User login
Drug overdose deaths declined in 2018
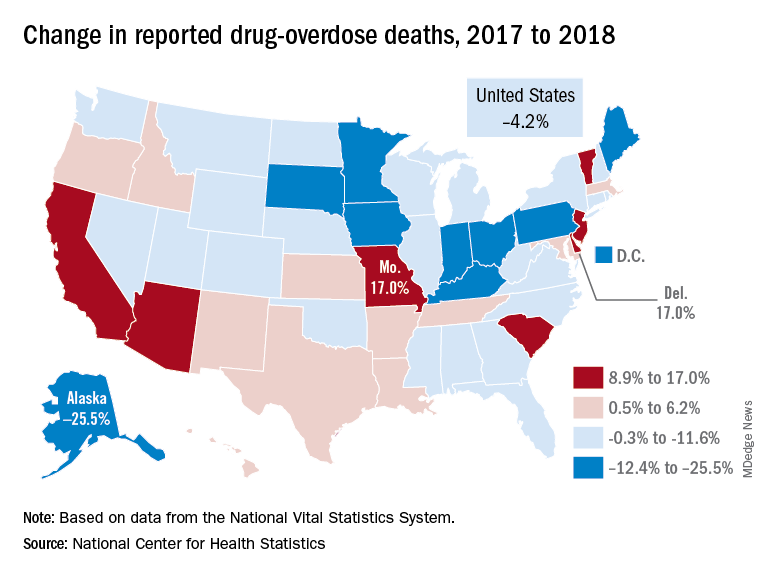
Reported drug overdose deaths in the United States declined by 4.2% from December 2017 to December 2018, the Centers for Disease Control and Prevention reported on July 17.
“The latest provisional data on overdose deaths show that America’s united efforts to curb opioid use disorder and addiction are working. Lives are being saved, and we’re beginning to win the fight against this crisis,” Health & Human Services Secretary Alex Azar said in a written statement. “Under President Trump’s leadership, and thanks to efforts on the ground by communities across America, the number of patients receiving medication-assisted treatment has risen, distribution of overdose-reversing drugs is up, and nationwide opioid prescriptions are down.”
The new data show that total drug overdose deaths were down from 70,699 in 2017 to 67,744 in 2018, a drop of 4.2%, the CDC said.
States, of course, fell on both sides of that national figure. Delaware and Missouri wound up on the other end of the scale with increases of 17.0% from 2017 to 2018. Deaths in Vermont, Arizona, and South Carolina also rose by double digits, data from the National Vital Statistics System show.
“While the declining trend of overdose deaths is an encouraging sign, by no means have we declared victory against the epidemic or addiction in general,” Secretary Azar said. “This crisis developed over 2 decades and it will not be solved overnight. We also face other emerging threats, like concerning trends in cocaine and methamphetamine overdoses.”
Reported drug overdose deaths in the United States declined by 4.2% from December 2017 to December 2018, the Centers for Disease Control and Prevention reported on July 17.

“The latest provisional data on overdose deaths show that America’s united efforts to curb opioid use disorder and addiction are working. Lives are being saved, and we’re beginning to win the fight against this crisis,” Health & Human Services Secretary Alex Azar said in a written statement. “Under President Trump’s leadership, and thanks to efforts on the ground by communities across America, the number of patients receiving medication-assisted treatment has risen, distribution of overdose-reversing drugs is up, and nationwide opioid prescriptions are down.”
The new data show that total drug overdose deaths were down from 70,699 in 2017 to 67,744 in 2018, a drop of 4.2%, the CDC said.
States, of course, fell on both sides of that national figure. Delaware and Missouri wound up on the other end of the scale with increases of 17.0% from 2017 to 2018. Deaths in Vermont, Arizona, and South Carolina also rose by double digits, data from the National Vital Statistics System show.
“While the declining trend of overdose deaths is an encouraging sign, by no means have we declared victory against the epidemic or addiction in general,” Secretary Azar said. “This crisis developed over 2 decades and it will not be solved overnight. We also face other emerging threats, like concerning trends in cocaine and methamphetamine overdoses.”
Reported drug overdose deaths in the United States declined by 4.2% from December 2017 to December 2018, the Centers for Disease Control and Prevention reported on July 17.

“The latest provisional data on overdose deaths show that America’s united efforts to curb opioid use disorder and addiction are working. Lives are being saved, and we’re beginning to win the fight against this crisis,” Health & Human Services Secretary Alex Azar said in a written statement. “Under President Trump’s leadership, and thanks to efforts on the ground by communities across America, the number of patients receiving medication-assisted treatment has risen, distribution of overdose-reversing drugs is up, and nationwide opioid prescriptions are down.”
The new data show that total drug overdose deaths were down from 70,699 in 2017 to 67,744 in 2018, a drop of 4.2%, the CDC said.
States, of course, fell on both sides of that national figure. Delaware and Missouri wound up on the other end of the scale with increases of 17.0% from 2017 to 2018. Deaths in Vermont, Arizona, and South Carolina also rose by double digits, data from the National Vital Statistics System show.
“While the declining trend of overdose deaths is an encouraging sign, by no means have we declared victory against the epidemic or addiction in general,” Secretary Azar said. “This crisis developed over 2 decades and it will not be solved overnight. We also face other emerging threats, like concerning trends in cocaine and methamphetamine overdoses.”
Weaponized ticks, pothead parents, and spider smoothies
Spider smoothie, anyone?
Eating spiders, grasshoppers, and cicadas – does that idea BUG you? Plenty of people around the world chomp down on insects as part of a balanced diet. But here in the United States, there’s definitely still an “ick” factor – as in, “ick, that thing has far too many legs to put in my mouth.”
These creepy crawlers, however, could be the key to a healthier lifestyle. Scientists have been recently looking at insects as a source of protein and antioxidants, among other health buzzwords.
Researchers from the University of Teremo in Italy took a look at commercially available insects (ever heard that phrase before?) and found that certain bugs, such as crickets, silkworms, caterpillars, and cicadas, displayed two or three times as much antioxidant activity as orange juice and olive oil. Perhaps you’d like a nice glass of cicada juice to go with your morning cereal?
Barclays also recently predicted that the insect protein market could be worth as much as $8 billion in the next decade, because of several factors. The earth’s population is rising, and people need new sources of food. We know bugs are plentiful – just go outside in the summer and stand with your mouth open, and you’ve got a three-course meal in 5 minutes.
We can also thank the teens: Barclays stated that Gen Z is the “most health-aware and environmentally conscious” generation yet, and therefore are all aboard the bug-eating train.
So, are you ready to start making eggs with a side of fried crickets? Perhaps a nice plant-based burger topped with cicada crumbles? Just make sure to stay away from the zombie ones.
Mom, put the joint down!
Way to harsh my mellow. You might think that smoking a little Mary Jane would chill parents out a bit. Turns out, that’s not the case.
A study from Ohio State University examined California parents, their substance use, and their disciplinarian styles. This is one of the first studies to look at how substance use relates to parenting, and the news is not good for the kids.
If you’re thinking about getting your parents into pot, think again. Parents who used marijuana in the past year tended to discipline their children more than parents who hadn’t used. Researchers also found similar trends with parents who used alcohol in the past.
Kids, if you’ve got a no-smoking, no-drinking square of a parent, be happy. It might save you a few groundings.
The true power of the dark side
When people go the grocery store, the produce department is – for many people – a big waste of space. It’s something to be skipped over. Who wants to buy fruits or vegetables anyway? Sure, they’re healthy, but they’re totally gross.
Turns out, there may be a way to get even your stubborn Uncle Joe, who probably thinks the food pyramid is a communist plot, to buy veggies. The key is a little black dress.
Okay, we’re not literally talking about sticking pineapples or broccoli in actual black dresses. But according to a study published in Food Quality and Preference, placing various types of produce against a black background made them more attractive to consumers over white or shades of gray. Even the lowly carrot, rated least attractive by the study participants when placed in front of every other color, got a big boost when placed on a black background. Something about black just brought out its natural shine.
So, next time you go through the produce department and it looks like it’s been designed by a teenager going through a particularly rough Goth phase, now you’ll know why.
The Lyme disease truth is out there
The setting is Rocky Mountain Laboratories in Hamilton, Mont. A man and a woman, both wearing dark suits, are standing in a large, poorly lit room full of file cabinets. Each is holding a flashlight.
Sculder: Explain to me again, Mully, why we couldn’t just ask to see these files?
Mully: C’mon, Sculder. My very secret and very reliable source said that Dr. Willy Burgdorfer, the scientist who discovered Lyme disease and worked in this lab, was actually a bioweapons specialist. My source said that Burgdorfer and “other bioweapons specialists stuffed ticks with pathogens to cause severe disability, disease – even death – to potential enemies.” If we had asked to see the records, they would have been destroyed by operatives of the shadow government.
Sculder: Where did you find this secret and reliable source, Mully?
Mully: I read his press release.
Sculder: Did the press release say anything about releases of diseased ticks, either accidental or by design?
Mully: Not until I ran it past a Navajo code talker.
Sculder stares at him blankly for several seconds.
Mully: Okay, okay. There’s this congressman, Rep. Chris Smith from New Jersey. The House of Representatives just approved his amendment to the 2020 National Defense Authorization Act. The amendment “directs the Inspector General of the Department of Defense to investigate the possible involvement of DOD biowarfare labs in the weaponization of Lyme disease in ticks and other insects from 1950 to 1975.” Honestly, it does.
Sculder turns around and quickly walks away. Mully follows her.
Mully: It could’ve happened! What if it’s another in the long line of government conspiracies that were too crazy to be true? Like Roswell. Or birtherism. Or New Coke. I suppose you’re going to tell me that Elvis and J. Edgar Hoover didn’t help NASA fake the moon landings?

Spider smoothie, anyone?
Eating spiders, grasshoppers, and cicadas – does that idea BUG you? Plenty of people around the world chomp down on insects as part of a balanced diet. But here in the United States, there’s definitely still an “ick” factor – as in, “ick, that thing has far too many legs to put in my mouth.”
These creepy crawlers, however, could be the key to a healthier lifestyle. Scientists have been recently looking at insects as a source of protein and antioxidants, among other health buzzwords.
Researchers from the University of Teremo in Italy took a look at commercially available insects (ever heard that phrase before?) and found that certain bugs, such as crickets, silkworms, caterpillars, and cicadas, displayed two or three times as much antioxidant activity as orange juice and olive oil. Perhaps you’d like a nice glass of cicada juice to go with your morning cereal?
Barclays also recently predicted that the insect protein market could be worth as much as $8 billion in the next decade, because of several factors. The earth’s population is rising, and people need new sources of food. We know bugs are plentiful – just go outside in the summer and stand with your mouth open, and you’ve got a three-course meal in 5 minutes.
We can also thank the teens: Barclays stated that Gen Z is the “most health-aware and environmentally conscious” generation yet, and therefore are all aboard the bug-eating train.
So, are you ready to start making eggs with a side of fried crickets? Perhaps a nice plant-based burger topped with cicada crumbles? Just make sure to stay away from the zombie ones.
Mom, put the joint down!
Way to harsh my mellow. You might think that smoking a little Mary Jane would chill parents out a bit. Turns out, that’s not the case.
A study from Ohio State University examined California parents, their substance use, and their disciplinarian styles. This is one of the first studies to look at how substance use relates to parenting, and the news is not good for the kids.
If you’re thinking about getting your parents into pot, think again. Parents who used marijuana in the past year tended to discipline their children more than parents who hadn’t used. Researchers also found similar trends with parents who used alcohol in the past.
Kids, if you’ve got a no-smoking, no-drinking square of a parent, be happy. It might save you a few groundings.
The true power of the dark side
When people go the grocery store, the produce department is – for many people – a big waste of space. It’s something to be skipped over. Who wants to buy fruits or vegetables anyway? Sure, they’re healthy, but they’re totally gross.
Turns out, there may be a way to get even your stubborn Uncle Joe, who probably thinks the food pyramid is a communist plot, to buy veggies. The key is a little black dress.
Okay, we’re not literally talking about sticking pineapples or broccoli in actual black dresses. But according to a study published in Food Quality and Preference, placing various types of produce against a black background made them more attractive to consumers over white or shades of gray. Even the lowly carrot, rated least attractive by the study participants when placed in front of every other color, got a big boost when placed on a black background. Something about black just brought out its natural shine.
So, next time you go through the produce department and it looks like it’s been designed by a teenager going through a particularly rough Goth phase, now you’ll know why.
The Lyme disease truth is out there
The setting is Rocky Mountain Laboratories in Hamilton, Mont. A man and a woman, both wearing dark suits, are standing in a large, poorly lit room full of file cabinets. Each is holding a flashlight.
Sculder: Explain to me again, Mully, why we couldn’t just ask to see these files?
Mully: C’mon, Sculder. My very secret and very reliable source said that Dr. Willy Burgdorfer, the scientist who discovered Lyme disease and worked in this lab, was actually a bioweapons specialist. My source said that Burgdorfer and “other bioweapons specialists stuffed ticks with pathogens to cause severe disability, disease – even death – to potential enemies.” If we had asked to see the records, they would have been destroyed by operatives of the shadow government.
Sculder: Where did you find this secret and reliable source, Mully?
Mully: I read his press release.
Sculder: Did the press release say anything about releases of diseased ticks, either accidental or by design?
Mully: Not until I ran it past a Navajo code talker.
Sculder stares at him blankly for several seconds.
Mully: Okay, okay. There’s this congressman, Rep. Chris Smith from New Jersey. The House of Representatives just approved his amendment to the 2020 National Defense Authorization Act. The amendment “directs the Inspector General of the Department of Defense to investigate the possible involvement of DOD biowarfare labs in the weaponization of Lyme disease in ticks and other insects from 1950 to 1975.” Honestly, it does.
Sculder turns around and quickly walks away. Mully follows her.
Mully: It could’ve happened! What if it’s another in the long line of government conspiracies that were too crazy to be true? Like Roswell. Or birtherism. Or New Coke. I suppose you’re going to tell me that Elvis and J. Edgar Hoover didn’t help NASA fake the moon landings?

Spider smoothie, anyone?
Eating spiders, grasshoppers, and cicadas – does that idea BUG you? Plenty of people around the world chomp down on insects as part of a balanced diet. But here in the United States, there’s definitely still an “ick” factor – as in, “ick, that thing has far too many legs to put in my mouth.”
These creepy crawlers, however, could be the key to a healthier lifestyle. Scientists have been recently looking at insects as a source of protein and antioxidants, among other health buzzwords.
