User login
Expert shares contact dermatitis trends
AUSTIN – Not long ago, Rajani Katta, MD, received a text message from a friend who expressed concern about a rash that developed in the underarm of her teenage daughter.
The culprit turned out to be the lavender essential oil contained in an “all natural” deodorant that her daughter had recently switched to – a storyline that Dr. Katta encounters with increasing frequency in her role as clinical professor of dermatology at the University of Texas Health Science Center at Houston.
Dr. Katta said at the annual meeting of the Society for Pediatric Dermatology. “When you talk about a natural allergy, it is more likely to occur if your skin barrier is compromised, so I think that’s why we’re seeing it, especially in young girls in the underarm area. If you shave the underarm, you impair that skin barrier and you’re more likely to develop a reaction to something you’re using over it.”
Her list of recommended deodorants includes Almay Roll-On Antiperspirant & Deodorant, Crystal Body Deodorant Stick, Crystal Roll-On Body Deodorant, Vanicream Deodorant for Sensitive Skin (aluminum-free), Vanicream Antiperspirant/Deodorant, and CertainDri Clinical Strength Roll-On. They are fragrance-free and lack propylene glycol, which is a common allergen.
Increasingly, essential oils are being added to lip balms and toothpastes, said Dr. Katta, who is also author of the 2018 book “Glow: The Dermatologist’s Guide to a Whole Foods Younger Skin Diet.” She recalled one patient who presented with chronic chapped lips. “It doesn’t matter how many lip glosses I try; it just keeps getting worse,” the patient told her. The likely culprit turned out to be ingredients contained in flavored lip balm from EOS. Reports of blistering and cracking of the lips from use of the products prompted a class action lawsuit and a notice to consumers from the Food and Drug Administration.
Another patient presented with cracked lips after switching to an “all natural” toothpaste that was labeled “gluten free.”
“It looked great,” Dr. Katta recalled. “Unfortunately it was not flavoring free. She reacted to multiple essential oils, including tea tree oil, contained in the toothpaste. This is being added to a number of toothpastes, and I think we’re going to see more of these types of reactions.”
Other toothpastes contain balsam of Peru, “which is consistently one of the top allergens in patch test clinics,” she said. “One of the components of balsam of Peru is a cinnamon compound, which can be an issue.”
Dr. Katta advises her patients to use Vaseline petroleum jelly as a lip balm and recommends Tom’s of Maine Silly Strawberry Flavor (this flavor only) toothpaste for children.
A few years ago, a teenager presented to Dr. Katta with intense bullae on the dorsum of the foot after wearing shoes without socks. “She was wearing white canvas Keds, which looked very innocuous,” she said. Patch testing revealed that the teen reacted to four different rubber accelerators. “When we contacted the company, they [acknowledged] using rubber cement to make the canvas Keds,” Dr. Katta said. “Rubber cement is an adhesive and it does contain rubber accelerators. Later, I saw two cases of children who had walked around all day at the amusement park wearing their Sperry Topsiders without any socks. We couldn’t get any information from that manufacturer, but I suspect that they also use a rubber-based glue to make those shoes.” She characterized shoes as “a real setup for a foot allergy because you have friction, sweat that’s pulling allergen out of an object, and sweat is carrying it over, especially to the dorsum of the foot.”
Dr. Katta has also noticed an uptick in the number of young patients who develop allergic reactions to dyes used to make workout clothing. “If you ever see rashes that do not involve the axillary vault but do have peraxillary accentuation, think textile allergy,” she said. “We’re seeing a lot of reactions to disperse blue clothing dyes. When you think about textile allergy from the dyes, it tends to be the blue and black clothing. It’s more likely in the setting of synthetic fabrics because they leach out dyes more easily, and it’s more likely in the setting of sweat because sweat helps pull allergen out. I’m seeing it a lot from sports uniforms and tight black leggings and tight sports bras that people are wearing. I’m also seeing some from bathing suits and swim shirts.”
Exposure to products containing the preservative methylisothiazolinone (MI) is also on the rise. It ranks as the second most frequent allergen for which the North American Contact Dermatitis Group is seeing positive results on patch testing, with rates of 13.4%. MI can be found in many skin care products and “probably about half of school glues, fabric glues, and craft glues,” Dr. Katta said. “Stick versus liquid doesn’t make a difference.” Children and teens often use craft glues, laundry detergents, and other products to create “slime” as a way to learn about viscosity, polymers, and chemical reactions. “Sometimes these children have sensitive skin, or they’re using it with prolonged contact, so they may be sensitizing themselves to the MI,” she said.
She concluded her remarks by noting that an increasing number of young patients are developing reactions to wearable medical devices such as insulin pumps and glucose monitors. “With this, the first thing to think about is frictional irritant dermatitis,” she said. “You can put Scanpor medical paper tape on people’s back for 48 hours straight to patch test them. Some people are incredibly reactive to the friction of just that tape. You also have to think about trapped allergen. One of my patients reacted to colophony, fragrance mix, and propylene glycol, all of which were contained in his skin care products. Some people are getting advice from other patients to use Mastisol liquid adhesive to help their glucose monitors stick better. Mastisol has a high rate of cross-reactivity with balsam of Peru, so it’s a fragrance allergen. That’s the first thing you want to ask patients about: what products they’re using.”
One of her patients thought she was reacting to adhesive tape on her skin, but in fact she was reacting to two different acrylates: ethylene glycol dimethacrylate (EGDMA) and hydroxyethyl methacrylate (HEMA). “I know about these allergens because I see reactions from butterfly needles in dialysis patients,” Dr. Katta explained. “What happens is, these acrylates are glues or plastics used somewhere else on the device, and they can migrate through barriers.”
In one published case, a 9-year-old boy developed a reaction to ethyl cyanoacrylate contained in a glucose sensor adhesive (Dermatitis. 2017; 28[4]:289-91). It never touched the boy’s skin directly but was presumed to migrate through that tape. “The bottom line is that acrylates may induce contact dermatitis even through perceived barriers,” she said. “Their use anywhere in medical devices may prove problematic.”
Dr. Katta reported that she is a member of the advisory board for Vichy Laboratories.
AUSTIN – Not long ago, Rajani Katta, MD, received a text message from a friend who expressed concern about a rash that developed in the underarm of her teenage daughter.
The culprit turned out to be the lavender essential oil contained in an “all natural” deodorant that her daughter had recently switched to – a storyline that Dr. Katta encounters with increasing frequency in her role as clinical professor of dermatology at the University of Texas Health Science Center at Houston.
Dr. Katta said at the annual meeting of the Society for Pediatric Dermatology. “When you talk about a natural allergy, it is more likely to occur if your skin barrier is compromised, so I think that’s why we’re seeing it, especially in young girls in the underarm area. If you shave the underarm, you impair that skin barrier and you’re more likely to develop a reaction to something you’re using over it.”
Her list of recommended deodorants includes Almay Roll-On Antiperspirant & Deodorant, Crystal Body Deodorant Stick, Crystal Roll-On Body Deodorant, Vanicream Deodorant for Sensitive Skin (aluminum-free), Vanicream Antiperspirant/Deodorant, and CertainDri Clinical Strength Roll-On. They are fragrance-free and lack propylene glycol, which is a common allergen.
Increasingly, essential oils are being added to lip balms and toothpastes, said Dr. Katta, who is also author of the 2018 book “Glow: The Dermatologist’s Guide to a Whole Foods Younger Skin Diet.” She recalled one patient who presented with chronic chapped lips. “It doesn’t matter how many lip glosses I try; it just keeps getting worse,” the patient told her. The likely culprit turned out to be ingredients contained in flavored lip balm from EOS. Reports of blistering and cracking of the lips from use of the products prompted a class action lawsuit and a notice to consumers from the Food and Drug Administration.
Another patient presented with cracked lips after switching to an “all natural” toothpaste that was labeled “gluten free.”
“It looked great,” Dr. Katta recalled. “Unfortunately it was not flavoring free. She reacted to multiple essential oils, including tea tree oil, contained in the toothpaste. This is being added to a number of toothpastes, and I think we’re going to see more of these types of reactions.”
Other toothpastes contain balsam of Peru, “which is consistently one of the top allergens in patch test clinics,” she said. “One of the components of balsam of Peru is a cinnamon compound, which can be an issue.”
Dr. Katta advises her patients to use Vaseline petroleum jelly as a lip balm and recommends Tom’s of Maine Silly Strawberry Flavor (this flavor only) toothpaste for children.
A few years ago, a teenager presented to Dr. Katta with intense bullae on the dorsum of the foot after wearing shoes without socks. “She was wearing white canvas Keds, which looked very innocuous,” she said. Patch testing revealed that the teen reacted to four different rubber accelerators. “When we contacted the company, they [acknowledged] using rubber cement to make the canvas Keds,” Dr. Katta said. “Rubber cement is an adhesive and it does contain rubber accelerators. Later, I saw two cases of children who had walked around all day at the amusement park wearing their Sperry Topsiders without any socks. We couldn’t get any information from that manufacturer, but I suspect that they also use a rubber-based glue to make those shoes.” She characterized shoes as “a real setup for a foot allergy because you have friction, sweat that’s pulling allergen out of an object, and sweat is carrying it over, especially to the dorsum of the foot.”
Dr. Katta has also noticed an uptick in the number of young patients who develop allergic reactions to dyes used to make workout clothing. “If you ever see rashes that do not involve the axillary vault but do have peraxillary accentuation, think textile allergy,” she said. “We’re seeing a lot of reactions to disperse blue clothing dyes. When you think about textile allergy from the dyes, it tends to be the blue and black clothing. It’s more likely in the setting of synthetic fabrics because they leach out dyes more easily, and it’s more likely in the setting of sweat because sweat helps pull allergen out. I’m seeing it a lot from sports uniforms and tight black leggings and tight sports bras that people are wearing. I’m also seeing some from bathing suits and swim shirts.”
Exposure to products containing the preservative methylisothiazolinone (MI) is also on the rise. It ranks as the second most frequent allergen for which the North American Contact Dermatitis Group is seeing positive results on patch testing, with rates of 13.4%. MI can be found in many skin care products and “probably about half of school glues, fabric glues, and craft glues,” Dr. Katta said. “Stick versus liquid doesn’t make a difference.” Children and teens often use craft glues, laundry detergents, and other products to create “slime” as a way to learn about viscosity, polymers, and chemical reactions. “Sometimes these children have sensitive skin, or they’re using it with prolonged contact, so they may be sensitizing themselves to the MI,” she said.
She concluded her remarks by noting that an increasing number of young patients are developing reactions to wearable medical devices such as insulin pumps and glucose monitors. “With this, the first thing to think about is frictional irritant dermatitis,” she said. “You can put Scanpor medical paper tape on people’s back for 48 hours straight to patch test them. Some people are incredibly reactive to the friction of just that tape. You also have to think about trapped allergen. One of my patients reacted to colophony, fragrance mix, and propylene glycol, all of which were contained in his skin care products. Some people are getting advice from other patients to use Mastisol liquid adhesive to help their glucose monitors stick better. Mastisol has a high rate of cross-reactivity with balsam of Peru, so it’s a fragrance allergen. That’s the first thing you want to ask patients about: what products they’re using.”
One of her patients thought she was reacting to adhesive tape on her skin, but in fact she was reacting to two different acrylates: ethylene glycol dimethacrylate (EGDMA) and hydroxyethyl methacrylate (HEMA). “I know about these allergens because I see reactions from butterfly needles in dialysis patients,” Dr. Katta explained. “What happens is, these acrylates are glues or plastics used somewhere else on the device, and they can migrate through barriers.”
In one published case, a 9-year-old boy developed a reaction to ethyl cyanoacrylate contained in a glucose sensor adhesive (Dermatitis. 2017; 28[4]:289-91). It never touched the boy’s skin directly but was presumed to migrate through that tape. “The bottom line is that acrylates may induce contact dermatitis even through perceived barriers,” she said. “Their use anywhere in medical devices may prove problematic.”
Dr. Katta reported that she is a member of the advisory board for Vichy Laboratories.
AUSTIN – Not long ago, Rajani Katta, MD, received a text message from a friend who expressed concern about a rash that developed in the underarm of her teenage daughter.
The culprit turned out to be the lavender essential oil contained in an “all natural” deodorant that her daughter had recently switched to – a storyline that Dr. Katta encounters with increasing frequency in her role as clinical professor of dermatology at the University of Texas Health Science Center at Houston.
Dr. Katta said at the annual meeting of the Society for Pediatric Dermatology. “When you talk about a natural allergy, it is more likely to occur if your skin barrier is compromised, so I think that’s why we’re seeing it, especially in young girls in the underarm area. If you shave the underarm, you impair that skin barrier and you’re more likely to develop a reaction to something you’re using over it.”
Her list of recommended deodorants includes Almay Roll-On Antiperspirant & Deodorant, Crystal Body Deodorant Stick, Crystal Roll-On Body Deodorant, Vanicream Deodorant for Sensitive Skin (aluminum-free), Vanicream Antiperspirant/Deodorant, and CertainDri Clinical Strength Roll-On. They are fragrance-free and lack propylene glycol, which is a common allergen.
