User login
Meeting just 2 of 7 ‘simple’ goals lowers HF risk
Turns out the American Heart Association is onto something when it urges people to embrace its “Life’s Simple 7” (LS7) recommendations, a series of strategies designed to boost cardiovascular health. A new European study finds that people who follow the recommendations were more than half as likely to develop heart failure (HF) and that mastering just two of the seven criteria makes a big difference, compared with mastering none at all.
“Focusing on particular components of the American Heart Association LS7 could be seen as a way to improve cardiovascular health,” wrote the authors of the study, which appears in JACC: Heart Failure.
The LS7 encourages the following strategies:
- Manage blood pressure.
- Control cholesterol.
- Reduce blood sugar.
- Get active.
- Eat better.
- Lose weight.
- Stop smoking.
For the new study, researchers led by Alicia Uijl, MSc, of University College London and University Medical Center Utrecht (the Netherlands) retrospectively tracked 37,803 participants in a prospective Dutch study of cancer and nutrition.
The subjects, 75% women, had a mean age of 49 years. The group was much thinner, with a mean body mass index of 25 kg/m2, than typical American men and women, whose mean BMIs are 29 and 30, per CDC statistics (Natl Health Stat Report. 2018 Dec;122:1-16)
Researchers gave the subjects an LS7 score (0-14) at baseline from 1993-1997. The score was based on whether they fully (2 points), partially (1) or not at all (0) met each of the LC7 criteria.
Most of the subjects failed to reach the ideal level of healthiness, which was defined as scores 11-14 and was achieved by 23%. The others were in the intermediate group (scores, 9-10 points; 35%) and inadequate group (scores, 0-8; 42%).
Over a median follow-up of 15 years, 2% of participants (690) developed HF. In an adjusted model, subjects in the top two groups (ideal and intermediate) were less likely to develop HF than were those in the lowest group (hazard ratios, 0.45 and 0.53, respectively).
The researchers found that diet, exercise, and cholesterol had lesser impacts on risk of HF than did the other elements. And they discovered that meeting the ideal level for just 2 of the 7 strategies would lower HF risk by 52%, compared with reaching no ideal levels.
What now? The high number of subjects in the lowest category suggests “there is ample room for improvements in healthy lifestyle behavior that may reduce HF in the general population,” the researchers wrote. “Given the robust associations between a healthy lifestyle and reduced incidence of HF, this study provides evidence that prevention of incident HF could be accomplished by implementing healthy lifestyle patterns.”
The study is funded by the European Commission, European Union/European Federation of Pharmaceutical Industries and Associations, and several other research organizations. The study authors reported no relevant disclosures.
SOURCE: Uijl A et al. JACC: Heart Fail. 2019 Jul 10. doi: 10.1016/j.jchf.2019.03.009
Christie M. Ballantyne, MD, and Vijay Nambi, MD, PhD, of Baylor College of Medicine, Houston, made these comments in an accompanying editorial. Dr. Ballantyne discloses grant/research support/consulting for Abbott and Roche and a provisional patent. Dr. Nambi discloses research site primary investigator work for Merck and a provisional patent.
Christie M. Ballantyne, MD, and Vijay Nambi, MD, PhD, of Baylor College of Medicine, Houston, made these comments in an accompanying editorial. Dr. Ballantyne discloses grant/research support/consulting for Abbott and Roche and a provisional patent. Dr. Nambi discloses research site primary investigator work for Merck and a provisional patent.
Christie M. Ballantyne, MD, and Vijay Nambi, MD, PhD, of Baylor College of Medicine, Houston, made these comments in an accompanying editorial. Dr. Ballantyne discloses grant/research support/consulting for Abbott and Roche and a provisional patent. Dr. Nambi discloses research site primary investigator work for Merck and a provisional patent.
Turns out the American Heart Association is onto something when it urges people to embrace its “Life’s Simple 7” (LS7) recommendations, a series of strategies designed to boost cardiovascular health. A new European study finds that people who follow the recommendations were more than half as likely to develop heart failure (HF) and that mastering just two of the seven criteria makes a big difference, compared with mastering none at all.
“Focusing on particular components of the American Heart Association LS7 could be seen as a way to improve cardiovascular health,” wrote the authors of the study, which appears in JACC: Heart Failure.
The LS7 encourages the following strategies:
- Manage blood pressure.
- Control cholesterol.
- Reduce blood sugar.
- Get active.
- Eat better.
- Lose weight.
- Stop smoking.
For the new study, researchers led by Alicia Uijl, MSc, of University College London and University Medical Center Utrecht (the Netherlands) retrospectively tracked 37,803 participants in a prospective Dutch study of cancer and nutrition.
The subjects, 75% women, had a mean age of 49 years. The group was much thinner, with a mean body mass index of 25 kg/m2, than typical American men and women, whose mean BMIs are 29 and 30, per CDC statistics (Natl Health Stat Report. 2018 Dec;122:1-16)
Researchers gave the subjects an LS7 score (0-14) at baseline from 1993-1997. The score was based on whether they fully (2 points), partially (1) or not at all (0) met each of the LC7 criteria.
Most of the subjects failed to reach the ideal level of healthiness, which was defined as scores 11-14 and was achieved by 23%. The others were in the intermediate group (scores, 9-10 points; 35%) and inadequate group (scores, 0-8; 42%).
Over a median follow-up of 15 years, 2% of participants (690) developed HF. In an adjusted model, subjects in the top two groups (ideal and intermediate) were less likely to develop HF than were those in the lowest group (hazard ratios, 0.45 and 0.53, respectively).
The researchers found that diet, exercise, and cholesterol had lesser impacts on risk of HF than did the other elements. And they discovered that meeting the ideal level for just 2 of the 7 strategies would lower HF risk by 52%, compared with reaching no ideal levels.
What now? The high number of subjects in the lowest category suggests “there is ample room for improvements in healthy lifestyle behavior that may reduce HF in the general population,” the researchers wrote. “Given the robust associations between a healthy lifestyle and reduced incidence of HF, this study provides evidence that prevention of incident HF could be accomplished by implementing healthy lifestyle patterns.”
The study is funded by the European Commission, European Union/European Federation of Pharmaceutical Industries and Associations, and several other research organizations. The study authors reported no relevant disclosures.
SOURCE: Uijl A et al. JACC: Heart Fail. 2019 Jul 10. doi: 10.1016/j.jchf.2019.03.009
Turns out the American Heart Association is onto something when it urges people to embrace its “Life’s Simple 7” (LS7) recommendations, a series of strategies designed to boost cardiovascular health. A new European study finds that people who follow the recommendations were more than half as likely to develop heart failure (HF) and that mastering just two of the seven criteria makes a big difference, compared with mastering none at all.
“Focusing on particular components of the American Heart Association LS7 could be seen as a way to improve cardiovascular health,” wrote the authors of the study, which appears in JACC: Heart Failure.
The LS7 encourages the following strategies:
- Manage blood pressure.
- Control cholesterol.
- Reduce blood sugar.
- Get active.
- Eat better.
- Lose weight.
- Stop smoking.
For the new study, researchers led by Alicia Uijl, MSc, of University College London and University Medical Center Utrecht (the Netherlands) retrospectively tracked 37,803 participants in a prospective Dutch study of cancer and nutrition.
The subjects, 75% women, had a mean age of 49 years. The group was much thinner, with a mean body mass index of 25 kg/m2, than typical American men and women, whose mean BMIs are 29 and 30, per CDC statistics (Natl Health Stat Report. 2018 Dec;122:1-16)
Researchers gave the subjects an LS7 score (0-14) at baseline from 1993-1997. The score was based on whether they fully (2 points), partially (1) or not at all (0) met each of the LC7 criteria.
Most of the subjects failed to reach the ideal level of healthiness, which was defined as scores 11-14 and was achieved by 23%. The others were in the intermediate group (scores, 9-10 points; 35%) and inadequate group (scores, 0-8; 42%).
Over a median follow-up of 15 years, 2% of participants (690) developed HF. In an adjusted model, subjects in the top two groups (ideal and intermediate) were less likely to develop HF than were those in the lowest group (hazard ratios, 0.45 and 0.53, respectively).
The researchers found that diet, exercise, and cholesterol had lesser impacts on risk of HF than did the other elements. And they discovered that meeting the ideal level for just 2 of the 7 strategies would lower HF risk by 52%, compared with reaching no ideal levels.
What now? The high number of subjects in the lowest category suggests “there is ample room for improvements in healthy lifestyle behavior that may reduce HF in the general population,” the researchers wrote. “Given the robust associations between a healthy lifestyle and reduced incidence of HF, this study provides evidence that prevention of incident HF could be accomplished by implementing healthy lifestyle patterns.”
The study is funded by the European Commission, European Union/European Federation of Pharmaceutical Industries and Associations, and several other research organizations. The study authors reported no relevant disclosures.
SOURCE: Uijl A et al. JACC: Heart Fail. 2019 Jul 10. doi: 10.1016/j.jchf.2019.03.009
FROM JACC: HEART FAILURE
Postpartum care: State scorecard for Medicaid enrollees
Timely postpartum care for Medicaid enrollees varies considerably, ranging from 17% to 74% among the states, according to the Centers for Medicare & Medicaid Services.
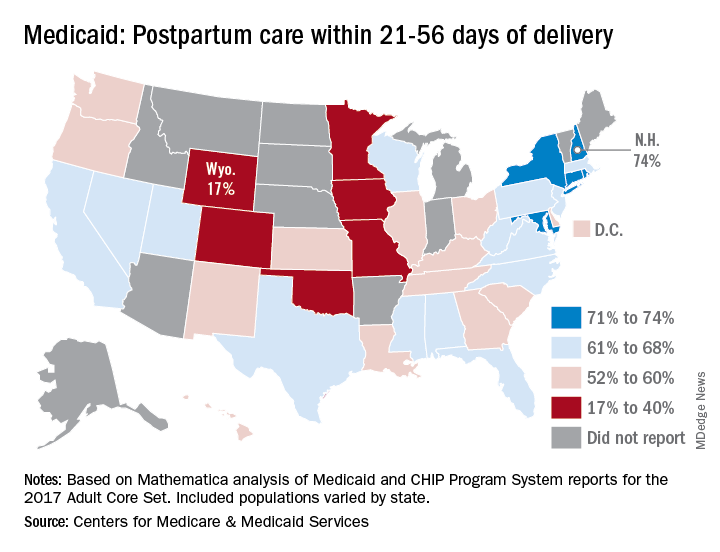
A national median of 60% of women saw a health care provider within 21-56 days of their delivery, CMS reported in its Medicaid and Children’s Health Insurance Program Scorecard.
“Medicaid is the largest payer for maternity care in the United States. The program has an important role to play in improving maternal and perinatal health outcomes,” the CMS noted.
New Hampshire’s rate of 74% was the highest of any state, just edging out Maryland and Rhode Island, each at 73%. The only other states over 70% were Connecticut and New York, which both reported rates of 71%, scorecard data show.
In Wyoming, the state with the lowest rate, 17% of Medicaid enrollees received timely postpartum care. The other four states with rates below 40% were Oklahoma (22%), Colorado (35%), Iowa (37%), and Missouri (38%), the CMS said after a recent refresh of data in the scorecard.
“The included populations … can vary by state. For example, some states report data on certain populations such as those covered under managed care but not those covered under fee for service. This variation in data and calculation methods can affect measure performance and comparisons between states,” the CMS said.
The scorecard is based on Mathematica analysis of Medicaid and CHIP Program System reports for the 2017 Adult Core Set, and the measurement period was Nov. 6, 2015, to Nov. 5, 2016. Twelve states did not report data on postpartum care to the CMS.
“More and more states are voluntarily reporting their health outcomes in the scorecard, and the new data is leading us into an era of increased transparency and accountability, so that together we can improve the quality of care we give to the vulnerable Americans that depend on this vital program,” CMS Administrator Seema Verma said in a written statement.