Researchers from the University of Teremo in Italy took a look at commercially available insects (ever heard that phrase before?) and found that certain bugs, such as crickets, silkworms, caterpillars, and cicadas, displayed two or three times as much antioxidant activity as orange juice and olive oil. Perhaps you’d like a nice glass of cicada juice to go with your morning cereal?
Barclays also recently predicted that the insect protein market could be worth as much as $8 billion in the next decade, because of several factors. The earth’s population is rising, and people need new sources of food. We know bugs are plentiful – just go outside in the summer and stand with your mouth open, and you’ve got a three-course meal in 5 minutes.
We can also thank the teens: Barclays stated that Gen Z is the “most health-aware and environmentally conscious” generation yet, and therefore are all aboard the bug-eating train.
So, are you ready to start making eggs with a side of fried crickets? Perhaps a nice plant-based burger topped with cicada crumbles? Just make sure to stay away from the zombie ones.
Mom, put the joint down!
Way to harsh my mellow. You might think that smoking a little Mary Jane would chill parents out a bit. Turns out, that’s not the case.
A study from Ohio State University examined California parents, their substance use, and their disciplinarian styles. This is one of the first studies to look at how substance use relates to parenting, and the news is not good for the kids.
If you’re thinking about getting your parents into pot, think again. Parents who used marijuana in the past year tended to discipline their children more than parents who hadn’t used. Researchers also found similar trends with parents who used alcohol in the past.
Kids, if you’ve got a no-smoking, no-drinking square of a parent, be happy. It might save you a few groundings.
The true power of the dark side
When people go the grocery store, the produce department is – for many people – a big waste of space. It’s something to be skipped over. Who wants to buy fruits or vegetables anyway? Sure, they’re healthy, but they’re totally gross.
Turns out, there may be a way to get even your stubborn Uncle Joe, who probably thinks the food pyramid is a communist plot, to buy veggies. The key is a little black dress.
Okay, we’re not literally talking about sticking pineapples or broccoli in actual black dresses. But according to a study published in Food Quality and Preference, placing various types of produce against a black background made them more attractive to consumers over white or shades of gray. Even the lowly carrot, rated least attractive by the study participants when placed in front of every other color, got a big boost when placed on a black background. Something about black just brought out its natural shine.
So, next time you go through the produce department and it looks like it’s been designed by a teenager going through a particularly rough Goth phase, now you’ll know why.
The Lyme disease truth is out there
The setting is Rocky Mountain Laboratories in Hamilton, Mont. A man and a woman, both wearing dark suits, are standing in a large, poorly lit room full of file cabinets. Each is holding a flashlight.
Sculder: Explain to me again, Mully, why we couldn’t just ask to see these files?
Mully: C’mon, Sculder. My very secret and very reliable source said that Dr. Willy Burgdorfer, the scientist who discovered Lyme disease and worked in this lab, was actually a bioweapons specialist. My source said that Burgdorfer and “other bioweapons specialists stuffed ticks with pathogens to cause severe disability, disease – even death – to potential enemies.” If we had asked to see the records, they would have been destroyed by operatives of the shadow government.
Sculder: Where did you find this secret and reliable source, Mully?
Mully: I read his press release.
Sculder: Did the press release say anything about releases of diseased ticks, either accidental or by design?
Mully: Not until I ran it past a Navajo code talker.
Sculder stares at him blankly for several seconds.
Mully: Okay, okay. There’s this congressman, Rep. Chris Smith from New Jersey. The House of Representatives just approved his amendment to the 2020 National Defense Authorization Act. The amendment “directs the Inspector General of the Department of Defense to investigate the possible involvement of DOD biowarfare labs in the weaponization of Lyme disease in ticks and other insects from 1950 to 1975.” Honestly, it does.
Sculder turns around and quickly walks away. Mully follows her.
Mully: It could’ve happened! What if it’s another in the long line of government conspiracies that were too crazy to be true? Like Roswell. Or birtherism. Or New Coke. I suppose you’re going to tell me that Elvis and J. Edgar Hoover didn’t help NASA fake the moon landings?

New scale could measure vaccine hesitancy in developing countries
By measuring parents’ attitudes regarding disease salience and community benefit, high scores on a four-item scale was associated with fivefold greater likelihood of not fully vaccinating their children, according to a study published in the Pediatric Infectious Disease Journal.
Mohammad Tahir Yousafzai, MPH, of Aga Khan University, Karachi, Pakistan, and colleagues developed a larger 14-item scale to measure parental attitudes and surveyed 901 households in the Sindh province of Pakistan during 2014. Part of this scale was a short 4-item subscale focusing on disease salience and community benefit, whereas the remaining 10 items form another subscale that measures parents’ perceptions and concerns regarding vaccines directly. The items are presented as 1-5 Likert scales, and scoring higher represents holding more negative attitudes regarding vaccines.
Of the 901 households surveyed, 25% of children were fully vaccinated, which meant children received all primary vaccines up to 14 weeks of age, and 54% were partially vaccinated, which meant at least one of those primary vaccines had been missed. The remaining 21% were unvaccinated.
High scores on the full 14-item scale showed some correlation with no vaccination versus partial or full vaccination (odds ratio, 3.05; 95% confidence interval, 1.75-5.31); the association disappeared after adjustment for children’s age and gender. The subscales performed better after adjustment: The adjusted ORs were 1.52 (95% CI, 1.05-2.21) for the longer subscale and 5.21 (95% CI, 3.60-7.55) for the shorter subscale. The data also showed high association between high scores on the shorter subscale and the likelihood of no or only partial vaccination (aOR, 9.65; 95% CI, 4.81-19.37).
The researchers noted that most similar scales used in developed countries are longer (10 or more items) and have lower internal consistency. This four-item scale, on the other hand, may be especially useful among lower-income populations and those in developing areas that have lower literacy rates.
One of the limitations of the study was that it was conducted only in Pakistan and not multiple developing countries; the researchers acknowledged this could limit generalizability. Another limitation is that the study size was too small for subdomain analysis; the researchers wrote that, although a lack of such analysis is unfortunate, it shouldn’t hamper the shorter subscale’s usability. A further limitation is that there was little variability across Likert scores – mostly answers at the extreme ends rather than a mix. This suggests that interviewees may not have understood how Likert scales work and therefore may not have answered accurately, noted the researchers, who employed a visual chart, trained interviewers, and field monitoring to mitigate this possibility.
“Measurement of the parental attitudes toward childhood vaccination is very important for the appropriate planning of strategies for increasing vaccine coverage and for monitoring,” they wrote.
The study was sponsored by Gavi, The Vaccine Alliance. The authors had no funding or conflicts of interest to disclose.
SOURCE: Yousafzai MT et al. Pediatr Infect Dis J. 2019 Jul;38(7):e143-8.
By measuring parents’ attitudes regarding disease salience and community benefit, high scores on a four-item scale was associated with fivefold greater likelihood of not fully vaccinating their children, according to a study published in the Pediatric Infectious Disease Journal.
Mohammad Tahir Yousafzai, MPH, of Aga Khan University, Karachi, Pakistan, and colleagues developed a larger 14-item scale to measure parental attitudes and surveyed 901 households in the Sindh province of Pakistan during 2014. Part of this scale was a short 4-item subscale focusing on disease salience and community benefit, whereas the remaining 10 items form another subscale that measures parents’ perceptions and concerns regarding vaccines directly. The items are presented as 1-5 Likert scales, and scoring higher represents holding more negative attitudes regarding vaccines.
Of the 901 households surveyed, 25% of children were fully vaccinated, which meant children received all primary vaccines up to 14 weeks of age, and 54% were partially vaccinated, which meant at least one of those primary vaccines had been missed. The remaining 21% were unvaccinated.
High scores on the full 14-item scale showed some correlation with no vaccination versus partial or full vaccination (odds ratio, 3.05; 95% confidence interval, 1.75-5.31); the association disappeared after adjustment for children’s age and gender. The subscales performed better after adjustment: The adjusted ORs were 1.52 (95% CI, 1.05-2.21) for the longer subscale and 5.21 (95% CI, 3.60-7.55) for the shorter subscale. The data also showed high association between high scores on the shorter subscale and the likelihood of no or only partial vaccination (aOR, 9.65; 95% CI, 4.81-19.37).
The researchers noted that most similar scales used in developed countries are longer (10 or more items) and have lower internal consistency. This four-item scale, on the other hand, may be especially useful among lower-income populations and those in developing areas that have lower literacy rates.
One of the limitations of the study was that it was conducted only in Pakistan and not multiple developing countries; the researchers acknowledged this could limit generalizability. Another limitation is that the study size was too small for subdomain analysis; the researchers wrote that, although a lack of such analysis is unfortunate, it shouldn’t hamper the shorter subscale’s usability. A further limitation is that there was little variability across Likert scores – mostly answers at the extreme ends rather than a mix. This suggests that interviewees may not have understood how Likert scales work and therefore may not have answered accurately, noted the researchers, who employed a visual chart, trained interviewers, and field monitoring to mitigate this possibility.
“Measurement of the parental attitudes toward childhood vaccination is very important for the appropriate planning of strategies for increasing vaccine coverage and for monitoring,” they wrote.
The study was sponsored by Gavi, The Vaccine Alliance. The authors had no funding or conflicts of interest to disclose.
SOURCE: Yousafzai MT et al. Pediatr Infect Dis J. 2019 Jul;38(7):e143-8.
By measuring parents’ attitudes regarding disease salience and community benefit, high scores on a four-item scale was associated with fivefold greater likelihood of not fully vaccinating their children, according to a study published in the Pediatric Infectious Disease Journal.
Mohammad Tahir Yousafzai, MPH, of Aga Khan University, Karachi, Pakistan, and colleagues developed a larger 14-item scale to measure parental attitudes and surveyed 901 households in the Sindh province of Pakistan during 2014. Part of this scale was a short 4-item subscale focusing on disease salience and community benefit, whereas the remaining 10 items form another subscale that measures parents’ perceptions and concerns regarding vaccines directly. The items are presented as 1-5 Likert scales, and scoring higher represents holding more negative attitudes regarding vaccines.
Of the 901 households surveyed, 25% of children were fully vaccinated, which meant children received all primary vaccines up to 14 weeks of age, and 54% were partially vaccinated, which meant at least one of those primary vaccines had been missed. The remaining 21% were unvaccinated.
High scores on the full 14-item scale showed some correlation with no vaccination versus partial or full vaccination (odds ratio, 3.05; 95% confidence interval, 1.75-5.31); the association disappeared after adjustment for children’s age and gender. The subscales performed better after adjustment: The adjusted ORs were 1.52 (95% CI, 1.05-2.21) for the longer subscale and 5.21 (95% CI, 3.60-7.55) for the shorter subscale. The data also showed high association between high scores on the shorter subscale and the likelihood of no or only partial vaccination (aOR, 9.65; 95% CI, 4.81-19.37).
The researchers noted that most similar scales used in developed countries are longer (10 or more items) and have lower internal consistency. This four-item scale, on the other hand, may be especially useful among lower-income populations and those in developing areas that have lower literacy rates.
One of the limitations of the study was that it was conducted only in Pakistan and not multiple developing countries; the researchers acknowledged this could limit generalizability. Another limitation is that the study size was too small for subdomain analysis; the researchers wrote that, although a lack of such analysis is unfortunate, it shouldn’t hamper the shorter subscale’s usability. A further limitation is that there was little variability across Likert scores – mostly answers at the extreme ends rather than a mix. This suggests that interviewees may not have understood how Likert scales work and therefore may not have answered accurately, noted the researchers, who employed a visual chart, trained interviewers, and field monitoring to mitigate this possibility.
“Measurement of the parental attitudes toward childhood vaccination is very important for the appropriate planning of strategies for increasing vaccine coverage and for monitoring,” they wrote.
The study was sponsored by Gavi, The Vaccine Alliance. The authors had no funding or conflicts of interest to disclose.
SOURCE: Yousafzai MT et al. Pediatr Infect Dis J. 2019 Jul;38(7):e143-8.
FROM THE PEDIATRIC INFECTIOUS DISEASE JOURNAL
New analysis challenges fluid resuscitation guidelines for patients in shock
Although guideline recommended, according to a detailed analysis of available data, including a randomized trial.
Several sets of guidelines for resuscitation of patients in shock have advocated volume expansion with bolus intravenous fluid, but that recommendation was based on expected physiologic benefits not a randomized trial. The only randomized trial associated this approach showed increased mortality, and a new analysis of these and other data appears to explain why.
According to the findings of a study lead by Michael Levin, MD, of the department of medicine at Imperial College London and colleagues, “volume resuscitation is associated with deterioration of respiratory function and neurological function in some patients.” Their study was published in Lancet Respiratory Medicine. The authors stated that saline-induced hyperchloremic acidosis appears to have been “a major contributor” to the observed increase in adverse outcomes.
The key take home message is that “normal saline and other unbuffered crystalloid solutions should be avoided in resuscitating seriously ill patients,” according to the authors, who believe the findings might be relevant to adults as well as children.
The controversy about the role of volume expansion for management of shock was ignited by a 2011 trial called FEAST (N Engl J Med. 2011;364:2483-95). That trial, which randomized African children with severe febrile illness to a bolus of 20-40 mg of 5% albumin solution, a bolus of 0.9% saline solution, or no bolus, was halted early when 48-hour mortality data showed a lower death rate in the no bolus group (7.3%) than either the albumin (10.6%) or saline (10.5%) bolus groups.
The FEAST result was unexpected and so contrary to accepted thinking that it prompted widespread debate, including whether findings in the resource-poor area of the world where the FEAST trial was conducted could be extrapolated to centers elsewhere in the world. Arguing for benefit, fluid resuscitation is known to increase pulse pressure and urinary output. Arguing against benefit, pulmonary edema is a known complication of bolus fluid replacement.
In an attempt to address and potentially resolve this controversy, data collected in the FEAST trial along with four other sets of data involving volume expansion in critically ill children were evaluated with a focus on changes in cardiovascular, neurological, and respiratory function. Analysis of blood biochemistry and blood oxygen transport were also conducted.
The cardiovascular, respiratory, and neurologic functions were scored on the basis of objective measurements, such as heart rate, respiratory rate, and blood pressure. These measures were evaluated prior to fluid administration and at 1 hour, 4 hours, 8 hours, 24 hours, and 48 hours after fluid administration. Odds ratio (OR) of an adverse outcome were evaluated in the context of each 10-unit change in these scores.
