User login
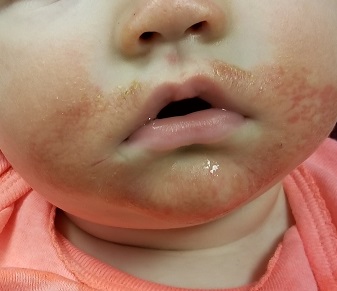
Mothers, migraine, colic ... and sleep
In a recent article on this website, Jake Remaly reports on a study suggesting that maternal migraine is associated with infant colic. In a presentation at the annual meeting of the American Headache Society, Amy Gelfand, MD, a neurologist at the University of California, San Francisco, reported the results of a national survey of more than 1,400 parents (827 mothers, 592 fathers) collected via social media. She and her colleagues found that mothers with migraine were more likely to have an infant with colic, with an odds ratio of 1.7 that increased to 2.5 for mothers with more frequent migraine. Fathers with migraine were no more likely to have an infant with colic.
In a video clip included in the article, Dr. Gelfand discusses the possibilities that she and her group considered as they attempted to explain the study’s findings. Are there such things as “migraine genes?” If so, the failure to discover a paternal association might suggest that these would be mitochondrial genes. The researchers wondered if a substance in breast milk was acting as trigger, but they found that the association between colic and migraine was unrelated to whether the baby was fed by breast or bottle.
In full disclosure, I was not one of the investigators. Neither my wife nor I have migraine, and although our children cried as infants, they wouldn’t have qualified as having colic. However, I spent more than 40 years immersed in more than 300,000 patient encounters and can claim membership in the International Brother/Sisterhood of Anecdotal Observers. And, as such will offer up my explanation for Dr. Gelfand’s findings.
It is clear to me that most, if not all, children with migraine have their headaches when they are sleep deprived. While my sample size is smaller, I believe the same association also is true for many of the adults I know who have migraine. At least in children, restorative sleep ends the migraine much as it does for an epileptic seizure.
Traditionally, colic has been thought to be somehow related to a gastrointestinal phenomenon by many extended family members and some physicians. However, in my experience, it is usually a symptom of sleep deprivation compounded by the failure of those around the children to realize the obvious and take appropriate action. Of course, some babies are reacting to sore tummies, but my guess is that most are having headaches. We may never know. Dr. Gelfand also shares my observation that colicky crying is more likely to occur “at the end of the day,” a time when we are tired and are less tolerant of overstimulation.
However, the presentation varies depending on the age of the patients. Remember, infants can’t talk. It already has been shown that adults with migraine often were more likely to have been colicky infants. (Dr. Gelfand mentions this as well.) These unfortunate individuals probably have inherited a vulnerability to sleep deprivation that manifests itself as a headache. I hope to live long enough to be around when someone discovers the wrinkle in the genome that creates this vulnerability.
So, why did the researchers fail to find an association between fathers and colic? The answer is simple. We fathers are beginning to take on a larger role in parenting of infants and like to complain about how difficult it is. However, it is mothers who still have the lioness’ share of the work. They lose the most sleep and are starting off parenthood with 9 months of less than optimal sleep followed by who knows how many hours of energy-sapping labor. It’s surprising they all don’t have migraines.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at pdnews@mdedge.com.
In a recent article on this website, Jake Remaly reports on a study suggesting that maternal migraine is associated with infant colic. In a presentation at the annual meeting of the American Headache Society, Amy Gelfand, MD, a neurologist at the University of California, San Francisco, reported the results of a national survey of more than 1,400 parents (827 mothers, 592 fathers) collected via social media. She and her colleagues found that mothers with migraine were more likely to have an infant with colic, with an odds ratio of 1.7 that increased to 2.5 for mothers with more frequent migraine. Fathers with migraine were no more likely to have an infant with colic.
In a video clip included in the article, Dr. Gelfand discusses the possibilities that she and her group considered as they attempted to explain the study’s findings. Are there such things as “migraine genes?” If so, the failure to discover a paternal association might suggest that these would be mitochondrial genes. The researchers wondered if a substance in breast milk was acting as trigger, but they found that the association between colic and migraine was unrelated to whether the baby was fed by breast or bottle.
In full disclosure, I was not one of the investigators. Neither my wife nor I have migraine, and although our children cried as infants, they wouldn’t have qualified as having colic. However, I spent more than 40 years immersed in more than 300,000 patient encounters and can claim membership in the International Brother/Sisterhood of Anecdotal Observers. And, as such will offer up my explanation for Dr. Gelfand’s findings.
It is clear to me that most, if not all, children with migraine have their headaches when they are sleep deprived. While my sample size is smaller, I believe the same association also is true for many of the adults I know who have migraine. At least in children, restorative sleep ends the migraine much as it does for an epileptic seizure.
Traditionally, colic has been thought to be somehow related to a gastrointestinal phenomenon by many extended family members and some physicians. However, in my experience, it is usually a symptom of sleep deprivation compounded by the failure of those around the children to realize the obvious and take appropriate action. Of course, some babies are reacting to sore tummies, but my guess is that most are having headaches. We may never know. Dr. Gelfand also shares my observation that colicky crying is more likely to occur “at the end of the day,” a time when we are tired and are less tolerant of overstimulation.
However, the presentation varies depending on the age of the patients. Remember, infants can’t talk. It already has been shown that adults with migraine often were more likely to have been colicky infants. (Dr. Gelfand mentions this as well.) These unfortunate individuals probably have inherited a vulnerability to sleep deprivation that manifests itself as a headache. I hope to live long enough to be around when someone discovers the wrinkle in the genome that creates this vulnerability.
So, why did the researchers fail to find an association between fathers and colic? The answer is simple. We fathers are beginning to take on a larger role in parenting of infants and like to complain about how difficult it is. However, it is mothers who still have the lioness’ share of the work. They lose the most sleep and are starting off parenthood with 9 months of less than optimal sleep followed by who knows how many hours of energy-sapping labor. It’s surprising they all don’t have migraines.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at pdnews@mdedge.com.
In a recent article on this website, Jake Remaly reports on a study suggesting that maternal migraine is associated with infant colic. In a presentation at the annual meeting of the American Headache Society, Amy Gelfand, MD, a neurologist at the University of California, San Francisco, reported the results of a national survey of more than 1,400 parents (827 mothers, 592 fathers) collected via social media. She and her colleagues found that mothers with migraine were more likely to have an infant with colic, with an odds ratio of 1.7 that increased to 2.5 for mothers with more frequent migraine. Fathers with migraine were no more likely to have an infant with colic.
In a video clip included in the article, Dr. Gelfand discusses the possibilities that she and her group considered as they attempted to explain the study’s findings. Are there such things as “migraine genes?” If so, the failure to discover a paternal association might suggest that these would be mitochondrial genes. The researchers wondered if a substance in breast milk was acting as trigger, but they found that the association between colic and migraine was unrelated to whether the baby was fed by breast or bottle.
In full disclosure, I was not one of the investigators. Neither my wife nor I have migraine, and although our children cried as infants, they wouldn’t have qualified as having colic. However, I spent more than 40 years immersed in more than 300,000 patient encounters and can claim membership in the International Brother/Sisterhood of Anecdotal Observers. And, as such will offer up my explanation for Dr. Gelfand’s findings.
It is clear to me that most, if not all, children with migraine have their headaches when they are sleep deprived. While my sample size is smaller, I believe the same association also is true for many of the adults I know who have migraine. At least in children, restorative sleep ends the migraine much as it does for an epileptic seizure.
Traditionally, colic has been thought to be somehow related to a gastrointestinal phenomenon by many extended family members and some physicians. However, in my experience, it is usually a symptom of sleep deprivation compounded by the failure of those around the children to realize the obvious and take appropriate action. Of course, some babies are reacting to sore tummies, but my guess is that most are having headaches. We may never know. Dr. Gelfand also shares my observation that colicky crying is more likely to occur “at the end of the day,” a time when we are tired and are less tolerant of overstimulation.
However, the presentation varies depending on the age of the patients. Remember, infants can’t talk. It already has been shown that adults with migraine often were more likely to have been colicky infants. (Dr. Gelfand mentions this as well.) These unfortunate individuals probably have inherited a vulnerability to sleep deprivation that manifests itself as a headache. I hope to live long enough to be around when someone discovers the wrinkle in the genome that creates this vulnerability.
So, why did the researchers fail to find an association between fathers and colic? The answer is simple. We fathers are beginning to take on a larger role in parenting of infants and like to complain about how difficult it is. However, it is mothers who still have the lioness’ share of the work. They lose the most sleep and are starting off parenthood with 9 months of less than optimal sleep followed by who knows how many hours of energy-sapping labor. It’s surprising they all don’t have migraines.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at pdnews@mdedge.com.
FDA declines dapagliflozin indication as adjunct for type 1 diabetes
The Food and Drug Administration has rejected AstraZeneca’s supplemental New Drug Application for the sodium-glucose cotransporter 2 inhibitor dapagliflozin (Farxiga) as an adjunct treatment to insulin in adult patients with type 1 diabetes.
The company said in a press statement that the FDA had issued a complete response letter regarding the application. No reason was given for the decision, but the company said it would work with the agency to discuss the next steps.
The once-daily therapy has been approved as both a monotherapy and combination therapy, as an adjunct to diet and exercise, for improving glycemic control in adults with type 2 diabetes who cannot achieve control with insulin alone. It also has additional demonstrated benefits of weight loss and reduction in blood pressure.
On March 25, 2019, the drug received its first approval for treatment of patients with type 1 diabetes when the European Commission gave it the green light for use in patients with a body mass index of 27 kg/m2 or more when insulin alone does not provide adequate glycemic control. Japan followed a few days later with its approval of the sodium-glucose cotransporter 2 inhibitor, also for type 1 disease in adults.
The approvals for type 1 diabetes in the European Union and Japan were based on data from the phase 3 DEPICT (Dapagliflozin Evaluation in Patients With Inadequately Controlled Type 1 Diabetes) trial program (DEPICT-1 and DEPICT-2), which showed that 5 mg dapagliflozin, taken daily as an oral adjunct to insulin in patients with hard-to-control type 1 disease, reduced blood glucose levels from baseline (the primary endpoint). Secondary endpoints – reductions in weight and total daily insulin use – were also achieved.
Dapagliflozin’s safety profile in the trials in patients with type 1 diabetes was consistent with that established in patients with type 2 disease. However, there was a higher number of cases of diabetic ketoacidosis events in patients who received dapagliflozin. Diabetic ketoacidosis is a known complication for adults with type 1 diabetes and is more prevalent in patients with type 1 disease than in those with type 2.
The Food and Drug Administration has rejected AstraZeneca’s supplemental New Drug Application for the sodium-glucose cotransporter 2 inhibitor dapagliflozin (Farxiga) as an adjunct treatment to insulin in adult patients with type 1 diabetes.
The company said in a press statement that the FDA had issued a complete response letter regarding the application. No reason was given for the decision, but the company said it would work with the agency to discuss the next steps.
The once-daily therapy has been approved as both a monotherapy and combination therapy, as an adjunct to diet and exercise, for improving glycemic control in adults with type 2 diabetes who cannot achieve control with insulin alone. It also has additional demonstrated benefits of weight loss and reduction in blood pressure.
On March 25, 2019, the drug received its first approval for treatment of patients with type 1 diabetes when the European Commission gave it the green light for use in patients with a body mass index of 27 kg/m2 or more when insulin alone does not provide adequate glycemic control. Japan followed a few days later with its approval of the sodium-glucose cotransporter 2 inhibitor, also for type 1 disease in adults.
The approvals for type 1 diabetes in the European Union and Japan were based on data from the phase 3 DEPICT (Dapagliflozin Evaluation in Patients With Inadequately Controlled Type 1 Diabetes) trial program (DEPICT-1 and DEPICT-2), which showed that 5 mg dapagliflozin, taken daily as an oral adjunct to insulin in patients with hard-to-control type 1 disease, reduced blood glucose levels from baseline (the primary endpoint). Secondary endpoints – reductions in weight and total daily insulin use – were also achieved.