Timely postpartum care for Medicaid enrollees varies considerably, ranging from 17% to 74% among the states, according to the Centers for Medicare & Medicaid Services.

A national median of 60% of women saw a health care provider within 21-56 days of their delivery, CMS reported in its Medicaid and Children’s Health Insurance Program Scorecard.
“Medicaid is the largest payer for maternity care in the United States. The program has an important role to play in improving maternal and perinatal health outcomes,” the CMS noted.
New Hampshire’s rate of 74% was the highest of any state, just edging out Maryland and Rhode Island, each at 73%. The only other states over 70% were Connecticut and New York, which both reported rates of 71%, scorecard data show.
In Wyoming, the state with the lowest rate, 17% of Medicaid enrollees received timely postpartum care. The other four states with rates below 40% were Oklahoma (22%), Colorado (35%), Iowa (37%), and Missouri (38%), the CMS said after a recent refresh of data in the scorecard.
“The included populations … can vary by state. For example, some states report data on certain populations such as those covered under managed care but not those covered under fee for service. This variation in data and calculation methods can affect measure performance and comparisons between states,” the CMS said.
The scorecard is based on Mathematica analysis of Medicaid and CHIP Program System reports for the 2017 Adult Core Set, and the measurement period was Nov. 6, 2015, to Nov. 5, 2016. Twelve states did not report data on postpartum care to the CMS.
“More and more states are voluntarily reporting their health outcomes in the scorecard, and the new data is leading us into an era of increased transparency and accountability, so that together we can improve the quality of care we give to the vulnerable Americans that depend on this vital program,” CMS Administrator Seema Verma said in a written statement.
Timely postpartum care for Medicaid enrollees varies considerably, ranging from 17% to 74% among the states, according to the Centers for Medicare & Medicaid Services.

A national median of 60% of women saw a health care provider within 21-56 days of their delivery, CMS reported in its Medicaid and Children’s Health Insurance Program Scorecard.
“Medicaid is the largest payer for maternity care in the United States. The program has an important role to play in improving maternal and perinatal health outcomes,” the CMS noted.
New Hampshire’s rate of 74% was the highest of any state, just edging out Maryland and Rhode Island, each at 73%. The only other states over 70% were Connecticut and New York, which both reported rates of 71%, scorecard data show.
In Wyoming, the state with the lowest rate, 17% of Medicaid enrollees received timely postpartum care. The other four states with rates below 40% were Oklahoma (22%), Colorado (35%), Iowa (37%), and Missouri (38%), the CMS said after a recent refresh of data in the scorecard.
“The included populations … can vary by state. For example, some states report data on certain populations such as those covered under managed care but not those covered under fee for service. This variation in data and calculation methods can affect measure performance and comparisons between states,” the CMS said.
The scorecard is based on Mathematica analysis of Medicaid and CHIP Program System reports for the 2017 Adult Core Set, and the measurement period was Nov. 6, 2015, to Nov. 5, 2016. Twelve states did not report data on postpartum care to the CMS.
“More and more states are voluntarily reporting their health outcomes in the scorecard, and the new data is leading us into an era of increased transparency and accountability, so that together we can improve the quality of care we give to the vulnerable Americans that depend on this vital program,” CMS Administrator Seema Verma said in a written statement.
How common is accelerated knee OA?
TORONTO – Accelerated knee osteoarthritis – a particularly noxious form of the joint disease – occurred in more than one in seven women who developed knee osteoarthritis in the prospective, long-term Chingford Cohort Study, Jeffrey B. Driban, PhD, reported at the OARSI 2019 World Congress.
This finding from a unique prospective study of 1,003 middle-aged U.K. women who were followed for the development of knee osteoarthritis (OA) for 15 years is important because the participants represented a typical community-based population sample. And yet the Chingford results are consistent with and confirmatory of those found earlier in the Osteoarthritis Initiative, a U.S. cohort study of nearly 4,800 individuals, even though the Osteoarthritis Initiative featured a population enriched with established risk factors for knee OA, Dr. Driban explained at the meeting, sponsored by the Osteoarthritis Research Society International.
In Chingford, accelerated knee OA accounted for 15% of all incident cases of knee OA during follow-up, and for 17% of all newly affected knees, whereas 20% of incident knee OA in the Osteoarthritis Initiative was accelerated knee OA, noted Dr. Driban of Tufts University, Boston.
Accelerated knee OA is defined by rapidly progressive structural damage. Affected individuals streak from no radiographic evidence of knee OA to advanced-stage disease marked by a Kellgren-Lawrence score of 3 or more within 4 years, whereas the typical form of knee OA follows a more gradual course. Also, accelerated knee OA features greater pain and disability.
In the Chingford study, the cumulative incidence of accelerated knee OA was 3.9%, while typical knee OA occurred in 21.7% of women. During years 6-15 of follow-up, 21% of women with accelerated knee OA underwent total knee replacement, compared with 2% of those with typical knee OA and 0.9% of women without knee OA.
Dr. Driban reported having no financial conflicts regarding his analysis of the Chingford Cohort Study and the Osteoarthritis Initiative, supported by Arthritis Research UK and the National Institutes of Health, respectively.
SOURCE: Driban JB et al. Osteoarthritis Cartilage. 2019 Apr;27[suppl 1]:S250-S251, Abstract 352.
TORONTO – Accelerated knee osteoarthritis – a particularly noxious form of the joint disease – occurred in more than one in seven women who developed knee osteoarthritis in the prospective, long-term Chingford Cohort Study, Jeffrey B. Driban, PhD, reported at the OARSI 2019 World Congress.
This finding from a unique prospective study of 1,003 middle-aged U.K. women who were followed for the development of knee osteoarthritis (OA) for 15 years is important because the participants represented a typical community-based population sample. And yet the Chingford results are consistent with and confirmatory of those found earlier in the Osteoarthritis Initiative, a U.S. cohort study of nearly 4,800 individuals, even though the Osteoarthritis Initiative featured a population enriched with established risk factors for knee OA, Dr. Driban explained at the meeting, sponsored by the Osteoarthritis Research Society International.
In Chingford, accelerated knee OA accounted for 15% of all incident cases of knee OA during follow-up, and for 17% of all newly affected knees, whereas 20% of incident knee OA in the Osteoarthritis Initiative was accelerated knee OA, noted Dr. Driban of Tufts University, Boston.
Accelerated knee OA is defined by rapidly progressive structural damage. Affected individuals streak from no radiographic evidence of knee OA to advanced-stage disease marked by a Kellgren-Lawrence score of 3 or more within 4 years, whereas the typical form of knee OA follows a more gradual course. Also, accelerated knee OA features greater pain and disability.
In the Chingford study, the cumulative incidence of accelerated knee OA was 3.9%, while typical knee OA occurred in 21.7% of women. During years 6-15 of follow-up, 21% of women with accelerated knee OA underwent total knee replacement, compared with 2% of those with typical knee OA and 0.9% of women without knee OA.
Dr. Driban reported having no financial conflicts regarding his analysis of the Chingford Cohort Study and the Osteoarthritis Initiative, supported by Arthritis Research UK and the National Institutes of Health, respectively.
SOURCE: Driban JB et al. Osteoarthritis Cartilage. 2019 Apr;27[suppl 1]:S250-S251, Abstract 352.
TORONTO – Accelerated knee osteoarthritis – a particularly noxious form of the joint disease – occurred in more than one in seven women who developed knee osteoarthritis in the prospective, long-term Chingford Cohort Study, Jeffrey B. Driban, PhD, reported at the OARSI 2019 World Congress.
This finding from a unique prospective study of 1,003 middle-aged U.K. women who were followed for the development of knee osteoarthritis (OA) for 15 years is important because the participants represented a typical community-based population sample. And yet the Chingford results are consistent with and confirmatory of those found earlier in the Osteoarthritis Initiative, a U.S. cohort study of nearly 4,800 individuals, even though the Osteoarthritis Initiative featured a population enriched with established risk factors for knee OA, Dr. Driban explained at the meeting, sponsored by the Osteoarthritis Research Society International.
In Chingford, accelerated knee OA accounted for 15% of all incident cases of knee OA during follow-up, and for 17% of all newly affected knees, whereas 20% of incident knee OA in the Osteoarthritis Initiative was accelerated knee OA, noted Dr. Driban of Tufts University, Boston.
Accelerated knee OA is defined by rapidly progressive structural damage. Affected individuals streak from no radiographic evidence of knee OA to advanced-stage disease marked by a Kellgren-Lawrence score of 3 or more within 4 years, whereas the typical form of knee OA follows a more gradual course. Also, accelerated knee OA features greater pain and disability.
In the Chingford study, the cumulative incidence of accelerated knee OA was 3.9%, while typical knee OA occurred in 21.7% of women. During years 6-15 of follow-up, 21% of women with accelerated knee OA underwent total knee replacement, compared with 2% of those with typical knee OA and 0.9% of women without knee OA.
Dr. Driban reported having no financial conflicts regarding his analysis of the Chingford Cohort Study and the Osteoarthritis Initiative, supported by Arthritis Research UK and the National Institutes of Health, respectively.
SOURCE: Driban JB et al. Osteoarthritis Cartilage. 2019 Apr;27[suppl 1]:S250-S251, Abstract 352.
REPORTING FROM OARSI 2019
Impact of Psoriasis Treatment on Comorbidities
1. Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113.
2. Davidovici BB, Sattar N, Prinz J, et al. Psoriasis and systemic inflammatory diseases: potential mechanistic links between skin disease and co-morbid conditions. J Invest Dermatol. 2010;130:1785-1796.
3. Oliveira Mde F, Rocha Bde O, Duarte GV. Psoriasis: classical and emerging comorbidities. An Bras Dermatol. 2015;90:9-20.
4. Shah K, Mellars L, Changolkar A, Feldman SR. Real-world burden of comorbidities in US patients with psoriasis. J Am Acad Dermatol. 2017;77:287-292.
5. Hu SC, Lan CE. Psoriasis and cardiovascular comorbidities: focusing on severe vascular events, cardiovascular risk factors and implications for treatment [published online October 21, 2017]. Int J Mol Sci. doi:10.3390/ijms18102211.
6. Hugh J, Van Voorhees AS, Nijhawan RI, et al. From the Medical Board of The National Psoriasis Foundation: the risk of cardiovascular disease in individuals with psoriasis and the potential impact of current therapies. J Am Acad Dermatol. 2014;70:168-177.
7. Churton S, Brown L, Shin TM, et al. Does treatment of psoriasis reduce the risk of cardiovascular disease? Drugs. 2014;74:169-182.
8. Prodanovich S, Ma F, Taylor J, et al. Methotrexate reduces incidence of vascular diseases in veterans with psoriasis or rheumatoid arthritis. J Am Acad Dermatol. 2005;52:262-226.
9. Gulliver WP, Young HM, Bachelez H, et al. Psoriasis patients treated with biologics and methotrexate have a reduced rate of myocardial infarction: a collaborative analysis using international cohorts. J Cutan Med Surg. 2016;20:550-554.
10. Ahlehoff O, Skov L, Gislason G, et al. Cardiovascular disease event rates in patients with severe psoriasis treated with systemic anti-inflammatory drugs: a Danish real-world cohort study. J Intern Med. 2013;273:197-204.
11. Wu JJ, Poon KY, Channual JC, et al. Association between tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. Arch Dermatol. 2012;148:1244-1250.
12. Wu JJ, Poon KY. Association of ethnicity, tumor necrosis factor inhibitor therapy, and myocardial infarction risk in patients with psoriasis. J Am Acad Dermatol. 2013;69:167-168.
13. Wu JJ, Poon KY, Bebchuk JD. Association between the type and length of tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. J Drugs Dermatol. 2013;12:899-903.
14. Wu JJ, Poon KY, Bebchuk JD. Tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis, psoriatic arthritis, or both. J Drugs Dermatol. 2014;13:932-934.