Increasingly, essential oils are being added to lip balms and toothpastes, said Dr. Katta, who is also author of the 2018 book “Glow: The Dermatologist’s Guide to a Whole Foods Younger Skin Diet.” She recalled one patient who presented with chronic chapped lips. “It doesn’t matter how many lip glosses I try; it just keeps getting worse,” the patient told her. The likely culprit turned out to be ingredients contained in flavored lip balm from EOS. Reports of blistering and cracking of the lips from use of the products prompted a class action lawsuit and a notice to consumers from the Food and Drug Administration.
Another patient presented with cracked lips after switching to an “all natural” toothpaste that was labeled “gluten free.”
“It looked great,” Dr. Katta recalled. “Unfortunately it was not flavoring free. She reacted to multiple essential oils, including tea tree oil, contained in the toothpaste. This is being added to a number of toothpastes, and I think we’re going to see more of these types of reactions.”
Other toothpastes contain balsam of Peru, “which is consistently one of the top allergens in patch test clinics,” she said. “One of the components of balsam of Peru is a cinnamon compound, which can be an issue.”
Dr. Katta advises her patients to use Vaseline petroleum jelly as a lip balm and recommends Tom’s of Maine Silly Strawberry Flavor (this flavor only) toothpaste for children.
A few years ago, a teenager presented to Dr. Katta with intense bullae on the dorsum of the foot after wearing shoes without socks. “She was wearing white canvas Keds, which looked very innocuous,” she said. Patch testing revealed that the teen reacted to four different rubber accelerators. “When we contacted the company, they [acknowledged] using rubber cement to make the canvas Keds,” Dr. Katta said. “Rubber cement is an adhesive and it does contain rubber accelerators. Later, I saw two cases of children who had walked around all day at the amusement park wearing their Sperry Topsiders without any socks. We couldn’t get any information from that manufacturer, but I suspect that they also use a rubber-based glue to make those shoes.” She characterized shoes as “a real setup for a foot allergy because you have friction, sweat that’s pulling allergen out of an object, and sweat is carrying it over, especially to the dorsum of the foot.”
Dr. Katta has also noticed an uptick in the number of young patients who develop allergic reactions to dyes used to make workout clothing. “If you ever see rashes that do not involve the axillary vault but do have peraxillary accentuation, think textile allergy,” she said. “We’re seeing a lot of reactions to disperse blue clothing dyes. When you think about textile allergy from the dyes, it tends to be the blue and black clothing. It’s more likely in the setting of synthetic fabrics because they leach out dyes more easily, and it’s more likely in the setting of sweat because sweat helps pull allergen out. I’m seeing it a lot from sports uniforms and tight black leggings and tight sports bras that people are wearing. I’m also seeing some from bathing suits and swim shirts.”
Exposure to products containing the preservative methylisothiazolinone (MI) is also on the rise. It ranks as the second most frequent allergen for which the North American Contact Dermatitis Group is seeing positive results on patch testing, with rates of 13.4%. MI can be found in many skin care products and “probably about half of school glues, fabric glues, and craft glues,” Dr. Katta said. “Stick versus liquid doesn’t make a difference.” Children and teens often use craft glues, laundry detergents, and other products to create “slime” as a way to learn about viscosity, polymers, and chemical reactions. “Sometimes these children have sensitive skin, or they’re using it with prolonged contact, so they may be sensitizing themselves to the MI,” she said.
She concluded her remarks by noting that an increasing number of young patients are developing reactions to wearable medical devices such as insulin pumps and glucose monitors. “With this, the first thing to think about is frictional irritant dermatitis,” she said. “You can put Scanpor medical paper tape on people’s back for 48 hours straight to patch test them. Some people are incredibly reactive to the friction of just that tape. You also have to think about trapped allergen. One of my patients reacted to colophony, fragrance mix, and propylene glycol, all of which were contained in his skin care products. Some people are getting advice from other patients to use Mastisol liquid adhesive to help their glucose monitors stick better. Mastisol has a high rate of cross-reactivity with balsam of Peru, so it’s a fragrance allergen. That’s the first thing you want to ask patients about: what products they’re using.”
One of her patients thought she was reacting to adhesive tape on her skin, but in fact she was reacting to two different acrylates: ethylene glycol dimethacrylate (EGDMA) and hydroxyethyl methacrylate (HEMA). “I know about these allergens because I see reactions from butterfly needles in dialysis patients,” Dr. Katta explained. “What happens is, these acrylates are glues or plastics used somewhere else on the device, and they can migrate through barriers.”
In one published case, a 9-year-old boy developed a reaction to ethyl cyanoacrylate contained in a glucose sensor adhesive (Dermatitis. 2017; 28[4]:289-91). It never touched the boy’s skin directly but was presumed to migrate through that tape. “The bottom line is that acrylates may induce contact dermatitis even through perceived barriers,” she said. “Their use anywhere in medical devices may prove problematic.”
Dr. Katta reported that she is a member of the advisory board for Vichy Laboratories.
EXPERT ANALYSIS FROM SPD 2019
Ibrutinib-venetoclax found highly active in hard-to-treat CLL
The strategy of simultaneously inhibiting proliferation and reactivating apoptosis can eradicate chronic lymphocytic leukemia (CLL) in a large share of patients, suggest results from the phase 2 CLARITY trial.
“Both ibrutinib and venetoclax are active in CLL with improved survival; however, as monotherapies, both currently are given until disease progression,” wrote Peter Hillmen, MBChB, PhD, St. James’s University Hospital, Leeds, England, and his colleagues.
In the single-arm, open-label trial, the investigators treated 53 patients with relapsed or refractory CLL with combination ibrutinib (Imbruvica), a small-molecule inhibitor of Bruton’s tyrosine kinase, and venetoclax (Venclexta), a small molecule inhibitor of the anti-apoptotic protein Bcl-2. The primary endpoint was MRD negativity, defined as presence of fewer than one CLL cell in 10,000 leukocytes, after 12 months of combination therapy.
Results reported in the Journal of Clinical Oncology showed that the combination was highly active, with 53% of patients achieving MRD negativity in the blood and 36% achieving MRD negativity in the marrow.
Most patients, 89%, had a treatment response, and slightly more than half, 51%, achieved a complete remission. With a median 21.1-month follow-up, only a single patient experienced progression and all were still alive.
Adverse effects were generally manageable. Grade 3-4 adverse events of special interest included 34 cases of neutropenia and 1 case of biochemical tumor lysis syndrome that was managed by delaying venetoclax.
“We have demonstrated promising efficacy that indicates potent synergy between ibrutinib and venetoclax for inducing MRD-negative responses with manageable adverse effects,” the investigators wrote. “The observation that a significant proportion of patients experience MRD-negative remission indicates that this combination can be given for a limited period and then stopped after patients achieve a deep remission.”
Whether the combination leads to permanent disease eradication in certain patients is still unclear, the investigators added.
The trial was supported by Bloodwise under the Trials Acceleration Programme, by the National Institute for Health Research Leeds Clinical Research Facility, and by an unrestricted educational grant from Janssen-Cilag and AbbVie. Ibrutinib was provided free of charge by Janssen-Cilag, and venetoclax was provided free of charge by AbbVie. Dr. Hillman reported financial relationships with Janssen, AbbVie, Roche, Pharmacyclics, and Gilead Sciences.
SOURCE: Hillmen P et al. J Clin Oncol. 2019 Jul 11. doi: 10.1200/JCO.19.00894.
The strategy of simultaneously inhibiting proliferation and reactivating apoptosis can eradicate chronic lymphocytic leukemia (CLL) in a large share of patients, suggest results from the phase 2 CLARITY trial.
“Both ibrutinib and venetoclax are active in CLL with improved survival; however, as monotherapies, both currently are given until disease progression,” wrote Peter Hillmen, MBChB, PhD, St. James’s University Hospital, Leeds, England, and his colleagues.
In the single-arm, open-label trial, the investigators treated 53 patients with relapsed or refractory CLL with combination ibrutinib (Imbruvica), a small-molecule inhibitor of Bruton’s tyrosine kinase, and venetoclax (Venclexta), a small molecule inhibitor of the anti-apoptotic protein Bcl-2. The primary endpoint was MRD negativity, defined as presence of fewer than one CLL cell in 10,000 leukocytes, after 12 months of combination therapy.
Results reported in the Journal of Clinical Oncology showed that the combination was highly active, with 53% of patients achieving MRD negativity in the blood and 36% achieving MRD negativity in the marrow.
Most patients, 89%, had a treatment response, and slightly more than half, 51%, achieved a complete remission. With a median 21.1-month follow-up, only a single patient experienced progression and all were still alive.
Adverse effects were generally manageable. Grade 3-4 adverse events of special interest included 34 cases of neutropenia and 1 case of biochemical tumor lysis syndrome that was managed by delaying venetoclax.
“We have demonstrated promising efficacy that indicates potent synergy between ibrutinib and venetoclax for inducing MRD-negative responses with manageable adverse effects,” the investigators wrote. “The observation that a significant proportion of patients experience MRD-negative remission indicates that this combination can be given for a limited period and then stopped after patients achieve a deep remission.”
Whether the combination leads to permanent disease eradication in certain patients is still unclear, the investigators added.
The trial was supported by Bloodwise under the Trials Acceleration Programme, by the National Institute for Health Research Leeds Clinical Research Facility, and by an unrestricted educational grant from Janssen-Cilag and AbbVie. Ibrutinib was provided free of charge by Janssen-Cilag, and venetoclax was provided free of charge by AbbVie. Dr. Hillman reported financial relationships with Janssen, AbbVie, Roche, Pharmacyclics, and Gilead Sciences.
SOURCE: Hillmen P et al. J Clin Oncol. 2019 Jul 11. doi: 10.1200/JCO.19.00894.
The strategy of simultaneously inhibiting proliferation and reactivating apoptosis can eradicate chronic lymphocytic leukemia (CLL) in a large share of patients, suggest results from the phase 2 CLARITY trial.
“Both ibrutinib and venetoclax are active in CLL with improved survival; however, as monotherapies, both currently are given until disease progression,” wrote Peter Hillmen, MBChB, PhD, St. James’s University Hospital, Leeds, England, and his colleagues.
In the single-arm, open-label trial, the investigators treated 53 patients with relapsed or refractory CLL with combination ibrutinib (Imbruvica), a small-molecule inhibitor of Bruton’s tyrosine kinase, and venetoclax (Venclexta), a small molecule inhibitor of the anti-apoptotic protein Bcl-2. The primary endpoint was MRD negativity, defined as presence of fewer than one CLL cell in 10,000 leukocytes, after 12 months of combination therapy.
Results reported in the Journal of Clinical Oncology showed that the combination was highly active, with 53% of patients achieving MRD negativity in the blood and 36% achieving MRD negativity in the marrow.
Most patients, 89%, had a treatment response, and slightly more than half, 51%, achieved a complete remission. With a median 21.1-month follow-up, only a single patient experienced progression and all were still alive.
Adverse effects were generally manageable. Grade 3-4 adverse events of special interest included 34 cases of neutropenia and 1 case of biochemical tumor lysis syndrome that was managed by delaying venetoclax.
“We have demonstrated promising efficacy that indicates potent synergy between ibrutinib and venetoclax for inducing MRD-negative responses with manageable adverse effects,” the investigators wrote. “The observation that a significant proportion of patients experience MRD-negative remission indicates that this combination can be given for a limited period and then stopped after patients achieve a deep remission.”
Whether the combination leads to permanent disease eradication in certain patients is still unclear, the investigators added.
The trial was supported by Bloodwise under the Trials Acceleration Programme, by the National Institute for Health Research Leeds Clinical Research Facility, and by an unrestricted educational grant from Janssen-Cilag and AbbVie. Ibrutinib was provided free of charge by Janssen-Cilag, and venetoclax was provided free of charge by AbbVie. Dr. Hillman reported financial relationships with Janssen, AbbVie, Roche, Pharmacyclics, and Gilead Sciences.
SOURCE: Hillmen P et al. J Clin Oncol. 2019 Jul 11. doi: 10.1200/JCO.19.00894.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
CAR T-cell therapy less effective in transformed follicular lymphoma
All complete responders with FL were still in remission at a median follow-up of 24 months, but the median duration of response was 10.2 months for patients with tFL.
Alexandre V. Hirayama, MD, of the Fred Hutchinson Cancer Research Center in Seattle, and colleagues reported these results in Blood.
The trial enrolled 21 adults with relapsed/refractory CD19+ B-cell malignancies, including 8 patients with FL and 13 with tFL. At baseline, the FL/tFL patients had a median age of 56 years (range, 51-62), and 67% were male. Most patients (n = 19) had stage III/IV disease, 17 had extranodal disease, 8 had bulky disease, and 6 had bone marrow involvement. The patients had received a median of 5 prior therapies (range, 2-8), and 13 had received a transplant.
In this study, patients received a lymphodepleting regimen of cyclophosphamide and fludarabine, followed by 2 x 106 CD19 CAR T cells/kg. Five patients (one with FL and four with tFL) also received bridging chemotherapy between leukapheresis and lymphodepletion.
Grade 1-2 cytokine release syndrome occurred in 50% of FL patients and 39% of tFL patients (P = .35). Grade 1-2 neurotoxicity occurred in 50% and 23%, respectively (P = .67). There were no cases of grade 3 or higher cytokine release syndrome or neurotoxicity.
Most FL patients (7 of 8; 88%) achieved a complete response (CR) to treatment, and all of these patients were still in CR at a median follow-up of 24 months (range, 5-37 months). One FL patient received a transplant while in CR.
