User login
A Unique Presentation of Lupus Erythematosus Tumidus in an Adolescent Boy
To the Editor:
Lupus erythematosus tumidus (LET) is a rarely diagnosed condition that was first described in 1909 by Hoffmann.1 Limited cases have been reported in the literature, with few documenting the disease in children.2 We report a unique clinical case of LET in a 14-year-old adolescent boy that was distributed solely on the hands. With slight heterogeneity in regards to clinical presentation and histopathology, there is a need for further exploration with regard to LET.
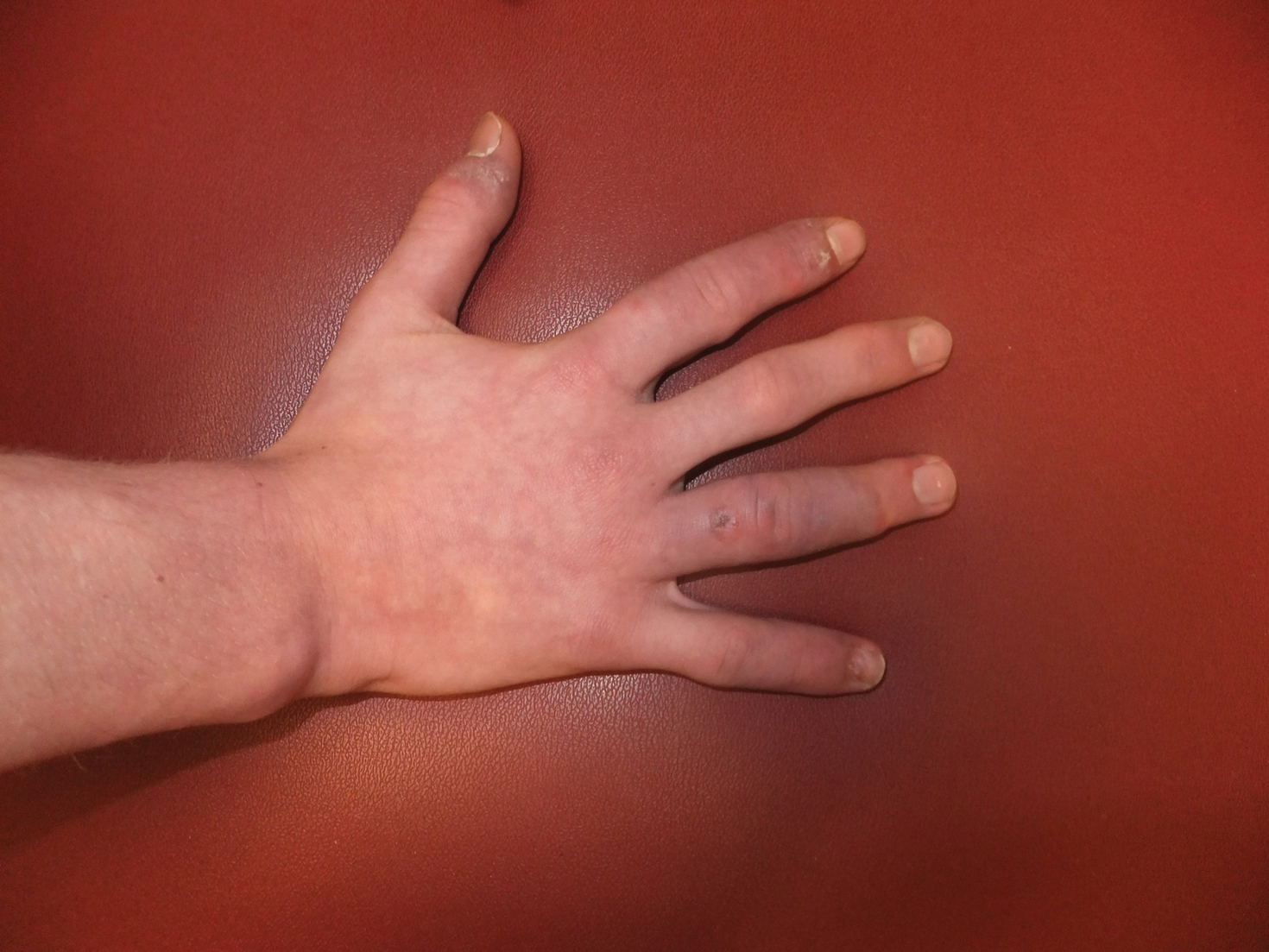
A 14-year-old adolescent boy presented to the dermatology clinic with progressive bilateral edema of 1 year’s duration with plaques and some scaling on the dorsal aspects of the digits and the nail bases predominantly on the right hand (Figure 1) and to a lesser extent on the left hand. The edema, erythema, and tenderness started in the right fifth digit; soon after the edema appeared, plaques began to form at the base of each nail bed, and the edema and erythema progressively spread to the other digits. He denied worsening of symptoms when exposed to cold temperatures. A complete review of systems was negative. The differential diagnoses included chilblain lupus erythematosus, perniosis, dermatomyositis, and polymorphous light eruption. A punch biopsy from the right fourth digit was performed.
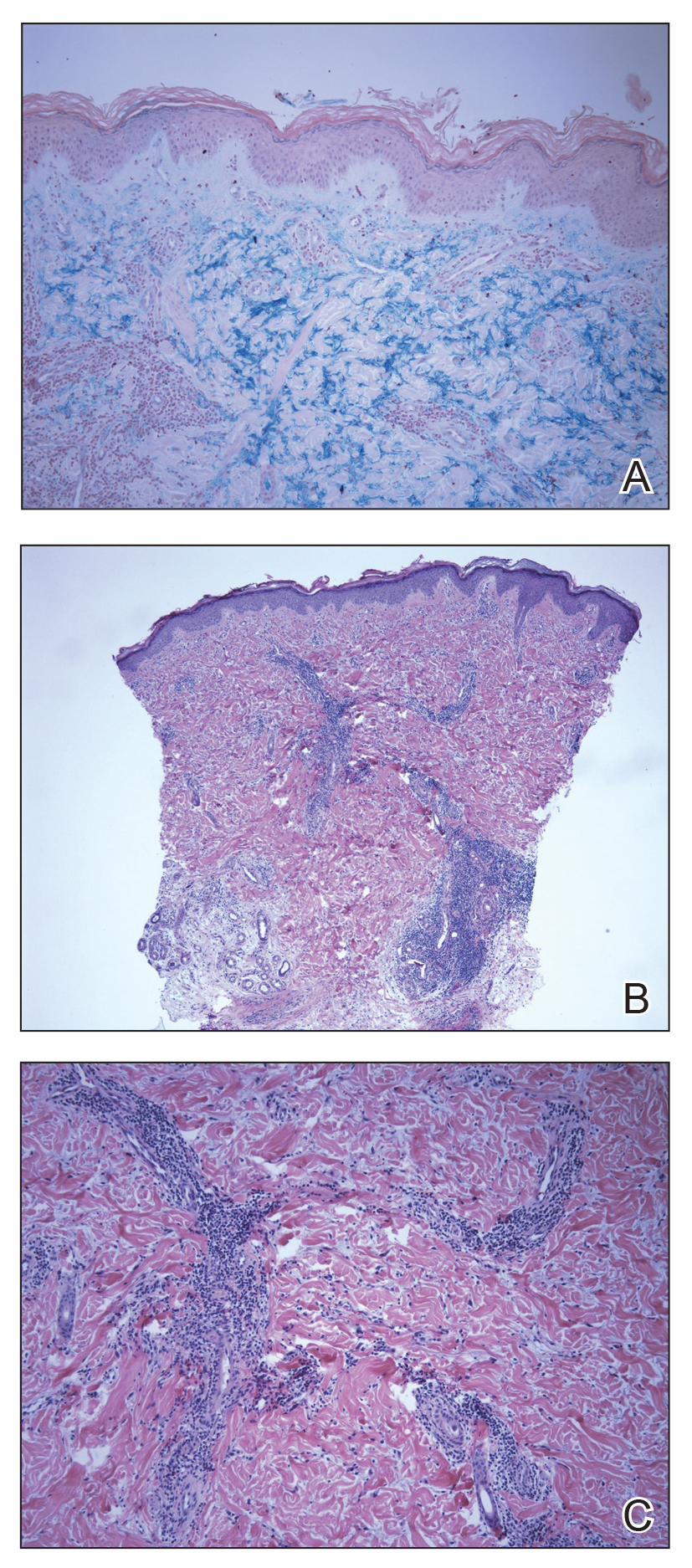
The biopsy showed superficial and deep perivascular and periadnexal mononuclear inflammation with large amounts of interstitial mucin deposition (Figure 2). The epidermis exhibited a loose orthokeratotic scale with no signs of interface damage. A diagnosis of perniosis was entertained but was ruled out due to the lack of papillary dermal edema and large amounts of mucin. With the lack of interface change and large amounts of mucin, a diagnosis of LET was favored over chilblain lupus erythematosus, as the latter diagnosis typically demonstrates interface change. The patient was started on hydroxychloroquine 200 mg twice daily and a short course of prednisone, and improvement of the lesions/plaques was noted at follow-up 6 weeks later. Continued improvement was noted 2 years after the initial presentation. His condition recurred when the hydroxychloroquine dosage was reduced to 200 mg once daily after 1 year. The patient did not report any adverse sequelae to treatment.
Histopathologic findings of superficial and deep perivascular and periadnexal lymphocytic infiltrates and interstitial dermal deposition of mucin in LET have remained consistent in the literature. Direct immunofluorescence has not revealed any complement or immunoglobulin deposition on the basement membrane.3,4 The epidermal characteristics are not as uniform, with the majority of cases in one review showing no epidermal changes and a minority showing minimal epidermal changes (eg, epidermal atrophy, hyperkeratosis, parakeratosis, acanthosis, spongiosis).5 When working up patients for LET, blood work usually is unremarkable, as LET rarely is associated with antinuclear antibodies or anti-Ro, anti-La, and anti-DNA antibodies.3,4 Lupus erythematosus tumidus generally is an independent process, but it has been reported to coexist with discoid lupus erythematosus and systemic lupus erythematosus in rare cases.6
The lesions of LET have been consistently described in the literature as photosensitive, erythematous, non-scarring, annular plaques and papules commonly occurring on the head/neck and other sun-exposed areas that do not cause hypopigmentation.3 Treatment of LET consists of systemic treatment with antimalarial drugs, sunscreens, and topical steroids for flares.
Lupus erythematosus tumidus is rare in children, with few case reports noted in the literature. Sonntag et al2 documented the disease in 3 children ranging from 3 to 8 years of age. Furthermore, Ruiz and Sanchez7 reported a case of LET in a 16-year-old adolescent girl. Our case is unique in that the lesions only occurred on the hands, whereas most case reports document distribution of the lesions on the head, neck, face, arms, back, and chest. Our patient’s age and the location of the lesions make it a unique clinical presentation of LET.
Reports in the literature show evidence of heterogeneity in the presentation, classification, and some of the histopathologic features of LET; however, there are minimal data on childhood LET. Further research and investigations are needed to more precisely define this condition.
Acknowledgment
The authors acknowledge Richard Schwartz, MD (Akron, Ohio), for reading the biopsy reports and assisting with photomicrographs.
- Hoffmann E. Demonstrationen: lupus erythematosus tumidus. Derm Zeitschr. 1909;16:159-160.
- Sonntag M, Lehmann P, Megahed M, et al. Lupus erythematosus tumidus in childhood. Dermatology. 2003;207:188-192.
- Schmitt V, Meuth AM, Amler S, et al. Lupus erythematosus tumidus is a separate subtype of cutaneous lupus erythematosus. Br J Dermatol. 2010;162:64-73.
- Vieira V, Del Pozo J, Yebra-Pimentel MT, et al. Lupus erythematosus tumidus: a series of 26 cases. Int J Dermatol. 2006;45:512-517.
- Kuhn A, Richter-Hintz D, Oslislo C, et al. Lupus erythematosus tumidus—a neglected subset of cutaneous lupus erythematosus: report of 40 cases. Arch Dermatol. 2000;136:1033-1041.
- Chen X, Wang S, Li L. A case report of lupus erythematosus tumidus converted from discoid lupus erythematosus. Medicine (Baltimore). 2018;97:e0375.
- Ruiz H, Sanchez J. Tumid lupus erythematosus. Am J Dermatopathol. 1999;21:356-360.
To the Editor:
Lupus erythematosus tumidus (LET) is a rarely diagnosed condition that was first described in 1909 by Hoffmann.1 Limited cases have been reported in the literature, with few documenting the disease in children.2 We report a unique clinical case of LET in a 14-year-old adolescent boy that was distributed solely on the hands. With slight heterogeneity in regards to clinical presentation and histopathology, there is a need for further exploration with regard to LET.
A 14-year-old adolescent boy presented to the dermatology clinic with progressive bilateral edema of 1 year’s duration with plaques and some scaling on the dorsal aspects of the digits and the nail bases predominantly on the right hand (Figure 1) and to a lesser extent on the left hand. The edema, erythema, and tenderness started in the right fifth digit; soon after the edema appeared, plaques began to form at the base of each nail bed, and the edema and erythema progressively spread to the other digits. He denied worsening of symptoms when exposed to cold temperatures. A complete review of systems was negative. The differential diagnoses included chilblain lupus erythematosus, perniosis, dermatomyositis, and polymorphous light eruption. A punch biopsy from the right fourth digit was performed.
The biopsy showed superficial and deep perivascular and periadnexal mononuclear inflammation with large amounts of interstitial mucin deposition (Figure 2). The epidermis exhibited a loose orthokeratotic scale with no signs of interface damage. A diagnosis of perniosis was entertained but was ruled out due to the lack of papillary dermal edema and large amounts of mucin. With the lack of interface change and large amounts of mucin, a diagnosis of LET was favored over chilblain lupus erythematosus, as the latter diagnosis typically demonstrates interface change. The patient was started on hydroxychloroquine 200 mg twice daily and a short course of prednisone, and improvement of the lesions/plaques was noted at follow-up 6 weeks later. Continued improvement was noted 2 years after the initial presentation. His condition recurred when the hydroxychloroquine dosage was reduced to 200 mg once daily after 1 year. The patient did not report any adverse sequelae to treatment.