15. Famenini S, Sako EY, Wu JJ. Effect of treating psoriasis on cardiovascular co-morbidities: focus on TNF inhibitors. Am J Clin Dermatol. 2014;15:45-50.
16. Nguyen T, Wu JJ. Relationship between tumor necrosis factor-alpha inhibitors and cardiovascular disease in psoriasis: a review. Perm J. 2014;18:49-54.
17. Shaaban D, Al-Mutairi N. The effect of tumour necrosis factor inhibitor therapy on the incidence of myocardial infarction in patients with psoriasis: a retrospective study [published online November 17, 2017]. J Dermatol Treat. doi:10.1080/09546634.2016.1254145.
18. Wu D, Hou SY, Zhao S, et al. Efficacy and safety of interleukin-17 antagonists in patients with plaque psoriasis: A meta-analysis from phase 3 randomized controlled trials. J Eur Acad Dermatol Venereol. 2017;31:992-100.
19. Yang ZS, Lin NN, Li L, et al. The effect of TNF inhibitors on cardiovascular events in psoriasis and psoriatic arthritis: an updated meta-analysis. Clin Rev Allergy Immunol. 2016;51:240-247.
20. Heredi E, Vegh J, Pogacsas L, et al. Subclinical cardiovascular disease and it’s improvement after long-term TNF-alpha inhibitor therapy in severe psoriatic patients. J Eur Acad Dermatol Venereol. 2016;30:1531-1536.
21. Pina T, Corrales A, Lopez-Mejias R, et al. Anti-tumor necrosis factor-alpha therapy improves endothelial function and arterial stiffness in patients with moderate to severe psoriasis: a 6-month prospective study. J Dermatol. 2016;43:1267-1272.
22. Piaserico S, Osto E, Famoso G, et al. Treatment with tumor necrosis factor inhibitors restores coronary microvascular function in young patients with severe psoriasis. Atherosclerosis. 2016;251:25-30.
23. Van de Kerkhof PC, Griffiths CE, Reich K, et al. Secukinumab long-term safety experience: a pooled analysis of 10 phase II and III clinical studies in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2016;75:83-98.
24. Wu JJ, Guerin A, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus methotrexate. J Am Acad Dermatol. 2017;76:81-90.
25. Torres T, Raposo I, Selores M. IL-17 blockade in psoriasis: friend or foe in cardiovascular risk? Am J Clin Dermatol. 2016;17:107-112.
26. Deeks ED. Apremilast: a review in psoriasis and psoriatic arthritis. Drugs. 2015;75:1393-1403.
27. Crowley J, Thaci D, Joly P, et al. Long-term safety and tolerability of apremilast in patients with psoriasis: pooled safety analysis for >/= 156 weeks from 2 phase 3, randomized, controlled trials (ESTEEM 1 and 2). J Am Acad Dermatol. 2017;77:310-317.
28. Kavanaugh A, Mease PJ, Gomez-Reino JJ, et al. Treatment of psoriatic arthritis in a phase 3 randomised, placebo-controlled trial with apremilast, an oral phosphodiesterase 4 inhibitor. Ann Rheum Dis. 2014;73:1020-1026.
29. Daudén E, Griffiths CE, Ortonne JP, et al. Improvements in patient-reported outcomes in moderate-to-severe psoriasis patients receiving continuous or paused etanercept treatment over 54 weeks: the CRYSTEL study. J Eur Acad Dermatol Venereol. 2009;23:1374-1382.
30. Menter A, Augustin M, Signorovitch J, et al. The effect of adalimumab on reducing depression symptoms in patients with moderate to severe psoriasis: a randomized clinical trial. J Am Acad Dermatol. 2010;62:812-818.
31. Tyring S, Gottlieb A, Papp K, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29-35.
32. Strober B, Gooderham M, de Jong EMGJ, et al. Depressive symptoms, depression, and the effect of biologic therapy among patients in Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Am Acad Dermatol. 2018;78:70-80.
33. Egeberg A, Khalid U, Gislason GH, et al. Association of psoriatic disease with uveitis: a Danish nationwide cohort study. JAMA Dermatol. 2015;151:1200-1205.
34. Huynh N, Cervantes-Castaneda RA, Bhat P, et al. Biologic response modifier therapy for psoriatic ocular inflammatory disease. Ocul Immunol Inflamm. 2008;16:89-93.
35. Pulusani S, McMurray SL, Jensen K, et al. Psoriasis treatment in patients with sickle cell disease Cutis. 2019;103:93-94.
36. Nnodim J, Meludu SC, Dioka CE, et al. Cytokine expression in homozygous sickle cell anaemia. JKIMSU. 2015;4:34-37.
1. Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113.
2. Davidovici BB, Sattar N, Prinz J, et al. Psoriasis and systemic inflammatory diseases: potential mechanistic links between skin disease and co-morbid conditions. J Invest Dermatol. 2010;130:1785-1796.
3. Oliveira Mde F, Rocha Bde O, Duarte GV. Psoriasis: classical and emerging comorbidities. An Bras Dermatol. 2015;90:9-20.
4. Shah K, Mellars L, Changolkar A, Feldman SR. Real-world burden of comorbidities in US patients with psoriasis. J Am Acad Dermatol. 2017;77:287-292.
5. Hu SC, Lan CE. Psoriasis and cardiovascular comorbidities: focusing on severe vascular events, cardiovascular risk factors and implications for treatment [published online October 21, 2017]. Int J Mol Sci. doi:10.3390/ijms18102211.
6. Hugh J, Van Voorhees AS, Nijhawan RI, et al. From the Medical Board of The National Psoriasis Foundation: the risk of cardiovascular disease in individuals with psoriasis and the potential impact of current therapies. J Am Acad Dermatol. 2014;70:168-177.
7. Churton S, Brown L, Shin TM, et al. Does treatment of psoriasis reduce the risk of cardiovascular disease? Drugs. 2014;74:169-182.
8. Prodanovich S, Ma F, Taylor J, et al. Methotrexate reduces incidence of vascular diseases in veterans with psoriasis or rheumatoid arthritis. J Am Acad Dermatol. 2005;52:262-226.
9. Gulliver WP, Young HM, Bachelez H, et al. Psoriasis patients treated with biologics and methotrexate have a reduced rate of myocardial infarction: a collaborative analysis using international cohorts. J Cutan Med Surg. 2016;20:550-554.
10. Ahlehoff O, Skov L, Gislason G, et al. Cardiovascular disease event rates in patients with severe psoriasis treated with systemic anti-inflammatory drugs: a Danish real-world cohort study. J Intern Med. 2013;273:197-204.
11. Wu JJ, Poon KY, Channual JC, et al. Association between tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. Arch Dermatol. 2012;148:1244-1250.
12. Wu JJ, Poon KY. Association of ethnicity, tumor necrosis factor inhibitor therapy, and myocardial infarction risk in patients with psoriasis. J Am Acad Dermatol. 2013;69:167-168.
13. Wu JJ, Poon KY, Bebchuk JD. Association between the type and length of tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. J Drugs Dermatol. 2013;12:899-903.
14. Wu JJ, Poon KY, Bebchuk JD. Tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis, psoriatic arthritis, or both. J Drugs Dermatol. 2014;13:932-934.
15. Famenini S, Sako EY, Wu JJ. Effect of treating psoriasis on cardiovascular co-morbidities: focus on TNF inhibitors. Am J Clin Dermatol. 2014;15:45-50.
16. Nguyen T, Wu JJ. Relationship between tumor necrosis factor-alpha inhibitors and cardiovascular disease in psoriasis: a review. Perm J. 2014;18:49-54.
17. Shaaban D, Al-Mutairi N. The effect of tumour necrosis factor inhibitor therapy on the incidence of myocardial infarction in patients with psoriasis: a retrospective study [published online November 17, 2017]. J Dermatol Treat. doi:10.1080/09546634.2016.1254145.
18. Wu D, Hou SY, Zhao S, et al. Efficacy and safety of interleukin-17 antagonists in patients with plaque psoriasis: A meta-analysis from phase 3 randomized controlled trials. J Eur Acad Dermatol Venereol. 2017;31:992-100.
19. Yang ZS, Lin NN, Li L, et al. The effect of TNF inhibitors on cardiovascular events in psoriasis and psoriatic arthritis: an updated meta-analysis. Clin Rev Allergy Immunol. 2016;51:240-247.
20. Heredi E, Vegh J, Pogacsas L, et al. Subclinical cardiovascular disease and it’s improvement after long-term TNF-alpha inhibitor therapy in severe psoriatic patients. J Eur Acad Dermatol Venereol. 2016;30:1531-1536.
21. Pina T, Corrales A, Lopez-Mejias R, et al. Anti-tumor necrosis factor-alpha therapy improves endothelial function and arterial stiffness in patients with moderate to severe psoriasis: a 6-month prospective study. J Dermatol. 2016;43:1267-1272.
22. Piaserico S, Osto E, Famoso G, et al. Treatment with tumor necrosis factor inhibitors restores coronary microvascular function in young patients with severe psoriasis. Atherosclerosis. 2016;251:25-30.
23. Van de Kerkhof PC, Griffiths CE, Reich K, et al. Secukinumab long-term safety experience: a pooled analysis of 10 phase II and III clinical studies in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2016;75:83-98.
24. Wu JJ, Guerin A, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus methotrexate. J Am Acad Dermatol. 2017;76:81-90.
25. Torres T, Raposo I, Selores M. IL-17 blockade in psoriasis: friend or foe in cardiovascular risk? Am J Clin Dermatol. 2016;17:107-112.
26. Deeks ED. Apremilast: a review in psoriasis and psoriatic arthritis. Drugs. 2015;75:1393-1403.
27. Crowley J, Thaci D, Joly P, et al. Long-term safety and tolerability of apremilast in patients with psoriasis: pooled safety analysis for >/= 156 weeks from 2 phase 3, randomized, controlled trials (ESTEEM 1 and 2). J Am Acad Dermatol. 2017;77:310-317.
28. Kavanaugh A, Mease PJ, Gomez-Reino JJ, et al. Treatment of psoriatic arthritis in a phase 3 randomised, placebo-controlled trial with apremilast, an oral phosphodiesterase 4 inhibitor. Ann Rheum Dis. 2014;73:1020-1026.
29. Daudén E, Griffiths CE, Ortonne JP, et al. Improvements in patient-reported outcomes in moderate-to-severe psoriasis patients receiving continuous or paused etanercept treatment over 54 weeks: the CRYSTEL study. J Eur Acad Dermatol Venereol. 2009;23:1374-1382.
30. Menter A, Augustin M, Signorovitch J, et al. The effect of adalimumab on reducing depression symptoms in patients with moderate to severe psoriasis: a randomized clinical trial. J Am Acad Dermatol. 2010;62:812-818.
31. Tyring S, Gottlieb A, Papp K, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29-35.
32. Strober B, Gooderham M, de Jong EMGJ, et al. Depressive symptoms, depression, and the effect of biologic therapy among patients in Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Am Acad Dermatol. 2018;78:70-80.
33. Egeberg A, Khalid U, Gislason GH, et al. Association of psoriatic disease with uveitis: a Danish nationwide cohort study. JAMA Dermatol. 2015;151:1200-1205.
34. Huynh N, Cervantes-Castaneda RA, Bhat P, et al. Biologic response modifier therapy for psoriatic ocular inflammatory disease. Ocul Immunol Inflamm. 2008;16:89-93.
35. Pulusani S, McMurray SL, Jensen K, et al. Psoriasis treatment in patients with sickle cell disease Cutis. 2019;103:93-94.
36. Nnodim J, Meludu SC, Dioka CE, et al. Cytokine expression in homozygous sickle cell anaemia. JKIMSU. 2015;4:34-37.
1. Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113.
2. Davidovici BB, Sattar N, Prinz J, et al. Psoriasis and systemic inflammatory diseases: potential mechanistic links between skin disease and co-morbid conditions. J Invest Dermatol. 2010;130:1785-1796.