Dapagliflozin’s safety profile in the trials in patients with type 1 diabetes was consistent with that established in patients with type 2 disease. However, there was a higher number of cases of diabetic ketoacidosis events in patients who received dapagliflozin. Diabetic ketoacidosis is a known complication for adults with type 1 diabetes and is more prevalent in patients with type 1 disease than in those with type 2.
The Food and Drug Administration has rejected AstraZeneca’s supplemental New Drug Application for the sodium-glucose cotransporter 2 inhibitor dapagliflozin (Farxiga) as an adjunct treatment to insulin in adult patients with type 1 diabetes.
The company said in a press statement that the FDA had issued a complete response letter regarding the application. No reason was given for the decision, but the company said it would work with the agency to discuss the next steps.
The once-daily therapy has been approved as both a monotherapy and combination therapy, as an adjunct to diet and exercise, for improving glycemic control in adults with type 2 diabetes who cannot achieve control with insulin alone. It also has additional demonstrated benefits of weight loss and reduction in blood pressure.
On March 25, 2019, the drug received its first approval for treatment of patients with type 1 diabetes when the European Commission gave it the green light for use in patients with a body mass index of 27 kg/m2 or more when insulin alone does not provide adequate glycemic control. Japan followed a few days later with its approval of the sodium-glucose cotransporter 2 inhibitor, also for type 1 disease in adults.
The approvals for type 1 diabetes in the European Union and Japan were based on data from the phase 3 DEPICT (Dapagliflozin Evaluation in Patients With Inadequately Controlled Type 1 Diabetes) trial program (DEPICT-1 and DEPICT-2), which showed that 5 mg dapagliflozin, taken daily as an oral adjunct to insulin in patients with hard-to-control type 1 disease, reduced blood glucose levels from baseline (the primary endpoint). Secondary endpoints – reductions in weight and total daily insulin use – were also achieved.
Dapagliflozin’s safety profile in the trials in patients with type 1 diabetes was consistent with that established in patients with type 2 disease. However, there was a higher number of cases of diabetic ketoacidosis events in patients who received dapagliflozin. Diabetic ketoacidosis is a known complication for adults with type 1 diabetes and is more prevalent in patients with type 1 disease than in those with type 2.
Gum bacteria and Alzheimer’s: A hypothesis inches forward
LOS ANGELES – Scientists testing an unusual hypothesis about the pathogenesis of Alzheimer’s disease say that use of an experimental antimicrobial drug to target a common oral infection was associated with biomarker improvements in people with the disease.
At the Alzheimer’s Association International Conference, Michael Detke, MD, PhD, of Cortexyme in South San Francisco, Calif., presented findings from a small cohort (n = 9) of people with mild to moderate Alzheimer’s disease (AD).
Six patients, mean age 72, were randomized to 4 weeks’ treatment with an agent called COR388, which inhibits toxic proteases produced by Porphyromonas gingivalis, a bacterium that colonizes the mouth and gums and has been found in the brains and cerebrospinal fluid of people with AD more than in non-AD patients. Another three subjects were randomized to placebo.
At 28 days, the CSF levels of an Alzheimer’s-associated apolipoprotein E (ApoE) fragment and serum levels of RANTES, a chemokine associated with chronic inflammation, were reduced from baseline by about one-third in treated subjects, compared with placebo subjects (P less than .05).
“In AD, ApoE is known to be fragmented and the fragments are known to be neurotoxic,” Dr. Detke, the lead study author, said in an interview. “We hypothesized that these P. gingivalis proteases may be acting on ApoE cleavage – so if you bind the proteases you should reduce the fragments. We saw in this study that ApoE fragment was reduced by 30%, or back to about normal levels.”
It is difficult to eradicate P. gingivalis infection using conventional antibiotics. The experimental agent COR388 “does not kill the bacteria but rather neutralizes it by binding to the proteases it produces, making the bacteria benign,” Dr. Detke said.
While hypotheses involving infectious causes of Alzheimer’s remain on the periphery of dementia research, Dr. Detke and his colleagues will soon be able to test theirs in a more meaningful way. At the conference, Dr. Detke presented detailed plans for a phase 2/3, placebo-controlled, clinical trial of COR388 in 570 patients aged 55-80 with mild to moderate AD.
Patients in the study are currently being randomized to 48 weeks of treatment with one of two doses, or placebo, with cognitive and biomarker endpoints planned, including for amyloid-beta, tau, and serum, plasma, and CSF markers of neuroinflammation.
Dr. Detke is an employee of Cortexyme, as are another 8 of the study’s 11 authors.
LOS ANGELES – Scientists testing an unusual hypothesis about the pathogenesis of Alzheimer’s disease say that use of an experimental antimicrobial drug to target a common oral infection was associated with biomarker improvements in people with the disease.
At the Alzheimer’s Association International Conference, Michael Detke, MD, PhD, of Cortexyme in South San Francisco, Calif., presented findings from a small cohort (n = 9) of people with mild to moderate Alzheimer’s disease (AD).
Six patients, mean age 72, were randomized to 4 weeks’ treatment with an agent called COR388, which inhibits toxic proteases produced by Porphyromonas gingivalis, a bacterium that colonizes the mouth and gums and has been found in the brains and cerebrospinal fluid of people with AD more than in non-AD patients. Another three subjects were randomized to placebo.
At 28 days, the CSF levels of an Alzheimer’s-associated apolipoprotein E (ApoE) fragment and serum levels of RANTES, a chemokine associated with chronic inflammation, were reduced from baseline by about one-third in treated subjects, compared with placebo subjects (P less than .05).
“In AD, ApoE is known to be fragmented and the fragments are known to be neurotoxic,” Dr. Detke, the lead study author, said in an interview. “We hypothesized that these P. gingivalis proteases may be acting on ApoE cleavage – so if you bind the proteases you should reduce the fragments. We saw in this study that ApoE fragment was reduced by 30%, or back to about normal levels.”
It is difficult to eradicate P. gingivalis infection using conventional antibiotics. The experimental agent COR388 “does not kill the bacteria but rather neutralizes it by binding to the proteases it produces, making the bacteria benign,” Dr. Detke said.
While hypotheses involving infectious causes of Alzheimer’s remain on the periphery of dementia research, Dr. Detke and his colleagues will soon be able to test theirs in a more meaningful way. At the conference, Dr. Detke presented detailed plans for a phase 2/3, placebo-controlled, clinical trial of COR388 in 570 patients aged 55-80 with mild to moderate AD.
Patients in the study are currently being randomized to 48 weeks of treatment with one of two doses, or placebo, with cognitive and biomarker endpoints planned, including for amyloid-beta, tau, and serum, plasma, and CSF markers of neuroinflammation.
Dr. Detke is an employee of Cortexyme, as are another 8 of the study’s 11 authors.
LOS ANGELES – Scientists testing an unusual hypothesis about the pathogenesis of Alzheimer’s disease say that use of an experimental antimicrobial drug to target a common oral infection was associated with biomarker improvements in people with the disease.
At the Alzheimer’s Association International Conference, Michael Detke, MD, PhD, of Cortexyme in South San Francisco, Calif., presented findings from a small cohort (n = 9) of people with mild to moderate Alzheimer’s disease (AD).
Six patients, mean age 72, were randomized to 4 weeks’ treatment with an agent called COR388, which inhibits toxic proteases produced by Porphyromonas gingivalis, a bacterium that colonizes the mouth and gums and has been found in the brains and cerebrospinal fluid of people with AD more than in non-AD patients. Another three subjects were randomized to placebo.
At 28 days, the CSF levels of an Alzheimer’s-associated apolipoprotein E (ApoE) fragment and serum levels of RANTES, a chemokine associated with chronic inflammation, were reduced from baseline by about one-third in treated subjects, compared with placebo subjects (P less than .05).
“In AD, ApoE is known to be fragmented and the fragments are known to be neurotoxic,” Dr. Detke, the lead study author, said in an interview. “We hypothesized that these P. gingivalis proteases may be acting on ApoE cleavage – so if you bind the proteases you should reduce the fragments. We saw in this study that ApoE fragment was reduced by 30%, or back to about normal levels.”
It is difficult to eradicate P. gingivalis infection using conventional antibiotics. The experimental agent COR388 “does not kill the bacteria but rather neutralizes it by binding to the proteases it produces, making the bacteria benign,” Dr. Detke said.
While hypotheses involving infectious causes of Alzheimer’s remain on the periphery of dementia research, Dr. Detke and his colleagues will soon be able to test theirs in a more meaningful way. At the conference, Dr. Detke presented detailed plans for a phase 2/3, placebo-controlled, clinical trial of COR388 in 570 patients aged 55-80 with mild to moderate AD.
Patients in the study are currently being randomized to 48 weeks of treatment with one of two doses, or placebo, with cognitive and biomarker endpoints planned, including for amyloid-beta, tau, and serum, plasma, and CSF markers of neuroinflammation.
Dr. Detke is an employee of Cortexyme, as are another 8 of the study’s 11 authors.
FROM AAIC 2019
Combo RT/pembro may show synergy in NSCLC
Combination radiotherapy and pembrolizumab may improve clinical outcomes by means of synergy in the treatment of patients with non–small cell lung cancer (NSCLC), according to results from two recent studies.
“The best way to combine immunotherapy with ablative therapies in the curative setting is an area of active investigation,” wrote Joshua M. Bauml, MD, of the University of Pennsylvania, Philadelphia, and colleagues.
Dr. Bauml and colleagues recently reported the results of a phase 2 study exploring the addition of pembrolizumab following the completion of locally ablative therapy in patients with oligometastatic NSCLC in JAMA Oncology.
The single-arm trial included 51 patients who received intravenous pembrolizumab (200 mg every 21 days) for a total of 8 cycles within 4-12 weeks of local ablative therapy completion. Study participants were administered locally ablative therapy to all recognized sites of malignancy.
The researchers measured two primary efficacy outcomes: progression-free survival (PFS) from the initiation of ablative therapy and the PFS from initiation of pembrolizumab. Secondary endpoints were safety, quality of life, and overall survival (OS).
Among patients who received pembrolizumab after ablative therapy, the median PFS was 19.1 months (95% CI, 9.4-28.7 months), which was significantly longer than the historical outcome (median PFS, 6.6 months; P = .005). In addition, the 24-month OS was 77.5%. With respect to safety, no decrease in quality of life or new safety signals were reported in the study.
One key limitation of the study was the single-arm design. As a result, distinguishing the effects of pembrolizumab over ablative therapy alone is not possible with the present data.
“This study is the first to show improved outcomes for immunotherapy after locally ablative therapy in patients with oligometastatic NSCLC,” Dr. Bauml and his colleagues wrote.
In another phase 2 trial (PEMBRO-RT study) reported in the same issue, Willemijn S.M.E. Theelen, MD, of the Netherlands Cancer Institute in Amsterdam and colleagues examined the use of pembrolizumab after stereotactic body radiotherapy or pembrolizumab alone in patients with recurrent metastatic NSCLC.
“This study evaluates whether stereotactic body radiotherapy enhances the effect of immune checkpoint blockade,” wrote Dr. Theelen and colleagues.
The PEMBRO-RT study included 76 patients with recurrent metastatic NSCLC who were randomized to pembrolizumab following radiotherapy or pembrolizumab alone. Intravenous pembrolizumab was administered at 200 mg/kg every 3 weeks, with the first dose given within 7 days after completion of radiotherapy.
The primary outcome was the overall response rate (ORR) at 12 weeks. Secondary outcomes included OS, PFS, and safety.
Among patients who received pembrolizumab after radiotherapy versus pembrolizumab alone, the ORR at 12 weeks was 36% and 18%, respectively (P = .07). In addition, the median PFS and OS were not statistically significant (P = .19 and P = .16, respectively).
“Positive results were largely influenced by the PD-L1–negative subgroup, which had significantly improved progression-free survival and overall survival,” the researchers wrote.
With respect to safety, no differences in grade 3-5 adverse effects were observed between the treatment groups. In addition, no significant differences were seen in pulmonary toxicities.
One key limitation of the study was the lack of information regarding optimal radiotherapy dosing and schedule.
“The results of this study are encouraging, and further evaluation in a larger phase 2/3 trial is recommended,” Dr. Theelen and colleagues wrote.
Further studies are needed to fully understand the links between combination radiotherapy and pembrolizumab in patients with NSCLC.
