User login
Trial follow-up spotlights survival gains in follicular lymphoma
Results from a 13-year follow-up of a trial comparing two types of rituximab-based chemotherapy as upfront treatment for patients with follicular lymphoma show an “extraordinary improvement” in overall survival, compared with the prerituximab era.
In a finding that the researchers termed “somewhat unexpected,” complete remission was the strongest factor in long-term survival.
Between March 2000 and May 2005, researchers enrolled 136 high-risk follicular lymphoma patients in the GITMO-IIL trial to evaluate the superiority of high-dose chemotherapy with rituximab and autograft (R-HDS) versus conventional chemotherapy with rituximab (R-CHOP) as frontline therapy. At a median follow-up of 4 years, there was no survival advantage for R-HDS.
In the current analysis – that had a 13-year median follow-up – median survival had not yet been reached. As of February 2017, two-thirds of all patients were alive at their last follow-up. Overall survival was 68.5% in the R-CHOP arm and 64.5% in the R-HDS arm, Riccardo Bruna, MD, of the European Institute of Oncology in Milano, Italy, and his colleagues, reported in Haematologica.
The main causes of death for the 46 patients who had died as of long-term follow-up were disease progression and secondary malignancies. Other causes included cardiovascular events, infections, graft failure following autograft, anaphylactic shock, and late sudden death.
Complete remission was seen in 98 patients (73.1%) overall – 59.1% in the R-CHOP arm and 86.7% in the R-HDS arm. Achieving durable complete remission was associated with prolonged survival, the researchers reported. Of the 79 patients in complete remission at 2 years after the start of treatment, 65 (82.3%) were alive at 13 years, compared with 21 (58.3%) of 36 patients who had an early relapse (P = .003).
“[Complete remission] achievement was the most important factor for prolonged survival,” the researchers wrote. “The importance of disease response is further emphasized by the first-time observation that [molecular remission] achievement is associated with survival duration and a high proportion of patients had prolonged survival in the absence of disease recurrence.”
The researchers reported that this is longest ever follow-up reported for first-line treatment of follicular lymphoma with rituximab-based chemotherapy.
This work was supported in part by the Ministero Italiano Università e Ricerca and by Banca del Piemonte. The initial clinical trial was supported by Compagnia di San Paolo, Regione Piemonte, and Roche, which provided rituximab for the study.
SOURCE: Bruna R et al. Haematologica. 2019 Apr 11. doi: 10.3324/haematol.2018.209932.
Results from a 13-year follow-up of a trial comparing two types of rituximab-based chemotherapy as upfront treatment for patients with follicular lymphoma show an “extraordinary improvement” in overall survival, compared with the prerituximab era.
In a finding that the researchers termed “somewhat unexpected,” complete remission was the strongest factor in long-term survival.
Between March 2000 and May 2005, researchers enrolled 136 high-risk follicular lymphoma patients in the GITMO-IIL trial to evaluate the superiority of high-dose chemotherapy with rituximab and autograft (R-HDS) versus conventional chemotherapy with rituximab (R-CHOP) as frontline therapy. At a median follow-up of 4 years, there was no survival advantage for R-HDS.
In the current analysis – that had a 13-year median follow-up – median survival had not yet been reached. As of February 2017, two-thirds of all patients were alive at their last follow-up. Overall survival was 68.5% in the R-CHOP arm and 64.5% in the R-HDS arm, Riccardo Bruna, MD, of the European Institute of Oncology in Milano, Italy, and his colleagues, reported in Haematologica.
The main causes of death for the 46 patients who had died as of long-term follow-up were disease progression and secondary malignancies. Other causes included cardiovascular events, infections, graft failure following autograft, anaphylactic shock, and late sudden death.
Complete remission was seen in 98 patients (73.1%) overall – 59.1% in the R-CHOP arm and 86.7% in the R-HDS arm. Achieving durable complete remission was associated with prolonged survival, the researchers reported. Of the 79 patients in complete remission at 2 years after the start of treatment, 65 (82.3%) were alive at 13 years, compared with 21 (58.3%) of 36 patients who had an early relapse (P = .003).
“[Complete remission] achievement was the most important factor for prolonged survival,” the researchers wrote. “The importance of disease response is further emphasized by the first-time observation that [molecular remission] achievement is associated with survival duration and a high proportion of patients had prolonged survival in the absence of disease recurrence.”
The researchers reported that this is longest ever follow-up reported for first-line treatment of follicular lymphoma with rituximab-based chemotherapy.
This work was supported in part by the Ministero Italiano Università e Ricerca and by Banca del Piemonte. The initial clinical trial was supported by Compagnia di San Paolo, Regione Piemonte, and Roche, which provided rituximab for the study.
SOURCE: Bruna R et al. Haematologica. 2019 Apr 11. doi: 10.3324/haematol.2018.209932.
Results from a 13-year follow-up of a trial comparing two types of rituximab-based chemotherapy as upfront treatment for patients with follicular lymphoma show an “extraordinary improvement” in overall survival, compared with the prerituximab era.
In a finding that the researchers termed “somewhat unexpected,” complete remission was the strongest factor in long-term survival.
Between March 2000 and May 2005, researchers enrolled 136 high-risk follicular lymphoma patients in the GITMO-IIL trial to evaluate the superiority of high-dose chemotherapy with rituximab and autograft (R-HDS) versus conventional chemotherapy with rituximab (R-CHOP) as frontline therapy. At a median follow-up of 4 years, there was no survival advantage for R-HDS.
In the current analysis – that had a 13-year median follow-up – median survival had not yet been reached. As of February 2017, two-thirds of all patients were alive at their last follow-up. Overall survival was 68.5% in the R-CHOP arm and 64.5% in the R-HDS arm, Riccardo Bruna, MD, of the European Institute of Oncology in Milano, Italy, and his colleagues, reported in Haematologica.
The main causes of death for the 46 patients who had died as of long-term follow-up were disease progression and secondary malignancies. Other causes included cardiovascular events, infections, graft failure following autograft, anaphylactic shock, and late sudden death.
Complete remission was seen in 98 patients (73.1%) overall – 59.1% in the R-CHOP arm and 86.7% in the R-HDS arm. Achieving durable complete remission was associated with prolonged survival, the researchers reported. Of the 79 patients in complete remission at 2 years after the start of treatment, 65 (82.3%) were alive at 13 years, compared with 21 (58.3%) of 36 patients who had an early relapse (P = .003).
“[Complete remission] achievement was the most important factor for prolonged survival,” the researchers wrote. “The importance of disease response is further emphasized by the first-time observation that [molecular remission] achievement is associated with survival duration and a high proportion of patients had prolonged survival in the absence of disease recurrence.”
The researchers reported that this is longest ever follow-up reported for first-line treatment of follicular lymphoma with rituximab-based chemotherapy.
This work was supported in part by the Ministero Italiano Università e Ricerca and by Banca del Piemonte. The initial clinical trial was supported by Compagnia di San Paolo, Regione Piemonte, and Roche, which provided rituximab for the study.
SOURCE: Bruna R et al. Haematologica. 2019 Apr 11. doi: 10.3324/haematol.2018.209932.
FROM HAEMATOLOGICA
Antiabortion measures may lead to Supreme Court showdown
The debate over abortion rights could be headed to the U.S. Supreme Court after two recent state measures that aim to restrict access to abortion.
On May 7, 2019, Georgia Gov. Brian Kemp (R) signed into law a statute that bars physicians from performing an abortion after a heartbeat is detected – usually at about 6 weeks of pregnancy. The law allows exceptions if the pregnancy poses death or serious harm to the woman, and in cases of rape or incest.
A week later, the Alabama Senate on May 14 approved a measure that would ban abortion at every pregnancy stage and penalize physicians with a Class A felony for performing an abortion and charge them with a Class C felony for attempting to perform an abortion. The Alabama bill includes an exception if a woman’s life is at risk, but not for cases of rape or incest. Alabama Gov. Kay Ivey (R) signed the bill into law on May 15.
Both measures will likely be challenged in court; the Alabama law, in particular, could land in front of the U.S. Supreme Court as a direct challenge to Roe v. Wade.
Abortion critics have been encouraged by the Supreme Court appointment of right-leaning Associate Justice Brett M. Kavanaugh and hope the Alabama measure will drive the Supreme Court to reconsider its central holding in Roe, according to Fatima Goss Graves, president of the National Women’s Law Center, a liberal-leaning legal firm that advocates gender equality.
“After decades of chipping away at Roe, antiabortion legislators and advocates are feeling emboldened with the addition of Justice Kavanaugh on the Supreme Court and they are exposing their true goal – to ban abortion altogether,” Ms. Graves said in a statement. “And make no mistake, [passage of the Alabama bill] is extreme overreach: The vast majority of people in this country do not support criminalizing women or doctors and want abortion to be safe and legal.”
National antiabortion group Susan B. Anthony List called Alabama’s measure a landmark victory and indicated that the bill passage may ultimately change the outcome of Roe v. Wade.
“Across the nation there is growing momentum, informed by science and compassion, and spurred on in reaction to abortion extremism in New York and Virginia, to recognize the humanity of the unborn child in the law,” Susan B. Anthony List President Marjorie Dannenfelser said in a statement. “It is clearer than ever that Roe is far from being settled law in the eyes and hearts of the American people, and this is increasingly reflected in state legislatures. ... The time is coming for the Supreme Court to let that debate go forward.”
Other states have recently passed laws similar to Georgia’s measure, dubbed the “fetal heartbeat bills,” including Kentucky, Mississippi, and Ohio.
On May 15, the American Civil Liberties Union and Planned Parenthood of Greater Ohio issued a joint legal challenge against Ohio’s law, calling it blatantly unconstitutional. Meanwhile, the Center for Reproductive Rights has pledged to sue Georgia over its law.
Ted Anderson, MD, president of the American College of Obstetricians and Gynecologists, said the many recent restrictions to abortion access across the country are harmful to women’s health and detrimental to the physician-patient relationship.