Histopathologic findings of superficial and deep perivascular and periadnexal lymphocytic infiltrates and interstitial dermal deposition of mucin in LET have remained consistent in the literature. Direct immunofluorescence has not revealed any complement or immunoglobulin deposition on the basement membrane.3,4 The epidermal characteristics are not as uniform, with the majority of cases in one review showing no epidermal changes and a minority showing minimal epidermal changes (eg, epidermal atrophy, hyperkeratosis, parakeratosis, acanthosis, spongiosis).5 When working up patients for LET, blood work usually is unremarkable, as LET rarely is associated with antinuclear antibodies or anti-Ro, anti-La, and anti-DNA antibodies.3,4 Lupus erythematosus tumidus generally is an independent process, but it has been reported to coexist with discoid lupus erythematosus and systemic lupus erythematosus in rare cases.6
The lesions of LET have been consistently described in the literature as photosensitive, erythematous, non-scarring, annular plaques and papules commonly occurring on the head/neck and other sun-exposed areas that do not cause hypopigmentation.3 Treatment of LET consists of systemic treatment with antimalarial drugs, sunscreens, and topical steroids for flares.
Lupus erythematosus tumidus is rare in children, with few case reports noted in the literature. Sonntag et al2 documented the disease in 3 children ranging from 3 to 8 years of age. Furthermore, Ruiz and Sanchez7 reported a case of LET in a 16-year-old adolescent girl. Our case is unique in that the lesions only occurred on the hands, whereas most case reports document distribution of the lesions on the head, neck, face, arms, back, and chest. Our patient’s age and the location of the lesions make it a unique clinical presentation of LET.
Reports in the literature show evidence of heterogeneity in the presentation, classification, and some of the histopathologic features of LET; however, there are minimal data on childhood LET. Further research and investigations are needed to more precisely define this condition.
Acknowledgment
The authors acknowledge Richard Schwartz, MD (Akron, Ohio), for reading the biopsy reports and assisting with photomicrographs.
To the Editor:
Lupus erythematosus tumidus (LET) is a rarely diagnosed condition that was first described in 1909 by Hoffmann.1 Limited cases have been reported in the literature, with few documenting the disease in children.2 We report a unique clinical case of LET in a 14-year-old adolescent boy that was distributed solely on the hands. With slight heterogeneity in regards to clinical presentation and histopathology, there is a need for further exploration with regard to LET.
A 14-year-old adolescent boy presented to the dermatology clinic with progressive bilateral edema of 1 year’s duration with plaques and some scaling on the dorsal aspects of the digits and the nail bases predominantly on the right hand (Figure 1) and to a lesser extent on the left hand. The edema, erythema, and tenderness started in the right fifth digit; soon after the edema appeared, plaques began to form at the base of each nail bed, and the edema and erythema progressively spread to the other digits. He denied worsening of symptoms when exposed to cold temperatures. A complete review of systems was negative. The differential diagnoses included chilblain lupus erythematosus, perniosis, dermatomyositis, and polymorphous light eruption. A punch biopsy from the right fourth digit was performed.
The biopsy showed superficial and deep perivascular and periadnexal mononuclear inflammation with large amounts of interstitial mucin deposition (Figure 2). The epidermis exhibited a loose orthokeratotic scale with no signs of interface damage. A diagnosis of perniosis was entertained but was ruled out due to the lack of papillary dermal edema and large amounts of mucin. With the lack of interface change and large amounts of mucin, a diagnosis of LET was favored over chilblain lupus erythematosus, as the latter diagnosis typically demonstrates interface change. The patient was started on hydroxychloroquine 200 mg twice daily and a short course of prednisone, and improvement of the lesions/plaques was noted at follow-up 6 weeks later. Continued improvement was noted 2 years after the initial presentation. His condition recurred when the hydroxychloroquine dosage was reduced to 200 mg once daily after 1 year. The patient did not report any adverse sequelae to treatment.
Histopathologic findings of superficial and deep perivascular and periadnexal lymphocytic infiltrates and interstitial dermal deposition of mucin in LET have remained consistent in the literature. Direct immunofluorescence has not revealed any complement or immunoglobulin deposition on the basement membrane.3,4 The epidermal characteristics are not as uniform, with the majority of cases in one review showing no epidermal changes and a minority showing minimal epidermal changes (eg, epidermal atrophy, hyperkeratosis, parakeratosis, acanthosis, spongiosis).5 When working up patients for LET, blood work usually is unremarkable, as LET rarely is associated with antinuclear antibodies or anti-Ro, anti-La, and anti-DNA antibodies.3,4 Lupus erythematosus tumidus generally is an independent process, but it has been reported to coexist with discoid lupus erythematosus and systemic lupus erythematosus in rare cases.6
The lesions of LET have been consistently described in the literature as photosensitive, erythematous, non-scarring, annular plaques and papules commonly occurring on the head/neck and other sun-exposed areas that do not cause hypopigmentation.3 Treatment of LET consists of systemic treatment with antimalarial drugs, sunscreens, and topical steroids for flares.
Lupus erythematosus tumidus is rare in children, with few case reports noted in the literature. Sonntag et al2 documented the disease in 3 children ranging from 3 to 8 years of age. Furthermore, Ruiz and Sanchez7 reported a case of LET in a 16-year-old adolescent girl. Our case is unique in that the lesions only occurred on the hands, whereas most case reports document distribution of the lesions on the head, neck, face, arms, back, and chest. Our patient’s age and the location of the lesions make it a unique clinical presentation of LET.
Reports in the literature show evidence of heterogeneity in the presentation, classification, and some of the histopathologic features of LET; however, there are minimal data on childhood LET. Further research and investigations are needed to more precisely define this condition.
Acknowledgment
The authors acknowledge Richard Schwartz, MD (Akron, Ohio), for reading the biopsy reports and assisting with photomicrographs.
- Hoffmann E. Demonstrationen: lupus erythematosus tumidus. Derm Zeitschr. 1909;16:159-160.
- Sonntag M, Lehmann P, Megahed M, et al. Lupus erythematosus tumidus in childhood. Dermatology. 2003;207:188-192.
- Schmitt V, Meuth AM, Amler S, et al. Lupus erythematosus tumidus is a separate subtype of cutaneous lupus erythematosus. Br J Dermatol. 2010;162:64-73.
- Vieira V, Del Pozo J, Yebra-Pimentel MT, et al. Lupus erythematosus tumidus: a series of 26 cases. Int J Dermatol. 2006;45:512-517.
- Kuhn A, Richter-Hintz D, Oslislo C, et al. Lupus erythematosus tumidus—a neglected subset of cutaneous lupus erythematosus: report of 40 cases. Arch Dermatol. 2000;136:1033-1041.
- Chen X, Wang S, Li L. A case report of lupus erythematosus tumidus converted from discoid lupus erythematosus. Medicine (Baltimore). 2018;97:e0375.
- Ruiz H, Sanchez J. Tumid lupus erythematosus. Am J Dermatopathol. 1999;21:356-360.
- Hoffmann E. Demonstrationen: lupus erythematosus tumidus. Derm Zeitschr. 1909;16:159-160.
- Sonntag M, Lehmann P, Megahed M, et al. Lupus erythematosus tumidus in childhood. Dermatology. 2003;207:188-192.
- Schmitt V, Meuth AM, Amler S, et al. Lupus erythematosus tumidus is a separate subtype of cutaneous lupus erythematosus. Br J Dermatol. 2010;162:64-73.
- Vieira V, Del Pozo J, Yebra-Pimentel MT, et al. Lupus erythematosus tumidus: a series of 26 cases. Int J Dermatol. 2006;45:512-517.
- Kuhn A, Richter-Hintz D, Oslislo C, et al. Lupus erythematosus tumidus—a neglected subset of cutaneous lupus erythematosus: report of 40 cases. Arch Dermatol. 2000;136:1033-1041.
- Chen X, Wang S, Li L. A case report of lupus erythematosus tumidus converted from discoid lupus erythematosus. Medicine (Baltimore). 2018;97:e0375.
- Ruiz H, Sanchez J. Tumid lupus erythematosus. Am J Dermatopathol. 1999;21:356-360.
Practice Points
- Lupus erythematosus tumidus rarely occurs in the pediatric population.
- Lupus erythematosus tumidus is a unique subset of lupus associated with lack of interface change on histology and large amounts of mucin.
- Lesions typically present on the face and trunk but can very rarely present on the extremities and hands.
Would a universal basic income help our patients?
If you take a nighttime stroll through the downtown district in any major U.S. city – and certainly in my hometown of Baltimore – you’ll find people sleeping on the streets. Advocates talk about “the homeless mentally ill,” and it’s estimated that a quarter of homeless persons suffer from psychiatric conditions. As psychiatrists, it’s good that we care about the homeless mentally ill. As human beings, shouldn’t we also care about the addicted and indigent homeless? In a country of wealth, it continues to be a disgrace that we have people who live in tent encampments, or who literally sleep on the ground in public places with all their belongings gathered around them.
With 25 contenders for the Democratic presidential nomination, Andrew Yang has caught my attention with his platform for a universal basic income (UBI), or “freedom dividend,” for all adults. Mr. Yang’s premise is a simple one: He’d like to give every person over the age of 18 a $1,000-a-month government-supplied income, funded by a new Value Added Tax (VAT), with no stipulations. I find the concept intriguing. It is the one suggestion that might make a drastic dent in extreme poverty in our country. In theory, every adult would have enough money to afford a place to sleep.
Paul Nestadt, MD, a psychiatrist at Johns Hopkins in Baltimore, agrees with the concept of a universal basic income. “The UBI seems like a humane and egalitarian way to eliminate starvation; it provides a ‘floor’ for poverty. It is a reasonable bare minimum, especially for our patients with serious mental illness who struggle to navigate the bureaucratic steeplechase required to have their basic needs met.” Dr. Nestadt believes that some of the other presidential candidates are supporting policies that would also accomplish this goal.
So who is Andrew Yang? With no political experience, he bills himself as a technology entrepreneur who started a national education company, then went on to found Venture for America, an organization designed to create jobs. During the first round of Democratic debates, few questions were directed to him, and he was the lone gentleman on the stage without a tie. In addition to the UBI, he supports Medicare-for-all and “human-centered capitalism.” His slogan is “Humanity First,” and his website includes policy statements on a vast number of topics: everything from robocalls to wildfires to free marital counseling for all. His supporters call themselves the Yang Gang, and as of this writing, he polls at number 8 – with just 2% of the projected vote – among the 25 Democratic candidates.
While I find the prospect of a universal basic income appealing from the perspective of making a dent in extreme poverty, this is not the demographic Mr. Yang is targeting. His platform is based on the prediction that automation will continue to eliminate jobs at a rate that will devastate our people and our economy. In his book, The War on Normal People (Hachette Book Group, 2018), Yang writes about the UBI: “It’s simple, it’s fair, it’s equitable, it’s easy to understand, it benefits at least 80 percent of the population, and it will be necessary to maintain the fabric of society during the automation wave.” Mr. Yang contends that at least one-third of Americans are at risk of losing their jobs to automation.
It’s difficult to imagine that it would not be helpful to everyone’s mental health – not just those individuals with psychiatric disorders – to be freed from the worry of earning enough money to survive. While our welfare, disability, and Medicaid systems provide a safety net to many Americans, they certainly don’t cover everyone, and they engender a sense of unfairness and anger. Our current system allows that some people work hard and struggle to meet their basic needs and pay medical bills while others – usually the disabled or the poor – receive government benefits and Medicaid and/or Medicare.
In the “Making Sense” podcast hosted by Sam Harris, “A conversation with Andrew Yang,” Mr. Yang made the point that a UBI is not a new concept – economists and politicians, including Richard Nixon, have supported the idea since the 1960s. A bill proposing a basic income passed in the House of Representatives in 1971 but did not pass in the Senate. In the podcast, Mr. Yang addresses the question of whether people are responsible for their own success, and whether, as Mr. Harris puts it, “it’s just simply wrong to hand out money to people.”
“It’s not as if the truck drivers are about to get dumber and lazier overnight,” Mr. Yang responded. “It’s just that their trucks are going to start driving themselves ... it has nothing to do with their character and work ethic.” He goes on to discuss how workers who lose jobs often leave the workforce and many go on disability.
“Right now, the country’s locked in a struggle between functioning and dysfunction, between reason and unreason, and scarcity and abundance, and scarcity is winning and that’s what we have to reverse through universal basic income; it’s our best way forward.”
If we are optimistic that our government could afford to provide everyone with both a UBI and a universal health plan, such as Medicare-for-all, I still wonder if there might be a societal downside. For self-motivated individuals, a sustenance allowance is unlikely to weaken a drive to achieve. But might there be people who decide they can live on this income, who choose instead to pursue leisure activities rather than pursue education and vocation? Mr. Harris asked Mr. Yang if we would be “subsidizing all the people in their mothers’ basement playing video games.”
Mr. Yang responded, “If you’re getting a thousand dollars a month, then you’re much more likely to get out of your parents’ basement and visit friends and find things to do that are somewhat more social and external-facing. A lot of the reason a lot of these men are retreating is because there’s no real economic security or path forward for them, and they feel much better served by going online and hanging out with their friends and making measurable progress in their gaming environment.”
I like to think that an automatic income would not crush a society’s motivation and productivity, and that money provided to people would fuel education, our economy, an ability to save, and entrepreneurial endeavors, but the truth is that we just don’t know. I would love to see such an experiment done as a large-scale pilot, with the ability to undo the experiment if it fails.
Andrew Yang remains a long shot as the Democratic presidential nominee. His platform, however, is enticing, and he takes on the imminent automation crisis in a way that no one else is actively addressing. His concepts include a degree of humanity that feels welcome when our current president is tweeting that those who are unhappy here should leave. While Mr. Yang is a bit lost in the fray, I do hope his innovative spirit gains some traction.
Dr. Miller is the coauthor with Annette Hanson, MD, of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016) and has a private practice in Baltimore.
