User login
Improving the Transition of Intravenous to Enteral Antibiotics in Pediatric Patients with Pneumonia or Skin and Soft Tissue Infections
Intravenous (IV) antibiotics are commonly used in hospitalized pediatric patients to treat bacterial infections. Antimicrobial stewardship guidelines published by the Infectious Diseases Society of America (IDSA) recommend institutions develop a systematic plan to convert from IV to enteral antibiotics, as early transition may reduce healthcare costs, decrease length of stay (LOS), and avoid prolonged IV access complications1 such as extravasation, thrombosis, and catheter-associated infections.2-5
Pediatric patients with community-acquired pneumonia (CAP) and mild skin and soft tissue infections (SSTI) may not require IV antibiotics, even if the patient is hospitalized.6 Although national guidelines for pediatric CAP and SSTI recommend IV antibiotics for hospitalized patients, these guidelines state that mild infections may be treated with enteral antibiotics and emphasize discontinuation of IV antibiotics when the patient meets discharge criteria.7,8 Furthermore, several enteral antibiotics used for the treatment of CAP and SSTI, such as cephalexin and clindamycin,9 have excellent bioavailability (>90%) or can achieve sufficient concentrations to attain the pharmacodynamic target (ie, amoxicillin and trimethoprim–sulfamethoxazole).10,11 Nonetheless, the guidelines do not explicitly outline criteria regarding the transition from IV to enteral antibiotics.7,8
At our institution, patients admitted to Hospital Medicine (HM) often remained on IV antibiotics until discharge. Data review revealed that antibiotic treatment of CAP and SSTI posed the greatest opportunity for early conversion to enteral therapy based on the high frequency of admissions and the ability of commonly used enteral antibiotics to attain pharmacodynamic targets. We sought to change practice culture by decoupling transition to enteral antibiotics from discharge and use administration of other enteral medications as an objective indicator for transition. Our aim was to increase the proportion of enterally administered antibiotic doses for HM patients aged >60 days admitted with uncomplicated CAP or SSTI from 44% to 75% in eight months.
METHODS
Context
Cincinnati Children’s Hospital Medical Center (CCHMC) is a large, urban, academic hospital. The HM division has 45 attendings and admits >8,000 general pediatric patients annually. The five HM teams at the main campus consist of attendings, fellows, residents, and medical students. One HM team serves as the resident quality improvement (QI) team where residents collaborate in a longitudinal study under the guidance of QI-trained coaches. The focus of this QI initiative was determined by resident consensus and aligned with a high-value care curriculum.12
To identify the target patient population, we investigated IV antimicrobials frequently used in HM patients. Ampicillin and clindamycin are commonly used IV antibiotics, most frequently corresponding with the diagnoses of CAP and SSTI, respectively, accounting for half of all antibiotic use on the HM service. Amoxicillin, the enteral equivalent of ampicillin, can achieve sufficient concentrations to attain the pharmacodynamic target at infection sites, and clindamycin has high bioavailability, making them ideal options for early transition. Our institution’s robust antimicrobial stewardship program has published local guidelines on using amoxicillin as the enteral antibiotic of choice for uncomplicated CAP, but it does not provide guidance on the timing of transition for either CAP or SSTI; the clinical team makes this decision.
HM attendings were surveyed to determine the criteria used to transition from IV to enteral antibiotics for patients with CAP or SSTI. The survey illustrated practice variability with providers using differing clinical criteria to signal the timing of transition. Additionally, only 49% of respondents (n = 37) rated themselves as “very comfortable” with residents making autonomous decisions to transition to enteral antibiotics. We chose to use the administration of other enteral medications, instead of discharge readiness, as an objective indicator of a patient’s readiness to transition to enteral antibiotics, given the low-risk patient population and the ability of the enteral antibiotics commonly used for CAP and SSTI to achieve pharmacodynamic targets.
The study population included patients aged >60 days admitted to HM with CAP or SSTI treated with any antibiotic. We excluded patients with potential complications or significant progression of their disease process, including patients with parapneumonic effusions or chest tubes, patients who underwent bronchoscopy, and patients with osteomyelitis, septic arthritis, or preseptal or orbital cellulitis. Past medical history and clinical status on admission were not used to exclude patients.
Interventions
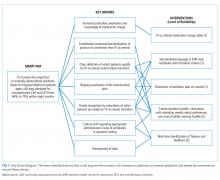
Our multidisciplinary team, formed in January 2017, included HM attendings, HM fellows, pediatric residents, a critical care attending, a pharmacy resident, and an antimicrobial stewardship pharmacist. Under the guidance of QI coaches, the residents on the HM QI team developed and tested all interventions on their team and then determined which interventions would spread to the other four teams. The nursing director of our primary HM unit disseminated project updates to bedside nurses. A simplified failure mode and effects analysis identified areas for improvement and potential interventions. Interventions focused on the following key drivers (Figure 1): increased prescriber awareness of medication charge, standardization of conversion from IV to enteral antibiotics, clear definition of the patients ready for transition, ongoing evaluation of the antimicrobial plan, timely recognition by prescribers of patients ready for transition, culture shift regarding the appropriate administration route in the inpatient setting, and transparency of data. The team implemented sequential Plan-Do-Study-Act (PDSA) cycles13 to test the interventions.
Charge Table
To improve knowledge about the increased charge for commonly used IV medications compared with enteral formulations, a table comparing relative charges was shared during monthly resident morning conferences and at an HM faculty meeting. The table included charge comparisons between ampicillin and amoxicillin and IV and enteral clindamycin.
Standardized Language in Electronic Health Record (EHR) Antibiotic Plan on Rounds
Standardized language to document antibiotic transition plans was added to admission and progress note templates in the EHR. The standard template prompted residents to (1) define clinical transition criteria, (2) discuss attending comfort with transition overnight (based on survey results), and (3) document patient preference of solid or liquid dosage forms. Plans were reviewed and updated daily. We hypothesized that since residents use the information in the daily progress notes, including assessments and plans, to present on rounds, inclusion of the transition criteria in the note would prompt transition plan discussions.
Communication Bundle
To promote early transition to enteral antibiotics, we standardized the discussion about antibiotic transition between residents and attendings. During a weekly preexisting meeting, the resident QI team reviewed preferences for transitions with the new service attending. By identifying attending preferences early, residents were able to proactively transition patients who met the criteria (eg, antibiotic transition in the evening instead of waiting until morning rounds). This discussion also provided an opportunity to engage service attendings in the QI efforts, which were also shared at HM faculty meetings quarterly.
Recognizing that in times of high census, discussion of patient plans may be abbreviated during rounds, residents were asked to identify all patients on IV antibiotics while reviewing patient medication orders prior to rounds. As part of an existing daily prerounds huddle to discuss rounding logistics, residents listed all patients on IV antibiotics and discussed which patients were ready for transition. If patients could not be transitioned immediately, the team identified the transition criteria.
At preexisting evening huddles between overnight shift HM residents and the evening HM attending, residents identified patients who were prescribed IV antibiotics and discussed readiness for enteral transition. If a patient could be transitioned overnight, enteral antibiotic orders were placed. Overnight residents were also encouraged to review the transition criteria with families upon admission.
Real-time Identification of Failures and Feedback
For two weeks, the EHR was queried daily to identify patients admitted for uncomplicated CAP and SSTI who were on antibiotics as well as other enteral medications. A failure was defined as an IV antibiotic dose given to a patient who was administered any enteral medication. Residents on the QI team approached residents on other HM teams whenever patients were identified as a failed transition to learn about failure reasons.
Study of the Interventions
Data for HM patients who met the inclusion criteria were collected weekly from January 2016 through June 2018 via EHR query. We initially searched for diagnoses that fit under the disease categories of pneumonia and SSTI in the EHR, which generated a list of International Classification of Disease-9 and -10 Diagnosis codes (Appendix Figure 1). The query identified patients based on these codes and reported whether the identified patients took a dose of any enteral medication, excluding nystatin, sildenafil, tacrolimus, and mouthwashes, which are commonly continued during NPO status due to no need for absorption or limited parenteral options. It also reported the ordered route of administration for the queried antibiotics (Appendix Figure 1).
The 2016 calendar year established our baseline to account for seasonal variability. Data were reported weekly and reviewed to evaluate the impact of PDSA cycles and inform new interventions.
Measures
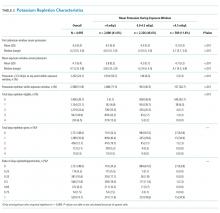
Our process measure was the total number of enteral antibiotic doses divided by all antibiotic doses in patients receiving any enteral medication. We reasoned that if patients were well enough to take medications enterally, they could be given an enteral antibiotic that is highly bioavailable or readily achieves concentrations that attain pharmacodynamic targets. This practice change was a culture shift, decoupling the switch to enteral antibiotics from discharge readiness. Our EHR query reported only the antibiotic doses given to patients who took an enteral medication on the day of antibiotic administration and excluded patients who received only IV medications.
Outcome measures included antimicrobial costs per patient encounter using average wholesale prices, which were reported in our EHR query, and LOS. To ensure that transitions of IV to enteral antibiotics were not negatively impacting patient outcomes, patient readmissions within seven days served as a balancing measure.
Analysis
An annotated statistical process control p-chart tracked the impact of interventions on the proportion of antibiotic doses that were enterally administered during hospitalization. An x-bar and an s-chart tracked the impact of interventions on antimicrobial costs per patient encounter and on LOS. A p-chart and an encounters-between g-chart were used to evaluate the impact of our interventions on readmissions. Control chart rules for identifying special cause were used for center line shifts.14
Ethical Considerations
This study was part of a larger study of the residency high-value care curriculum,12 which was deemed exempt by the CCHMC IRB.
RESULTS
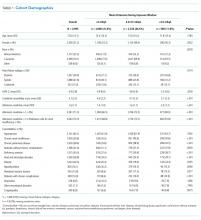
The baseline data collected included 372 patients and the postintervention period in 2017 included 326 patients (Table). Approximately two-thirds of patients had a diagnosis of CAP.
The percentage of antibiotic doses given enterally increased from 44% to 80% within eight months (Figure 2). When studying the impact of interventions, residents on the HM QI team found that the standard EHR template added to daily notes did not consistently prompt residents to discuss antibiotic plans and thus was abandoned. Initial improvement coincided with standardizing discussions between residents and attendings regarding transitions. Furthermore, discussion of all patients on IV antibiotics during the prerounds huddle allowed for reliable, daily communication about antibiotic plans and was subsequently spread to and adopted by all HM teams. The percentage of enterally administered antibiotic doses increased to >75% after the evening huddle, which involved all HM teams, and real-time identification of failures on all HM teams with provider feedback. Despite variability when the total number of antibiotic doses prescribed per week was low (<10), we demonstrated sustainability for 11 months (Figure 2), during which the prerounds and evening huddle discussions were continued and an updated control chart was shown monthly to residents during their educational conferences.
Residents on the QI team spoke directly with other HM residents when there were missed opportunities for transition. Based on these discussions and intermittent chart reviews, common reasons for failure to transition in patients with CAP included admission for failed outpatient enteral treatment, recent evaluation by critical care physicians for possible transfer to the intensive care unit, and difficulty weaning oxygen. For patients with SSTI, hand abscesses requiring drainage by surgery and treatment failure with other antibiotics constituted many of the IV antibiotic doses given to patients on enteral medications.
Antimicrobial costs per patient encounter decreased by 70% over one year; the shift in costs coincided with the second shift in our process measure (Appendix Figure 2A). Based on an estimate of 350 patients admitted per year for uncomplicated CAP or SSTI, this translates to an annual cost savings of approximately $29,000. The standard deviation of costs per patient encounter decreased by 84% (Appendix Figure 2B), suggesting a decrease in the variability of prescribing practices.
The average LOS in our patient population prior to intervention was 2.1 days and did not change (Appendix Figure 2C), but the standard deviation decreased by >50% (Appendix Figure 2D). There was no shift in the mean seven-day readmission rate or the number of encounters between readmissions (2.6% and 26, respectively; Appendix Figure 3). In addition, the hospital billing department did not identify an increase in insurance denials related to the route of antibiotic administration.
DISCUSSION
Summary
Using improvement science, we promoted earlier transition to enteral antibiotics for children hospitalized with uncomplicated CAP and SSTI by linking the decision for transition to the ability to take other enteral medications, rather than to discharge readiness. We increased the percentage of enterally administered antibiotic doses in this patient population from 44% to 80% in eight months. Although we did not observe a decrease in LOS as previously noted in a cost analysis study comparing pediatric patients with CAP treated with oral antibiotics versus those treated with IV antibiotics,15 we did find a decrease in LOS variability and in antimicrobial costs to our patients. These cost savings did not include potential savings from nursing or pharmacy labor. In addition, we noted a decrease in the variability in antibiotic prescribing practice, which demonstrates provider ability and willingness to couple antibiotic route transition to an objective characteristic (administration of other enteral medications).
A strength of our study was that residents, the most frequent prescribers of antibiotics on our HM service, were highly involved in the QI initiative, including defining the SMART aim, identifying key drivers, developing interventions, and completing sequential PDSA cycles. Under the guidance of QI-trained coaches, residents developed feasible interventions and assessed their success in real time. Consistent with other studies,16,17 resident buy-in and involvement led to the success of our improvement study.
Interpretation
Despite emerging evidence regarding the timing of transition to enteral antibiotics, several factors impeded early transition at our institution, including physician culture, variable practice habits, and hospital workflow. Evidence supports the use of enteral antibiotics in immunocompetent children hospitalized for uncomplicated CAP who do not have chronic lung disease, are not in shock, and have oxygen saturations >85%.6 Although existing literature suggests that in pediatric patients admitted for SSTIs not involving the eye or bone, IV antibiotics may be transitioned when clinical improvement, evidenced by a reduction in fever or erythema, is noted,6 enteral antibiotics that achieve appropriate concentrations to attain pharmacodynamic targets should have the same efficacy as that of IV antibiotics.9 Using the criterion of administration of any medication enterally to identify a patient’s readiness to transition, we were able to overcome practice variation among providers who may have differing opinions of what constitutes clinical improvement. Of note, new evidence is emerging on predictors of enteral antibiotic treatment failure in patients with CAP and SSTI to guide transition timing, but these studies have largely focused on the adult population or were performed in the outpatient and emergency department (ED) settings.18,19 Regardless, the stable number of encounters between readmissions in our patient population likely indicates that treatment failure in these patients was rare.
Rising healthcare costs have led to concerns around sustainability of the healthcare system;20,21 tackling overuse in clinical practice, as in our study, is one mitigation strategy. Several studies have used QI methods to facilitate the provision of high-value care through the decrease of continuous monitor overuse and extraneous ordering of electrolytes.22,23 Our QI study adds to the high-value care literature by safely decreasing the use of IV antibiotics. One retrospective study demonstrated that a one-day decrease in the use of IV antibiotics in pneumonia resulted in decreased costs without an increase in readmissions, similar to our findings.24 In adults, QI initiatives aimed at improving early transition of antibiotics utilized electronic trigger tools.25,26 Fischer et al. used active orders for scheduled enteral medications or an enteral diet as indication that a patient’s IV medications could be converted to enteral form.26
Our work is not without limitations. The list of ICD-9 and -10 codes used to query the EHR did not capture all diagnoses that would be considered as uncomplicated CAP or SSTI. However, we included an extensive list of diagnoses to ensure that the majority of patients meeting our inclusion criteria were captured. Our process measure did not account for patients on IV antibiotics who were not administered other enteral medications but tolerating an enteral diet. These patients were not identified in our EHR query and were not included in our process measure as a failure. However, in latter interventions, residents identified all patients on IV antibiotics, so that patients not identified by our EHR query benefited from our work. Furthermore, this QI study was conducted at a single institution and several interventions took advantage of preexisting structured huddles and a resident QI curriculum, which may not exist at other institutions. Our study does highlight that engaging frontline providers, such as residents, to review antibiotic orders consistently and question the appropriateness of the administration route is key to making incremental changes in prescribing practices.
CONCLUSIONS
Through a partnership between HM and Pharmacy and with substantial resident involvement, we improved the transition of IV antibiotics in patients with CAP or SSTI by increasing the percentage of enterally administered antibiotic doses and reducing antimicrobial costs and variability in antibiotic prescribing practices. This work illustrates how reducing overuse of IV antibiotics promotes high-value care and aligns with initiatives to prevent avoidable harm.27 Our work highlights that standardized discussions about medication orders to create consensus around enteral antibiotic transitions, real-time feedback, and challenging the status quo can influence practice habits and effect change.
Next steps include testing automated methods to notify providers of opportunities for transition from IV to enteral antibiotics through embedded clinical decision support, a method similar to the electronic trigger tools used in adult QI studies.25,26 Since our prerounds huddle includes identifying all patients on IV antibiotics, studying the transition to enteral antibiotics and its effect on prescribing practices in other diagnoses (ie, urinary tract infection and osteomyelitis) may contribute to spreading these efforts. Partnering with our ED colleagues may be an important next step, as several patients admitted to HM on IV antibiotics are given their first dose in the ED.
Acknowledgments
The authors would like to thank the faculty of the James M. Anderson Center for Health Systems Excellence Intermediate Improvement Science Series for their guidance in the planning of this project. The authors would also like to thank Ms. Ursula Bradshaw and Mr. Michael Ponti-Zins for obtaining the hospital data on length of stay and readmissions. The authors acknowledge Dr. Philip Hagedorn for his assistance with the software that queries the electronic health record and Dr. Laura Brower and Dr. Joanna Thomson for their assistance with statistical analysis. The authors are grateful to all the residents and coaches on the QI Hospital Medicine team who contributed ideas on study design and interventions.
1. Dellit TH, Owens RC, McGowan JE, Jr, et al. Infectious diseases society of America and the society for healthcare epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44(2):159-177. https://doi.org/10.1086/510393.
2. Shah SS, Srivastava R, Wu S, et al. Intravenous Versus oral antibiotics for postdischarge treatment of complicated pneumonia. Pediatrics. 2016;138(6). https://doi.org/10.1542/peds.2016-1692.
3. Keren R, Shah SS, Srivastava R, et al. Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomyelitis in children. JAMA Pediatr. 2015;169(2):120-128. https://doi.org/10.1001/jamapediatrics.2014.2822.
4. Jumani K, Advani S, Reich NG, Gosey L, Milstone AM. Risk factors for peripherally inserted central venous catheter complications in children. JAMA Pediatr. 2013;167(5):429-435.https://doi.org/10.1001/jamapediatrics.2013.775.
5. Zaoutis T, Localio AR, Leckerman K, et al. Prolonged intravenous therapy versus early transition to oral antimicrobial therapy for acute osteomyelitis in children. Pediatrics. 2009;123(2):636-642. https://doi.org/10.1542/peds.2008-0596.
6. McMullan BJ, Andresen D, Blyth CC, et al. Antibiotic duration and timing of the switch from intravenous to oral route for bacterial infections in children: systematic review and guidelines. Lancet Infect Dis. 2016;16(8):e139-e152. https://doi.org/10.1016/S1473-3099(16)30024-X.
7. Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):e25-e76. https://doi.org/10.1093/cid/cir531.
8. Stevens DL, Bisno AL, Chambers HF, et al. Executive summary: practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the infectious diseases society of America. Clin Infect Dis. 2014;59(2):147-159. https://doi.org/10.1093/cid/ciu444.
9. MacGregor RR, Graziani AL. Oral administration of antibiotics: a rational alternative to the parenteral route. Clin Infect Dis. 1997;24(3):457-467. https://doi.org/10.1093/clinids/24.3.457.
10. Downes KJ, Hahn A, Wiles J, Courter JD, Vinks AA. Dose optimisation of antibiotics in children: application of pharmacokinetics/pharmacodynamics in paediatrics. Int J Antimicrob Agents. 2014;43(3):223-230. https://doi.org/10.1016/j.ijantimicag.2013.11.006.
11. Autmizguine J, Melloni C, Hornik CP, et al. Population pharmacokinetics of trimethoprim-sulfamethoxazole in infants and children. Antimicrob Agents Chemother. 2018;62(1):e01813-e01817. https://doi.org/10.1128/AAC.01813-17.
12. Dewan M, Herrmann LE, Tchou MJ, et al. Development and evaluation of high-value pediatrics: a high-value care pediatric resident curriculum. Hosp Pediatr. 2018;8(12):785-792. https://doi.org/10.1542/hpeds.2018-0115
13. Langley GJ, Moen RD, Nolan KM, Nolan TW, Norman CL, Provost LP. The Improvement Guide: A Practical Approach to Enhancing Organizational Performance. New Jersey, US: John Wiley & Sons; 2009.
14. Benneyan JC. Use and interpretation of statistical quality control charts. Int J Qual Health Care. 1998;10(1):69-73. https://doi.org/10.1093/intqhc/10.1.69.
15. Lorgelly PK, Atkinson M, Lakhanpaul M, et al. Oral versus i.v. antibiotics for community-acquired pneumonia in children: a cost-minimisation analysis. Eur Respir J. 2010;35(4):858-864. https://doi.org/10.1183/09031936.00087209.
16. Vidyarthi AR, Green AL, Rosenbluth G, Baron RB. Engaging residents and fellows to improve institution-wide quality: the first six years of a novel financial incentive program. Acad Med. 2014;89(3):460-468. https://doi.org/10.1097/ACM.0000000000000159.
17. Stinnett-Donnelly JM, Stevens PG, Hood VL. Developing a high value care programme from the bottom up: a programme of faculty-resident improvement projects targeting harmful or unnecessary care. BMJ Qual Saf. 2016;25(11):901-908. https://doi.org/10.1136/bmjqs-2015-004546.
18. Peterson D, McLeod S, Woolfrey K, McRae A. Predictors of failure of empiric outpatient antibiotic therapy in emergency department patients with uncomplicated cellulitis. Acad Emerg Med. 2014;21(5):526-531. https://doi.org/10.1111/acem.12371.
19. Yadav K, Suh KN, Eagles D, et al. Predictors of oral antibiotic treatment failure for non-purulent skin and soft tissue infections in the emergency department. Acad Emerg Med. 2018;20(S1):S24-S25. https://doi.org/10.1017/cem.2018.114.
20. Organisation for Economic Co-operation and Development. Healthcare costs unsustainable in advanced economies without reform. http://www.oecd.org/health/healthcarecostsunsustainableinadvancedeconomieswithoutreform.htm. Accessed June 28, 2018; 2015.
21. Berwick DM, Hackbarth AD. Eliminating waste in US health care. JAMA. 2012;307(14):1513-1516. https://doi.org/10.1001/jama.2012.362.
22. Schondelmeyer AC, Simmons JM, Statile AM, et al. Using quality improvement to reduce continuous pulse oximetry use in children with wheezing. Pediatrics. 2015;135(4):e1044-e1051. https://doi.org/10.1542/peds.2014-2295.
23. Tchou MJ, Tang Girdwood S, Wormser B, et al. Reducing electrolyte testing in hospitalized children by using quality improvement methods. Pediatrics. 2018;141(5). https://doi.org/10.1542/peds.2017-3187.
24. Christensen EW, Spaulding AB, Pomputius WF, Grapentine SP. Effects of hospital practice patterns for antibiotic administration for pneumonia on hospital lengths of stay and costs. J Pediatr Infect Dis Soc. 2019;8(2):115-121. https://doi.org/10.1093/jpids/piy003.
25. Berrevoets MAH, Pot JHLW, Houterman AE, et al. An electronic trigger tool to optimise intravenous to oral antibiotic switch: a controlled, interrupted time series study. Antimicrob Resist Infect Control. 2017;6:81. https://doi.org/10.1186/s13756-017-0239-3.
26. Fischer MA, Solomon DH, Teich JM, Avorn J. Conversion from intravenous to oral medications: assessment of a computerized intervention for hospitalized patients. Arch Intern Med. 2003;163(21):2585-2589. https://doi.org/10.1001/archinte.163.21.2585.
27. Schroeder AR, Harris SJ, Newman TB. Safely doing less: a missing component of the patient safety dialogue. Pediatrics. 2011;128(6):e1596-e1597. https://doi.org/10.1542/peds.2011-2726.
Intravenous (IV) antibiotics are commonly used in hospitalized pediatric patients to treat bacterial infections. Antimicrobial stewardship guidelines published by the Infectious Diseases Society of America (IDSA) recommend institutions develop a systematic plan to convert from IV to enteral antibiotics, as early transition may reduce healthcare costs, decrease length of stay (LOS), and avoid prolonged IV access complications1 such as extravasation, thrombosis, and catheter-associated infections.2-5
Pediatric patients with community-acquired pneumonia (CAP) and mild skin and soft tissue infections (SSTI) may not require IV antibiotics, even if the patient is hospitalized.6 Although national guidelines for pediatric CAP and SSTI recommend IV antibiotics for hospitalized patients, these guidelines state that mild infections may be treated with enteral antibiotics and emphasize discontinuation of IV antibiotics when the patient meets discharge criteria.7,8 Furthermore, several enteral antibiotics used for the treatment of CAP and SSTI, such as cephalexin and clindamycin,9 have excellent bioavailability (>90%) or can achieve sufficient concentrations to attain the pharmacodynamic target (ie, amoxicillin and trimethoprim–sulfamethoxazole).10,11 Nonetheless, the guidelines do not explicitly outline criteria regarding the transition from IV to enteral antibiotics.7,8
At our institution, patients admitted to Hospital Medicine (HM) often remained on IV antibiotics until discharge. Data review revealed that antibiotic treatment of CAP and SSTI posed the greatest opportunity for early conversion to enteral therapy based on the high frequency of admissions and the ability of commonly used enteral antibiotics to attain pharmacodynamic targets. We sought to change practice culture by decoupling transition to enteral antibiotics from discharge and use administration of other enteral medications as an objective indicator for transition. Our aim was to increase the proportion of enterally administered antibiotic doses for HM patients aged >60 days admitted with uncomplicated CAP or SSTI from 44% to 75% in eight months.
METHODS
Context
Cincinnati Children’s Hospital Medical Center (CCHMC) is a large, urban, academic hospital. The HM division has 45 attendings and admits >8,000 general pediatric patients annually. The five HM teams at the main campus consist of attendings, fellows, residents, and medical students. One HM team serves as the resident quality improvement (QI) team where residents collaborate in a longitudinal study under the guidance of QI-trained coaches. The focus of this QI initiative was determined by resident consensus and aligned with a high-value care curriculum.12
To identify the target patient population, we investigated IV antimicrobials frequently used in HM patients. Ampicillin and clindamycin are commonly used IV antibiotics, most frequently corresponding with the diagnoses of CAP and SSTI, respectively, accounting for half of all antibiotic use on the HM service. Amoxicillin, the enteral equivalent of ampicillin, can achieve sufficient concentrations to attain the pharmacodynamic target at infection sites, and clindamycin has high bioavailability, making them ideal options for early transition. Our institution’s robust antimicrobial stewardship program has published local guidelines on using amoxicillin as the enteral antibiotic of choice for uncomplicated CAP, but it does not provide guidance on the timing of transition for either CAP or SSTI; the clinical team makes this decision.
HM attendings were surveyed to determine the criteria used to transition from IV to enteral antibiotics for patients with CAP or SSTI. The survey illustrated practice variability with providers using differing clinical criteria to signal the timing of transition. Additionally, only 49% of respondents (n = 37) rated themselves as “very comfortable” with residents making autonomous decisions to transition to enteral antibiotics. We chose to use the administration of other enteral medications, instead of discharge readiness, as an objective indicator of a patient’s readiness to transition to enteral antibiotics, given the low-risk patient population and the ability of the enteral antibiotics commonly used for CAP and SSTI to achieve pharmacodynamic targets.
The study population included patients aged >60 days admitted to HM with CAP or SSTI treated with any antibiotic. We excluded patients with potential complications or significant progression of their disease process, including patients with parapneumonic effusions or chest tubes, patients who underwent bronchoscopy, and patients with osteomyelitis, septic arthritis, or preseptal or orbital cellulitis. Past medical history and clinical status on admission were not used to exclude patients.
Interventions
Our multidisciplinary team, formed in January 2017, included HM attendings, HM fellows, pediatric residents, a critical care attending, a pharmacy resident, and an antimicrobial stewardship pharmacist. Under the guidance of QI coaches, the residents on the HM QI team developed and tested all interventions on their team and then determined which interventions would spread to the other four teams. The nursing director of our primary HM unit disseminated project updates to bedside nurses. A simplified failure mode and effects analysis identified areas for improvement and potential interventions. Interventions focused on the following key drivers (Figure 1): increased prescriber awareness of medication charge, standardization of conversion from IV to enteral antibiotics, clear definition of the patients ready for transition, ongoing evaluation of the antimicrobial plan, timely recognition by prescribers of patients ready for transition, culture shift regarding the appropriate administration route in the inpatient setting, and transparency of data. The team implemented sequential Plan-Do-Study-Act (PDSA) cycles13 to test the interventions.
Charge Table
To improve knowledge about the increased charge for commonly used IV medications compared with enteral formulations, a table comparing relative charges was shared during monthly resident morning conferences and at an HM faculty meeting. The table included charge comparisons between ampicillin and amoxicillin and IV and enteral clindamycin.
Standardized Language in Electronic Health Record (EHR) Antibiotic Plan on Rounds
Standardized language to document antibiotic transition plans was added to admission and progress note templates in the EHR. The standard template prompted residents to (1) define clinical transition criteria, (2) discuss attending comfort with transition overnight (based on survey results), and (3) document patient preference of solid or liquid dosage forms. Plans were reviewed and updated daily. We hypothesized that since residents use the information in the daily progress notes, including assessments and plans, to present on rounds, inclusion of the transition criteria in the note would prompt transition plan discussions.
Communication Bundle
To promote early transition to enteral antibiotics, we standardized the discussion about antibiotic transition between residents and attendings. During a weekly preexisting meeting, the resident QI team reviewed preferences for transitions with the new service attending. By identifying attending preferences early, residents were able to proactively transition patients who met the criteria (eg, antibiotic transition in the evening instead of waiting until morning rounds). This discussion also provided an opportunity to engage service attendings in the QI efforts, which were also shared at HM faculty meetings quarterly.
Recognizing that in times of high census, discussion of patient plans may be abbreviated during rounds, residents were asked to identify all patients on IV antibiotics while reviewing patient medication orders prior to rounds. As part of an existing daily prerounds huddle to discuss rounding logistics, residents listed all patients on IV antibiotics and discussed which patients were ready for transition. If patients could not be transitioned immediately, the team identified the transition criteria.
At preexisting evening huddles between overnight shift HM residents and the evening HM attending, residents identified patients who were prescribed IV antibiotics and discussed readiness for enteral transition. If a patient could be transitioned overnight, enteral antibiotic orders were placed. Overnight residents were also encouraged to review the transition criteria with families upon admission.
Real-time Identification of Failures and Feedback
For two weeks, the EHR was queried daily to identify patients admitted for uncomplicated CAP and SSTI who were on antibiotics as well as other enteral medications. A failure was defined as an IV antibiotic dose given to a patient who was administered any enteral medication. Residents on the QI team approached residents on other HM teams whenever patients were identified as a failed transition to learn about failure reasons.
Study of the Interventions
Data for HM patients who met the inclusion criteria were collected weekly from January 2016 through June 2018 via EHR query. We initially searched for diagnoses that fit under the disease categories of pneumonia and SSTI in the EHR, which generated a list of International Classification of Disease-9 and -10 Diagnosis codes (Appendix Figure 1). The query identified patients based on these codes and reported whether the identified patients took a dose of any enteral medication, excluding nystatin, sildenafil, tacrolimus, and mouthwashes, which are commonly continued during NPO status due to no need for absorption or limited parenteral options. It also reported the ordered route of administration for the queried antibiotics (Appendix Figure 1).
The 2016 calendar year established our baseline to account for seasonal variability. Data were reported weekly and reviewed to evaluate the impact of PDSA cycles and inform new interventions.
Measures
Our process measure was the total number of enteral antibiotic doses divided by all antibiotic doses in patients receiving any enteral medication. We reasoned that if patients were well enough to take medications enterally, they could be given an enteral antibiotic that is highly bioavailable or readily achieves concentrations that attain pharmacodynamic targets. This practice change was a culture shift, decoupling the switch to enteral antibiotics from discharge readiness. Our EHR query reported only the antibiotic doses given to patients who took an enteral medication on the day of antibiotic administration and excluded patients who received only IV medications.
Outcome measures included antimicrobial costs per patient encounter using average wholesale prices, which were reported in our EHR query, and LOS. To ensure that transitions of IV to enteral antibiotics were not negatively impacting patient outcomes, patient readmissions within seven days served as a balancing measure.
Analysis
An annotated statistical process control p-chart tracked the impact of interventions on the proportion of antibiotic doses that were enterally administered during hospitalization. An x-bar and an s-chart tracked the impact of interventions on antimicrobial costs per patient encounter and on LOS. A p-chart and an encounters-between g-chart were used to evaluate the impact of our interventions on readmissions. Control chart rules for identifying special cause were used for center line shifts.14
Ethical Considerations
This study was part of a larger study of the residency high-value care curriculum,12 which was deemed exempt by the CCHMC IRB.
RESULTS
The baseline data collected included 372 patients and the postintervention period in 2017 included 326 patients (Table). Approximately two-thirds of patients had a diagnosis of CAP.
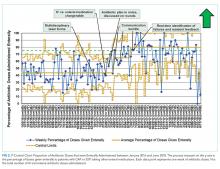
The percentage of antibiotic doses given enterally increased from 44% to 80% within eight months (Figure 2). When studying the impact of interventions, residents on the HM QI team found that the standard EHR template added to daily notes did not consistently prompt residents to discuss antibiotic plans and thus was abandoned. Initial improvement coincided with standardizing discussions between residents and attendings regarding transitions. Furthermore, discussion of all patients on IV antibiotics during the prerounds huddle allowed for reliable, daily communication about antibiotic plans and was subsequently spread to and adopted by all HM teams. The percentage of enterally administered antibiotic doses increased to >75% after the evening huddle, which involved all HM teams, and real-time identification of failures on all HM teams with provider feedback. Despite variability when the total number of antibiotic doses prescribed per week was low (<10), we demonstrated sustainability for 11 months (Figure 2), during which the prerounds and evening huddle discussions were continued and an updated control chart was shown monthly to residents during their educational conferences.
Residents on the QI team spoke directly with other HM residents when there were missed opportunities for transition. Based on these discussions and intermittent chart reviews, common reasons for failure to transition in patients with CAP included admission for failed outpatient enteral treatment, recent evaluation by critical care physicians for possible transfer to the intensive care unit, and difficulty weaning oxygen. For patients with SSTI, hand abscesses requiring drainage by surgery and treatment failure with other antibiotics constituted many of the IV antibiotic doses given to patients on enteral medications.
Antimicrobial costs per patient encounter decreased by 70% over one year; the shift in costs coincided with the second shift in our process measure (Appendix Figure 2A). Based on an estimate of 350 patients admitted per year for uncomplicated CAP or SSTI, this translates to an annual cost savings of approximately $29,000. The standard deviation of costs per patient encounter decreased by 84% (Appendix Figure 2B), suggesting a decrease in the variability of prescribing practices.
The average LOS in our patient population prior to intervention was 2.1 days and did not change (Appendix Figure 2C), but the standard deviation decreased by >50% (Appendix Figure 2D). There was no shift in the mean seven-day readmission rate or the number of encounters between readmissions (2.6% and 26, respectively; Appendix Figure 3). In addition, the hospital billing department did not identify an increase in insurance denials related to the route of antibiotic administration.
DISCUSSION
Summary
Using improvement science, we promoted earlier transition to enteral antibiotics for children hospitalized with uncomplicated CAP and SSTI by linking the decision for transition to the ability to take other enteral medications, rather than to discharge readiness. We increased the percentage of enterally administered antibiotic doses in this patient population from 44% to 80% in eight months. Although we did not observe a decrease in LOS as previously noted in a cost analysis study comparing pediatric patients with CAP treated with oral antibiotics versus those treated with IV antibiotics,15 we did find a decrease in LOS variability and in antimicrobial costs to our patients. These cost savings did not include potential savings from nursing or pharmacy labor. In addition, we noted a decrease in the variability in antibiotic prescribing practice, which demonstrates provider ability and willingness to couple antibiotic route transition to an objective characteristic (administration of other enteral medications).
A strength of our study was that residents, the most frequent prescribers of antibiotics on our HM service, were highly involved in the QI initiative, including defining the SMART aim, identifying key drivers, developing interventions, and completing sequential PDSA cycles. Under the guidance of QI-trained coaches, residents developed feasible interventions and assessed their success in real time. Consistent with other studies,16,17 resident buy-in and involvement led to the success of our improvement study.
Interpretation
Despite emerging evidence regarding the timing of transition to enteral antibiotics, several factors impeded early transition at our institution, including physician culture, variable practice habits, and hospital workflow. Evidence supports the use of enteral antibiotics in immunocompetent children hospitalized for uncomplicated CAP who do not have chronic lung disease, are not in shock, and have oxygen saturations >85%.6 Although existing literature suggests that in pediatric patients admitted for SSTIs not involving the eye or bone, IV antibiotics may be transitioned when clinical improvement, evidenced by a reduction in fever or erythema, is noted,6 enteral antibiotics that achieve appropriate concentrations to attain pharmacodynamic targets should have the same efficacy as that of IV antibiotics.9 Using the criterion of administration of any medication enterally to identify a patient’s readiness to transition, we were able to overcome practice variation among providers who may have differing opinions of what constitutes clinical improvement. Of note, new evidence is emerging on predictors of enteral antibiotic treatment failure in patients with CAP and SSTI to guide transition timing, but these studies have largely focused on the adult population or were performed in the outpatient and emergency department (ED) settings.18,19 Regardless, the stable number of encounters between readmissions in our patient population likely indicates that treatment failure in these patients was rare.
Rising healthcare costs have led to concerns around sustainability of the healthcare system;20,21 tackling overuse in clinical practice, as in our study, is one mitigation strategy. Several studies have used QI methods to facilitate the provision of high-value care through the decrease of continuous monitor overuse and extraneous ordering of electrolytes.22,23 Our QI study adds to the high-value care literature by safely decreasing the use of IV antibiotics. One retrospective study demonstrated that a one-day decrease in the use of IV antibiotics in pneumonia resulted in decreased costs without an increase in readmissions, similar to our findings.24 In adults, QI initiatives aimed at improving early transition of antibiotics utilized electronic trigger tools.25,26 Fischer et al. used active orders for scheduled enteral medications or an enteral diet as indication that a patient’s IV medications could be converted to enteral form.26
Our work is not without limitations. The list of ICD-9 and -10 codes used to query the EHR did not capture all diagnoses that would be considered as uncomplicated CAP or SSTI. However, we included an extensive list of diagnoses to ensure that the majority of patients meeting our inclusion criteria were captured. Our process measure did not account for patients on IV antibiotics who were not administered other enteral medications but tolerating an enteral diet. These patients were not identified in our EHR query and were not included in our process measure as a failure. However, in latter interventions, residents identified all patients on IV antibiotics, so that patients not identified by our EHR query benefited from our work. Furthermore, this QI study was conducted at a single institution and several interventions took advantage of preexisting structured huddles and a resident QI curriculum, which may not exist at other institutions. Our study does highlight that engaging frontline providers, such as residents, to review antibiotic orders consistently and question the appropriateness of the administration route is key to making incremental changes in prescribing practices.
CONCLUSIONS
Through a partnership between HM and Pharmacy and with substantial resident involvement, we improved the transition of IV antibiotics in patients with CAP or SSTI by increasing the percentage of enterally administered antibiotic doses and reducing antimicrobial costs and variability in antibiotic prescribing practices. This work illustrates how reducing overuse of IV antibiotics promotes high-value care and aligns with initiatives to prevent avoidable harm.27 Our work highlights that standardized discussions about medication orders to create consensus around enteral antibiotic transitions, real-time feedback, and challenging the status quo can influence practice habits and effect change.
Next steps include testing automated methods to notify providers of opportunities for transition from IV to enteral antibiotics through embedded clinical decision support, a method similar to the electronic trigger tools used in adult QI studies.25,26 Since our prerounds huddle includes identifying all patients on IV antibiotics, studying the transition to enteral antibiotics and its effect on prescribing practices in other diagnoses (ie, urinary tract infection and osteomyelitis) may contribute to spreading these efforts. Partnering with our ED colleagues may be an important next step, as several patients admitted to HM on IV antibiotics are given their first dose in the ED.
Acknowledgments
The authors would like to thank the faculty of the James M. Anderson Center for Health Systems Excellence Intermediate Improvement Science Series for their guidance in the planning of this project. The authors would also like to thank Ms. Ursula Bradshaw and Mr. Michael Ponti-Zins for obtaining the hospital data on length of stay and readmissions. The authors acknowledge Dr. Philip Hagedorn for his assistance with the software that queries the electronic health record and Dr. Laura Brower and Dr. Joanna Thomson for their assistance with statistical analysis. The authors are grateful to all the residents and coaches on the QI Hospital Medicine team who contributed ideas on study design and interventions.
Intravenous (IV) antibiotics are commonly used in hospitalized pediatric patients to treat bacterial infections. Antimicrobial stewardship guidelines published by the Infectious Diseases Society of America (IDSA) recommend institutions develop a systematic plan to convert from IV to enteral antibiotics, as early transition may reduce healthcare costs, decrease length of stay (LOS), and avoid prolonged IV access complications1 such as extravasation, thrombosis, and catheter-associated infections.2-5
Pediatric patients with community-acquired pneumonia (CAP) and mild skin and soft tissue infections (SSTI) may not require IV antibiotics, even if the patient is hospitalized.6 Although national guidelines for pediatric CAP and SSTI recommend IV antibiotics for hospitalized patients, these guidelines state that mild infections may be treated with enteral antibiotics and emphasize discontinuation of IV antibiotics when the patient meets discharge criteria.7,8 Furthermore, several enteral antibiotics used for the treatment of CAP and SSTI, such as cephalexin and clindamycin,9 have excellent bioavailability (>90%) or can achieve sufficient concentrations to attain the pharmacodynamic target (ie, amoxicillin and trimethoprim–sulfamethoxazole).10,11 Nonetheless, the guidelines do not explicitly outline criteria regarding the transition from IV to enteral antibiotics.7,8
At our institution, patients admitted to Hospital Medicine (HM) often remained on IV antibiotics until discharge. Data review revealed that antibiotic treatment of CAP and SSTI posed the greatest opportunity for early conversion to enteral therapy based on the high frequency of admissions and the ability of commonly used enteral antibiotics to attain pharmacodynamic targets. We sought to change practice culture by decoupling transition to enteral antibiotics from discharge and use administration of other enteral medications as an objective indicator for transition. Our aim was to increase the proportion of enterally administered antibiotic doses for HM patients aged >60 days admitted with uncomplicated CAP or SSTI from 44% to 75% in eight months.
METHODS
Context
Cincinnati Children’s Hospital Medical Center (CCHMC) is a large, urban, academic hospital. The HM division has 45 attendings and admits >8,000 general pediatric patients annually. The five HM teams at the main campus consist of attendings, fellows, residents, and medical students. One HM team serves as the resident quality improvement (QI) team where residents collaborate in a longitudinal study under the guidance of QI-trained coaches. The focus of this QI initiative was determined by resident consensus and aligned with a high-value care curriculum.12
To identify the target patient population, we investigated IV antimicrobials frequently used in HM patients. Ampicillin and clindamycin are commonly used IV antibiotics, most frequently corresponding with the diagnoses of CAP and SSTI, respectively, accounting for half of all antibiotic use on the HM service. Amoxicillin, the enteral equivalent of ampicillin, can achieve sufficient concentrations to attain the pharmacodynamic target at infection sites, and clindamycin has high bioavailability, making them ideal options for early transition. Our institution’s robust antimicrobial stewardship program has published local guidelines on using amoxicillin as the enteral antibiotic of choice for uncomplicated CAP, but it does not provide guidance on the timing of transition for either CAP or SSTI; the clinical team makes this decision.
HM attendings were surveyed to determine the criteria used to transition from IV to enteral antibiotics for patients with CAP or SSTI. The survey illustrated practice variability with providers using differing clinical criteria to signal the timing of transition. Additionally, only 49% of respondents (n = 37) rated themselves as “very comfortable” with residents making autonomous decisions to transition to enteral antibiotics. We chose to use the administration of other enteral medications, instead of discharge readiness, as an objective indicator of a patient’s readiness to transition to enteral antibiotics, given the low-risk patient population and the ability of the enteral antibiotics commonly used for CAP and SSTI to achieve pharmacodynamic targets.
The study population included patients aged >60 days admitted to HM with CAP or SSTI treated with any antibiotic. We excluded patients with potential complications or significant progression of their disease process, including patients with parapneumonic effusions or chest tubes, patients who underwent bronchoscopy, and patients with osteomyelitis, septic arthritis, or preseptal or orbital cellulitis. Past medical history and clinical status on admission were not used to exclude patients.