“Lawmakers must support health policies based on sound science and evidence. Politicians must seek to improve access to care, not restrict it,” Dr. Anderson said in a statement. “Legislative restrictions fundamentally interfere with the patient-provider relationship and decrease access to necessary care for all women, and particularly for low-income women and those living long distances from health care providers. Health care decisions should be made jointly only by patients and their trusted health care professionals, not by politicians.”
The debate over abortion rights could be headed to the U.S. Supreme Court after two recent state measures that aim to restrict access to abortion.
On May 7, 2019, Georgia Gov. Brian Kemp (R) signed into law a statute that bars physicians from performing an abortion after a heartbeat is detected – usually at about 6 weeks of pregnancy. The law allows exceptions if the pregnancy poses death or serious harm to the woman, and in cases of rape or incest.
A week later, the Alabama Senate on May 14 approved a measure that would ban abortion at every pregnancy stage and penalize physicians with a Class A felony for performing an abortion and charge them with a Class C felony for attempting to perform an abortion. The Alabama bill includes an exception if a woman’s life is at risk, but not for cases of rape or incest. Alabama Gov. Kay Ivey (R) signed the bill into law on May 15.
Both measures will likely be challenged in court; the Alabama law, in particular, could land in front of the U.S. Supreme Court as a direct challenge to Roe v. Wade.
Abortion critics have been encouraged by the Supreme Court appointment of right-leaning Associate Justice Brett M. Kavanaugh and hope the Alabama measure will drive the Supreme Court to reconsider its central holding in Roe, according to Fatima Goss Graves, president of the National Women’s Law Center, a liberal-leaning legal firm that advocates gender equality.
“After decades of chipping away at Roe, antiabortion legislators and advocates are feeling emboldened with the addition of Justice Kavanaugh on the Supreme Court and they are exposing their true goal – to ban abortion altogether,” Ms. Graves said in a statement. “And make no mistake, [passage of the Alabama bill] is extreme overreach: The vast majority of people in this country do not support criminalizing women or doctors and want abortion to be safe and legal.”
National antiabortion group Susan B. Anthony List called Alabama’s measure a landmark victory and indicated that the bill passage may ultimately change the outcome of Roe v. Wade.
“Across the nation there is growing momentum, informed by science and compassion, and spurred on in reaction to abortion extremism in New York and Virginia, to recognize the humanity of the unborn child in the law,” Susan B. Anthony List President Marjorie Dannenfelser said in a statement. “It is clearer than ever that Roe is far from being settled law in the eyes and hearts of the American people, and this is increasingly reflected in state legislatures. ... The time is coming for the Supreme Court to let that debate go forward.”
Other states have recently passed laws similar to Georgia’s measure, dubbed the “fetal heartbeat bills,” including Kentucky, Mississippi, and Ohio.
On May 15, the American Civil Liberties Union and Planned Parenthood of Greater Ohio issued a joint legal challenge against Ohio’s law, calling it blatantly unconstitutional. Meanwhile, the Center for Reproductive Rights has pledged to sue Georgia over its law.
Ted Anderson, MD, president of the American College of Obstetricians and Gynecologists, said the many recent restrictions to abortion access across the country are harmful to women’s health and detrimental to the physician-patient relationship.
“Lawmakers must support health policies based on sound science and evidence. Politicians must seek to improve access to care, not restrict it,” Dr. Anderson said in a statement. “Legislative restrictions fundamentally interfere with the patient-provider relationship and decrease access to necessary care for all women, and particularly for low-income women and those living long distances from health care providers. Health care decisions should be made jointly only by patients and their trusted health care professionals, not by politicians.”
The debate over abortion rights could be headed to the U.S. Supreme Court after two recent state measures that aim to restrict access to abortion.
On May 7, 2019, Georgia Gov. Brian Kemp (R) signed into law a statute that bars physicians from performing an abortion after a heartbeat is detected – usually at about 6 weeks of pregnancy. The law allows exceptions if the pregnancy poses death or serious harm to the woman, and in cases of rape or incest.
A week later, the Alabama Senate on May 14 approved a measure that would ban abortion at every pregnancy stage and penalize physicians with a Class A felony for performing an abortion and charge them with a Class C felony for attempting to perform an abortion. The Alabama bill includes an exception if a woman’s life is at risk, but not for cases of rape or incest. Alabama Gov. Kay Ivey (R) signed the bill into law on May 15.
Both measures will likely be challenged in court; the Alabama law, in particular, could land in front of the U.S. Supreme Court as a direct challenge to Roe v. Wade.
Abortion critics have been encouraged by the Supreme Court appointment of right-leaning Associate Justice Brett M. Kavanaugh and hope the Alabama measure will drive the Supreme Court to reconsider its central holding in Roe, according to Fatima Goss Graves, president of the National Women’s Law Center, a liberal-leaning legal firm that advocates gender equality.
“After decades of chipping away at Roe, antiabortion legislators and advocates are feeling emboldened with the addition of Justice Kavanaugh on the Supreme Court and they are exposing their true goal – to ban abortion altogether,” Ms. Graves said in a statement. “And make no mistake, [passage of the Alabama bill] is extreme overreach: The vast majority of people in this country do not support criminalizing women or doctors and want abortion to be safe and legal.”
National antiabortion group Susan B. Anthony List called Alabama’s measure a landmark victory and indicated that the bill passage may ultimately change the outcome of Roe v. Wade.
“Across the nation there is growing momentum, informed by science and compassion, and spurred on in reaction to abortion extremism in New York and Virginia, to recognize the humanity of the unborn child in the law,” Susan B. Anthony List President Marjorie Dannenfelser said in a statement. “It is clearer than ever that Roe is far from being settled law in the eyes and hearts of the American people, and this is increasingly reflected in state legislatures. ... The time is coming for the Supreme Court to let that debate go forward.”
Other states have recently passed laws similar to Georgia’s measure, dubbed the “fetal heartbeat bills,” including Kentucky, Mississippi, and Ohio.
On May 15, the American Civil Liberties Union and Planned Parenthood of Greater Ohio issued a joint legal challenge against Ohio’s law, calling it blatantly unconstitutional. Meanwhile, the Center for Reproductive Rights has pledged to sue Georgia over its law.
Ted Anderson, MD, president of the American College of Obstetricians and Gynecologists, said the many recent restrictions to abortion access across the country are harmful to women’s health and detrimental to the physician-patient relationship.
“Lawmakers must support health policies based on sound science and evidence. Politicians must seek to improve access to care, not restrict it,” Dr. Anderson said in a statement. “Legislative restrictions fundamentally interfere with the patient-provider relationship and decrease access to necessary care for all women, and particularly for low-income women and those living long distances from health care providers. Health care decisions should be made jointly only by patients and their trusted health care professionals, not by politicians.”
Minimize iatrogenic neonatal abstinence syndrome
BALTIMORE – Some infants, especially among those with persistent pulmonary hypertension, are at risk for developing iatrogenic neonatal abstinence syndrome, according to Amber Dave, MD, a neonatal-perinatal medicine fellow at Georgetown University Hospital in Washington.
Of 70 infants administered morphine or fentanyl for longer than a day in the neonatal ICU, almost a third (22, or 31%) developed iatrogenic neonatal abstinence syndrome (INAS). As a result, they needed prolonged respiratory support, more time to reach full feeds, and extended lengths of stay. Children exposed to opioids before birth were excluded from the analysis.
The greatest risk was in infants with persistent pulmonary hypertension; INAS was diagnosed in 13 of 22 (57%).
Opioid dosing also was all over the map for a given Neonatal Pain, Agitation, and Sedation Scale (N-PASS) score, Dr. Dave said. Some infants with an N-PASS pain score of 2, for instance, received no opioids, while others received up to 1,500 mg/kg morphine equivalents.
N-PASS is used in NICUs nationwide to guide dosing, but the variability seen in the study suggests that there’s need for a more objective measure of neonatal distress and for neonatologists to establish ground rules for NICU opioid use, she added.
The use of opioids has been increasing in NICUs for years (J Opioid Manag. 2015 Jul-Aug;11[4]:305-12), and at least one institution (J Perinatol. 2017 Sep;37[9]:1038-42) already has established guidelines to curb overuse. Dr. Dave said that several neonatologists, after viewing her poster at the Pediatric Academic Societies annual meeting, told her that they probably had the same problem at their NICUs but had not examined their data.
“We are using” these medications more in the NICU, “but how much is too much? We need to find that balance. We need to improve our practice.”
“The overarching question is if there are better alternatives for treating pain and stress in critically ill neonates.” Dexmedetomidine, an opioid-sparing alpha-2 agonist adrenoreceptor sedative, analgesic, and anxiolytic, is one of several options “being looked at closely in this population. We also need to think of nonpharmacologic measures,” Dr. Dave said.
In addition to infants with persistent pulmonary hypertension, the 22 INAS cases at the study site included, among others, three children on extracorporeal membrane oxygenation, one with meconium aspiration syndrome, and one surgical case, out of the 15 included in the study. The common denominator was the need to keep infants calm and comfortable during prolonged intubation, which was a mean of 10.5 days among INAS infants versus 5 among children who didn’t go into opioid withdrawal.
INAS infants had a daily mean morphine-equivalent dose of 106.6 mg/kg, with a mean exposure of 17 days and mean cumulative dose of 1,515 mg/kg. The daily mean morphine-equivalent dose among infants who didn’t develop INAS was 42.4 mg/kg, with a mean exposure of 4 days and mean cumulative dose of 246 mg/kg.
INAS infants spent a mean of 27 days in the hospital, and it took them a mean of almost 6 days to reach full feeds, versus 15 days for the other infants full feeds by day 4. Over half of the INAS infants (12) also were on midazolam, and they had higher cumulative doses of the sedative than infants who didn’t develop INAS (mean, 2.64 mg/kg vs. 0.19 mg/kg). The findings all were statistically significant.