The study by Dr. Bauml and colleagues was funded by the Abramson Cancer Center and Merck & Co. The authors reported financial affiliations with Amgen, AstraZeneca, Bristol-Myers Squibb, Celgene, Janssen, Takeda, and several others.
The study by Dr. Theelen and colleagues was funded by Merck Sharp & Dohme. The authors reported financial affiliations with AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Merck Sharp & Dohme, Roche, Takeda, and several others.
SOURCE: Bauml JM et al. JAMA Oncol. 2019 Jul 11. doi: 10.1001/jamaoncol.2019.1449. Theelen WSME et al. JAMA Oncol. 2019 Jul 11. doi: 10.1001/jamaoncol.2019.1478.
Over the last 20 years, significant advances have been made in the development of ablative radiotherapy and immunotherapy, particularly in the oncology setting. More recently, combination radiation therapy (RT) and immuno-oncology (IO) approaches have emerged, and the body of evidence for this novel treatment strategy continues to grow.
Recent studies have suggested that combined RT/IO therapy may confer survival benefit for patients with non–small cell lung cancer (NSCLC). Limitations of these studies include design, which have been largely case reports and single-center studies. The recent findings reported by Bauml et al. and Theelen et al. provide insight into the combined use of immune checkpoint blockade and radiotherapy in patients with NSCLC.
While the study by Dr. Theelen and colleagues did not reach its prespecified endpoint, the findings showed promise in some patient subpopulations. Dr. Bauml and colleagues reported favorable survival outcomes in their study, notably progression-free survival, following radical local therapy, when compared with historical outcomes. Intriguingly, the combination approach in both studies was well tolerated, with little to no grade 3-5 toxicities reported.
Taken together, these data constitute early evidence indicative of possible synergy between both therapies. In response, well-designed phase 3 studies are warranted to further explore these effects.
Joshua Walker, MD, PhD, is affiliated with Oregon Health & Science University in Portland. Billy W. Loo Jr., MD, PhD, is with Stanford (Calif.) University. Dr. Walker reported no conflicts of interest. Dr. Loo reported receiving research support from Varian Medical Systems and is a board member of TibaRay. These comments are adapted from their editorial (JAMA Oncol. 2019 Jul 11. doi: 10.1001/jamaoncol.2019.1448 ).
Over the last 20 years, significant advances have been made in the development of ablative radiotherapy and immunotherapy, particularly in the oncology setting. More recently, combination radiation therapy (RT) and immuno-oncology (IO) approaches have emerged, and the body of evidence for this novel treatment strategy continues to grow.
Recent studies have suggested that combined RT/IO therapy may confer survival benefit for patients with non–small cell lung cancer (NSCLC). Limitations of these studies include design, which have been largely case reports and single-center studies. The recent findings reported by Bauml et al. and Theelen et al. provide insight into the combined use of immune checkpoint blockade and radiotherapy in patients with NSCLC.
While the study by Dr. Theelen and colleagues did not reach its prespecified endpoint, the findings showed promise in some patient subpopulations. Dr. Bauml and colleagues reported favorable survival outcomes in their study, notably progression-free survival, following radical local therapy, when compared with historical outcomes. Intriguingly, the combination approach in both studies was well tolerated, with little to no grade 3-5 toxicities reported.
Taken together, these data constitute early evidence indicative of possible synergy between both therapies. In response, well-designed phase 3 studies are warranted to further explore these effects.
Joshua Walker, MD, PhD, is affiliated with Oregon Health & Science University in Portland. Billy W. Loo Jr., MD, PhD, is with Stanford (Calif.) University. Dr. Walker reported no conflicts of interest. Dr. Loo reported receiving research support from Varian Medical Systems and is a board member of TibaRay. These comments are adapted from their editorial (JAMA Oncol. 2019 Jul 11. doi: 10.1001/jamaoncol.2019.1448 ).
Over the last 20 years, significant advances have been made in the development of ablative radiotherapy and immunotherapy, particularly in the oncology setting. More recently, combination radiation therapy (RT) and immuno-oncology (IO) approaches have emerged, and the body of evidence for this novel treatment strategy continues to grow.
Recent studies have suggested that combined RT/IO therapy may confer survival benefit for patients with non–small cell lung cancer (NSCLC). Limitations of these studies include design, which have been largely case reports and single-center studies. The recent findings reported by Bauml et al. and Theelen et al. provide insight into the combined use of immune checkpoint blockade and radiotherapy in patients with NSCLC.
While the study by Dr. Theelen and colleagues did not reach its prespecified endpoint, the findings showed promise in some patient subpopulations. Dr. Bauml and colleagues reported favorable survival outcomes in their study, notably progression-free survival, following radical local therapy, when compared with historical outcomes. Intriguingly, the combination approach in both studies was well tolerated, with little to no grade 3-5 toxicities reported.
Taken together, these data constitute early evidence indicative of possible synergy between both therapies. In response, well-designed phase 3 studies are warranted to further explore these effects.
Joshua Walker, MD, PhD, is affiliated with Oregon Health & Science University in Portland. Billy W. Loo Jr., MD, PhD, is with Stanford (Calif.) University. Dr. Walker reported no conflicts of interest. Dr. Loo reported receiving research support from Varian Medical Systems and is a board member of TibaRay. These comments are adapted from their editorial (JAMA Oncol. 2019 Jul 11. doi: 10.1001/jamaoncol.2019.1448 ).
Combination radiotherapy and pembrolizumab may improve clinical outcomes by means of synergy in the treatment of patients with non–small cell lung cancer (NSCLC), according to results from two recent studies.
“The best way to combine immunotherapy with ablative therapies in the curative setting is an area of active investigation,” wrote Joshua M. Bauml, MD, of the University of Pennsylvania, Philadelphia, and colleagues.
Dr. Bauml and colleagues recently reported the results of a phase 2 study exploring the addition of pembrolizumab following the completion of locally ablative therapy in patients with oligometastatic NSCLC in JAMA Oncology.
The single-arm trial included 51 patients who received intravenous pembrolizumab (200 mg every 21 days) for a total of 8 cycles within 4-12 weeks of local ablative therapy completion. Study participants were administered locally ablative therapy to all recognized sites of malignancy.
The researchers measured two primary efficacy outcomes: progression-free survival (PFS) from the initiation of ablative therapy and the PFS from initiation of pembrolizumab. Secondary endpoints were safety, quality of life, and overall survival (OS).
Among patients who received pembrolizumab after ablative therapy, the median PFS was 19.1 months (95% CI, 9.4-28.7 months), which was significantly longer than the historical outcome (median PFS, 6.6 months; P = .005). In addition, the 24-month OS was 77.5%. With respect to safety, no decrease in quality of life or new safety signals were reported in the study.
One key limitation of the study was the single-arm design. As a result, distinguishing the effects of pembrolizumab over ablative therapy alone is not possible with the present data.
“This study is the first to show improved outcomes for immunotherapy after locally ablative therapy in patients with oligometastatic NSCLC,” Dr. Bauml and his colleagues wrote.
In another phase 2 trial (PEMBRO-RT study) reported in the same issue, Willemijn S.M.E. Theelen, MD, of the Netherlands Cancer Institute in Amsterdam and colleagues examined the use of pembrolizumab after stereotactic body radiotherapy or pembrolizumab alone in patients with recurrent metastatic NSCLC.
“This study evaluates whether stereotactic body radiotherapy enhances the effect of immune checkpoint blockade,” wrote Dr. Theelen and colleagues.
The PEMBRO-RT study included 76 patients with recurrent metastatic NSCLC who were randomized to pembrolizumab following radiotherapy or pembrolizumab alone. Intravenous pembrolizumab was administered at 200 mg/kg every 3 weeks, with the first dose given within 7 days after completion of radiotherapy.
The primary outcome was the overall response rate (ORR) at 12 weeks. Secondary outcomes included OS, PFS, and safety.
Among patients who received pembrolizumab after radiotherapy versus pembrolizumab alone, the ORR at 12 weeks was 36% and 18%, respectively (P = .07). In addition, the median PFS and OS were not statistically significant (P = .19 and P = .16, respectively).
“Positive results were largely influenced by the PD-L1–negative subgroup, which had significantly improved progression-free survival and overall survival,” the researchers wrote.
With respect to safety, no differences in grade 3-5 adverse effects were observed between the treatment groups. In addition, no significant differences were seen in pulmonary toxicities.
One key limitation of the study was the lack of information regarding optimal radiotherapy dosing and schedule.
“The results of this study are encouraging, and further evaluation in a larger phase 2/3 trial is recommended,” Dr. Theelen and colleagues wrote.
Further studies are needed to fully understand the links between combination radiotherapy and pembrolizumab in patients with NSCLC.
The study by Dr. Bauml and colleagues was funded by the Abramson Cancer Center and Merck & Co. The authors reported financial affiliations with Amgen, AstraZeneca, Bristol-Myers Squibb, Celgene, Janssen, Takeda, and several others.
The study by Dr. Theelen and colleagues was funded by Merck Sharp & Dohme. The authors reported financial affiliations with AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Merck Sharp & Dohme, Roche, Takeda, and several others.
SOURCE: Bauml JM et al. JAMA Oncol. 2019 Jul 11. doi: 10.1001/jamaoncol.2019.1449. Theelen WSME et al. JAMA Oncol. 2019 Jul 11. doi: 10.1001/jamaoncol.2019.1478.
Combination radiotherapy and pembrolizumab may improve clinical outcomes by means of synergy in the treatment of patients with non–small cell lung cancer (NSCLC), according to results from two recent studies.
“The best way to combine immunotherapy with ablative therapies in the curative setting is an area of active investigation,” wrote Joshua M. Bauml, MD, of the University of Pennsylvania, Philadelphia, and colleagues.
Dr. Bauml and colleagues recently reported the results of a phase 2 study exploring the addition of pembrolizumab following the completion of locally ablative therapy in patients with oligometastatic NSCLC in JAMA Oncology.
The single-arm trial included 51 patients who received intravenous pembrolizumab (200 mg every 21 days) for a total of 8 cycles within 4-12 weeks of local ablative therapy completion. Study participants were administered locally ablative therapy to all recognized sites of malignancy.
The researchers measured two primary efficacy outcomes: progression-free survival (PFS) from the initiation of ablative therapy and the PFS from initiation of pembrolizumab. Secondary endpoints were safety, quality of life, and overall survival (OS).
Among patients who received pembrolizumab after ablative therapy, the median PFS was 19.1 months (95% CI, 9.4-28.7 months), which was significantly longer than the historical outcome (median PFS, 6.6 months; P = .005). In addition, the 24-month OS was 77.5%. With respect to safety, no decrease in quality of life or new safety signals were reported in the study.
One key limitation of the study was the single-arm design. As a result, distinguishing the effects of pembrolizumab over ablative therapy alone is not possible with the present data.
“This study is the first to show improved outcomes for immunotherapy after locally ablative therapy in patients with oligometastatic NSCLC,” Dr. Bauml and his colleagues wrote.
In another phase 2 trial (PEMBRO-RT study) reported in the same issue, Willemijn S.M.E. Theelen, MD, of the Netherlands Cancer Institute in Amsterdam and colleagues examined the use of pembrolizumab after stereotactic body radiotherapy or pembrolizumab alone in patients with recurrent metastatic NSCLC.
“This study evaluates whether stereotactic body radiotherapy enhances the effect of immune checkpoint blockade,” wrote Dr. Theelen and colleagues.
The PEMBRO-RT study included 76 patients with recurrent metastatic NSCLC who were randomized to pembrolizumab following radiotherapy or pembrolizumab alone. Intravenous pembrolizumab was administered at 200 mg/kg every 3 weeks, with the first dose given within 7 days after completion of radiotherapy.
The primary outcome was the overall response rate (ORR) at 12 weeks. Secondary outcomes included OS, PFS, and safety.
Among patients who received pembrolizumab after radiotherapy versus pembrolizumab alone, the ORR at 12 weeks was 36% and 18%, respectively (P = .07). In addition, the median PFS and OS were not statistically significant (P = .19 and P = .16, respectively).
“Positive results were largely influenced by the PD-L1–negative subgroup, which had significantly improved progression-free survival and overall survival,” the researchers wrote.