Interventions
Our multidisciplinary team, formed in January 2017, included HM attendings, HM fellows, pediatric residents, a critical care attending, a pharmacy resident, and an antimicrobial stewardship pharmacist. Under the guidance of QI coaches, the residents on the HM QI team developed and tested all interventions on their team and then determined which interventions would spread to the other four teams. The nursing director of our primary HM unit disseminated project updates to bedside nurses. A simplified failure mode and effects analysis identified areas for improvement and potential interventions. Interventions focused on the following key drivers (Figure 1): increased prescriber awareness of medication charge, standardization of conversion from IV to enteral antibiotics, clear definition of the patients ready for transition, ongoing evaluation of the antimicrobial plan, timely recognition by prescribers of patients ready for transition, culture shift regarding the appropriate administration route in the inpatient setting, and transparency of data. The team implemented sequential Plan-Do-Study-Act (PDSA) cycles13 to test the interventions.
Charge Table
To improve knowledge about the increased charge for commonly used IV medications compared with enteral formulations, a table comparing relative charges was shared during monthly resident morning conferences and at an HM faculty meeting. The table included charge comparisons between ampicillin and amoxicillin and IV and enteral clindamycin.
Standardized Language in Electronic Health Record (EHR) Antibiotic Plan on Rounds
Standardized language to document antibiotic transition plans was added to admission and progress note templates in the EHR. The standard template prompted residents to (1) define clinical transition criteria, (2) discuss attending comfort with transition overnight (based on survey results), and (3) document patient preference of solid or liquid dosage forms. Plans were reviewed and updated daily. We hypothesized that since residents use the information in the daily progress notes, including assessments and plans, to present on rounds, inclusion of the transition criteria in the note would prompt transition plan discussions.
Communication Bundle
To promote early transition to enteral antibiotics, we standardized the discussion about antibiotic transition between residents and attendings. During a weekly preexisting meeting, the resident QI team reviewed preferences for transitions with the new service attending. By identifying attending preferences early, residents were able to proactively transition patients who met the criteria (eg, antibiotic transition in the evening instead of waiting until morning rounds). This discussion also provided an opportunity to engage service attendings in the QI efforts, which were also shared at HM faculty meetings quarterly.
Recognizing that in times of high census, discussion of patient plans may be abbreviated during rounds, residents were asked to identify all patients on IV antibiotics while reviewing patient medication orders prior to rounds. As part of an existing daily prerounds huddle to discuss rounding logistics, residents listed all patients on IV antibiotics and discussed which patients were ready for transition. If patients could not be transitioned immediately, the team identified the transition criteria.
At preexisting evening huddles between overnight shift HM residents and the evening HM attending, residents identified patients who were prescribed IV antibiotics and discussed readiness for enteral transition. If a patient could be transitioned overnight, enteral antibiotic orders were placed. Overnight residents were also encouraged to review the transition criteria with families upon admission.
Real-time Identification of Failures and Feedback
For two weeks, the EHR was queried daily to identify patients admitted for uncomplicated CAP and SSTI who were on antibiotics as well as other enteral medications. A failure was defined as an IV antibiotic dose given to a patient who was administered any enteral medication. Residents on the QI team approached residents on other HM teams whenever patients were identified as a failed transition to learn about failure reasons.
Study of the Interventions
Data for HM patients who met the inclusion criteria were collected weekly from January 2016 through June 2018 via EHR query. We initially searched for diagnoses that fit under the disease categories of pneumonia and SSTI in the EHR, which generated a list of International Classification of Disease-9 and -10 Diagnosis codes (Appendix Figure 1). The query identified patients based on these codes and reported whether the identified patients took a dose of any enteral medication, excluding nystatin, sildenafil, tacrolimus, and mouthwashes, which are commonly continued during NPO status due to no need for absorption or limited parenteral options. It also reported the ordered route of administration for the queried antibiotics (Appendix Figure 1).
The 2016 calendar year established our baseline to account for seasonal variability. Data were reported weekly and reviewed to evaluate the impact of PDSA cycles and inform new interventions.
Measures
Our process measure was the total number of enteral antibiotic doses divided by all antibiotic doses in patients receiving any enteral medication. We reasoned that if patients were well enough to take medications enterally, they could be given an enteral antibiotic that is highly bioavailable or readily achieves concentrations that attain pharmacodynamic targets. This practice change was a culture shift, decoupling the switch to enteral antibiotics from discharge readiness. Our EHR query reported only the antibiotic doses given to patients who took an enteral medication on the day of antibiotic administration and excluded patients who received only IV medications.
Outcome measures included antimicrobial costs per patient encounter using average wholesale prices, which were reported in our EHR query, and LOS. To ensure that transitions of IV to enteral antibiotics were not negatively impacting patient outcomes, patient readmissions within seven days served as a balancing measure.
Analysis
An annotated statistical process control p-chart tracked the impact of interventions on the proportion of antibiotic doses that were enterally administered during hospitalization. An x-bar and an s-chart tracked the impact of interventions on antimicrobial costs per patient encounter and on LOS. A p-chart and an encounters-between g-chart were used to evaluate the impact of our interventions on readmissions. Control chart rules for identifying special cause were used for center line shifts.14
Ethical Considerations
This study was part of a larger study of the residency high-value care curriculum,12 which was deemed exempt by the CCHMC IRB.
RESULTS
The baseline data collected included 372 patients and the postintervention period in 2017 included 326 patients (Table). Approximately two-thirds of patients had a diagnosis of CAP.
The percentage of antibiotic doses given enterally increased from 44% to 80% within eight months (Figure 2). When studying the impact of interventions, residents on the HM QI team found that the standard EHR template added to daily notes did not consistently prompt residents to discuss antibiotic plans and thus was abandoned. Initial improvement coincided with standardizing discussions between residents and attendings regarding transitions. Furthermore, discussion of all patients on IV antibiotics during the prerounds huddle allowed for reliable, daily communication about antibiotic plans and was subsequently spread to and adopted by all HM teams. The percentage of enterally administered antibiotic doses increased to >75% after the evening huddle, which involved all HM teams, and real-time identification of failures on all HM teams with provider feedback. Despite variability when the total number of antibiotic doses prescribed per week was low (<10), we demonstrated sustainability for 11 months (Figure 2), during which the prerounds and evening huddle discussions were continued and an updated control chart was shown monthly to residents during their educational conferences.
Residents on the QI team spoke directly with other HM residents when there were missed opportunities for transition. Based on these discussions and intermittent chart reviews, common reasons for failure to transition in patients with CAP included admission for failed outpatient enteral treatment, recent evaluation by critical care physicians for possible transfer to the intensive care unit, and difficulty weaning oxygen. For patients with SSTI, hand abscesses requiring drainage by surgery and treatment failure with other antibiotics constituted many of the IV antibiotic doses given to patients on enteral medications.
Antimicrobial costs per patient encounter decreased by 70% over one year; the shift in costs coincided with the second shift in our process measure (Appendix Figure 2A). Based on an estimate of 350 patients admitted per year for uncomplicated CAP or SSTI, this translates to an annual cost savings of approximately $29,000. The standard deviation of costs per patient encounter decreased by 84% (Appendix Figure 2B), suggesting a decrease in the variability of prescribing practices.
The average LOS in our patient population prior to intervention was 2.1 days and did not change (Appendix Figure 2C), but the standard deviation decreased by >50% (Appendix Figure 2D). There was no shift in the mean seven-day readmission rate or the number of encounters between readmissions (2.6% and 26, respectively; Appendix Figure 3). In addition, the hospital billing department did not identify an increase in insurance denials related to the route of antibiotic administration.
DISCUSSION
Summary
Using improvement science, we promoted earlier transition to enteral antibiotics for children hospitalized with uncomplicated CAP and SSTI by linking the decision for transition to the ability to take other enteral medications, rather than to discharge readiness. We increased the percentage of enterally administered antibiotic doses in this patient population from 44% to 80% in eight months. Although we did not observe a decrease in LOS as previously noted in a cost analysis study comparing pediatric patients with CAP treated with oral antibiotics versus those treated with IV antibiotics,15 we did find a decrease in LOS variability and in antimicrobial costs to our patients. These cost savings did not include potential savings from nursing or pharmacy labor. In addition, we noted a decrease in the variability in antibiotic prescribing practice, which demonstrates provider ability and willingness to couple antibiotic route transition to an objective characteristic (administration of other enteral medications).
A strength of our study was that residents, the most frequent prescribers of antibiotics on our HM service, were highly involved in the QI initiative, including defining the SMART aim, identifying key drivers, developing interventions, and completing sequential PDSA cycles. Under the guidance of QI-trained coaches, residents developed feasible interventions and assessed their success in real time. Consistent with other studies,16,17 resident buy-in and involvement led to the success of our improvement study.
Interpretation
Despite emerging evidence regarding the timing of transition to enteral antibiotics, several factors impeded early transition at our institution, including physician culture, variable practice habits, and hospital workflow. Evidence supports the use of enteral antibiotics in immunocompetent children hospitalized for uncomplicated CAP who do not have chronic lung disease, are not in shock, and have oxygen saturations >85%.6 Although existing literature suggests that in pediatric patients admitted for SSTIs not involving the eye or bone, IV antibiotics may be transitioned when clinical improvement, evidenced by a reduction in fever or erythema, is noted,6 enteral antibiotics that achieve appropriate concentrations to attain pharmacodynamic targets should have the same efficacy as that of IV antibiotics.9 Using the criterion of administration of any medication enterally to identify a patient’s readiness to transition, we were able to overcome practice variation among providers who may have differing opinions of what constitutes clinical improvement. Of note, new evidence is emerging on predictors of enteral antibiotic treatment failure in patients with CAP and SSTI to guide transition timing, but these studies have largely focused on the adult population or were performed in the outpatient and emergency department (ED) settings.18,19 Regardless, the stable number of encounters between readmissions in our patient population likely indicates that treatment failure in these patients was rare.
Rising healthcare costs have led to concerns around sustainability of the healthcare system;20,21 tackling overuse in clinical practice, as in our study, is one mitigation strategy. Several studies have used QI methods to facilitate the provision of high-value care through the decrease of continuous monitor overuse and extraneous ordering of electrolytes.22,23 Our QI study adds to the high-value care literature by safely decreasing the use of IV antibiotics. One retrospective study demonstrated that a one-day decrease in the use of IV antibiotics in pneumonia resulted in decreased costs without an increase in readmissions, similar to our findings.24 In adults, QI initiatives aimed at improving early transition of antibiotics utilized electronic trigger tools.25,26 Fischer et al. used active orders for scheduled enteral medications or an enteral diet as indication that a patient’s IV medications could be converted to enteral form.26
Our work is not without limitations. The list of ICD-9 and -10 codes used to query the EHR did not capture all diagnoses that would be considered as uncomplicated CAP or SSTI. However, we included an extensive list of diagnoses to ensure that the majority of patients meeting our inclusion criteria were captured. Our process measure did not account for patients on IV antibiotics who were not administered other enteral medications but tolerating an enteral diet. These patients were not identified in our EHR query and were not included in our process measure as a failure. However, in latter interventions, residents identified all patients on IV antibiotics, so that patients not identified by our EHR query benefited from our work. Furthermore, this QI study was conducted at a single institution and several interventions took advantage of preexisting structured huddles and a resident QI curriculum, which may not exist at other institutions. Our study does highlight that engaging frontline providers, such as residents, to review antibiotic orders consistently and question the appropriateness of the administration route is key to making incremental changes in prescribing practices.
CONCLUSIONS
Through a partnership between HM and Pharmacy and with substantial resident involvement, we improved the transition of IV antibiotics in patients with CAP or SSTI by increasing the percentage of enterally administered antibiotic doses and reducing antimicrobial costs and variability in antibiotic prescribing practices. This work illustrates how reducing overuse of IV antibiotics promotes high-value care and aligns with initiatives to prevent avoidable harm.27 Our work highlights that standardized discussions about medication orders to create consensus around enteral antibiotic transitions, real-time feedback, and challenging the status quo can influence practice habits and effect change.
Next steps include testing automated methods to notify providers of opportunities for transition from IV to enteral antibiotics through embedded clinical decision support, a method similar to the electronic trigger tools used in adult QI studies.25,26 Since our prerounds huddle includes identifying all patients on IV antibiotics, studying the transition to enteral antibiotics and its effect on prescribing practices in other diagnoses (ie, urinary tract infection and osteomyelitis) may contribute to spreading these efforts. Partnering with our ED colleagues may be an important next step, as several patients admitted to HM on IV antibiotics are given their first dose in the ED.
Acknowledgments
The authors would like to thank the faculty of the James M. Anderson Center for Health Systems Excellence Intermediate Improvement Science Series for their guidance in the planning of this project. The authors would also like to thank Ms. Ursula Bradshaw and Mr. Michael Ponti-Zins for obtaining the hospital data on length of stay and readmissions. The authors acknowledge Dr. Philip Hagedorn for his assistance with the software that queries the electronic health record and Dr. Laura Brower and Dr. Joanna Thomson for their assistance with statistical analysis. The authors are grateful to all the residents and coaches on the QI Hospital Medicine team who contributed ideas on study design and interventions.
1. Dellit TH, Owens RC, McGowan JE, Jr, et al. Infectious diseases society of America and the society for healthcare epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44(2):159-177. https://doi.org/10.1086/510393.
2. Shah SS, Srivastava R, Wu S, et al. Intravenous Versus oral antibiotics for postdischarge treatment of complicated pneumonia. Pediatrics. 2016;138(6). https://doi.org/10.1542/peds.2016-1692.
3. Keren R, Shah SS, Srivastava R, et al. Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomyelitis in children. JAMA Pediatr. 2015;169(2):120-128. https://doi.org/10.1001/jamapediatrics.2014.2822.
4. Jumani K, Advani S, Reich NG, Gosey L, Milstone AM. Risk factors for peripherally inserted central venous catheter complications in children. JAMA Pediatr. 2013;167(5):429-435.https://doi.org/10.1001/jamapediatrics.2013.775.
5. Zaoutis T, Localio AR, Leckerman K, et al. Prolonged intravenous therapy versus early transition to oral antimicrobial therapy for acute osteomyelitis in children. Pediatrics. 2009;123(2):636-642. https://doi.org/10.1542/peds.2008-0596.
6. McMullan BJ, Andresen D, Blyth CC, et al. Antibiotic duration and timing of the switch from intravenous to oral route for bacterial infections in children: systematic review and guidelines. Lancet Infect Dis. 2016;16(8):e139-e152. https://doi.org/10.1016/S1473-3099(16)30024-X.
7. Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):e25-e76. https://doi.org/10.1093/cid/cir531.
8. Stevens DL, Bisno AL, Chambers HF, et al. Executive summary: practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the infectious diseases society of America. Clin Infect Dis. 2014;59(2):147-159. https://doi.org/10.1093/cid/ciu444.
9. MacGregor RR, Graziani AL. Oral administration of antibiotics: a rational alternative to the parenteral route. Clin Infect Dis. 1997;24(3):457-467. https://doi.org/10.1093/clinids/24.3.457.
10. Downes KJ, Hahn A, Wiles J, Courter JD, Vinks AA. Dose optimisation of antibiotics in children: application of pharmacokinetics/pharmacodynamics in paediatrics. Int J Antimicrob Agents. 2014;43(3):223-230. https://doi.org/10.1016/j.ijantimicag.2013.11.006.
11. Autmizguine J, Melloni C, Hornik CP, et al. Population pharmacokinetics of trimethoprim-sulfamethoxazole in infants and children. Antimicrob Agents Chemother. 2018;62(1):e01813-e01817. https://doi.org/10.1128/AAC.01813-17.
12. Dewan M, Herrmann LE, Tchou MJ, et al. Development and evaluation of high-value pediatrics: a high-value care pediatric resident curriculum. Hosp Pediatr. 2018;8(12):785-792. https://doi.org/10.1542/hpeds.2018-0115
13. Langley GJ, Moen RD, Nolan KM, Nolan TW, Norman CL, Provost LP. The Improvement Guide: A Practical Approach to Enhancing Organizational Performance. New Jersey, US: John Wiley & Sons; 2009.
14. Benneyan JC. Use and interpretation of statistical quality control charts. Int J Qual Health Care. 1998;10(1):69-73. https://doi.org/10.1093/intqhc/10.1.69.
15. Lorgelly PK, Atkinson M, Lakhanpaul M, et al. Oral versus i.v. antibiotics for community-acquired pneumonia in children: a cost-minimisation analysis. Eur Respir J. 2010;35(4):858-864. https://doi.org/10.1183/09031936.00087209.
16. Vidyarthi AR, Green AL, Rosenbluth G, Baron RB. Engaging residents and fellows to improve institution-wide quality: the first six years of a novel financial incentive program. Acad Med. 2014;89(3):460-468. https://doi.org/10.1097/ACM.0000000000000159.
17. Stinnett-Donnelly JM, Stevens PG, Hood VL. Developing a high value care programme from the bottom up: a programme of faculty-resident improvement projects targeting harmful or unnecessary care. BMJ Qual Saf. 2016;25(11):901-908. https://doi.org/10.1136/bmjqs-2015-004546.
18. Peterson D, McLeod S, Woolfrey K, McRae A. Predictors of failure of empiric outpatient antibiotic therapy in emergency department patients with uncomplicated cellulitis. Acad Emerg Med. 2014;21(5):526-531. https://doi.org/10.1111/acem.12371.
19. Yadav K, Suh KN, Eagles D, et al. Predictors of oral antibiotic treatment failure for non-purulent skin and soft tissue infections in the emergency department. Acad Emerg Med. 2018;20(S1):S24-S25. https://doi.org/10.1017/cem.2018.114.
20. Organisation for Economic Co-operation and Development. Healthcare costs unsustainable in advanced economies without reform. http://www.oecd.org/health/healthcarecostsunsustainableinadvancedeconomieswithoutreform.htm. Accessed June 28, 2018; 2015.
21. Berwick DM, Hackbarth AD. Eliminating waste in US health care. JAMA. 2012;307(14):1513-1516. https://doi.org/10.1001/jama.2012.362.
22. Schondelmeyer AC, Simmons JM, Statile AM, et al. Using quality improvement to reduce continuous pulse oximetry use in children with wheezing. Pediatrics. 2015;135(4):e1044-e1051. https://doi.org/10.1542/peds.2014-2295.
23. Tchou MJ, Tang Girdwood S, Wormser B, et al. Reducing electrolyte testing in hospitalized children by using quality improvement methods. Pediatrics. 2018;141(5). https://doi.org/10.1542/peds.2017-3187.
24. Christensen EW, Spaulding AB, Pomputius WF, Grapentine SP. Effects of hospital practice patterns for antibiotic administration for pneumonia on hospital lengths of stay and costs. J Pediatr Infect Dis Soc. 2019;8(2):115-121. https://doi.org/10.1093/jpids/piy003.
25. Berrevoets MAH, Pot JHLW, Houterman AE, et al. An electronic trigger tool to optimise intravenous to oral antibiotic switch: a controlled, interrupted time series study. Antimicrob Resist Infect Control. 2017;6:81. https://doi.org/10.1186/s13756-017-0239-3.
26. Fischer MA, Solomon DH, Teich JM, Avorn J. Conversion from intravenous to oral medications: assessment of a computerized intervention for hospitalized patients. Arch Intern Med. 2003;163(21):2585-2589. https://doi.org/10.1001/archinte.163.21.2585.
27. Schroeder AR, Harris SJ, Newman TB. Safely doing less: a missing component of the patient safety dialogue. Pediatrics. 2011;128(6):e1596-e1597. https://doi.org/10.1542/peds.2011-2726.
1. Dellit TH, Owens RC, McGowan JE, Jr, et al. Infectious diseases society of America and the society for healthcare epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44(2):159-177. https://doi.org/10.1086/510393.
2. Shah SS, Srivastava R, Wu S, et al. Intravenous Versus oral antibiotics for postdischarge treatment of complicated pneumonia. Pediatrics. 2016;138(6). https://doi.org/10.1542/peds.2016-1692.
3. Keren R, Shah SS, Srivastava R, et al. Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomyelitis in children. JAMA Pediatr. 2015;169(2):120-128. https://doi.org/10.1001/jamapediatrics.2014.2822.
4. Jumani K, Advani S, Reich NG, Gosey L, Milstone AM. Risk factors for peripherally inserted central venous catheter complications in children. JAMA Pediatr. 2013;167(5):429-435.https://doi.org/10.1001/jamapediatrics.2013.775.
5. Zaoutis T, Localio AR, Leckerman K, et al. Prolonged intravenous therapy versus early transition to oral antimicrobial therapy for acute osteomyelitis in children. Pediatrics. 2009;123(2):636-642. https://doi.org/10.1542/peds.2008-0596.
6. McMullan BJ, Andresen D, Blyth CC, et al. Antibiotic duration and timing of the switch from intravenous to oral route for bacterial infections in children: systematic review and guidelines. Lancet Infect Dis. 2016;16(8):e139-e152. https://doi.org/10.1016/S1473-3099(16)30024-X.
7. Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):e25-e76. https://doi.org/10.1093/cid/cir531.
8. Stevens DL, Bisno AL, Chambers HF, et al. Executive summary: practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the infectious diseases society of America. Clin Infect Dis. 2014;59(2):147-159. https://doi.org/10.1093/cid/ciu444.
9. MacGregor RR, Graziani AL. Oral administration of antibiotics: a rational alternative to the parenteral route. Clin Infect Dis. 1997;24(3):457-467. https://doi.org/10.1093/clinids/24.3.457.
10. Downes KJ, Hahn A, Wiles J, Courter JD, Vinks AA. Dose optimisation of antibiotics in children: application of pharmacokinetics/pharmacodynamics in paediatrics. Int J Antimicrob Agents. 2014;43(3):223-230. https://doi.org/10.1016/j.ijantimicag.2013.11.006.
11. Autmizguine J, Melloni C, Hornik CP, et al. Population pharmacokinetics of trimethoprim-sulfamethoxazole in infants and children. Antimicrob Agents Chemother. 2018;62(1):e01813-e01817. https://doi.org/10.1128/AAC.01813-17.
12. Dewan M, Herrmann LE, Tchou MJ, et al. Development and evaluation of high-value pediatrics: a high-value care pediatric resident curriculum. Hosp Pediatr. 2018;8(12):785-792. https://doi.org/10.1542/hpeds.2018-0115
13. Langley GJ, Moen RD, Nolan KM, Nolan TW, Norman CL, Provost LP. The Improvement Guide: A Practical Approach to Enhancing Organizational Performance. New Jersey, US: John Wiley & Sons; 2009.
14. Benneyan JC. Use and interpretation of statistical quality control charts. Int J Qual Health Care. 1998;10(1):69-73. https://doi.org/10.1093/intqhc/10.1.69.
15. Lorgelly PK, Atkinson M, Lakhanpaul M, et al. Oral versus i.v. antibiotics for community-acquired pneumonia in children: a cost-minimisation analysis. Eur Respir J. 2010;35(4):858-864. https://doi.org/10.1183/09031936.00087209.
16. Vidyarthi AR, Green AL, Rosenbluth G, Baron RB. Engaging residents and fellows to improve institution-wide quality: the first six years of a novel financial incentive program. Acad Med. 2014;89(3):460-468. https://doi.org/10.1097/ACM.0000000000000159.
17. Stinnett-Donnelly JM, Stevens PG, Hood VL. Developing a high value care programme from the bottom up: a programme of faculty-resident improvement projects targeting harmful or unnecessary care. BMJ Qual Saf. 2016;25(11):901-908. https://doi.org/10.1136/bmjqs-2015-004546.
18. Peterson D, McLeod S, Woolfrey K, McRae A. Predictors of failure of empiric outpatient antibiotic therapy in emergency department patients with uncomplicated cellulitis. Acad Emerg Med. 2014;21(5):526-531. https://doi.org/10.1111/acem.12371.
19. Yadav K, Suh KN, Eagles D, et al. Predictors of oral antibiotic treatment failure for non-purulent skin and soft tissue infections in the emergency department. Acad Emerg Med. 2018;20(S1):S24-S25. https://doi.org/10.1017/cem.2018.114.
20. Organisation for Economic Co-operation and Development. Healthcare costs unsustainable in advanced economies without reform. http://www.oecd.org/health/healthcarecostsunsustainableinadvancedeconomieswithoutreform.htm. Accessed June 28, 2018; 2015.
21. Berwick DM, Hackbarth AD. Eliminating waste in US health care. JAMA. 2012;307(14):1513-1516. https://doi.org/10.1001/jama.2012.362.
22. Schondelmeyer AC, Simmons JM, Statile AM, et al. Using quality improvement to reduce continuous pulse oximetry use in children with wheezing. Pediatrics. 2015;135(4):e1044-e1051. https://doi.org/10.1542/peds.2014-2295.
23. Tchou MJ, Tang Girdwood S, Wormser B, et al. Reducing electrolyte testing in hospitalized children by using quality improvement methods. Pediatrics. 2018;141(5). https://doi.org/10.1542/peds.2017-3187.
24. Christensen EW, Spaulding AB, Pomputius WF, Grapentine SP. Effects of hospital practice patterns for antibiotic administration for pneumonia on hospital lengths of stay and costs. J Pediatr Infect Dis Soc. 2019;8(2):115-121. https://doi.org/10.1093/jpids/piy003.
25. Berrevoets MAH, Pot JHLW, Houterman AE, et al. An electronic trigger tool to optimise intravenous to oral antibiotic switch: a controlled, interrupted time series study. Antimicrob Resist Infect Control. 2017;6:81. https://doi.org/10.1186/s13756-017-0239-3.
26. Fischer MA, Solomon DH, Teich JM, Avorn J. Conversion from intravenous to oral medications: assessment of a computerized intervention for hospitalized patients. Arch Intern Med. 2003;163(21):2585-2589. https://doi.org/10.1001/archinte.163.21.2585.
27. Schroeder AR, Harris SJ, Newman TB. Safely doing less: a missing component of the patient safety dialogue. Pediatrics. 2011;128(6):e1596-e1597. https://doi.org/10.1542/peds.2011-2726.
© 2019 Society of Hospital Medicine
Examining the “Repletion Reflex”: The Association between Serum Potassium and Outcomes in Hospitalized Patients with Heart Failure
Heart failure (HF) is a leading cause of hospital admission and mortality, accounting for approximately 900,000 discharges in 2014.1 One-year all-cause mortality risk has been estimated at 17% after hospitalization,2 and roughly 50% of deaths are related to sudden cardiac death, mostly due to ventricular arrhythmia.
The principles underlying potassium management in acute HF are complex. Both low and high values have been linked to fatal arrhythmias, notably ventricular fibrillation, and small serum changes often reflect large total body potassium fluctuations.11 Recent literature links hypokalemia to general membrane hypoexcitability, skeletal muscle hyporeflexia, and arrhythmias initiated by reduced sodium-potassium adenosine triphosphatase activity, leading to increased intracellular calcium and regional variations in action potential duration.12 Potassium abnormalities are common at admission and may be exacerbated by both acute illness and treatments given during hospitalization, including baseline potassium, acute kidney injury, aggressive diuretic therapy, or other potassium-related treatments and conditions.13 The success of potassium repletion may also be affected by the choice of HF therapies.14
The belief that patients with HF must maintain a potassium >4.0 mEq/L remains pervasive, with at least one family medicine guideline recommending that patients with HF maintain a serum potassium level >4.0 mEq/L.
METHODS
Data Sources and Cohort Definition
The Institutional Review Board at Baystate Medical Center approved this study. We identified patients with HF who were admitted for more than 72 hours between January 2010 and December 2012 to hospitals contributing to the HealthFacts database, a multihospital dataset derived from the comprehensive electronic health records of 116 geographically and structurally diverse hospitals throughout the United States (Cerner Corp.). HealthFacts—which includes date-stamped pharmacy, laboratory, and billing information—contains records of more than 84 million acute admissions, emergency room visits, and ambulatory visits. We limited the sample to hospitals that contributed to the pharmacy, laboratory, and diagnosis segments.
We included patients who had a principal International Classification of Disease (ICD-9-CM) diagnosis of HF or a principal diagnosis of respiratory failure with secondary diagnosis of HF (ICD-9-CM codes for HF: 402.01, 402.11, 402.91, 404.01, 404.03, 404.11, 404.13, 404.91, 404.93, 428.xx16 and for respiratory failure: 518.81, 518.82, 518.84) and were 18 years or older. We ensured that patients were treated for acute decompensated HF during the hospitalization by restricting the cohort to patients in whom at least one HF therapy (eg, loop diuretics, metolazone, inotropes, and intra-aortic balloon pump) was initiated within the first two days of hospitalization. We excluded patients with a pediatric or psychiatric attending physician, those with elective admissions, and those who were transferred from or to another acute care facility because we could not accurately determine the onset or subsequent course of their illness.
Definition of Variables Describing Serum Potassium Levels
We limited the sample to patients hospitalized for longer than 72 hours in order to observe how initial potassium values influenced outcomes over the course of hospitalization. We chose an exposure window of 72 hours because this allowed, on average, three potential observations of serum potassium per patient. We further restricted the sample to those who had a normal potassium value (3.5-5.0 mEq/L) at admission (defined as 24 hours prior to admission through midnight of the day of admission) to ensure that the included patients did not have abnormal potassium values upon presentation. We identified the period of time from 24 hours prior to admission through 72 hours following admission as “the exposure window” (the time during which patients were eligible to be classified into average serum potassium levels of <4.0, 4.0-4.5, or >4.5 mEq/L). We excluded patients who, during this window, had fewer than three serum potassium levels drawn (“exposure” levels could be disproportionately influenced by a single value) or received sodium polystyrene (as this would indicate that the physicians felt the potassium was dangerously high). For patients with repeated hospitalizations, we randomly selected one visit for inclusion to reduce the risk of survivor bias. We calculated the mean of all serum potassium levels during the exposure window, including the admission value, and then evaluated two different categorizations of mean serum potassium, based on categories of risk previously reported in the literature:8,17,18: (1) <4.0, 4.0-4.5, or >4.5 mEq/L and (2) <4.0 versus ≥4.0 mEq/L.
Outcomes
We assessed three outcomes: in-hospital mortality, transfer to an intensive care unit (ICU), and length of stay (LOS). Admission to the ICU was defined as any evidence, after the exposure window, that the patient received care in the ICU. We excluded patients with ICU admissions during the exposure window from the analysis of this outcome. We calculated LOS as the difference between discharge date/time and the admission date/time.
Covariates and Comorbidity Adjustment
We obtained information on patient demographics (age and race) and identified the presence of comorbid conditions using previously derived and validated models.19,20 We then further quantified these conditions into a single combined score to adjust for differences in presenting illness severity (including kidney disease) and help reduce confounding.21 To account for presenting severity of illness, we calculated the Laboratory-based Acute Physiology Score (LAPS-2).22,23 LAPS-2 was developed for predicting mortality risk in general medical patients, but we previously externally validated it against other published clinical HF models in a cohort of patients hospitalized with acute decompensated HF.5
Potassium Repletion
Analysis
We evaluated the differences in patient characteristics across serum potassium categories. Categorical variables are presented as frequencies and percentages, whereas continuous variables are presented as means and standard deviations. For binary outcomes, we used generalized estimating equations (with a binomial family and logit link and clustering by hospital) to estimate incidence and calculate unadjusted and adjusted odds ratios (ORs) and 95% confidence intervals (CIs). For LOS, we estimated the median and 95% CIs using quantile regression with clustered standard errors.24 We calculated all models using both a binary exposure (<4.0 versus ≥4.0 mEq/L) and a three-level categorization (<4.0, 4.0-4.5, and >4.5 mEq/L) to explore the effects at the highest potassium level. We adjusted all models for age, race, LAPS-2 score, and combined comorbidity score. We conducted two sensitivity analyses. First, we restricted our sample to those who never received potassium during the exposure window, as these patients may be different than patients who required potassium repletion. Second, we stratified our findings by the presence or absence of acute or chronic renal insufficiency (defined as an admission creatinine >1 or the presence of a diagnostic code for renal insufficiency, as defined by Elixhauser et al.).19,21 Statistical significance was set at an alpha of 0.05. Analysis was completed using Stata v15.1, StataCorp LP, College Station, Texas.
RESULTS
Cohort Description
We identified patients from 56 geographically diverse US hospitals, although most were located in either the northeast (n = 21; 38%) or south (n = 18; 32%). A total of 59% of the hospitals were teaching hospitals, and nearly 95% were in an urban setting. We identified 13,163 patients with HF, of which 4,995 (38.0%) met the inclusion criteria. We excluded 3,744 (28.4%) patients with LOS < 72 hours, 2,210 (16.8%) with admission potassium values outside of the defined range, and 896 (6.8%) with fewer than three potassium values during the exposure window. Of the patients who met the inclusion criteria, 2,080 (41.6%), 2,326 (46.6%), and 589 (11.8%) were categorized in the <4.0, 4.0-4.5, and >4.5 mEq/L groups, respectively (Table 1). The groups were clinically similar in terms of age, sex, illness severity (LAPS-2), and comorbidity score. Compared with other racial groups, black patients had higher potassium values. While the <4.0 and 4.0-4.5 mEq/L groups were relatively similar, the group with mean potassium >4.5 mEq/L had higher admission creatinine and a greater prevalence of chronic kidney disease, deficiency anemias, and chronic obstructive pulmonary disease (Table 1).
Serum Potassium Values
Individuals’ mean serum potassium within the 72-hour exposure window ranged from 2.9 to 5.8 mEq/L (Table 2). In the <4.0, 4-4.5, and >4.5 mEq/L cohorts respectively, patients had a median serum potassium of 3.8 mEq/L (2.9-3.9), 4.2 mEq/L (4.0-4.5), and 4.7 mEq/L (4.5-5.8) during the exposure window. Approximately half of the patients in the <4.0 mEq/L group had a serum potassium <3.5 mEq/L at some point during the exposure window. In contrast, <10% of the other groups had this low value during the exposure window.
Potassium Repletion
Patients in the <4.0 mEq/L group were much more likely to receive potassium repletion during the exposure window when compared with the 4.0-4.5 mEq/L (71.5% vs 40.5%) and >4.5 mEq/L (71.5% vs 26.7%) groups. On days that they were eligible for repletion (defined as a daily potassium value <4.0 mEq/L), patients with mean serum potassium >4.0 mEq/L were less likely to receive potassium repletion compared with those with values <4.0 mEq/L. There were 592 (28.5%), 1,383 (59.5%), and 432 (73.3%) patients in the <4.0, 4-4.5, and >4,5 mEq/L groups, respectively, who did not receive potassium repletion therapy during the exposure window.
Relationship of Serum Potassium Levels and Outcomes
Overall, 3.7% (n = 187) of patients died during the hospitalization, 2.4% (n = 98) were admitted to the ICU after the exposure window, and the median LOS was 5.6 days. We did not observe a significant association between mean serum potassium of <4.0 or 4.0-4.5 mEq/L and increased risk of mortality, ICU transfer, or LOS (Table 3). Our unadjusted analysis showed that patients with values >4.5 mEq/L had worse outcomes, including more deaths (5.3%; OR = 1.55; 95% CI: 1.01 to 2.39) and ICU admission (3.8%; OR = 2.10; 95% CI: 1.16 to 3.80) compared with those with values <4.0 mEq/L (Table 3). We also found that, compared with the <4.0 mEq/L group, the >4.5 mEq/L group showed just over a half-day longer LOS (0.6 days; 95% CI: 0.0 to 1.0; Table 3). However, we found that mortality and ICU admission results were attenuated after adjustment for age, race, comorbidity score, and LAPS-2 and were no longer statistically significant, whereas the association with LOS was consistent after adjustment. When using a binary exposure (<4.0 versus ≥4.0 mEq/L), we observed no association between mean potassium value and increased risk of mortality, ICU transfer, or LOS both before and after adjustment for age, race, LAPS-2, and comorbidity score (data not shown).
Sensitivity Analyses
In the sensitivity analysis restricted to those who did not receive potassium repletion during the exposure window, we continued to observe no association between the <4.0 and 4.0-4.5 mEq/L groups and outcomes (Table 3). In adjusted models for the >4.5 versus <4.0 mEq/L groups, risk estimates for mortality were similar to the full sample, but statistical significance was lost (OR = 1.56; 95% CI: 0.81 to 3.01). Adjusted risk estimates for ICU transfer were attenuated and not statistically significant (OR = 1.40; 95% CI: 0.60 to 3.26). However, LOS estimates were very similar to that observed in the full dataset (0.6 days; 95% CI: 0.1 to 1.2).
When stratifying our results by the presence or absence of acute or chronic renal insufficiency, we continued to observe no increased risk of any outcome in the 4.0-4.5 mEq/L compared with the <4.0 mEq/L groups across all strata (Table 4). Interestingly, even after adjustment, we did find that most of the increased risk of mortality and ICU admission in the >4.5 versus <4.0 mEq/L groups was among those without renal insufficiency (mortality OR = 3.03; ICU admission OR = 3.00) and was not statistically significant in those with renal insufficiency (mortality OR = 1.27; ICU admission OR = 1.63). Adjusted LOS estimates remained relatively similar in this stratified analysis.
DISCUSSION
The best approach to mild serum potassium value abnormalities in patients hospitalized with HF remains unclear. Many physicians reflexively replete potassium to ensure all patients maintain a serum value of >4.0 mEq/L.15 Yet, in this large observational study of patients hospitalized with an acute HF exacerbation, we found little evidence of association between serum potassium <4.0 mEq/L and negative outcomes.
Compared with those with mean potassium values <4.0 mEq/L (in unadjusted models), there was an association between potassium values of >4.5 mEq/L and increased risk of mortality and ICU transfer. This association was attenuated after adjustment, suggesting that factors beyond potassium values influenced the observed relationship. These findings seem to suggest that unobserved differences in the >4.5 mEq/L group (there were observed differences in this group, eg, greater presenting severity and higher comorbidity scores, suggesting that there were also unobserved differences), and not average potassium value, were the reasons for the observed differences in outcomes. However, we cannot rule out the possibility that potassium >4.5 mEq/L has some associated increased risk compared with mean potassium values of <4.0 mEq/L for patients hospitalized with acute decompensated HF.
Patients in our study routinely received exogenous potassium: more than 70% of patients received repletion at least once, although it is notable that the majority of patients in the 4.0-4.5 and >4.5 mEq/L groups did not receive repletion. Despite this practice, the data supporting this approach to potassium management for patients hospitalized with HF remain mixed. A serum potassium decline of >15% during an acute HF hospital stay has been reported as a predictor of all-cause mortality after controlling for disease severity and associated comorbidities, including renal function.25 However, this study was focused on decline in admission potassium rather than an absolute cut-off (eg, >4.0 mEq/L). Additionally, potassium levels <3.9 mEq/L were associated with increased mortality in patients with acute HF following a myocardial infarction, but this study was not focused on patients with HF.26 Most of the prior literature in patients with HF was conducted in patients in outpatient settings and examined patients who were not experiencing acute exacerbations. MacDonald and Struthers advocate that patients with HF have their potassium maintained above 4.0 mEq/L but did not specify whether this included patients with acute HF exacerbations.10 Additionally, many studies evaluating potassium repletion were conducted before widespread availability of angiotensin-converting enzyme (ACE) inhibitors or potassium-sparing diuretics, including spironolactone. Prior work has consistently reported that hyperkalemia, defined as serum potassium >4.5 mEq/L, is associated with mortality in patients with acute HF over the course of hospitalization (which aligned with the results from our sensitivity analysis), but concurrent medication regimens and underlying impaired renal function likely accounted for most of this association.17 The picture is further complicated as patients with acute HF presenting with hypokalemia may be at risk for subsequent hyperkalemia, and potassium repletion can stimulate aldosterone secretion, potentially exacerbating underlying HF.27,28
These data are observational and are unlikely to change practice. However, daily potassium repletion represents a huge cost in time, money, and effort to the health system. Furthermore, the greatest burden occurs for the patients, who have labs drawn and values checked routinely and potassium administered orally or parenterally. While future randomized clinical trials (RCTs) would best examine the benefits of repletion, future pragmatic trials could attempt to disentangle the associated risks and benefits of potassium repletion in the absence of RCTs. Additionally, such studies could better take into account the role of concurrent medication use (like ACEs or angiotensin II receptor blockers), as well as assess the role of chronic renal insufficiency, acute kidney injury, and magnesium levels.29
This study has limitations. Its retrospective design leads to unmeasured confounding; however, we adjusted for multiple variables (including LAPS-2), which reflect the severity of disease at admission and underlying kidney function at presentation, as well as other comorbid conditions. In addition, data from the cohort only extend to 2012, so more recent changes in practice may not be completely reflected. The nature of the data did not allow us to directly investigate the relationship between serum potassium and arrhythmias, although ICU transfer and mortality were used as surrogates.
In conclusion, the benefit of a serum potassium level >4.0 mEq/L in patients admitted with HF remains unclear. We did not observe that mean potassium values <4.0 mEq/L were associated with worse outcomes, and, more concerning, there may be some risk for patients with mean values >4.5 mEq/L.
Acknowledgments
Dr. Lagu had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Disclosures
The authors report no potential conflicts of interest. Dr. Lagu has served as a consultant for the Yale Center for Outcomes Research and Evaluation, under contract to the Centers for Medicare and Medicaid Services, for which she has provided clinical and methodological expertise and input on the development, reevaluation, and implementation of hospital outcome and efficiency measures.
Funding
Dr. Lagu is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number K01HL114745 and R01 HL139985-01A1. Dr. Stefan is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number K01HL114631-01A1. Dr. Pack is supported by NHLBI 1K23HL135440. Dr. Lindenauer is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number 1K24HL132008.
Disclaimer
The views expressed in this manuscript do not necessarily reflect those of the Yale Center for Outcomes Research and Evaluation or the Centers for Medicare and Medicaid Services.
1. Benjamin EJ, Virani SS, Callaway CW, et al. Heart disease and stroke statistics–2018 update: a report from the American Heart Association. Circulation. 2018;137(12):e67-e492. https://doi.org/10.1161/CIR.0000000000000558.
2. Maggioni AP, Dahlström U, Filippatos G, et al. EURObservational Research Programme: regional differences and 1-year follow-up results of the Heart Failure Pilot Survey (ESC-HF Pilot). Eur J Heart Fail. 2013;15(7):808-817. https://doi.org/10.1093/eurjhf/hft050.
3. Tomaselli GF, Zipes DP. What causes sudden death in heart failure? Circ Res. 2004;95(8):754-763. https://doi.org/10.1161/01.RES.0000145047.
4. Bowen GS, Diop MS, Jiang L, Wu W-C, Rudolph JL. A multivariable prediction model for mortality in individuals admitted for heart failure. J Am Geriatr Soc. 2018;66(5):902-908. https://doi.org/10.1111/jgs.15319.
5. Lagu T, Pekow PS, Shieh M-S, et al. Validation and comparison of seven mortality prediction models for hospitalized patients with acute decompensated heart failure. Circ Heart Fail. 2016;9(8). https://doi.org/10.1161/CIRCHEARTFAILURE.115.002912.
6. Núñez J, Bayés-Genís A, Zannad F, et al. Long-term potassium monitoring and dynamics in heart failure and risk of mortality. Circulation. 2018;137(13):1320-1330. https://doi.org/10.1161/CIRCULATIONAHA.117.030576.
7. Vardeny O, Claggett B, Anand I, et al. Incidence, predictors, and outcomes related to hypo- and hyperkalemia in patients with severe heart failure treated with a mineralocorticoid receptor antagonist. Circ Heart Fail. 2014;7(4):573-579. https://doi.org/10.1161/CIRCHEARTFAILURE.114.00110.
8. Aldahl M, Jensen A-SC, Davidsen L, et al. Associations of serum potassium levels with mortality in chronic heart failure patients. Eur Heart J. 2017;38(38):2890-2896. https://doi.org/10.1093/eurheartj/ehx460.
9. Hoppe LK, Muhlack DC, Koenig W, Carr PR, Brenner H, Schöttker B. Association of abnormal serum potassium levels with arrhythmias and cardiovascular mortality: a systematic review and meta-analysis of observational studies. Cardiovasc Drugs Ther. 2018;32(2):197-212. https://doi.org/10.1007/s10557-018-6783-0.
10. Macdonald JE, Struthers AD. What is the optimal serum potassium level in cardiovascular patients? J Am Coll Cardiol. 2004;43(2):155-161. https://doi.org/10.1016/j.jacc.2003.06.021.
11. Hulting J. In-hospital ventricular fibrillation and its relation to serum potassium. Acta Med Scand Suppl. 1981;647(647):109-116. https://doi.org/10.1111/j.0954-6820.1981.tb02646.x.
12. Skogestad J, Aronsen JM. Hypokalemia-induced arrhythmias and heart failure: new insights and implications for therapy. Front Physiol. 2018;9:1500. https://doi.org/10.3389/fphys.2018.01500.