Dr. Dave said the most surprising finding was the variability in opioid dosing. In another example, some infants received up to 1,400 mg/kg morphine equivalents even when their fraction of inspired oxygen requirement fell below 60%, which meant that they were getting better. Other infants by that point were off opioids altogether.
“This has definitely brought awareness to my practice. Before I would say, ‘Okay, let’s just go up,’ ” when a nurse requested an opioid increase based on N-PASS scores. Now, “I try to really figure out why they think the baby needs an increase, and I may say ‘Actually, we are turning a corner now, and maybe the baby can be a little bit more awake. How do you feel about that?’ ” she said.
“My long-term goal for this project is putting some guidelines in place,” she said.
There was no industry funding for the work, and Dr. Dave didn’t have any disclosures.
BALTIMORE – Some infants, especially among those with persistent pulmonary hypertension, are at risk for developing iatrogenic neonatal abstinence syndrome, according to Amber Dave, MD, a neonatal-perinatal medicine fellow at Georgetown University Hospital in Washington.
Of 70 infants administered morphine or fentanyl for longer than a day in the neonatal ICU, almost a third (22, or 31%) developed iatrogenic neonatal abstinence syndrome (INAS). As a result, they needed prolonged respiratory support, more time to reach full feeds, and extended lengths of stay. Children exposed to opioids before birth were excluded from the analysis.
The greatest risk was in infants with persistent pulmonary hypertension; INAS was diagnosed in 13 of 22 (57%).
Opioid dosing also was all over the map for a given Neonatal Pain, Agitation, and Sedation Scale (N-PASS) score, Dr. Dave said. Some infants with an N-PASS pain score of 2, for instance, received no opioids, while others received up to 1,500 mg/kg morphine equivalents.
N-PASS is used in NICUs nationwide to guide dosing, but the variability seen in the study suggests that there’s need for a more objective measure of neonatal distress and for neonatologists to establish ground rules for NICU opioid use, she added.
The use of opioids has been increasing in NICUs for years (J Opioid Manag. 2015 Jul-Aug;11[4]:305-12), and at least one institution (J Perinatol. 2017 Sep;37[9]:1038-42) already has established guidelines to curb overuse. Dr. Dave said that several neonatologists, after viewing her poster at the Pediatric Academic Societies annual meeting, told her that they probably had the same problem at their NICUs but had not examined their data.
“We are using” these medications more in the NICU, “but how much is too much? We need to find that balance. We need to improve our practice.”
“The overarching question is if there are better alternatives for treating pain and stress in critically ill neonates.” Dexmedetomidine, an opioid-sparing alpha-2 agonist adrenoreceptor sedative, analgesic, and anxiolytic, is one of several options “being looked at closely in this population. We also need to think of nonpharmacologic measures,” Dr. Dave said.
In addition to infants with persistent pulmonary hypertension, the 22 INAS cases at the study site included, among others, three children on extracorporeal membrane oxygenation, one with meconium aspiration syndrome, and one surgical case, out of the 15 included in the study. The common denominator was the need to keep infants calm and comfortable during prolonged intubation, which was a mean of 10.5 days among INAS infants versus 5 among children who didn’t go into opioid withdrawal.
INAS infants had a daily mean morphine-equivalent dose of 106.6 mg/kg, with a mean exposure of 17 days and mean cumulative dose of 1,515 mg/kg. The daily mean morphine-equivalent dose among infants who didn’t develop INAS was 42.4 mg/kg, with a mean exposure of 4 days and mean cumulative dose of 246 mg/kg.
INAS infants spent a mean of 27 days in the hospital, and it took them a mean of almost 6 days to reach full feeds, versus 15 days for the other infants full feeds by day 4. Over half of the INAS infants (12) also were on midazolam, and they had higher cumulative doses of the sedative than infants who didn’t develop INAS (mean, 2.64 mg/kg vs. 0.19 mg/kg). The findings all were statistically significant.
Dr. Dave said the most surprising finding was the variability in opioid dosing. In another example, some infants received up to 1,400 mg/kg morphine equivalents even when their fraction of inspired oxygen requirement fell below 60%, which meant that they were getting better. Other infants by that point were off opioids altogether.
“This has definitely brought awareness to my practice. Before I would say, ‘Okay, let’s just go up,’ ” when a nurse requested an opioid increase based on N-PASS scores. Now, “I try to really figure out why they think the baby needs an increase, and I may say ‘Actually, we are turning a corner now, and maybe the baby can be a little bit more awake. How do you feel about that?’ ” she said.
“My long-term goal for this project is putting some guidelines in place,” she said.
There was no industry funding for the work, and Dr. Dave didn’t have any disclosures.
BALTIMORE – Some infants, especially among those with persistent pulmonary hypertension, are at risk for developing iatrogenic neonatal abstinence syndrome, according to Amber Dave, MD, a neonatal-perinatal medicine fellow at Georgetown University Hospital in Washington.
Of 70 infants administered morphine or fentanyl for longer than a day in the neonatal ICU, almost a third (22, or 31%) developed iatrogenic neonatal abstinence syndrome (INAS). As a result, they needed prolonged respiratory support, more time to reach full feeds, and extended lengths of stay. Children exposed to opioids before birth were excluded from the analysis.
The greatest risk was in infants with persistent pulmonary hypertension; INAS was diagnosed in 13 of 22 (57%).
Opioid dosing also was all over the map for a given Neonatal Pain, Agitation, and Sedation Scale (N-PASS) score, Dr. Dave said. Some infants with an N-PASS pain score of 2, for instance, received no opioids, while others received up to 1,500 mg/kg morphine equivalents.
N-PASS is used in NICUs nationwide to guide dosing, but the variability seen in the study suggests that there’s need for a more objective measure of neonatal distress and for neonatologists to establish ground rules for NICU opioid use, she added.
The use of opioids has been increasing in NICUs for years (J Opioid Manag. 2015 Jul-Aug;11[4]:305-12), and at least one institution (J Perinatol. 2017 Sep;37[9]:1038-42) already has established guidelines to curb overuse. Dr. Dave said that several neonatologists, after viewing her poster at the Pediatric Academic Societies annual meeting, told her that they probably had the same problem at their NICUs but had not examined their data.
“We are using” these medications more in the NICU, “but how much is too much? We need to find that balance. We need to improve our practice.”
“The overarching question is if there are better alternatives for treating pain and stress in critically ill neonates.” Dexmedetomidine, an opioid-sparing alpha-2 agonist adrenoreceptor sedative, analgesic, and anxiolytic, is one of several options “being looked at closely in this population. We also need to think of nonpharmacologic measures,” Dr. Dave said.
In addition to infants with persistent pulmonary hypertension, the 22 INAS cases at the study site included, among others, three children on extracorporeal membrane oxygenation, one with meconium aspiration syndrome, and one surgical case, out of the 15 included in the study. The common denominator was the need to keep infants calm and comfortable during prolonged intubation, which was a mean of 10.5 days among INAS infants versus 5 among children who didn’t go into opioid withdrawal.
INAS infants had a daily mean morphine-equivalent dose of 106.6 mg/kg, with a mean exposure of 17 days and mean cumulative dose of 1,515 mg/kg. The daily mean morphine-equivalent dose among infants who didn’t develop INAS was 42.4 mg/kg, with a mean exposure of 4 days and mean cumulative dose of 246 mg/kg.
INAS infants spent a mean of 27 days in the hospital, and it took them a mean of almost 6 days to reach full feeds, versus 15 days for the other infants full feeds by day 4. Over half of the INAS infants (12) also were on midazolam, and they had higher cumulative doses of the sedative than infants who didn’t develop INAS (mean, 2.64 mg/kg vs. 0.19 mg/kg). The findings all were statistically significant.
Dr. Dave said the most surprising finding was the variability in opioid dosing. In another example, some infants received up to 1,400 mg/kg morphine equivalents even when their fraction of inspired oxygen requirement fell below 60%, which meant that they were getting better. Other infants by that point were off opioids altogether.
“This has definitely brought awareness to my practice. Before I would say, ‘Okay, let’s just go up,’ ” when a nurse requested an opioid increase based on N-PASS scores. Now, “I try to really figure out why they think the baby needs an increase, and I may say ‘Actually, we are turning a corner now, and maybe the baby can be a little bit more awake. How do you feel about that?’ ” she said.
“My long-term goal for this project is putting some guidelines in place,” she said.
There was no industry funding for the work, and Dr. Dave didn’t have any disclosures.
REPORTING FROM PAS 2019
Key clinical point: Some infants in the NICU, especially those with persistent pulmonary hypertension, are at risk for iatrogenic neonatal abstinence syndrome.
Major finding: Of 70 infants administered morphine or fentanyl for longer than a day, almost a third (22) developed iatrogenic neonatal abstinence syndrome.
Study details: Single-center NICU chart review.
Disclosures: There was no industry funding, and the lead investigator didn’t have any relevant financial disclosures.
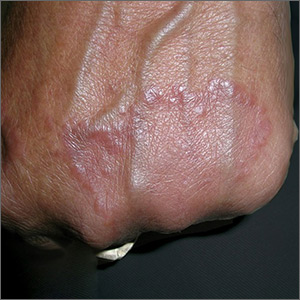
Raised lesion on hand
The FP diagnosed granuloma annulare (GA) in this patient, based on the typical clinical appearance of a raised ring on the back of the hand with no scale.
GA is a common benign cutaneous, inflammatory disorder of unknown origin. It affects twice as many women as men. It features annular lesions that have raised borders and are skin-colored to erythematous. The rings may become hyperpigmented and often feature a central depression. These lesions are typically 1 to 5 cm wide. Although the classical appearance of GA is annular, the rings may not always be complete. Most importantly, there is no scaling, which one would expect to see in tinea infections, also known as “ringworm.”
The most common form of granuloma annulare is localized, as it was in this case. It typically presents on the dorsal surfaces of extremities, especially of the hands and feet. When the presentation is classic, there is no need for a biopsy to confirm the diagnosis.