3. Oliveira Mde F, Rocha Bde O, Duarte GV. Psoriasis: classical and emerging comorbidities. An Bras Dermatol. 2015;90:9-20.
4. Shah K, Mellars L, Changolkar A, Feldman SR. Real-world burden of comorbidities in US patients with psoriasis. J Am Acad Dermatol. 2017;77:287-292.
5. Hu SC, Lan CE. Psoriasis and cardiovascular comorbidities: focusing on severe vascular events, cardiovascular risk factors and implications for treatment [published online October 21, 2017]. Int J Mol Sci. doi:10.3390/ijms18102211.
6. Hugh J, Van Voorhees AS, Nijhawan RI, et al. From the Medical Board of The National Psoriasis Foundation: the risk of cardiovascular disease in individuals with psoriasis and the potential impact of current therapies. J Am Acad Dermatol. 2014;70:168-177.
7. Churton S, Brown L, Shin TM, et al. Does treatment of psoriasis reduce the risk of cardiovascular disease? Drugs. 2014;74:169-182.
8. Prodanovich S, Ma F, Taylor J, et al. Methotrexate reduces incidence of vascular diseases in veterans with psoriasis or rheumatoid arthritis. J Am Acad Dermatol. 2005;52:262-226.
9. Gulliver WP, Young HM, Bachelez H, et al. Psoriasis patients treated with biologics and methotrexate have a reduced rate of myocardial infarction: a collaborative analysis using international cohorts. J Cutan Med Surg. 2016;20:550-554.
10. Ahlehoff O, Skov L, Gislason G, et al. Cardiovascular disease event rates in patients with severe psoriasis treated with systemic anti-inflammatory drugs: a Danish real-world cohort study. J Intern Med. 2013;273:197-204.
11. Wu JJ, Poon KY, Channual JC, et al. Association between tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. Arch Dermatol. 2012;148:1244-1250.
12. Wu JJ, Poon KY. Association of ethnicity, tumor necrosis factor inhibitor therapy, and myocardial infarction risk in patients with psoriasis. J Am Acad Dermatol. 2013;69:167-168.
13. Wu JJ, Poon KY, Bebchuk JD. Association between the type and length of tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. J Drugs Dermatol. 2013;12:899-903.
14. Wu JJ, Poon KY, Bebchuk JD. Tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis, psoriatic arthritis, or both. J Drugs Dermatol. 2014;13:932-934.
15. Famenini S, Sako EY, Wu JJ. Effect of treating psoriasis on cardiovascular co-morbidities: focus on TNF inhibitors. Am J Clin Dermatol. 2014;15:45-50.
16. Nguyen T, Wu JJ. Relationship between tumor necrosis factor-alpha inhibitors and cardiovascular disease in psoriasis: a review. Perm J. 2014;18:49-54.
17. Shaaban D, Al-Mutairi N. The effect of tumour necrosis factor inhibitor therapy on the incidence of myocardial infarction in patients with psoriasis: a retrospective study [published online November 17, 2017]. J Dermatol Treat. doi:10.1080/09546634.2016.1254145.
18. Wu D, Hou SY, Zhao S, et al. Efficacy and safety of interleukin-17 antagonists in patients with plaque psoriasis: A meta-analysis from phase 3 randomized controlled trials. J Eur Acad Dermatol Venereol. 2017;31:992-100.
19. Yang ZS, Lin NN, Li L, et al. The effect of TNF inhibitors on cardiovascular events in psoriasis and psoriatic arthritis: an updated meta-analysis. Clin Rev Allergy Immunol. 2016;51:240-247.
20. Heredi E, Vegh J, Pogacsas L, et al. Subclinical cardiovascular disease and it’s improvement after long-term TNF-alpha inhibitor therapy in severe psoriatic patients. J Eur Acad Dermatol Venereol. 2016;30:1531-1536.
21. Pina T, Corrales A, Lopez-Mejias R, et al. Anti-tumor necrosis factor-alpha therapy improves endothelial function and arterial stiffness in patients with moderate to severe psoriasis: a 6-month prospective study. J Dermatol. 2016;43:1267-1272.
22. Piaserico S, Osto E, Famoso G, et al. Treatment with tumor necrosis factor inhibitors restores coronary microvascular function in young patients with severe psoriasis. Atherosclerosis. 2016;251:25-30.
23. Van de Kerkhof PC, Griffiths CE, Reich K, et al. Secukinumab long-term safety experience: a pooled analysis of 10 phase II and III clinical studies in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2016;75:83-98.
24. Wu JJ, Guerin A, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus methotrexate. J Am Acad Dermatol. 2017;76:81-90.
25. Torres T, Raposo I, Selores M. IL-17 blockade in psoriasis: friend or foe in cardiovascular risk? Am J Clin Dermatol. 2016;17:107-112.
26. Deeks ED. Apremilast: a review in psoriasis and psoriatic arthritis. Drugs. 2015;75:1393-1403.
27. Crowley J, Thaci D, Joly P, et al. Long-term safety and tolerability of apremilast in patients with psoriasis: pooled safety analysis for >/= 156 weeks from 2 phase 3, randomized, controlled trials (ESTEEM 1 and 2). J Am Acad Dermatol. 2017;77:310-317.
28. Kavanaugh A, Mease PJ, Gomez-Reino JJ, et al. Treatment of psoriatic arthritis in a phase 3 randomised, placebo-controlled trial with apremilast, an oral phosphodiesterase 4 inhibitor. Ann Rheum Dis. 2014;73:1020-1026.
29. Daudén E, Griffiths CE, Ortonne JP, et al. Improvements in patient-reported outcomes in moderate-to-severe psoriasis patients receiving continuous or paused etanercept treatment over 54 weeks: the CRYSTEL study. J Eur Acad Dermatol Venereol. 2009;23:1374-1382.
30. Menter A, Augustin M, Signorovitch J, et al. The effect of adalimumab on reducing depression symptoms in patients with moderate to severe psoriasis: a randomized clinical trial. J Am Acad Dermatol. 2010;62:812-818.
31. Tyring S, Gottlieb A, Papp K, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29-35.
32. Strober B, Gooderham M, de Jong EMGJ, et al. Depressive symptoms, depression, and the effect of biologic therapy among patients in Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Am Acad Dermatol. 2018;78:70-80.
33. Egeberg A, Khalid U, Gislason GH, et al. Association of psoriatic disease with uveitis: a Danish nationwide cohort study. JAMA Dermatol. 2015;151:1200-1205.
34. Huynh N, Cervantes-Castaneda RA, Bhat P, et al. Biologic response modifier therapy for psoriatic ocular inflammatory disease. Ocul Immunol Inflamm. 2008;16:89-93.
35. Pulusani S, McMurray SL, Jensen K, et al. Psoriasis treatment in patients with sickle cell disease Cutis. 2019;103:93-94.
36. Nnodim J, Meludu SC, Dioka CE, et al. Cytokine expression in homozygous sickle cell anaemia. JKIMSU. 2015;4:34-37.
NIH launches 5-year, $10 million study on acute flaccid myelitis
Researchers at the University of Alabama at Birmingham will lead a 5-year, federally-funded study of acute flaccid myelitis (AFM) – a rare pediatric neurologic disease.
The National Institute of Allergy and Infectious Diseases (NIAID) awarded the $10 million grant to primary investigator David Kimberlin, MD, a UAB professor of pediatrics. Carlos Pardo-Villamizar, MD, professor of neurology and pathology at Johns Hopkins University, Baltimore, is the co-principal investigator.
The university will organize and implement the international, multisite study. Its primary goal is to examine the incidence and distribution of AFM, and its pathogenesis and progression. Enrollment is expected to commence next fall. Investigators will enroll children with symptoms of AFM and follow them for 1 year. Household contacts of the subjects will serve as comparators.
In addition to collecting data about risk factors and disease progression, the researchers will collect clinical specimens, including blood and cerebrospinal fluid. More details about the design and study sites will be released then, according to a press statement issued by NIAID.
AFM targets spinal nerves and often develops after a mild respiratory illness. The disease mounted a global epidemic comeback in 2014, primarily affecting children; it has occurred concurrently with enterovirus outbreaks.
“Growing epidemiological evidence suggests that enterovirus-D68 [EV-D68] could play a role,” the statement noted. “Most people who become infected with EV-D68 are asymptomatic or experience mild, cold-like symptoms. Researchers and physicians are working to understand if there is a connection between these viral outbreaks and AFM, and if so, why some children but not others experience this sudden muscle weakness and paralysis.”
The study will draw on the expertise of the AFM Task Force, established last fall. The group comprises physicians, scientists, and public health experts from diverse disciplines and institutions who will assist in the ongoing investigation.
The AFM natural history study is funded under contract HHSN272201600018C.
Researchers at the University of Alabama at Birmingham will lead a 5-year, federally-funded study of acute flaccid myelitis (AFM) – a rare pediatric neurologic disease.
The National Institute of Allergy and Infectious Diseases (NIAID) awarded the $10 million grant to primary investigator David Kimberlin, MD, a UAB professor of pediatrics. Carlos Pardo-Villamizar, MD, professor of neurology and pathology at Johns Hopkins University, Baltimore, is the co-principal investigator.
The university will organize and implement the international, multisite study. Its primary goal is to examine the incidence and distribution of AFM, and its pathogenesis and progression. Enrollment is expected to commence next fall. Investigators will enroll children with symptoms of AFM and follow them for 1 year. Household contacts of the subjects will serve as comparators.
In addition to collecting data about risk factors and disease progression, the researchers will collect clinical specimens, including blood and cerebrospinal fluid. More details about the design and study sites will be released then, according to a press statement issued by NIAID.
AFM targets spinal nerves and often develops after a mild respiratory illness. The disease mounted a global epidemic comeback in 2014, primarily affecting children; it has occurred concurrently with enterovirus outbreaks.
“Growing epidemiological evidence suggests that enterovirus-D68 [EV-D68] could play a role,” the statement noted. “Most people who become infected with EV-D68 are asymptomatic or experience mild, cold-like symptoms. Researchers and physicians are working to understand if there is a connection between these viral outbreaks and AFM, and if so, why some children but not others experience this sudden muscle weakness and paralysis.”
The study will draw on the expertise of the AFM Task Force, established last fall. The group comprises physicians, scientists, and public health experts from diverse disciplines and institutions who will assist in the ongoing investigation.
The AFM natural history study is funded under contract HHSN272201600018C.
Researchers at the University of Alabama at Birmingham will lead a 5-year, federally-funded study of acute flaccid myelitis (AFM) – a rare pediatric neurologic disease.
The National Institute of Allergy and Infectious Diseases (NIAID) awarded the $10 million grant to primary investigator David Kimberlin, MD, a UAB professor of pediatrics. Carlos Pardo-Villamizar, MD, professor of neurology and pathology at Johns Hopkins University, Baltimore, is the co-principal investigator.
The university will organize and implement the international, multisite study. Its primary goal is to examine the incidence and distribution of AFM, and its pathogenesis and progression. Enrollment is expected to commence next fall. Investigators will enroll children with symptoms of AFM and follow them for 1 year. Household contacts of the subjects will serve as comparators.
In addition to collecting data about risk factors and disease progression, the researchers will collect clinical specimens, including blood and cerebrospinal fluid. More details about the design and study sites will be released then, according to a press statement issued by NIAID.
AFM targets spinal nerves and often develops after a mild respiratory illness. The disease mounted a global epidemic comeback in 2014, primarily affecting children; it has occurred concurrently with enterovirus outbreaks.
“Growing epidemiological evidence suggests that enterovirus-D68 [EV-D68] could play a role,” the statement noted. “Most people who become infected with EV-D68 are asymptomatic or experience mild, cold-like symptoms. Researchers and physicians are working to understand if there is a connection between these viral outbreaks and AFM, and if so, why some children but not others experience this sudden muscle weakness and paralysis.”