13. Tromp J, Ter Maaten JM, Damman K, et al. Serum potassium levels and outcome in acute heart failure (data from the PROTECT and COACH trials). Am J Cardiol. 2017;119(2):290-296. https://doi.org/10.1016/j.amjcard.2016.09.038.
14. Khan SS, Campia U, Chioncel O, et al. Changes in serum potassium levels during hospitalization in patients with worsening heart failure and reduced ejection fraction (from the EVEREST trial). Am J Cardiol. 2015;115(6):790-796. https://doi.org/10.1016/j.amjcard.2014.12.045
15. Viera AJ, Wouk N. Potassium disorders: hypokalemia and hyperkalemia. Am Fam Physician. 2015;92(6):487-495.
16. Krumholz HM, Wang Y, Mattera JA, et al. An administrative claims model suitable for profiling hospital performance based on 30-day mortality rates among patients with heart failure. Circulation. 2006;113(13):1693-1701. https://doi.org/10.1161/CIRCULATIONAHA.105.611194.
17. Legrand M, Ludes P-O, Massy Z, et al. Association between hypo- and hyperkalemia and outcome in acute heart failure patients: the role of medications. Clin Res Cardiol. 2018;107(3):214-221. https://doi.org/10.1007/s00392-017-1173-3.
18. Kok W, Salah K, Stienen S. Are changes in serum potassium levels during admissions for acute decompensated heart failure irrelevant for prognosis: the end of the story? Am J Cardiol. 2015;116(5):825. https://doi.org/10.1016/j.amjcard.2015.05.059.
19. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. https://doi.org/10.1097/00005650-199801000-00004.
20. Quan H, Parsons GA, Ghali WA. Validity of information on comorbidity derived from ICD-9-CCM administrative data. Med Care. 2002;40(8):675-685. https://doi.org/10.1097/01.MLR.0000020927.46398.5D.
21. Gagne JJ, Glynn RJ, Avorn J, Levin R, Schneeweiss S. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol. 2011;64(7):749-759. https://doi.org/10.1016/j.jclinepi.2010.10.004.
22. Escobar GJ, Gardner MN, Greene JD, Draper D, Kipnis P. Risk-adjusting hospital mortality using a comprehensive electronic record in an integrated health care delivery system. Med Care. 2013;51(5):446-453. https://doi.org/10.1097/MLR.0b013e3182881c8e.
23. Escobar GJ, Greene JD, Scheirer P, Gardner MN, Draper D, Kipnis P. Risk-adjusting hospital inpatient mortality using automated inpatient, outpatient, and laboratory databases. Med Care. 2008;46(3):232-239. https://doi.org/10.1097/MLR.0b013e3181589bb6.
24. Parente PMDC, Santos Silva JMC. Quantile regression with clustered data. J Econom Method. 2016;5(1):1-15. https://doi.org/10.1515/jem-2014-0011.
25. Salah K, Pinto YM, Eurlings LW, et al. Serum potassium decline during hospitalization for acute decompensated heart failure is a predictor of 6-month mortality, independent of N-terminal pro-B-type natriuretic peptide levels: An individual patient data analysis. Am Heart J. 2015;170(3):531-542.e1. https://doi.org/10.1016/j.ahj.2015.06.003.
26. Krogager ML, Eggers-Kaas L, Aasbjerg K, et al. Short-term mortality risk of serum potassium levels in acute heart failure following myocardial infarction. Eur Heart J Cardiovasc Pharmacother. 2015;1(4):245-251. https://doi.org/10.1093/ehjcvp/pvv026.
27. Crop MJ, Hoorn EJ, Lindemans J, Zietse R. Hypokalaemia and subsequent hyperkalaemia in hospitalized patients. Nephrol Dial Transplant. 2007;22(12):3471-3477.https://doi.org/10.1093/ndt/gfm471.
28. Kok W, Salah K, Stienen S. Serum potassium levels during admissions for acute decompensated heart failure: identifying possible threats to outcome. Am J Cardiol. 2018;121(1):141. https://doi.org/10.1016/j.amjcard.2017.09.032.
29. Freda BJ, Knee AB, Braden GL, Visintainer PF, Thakar CV. Effect of transient and sustained acute kidney injury on readmissions in acute decompensated heart failure. Am J Cardiol. 2017;119(11):1809-1814. https://doi.org/10.1016/j.amjcard.2017.02.044.
Heart failure (HF) is a leading cause of hospital admission and mortality, accounting for approximately 900,000 discharges in 2014.1 One-year all-cause mortality risk has been estimated at 17% after hospitalization,2 and roughly 50% of deaths are related to sudden cardiac death, mostly due to ventricular arrhythmia.
The principles underlying potassium management in acute HF are complex. Both low and high values have been linked to fatal arrhythmias, notably ventricular fibrillation, and small serum changes often reflect large total body potassium fluctuations.11 Recent literature links hypokalemia to general membrane hypoexcitability, skeletal muscle hyporeflexia, and arrhythmias initiated by reduced sodium-potassium adenosine triphosphatase activity, leading to increased intracellular calcium and regional variations in action potential duration.12 Potassium abnormalities are common at admission and may be exacerbated by both acute illness and treatments given during hospitalization, including baseline potassium, acute kidney injury, aggressive diuretic therapy, or other potassium-related treatments and conditions.13 The success of potassium repletion may also be affected by the choice of HF therapies.14
The belief that patients with HF must maintain a potassium >4.0 mEq/L remains pervasive, with at least one family medicine guideline recommending that patients with HF maintain a serum potassium level >4.0 mEq/L.
METHODS
Data Sources and Cohort Definition
The Institutional Review Board at Baystate Medical Center approved this study. We identified patients with HF who were admitted for more than 72 hours between January 2010 and December 2012 to hospitals contributing to the HealthFacts database, a multihospital dataset derived from the comprehensive electronic health records of 116 geographically and structurally diverse hospitals throughout the United States (Cerner Corp.). HealthFacts—which includes date-stamped pharmacy, laboratory, and billing information—contains records of more than 84 million acute admissions, emergency room visits, and ambulatory visits. We limited the sample to hospitals that contributed to the pharmacy, laboratory, and diagnosis segments.
We included patients who had a principal International Classification of Disease (ICD-9-CM) diagnosis of HF or a principal diagnosis of respiratory failure with secondary diagnosis of HF (ICD-9-CM codes for HF: 402.01, 402.11, 402.91, 404.01, 404.03, 404.11, 404.13, 404.91, 404.93, 428.xx16 and for respiratory failure: 518.81, 518.82, 518.84) and were 18 years or older. We ensured that patients were treated for acute decompensated HF during the hospitalization by restricting the cohort to patients in whom at least one HF therapy (eg, loop diuretics, metolazone, inotropes, and intra-aortic balloon pump) was initiated within the first two days of hospitalization. We excluded patients with a pediatric or psychiatric attending physician, those with elective admissions, and those who were transferred from or to another acute care facility because we could not accurately determine the onset or subsequent course of their illness.
Definition of Variables Describing Serum Potassium Levels
We limited the sample to patients hospitalized for longer than 72 hours in order to observe how initial potassium values influenced outcomes over the course of hospitalization. We chose an exposure window of 72 hours because this allowed, on average, three potential observations of serum potassium per patient. We further restricted the sample to those who had a normal potassium value (3.5-5.0 mEq/L) at admission (defined as 24 hours prior to admission through midnight of the day of admission) to ensure that the included patients did not have abnormal potassium values upon presentation. We identified the period of time from 24 hours prior to admission through 72 hours following admission as “the exposure window” (the time during which patients were eligible to be classified into average serum potassium levels of <4.0, 4.0-4.5, or >4.5 mEq/L). We excluded patients who, during this window, had fewer than three serum potassium levels drawn (“exposure” levels could be disproportionately influenced by a single value) or received sodium polystyrene (as this would indicate that the physicians felt the potassium was dangerously high). For patients with repeated hospitalizations, we randomly selected one visit for inclusion to reduce the risk of survivor bias. We calculated the mean of all serum potassium levels during the exposure window, including the admission value, and then evaluated two different categorizations of mean serum potassium, based on categories of risk previously reported in the literature:8,17,18: (1) <4.0, 4.0-4.5, or >4.5 mEq/L and (2) <4.0 versus ≥4.0 mEq/L.
Outcomes
We assessed three outcomes: in-hospital mortality, transfer to an intensive care unit (ICU), and length of stay (LOS). Admission to the ICU was defined as any evidence, after the exposure window, that the patient received care in the ICU. We excluded patients with ICU admissions during the exposure window from the analysis of this outcome. We calculated LOS as the difference between discharge date/time and the admission date/time.
Covariates and Comorbidity Adjustment
We obtained information on patient demographics (age and race) and identified the presence of comorbid conditions using previously derived and validated models.19,20 We then further quantified these conditions into a single combined score to adjust for differences in presenting illness severity (including kidney disease) and help reduce confounding.21 To account for presenting severity of illness, we calculated the Laboratory-based Acute Physiology Score (LAPS-2).22,23 LAPS-2 was developed for predicting mortality risk in general medical patients, but we previously externally validated it against other published clinical HF models in a cohort of patients hospitalized with acute decompensated HF.5
Potassium Repletion
Analysis
We evaluated the differences in patient characteristics across serum potassium categories. Categorical variables are presented as frequencies and percentages, whereas continuous variables are presented as means and standard deviations. For binary outcomes, we used generalized estimating equations (with a binomial family and logit link and clustering by hospital) to estimate incidence and calculate unadjusted and adjusted odds ratios (ORs) and 95% confidence intervals (CIs). For LOS, we estimated the median and 95% CIs using quantile regression with clustered standard errors.24 We calculated all models using both a binary exposure (<4.0 versus ≥4.0 mEq/L) and a three-level categorization (<4.0, 4.0-4.5, and >4.5 mEq/L) to explore the effects at the highest potassium level. We adjusted all models for age, race, LAPS-2 score, and combined comorbidity score. We conducted two sensitivity analyses. First, we restricted our sample to those who never received potassium during the exposure window, as these patients may be different than patients who required potassium repletion. Second, we stratified our findings by the presence or absence of acute or chronic renal insufficiency (defined as an admission creatinine >1 or the presence of a diagnostic code for renal insufficiency, as defined by Elixhauser et al.).19,21 Statistical significance was set at an alpha of 0.05. Analysis was completed using Stata v15.1, StataCorp LP, College Station, Texas.
RESULTS
Cohort Description
We identified patients from 56 geographically diverse US hospitals, although most were located in either the northeast (n = 21; 38%) or south (n = 18; 32%). A total of 59% of the hospitals were teaching hospitals, and nearly 95% were in an urban setting. We identified 13,163 patients with HF, of which 4,995 (38.0%) met the inclusion criteria. We excluded 3,744 (28.4%) patients with LOS < 72 hours, 2,210 (16.8%) with admission potassium values outside of the defined range, and 896 (6.8%) with fewer than three potassium values during the exposure window. Of the patients who met the inclusion criteria, 2,080 (41.6%), 2,326 (46.6%), and 589 (11.8%) were categorized in the <4.0, 4.0-4.5, and >4.5 mEq/L groups, respectively (Table 1). The groups were clinically similar in terms of age, sex, illness severity (LAPS-2), and comorbidity score. Compared with other racial groups, black patients had higher potassium values. While the <4.0 and 4.0-4.5 mEq/L groups were relatively similar, the group with mean potassium >4.5 mEq/L had higher admission creatinine and a greater prevalence of chronic kidney disease, deficiency anemias, and chronic obstructive pulmonary disease (Table 1).
Serum Potassium Values
Individuals’ mean serum potassium within the 72-hour exposure window ranged from 2.9 to 5.8 mEq/L (Table 2). In the <4.0, 4-4.5, and >4.5 mEq/L cohorts respectively, patients had a median serum potassium of 3.8 mEq/L (2.9-3.9), 4.2 mEq/L (4.0-4.5), and 4.7 mEq/L (4.5-5.8) during the exposure window. Approximately half of the patients in the <4.0 mEq/L group had a serum potassium <3.5 mEq/L at some point during the exposure window. In contrast, <10% of the other groups had this low value during the exposure window.
Potassium Repletion
Patients in the <4.0 mEq/L group were much more likely to receive potassium repletion during the exposure window when compared with the 4.0-4.5 mEq/L (71.5% vs 40.5%) and >4.5 mEq/L (71.5% vs 26.7%) groups. On days that they were eligible for repletion (defined as a daily potassium value <4.0 mEq/L), patients with mean serum potassium >4.0 mEq/L were less likely to receive potassium repletion compared with those with values <4.0 mEq/L. There were 592 (28.5%), 1,383 (59.5%), and 432 (73.3%) patients in the <4.0, 4-4.5, and >4,5 mEq/L groups, respectively, who did not receive potassium repletion therapy during the exposure window.
Relationship of Serum Potassium Levels and Outcomes
Overall, 3.7% (n = 187) of patients died during the hospitalization, 2.4% (n = 98) were admitted to the ICU after the exposure window, and the median LOS was 5.6 days. We did not observe a significant association between mean serum potassium of <4.0 or 4.0-4.5 mEq/L and increased risk of mortality, ICU transfer, or LOS (Table 3). Our unadjusted analysis showed that patients with values >4.5 mEq/L had worse outcomes, including more deaths (5.3%; OR = 1.55; 95% CI: 1.01 to 2.39) and ICU admission (3.8%; OR = 2.10; 95% CI: 1.16 to 3.80) compared with those with values <4.0 mEq/L (Table 3). We also found that, compared with the <4.0 mEq/L group, the >4.5 mEq/L group showed just over a half-day longer LOS (0.6 days; 95% CI: 0.0 to 1.0; Table 3). However, we found that mortality and ICU admission results were attenuated after adjustment for age, race, comorbidity score, and LAPS-2 and were no longer statistically significant, whereas the association with LOS was consistent after adjustment. When using a binary exposure (<4.0 versus ≥4.0 mEq/L), we observed no association between mean potassium value and increased risk of mortality, ICU transfer, or LOS both before and after adjustment for age, race, LAPS-2, and comorbidity score (data not shown).
Sensitivity Analyses
In the sensitivity analysis restricted to those who did not receive potassium repletion during the exposure window, we continued to observe no association between the <4.0 and 4.0-4.5 mEq/L groups and outcomes (Table 3). In adjusted models for the >4.5 versus <4.0 mEq/L groups, risk estimates for mortality were similar to the full sample, but statistical significance was lost (OR = 1.56; 95% CI: 0.81 to 3.01). Adjusted risk estimates for ICU transfer were attenuated and not statistically significant (OR = 1.40; 95% CI: 0.60 to 3.26). However, LOS estimates were very similar to that observed in the full dataset (0.6 days; 95% CI: 0.1 to 1.2).
When stratifying our results by the presence or absence of acute or chronic renal insufficiency, we continued to observe no increased risk of any outcome in the 4.0-4.5 mEq/L compared with the <4.0 mEq/L groups across all strata (Table 4). Interestingly, even after adjustment, we did find that most of the increased risk of mortality and ICU admission in the >4.5 versus <4.0 mEq/L groups was among those without renal insufficiency (mortality OR = 3.03; ICU admission OR = 3.00) and was not statistically significant in those with renal insufficiency (mortality OR = 1.27; ICU admission OR = 1.63). Adjusted LOS estimates remained relatively similar in this stratified analysis.
DISCUSSION
The best approach to mild serum potassium value abnormalities in patients hospitalized with HF remains unclear. Many physicians reflexively replete potassium to ensure all patients maintain a serum value of >4.0 mEq/L.15 Yet, in this large observational study of patients hospitalized with an acute HF exacerbation, we found little evidence of association between serum potassium <4.0 mEq/L and negative outcomes.
Compared with those with mean potassium values <4.0 mEq/L (in unadjusted models), there was an association between potassium values of >4.5 mEq/L and increased risk of mortality and ICU transfer. This association was attenuated after adjustment, suggesting that factors beyond potassium values influenced the observed relationship. These findings seem to suggest that unobserved differences in the >4.5 mEq/L group (there were observed differences in this group, eg, greater presenting severity and higher comorbidity scores, suggesting that there were also unobserved differences), and not average potassium value, were the reasons for the observed differences in outcomes. However, we cannot rule out the possibility that potassium >4.5 mEq/L has some associated increased risk compared with mean potassium values of <4.0 mEq/L for patients hospitalized with acute decompensated HF.
Patients in our study routinely received exogenous potassium: more than 70% of patients received repletion at least once, although it is notable that the majority of patients in the 4.0-4.5 and >4.5 mEq/L groups did not receive repletion. Despite this practice, the data supporting this approach to potassium management for patients hospitalized with HF remain mixed. A serum potassium decline of >15% during an acute HF hospital stay has been reported as a predictor of all-cause mortality after controlling for disease severity and associated comorbidities, including renal function.25 However, this study was focused on decline in admission potassium rather than an absolute cut-off (eg, >4.0 mEq/L). Additionally, potassium levels <3.9 mEq/L were associated with increased mortality in patients with acute HF following a myocardial infarction, but this study was not focused on patients with HF.26 Most of the prior literature in patients with HF was conducted in patients in outpatient settings and examined patients who were not experiencing acute exacerbations. MacDonald and Struthers advocate that patients with HF have their potassium maintained above 4.0 mEq/L but did not specify whether this included patients with acute HF exacerbations.10 Additionally, many studies evaluating potassium repletion were conducted before widespread availability of angiotensin-converting enzyme (ACE) inhibitors or potassium-sparing diuretics, including spironolactone. Prior work has consistently reported that hyperkalemia, defined as serum potassium >4.5 mEq/L, is associated with mortality in patients with acute HF over the course of hospitalization (which aligned with the results from our sensitivity analysis), but concurrent medication regimens and underlying impaired renal function likely accounted for most of this association.17 The picture is further complicated as patients with acute HF presenting with hypokalemia may be at risk for subsequent hyperkalemia, and potassium repletion can stimulate aldosterone secretion, potentially exacerbating underlying HF.27,28
These data are observational and are unlikely to change practice. However, daily potassium repletion represents a huge cost in time, money, and effort to the health system. Furthermore, the greatest burden occurs for the patients, who have labs drawn and values checked routinely and potassium administered orally or parenterally. While future randomized clinical trials (RCTs) would best examine the benefits of repletion, future pragmatic trials could attempt to disentangle the associated risks and benefits of potassium repletion in the absence of RCTs. Additionally, such studies could better take into account the role of concurrent medication use (like ACEs or angiotensin II receptor blockers), as well as assess the role of chronic renal insufficiency, acute kidney injury, and magnesium levels.29
This study has limitations. Its retrospective design leads to unmeasured confounding; however, we adjusted for multiple variables (including LAPS-2), which reflect the severity of disease at admission and underlying kidney function at presentation, as well as other comorbid conditions. In addition, data from the cohort only extend to 2012, so more recent changes in practice may not be completely reflected. The nature of the data did not allow us to directly investigate the relationship between serum potassium and arrhythmias, although ICU transfer and mortality were used as surrogates.
In conclusion, the benefit of a serum potassium level >4.0 mEq/L in patients admitted with HF remains unclear. We did not observe that mean potassium values <4.0 mEq/L were associated with worse outcomes, and, more concerning, there may be some risk for patients with mean values >4.5 mEq/L.
Acknowledgments
Dr. Lagu had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Disclosures
The authors report no potential conflicts of interest. Dr. Lagu has served as a consultant for the Yale Center for Outcomes Research and Evaluation, under contract to the Centers for Medicare and Medicaid Services, for which she has provided clinical and methodological expertise and input on the development, reevaluation, and implementation of hospital outcome and efficiency measures.
Funding
Dr. Lagu is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number K01HL114745 and R01 HL139985-01A1. Dr. Stefan is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number K01HL114631-01A1. Dr. Pack is supported by NHLBI 1K23HL135440. Dr. Lindenauer is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number 1K24HL132008.
Disclaimer
The views expressed in this manuscript do not necessarily reflect those of the Yale Center for Outcomes Research and Evaluation or the Centers for Medicare and Medicaid Services.
Heart failure (HF) is a leading cause of hospital admission and mortality, accounting for approximately 900,000 discharges in 2014.1 One-year all-cause mortality risk has been estimated at 17% after hospitalization,2 and roughly 50% of deaths are related to sudden cardiac death, mostly due to ventricular arrhythmia.
The principles underlying potassium management in acute HF are complex. Both low and high values have been linked to fatal arrhythmias, notably ventricular fibrillation, and small serum changes often reflect large total body potassium fluctuations.11 Recent literature links hypokalemia to general membrane hypoexcitability, skeletal muscle hyporeflexia, and arrhythmias initiated by reduced sodium-potassium adenosine triphosphatase activity, leading to increased intracellular calcium and regional variations in action potential duration.12 Potassium abnormalities are common at admission and may be exacerbated by both acute illness and treatments given during hospitalization, including baseline potassium, acute kidney injury, aggressive diuretic therapy, or other potassium-related treatments and conditions.13 The success of potassium repletion may also be affected by the choice of HF therapies.14
The belief that patients with HF must maintain a potassium >4.0 mEq/L remains pervasive, with at least one family medicine guideline recommending that patients with HF maintain a serum potassium level >4.0 mEq/L.
METHODS
Data Sources and Cohort Definition
The Institutional Review Board at Baystate Medical Center approved this study. We identified patients with HF who were admitted for more than 72 hours between January 2010 and December 2012 to hospitals contributing to the HealthFacts database, a multihospital dataset derived from the comprehensive electronic health records of 116 geographically and structurally diverse hospitals throughout the United States (Cerner Corp.). HealthFacts—which includes date-stamped pharmacy, laboratory, and billing information—contains records of more than 84 million acute admissions, emergency room visits, and ambulatory visits. We limited the sample to hospitals that contributed to the pharmacy, laboratory, and diagnosis segments.
We included patients who had a principal International Classification of Disease (ICD-9-CM) diagnosis of HF or a principal diagnosis of respiratory failure with secondary diagnosis of HF (ICD-9-CM codes for HF: 402.01, 402.11, 402.91, 404.01, 404.03, 404.11, 404.13, 404.91, 404.93, 428.xx16 and for respiratory failure: 518.81, 518.82, 518.84) and were 18 years or older. We ensured that patients were treated for acute decompensated HF during the hospitalization by restricting the cohort to patients in whom at least one HF therapy (eg, loop diuretics, metolazone, inotropes, and intra-aortic balloon pump) was initiated within the first two days of hospitalization. We excluded patients with a pediatric or psychiatric attending physician, those with elective admissions, and those who were transferred from or to another acute care facility because we could not accurately determine the onset or subsequent course of their illness.
Definition of Variables Describing Serum Potassium Levels
We limited the sample to patients hospitalized for longer than 72 hours in order to observe how initial potassium values influenced outcomes over the course of hospitalization. We chose an exposure window of 72 hours because this allowed, on average, three potential observations of serum potassium per patient. We further restricted the sample to those who had a normal potassium value (3.5-5.0 mEq/L) at admission (defined as 24 hours prior to admission through midnight of the day of admission) to ensure that the included patients did not have abnormal potassium values upon presentation. We identified the period of time from 24 hours prior to admission through 72 hours following admission as “the exposure window” (the time during which patients were eligible to be classified into average serum potassium levels of <4.0, 4.0-4.5, or >4.5 mEq/L). We excluded patients who, during this window, had fewer than three serum potassium levels drawn (“exposure” levels could be disproportionately influenced by a single value) or received sodium polystyrene (as this would indicate that the physicians felt the potassium was dangerously high). For patients with repeated hospitalizations, we randomly selected one visit for inclusion to reduce the risk of survivor bias. We calculated the mean of all serum potassium levels during the exposure window, including the admission value, and then evaluated two different categorizations of mean serum potassium, based on categories of risk previously reported in the literature:8,17,18: (1) <4.0, 4.0-4.5, or >4.5 mEq/L and (2) <4.0 versus ≥4.0 mEq/L.
Outcomes
We assessed three outcomes: in-hospital mortality, transfer to an intensive care unit (ICU), and length of stay (LOS). Admission to the ICU was defined as any evidence, after the exposure window, that the patient received care in the ICU. We excluded patients with ICU admissions during the exposure window from the analysis of this outcome. We calculated LOS as the difference between discharge date/time and the admission date/time.
Covariates and Comorbidity Adjustment
We obtained information on patient demographics (age and race) and identified the presence of comorbid conditions using previously derived and validated models.19,20 We then further quantified these conditions into a single combined score to adjust for differences in presenting illness severity (including kidney disease) and help reduce confounding.21 To account for presenting severity of illness, we calculated the Laboratory-based Acute Physiology Score (LAPS-2).22,23 LAPS-2 was developed for predicting mortality risk in general medical patients, but we previously externally validated it against other published clinical HF models in a cohort of patients hospitalized with acute decompensated HF.5
Potassium Repletion
Analysis
We evaluated the differences in patient characteristics across serum potassium categories. Categorical variables are presented as frequencies and percentages, whereas continuous variables are presented as means and standard deviations. For binary outcomes, we used generalized estimating equations (with a binomial family and logit link and clustering by hospital) to estimate incidence and calculate unadjusted and adjusted odds ratios (ORs) and 95% confidence intervals (CIs). For LOS, we estimated the median and 95% CIs using quantile regression with clustered standard errors.24 We calculated all models using both a binary exposure (<4.0 versus ≥4.0 mEq/L) and a three-level categorization (<4.0, 4.0-4.5, and >4.5 mEq/L) to explore the effects at the highest potassium level. We adjusted all models for age, race, LAPS-2 score, and combined comorbidity score. We conducted two sensitivity analyses. First, we restricted our sample to those who never received potassium during the exposure window, as these patients may be different than patients who required potassium repletion. Second, we stratified our findings by the presence or absence of acute or chronic renal insufficiency (defined as an admission creatinine >1 or the presence of a diagnostic code for renal insufficiency, as defined by Elixhauser et al.).19,21 Statistical significance was set at an alpha of 0.05. Analysis was completed using Stata v15.1, StataCorp LP, College Station, Texas.
RESULTS
Cohort Description
We identified patients from 56 geographically diverse US hospitals, although most were located in either the northeast (n = 21; 38%) or south (n = 18; 32%). A total of 59% of the hospitals were teaching hospitals, and nearly 95% were in an urban setting. We identified 13,163 patients with HF, of which 4,995 (38.0%) met the inclusion criteria. We excluded 3,744 (28.4%) patients with LOS < 72 hours, 2,210 (16.8%) with admission potassium values outside of the defined range, and 896 (6.8%) with fewer than three potassium values during the exposure window. Of the patients who met the inclusion criteria, 2,080 (41.6%), 2,326 (46.6%), and 589 (11.8%) were categorized in the <4.0, 4.0-4.5, and >4.5 mEq/L groups, respectively (Table 1). The groups were clinically similar in terms of age, sex, illness severity (LAPS-2), and comorbidity score. Compared with other racial groups, black patients had higher potassium values. While the <4.0 and 4.0-4.5 mEq/L groups were relatively similar, the group with mean potassium >4.5 mEq/L had higher admission creatinine and a greater prevalence of chronic kidney disease, deficiency anemias, and chronic obstructive pulmonary disease (Table 1).
Serum Potassium Values
Individuals’ mean serum potassium within the 72-hour exposure window ranged from 2.9 to 5.8 mEq/L (Table 2). In the <4.0, 4-4.5, and >4.5 mEq/L cohorts respectively, patients had a median serum potassium of 3.8 mEq/L (2.9-3.9), 4.2 mEq/L (4.0-4.5), and 4.7 mEq/L (4.5-5.8) during the exposure window. Approximately half of the patients in the <4.0 mEq/L group had a serum potassium <3.5 mEq/L at some point during the exposure window. In contrast, <10% of the other groups had this low value during the exposure window.
Potassium Repletion
Patients in the <4.0 mEq/L group were much more likely to receive potassium repletion during the exposure window when compared with the 4.0-4.5 mEq/L (71.5% vs 40.5%) and >4.5 mEq/L (71.5% vs 26.7%) groups. On days that they were eligible for repletion (defined as a daily potassium value <4.0 mEq/L), patients with mean serum potassium >4.0 mEq/L were less likely to receive potassium repletion compared with those with values <4.0 mEq/L. There were 592 (28.5%), 1,383 (59.5%), and 432 (73.3%) patients in the <4.0, 4-4.5, and >4,5 mEq/L groups, respectively, who did not receive potassium repletion therapy during the exposure window.
Relationship of Serum Potassium Levels and Outcomes
Overall, 3.7% (n = 187) of patients died during the hospitalization, 2.4% (n = 98) were admitted to the ICU after the exposure window, and the median LOS was 5.6 days. We did not observe a significant association between mean serum potassium of <4.0 or 4.0-4.5 mEq/L and increased risk of mortality, ICU transfer, or LOS (Table 3). Our unadjusted analysis showed that patients with values >4.5 mEq/L had worse outcomes, including more deaths (5.3%; OR = 1.55; 95% CI: 1.01 to 2.39) and ICU admission (3.8%; OR = 2.10; 95% CI: 1.16 to 3.80) compared with those with values <4.0 mEq/L (Table 3). We also found that, compared with the <4.0 mEq/L group, the >4.5 mEq/L group showed just over a half-day longer LOS (0.6 days; 95% CI: 0.0 to 1.0; Table 3). However, we found that mortality and ICU admission results were attenuated after adjustment for age, race, comorbidity score, and LAPS-2 and were no longer statistically significant, whereas the association with LOS was consistent after adjustment. When using a binary exposure (<4.0 versus ≥4.0 mEq/L), we observed no association between mean potassium value and increased risk of mortality, ICU transfer, or LOS both before and after adjustment for age, race, LAPS-2, and comorbidity score (data not shown).
Sensitivity Analyses
In the sensitivity analysis restricted to those who did not receive potassium repletion during the exposure window, we continued to observe no association between the <4.0 and 4.0-4.5 mEq/L groups and outcomes (Table 3). In adjusted models for the >4.5 versus <4.0 mEq/L groups, risk estimates for mortality were similar to the full sample, but statistical significance was lost (OR = 1.56; 95% CI: 0.81 to 3.01). Adjusted risk estimates for ICU transfer were attenuated and not statistically significant (OR = 1.40; 95% CI: 0.60 to 3.26). However, LOS estimates were very similar to that observed in the full dataset (0.6 days; 95% CI: 0.1 to 1.2).
When stratifying our results by the presence or absence of acute or chronic renal insufficiency, we continued to observe no increased risk of any outcome in the 4.0-4.5 mEq/L compared with the <4.0 mEq/L groups across all strata (Table 4). Interestingly, even after adjustment, we did find that most of the increased risk of mortality and ICU admission in the >4.5 versus <4.0 mEq/L groups was among those without renal insufficiency (mortality OR = 3.03; ICU admission OR = 3.00) and was not statistically significant in those with renal insufficiency (mortality OR = 1.27; ICU admission OR = 1.63). Adjusted LOS estimates remained relatively similar in this stratified analysis.
DISCUSSION
The best approach to mild serum potassium value abnormalities in patients hospitalized with HF remains unclear. Many physicians reflexively replete potassium to ensure all patients maintain a serum value of >4.0 mEq/L.15 Yet, in this large observational study of patients hospitalized with an acute HF exacerbation, we found little evidence of association between serum potassium <4.0 mEq/L and negative outcomes.
Compared with those with mean potassium values <4.0 mEq/L (in unadjusted models), there was an association between potassium values of >4.5 mEq/L and increased risk of mortality and ICU transfer. This association was attenuated after adjustment, suggesting that factors beyond potassium values influenced the observed relationship. These findings seem to suggest that unobserved differences in the >4.5 mEq/L group (there were observed differences in this group, eg, greater presenting severity and higher comorbidity scores, suggesting that there were also unobserved differences), and not average potassium value, were the reasons for the observed differences in outcomes. However, we cannot rule out the possibility that potassium >4.5 mEq/L has some associated increased risk compared with mean potassium values of <4.0 mEq/L for patients hospitalized with acute decompensated HF.
Patients in our study routinely received exogenous potassium: more than 70% of patients received repletion at least once, although it is notable that the majority of patients in the 4.0-4.5 and >4.5 mEq/L groups did not receive repletion. Despite this practice, the data supporting this approach to potassium management for patients hospitalized with HF remain mixed. A serum potassium decline of >15% during an acute HF hospital stay has been reported as a predictor of all-cause mortality after controlling for disease severity and associated comorbidities, including renal function.25 However, this study was focused on decline in admission potassium rather than an absolute cut-off (eg, >4.0 mEq/L). Additionally, potassium levels <3.9 mEq/L were associated with increased mortality in patients with acute HF following a myocardial infarction, but this study was not focused on patients with HF.26 Most of the prior literature in patients with HF was conducted in patients in outpatient settings and examined patients who were not experiencing acute exacerbations. MacDonald and Struthers advocate that patients with HF have their potassium maintained above 4.0 mEq/L but did not specify whether this included patients with acute HF exacerbations.10 Additionally, many studies evaluating potassium repletion were conducted before widespread availability of angiotensin-converting enzyme (ACE) inhibitors or potassium-sparing diuretics, including spironolactone. Prior work has consistently reported that hyperkalemia, defined as serum potassium >4.5 mEq/L, is associated with mortality in patients with acute HF over the course of hospitalization (which aligned with the results from our sensitivity analysis), but concurrent medication regimens and underlying impaired renal function likely accounted for most of this association.17 The picture is further complicated as patients with acute HF presenting with hypokalemia may be at risk for subsequent hyperkalemia, and potassium repletion can stimulate aldosterone secretion, potentially exacerbating underlying HF.27,28
These data are observational and are unlikely to change practice. However, daily potassium repletion represents a huge cost in time, money, and effort to the health system. Furthermore, the greatest burden occurs for the patients, who have labs drawn and values checked routinely and potassium administered orally or parenterally. While future randomized clinical trials (RCTs) would best examine the benefits of repletion, future pragmatic trials could attempt to disentangle the associated risks and benefits of potassium repletion in the absence of RCTs. Additionally, such studies could better take into account the role of concurrent medication use (like ACEs or angiotensin II receptor blockers), as well as assess the role of chronic renal insufficiency, acute kidney injury, and magnesium levels.29
This study has limitations. Its retrospective design leads to unmeasured confounding; however, we adjusted for multiple variables (including LAPS-2), which reflect the severity of disease at admission and underlying kidney function at presentation, as well as other comorbid conditions. In addition, data from the cohort only extend to 2012, so more recent changes in practice may not be completely reflected. The nature of the data did not allow us to directly investigate the relationship between serum potassium and arrhythmias, although ICU transfer and mortality were used as surrogates.
In conclusion, the benefit of a serum potassium level >4.0 mEq/L in patients admitted with HF remains unclear. We did not observe that mean potassium values <4.0 mEq/L were associated with worse outcomes, and, more concerning, there may be some risk for patients with mean values >4.5 mEq/L.
Acknowledgments
Dr. Lagu had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Disclosures
The authors report no potential conflicts of interest. Dr. Lagu has served as a consultant for the Yale Center for Outcomes Research and Evaluation, under contract to the Centers for Medicare and Medicaid Services, for which she has provided clinical and methodological expertise and input on the development, reevaluation, and implementation of hospital outcome and efficiency measures.
Funding
Dr. Lagu is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number K01HL114745 and R01 HL139985-01A1. Dr. Stefan is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number K01HL114631-01A1. Dr. Pack is supported by NHLBI 1K23HL135440. Dr. Lindenauer is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number 1K24HL132008.
Disclaimer
The views expressed in this manuscript do not necessarily reflect those of the Yale Center for Outcomes Research and Evaluation or the Centers for Medicare and Medicaid Services.
1. Benjamin EJ, Virani SS, Callaway CW, et al. Heart disease and stroke statistics–2018 update: a report from the American Heart Association. Circulation. 2018;137(12):e67-e492. https://doi.org/10.1161/CIR.0000000000000558.
2. Maggioni AP, Dahlström U, Filippatos G, et al. EURObservational Research Programme: regional differences and 1-year follow-up results of the Heart Failure Pilot Survey (ESC-HF Pilot). Eur J Heart Fail. 2013;15(7):808-817. https://doi.org/10.1093/eurjhf/hft050.
3. Tomaselli GF, Zipes DP. What causes sudden death in heart failure? Circ Res. 2004;95(8):754-763. https://doi.org/10.1161/01.RES.0000145047.
4. Bowen GS, Diop MS, Jiang L, Wu W-C, Rudolph JL. A multivariable prediction model for mortality in individuals admitted for heart failure. J Am Geriatr Soc. 2018;66(5):902-908. https://doi.org/10.1111/jgs.15319.
5. Lagu T, Pekow PS, Shieh M-S, et al. Validation and comparison of seven mortality prediction models for hospitalized patients with acute decompensated heart failure. Circ Heart Fail. 2016;9(8). https://doi.org/10.1161/CIRCHEARTFAILURE.115.002912.
6. Núñez J, Bayés-Genís A, Zannad F, et al. Long-term potassium monitoring and dynamics in heart failure and risk of mortality. Circulation. 2018;137(13):1320-1330. https://doi.org/10.1161/CIRCULATIONAHA.117.030576.
7. Vardeny O, Claggett B, Anand I, et al. Incidence, predictors, and outcomes related to hypo- and hyperkalemia in patients with severe heart failure treated with a mineralocorticoid receptor antagonist. Circ Heart Fail. 2014;7(4):573-579. https://doi.org/10.1161/CIRCHEARTFAILURE.114.00110.
8. Aldahl M, Jensen A-SC, Davidsen L, et al. Associations of serum potassium levels with mortality in chronic heart failure patients. Eur Heart J. 2017;38(38):2890-2896. https://doi.org/10.1093/eurheartj/ehx460.
9. Hoppe LK, Muhlack DC, Koenig W, Carr PR, Brenner H, Schöttker B. Association of abnormal serum potassium levels with arrhythmias and cardiovascular mortality: a systematic review and meta-analysis of observational studies. Cardiovasc Drugs Ther. 2018;32(2):197-212. https://doi.org/10.1007/s10557-018-6783-0.
10. Macdonald JE, Struthers AD. What is the optimal serum potassium level in cardiovascular patients? J Am Coll Cardiol. 2004;43(2):155-161. https://doi.org/10.1016/j.jacc.2003.06.021.
11. Hulting J. In-hospital ventricular fibrillation and its relation to serum potassium. Acta Med Scand Suppl. 1981;647(647):109-116. https://doi.org/10.1111/j.0954-6820.1981.tb02646.x.
12. Skogestad J, Aronsen JM. Hypokalemia-induced arrhythmias and heart failure: new insights and implications for therapy. Front Physiol. 2018;9:1500. https://doi.org/10.3389/fphys.2018.01500.
13. Tromp J, Ter Maaten JM, Damman K, et al. Serum potassium levels and outcome in acute heart failure (data from the PROTECT and COACH trials). Am J Cardiol. 2017;119(2):290-296. https://doi.org/10.1016/j.amjcard.2016.09.038.
14. Khan SS, Campia U, Chioncel O, et al. Changes in serum potassium levels during hospitalization in patients with worsening heart failure and reduced ejection fraction (from the EVEREST trial). Am J Cardiol. 2015;115(6):790-796. https://doi.org/10.1016/j.amjcard.2014.12.045
15. Viera AJ, Wouk N. Potassium disorders: hypokalemia and hyperkalemia. Am Fam Physician. 2015;92(6):487-495.
16. Krumholz HM, Wang Y, Mattera JA, et al. An administrative claims model suitable for profiling hospital performance based on 30-day mortality rates among patients with heart failure. Circulation. 2006;113(13):1693-1701. https://doi.org/10.1161/CIRCULATIONAHA.105.611194.
17. Legrand M, Ludes P-O, Massy Z, et al. Association between hypo- and hyperkalemia and outcome in acute heart failure patients: the role of medications. Clin Res Cardiol. 2018;107(3):214-221. https://doi.org/10.1007/s00392-017-1173-3.
18. Kok W, Salah K, Stienen S. Are changes in serum potassium levels during admissions for acute decompensated heart failure irrelevant for prognosis: the end of the story? Am J Cardiol. 2015;116(5):825. https://doi.org/10.1016/j.amjcard.2015.05.059.
19. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. https://doi.org/10.1097/00005650-199801000-00004.
20. Quan H, Parsons GA, Ghali WA. Validity of information on comorbidity derived from ICD-9-CCM administrative data. Med Care. 2002;40(8):675-685. https://doi.org/10.1097/01.MLR.0000020927.46398.5D.
21. Gagne JJ, Glynn RJ, Avorn J, Levin R, Schneeweiss S. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol. 2011;64(7):749-759. https://doi.org/10.1016/j.jclinepi.2010.10.004.
22. Escobar GJ, Gardner MN, Greene JD, Draper D, Kipnis P. Risk-adjusting hospital mortality using a comprehensive electronic record in an integrated health care delivery system. Med Care. 2013;51(5):446-453. https://doi.org/10.1097/MLR.0b013e3182881c8e.
23. Escobar GJ, Greene JD, Scheirer P, Gardner MN, Draper D, Kipnis P. Risk-adjusting hospital inpatient mortality using automated inpatient, outpatient, and laboratory databases. Med Care. 2008;46(3):232-239. https://doi.org/10.1097/MLR.0b013e3181589bb6.
24. Parente PMDC, Santos Silva JMC. Quantile regression with clustered data. J Econom Method. 2016;5(1):1-15. https://doi.org/10.1515/jem-2014-0011.
25. Salah K, Pinto YM, Eurlings LW, et al. Serum potassium decline during hospitalization for acute decompensated heart failure is a predictor of 6-month mortality, independent of N-terminal pro-B-type natriuretic peptide levels: An individual patient data analysis. Am Heart J. 2015;170(3):531-542.e1. https://doi.org/10.1016/j.ahj.2015.06.003.
26. Krogager ML, Eggers-Kaas L, Aasbjerg K, et al. Short-term mortality risk of serum potassium levels in acute heart failure following myocardial infarction. Eur Heart J Cardiovasc Pharmacother. 2015;1(4):245-251. https://doi.org/10.1093/ehjcvp/pvv026.
27. Crop MJ, Hoorn EJ, Lindemans J, Zietse R. Hypokalaemia and subsequent hyperkalaemia in hospitalized patients. Nephrol Dial Transplant. 2007;22(12):3471-3477.https://doi.org/10.1093/ndt/gfm471.
28. Kok W, Salah K, Stienen S. Serum potassium levels during admissions for acute decompensated heart failure: identifying possible threats to outcome. Am J Cardiol. 2018;121(1):141. https://doi.org/10.1016/j.amjcard.2017.09.032.
29. Freda BJ, Knee AB, Braden GL, Visintainer PF, Thakar CV. Effect of transient and sustained acute kidney injury on readmissions in acute decompensated heart failure. Am J Cardiol. 2017;119(11):1809-1814. https://doi.org/10.1016/j.amjcard.2017.02.044.
1. Benjamin EJ, Virani SS, Callaway CW, et al. Heart disease and stroke statistics–2018 update: a report from the American Heart Association. Circulation. 2018;137(12):e67-e492. https://doi.org/10.1161/CIR.0000000000000558.
2. Maggioni AP, Dahlström U, Filippatos G, et al. EURObservational Research Programme: regional differences and 1-year follow-up results of the Heart Failure Pilot Survey (ESC-HF Pilot). Eur J Heart Fail. 2013;15(7):808-817. https://doi.org/10.1093/eurjhf/hft050.
3. Tomaselli GF, Zipes DP. What causes sudden death in heart failure? Circ Res. 2004;95(8):754-763. https://doi.org/10.1161/01.RES.0000145047.
4. Bowen GS, Diop MS, Jiang L, Wu W-C, Rudolph JL. A multivariable prediction model for mortality in individuals admitted for heart failure. J Am Geriatr Soc. 2018;66(5):902-908. https://doi.org/10.1111/jgs.15319.
5. Lagu T, Pekow PS, Shieh M-S, et al. Validation and comparison of seven mortality prediction models for hospitalized patients with acute decompensated heart failure. Circ Heart Fail. 2016;9(8). https://doi.org/10.1161/CIRCHEARTFAILURE.115.002912.
6. Núñez J, Bayés-Genís A, Zannad F, et al. Long-term potassium monitoring and dynamics in heart failure and risk of mortality. Circulation. 2018;137(13):1320-1330. https://doi.org/10.1161/CIRCULATIONAHA.117.030576.
7. Vardeny O, Claggett B, Anand I, et al. Incidence, predictors, and outcomes related to hypo- and hyperkalemia in patients with severe heart failure treated with a mineralocorticoid receptor antagonist. Circ Heart Fail. 2014;7(4):573-579. https://doi.org/10.1161/CIRCHEARTFAILURE.114.00110.
8. Aldahl M, Jensen A-SC, Davidsen L, et al. Associations of serum potassium levels with mortality in chronic heart failure patients. Eur Heart J. 2017;38(38):2890-2896. https://doi.org/10.1093/eurheartj/ehx460.