The most effective treatment for local disease is intralesional triamcinolone (5mg/mL). (See Watch & Learn: Intralesional injections.) The FP offered this treatment to the patient, and she tolerated the procedure well. The GA resolved over the weeks that followed with some faint hypopigmentation that lasted for several months. The patient was happy with the results.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mauskar M, Usatine R. Granuloma annulare. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1141-1146.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com
The FP diagnosed granuloma annulare (GA) in this patient, based on the typical clinical appearance of a raised ring on the back of the hand with no scale.
GA is a common benign cutaneous, inflammatory disorder of unknown origin. It affects twice as many women as men. It features annular lesions that have raised borders and are skin-colored to erythematous. The rings may become hyperpigmented and often feature a central depression. These lesions are typically 1 to 5 cm wide. Although the classical appearance of GA is annular, the rings may not always be complete. Most importantly, there is no scaling, which one would expect to see in tinea infections, also known as “ringworm.”
The most common form of granuloma annulare is localized, as it was in this case. It typically presents on the dorsal surfaces of extremities, especially of the hands and feet. When the presentation is classic, there is no need for a biopsy to confirm the diagnosis.
The most effective treatment for local disease is intralesional triamcinolone (5mg/mL). (See Watch & Learn: Intralesional injections.) The FP offered this treatment to the patient, and she tolerated the procedure well. The GA resolved over the weeks that followed with some faint hypopigmentation that lasted for several months. The patient was happy with the results.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mauskar M, Usatine R. Granuloma annulare. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1141-1146.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com
The FP diagnosed granuloma annulare (GA) in this patient, based on the typical clinical appearance of a raised ring on the back of the hand with no scale.
GA is a common benign cutaneous, inflammatory disorder of unknown origin. It affects twice as many women as men. It features annular lesions that have raised borders and are skin-colored to erythematous. The rings may become hyperpigmented and often feature a central depression. These lesions are typically 1 to 5 cm wide. Although the classical appearance of GA is annular, the rings may not always be complete. Most importantly, there is no scaling, which one would expect to see in tinea infections, also known as “ringworm.”
The most common form of granuloma annulare is localized, as it was in this case. It typically presents on the dorsal surfaces of extremities, especially of the hands and feet. When the presentation is classic, there is no need for a biopsy to confirm the diagnosis.
The most effective treatment for local disease is intralesional triamcinolone (5mg/mL). (See Watch & Learn: Intralesional injections.) The FP offered this treatment to the patient, and she tolerated the procedure well. The GA resolved over the weeks that followed with some faint hypopigmentation that lasted for several months. The patient was happy with the results.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mauskar M, Usatine R. Granuloma annulare. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1141-1146.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com
More than 40% of U.K. physicians report binge drinking
Many doctors say they cope with job-related stress by drinking alcohol or taking drugs
Occupational distress among physicians is tied to increased odds of substance use, sleep disturbance, binge eating, and poor health in general, a cross-sectional study of 417 U.K. doctors shows.
Burned-out or depressed doctors had higher risks of those health problems regardless of whether or not they worked in a hospital setting, according to Asta Medisauskaite, PhD, of University College London and Caroline Kamau, PhD, of the University of London. The study was published in BMJ Open.
The investigators asked the participants to answer a battery of validated questionnaires online, including the Alcohol Use Disorders Identification Test, the Eating Disorder Diagnostic Scale, and the Insomnia Severity Index.
The odds of many health problems were increased among the physicians with emotional exhaustion, as indicated by the Maslach Burnout Inventory, as was also the case with psychiatric disorders, according to the General Health Questionnaire-12, which investigators noted has been used extensively to examine medical doctors and other working populations.
Sleep disturbances were, for example, more likely in physicians with burnout or psychiatric morbidity, with odds ratios ranging from 1.344 to 3.826, the investigators reported. Likewise, these indicators of occupational distress increased the risk of suffering from frequent poor health, with odds ratios from 1.050 to 3.544, and of binge eating, with odds ratios from 1.311 to 1.841, Dr. Medisauskaite and Dr. Kamau reported.
Distressed doctors more often used alcohol, according to the researchers, who said that they found a higher risk of alcohol dependence (odds ratio, 6.165) among physicians reporting that they used substances to feel better or cope with stress. Those doctors also had higher risk of binge drinking, drinking larger quantities, and using alcohol more often, the data show. In fact, 44% of the physicians reported binge drinking, and 5% met the criteria for alcohol dependence, Dr. Medisauskaite and Dr. Kamau wrote. Binge drinking was defined as consuming more than six drinks on a single occasion.
Previous studies have indicated that occupational distress among physicians has negative effects on quality of care and patient safety, the authors noted. This latest cross-sectional study builds on those findings by showing that occupational distress increases risk of health problems among doctors. “The impact of occupational distress or ill health could increase levels of sickness-absence among doctors, thus reducing patient safety because of understaffing,” Dr. Medisauskaite and Dr. Kamau wrote.
Similarly, physicians with sleep problems or substance use related to occupational distress could perform poorly on the job because of being groggy, intoxicated, or hung over, they said.
“We recommend that doctors’ mentors, supervisors, peers, and occupational health support services recognize and act on (1) the prevalence of occupational distress and health problems among doctors; (2) the possibility that occupational distress raises the risk of several health problems; and (3) the need to provide early interventions,” Dr. Medisauskaite and Dr. Kamau wrote. Such interventions could help prevent physicians who are experiencing occupational distress from suffering the long-term health effects from sleep disturbances, binge drinking and binge eating, and ill health, they suggested.
One limitation cited was the study’s cross-sectional design, which makes it impossible to draw conclusions about causation. The researchers also conceded that some participants might not have been comfortable answering questions about illicit use of drugs or alcohol. Nevertheless, they said,
Dr. Medisauskaite and Dr. Kamau declared no competing interests related to the study.
SOURCE: Medisauskaite A, Kamau C. BMJ Open. 2019 May 15. doi: 10.1136/bmjopen-2018-027362.
Many doctors say they cope with job-related stress by drinking alcohol or taking drugs
Many doctors say they cope with job-related stress by drinking alcohol or taking drugs
Occupational distress among physicians is tied to increased odds of substance use, sleep disturbance, binge eating, and poor health in general, a cross-sectional study of 417 U.K. doctors shows.
Burned-out or depressed doctors had higher risks of those health problems regardless of whether or not they worked in a hospital setting, according to Asta Medisauskaite, PhD, of University College London and Caroline Kamau, PhD, of the University of London. The study was published in BMJ Open.
The investigators asked the participants to answer a battery of validated questionnaires online, including the Alcohol Use Disorders Identification Test, the Eating Disorder Diagnostic Scale, and the Insomnia Severity Index.
The odds of many health problems were increased among the physicians with emotional exhaustion, as indicated by the Maslach Burnout Inventory, as was also the case with psychiatric disorders, according to the General Health Questionnaire-12, which investigators noted has been used extensively to examine medical doctors and other working populations.
Sleep disturbances were, for example, more likely in physicians with burnout or psychiatric morbidity, with odds ratios ranging from 1.344 to 3.826, the investigators reported. Likewise, these indicators of occupational distress increased the risk of suffering from frequent poor health, with odds ratios from 1.050 to 3.544, and of binge eating, with odds ratios from 1.311 to 1.841, Dr. Medisauskaite and Dr. Kamau reported.
Distressed doctors more often used alcohol, according to the researchers, who said that they found a higher risk of alcohol dependence (odds ratio, 6.165) among physicians reporting that they used substances to feel better or cope with stress. Those doctors also had higher risk of binge drinking, drinking larger quantities, and using alcohol more often, the data show. In fact, 44% of the physicians reported binge drinking, and 5% met the criteria for alcohol dependence, Dr. Medisauskaite and Dr. Kamau wrote. Binge drinking was defined as consuming more than six drinks on a single occasion.
Previous studies have indicated that occupational distress among physicians has negative effects on quality of care and patient safety, the authors noted. This latest cross-sectional study builds on those findings by showing that occupational distress increases risk of health problems among doctors. “The impact of occupational distress or ill health could increase levels of sickness-absence among doctors, thus reducing patient safety because of understaffing,” Dr. Medisauskaite and Dr. Kamau wrote.
Similarly, physicians with sleep problems or substance use related to occupational distress could perform poorly on the job because of being groggy, intoxicated, or hung over, they said.
“We recommend that doctors’ mentors, supervisors, peers, and occupational health support services recognize and act on (1) the prevalence of occupational distress and health problems among doctors; (2) the possibility that occupational distress raises the risk of several health problems; and (3) the need to provide early interventions,” Dr. Medisauskaite and Dr. Kamau wrote. Such interventions could help prevent physicians who are experiencing occupational distress from suffering the long-term health effects from sleep disturbances, binge drinking and binge eating, and ill health, they suggested.
One limitation cited was the study’s cross-sectional design, which makes it impossible to draw conclusions about causation. The researchers also conceded that some participants might not have been comfortable answering questions about illicit use of drugs or alcohol. Nevertheless, they said,
Dr. Medisauskaite and Dr. Kamau declared no competing interests related to the study.
SOURCE: Medisauskaite A, Kamau C. BMJ Open. 2019 May 15. doi: 10.1136/bmjopen-2018-027362.
Occupational distress among physicians is tied to increased odds of substance use, sleep disturbance, binge eating, and poor health in general, a cross-sectional study of 417 U.K. doctors shows.
Burned-out or depressed doctors had higher risks of those health problems regardless of whether or not they worked in a hospital setting, according to Asta Medisauskaite, PhD, of University College London and Caroline Kamau, PhD, of the University of London. The study was published in BMJ Open.
The investigators asked the participants to answer a battery of validated questionnaires online, including the Alcohol Use Disorders Identification Test, the Eating Disorder Diagnostic Scale, and the Insomnia Severity Index.
The odds of many health problems were increased among the physicians with emotional exhaustion, as indicated by the Maslach Burnout Inventory, as was also the case with psychiatric disorders, according to the General Health Questionnaire-12, which investigators noted has been used extensively to examine medical doctors and other working populations.