Six of 13 tFL patients (46%) achieved a CR. At a median follow-up of 38 months (range, 3-39 months), the median duration of response was 10.2 months. The median progression-free survival was 11.2 months in patients who achieved a CR and 1.4 months in all tFL patients.
The researchers noted that peak CAR T-cell counts and the duration of CAR T-cell detection were similar between FL and tFL patients. However, tFL patients had higher serum interleukin-8 concentrations and higher lactate dehydrogenase levels before treatment.
Past research suggested that IL-8 mediates the recruitment of tumor-associated neutrophils, promotes diffuse large B-cell lymphoma progression, and can contribute to local immune suppression. Other studies have linked elevated lactate dehydrogenase to aggressive disease and a more immunosuppressive tumor microenvironment.
“Although these data raise the possibility that differences in the tumor microenvironment may, in part, contribute to differences in outcomes after CAR T-cell immunotherapy in FL and tFL patients, additional studies are required,” the researchers wrote.
This research was supported by the National Institutes of Health, the Life Science Discovery Fund, the Bezos family, the University of British Columbia Clinician Investigator Program, the Fred Hutchinson Cancer Research Center’s Immunotherapy Integrated Research Center, and Juno Therapeutics/Celgene.
The researchers disclosed relationships with Celgene, Juno Therapeutics, Lyell Immunopharma, Adaptive Biotechnologies, Nohla, Kite Pharma, Gilead, Genentech, Novartis, Eureka Therapeutics, Nektar Therapeutics, Caribou Biosciences, Precision Biosciences, Aptevo, Humanigen, and Allogene.
SOURCE: Hirayama AV et al. Blood. 2019 Jun 26. doi: 10.1182/blood.2019000905
All complete responders with FL were still in remission at a median follow-up of 24 months, but the median duration of response was 10.2 months for patients with tFL.
Alexandre V. Hirayama, MD, of the Fred Hutchinson Cancer Research Center in Seattle, and colleagues reported these results in Blood.
The trial enrolled 21 adults with relapsed/refractory CD19+ B-cell malignancies, including 8 patients with FL and 13 with tFL. At baseline, the FL/tFL patients had a median age of 56 years (range, 51-62), and 67% were male. Most patients (n = 19) had stage III/IV disease, 17 had extranodal disease, 8 had bulky disease, and 6 had bone marrow involvement. The patients had received a median of 5 prior therapies (range, 2-8), and 13 had received a transplant.
In this study, patients received a lymphodepleting regimen of cyclophosphamide and fludarabine, followed by 2 x 106 CD19 CAR T cells/kg. Five patients (one with FL and four with tFL) also received bridging chemotherapy between leukapheresis and lymphodepletion.
Grade 1-2 cytokine release syndrome occurred in 50% of FL patients and 39% of tFL patients (P = .35). Grade 1-2 neurotoxicity occurred in 50% and 23%, respectively (P = .67). There were no cases of grade 3 or higher cytokine release syndrome or neurotoxicity.
Most FL patients (7 of 8; 88%) achieved a complete response (CR) to treatment, and all of these patients were still in CR at a median follow-up of 24 months (range, 5-37 months). One FL patient received a transplant while in CR.
Six of 13 tFL patients (46%) achieved a CR. At a median follow-up of 38 months (range, 3-39 months), the median duration of response was 10.2 months. The median progression-free survival was 11.2 months in patients who achieved a CR and 1.4 months in all tFL patients.
The researchers noted that peak CAR T-cell counts and the duration of CAR T-cell detection were similar between FL and tFL patients. However, tFL patients had higher serum interleukin-8 concentrations and higher lactate dehydrogenase levels before treatment.
Past research suggested that IL-8 mediates the recruitment of tumor-associated neutrophils, promotes diffuse large B-cell lymphoma progression, and can contribute to local immune suppression. Other studies have linked elevated lactate dehydrogenase to aggressive disease and a more immunosuppressive tumor microenvironment.
“Although these data raise the possibility that differences in the tumor microenvironment may, in part, contribute to differences in outcomes after CAR T-cell immunotherapy in FL and tFL patients, additional studies are required,” the researchers wrote.
This research was supported by the National Institutes of Health, the Life Science Discovery Fund, the Bezos family, the University of British Columbia Clinician Investigator Program, the Fred Hutchinson Cancer Research Center’s Immunotherapy Integrated Research Center, and Juno Therapeutics/Celgene.
The researchers disclosed relationships with Celgene, Juno Therapeutics, Lyell Immunopharma, Adaptive Biotechnologies, Nohla, Kite Pharma, Gilead, Genentech, Novartis, Eureka Therapeutics, Nektar Therapeutics, Caribou Biosciences, Precision Biosciences, Aptevo, Humanigen, and Allogene.
SOURCE: Hirayama AV et al. Blood. 2019 Jun 26. doi: 10.1182/blood.2019000905
All complete responders with FL were still in remission at a median follow-up of 24 months, but the median duration of response was 10.2 months for patients with tFL.
Alexandre V. Hirayama, MD, of the Fred Hutchinson Cancer Research Center in Seattle, and colleagues reported these results in Blood.
The trial enrolled 21 adults with relapsed/refractory CD19+ B-cell malignancies, including 8 patients with FL and 13 with tFL. At baseline, the FL/tFL patients had a median age of 56 years (range, 51-62), and 67% were male. Most patients (n = 19) had stage III/IV disease, 17 had extranodal disease, 8 had bulky disease, and 6 had bone marrow involvement. The patients had received a median of 5 prior therapies (range, 2-8), and 13 had received a transplant.
In this study, patients received a lymphodepleting regimen of cyclophosphamide and fludarabine, followed by 2 x 106 CD19 CAR T cells/kg. Five patients (one with FL and four with tFL) also received bridging chemotherapy between leukapheresis and lymphodepletion.
Grade 1-2 cytokine release syndrome occurred in 50% of FL patients and 39% of tFL patients (P = .35). Grade 1-2 neurotoxicity occurred in 50% and 23%, respectively (P = .67). There were no cases of grade 3 or higher cytokine release syndrome or neurotoxicity.
Most FL patients (7 of 8; 88%) achieved a complete response (CR) to treatment, and all of these patients were still in CR at a median follow-up of 24 months (range, 5-37 months). One FL patient received a transplant while in CR.
Six of 13 tFL patients (46%) achieved a CR. At a median follow-up of 38 months (range, 3-39 months), the median duration of response was 10.2 months. The median progression-free survival was 11.2 months in patients who achieved a CR and 1.4 months in all tFL patients.
The researchers noted that peak CAR T-cell counts and the duration of CAR T-cell detection were similar between FL and tFL patients. However, tFL patients had higher serum interleukin-8 concentrations and higher lactate dehydrogenase levels before treatment.
Past research suggested that IL-8 mediates the recruitment of tumor-associated neutrophils, promotes diffuse large B-cell lymphoma progression, and can contribute to local immune suppression. Other studies have linked elevated lactate dehydrogenase to aggressive disease and a more immunosuppressive tumor microenvironment.
“Although these data raise the possibility that differences in the tumor microenvironment may, in part, contribute to differences in outcomes after CAR T-cell immunotherapy in FL and tFL patients, additional studies are required,” the researchers wrote.
This research was supported by the National Institutes of Health, the Life Science Discovery Fund, the Bezos family, the University of British Columbia Clinician Investigator Program, the Fred Hutchinson Cancer Research Center’s Immunotherapy Integrated Research Center, and Juno Therapeutics/Celgene.
The researchers disclosed relationships with Celgene, Juno Therapeutics, Lyell Immunopharma, Adaptive Biotechnologies, Nohla, Kite Pharma, Gilead, Genentech, Novartis, Eureka Therapeutics, Nektar Therapeutics, Caribou Biosciences, Precision Biosciences, Aptevo, Humanigen, and Allogene.
SOURCE: Hirayama AV et al. Blood. 2019 Jun 26. doi: 10.1182/blood.2019000905
FROM BLOOD
Cases of pediatric invasive melanoma have declined since 2002, study finds
AUSTIN – The compared with females. The risk of death is also significantly increased in black patients, other nonwhite patients, and in cases where surgery was not performed.
Those are key findings from a study that set out to investigate the incidence of pediatric melanoma over the last 2 decades and factors influencing survival. At the annual meeting of the Society for Pediatric Dermatology, one of the study authors, Spandana Maddukuri, said that pediatric melanoma is the most common skin cancer in the pediatric population, accounting for 1-3% of all pediatric malignancies and 1%-4% of all cases of melanoma (Pediatr Surg. 2013;48[11]:2207-13).
“Nonmodifiable risk factors are similar to those in adult melanoma and include fair skin, light hair and eye color, increased number of congenital nevi, and family history of melanoma,” said Ms. Maddukuri, a third-year student at New Jersey Medical School, Newark. “Environmental risk factors are similar to those in adult melanoma and include exposure to UV radiation. About 60% of children do not meet standard ABCDE [asymmetrical, border, color, diameter, evolving] diagnosis criteria, which often leads to delayed diagnosis.”
Some of the characteristics that are more commonly found in pediatric lesions include amelanosis, bleeding, uniform color, and variable diameter (J Am Acad Dermatol. 2013; 68[6]:913-25).
Ms. Maddukuri and colleagues queried the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) database for cases of malignant melanoma that were diagnosed in individuals aged younger than 20 years between 2002 and 2015. After excluding all cases of adult melanoma and all cases of in situ melanoma, they included 1,620 patients in the final analysis and divided them into five age groups: less than 1 year, 1-4 years, 5-9 years, 10-14 years, and 15-19 years. They calculated the overall incidence rate per 100,000 population of pediatric melanoma based on data from the 2000 U.S. Census. Age-, sex-, and race-specific incidence rates were also calculated. Kaplan-Meier and Cox regression analyses to investigate disease-specific survival and risk factors.
With each successive age group, the investigators observed that incidence rate was significantly higher than that of the previous age group (P less than .005). “However, the most striking increase in incidence occurs between the age group of 10-14 and 15-19,” she said. “Sex also influenced incidence rates. Males had an incidence rate of 0.396 per 100,000 population while females had an incidence rate of 0.579 per 100,000 population.”
Race also influenced incidence rates. White patients had the highest incidence rate of 0.605 per 100,000 population, while blacks had the lowest incident rate at 0.042 per 100,000 population. American Indian and Alaska Native patients had incidence rates of 0.046 per 100,000 population, while Asians and Pacific Islanders had an incidence rate of 0.127 per 100,000 population.
The researchers found that increased survival was associated with white race, female sex, treatment with surgical intervention, and age older than 5 years. No differences in survival were observed regarding the primary anatomic location or extent of disease. The hazard ratio of death from invasive melanoma was significantly increased in males (HR, 2.34), black patients (HR, 3.96), other nonwhite patients (HR, 3.64), and in cases where surgery was not performed (HR, 6.04).
“It is surprising that, although incidence is significantly higher in white patients and females, compared to black patients and males, respectively, the risk of dying from melanoma is much higher in black patients and males,” Ms. Maddukuri said in an interview at the meeting. “Overall, the dermatologic community is on the right track in screening and diagnosing pediatric melanoma, as seen by the decreased incidence over the last 2 decades. However, increased awareness regarding pediatric melanoma is still encouraged. I believe we were able to identify certain populations that need more attention in terms of screening, diagnosis, and treatment, which are patients less than 5 years old, black and other nonwhite patients, and males.”
She acknowledged certain shortcomings of the study, including a limited clinical history of the patient population because of the nature of the database. She also said that further studies are required to investigate the contributing factors to decreasing incidence and to evaluate the relationship of the favorable prognostic factors to increased survival. The researchers are currently working on correlating incidence rates with UV exposure and geographical location.
They reported having no financial disclosures.
AUSTIN – The compared with females. The risk of death is also significantly increased in black patients, other nonwhite patients, and in cases where surgery was not performed.
Those are key findings from a study that set out to investigate the incidence of pediatric melanoma over the last 2 decades and factors influencing survival. At the annual meeting of the Society for Pediatric Dermatology, one of the study authors, Spandana Maddukuri, said that pediatric melanoma is the most common skin cancer in the pediatric population, accounting for 1-3% of all pediatric malignancies and 1%-4% of all cases of melanoma (Pediatr Surg. 2013;48[11]:2207-13).
“Nonmodifiable risk factors are similar to those in adult melanoma and include fair skin, light hair and eye color, increased number of congenital nevi, and family history of melanoma,” said Ms. Maddukuri, a third-year student at New Jersey Medical School, Newark. “Environmental risk factors are similar to those in adult melanoma and include exposure to UV radiation. About 60% of children do not meet standard ABCDE [asymmetrical, border, color, diameter, evolving] diagnosis criteria, which often leads to delayed diagnosis.”
Some of the characteristics that are more commonly found in pediatric lesions include amelanosis, bleeding, uniform color, and variable diameter (J Am Acad Dermatol. 2013; 68[6]:913-25).
Ms. Maddukuri and colleagues queried the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) database for cases of malignant melanoma that were diagnosed in individuals aged younger than 20 years between 2002 and 2015. After excluding all cases of adult melanoma and all cases of in situ melanoma, they included 1,620 patients in the final analysis and divided them into five age groups: less than 1 year, 1-4 years, 5-9 years, 10-14 years, and 15-19 years. They calculated the overall incidence rate per 100,000 population of pediatric melanoma based on data from the 2000 U.S. Census. Age-, sex-, and race-specific incidence rates were also calculated. Kaplan-Meier and Cox regression analyses to investigate disease-specific survival and risk factors.