9. Hoppe LK, Muhlack DC, Koenig W, Carr PR, Brenner H, Schöttker B. Association of abnormal serum potassium levels with arrhythmias and cardiovascular mortality: a systematic review and meta-analysis of observational studies. Cardiovasc Drugs Ther. 2018;32(2):197-212. https://doi.org/10.1007/s10557-018-6783-0.
10. Macdonald JE, Struthers AD. What is the optimal serum potassium level in cardiovascular patients? J Am Coll Cardiol. 2004;43(2):155-161. https://doi.org/10.1016/j.jacc.2003.06.021.
11. Hulting J. In-hospital ventricular fibrillation and its relation to serum potassium. Acta Med Scand Suppl. 1981;647(647):109-116. https://doi.org/10.1111/j.0954-6820.1981.tb02646.x.
12. Skogestad J, Aronsen JM. Hypokalemia-induced arrhythmias and heart failure: new insights and implications for therapy. Front Physiol. 2018;9:1500. https://doi.org/10.3389/fphys.2018.01500.
13. Tromp J, Ter Maaten JM, Damman K, et al. Serum potassium levels and outcome in acute heart failure (data from the PROTECT and COACH trials). Am J Cardiol. 2017;119(2):290-296. https://doi.org/10.1016/j.amjcard.2016.09.038.
14. Khan SS, Campia U, Chioncel O, et al. Changes in serum potassium levels during hospitalization in patients with worsening heart failure and reduced ejection fraction (from the EVEREST trial). Am J Cardiol. 2015;115(6):790-796. https://doi.org/10.1016/j.amjcard.2014.12.045
15. Viera AJ, Wouk N. Potassium disorders: hypokalemia and hyperkalemia. Am Fam Physician. 2015;92(6):487-495.
16. Krumholz HM, Wang Y, Mattera JA, et al. An administrative claims model suitable for profiling hospital performance based on 30-day mortality rates among patients with heart failure. Circulation. 2006;113(13):1693-1701. https://doi.org/10.1161/CIRCULATIONAHA.105.611194.
17. Legrand M, Ludes P-O, Massy Z, et al. Association between hypo- and hyperkalemia and outcome in acute heart failure patients: the role of medications. Clin Res Cardiol. 2018;107(3):214-221. https://doi.org/10.1007/s00392-017-1173-3.
18. Kok W, Salah K, Stienen S. Are changes in serum potassium levels during admissions for acute decompensated heart failure irrelevant for prognosis: the end of the story? Am J Cardiol. 2015;116(5):825. https://doi.org/10.1016/j.amjcard.2015.05.059.
19. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. https://doi.org/10.1097/00005650-199801000-00004.
20. Quan H, Parsons GA, Ghali WA. Validity of information on comorbidity derived from ICD-9-CCM administrative data. Med Care. 2002;40(8):675-685. https://doi.org/10.1097/01.MLR.0000020927.46398.5D.
21. Gagne JJ, Glynn RJ, Avorn J, Levin R, Schneeweiss S. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol. 2011;64(7):749-759. https://doi.org/10.1016/j.jclinepi.2010.10.004.
22. Escobar GJ, Gardner MN, Greene JD, Draper D, Kipnis P. Risk-adjusting hospital mortality using a comprehensive electronic record in an integrated health care delivery system. Med Care. 2013;51(5):446-453. https://doi.org/10.1097/MLR.0b013e3182881c8e.
23. Escobar GJ, Greene JD, Scheirer P, Gardner MN, Draper D, Kipnis P. Risk-adjusting hospital inpatient mortality using automated inpatient, outpatient, and laboratory databases. Med Care. 2008;46(3):232-239. https://doi.org/10.1097/MLR.0b013e3181589bb6.
24. Parente PMDC, Santos Silva JMC. Quantile regression with clustered data. J Econom Method. 2016;5(1):1-15. https://doi.org/10.1515/jem-2014-0011.
25. Salah K, Pinto YM, Eurlings LW, et al. Serum potassium decline during hospitalization for acute decompensated heart failure is a predictor of 6-month mortality, independent of N-terminal pro-B-type natriuretic peptide levels: An individual patient data analysis. Am Heart J. 2015;170(3):531-542.e1. https://doi.org/10.1016/j.ahj.2015.06.003.
26. Krogager ML, Eggers-Kaas L, Aasbjerg K, et al. Short-term mortality risk of serum potassium levels in acute heart failure following myocardial infarction. Eur Heart J Cardiovasc Pharmacother. 2015;1(4):245-251. https://doi.org/10.1093/ehjcvp/pvv026.
27. Crop MJ, Hoorn EJ, Lindemans J, Zietse R. Hypokalaemia and subsequent hyperkalaemia in hospitalized patients. Nephrol Dial Transplant. 2007;22(12):3471-3477.https://doi.org/10.1093/ndt/gfm471.
28. Kok W, Salah K, Stienen S. Serum potassium levels during admissions for acute decompensated heart failure: identifying possible threats to outcome. Am J Cardiol. 2018;121(1):141. https://doi.org/10.1016/j.amjcard.2017.09.032.
29. Freda BJ, Knee AB, Braden GL, Visintainer PF, Thakar CV. Effect of transient and sustained acute kidney injury on readmissions in acute decompensated heart failure. Am J Cardiol. 2017;119(11):1809-1814. https://doi.org/10.1016/j.amjcard.2017.02.044.
© 2019 Society of Hospital Medicine
Collaboration, Not Calculation: A Qualitative Study of How Hospital Executives Value Hospital Medicine Groups
The field of hospital medicine has expanded rapidly since its inception in the late 1990s, and currently, most hospitals in the United States employ or contract with hospital medicine groups (HMGs).1-4 This dramatic growth began in response to several factors: primary care physicians (PCPs) opting out of inpatient care, the increasing acuity and complexity of inpatient care, and cost pressures on hospitals.5,6 Recent studies associate greater use of hospitalists with increased hospital revenues and modest improvements in hospital financial performance.7 However, funding the hospitalist delivery model required hospitals to share the savings hospitalists generate through facility billing and quality incentives.
Hospitalists’ professional fee revenues alone generally do not fund their salaries. An average HMG serving adult patients requires $176,658 from the hospital to support a full-time physician.8 Determining the appropriate level of HMG support typically occurs through negotiation with hospital executives. During the last 10 years, HMG size and hospitalist compensation have risen steadily, combining to increase the hospitalist labor costs borne by hospitals.4,8 Accordingly, hospital executives in challenging economic environments may pressure HMG leaders to accept diminished support or to demonstrate a better return on the hospital’s investment.
These negotiations are influenced by the beliefs of hospital executives about the value of the hospitalist labor model. Little is known about how hospital and health system executive leadership assess the value of hospitalists. A deeper understanding of executive attitudes and beliefs could inform HMG leaders seeking integrative (“win-win”) outcomes in contract and compensation negotiations. Members of the Society of Hospital Medicine (SHM) Practice Management Committee surveyed hospital executives to guide SHM program development. We sought to analyze transcripts from these interviews to describe how executives assess HMGs and to test the hypothesis that hospital executives apply specific financial models when determining the return on investment (ROI) from subsidizing an HMG.
METHODS
Study Design, Setting, and Participants
Members of the SHM Practice Management Committee conducted interviews with a convenience sample of 24 key informants representing the following stakeholders at hospitals employing hospitalists: Chief Executive Officers (CEOs), Presidents, Vice Presidents, Chief Medical Officers (CMOs), and Chief Financial Officers (CFOs). Participants were recruited from 17 fee-for-service healthcare organizations, including rural, suburban, urban, community, and academic medical centers. The semi-structured interviews occurred in person between January and March 2018; each one lasted an average of 45 minutes and were designed to guide SHM program and product development. Twenty-eight executives were recruited by e-mail, and four did not complete the interview due to scheduling difficulty. All the participants provided informed consent. The University of Washington Institutional Review Board approved the secondary analysis of deidentified transcripts.
Interview Guide and Data Collection
All interviews followed a guide with eight demographic questions and 10 open-ended questions (Appendix). Cognitive interviews were performed with two hospital executives outside the study cohort, resulting in the addition of one question and rewording one question for clarity. One-on-one interviews were performed by 10 committee members (range, 1-3 interviews). All interview audios were recorded, and no field notes were kept. The goal of the interviews was to obtain an understanding of how hospital executives value the contributions and costs of hospitalist groups.
The interviews began with questions about the informant’s current interactions with hospitalists and the origin of the hospitalist group at their facility. Informants then described the value they feel hospitalists bring to their hospital and occasions they were surprised or dissatisfied with the clinical or financial value delivered by the hospitalists. Participants described how they calculate a return on investment (ROI) for their hospitalist group, nonfinancial benefits and disadvantages to hospitalists, and how they believe hospitalists should participate in risk-sharing contracts.
Data Analysis
The interview audiotapes were transcribed and deidentified. A sample of eight transcripts was verified by participants to ensure accuracy. Three investigators (AAW, RC, CC) reviewed a random sample of five transcripts to identify and codify preliminary themes. We applied a general inductive framework with a content analysis approach. Two investigators (TM and MC) read all transcripts independently, coding the presence of each theme and quotations exemplifying these themes using qualitative analysis software (Dedoose Version 7.0.23, SocioCultural Research Consultants). A third investigator (AAW) read all the transcripts and resolved differences of opinion. Themes and code application were discussed among the study team after the second and fifth transcripts to add or clarify codes. No new codes were identified after the first review of the preliminary codebook, although investigators intermittently used an “unknown” code through the 20th transcript. After discussion to reach consensus, excerpts initially coded “unknown” were assigned existing codes; the 20th transcript represents the approximate point of reaching thematic saturation.
RESULTS
Of the 24 participants, 18 (75%) were male, representing a variety of roles: 7 (29.2%) CMOs, 5 (20.8%) Presidents, 5 (20.8%) CFOs, 4 (16.7%) CEOs, and 3 (12.5%) Vice Presidents. The participants represented all regions (Midwest 12 [50%], South 6 [25%], West 4 [16.7%], and East 2 [8.3%], community size (Urban 11 [45.8%], Suburban 8 [33.3%], and Rural 5 [20.8%]), and Hospital Types (Community 11 [45.8%], Multihospital System 5 [20.8%], Academic 5 [20.8%], Safety Net 2 [8.3%], and Critical Access 1 [4.2%]). We present specific themes below and supporting quotations in Tables 1 and 2.
Current Value of the HMG at the Respondent’s Hospital
Most executives reported their hospital’s HMG had operated for over a decade and had developed an earlier, outdated value framework. Interviewees described an initial mix of financial pressures, shifts in physician work preferences, increasing patient acuity, resident labor shortages, and unsolved hospital throughput needs that triggered a reactive conversion from community PCP staffing to hospitalist care teams, followed by refinements to realize value.
“I think initially here it was to deal with the resident caps, right? So, at that moment, the solution that was put in place probably made a lot of sense. If that’s all someone came in with, now I’d be scratching my head and said, what are you thinking?” (President, #2)
Respondents perceived that HMGs provide value in many domains, including financial contributions, high-quality care, organizational efficiency, academics, leadership of interprofessional teams, effective communication, system improvement, and beneficial influence on the care environment and other employees. Regarding the measurable generation of financial benefit, documentation for improved billing accuracy, increased hospital efficiency (eg, lower length of stay, early discharges), and comanagement arrangements were commonly identified.
“I don’t want a urologist with a stethoscope, so I’m happy to have the hospitalists say, ‘Look, I’ll take care of the patient. You do the procedure.’ Well, that’s inherently valuable, whether we measure it or whether we don’t.” (CMO, #21)
Executives generally expressed satisfaction with their HMG’s quality of care and the related pay-for-performance financial benefits from payers, attributing success to hospitalists’ familiarity with inpatient systems and willingness to standardize.
“I just think it’s having one structure, one group to go to, a standard rather than trying to push it through the medical staff.” (VP, #18)
Executives reported that HMGs generate substantial value that is difficult to measure financially. For example, a large bundle of excerpts organized around communication with patients, nurses, and other providers.
“If we have the right hospitalist staff, to engage them with the nursing staff would help to reduce my turnover rate…and create a very positive morale within the nursing units. That’s huge. That’s nonfinancial” (President, #15)
Executives particularly appreciated hospitalists’ work to aggregate input from multiple specialists and present a cohesive explanation to patients. Executives also felt that HMGs create significant unmeasured value by improving processes and outcomes on service lines beyond hospital medicine, achieving this through culture change, involvement in leadership, hospital-wide process redesign, and running rapid response teams. Some executives expressed a desire for hospitalists to assume this global quality responsibility more explicitly as a job expectation.
Executives described how they would evaluate a de novo proposal for hospitalist services, usually enumerating key general domains without explaining specifically how they would measure each element. The following priorities emerged: clinical excellence, capacity to collaborate with hospital leadership, the scope of services provided, cultural fit/alignment, financial performance, contract cost, pay-for-performance measures, and turnover. Regarding financial performance, respondents expected to know the cost of the proposal but lacked a specific price threshold. Instead, they sought to understand the total value of the proposal through its effect on metrics such as facility fees or resource use. Nonetheless, cultural fit was a critical, overriding driver of the hypothetical decision, despite difficulty defining beyond estimates of teamwork, alignment with hospital priorities, and qualities of the group leader.
“For us, it usually ends being how do we mix personally, do we like them?” (CMO, #5)
Alignment and Collaboration
The related concepts of “collaboration” and “alignment” emerged as prominent themes during all interviews. Executives highly valued hospitalist groups that could demonstrate alignment with hospital priorities and often used this concept to summarize the HMG’s success or failure across a group of value domains.
“If you’re just coming in to fill a shift and see 10 patients, you have much less value than somebody who’s going to play that active partnership role… hospitalist services need to partner with hospitals and be intimately involved with the success of the hospital.” (CMO, #20)
Alignment sometimes manifested in a quantified, explicit way, through incentive plans or shared savings plans. However, it most often manifested as a broader sense that the hospitalists’ work targeted the same priorities as the executive leaders and that hospitalists genuinely cared about those priorities. A “shift-work mentality” was expressed by some as the antithesis of alignment. Incorporating hospitalist leaders in hospital leadership and frequent communication arose as mechanisms to increase alignment.
Ways HMGs Fail to Meet Expectations
Respondents described unresolved disadvantages to the hospitalist care model.
“I mean, OPPE, how do you do that for a hospitalist? How can you do it? It’s hard to attribute a patient to someone….it is a weakness and I think we all know it.” (CMO, #21)
Executives also worried about inconsistent handoffs with primary care providers and the field’s demographics, finding it disproportionately comprised of junior or transient physicians. They also hoped that hospitalist innovators would solve clinician burnout and the high cost of inpatient care. Disappointments specific to the local HMG revolved around difficulty developing shared models of value and mechanisms to achieve them.
“I would like to have more dialog between the hospital leadership team and the hospitalist group…I would like to see a little bit more collaboration.” (President, #13)
These challenges emerged not as a deficiency with hospital medicine as a specialty, but a failure at their specific facility to achieve the goal of alignment through joint strategic planning.
Calculating Value
When asked if their hospital had a formal process to evaluate ROI for their HMG, two dominant answers emerged: (1) the executive lacked a formal process for determining ROI and was unaware of one used at their facility or (2) the executive evaluated HMG performance based on multiple measures, including cost, but did not attempt to calculate ROI or a summary value. Several described the financial evaluation process as too difficult or unnecessary.
“No. It’s too difficult to extract that data. I would say the best proxy that we could do it is our case mix index on our medicine service line.” (CMO, #20)
“No, not a formal process, no… I question the value of some of the other things we do with the medical group…but not the value of the hospitalists… I don’t think we’ve done a formal assessment. I appreciate the flexibility, especially in a small hospital.” (President, #10)
Rarely, executives described specific financial calculations that served as a proxy for ROI. These included calculating a contribution margin to compare against the cost of salary support or the application of external survey benchmarking comparisons for productivity and salary to evaluate the appropriateness of a limited set of financial indicators. Twice respondents alluded to more sophisticated measurements conducted by the finance department but lacked familiarity with the process. Several executives described ROI calculations for specific projects and discrete business decisions involving hospitalists, particularly considering hiring an additional hospitalist.
Executives generally struggled to recall specific ways that the nonfinancial contributions of hospitalists were incorporated into executive decisions regarding the hospitalist group. Two related themes emerged: first, the belief that hospitals could not function effectively without hospitalists, making their presence an expected cost of doing business. Second, absent measures of HMG ROI, executives appeared to determine an approximate overall value of hospitalists, rather than parsing the various contributions. A few respondents expressed alarm at the rise in hospitalist salaries, whereas others acknowledged market forces beyond their control.
“… there is going to be more of a demand for hospitalists, which is definitely going to drive up the compensation. So, I don’t worry that the compensation will be driven up so high that there won’t be a return [on investment].” (CFO, #16)
Some urged individual hospitalists to develop a deeper understanding of what supports their salary to avoid strained relationships with executives.
Evolution and Risk-Sharing Contracts
Respondents described an evolving conceptualization of the hospitalist’s value, occurring at both a broad, long-term scale and at an incremental, annual scale through minor modifications to incentive pay schemes. For most executives, hiring hospitalists as replacements for PCPs had become necessary and not a source of novel value; many executives described it as “the cost of doing business.” Some described gradually deemphasizing relative value unit (RVU) production to recognize other contributions. Several reported their general appreciation of hospitalists evolved as specific hospitalists matured and demonstrated new contributions to hospital function. Some leaders tried to speculate about future phases of this evolution, although details were sparse.
Among respondents with greater implementation of risk-sharing contracts or ACOs, executives did not describe significantly different goals for hospitalists; executives emphasized that hospitalists should accelerate existing efforts to reduce inpatient costs, length of stay, healthcare-acquired conditions, unnecessary testing, and readmissions. A theme emerged around hospitalists supporting the continuum of care, through improved communication and increased alignment with health systems.
“Where I see the real benefit…is to figure out a way to use hospitalists and match them up with the primary care physicians on the outpatient side to truly develop an integrated population-based medicine practice for all our patients.” (President, #15)
Executives believed that communication and collaboration with PCPs and postacute care providers would attract more measurement.
DISCUSSION
Our findings provide hospitalists with insight into the approach hospital executives may follow when determining the rationale for and extent of financial support for HMGs. The results did not support our hypothesis that executives commonly determine the appropriate support by summing detailed quantitative models for various HMG contributions. Instead, most hospital executives appear to make decisions about the appropriateness of financial support based on a small number of basic financial or care quality metrics combined with a subjective assessment of the HMG’s broader alignment with hospital priorities. However, we did find substantial evidence that hospital executives’ expectations of hospitalists have evolved in the last decade, creating the potential for dissociation from how hospitalists prioritize and value their own efforts. Together, our findings suggest that enhanced communication, relationship building, and collaboration with hospital leaders may help HMGs to maintain a shared model of value with hospital executives.
The general absence of summary value calculations suggests specific opportunities, benefits, and risks for HMG group leaders (Table 3). An important opportunity relates to the communication agenda about unmeasured or nonfinancial contributions. Although executives recognized many of these, our data suggest a need for HMG leaders to educate hospital leaders about their unmeasured contributions proactively. Although some might recommend doing so by quantifying and financially rewarding key intangible contributions (eg, measuring leadership in culture change9), this entails important risks.10 Some experts propose that the proliferation of physician pay-for-performance schemes threatens medical professionalism, fails patients, and misunderstands what motivates physicians.11 HMG groups that feel undervalued should hesitate before monetizing all aspects of their work, and consider emphasizing relationship-building as a platform for communication about their performance. Achieving better alignment with executives is not just an opportunity for HMG leaders, but for each hospitalist within the group. Although executives wanted to have deeper relationships with group members, this may not be feasible in large organizations. Instead, it is incumbent for HMG leaders to translate executives’ expectations and forge better alignment.
Residency may not adequately prepare hospitalists to meet key expectations hospital executives hold related to system leadership and interprofessional team leadership. For example, hospital leaders particularly valued HMGs’ perceived ability to improve nurse retention and morale. Unfortunately, residency curricula generally lack concerted instruction on the skills required to produce such interprofessional inpatient teams reliably. Similarly, executives strongly wanted HMGs to acknowledge a role as partners in running the quality, stewardship, and safety missions of the entire hospital. While residency training builds clinical competence through the care of individual patients, many residents do not receive experiential education in system design and leadership. This suggests a need for HMGs to provide early career training or mentorship in quality improvement and interprofessional teamwork. Executives and HMG leaders seeking a stable, mature workforce, should allocate resources to retaining mid and late career hospitalists through leadership roles or financial incentives for longevity.
As with many qualitative studies, the generalizability of our findings may be limited, particularly outside the US healthcare system. We invited executives from diverse practice settings but may not have captured all the relevant viewpoints. This study did not include Veterans Affairs hospitals, safety net hospitals were underrepresented, Midwestern hospitals were overrepresented and the participants were predominantly male. We were unable to determine the influence of employment model on participant beliefs about HMGs, nor did we elicit comparisons to other physician specialties that would highlight a distinct approach to negotiating with HMGs. Because we used hospitalists as interviewers, including some from the same institution as the interviewee, respondents may have dampened critiques or descriptions of unmet expectations. Our data do not provide quantitative support for any approach to determining or negotiating appropriate financial support for an HMG.
CONCLUSIONS
This work contributes new understanding of the expectations executives have for HMGs and individual hospitalists. This highlights opportunities for group leaders, hospitalists, medical educators, and quality improvement experts to produce a hospitalist labor force that can engage in productive and mutually satisfying relationships with hospital leaders. Hospitalists should strive to improve alignment and communication with executive groups.
Disclosures
The authors report no potential conflict of interest.
1. Lapps J, Flansbaum B, Leykum L, et al. Updating threshold-based identification of hospitalists in 2012 Medicare pay data. J Hosp Med. 2016;11(1):45-47. https://doi.org/10.1002/jhm.2480.
2. Wachter RM, Goldman L. Zero to 50,000–the 20th Anniversary of the hospitalist. NEJM. 2016;375(11):1009-1011. https://doi.org/10.1056/nejmp1607958.
3. Stevens JP, Nyweide DJ, Maresh S, et al. Comparison of hospital resource use and outcomes among hospitalists, primary care physicians, and other generalists. JAMA Intern Med. 2017;177(12):1781-1787. https://doi.org/10.1001/jamainternmed.2017.5824.
4. American Hospital Association (AHA) (2017), Hospital Statistics, AHA, Chicago, IL.
5. Wachter RM, Goldman L. The emerging role of “hospitalists” in the American health care system. NEJM. 1996;335(7):514-517. https://doi.org/10.1093/ajhp/53.20.2389a.
6. Pham HH, Devers KJ, Kuo S, et al. Health care market trends and the evolution of hospitalist use and roles. J Gen Intern Med. 2005;20(2):101-107. https://doi.org/10.1111/j.1525-1497.2005.40184.x.
7. Epané JP, Weech-Maldonado R, Hearld L, et al. Hospitals’ use of hospitalistas: implications for financial performance. Health Care Manage Rev. 2019;44(1):10-18. https://doi.org/10.1097/hmr.0000000000000170.
8. State of Hospital Medicine: 2018 Report Based on 2017 Data. Society of Hospital Medicine. https://sohm.hospitalmedicine.org/ Accessed December 9, 2018.
9. Carmeli A, Tishler A. The relationships between intangible organizational elements and organizational performance. Strategic Manag J. 2004;25(13):1257-1278. https://doi.org/10.1002/smj.428.
10. Bernard M. Strategic performance management: leveraging and measuring your intangible value drivers. Amsterdam: Butterworth-Heinemann, 2006.
11. Khullar D, Wolfson D, Casalino LP. Professionalism, performance, and the future of physician incentives. JAMA. 2018;320(23):2419-2420. https://doi.org/10.1001/jama.2018.17719.
The field of hospital medicine has expanded rapidly since its inception in the late 1990s, and currently, most hospitals in the United States employ or contract with hospital medicine groups (HMGs).1-4 This dramatic growth began in response to several factors: primary care physicians (PCPs) opting out of inpatient care, the increasing acuity and complexity of inpatient care, and cost pressures on hospitals.5,6 Recent studies associate greater use of hospitalists with increased hospital revenues and modest improvements in hospital financial performance.7 However, funding the hospitalist delivery model required hospitals to share the savings hospitalists generate through facility billing and quality incentives.
Hospitalists’ professional fee revenues alone generally do not fund their salaries. An average HMG serving adult patients requires $176,658 from the hospital to support a full-time physician.8 Determining the appropriate level of HMG support typically occurs through negotiation with hospital executives. During the last 10 years, HMG size and hospitalist compensation have risen steadily, combining to increase the hospitalist labor costs borne by hospitals.4,8 Accordingly, hospital executives in challenging economic environments may pressure HMG leaders to accept diminished support or to demonstrate a better return on the hospital’s investment.
These negotiations are influenced by the beliefs of hospital executives about the value of the hospitalist labor model. Little is known about how hospital and health system executive leadership assess the value of hospitalists. A deeper understanding of executive attitudes and beliefs could inform HMG leaders seeking integrative (“win-win”) outcomes in contract and compensation negotiations. Members of the Society of Hospital Medicine (SHM) Practice Management Committee surveyed hospital executives to guide SHM program development. We sought to analyze transcripts from these interviews to describe how executives assess HMGs and to test the hypothesis that hospital executives apply specific financial models when determining the return on investment (ROI) from subsidizing an HMG.
METHODS
Study Design, Setting, and Participants
Members of the SHM Practice Management Committee conducted interviews with a convenience sample of 24 key informants representing the following stakeholders at hospitals employing hospitalists: Chief Executive Officers (CEOs), Presidents, Vice Presidents, Chief Medical Officers (CMOs), and Chief Financial Officers (CFOs). Participants were recruited from 17 fee-for-service healthcare organizations, including rural, suburban, urban, community, and academic medical centers. The semi-structured interviews occurred in person between January and March 2018; each one lasted an average of 45 minutes and were designed to guide SHM program and product development. Twenty-eight executives were recruited by e-mail, and four did not complete the interview due to scheduling difficulty. All the participants provided informed consent. The University of Washington Institutional Review Board approved the secondary analysis of deidentified transcripts.
Interview Guide and Data Collection
All interviews followed a guide with eight demographic questions and 10 open-ended questions (Appendix). Cognitive interviews were performed with two hospital executives outside the study cohort, resulting in the addition of one question and rewording one question for clarity. One-on-one interviews were performed by 10 committee members (range, 1-3 interviews). All interview audios were recorded, and no field notes were kept. The goal of the interviews was to obtain an understanding of how hospital executives value the contributions and costs of hospitalist groups.
The interviews began with questions about the informant’s current interactions with hospitalists and the origin of the hospitalist group at their facility. Informants then described the value they feel hospitalists bring to their hospital and occasions they were surprised or dissatisfied with the clinical or financial value delivered by the hospitalists. Participants described how they calculate a return on investment (ROI) for their hospitalist group, nonfinancial benefits and disadvantages to hospitalists, and how they believe hospitalists should participate in risk-sharing contracts.
Data Analysis
The interview audiotapes were transcribed and deidentified. A sample of eight transcripts was verified by participants to ensure accuracy. Three investigators (AAW, RC, CC) reviewed a random sample of five transcripts to identify and codify preliminary themes. We applied a general inductive framework with a content analysis approach. Two investigators (TM and MC) read all transcripts independently, coding the presence of each theme and quotations exemplifying these themes using qualitative analysis software (Dedoose Version 7.0.23, SocioCultural Research Consultants). A third investigator (AAW) read all the transcripts and resolved differences of opinion. Themes and code application were discussed among the study team after the second and fifth transcripts to add or clarify codes. No new codes were identified after the first review of the preliminary codebook, although investigators intermittently used an “unknown” code through the 20th transcript. After discussion to reach consensus, excerpts initially coded “unknown” were assigned existing codes; the 20th transcript represents the approximate point of reaching thematic saturation.
RESULTS
Of the 24 participants, 18 (75%) were male, representing a variety of roles: 7 (29.2%) CMOs, 5 (20.8%) Presidents, 5 (20.8%) CFOs, 4 (16.7%) CEOs, and 3 (12.5%) Vice Presidents. The participants represented all regions (Midwest 12 [50%], South 6 [25%], West 4 [16.7%], and East 2 [8.3%], community size (Urban 11 [45.8%], Suburban 8 [33.3%], and Rural 5 [20.8%]), and Hospital Types (Community 11 [45.8%], Multihospital System 5 [20.8%], Academic 5 [20.8%], Safety Net 2 [8.3%], and Critical Access 1 [4.2%]). We present specific themes below and supporting quotations in Tables 1 and 2.
Current Value of the HMG at the Respondent’s Hospital
Most executives reported their hospital’s HMG had operated for over a decade and had developed an earlier, outdated value framework. Interviewees described an initial mix of financial pressures, shifts in physician work preferences, increasing patient acuity, resident labor shortages, and unsolved hospital throughput needs that triggered a reactive conversion from community PCP staffing to hospitalist care teams, followed by refinements to realize value.
“I think initially here it was to deal with the resident caps, right? So, at that moment, the solution that was put in place probably made a lot of sense. If that’s all someone came in with, now I’d be scratching my head and said, what are you thinking?” (President, #2)
Respondents perceived that HMGs provide value in many domains, including financial contributions, high-quality care, organizational efficiency, academics, leadership of interprofessional teams, effective communication, system improvement, and beneficial influence on the care environment and other employees. Regarding the measurable generation of financial benefit, documentation for improved billing accuracy, increased hospital efficiency (eg, lower length of stay, early discharges), and comanagement arrangements were commonly identified.
“I don’t want a urologist with a stethoscope, so I’m happy to have the hospitalists say, ‘Look, I’ll take care of the patient. You do the procedure.’ Well, that’s inherently valuable, whether we measure it or whether we don’t.” (CMO, #21)
Executives generally expressed satisfaction with their HMG’s quality of care and the related pay-for-performance financial benefits from payers, attributing success to hospitalists’ familiarity with inpatient systems and willingness to standardize.
“I just think it’s having one structure, one group to go to, a standard rather than trying to push it through the medical staff.” (VP, #18)
Executives reported that HMGs generate substantial value that is difficult to measure financially. For example, a large bundle of excerpts organized around communication with patients, nurses, and other providers.
“If we have the right hospitalist staff, to engage them with the nursing staff would help to reduce my turnover rate…and create a very positive morale within the nursing units. That’s huge. That’s nonfinancial” (President, #15)
Executives particularly appreciated hospitalists’ work to aggregate input from multiple specialists and present a cohesive explanation to patients. Executives also felt that HMGs create significant unmeasured value by improving processes and outcomes on service lines beyond hospital medicine, achieving this through culture change, involvement in leadership, hospital-wide process redesign, and running rapid response teams. Some executives expressed a desire for hospitalists to assume this global quality responsibility more explicitly as a job expectation.
Executives described how they would evaluate a de novo proposal for hospitalist services, usually enumerating key general domains without explaining specifically how they would measure each element. The following priorities emerged: clinical excellence, capacity to collaborate with hospital leadership, the scope of services provided, cultural fit/alignment, financial performance, contract cost, pay-for-performance measures, and turnover. Regarding financial performance, respondents expected to know the cost of the proposal but lacked a specific price threshold. Instead, they sought to understand the total value of the proposal through its effect on metrics such as facility fees or resource use. Nonetheless, cultural fit was a critical, overriding driver of the hypothetical decision, despite difficulty defining beyond estimates of teamwork, alignment with hospital priorities, and qualities of the group leader.
“For us, it usually ends being how do we mix personally, do we like them?” (CMO, #5)
Alignment and Collaboration
The related concepts of “collaboration” and “alignment” emerged as prominent themes during all interviews. Executives highly valued hospitalist groups that could demonstrate alignment with hospital priorities and often used this concept to summarize the HMG’s success or failure across a group of value domains.
“If you’re just coming in to fill a shift and see 10 patients, you have much less value than somebody who’s going to play that active partnership role… hospitalist services need to partner with hospitals and be intimately involved with the success of the hospital.” (CMO, #20)
Alignment sometimes manifested in a quantified, explicit way, through incentive plans or shared savings plans. However, it most often manifested as a broader sense that the hospitalists’ work targeted the same priorities as the executive leaders and that hospitalists genuinely cared about those priorities. A “shift-work mentality” was expressed by some as the antithesis of alignment. Incorporating hospitalist leaders in hospital leadership and frequent communication arose as mechanisms to increase alignment.
Ways HMGs Fail to Meet Expectations
Respondents described unresolved disadvantages to the hospitalist care model.
“I mean, OPPE, how do you do that for a hospitalist? How can you do it? It’s hard to attribute a patient to someone….it is a weakness and I think we all know it.” (CMO, #21)
Executives also worried about inconsistent handoffs with primary care providers and the field’s demographics, finding it disproportionately comprised of junior or transient physicians. They also hoped that hospitalist innovators would solve clinician burnout and the high cost of inpatient care. Disappointments specific to the local HMG revolved around difficulty developing shared models of value and mechanisms to achieve them.
“I would like to have more dialog between the hospital leadership team and the hospitalist group…I would like to see a little bit more collaboration.” (President, #13)
These challenges emerged not as a deficiency with hospital medicine as a specialty, but a failure at their specific facility to achieve the goal of alignment through joint strategic planning.
Calculating Value
When asked if their hospital had a formal process to evaluate ROI for their HMG, two dominant answers emerged: (1) the executive lacked a formal process for determining ROI and was unaware of one used at their facility or (2) the executive evaluated HMG performance based on multiple measures, including cost, but did not attempt to calculate ROI or a summary value. Several described the financial evaluation process as too difficult or unnecessary.
“No. It’s too difficult to extract that data. I would say the best proxy that we could do it is our case mix index on our medicine service line.” (CMO, #20)
“No, not a formal process, no… I question the value of some of the other things we do with the medical group…but not the value of the hospitalists… I don’t think we’ve done a formal assessment. I appreciate the flexibility, especially in a small hospital.” (President, #10)
Rarely, executives described specific financial calculations that served as a proxy for ROI. These included calculating a contribution margin to compare against the cost of salary support or the application of external survey benchmarking comparisons for productivity and salary to evaluate the appropriateness of a limited set of financial indicators. Twice respondents alluded to more sophisticated measurements conducted by the finance department but lacked familiarity with the process. Several executives described ROI calculations for specific projects and discrete business decisions involving hospitalists, particularly considering hiring an additional hospitalist.
Executives generally struggled to recall specific ways that the nonfinancial contributions of hospitalists were incorporated into executive decisions regarding the hospitalist group. Two related themes emerged: first, the belief that hospitals could not function effectively without hospitalists, making their presence an expected cost of doing business. Second, absent measures of HMG ROI, executives appeared to determine an approximate overall value of hospitalists, rather than parsing the various contributions. A few respondents expressed alarm at the rise in hospitalist salaries, whereas others acknowledged market forces beyond their control.
“… there is going to be more of a demand for hospitalists, which is definitely going to drive up the compensation. So, I don’t worry that the compensation will be driven up so high that there won’t be a return [on investment].” (CFO, #16)
Some urged individual hospitalists to develop a deeper understanding of what supports their salary to avoid strained relationships with executives.
Evolution and Risk-Sharing Contracts
Respondents described an evolving conceptualization of the hospitalist’s value, occurring at both a broad, long-term scale and at an incremental, annual scale through minor modifications to incentive pay schemes. For most executives, hiring hospitalists as replacements for PCPs had become necessary and not a source of novel value; many executives described it as “the cost of doing business.” Some described gradually deemphasizing relative value unit (RVU) production to recognize other contributions. Several reported their general appreciation of hospitalists evolved as specific hospitalists matured and demonstrated new contributions to hospital function. Some leaders tried to speculate about future phases of this evolution, although details were sparse.
Among respondents with greater implementation of risk-sharing contracts or ACOs, executives did not describe significantly different goals for hospitalists; executives emphasized that hospitalists should accelerate existing efforts to reduce inpatient costs, length of stay, healthcare-acquired conditions, unnecessary testing, and readmissions. A theme emerged around hospitalists supporting the continuum of care, through improved communication and increased alignment with health systems.
“Where I see the real benefit…is to figure out a way to use hospitalists and match them up with the primary care physicians on the outpatient side to truly develop an integrated population-based medicine practice for all our patients.” (President, #15)
Executives believed that communication and collaboration with PCPs and postacute care providers would attract more measurement.
DISCUSSION
Our findings provide hospitalists with insight into the approach hospital executives may follow when determining the rationale for and extent of financial support for HMGs. The results did not support our hypothesis that executives commonly determine the appropriate support by summing detailed quantitative models for various HMG contributions. Instead, most hospital executives appear to make decisions about the appropriateness of financial support based on a small number of basic financial or care quality metrics combined with a subjective assessment of the HMG’s broader alignment with hospital priorities. However, we did find substantial evidence that hospital executives’ expectations of hospitalists have evolved in the last decade, creating the potential for dissociation from how hospitalists prioritize and value their own efforts. Together, our findings suggest that enhanced communication, relationship building, and collaboration with hospital leaders may help HMGs to maintain a shared model of value with hospital executives.
The general absence of summary value calculations suggests specific opportunities, benefits, and risks for HMG group leaders (Table 3). An important opportunity relates to the communication agenda about unmeasured or nonfinancial contributions. Although executives recognized many of these, our data suggest a need for HMG leaders to educate hospital leaders about their unmeasured contributions proactively. Although some might recommend doing so by quantifying and financially rewarding key intangible contributions (eg, measuring leadership in culture change9), this entails important risks.10 Some experts propose that the proliferation of physician pay-for-performance schemes threatens medical professionalism, fails patients, and misunderstands what motivates physicians.11 HMG groups that feel undervalued should hesitate before monetizing all aspects of their work, and consider emphasizing relationship-building as a platform for communication about their performance. Achieving better alignment with executives is not just an opportunity for HMG leaders, but for each hospitalist within the group. Although executives wanted to have deeper relationships with group members, this may not be feasible in large organizations. Instead, it is incumbent for HMG leaders to translate executives’ expectations and forge better alignment.
Residency may not adequately prepare hospitalists to meet key expectations hospital executives hold related to system leadership and interprofessional team leadership. For example, hospital leaders particularly valued HMGs’ perceived ability to improve nurse retention and morale. Unfortunately, residency curricula generally lack concerted instruction on the skills required to produce such interprofessional inpatient teams reliably. Similarly, executives strongly wanted HMGs to acknowledge a role as partners in running the quality, stewardship, and safety missions of the entire hospital. While residency training builds clinical competence through the care of individual patients, many residents do not receive experiential education in system design and leadership. This suggests a need for HMGs to provide early career training or mentorship in quality improvement and interprofessional teamwork. Executives and HMG leaders seeking a stable, mature workforce, should allocate resources to retaining mid and late career hospitalists through leadership roles or financial incentives for longevity.
As with many qualitative studies, the generalizability of our findings may be limited, particularly outside the US healthcare system. We invited executives from diverse practice settings but may not have captured all the relevant viewpoints. This study did not include Veterans Affairs hospitals, safety net hospitals were underrepresented, Midwestern hospitals were overrepresented and the participants were predominantly male. We were unable to determine the influence of employment model on participant beliefs about HMGs, nor did we elicit comparisons to other physician specialties that would highlight a distinct approach to negotiating with HMGs. Because we used hospitalists as interviewers, including some from the same institution as the interviewee, respondents may have dampened critiques or descriptions of unmet expectations. Our data do not provide quantitative support for any approach to determining or negotiating appropriate financial support for an HMG.
CONCLUSIONS
This work contributes new understanding of the expectations executives have for HMGs and individual hospitalists. This highlights opportunities for group leaders, hospitalists, medical educators, and quality improvement experts to produce a hospitalist labor force that can engage in productive and mutually satisfying relationships with hospital leaders. Hospitalists should strive to improve alignment and communication with executive groups.
Disclosures
The authors report no potential conflict of interest.
The field of hospital medicine has expanded rapidly since its inception in the late 1990s, and currently, most hospitals in the United States employ or contract with hospital medicine groups (HMGs).1-4 This dramatic growth began in response to several factors: primary care physicians (PCPs) opting out of inpatient care, the increasing acuity and complexity of inpatient care, and cost pressures on hospitals.5,6 Recent studies associate greater use of hospitalists with increased hospital revenues and modest improvements in hospital financial performance.7 However, funding the hospitalist delivery model required hospitals to share the savings hospitalists generate through facility billing and quality incentives.
Hospitalists’ professional fee revenues alone generally do not fund their salaries. An average HMG serving adult patients requires $176,658 from the hospital to support a full-time physician.8 Determining the appropriate level of HMG support typically occurs through negotiation with hospital executives. During the last 10 years, HMG size and hospitalist compensation have risen steadily, combining to increase the hospitalist labor costs borne by hospitals.4,8 Accordingly, hospital executives in challenging economic environments may pressure HMG leaders to accept diminished support or to demonstrate a better return on the hospital’s investment.
These negotiations are influenced by the beliefs of hospital executives about the value of the hospitalist labor model. Little is known about how hospital and health system executive leadership assess the value of hospitalists. A deeper understanding of executive attitudes and beliefs could inform HMG leaders seeking integrative (“win-win”) outcomes in contract and compensation negotiations. Members of the Society of Hospital Medicine (SHM) Practice Management Committee surveyed hospital executives to guide SHM program development. We sought to analyze transcripts from these interviews to describe how executives assess HMGs and to test the hypothesis that hospital executives apply specific financial models when determining the return on investment (ROI) from subsidizing an HMG.
METHODS
Study Design, Setting, and Participants
Members of the SHM Practice Management Committee conducted interviews with a convenience sample of 24 key informants representing the following stakeholders at hospitals employing hospitalists: Chief Executive Officers (CEOs), Presidents, Vice Presidents, Chief Medical Officers (CMOs), and Chief Financial Officers (CFOs). Participants were recruited from 17 fee-for-service healthcare organizations, including rural, suburban, urban, community, and academic medical centers. The semi-structured interviews occurred in person between January and March 2018; each one lasted an average of 45 minutes and were designed to guide SHM program and product development. Twenty-eight executives were recruited by e-mail, and four did not complete the interview due to scheduling difficulty. All the participants provided informed consent. The University of Washington Institutional Review Board approved the secondary analysis of deidentified transcripts.
Interview Guide and Data Collection
All interviews followed a guide with eight demographic questions and 10 open-ended questions (Appendix). Cognitive interviews were performed with two hospital executives outside the study cohort, resulting in the addition of one question and rewording one question for clarity. One-on-one interviews were performed by 10 committee members (range, 1-3 interviews). All interview audios were recorded, and no field notes were kept. The goal of the interviews was to obtain an understanding of how hospital executives value the contributions and costs of hospitalist groups.
The interviews began with questions about the informant’s current interactions with hospitalists and the origin of the hospitalist group at their facility. Informants then described the value they feel hospitalists bring to their hospital and occasions they were surprised or dissatisfied with the clinical or financial value delivered by the hospitalists. Participants described how they calculate a return on investment (ROI) for their hospitalist group, nonfinancial benefits and disadvantages to hospitalists, and how they believe hospitalists should participate in risk-sharing contracts.
Data Analysis
The interview audiotapes were transcribed and deidentified. A sample of eight transcripts was verified by participants to ensure accuracy. Three investigators (AAW, RC, CC) reviewed a random sample of five transcripts to identify and codify preliminary themes. We applied a general inductive framework with a content analysis approach. Two investigators (TM and MC) read all transcripts independently, coding the presence of each theme and quotations exemplifying these themes using qualitative analysis software (Dedoose Version 7.0.23, SocioCultural Research Consultants). A third investigator (AAW) read all the transcripts and resolved differences of opinion. Themes and code application were discussed among the study team after the second and fifth transcripts to add or clarify codes. No new codes were identified after the first review of the preliminary codebook, although investigators intermittently used an “unknown” code through the 20th transcript. After discussion to reach consensus, excerpts initially coded “unknown” were assigned existing codes; the 20th transcript represents the approximate point of reaching thematic saturation.
RESULTS
Of the 24 participants, 18 (75%) were male, representing a variety of roles: 7 (29.2%) CMOs, 5 (20.8%) Presidents, 5 (20.8%) CFOs, 4 (16.7%) CEOs, and 3 (12.5%) Vice Presidents. The participants represented all regions (Midwest 12 [50%], South 6 [25%], West 4 [16.7%], and East 2 [8.3%], community size (Urban 11 [45.8%], Suburban 8 [33.3%], and Rural 5 [20.8%]), and Hospital Types (Community 11 [45.8%], Multihospital System 5 [20.8%], Academic 5 [20.8%], Safety Net 2 [8.3%], and Critical Access 1 [4.2%]). We present specific themes below and supporting quotations in Tables 1 and 2.
Current Value of the HMG at the Respondent’s Hospital
Most executives reported their hospital’s HMG had operated for over a decade and had developed an earlier, outdated value framework. Interviewees described an initial mix of financial pressures, shifts in physician work preferences, increasing patient acuity, resident labor shortages, and unsolved hospital throughput needs that triggered a reactive conversion from community PCP staffing to hospitalist care teams, followed by refinements to realize value.