Sleep disturbances were, for example, more likely in physicians with burnout or psychiatric morbidity, with odds ratios ranging from 1.344 to 3.826, the investigators reported. Likewise, these indicators of occupational distress increased the risk of suffering from frequent poor health, with odds ratios from 1.050 to 3.544, and of binge eating, with odds ratios from 1.311 to 1.841, Dr. Medisauskaite and Dr. Kamau reported.
Distressed doctors more often used alcohol, according to the researchers, who said that they found a higher risk of alcohol dependence (odds ratio, 6.165) among physicians reporting that they used substances to feel better or cope with stress. Those doctors also had higher risk of binge drinking, drinking larger quantities, and using alcohol more often, the data show. In fact, 44% of the physicians reported binge drinking, and 5% met the criteria for alcohol dependence, Dr. Medisauskaite and Dr. Kamau wrote. Binge drinking was defined as consuming more than six drinks on a single occasion.
Previous studies have indicated that occupational distress among physicians has negative effects on quality of care and patient safety, the authors noted. This latest cross-sectional study builds on those findings by showing that occupational distress increases risk of health problems among doctors. “The impact of occupational distress or ill health could increase levels of sickness-absence among doctors, thus reducing patient safety because of understaffing,” Dr. Medisauskaite and Dr. Kamau wrote.
Similarly, physicians with sleep problems or substance use related to occupational distress could perform poorly on the job because of being groggy, intoxicated, or hung over, they said.
“We recommend that doctors’ mentors, supervisors, peers, and occupational health support services recognize and act on (1) the prevalence of occupational distress and health problems among doctors; (2) the possibility that occupational distress raises the risk of several health problems; and (3) the need to provide early interventions,” Dr. Medisauskaite and Dr. Kamau wrote. Such interventions could help prevent physicians who are experiencing occupational distress from suffering the long-term health effects from sleep disturbances, binge drinking and binge eating, and ill health, they suggested.
One limitation cited was the study’s cross-sectional design, which makes it impossible to draw conclusions about causation. The researchers also conceded that some participants might not have been comfortable answering questions about illicit use of drugs or alcohol. Nevertheless, they said,
Dr. Medisauskaite and Dr. Kamau declared no competing interests related to the study.
SOURCE: Medisauskaite A, Kamau C. BMJ Open. 2019 May 15. doi: 10.1136/bmjopen-2018-027362.
FROM BMJ OPEN
Key clinical point: Occupational distress increased the odds of substance use, sleep disturbance, binge eating, and poor health among medical doctors in the United Kingdom.
Major finding: Distressed medical doctors had a higher risk of binge drinking. In fact, 44% reported binge drinking, defined as consuming more than six drinks on a single occasion.
Study details: A U.K. cross-sectional study of 417 doctors who answered a series of validated health-related questionnaires online.
Disclosures: The authors declared no competing interests related to the study.
Source: Medisauskaite A, Kamau C. BMJ Open. 2019 May 15. doi: 10.1136/bmjopen-2018-027362.
FDA approves venetoclax/obinutuzumab combo for CLL
The Food and Drug Administration has approved the combination of venetoclax (Venclexta) plus obinutuzumab (Gazyva) for patients with previously untreated chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma.
The approval provides a chemotherapy-free, fixed duration treatment. The FDA based the approval on the results of the phase 3 CLL14 trial, which will be presented at the 2019 annual meeting of the American Society of Clinical Oncology.
Researchers randomized 432 patients to either a 12-month duration of venetoclax with a 6-month duration of obinutuzumab or to a 6-month duration of obinutuzumab plus chlorambucil and another 6-month duration of chlorambucil.
The newly approved combination reduced the risk of disease progression or death (progression-free survival as assessed by an independent review committee) by 67%, compared with obinutuzumab/chlorambucil (hazard ratio, 0.33; P less than .0001).
Venetoclax/obinutuzumab also had a higher rate of minimal residual disease negativity in bone marrow and peripheral blood, compared to the other combination, according to Genentech.
The most common adverse reactions of any grade reported for venetoclax/obinutuzumab were neutropenia, diarrhea, fatigue, nausea, anemia, and upper respiratory tract infection.
The Food and Drug Administration has approved the combination of venetoclax (Venclexta) plus obinutuzumab (Gazyva) for patients with previously untreated chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma.
The approval provides a chemotherapy-free, fixed duration treatment. The FDA based the approval on the results of the phase 3 CLL14 trial, which will be presented at the 2019 annual meeting of the American Society of Clinical Oncology.
Researchers randomized 432 patients to either a 12-month duration of venetoclax with a 6-month duration of obinutuzumab or to a 6-month duration of obinutuzumab plus chlorambucil and another 6-month duration of chlorambucil.
The newly approved combination reduced the risk of disease progression or death (progression-free survival as assessed by an independent review committee) by 67%, compared with obinutuzumab/chlorambucil (hazard ratio, 0.33; P less than .0001).
Venetoclax/obinutuzumab also had a higher rate of minimal residual disease negativity in bone marrow and peripheral blood, compared to the other combination, according to Genentech.
The most common adverse reactions of any grade reported for venetoclax/obinutuzumab were neutropenia, diarrhea, fatigue, nausea, anemia, and upper respiratory tract infection.
The Food and Drug Administration has approved the combination of venetoclax (Venclexta) plus obinutuzumab (Gazyva) for patients with previously untreated chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma.
The approval provides a chemotherapy-free, fixed duration treatment. The FDA based the approval on the results of the phase 3 CLL14 trial, which will be presented at the 2019 annual meeting of the American Society of Clinical Oncology.
Researchers randomized 432 patients to either a 12-month duration of venetoclax with a 6-month duration of obinutuzumab or to a 6-month duration of obinutuzumab plus chlorambucil and another 6-month duration of chlorambucil.
The newly approved combination reduced the risk of disease progression or death (progression-free survival as assessed by an independent review committee) by 67%, compared with obinutuzumab/chlorambucil (hazard ratio, 0.33; P less than .0001).
Venetoclax/obinutuzumab also had a higher rate of minimal residual disease negativity in bone marrow and peripheral blood, compared to the other combination, according to Genentech.
The most common adverse reactions of any grade reported for venetoclax/obinutuzumab were neutropenia, diarrhea, fatigue, nausea, anemia, and upper respiratory tract infection.
Survey: Physicians predict increase in measles deaths
by real-time market insights technology firm InCrowd.
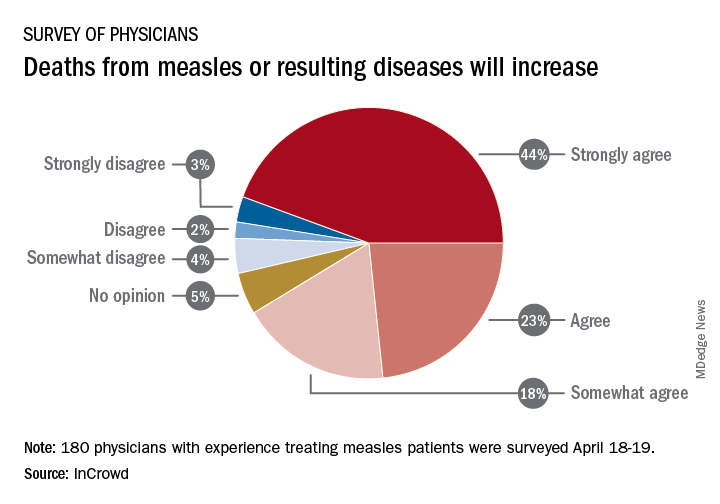
Among the 180 physicians with experience treating measles, 23% agreed and 44% said that they strongly agreed with the statement that measles deaths would increase, and another 18% said that they somewhat agreed. Only 9% expressed some level of disagreement, InCrowd said.
Most of those respondents also believe that summer travel will increase measles outbreaks (29% agreed and 30% strongly agreed) and that more communities will adopt requirements for measles vaccinations (26% and 36%). A majority also said that education about vaccinations will improve (26% agreed and 29% strongly agreed), but almost half of the physicians surveyed also expect vaccination misinformation to get worse (29% and 19%), InCrowd reported.
“With 44% of respondents predicting a high likelihood that deaths caused by measles will increase, the data show the imperative for physicians and patients to keep up the dialogue. … We have a long way to go before declaring victory,” said Diane Hayes, PhD, president and cofounder of InCrowd.
The InCrowd 5-minute microsurvey was conducted on April 18-19, 2019, and included 455 primary care physicians, of whom 40% said that they have treated or knew of colleagues in their facility or community who have treated patients with measles. Of those 180 respondents, 89 were pediatricians and 91 were in other primary care specialties.
by real-time market insights technology firm InCrowd.

Among the 180 physicians with experience treating measles, 23% agreed and 44% said that they strongly agreed with the statement that measles deaths would increase, and another 18% said that they somewhat agreed. Only 9% expressed some level of disagreement, InCrowd said.
Most of those respondents also believe that summer travel will increase measles outbreaks (29% agreed and 30% strongly agreed) and that more communities will adopt requirements for measles vaccinations (26% and 36%). A majority also said that education about vaccinations will improve (26% agreed and 29% strongly agreed), but almost half of the physicians surveyed also expect vaccination misinformation to get worse (29% and 19%), InCrowd reported.
“With 44% of respondents predicting a high likelihood that deaths caused by measles will increase, the data show the imperative for physicians and patients to keep up the dialogue. … We have a long way to go before declaring victory,” said Diane Hayes, PhD, president and cofounder of InCrowd.
The InCrowd 5-minute microsurvey was conducted on April 18-19, 2019, and included 455 primary care physicians, of whom 40% said that they have treated or knew of colleagues in their facility or community who have treated patients with measles. Of those 180 respondents, 89 were pediatricians and 91 were in other primary care specialties.
by real-time market insights technology firm InCrowd.

Among the 180 physicians with experience treating measles, 23% agreed and 44% said that they strongly agreed with the statement that measles deaths would increase, and another 18% said that they somewhat agreed. Only 9% expressed some level of disagreement, InCrowd said.
