonly used in yeast culture preparation and can be a by-product of fermentation, then it is present in trace amounts in many beers and breads and is not specific to these two products.
Contact Allergy
There are few prior reports of ACD due to beer. A case series in 1969 described 4 patients with positive patch testing to ethanol and alcohol by-products and clinical resolution with avoidance of alcohol.8 Another case from 1985 described ACD to beer where patch testing was positive to the beer itself.9 Other published cases of cutaneous reactions to beer demonstrated immediate-type hypersensitivity resulting from both ingestion and skin contact, which is thought to be caused by IgE antibodies to malt and barley proteins.10,11
It is important to distinguish between systemic ACD and oral allergy syndrome (OAS). Although the defining features and criteria for diagnosing OAS have not been officially established, OAS is an IgE-mediated immune reaction commonly described as itching, tingling, or swelling, usually confined to the oral cavity after recent consumption of foods such as raw fruits, vegetables, and nuts.12 Oral allergy syndrome is treated with antihistamines and avoidance of known food allergens. In comparison, ACD is a type IV hypersensitivity, delayed cell-mediated reaction, commonly presenting with widespread rash.
Occupational contact dermatitis is common in bakers and food handlers and is more often irritant than allergic. Several relevant allergens have been identified in these groups13,14 and do not include sorbitans; our patient tested positive to both SSO and SMO. Sorbitan sesquioleate and SMO have been increasingly recognized as contact allergens over the last several years, both as standalone allergens and as potential cross-reactors.1 Sorbitan sesquioleate, SMO, and other sorbitol derivatives are found in cosmetics, topical and oral medications, topical emollients, produce, and other commercial products, including but not limited to topical clindamycin, topical metronidazole, topical ketoconazole, tazarotene cream 0.05% and 0.1%, toothpastes, acetaminophen maximum strength liquid, apples, berries, and sucrose-free cakes and cookies.1,15,16
In 2014, a study evaluated 12 oral antihistamines as potential sources for systemic contact allergens; 55% of these 12 oral antihistamine preparations included at least 1 of 10 allergen groups specifically identified. The sorbitans and sorbitol derivatives group ranked highest among the group of allergens found listed in these oral medications.17
Most patients found to have a contact allergy to the products containing SSO, SMO, or sorbitol derivatives reported notable improvement with discontinuation and change to sorbitol-free product use.1,18 It should be noted that SSO is added as an emulsifier to many of the fragrances used for patch testing. A positive patch test to fragrance mix without concomitant sorbitan testing may incorrectly diagnose the allergen.19
Patients with atopic dermatitis, particularly those with a filaggrin mutation, are at increased risk for ACD to sorbitans due to a compromised skin barrier and frequent use of topical steroids. In one study, 75% of patients (n=12) with a positive patch test to SSO were using a topical steroid emulsified with sorbitol or sorbitan derivatives.19
Conclusion
Sorbitan sesquioleate and SMO are increasingly relevant contact allergens. Sorbitol and related substances have been identified in numerous products and may be present in yeast-fermented and leavened goods. When patch testing is positive to SSO and SMO, the dermatologist should inquire about dietary habits with specific attention to beer and bread, in addition to inventorying other dietary preferences, prescription and over-the-counter medications, and personal care products. We suggest dietary considerations only if topical exposures have been eliminated and the rash has not improved.
- Asarch A, Scheinman PL. Sorbitan sesquioleate: an emerging contact allergen. Dermatitis. 2008;19:339-341.
- Lundblad V, Struhl K. Yeast. In: Adelman K, Ausubel F, Brent R, et al. Current Protocols in Molecular Biology, Supplement 64. New York, NY: John Wiley & Sons, Inc; 2008:13.0.1-13.0.4. https://onlinelibrary.wiley.com. Accessed August 19, 2019.
- Spitaels F, Wieme A, Balzarini T, et al. Gluconobacter cerevisiae sp. nov., isolated from the brewery environment. Int J Sys Evol Microbiol. 2014;64(pt 4):1134-1141.
- Fleischmann’s, n.d. Product Label for Rapid Rise Instant Yeast. Memphis, TN. 2017.
- Fleischmann’s, n.d. Product Label for Active Dry Yeast. Memphis, TN. 2017.
- Red Star, n.d. Product Label for Quick-Rise. Milwaukee, WI. 2017.
- Red Star, n.d. Product Label for Platinum Superior Baking Yeast. Milwaukee, WI. 2017.
- Fregert S, Groth O, Hjorth N, et al. Alcohol dermatitis. Acta Derm Venereol. 1969;49:493-497.
- Clarke P. Contact dermatitis due to beer. Med J Aust. 1985;143:92.
- Koelemij I, Van Zuuren EJ. Contact urticaria from beer. Clin Exp Dermatol. 2014;39:395-407.
- Santucci B, Cristaudo A, Cannistraci C, et al. Urticaria from beer in 3 patients. Contact Dermatitis. 1996;34:368.
- Kohn JB. What is oral allergy syndrome? J Acad Nutr Diet. 2017;117:988.
- Vincenzi C, Stinchi C, Ricci C, et al. Contact dermatitis due to an emulsifying agent in a baker. Contact Dermatitis. 1995;32:57.
- Nethercott JR, Holness DL. Occupational dermatitis in food handlers and bakers. J Am Acad Dermatol. 1989;21:485-490.
- Pereira F, Cunha H, Dias M. Contact dermatitis due to emulsifiers. Contact Dermatitis. 1997;36:114.
- Gao Z, Maurousset L, Lemoine R, et al. Cloning, expression, and characterization of sorbitol transporters from developing sour cherry fruit and leaf sink tissues. Plant Physiol. 2003;131:1566-1575.
- McEnery-Stonelake M, Silvestri DL. Contact allergens in oral antihistamines. Dermatitis. 2014;25:83-88.
- Asarch A, Scheinman PL. Sorbitan sesquioleate, a common emulsifier in topical steroids, is an important contact allergen. Dermatitis. 2008;19:323-327.
- Hald M, Menné T, Johansen JD, et al. Allergic contact dermatitis caused by sorbitan sesquioleate imitating severe glove dermatitis in a patient with filaggrin mutation. Contact Dermatitis. 2013;69:311-322.
- Asarch A, Scheinman PL. Sorbitan sesquioleate: an emerging contact allergen. Dermatitis. 2008;19:339-341.
- Lundblad V, Struhl K. Yeast. In: Adelman K, Ausubel F, Brent R, et al. Current Protocols in Molecular Biology, Supplement 64. New York, NY: John Wiley & Sons, Inc; 2008:13.0.1-13.0.4. https://onlinelibrary.wiley.com. Accessed August 19, 2019.
- Spitaels F, Wieme A, Balzarini T, et al. Gluconobacter cerevisiae sp. nov., isolated from the brewery environment. Int J Sys Evol Microbiol. 2014;64(pt 4):1134-1141.
- Fleischmann’s, n.d. Product Label for Rapid Rise Instant Yeast. Memphis, TN. 2017.
- Fleischmann’s, n.d. Product Label for Active Dry Yeast. Memphis, TN. 2017.
- Red Star, n.d. Product Label for Quick-Rise. Milwaukee, WI. 2017.
- Red Star, n.d. Product Label for Platinum Superior Baking Yeast. Milwaukee, WI. 2017.
- Fregert S, Groth O, Hjorth N, et al. Alcohol dermatitis. Acta Derm Venereol. 1969;49:493-497.
- Clarke P. Contact dermatitis due to beer. Med J Aust. 1985;143:92.
- Koelemij I, Van Zuuren EJ. Contact urticaria from beer. Clin Exp Dermatol. 2014;39:395-407.
- Santucci B, Cristaudo A, Cannistraci C, et al. Urticaria from beer in 3 patients. Contact Dermatitis. 1996;34:368.
- Kohn JB. What is oral allergy syndrome? J Acad Nutr Diet. 2017;117:988.
- Vincenzi C, Stinchi C, Ricci C, et al. Contact dermatitis due to an emulsifying agent in a baker. Contact Dermatitis. 1995;32:57.
- Nethercott JR, Holness DL. Occupational dermatitis in food handlers and bakers. J Am Acad Dermatol. 1989;21:485-490.
- Pereira F, Cunha H, Dias M. Contact dermatitis due to emulsifiers. Contact Dermatitis. 1997;36:114.
- Gao Z, Maurousset L, Lemoine R, et al. Cloning, expression, and characterization of sorbitol transporters from developing sour cherry fruit and leaf sink tissues. Plant Physiol. 2003;131:1566-1575.
- McEnery-Stonelake M, Silvestri DL. Contact allergens in oral antihistamines. Dermatitis. 2014;25:83-88.
- Asarch A, Scheinman PL. Sorbitan sesquioleate, a common emulsifier in topical steroids, is an important contact allergen. Dermatitis. 2008;19:323-327.
- Hald M, Menné T, Johansen JD, et al. Allergic contact dermatitis caused by sorbitan sesquioleate imitating severe glove dermatitis in a patient with filaggrin mutation. Contact Dermatitis. 2013;69:311-322.
Practice Points
- Sorbitan sesquioleate (SSO) and sorbitan monooleate (SMO) are increasingly relevant contact allergens that may be present in yeast-fermented and leavened products.
- When patch testing is positive to SSO and SMO, the dermatologist should inquire about dietary habits with specific attention to beer and bread.
- Consider elimination of beer, bread, and other leavened products when rash persists after avoidance of topical exposures.
What’s Eating You? Cat Flea (Ctenocephalides felis) Revisited
Fleas of the order Siphonaptera are insects that feed on the blood of a mammalian host. They have no wings but jump to near 150 times their body lengths to reach potential hosts.1 An epidemiologic survey performed in 2016 demonstrated that 96% of fleas in the United States are cat fleas (Ctenocephalides felis).2 The bites often present as pruritic, nonfollicular-based, excoriated papules; papular urticaria; or vesiculobullous lesions distributed across the lower legs. Antihistamines and topical steroids may be helpful for symptomatic relief, but flea eradication is key.
Identification
Ctenocephalides fleas, including the common cat flea and the dog flea, have a characteristic pronotal comb that resembles a mane of hair (Figure 1) and genal comb that resembles a mustache. Compared to the dog flea (Ctenocephalides canis), cat fleas have a flatter head and fewer hair-bearing notches on the dorsal hind tibia (the dog flea has 8 notches and the cat flea has 6 notches)(Figure 2).
Flea Prevention and Eradication
Effective management of flea bites requires avoidance of infested areas and eradication of fleas from the home and pets. Home treatment should be performed by a qualified specialist and a veterinarian should treat the pet, but the dermatologist must be knowledgeable about treatment options. Flea pupae can lie dormant between floorboards for extended periods of time and hatch rapidly when new tenants enter a house or apartment. Insecticidal dusts and spray formulations frequently are used to treat infested homes. It also is important to reduce flea egg numbers by vacuuming carpets and areas where pets sleep.3 Rodents often introduce fleas to households and pets, so eliminating them from the area may play an important role in flea control. Consulting with a veterinarian is important, as treatment directed at pets is critical to control flea populations. Oral agents, including fluralaner, afoxolaner, sarolaner, and spinosad, can reduce flea populations on animals by as much as 99.3% after 7 days.4,5 Fast-acting pulicidal agents, such as the combination of dinotefuran and fipronil, demonstrate curative activity as soon as 3 hours after treatment, which also may prevent reinfestation for as long as 6 weeks after treatment.6
Vector-Borne Disease
Fleas living on animals in close contact with humans, such as cats and dogs, can transmit zoonotic pathogens. Around 12,000 outpatients and 500 inpatients are diagnosed with cat scratch disease, a form of bartonellosis, annually. Ctenocephalides felis transmits Bartonella henselae from cat-to-cat and often cat-to-human through infected flea feces, causing a primary inoculation lesion and lymphadenitis. Of 3011 primary care providers surveyed from 2014 to 2015, 37.2% had treated at least 1 patient with cat scratch disease, yet knowledge gaps remain regarding the proper treatment and preventative measures for the disease.7 Current recommendations for the treatment of lymphadenitis caused by B henselae include a 5-day course of oral azithromycin.8 The preferred dosing regimen in adults is 500 mg on day 1 and 250 mg on days 2 through 5. Pediatric patients weighing less than 45.5 kg should receive 10 mg/kg on day 1 and 5 mg/kg on days 2 through 5.8 Additionally, less than one-third of the primary care providers surveyed from 2014 to 2015 said they would discuss the importance of pet flea control with immunocompromised patients who own cats, despite evidence implicating fleas in disease transmission.7 Pet-directed topical therapy with agents such as selamectin prescribed by a qualified veterinarian can prevent transmission of B henselae in cats exposed to fleas infected with the bacteria,9 which supports the importance of patient education and flea control, especially in pets owned by immunocompromised patients. Patients who are immunocompromised are at increased risk for persistent or disseminated bartonellosis, including endocarditis, in addition to cat scratch disease. Although arriving at a diagnosis may be difficult, one study found that bartonellosis in 13 renal transplant recipients was best diagnosed using both serology and polymerase chain reaction via DNA extraction of tissue specimens.10 These findings may enhance diagnostic yield for similar patients when bartonellosis is suspected.
Flea-borne typhus is endemic to Texas and Southern California.11,12 Evidence suggests that the pathogenic bacteria, Rickettsia typhi and Rickettsia felis, also commonly infect fleas in the Great Plains area.13 Opossums carry R felis, and the fleas transmit murine or endemic typhus. A retrospective case series in Texas identified 11 cases of fatal flea-borne typhus from 1985 to 2015.11 More than half of the patients reported contact with animals or fleas prior to the illness. Patients with typhus may present with fever, nausea, vomiting, rash (macular, maculopapular, papular, petechial, or morbilliform), respiratory or neurologic symptoms, thrombocytopenia, and elevated hepatic liver enzymes. Unfortunately, there often is a notable delay in initiation of treatment with the appropriate class of antibiotics—tetracyclines—and such delays can prove fatal.11 The current recommendation for nonpregnant adults is oral doxycycline 100 mg twice daily continued 48 hours after the patient becomes afebrile or for 7 days, whichever therapy duration is longer.14 Because of the consequences of delayed treatment, it is important for clinicians to consider a diagnosis of vector-borne illness in a febrile patient with other associated gastrointestinal, cutaneous, respiratory, or neurologic symptoms, especially if they have animal or flea exposures. Flea control and exposure awareness remains paramount in preventing and treating this illness.
Yersinia pestis causes the plague, an important re-emerging disease that causes infection through flea bites, inhalation, or ingestion.15 From 2000 to 2009, 56 cases and 7 deaths in the United States—New Mexico, Arizona, Colorado, California, and Texas—and 21,725 cases and 1612 deaths worldwide were attributed to Y pestis. Most patients present with the bubonic form of the disease, with fever and an enlarging painful femoral or inguinal lymph node due to leg flea bites.16 Other forms of disease, including septicemic and pneumonic plague, are less common but relevant, as one-third of cases in the United States present with septicemia.15,17,18 Although molecular diagnosis and immunohistochemistry play important roles, the diagnosis of Y pestis infection often is still accomplished with culture. A 2012 survey of 392 strains from 17 countries demonstrated that Y pestis remained susceptible to the antibiotics currently used to treat the disease, including doxycycline, streptomycin, gentamicin, tetracycline, trimethoprim-sulfamethoxazole, and ciprofloxacin.19
Human infection with Dipylidium caninum, a dog tapeworm, has been reported after suspected accidental ingestion of cat fleas carrying the parasite.20 Children, who may present with diarrhea or white worms in their feces, are more susceptible to the infection, perhaps due to accidental flea consumption while being licked by the pet.20,21
Conclusion
Cat fleas may act as a pruritic nuisance for pet owners and even deliver deadly pathogens to immunocompromised patients. Providers can minimize their impact by educating patients on flea prevention and eradication as well as astutely recognizing and treating flea-borne diseases.
- Cadiergues MC. A comparison of jump performances of the dog flea, Ctenocephalides canis (Curtis, 1826) and the cat flea, Ctenocephalides felis (Bouché, 1835). Vet Parasitol. 2000;92:239-241.
- Blagburn B, Butler J, Land T, et al. Who’s who and where: prevalence of Ctenocephalides felis and Ctenocephalides canis in shelter dogs and cats in the United States. Presented at: American Association of Veterinary Parasitologists 61st Annual Meeting; August 6-9, 2016; San Antonio, TX. P9.
- Bitam I, Dittmar K, Parola P, et al. Fleas and flea-borne diseases. Int J Infect Dis. 2010;14:E667-E676.
- Dryden MW, Canfield MS, Niedfeldt E, et al. Evaluation of sarolaner and spinosad oral treatments to eliminate fleas, reduce dermatologic lesions and minimize pruritus in naturally infested dogs in west Central Florida, USA. Parasit Vectors. 2017;10:389.
- Dryden MW, Canfield MS, Kalosy K, et al. Evaluation of fluralaner and afoxolaner treatments to control flea populations, reduce pruritus and minimize dermatologic lesions in naturally infested dogs in private residences in west Central Florida, USA. Parasit Vectors. 2016;9:365.
- Delcombel R, Karembe H, Nare B, et al. Synergy between dinotefuran and fipronil against the cat flea (Ctenocephalides felis): improved onset of action and residual speed of kill in adult cats. Parasit Vectors. 2017;10:341.
- Nelson CA, Moore AR, Perea AE, et al. Cat scratch disease: U.S. clinicians’ experience and knowledge. Zoonoses Public Health. 2018;65:67-73.
- Spach DH, Kaplan SL. Treatment of cat scratch disease. UpToDate. https://www.uptodate.com/contents/treatment-of-cat-scratch-disease?search=treatment%20of%20cat%20scratch&source=search_result&selectedTitle=1~59&usage_type=default&display_rank=1.Updated June 12, 2019. Accessed August 15, 2019.
- Bouhsira E, Franc M, Lienard E, et al. The efficacy of a selamectin (Stronghold®) spot on treatment in the prevention of Bartonella henselae transmission by Ctenocephalides felis in cats, using a new high-challenge model. Parasitol Res. 2015;114:1045-1050.
- Shamekhi Amiri F. Bartonellosis in chronic kidney disease: an unrecognized and unsuspected diagnosis. Ther Apher Dial. 2017;21:430-440.
- Pieracci EG, Evert N, Drexler NA, et al. Fatal flea-borne typhus in Texas: a retrospective case series, 1985-2015. American J Trop Med Hyg. 2017;96:1088-1093.
- Maina AN, Fogarty C, Krueger L, et al. Rickettsial infections among Ctenocephalides felis and host animals during a flea-borne rickettsioses outbreak in Orange County, California. PLoS One. 2016;11:e0160604.
- Noden BH, Davidson S, Smith JL, et al. First detection of Rickettsia typhi and Rickettsia felis in fleas collected from client-owned companion animals in the Southern Great Plains. J Med Entomol. 2017;54:1093-1097.
- Sexton DJ. Murine typhus. UpToDate. https://www.uptodate.com/contents/murine-typhus?search=diagnosis-and-treatment-of-murine-typhus&source=search_result&selectedTitle=1~21&usage_type=default&display_rank=1. Updated January 17, 2019. Accessed August 15, 2019.
- Riehm JM, Löscher T. Human plague and pneumonic plague: pathogenicity, epidemiology, clinical presentations and therapy [in German]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2015;58:721-729.
- Butler T. Plague gives surprises in the first decade of the 21st century in the United States and worldwide. Am J Trop Med Hyg. 2013;89:788-793.
- Gould LH, Pape J, Ettestad P, Griffith KS, et al. Dog-associated risk factors for human plague. Zoonoses Public Health. 2008;55:448-454.
- Margolis DA, Burns J, Reed SL, et al. Septicemic plague in a community hospital in California. Am J Trop Med Hyg. 2008;78:868-871.
- Urich SK, Chalcraft L, Schriefer ME, et al. Lack of antimicrobial resistance in Yersinia pestis isolates from 17 countries in the Americas, Africa, and Asia. Antimicrob Agents Chemother. 2012;56:555-558.
- Jiang P, Zhang X, Liu RD, et al. A human case of zoonotic dog tapeworm, Dipylidium caninum (Eucestoda: Dilepidiidae), in China. Korean J Parasitol. 2017;55:61-64.
- Roberts LS, Janovy J Jr, eds. Foundations of Parasitology. 8th ed. New York, NY: McGraw-Hill; 2009.
Fleas of the order Siphonaptera are insects that feed on the blood of a mammalian host. They have no wings but jump to near 150 times their body lengths to reach potential hosts.1 An epidemiologic survey performed in 2016 demonstrated that 96% of fleas in the United States are cat fleas (Ctenocephalides felis).2 The bites often present as pruritic, nonfollicular-based, excoriated papules; papular urticaria; or vesiculobullous lesions distributed across the lower legs. Antihistamines and topical steroids may be helpful for symptomatic relief, but flea eradication is key.
Identification
Ctenocephalides fleas, including the common cat flea and the dog flea, have a characteristic pronotal comb that resembles a mane of hair (Figure 1) and genal comb that resembles a mustache. Compared to the dog flea (Ctenocephalides canis), cat fleas have a flatter head and fewer hair-bearing notches on the dorsal hind tibia (the dog flea has 8 notches and the cat flea has 6 notches)(Figure 2).
Flea Prevention and Eradication
Effective management of flea bites requires avoidance of infested areas and eradication of fleas from the home and pets. Home treatment should be performed by a qualified specialist and a veterinarian should treat the pet, but the dermatologist must be knowledgeable about treatment options. Flea pupae can lie dormant between floorboards for extended periods of time and hatch rapidly when new tenants enter a house or apartment. Insecticidal dusts and spray formulations frequently are used to treat infested homes. It also is important to reduce flea egg numbers by vacuuming carpets and areas where pets sleep.3 Rodents often introduce fleas to households and pets, so eliminating them from the area may play an important role in flea control. Consulting with a veterinarian is important, as treatment directed at pets is critical to control flea populations. Oral agents, including fluralaner, afoxolaner, sarolaner, and spinosad, can reduce flea populations on animals by as much as 99.3% after 7 days.4,5 Fast-acting pulicidal agents, such as the combination of dinotefuran and fipronil, demonstrate curative activity as soon as 3 hours after treatment, which also may prevent reinfestation for as long as 6 weeks after treatment.6
Vector-Borne Disease
Fleas living on animals in close contact with humans, such as cats and dogs, can transmit zoonotic pathogens. Around 12,000 outpatients and 500 inpatients are diagnosed with cat scratch disease, a form of bartonellosis, annually. Ctenocephalides felis transmits Bartonella henselae from cat-to-cat and often cat-to-human through infected flea feces, causing a primary inoculation lesion and lymphadenitis. Of 3011 primary care providers surveyed from 2014 to 2015, 37.2% had treated at least 1 patient with cat scratch disease, yet knowledge gaps remain regarding the proper treatment and preventative measures for the disease.7 Current recommendations for the treatment of lymphadenitis caused by B henselae include a 5-day course of oral azithromycin.8 The preferred dosing regimen in adults is 500 mg on day 1 and 250 mg on days 2 through 5. Pediatric patients weighing less than 45.5 kg should receive 10 mg/kg on day 1 and 5 mg/kg on days 2 through 5.8 Additionally, less than one-third of the primary care providers surveyed from 2014 to 2015 said they would discuss the importance of pet flea control with immunocompromised patients who own cats, despite evidence implicating fleas in disease transmission.7 Pet-directed topical therapy with agents such as selamectin prescribed by a qualified veterinarian can prevent transmission of B henselae in cats exposed to fleas infected with the bacteria,9 which supports the importance of patient education and flea control, especially in pets owned by immunocompromised patients. Patients who are immunocompromised are at increased risk for persistent or disseminated bartonellosis, including endocarditis, in addition to cat scratch disease. Although arriving at a diagnosis may be difficult, one study found that bartonellosis in 13 renal transplant recipients was best diagnosed using both serology and polymerase chain reaction via DNA extraction of tissue specimens.10 These findings may enhance diagnostic yield for similar patients when bartonellosis is suspected.
Flea-borne typhus is endemic to Texas and Southern California.11,12 Evidence suggests that the pathogenic bacteria, Rickettsia typhi and Rickettsia felis, also commonly infect fleas in the Great Plains area.13 Opossums carry R felis, and the fleas transmit murine or endemic typhus. A retrospective case series in Texas identified 11 cases of fatal flea-borne typhus from 1985 to 2015.11 More than half of the patients reported contact with animals or fleas prior to the illness. Patients with typhus may present with fever, nausea, vomiting, rash (macular, maculopapular, papular, petechial, or morbilliform), respiratory or neurologic symptoms, thrombocytopenia, and elevated hepatic liver enzymes. Unfortunately, there often is a notable delay in initiation of treatment with the appropriate class of antibiotics—tetracyclines—and such delays can prove fatal.11 The current recommendation for nonpregnant adults is oral doxycycline 100 mg twice daily continued 48 hours after the patient becomes afebrile or for 7 days, whichever therapy duration is longer.14 Because of the consequences of delayed treatment, it is important for clinicians to consider a diagnosis of vector-borne illness in a febrile patient with other associated gastrointestinal, cutaneous, respiratory, or neurologic symptoms, especially if they have animal or flea exposures. Flea control and exposure awareness remains paramount in preventing and treating this illness.
Yersinia pestis causes the plague, an important re-emerging disease that causes infection through flea bites, inhalation, or ingestion.15 From 2000 to 2009, 56 cases and 7 deaths in the United States—New Mexico, Arizona, Colorado, California, and Texas—and 21,725 cases and 1612 deaths worldwide were attributed to Y pestis. Most patients present with the bubonic form of the disease, with fever and an enlarging painful femoral or inguinal lymph node due to leg flea bites.16 Other forms of disease, including septicemic and pneumonic plague, are less common but relevant, as one-third of cases in the United States present with septicemia.15,17,18 Although molecular diagnosis and immunohistochemistry play important roles, the diagnosis of Y pestis infection often is still accomplished with culture. A 2012 survey of 392 strains from 17 countries demonstrated that Y pestis remained susceptible to the antibiotics currently used to treat the disease, including doxycycline, streptomycin, gentamicin, tetracycline, trimethoprim-sulfamethoxazole, and ciprofloxacin.19
Human infection with Dipylidium caninum, a dog tapeworm, has been reported after suspected accidental ingestion of cat fleas carrying the parasite.20 Children, who may present with diarrhea or white worms in their feces, are more susceptible to the infection, perhaps due to accidental flea consumption while being licked by the pet.20,21
Conclusion
Cat fleas may act as a pruritic nuisance for pet owners and even deliver deadly pathogens to immunocompromised patients. Providers can minimize their impact by educating patients on flea prevention and eradication as well as astutely recognizing and treating flea-borne diseases.
Fleas of the order Siphonaptera are insects that feed on the blood of a mammalian host. They have no wings but jump to near 150 times their body lengths to reach potential hosts.1 An epidemiologic survey performed in 2016 demonstrated that 96% of fleas in the United States are cat fleas (Ctenocephalides felis).2 The bites often present as pruritic, nonfollicular-based, excoriated papules; papular urticaria; or vesiculobullous lesions distributed across the lower legs. Antihistamines and topical steroids may be helpful for symptomatic relief, but flea eradication is key.
Identification
Ctenocephalides fleas, including the common cat flea and the dog flea, have a characteristic pronotal comb that resembles a mane of hair (Figure 1) and genal comb that resembles a mustache. Compared to the dog flea (Ctenocephalides canis), cat fleas have a flatter head and fewer hair-bearing notches on the dorsal hind tibia (the dog flea has 8 notches and the cat flea has 6 notches)(Figure 2).
Flea Prevention and Eradication
Effective management of flea bites requires avoidance of infested areas and eradication of fleas from the home and pets. Home treatment should be performed by a qualified specialist and a veterinarian should treat the pet, but the dermatologist must be knowledgeable about treatment options. Flea pupae can lie dormant between floorboards for extended periods of time and hatch rapidly when new tenants enter a house or apartment. Insecticidal dusts and spray formulations frequently are used to treat infested homes. It also is important to reduce flea egg numbers by vacuuming carpets and areas where pets sleep.3 Rodents often introduce fleas to households and pets, so eliminating them from the area may play an important role in flea control. Consulting with a veterinarian is important, as treatment directed at pets is critical to control flea populations. Oral agents, including fluralaner, afoxolaner, sarolaner, and spinosad, can reduce flea populations on animals by as much as 99.3% after 7 days.4,5 Fast-acting pulicidal agents, such as the combination of dinotefuran and fipronil, demonstrate curative activity as soon as 3 hours after treatment, which also may prevent reinfestation for as long as 6 weeks after treatment.6
Vector-Borne Disease
Fleas living on animals in close contact with humans, such as cats and dogs, can transmit zoonotic pathogens. Around 12,000 outpatients and 500 inpatients are diagnosed with cat scratch disease, a form of bartonellosis, annually. Ctenocephalides felis transmits Bartonella henselae from cat-to-cat and often cat-to-human through infected flea feces, causing a primary inoculation lesion and lymphadenitis. Of 3011 primary care providers surveyed from 2014 to 2015, 37.2% had treated at least 1 patient with cat scratch disease, yet knowledge gaps remain regarding the proper treatment and preventative measures for the disease.7 Current recommendations for the treatment of lymphadenitis caused by B henselae include a 5-day course of oral azithromycin.8 The preferred dosing regimen in adults is 500 mg on day 1 and 250 mg on days 2 through 5. Pediatric patients weighing less than 45.5 kg should receive 10 mg/kg on day 1 and 5 mg/kg on days 2 through 5.8 Additionally, less than one-third of the primary care providers surveyed from 2014 to 2015 said they would discuss the importance of pet flea control with immunocompromised patients who own cats, despite evidence implicating fleas in disease transmission.7 Pet-directed topical therapy with agents such as selamectin prescribed by a qualified veterinarian can prevent transmission of B henselae in cats exposed to fleas infected with the bacteria,9 which supports the importance of patient education and flea control, especially in pets owned by immunocompromised patients. Patients who are immunocompromised are at increased risk for persistent or disseminated bartonellosis, including endocarditis, in addition to cat scratch disease. Although arriving at a diagnosis may be difficult, one study found that bartonellosis in 13 renal transplant recipients was best diagnosed using both serology and polymerase chain reaction via DNA extraction of tissue specimens.10 These findings may enhance diagnostic yield for similar patients when bartonellosis is suspected.
Flea-borne typhus is endemic to Texas and Southern California.11,12 Evidence suggests that the pathogenic bacteria, Rickettsia typhi and Rickettsia felis, also commonly infect fleas in the Great Plains area.13 Opossums carry R felis, and the fleas transmit murine or endemic typhus. A retrospective case series in Texas identified 11 cases of fatal flea-borne typhus from 1985 to 2015.11 More than half of the patients reported contact with animals or fleas prior to the illness. Patients with typhus may present with fever, nausea, vomiting, rash (macular, maculopapular, papular, petechial, or morbilliform), respiratory or neurologic symptoms, thrombocytopenia, and elevated hepatic liver enzymes. Unfortunately, there often is a notable delay in initiation of treatment with the appropriate class of antibiotics—tetracyclines—and such delays can prove fatal.11 The current recommendation for nonpregnant adults is oral doxycycline 100 mg twice daily continued 48 hours after the patient becomes afebrile or for 7 days, whichever therapy duration is longer.14 Because of the consequences of delayed treatment, it is important for clinicians to consider a diagnosis of vector-borne illness in a febrile patient with other associated gastrointestinal, cutaneous, respiratory, or neurologic symptoms, especially if they have animal or flea exposures. Flea control and exposure awareness remains paramount in preventing and treating this illness.
Yersinia pestis causes the plague, an important re-emerging disease that causes infection through flea bites, inhalation, or ingestion.15 From 2000 to 2009, 56 cases and 7 deaths in the United States—New Mexico, Arizona, Colorado, California, and Texas—and 21,725 cases and 1612 deaths worldwide were attributed to Y pestis. Most patients present with the bubonic form of the disease, with fever and an enlarging painful femoral or inguinal lymph node due to leg flea bites.16 Other forms of disease, including septicemic and pneumonic plague, are less common but relevant, as one-third of cases in the United States present with septicemia.15,17,18 Although molecular diagnosis and immunohistochemistry play important roles, the diagnosis of Y pestis infection often is still accomplished with culture. A 2012 survey of 392 strains from 17 countries demonstrated that Y pestis remained susceptible to the antibiotics currently used to treat the disease, including doxycycline, streptomycin, gentamicin, tetracycline, trimethoprim-sulfamethoxazole, and ciprofloxacin.19
Human infection with Dipylidium caninum, a dog tapeworm, has been reported after suspected accidental ingestion of cat fleas carrying the parasite.20 Children, who may present with diarrhea or white worms in their feces, are more susceptible to the infection, perhaps due to accidental flea consumption while being licked by the pet.20,21
Conclusion
Cat fleas may act as a pruritic nuisance for pet owners and even deliver deadly pathogens to immunocompromised patients. Providers can minimize their impact by educating patients on flea prevention and eradication as well as astutely recognizing and treating flea-borne diseases.