With each successive age group, the investigators observed that incidence rate was significantly higher than that of the previous age group (P less than .005). “However, the most striking increase in incidence occurs between the age group of 10-14 and 15-19,” she said. “Sex also influenced incidence rates. Males had an incidence rate of 0.396 per 100,000 population while females had an incidence rate of 0.579 per 100,000 population.”
Race also influenced incidence rates. White patients had the highest incidence rate of 0.605 per 100,000 population, while blacks had the lowest incident rate at 0.042 per 100,000 population. American Indian and Alaska Native patients had incidence rates of 0.046 per 100,000 population, while Asians and Pacific Islanders had an incidence rate of 0.127 per 100,000 population.
The researchers found that increased survival was associated with white race, female sex, treatment with surgical intervention, and age older than 5 years. No differences in survival were observed regarding the primary anatomic location or extent of disease. The hazard ratio of death from invasive melanoma was significantly increased in males (HR, 2.34), black patients (HR, 3.96), other nonwhite patients (HR, 3.64), and in cases where surgery was not performed (HR, 6.04).
“It is surprising that, although incidence is significantly higher in white patients and females, compared to black patients and males, respectively, the risk of dying from melanoma is much higher in black patients and males,” Ms. Maddukuri said in an interview at the meeting. “Overall, the dermatologic community is on the right track in screening and diagnosing pediatric melanoma, as seen by the decreased incidence over the last 2 decades. However, increased awareness regarding pediatric melanoma is still encouraged. I believe we were able to identify certain populations that need more attention in terms of screening, diagnosis, and treatment, which are patients less than 5 years old, black and other nonwhite patients, and males.”
She acknowledged certain shortcomings of the study, including a limited clinical history of the patient population because of the nature of the database. She also said that further studies are required to investigate the contributing factors to decreasing incidence and to evaluate the relationship of the favorable prognostic factors to increased survival. The researchers are currently working on correlating incidence rates with UV exposure and geographical location.
They reported having no financial disclosures.
AUSTIN – The compared with females. The risk of death is also significantly increased in black patients, other nonwhite patients, and in cases where surgery was not performed.
Those are key findings from a study that set out to investigate the incidence of pediatric melanoma over the last 2 decades and factors influencing survival. At the annual meeting of the Society for Pediatric Dermatology, one of the study authors, Spandana Maddukuri, said that pediatric melanoma is the most common skin cancer in the pediatric population, accounting for 1-3% of all pediatric malignancies and 1%-4% of all cases of melanoma (Pediatr Surg. 2013;48[11]:2207-13).
“Nonmodifiable risk factors are similar to those in adult melanoma and include fair skin, light hair and eye color, increased number of congenital nevi, and family history of melanoma,” said Ms. Maddukuri, a third-year student at New Jersey Medical School, Newark. “Environmental risk factors are similar to those in adult melanoma and include exposure to UV radiation. About 60% of children do not meet standard ABCDE [asymmetrical, border, color, diameter, evolving] diagnosis criteria, which often leads to delayed diagnosis.”
Some of the characteristics that are more commonly found in pediatric lesions include amelanosis, bleeding, uniform color, and variable diameter (J Am Acad Dermatol. 2013; 68[6]:913-25).
Ms. Maddukuri and colleagues queried the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) database for cases of malignant melanoma that were diagnosed in individuals aged younger than 20 years between 2002 and 2015. After excluding all cases of adult melanoma and all cases of in situ melanoma, they included 1,620 patients in the final analysis and divided them into five age groups: less than 1 year, 1-4 years, 5-9 years, 10-14 years, and 15-19 years. They calculated the overall incidence rate per 100,000 population of pediatric melanoma based on data from the 2000 U.S. Census. Age-, sex-, and race-specific incidence rates were also calculated. Kaplan-Meier and Cox regression analyses to investigate disease-specific survival and risk factors.
With each successive age group, the investigators observed that incidence rate was significantly higher than that of the previous age group (P less than .005). “However, the most striking increase in incidence occurs between the age group of 10-14 and 15-19,” she said. “Sex also influenced incidence rates. Males had an incidence rate of 0.396 per 100,000 population while females had an incidence rate of 0.579 per 100,000 population.”
Race also influenced incidence rates. White patients had the highest incidence rate of 0.605 per 100,000 population, while blacks had the lowest incident rate at 0.042 per 100,000 population. American Indian and Alaska Native patients had incidence rates of 0.046 per 100,000 population, while Asians and Pacific Islanders had an incidence rate of 0.127 per 100,000 population.
The researchers found that increased survival was associated with white race, female sex, treatment with surgical intervention, and age older than 5 years. No differences in survival were observed regarding the primary anatomic location or extent of disease. The hazard ratio of death from invasive melanoma was significantly increased in males (HR, 2.34), black patients (HR, 3.96), other nonwhite patients (HR, 3.64), and in cases where surgery was not performed (HR, 6.04).
“It is surprising that, although incidence is significantly higher in white patients and females, compared to black patients and males, respectively, the risk of dying from melanoma is much higher in black patients and males,” Ms. Maddukuri said in an interview at the meeting. “Overall, the dermatologic community is on the right track in screening and diagnosing pediatric melanoma, as seen by the decreased incidence over the last 2 decades. However, increased awareness regarding pediatric melanoma is still encouraged. I believe we were able to identify certain populations that need more attention in terms of screening, diagnosis, and treatment, which are patients less than 5 years old, black and other nonwhite patients, and males.”
She acknowledged certain shortcomings of the study, including a limited clinical history of the patient population because of the nature of the database. She also said that further studies are required to investigate the contributing factors to decreasing incidence and to evaluate the relationship of the favorable prognostic factors to increased survival. The researchers are currently working on correlating incidence rates with UV exposure and geographical location.
They reported having no financial disclosures.
REPORTING FROM SPD 2019
Colorectal cancer diagnoses still moving up in younger adults, with no sign of plateau
Colorectal cancer (CRC) incidence continues to rise in younger adults, with no signs of plateauing, according to investigators who recently conducted an analysis of a large US cancer registry.
Adults aged 50 years and younger accounted for about 12% of colorectal cancer diagnoses in 2015, up from 10% in 2004, and significantly more of those younger patients had advanced disease at diagnosis as compared to older adults, according to the analysis of the National Cancer Database (NCDB) by Boone Goodgame, MD, of the University of Texas at Austin, and colleagues.
“These results may provide support for adjusting CRC screening guidelines to identify patients before the age of 50 years,” said Dr. Goodgame and study coauthors in Cancer.
Only 5.8% of colorectal cancer cases were diagnosed in individuals younger than 45 years, suggesting that age may be an “appropriate target” for the screening age, the authors said in their report, alluding to the 2018 qualified recommendation from the American Cancer Society to begin screening at age 45.
However, a member of the U.S. Preventive Services Task Force – which continues to recommend screening of asymptomatic adults starting at 50 years – said in an editorial that it remains “unknown” whether the harms of screening for sporadic cases of colorectal cancer in younger individuals would outweigh the benefits.
The study by Dr. Goodgame and colleagues included a total of 1,185,763 colorectal cancer cases in the NCDB during 2004-2015, of which 89% were diagnosed at the age of 50 or older, and 11% were diagnosed in younger individuals.
The proportion of colorectal cancer cases diagnosed in people aged 50 years and younger increased from 10.0% to 12.2% during the study period (P less than .0001), with comparable increases for both rectal and colon primary tumors, according to the the journal article.
Younger patients were more likely to have stage III and stage IV disease than older patients were, according to the investigators. Stage III disease was reported for 28.1% and 23.1% of younger individuals and those over 50 years, respectively, while stage IV disease was reported for 23.5% and 16.9% (P less than .0001 for both comparisons).
Race and sex differences were seen in proportions of patients younger than 50 years with colorectal cancer, further analysis of the NCDB data showed.
Among men, only non-Hispanic whites had a significant increase in colorectal cancer diagnoses under the age of 50 years over the study period, while in women, significant increases were seen in Hispanic and non-Hispanic whites, according to the report.
It’s unclear exactly what’s behind the increase in colorectal cancer diagnosis, the authors acknowledged in their report, citing a host of potentially explanatory factors, such as access to health care, lifestyle factors such as obesity, or increased antibiotic use.
Some say the increase could simply be from the more liberal use of colonoscopy, resulting in a lead time bias, the authors noted.
“However, a change in the lead-time bias should also increase the proportion of earlier stage disease in younger adults, and we did not see this in our study,” they said in the report. “Therefore, increasing colonoscopy use does not appear to be a sufficient explanation for this association.”
In any case, more studies are needed to better determine the risks, benefits, and costs of screening individuals younger than 50 years for colorectal cancer, they concluded, saying that their data should be included in an “ongoing discussion” of screening guidelines.
Dr. Goodgame and coauthors made no conflict of interest disclosures related to the study, which was supported by the National Cancer Institute and the Cancer Prevention and Research Institute of Texas.
SOURCE: Virostko J et al. Cancer. 2019 Jul 22. doi: 10.1002/cncr.32347.
This study of the U.S. National Cancer Data Base by Virostko et al. shows that the proportion of colorectal cancer cases diagnosed before the age of 50 years continues to increase. According to investigators, those findings may provide support for adjusting screening guidelines downward.
While those findings are “provocative,” colorectal cancer remains a very rare condition in younger individuals, in whom heritable risks play a larger role than in older individuals, according to Chyke A. Doubeni, MD, MPH, a member of the U.S. Preventive Services Task Force.
“Because the number of colorectal cancer cases from inherited causes is much higher in younger individuals, it is unknown whether screening for sporadic cases in a group with such low disease rates would result in a favorable balance of harms and benefits,” he wrote in an editorial accompanying the report.
To determine whether changing the screening age for individuals not at risk is the most appropriate public health response, multiple hypotheses need to be tested as to why colorectal cancer incidence is increasing in younger people, according to Dr. Doubeni.
“A core principle of population-based screening is to do no more harm around the time of screening than the potential future health benefits,” he said.
While evidence is needed to show that routine screening is as effective in preventing colorectal cancer deaths in younger individuals as it is in older individuals, it’s unlikely that empirical studies could be designed to adequately assess that endpoint, according to Dr. Doubeni.
“At a population level, a decrease in the average age at diagnosis or in the proportion of colorectal cancers in people 50 years and older and a shift to an earlier stage at diagnosis may all suggest that current preventive interventions are effective,” he said in the editorial.
Dr. Doubeni is a member of the U.S. Preventive Services Task Force. He reported no specific funding support related to his editorial, which appears in the journal Cancer .
This study of the U.S. National Cancer Data Base by Virostko et al. shows that the proportion of colorectal cancer cases diagnosed before the age of 50 years continues to increase. According to investigators, those findings may provide support for adjusting screening guidelines downward.
While those findings are “provocative,” colorectal cancer remains a very rare condition in younger individuals, in whom heritable risks play a larger role than in older individuals, according to Chyke A. Doubeni, MD, MPH, a member of the U.S. Preventive Services Task Force.
“Because the number of colorectal cancer cases from inherited causes is much higher in younger individuals, it is unknown whether screening for sporadic cases in a group with such low disease rates would result in a favorable balance of harms and benefits,” he wrote in an editorial accompanying the report.
To determine whether changing the screening age for individuals not at risk is the most appropriate public health response, multiple hypotheses need to be tested as to why colorectal cancer incidence is increasing in younger people, according to Dr. Doubeni.
“A core principle of population-based screening is to do no more harm around the time of screening than the potential future health benefits,” he said.
While evidence is needed to show that routine screening is as effective in preventing colorectal cancer deaths in younger individuals as it is in older individuals, it’s unlikely that empirical studies could be designed to adequately assess that endpoint, according to Dr. Doubeni.
“At a population level, a decrease in the average age at diagnosis or in the proportion of colorectal cancers in people 50 years and older and a shift to an earlier stage at diagnosis may all suggest that current preventive interventions are effective,” he said in the editorial.
Dr. Doubeni is a member of the U.S. Preventive Services Task Force. He reported no specific funding support related to his editorial, which appears in the journal Cancer .
This study of the U.S. National Cancer Data Base by Virostko et al. shows that the proportion of colorectal cancer cases diagnosed before the age of 50 years continues to increase. According to investigators, those findings may provide support for adjusting screening guidelines downward.
While those findings are “provocative,” colorectal cancer remains a very rare condition in younger individuals, in whom heritable risks play a larger role than in older individuals, according to Chyke A. Doubeni, MD, MPH, a member of the U.S. Preventive Services Task Force.
“Because the number of colorectal cancer cases from inherited causes is much higher in younger individuals, it is unknown whether screening for sporadic cases in a group with such low disease rates would result in a favorable balance of harms and benefits,” he wrote in an editorial accompanying the report.
To determine whether changing the screening age for individuals not at risk is the most appropriate public health response, multiple hypotheses need to be tested as to why colorectal cancer incidence is increasing in younger people, according to Dr. Doubeni.
“A core principle of population-based screening is to do no more harm around the time of screening than the potential future health benefits,” he said.
While evidence is needed to show that routine screening is as effective in preventing colorectal cancer deaths in younger individuals as it is in older individuals, it’s unlikely that empirical studies could be designed to adequately assess that endpoint, according to Dr. Doubeni.