Relative to baseline, there was worsening respiratory and neurological function after fluid administration. Although cardiovascular function improved, hemoglobin concentrations were lower in those who received fluid than in those who did not. Fluid resuscitation was also associated with lower bicarbonate and increased base deficit and chloride at 24 hours.
Regression modeling with physiological variables suggests “that the increased mortality in FEAST can be explained by bolus-induced worsening in respiratory and neurological function, hemodilution, and hyperchloremic acidosis,” according to the authors.
Analyses of the four other sets of data, which included children treated for meningococcal sepsis in the United Kingdom, acutely ill with malaria treated in Malawi, and cohorts of children in South Africa and a London hospital for acute illnesses, provided supportive data.
Although this analysis does not address the value of administering buffered solutions in low volumes, the authors concluded that the data from the FEAST trial are generalizable. They challenge the routine use of bolus infusions of saline or albumin in the initial management of shock, which has been guideline recommended. The risks of fluid resuscitation might be particularly high among children who already have compromised respiratory or neurologic function.
SOURCE: Levin M et al. Lancet Respir Med. 2019;7:581-93.
Although guideline recommended, according to a detailed analysis of available data, including a randomized trial.
Several sets of guidelines for resuscitation of patients in shock have advocated volume expansion with bolus intravenous fluid, but that recommendation was based on expected physiologic benefits not a randomized trial. The only randomized trial associated this approach showed increased mortality, and a new analysis of these and other data appears to explain why.
According to the findings of a study lead by Michael Levin, MD, of the department of medicine at Imperial College London and colleagues, “volume resuscitation is associated with deterioration of respiratory function and neurological function in some patients.” Their study was published in Lancet Respiratory Medicine. The authors stated that saline-induced hyperchloremic acidosis appears to have been “a major contributor” to the observed increase in adverse outcomes.
The key take home message is that “normal saline and other unbuffered crystalloid solutions should be avoided in resuscitating seriously ill patients,” according to the authors, who believe the findings might be relevant to adults as well as children.
The controversy about the role of volume expansion for management of shock was ignited by a 2011 trial called FEAST (N Engl J Med. 2011;364:2483-95). That trial, which randomized African children with severe febrile illness to a bolus of 20-40 mg of 5% albumin solution, a bolus of 0.9% saline solution, or no bolus, was halted early when 48-hour mortality data showed a lower death rate in the no bolus group (7.3%) than either the albumin (10.6%) or saline (10.5%) bolus groups.
The FEAST result was unexpected and so contrary to accepted thinking that it prompted widespread debate, including whether findings in the resource-poor area of the world where the FEAST trial was conducted could be extrapolated to centers elsewhere in the world. Arguing for benefit, fluid resuscitation is known to increase pulse pressure and urinary output. Arguing against benefit, pulmonary edema is a known complication of bolus fluid replacement.
In an attempt to address and potentially resolve this controversy, data collected in the FEAST trial along with four other sets of data involving volume expansion in critically ill children were evaluated with a focus on changes in cardiovascular, neurological, and respiratory function. Analysis of blood biochemistry and blood oxygen transport were also conducted.
The cardiovascular, respiratory, and neurologic functions were scored on the basis of objective measurements, such as heart rate, respiratory rate, and blood pressure. These measures were evaluated prior to fluid administration and at 1 hour, 4 hours, 8 hours, 24 hours, and 48 hours after fluid administration. Odds ratio (OR) of an adverse outcome were evaluated in the context of each 10-unit change in these scores.
Relative to baseline, there was worsening respiratory and neurological function after fluid administration. Although cardiovascular function improved, hemoglobin concentrations were lower in those who received fluid than in those who did not. Fluid resuscitation was also associated with lower bicarbonate and increased base deficit and chloride at 24 hours.
Regression modeling with physiological variables suggests “that the increased mortality in FEAST can be explained by bolus-induced worsening in respiratory and neurological function, hemodilution, and hyperchloremic acidosis,” according to the authors.
Analyses of the four other sets of data, which included children treated for meningococcal sepsis in the United Kingdom, acutely ill with malaria treated in Malawi, and cohorts of children in South Africa and a London hospital for acute illnesses, provided supportive data.
Although this analysis does not address the value of administering buffered solutions in low volumes, the authors concluded that the data from the FEAST trial are generalizable. They challenge the routine use of bolus infusions of saline or albumin in the initial management of shock, which has been guideline recommended. The risks of fluid resuscitation might be particularly high among children who already have compromised respiratory or neurologic function.
SOURCE: Levin M et al. Lancet Respir Med. 2019;7:581-93.
Although guideline recommended, according to a detailed analysis of available data, including a randomized trial.
Several sets of guidelines for resuscitation of patients in shock have advocated volume expansion with bolus intravenous fluid, but that recommendation was based on expected physiologic benefits not a randomized trial. The only randomized trial associated this approach showed increased mortality, and a new analysis of these and other data appears to explain why.
According to the findings of a study lead by Michael Levin, MD, of the department of medicine at Imperial College London and colleagues, “volume resuscitation is associated with deterioration of respiratory function and neurological function in some patients.” Their study was published in Lancet Respiratory Medicine. The authors stated that saline-induced hyperchloremic acidosis appears to have been “a major contributor” to the observed increase in adverse outcomes.
The key take home message is that “normal saline and other unbuffered crystalloid solutions should be avoided in resuscitating seriously ill patients,” according to the authors, who believe the findings might be relevant to adults as well as children.
The controversy about the role of volume expansion for management of shock was ignited by a 2011 trial called FEAST (N Engl J Med. 2011;364:2483-95). That trial, which randomized African children with severe febrile illness to a bolus of 20-40 mg of 5% albumin solution, a bolus of 0.9% saline solution, or no bolus, was halted early when 48-hour mortality data showed a lower death rate in the no bolus group (7.3%) than either the albumin (10.6%) or saline (10.5%) bolus groups.
The FEAST result was unexpected and so contrary to accepted thinking that it prompted widespread debate, including whether findings in the resource-poor area of the world where the FEAST trial was conducted could be extrapolated to centers elsewhere in the world. Arguing for benefit, fluid resuscitation is known to increase pulse pressure and urinary output. Arguing against benefit, pulmonary edema is a known complication of bolus fluid replacement.
In an attempt to address and potentially resolve this controversy, data collected in the FEAST trial along with four other sets of data involving volume expansion in critically ill children were evaluated with a focus on changes in cardiovascular, neurological, and respiratory function. Analysis of blood biochemistry and blood oxygen transport were also conducted.
The cardiovascular, respiratory, and neurologic functions were scored on the basis of objective measurements, such as heart rate, respiratory rate, and blood pressure. These measures were evaluated prior to fluid administration and at 1 hour, 4 hours, 8 hours, 24 hours, and 48 hours after fluid administration. Odds ratio (OR) of an adverse outcome were evaluated in the context of each 10-unit change in these scores.
Relative to baseline, there was worsening respiratory and neurological function after fluid administration. Although cardiovascular function improved, hemoglobin concentrations were lower in those who received fluid than in those who did not. Fluid resuscitation was also associated with lower bicarbonate and increased base deficit and chloride at 24 hours.
Regression modeling with physiological variables suggests “that the increased mortality in FEAST can be explained by bolus-induced worsening in respiratory and neurological function, hemodilution, and hyperchloremic acidosis,” according to the authors.
Analyses of the four other sets of data, which included children treated for meningococcal sepsis in the United Kingdom, acutely ill with malaria treated in Malawi, and cohorts of children in South Africa and a London hospital for acute illnesses, provided supportive data.
Although this analysis does not address the value of administering buffered solutions in low volumes, the authors concluded that the data from the FEAST trial are generalizable. They challenge the routine use of bolus infusions of saline or albumin in the initial management of shock, which has been guideline recommended. The risks of fluid resuscitation might be particularly high among children who already have compromised respiratory or neurologic function.
SOURCE: Levin M et al. Lancet Respir Med. 2019;7:581-93.
FROM THE LANCET RESPIRATORY MEDICINE
Noninvasive EEG may speed diagnosis of West syndrome
BANGKOK – , Hiroki Nariai, MD, reported at the International Epilepsy Congress.
Among the most promising of these potential EEG biomarkers for development of epilepsy are ictal or interictal high-frequency oscillations (HFOs) at 80 Hz or more, along with cross-frequency coupling of HFOs and delta wave activity, according to Dr. Nariai, a pediatric neurologist at the University of California, Los Angeles.
West syndrome, the most common epileptic encephalopathy during the first 2 years of life, has diverse etiologies. For example, in 250 infants with West syndrome enrolled in the United Kingdom National Infantile Spasms Consortium, a cause was identified in 64%. The etiology was genetic in 14% of subjects, a structural-congenital anomaly in 11%, tuberous sclerosis – a genetic-structural abnormality – in 10%, stroke in 22%, a metabolic defect in 5%, and infection in 2% (Epilepsia. 2015 Apr;56[4]:617-25).
West syndrome is rare, with an estimated prevalence of roughly 1 per 6,000 live births, but the associated mortality is high: 31% on average. And West syndrome often brings severe neurodevelopmental morbidity, with normal or near-normal intelligence present in only 25% of survivors.
An intensive search is on for an objective, reliable diagnostic biomarker – be it electroencephalographic, biochemical, or perhaps a neuroimaging finding – because the clinical diagnosis of West syndrome is highly subjective. It relies upon the triad of epileptic spasms, developmental regression or psychomotor delay, and hypsarrhythmia, which is a chaotic, disorganized, patternless form of brain electrical activity. And while that description makes hypsarrhythmia sound as if it should be easily recognizable, in fact that’s often not the case: Interrater reliability was poor in a study of six pediatric EEG experts at four centers who viewed 5-minute-long EEG samples obtained from 22 patients with infantile spasms (Epilepsia. 2015 Jan;56[1]:77-81), Dr. Nariai noted at the congress, sponsored by the International League Against Epilepsy.
“The clinical trial is maybe not so useful,” he observed.
The hunt for a reliable biomarker is further fueled by evidence that early diagnosis and treatment of West syndrome and other etiologies of infantile spasms makes a real difference. Indeed, investigators found in the United Kingdom Infantile Spasms Study that increasing lag time from onset of spasms to initiation of treatment was associated in stepwise fashion with significantly lower IQ at 4 years of age. While infants who started treatment within 7 days of onset of the seizure disorder had a mean IQ of 76.2 at age 4 years, those with an 8- to 14-day lag time between symptom onset and treatment averaged an additional 3.9-point decrement in IQ. A 15- to 30-day delay was associated with a 7.8-point reduction in IQ, compared with the reference group, while the decrease in IQ averaged 11.7 points in infants with a 1- to 2-month lag time and 15.6 points in those with a lag time of more than 2 months (Epilepsia. 2011 Jul;52[7]:1359-64).
At the start of the decade, Dr. Nariai and other investigators demonstrated that pathologic HFOs recorded during invasive EEG monitoring in conjunction with epilepsy surgery served as a reliable biomarker of epilepsy. While this was an important observation, a biomarker obtained through invasive monitoring during brain surgery clearly has very limited clinical applicability. But more recently, Dr. Nariai and his coinvestigators in the Tuberous Sclerosis Complex Autism Center of Excellence Network reported that noninvasive detection of interictal HFO fast ripples in the 250-500 Hz range via scalp EEG showed promise as a biomarker of epilepsy. Sensitivity of this far more practical approach to the detection of fast ripples was excellent, whether analyzed visually or by automatic detector (Clin Neurophysiol. 2018 Jul;129[7]:1458-66).
Moreover, in a recent, not-yet published study that Dr. Nariai and coworkers conducted in 24 infants with active epileptic spasms and 6 controls, noninvasive objective measurement of HFO rate using scalp EEG had an 83% sensitivity and 100% specificity for active epileptic spasms, while the modulation index of HFO and delta coupling in the 3-4 Hz range showed 74% sensitivity and 86% specificity.
If future studies validate the utility of detection of HFOs above a defined threshold or another noninvasively obtained EEG biomarker for diagnosis of epilepsy, the same strategy would presumably also be applicable for monitoring response to antiepileptic therapies, thereby eliminating the traditional trial-and-error approach to treatment. This would be a particularly important application in patients with West syndrome, where it’s believed that the electrical activity itself is contributing to the progressive – and often rapid – loss of cognitive function and behavioral disturbances. Thus, unlike in most other forms of epilepsy, the treatment goal isn’t merely to suppress the seizures, but also to achieve disease modification by eliminating the underlying subclinical EEG abnormalities, he explained.
A reliable biomarker would also be a boon in selecting the best participants for clinical trials of new antiseizure therapies.
Dr. Nariai reported having no financial conflicts regarding his presentation. His work is funded by research foundations and the National Institutes of Health.
BANGKOK – , Hiroki Nariai, MD, reported at the International Epilepsy Congress.
Among the most promising of these potential EEG biomarkers for development of epilepsy are ictal or interictal high-frequency oscillations (HFOs) at 80 Hz or more, along with cross-frequency coupling of HFOs and delta wave activity, according to Dr. Nariai, a pediatric neurologist at the University of California, Los Angeles.
West syndrome, the most common epileptic encephalopathy during the first 2 years of life, has diverse etiologies. For example, in 250 infants with West syndrome enrolled in the United Kingdom National Infantile Spasms Consortium, a cause was identified in 64%. The etiology was genetic in 14% of subjects, a structural-congenital anomaly in 11%, tuberous sclerosis – a genetic-structural abnormality – in 10%, stroke in 22%, a metabolic defect in 5%, and infection in 2% (Epilepsia. 2015 Apr;56[4]:617-25).
West syndrome is rare, with an estimated prevalence of roughly 1 per 6,000 live births, but the associated mortality is high: 31% on average. And West syndrome often brings severe neurodevelopmental morbidity, with normal or near-normal intelligence present in only 25% of survivors.
An intensive search is on for an objective, reliable diagnostic biomarker – be it electroencephalographic, biochemical, or perhaps a neuroimaging finding – because the clinical diagnosis of West syndrome is highly subjective. It relies upon the triad of epileptic spasms, developmental regression or psychomotor delay, and hypsarrhythmia, which is a chaotic, disorganized, patternless form of brain electrical activity. And while that description makes hypsarrhythmia sound as if it should be easily recognizable, in fact that’s often not the case: Interrater reliability was poor in a study of six pediatric EEG experts at four centers who viewed 5-minute-long EEG samples obtained from 22 patients with infantile spasms (Epilepsia. 2015 Jan;56[1]:77-81), Dr. Nariai noted at the congress, sponsored by the International League Against Epilepsy.