“I think initially here it was to deal with the resident caps, right? So, at that moment, the solution that was put in place probably made a lot of sense. If that’s all someone came in with, now I’d be scratching my head and said, what are you thinking?” (President, #2)
Respondents perceived that HMGs provide value in many domains, including financial contributions, high-quality care, organizational efficiency, academics, leadership of interprofessional teams, effective communication, system improvement, and beneficial influence on the care environment and other employees. Regarding the measurable generation of financial benefit, documentation for improved billing accuracy, increased hospital efficiency (eg, lower length of stay, early discharges), and comanagement arrangements were commonly identified.
“I don’t want a urologist with a stethoscope, so I’m happy to have the hospitalists say, ‘Look, I’ll take care of the patient. You do the procedure.’ Well, that’s inherently valuable, whether we measure it or whether we don’t.” (CMO, #21)
Executives generally expressed satisfaction with their HMG’s quality of care and the related pay-for-performance financial benefits from payers, attributing success to hospitalists’ familiarity with inpatient systems and willingness to standardize.
“I just think it’s having one structure, one group to go to, a standard rather than trying to push it through the medical staff.” (VP, #18)
Executives reported that HMGs generate substantial value that is difficult to measure financially. For example, a large bundle of excerpts organized around communication with patients, nurses, and other providers.
“If we have the right hospitalist staff, to engage them with the nursing staff would help to reduce my turnover rate…and create a very positive morale within the nursing units. That’s huge. That’s nonfinancial” (President, #15)
Executives particularly appreciated hospitalists’ work to aggregate input from multiple specialists and present a cohesive explanation to patients. Executives also felt that HMGs create significant unmeasured value by improving processes and outcomes on service lines beyond hospital medicine, achieving this through culture change, involvement in leadership, hospital-wide process redesign, and running rapid response teams. Some executives expressed a desire for hospitalists to assume this global quality responsibility more explicitly as a job expectation.
Executives described how they would evaluate a de novo proposal for hospitalist services, usually enumerating key general domains without explaining specifically how they would measure each element. The following priorities emerged: clinical excellence, capacity to collaborate with hospital leadership, the scope of services provided, cultural fit/alignment, financial performance, contract cost, pay-for-performance measures, and turnover. Regarding financial performance, respondents expected to know the cost of the proposal but lacked a specific price threshold. Instead, they sought to understand the total value of the proposal through its effect on metrics such as facility fees or resource use. Nonetheless, cultural fit was a critical, overriding driver of the hypothetical decision, despite difficulty defining beyond estimates of teamwork, alignment with hospital priorities, and qualities of the group leader.
“For us, it usually ends being how do we mix personally, do we like them?” (CMO, #5)
Alignment and Collaboration
The related concepts of “collaboration” and “alignment” emerged as prominent themes during all interviews. Executives highly valued hospitalist groups that could demonstrate alignment with hospital priorities and often used this concept to summarize the HMG’s success or failure across a group of value domains.
“If you’re just coming in to fill a shift and see 10 patients, you have much less value than somebody who’s going to play that active partnership role… hospitalist services need to partner with hospitals and be intimately involved with the success of the hospital.” (CMO, #20)
Alignment sometimes manifested in a quantified, explicit way, through incentive plans or shared savings plans. However, it most often manifested as a broader sense that the hospitalists’ work targeted the same priorities as the executive leaders and that hospitalists genuinely cared about those priorities. A “shift-work mentality” was expressed by some as the antithesis of alignment. Incorporating hospitalist leaders in hospital leadership and frequent communication arose as mechanisms to increase alignment.
Ways HMGs Fail to Meet Expectations
Respondents described unresolved disadvantages to the hospitalist care model.
“I mean, OPPE, how do you do that for a hospitalist? How can you do it? It’s hard to attribute a patient to someone….it is a weakness and I think we all know it.” (CMO, #21)
Executives also worried about inconsistent handoffs with primary care providers and the field’s demographics, finding it disproportionately comprised of junior or transient physicians. They also hoped that hospitalist innovators would solve clinician burnout and the high cost of inpatient care. Disappointments specific to the local HMG revolved around difficulty developing shared models of value and mechanisms to achieve them.
“I would like to have more dialog between the hospital leadership team and the hospitalist group…I would like to see a little bit more collaboration.” (President, #13)
These challenges emerged not as a deficiency with hospital medicine as a specialty, but a failure at their specific facility to achieve the goal of alignment through joint strategic planning.
Calculating Value
When asked if their hospital had a formal process to evaluate ROI for their HMG, two dominant answers emerged: (1) the executive lacked a formal process for determining ROI and was unaware of one used at their facility or (2) the executive evaluated HMG performance based on multiple measures, including cost, but did not attempt to calculate ROI or a summary value. Several described the financial evaluation process as too difficult or unnecessary.
“No. It’s too difficult to extract that data. I would say the best proxy that we could do it is our case mix index on our medicine service line.” (CMO, #20)
“No, not a formal process, no… I question the value of some of the other things we do with the medical group…but not the value of the hospitalists… I don’t think we’ve done a formal assessment. I appreciate the flexibility, especially in a small hospital.” (President, #10)
Rarely, executives described specific financial calculations that served as a proxy for ROI. These included calculating a contribution margin to compare against the cost of salary support or the application of external survey benchmarking comparisons for productivity and salary to evaluate the appropriateness of a limited set of financial indicators. Twice respondents alluded to more sophisticated measurements conducted by the finance department but lacked familiarity with the process. Several executives described ROI calculations for specific projects and discrete business decisions involving hospitalists, particularly considering hiring an additional hospitalist.
Executives generally struggled to recall specific ways that the nonfinancial contributions of hospitalists were incorporated into executive decisions regarding the hospitalist group. Two related themes emerged: first, the belief that hospitals could not function effectively without hospitalists, making their presence an expected cost of doing business. Second, absent measures of HMG ROI, executives appeared to determine an approximate overall value of hospitalists, rather than parsing the various contributions. A few respondents expressed alarm at the rise in hospitalist salaries, whereas others acknowledged market forces beyond their control.
“… there is going to be more of a demand for hospitalists, which is definitely going to drive up the compensation. So, I don’t worry that the compensation will be driven up so high that there won’t be a return [on investment].” (CFO, #16)
Some urged individual hospitalists to develop a deeper understanding of what supports their salary to avoid strained relationships with executives.
Evolution and Risk-Sharing Contracts
Respondents described an evolving conceptualization of the hospitalist’s value, occurring at both a broad, long-term scale and at an incremental, annual scale through minor modifications to incentive pay schemes. For most executives, hiring hospitalists as replacements for PCPs had become necessary and not a source of novel value; many executives described it as “the cost of doing business.” Some described gradually deemphasizing relative value unit (RVU) production to recognize other contributions. Several reported their general appreciation of hospitalists evolved as specific hospitalists matured and demonstrated new contributions to hospital function. Some leaders tried to speculate about future phases of this evolution, although details were sparse.
Among respondents with greater implementation of risk-sharing contracts or ACOs, executives did not describe significantly different goals for hospitalists; executives emphasized that hospitalists should accelerate existing efforts to reduce inpatient costs, length of stay, healthcare-acquired conditions, unnecessary testing, and readmissions. A theme emerged around hospitalists supporting the continuum of care, through improved communication and increased alignment with health systems.
“Where I see the real benefit…is to figure out a way to use hospitalists and match them up with the primary care physicians on the outpatient side to truly develop an integrated population-based medicine practice for all our patients.” (President, #15)
Executives believed that communication and collaboration with PCPs and postacute care providers would attract more measurement.
DISCUSSION
Our findings provide hospitalists with insight into the approach hospital executives may follow when determining the rationale for and extent of financial support for HMGs. The results did not support our hypothesis that executives commonly determine the appropriate support by summing detailed quantitative models for various HMG contributions. Instead, most hospital executives appear to make decisions about the appropriateness of financial support based on a small number of basic financial or care quality metrics combined with a subjective assessment of the HMG’s broader alignment with hospital priorities. However, we did find substantial evidence that hospital executives’ expectations of hospitalists have evolved in the last decade, creating the potential for dissociation from how hospitalists prioritize and value their own efforts. Together, our findings suggest that enhanced communication, relationship building, and collaboration with hospital leaders may help HMGs to maintain a shared model of value with hospital executives.
The general absence of summary value calculations suggests specific opportunities, benefits, and risks for HMG group leaders (Table 3). An important opportunity relates to the communication agenda about unmeasured or nonfinancial contributions. Although executives recognized many of these, our data suggest a need for HMG leaders to educate hospital leaders about their unmeasured contributions proactively. Although some might recommend doing so by quantifying and financially rewarding key intangible contributions (eg, measuring leadership in culture change9), this entails important risks.10 Some experts propose that the proliferation of physician pay-for-performance schemes threatens medical professionalism, fails patients, and misunderstands what motivates physicians.11 HMG groups that feel undervalued should hesitate before monetizing all aspects of their work, and consider emphasizing relationship-building as a platform for communication about their performance. Achieving better alignment with executives is not just an opportunity for HMG leaders, but for each hospitalist within the group. Although executives wanted to have deeper relationships with group members, this may not be feasible in large organizations. Instead, it is incumbent for HMG leaders to translate executives’ expectations and forge better alignment.
Residency may not adequately prepare hospitalists to meet key expectations hospital executives hold related to system leadership and interprofessional team leadership. For example, hospital leaders particularly valued HMGs’ perceived ability to improve nurse retention and morale. Unfortunately, residency curricula generally lack concerted instruction on the skills required to produce such interprofessional inpatient teams reliably. Similarly, executives strongly wanted HMGs to acknowledge a role as partners in running the quality, stewardship, and safety missions of the entire hospital. While residency training builds clinical competence through the care of individual patients, many residents do not receive experiential education in system design and leadership. This suggests a need for HMGs to provide early career training or mentorship in quality improvement and interprofessional teamwork. Executives and HMG leaders seeking a stable, mature workforce, should allocate resources to retaining mid and late career hospitalists through leadership roles or financial incentives for longevity.
As with many qualitative studies, the generalizability of our findings may be limited, particularly outside the US healthcare system. We invited executives from diverse practice settings but may not have captured all the relevant viewpoints. This study did not include Veterans Affairs hospitals, safety net hospitals were underrepresented, Midwestern hospitals were overrepresented and the participants were predominantly male. We were unable to determine the influence of employment model on participant beliefs about HMGs, nor did we elicit comparisons to other physician specialties that would highlight a distinct approach to negotiating with HMGs. Because we used hospitalists as interviewers, including some from the same institution as the interviewee, respondents may have dampened critiques or descriptions of unmet expectations. Our data do not provide quantitative support for any approach to determining or negotiating appropriate financial support for an HMG.
CONCLUSIONS
This work contributes new understanding of the expectations executives have for HMGs and individual hospitalists. This highlights opportunities for group leaders, hospitalists, medical educators, and quality improvement experts to produce a hospitalist labor force that can engage in productive and mutually satisfying relationships with hospital leaders. Hospitalists should strive to improve alignment and communication with executive groups.
Disclosures
The authors report no potential conflict of interest.
1. Lapps J, Flansbaum B, Leykum L, et al. Updating threshold-based identification of hospitalists in 2012 Medicare pay data. J Hosp Med. 2016;11(1):45-47. https://doi.org/10.1002/jhm.2480.
2. Wachter RM, Goldman L. Zero to 50,000–the 20th Anniversary of the hospitalist. NEJM. 2016;375(11):1009-1011. https://doi.org/10.1056/nejmp1607958.
3. Stevens JP, Nyweide DJ, Maresh S, et al. Comparison of hospital resource use and outcomes among hospitalists, primary care physicians, and other generalists. JAMA Intern Med. 2017;177(12):1781-1787. https://doi.org/10.1001/jamainternmed.2017.5824.
4. American Hospital Association (AHA) (2017), Hospital Statistics, AHA, Chicago, IL.
5. Wachter RM, Goldman L. The emerging role of “hospitalists” in the American health care system. NEJM. 1996;335(7):514-517. https://doi.org/10.1093/ajhp/53.20.2389a.
6. Pham HH, Devers KJ, Kuo S, et al. Health care market trends and the evolution of hospitalist use and roles. J Gen Intern Med. 2005;20(2):101-107. https://doi.org/10.1111/j.1525-1497.2005.40184.x.
7. Epané JP, Weech-Maldonado R, Hearld L, et al. Hospitals’ use of hospitalistas: implications for financial performance. Health Care Manage Rev. 2019;44(1):10-18. https://doi.org/10.1097/hmr.0000000000000170.
8. State of Hospital Medicine: 2018 Report Based on 2017 Data. Society of Hospital Medicine. https://sohm.hospitalmedicine.org/ Accessed December 9, 2018.
9. Carmeli A, Tishler A. The relationships between intangible organizational elements and organizational performance. Strategic Manag J. 2004;25(13):1257-1278. https://doi.org/10.1002/smj.428.
10. Bernard M. Strategic performance management: leveraging and measuring your intangible value drivers. Amsterdam: Butterworth-Heinemann, 2006.
11. Khullar D, Wolfson D, Casalino LP. Professionalism, performance, and the future of physician incentives. JAMA. 2018;320(23):2419-2420. https://doi.org/10.1001/jama.2018.17719.
1. Lapps J, Flansbaum B, Leykum L, et al. Updating threshold-based identification of hospitalists in 2012 Medicare pay data. J Hosp Med. 2016;11(1):45-47. https://doi.org/10.1002/jhm.2480.
2. Wachter RM, Goldman L. Zero to 50,000–the 20th Anniversary of the hospitalist. NEJM. 2016;375(11):1009-1011. https://doi.org/10.1056/nejmp1607958.
3. Stevens JP, Nyweide DJ, Maresh S, et al. Comparison of hospital resource use and outcomes among hospitalists, primary care physicians, and other generalists. JAMA Intern Med. 2017;177(12):1781-1787. https://doi.org/10.1001/jamainternmed.2017.5824.
4. American Hospital Association (AHA) (2017), Hospital Statistics, AHA, Chicago, IL.
5. Wachter RM, Goldman L. The emerging role of “hospitalists” in the American health care system. NEJM. 1996;335(7):514-517. https://doi.org/10.1093/ajhp/53.20.2389a.
6. Pham HH, Devers KJ, Kuo S, et al. Health care market trends and the evolution of hospitalist use and roles. J Gen Intern Med. 2005;20(2):101-107. https://doi.org/10.1111/j.1525-1497.2005.40184.x.
7. Epané JP, Weech-Maldonado R, Hearld L, et al. Hospitals’ use of hospitalistas: implications for financial performance. Health Care Manage Rev. 2019;44(1):10-18. https://doi.org/10.1097/hmr.0000000000000170.
8. State of Hospital Medicine: 2018 Report Based on 2017 Data. Society of Hospital Medicine. https://sohm.hospitalmedicine.org/ Accessed December 9, 2018.
9. Carmeli A, Tishler A. The relationships between intangible organizational elements and organizational performance. Strategic Manag J. 2004;25(13):1257-1278. https://doi.org/10.1002/smj.428.
10. Bernard M. Strategic performance management: leveraging and measuring your intangible value drivers. Amsterdam: Butterworth-Heinemann, 2006.
11. Khullar D, Wolfson D, Casalino LP. Professionalism, performance, and the future of physician incentives. JAMA. 2018;320(23):2419-2420. https://doi.org/10.1001/jama.2018.17719.
© 2019 Society of Hospital Medicine
Inpatient Communication Barriers and Drivers When Caring for Limited English Proficiency Children
Immigrant children make up the fastest growing segment of the population in the United States.1 While most immigrant children are fluent in English, approximately 40% live with a parent who has limited English proficiency (LEP; ie, speaks English less than “very well”).2,3 In pediatrics, LEP status has been associated with longer hospitalizations,4 higher hospitalization costs,5 increased risk for serious adverse medical events,4,6 and more frequent emergency department reutilization.7 In the inpatient setting, multiple aspects of care present a variety of communication challenges,8 which are amplified by shift work and workflow complexity that result in patients and families interacting with numerous providers over the course of an inpatient stay.
Increasing access to trained professional interpreters when caring for LEP patients improves communication, patient satisfaction, adherence, and mortality.9-12 However, even when access to interpreter services is established, effective use is not guaranteed.13 Up to 57% of pediatricians report relying on family members to communicate with LEP patients and their caregivers;9 23% of pediatric residents categorized LEP encounters as frustrating while 78% perceived care of LEP patients to be “misdirected” (eg, delay in diagnosis or discharge) because of associated language barriers.14
Understanding experiences of frontline inpatient medical providers and interpreters is crucial in identifying challenges and ways to optimize communication for hospitalized LEP patients and families. However, there is a paucity of literature exploring the perspectives of medical providers and interpreters as it relates to communication with hospitalized LEP children and families. In this study, we sought to identify barriers and drivers of effective communication with pediatric patients and families with LEP in the inpatient setting from the perspective of frontline medical providers and interpreters.
METHODS
Study Design
This qualitative study used Group Level Assessment (GLA), a structured participatory methodology that allows diverse groups of stakeholders to generate and evaluate data in interactive sessions.15-18 GLA structure promotes active participation, group problem-solving, and development of actionable plans, distinguishing it from focus groups and in-depth semistructured interviews.15,19 This study received a human subject research exemption by the institutional review board.
Study Setting
Cincinnati Children’s Hospital Medical Center (CCHMC) is a large quaternary care center with ~200 patient encounters each day who require the use of interpreter services. Interpreters (in-person, video, and phone) are utilized during admission, formal family-centered rounds, hospital discharge, and other encounters with physicians, nurses, and other healthcare professionals. In-person interpreters are available in-house for Spanish and Arabic, with 18 additional languages available through regional vendors. Despite available resources, there is no standard way in which medical providers and interpreters work with one another.
Study Participants and Recruitment
Medical providers who care for hospitalized general pediatric patients were eligible to participate, including attending physicians, resident physicians, bedside nurses, and inpatient ancillary staff (eg, respiratory therapists, physical therapists). Interpreters employed by CCHMC with experience in the inpatient setting were also eligible. Individuals were recruited based on published recommendations to optimize discussion and group-thinking.15 Each participant was asked to take part in one GLA session. Participants were assigned to specific sessions based on roles (ie, physicians, nurses, and interpreters) to maximize engagement and minimize the impact of hierarchy.
Study Procedure
GLA involves a seven-step structured process (Appendix 1): climate setting, generating, appreciating, reflecting, understanding, selecting, and action.15,18 Qualitative data were generated individually and anonymously by participants on flip charts in response to prompts such as: “I worry that LEP families___,” “The biggest challenge when using interpreter services is___,” and “I find___ works well in providing care for LEP families.” Prompts were developed by study investigators, modified based on input from nursing and interpreter services leadership, and finalized by GLA facilitators. Fifty-one unique prompts were utilized (Appendix 2); the number of prompts used (ranging from 15 to 32 prompts) per session was based on published recommendations.15 During sessions, study investigators took detailed notes, including verbatim transcription of participant quotes. Upon conclusion of the session, each participant completed a demographic survey, including years of experience, languages spoken and perceived fluency,20 and ethnicity.
Data Analysis
Within each session, under the guidance of trained and experienced GLA facilitators (WB, HV), participants distilled and summarized qualitative data into themes, discussed and prioritized themes, and generated action items. Following completion of all sessions, analyzed data was compiled by the research team to determine similarities and differences across groups based on participant roles, consolidate themes into barriers and drivers of communication with LEP families, and determine any overlap of priorities for action. Findings were shared back with each group to ensure accuracy and relevance.
RESULTS
Participants
A total of 64 individuals participated (Table 1): hospital medicine physicians and residents (56%), inpatient nurses and ancillary staff (16%), and interpreters (28%). While 81% of physicians spoke multiple languages, only 25% reported speaking them well; two physicians were certified to communicate medical information without an interpreter present.
Themes Resulting from GLA Sessions
A total of four barriers (Table 2) and four drivers (Table 3) of effective communication with pediatric LEP patients and their families in the inpatient setting were identified by participants. Participants across all groups, despite enthusiasm around improving communication, were concerned about quality of care LEP families received, noting that the system is “designed to deliver less-good care” and that “we really haven’t figured out how to care for [LEP patients and families] in a [high-]quality and reliable way.” Variation in theme discussion was noted between groups based on participant role: physicians voiced concern about rapport with LEP families, nurses emphasized actionable tasks, and interpreters focused on heightened challenges in times of stress.
Barrier 1: Difficulties Accessing Interpreter Services
Medical providers (physicians and nurses) identified the “opaque process to access [interpreter] services” as one of their biggest challenges when communicating with LEP families. In particular, the process of scheduling interpreters was described as a “black box,” with physicians and nurses expressing difficulty determining if and when in-person interpreters were scheduled and uncertainty about when to use modalities other than in-person interpretation. Participants across groups highlighted the lack of systems knowledge from medical providers and limitations within the system that make predictable, timely, and reliable access to interpreters challenging, especially for uncommon languages. Medical providers desired more in-person interpreters who can “stay as long as clinically indicated,” citing frustration associated with using phone- and video-interpretation (eg, challenges locating technology, unfamiliarity with use, unreliable functionality of equipment). Interpreters voiced wanting to take time to finish each encounter fully without “being in a hurry because the next appointment is coming soon” or “rushing… in [to the next] session sweating.”
Barrier 2: Uncertainty in Communication with LEP Families
Participants across all groups described three areas of uncertainty as detailed in Table 2: (1) what to share and how to prioritize information during encounters with LEP patients and families, (2) what is communicated during interpretation, and (3) what LEP patients and families understand.
Barrier 3: Unclear and Inconsistent Expectations and Roles of Team Members
Given the complexity involved in communication between medical providers, interpreters, and families, participants across all groups reported feeling ill-prepared when navigating hospital encounters with LEP patients and families. Interpreters reported having little to no clinical context, medical providers reported having no knowledge of the assigned interpreter’s style, and both interpreters and medical providers reported that families have little idea of what to expect or how to engage. All groups voiced frustration about the lack of clarity regarding specific roles and scope of practice for each team member during an encounter, where multiple people end up “talking [or] using the interpreter at once.” Interpreters shared their expectations of medical providers to set the pace and lead conversations with LEP families. On the other hand, medical providers expressed a desire for interpreters to provide cultural context to the team without prompting and to interrupt during encounters when necessary to voice concerns or redirect conversations.
Barrier 4: Unmet Family Engagement Expectations
Participants across all groups articulated challenges with establishing rapport with LEP patients and families, sharing concerns that “inadequate communication” due to “cultural or language barriers” ultimately impacts quality of care. Participants reported decreased bidirectional engagement with and from LEP families. Medical providers not only noted difficulty in connecting with LEP families “on a more personal level” and providing frequent medical updates, but also felt that LEP families do not ask questions even when uncertain. Interpreters expressed concerns about medical providers “not [having] enough patience to answer families’ questions” while LEP families “shy away from asking questions.”
Driver 1: Utilizing a Team-Based Approach between Medical Providers and Interpreters
Participants from all groups emphasized that a mutual understanding of roles and shared expectations regarding communication and interpretation style, clinical context, and time constraints would establish a foundation for respect between medical providers and interpreters. They reported that a team-based approach to LEP patient and family encounters were crucial to achieving effective communication.
Driver 2: Understanding the Role of Cultural Context in Providing Culturally Effective Care.
Participants across all groups highlighted three different aspects of cultural context that drive effective communication: (1) medical providers’ perception of the family’s culture; (2) LEP families’ knowledge about the culture and healthcare system in the US, and (3) medical providers insight into their own preconceived ideas about LEP families.
Driver 3: Practicing Empathy for Patients and Families
All participants reported that respect for diversity and consideration of the backgrounds and perspectives of LEP patients and families are necessary. Furthermore, both medical providers and interpreters articulated a need to remain patient and mindful when interacting with LEP families despite challenges, especially since, as noted by interpreters, encounters may “take longer, but it’s for a reason.”
Driver 4: Using Effective Family-Centered Communication Strategies
Participants identified the use of effective family-centered communication principles as a driver to optimal communication. Many of the principles identified by medical providers and interpreters are generally applicable to all hospitalized patients and families regardless of English proficiency: optimizing verbal communication (eg, using shorter sentences, pausing to allow for interpretation), optimizing nonverbal communication (eg, setting, position, and body language), and assessment of family understanding and engagement (eg, use of teach back).
DISCUSSION
Frontline medical providers and interpreters identified barriers and drivers that impact communication with LEP patients and families during hospitalization. To our knowledge, this is the first study that uses a participatory method to explore the perspectives of medical providers and interpreters who care for LEP children and families in the inpatient setting. Despite existing difficulties and concerns regarding language barriers and its impact on quality of care for hospitalized LEP patients and families, participants were enthusiastic about how identified barriers and drivers may inform future improvement efforts. Notable action steps for future improvement discussed by our participants included: increased use and functionality of technology for timely and predictable access to interpreters, deliberate training for providers focused on delivery of culturally-effective care, consistent use of family-centered communication strategies including teach-back, and implementing interdisciplinary expectation setting through “presessions” before encounters with LEP families.
Participants elaborated on several barriers previously described in the literature including time constraints and technical problems.14,21,22 Such barriers may serve as deterrents to consistent and appropriate use of interpreters in healthcare settings.9 A heavy reliance on off-site interpreters (including phone- or video-interpreters) and lack of knowledge regarding resource availability likely amplified frustration for medical providers. Communication with LEP families can be daunting, especially when medical providers do not care for LEP families or work with interpreters on a regular basis.14 Standardizing the education of medical providers regarding available resources, as well as the logistics, process, and parameters for scheduling interpreters and using technology, was an action step identified by our GLA participants. Targeted education about the logistics of accessing interpreter services and having standardized ways to make technology use easier (ie, one-touch dialing in hospital rooms) has been associated with increased interpreter use and decreased interpreter-related delays in care.23
Our frontline medical providers expressed added concern about not spending as much time with LEP families. In fact, LEP families in the literature have perceived medical providers to spend less time with their children compared to their English-proficient counterparts.24 Language and cultural barriers, both perceived and real, may limit medical provider rapport with LEP patients and families14 and likely contribute to medical providers relying on their preconceived assumptions instead.25 Cultural competency education for medical providers, as highlighted by our GLA participants as an action item, can be used to provide more comprehensive and effective care.26,27
In addition to enhancing cultural humility through education, our participants emphasized the use of family-centered communication strategies as a driver of optimal family engagement and understanding. Actively inviting questions from families and utilizing teach-back, an established evidence-based strategy28-30 discussed by our participants, can be particularly powerful in assessing family understanding and engagement. While information should be presented in plain language for families in all encounters,31 these evidence-based practices are of particular importance when communicating with LEP families. They promote effective communication, empower families to share concerns in a structured manner, and allow medical providers to address matters in real-time with interpreters present.
Finally, our participants highlighted the need for partnerships between providers and interpreter services, noting unclear roles and expectations among interpreters and medical providers as a major barrier. Specifically, physicians noted confusion regarding the scope of an interpreter’s practice. Participants from GLA sessions discussed the importance of a team-based approach and suggested implementing a “presession” prior to encounters with LEP patients and families. Presessions—a concept well accepted among interpreters and recommended by consensus-based practice guidelines—enable medical providers and interpreters to establish shared expectations about scope of practice, communication, interpretation style, time constraints, and medical context prior to patient encounters.32,33
There are several limitations to our study. First, individuals who chose to participate were likely highly motivated by their clinical experiences with LEP patients and invested in improving communication with LEP families. Second, the study is limited in generalizability, as it was conducted at a single academic institution in a Midwestern city. Despite regional variations in available resources as well as patient and workforce demographics, our findings regarding major themes are in agreement with previously published literature and further add to our understanding of ways to improve communication with this vulnerable population across the care spectrum. Lastly, we were logistically limited in our ability to elicit the perspectives of LEP families due to the participatory nature of GLA; the need for multiple interpreters to simultaneously interact with LEP individuals would have not only hindered active LEP family participation but may have also biased the data generated by patients and families, as the services interpreters provide during their inpatient stay was the focus of our study. Engaging LEP families in their preferred language using participatory methods should be considered for future studies.
In conclusion, frontline providers of medical and language services identified barriers and drivers impacting the effective use of interpreter services when communicating with LEP families during hospitalization. Our enhanced understanding of barriers and drivers, as well as identified actionable interventions, will inform future improvement of communication and interactions with LEP families that contributes to effective and efficient family centered care. A framework for the development and implementation of organizational strategies aimed at improving communication with LEP families must include a thorough assessment of impact, feasibility, stakeholder involvement, and sustainability of specific interventions. While there is no simple formula to improve language services, health systems should establish and adopt language access policies, standardize communication practices, and develop processes to optimize the use of language services in the hospital. Furthermore, engagement with LEP families to better understand their perceptions and experiences with the healthcare system is crucial to improve communication between medical providers and LEP families in the inpatient setting and should be the subject of future studies.
Disclosures
The authors have no conflicts of interest to disclose.
Funding
No external funding was secured for this study. Dr. Joanna Thomson is supported by the Agency for Healthcare Research and Quality (Grant #K08 HS025138). Dr. Raglin Bignall was supported through a Ruth L. Kirschstein National Research Service Award (T32HP10027) when the study was conducted. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding organizations. The funding organizations had no role in the design, preparation, review, or approval of this paper.
1. The American Academy of Pediatrics Council on Community Pediatrics. Providing care for immigrant, migrant, and border children. Pediatrics. 2013;131(6):e2028-e2034. PubMed
2. Meneses C, Chilton L, Duffee J, et al. Council on Community Pediatrics Immigrant Health Tool Kit. The American Academy of Pediatrics. https://www.aap.org/en-us/Documents/cocp_toolkit_full.pdf. Accessed May 13, 2019.
3. Office for Civil Rights. Guidance to Federal Financial Assistance Recipients Regarding Title VI and the Prohibition Against National Origin Discrimination Affecting Limited English Proficient Persons. https://www.hhs.gov/civil-rights/for-individuals/special-topics/limited-english-proficiency/guidance-federal-financial-assistance-recipients-title-vi/index.html. Accessed May 13, 2019.
4. Lion KC, Rafton SA, Shafii J, et al. Association between language, serious adverse events, and length of stay Among hospitalized children. Hosp Pediatr. 2013;3(3):219-225. https://doi.org/10.1542/hpeds.2012-0091.
5. Lion KC, Wright DR, Desai AD, Mangione-Smith R. Costs of care for hospitalized children associated With preferred language and insurance type. Hosp Pediatr. 2017;7(2):70-78. https://doi.org/10.1542/hpeds.2016-0051.
6. Cohen AL, Rivara F, Marcuse EK, McPhillips H, Davis R. Are language barriers associated with serious medical events in hospitalized pediatric patients? Pediatrics. 2005;116(3):575-579. https://doi.org/10.1542/peds.2005-0521.
7. Samuels-Kalow ME, Stack AM, Amico K, Porter SC. Parental language and return visits to the Emergency Department After discharge. Pediatr Emerg Care. 2017;33(6):402-404. https://doi.org/10.1097/PEC.0000000000000592.
8. Unaka NI, Statile AM, Choe A, Shonna Yin H. Addressing health literacy in the inpatient setting. Curr Treat Options Pediatr. 2018;4(2):283-299. https://doi.org/10.1007/s40746-018-0122-3.
9. DeCamp LR, Kuo DZ, Flores G, O’Connor K, Minkovitz CS. Changes in language services use by US pediatricians. Pediatrics. 2013;132(2):e396-e406. https://doi.org/10.1542/peds.2012-2909.
10. Flores G. The impact of medical interpreter services on the quality of health care: A systematic review. Med Care Res Rev. 2005;62(3):255-299. https://doi.org/10.1177/1077558705275416.
11. Flores G, Abreu M, Barone CP, Bachur R, Lin H. Errors of medical interpretation and their potential clinical consequences: A comparison of professional versus hoc versus no interpreters. Ann Emerg Med. 2012;60(5):545-553. https://doi.org/10.1016/j.annemergmed.2012.01.025.
12. Anand KJ, Sepanski RJ, Giles K, Shah SH, Juarez PD. Pediatric intensive care unit mortality among Latino children before and after a multilevel health care delivery intervention. JAMA Pediatr. 2015;169(4):383-390. https://doi.org/10.1001/jamapediatrics.2014.3789.
13. The Joint Commission. Advancing Effective Communication, Cultural Competence, and Patient- and Family-Centered Care: A Roadmap for Hospitals. Oakbrook Terrace, IL: The Joint Commission; 2010.
14. Hernandez RG, Cowden JD, Moon M et al. Predictors of resident satisfaction in caring for limited English proficient families: a multisite study. Acad Pediatr. 2014;14(2):173-180. https://doi.org/10.1016/j.acap.2013.12.002.
15. Vaughn LM, Lohmueller M. Calling all stakeholders: group-level assessment (GLA)-a qualitative and participatory method for large groups. Eval Rev. 2014;38(4):336-355. https://doi.org/10.1177/0193841X14544903.
16. Vaughn LM, Jacquez F, Zhao J, Lang M. Partnering with students to explore the health needs of an ethnically diverse, low-resource school: an innovative large group assessment approach. Fam Commun Health. 2011;34(1):72-84. https://doi.org/10.1097/FCH.0b013e3181fded12.
17. Gosdin CH, Vaughn L. Perceptions of physician bedside handoff with nurse and family involvement. Hosp Pediatr. 2012;2(1):34-38. https://doi.org/10.1542/hpeds.2011-0008-2.
18. Graham KE, Schellinger AR, Vaughn LM. Developing strategies for positive change: transitioning foster youth to adulthood. Child Youth Serv Rev. 2015;54:71-79. https://doi.org/10.1016/j.childyouth.2015.04.014.
19. Vaughn LM. Group level assessment: A Large Group Method for Identifying Primary Issues and Needs within a community. London2014. http://methods.sagepub.com/case/group-level-assessment-large-group-primary-issues-needs-community. Accessed 2017/07/26.
20. Association of American Medical Colleges Electronic Residency Application Service. ERAS 2018 MyERAS Application Worksheet: Language Fluency. Washington, DC:: Association of American Medical Colleges; 2018:5.
21. Brisset C, Leanza Y, Laforest K. Working with interpreters in health care: A systematic review and meta-ethnography of qualitative studies. Patient Educ Couns. 2013;91(2):131-140. https://doi.org/10.1016/j.pec.2012.11.008.
22. Wiking E, Saleh-Stattin N, Johansson SE, Sundquist J. A description of some aspects of the triangular meeting between immigrant patients, their interpreters and GPs in primary health care in Stockholm, Sweden. Fam Pract. 2009;26(5):377-383. https://doi.org/10.1093/fampra/cmp052.
23. Lion KC, Ebel BE, Rafton S et al. Evaluation of a quality improvement intervention to increase use of telephonic interpretation. Pediatrics. 2015;135(3):e709-e716. https://doi.org/10.1542/peds.2014-2024.
24. Zurca AD, Fisher KR, Flor RJ, et al. Communication with limited English-proficient families in the PICU. Hosp Pediatr. 2017;7(1):9-15. https://doi.org/10.1542/hpeds.2016-0071.
25. Kodjo C. Cultural competence in clinician communication. Pediatr Rev. 2009;30(2):57-64. https://doi.org/10.1542/pir.30-2-57.
26. Britton CV, American Academy of Pediatrics Committee on Pediatric Workforce. Ensuring culturally effective pediatric care: implications for education and health policy. Pediatrics. 2004;114(6):1677-1685. https://doi.org/10.1542/peds.2004-2091.
27. The American Academy of Pediatrics. Culturally Effective Care Toolkit: Providing Cuturally Effective Pediatric Care; 2018. https://www.aap.org/en-us/professional-resources/practice-transformation/managing-patients/Pages/effective-care.aspx. Accessed May 13, 2019.
28. Starmer AJ, Spector ND, Srivastava R, et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):1803-1812. https://doi.org/10.1056/NEJMsa1405556.
29. Jager AJ, Wynia MK. Who gets a teach-back? Patient-reported incidence of experiencing a teach-back. J Health Commun. 2012;17 Supplement 3:294-302. https://doi.org/10.1080/10810730.2012.712624.
30. Kornburger C, Gibson C, Sadowski S, Maletta K, Klingbeil C. Using “teach-back” to promote a safe transition from hospital to home: an evidence-based approach to improving the discharge process. J Pediatr Nurs. 2013;28(3):282-291. https://doi.org/10.1016/j.pedn.2012.10.007.
31. Abrams MA, Klass P, Dreyer BP. Health literacy and children: recommendations for action. Pediatrics. 2009;124 Supplement 3:S327-S331. https://doi.org/10.1542/peds.2009-1162I.
32. Betancourt JR, Renfrew MR, Green AR, Lopez L, Wasserman M. Improving Patient Safety Systems for Patients with Limited English Proficiency: a Guide for Hospitals. Agency for Healthcare Research and Quality; 2012. 33. The National Council on Interpreting in Health Care. Best Practices for Communicating Through an Interpreter . https://refugeehealthta.org/access-to-care/language-access/best-practices-communicating-through-an-interpreter/. Accessed May 19, 2019.
33. The National Council on Interpreting in Health Care. Best Practices for Communicating Through an Interpreter . https://refugeehealthta.org/access-to-care/language-access/best-practices-communicating-through-an-interpreter/. Accessed May 19, 2019.
Immigrant children make up the fastest growing segment of the population in the United States.1 While most immigrant children are fluent in English, approximately 40% live with a parent who has limited English proficiency (LEP; ie, speaks English less than “very well”).2,3 In pediatrics, LEP status has been associated with longer hospitalizations,4 higher hospitalization costs,5 increased risk for serious adverse medical events,4,6 and more frequent emergency department reutilization.7 In the inpatient setting, multiple aspects of care present a variety of communication challenges,8 which are amplified by shift work and workflow complexity that result in patients and families interacting with numerous providers over the course of an inpatient stay.
Increasing access to trained professional interpreters when caring for LEP patients improves communication, patient satisfaction, adherence, and mortality.9-12 However, even when access to interpreter services is established, effective use is not guaranteed.13 Up to 57% of pediatricians report relying on family members to communicate with LEP patients and their caregivers;9 23% of pediatric residents categorized LEP encounters as frustrating while 78% perceived care of LEP patients to be “misdirected” (eg, delay in diagnosis or discharge) because of associated language barriers.14
Understanding experiences of frontline inpatient medical providers and interpreters is crucial in identifying challenges and ways to optimize communication for hospitalized LEP patients and families. However, there is a paucity of literature exploring the perspectives of medical providers and interpreters as it relates to communication with hospitalized LEP children and families. In this study, we sought to identify barriers and drivers of effective communication with pediatric patients and families with LEP in the inpatient setting from the perspective of frontline medical providers and interpreters.
METHODS
Study Design
This qualitative study used Group Level Assessment (GLA), a structured participatory methodology that allows diverse groups of stakeholders to generate and evaluate data in interactive sessions.15-18 GLA structure promotes active participation, group problem-solving, and development of actionable plans, distinguishing it from focus groups and in-depth semistructured interviews.15,19 This study received a human subject research exemption by the institutional review board.
Study Setting
Cincinnati Children’s Hospital Medical Center (CCHMC) is a large quaternary care center with ~200 patient encounters each day who require the use of interpreter services. Interpreters (in-person, video, and phone) are utilized during admission, formal family-centered rounds, hospital discharge, and other encounters with physicians, nurses, and other healthcare professionals. In-person interpreters are available in-house for Spanish and Arabic, with 18 additional languages available through regional vendors. Despite available resources, there is no standard way in which medical providers and interpreters work with one another.
Study Participants and Recruitment
Medical providers who care for hospitalized general pediatric patients were eligible to participate, including attending physicians, resident physicians, bedside nurses, and inpatient ancillary staff (eg, respiratory therapists, physical therapists). Interpreters employed by CCHMC with experience in the inpatient setting were also eligible. Individuals were recruited based on published recommendations to optimize discussion and group-thinking.15 Each participant was asked to take part in one GLA session. Participants were assigned to specific sessions based on roles (ie, physicians, nurses, and interpreters) to maximize engagement and minimize the impact of hierarchy.
Study Procedure
GLA involves a seven-step structured process (Appendix 1): climate setting, generating, appreciating, reflecting, understanding, selecting, and action.15,18 Qualitative data were generated individually and anonymously by participants on flip charts in response to prompts such as: “I worry that LEP families___,” “The biggest challenge when using interpreter services is___,” and “I find___ works well in providing care for LEP families.” Prompts were developed by study investigators, modified based on input from nursing and interpreter services leadership, and finalized by GLA facilitators. Fifty-one unique prompts were utilized (Appendix 2); the number of prompts used (ranging from 15 to 32 prompts) per session was based on published recommendations.15 During sessions, study investigators took detailed notes, including verbatim transcription of participant quotes. Upon conclusion of the session, each participant completed a demographic survey, including years of experience, languages spoken and perceived fluency,20 and ethnicity.
Data Analysis
Within each session, under the guidance of trained and experienced GLA facilitators (WB, HV), participants distilled and summarized qualitative data into themes, discussed and prioritized themes, and generated action items. Following completion of all sessions, analyzed data was compiled by the research team to determine similarities and differences across groups based on participant roles, consolidate themes into barriers and drivers of communication with LEP families, and determine any overlap of priorities for action. Findings were shared back with each group to ensure accuracy and relevance.
RESULTS
Participants
A total of 64 individuals participated (Table 1): hospital medicine physicians and residents (56%), inpatient nurses and ancillary staff (16%), and interpreters (28%). While 81% of physicians spoke multiple languages, only 25% reported speaking them well; two physicians were certified to communicate medical information without an interpreter present.
Themes Resulting from GLA Sessions
A total of four barriers (Table 2) and four drivers (Table 3) of effective communication with pediatric LEP patients and their families in the inpatient setting were identified by participants. Participants across all groups, despite enthusiasm around improving communication, were concerned about quality of care LEP families received, noting that the system is “designed to deliver less-good care” and that “we really haven’t figured out how to care for [LEP patients and families] in a [high-]quality and reliable way.” Variation in theme discussion was noted between groups based on participant role: physicians voiced concern about rapport with LEP families, nurses emphasized actionable tasks, and interpreters focused on heightened challenges in times of stress.
Barrier 1: Difficulties Accessing Interpreter Services
Medical providers (physicians and nurses) identified the “opaque process to access [interpreter] services” as one of their biggest challenges when communicating with LEP families. In particular, the process of scheduling interpreters was described as a “black box,” with physicians and nurses expressing difficulty determining if and when in-person interpreters were scheduled and uncertainty about when to use modalities other than in-person interpretation. Participants across groups highlighted the lack of systems knowledge from medical providers and limitations within the system that make predictable, timely, and reliable access to interpreters challenging, especially for uncommon languages. Medical providers desired more in-person interpreters who can “stay as long as clinically indicated,” citing frustration associated with using phone- and video-interpretation (eg, challenges locating technology, unfamiliarity with use, unreliable functionality of equipment). Interpreters voiced wanting to take time to finish each encounter fully without “being in a hurry because the next appointment is coming soon” or “rushing… in [to the next] session sweating.”
Barrier 2: Uncertainty in Communication with LEP Families
Participants across all groups described three areas of uncertainty as detailed in Table 2: (1) what to share and how to prioritize information during encounters with LEP patients and families, (2) what is communicated during interpretation, and (3) what LEP patients and families understand.
Barrier 3: Unclear and Inconsistent Expectations and Roles of Team Members
Given the complexity involved in communication between medical providers, interpreters, and families, participants across all groups reported feeling ill-prepared when navigating hospital encounters with LEP patients and families. Interpreters reported having little to no clinical context, medical providers reported having no knowledge of the assigned interpreter’s style, and both interpreters and medical providers reported that families have little idea of what to expect or how to engage. All groups voiced frustration about the lack of clarity regarding specific roles and scope of practice for each team member during an encounter, where multiple people end up “talking [or] using the interpreter at once.” Interpreters shared their expectations of medical providers to set the pace and lead conversations with LEP families. On the other hand, medical providers expressed a desire for interpreters to provide cultural context to the team without prompting and to interrupt during encounters when necessary to voice concerns or redirect conversations.
Barrier 4: Unmet Family Engagement Expectations
Participants across all groups articulated challenges with establishing rapport with LEP patients and families, sharing concerns that “inadequate communication” due to “cultural or language barriers” ultimately impacts quality of care. Participants reported decreased bidirectional engagement with and from LEP families. Medical providers not only noted difficulty in connecting with LEP families “on a more personal level” and providing frequent medical updates, but also felt that LEP families do not ask questions even when uncertain. Interpreters expressed concerns about medical providers “not [having] enough patience to answer families’ questions” while LEP families “shy away from asking questions.”
Driver 1: Utilizing a Team-Based Approach between Medical Providers and Interpreters
Participants from all groups emphasized that a mutual understanding of roles and shared expectations regarding communication and interpretation style, clinical context, and time constraints would establish a foundation for respect between medical providers and interpreters. They reported that a team-based approach to LEP patient and family encounters were crucial to achieving effective communication.