“At a population level, a decrease in the average age at diagnosis or in the proportion of colorectal cancers in people 50 years and older and a shift to an earlier stage at diagnosis may all suggest that current preventive interventions are effective,” he said in the editorial.
Dr. Doubeni is a member of the U.S. Preventive Services Task Force. He reported no specific funding support related to his editorial, which appears in the journal Cancer .
Colorectal cancer (CRC) incidence continues to rise in younger adults, with no signs of plateauing, according to investigators who recently conducted an analysis of a large US cancer registry.
Adults aged 50 years and younger accounted for about 12% of colorectal cancer diagnoses in 2015, up from 10% in 2004, and significantly more of those younger patients had advanced disease at diagnosis as compared to older adults, according to the analysis of the National Cancer Database (NCDB) by Boone Goodgame, MD, of the University of Texas at Austin, and colleagues.
“These results may provide support for adjusting CRC screening guidelines to identify patients before the age of 50 years,” said Dr. Goodgame and study coauthors in Cancer.
Only 5.8% of colorectal cancer cases were diagnosed in individuals younger than 45 years, suggesting that age may be an “appropriate target” for the screening age, the authors said in their report, alluding to the 2018 qualified recommendation from the American Cancer Society to begin screening at age 45.
However, a member of the U.S. Preventive Services Task Force – which continues to recommend screening of asymptomatic adults starting at 50 years – said in an editorial that it remains “unknown” whether the harms of screening for sporadic cases of colorectal cancer in younger individuals would outweigh the benefits.
The study by Dr. Goodgame and colleagues included a total of 1,185,763 colorectal cancer cases in the NCDB during 2004-2015, of which 89% were diagnosed at the age of 50 or older, and 11% were diagnosed in younger individuals.
The proportion of colorectal cancer cases diagnosed in people aged 50 years and younger increased from 10.0% to 12.2% during the study period (P less than .0001), with comparable increases for both rectal and colon primary tumors, according to the the journal article.
Younger patients were more likely to have stage III and stage IV disease than older patients were, according to the investigators. Stage III disease was reported for 28.1% and 23.1% of younger individuals and those over 50 years, respectively, while stage IV disease was reported for 23.5% and 16.9% (P less than .0001 for both comparisons).
Race and sex differences were seen in proportions of patients younger than 50 years with colorectal cancer, further analysis of the NCDB data showed.
Among men, only non-Hispanic whites had a significant increase in colorectal cancer diagnoses under the age of 50 years over the study period, while in women, significant increases were seen in Hispanic and non-Hispanic whites, according to the report.
It’s unclear exactly what’s behind the increase in colorectal cancer diagnosis, the authors acknowledged in their report, citing a host of potentially explanatory factors, such as access to health care, lifestyle factors such as obesity, or increased antibiotic use.
Some say the increase could simply be from the more liberal use of colonoscopy, resulting in a lead time bias, the authors noted.
“However, a change in the lead-time bias should also increase the proportion of earlier stage disease in younger adults, and we did not see this in our study,” they said in the report. “Therefore, increasing colonoscopy use does not appear to be a sufficient explanation for this association.”
In any case, more studies are needed to better determine the risks, benefits, and costs of screening individuals younger than 50 years for colorectal cancer, they concluded, saying that their data should be included in an “ongoing discussion” of screening guidelines.
Dr. Goodgame and coauthors made no conflict of interest disclosures related to the study, which was supported by the National Cancer Institute and the Cancer Prevention and Research Institute of Texas.
SOURCE: Virostko J et al. Cancer. 2019 Jul 22. doi: 10.1002/cncr.32347.
Colorectal cancer (CRC) incidence continues to rise in younger adults, with no signs of plateauing, according to investigators who recently conducted an analysis of a large US cancer registry.
Adults aged 50 years and younger accounted for about 12% of colorectal cancer diagnoses in 2015, up from 10% in 2004, and significantly more of those younger patients had advanced disease at diagnosis as compared to older adults, according to the analysis of the National Cancer Database (NCDB) by Boone Goodgame, MD, of the University of Texas at Austin, and colleagues.
“These results may provide support for adjusting CRC screening guidelines to identify patients before the age of 50 years,” said Dr. Goodgame and study coauthors in Cancer.
Only 5.8% of colorectal cancer cases were diagnosed in individuals younger than 45 years, suggesting that age may be an “appropriate target” for the screening age, the authors said in their report, alluding to the 2018 qualified recommendation from the American Cancer Society to begin screening at age 45.
However, a member of the U.S. Preventive Services Task Force – which continues to recommend screening of asymptomatic adults starting at 50 years – said in an editorial that it remains “unknown” whether the harms of screening for sporadic cases of colorectal cancer in younger individuals would outweigh the benefits.
The study by Dr. Goodgame and colleagues included a total of 1,185,763 colorectal cancer cases in the NCDB during 2004-2015, of which 89% were diagnosed at the age of 50 or older, and 11% were diagnosed in younger individuals.
The proportion of colorectal cancer cases diagnosed in people aged 50 years and younger increased from 10.0% to 12.2% during the study period (P less than .0001), with comparable increases for both rectal and colon primary tumors, according to the the journal article.
Younger patients were more likely to have stage III and stage IV disease than older patients were, according to the investigators. Stage III disease was reported for 28.1% and 23.1% of younger individuals and those over 50 years, respectively, while stage IV disease was reported for 23.5% and 16.9% (P less than .0001 for both comparisons).
Race and sex differences were seen in proportions of patients younger than 50 years with colorectal cancer, further analysis of the NCDB data showed.
Among men, only non-Hispanic whites had a significant increase in colorectal cancer diagnoses under the age of 50 years over the study period, while in women, significant increases were seen in Hispanic and non-Hispanic whites, according to the report.
It’s unclear exactly what’s behind the increase in colorectal cancer diagnosis, the authors acknowledged in their report, citing a host of potentially explanatory factors, such as access to health care, lifestyle factors such as obesity, or increased antibiotic use.
Some say the increase could simply be from the more liberal use of colonoscopy, resulting in a lead time bias, the authors noted.
“However, a change in the lead-time bias should also increase the proportion of earlier stage disease in younger adults, and we did not see this in our study,” they said in the report. “Therefore, increasing colonoscopy use does not appear to be a sufficient explanation for this association.”
In any case, more studies are needed to better determine the risks, benefits, and costs of screening individuals younger than 50 years for colorectal cancer, they concluded, saying that their data should be included in an “ongoing discussion” of screening guidelines.
Dr. Goodgame and coauthors made no conflict of interest disclosures related to the study, which was supported by the National Cancer Institute and the Cancer Prevention and Research Institute of Texas.
SOURCE: Virostko J et al. Cancer. 2019 Jul 22. doi: 10.1002/cncr.32347.
FROM CANCER
Key clinical point: Colorectal cancer incidence continues to rise in younger adults, with no signs of plateauing.
Major finding: Adults aged 50 years and younger accounted for about 12% of colorectal cancer diagnoses in 2015, up from 10% in 2004. Significantly more younger patients had advanced disease at diagnosis, compared with older adults.
Study details: Analysis of the National Cancer Data Base including 1,185,763 colorectal cancer cases diagnosed during 2004-2015.
Disclosures: Authors made no conflict of interest disclosures related to the study, which was supported by the National Cancer Institute and the Cancer Prevention and Research Institute of Texas.
Source: Virostko J et al. Cancer. 2019 Jul 22. doi: 10.1002/cncr.32347.
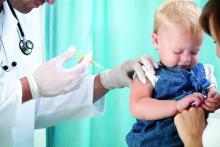
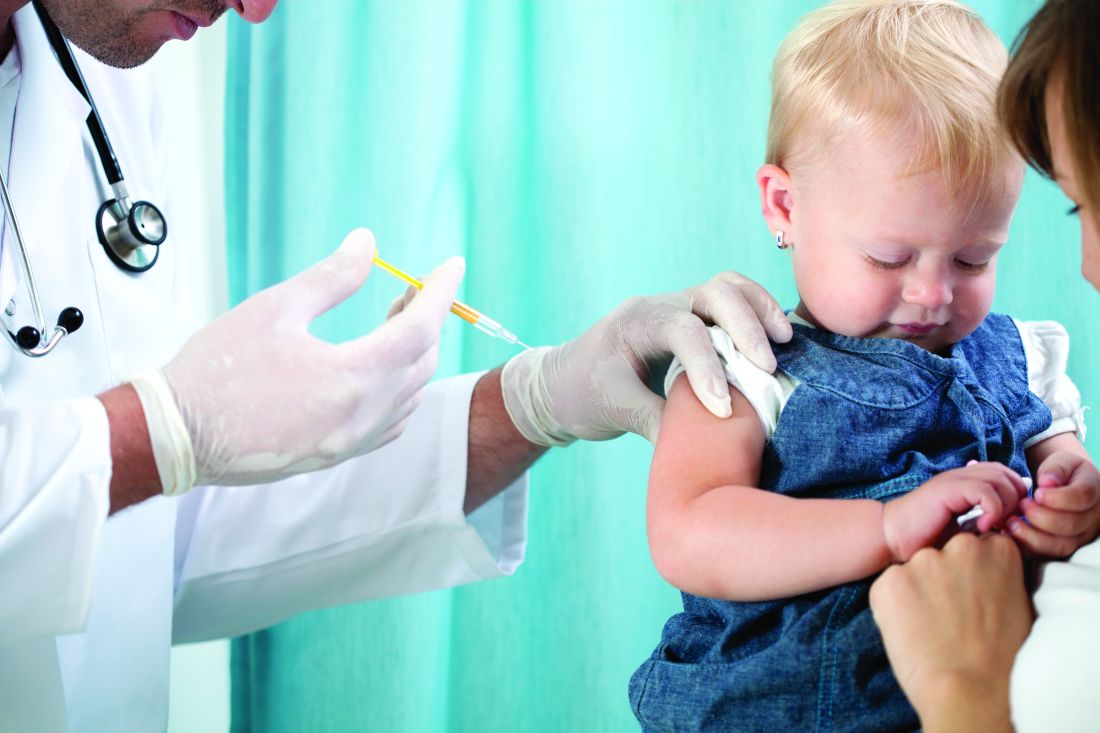
Adjuvanted flu vaccine performs better than others in young children
according to an industry-funded synthesis of six studies.
The vaccine “offers significant advances over conventional inactivated influenza vaccines and presents an acceptable safety profile in children 6 months through 5 years of age,” Sanjay S. Patel, PhD, of Novartis Vaccines and Diagnostics, Cambridge, Mass., and associates wrote in the analysis, published in the International Journal of Infectious Diseases. “The noteworthy increases in antibody responses and decreases in influenza cases following vaccination suggest an alternative for use in a population that is heavily impacted by influenza disease.”
Children are, of course, vulnerable to flu. The Centers for Disease Control and Prevention reported that 186 children died of flu during the landmark 2017-2018 flu season. That’s the highest number of pediatric flu deaths since they became a notifiable condition in 2004 (exclusive of the 2009 pandemic, when 358 pediatric deaths were reported from April 15, 2009, to October 2, 2010).The CDC said the vaccine during that flu season had an overall effectiveness level of 40%. According to research of others, however, flu vaccines are less effective in younger children than in adolescents and adults (Vaccine. 2014;32[31]:3886-94; Cochrane Database Syst Rev. 2008. doi: 10.1002/14651858.CD004879.pub3).
Fluad – a MF59-adjuvanted inactivated trivalent seasonal influenza vaccine – is used in adults over 65 in the United States and 29 other countries, and it is approved for children aged 6 months through 23 months in Canada.
Dr. Patel and associates examined the results of six studies – one phase 1b, three phase 2, and two phase 3 – that tested Fluad with or without other vaccines in 11,942 children aged 6 months to 5 years. The studies, mostly multicenter, were conducted in various countries, mainly in Europe and South and Central America, from 2006 to 2012.
In general, children in the intervention groups in the studies received two doses of the Fluad vaccine 4 weeks apart: two 0.25-mL doses for children aged 6-35 months and two 0.5-mL doses for those aged 3 years or older. In most of the studies, parallel control groups received nonadjuvanted trivalent or quadrivalent influenza vaccines.
Most participants (93%-94%) completed the studies. Solicited adverse effects were common in all groups (72% in the Fluad group vs. 67% who received IIV3 vaccines), and generally mild to moderate and resolved in 1-3 days. Unsolicited adverse effects were similar (55% and 62%, respectively) in the two flu vaccine groups. The authors wrote that “these data reflect a safety profile consistent with other licensed inactivated influenza vaccines administered to children.”
As for results, Dr. Patel and colleagues said, “HI [hemagglutination inhibition] antibody responses to both homologous and heterologous influenza strains are higher following vaccination with aIIV3, and this increase in immunogenicity is observed across all age subgroups in children aged 6 months through 5 years, and most profound in the children 6 to 36 months.”
For example, in one of the phase 3 studies when the influenza viruses were antigenically matched (homologous) for A/H1N1 among the children aged 6-35 months seroconversion was 100% for allV3 (Fluad) and 38% for IIV3-1/IIV3-4 (trivalent/quadrivalent flu vaccines); among children aged 3-5 years seroconversion was 100% for allV3 and 82% for IIV3-1/IIV3-4. For AH3N2 homologous among children aged 6-35 months, seroconversion was 98% for allV3 and 44% for IIV3-1/IIV3-4. For the B strain homologous among children aged 6-35 months, seroconversion was 88% for allV3 and 19% for IIV3-1/IIV3-4; among children aged 3-5 years seroconversion for B was 99% for allV3 and 59% for IIV3-1/IIV3-4.