- Cadiergues MC. A comparison of jump performances of the dog flea, Ctenocephalides canis (Curtis, 1826) and the cat flea, Ctenocephalides felis (Bouché, 1835). Vet Parasitol. 2000;92:239-241.
- Blagburn B, Butler J, Land T, et al. Who’s who and where: prevalence of Ctenocephalides felis and Ctenocephalides canis in shelter dogs and cats in the United States. Presented at: American Association of Veterinary Parasitologists 61st Annual Meeting; August 6-9, 2016; San Antonio, TX. P9.
- Bitam I, Dittmar K, Parola P, et al. Fleas and flea-borne diseases. Int J Infect Dis. 2010;14:E667-E676.
- Dryden MW, Canfield MS, Niedfeldt E, et al. Evaluation of sarolaner and spinosad oral treatments to eliminate fleas, reduce dermatologic lesions and minimize pruritus in naturally infested dogs in west Central Florida, USA. Parasit Vectors. 2017;10:389.
- Dryden MW, Canfield MS, Kalosy K, et al. Evaluation of fluralaner and afoxolaner treatments to control flea populations, reduce pruritus and minimize dermatologic lesions in naturally infested dogs in private residences in west Central Florida, USA. Parasit Vectors. 2016;9:365.
- Delcombel R, Karembe H, Nare B, et al. Synergy between dinotefuran and fipronil against the cat flea (Ctenocephalides felis): improved onset of action and residual speed of kill in adult cats. Parasit Vectors. 2017;10:341.
- Nelson CA, Moore AR, Perea AE, et al. Cat scratch disease: U.S. clinicians’ experience and knowledge. Zoonoses Public Health. 2018;65:67-73.
- Spach DH, Kaplan SL. Treatment of cat scratch disease. UpToDate. https://www.uptodate.com/contents/treatment-of-cat-scratch-disease?search=treatment%20of%20cat%20scratch&source=search_result&selectedTitle=1~59&usage_type=default&display_rank=1.Updated June 12, 2019. Accessed August 15, 2019.
- Bouhsira E, Franc M, Lienard E, et al. The efficacy of a selamectin (Stronghold®) spot on treatment in the prevention of Bartonella henselae transmission by Ctenocephalides felis in cats, using a new high-challenge model. Parasitol Res. 2015;114:1045-1050.
- Shamekhi Amiri F. Bartonellosis in chronic kidney disease: an unrecognized and unsuspected diagnosis. Ther Apher Dial. 2017;21:430-440.
- Pieracci EG, Evert N, Drexler NA, et al. Fatal flea-borne typhus in Texas: a retrospective case series, 1985-2015. American J Trop Med Hyg. 2017;96:1088-1093.
- Maina AN, Fogarty C, Krueger L, et al. Rickettsial infections among Ctenocephalides felis and host animals during a flea-borne rickettsioses outbreak in Orange County, California. PLoS One. 2016;11:e0160604.
- Noden BH, Davidson S, Smith JL, et al. First detection of Rickettsia typhi and Rickettsia felis in fleas collected from client-owned companion animals in the Southern Great Plains. J Med Entomol. 2017;54:1093-1097.
- Sexton DJ. Murine typhus. UpToDate. https://www.uptodate.com/contents/murine-typhus?search=diagnosis-and-treatment-of-murine-typhus&source=search_result&selectedTitle=1~21&usage_type=default&display_rank=1. Updated January 17, 2019. Accessed August 15, 2019.
- Riehm JM, Löscher T. Human plague and pneumonic plague: pathogenicity, epidemiology, clinical presentations and therapy [in German]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2015;58:721-729.
- Butler T. Plague gives surprises in the first decade of the 21st century in the United States and worldwide. Am J Trop Med Hyg. 2013;89:788-793.
- Gould LH, Pape J, Ettestad P, Griffith KS, et al. Dog-associated risk factors for human plague. Zoonoses Public Health. 2008;55:448-454.
- Margolis DA, Burns J, Reed SL, et al. Septicemic plague in a community hospital in California. Am J Trop Med Hyg. 2008;78:868-871.
- Urich SK, Chalcraft L, Schriefer ME, et al. Lack of antimicrobial resistance in Yersinia pestis isolates from 17 countries in the Americas, Africa, and Asia. Antimicrob Agents Chemother. 2012;56:555-558.
- Jiang P, Zhang X, Liu RD, et al. A human case of zoonotic dog tapeworm, Dipylidium caninum (Eucestoda: Dilepidiidae), in China. Korean J Parasitol. 2017;55:61-64.
- Roberts LS, Janovy J Jr, eds. Foundations of Parasitology. 8th ed. New York, NY: McGraw-Hill; 2009.
- Cadiergues MC. A comparison of jump performances of the dog flea, Ctenocephalides canis (Curtis, 1826) and the cat flea, Ctenocephalides felis (Bouché, 1835). Vet Parasitol. 2000;92:239-241.
- Blagburn B, Butler J, Land T, et al. Who’s who and where: prevalence of Ctenocephalides felis and Ctenocephalides canis in shelter dogs and cats in the United States. Presented at: American Association of Veterinary Parasitologists 61st Annual Meeting; August 6-9, 2016; San Antonio, TX. P9.
- Bitam I, Dittmar K, Parola P, et al. Fleas and flea-borne diseases. Int J Infect Dis. 2010;14:E667-E676.
- Dryden MW, Canfield MS, Niedfeldt E, et al. Evaluation of sarolaner and spinosad oral treatments to eliminate fleas, reduce dermatologic lesions and minimize pruritus in naturally infested dogs in west Central Florida, USA. Parasit Vectors. 2017;10:389.
- Dryden MW, Canfield MS, Kalosy K, et al. Evaluation of fluralaner and afoxolaner treatments to control flea populations, reduce pruritus and minimize dermatologic lesions in naturally infested dogs in private residences in west Central Florida, USA. Parasit Vectors. 2016;9:365.
- Delcombel R, Karembe H, Nare B, et al. Synergy between dinotefuran and fipronil against the cat flea (Ctenocephalides felis): improved onset of action and residual speed of kill in adult cats. Parasit Vectors. 2017;10:341.
- Nelson CA, Moore AR, Perea AE, et al. Cat scratch disease: U.S. clinicians’ experience and knowledge. Zoonoses Public Health. 2018;65:67-73.
- Spach DH, Kaplan SL. Treatment of cat scratch disease. UpToDate. https://www.uptodate.com/contents/treatment-of-cat-scratch-disease?search=treatment%20of%20cat%20scratch&source=search_result&selectedTitle=1~59&usage_type=default&display_rank=1.Updated June 12, 2019. Accessed August 15, 2019.
- Bouhsira E, Franc M, Lienard E, et al. The efficacy of a selamectin (Stronghold®) spot on treatment in the prevention of Bartonella henselae transmission by Ctenocephalides felis in cats, using a new high-challenge model. Parasitol Res. 2015;114:1045-1050.
- Shamekhi Amiri F. Bartonellosis in chronic kidney disease: an unrecognized and unsuspected diagnosis. Ther Apher Dial. 2017;21:430-440.
- Pieracci EG, Evert N, Drexler NA, et al. Fatal flea-borne typhus in Texas: a retrospective case series, 1985-2015. American J Trop Med Hyg. 2017;96:1088-1093.
- Maina AN, Fogarty C, Krueger L, et al. Rickettsial infections among Ctenocephalides felis and host animals during a flea-borne rickettsioses outbreak in Orange County, California. PLoS One. 2016;11:e0160604.
- Noden BH, Davidson S, Smith JL, et al. First detection of Rickettsia typhi and Rickettsia felis in fleas collected from client-owned companion animals in the Southern Great Plains. J Med Entomol. 2017;54:1093-1097.
- Sexton DJ. Murine typhus. UpToDate. https://www.uptodate.com/contents/murine-typhus?search=diagnosis-and-treatment-of-murine-typhus&source=search_result&selectedTitle=1~21&usage_type=default&display_rank=1. Updated January 17, 2019. Accessed August 15, 2019.
- Riehm JM, Löscher T. Human plague and pneumonic plague: pathogenicity, epidemiology, clinical presentations and therapy [in German]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2015;58:721-729.
- Butler T. Plague gives surprises in the first decade of the 21st century in the United States and worldwide. Am J Trop Med Hyg. 2013;89:788-793.
- Gould LH, Pape J, Ettestad P, Griffith KS, et al. Dog-associated risk factors for human plague. Zoonoses Public Health. 2008;55:448-454.
- Margolis DA, Burns J, Reed SL, et al. Septicemic plague in a community hospital in California. Am J Trop Med Hyg. 2008;78:868-871.
- Urich SK, Chalcraft L, Schriefer ME, et al. Lack of antimicrobial resistance in Yersinia pestis isolates from 17 countries in the Americas, Africa, and Asia. Antimicrob Agents Chemother. 2012;56:555-558.
- Jiang P, Zhang X, Liu RD, et al. A human case of zoonotic dog tapeworm, Dipylidium caninum (Eucestoda: Dilepidiidae), in China. Korean J Parasitol. 2017;55:61-64.
- Roberts LS, Janovy J Jr, eds. Foundations of Parasitology. 8th ed. New York, NY: McGraw-Hill; 2009.
Practice Points
- Cat fleas classically cause pruritic grouped papulovesicles on the lower legs of pet owners.
- Affected patients require thorough education on flea eradication.
- Cat fleas can transmit endemic typhus, cat scratch disease, and bubonic plague.
Quality of Life in Patients With Atopic Dermatitis
Atopic dermatitis (AD) is a chronic, relapsing, inflammatory skin disease typically with childhood onset. In some cases, the condition persists, but AD usually resolves by the time a child reaches adulthood. Prevalence is difficult to estimate but, in developed countries, is approximately 15% to 30% among children and 2% to 10% among adults.1
Atopic dermatitis is characterized by chronically itchy dry skin, weeping erythematous papules and plaques, and lichenification. Furthermore, AD often is associated with other atopic diseases, such as food allergy, allergic rhinitis, and bronchial asthma.
In this article, we review the literature on the quality of life (QOL) of patients with AD. Our goals are to discuss the most common methods for measuring QOL in AD and how to use them; highlight specific alterations of QOL in AD; and review data about QOL of children with AD, which is underrepresented in the medical literature, as studies tend to focus on adults. In addition, we address the importance of assessing QOL in patients with AD due to the psychological burden of the disease.
Quality of Life
The harmful effects of AD can include a range of areas, including
Because QOL is an important instrument used in many AD studies, we call attention to the work of the Harmonising Outcome Measures for Eczema (HOME) initiative, which established a core outcome set for all AD clinical trials to enable comparison of results of individual studies.2 Quality of life was identified in HOME as one of 4 basic outcome measures that should be included in every AD trial (the others are clinician-reported signs, patient-reported symptoms, and long-term control).3 According to the recent agreement, the following QOL instruments should be used: Dermatology Life Quality Index (DLQI) for adults, Children’s Dermatology Life Quality Index (CDLQI) for children, and Infants’ Dermatitis Quality of Life Index (IDQOL) for infants.4
In dermatology, these instruments can be divided into 3 basic categories: generic, dermatology specific, and disease specific.5 Generic QOL questionnaires are beneficial when comparing the QOL of an AD patient to patients with other conditions or to healthy individuals. On the other hand, dermatology-specific and AD-specific methods are more effective instruments for detecting impairments linked directly to the disease and, therefore, are more sensitive to changes in QOL.5 Some of the most frequently used QOL measures5,6 for AD along with their key attributes are
Given that AD is a chronic disease that requires constant care, parents/guardians or the partner of the patient usually are affected as well. To detect this effect, the Family Dermatology Life Quality Index (FDLQI), a dermatology-specific instrument, measures the QOL in family members of dermatology patients.7 The Dermatitis Family Impact (DFI)8 is a disease-specific method for assessing how having a child with AD can impact the QOL of family members; it is a 10-item questionnaire completed by an adult family member. The FDLQI7 and DFI8 both help to understand the secondary impact of the disease.
In contrast, several other methods that also are administered by a parent/guardian assess how the parent perceives the QOL of their child with AD; these methods are essential for small children and infants who cannot answer questions themselves. The IDQOL9 was designed to assess the QOL of patients younger than 4 years using a parent-completed questionnaire. For older children and adolescents aged 4 to 16 years, the CDLQI10 is a widely used instrument; the questionnaire is completed by the child and is available in a cartoon format.10
For patients older than 16 years, 2 important instruments are the DLQI, a generic dermatology instrument, and the Quality of Life Index for Atopic Dermatitis (QoLIAD).11
Clearly it can be troublesome for researchers and clinicians to find the most suitable instrument to evaluate QOL in AD patients. To make this task easier, the European Academy of Dermatology and Venereology Task Force released a position paper with the following recommendations: (1) only validated instruments should be used, and (2) their use should be based on the age of the patients for which the instruments were designed. It is reommended that researchers use a combination of a generic and a dermatology-specific or AD-specific instrument, whereas clinicians should apply a dermatology-specific or AD-specific method, or both.5
Alterations of QOL in AD
Sleep Disturbance in AD
Sleep disorders observed in AD include difficulty falling asleep, frequent waking episodes, shorter sleep duration, and feelings of inadequate sleep, which often result in impairment of daily activity.12,13 Correlation has been found between sleep quality and QOL in both children and adults.14 Approximately 60% of children affected by AD experience a sleep disturbance,15 which seems to correlate well with disease severity.16 A US study found that adults with AD are more likely to experience a sleep disturbance, which often affects daytime functioning and work productivity.13
Financial Aspects and Impact on Work
The financial burden of AD is extensive.17 There are direct medical costs, including medication, visits to the physician, alternative therapies, and nonprescription products. Patients tend to spend relevant money on such items as moisturizers, bath products, antihistamines, topical steroids, and topical antibiotics.18,19 However, it seems that most of the cost of AD is due to indirect and nonmedical costs, including transportation to medical visits; loss of work days; extra childcare; and expenditures associated with lifestyle changes,19,20 such as modifying diet, wearing special clothes, using special bed linens, and purchasing special household items (eg, anti–dust mite vacuum cleaner, humidifier, new carpeting).17,19
Absenteeism from work often is a consequence of physician appointments; in addition, parents/guardians of a child with AD often miss work due to medical care. Even at work, patients (or parents/guardians) often experience decreased work productivity (so-called presenteeism) due to loss of sleep and anxiety.21 In addressing the effects of AD on work life, a systematic literature review found that AD strongly affects sick leave and might have an impact on job choice and change or loss of job.22
Furthermore, according to Su et al,23 the costs of AD are related to disease severity. Moreover, their data suggest that among chronic childhood diseases, the financial burden of AD is greater than the cost of asthma and similar to the cost of diabetes mellitus.23
Association Between QOL and Disease Severity
A large observational study found that improvement in AD severity was followed by an increase in QOL.24 A positive correlation between disease severity and QOL has been found in other studies,25,26 though no correlation or only moderate correlation also has been reported.27 Apparently, in addition to QOL, disease severity scores are substantial parameters in the evaluation of distress caused by AD; the HOME initiative has identified clinician-reported signs and patient-reported symptoms as 2 of 4 core outcomes domains to include in all future AD clinical trials.3 For measuring symptoms, the Patient-Oriented Eczema Measure (POEM) is the recommended instrument.28 Regarding clinical signs, the HOME group named the Eczema Area and Severity Index (EASI) as the preferred instrument.29
Psychological Burden
Stress is a triggering factor for AD, but the connection between skin and mind appears bidirectional. The biological reaction to stress probably lowers the itch threshold and disrupts the skin barrier.30 The Global Burden of Disease Study showed that skin diseases are the fourth leading cause of nonfatal disease burden.31 There are several factors—pruritus, scratch, and pain—that can all lead to sleep deprivation and daytime fatigue. Based on our experience, if lesions develop on visible areas, patients can feel stigmatized, which restricts their social life.
The most common psychological comorbidities of AD are anxiety and depression. In a cross-sectional, multicenter study, there was a significantly higher prevalence of depression (P<.001) and anxiety disorder (P=.02) among patients with common skin diseases compared to a control group.32 In a study that assessed AD patients, researchers found a higher risk of depression and anxiety.33 Suicidal ideation also is more common in the population with AD32,34; a study showed that the risk of suicidal ideation in adolescents was nearly 4-fold in patients with itching skin lesions compared to those without itch.34
According to Linnet and Jemec,35 mental and psychological comorbidities of AD are associated with lower QOL, not with clinical severity. As a result, to improve QOL in AD, one should take care of both dermatological and psychological problems. It has been demonstrated that psychological interventions, such as autogenic training, cognitive-behavioral therapy, relaxation techniques, habit reversal training,36 and hypnotherapy37 might be helpful in individual cases; educational interventions also are recommended.36 With these adjuvant therapies, psychological status, unpleasant clinical symptoms, and QOL could be improved, though further studies are needed to confirm these benefits.
Conclusion
Atopic dermatitis places a notable burden on patients and their families. The degree of burden is probably related to disease severity. For measuring QOL, researchers and clinicians should use validated methods suited to the age of the patients for which they were designed. More studies are needed to assess the effects of different treatments on QOL. Besides pharmacotherapy, psychotherapy and educational programs might be beneficial for improving QOL, another important area to be studied.
- Bieber T. Atopic dermatitis. N Engl J Med. 2008;358:1483-1494.
- Schmitt J, Williams H; HOME Development Group. Harmonising Outcome Measures for Eczema (HOME). report from the First International Consensus Meeting (HOME 1), 24 July 2010, Munich, Germany. Br J Dermatol. 2010;163:1166-1168.
- Schmitt J, Spuls P, Boers M, et al. Towards global consensus on outcome measures for atopic eczema research: results of the HOME II meeting. Allergy. 2012;67:1111-1117.
- Quality of Life (QoL). Harmonising Outcome Measures for Eczema (HOME) website. http://www.homeforeczema.org/research/quality-of-life.aspx. Accessed August 18, 2019.
Chernyshov PV, Tomas-Aragones L, Manolache L, et al; EADV Quality of Life Task Force. Quality of life measurement in atopic dermatitis. Position paper of the European Academy of Dermatology and Venereology (EADV) Task Force on quality of life. J Eur Acad Dermatol Venereol. 2017;31:576-593. - Hill MK, Kheirandish Pishkenari A, Braunberger TL, et al. Recent trends in disease severity and quality of life instruments for patients with atopic dermatitis: a systematic review. J Am Acad Dermatol. 2016;75:906-917.
- Basra MK, Sue-Ho R, Finlay AY. The Family Dermatology Life Quality Index: measuring the secondary impact of skin disease. Br J Dermatol. 2007;156:528-538.
- Dodington SR, Basra MK, Finlay AY, et al. The Dermatitis Family Impact questionnaire: a review of its measurement properties and clinical application. Br J Dermatol. 2013;169:31-46.
- Lewis-Jones MS, Finlay AY, Dykes PJ. The Infants’ Dermatitis Quality of Life Index. Br J Dermatol. 2001;144:104-110.
- Holme SA, Man I, Sharpe JL, et al. The Children’s Dermatology Life Quality Index: validation of the cartoon version. Br J Dermatol. 2003;148:285-290.
- Whalley D, McKenna SP, Dewar AL, et al. A new instrument for assessing quality of life in atopic dermatitis: international development of the Quality of Life Index for Atopic Dermatitis (QoLIAD). Br J Dermatol. 2004;150:274-283.
- Jeon C, Yan D, Nakamura M, et al. Frequency and management of sleep disturbance in adults with atopic dermatitis: a systematic review. Dermatol Ther (Heidelb). 2017;7:349-364.
- Yu SH, Attarian H, Zee P, et al. Burden of sleep and fatigue in US adults with atopic dermatitis. Dermatitis. 2016;27:50-58.
- Kong TS, Han TY, Lee JH, et al. Correlation between severity of atopic dermatitis and sleep quality in children and adults. Ann Dermatol. 2016;28:321-326.
- Fishbein AB, Mueller K, Kruse L, et al. Sleep disturbance in children with moderate/severe atopic dermatitis: a case-control study. J Am Acad Dermatol. 2018;78:336-341.
- Chamlin SL, Mattson CL, Frieden IJ, et al. The price of pruritus: sleep disturbance and cosleeping in atopic dermatitis. Arch Pediatr Adolesc Med. 2005;159:745-750.
- Emerson RM, Williams HC, Allen BR. What is the cost of atopic dermatitis in preschool children? Br J Dermatol. 2001;144:514-522.
- Filanovsky MG, Pootongkam S, Tamburro JE, et al. The financial and emotional impact of atopic dermatitis on children and their families. J Pediatr. 2016;169:284-290.
- Fivenson D, Arnold RJ, Kaniecki DJ, et al. The effect of atopic dermatitis on total burden of illness and quality of life on adults and children in a large managed care organization. J Manag Care Pharm. 2002;8:333-342.
- Carroll CL, Balkrishnan R, Feldman SR, et al. The burden of atopic dermatitis: impact on the patient, family, and society. Pediatr Dermatol. 2005;22:192-199.
- Drucker AM, Wang AR, Qureshi AA. Research gaps in quality of life and economic burden of atopic dermatitis: the National Eczema Association Burden of Disease Audit. JAMA Dermatol. 2016;152:873-874.
- Nørreslet LB, Ebbehøj NE, Ellekilde Bonde JP, et al. The impact of atopic dermatitis on work life—a systematic review. J Eur Acad Dermatol Venereol. 2018;32:23-38.
- Su JC, Kemp AS, Varigos GA, et al. Atopic eczema: its impact on the family and financial cost. Arch Dis Child. 1997;76:159-162.
- Coutanceau C, Stalder JF. Analysis of correlations between patient-oriented SCORAD (PO-SCORAD) and other assessment scores of atopic dermatitis severity and quality of life. Dermatology. 2014;229:248-255.
- Ben-Gashir MA, Seed PT, Hay RJ. Quality of life and disease severity are correlated in children with atopic dermatitis. Br J Dermatol. 2004;150:284-290.
- van Valburg RW, Willemsen MG, Dirven-Meijer PC, et al. Quality of life measurement and its relationship to disease severity in children with atopic dermatitis in general practice. Acta Derm Venereol. 2011;91:147-151.
- Haeck IM, ten Berge O, van Velsen SG, et al. Moderate correlation between quality of life and disease activity in adult patients with atopic dermatitis. J Eur Acad Dermatol Venereol. 2012;26:236-241.
- Spuls PI, Gerbens LAA, Simpson E, et al; HOME initiative collaborators. Patient-Oriented Eczema Measure (POEM), a core instrument to measure symptoms in clinical trials: a Harmonising Outcome Measures for Eczema (HOME) statement. Br J Dermatol. 2017;176:979-984.
- Schmitt J, Spuls PI, Thomas KS, et al; HOME initiative collaborators. The Harmonising Outcome Measures for Eczema (HOME) statement to assess clinical signs of atopic eczema in trials. J Allergy Clin Immunol. 2014;134:800-807.
- Oh SH, Bae BG, Park CO, et al. Association of stress with symptoms of atopic dermatitis. Acta Derm Venereol. 2010;90:582-588.
- Hay RJ, Johns NE, Williams HC, et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol. 2014;134:1527-1534.
- Dalgard FJ, Gieler U, Tomas-Aragones L, et al. The psychological burden of skin diseases: a cross-sectional multicenter study among dermatological out-patients in 13 European countries. J Invest Dermatol. 2015;135:984-991.
- Cheng CM, Hsu JW, Huang KL, et al. Risk of developing major depressive disorder and anxiety disorders among adolescents and adults with atopic dermatitis: a nationwide longitudinal study. J Affect Disord. 2015;178:60-65.
Halvorsen JA, Lien L, Dalgard F, et al. Suicidal ideation, mental health problems, and social function in adolescents with eczema: a population-based study. J Invest Dermatol. 2014;134:1847-1854. - Linnet J, Jemec GB. An assessment of anxiety and dermatology life quality in patients with atopic dermatitis. Br J Dermatol. 1999;140:268-272.
- Ring J, Alomar A, Bieber T, et al; European Dermatology Forum; European Academy of Dermatology and Venereology; European Task Force on Atopic Dermatitis; European Federation of Allergy; European Society of Pediatric Dermatology; Global Allergy and Asthma European Network. Guidelines for treatment of atopic eczema (atopic dermatitis) Part II. J Eur Acad Dermatol Venereol. 2012;26:1176-1193.
- Perczel K, Gál J. Hypnotherapy of atopic dermatitis in an adult. case report. Orv Hetil. 2016;157:111-115.
Atopic dermatitis (AD) is a chronic, relapsing, inflammatory skin disease typically with childhood onset. In some cases, the condition persists, but AD usually resolves by the time a child reaches adulthood. Prevalence is difficult to estimate but, in developed countries, is approximately 15% to 30% among children and 2% to 10% among adults.1
Atopic dermatitis is characterized by chronically itchy dry skin, weeping erythematous papules and plaques, and lichenification. Furthermore, AD often is associated with other atopic diseases, such as food allergy, allergic rhinitis, and bronchial asthma.
In this article, we review the literature on the quality of life (QOL) of patients with AD. Our goals are to discuss the most common methods for measuring QOL in AD and how to use them; highlight specific alterations of QOL in AD; and review data about QOL of children with AD, which is underrepresented in the medical literature, as studies tend to focus on adults. In addition, we address the importance of assessing QOL in patients with AD due to the psychological burden of the disease.
Quality of Life
The harmful effects of AD can include a range of areas, including
Because QOL is an important instrument used in many AD studies, we call attention to the work of the Harmonising Outcome Measures for Eczema (HOME) initiative, which established a core outcome set for all AD clinical trials to enable comparison of results of individual studies.2 Quality of life was identified in HOME as one of 4 basic outcome measures that should be included in every AD trial (the others are clinician-reported signs, patient-reported symptoms, and long-term control).3 According to the recent agreement, the following QOL instruments should be used: Dermatology Life Quality Index (DLQI) for adults, Children’s Dermatology Life Quality Index (CDLQI) for children, and Infants’ Dermatitis Quality of Life Index (IDQOL) for infants.4
In dermatology, these instruments can be divided into 3 basic categories: generic, dermatology specific, and disease specific.5 Generic QOL questionnaires are beneficial when comparing the QOL of an AD patient to patients with other conditions or to healthy individuals. On the other hand, dermatology-specific and AD-specific methods are more effective instruments for detecting impairments linked directly to the disease and, therefore, are more sensitive to changes in QOL.5 Some of the most frequently used QOL measures5,6 for AD along with their key attributes are
Given that AD is a chronic disease that requires constant care, parents/guardians or the partner of the patient usually are affected as well. To detect this effect, the Family Dermatology Life Quality Index (FDLQI), a dermatology-specific instrument, measures the QOL in family members of dermatology patients.7 The Dermatitis Family Impact (DFI)8 is a disease-specific method for assessing how having a child with AD can impact the QOL of family members; it is a 10-item questionnaire completed by an adult family member. The FDLQI7 and DFI8 both help to understand the secondary impact of the disease.
In contrast, several other methods that also are administered by a parent/guardian assess how the parent perceives the QOL of their child with AD; these methods are essential for small children and infants who cannot answer questions themselves. The IDQOL9 was designed to assess the QOL of patients younger than 4 years using a parent-completed questionnaire. For older children and adolescents aged 4 to 16 years, the CDLQI10 is a widely used instrument; the questionnaire is completed by the child and is available in a cartoon format.10
For patients older than 16 years, 2 important instruments are the DLQI, a generic dermatology instrument, and the Quality of Life Index for Atopic Dermatitis (QoLIAD).11
Clearly it can be troublesome for researchers and clinicians to find the most suitable instrument to evaluate QOL in AD patients. To make this task easier, the European Academy of Dermatology and Venereology Task Force released a position paper with the following recommendations: (1) only validated instruments should be used, and (2) their use should be based on the age of the patients for which the instruments were designed. It is reommended that researchers use a combination of a generic and a dermatology-specific or AD-specific instrument, whereas clinicians should apply a dermatology-specific or AD-specific method, or both.5
Alterations of QOL in AD
Sleep Disturbance in AD
Sleep disorders observed in AD include difficulty falling asleep, frequent waking episodes, shorter sleep duration, and feelings of inadequate sleep, which often result in impairment of daily activity.12,13 Correlation has been found between sleep quality and QOL in both children and adults.14 Approximately 60% of children affected by AD experience a sleep disturbance,15 which seems to correlate well with disease severity.16 A US study found that adults with AD are more likely to experience a sleep disturbance, which often affects daytime functioning and work productivity.13
Financial Aspects and Impact on Work
The financial burden of AD is extensive.17 There are direct medical costs, including medication, visits to the physician, alternative therapies, and nonprescription products. Patients tend to spend relevant money on such items as moisturizers, bath products, antihistamines, topical steroids, and topical antibiotics.18,19 However, it seems that most of the cost of AD is due to indirect and nonmedical costs, including transportation to medical visits; loss of work days; extra childcare; and expenditures associated with lifestyle changes,19,20 such as modifying diet, wearing special clothes, using special bed linens, and purchasing special household items (eg, anti–dust mite vacuum cleaner, humidifier, new carpeting).17,19
Absenteeism from work often is a consequence of physician appointments; in addition, parents/guardians of a child with AD often miss work due to medical care. Even at work, patients (or parents/guardians) often experience decreased work productivity (so-called presenteeism) due to loss of sleep and anxiety.21 In addressing the effects of AD on work life, a systematic literature review found that AD strongly affects sick leave and might have an impact on job choice and change or loss of job.22
Furthermore, according to Su et al,23 the costs of AD are related to disease severity. Moreover, their data suggest that among chronic childhood diseases, the financial burden of AD is greater than the cost of asthma and similar to the cost of diabetes mellitus.23
Association Between QOL and Disease Severity
A large observational study found that improvement in AD severity was followed by an increase in QOL.24 A positive correlation between disease severity and QOL has been found in other studies,25,26 though no correlation or only moderate correlation also has been reported.27 Apparently, in addition to QOL, disease severity scores are substantial parameters in the evaluation of distress caused by AD; the HOME initiative has identified clinician-reported signs and patient-reported symptoms as 2 of 4 core outcomes domains to include in all future AD clinical trials.3 For measuring symptoms, the Patient-Oriented Eczema Measure (POEM) is the recommended instrument.28 Regarding clinical signs, the HOME group named the Eczema Area and Severity Index (EASI) as the preferred instrument.29
Psychological Burden
Stress is a triggering factor for AD, but the connection between skin and mind appears bidirectional. The biological reaction to stress probably lowers the itch threshold and disrupts the skin barrier.30 The Global Burden of Disease Study showed that skin diseases are the fourth leading cause of nonfatal disease burden.31 There are several factors—pruritus, scratch, and pain—that can all lead to sleep deprivation and daytime fatigue. Based on our experience, if lesions develop on visible areas, patients can feel stigmatized, which restricts their social life.
The most common psychological comorbidities of AD are anxiety and depression. In a cross-sectional, multicenter study, there was a significantly higher prevalence of depression (P<.001) and anxiety disorder (P=.02) among patients with common skin diseases compared to a control group.32 In a study that assessed AD patients, researchers found a higher risk of depression and anxiety.33 Suicidal ideation also is more common in the population with AD32,34; a study showed that the risk of suicidal ideation in adolescents was nearly 4-fold in patients with itching skin lesions compared to those without itch.34
According to Linnet and Jemec,35 mental and psychological comorbidities of AD are associated with lower QOL, not with clinical severity. As a result, to improve QOL in AD, one should take care of both dermatological and psychological problems. It has been demonstrated that psychological interventions, such as autogenic training, cognitive-behavioral therapy, relaxation techniques, habit reversal training,36 and hypnotherapy37 might be helpful in individual cases; educational interventions also are recommended.36 With these adjuvant therapies, psychological status, unpleasant clinical symptoms, and QOL could be improved, though further studies are needed to confirm these benefits.
Conclusion
Atopic dermatitis places a notable burden on patients and their families. The degree of burden is probably related to disease severity. For measuring QOL, researchers and clinicians should use validated methods suited to the age of the patients for which they were designed. More studies are needed to assess the effects of different treatments on QOL. Besides pharmacotherapy, psychotherapy and educational programs might be beneficial for improving QOL, another important area to be studied.
Atopic dermatitis (AD) is a chronic, relapsing, inflammatory skin disease typically with childhood onset. In some cases, the condition persists, but AD usually resolves by the time a child reaches adulthood. Prevalence is difficult to estimate but, in developed countries, is approximately 15% to 30% among children and 2% to 10% among adults.1
Atopic dermatitis is characterized by chronically itchy dry skin, weeping erythematous papules and plaques, and lichenification. Furthermore, AD often is associated with other atopic diseases, such as food allergy, allergic rhinitis, and bronchial asthma.
In this article, we review the literature on the quality of life (QOL) of patients with AD. Our goals are to discuss the most common methods for measuring QOL in AD and how to use them; highlight specific alterations of QOL in AD; and review data about QOL of children with AD, which is underrepresented in the medical literature, as studies tend to focus on adults. In addition, we address the importance of assessing QOL in patients with AD due to the psychological burden of the disease.