“The clinical trial is maybe not so useful,” he observed.
The hunt for a reliable biomarker is further fueled by evidence that early diagnosis and treatment of West syndrome and other etiologies of infantile spasms makes a real difference. Indeed, investigators found in the United Kingdom Infantile Spasms Study that increasing lag time from onset of spasms to initiation of treatment was associated in stepwise fashion with significantly lower IQ at 4 years of age. While infants who started treatment within 7 days of onset of the seizure disorder had a mean IQ of 76.2 at age 4 years, those with an 8- to 14-day lag time between symptom onset and treatment averaged an additional 3.9-point decrement in IQ. A 15- to 30-day delay was associated with a 7.8-point reduction in IQ, compared with the reference group, while the decrease in IQ averaged 11.7 points in infants with a 1- to 2-month lag time and 15.6 points in those with a lag time of more than 2 months (Epilepsia. 2011 Jul;52[7]:1359-64).
At the start of the decade, Dr. Nariai and other investigators demonstrated that pathologic HFOs recorded during invasive EEG monitoring in conjunction with epilepsy surgery served as a reliable biomarker of epilepsy. While this was an important observation, a biomarker obtained through invasive monitoring during brain surgery clearly has very limited clinical applicability. But more recently, Dr. Nariai and his coinvestigators in the Tuberous Sclerosis Complex Autism Center of Excellence Network reported that noninvasive detection of interictal HFO fast ripples in the 250-500 Hz range via scalp EEG showed promise as a biomarker of epilepsy. Sensitivity of this far more practical approach to the detection of fast ripples was excellent, whether analyzed visually or by automatic detector (Clin Neurophysiol. 2018 Jul;129[7]:1458-66).
Moreover, in a recent, not-yet published study that Dr. Nariai and coworkers conducted in 24 infants with active epileptic spasms and 6 controls, noninvasive objective measurement of HFO rate using scalp EEG had an 83% sensitivity and 100% specificity for active epileptic spasms, while the modulation index of HFO and delta coupling in the 3-4 Hz range showed 74% sensitivity and 86% specificity.
If future studies validate the utility of detection of HFOs above a defined threshold or another noninvasively obtained EEG biomarker for diagnosis of epilepsy, the same strategy would presumably also be applicable for monitoring response to antiepileptic therapies, thereby eliminating the traditional trial-and-error approach to treatment. This would be a particularly important application in patients with West syndrome, where it’s believed that the electrical activity itself is contributing to the progressive – and often rapid – loss of cognitive function and behavioral disturbances. Thus, unlike in most other forms of epilepsy, the treatment goal isn’t merely to suppress the seizures, but also to achieve disease modification by eliminating the underlying subclinical EEG abnormalities, he explained.
A reliable biomarker would also be a boon in selecting the best participants for clinical trials of new antiseizure therapies.
Dr. Nariai reported having no financial conflicts regarding his presentation. His work is funded by research foundations and the National Institutes of Health.
BANGKOK – , Hiroki Nariai, MD, reported at the International Epilepsy Congress.
Among the most promising of these potential EEG biomarkers for development of epilepsy are ictal or interictal high-frequency oscillations (HFOs) at 80 Hz or more, along with cross-frequency coupling of HFOs and delta wave activity, according to Dr. Nariai, a pediatric neurologist at the University of California, Los Angeles.
West syndrome, the most common epileptic encephalopathy during the first 2 years of life, has diverse etiologies. For example, in 250 infants with West syndrome enrolled in the United Kingdom National Infantile Spasms Consortium, a cause was identified in 64%. The etiology was genetic in 14% of subjects, a structural-congenital anomaly in 11%, tuberous sclerosis – a genetic-structural abnormality – in 10%, stroke in 22%, a metabolic defect in 5%, and infection in 2% (Epilepsia. 2015 Apr;56[4]:617-25).
West syndrome is rare, with an estimated prevalence of roughly 1 per 6,000 live births, but the associated mortality is high: 31% on average. And West syndrome often brings severe neurodevelopmental morbidity, with normal or near-normal intelligence present in only 25% of survivors.
An intensive search is on for an objective, reliable diagnostic biomarker – be it electroencephalographic, biochemical, or perhaps a neuroimaging finding – because the clinical diagnosis of West syndrome is highly subjective. It relies upon the triad of epileptic spasms, developmental regression or psychomotor delay, and hypsarrhythmia, which is a chaotic, disorganized, patternless form of brain electrical activity. And while that description makes hypsarrhythmia sound as if it should be easily recognizable, in fact that’s often not the case: Interrater reliability was poor in a study of six pediatric EEG experts at four centers who viewed 5-minute-long EEG samples obtained from 22 patients with infantile spasms (Epilepsia. 2015 Jan;56[1]:77-81), Dr. Nariai noted at the congress, sponsored by the International League Against Epilepsy.
“The clinical trial is maybe not so useful,” he observed.
The hunt for a reliable biomarker is further fueled by evidence that early diagnosis and treatment of West syndrome and other etiologies of infantile spasms makes a real difference. Indeed, investigators found in the United Kingdom Infantile Spasms Study that increasing lag time from onset of spasms to initiation of treatment was associated in stepwise fashion with significantly lower IQ at 4 years of age. While infants who started treatment within 7 days of onset of the seizure disorder had a mean IQ of 76.2 at age 4 years, those with an 8- to 14-day lag time between symptom onset and treatment averaged an additional 3.9-point decrement in IQ. A 15- to 30-day delay was associated with a 7.8-point reduction in IQ, compared with the reference group, while the decrease in IQ averaged 11.7 points in infants with a 1- to 2-month lag time and 15.6 points in those with a lag time of more than 2 months (Epilepsia. 2011 Jul;52[7]:1359-64).
At the start of the decade, Dr. Nariai and other investigators demonstrated that pathologic HFOs recorded during invasive EEG monitoring in conjunction with epilepsy surgery served as a reliable biomarker of epilepsy. While this was an important observation, a biomarker obtained through invasive monitoring during brain surgery clearly has very limited clinical applicability. But more recently, Dr. Nariai and his coinvestigators in the Tuberous Sclerosis Complex Autism Center of Excellence Network reported that noninvasive detection of interictal HFO fast ripples in the 250-500 Hz range via scalp EEG showed promise as a biomarker of epilepsy. Sensitivity of this far more practical approach to the detection of fast ripples was excellent, whether analyzed visually or by automatic detector (Clin Neurophysiol. 2018 Jul;129[7]:1458-66).
Moreover, in a recent, not-yet published study that Dr. Nariai and coworkers conducted in 24 infants with active epileptic spasms and 6 controls, noninvasive objective measurement of HFO rate using scalp EEG had an 83% sensitivity and 100% specificity for active epileptic spasms, while the modulation index of HFO and delta coupling in the 3-4 Hz range showed 74% sensitivity and 86% specificity.
If future studies validate the utility of detection of HFOs above a defined threshold or another noninvasively obtained EEG biomarker for diagnosis of epilepsy, the same strategy would presumably also be applicable for monitoring response to antiepileptic therapies, thereby eliminating the traditional trial-and-error approach to treatment. This would be a particularly important application in patients with West syndrome, where it’s believed that the electrical activity itself is contributing to the progressive – and often rapid – loss of cognitive function and behavioral disturbances. Thus, unlike in most other forms of epilepsy, the treatment goal isn’t merely to suppress the seizures, but also to achieve disease modification by eliminating the underlying subclinical EEG abnormalities, he explained.
A reliable biomarker would also be a boon in selecting the best participants for clinical trials of new antiseizure therapies.
Dr. Nariai reported having no financial conflicts regarding his presentation. His work is funded by research foundations and the National Institutes of Health.
REPORTING FROM IEC 2019
FDA approves Recarbrio for cUTI, cIAI treatment in adults
The Recarbrio is a three-drug combo injection containing imipenem/cilastatin, an antibiotic previously approved by the FDA, and relebactam, a beta-lactamase inhibitor.
The efficacy of Recarbrio was supported by data on the efficacy of imipenem/cilastatin in the treatment of cUTI and cIAI and by in vitro studies and animal models of infection with treatment by relebactam. The safety was assessed in a pair of clinical studies, one that assessed cUTI patients and another that assessed cIAI patients.
The most common adverse events reported were nausea, diarrhea, headache, fever, and increased liver enzymes. Treatment with Recarbrio is not recommended in patients taking ganciclovir, valproic acid, or divalproex sodium because there is an increased risk of seizures, according to the FDA.
“The FDA remains focused on facilitating the development of safe and effective new antibacterial drugs to give patients more options to fight serious infections. It is important that the use of Recarbrio be reserved for situations when there are limited or no alternative antibacterial drugs for treating a patient’s infection,” Ed Cox, MD, MPH, director for the Office of Antimicrobial Products in FDA’s Center for Drug Evaluation and Research, said in the press release.
Find the full press release on the FDA website.
The Recarbrio is a three-drug combo injection containing imipenem/cilastatin, an antibiotic previously approved by the FDA, and relebactam, a beta-lactamase inhibitor.
The efficacy of Recarbrio was supported by data on the efficacy of imipenem/cilastatin in the treatment of cUTI and cIAI and by in vitro studies and animal models of infection with treatment by relebactam. The safety was assessed in a pair of clinical studies, one that assessed cUTI patients and another that assessed cIAI patients.
The most common adverse events reported were nausea, diarrhea, headache, fever, and increased liver enzymes. Treatment with Recarbrio is not recommended in patients taking ganciclovir, valproic acid, or divalproex sodium because there is an increased risk of seizures, according to the FDA.
“The FDA remains focused on facilitating the development of safe and effective new antibacterial drugs to give patients more options to fight serious infections. It is important that the use of Recarbrio be reserved for situations when there are limited or no alternative antibacterial drugs for treating a patient’s infection,” Ed Cox, MD, MPH, director for the Office of Antimicrobial Products in FDA’s Center for Drug Evaluation and Research, said in the press release.
Find the full press release on the FDA website.
The Recarbrio is a three-drug combo injection containing imipenem/cilastatin, an antibiotic previously approved by the FDA, and relebactam, a beta-lactamase inhibitor.
The efficacy of Recarbrio was supported by data on the efficacy of imipenem/cilastatin in the treatment of cUTI and cIAI and by in vitro studies and animal models of infection with treatment by relebactam. The safety was assessed in a pair of clinical studies, one that assessed cUTI patients and another that assessed cIAI patients.
The most common adverse events reported were nausea, diarrhea, headache, fever, and increased liver enzymes. Treatment with Recarbrio is not recommended in patients taking ganciclovir, valproic acid, or divalproex sodium because there is an increased risk of seizures, according to the FDA.
“The FDA remains focused on facilitating the development of safe and effective new antibacterial drugs to give patients more options to fight serious infections. It is important that the use of Recarbrio be reserved for situations when there are limited or no alternative antibacterial drugs for treating a patient’s infection,” Ed Cox, MD, MPH, director for the Office of Antimicrobial Products in FDA’s Center for Drug Evaluation and Research, said in the press release.
Find the full press release on the FDA website.
Cutaneous reaction to AEDs? Think autoimmune epilepsy
BANGKOK – Cutaneous reactions to antiepileptic drugs in patients with chronic epilepsy suggest increased likelihood of an autoimmune element to their seizure disorder, Fernando Cendes, MD, PhD, reported at the International Epilepsy Congress.
“My recommendation based on our findings is that if you have a patient who has a history of skin reactions to AEDs [antiepileptic drugs], or who has psychosis, or who has a very strange response to antiepileptic medication – meaning that at some points they are refractory and at other points they are very well controlled – I think those patients are probably at risk for having an autoantibody,” he said at the congress sponsored by the International League Against Epilepsy.
Screening for autoantibodies in such patients is appropriate. However, there’s a caveat: “The thing is, we don’t have evidence that treating these autoantibodies with immunotherapy will have any benefit on seizure control in these patients. We don’t have that data yet, but we are looking into it,” according to Dr. Cendes, professor of neurology at the State University of Campinas (Brazil).
He presented a study of 221 consecutive adults with severe chronic refractory epilepsy as evidenced by a mean disease duration of nearly 29 years, with an average of 5.93 seizures per month. A total of 77% had a structural etiology for their epilepsy, in most cases hippocampal sclerosis. In 19% of patients, the etiology was unknown. Overall, 95% of subjects had focal epilepsy, and the remainder had generalized epilepsy. All underwent serum testing for a variety of antibodies against neuronal surface antigens that have been implicated in encephalitis, seizures, and/or psychosis. Those who tested positive then underwent confirmatory testing of their cerebrospinal fluid.
The impetus for this study, the neurologist explained, is that although it’s now well established that seizures are a common clinical expression of acute- and subacute-phase autoimmune encephalitis marked by neuronal autoantibodies, little is known about the relationship between chronic epilepsy and such antibodies.
Only five Brazilian patients with chronic epilepsy, or 2.2%, tested positive for autoantibodies, all of whom had mesial temporal lobe epilepsy with hippocampal sclerosis. This suggests a possible autoimmune etiology for hippocampal sclerosis. Three of the five patients had anti-N-methyl-D-aspartate receptor antibodies (anti-NMDA) and two had antiglutamic acid decarboxylate antibodies (anti-GAD). No one was positive for anti–leucine-rich glioma-inactivated 1 antibodies (anti-LGI1), anti–contactin-associated proteinlike 2 (anti-caspr2), anti-glutamate receptor antibodies (anti-AMPAr), or anti–gamma-aminobutyric acid receptor antibodies (anti-GABAr).
The autoantibody-negative and the much smaller autoantibody-positive groups didn’t differ significantly in terms of demographics, seizure frequency, disease duration, drug resistance, cognitive impairment, comorbid autoimmune conditions, or history of status epilepticus. Indeed, only two between-group differences were found: fluctuation in seizure control was an issue in 10.6% of autoantibody-negative and 40% of autoantibody-positive patients, and cutaneous adverse reactions to antiepileptic drugs were noted in 10.6% of antibody-negative and 60% of antibody-positive patients. Psychiatric comorbidities were present in 49.5% of autoantibody-negative patients as compared with 80% – that is, four of five – who were autoantibody-positive, a trend that didn’t achieve statistical significance.
Asked if he thinks the autoantibodies found in a small subset of patients with chronic epilepsy were a cause or an effect of repeated seizures for so long, Dr. Cendes replied, “That’s a very interesting question, and I don’t have an answer, actually. But if seizures trigger development of these antibodies – and remember, this population we’re talking about had many, many seizures over the years – I would expect antibodies to be more frequent than the figure we found.”
He reported having no financial conflicts regarding his study.
SOURCE: Watanabe N et al. IEC 2019, Abstract P004.
BANGKOK – Cutaneous reactions to antiepileptic drugs in patients with chronic epilepsy suggest increased likelihood of an autoimmune element to their seizure disorder, Fernando Cendes, MD, PhD, reported at the International Epilepsy Congress.
“My recommendation based on our findings is that if you have a patient who has a history of skin reactions to AEDs [antiepileptic drugs], or who has psychosis, or who has a very strange response to antiepileptic medication – meaning that at some points they are refractory and at other points they are very well controlled – I think those patients are probably at risk for having an autoantibody,” he said at the congress sponsored by the International League Against Epilepsy.
Screening for autoantibodies in such patients is appropriate. However, there’s a caveat: “The thing is, we don’t have evidence that treating these autoantibodies with immunotherapy will have any benefit on seizure control in these patients. We don’t have that data yet, but we are looking into it,” according to Dr. Cendes, professor of neurology at the State University of Campinas (Brazil).
He presented a study of 221 consecutive adults with severe chronic refractory epilepsy as evidenced by a mean disease duration of nearly 29 years, with an average of 5.93 seizures per month. A total of 77% had a structural etiology for their epilepsy, in most cases hippocampal sclerosis. In 19% of patients, the etiology was unknown. Overall, 95% of subjects had focal epilepsy, and the remainder had generalized epilepsy. All underwent serum testing for a variety of antibodies against neuronal surface antigens that have been implicated in encephalitis, seizures, and/or psychosis. Those who tested positive then underwent confirmatory testing of their cerebrospinal fluid.
The impetus for this study, the neurologist explained, is that although it’s now well established that seizures are a common clinical expression of acute- and subacute-phase autoimmune encephalitis marked by neuronal autoantibodies, little is known about the relationship between chronic epilepsy and such antibodies.
Only five Brazilian patients with chronic epilepsy, or 2.2%, tested positive for autoantibodies, all of whom had mesial temporal lobe epilepsy with hippocampal sclerosis. This suggests a possible autoimmune etiology for hippocampal sclerosis. Three of the five patients had anti-N-methyl-D-aspartate receptor antibodies (anti-NMDA) and two had antiglutamic acid decarboxylate antibodies (anti-GAD). No one was positive for anti–leucine-rich glioma-inactivated 1 antibodies (anti-LGI1), anti–contactin-associated proteinlike 2 (anti-caspr2), anti-glutamate receptor antibodies (anti-AMPAr), or anti–gamma-aminobutyric acid receptor antibodies (anti-GABAr).
The autoantibody-negative and the much smaller autoantibody-positive groups didn’t differ significantly in terms of demographics, seizure frequency, disease duration, drug resistance, cognitive impairment, comorbid autoimmune conditions, or history of status epilepticus. Indeed, only two between-group differences were found: fluctuation in seizure control was an issue in 10.6% of autoantibody-negative and 40% of autoantibody-positive patients, and cutaneous adverse reactions to antiepileptic drugs were noted in 10.6% of antibody-negative and 60% of antibody-positive patients. Psychiatric comorbidities were present in 49.5% of autoantibody-negative patients as compared with 80% – that is, four of five – who were autoantibody-positive, a trend that didn’t achieve statistical significance.
Asked if he thinks the autoantibodies found in a small subset of patients with chronic epilepsy were a cause or an effect of repeated seizures for so long, Dr. Cendes replied, “That’s a very interesting question, and I don’t have an answer, actually. But if seizures trigger development of these antibodies – and remember, this population we’re talking about had many, many seizures over the years – I would expect antibodies to be more frequent than the figure we found.”
He reported having no financial conflicts regarding his study.
SOURCE: Watanabe N et al. IEC 2019, Abstract P004.
BANGKOK – Cutaneous reactions to antiepileptic drugs in patients with chronic epilepsy suggest increased likelihood of an autoimmune element to their seizure disorder, Fernando Cendes, MD, PhD, reported at the International Epilepsy Congress.
“My recommendation based on our findings is that if you have a patient who has a history of skin reactions to AEDs [antiepileptic drugs], or who has psychosis, or who has a very strange response to antiepileptic medication – meaning that at some points they are refractory and at other points they are very well controlled – I think those patients are probably at risk for having an autoantibody,” he said at the congress sponsored by the International League Against Epilepsy.
Screening for autoantibodies in such patients is appropriate. However, there’s a caveat: “The thing is, we don’t have evidence that treating these autoantibodies with immunotherapy will have any benefit on seizure control in these patients. We don’t have that data yet, but we are looking into it,” according to Dr. Cendes, professor of neurology at the State University of Campinas (Brazil).
He presented a study of 221 consecutive adults with severe chronic refractory epilepsy as evidenced by a mean disease duration of nearly 29 years, with an average of 5.93 seizures per month. A total of 77% had a structural etiology for their epilepsy, in most cases hippocampal sclerosis. In 19% of patients, the etiology was unknown. Overall, 95% of subjects had focal epilepsy, and the remainder had generalized epilepsy. All underwent serum testing for a variety of antibodies against neuronal surface antigens that have been implicated in encephalitis, seizures, and/or psychosis. Those who tested positive then underwent confirmatory testing of their cerebrospinal fluid.
The impetus for this study, the neurologist explained, is that although it’s now well established that seizures are a common clinical expression of acute- and subacute-phase autoimmune encephalitis marked by neuronal autoantibodies, little is known about the relationship between chronic epilepsy and such antibodies.
Only five Brazilian patients with chronic epilepsy, or 2.2%, tested positive for autoantibodies, all of whom had mesial temporal lobe epilepsy with hippocampal sclerosis. This suggests a possible autoimmune etiology for hippocampal sclerosis. Three of the five patients had anti-N-methyl-D-aspartate receptor antibodies (anti-NMDA) and two had antiglutamic acid decarboxylate antibodies (anti-GAD). No one was positive for anti–leucine-rich glioma-inactivated 1 antibodies (anti-LGI1), anti–contactin-associated proteinlike 2 (anti-caspr2), anti-glutamate receptor antibodies (anti-AMPAr), or anti–gamma-aminobutyric acid receptor antibodies (anti-GABAr).
The autoantibody-negative and the much smaller autoantibody-positive groups didn’t differ significantly in terms of demographics, seizure frequency, disease duration, drug resistance, cognitive impairment, comorbid autoimmune conditions, or history of status epilepticus. Indeed, only two between-group differences were found: fluctuation in seizure control was an issue in 10.6% of autoantibody-negative and 40% of autoantibody-positive patients, and cutaneous adverse reactions to antiepileptic drugs were noted in 10.6% of antibody-negative and 60% of antibody-positive patients. Psychiatric comorbidities were present in 49.5% of autoantibody-negative patients as compared with 80% – that is, four of five – who were autoantibody-positive, a trend that didn’t achieve statistical significance.
Asked if he thinks the autoantibodies found in a small subset of patients with chronic epilepsy were a cause or an effect of repeated seizures for so long, Dr. Cendes replied, “That’s a very interesting question, and I don’t have an answer, actually. But if seizures trigger development of these antibodies – and remember, this population we’re talking about had many, many seizures over the years – I would expect antibodies to be more frequent than the figure we found.”
He reported having no financial conflicts regarding his study.
SOURCE: Watanabe N et al. IEC 2019, Abstract P004.
REPORTING FROM IEC 2019
Test could inform care of patients with pancreatic cysts
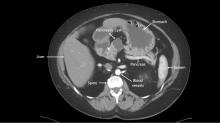
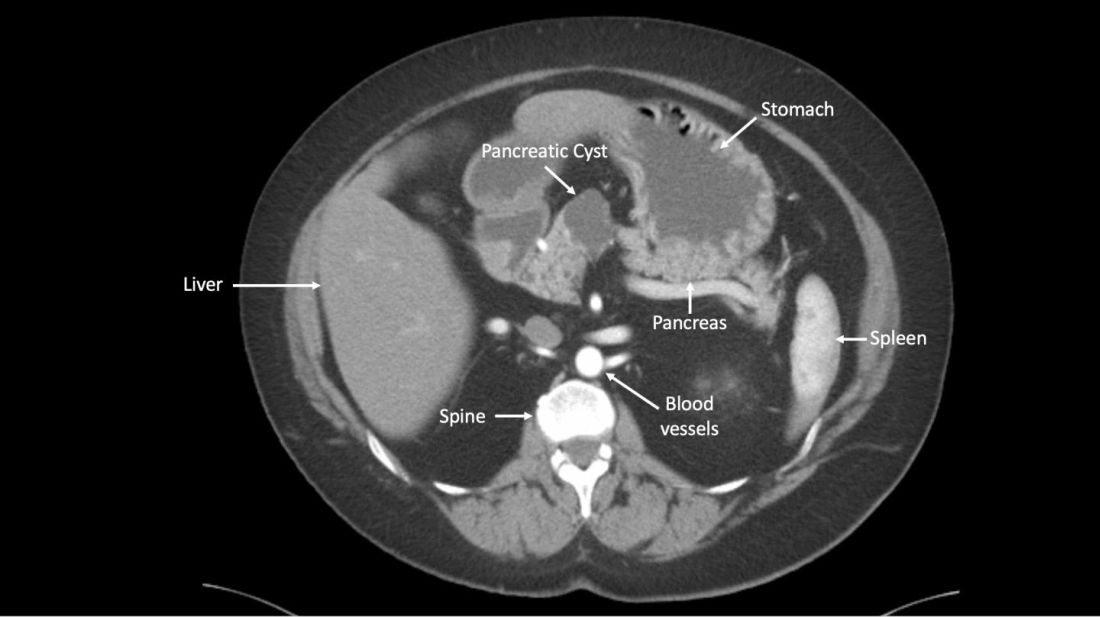
A newly developed test could help clinicians more accurately identify which patients with pancreatic cysts require surgery, according to researchers.
The test, CompCyst, incorporates clinical and imaging data as well as data on genetic and biochemical markers associated with pancreatic cancer.
CompCyst proved more effective than standard practice in estimating the risk of cancer so as to differentiate patients who should undergo surgery from patients who require monitoring and those who need no additional care.
Simeon Springer, PhD, of Ring Therapeutics in Cambridge, Mass., and colleagues described the development and testing of CompCyst in Science Translational Medicine.
The researchers collected data from 875 patients who had undergone surgical resection of pancreatic cysts. The team used clinical, imaging, and molecular data from 436 of those patients to train CompCyst to classify patients into three categories.
- Patients with benign, nonmucin-producing cysts who do not require surgery or monitoring
- Patients who require monitoring because they have mucin-producing cysts with low- or intermediate-grade dysplasia
- Patients who have invasive cancer or high-grade dysplasia and require surgery.
The researchers then tested CompCyst in the remaining 426 patients (the validation cohort), comparing CompCyst with standard practice, which involves use of clinical and imaging criteria only.
“Our aim [in developing CompCyst] was not to replace current knowledge derived from clinical data and imaging characteristics with molecular testing but, rather, to integrate all these aspects together,” study author Marco Dal Molin, MD, of Johns Hopkins University, Baltimore, said in a press conference.
“An important aspect of this paper is the comparison between the performance of our test and current clinical practice. Because the histopathology of all cysts was known from surgical specimens, we could determine, in retrospect, what the optimal treatment should have been.”
Histopathology showed that 53 patients in the validation cohort had a benign, nonmucin-producing cyst and did not require any additional intervention. Standard practice correctly identified 19% (n =10) of these patients, while CompCyst correctly identified 60% (n = 32).
There were 140 patients who had mucin-producing cysts with low- or intermediate-grade dysplasia. Standard practice correctly identified 34% (n = 48) of these patients, while CompCyst correctly identified 49% (n = 68).
“Overall, the use of CompCyst would have avoided unnecessary surgery in 60% of the patients in this study,” Dr. Dal Molin said.
Surgery was needed in 152 patients in the validation cohort. Standard practice correctly identified 89% (n = 135) of these patients, while CompCyst correctly identified 91% (n = 138). Neither method would have recommended discharge for any patient who actually required surgery, the researchers noted.
Based on these results, the researchers are hoping to make CompCyst available to patients at Johns Hopkins within the next 6-12 months.
“In the long term, we hope that a new, prospective study will be carried out, which will gain approval of this test by the FDA [Food and Drug Administration],” study author Bert Vogelstein, MD, of Johns Hopkins, said at the press conference.
“Then, at that point, we hope the technology can be commercialized and offered to the public through a company called Thrive [Earlier Detection], which has licensed the technology from Johns Hopkins.”
This research was supported by the Lustgarten Foundation for Pancreatic Cancer Research, the Virginia and D.K. Ludwig Fund for Cancer Research, the Sol Goldman Pancreatic Cancer Research Center, the Michael Rolfe Pancreatic Cancer Foundation, the Benjamin Baker Scholarship, and the National Institutes of Health. The researchers reported relationships with Thrive Earlier Detection, Personal Genome Diagnostics, Eisai-Morphotek, Sysmex Inostics, Nexus Strategy (Camden Partners), NeoPhore, and CAGE.
SOURCE: Springer S et al. Sci Transl Med. 2019 Jul 17. doi: 10.1126/scitranslmed.aav477.
A newly developed test could help clinicians more accurately identify which patients with pancreatic cysts require surgery, according to researchers.
The test, CompCyst, incorporates clinical and imaging data as well as data on genetic and biochemical markers associated with pancreatic cancer.
CompCyst proved more effective than standard practice in estimating the risk of cancer so as to differentiate patients who should undergo surgery from patients who require monitoring and those who need no additional care.
Simeon Springer, PhD, of Ring Therapeutics in Cambridge, Mass., and colleagues described the development and testing of CompCyst in Science Translational Medicine.
The researchers collected data from 875 patients who had undergone surgical resection of pancreatic cysts. The team used clinical, imaging, and molecular data from 436 of those patients to train CompCyst to classify patients into three categories.
- Patients with benign, nonmucin-producing cysts who do not require surgery or monitoring
- Patients who require monitoring because they have mucin-producing cysts with low- or intermediate-grade dysplasia
- Patients who have invasive cancer or high-grade dysplasia and require surgery.
The researchers then tested CompCyst in the remaining 426 patients (the validation cohort), comparing CompCyst with standard practice, which involves use of clinical and imaging criteria only.
“Our aim [in developing CompCyst] was not to replace current knowledge derived from clinical data and imaging characteristics with molecular testing but, rather, to integrate all these aspects together,” study author Marco Dal Molin, MD, of Johns Hopkins University, Baltimore, said in a press conference.
“An important aspect of this paper is the comparison between the performance of our test and current clinical practice. Because the histopathology of all cysts was known from surgical specimens, we could determine, in retrospect, what the optimal treatment should have been.”
Histopathology showed that 53 patients in the validation cohort had a benign, nonmucin-producing cyst and did not require any additional intervention. Standard practice correctly identified 19% (n =10) of these patients, while CompCyst correctly identified 60% (n = 32).
There were 140 patients who had mucin-producing cysts with low- or intermediate-grade dysplasia. Standard practice correctly identified 34% (n = 48) of these patients, while CompCyst correctly identified 49% (n = 68).
“Overall, the use of CompCyst would have avoided unnecessary surgery in 60% of the patients in this study,” Dr. Dal Molin said.
Surgery was needed in 152 patients in the validation cohort. Standard practice correctly identified 89% (n = 135) of these patients, while CompCyst correctly identified 91% (n = 138). Neither method would have recommended discharge for any patient who actually required surgery, the researchers noted.
Based on these results, the researchers are hoping to make CompCyst available to patients at Johns Hopkins within the next 6-12 months.
“In the long term, we hope that a new, prospective study will be carried out, which will gain approval of this test by the FDA [Food and Drug Administration],” study author Bert Vogelstein, MD, of Johns Hopkins, said at the press conference.
“Then, at that point, we hope the technology can be commercialized and offered to the public through a company called Thrive [Earlier Detection], which has licensed the technology from Johns Hopkins.”
This research was supported by the Lustgarten Foundation for Pancreatic Cancer Research, the Virginia and D.K. Ludwig Fund for Cancer Research, the Sol Goldman Pancreatic Cancer Research Center, the Michael Rolfe Pancreatic Cancer Foundation, the Benjamin Baker Scholarship, and the National Institutes of Health. The researchers reported relationships with Thrive Earlier Detection, Personal Genome Diagnostics, Eisai-Morphotek, Sysmex Inostics, Nexus Strategy (Camden Partners), NeoPhore, and CAGE.
SOURCE: Springer S et al. Sci Transl Med. 2019 Jul 17. doi: 10.1126/scitranslmed.aav477.
A newly developed test could help clinicians more accurately identify which patients with pancreatic cysts require surgery, according to researchers.
The test, CompCyst, incorporates clinical and imaging data as well as data on genetic and biochemical markers associated with pancreatic cancer.
CompCyst proved more effective than standard practice in estimating the risk of cancer so as to differentiate patients who should undergo surgery from patients who require monitoring and those who need no additional care.
Simeon Springer, PhD, of Ring Therapeutics in Cambridge, Mass., and colleagues described the development and testing of CompCyst in Science Translational Medicine.
The researchers collected data from 875 patients who had undergone surgical resection of pancreatic cysts. The team used clinical, imaging, and molecular data from 436 of those patients to train CompCyst to classify patients into three categories.
- Patients with benign, nonmucin-producing cysts who do not require surgery or monitoring
- Patients who require monitoring because they have mucin-producing cysts with low- or intermediate-grade dysplasia
- Patients who have invasive cancer or high-grade dysplasia and require surgery.
The researchers then tested CompCyst in the remaining 426 patients (the validation cohort), comparing CompCyst with standard practice, which involves use of clinical and imaging criteria only.
“Our aim [in developing CompCyst] was not to replace current knowledge derived from clinical data and imaging characteristics with molecular testing but, rather, to integrate all these aspects together,” study author Marco Dal Molin, MD, of Johns Hopkins University, Baltimore, said in a press conference.
“An important aspect of this paper is the comparison between the performance of our test and current clinical practice. Because the histopathology of all cysts was known from surgical specimens, we could determine, in retrospect, what the optimal treatment should have been.”
Histopathology showed that 53 patients in the validation cohort had a benign, nonmucin-producing cyst and did not require any additional intervention. Standard practice correctly identified 19% (n =10) of these patients, while CompCyst correctly identified 60% (n = 32).
There were 140 patients who had mucin-producing cysts with low- or intermediate-grade dysplasia. Standard practice correctly identified 34% (n = 48) of these patients, while CompCyst correctly identified 49% (n = 68).
“Overall, the use of CompCyst would have avoided unnecessary surgery in 60% of the patients in this study,” Dr. Dal Molin said.
Surgery was needed in 152 patients in the validation cohort. Standard practice correctly identified 89% (n = 135) of these patients, while CompCyst correctly identified 91% (n = 138). Neither method would have recommended discharge for any patient who actually required surgery, the researchers noted.
Based on these results, the researchers are hoping to make CompCyst available to patients at Johns Hopkins within the next 6-12 months.
“In the long term, we hope that a new, prospective study will be carried out, which will gain approval of this test by the FDA [Food and Drug Administration],” study author Bert Vogelstein, MD, of Johns Hopkins, said at the press conference.
“Then, at that point, we hope the technology can be commercialized and offered to the public through a company called Thrive [Earlier Detection], which has licensed the technology from Johns Hopkins.”
This research was supported by the Lustgarten Foundation for Pancreatic Cancer Research, the Virginia and D.K. Ludwig Fund for Cancer Research, the Sol Goldman Pancreatic Cancer Research Center, the Michael Rolfe Pancreatic Cancer Foundation, the Benjamin Baker Scholarship, and the National Institutes of Health. The researchers reported relationships with Thrive Earlier Detection, Personal Genome Diagnostics, Eisai-Morphotek, Sysmex Inostics, Nexus Strategy (Camden Partners), NeoPhore, and CAGE.
SOURCE: Springer S et al. Sci Transl Med. 2019 Jul 17. doi: 10.1126/scitranslmed.aav477.
FROM SCIENCE TRANSLATIONAL MEDICINE
Key clinical point: A test called CompCyst could help clinicians more accurately identify which patients with pancreatic cysts require surgery.
Major finding: CompCyst correctly identified 91% of patients who required surgery, 49% of those who required monitoring, and 60% of patients who required no further care.
Study details: A retrospective analysis of 875 patients with pancreatic cysts
Disclosures: The research was supported by the Lustgarten Foundation for Pancreatic Cancer Research, the Virginia and D.K. Ludwig Fund for Cancer Research, the Sol Goldman Pancreatic Cancer Research Center, the Michael Rolfe Pancreatic Cancer Foundation, the Benjamin Baker Scholarship, and the National Institutes of Health. The researchers reported relationships with Thrive Earlier Detection, Personal Genome Diagnostics, Eisai-Morphotek, Sysmex Inostics, Nexus Strategy (Camden Partners), NeoPhore, and CAGE.
Source: Springer S et al. Sci Transl Med. 2019 Jul 17. doi: 10.1126/scitranslmed.aav477.
Intravenous CNS chemo looks best for testicular DLBCL
For patients with testicular diffuse large B-cell lymphoma (T-DLBCL), intravenous central nervous system (CNS)–directed chemotherapy and prophylactic treatment of the contralateral testis offer the best survival outcomes, according to findings from a recent retrospective analysis.
In contrast, intrathecal chemotherapy offered no benefit, reported lead author Susanna Mannisto, MD, of Helsinki University Hospital in Finland, and her colleagues. Survival advantages gained by CNS-directed chemotherapy were generally due to control of systemic disease rather than prevention of CNS progression, they noted.
There have not been randomized trials conducted specifically in T-DLBCL and treatment guidelines are currently based on phase 2 trials. Treatment for T-DLBCL typically involves cyclophosphamide, doxorubicin, vincristine, prednisolone plus rituximab (R–CHOP), with the addition of CNS prophylaxis with either intravenous or intrathecal methotrexate or cytarabine (AraC) in eligible patients. “Thus far, however, no prospective randomised studies on the benefit of this approach have been published,” the investigators wrote. The study is in the European Journal of Cancer.
They drew data from the Danish Lymphoma Registry and three Southern Finland University Hospitals. Out of 235 patients diagnosed with T-DLBCL between 1987 and 2013, 192 (82%) were treated with curative anthracycline-based chemotherapy. As some cases dated back to 1987, about one-third (36%) were treated before rituximab was available. A slightly higher proportion received intravenous CNS-directed chemotherapy (40%). Due to data availability, 189 patients were included in the survival analysis.
Results of multivariate analyses showed that CNS-directed chemotherapy (intravenous methotrexate, high-dose AraC, or both), was associated with an approximately 58% decreased risk of death across the entire population (hazard ratio [HR] for overall survival, 0.419; 95% confidence interval, 0.256-0.686; P = .001), with elderly patients realizing the greatest benefit.
“[I]t is important to note that we did not observe reduction in the CNS recurrence rate, but rather a better control of the systemic disease, suggesting that R–CHOP alone may not be a sufficient therapy for the patients with testicular DLBCL involvement,” the investigators wrote.
Intrathecal CNS-targeted therapy did not correlate with improved survival in the study. Likewise, for the entire population, rituximab did not boost survival; however, for high risk patients (International Prognostic Index, 3-5), immunochemotherapy did provide a better rate of 5-year disease-specific survival than that of conventional chemotherapy (44% vs 14%; P = .019).
Treatment of the contralateral testis was worthwhile for the entire population, with a 46% decreased risk of death (HR for overall survival, 0.514, 95% CI, 0.338-0.782; P = .002).
Shortest overall survival times, regardless of treatment type, were reported among patients with poor performance status, elevated lactate dehydrogenase (LDH), primary T-DLBCL, more extranodal sites, higher International Prognostic Index, and patients older than 70 years.
“[O]ur results recommend aggressive chemotherapy with [intravenous high-dose methotrexate and/or high-dose AraC] for better control of systemic disease for eligible patients,” the investigators concluded. “In addition, treatment of the contralateral testis with either radiotherapy or orchiectomy should be included in the management strategy of patients with T-DLBCL.”
The study was funded by the Academy of Finland, the Finnish Cancer Foundation, the Sigrid Juselius Foundation, and others. The investigators reported additional relationships with Pfizer, Takeda, Roche, Sanofi, and others.
SOURCE: Mannisto S et al. Eur J Cancer. 2019 May 10. doi: 10.1016/j.ejca.2019.04.004.
For patients with testicular diffuse large B-cell lymphoma (T-DLBCL), intravenous central nervous system (CNS)–directed chemotherapy and prophylactic treatment of the contralateral testis offer the best survival outcomes, according to findings from a recent retrospective analysis.
In contrast, intrathecal chemotherapy offered no benefit, reported lead author Susanna Mannisto, MD, of Helsinki University Hospital in Finland, and her colleagues. Survival advantages gained by CNS-directed chemotherapy were generally due to control of systemic disease rather than prevention of CNS progression, they noted.
There have not been randomized trials conducted specifically in T-DLBCL and treatment guidelines are currently based on phase 2 trials. Treatment for T-DLBCL typically involves cyclophosphamide, doxorubicin, vincristine, prednisolone plus rituximab (R–CHOP), with the addition of CNS prophylaxis with either intravenous or intrathecal methotrexate or cytarabine (AraC) in eligible patients. “Thus far, however, no prospective randomised studies on the benefit of this approach have been published,” the investigators wrote. The study is in the European Journal of Cancer.
They drew data from the Danish Lymphoma Registry and three Southern Finland University Hospitals. Out of 235 patients diagnosed with T-DLBCL between 1987 and 2013, 192 (82%) were treated with curative anthracycline-based chemotherapy. As some cases dated back to 1987, about one-third (36%) were treated before rituximab was available. A slightly higher proportion received intravenous CNS-directed chemotherapy (40%). Due to data availability, 189 patients were included in the survival analysis.
Results of multivariate analyses showed that CNS-directed chemotherapy (intravenous methotrexate, high-dose AraC, or both), was associated with an approximately 58% decreased risk of death across the entire population (hazard ratio [HR] for overall survival, 0.419; 95% confidence interval, 0.256-0.686; P = .001), with elderly patients realizing the greatest benefit.
“[I]t is important to note that we did not observe reduction in the CNS recurrence rate, but rather a better control of the systemic disease, suggesting that R–CHOP alone may not be a sufficient therapy for the patients with testicular DLBCL involvement,” the investigators wrote.
Intrathecal CNS-targeted therapy did not correlate with improved survival in the study. Likewise, for the entire population, rituximab did not boost survival; however, for high risk patients (International Prognostic Index, 3-5), immunochemotherapy did provide a better rate of 5-year disease-specific survival than that of conventional chemotherapy (44% vs 14%; P = .019).
Treatment of the contralateral testis was worthwhile for the entire population, with a 46% decreased risk of death (HR for overall survival, 0.514, 95% CI, 0.338-0.782; P = .002).
Shortest overall survival times, regardless of treatment type, were reported among patients with poor performance status, elevated lactate dehydrogenase (LDH), primary T-DLBCL, more extranodal sites, higher International Prognostic Index, and patients older than 70 years.
“[O]ur results recommend aggressive chemotherapy with [intravenous high-dose methotrexate and/or high-dose AraC] for better control of systemic disease for eligible patients,” the investigators concluded. “In addition, treatment of the contralateral testis with either radiotherapy or orchiectomy should be included in the management strategy of patients with T-DLBCL.”
The study was funded by the Academy of Finland, the Finnish Cancer Foundation, the Sigrid Juselius Foundation, and others. The investigators reported additional relationships with Pfizer, Takeda, Roche, Sanofi, and others.
SOURCE: Mannisto S et al. Eur J Cancer. 2019 May 10. doi: 10.1016/j.ejca.2019.04.004.
For patients with testicular diffuse large B-cell lymphoma (T-DLBCL), intravenous central nervous system (CNS)–directed chemotherapy and prophylactic treatment of the contralateral testis offer the best survival outcomes, according to findings from a recent retrospective analysis.
In contrast, intrathecal chemotherapy offered no benefit, reported lead author Susanna Mannisto, MD, of Helsinki University Hospital in Finland, and her colleagues. Survival advantages gained by CNS-directed chemotherapy were generally due to control of systemic disease rather than prevention of CNS progression, they noted.