The study will draw on the expertise of the AFM Task Force, established last fall. The group comprises physicians, scientists, and public health experts from diverse disciplines and institutions who will assist in the ongoing investigation.
The AFM natural history study is funded under contract HHSN272201600018C.
Depression, anxiety among elderly breast cancer survivors linked to increased opioid use, death
Mental health comorbidities increase the rates of opioid use and mortality among breast cancer survivors on endocrine therapy, based on a retrospective study of more than 10,000 patients in a Medicare-linked database.
Screen for mental health conditions in the early stages of cancer care and lean toward opioid alternatives for pain management, advised lead author Raj Desai, MS, of the University of Florida, Gainesville, and colleagues.
“The complex relationship among breast cancer, mental health problems, and the use of opioids is not well understood, despite the high prevalence of mental health comorbidities like depression and anxiety in breast cancer survivors, and the high rate of opioid use in those on AET [adjuvant endocrine therapy],” the investigators wrote in the Journal of Oncology Practice.
“Therefore, this study aimed to determine whether breast cancer survivors with varying levels of mental health comorbidities, such as depression and anxiety, are more likely to use opioids for AET-related pain,” they added.
The study involved 10,452 breast cancer survivors who first filled an AET prescription from 2006 to 2012 and had follow-up records available for at least 2 years. All patients had a diagnosis of incident, primary, hormone receptor–positive, stage I-III breast cancer. Data were drawn from the Surveillance, Epidemiology, and End Results–Medicare linked database. Records were evaluated for diagnoses of mental health conditions such as depression and anxiety, opioid use, and survival.
Analysis showed that the most common mental health conditions were depression and anxiety, diagnosed in 554 and 246 women, respectively. Patients with mental health comorbidities were compared with patients who did not have such problems, using both unmatched and matched cohorts. While unmatched comparison for opioid use was not statistically significant, matched comparison showed that survivors with mental health comorbidities were 33% more likely to use opioids than those without mental health comorbidities (95% confidence interval, 1.06-1.68). Similarly, greater adjusted probabilities of opioid use were reported in the mental health comorbidity cohort (72.5% vs. 66.9%; P = .01).
Concerning survival, unmatched comparison revealed a 44% higher risk of death among women with depression and a 32% increase associated with anxiety. Matched comparison showed an even higher increased risk of mortality among women with any mental health comorbidity (49%; P less than .05).
The investigators concluded that opioid use among breast cancer survivors with mental health comorbidities “remains a significant problem.”
“A need exists for collaborative care in the management of mental health comorbidities in women with breast cancer, which could improve symptoms, adherence to treatment, and recovery from these mental conditions,” the investigators wrote. “Mental health treatments also are recommended to be offered in primary care, which not only would be convenient for patients, but also would reduce the stigma associated with treatments for mental health comorbidities and improve the patient-provider relationship.”
The investigators reported financial relationships with Merck.
SOURCE: Desai R et al. J Oncol Pract. 2019 Jul 19. doi: 10.1200/JOP.18.00781.
Mental health comorbidities increase the rates of opioid use and mortality among breast cancer survivors on endocrine therapy, based on a retrospective study of more than 10,000 patients in a Medicare-linked database.
Screen for mental health conditions in the early stages of cancer care and lean toward opioid alternatives for pain management, advised lead author Raj Desai, MS, of the University of Florida, Gainesville, and colleagues.
“The complex relationship among breast cancer, mental health problems, and the use of opioids is not well understood, despite the high prevalence of mental health comorbidities like depression and anxiety in breast cancer survivors, and the high rate of opioid use in those on AET [adjuvant endocrine therapy],” the investigators wrote in the Journal of Oncology Practice.
“Therefore, this study aimed to determine whether breast cancer survivors with varying levels of mental health comorbidities, such as depression and anxiety, are more likely to use opioids for AET-related pain,” they added.
The study involved 10,452 breast cancer survivors who first filled an AET prescription from 2006 to 2012 and had follow-up records available for at least 2 years. All patients had a diagnosis of incident, primary, hormone receptor–positive, stage I-III breast cancer. Data were drawn from the Surveillance, Epidemiology, and End Results–Medicare linked database. Records were evaluated for diagnoses of mental health conditions such as depression and anxiety, opioid use, and survival.
Analysis showed that the most common mental health conditions were depression and anxiety, diagnosed in 554 and 246 women, respectively. Patients with mental health comorbidities were compared with patients who did not have such problems, using both unmatched and matched cohorts. While unmatched comparison for opioid use was not statistically significant, matched comparison showed that survivors with mental health comorbidities were 33% more likely to use opioids than those without mental health comorbidities (95% confidence interval, 1.06-1.68). Similarly, greater adjusted probabilities of opioid use were reported in the mental health comorbidity cohort (72.5% vs. 66.9%; P = .01).
Concerning survival, unmatched comparison revealed a 44% higher risk of death among women with depression and a 32% increase associated with anxiety. Matched comparison showed an even higher increased risk of mortality among women with any mental health comorbidity (49%; P less than .05).
The investigators concluded that opioid use among breast cancer survivors with mental health comorbidities “remains a significant problem.”
“A need exists for collaborative care in the management of mental health comorbidities in women with breast cancer, which could improve symptoms, adherence to treatment, and recovery from these mental conditions,” the investigators wrote. “Mental health treatments also are recommended to be offered in primary care, which not only would be convenient for patients, but also would reduce the stigma associated with treatments for mental health comorbidities and improve the patient-provider relationship.”
The investigators reported financial relationships with Merck.
SOURCE: Desai R et al. J Oncol Pract. 2019 Jul 19. doi: 10.1200/JOP.18.00781.
Mental health comorbidities increase the rates of opioid use and mortality among breast cancer survivors on endocrine therapy, based on a retrospective study of more than 10,000 patients in a Medicare-linked database.
Screen for mental health conditions in the early stages of cancer care and lean toward opioid alternatives for pain management, advised lead author Raj Desai, MS, of the University of Florida, Gainesville, and colleagues.
“The complex relationship among breast cancer, mental health problems, and the use of opioids is not well understood, despite the high prevalence of mental health comorbidities like depression and anxiety in breast cancer survivors, and the high rate of opioid use in those on AET [adjuvant endocrine therapy],” the investigators wrote in the Journal of Oncology Practice.
“Therefore, this study aimed to determine whether breast cancer survivors with varying levels of mental health comorbidities, such as depression and anxiety, are more likely to use opioids for AET-related pain,” they added.
The study involved 10,452 breast cancer survivors who first filled an AET prescription from 2006 to 2012 and had follow-up records available for at least 2 years. All patients had a diagnosis of incident, primary, hormone receptor–positive, stage I-III breast cancer. Data were drawn from the Surveillance, Epidemiology, and End Results–Medicare linked database. Records were evaluated for diagnoses of mental health conditions such as depression and anxiety, opioid use, and survival.
Analysis showed that the most common mental health conditions were depression and anxiety, diagnosed in 554 and 246 women, respectively. Patients with mental health comorbidities were compared with patients who did not have such problems, using both unmatched and matched cohorts. While unmatched comparison for opioid use was not statistically significant, matched comparison showed that survivors with mental health comorbidities were 33% more likely to use opioids than those without mental health comorbidities (95% confidence interval, 1.06-1.68). Similarly, greater adjusted probabilities of opioid use were reported in the mental health comorbidity cohort (72.5% vs. 66.9%; P = .01).
Concerning survival, unmatched comparison revealed a 44% higher risk of death among women with depression and a 32% increase associated with anxiety. Matched comparison showed an even higher increased risk of mortality among women with any mental health comorbidity (49%; P less than .05).
The investigators concluded that opioid use among breast cancer survivors with mental health comorbidities “remains a significant problem.”
“A need exists for collaborative care in the management of mental health comorbidities in women with breast cancer, which could improve symptoms, adherence to treatment, and recovery from these mental conditions,” the investigators wrote. “Mental health treatments also are recommended to be offered in primary care, which not only would be convenient for patients, but also would reduce the stigma associated with treatments for mental health comorbidities and improve the patient-provider relationship.”
The investigators reported financial relationships with Merck.
SOURCE: Desai R et al. J Oncol Pract. 2019 Jul 19. doi: 10.1200/JOP.18.00781.
FROM THE JOURNAL OF ONCOLOGY PRACTICE
Do prophylactic PPIs improve mortality in critically ill patients?
Background: Prophylactic proton pump inhibitors (PPIs) are used frequently in an ICU setting for acid suppression, but this is an off-label use and the evidence in support of using PPI prophylactically is limited. In fact, PPIs have been associated with adverse effects in recent literature including Clostridium difficile infection, myocardial ischemia, and pneumonia.
Study design: Multicenter, parallel group, blinded clinical trial that compared PPI with placebo.
Setting: 78 sites in the United States and Canada.
Synopsis: Among 3,298 total participants, 90-day mortality was 31.1% in the pantoprazole group and 30.4% in the placebo group, which is a relative risk of 1.02 (95% confidence interval, 0.91-1.13; P = .76).
The researchers also used a composite outcome comprising clinically important gastrointestinal bleeding, Clostridium difficile infection, new onset pneumonia, and acute myocardial ischemia. Overall, 21.9% in the pantoprazole group and 22.6% participants in the placebo group had the composite outcome – a relative risk of 0.96 (95% CI, 0.83-1.11). Clinically important gastrointestinal bleeding was the only component of the composite outcome that was significantly different between groups, occurring less often in the pantoprazole group – the relative risk was 0.58 (95% CI, 0.40-0.86).
Bottom line: Pantoprazole does not differ significantly, compared with placebo, with regard to 90-day mortality and a composite outcome of clinically significant events.
Citation: Krag M et al. Pantoprazole in patients at risk of gastrointestinal bleeding in the ICU. N Eng J Med. 2018 Dec 6;379(23):2199-208.
Dr. Puri is assistant professor of medicine at Northwestern University Feinberg School of Medicine and a hospitalist at Northwestern Memorial Hospital, both in Chicago.
Background: Prophylactic proton pump inhibitors (PPIs) are used frequently in an ICU setting for acid suppression, but this is an off-label use and the evidence in support of using PPI prophylactically is limited. In fact, PPIs have been associated with adverse effects in recent literature including Clostridium difficile infection, myocardial ischemia, and pneumonia.
Study design: Multicenter, parallel group, blinded clinical trial that compared PPI with placebo.
Setting: 78 sites in the United States and Canada.
Synopsis: Among 3,298 total participants, 90-day mortality was 31.1% in the pantoprazole group and 30.4% in the placebo group, which is a relative risk of 1.02 (95% confidence interval, 0.91-1.13; P = .76).
The researchers also used a composite outcome comprising clinically important gastrointestinal bleeding, Clostridium difficile infection, new onset pneumonia, and acute myocardial ischemia. Overall, 21.9% in the pantoprazole group and 22.6% participants in the placebo group had the composite outcome – a relative risk of 0.96 (95% CI, 0.83-1.11). Clinically important gastrointestinal bleeding was the only component of the composite outcome that was significantly different between groups, occurring less often in the pantoprazole group – the relative risk was 0.58 (95% CI, 0.40-0.86).
Bottom line: Pantoprazole does not differ significantly, compared with placebo, with regard to 90-day mortality and a composite outcome of clinically significant events.
Citation: Krag M et al. Pantoprazole in patients at risk of gastrointestinal bleeding in the ICU. N Eng J Med. 2018 Dec 6;379(23):2199-208.
Dr. Puri is assistant professor of medicine at Northwestern University Feinberg School of Medicine and a hospitalist at Northwestern Memorial Hospital, both in Chicago.
Background: Prophylactic proton pump inhibitors (PPIs) are used frequently in an ICU setting for acid suppression, but this is an off-label use and the evidence in support of using PPI prophylactically is limited. In fact, PPIs have been associated with adverse effects in recent literature including Clostridium difficile infection, myocardial ischemia, and pneumonia.
Study design: Multicenter, parallel group, blinded clinical trial that compared PPI with placebo.