Driver 2: Understanding the Role of Cultural Context in Providing Culturally Effective Care.
Participants across all groups highlighted three different aspects of cultural context that drive effective communication: (1) medical providers’ perception of the family’s culture; (2) LEP families’ knowledge about the culture and healthcare system in the US, and (3) medical providers insight into their own preconceived ideas about LEP families.
Driver 3: Practicing Empathy for Patients and Families
All participants reported that respect for diversity and consideration of the backgrounds and perspectives of LEP patients and families are necessary. Furthermore, both medical providers and interpreters articulated a need to remain patient and mindful when interacting with LEP families despite challenges, especially since, as noted by interpreters, encounters may “take longer, but it’s for a reason.”
Driver 4: Using Effective Family-Centered Communication Strategies
Participants identified the use of effective family-centered communication principles as a driver to optimal communication. Many of the principles identified by medical providers and interpreters are generally applicable to all hospitalized patients and families regardless of English proficiency: optimizing verbal communication (eg, using shorter sentences, pausing to allow for interpretation), optimizing nonverbal communication (eg, setting, position, and body language), and assessment of family understanding and engagement (eg, use of teach back).
DISCUSSION
Frontline medical providers and interpreters identified barriers and drivers that impact communication with LEP patients and families during hospitalization. To our knowledge, this is the first study that uses a participatory method to explore the perspectives of medical providers and interpreters who care for LEP children and families in the inpatient setting. Despite existing difficulties and concerns regarding language barriers and its impact on quality of care for hospitalized LEP patients and families, participants were enthusiastic about how identified barriers and drivers may inform future improvement efforts. Notable action steps for future improvement discussed by our participants included: increased use and functionality of technology for timely and predictable access to interpreters, deliberate training for providers focused on delivery of culturally-effective care, consistent use of family-centered communication strategies including teach-back, and implementing interdisciplinary expectation setting through “presessions” before encounters with LEP families.
Participants elaborated on several barriers previously described in the literature including time constraints and technical problems.14,21,22 Such barriers may serve as deterrents to consistent and appropriate use of interpreters in healthcare settings.9 A heavy reliance on off-site interpreters (including phone- or video-interpreters) and lack of knowledge regarding resource availability likely amplified frustration for medical providers. Communication with LEP families can be daunting, especially when medical providers do not care for LEP families or work with interpreters on a regular basis.14 Standardizing the education of medical providers regarding available resources, as well as the logistics, process, and parameters for scheduling interpreters and using technology, was an action step identified by our GLA participants. Targeted education about the logistics of accessing interpreter services and having standardized ways to make technology use easier (ie, one-touch dialing in hospital rooms) has been associated with increased interpreter use and decreased interpreter-related delays in care.23
Our frontline medical providers expressed added concern about not spending as much time with LEP families. In fact, LEP families in the literature have perceived medical providers to spend less time with their children compared to their English-proficient counterparts.24 Language and cultural barriers, both perceived and real, may limit medical provider rapport with LEP patients and families14 and likely contribute to medical providers relying on their preconceived assumptions instead.25 Cultural competency education for medical providers, as highlighted by our GLA participants as an action item, can be used to provide more comprehensive and effective care.26,27
In addition to enhancing cultural humility through education, our participants emphasized the use of family-centered communication strategies as a driver of optimal family engagement and understanding. Actively inviting questions from families and utilizing teach-back, an established evidence-based strategy28-30 discussed by our participants, can be particularly powerful in assessing family understanding and engagement. While information should be presented in plain language for families in all encounters,31 these evidence-based practices are of particular importance when communicating with LEP families. They promote effective communication, empower families to share concerns in a structured manner, and allow medical providers to address matters in real-time with interpreters present.
Finally, our participants highlighted the need for partnerships between providers and interpreter services, noting unclear roles and expectations among interpreters and medical providers as a major barrier. Specifically, physicians noted confusion regarding the scope of an interpreter’s practice. Participants from GLA sessions discussed the importance of a team-based approach and suggested implementing a “presession” prior to encounters with LEP patients and families. Presessions—a concept well accepted among interpreters and recommended by consensus-based practice guidelines—enable medical providers and interpreters to establish shared expectations about scope of practice, communication, interpretation style, time constraints, and medical context prior to patient encounters.32,33
There are several limitations to our study. First, individuals who chose to participate were likely highly motivated by their clinical experiences with LEP patients and invested in improving communication with LEP families. Second, the study is limited in generalizability, as it was conducted at a single academic institution in a Midwestern city. Despite regional variations in available resources as well as patient and workforce demographics, our findings regarding major themes are in agreement with previously published literature and further add to our understanding of ways to improve communication with this vulnerable population across the care spectrum. Lastly, we were logistically limited in our ability to elicit the perspectives of LEP families due to the participatory nature of GLA; the need for multiple interpreters to simultaneously interact with LEP individuals would have not only hindered active LEP family participation but may have also biased the data generated by patients and families, as the services interpreters provide during their inpatient stay was the focus of our study. Engaging LEP families in their preferred language using participatory methods should be considered for future studies.
In conclusion, frontline providers of medical and language services identified barriers and drivers impacting the effective use of interpreter services when communicating with LEP families during hospitalization. Our enhanced understanding of barriers and drivers, as well as identified actionable interventions, will inform future improvement of communication and interactions with LEP families that contributes to effective and efficient family centered care. A framework for the development and implementation of organizational strategies aimed at improving communication with LEP families must include a thorough assessment of impact, feasibility, stakeholder involvement, and sustainability of specific interventions. While there is no simple formula to improve language services, health systems should establish and adopt language access policies, standardize communication practices, and develop processes to optimize the use of language services in the hospital. Furthermore, engagement with LEP families to better understand their perceptions and experiences with the healthcare system is crucial to improve communication between medical providers and LEP families in the inpatient setting and should be the subject of future studies.
Disclosures
The authors have no conflicts of interest to disclose.
Funding
No external funding was secured for this study. Dr. Joanna Thomson is supported by the Agency for Healthcare Research and Quality (Grant #K08 HS025138). Dr. Raglin Bignall was supported through a Ruth L. Kirschstein National Research Service Award (T32HP10027) when the study was conducted. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding organizations. The funding organizations had no role in the design, preparation, review, or approval of this paper.
Immigrant children make up the fastest growing segment of the population in the United States.1 While most immigrant children are fluent in English, approximately 40% live with a parent who has limited English proficiency (LEP; ie, speaks English less than “very well”).2,3 In pediatrics, LEP status has been associated with longer hospitalizations,4 higher hospitalization costs,5 increased risk for serious adverse medical events,4,6 and more frequent emergency department reutilization.7 In the inpatient setting, multiple aspects of care present a variety of communication challenges,8 which are amplified by shift work and workflow complexity that result in patients and families interacting with numerous providers over the course of an inpatient stay.
Increasing access to trained professional interpreters when caring for LEP patients improves communication, patient satisfaction, adherence, and mortality.9-12 However, even when access to interpreter services is established, effective use is not guaranteed.13 Up to 57% of pediatricians report relying on family members to communicate with LEP patients and their caregivers;9 23% of pediatric residents categorized LEP encounters as frustrating while 78% perceived care of LEP patients to be “misdirected” (eg, delay in diagnosis or discharge) because of associated language barriers.14
Understanding experiences of frontline inpatient medical providers and interpreters is crucial in identifying challenges and ways to optimize communication for hospitalized LEP patients and families. However, there is a paucity of literature exploring the perspectives of medical providers and interpreters as it relates to communication with hospitalized LEP children and families. In this study, we sought to identify barriers and drivers of effective communication with pediatric patients and families with LEP in the inpatient setting from the perspective of frontline medical providers and interpreters.
METHODS
Study Design
This qualitative study used Group Level Assessment (GLA), a structured participatory methodology that allows diverse groups of stakeholders to generate and evaluate data in interactive sessions.15-18 GLA structure promotes active participation, group problem-solving, and development of actionable plans, distinguishing it from focus groups and in-depth semistructured interviews.15,19 This study received a human subject research exemption by the institutional review board.
Study Setting
Cincinnati Children’s Hospital Medical Center (CCHMC) is a large quaternary care center with ~200 patient encounters each day who require the use of interpreter services. Interpreters (in-person, video, and phone) are utilized during admission, formal family-centered rounds, hospital discharge, and other encounters with physicians, nurses, and other healthcare professionals. In-person interpreters are available in-house for Spanish and Arabic, with 18 additional languages available through regional vendors. Despite available resources, there is no standard way in which medical providers and interpreters work with one another.
Study Participants and Recruitment
Medical providers who care for hospitalized general pediatric patients were eligible to participate, including attending physicians, resident physicians, bedside nurses, and inpatient ancillary staff (eg, respiratory therapists, physical therapists). Interpreters employed by CCHMC with experience in the inpatient setting were also eligible. Individuals were recruited based on published recommendations to optimize discussion and group-thinking.15 Each participant was asked to take part in one GLA session. Participants were assigned to specific sessions based on roles (ie, physicians, nurses, and interpreters) to maximize engagement and minimize the impact of hierarchy.
Study Procedure
GLA involves a seven-step structured process (Appendix 1): climate setting, generating, appreciating, reflecting, understanding, selecting, and action.15,18 Qualitative data were generated individually and anonymously by participants on flip charts in response to prompts such as: “I worry that LEP families___,” “The biggest challenge when using interpreter services is___,” and “I find___ works well in providing care for LEP families.” Prompts were developed by study investigators, modified based on input from nursing and interpreter services leadership, and finalized by GLA facilitators. Fifty-one unique prompts were utilized (Appendix 2); the number of prompts used (ranging from 15 to 32 prompts) per session was based on published recommendations.15 During sessions, study investigators took detailed notes, including verbatim transcription of participant quotes. Upon conclusion of the session, each participant completed a demographic survey, including years of experience, languages spoken and perceived fluency,20 and ethnicity.
Data Analysis
Within each session, under the guidance of trained and experienced GLA facilitators (WB, HV), participants distilled and summarized qualitative data into themes, discussed and prioritized themes, and generated action items. Following completion of all sessions, analyzed data was compiled by the research team to determine similarities and differences across groups based on participant roles, consolidate themes into barriers and drivers of communication with LEP families, and determine any overlap of priorities for action. Findings were shared back with each group to ensure accuracy and relevance.
RESULTS
Participants
A total of 64 individuals participated (Table 1): hospital medicine physicians and residents (56%), inpatient nurses and ancillary staff (16%), and interpreters (28%). While 81% of physicians spoke multiple languages, only 25% reported speaking them well; two physicians were certified to communicate medical information without an interpreter present.
Themes Resulting from GLA Sessions
A total of four barriers (Table 2) and four drivers (Table 3) of effective communication with pediatric LEP patients and their families in the inpatient setting were identified by participants. Participants across all groups, despite enthusiasm around improving communication, were concerned about quality of care LEP families received, noting that the system is “designed to deliver less-good care” and that “we really haven’t figured out how to care for [LEP patients and families] in a [high-]quality and reliable way.” Variation in theme discussion was noted between groups based on participant role: physicians voiced concern about rapport with LEP families, nurses emphasized actionable tasks, and interpreters focused on heightened challenges in times of stress.
Barrier 1: Difficulties Accessing Interpreter Services
Medical providers (physicians and nurses) identified the “opaque process to access [interpreter] services” as one of their biggest challenges when communicating with LEP families. In particular, the process of scheduling interpreters was described as a “black box,” with physicians and nurses expressing difficulty determining if and when in-person interpreters were scheduled and uncertainty about when to use modalities other than in-person interpretation. Participants across groups highlighted the lack of systems knowledge from medical providers and limitations within the system that make predictable, timely, and reliable access to interpreters challenging, especially for uncommon languages. Medical providers desired more in-person interpreters who can “stay as long as clinically indicated,” citing frustration associated with using phone- and video-interpretation (eg, challenges locating technology, unfamiliarity with use, unreliable functionality of equipment). Interpreters voiced wanting to take time to finish each encounter fully without “being in a hurry because the next appointment is coming soon” or “rushing… in [to the next] session sweating.”
Barrier 2: Uncertainty in Communication with LEP Families
Participants across all groups described three areas of uncertainty as detailed in Table 2: (1) what to share and how to prioritize information during encounters with LEP patients and families, (2) what is communicated during interpretation, and (3) what LEP patients and families understand.
Barrier 3: Unclear and Inconsistent Expectations and Roles of Team Members
Given the complexity involved in communication between medical providers, interpreters, and families, participants across all groups reported feeling ill-prepared when navigating hospital encounters with LEP patients and families. Interpreters reported having little to no clinical context, medical providers reported having no knowledge of the assigned interpreter’s style, and both interpreters and medical providers reported that families have little idea of what to expect or how to engage. All groups voiced frustration about the lack of clarity regarding specific roles and scope of practice for each team member during an encounter, where multiple people end up “talking [or] using the interpreter at once.” Interpreters shared their expectations of medical providers to set the pace and lead conversations with LEP families. On the other hand, medical providers expressed a desire for interpreters to provide cultural context to the team without prompting and to interrupt during encounters when necessary to voice concerns or redirect conversations.
Barrier 4: Unmet Family Engagement Expectations
Participants across all groups articulated challenges with establishing rapport with LEP patients and families, sharing concerns that “inadequate communication” due to “cultural or language barriers” ultimately impacts quality of care. Participants reported decreased bidirectional engagement with and from LEP families. Medical providers not only noted difficulty in connecting with LEP families “on a more personal level” and providing frequent medical updates, but also felt that LEP families do not ask questions even when uncertain. Interpreters expressed concerns about medical providers “not [having] enough patience to answer families’ questions” while LEP families “shy away from asking questions.”
Driver 1: Utilizing a Team-Based Approach between Medical Providers and Interpreters
Participants from all groups emphasized that a mutual understanding of roles and shared expectations regarding communication and interpretation style, clinical context, and time constraints would establish a foundation for respect between medical providers and interpreters. They reported that a team-based approach to LEP patient and family encounters were crucial to achieving effective communication.
Driver 2: Understanding the Role of Cultural Context in Providing Culturally Effective Care.
Participants across all groups highlighted three different aspects of cultural context that drive effective communication: (1) medical providers’ perception of the family’s culture; (2) LEP families’ knowledge about the culture and healthcare system in the US, and (3) medical providers insight into their own preconceived ideas about LEP families.
Driver 3: Practicing Empathy for Patients and Families
All participants reported that respect for diversity and consideration of the backgrounds and perspectives of LEP patients and families are necessary. Furthermore, both medical providers and interpreters articulated a need to remain patient and mindful when interacting with LEP families despite challenges, especially since, as noted by interpreters, encounters may “take longer, but it’s for a reason.”
Driver 4: Using Effective Family-Centered Communication Strategies
Participants identified the use of effective family-centered communication principles as a driver to optimal communication. Many of the principles identified by medical providers and interpreters are generally applicable to all hospitalized patients and families regardless of English proficiency: optimizing verbal communication (eg, using shorter sentences, pausing to allow for interpretation), optimizing nonverbal communication (eg, setting, position, and body language), and assessment of family understanding and engagement (eg, use of teach back).
DISCUSSION
Frontline medical providers and interpreters identified barriers and drivers that impact communication with LEP patients and families during hospitalization. To our knowledge, this is the first study that uses a participatory method to explore the perspectives of medical providers and interpreters who care for LEP children and families in the inpatient setting. Despite existing difficulties and concerns regarding language barriers and its impact on quality of care for hospitalized LEP patients and families, participants were enthusiastic about how identified barriers and drivers may inform future improvement efforts. Notable action steps for future improvement discussed by our participants included: increased use and functionality of technology for timely and predictable access to interpreters, deliberate training for providers focused on delivery of culturally-effective care, consistent use of family-centered communication strategies including teach-back, and implementing interdisciplinary expectation setting through “presessions” before encounters with LEP families.
Participants elaborated on several barriers previously described in the literature including time constraints and technical problems.14,21,22 Such barriers may serve as deterrents to consistent and appropriate use of interpreters in healthcare settings.9 A heavy reliance on off-site interpreters (including phone- or video-interpreters) and lack of knowledge regarding resource availability likely amplified frustration for medical providers. Communication with LEP families can be daunting, especially when medical providers do not care for LEP families or work with interpreters on a regular basis.14 Standardizing the education of medical providers regarding available resources, as well as the logistics, process, and parameters for scheduling interpreters and using technology, was an action step identified by our GLA participants. Targeted education about the logistics of accessing interpreter services and having standardized ways to make technology use easier (ie, one-touch dialing in hospital rooms) has been associated with increased interpreter use and decreased interpreter-related delays in care.23
Our frontline medical providers expressed added concern about not spending as much time with LEP families. In fact, LEP families in the literature have perceived medical providers to spend less time with their children compared to their English-proficient counterparts.24 Language and cultural barriers, both perceived and real, may limit medical provider rapport with LEP patients and families14 and likely contribute to medical providers relying on their preconceived assumptions instead.25 Cultural competency education for medical providers, as highlighted by our GLA participants as an action item, can be used to provide more comprehensive and effective care.26,27
In addition to enhancing cultural humility through education, our participants emphasized the use of family-centered communication strategies as a driver of optimal family engagement and understanding. Actively inviting questions from families and utilizing teach-back, an established evidence-based strategy28-30 discussed by our participants, can be particularly powerful in assessing family understanding and engagement. While information should be presented in plain language for families in all encounters,31 these evidence-based practices are of particular importance when communicating with LEP families. They promote effective communication, empower families to share concerns in a structured manner, and allow medical providers to address matters in real-time with interpreters present.
Finally, our participants highlighted the need for partnerships between providers and interpreter services, noting unclear roles and expectations among interpreters and medical providers as a major barrier. Specifically, physicians noted confusion regarding the scope of an interpreter’s practice. Participants from GLA sessions discussed the importance of a team-based approach and suggested implementing a “presession” prior to encounters with LEP patients and families. Presessions—a concept well accepted among interpreters and recommended by consensus-based practice guidelines—enable medical providers and interpreters to establish shared expectations about scope of practice, communication, interpretation style, time constraints, and medical context prior to patient encounters.32,33
There are several limitations to our study. First, individuals who chose to participate were likely highly motivated by their clinical experiences with LEP patients and invested in improving communication with LEP families. Second, the study is limited in generalizability, as it was conducted at a single academic institution in a Midwestern city. Despite regional variations in available resources as well as patient and workforce demographics, our findings regarding major themes are in agreement with previously published literature and further add to our understanding of ways to improve communication with this vulnerable population across the care spectrum. Lastly, we were logistically limited in our ability to elicit the perspectives of LEP families due to the participatory nature of GLA; the need for multiple interpreters to simultaneously interact with LEP individuals would have not only hindered active LEP family participation but may have also biased the data generated by patients and families, as the services interpreters provide during their inpatient stay was the focus of our study. Engaging LEP families in their preferred language using participatory methods should be considered for future studies.
In conclusion, frontline providers of medical and language services identified barriers and drivers impacting the effective use of interpreter services when communicating with LEP families during hospitalization. Our enhanced understanding of barriers and drivers, as well as identified actionable interventions, will inform future improvement of communication and interactions with LEP families that contributes to effective and efficient family centered care. A framework for the development and implementation of organizational strategies aimed at improving communication with LEP families must include a thorough assessment of impact, feasibility, stakeholder involvement, and sustainability of specific interventions. While there is no simple formula to improve language services, health systems should establish and adopt language access policies, standardize communication practices, and develop processes to optimize the use of language services in the hospital. Furthermore, engagement with LEP families to better understand their perceptions and experiences with the healthcare system is crucial to improve communication between medical providers and LEP families in the inpatient setting and should be the subject of future studies.
Disclosures
The authors have no conflicts of interest to disclose.
Funding
No external funding was secured for this study. Dr. Joanna Thomson is supported by the Agency for Healthcare Research and Quality (Grant #K08 HS025138). Dr. Raglin Bignall was supported through a Ruth L. Kirschstein National Research Service Award (T32HP10027) when the study was conducted. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding organizations. The funding organizations had no role in the design, preparation, review, or approval of this paper.
1. The American Academy of Pediatrics Council on Community Pediatrics. Providing care for immigrant, migrant, and border children. Pediatrics. 2013;131(6):e2028-e2034. PubMed
2. Meneses C, Chilton L, Duffee J, et al. Council on Community Pediatrics Immigrant Health Tool Kit. The American Academy of Pediatrics. https://www.aap.org/en-us/Documents/cocp_toolkit_full.pdf. Accessed May 13, 2019.
3. Office for Civil Rights. Guidance to Federal Financial Assistance Recipients Regarding Title VI and the Prohibition Against National Origin Discrimination Affecting Limited English Proficient Persons. https://www.hhs.gov/civil-rights/for-individuals/special-topics/limited-english-proficiency/guidance-federal-financial-assistance-recipients-title-vi/index.html. Accessed May 13, 2019.
4. Lion KC, Rafton SA, Shafii J, et al. Association between language, serious adverse events, and length of stay Among hospitalized children. Hosp Pediatr. 2013;3(3):219-225. https://doi.org/10.1542/hpeds.2012-0091.
5. Lion KC, Wright DR, Desai AD, Mangione-Smith R. Costs of care for hospitalized children associated With preferred language and insurance type. Hosp Pediatr. 2017;7(2):70-78. https://doi.org/10.1542/hpeds.2016-0051.
6. Cohen AL, Rivara F, Marcuse EK, McPhillips H, Davis R. Are language barriers associated with serious medical events in hospitalized pediatric patients? Pediatrics. 2005;116(3):575-579. https://doi.org/10.1542/peds.2005-0521.
7. Samuels-Kalow ME, Stack AM, Amico K, Porter SC. Parental language and return visits to the Emergency Department After discharge. Pediatr Emerg Care. 2017;33(6):402-404. https://doi.org/10.1097/PEC.0000000000000592.
8. Unaka NI, Statile AM, Choe A, Shonna Yin H. Addressing health literacy in the inpatient setting. Curr Treat Options Pediatr. 2018;4(2):283-299. https://doi.org/10.1007/s40746-018-0122-3.
9. DeCamp LR, Kuo DZ, Flores G, O’Connor K, Minkovitz CS. Changes in language services use by US pediatricians. Pediatrics. 2013;132(2):e396-e406. https://doi.org/10.1542/peds.2012-2909.
10. Flores G. The impact of medical interpreter services on the quality of health care: A systematic review. Med Care Res Rev. 2005;62(3):255-299. https://doi.org/10.1177/1077558705275416.
11. Flores G, Abreu M, Barone CP, Bachur R, Lin H. Errors of medical interpretation and their potential clinical consequences: A comparison of professional versus hoc versus no interpreters. Ann Emerg Med. 2012;60(5):545-553. https://doi.org/10.1016/j.annemergmed.2012.01.025.
12. Anand KJ, Sepanski RJ, Giles K, Shah SH, Juarez PD. Pediatric intensive care unit mortality among Latino children before and after a multilevel health care delivery intervention. JAMA Pediatr. 2015;169(4):383-390. https://doi.org/10.1001/jamapediatrics.2014.3789.
13. The Joint Commission. Advancing Effective Communication, Cultural Competence, and Patient- and Family-Centered Care: A Roadmap for Hospitals. Oakbrook Terrace, IL: The Joint Commission; 2010.
14. Hernandez RG, Cowden JD, Moon M et al. Predictors of resident satisfaction in caring for limited English proficient families: a multisite study. Acad Pediatr. 2014;14(2):173-180. https://doi.org/10.1016/j.acap.2013.12.002.
15. Vaughn LM, Lohmueller M. Calling all stakeholders: group-level assessment (GLA)-a qualitative and participatory method for large groups. Eval Rev. 2014;38(4):336-355. https://doi.org/10.1177/0193841X14544903.
16. Vaughn LM, Jacquez F, Zhao J, Lang M. Partnering with students to explore the health needs of an ethnically diverse, low-resource school: an innovative large group assessment approach. Fam Commun Health. 2011;34(1):72-84. https://doi.org/10.1097/FCH.0b013e3181fded12.
17. Gosdin CH, Vaughn L. Perceptions of physician bedside handoff with nurse and family involvement. Hosp Pediatr. 2012;2(1):34-38. https://doi.org/10.1542/hpeds.2011-0008-2.
18. Graham KE, Schellinger AR, Vaughn LM. Developing strategies for positive change: transitioning foster youth to adulthood. Child Youth Serv Rev. 2015;54:71-79. https://doi.org/10.1016/j.childyouth.2015.04.014.
19. Vaughn LM. Group level assessment: A Large Group Method for Identifying Primary Issues and Needs within a community. London2014. http://methods.sagepub.com/case/group-level-assessment-large-group-primary-issues-needs-community. Accessed 2017/07/26.
20. Association of American Medical Colleges Electronic Residency Application Service. ERAS 2018 MyERAS Application Worksheet: Language Fluency. Washington, DC:: Association of American Medical Colleges; 2018:5.
21. Brisset C, Leanza Y, Laforest K. Working with interpreters in health care: A systematic review and meta-ethnography of qualitative studies. Patient Educ Couns. 2013;91(2):131-140. https://doi.org/10.1016/j.pec.2012.11.008.
22. Wiking E, Saleh-Stattin N, Johansson SE, Sundquist J. A description of some aspects of the triangular meeting between immigrant patients, their interpreters and GPs in primary health care in Stockholm, Sweden. Fam Pract. 2009;26(5):377-383. https://doi.org/10.1093/fampra/cmp052.
23. Lion KC, Ebel BE, Rafton S et al. Evaluation of a quality improvement intervention to increase use of telephonic interpretation. Pediatrics. 2015;135(3):e709-e716. https://doi.org/10.1542/peds.2014-2024.
24. Zurca AD, Fisher KR, Flor RJ, et al. Communication with limited English-proficient families in the PICU. Hosp Pediatr. 2017;7(1):9-15. https://doi.org/10.1542/hpeds.2016-0071.
25. Kodjo C. Cultural competence in clinician communication. Pediatr Rev. 2009;30(2):57-64. https://doi.org/10.1542/pir.30-2-57.
26. Britton CV, American Academy of Pediatrics Committee on Pediatric Workforce. Ensuring culturally effective pediatric care: implications for education and health policy. Pediatrics. 2004;114(6):1677-1685. https://doi.org/10.1542/peds.2004-2091.
27. The American Academy of Pediatrics. Culturally Effective Care Toolkit: Providing Cuturally Effective Pediatric Care; 2018. https://www.aap.org/en-us/professional-resources/practice-transformation/managing-patients/Pages/effective-care.aspx. Accessed May 13, 2019.
28. Starmer AJ, Spector ND, Srivastava R, et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):1803-1812. https://doi.org/10.1056/NEJMsa1405556.
29. Jager AJ, Wynia MK. Who gets a teach-back? Patient-reported incidence of experiencing a teach-back. J Health Commun. 2012;17 Supplement 3:294-302. https://doi.org/10.1080/10810730.2012.712624.
30. Kornburger C, Gibson C, Sadowski S, Maletta K, Klingbeil C. Using “teach-back” to promote a safe transition from hospital to home: an evidence-based approach to improving the discharge process. J Pediatr Nurs. 2013;28(3):282-291. https://doi.org/10.1016/j.pedn.2012.10.007.
31. Abrams MA, Klass P, Dreyer BP. Health literacy and children: recommendations for action. Pediatrics. 2009;124 Supplement 3:S327-S331. https://doi.org/10.1542/peds.2009-1162I.
32. Betancourt JR, Renfrew MR, Green AR, Lopez L, Wasserman M. Improving Patient Safety Systems for Patients with Limited English Proficiency: a Guide for Hospitals. Agency for Healthcare Research and Quality; 2012. 33. The National Council on Interpreting in Health Care. Best Practices for Communicating Through an Interpreter . https://refugeehealthta.org/access-to-care/language-access/best-practices-communicating-through-an-interpreter/. Accessed May 19, 2019.
33. The National Council on Interpreting in Health Care. Best Practices for Communicating Through an Interpreter . https://refugeehealthta.org/access-to-care/language-access/best-practices-communicating-through-an-interpreter/. Accessed May 19, 2019.
1. The American Academy of Pediatrics Council on Community Pediatrics. Providing care for immigrant, migrant, and border children. Pediatrics. 2013;131(6):e2028-e2034. PubMed
2. Meneses C, Chilton L, Duffee J, et al. Council on Community Pediatrics Immigrant Health Tool Kit. The American Academy of Pediatrics. https://www.aap.org/en-us/Documents/cocp_toolkit_full.pdf. Accessed May 13, 2019.
3. Office for Civil Rights. Guidance to Federal Financial Assistance Recipients Regarding Title VI and the Prohibition Against National Origin Discrimination Affecting Limited English Proficient Persons. https://www.hhs.gov/civil-rights/for-individuals/special-topics/limited-english-proficiency/guidance-federal-financial-assistance-recipients-title-vi/index.html. Accessed May 13, 2019.
4. Lion KC, Rafton SA, Shafii J, et al. Association between language, serious adverse events, and length of stay Among hospitalized children. Hosp Pediatr. 2013;3(3):219-225. https://doi.org/10.1542/hpeds.2012-0091.
5. Lion KC, Wright DR, Desai AD, Mangione-Smith R. Costs of care for hospitalized children associated With preferred language and insurance type. Hosp Pediatr. 2017;7(2):70-78. https://doi.org/10.1542/hpeds.2016-0051.
6. Cohen AL, Rivara F, Marcuse EK, McPhillips H, Davis R. Are language barriers associated with serious medical events in hospitalized pediatric patients? Pediatrics. 2005;116(3):575-579. https://doi.org/10.1542/peds.2005-0521.
7. Samuels-Kalow ME, Stack AM, Amico K, Porter SC. Parental language and return visits to the Emergency Department After discharge. Pediatr Emerg Care. 2017;33(6):402-404. https://doi.org/10.1097/PEC.0000000000000592.
8. Unaka NI, Statile AM, Choe A, Shonna Yin H. Addressing health literacy in the inpatient setting. Curr Treat Options Pediatr. 2018;4(2):283-299. https://doi.org/10.1007/s40746-018-0122-3.
9. DeCamp LR, Kuo DZ, Flores G, O’Connor K, Minkovitz CS. Changes in language services use by US pediatricians. Pediatrics. 2013;132(2):e396-e406. https://doi.org/10.1542/peds.2012-2909.
10. Flores G. The impact of medical interpreter services on the quality of health care: A systematic review. Med Care Res Rev. 2005;62(3):255-299. https://doi.org/10.1177/1077558705275416.
11. Flores G, Abreu M, Barone CP, Bachur R, Lin H. Errors of medical interpretation and their potential clinical consequences: A comparison of professional versus hoc versus no interpreters. Ann Emerg Med. 2012;60(5):545-553. https://doi.org/10.1016/j.annemergmed.2012.01.025.
12. Anand KJ, Sepanski RJ, Giles K, Shah SH, Juarez PD. Pediatric intensive care unit mortality among Latino children before and after a multilevel health care delivery intervention. JAMA Pediatr. 2015;169(4):383-390. https://doi.org/10.1001/jamapediatrics.2014.3789.
13. The Joint Commission. Advancing Effective Communication, Cultural Competence, and Patient- and Family-Centered Care: A Roadmap for Hospitals. Oakbrook Terrace, IL: The Joint Commission; 2010.
14. Hernandez RG, Cowden JD, Moon M et al. Predictors of resident satisfaction in caring for limited English proficient families: a multisite study. Acad Pediatr. 2014;14(2):173-180. https://doi.org/10.1016/j.acap.2013.12.002.
15. Vaughn LM, Lohmueller M. Calling all stakeholders: group-level assessment (GLA)-a qualitative and participatory method for large groups. Eval Rev. 2014;38(4):336-355. https://doi.org/10.1177/0193841X14544903.
16. Vaughn LM, Jacquez F, Zhao J, Lang M. Partnering with students to explore the health needs of an ethnically diverse, low-resource school: an innovative large group assessment approach. Fam Commun Health. 2011;34(1):72-84. https://doi.org/10.1097/FCH.0b013e3181fded12.
17. Gosdin CH, Vaughn L. Perceptions of physician bedside handoff with nurse and family involvement. Hosp Pediatr. 2012;2(1):34-38. https://doi.org/10.1542/hpeds.2011-0008-2.
18. Graham KE, Schellinger AR, Vaughn LM. Developing strategies for positive change: transitioning foster youth to adulthood. Child Youth Serv Rev. 2015;54:71-79. https://doi.org/10.1016/j.childyouth.2015.04.014.
19. Vaughn LM. Group level assessment: A Large Group Method for Identifying Primary Issues and Needs within a community. London2014. http://methods.sagepub.com/case/group-level-assessment-large-group-primary-issues-needs-community. Accessed 2017/07/26.
20. Association of American Medical Colleges Electronic Residency Application Service. ERAS 2018 MyERAS Application Worksheet: Language Fluency. Washington, DC:: Association of American Medical Colleges; 2018:5.
21. Brisset C, Leanza Y, Laforest K. Working with interpreters in health care: A systematic review and meta-ethnography of qualitative studies. Patient Educ Couns. 2013;91(2):131-140. https://doi.org/10.1016/j.pec.2012.11.008.
22. Wiking E, Saleh-Stattin N, Johansson SE, Sundquist J. A description of some aspects of the triangular meeting between immigrant patients, their interpreters and GPs in primary health care in Stockholm, Sweden. Fam Pract. 2009;26(5):377-383. https://doi.org/10.1093/fampra/cmp052.
23. Lion KC, Ebel BE, Rafton S et al. Evaluation of a quality improvement intervention to increase use of telephonic interpretation. Pediatrics. 2015;135(3):e709-e716. https://doi.org/10.1542/peds.2014-2024.
24. Zurca AD, Fisher KR, Flor RJ, et al. Communication with limited English-proficient families in the PICU. Hosp Pediatr. 2017;7(1):9-15. https://doi.org/10.1542/hpeds.2016-0071.
25. Kodjo C. Cultural competence in clinician communication. Pediatr Rev. 2009;30(2):57-64. https://doi.org/10.1542/pir.30-2-57.
26. Britton CV, American Academy of Pediatrics Committee on Pediatric Workforce. Ensuring culturally effective pediatric care: implications for education and health policy. Pediatrics. 2004;114(6):1677-1685. https://doi.org/10.1542/peds.2004-2091.
27. The American Academy of Pediatrics. Culturally Effective Care Toolkit: Providing Cuturally Effective Pediatric Care; 2018. https://www.aap.org/en-us/professional-resources/practice-transformation/managing-patients/Pages/effective-care.aspx. Accessed May 13, 2019.
28. Starmer AJ, Spector ND, Srivastava R, et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):1803-1812. https://doi.org/10.1056/NEJMsa1405556.
29. Jager AJ, Wynia MK. Who gets a teach-back? Patient-reported incidence of experiencing a teach-back. J Health Commun. 2012;17 Supplement 3:294-302. https://doi.org/10.1080/10810730.2012.712624.
30. Kornburger C, Gibson C, Sadowski S, Maletta K, Klingbeil C. Using “teach-back” to promote a safe transition from hospital to home: an evidence-based approach to improving the discharge process. J Pediatr Nurs. 2013;28(3):282-291. https://doi.org/10.1016/j.pedn.2012.10.007.
31. Abrams MA, Klass P, Dreyer BP. Health literacy and children: recommendations for action. Pediatrics. 2009;124 Supplement 3:S327-S331. https://doi.org/10.1542/peds.2009-1162I.
32. Betancourt JR, Renfrew MR, Green AR, Lopez L, Wasserman M. Improving Patient Safety Systems for Patients with Limited English Proficiency: a Guide for Hospitals. Agency for Healthcare Research and Quality; 2012. 33. The National Council on Interpreting in Health Care. Best Practices for Communicating Through an Interpreter . https://refugeehealthta.org/access-to-care/language-access/best-practices-communicating-through-an-interpreter/. Accessed May 19, 2019.
33. The National Council on Interpreting in Health Care. Best Practices for Communicating Through an Interpreter . https://refugeehealthta.org/access-to-care/language-access/best-practices-communicating-through-an-interpreter/. Accessed May 19, 2019.
© 2019 Society of Hospital Medicine
Things We Do for No Reason: Systemic Corticosteroids for Wheezing in Preschool-Aged Children
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason” series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.

CASE PRESENTATION
A four-year-old girl, with a history of one wheezing episode, presents to the emergency department (ED) with wheezing, tachypnea, and respiratory distress. She receives three successive treatments of short-acting bronchodilators and is given one dose of dexamethasone, after which she improves significantly. Because of persistent tachypnea and wheezing, she is admitted for further management. By the next day she is much improved, now requiring bronchodilator treatment every four hours. She receives a second dose of dexamethasone to complete her steroid burst. Was the trajectory of this patient’s illness altered by treatment with systemic corticosteroids (SCS)? Is there any benefit to SCS treatment in a wheezing preschool-aged patient?
BACKGROUND
Wheezing is common in preschool-aged children (ages 2-5 years), with up to half in this age group having experienced a wheezing episode and up to one-third, recurrent wheezing.1,2 Young children with wheezing require ED visits and hospitalizations at much higher rates than older children and adults.3 Several studies have also demonstrated that children in this age group have higher rates of SCS prescriptions compared with older children.4,5 Despite the high prevalence of wheezing in this age group, there is great heterogeneity in the etiology and clinical progression of early childhood wheezing, with up to six described phenotypes each with varying levels of association with the development of asthma.6 Given the high frequency of asthma, preschool-aged children admitted with wheezing are often treated with SCS, as this is the standard of care for an acute asthma exacerbation.7
WHY YOU MIGHT THINK SYSTEMIC CORTICOSTEROIDS WOULD BE HELPFUL IN TREATING PRESCHOOL WHEEZE
The benefit of SCS in school-aged children and adolescents with multitrigger asthma exacerbation is well established and includes shorter time to resolution of acute illness and reduction in relapses.8 Because of these benefits, expert panels and regulatory agencies often include preschool-aged children in treatment recommendations for the older age groups.7,9,10 Consequently, apart from infants diagnosed with bronchiolitis, SCS remain a common and accepted treatment for young children presenting with asthma-like symptoms.4,5
Some data suggest that there may be clinical benefit from treatment with SCS in preschool children who wheeze. A recent trial by Foster et al. included 605 children, aged 24-72 months, presenting to a pediatric ED with wheeze plus viral upper respiratory symptoms.11 Patients were randomized to receive a three-day course of prednisolone (1 mg/kg) or placebo. The primary outcome was length of hospital stay until ready for discharge, which they found was significantly longer for placebo-treated patients (540 minutes) versus prednisolone (370 minutes).
WHY SYSTEMIC CORTICOSTEROIDS ARE NOT ROUTINELY HELPFUL IN PRESCHOOL CHILDREN WHO WHEEZE
There are few randomized controlled trials evaluating the efficacy of SCS in preschool-aged children with viral-induced wheezing, and these children are often grouped with younger or older children in studies. While limited in number, these studies have evaluated SCS efficacy with acute wheezing in preschool-aged children in outpatient, ED, and inpatient settings (Appendix Table).12-16 The majority of trials of SCS in this age group have shown mixed or negative results.
Admission rates for preschoolers with viral wheezing were not statistically different in those receiving oral prednisolone versus placebo in a study conducted by Oomen et al. evaluating outpatient, parent-initiated prednisolone.14 Tal et al. found overall benefit with reduced admission rate for patients treated in the ED with methylprednisolone versus placebo; however, this finding was not statistically significant in patients 24-54 months old.16
For those requiring hospitalization, length of hospital stay and time until readiness to discharge were the primary outcomes assessed by Panickar et al. and Jartti et al. Neither study found a statistically significant difference between groups who received oral prednisolone versus placebo for 3 or 5 days. Secondary outcomes such as symptom scores, symptom duration, albuterol use, and 60-day relapse rate were also not improved in those taking oral prednisolone compared with placebo.14,15
The mixed results of studies assessing the efficacy of SCS in preschool-aged wheezing children may be attributed to the fact that wheezing in this age group likely represents multiple underlying processes. Most acute wheezing at this age is not associated with atopy and is often triggered by viral respiratory tract infections.17 Furthermore, 90% of wheezing in children under the age of five years does not persist to the asthma phenotype (recurrent episodes with multiple triggers, airway obstruction, and hyper-responsiveness) once they reach school age.18
While SCS are generally not expensive, their use is not without cost. Studies of oral corticosteroid use in children with asthma have shown adverse effects including vomiting, hypertension, and impaired growth.19 Children with recurrent wheeze receiving SCS may demonstrate biochemical hypothalamic-pituitary-axis dysfunction.20 Given the high utilization and SCS prescription rates in this age group, reducing the use of SCS with wheezing episodes could have a large clinical and financial impact.3,4 These medications should be used judiciously in order to balance benefit with potential risks.
WHEN MIGHT SYSTEMIC CORTICOSTEROIDS BE HELPFUL IN WHEEZING PRESCHOOLERS
Given that there is diversity in the phenotype of preschool-aged children who wheeze, it is possible that a subset of these children would benefit from SCS. Some studies have shown that certain groups of patients derive benefit, including those with rhinovirus infection, eczema, and children at higher risk for multitrigger asthma.11,13 Children who have atopic wheeze are more likely to have persistent symptoms that may eventually be diagnosed as asthma.18 These children will have airway inflammation secondary to eosinophilic infiltration and may be responsive to SCS at times of exacerbation. However, attempts to classify preschool children based on risk of asthma have not shown consistent results.
The Asthma Predictive Index (API), a tool developed as a part of the Tucson Children’s Respiratory Study, uses clinical factors including history of wheeze, atopic dermatitis, and allergic rhinitis to determine a young child’s risk of having asthma symptoms after age six years.21 Jartti et al. and Panickar et al. used the API to stratify patients based on future asthma risk.13,15 The high risk group in the Jartti et al. study showed the benefit of SCS, while there was no benefit in the Panickar et al. study. When Oommen et al. also attempted to stratify asthma risk using levels of blood eosinophil proteins, which when elevated, are predictive of persistent wheeze.14 There was no difference in drug efficacy between patients with high and low blood eosinophil proteins. Although Foster et al. demonstrated shorter length of stay (LOS) with SCS overall, this was only seen in the subgroup with a previous diagnosis of asthma.
Patients presenting with severe disease (including those requiring critical care or with the highest symptom scores) have mostly been excluded from these studies. Although patients with severe disease often receive steroids, there is insufficient evidence of the efficacy of SCS in this population.12,13,15,22 Foster et al. did include patients with high symptom scores (although they excluded patients with “critical wheeze”) and found that the efficacy of SCS was clearest for those with severe presentations.11
Finally, some studies have demonstrated a virus-specific effect, with a reduction in time to readiness for discharge and reduction in recurrent wheeze in children treated with prednisolone who were positive for rhinovirus.12,23 Rhinovirus infection has also been associated with allergic sensitization and recurrent wheezing.23,24 However, rhinovirus-specific steroid responsiveness has not been consistently replicated by other investigators.11
WHAT YOU SHOULD DO INSTEAD
The majority of preschool-aged children presenting with wheeze in the care of hospitalists have mild to moderate symptoms, triggered by viral infections.22 It can be helpful to categorize the wheezing child as atopic or nonatopic. Laboratory studies such as allergen-specific IgE, peripheral eosinophil count, and exhaled nitric oxide can aid in predicting response to asthma medications and progression to the classic asthma phenotype.25 In the absence of these diagnostic studies, which are often costly and challenging to obtain in young children, a clinical score such as the API, or the recently validated Pediatric Asthma Risk Score (PARS), can help to assess future risk of developing multitrigger asthma.21,26 A positive API requires a history of more than three episodes of wheeze over the past year as well as one major (physician-diagnosed atopic dermatitis or parental asthma) or two minor (peripheral blood eosinophilia, physician-diagnosed allergic rhinitis, or wheezing apart from colds) criteria.17 It has a sensitivity of 57% and specificity of 81%.26 The PARS uses the presence of parental asthma, eczema, early wheezing, wheezing apart from colds, African-American race, and ≥2 positive skin prick tests to predict asthma. The sensitivity and specificity of PARS are similar to the API at 68% and 79%, respectively.26
Given the mixed results from studies evaluating the benefit of SCS in preschoolers with atopic symptoms and/or a positive API, evidence is lacking to guide decision-making in these children.13-15 However, it is reasonable to treat those at higher risk for future multitrigger asthma with SCS. There is also insufficient evidence to determine whether those with more severe disease or those infected with particular viral pathogens may benefit. Therefore, for the majority of children presenting with viral-induced wheezing, with a negative API or low PARS, there is no evidence that treatment with an SCS provides benefit in the form of reduced LOS, reduction in clinical symptoms, treatment failure, or relapse rate.
RECOMMENDATIONS
- Do not routinely treat with SCS preschool-aged children who have episodic wheezing triggered by viral respiratory tract infections and who do not have risk factors for persistent asthma.
- For preschool-aged children with a history of atopy, a positive API, or elevated PARS, SCS can be considered during admissions for respiratory distress and wheezing.