With respect to safety, no differences in grade 3-5 adverse effects were observed between the treatment groups. In addition, no significant differences were seen in pulmonary toxicities.
One key limitation of the study was the lack of information regarding optimal radiotherapy dosing and schedule.
“The results of this study are encouraging, and further evaluation in a larger phase 2/3 trial is recommended,” Dr. Theelen and colleagues wrote.
Further studies are needed to fully understand the links between combination radiotherapy and pembrolizumab in patients with NSCLC.
The study by Dr. Bauml and colleagues was funded by the Abramson Cancer Center and Merck & Co. The authors reported financial affiliations with Amgen, AstraZeneca, Bristol-Myers Squibb, Celgene, Janssen, Takeda, and several others.
The study by Dr. Theelen and colleagues was funded by Merck Sharp & Dohme. The authors reported financial affiliations with AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Merck Sharp & Dohme, Roche, Takeda, and several others.
SOURCE: Bauml JM et al. JAMA Oncol. 2019 Jul 11. doi: 10.1001/jamaoncol.2019.1449. Theelen WSME et al. JAMA Oncol. 2019 Jul 11. doi: 10.1001/jamaoncol.2019.1478.
FROM JAMA ONCOLOGY
Motivational interviewing may beat relaxation for OCD symptoms
Benefits of the intervention appear to end at 12-month follow-up
Patients with obsessive-compulsive disorder who participate in a motivational interviewing (MI) intervention before treatment with exposure and response prevention (ERP) get better short-term results, compared with those who participate in a relaxation intervention before ERP, a small study shows.
“These findings suggest that MI prior to ERP may confer a small but meaningful benefit for enhancing treatment outcome post ERP,” wrote Randi E. McCabe, PhD, of the department of psychiatry and behavioral neurosciences at McMaster University, Hamilton, Ont., and associates. The study was published in the Journal of Obsessive-Compulsive and Related Disorders.
Dr. McCabe and associates randomized 40 patients aged 18-65 years to the MI intervention and relaxation groups. All participants had a diagnosis of OCD as defined by the Structured Clinical Interview for DSM-IV. They also scored 16 or higher on the Yale-Brown Obsessive-Compulsive Scale (Y-BOCS) and said they were interested in the ERP treatment. After a few participants dropped out, 18 were left in the MI group and 17 were in the relaxation group.
The MI intervention consisted of three sessions that focused on raising awareness about the emotional and financial impact of the illness on patients’ lives, and addressing their concerns about ERP – which is a form of cognitive-behavioral therapy tailored to meet the needs of people with OCD. Meanwhile, the relaxation therapy was a three-session protocol consisting of progressive muscle relaxation.
Participants in both groups experienced reductions in symptoms in the short term, but the symptom reductions were more significant for participants in the MI group. For example, Y-BOCS scores were significantly lower among participants in the MI group posttreatment, compared with those in the relaxation intervention (13.72 vs. 16.20 at 3-month follow-up and 13.81 vs. 14.00 at 6-month follow-up). “Whereas Y-BOCS scores decreased from the severe range to the moderate range in the relaxation group, scores decreased from the severe to the mild range in the MI group,” the investigators wrote. Similar trends were found on other measures, including the DASS-21 depression scale and the DASS-21 anxiety stress scale. At the 12-month follow-up, however, “both groups looked similar,” Dr. McCabe and associates reported.
Several limitations were cited, including the small study size and the baseline differences in the participants’ self-reported OCD symptoms.
Nevertheless, the results suggest that intervening with they wrote.
Dr. McCabe and her associates reported no disclosures.
SOURCE: McCabe RE et al. J Obsessive Compuls Relat Disord. 2019. doi: 10.1016/j.jocrd.2019.1004466.
Benefits of the intervention appear to end at 12-month follow-up
Benefits of the intervention appear to end at 12-month follow-up
Patients with obsessive-compulsive disorder who participate in a motivational interviewing (MI) intervention before treatment with exposure and response prevention (ERP) get better short-term results, compared with those who participate in a relaxation intervention before ERP, a small study shows.
“These findings suggest that MI prior to ERP may confer a small but meaningful benefit for enhancing treatment outcome post ERP,” wrote Randi E. McCabe, PhD, of the department of psychiatry and behavioral neurosciences at McMaster University, Hamilton, Ont., and associates. The study was published in the Journal of Obsessive-Compulsive and Related Disorders.
Dr. McCabe and associates randomized 40 patients aged 18-65 years to the MI intervention and relaxation groups. All participants had a diagnosis of OCD as defined by the Structured Clinical Interview for DSM-IV. They also scored 16 or higher on the Yale-Brown Obsessive-Compulsive Scale (Y-BOCS) and said they were interested in the ERP treatment. After a few participants dropped out, 18 were left in the MI group and 17 were in the relaxation group.
The MI intervention consisted of three sessions that focused on raising awareness about the emotional and financial impact of the illness on patients’ lives, and addressing their concerns about ERP – which is a form of cognitive-behavioral therapy tailored to meet the needs of people with OCD. Meanwhile, the relaxation therapy was a three-session protocol consisting of progressive muscle relaxation.
Participants in both groups experienced reductions in symptoms in the short term, but the symptom reductions were more significant for participants in the MI group. For example, Y-BOCS scores were significantly lower among participants in the MI group posttreatment, compared with those in the relaxation intervention (13.72 vs. 16.20 at 3-month follow-up and 13.81 vs. 14.00 at 6-month follow-up). “Whereas Y-BOCS scores decreased from the severe range to the moderate range in the relaxation group, scores decreased from the severe to the mild range in the MI group,” the investigators wrote. Similar trends were found on other measures, including the DASS-21 depression scale and the DASS-21 anxiety stress scale. At the 12-month follow-up, however, “both groups looked similar,” Dr. McCabe and associates reported.
Several limitations were cited, including the small study size and the baseline differences in the participants’ self-reported OCD symptoms.
Nevertheless, the results suggest that intervening with they wrote.
Dr. McCabe and her associates reported no disclosures.
SOURCE: McCabe RE et al. J Obsessive Compuls Relat Disord. 2019. doi: 10.1016/j.jocrd.2019.1004466.
Patients with obsessive-compulsive disorder who participate in a motivational interviewing (MI) intervention before treatment with exposure and response prevention (ERP) get better short-term results, compared with those who participate in a relaxation intervention before ERP, a small study shows.
“These findings suggest that MI prior to ERP may confer a small but meaningful benefit for enhancing treatment outcome post ERP,” wrote Randi E. McCabe, PhD, of the department of psychiatry and behavioral neurosciences at McMaster University, Hamilton, Ont., and associates. The study was published in the Journal of Obsessive-Compulsive and Related Disorders.
Dr. McCabe and associates randomized 40 patients aged 18-65 years to the MI intervention and relaxation groups. All participants had a diagnosis of OCD as defined by the Structured Clinical Interview for DSM-IV. They also scored 16 or higher on the Yale-Brown Obsessive-Compulsive Scale (Y-BOCS) and said they were interested in the ERP treatment. After a few participants dropped out, 18 were left in the MI group and 17 were in the relaxation group.
The MI intervention consisted of three sessions that focused on raising awareness about the emotional and financial impact of the illness on patients’ lives, and addressing their concerns about ERP – which is a form of cognitive-behavioral therapy tailored to meet the needs of people with OCD. Meanwhile, the relaxation therapy was a three-session protocol consisting of progressive muscle relaxation.
Participants in both groups experienced reductions in symptoms in the short term, but the symptom reductions were more significant for participants in the MI group. For example, Y-BOCS scores were significantly lower among participants in the MI group posttreatment, compared with those in the relaxation intervention (13.72 vs. 16.20 at 3-month follow-up and 13.81 vs. 14.00 at 6-month follow-up). “Whereas Y-BOCS scores decreased from the severe range to the moderate range in the relaxation group, scores decreased from the severe to the mild range in the MI group,” the investigators wrote. Similar trends were found on other measures, including the DASS-21 depression scale and the DASS-21 anxiety stress scale. At the 12-month follow-up, however, “both groups looked similar,” Dr. McCabe and associates reported.
Several limitations were cited, including the small study size and the baseline differences in the participants’ self-reported OCD symptoms.
Nevertheless, the results suggest that intervening with they wrote.
Dr. McCabe and her associates reported no disclosures.
SOURCE: McCabe RE et al. J Obsessive Compuls Relat Disord. 2019. doi: 10.1016/j.jocrd.2019.1004466.
FROM THE JOURNAL OF OBSESSIVE-COMPULSIVE AND RELATED DISORDERS
Ebola outbreak: WHO/OCHA call for more aid, better security
The continuing outbreak of Ebola in the Democratic Republic of the Congo (DRC) was the subject of a special United Nations high-level event organized by the World Health Organization (WHO) and the United Nations Office for the Coordination of Humanitarian Affairs (OCHA). It was comoderated by Tedros Adhanom Ghebreyesus, Director-General of the World Health Organization, and Mark Lowcock, the UN Under-Secretary-General for Humanitarian Affairs and Emergency Relief Coordinator.

The DRC Ebola outbreak has drawn continuing concern and was highlighted by an even greater feeling of urgency as “on the eve of the conference,” according to one speaker.
That same infected individual – a priest arriving by bus to the city from an affected area – died of the disease the day after the conference concluded, according to the DRC authorities.
In his opening remarks, Mr. Lowcock stressed the importance of coordinating international efforts with the on-the-ground responses being carried out under the direction of Oly Ilunga Kalenga, MD, the DRC’s Minister of Public Health, who was also present and spoke at the meeting. Dr. Kalenga resigned his post on July 22, 2019.*
Mr. Lowcock stressed three points in particular that make for unique changes to the current response, compared with the earlier outbreak of 2014-2016.
First, in the previous outbreak in West Africa, “we didn’t have the vaccine and we didn’t have some of the successful treatments” that are currently available. Furthermore, more than 160,000 people have now been vaccinated and “the vaccine has a high degree of effectiveness.” This is an asset compared to the previous situation, he stated.
In his second point, he warned that the outbreak in the DRC “is taking place in an insecure and complex area with multiple armed groups present and large-scale preexisting humanitarian needs. Special interests distort the context. A history of disaffection with national authorities and foreigners generates distrust and makes the response more complicated. And one manifestation of that is attacks against health facilities and health care workers.” He added that “two more of our colleagues, trying to be part of the solution,” were killed in the past few days before the meeting. “Therefore, security for the response is of absolutely paramount importance, and we are trying to strengthen the way the UN family supports the government’s own security.”
The third major difference from the West Africa outbreak, Mr. Lowcock pointed out, was the issue of money. There was more than $2 billion in international support available for that earlier response. However, “what we have available for us in the DRC is just a small fraction of that. Donors released funds early on ... but much more is needed.” He warned that the cost of reaching zero cases must not be underestimated, and that the fourth strategic response plan for this outbreak, currently under development, “will be budgeted at a much higher level than the previous three plans, and that’s because it’s our assessment that we need a bigger, more comprehensive response if we’re to get to zero cases than we’ve had hitherto.”
In fact, he said, “unless there is a big scale up in the response, we’re unlikely to get to zero cases.”
The meeting also featured speakers outlining more local aspects of the response and discussing how international workers were coordinating more and more with local authorities and health practitioners in order to deliver health care on the ground while attempting to avoid the distrust created in the past, while still ensuring security for foreign personnel.
Commenting on the issue of security, Rory Stewart, the United Kingdom’s Secretary of State for International Development, described how a major DRC Ebola treatment center was attacked and burned by military insurgents, but is now rebuilt. He said that, while there have been some improvements and reasons to be hopeful, “this isn’t a moment for complacency; [the situation] is literally on the knife-edge.” He added: “If you go into that treatment center now, you will see that, although there are very good medical procedures, there are really, really worrying security procedures. The entire protection for the medical staff consists of a small square of sandbags about the height of this table [he raised his hand just above the standard conference table he was sitting at], behind which the doctors and nurses are supposed to hide if armed men get into the compound.”
In his presentation, David Gressly, the UN Emergency Ebola Response Coordinator (speaking by video from the DRC), stressed the need to cooperate with local authorities and to build local trust, stating that “the UN is putting together a tight, disciplined, coordinated system for rapid response and operational adjustments so that we can shift from chasing the disease to getting ahead of it. We need to quickly detect cases ... that have moved into areas of risk to stop the transmission early.”