Quality of Life
The harmful effects of AD can include a range of areas, including
Because QOL is an important instrument used in many AD studies, we call attention to the work of the Harmonising Outcome Measures for Eczema (HOME) initiative, which established a core outcome set for all AD clinical trials to enable comparison of results of individual studies.2 Quality of life was identified in HOME as one of 4 basic outcome measures that should be included in every AD trial (the others are clinician-reported signs, patient-reported symptoms, and long-term control).3 According to the recent agreement, the following QOL instruments should be used: Dermatology Life Quality Index (DLQI) for adults, Children’s Dermatology Life Quality Index (CDLQI) for children, and Infants’ Dermatitis Quality of Life Index (IDQOL) for infants.4
In dermatology, these instruments can be divided into 3 basic categories: generic, dermatology specific, and disease specific.5 Generic QOL questionnaires are beneficial when comparing the QOL of an AD patient to patients with other conditions or to healthy individuals. On the other hand, dermatology-specific and AD-specific methods are more effective instruments for detecting impairments linked directly to the disease and, therefore, are more sensitive to changes in QOL.5 Some of the most frequently used QOL measures5,6 for AD along with their key attributes are
Given that AD is a chronic disease that requires constant care, parents/guardians or the partner of the patient usually are affected as well. To detect this effect, the Family Dermatology Life Quality Index (FDLQI), a dermatology-specific instrument, measures the QOL in family members of dermatology patients.7 The Dermatitis Family Impact (DFI)8 is a disease-specific method for assessing how having a child with AD can impact the QOL of family members; it is a 10-item questionnaire completed by an adult family member. The FDLQI7 and DFI8 both help to understand the secondary impact of the disease.
In contrast, several other methods that also are administered by a parent/guardian assess how the parent perceives the QOL of their child with AD; these methods are essential for small children and infants who cannot answer questions themselves. The IDQOL9 was designed to assess the QOL of patients younger than 4 years using a parent-completed questionnaire. For older children and adolescents aged 4 to 16 years, the CDLQI10 is a widely used instrument; the questionnaire is completed by the child and is available in a cartoon format.10
For patients older than 16 years, 2 important instruments are the DLQI, a generic dermatology instrument, and the Quality of Life Index for Atopic Dermatitis (QoLIAD).11
Clearly it can be troublesome for researchers and clinicians to find the most suitable instrument to evaluate QOL in AD patients. To make this task easier, the European Academy of Dermatology and Venereology Task Force released a position paper with the following recommendations: (1) only validated instruments should be used, and (2) their use should be based on the age of the patients for which the instruments were designed. It is reommended that researchers use a combination of a generic and a dermatology-specific or AD-specific instrument, whereas clinicians should apply a dermatology-specific or AD-specific method, or both.5
Alterations of QOL in AD
Sleep Disturbance in AD
Sleep disorders observed in AD include difficulty falling asleep, frequent waking episodes, shorter sleep duration, and feelings of inadequate sleep, which often result in impairment of daily activity.12,13 Correlation has been found between sleep quality and QOL in both children and adults.14 Approximately 60% of children affected by AD experience a sleep disturbance,15 which seems to correlate well with disease severity.16 A US study found that adults with AD are more likely to experience a sleep disturbance, which often affects daytime functioning and work productivity.13
Financial Aspects and Impact on Work
The financial burden of AD is extensive.17 There are direct medical costs, including medication, visits to the physician, alternative therapies, and nonprescription products. Patients tend to spend relevant money on such items as moisturizers, bath products, antihistamines, topical steroids, and topical antibiotics.18,19 However, it seems that most of the cost of AD is due to indirect and nonmedical costs, including transportation to medical visits; loss of work days; extra childcare; and expenditures associated with lifestyle changes,19,20 such as modifying diet, wearing special clothes, using special bed linens, and purchasing special household items (eg, anti–dust mite vacuum cleaner, humidifier, new carpeting).17,19
Absenteeism from work often is a consequence of physician appointments; in addition, parents/guardians of a child with AD often miss work due to medical care. Even at work, patients (or parents/guardians) often experience decreased work productivity (so-called presenteeism) due to loss of sleep and anxiety.21 In addressing the effects of AD on work life, a systematic literature review found that AD strongly affects sick leave and might have an impact on job choice and change or loss of job.22
Furthermore, according to Su et al,23 the costs of AD are related to disease severity. Moreover, their data suggest that among chronic childhood diseases, the financial burden of AD is greater than the cost of asthma and similar to the cost of diabetes mellitus.23
Association Between QOL and Disease Severity
A large observational study found that improvement in AD severity was followed by an increase in QOL.24 A positive correlation between disease severity and QOL has been found in other studies,25,26 though no correlation or only moderate correlation also has been reported.27 Apparently, in addition to QOL, disease severity scores are substantial parameters in the evaluation of distress caused by AD; the HOME initiative has identified clinician-reported signs and patient-reported symptoms as 2 of 4 core outcomes domains to include in all future AD clinical trials.3 For measuring symptoms, the Patient-Oriented Eczema Measure (POEM) is the recommended instrument.28 Regarding clinical signs, the HOME group named the Eczema Area and Severity Index (EASI) as the preferred instrument.29
Psychological Burden
Stress is a triggering factor for AD, but the connection between skin and mind appears bidirectional. The biological reaction to stress probably lowers the itch threshold and disrupts the skin barrier.30 The Global Burden of Disease Study showed that skin diseases are the fourth leading cause of nonfatal disease burden.31 There are several factors—pruritus, scratch, and pain—that can all lead to sleep deprivation and daytime fatigue. Based on our experience, if lesions develop on visible areas, patients can feel stigmatized, which restricts their social life.
The most common psychological comorbidities of AD are anxiety and depression. In a cross-sectional, multicenter study, there was a significantly higher prevalence of depression (P<.001) and anxiety disorder (P=.02) among patients with common skin diseases compared to a control group.32 In a study that assessed AD patients, researchers found a higher risk of depression and anxiety.33 Suicidal ideation also is more common in the population with AD32,34; a study showed that the risk of suicidal ideation in adolescents was nearly 4-fold in patients with itching skin lesions compared to those without itch.34
According to Linnet and Jemec,35 mental and psychological comorbidities of AD are associated with lower QOL, not with clinical severity. As a result, to improve QOL in AD, one should take care of both dermatological and psychological problems. It has been demonstrated that psychological interventions, such as autogenic training, cognitive-behavioral therapy, relaxation techniques, habit reversal training,36 and hypnotherapy37 might be helpful in individual cases; educational interventions also are recommended.36 With these adjuvant therapies, psychological status, unpleasant clinical symptoms, and QOL could be improved, though further studies are needed to confirm these benefits.
Conclusion
Atopic dermatitis places a notable burden on patients and their families. The degree of burden is probably related to disease severity. For measuring QOL, researchers and clinicians should use validated methods suited to the age of the patients for which they were designed. More studies are needed to assess the effects of different treatments on QOL. Besides pharmacotherapy, psychotherapy and educational programs might be beneficial for improving QOL, another important area to be studied.
- Bieber T. Atopic dermatitis. N Engl J Med. 2008;358:1483-1494.
- Schmitt J, Williams H; HOME Development Group. Harmonising Outcome Measures for Eczema (HOME). report from the First International Consensus Meeting (HOME 1), 24 July 2010, Munich, Germany. Br J Dermatol. 2010;163:1166-1168.
- Schmitt J, Spuls P, Boers M, et al. Towards global consensus on outcome measures for atopic eczema research: results of the HOME II meeting. Allergy. 2012;67:1111-1117.
- Quality of Life (QoL). Harmonising Outcome Measures for Eczema (HOME) website. http://www.homeforeczema.org/research/quality-of-life.aspx. Accessed August 18, 2019.
Chernyshov PV, Tomas-Aragones L, Manolache L, et al; EADV Quality of Life Task Force. Quality of life measurement in atopic dermatitis. Position paper of the European Academy of Dermatology and Venereology (EADV) Task Force on quality of life. J Eur Acad Dermatol Venereol. 2017;31:576-593. - Hill MK, Kheirandish Pishkenari A, Braunberger TL, et al. Recent trends in disease severity and quality of life instruments for patients with atopic dermatitis: a systematic review. J Am Acad Dermatol. 2016;75:906-917.
- Basra MK, Sue-Ho R, Finlay AY. The Family Dermatology Life Quality Index: measuring the secondary impact of skin disease. Br J Dermatol. 2007;156:528-538.
- Dodington SR, Basra MK, Finlay AY, et al. The Dermatitis Family Impact questionnaire: a review of its measurement properties and clinical application. Br J Dermatol. 2013;169:31-46.
- Lewis-Jones MS, Finlay AY, Dykes PJ. The Infants’ Dermatitis Quality of Life Index. Br J Dermatol. 2001;144:104-110.
- Holme SA, Man I, Sharpe JL, et al. The Children’s Dermatology Life Quality Index: validation of the cartoon version. Br J Dermatol. 2003;148:285-290.
- Whalley D, McKenna SP, Dewar AL, et al. A new instrument for assessing quality of life in atopic dermatitis: international development of the Quality of Life Index for Atopic Dermatitis (QoLIAD). Br J Dermatol. 2004;150:274-283.
- Jeon C, Yan D, Nakamura M, et al. Frequency and management of sleep disturbance in adults with atopic dermatitis: a systematic review. Dermatol Ther (Heidelb). 2017;7:349-364.
- Yu SH, Attarian H, Zee P, et al. Burden of sleep and fatigue in US adults with atopic dermatitis. Dermatitis. 2016;27:50-58.
- Kong TS, Han TY, Lee JH, et al. Correlation between severity of atopic dermatitis and sleep quality in children and adults. Ann Dermatol. 2016;28:321-326.
- Fishbein AB, Mueller K, Kruse L, et al. Sleep disturbance in children with moderate/severe atopic dermatitis: a case-control study. J Am Acad Dermatol. 2018;78:336-341.
- Chamlin SL, Mattson CL, Frieden IJ, et al. The price of pruritus: sleep disturbance and cosleeping in atopic dermatitis. Arch Pediatr Adolesc Med. 2005;159:745-750.
- Emerson RM, Williams HC, Allen BR. What is the cost of atopic dermatitis in preschool children? Br J Dermatol. 2001;144:514-522.
- Filanovsky MG, Pootongkam S, Tamburro JE, et al. The financial and emotional impact of atopic dermatitis on children and their families. J Pediatr. 2016;169:284-290.
- Fivenson D, Arnold RJ, Kaniecki DJ, et al. The effect of atopic dermatitis on total burden of illness and quality of life on adults and children in a large managed care organization. J Manag Care Pharm. 2002;8:333-342.
- Carroll CL, Balkrishnan R, Feldman SR, et al. The burden of atopic dermatitis: impact on the patient, family, and society. Pediatr Dermatol. 2005;22:192-199.
- Drucker AM, Wang AR, Qureshi AA. Research gaps in quality of life and economic burden of atopic dermatitis: the National Eczema Association Burden of Disease Audit. JAMA Dermatol. 2016;152:873-874.
- Nørreslet LB, Ebbehøj NE, Ellekilde Bonde JP, et al. The impact of atopic dermatitis on work life—a systematic review. J Eur Acad Dermatol Venereol. 2018;32:23-38.
- Su JC, Kemp AS, Varigos GA, et al. Atopic eczema: its impact on the family and financial cost. Arch Dis Child. 1997;76:159-162.
- Coutanceau C, Stalder JF. Analysis of correlations between patient-oriented SCORAD (PO-SCORAD) and other assessment scores of atopic dermatitis severity and quality of life. Dermatology. 2014;229:248-255.
- Ben-Gashir MA, Seed PT, Hay RJ. Quality of life and disease severity are correlated in children with atopic dermatitis. Br J Dermatol. 2004;150:284-290.
- van Valburg RW, Willemsen MG, Dirven-Meijer PC, et al. Quality of life measurement and its relationship to disease severity in children with atopic dermatitis in general practice. Acta Derm Venereol. 2011;91:147-151.
- Haeck IM, ten Berge O, van Velsen SG, et al. Moderate correlation between quality of life and disease activity in adult patients with atopic dermatitis. J Eur Acad Dermatol Venereol. 2012;26:236-241.
- Spuls PI, Gerbens LAA, Simpson E, et al; HOME initiative collaborators. Patient-Oriented Eczema Measure (POEM), a core instrument to measure symptoms in clinical trials: a Harmonising Outcome Measures for Eczema (HOME) statement. Br J Dermatol. 2017;176:979-984.
- Schmitt J, Spuls PI, Thomas KS, et al; HOME initiative collaborators. The Harmonising Outcome Measures for Eczema (HOME) statement to assess clinical signs of atopic eczema in trials. J Allergy Clin Immunol. 2014;134:800-807.
- Oh SH, Bae BG, Park CO, et al. Association of stress with symptoms of atopic dermatitis. Acta Derm Venereol. 2010;90:582-588.
- Hay RJ, Johns NE, Williams HC, et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol. 2014;134:1527-1534.
- Dalgard FJ, Gieler U, Tomas-Aragones L, et al. The psychological burden of skin diseases: a cross-sectional multicenter study among dermatological out-patients in 13 European countries. J Invest Dermatol. 2015;135:984-991.
- Cheng CM, Hsu JW, Huang KL, et al. Risk of developing major depressive disorder and anxiety disorders among adolescents and adults with atopic dermatitis: a nationwide longitudinal study. J Affect Disord. 2015;178:60-65.
Halvorsen JA, Lien L, Dalgard F, et al. Suicidal ideation, mental health problems, and social function in adolescents with eczema: a population-based study. J Invest Dermatol. 2014;134:1847-1854. - Linnet J, Jemec GB. An assessment of anxiety and dermatology life quality in patients with atopic dermatitis. Br J Dermatol. 1999;140:268-272.
- Ring J, Alomar A, Bieber T, et al; European Dermatology Forum; European Academy of Dermatology and Venereology; European Task Force on Atopic Dermatitis; European Federation of Allergy; European Society of Pediatric Dermatology; Global Allergy and Asthma European Network. Guidelines for treatment of atopic eczema (atopic dermatitis) Part II. J Eur Acad Dermatol Venereol. 2012;26:1176-1193.
- Perczel K, Gál J. Hypnotherapy of atopic dermatitis in an adult. case report. Orv Hetil. 2016;157:111-115.
- Bieber T. Atopic dermatitis. N Engl J Med. 2008;358:1483-1494.
- Schmitt J, Williams H; HOME Development Group. Harmonising Outcome Measures for Eczema (HOME). report from the First International Consensus Meeting (HOME 1), 24 July 2010, Munich, Germany. Br J Dermatol. 2010;163:1166-1168.
- Schmitt J, Spuls P, Boers M, et al. Towards global consensus on outcome measures for atopic eczema research: results of the HOME II meeting. Allergy. 2012;67:1111-1117.
- Quality of Life (QoL). Harmonising Outcome Measures for Eczema (HOME) website. http://www.homeforeczema.org/research/quality-of-life.aspx. Accessed August 18, 2019.
Chernyshov PV, Tomas-Aragones L, Manolache L, et al; EADV Quality of Life Task Force. Quality of life measurement in atopic dermatitis. Position paper of the European Academy of Dermatology and Venereology (EADV) Task Force on quality of life. J Eur Acad Dermatol Venereol. 2017;31:576-593. - Hill MK, Kheirandish Pishkenari A, Braunberger TL, et al. Recent trends in disease severity and quality of life instruments for patients with atopic dermatitis: a systematic review. J Am Acad Dermatol. 2016;75:906-917.
- Basra MK, Sue-Ho R, Finlay AY. The Family Dermatology Life Quality Index: measuring the secondary impact of skin disease. Br J Dermatol. 2007;156:528-538.
- Dodington SR, Basra MK, Finlay AY, et al. The Dermatitis Family Impact questionnaire: a review of its measurement properties and clinical application. Br J Dermatol. 2013;169:31-46.
- Lewis-Jones MS, Finlay AY, Dykes PJ. The Infants’ Dermatitis Quality of Life Index. Br J Dermatol. 2001;144:104-110.
- Holme SA, Man I, Sharpe JL, et al. The Children’s Dermatology Life Quality Index: validation of the cartoon version. Br J Dermatol. 2003;148:285-290.
- Whalley D, McKenna SP, Dewar AL, et al. A new instrument for assessing quality of life in atopic dermatitis: international development of the Quality of Life Index for Atopic Dermatitis (QoLIAD). Br J Dermatol. 2004;150:274-283.
- Jeon C, Yan D, Nakamura M, et al. Frequency and management of sleep disturbance in adults with atopic dermatitis: a systematic review. Dermatol Ther (Heidelb). 2017;7:349-364.
- Yu SH, Attarian H, Zee P, et al. Burden of sleep and fatigue in US adults with atopic dermatitis. Dermatitis. 2016;27:50-58.
- Kong TS, Han TY, Lee JH, et al. Correlation between severity of atopic dermatitis and sleep quality in children and adults. Ann Dermatol. 2016;28:321-326.
- Fishbein AB, Mueller K, Kruse L, et al. Sleep disturbance in children with moderate/severe atopic dermatitis: a case-control study. J Am Acad Dermatol. 2018;78:336-341.
- Chamlin SL, Mattson CL, Frieden IJ, et al. The price of pruritus: sleep disturbance and cosleeping in atopic dermatitis. Arch Pediatr Adolesc Med. 2005;159:745-750.
- Emerson RM, Williams HC, Allen BR. What is the cost of atopic dermatitis in preschool children? Br J Dermatol. 2001;144:514-522.
- Filanovsky MG, Pootongkam S, Tamburro JE, et al. The financial and emotional impact of atopic dermatitis on children and their families. J Pediatr. 2016;169:284-290.
- Fivenson D, Arnold RJ, Kaniecki DJ, et al. The effect of atopic dermatitis on total burden of illness and quality of life on adults and children in a large managed care organization. J Manag Care Pharm. 2002;8:333-342.
- Carroll CL, Balkrishnan R, Feldman SR, et al. The burden of atopic dermatitis: impact on the patient, family, and society. Pediatr Dermatol. 2005;22:192-199.
- Drucker AM, Wang AR, Qureshi AA. Research gaps in quality of life and economic burden of atopic dermatitis: the National Eczema Association Burden of Disease Audit. JAMA Dermatol. 2016;152:873-874.
- Nørreslet LB, Ebbehøj NE, Ellekilde Bonde JP, et al. The impact of atopic dermatitis on work life—a systematic review. J Eur Acad Dermatol Venereol. 2018;32:23-38.
- Su JC, Kemp AS, Varigos GA, et al. Atopic eczema: its impact on the family and financial cost. Arch Dis Child. 1997;76:159-162.
- Coutanceau C, Stalder JF. Analysis of correlations between patient-oriented SCORAD (PO-SCORAD) and other assessment scores of atopic dermatitis severity and quality of life. Dermatology. 2014;229:248-255.
- Ben-Gashir MA, Seed PT, Hay RJ. Quality of life and disease severity are correlated in children with atopic dermatitis. Br J Dermatol. 2004;150:284-290.
- van Valburg RW, Willemsen MG, Dirven-Meijer PC, et al. Quality of life measurement and its relationship to disease severity in children with atopic dermatitis in general practice. Acta Derm Venereol. 2011;91:147-151.
- Haeck IM, ten Berge O, van Velsen SG, et al. Moderate correlation between quality of life and disease activity in adult patients with atopic dermatitis. J Eur Acad Dermatol Venereol. 2012;26:236-241.
- Spuls PI, Gerbens LAA, Simpson E, et al; HOME initiative collaborators. Patient-Oriented Eczema Measure (POEM), a core instrument to measure symptoms in clinical trials: a Harmonising Outcome Measures for Eczema (HOME) statement. Br J Dermatol. 2017;176:979-984.
- Schmitt J, Spuls PI, Thomas KS, et al; HOME initiative collaborators. The Harmonising Outcome Measures for Eczema (HOME) statement to assess clinical signs of atopic eczema in trials. J Allergy Clin Immunol. 2014;134:800-807.
- Oh SH, Bae BG, Park CO, et al. Association of stress with symptoms of atopic dermatitis. Acta Derm Venereol. 2010;90:582-588.
- Hay RJ, Johns NE, Williams HC, et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol. 2014;134:1527-1534.
- Dalgard FJ, Gieler U, Tomas-Aragones L, et al. The psychological burden of skin diseases: a cross-sectional multicenter study among dermatological out-patients in 13 European countries. J Invest Dermatol. 2015;135:984-991.
- Cheng CM, Hsu JW, Huang KL, et al. Risk of developing major depressive disorder and anxiety disorders among adolescents and adults with atopic dermatitis: a nationwide longitudinal study. J Affect Disord. 2015;178:60-65.
Halvorsen JA, Lien L, Dalgard F, et al. Suicidal ideation, mental health problems, and social function in adolescents with eczema: a population-based study. J Invest Dermatol. 2014;134:1847-1854. - Linnet J, Jemec GB. An assessment of anxiety and dermatology life quality in patients with atopic dermatitis. Br J Dermatol. 1999;140:268-272.
- Ring J, Alomar A, Bieber T, et al; European Dermatology Forum; European Academy of Dermatology and Venereology; European Task Force on Atopic Dermatitis; European Federation of Allergy; European Society of Pediatric Dermatology; Global Allergy and Asthma European Network. Guidelines for treatment of atopic eczema (atopic dermatitis) Part II. J Eur Acad Dermatol Venereol. 2012;26:1176-1193.
- Perczel K, Gál J. Hypnotherapy of atopic dermatitis in an adult. case report. Orv Hetil. 2016;157:111-115.
Practice Points
- For assessing quality of life (QOL) in atopic dermatitis (AD), it is recommended that researchers use a combination of a generic and a dermatology-specific or AD-specific instrument, whereas clinicians should apply a dermatology-specific or an AD-specific method or both.
- Anxiety and depression are common comorbidities in AD; patients also may need psychological support.
- Patient education is key for improving QOL in AD.
- Financial aspects of the treatment of AD should be taken into consideration because AD requires constant care, which puts a financial burden on patients.
Atopic Dermatitis in Adolescents With Skin of Color
Data are limited on the management of atopic dermatitis (AD) in adolescents, particularly in patients with skin of color, making it important to identify factors that may improve AD management in this population. Comorbid conditions (eg, acne, postinflammatory hyperpigmentation [PIH]), extracurricular activities (eg, athletics), and experimentation with cosmetics in adolescents, all of which can undermine treatment efficacy and medication adherence, make it particularly challenging to devise a therapeutic regimen in this patient population. We review the management of AD in black adolescents, with special consideration of concomitant treatment of acne vulgaris (AV) as well as lifestyle and social choices (Table).
Prevalence and Epidemiology
Atopic dermatitis affects 13% to 25% of children and 2% to 10% of adults.1,2 Population‐based studies in the United States show a higher prevalence of AD in black children (19.3%) compared to European American (EA) children (16.1%).3,4
AD in Black Adolescents
Atopic dermatitis is a common skin condition that is defined as a chronic, pruritic, inflammatory dermatosis with recurrent scaling, papules, and plaques (Figure) that usually develop during infancy and early childhood.3 Although AD severity improves for some patients in adolescence, it can be a lifelong issue affecting performance in academic and occupational settings.5 One US study of 8015 children found that there are racial and ethnic disparities in school absences among children (age range, 2–17 years) with AD, with children with skin of color being absent more often than white children.6 The same study noted that black children had a 1.5-fold higher chance of being absent 6 days over a 6-month school period compared to white children. It is postulated that AD has a greater impact on quality of life (QOL) in children with skin of color, resulting in the increased number of school absences in this population.6
The origin of AD currently is thought to be complex and can involve skin barrier dysfunction, environmental factors, microbiome effects, genetic predisposition, and immune dysregulation.1,4 Atopic dermatitis is a heterogeneous disease with variations in the prevalence, genetic background, and immune activation patterns across racial groups.4 It is now understood to be an immune-mediated disease with multiple inflammatory pathways, with type 2–associated inflammation being a primary pathway. Patients with AD have strong helper T cell (TH2) activation, and black patients with AD have higher IgE serum levels as well as absent TH17/TH1 activation.4
Atopic dermatitis currently is seen as a defect of the epidermal barrier, with variable clinical manifestations and expressivity.7 Filaggrin is an epidermal barrier protein, encoded by the FLG gene, and plays a major role in barrier function by regulating pH and promoting hydration of the skin.4 Loss of function of the FLG gene is the most well-studied genetic risk factor for developing AD, and this mutation is seen in patients with more severe and persistent AD in addition to patients with more skin infections and allergic sensitizations.3,4 However, in the skin of color population, FLG mutations are 6 times less common than in the EA population, despite the fact that AD is more prevalent in patients of African descent.4 Therefore, the role of the FLG loss-of-function mutation and AD is not as well defined in black patients, and some researchers have found no association.3 The FLG loss-of-function mutation seems to play a smaller role in black patients than in EA patients, and other genes may be involved in skin barrier dysfunction.3,4 In a small study of patients with mild AD compared to nonaffected patients, those with AD had lower total ceramide levels in the stratum corneum of affected sites than normal skin sites in healthy individuals.8
Particular disturbances in the gut microbiome have the possibility of impacting the development of AD.9 Additionally, the development of AD may be influenced by the skin microbiome, which can change depending on body site, with fungal organisms thought to make up a large proportion of the microbiome of patients with AD. In patients with AD, there is a lack of microbial diversity and an overgrowth of Staphylococcus aureus.9
Diagnosis
Clinicians diagnose AD based on clinical characteristics, and the lack of objective criteria can hinder diagnosis.1 Thus, diagnosing AD in children with dark skin can pose a particular challenge given the varied clinical presentation of AD across skin types. Severe cases of AD may not be diagnosed or treated adequately in deeply pigmented children because erythema, a defining characteristic of AD, may be hard to identify in darker skin types.10 Furthermore, clinical erythema scores among black children may be “strongly” underestimated using scoring systems such as Eczema Area and Severity Index and SCORing Atopic Dermatitis.4 It is estimated that the risk for severe AD may be 6 times higher in black children compared to white children.10 Additionally, patients with skin of color can present with more treatment-resistant AD.4
Treatment of AD
Current treatment is focused on restoring epidermal barrier function, often with topical agents, such as moisturizers containing different amounts of emollients, occlusives, and humectants; corticosteroids; calcineurin inhibitors; and antimicrobials. Emollients such as glycol stearate, glyceryl stearate, and soy sterols function as lubricants, softening the skin. Occlusive agents include petrolatum, dimethicone, and mineral oil; they act by forming a layer to slow evaporation of water. Humectants including glycerol, lactic acid, and urea function by promoting water retention.11 For acute flares, mid- to high-potency topical corticosteroids are recommended. Also, topical calcineurin inhibitors such as tacrolimus and pimecrolimus may be used alone or in combination with topical steroids. Finally, bleach baths and topical mupirocin applied to the nares also have proved helpful in moderate to severe AD with secondary bacterial infections.11 Phototherapy can be used in adult and pediatric patients with acute and chronic AD if traditional treatments have failed.2
Systemic agents are indicated and recommended for the subset of adult and pediatric patients in whom optimized topical regimens and/or phototherapy do not adequately provide disease control or when QOL is substantially impacted. The systemic agents effective in the pediatric population include cyclosporine, azathioprine, mycophenolate mofetil, and possibly methotrexate.11 Dupilumab recently was approved by the US Food and Drug Administration for patients 12 years and older with moderate to severe AD whose disease is not well controlled with topical medications.
Patients with AD are predisposed to secondary bacterial and viral infections because of their dysfunctional skin barrier; these infections most commonly are caused by S aureus and herpes simplex virus, respectively.2 Systemic antibiotics are only recommended for patients with AD when there is clinical evidence of bacterial infection. In patients with evidence of eczema herpeticum, systemic antiviral agents should be used to treat the underlying herpes simplex virus infection.2 Atopic dermatitis typically has been studied in white patients; however, patients with skin of color have higher frequencies of treatment-resistant AD. Further research on treatment efficacy for AD in this patient population is needed, as data are limited.4
Treatment of AV in Patients With AD
Two of the most prevalent skin diseases affecting the pediatric population are AD and AV, and both can remarkably impact QOL.12 Acne is one of the most common reasons for adolescent patients to seek dermatologic care, including patients with skin of color (Fitzpatrick skin types IV to VI).13 Thus, it is to be expected that many black adolescents with AD also will have AV. For mild to moderate acne in patients with skin of color, topical retinoids and benzoyl peroxide typically are first line.13 These medications can be problematic for patients with AD, as retinoids and many other acne treatments can cause dryness, which may exacerbate AD.
Moisturizers containing ceramide can be a helpful adjunctive therapy in treating acne,14 especially in patients with AD. Modifications to application of acne medications, such as using topical retinoids every other night or mixing them with moisturizers to minimize dryness, may be beneficial to these patients. Dapsone gel 7.5% used daily also may be an option for adolescents with AD and AV. A double-blind, vehicle-controlled study demonstrated that dapsone is safe and effective for patients 12 years and older with moderate acne, and patients with Fitzpatrick skin types IV to VI rated local scaling, erythema, dryness, and stinging/burning as “none” in the study.15 Another potentially helpful topical agent in patients with AD and AV is sulfacetamide, as it is not likely to cause dryness of the skin. In a small study, sodium sulfacetamide 10% and sulfur 5% in an emollient foam vehicle showed no residual film or sulfur smell and resulted in acne reduction of 50%.16
Patients with skin of color often experience PIH in AD and acne or hypopigmentation from inflammatory dermatoses including AD.17,18 In addition to the dryness from AD and topical retinoid use, patients with skin of color may develop irritant contact dermatitis, thus leading to PIH.13 Dryness and irritant contact dermatitis also can be seen with the use of benzoyl peroxide in black patients. Because of these effects, gentle moisturizers are recommended, and both benzoyl peroxide and retinoids should be initiated at lower doses in patients with skin of color.13
For patients with severe nodulocystic acne, isotretinoin is the treatment of choice in patients with skin of color,13 but there is a dearth of clinical studies addressing complications seen in black adolescents on this treatment, especially with respect to those with AD. Of note, systemic antibiotics typically are initiated before isotretinoin; however, this strategy is falling out of favor due to concern for antibiotic resistance with long-term use.19
Impact of Athletics on AD in Black Adolescents
Because of the exacerbating effects of perspiration and heat causing itch and irritation in patients with AD, it is frequently advised that pediatric patients limit their participation in athletics because of the exacerbating effects of strenuous physical exercise on their disease.12 In one study, 429 pediatric patients or their parents/guardians completed QOL questionnaires; 89% of patients 15 years and younger with severe AD reported that their disease was impacted by athletics and outdoor activities, and 86% of these pediatric patients with severe AD responded that their social lives and leisure activities were impacted.20 Because adolescents often are involved in athletics or have mandatory physical education classes, AD may be isolating and may have a severe impact on self-esteem.
Aggressive treatment of AD with topical and systemic medications may be helpful in adolescents who may be reluctant to participate in sports because of teasing, bullying, or worsening of symptoms with heat or sweating.21 Now that dupilumab is available for adolescents, there is a chance that patients with severe and/or recalcitrant disease managed on this medication can achieve better control of their symptoms without the laboratory requirement of methotrexate and the difficulties of topical medication application, allowing them to engage in mandatory athletic classes as well as desired organized sports.
Use of Cosmetics for AD
Many adolescents experiment with cosmetics, and those with AD may use cosmetic products to cover hyperpigmented or hypopigmented lesions.18 In patients with active AD or increased sensitivity to allergens in cosmetic products, use of makeup can be a contributing factor for AD flares. Acne associated with cosmetics is especially important to consider in darker-skinned patients who may use makeup that is opaque and contains oil to conceal acne or PIH.
Allergens can be present in both cosmetics and pharmaceutical topical agents, and a Brazilian study found that approximately 89% of 813 prescription and nonprescription products (eg, topical drugs, sunscreens, moisturizers, soaps, cleansing lotions, shampoos, cosmeceuticals) contained allergens.22 Patients with AD have a higher prevalence of contact sensitization to fragrances, including balsam of Peru.23 Some AD treatments that contain fragrances have caused further skin issues in a few patients. In one case series, 3 pediatric patients developed allergic contact dermatitis to Myroxylon pereirae (balsam of Peru) when using topical treatments for their AD, and their symptoms of scalp inflammation and alopecia resolved with discontinuation.23
In a Dutch study, sensitization to Fragrance Mix I and M pereirae as well as other ingredients (eg, lanolin alcohol, Amerchol™ L 101 [a lanolin product]) was notably more common in pediatric patients with AD than in patients without AD; however, no data on patients with skin of color were included in this study.24
Because of the increased risk of sensitization to fragrances and other ingredients in patients with AD as well as the high percentage of allergens in prescription and nonprescription products, it is important to discuss all personal care products that patients may be using, not just their cosmetic products. Also, patch testing may be helpful in determining true allergens in some patients. Patch testing is recommended for patients with treatment-resistant AD, and a recent study suggested it should be done prior to long-term use of immunosuppressive agents.25 Increased steroid phobia and a push toward alternative medicines are leading both patients with AD and guardians of children with AD to look for other forms of moisturization, such as olive oil, coconut oil, sunflower seed oil, and shea butter, to decrease transepidermal water loss.26,27 An important factor in AD treatment efficacy is patient acceptability in using what is recommended.27 One study showed there was no difference in efficacy or acceptability in using a cream containing shea butter extract vs the ceramide-precursor product.27 Current data show olive oil may exacerbate dry skin and AD,26 and recommendation of any over-the-counter oils and butters in patients with AD should be made with great caution, as many of these products contain fragrances and other potential allergens.