Setting: 78 sites in the United States and Canada.
Synopsis: Among 3,298 total participants, 90-day mortality was 31.1% in the pantoprazole group and 30.4% in the placebo group, which is a relative risk of 1.02 (95% confidence interval, 0.91-1.13; P = .76).
The researchers also used a composite outcome comprising clinically important gastrointestinal bleeding, Clostridium difficile infection, new onset pneumonia, and acute myocardial ischemia. Overall, 21.9% in the pantoprazole group and 22.6% participants in the placebo group had the composite outcome – a relative risk of 0.96 (95% CI, 0.83-1.11). Clinically important gastrointestinal bleeding was the only component of the composite outcome that was significantly different between groups, occurring less often in the pantoprazole group – the relative risk was 0.58 (95% CI, 0.40-0.86).
Bottom line: Pantoprazole does not differ significantly, compared with placebo, with regard to 90-day mortality and a composite outcome of clinically significant events.
Citation: Krag M et al. Pantoprazole in patients at risk of gastrointestinal bleeding in the ICU. N Eng J Med. 2018 Dec 6;379(23):2199-208.
Dr. Puri is assistant professor of medicine at Northwestern University Feinberg School of Medicine and a hospitalist at Northwestern Memorial Hospital, both in Chicago.
Summary: American Medical Society for Sports Medicine position statement on concussion in sport
An estimated 1-1.8 million sport-related concussions (SRC) occur per year in patients younger than 18 years of age. Concussion is defined as “a traumatically induced transient disturbance of brain function.” More than 50% of concussions among high school youth are not related to organized sports and between 2% and 15% of athletes in organized sports will sustain a concussion during a season of play.
which will be described in this article. The guidelines include recommendations for imaging, treatment, and decision making regarding when as well as whether to return to play. Here is a brief summary of those recommendations.
Preseason: Preseason evaluation includes a preparticipation physical evaluation and discussion of concussion history as well as risk factors associated with prolonged concussion recovery. Neurocognitive tests are available for baseline evaluation. While these may assist with diagnosis and return-to-play decisions, there can be considerable variation in an individual’s baseline score as well as the possibility of changes in that baseline over time. Because of this potential for variability, these tests are not required or accepted as the standard of care.
Sideline assessment: Familiarity with the athlete is the best way to detect subtle changes in personality or performance. Looking at symptoms is still the most sensitive way to diagnose a concussion. Loss of consciousness, seizure, tonic posturing, lack of motor coordination, confusion, amnesia, difficulty with balance, or any cognitive difficulty should prompt removal from play for possible concussion. Once a potential injury is identified, how the athlete responds to the elements of orientation, memory, concentration, speech pattern, and balance should be evaluated. If an athlete has a probable or definite concussion, the athlete needs to be removed from play and cannot return to same-day play, and a more detailed evaluation needs to be done.
Office assessment: It is not unusual for symptoms and testing to normalize by the time an office visit occurs. If this is the case, the visit should focus on recommendations for safe return to school and sport. A standard office evaluation should include taking a history with details of the mechanism of injury and preexisting conditions – such as depression and prior concussion – that can affect concussion recovery. The history should focus on detecting symptoms that typically cause impairment from concussion: headache, ocular-vestibular issues leading to problems with balance, and cognitive issues with difficulty concentrating and remembering, as well as fatigue and mood issues such as anxiety, irritability, and depression. The physical exam should include assessment of ocular and vestibular function, gait, and balance in addition to a neurological exam.
Imaging: Head CT or MRI are rarely indicated. Intracranial bleeds are rare in the context of SRC but can occur. If there is concern for a bleed, then CT scan is the imaging test of choice. MRI may have value for evaluation for atypical or prolonged recovery.
Recovery time: The large majority (80%-90%) of concussed older adolescents and adults return to preinjury levels of function within 2 weeks; in younger athletes, clinical recovery may take up to 4 weeks. The best predictor of recovery from SRC is the number and severity of symptoms.
Treatment: For decades, cognitive and physical rest has been the standard of treatment. However, this is no longer the “gold standard” as it has been shown that strict rest (“cocoon therapy”) after SRC slows recovery and leads to an increased chance of prolonged symptoms. Current consensus guidelines support 24-48 hours of symptom-limited rest, both cognitive and physical, followed by a gradual increase in activity, staying below symptom-exacerbation thresholds. Activity, along with good sleep hygiene, appears to be helpful in facilitating recovery from SRC. In athletes with persistent post concussive symptoms that continue beyond the expected recovery time frame, activities of daily living, school, and exercise that do not significantly exacerbate symptoms are recommended.
Return to learning/play: A concussion can cause temporary deficits in attention, cognitive processing, short-term memory, and executive functioning. School personnel should be informed of the injury and assist in employing an individualized return to learn plan, including academic accommodations. Ultimately, return to sports activities should follow a successful return to the classroom. Return to play involves a stepwise increase in physical demands/activity without symptoms before a student is allowed to participate in full contact play.
Concussion-related risks: Continuing to participate in sports before resolution of concussion can worsen and prolong symptoms of SRC. Returning too early after concussion, before full recovery, increases the risk of recurrent SRC. During the initial post-injury period, returning to sports too early increases the risk for a rare but devastating possibility of second impact syndrome that can be a life-threatening repeat head injury. Studies of long-term mental health diagnoses are conflicting and inconsistent. Chronic traumatic encephalopathy has been described in athletes with a long history of concussions and repetitive sub-symptom head impacts. The degree of exposure needed appears to be variable and dependent on the individual.
Disqualification from play: Because each athlete is individually assessed after SRC, there are no evidence-based studies indicating how many concussions are “safe” for an athlete to have in a lifetime. The decision to stop playing sports is both serious and difficult for most athletes and requires shared decision making between clinician, the athlete, and the athlete’s parents. Factors to consider when determining if disqualification from play is warranted include:
- The total number of concussions experienced by a patient.
- Whether a patient has sustained subsequent concussions with progressively less forceful blows to the head.
- If a patient has sustained multiple concussions,whether the time to complete a full recovery after each concussion event increased.
The bottom line: “Cocoon therapy” is no longer recommended. Consensus guidelines endorse 24-48 hours of symptom-limited cognitive and physical rest followed by a gradual increase in activity, including noncontact physical activity that does not provoke symptoms.
Dr. Belogorodsky is a second-year resident and Dr. Fidler is an associate director in the Family Medicine Residency Program at Abington (Pa.) Jefferson Health. Dr. Skolnik is professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington Jefferson Health.
Reference
Harmon KG et al. American Medical Society for Sports Medicine position statement on concussion in sport. Br J Sports Med. 2019;53:213-25.
An estimated 1-1.8 million sport-related concussions (SRC) occur per year in patients younger than 18 years of age. Concussion is defined as “a traumatically induced transient disturbance of brain function.” More than 50% of concussions among high school youth are not related to organized sports and between 2% and 15% of athletes in organized sports will sustain a concussion during a season of play.
which will be described in this article. The guidelines include recommendations for imaging, treatment, and decision making regarding when as well as whether to return to play. Here is a brief summary of those recommendations.
Preseason: Preseason evaluation includes a preparticipation physical evaluation and discussion of concussion history as well as risk factors associated with prolonged concussion recovery. Neurocognitive tests are available for baseline evaluation. While these may assist with diagnosis and return-to-play decisions, there can be considerable variation in an individual’s baseline score as well as the possibility of changes in that baseline over time. Because of this potential for variability, these tests are not required or accepted as the standard of care.
Sideline assessment: Familiarity with the athlete is the best way to detect subtle changes in personality or performance. Looking at symptoms is still the most sensitive way to diagnose a concussion. Loss of consciousness, seizure, tonic posturing, lack of motor coordination, confusion, amnesia, difficulty with balance, or any cognitive difficulty should prompt removal from play for possible concussion. Once a potential injury is identified, how the athlete responds to the elements of orientation, memory, concentration, speech pattern, and balance should be evaluated. If an athlete has a probable or definite concussion, the athlete needs to be removed from play and cannot return to same-day play, and a more detailed evaluation needs to be done.
Office assessment: It is not unusual for symptoms and testing to normalize by the time an office visit occurs. If this is the case, the visit should focus on recommendations for safe return to school and sport. A standard office evaluation should include taking a history with details of the mechanism of injury and preexisting conditions – such as depression and prior concussion – that can affect concussion recovery. The history should focus on detecting symptoms that typically cause impairment from concussion: headache, ocular-vestibular issues leading to problems with balance, and cognitive issues with difficulty concentrating and remembering, as well as fatigue and mood issues such as anxiety, irritability, and depression. The physical exam should include assessment of ocular and vestibular function, gait, and balance in addition to a neurological exam.
Imaging: Head CT or MRI are rarely indicated. Intracranial bleeds are rare in the context of SRC but can occur. If there is concern for a bleed, then CT scan is the imaging test of choice. MRI may have value for evaluation for atypical or prolonged recovery.
Recovery time: The large majority (80%-90%) of concussed older adolescents and adults return to preinjury levels of function within 2 weeks; in younger athletes, clinical recovery may take up to 4 weeks. The best predictor of recovery from SRC is the number and severity of symptoms.
Treatment: For decades, cognitive and physical rest has been the standard of treatment. However, this is no longer the “gold standard” as it has been shown that strict rest (“cocoon therapy”) after SRC slows recovery and leads to an increased chance of prolonged symptoms. Current consensus guidelines support 24-48 hours of symptom-limited rest, both cognitive and physical, followed by a gradual increase in activity, staying below symptom-exacerbation thresholds. Activity, along with good sleep hygiene, appears to be helpful in facilitating recovery from SRC. In athletes with persistent post concussive symptoms that continue beyond the expected recovery time frame, activities of daily living, school, and exercise that do not significantly exacerbate symptoms are recommended.
Return to learning/play: A concussion can cause temporary deficits in attention, cognitive processing, short-term memory, and executive functioning. School personnel should be informed of the injury and assist in employing an individualized return to learn plan, including academic accommodations. Ultimately, return to sports activities should follow a successful return to the classroom. Return to play involves a stepwise increase in physical demands/activity without symptoms before a student is allowed to participate in full contact play.
Concussion-related risks: Continuing to participate in sports before resolution of concussion can worsen and prolong symptoms of SRC. Returning too early after concussion, before full recovery, increases the risk of recurrent SRC. During the initial post-injury period, returning to sports too early increases the risk for a rare but devastating possibility of second impact syndrome that can be a life-threatening repeat head injury. Studies of long-term mental health diagnoses are conflicting and inconsistent. Chronic traumatic encephalopathy has been described in athletes with a long history of concussions and repetitive sub-symptom head impacts. The degree of exposure needed appears to be variable and dependent on the individual.
Disqualification from play: Because each athlete is individually assessed after SRC, there are no evidence-based studies indicating how many concussions are “safe” for an athlete to have in a lifetime. The decision to stop playing sports is both serious and difficult for most athletes and requires shared decision making between clinician, the athlete, and the athlete’s parents. Factors to consider when determining if disqualification from play is warranted include:
- The total number of concussions experienced by a patient.
- Whether a patient has sustained subsequent concussions with progressively less forceful blows to the head.
- If a patient has sustained multiple concussions,whether the time to complete a full recovery after each concussion event increased.
The bottom line: “Cocoon therapy” is no longer recommended. Consensus guidelines endorse 24-48 hours of symptom-limited cognitive and physical rest followed by a gradual increase in activity, including noncontact physical activity that does not provoke symptoms.
Dr. Belogorodsky is a second-year resident and Dr. Fidler is an associate director in the Family Medicine Residency Program at Abington (Pa.) Jefferson Health. Dr. Skolnik is professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington Jefferson Health.
Reference
Harmon KG et al. American Medical Society for Sports Medicine position statement on concussion in sport. Br J Sports Med. 2019;53:213-25.