In the same study when the influenza viruses were antigenically mismatched (heterologous) for A/H1N1 among children of all ages 6 months to greater than 72 months, seroconversion was 96% for allV3 (Fluad) and 44% for IIV3-1/IIV3-4; for A/H3N2 it was 98% for allV3 and 49% for IIV3-1/IIV3-4, and for the B strain it was 10% for allV3 and 3% for IIV3-1/IIV3-4.
They added that “in addition, aIIV3 had the fastest onset of immunogenicity and longest persistence of immune response, which has implications for the real-world clinical setting, where the influenza season might start earlier than expected or last longer, and second (follow-up) vaccinations may be missed.”
Dr. Patel and associates said the MF59 adjuvant in Fluad “recruits immune cells (primarily monocytes, macrophages, neutrophils, and dendritic cells) at the site of injection and differentiates them into antigen-presenting cells. With an MF59-adjuvanted vaccine, more antigen is transported from the injection site to the draining lymph node, wherein MF59 leads to T-cell activation and an increased B-cell expansion and a greater number and diversity of antibodies.”
According to goodrx.com, one syringe of Fluad 0.5 mL costs $45-$74 with coupon. The same dose of Fluzone Quadrivalent, a flu vaccine recently approved by the Food and Drug Administration for use in young children aged 6-35 months, costs $31 with coupon.
The study was funded by Novartis Vaccines and Diagnostics and Seqirus (formerly part of Novartis Vaccines and Diagnostics). The study authors disclosed employment by Novartis and Seqirus.
SOURCE: Patel SS et al. Int J Infect Dis. 2019. doi: 10.1016/j.ijid.2019.05.009.
according to an industry-funded synthesis of six studies.
The vaccine “offers significant advances over conventional inactivated influenza vaccines and presents an acceptable safety profile in children 6 months through 5 years of age,” Sanjay S. Patel, PhD, of Novartis Vaccines and Diagnostics, Cambridge, Mass., and associates wrote in the analysis, published in the International Journal of Infectious Diseases. “The noteworthy increases in antibody responses and decreases in influenza cases following vaccination suggest an alternative for use in a population that is heavily impacted by influenza disease.”
Children are, of course, vulnerable to flu. The Centers for Disease Control and Prevention reported that 186 children died of flu during the landmark 2017-2018 flu season. That’s the highest number of pediatric flu deaths since they became a notifiable condition in 2004 (exclusive of the 2009 pandemic, when 358 pediatric deaths were reported from April 15, 2009, to October 2, 2010).The CDC said the vaccine during that flu season had an overall effectiveness level of 40%. According to research of others, however, flu vaccines are less effective in younger children than in adolescents and adults (Vaccine. 2014;32[31]:3886-94; Cochrane Database Syst Rev. 2008. doi: 10.1002/14651858.CD004879.pub3).
Fluad – a MF59-adjuvanted inactivated trivalent seasonal influenza vaccine – is used in adults over 65 in the United States and 29 other countries, and it is approved for children aged 6 months through 23 months in Canada.
Dr. Patel and associates examined the results of six studies – one phase 1b, three phase 2, and two phase 3 – that tested Fluad with or without other vaccines in 11,942 children aged 6 months to 5 years. The studies, mostly multicenter, were conducted in various countries, mainly in Europe and South and Central America, from 2006 to 2012.
In general, children in the intervention groups in the studies received two doses of the Fluad vaccine 4 weeks apart: two 0.25-mL doses for children aged 6-35 months and two 0.5-mL doses for those aged 3 years or older. In most of the studies, parallel control groups received nonadjuvanted trivalent or quadrivalent influenza vaccines.
Most participants (93%-94%) completed the studies. Solicited adverse effects were common in all groups (72% in the Fluad group vs. 67% who received IIV3 vaccines), and generally mild to moderate and resolved in 1-3 days. Unsolicited adverse effects were similar (55% and 62%, respectively) in the two flu vaccine groups. The authors wrote that “these data reflect a safety profile consistent with other licensed inactivated influenza vaccines administered to children.”
As for results, Dr. Patel and colleagues said, “HI [hemagglutination inhibition] antibody responses to both homologous and heterologous influenza strains are higher following vaccination with aIIV3, and this increase in immunogenicity is observed across all age subgroups in children aged 6 months through 5 years, and most profound in the children 6 to 36 months.”
For example, in one of the phase 3 studies when the influenza viruses were antigenically matched (homologous) for A/H1N1 among the children aged 6-35 months seroconversion was 100% for allV3 (Fluad) and 38% for IIV3-1/IIV3-4 (trivalent/quadrivalent flu vaccines); among children aged 3-5 years seroconversion was 100% for allV3 and 82% for IIV3-1/IIV3-4. For AH3N2 homologous among children aged 6-35 months, seroconversion was 98% for allV3 and 44% for IIV3-1/IIV3-4. For the B strain homologous among children aged 6-35 months, seroconversion was 88% for allV3 and 19% for IIV3-1/IIV3-4; among children aged 3-5 years seroconversion for B was 99% for allV3 and 59% for IIV3-1/IIV3-4.
In the same study when the influenza viruses were antigenically mismatched (heterologous) for A/H1N1 among children of all ages 6 months to greater than 72 months, seroconversion was 96% for allV3 (Fluad) and 44% for IIV3-1/IIV3-4; for A/H3N2 it was 98% for allV3 and 49% for IIV3-1/IIV3-4, and for the B strain it was 10% for allV3 and 3% for IIV3-1/IIV3-4.
They added that “in addition, aIIV3 had the fastest onset of immunogenicity and longest persistence of immune response, which has implications for the real-world clinical setting, where the influenza season might start earlier than expected or last longer, and second (follow-up) vaccinations may be missed.”
Dr. Patel and associates said the MF59 adjuvant in Fluad “recruits immune cells (primarily monocytes, macrophages, neutrophils, and dendritic cells) at the site of injection and differentiates them into antigen-presenting cells. With an MF59-adjuvanted vaccine, more antigen is transported from the injection site to the draining lymph node, wherein MF59 leads to T-cell activation and an increased B-cell expansion and a greater number and diversity of antibodies.”
According to goodrx.com, one syringe of Fluad 0.5 mL costs $45-$74 with coupon. The same dose of Fluzone Quadrivalent, a flu vaccine recently approved by the Food and Drug Administration for use in young children aged 6-35 months, costs $31 with coupon.
The study was funded by Novartis Vaccines and Diagnostics and Seqirus (formerly part of Novartis Vaccines and Diagnostics). The study authors disclosed employment by Novartis and Seqirus.
SOURCE: Patel SS et al. Int J Infect Dis. 2019. doi: 10.1016/j.ijid.2019.05.009.
according to an industry-funded synthesis of six studies.
The vaccine “offers significant advances over conventional inactivated influenza vaccines and presents an acceptable safety profile in children 6 months through 5 years of age,” Sanjay S. Patel, PhD, of Novartis Vaccines and Diagnostics, Cambridge, Mass., and associates wrote in the analysis, published in the International Journal of Infectious Diseases. “The noteworthy increases in antibody responses and decreases in influenza cases following vaccination suggest an alternative for use in a population that is heavily impacted by influenza disease.”
Children are, of course, vulnerable to flu. The Centers for Disease Control and Prevention reported that 186 children died of flu during the landmark 2017-2018 flu season. That’s the highest number of pediatric flu deaths since they became a notifiable condition in 2004 (exclusive of the 2009 pandemic, when 358 pediatric deaths were reported from April 15, 2009, to October 2, 2010).The CDC said the vaccine during that flu season had an overall effectiveness level of 40%. According to research of others, however, flu vaccines are less effective in younger children than in adolescents and adults (Vaccine. 2014;32[31]:3886-94; Cochrane Database Syst Rev. 2008. doi: 10.1002/14651858.CD004879.pub3).
Fluad – a MF59-adjuvanted inactivated trivalent seasonal influenza vaccine – is used in adults over 65 in the United States and 29 other countries, and it is approved for children aged 6 months through 23 months in Canada.
Dr. Patel and associates examined the results of six studies – one phase 1b, three phase 2, and two phase 3 – that tested Fluad with or without other vaccines in 11,942 children aged 6 months to 5 years. The studies, mostly multicenter, were conducted in various countries, mainly in Europe and South and Central America, from 2006 to 2012.
In general, children in the intervention groups in the studies received two doses of the Fluad vaccine 4 weeks apart: two 0.25-mL doses for children aged 6-35 months and two 0.5-mL doses for those aged 3 years or older. In most of the studies, parallel control groups received nonadjuvanted trivalent or quadrivalent influenza vaccines.
Most participants (93%-94%) completed the studies. Solicited adverse effects were common in all groups (72% in the Fluad group vs. 67% who received IIV3 vaccines), and generally mild to moderate and resolved in 1-3 days. Unsolicited adverse effects were similar (55% and 62%, respectively) in the two flu vaccine groups. The authors wrote that “these data reflect a safety profile consistent with other licensed inactivated influenza vaccines administered to children.”
As for results, Dr. Patel and colleagues said, “HI [hemagglutination inhibition] antibody responses to both homologous and heterologous influenza strains are higher following vaccination with aIIV3, and this increase in immunogenicity is observed across all age subgroups in children aged 6 months through 5 years, and most profound in the children 6 to 36 months.”
For example, in one of the phase 3 studies when the influenza viruses were antigenically matched (homologous) for A/H1N1 among the children aged 6-35 months seroconversion was 100% for allV3 (Fluad) and 38% for IIV3-1/IIV3-4 (trivalent/quadrivalent flu vaccines); among children aged 3-5 years seroconversion was 100% for allV3 and 82% for IIV3-1/IIV3-4. For AH3N2 homologous among children aged 6-35 months, seroconversion was 98% for allV3 and 44% for IIV3-1/IIV3-4. For the B strain homologous among children aged 6-35 months, seroconversion was 88% for allV3 and 19% for IIV3-1/IIV3-4; among children aged 3-5 years seroconversion for B was 99% for allV3 and 59% for IIV3-1/IIV3-4.
In the same study when the influenza viruses were antigenically mismatched (heterologous) for A/H1N1 among children of all ages 6 months to greater than 72 months, seroconversion was 96% for allV3 (Fluad) and 44% for IIV3-1/IIV3-4; for A/H3N2 it was 98% for allV3 and 49% for IIV3-1/IIV3-4, and for the B strain it was 10% for allV3 and 3% for IIV3-1/IIV3-4.
They added that “in addition, aIIV3 had the fastest onset of immunogenicity and longest persistence of immune response, which has implications for the real-world clinical setting, where the influenza season might start earlier than expected or last longer, and second (follow-up) vaccinations may be missed.”
Dr. Patel and associates said the MF59 adjuvant in Fluad “recruits immune cells (primarily monocytes, macrophages, neutrophils, and dendritic cells) at the site of injection and differentiates them into antigen-presenting cells. With an MF59-adjuvanted vaccine, more antigen is transported from the injection site to the draining lymph node, wherein MF59 leads to T-cell activation and an increased B-cell expansion and a greater number and diversity of antibodies.”
According to goodrx.com, one syringe of Fluad 0.5 mL costs $45-$74 with coupon. The same dose of Fluzone Quadrivalent, a flu vaccine recently approved by the Food and Drug Administration for use in young children aged 6-35 months, costs $31 with coupon.
The study was funded by Novartis Vaccines and Diagnostics and Seqirus (formerly part of Novartis Vaccines and Diagnostics). The study authors disclosed employment by Novartis and Seqirus.
SOURCE: Patel SS et al. Int J Infect Dis. 2019. doi: 10.1016/j.ijid.2019.05.009.
FROM INTERNATIONAL JOURNAL OF INFECTIOUS DISEASES
Plant-based foods could keep type 2 diabetes at bay
according to a new systematic review and meta-analysis of nine observational studies.
The findings aren’t conclusive, but the study authors wrote in JAMA Internal Medicine that they suggest that “plant-based dietary patterns were associated with lower risk of type 2 diabetes, even after adjustment for [body mass index].”
According to 2015 figures provided by the American Diabetes Association, about 29 million people in the United States have type 2 diabetes. Overall, diabetes contributes to hundreds of thousands of deaths each year, which makes it the seventh-leading cause of death in the nation.
For the new analysis, researchers led by Frank Qian, MPH, of Harvard T.H. Chan School of Public Health included nine studies that examined diet and type 2 diabetes. In all, the studies included 307,099 participants, and there were 23,544 cases of incident type 2 diabetes. They were conducted in five countries, including the United States, and tracked participants for 2-28 years; the studies were all published within the last 11 years. The mean ages of participants ranged from 36-65 years.
The meta-analysis linked higher consumption of plant-based foods to a 23% reduced risk of type 2 diabetes, compared with lower consumption (relative risk, 0.77; 95% confidence interval, 0.71-0.84; P = .07 for heterogeneity). The risk dipped even further (down to 30%) when the researchers analyzed four studies that focused on healthy plant-based foods, such as fruits and vegetables, instead of foods such as refined grains, starches, and sugars (RR, 0.70; 95% CI, 0.62-0.79).
The researchers suggested that plant-based diets may lower the risk of diabetes type 2 by limiting weight gain. They also noted various limitations to their analysis, such as the reliance on self-reports and the observational nature of the studies.