Alternative Therapies for AD
Patients with AD often seek alternative or integrative treatment options, including dietary modifications and holistic remedies. Studies investigating the role of vitamins and supplements in treating AD are limited by sample size.28 However, there is some evidence that may support supplementation with vitamins D and E in addressing AD symptoms. The use of probiotics in treating AD is controversial, but there are studies suggesting that the use of probiotics may prove beneficial in preventing infantile AD.28 Additionally, findings from an ex vivo and in vitro study show that some conditions, including AD and acne, may benefit from the same probiotics, despite the differences in these two diseases. Both AD and acne have inflammatory and skin dysbiosis characteristics, which may be the common thread leading to both conditions potentially responding to treatment with probiotics.29
Preliminary evidence indicates that supplements containing fatty acids such as docosahexaenoic acid, sea buckthorn oil, and hemp seed oil may decrease the severity of AD.28 In a 20-week, randomized, single-blind, crossover study published in 2005, dietary hemp seed oil showed an improvement of clinical symptoms, including dry skin and itchiness, in patients with AD.30
In light of recent legalization in several states, patients may turn to use of cannabinoid products to manage AD. In a systematic review, cannabinoid use was reportedly a therapeutic option in the treatment of AD and AV; however, the data are based on preclinical work, and there are no randomized, placebo-controlled studies to support the use of cannabinoids.31 Furthermore, there is great concern that use of these products in adolescents is an even larger unknown.
Final Thoughts
Eighty percent of children diagnosed with AD experience symptom improvement before their early teens32; for those with AD during their preteen and teenage years, there can be psychological ramifications, as teenagers with AD report having fewer friends, are less socially involved, participate in fewer sports, and are absent from classes more often than their peers.5 In black patients with AD, school absences are even more common.6 Given the social and emotional impact of AD on patients with skin of color, it is imperative to treat the condition appropriately.33 There are areas of opportunity for further research on alternate dosing of existing treatments for AV in patients with AD, further recommendations for adolescent athletes with AD, and which cosmetic and alternative medicine products may be beneficial for this population to improve their QOL.
Providers should discuss medical management in a broader context considering patients’ extracurricular activities, treatment vehicle preferences, expectations, and personal care habits. It also is important to address the many possible factors that may influence treatment adherence early on, particularly in adolescents, as these could be barriers to treatment. This article highlights considerations for treating AD and comorbid conditions that may further complicate treatment in adolescent patients with skin of color. The information provided should serve as a guide in initial counseling and management of AD in adolescents with skin of color.
- Feldman SR, Cox LS, Strowd LC, et al. The challenge of managing atopic dermatitis in the United States. Am Health Drug Benefits. 2019;12:83-93.
- Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: section 3. management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-349.
- Kaufman BP, Guttman-Yassky E, Alexis AF. Atopic dermatitis in diverse racial and ethnic groups—variations in epidemiology, genetics, clinical presentation and treatment. Exp Dermatol. 2018;27:340-357.
- Brunner PM, Guttman-Yassky E. Racial differences in atopic dermatitis. Ann Allergy Asthma Immunol. 2019;122:449-455.
- Vivar KL, Kruse L. The impact of pediatric skin disease on self-esteem. Int J Womens Dermatol. 2018;4:27-31.
- Wan J, Margolis DJ, Mitra N, et al. Racial and ethnic differences in atopic dermatitis–related school absences among US children [published online May 22, 2019]. JAMA Dermatol. doi:10.1001/jamadermatol.2019.0597.
- Weidinger S, Novak N. Atopic dermatitis. Lancet. 2016;387:1109-1122.
- Ishikawa J, Narita H, Kondo N, et al. Changes in the ceramide profile of atopic dermatitis patients. J Invest Dermatol. 2010;130:2511-2514.
- Chernikova D, Yuan I, Shaker M. Prevention of allergy with diverse and healthy microbiota: an update. Curr Opin Pediatr. 2019;31:418-425.
- Ben-Gashir MA, Hay RJ. Reliance on erythema scores may mask severe atopic dermatitis in black children compared with their white counterparts. Br J Dermatol. 2002;147:920-925.
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2. management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132.
- Nguyen CM, Koo J, Cordoro KM. Psychodermatologic effects of atopic dermatitis and acne: a review on self-esteem and identity. Pediatr Dermatol. 2016;33:129-135.
- Davis EC, Callender VD. A review of acne in ethnic skin: pathogenesis, clinical manifestations, and management strategies. J Clin Aesthet Dermatol. 2010;3:24-38.
- Lynde CW, Andriessen A, Barankin B, et al. Moisturizers and ceramide-containing moisturizers may offer concomitant therapy with benefits. J Clin Aesthet Dermatol. 2014;7:18-26.
- Taylor SC, Cook-Bolden FE, McMichael A, et al. Efficacy, safety, and tolerability of topical dapsone gel, 7.5% for treatment of acne vulgaris by Fitzpatrick skin phototype. J Drugs Dermatol. 2018;17:160-167.
- Draelos ZD. The multifunctionality of 10% sodium sulfacetamide, 5% sulfur emollient foam in the treatment of inflammatory facial dermatoses. J Drugs Dermatol. 2010;9:234-236.
- Vachiramon V, Tey HL, Thompson AE, et al. Atopic dermatitis in African American children: addressing unmet needs of a common disease. Pediatr Dermatol. 2012;29:395-402.
- Heath CR. Managing postinflammatory hyperpigmentation in pediatric patients with skin of color. Cutis. 2018;102:71-73.
- Nagler AR, Milam EC, Orlow SJ. The use of oral antibiotics before isotretinoin therapy in patients with acne. J Am Acad Dermatol. 2016;74:273-279.
- Paller AS, McAlister RO, Doyle JJ, et al. Perceptions of physicians and pediatric patients about atopic dermatitis, its impact, and its treatment. Clin Pediatr. 2002;41:323-332.
- Sibbald C, Drucker AM. Patient burden of atopic dermatitis. Dermatol Clin. 2017;35:303-316.
- Rocha VB, Machado CJ, Bittencourt FV. Presence of allergens in the vehicles of Brazilian dermatological products. Contact Dermatitis. 2017;76:126-128.
- Admani S, Goldenberg A, Jacob SE. Contact alopecia: improvement of alopecia with discontinuation of fluocinolone oil in individuals allergic to balsam fragrance. Pediatr Dermatol. 2017;34:e57-e60.
- Uter W, Werfel T, White IR, et al. Contact allergy: a review of current problems from a clinical perspective. Int J Environ Res Public Health. 2018;15:E1108.
- López-Jiménez EC, Marrero-Alemán G, Borrego L. One-third of patients with therapy-resistant atopic dermatitis may benefit after patch testing [published online May 13, 2019]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.15672.
- Karagounis TK, Gittler JK, Rotemberg V, et al. Use of “natural” oils for moisturization: review of olive, coconut, and sunflower seed oil. Pediatr Dermatol. 2019;36:9-15.
- Hon KL, Tsang YC, Pong NH, et al. Patient acceptability, efficacy, and skin biophysiology of a cream and cleanser containing lipid complex with shea butter extract versus a ceramide product for eczema. Hong Kong Med J. 2015;21:417-425.
- Reynolds KA, Juhasz MLW, Mesinkovska NA. The role of oral vitamins and supplements in the management of atopic dermatitis: a systematic review [published online March 20, 2019]. Int J Dermatol. doi:10.1111/ijd.14404.
- Mottin VHM, Suyenaga ES. An approach on the potential use of probiotics in the treatment of skin conditions: acne and atopic dermatitis. Int J Dermatol. 2018;57:1425-1432.
- Callaway J, Schwab U, Harvima I, et al. Efficacy of dietary hempseed oil in patients with atopic dermatitis. J Dermatol Treat. 2005;16:87-94.
- Eagleston LRM, Kalani NK, Patel RR, et al. Cannabinoids in dermatology: a scoping review [published June 15, 2018]. Dermatol Online J. 2018;24.
- Kim JP, Chao LX, Simpson EL, et al. Persistence of atopic dermatitis (AD): a systematic review and meta-analysis. J Am Acad Dermatol. 2016;75:681-687.e611.
- de María Díaz Granados L, Quijano MA, Ramírez PA, et al. Quality assessment of atopic dermatitis clinical practice guidelines in ≤ 18 years. Arch Dermatol Res. 2018;310:29-37.
Data are limited on the management of atopic dermatitis (AD) in adolescents, particularly in patients with skin of color, making it important to identify factors that may improve AD management in this population. Comorbid conditions (eg, acne, postinflammatory hyperpigmentation [PIH]), extracurricular activities (eg, athletics), and experimentation with cosmetics in adolescents, all of which can undermine treatment efficacy and medication adherence, make it particularly challenging to devise a therapeutic regimen in this patient population. We review the management of AD in black adolescents, with special consideration of concomitant treatment of acne vulgaris (AV) as well as lifestyle and social choices (Table).
Prevalence and Epidemiology
Atopic dermatitis affects 13% to 25% of children and 2% to 10% of adults.1,2 Population‐based studies in the United States show a higher prevalence of AD in black children (19.3%) compared to European American (EA) children (16.1%).3,4
AD in Black Adolescents
Atopic dermatitis is a common skin condition that is defined as a chronic, pruritic, inflammatory dermatosis with recurrent scaling, papules, and plaques (Figure) that usually develop during infancy and early childhood.3 Although AD severity improves for some patients in adolescence, it can be a lifelong issue affecting performance in academic and occupational settings.5 One US study of 8015 children found that there are racial and ethnic disparities in school absences among children (age range, 2–17 years) with AD, with children with skin of color being absent more often than white children.6 The same study noted that black children had a 1.5-fold higher chance of being absent 6 days over a 6-month school period compared to white children. It is postulated that AD has a greater impact on quality of life (QOL) in children with skin of color, resulting in the increased number of school absences in this population.6
The origin of AD currently is thought to be complex and can involve skin barrier dysfunction, environmental factors, microbiome effects, genetic predisposition, and immune dysregulation.1,4 Atopic dermatitis is a heterogeneous disease with variations in the prevalence, genetic background, and immune activation patterns across racial groups.4 It is now understood to be an immune-mediated disease with multiple inflammatory pathways, with type 2–associated inflammation being a primary pathway. Patients with AD have strong helper T cell (TH2) activation, and black patients with AD have higher IgE serum levels as well as absent TH17/TH1 activation.4
Atopic dermatitis currently is seen as a defect of the epidermal barrier, with variable clinical manifestations and expressivity.7 Filaggrin is an epidermal barrier protein, encoded by the FLG gene, and plays a major role in barrier function by regulating pH and promoting hydration of the skin.4 Loss of function of the FLG gene is the most well-studied genetic risk factor for developing AD, and this mutation is seen in patients with more severe and persistent AD in addition to patients with more skin infections and allergic sensitizations.3,4 However, in the skin of color population, FLG mutations are 6 times less common than in the EA population, despite the fact that AD is more prevalent in patients of African descent.4 Therefore, the role of the FLG loss-of-function mutation and AD is not as well defined in black patients, and some researchers have found no association.3 The FLG loss-of-function mutation seems to play a smaller role in black patients than in EA patients, and other genes may be involved in skin barrier dysfunction.3,4 In a small study of patients with mild AD compared to nonaffected patients, those with AD had lower total ceramide levels in the stratum corneum of affected sites than normal skin sites in healthy individuals.8
Particular disturbances in the gut microbiome have the possibility of impacting the development of AD.9 Additionally, the development of AD may be influenced by the skin microbiome, which can change depending on body site, with fungal organisms thought to make up a large proportion of the microbiome of patients with AD. In patients with AD, there is a lack of microbial diversity and an overgrowth of Staphylococcus aureus.9
Diagnosis
Clinicians diagnose AD based on clinical characteristics, and the lack of objective criteria can hinder diagnosis.1 Thus, diagnosing AD in children with dark skin can pose a particular challenge given the varied clinical presentation of AD across skin types. Severe cases of AD may not be diagnosed or treated adequately in deeply pigmented children because erythema, a defining characteristic of AD, may be hard to identify in darker skin types.10 Furthermore, clinical erythema scores among black children may be “strongly” underestimated using scoring systems such as Eczema Area and Severity Index and SCORing Atopic Dermatitis.4 It is estimated that the risk for severe AD may be 6 times higher in black children compared to white children.10 Additionally, patients with skin of color can present with more treatment-resistant AD.4
Treatment of AD
Current treatment is focused on restoring epidermal barrier function, often with topical agents, such as moisturizers containing different amounts of emollients, occlusives, and humectants; corticosteroids; calcineurin inhibitors; and antimicrobials. Emollients such as glycol stearate, glyceryl stearate, and soy sterols function as lubricants, softening the skin. Occlusive agents include petrolatum, dimethicone, and mineral oil; they act by forming a layer to slow evaporation of water. Humectants including glycerol, lactic acid, and urea function by promoting water retention.11 For acute flares, mid- to high-potency topical corticosteroids are recommended. Also, topical calcineurin inhibitors such as tacrolimus and pimecrolimus may be used alone or in combination with topical steroids. Finally, bleach baths and topical mupirocin applied to the nares also have proved helpful in moderate to severe AD with secondary bacterial infections.11 Phototherapy can be used in adult and pediatric patients with acute and chronic AD if traditional treatments have failed.2
Systemic agents are indicated and recommended for the subset of adult and pediatric patients in whom optimized topical regimens and/or phototherapy do not adequately provide disease control or when QOL is substantially impacted. The systemic agents effective in the pediatric population include cyclosporine, azathioprine, mycophenolate mofetil, and possibly methotrexate.11 Dupilumab recently was approved by the US Food and Drug Administration for patients 12 years and older with moderate to severe AD whose disease is not well controlled with topical medications.
Patients with AD are predisposed to secondary bacterial and viral infections because of their dysfunctional skin barrier; these infections most commonly are caused by S aureus and herpes simplex virus, respectively.2 Systemic antibiotics are only recommended for patients with AD when there is clinical evidence of bacterial infection. In patients with evidence of eczema herpeticum, systemic antiviral agents should be used to treat the underlying herpes simplex virus infection.2 Atopic dermatitis typically has been studied in white patients; however, patients with skin of color have higher frequencies of treatment-resistant AD. Further research on treatment efficacy for AD in this patient population is needed, as data are limited.4
Treatment of AV in Patients With AD
Two of the most prevalent skin diseases affecting the pediatric population are AD and AV, and both can remarkably impact QOL.12 Acne is one of the most common reasons for adolescent patients to seek dermatologic care, including patients with skin of color (Fitzpatrick skin types IV to VI).13 Thus, it is to be expected that many black adolescents with AD also will have AV. For mild to moderate acne in patients with skin of color, topical retinoids and benzoyl peroxide typically are first line.13 These medications can be problematic for patients with AD, as retinoids and many other acne treatments can cause dryness, which may exacerbate AD.
Moisturizers containing ceramide can be a helpful adjunctive therapy in treating acne,14 especially in patients with AD. Modifications to application of acne medications, such as using topical retinoids every other night or mixing them with moisturizers to minimize dryness, may be beneficial to these patients. Dapsone gel 7.5% used daily also may be an option for adolescents with AD and AV. A double-blind, vehicle-controlled study demonstrated that dapsone is safe and effective for patients 12 years and older with moderate acne, and patients with Fitzpatrick skin types IV to VI rated local scaling, erythema, dryness, and stinging/burning as “none” in the study.15 Another potentially helpful topical agent in patients with AD and AV is sulfacetamide, as it is not likely to cause dryness of the skin. In a small study, sodium sulfacetamide 10% and sulfur 5% in an emollient foam vehicle showed no residual film or sulfur smell and resulted in acne reduction of 50%.16
Patients with skin of color often experience PIH in AD and acne or hypopigmentation from inflammatory dermatoses including AD.17,18 In addition to the dryness from AD and topical retinoid use, patients with skin of color may develop irritant contact dermatitis, thus leading to PIH.13 Dryness and irritant contact dermatitis also can be seen with the use of benzoyl peroxide in black patients. Because of these effects, gentle moisturizers are recommended, and both benzoyl peroxide and retinoids should be initiated at lower doses in patients with skin of color.13
For patients with severe nodulocystic acne, isotretinoin is the treatment of choice in patients with skin of color,13 but there is a dearth of clinical studies addressing complications seen in black adolescents on this treatment, especially with respect to those with AD. Of note, systemic antibiotics typically are initiated before isotretinoin; however, this strategy is falling out of favor due to concern for antibiotic resistance with long-term use.19
Impact of Athletics on AD in Black Adolescents
Because of the exacerbating effects of perspiration and heat causing itch and irritation in patients with AD, it is frequently advised that pediatric patients limit their participation in athletics because of the exacerbating effects of strenuous physical exercise on their disease.12 In one study, 429 pediatric patients or their parents/guardians completed QOL questionnaires; 89% of patients 15 years and younger with severe AD reported that their disease was impacted by athletics and outdoor activities, and 86% of these pediatric patients with severe AD responded that their social lives and leisure activities were impacted.20 Because adolescents often are involved in athletics or have mandatory physical education classes, AD may be isolating and may have a severe impact on self-esteem.
Aggressive treatment of AD with topical and systemic medications may be helpful in adolescents who may be reluctant to participate in sports because of teasing, bullying, or worsening of symptoms with heat or sweating.21 Now that dupilumab is available for adolescents, there is a chance that patients with severe and/or recalcitrant disease managed on this medication can achieve better control of their symptoms without the laboratory requirement of methotrexate and the difficulties of topical medication application, allowing them to engage in mandatory athletic classes as well as desired organized sports.
Use of Cosmetics for AD
Many adolescents experiment with cosmetics, and those with AD may use cosmetic products to cover hyperpigmented or hypopigmented lesions.18 In patients with active AD or increased sensitivity to allergens in cosmetic products, use of makeup can be a contributing factor for AD flares. Acne associated with cosmetics is especially important to consider in darker-skinned patients who may use makeup that is opaque and contains oil to conceal acne or PIH.
Allergens can be present in both cosmetics and pharmaceutical topical agents, and a Brazilian study found that approximately 89% of 813 prescription and nonprescription products (eg, topical drugs, sunscreens, moisturizers, soaps, cleansing lotions, shampoos, cosmeceuticals) contained allergens.22 Patients with AD have a higher prevalence of contact sensitization to fragrances, including balsam of Peru.23 Some AD treatments that contain fragrances have caused further skin issues in a few patients. In one case series, 3 pediatric patients developed allergic contact dermatitis to Myroxylon pereirae (balsam of Peru) when using topical treatments for their AD, and their symptoms of scalp inflammation and alopecia resolved with discontinuation.23
In a Dutch study, sensitization to Fragrance Mix I and M pereirae as well as other ingredients (eg, lanolin alcohol, Amerchol™ L 101 [a lanolin product]) was notably more common in pediatric patients with AD than in patients without AD; however, no data on patients with skin of color were included in this study.24
Because of the increased risk of sensitization to fragrances and other ingredients in patients with AD as well as the high percentage of allergens in prescription and nonprescription products, it is important to discuss all personal care products that patients may be using, not just their cosmetic products. Also, patch testing may be helpful in determining true allergens in some patients. Patch testing is recommended for patients with treatment-resistant AD, and a recent study suggested it should be done prior to long-term use of immunosuppressive agents.25 Increased steroid phobia and a push toward alternative medicines are leading both patients with AD and guardians of children with AD to look for other forms of moisturization, such as olive oil, coconut oil, sunflower seed oil, and shea butter, to decrease transepidermal water loss.26,27 An important factor in AD treatment efficacy is patient acceptability in using what is recommended.27 One study showed there was no difference in efficacy or acceptability in using a cream containing shea butter extract vs the ceramide-precursor product.27 Current data show olive oil may exacerbate dry skin and AD,26 and recommendation of any over-the-counter oils and butters in patients with AD should be made with great caution, as many of these products contain fragrances and other potential allergens.
Alternative Therapies for AD
Patients with AD often seek alternative or integrative treatment options, including dietary modifications and holistic remedies. Studies investigating the role of vitamins and supplements in treating AD are limited by sample size.28 However, there is some evidence that may support supplementation with vitamins D and E in addressing AD symptoms. The use of probiotics in treating AD is controversial, but there are studies suggesting that the use of probiotics may prove beneficial in preventing infantile AD.28 Additionally, findings from an ex vivo and in vitro study show that some conditions, including AD and acne, may benefit from the same probiotics, despite the differences in these two diseases. Both AD and acne have inflammatory and skin dysbiosis characteristics, which may be the common thread leading to both conditions potentially responding to treatment with probiotics.29
Preliminary evidence indicates that supplements containing fatty acids such as docosahexaenoic acid, sea buckthorn oil, and hemp seed oil may decrease the severity of AD.28 In a 20-week, randomized, single-blind, crossover study published in 2005, dietary hemp seed oil showed an improvement of clinical symptoms, including dry skin and itchiness, in patients with AD.30
In light of recent legalization in several states, patients may turn to use of cannabinoid products to manage AD. In a systematic review, cannabinoid use was reportedly a therapeutic option in the treatment of AD and AV; however, the data are based on preclinical work, and there are no randomized, placebo-controlled studies to support the use of cannabinoids.31 Furthermore, there is great concern that use of these products in adolescents is an even larger unknown.
Final Thoughts
Eighty percent of children diagnosed with AD experience symptom improvement before their early teens32; for those with AD during their preteen and teenage years, there can be psychological ramifications, as teenagers with AD report having fewer friends, are less socially involved, participate in fewer sports, and are absent from classes more often than their peers.5 In black patients with AD, school absences are even more common.6 Given the social and emotional impact of AD on patients with skin of color, it is imperative to treat the condition appropriately.33 There are areas of opportunity for further research on alternate dosing of existing treatments for AV in patients with AD, further recommendations for adolescent athletes with AD, and which cosmetic and alternative medicine products may be beneficial for this population to improve their QOL.
Providers should discuss medical management in a broader context considering patients’ extracurricular activities, treatment vehicle preferences, expectations, and personal care habits. It also is important to address the many possible factors that may influence treatment adherence early on, particularly in adolescents, as these could be barriers to treatment. This article highlights considerations for treating AD and comorbid conditions that may further complicate treatment in adolescent patients with skin of color. The information provided should serve as a guide in initial counseling and management of AD in adolescents with skin of color.
Data are limited on the management of atopic dermatitis (AD) in adolescents, particularly in patients with skin of color, making it important to identify factors that may improve AD management in this population. Comorbid conditions (eg, acne, postinflammatory hyperpigmentation [PIH]), extracurricular activities (eg, athletics), and experimentation with cosmetics in adolescents, all of which can undermine treatment efficacy and medication adherence, make it particularly challenging to devise a therapeutic regimen in this patient population. We review the management of AD in black adolescents, with special consideration of concomitant treatment of acne vulgaris (AV) as well as lifestyle and social choices (Table).
Prevalence and Epidemiology
Atopic dermatitis affects 13% to 25% of children and 2% to 10% of adults.1,2 Population‐based studies in the United States show a higher prevalence of AD in black children (19.3%) compared to European American (EA) children (16.1%).3,4
AD in Black Adolescents
Atopic dermatitis is a common skin condition that is defined as a chronic, pruritic, inflammatory dermatosis with recurrent scaling, papules, and plaques (Figure) that usually develop during infancy and early childhood.3 Although AD severity improves for some patients in adolescence, it can be a lifelong issue affecting performance in academic and occupational settings.5 One US study of 8015 children found that there are racial and ethnic disparities in school absences among children (age range, 2–17 years) with AD, with children with skin of color being absent more often than white children.6 The same study noted that black children had a 1.5-fold higher chance of being absent 6 days over a 6-month school period compared to white children. It is postulated that AD has a greater impact on quality of life (QOL) in children with skin of color, resulting in the increased number of school absences in this population.6
The origin of AD currently is thought to be complex and can involve skin barrier dysfunction, environmental factors, microbiome effects, genetic predisposition, and immune dysregulation.1,4 Atopic dermatitis is a heterogeneous disease with variations in the prevalence, genetic background, and immune activation patterns across racial groups.4 It is now understood to be an immune-mediated disease with multiple inflammatory pathways, with type 2–associated inflammation being a primary pathway. Patients with AD have strong helper T cell (TH2) activation, and black patients with AD have higher IgE serum levels as well as absent TH17/TH1 activation.4
Atopic dermatitis currently is seen as a defect of the epidermal barrier, with variable clinical manifestations and expressivity.7 Filaggrin is an epidermal barrier protein, encoded by the FLG gene, and plays a major role in barrier function by regulating pH and promoting hydration of the skin.4 Loss of function of the FLG gene is the most well-studied genetic risk factor for developing AD, and this mutation is seen in patients with more severe and persistent AD in addition to patients with more skin infections and allergic sensitizations.3,4 However, in the skin of color population, FLG mutations are 6 times less common than in the EA population, despite the fact that AD is more prevalent in patients of African descent.4 Therefore, the role of the FLG loss-of-function mutation and AD is not as well defined in black patients, and some researchers have found no association.3 The FLG loss-of-function mutation seems to play a smaller role in black patients than in EA patients, and other genes may be involved in skin barrier dysfunction.3,4 In a small study of patients with mild AD compared to nonaffected patients, those with AD had lower total ceramide levels in the stratum corneum of affected sites than normal skin sites in healthy individuals.8
Particular disturbances in the gut microbiome have the possibility of impacting the development of AD.9 Additionally, the development of AD may be influenced by the skin microbiome, which can change depending on body site, with fungal organisms thought to make up a large proportion of the microbiome of patients with AD. In patients with AD, there is a lack of microbial diversity and an overgrowth of Staphylococcus aureus.9
Diagnosis
Clinicians diagnose AD based on clinical characteristics, and the lack of objective criteria can hinder diagnosis.1 Thus, diagnosing AD in children with dark skin can pose a particular challenge given the varied clinical presentation of AD across skin types. Severe cases of AD may not be diagnosed or treated adequately in deeply pigmented children because erythema, a defining characteristic of AD, may be hard to identify in darker skin types.10 Furthermore, clinical erythema scores among black children may be “strongly” underestimated using scoring systems such as Eczema Area and Severity Index and SCORing Atopic Dermatitis.4 It is estimated that the risk for severe AD may be 6 times higher in black children compared to white children.10 Additionally, patients with skin of color can present with more treatment-resistant AD.4
Treatment of AD
Current treatment is focused on restoring epidermal barrier function, often with topical agents, such as moisturizers containing different amounts of emollients, occlusives, and humectants; corticosteroids; calcineurin inhibitors; and antimicrobials. Emollients such as glycol stearate, glyceryl stearate, and soy sterols function as lubricants, softening the skin. Occlusive agents include petrolatum, dimethicone, and mineral oil; they act by forming a layer to slow evaporation of water. Humectants including glycerol, lactic acid, and urea function by promoting water retention.11 For acute flares, mid- to high-potency topical corticosteroids are recommended. Also, topical calcineurin inhibitors such as tacrolimus and pimecrolimus may be used alone or in combination with topical steroids. Finally, bleach baths and topical mupirocin applied to the nares also have proved helpful in moderate to severe AD with secondary bacterial infections.11 Phototherapy can be used in adult and pediatric patients with acute and chronic AD if traditional treatments have failed.2
Systemic agents are indicated and recommended for the subset of adult and pediatric patients in whom optimized topical regimens and/or phototherapy do not adequately provide disease control or when QOL is substantially impacted. The systemic agents effective in the pediatric population include cyclosporine, azathioprine, mycophenolate mofetil, and possibly methotrexate.11 Dupilumab recently was approved by the US Food and Drug Administration for patients 12 years and older with moderate to severe AD whose disease is not well controlled with topical medications.
Patients with AD are predisposed to secondary bacterial and viral infections because of their dysfunctional skin barrier; these infections most commonly are caused by S aureus and herpes simplex virus, respectively.2 Systemic antibiotics are only recommended for patients with AD when there is clinical evidence of bacterial infection. In patients with evidence of eczema herpeticum, systemic antiviral agents should be used to treat the underlying herpes simplex virus infection.2 Atopic dermatitis typically has been studied in white patients; however, patients with skin of color have higher frequencies of treatment-resistant AD. Further research on treatment efficacy for AD in this patient population is needed, as data are limited.4
Treatment of AV in Patients With AD
Two of the most prevalent skin diseases affecting the pediatric population are AD and AV, and both can remarkably impact QOL.12 Acne is one of the most common reasons for adolescent patients to seek dermatologic care, including patients with skin of color (Fitzpatrick skin types IV to VI).13 Thus, it is to be expected that many black adolescents with AD also will have AV. For mild to moderate acne in patients with skin of color, topical retinoids and benzoyl peroxide typically are first line.13 These medications can be problematic for patients with AD, as retinoids and many other acne treatments can cause dryness, which may exacerbate AD.
Moisturizers containing ceramide can be a helpful adjunctive therapy in treating acne,14 especially in patients with AD. Modifications to application of acne medications, such as using topical retinoids every other night or mixing them with moisturizers to minimize dryness, may be beneficial to these patients. Dapsone gel 7.5% used daily also may be an option for adolescents with AD and AV. A double-blind, vehicle-controlled study demonstrated that dapsone is safe and effective for patients 12 years and older with moderate acne, and patients with Fitzpatrick skin types IV to VI rated local scaling, erythema, dryness, and stinging/burning as “none” in the study.15 Another potentially helpful topical agent in patients with AD and AV is sulfacetamide, as it is not likely to cause dryness of the skin. In a small study, sodium sulfacetamide 10% and sulfur 5% in an emollient foam vehicle showed no residual film or sulfur smell and resulted in acne reduction of 50%.16
Patients with skin of color often experience PIH in AD and acne or hypopigmentation from inflammatory dermatoses including AD.17,18 In addition to the dryness from AD and topical retinoid use, patients with skin of color may develop irritant contact dermatitis, thus leading to PIH.13 Dryness and irritant contact dermatitis also can be seen with the use of benzoyl peroxide in black patients. Because of these effects, gentle moisturizers are recommended, and both benzoyl peroxide and retinoids should be initiated at lower doses in patients with skin of color.13
For patients with severe nodulocystic acne, isotretinoin is the treatment of choice in patients with skin of color,13 but there is a dearth of clinical studies addressing complications seen in black adolescents on this treatment, especially with respect to those with AD. Of note, systemic antibiotics typically are initiated before isotretinoin; however, this strategy is falling out of favor due to concern for antibiotic resistance with long-term use.19
Impact of Athletics on AD in Black Adolescents
Because of the exacerbating effects of perspiration and heat causing itch and irritation in patients with AD, it is frequently advised that pediatric patients limit their participation in athletics because of the exacerbating effects of strenuous physical exercise on their disease.12 In one study, 429 pediatric patients or their parents/guardians completed QOL questionnaires; 89% of patients 15 years and younger with severe AD reported that their disease was impacted by athletics and outdoor activities, and 86% of these pediatric patients with severe AD responded that their social lives and leisure activities were impacted.20 Because adolescents often are involved in athletics or have mandatory physical education classes, AD may be isolating and may have a severe impact on self-esteem.
Aggressive treatment of AD with topical and systemic medications may be helpful in adolescents who may be reluctant to participate in sports because of teasing, bullying, or worsening of symptoms with heat or sweating.21 Now that dupilumab is available for adolescents, there is a chance that patients with severe and/or recalcitrant disease managed on this medication can achieve better control of their symptoms without the laboratory requirement of methotrexate and the difficulties of topical medication application, allowing them to engage in mandatory athletic classes as well as desired organized sports.
Use of Cosmetics for AD
Many adolescents experiment with cosmetics, and those with AD may use cosmetic products to cover hyperpigmented or hypopigmented lesions.18 In patients with active AD or increased sensitivity to allergens in cosmetic products, use of makeup can be a contributing factor for AD flares. Acne associated with cosmetics is especially important to consider in darker-skinned patients who may use makeup that is opaque and contains oil to conceal acne or PIH.