Matshidiso Moeti, MD, WHO Regional Director for Africa, added in her presentation: “We’ve identified nine high-risk countries. Among those, Burundi, South Sudan, and Uganda face the highest risk and require our concerted and continued efforts.” She said that more than 10,000 health care workers in areas of high risk have so far been vaccinated against the disease.
In his concluding statement, Dr. Kalenga described the current state of affairs in his country with a modicum of hope. “A community that has been told it has a case of Ebola is a community that is traumatized by the very announcement of this epidemic. With time, the community has learned to face up to this epidemic differently. In some villages we are given a very different welcome than before. ... The villagers ask: ‘What should we do to make sure the Ebola case is the only one? The first and the last one?’ Throughout this epidemic, we have seen the people become more aware, and a certain acceptance of the very difficult and lethal diagnosis. ... So we have seen that work in the community has been maturing and bearing fruit.”
However, he pointed out, “there is a whole debate around the area of vaccinations, and we do need to close down this debate. At this point in time, we have a vaccine that is highly effective, a vaccine that is accepted by the population, after whole periods of mistrust. So we’ve come to a point in time when the population is accepting a vaccine, a vaccine that works, so we decided to no longer open the debate on vaccines and vaccination. ....We don’t want contradictory messages going out here, we don’t want different schemes going out. ...We have an effective weapon, we have an effective molecule. Let’s focus on that. Let’s all go in the same direction,” he concluded.
On July 11, an announcement by DRC officials stated that Merck’s rVSV-ZEBOV would be the only vaccine that will be used during the current Ebola outbreak in North Kivu and Ituri provinces, and that no other clinical vaccine trials to be allowed in the country so as not to confuse the population.
In that same announcement, the DRC reported that, since the beginning of the epidemic, the cumulative number of Ebola cases was 2,451, of which 2,357 were confirmed and 94 probable. There were 1,647 deaths (1,553 confirmed and 94 probable) and 683 people who survived. An additional 364 suspected cases were under investigation.
*Updated Aug. 1, 2019.
SOURCE: United Nations WHO/OCHA Webcast and Media Stakeout. July 15, 2019.
The continuing outbreak of Ebola in the Democratic Republic of the Congo (DRC) was the subject of a special United Nations high-level event organized by the World Health Organization (WHO) and the United Nations Office for the Coordination of Humanitarian Affairs (OCHA). It was comoderated by Tedros Adhanom Ghebreyesus, Director-General of the World Health Organization, and Mark Lowcock, the UN Under-Secretary-General for Humanitarian Affairs and Emergency Relief Coordinator.

The DRC Ebola outbreak has drawn continuing concern and was highlighted by an even greater feeling of urgency as “on the eve of the conference,” according to one speaker.
That same infected individual – a priest arriving by bus to the city from an affected area – died of the disease the day after the conference concluded, according to the DRC authorities.
In his opening remarks, Mr. Lowcock stressed the importance of coordinating international efforts with the on-the-ground responses being carried out under the direction of Oly Ilunga Kalenga, MD, the DRC’s Minister of Public Health, who was also present and spoke at the meeting. Dr. Kalenga resigned his post on July 22, 2019.*
Mr. Lowcock stressed three points in particular that make for unique changes to the current response, compared with the earlier outbreak of 2014-2016.
First, in the previous outbreak in West Africa, “we didn’t have the vaccine and we didn’t have some of the successful treatments” that are currently available. Furthermore, more than 160,000 people have now been vaccinated and “the vaccine has a high degree of effectiveness.” This is an asset compared to the previous situation, he stated.
In his second point, he warned that the outbreak in the DRC “is taking place in an insecure and complex area with multiple armed groups present and large-scale preexisting humanitarian needs. Special interests distort the context. A history of disaffection with national authorities and foreigners generates distrust and makes the response more complicated. And one manifestation of that is attacks against health facilities and health care workers.” He added that “two more of our colleagues, trying to be part of the solution,” were killed in the past few days before the meeting. “Therefore, security for the response is of absolutely paramount importance, and we are trying to strengthen the way the UN family supports the government’s own security.”
The third major difference from the West Africa outbreak, Mr. Lowcock pointed out, was the issue of money. There was more than $2 billion in international support available for that earlier response. However, “what we have available for us in the DRC is just a small fraction of that. Donors released funds early on ... but much more is needed.” He warned that the cost of reaching zero cases must not be underestimated, and that the fourth strategic response plan for this outbreak, currently under development, “will be budgeted at a much higher level than the previous three plans, and that’s because it’s our assessment that we need a bigger, more comprehensive response if we’re to get to zero cases than we’ve had hitherto.”
In fact, he said, “unless there is a big scale up in the response, we’re unlikely to get to zero cases.”
The meeting also featured speakers outlining more local aspects of the response and discussing how international workers were coordinating more and more with local authorities and health practitioners in order to deliver health care on the ground while attempting to avoid the distrust created in the past, while still ensuring security for foreign personnel.
Commenting on the issue of security, Rory Stewart, the United Kingdom’s Secretary of State for International Development, described how a major DRC Ebola treatment center was attacked and burned by military insurgents, but is now rebuilt. He said that, while there have been some improvements and reasons to be hopeful, “this isn’t a moment for complacency; [the situation] is literally on the knife-edge.” He added: “If you go into that treatment center now, you will see that, although there are very good medical procedures, there are really, really worrying security procedures. The entire protection for the medical staff consists of a small square of sandbags about the height of this table [he raised his hand just above the standard conference table he was sitting at], behind which the doctors and nurses are supposed to hide if armed men get into the compound.”
In his presentation, David Gressly, the UN Emergency Ebola Response Coordinator (speaking by video from the DRC), stressed the need to cooperate with local authorities and to build local trust, stating that “the UN is putting together a tight, disciplined, coordinated system for rapid response and operational adjustments so that we can shift from chasing the disease to getting ahead of it. We need to quickly detect cases ... that have moved into areas of risk to stop the transmission early.”
Matshidiso Moeti, MD, WHO Regional Director for Africa, added in her presentation: “We’ve identified nine high-risk countries. Among those, Burundi, South Sudan, and Uganda face the highest risk and require our concerted and continued efforts.” She said that more than 10,000 health care workers in areas of high risk have so far been vaccinated against the disease.
In his concluding statement, Dr. Kalenga described the current state of affairs in his country with a modicum of hope. “A community that has been told it has a case of Ebola is a community that is traumatized by the very announcement of this epidemic. With time, the community has learned to face up to this epidemic differently. In some villages we are given a very different welcome than before. ... The villagers ask: ‘What should we do to make sure the Ebola case is the only one? The first and the last one?’ Throughout this epidemic, we have seen the people become more aware, and a certain acceptance of the very difficult and lethal diagnosis. ... So we have seen that work in the community has been maturing and bearing fruit.”
However, he pointed out, “there is a whole debate around the area of vaccinations, and we do need to close down this debate. At this point in time, we have a vaccine that is highly effective, a vaccine that is accepted by the population, after whole periods of mistrust. So we’ve come to a point in time when the population is accepting a vaccine, a vaccine that works, so we decided to no longer open the debate on vaccines and vaccination. ....We don’t want contradictory messages going out here, we don’t want different schemes going out. ...We have an effective weapon, we have an effective molecule. Let’s focus on that. Let’s all go in the same direction,” he concluded.
On July 11, an announcement by DRC officials stated that Merck’s rVSV-ZEBOV would be the only vaccine that will be used during the current Ebola outbreak in North Kivu and Ituri provinces, and that no other clinical vaccine trials to be allowed in the country so as not to confuse the population.
In that same announcement, the DRC reported that, since the beginning of the epidemic, the cumulative number of Ebola cases was 2,451, of which 2,357 were confirmed and 94 probable. There were 1,647 deaths (1,553 confirmed and 94 probable) and 683 people who survived. An additional 364 suspected cases were under investigation.
*Updated Aug. 1, 2019.
SOURCE: United Nations WHO/OCHA Webcast and Media Stakeout. July 15, 2019.
The continuing outbreak of Ebola in the Democratic Republic of the Congo (DRC) was the subject of a special United Nations high-level event organized by the World Health Organization (WHO) and the United Nations Office for the Coordination of Humanitarian Affairs (OCHA). It was comoderated by Tedros Adhanom Ghebreyesus, Director-General of the World Health Organization, and Mark Lowcock, the UN Under-Secretary-General for Humanitarian Affairs and Emergency Relief Coordinator.

The DRC Ebola outbreak has drawn continuing concern and was highlighted by an even greater feeling of urgency as “on the eve of the conference,” according to one speaker.
That same infected individual – a priest arriving by bus to the city from an affected area – died of the disease the day after the conference concluded, according to the DRC authorities.
In his opening remarks, Mr. Lowcock stressed the importance of coordinating international efforts with the on-the-ground responses being carried out under the direction of Oly Ilunga Kalenga, MD, the DRC’s Minister of Public Health, who was also present and spoke at the meeting. Dr. Kalenga resigned his post on July 22, 2019.*
Mr. Lowcock stressed three points in particular that make for unique changes to the current response, compared with the earlier outbreak of 2014-2016.
First, in the previous outbreak in West Africa, “we didn’t have the vaccine and we didn’t have some of the successful treatments” that are currently available. Furthermore, more than 160,000 people have now been vaccinated and “the vaccine has a high degree of effectiveness.” This is an asset compared to the previous situation, he stated.
In his second point, he warned that the outbreak in the DRC “is taking place in an insecure and complex area with multiple armed groups present and large-scale preexisting humanitarian needs. Special interests distort the context. A history of disaffection with national authorities and foreigners generates distrust and makes the response more complicated. And one manifestation of that is attacks against health facilities and health care workers.” He added that “two more of our colleagues, trying to be part of the solution,” were killed in the past few days before the meeting. “Therefore, security for the response is of absolutely paramount importance, and we are trying to strengthen the way the UN family supports the government’s own security.”
The third major difference from the West Africa outbreak, Mr. Lowcock pointed out, was the issue of money. There was more than $2 billion in international support available for that earlier response. However, “what we have available for us in the DRC is just a small fraction of that. Donors released funds early on ... but much more is needed.” He warned that the cost of reaching zero cases must not be underestimated, and that the fourth strategic response plan for this outbreak, currently under development, “will be budgeted at a much higher level than the previous three plans, and that’s because it’s our assessment that we need a bigger, more comprehensive response if we’re to get to zero cases than we’ve had hitherto.”
In fact, he said, “unless there is a big scale up in the response, we’re unlikely to get to zero cases.”
The meeting also featured speakers outlining more local aspects of the response and discussing how international workers were coordinating more and more with local authorities and health practitioners in order to deliver health care on the ground while attempting to avoid the distrust created in the past, while still ensuring security for foreign personnel.
Commenting on the issue of security, Rory Stewart, the United Kingdom’s Secretary of State for International Development, described how a major DRC Ebola treatment center was attacked and burned by military insurgents, but is now rebuilt. He said that, while there have been some improvements and reasons to be hopeful, “this isn’t a moment for complacency; [the situation] is literally on the knife-edge.” He added: “If you go into that treatment center now, you will see that, although there are very good medical procedures, there are really, really worrying security procedures. The entire protection for the medical staff consists of a small square of sandbags about the height of this table [he raised his hand just above the standard conference table he was sitting at], behind which the doctors and nurses are supposed to hide if armed men get into the compound.”
In his presentation, David Gressly, the UN Emergency Ebola Response Coordinator (speaking by video from the DRC), stressed the need to cooperate with local authorities and to build local trust, stating that “the UN is putting together a tight, disciplined, coordinated system for rapid response and operational adjustments so that we can shift from chasing the disease to getting ahead of it. We need to quickly detect cases ... that have moved into areas of risk to stop the transmission early.”
Matshidiso Moeti, MD, WHO Regional Director for Africa, added in her presentation: “We’ve identified nine high-risk countries. Among those, Burundi, South Sudan, and Uganda face the highest risk and require our concerted and continued efforts.” She said that more than 10,000 health care workers in areas of high risk have so far been vaccinated against the disease.