There have not been randomized trials conducted specifically in T-DLBCL and treatment guidelines are currently based on phase 2 trials. Treatment for T-DLBCL typically involves cyclophosphamide, doxorubicin, vincristine, prednisolone plus rituximab (R–CHOP), with the addition of CNS prophylaxis with either intravenous or intrathecal methotrexate or cytarabine (AraC) in eligible patients. “Thus far, however, no prospective randomised studies on the benefit of this approach have been published,” the investigators wrote. The study is in the European Journal of Cancer.
They drew data from the Danish Lymphoma Registry and three Southern Finland University Hospitals. Out of 235 patients diagnosed with T-DLBCL between 1987 and 2013, 192 (82%) were treated with curative anthracycline-based chemotherapy. As some cases dated back to 1987, about one-third (36%) were treated before rituximab was available. A slightly higher proportion received intravenous CNS-directed chemotherapy (40%). Due to data availability, 189 patients were included in the survival analysis.
Results of multivariate analyses showed that CNS-directed chemotherapy (intravenous methotrexate, high-dose AraC, or both), was associated with an approximately 58% decreased risk of death across the entire population (hazard ratio [HR] for overall survival, 0.419; 95% confidence interval, 0.256-0.686; P = .001), with elderly patients realizing the greatest benefit.
“[I]t is important to note that we did not observe reduction in the CNS recurrence rate, but rather a better control of the systemic disease, suggesting that R–CHOP alone may not be a sufficient therapy for the patients with testicular DLBCL involvement,” the investigators wrote.
Intrathecal CNS-targeted therapy did not correlate with improved survival in the study. Likewise, for the entire population, rituximab did not boost survival; however, for high risk patients (International Prognostic Index, 3-5), immunochemotherapy did provide a better rate of 5-year disease-specific survival than that of conventional chemotherapy (44% vs 14%; P = .019).
Treatment of the contralateral testis was worthwhile for the entire population, with a 46% decreased risk of death (HR for overall survival, 0.514, 95% CI, 0.338-0.782; P = .002).
Shortest overall survival times, regardless of treatment type, were reported among patients with poor performance status, elevated lactate dehydrogenase (LDH), primary T-DLBCL, more extranodal sites, higher International Prognostic Index, and patients older than 70 years.
“[O]ur results recommend aggressive chemotherapy with [intravenous high-dose methotrexate and/or high-dose AraC] for better control of systemic disease for eligible patients,” the investigators concluded. “In addition, treatment of the contralateral testis with either radiotherapy or orchiectomy should be included in the management strategy of patients with T-DLBCL.”
The study was funded by the Academy of Finland, the Finnish Cancer Foundation, the Sigrid Juselius Foundation, and others. The investigators reported additional relationships with Pfizer, Takeda, Roche, Sanofi, and others.
SOURCE: Mannisto S et al. Eur J Cancer. 2019 May 10. doi: 10.1016/j.ejca.2019.04.004.
FROM EUROPEAN JOURNAL OF CANCER
Tips to improve immunization rates in your office
In October 2018 the US Food and Drug Administration expanded the approved use of the human papillomavirus (HPV) vaccine (Gardasil 9) to adults aged 27 through 45.1 In June 2019, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP) voted to extend catch-up HPV vaccination to include all individuals through age 26 and to catch-up HPV vaccination, based on shared clinical decision making, for all adults aged 27 through 45.2 HPV viruses are associated with cervical cancer, as well as several other forms of cancer that affect both women and men. Approval for the expanded use of the HPV vaccine was based on data of the vaccine’s use in women.1,3
Unfortunately, adult immunization rates, including among pregnant women, do not equal the higher rates in childhood vaccine uptake, according to Kevin A. Ault, MD, and colleagues. Less than half of women (46.6%) receive influenza vaccination prior to and during pregnancy, for instance.4 Dr. Ault has identified the need for an “immunization champion”—someone who can manage one-on-one conversations with patients in the office setting to enhance the acceptance and uptake of adult and maternal vaccines.
OBG Management:
Dr. Kevin A. Ault, MD: The main thing a practice needs to do is to identify someone who is interested, and this person does not have to be a physician. In fact, he or she can frequently be a member of your nursing staff or office staff. And the word “champion” involves a lot of nuts and bolts: such details as how do you store the vaccine, how do you keep track of it, where are the vaccine information statements filed, where can the provider get more information if there is a question about contraindications? One person should organize all these details. The mechanics of vaccine administration are important as well, as the research shows that the more automated the process is, the better and more smoothly it is carried out. There is certainly a role for “standing orders” for adult vaccines.
OBG Management: What communication approach do you take with patients to enhance vaccination acceptance and uptake?
Dr. Ault: There are multiple research studies that show that provider recommendation is the most important way to get both nonpregnant and pregnant adults to receive vaccinations. Take the pertussis vaccine (the whooping cough booster) as an example. It is a relatively new vaccine recommendation during pregnancy. Your approach is relatively straightforward when explaining it to pregnant women. Make the point that we do not want your newborn to have whooping cough in those first few months of life before the newborn or infant vaccine becomes effective. Most people know they had a whooping cough, or pertussis, vaccine when they were younger, and the concept of the booster is well-known to patients. You should explain that the maternal antibodies pass through the placenta to the fetus, and they provide benefit for the first few months of life after birth.
The pertussis vaccine does not have all the “baggage” of the influenza vaccine. Talking with patients about the flu vaccine may present more challenges. Typically, each fall there is a popular press publication that explains “the 10, or 20, most common myths about influenza vaccine.” Every fall I try to find one of those articles, print it out, and even carry it in my jacket pocket and talk about all the myths. For example, there is a myth that “I always get sick when I get the flu shot.” Obstetricians should be giving patients an inactivated vaccine that does not contain any live flu virus. We should be able to explain to patients, your arm will be sore, and you may have some muscle aches, but you will not have the flu from your flu vaccine.
I think another reason that pregnant women do not always take the flu vaccine is that we do not yet have normalized influenza vaccination in the adult population. Women in their twenties and thirties are generally very healthy and have other concerns when they are pregnant, and they perhaps do not realize that they are more vulnerable to devastating effects of influenza while pregnant. Additionally, maternal influenza vaccination does protect the newborn from flu for the first few months. It is vital that those patients who are due during the dark winter months, when the flu is in season, get vaccinated.
Combat the myths and tell your patients the reasons for flu vaccination. Also tell them that you got your flu shot, like most health care professionals do every fall. You should be prepared to talk about safety. There are wonderful safety data, even some published in 2017 and 2018, about pertussis vaccine safety during pregnancy, and it is very reassuring to patients. For flu, the idea of vaccinating women against influenza has been around for decades, and so we have reliable information about that as well. Certainly, the risks are very minor, and the benefits are potentially huge for the pregnant woman and for the newborn.
OBG Management: When do you recommend that ObGyns administer the flu vaccine for pregnant women?
Dr. Ault: There are 2 issues to this question: when throughout the year and when during the pregnancy to administer the vaccine. First, you want to give the flu vaccine during the usual influenza season during the fall. As soon as the vaccine is available, you will recommend that pregnant women, even in their late pregnancy, get vaccinated so that their newborns who are 3 and 4 months old in the peak flu season are protected. The patients who deliver over the summer, who are coming in for their postpartum visit during the fall, should be getting vaccinated as well, because they are still vulnerable to influenza and pneumonia for several months postpartum.
If you have patients that come in for preconception visits, you could say: “Let’s get this out of the way. You could be pregnant by the time flu season really gets cranked up.”
Because we see patients 10 or 12 times during pregnancy, we certainly have plenty of opportunities to educate patients about and administer the flu vaccine. There are older data that demonstrate if patients do not get the flu vaccine done during early pregnancy, the opportunity may be lost. It is different now because there is more emphasis on vaccinating all adults. Your patients certainly can get their vaccine at the pharmacy or at their primary care doctor; however, delaying until later pregnancy usually means not getting the vaccine.
I would like to address one recent study from Donahue and colleagues that showed a potentially increased risk of miscarriage with flu vaccination.5 That study was an anomaly, as there are many other studies into the issue. Yes, there are not a lot of first trimester data, but there are other studies, including studies by the same authors, that did not find this to be the case.6-10
The 2017 study by Donahue and colleagues was an anomaly because the group of women they were vaccinating were already at high risk for miscarriage. The women were older, had diabetes, or a history of miscarriages. There is selection bias in the study because the pregnant women who were vaccinated were already at higher risk for miscarriage. The Centers for Disease Control and Prevention and the American College of Obstetricians and Gynecologists are not going to change any of their recommendations based on a single study that is different than our previous data.11
Current recommended adult (anyone over 18 years old) immunization schedule
ACOG Immunization Champions (ACOG members who have demonstrated exceptional progress in increasing immunization rates among adults and pregnant women in their communities through leadership, innovation, collaboration, and educational activities aimed at following ACOG and CDC guidance.)
Summary of Maternal Immunization Recommendations is a provider resource from ACOG and the Centers for Disease Control and Prevention.
Maternal Immunization Toolkit contains materials, including the Vaccines During Pregnancy Poster, to support ObGyns on recommending the influenza vaccine and the Tdap vaccine to all pregnant patients.
Influenza Immunization During Pregnancy Toolkit
CDC vaccine schedules app for health care providers
CDC Vaccine Information Statements (available for clinician or patient download)
- FDA approves expanded use of Gardasil 9 to include individuals 27 through 45 years old [press release]. Washington, DC: Food and Drug Administration; October 5, 2018.
- Color/Blue2. Splete H. ACIP extends HPV vaccine coverage. June 27, 2019. https://www.mdedge.com/obgyn/article/203656/vaccines/acip-extends-hpv-vaccine-coverage. Accessed July 5, 2019.
- Levy BS, Downs Jr L. The HPV vaccine is now recommended for adults aged 27–45: Counseling implications. OBG Manag. 2019;31(1):9-11.
- Frew PM, Randall LA, Malik F, et al. Clinician perspectives on strategies to improve patient maternal immunization acceptability in obstetrics and gynecology practice settings. Hum Vaccin Immunother. 2018;14(7):1548–1557.
- Donahue JG, Kieke BA, King JP, et al. Association of spontaneous abortion with receipt of inactivated influenza vaccine containing H1N1pdm09 in 2010-11 and 2011-12. Vaccine. 2017;35(40):5314-5322.
- Moro PL, Broder K, Zheteyeva Y, et al. Adverse events in pregnant women following administration of trivalent inactivated influenza vaccine and live attenuated influenza vaccine in the Vaccine Adverse Event Reporting System, 1990-2009. Am J Obstet Gynecol. 2011;204:146.e1-146.e7.
- Irving SA, Kieke BA, Donahue JG, et al; Vaccine Safety Datalink. Trivalent inactivated influenza vaccine and spontaneous abortion. 2013;121:159-165.
- Kharbanda EO, Vazquez-Benitez G, Lipkind H, et al; Vaccine Safety Datalink Team. Inactivated influenza vaccine during pregnancy and risks for adverse obstetric events. Obstet Gynecol. 2013;122:659-667.
- Nordin JD, Kharbanda EO, Vazquez-Benitez G, et al; Vaccine Safety Datalink. Maternal Influenza vaccine and risks for preterm or small for gestational age birth. J Pediatrics. 2014;164:1051-1057.e2.
- Kharbanda EO, Vazquez-Benitez G, Romitti PA, et al; Vaccine Safety Datalink. First trimester influenza vaccination and risks for major structural birth defects in offspring. 2017;187:234-239.e4.
- Flu vaccination and possible safety signal. CDC website. https://www.cdc.gov/flu/professionals/vaccination/vaccination-possible-safety-signal.html. Last reviewed September 13, 2017. Accessed May 15, 2019.
In October 2018 the US Food and Drug Administration expanded the approved use of the human papillomavirus (HPV) vaccine (Gardasil 9) to adults aged 27 through 45.1 In June 2019, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP) voted to extend catch-up HPV vaccination to include all individuals through age 26 and to catch-up HPV vaccination, based on shared clinical decision making, for all adults aged 27 through 45.2 HPV viruses are associated with cervical cancer, as well as several other forms of cancer that affect both women and men. Approval for the expanded use of the HPV vaccine was based on data of the vaccine’s use in women.1,3
Unfortunately, adult immunization rates, including among pregnant women, do not equal the higher rates in childhood vaccine uptake, according to Kevin A. Ault, MD, and colleagues. Less than half of women (46.6%) receive influenza vaccination prior to and during pregnancy, for instance.4 Dr. Ault has identified the need for an “immunization champion”—someone who can manage one-on-one conversations with patients in the office setting to enhance the acceptance and uptake of adult and maternal vaccines.
OBG Management:
Dr. Kevin A. Ault, MD: The main thing a practice needs to do is to identify someone who is interested, and this person does not have to be a physician. In fact, he or she can frequently be a member of your nursing staff or office staff. And the word “champion” involves a lot of nuts and bolts: such details as how do you store the vaccine, how do you keep track of it, where are the vaccine information statements filed, where can the provider get more information if there is a question about contraindications? One person should organize all these details. The mechanics of vaccine administration are important as well, as the research shows that the more automated the process is, the better and more smoothly it is carried out. There is certainly a role for “standing orders” for adult vaccines.
OBG Management: What communication approach do you take with patients to enhance vaccination acceptance and uptake?
Dr. Ault: There are multiple research studies that show that provider recommendation is the most important way to get both nonpregnant and pregnant adults to receive vaccinations. Take the pertussis vaccine (the whooping cough booster) as an example. It is a relatively new vaccine recommendation during pregnancy. Your approach is relatively straightforward when explaining it to pregnant women. Make the point that we do not want your newborn to have whooping cough in those first few months of life before the newborn or infant vaccine becomes effective. Most people know they had a whooping cough, or pertussis, vaccine when they were younger, and the concept of the booster is well-known to patients. You should explain that the maternal antibodies pass through the placenta to the fetus, and they provide benefit for the first few months of life after birth.
The pertussis vaccine does not have all the “baggage” of the influenza vaccine. Talking with patients about the flu vaccine may present more challenges. Typically, each fall there is a popular press publication that explains “the 10, or 20, most common myths about influenza vaccine.” Every fall I try to find one of those articles, print it out, and even carry it in my jacket pocket and talk about all the myths. For example, there is a myth that “I always get sick when I get the flu shot.” Obstetricians should be giving patients an inactivated vaccine that does not contain any live flu virus. We should be able to explain to patients, your arm will be sore, and you may have some muscle aches, but you will not have the flu from your flu vaccine.
I think another reason that pregnant women do not always take the flu vaccine is that we do not yet have normalized influenza vaccination in the adult population. Women in their twenties and thirties are generally very healthy and have other concerns when they are pregnant, and they perhaps do not realize that they are more vulnerable to devastating effects of influenza while pregnant. Additionally, maternal influenza vaccination does protect the newborn from flu for the first few months. It is vital that those patients who are due during the dark winter months, when the flu is in season, get vaccinated.
Combat the myths and tell your patients the reasons for flu vaccination. Also tell them that you got your flu shot, like most health care professionals do every fall. You should be prepared to talk about safety. There are wonderful safety data, even some published in 2017 and 2018, about pertussis vaccine safety during pregnancy, and it is very reassuring to patients. For flu, the idea of vaccinating women against influenza has been around for decades, and so we have reliable information about that as well. Certainly, the risks are very minor, and the benefits are potentially huge for the pregnant woman and for the newborn.
OBG Management: When do you recommend that ObGyns administer the flu vaccine for pregnant women?
Dr. Ault: There are 2 issues to this question: when throughout the year and when during the pregnancy to administer the vaccine. First, you want to give the flu vaccine during the usual influenza season during the fall. As soon as the vaccine is available, you will recommend that pregnant women, even in their late pregnancy, get vaccinated so that their newborns who are 3 and 4 months old in the peak flu season are protected. The patients who deliver over the summer, who are coming in for their postpartum visit during the fall, should be getting vaccinated as well, because they are still vulnerable to influenza and pneumonia for several months postpartum.
If you have patients that come in for preconception visits, you could say: “Let’s get this out of the way. You could be pregnant by the time flu season really gets cranked up.”
Because we see patients 10 or 12 times during pregnancy, we certainly have plenty of opportunities to educate patients about and administer the flu vaccine. There are older data that demonstrate if patients do not get the flu vaccine done during early pregnancy, the opportunity may be lost. It is different now because there is more emphasis on vaccinating all adults. Your patients certainly can get their vaccine at the pharmacy or at their primary care doctor; however, delaying until later pregnancy usually means not getting the vaccine.
I would like to address one recent study from Donahue and colleagues that showed a potentially increased risk of miscarriage with flu vaccination.5 That study was an anomaly, as there are many other studies into the issue. Yes, there are not a lot of first trimester data, but there are other studies, including studies by the same authors, that did not find this to be the case.6-10
The 2017 study by Donahue and colleagues was an anomaly because the group of women they were vaccinating were already at high risk for miscarriage. The women were older, had diabetes, or a history of miscarriages. There is selection bias in the study because the pregnant women who were vaccinated were already at higher risk for miscarriage. The Centers for Disease Control and Prevention and the American College of Obstetricians and Gynecologists are not going to change any of their recommendations based on a single study that is different than our previous data.11
Current recommended adult (anyone over 18 years old) immunization schedule
ACOG Immunization Champions (ACOG members who have demonstrated exceptional progress in increasing immunization rates among adults and pregnant women in their communities through leadership, innovation, collaboration, and educational activities aimed at following ACOG and CDC guidance.)
Summary of Maternal Immunization Recommendations is a provider resource from ACOG and the Centers for Disease Control and Prevention.
Maternal Immunization Toolkit contains materials, including the Vaccines During Pregnancy Poster, to support ObGyns on recommending the influenza vaccine and the Tdap vaccine to all pregnant patients.
Influenza Immunization During Pregnancy Toolkit
CDC vaccine schedules app for health care providers
CDC Vaccine Information Statements (available for clinician or patient download)
In October 2018 the US Food and Drug Administration expanded the approved use of the human papillomavirus (HPV) vaccine (Gardasil 9) to adults aged 27 through 45.1 In June 2019, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP) voted to extend catch-up HPV vaccination to include all individuals through age 26 and to catch-up HPV vaccination, based on shared clinical decision making, for all adults aged 27 through 45.2 HPV viruses are associated with cervical cancer, as well as several other forms of cancer that affect both women and men. Approval for the expanded use of the HPV vaccine was based on data of the vaccine’s use in women.1,3
Unfortunately, adult immunization rates, including among pregnant women, do not equal the higher rates in childhood vaccine uptake, according to Kevin A. Ault, MD, and colleagues. Less than half of women (46.6%) receive influenza vaccination prior to and during pregnancy, for instance.4 Dr. Ault has identified the need for an “immunization champion”—someone who can manage one-on-one conversations with patients in the office setting to enhance the acceptance and uptake of adult and maternal vaccines.
OBG Management:
Dr. Kevin A. Ault, MD: The main thing a practice needs to do is to identify someone who is interested, and this person does not have to be a physician. In fact, he or she can frequently be a member of your nursing staff or office staff. And the word “champion” involves a lot of nuts and bolts: such details as how do you store the vaccine, how do you keep track of it, where are the vaccine information statements filed, where can the provider get more information if there is a question about contraindications? One person should organize all these details. The mechanics of vaccine administration are important as well, as the research shows that the more automated the process is, the better and more smoothly it is carried out. There is certainly a role for “standing orders” for adult vaccines.
OBG Management: What communication approach do you take with patients to enhance vaccination acceptance and uptake?
Dr. Ault: There are multiple research studies that show that provider recommendation is the most important way to get both nonpregnant and pregnant adults to receive vaccinations. Take the pertussis vaccine (the whooping cough booster) as an example. It is a relatively new vaccine recommendation during pregnancy. Your approach is relatively straightforward when explaining it to pregnant women. Make the point that we do not want your newborn to have whooping cough in those first few months of life before the newborn or infant vaccine becomes effective. Most people know they had a whooping cough, or pertussis, vaccine when they were younger, and the concept of the booster is well-known to patients. You should explain that the maternal antibodies pass through the placenta to the fetus, and they provide benefit for the first few months of life after birth.
The pertussis vaccine does not have all the “baggage” of the influenza vaccine. Talking with patients about the flu vaccine may present more challenges. Typically, each fall there is a popular press publication that explains “the 10, or 20, most common myths about influenza vaccine.” Every fall I try to find one of those articles, print it out, and even carry it in my jacket pocket and talk about all the myths. For example, there is a myth that “I always get sick when I get the flu shot.” Obstetricians should be giving patients an inactivated vaccine that does not contain any live flu virus. We should be able to explain to patients, your arm will be sore, and you may have some muscle aches, but you will not have the flu from your flu vaccine.
I think another reason that pregnant women do not always take the flu vaccine is that we do not yet have normalized influenza vaccination in the adult population. Women in their twenties and thirties are generally very healthy and have other concerns when they are pregnant, and they perhaps do not realize that they are more vulnerable to devastating effects of influenza while pregnant. Additionally, maternal influenza vaccination does protect the newborn from flu for the first few months. It is vital that those patients who are due during the dark winter months, when the flu is in season, get vaccinated.
Combat the myths and tell your patients the reasons for flu vaccination. Also tell them that you got your flu shot, like most health care professionals do every fall. You should be prepared to talk about safety. There are wonderful safety data, even some published in 2017 and 2018, about pertussis vaccine safety during pregnancy, and it is very reassuring to patients. For flu, the idea of vaccinating women against influenza has been around for decades, and so we have reliable information about that as well. Certainly, the risks are very minor, and the benefits are potentially huge for the pregnant woman and for the newborn.
OBG Management: When do you recommend that ObGyns administer the flu vaccine for pregnant women?
Dr. Ault: There are 2 issues to this question: when throughout the year and when during the pregnancy to administer the vaccine. First, you want to give the flu vaccine during the usual influenza season during the fall. As soon as the vaccine is available, you will recommend that pregnant women, even in their late pregnancy, get vaccinated so that their newborns who are 3 and 4 months old in the peak flu season are protected. The patients who deliver over the summer, who are coming in for their postpartum visit during the fall, should be getting vaccinated as well, because they are still vulnerable to influenza and pneumonia for several months postpartum.
If you have patients that come in for preconception visits, you could say: “Let’s get this out of the way. You could be pregnant by the time flu season really gets cranked up.”
Because we see patients 10 or 12 times during pregnancy, we certainly have plenty of opportunities to educate patients about and administer the flu vaccine. There are older data that demonstrate if patients do not get the flu vaccine done during early pregnancy, the opportunity may be lost. It is different now because there is more emphasis on vaccinating all adults. Your patients certainly can get their vaccine at the pharmacy or at their primary care doctor; however, delaying until later pregnancy usually means not getting the vaccine.
I would like to address one recent study from Donahue and colleagues that showed a potentially increased risk of miscarriage with flu vaccination.5 That study was an anomaly, as there are many other studies into the issue. Yes, there are not a lot of first trimester data, but there are other studies, including studies by the same authors, that did not find this to be the case.6-10
The 2017 study by Donahue and colleagues was an anomaly because the group of women they were vaccinating were already at high risk for miscarriage. The women were older, had diabetes, or a history of miscarriages. There is selection bias in the study because the pregnant women who were vaccinated were already at higher risk for miscarriage. The Centers for Disease Control and Prevention and the American College of Obstetricians and Gynecologists are not going to change any of their recommendations based on a single study that is different than our previous data.11
Current recommended adult (anyone over 18 years old) immunization schedule
ACOG Immunization Champions (ACOG members who have demonstrated exceptional progress in increasing immunization rates among adults and pregnant women in their communities through leadership, innovation, collaboration, and educational activities aimed at following ACOG and CDC guidance.)
Summary of Maternal Immunization Recommendations is a provider resource from ACOG and the Centers for Disease Control and Prevention.
Maternal Immunization Toolkit contains materials, including the Vaccines During Pregnancy Poster, to support ObGyns on recommending the influenza vaccine and the Tdap vaccine to all pregnant patients.
Influenza Immunization During Pregnancy Toolkit
CDC vaccine schedules app for health care providers
CDC Vaccine Information Statements (available for clinician or patient download)
- FDA approves expanded use of Gardasil 9 to include individuals 27 through 45 years old [press release]. Washington, DC: Food and Drug Administration; October 5, 2018.
- Color/Blue2. Splete H. ACIP extends HPV vaccine coverage. June 27, 2019. https://www.mdedge.com/obgyn/article/203656/vaccines/acip-extends-hpv-vaccine-coverage. Accessed July 5, 2019.
- Levy BS, Downs Jr L. The HPV vaccine is now recommended for adults aged 27–45: Counseling implications. OBG Manag. 2019;31(1):9-11.
- Frew PM, Randall LA, Malik F, et al. Clinician perspectives on strategies to improve patient maternal immunization acceptability in obstetrics and gynecology practice settings. Hum Vaccin Immunother. 2018;14(7):1548–1557.
- Donahue JG, Kieke BA, King JP, et al. Association of spontaneous abortion with receipt of inactivated influenza vaccine containing H1N1pdm09 in 2010-11 and 2011-12. Vaccine. 2017;35(40):5314-5322.
- Moro PL, Broder K, Zheteyeva Y, et al. Adverse events in pregnant women following administration of trivalent inactivated influenza vaccine and live attenuated influenza vaccine in the Vaccine Adverse Event Reporting System, 1990-2009. Am J Obstet Gynecol. 2011;204:146.e1-146.e7.
- Irving SA, Kieke BA, Donahue JG, et al; Vaccine Safety Datalink. Trivalent inactivated influenza vaccine and spontaneous abortion. 2013;121:159-165.
- Kharbanda EO, Vazquez-Benitez G, Lipkind H, et al; Vaccine Safety Datalink Team. Inactivated influenza vaccine during pregnancy and risks for adverse obstetric events. Obstet Gynecol. 2013;122:659-667.
- Nordin JD, Kharbanda EO, Vazquez-Benitez G, et al; Vaccine Safety Datalink. Maternal Influenza vaccine and risks for preterm or small for gestational age birth. J Pediatrics. 2014;164:1051-1057.e2.
- Kharbanda EO, Vazquez-Benitez G, Romitti PA, et al; Vaccine Safety Datalink. First trimester influenza vaccination and risks for major structural birth defects in offspring. 2017;187:234-239.e4.
- Flu vaccination and possible safety signal. CDC website. https://www.cdc.gov/flu/professionals/vaccination/vaccination-possible-safety-signal.html. Last reviewed September 13, 2017. Accessed May 15, 2019.
- FDA approves expanded use of Gardasil 9 to include individuals 27 through 45 years old [press release]. Washington, DC: Food and Drug Administration; October 5, 2018.
- Color/Blue2. Splete H. ACIP extends HPV vaccine coverage. June 27, 2019. https://www.mdedge.com/obgyn/article/203656/vaccines/acip-extends-hpv-vaccine-coverage. Accessed July 5, 2019.
- Levy BS, Downs Jr L. The HPV vaccine is now recommended for adults aged 27–45: Counseling implications. OBG Manag. 2019;31(1):9-11.
- Frew PM, Randall LA, Malik F, et al. Clinician perspectives on strategies to improve patient maternal immunization acceptability in obstetrics and gynecology practice settings. Hum Vaccin Immunother. 2018;14(7):1548–1557.
- Donahue JG, Kieke BA, King JP, et al. Association of spontaneous abortion with receipt of inactivated influenza vaccine containing H1N1pdm09 in 2010-11 and 2011-12. Vaccine. 2017;35(40):5314-5322.
- Moro PL, Broder K, Zheteyeva Y, et al. Adverse events in pregnant women following administration of trivalent inactivated influenza vaccine and live attenuated influenza vaccine in the Vaccine Adverse Event Reporting System, 1990-2009. Am J Obstet Gynecol. 2011;204:146.e1-146.e7.
- Irving SA, Kieke BA, Donahue JG, et al; Vaccine Safety Datalink. Trivalent inactivated influenza vaccine and spontaneous abortion. 2013;121:159-165.
- Kharbanda EO, Vazquez-Benitez G, Lipkind H, et al; Vaccine Safety Datalink Team. Inactivated influenza vaccine during pregnancy and risks for adverse obstetric events. Obstet Gynecol. 2013;122:659-667.
- Nordin JD, Kharbanda EO, Vazquez-Benitez G, et al; Vaccine Safety Datalink. Maternal Influenza vaccine and risks for preterm or small for gestational age birth. J Pediatrics. 2014;164:1051-1057.e2.
- Kharbanda EO, Vazquez-Benitez G, Romitti PA, et al; Vaccine Safety Datalink. First trimester influenza vaccination and risks for major structural birth defects in offspring. 2017;187:234-239.e4.
- Flu vaccination and possible safety signal. CDC website. https://www.cdc.gov/flu/professionals/vaccination/vaccination-possible-safety-signal.html. Last reviewed September 13, 2017. Accessed May 15, 2019.