Allergens can be present in both cosmetics and pharmaceutical topical agents, and a Brazilian study found that approximately 89% of 813 prescription and nonprescription products (eg, topical drugs, sunscreens, moisturizers, soaps, cleansing lotions, shampoos, cosmeceuticals) contained allergens.22 Patients with AD have a higher prevalence of contact sensitization to fragrances, including balsam of Peru.23 Some AD treatments that contain fragrances have caused further skin issues in a few patients. In one case series, 3 pediatric patients developed allergic contact dermatitis to Myroxylon pereirae (balsam of Peru) when using topical treatments for their AD, and their symptoms of scalp inflammation and alopecia resolved with discontinuation.23
In a Dutch study, sensitization to Fragrance Mix I and M pereirae as well as other ingredients (eg, lanolin alcohol, Amerchol™ L 101 [a lanolin product]) was notably more common in pediatric patients with AD than in patients without AD; however, no data on patients with skin of color were included in this study.24
Because of the increased risk of sensitization to fragrances and other ingredients in patients with AD as well as the high percentage of allergens in prescription and nonprescription products, it is important to discuss all personal care products that patients may be using, not just their cosmetic products. Also, patch testing may be helpful in determining true allergens in some patients. Patch testing is recommended for patients with treatment-resistant AD, and a recent study suggested it should be done prior to long-term use of immunosuppressive agents.25 Increased steroid phobia and a push toward alternative medicines are leading both patients with AD and guardians of children with AD to look for other forms of moisturization, such as olive oil, coconut oil, sunflower seed oil, and shea butter, to decrease transepidermal water loss.26,27 An important factor in AD treatment efficacy is patient acceptability in using what is recommended.27 One study showed there was no difference in efficacy or acceptability in using a cream containing shea butter extract vs the ceramide-precursor product.27 Current data show olive oil may exacerbate dry skin and AD,26 and recommendation of any over-the-counter oils and butters in patients with AD should be made with great caution, as many of these products contain fragrances and other potential allergens.
Alternative Therapies for AD
Patients with AD often seek alternative or integrative treatment options, including dietary modifications and holistic remedies. Studies investigating the role of vitamins and supplements in treating AD are limited by sample size.28 However, there is some evidence that may support supplementation with vitamins D and E in addressing AD symptoms. The use of probiotics in treating AD is controversial, but there are studies suggesting that the use of probiotics may prove beneficial in preventing infantile AD.28 Additionally, findings from an ex vivo and in vitro study show that some conditions, including AD and acne, may benefit from the same probiotics, despite the differences in these two diseases. Both AD and acne have inflammatory and skin dysbiosis characteristics, which may be the common thread leading to both conditions potentially responding to treatment with probiotics.29
Preliminary evidence indicates that supplements containing fatty acids such as docosahexaenoic acid, sea buckthorn oil, and hemp seed oil may decrease the severity of AD.28 In a 20-week, randomized, single-blind, crossover study published in 2005, dietary hemp seed oil showed an improvement of clinical symptoms, including dry skin and itchiness, in patients with AD.30
In light of recent legalization in several states, patients may turn to use of cannabinoid products to manage AD. In a systematic review, cannabinoid use was reportedly a therapeutic option in the treatment of AD and AV; however, the data are based on preclinical work, and there are no randomized, placebo-controlled studies to support the use of cannabinoids.31 Furthermore, there is great concern that use of these products in adolescents is an even larger unknown.
Final Thoughts
Eighty percent of children diagnosed with AD experience symptom improvement before their early teens32; for those with AD during their preteen and teenage years, there can be psychological ramifications, as teenagers with AD report having fewer friends, are less socially involved, participate in fewer sports, and are absent from classes more often than their peers.5 In black patients with AD, school absences are even more common.6 Given the social and emotional impact of AD on patients with skin of color, it is imperative to treat the condition appropriately.33 There are areas of opportunity for further research on alternate dosing of existing treatments for AV in patients with AD, further recommendations for adolescent athletes with AD, and which cosmetic and alternative medicine products may be beneficial for this population to improve their QOL.
Providers should discuss medical management in a broader context considering patients’ extracurricular activities, treatment vehicle preferences, expectations, and personal care habits. It also is important to address the many possible factors that may influence treatment adherence early on, particularly in adolescents, as these could be barriers to treatment. This article highlights considerations for treating AD and comorbid conditions that may further complicate treatment in adolescent patients with skin of color. The information provided should serve as a guide in initial counseling and management of AD in adolescents with skin of color.
- Feldman SR, Cox LS, Strowd LC, et al. The challenge of managing atopic dermatitis in the United States. Am Health Drug Benefits. 2019;12:83-93.
- Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: section 3. management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-349.
- Kaufman BP, Guttman-Yassky E, Alexis AF. Atopic dermatitis in diverse racial and ethnic groups—variations in epidemiology, genetics, clinical presentation and treatment. Exp Dermatol. 2018;27:340-357.
- Brunner PM, Guttman-Yassky E. Racial differences in atopic dermatitis. Ann Allergy Asthma Immunol. 2019;122:449-455.
- Vivar KL, Kruse L. The impact of pediatric skin disease on self-esteem. Int J Womens Dermatol. 2018;4:27-31.
- Wan J, Margolis DJ, Mitra N, et al. Racial and ethnic differences in atopic dermatitis–related school absences among US children [published online May 22, 2019]. JAMA Dermatol. doi:10.1001/jamadermatol.2019.0597.
- Weidinger S, Novak N. Atopic dermatitis. Lancet. 2016;387:1109-1122.
- Ishikawa J, Narita H, Kondo N, et al. Changes in the ceramide profile of atopic dermatitis patients. J Invest Dermatol. 2010;130:2511-2514.
- Chernikova D, Yuan I, Shaker M. Prevention of allergy with diverse and healthy microbiota: an update. Curr Opin Pediatr. 2019;31:418-425.
- Ben-Gashir MA, Hay RJ. Reliance on erythema scores may mask severe atopic dermatitis in black children compared with their white counterparts. Br J Dermatol. 2002;147:920-925.
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2. management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132.
- Nguyen CM, Koo J, Cordoro KM. Psychodermatologic effects of atopic dermatitis and acne: a review on self-esteem and identity. Pediatr Dermatol. 2016;33:129-135.
- Davis EC, Callender VD. A review of acne in ethnic skin: pathogenesis, clinical manifestations, and management strategies. J Clin Aesthet Dermatol. 2010;3:24-38.
- Lynde CW, Andriessen A, Barankin B, et al. Moisturizers and ceramide-containing moisturizers may offer concomitant therapy with benefits. J Clin Aesthet Dermatol. 2014;7:18-26.
- Taylor SC, Cook-Bolden FE, McMichael A, et al. Efficacy, safety, and tolerability of topical dapsone gel, 7.5% for treatment of acne vulgaris by Fitzpatrick skin phototype. J Drugs Dermatol. 2018;17:160-167.
- Draelos ZD. The multifunctionality of 10% sodium sulfacetamide, 5% sulfur emollient foam in the treatment of inflammatory facial dermatoses. J Drugs Dermatol. 2010;9:234-236.
- Vachiramon V, Tey HL, Thompson AE, et al. Atopic dermatitis in African American children: addressing unmet needs of a common disease. Pediatr Dermatol. 2012;29:395-402.
- Heath CR. Managing postinflammatory hyperpigmentation in pediatric patients with skin of color. Cutis. 2018;102:71-73.
- Nagler AR, Milam EC, Orlow SJ. The use of oral antibiotics before isotretinoin therapy in patients with acne. J Am Acad Dermatol. 2016;74:273-279.
- Paller AS, McAlister RO, Doyle JJ, et al. Perceptions of physicians and pediatric patients about atopic dermatitis, its impact, and its treatment. Clin Pediatr. 2002;41:323-332.
- Sibbald C, Drucker AM. Patient burden of atopic dermatitis. Dermatol Clin. 2017;35:303-316.
- Rocha VB, Machado CJ, Bittencourt FV. Presence of allergens in the vehicles of Brazilian dermatological products. Contact Dermatitis. 2017;76:126-128.
- Admani S, Goldenberg A, Jacob SE. Contact alopecia: improvement of alopecia with discontinuation of fluocinolone oil in individuals allergic to balsam fragrance. Pediatr Dermatol. 2017;34:e57-e60.
- Uter W, Werfel T, White IR, et al. Contact allergy: a review of current problems from a clinical perspective. Int J Environ Res Public Health. 2018;15:E1108.
- López-Jiménez EC, Marrero-Alemán G, Borrego L. One-third of patients with therapy-resistant atopic dermatitis may benefit after patch testing [published online May 13, 2019]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.15672.
- Karagounis TK, Gittler JK, Rotemberg V, et al. Use of “natural” oils for moisturization: review of olive, coconut, and sunflower seed oil. Pediatr Dermatol. 2019;36:9-15.
- Hon KL, Tsang YC, Pong NH, et al. Patient acceptability, efficacy, and skin biophysiology of a cream and cleanser containing lipid complex with shea butter extract versus a ceramide product for eczema. Hong Kong Med J. 2015;21:417-425.
- Reynolds KA, Juhasz MLW, Mesinkovska NA. The role of oral vitamins and supplements in the management of atopic dermatitis: a systematic review [published online March 20, 2019]. Int J Dermatol. doi:10.1111/ijd.14404.
- Mottin VHM, Suyenaga ES. An approach on the potential use of probiotics in the treatment of skin conditions: acne and atopic dermatitis. Int J Dermatol. 2018;57:1425-1432.
- Callaway J, Schwab U, Harvima I, et al. Efficacy of dietary hempseed oil in patients with atopic dermatitis. J Dermatol Treat. 2005;16:87-94.
- Eagleston LRM, Kalani NK, Patel RR, et al. Cannabinoids in dermatology: a scoping review [published June 15, 2018]. Dermatol Online J. 2018;24.
- Kim JP, Chao LX, Simpson EL, et al. Persistence of atopic dermatitis (AD): a systematic review and meta-analysis. J Am Acad Dermatol. 2016;75:681-687.e611.
- de María Díaz Granados L, Quijano MA, Ramírez PA, et al. Quality assessment of atopic dermatitis clinical practice guidelines in ≤ 18 years. Arch Dermatol Res. 2018;310:29-37.
- Feldman SR, Cox LS, Strowd LC, et al. The challenge of managing atopic dermatitis in the United States. Am Health Drug Benefits. 2019;12:83-93.
- Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: section 3. management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-349.
- Kaufman BP, Guttman-Yassky E, Alexis AF. Atopic dermatitis in diverse racial and ethnic groups—variations in epidemiology, genetics, clinical presentation and treatment. Exp Dermatol. 2018;27:340-357.
- Brunner PM, Guttman-Yassky E. Racial differences in atopic dermatitis. Ann Allergy Asthma Immunol. 2019;122:449-455.
- Vivar KL, Kruse L. The impact of pediatric skin disease on self-esteem. Int J Womens Dermatol. 2018;4:27-31.
- Wan J, Margolis DJ, Mitra N, et al. Racial and ethnic differences in atopic dermatitis–related school absences among US children [published online May 22, 2019]. JAMA Dermatol. doi:10.1001/jamadermatol.2019.0597.
- Weidinger S, Novak N. Atopic dermatitis. Lancet. 2016;387:1109-1122.
- Ishikawa J, Narita H, Kondo N, et al. Changes in the ceramide profile of atopic dermatitis patients. J Invest Dermatol. 2010;130:2511-2514.
- Chernikova D, Yuan I, Shaker M. Prevention of allergy with diverse and healthy microbiota: an update. Curr Opin Pediatr. 2019;31:418-425.
- Ben-Gashir MA, Hay RJ. Reliance on erythema scores may mask severe atopic dermatitis in black children compared with their white counterparts. Br J Dermatol. 2002;147:920-925.
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2. management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132.
- Nguyen CM, Koo J, Cordoro KM. Psychodermatologic effects of atopic dermatitis and acne: a review on self-esteem and identity. Pediatr Dermatol. 2016;33:129-135.
- Davis EC, Callender VD. A review of acne in ethnic skin: pathogenesis, clinical manifestations, and management strategies. J Clin Aesthet Dermatol. 2010;3:24-38.
- Lynde CW, Andriessen A, Barankin B, et al. Moisturizers and ceramide-containing moisturizers may offer concomitant therapy with benefits. J Clin Aesthet Dermatol. 2014;7:18-26.
- Taylor SC, Cook-Bolden FE, McMichael A, et al. Efficacy, safety, and tolerability of topical dapsone gel, 7.5% for treatment of acne vulgaris by Fitzpatrick skin phototype. J Drugs Dermatol. 2018;17:160-167.
- Draelos ZD. The multifunctionality of 10% sodium sulfacetamide, 5% sulfur emollient foam in the treatment of inflammatory facial dermatoses. J Drugs Dermatol. 2010;9:234-236.
- Vachiramon V, Tey HL, Thompson AE, et al. Atopic dermatitis in African American children: addressing unmet needs of a common disease. Pediatr Dermatol. 2012;29:395-402.
- Heath CR. Managing postinflammatory hyperpigmentation in pediatric patients with skin of color. Cutis. 2018;102:71-73.
- Nagler AR, Milam EC, Orlow SJ. The use of oral antibiotics before isotretinoin therapy in patients with acne. J Am Acad Dermatol. 2016;74:273-279.
- Paller AS, McAlister RO, Doyle JJ, et al. Perceptions of physicians and pediatric patients about atopic dermatitis, its impact, and its treatment. Clin Pediatr. 2002;41:323-332.
- Sibbald C, Drucker AM. Patient burden of atopic dermatitis. Dermatol Clin. 2017;35:303-316.
- Rocha VB, Machado CJ, Bittencourt FV. Presence of allergens in the vehicles of Brazilian dermatological products. Contact Dermatitis. 2017;76:126-128.
- Admani S, Goldenberg A, Jacob SE. Contact alopecia: improvement of alopecia with discontinuation of fluocinolone oil in individuals allergic to balsam fragrance. Pediatr Dermatol. 2017;34:e57-e60.
- Uter W, Werfel T, White IR, et al. Contact allergy: a review of current problems from a clinical perspective. Int J Environ Res Public Health. 2018;15:E1108.
- López-Jiménez EC, Marrero-Alemán G, Borrego L. One-third of patients with therapy-resistant atopic dermatitis may benefit after patch testing [published online May 13, 2019]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.15672.
- Karagounis TK, Gittler JK, Rotemberg V, et al. Use of “natural” oils for moisturization: review of olive, coconut, and sunflower seed oil. Pediatr Dermatol. 2019;36:9-15.
- Hon KL, Tsang YC, Pong NH, et al. Patient acceptability, efficacy, and skin biophysiology of a cream and cleanser containing lipid complex with shea butter extract versus a ceramide product for eczema. Hong Kong Med J. 2015;21:417-425.
- Reynolds KA, Juhasz MLW, Mesinkovska NA. The role of oral vitamins and supplements in the management of atopic dermatitis: a systematic review [published online March 20, 2019]. Int J Dermatol. doi:10.1111/ijd.14404.
- Mottin VHM, Suyenaga ES. An approach on the potential use of probiotics in the treatment of skin conditions: acne and atopic dermatitis. Int J Dermatol. 2018;57:1425-1432.
- Callaway J, Schwab U, Harvima I, et al. Efficacy of dietary hempseed oil in patients with atopic dermatitis. J Dermatol Treat. 2005;16:87-94.
- Eagleston LRM, Kalani NK, Patel RR, et al. Cannabinoids in dermatology: a scoping review [published June 15, 2018]. Dermatol Online J. 2018;24.
- Kim JP, Chao LX, Simpson EL, et al. Persistence of atopic dermatitis (AD): a systematic review and meta-analysis. J Am Acad Dermatol. 2016;75:681-687.e611.
- de María Díaz Granados L, Quijano MA, Ramírez PA, et al. Quality assessment of atopic dermatitis clinical practice guidelines in ≤ 18 years. Arch Dermatol Res. 2018;310:29-37.
Practice Points
- Atopic dermatitis (AD) can be a lifelong issue that affects academic and occupational performance, with higher rates of absenteeism seen in black patients.
- The FLG loss-of-function mutation seems to play a smaller role in black patients, and other genes may be involved in skin barrier dysfunction, which could be why there is a higher rate of skin of color patients with treatment-resistant AD.
- Diagnosing AD in skin of color patients can pose a particular challenge, and severe cases of AD may not be diagnosed or treated adequately in deeply pigmented children because erythema, a defining characteristic of AD, may be hard to identify in darker skin tones.
- There are several areas of opportunity for further research to better treat AD in this patient population and improve
quality of life.
Atopic Dermatitis in the US Military
Dermatologic conditions historically have affected military members’ ability to serve during times of peace and conflict. These conditions range from chronic dermatologic diseases to environment- or occupation-related dermatologic diseases. Mild to moderate atopic dermatitis (AD) typically is a manageable skin condition. However, in a deployed setting, a flare of AD can result in the inability of a member to perform their military duty, which directly compromises mission safety and effectiveness. The military developed and updates medical standards for entry and retention of service members. These standards are designed to ensure the greatest potential for a military member to successfully serve at home station and during combat operations.
Impact of Injuries in Military
Historically, disease and nonbattle injuries have resulted in notably more hospitalizations and time lost than injuries sustained on the battlefield.1 A review of major conflicts dating from World War II shows approximately 10% of all dermatologic concerns were related to eczematous dermatitis, with 2% specifically related to AD. These numbers varied remarkably depending on the location and environment of the conflict, with eczema accounting for 25% of dermatologic concerns during the Gulf War.2 During the initial phases of Operation Iraqi Freedom, approximately 75% of hospitalizations were from disease and nonbattle injuries, of which dermatologic disease accounted for 3%.1 From 2003 to 2006 in Iraq, 35 service members were evacuated from combat zones specifically for uncontrolled AD.3 In a deployed environment, each member is critical to the unit’s success in completing their mission. A single member of a unit often is the only person qualified to perform a function for that team. There are rarely extra people with similar skills to replace a member unable to complete his/her duties. The loss of a single member compromises the effectiveness and safety of the team and can lead to mission failure. Therefore, AD can have a profound impact on military operations in a deployed environment.
Military Medical Standards for Accession and Retention
There are 2 main goals of the military medical standards. First, the individual health of the applicant or military member is of utmost importance. Applicants with medical conditions that will be exacerbated by military service or that limit the ability for successful military operations are not accepted for military service. Once an active-duty member is diagnosed with a medical condition, the military determines if limitations are needed for military assignments and deployments based on available medical care in those locations. Second, mission accomplishment in combat operations requires that healthy military members are able to complete their jobs in extreme environments and under notable stress. If an applicant has a medical condition unsuitable for military service, it is in the best interest of the applicant and the military to deny entry.
The Medical Standards for Appointment, Enlistment, or Induction Into the Military Services (DoD Instruction 6130.03) lists conditions that are disqualifying for military service.4 Section 5.21 lists the following as disqualifying for military service in relation to eczematous dermatitis:
d. History of AD or eczema after the 12th birthday. History of residual or recurrent lesions in characteristic areas (face, neck, antecubital or popliteal fossae, occasionally wrists and hands).
e. History of recurrent or chronic nonspecific dermatitis within the past 2 years to include contact (irritant or allergic) or dyshidrotic dermatitis requiring more than treatment with topical corticosteroid.4
Although cases of incorrect diagnosis or very mild AD can be considered for a waiver, the process can be laborious and consideration or approval is not guaranteed. For current military members with new chronic eczematous dermatitis, each service has a process for evaluation and treatment. Some special operational jobs, such as aircrew, missile operators, and divers, have more restrictive medical requirements that are monitored by physicians with special training in these populations.
Atopic dermatitis affects 25% of children and 2% to 3% of adults.5 Approximately 60% of patients with AD will develop their first eruption by 1 year of age, and 90% by 5 years of age. Although the majority of patients will have resolution of their disease during childhood, 10% to 30% will have persistent disease into adulthood.5 Because the majority of AD resolves in childhood, it is understandable that asymptomatic individuals with a history of AD before 12 years of age meet military entrance medical standards.
Provoking Factors
The US Military maintains stringent medical standards because of the nature of the dynamic, rapidly changing military environment and its demands. Whether training for readiness in an austere location, deploying to extreme climates, or being stationed overseas, service members must be prepared to encounter a myriad of environmental extremes, physical stress, and psychological stressors. Environmental factors commonly experienced in the military can provoke or exacerbate symptoms of AD (Figures 1 and 2). Ideally, an individual with AD lives in a stable climate, has access to moisturizers and topical steroids, bathes regularly to remove dust and debris, wears 100% cotton garments to avoid irritation, and avoids using gear that would cause exacerbations. Service members rarely have such accommodations in deployed settings. A recent article in Military Medicine explained quite well, “If someone wanted to design an experience with the explicit goal to flare a person with otherwise well controlled atopic dermatitis it would probably look like a military deployment.”3
The United States has a military presence in countries with extreme temperature and humidity variations all over the world. Uniforms are standardized, and members are required to wear prescribed clothing with no alternatives. Uniforms are made of durable sturdy material. If uniforms can be laundered, they often are grouped together, and sensitive detergent cannot be specified. Bathing is challenging in deployed locations, with troops often going weeks using baby wipes for self-hygiene. These conditions increase risk for development of contact allergens, and little access to proper hygiene practices also increases risk for secondary infections in members with AD.
In addition to environmental challenges, the military gear and equipment used can flare AD. Service members must wear protective gear such as body armor. These heavy hard pieces of material are bulky; difficult to wash; and cause friction, sweating, and irritation. The military prepares for operations in chemical, biological, radiological, or nuclear environments, which requires wearing a rubber mask, multiple layers of boots and gloves, and thick charcoal impregnated over garments for many hours. Such conditions may flare AD or make it intolerable.
Although stress is a part of any deployment experience, excessive or prolonged stress can lead to combat operational stress reactions that inhibit a service member’s ability to function.6 Stressors during deployment can accumulate and may be caused by the operational environment, loss of fellow service members to injury or death, illness, leadership demands, personal choices, issues on the home front, interpersonal conflicts, and sleep loss.7 Atopic dermatitis can be exacerbated by such stress, leading to increased pruritus and scratching.7-9 Symptomatic AD also can play a role in worsening combat stress. Although severe pruritus may affect attentiveness to job duties during the day, these symptoms, if uncontrolled, also can negatively affect sleep. As many as 60% of patients with AD at baseline and 83% of patients with exacerbations experience sleep disturbance due to their disease.5 These stressors experienced by deployed military personnel can contribute to combat stress reactions, which may vary from simple inattentiveness to more serious behaviors such as suicidal ideation.6 Combat stress reactions inhibit a military member’s ability to function properly in the deployed environment and can lead to notable safety concerns and potential mission failure.
Vaccinations
Military members deploying overseas are required to receive specific vaccinations, including the smallpox vaccine. Although the virus was eradicated in 1980, the concern for smallpox to be used as a biological weapon in certain areas of the world necessitates continued vaccination of military populations. According to the Centers for Disease Control and Prevention, the only known reservoir for the virus is humans, and the disease has a mortality rate of 30%.10 A history of or present AD is a contraindication for primary smallpox vaccination and revaccination for nonemergency use because of the risk for eczema vaccinatum.11 The risk also applies to close contacts of vaccinated members. For 30 days after vaccination, service members must avoid skin-to-skin contact with anyone who has active AD.12 Eczema vaccinatum in vaccinated individuals is typically self-limited; however, eczema vaccinatum in nonvaccinated contacts can be severe. One case report described a 28-month-old child with refractory AD who developed severe eczema vaccinatum after contact with her recently vaccinated military parent. The child required a 48-day admission to the intensive care unit and multiple skin grafts; fortunately, the child did not develop any apparent long-term sequelae.13 This case highlights the importance of understanding the risks associated with smallpox vaccination in military members with AD and the responsibility of health care providers to properly screen and counsel individuals prior to administering smallpox vaccines.
Treatment
Treatment of mild to moderate AD is relatively straightforward in developed countries with good access to medical care. The most recent American Academy of Dermatology clinical guidelines for AD focus on minimizing irritants and triggers, regularly using moisturizers soon after bathing, and using topical steroids as needed.5 Military members face specific challenges regarding treatment of AD, particularly when deployed to remote locations without access to treatment facilities or medications. Military members are required to carry all necessary personal medications with them for at least 6 months and preferably the duration of the deployment, sometimes up to 1 year. Military members carry a large amount of gear for deployments, and it is not feasible to pack an additional 10 to 20 lb worth of emollients and topical steroids to last the entire deployment. Routine laboratory monitoring is limited or completely unavailable. Refrigeration typically is not available, making use of systemic medications nearly impossible during deployments. In the event of complications such as eczema herpeticum or secondary bacterial infection, service members could require evacuation from the deployed location to a larger field hospital or to the United States, which is costly and also removes a valuable team member from the deployed unit. These limitations in access to care, medications, and treatment options make AD a difficult condition to treat in the deployed setting.
Nonmilitary Medical Providers
Civilian providers play an important role in diagnosing and treating AD. It is vital to completely and accurately document treatment of all skin diseases; however, it is especially important for those who desire to or currently serve in the military. Military primary care providers or military dermatologists must review the information from civilian providers to aid in determining suitability for entry or retention in the military. Clearly documenting the morphology, extent of disease involvement (eg, body surface area), treatment plan, response to treatment, and exacerbating factors will aid in ensuring the patient’s medical record accurately reflects their skin disease. Ultimately, this record often is the only information available to make health determinations regarding military service.
Conclusion
A career in the military is challenging and rewarding for those who volunteer to serve. Because of the demanding and unpredictable lifestyle inherent with military service, the Department of Defense maintains strict medical standards for entrance and retention. These standards ensure members are capable of safely completing training and deploying anywhere in the world. Although AD is a relatively common and treatable skin disease in locations with well-established medical care, it can pose a notable problem for service members while deployed to austere locations with variable environments around the world. Environmental factors and gear requirements, coupled with limited access to treatment facilities and medications, render AD a potentially serious issue. Atopic dermatitis in military members can affect individual medical readiness and unit success. It is important that all providers understand the myriad effects that AD can have on an individual who wishes to join or continue service in the military.
- Zouris JM, Wade AL, Magno CP. Injury and illness casualty distributions among U.S. Army and Marine Corps personnel during Operation Iraqi Freedom. Mil Med. 2008;173:247-252.
- Gelman, AB, Norton SA, Valdes-Rodriguez R, et al. A review of skin conditions in modern warfare and peacekeeping operations. Mil Med. 2015;180:32-37.
- Jeter J, Bowen C. Atopic dermatitis and implications for military service. Mil Med. 2019;184:177-182.
- Medical Standards for Appointment, Enlistment, or Induction Into the Military Services (DoD Instruction 6130.03). Washington, DC: Department of Defense; May 6, 2018. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003p.pdf?ver=2018-05-04-113917-883. Accessed May 8, 2019.
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351.
- Force Health Protection (Army Techniques Publication No. 4-02.8). Washington, DC: Department of the Army; March 2016. https://armypubs.army.mil/epubs/DR_pubs/DR_a/pdf/web/atp4_02x8.pdf. Accessed August 19, 2019.
- Judkins JL, Bradley DL. A review of the effectiveness of a combat and operational stress control restoration center in Afghanistan. Mil Med. 2017;182:1755-1762.
- Suarez AL, Feramisco JD, Koo J, et al. Psychoneuroimmunology of psychological stress and atopic dermatitis: pathophysiologic and therapeutic updates. Acta Dermatol Venereol. 2012;92:7-15.
- Mochizuki H, Lavery MJ, Nattkemper LA, et al. Impact of acute stress on itch sensation and scratching behaviour in patients with atopic dermatitis and healthy controls. Br J Dermatol. 2019;180:821-827.
- Centers for Disease Control and Prevention. Smallpox: contraindications to vaccination. https://www.cdc.gov/smallpox/clinicians/vaccination-contraindications1.html. Updated December 5, 2016. Accessed August 19, 2019.
- Kemper AR, Davis MM, Freed GL. Expected adverse events in a mass smallpox vaccination campaign. Eff Clin Pract. 2002;5:84-90.
- Reed JL, Scott DE, Bray M. Eczema vaccinatum. Clin Infect Dis. 2012;54:832-840.
- Vora S, Damon I, Fulginiti V, et al. Severe eczema vaccinatum in a household contact of a smallpox vaccine. Clin Infect Dis. 2008;46:1555-1561.
Dermatologic conditions historically have affected military members’ ability to serve during times of peace and conflict. These conditions range from chronic dermatologic diseases to environment- or occupation-related dermatologic diseases. Mild to moderate atopic dermatitis (AD) typically is a manageable skin condition. However, in a deployed setting, a flare of AD can result in the inability of a member to perform their military duty, which directly compromises mission safety and effectiveness. The military developed and updates medical standards for entry and retention of service members. These standards are designed to ensure the greatest potential for a military member to successfully serve at home station and during combat operations.
Impact of Injuries in Military
Historically, disease and nonbattle injuries have resulted in notably more hospitalizations and time lost than injuries sustained on the battlefield.1 A review of major conflicts dating from World War II shows approximately 10% of all dermatologic concerns were related to eczematous dermatitis, with 2% specifically related to AD. These numbers varied remarkably depending on the location and environment of the conflict, with eczema accounting for 25% of dermatologic concerns during the Gulf War.2 During the initial phases of Operation Iraqi Freedom, approximately 75% of hospitalizations were from disease and nonbattle injuries, of which dermatologic disease accounted for 3%.1 From 2003 to 2006 in Iraq, 35 service members were evacuated from combat zones specifically for uncontrolled AD.3 In a deployed environment, each member is critical to the unit’s success in completing their mission. A single member of a unit often is the only person qualified to perform a function for that team. There are rarely extra people with similar skills to replace a member unable to complete his/her duties. The loss of a single member compromises the effectiveness and safety of the team and can lead to mission failure. Therefore, AD can have a profound impact on military operations in a deployed environment.
Military Medical Standards for Accession and Retention
There are 2 main goals of the military medical standards. First, the individual health of the applicant or military member is of utmost importance. Applicants with medical conditions that will be exacerbated by military service or that limit the ability for successful military operations are not accepted for military service. Once an active-duty member is diagnosed with a medical condition, the military determines if limitations are needed for military assignments and deployments based on available medical care in those locations. Second, mission accomplishment in combat operations requires that healthy military members are able to complete their jobs in extreme environments and under notable stress. If an applicant has a medical condition unsuitable for military service, it is in the best interest of the applicant and the military to deny entry.
The Medical Standards for Appointment, Enlistment, or Induction Into the Military Services (DoD Instruction 6130.03) lists conditions that are disqualifying for military service.4 Section 5.21 lists the following as disqualifying for military service in relation to eczematous dermatitis:
d. History of AD or eczema after the 12th birthday. History of residual or recurrent lesions in characteristic areas (face, neck, antecubital or popliteal fossae, occasionally wrists and hands).
e. History of recurrent or chronic nonspecific dermatitis within the past 2 years to include contact (irritant or allergic) or dyshidrotic dermatitis requiring more than treatment with topical corticosteroid.4
Although cases of incorrect diagnosis or very mild AD can be considered for a waiver, the process can be laborious and consideration or approval is not guaranteed. For current military members with new chronic eczematous dermatitis, each service has a process for evaluation and treatment. Some special operational jobs, such as aircrew, missile operators, and divers, have more restrictive medical requirements that are monitored by physicians with special training in these populations.
Atopic dermatitis affects 25% of children and 2% to 3% of adults.5 Approximately 60% of patients with AD will develop their first eruption by 1 year of age, and 90% by 5 years of age. Although the majority of patients will have resolution of their disease during childhood, 10% to 30% will have persistent disease into adulthood.5 Because the majority of AD resolves in childhood, it is understandable that asymptomatic individuals with a history of AD before 12 years of age meet military entrance medical standards.
Provoking Factors
The US Military maintains stringent medical standards because of the nature of the dynamic, rapidly changing military environment and its demands. Whether training for readiness in an austere location, deploying to extreme climates, or being stationed overseas, service members must be prepared to encounter a myriad of environmental extremes, physical stress, and psychological stressors. Environmental factors commonly experienced in the military can provoke or exacerbate symptoms of AD (Figures 1 and 2). Ideally, an individual with AD lives in a stable climate, has access to moisturizers and topical steroids, bathes regularly to remove dust and debris, wears 100% cotton garments to avoid irritation, and avoids using gear that would cause exacerbations. Service members rarely have such accommodations in deployed settings. A recent article in Military Medicine explained quite well, “If someone wanted to design an experience with the explicit goal to flare a person with otherwise well controlled atopic dermatitis it would probably look like a military deployment.”3
The United States has a military presence in countries with extreme temperature and humidity variations all over the world. Uniforms are standardized, and members are required to wear prescribed clothing with no alternatives. Uniforms are made of durable sturdy material. If uniforms can be laundered, they often are grouped together, and sensitive detergent cannot be specified. Bathing is challenging in deployed locations, with troops often going weeks using baby wipes for self-hygiene. These conditions increase risk for development of contact allergens, and little access to proper hygiene practices also increases risk for secondary infections in members with AD.
In addition to environmental challenges, the military gear and equipment used can flare AD. Service members must wear protective gear such as body armor. These heavy hard pieces of material are bulky; difficult to wash; and cause friction, sweating, and irritation. The military prepares for operations in chemical, biological, radiological, or nuclear environments, which requires wearing a rubber mask, multiple layers of boots and gloves, and thick charcoal impregnated over garments for many hours. Such conditions may flare AD or make it intolerable.
Although stress is a part of any deployment experience, excessive or prolonged stress can lead to combat operational stress reactions that inhibit a service member’s ability to function.6 Stressors during deployment can accumulate and may be caused by the operational environment, loss of fellow service members to injury or death, illness, leadership demands, personal choices, issues on the home front, interpersonal conflicts, and sleep loss.7 Atopic dermatitis can be exacerbated by such stress, leading to increased pruritus and scratching.7-9 Symptomatic AD also can play a role in worsening combat stress. Although severe pruritus may affect attentiveness to job duties during the day, these symptoms, if uncontrolled, also can negatively affect sleep. As many as 60% of patients with AD at baseline and 83% of patients with exacerbations experience sleep disturbance due to their disease.5 These stressors experienced by deployed military personnel can contribute to combat stress reactions, which may vary from simple inattentiveness to more serious behaviors such as suicidal ideation.6 Combat stress reactions inhibit a military member’s ability to function properly in the deployed environment and can lead to notable safety concerns and potential mission failure.
Vaccinations
Military members deploying overseas are required to receive specific vaccinations, including the smallpox vaccine. Although the virus was eradicated in 1980, the concern for smallpox to be used as a biological weapon in certain areas of the world necessitates continued vaccination of military populations. According to the Centers for Disease Control and Prevention, the only known reservoir for the virus is humans, and the disease has a mortality rate of 30%.10 A history of or present AD is a contraindication for primary smallpox vaccination and revaccination for nonemergency use because of the risk for eczema vaccinatum.11 The risk also applies to close contacts of vaccinated members. For 30 days after vaccination, service members must avoid skin-to-skin contact with anyone who has active AD.12 Eczema vaccinatum in vaccinated individuals is typically self-limited; however, eczema vaccinatum in nonvaccinated contacts can be severe. One case report described a 28-month-old child with refractory AD who developed severe eczema vaccinatum after contact with her recently vaccinated military parent. The child required a 48-day admission to the intensive care unit and multiple skin grafts; fortunately, the child did not develop any apparent long-term sequelae.13 This case highlights the importance of understanding the risks associated with smallpox vaccination in military members with AD and the responsibility of health care providers to properly screen and counsel individuals prior to administering smallpox vaccines.
Treatment
Treatment of mild to moderate AD is relatively straightforward in developed countries with good access to medical care. The most recent American Academy of Dermatology clinical guidelines for AD focus on minimizing irritants and triggers, regularly using moisturizers soon after bathing, and using topical steroids as needed.5 Military members face specific challenges regarding treatment of AD, particularly when deployed to remote locations without access to treatment facilities or medications. Military members are required to carry all necessary personal medications with them for at least 6 months and preferably the duration of the deployment, sometimes up to 1 year. Military members carry a large amount of gear for deployments, and it is not feasible to pack an additional 10 to 20 lb worth of emollients and topical steroids to last the entire deployment. Routine laboratory monitoring is limited or completely unavailable. Refrigeration typically is not available, making use of systemic medications nearly impossible during deployments. In the event of complications such as eczema herpeticum or secondary bacterial infection, service members could require evacuation from the deployed location to a larger field hospital or to the United States, which is costly and also removes a valuable team member from the deployed unit. These limitations in access to care, medications, and treatment options make AD a difficult condition to treat in the deployed setting.
Nonmilitary Medical Providers
Civilian providers play an important role in diagnosing and treating AD. It is vital to completely and accurately document treatment of all skin diseases; however, it is especially important for those who desire to or currently serve in the military. Military primary care providers or military dermatologists must review the information from civilian providers to aid in determining suitability for entry or retention in the military. Clearly documenting the morphology, extent of disease involvement (eg, body surface area), treatment plan, response to treatment, and exacerbating factors will aid in ensuring the patient’s medical record accurately reflects their skin disease. Ultimately, this record often is the only information available to make health determinations regarding military service.
Conclusion
A career in the military is challenging and rewarding for those who volunteer to serve. Because of the demanding and unpredictable lifestyle inherent with military service, the Department of Defense maintains strict medical standards for entrance and retention. These standards ensure members are capable of safely completing training and deploying anywhere in the world. Although AD is a relatively common and treatable skin disease in locations with well-established medical care, it can pose a notable problem for service members while deployed to austere locations with variable environments around the world. Environmental factors and gear requirements, coupled with limited access to treatment facilities and medications, render AD a potentially serious issue. Atopic dermatitis in military members can affect individual medical readiness and unit success. It is important that all providers understand the myriad effects that AD can have on an individual who wishes to join or continue service in the military.
Dermatologic conditions historically have affected military members’ ability to serve during times of peace and conflict. These conditions range from chronic dermatologic diseases to environment- or occupation-related dermatologic diseases. Mild to moderate atopic dermatitis (AD) typically is a manageable skin condition. However, in a deployed setting, a flare of AD can result in the inability of a member to perform their military duty, which directly compromises mission safety and effectiveness. The military developed and updates medical standards for entry and retention of service members. These standards are designed to ensure the greatest potential for a military member to successfully serve at home station and during combat operations.
Impact of Injuries in Military
Historically, disease and nonbattle injuries have resulted in notably more hospitalizations and time lost than injuries sustained on the battlefield.1 A review of major conflicts dating from World War II shows approximately 10% of all dermatologic concerns were related to eczematous dermatitis, with 2% specifically related to AD. These numbers varied remarkably depending on the location and environment of the conflict, with eczema accounting for 25% of dermatologic concerns during the Gulf War.2 During the initial phases of Operation Iraqi Freedom, approximately 75% of hospitalizations were from disease and nonbattle injuries, of which dermatologic disease accounted for 3%.1 From 2003 to 2006 in Iraq, 35 service members were evacuated from combat zones specifically for uncontrolled AD.3 In a deployed environment, each member is critical to the unit’s success in completing their mission. A single member of a unit often is the only person qualified to perform a function for that team. There are rarely extra people with similar skills to replace a member unable to complete his/her duties. The loss of a single member compromises the effectiveness and safety of the team and can lead to mission failure. Therefore, AD can have a profound impact on military operations in a deployed environment.
Military Medical Standards for Accession and Retention
There are 2 main goals of the military medical standards. First, the individual health of the applicant or military member is of utmost importance. Applicants with medical conditions that will be exacerbated by military service or that limit the ability for successful military operations are not accepted for military service. Once an active-duty member is diagnosed with a medical condition, the military determines if limitations are needed for military assignments and deployments based on available medical care in those locations. Second, mission accomplishment in combat operations requires that healthy military members are able to complete their jobs in extreme environments and under notable stress. If an applicant has a medical condition unsuitable for military service, it is in the best interest of the applicant and the military to deny entry.
The Medical Standards for Appointment, Enlistment, or Induction Into the Military Services (DoD Instruction 6130.03) lists conditions that are disqualifying for military service.4 Section 5.21 lists the following as disqualifying for military service in relation to eczematous dermatitis:
d. History of AD or eczema after the 12th birthday. History of residual or recurrent lesions in characteristic areas (face, neck, antecubital or popliteal fossae, occasionally wrists and hands).
e. History of recurrent or chronic nonspecific dermatitis within the past 2 years to include contact (irritant or allergic) or dyshidrotic dermatitis requiring more than treatment with topical corticosteroid.4
Although cases of incorrect diagnosis or very mild AD can be considered for a waiver, the process can be laborious and consideration or approval is not guaranteed. For current military members with new chronic eczematous dermatitis, each service has a process for evaluation and treatment. Some special operational jobs, such as aircrew, missile operators, and divers, have more restrictive medical requirements that are monitored by physicians with special training in these populations.
Atopic dermatitis affects 25% of children and 2% to 3% of adults.5 Approximately 60% of patients with AD will develop their first eruption by 1 year of age, and 90% by 5 years of age. Although the majority of patients will have resolution of their disease during childhood, 10% to 30% will have persistent disease into adulthood.5 Because the majority of AD resolves in childhood, it is understandable that asymptomatic individuals with a history of AD before 12 years of age meet military entrance medical standards.
Provoking Factors
The US Military maintains stringent medical standards because of the nature of the dynamic, rapidly changing military environment and its demands. Whether training for readiness in an austere location, deploying to extreme climates, or being stationed overseas, service members must be prepared to encounter a myriad of environmental extremes, physical stress, and psychological stressors. Environmental factors commonly experienced in the military can provoke or exacerbate symptoms of AD (Figures 1 and 2). Ideally, an individual with AD lives in a stable climate, has access to moisturizers and topical steroids, bathes regularly to remove dust and debris, wears 100% cotton garments to avoid irritation, and avoids using gear that would cause exacerbations. Service members rarely have such accommodations in deployed settings. A recent article in Military Medicine explained quite well, “If someone wanted to design an experience with the explicit goal to flare a person with otherwise well controlled atopic dermatitis it would probably look like a military deployment.”3
The United States has a military presence in countries with extreme temperature and humidity variations all over the world. Uniforms are standardized, and members are required to wear prescribed clothing with no alternatives. Uniforms are made of durable sturdy material. If uniforms can be laundered, they often are grouped together, and sensitive detergent cannot be specified. Bathing is challenging in deployed locations, with troops often going weeks using baby wipes for self-hygiene. These conditions increase risk for development of contact allergens, and little access to proper hygiene practices also increases risk for secondary infections in members with AD.
In addition to environmental challenges, the military gear and equipment used can flare AD. Service members must wear protective gear such as body armor. These heavy hard pieces of material are bulky; difficult to wash; and cause friction, sweating, and irritation. The military prepares for operations in chemical, biological, radiological, or nuclear environments, which requires wearing a rubber mask, multiple layers of boots and gloves, and thick charcoal impregnated over garments for many hours. Such conditions may flare AD or make it intolerable.
Although stress is a part of any deployment experience, excessive or prolonged stress can lead to combat operational stress reactions that inhibit a service member’s ability to function.6 Stressors during deployment can accumulate and may be caused by the operational environment, loss of fellow service members to injury or death, illness, leadership demands, personal choices, issues on the home front, interpersonal conflicts, and sleep loss.7 Atopic dermatitis can be exacerbated by such stress, leading to increased pruritus and scratching.7-9 Symptomatic AD also can play a role in worsening combat stress. Although severe pruritus may affect attentiveness to job duties during the day, these symptoms, if uncontrolled, also can negatively affect sleep. As many as 60% of patients with AD at baseline and 83% of patients with exacerbations experience sleep disturbance due to their disease.5 These stressors experienced by deployed military personnel can contribute to combat stress reactions, which may vary from simple inattentiveness to more serious behaviors such as suicidal ideation.6 Combat stress reactions inhibit a military member’s ability to function properly in the deployed environment and can lead to notable safety concerns and potential mission failure.
Vaccinations
Military members deploying overseas are required to receive specific vaccinations, including the smallpox vaccine. Although the virus was eradicated in 1980, the concern for smallpox to be used as a biological weapon in certain areas of the world necessitates continued vaccination of military populations. According to the Centers for Disease Control and Prevention, the only known reservoir for the virus is humans, and the disease has a mortality rate of 30%.10 A history of or present AD is a contraindication for primary smallpox vaccination and revaccination for nonemergency use because of the risk for eczema vaccinatum.11 The risk also applies to close contacts of vaccinated members. For 30 days after vaccination, service members must avoid skin-to-skin contact with anyone who has active AD.12 Eczema vaccinatum in vaccinated individuals is typically self-limited; however, eczema vaccinatum in nonvaccinated contacts can be severe. One case report described a 28-month-old child with refractory AD who developed severe eczema vaccinatum after contact with her recently vaccinated military parent. The child required a 48-day admission to the intensive care unit and multiple skin grafts; fortunately, the child did not develop any apparent long-term sequelae.13 This case highlights the importance of understanding the risks associated with smallpox vaccination in military members with AD and the responsibility of health care providers to properly screen and counsel individuals prior to administering smallpox vaccines.
Treatment
Treatment of mild to moderate AD is relatively straightforward in developed countries with good access to medical care. The most recent American Academy of Dermatology clinical guidelines for AD focus on minimizing irritants and triggers, regularly using moisturizers soon after bathing, and using topical steroids as needed.5 Military members face specific challenges regarding treatment of AD, particularly when deployed to remote locations without access to treatment facilities or medications. Military members are required to carry all necessary personal medications with them for at least 6 months and preferably the duration of the deployment, sometimes up to 1 year. Military members carry a large amount of gear for deployments, and it is not feasible to pack an additional 10 to 20 lb worth of emollients and topical steroids to last the entire deployment. Routine laboratory monitoring is limited or completely unavailable. Refrigeration typically is not available, making use of systemic medications nearly impossible during deployments. In the event of complications such as eczema herpeticum or secondary bacterial infection, service members could require evacuation from the deployed location to a larger field hospital or to the United States, which is costly and also removes a valuable team member from the deployed unit. These limitations in access to care, medications, and treatment options make AD a difficult condition to treat in the deployed setting.
Nonmilitary Medical Providers
Civilian providers play an important role in diagnosing and treating AD. It is vital to completely and accurately document treatment of all skin diseases; however, it is especially important for those who desire to or currently serve in the military. Military primary care providers or military dermatologists must review the information from civilian providers to aid in determining suitability for entry or retention in the military. Clearly documenting the morphology, extent of disease involvement (eg, body surface area), treatment plan, response to treatment, and exacerbating factors will aid in ensuring the patient’s medical record accurately reflects their skin disease. Ultimately, this record often is the only information available to make health determinations regarding military service.
Conclusion
A career in the military is challenging and rewarding for those who volunteer to serve. Because of the demanding and unpredictable lifestyle inherent with military service, the Department of Defense maintains strict medical standards for entrance and retention. These standards ensure members are capable of safely completing training and deploying anywhere in the world. Although AD is a relatively common and treatable skin disease in locations with well-established medical care, it can pose a notable problem for service members while deployed to austere locations with variable environments around the world. Environmental factors and gear requirements, coupled with limited access to treatment facilities and medications, render AD a potentially serious issue. Atopic dermatitis in military members can affect individual medical readiness and unit success. It is important that all providers understand the myriad effects that AD can have on an individual who wishes to join or continue service in the military.
- Zouris JM, Wade AL, Magno CP. Injury and illness casualty distributions among U.S. Army and Marine Corps personnel during Operation Iraqi Freedom. Mil Med. 2008;173:247-252.
- Gelman, AB, Norton SA, Valdes-Rodriguez R, et al. A review of skin conditions in modern warfare and peacekeeping operations. Mil Med. 2015;180:32-37.
- Jeter J, Bowen C. Atopic dermatitis and implications for military service. Mil Med. 2019;184:177-182.
- Medical Standards for Appointment, Enlistment, or Induction Into the Military Services (DoD Instruction 6130.03). Washington, DC: Department of Defense; May 6, 2018. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003p.pdf?ver=2018-05-04-113917-883. Accessed May 8, 2019.
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351.
- Force Health Protection (Army Techniques Publication No. 4-02.8). Washington, DC: Department of the Army; March 2016. https://armypubs.army.mil/epubs/DR_pubs/DR_a/pdf/web/atp4_02x8.pdf. Accessed August 19, 2019.
- Judkins JL, Bradley DL. A review of the effectiveness of a combat and operational stress control restoration center in Afghanistan. Mil Med. 2017;182:1755-1762.
- Suarez AL, Feramisco JD, Koo J, et al. Psychoneuroimmunology of psychological stress and atopic dermatitis: pathophysiologic and therapeutic updates. Acta Dermatol Venereol. 2012;92:7-15.
- Mochizuki H, Lavery MJ, Nattkemper LA, et al. Impact of acute stress on itch sensation and scratching behaviour in patients with atopic dermatitis and healthy controls. Br J Dermatol. 2019;180:821-827.
- Centers for Disease Control and Prevention. Smallpox: contraindications to vaccination. https://www.cdc.gov/smallpox/clinicians/vaccination-contraindications1.html. Updated December 5, 2016. Accessed August 19, 2019.
- Kemper AR, Davis MM, Freed GL. Expected adverse events in a mass smallpox vaccination campaign. Eff Clin Pract. 2002;5:84-90.
- Reed JL, Scott DE, Bray M. Eczema vaccinatum. Clin Infect Dis. 2012;54:832-840.
- Vora S, Damon I, Fulginiti V, et al. Severe eczema vaccinatum in a household contact of a smallpox vaccine. Clin Infect Dis. 2008;46:1555-1561.
- Zouris JM, Wade AL, Magno CP. Injury and illness casualty distributions among U.S. Army and Marine Corps personnel during Operation Iraqi Freedom. Mil Med. 2008;173:247-252.
- Gelman, AB, Norton SA, Valdes-Rodriguez R, et al. A review of skin conditions in modern warfare and peacekeeping operations. Mil Med. 2015;180:32-37.
- Jeter J, Bowen C. Atopic dermatitis and implications for military service. Mil Med. 2019;184:177-182.
- Medical Standards for Appointment, Enlistment, or Induction Into the Military Services (DoD Instruction 6130.03). Washington, DC: Department of Defense; May 6, 2018. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003p.pdf?ver=2018-05-04-113917-883. Accessed May 8, 2019.
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351.
- Force Health Protection (Army Techniques Publication No. 4-02.8). Washington, DC: Department of the Army; March 2016. https://armypubs.army.mil/epubs/DR_pubs/DR_a/pdf/web/atp4_02x8.pdf. Accessed August 19, 2019.
- Judkins JL, Bradley DL. A review of the effectiveness of a combat and operational stress control restoration center in Afghanistan. Mil Med. 2017;182:1755-1762.
- Suarez AL, Feramisco JD, Koo J, et al. Psychoneuroimmunology of psychological stress and atopic dermatitis: pathophysiologic and therapeutic updates. Acta Dermatol Venereol. 2012;92:7-15.
- Mochizuki H, Lavery MJ, Nattkemper LA, et al. Impact of acute stress on itch sensation and scratching behaviour in patients with atopic dermatitis and healthy controls. Br J Dermatol. 2019;180:821-827.
- Centers for Disease Control and Prevention. Smallpox: contraindications to vaccination. https://www.cdc.gov/smallpox/clinicians/vaccination-contraindications1.html. Updated December 5, 2016. Accessed August 19, 2019.
- Kemper AR, Davis MM, Freed GL. Expected adverse events in a mass smallpox vaccination campaign. Eff Clin Pract. 2002;5:84-90.
- Reed JL, Scott DE, Bray M. Eczema vaccinatum. Clin Infect Dis. 2012;54:832-840.
- Vora S, Damon I, Fulginiti V, et al. Severe eczema vaccinatum in a household contact of a smallpox vaccine. Clin Infect Dis. 2008;46:1555-1561.
Practice Points
- The US Military follows strict medical eligibility requirements for enlistment and retention. Atopic dermatitis (AD) and chronic eczematous conditions after 12 years of age is disqualifying for military service, but waivers may be possible for mild cases.
- Unpredictable and rigorous environmental and occupational stressors associated with military service as well as limited access to medical care make AD a challenging condition to manage for service members, particularly during military deployment.
- Accurate diagnosis and documentation of AD in childhood and adolescence by nonmilitary providers are essential, as they will aid in appropriately determining an applicant’s potential to successfully serve in the military.
- For current service members, nonmilitary providers play a vital role in diagnosis and management where military dermatologists are not readily available.
ID Blog: The story of syphilis, part I
Rise of a global scourge
The Great Pox, the French Disease, Cupid’s Disease – syphilis has had many names throughout history.
Why should we care about the history of syphilis? Surely syphilis has reached the status of a nonentity disease – in-and-out of the doctor’s office with a course of antibiotics and farewell to the problem. And on the surface, that is certainly true. For now. In the developed world. For those with access to reasonable health care.
But that is all the shiny surface of modern medical triumph. Despite successes in prevention throughout the late 20th and early 21st century, syphilis is making comeback. A growing reservoir of syphilis, often untreated, lies hidden by the invisibility of poorer nations and increasingly in the lower economic strata of the developed world. And the danger is increased by the rise of antibiotic-resistant strains of the disease.
Over the last decade, the European Union and several other high-income countries observed an increasing syphilis trend, according to a recent report by the European Centre for Disease Prevention and Control. And in the United States, the Centers for Disease Control and Prevention has expressed concern over “the rising tide of syphilis” and a “devastating surge in congenital syphilis.” Many reasons have been suggested for this resurgence of syphilis, including the prevalence of unprotected sex and the overall increase in multiple sexual partners in the sexually active population. This trend has been ascribed to a reduced fear of acquiring HIV from condomless sex because of the rise of antiretroviral therapies, which make HIV infection no longer a death sentence for those who have access to and can afford the drugs.
Men who have sex with men are the most affected population cited, which may in part be related to the trend in unprotected sex that has accompanied the decreasing fear of HIV. But in some countries, syphilis rates among heterosexual populations are on the increase as well. Even more troubling were the increases in syphilis diagnosed among pregnant women that were reported in high-income settings outside of the European Union, which led to increases in congenital syphilis infections.
According to a 2018 update on the global epidemiology of syphilis, each year an estimated 6 million new cases are diagnosed in people aged 15-49 years, with more than 300,000 fetal and neonatal deaths attributed to the disease. An additional 215,000 infants are at increased risk of early death because of prenatal infection.
For syphilis is indeed a nasty disease. But a remarkable one as well. It presents an almost textbook example of disease evolution and adaptation writ large. It is also a disease with equally remarkable properties – acute, systemic, latent, eruptive, and congenital in its various manifestations. As Sir William Osler, one of the brightest lights of medical education of his time, said in 1897: “I often tell my students that it [syphilis] is the only disease which they require to know thoroughly. Know syphilis in all its manifestations and relations, and all other things clinical will be added unto you.”
Syphilis is caused by the spirochete Treponema pallidum subspecies pallidum and is generally acquired by sexual contact. Congenital syphilis infection occurs by transplacental transmission.
In its modern manifestation, the disease evolves through several stages – primary, secondary, and tertiary. Primary, noncongenital infection is characterized by a lesion. This chancre, as it is called, occurs at the original site of infection, typically between 10 days and 3 months after exposure. The chancre usually appears on the genitals, but given the variety of sexual behaviors, chancres can also occur on the rectum, tongue, pharynx, breast, and so on. The myth of only choosing “a clean partner,” one without visible lesions, is misleading because vaginal and rectal lesions may not be easy to spot yet still remain profoundly infectious.
The secondary stage of an untreated infection occurs 2-3 months after the onset of chancre, and results in multisystem involvement as the spirochetes spread through the bloodstream. Symptoms include skin rash (involving the palms and the soles of the feet) and potentially a variety of other dermatologic manifestations. Fever and swollen lymph nodes may also be present before the disease moves into a latent stage, in which no clinical symptoms are evident. Following this, tertiary syphilis can occur 10-30 years after the initial infection in about 30% of the untreated population, resulting in neurosyphilis, cardiovascular syphilis, or late benign syphilis. Disease progression in tertiary syphilis can lead to dementia, disfigurement, and death.
Sounds bad, doesn’t it? But what we’ve just recounted is the relatively benign disease that modern syphilis has become. Syphilis began as a sweeping, lethal epidemic in the late 15th century spreading dread across the world from the Americas to Europe and then to Asia at a speed equal to the fastest sailing ships of the era.
Syphilis first appeared in Naples in its epidemic form in 1495. Recent anthropological and historical consensus has suggested that syphilis, as we know it today, like tobacco, potatoes, and maize is a product of the Americas that was brought to the Old World by the intrepid exploits of one Christopher Columbus in 1493. Just as the Spanish inadvertently brought smallpox to devastate the population of the New World, Christopher Columbus appears to have brought epidemic syphilis to the Old World in an ironic twist of fate.
Ruy Diaz de Isla, one of two Spanish physicians present when Christopher Columbus returned from his first voyage to America, wrote in a manuscript that Pinzon de Palos, the pilot of Columbus, and also other members of the crew already suffered from symptoms of what was likely syphilis on their return from the New World
Although there has been some controversy regarding the origin of the syphilis epidemic, a recent molecular study using a large collection of pathogenic Treponema strains indicated that venereal syphilis arose relatively recently in human history, and that the closest related syphilis-causing strains were found in South America, providing support for the Columbian theory of syphilis’s origin.
Syphilis flamed across Europe like wildfire, lit by a series of small wars that started shortly after Columbus’s return. Soldiers throughout history have indulged themselves in activities well primed for the spread of venereal disease, and the doughty warriors of the late 15th century were no exception. And throughout the next 500-plus years, syphilis and war rode across the world in tandem, like the white and red horsemen of the Apocalypse.
In its initial launch, syphilis had the help of Charles VIII, the King of France, who had invaded Italy in early 1495 with an army of more than 30,000 mercenaries recruited from across Europe. His forces conquered Naples, which was primarily defended by Spanish mercenaries.
When Charles VIII broke up his army, “mercenaries, infected with a mysterious, serious disease, returned to their native lands or moved elsewhere to wage war, spreading the disease across Europe.” The “Great Pox” initially struck Italy, France, Germany, and Switzerland in 1495; then Holland and Greece in the following year, reaching England and Scotland by 1497; and then Hungary, Poland, and the Scandinavian countries by 1500.
As this period was the Age of Exploration, French, Dutch, and English sailors soon carried syphilis across the rest of an unsuspecting world, with the disease reaching India in 1498 before moving also to Africa and then throughout the rest of Asia in the early 16th century.
And yet, one of the most remarkable parts of the story is the rapid transformation of syphilis from a deadly virulent epidemic to a (comparatively) benign endemic status. Which will be the subject of my next posting.

Mark Lesney is the managing editor of MDedge.com/IDPractioner . He has a PhD in Plant Virology and a PhD in the History of Science, with a focus on the history of biotechnology and medicine. He has served as an adjunct assistant professor at the Georgetown University School of Medicine, Department of Biochemistry and Molecular & Cellular Biology, Washington, DC.
Rise of a global scourge
Rise of a global scourge
The Great Pox, the French Disease, Cupid’s Disease – syphilis has had many names throughout history.
Why should we care about the history of syphilis? Surely syphilis has reached the status of a nonentity disease – in-and-out of the doctor’s office with a course of antibiotics and farewell to the problem. And on the surface, that is certainly true. For now. In the developed world. For those with access to reasonable health care.
But that is all the shiny surface of modern medical triumph. Despite successes in prevention throughout the late 20th and early 21st century, syphilis is making comeback. A growing reservoir of syphilis, often untreated, lies hidden by the invisibility of poorer nations and increasingly in the lower economic strata of the developed world. And the danger is increased by the rise of antibiotic-resistant strains of the disease.
Over the last decade, the European Union and several other high-income countries observed an increasing syphilis trend, according to a recent report by the European Centre for Disease Prevention and Control. And in the United States, the Centers for Disease Control and Prevention has expressed concern over “the rising tide of syphilis” and a “devastating surge in congenital syphilis.” Many reasons have been suggested for this resurgence of syphilis, including the prevalence of unprotected sex and the overall increase in multiple sexual partners in the sexually active population. This trend has been ascribed to a reduced fear of acquiring HIV from condomless sex because of the rise of antiretroviral therapies, which make HIV infection no longer a death sentence for those who have access to and can afford the drugs.
Men who have sex with men are the most affected population cited, which may in part be related to the trend in unprotected sex that has accompanied the decreasing fear of HIV. But in some countries, syphilis rates among heterosexual populations are on the increase as well. Even more troubling were the increases in syphilis diagnosed among pregnant women that were reported in high-income settings outside of the European Union, which led to increases in congenital syphilis infections.
According to a 2018 update on the global epidemiology of syphilis, each year an estimated 6 million new cases are diagnosed in people aged 15-49 years, with more than 300,000 fetal and neonatal deaths attributed to the disease. An additional 215,000 infants are at increased risk of early death because of prenatal infection.
For syphilis is indeed a nasty disease. But a remarkable one as well. It presents an almost textbook example of disease evolution and adaptation writ large. It is also a disease with equally remarkable properties – acute, systemic, latent, eruptive, and congenital in its various manifestations. As Sir William Osler, one of the brightest lights of medical education of his time, said in 1897: “I often tell my students that it [syphilis] is the only disease which they require to know thoroughly. Know syphilis in all its manifestations and relations, and all other things clinical will be added unto you.”
Syphilis is caused by the spirochete Treponema pallidum subspecies pallidum and is generally acquired by sexual contact. Congenital syphilis infection occurs by transplacental transmission.
In its modern manifestation, the disease evolves through several stages – primary, secondary, and tertiary. Primary, noncongenital infection is characterized by a lesion. This chancre, as it is called, occurs at the original site of infection, typically between 10 days and 3 months after exposure. The chancre usually appears on the genitals, but given the variety of sexual behaviors, chancres can also occur on the rectum, tongue, pharynx, breast, and so on. The myth of only choosing “a clean partner,” one without visible lesions, is misleading because vaginal and rectal lesions may not be easy to spot yet still remain profoundly infectious.
The secondary stage of an untreated infection occurs 2-3 months after the onset of chancre, and results in multisystem involvement as the spirochetes spread through the bloodstream. Symptoms include skin rash (involving the palms and the soles of the feet) and potentially a variety of other dermatologic manifestations. Fever and swollen lymph nodes may also be present before the disease moves into a latent stage, in which no clinical symptoms are evident. Following this, tertiary syphilis can occur 10-30 years after the initial infection in about 30% of the untreated population, resulting in neurosyphilis, cardiovascular syphilis, or late benign syphilis. Disease progression in tertiary syphilis can lead to dementia, disfigurement, and death.
Sounds bad, doesn’t it? But what we’ve just recounted is the relatively benign disease that modern syphilis has become. Syphilis began as a sweeping, lethal epidemic in the late 15th century spreading dread across the world from the Americas to Europe and then to Asia at a speed equal to the fastest sailing ships of the era.
Syphilis first appeared in Naples in its epidemic form in 1495. Recent anthropological and historical consensus has suggested that syphilis, as we know it today, like tobacco, potatoes, and maize is a product of the Americas that was brought to the Old World by the intrepid exploits of one Christopher Columbus in 1493. Just as the Spanish inadvertently brought smallpox to devastate the population of the New World, Christopher Columbus appears to have brought epidemic syphilis to the Old World in an ironic twist of fate.
Ruy Diaz de Isla, one of two Spanish physicians present when Christopher Columbus returned from his first voyage to America, wrote in a manuscript that Pinzon de Palos, the pilot of Columbus, and also other members of the crew already suffered from symptoms of what was likely syphilis on their return from the New World
Although there has been some controversy regarding the origin of the syphilis epidemic, a recent molecular study using a large collection of pathogenic Treponema strains indicated that venereal syphilis arose relatively recently in human history, and that the closest related syphilis-causing strains were found in South America, providing support for the Columbian theory of syphilis’s origin.
Syphilis flamed across Europe like wildfire, lit by a series of small wars that started shortly after Columbus’s return. Soldiers throughout history have indulged themselves in activities well primed for the spread of venereal disease, and the doughty warriors of the late 15th century were no exception. And throughout the next 500-plus years, syphilis and war rode across the world in tandem, like the white and red horsemen of the Apocalypse.
In its initial launch, syphilis had the help of Charles VIII, the King of France, who had invaded Italy in early 1495 with an army of more than 30,000 mercenaries recruited from across Europe. His forces conquered Naples, which was primarily defended by Spanish mercenaries.
When Charles VIII broke up his army, “mercenaries, infected with a mysterious, serious disease, returned to their native lands or moved elsewhere to wage war, spreading the disease across Europe.” The “Great Pox” initially struck Italy, France, Germany, and Switzerland in 1495; then Holland and Greece in the following year, reaching England and Scotland by 1497; and then Hungary, Poland, and the Scandinavian countries by 1500.
As this period was the Age of Exploration, French, Dutch, and English sailors soon carried syphilis across the rest of an unsuspecting world, with the disease reaching India in 1498 before moving also to Africa and then throughout the rest of Asia in the early 16th century.
And yet, one of the most remarkable parts of the story is the rapid transformation of syphilis from a deadly virulent epidemic to a (comparatively) benign endemic status. Which will be the subject of my next posting.

Mark Lesney is the managing editor of MDedge.com/IDPractioner . He has a PhD in Plant Virology and a PhD in the History of Science, with a focus on the history of biotechnology and medicine. He has served as an adjunct assistant professor at the Georgetown University School of Medicine, Department of Biochemistry and Molecular & Cellular Biology, Washington, DC.
The Great Pox, the French Disease, Cupid’s Disease – syphilis has had many names throughout history.
Why should we care about the history of syphilis? Surely syphilis has reached the status of a nonentity disease – in-and-out of the doctor’s office with a course of antibiotics and farewell to the problem. And on the surface, that is certainly true. For now. In the developed world. For those with access to reasonable health care.
But that is all the shiny surface of modern medical triumph. Despite successes in prevention throughout the late 20th and early 21st century, syphilis is making comeback. A growing reservoir of syphilis, often untreated, lies hidden by the invisibility of poorer nations and increasingly in the lower economic strata of the developed world. And the danger is increased by the rise of antibiotic-resistant strains of the disease.
Over the last decade, the European Union and several other high-income countries observed an increasing syphilis trend, according to a recent report by the European Centre for Disease Prevention and Control. And in the United States, the Centers for Disease Control and Prevention has expressed concern over “the rising tide of syphilis” and a “devastating surge in congenital syphilis.” Many reasons have been suggested for this resurgence of syphilis, including the prevalence of unprotected sex and the overall increase in multiple sexual partners in the sexually active population. This trend has been ascribed to a reduced fear of acquiring HIV from condomless sex because of the rise of antiretroviral therapies, which make HIV infection no longer a death sentence for those who have access to and can afford the drugs.