If you take a nighttime stroll through the downtown district in any major U.S. city – and certainly in my hometown of Baltimore – you’ll find people sleeping on the streets. Advocates talk about “the homeless mentally ill,” and it’s estimated that a quarter of homeless persons suffer from psychiatric conditions. As psychiatrists, it’s good that we care about the homeless mentally ill. As human beings, shouldn’t we also care about the addicted and indigent homeless? In a country of wealth, it continues to be a disgrace that we have people who live in tent encampments, or who literally sleep on the ground in public places with all their belongings gathered around them.
With 25 contenders for the Democratic presidential nomination, Andrew Yang has caught my attention with his platform for a universal basic income (UBI), or “freedom dividend,” for all adults. Mr. Yang’s premise is a simple one: He’d like to give every person over the age of 18 a $1,000-a-month government-supplied income, funded by a new Value Added Tax (VAT), with no stipulations. I find the concept intriguing. It is the one suggestion that might make a drastic dent in extreme poverty in our country. In theory, every adult would have enough money to afford a place to sleep.
Paul Nestadt, MD, a psychiatrist at Johns Hopkins in Baltimore, agrees with the concept of a universal basic income. “The UBI seems like a humane and egalitarian way to eliminate starvation; it provides a ‘floor’ for poverty. It is a reasonable bare minimum, especially for our patients with serious mental illness who struggle to navigate the bureaucratic steeplechase required to have their basic needs met.” Dr. Nestadt believes that some of the other presidential candidates are supporting policies that would also accomplish this goal.
So who is Andrew Yang? With no political experience, he bills himself as a technology entrepreneur who started a national education company, then went on to found Venture for America, an organization designed to create jobs. During the first round of Democratic debates, few questions were directed to him, and he was the lone gentleman on the stage without a tie. In addition to the UBI, he supports Medicare-for-all and “human-centered capitalism.” His slogan is “Humanity First,” and his website includes policy statements on a vast number of topics: everything from robocalls to wildfires to free marital counseling for all. His supporters call themselves the Yang Gang, and as of this writing, he polls at number 8 – with just 2% of the projected vote – among the 25 Democratic candidates.
While I find the prospect of a universal basic income appealing from the perspective of making a dent in extreme poverty, this is not the demographic Mr. Yang is targeting. His platform is based on the prediction that automation will continue to eliminate jobs at a rate that will devastate our people and our economy. In his book, The War on Normal People (Hachette Book Group, 2018), Yang writes about the UBI: “It’s simple, it’s fair, it’s equitable, it’s easy to understand, it benefits at least 80 percent of the population, and it will be necessary to maintain the fabric of society during the automation wave.” Mr. Yang contends that at least one-third of Americans are at risk of losing their jobs to automation.
It’s difficult to imagine that it would not be helpful to everyone’s mental health – not just those individuals with psychiatric disorders – to be freed from the worry of earning enough money to survive. While our welfare, disability, and Medicaid systems provide a safety net to many Americans, they certainly don’t cover everyone, and they engender a sense of unfairness and anger. Our current system allows that some people work hard and struggle to meet their basic needs and pay medical bills while others – usually the disabled or the poor – receive government benefits and Medicaid and/or Medicare.
In the “Making Sense” podcast hosted by Sam Harris, “A conversation with Andrew Yang,” Mr. Yang made the point that a UBI is not a new concept – economists and politicians, including Richard Nixon, have supported the idea since the 1960s. A bill proposing a basic income passed in the House of Representatives in 1971 but did not pass in the Senate. In the podcast, Mr. Yang addresses the question of whether people are responsible for their own success, and whether, as Mr. Harris puts it, “it’s just simply wrong to hand out money to people.”
“It’s not as if the truck drivers are about to get dumber and lazier overnight,” Mr. Yang responded. “It’s just that their trucks are going to start driving themselves ... it has nothing to do with their character and work ethic.” He goes on to discuss how workers who lose jobs often leave the workforce and many go on disability.
“Right now, the country’s locked in a struggle between functioning and dysfunction, between reason and unreason, and scarcity and abundance, and scarcity is winning and that’s what we have to reverse through universal basic income; it’s our best way forward.”
If we are optimistic that our government could afford to provide everyone with both a UBI and a universal health plan, such as Medicare-for-all, I still wonder if there might be a societal downside. For self-motivated individuals, a sustenance allowance is unlikely to weaken a drive to achieve. But might there be people who decide they can live on this income, who choose instead to pursue leisure activities rather than pursue education and vocation? Mr. Harris asked Mr. Yang if we would be “subsidizing all the people in their mothers’ basement playing video games.”
Mr. Yang responded, “If you’re getting a thousand dollars a month, then you’re much more likely to get out of your parents’ basement and visit friends and find things to do that are somewhat more social and external-facing. A lot of the reason a lot of these men are retreating is because there’s no real economic security or path forward for them, and they feel much better served by going online and hanging out with their friends and making measurable progress in their gaming environment.”
I like to think that an automatic income would not crush a society’s motivation and productivity, and that money provided to people would fuel education, our economy, an ability to save, and entrepreneurial endeavors, but the truth is that we just don’t know. I would love to see such an experiment done as a large-scale pilot, with the ability to undo the experiment if it fails.
Andrew Yang remains a long shot as the Democratic presidential nominee. His platform, however, is enticing, and he takes on the imminent automation crisis in a way that no one else is actively addressing. His concepts include a degree of humanity that feels welcome when our current president is tweeting that those who are unhappy here should leave. While Mr. Yang is a bit lost in the fray, I do hope his innovative spirit gains some traction.
Dr. Miller is the coauthor with Annette Hanson, MD, of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016) and has a private practice in Baltimore.
If you take a nighttime stroll through the downtown district in any major U.S. city – and certainly in my hometown of Baltimore – you’ll find people sleeping on the streets. Advocates talk about “the homeless mentally ill,” and it’s estimated that a quarter of homeless persons suffer from psychiatric conditions. As psychiatrists, it’s good that we care about the homeless mentally ill. As human beings, shouldn’t we also care about the addicted and indigent homeless? In a country of wealth, it continues to be a disgrace that we have people who live in tent encampments, or who literally sleep on the ground in public places with all their belongings gathered around them.
With 25 contenders for the Democratic presidential nomination, Andrew Yang has caught my attention with his platform for a universal basic income (UBI), or “freedom dividend,” for all adults. Mr. Yang’s premise is a simple one: He’d like to give every person over the age of 18 a $1,000-a-month government-supplied income, funded by a new Value Added Tax (VAT), with no stipulations. I find the concept intriguing. It is the one suggestion that might make a drastic dent in extreme poverty in our country. In theory, every adult would have enough money to afford a place to sleep.
Paul Nestadt, MD, a psychiatrist at Johns Hopkins in Baltimore, agrees with the concept of a universal basic income. “The UBI seems like a humane and egalitarian way to eliminate starvation; it provides a ‘floor’ for poverty. It is a reasonable bare minimum, especially for our patients with serious mental illness who struggle to navigate the bureaucratic steeplechase required to have their basic needs met.” Dr. Nestadt believes that some of the other presidential candidates are supporting policies that would also accomplish this goal.
So who is Andrew Yang? With no political experience, he bills himself as a technology entrepreneur who started a national education company, then went on to found Venture for America, an organization designed to create jobs. During the first round of Democratic debates, few questions were directed to him, and he was the lone gentleman on the stage without a tie. In addition to the UBI, he supports Medicare-for-all and “human-centered capitalism.” His slogan is “Humanity First,” and his website includes policy statements on a vast number of topics: everything from robocalls to wildfires to free marital counseling for all. His supporters call themselves the Yang Gang, and as of this writing, he polls at number 8 – with just 2% of the projected vote – among the 25 Democratic candidates.
While I find the prospect of a universal basic income appealing from the perspective of making a dent in extreme poverty, this is not the demographic Mr. Yang is targeting. His platform is based on the prediction that automation will continue to eliminate jobs at a rate that will devastate our people and our economy. In his book, The War on Normal People (Hachette Book Group, 2018), Yang writes about the UBI: “It’s simple, it’s fair, it’s equitable, it’s easy to understand, it benefits at least 80 percent of the population, and it will be necessary to maintain the fabric of society during the automation wave.” Mr. Yang contends that at least one-third of Americans are at risk of losing their jobs to automation.
It’s difficult to imagine that it would not be helpful to everyone’s mental health – not just those individuals with psychiatric disorders – to be freed from the worry of earning enough money to survive. While our welfare, disability, and Medicaid systems provide a safety net to many Americans, they certainly don’t cover everyone, and they engender a sense of unfairness and anger. Our current system allows that some people work hard and struggle to meet their basic needs and pay medical bills while others – usually the disabled or the poor – receive government benefits and Medicaid and/or Medicare.
In the “Making Sense” podcast hosted by Sam Harris, “A conversation with Andrew Yang,” Mr. Yang made the point that a UBI is not a new concept – economists and politicians, including Richard Nixon, have supported the idea since the 1960s. A bill proposing a basic income passed in the House of Representatives in 1971 but did not pass in the Senate. In the podcast, Mr. Yang addresses the question of whether people are responsible for their own success, and whether, as Mr. Harris puts it, “it’s just simply wrong to hand out money to people.”
“It’s not as if the truck drivers are about to get dumber and lazier overnight,” Mr. Yang responded. “It’s just that their trucks are going to start driving themselves ... it has nothing to do with their character and work ethic.” He goes on to discuss how workers who lose jobs often leave the workforce and many go on disability.
“Right now, the country’s locked in a struggle between functioning and dysfunction, between reason and unreason, and scarcity and abundance, and scarcity is winning and that’s what we have to reverse through universal basic income; it’s our best way forward.”
If we are optimistic that our government could afford to provide everyone with both a UBI and a universal health plan, such as Medicare-for-all, I still wonder if there might be a societal downside. For self-motivated individuals, a sustenance allowance is unlikely to weaken a drive to achieve. But might there be people who decide they can live on this income, who choose instead to pursue leisure activities rather than pursue education and vocation? Mr. Harris asked Mr. Yang if we would be “subsidizing all the people in their mothers’ basement playing video games.”
Mr. Yang responded, “If you’re getting a thousand dollars a month, then you’re much more likely to get out of your parents’ basement and visit friends and find things to do that are somewhat more social and external-facing. A lot of the reason a lot of these men are retreating is because there’s no real economic security or path forward for them, and they feel much better served by going online and hanging out with their friends and making measurable progress in their gaming environment.”
I like to think that an automatic income would not crush a society’s motivation and productivity, and that money provided to people would fuel education, our economy, an ability to save, and entrepreneurial endeavors, but the truth is that we just don’t know. I would love to see such an experiment done as a large-scale pilot, with the ability to undo the experiment if it fails.
Andrew Yang remains a long shot as the Democratic presidential nominee. His platform, however, is enticing, and he takes on the imminent automation crisis in a way that no one else is actively addressing. His concepts include a degree of humanity that feels welcome when our current president is tweeting that those who are unhappy here should leave. While Mr. Yang is a bit lost in the fray, I do hope his innovative spirit gains some traction.
Dr. Miller is the coauthor with Annette Hanson, MD, of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016) and has a private practice in Baltimore.
Hurricanes delay RT, worsening survival of NSCLC
suggests a retrospective cohort study.
“Radiotherapy is particularly vulnerable because it requires dependable electrical power and daily treatment,” lead investigator Leticia M. Nogueira, PhD, Surveillance and Health Services Research Program, American Cancer Society, and colleagues noted. “Disruptions are especially concerning for patients undergoing treatment for locally advanced ... NSCLC because treatment delays as little as 2 days negatively affect survival.”
The investigators used the National Cancer Database to identify patients receiving definitive radiotherapy for nonoperative locally advanced NSCLC between 2004 and 2014 who had at least 1 year of follow-up for vital status.
Each patient undergoing radiotherapy when a hurricane disaster was declared for their facility’s area was matched through propensity scoring with a patient treated during a declaration-free period having similar start month, sex, age, stage, nodal status, and income. Analyses compared 1,734 exposed patients with 1,734 unexposed patients.
Study results reported in JAMA showed that 101 hurricane disaster declarations were made during the study period, and they lasted from 1 day to 69 days. The radiation treatment duration was about 21 days (45%) longer for patients exposed to these declarations than for unexposed counterparts (66.9 vs. 46.2 days; P less than .001).
Over a median follow-up of 15 months, exposed patients were more likely to die (adjusted hazard ratio, 1.19; P = .001). Moreover, risk generally rose with the duration of the declaration, peaking for patients exposed to those lasting 27 days (adjusted relative risk, 1.27).
“Because data on other potentially explanatory factors are lacking, the relative contribution of treatment delay to the observed association cannot be quantified. However, treatment delay is one of the few hurricane-related disruptions that can be prevented,” Dr. Nogueira and colleagues maintain.
“Because no recommended correction for radiotherapy delays exists ... strategies for identifying patients, arranging for transferring treatment, and eliminating patient out-of-network insurance charges should be considered in disaster mitigation planning,” they recommend.
Dr. Nogueira disclosed no relevant conflicts of interest. The investigators conducted the study as part of the in-tramural research program at the American Cancer Society or contributed their time.
SOURCE: Nogueira LM et al. JAMA. 2019 Jul 16;322(3):269-71.
suggests a retrospective cohort study.
“Radiotherapy is particularly vulnerable because it requires dependable electrical power and daily treatment,” lead investigator Leticia M. Nogueira, PhD, Surveillance and Health Services Research Program, American Cancer Society, and colleagues noted. “Disruptions are especially concerning for patients undergoing treatment for locally advanced ... NSCLC because treatment delays as little as 2 days negatively affect survival.”
The investigators used the National Cancer Database to identify patients receiving definitive radiotherapy for nonoperative locally advanced NSCLC between 2004 and 2014 who had at least 1 year of follow-up for vital status.
Each patient undergoing radiotherapy when a hurricane disaster was declared for their facility’s area was matched through propensity scoring with a patient treated during a declaration-free period having similar start month, sex, age, stage, nodal status, and income. Analyses compared 1,734 exposed patients with 1,734 unexposed patients.
Study results reported in JAMA showed that 101 hurricane disaster declarations were made during the study period, and they lasted from 1 day to 69 days. The radiation treatment duration was about 21 days (45%) longer for patients exposed to these declarations than for unexposed counterparts (66.9 vs. 46.2 days; P less than .001).
Over a median follow-up of 15 months, exposed patients were more likely to die (adjusted hazard ratio, 1.19; P = .001). Moreover, risk generally rose with the duration of the declaration, peaking for patients exposed to those lasting 27 days (adjusted relative risk, 1.27).
“Because data on other potentially explanatory factors are lacking, the relative contribution of treatment delay to the observed association cannot be quantified. However, treatment delay is one of the few hurricane-related disruptions that can be prevented,” Dr. Nogueira and colleagues maintain.
“Because no recommended correction for radiotherapy delays exists ... strategies for identifying patients, arranging for transferring treatment, and eliminating patient out-of-network insurance charges should be considered in disaster mitigation planning,” they recommend.
Dr. Nogueira disclosed no relevant conflicts of interest. The investigators conducted the study as part of the in-tramural research program at the American Cancer Society or contributed their time.
SOURCE: Nogueira LM et al. JAMA. 2019 Jul 16;322(3):269-71.
suggests a retrospective cohort study.
“Radiotherapy is particularly vulnerable because it requires dependable electrical power and daily treatment,” lead investigator Leticia M. Nogueira, PhD, Surveillance and Health Services Research Program, American Cancer Society, and colleagues noted. “Disruptions are especially concerning for patients undergoing treatment for locally advanced ... NSCLC because treatment delays as little as 2 days negatively affect survival.”
The investigators used the National Cancer Database to identify patients receiving definitive radiotherapy for nonoperative locally advanced NSCLC between 2004 and 2014 who had at least 1 year of follow-up for vital status.
Each patient undergoing radiotherapy when a hurricane disaster was declared for their facility’s area was matched through propensity scoring with a patient treated during a declaration-free period having similar start month, sex, age, stage, nodal status, and income. Analyses compared 1,734 exposed patients with 1,734 unexposed patients.
Study results reported in JAMA showed that 101 hurricane disaster declarations were made during the study period, and they lasted from 1 day to 69 days. The radiation treatment duration was about 21 days (45%) longer for patients exposed to these declarations than for unexposed counterparts (66.9 vs. 46.2 days; P less than .001).
Over a median follow-up of 15 months, exposed patients were more likely to die (adjusted hazard ratio, 1.19; P = .001). Moreover, risk generally rose with the duration of the declaration, peaking for patients exposed to those lasting 27 days (adjusted relative risk, 1.27).
“Because data on other potentially explanatory factors are lacking, the relative contribution of treatment delay to the observed association cannot be quantified. However, treatment delay is one of the few hurricane-related disruptions that can be prevented,” Dr. Nogueira and colleagues maintain.
“Because no recommended correction for radiotherapy delays exists ... strategies for identifying patients, arranging for transferring treatment, and eliminating patient out-of-network insurance charges should be considered in disaster mitigation planning,” they recommend.
Dr. Nogueira disclosed no relevant conflicts of interest. The investigators conducted the study as part of the in-tramural research program at the American Cancer Society or contributed their time.
SOURCE: Nogueira LM et al. JAMA. 2019 Jul 16;322(3):269-71.
FROM JAMA
USPSTF updates, reaffirms recommendation for HBV screening in pregnant women
The according to task force member Douglas K. Owens, MD, of the Veterans Affairs Palo Alto (Calif.) Health Care System and other members of the task force.
The recommendation statement, published in JAMA, was based on an evidence report and systematic review also published in JAMA. In that review, two studies of fair quality were identified; one included 155,081 infants born to HBV-positive women identified for case management through the national Perinatal Hepatitis B Prevention Program from 1994 to 2008, and the other included 4,446 infants born in a large, regional health care organization in the United States between 1997 and 2010. In both, low rates of perinatal transmission were reported for those periods – between 0.5% and 1.9% – with the rate falling over time.
In the 2009 recommendation, the USPSTF found adequate evidence that serologic testing for hepatitis B surface antigen accurately identifies HBV infection, and that interventions were effective in preventing perinatal transmission. That recommendation has been reaffirmed in the current update, with HBV screening receiving a grade of A, which is the strongest the USPSTF offers.
In a related editorial, Neil S. Silverman, MD, of the Center for Fetal Medicine and Women’s Ultrasound in Los Angeles noted several improvements in maternal HBV therapy since the publication of the original 2009 recommendation, including maternal HBV-targeted antiviral therapy during pregnancy as an adjunct to neonatal immunoprophylaxis and the ability to refer women for chronic treatment of their HBV disease to prevent long-term infection complications. The task forces also noted that HBV screening of all pregnant women is mandated by law in 26 states.
One member of the task force reported receiving grants and/or personal fees from Healthwise, another member reported receiving personal fees from UpToDate; a third reported participating in the American Association for the Study of Liver Diseases’ Hepatitis B Guidance and Hepatitis B Systematic Review Group. The evidence and review study was funded by the Agency for Healthcare Research and Quality. Dr. Silverman reported no disclosures.
SOURCes: Owens DK et al. JAMA. 2019 Jul 23. doi: 10.1001/jama.2019.9365; Henderson JT et al. JAMA. 2019 Jul 23;32(4):360-2; Silverman NS. JAMA. 2019 Jul 23;32(4):312-14.
The according to task force member Douglas K. Owens, MD, of the Veterans Affairs Palo Alto (Calif.) Health Care System and other members of the task force.
The recommendation statement, published in JAMA, was based on an evidence report and systematic review also published in JAMA. In that review, two studies of fair quality were identified; one included 155,081 infants born to HBV-positive women identified for case management through the national Perinatal Hepatitis B Prevention Program from 1994 to 2008, and the other included 4,446 infants born in a large, regional health care organization in the United States between 1997 and 2010. In both, low rates of perinatal transmission were reported for those periods – between 0.5% and 1.9% – with the rate falling over time.
In the 2009 recommendation, the USPSTF found adequate evidence that serologic testing for hepatitis B surface antigen accurately identifies HBV infection, and that interventions were effective in preventing perinatal transmission. That recommendation has been reaffirmed in the current update, with HBV screening receiving a grade of A, which is the strongest the USPSTF offers.
In a related editorial, Neil S. Silverman, MD, of the Center for Fetal Medicine and Women’s Ultrasound in Los Angeles noted several improvements in maternal HBV therapy since the publication of the original 2009 recommendation, including maternal HBV-targeted antiviral therapy during pregnancy as an adjunct to neonatal immunoprophylaxis and the ability to refer women for chronic treatment of their HBV disease to prevent long-term infection complications. The task forces also noted that HBV screening of all pregnant women is mandated by law in 26 states.
One member of the task force reported receiving grants and/or personal fees from Healthwise, another member reported receiving personal fees from UpToDate; a third reported participating in the American Association for the Study of Liver Diseases’ Hepatitis B Guidance and Hepatitis B Systematic Review Group. The evidence and review study was funded by the Agency for Healthcare Research and Quality. Dr. Silverman reported no disclosures.
SOURCes: Owens DK et al. JAMA. 2019 Jul 23. doi: 10.1001/jama.2019.9365; Henderson JT et al. JAMA. 2019 Jul 23;32(4):360-2; Silverman NS. JAMA. 2019 Jul 23;32(4):312-14.
The according to task force member Douglas K. Owens, MD, of the Veterans Affairs Palo Alto (Calif.) Health Care System and other members of the task force.
The recommendation statement, published in JAMA, was based on an evidence report and systematic review also published in JAMA. In that review, two studies of fair quality were identified; one included 155,081 infants born to HBV-positive women identified for case management through the national Perinatal Hepatitis B Prevention Program from 1994 to 2008, and the other included 4,446 infants born in a large, regional health care organization in the United States between 1997 and 2010. In both, low rates of perinatal transmission were reported for those periods – between 0.5% and 1.9% – with the rate falling over time.
In the 2009 recommendation, the USPSTF found adequate evidence that serologic testing for hepatitis B surface antigen accurately identifies HBV infection, and that interventions were effective in preventing perinatal transmission. That recommendation has been reaffirmed in the current update, with HBV screening receiving a grade of A, which is the strongest the USPSTF offers.
In a related editorial, Neil S. Silverman, MD, of the Center for Fetal Medicine and Women’s Ultrasound in Los Angeles noted several improvements in maternal HBV therapy since the publication of the original 2009 recommendation, including maternal HBV-targeted antiviral therapy during pregnancy as an adjunct to neonatal immunoprophylaxis and the ability to refer women for chronic treatment of their HBV disease to prevent long-term infection complications. The task forces also noted that HBV screening of all pregnant women is mandated by law in 26 states.
One member of the task force reported receiving grants and/or personal fees from Healthwise, another member reported receiving personal fees from UpToDate; a third reported participating in the American Association for the Study of Liver Diseases’ Hepatitis B Guidance and Hepatitis B Systematic Review Group. The evidence and review study was funded by the Agency for Healthcare Research and Quality. Dr. Silverman reported no disclosures.
SOURCes: Owens DK et al. JAMA. 2019 Jul 23. doi: 10.1001/jama.2019.9365; Henderson JT et al. JAMA. 2019 Jul 23;32(4):360-2; Silverman NS. JAMA. 2019 Jul 23;32(4):312-14.
FROM JAMA
Solar Urticaria Treated With Omalizumab
To the Editor:
First documented in 1904,1 solar urticaria is an IgE-induced condition that predominantly occurs in women aged 20 to 50 years. Worldwide prevalence and incidence information is lacking, but it is known to occur in up to 0.4% of urticaria cases.2 Solar urticaria is characterized by pruritus of the skin with erythematous wheals and flares in reaction to sunlight exposure, even despite partial protection by barriers such as glass or clothing.2,3 It can have an acute or chronic presentation caused by visible or UV light wavelengths. Solar urticaria can lead to debilitating symptoms and psychological stressors that can severely impact a patient’s well-being and also may be accompanied by conditions such as polymorphous light eruption, angioedema, or vasculitis.4 Standard treatments include first- and second-generation antihistamines, which are efficacious approximately 50% of the time, as well as phototherapy, which can be time consuming and a burden on patients who work or go to school full time.2 Other possible treatment modalities include plasmapheresis, intravenous immunoglobulins, steroids, cyclosporine, and anti-IgE recombinant monoclonal antibody injections.5,6 We present the case of a patient who was successfully treated with subcutaneous injections of omalizumab every 3 weeks to add to the growing number of case reports of treatment of solar urticaria.
A 30-year-old woman with Fitzpatrick skin type III and a 9-year history of solar urticaria was referred to the Department of Allergy and Immunology by her primary care physician. The patient reported that redness, swelling, and itching would occur on sun-exposed areas of the skin after approximately 10 minutes of exposure despite daily sunscreen application. She had been successfully treated with hydroxychloroquine 400 mg once daily after her first formal evaluation by dermatology 4 years prior to the current presentation. She subsequently self-discontinued treatment after 8 months of treatment due to resolution of symptoms. She noted the symptoms had returned upon relocating to Hawaii after living in the continental United States and Italy. Initially she was restarted on hydroxychloroquine 200 mg once daily and 4-times the recommended daily dose of second-generation antihistamines without relief. The hydroxychloroquine dosage subsequently was increased to 400 mg once daily, but her symptoms did not resolve.
On physical examination, sun-exposed areas of the skin showed marked macular erythema with discrete erythematous lines of demarcation observed between exposed and unexposed skin. The patient also reported concomitant pruritus, which antihistamines did not alleviate. A maximum 1-year course of cyclosporine 300 mg once daily initially was planned but was discontinued due to immediate onset of severe nausea and emesis after the first dose as well as continued outbreaks of urticaria for 1 month after incrementally increasing by 100 mg from a starting dose of 100 mg.
After discussion with the dermatology department, a trial of omalizumab was started because the daily impact of a UV light sensitization course was not feasible with her work schedule, and serum IgE blood level was 560.4 µg/L (reference range, 0–1500 µg/L). The patient was started on a regimen of omalizumab 300 mg (subcutaneous injections) every 2 weeks with noted improvement after the third dose, with no urticarial symptoms after sun exposure. After 2 months, the dosage interval was increased to every 4 weeks given her level of improvement, but her symptoms recurred. The treatment regimen was then changed to every 3 weeks. The patient was symptom free for a period of 10 months on this regimen, followed by only 1 outbreak of erythema and urticaria, which occurred 1 day prior to a scheduled omalizumab injection. Symptoms have otherwise been well controlled to date on omalizumab.
Solar urticaria is a poorly understood phenomenon that has no clear prognostic indicators; therefore, diagnosis often is made based on the patient’s history and physical examination. Further testing to confirm the diagnosis can be performed using specific wavelengths of UV light to determine which band of light affects patients most; however, the wavelength can change over time, leading to less clinical significance, and may decrease efficacy of phototherapy.2 Solar urticaria has no clear predisposing factors, and treatments to date have been moderately successful. Exposure to sunlight is thought to initiate an alteration in a skin or serum chromophore or photoallergen, which then causes subsequent cross-linking and IgE-dependent release of histamine as well as other mediators such as cytokines, eicosanoids, and proteases with mast cell degranulation.7
Omalizumab is a recombinant humanized monoclonal IgG1 antibody targeting the methylated IgE Cε3 domain that initially was marketed toward controlling IgE-mediated moderate to severe asthma recalcitrant to standard treatments. It has since received approval from the US Food and Drug Administration for treatment of chronic idiopathic urticaria after first being noticed to serendipitously treat a patient with cold urticaria and asthma in 2006.4,7,8 It was then first documented to successfully treat solar urticaria in 2008.6 The safety profile of omalizumab makes it a more favorable choice when compared to other immunomodulating treatments, with the most serious adverse reaction being anaphylaxis, occurring in 0.2% of patients in a postmarketing study.9 It functions through binding to free IgE at a region necessary for IgE to bind at low- and high-affinity receptors but not to immunoglobulins already bound to cells, thus theoretically preventing activation of mast cells or basophils.10 It also has been suggested that low steady-state values are needed to see continued benefit from the drug,10 which may have been seen in our patient after having an outbreak just prior to receiving an injection; however, prior reports have shown benefit unrelated to total IgE levels, with improvement after days to 4 months.4,10,11 One case report showed no response after 4 doses; it is unknown if this patient was tested for clinical improvement to omalizumab through further immunoglobulin analysis, but treatment response is important to consider when deciding on whether to use this drug in future patients.12 It is unknown why some patients will respond to omalizumab, others will partially respond, and others will not respond, which can be ascertained either through quality-of-life improvement or lack thereof.