An estimated 1-1.8 million sport-related concussions (SRC) occur per year in patients younger than 18 years of age. Concussion is defined as “a traumatically induced transient disturbance of brain function.” More than 50% of concussions among high school youth are not related to organized sports and between 2% and 15% of athletes in organized sports will sustain a concussion during a season of play.
which will be described in this article. The guidelines include recommendations for imaging, treatment, and decision making regarding when as well as whether to return to play. Here is a brief summary of those recommendations.
Preseason: Preseason evaluation includes a preparticipation physical evaluation and discussion of concussion history as well as risk factors associated with prolonged concussion recovery. Neurocognitive tests are available for baseline evaluation. While these may assist with diagnosis and return-to-play decisions, there can be considerable variation in an individual’s baseline score as well as the possibility of changes in that baseline over time. Because of this potential for variability, these tests are not required or accepted as the standard of care.
Sideline assessment: Familiarity with the athlete is the best way to detect subtle changes in personality or performance. Looking at symptoms is still the most sensitive way to diagnose a concussion. Loss of consciousness, seizure, tonic posturing, lack of motor coordination, confusion, amnesia, difficulty with balance, or any cognitive difficulty should prompt removal from play for possible concussion. Once a potential injury is identified, how the athlete responds to the elements of orientation, memory, concentration, speech pattern, and balance should be evaluated. If an athlete has a probable or definite concussion, the athlete needs to be removed from play and cannot return to same-day play, and a more detailed evaluation needs to be done.
Office assessment: It is not unusual for symptoms and testing to normalize by the time an office visit occurs. If this is the case, the visit should focus on recommendations for safe return to school and sport. A standard office evaluation should include taking a history with details of the mechanism of injury and preexisting conditions – such as depression and prior concussion – that can affect concussion recovery. The history should focus on detecting symptoms that typically cause impairment from concussion: headache, ocular-vestibular issues leading to problems with balance, and cognitive issues with difficulty concentrating and remembering, as well as fatigue and mood issues such as anxiety, irritability, and depression. The physical exam should include assessment of ocular and vestibular function, gait, and balance in addition to a neurological exam.
Imaging: Head CT or MRI are rarely indicated. Intracranial bleeds are rare in the context of SRC but can occur. If there is concern for a bleed, then CT scan is the imaging test of choice. MRI may have value for evaluation for atypical or prolonged recovery.
Recovery time: The large majority (80%-90%) of concussed older adolescents and adults return to preinjury levels of function within 2 weeks; in younger athletes, clinical recovery may take up to 4 weeks. The best predictor of recovery from SRC is the number and severity of symptoms.
Treatment: For decades, cognitive and physical rest has been the standard of treatment. However, this is no longer the “gold standard” as it has been shown that strict rest (“cocoon therapy”) after SRC slows recovery and leads to an increased chance of prolonged symptoms. Current consensus guidelines support 24-48 hours of symptom-limited rest, both cognitive and physical, followed by a gradual increase in activity, staying below symptom-exacerbation thresholds. Activity, along with good sleep hygiene, appears to be helpful in facilitating recovery from SRC. In athletes with persistent post concussive symptoms that continue beyond the expected recovery time frame, activities of daily living, school, and exercise that do not significantly exacerbate symptoms are recommended.
Return to learning/play: A concussion can cause temporary deficits in attention, cognitive processing, short-term memory, and executive functioning. School personnel should be informed of the injury and assist in employing an individualized return to learn plan, including academic accommodations. Ultimately, return to sports activities should follow a successful return to the classroom. Return to play involves a stepwise increase in physical demands/activity without symptoms before a student is allowed to participate in full contact play.
Concussion-related risks: Continuing to participate in sports before resolution of concussion can worsen and prolong symptoms of SRC. Returning too early after concussion, before full recovery, increases the risk of recurrent SRC. During the initial post-injury period, returning to sports too early increases the risk for a rare but devastating possibility of second impact syndrome that can be a life-threatening repeat head injury. Studies of long-term mental health diagnoses are conflicting and inconsistent. Chronic traumatic encephalopathy has been described in athletes with a long history of concussions and repetitive sub-symptom head impacts. The degree of exposure needed appears to be variable and dependent on the individual.
Disqualification from play: Because each athlete is individually assessed after SRC, there are no evidence-based studies indicating how many concussions are “safe” for an athlete to have in a lifetime. The decision to stop playing sports is both serious and difficult for most athletes and requires shared decision making between clinician, the athlete, and the athlete’s parents. Factors to consider when determining if disqualification from play is warranted include:
- The total number of concussions experienced by a patient.
- Whether a patient has sustained subsequent concussions with progressively less forceful blows to the head.
- If a patient has sustained multiple concussions,whether the time to complete a full recovery after each concussion event increased.
The bottom line: “Cocoon therapy” is no longer recommended. Consensus guidelines endorse 24-48 hours of symptom-limited cognitive and physical rest followed by a gradual increase in activity, including noncontact physical activity that does not provoke symptoms.
Dr. Belogorodsky is a second-year resident and Dr. Fidler is an associate director in the Family Medicine Residency Program at Abington (Pa.) Jefferson Health. Dr. Skolnik is professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington Jefferson Health.
Reference
Harmon KG et al. American Medical Society for Sports Medicine position statement on concussion in sport. Br J Sports Med. 2019;53:213-25.
Atogepant shows safety, efficacy for migraine prevention
PHILADELPHIA – Atogepant, an oral small-molecule migraine drug from the “gepant” class, showed safety and efficacy for preventing migraine headaches in a phase 2/3, dose-ranging trial with 825 evaluable patients.
Atogepant is the first drug from this novel class to undergo efficacy testing in an advanced-phase trial for prevention of migraine headaches, David W. Dodick, MD, said at the annual meeting of the American Headache Society. Two other agents from the gepant class, rimegepant and ubrogepant, have undergone phase 3 testing for acute treatment of migraine headache, and both drugs are now under Food and Drug Administration consideration for an acute-treatment indication.
Three monoclonal antibodies to the calcitonin gene–related peptide (CGRP) receptor have received FDA marketing approval since 2018 for migraine headache prevention. The small-molecule antagonists to the CGRP receptor in the gepant class have an effect similar to the monoclonal antibodies, but with different dosage schedules, an oral route of administration, and with half-lives measured in hours, compared with days and weeks.
“Historically the [gepant] class of drugs was designed for acute treatment, and a couple of drugs from this class didn’t make it because of liver toxicity, but the liver toxicity is not a class effect.” The more recent candidate agents – atogepant, ubrogepant, and rimegepant – have shown hepatic safety as well as overall safety, noted Dr. Dodick, a neurologist and headache specialist at the Mayo Clinic in Phoenix.
If these gepant agents eventually receive FDA approval, which seems likely based on the evidence collected so far, they would collectively form “the only class to have both acute and preventive indications” for migraine, Dr. Dodick said in an interview. The preventive efficacy seen with atogepant in the current study is likely a class effect that has not yet been tested in ubrogepant and rimegepant.
In the multicenter study he reported, 834 patients, 86% of them women, were randomized to placebo or to any of five dosages of atogepant, ranging from 10 mg once daily to 60 mg b.i.d. Study participants had a history of episodic migraine with an average of nearly eight migraine-headache days per month and an average disease duration of about 19 years. The patients’ average age was about 40 years, and 72% had never before used a migraine-preventive treatment. Half had migraine without aura, about a quarter had migraine with aura, and a quarter had migraines of both types.
The researchers had safety data for 825 patients, and efficacy data for 795.
The study’s primary efficacy endpoint was the reduction from baseline in average migraine headache days per month after 12 weeks on treatment. Average migraine headache days fell by 2.85 days in the placebo group and by 3.55-4.23 days in the atogepant treatment groups, a significant difference. The reduction depended on the dosage patients received, but there was no clear dose-response relationship.
At least a 50% drop in average monthly headache day totals was seen in 40% of the placebo patients and in 52%-62% of the patients on atogepant, depending on their dosage. In this case, a signal appeared for a dose-response relationship as only the two subgroups with the patients who received the largest atogepant dosages showed statistically significant improvements in response, compared with placebo.
All patients on atogepant, regardless of their dosage, also had statistically significant reductions in their use of acute migraine medications, compared with placebo patients.
The level of benefit beyond placebo seen in these results was “clinically meaningful,” and roughly comparable with the preventive benefit seen with both the CGRP receptor antagonist monoclonal antibodies, as well as with the three conventional agents most commonly used for migraine headache prevention in current U.S. practice: topiramate, amitriptyline, and propranolol, Dr. Dodick said. Amitriptyline is not approved for this indication.
The adverse event profile among patients who received atogepant was about the same as it was among the placebo recipients. Seven study patients had serious treatment-related adverse effects; two of these patients were in the placebo arm of 186 patients. The most common adverse events were nausea, constipation, and fatigue, and were seen mostly at the highest dosage of atogepant. The incidence of elevated liver enzymes was low and similar in the placebo and drug-treated patients. Two patients, one on placebo and one on atogepant, had their liver enzymes reach at least five times the upper limit of normal. None had their enzymes reach at least 10 times the upper limit of normal, and no patients in the study had a response that fulfilled “Hy’s law,” which flags drug-induced liver injury as patients who develop the combination of elevated liver enzymes, elevated bilirubin, and depressed alkaline phosphatase.
The study was sponsored by Allergan, the company developing atogepant and ubrogepant. Dr. Dodick has been a consultant to Allergan and to several other drug companies.
PHILADELPHIA – Atogepant, an oral small-molecule migraine drug from the “gepant” class, showed safety and efficacy for preventing migraine headaches in a phase 2/3, dose-ranging trial with 825 evaluable patients.
Atogepant is the first drug from this novel class to undergo efficacy testing in an advanced-phase trial for prevention of migraine headaches, David W. Dodick, MD, said at the annual meeting of the American Headache Society. Two other agents from the gepant class, rimegepant and ubrogepant, have undergone phase 3 testing for acute treatment of migraine headache, and both drugs are now under Food and Drug Administration consideration for an acute-treatment indication.
Three monoclonal antibodies to the calcitonin gene–related peptide (CGRP) receptor have received FDA marketing approval since 2018 for migraine headache prevention. The small-molecule antagonists to the CGRP receptor in the gepant class have an effect similar to the monoclonal antibodies, but with different dosage schedules, an oral route of administration, and with half-lives measured in hours, compared with days and weeks.
“Historically the [gepant] class of drugs was designed for acute treatment, and a couple of drugs from this class didn’t make it because of liver toxicity, but the liver toxicity is not a class effect.” The more recent candidate agents – atogepant, ubrogepant, and rimegepant – have shown hepatic safety as well as overall safety, noted Dr. Dodick, a neurologist and headache specialist at the Mayo Clinic in Phoenix.
If these gepant agents eventually receive FDA approval, which seems likely based on the evidence collected so far, they would collectively form “the only class to have both acute and preventive indications” for migraine, Dr. Dodick said in an interview. The preventive efficacy seen with atogepant in the current study is likely a class effect that has not yet been tested in ubrogepant and rimegepant.
In the multicenter study he reported, 834 patients, 86% of them women, were randomized to placebo or to any of five dosages of atogepant, ranging from 10 mg once daily to 60 mg b.i.d. Study participants had a history of episodic migraine with an average of nearly eight migraine-headache days per month and an average disease duration of about 19 years. The patients’ average age was about 40 years, and 72% had never before used a migraine-preventive treatment. Half had migraine without aura, about a quarter had migraine with aura, and a quarter had migraines of both types.
The researchers had safety data for 825 patients, and efficacy data for 795.
The study’s primary efficacy endpoint was the reduction from baseline in average migraine headache days per month after 12 weeks on treatment. Average migraine headache days fell by 2.85 days in the placebo group and by 3.55-4.23 days in the atogepant treatment groups, a significant difference. The reduction depended on the dosage patients received, but there was no clear dose-response relationship.