Men who have sex with men are the most affected population cited, which may in part be related to the trend in unprotected sex that has accompanied the decreasing fear of HIV. But in some countries, syphilis rates among heterosexual populations are on the increase as well. Even more troubling were the increases in syphilis diagnosed among pregnant women that were reported in high-income settings outside of the European Union, which led to increases in congenital syphilis infections.
According to a 2018 update on the global epidemiology of syphilis, each year an estimated 6 million new cases are diagnosed in people aged 15-49 years, with more than 300,000 fetal and neonatal deaths attributed to the disease. An additional 215,000 infants are at increased risk of early death because of prenatal infection.
For syphilis is indeed a nasty disease. But a remarkable one as well. It presents an almost textbook example of disease evolution and adaptation writ large. It is also a disease with equally remarkable properties – acute, systemic, latent, eruptive, and congenital in its various manifestations. As Sir William Osler, one of the brightest lights of medical education of his time, said in 1897: “I often tell my students that it [syphilis] is the only disease which they require to know thoroughly. Know syphilis in all its manifestations and relations, and all other things clinical will be added unto you.”
Syphilis is caused by the spirochete Treponema pallidum subspecies pallidum and is generally acquired by sexual contact. Congenital syphilis infection occurs by transplacental transmission.
In its modern manifestation, the disease evolves through several stages – primary, secondary, and tertiary. Primary, noncongenital infection is characterized by a lesion. This chancre, as it is called, occurs at the original site of infection, typically between 10 days and 3 months after exposure. The chancre usually appears on the genitals, but given the variety of sexual behaviors, chancres can also occur on the rectum, tongue, pharynx, breast, and so on. The myth of only choosing “a clean partner,” one without visible lesions, is misleading because vaginal and rectal lesions may not be easy to spot yet still remain profoundly infectious.
The secondary stage of an untreated infection occurs 2-3 months after the onset of chancre, and results in multisystem involvement as the spirochetes spread through the bloodstream. Symptoms include skin rash (involving the palms and the soles of the feet) and potentially a variety of other dermatologic manifestations. Fever and swollen lymph nodes may also be present before the disease moves into a latent stage, in which no clinical symptoms are evident. Following this, tertiary syphilis can occur 10-30 years after the initial infection in about 30% of the untreated population, resulting in neurosyphilis, cardiovascular syphilis, or late benign syphilis. Disease progression in tertiary syphilis can lead to dementia, disfigurement, and death.
Sounds bad, doesn’t it? But what we’ve just recounted is the relatively benign disease that modern syphilis has become. Syphilis began as a sweeping, lethal epidemic in the late 15th century spreading dread across the world from the Americas to Europe and then to Asia at a speed equal to the fastest sailing ships of the era.
Syphilis first appeared in Naples in its epidemic form in 1495. Recent anthropological and historical consensus has suggested that syphilis, as we know it today, like tobacco, potatoes, and maize is a product of the Americas that was brought to the Old World by the intrepid exploits of one Christopher Columbus in 1493. Just as the Spanish inadvertently brought smallpox to devastate the population of the New World, Christopher Columbus appears to have brought epidemic syphilis to the Old World in an ironic twist of fate.
Ruy Diaz de Isla, one of two Spanish physicians present when Christopher Columbus returned from his first voyage to America, wrote in a manuscript that Pinzon de Palos, the pilot of Columbus, and also other members of the crew already suffered from symptoms of what was likely syphilis on their return from the New World
Although there has been some controversy regarding the origin of the syphilis epidemic, a recent molecular study using a large collection of pathogenic Treponema strains indicated that venereal syphilis arose relatively recently in human history, and that the closest related syphilis-causing strains were found in South America, providing support for the Columbian theory of syphilis’s origin.
Syphilis flamed across Europe like wildfire, lit by a series of small wars that started shortly after Columbus’s return. Soldiers throughout history have indulged themselves in activities well primed for the spread of venereal disease, and the doughty warriors of the late 15th century were no exception. And throughout the next 500-plus years, syphilis and war rode across the world in tandem, like the white and red horsemen of the Apocalypse.
In its initial launch, syphilis had the help of Charles VIII, the King of France, who had invaded Italy in early 1495 with an army of more than 30,000 mercenaries recruited from across Europe. His forces conquered Naples, which was primarily defended by Spanish mercenaries.
When Charles VIII broke up his army, “mercenaries, infected with a mysterious, serious disease, returned to their native lands or moved elsewhere to wage war, spreading the disease across Europe.” The “Great Pox” initially struck Italy, France, Germany, and Switzerland in 1495; then Holland and Greece in the following year, reaching England and Scotland by 1497; and then Hungary, Poland, and the Scandinavian countries by 1500.
As this period was the Age of Exploration, French, Dutch, and English sailors soon carried syphilis across the rest of an unsuspecting world, with the disease reaching India in 1498 before moving also to Africa and then throughout the rest of Asia in the early 16th century.
And yet, one of the most remarkable parts of the story is the rapid transformation of syphilis from a deadly virulent epidemic to a (comparatively) benign endemic status. Which will be the subject of my next posting.

Mark Lesney is the managing editor of MDedge.com/IDPractioner . He has a PhD in Plant Virology and a PhD in the History of Science, with a focus on the history of biotechnology and medicine. He has served as an adjunct assistant professor at the Georgetown University School of Medicine, Department of Biochemistry and Molecular & Cellular Biology, Washington, DC.
Telehealth Pulmonary Rehabilitation for Patients With Severe Chronic Obstructive Pulmonary Disease
According to World Health Organization estimates, 65 million people have moderate-to-severe chronic obstructive pulmonary disease (COPD) globally, and > 20 million patients with COPD are living in the US.1 COPD is a progressive respiratory disease with a poor prognosis and a significant cause of morbidity and mortality in the US, especially within the Veterans Health Administration (VHA).2 The prevalence of COPD is higher in veterans than it is in the general population. COPD prevalence in the adult US population has been estimated to be between 5% and 15%, whereas in veterans, prevalence estimates have ranged from about 5% to 43%.3-5
COPD is associated with disabling dyspnea, muscle weakness, exercise intolerance, morbidity, and mortality. These symptoms and complications gradually and progressively compromise mobility, ability to perform daily functions, and decrease quality of life (QOL). Dyspnea, fatigue, and discomfort are the principal symptoms that negatively impact exercise tolerance.6,7 Therefore, patients often intentionally limit their activities to avoid these uncomfortable feelings and adopt a more sedentary behavior. As the disease progresses, individuals with COPD will gradually need assistance in performing activities of daily living, which eventually leads to functional dependence.
Pulmonary rehabilitation (PR) is an essential component of the management of symptomatic patients with COPD. PR is an evidence-based, multidisciplinary, comprehensive intervention that includes exercise and education for patients with chronic respiratory disease.8 The key benefits of PR are clinical improvements in dyspnea, physical capacity, QOL, and reduced disability in patients with COPD and other respiratory diseases.9-11 PR was found to improve respiratory health in veterans with COPD and decrease respiratory-related health care utilization.12
Despite the known benefits of PR, many patients with chronic respiratory diseases are not referred or do not have access to rehabilitation. Also, uptake of PR is low due to patient frailty, transportation issues, and other health care access problems.13-15 Unfortunately, in the US health care system, access to PR and other nonpharmacologic treatments can be challenging due to a shortage of available PR programs, limited physician referral to existing programs, and lack of family and social support.16
There are only a few accredited PR programs in VHA facilities, and they tend to be located in urban areas.12,17 Many patients have limited access to the PR programs due to geographic distance to the programs and transportation challenges (eg, limited ability to drive, cost of transportation). Moreover, veterans with COPD are likely to have limited mobility or are homebound due to experiencing shortness of breath with minimal exertion. Given the clear benefits of PR and the increasing impact of COPD on morbidity and mortality of the patients with COPD, strategies to improve the access and capacity of PR are needed. VA telehealth services allow for distribution of health care services in different geographic locations by providing access for the veterans who live in rural and highly rural areas. The most recent implementation of VA Video Connect (VVC) by the VHA provides a new avenue for clinicians to deliver much needed medical care into the veterans’ home.
COPD Telehealth Program
In this article, we describe the processes for developing and delivering an in-home, interactive, supervised PR program for veterans with severe COPD through VA telehealth service. The program consists of 18 sessions delivered over 6 weeks by a licensed physical therapist (PT) and a respiratory therapist (RT). The aims of the telehealth PR are to improve exercise tolerance, reduce dyspnea and fatigue, improve QOL, improve accessibility, and decrease costs and transportation burdens for patients with COPD. The program was developed, implemented and delivered by an interdisciplinary team, including a pulmonologist, PT, RT, physiatrist, and nonclinical supporting staff.
Patient Assessment
To be eligible to participate in the program the patient must: (1) have a forced expiratory volume (FEV1) < 60%; ( 2) be medically stable and be receiving optimal medical management; (3) have no severe cognitive impairments; (4) be able to use a computer and e-mail; (5) be able to ambulate with or without a walking device; (6) be willing to enroll in a smoking cessation program or to stop smoking; (7) be willing to participate without prolonged interruption; and (8) have all visual and auditory impairments corrected with medical devices.
After referral and enrollment, patients receive medical and physical examinations by the PR team, including a pulmonologist, a PT, and a RT, to ensure that the patients are medically stable to undergo rehabilitation and to develop a tailored exercise program while being mindful of the comorbidities, limitations, and precautions, (eg, loss of balance, risk of fall, limited range of motion). The preprogram assessment includes a pulmonary function test, arterial blood gas test, Montreal Cognitive Assessment, Modified Medical Research Council Scale, St. George Respiratory Questionnaire, the COPD Assessment Test, Patient Health Questionnaire-9,Generalized Anxiety Disorder Assessment-7, Epworth Sleepiness Scale, Katz Index of Independence of Activities of Daily Living, medications and inhaler use, oxygen use, breathing pattern, coughing, 6-minute walk test, Modified Borg Dyspnea Scale, grip strength, 5 Times Sit to Stand Test, manual muscle test, gait measure, Timed Up & Go test, clinical balance tests, range of motion, flexibility, sensation, pain, and fall history.18-32 Educational needs (eg, respiratory hygiene, nutrition, infection control, sleep, disease/symptom management) also are evaluated.
This thorough assessment is performed in a face-to-face outpatient visit. During the program participation, a physiatrist may be consulted for additional needs (eg, wheelchair assessment, home safety evaluation/ modifications, and mobility/disability issues). After completing the 6-week program, patients are scheduled for the postprogram evaluation in a face-to-face outpatient visit with the clinicians.
Equipment
Both clinician and the patient are equipped with a computer with Wi-Fi connectivity, a webcam, and a microphone. Patients are provided an exercise pictorial booklet, an exercise compact disk (audio and video), small exercise apparatuses (eg, assorted colors of resistance bands, hand grip exerciser, hand putty, ergometer, harmonica, and pedometer), incentive spirometer, pulse oximeter, cough assistive device (as needed), blood pressure monitor, COPD information booklets, and a diary to use at home during the program.33
Technology Preparation
Prior to starting the telehealth program, the patient is contacted 1 or 2 days before the first session for technical preparation and familiarization of the VA telehealth connection process. Either the PT or RT provides step-by-step instructions for the patient to practice connecting through VVC during this preparatory phone call. The patient also practices using the computer webcam, speaker, and microphone; checks the telehealth scheduling e-mail; and learns how to solve possible common technical issues (eg, adjusting volume and position of webcam). The patient is asked to set up a table close to the computer and to place all exercise apparatuses and respiratory devices on the table surface.
Program Delivery
A secure online VVC is used for connection during the telehealth session. The patient received an e-mail from the telehealth scheduling system with a link for VVC before each session. During the 6-week program, each telehealth session is conducted by a PT and a RT concurrently for 120 minutes, 3 days per week. The PT provides exercises for the patient to attempt, and the RT provides breathing training and monitoring during the session. After a successful connection to VVC, the therapist verifies the patient’s identity and confirms patient consent for the telehealth session.
After this check-in process, the patient performs a self-measure of resting blood pressure (BP), heart rate, respiratory rate, and blood oxygen saturation and reports to the therapists. During the exercise session, fatigue/exertion, dyspnea (Modified Borg Dyspnea Scale; Borg CR10 Scale), BP, heart rate, oxygen saturation, and other clinical symptoms and responses to exercise are monitored by the therapists, using both patient-reported measures and clinical observation by the therapists.34,35 Any medical emergency during the session is reported immediately to the pulmonologist for further management.
Structure
Prior to each exercise session, exercise precautions, fall prevention, good posture, pursed-lip breathing, pacing, and coordinated breathing are discussed with the patient. The PT demonstrates stretching and warm-up exercises in front of the webcam for the patient to follow. Then the patient performs all exercises in view of the webcam during the session (Figure 1). A RT monitors breathing patterns and corrects with verbal instructions if not properly performed.
Loss of skeletal muscle mass and cachexia are highly prevalent comorbidities of COPD and have been associated with breathlessness, functional limitation, and poor prognosis.36 To address these comorbidities, our program consists of progressive strengthening, aerobic, balance, and flexibility exercises. Resistance bands and tubes are used for strengthening exercises. Callisthenic exercises (eg, chair squat, chair stand, knee marching, bridging, single limb stances, and lunge) are used for progressive strengthening and balance exercises. Progression of strengthening and balance exercises are done through increasing the volume of exercise (ie, numbers of sets and repetitions) and increased load and level of difficulty based on the patient’s progress and comorbidity. The exercise program focuses on strengthening muscles, especially large muscle groups, to improve overall muscle strength and performance of functional activities.37
Arm/pedal ergometer and daily walking are used for daily aerobic exercise. In a study of patients with COPD by the PAC-COPD Study Group, step counter use was found to increase physical activity and improve exercise capacity, which supports its use in COPD management.38 During program participation, the patient is asked to wear a pedometer to monitor the number of steps taken per day and to report step data to the therapists during the telehealth session. The pedometer stores the previous 41 calendar days of data and displays the most recent 7 calendar days of data.
The patient is encouraged to set a realistic daily step goal. The general program goal is to increase at least 1000 steps per day. However, this goal can be adjusted depending on the patient’s health status and comorbid conditions. The PAC-COPD Study Group found that for every additional 1000 daily steps at low intensity, COPD hospitalization risk decreased by 20%.39 A magnitude of 2000 steps or about 1 mile of walking per day was found to be associated with increased physical activity and health benefits in the general population.40
Respiratory muscle training and breathing exercise are provided by the RT, using breathing and incentive spirometer techniques (Figure 2). Huff coughing, diaphragmatic deep breathing, and pursed-lip breathing are instructed by the RT during the session. Effective coughing technique with a cough assistive device is also provided during breathing training if needed.
Patient Education
In patients with COPD, there are numerous positive health benefits associated with education, including assisting the patients to become active participants in the PR program leading to satisfying outcomes; assisting the patients to better understand the lung health, disease processes, physical and psychological changes that occur with COPD; assisting the patients to explore coping strategies for those changes; building lifelong behavioral changes; and developing the self-management skills for sustainability. Through the educational process, patients with COPD can become more skilled at collaborative self-management and improve adherence to their treatment plan, which in turn can result in a reduction in hospital admissions and reduced health care costs.8,41
Education is provided with every session after the patient completes the exercise. Patients are required to record their COPD symptoms, daily activity, home exercise program, sleep, food intake, and additional physical or social activity in their COPD diary and to report during the session (Figure 3). A COPD diary assists patients in self-monitoring their COPD symptoms and provides the therapists with information about clinical changes, behavioral changes, and/or specific unmet needs for education. Several topics related to COPD are included in the education session: lung or respiratory disease/condition and self-management; smoking cessation; physical activity; energy-conserving techniques; breathing and coughing techniques; smoking cessation; nutrition/healthy eating and weight counseling; sex and intimacy; psychological counseling and/or group support; emergency planning (eg, medical, travel, and inclement weather); correct use of inhaler and medications; home oxygen; sleep and sleep hygiene; palliative care and advanced directive; infection control; and sputum clearance.42,43
Program Maintenance
After successfully completing the 6-week program, patients are referred to the VA TeleMOVE! Program or MOVE! Weight Management Program for continuous, long-term management of weight, nutrition, physical activity/exercise, and social activity needs or goals. The patients are scheduled for monthly follow-up phone visits for 6 months with the telerehabilitation team for enforcing sustainability. The phone call visit consists of reviewing breathing techniques, exercise program, physical activity, education, encouragement, and addressing any issues that arise during the self-maintained period.
Limitations
There are several issues of concern and precautions when delivering PR through telehealth into the home. First, the patient performs exercises independently without being manually guarded by the therapists. Risk of falls are a major concern due to impaired balance, poor vision, and other possible unusual physiologic responses to exercise (eg, drop in BP, dizziness, loss of balance). The area in front of the computer needs to be cleared of fall hazards (ie, area rug, wires, objects on the floor). The patient also needs to be educated on self-measurements of BP and oxygen saturation and reports to the therapists. The therapists provide detailed instructions on how to obtain these measures correctly; otherwise, the values may not be valid for a clinical judgment during the exercise session or for other clinical management. In a home environment, there is a limited use of exercise apparatuses. For this program, we only used resistance bands/tubes, small arm/leg ergometer, hand grip, and hand putty for the exercise program. We feel that dumbbell and weight plates are not suitable due to a possible risk of injury if the patient accidently drops them.
Advanced balance training is not suitable due to an increased risk for falls. Without the presence of the PT, level of challenge/difficulty is somewhat limited for this telehealth supervision exercise program. In addition, visual and audio quality are necessary for the session. The patient and the therapists need to see each other clearly to ensure correct methods and forms of each exercise. Furthermore, rehearsal of technical skills with the therapists is very important because this population is older and often has limited computer skills. Any technical difficulty or failure can lead to undesirable situations (eg, anxiety episodes, worries, shortness of breath, upset), which compromise exercise performance during the session. Finally, a phone is needed as an alternative in case of a poor VVC connection.
Conclusion
COPD symptoms and complications greatly affect patients’ ability to perform daily activities, decrease QOL and functional ability, and result in extensive use of health services. Many patients have limited access to a PR program at hospitals or rehabilitation centers due to health conditions, lack of transportation, and/or family support. This home-based, interactive telehealth PR program can break down the geographic barriers, solve poor program accessibility, potentially increase the utilization of PR, and reduce the cost and travel required by the patients.
Acknowledgments
The Telehealth Pulmonary Rehabilitation Program was originally funded by the Veterans Health Administration VA ACCESS Program (AS, CL, HKH). We thank all the veterans for their time and effort in participating in this newly developed rehabilitation program.
1. World Health Organization. Chronic obstructive pulmonary disease (COPD). http://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd). Published December 1, 2017. Accessed August 7, 2019.
2. Yu W, Ravelo A, Wagner TH, et al. Prevalence and costs of chronic conditions in the VA health care system. Med Care Res Rev. 2003;60(suppl 3):146S-167S.
3. Doney B, Hnizdo E, Dillon CF, et al. Prevalence of airflow obstruction in U.S. adults aged 40-79 years: NHANES data 1988-1994 and 2007-2010. COPD. 2015;12(4):355-365.
4. Murphy DE, Chaudhry Z, Almoosa KF, Panos RJ. High prevalence of chronic obstructive pulmonary disease among veterans in the urban midwest. Mil Med. 2011;176(5):552-560.
5. Cypel YS, Hines SE, Davey VJ, Eber SM, Schneiderman AI. Self-reported physician-diagnosed chronic obstructive pulmonary disease and spirometry patterns in Vietnam Era US Army Chemical Corps veterans: a retrospective cohort study. Am J Ind Med. 2018;61(10):802-814.
6. Rochester CL. Exercise training in chronic obstructive pulmonary disease. J Rehabil Res Dev. 2003;40(5)(suppl 2):59-80.
7. Cortopassi F, Gurung P, Pinto-Plata V. Chronic obstructive pulmonary disease in elderly patients. Clin Geriatr Med. 2017;33(4):539-552.
8. Spruit MA, Singh SJ, Garvey C, et al; ATS/ERS Task Force on Pulmonary Rehabilitation. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188(8):e13-e64.
9. Robinson H, Williams V, Curtis F, Bridle C, Jones AW. Facilitators and barriers to physical activity following pulmonary rehabilitation in COPD: a systematic review of qualitative studies. NPJ Prim Care Respir Med. 2018;28(1):19.
10. McCarthy B, Casey D, Devane D, Murphy K, Murphy E, Lacasse Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015;(2):CD003793.
11. Ries AL, Bauldoff GS, Carlin BW, et al. Pulmonary rehabilitation: joint AACP/AACVPR evidence-based clinical practice guidelines. Chest. 2007;131(suppl 5):4S-42S.
12. Major S, Moreno M, Shelton J, Panos RJ. Veterans with chronic obstructive pulmonary disease achieve clinically relevant improvements in respiratory health after pulmonary rehabilitation. J Cardiopulm Rehabil Prev. 2014;34(6):420-429.
13. Liu Y, Dickerson T, Early F, Fuld J, Clarkson PJ. Understanding influences on the uptake of pulmonary rehabilitation in the East of England: an inclusive design/mixed methods study protocol. BMJ Open. 2018;8(4):e020750.
14. Harris D, Hayter M, Allender S. Factors affecting the offer of pulmonary rehabilitation to patients with chronic obstructive pulmonary disease by primary care professionals: a qualitative study. Prim Health Care Res Dev. 2008;9(4):280-290.
15. Mathar H, Fastholm P, Hansen IR, Larsen NS. Why do patients with COPD decline rehabilitation. Scand J Caring Sci. 2016;30(3):432-441.
16. Han MK, Martinez CH, Au DH, et al. Meeting the challenge of COPD care delivery in the USA: a multiprovider perspective. Lancet Respir Med. 2016;4(6):473-526.
17. American Association of Cardiovascular and Pulmonary Rehabilitation (AACVPR). Online searchable program directory. https://www.aacvpr.org/Resources/Program-Directory Accessed July 19, 2018.
18. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695-699.
19. Fletcher CM, Elmes PC, Fairbairn AS, Wood CH. The significance of respiratory symptoms and the diagnosis of chronic bronchitis in a working population. Br Med J. 1959;2(5147):257-266.
20. O’Donnell DE, Aaron S, Bourbeau J, et al. Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease—2007 update. Can Respir J. 2007;14(suppl B):5B-32B.
21. Jones PW, Quirk FH, Baveystock CM. The St George’s Respiratory Questionnaire. Respir Med. 1991;85(suppl B):25-31.
22. Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34(3):648-654.
23. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613.
24. Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092-1097.
25. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540-545.
26. Katz S. Assessing self-maintenance: activities of daily living, mobility and instrumental activities of daily living. J Am Geriatr Soc. 1983;31(12):721-727.
27. Holland AE, Spruit MA, Troosters T, et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J. 2014;44(6):1428-1446.
28. Mahler DA, Horowitz MB. Perception of breathlessness during exercise in patients with respiratory disease. Med Sci Sports Exerc. 1994;26(9):1078-1081.
29. Liao WC, Wang CH, Yu SY, Chen LY, Wang CY. Grip strength measurement in older adults in Taiwan: a comparison of three testing positions. Australas J Ageing. 2014;33(4):278-282.
30. Buatois S, Miljkovic D, Manckoundia P, et al. Five times sit to stand test is a predictor of recurrent falls in healthy community-living subjects aged 65 and older. J Am Geriatr Soc. 2008;56(8):1575-1577.
31. Bryant MS, Workman CD, Jackson GR. Multidirectional walk test in persons with Parkinson’s disease: a validity study. Int J Rehabil Res. 2015;38(1):88-91.
32. Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142-148.
33. University of Nebraska Medical Center. Timed Up and Go (TUG) Test. https://www.unmc.edu/media/intmed/geriatrics/nebgec/pdf/frailelderlyjuly09/toolkits/timedupandgo_w_norms.pdf. Accessed August 13, 2019.
34. Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377-381.
35. Mahler DA, Horowitz MB. Clinical evaluation of exertional dyspnea. Clin Chest Med. 1994;15(2):259-269.
36. Dudgeon D, Baracos VE. Physiological and functional failure in chronic obstructive pulmonary disease, congestive heart failure and cancer: a debilitating intersection of sarcopenia, cachexia and breathlessness. Curr Opin Support Palliat Care. 2016;10(3):236-241.
37. Lee AL, Holland AE. Time to adapt exercise training regimens in pulmonary rehabilitation—a review of the literature. Int J Chron Obstruct Pulmon Dis. 2014;9:1275-1288.
38. Qiu S, Cai X, Wang X, et al. Using step counters to promote physical activity and exercise capacity in patients with chronic obstructive pulmonary disease: a meta-analysis. Ther Adv Respir Dis. 2018;12:1753466618787386.
39. Donaire-Gonzalez D, Gimeno-Santos E, Balcells E, et al; PAC-COPD Study Group. Benefits of physical activity on COPD hospitalization depend on intensity. Eur Respir J. 2015;46(5):1281-1289.
40. Bravata DM, Smith-Spangler C, Sundaram V, et al. Using pedometers to increase physical activity and improve health: a systematic review. JAMA. 2007;298(19):2296-2304.
41. Zwerink M, Brusse-Keizer M, van der Valk PD, et al. Self-management for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014;19(3):CD002990.
42. Wilson JS, O’Neill B, Reilly J, MacMahon J, Bradley JM. Education in pulmonary rehabilitation: the patient’s perspective. Arch Phys Med Rehabil. 2007;88(12):1704-1709.
43. Bourbeau J, Nault D, Dang-Tan T. Self-management and behaviour modification in COPD. Patient Educ Couns. 2004;52(3):271-277.
According to World Health Organization estimates, 65 million people have moderate-to-severe chronic obstructive pulmonary disease (COPD) globally, and > 20 million patients with COPD are living in the US.1 COPD is a progressive respiratory disease with a poor prognosis and a significant cause of morbidity and mortality in the US, especially within the Veterans Health Administration (VHA).2 The prevalence of COPD is higher in veterans than it is in the general population. COPD prevalence in the adult US population has been estimated to be between 5% and 15%, whereas in veterans, prevalence estimates have ranged from about 5% to 43%.3-5
COPD is associated with disabling dyspnea, muscle weakness, exercise intolerance, morbidity, and mortality. These symptoms and complications gradually and progressively compromise mobility, ability to perform daily functions, and decrease quality of life (QOL). Dyspnea, fatigue, and discomfort are the principal symptoms that negatively impact exercise tolerance.6,7 Therefore, patients often intentionally limit their activities to avoid these uncomfortable feelings and adopt a more sedentary behavior. As the disease progresses, individuals with COPD will gradually need assistance in performing activities of daily living, which eventually leads to functional dependence.
Pulmonary rehabilitation (PR) is an essential component of the management of symptomatic patients with COPD. PR is an evidence-based, multidisciplinary, comprehensive intervention that includes exercise and education for patients with chronic respiratory disease.8 The key benefits of PR are clinical improvements in dyspnea, physical capacity, QOL, and reduced disability in patients with COPD and other respiratory diseases.9-11 PR was found to improve respiratory health in veterans with COPD and decrease respiratory-related health care utilization.12
Despite the known benefits of PR, many patients with chronic respiratory diseases are not referred or do not have access to rehabilitation. Also, uptake of PR is low due to patient frailty, transportation issues, and other health care access problems.13-15 Unfortunately, in the US health care system, access to PR and other nonpharmacologic treatments can be challenging due to a shortage of available PR programs, limited physician referral to existing programs, and lack of family and social support.16
There are only a few accredited PR programs in VHA facilities, and they tend to be located in urban areas.12,17 Many patients have limited access to the PR programs due to geographic distance to the programs and transportation challenges (eg, limited ability to drive, cost of transportation). Moreover, veterans with COPD are likely to have limited mobility or are homebound due to experiencing shortness of breath with minimal exertion. Given the clear benefits of PR and the increasing impact of COPD on morbidity and mortality of the patients with COPD, strategies to improve the access and capacity of PR are needed. VA telehealth services allow for distribution of health care services in different geographic locations by providing access for the veterans who live in rural and highly rural areas. The most recent implementation of VA Video Connect (VVC) by the VHA provides a new avenue for clinicians to deliver much needed medical care into the veterans’ home.
COPD Telehealth Program
In this article, we describe the processes for developing and delivering an in-home, interactive, supervised PR program for veterans with severe COPD through VA telehealth service. The program consists of 18 sessions delivered over 6 weeks by a licensed physical therapist (PT) and a respiratory therapist (RT). The aims of the telehealth PR are to improve exercise tolerance, reduce dyspnea and fatigue, improve QOL, improve accessibility, and decrease costs and transportation burdens for patients with COPD. The program was developed, implemented and delivered by an interdisciplinary team, including a pulmonologist, PT, RT, physiatrist, and nonclinical supporting staff.
Patient Assessment
To be eligible to participate in the program the patient must: (1) have a forced expiratory volume (FEV1) < 60%; ( 2) be medically stable and be receiving optimal medical management; (3) have no severe cognitive impairments; (4) be able to use a computer and e-mail; (5) be able to ambulate with or without a walking device; (6) be willing to enroll in a smoking cessation program or to stop smoking; (7) be willing to participate without prolonged interruption; and (8) have all visual and auditory impairments corrected with medical devices.
After referral and enrollment, patients receive medical and physical examinations by the PR team, including a pulmonologist, a PT, and a RT, to ensure that the patients are medically stable to undergo rehabilitation and to develop a tailored exercise program while being mindful of the comorbidities, limitations, and precautions, (eg, loss of balance, risk of fall, limited range of motion). The preprogram assessment includes a pulmonary function test, arterial blood gas test, Montreal Cognitive Assessment, Modified Medical Research Council Scale, St. George Respiratory Questionnaire, the COPD Assessment Test, Patient Health Questionnaire-9,Generalized Anxiety Disorder Assessment-7, Epworth Sleepiness Scale, Katz Index of Independence of Activities of Daily Living, medications and inhaler use, oxygen use, breathing pattern, coughing, 6-minute walk test, Modified Borg Dyspnea Scale, grip strength, 5 Times Sit to Stand Test, manual muscle test, gait measure, Timed Up & Go test, clinical balance tests, range of motion, flexibility, sensation, pain, and fall history.18-32 Educational needs (eg, respiratory hygiene, nutrition, infection control, sleep, disease/symptom management) also are evaluated.
This thorough assessment is performed in a face-to-face outpatient visit. During the program participation, a physiatrist may be consulted for additional needs (eg, wheelchair assessment, home safety evaluation/ modifications, and mobility/disability issues). After completing the 6-week program, patients are scheduled for the postprogram evaluation in a face-to-face outpatient visit with the clinicians.
Equipment
Both clinician and the patient are equipped with a computer with Wi-Fi connectivity, a webcam, and a microphone. Patients are provided an exercise pictorial booklet, an exercise compact disk (audio and video), small exercise apparatuses (eg, assorted colors of resistance bands, hand grip exerciser, hand putty, ergometer, harmonica, and pedometer), incentive spirometer, pulse oximeter, cough assistive device (as needed), blood pressure monitor, COPD information booklets, and a diary to use at home during the program.33
Technology Preparation
Prior to starting the telehealth program, the patient is contacted 1 or 2 days before the first session for technical preparation and familiarization of the VA telehealth connection process. Either the PT or RT provides step-by-step instructions for the patient to practice connecting through VVC during this preparatory phone call. The patient also practices using the computer webcam, speaker, and microphone; checks the telehealth scheduling e-mail; and learns how to solve possible common technical issues (eg, adjusting volume and position of webcam). The patient is asked to set up a table close to the computer and to place all exercise apparatuses and respiratory devices on the table surface.
Program Delivery
A secure online VVC is used for connection during the telehealth session. The patient received an e-mail from the telehealth scheduling system with a link for VVC before each session. During the 6-week program, each telehealth session is conducted by a PT and a RT concurrently for 120 minutes, 3 days per week. The PT provides exercises for the patient to attempt, and the RT provides breathing training and monitoring during the session. After a successful connection to VVC, the therapist verifies the patient’s identity and confirms patient consent for the telehealth session.
After this check-in process, the patient performs a self-measure of resting blood pressure (BP), heart rate, respiratory rate, and blood oxygen saturation and reports to the therapists. During the exercise session, fatigue/exertion, dyspnea (Modified Borg Dyspnea Scale; Borg CR10 Scale), BP, heart rate, oxygen saturation, and other clinical symptoms and responses to exercise are monitored by the therapists, using both patient-reported measures and clinical observation by the therapists.34,35 Any medical emergency during the session is reported immediately to the pulmonologist for further management.
Structure
Prior to each exercise session, exercise precautions, fall prevention, good posture, pursed-lip breathing, pacing, and coordinated breathing are discussed with the patient. The PT demonstrates stretching and warm-up exercises in front of the webcam for the patient to follow. Then the patient performs all exercises in view of the webcam during the session (Figure 1). A RT monitors breathing patterns and corrects with verbal instructions if not properly performed.