In his concluding statement, Dr. Kalenga described the current state of affairs in his country with a modicum of hope. “A community that has been told it has a case of Ebola is a community that is traumatized by the very announcement of this epidemic. With time, the community has learned to face up to this epidemic differently. In some villages we are given a very different welcome than before. ... The villagers ask: ‘What should we do to make sure the Ebola case is the only one? The first and the last one?’ Throughout this epidemic, we have seen the people become more aware, and a certain acceptance of the very difficult and lethal diagnosis. ... So we have seen that work in the community has been maturing and bearing fruit.”
However, he pointed out, “there is a whole debate around the area of vaccinations, and we do need to close down this debate. At this point in time, we have a vaccine that is highly effective, a vaccine that is accepted by the population, after whole periods of mistrust. So we’ve come to a point in time when the population is accepting a vaccine, a vaccine that works, so we decided to no longer open the debate on vaccines and vaccination. ....We don’t want contradictory messages going out here, we don’t want different schemes going out. ...We have an effective weapon, we have an effective molecule. Let’s focus on that. Let’s all go in the same direction,” he concluded.
On July 11, an announcement by DRC officials stated that Merck’s rVSV-ZEBOV would be the only vaccine that will be used during the current Ebola outbreak in North Kivu and Ituri provinces, and that no other clinical vaccine trials to be allowed in the country so as not to confuse the population.
In that same announcement, the DRC reported that, since the beginning of the epidemic, the cumulative number of Ebola cases was 2,451, of which 2,357 were confirmed and 94 probable. There were 1,647 deaths (1,553 confirmed and 94 probable) and 683 people who survived. An additional 364 suspected cases were under investigation.
*Updated Aug. 1, 2019.
SOURCE: United Nations WHO/OCHA Webcast and Media Stakeout. July 15, 2019.
REPORTING FROM A UN MEETING LIVE WEBCAST
Minor surgeries appear safe for hemophilia patients on emicizumab
MELBOURNE – A majority of minor surgeries can be performed in hemophilia A patients receiving emicizumab therapy without requiring prophylactic treatment with coagulation factors, according to data presented at the International Society on Thrombosis and Haemostasis congress.
Elena Santagostino, MD, PhD, from the Hemophilia and Thrombosis Center at Ospedale Maggiore Policlinico in Milan presented data from 399 patients involved in the four HAVEN trials of the humanized bispecific monoclonal antibody emicizumab (Hemlibra), which is Food and Drug Administration–approved for the prevention of bleeding episodes in individuals with hemophilia A, with or without inhibitors.
The analysis focused on the 126 patients (31.6%) who underwent at least one surgical procedure during the studies. Of the 233 surgeries, there were 215 minor procedures performed in 115 patients, and 18 major surgeries in 18 patients. All patients were receiving ongoing treatment with emicizumab, and there was no change to that treatment regimen during surgery.
“It is clear that surgery is a challenge for hemophilia,” Dr. Santagostino said. “It is a challenge for bleeding, it is a challenge for thrombosis, it is a challenge for any new drug, and this is why there is a lot of interest around this topic.”
Overall, 65.6% of minor surgeries were performed without any prophylactic coagulation factor treatment, and 90.8% of minor surgeries were conducted without postoperative bleeds requiring treatment. There were no cases of thrombosis reported.
The surgeries that did not require prophylactic coagulation factor included 42 dental procedures, 25 central venous access devices, 17 endoscopic procedures, and 12 joint procedures.
While the HAVEN studies did not allow for elective major surgery, there were still 18 unplanned major surgical situations that arose during the course of the studies. These included three hip, one knee, and one ankle arthroplasties; three synovectomies; and some dental, central venous line, and endoscopic biopsy procedures.
Of these, 15 involved prophylactic coagulant factor administration, but three procedures – including one synovectomy – were performed without prophylaxis and none resulted in a bleed.
There was one complicated bleed that occurred in a patient undergoing multiple procedures including a synovectomy, joint debridement and chondroplasty, who received prolonged treatment with recombinant Factor VIIa.
Dr. Santagostino said the findings showed surgery could be safely performed in patients who were being treated with emicizumab, both with and without inhibitors.
“A large number of minor procedures can be done without adding coagulation factors,” she said in an interview. “This is true for less invasive surgeries, such as catheter-related central venous line procedures. Even several endoscopic procedures, like a single biopsy, can be done reasonably safely.”
However she said there was still a lack of experience in dealing with hemophilia A patients who were undergoing cancer surgery, or who had significant comorbidities that might put them at higher risk of thrombosis.
“These are special patients populations that are still not investigated in the trial setting,” she said.
Commenting on the data, session cochair Liane Khoo, MD, from the Haemophilia Treatment Centre at Royal Prince Alfred Hospital in Sydney, said the results showed surgery could be performed in hemophilia A patients with and without inhibitors.
“The more we have the medication and the more experience we have, then we become more confident in using it,” she said.
The study was funded by F. Hoffman-La Roche and Chugai Pharmaceutical. Dr. Santagostino reported consultancies and speakers bureau engagements with the pharmaceutical sector.
SOURCE: Santagostino E et al. 2019 ISTH Congress, Abstract OC 60.1.
MELBOURNE – A majority of minor surgeries can be performed in hemophilia A patients receiving emicizumab therapy without requiring prophylactic treatment with coagulation factors, according to data presented at the International Society on Thrombosis and Haemostasis congress.
Elena Santagostino, MD, PhD, from the Hemophilia and Thrombosis Center at Ospedale Maggiore Policlinico in Milan presented data from 399 patients involved in the four HAVEN trials of the humanized bispecific monoclonal antibody emicizumab (Hemlibra), which is Food and Drug Administration–approved for the prevention of bleeding episodes in individuals with hemophilia A, with or without inhibitors.
The analysis focused on the 126 patients (31.6%) who underwent at least one surgical procedure during the studies. Of the 233 surgeries, there were 215 minor procedures performed in 115 patients, and 18 major surgeries in 18 patients. All patients were receiving ongoing treatment with emicizumab, and there was no change to that treatment regimen during surgery.
“It is clear that surgery is a challenge for hemophilia,” Dr. Santagostino said. “It is a challenge for bleeding, it is a challenge for thrombosis, it is a challenge for any new drug, and this is why there is a lot of interest around this topic.”
Overall, 65.6% of minor surgeries were performed without any prophylactic coagulation factor treatment, and 90.8% of minor surgeries were conducted without postoperative bleeds requiring treatment. There were no cases of thrombosis reported.
The surgeries that did not require prophylactic coagulation factor included 42 dental procedures, 25 central venous access devices, 17 endoscopic procedures, and 12 joint procedures.
While the HAVEN studies did not allow for elective major surgery, there were still 18 unplanned major surgical situations that arose during the course of the studies. These included three hip, one knee, and one ankle arthroplasties; three synovectomies; and some dental, central venous line, and endoscopic biopsy procedures.
Of these, 15 involved prophylactic coagulant factor administration, but three procedures – including one synovectomy – were performed without prophylaxis and none resulted in a bleed.
There was one complicated bleed that occurred in a patient undergoing multiple procedures including a synovectomy, joint debridement and chondroplasty, who received prolonged treatment with recombinant Factor VIIa.
Dr. Santagostino said the findings showed surgery could be safely performed in patients who were being treated with emicizumab, both with and without inhibitors.
“A large number of minor procedures can be done without adding coagulation factors,” she said in an interview. “This is true for less invasive surgeries, such as catheter-related central venous line procedures. Even several endoscopic procedures, like a single biopsy, can be done reasonably safely.”
However she said there was still a lack of experience in dealing with hemophilia A patients who were undergoing cancer surgery, or who had significant comorbidities that might put them at higher risk of thrombosis.
“These are special patients populations that are still not investigated in the trial setting,” she said.
Commenting on the data, session cochair Liane Khoo, MD, from the Haemophilia Treatment Centre at Royal Prince Alfred Hospital in Sydney, said the results showed surgery could be performed in hemophilia A patients with and without inhibitors.
“The more we have the medication and the more experience we have, then we become more confident in using it,” she said.
The study was funded by F. Hoffman-La Roche and Chugai Pharmaceutical. Dr. Santagostino reported consultancies and speakers bureau engagements with the pharmaceutical sector.
SOURCE: Santagostino E et al. 2019 ISTH Congress, Abstract OC 60.1.
MELBOURNE – A majority of minor surgeries can be performed in hemophilia A patients receiving emicizumab therapy without requiring prophylactic treatment with coagulation factors, according to data presented at the International Society on Thrombosis and Haemostasis congress.
Elena Santagostino, MD, PhD, from the Hemophilia and Thrombosis Center at Ospedale Maggiore Policlinico in Milan presented data from 399 patients involved in the four HAVEN trials of the humanized bispecific monoclonal antibody emicizumab (Hemlibra), which is Food and Drug Administration–approved for the prevention of bleeding episodes in individuals with hemophilia A, with or without inhibitors.
The analysis focused on the 126 patients (31.6%) who underwent at least one surgical procedure during the studies. Of the 233 surgeries, there were 215 minor procedures performed in 115 patients, and 18 major surgeries in 18 patients. All patients were receiving ongoing treatment with emicizumab, and there was no change to that treatment regimen during surgery.
“It is clear that surgery is a challenge for hemophilia,” Dr. Santagostino said. “It is a challenge for bleeding, it is a challenge for thrombosis, it is a challenge for any new drug, and this is why there is a lot of interest around this topic.”
Overall, 65.6% of minor surgeries were performed without any prophylactic coagulation factor treatment, and 90.8% of minor surgeries were conducted without postoperative bleeds requiring treatment. There were no cases of thrombosis reported.
The surgeries that did not require prophylactic coagulation factor included 42 dental procedures, 25 central venous access devices, 17 endoscopic procedures, and 12 joint procedures.
While the HAVEN studies did not allow for elective major surgery, there were still 18 unplanned major surgical situations that arose during the course of the studies. These included three hip, one knee, and one ankle arthroplasties; three synovectomies; and some dental, central venous line, and endoscopic biopsy procedures.
Of these, 15 involved prophylactic coagulant factor administration, but three procedures – including one synovectomy – were performed without prophylaxis and none resulted in a bleed.
There was one complicated bleed that occurred in a patient undergoing multiple procedures including a synovectomy, joint debridement and chondroplasty, who received prolonged treatment with recombinant Factor VIIa.
Dr. Santagostino said the findings showed surgery could be safely performed in patients who were being treated with emicizumab, both with and without inhibitors.
“A large number of minor procedures can be done without adding coagulation factors,” she said in an interview. “This is true for less invasive surgeries, such as catheter-related central venous line procedures. Even several endoscopic procedures, like a single biopsy, can be done reasonably safely.”
However she said there was still a lack of experience in dealing with hemophilia A patients who were undergoing cancer surgery, or who had significant comorbidities that might put them at higher risk of thrombosis.
“These are special patients populations that are still not investigated in the trial setting,” she said.
Commenting on the data, session cochair Liane Khoo, MD, from the Haemophilia Treatment Centre at Royal Prince Alfred Hospital in Sydney, said the results showed surgery could be performed in hemophilia A patients with and without inhibitors.
“The more we have the medication and the more experience we have, then we become more confident in using it,” she said.
The study was funded by F. Hoffman-La Roche and Chugai Pharmaceutical. Dr. Santagostino reported consultancies and speakers bureau engagements with the pharmaceutical sector.
SOURCE: Santagostino E et al. 2019 ISTH Congress, Abstract OC 60.1.
REPORTING FROM 2019 ISTH CONGRESS
Baby’s Rash Causes Family Feud
Since birth, this 5-month-old boy has had a facial rash that comes and goes. At times severe, it is the source of much familial disagreement about its cause: Some say the problem is related to food, while others are sure it represents infection.
Several medications, including triple-antibiotic ointment and nystatin cream, have been tried. None have had much effect.
The child is well in all other respects—gaining weight as expected and experiencing normal growth and development. The rash does not appear to bother him as much as it bothers his family to see.
Further questioning reveals a strong family history of seasonal allergies, eczema, and asthma. Notably, all affected individuals have long since outgrown those problems.