Still, “in general populations that do not practice strict vegetarian or vegan diets, replacing animal products with healthful plant-based foods is likely to exert a significant reduction in the risk of diabetes,” the authors wrote.
The study was funded by the National Institutes of Health, and one author received support from the National Institute of Diabetes and Digestive and Kidney Diseases. The remaining authors reported no conflicts of interest withing the scope of this study.
SOURCE: Qian F et al. JAMA Internal Medicine. 2019 Jul 22. doi: 10.1001/jamainternmed.2019.2195.
according to a new systematic review and meta-analysis of nine observational studies.
The findings aren’t conclusive, but the study authors wrote in JAMA Internal Medicine that they suggest that “plant-based dietary patterns were associated with lower risk of type 2 diabetes, even after adjustment for [body mass index].”
According to 2015 figures provided by the American Diabetes Association, about 29 million people in the United States have type 2 diabetes. Overall, diabetes contributes to hundreds of thousands of deaths each year, which makes it the seventh-leading cause of death in the nation.
For the new analysis, researchers led by Frank Qian, MPH, of Harvard T.H. Chan School of Public Health included nine studies that examined diet and type 2 diabetes. In all, the studies included 307,099 participants, and there were 23,544 cases of incident type 2 diabetes. They were conducted in five countries, including the United States, and tracked participants for 2-28 years; the studies were all published within the last 11 years. The mean ages of participants ranged from 36-65 years.
The meta-analysis linked higher consumption of plant-based foods to a 23% reduced risk of type 2 diabetes, compared with lower consumption (relative risk, 0.77; 95% confidence interval, 0.71-0.84; P = .07 for heterogeneity). The risk dipped even further (down to 30%) when the researchers analyzed four studies that focused on healthy plant-based foods, such as fruits and vegetables, instead of foods such as refined grains, starches, and sugars (RR, 0.70; 95% CI, 0.62-0.79).
The researchers suggested that plant-based diets may lower the risk of diabetes type 2 by limiting weight gain. They also noted various limitations to their analysis, such as the reliance on self-reports and the observational nature of the studies.
Still, “in general populations that do not practice strict vegetarian or vegan diets, replacing animal products with healthful plant-based foods is likely to exert a significant reduction in the risk of diabetes,” the authors wrote.
The study was funded by the National Institutes of Health, and one author received support from the National Institute of Diabetes and Digestive and Kidney Diseases. The remaining authors reported no conflicts of interest withing the scope of this study.
SOURCE: Qian F et al. JAMA Internal Medicine. 2019 Jul 22. doi: 10.1001/jamainternmed.2019.2195.
according to a new systematic review and meta-analysis of nine observational studies.
The findings aren’t conclusive, but the study authors wrote in JAMA Internal Medicine that they suggest that “plant-based dietary patterns were associated with lower risk of type 2 diabetes, even after adjustment for [body mass index].”
According to 2015 figures provided by the American Diabetes Association, about 29 million people in the United States have type 2 diabetes. Overall, diabetes contributes to hundreds of thousands of deaths each year, which makes it the seventh-leading cause of death in the nation.
For the new analysis, researchers led by Frank Qian, MPH, of Harvard T.H. Chan School of Public Health included nine studies that examined diet and type 2 diabetes. In all, the studies included 307,099 participants, and there were 23,544 cases of incident type 2 diabetes. They were conducted in five countries, including the United States, and tracked participants for 2-28 years; the studies were all published within the last 11 years. The mean ages of participants ranged from 36-65 years.
The meta-analysis linked higher consumption of plant-based foods to a 23% reduced risk of type 2 diabetes, compared with lower consumption (relative risk, 0.77; 95% confidence interval, 0.71-0.84; P = .07 for heterogeneity). The risk dipped even further (down to 30%) when the researchers analyzed four studies that focused on healthy plant-based foods, such as fruits and vegetables, instead of foods such as refined grains, starches, and sugars (RR, 0.70; 95% CI, 0.62-0.79).
The researchers suggested that plant-based diets may lower the risk of diabetes type 2 by limiting weight gain. They also noted various limitations to their analysis, such as the reliance on self-reports and the observational nature of the studies.
Still, “in general populations that do not practice strict vegetarian or vegan diets, replacing animal products with healthful plant-based foods is likely to exert a significant reduction in the risk of diabetes,” the authors wrote.
The study was funded by the National Institutes of Health, and one author received support from the National Institute of Diabetes and Digestive and Kidney Diseases. The remaining authors reported no conflicts of interest withing the scope of this study.
SOURCE: Qian F et al. JAMA Internal Medicine. 2019 Jul 22. doi: 10.1001/jamainternmed.2019.2195.
FROM JAMA INTERNAL MEDICINE
Adjuvanted influenza vaccine appears safe for at-risk children
according to a study in the International Journal of Infectious Diseases.
Sanjay S. Patel, PhD, of Novartis Vaccines and Diagnostics, Cambridge, Mass., and colleagues performed a retrospective analysis on an integrated dataset that drew from six randomized clinical trials comparing aIIV3 with nonadjuvanted trivalent inactivated influenza vaccine (IIV3). The dataset comprised 10,794 patients aged 6 months through 5 years, of whom 373 (3%) were deemed at risk of influenza complications after review of their medical history for conditions such as heart disease, asthma, and endocrine disorders.
The rates of solicited adverse events (such as erythema, diarrhea, fever, and localized swelling) were 74% in the aIIV3 group and 73% in the IIV3 group. The rates for any unsolicited adverse events (such as upper respiratory tract infection) for aIIV3 and IIV3 were 54% and 59%, respectively (Int J Infect Dis. 2019. doi: 10.1016/j.ijid.2019.04.023).
One of the six studies included in the dataset randomized 2,655 children for immunogenicity analyses, of whom 103 (4%) were deemed at risk. Hemagglutination inhibition assay geometric mean titers against homologous A/H1N1, A/H3N2, and B strains 21 days after the second of two doses of vaccines were two to three times higher in the aIIV3 than in the IIV3 group, which suggests that aIIV3 is more immunogenic than IIV3. As the investigators noted, this is likely because the adjuvanted vaccine induces a greater magnitude of immune response to the vaccine, something already lower in children than in adults, as well as more breadth of response, meaning the response goes beyond strains included in the vaccines.
The small number of at-risk children in the study poses a limitation on its findings. Dr. Patel and associates said that, regardless, the results of immunogenicity analyses were strong. “Overall, this analysis indicates that aIIV3 has a similar safety profile in young children with underlying medical conditions, consistent with other licensed inactivated influenza vaccines.”
Novartis Vaccines and Diagnostics originally funded the study, but was later acquired by CSL Group and now operates as Seqirus, which continued funding for the study. The authors were employees of one or the other of these companies.
according to a study in the International Journal of Infectious Diseases.
Sanjay S. Patel, PhD, of Novartis Vaccines and Diagnostics, Cambridge, Mass., and colleagues performed a retrospective analysis on an integrated dataset that drew from six randomized clinical trials comparing aIIV3 with nonadjuvanted trivalent inactivated influenza vaccine (IIV3). The dataset comprised 10,794 patients aged 6 months through 5 years, of whom 373 (3%) were deemed at risk of influenza complications after review of their medical history for conditions such as heart disease, asthma, and endocrine disorders.
The rates of solicited adverse events (such as erythema, diarrhea, fever, and localized swelling) were 74% in the aIIV3 group and 73% in the IIV3 group. The rates for any unsolicited adverse events (such as upper respiratory tract infection) for aIIV3 and IIV3 were 54% and 59%, respectively (Int J Infect Dis. 2019. doi: 10.1016/j.ijid.2019.04.023).
One of the six studies included in the dataset randomized 2,655 children for immunogenicity analyses, of whom 103 (4%) were deemed at risk. Hemagglutination inhibition assay geometric mean titers against homologous A/H1N1, A/H3N2, and B strains 21 days after the second of two doses of vaccines were two to three times higher in the aIIV3 than in the IIV3 group, which suggests that aIIV3 is more immunogenic than IIV3. As the investigators noted, this is likely because the adjuvanted vaccine induces a greater magnitude of immune response to the vaccine, something already lower in children than in adults, as well as more breadth of response, meaning the response goes beyond strains included in the vaccines.
The small number of at-risk children in the study poses a limitation on its findings. Dr. Patel and associates said that, regardless, the results of immunogenicity analyses were strong. “Overall, this analysis indicates that aIIV3 has a similar safety profile in young children with underlying medical conditions, consistent with other licensed inactivated influenza vaccines.”
Novartis Vaccines and Diagnostics originally funded the study, but was later acquired by CSL Group and now operates as Seqirus, which continued funding for the study. The authors were employees of one or the other of these companies.
according to a study in the International Journal of Infectious Diseases.
Sanjay S. Patel, PhD, of Novartis Vaccines and Diagnostics, Cambridge, Mass., and colleagues performed a retrospective analysis on an integrated dataset that drew from six randomized clinical trials comparing aIIV3 with nonadjuvanted trivalent inactivated influenza vaccine (IIV3). The dataset comprised 10,794 patients aged 6 months through 5 years, of whom 373 (3%) were deemed at risk of influenza complications after review of their medical history for conditions such as heart disease, asthma, and endocrine disorders.
The rates of solicited adverse events (such as erythema, diarrhea, fever, and localized swelling) were 74% in the aIIV3 group and 73% in the IIV3 group. The rates for any unsolicited adverse events (such as upper respiratory tract infection) for aIIV3 and IIV3 were 54% and 59%, respectively (Int J Infect Dis. 2019. doi: 10.1016/j.ijid.2019.04.023).
One of the six studies included in the dataset randomized 2,655 children for immunogenicity analyses, of whom 103 (4%) were deemed at risk. Hemagglutination inhibition assay geometric mean titers against homologous A/H1N1, A/H3N2, and B strains 21 days after the second of two doses of vaccines were two to three times higher in the aIIV3 than in the IIV3 group, which suggests that aIIV3 is more immunogenic than IIV3. As the investigators noted, this is likely because the adjuvanted vaccine induces a greater magnitude of immune response to the vaccine, something already lower in children than in adults, as well as more breadth of response, meaning the response goes beyond strains included in the vaccines.
The small number of at-risk children in the study poses a limitation on its findings. Dr. Patel and associates said that, regardless, the results of immunogenicity analyses were strong. “Overall, this analysis indicates that aIIV3 has a similar safety profile in young children with underlying medical conditions, consistent with other licensed inactivated influenza vaccines.”
Novartis Vaccines and Diagnostics originally funded the study, but was later acquired by CSL Group and now operates as Seqirus, which continued funding for the study. The authors were employees of one or the other of these companies.
FROM THE INTERNATIONAL JOURNAL OF INFECTIOUS DISEASES
Filgotinib fares well in RA trial of inadequate methotrexate responders
MADRID – (cDMARD) in a phase 3 study.
The primary outcome results of the FINCH 1 study, which were presented at the European Congress of Rheumatology, showed that significantly more patients treated with filgotinib than placebo were able to achieve 20% improvement in American College of Rheumatology response criteria (ACR20).
At week 12, an ACR20 response was achieved by 69.8% of 480 patients treated with filgotinib 100 mg/day, 76.6% of 475 treated with filgotinib 200 mg/day, and 49.9% of 475 given a matching daily placebo (P less than .0001 for both comparisons). Adalimumab (Humira; 40 mg every 2 weeks) was used as an active comparator in the trial, and 70.8% of 325 patients treated with this biologic drug achieved an ACR20.
Similar patterns were seen for the ACR50 and ACR70 responses: more than 50% of patients treated with filgotinib or adalimumab achieved an ACR50 versus 33.3% of patients treated with placebo. The ACR70 response rate was more than 30% in patients treated with either biologic, compared against 14.9% with placebo.
The percentages of patients in the filgotinib 100-mg, filgotinib 200-mg, adalimumab, and placebo arms who achieved a 28-joint Disease Activity Score using C-reactive protein (DAS28-CRP) of 3.2 or less at 12 weeks were 38.8%, 49.9%, 43.4%, and 23.4%. At 24 weeks, the rates were 53.1%, 60.6%, 50.5%, and 33.7%.
At 12 weeks, analysis showed that filgotinib 200 mg was noninferior to adalimumab in achieving a DAS28-CRP of 3.2 or less.
These study data, together with the results of two other phase 3 studies – FINCH 2 and FINCH 3 – will be used to submit a new drug application to the Food and Drug Administration for the use of filgotinib in the treatment of RA later this year, the drug’s developer, Gilead Sciences, announced on July 1. Each of the trials has addressed a different population of RA patients; while FINCH 1 looked at inadequate responders to methotrexate, FINCH 2 looked at those with an inadequate response to biologic DMARDs, and FINCH 3 recruited RA patients who were naive to methotrexate therapy.
FINCH 1 was a 1-year study, said presenting study investigator Bernard Combe, MD, PhD, professor of rheumatology at Montpellier (France) University and head of the bone and joint diseases department at the university. A total of 1,759 patients were randomized and 1,755 received study treatment with filgotinib, adalimumab, or placebo in addition to methotrexate. Data for the first 24 weeks were presented.
Dr. Combe and coauthors used hierarchical statistical testing to first compare the 200-mg dose versus placebo for the primary endpoint, and then, if positive, the percentage of patients at 12 weeks achieving a DAS28-CRP score of 3.2 or less and the score at 12 weeks on the Health Assessment Questionnaire – Disability Index (HAQ-DI), and then the DAS28-CRP again at 24 weeks. This was repeated with the 100-mg dose until finally noninferiority of the 200-mg dose versus adalimumab in DAS28-CRP at 12 weeks was tested.
Other findings included a significant reduction in radiographic progression at week 24 with both doses of filgotinib versus placebo; improvements in HAQ-DI and Functional Assessment of Chronic Illness Therapy-Fatigue scores also were seen at 12 and 24 weeks.
“The selective JAK1 inhibitor filgotinib, at doses of 200 and 100 mg per day, led to significant improvement in symptoms of RA patients with inadequate response to methotrexate,” Dr. Combe concluded. It “prevented radiographic progression, and improved physical function compared to placebo.”
Importantly, the drug was “well tolerated” and “a low frequency of venous thrombotic events, serious infections, and other adverse events of interest was observed.”
Commenting on the study during the Q&A session that followed Dr. Combe’s presentation, Roy M. Fleischmann, MD, clinical professor of medicine at the University of Texas Southwestern Medical Center in Dallas, noted that the choice of DAS28-CRP was “very unusual” as an endpoint after the ACR20 as it’s “almost always an ACR50 or 70.” There was also a “very high placebo response.”
Dr. Combe responded that he “wasn’t so surprised by the high placebo response. You know that this has been shown previously in some other trials.” As to why, he noted that there was an ongoing analysis but also proposed two reasons: First, the geographic region – with more than 1,700 patients from all over the world included in the trial, there could be variation in the placebo responses. Second, methotrexate might still be having a minor effect when the trial started.
The study was sponsored by Gilead Sciences in collaboration with Galapagos NV. Dr. Combe has received honoraria from AbbVie, Bristol-Myers Squibb, Gilead, Janssen, Eli Lilly, Merck Sharp & Dohme, Novartis, Pfizer, UCB, Genzyme, Sanofi, Regeneron, Sun Pharma Advanced Research, Boehringer Ingelheim, and Flexion. Dr. Combe is a shareholder in Novartis.
SOURCE: Combe B et al. Ann Rheum Dis. Jun 2019;78(Suppl 2):77-8. Abstract LB0001, doi: 10.1136/annrheumdis-2019-eular.8676
MADRID – (cDMARD) in a phase 3 study.
The primary outcome results of the FINCH 1 study, which were presented at the European Congress of Rheumatology, showed that significantly more patients treated with filgotinib than placebo were able to achieve 20% improvement in American College of Rheumatology response criteria (ACR20).
At week 12, an ACR20 response was achieved by 69.8% of 480 patients treated with filgotinib 100 mg/day, 76.6% of 475 treated with filgotinib 200 mg/day, and 49.9% of 475 given a matching daily placebo (P less than .0001 for both comparisons). Adalimumab (Humira; 40 mg every 2 weeks) was used as an active comparator in the trial, and 70.8% of 325 patients treated with this biologic drug achieved an ACR20.
Similar patterns were seen for the ACR50 and ACR70 responses: more than 50% of patients treated with filgotinib or adalimumab achieved an ACR50 versus 33.3% of patients treated with placebo. The ACR70 response rate was more than 30% in patients treated with either biologic, compared against 14.9% with placebo.
The percentages of patients in the filgotinib 100-mg, filgotinib 200-mg, adalimumab, and placebo arms who achieved a 28-joint Disease Activity Score using C-reactive protein (DAS28-CRP) of 3.2 or less at 12 weeks were 38.8%, 49.9%, 43.4%, and 23.4%. At 24 weeks, the rates were 53.1%, 60.6%, 50.5%, and 33.7%.
At 12 weeks, analysis showed that filgotinib 200 mg was noninferior to adalimumab in achieving a DAS28-CRP of 3.2 or less.
These study data, together with the results of two other phase 3 studies – FINCH 2 and FINCH 3 – will be used to submit a new drug application to the Food and Drug Administration for the use of filgotinib in the treatment of RA later this year, the drug’s developer, Gilead Sciences, announced on July 1. Each of the trials has addressed a different population of RA patients; while FINCH 1 looked at inadequate responders to methotrexate, FINCH 2 looked at those with an inadequate response to biologic DMARDs, and FINCH 3 recruited RA patients who were naive to methotrexate therapy.
FINCH 1 was a 1-year study, said presenting study investigator Bernard Combe, MD, PhD, professor of rheumatology at Montpellier (France) University and head of the bone and joint diseases department at the university. A total of 1,759 patients were randomized and 1,755 received study treatment with filgotinib, adalimumab, or placebo in addition to methotrexate. Data for the first 24 weeks were presented.
Dr. Combe and coauthors used hierarchical statistical testing to first compare the 200-mg dose versus placebo for the primary endpoint, and then, if positive, the percentage of patients at 12 weeks achieving a DAS28-CRP score of 3.2 or less and the score at 12 weeks on the Health Assessment Questionnaire – Disability Index (HAQ-DI), and then the DAS28-CRP again at 24 weeks. This was repeated with the 100-mg dose until finally noninferiority of the 200-mg dose versus adalimumab in DAS28-CRP at 12 weeks was tested.
Other findings included a significant reduction in radiographic progression at week 24 with both doses of filgotinib versus placebo; improvements in HAQ-DI and Functional Assessment of Chronic Illness Therapy-Fatigue scores also were seen at 12 and 24 weeks.
“The selective JAK1 inhibitor filgotinib, at doses of 200 and 100 mg per day, led to significant improvement in symptoms of RA patients with inadequate response to methotrexate,” Dr. Combe concluded. It “prevented radiographic progression, and improved physical function compared to placebo.”
Importantly, the drug was “well tolerated” and “a low frequency of venous thrombotic events, serious infections, and other adverse events of interest was observed.”
Commenting on the study during the Q&A session that followed Dr. Combe’s presentation, Roy M. Fleischmann, MD, clinical professor of medicine at the University of Texas Southwestern Medical Center in Dallas, noted that the choice of DAS28-CRP was “very unusual” as an endpoint after the ACR20 as it’s “almost always an ACR50 or 70.” There was also a “very high placebo response.”
Dr. Combe responded that he “wasn’t so surprised by the high placebo response. You know that this has been shown previously in some other trials.” As to why, he noted that there was an ongoing analysis but also proposed two reasons: First, the geographic region – with more than 1,700 patients from all over the world included in the trial, there could be variation in the placebo responses. Second, methotrexate might still be having a minor effect when the trial started.
The study was sponsored by Gilead Sciences in collaboration with Galapagos NV. Dr. Combe has received honoraria from AbbVie, Bristol-Myers Squibb, Gilead, Janssen, Eli Lilly, Merck Sharp & Dohme, Novartis, Pfizer, UCB, Genzyme, Sanofi, Regeneron, Sun Pharma Advanced Research, Boehringer Ingelheim, and Flexion. Dr. Combe is a shareholder in Novartis.
SOURCE: Combe B et al. Ann Rheum Dis. Jun 2019;78(Suppl 2):77-8. Abstract LB0001, doi: 10.1136/annrheumdis-2019-eular.8676
MADRID – (cDMARD) in a phase 3 study.
The primary outcome results of the FINCH 1 study, which were presented at the European Congress of Rheumatology, showed that significantly more patients treated with filgotinib than placebo were able to achieve 20% improvement in American College of Rheumatology response criteria (ACR20).
At week 12, an ACR20 response was achieved by 69.8% of 480 patients treated with filgotinib 100 mg/day, 76.6% of 475 treated with filgotinib 200 mg/day, and 49.9% of 475 given a matching daily placebo (P less than .0001 for both comparisons). Adalimumab (Humira; 40 mg every 2 weeks) was used as an active comparator in the trial, and 70.8% of 325 patients treated with this biologic drug achieved an ACR20.
Similar patterns were seen for the ACR50 and ACR70 responses: more than 50% of patients treated with filgotinib or adalimumab achieved an ACR50 versus 33.3% of patients treated with placebo. The ACR70 response rate was more than 30% in patients treated with either biologic, compared against 14.9% with placebo.
The percentages of patients in the filgotinib 100-mg, filgotinib 200-mg, adalimumab, and placebo arms who achieved a 28-joint Disease Activity Score using C-reactive protein (DAS28-CRP) of 3.2 or less at 12 weeks were 38.8%, 49.9%, 43.4%, and 23.4%. At 24 weeks, the rates were 53.1%, 60.6%, 50.5%, and 33.7%.
At 12 weeks, analysis showed that filgotinib 200 mg was noninferior to adalimumab in achieving a DAS28-CRP of 3.2 or less.
These study data, together with the results of two other phase 3 studies – FINCH 2 and FINCH 3 – will be used to submit a new drug application to the Food and Drug Administration for the use of filgotinib in the treatment of RA later this year, the drug’s developer, Gilead Sciences, announced on July 1. Each of the trials has addressed a different population of RA patients; while FINCH 1 looked at inadequate responders to methotrexate, FINCH 2 looked at those with an inadequate response to biologic DMARDs, and FINCH 3 recruited RA patients who were naive to methotrexate therapy.
FINCH 1 was a 1-year study, said presenting study investigator Bernard Combe, MD, PhD, professor of rheumatology at Montpellier (France) University and head of the bone and joint diseases department at the university. A total of 1,759 patients were randomized and 1,755 received study treatment with filgotinib, adalimumab, or placebo in addition to methotrexate. Data for the first 24 weeks were presented.
Dr. Combe and coauthors used hierarchical statistical testing to first compare the 200-mg dose versus placebo for the primary endpoint, and then, if positive, the percentage of patients at 12 weeks achieving a DAS28-CRP score of 3.2 or less and the score at 12 weeks on the Health Assessment Questionnaire – Disability Index (HAQ-DI), and then the DAS28-CRP again at 24 weeks. This was repeated with the 100-mg dose until finally noninferiority of the 200-mg dose versus adalimumab in DAS28-CRP at 12 weeks was tested.
Other findings included a significant reduction in radiographic progression at week 24 with both doses of filgotinib versus placebo; improvements in HAQ-DI and Functional Assessment of Chronic Illness Therapy-Fatigue scores also were seen at 12 and 24 weeks.
“The selective JAK1 inhibitor filgotinib, at doses of 200 and 100 mg per day, led to significant improvement in symptoms of RA patients with inadequate response to methotrexate,” Dr. Combe concluded. It “prevented radiographic progression, and improved physical function compared to placebo.”
Importantly, the drug was “well tolerated” and “a low frequency of venous thrombotic events, serious infections, and other adverse events of interest was observed.”
Commenting on the study during the Q&A session that followed Dr. Combe’s presentation, Roy M. Fleischmann, MD, clinical professor of medicine at the University of Texas Southwestern Medical Center in Dallas, noted that the choice of DAS28-CRP was “very unusual” as an endpoint after the ACR20 as it’s “almost always an ACR50 or 70.” There was also a “very high placebo response.”
Dr. Combe responded that he “wasn’t so surprised by the high placebo response. You know that this has been shown previously in some other trials.” As to why, he noted that there was an ongoing analysis but also proposed two reasons: First, the geographic region – with more than 1,700 patients from all over the world included in the trial, there could be variation in the placebo responses. Second, methotrexate might still be having a minor effect when the trial started.
The study was sponsored by Gilead Sciences in collaboration with Galapagos NV. Dr. Combe has received honoraria from AbbVie, Bristol-Myers Squibb, Gilead, Janssen, Eli Lilly, Merck Sharp & Dohme, Novartis, Pfizer, UCB, Genzyme, Sanofi, Regeneron, Sun Pharma Advanced Research, Boehringer Ingelheim, and Flexion. Dr. Combe is a shareholder in Novartis.
SOURCE: Combe B et al. Ann Rheum Dis. Jun 2019;78(Suppl 2):77-8. Abstract LB0001, doi: 10.1136/annrheumdis-2019-eular.8676
REPORTING FROM EULAR 2019 CONGRESS
Consider a Donation to the SVS PAC
The SVS Political Action Committee has set an aggressive fundraising goal for this current 2020 election cycle, and your contribution is critical to helping the PAC to meet this goal. The SVS PAC is one of the most effective tools for communicating with policymakers on Capitol Hill to make the greatest impact on legislative issues that affect your patients and your practices. Contributions allow the PAC Committee to establish strong relationships with members of Congress and cultivate congressional champions for our legislative and regulatory priorities. Make your donation or enhance your previously made donation to the SVS PAC here. Want to learn more? Click here.
The SVS Political Action Committee has set an aggressive fundraising goal for this current 2020 election cycle, and your contribution is critical to helping the PAC to meet this goal. The SVS PAC is one of the most effective tools for communicating with policymakers on Capitol Hill to make the greatest impact on legislative issues that affect your patients and your practices. Contributions allow the PAC Committee to establish strong relationships with members of Congress and cultivate congressional champions for our legislative and regulatory priorities. Make your donation or enhance your previously made donation to the SVS PAC here. Want to learn more? Click here.
The SVS Political Action Committee has set an aggressive fundraising goal for this current 2020 election cycle, and your contribution is critical to helping the PAC to meet this goal. The SVS PAC is one of the most effective tools for communicating with policymakers on Capitol Hill to make the greatest impact on legislative issues that affect your patients and your practices. Contributions allow the PAC Committee to establish strong relationships with members of Congress and cultivate congressional champions for our legislative and regulatory priorities. Make your donation or enhance your previously made donation to the SVS PAC here. Want to learn more? Click here.