- Preschool-aged children presenting with severe disease or requiring intensive care may benefit from SCS, but there is insufficient evidence to conclude whether this practice provides benefit.
CONCLUSIONS
Current evidence does not support the routine use of SCS for preschool-aged children admitted for mild to moderate wheezing episodes. The majority of these children have viral episodic wheeze that does not develop into the asthma phenotype. For children with severe disease or at higher risk for asthma, SCS may be considered, although there remains insufficient evidence as to their efficacy. The patient in the introductory case lacks risk factors that would suggest SCS responsiveness (eg, positive API, previous asthma diagnosis, inhaled corticosteroid use, or severe disease) and would likely receive no clinical benefit from dexamethasone treatment.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing TWDFNR@hospitalmedicine.org.
Disclosures
Dr. Jennifer O’Toole consulted with and received honoraria payment from the I-PASS Patient Safety Institute. She also holds stock options in the I-PASS Patient Safety Institute, a nonpublicly traded company. Drs. Jones and Hubbell have nothing to disclose.
Funding
Dr. Thomson was supported by the Agency for Healthcare Research and Quality under award number K08HS025138.
1. Mallol J, Garcia-Marcos L, Sole D, Brand P, EISL Study Group. International prevalence of recurrent wheezing during the first year of life: variability, treatment patterns and use of health resources. Thorax. 2010;65(11):1004-1009. https://doi.org/10.1136/thx.2009.115188.
2. Bisgaard H, Szefler S. Prevalence of asthma-like symptoms in young children. Pediatric Pulmonol. 2007;48(8):723-728. https://doi.org/10.1002/ppul.20644.
3. Zahran HS, Bailey CM, Damon SA, Garbe PL, Breysse PN. Vital signs: asthma in children - United States, 2001-2016. MMWR Morb Mortal Wkly Rep. 2018;67(5):149-155. https://doi.org/10.15585/mmwr.mm6705e1.
4. Arabkhazaeli A, Vijverberg SJ, van der Ent CK, Raaijmakers JA, Maitland-van der Zee AH. High incidence of oral corticosteroids prescriptions in children with asthma in early childhood. J Asthma. 2016;53(10):1012-1017. https://doi.org/10.1080/02770903.2016.1185439.
5. Farber HJ, Silveira EA, Vicere DR, Kothari VD, Giardino AP. Oral corticosteroid prescribing for children with asthma in a medicaid managed care program. Pediatrics. 2017;139(5):139. https://doi.org/10.1542/peds.2016-4146.
6. Henderson J, Granell R, Heron J, et al. Associations of wheezing phenotypes in the first 6 years of life with atopy, lung function and airway responsiveness in mid-childhood. Thorax. 2008;63(11):974-980. https://doi.org/10.1136/thx.2007.093187.
7. National Asthma Education and Prevention Program. Expert Panel Report 3(EPR-3): Guidelines for the Diagnosis and Management of Asthma- Summary Report 2007. J Allergy Clin Immunol. 2007;120(5):S94-S138. https://doi.org/10.1016/j.jaci.2007.09.043.
8. Smith M, Iqbal S, Elliott TM, Everard M, Rowe BH. Corticosteroids for hospitalised children with acute asthma. Cochrane Database Syst Rev. 2003(2):CD002886. https://doi.org/10.1002/14651858.CD002886.
9. Pedersen SE, Hurd SS, Lemanske Rf Jr., et al. Global strategy for the diagnosis and management of asthma in children 5 years and younger. Pediatr Pulmonol. 2011;46(1):1-7. https://doi.org/10.1002/ppul.21321.
10. Bacharier LB, Boner A, Carlsen KH, et al. Diagnosis and treatment of asthma in childhood: a PRACTALL consensus report. Allergy. 2008;63(1):5-34. https://doi.org/10.1111/j.1398-9995.2007.01586.x.
11. Foster SJ, Cooper MN, Oosterhof S, Borland ML. Oral prednisolone in preschool children with virus-associated wheeze: a prospective, randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2018;6(2):97-106. https://doi.org/10.1016/S2213-2600(18)30008-0.
12. Jartti T, Lehtinen P, Vanto T, et al. Evaluation of the efficacy of prednisolone in early wheezing induced by rhinovirus or respiratory syncytial virus. Pediatr Infect Dis J. 2006;25(6):482-488. https://doi.org/10.1097/01.inf.0000215226.69696.0c.
13. Jartti T, Lehtinen P, Vanto T, et al. Atopic characteristics of wheezing children and responses to prednisolone. Pediatr Pulmonol. 2007;42(12):1125-1133. https://doi.org/10.1002/ppul.20706.
14. Oommen A, Lambert PC, Grigg J. Efficacy of a short course of parent-initiated oral prednisolone for viral wheeze in children aged 1–5 years: randomised controlled trial. Lancet. 2003;362(9394):1433-1438. https://doi.org/10.1016/S0140-6736(03)14685-5.
15. Panickar J, Lakhanpaul M, Lambert PC, et al. Oral prednisolone for preschool children with acute virus-induced wheezing. N Engl J Med. 2009;360(4):329-338. https://doi.org/10.1056/NEJMoa0804897.
16. Tal A, Levy N, Bearman JE. Methylprednisolone therapy for acute asthma in infants and toddlers: a controlled clinical trial. Pediatrics. 1990;86(3):350-356 .
17. Taussig LM, Wright AL, Holberg CJ, Halonen M, Morgan WJ, Martinez FD. Tucson children’s respiratory study: 1980 to present. J Allergy Clin Immunol. 2003;111(4):661-675. https://doi.org/10.1067/mai.2003.162.
18. Illi S, von Mutius E, Lau S, Niggemann B, Grüber C, Wahn U, Multicentre Allergy Study (MAS) group. Perennial allergen sensitisation early in life and chronic asthma in children: a birth cohort study. Lancet. 2006;368(9537):763-770. https://doi.org/10.1016/S0140-6736(06)69286-6.
19. Manson SC, Brown RE, Cerulli A, Vidaurre CF. The cumulative burden of oral corticosteroid side effects and the economic implications of steroid use. Respir Med. 2009;103(7):975-994. https://doi.org/10.1016/j.rmed.2009.01.003.
20. Barra CB, Fontes MJF, Cintra MTG, et al. Oral corticosteroids for asthma exacerbations might be associated with adrenal suppression: are physicians aware of that? Rev Assoc Med Bras. 2017;63(10):899-903. https://doi.org/10.1590/1806-9282.63.10.899..
21. Castro-Rodriguez JA, Holberg CJ, Wright AL, Martinez FD. A clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med. 2000;162(4):1403-1406. https://doi.org/10.1164/ajrccm.162.4.9912111.
22. Bush A, Grigg J, Saglani S. Managing wheeze in preschool children. BMJ. 2014;348:g15. https://doi.org/10.1136/bmj.g15.
23. Lukkarinen M, Lukkarinen H, Lehtinen P, Vuorinen T, Ruuskanen O, Jartti T. Prednisolone reduces recurrent wheezing after first rhinovirus wheeze: a 7-year follow-up. Pediatr Allergy Immunol. 2013;24(3):237-243. (1399-3038. https://doi.org/10.1111/pai.12046.
24. Jartti T, Kuusipalo H, Vuorinen T, et al. Allergic sensitization is associated with rhinovirus-, but not other virus-, induced wheezing in children. Pediatr Allergy Immunol. 2010;21(7):1008-1014. https://doi.org/10.1111/j.1399-3038.2010.01059.x.
25. Burbank AJ, Szefler SJ. Current and future management of the young child with early onset wheezing. Curr Opin Allergy Clin Immunol. 2017;17(2):146-152. https://doi.org/10.1097/ACI.0000000000000341
26. Myers JM, Schauberger E, He H, et al. A Pediatric Asthma Risk Score (PARS) to better predict asthma development in young children. J Allergy Clin Immunol. 2018;143(5):1803-1810.e2. https://doi.org/10.1016/j.jaci.2018.09.037.
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason” series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.

CASE PRESENTATION
A four-year-old girl, with a history of one wheezing episode, presents to the emergency department (ED) with wheezing, tachypnea, and respiratory distress. She receives three successive treatments of short-acting bronchodilators and is given one dose of dexamethasone, after which she improves significantly. Because of persistent tachypnea and wheezing, she is admitted for further management. By the next day she is much improved, now requiring bronchodilator treatment every four hours. She receives a second dose of dexamethasone to complete her steroid burst. Was the trajectory of this patient’s illness altered by treatment with systemic corticosteroids (SCS)? Is there any benefit to SCS treatment in a wheezing preschool-aged patient?
BACKGROUND
Wheezing is common in preschool-aged children (ages 2-5 years), with up to half in this age group having experienced a wheezing episode and up to one-third, recurrent wheezing.1,2 Young children with wheezing require ED visits and hospitalizations at much higher rates than older children and adults.3 Several studies have also demonstrated that children in this age group have higher rates of SCS prescriptions compared with older children.4,5 Despite the high prevalence of wheezing in this age group, there is great heterogeneity in the etiology and clinical progression of early childhood wheezing, with up to six described phenotypes each with varying levels of association with the development of asthma.6 Given the high frequency of asthma, preschool-aged children admitted with wheezing are often treated with SCS, as this is the standard of care for an acute asthma exacerbation.7
WHY YOU MIGHT THINK SYSTEMIC CORTICOSTEROIDS WOULD BE HELPFUL IN TREATING PRESCHOOL WHEEZE
The benefit of SCS in school-aged children and adolescents with multitrigger asthma exacerbation is well established and includes shorter time to resolution of acute illness and reduction in relapses.8 Because of these benefits, expert panels and regulatory agencies often include preschool-aged children in treatment recommendations for the older age groups.7,9,10 Consequently, apart from infants diagnosed with bronchiolitis, SCS remain a common and accepted treatment for young children presenting with asthma-like symptoms.4,5
Some data suggest that there may be clinical benefit from treatment with SCS in preschool children who wheeze. A recent trial by Foster et al. included 605 children, aged 24-72 months, presenting to a pediatric ED with wheeze plus viral upper respiratory symptoms.11 Patients were randomized to receive a three-day course of prednisolone (1 mg/kg) or placebo. The primary outcome was length of hospital stay until ready for discharge, which they found was significantly longer for placebo-treated patients (540 minutes) versus prednisolone (370 minutes).
WHY SYSTEMIC CORTICOSTEROIDS ARE NOT ROUTINELY HELPFUL IN PRESCHOOL CHILDREN WHO WHEEZE
There are few randomized controlled trials evaluating the efficacy of SCS in preschool-aged children with viral-induced wheezing, and these children are often grouped with younger or older children in studies. While limited in number, these studies have evaluated SCS efficacy with acute wheezing in preschool-aged children in outpatient, ED, and inpatient settings (Appendix Table).12-16 The majority of trials of SCS in this age group have shown mixed or negative results.
Admission rates for preschoolers with viral wheezing were not statistically different in those receiving oral prednisolone versus placebo in a study conducted by Oomen et al. evaluating outpatient, parent-initiated prednisolone.14 Tal et al. found overall benefit with reduced admission rate for patients treated in the ED with methylprednisolone versus placebo; however, this finding was not statistically significant in patients 24-54 months old.16
For those requiring hospitalization, length of hospital stay and time until readiness to discharge were the primary outcomes assessed by Panickar et al. and Jartti et al. Neither study found a statistically significant difference between groups who received oral prednisolone versus placebo for 3 or 5 days. Secondary outcomes such as symptom scores, symptom duration, albuterol use, and 60-day relapse rate were also not improved in those taking oral prednisolone compared with placebo.14,15
The mixed results of studies assessing the efficacy of SCS in preschool-aged wheezing children may be attributed to the fact that wheezing in this age group likely represents multiple underlying processes. Most acute wheezing at this age is not associated with atopy and is often triggered by viral respiratory tract infections.17 Furthermore, 90% of wheezing in children under the age of five years does not persist to the asthma phenotype (recurrent episodes with multiple triggers, airway obstruction, and hyper-responsiveness) once they reach school age.18
While SCS are generally not expensive, their use is not without cost. Studies of oral corticosteroid use in children with asthma have shown adverse effects including vomiting, hypertension, and impaired growth.19 Children with recurrent wheeze receiving SCS may demonstrate biochemical hypothalamic-pituitary-axis dysfunction.20 Given the high utilization and SCS prescription rates in this age group, reducing the use of SCS with wheezing episodes could have a large clinical and financial impact.3,4 These medications should be used judiciously in order to balance benefit with potential risks.
WHEN MIGHT SYSTEMIC CORTICOSTEROIDS BE HELPFUL IN WHEEZING PRESCHOOLERS
Given that there is diversity in the phenotype of preschool-aged children who wheeze, it is possible that a subset of these children would benefit from SCS. Some studies have shown that certain groups of patients derive benefit, including those with rhinovirus infection, eczema, and children at higher risk for multitrigger asthma.11,13 Children who have atopic wheeze are more likely to have persistent symptoms that may eventually be diagnosed as asthma.18 These children will have airway inflammation secondary to eosinophilic infiltration and may be responsive to SCS at times of exacerbation. However, attempts to classify preschool children based on risk of asthma have not shown consistent results.
The Asthma Predictive Index (API), a tool developed as a part of the Tucson Children’s Respiratory Study, uses clinical factors including history of wheeze, atopic dermatitis, and allergic rhinitis to determine a young child’s risk of having asthma symptoms after age six years.21 Jartti et al. and Panickar et al. used the API to stratify patients based on future asthma risk.13,15 The high risk group in the Jartti et al. study showed the benefit of SCS, while there was no benefit in the Panickar et al. study. When Oommen et al. also attempted to stratify asthma risk using levels of blood eosinophil proteins, which when elevated, are predictive of persistent wheeze.14 There was no difference in drug efficacy between patients with high and low blood eosinophil proteins. Although Foster et al. demonstrated shorter length of stay (LOS) with SCS overall, this was only seen in the subgroup with a previous diagnosis of asthma.
Patients presenting with severe disease (including those requiring critical care or with the highest symptom scores) have mostly been excluded from these studies. Although patients with severe disease often receive steroids, there is insufficient evidence of the efficacy of SCS in this population.12,13,15,22 Foster et al. did include patients with high symptom scores (although they excluded patients with “critical wheeze”) and found that the efficacy of SCS was clearest for those with severe presentations.11
Finally, some studies have demonstrated a virus-specific effect, with a reduction in time to readiness for discharge and reduction in recurrent wheeze in children treated with prednisolone who were positive for rhinovirus.12,23 Rhinovirus infection has also been associated with allergic sensitization and recurrent wheezing.23,24 However, rhinovirus-specific steroid responsiveness has not been consistently replicated by other investigators.11
WHAT YOU SHOULD DO INSTEAD
The majority of preschool-aged children presenting with wheeze in the care of hospitalists have mild to moderate symptoms, triggered by viral infections.22 It can be helpful to categorize the wheezing child as atopic or nonatopic. Laboratory studies such as allergen-specific IgE, peripheral eosinophil count, and exhaled nitric oxide can aid in predicting response to asthma medications and progression to the classic asthma phenotype.25 In the absence of these diagnostic studies, which are often costly and challenging to obtain in young children, a clinical score such as the API, or the recently validated Pediatric Asthma Risk Score (PARS), can help to assess future risk of developing multitrigger asthma.21,26 A positive API requires a history of more than three episodes of wheeze over the past year as well as one major (physician-diagnosed atopic dermatitis or parental asthma) or two minor (peripheral blood eosinophilia, physician-diagnosed allergic rhinitis, or wheezing apart from colds) criteria.17 It has a sensitivity of 57% and specificity of 81%.26 The PARS uses the presence of parental asthma, eczema, early wheezing, wheezing apart from colds, African-American race, and ≥2 positive skin prick tests to predict asthma. The sensitivity and specificity of PARS are similar to the API at 68% and 79%, respectively.26
Given the mixed results from studies evaluating the benefit of SCS in preschoolers with atopic symptoms and/or a positive API, evidence is lacking to guide decision-making in these children.13-15 However, it is reasonable to treat those at higher risk for future multitrigger asthma with SCS. There is also insufficient evidence to determine whether those with more severe disease or those infected with particular viral pathogens may benefit. Therefore, for the majority of children presenting with viral-induced wheezing, with a negative API or low PARS, there is no evidence that treatment with an SCS provides benefit in the form of reduced LOS, reduction in clinical symptoms, treatment failure, or relapse rate.
RECOMMENDATIONS
- Do not routinely treat with SCS preschool-aged children who have episodic wheezing triggered by viral respiratory tract infections and who do not have risk factors for persistent asthma.
- For preschool-aged children with a history of atopy, a positive API, or elevated PARS, SCS can be considered during admissions for respiratory distress and wheezing.
- Preschool-aged children presenting with severe disease or requiring intensive care may benefit from SCS, but there is insufficient evidence to conclude whether this practice provides benefit.
CONCLUSIONS
Current evidence does not support the routine use of SCS for preschool-aged children admitted for mild to moderate wheezing episodes. The majority of these children have viral episodic wheeze that does not develop into the asthma phenotype. For children with severe disease or at higher risk for asthma, SCS may be considered, although there remains insufficient evidence as to their efficacy. The patient in the introductory case lacks risk factors that would suggest SCS responsiveness (eg, positive API, previous asthma diagnosis, inhaled corticosteroid use, or severe disease) and would likely receive no clinical benefit from dexamethasone treatment.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing TWDFNR@hospitalmedicine.org.
Disclosures
Dr. Jennifer O’Toole consulted with and received honoraria payment from the I-PASS Patient Safety Institute. She also holds stock options in the I-PASS Patient Safety Institute, a nonpublicly traded company. Drs. Jones and Hubbell have nothing to disclose.
Funding
Dr. Thomson was supported by the Agency for Healthcare Research and Quality under award number K08HS025138.
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason” series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.

CASE PRESENTATION
A four-year-old girl, with a history of one wheezing episode, presents to the emergency department (ED) with wheezing, tachypnea, and respiratory distress. She receives three successive treatments of short-acting bronchodilators and is given one dose of dexamethasone, after which she improves significantly. Because of persistent tachypnea and wheezing, she is admitted for further management. By the next day she is much improved, now requiring bronchodilator treatment every four hours. She receives a second dose of dexamethasone to complete her steroid burst. Was the trajectory of this patient’s illness altered by treatment with systemic corticosteroids (SCS)? Is there any benefit to SCS treatment in a wheezing preschool-aged patient?
BACKGROUND
Wheezing is common in preschool-aged children (ages 2-5 years), with up to half in this age group having experienced a wheezing episode and up to one-third, recurrent wheezing.1,2 Young children with wheezing require ED visits and hospitalizations at much higher rates than older children and adults.3 Several studies have also demonstrated that children in this age group have higher rates of SCS prescriptions compared with older children.4,5 Despite the high prevalence of wheezing in this age group, there is great heterogeneity in the etiology and clinical progression of early childhood wheezing, with up to six described phenotypes each with varying levels of association with the development of asthma.6 Given the high frequency of asthma, preschool-aged children admitted with wheezing are often treated with SCS, as this is the standard of care for an acute asthma exacerbation.7
WHY YOU MIGHT THINK SYSTEMIC CORTICOSTEROIDS WOULD BE HELPFUL IN TREATING PRESCHOOL WHEEZE
The benefit of SCS in school-aged children and adolescents with multitrigger asthma exacerbation is well established and includes shorter time to resolution of acute illness and reduction in relapses.8 Because of these benefits, expert panels and regulatory agencies often include preschool-aged children in treatment recommendations for the older age groups.7,9,10 Consequently, apart from infants diagnosed with bronchiolitis, SCS remain a common and accepted treatment for young children presenting with asthma-like symptoms.4,5
Some data suggest that there may be clinical benefit from treatment with SCS in preschool children who wheeze. A recent trial by Foster et al. included 605 children, aged 24-72 months, presenting to a pediatric ED with wheeze plus viral upper respiratory symptoms.11 Patients were randomized to receive a three-day course of prednisolone (1 mg/kg) or placebo. The primary outcome was length of hospital stay until ready for discharge, which they found was significantly longer for placebo-treated patients (540 minutes) versus prednisolone (370 minutes).
WHY SYSTEMIC CORTICOSTEROIDS ARE NOT ROUTINELY HELPFUL IN PRESCHOOL CHILDREN WHO WHEEZE
There are few randomized controlled trials evaluating the efficacy of SCS in preschool-aged children with viral-induced wheezing, and these children are often grouped with younger or older children in studies. While limited in number, these studies have evaluated SCS efficacy with acute wheezing in preschool-aged children in outpatient, ED, and inpatient settings (Appendix Table).12-16 The majority of trials of SCS in this age group have shown mixed or negative results.
Admission rates for preschoolers with viral wheezing were not statistically different in those receiving oral prednisolone versus placebo in a study conducted by Oomen et al. evaluating outpatient, parent-initiated prednisolone.14 Tal et al. found overall benefit with reduced admission rate for patients treated in the ED with methylprednisolone versus placebo; however, this finding was not statistically significant in patients 24-54 months old.16
For those requiring hospitalization, length of hospital stay and time until readiness to discharge were the primary outcomes assessed by Panickar et al. and Jartti et al. Neither study found a statistically significant difference between groups who received oral prednisolone versus placebo for 3 or 5 days. Secondary outcomes such as symptom scores, symptom duration, albuterol use, and 60-day relapse rate were also not improved in those taking oral prednisolone compared with placebo.14,15
The mixed results of studies assessing the efficacy of SCS in preschool-aged wheezing children may be attributed to the fact that wheezing in this age group likely represents multiple underlying processes. Most acute wheezing at this age is not associated with atopy and is often triggered by viral respiratory tract infections.17 Furthermore, 90% of wheezing in children under the age of five years does not persist to the asthma phenotype (recurrent episodes with multiple triggers, airway obstruction, and hyper-responsiveness) once they reach school age.18
While SCS are generally not expensive, their use is not without cost. Studies of oral corticosteroid use in children with asthma have shown adverse effects including vomiting, hypertension, and impaired growth.19 Children with recurrent wheeze receiving SCS may demonstrate biochemical hypothalamic-pituitary-axis dysfunction.20 Given the high utilization and SCS prescription rates in this age group, reducing the use of SCS with wheezing episodes could have a large clinical and financial impact.3,4 These medications should be used judiciously in order to balance benefit with potential risks.
WHEN MIGHT SYSTEMIC CORTICOSTEROIDS BE HELPFUL IN WHEEZING PRESCHOOLERS
Given that there is diversity in the phenotype of preschool-aged children who wheeze, it is possible that a subset of these children would benefit from SCS. Some studies have shown that certain groups of patients derive benefit, including those with rhinovirus infection, eczema, and children at higher risk for multitrigger asthma.11,13 Children who have atopic wheeze are more likely to have persistent symptoms that may eventually be diagnosed as asthma.18 These children will have airway inflammation secondary to eosinophilic infiltration and may be responsive to SCS at times of exacerbation. However, attempts to classify preschool children based on risk of asthma have not shown consistent results.
The Asthma Predictive Index (API), a tool developed as a part of the Tucson Children’s Respiratory Study, uses clinical factors including history of wheeze, atopic dermatitis, and allergic rhinitis to determine a young child’s risk of having asthma symptoms after age six years.21 Jartti et al. and Panickar et al. used the API to stratify patients based on future asthma risk.13,15 The high risk group in the Jartti et al. study showed the benefit of SCS, while there was no benefit in the Panickar et al. study. When Oommen et al. also attempted to stratify asthma risk using levels of blood eosinophil proteins, which when elevated, are predictive of persistent wheeze.14 There was no difference in drug efficacy between patients with high and low blood eosinophil proteins. Although Foster et al. demonstrated shorter length of stay (LOS) with SCS overall, this was only seen in the subgroup with a previous diagnosis of asthma.
Patients presenting with severe disease (including those requiring critical care or with the highest symptom scores) have mostly been excluded from these studies. Although patients with severe disease often receive steroids, there is insufficient evidence of the efficacy of SCS in this population.12,13,15,22 Foster et al. did include patients with high symptom scores (although they excluded patients with “critical wheeze”) and found that the efficacy of SCS was clearest for those with severe presentations.11
Finally, some studies have demonstrated a virus-specific effect, with a reduction in time to readiness for discharge and reduction in recurrent wheeze in children treated with prednisolone who were positive for rhinovirus.12,23 Rhinovirus infection has also been associated with allergic sensitization and recurrent wheezing.23,24 However, rhinovirus-specific steroid responsiveness has not been consistently replicated by other investigators.11
WHAT YOU SHOULD DO INSTEAD
The majority of preschool-aged children presenting with wheeze in the care of hospitalists have mild to moderate symptoms, triggered by viral infections.22 It can be helpful to categorize the wheezing child as atopic or nonatopic. Laboratory studies such as allergen-specific IgE, peripheral eosinophil count, and exhaled nitric oxide can aid in predicting response to asthma medications and progression to the classic asthma phenotype.25 In the absence of these diagnostic studies, which are often costly and challenging to obtain in young children, a clinical score such as the API, or the recently validated Pediatric Asthma Risk Score (PARS), can help to assess future risk of developing multitrigger asthma.21,26 A positive API requires a history of more than three episodes of wheeze over the past year as well as one major (physician-diagnosed atopic dermatitis or parental asthma) or two minor (peripheral blood eosinophilia, physician-diagnosed allergic rhinitis, or wheezing apart from colds) criteria.17 It has a sensitivity of 57% and specificity of 81%.26 The PARS uses the presence of parental asthma, eczema, early wheezing, wheezing apart from colds, African-American race, and ≥2 positive skin prick tests to predict asthma. The sensitivity and specificity of PARS are similar to the API at 68% and 79%, respectively.26
Given the mixed results from studies evaluating the benefit of SCS in preschoolers with atopic symptoms and/or a positive API, evidence is lacking to guide decision-making in these children.13-15 However, it is reasonable to treat those at higher risk for future multitrigger asthma with SCS. There is also insufficient evidence to determine whether those with more severe disease or those infected with particular viral pathogens may benefit. Therefore, for the majority of children presenting with viral-induced wheezing, with a negative API or low PARS, there is no evidence that treatment with an SCS provides benefit in the form of reduced LOS, reduction in clinical symptoms, treatment failure, or relapse rate.
RECOMMENDATIONS
- Do not routinely treat with SCS preschool-aged children who have episodic wheezing triggered by viral respiratory tract infections and who do not have risk factors for persistent asthma.
- For preschool-aged children with a history of atopy, a positive API, or elevated PARS, SCS can be considered during admissions for respiratory distress and wheezing.
- Preschool-aged children presenting with severe disease or requiring intensive care may benefit from SCS, but there is insufficient evidence to conclude whether this practice provides benefit.
CONCLUSIONS
Current evidence does not support the routine use of SCS for preschool-aged children admitted for mild to moderate wheezing episodes. The majority of these children have viral episodic wheeze that does not develop into the asthma phenotype. For children with severe disease or at higher risk for asthma, SCS may be considered, although there remains insufficient evidence as to their efficacy. The patient in the introductory case lacks risk factors that would suggest SCS responsiveness (eg, positive API, previous asthma diagnosis, inhaled corticosteroid use, or severe disease) and would likely receive no clinical benefit from dexamethasone treatment.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing TWDFNR@hospitalmedicine.org.
Disclosures
Dr. Jennifer O’Toole consulted with and received honoraria payment from the I-PASS Patient Safety Institute. She also holds stock options in the I-PASS Patient Safety Institute, a nonpublicly traded company. Drs. Jones and Hubbell have nothing to disclose.
Funding
Dr. Thomson was supported by the Agency for Healthcare Research and Quality under award number K08HS025138.
1. Mallol J, Garcia-Marcos L, Sole D, Brand P, EISL Study Group. International prevalence of recurrent wheezing during the first year of life: variability, treatment patterns and use of health resources. Thorax. 2010;65(11):1004-1009. https://doi.org/10.1136/thx.2009.115188.
2. Bisgaard H, Szefler S. Prevalence of asthma-like symptoms in young children. Pediatric Pulmonol. 2007;48(8):723-728. https://doi.org/10.1002/ppul.20644.
3. Zahran HS, Bailey CM, Damon SA, Garbe PL, Breysse PN. Vital signs: asthma in children - United States, 2001-2016. MMWR Morb Mortal Wkly Rep. 2018;67(5):149-155. https://doi.org/10.15585/mmwr.mm6705e1.
4. Arabkhazaeli A, Vijverberg SJ, van der Ent CK, Raaijmakers JA, Maitland-van der Zee AH. High incidence of oral corticosteroids prescriptions in children with asthma in early childhood. J Asthma. 2016;53(10):1012-1017. https://doi.org/10.1080/02770903.2016.1185439.
5. Farber HJ, Silveira EA, Vicere DR, Kothari VD, Giardino AP. Oral corticosteroid prescribing for children with asthma in a medicaid managed care program. Pediatrics. 2017;139(5):139. https://doi.org/10.1542/peds.2016-4146.
6. Henderson J, Granell R, Heron J, et al. Associations of wheezing phenotypes in the first 6 years of life with atopy, lung function and airway responsiveness in mid-childhood. Thorax. 2008;63(11):974-980. https://doi.org/10.1136/thx.2007.093187.
7. National Asthma Education and Prevention Program. Expert Panel Report 3(EPR-3): Guidelines for the Diagnosis and Management of Asthma- Summary Report 2007. J Allergy Clin Immunol. 2007;120(5):S94-S138. https://doi.org/10.1016/j.jaci.2007.09.043.
8. Smith M, Iqbal S, Elliott TM, Everard M, Rowe BH. Corticosteroids for hospitalised children with acute asthma. Cochrane Database Syst Rev. 2003(2):CD002886. https://doi.org/10.1002/14651858.CD002886.
9. Pedersen SE, Hurd SS, Lemanske Rf Jr., et al. Global strategy for the diagnosis and management of asthma in children 5 years and younger. Pediatr Pulmonol. 2011;46(1):1-7. https://doi.org/10.1002/ppul.21321.
10. Bacharier LB, Boner A, Carlsen KH, et al. Diagnosis and treatment of asthma in childhood: a PRACTALL consensus report. Allergy. 2008;63(1):5-34. https://doi.org/10.1111/j.1398-9995.2007.01586.x.
11. Foster SJ, Cooper MN, Oosterhof S, Borland ML. Oral prednisolone in preschool children with virus-associated wheeze: a prospective, randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2018;6(2):97-106. https://doi.org/10.1016/S2213-2600(18)30008-0.
12. Jartti T, Lehtinen P, Vanto T, et al. Evaluation of the efficacy of prednisolone in early wheezing induced by rhinovirus or respiratory syncytial virus. Pediatr Infect Dis J. 2006;25(6):482-488. https://doi.org/10.1097/01.inf.0000215226.69696.0c.
13. Jartti T, Lehtinen P, Vanto T, et al. Atopic characteristics of wheezing children and responses to prednisolone. Pediatr Pulmonol. 2007;42(12):1125-1133. https://doi.org/10.1002/ppul.20706.
14. Oommen A, Lambert PC, Grigg J. Efficacy of a short course of parent-initiated oral prednisolone for viral wheeze in children aged 1–5 years: randomised controlled trial. Lancet. 2003;362(9394):1433-1438. https://doi.org/10.1016/S0140-6736(03)14685-5.
15. Panickar J, Lakhanpaul M, Lambert PC, et al. Oral prednisolone for preschool children with acute virus-induced wheezing. N Engl J Med. 2009;360(4):329-338. https://doi.org/10.1056/NEJMoa0804897.
16. Tal A, Levy N, Bearman JE. Methylprednisolone therapy for acute asthma in infants and toddlers: a controlled clinical trial. Pediatrics. 1990;86(3):350-356 .
17. Taussig LM, Wright AL, Holberg CJ, Halonen M, Morgan WJ, Martinez FD. Tucson children’s respiratory study: 1980 to present. J Allergy Clin Immunol. 2003;111(4):661-675. https://doi.org/10.1067/mai.2003.162.
18. Illi S, von Mutius E, Lau S, Niggemann B, Grüber C, Wahn U, Multicentre Allergy Study (MAS) group. Perennial allergen sensitisation early in life and chronic asthma in children: a birth cohort study. Lancet. 2006;368(9537):763-770. https://doi.org/10.1016/S0140-6736(06)69286-6.
19. Manson SC, Brown RE, Cerulli A, Vidaurre CF. The cumulative burden of oral corticosteroid side effects and the economic implications of steroid use. Respir Med. 2009;103(7):975-994. https://doi.org/10.1016/j.rmed.2009.01.003.
20. Barra CB, Fontes MJF, Cintra MTG, et al. Oral corticosteroids for asthma exacerbations might be associated with adrenal suppression: are physicians aware of that? Rev Assoc Med Bras. 2017;63(10):899-903. https://doi.org/10.1590/1806-9282.63.10.899..
21. Castro-Rodriguez JA, Holberg CJ, Wright AL, Martinez FD. A clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med. 2000;162(4):1403-1406. https://doi.org/10.1164/ajrccm.162.4.9912111.
22. Bush A, Grigg J, Saglani S. Managing wheeze in preschool children. BMJ. 2014;348:g15. https://doi.org/10.1136/bmj.g15.
23. Lukkarinen M, Lukkarinen H, Lehtinen P, Vuorinen T, Ruuskanen O, Jartti T. Prednisolone reduces recurrent wheezing after first rhinovirus wheeze: a 7-year follow-up. Pediatr Allergy Immunol. 2013;24(3):237-243. (1399-3038. https://doi.org/10.1111/pai.12046.
24. Jartti T, Kuusipalo H, Vuorinen T, et al. Allergic sensitization is associated with rhinovirus-, but not other virus-, induced wheezing in children. Pediatr Allergy Immunol. 2010;21(7):1008-1014. https://doi.org/10.1111/j.1399-3038.2010.01059.x.
25. Burbank AJ, Szefler SJ. Current and future management of the young child with early onset wheezing. Curr Opin Allergy Clin Immunol. 2017;17(2):146-152. https://doi.org/10.1097/ACI.0000000000000341
26. Myers JM, Schauberger E, He H, et al. A Pediatric Asthma Risk Score (PARS) to better predict asthma development in young children. J Allergy Clin Immunol. 2018;143(5):1803-1810.e2. https://doi.org/10.1016/j.jaci.2018.09.037.
1. Mallol J, Garcia-Marcos L, Sole D, Brand P, EISL Study Group. International prevalence of recurrent wheezing during the first year of life: variability, treatment patterns and use of health resources. Thorax. 2010;65(11):1004-1009. https://doi.org/10.1136/thx.2009.115188.
2. Bisgaard H, Szefler S. Prevalence of asthma-like symptoms in young children. Pediatric Pulmonol. 2007;48(8):723-728. https://doi.org/10.1002/ppul.20644.
3. Zahran HS, Bailey CM, Damon SA, Garbe PL, Breysse PN. Vital signs: asthma in children - United States, 2001-2016. MMWR Morb Mortal Wkly Rep. 2018;67(5):149-155. https://doi.org/10.15585/mmwr.mm6705e1.
4. Arabkhazaeli A, Vijverberg SJ, van der Ent CK, Raaijmakers JA, Maitland-van der Zee AH. High incidence of oral corticosteroids prescriptions in children with asthma in early childhood. J Asthma. 2016;53(10):1012-1017. https://doi.org/10.1080/02770903.2016.1185439.
5. Farber HJ, Silveira EA, Vicere DR, Kothari VD, Giardino AP. Oral corticosteroid prescribing for children with asthma in a medicaid managed care program. Pediatrics. 2017;139(5):139. https://doi.org/10.1542/peds.2016-4146.
6. Henderson J, Granell R, Heron J, et al. Associations of wheezing phenotypes in the first 6 years of life with atopy, lung function and airway responsiveness in mid-childhood. Thorax. 2008;63(11):974-980. https://doi.org/10.1136/thx.2007.093187.
7. National Asthma Education and Prevention Program. Expert Panel Report 3(EPR-3): Guidelines for the Diagnosis and Management of Asthma- Summary Report 2007. J Allergy Clin Immunol. 2007;120(5):S94-S138. https://doi.org/10.1016/j.jaci.2007.09.043.
8. Smith M, Iqbal S, Elliott TM, Everard M, Rowe BH. Corticosteroids for hospitalised children with acute asthma. Cochrane Database Syst Rev. 2003(2):CD002886. https://doi.org/10.1002/14651858.CD002886.
9. Pedersen SE, Hurd SS, Lemanske Rf Jr., et al. Global strategy for the diagnosis and management of asthma in children 5 years and younger. Pediatr Pulmonol. 2011;46(1):1-7. https://doi.org/10.1002/ppul.21321.
10. Bacharier LB, Boner A, Carlsen KH, et al. Diagnosis and treatment of asthma in childhood: a PRACTALL consensus report. Allergy. 2008;63(1):5-34. https://doi.org/10.1111/j.1398-9995.2007.01586.x.
11. Foster SJ, Cooper MN, Oosterhof S, Borland ML. Oral prednisolone in preschool children with virus-associated wheeze: a prospective, randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2018;6(2):97-106. https://doi.org/10.1016/S2213-2600(18)30008-0.
12. Jartti T, Lehtinen P, Vanto T, et al. Evaluation of the efficacy of prednisolone in early wheezing induced by rhinovirus or respiratory syncytial virus. Pediatr Infect Dis J. 2006;25(6):482-488. https://doi.org/10.1097/01.inf.0000215226.69696.0c.
13. Jartti T, Lehtinen P, Vanto T, et al. Atopic characteristics of wheezing children and responses to prednisolone. Pediatr Pulmonol. 2007;42(12):1125-1133. https://doi.org/10.1002/ppul.20706.
14. Oommen A, Lambert PC, Grigg J. Efficacy of a short course of parent-initiated oral prednisolone for viral wheeze in children aged 1–5 years: randomised controlled trial. Lancet. 2003;362(9394):1433-1438. https://doi.org/10.1016/S0140-6736(03)14685-5.
15. Panickar J, Lakhanpaul M, Lambert PC, et al. Oral prednisolone for preschool children with acute virus-induced wheezing. N Engl J Med. 2009;360(4):329-338. https://doi.org/10.1056/NEJMoa0804897.
16. Tal A, Levy N, Bearman JE. Methylprednisolone therapy for acute asthma in infants and toddlers: a controlled clinical trial. Pediatrics. 1990;86(3):350-356 .
17. Taussig LM, Wright AL, Holberg CJ, Halonen M, Morgan WJ, Martinez FD. Tucson children’s respiratory study: 1980 to present. J Allergy Clin Immunol. 2003;111(4):661-675. https://doi.org/10.1067/mai.2003.162.
18. Illi S, von Mutius E, Lau S, Niggemann B, Grüber C, Wahn U, Multicentre Allergy Study (MAS) group. Perennial allergen sensitisation early in life and chronic asthma in children: a birth cohort study. Lancet. 2006;368(9537):763-770. https://doi.org/10.1016/S0140-6736(06)69286-6.
19. Manson SC, Brown RE, Cerulli A, Vidaurre CF. The cumulative burden of oral corticosteroid side effects and the economic implications of steroid use. Respir Med. 2009;103(7):975-994. https://doi.org/10.1016/j.rmed.2009.01.003.
20. Barra CB, Fontes MJF, Cintra MTG, et al. Oral corticosteroids for asthma exacerbations might be associated with adrenal suppression: are physicians aware of that? Rev Assoc Med Bras. 2017;63(10):899-903. https://doi.org/10.1590/1806-9282.63.10.899..
21. Castro-Rodriguez JA, Holberg CJ, Wright AL, Martinez FD. A clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med. 2000;162(4):1403-1406. https://doi.org/10.1164/ajrccm.162.4.9912111.
22. Bush A, Grigg J, Saglani S. Managing wheeze in preschool children. BMJ. 2014;348:g15. https://doi.org/10.1136/bmj.g15.
23. Lukkarinen M, Lukkarinen H, Lehtinen P, Vuorinen T, Ruuskanen O, Jartti T. Prednisolone reduces recurrent wheezing after first rhinovirus wheeze: a 7-year follow-up. Pediatr Allergy Immunol. 2013;24(3):237-243. (1399-3038. https://doi.org/10.1111/pai.12046.
24. Jartti T, Kuusipalo H, Vuorinen T, et al. Allergic sensitization is associated with rhinovirus-, but not other virus-, induced wheezing in children. Pediatr Allergy Immunol. 2010;21(7):1008-1014. https://doi.org/10.1111/j.1399-3038.2010.01059.x.
25. Burbank AJ, Szefler SJ. Current and future management of the young child with early onset wheezing. Curr Opin Allergy Clin Immunol. 2017;17(2):146-152. https://doi.org/10.1097/ACI.0000000000000341
26. Myers JM, Schauberger E, He H, et al. A Pediatric Asthma Risk Score (PARS) to better predict asthma development in young children. J Allergy Clin Immunol. 2018;143(5):1803-1810.e2. https://doi.org/10.1016/j.jaci.2018.09.037.
© 2019 Society of Hospital Medicine
High-Goal ‘Lytes: Repletion Gone Awry?
Electrolyte imbalances, per se, predispose to ventricular ectopy and, in extreme cases, sudden cardiac death.1 As these outcomes are more common in the presence of intrinsic heart disease, serum electrolytes—particularly potassium and magnesium—are routinely monitored and made replete in patients with myocardial infarction (MI) or acute decompensated heart failure (ADHF).
Patients hospitalized with ADHF often present with metabolic derangements and varying degrees of chronic adaptations in their renin–angiotensin–aldosterone system.1,2 In addition, during an ADHF hospitalization, they are subjected to guideline-directed medical therapy (GDMT), commonly in escalating doses, that exhibit well-established effects on serum potassium levels, including diuretics, angiotensin-converting-enzyme inhibitors, angiotensin receptor blockers, beta blockers, and mineralocorticoid receptor antagonists. Thus, there are myriad ways patients hospitalized for ADHF might experience electrolyte abnormalities.
In this issue of the Journal of Hospital Medicine, O’Sullivan et al. explore the associations between mean 72-hour serum potassium and important clinical outcomes—in-hospital mortality, transfer to an intensive care unit (ICU), and length of stay (LOS)—among patients with normal admission serum potassium hospitalized for ADHF.3 Through a retrospective review of electronic records from 116 hospitals, the authors identified 4,995 initially normokalemic heart failure (HF; identified by ICD-9 codes) patients and grouped them into low-normal (3.5-4.0 mEq/L), normal (4.0-4.5 mEq/L), and high-normal (4.5-5.0 mEq/L) potassium groups.3 Adjustments were made for composite scores encapsulating other lab abnormalities and comorbidities.
Over the 72-hour exposure window, the authors observed no statistically significant difference in mortality, ICU transfer, or LOS between the low-normal and normal potassium groups.3 Moreover, in a sensitivity analysis of patients who did not receive potassium supplementation, there remained statistically similar rates of mortality, ICU transfer, and LOS.3 Together, these findings suggest that maintenance of potassium >4 mEq/L may not be efficacious for preventing in-hospital complications of ADHF.3 In fact, they observed more frequent mortality and ICU transfer in patients who had high-normal potassium. This group, however, had a higher burden of chronic kidney disease and illness severity on presentation and was less likely to receive supplemental potassium.3
ADHF accounts for more than one million hospital admissions annually with one in four patients readmitted within 30 days; estimated costs surpass $30 billion.2 Reducing unnecessary expenditures in the management of HF through evidence-based guidelines is paramount. Electrolyte repletion in the setting of ADHF may represent one such opportunity by reducing excess phlebotomy, laboratory services, and potassium supplementation. Patient experience may also improve from curbing these cumbersome practices. While society guidelines endorse potassium repletion in MI to reduce the risk of ventricular arrhythmia,4 there is no uniform consensus in ADHF. As the authors cite, existing data regarding ideal potassium levels in patients with ADHF is lacking, with current evidence drawn from small observational studies. The present study, being much larger in size and being linked with observed rates of active potassium supplementation, provides some of the strongest evidence to date that a potassium goal of >4 mEq/L may not be efficacious at reducing ADHF-related complications in the generalized HF population.
While it remains uncertain if avoiding low-normal potassium levels in ADHF is beneficial, over the long term, intermediate-range potassium levels are clearly associated with the lowest HF-related mortality. In a study of over 2,000 HF patients who underwent longitudinal potassium monitoring, mortality was distributed along a U-shaped curve with highest mortality at the extremes of kalemia and a nadir at a level of 4.3 mEq/L.5
A major limitation of the present study is that it does not account for variability within the ADHF population. Firstly, knowledge regarding the use of GDMT, which not only affects serum potassium (all GDMTs) but also reduces the likelihood of arrhythmias (beta blockers), would have been informative. Moreover, the authors do not have access to data regarding incident arrhythmia and instead use ICU admission as a surrogate. In addition, ADHF patients in this study varied greatly in illness severity, ranging from those receiving initial therapy with loop diuretics alone to those requiring augmentation with thiazides and even the use of temporary mechanical circulatory support.3 Escalating loop diuretic or metolazone use not only is associated with increased mortality6 but often results in impressive natriuresis and, potentially dangerous, kaliuresis secondary to the sequential nephron blockade.7 Those who underwent extensive potassium swings in the study may not be appropriately captured using 72-hour serum potassium averages. Additionally, this study did not assess for quantity of diuresis, which is known to affect serum potassium values. It is possible that those with low-normal potassium represent patients who underwent more effective diuresis and therefore were discharged sooner. Adding to the variability, ADHF in this study encompassed both systolic (HF with a reduced ejection fraction) and diastolic (HF with a preserved ejection fraction) HF although, perhaps not surprisingly, there were marked differences in the HF subtype by potassium group—the proportions with only diastolic dysfunction were 37.1%, 39.0%, and 45.8% in the low-normal, normal, and high-normal groups, respectively (P = .0174).3 Given the known heterogeneity between these two HF subtypes,8 particularly with respect to their response to mortality-reducing GDMT,2,8 the results may be significantly confounded.