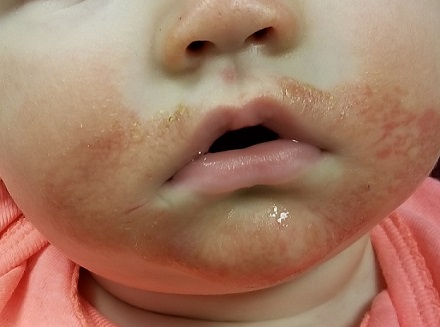
EXAMINATION
The child is in no apparent distress but is noted to have nasal congestion, with continual mouth breathing. Overall, his skin is quite dry and fair.
The rash itself is rather florid, affecting the perioral area and spreading onto the cheeks in a symmetrical configuration. The skin in these areas is focally erythematous, though not swollen. It is also quite scaly in places, giving the appearance of, as his parents note, “chapped” skin. Examination of the diaper area reveals a similar look focally.
What’s the diagnosis?
DISCUSSION
This case is typical of those seen multiple times daily in primary care and dermatology offices—hardly surprising, since atopic dermatitis (AD) affects about 20% of all newborns in this and other developed countries. In very young children, AD primarily affects the face and diaper area, as well as the trunk. About 50% of affected patients will have cradle cap—as did this child in his first month of life, we subsequently learned.
The tendency to develop AD is inherited. It is not related to food, although children with AD could develop a food allergy. However, it would more likely manifest with gastrointestinal symptoms. Another myth embedded in Western culture is that AD is caused by exposure to a particular laundry detergent.
Rather, this child and others like him have inherited dry, thin, overreactive skin that is bathed early on with nasal secretions, bacteria, and saliva. Later, their eczema will migrate to areas that stay moist, such as the antecubital and popliteal folds, or to the area around the neck, where the itching can be intense—which of course causes the child to scratch, in turn worsening the problem.
Patient/parent education is the key to dealing with AD and can be bolstered by handouts or direction to reliable websites. Besides objectifying the problem, these resources detail its nature and outline the need for daily bathing with mild cleansers, generous application of heavy moisturizers, careful use of topical corticosteroid creams or ointments (eg, 2.5% hydrocortisone), and avoidance of woolen clothing or bedding.
Another important component of patient/parent education is the reassurance that eczema will not scar the patient. It does occasionally become severe enough to require a short course of oral antibiotics (eg, cephalexin) or even the use of oral prednisolone. Since AD is not a histamine-driven process, antihistamines are ineffective for eczema.
TAKE-HOME LEARNING POINTS
- Atopic dermatitis (AD) is extremely common, affecting 20% of all newborns in developed countries.
- Infantile eczema typically centers on the face, particularly the perioral area.
- Later, it begins to involve the antecubital and popliteal areas, which stay moist a good part of the time.
- Most children outgrow the worst of the problem—and go on to have children who develop it.
Since birth, this 5-month-old boy has had a facial rash that comes and goes. At times severe, it is the source of much familial disagreement about its cause: Some say the problem is related to food, while others are sure it represents infection.
Several medications, including triple-antibiotic ointment and nystatin cream, have been tried. None have had much effect.
The child is well in all other respects—gaining weight as expected and experiencing normal growth and development. The rash does not appear to bother him as much as it bothers his family to see.
Further questioning reveals a strong family history of seasonal allergies, eczema, and asthma. Notably, all affected individuals have long since outgrown those problems.

EXAMINATION
The child is in no apparent distress but is noted to have nasal congestion, with continual mouth breathing. Overall, his skin is quite dry and fair.
The rash itself is rather florid, affecting the perioral area and spreading onto the cheeks in a symmetrical configuration. The skin in these areas is focally erythematous, though not swollen. It is also quite scaly in places, giving the appearance of, as his parents note, “chapped” skin. Examination of the diaper area reveals a similar look focally.
What’s the diagnosis?
DISCUSSION
This case is typical of those seen multiple times daily in primary care and dermatology offices—hardly surprising, since atopic dermatitis (AD) affects about 20% of all newborns in this and other developed countries. In very young children, AD primarily affects the face and diaper area, as well as the trunk. About 50% of affected patients will have cradle cap—as did this child in his first month of life, we subsequently learned.
The tendency to develop AD is inherited. It is not related to food, although children with AD could develop a food allergy. However, it would more likely manifest with gastrointestinal symptoms. Another myth embedded in Western culture is that AD is caused by exposure to a particular laundry detergent.
Rather, this child and others like him have inherited dry, thin, overreactive skin that is bathed early on with nasal secretions, bacteria, and saliva. Later, their eczema will migrate to areas that stay moist, such as the antecubital and popliteal folds, or to the area around the neck, where the itching can be intense—which of course causes the child to scratch, in turn worsening the problem.
Patient/parent education is the key to dealing with AD and can be bolstered by handouts or direction to reliable websites. Besides objectifying the problem, these resources detail its nature and outline the need for daily bathing with mild cleansers, generous application of heavy moisturizers, careful use of topical corticosteroid creams or ointments (eg, 2.5% hydrocortisone), and avoidance of woolen clothing or bedding.
Another important component of patient/parent education is the reassurance that eczema will not scar the patient. It does occasionally become severe enough to require a short course of oral antibiotics (eg, cephalexin) or even the use of oral prednisolone. Since AD is not a histamine-driven process, antihistamines are ineffective for eczema.
TAKE-HOME LEARNING POINTS
- Atopic dermatitis (AD) is extremely common, affecting 20% of all newborns in developed countries.
- Infantile eczema typically centers on the face, particularly the perioral area.
- Later, it begins to involve the antecubital and popliteal areas, which stay moist a good part of the time.
- Most children outgrow the worst of the problem—and go on to have children who develop it.
Since birth, this 5-month-old boy has had a facial rash that comes and goes. At times severe, it is the source of much familial disagreement about its cause: Some say the problem is related to food, while others are sure it represents infection.
Several medications, including triple-antibiotic ointment and nystatin cream, have been tried. None have had much effect.
The child is well in all other respects—gaining weight as expected and experiencing normal growth and development. The rash does not appear to bother him as much as it bothers his family to see.
Further questioning reveals a strong family history of seasonal allergies, eczema, and asthma. Notably, all affected individuals have long since outgrown those problems.

EXAMINATION
The child is in no apparent distress but is noted to have nasal congestion, with continual mouth breathing. Overall, his skin is quite dry and fair.
The rash itself is rather florid, affecting the perioral area and spreading onto the cheeks in a symmetrical configuration. The skin in these areas is focally erythematous, though not swollen. It is also quite scaly in places, giving the appearance of, as his parents note, “chapped” skin. Examination of the diaper area reveals a similar look focally.
What’s the diagnosis?
DISCUSSION
This case is typical of those seen multiple times daily in primary care and dermatology offices—hardly surprising, since atopic dermatitis (AD) affects about 20% of all newborns in this and other developed countries. In very young children, AD primarily affects the face and diaper area, as well as the trunk. About 50% of affected patients will have cradle cap—as did this child in his first month of life, we subsequently learned.
The tendency to develop AD is inherited. It is not related to food, although children with AD could develop a food allergy. However, it would more likely manifest with gastrointestinal symptoms. Another myth embedded in Western culture is that AD is caused by exposure to a particular laundry detergent.
Rather, this child and others like him have inherited dry, thin, overreactive skin that is bathed early on with nasal secretions, bacteria, and saliva. Later, their eczema will migrate to areas that stay moist, such as the antecubital and popliteal folds, or to the area around the neck, where the itching can be intense—which of course causes the child to scratch, in turn worsening the problem.
Patient/parent education is the key to dealing with AD and can be bolstered by handouts or direction to reliable websites. Besides objectifying the problem, these resources detail its nature and outline the need for daily bathing with mild cleansers, generous application of heavy moisturizers, careful use of topical corticosteroid creams or ointments (eg, 2.5% hydrocortisone), and avoidance of woolen clothing or bedding.
Another important component of patient/parent education is the reassurance that eczema will not scar the patient. It does occasionally become severe enough to require a short course of oral antibiotics (eg, cephalexin) or even the use of oral prednisolone. Since AD is not a histamine-driven process, antihistamines are ineffective for eczema.
TAKE-HOME LEARNING POINTS
- Atopic dermatitis (AD) is extremely common, affecting 20% of all newborns in developed countries.
- Infantile eczema typically centers on the face, particularly the perioral area.
- Later, it begins to involve the antecubital and popliteal areas, which stay moist a good part of the time.
- Most children outgrow the worst of the problem—and go on to have children who develop it.
Rash on elbows and hands
Based on the target lesions with central epithelial disruption, the FP diagnosed erythema multiforme (EM) in this patient. He also diagnosed genital herpes simplex in the crusting stage and suspected that it was the inciting event for the EM.
EM is considered a hypersensitivity reaction that is often secondary to infections or medications. Herpes simplex viruses (HSVI and HSV2) are the most common causative agents and have been implicated in at least 60% of cases.
The patient did not know that she had genital herpes simplex but remembered having sores in the genital area that preceded the similar rash a year earlier. This was a case of recurrent EM responding to recurrent herpes simplex.
The patient suspected that she had genital herpes simplex on and off for the past 10 years but lacked health insurance, so she never visited a doctor for treatment. She recently obtained health insurance and was willing to be tested for other sexually transmitted diseases. She also wanted to have a test done to confirm this was herpes simplex. The FP explained that it was unlikely that an antiviral agent would help the current case of herpes simplex and the associated EM because of the late timing, but antiviral medication could help to prevent further outbreaks and decrease transmission to her husband.
The patient was enthusiastic to start valacyclovir 500 mg/d for prophylaxis. For symptomatic relief, the FP prescribed a 30-g tube of 0.1% triamcinolone appointment and instructed the patient to apply it to the EM twice daily. The patient was sent for blood tests for syphilis and HIV; fortunately, both came back negative. The herpes simplex PCR test was positive for HSV2.
On a 1-week follow-up visit, the skin lesions were resolving, and the patient had no further symptoms. The patient said she understood that she should refrain from sexual intercourse if she developed lesions while on prophylaxis.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Milana C, Smith M. Erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1161-1168.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com
Based on the target lesions with central epithelial disruption, the FP diagnosed erythema multiforme (EM) in this patient. He also diagnosed genital herpes simplex in the crusting stage and suspected that it was the inciting event for the EM.
EM is considered a hypersensitivity reaction that is often secondary to infections or medications. Herpes simplex viruses (HSVI and HSV2) are the most common causative agents and have been implicated in at least 60% of cases.
The patient did not know that she had genital herpes simplex but remembered having sores in the genital area that preceded the similar rash a year earlier. This was a case of recurrent EM responding to recurrent herpes simplex.
The patient suspected that she had genital herpes simplex on and off for the past 10 years but lacked health insurance, so she never visited a doctor for treatment. She recently obtained health insurance and was willing to be tested for other sexually transmitted diseases. She also wanted to have a test done to confirm this was herpes simplex. The FP explained that it was unlikely that an antiviral agent would help the current case of herpes simplex and the associated EM because of the late timing, but antiviral medication could help to prevent further outbreaks and decrease transmission to her husband.
The patient was enthusiastic to start valacyclovir 500 mg/d for prophylaxis. For symptomatic relief, the FP prescribed a 30-g tube of 0.1% triamcinolone appointment and instructed the patient to apply it to the EM twice daily. The patient was sent for blood tests for syphilis and HIV; fortunately, both came back negative. The herpes simplex PCR test was positive for HSV2.
On a 1-week follow-up visit, the skin lesions were resolving, and the patient had no further symptoms. The patient said she understood that she should refrain from sexual intercourse if she developed lesions while on prophylaxis.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Milana C, Smith M. Erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1161-1168.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com
Based on the target lesions with central epithelial disruption, the FP diagnosed erythema multiforme (EM) in this patient. He also diagnosed genital herpes simplex in the crusting stage and suspected that it was the inciting event for the EM.
EM is considered a hypersensitivity reaction that is often secondary to infections or medications. Herpes simplex viruses (HSVI and HSV2) are the most common causative agents and have been implicated in at least 60% of cases.
The patient did not know that she had genital herpes simplex but remembered having sores in the genital area that preceded the similar rash a year earlier. This was a case of recurrent EM responding to recurrent herpes simplex.