In our experience, omalizumab is a viable option to consider in patients with solar urticaria that is recalcitrant to standard treatments and elevated IgE levels for whom other treatments are either too time consuming or have side-effect profiles that are not tolerable to the patient. If the patient has concomitant asthma, there may be additional therapeutic benefit. Further research is needed with regard to a cost-benefit analysis of omalizumab and whether using such a costly drug outweighs the cost associated with time and resources utilized with repeat clinic visits if other standard treatments are not effective.13
- Merkin P. Pratique Dermatologique. Paris, France: Masso; 1904.
- Beattie PE, Dawe RS, Ibbotson SH, et al. Characteristics and prognosis of idiopathic solar urticaria: a cohort of 87 cases. Arch Dermatol. 2003;139:1149-1154.
- Kaplan AP. Therapy of chronic urticaria: a simple, modern approach. Ann Allergy Asthma Immunol. 2014;112:419-425.
- Metz M, Maurer M. Omalizumab in chronic urticaria. Curr Opin Allergy Clin Immunol. 2012;12:406-410.
- Aubin F, Porcher R, Jeanmougin M, et al. Severe and refractory solar urticaria treated with intravenous immunoglobulins: a phase II multicenter study. J Am Acad Dermatol. 2014;71:948-953.e1.
- Güzelbey O, Ardelean E, Magerl M, et al. Successful treatment of solar urticaria with anti-immunoglobulin E therapy. Allergy. 2008;63:1563-1565.
- Wu K, Jabbar-Lopez Z. Omalizumab, an anti-IgE mAb, receives approval for the treatment of chronic idiopathic/spontaneous urticaria. J Invest Dermatol. 2015;135:13-15.
- Boyce JA. Successful treatment of cold-induced urticaria/anaphylaxis with anti-IgE. J Allergy Clin Immunol. 2006;117:1415-1418.
- Corren J, Casale TB, Lanier B, et al. Safety and tolerability of omalizumab. Clin Exp Allergy. 2009;39:788-797.
- Wu K, Long H. Omalizumab for chronic urticaria. N Engl J Med. 2013;368:2527-2528.
- Morgado-Carrasco D, Giacaman-Von der Weth M, Fusta-Novell X, et al. Clinical response and long-term follow-up of 20 patients with refractory solar urticarial under treatment with omalizumab [published online May 28, 2019]. J Am Acad Dermatol. doi:10.1016/j.jaad.2019.05.070.
- Duchini G, Bäumler W, Bircher AJ, et al. Failure of omalizumab (Xolair®) in the treatment of a case of solar urticaria caused by ultraviolet A and visible light. Photodermatol Photoimmunol Photomed. 2011;27:336-337.
- Bernstein JA, Lang DM, Khan DA, et al. The diagnosis and management of acute and chronic urticaria: 2014 update. J Allergy Clin Immunol. 2014;133:1270-1277.
To the Editor:
First documented in 1904,1 solar urticaria is an IgE-induced condition that predominantly occurs in women aged 20 to 50 years. Worldwide prevalence and incidence information is lacking, but it is known to occur in up to 0.4% of urticaria cases.2 Solar urticaria is characterized by pruritus of the skin with erythematous wheals and flares in reaction to sunlight exposure, even despite partial protection by barriers such as glass or clothing.2,3 It can have an acute or chronic presentation caused by visible or UV light wavelengths. Solar urticaria can lead to debilitating symptoms and psychological stressors that can severely impact a patient’s well-being and also may be accompanied by conditions such as polymorphous light eruption, angioedema, or vasculitis.4 Standard treatments include first- and second-generation antihistamines, which are efficacious approximately 50% of the time, as well as phototherapy, which can be time consuming and a burden on patients who work or go to school full time.2 Other possible treatment modalities include plasmapheresis, intravenous immunoglobulins, steroids, cyclosporine, and anti-IgE recombinant monoclonal antibody injections.5,6 We present the case of a patient who was successfully treated with subcutaneous injections of omalizumab every 3 weeks to add to the growing number of case reports of treatment of solar urticaria.
A 30-year-old woman with Fitzpatrick skin type III and a 9-year history of solar urticaria was referred to the Department of Allergy and Immunology by her primary care physician. The patient reported that redness, swelling, and itching would occur on sun-exposed areas of the skin after approximately 10 minutes of exposure despite daily sunscreen application. She had been successfully treated with hydroxychloroquine 400 mg once daily after her first formal evaluation by dermatology 4 years prior to the current presentation. She subsequently self-discontinued treatment after 8 months of treatment due to resolution of symptoms. She noted the symptoms had returned upon relocating to Hawaii after living in the continental United States and Italy. Initially she was restarted on hydroxychloroquine 200 mg once daily and 4-times the recommended daily dose of second-generation antihistamines without relief. The hydroxychloroquine dosage subsequently was increased to 400 mg once daily, but her symptoms did not resolve.
On physical examination, sun-exposed areas of the skin showed marked macular erythema with discrete erythematous lines of demarcation observed between exposed and unexposed skin. The patient also reported concomitant pruritus, which antihistamines did not alleviate. A maximum 1-year course of cyclosporine 300 mg once daily initially was planned but was discontinued due to immediate onset of severe nausea and emesis after the first dose as well as continued outbreaks of urticaria for 1 month after incrementally increasing by 100 mg from a starting dose of 100 mg.
After discussion with the dermatology department, a trial of omalizumab was started because the daily impact of a UV light sensitization course was not feasible with her work schedule, and serum IgE blood level was 560.4 µg/L (reference range, 0–1500 µg/L). The patient was started on a regimen of omalizumab 300 mg (subcutaneous injections) every 2 weeks with noted improvement after the third dose, with no urticarial symptoms after sun exposure. After 2 months, the dosage interval was increased to every 4 weeks given her level of improvement, but her symptoms recurred. The treatment regimen was then changed to every 3 weeks. The patient was symptom free for a period of 10 months on this regimen, followed by only 1 outbreak of erythema and urticaria, which occurred 1 day prior to a scheduled omalizumab injection. Symptoms have otherwise been well controlled to date on omalizumab.
Solar urticaria is a poorly understood phenomenon that has no clear prognostic indicators; therefore, diagnosis often is made based on the patient’s history and physical examination. Further testing to confirm the diagnosis can be performed using specific wavelengths of UV light to determine which band of light affects patients most; however, the wavelength can change over time, leading to less clinical significance, and may decrease efficacy of phototherapy.2 Solar urticaria has no clear predisposing factors, and treatments to date have been moderately successful. Exposure to sunlight is thought to initiate an alteration in a skin or serum chromophore or photoallergen, which then causes subsequent cross-linking and IgE-dependent release of histamine as well as other mediators such as cytokines, eicosanoids, and proteases with mast cell degranulation.7
Omalizumab is a recombinant humanized monoclonal IgG1 antibody targeting the methylated IgE Cε3 domain that initially was marketed toward controlling IgE-mediated moderate to severe asthma recalcitrant to standard treatments. It has since received approval from the US Food and Drug Administration for treatment of chronic idiopathic urticaria after first being noticed to serendipitously treat a patient with cold urticaria and asthma in 2006.4,7,8 It was then first documented to successfully treat solar urticaria in 2008.6 The safety profile of omalizumab makes it a more favorable choice when compared to other immunomodulating treatments, with the most serious adverse reaction being anaphylaxis, occurring in 0.2% of patients in a postmarketing study.9 It functions through binding to free IgE at a region necessary for IgE to bind at low- and high-affinity receptors but not to immunoglobulins already bound to cells, thus theoretically preventing activation of mast cells or basophils.10 It also has been suggested that low steady-state values are needed to see continued benefit from the drug,10 which may have been seen in our patient after having an outbreak just prior to receiving an injection; however, prior reports have shown benefit unrelated to total IgE levels, with improvement after days to 4 months.4,10,11 One case report showed no response after 4 doses; it is unknown if this patient was tested for clinical improvement to omalizumab through further immunoglobulin analysis, but treatment response is important to consider when deciding on whether to use this drug in future patients.12 It is unknown why some patients will respond to omalizumab, others will partially respond, and others will not respond, which can be ascertained either through quality-of-life improvement or lack thereof.
In our experience, omalizumab is a viable option to consider in patients with solar urticaria that is recalcitrant to standard treatments and elevated IgE levels for whom other treatments are either too time consuming or have side-effect profiles that are not tolerable to the patient. If the patient has concomitant asthma, there may be additional therapeutic benefit. Further research is needed with regard to a cost-benefit analysis of omalizumab and whether using such a costly drug outweighs the cost associated with time and resources utilized with repeat clinic visits if other standard treatments are not effective.13
To the Editor:
First documented in 1904,1 solar urticaria is an IgE-induced condition that predominantly occurs in women aged 20 to 50 years. Worldwide prevalence and incidence information is lacking, but it is known to occur in up to 0.4% of urticaria cases.2 Solar urticaria is characterized by pruritus of the skin with erythematous wheals and flares in reaction to sunlight exposure, even despite partial protection by barriers such as glass or clothing.2,3 It can have an acute or chronic presentation caused by visible or UV light wavelengths. Solar urticaria can lead to debilitating symptoms and psychological stressors that can severely impact a patient’s well-being and also may be accompanied by conditions such as polymorphous light eruption, angioedema, or vasculitis.4 Standard treatments include first- and second-generation antihistamines, which are efficacious approximately 50% of the time, as well as phototherapy, which can be time consuming and a burden on patients who work or go to school full time.2 Other possible treatment modalities include plasmapheresis, intravenous immunoglobulins, steroids, cyclosporine, and anti-IgE recombinant monoclonal antibody injections.5,6 We present the case of a patient who was successfully treated with subcutaneous injections of omalizumab every 3 weeks to add to the growing number of case reports of treatment of solar urticaria.
A 30-year-old woman with Fitzpatrick skin type III and a 9-year history of solar urticaria was referred to the Department of Allergy and Immunology by her primary care physician. The patient reported that redness, swelling, and itching would occur on sun-exposed areas of the skin after approximately 10 minutes of exposure despite daily sunscreen application. She had been successfully treated with hydroxychloroquine 400 mg once daily after her first formal evaluation by dermatology 4 years prior to the current presentation. She subsequently self-discontinued treatment after 8 months of treatment due to resolution of symptoms. She noted the symptoms had returned upon relocating to Hawaii after living in the continental United States and Italy. Initially she was restarted on hydroxychloroquine 200 mg once daily and 4-times the recommended daily dose of second-generation antihistamines without relief. The hydroxychloroquine dosage subsequently was increased to 400 mg once daily, but her symptoms did not resolve.
On physical examination, sun-exposed areas of the skin showed marked macular erythema with discrete erythematous lines of demarcation observed between exposed and unexposed skin. The patient also reported concomitant pruritus, which antihistamines did not alleviate. A maximum 1-year course of cyclosporine 300 mg once daily initially was planned but was discontinued due to immediate onset of severe nausea and emesis after the first dose as well as continued outbreaks of urticaria for 1 month after incrementally increasing by 100 mg from a starting dose of 100 mg.
After discussion with the dermatology department, a trial of omalizumab was started because the daily impact of a UV light sensitization course was not feasible with her work schedule, and serum IgE blood level was 560.4 µg/L (reference range, 0–1500 µg/L). The patient was started on a regimen of omalizumab 300 mg (subcutaneous injections) every 2 weeks with noted improvement after the third dose, with no urticarial symptoms after sun exposure. After 2 months, the dosage interval was increased to every 4 weeks given her level of improvement, but her symptoms recurred. The treatment regimen was then changed to every 3 weeks. The patient was symptom free for a period of 10 months on this regimen, followed by only 1 outbreak of erythema and urticaria, which occurred 1 day prior to a scheduled omalizumab injection. Symptoms have otherwise been well controlled to date on omalizumab.
Solar urticaria is a poorly understood phenomenon that has no clear prognostic indicators; therefore, diagnosis often is made based on the patient’s history and physical examination. Further testing to confirm the diagnosis can be performed using specific wavelengths of UV light to determine which band of light affects patients most; however, the wavelength can change over time, leading to less clinical significance, and may decrease efficacy of phototherapy.2 Solar urticaria has no clear predisposing factors, and treatments to date have been moderately successful. Exposure to sunlight is thought to initiate an alteration in a skin or serum chromophore or photoallergen, which then causes subsequent cross-linking and IgE-dependent release of histamine as well as other mediators such as cytokines, eicosanoids, and proteases with mast cell degranulation.7
Omalizumab is a recombinant humanized monoclonal IgG1 antibody targeting the methylated IgE Cε3 domain that initially was marketed toward controlling IgE-mediated moderate to severe asthma recalcitrant to standard treatments. It has since received approval from the US Food and Drug Administration for treatment of chronic idiopathic urticaria after first being noticed to serendipitously treat a patient with cold urticaria and asthma in 2006.4,7,8 It was then first documented to successfully treat solar urticaria in 2008.6 The safety profile of omalizumab makes it a more favorable choice when compared to other immunomodulating treatments, with the most serious adverse reaction being anaphylaxis, occurring in 0.2% of patients in a postmarketing study.9 It functions through binding to free IgE at a region necessary for IgE to bind at low- and high-affinity receptors but not to immunoglobulins already bound to cells, thus theoretically preventing activation of mast cells or basophils.10 It also has been suggested that low steady-state values are needed to see continued benefit from the drug,10 which may have been seen in our patient after having an outbreak just prior to receiving an injection; however, prior reports have shown benefit unrelated to total IgE levels, with improvement after days to 4 months.4,10,11 One case report showed no response after 4 doses; it is unknown if this patient was tested for clinical improvement to omalizumab through further immunoglobulin analysis, but treatment response is important to consider when deciding on whether to use this drug in future patients.12 It is unknown why some patients will respond to omalizumab, others will partially respond, and others will not respond, which can be ascertained either through quality-of-life improvement or lack thereof.
In our experience, omalizumab is a viable option to consider in patients with solar urticaria that is recalcitrant to standard treatments and elevated IgE levels for whom other treatments are either too time consuming or have side-effect profiles that are not tolerable to the patient. If the patient has concomitant asthma, there may be additional therapeutic benefit. Further research is needed with regard to a cost-benefit analysis of omalizumab and whether using such a costly drug outweighs the cost associated with time and resources utilized with repeat clinic visits if other standard treatments are not effective.13
- Merkin P. Pratique Dermatologique. Paris, France: Masso; 1904.
- Beattie PE, Dawe RS, Ibbotson SH, et al. Characteristics and prognosis of idiopathic solar urticaria: a cohort of 87 cases. Arch Dermatol. 2003;139:1149-1154.
- Kaplan AP. Therapy of chronic urticaria: a simple, modern approach. Ann Allergy Asthma Immunol. 2014;112:419-425.
- Metz M, Maurer M. Omalizumab in chronic urticaria. Curr Opin Allergy Clin Immunol. 2012;12:406-410.
- Aubin F, Porcher R, Jeanmougin M, et al. Severe and refractory solar urticaria treated with intravenous immunoglobulins: a phase II multicenter study. J Am Acad Dermatol. 2014;71:948-953.e1.
- Güzelbey O, Ardelean E, Magerl M, et al. Successful treatment of solar urticaria with anti-immunoglobulin E therapy. Allergy. 2008;63:1563-1565.
- Wu K, Jabbar-Lopez Z. Omalizumab, an anti-IgE mAb, receives approval for the treatment of chronic idiopathic/spontaneous urticaria. J Invest Dermatol. 2015;135:13-15.
- Boyce JA. Successful treatment of cold-induced urticaria/anaphylaxis with anti-IgE. J Allergy Clin Immunol. 2006;117:1415-1418.
- Corren J, Casale TB, Lanier B, et al. Safety and tolerability of omalizumab. Clin Exp Allergy. 2009;39:788-797.
- Wu K, Long H. Omalizumab for chronic urticaria. N Engl J Med. 2013;368:2527-2528.
- Morgado-Carrasco D, Giacaman-Von der Weth M, Fusta-Novell X, et al. Clinical response and long-term follow-up of 20 patients with refractory solar urticarial under treatment with omalizumab [published online May 28, 2019]. J Am Acad Dermatol. doi:10.1016/j.jaad.2019.05.070.
- Duchini G, Bäumler W, Bircher AJ, et al. Failure of omalizumab (Xolair®) in the treatment of a case of solar urticaria caused by ultraviolet A and visible light. Photodermatol Photoimmunol Photomed. 2011;27:336-337.
- Bernstein JA, Lang DM, Khan DA, et al. The diagnosis and management of acute and chronic urticaria: 2014 update. J Allergy Clin Immunol. 2014;133:1270-1277.
- Merkin P. Pratique Dermatologique. Paris, France: Masso; 1904.
- Beattie PE, Dawe RS, Ibbotson SH, et al. Characteristics and prognosis of idiopathic solar urticaria: a cohort of 87 cases. Arch Dermatol. 2003;139:1149-1154.
- Kaplan AP. Therapy of chronic urticaria: a simple, modern approach. Ann Allergy Asthma Immunol. 2014;112:419-425.
- Metz M, Maurer M. Omalizumab in chronic urticaria. Curr Opin Allergy Clin Immunol. 2012;12:406-410.
- Aubin F, Porcher R, Jeanmougin M, et al. Severe and refractory solar urticaria treated with intravenous immunoglobulins: a phase II multicenter study. J Am Acad Dermatol. 2014;71:948-953.e1.
- Güzelbey O, Ardelean E, Magerl M, et al. Successful treatment of solar urticaria with anti-immunoglobulin E therapy. Allergy. 2008;63:1563-1565.
- Wu K, Jabbar-Lopez Z. Omalizumab, an anti-IgE mAb, receives approval for the treatment of chronic idiopathic/spontaneous urticaria. J Invest Dermatol. 2015;135:13-15.
- Boyce JA. Successful treatment of cold-induced urticaria/anaphylaxis with anti-IgE. J Allergy Clin Immunol. 2006;117:1415-1418.
- Corren J, Casale TB, Lanier B, et al. Safety and tolerability of omalizumab. Clin Exp Allergy. 2009;39:788-797.
- Wu K, Long H. Omalizumab for chronic urticaria. N Engl J Med. 2013;368:2527-2528.
- Morgado-Carrasco D, Giacaman-Von der Weth M, Fusta-Novell X, et al. Clinical response and long-term follow-up of 20 patients with refractory solar urticarial under treatment with omalizumab [published online May 28, 2019]. J Am Acad Dermatol. doi:10.1016/j.jaad.2019.05.070.
- Duchini G, Bäumler W, Bircher AJ, et al. Failure of omalizumab (Xolair®) in the treatment of a case of solar urticaria caused by ultraviolet A and visible light. Photodermatol Photoimmunol Photomed. 2011;27:336-337.
- Bernstein JA, Lang DM, Khan DA, et al. The diagnosis and management of acute and chronic urticaria: 2014 update. J Allergy Clin Immunol. 2014;133:1270-1277.
Practice Points
- Recurrent solar urticaria can be recalcitrant to treatment.
- Omalizumab may be an effective treatment option for solar urticaria, especially in patients with a concomitant asthma diagnosis.
MIPS: Nearly all eligible clinicians got a bonus for 2018
Nearly all clinicians who are eligible to participate in the Merit-Based Incentive Payment System (MIPS) track of the Quality Payment Program did so in 2018; most scored above the performance threshold and got a bonus.
According to the most recent data released this month by the Centers for Medicare & Medicaid Services, 98.37% of MIPS-eligible clinicians participated in the program. In the small/solo practice space, 89.20% of MIPS-eligible clinicians participated.
But more importantly, the clinicians are performing better one year later with the program, even though fewer are participating.
In 2018, 97.63% of clinicians scored above the performance threshold, up from 93.12% in 2017. There also were fewer clinicians performing at the threshold (0.42% in 2018, down from 2.01% in the previous year) and fewer clinicians scoring below the threshold (1.95%, down from 4.87%).
Exceeding the performance threshold resulted in a bonus to fee schedule payments in 2018, although the agency did not disclose how much money was paid out in performance bonuses.
MIPS scored “improved across performance categories, with the biggest gain in the Quality performance category, which highlights the program’s effectiveness in measuring outcomes for beneficiaries,” CMS Administrator Seema Verma wrote in a blog post.
The total number of eligible clinicians decreased in 2018 to 916,058, down from 1,057,824 in 2017 because CMS broadened the low-volume threshold to exempt providers from participation requirements.
Participants in a MIPS alternative payment model saw even more success in 2018. Participation increased from 341,220 clinicians in 2017 to 356,828 clinicians in 2018, while virtually all performed above the performance threshold (100% in 2017 and 99.99% in 2018). The 0.01% that was not above the threshold still met it, while no clinicians in either year that participated in a MIPS alternative payment model performed below the threshold.
Participation in the advanced alternative payment model track increased as well, going from 99,026 in 2017 to 183,306 in 2018.
Nearly all clinicians who are eligible to participate in the Merit-Based Incentive Payment System (MIPS) track of the Quality Payment Program did so in 2018; most scored above the performance threshold and got a bonus.
According to the most recent data released this month by the Centers for Medicare & Medicaid Services, 98.37% of MIPS-eligible clinicians participated in the program. In the small/solo practice space, 89.20% of MIPS-eligible clinicians participated.
But more importantly, the clinicians are performing better one year later with the program, even though fewer are participating.
In 2018, 97.63% of clinicians scored above the performance threshold, up from 93.12% in 2017. There also were fewer clinicians performing at the threshold (0.42% in 2018, down from 2.01% in the previous year) and fewer clinicians scoring below the threshold (1.95%, down from 4.87%).
Exceeding the performance threshold resulted in a bonus to fee schedule payments in 2018, although the agency did not disclose how much money was paid out in performance bonuses.
MIPS scored “improved across performance categories, with the biggest gain in the Quality performance category, which highlights the program’s effectiveness in measuring outcomes for beneficiaries,” CMS Administrator Seema Verma wrote in a blog post.
The total number of eligible clinicians decreased in 2018 to 916,058, down from 1,057,824 in 2017 because CMS broadened the low-volume threshold to exempt providers from participation requirements.
Participants in a MIPS alternative payment model saw even more success in 2018. Participation increased from 341,220 clinicians in 2017 to 356,828 clinicians in 2018, while virtually all performed above the performance threshold (100% in 2017 and 99.99% in 2018). The 0.01% that was not above the threshold still met it, while no clinicians in either year that participated in a MIPS alternative payment model performed below the threshold.
Participation in the advanced alternative payment model track increased as well, going from 99,026 in 2017 to 183,306 in 2018.
Nearly all clinicians who are eligible to participate in the Merit-Based Incentive Payment System (MIPS) track of the Quality Payment Program did so in 2018; most scored above the performance threshold and got a bonus.
According to the most recent data released this month by the Centers for Medicare & Medicaid Services, 98.37% of MIPS-eligible clinicians participated in the program. In the small/solo practice space, 89.20% of MIPS-eligible clinicians participated.
But more importantly, the clinicians are performing better one year later with the program, even though fewer are participating.
In 2018, 97.63% of clinicians scored above the performance threshold, up from 93.12% in 2017. There also were fewer clinicians performing at the threshold (0.42% in 2018, down from 2.01% in the previous year) and fewer clinicians scoring below the threshold (1.95%, down from 4.87%).
Exceeding the performance threshold resulted in a bonus to fee schedule payments in 2018, although the agency did not disclose how much money was paid out in performance bonuses.
MIPS scored “improved across performance categories, with the biggest gain in the Quality performance category, which highlights the program’s effectiveness in measuring outcomes for beneficiaries,” CMS Administrator Seema Verma wrote in a blog post.
The total number of eligible clinicians decreased in 2018 to 916,058, down from 1,057,824 in 2017 because CMS broadened the low-volume threshold to exempt providers from participation requirements.
Participants in a MIPS alternative payment model saw even more success in 2018. Participation increased from 341,220 clinicians in 2017 to 356,828 clinicians in 2018, while virtually all performed above the performance threshold (100% in 2017 and 99.99% in 2018). The 0.01% that was not above the threshold still met it, while no clinicians in either year that participated in a MIPS alternative payment model performed below the threshold.
Participation in the advanced alternative payment model track increased as well, going from 99,026 in 2017 to 183,306 in 2018.
Chemo-free combo gets high response rate in relapsed or refractory DLBCL
LUGANO, Switzerland – A chemotherapy-free combination of lenalidomide (Revlimid) and the novel anti-CD19 antibody tafasitamab (MOR208) continues to show encouraging clinical activity against relapsed/refractory diffuse large B cell lymphoma, with durable responses and promising progression-free and overall survival, investigators in the phase 2 L-MIND study reported.
After a median follow-up of 17.3 months, the overall response rate (ORR) – the primary endpoint in the single arm trial – was 60%, consisting of 42.5% complete responses (CR) and 17.5% partial responses (PR), reported Giles Salles, MD, PhD, of Claude Bernard University in Lyon, France.
“We see consistently high activity in transplant-ineligible subgroups, patients who have limited treatment options and who have really poor prognosis,” he said at the International Conference on Malignant Lymphoma (15-ICML).
In a preclinical study, a combination of MOR208 and lenalidomide showed synergistic antileukemic and antilymphoma activity both in vivo and in vitro. In addition, both lenalidomide and MOR208 have shown significant activity against relapsed, refractory B-cell non-Hodgkin lymphomas.
At the previous ICML meeting in 2017, Dr. Salles reported early interim results from the study, which showed that among 34 patients evaluable for response, the ORR was 56%, including complete responses in 32% of patients.
The L-MIND investigators enrolled transplant-ineligible patients 18 years and older with relapsed/refractory DLBCL, Eastern Cooperative Oncology Group performance status 0-2, and adequate organ function who had disease progression after 1-3 prior lines of therapy.
Patients with primary refractory DLBCL, double-hit or triple-hit DLBCL (i.e., mutations in Myc, BCL2, and/or BCL6), other non-Hodgkin lymphoma histological subtypes, or central nervous system lymphoma involvement were excluded.
Patients received tafasitamab 12 mg/kg intravenously on days 1, 8, 15, and 22 for cycles 1-3 and on days 1 and 15 of cycles 4-12. Lenalidomide 25 mg orally was delivered on days 1-21 of each cycle. Patients with stable disease or better at the end of 12 cycles could be maintained on tafasitamab at the same dose on days 1 and 15.
As noted, the combination was associated with an ORR among 80 patients of 60%, consisting of 34 CR (42.5%) complete responses and 14 (17.5%) PR. An additional 11 patients (13.75%) had stable disease, 13 (16.25%) had disease progression, and eight (10%) were not evaluable because of missing post-baseline tumor assessments.
The median duration of response in the entire cohort was 21.7 months. For patients with a CR, the median duration of response had not been reached at the time of data cutoff. For patients with a PR, the median duration of response was 4.4 months.
Hematologic treatment-emergent toxicities occurring in 10% or more of patients included (in descending order of frequency) neutropenia, anemia, thrombocytopenia, leukopenia, and febrile neutropenia.
Nonhematologic treatment-emergent events occurring in at least 10% of patients included diarrhea, asthenia, peripheral edema, pyrexia, rash, decreased appetite, hypokalemia, fatigue, and similar events, the majority of which were grade 1 or 2 in severity.
“The durable responses and favorable overall survival I would say represent a remarkable outcome, and this combination of lenalidomide with tafasitamab results in a new chemo-free immunotherapy for patients with relapsed/refractory DLBCL,” Dr. Salles said.
The L-MIND study is funded by MorphoSys Ag. Dr. Salles reported receiving fees for advisory board/consulting activities and educational activities from MorphoSys and other companies.
SOURCE: Salles G et al. 15-ICML, Abstract 124.
LUGANO, Switzerland – A chemotherapy-free combination of lenalidomide (Revlimid) and the novel anti-CD19 antibody tafasitamab (MOR208) continues to show encouraging clinical activity against relapsed/refractory diffuse large B cell lymphoma, with durable responses and promising progression-free and overall survival, investigators in the phase 2 L-MIND study reported.
After a median follow-up of 17.3 months, the overall response rate (ORR) – the primary endpoint in the single arm trial – was 60%, consisting of 42.5% complete responses (CR) and 17.5% partial responses (PR), reported Giles Salles, MD, PhD, of Claude Bernard University in Lyon, France.
“We see consistently high activity in transplant-ineligible subgroups, patients who have limited treatment options and who have really poor prognosis,” he said at the International Conference on Malignant Lymphoma (15-ICML).
In a preclinical study, a combination of MOR208 and lenalidomide showed synergistic antileukemic and antilymphoma activity both in vivo and in vitro. In addition, both lenalidomide and MOR208 have shown significant activity against relapsed, refractory B-cell non-Hodgkin lymphomas.
At the previous ICML meeting in 2017, Dr. Salles reported early interim results from the study, which showed that among 34 patients evaluable for response, the ORR was 56%, including complete responses in 32% of patients.
The L-MIND investigators enrolled transplant-ineligible patients 18 years and older with relapsed/refractory DLBCL, Eastern Cooperative Oncology Group performance status 0-2, and adequate organ function who had disease progression after 1-3 prior lines of therapy.
Patients with primary refractory DLBCL, double-hit or triple-hit DLBCL (i.e., mutations in Myc, BCL2, and/or BCL6), other non-Hodgkin lymphoma histological subtypes, or central nervous system lymphoma involvement were excluded.
Patients received tafasitamab 12 mg/kg intravenously on days 1, 8, 15, and 22 for cycles 1-3 and on days 1 and 15 of cycles 4-12. Lenalidomide 25 mg orally was delivered on days 1-21 of each cycle. Patients with stable disease or better at the end of 12 cycles could be maintained on tafasitamab at the same dose on days 1 and 15.
As noted, the combination was associated with an ORR among 80 patients of 60%, consisting of 34 CR (42.5%) complete responses and 14 (17.5%) PR. An additional 11 patients (13.75%) had stable disease, 13 (16.25%) had disease progression, and eight (10%) were not evaluable because of missing post-baseline tumor assessments.
The median duration of response in the entire cohort was 21.7 months. For patients with a CR, the median duration of response had not been reached at the time of data cutoff. For patients with a PR, the median duration of response was 4.4 months.
Hematologic treatment-emergent toxicities occurring in 10% or more of patients included (in descending order of frequency) neutropenia, anemia, thrombocytopenia, leukopenia, and febrile neutropenia.
Nonhematologic treatment-emergent events occurring in at least 10% of patients included diarrhea, asthenia, peripheral edema, pyrexia, rash, decreased appetite, hypokalemia, fatigue, and similar events, the majority of which were grade 1 or 2 in severity.
“The durable responses and favorable overall survival I would say represent a remarkable outcome, and this combination of lenalidomide with tafasitamab results in a new chemo-free immunotherapy for patients with relapsed/refractory DLBCL,” Dr. Salles said.
The L-MIND study is funded by MorphoSys Ag. Dr. Salles reported receiving fees for advisory board/consulting activities and educational activities from MorphoSys and other companies.
SOURCE: Salles G et al. 15-ICML, Abstract 124.
LUGANO, Switzerland – A chemotherapy-free combination of lenalidomide (Revlimid) and the novel anti-CD19 antibody tafasitamab (MOR208) continues to show encouraging clinical activity against relapsed/refractory diffuse large B cell lymphoma, with durable responses and promising progression-free and overall survival, investigators in the phase 2 L-MIND study reported.
After a median follow-up of 17.3 months, the overall response rate (ORR) – the primary endpoint in the single arm trial – was 60%, consisting of 42.5% complete responses (CR) and 17.5% partial responses (PR), reported Giles Salles, MD, PhD, of Claude Bernard University in Lyon, France.
“We see consistently high activity in transplant-ineligible subgroups, patients who have limited treatment options and who have really poor prognosis,” he said at the International Conference on Malignant Lymphoma (15-ICML).
In a preclinical study, a combination of MOR208 and lenalidomide showed synergistic antileukemic and antilymphoma activity both in vivo and in vitro. In addition, both lenalidomide and MOR208 have shown significant activity against relapsed, refractory B-cell non-Hodgkin lymphomas.
At the previous ICML meeting in 2017, Dr. Salles reported early interim results from the study, which showed that among 34 patients evaluable for response, the ORR was 56%, including complete responses in 32% of patients.
The L-MIND investigators enrolled transplant-ineligible patients 18 years and older with relapsed/refractory DLBCL, Eastern Cooperative Oncology Group performance status 0-2, and adequate organ function who had disease progression after 1-3 prior lines of therapy.
Patients with primary refractory DLBCL, double-hit or triple-hit DLBCL (i.e., mutations in Myc, BCL2, and/or BCL6), other non-Hodgkin lymphoma histological subtypes, or central nervous system lymphoma involvement were excluded.
Patients received tafasitamab 12 mg/kg intravenously on days 1, 8, 15, and 22 for cycles 1-3 and on days 1 and 15 of cycles 4-12. Lenalidomide 25 mg orally was delivered on days 1-21 of each cycle. Patients with stable disease or better at the end of 12 cycles could be maintained on tafasitamab at the same dose on days 1 and 15.
As noted, the combination was associated with an ORR among 80 patients of 60%, consisting of 34 CR (42.5%) complete responses and 14 (17.5%) PR. An additional 11 patients (13.75%) had stable disease, 13 (16.25%) had disease progression, and eight (10%) were not evaluable because of missing post-baseline tumor assessments.
The median duration of response in the entire cohort was 21.7 months. For patients with a CR, the median duration of response had not been reached at the time of data cutoff. For patients with a PR, the median duration of response was 4.4 months.
Hematologic treatment-emergent toxicities occurring in 10% or more of patients included (in descending order of frequency) neutropenia, anemia, thrombocytopenia, leukopenia, and febrile neutropenia.
Nonhematologic treatment-emergent events occurring in at least 10% of patients included diarrhea, asthenia, peripheral edema, pyrexia, rash, decreased appetite, hypokalemia, fatigue, and similar events, the majority of which were grade 1 or 2 in severity.
“The durable responses and favorable overall survival I would say represent a remarkable outcome, and this combination of lenalidomide with tafasitamab results in a new chemo-free immunotherapy for patients with relapsed/refractory DLBCL,” Dr. Salles said.
The L-MIND study is funded by MorphoSys Ag. Dr. Salles reported receiving fees for advisory board/consulting activities and educational activities from MorphoSys and other companies.
SOURCE: Salles G et al. 15-ICML, Abstract 124.
REPORTING FROM 15-ICML
Amitriptyline for chronic low back pain
Clinical question: Is a low-dose tricyclic antidepressant effective in the treatment of chronic low back pain?
Background: Lower back pain is the leading cause of disability globally and effective treatments are limited. Furthermore, opioid usage for lower back pain is a large contributor to the current opioid epidemic. A recent Cochrane review showed no clear evidence that antidepressant use in the treatment of back pain is effective, but it did note a lack of high-quality trials of sufficient rigor or length.
Study design: Double-blind, randomized controlled trial.
Setting: Single center trial in Melbourne.
Synopsis: Overall, 146 patients aged 18-75 with chronic lower back pain of no specific cause for more than 3 months were included. Exclusions included pathological cause, major coexisting illness, psychosis, or diagnosed depression. Patients were given amitriptyline 25 mg daily or benztropine mesylate 1 mg daily. Benztropine has similar anticholinergic side effects, without the antidepressant effect. Participants were assessed and followed by calls at 2 weeks, 1-2 months, 3 months, 4-5 months, and 6 months. Adherence was noted by the return of empty medication bottles at 6 months. Six-month surveys were completed by 81% and found that 70% of each group was adherent and 12% in each group withdrew because of adverse effects.
The primary outcome was level of pain at 6 months using a visual analog and descriptor scales. Secondary outcomes were measurement of disability, work missed, global improvement, general health, fear of movement, and depression.
The primary outcome was reduction in pain intensity of 12.6 (standard error, 2.7) with amitriptyline at 6 months, compared with 4.8 (SE 2.9) with benztropine, which did not meet statistical significance. There was a statistically significant difference in disability at 3 months, but not at 6 months.
Bottom line: This trial suggests that there may be a place for prescribed amitriptyline for chronic lower back pain, but it failed to show statistical significance. The study may not have been sufficiently powered to detect the difference.
Citations: Urquhart DM et al. Efficacy of low-dose amitriptyline for chronic low back pain: A randomized clinical trial. JAMA Intern Med. 2018;178(11):1474-81.
Dr. Lennon is an instructor of medicine at Northwestern University Feinberg School of Medicine and a hospitalist at Northwestern Memorial Hospital, both in Chicago.
Clinical question: Is a low-dose tricyclic antidepressant effective in the treatment of chronic low back pain?
Background: Lower back pain is the leading cause of disability globally and effective treatments are limited. Furthermore, opioid usage for lower back pain is a large contributor to the current opioid epidemic. A recent Cochrane review showed no clear evidence that antidepressant use in the treatment of back pain is effective, but it did note a lack of high-quality trials of sufficient rigor or length.
Study design: Double-blind, randomized controlled trial.
Setting: Single center trial in Melbourne.
Synopsis: Overall, 146 patients aged 18-75 with chronic lower back pain of no specific cause for more than 3 months were included. Exclusions included pathological cause, major coexisting illness, psychosis, or diagnosed depression. Patients were given amitriptyline 25 mg daily or benztropine mesylate 1 mg daily. Benztropine has similar anticholinergic side effects, without the antidepressant effect. Participants were assessed and followed by calls at 2 weeks, 1-2 months, 3 months, 4-5 months, and 6 months. Adherence was noted by the return of empty medication bottles at 6 months. Six-month surveys were completed by 81% and found that 70% of each group was adherent and 12% in each group withdrew because of adverse effects.
The primary outcome was level of pain at 6 months using a visual analog and descriptor scales. Secondary outcomes were measurement of disability, work missed, global improvement, general health, fear of movement, and depression.
The primary outcome was reduction in pain intensity of 12.6 (standard error, 2.7) with amitriptyline at 6 months, compared with 4.8 (SE 2.9) with benztropine, which did not meet statistical significance. There was a statistically significant difference in disability at 3 months, but not at 6 months.
Bottom line: This trial suggests that there may be a place for prescribed amitriptyline for chronic lower back pain, but it failed to show statistical significance. The study may not have been sufficiently powered to detect the difference.
Citations: Urquhart DM et al. Efficacy of low-dose amitriptyline for chronic low back pain: A randomized clinical trial. JAMA Intern Med. 2018;178(11):1474-81.
Dr. Lennon is an instructor of medicine at Northwestern University Feinberg School of Medicine and a hospitalist at Northwestern Memorial Hospital, both in Chicago.
Clinical question: Is a low-dose tricyclic antidepressant effective in the treatment of chronic low back pain?
Background: Lower back pain is the leading cause of disability globally and effective treatments are limited. Furthermore, opioid usage for lower back pain is a large contributor to the current opioid epidemic. A recent Cochrane review showed no clear evidence that antidepressant use in the treatment of back pain is effective, but it did note a lack of high-quality trials of sufficient rigor or length.
Study design: Double-blind, randomized controlled trial.
Setting: Single center trial in Melbourne.
Synopsis: Overall, 146 patients aged 18-75 with chronic lower back pain of no specific cause for more than 3 months were included. Exclusions included pathological cause, major coexisting illness, psychosis, or diagnosed depression. Patients were given amitriptyline 25 mg daily or benztropine mesylate 1 mg daily. Benztropine has similar anticholinergic side effects, without the antidepressant effect. Participants were assessed and followed by calls at 2 weeks, 1-2 months, 3 months, 4-5 months, and 6 months. Adherence was noted by the return of empty medication bottles at 6 months. Six-month surveys were completed by 81% and found that 70% of each group was adherent and 12% in each group withdrew because of adverse effects.
The primary outcome was level of pain at 6 months using a visual analog and descriptor scales. Secondary outcomes were measurement of disability, work missed, global improvement, general health, fear of movement, and depression.
The primary outcome was reduction in pain intensity of 12.6 (standard error, 2.7) with amitriptyline at 6 months, compared with 4.8 (SE 2.9) with benztropine, which did not meet statistical significance. There was a statistically significant difference in disability at 3 months, but not at 6 months.
Bottom line: This trial suggests that there may be a place for prescribed amitriptyline for chronic lower back pain, but it failed to show statistical significance. The study may not have been sufficiently powered to detect the difference.
Citations: Urquhart DM et al. Efficacy of low-dose amitriptyline for chronic low back pain: A randomized clinical trial. JAMA Intern Med. 2018;178(11):1474-81.
Dr. Lennon is an instructor of medicine at Northwestern University Feinberg School of Medicine and a hospitalist at Northwestern Memorial Hospital, both in Chicago.
An Algorithm to Identify PrEP-Potential Patients
Health care providers who do not have the time or tools to screen patients for HIV risk also may not be prescribing preexposure prophylaxis (PrEP). But help is on the way: NIH-funded researchers have come up with novel computerized methods to identify the patients PrEP could benefit.
In 2 separate studies, the researchers developed and validated algorithms that analyze electronic health records (EHR). In the first study, Harvard researchers used machine learning to create an HIV prediction algorithm using 2007 to 2015 data from > 1 million patients in Massachusetts. The model included variables such as diagnosis codes for HIV counseling or sexually transmitted infections (STIs), laboratory tests for HIV or STIs, and prescriptions for medications related to treating STIs.
The model was validated using data from nearly 600,000 other patients treated between 2011 and 2016. The prediction algorithm successfully distinguished with high precision between patients who did or did not acquire HIV and between those who did or did not receive a PrEP prescription.
The researchers found hundreds of potential missed opportunities. They point to > 9,500 people in the 2016 dataset with particularly high-risk scores who were not prescribed PrEP. A “striking outcome,” the researchers say, is that their analysis suggests that nearly 40% of new HIV cases might have been averted had clinicians received alerts to discuss and offer PrEP to patients with the highest 2% of risk scores.
In the second study, researchers used the EHRs of > 3.7 million patients who entered the Kaiser Permanente System Northern California between 2007 and 2014 to develop a model to predict HIV incidence, then validated the model with data from between 2015 and 2017. Of the original patient group, 784 developed HIV within 3 years of baseline. The study found that the model identified nearly half of the incident HIV cases among males by flagging only 2% of the general patient population.
Embedding the algorithm into the EHR, the lead investigator says, “could prompt providers to discuss PrEP with patients who are most likely to benefit.”
Health care providers who do not have the time or tools to screen patients for HIV risk also may not be prescribing preexposure prophylaxis (PrEP). But help is on the way: NIH-funded researchers have come up with novel computerized methods to identify the patients PrEP could benefit.
In 2 separate studies, the researchers developed and validated algorithms that analyze electronic health records (EHR). In the first study, Harvard researchers used machine learning to create an HIV prediction algorithm using 2007 to 2015 data from > 1 million patients in Massachusetts. The model included variables such as diagnosis codes for HIV counseling or sexually transmitted infections (STIs), laboratory tests for HIV or STIs, and prescriptions for medications related to treating STIs.
The model was validated using data from nearly 600,000 other patients treated between 2011 and 2016. The prediction algorithm successfully distinguished with high precision between patients who did or did not acquire HIV and between those who did or did not receive a PrEP prescription.
The researchers found hundreds of potential missed opportunities. They point to > 9,500 people in the 2016 dataset with particularly high-risk scores who were not prescribed PrEP. A “striking outcome,” the researchers say, is that their analysis suggests that nearly 40% of new HIV cases might have been averted had clinicians received alerts to discuss and offer PrEP to patients with the highest 2% of risk scores.
In the second study, researchers used the EHRs of > 3.7 million patients who entered the Kaiser Permanente System Northern California between 2007 and 2014 to develop a model to predict HIV incidence, then validated the model with data from between 2015 and 2017. Of the original patient group, 784 developed HIV within 3 years of baseline. The study found that the model identified nearly half of the incident HIV cases among males by flagging only 2% of the general patient population.
Embedding the algorithm into the EHR, the lead investigator says, “could prompt providers to discuss PrEP with patients who are most likely to benefit.”
Health care providers who do not have the time or tools to screen patients for HIV risk also may not be prescribing preexposure prophylaxis (PrEP). But help is on the way: NIH-funded researchers have come up with novel computerized methods to identify the patients PrEP could benefit.
In 2 separate studies, the researchers developed and validated algorithms that analyze electronic health records (EHR). In the first study, Harvard researchers used machine learning to create an HIV prediction algorithm using 2007 to 2015 data from > 1 million patients in Massachusetts. The model included variables such as diagnosis codes for HIV counseling or sexually transmitted infections (STIs), laboratory tests for HIV or STIs, and prescriptions for medications related to treating STIs.
The model was validated using data from nearly 600,000 other patients treated between 2011 and 2016. The prediction algorithm successfully distinguished with high precision between patients who did or did not acquire HIV and between those who did or did not receive a PrEP prescription.
The researchers found hundreds of potential missed opportunities. They point to > 9,500 people in the 2016 dataset with particularly high-risk scores who were not prescribed PrEP. A “striking outcome,” the researchers say, is that their analysis suggests that nearly 40% of new HIV cases might have been averted had clinicians received alerts to discuss and offer PrEP to patients with the highest 2% of risk scores.
In the second study, researchers used the EHRs of > 3.7 million patients who entered the Kaiser Permanente System Northern California between 2007 and 2014 to develop a model to predict HIV incidence, then validated the model with data from between 2015 and 2017. Of the original patient group, 784 developed HIV within 3 years of baseline. The study found that the model identified nearly half of the incident HIV cases among males by flagging only 2% of the general patient population.
Embedding the algorithm into the EHR, the lead investigator says, “could prompt providers to discuss PrEP with patients who are most likely to benefit.”
Liberalized low–glycemic-index diet effective for seizure reduction
BANGKOK – in a randomized, double-blind, 24-week, noninferiority study.
The low–glycemic-index diet (LGID) was introduced as a kinder, gentler, variant of the classic ketogenic diet for seizure frequency reduction. The ketogenic diet’s efficacy for this purpose is well established, but compliance is a problem and discontinuation rates are high. Yet even though the LGID was designed to be less onerous than the ketogenic diet, many children and parents also find the 7-days-a-week LGID to be excessively burdensome. This was the impetus for pitting the daily LGID against an intermittent version – 5 days on, 2 days off – in a randomized trial, Prateek K. Panda, MD, explained at the International Epilepsy Congress.
The hypothesis of this noninferiority trial was that adherence to the liberalized LGID would be similar to or better than that with the daily LGID regimen, with resultant similar reductions in seizure frequency. And further, that patients on the intermittent LGID would feel better because it would help improve depleted glycogen stores important for daily activity and that the liberalized diet would also be rated more favorably by caregivers, Dr. Panda said at the congress sponsored by the International League Against Epilepsy.
The 24-week, single-center trial included 122 children ages 1-15 years with drug-resistant epilepsy. At baseline they averaged 99 seizures per week by parental diary despite being on a median of four antiepileptic drugs. A total of 88% of participants had some form of structural epilepsy; the rest had a probable or confirmed genetic cause for their seizure disorder, according to Dr. Panda of the All-India Institute of Medical Sciences in New Delhi.
The standard daily LGID was comprised of 10% carbohydrate, 30% protein, and 60% fat, with only low–glycemic-index foods permitted. The cohort randomized to the liberalized diet ate that way on weekdays; however, on Saturdays and Sundays their diet was 20% carbohydrate, 30% protein, and 50% fat, with both medium- and low–glycemic-index foods allowed.
The primary outcome was the mean reduction in seizures per week by caregiver records at 24 weeks. The reduction from baseline was 54% in the strict LGID group and not significantly different at 49% in the intermittent LGID patients. Overall, 54% of patients in the strict LGID arm experienced a greater than 50% reduction in weekly seizure frequency, as did 50% on the liberalized diet, a nonsignificant difference.
There were five study dropouts in the strict LGID group and three in the liberalized LGID cohort. The two groups showed similar improvements over baseline in measures of social function, behavior, and cognition. Parents of children in the liberalized LGID group rated that diet as significantly less difficult to administer than those randomized to the strict LGID therapy.
Mean hemoglobin A1c improved in the strict LGID patients from 5.7% at baseline to 5.1% at both 12 and 24 weeks. The intermittent LGID group went from 5.6% to 5.0% and then to 5.2%. There was no correlation between HbA1c and reduction in seizure frequency. In contrast, serum beta-hydroxybutyrate levels showed a moderate correlation with seizure frequency, a novel finding which if confirmed might render beta-hydroxybutyrate useful as a biomarker, according to Dr. Panda.
Adverse events – mostly dyslipidemia and GI complaints such as vomiting or constipation – occurred in 25% of the strict LGID group and 13% with the intermittent LGID. All adverse events were mild.
Dr. Panda reported having no financial conflicts regarding the study, sponsored by the All-India Institute of Medical Sciences.
SOURCE: Panda PK et al. IEC 2019, Abstract P056.
BANGKOK – in a randomized, double-blind, 24-week, noninferiority study.
The low–glycemic-index diet (LGID) was introduced as a kinder, gentler, variant of the classic ketogenic diet for seizure frequency reduction. The ketogenic diet’s efficacy for this purpose is well established, but compliance is a problem and discontinuation rates are high. Yet even though the LGID was designed to be less onerous than the ketogenic diet, many children and parents also find the 7-days-a-week LGID to be excessively burdensome. This was the impetus for pitting the daily LGID against an intermittent version – 5 days on, 2 days off – in a randomized trial, Prateek K. Panda, MD, explained at the International Epilepsy Congress.
The hypothesis of this noninferiority trial was that adherence to the liberalized LGID would be similar to or better than that with the daily LGID regimen, with resultant similar reductions in seizure frequency. And further, that patients on the intermittent LGID would feel better because it would help improve depleted glycogen stores important for daily activity and that the liberalized diet would also be rated more favorably by caregivers, Dr. Panda said at the congress sponsored by the International League Against Epilepsy.
The 24-week, single-center trial included 122 children ages 1-15 years with drug-resistant epilepsy. At baseline they averaged 99 seizures per week by parental diary despite being on a median of four antiepileptic drugs. A total of 88% of participants had some form of structural epilepsy; the rest had a probable or confirmed genetic cause for their seizure disorder, according to Dr. Panda of the All-India Institute of Medical Sciences in New Delhi.
The standard daily LGID was comprised of 10% carbohydrate, 30% protein, and 60% fat, with only low–glycemic-index foods permitted. The cohort randomized to the liberalized diet ate that way on weekdays; however, on Saturdays and Sundays their diet was 20% carbohydrate, 30% protein, and 50% fat, with both medium- and low–glycemic-index foods allowed.
The primary outcome was the mean reduction in seizures per week by caregiver records at 24 weeks. The reduction from baseline was 54% in the strict LGID group and not significantly different at 49% in the intermittent LGID patients. Overall, 54% of patients in the strict LGID arm experienced a greater than 50% reduction in weekly seizure frequency, as did 50% on the liberalized diet, a nonsignificant difference.
There were five study dropouts in the strict LGID group and three in the liberalized LGID cohort. The two groups showed similar improvements over baseline in measures of social function, behavior, and cognition. Parents of children in the liberalized LGID group rated that diet as significantly less difficult to administer than those randomized to the strict LGID therapy.
Mean hemoglobin A1c improved in the strict LGID patients from 5.7% at baseline to 5.1% at both 12 and 24 weeks. The intermittent LGID group went from 5.6% to 5.0% and then to 5.2%. There was no correlation between HbA1c and reduction in seizure frequency. In contrast, serum beta-hydroxybutyrate levels showed a moderate correlation with seizure frequency, a novel finding which if confirmed might render beta-hydroxybutyrate useful as a biomarker, according to Dr. Panda.
Adverse events – mostly dyslipidemia and GI complaints such as vomiting or constipation – occurred in 25% of the strict LGID group and 13% with the intermittent LGID. All adverse events were mild.
Dr. Panda reported having no financial conflicts regarding the study, sponsored by the All-India Institute of Medical Sciences.
SOURCE: Panda PK et al. IEC 2019, Abstract P056.
BANGKOK – in a randomized, double-blind, 24-week, noninferiority study.
The low–glycemic-index diet (LGID) was introduced as a kinder, gentler, variant of the classic ketogenic diet for seizure frequency reduction. The ketogenic diet’s efficacy for this purpose is well established, but compliance is a problem and discontinuation rates are high. Yet even though the LGID was designed to be less onerous than the ketogenic diet, many children and parents also find the 7-days-a-week LGID to be excessively burdensome. This was the impetus for pitting the daily LGID against an intermittent version – 5 days on, 2 days off – in a randomized trial, Prateek K. Panda, MD, explained at the International Epilepsy Congress.
The hypothesis of this noninferiority trial was that adherence to the liberalized LGID would be similar to or better than that with the daily LGID regimen, with resultant similar reductions in seizure frequency. And further, that patients on the intermittent LGID would feel better because it would help improve depleted glycogen stores important for daily activity and that the liberalized diet would also be rated more favorably by caregivers, Dr. Panda said at the congress sponsored by the International League Against Epilepsy.
The 24-week, single-center trial included 122 children ages 1-15 years with drug-resistant epilepsy. At baseline they averaged 99 seizures per week by parental diary despite being on a median of four antiepileptic drugs. A total of 88% of participants had some form of structural epilepsy; the rest had a probable or confirmed genetic cause for their seizure disorder, according to Dr. Panda of the All-India Institute of Medical Sciences in New Delhi.
The standard daily LGID was comprised of 10% carbohydrate, 30% protein, and 60% fat, with only low–glycemic-index foods permitted. The cohort randomized to the liberalized diet ate that way on weekdays; however, on Saturdays and Sundays their diet was 20% carbohydrate, 30% protein, and 50% fat, with both medium- and low–glycemic-index foods allowed.
The primary outcome was the mean reduction in seizures per week by caregiver records at 24 weeks. The reduction from baseline was 54% in the strict LGID group and not significantly different at 49% in the intermittent LGID patients. Overall, 54% of patients in the strict LGID arm experienced a greater than 50% reduction in weekly seizure frequency, as did 50% on the liberalized diet, a nonsignificant difference.
There were five study dropouts in the strict LGID group and three in the liberalized LGID cohort. The two groups showed similar improvements over baseline in measures of social function, behavior, and cognition. Parents of children in the liberalized LGID group rated that diet as significantly less difficult to administer than those randomized to the strict LGID therapy.
Mean hemoglobin A1c improved in the strict LGID patients from 5.7% at baseline to 5.1% at both 12 and 24 weeks. The intermittent LGID group went from 5.6% to 5.0% and then to 5.2%. There was no correlation between HbA1c and reduction in seizure frequency. In contrast, serum beta-hydroxybutyrate levels showed a moderate correlation with seizure frequency, a novel finding which if confirmed might render beta-hydroxybutyrate useful as a biomarker, according to Dr. Panda.
Adverse events – mostly dyslipidemia and GI complaints such as vomiting or constipation – occurred in 25% of the strict LGID group and 13% with the intermittent LGID. All adverse events were mild.
Dr. Panda reported having no financial conflicts regarding the study, sponsored by the All-India Institute of Medical Sciences.
SOURCE: Panda PK et al. IEC 2019, Abstract P056.
REPORTING FROM IEC 2019