Most of those respondents also believe that summer travel will increase measles outbreaks (29% agreed and 30% strongly agreed) and that more communities will adopt requirements for measles vaccinations (26% and 36%). A majority also said that education about vaccinations will improve (26% agreed and 29% strongly agreed), but almost half of the physicians surveyed also expect vaccination misinformation to get worse (29% and 19%), InCrowd reported.
“With 44% of respondents predicting a high likelihood that deaths caused by measles will increase, the data show the imperative for physicians and patients to keep up the dialogue. … We have a long way to go before declaring victory,” said Diane Hayes, PhD, president and cofounder of InCrowd.
The InCrowd 5-minute microsurvey was conducted on April 18-19, 2019, and included 455 primary care physicians, of whom 40% said that they have treated or knew of colleagues in their facility or community who have treated patients with measles. Of those 180 respondents, 89 were pediatricians and 91 were in other primary care specialties.
New risk score predicts cardiac-device infection
SAN FRANCISCO – Researchers have devised a five-item scoring formula to quantify the risk for infection in patients undergoing placement, revision, or removal of a cardiac-rhythm device based on data from nearly 20,000 patients enrolled in a recent infection-prophylaxis trial.
The risk score can help identify patients who might benefit from intensified antibiotic prophylaxis, and it can also help during shared decision making with patients to better understand the risk a patient faces from infection, compared with their predicted device benefit, David H. Birnie, MD, said at the annual scientific sessions of the Heart Rhythm Society.
The new risk score produced a concordance statistic, the area under the receiver-operator characteristic curve, of 0.704. It showed that, although it could use further validation, the score as it currently stands has substantial predictive value, said Dr. Birnie, professor of medicine at the University of Ottawa and deputy chief of cardiology at the University of Ottawa Heart Institute. “It’s certainly better than anything we have now,” he said in a video interview.
Dr. Birnie and his associates used data they collected on baseline characteristics and infection outcomes of the 19,603 patients enrolled in PADIT (Prevention of Arrhythmia Device Infection Trial) who underwent a rhythm-device procedure at 1 of 28 participating Canadian centers. The primary aim of PADIT was to assess the safety and efficacy of an intensified antibiotic-prophylaxis regimen, compared with a standard regimen of a cefazolin infusion just before the procedure. The study’s primary endpoint was the incidence of hospitalization for device infection during 1-year follow-up, and while the intensified prophylactic regimen linked with a 23% relative reduction in the hospitalization rate, compared with standard treatment, the difference was not statistically significant (J Am Coll Cardiol. 2018 Dec 18;72[24]:3098-109).
The researchers analyzed the baseline patient data and the blindly adjudicated infection outcomes and identified five factors that were independently associated with an increased infection rate. They organized the five factors and produced a formula they call the PADIT score (see chart). Those five factors are: prior procedures (the greater the number the greater the risk), age (which unexpectedly had an inverse relationship with infection incidence), depressed renal function, immuno-compromised status, and type of procedure. A patient can potentially score 0-15 points.
Among the PADIT patients a score of 0 correlated with about a 0.3% rate of hospitalization for a device-related infection during 1 year of follow-up, a score of 5 with about a 1.1% rate, a score of 6 with about a 1.8% rate, and a score of seven or more with a 3.4% infection rate over the following year. About 5% of patients had a score of 7 or more, and roughly another 5% had a score of 5 or 6, Dr. Birnie said. At his center, clinicians have begun routinely calculating scores for patients scheduled for an arrhythmia-device procedure, and they are considering routinely administering added antibiotic prophylaxis to patients with a preprocedural score of 6 or higher. They may also use the score to determine whether to use the antibacterial envelope recently reported to prevent cardiac-device infections (N Engl J Med. 2019 May 16;380[20]:1895-905).
“It’s very easy for patients to get to a PADIT score of 7 or higher,” Dr. Birnie noted. As an example, he cited a common patient, an 85-year-old with renal dysfunction who is under consideration for a second replacement of an implantable cardioverter defibrillator. The patient would score 1 point for renal insufficiency, 2 points for the type of device, and 4 points for having a prior history of two devices, and the consequent 3.4% risk for infection might counterbalance the potential benefit this elderly patient could expect from the new device. The score will be very important for targeting treatment, shared decision making, and selection of patients for future intervention trials, he concluded.
“I think this risk score will change practice by giving clinicians a better idea of a patient’s risk for infection,” commented Fred M. Kusumoto, MD, professor of medicine at the Mayo Medical School, Rochester, Minn., and director of heart rhythm services at the Mayo Clinic in Jacksonville, Fla. The PADIT score will help identify patients for whom leaving a device in place is a better option than taking it out because of their infection risk. The risk score could also help improve the cost effectiveness of preventive treatments, such as antibiotic-eluting envelopes, by targeting treatment to higher-risk patients, Dr. Kusumoto said during a press briefing.
SOURCE: Birnie DH. Heart Rhythm 2019, Absract S-LCT02-01.
I like this new infection risk score. It addresses a very clinically relevant issue. It’s important for the electrophysiology community to better understand how to best manage infections related to cardiac rhythm devices and ideally prevent them from happening.
I’m not a big fan of risk scores in general because they can sometimes detract from independent thinking about how to manage a patient. However, it is also helpful to have this type of risk-assessment information when discussing management options with a patient.
The PADIT risk score may also help identify which patients could potentially benefit the most from an antibiotic-eluting envelope when receiving an implanted cardiac-rhythm device. Recently reported results from WRAP-IT showed that routinely using envelopes cut the incidence of major infections by a relative 40%, but in absolute terms, the number needed to treat with the envelop to prevent one major infection was about 200 patients, a big number given the high cost of the envelope (N Engl J Med. 2019 May 16;380[20]:1895-905). It is therefore very interesting to think about using the PADIT risk score to better target an effective but expensive preventive measure like an antibiotic-eluting envelop to patients at the highest risk for infection.
Ulrika Birgersdotter-Green, MD , professor of medicine and director of pacemaker and ICD services at the University of California, San Diego, made these comments as a designated discussant for the report. She has been a consultant to and received honoraria from Abbott, Boston Scientific, and Medtronic.
I like this new infection risk score. It addresses a very clinically relevant issue. It’s important for the electrophysiology community to better understand how to best manage infections related to cardiac rhythm devices and ideally prevent them from happening.
I’m not a big fan of risk scores in general because they can sometimes detract from independent thinking about how to manage a patient. However, it is also helpful to have this type of risk-assessment information when discussing management options with a patient.
The PADIT risk score may also help identify which patients could potentially benefit the most from an antibiotic-eluting envelope when receiving an implanted cardiac-rhythm device. Recently reported results from WRAP-IT showed that routinely using envelopes cut the incidence of major infections by a relative 40%, but in absolute terms, the number needed to treat with the envelop to prevent one major infection was about 200 patients, a big number given the high cost of the envelope (N Engl J Med. 2019 May 16;380[20]:1895-905). It is therefore very interesting to think about using the PADIT risk score to better target an effective but expensive preventive measure like an antibiotic-eluting envelop to patients at the highest risk for infection.
Ulrika Birgersdotter-Green, MD , professor of medicine and director of pacemaker and ICD services at the University of California, San Diego, made these comments as a designated discussant for the report. She has been a consultant to and received honoraria from Abbott, Boston Scientific, and Medtronic.
I like this new infection risk score. It addresses a very clinically relevant issue. It’s important for the electrophysiology community to better understand how to best manage infections related to cardiac rhythm devices and ideally prevent them from happening.
I’m not a big fan of risk scores in general because they can sometimes detract from independent thinking about how to manage a patient. However, it is also helpful to have this type of risk-assessment information when discussing management options with a patient.
The PADIT risk score may also help identify which patients could potentially benefit the most from an antibiotic-eluting envelope when receiving an implanted cardiac-rhythm device. Recently reported results from WRAP-IT showed that routinely using envelopes cut the incidence of major infections by a relative 40%, but in absolute terms, the number needed to treat with the envelop to prevent one major infection was about 200 patients, a big number given the high cost of the envelope (N Engl J Med. 2019 May 16;380[20]:1895-905). It is therefore very interesting to think about using the PADIT risk score to better target an effective but expensive preventive measure like an antibiotic-eluting envelop to patients at the highest risk for infection.
Ulrika Birgersdotter-Green, MD , professor of medicine and director of pacemaker and ICD services at the University of California, San Diego, made these comments as a designated discussant for the report. She has been a consultant to and received honoraria from Abbott, Boston Scientific, and Medtronic.
SAN FRANCISCO – Researchers have devised a five-item scoring formula to quantify the risk for infection in patients undergoing placement, revision, or removal of a cardiac-rhythm device based on data from nearly 20,000 patients enrolled in a recent infection-prophylaxis trial.
The risk score can help identify patients who might benefit from intensified antibiotic prophylaxis, and it can also help during shared decision making with patients to better understand the risk a patient faces from infection, compared with their predicted device benefit, David H. Birnie, MD, said at the annual scientific sessions of the Heart Rhythm Society.
The new risk score produced a concordance statistic, the area under the receiver-operator characteristic curve, of 0.704. It showed that, although it could use further validation, the score as it currently stands has substantial predictive value, said Dr. Birnie, professor of medicine at the University of Ottawa and deputy chief of cardiology at the University of Ottawa Heart Institute. “It’s certainly better than anything we have now,” he said in a video interview.
Dr. Birnie and his associates used data they collected on baseline characteristics and infection outcomes of the 19,603 patients enrolled in PADIT (Prevention of Arrhythmia Device Infection Trial) who underwent a rhythm-device procedure at 1 of 28 participating Canadian centers. The primary aim of PADIT was to assess the safety and efficacy of an intensified antibiotic-prophylaxis regimen, compared with a standard regimen of a cefazolin infusion just before the procedure. The study’s primary endpoint was the incidence of hospitalization for device infection during 1-year follow-up, and while the intensified prophylactic regimen linked with a 23% relative reduction in the hospitalization rate, compared with standard treatment, the difference was not statistically significant (J Am Coll Cardiol. 2018 Dec 18;72[24]:3098-109).