The patient suspected that she had genital herpes simplex on and off for the past 10 years but lacked health insurance, so she never visited a doctor for treatment. She recently obtained health insurance and was willing to be tested for other sexually transmitted diseases. She also wanted to have a test done to confirm this was herpes simplex. The FP explained that it was unlikely that an antiviral agent would help the current case of herpes simplex and the associated EM because of the late timing, but antiviral medication could help to prevent further outbreaks and decrease transmission to her husband.
The patient was enthusiastic to start valacyclovir 500 mg/d for prophylaxis. For symptomatic relief, the FP prescribed a 30-g tube of 0.1% triamcinolone appointment and instructed the patient to apply it to the EM twice daily. The patient was sent for blood tests for syphilis and HIV; fortunately, both came back negative. The herpes simplex PCR test was positive for HSV2.
On a 1-week follow-up visit, the skin lesions were resolving, and the patient had no further symptoms. The patient said she understood that she should refrain from sexual intercourse if she developed lesions while on prophylaxis.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Milana C, Smith M. Erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1161-1168.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com
Systolic, diastolic BP each tied to adverse CV outcomes
Both systolic and diastolic hypertension independently predict myocardial infarction and strokes, but systolic blood pressure is more strongly linked to adverse outcomes.
That’s according to a study of more than 1 million patients and 36 million outpatient blood pressure measurements published in the New England Journal of Medicine.
Systolic and diastolic hypertension predicted adverse outcomes at cutpoints of 140/90 and 130/80 mm Hg in the large retrospective cohort study, supporting the recent guideline changes that made blood pressure targets more stringent for higher-risk patients, said lead investigator Alexander C. Flint, MD, of Kaiser Permanente Northern California (KPNC) in Oakland.
“While systolic does count for more, in the fact that it is a stronger driver of the risk of heart attack and stroke, diastolic absolutely does as well, and it does so independently. So we ignore our diastolic hypertension at our own peril,” Dr. Flint said in an interview.
Systolic hypertension began to overshadow diastolic after the Framingham Heart Study and others that suggested it is a more important predictor of adverse cardiovascular outcomes, Dr. Flint and coauthors said in a report on their study.
Those findings caused some to say diastole should be abandoned, and led to a “near-exclusive focus” on systolic hypertension in a 2000 advisory statement from the National High Blood Pressure Education Program, they say in their report.
While current guidelines emphasize the importance of both systolic and diastolic targets, many clinicians today often assign little importance to diastolic blood pressure values, the report adds.
“The pendulum needs to swing back, right down in the middle,” Dr. Flint said in the interview.
The study by Dr. Flint and colleagues comprised a cohort of approximately 1.3 million outpatients from KPNC who had at least one baseline blood pressure reading in during 2007-2008, and two or more follow-up measurements between 2009 and 2016, for a total of about 36.8 million data points.
Systolic hypertension burden was linked to the composite of MI or stroke, with a hazard ratio of 1.18 (95% confidence interval, 1.17-1.18; P less than .001) per unit increase in z score, according to results of a multivariable regression analysis. Likewise, diastolic hypertension burden was linked to those adverse outcomes, with a hazard ratio of 1.06 (95% CI, 1.06-1.07; P less than .001).
Put in terms of estimated risk of MI or stroke, patients with a systolic blood pressure around 160 mm Hg – 3 standard deviations from the mean – was 4.8%, compared to a predicted risk of just 1.9% for a systolic blood pressure near 136 mm Hg, the investigators said in their report.
Similarly, predicted risk was 3.6% for a diastolic pressure of about 96 mm Hg, also 3 standard deviations from the mean, and 1.9% for a diastolic BP near 81 mm Hg.
“The two are not that separate,” Dr. Flint said of the risks associated with systolic and diastolic hypertension at that 3-standard-deviation point. Beyond that, increased systolic blood pressure is associated with more risk relative to increased diastolic blood pressure, the logistic regression modeling shows.
Taken together, findings from this large cohort study emphasize the importance of making lifestyle modifications and adjusting medication to ensure that both systolic and diastolic targets are met, according to Dr. Flint.
“Rises in systolic blood pressure count for more in influencing the risk of heart attack and stroke,” he said, “but diastolic independently counts for quite a lot. It’s a close second.”
Dr. Flint reported no disclosures. Senior author Deepak L. Bhatt, MD, MPH, reported disclosures with Amarin, AstraZeneca, Bristol-Myers Squibb, Eisai, Ethicon, Medtronic, Sanofi Aventis, Takeda, The Medicines Company, and others. The remaining authors had no disclosures.
SOURCE: Flint AC et al. N Engl J Med. 2019 Jul 18. doi: 10.1056/NEJMoa1803180.
Both systolic and diastolic hypertension independently predict myocardial infarction and strokes, but systolic blood pressure is more strongly linked to adverse outcomes.
That’s according to a study of more than 1 million patients and 36 million outpatient blood pressure measurements published in the New England Journal of Medicine.
Systolic and diastolic hypertension predicted adverse outcomes at cutpoints of 140/90 and 130/80 mm Hg in the large retrospective cohort study, supporting the recent guideline changes that made blood pressure targets more stringent for higher-risk patients, said lead investigator Alexander C. Flint, MD, of Kaiser Permanente Northern California (KPNC) in Oakland.
“While systolic does count for more, in the fact that it is a stronger driver of the risk of heart attack and stroke, diastolic absolutely does as well, and it does so independently. So we ignore our diastolic hypertension at our own peril,” Dr. Flint said in an interview.
Systolic hypertension began to overshadow diastolic after the Framingham Heart Study and others that suggested it is a more important predictor of adverse cardiovascular outcomes, Dr. Flint and coauthors said in a report on their study.
Those findings caused some to say diastole should be abandoned, and led to a “near-exclusive focus” on systolic hypertension in a 2000 advisory statement from the National High Blood Pressure Education Program, they say in their report.
While current guidelines emphasize the importance of both systolic and diastolic targets, many clinicians today often assign little importance to diastolic blood pressure values, the report adds.
“The pendulum needs to swing back, right down in the middle,” Dr. Flint said in the interview.
The study by Dr. Flint and colleagues comprised a cohort of approximately 1.3 million outpatients from KPNC who had at least one baseline blood pressure reading in during 2007-2008, and two or more follow-up measurements between 2009 and 2016, for a total of about 36.8 million data points.
Systolic hypertension burden was linked to the composite of MI or stroke, with a hazard ratio of 1.18 (95% confidence interval, 1.17-1.18; P less than .001) per unit increase in z score, according to results of a multivariable regression analysis. Likewise, diastolic hypertension burden was linked to those adverse outcomes, with a hazard ratio of 1.06 (95% CI, 1.06-1.07; P less than .001).
Put in terms of estimated risk of MI or stroke, patients with a systolic blood pressure around 160 mm Hg – 3 standard deviations from the mean – was 4.8%, compared to a predicted risk of just 1.9% for a systolic blood pressure near 136 mm Hg, the investigators said in their report.
Similarly, predicted risk was 3.6% for a diastolic pressure of about 96 mm Hg, also 3 standard deviations from the mean, and 1.9% for a diastolic BP near 81 mm Hg.
“The two are not that separate,” Dr. Flint said of the risks associated with systolic and diastolic hypertension at that 3-standard-deviation point. Beyond that, increased systolic blood pressure is associated with more risk relative to increased diastolic blood pressure, the logistic regression modeling shows.
Taken together, findings from this large cohort study emphasize the importance of making lifestyle modifications and adjusting medication to ensure that both systolic and diastolic targets are met, according to Dr. Flint.
“Rises in systolic blood pressure count for more in influencing the risk of heart attack and stroke,” he said, “but diastolic independently counts for quite a lot. It’s a close second.”
Dr. Flint reported no disclosures. Senior author Deepak L. Bhatt, MD, MPH, reported disclosures with Amarin, AstraZeneca, Bristol-Myers Squibb, Eisai, Ethicon, Medtronic, Sanofi Aventis, Takeda, The Medicines Company, and others. The remaining authors had no disclosures.
SOURCE: Flint AC et al. N Engl J Med. 2019 Jul 18. doi: 10.1056/NEJMoa1803180.
Both systolic and diastolic hypertension independently predict myocardial infarction and strokes, but systolic blood pressure is more strongly linked to adverse outcomes.
That’s according to a study of more than 1 million patients and 36 million outpatient blood pressure measurements published in the New England Journal of Medicine.
Systolic and diastolic hypertension predicted adverse outcomes at cutpoints of 140/90 and 130/80 mm Hg in the large retrospective cohort study, supporting the recent guideline changes that made blood pressure targets more stringent for higher-risk patients, said lead investigator Alexander C. Flint, MD, of Kaiser Permanente Northern California (KPNC) in Oakland.
“While systolic does count for more, in the fact that it is a stronger driver of the risk of heart attack and stroke, diastolic absolutely does as well, and it does so independently. So we ignore our diastolic hypertension at our own peril,” Dr. Flint said in an interview.
Systolic hypertension began to overshadow diastolic after the Framingham Heart Study and others that suggested it is a more important predictor of adverse cardiovascular outcomes, Dr. Flint and coauthors said in a report on their study.
Those findings caused some to say diastole should be abandoned, and led to a “near-exclusive focus” on systolic hypertension in a 2000 advisory statement from the National High Blood Pressure Education Program, they say in their report.
While current guidelines emphasize the importance of both systolic and diastolic targets, many clinicians today often assign little importance to diastolic blood pressure values, the report adds.
“The pendulum needs to swing back, right down in the middle,” Dr. Flint said in the interview.
The study by Dr. Flint and colleagues comprised a cohort of approximately 1.3 million outpatients from KPNC who had at least one baseline blood pressure reading in during 2007-2008, and two or more follow-up measurements between 2009 and 2016, for a total of about 36.8 million data points.
Systolic hypertension burden was linked to the composite of MI or stroke, with a hazard ratio of 1.18 (95% confidence interval, 1.17-1.18; P less than .001) per unit increase in z score, according to results of a multivariable regression analysis. Likewise, diastolic hypertension burden was linked to those adverse outcomes, with a hazard ratio of 1.06 (95% CI, 1.06-1.07; P less than .001).
Put in terms of estimated risk of MI or stroke, patients with a systolic blood pressure around 160 mm Hg – 3 standard deviations from the mean – was 4.8%, compared to a predicted risk of just 1.9% for a systolic blood pressure near 136 mm Hg, the investigators said in their report.
Similarly, predicted risk was 3.6% for a diastolic pressure of about 96 mm Hg, also 3 standard deviations from the mean, and 1.9% for a diastolic BP near 81 mm Hg.
“The two are not that separate,” Dr. Flint said of the risks associated with systolic and diastolic hypertension at that 3-standard-deviation point. Beyond that, increased systolic blood pressure is associated with more risk relative to increased diastolic blood pressure, the logistic regression modeling shows.
Taken together, findings from this large cohort study emphasize the importance of making lifestyle modifications and adjusting medication to ensure that both systolic and diastolic targets are met, according to Dr. Flint.
“Rises in systolic blood pressure count for more in influencing the risk of heart attack and stroke,” he said, “but diastolic independently counts for quite a lot. It’s a close second.”
Dr. Flint reported no disclosures. Senior author Deepak L. Bhatt, MD, MPH, reported disclosures with Amarin, AstraZeneca, Bristol-Myers Squibb, Eisai, Ethicon, Medtronic, Sanofi Aventis, Takeda, The Medicines Company, and others. The remaining authors had no disclosures.
SOURCE: Flint AC et al. N Engl J Med. 2019 Jul 18. doi: 10.1056/NEJMoa1803180.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: .
Major finding: Systolic and diastolic hypertension burden were linked to the composite endpoint with hazard ratios of 1.18 and 1.06 per unit increase in z score, respectively.
Study details: A retrospective cohort study of roughly 1.3 million outpatients with 36.8 million BP measurements.
Disclosures: The senior author of the study reported disclosures with Amarin, AstraZeneca, Bristol Myers Squibb, Eisai, Ethicon, Medtronic, Sanofi Aventis, Takeda, The Medicines Company, and others. The remaining authors had no disclosures.
Source: Flint AC et al. N Engl J Med. 2019 Jul 18. doi: 10.1056/NEJMoa1803180.