Loss of skeletal muscle mass and cachexia are highly prevalent comorbidities of COPD and have been associated with breathlessness, functional limitation, and poor prognosis.36 To address these comorbidities, our program consists of progressive strengthening, aerobic, balance, and flexibility exercises. Resistance bands and tubes are used for strengthening exercises. Callisthenic exercises (eg, chair squat, chair stand, knee marching, bridging, single limb stances, and lunge) are used for progressive strengthening and balance exercises. Progression of strengthening and balance exercises are done through increasing the volume of exercise (ie, numbers of sets and repetitions) and increased load and level of difficulty based on the patient’s progress and comorbidity. The exercise program focuses on strengthening muscles, especially large muscle groups, to improve overall muscle strength and performance of functional activities.37
Arm/pedal ergometer and daily walking are used for daily aerobic exercise. In a study of patients with COPD by the PAC-COPD Study Group, step counter use was found to increase physical activity and improve exercise capacity, which supports its use in COPD management.38 During program participation, the patient is asked to wear a pedometer to monitor the number of steps taken per day and to report step data to the therapists during the telehealth session. The pedometer stores the previous 41 calendar days of data and displays the most recent 7 calendar days of data.
The patient is encouraged to set a realistic daily step goal. The general program goal is to increase at least 1000 steps per day. However, this goal can be adjusted depending on the patient’s health status and comorbid conditions. The PAC-COPD Study Group found that for every additional 1000 daily steps at low intensity, COPD hospitalization risk decreased by 20%.39 A magnitude of 2000 steps or about 1 mile of walking per day was found to be associated with increased physical activity and health benefits in the general population.40
Respiratory muscle training and breathing exercise are provided by the RT, using breathing and incentive spirometer techniques (Figure 2). Huff coughing, diaphragmatic deep breathing, and pursed-lip breathing are instructed by the RT during the session. Effective coughing technique with a cough assistive device is also provided during breathing training if needed.
Patient Education
In patients with COPD, there are numerous positive health benefits associated with education, including assisting the patients to become active participants in the PR program leading to satisfying outcomes; assisting the patients to better understand the lung health, disease processes, physical and psychological changes that occur with COPD; assisting the patients to explore coping strategies for those changes; building lifelong behavioral changes; and developing the self-management skills for sustainability. Through the educational process, patients with COPD can become more skilled at collaborative self-management and improve adherence to their treatment plan, which in turn can result in a reduction in hospital admissions and reduced health care costs.8,41
Education is provided with every session after the patient completes the exercise. Patients are required to record their COPD symptoms, daily activity, home exercise program, sleep, food intake, and additional physical or social activity in their COPD diary and to report during the session (Figure 3). A COPD diary assists patients in self-monitoring their COPD symptoms and provides the therapists with information about clinical changes, behavioral changes, and/or specific unmet needs for education. Several topics related to COPD are included in the education session: lung or respiratory disease/condition and self-management; smoking cessation; physical activity; energy-conserving techniques; breathing and coughing techniques; smoking cessation; nutrition/healthy eating and weight counseling; sex and intimacy; psychological counseling and/or group support; emergency planning (eg, medical, travel, and inclement weather); correct use of inhaler and medications; home oxygen; sleep and sleep hygiene; palliative care and advanced directive; infection control; and sputum clearance.42,43
Program Maintenance
After successfully completing the 6-week program, patients are referred to the VA TeleMOVE! Program or MOVE! Weight Management Program for continuous, long-term management of weight, nutrition, physical activity/exercise, and social activity needs or goals. The patients are scheduled for monthly follow-up phone visits for 6 months with the telerehabilitation team for enforcing sustainability. The phone call visit consists of reviewing breathing techniques, exercise program, physical activity, education, encouragement, and addressing any issues that arise during the self-maintained period.
Limitations
There are several issues of concern and precautions when delivering PR through telehealth into the home. First, the patient performs exercises independently without being manually guarded by the therapists. Risk of falls are a major concern due to impaired balance, poor vision, and other possible unusual physiologic responses to exercise (eg, drop in BP, dizziness, loss of balance). The area in front of the computer needs to be cleared of fall hazards (ie, area rug, wires, objects on the floor). The patient also needs to be educated on self-measurements of BP and oxygen saturation and reports to the therapists. The therapists provide detailed instructions on how to obtain these measures correctly; otherwise, the values may not be valid for a clinical judgment during the exercise session or for other clinical management. In a home environment, there is a limited use of exercise apparatuses. For this program, we only used resistance bands/tubes, small arm/leg ergometer, hand grip, and hand putty for the exercise program. We feel that dumbbell and weight plates are not suitable due to a possible risk of injury if the patient accidently drops them.
Advanced balance training is not suitable due to an increased risk for falls. Without the presence of the PT, level of challenge/difficulty is somewhat limited for this telehealth supervision exercise program. In addition, visual and audio quality are necessary for the session. The patient and the therapists need to see each other clearly to ensure correct methods and forms of each exercise. Furthermore, rehearsal of technical skills with the therapists is very important because this population is older and often has limited computer skills. Any technical difficulty or failure can lead to undesirable situations (eg, anxiety episodes, worries, shortness of breath, upset), which compromise exercise performance during the session. Finally, a phone is needed as an alternative in case of a poor VVC connection.
Conclusion
COPD symptoms and complications greatly affect patients’ ability to perform daily activities, decrease QOL and functional ability, and result in extensive use of health services. Many patients have limited access to a PR program at hospitals or rehabilitation centers due to health conditions, lack of transportation, and/or family support. This home-based, interactive telehealth PR program can break down the geographic barriers, solve poor program accessibility, potentially increase the utilization of PR, and reduce the cost and travel required by the patients.
Acknowledgments
The Telehealth Pulmonary Rehabilitation Program was originally funded by the Veterans Health Administration VA ACCESS Program (AS, CL, HKH). We thank all the veterans for their time and effort in participating in this newly developed rehabilitation program.
According to World Health Organization estimates, 65 million people have moderate-to-severe chronic obstructive pulmonary disease (COPD) globally, and > 20 million patients with COPD are living in the US.1 COPD is a progressive respiratory disease with a poor prognosis and a significant cause of morbidity and mortality in the US, especially within the Veterans Health Administration (VHA).2 The prevalence of COPD is higher in veterans than it is in the general population. COPD prevalence in the adult US population has been estimated to be between 5% and 15%, whereas in veterans, prevalence estimates have ranged from about 5% to 43%.3-5
COPD is associated with disabling dyspnea, muscle weakness, exercise intolerance, morbidity, and mortality. These symptoms and complications gradually and progressively compromise mobility, ability to perform daily functions, and decrease quality of life (QOL). Dyspnea, fatigue, and discomfort are the principal symptoms that negatively impact exercise tolerance.6,7 Therefore, patients often intentionally limit their activities to avoid these uncomfortable feelings and adopt a more sedentary behavior. As the disease progresses, individuals with COPD will gradually need assistance in performing activities of daily living, which eventually leads to functional dependence.
Pulmonary rehabilitation (PR) is an essential component of the management of symptomatic patients with COPD. PR is an evidence-based, multidisciplinary, comprehensive intervention that includes exercise and education for patients with chronic respiratory disease.8 The key benefits of PR are clinical improvements in dyspnea, physical capacity, QOL, and reduced disability in patients with COPD and other respiratory diseases.9-11 PR was found to improve respiratory health in veterans with COPD and decrease respiratory-related health care utilization.12
Despite the known benefits of PR, many patients with chronic respiratory diseases are not referred or do not have access to rehabilitation. Also, uptake of PR is low due to patient frailty, transportation issues, and other health care access problems.13-15 Unfortunately, in the US health care system, access to PR and other nonpharmacologic treatments can be challenging due to a shortage of available PR programs, limited physician referral to existing programs, and lack of family and social support.16
There are only a few accredited PR programs in VHA facilities, and they tend to be located in urban areas.12,17 Many patients have limited access to the PR programs due to geographic distance to the programs and transportation challenges (eg, limited ability to drive, cost of transportation). Moreover, veterans with COPD are likely to have limited mobility or are homebound due to experiencing shortness of breath with minimal exertion. Given the clear benefits of PR and the increasing impact of COPD on morbidity and mortality of the patients with COPD, strategies to improve the access and capacity of PR are needed. VA telehealth services allow for distribution of health care services in different geographic locations by providing access for the veterans who live in rural and highly rural areas. The most recent implementation of VA Video Connect (VVC) by the VHA provides a new avenue for clinicians to deliver much needed medical care into the veterans’ home.
COPD Telehealth Program
In this article, we describe the processes for developing and delivering an in-home, interactive, supervised PR program for veterans with severe COPD through VA telehealth service. The program consists of 18 sessions delivered over 6 weeks by a licensed physical therapist (PT) and a respiratory therapist (RT). The aims of the telehealth PR are to improve exercise tolerance, reduce dyspnea and fatigue, improve QOL, improve accessibility, and decrease costs and transportation burdens for patients with COPD. The program was developed, implemented and delivered by an interdisciplinary team, including a pulmonologist, PT, RT, physiatrist, and nonclinical supporting staff.
Patient Assessment
To be eligible to participate in the program the patient must: (1) have a forced expiratory volume (FEV1) < 60%; ( 2) be medically stable and be receiving optimal medical management; (3) have no severe cognitive impairments; (4) be able to use a computer and e-mail; (5) be able to ambulate with or without a walking device; (6) be willing to enroll in a smoking cessation program or to stop smoking; (7) be willing to participate without prolonged interruption; and (8) have all visual and auditory impairments corrected with medical devices.
After referral and enrollment, patients receive medical and physical examinations by the PR team, including a pulmonologist, a PT, and a RT, to ensure that the patients are medically stable to undergo rehabilitation and to develop a tailored exercise program while being mindful of the comorbidities, limitations, and precautions, (eg, loss of balance, risk of fall, limited range of motion). The preprogram assessment includes a pulmonary function test, arterial blood gas test, Montreal Cognitive Assessment, Modified Medical Research Council Scale, St. George Respiratory Questionnaire, the COPD Assessment Test, Patient Health Questionnaire-9,Generalized Anxiety Disorder Assessment-7, Epworth Sleepiness Scale, Katz Index of Independence of Activities of Daily Living, medications and inhaler use, oxygen use, breathing pattern, coughing, 6-minute walk test, Modified Borg Dyspnea Scale, grip strength, 5 Times Sit to Stand Test, manual muscle test, gait measure, Timed Up & Go test, clinical balance tests, range of motion, flexibility, sensation, pain, and fall history.18-32 Educational needs (eg, respiratory hygiene, nutrition, infection control, sleep, disease/symptom management) also are evaluated.
This thorough assessment is performed in a face-to-face outpatient visit. During the program participation, a physiatrist may be consulted for additional needs (eg, wheelchair assessment, home safety evaluation/ modifications, and mobility/disability issues). After completing the 6-week program, patients are scheduled for the postprogram evaluation in a face-to-face outpatient visit with the clinicians.
Equipment
Both clinician and the patient are equipped with a computer with Wi-Fi connectivity, a webcam, and a microphone. Patients are provided an exercise pictorial booklet, an exercise compact disk (audio and video), small exercise apparatuses (eg, assorted colors of resistance bands, hand grip exerciser, hand putty, ergometer, harmonica, and pedometer), incentive spirometer, pulse oximeter, cough assistive device (as needed), blood pressure monitor, COPD information booklets, and a diary to use at home during the program.33
Technology Preparation
Prior to starting the telehealth program, the patient is contacted 1 or 2 days before the first session for technical preparation and familiarization of the VA telehealth connection process. Either the PT or RT provides step-by-step instructions for the patient to practice connecting through VVC during this preparatory phone call. The patient also practices using the computer webcam, speaker, and microphone; checks the telehealth scheduling e-mail; and learns how to solve possible common technical issues (eg, adjusting volume and position of webcam). The patient is asked to set up a table close to the computer and to place all exercise apparatuses and respiratory devices on the table surface.
Program Delivery
A secure online VVC is used for connection during the telehealth session. The patient received an e-mail from the telehealth scheduling system with a link for VVC before each session. During the 6-week program, each telehealth session is conducted by a PT and a RT concurrently for 120 minutes, 3 days per week. The PT provides exercises for the patient to attempt, and the RT provides breathing training and monitoring during the session. After a successful connection to VVC, the therapist verifies the patient’s identity and confirms patient consent for the telehealth session.
After this check-in process, the patient performs a self-measure of resting blood pressure (BP), heart rate, respiratory rate, and blood oxygen saturation and reports to the therapists. During the exercise session, fatigue/exertion, dyspnea (Modified Borg Dyspnea Scale; Borg CR10 Scale), BP, heart rate, oxygen saturation, and other clinical symptoms and responses to exercise are monitored by the therapists, using both patient-reported measures and clinical observation by the therapists.34,35 Any medical emergency during the session is reported immediately to the pulmonologist for further management.
Structure
Prior to each exercise session, exercise precautions, fall prevention, good posture, pursed-lip breathing, pacing, and coordinated breathing are discussed with the patient. The PT demonstrates stretching and warm-up exercises in front of the webcam for the patient to follow. Then the patient performs all exercises in view of the webcam during the session (Figure 1). A RT monitors breathing patterns and corrects with verbal instructions if not properly performed.
Loss of skeletal muscle mass and cachexia are highly prevalent comorbidities of COPD and have been associated with breathlessness, functional limitation, and poor prognosis.36 To address these comorbidities, our program consists of progressive strengthening, aerobic, balance, and flexibility exercises. Resistance bands and tubes are used for strengthening exercises. Callisthenic exercises (eg, chair squat, chair stand, knee marching, bridging, single limb stances, and lunge) are used for progressive strengthening and balance exercises. Progression of strengthening and balance exercises are done through increasing the volume of exercise (ie, numbers of sets and repetitions) and increased load and level of difficulty based on the patient’s progress and comorbidity. The exercise program focuses on strengthening muscles, especially large muscle groups, to improve overall muscle strength and performance of functional activities.37
Arm/pedal ergometer and daily walking are used for daily aerobic exercise. In a study of patients with COPD by the PAC-COPD Study Group, step counter use was found to increase physical activity and improve exercise capacity, which supports its use in COPD management.38 During program participation, the patient is asked to wear a pedometer to monitor the number of steps taken per day and to report step data to the therapists during the telehealth session. The pedometer stores the previous 41 calendar days of data and displays the most recent 7 calendar days of data.
The patient is encouraged to set a realistic daily step goal. The general program goal is to increase at least 1000 steps per day. However, this goal can be adjusted depending on the patient’s health status and comorbid conditions. The PAC-COPD Study Group found that for every additional 1000 daily steps at low intensity, COPD hospitalization risk decreased by 20%.39 A magnitude of 2000 steps or about 1 mile of walking per day was found to be associated with increased physical activity and health benefits in the general population.40
Respiratory muscle training and breathing exercise are provided by the RT, using breathing and incentive spirometer techniques (Figure 2). Huff coughing, diaphragmatic deep breathing, and pursed-lip breathing are instructed by the RT during the session. Effective coughing technique with a cough assistive device is also provided during breathing training if needed.
Patient Education
In patients with COPD, there are numerous positive health benefits associated with education, including assisting the patients to become active participants in the PR program leading to satisfying outcomes; assisting the patients to better understand the lung health, disease processes, physical and psychological changes that occur with COPD; assisting the patients to explore coping strategies for those changes; building lifelong behavioral changes; and developing the self-management skills for sustainability. Through the educational process, patients with COPD can become more skilled at collaborative self-management and improve adherence to their treatment plan, which in turn can result in a reduction in hospital admissions and reduced health care costs.8,41
Education is provided with every session after the patient completes the exercise. Patients are required to record their COPD symptoms, daily activity, home exercise program, sleep, food intake, and additional physical or social activity in their COPD diary and to report during the session (Figure 3). A COPD diary assists patients in self-monitoring their COPD symptoms and provides the therapists with information about clinical changes, behavioral changes, and/or specific unmet needs for education. Several topics related to COPD are included in the education session: lung or respiratory disease/condition and self-management; smoking cessation; physical activity; energy-conserving techniques; breathing and coughing techniques; smoking cessation; nutrition/healthy eating and weight counseling; sex and intimacy; psychological counseling and/or group support; emergency planning (eg, medical, travel, and inclement weather); correct use of inhaler and medications; home oxygen; sleep and sleep hygiene; palliative care and advanced directive; infection control; and sputum clearance.42,43
Program Maintenance
After successfully completing the 6-week program, patients are referred to the VA TeleMOVE! Program or MOVE! Weight Management Program for continuous, long-term management of weight, nutrition, physical activity/exercise, and social activity needs or goals. The patients are scheduled for monthly follow-up phone visits for 6 months with the telerehabilitation team for enforcing sustainability. The phone call visit consists of reviewing breathing techniques, exercise program, physical activity, education, encouragement, and addressing any issues that arise during the self-maintained period.
Limitations
There are several issues of concern and precautions when delivering PR through telehealth into the home. First, the patient performs exercises independently without being manually guarded by the therapists. Risk of falls are a major concern due to impaired balance, poor vision, and other possible unusual physiologic responses to exercise (eg, drop in BP, dizziness, loss of balance). The area in front of the computer needs to be cleared of fall hazards (ie, area rug, wires, objects on the floor). The patient also needs to be educated on self-measurements of BP and oxygen saturation and reports to the therapists. The therapists provide detailed instructions on how to obtain these measures correctly; otherwise, the values may not be valid for a clinical judgment during the exercise session or for other clinical management. In a home environment, there is a limited use of exercise apparatuses. For this program, we only used resistance bands/tubes, small arm/leg ergometer, hand grip, and hand putty for the exercise program. We feel that dumbbell and weight plates are not suitable due to a possible risk of injury if the patient accidently drops them.
Advanced balance training is not suitable due to an increased risk for falls. Without the presence of the PT, level of challenge/difficulty is somewhat limited for this telehealth supervision exercise program. In addition, visual and audio quality are necessary for the session. The patient and the therapists need to see each other clearly to ensure correct methods and forms of each exercise. Furthermore, rehearsal of technical skills with the therapists is very important because this population is older and often has limited computer skills. Any technical difficulty or failure can lead to undesirable situations (eg, anxiety episodes, worries, shortness of breath, upset), which compromise exercise performance during the session. Finally, a phone is needed as an alternative in case of a poor VVC connection.
Conclusion
COPD symptoms and complications greatly affect patients’ ability to perform daily activities, decrease QOL and functional ability, and result in extensive use of health services. Many patients have limited access to a PR program at hospitals or rehabilitation centers due to health conditions, lack of transportation, and/or family support. This home-based, interactive telehealth PR program can break down the geographic barriers, solve poor program accessibility, potentially increase the utilization of PR, and reduce the cost and travel required by the patients.
Acknowledgments
The Telehealth Pulmonary Rehabilitation Program was originally funded by the Veterans Health Administration VA ACCESS Program (AS, CL, HKH). We thank all the veterans for their time and effort in participating in this newly developed rehabilitation program.
1. World Health Organization. Chronic obstructive pulmonary disease (COPD). http://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd). Published December 1, 2017. Accessed August 7, 2019.
2. Yu W, Ravelo A, Wagner TH, et al. Prevalence and costs of chronic conditions in the VA health care system. Med Care Res Rev. 2003;60(suppl 3):146S-167S.
3. Doney B, Hnizdo E, Dillon CF, et al. Prevalence of airflow obstruction in U.S. adults aged 40-79 years: NHANES data 1988-1994 and 2007-2010. COPD. 2015;12(4):355-365.
4. Murphy DE, Chaudhry Z, Almoosa KF, Panos RJ. High prevalence of chronic obstructive pulmonary disease among veterans in the urban midwest. Mil Med. 2011;176(5):552-560.
5. Cypel YS, Hines SE, Davey VJ, Eber SM, Schneiderman AI. Self-reported physician-diagnosed chronic obstructive pulmonary disease and spirometry patterns in Vietnam Era US Army Chemical Corps veterans: a retrospective cohort study. Am J Ind Med. 2018;61(10):802-814.
6. Rochester CL. Exercise training in chronic obstructive pulmonary disease. J Rehabil Res Dev. 2003;40(5)(suppl 2):59-80.
7. Cortopassi F, Gurung P, Pinto-Plata V. Chronic obstructive pulmonary disease in elderly patients. Clin Geriatr Med. 2017;33(4):539-552.
8. Spruit MA, Singh SJ, Garvey C, et al; ATS/ERS Task Force on Pulmonary Rehabilitation. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188(8):e13-e64.
9. Robinson H, Williams V, Curtis F, Bridle C, Jones AW. Facilitators and barriers to physical activity following pulmonary rehabilitation in COPD: a systematic review of qualitative studies. NPJ Prim Care Respir Med. 2018;28(1):19.
10. McCarthy B, Casey D, Devane D, Murphy K, Murphy E, Lacasse Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015;(2):CD003793.
11. Ries AL, Bauldoff GS, Carlin BW, et al. Pulmonary rehabilitation: joint AACP/AACVPR evidence-based clinical practice guidelines. Chest. 2007;131(suppl 5):4S-42S.
12. Major S, Moreno M, Shelton J, Panos RJ. Veterans with chronic obstructive pulmonary disease achieve clinically relevant improvements in respiratory health after pulmonary rehabilitation. J Cardiopulm Rehabil Prev. 2014;34(6):420-429.
13. Liu Y, Dickerson T, Early F, Fuld J, Clarkson PJ. Understanding influences on the uptake of pulmonary rehabilitation in the East of England: an inclusive design/mixed methods study protocol. BMJ Open. 2018;8(4):e020750.
14. Harris D, Hayter M, Allender S. Factors affecting the offer of pulmonary rehabilitation to patients with chronic obstructive pulmonary disease by primary care professionals: a qualitative study. Prim Health Care Res Dev. 2008;9(4):280-290.
15. Mathar H, Fastholm P, Hansen IR, Larsen NS. Why do patients with COPD decline rehabilitation. Scand J Caring Sci. 2016;30(3):432-441.
16. Han MK, Martinez CH, Au DH, et al. Meeting the challenge of COPD care delivery in the USA: a multiprovider perspective. Lancet Respir Med. 2016;4(6):473-526.
17. American Association of Cardiovascular and Pulmonary Rehabilitation (AACVPR). Online searchable program directory. https://www.aacvpr.org/Resources/Program-Directory Accessed July 19, 2018.
18. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695-699.
19. Fletcher CM, Elmes PC, Fairbairn AS, Wood CH. The significance of respiratory symptoms and the diagnosis of chronic bronchitis in a working population. Br Med J. 1959;2(5147):257-266.
20. O’Donnell DE, Aaron S, Bourbeau J, et al. Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease—2007 update. Can Respir J. 2007;14(suppl B):5B-32B.
21. Jones PW, Quirk FH, Baveystock CM. The St George’s Respiratory Questionnaire. Respir Med. 1991;85(suppl B):25-31.
22. Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34(3):648-654.
23. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613.
24. Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092-1097.
25. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540-545.
26. Katz S. Assessing self-maintenance: activities of daily living, mobility and instrumental activities of daily living. J Am Geriatr Soc. 1983;31(12):721-727.
27. Holland AE, Spruit MA, Troosters T, et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J. 2014;44(6):1428-1446.
28. Mahler DA, Horowitz MB. Perception of breathlessness during exercise in patients with respiratory disease. Med Sci Sports Exerc. 1994;26(9):1078-1081.
29. Liao WC, Wang CH, Yu SY, Chen LY, Wang CY. Grip strength measurement in older adults in Taiwan: a comparison of three testing positions. Australas J Ageing. 2014;33(4):278-282.
30. Buatois S, Miljkovic D, Manckoundia P, et al. Five times sit to stand test is a predictor of recurrent falls in healthy community-living subjects aged 65 and older. J Am Geriatr Soc. 2008;56(8):1575-1577.
31. Bryant MS, Workman CD, Jackson GR. Multidirectional walk test in persons with Parkinson’s disease: a validity study. Int J Rehabil Res. 2015;38(1):88-91.
32. Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142-148.
33. University of Nebraska Medical Center. Timed Up and Go (TUG) Test. https://www.unmc.edu/media/intmed/geriatrics/nebgec/pdf/frailelderlyjuly09/toolkits/timedupandgo_w_norms.pdf. Accessed August 13, 2019.
34. Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377-381.
35. Mahler DA, Horowitz MB. Clinical evaluation of exertional dyspnea. Clin Chest Med. 1994;15(2):259-269.
36. Dudgeon D, Baracos VE. Physiological and functional failure in chronic obstructive pulmonary disease, congestive heart failure and cancer: a debilitating intersection of sarcopenia, cachexia and breathlessness. Curr Opin Support Palliat Care. 2016;10(3):236-241.
37. Lee AL, Holland AE. Time to adapt exercise training regimens in pulmonary rehabilitation—a review of the literature. Int J Chron Obstruct Pulmon Dis. 2014;9:1275-1288.
38. Qiu S, Cai X, Wang X, et al. Using step counters to promote physical activity and exercise capacity in patients with chronic obstructive pulmonary disease: a meta-analysis. Ther Adv Respir Dis. 2018;12:1753466618787386.
39. Donaire-Gonzalez D, Gimeno-Santos E, Balcells E, et al; PAC-COPD Study Group. Benefits of physical activity on COPD hospitalization depend on intensity. Eur Respir J. 2015;46(5):1281-1289.
40. Bravata DM, Smith-Spangler C, Sundaram V, et al. Using pedometers to increase physical activity and improve health: a systematic review. JAMA. 2007;298(19):2296-2304.
41. Zwerink M, Brusse-Keizer M, van der Valk PD, et al. Self-management for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014;19(3):CD002990.
42. Wilson JS, O’Neill B, Reilly J, MacMahon J, Bradley JM. Education in pulmonary rehabilitation: the patient’s perspective. Arch Phys Med Rehabil. 2007;88(12):1704-1709.
43. Bourbeau J, Nault D, Dang-Tan T. Self-management and behaviour modification in COPD. Patient Educ Couns. 2004;52(3):271-277.
1. World Health Organization. Chronic obstructive pulmonary disease (COPD). http://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd). Published December 1, 2017. Accessed August 7, 2019.
2. Yu W, Ravelo A, Wagner TH, et al. Prevalence and costs of chronic conditions in the VA health care system. Med Care Res Rev. 2003;60(suppl 3):146S-167S.
3. Doney B, Hnizdo E, Dillon CF, et al. Prevalence of airflow obstruction in U.S. adults aged 40-79 years: NHANES data 1988-1994 and 2007-2010. COPD. 2015;12(4):355-365.
4. Murphy DE, Chaudhry Z, Almoosa KF, Panos RJ. High prevalence of chronic obstructive pulmonary disease among veterans in the urban midwest. Mil Med. 2011;176(5):552-560.
5. Cypel YS, Hines SE, Davey VJ, Eber SM, Schneiderman AI. Self-reported physician-diagnosed chronic obstructive pulmonary disease and spirometry patterns in Vietnam Era US Army Chemical Corps veterans: a retrospective cohort study. Am J Ind Med. 2018;61(10):802-814.
6. Rochester CL. Exercise training in chronic obstructive pulmonary disease. J Rehabil Res Dev. 2003;40(5)(suppl 2):59-80.
7. Cortopassi F, Gurung P, Pinto-Plata V. Chronic obstructive pulmonary disease in elderly patients. Clin Geriatr Med. 2017;33(4):539-552.
8. Spruit MA, Singh SJ, Garvey C, et al; ATS/ERS Task Force on Pulmonary Rehabilitation. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188(8):e13-e64.
9. Robinson H, Williams V, Curtis F, Bridle C, Jones AW. Facilitators and barriers to physical activity following pulmonary rehabilitation in COPD: a systematic review of qualitative studies. NPJ Prim Care Respir Med. 2018;28(1):19.
10. McCarthy B, Casey D, Devane D, Murphy K, Murphy E, Lacasse Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015;(2):CD003793.
11. Ries AL, Bauldoff GS, Carlin BW, et al. Pulmonary rehabilitation: joint AACP/AACVPR evidence-based clinical practice guidelines. Chest. 2007;131(suppl 5):4S-42S.
12. Major S, Moreno M, Shelton J, Panos RJ. Veterans with chronic obstructive pulmonary disease achieve clinically relevant improvements in respiratory health after pulmonary rehabilitation. J Cardiopulm Rehabil Prev. 2014;34(6):420-429.
13. Liu Y, Dickerson T, Early F, Fuld J, Clarkson PJ. Understanding influences on the uptake of pulmonary rehabilitation in the East of England: an inclusive design/mixed methods study protocol. BMJ Open. 2018;8(4):e020750.
14. Harris D, Hayter M, Allender S. Factors affecting the offer of pulmonary rehabilitation to patients with chronic obstructive pulmonary disease by primary care professionals: a qualitative study. Prim Health Care Res Dev. 2008;9(4):280-290.
15. Mathar H, Fastholm P, Hansen IR, Larsen NS. Why do patients with COPD decline rehabilitation. Scand J Caring Sci. 2016;30(3):432-441.
16. Han MK, Martinez CH, Au DH, et al. Meeting the challenge of COPD care delivery in the USA: a multiprovider perspective. Lancet Respir Med. 2016;4(6):473-526.
17. American Association of Cardiovascular and Pulmonary Rehabilitation (AACVPR). Online searchable program directory. https://www.aacvpr.org/Resources/Program-Directory Accessed July 19, 2018.
18. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695-699.
19. Fletcher CM, Elmes PC, Fairbairn AS, Wood CH. The significance of respiratory symptoms and the diagnosis of chronic bronchitis in a working population. Br Med J. 1959;2(5147):257-266.
20. O’Donnell DE, Aaron S, Bourbeau J, et al. Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease—2007 update. Can Respir J. 2007;14(suppl B):5B-32B.
21. Jones PW, Quirk FH, Baveystock CM. The St George’s Respiratory Questionnaire. Respir Med. 1991;85(suppl B):25-31.
22. Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34(3):648-654.
23. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613.
24. Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092-1097.
25. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540-545.
26. Katz S. Assessing self-maintenance: activities of daily living, mobility and instrumental activities of daily living. J Am Geriatr Soc. 1983;31(12):721-727.
27. Holland AE, Spruit MA, Troosters T, et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J. 2014;44(6):1428-1446.
28. Mahler DA, Horowitz MB. Perception of breathlessness during exercise in patients with respiratory disease. Med Sci Sports Exerc. 1994;26(9):1078-1081.
29. Liao WC, Wang CH, Yu SY, Chen LY, Wang CY. Grip strength measurement in older adults in Taiwan: a comparison of three testing positions. Australas J Ageing. 2014;33(4):278-282.
30. Buatois S, Miljkovic D, Manckoundia P, et al. Five times sit to stand test is a predictor of recurrent falls in healthy community-living subjects aged 65 and older. J Am Geriatr Soc. 2008;56(8):1575-1577.
31. Bryant MS, Workman CD, Jackson GR. Multidirectional walk test in persons with Parkinson’s disease: a validity study. Int J Rehabil Res. 2015;38(1):88-91.
32. Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142-148.
33. University of Nebraska Medical Center. Timed Up and Go (TUG) Test. https://www.unmc.edu/media/intmed/geriatrics/nebgec/pdf/frailelderlyjuly09/toolkits/timedupandgo_w_norms.pdf. Accessed August 13, 2019.
34. Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377-381.
35. Mahler DA, Horowitz MB. Clinical evaluation of exertional dyspnea. Clin Chest Med. 1994;15(2):259-269.
36. Dudgeon D, Baracos VE. Physiological and functional failure in chronic obstructive pulmonary disease, congestive heart failure and cancer: a debilitating intersection of sarcopenia, cachexia and breathlessness. Curr Opin Support Palliat Care. 2016;10(3):236-241.
37. Lee AL, Holland AE. Time to adapt exercise training regimens in pulmonary rehabilitation—a review of the literature. Int J Chron Obstruct Pulmon Dis. 2014;9:1275-1288.
38. Qiu S, Cai X, Wang X, et al. Using step counters to promote physical activity and exercise capacity in patients with chronic obstructive pulmonary disease: a meta-analysis. Ther Adv Respir Dis. 2018;12:1753466618787386.
39. Donaire-Gonzalez D, Gimeno-Santos E, Balcells E, et al; PAC-COPD Study Group. Benefits of physical activity on COPD hospitalization depend on intensity. Eur Respir J. 2015;46(5):1281-1289.
40. Bravata DM, Smith-Spangler C, Sundaram V, et al. Using pedometers to increase physical activity and improve health: a systematic review. JAMA. 2007;298(19):2296-2304.
41. Zwerink M, Brusse-Keizer M, van der Valk PD, et al. Self-management for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014;19(3):CD002990.
42. Wilson JS, O’Neill B, Reilly J, MacMahon J, Bradley JM. Education in pulmonary rehabilitation: the patient’s perspective. Arch Phys Med Rehabil. 2007;88(12):1704-1709.
43. Bourbeau J, Nault D, Dang-Tan T. Self-management and behaviour modification in COPD. Patient Educ Couns. 2004;52(3):271-277.