The researchers analyzed the baseline patient data and the blindly adjudicated infection outcomes and identified five factors that were independently associated with an increased infection rate. They organized the five factors and produced a formula they call the PADIT score (see chart). Those five factors are: prior procedures (the greater the number the greater the risk), age (which unexpectedly had an inverse relationship with infection incidence), depressed renal function, immuno-compromised status, and type of procedure. A patient can potentially score 0-15 points.
Among the PADIT patients a score of 0 correlated with about a 0.3% rate of hospitalization for a device-related infection during 1 year of follow-up, a score of 5 with about a 1.1% rate, a score of 6 with about a 1.8% rate, and a score of seven or more with a 3.4% infection rate over the following year. About 5% of patients had a score of 7 or more, and roughly another 5% had a score of 5 or 6, Dr. Birnie said. At his center, clinicians have begun routinely calculating scores for patients scheduled for an arrhythmia-device procedure, and they are considering routinely administering added antibiotic prophylaxis to patients with a preprocedural score of 6 or higher. They may also use the score to determine whether to use the antibacterial envelope recently reported to prevent cardiac-device infections (N Engl J Med. 2019 May 16;380[20]:1895-905).
“It’s very easy for patients to get to a PADIT score of 7 or higher,” Dr. Birnie noted. As an example, he cited a common patient, an 85-year-old with renal dysfunction who is under consideration for a second replacement of an implantable cardioverter defibrillator. The patient would score 1 point for renal insufficiency, 2 points for the type of device, and 4 points for having a prior history of two devices, and the consequent 3.4% risk for infection might counterbalance the potential benefit this elderly patient could expect from the new device. The score will be very important for targeting treatment, shared decision making, and selection of patients for future intervention trials, he concluded.
“I think this risk score will change practice by giving clinicians a better idea of a patient’s risk for infection,” commented Fred M. Kusumoto, MD, professor of medicine at the Mayo Medical School, Rochester, Minn., and director of heart rhythm services at the Mayo Clinic in Jacksonville, Fla. The PADIT score will help identify patients for whom leaving a device in place is a better option than taking it out because of their infection risk. The risk score could also help improve the cost effectiveness of preventive treatments, such as antibiotic-eluting envelopes, by targeting treatment to higher-risk patients, Dr. Kusumoto said during a press briefing.
SOURCE: Birnie DH. Heart Rhythm 2019, Absract S-LCT02-01.
SAN FRANCISCO – Researchers have devised a five-item scoring formula to quantify the risk for infection in patients undergoing placement, revision, or removal of a cardiac-rhythm device based on data from nearly 20,000 patients enrolled in a recent infection-prophylaxis trial.
The risk score can help identify patients who might benefit from intensified antibiotic prophylaxis, and it can also help during shared decision making with patients to better understand the risk a patient faces from infection, compared with their predicted device benefit, David H. Birnie, MD, said at the annual scientific sessions of the Heart Rhythm Society.
The new risk score produced a concordance statistic, the area under the receiver-operator characteristic curve, of 0.704. It showed that, although it could use further validation, the score as it currently stands has substantial predictive value, said Dr. Birnie, professor of medicine at the University of Ottawa and deputy chief of cardiology at the University of Ottawa Heart Institute. “It’s certainly better than anything we have now,” he said in a video interview.
Dr. Birnie and his associates used data they collected on baseline characteristics and infection outcomes of the 19,603 patients enrolled in PADIT (Prevention of Arrhythmia Device Infection Trial) who underwent a rhythm-device procedure at 1 of 28 participating Canadian centers. The primary aim of PADIT was to assess the safety and efficacy of an intensified antibiotic-prophylaxis regimen, compared with a standard regimen of a cefazolin infusion just before the procedure. The study’s primary endpoint was the incidence of hospitalization for device infection during 1-year follow-up, and while the intensified prophylactic regimen linked with a 23% relative reduction in the hospitalization rate, compared with standard treatment, the difference was not statistically significant (J Am Coll Cardiol. 2018 Dec 18;72[24]:3098-109).
The researchers analyzed the baseline patient data and the blindly adjudicated infection outcomes and identified five factors that were independently associated with an increased infection rate. They organized the five factors and produced a formula they call the PADIT score (see chart). Those five factors are: prior procedures (the greater the number the greater the risk), age (which unexpectedly had an inverse relationship with infection incidence), depressed renal function, immuno-compromised status, and type of procedure. A patient can potentially score 0-15 points.
Among the PADIT patients a score of 0 correlated with about a 0.3% rate of hospitalization for a device-related infection during 1 year of follow-up, a score of 5 with about a 1.1% rate, a score of 6 with about a 1.8% rate, and a score of seven or more with a 3.4% infection rate over the following year. About 5% of patients had a score of 7 or more, and roughly another 5% had a score of 5 or 6, Dr. Birnie said. At his center, clinicians have begun routinely calculating scores for patients scheduled for an arrhythmia-device procedure, and they are considering routinely administering added antibiotic prophylaxis to patients with a preprocedural score of 6 or higher. They may also use the score to determine whether to use the antibacterial envelope recently reported to prevent cardiac-device infections (N Engl J Med. 2019 May 16;380[20]:1895-905).
“It’s very easy for patients to get to a PADIT score of 7 or higher,” Dr. Birnie noted. As an example, he cited a common patient, an 85-year-old with renal dysfunction who is under consideration for a second replacement of an implantable cardioverter defibrillator. The patient would score 1 point for renal insufficiency, 2 points for the type of device, and 4 points for having a prior history of two devices, and the consequent 3.4% risk for infection might counterbalance the potential benefit this elderly patient could expect from the new device. The score will be very important for targeting treatment, shared decision making, and selection of patients for future intervention trials, he concluded.
“I think this risk score will change practice by giving clinicians a better idea of a patient’s risk for infection,” commented Fred M. Kusumoto, MD, professor of medicine at the Mayo Medical School, Rochester, Minn., and director of heart rhythm services at the Mayo Clinic in Jacksonville, Fla. The PADIT score will help identify patients for whom leaving a device in place is a better option than taking it out because of their infection risk. The risk score could also help improve the cost effectiveness of preventive treatments, such as antibiotic-eluting envelopes, by targeting treatment to higher-risk patients, Dr. Kusumoto said during a press briefing.
SOURCE: Birnie DH. Heart Rhythm 2019, Absract S-LCT02-01.
REPORTING FROM HEART RHYTHM 2019
Key clinical point: Researchers have devised a five-item scoring formula to predict a patient’s risk for infection from an cardiac rhythm–device procedure.
Major finding: The risk score had an optimism-corrected concordance statistic of 0.704.
Study details: Investigators developed the risk score using data from PADIT, a multicenter, randomized trial with 19,603 patients.
Disclosures: PADIT received no commercial funding. Dr. Birnie had no relevant disclosures.
Source: Birnie DH. Heart Rhythm 2019, Absract S-LCT02-01.
Texting a stroke, game-show grants, and, um, The Beast
Autocorrect, or worse?
Is it just fat thumbs, or something more serious? Incoherent text messages could be the first sign of a stroke for adults, as displayed in two case reports presented at the annual meeting of the American Academy of Neurology.
It makes sense that stroke can cause dystextia: Typing on a phone involves some fine motor, language, and vision skills. Stroke can affect all these functions, leading to bizarre typos or angry political rants on Facebook.
Just kidding; those are equally as concerning but require a whole different diagnosis.
‘Research Funds’ for $11,000, Alex
In the universal struggle for research funding, many a scientist has resorted to desperate measures to keep the lab assistants paid, the JAX Mice fed, and the frontiers of medical science expanding ever outward.
Marina Simian is a biologist for Argentina’s National Scientific and Technical Research Council. She runs a research lab focused on oncology treatments for breast cancer. Faced with a national economic crisis and cuts to research funding, Simian says government funding has nearly dried up. Where did she turn instead?
Enter the local version of the TV game show “Who Wants to be a Millionaire?” Simian went on the show as a contestant, explaining that she needed money to support her cancer research. And when the camera cut away to commercials, Simian walked away with 500,000 pesos ($11,000) in winnings. Which she used to buy more lab supplies.
Admittedly, the “Slumdog Millionaire” approach may not save every cash-strapped scientist’s pet project, but it does give us an idea for game show super champ James Holzhauer’s career when he finally wears out his “Jeopardy!” buzzer: medical research funding consultant.
FDA tames THE BEAST
The LOTME staff had a meeting the other day with our editor (we think he might be the love child of Lois Lane and Ron Burgundy), who said that lately we’ve been “too juvenile” and “not medical enough” and told us to get our “dirty little minds out of the gutter.”
After we stopped crying, he offered up this item from the Food and Drug Administration:
“STIFF BOY LLC. Issues Voluntary Nationwide Recall of THE BEAST Capsules Due to Presence of Undeclared Sildenafil” (no, we did not add the all-caps).
Okay, here goes.
THE BEAST was marketed as a dietary supplement for “male enhancement” (add nonjuvenile but hilarious remark about tumescence), which would not be regulated by the FDA. The presence of Viagra’s active ingredient in the capsules, however, “renders it an unapproved drug for which safety and efficacy have not been established and, therefore, subject to recall,” the FDA said (insert comment about seemingly serious but totally fictitious side effects).
Although the sildenafil in THE BEAST may interact with nitrates found in some prescription drugs, STIFF BOY said that it had not received any reports of adverse events before the recall (imagine a guy working on the engine of his pickup truck).
Seek immediate medical attention if your laughter lasts for more than 4 hours after reading this.
Time flies when you’re having ‘fun’
Match Day is one of the most exciting times in any young, prospective doctor’s life. Finally, the specialty of your dreams is yours. You know the training will be stressful and the hours will be long, but how bad could it be?
It’s not like it’ll take years off your life, right?