Relatedly, by excluding initially hypokalemic patients, the authors have lost considerable power and broad generalizability as these patients likely represent those at greatest risk of recurrent hypokalemia and its attendant complications during admission.
This study should be lauded for critically appraising the ubiquitous practice of electrolyte repletion. The authors present compelling preliminary data suggesting that maintenance of potassium >4 mEq/L in the general ADHF population is not efficacious at preventing ADHF complications and, as a corollary, is likely not cost-effective. However, we agree with the authors that a randomized controlled trial will be needed to change clinical practice. Ideally, such a study would account for HF subtype and GDMT use and could compare rates of arrhythmia, AHDF-related death, and all-cause mortality in patients maintained to goal normokalemia (>3.5 mEq/L) versus “high
Disclosures
Dr. Blaha reports grants from NIH, grants from FDA, grants from AHA, grants and personal fees from Amgen Foundation, grants from Aetna Foundation, personal fees from Sanofi, personal fees from Regeneron, and personal fees from Novartis, from Novo Nordisk, and from Bayer, outside the submitted work. Dr. Dudum and Dr. Lahti have nothing to disclose.
1. Packer M, Gottlieb SS, Blum MA. Immediate and long-term pathophysiologic mechanisms underlying the genesis of sudden cardiac death in patients with congestive heart failure. Am J Med. 1987;82(3):4-10. https://doi.org/10.1016/0002-9343(87)90126-4.
2. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147-e239. https://doi.org/10.1016/j.jacc.2013.05.019.
3. O’Sullivan KF, Kashef MA, Knee AB, et al. Examining the “Repletion Reflex”: the association between serum potassium and outcomes in hospitalized patients with HF. J Hosp Med. 14(12);729-736. https://doi.org/10.12788/jhm.3270.
4. Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction--executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1999 Guidelines for the Management of Patients With Acute Myocardial Infarction). Circulation 2004;110(5):588-636. https://doi.org/10.1161/01.CIR.0000134791.68010.FA
5. Nunez J, Bayes-Genis A, Zannad F, et al. Long-Term Potassium Monitoring and Dynamics in Heart Failure and Risk of Mortality. Circulation 2018;137(13):1320-1330. https://doi.org/10.1161/CIRCULATIONAHA.117.030576.
6. Neuberg GW, Miller AB, O’Connor CM, et al. Diuretic resistance predicts mortality in patients with advanced heart failure. Am Heart J. 2002;144(1):31-38. https://doi.org/10.1067/mhj.2002.123144
7. Jentzer JC, DeWald TA, Hernandez AF. Combination of loop diuretics with thiazide-type diuretics in heart failure. J Am Coll Cardiol. 2010;56(19):1527-1534. https://doi.org/10.1016/j.jacc.2010.06.034.
8. Triposkiadis F, Butler J, Abboud FM, et al. The continuous heart failure spectrum: moving beyond an ejection fraction classification. Eur Heart J. 40(26):2155-2163. https://doi.org/10.1093/eurheartj/ehz158.
Electrolyte imbalances, per se, predispose to ventricular ectopy and, in extreme cases, sudden cardiac death.1 As these outcomes are more common in the presence of intrinsic heart disease, serum electrolytes—particularly potassium and magnesium—are routinely monitored and made replete in patients with myocardial infarction (MI) or acute decompensated heart failure (ADHF).
Patients hospitalized with ADHF often present with metabolic derangements and varying degrees of chronic adaptations in their renin–angiotensin–aldosterone system.1,2 In addition, during an ADHF hospitalization, they are subjected to guideline-directed medical therapy (GDMT), commonly in escalating doses, that exhibit well-established effects on serum potassium levels, including diuretics, angiotensin-converting-enzyme inhibitors, angiotensin receptor blockers, beta blockers, and mineralocorticoid receptor antagonists. Thus, there are myriad ways patients hospitalized for ADHF might experience electrolyte abnormalities.
In this issue of the Journal of Hospital Medicine, O’Sullivan et al. explore the associations between mean 72-hour serum potassium and important clinical outcomes—in-hospital mortality, transfer to an intensive care unit (ICU), and length of stay (LOS)—among patients with normal admission serum potassium hospitalized for ADHF.3 Through a retrospective review of electronic records from 116 hospitals, the authors identified 4,995 initially normokalemic heart failure (HF; identified by ICD-9 codes) patients and grouped them into low-normal (3.5-4.0 mEq/L), normal (4.0-4.5 mEq/L), and high-normal (4.5-5.0 mEq/L) potassium groups.3 Adjustments were made for composite scores encapsulating other lab abnormalities and comorbidities.
Over the 72-hour exposure window, the authors observed no statistically significant difference in mortality, ICU transfer, or LOS between the low-normal and normal potassium groups.3 Moreover, in a sensitivity analysis of patients who did not receive potassium supplementation, there remained statistically similar rates of mortality, ICU transfer, and LOS.3 Together, these findings suggest that maintenance of potassium >4 mEq/L may not be efficacious for preventing in-hospital complications of ADHF.3 In fact, they observed more frequent mortality and ICU transfer in patients who had high-normal potassium. This group, however, had a higher burden of chronic kidney disease and illness severity on presentation and was less likely to receive supplemental potassium.3
ADHF accounts for more than one million hospital admissions annually with one in four patients readmitted within 30 days; estimated costs surpass $30 billion.2 Reducing unnecessary expenditures in the management of HF through evidence-based guidelines is paramount. Electrolyte repletion in the setting of ADHF may represent one such opportunity by reducing excess phlebotomy, laboratory services, and potassium supplementation. Patient experience may also improve from curbing these cumbersome practices. While society guidelines endorse potassium repletion in MI to reduce the risk of ventricular arrhythmia,4 there is no uniform consensus in ADHF. As the authors cite, existing data regarding ideal potassium levels in patients with ADHF is lacking, with current evidence drawn from small observational studies. The present study, being much larger in size and being linked with observed rates of active potassium supplementation, provides some of the strongest evidence to date that a potassium goal of >4 mEq/L may not be efficacious at reducing ADHF-related complications in the generalized HF population.
While it remains uncertain if avoiding low-normal potassium levels in ADHF is beneficial, over the long term, intermediate-range potassium levels are clearly associated with the lowest HF-related mortality. In a study of over 2,000 HF patients who underwent longitudinal potassium monitoring, mortality was distributed along a U-shaped curve with highest mortality at the extremes of kalemia and a nadir at a level of 4.3 mEq/L.5
A major limitation of the present study is that it does not account for variability within the ADHF population. Firstly, knowledge regarding the use of GDMT, which not only affects serum potassium (all GDMTs) but also reduces the likelihood of arrhythmias (beta blockers), would have been informative. Moreover, the authors do not have access to data regarding incident arrhythmia and instead use ICU admission as a surrogate. In addition, ADHF patients in this study varied greatly in illness severity, ranging from those receiving initial therapy with loop diuretics alone to those requiring augmentation with thiazides and even the use of temporary mechanical circulatory support.3 Escalating loop diuretic or metolazone use not only is associated with increased mortality6 but often results in impressive natriuresis and, potentially dangerous, kaliuresis secondary to the sequential nephron blockade.7 Those who underwent extensive potassium swings in the study may not be appropriately captured using 72-hour serum potassium averages. Additionally, this study did not assess for quantity of diuresis, which is known to affect serum potassium values. It is possible that those with low-normal potassium represent patients who underwent more effective diuresis and therefore were discharged sooner. Adding to the variability, ADHF in this study encompassed both systolic (HF with a reduced ejection fraction) and diastolic (HF with a preserved ejection fraction) HF although, perhaps not surprisingly, there were marked differences in the HF subtype by potassium group—the proportions with only diastolic dysfunction were 37.1%, 39.0%, and 45.8% in the low-normal, normal, and high-normal groups, respectively (P = .0174).3 Given the known heterogeneity between these two HF subtypes,8 particularly with respect to their response to mortality-reducing GDMT,2,8 the results may be significantly confounded.
Relatedly, by excluding initially hypokalemic patients, the authors have lost considerable power and broad generalizability as these patients likely represent those at greatest risk of recurrent hypokalemia and its attendant complications during admission.
This study should be lauded for critically appraising the ubiquitous practice of electrolyte repletion. The authors present compelling preliminary data suggesting that maintenance of potassium >4 mEq/L in the general ADHF population is not efficacious at preventing ADHF complications and, as a corollary, is likely not cost-effective. However, we agree with the authors that a randomized controlled trial will be needed to change clinical practice. Ideally, such a study would account for HF subtype and GDMT use and could compare rates of arrhythmia, AHDF-related death, and all-cause mortality in patients maintained to goal normokalemia (>3.5 mEq/L) versus “high
Disclosures
Dr. Blaha reports grants from NIH, grants from FDA, grants from AHA, grants and personal fees from Amgen Foundation, grants from Aetna Foundation, personal fees from Sanofi, personal fees from Regeneron, and personal fees from Novartis, from Novo Nordisk, and from Bayer, outside the submitted work. Dr. Dudum and Dr. Lahti have nothing to disclose.
Electrolyte imbalances, per se, predispose to ventricular ectopy and, in extreme cases, sudden cardiac death.1 As these outcomes are more common in the presence of intrinsic heart disease, serum electrolytes—particularly potassium and magnesium—are routinely monitored and made replete in patients with myocardial infarction (MI) or acute decompensated heart failure (ADHF).
Patients hospitalized with ADHF often present with metabolic derangements and varying degrees of chronic adaptations in their renin–angiotensin–aldosterone system.1,2 In addition, during an ADHF hospitalization, they are subjected to guideline-directed medical therapy (GDMT), commonly in escalating doses, that exhibit well-established effects on serum potassium levels, including diuretics, angiotensin-converting-enzyme inhibitors, angiotensin receptor blockers, beta blockers, and mineralocorticoid receptor antagonists. Thus, there are myriad ways patients hospitalized for ADHF might experience electrolyte abnormalities.
In this issue of the Journal of Hospital Medicine, O’Sullivan et al. explore the associations between mean 72-hour serum potassium and important clinical outcomes—in-hospital mortality, transfer to an intensive care unit (ICU), and length of stay (LOS)—among patients with normal admission serum potassium hospitalized for ADHF.3 Through a retrospective review of electronic records from 116 hospitals, the authors identified 4,995 initially normokalemic heart failure (HF; identified by ICD-9 codes) patients and grouped them into low-normal (3.5-4.0 mEq/L), normal (4.0-4.5 mEq/L), and high-normal (4.5-5.0 mEq/L) potassium groups.3 Adjustments were made for composite scores encapsulating other lab abnormalities and comorbidities.
Over the 72-hour exposure window, the authors observed no statistically significant difference in mortality, ICU transfer, or LOS between the low-normal and normal potassium groups.3 Moreover, in a sensitivity analysis of patients who did not receive potassium supplementation, there remained statistically similar rates of mortality, ICU transfer, and LOS.3 Together, these findings suggest that maintenance of potassium >4 mEq/L may not be efficacious for preventing in-hospital complications of ADHF.3 In fact, they observed more frequent mortality and ICU transfer in patients who had high-normal potassium. This group, however, had a higher burden of chronic kidney disease and illness severity on presentation and was less likely to receive supplemental potassium.3
ADHF accounts for more than one million hospital admissions annually with one in four patients readmitted within 30 days; estimated costs surpass $30 billion.2 Reducing unnecessary expenditures in the management of HF through evidence-based guidelines is paramount. Electrolyte repletion in the setting of ADHF may represent one such opportunity by reducing excess phlebotomy, laboratory services, and potassium supplementation. Patient experience may also improve from curbing these cumbersome practices. While society guidelines endorse potassium repletion in MI to reduce the risk of ventricular arrhythmia,4 there is no uniform consensus in ADHF. As the authors cite, existing data regarding ideal potassium levels in patients with ADHF is lacking, with current evidence drawn from small observational studies. The present study, being much larger in size and being linked with observed rates of active potassium supplementation, provides some of the strongest evidence to date that a potassium goal of >4 mEq/L may not be efficacious at reducing ADHF-related complications in the generalized HF population.
While it remains uncertain if avoiding low-normal potassium levels in ADHF is beneficial, over the long term, intermediate-range potassium levels are clearly associated with the lowest HF-related mortality. In a study of over 2,000 HF patients who underwent longitudinal potassium monitoring, mortality was distributed along a U-shaped curve with highest mortality at the extremes of kalemia and a nadir at a level of 4.3 mEq/L.5
A major limitation of the present study is that it does not account for variability within the ADHF population. Firstly, knowledge regarding the use of GDMT, which not only affects serum potassium (all GDMTs) but also reduces the likelihood of arrhythmias (beta blockers), would have been informative. Moreover, the authors do not have access to data regarding incident arrhythmia and instead use ICU admission as a surrogate. In addition, ADHF patients in this study varied greatly in illness severity, ranging from those receiving initial therapy with loop diuretics alone to those requiring augmentation with thiazides and even the use of temporary mechanical circulatory support.3 Escalating loop diuretic or metolazone use not only is associated with increased mortality6 but often results in impressive natriuresis and, potentially dangerous, kaliuresis secondary to the sequential nephron blockade.7 Those who underwent extensive potassium swings in the study may not be appropriately captured using 72-hour serum potassium averages. Additionally, this study did not assess for quantity of diuresis, which is known to affect serum potassium values. It is possible that those with low-normal potassium represent patients who underwent more effective diuresis and therefore were discharged sooner. Adding to the variability, ADHF in this study encompassed both systolic (HF with a reduced ejection fraction) and diastolic (HF with a preserved ejection fraction) HF although, perhaps not surprisingly, there were marked differences in the HF subtype by potassium group—the proportions with only diastolic dysfunction were 37.1%, 39.0%, and 45.8% in the low-normal, normal, and high-normal groups, respectively (P = .0174).3 Given the known heterogeneity between these two HF subtypes,8 particularly with respect to their response to mortality-reducing GDMT,2,8 the results may be significantly confounded.
Relatedly, by excluding initially hypokalemic patients, the authors have lost considerable power and broad generalizability as these patients likely represent those at greatest risk of recurrent hypokalemia and its attendant complications during admission.
This study should be lauded for critically appraising the ubiquitous practice of electrolyte repletion. The authors present compelling preliminary data suggesting that maintenance of potassium >4 mEq/L in the general ADHF population is not efficacious at preventing ADHF complications and, as a corollary, is likely not cost-effective. However, we agree with the authors that a randomized controlled trial will be needed to change clinical practice. Ideally, such a study would account for HF subtype and GDMT use and could compare rates of arrhythmia, AHDF-related death, and all-cause mortality in patients maintained to goal normokalemia (>3.5 mEq/L) versus “high
Disclosures
Dr. Blaha reports grants from NIH, grants from FDA, grants from AHA, grants and personal fees from Amgen Foundation, grants from Aetna Foundation, personal fees from Sanofi, personal fees from Regeneron, and personal fees from Novartis, from Novo Nordisk, and from Bayer, outside the submitted work. Dr. Dudum and Dr. Lahti have nothing to disclose.
1. Packer M, Gottlieb SS, Blum MA. Immediate and long-term pathophysiologic mechanisms underlying the genesis of sudden cardiac death in patients with congestive heart failure. Am J Med. 1987;82(3):4-10. https://doi.org/10.1016/0002-9343(87)90126-4.
2. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147-e239. https://doi.org/10.1016/j.jacc.2013.05.019.
3. O’Sullivan KF, Kashef MA, Knee AB, et al. Examining the “Repletion Reflex”: the association between serum potassium and outcomes in hospitalized patients with HF. J Hosp Med. 14(12);729-736. https://doi.org/10.12788/jhm.3270.
4. Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction--executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1999 Guidelines for the Management of Patients With Acute Myocardial Infarction). Circulation 2004;110(5):588-636. https://doi.org/10.1161/01.CIR.0000134791.68010.FA
5. Nunez J, Bayes-Genis A, Zannad F, et al. Long-Term Potassium Monitoring and Dynamics in Heart Failure and Risk of Mortality. Circulation 2018;137(13):1320-1330. https://doi.org/10.1161/CIRCULATIONAHA.117.030576.
6. Neuberg GW, Miller AB, O’Connor CM, et al. Diuretic resistance predicts mortality in patients with advanced heart failure. Am Heart J. 2002;144(1):31-38. https://doi.org/10.1067/mhj.2002.123144
7. Jentzer JC, DeWald TA, Hernandez AF. Combination of loop diuretics with thiazide-type diuretics in heart failure. J Am Coll Cardiol. 2010;56(19):1527-1534. https://doi.org/10.1016/j.jacc.2010.06.034.
8. Triposkiadis F, Butler J, Abboud FM, et al. The continuous heart failure spectrum: moving beyond an ejection fraction classification. Eur Heart J. 40(26):2155-2163. https://doi.org/10.1093/eurheartj/ehz158.
1. Packer M, Gottlieb SS, Blum MA. Immediate and long-term pathophysiologic mechanisms underlying the genesis of sudden cardiac death in patients with congestive heart failure. Am J Med. 1987;82(3):4-10. https://doi.org/10.1016/0002-9343(87)90126-4.
2. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147-e239. https://doi.org/10.1016/j.jacc.2013.05.019.
3. O’Sullivan KF, Kashef MA, Knee AB, et al. Examining the “Repletion Reflex”: the association between serum potassium and outcomes in hospitalized patients with HF. J Hosp Med. 14(12);729-736. https://doi.org/10.12788/jhm.3270.
4. Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction--executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1999 Guidelines for the Management of Patients With Acute Myocardial Infarction). Circulation 2004;110(5):588-636. https://doi.org/10.1161/01.CIR.0000134791.68010.FA
5. Nunez J, Bayes-Genis A, Zannad F, et al. Long-Term Potassium Monitoring and Dynamics in Heart Failure and Risk of Mortality. Circulation 2018;137(13):1320-1330. https://doi.org/10.1161/CIRCULATIONAHA.117.030576.
6. Neuberg GW, Miller AB, O’Connor CM, et al. Diuretic resistance predicts mortality in patients with advanced heart failure. Am Heart J. 2002;144(1):31-38. https://doi.org/10.1067/mhj.2002.123144
7. Jentzer JC, DeWald TA, Hernandez AF. Combination of loop diuretics with thiazide-type diuretics in heart failure. J Am Coll Cardiol. 2010;56(19):1527-1534. https://doi.org/10.1016/j.jacc.2010.06.034.
8. Triposkiadis F, Butler J, Abboud FM, et al. The continuous heart failure spectrum: moving beyond an ejection fraction classification. Eur Heart J. 40(26):2155-2163. https://doi.org/10.1093/eurheartj/ehz158.
© 2019 Society of Hospital Medicine
Hadlima approved as fourth adalimumab biosimilar in U.S.
The Food and Drug Administration has approved the Humira biosimilar Hadlima (adalimumab-bwwd), making it the fourth adalimumab biosimilar approved in the United States, the agency announced.
Hadlima is approved for seven of the reference product’s indications, which include rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, plaque psoriasis, psoriatic arthritis, ankylosing spondylitis, adult Crohn’s disease, and ulcerative colitis.
The product will launch in the United States on June 30, 2023. Other FDA-approved adalimumab biosimilars – Amjevita (adalimunab-atto), Cyltezo (adalimumab-adbm), Hyrimoz (adalimumab-adaz) – similarly will not reach the U.S. market until 2023.
Hadlima is developed by Samsung Bioepis and commercialized by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co.
*This article was updated on July 24, 2019.
The Food and Drug Administration has approved the Humira biosimilar Hadlima (adalimumab-bwwd), making it the fourth adalimumab biosimilar approved in the United States, the agency announced.
Hadlima is approved for seven of the reference product’s indications, which include rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, plaque psoriasis, psoriatic arthritis, ankylosing spondylitis, adult Crohn’s disease, and ulcerative colitis.
The product will launch in the United States on June 30, 2023. Other FDA-approved adalimumab biosimilars – Amjevita (adalimunab-atto), Cyltezo (adalimumab-adbm), Hyrimoz (adalimumab-adaz) – similarly will not reach the U.S. market until 2023.
Hadlima is developed by Samsung Bioepis and commercialized by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co.
*This article was updated on July 24, 2019.
The Food and Drug Administration has approved the Humira biosimilar Hadlima (adalimumab-bwwd), making it the fourth adalimumab biosimilar approved in the United States, the agency announced.
Hadlima is approved for seven of the reference product’s indications, which include rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, plaque psoriasis, psoriatic arthritis, ankylosing spondylitis, adult Crohn’s disease, and ulcerative colitis.
The product will launch in the United States on June 30, 2023. Other FDA-approved adalimumab biosimilars – Amjevita (adalimunab-atto), Cyltezo (adalimumab-adbm), Hyrimoz (adalimumab-adaz) – similarly will not reach the U.S. market until 2023.
Hadlima is developed by Samsung Bioepis and commercialized by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co.
*This article was updated on July 24, 2019.
More awareness needed of compensation in autism
Understanding benefits, costs of strategies could guide diagnoses
Individuals with undiagnosed autism spectrum disorders and those with a formal diagnosis employ similar compensation behaviors to manage social and cognitive difficulties, and undiagnosed individuals may go unrecognized, data from an online survey of 136 adults suggest.
Unlike other adaptive behaviors in psychiatry, “autistic compensators, despite apparent lack of observable autistic behavior, continue being autistic at the neurocognitive level,” wrote Lucy Anne Livingston of Kings College London, and colleagues. “Because autism spectrum disorder is diagnosed by behavior alone, compensators might not receive a diagnosis and support until later in life, if at all. “
The researchers compared compensation behaviors in adults with and without an autism diagnosis. They recruited adults aged 18 years and older through an online advertisement distributed through social media and the U.K. National Autistic Society. Participants completed an online survey during Oct. 19, 2017–Jan. 2, 2018. The study was published in the Lancet Psychiatry.
preplanning social niceties (e.g., asking others questions about themselves), and switching between social rules,” the researchers wrote.
The study population included 58 individuals with a clinical diagnosis of autism, 19 of whom self-identified as autistic but were not formally diagnosed, and 59 of whom reported social difficulties but had no formal diagnosis and did not self-identify as autistic. The average age of the three groups was 36 years, 40 years, and 34 years, respectively, and the average age at diagnosis for the diagnosed group was 30 years. Most of the individuals in each group were women (64%, 47%, and 86%, respectively). Responses were examined using a thematic analysis and thematic map.
In general, participants reported that compensation was a cognitively taxing process that served as a secondary route for social interaction because the primary route was unavailable, but that compensation strategies fell short in certain situations, such as unexpected turns of conversation. Overall, 38% of the respondents said their compensation strategies were “extremely successful” and 56% reported “somewhat successful.” However, 12% also reported their strategies were “extremely tiring,” and 36% reported “somewhat tiring.”
Factors affecting the participants’ abilities to compensate included environmental and sensory factors such as bright lights, loud noise, and large groups with unstructured social settings, such as parties. Also, transition to living independently as an adult led to problems, because compensation had allowed individuals to grow up appearing normal but lacking in additional support and strategies, the researchers noted.
Factors that contributed to successful interactions included similar interests with an interaction partner, and motivation to develop meaningful relationships. Participants also said they viewed compensation as a way to avoid ostracism and bullying. In addition, “fitting neurotypical peoples’ interaction style (e.g., eye contact or small talk) was viewed as vital for achieving life goals (e.g., independence and employment),” the researchers wrote.
In an accompanying editorial, Julia Parish-Morris, PhD, suggested the observation made by Ms. Livingston, a researcher at the Institute of Psychiatry, Psychology and Neuroscience at the college, and associates that compensation also occurs in people who do not have autism suggests that compensation might be a “general social lubricant that facilitates community living and is therefore a potentially useful tool.”
“In other words, perhaps raising awareness about compensation in autism spectrum disorder is an important first step toward eliminating the need for it,” wrote Dr. Parish-Morris, of the Center for Autism Research at Children’s Hospital of Philadelphia (Lancet Psychiatry. 2019 Jul 23. doi: 10.1016/S2215-0366[19]30294-9).
The study data were limited by the prevalence of female, well-educated, late-diagnosed individuals in the study population, which might limit the generalizability of the findings, and the lack of data on subconscious compensation because of the use of self-reports, the researchers noted.
However, “Given the individual differences found in this study, we tentatively suggest that clinicians take an individualized approach when assessing and discussing compensatory strategies with people with autism,” they said. “It will be important to establish which compensatory strategies are most beneficial, and how their success might be maximized with changes to external environments irrespective of clinical intervention.”
The researchers had no financial interests to disclose.
SOURCE: Livingston LA et al. Lancet Psychiatry. 2019 Jul 23. doi. org/10.1016/S2215-0366(19)30224-X.
Understanding benefits, costs of strategies could guide diagnoses
Understanding benefits, costs of strategies could guide diagnoses
Individuals with undiagnosed autism spectrum disorders and those with a formal diagnosis employ similar compensation behaviors to manage social and cognitive difficulties, and undiagnosed individuals may go unrecognized, data from an online survey of 136 adults suggest.
Unlike other adaptive behaviors in psychiatry, “autistic compensators, despite apparent lack of observable autistic behavior, continue being autistic at the neurocognitive level,” wrote Lucy Anne Livingston of Kings College London, and colleagues. “Because autism spectrum disorder is diagnosed by behavior alone, compensators might not receive a diagnosis and support until later in life, if at all. “
The researchers compared compensation behaviors in adults with and without an autism diagnosis. They recruited adults aged 18 years and older through an online advertisement distributed through social media and the U.K. National Autistic Society. Participants completed an online survey during Oct. 19, 2017–Jan. 2, 2018. The study was published in the Lancet Psychiatry.
preplanning social niceties (e.g., asking others questions about themselves), and switching between social rules,” the researchers wrote.
The study population included 58 individuals with a clinical diagnosis of autism, 19 of whom self-identified as autistic but were not formally diagnosed, and 59 of whom reported social difficulties but had no formal diagnosis and did not self-identify as autistic. The average age of the three groups was 36 years, 40 years, and 34 years, respectively, and the average age at diagnosis for the diagnosed group was 30 years. Most of the individuals in each group were women (64%, 47%, and 86%, respectively). Responses were examined using a thematic analysis and thematic map.
In general, participants reported that compensation was a cognitively taxing process that served as a secondary route for social interaction because the primary route was unavailable, but that compensation strategies fell short in certain situations, such as unexpected turns of conversation. Overall, 38% of the respondents said their compensation strategies were “extremely successful” and 56% reported “somewhat successful.” However, 12% also reported their strategies were “extremely tiring,” and 36% reported “somewhat tiring.”
Factors affecting the participants’ abilities to compensate included environmental and sensory factors such as bright lights, loud noise, and large groups with unstructured social settings, such as parties. Also, transition to living independently as an adult led to problems, because compensation had allowed individuals to grow up appearing normal but lacking in additional support and strategies, the researchers noted.
Factors that contributed to successful interactions included similar interests with an interaction partner, and motivation to develop meaningful relationships. Participants also said they viewed compensation as a way to avoid ostracism and bullying. In addition, “fitting neurotypical peoples’ interaction style (e.g., eye contact or small talk) was viewed as vital for achieving life goals (e.g., independence and employment),” the researchers wrote.
In an accompanying editorial, Julia Parish-Morris, PhD, suggested the observation made by Ms. Livingston, a researcher at the Institute of Psychiatry, Psychology and Neuroscience at the college, and associates that compensation also occurs in people who do not have autism suggests that compensation might be a “general social lubricant that facilitates community living and is therefore a potentially useful tool.”
“In other words, perhaps raising awareness about compensation in autism spectrum disorder is an important first step toward eliminating the need for it,” wrote Dr. Parish-Morris, of the Center for Autism Research at Children’s Hospital of Philadelphia (Lancet Psychiatry. 2019 Jul 23. doi: 10.1016/S2215-0366[19]30294-9).
The study data were limited by the prevalence of female, well-educated, late-diagnosed individuals in the study population, which might limit the generalizability of the findings, and the lack of data on subconscious compensation because of the use of self-reports, the researchers noted.
However, “Given the individual differences found in this study, we tentatively suggest that clinicians take an individualized approach when assessing and discussing compensatory strategies with people with autism,” they said. “It will be important to establish which compensatory strategies are most beneficial, and how their success might be maximized with changes to external environments irrespective of clinical intervention.”
The researchers had no financial interests to disclose.
SOURCE: Livingston LA et al. Lancet Psychiatry. 2019 Jul 23. doi. org/10.1016/S2215-0366(19)30224-X.
Individuals with undiagnosed autism spectrum disorders and those with a formal diagnosis employ similar compensation behaviors to manage social and cognitive difficulties, and undiagnosed individuals may go unrecognized, data from an online survey of 136 adults suggest.
Unlike other adaptive behaviors in psychiatry, “autistic compensators, despite apparent lack of observable autistic behavior, continue being autistic at the neurocognitive level,” wrote Lucy Anne Livingston of Kings College London, and colleagues. “Because autism spectrum disorder is diagnosed by behavior alone, compensators might not receive a diagnosis and support until later in life, if at all. “
The researchers compared compensation behaviors in adults with and without an autism diagnosis. They recruited adults aged 18 years and older through an online advertisement distributed through social media and the U.K. National Autistic Society. Participants completed an online survey during Oct. 19, 2017–Jan. 2, 2018. The study was published in the Lancet Psychiatry.
preplanning social niceties (e.g., asking others questions about themselves), and switching between social rules,” the researchers wrote.
The study population included 58 individuals with a clinical diagnosis of autism, 19 of whom self-identified as autistic but were not formally diagnosed, and 59 of whom reported social difficulties but had no formal diagnosis and did not self-identify as autistic. The average age of the three groups was 36 years, 40 years, and 34 years, respectively, and the average age at diagnosis for the diagnosed group was 30 years. Most of the individuals in each group were women (64%, 47%, and 86%, respectively). Responses were examined using a thematic analysis and thematic map.
In general, participants reported that compensation was a cognitively taxing process that served as a secondary route for social interaction because the primary route was unavailable, but that compensation strategies fell short in certain situations, such as unexpected turns of conversation. Overall, 38% of the respondents said their compensation strategies were “extremely successful” and 56% reported “somewhat successful.” However, 12% also reported their strategies were “extremely tiring,” and 36% reported “somewhat tiring.”
Factors affecting the participants’ abilities to compensate included environmental and sensory factors such as bright lights, loud noise, and large groups with unstructured social settings, such as parties. Also, transition to living independently as an adult led to problems, because compensation had allowed individuals to grow up appearing normal but lacking in additional support and strategies, the researchers noted.
Factors that contributed to successful interactions included similar interests with an interaction partner, and motivation to develop meaningful relationships. Participants also said they viewed compensation as a way to avoid ostracism and bullying. In addition, “fitting neurotypical peoples’ interaction style (e.g., eye contact or small talk) was viewed as vital for achieving life goals (e.g., independence and employment),” the researchers wrote.
In an accompanying editorial, Julia Parish-Morris, PhD, suggested the observation made by Ms. Livingston, a researcher at the Institute of Psychiatry, Psychology and Neuroscience at the college, and associates that compensation also occurs in people who do not have autism suggests that compensation might be a “general social lubricant that facilitates community living and is therefore a potentially useful tool.”
“In other words, perhaps raising awareness about compensation in autism spectrum disorder is an important first step toward eliminating the need for it,” wrote Dr. Parish-Morris, of the Center for Autism Research at Children’s Hospital of Philadelphia (Lancet Psychiatry. 2019 Jul 23. doi: 10.1016/S2215-0366[19]30294-9).
The study data were limited by the prevalence of female, well-educated, late-diagnosed individuals in the study population, which might limit the generalizability of the findings, and the lack of data on subconscious compensation because of the use of self-reports, the researchers noted.
However, “Given the individual differences found in this study, we tentatively suggest that clinicians take an individualized approach when assessing and discussing compensatory strategies with people with autism,” they said. “It will be important to establish which compensatory strategies are most beneficial, and how their success might be maximized with changes to external environments irrespective of clinical intervention.”
The researchers had no financial interests to disclose.
SOURCE: Livingston LA et al. Lancet Psychiatry. 2019 Jul 23. doi. org/10.1016/S2215-0366(19)30224-X.
FROM THE LANCET PSYCHIATRY
Key clinical point: Individuals with social difficulties use similar compensation strategies to manage social situations whether or not they have an autism diagnosis.
Major finding: A total of 38% of respondents said their compensation behaviors were “extremely successful,” but 12% also reported those strategies were “extremely tiring.”
Study details: The data come from 136 adults who responded to an online survey; 58 diagnosed with autism, 19 self-identified, and 59 reported social difficulties without self-identification or diagnosis.
Disclosures: The researchers had no financial conflicts to disclose.
Source: Livingston LA et al. Lancet Psychiatry. 2019 Jul 23. doi: 10.1016/S2215-0366(19)30224-X.
Most preschoolers with signs of ADHD aren’t ready for primary school
Are preschoolers with signs of ADHD ready for school? A new study suggests they’re far from prepared.
A small sample of children with symptoms of moderate to severe ADHD scored markedly lower than comparable children on 8 of 10 measures of readiness for primary education in a study published in Pediatrics.
These children require early identification and intervention,” Hannah T. Perrin, MD, of Stanford University and associates wrote.
There’s sparse research into the prevalence of ADHD symptoms in preschoolers, but the Centers for Disease Control and Prevention reports that nearly half of children aged 4-5 years with the condition got no behavioral therapy from 2009 to 2010. About 25% received only medical treatment.
Dr. Perrin and colleagues recruited 93 children aged 4-6 years from the community. Their parents, who were compensated, took the Early Childhood Inventory-4 (ECI-4) questionnaire. It revealed that 80% (n = 45) of those diagnosed with ADHD had scores considered signs of moderate or severe ADHD symptom severity based on the parent ratings. Those with lower scores made up the comparison group (n = 48).
The groups were similar, about 60% male and more than 50% white; neither difference between groups was statistically significant. However, those in the comparison group were much more likely to have non-Latino/non-Hispanic ethnicity; 61% in ADHD group vs. 91% in comparison group, P = .001.
The children were tested for school readiness through several measures in two 1- to 1.5-hour sessions.
The researchers reported that 79% of children in the ADHD group were not ready for school (impaired) vs. 13% of the comparison group. (odds ratio, 21, 95% confidence interval, 5.67-77.77, P = .001).
“We found that preschool-aged children with ADHD symptoms demonstrated significantly worse performance on 8 of 10 school readiness measures,” the authors added, “and significantly greater odds of impairment in four of five domains and overall school readiness.”
Dr. Perrin and associates cautioned that the findings rely on a convenience sample, are based on parent – but not teacher – input, do not include Spanish speakers, and do not follow children over the long term.
Going forward, they wrote, “family dynamics and social-emotional functioning should be assessed for each preschool-aged child with ADHD symptoms, and appropriate therapeutic interventions and community supports should be prescribed to enhance school readiness.”
The study authors had no disclosures. Study funders include the Maternal and Child Health Bureau, the Katharine McCormick Faculty Scholar Award, Stanford Children’s Health and Child Health Research Institute Pilot Early Career Award, and the National Institutes of Health.
SOURCE: Perrin HT et al. Pediatrics. 2019 Aug. doi: 10.1542/peds.2019-0038.
Are preschoolers with signs of ADHD ready for school? A new study suggests they’re far from prepared.
A small sample of children with symptoms of moderate to severe ADHD scored markedly lower than comparable children on 8 of 10 measures of readiness for primary education in a study published in Pediatrics.
These children require early identification and intervention,” Hannah T. Perrin, MD, of Stanford University and associates wrote.
There’s sparse research into the prevalence of ADHD symptoms in preschoolers, but the Centers for Disease Control and Prevention reports that nearly half of children aged 4-5 years with the condition got no behavioral therapy from 2009 to 2010. About 25% received only medical treatment.
Dr. Perrin and colleagues recruited 93 children aged 4-6 years from the community. Their parents, who were compensated, took the Early Childhood Inventory-4 (ECI-4) questionnaire. It revealed that 80% (n = 45) of those diagnosed with ADHD had scores considered signs of moderate or severe ADHD symptom severity based on the parent ratings. Those with lower scores made up the comparison group (n = 48).
The groups were similar, about 60% male and more than 50% white; neither difference between groups was statistically significant. However, those in the comparison group were much more likely to have non-Latino/non-Hispanic ethnicity; 61% in ADHD group vs. 91% in comparison group, P = .001.
The children were tested for school readiness through several measures in two 1- to 1.5-hour sessions.
The researchers reported that 79% of children in the ADHD group were not ready for school (impaired) vs. 13% of the comparison group. (odds ratio, 21, 95% confidence interval, 5.67-77.77, P = .001).
“We found that preschool-aged children with ADHD symptoms demonstrated significantly worse performance on 8 of 10 school readiness measures,” the authors added, “and significantly greater odds of impairment in four of five domains and overall school readiness.”
Dr. Perrin and associates cautioned that the findings rely on a convenience sample, are based on parent – but not teacher – input, do not include Spanish speakers, and do not follow children over the long term.
Going forward, they wrote, “family dynamics and social-emotional functioning should be assessed for each preschool-aged child with ADHD symptoms, and appropriate therapeutic interventions and community supports should be prescribed to enhance school readiness.”
The study authors had no disclosures. Study funders include the Maternal and Child Health Bureau, the Katharine McCormick Faculty Scholar Award, Stanford Children’s Health and Child Health Research Institute Pilot Early Career Award, and the National Institutes of Health.
SOURCE: Perrin HT et al. Pediatrics. 2019 Aug. doi: 10.1542/peds.2019-0038.
Are preschoolers with signs of ADHD ready for school? A new study suggests they’re far from prepared.
A small sample of children with symptoms of moderate to severe ADHD scored markedly lower than comparable children on 8 of 10 measures of readiness for primary education in a study published in Pediatrics.
These children require early identification and intervention,” Hannah T. Perrin, MD, of Stanford University and associates wrote.
There’s sparse research into the prevalence of ADHD symptoms in preschoolers, but the Centers for Disease Control and Prevention reports that nearly half of children aged 4-5 years with the condition got no behavioral therapy from 2009 to 2010. About 25% received only medical treatment.
Dr. Perrin and colleagues recruited 93 children aged 4-6 years from the community. Their parents, who were compensated, took the Early Childhood Inventory-4 (ECI-4) questionnaire. It revealed that 80% (n = 45) of those diagnosed with ADHD had scores considered signs of moderate or severe ADHD symptom severity based on the parent ratings. Those with lower scores made up the comparison group (n = 48).
The groups were similar, about 60% male and more than 50% white; neither difference between groups was statistically significant. However, those in the comparison group were much more likely to have non-Latino/non-Hispanic ethnicity; 61% in ADHD group vs. 91% in comparison group, P = .001.
The children were tested for school readiness through several measures in two 1- to 1.5-hour sessions.
The researchers reported that 79% of children in the ADHD group were not ready for school (impaired) vs. 13% of the comparison group. (odds ratio, 21, 95% confidence interval, 5.67-77.77, P = .001).
“We found that preschool-aged children with ADHD symptoms demonstrated significantly worse performance on 8 of 10 school readiness measures,” the authors added, “and significantly greater odds of impairment in four of five domains and overall school readiness.”
Dr. Perrin and associates cautioned that the findings rely on a convenience sample, are based on parent – but not teacher – input, do not include Spanish speakers, and do not follow children over the long term.
Going forward, they wrote, “family dynamics and social-emotional functioning should be assessed for each preschool-aged child with ADHD symptoms, and appropriate therapeutic interventions and community supports should be prescribed to enhance school readiness.”
The study authors had no disclosures. Study funders include the Maternal and Child Health Bureau, the Katharine McCormick Faculty Scholar Award, Stanford Children’s Health and Child Health Research Institute Pilot Early Career Award, and the National Institutes of Health.
SOURCE: Perrin HT et al. Pediatrics. 2019 Aug. doi: 10.1542/peds.2019-0038.
FROM PEDIATRICS
FDA approves rituximab biosimilar for cancer, autoimmune disorders
The Food and Drug Administration has approved rituximab-pvvr (Ruxience) for adults with non-Hodgkin lymphoma, chronic lymphocytic leukemia (CLL), and granulomatosis with polyangiitis and microscopic polyangiitis. It is the first biosimilar approved to treat these two rare autoimmune conditions.
Specifically, the biosimilar product is approved as single-agent therapy for relapsed or refractory, low grade or follicular, CD20-positive B-cell non-Hodgkin lymphoma; in combination with chemotherapy for other types of previously untreated CD20-positive B-cell non-Hodgkin lymphoma; and as a single agent for nonprogressing, low-grade, CD20-positive B-cell non-Hodgkin lymphoma after first-line chemotherapy treatment. It is also approved for both previously untreated and previously treated CD20-positive CLL in combination with chemotherapy. And it is approved for granulomatosis with polyangiitis and microscopic polyangiitis in combination with glucocorticoids.
The approval is based on demonstration that rituximab-pvvr had no clinically meaningful differences in safety or efficacy when compared with the reference drug, rituximab (Rituxan), according to a release from the biosimilar’s developer. As with rituximab, rituximab-pvvr’s label comes with an FDA boxed warning. In the biosimilar’s case, it warns against fatal infusion-related reactions, severe mucocutaneous reactions, hepatitis B virus reactivation, and progressive multifocal leukoencephalopathy. Other adverse reactions include fever, headache, neutropenia, and lymphopenia.
The Food and Drug Administration has approved rituximab-pvvr (Ruxience) for adults with non-Hodgkin lymphoma, chronic lymphocytic leukemia (CLL), and granulomatosis with polyangiitis and microscopic polyangiitis. It is the first biosimilar approved to treat these two rare autoimmune conditions.
Specifically, the biosimilar product is approved as single-agent therapy for relapsed or refractory, low grade or follicular, CD20-positive B-cell non-Hodgkin lymphoma; in combination with chemotherapy for other types of previously untreated CD20-positive B-cell non-Hodgkin lymphoma; and as a single agent for nonprogressing, low-grade, CD20-positive B-cell non-Hodgkin lymphoma after first-line chemotherapy treatment. It is also approved for both previously untreated and previously treated CD20-positive CLL in combination with chemotherapy. And it is approved for granulomatosis with polyangiitis and microscopic polyangiitis in combination with glucocorticoids.
The approval is based on demonstration that rituximab-pvvr had no clinically meaningful differences in safety or efficacy when compared with the reference drug, rituximab (Rituxan), according to a release from the biosimilar’s developer. As with rituximab, rituximab-pvvr’s label comes with an FDA boxed warning. In the biosimilar’s case, it warns against fatal infusion-related reactions, severe mucocutaneous reactions, hepatitis B virus reactivation, and progressive multifocal leukoencephalopathy. Other adverse reactions include fever, headache, neutropenia, and lymphopenia.
The Food and Drug Administration has approved rituximab-pvvr (Ruxience) for adults with non-Hodgkin lymphoma, chronic lymphocytic leukemia (CLL), and granulomatosis with polyangiitis and microscopic polyangiitis. It is the first biosimilar approved to treat these two rare autoimmune conditions.
Specifically, the biosimilar product is approved as single-agent therapy for relapsed or refractory, low grade or follicular, CD20-positive B-cell non-Hodgkin lymphoma; in combination with chemotherapy for other types of previously untreated CD20-positive B-cell non-Hodgkin lymphoma; and as a single agent for nonprogressing, low-grade, CD20-positive B-cell non-Hodgkin lymphoma after first-line chemotherapy treatment. It is also approved for both previously untreated and previously treated CD20-positive CLL in combination with chemotherapy. And it is approved for granulomatosis with polyangiitis and microscopic polyangiitis in combination with glucocorticoids.
The approval is based on demonstration that rituximab-pvvr had no clinically meaningful differences in safety or efficacy when compared with the reference drug, rituximab (Rituxan), according to a release from the biosimilar’s developer. As with rituximab, rituximab-pvvr’s label comes with an FDA boxed warning. In the biosimilar’s case, it warns against fatal infusion-related reactions, severe mucocutaneous reactions, hepatitis B virus reactivation, and progressive multifocal leukoencephalopathy. Other adverse reactions include fever, headache, neutropenia, and lymphopenia.