At least a 50% drop in average monthly headache day totals was seen in 40% of the placebo patients and in 52%-62% of the patients on atogepant, depending on their dosage. In this case, a signal appeared for a dose-response relationship as only the two subgroups with the patients who received the largest atogepant dosages showed statistically significant improvements in response, compared with placebo.
All patients on atogepant, regardless of their dosage, also had statistically significant reductions in their use of acute migraine medications, compared with placebo patients.
The level of benefit beyond placebo seen in these results was “clinically meaningful,” and roughly comparable with the preventive benefit seen with both the CGRP receptor antagonist monoclonal antibodies, as well as with the three conventional agents most commonly used for migraine headache prevention in current U.S. practice: topiramate, amitriptyline, and propranolol, Dr. Dodick said. Amitriptyline is not approved for this indication.
The adverse event profile among patients who received atogepant was about the same as it was among the placebo recipients. Seven study patients had serious treatment-related adverse effects; two of these patients were in the placebo arm of 186 patients. The most common adverse events were nausea, constipation, and fatigue, and were seen mostly at the highest dosage of atogepant. The incidence of elevated liver enzymes was low and similar in the placebo and drug-treated patients. Two patients, one on placebo and one on atogepant, had their liver enzymes reach at least five times the upper limit of normal. None had their enzymes reach at least 10 times the upper limit of normal, and no patients in the study had a response that fulfilled “Hy’s law,” which flags drug-induced liver injury as patients who develop the combination of elevated liver enzymes, elevated bilirubin, and depressed alkaline phosphatase.
The study was sponsored by Allergan, the company developing atogepant and ubrogepant. Dr. Dodick has been a consultant to Allergan and to several other drug companies.
PHILADELPHIA – Atogepant, an oral small-molecule migraine drug from the “gepant” class, showed safety and efficacy for preventing migraine headaches in a phase 2/3, dose-ranging trial with 825 evaluable patients.
Atogepant is the first drug from this novel class to undergo efficacy testing in an advanced-phase trial for prevention of migraine headaches, David W. Dodick, MD, said at the annual meeting of the American Headache Society. Two other agents from the gepant class, rimegepant and ubrogepant, have undergone phase 3 testing for acute treatment of migraine headache, and both drugs are now under Food and Drug Administration consideration for an acute-treatment indication.
Three monoclonal antibodies to the calcitonin gene–related peptide (CGRP) receptor have received FDA marketing approval since 2018 for migraine headache prevention. The small-molecule antagonists to the CGRP receptor in the gepant class have an effect similar to the monoclonal antibodies, but with different dosage schedules, an oral route of administration, and with half-lives measured in hours, compared with days and weeks.
“Historically the [gepant] class of drugs was designed for acute treatment, and a couple of drugs from this class didn’t make it because of liver toxicity, but the liver toxicity is not a class effect.” The more recent candidate agents – atogepant, ubrogepant, and rimegepant – have shown hepatic safety as well as overall safety, noted Dr. Dodick, a neurologist and headache specialist at the Mayo Clinic in Phoenix.
If these gepant agents eventually receive FDA approval, which seems likely based on the evidence collected so far, they would collectively form “the only class to have both acute and preventive indications” for migraine, Dr. Dodick said in an interview. The preventive efficacy seen with atogepant in the current study is likely a class effect that has not yet been tested in ubrogepant and rimegepant.
In the multicenter study he reported, 834 patients, 86% of them women, were randomized to placebo or to any of five dosages of atogepant, ranging from 10 mg once daily to 60 mg b.i.d. Study participants had a history of episodic migraine with an average of nearly eight migraine-headache days per month and an average disease duration of about 19 years. The patients’ average age was about 40 years, and 72% had never before used a migraine-preventive treatment. Half had migraine without aura, about a quarter had migraine with aura, and a quarter had migraines of both types.
The researchers had safety data for 825 patients, and efficacy data for 795.
The study’s primary efficacy endpoint was the reduction from baseline in average migraine headache days per month after 12 weeks on treatment. Average migraine headache days fell by 2.85 days in the placebo group and by 3.55-4.23 days in the atogepant treatment groups, a significant difference. The reduction depended on the dosage patients received, but there was no clear dose-response relationship.
At least a 50% drop in average monthly headache day totals was seen in 40% of the placebo patients and in 52%-62% of the patients on atogepant, depending on their dosage. In this case, a signal appeared for a dose-response relationship as only the two subgroups with the patients who received the largest atogepant dosages showed statistically significant improvements in response, compared with placebo.
All patients on atogepant, regardless of their dosage, also had statistically significant reductions in their use of acute migraine medications, compared with placebo patients.
The level of benefit beyond placebo seen in these results was “clinically meaningful,” and roughly comparable with the preventive benefit seen with both the CGRP receptor antagonist monoclonal antibodies, as well as with the three conventional agents most commonly used for migraine headache prevention in current U.S. practice: topiramate, amitriptyline, and propranolol, Dr. Dodick said. Amitriptyline is not approved for this indication.
The adverse event profile among patients who received atogepant was about the same as it was among the placebo recipients. Seven study patients had serious treatment-related adverse effects; two of these patients were in the placebo arm of 186 patients. The most common adverse events were nausea, constipation, and fatigue, and were seen mostly at the highest dosage of atogepant. The incidence of elevated liver enzymes was low and similar in the placebo and drug-treated patients. Two patients, one on placebo and one on atogepant, had their liver enzymes reach at least five times the upper limit of normal. None had their enzymes reach at least 10 times the upper limit of normal, and no patients in the study had a response that fulfilled “Hy’s law,” which flags drug-induced liver injury as patients who develop the combination of elevated liver enzymes, elevated bilirubin, and depressed alkaline phosphatase.
The study was sponsored by Allergan, the company developing atogepant and ubrogepant. Dr. Dodick has been a consultant to Allergan and to several other drug companies.
REPORTING FROM AHS 2019
When Flu Goes to Work
When influenza season arrives, conventional morbidity and mortality statistics, health care encounters, and laboratory data might not “fully reflect the disruption caused to the social and economic life of the community,” say CDC researchers. That is the reason that the National Institute for Occupational Safety and Health (NIOSH) monitors health-related workplace absenteeism every month, and why the World Health Organization (WHO) uses those data to help determine the impact of influenza season worldwide.
The workplace is a prime area for transmission—people share workspace and equipment and interact with one another closely. Estimates of influenza attack rates for working-aged adults can be as high as 14.3% in a given influenza season, the CDC says.
According to NIOSH, absenteeism rose sharply in the 2017-2018 season, to a level significantly higher than that of the average during the previous 5 seasons. In October, 1.7% of workers were absent due to health issues. That figure began climbing in November, peaking in January at 3.0%, significantly exceeding the epidemic threshold. Absenteeism declined steadily after that to a low of 1.4%, then began rising again in August and September.
Male workers, people aged 45 to 64 years, and those working in certain occupations (including management, business, and repair services) were more likely to be out.
Regional absenteeism peaks corresponded to concurrent peaks in influenza-like illness (ILI) activity in those regions. The researchers say this is in line with a longtime recognition that health-related workplace absenteeism correlates well with the presence of ILI, which is why absenteeism data are used as a nonspecific indicator of ILI in the community. During the 2009-2010 influenza A (H1N1) pandemic, for instance, peak workplace absenteeism correlated with the highest occurrence of both ILI and influenza-positive laboratory tests, according to NIOSH.
The associations between ILI, absenteeism, and demographic characteristics are complex, the researchers say, and mediated by factors such as vaccination coverage and access to paid sick leave.
The usual recommendations—vaccination, covering coughs and sneezes, handwashing, and routinely cleaning frequently touched surfaces—are the most effective ways to prevent transmission, the researchers note. During a pandemic, other measures may be needed, such as “social distancing” in workplaces.
NIOSH makes absenteeism surveillance results available (https://www.cdc.gov/niosh/topics/absences/default.html). The researchers suggest that employers might wish to consult them when developing prevention messages.
When influenza season arrives, conventional morbidity and mortality statistics, health care encounters, and laboratory data might not “fully reflect the disruption caused to the social and economic life of the community,” say CDC researchers. That is the reason that the National Institute for Occupational Safety and Health (NIOSH) monitors health-related workplace absenteeism every month, and why the World Health Organization (WHO) uses those data to help determine the impact of influenza season worldwide.
The workplace is a prime area for transmission—people share workspace and equipment and interact with one another closely. Estimates of influenza attack rates for working-aged adults can be as high as 14.3% in a given influenza season, the CDC says.
According to NIOSH, absenteeism rose sharply in the 2017-2018 season, to a level significantly higher than that of the average during the previous 5 seasons. In October, 1.7% of workers were absent due to health issues. That figure began climbing in November, peaking in January at 3.0%, significantly exceeding the epidemic threshold. Absenteeism declined steadily after that to a low of 1.4%, then began rising again in August and September.
Male workers, people aged 45 to 64 years, and those working in certain occupations (including management, business, and repair services) were more likely to be out.
Regional absenteeism peaks corresponded to concurrent peaks in influenza-like illness (ILI) activity in those regions. The researchers say this is in line with a longtime recognition that health-related workplace absenteeism correlates well with the presence of ILI, which is why absenteeism data are used as a nonspecific indicator of ILI in the community. During the 2009-2010 influenza A (H1N1) pandemic, for instance, peak workplace absenteeism correlated with the highest occurrence of both ILI and influenza-positive laboratory tests, according to NIOSH.
The associations between ILI, absenteeism, and demographic characteristics are complex, the researchers say, and mediated by factors such as vaccination coverage and access to paid sick leave.
The usual recommendations—vaccination, covering coughs and sneezes, handwashing, and routinely cleaning frequently touched surfaces—are the most effective ways to prevent transmission, the researchers note. During a pandemic, other measures may be needed, such as “social distancing” in workplaces.
NIOSH makes absenteeism surveillance results available (https://www.cdc.gov/niosh/topics/absences/default.html). The researchers suggest that employers might wish to consult them when developing prevention messages.
When influenza season arrives, conventional morbidity and mortality statistics, health care encounters, and laboratory data might not “fully reflect the disruption caused to the social and economic life of the community,” say CDC researchers. That is the reason that the National Institute for Occupational Safety and Health (NIOSH) monitors health-related workplace absenteeism every month, and why the World Health Organization (WHO) uses those data to help determine the impact of influenza season worldwide.
The workplace is a prime area for transmission—people share workspace and equipment and interact with one another closely. Estimates of influenza attack rates for working-aged adults can be as high as 14.3% in a given influenza season, the CDC says.
According to NIOSH, absenteeism rose sharply in the 2017-2018 season, to a level significantly higher than that of the average during the previous 5 seasons. In October, 1.7% of workers were absent due to health issues. That figure began climbing in November, peaking in January at 3.0%, significantly exceeding the epidemic threshold. Absenteeism declined steadily after that to a low of 1.4%, then began rising again in August and September.
Male workers, people aged 45 to 64 years, and those working in certain occupations (including management, business, and repair services) were more likely to be out.
Regional absenteeism peaks corresponded to concurrent peaks in influenza-like illness (ILI) activity in those regions. The researchers say this is in line with a longtime recognition that health-related workplace absenteeism correlates well with the presence of ILI, which is why absenteeism data are used as a nonspecific indicator of ILI in the community. During the 2009-2010 influenza A (H1N1) pandemic, for instance, peak workplace absenteeism correlated with the highest occurrence of both ILI and influenza-positive laboratory tests, according to NIOSH.
The associations between ILI, absenteeism, and demographic characteristics are complex, the researchers say, and mediated by factors such as vaccination coverage and access to paid sick leave.
The usual recommendations—vaccination, covering coughs and sneezes, handwashing, and routinely cleaning frequently touched surfaces—are the most effective ways to prevent transmission, the researchers note. During a pandemic, other measures may be needed, such as “social distancing” in workplaces.
NIOSH makes absenteeism surveillance results available (https://www.cdc.gov/niosh/topics/absences/default.html). The researchers suggest that employers might wish to consult them when developing prevention messages.