Well, according to new research published in Biological Psychiatry, that’s almost exactly what medical residency will do to you.
The researchers took a group of medical students at the University of Michigan, Ann Arbor, entering their first year of residency and measured their telomere length both before and after their internship year, comparing it with a group of first-year undergraduates. Rapidly shrinking telomere length is a well-accepted sign of aging, and interns had their telomeres shrink at a rate six times faster than their nonmedical peers, who were apparently too busy doing upside-down kegstands to notice how stressful college can be.
Oh, don’t worry, there was most definitely an association between hours worked and increased telomere shrinkage. Those who had to work more than 80 hours a week aged most of all.
So, if you emerge from a particularly difficult internship with the sudden desire to yell at those darn kids for being on your lawn, we completely understand.
Autocorrect, or worse?
Is it just fat thumbs, or something more serious? Incoherent text messages could be the first sign of a stroke for adults, as displayed in two case reports presented at the annual meeting of the American Academy of Neurology.
It makes sense that stroke can cause dystextia: Typing on a phone involves some fine motor, language, and vision skills. Stroke can affect all these functions, leading to bizarre typos or angry political rants on Facebook.
Just kidding; those are equally as concerning but require a whole different diagnosis.
‘Research Funds’ for $11,000, Alex
In the universal struggle for research funding, many a scientist has resorted to desperate measures to keep the lab assistants paid, the JAX Mice fed, and the frontiers of medical science expanding ever outward.
Marina Simian is a biologist for Argentina’s National Scientific and Technical Research Council. She runs a research lab focused on oncology treatments for breast cancer. Faced with a national economic crisis and cuts to research funding, Simian says government funding has nearly dried up. Where did she turn instead?
Enter the local version of the TV game show “Who Wants to be a Millionaire?” Simian went on the show as a contestant, explaining that she needed money to support her cancer research. And when the camera cut away to commercials, Simian walked away with 500,000 pesos ($11,000) in winnings. Which she used to buy more lab supplies.
Admittedly, the “Slumdog Millionaire” approach may not save every cash-strapped scientist’s pet project, but it does give us an idea for game show super champ James Holzhauer’s career when he finally wears out his “Jeopardy!” buzzer: medical research funding consultant.
FDA tames THE BEAST
The LOTME staff had a meeting the other day with our editor (we think he might be the love child of Lois Lane and Ron Burgundy), who said that lately we’ve been “too juvenile” and “not medical enough” and told us to get our “dirty little minds out of the gutter.”
After we stopped crying, he offered up this item from the Food and Drug Administration:
“STIFF BOY LLC. Issues Voluntary Nationwide Recall of THE BEAST Capsules Due to Presence of Undeclared Sildenafil” (no, we did not add the all-caps).
Okay, here goes.
THE BEAST was marketed as a dietary supplement for “male enhancement” (add nonjuvenile but hilarious remark about tumescence), which would not be regulated by the FDA. The presence of Viagra’s active ingredient in the capsules, however, “renders it an unapproved drug for which safety and efficacy have not been established and, therefore, subject to recall,” the FDA said (insert comment about seemingly serious but totally fictitious side effects).
Although the sildenafil in THE BEAST may interact with nitrates found in some prescription drugs, STIFF BOY said that it had not received any reports of adverse events before the recall (imagine a guy working on the engine of his pickup truck).
Seek immediate medical attention if your laughter lasts for more than 4 hours after reading this.
Time flies when you’re having ‘fun’
Match Day is one of the most exciting times in any young, prospective doctor’s life. Finally, the specialty of your dreams is yours. You know the training will be stressful and the hours will be long, but how bad could it be?
It’s not like it’ll take years off your life, right?
Well, according to new research published in Biological Psychiatry, that’s almost exactly what medical residency will do to you.
The researchers took a group of medical students at the University of Michigan, Ann Arbor, entering their first year of residency and measured their telomere length both before and after their internship year, comparing it with a group of first-year undergraduates. Rapidly shrinking telomere length is a well-accepted sign of aging, and interns had their telomeres shrink at a rate six times faster than their nonmedical peers, who were apparently too busy doing upside-down kegstands to notice how stressful college can be.
Oh, don’t worry, there was most definitely an association between hours worked and increased telomere shrinkage. Those who had to work more than 80 hours a week aged most of all.
So, if you emerge from a particularly difficult internship with the sudden desire to yell at those darn kids for being on your lawn, we completely understand.

Autocorrect, or worse?
Is it just fat thumbs, or something more serious? Incoherent text messages could be the first sign of a stroke for adults, as displayed in two case reports presented at the annual meeting of the American Academy of Neurology.
It makes sense that stroke can cause dystextia: Typing on a phone involves some fine motor, language, and vision skills. Stroke can affect all these functions, leading to bizarre typos or angry political rants on Facebook.
Just kidding; those are equally as concerning but require a whole different diagnosis.
‘Research Funds’ for $11,000, Alex
In the universal struggle for research funding, many a scientist has resorted to desperate measures to keep the lab assistants paid, the JAX Mice fed, and the frontiers of medical science expanding ever outward.
Marina Simian is a biologist for Argentina’s National Scientific and Technical Research Council. She runs a research lab focused on oncology treatments for breast cancer. Faced with a national economic crisis and cuts to research funding, Simian says government funding has nearly dried up. Where did she turn instead?
Enter the local version of the TV game show “Who Wants to be a Millionaire?” Simian went on the show as a contestant, explaining that she needed money to support her cancer research. And when the camera cut away to commercials, Simian walked away with 500,000 pesos ($11,000) in winnings. Which she used to buy more lab supplies.
Admittedly, the “Slumdog Millionaire” approach may not save every cash-strapped scientist’s pet project, but it does give us an idea for game show super champ James Holzhauer’s career when he finally wears out his “Jeopardy!” buzzer: medical research funding consultant.
FDA tames THE BEAST
The LOTME staff had a meeting the other day with our editor (we think he might be the love child of Lois Lane and Ron Burgundy), who said that lately we’ve been “too juvenile” and “not medical enough” and told us to get our “dirty little minds out of the gutter.”
After we stopped crying, he offered up this item from the Food and Drug Administration:
“STIFF BOY LLC. Issues Voluntary Nationwide Recall of THE BEAST Capsules Due to Presence of Undeclared Sildenafil” (no, we did not add the all-caps).
Okay, here goes.
THE BEAST was marketed as a dietary supplement for “male enhancement” (add nonjuvenile but hilarious remark about tumescence), which would not be regulated by the FDA. The presence of Viagra’s active ingredient in the capsules, however, “renders it an unapproved drug for which safety and efficacy have not been established and, therefore, subject to recall,” the FDA said (insert comment about seemingly serious but totally fictitious side effects).
Although the sildenafil in THE BEAST may interact with nitrates found in some prescription drugs, STIFF BOY said that it had not received any reports of adverse events before the recall (imagine a guy working on the engine of his pickup truck).
Seek immediate medical attention if your laughter lasts for more than 4 hours after reading this.
Time flies when you’re having ‘fun’
Match Day is one of the most exciting times in any young, prospective doctor’s life. Finally, the specialty of your dreams is yours. You know the training will be stressful and the hours will be long, but how bad could it be?
It’s not like it’ll take years off your life, right?
Well, according to new research published in Biological Psychiatry, that’s almost exactly what medical residency will do to you.
The researchers took a group of medical students at the University of Michigan, Ann Arbor, entering their first year of residency and measured their telomere length both before and after their internship year, comparing it with a group of first-year undergraduates. Rapidly shrinking telomere length is a well-accepted sign of aging, and interns had their telomeres shrink at a rate six times faster than their nonmedical peers, who were apparently too busy doing upside-down kegstands to notice how stressful college can be.
Oh, don’t worry, there was most definitely an association between hours worked and increased telomere shrinkage. Those who had to work more than 80 hours a week aged most of all.
So, if you emerge from a particularly difficult internship with the sudden desire to yell at those darn kids for being on your lawn, we completely understand.

ICYMI: Dabigatran no better than aspirin for recurrent stroke prevention
Dabigatran was slightly but not significantly superior to aspirin at the prevention of recurring stroke in patients with a recent history of embolic stroke of undetermined source over a median follow-up of 19 months (6.6% rate of recurrent stroke for dabigatran vs. 7.7% for aspirin; hazard ratio, 0.85; 95% confidence interval, 0.69-1.03; P = 0.10), according to RE-SPECT ESUS, a multicenter, randomized, double-blind trial published in the New England Journal of Medicine (2019 May 15. doi: 10.1056/NEJMoa1813959).
We first reported on the results of this trial when they were presented at the World Stroke Congress by lead investigator Hans-Christoph Diener, MD. Find our coverage at the link below.
Dabigatran was slightly but not significantly superior to aspirin at the prevention of recurring stroke in patients with a recent history of embolic stroke of undetermined source over a median follow-up of 19 months (6.6% rate of recurrent stroke for dabigatran vs. 7.7% for aspirin; hazard ratio, 0.85; 95% confidence interval, 0.69-1.03; P = 0.10), according to RE-SPECT ESUS, a multicenter, randomized, double-blind trial published in the New England Journal of Medicine (2019 May 15. doi: 10.1056/NEJMoa1813959).
We first reported on the results of this trial when they were presented at the World Stroke Congress by lead investigator Hans-Christoph Diener, MD. Find our coverage at the link below.
Dabigatran was slightly but not significantly superior to aspirin at the prevention of recurring stroke in patients with a recent history of embolic stroke of undetermined source over a median follow-up of 19 months (6.6% rate of recurrent stroke for dabigatran vs. 7.7% for aspirin; hazard ratio, 0.85; 95% confidence interval, 0.69-1.03; P = 0.10), according to RE-SPECT ESUS, a multicenter, randomized, double-blind trial published in the New England Journal of Medicine (2019 May 15. doi: 10.1056/NEJMoa1813959).
We first reported on the results of this trial when they were presented at the World Stroke Congress by lead investigator Hans-Christoph Diener, MD. Find our coverage at the link below.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE