User login
Better screening needed to reduce pregnancy-related overdose, death
reported Marcela C. Smid, MD, of the University of Utah, Salt Lake City, and her associates.
The stressful demands of newborn care, postpartum depression and anxiety, sleep deprivation, and other factors “may result in the ‘perfect storm’ leading to drug use, relapse, overdose, and death,” they cautioned.
Dr. Smid and associates conducted a retrospective cohort study of all pregnancy-associated deaths occurring in Utah between January 2005 and December 2014 using data from the Utah Perinatal Mortality Review Committee database. The authors defined pregnancy-associated deaths as those occurring during or within 1 year of the end of pregnancy, but not pregnancy related. A total of 136 pregnancy-associated deaths, including 69 pregnancy-related deaths, were identified.
During the 10-year span of the study, the three most common causes of pregnancy-associated deaths were drugs (n = 35, 26%), thromboembolic disease (n = 18, 13%), and automobile accidents (n = 17, 12%). The remainder of deaths in this group (n = 66, 49%) were caused by cardiac conditions, hypertension, infection, homicide or suicide, hemorrhage, malignancy, or other unspecified causes.
Over the study period, the authors observed a 76% increase in the pregnancy-associated mortality ratio; overall drug-induced pregnancy-associated mortality increased by 200% – from 4 in 2005 to 12 in 2014. About 77% of the drug-induced deaths were caused by opioids. Of the 35 women with drug-induced deaths, 54% were accidental overdoses, 26% were intentional, and the remaining 20% could not be determined.
Of key interest, a detailed review of the records showed that women were not systematically screened for drug use with validated screening tools. In fact, most women received no mental health or drug treatment, nor were they prescribed pharmacotherapy for the treatment of opioid use disorder. Those who died primarily in the late postpartum period and were known to have a drug-induced, pregnancy-associated death already had discontinued obstetrical health care. These findings are consistent with other published studies in Maryland and Georgia.
A 2018 study by Schiff et al. in Massachusetts reported a corresponding decrease in the rate of overdose deaths among those who were receiving pharmacotherapy, especially during the late postpartum period (Obstet Gynecol. 2018 Aug;132[2]:466-74). Dr. Smid and colleagues characterized their findings, in which those with drug-induced deaths were not receiving any kind of treatment, as “a missed opportunity for potentially lifesaving interventions.”
The authors considered their assessment of awareness of drug misuse prior to drug-induced death among obstetric health care providers to be their “unique contribution.” While the majority of women included in the study had known drug misuse or substance abuse disorder, for the 46% of who experienced drug-induced death, this use was not noted at any time during their obstetric care.
The primary limitations of the study were the inability to systematically capture insurance status or coverage lapses. The investigators also could not identify those who carried insurance at the time of their death or to what extent, if any, insurance status proved a barrier to access of mental health or addiction specialty care.
Although the Utah Perinatal Mortality Review Committee characterized 85% of drug-induced pregnancy-associated deaths as unpreventable between 2005 and 2014, new onset or exacerbations of conditions such as depression, anxiety, chronic pain, and substance use disorders generally have been acknowledged to occur both during pregnancy and post partum. Beginning in 2015, the committee began classifying drug-induced deaths as pregnancy related.
Dr. Smid and colleagues speculated that, with ongoing discussion of this topic at national mortality meetings, this kind of important change in classification may be implemented in other states in the future. Such a move would aid in determining the preventability of deaths, thereby leading to a growing awareness of and screening for drug use, both during and after pregnancy. Improved access to mental health and addiction services, as well as increased support for new mothers beyond the traditional 6-week postpartum visit, especially, would be highly beneficial.
Recommendations also have been recently published by the American College of Obstetricians and Gynecologists and the Council on Patient Safety in Women’s Health safety bundle committee on the care of pregnant and postpartum women with opioid use disorders. Separately, ACOG published a committee opinion that reconceptualizes “postpartum care as the extended ‘fourth trimester.’ ” It has further urged that “continued engagement and coordinated care for women with preexisting conditions, including substance use disorder and mental health conditions, is imperative” for reducing severe maternal morbidity and mortality.
“Our results support the mounting evidence that pregnant, and particularly postpartum, women with history of drug use and overdose, psychiatric comorbidities, prior suicide attempt, and polysubstance use need enhanced and ongoing care,” Dr. Smid and associates wrote.
They suggested that additional studies also are needed to better comprehend in what context pregnant and postpartum women are experiencing drug use, relapse, and overdose. “These studies are urgently needed to develop effective strategies to reduce the catastrophic event of maternal death.”
Dr. Smid is supported by Women’s Reproductive Health Research Career Development Program. The authors reported no other financial relationships or potential conflicts of interest.
SOURCE: Smid M et al. Obstet Gynecol. 2019;133:1131-40.
reported Marcela C. Smid, MD, of the University of Utah, Salt Lake City, and her associates.
The stressful demands of newborn care, postpartum depression and anxiety, sleep deprivation, and other factors “may result in the ‘perfect storm’ leading to drug use, relapse, overdose, and death,” they cautioned.
Dr. Smid and associates conducted a retrospective cohort study of all pregnancy-associated deaths occurring in Utah between January 2005 and December 2014 using data from the Utah Perinatal Mortality Review Committee database. The authors defined pregnancy-associated deaths as those occurring during or within 1 year of the end of pregnancy, but not pregnancy related. A total of 136 pregnancy-associated deaths, including 69 pregnancy-related deaths, were identified.
During the 10-year span of the study, the three most common causes of pregnancy-associated deaths were drugs (n = 35, 26%), thromboembolic disease (n = 18, 13%), and automobile accidents (n = 17, 12%). The remainder of deaths in this group (n = 66, 49%) were caused by cardiac conditions, hypertension, infection, homicide or suicide, hemorrhage, malignancy, or other unspecified causes.
Over the study period, the authors observed a 76% increase in the pregnancy-associated mortality ratio; overall drug-induced pregnancy-associated mortality increased by 200% – from 4 in 2005 to 12 in 2014. About 77% of the drug-induced deaths were caused by opioids. Of the 35 women with drug-induced deaths, 54% were accidental overdoses, 26% were intentional, and the remaining 20% could not be determined.
Of key interest, a detailed review of the records showed that women were not systematically screened for drug use with validated screening tools. In fact, most women received no mental health or drug treatment, nor were they prescribed pharmacotherapy for the treatment of opioid use disorder. Those who died primarily in the late postpartum period and were known to have a drug-induced, pregnancy-associated death already had discontinued obstetrical health care. These findings are consistent with other published studies in Maryland and Georgia.
A 2018 study by Schiff et al. in Massachusetts reported a corresponding decrease in the rate of overdose deaths among those who were receiving pharmacotherapy, especially during the late postpartum period (Obstet Gynecol. 2018 Aug;132[2]:466-74). Dr. Smid and colleagues characterized their findings, in which those with drug-induced deaths were not receiving any kind of treatment, as “a missed opportunity for potentially lifesaving interventions.”
The authors considered their assessment of awareness of drug misuse prior to drug-induced death among obstetric health care providers to be their “unique contribution.” While the majority of women included in the study had known drug misuse or substance abuse disorder, for the 46% of who experienced drug-induced death, this use was not noted at any time during their obstetric care.
The primary limitations of the study were the inability to systematically capture insurance status or coverage lapses. The investigators also could not identify those who carried insurance at the time of their death or to what extent, if any, insurance status proved a barrier to access of mental health or addiction specialty care.
Although the Utah Perinatal Mortality Review Committee characterized 85% of drug-induced pregnancy-associated deaths as unpreventable between 2005 and 2014, new onset or exacerbations of conditions such as depression, anxiety, chronic pain, and substance use disorders generally have been acknowledged to occur both during pregnancy and post partum. Beginning in 2015, the committee began classifying drug-induced deaths as pregnancy related.
Dr. Smid and colleagues speculated that, with ongoing discussion of this topic at national mortality meetings, this kind of important change in classification may be implemented in other states in the future. Such a move would aid in determining the preventability of deaths, thereby leading to a growing awareness of and screening for drug use, both during and after pregnancy. Improved access to mental health and addiction services, as well as increased support for new mothers beyond the traditional 6-week postpartum visit, especially, would be highly beneficial.
Recommendations also have been recently published by the American College of Obstetricians and Gynecologists and the Council on Patient Safety in Women’s Health safety bundle committee on the care of pregnant and postpartum women with opioid use disorders. Separately, ACOG published a committee opinion that reconceptualizes “postpartum care as the extended ‘fourth trimester.’ ” It has further urged that “continued engagement and coordinated care for women with preexisting conditions, including substance use disorder and mental health conditions, is imperative” for reducing severe maternal morbidity and mortality.
“Our results support the mounting evidence that pregnant, and particularly postpartum, women with history of drug use and overdose, psychiatric comorbidities, prior suicide attempt, and polysubstance use need enhanced and ongoing care,” Dr. Smid and associates wrote.
They suggested that additional studies also are needed to better comprehend in what context pregnant and postpartum women are experiencing drug use, relapse, and overdose. “These studies are urgently needed to develop effective strategies to reduce the catastrophic event of maternal death.”
Dr. Smid is supported by Women’s Reproductive Health Research Career Development Program. The authors reported no other financial relationships or potential conflicts of interest.
SOURCE: Smid M et al. Obstet Gynecol. 2019;133:1131-40.
reported Marcela C. Smid, MD, of the University of Utah, Salt Lake City, and her associates.
The stressful demands of newborn care, postpartum depression and anxiety, sleep deprivation, and other factors “may result in the ‘perfect storm’ leading to drug use, relapse, overdose, and death,” they cautioned.
Dr. Smid and associates conducted a retrospective cohort study of all pregnancy-associated deaths occurring in Utah between January 2005 and December 2014 using data from the Utah Perinatal Mortality Review Committee database. The authors defined pregnancy-associated deaths as those occurring during or within 1 year of the end of pregnancy, but not pregnancy related. A total of 136 pregnancy-associated deaths, including 69 pregnancy-related deaths, were identified.
During the 10-year span of the study, the three most common causes of pregnancy-associated deaths were drugs (n = 35, 26%), thromboembolic disease (n = 18, 13%), and automobile accidents (n = 17, 12%). The remainder of deaths in this group (n = 66, 49%) were caused by cardiac conditions, hypertension, infection, homicide or suicide, hemorrhage, malignancy, or other unspecified causes.
Over the study period, the authors observed a 76% increase in the pregnancy-associated mortality ratio; overall drug-induced pregnancy-associated mortality increased by 200% – from 4 in 2005 to 12 in 2014. About 77% of the drug-induced deaths were caused by opioids. Of the 35 women with drug-induced deaths, 54% were accidental overdoses, 26% were intentional, and the remaining 20% could not be determined.
Of key interest, a detailed review of the records showed that women were not systematically screened for drug use with validated screening tools. In fact, most women received no mental health or drug treatment, nor were they prescribed pharmacotherapy for the treatment of opioid use disorder. Those who died primarily in the late postpartum period and were known to have a drug-induced, pregnancy-associated death already had discontinued obstetrical health care. These findings are consistent with other published studies in Maryland and Georgia.
A 2018 study by Schiff et al. in Massachusetts reported a corresponding decrease in the rate of overdose deaths among those who were receiving pharmacotherapy, especially during the late postpartum period (Obstet Gynecol. 2018 Aug;132[2]:466-74). Dr. Smid and colleagues characterized their findings, in which those with drug-induced deaths were not receiving any kind of treatment, as “a missed opportunity for potentially lifesaving interventions.”
The authors considered their assessment of awareness of drug misuse prior to drug-induced death among obstetric health care providers to be their “unique contribution.” While the majority of women included in the study had known drug misuse or substance abuse disorder, for the 46% of who experienced drug-induced death, this use was not noted at any time during their obstetric care.
The primary limitations of the study were the inability to systematically capture insurance status or coverage lapses. The investigators also could not identify those who carried insurance at the time of their death or to what extent, if any, insurance status proved a barrier to access of mental health or addiction specialty care.
Although the Utah Perinatal Mortality Review Committee characterized 85% of drug-induced pregnancy-associated deaths as unpreventable between 2005 and 2014, new onset or exacerbations of conditions such as depression, anxiety, chronic pain, and substance use disorders generally have been acknowledged to occur both during pregnancy and post partum. Beginning in 2015, the committee began classifying drug-induced deaths as pregnancy related.
Dr. Smid and colleagues speculated that, with ongoing discussion of this topic at national mortality meetings, this kind of important change in classification may be implemented in other states in the future. Such a move would aid in determining the preventability of deaths, thereby leading to a growing awareness of and screening for drug use, both during and after pregnancy. Improved access to mental health and addiction services, as well as increased support for new mothers beyond the traditional 6-week postpartum visit, especially, would be highly beneficial.
Recommendations also have been recently published by the American College of Obstetricians and Gynecologists and the Council on Patient Safety in Women’s Health safety bundle committee on the care of pregnant and postpartum women with opioid use disorders. Separately, ACOG published a committee opinion that reconceptualizes “postpartum care as the extended ‘fourth trimester.’ ” It has further urged that “continued engagement and coordinated care for women with preexisting conditions, including substance use disorder and mental health conditions, is imperative” for reducing severe maternal morbidity and mortality.
“Our results support the mounting evidence that pregnant, and particularly postpartum, women with history of drug use and overdose, psychiatric comorbidities, prior suicide attempt, and polysubstance use need enhanced and ongoing care,” Dr. Smid and associates wrote.
They suggested that additional studies also are needed to better comprehend in what context pregnant and postpartum women are experiencing drug use, relapse, and overdose. “These studies are urgently needed to develop effective strategies to reduce the catastrophic event of maternal death.”
Dr. Smid is supported by Women’s Reproductive Health Research Career Development Program. The authors reported no other financial relationships or potential conflicts of interest.
SOURCE: Smid M et al. Obstet Gynecol. 2019;133:1131-40.
FROM OBSTETRICS & GYNECOLOGY
Key clinical point: Drug-induced death is the leading cause of pregnancy-associated death in Utah, primarily occurring in the late postpartum period.
Major finding: Despite known history, pregnant women were not systematically screened for drug use or treated for mental health disorders or drug misuse.
Study details: A retrospective cohort study of 136 pregnancy-associated deaths, of which 26% were drug induced.
Disclosures: Dr. Smid is supported by the Women’s Reproductive Health Research Career Development Program. The authors reported no other financial relationships or potential conflicts of interest.
Source: Smid M et al. Obstet Gynecol. 2019;133:1131-40.
Benzodiazepines nearly double the odds of spontaneous abortion
Early-pregnancy spontaneous abortion was almost twice as common among women who used benzodiazepines, according to 17 years’ worth of data from the Quebec Pregnancy Cohort, which prospectively collects data on all pregnancies of women covered by the Quebec Public Prescription Drug Insurance Plan.
The findings suggest the need for caution before prescribing benzodiazepines to treat insomnia and mood or anxiety disorders in early pregnancy. “Alternative nonpharmacologic treatments exist and are recommended, but if benzodiazepines are needed, they should be prescribed for short durations,” wrote Odile Sheehy, MSc, of the Research Center at Centre Hospitalier Universitaire Sainte-Justine, Montreal, and colleagues, in a study published in JAMA Psychiatry.
The researchers evaluated data from 27,149 study-eligible women who had a spontaneous abortion after 6 weeks’ gestation and before 20 weeks’ gestation between Jan. 1, 1998, and Dec. 31, 2015. Among filled prescriptions, at least one benzodiazepine was used by 375 (1.4%) of the women. These women were matched with five randomly selected control pregnancies per case. The data were adjusted for diagnoses of mood and anxiety disorders and insomnia as well as for several documented proxies of these diseases, such as concomitant exposure to antidepressants or antipsychotics, visits to a psychiatrist, comorbidities, and hospitalizations.
The investigators found an adjusted odds ratio (aOR) of 1.85 (95% confidence interval, 1.61-2.12) for benzodiazepine use. The odds of spontaneous abortion was increased with use of all types of benzodiazepines evaluated in the study, with aORs as low as 1.13 and as high as 3.43, as well as similar aORs between long-acting and short-acting benzodiazepines (1.81 vs. 1.73, respectively).
While the information is accurate regarding filled prescriptions, the findings might not apply to women with private drug insurance as the study included only women in a prescription drug program, the researchers said. They noted, however, that pregnant women receiving medication insurance from Quebec’s public system have characteristics and comorbidities similar to those of women who are covered by private medication insurance.
One author reported being a consultant for plaintiffs in litigations involving antidepressants and birth defects. No other disclosures were reported.
SOURCE: Sheehy O et al. JAMA Psychiatry. 2019 May 15. doi: 10.1001/jamapsychiatry.2019.0963.
Early-pregnancy spontaneous abortion was almost twice as common among women who used benzodiazepines, according to 17 years’ worth of data from the Quebec Pregnancy Cohort, which prospectively collects data on all pregnancies of women covered by the Quebec Public Prescription Drug Insurance Plan.
The findings suggest the need for caution before prescribing benzodiazepines to treat insomnia and mood or anxiety disorders in early pregnancy. “Alternative nonpharmacologic treatments exist and are recommended, but if benzodiazepines are needed, they should be prescribed for short durations,” wrote Odile Sheehy, MSc, of the Research Center at Centre Hospitalier Universitaire Sainte-Justine, Montreal, and colleagues, in a study published in JAMA Psychiatry.
The researchers evaluated data from 27,149 study-eligible women who had a spontaneous abortion after 6 weeks’ gestation and before 20 weeks’ gestation between Jan. 1, 1998, and Dec. 31, 2015. Among filled prescriptions, at least one benzodiazepine was used by 375 (1.4%) of the women. These women were matched with five randomly selected control pregnancies per case. The data were adjusted for diagnoses of mood and anxiety disorders and insomnia as well as for several documented proxies of these diseases, such as concomitant exposure to antidepressants or antipsychotics, visits to a psychiatrist, comorbidities, and hospitalizations.
The investigators found an adjusted odds ratio (aOR) of 1.85 (95% confidence interval, 1.61-2.12) for benzodiazepine use. The odds of spontaneous abortion was increased with use of all types of benzodiazepines evaluated in the study, with aORs as low as 1.13 and as high as 3.43, as well as similar aORs between long-acting and short-acting benzodiazepines (1.81 vs. 1.73, respectively).
While the information is accurate regarding filled prescriptions, the findings might not apply to women with private drug insurance as the study included only women in a prescription drug program, the researchers said. They noted, however, that pregnant women receiving medication insurance from Quebec’s public system have characteristics and comorbidities similar to those of women who are covered by private medication insurance.
One author reported being a consultant for plaintiffs in litigations involving antidepressants and birth defects. No other disclosures were reported.
SOURCE: Sheehy O et al. JAMA Psychiatry. 2019 May 15. doi: 10.1001/jamapsychiatry.2019.0963.
Early-pregnancy spontaneous abortion was almost twice as common among women who used benzodiazepines, according to 17 years’ worth of data from the Quebec Pregnancy Cohort, which prospectively collects data on all pregnancies of women covered by the Quebec Public Prescription Drug Insurance Plan.
The findings suggest the need for caution before prescribing benzodiazepines to treat insomnia and mood or anxiety disorders in early pregnancy. “Alternative nonpharmacologic treatments exist and are recommended, but if benzodiazepines are needed, they should be prescribed for short durations,” wrote Odile Sheehy, MSc, of the Research Center at Centre Hospitalier Universitaire Sainte-Justine, Montreal, and colleagues, in a study published in JAMA Psychiatry.
The researchers evaluated data from 27,149 study-eligible women who had a spontaneous abortion after 6 weeks’ gestation and before 20 weeks’ gestation between Jan. 1, 1998, and Dec. 31, 2015. Among filled prescriptions, at least one benzodiazepine was used by 375 (1.4%) of the women. These women were matched with five randomly selected control pregnancies per case. The data were adjusted for diagnoses of mood and anxiety disorders and insomnia as well as for several documented proxies of these diseases, such as concomitant exposure to antidepressants or antipsychotics, visits to a psychiatrist, comorbidities, and hospitalizations.
The investigators found an adjusted odds ratio (aOR) of 1.85 (95% confidence interval, 1.61-2.12) for benzodiazepine use. The odds of spontaneous abortion was increased with use of all types of benzodiazepines evaluated in the study, with aORs as low as 1.13 and as high as 3.43, as well as similar aORs between long-acting and short-acting benzodiazepines (1.81 vs. 1.73, respectively).
While the information is accurate regarding filled prescriptions, the findings might not apply to women with private drug insurance as the study included only women in a prescription drug program, the researchers said. They noted, however, that pregnant women receiving medication insurance from Quebec’s public system have characteristics and comorbidities similar to those of women who are covered by private medication insurance.
One author reported being a consultant for plaintiffs in litigations involving antidepressants and birth defects. No other disclosures were reported.
SOURCE: Sheehy O et al. JAMA Psychiatry. 2019 May 15. doi: 10.1001/jamapsychiatry.2019.0963.
FROM JAMA PSYCHIATRY
Two cases of genital pruritus: What is the one diagnosis?
Lichen sclerosus
Lichen sclerosus is an inflammatory skin disease that primarily affects the genital and perianal skin of postmenopausal women. The mean age of onset is the mid- to late 50s; fewer than 15% of lichen sclerosus cases present in children.1,2 Case 1 represents presentation of vulvar lichen sclerosus in a premenopausal woman, which is uncommon.
The classic presentation of lichen sclerosus is a well-defined white, atrophic plaque with a wrinkled surface appearance located on the vulva, perineum, and perianal skin. Less commonly, examination may reveal white papules and macules, pallor with overlying edema, or hyperpigmentation. Loss of labia minora tissue and phimosis of the clitoral hood also are often present in patients with untreated lichen sclerosus.
Additionally, secondary changes, such as erosions, fissuring, and blisters, can be seen on examination. The most frequent symptom associated with lichen sclerosus is intense itching of the affected area. Other symptoms include dyspareunia, dysuria, sexual dysfunction, and bleeding. Occasionally, lichen sclerosus is asymptomatic.1 Like other autoimmune conditions, lichen sclerosus may persist indefinitely, highlighting the importance of effective treatment.
How should we evaluate and treat patients with these symptoms?
Perform a skin biopsy and start treatment with very high–potency topical corticosteroid ointment daily for at least 6 weeks.
Skin biopsy. Definitive diagnosis of lichen sclerosus is made based on a skin biopsy. Because treatment can impact the interpretation of a skin biopsy, a biopsy is optimally performed prior to treatment initiation.
The patient in Case 1 underwent biopsy of the left labia majora. Results were consistent with early lichen sclerosus. The patient in Case 2 was reluctant to proceed with vulvar biopsy.
A biopsy specimen should be taken from the affected area that is most white in appearance.1
Topical treatment. To induce remission, twice-daily application of very high–potency topical corticosteroid ointment to the affected area for at least 6 weeks is recommended. Once the skin color and texture have normalized, the topical corticosteroid strength (and frequency of application) can slowly be reduced to the lowest potency/frequency at which the patient remains in remission. Examples of very high–, high-, moderate-, and low-potency corticosteroid ointments are listed in the TABLE.
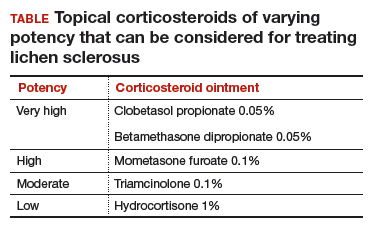
Follow-up. Evaluate the patient every 3 months until the topical steroid potency remains stable and the skin appearance is normal.
Treat early, and aggressively, to prevent complications
Early diagnosis and aggressive intervention are important in managing this disease process. If diagnosis and treatment are delayed, significant scarring and deformation of the vulva can occur.1
Neoplastic transformation of lichen sclerosus into vulvar intraepithelial neoplasia and squamous cell carcinoma can occur (mean incidence, 2.8%). However, the literature reports significant variability in the incidence, ranging between 0% and 31%.3 Published reports support decreased scarring and future development of malignancies in patients who adhere to treatment recommendations.4
Symptoms resolved
In both cases described here, the patients were treated with clobetasol 0.05% ointment twice daily for 6 weeks. Both women reported complete resolution of pruritus after treatment. As can be seen in the posttreatment photo of the patient described in Case 1, her vulvar inflammation resolved (FIGURE 4).
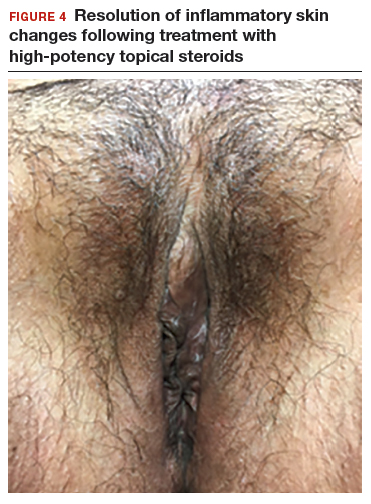
These cases represent the varied exam findings in patients experiencing vulvar pruritus with early (Case 1) versus more advanced (Case 2) lichen sclerosus. In addition, they underscore that appropriate evaluation and management of lichen sclerosus can produce excellent treatment results.
- Lee A, Fischer G. Diagnosis and treatment of vulvar lichen sclerosus: an update for dermatologists. Am J Clin Dermatol. 2018;19:695.
- Tong LX, Sun GS, Teng JM. Pediatric lichen sclerosus: a review of the epidemiology and treatment options. Pediatr Dermatol. 2015;32:593-599.
- Micheletti L, Preti M, Radici G, et al. Vulvar lichen sclerosus and neoplastic transformation: a retrospective study of 976 cases. J Low Genit Tract Dis. 2016;20:180-183.
- Lee A, Bradford J, Fischer G. Long-term management of adult vulvar lichen sclerosus: a prospective cohort study of 507 women. JAMA Dermatol. 2015;151:1061-1067.
Lichen sclerosus
Lichen sclerosus is an inflammatory skin disease that primarily affects the genital and perianal skin of postmenopausal women. The mean age of onset is the mid- to late 50s; fewer than 15% of lichen sclerosus cases present in children.1,2 Case 1 represents presentation of vulvar lichen sclerosus in a premenopausal woman, which is uncommon.
The classic presentation of lichen sclerosus is a well-defined white, atrophic plaque with a wrinkled surface appearance located on the vulva, perineum, and perianal skin. Less commonly, examination may reveal white papules and macules, pallor with overlying edema, or hyperpigmentation. Loss of labia minora tissue and phimosis of the clitoral hood also are often present in patients with untreated lichen sclerosus.
Additionally, secondary changes, such as erosions, fissuring, and blisters, can be seen on examination. The most frequent symptom associated with lichen sclerosus is intense itching of the affected area. Other symptoms include dyspareunia, dysuria, sexual dysfunction, and bleeding. Occasionally, lichen sclerosus is asymptomatic.1 Like other autoimmune conditions, lichen sclerosus may persist indefinitely, highlighting the importance of effective treatment.
How should we evaluate and treat patients with these symptoms?
Perform a skin biopsy and start treatment with very high–potency topical corticosteroid ointment daily for at least 6 weeks.
Skin biopsy. Definitive diagnosis of lichen sclerosus is made based on a skin biopsy. Because treatment can impact the interpretation of a skin biopsy, a biopsy is optimally performed prior to treatment initiation.
The patient in Case 1 underwent biopsy of the left labia majora. Results were consistent with early lichen sclerosus. The patient in Case 2 was reluctant to proceed with vulvar biopsy.
A biopsy specimen should be taken from the affected area that is most white in appearance.1
Topical treatment. To induce remission, twice-daily application of very high–potency topical corticosteroid ointment to the affected area for at least 6 weeks is recommended. Once the skin color and texture have normalized, the topical corticosteroid strength (and frequency of application) can slowly be reduced to the lowest potency/frequency at which the patient remains in remission. Examples of very high–, high-, moderate-, and low-potency corticosteroid ointments are listed in the TABLE.

Follow-up. Evaluate the patient every 3 months until the topical steroid potency remains stable and the skin appearance is normal.
Treat early, and aggressively, to prevent complications
Early diagnosis and aggressive intervention are important in managing this disease process. If diagnosis and treatment are delayed, significant scarring and deformation of the vulva can occur.1
Neoplastic transformation of lichen sclerosus into vulvar intraepithelial neoplasia and squamous cell carcinoma can occur (mean incidence, 2.8%). However, the literature reports significant variability in the incidence, ranging between 0% and 31%.3 Published reports support decreased scarring and future development of malignancies in patients who adhere to treatment recommendations.4
Symptoms resolved
In both cases described here, the patients were treated with clobetasol 0.05% ointment twice daily for 6 weeks. Both women reported complete resolution of pruritus after treatment. As can be seen in the posttreatment photo of the patient described in Case 1, her vulvar inflammation resolved (FIGURE 4).

These cases represent the varied exam findings in patients experiencing vulvar pruritus with early (Case 1) versus more advanced (Case 2) lichen sclerosus. In addition, they underscore that appropriate evaluation and management of lichen sclerosus can produce excellent treatment results.
Lichen sclerosus
Lichen sclerosus is an inflammatory skin disease that primarily affects the genital and perianal skin of postmenopausal women. The mean age of onset is the mid- to late 50s; fewer than 15% of lichen sclerosus cases present in children.1,2 Case 1 represents presentation of vulvar lichen sclerosus in a premenopausal woman, which is uncommon.
The classic presentation of lichen sclerosus is a well-defined white, atrophic plaque with a wrinkled surface appearance located on the vulva, perineum, and perianal skin. Less commonly, examination may reveal white papules and macules, pallor with overlying edema, or hyperpigmentation. Loss of labia minora tissue and phimosis of the clitoral hood also are often present in patients with untreated lichen sclerosus.
Additionally, secondary changes, such as erosions, fissuring, and blisters, can be seen on examination. The most frequent symptom associated with lichen sclerosus is intense itching of the affected area. Other symptoms include dyspareunia, dysuria, sexual dysfunction, and bleeding. Occasionally, lichen sclerosus is asymptomatic.1 Like other autoimmune conditions, lichen sclerosus may persist indefinitely, highlighting the importance of effective treatment.
How should we evaluate and treat patients with these symptoms?
Perform a skin biopsy and start treatment with very high–potency topical corticosteroid ointment daily for at least 6 weeks.
Skin biopsy. Definitive diagnosis of lichen sclerosus is made based on a skin biopsy. Because treatment can impact the interpretation of a skin biopsy, a biopsy is optimally performed prior to treatment initiation.
The patient in Case 1 underwent biopsy of the left labia majora. Results were consistent with early lichen sclerosus. The patient in Case 2 was reluctant to proceed with vulvar biopsy.
A biopsy specimen should be taken from the affected area that is most white in appearance.1
Topical treatment. To induce remission, twice-daily application of very high–potency topical corticosteroid ointment to the affected area for at least 6 weeks is recommended. Once the skin color and texture have normalized, the topical corticosteroid strength (and frequency of application) can slowly be reduced to the lowest potency/frequency at which the patient remains in remission. Examples of very high–, high-, moderate-, and low-potency corticosteroid ointments are listed in the TABLE.

Follow-up. Evaluate the patient every 3 months until the topical steroid potency remains stable and the skin appearance is normal.
Treat early, and aggressively, to prevent complications
Early diagnosis and aggressive intervention are important in managing this disease process. If diagnosis and treatment are delayed, significant scarring and deformation of the vulva can occur.1
Neoplastic transformation of lichen sclerosus into vulvar intraepithelial neoplasia and squamous cell carcinoma can occur (mean incidence, 2.8%). However, the literature reports significant variability in the incidence, ranging between 0% and 31%.3 Published reports support decreased scarring and future development of malignancies in patients who adhere to treatment recommendations.4
Symptoms resolved
In both cases described here, the patients were treated with clobetasol 0.05% ointment twice daily for 6 weeks. Both women reported complete resolution of pruritus after treatment. As can be seen in the posttreatment photo of the patient described in Case 1, her vulvar inflammation resolved (FIGURE 4).

These cases represent the varied exam findings in patients experiencing vulvar pruritus with early (Case 1) versus more advanced (Case 2) lichen sclerosus. In addition, they underscore that appropriate evaluation and management of lichen sclerosus can produce excellent treatment results.
- Lee A, Fischer G. Diagnosis and treatment of vulvar lichen sclerosus: an update for dermatologists. Am J Clin Dermatol. 2018;19:695.
- Tong LX, Sun GS, Teng JM. Pediatric lichen sclerosus: a review of the epidemiology and treatment options. Pediatr Dermatol. 2015;32:593-599.
- Micheletti L, Preti M, Radici G, et al. Vulvar lichen sclerosus and neoplastic transformation: a retrospective study of 976 cases. J Low Genit Tract Dis. 2016;20:180-183.
- Lee A, Bradford J, Fischer G. Long-term management of adult vulvar lichen sclerosus: a prospective cohort study of 507 women. JAMA Dermatol. 2015;151:1061-1067.
- Lee A, Fischer G. Diagnosis and treatment of vulvar lichen sclerosus: an update for dermatologists. Am J Clin Dermatol. 2018;19:695.
- Tong LX, Sun GS, Teng JM. Pediatric lichen sclerosus: a review of the epidemiology and treatment options. Pediatr Dermatol. 2015;32:593-599.
- Micheletti L, Preti M, Radici G, et al. Vulvar lichen sclerosus and neoplastic transformation: a retrospective study of 976 cases. J Low Genit Tract Dis. 2016;20:180-183.
- Lee A, Bradford J, Fischer G. Long-term management of adult vulvar lichen sclerosus: a prospective cohort study of 507 women. JAMA Dermatol. 2015;151:1061-1067.
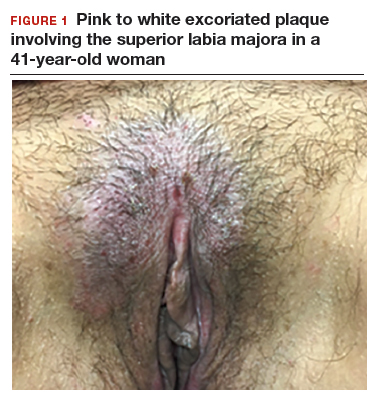
CASE 1: Vulvar pruritus affecting a woman’s quality of life
A 41-year-old premenopausal white woman presented to her gynecologist with intense vulvar pruritus for a 6-month duration, with a recent increase in severity (FIGURE 1). She tried treating it with topical antifungal cream, hydrocortisone ointment, and coconut oil, with no improvement. She noted that the intense itching was interfering with her sleep and marriage. The patient denied having an increase in urinary frequency or urgency, dysuria, hematochezia, or bowel changes.
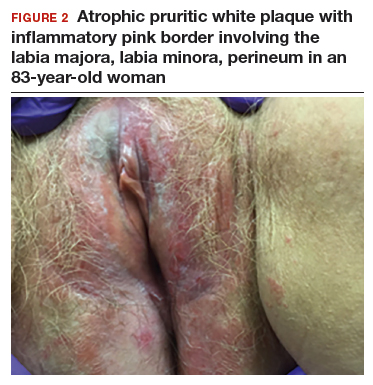
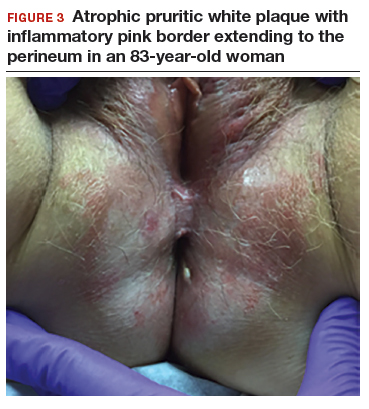
CASE 2: Older woman with long-term persistent genital pruritus
An 83-year-old postmenopausal white woman presented to the dermatology clinic for a regular skin examination. The patient endorsed symptoms of vulvar and perianal pruritus that had persisted for more than 6 months (FIGURES 2 and 3). The genital itching occurred throughout most of the day. The patient previously treated her symptoms with an over-the-counter antifungal cream, which minimally improved the itching.
Advanced Melanoma: First-line Therapy
Malignant melanoma is the most serious form of primary skin cancer and one of the only malignancies in which the incidence rate has been rising. It is estimated that in 2018 there were 91,270 newly diagnosed cases and 9320 deaths from advanced melanoma in the United States. Melanoma is the fifth most common cancer type in males and the sixth most common in females. Despite rising incidence rates, improvement in the treatment of advanced melanoma has resulted in declining death rates over the past decade.1 Although most melanoma is diagnosed at an early stage and can be cured with surgical excision, the prognosis for metastatic melanoma had been historically poor prior to recent advancements in treatment. Conventional chemotherapy treatment with dacarbazine or temozolomide resulted in response rates ranging from 7.5% to 12.1%, but without much impact on median overall survival (OS), with reported OS ranging from 6.4 to 7.8 months. Combination approaches with interferon alfa-2B and low-dose interleukin-2 resulted in improved response rates compared with traditional chemotherapy, but again without survival benefit.2
Immunotherapy in the form of high-dose interleukin-2 emerged as the first therapy to alter the natural history of advanced melanoma, with both improved response rates (objective response rate [ORR], 16%) and median OS (2 months), with some patients achieving durable responses lasting more than 30 months. However, significant systemic toxicity limited its application to carefully selected patients.3 The past decade has brought rapid advancements in treatment with immune checkpoint inhibitors and molecularly targeted agents, which have significantly improved ORRs, progression-free survival (PFS), and OS for patients with metastatic melanoma.4-8
This review is the first of 2 articles focusing on the treatment and sequencing of therapies in advanced melanoma. Here, we review the selection of first-line therapy for metastatic melanoma. Current evidence for immune checkpoint blockade and molecularly targeted agents in the treatment of metastatic melanoma after progression on first-line therapy is discussed in a separate article.
Pathogenesis
The incidence of melanoma is strongly associated with ultraviolet light–mediated DNA damage related to sun exposure. Specifically, melanoma is associated to a greater degree with intense intermittent sun exposure and sunburn, but not associated with higher occupational exposure.9 Ultraviolet radiation can induce DNA damage by a number of mechanisms, and deficient DNA repair leads to somatic mutations that drive the progression from normal melanocyte to melanoma.10
The most commonly identified genetic mutations in cutaneous melanomas are alterations in the mitogen-activated protein kinase (MAPK) pathway. Typically, an extracellular growth factor causes dimerization of the growth factor receptor, which activates the intracellular RAS GTPase protein. Subsequently BRAF is phosphorylated within the kinase domain, which leads to downstream activation of the MEK and ERK kinases through phosphorylation. Activated ERK leads to phosphorylation of various cytoplasmic and nuclear targets, and the downstream effects of these changes promote cellular proliferation. While activation of this pathway usually requires phosphorylation of BRAF by RAS, mutations placing an acidic amino acid near the kinase domain mimics phosphorylation and leads to constitutive activation of the BRAF serine/threonine kinase in the absence of upstream signaling from extracellular growth factors mediated through RAS.11 One study of tumor samples of 71 patients with cutaneous melanoma detected NRAS mutations in 30% and BRAF mutations in 59% of all tumors tested. Of the BRAF mutation–positive tumors, 88% harbored the Val599Glu mutation, now commonly referred to as the BRAF V600E mutation. The same study demonstrated that the vast majority of BRAF mutations were seen in the primary tumor and were preserved when metastases were analyzed. Additionally, both NRAS and BRAF mutations were detected in the radial growth phase of the melanoma tumor. These findings indicate that alterations in the MAPK pathway occur early in the pathogenesis of advanced melanoma.11 Another group demonstrated that 66% of malignant melanoma tumor samples harbored BRAF mutations, of which 80% were specifically the V600E mutation. In vitro assays showed that the BRAF V600E–mutated kinase had greater than 10-fold kinase activity compared to wild-type BRAF, and that this kinase enhanced cellular proliferation even when upstream NRAS signaling was inhibited.12
The Cancer Genome Atlas Network performed a large analysis of tumor samples from 331 different melanoma patients and studied variations at the DNA, RNA, and protein levels. The study established a framework of 4 notable genomic subtypes, including mutant BRAF (52%), mutant RAS (28%), mutant NF1 (14%), and triple wild-type (6%). Additionally, mRNA transcriptomic analysis of overexpressed genes identified 3 different subclasses, which were labeled as “immune,” “keratin,” and “MITF-low.” The immune subclass was characterized by increased expression of proteins found in immune cells, immune signaling molecules, immune checkpoint proteins, cytokines, and chemokines, and correlated with increased lymphocyte invasion within the tumor. Interestingly, in the post-accession survival analysis, the “immune” transcriptomic subclass was statistically correlated with an improved prognosis.13 Having an understanding of the molecular pathogenesis of advanced melanoma helps to create a framework for understanding the mechanisms of current standard of care therapies for the disease.
Case Presentation
A 62-year-old Caucasian man with a history of well-controlled type 2 diabetes mellitus and hypertension is being followed by his dermatologist for surveillance of melanocytic nevi. On follow-up he is noted to have an asymmetrical melanocytic lesion over the right scalp with irregular borders and variegated color. He is asymptomatic and the remainder of physical examination is unremarkable, as he has no other concerning skin lesions and no cervical, axillary, or inguinal lymphadenopathy.
How is melanoma diagnosed?
Detailed discussion about diagnosis and staging will be deferred in this review of treatment of advanced melanoma. In brief, melanoma is best diagnosed by excisional biopsy and histopathology. Staging of melanoma is done according to the American Joint Committee on Cancer’s (AJCC) Cancer Staging Manual, 8th edition, using a TNM staging system that incorporates tumor thickness (Breslow depth); ulceration; number of involved regional lymph nodes; presence of in-transit, satellite, and/or microsatellite metastases; distant metastases; and serum lactate dehydrogenase level.14
Case Continued
The patient undergoes a wide excisional biopsy of the right scalp lesion, which is consistent with malignant melanoma. Pathology demonstrates a Breslow depth of 2.6 mm, 2 mitotic figures/mm2, and no evidence of ulceration. He subsequently undergoes wide local excision with 0/3 sentinel lymph nodes positive for malignancy. His final staging is consistent with pT3aN0M0, stage IIA melanoma.
He is seen in follow-up with medical oncology for the next 3.5 years without any evidence of disease recurrence. He then develops symptoms of vertigo, diplopia, and recurrent falls, prompting medical attention. Magnetic resonance imaging (MRI) brain reveals multiple supratentorial and infratentorial lesions concerning for intracranial metastases. Further imaging with computed tomography (CT) chest/abdomen/pelvis reveals a right lower lobe pulmonary mass with right hilar and subcarinal lymphadenopathy. He is admitted for treatment with intravenous dexamethasone and further evaluation with endobronchial ultrasound-guided fine-needle aspiration of the right lower lobe mass, which reveals metastatic melanoma. Given the extent of his intracranial metastases, he is treated with whole brain radiation therapy for symptomatic relief prior to initiating systemic therapy.
What is the general approach to first-line treatment for metastatic melanoma?
The past decade has brought an abundance of data supporting the use of immunotherapy with immune checkpoint inhibitors or molecularly targeted therapy with combined BRAF/MEK inhibitors in the first-line setting.4-8 After the diagnosis of metastatic melanoma has been made, molecular testing is recommended to determine the BRAF status of the tumor. Immunotherapy is the clear choice for first-line therapy in the absence of an activating BRAF V600 mutation. When a BRAF V600 mutation is present, current evidence supports the use of either immunotherapy or molecularly targeted therapy as first-line therapy.
To date, there have been no prospective clinical trials comparing the sequencing of immunotherapy and molecularly targeted therapy in the first-line setting. An ongoing clinical trial (NCT02224781) is comparing dabrafenib and trametinib followed by ipilimumab and nivolumab at time of progression to ipilimumab and nivolumab followed by dabrafenib and trametinib in patients with newly diagnosed stage III/IV BRAF V600 mutation–positive melanoma. The primary outcome measure is 2-year OS. Until completion of that trial, current practice regarding which type of therapy to use in the first-line setting is based on a number of factors including clinical characteristics and provider preferences.
Data suggest that immunotherapies can produce durable responses, especially after treatment completion or discontinuation, albeit at the expense of taking a longer time to achieve clinical benefit and the risk of potentially serious immune-related adverse effects. This idea of a durable, off-treatment response is highlighted by a study that followed 105 patients who had achieved a complete response (CR) and found that 24-month disease-free survival from the time of CR was 90.9% in all patients and 89.9% in the 67 patients who had discontinued pembrolizumab after attaining CR.15 BRAF/MEK inhibition has the potential for rapid clinical responses, though concerns exist about the development of resistance to therapy. The following sections explore the evidence supporting the use of these therapies.
Immunotherapy with Immune Checkpoint Inhibitors
Immunotherapy via immune checkpoint blockade has revolutionized the treatment of many solid tumors over the past decade. The promise of immunotherapy revolves around the potential for achieving a dynamic and durable systemic response against cancer by augmenting the antitumor effects of the immune system. T-cells are central to mounting a systemic antitumor response, and, in addition to antigen recognition, their function depends heavily on fine tuning between co-stimulatory and co-inhibitory signaling. The cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) expressed on T-cells was the first discovered co-inhibitory receptor of T-cell activation.16 Later, it was discovered that the programmed cell death 1 receptor (PD-1), expressed on T-cells, and its ligands PD-L1 and PD-L2, expressed on antigen presenting cells, tumor cells, or other cells in the tumor microenvironment, also served as a potent negative regulator of T-cell function.17
Together, these 2 signaling pathways help to maintain peripheral immune tolerance, whereby autoreactive T-cells that have escaped from the thymus are silenced to prevent autoimmunity. However, these pathways can also be utilized by cancer cells to escape immune surveillance. Monoclonal antibodies that inhibit the aforementioned co-inhibitory signaling pathways, and thus augment the immune response, have proven to be an effective anticancer therapy capable of producing profound and durable responses in certain malignancies.16,17
Ipilimumab
Ipilimumab is a monoclonal antibody that inhibits the function of the CTLA-4 co-inhibitory immune checkpoint. In a phase 3 randomized controlled trial of 676 patients with previously treated metastatic melanoma, ipilimumab at a dose of 3 mg/kg every 3 weeks for 4 cycles, with or without a gp100 peptide vaccine, resulted in an improved median OS of 10.0 and 10.1 months, respectively, compared to 6.4 months in those receiving the peptide vaccine alone, meeting the primary endpoint.4 Subsequently, a phase 3 trial of 502 patients with untreated metastatic melanoma compared ipilimumab at a dose of 10 mg/kg every 3 weeks for 4 cycles plus dacarbazine to dacarbazine plus placebo and found a significant increase in median OS (11.2 months vs 9.1 months), with no additive benefit of chemotherapy. There was a higher reported rate of grade 3 or 4 adverse events in this trial with ipilimumab dosed at 10 mg/kg, which was felt to be dose-related.18 These trials were the first to show improved OS with any systemic therapy in metastatic melanoma and led to US Food and Drug Administration approval of ipilimumab for this indication in 2011.
PD-1 Inhibitor Monotherapy
The PD-1 inhibitors nivolumab and pembrolizumab were initially approved for metastatic melanoma after progression on ipilimumab. In the phase 1 trial of patients with previously treated metastatic melanoma, nivolumab therapy resulted in an ORR of 28%.19 The subsequent phase 2 trial conducted in pretreated patients, including patients who had progressed on ipilimumab, confirmed a similar ORR of 31%, as well as a median PFS of 3.7 months and a median OS of 16.8 months. The estimated response duration in patients who did achieve a response to therapy was 2 years.20 A phase 3 trial (CheckMate 037) comparing nivolumab (n = 120) to investigator’s choice chemotherapy (n = 47) in those with melanoma refractory to ipilimumab demonstrated that nivolumab was superior for the primary endpoint of ORR (31.7% vs 10.6%), had less toxicity (5% rate of grade 3 or 4 adverse events versus 9%), and increased median duration of response (32 months vs 13 months).21
The phase 1 trial (KEYNOTE-001) testing the efficacy of pembrolizumab demonstrated an ORR of 33% in the total population of patients treated and an ORR of 45% in those who were treatment-naive. Additionally, the median OS was 23 months for the total population and 31 months for treatment-naive patients, with only 14% of patients experiencing a grade 3 or 4 adverse event.22 The KEYNOTE-002 phase 2 trial compared 2 different pembrolizumab doses (2 mg/kg and 10 mg/kg every 3 weeks) to investigator’s choice chemotherapy (paclitaxel plus carboplatin, paclitaxel, carboplatin, dacarbazine, or oral temozolomide) in 540 patients with advanced melanoma with documented progression on ipilimumab with or without prior progression on molecularly targeted therapy if positive for a BRAF V600 mutation. The final analysis demonstrated significantly improved ORR with pembrolizumab (22% at 2 mg/kg vs 26% at 10 mg/kg vs 4% chemotherapy) and significantly improved 24-month PFS (16% vs 22% vs 0.6%, respectively). There was a nonstatistically significant improvement in median OS (13.4 months vs 14.7 months vs 10 months), although 55% of the patients initially assigned to the chemotherapy arm crossed over and received pembrolizumab after documentation of progressive disease.23,24
Because PD-1 inhibition improved efficacy with less toxicity than chemotherapy when studied in progressive disease, subsequent studies focused on PD-1 inhibition in the frontline setting. CheckMate 066 was a phase 3 trial comparing nivolumab to dacarbazine as first-line therapy for 418 patients with untreated metastatic melanoma who did not have a BRAF mutation. For the primary end point of 1-year OS, nivolumab was superior to dacarbazine (72.9% vs 42.1%; hazard ratio [HR], 0.42; P < 0.001). Treatment with nivolumab also resulted in superior ORR (40% vs 14%) and PFS (5.1 months vs 2.2 months). Additionally, nivolumab therapy had a lower rate of grade 3 or 4 toxicity compared to dacarbazine (11.7% vs 17.6%).25
The KEYNOTE-006 trial compared 2 separate dosing schedules of pembrolizumab (10 mg/kg every 2 weeks versus every 3 weeks) to ipilimumab (3 mg/kg every 3 weeks for 4 cycles) in a 1:1:1 ratio in 834 patients with metastatic melanoma who had received up to 1 prior systemic therapy, but no prior CTLA-4 or PD-1 inhibitors. The first published data reported statistically significant outcomes for the co-primary end points of 6-month PFS (47.3% for pembrolizumab every 2 weeks vs 46.4% for pembrolizumab every 3 weeks vs 26.5% for ipilimumab; HR, 0.58 for both pembrolizumab groups compared to ipilimumab; P < 0.001) and 12-month OS (74.1% vs 68.4% vs 58.2%) with pembrolizumab compared to ipilimumab. Compared to ipilimumab, pembrolizumab every 2 weeks had a hazard ratio of 0.63 (P = 0.0005) and pembrolizumab every 3 weeks had a hazard ratio of 0.69 (P = 0.0036). The pembrolizumab groups was also had lower rates of grade 3 to 5 toxicity (13.3% vs 10.1% vs 19.9%).5 Updated outcomes demonstrated improved ORR compared to the first analysis (37% vs 36% vs 13%), and improved OS (median OS, not reached for the pembrolizumab groups vs 16.0 months for the ipilimumab group; HR, 0.68, P = 0.0009 for pembrolizumab every 2 weeks versus HR 0.68, P = 0.0008 for pembrolizumab every 3 weeks).26 In addition, 24-month OS was 55% in both pembrolizumab groups compared to 43% in the ipilimumab group. Grade 3 or 4 toxicity occurred less frequently with pembrolizumab (17% vs 17% vs 20%).
Further analysis from the KEYNOTE-006 trial data demonstrated improved ORR, PFS, and OS with pembrolizumab compared to ipilimumab in tumors positive for PD-L1 expression. For PD-L1-negative tumors, response rate was higher, and PFS and OS rates were similar with pembrolizumab compared to ipilimumab. Given that pembrolizumab was associated with similar survival outcomes in PD-L1-negative tumors and with less toxicity than ipilimumab, the superiority of PD-L1 inhibitors over ipilimumab was further supported, regardless of tumor PD-L1 status.27
In sum, PD-1 inhibition should be considered the first-line immunotherapy in advanced melanoma, either alone or in combination with ipilimumab, as discussed in the following section. There is no longer a role for ipilimumab monotherapy in the first-line setting, based on evidence from direct comparison to single-agent PD-1 inhibition in clinical trials that demonstrated superior efficacy and less serious toxicity with PD-1 inhibitors.5,26 The finding that ORR and OS outcomes with single-agent PD-1 inhibitors are higher in treatment-naive patients compared to those receiving prior therapies also supports this approach.22
Combination CTLA-4 and PD-1 Therapy
Despite the potential for durable responses, the majority of patients fail to respond to single-agent PD-1 therapy. Given that preclinical data had suggested the potential for synergy between dual inhibition of CTLA-4 and PD-1, clinical trials were designed to test this approach. The first randomized phase 2 trial that established superior efficacy with combination therapy was the CheckMate 069 trial comparing nivolumab plus ipilimumab to ipilimumab monotherapy. Combination therapy resulted in increased ORR (59% vs 11%), median PFS (not reached vs 3.0 months), 2-year PFS (51.3% vs 12.0%), and 2-year OS (63.8% vs 53.6%).28 Similarly, a phase 1b trial of pembrolizumab plus reduced-dose ipilimumab demonstrated an ORR of 61%, with a 1-year PFS of 69% and 1-year OS of 89%.29
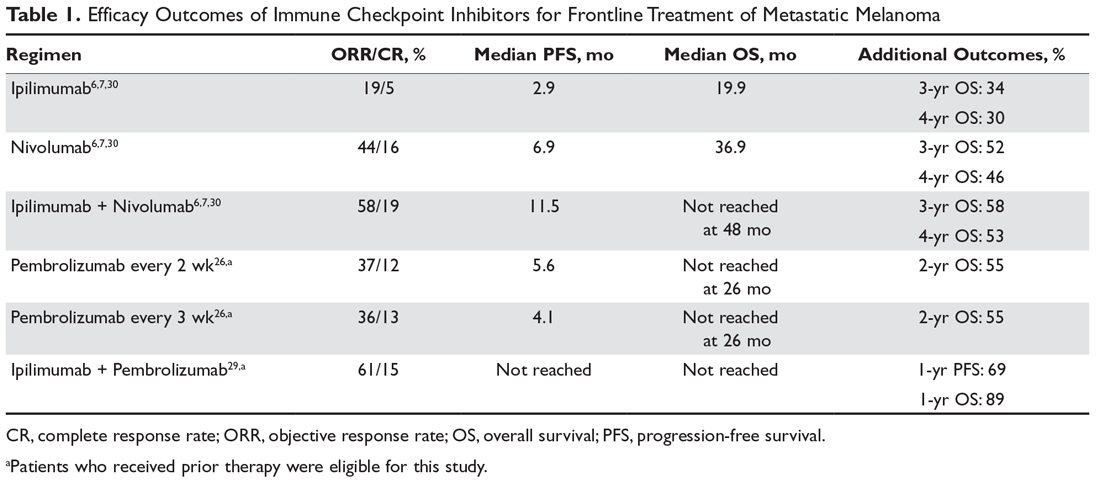
The landmark phase 3 CheckMate 067 trial analyzed efficacy outcomes for 3 different treatment regimens including nivolumab plus ipilimumab, nivolumab monotherapy, and ipilimumab monotherapy in previously untreated patients with unresectable stage III or IV melanoma. The trial was powered to compare survival outcomes for both the combination therapy arm against ipilimumab and the nivolumab monotherapy arm against ipilimumab, but not to compare combination therapy to nivolumab monotherapy. The initial analysis demonstrated a median PFS of 11.5 months with combination therapy versus 6.9 months with nivolumab and 2.9 months with ipilimumab, as well as an ORR of 58% versus 44% and 19%, respectively (Table 1).6 The updated 3-year survival outcomes from CheckMate 067 were notable for superior median OS with combination therapy (not reached in combination vs 37.6 months for nivolumab vs 19.9 months ipilimumab), improved 3-year OS (58% vs 52% vs 34%), and improved 3-year PFS (39% vs 32% vs 10%).7 In the reported 4-year survival outcomes, median OS was not reached in the combination therapy group, and was 36.9 months in the nivolumab monotherapy group and 19.9 months in the ipilimumab monotherapy group. Rates of grade 3 or 4 adverse events were significantly higher in the combination therapy group, at 59% compared to 22% with nivolumab monotherapy and 28% with ipilimumab alone.30 The 3- and 4-year OS outcomes (58% and 54%, respectively) with combination therapy were the highest seen in any phase 3 trial for treatment of advanced melanoma, supporting its use as the best approved first-line therapy in those who can tolerate the potential toxicity of combination therapy7,30 The conclusions from this landmark trial were that both combination therapy and nivolumab monotherapy resulted in statistically significant improvement in OS compared to ipilimumab.
Toxicity Associated with Immune Checkpoint Inhibitors
While immune checkpoint inhibitors have revolutionized the treatment of many solid tumor malignancies, this new class of cancer therapy has brought about a new type of toxicity for clinicians to be aware of, termed immune-related adverse events (irAEs). As immune checkpoint inhibitors amplify the immune response against malignancy, they also increase the likelihood that autoreactive T-cells persist and proliferate within the circulation. Therefore, these therapies can result in almost any type of autoimmune side effect. The most commonly reported irAEs in large clinical trials studying CTLA-4 and PD-1 inhibitors include rash/pruritus, diarrhea/colitis, hepatitis, endocrinopathies (thyroiditis, hypophysitis, adrenalitis), and pneumonitis. Other more rare toxicities include pancreatitis, autoimmune hematologic toxicities, cardiac toxicity (myocarditis, heart failure), and neurologic toxicities (neuropathies, myasthenia gravis-like syndrome, Guillain-Barré syndrome). It has been observed that PD-1 inhibitors have a lower incidence of irAEs than CTLA-4 inhibitors, and that the combined use of PD-1 and CTLA-4 inhibitors is associated with a greater incidence of irAEs compared to monotherapy with either agent.31 Toxicities associated with ipilimumab have been noted to be dose dependent.18 Generally, these toxicities are treated with immunosuppression in the form of glucocorticoids and are often reversible.31 There are several published guidelines that include algorithms for the management of irAEs by organizations such as the National Comprehensive Cancer Network.32
For example, previously untreated patients treated with ipilimumab plus dacarbazine as compared to dacarbazine plus placebo had greater grade 3 or 4 adverse events (56.3% vs 27.5%), and 77.7% of patients experiencing an irAE of any grade.18 In the CheckMate 066 trial comparing frontline nivolumab to dacarbazine, nivolumab had a lower rate of grade 3 or 4 toxicity (11.7% vs 17.6%) and irAEs were relatively infrequent, with diarrhea and elevated alanine aminotransferase level each being the most prominent irAE (affecting 1.0% of patients).25 In the KEYNOTE-006 trial, irAEs seen in more than 1% of patients treated with pembrolizumab included colitis, hepatitis, hypothyroidism, and hyperthyroidism, whereas those occurring in more than 1% of patients treated with ipilimumab included colitis and hypophysitis. Overall, there were lower rates of grade 3 to 5 toxicity with the 2 pembrolizumab doses compared to ipilimumab (13.3% pembrolizumab every 2 weeks vs 10.1% pembrolizumab every 3 weeks vs 19.9% ipilimumab).5 In the CheckMate 067 trial comparing nivolumab plus ipilimumab, nivolumab monotherapy, and ipilimumab monotherapy, rates of treatment-related adverse events of any grade were higher in the combination group (96% combination vs 86% nivolumab vs 86% ipilimumab), as were rates of grade 3 or 4 adverse events (59% vs 21% vs 28%, respectively). The irAE profile was similar to that demonstrated in prior studies: rash/pruritus were the most common, and diarrhea/colitis, elevated aminotransferases, and endocrinopathies were among the more common irAEs.7
Alternative dosing strategies have been investigated in an effort to preserve efficacy and minimize toxicity. A phase 1b trial of pembrolizumab plus reduced-dose ipilimumab demonstrated an ORR of 61%, with a 1-year PFS of 69% and a 1-year OS of 89%. This combination led to 45% of patients having a grade 3 or 4 adverse event, 60% having irAEs of any grade, and only 27% having grade 3 or 4 irAEs.29 The CheckMate 067 trial studied the combination of nivolumab 1 mg/kg plus ipilimumab 3 mg/kg.6 The CheckMate 511 trial compared different combination dosing strategies (nivolumab 3 mg/kg + ipilimumab 1 mg/kg versus nivolumab 1 mg/kg + ipilimumab 3 mg/kg) to assess for safety benefit. In the results published in abstract form, the reduced ipilimumab dose (nivolumab 3 mg/kg + ipilimumab 1 mg/kg arm) resulted in significantly decreased grade 3 to 5 adverse events (33.9% vs 48.3%) without significant differences in ORR, PFS, or OS.33
The question about the efficacy of checkpoint inhibitors in patients who discontinue treatment due to irAEs has been raised, as one hypothesis suggests that such toxicities may also indicate that the antitumor immune response has been activated. In a retrospective pooled analysis of phase 2 and 3 trials where patients received combination therapy with ipilimumab and nivolumab and discontinued therapy during the induction phase due to irAEs, outcomes did not appear to be inferior. Median PFS was 8.4 months in those who discontinued therapy compared to 10.8 months in those who continued therapy, but this did not reach statistical significance. Median OS had not been reached in either group and ORR was actually higher in those who discontinued due to adverse events (58.3% vs 50.2%). While this retrospective analysis needs to be validated, it does suggest that patients likely derive antitumor benefit from immunotherapy even if they have to discontinue therapy due to irAEs. Of note, patients in this analysis were not trialed on nivolumab monotherapy after receiving immunosuppressive treatment for toxicity related to combination therapy, which has since been deemed a reasonable treatment option.34
Molecularly Targeted Therapy for Metastatic Melanoma
As previously mentioned, the MAPK pathway is frequently altered in metastatic melanoma and thus serves as a target for therapy. Mutations in BRAF can cause constitutive activation of the protein’s kinase function, which subsequently phosphorylates/activates MEK in the absence of extracellular growth signals and causes increased cellular proliferation. For the roughly half of patients diagnosed with metastatic melanoma who harbor a BRAF V600 mutation, molecularly targeted therapy with BRAF/MEK inhibitors has emerged as a standard of care treatment option. As such, all patients with advanced disease should be tested for BRAF mutations.
After early phase 1 studies of the BRAF inhibitor vemurafenib demonstrated successful inhibition of mutated BRAF,35 subsequent studies confirmed the benefit of BRAF targeted therapy. In the phase 3 randomized controlled BRIM-3 trial comparing vemurafenib with dacarbazine for treatment of 675 patients with previously untreated metastatic melanoma positive for a BRAF V600E mutation, the vemurafenib group had superior ORR and 6-month OS during the first analysis.36 In a subsequent analysis, median PFS and median OS were also superior with vemurafenib compared to dacarbazine, as vemurafenib had a median OS of 13.6 months compared to 9.7 months with dacarbazine (HR, 0.70; P = 0.0008).37 Dabrafenib was the next BRAF inhibitor to demonstrate clinical efficacy with superior PFS compared to dacarbazine.38
Despite tumor shrinkage in the majority of patients, the development of resistance to therapy was an issue early on. The development of acquired resistance emerged as a heterogeneous process, though many of the identified resistance mechanisms involved reactivation of the MAPK pathway.39 A phase 3 trial of 322 patients with metastatic melanoma comparing the MEK inhibitor trametinib as monotherapy against chemotherapy demonstrated a modest improvement in both median PFS and OS.40 As a result, subsequent efforts focused on a strategy of concurrent MEK inhibition as a means to overcome resistance to molecularly targeted monotherapy
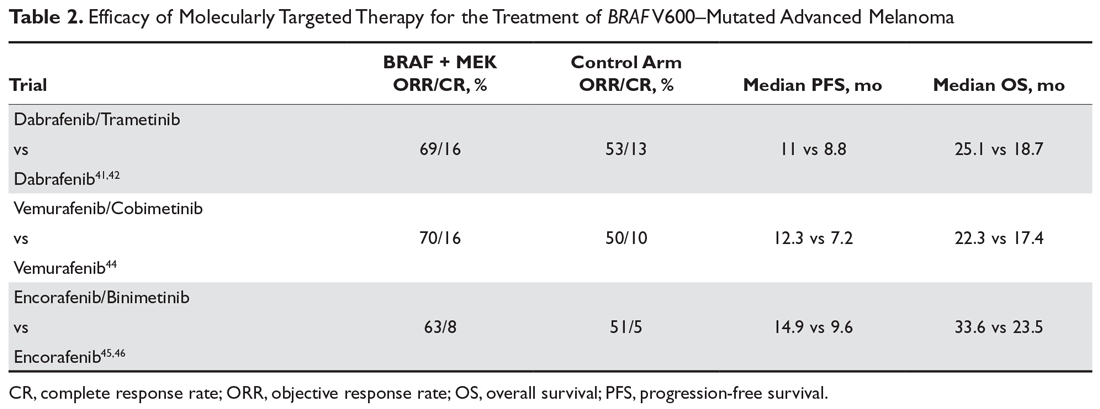
At least 4 large phase 3 randomized controlled trials of combination therapy with BRAF plus MEK inhibitors showed an improved ORR, PFS, and OS when compared to BRAF inhibition alone. The COMBI-d trial comparing dabrafenib plus trametinib versus dabrafenib alone was the first to demonstrate the superiority of combined BRAF/MEK inhibition and made combination therapy the current standard of care for patients with metastatic melanoma and a BRAF V600 mutation. In the final analysis of this trial, 3-year PFS was 22% with combination therapy compared to 12% with dabrafenib alone, and 3-year OS was 44% compared to 32%.8,41,42 A second trial with the combination of dabrafenib and trametinib (COMBI-V) also demonstrated superior efficacy when compared to single-agent vemurafenib without increased toxicity.43 Subsequently, the combination of vemurafenib with MEK inhibitor cobimetinib demonstrated superiority compared to vemurafenib alone,44 followed by the newest combination encorafenib (BRAF inhibitor) and binimetinib (MEK inhibitor) proving superior to either vemurafenib or encorafenib alone.45,46
It is important to note that there have been no studies directly comparing the efficacy of the 3 approved BRAF/MEK inhibitor combinations, but the 3 different regimens have some differences in their toxicity profiles (Table 2). Of note, single-agent BRAF inhibition was associated with increased cutaneous toxicity, including secondary squamous cell carcinoma and keratoacanthoma,47 which was demonstrated to be driven by paradoxical activation of the MAPK pathway.48 The concerning cutaneous toxicities such as squamous cell carcinoma were substantially reduced by combination BRAF/MEK inhibitor therapy.47 Collectively, the higher efficacy along with manageable toxicity profile established combination BRAF/MEK inhibition as the preferred regimen for patients with BRAF-mutated metastatic melanoma who are being considered for molecularly targeted therapy. BRAF inhibitor monotherapy should only be used when there is a specific concern regarding the use of a MEK inhibitor in certain clinical circumstances.
Other driver mutations associated with metastatic melanoma such as NRAS-mutated tumors have proven more difficult to effectively treat with molecularly targeted therapy, with one study showing that the MEK inhibitor binimetinib resulted in a modest improvement in ORR and median PFS without OS benefit compared to dacarbazine.49 Several phase 2 trials involving metastatic melanoma harboring a c-Kit alteration have demonstrated some efficacy with the tyrosine kinase inhibitor imatinib. The largest phase 2 trial of 43 patients treated with imatinib resulted in a 53.5% disease control rate (23.3% partial response and 30.2% stable disease), with 9 of the 10 patients who achieved partial response having a mutation in either exon 11 or 13. Median PFS was 3.5 months and 1-year OS was 51.0%.50
Case Conclusion
Prior to initiation of systemic therapy, the patient’s melanoma is tested and is found to be positive for a BRAF V600K mutation. At his follow-up appointment, the patient continues to endorse generalized weakness, fatigue, issues with balance, and residual pulmonary symptoms after being treated for post-obstructive pneumonia. Given his current symptoms and extent of metastatic disease, immunotherapy is deferred and he is started on combination molecularly targeted therapy with dabrafenib and trametinib. He initially does well, with a partial response noted by resolution of symptoms and decreased size of his intracranial metastases and decreased size of the right lower lobe mass. Further follow-up of this patient is presented in the second article in this 2-part review of advanced melanoma.
1. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2018. CA Cancer J Clin. 2018;68:7-30.
2. Ives NJ, Stowe RL, Lorigan P, Wheatley K. Chemotherapy compared with biochemotherapy for the treatment of metastatic melanoma: a meta-analysis of 18 trials involving 2621 patients. J Clin Oncol. 2007;25:5426-34.
3. Atkins MB, Lotze MT, Dutcher JP, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17:2105-16.
4. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711-23.
5. Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372:2522-2532.
6. Larkin J, Chiarion-Sileni V, Gonazalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23-34.
7. Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2017;377:1345-1356.
8. Long GV, Stroyakovskiy D, Gogas H, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med. 2014;371:1877-1888.
9. Elwood JM, Jopson J. Melanoma and sun exposure: an overview of published studies. Int J Cancer. 1997;73:198-203.
10. Gilchrest BA, Eller MS, Geller AC, Yaar M. The pathogenesis of melanoma induced by ultraviolet radiation. N Engl J Med. 199;340:1341-1348.
11. Omholt K, Platz A, Kanter L, et al. NRAS and BRAF mutations arise early during melanoma pathogenesis and are preserved throughout tumor progression. Clin Cancer Res. 2003;9:6483-8.
12. Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949-54.
13. Cancer Genome Atlas Network. Genomic classification of cutaneous melanoma. Cell 2015;161:1681-96.
14. Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma staging: evidence-based changes in the American Joint Committee on Cancer Eighth Edition Cancer Staging Manual. CA Cancer J Clin. 2017;67:472-492.
15. Robert C, Ribas A, Hamid O, et al. Durable complete response after discontinuation of pembrolizumab in patients with metastatic melanoma. J Clin Oncol. 2018;36:1668-1674.
16. Salama AKS, Hodi FS. Cytotoxic T-lymphocyte-associated antigen-4. Clin Cancer Res. 2011;17:4622-8.
17. Boussiotis VA. Molecular and biochemical aspects of the PD-1 checkpoint pathway. N Engl J Med. 2016;375:1767-1778.
18. Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517-2526.
19. Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443-2454.
20. Topalian S, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32:1020-30.
21. Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375-84.
22. Ribas A, Hamid O, Daud A, et al. Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA. 2016;315:1600-1609.
23. Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16:908-18.
24. Hamid O, Puzanov I, Dummer R, et al. Final analysis of a randomised trial comparing pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory advanced melanoma. Eur J Cancer. 2017;86:37-45.
25. Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320-330.
26. Schachter J, Ribas A, Long GV, et al. Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicenter, randomised, open-label phase 3 study (KEYNOTE-006). Lancet Oncol. 2017;390:1853-1862.
27. Carlino MS, Long GV, Schadendorf D, et al. Outcomes by line of therapy and programmed death ligand 1 expression in patients with advanced melanoma treated with pembrolizumab or ipilimumab in KEYNOTE-006. A randomised clinical trial. Eur J Cancer. 2018;101:236-243.
28. Hodi FS, Chesney J, Pavlick AC, et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 2016;17:1558-1568.
29. Long GV, Atkinson V, Cebon JS, et al. Standard-dose pembrolizumab in combination with reduced-dose ipilimumab for patients with advanced melanoma (KEYNOTE-029): an open-label, phase 1b trial. Lancet Oncol. 2017;18:1202-10.
30. Hodi FS, Chiarion-Sileni V, Gonzalez R, et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19:1480-1492.
31. Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the immune-related adverse effects of immune checkpoint inhibitors: a review. JAMA Oncol. 2016;2:1346-1353.
32. National Comprehensive Cancer Network. Management of immunotherapy-related toxicities (version 2.2019). www.nccn.org/professionals/physician_gls/pdf/immunotherapy.pdf. Accessed April 8, 2019.
33. Lebbé C, Meyer N, Mortier L, et al. Initial results from a phase IIIb/IV study evaluating two dosing regimens of nivolumab (NIVO) in combination with ipilimumab (IPI) in patients with advanced melanoma (CheckMate 511) [Abstract LBA47]. Ann Oncol. 2018;29:mdy424.057.
34. Schadendorf D, Wolchok JD, Hodi FS, et al. Efficacy and safety outcomes in patients with advanced melanoma who discontinued treatment with nivolumab and ipilimumab because of adverse events: a pooled analysis of randomized phase ii and iii trials. J Clin Oncol. 2017;35:3807-3814.
35. Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809-819.
36. Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507-2516.
37. McArthur GA, Chapman PB, Robert C, et al. Safety and efficacy of vemurafenib in BRAFV600E and BRAFV600K mutation-positive melanoma (BRIM-3): extended follow up of a phase 3, randomised, open-label study. Lancet Oncol. 2014;15:323-332.
38. Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicenter, open-label, phase 3 randomised controlled trial. Lancet Oncol. 2012;380:358-365.
39. Rizos H, Menzies AM, Pupo GM, et al. BRAF inhibitor resistance mechanisms in metastatic melanoma: spectrum and clinical impact. Clin Cancer Res. 2014;20:1965-1977.
40. Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med. 2012;367:107-114.
41. Long GV, Stroyakovskiy D, Gogas H, et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: a multicenter, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2015;386:444-451.
42. Long GV, Flaherty KT, Stroyakovskiy D, et al. Dabrafenib plus trametinib versus dabrafenib monotherapy in patients with metastatic BRAF V600E/K-mutant melanoma: long-term survival and safety analysis of a phase 3 study. Ann Oncol. 2017;28:1631-1639.
43. Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30-39.
44. Ascierto PA, McArthur GA, Dréno B, et al. Cobimetinib combined with vemurafenib in advanced BRAFV600-mutant melanoma (coBRIM): updated efficacy results from a randomised, double-blind, phase 3 trial. Lancet Oncol. 2016;17:1248-260.
45. Dummer R, Ascierto PA, Gogas HJ, et al. Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF-mutant melanoma (COLUMBUS): a multicenter, open-label, randomised phase 3 trial. Lancet Oncol. 2018;19:603-615.
46. Dummer R, Ascierto PA, Gogas HJ, et al. Overall survival in patients with BRAF-mutant melanoma receiving encorafenib plus binimetinib versus vemurafenib or encorafenib (COLUMBUS): a multicenter, open-label, randomised, phase 3 trial. Lancet Oncol. 2018;19:1315-1327.
47. Carlos G, Anforth R, Clements A, et al. Cutaneous toxic effects of BRAF inhibitors alone and in combination with MEK inhibitors for metastatic melanoma. JAMA. Dermatol 2015;151:1103-1109.
48. Su F, Viros A, Milagre C, et al. RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. N Engl J Med. 2012;366:207-215.
49. Dummer R, Schadendorf D, Ascierto P, et al. Binimetinib versus dacarbazine in patients with advanced NRAS-mutant melanoma (NEMO): a multicenter, open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18:435-445.
50. Guo J, Si L, Kong Y, et al. Phase II, open-label, single-arm trial of imatinib mesylate in patients with metastatic melanoma harboring c-Kit mutation or amplification. J Clin Oncol. 2011;29:2904-2909.
Malignant melanoma is the most serious form of primary skin cancer and one of the only malignancies in which the incidence rate has been rising. It is estimated that in 2018 there were 91,270 newly diagnosed cases and 9320 deaths from advanced melanoma in the United States. Melanoma is the fifth most common cancer type in males and the sixth most common in females. Despite rising incidence rates, improvement in the treatment of advanced melanoma has resulted in declining death rates over the past decade.1 Although most melanoma is diagnosed at an early stage and can be cured with surgical excision, the prognosis for metastatic melanoma had been historically poor prior to recent advancements in treatment. Conventional chemotherapy treatment with dacarbazine or temozolomide resulted in response rates ranging from 7.5% to 12.1%, but without much impact on median overall survival (OS), with reported OS ranging from 6.4 to 7.8 months. Combination approaches with interferon alfa-2B and low-dose interleukin-2 resulted in improved response rates compared with traditional chemotherapy, but again without survival benefit.2
Immunotherapy in the form of high-dose interleukin-2 emerged as the first therapy to alter the natural history of advanced melanoma, with both improved response rates (objective response rate [ORR], 16%) and median OS (2 months), with some patients achieving durable responses lasting more than 30 months. However, significant systemic toxicity limited its application to carefully selected patients.3 The past decade has brought rapid advancements in treatment with immune checkpoint inhibitors and molecularly targeted agents, which have significantly improved ORRs, progression-free survival (PFS), and OS for patients with metastatic melanoma.4-8
This review is the first of 2 articles focusing on the treatment and sequencing of therapies in advanced melanoma. Here, we review the selection of first-line therapy for metastatic melanoma. Current evidence for immune checkpoint blockade and molecularly targeted agents in the treatment of metastatic melanoma after progression on first-line therapy is discussed in a separate article.
Pathogenesis
The incidence of melanoma is strongly associated with ultraviolet light–mediated DNA damage related to sun exposure. Specifically, melanoma is associated to a greater degree with intense intermittent sun exposure and sunburn, but not associated with higher occupational exposure.9 Ultraviolet radiation can induce DNA damage by a number of mechanisms, and deficient DNA repair leads to somatic mutations that drive the progression from normal melanocyte to melanoma.10
The most commonly identified genetic mutations in cutaneous melanomas are alterations in the mitogen-activated protein kinase (MAPK) pathway. Typically, an extracellular growth factor causes dimerization of the growth factor receptor, which activates the intracellular RAS GTPase protein. Subsequently BRAF is phosphorylated within the kinase domain, which leads to downstream activation of the MEK and ERK kinases through phosphorylation. Activated ERK leads to phosphorylation of various cytoplasmic and nuclear targets, and the downstream effects of these changes promote cellular proliferation. While activation of this pathway usually requires phosphorylation of BRAF by RAS, mutations placing an acidic amino acid near the kinase domain mimics phosphorylation and leads to constitutive activation of the BRAF serine/threonine kinase in the absence of upstream signaling from extracellular growth factors mediated through RAS.11 One study of tumor samples of 71 patients with cutaneous melanoma detected NRAS mutations in 30% and BRAF mutations in 59% of all tumors tested. Of the BRAF mutation–positive tumors, 88% harbored the Val599Glu mutation, now commonly referred to as the BRAF V600E mutation. The same study demonstrated that the vast majority of BRAF mutations were seen in the primary tumor and were preserved when metastases were analyzed. Additionally, both NRAS and BRAF mutations were detected in the radial growth phase of the melanoma tumor. These findings indicate that alterations in the MAPK pathway occur early in the pathogenesis of advanced melanoma.11 Another group demonstrated that 66% of malignant melanoma tumor samples harbored BRAF mutations, of which 80% were specifically the V600E mutation. In vitro assays showed that the BRAF V600E–mutated kinase had greater than 10-fold kinase activity compared to wild-type BRAF, and that this kinase enhanced cellular proliferation even when upstream NRAS signaling was inhibited.12
The Cancer Genome Atlas Network performed a large analysis of tumor samples from 331 different melanoma patients and studied variations at the DNA, RNA, and protein levels. The study established a framework of 4 notable genomic subtypes, including mutant BRAF (52%), mutant RAS (28%), mutant NF1 (14%), and triple wild-type (6%). Additionally, mRNA transcriptomic analysis of overexpressed genes identified 3 different subclasses, which were labeled as “immune,” “keratin,” and “MITF-low.” The immune subclass was characterized by increased expression of proteins found in immune cells, immune signaling molecules, immune checkpoint proteins, cytokines, and chemokines, and correlated with increased lymphocyte invasion within the tumor. Interestingly, in the post-accession survival analysis, the “immune” transcriptomic subclass was statistically correlated with an improved prognosis.13 Having an understanding of the molecular pathogenesis of advanced melanoma helps to create a framework for understanding the mechanisms of current standard of care therapies for the disease.
Case Presentation
A 62-year-old Caucasian man with a history of well-controlled type 2 diabetes mellitus and hypertension is being followed by his dermatologist for surveillance of melanocytic nevi. On follow-up he is noted to have an asymmetrical melanocytic lesion over the right scalp with irregular borders and variegated color. He is asymptomatic and the remainder of physical examination is unremarkable, as he has no other concerning skin lesions and no cervical, axillary, or inguinal lymphadenopathy.
How is melanoma diagnosed?
Detailed discussion about diagnosis and staging will be deferred in this review of treatment of advanced melanoma. In brief, melanoma is best diagnosed by excisional biopsy and histopathology. Staging of melanoma is done according to the American Joint Committee on Cancer’s (AJCC) Cancer Staging Manual, 8th edition, using a TNM staging system that incorporates tumor thickness (Breslow depth); ulceration; number of involved regional lymph nodes; presence of in-transit, satellite, and/or microsatellite metastases; distant metastases; and serum lactate dehydrogenase level.14
Case Continued
The patient undergoes a wide excisional biopsy of the right scalp lesion, which is consistent with malignant melanoma. Pathology demonstrates a Breslow depth of 2.6 mm, 2 mitotic figures/mm2, and no evidence of ulceration. He subsequently undergoes wide local excision with 0/3 sentinel lymph nodes positive for malignancy. His final staging is consistent with pT3aN0M0, stage IIA melanoma.
He is seen in follow-up with medical oncology for the next 3.5 years without any evidence of disease recurrence. He then develops symptoms of vertigo, diplopia, and recurrent falls, prompting medical attention. Magnetic resonance imaging (MRI) brain reveals multiple supratentorial and infratentorial lesions concerning for intracranial metastases. Further imaging with computed tomography (CT) chest/abdomen/pelvis reveals a right lower lobe pulmonary mass with right hilar and subcarinal lymphadenopathy. He is admitted for treatment with intravenous dexamethasone and further evaluation with endobronchial ultrasound-guided fine-needle aspiration of the right lower lobe mass, which reveals metastatic melanoma. Given the extent of his intracranial metastases, he is treated with whole brain radiation therapy for symptomatic relief prior to initiating systemic therapy.
What is the general approach to first-line treatment for metastatic melanoma?
The past decade has brought an abundance of data supporting the use of immunotherapy with immune checkpoint inhibitors or molecularly targeted therapy with combined BRAF/MEK inhibitors in the first-line setting.4-8 After the diagnosis of metastatic melanoma has been made, molecular testing is recommended to determine the BRAF status of the tumor. Immunotherapy is the clear choice for first-line therapy in the absence of an activating BRAF V600 mutation. When a BRAF V600 mutation is present, current evidence supports the use of either immunotherapy or molecularly targeted therapy as first-line therapy.
To date, there have been no prospective clinical trials comparing the sequencing of immunotherapy and molecularly targeted therapy in the first-line setting. An ongoing clinical trial (NCT02224781) is comparing dabrafenib and trametinib followed by ipilimumab and nivolumab at time of progression to ipilimumab and nivolumab followed by dabrafenib and trametinib in patients with newly diagnosed stage III/IV BRAF V600 mutation–positive melanoma. The primary outcome measure is 2-year OS. Until completion of that trial, current practice regarding which type of therapy to use in the first-line setting is based on a number of factors including clinical characteristics and provider preferences.
Data suggest that immunotherapies can produce durable responses, especially after treatment completion or discontinuation, albeit at the expense of taking a longer time to achieve clinical benefit and the risk of potentially serious immune-related adverse effects. This idea of a durable, off-treatment response is highlighted by a study that followed 105 patients who had achieved a complete response (CR) and found that 24-month disease-free survival from the time of CR was 90.9% in all patients and 89.9% in the 67 patients who had discontinued pembrolizumab after attaining CR.15 BRAF/MEK inhibition has the potential for rapid clinical responses, though concerns exist about the development of resistance to therapy. The following sections explore the evidence supporting the use of these therapies.
Immunotherapy with Immune Checkpoint Inhibitors
Immunotherapy via immune checkpoint blockade has revolutionized the treatment of many solid tumors over the past decade. The promise of immunotherapy revolves around the potential for achieving a dynamic and durable systemic response against cancer by augmenting the antitumor effects of the immune system. T-cells are central to mounting a systemic antitumor response, and, in addition to antigen recognition, their function depends heavily on fine tuning between co-stimulatory and co-inhibitory signaling. The cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) expressed on T-cells was the first discovered co-inhibitory receptor of T-cell activation.16 Later, it was discovered that the programmed cell death 1 receptor (PD-1), expressed on T-cells, and its ligands PD-L1 and PD-L2, expressed on antigen presenting cells, tumor cells, or other cells in the tumor microenvironment, also served as a potent negative regulator of T-cell function.17
Together, these 2 signaling pathways help to maintain peripheral immune tolerance, whereby autoreactive T-cells that have escaped from the thymus are silenced to prevent autoimmunity. However, these pathways can also be utilized by cancer cells to escape immune surveillance. Monoclonal antibodies that inhibit the aforementioned co-inhibitory signaling pathways, and thus augment the immune response, have proven to be an effective anticancer therapy capable of producing profound and durable responses in certain malignancies.16,17
Ipilimumab
Ipilimumab is a monoclonal antibody that inhibits the function of the CTLA-4 co-inhibitory immune checkpoint. In a phase 3 randomized controlled trial of 676 patients with previously treated metastatic melanoma, ipilimumab at a dose of 3 mg/kg every 3 weeks for 4 cycles, with or without a gp100 peptide vaccine, resulted in an improved median OS of 10.0 and 10.1 months, respectively, compared to 6.4 months in those receiving the peptide vaccine alone, meeting the primary endpoint.4 Subsequently, a phase 3 trial of 502 patients with untreated metastatic melanoma compared ipilimumab at a dose of 10 mg/kg every 3 weeks for 4 cycles plus dacarbazine to dacarbazine plus placebo and found a significant increase in median OS (11.2 months vs 9.1 months), with no additive benefit of chemotherapy. There was a higher reported rate of grade 3 or 4 adverse events in this trial with ipilimumab dosed at 10 mg/kg, which was felt to be dose-related.18 These trials were the first to show improved OS with any systemic therapy in metastatic melanoma and led to US Food and Drug Administration approval of ipilimumab for this indication in 2011.
PD-1 Inhibitor Monotherapy
The PD-1 inhibitors nivolumab and pembrolizumab were initially approved for metastatic melanoma after progression on ipilimumab. In the phase 1 trial of patients with previously treated metastatic melanoma, nivolumab therapy resulted in an ORR of 28%.19 The subsequent phase 2 trial conducted in pretreated patients, including patients who had progressed on ipilimumab, confirmed a similar ORR of 31%, as well as a median PFS of 3.7 months and a median OS of 16.8 months. The estimated response duration in patients who did achieve a response to therapy was 2 years.20 A phase 3 trial (CheckMate 037) comparing nivolumab (n = 120) to investigator’s choice chemotherapy (n = 47) in those with melanoma refractory to ipilimumab demonstrated that nivolumab was superior for the primary endpoint of ORR (31.7% vs 10.6%), had less toxicity (5% rate of grade 3 or 4 adverse events versus 9%), and increased median duration of response (32 months vs 13 months).21
The phase 1 trial (KEYNOTE-001) testing the efficacy of pembrolizumab demonstrated an ORR of 33% in the total population of patients treated and an ORR of 45% in those who were treatment-naive. Additionally, the median OS was 23 months for the total population and 31 months for treatment-naive patients, with only 14% of patients experiencing a grade 3 or 4 adverse event.22 The KEYNOTE-002 phase 2 trial compared 2 different pembrolizumab doses (2 mg/kg and 10 mg/kg every 3 weeks) to investigator’s choice chemotherapy (paclitaxel plus carboplatin, paclitaxel, carboplatin, dacarbazine, or oral temozolomide) in 540 patients with advanced melanoma with documented progression on ipilimumab with or without prior progression on molecularly targeted therapy if positive for a BRAF V600 mutation. The final analysis demonstrated significantly improved ORR with pembrolizumab (22% at 2 mg/kg vs 26% at 10 mg/kg vs 4% chemotherapy) and significantly improved 24-month PFS (16% vs 22% vs 0.6%, respectively). There was a nonstatistically significant improvement in median OS (13.4 months vs 14.7 months vs 10 months), although 55% of the patients initially assigned to the chemotherapy arm crossed over and received pembrolizumab after documentation of progressive disease.23,24
Because PD-1 inhibition improved efficacy with less toxicity than chemotherapy when studied in progressive disease, subsequent studies focused on PD-1 inhibition in the frontline setting. CheckMate 066 was a phase 3 trial comparing nivolumab to dacarbazine as first-line therapy for 418 patients with untreated metastatic melanoma who did not have a BRAF mutation. For the primary end point of 1-year OS, nivolumab was superior to dacarbazine (72.9% vs 42.1%; hazard ratio [HR], 0.42; P < 0.001). Treatment with nivolumab also resulted in superior ORR (40% vs 14%) and PFS (5.1 months vs 2.2 months). Additionally, nivolumab therapy had a lower rate of grade 3 or 4 toxicity compared to dacarbazine (11.7% vs 17.6%).25
The KEYNOTE-006 trial compared 2 separate dosing schedules of pembrolizumab (10 mg/kg every 2 weeks versus every 3 weeks) to ipilimumab (3 mg/kg every 3 weeks for 4 cycles) in a 1:1:1 ratio in 834 patients with metastatic melanoma who had received up to 1 prior systemic therapy, but no prior CTLA-4 or PD-1 inhibitors. The first published data reported statistically significant outcomes for the co-primary end points of 6-month PFS (47.3% for pembrolizumab every 2 weeks vs 46.4% for pembrolizumab every 3 weeks vs 26.5% for ipilimumab; HR, 0.58 for both pembrolizumab groups compared to ipilimumab; P < 0.001) and 12-month OS (74.1% vs 68.4% vs 58.2%) with pembrolizumab compared to ipilimumab. Compared to ipilimumab, pembrolizumab every 2 weeks had a hazard ratio of 0.63 (P = 0.0005) and pembrolizumab every 3 weeks had a hazard ratio of 0.69 (P = 0.0036). The pembrolizumab groups was also had lower rates of grade 3 to 5 toxicity (13.3% vs 10.1% vs 19.9%).5 Updated outcomes demonstrated improved ORR compared to the first analysis (37% vs 36% vs 13%), and improved OS (median OS, not reached for the pembrolizumab groups vs 16.0 months for the ipilimumab group; HR, 0.68, P = 0.0009 for pembrolizumab every 2 weeks versus HR 0.68, P = 0.0008 for pembrolizumab every 3 weeks).26 In addition, 24-month OS was 55% in both pembrolizumab groups compared to 43% in the ipilimumab group. Grade 3 or 4 toxicity occurred less frequently with pembrolizumab (17% vs 17% vs 20%).
Further analysis from the KEYNOTE-006 trial data demonstrated improved ORR, PFS, and OS with pembrolizumab compared to ipilimumab in tumors positive for PD-L1 expression. For PD-L1-negative tumors, response rate was higher, and PFS and OS rates were similar with pembrolizumab compared to ipilimumab. Given that pembrolizumab was associated with similar survival outcomes in PD-L1-negative tumors and with less toxicity than ipilimumab, the superiority of PD-L1 inhibitors over ipilimumab was further supported, regardless of tumor PD-L1 status.27
In sum, PD-1 inhibition should be considered the first-line immunotherapy in advanced melanoma, either alone or in combination with ipilimumab, as discussed in the following section. There is no longer a role for ipilimumab monotherapy in the first-line setting, based on evidence from direct comparison to single-agent PD-1 inhibition in clinical trials that demonstrated superior efficacy and less serious toxicity with PD-1 inhibitors.5,26 The finding that ORR and OS outcomes with single-agent PD-1 inhibitors are higher in treatment-naive patients compared to those receiving prior therapies also supports this approach.22
Combination CTLA-4 and PD-1 Therapy
Despite the potential for durable responses, the majority of patients fail to respond to single-agent PD-1 therapy. Given that preclinical data had suggested the potential for synergy between dual inhibition of CTLA-4 and PD-1, clinical trials were designed to test this approach. The first randomized phase 2 trial that established superior efficacy with combination therapy was the CheckMate 069 trial comparing nivolumab plus ipilimumab to ipilimumab monotherapy. Combination therapy resulted in increased ORR (59% vs 11%), median PFS (not reached vs 3.0 months), 2-year PFS (51.3% vs 12.0%), and 2-year OS (63.8% vs 53.6%).28 Similarly, a phase 1b trial of pembrolizumab plus reduced-dose ipilimumab demonstrated an ORR of 61%, with a 1-year PFS of 69% and 1-year OS of 89%.29
The landmark phase 3 CheckMate 067 trial analyzed efficacy outcomes for 3 different treatment regimens including nivolumab plus ipilimumab, nivolumab monotherapy, and ipilimumab monotherapy in previously untreated patients with unresectable stage III or IV melanoma. The trial was powered to compare survival outcomes for both the combination therapy arm against ipilimumab and the nivolumab monotherapy arm against ipilimumab, but not to compare combination therapy to nivolumab monotherapy. The initial analysis demonstrated a median PFS of 11.5 months with combination therapy versus 6.9 months with nivolumab and 2.9 months with ipilimumab, as well as an ORR of 58% versus 44% and 19%, respectively (Table 1).6 The updated 3-year survival outcomes from CheckMate 067 were notable for superior median OS with combination therapy (not reached in combination vs 37.6 months for nivolumab vs 19.9 months ipilimumab), improved 3-year OS (58% vs 52% vs 34%), and improved 3-year PFS (39% vs 32% vs 10%).7 In the reported 4-year survival outcomes, median OS was not reached in the combination therapy group, and was 36.9 months in the nivolumab monotherapy group and 19.9 months in the ipilimumab monotherapy group. Rates of grade 3 or 4 adverse events were significantly higher in the combination therapy group, at 59% compared to 22% with nivolumab monotherapy and 28% with ipilimumab alone.30 The 3- and 4-year OS outcomes (58% and 54%, respectively) with combination therapy were the highest seen in any phase 3 trial for treatment of advanced melanoma, supporting its use as the best approved first-line therapy in those who can tolerate the potential toxicity of combination therapy7,30 The conclusions from this landmark trial were that both combination therapy and nivolumab monotherapy resulted in statistically significant improvement in OS compared to ipilimumab.
Toxicity Associated with Immune Checkpoint Inhibitors
While immune checkpoint inhibitors have revolutionized the treatment of many solid tumor malignancies, this new class of cancer therapy has brought about a new type of toxicity for clinicians to be aware of, termed immune-related adverse events (irAEs). As immune checkpoint inhibitors amplify the immune response against malignancy, they also increase the likelihood that autoreactive T-cells persist and proliferate within the circulation. Therefore, these therapies can result in almost any type of autoimmune side effect. The most commonly reported irAEs in large clinical trials studying CTLA-4 and PD-1 inhibitors include rash/pruritus, diarrhea/colitis, hepatitis, endocrinopathies (thyroiditis, hypophysitis, adrenalitis), and pneumonitis. Other more rare toxicities include pancreatitis, autoimmune hematologic toxicities, cardiac toxicity (myocarditis, heart failure), and neurologic toxicities (neuropathies, myasthenia gravis-like syndrome, Guillain-Barré syndrome). It has been observed that PD-1 inhibitors have a lower incidence of irAEs than CTLA-4 inhibitors, and that the combined use of PD-1 and CTLA-4 inhibitors is associated with a greater incidence of irAEs compared to monotherapy with either agent.31 Toxicities associated with ipilimumab have been noted to be dose dependent.18 Generally, these toxicities are treated with immunosuppression in the form of glucocorticoids and are often reversible.31 There are several published guidelines that include algorithms for the management of irAEs by organizations such as the National Comprehensive Cancer Network.32
For example, previously untreated patients treated with ipilimumab plus dacarbazine as compared to dacarbazine plus placebo had greater grade 3 or 4 adverse events (56.3% vs 27.5%), and 77.7% of patients experiencing an irAE of any grade.18 In the CheckMate 066 trial comparing frontline nivolumab to dacarbazine, nivolumab had a lower rate of grade 3 or 4 toxicity (11.7% vs 17.6%) and irAEs were relatively infrequent, with diarrhea and elevated alanine aminotransferase level each being the most prominent irAE (affecting 1.0% of patients).25 In the KEYNOTE-006 trial, irAEs seen in more than 1% of patients treated with pembrolizumab included colitis, hepatitis, hypothyroidism, and hyperthyroidism, whereas those occurring in more than 1% of patients treated with ipilimumab included colitis and hypophysitis. Overall, there were lower rates of grade 3 to 5 toxicity with the 2 pembrolizumab doses compared to ipilimumab (13.3% pembrolizumab every 2 weeks vs 10.1% pembrolizumab every 3 weeks vs 19.9% ipilimumab).5 In the CheckMate 067 trial comparing nivolumab plus ipilimumab, nivolumab monotherapy, and ipilimumab monotherapy, rates of treatment-related adverse events of any grade were higher in the combination group (96% combination vs 86% nivolumab vs 86% ipilimumab), as were rates of grade 3 or 4 adverse events (59% vs 21% vs 28%, respectively). The irAE profile was similar to that demonstrated in prior studies: rash/pruritus were the most common, and diarrhea/colitis, elevated aminotransferases, and endocrinopathies were among the more common irAEs.7
Alternative dosing strategies have been investigated in an effort to preserve efficacy and minimize toxicity. A phase 1b trial of pembrolizumab plus reduced-dose ipilimumab demonstrated an ORR of 61%, with a 1-year PFS of 69% and a 1-year OS of 89%. This combination led to 45% of patients having a grade 3 or 4 adverse event, 60% having irAEs of any grade, and only 27% having grade 3 or 4 irAEs.29 The CheckMate 067 trial studied the combination of nivolumab 1 mg/kg plus ipilimumab 3 mg/kg.6 The CheckMate 511 trial compared different combination dosing strategies (nivolumab 3 mg/kg + ipilimumab 1 mg/kg versus nivolumab 1 mg/kg + ipilimumab 3 mg/kg) to assess for safety benefit. In the results published in abstract form, the reduced ipilimumab dose (nivolumab 3 mg/kg + ipilimumab 1 mg/kg arm) resulted in significantly decreased grade 3 to 5 adverse events (33.9% vs 48.3%) without significant differences in ORR, PFS, or OS.33
The question about the efficacy of checkpoint inhibitors in patients who discontinue treatment due to irAEs has been raised, as one hypothesis suggests that such toxicities may also indicate that the antitumor immune response has been activated. In a retrospective pooled analysis of phase 2 and 3 trials where patients received combination therapy with ipilimumab and nivolumab and discontinued therapy during the induction phase due to irAEs, outcomes did not appear to be inferior. Median PFS was 8.4 months in those who discontinued therapy compared to 10.8 months in those who continued therapy, but this did not reach statistical significance. Median OS had not been reached in either group and ORR was actually higher in those who discontinued due to adverse events (58.3% vs 50.2%). While this retrospective analysis needs to be validated, it does suggest that patients likely derive antitumor benefit from immunotherapy even if they have to discontinue therapy due to irAEs. Of note, patients in this analysis were not trialed on nivolumab monotherapy after receiving immunosuppressive treatment for toxicity related to combination therapy, which has since been deemed a reasonable treatment option.34
Molecularly Targeted Therapy for Metastatic Melanoma
As previously mentioned, the MAPK pathway is frequently altered in metastatic melanoma and thus serves as a target for therapy. Mutations in BRAF can cause constitutive activation of the protein’s kinase function, which subsequently phosphorylates/activates MEK in the absence of extracellular growth signals and causes increased cellular proliferation. For the roughly half of patients diagnosed with metastatic melanoma who harbor a BRAF V600 mutation, molecularly targeted therapy with BRAF/MEK inhibitors has emerged as a standard of care treatment option. As such, all patients with advanced disease should be tested for BRAF mutations.
After early phase 1 studies of the BRAF inhibitor vemurafenib demonstrated successful inhibition of mutated BRAF,35 subsequent studies confirmed the benefit of BRAF targeted therapy. In the phase 3 randomized controlled BRIM-3 trial comparing vemurafenib with dacarbazine for treatment of 675 patients with previously untreated metastatic melanoma positive for a BRAF V600E mutation, the vemurafenib group had superior ORR and 6-month OS during the first analysis.36 In a subsequent analysis, median PFS and median OS were also superior with vemurafenib compared to dacarbazine, as vemurafenib had a median OS of 13.6 months compared to 9.7 months with dacarbazine (HR, 0.70; P = 0.0008).37 Dabrafenib was the next BRAF inhibitor to demonstrate clinical efficacy with superior PFS compared to dacarbazine.38
Despite tumor shrinkage in the majority of patients, the development of resistance to therapy was an issue early on. The development of acquired resistance emerged as a heterogeneous process, though many of the identified resistance mechanisms involved reactivation of the MAPK pathway.39 A phase 3 trial of 322 patients with metastatic melanoma comparing the MEK inhibitor trametinib as monotherapy against chemotherapy demonstrated a modest improvement in both median PFS and OS.40 As a result, subsequent efforts focused on a strategy of concurrent MEK inhibition as a means to overcome resistance to molecularly targeted monotherapy
At least 4 large phase 3 randomized controlled trials of combination therapy with BRAF plus MEK inhibitors showed an improved ORR, PFS, and OS when compared to BRAF inhibition alone. The COMBI-d trial comparing dabrafenib plus trametinib versus dabrafenib alone was the first to demonstrate the superiority of combined BRAF/MEK inhibition and made combination therapy the current standard of care for patients with metastatic melanoma and a BRAF V600 mutation. In the final analysis of this trial, 3-year PFS was 22% with combination therapy compared to 12% with dabrafenib alone, and 3-year OS was 44% compared to 32%.8,41,42 A second trial with the combination of dabrafenib and trametinib (COMBI-V) also demonstrated superior efficacy when compared to single-agent vemurafenib without increased toxicity.43 Subsequently, the combination of vemurafenib with MEK inhibitor cobimetinib demonstrated superiority compared to vemurafenib alone,44 followed by the newest combination encorafenib (BRAF inhibitor) and binimetinib (MEK inhibitor) proving superior to either vemurafenib or encorafenib alone.45,46
It is important to note that there have been no studies directly comparing the efficacy of the 3 approved BRAF/MEK inhibitor combinations, but the 3 different regimens have some differences in their toxicity profiles (Table 2). Of note, single-agent BRAF inhibition was associated with increased cutaneous toxicity, including secondary squamous cell carcinoma and keratoacanthoma,47 which was demonstrated to be driven by paradoxical activation of the MAPK pathway.48 The concerning cutaneous toxicities such as squamous cell carcinoma were substantially reduced by combination BRAF/MEK inhibitor therapy.47 Collectively, the higher efficacy along with manageable toxicity profile established combination BRAF/MEK inhibition as the preferred regimen for patients with BRAF-mutated metastatic melanoma who are being considered for molecularly targeted therapy. BRAF inhibitor monotherapy should only be used when there is a specific concern regarding the use of a MEK inhibitor in certain clinical circumstances.
Other driver mutations associated with metastatic melanoma such as NRAS-mutated tumors have proven more difficult to effectively treat with molecularly targeted therapy, with one study showing that the MEK inhibitor binimetinib resulted in a modest improvement in ORR and median PFS without OS benefit compared to dacarbazine.49 Several phase 2 trials involving metastatic melanoma harboring a c-Kit alteration have demonstrated some efficacy with the tyrosine kinase inhibitor imatinib. The largest phase 2 trial of 43 patients treated with imatinib resulted in a 53.5% disease control rate (23.3% partial response and 30.2% stable disease), with 9 of the 10 patients who achieved partial response having a mutation in either exon 11 or 13. Median PFS was 3.5 months and 1-year OS was 51.0%.50
Case Conclusion
Prior to initiation of systemic therapy, the patient’s melanoma is tested and is found to be positive for a BRAF V600K mutation. At his follow-up appointment, the patient continues to endorse generalized weakness, fatigue, issues with balance, and residual pulmonary symptoms after being treated for post-obstructive pneumonia. Given his current symptoms and extent of metastatic disease, immunotherapy is deferred and he is started on combination molecularly targeted therapy with dabrafenib and trametinib. He initially does well, with a partial response noted by resolution of symptoms and decreased size of his intracranial metastases and decreased size of the right lower lobe mass. Further follow-up of this patient is presented in the second article in this 2-part review of advanced melanoma.
Malignant melanoma is the most serious form of primary skin cancer and one of the only malignancies in which the incidence rate has been rising. It is estimated that in 2018 there were 91,270 newly diagnosed cases and 9320 deaths from advanced melanoma in the United States. Melanoma is the fifth most common cancer type in males and the sixth most common in females. Despite rising incidence rates, improvement in the treatment of advanced melanoma has resulted in declining death rates over the past decade.1 Although most melanoma is diagnosed at an early stage and can be cured with surgical excision, the prognosis for metastatic melanoma had been historically poor prior to recent advancements in treatment. Conventional chemotherapy treatment with dacarbazine or temozolomide resulted in response rates ranging from 7.5% to 12.1%, but without much impact on median overall survival (OS), with reported OS ranging from 6.4 to 7.8 months. Combination approaches with interferon alfa-2B and low-dose interleukin-2 resulted in improved response rates compared with traditional chemotherapy, but again without survival benefit.2
Immunotherapy in the form of high-dose interleukin-2 emerged as the first therapy to alter the natural history of advanced melanoma, with both improved response rates (objective response rate [ORR], 16%) and median OS (2 months), with some patients achieving durable responses lasting more than 30 months. However, significant systemic toxicity limited its application to carefully selected patients.3 The past decade has brought rapid advancements in treatment with immune checkpoint inhibitors and molecularly targeted agents, which have significantly improved ORRs, progression-free survival (PFS), and OS for patients with metastatic melanoma.4-8
This review is the first of 2 articles focusing on the treatment and sequencing of therapies in advanced melanoma. Here, we review the selection of first-line therapy for metastatic melanoma. Current evidence for immune checkpoint blockade and molecularly targeted agents in the treatment of metastatic melanoma after progression on first-line therapy is discussed in a separate article.
Pathogenesis
The incidence of melanoma is strongly associated with ultraviolet light–mediated DNA damage related to sun exposure. Specifically, melanoma is associated to a greater degree with intense intermittent sun exposure and sunburn, but not associated with higher occupational exposure.9 Ultraviolet radiation can induce DNA damage by a number of mechanisms, and deficient DNA repair leads to somatic mutations that drive the progression from normal melanocyte to melanoma.10
The most commonly identified genetic mutations in cutaneous melanomas are alterations in the mitogen-activated protein kinase (MAPK) pathway. Typically, an extracellular growth factor causes dimerization of the growth factor receptor, which activates the intracellular RAS GTPase protein. Subsequently BRAF is phosphorylated within the kinase domain, which leads to downstream activation of the MEK and ERK kinases through phosphorylation. Activated ERK leads to phosphorylation of various cytoplasmic and nuclear targets, and the downstream effects of these changes promote cellular proliferation. While activation of this pathway usually requires phosphorylation of BRAF by RAS, mutations placing an acidic amino acid near the kinase domain mimics phosphorylation and leads to constitutive activation of the BRAF serine/threonine kinase in the absence of upstream signaling from extracellular growth factors mediated through RAS.11 One study of tumor samples of 71 patients with cutaneous melanoma detected NRAS mutations in 30% and BRAF mutations in 59% of all tumors tested. Of the BRAF mutation–positive tumors, 88% harbored the Val599Glu mutation, now commonly referred to as the BRAF V600E mutation. The same study demonstrated that the vast majority of BRAF mutations were seen in the primary tumor and were preserved when metastases were analyzed. Additionally, both NRAS and BRAF mutations were detected in the radial growth phase of the melanoma tumor. These findings indicate that alterations in the MAPK pathway occur early in the pathogenesis of advanced melanoma.11 Another group demonstrated that 66% of malignant melanoma tumor samples harbored BRAF mutations, of which 80% were specifically the V600E mutation. In vitro assays showed that the BRAF V600E–mutated kinase had greater than 10-fold kinase activity compared to wild-type BRAF, and that this kinase enhanced cellular proliferation even when upstream NRAS signaling was inhibited.12
The Cancer Genome Atlas Network performed a large analysis of tumor samples from 331 different melanoma patients and studied variations at the DNA, RNA, and protein levels. The study established a framework of 4 notable genomic subtypes, including mutant BRAF (52%), mutant RAS (28%), mutant NF1 (14%), and triple wild-type (6%). Additionally, mRNA transcriptomic analysis of overexpressed genes identified 3 different subclasses, which were labeled as “immune,” “keratin,” and “MITF-low.” The immune subclass was characterized by increased expression of proteins found in immune cells, immune signaling molecules, immune checkpoint proteins, cytokines, and chemokines, and correlated with increased lymphocyte invasion within the tumor. Interestingly, in the post-accession survival analysis, the “immune” transcriptomic subclass was statistically correlated with an improved prognosis.13 Having an understanding of the molecular pathogenesis of advanced melanoma helps to create a framework for understanding the mechanisms of current standard of care therapies for the disease.
Case Presentation
A 62-year-old Caucasian man with a history of well-controlled type 2 diabetes mellitus and hypertension is being followed by his dermatologist for surveillance of melanocytic nevi. On follow-up he is noted to have an asymmetrical melanocytic lesion over the right scalp with irregular borders and variegated color. He is asymptomatic and the remainder of physical examination is unremarkable, as he has no other concerning skin lesions and no cervical, axillary, or inguinal lymphadenopathy.
How is melanoma diagnosed?
Detailed discussion about diagnosis and staging will be deferred in this review of treatment of advanced melanoma. In brief, melanoma is best diagnosed by excisional biopsy and histopathology. Staging of melanoma is done according to the American Joint Committee on Cancer’s (AJCC) Cancer Staging Manual, 8th edition, using a TNM staging system that incorporates tumor thickness (Breslow depth); ulceration; number of involved regional lymph nodes; presence of in-transit, satellite, and/or microsatellite metastases; distant metastases; and serum lactate dehydrogenase level.14
Case Continued
The patient undergoes a wide excisional biopsy of the right scalp lesion, which is consistent with malignant melanoma. Pathology demonstrates a Breslow depth of 2.6 mm, 2 mitotic figures/mm2, and no evidence of ulceration. He subsequently undergoes wide local excision with 0/3 sentinel lymph nodes positive for malignancy. His final staging is consistent with pT3aN0M0, stage IIA melanoma.
He is seen in follow-up with medical oncology for the next 3.5 years without any evidence of disease recurrence. He then develops symptoms of vertigo, diplopia, and recurrent falls, prompting medical attention. Magnetic resonance imaging (MRI) brain reveals multiple supratentorial and infratentorial lesions concerning for intracranial metastases. Further imaging with computed tomography (CT) chest/abdomen/pelvis reveals a right lower lobe pulmonary mass with right hilar and subcarinal lymphadenopathy. He is admitted for treatment with intravenous dexamethasone and further evaluation with endobronchial ultrasound-guided fine-needle aspiration of the right lower lobe mass, which reveals metastatic melanoma. Given the extent of his intracranial metastases, he is treated with whole brain radiation therapy for symptomatic relief prior to initiating systemic therapy.
What is the general approach to first-line treatment for metastatic melanoma?
The past decade has brought an abundance of data supporting the use of immunotherapy with immune checkpoint inhibitors or molecularly targeted therapy with combined BRAF/MEK inhibitors in the first-line setting.4-8 After the diagnosis of metastatic melanoma has been made, molecular testing is recommended to determine the BRAF status of the tumor. Immunotherapy is the clear choice for first-line therapy in the absence of an activating BRAF V600 mutation. When a BRAF V600 mutation is present, current evidence supports the use of either immunotherapy or molecularly targeted therapy as first-line therapy.
To date, there have been no prospective clinical trials comparing the sequencing of immunotherapy and molecularly targeted therapy in the first-line setting. An ongoing clinical trial (NCT02224781) is comparing dabrafenib and trametinib followed by ipilimumab and nivolumab at time of progression to ipilimumab and nivolumab followed by dabrafenib and trametinib in patients with newly diagnosed stage III/IV BRAF V600 mutation–positive melanoma. The primary outcome measure is 2-year OS. Until completion of that trial, current practice regarding which type of therapy to use in the first-line setting is based on a number of factors including clinical characteristics and provider preferences.
Data suggest that immunotherapies can produce durable responses, especially after treatment completion or discontinuation, albeit at the expense of taking a longer time to achieve clinical benefit and the risk of potentially serious immune-related adverse effects. This idea of a durable, off-treatment response is highlighted by a study that followed 105 patients who had achieved a complete response (CR) and found that 24-month disease-free survival from the time of CR was 90.9% in all patients and 89.9% in the 67 patients who had discontinued pembrolizumab after attaining CR.15 BRAF/MEK inhibition has the potential for rapid clinical responses, though concerns exist about the development of resistance to therapy. The following sections explore the evidence supporting the use of these therapies.
Immunotherapy with Immune Checkpoint Inhibitors
Immunotherapy via immune checkpoint blockade has revolutionized the treatment of many solid tumors over the past decade. The promise of immunotherapy revolves around the potential for achieving a dynamic and durable systemic response against cancer by augmenting the antitumor effects of the immune system. T-cells are central to mounting a systemic antitumor response, and, in addition to antigen recognition, their function depends heavily on fine tuning between co-stimulatory and co-inhibitory signaling. The cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) expressed on T-cells was the first discovered co-inhibitory receptor of T-cell activation.16 Later, it was discovered that the programmed cell death 1 receptor (PD-1), expressed on T-cells, and its ligands PD-L1 and PD-L2, expressed on antigen presenting cells, tumor cells, or other cells in the tumor microenvironment, also served as a potent negative regulator of T-cell function.17
Together, these 2 signaling pathways help to maintain peripheral immune tolerance, whereby autoreactive T-cells that have escaped from the thymus are silenced to prevent autoimmunity. However, these pathways can also be utilized by cancer cells to escape immune surveillance. Monoclonal antibodies that inhibit the aforementioned co-inhibitory signaling pathways, and thus augment the immune response, have proven to be an effective anticancer therapy capable of producing profound and durable responses in certain malignancies.16,17
Ipilimumab
Ipilimumab is a monoclonal antibody that inhibits the function of the CTLA-4 co-inhibitory immune checkpoint. In a phase 3 randomized controlled trial of 676 patients with previously treated metastatic melanoma, ipilimumab at a dose of 3 mg/kg every 3 weeks for 4 cycles, with or without a gp100 peptide vaccine, resulted in an improved median OS of 10.0 and 10.1 months, respectively, compared to 6.4 months in those receiving the peptide vaccine alone, meeting the primary endpoint.4 Subsequently, a phase 3 trial of 502 patients with untreated metastatic melanoma compared ipilimumab at a dose of 10 mg/kg every 3 weeks for 4 cycles plus dacarbazine to dacarbazine plus placebo and found a significant increase in median OS (11.2 months vs 9.1 months), with no additive benefit of chemotherapy. There was a higher reported rate of grade 3 or 4 adverse events in this trial with ipilimumab dosed at 10 mg/kg, which was felt to be dose-related.18 These trials were the first to show improved OS with any systemic therapy in metastatic melanoma and led to US Food and Drug Administration approval of ipilimumab for this indication in 2011.
PD-1 Inhibitor Monotherapy
The PD-1 inhibitors nivolumab and pembrolizumab were initially approved for metastatic melanoma after progression on ipilimumab. In the phase 1 trial of patients with previously treated metastatic melanoma, nivolumab therapy resulted in an ORR of 28%.19 The subsequent phase 2 trial conducted in pretreated patients, including patients who had progressed on ipilimumab, confirmed a similar ORR of 31%, as well as a median PFS of 3.7 months and a median OS of 16.8 months. The estimated response duration in patients who did achieve a response to therapy was 2 years.20 A phase 3 trial (CheckMate 037) comparing nivolumab (n = 120) to investigator’s choice chemotherapy (n = 47) in those with melanoma refractory to ipilimumab demonstrated that nivolumab was superior for the primary endpoint of ORR (31.7% vs 10.6%), had less toxicity (5% rate of grade 3 or 4 adverse events versus 9%), and increased median duration of response (32 months vs 13 months).21
The phase 1 trial (KEYNOTE-001) testing the efficacy of pembrolizumab demonstrated an ORR of 33% in the total population of patients treated and an ORR of 45% in those who were treatment-naive. Additionally, the median OS was 23 months for the total population and 31 months for treatment-naive patients, with only 14% of patients experiencing a grade 3 or 4 adverse event.22 The KEYNOTE-002 phase 2 trial compared 2 different pembrolizumab doses (2 mg/kg and 10 mg/kg every 3 weeks) to investigator’s choice chemotherapy (paclitaxel plus carboplatin, paclitaxel, carboplatin, dacarbazine, or oral temozolomide) in 540 patients with advanced melanoma with documented progression on ipilimumab with or without prior progression on molecularly targeted therapy if positive for a BRAF V600 mutation. The final analysis demonstrated significantly improved ORR with pembrolizumab (22% at 2 mg/kg vs 26% at 10 mg/kg vs 4% chemotherapy) and significantly improved 24-month PFS (16% vs 22% vs 0.6%, respectively). There was a nonstatistically significant improvement in median OS (13.4 months vs 14.7 months vs 10 months), although 55% of the patients initially assigned to the chemotherapy arm crossed over and received pembrolizumab after documentation of progressive disease.23,24
Because PD-1 inhibition improved efficacy with less toxicity than chemotherapy when studied in progressive disease, subsequent studies focused on PD-1 inhibition in the frontline setting. CheckMate 066 was a phase 3 trial comparing nivolumab to dacarbazine as first-line therapy for 418 patients with untreated metastatic melanoma who did not have a BRAF mutation. For the primary end point of 1-year OS, nivolumab was superior to dacarbazine (72.9% vs 42.1%; hazard ratio [HR], 0.42; P < 0.001). Treatment with nivolumab also resulted in superior ORR (40% vs 14%) and PFS (5.1 months vs 2.2 months). Additionally, nivolumab therapy had a lower rate of grade 3 or 4 toxicity compared to dacarbazine (11.7% vs 17.6%).25
The KEYNOTE-006 trial compared 2 separate dosing schedules of pembrolizumab (10 mg/kg every 2 weeks versus every 3 weeks) to ipilimumab (3 mg/kg every 3 weeks for 4 cycles) in a 1:1:1 ratio in 834 patients with metastatic melanoma who had received up to 1 prior systemic therapy, but no prior CTLA-4 or PD-1 inhibitors. The first published data reported statistically significant outcomes for the co-primary end points of 6-month PFS (47.3% for pembrolizumab every 2 weeks vs 46.4% for pembrolizumab every 3 weeks vs 26.5% for ipilimumab; HR, 0.58 for both pembrolizumab groups compared to ipilimumab; P < 0.001) and 12-month OS (74.1% vs 68.4% vs 58.2%) with pembrolizumab compared to ipilimumab. Compared to ipilimumab, pembrolizumab every 2 weeks had a hazard ratio of 0.63 (P = 0.0005) and pembrolizumab every 3 weeks had a hazard ratio of 0.69 (P = 0.0036). The pembrolizumab groups was also had lower rates of grade 3 to 5 toxicity (13.3% vs 10.1% vs 19.9%).5 Updated outcomes demonstrated improved ORR compared to the first analysis (37% vs 36% vs 13%), and improved OS (median OS, not reached for the pembrolizumab groups vs 16.0 months for the ipilimumab group; HR, 0.68, P = 0.0009 for pembrolizumab every 2 weeks versus HR 0.68, P = 0.0008 for pembrolizumab every 3 weeks).26 In addition, 24-month OS was 55% in both pembrolizumab groups compared to 43% in the ipilimumab group. Grade 3 or 4 toxicity occurred less frequently with pembrolizumab (17% vs 17% vs 20%).
Further analysis from the KEYNOTE-006 trial data demonstrated improved ORR, PFS, and OS with pembrolizumab compared to ipilimumab in tumors positive for PD-L1 expression. For PD-L1-negative tumors, response rate was higher, and PFS and OS rates were similar with pembrolizumab compared to ipilimumab. Given that pembrolizumab was associated with similar survival outcomes in PD-L1-negative tumors and with less toxicity than ipilimumab, the superiority of PD-L1 inhibitors over ipilimumab was further supported, regardless of tumor PD-L1 status.27
In sum, PD-1 inhibition should be considered the first-line immunotherapy in advanced melanoma, either alone or in combination with ipilimumab, as discussed in the following section. There is no longer a role for ipilimumab monotherapy in the first-line setting, based on evidence from direct comparison to single-agent PD-1 inhibition in clinical trials that demonstrated superior efficacy and less serious toxicity with PD-1 inhibitors.5,26 The finding that ORR and OS outcomes with single-agent PD-1 inhibitors are higher in treatment-naive patients compared to those receiving prior therapies also supports this approach.22
Combination CTLA-4 and PD-1 Therapy
Despite the potential for durable responses, the majority of patients fail to respond to single-agent PD-1 therapy. Given that preclinical data had suggested the potential for synergy between dual inhibition of CTLA-4 and PD-1, clinical trials were designed to test this approach. The first randomized phase 2 trial that established superior efficacy with combination therapy was the CheckMate 069 trial comparing nivolumab plus ipilimumab to ipilimumab monotherapy. Combination therapy resulted in increased ORR (59% vs 11%), median PFS (not reached vs 3.0 months), 2-year PFS (51.3% vs 12.0%), and 2-year OS (63.8% vs 53.6%).28 Similarly, a phase 1b trial of pembrolizumab plus reduced-dose ipilimumab demonstrated an ORR of 61%, with a 1-year PFS of 69% and 1-year OS of 89%.29
The landmark phase 3 CheckMate 067 trial analyzed efficacy outcomes for 3 different treatment regimens including nivolumab plus ipilimumab, nivolumab monotherapy, and ipilimumab monotherapy in previously untreated patients with unresectable stage III or IV melanoma. The trial was powered to compare survival outcomes for both the combination therapy arm against ipilimumab and the nivolumab monotherapy arm against ipilimumab, but not to compare combination therapy to nivolumab monotherapy. The initial analysis demonstrated a median PFS of 11.5 months with combination therapy versus 6.9 months with nivolumab and 2.9 months with ipilimumab, as well as an ORR of 58% versus 44% and 19%, respectively (Table 1).6 The updated 3-year survival outcomes from CheckMate 067 were notable for superior median OS with combination therapy (not reached in combination vs 37.6 months for nivolumab vs 19.9 months ipilimumab), improved 3-year OS (58% vs 52% vs 34%), and improved 3-year PFS (39% vs 32% vs 10%).7 In the reported 4-year survival outcomes, median OS was not reached in the combination therapy group, and was 36.9 months in the nivolumab monotherapy group and 19.9 months in the ipilimumab monotherapy group. Rates of grade 3 or 4 adverse events were significantly higher in the combination therapy group, at 59% compared to 22% with nivolumab monotherapy and 28% with ipilimumab alone.30 The 3- and 4-year OS outcomes (58% and 54%, respectively) with combination therapy were the highest seen in any phase 3 trial for treatment of advanced melanoma, supporting its use as the best approved first-line therapy in those who can tolerate the potential toxicity of combination therapy7,30 The conclusions from this landmark trial were that both combination therapy and nivolumab monotherapy resulted in statistically significant improvement in OS compared to ipilimumab.
Toxicity Associated with Immune Checkpoint Inhibitors
While immune checkpoint inhibitors have revolutionized the treatment of many solid tumor malignancies, this new class of cancer therapy has brought about a new type of toxicity for clinicians to be aware of, termed immune-related adverse events (irAEs). As immune checkpoint inhibitors amplify the immune response against malignancy, they also increase the likelihood that autoreactive T-cells persist and proliferate within the circulation. Therefore, these therapies can result in almost any type of autoimmune side effect. The most commonly reported irAEs in large clinical trials studying CTLA-4 and PD-1 inhibitors include rash/pruritus, diarrhea/colitis, hepatitis, endocrinopathies (thyroiditis, hypophysitis, adrenalitis), and pneumonitis. Other more rare toxicities include pancreatitis, autoimmune hematologic toxicities, cardiac toxicity (myocarditis, heart failure), and neurologic toxicities (neuropathies, myasthenia gravis-like syndrome, Guillain-Barré syndrome). It has been observed that PD-1 inhibitors have a lower incidence of irAEs than CTLA-4 inhibitors, and that the combined use of PD-1 and CTLA-4 inhibitors is associated with a greater incidence of irAEs compared to monotherapy with either agent.31 Toxicities associated with ipilimumab have been noted to be dose dependent.18 Generally, these toxicities are treated with immunosuppression in the form of glucocorticoids and are often reversible.31 There are several published guidelines that include algorithms for the management of irAEs by organizations such as the National Comprehensive Cancer Network.32
For example, previously untreated patients treated with ipilimumab plus dacarbazine as compared to dacarbazine plus placebo had greater grade 3 or 4 adverse events (56.3% vs 27.5%), and 77.7% of patients experiencing an irAE of any grade.18 In the CheckMate 066 trial comparing frontline nivolumab to dacarbazine, nivolumab had a lower rate of grade 3 or 4 toxicity (11.7% vs 17.6%) and irAEs were relatively infrequent, with diarrhea and elevated alanine aminotransferase level each being the most prominent irAE (affecting 1.0% of patients).25 In the KEYNOTE-006 trial, irAEs seen in more than 1% of patients treated with pembrolizumab included colitis, hepatitis, hypothyroidism, and hyperthyroidism, whereas those occurring in more than 1% of patients treated with ipilimumab included colitis and hypophysitis. Overall, there were lower rates of grade 3 to 5 toxicity with the 2 pembrolizumab doses compared to ipilimumab (13.3% pembrolizumab every 2 weeks vs 10.1% pembrolizumab every 3 weeks vs 19.9% ipilimumab).5 In the CheckMate 067 trial comparing nivolumab plus ipilimumab, nivolumab monotherapy, and ipilimumab monotherapy, rates of treatment-related adverse events of any grade were higher in the combination group (96% combination vs 86% nivolumab vs 86% ipilimumab), as were rates of grade 3 or 4 adverse events (59% vs 21% vs 28%, respectively). The irAE profile was similar to that demonstrated in prior studies: rash/pruritus were the most common, and diarrhea/colitis, elevated aminotransferases, and endocrinopathies were among the more common irAEs.7
Alternative dosing strategies have been investigated in an effort to preserve efficacy and minimize toxicity. A phase 1b trial of pembrolizumab plus reduced-dose ipilimumab demonstrated an ORR of 61%, with a 1-year PFS of 69% and a 1-year OS of 89%. This combination led to 45% of patients having a grade 3 or 4 adverse event, 60% having irAEs of any grade, and only 27% having grade 3 or 4 irAEs.29 The CheckMate 067 trial studied the combination of nivolumab 1 mg/kg plus ipilimumab 3 mg/kg.6 The CheckMate 511 trial compared different combination dosing strategies (nivolumab 3 mg/kg + ipilimumab 1 mg/kg versus nivolumab 1 mg/kg + ipilimumab 3 mg/kg) to assess for safety benefit. In the results published in abstract form, the reduced ipilimumab dose (nivolumab 3 mg/kg + ipilimumab 1 mg/kg arm) resulted in significantly decreased grade 3 to 5 adverse events (33.9% vs 48.3%) without significant differences in ORR, PFS, or OS.33
The question about the efficacy of checkpoint inhibitors in patients who discontinue treatment due to irAEs has been raised, as one hypothesis suggests that such toxicities may also indicate that the antitumor immune response has been activated. In a retrospective pooled analysis of phase 2 and 3 trials where patients received combination therapy with ipilimumab and nivolumab and discontinued therapy during the induction phase due to irAEs, outcomes did not appear to be inferior. Median PFS was 8.4 months in those who discontinued therapy compared to 10.8 months in those who continued therapy, but this did not reach statistical significance. Median OS had not been reached in either group and ORR was actually higher in those who discontinued due to adverse events (58.3% vs 50.2%). While this retrospective analysis needs to be validated, it does suggest that patients likely derive antitumor benefit from immunotherapy even if they have to discontinue therapy due to irAEs. Of note, patients in this analysis were not trialed on nivolumab monotherapy after receiving immunosuppressive treatment for toxicity related to combination therapy, which has since been deemed a reasonable treatment option.34
Molecularly Targeted Therapy for Metastatic Melanoma
As previously mentioned, the MAPK pathway is frequently altered in metastatic melanoma and thus serves as a target for therapy. Mutations in BRAF can cause constitutive activation of the protein’s kinase function, which subsequently phosphorylates/activates MEK in the absence of extracellular growth signals and causes increased cellular proliferation. For the roughly half of patients diagnosed with metastatic melanoma who harbor a BRAF V600 mutation, molecularly targeted therapy with BRAF/MEK inhibitors has emerged as a standard of care treatment option. As such, all patients with advanced disease should be tested for BRAF mutations.
After early phase 1 studies of the BRAF inhibitor vemurafenib demonstrated successful inhibition of mutated BRAF,35 subsequent studies confirmed the benefit of BRAF targeted therapy. In the phase 3 randomized controlled BRIM-3 trial comparing vemurafenib with dacarbazine for treatment of 675 patients with previously untreated metastatic melanoma positive for a BRAF V600E mutation, the vemurafenib group had superior ORR and 6-month OS during the first analysis.36 In a subsequent analysis, median PFS and median OS were also superior with vemurafenib compared to dacarbazine, as vemurafenib had a median OS of 13.6 months compared to 9.7 months with dacarbazine (HR, 0.70; P = 0.0008).37 Dabrafenib was the next BRAF inhibitor to demonstrate clinical efficacy with superior PFS compared to dacarbazine.38
Despite tumor shrinkage in the majority of patients, the development of resistance to therapy was an issue early on. The development of acquired resistance emerged as a heterogeneous process, though many of the identified resistance mechanisms involved reactivation of the MAPK pathway.39 A phase 3 trial of 322 patients with metastatic melanoma comparing the MEK inhibitor trametinib as monotherapy against chemotherapy demonstrated a modest improvement in both median PFS and OS.40 As a result, subsequent efforts focused on a strategy of concurrent MEK inhibition as a means to overcome resistance to molecularly targeted monotherapy
At least 4 large phase 3 randomized controlled trials of combination therapy with BRAF plus MEK inhibitors showed an improved ORR, PFS, and OS when compared to BRAF inhibition alone. The COMBI-d trial comparing dabrafenib plus trametinib versus dabrafenib alone was the first to demonstrate the superiority of combined BRAF/MEK inhibition and made combination therapy the current standard of care for patients with metastatic melanoma and a BRAF V600 mutation. In the final analysis of this trial, 3-year PFS was 22% with combination therapy compared to 12% with dabrafenib alone, and 3-year OS was 44% compared to 32%.8,41,42 A second trial with the combination of dabrafenib and trametinib (COMBI-V) also demonstrated superior efficacy when compared to single-agent vemurafenib without increased toxicity.43 Subsequently, the combination of vemurafenib with MEK inhibitor cobimetinib demonstrated superiority compared to vemurafenib alone,44 followed by the newest combination encorafenib (BRAF inhibitor) and binimetinib (MEK inhibitor) proving superior to either vemurafenib or encorafenib alone.45,46
It is important to note that there have been no studies directly comparing the efficacy of the 3 approved BRAF/MEK inhibitor combinations, but the 3 different regimens have some differences in their toxicity profiles (Table 2). Of note, single-agent BRAF inhibition was associated with increased cutaneous toxicity, including secondary squamous cell carcinoma and keratoacanthoma,47 which was demonstrated to be driven by paradoxical activation of the MAPK pathway.48 The concerning cutaneous toxicities such as squamous cell carcinoma were substantially reduced by combination BRAF/MEK inhibitor therapy.47 Collectively, the higher efficacy along with manageable toxicity profile established combination BRAF/MEK inhibition as the preferred regimen for patients with BRAF-mutated metastatic melanoma who are being considered for molecularly targeted therapy. BRAF inhibitor monotherapy should only be used when there is a specific concern regarding the use of a MEK inhibitor in certain clinical circumstances.
Other driver mutations associated with metastatic melanoma such as NRAS-mutated tumors have proven more difficult to effectively treat with molecularly targeted therapy, with one study showing that the MEK inhibitor binimetinib resulted in a modest improvement in ORR and median PFS without OS benefit compared to dacarbazine.49 Several phase 2 trials involving metastatic melanoma harboring a c-Kit alteration have demonstrated some efficacy with the tyrosine kinase inhibitor imatinib. The largest phase 2 trial of 43 patients treated with imatinib resulted in a 53.5% disease control rate (23.3% partial response and 30.2% stable disease), with 9 of the 10 patients who achieved partial response having a mutation in either exon 11 or 13. Median PFS was 3.5 months and 1-year OS was 51.0%.50
Case Conclusion
Prior to initiation of systemic therapy, the patient’s melanoma is tested and is found to be positive for a BRAF V600K mutation. At his follow-up appointment, the patient continues to endorse generalized weakness, fatigue, issues with balance, and residual pulmonary symptoms after being treated for post-obstructive pneumonia. Given his current symptoms and extent of metastatic disease, immunotherapy is deferred and he is started on combination molecularly targeted therapy with dabrafenib and trametinib. He initially does well, with a partial response noted by resolution of symptoms and decreased size of his intracranial metastases and decreased size of the right lower lobe mass. Further follow-up of this patient is presented in the second article in this 2-part review of advanced melanoma.
1. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2018. CA Cancer J Clin. 2018;68:7-30.
2. Ives NJ, Stowe RL, Lorigan P, Wheatley K. Chemotherapy compared with biochemotherapy for the treatment of metastatic melanoma: a meta-analysis of 18 trials involving 2621 patients. J Clin Oncol. 2007;25:5426-34.
3. Atkins MB, Lotze MT, Dutcher JP, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17:2105-16.
4. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711-23.
5. Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372:2522-2532.
6. Larkin J, Chiarion-Sileni V, Gonazalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23-34.
7. Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2017;377:1345-1356.
8. Long GV, Stroyakovskiy D, Gogas H, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med. 2014;371:1877-1888.
9. Elwood JM, Jopson J. Melanoma and sun exposure: an overview of published studies. Int J Cancer. 1997;73:198-203.
10. Gilchrest BA, Eller MS, Geller AC, Yaar M. The pathogenesis of melanoma induced by ultraviolet radiation. N Engl J Med. 199;340:1341-1348.
11. Omholt K, Platz A, Kanter L, et al. NRAS and BRAF mutations arise early during melanoma pathogenesis and are preserved throughout tumor progression. Clin Cancer Res. 2003;9:6483-8.
12. Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949-54.
13. Cancer Genome Atlas Network. Genomic classification of cutaneous melanoma. Cell 2015;161:1681-96.
14. Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma staging: evidence-based changes in the American Joint Committee on Cancer Eighth Edition Cancer Staging Manual. CA Cancer J Clin. 2017;67:472-492.
15. Robert C, Ribas A, Hamid O, et al. Durable complete response after discontinuation of pembrolizumab in patients with metastatic melanoma. J Clin Oncol. 2018;36:1668-1674.
16. Salama AKS, Hodi FS. Cytotoxic T-lymphocyte-associated antigen-4. Clin Cancer Res. 2011;17:4622-8.
17. Boussiotis VA. Molecular and biochemical aspects of the PD-1 checkpoint pathway. N Engl J Med. 2016;375:1767-1778.
18. Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517-2526.
19. Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443-2454.
20. Topalian S, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32:1020-30.
21. Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375-84.
22. Ribas A, Hamid O, Daud A, et al. Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA. 2016;315:1600-1609.
23. Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16:908-18.
24. Hamid O, Puzanov I, Dummer R, et al. Final analysis of a randomised trial comparing pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory advanced melanoma. Eur J Cancer. 2017;86:37-45.
25. Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320-330.
26. Schachter J, Ribas A, Long GV, et al. Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicenter, randomised, open-label phase 3 study (KEYNOTE-006). Lancet Oncol. 2017;390:1853-1862.
27. Carlino MS, Long GV, Schadendorf D, et al. Outcomes by line of therapy and programmed death ligand 1 expression in patients with advanced melanoma treated with pembrolizumab or ipilimumab in KEYNOTE-006. A randomised clinical trial. Eur J Cancer. 2018;101:236-243.
28. Hodi FS, Chesney J, Pavlick AC, et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 2016;17:1558-1568.
29. Long GV, Atkinson V, Cebon JS, et al. Standard-dose pembrolizumab in combination with reduced-dose ipilimumab for patients with advanced melanoma (KEYNOTE-029): an open-label, phase 1b trial. Lancet Oncol. 2017;18:1202-10.
30. Hodi FS, Chiarion-Sileni V, Gonzalez R, et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19:1480-1492.
31. Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the immune-related adverse effects of immune checkpoint inhibitors: a review. JAMA Oncol. 2016;2:1346-1353.
32. National Comprehensive Cancer Network. Management of immunotherapy-related toxicities (version 2.2019). www.nccn.org/professionals/physician_gls/pdf/immunotherapy.pdf. Accessed April 8, 2019.
33. Lebbé C, Meyer N, Mortier L, et al. Initial results from a phase IIIb/IV study evaluating two dosing regimens of nivolumab (NIVO) in combination with ipilimumab (IPI) in patients with advanced melanoma (CheckMate 511) [Abstract LBA47]. Ann Oncol. 2018;29:mdy424.057.
34. Schadendorf D, Wolchok JD, Hodi FS, et al. Efficacy and safety outcomes in patients with advanced melanoma who discontinued treatment with nivolumab and ipilimumab because of adverse events: a pooled analysis of randomized phase ii and iii trials. J Clin Oncol. 2017;35:3807-3814.
35. Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809-819.
36. Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507-2516.
37. McArthur GA, Chapman PB, Robert C, et al. Safety and efficacy of vemurafenib in BRAFV600E and BRAFV600K mutation-positive melanoma (BRIM-3): extended follow up of a phase 3, randomised, open-label study. Lancet Oncol. 2014;15:323-332.
38. Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicenter, open-label, phase 3 randomised controlled trial. Lancet Oncol. 2012;380:358-365.
39. Rizos H, Menzies AM, Pupo GM, et al. BRAF inhibitor resistance mechanisms in metastatic melanoma: spectrum and clinical impact. Clin Cancer Res. 2014;20:1965-1977.
40. Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med. 2012;367:107-114.
41. Long GV, Stroyakovskiy D, Gogas H, et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: a multicenter, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2015;386:444-451.
42. Long GV, Flaherty KT, Stroyakovskiy D, et al. Dabrafenib plus trametinib versus dabrafenib monotherapy in patients with metastatic BRAF V600E/K-mutant melanoma: long-term survival and safety analysis of a phase 3 study. Ann Oncol. 2017;28:1631-1639.
43. Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30-39.
44. Ascierto PA, McArthur GA, Dréno B, et al. Cobimetinib combined with vemurafenib in advanced BRAFV600-mutant melanoma (coBRIM): updated efficacy results from a randomised, double-blind, phase 3 trial. Lancet Oncol. 2016;17:1248-260.
45. Dummer R, Ascierto PA, Gogas HJ, et al. Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF-mutant melanoma (COLUMBUS): a multicenter, open-label, randomised phase 3 trial. Lancet Oncol. 2018;19:603-615.
46. Dummer R, Ascierto PA, Gogas HJ, et al. Overall survival in patients with BRAF-mutant melanoma receiving encorafenib plus binimetinib versus vemurafenib or encorafenib (COLUMBUS): a multicenter, open-label, randomised, phase 3 trial. Lancet Oncol. 2018;19:1315-1327.
47. Carlos G, Anforth R, Clements A, et al. Cutaneous toxic effects of BRAF inhibitors alone and in combination with MEK inhibitors for metastatic melanoma. JAMA. Dermatol 2015;151:1103-1109.
48. Su F, Viros A, Milagre C, et al. RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. N Engl J Med. 2012;366:207-215.
49. Dummer R, Schadendorf D, Ascierto P, et al. Binimetinib versus dacarbazine in patients with advanced NRAS-mutant melanoma (NEMO): a multicenter, open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18:435-445.
50. Guo J, Si L, Kong Y, et al. Phase II, open-label, single-arm trial of imatinib mesylate in patients with metastatic melanoma harboring c-Kit mutation or amplification. J Clin Oncol. 2011;29:2904-2909.
1. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2018. CA Cancer J Clin. 2018;68:7-30.
2. Ives NJ, Stowe RL, Lorigan P, Wheatley K. Chemotherapy compared with biochemotherapy for the treatment of metastatic melanoma: a meta-analysis of 18 trials involving 2621 patients. J Clin Oncol. 2007;25:5426-34.
3. Atkins MB, Lotze MT, Dutcher JP, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17:2105-16.
4. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711-23.
5. Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372:2522-2532.
6. Larkin J, Chiarion-Sileni V, Gonazalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23-34.
7. Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2017;377:1345-1356.
8. Long GV, Stroyakovskiy D, Gogas H, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med. 2014;371:1877-1888.
9. Elwood JM, Jopson J. Melanoma and sun exposure: an overview of published studies. Int J Cancer. 1997;73:198-203.
10. Gilchrest BA, Eller MS, Geller AC, Yaar M. The pathogenesis of melanoma induced by ultraviolet radiation. N Engl J Med. 199;340:1341-1348.
11. Omholt K, Platz A, Kanter L, et al. NRAS and BRAF mutations arise early during melanoma pathogenesis and are preserved throughout tumor progression. Clin Cancer Res. 2003;9:6483-8.
12. Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949-54.
13. Cancer Genome Atlas Network. Genomic classification of cutaneous melanoma. Cell 2015;161:1681-96.
14. Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma staging: evidence-based changes in the American Joint Committee on Cancer Eighth Edition Cancer Staging Manual. CA Cancer J Clin. 2017;67:472-492.
15. Robert C, Ribas A, Hamid O, et al. Durable complete response after discontinuation of pembrolizumab in patients with metastatic melanoma. J Clin Oncol. 2018;36:1668-1674.
16. Salama AKS, Hodi FS. Cytotoxic T-lymphocyte-associated antigen-4. Clin Cancer Res. 2011;17:4622-8.
17. Boussiotis VA. Molecular and biochemical aspects of the PD-1 checkpoint pathway. N Engl J Med. 2016;375:1767-1778.
18. Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517-2526.
19. Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443-2454.
20. Topalian S, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32:1020-30.
21. Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375-84.
22. Ribas A, Hamid O, Daud A, et al. Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA. 2016;315:1600-1609.
23. Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16:908-18.
24. Hamid O, Puzanov I, Dummer R, et al. Final analysis of a randomised trial comparing pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory advanced melanoma. Eur J Cancer. 2017;86:37-45.
25. Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320-330.
26. Schachter J, Ribas A, Long GV, et al. Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicenter, randomised, open-label phase 3 study (KEYNOTE-006). Lancet Oncol. 2017;390:1853-1862.
27. Carlino MS, Long GV, Schadendorf D, et al. Outcomes by line of therapy and programmed death ligand 1 expression in patients with advanced melanoma treated with pembrolizumab or ipilimumab in KEYNOTE-006. A randomised clinical trial. Eur J Cancer. 2018;101:236-243.
28. Hodi FS, Chesney J, Pavlick AC, et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 2016;17:1558-1568.
29. Long GV, Atkinson V, Cebon JS, et al. Standard-dose pembrolizumab in combination with reduced-dose ipilimumab for patients with advanced melanoma (KEYNOTE-029): an open-label, phase 1b trial. Lancet Oncol. 2017;18:1202-10.
30. Hodi FS, Chiarion-Sileni V, Gonzalez R, et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19:1480-1492.
31. Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the immune-related adverse effects of immune checkpoint inhibitors: a review. JAMA Oncol. 2016;2:1346-1353.
32. National Comprehensive Cancer Network. Management of immunotherapy-related toxicities (version 2.2019). www.nccn.org/professionals/physician_gls/pdf/immunotherapy.pdf. Accessed April 8, 2019.
33. Lebbé C, Meyer N, Mortier L, et al. Initial results from a phase IIIb/IV study evaluating two dosing regimens of nivolumab (NIVO) in combination with ipilimumab (IPI) in patients with advanced melanoma (CheckMate 511) [Abstract LBA47]. Ann Oncol. 2018;29:mdy424.057.
34. Schadendorf D, Wolchok JD, Hodi FS, et al. Efficacy and safety outcomes in patients with advanced melanoma who discontinued treatment with nivolumab and ipilimumab because of adverse events: a pooled analysis of randomized phase ii and iii trials. J Clin Oncol. 2017;35:3807-3814.
35. Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809-819.
36. Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507-2516.
37. McArthur GA, Chapman PB, Robert C, et al. Safety and efficacy of vemurafenib in BRAFV600E and BRAFV600K mutation-positive melanoma (BRIM-3): extended follow up of a phase 3, randomised, open-label study. Lancet Oncol. 2014;15:323-332.
38. Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicenter, open-label, phase 3 randomised controlled trial. Lancet Oncol. 2012;380:358-365.
39. Rizos H, Menzies AM, Pupo GM, et al. BRAF inhibitor resistance mechanisms in metastatic melanoma: spectrum and clinical impact. Clin Cancer Res. 2014;20:1965-1977.
40. Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med. 2012;367:107-114.
41. Long GV, Stroyakovskiy D, Gogas H, et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: a multicenter, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2015;386:444-451.
42. Long GV, Flaherty KT, Stroyakovskiy D, et al. Dabrafenib plus trametinib versus dabrafenib monotherapy in patients with metastatic BRAF V600E/K-mutant melanoma: long-term survival and safety analysis of a phase 3 study. Ann Oncol. 2017;28:1631-1639.
43. Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30-39.
44. Ascierto PA, McArthur GA, Dréno B, et al. Cobimetinib combined with vemurafenib in advanced BRAFV600-mutant melanoma (coBRIM): updated efficacy results from a randomised, double-blind, phase 3 trial. Lancet Oncol. 2016;17:1248-260.
45. Dummer R, Ascierto PA, Gogas HJ, et al. Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF-mutant melanoma (COLUMBUS): a multicenter, open-label, randomised phase 3 trial. Lancet Oncol. 2018;19:603-615.
46. Dummer R, Ascierto PA, Gogas HJ, et al. Overall survival in patients with BRAF-mutant melanoma receiving encorafenib plus binimetinib versus vemurafenib or encorafenib (COLUMBUS): a multicenter, open-label, randomised, phase 3 trial. Lancet Oncol. 2018;19:1315-1327.
47. Carlos G, Anforth R, Clements A, et al. Cutaneous toxic effects of BRAF inhibitors alone and in combination with MEK inhibitors for metastatic melanoma. JAMA. Dermatol 2015;151:1103-1109.
48. Su F, Viros A, Milagre C, et al. RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. N Engl J Med. 2012;366:207-215.
49. Dummer R, Schadendorf D, Ascierto P, et al. Binimetinib versus dacarbazine in patients with advanced NRAS-mutant melanoma (NEMO): a multicenter, open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18:435-445.
50. Guo J, Si L, Kong Y, et al. Phase II, open-label, single-arm trial of imatinib mesylate in patients with metastatic melanoma harboring c-Kit mutation or amplification. J Clin Oncol. 2011;29:2904-2909.
PT beats steroid injections for knee OA
TORONTO – Eight physical therapy sessions spread over 4-6 weeks in patients with knee osteoarthritis provided significantly greater and longer-lasting improvements in both pain and function than an intra-articular corticosteroid injection in a randomized, multicenter trial with 12 months of prospective, blinded follow-up, Daniel I. Rhon, DPT, DSc, reported at the OARSI 2019 World Congress.
“Considering the very low utilization rate of physical therapy prior to arthroplasty, perhaps we should more often give it a try before declaring that conservative care has failed and moving on to surgical management,” concluded Dr. Rhon, director of the primary care musculoskeletal research center at Brooke Army Medical Center in San Antonio.
Various studies have shown that close to 50% of patients with knee OA receive one or more intra-articular corticosteroid injections within 5 years prior to undergoing total knee arthroplasty, compared with physical therapy in only about 10% of patients, even though most guidelines rate both as first-line therapies, he noted at the meeting sponsored by the Osteoarthritis Research Society International.
He presented a randomized trial of 156 patients who sought treatment for pain caused by knee OA at army medical center primary care clinics. To his knowledge, this was the first-ever randomized, head-to-head comparison of the effectiveness of a physical therapy regimen versus corticosteroid injections for knee OA. Because he and his coinvestigators wanted a pragmatic study giving each treatment strategy its due, booster therapy was available to patients who requested it. Patients in the corticosteroid arm were able to receive up to two additional spaced intra-articular injections as needed, while those assigned to the individualized manual physical therapy intervention, which utilized exercises targeting the typical strength and movement deficits found in patients with knee OA, could have up to three additional sessions. At the outset, all participants received education about the benefits of regular low-impact physical activity, weight reduction, and strength and flexibility exercises.
The two treatment groups were comparable except that the physical therapy group had a longer disease duration – a mean of 123 months as compared with 89 in the corticosteroid group – and more radiographically severe disease. Indeed, 60% of patients randomized to physical therapy were Kellgren-Lawrence scale grade III-IV, versus 45% of those assigned to intra-articular corticosteroid injection.
The primary outcome in the study was change in Western Ontario & McMaster Universities Arthritis Index (WOMAC) score at 12 months. As early as 4 weeks into the study, the physical therapy group showed significantly greater improvement than in the comparison arm: from a mean baseline WOMAC score of 115 to 42.9 at 4 weeks, 42.5 at 6 months, and 38.4 at 1 year. The comparable figures in the intra-articular corticosteroid group were 113.3 at baseline, 53.3 at 4 weeks, 57.9 at 6 months, and 53.8 at 1 year.
“Physical therapy provided clinically important benefit that was superior to corticosteroid injection out to 1 year, while also providing the short-term benefit typically sought from corticosteroid injection,” Dr. Rhon observed.
The median improvement in WOMAC score at 1 year was 52% in the corticosteroid group and 71% in the physical therapy arm. About 59% of the physical therapy group experienced at least a 50% reduction in WOMAC score at 12 months, as did 38% of intra-articular injection recipients. The number needed to treat with physical therapy instead of intra-articular corticosteroids in order for one additional patient to achieve at least a 50% improvement in WOMAC score through 1 year of follow-up was four.
“We were a little bit surprised that there was such a large effect size in the study. The effect size in the injection group was bigger than reported in some other trials,” according to Dr. Rhon.
In terms of downstream utilization of health care, there were two knee arthroplasties and one arthroscopy in the study population, all in the intra-articular corticosteroid group. Seven patients in the intra-articular steroid group received more than three injections, including one patient with nine. And 13 patients in the physical therapy arm went outside the study in order to receive at least one corticosteroid injection.
One audience member pointed out that the physical therapy approach offers an important side benefit: In addition to improving pain and function, the exercise regimen has a favorable effect on comorbid metabolic diseases commonly associated with knee OA.
“An injection doesn’t achieve that,” he noted.
Dr. Rhon reported having no financial conflicts of interest regarding the randomized trial, sponsored by Madigan Army Medical Center. He receives research funding from the National Institutes of Health and the Congressionally Directed Medical Research Programs.
SOURCE: Rhon DI et al. Osteoarthritis Cartilage. 2019 Apr;27[suppl 1]:S32, Abstract 13
TORONTO – Eight physical therapy sessions spread over 4-6 weeks in patients with knee osteoarthritis provided significantly greater and longer-lasting improvements in both pain and function than an intra-articular corticosteroid injection in a randomized, multicenter trial with 12 months of prospective, blinded follow-up, Daniel I. Rhon, DPT, DSc, reported at the OARSI 2019 World Congress.
“Considering the very low utilization rate of physical therapy prior to arthroplasty, perhaps we should more often give it a try before declaring that conservative care has failed and moving on to surgical management,” concluded Dr. Rhon, director of the primary care musculoskeletal research center at Brooke Army Medical Center in San Antonio.
Various studies have shown that close to 50% of patients with knee OA receive one or more intra-articular corticosteroid injections within 5 years prior to undergoing total knee arthroplasty, compared with physical therapy in only about 10% of patients, even though most guidelines rate both as first-line therapies, he noted at the meeting sponsored by the Osteoarthritis Research Society International.
He presented a randomized trial of 156 patients who sought treatment for pain caused by knee OA at army medical center primary care clinics. To his knowledge, this was the first-ever randomized, head-to-head comparison of the effectiveness of a physical therapy regimen versus corticosteroid injections for knee OA. Because he and his coinvestigators wanted a pragmatic study giving each treatment strategy its due, booster therapy was available to patients who requested it. Patients in the corticosteroid arm were able to receive up to two additional spaced intra-articular injections as needed, while those assigned to the individualized manual physical therapy intervention, which utilized exercises targeting the typical strength and movement deficits found in patients with knee OA, could have up to three additional sessions. At the outset, all participants received education about the benefits of regular low-impact physical activity, weight reduction, and strength and flexibility exercises.
The two treatment groups were comparable except that the physical therapy group had a longer disease duration – a mean of 123 months as compared with 89 in the corticosteroid group – and more radiographically severe disease. Indeed, 60% of patients randomized to physical therapy were Kellgren-Lawrence scale grade III-IV, versus 45% of those assigned to intra-articular corticosteroid injection.
The primary outcome in the study was change in Western Ontario & McMaster Universities Arthritis Index (WOMAC) score at 12 months. As early as 4 weeks into the study, the physical therapy group showed significantly greater improvement than in the comparison arm: from a mean baseline WOMAC score of 115 to 42.9 at 4 weeks, 42.5 at 6 months, and 38.4 at 1 year. The comparable figures in the intra-articular corticosteroid group were 113.3 at baseline, 53.3 at 4 weeks, 57.9 at 6 months, and 53.8 at 1 year.
“Physical therapy provided clinically important benefit that was superior to corticosteroid injection out to 1 year, while also providing the short-term benefit typically sought from corticosteroid injection,” Dr. Rhon observed.
The median improvement in WOMAC score at 1 year was 52% in the corticosteroid group and 71% in the physical therapy arm. About 59% of the physical therapy group experienced at least a 50% reduction in WOMAC score at 12 months, as did 38% of intra-articular injection recipients. The number needed to treat with physical therapy instead of intra-articular corticosteroids in order for one additional patient to achieve at least a 50% improvement in WOMAC score through 1 year of follow-up was four.
“We were a little bit surprised that there was such a large effect size in the study. The effect size in the injection group was bigger than reported in some other trials,” according to Dr. Rhon.
In terms of downstream utilization of health care, there were two knee arthroplasties and one arthroscopy in the study population, all in the intra-articular corticosteroid group. Seven patients in the intra-articular steroid group received more than three injections, including one patient with nine. And 13 patients in the physical therapy arm went outside the study in order to receive at least one corticosteroid injection.
One audience member pointed out that the physical therapy approach offers an important side benefit: In addition to improving pain and function, the exercise regimen has a favorable effect on comorbid metabolic diseases commonly associated with knee OA.
“An injection doesn’t achieve that,” he noted.
Dr. Rhon reported having no financial conflicts of interest regarding the randomized trial, sponsored by Madigan Army Medical Center. He receives research funding from the National Institutes of Health and the Congressionally Directed Medical Research Programs.
SOURCE: Rhon DI et al. Osteoarthritis Cartilage. 2019 Apr;27[suppl 1]:S32, Abstract 13
TORONTO – Eight physical therapy sessions spread over 4-6 weeks in patients with knee osteoarthritis provided significantly greater and longer-lasting improvements in both pain and function than an intra-articular corticosteroid injection in a randomized, multicenter trial with 12 months of prospective, blinded follow-up, Daniel I. Rhon, DPT, DSc, reported at the OARSI 2019 World Congress.
“Considering the very low utilization rate of physical therapy prior to arthroplasty, perhaps we should more often give it a try before declaring that conservative care has failed and moving on to surgical management,” concluded Dr. Rhon, director of the primary care musculoskeletal research center at Brooke Army Medical Center in San Antonio.
Various studies have shown that close to 50% of patients with knee OA receive one or more intra-articular corticosteroid injections within 5 years prior to undergoing total knee arthroplasty, compared with physical therapy in only about 10% of patients, even though most guidelines rate both as first-line therapies, he noted at the meeting sponsored by the Osteoarthritis Research Society International.
He presented a randomized trial of 156 patients who sought treatment for pain caused by knee OA at army medical center primary care clinics. To his knowledge, this was the first-ever randomized, head-to-head comparison of the effectiveness of a physical therapy regimen versus corticosteroid injections for knee OA. Because he and his coinvestigators wanted a pragmatic study giving each treatment strategy its due, booster therapy was available to patients who requested it. Patients in the corticosteroid arm were able to receive up to two additional spaced intra-articular injections as needed, while those assigned to the individualized manual physical therapy intervention, which utilized exercises targeting the typical strength and movement deficits found in patients with knee OA, could have up to three additional sessions. At the outset, all participants received education about the benefits of regular low-impact physical activity, weight reduction, and strength and flexibility exercises.
The two treatment groups were comparable except that the physical therapy group had a longer disease duration – a mean of 123 months as compared with 89 in the corticosteroid group – and more radiographically severe disease. Indeed, 60% of patients randomized to physical therapy were Kellgren-Lawrence scale grade III-IV, versus 45% of those assigned to intra-articular corticosteroid injection.
The primary outcome in the study was change in Western Ontario & McMaster Universities Arthritis Index (WOMAC) score at 12 months. As early as 4 weeks into the study, the physical therapy group showed significantly greater improvement than in the comparison arm: from a mean baseline WOMAC score of 115 to 42.9 at 4 weeks, 42.5 at 6 months, and 38.4 at 1 year. The comparable figures in the intra-articular corticosteroid group were 113.3 at baseline, 53.3 at 4 weeks, 57.9 at 6 months, and 53.8 at 1 year.
“Physical therapy provided clinically important benefit that was superior to corticosteroid injection out to 1 year, while also providing the short-term benefit typically sought from corticosteroid injection,” Dr. Rhon observed.
The median improvement in WOMAC score at 1 year was 52% in the corticosteroid group and 71% in the physical therapy arm. About 59% of the physical therapy group experienced at least a 50% reduction in WOMAC score at 12 months, as did 38% of intra-articular injection recipients. The number needed to treat with physical therapy instead of intra-articular corticosteroids in order for one additional patient to achieve at least a 50% improvement in WOMAC score through 1 year of follow-up was four.
“We were a little bit surprised that there was such a large effect size in the study. The effect size in the injection group was bigger than reported in some other trials,” according to Dr. Rhon.
In terms of downstream utilization of health care, there were two knee arthroplasties and one arthroscopy in the study population, all in the intra-articular corticosteroid group. Seven patients in the intra-articular steroid group received more than three injections, including one patient with nine. And 13 patients in the physical therapy arm went outside the study in order to receive at least one corticosteroid injection.
One audience member pointed out that the physical therapy approach offers an important side benefit: In addition to improving pain and function, the exercise regimen has a favorable effect on comorbid metabolic diseases commonly associated with knee OA.
“An injection doesn’t achieve that,” he noted.
Dr. Rhon reported having no financial conflicts of interest regarding the randomized trial, sponsored by Madigan Army Medical Center. He receives research funding from the National Institutes of Health and the Congressionally Directed Medical Research Programs.
SOURCE: Rhon DI et al. Osteoarthritis Cartilage. 2019 Apr;27[suppl 1]:S32, Abstract 13
REPORTING FROM OARSI 2019
Weekly ciprofloxacin as effective as daily norfloxacin in prevention of SBP
Clinical question: Does ciprofloxacin administered once weekly prevent spontaneous bacterial peritonitis (SBP) as effectively as daily norfloxacin?
Background: Studies have shown that daily administration of norfloxacin is effective for primary prophylaxis as well as secondary prevention of SBP in patients with cirrhosis and ascites. Prior studies have demonstrated efficacy of weekly ciprofloxacin, but no previous studies have compared the two antibiotics.
Study design: Investigator initiated open-label randomized, controlled trial.
Setting: Seven tertiary hospitals in South Korea.
Synopsis: The investigators enrolled 124 patients aged 20-75 with cirrhosis and ascites, ascitic cell count less than 250/mm3, and either ascitic protein less than 1.5g/dL or a history of spontaneous bacterial peritonitis. The patients were randomized to receive norfloxacin 400 mg daily or ciprofloxacin 750 mg weekly, with routine visits during the 12-month study period.
The primary end point of SBP prevention rates at 1 year were 92.7% (51/55) in the norfloxacin group and 96.5% (55/57) in the ciprofloxacin group (P = .712), which met criteria for noninferiority. Other outcomes included no difference in rates of liver transplantation, infectious complications, hepatorenal syndrome, hepatic encephalopathy, variceal bleeding, and hepatocellular carcinoma. A subgroup analysis of patients at higher risk of developing SBP showed 87% prevention rates for the norfloxacin group and 94% for the ciprofloxacin group, although this result was not statistically significant.
The major limitation of this study is that it was not double blinded, so patients were aware of which medication they were taking. Additionally, almost 10% of the cohort was lost to follow-up, but this was accounted for in the sample-size calculation.
Bottom line: Once weekly administration of ciprofloxacin is not inferior to daily norfloxacin for the prevention of SBP in patients with cirrhosis and low ascitic protein levels and may provide a more cost-effective therapy with greater patient compliance.
Citation: Yim HJ et al. Daily norfloxacin vs weekly ciprofloxacin to prevent spontaneous bacterial peritonitis: A randomized controlled trial. Am J Gastroenterol. 2018 Aug;113:1167-76.
Dr. Angeli is an assistant professor in the division of hospital medicine, University of New Mexico.
Clinical question: Does ciprofloxacin administered once weekly prevent spontaneous bacterial peritonitis (SBP) as effectively as daily norfloxacin?
Background: Studies have shown that daily administration of norfloxacin is effective for primary prophylaxis as well as secondary prevention of SBP in patients with cirrhosis and ascites. Prior studies have demonstrated efficacy of weekly ciprofloxacin, but no previous studies have compared the two antibiotics.
Study design: Investigator initiated open-label randomized, controlled trial.
Setting: Seven tertiary hospitals in South Korea.
Synopsis: The investigators enrolled 124 patients aged 20-75 with cirrhosis and ascites, ascitic cell count less than 250/mm3, and either ascitic protein less than 1.5g/dL or a history of spontaneous bacterial peritonitis. The patients were randomized to receive norfloxacin 400 mg daily or ciprofloxacin 750 mg weekly, with routine visits during the 12-month study period.
The primary end point of SBP prevention rates at 1 year were 92.7% (51/55) in the norfloxacin group and 96.5% (55/57) in the ciprofloxacin group (P = .712), which met criteria for noninferiority. Other outcomes included no difference in rates of liver transplantation, infectious complications, hepatorenal syndrome, hepatic encephalopathy, variceal bleeding, and hepatocellular carcinoma. A subgroup analysis of patients at higher risk of developing SBP showed 87% prevention rates for the norfloxacin group and 94% for the ciprofloxacin group, although this result was not statistically significant.
The major limitation of this study is that it was not double blinded, so patients were aware of which medication they were taking. Additionally, almost 10% of the cohort was lost to follow-up, but this was accounted for in the sample-size calculation.
Bottom line: Once weekly administration of ciprofloxacin is not inferior to daily norfloxacin for the prevention of SBP in patients with cirrhosis and low ascitic protein levels and may provide a more cost-effective therapy with greater patient compliance.
Citation: Yim HJ et al. Daily norfloxacin vs weekly ciprofloxacin to prevent spontaneous bacterial peritonitis: A randomized controlled trial. Am J Gastroenterol. 2018 Aug;113:1167-76.
Dr. Angeli is an assistant professor in the division of hospital medicine, University of New Mexico.
Clinical question: Does ciprofloxacin administered once weekly prevent spontaneous bacterial peritonitis (SBP) as effectively as daily norfloxacin?
Background: Studies have shown that daily administration of norfloxacin is effective for primary prophylaxis as well as secondary prevention of SBP in patients with cirrhosis and ascites. Prior studies have demonstrated efficacy of weekly ciprofloxacin, but no previous studies have compared the two antibiotics.
Study design: Investigator initiated open-label randomized, controlled trial.
Setting: Seven tertiary hospitals in South Korea.
Synopsis: The investigators enrolled 124 patients aged 20-75 with cirrhosis and ascites, ascitic cell count less than 250/mm3, and either ascitic protein less than 1.5g/dL or a history of spontaneous bacterial peritonitis. The patients were randomized to receive norfloxacin 400 mg daily or ciprofloxacin 750 mg weekly, with routine visits during the 12-month study period.
The primary end point of SBP prevention rates at 1 year were 92.7% (51/55) in the norfloxacin group and 96.5% (55/57) in the ciprofloxacin group (P = .712), which met criteria for noninferiority. Other outcomes included no difference in rates of liver transplantation, infectious complications, hepatorenal syndrome, hepatic encephalopathy, variceal bleeding, and hepatocellular carcinoma. A subgroup analysis of patients at higher risk of developing SBP showed 87% prevention rates for the norfloxacin group and 94% for the ciprofloxacin group, although this result was not statistically significant.
The major limitation of this study is that it was not double blinded, so patients were aware of which medication they were taking. Additionally, almost 10% of the cohort was lost to follow-up, but this was accounted for in the sample-size calculation.
Bottom line: Once weekly administration of ciprofloxacin is not inferior to daily norfloxacin for the prevention of SBP in patients with cirrhosis and low ascitic protein levels and may provide a more cost-effective therapy with greater patient compliance.
Citation: Yim HJ et al. Daily norfloxacin vs weekly ciprofloxacin to prevent spontaneous bacterial peritonitis: A randomized controlled trial. Am J Gastroenterol. 2018 Aug;113:1167-76.
Dr. Angeli is an assistant professor in the division of hospital medicine, University of New Mexico.
Treatment Guidelines for Pregnant Native Women With Opioid Use Disorder
To provide better “culturally responsive” care, the IHS and American College of Obstetricians and Gynecologists (ACOG) have announced new clinical recommendations for health care providers (HCPs) who treat Native American pregnant woman and women of childbearing age with opioid use disorder (OUD).
There are no current comprehensive guidelines to manage the care of pregnant women with opioid dependence who live in rural or remote communities, ACOG acknowledges. That absence, in addition to a lack of resources, lack of training in treating substance use disorder in pregnancy, and providers’ discomfort with opioid agonist therapy in pregnancy, has contributed to “wide variation in the quality of care these women receive.”
Disparities are particularly extreme for American Indian and Alaska Native women (AI/AN), ACOG notes. They have the highest risk of dying of prescription opioid overdose, and they face specific barriers to accessing treatment. For instance, there are few opioid treatment programs offering methadone treatment on tribal lands.
The new recommendations were developed in partnership with tribes and ACOG’s Committee on American Indian and Alaska Native Women’s Health, based on critical feedback from listening sessions and tribal consultations in the past year. The specific guidelines are tailored for Native women.
The committee recognizes, it says, the “necessary wide-ranging scope of treatment for OUD, especially among AI/AN childbearing women.” Key recommendations include strategies to avoid or minimize the use of opioids for pain management and encourage alternative pain therapies, such as physical therapy, acupuncture, and mindfulness-based therapy. In pregnancy, ACOG recommends that obstetric providers perform universal screening and brief intervention using a validated tool as early in prenatal care as possible.
Treatment may require management of co-occurring polysubstance use disorders; concomitant alcohol and methamphetamine use predominate in many tribal areas. HCPs also may need to offer personalized care that “acknowledges the contributions of intergenerational and personal trauma,” the guidelines say. Trauma-informed interdisciplinary approaches to posttraumatic stress disorder that engage tribal resources, social structures, and assets are “crucial to impactful care of opioid use disorder.”
The postpartum period is associated with a high rate of relapse, ACOG says. Histories of trauma, for instance, can exacerbate mood disorders. Moreover, substance use and overdose are increasingly being recognized as key contributors to pregnancy-associated death in the US; a disproportionate share of deaths are postpartum. Infants of untreated, depressed mothers demonstrate poor outcomes, including impaired motor adaptation and self-regulation, developmental delay, and higher arousal scores. The guidelines advise treating mothers and infants as dyads to improve the course of neonatal opioid withdrawal syndrome (NOWS). The proportion of infants with NOWS who need pharmacologic treatment has risen dramatically, the committee notes.
“[I]t is clear from our site visits and clinical experience,” the committee members note, “that adaptation of systems for integration and reach in rural settings is necessary, with potentially different needs and assets in Native and rural populations.” Native culture and traditions, they add, offer opportunities for community engagement and support that can be integrated into medical care for the women and their infants.
To provide better “culturally responsive” care, the IHS and American College of Obstetricians and Gynecologists (ACOG) have announced new clinical recommendations for health care providers (HCPs) who treat Native American pregnant woman and women of childbearing age with opioid use disorder (OUD).
There are no current comprehensive guidelines to manage the care of pregnant women with opioid dependence who live in rural or remote communities, ACOG acknowledges. That absence, in addition to a lack of resources, lack of training in treating substance use disorder in pregnancy, and providers’ discomfort with opioid agonist therapy in pregnancy, has contributed to “wide variation in the quality of care these women receive.”
Disparities are particularly extreme for American Indian and Alaska Native women (AI/AN), ACOG notes. They have the highest risk of dying of prescription opioid overdose, and they face specific barriers to accessing treatment. For instance, there are few opioid treatment programs offering methadone treatment on tribal lands.
The new recommendations were developed in partnership with tribes and ACOG’s Committee on American Indian and Alaska Native Women’s Health, based on critical feedback from listening sessions and tribal consultations in the past year. The specific guidelines are tailored for Native women.
The committee recognizes, it says, the “necessary wide-ranging scope of treatment for OUD, especially among AI/AN childbearing women.” Key recommendations include strategies to avoid or minimize the use of opioids for pain management and encourage alternative pain therapies, such as physical therapy, acupuncture, and mindfulness-based therapy. In pregnancy, ACOG recommends that obstetric providers perform universal screening and brief intervention using a validated tool as early in prenatal care as possible.
Treatment may require management of co-occurring polysubstance use disorders; concomitant alcohol and methamphetamine use predominate in many tribal areas. HCPs also may need to offer personalized care that “acknowledges the contributions of intergenerational and personal trauma,” the guidelines say. Trauma-informed interdisciplinary approaches to posttraumatic stress disorder that engage tribal resources, social structures, and assets are “crucial to impactful care of opioid use disorder.”
The postpartum period is associated with a high rate of relapse, ACOG says. Histories of trauma, for instance, can exacerbate mood disorders. Moreover, substance use and overdose are increasingly being recognized as key contributors to pregnancy-associated death in the US; a disproportionate share of deaths are postpartum. Infants of untreated, depressed mothers demonstrate poor outcomes, including impaired motor adaptation and self-regulation, developmental delay, and higher arousal scores. The guidelines advise treating mothers and infants as dyads to improve the course of neonatal opioid withdrawal syndrome (NOWS). The proportion of infants with NOWS who need pharmacologic treatment has risen dramatically, the committee notes.
“[I]t is clear from our site visits and clinical experience,” the committee members note, “that adaptation of systems for integration and reach in rural settings is necessary, with potentially different needs and assets in Native and rural populations.” Native culture and traditions, they add, offer opportunities for community engagement and support that can be integrated into medical care for the women and their infants.
To provide better “culturally responsive” care, the IHS and American College of Obstetricians and Gynecologists (ACOG) have announced new clinical recommendations for health care providers (HCPs) who treat Native American pregnant woman and women of childbearing age with opioid use disorder (OUD).
There are no current comprehensive guidelines to manage the care of pregnant women with opioid dependence who live in rural or remote communities, ACOG acknowledges. That absence, in addition to a lack of resources, lack of training in treating substance use disorder in pregnancy, and providers’ discomfort with opioid agonist therapy in pregnancy, has contributed to “wide variation in the quality of care these women receive.”
Disparities are particularly extreme for American Indian and Alaska Native women (AI/AN), ACOG notes. They have the highest risk of dying of prescription opioid overdose, and they face specific barriers to accessing treatment. For instance, there are few opioid treatment programs offering methadone treatment on tribal lands.
The new recommendations were developed in partnership with tribes and ACOG’s Committee on American Indian and Alaska Native Women’s Health, based on critical feedback from listening sessions and tribal consultations in the past year. The specific guidelines are tailored for Native women.
The committee recognizes, it says, the “necessary wide-ranging scope of treatment for OUD, especially among AI/AN childbearing women.” Key recommendations include strategies to avoid or minimize the use of opioids for pain management and encourage alternative pain therapies, such as physical therapy, acupuncture, and mindfulness-based therapy. In pregnancy, ACOG recommends that obstetric providers perform universal screening and brief intervention using a validated tool as early in prenatal care as possible.
Treatment may require management of co-occurring polysubstance use disorders; concomitant alcohol and methamphetamine use predominate in many tribal areas. HCPs also may need to offer personalized care that “acknowledges the contributions of intergenerational and personal trauma,” the guidelines say. Trauma-informed interdisciplinary approaches to posttraumatic stress disorder that engage tribal resources, social structures, and assets are “crucial to impactful care of opioid use disorder.”
The postpartum period is associated with a high rate of relapse, ACOG says. Histories of trauma, for instance, can exacerbate mood disorders. Moreover, substance use and overdose are increasingly being recognized as key contributors to pregnancy-associated death in the US; a disproportionate share of deaths are postpartum. Infants of untreated, depressed mothers demonstrate poor outcomes, including impaired motor adaptation and self-regulation, developmental delay, and higher arousal scores. The guidelines advise treating mothers and infants as dyads to improve the course of neonatal opioid withdrawal syndrome (NOWS). The proportion of infants with NOWS who need pharmacologic treatment has risen dramatically, the committee notes.
“[I]t is clear from our site visits and clinical experience,” the committee members note, “that adaptation of systems for integration and reach in rural settings is necessary, with potentially different needs and assets in Native and rural populations.” Native culture and traditions, they add, offer opportunities for community engagement and support that can be integrated into medical care for the women and their infants.
Ultrasound or biopsy for evaluation of endometrium? It depends
NASHVILLE, TENN. – Biopsy isn’t usually the first step in evaluating the endometrium of a reproductive-age woman who presents with abnormal uterine bleeding, but that’s not always the case, according to James M. Shwayder, MD.

“If we have young women come in, generally speaking, we don’t think much about doing biopsies, but there are those patients who really require a biopsy very early on: If they are obese and if they have long histories of oligomenorrhea ... they are at significantly greater risk for either endometrial hyperplasia or cancer, so in those patients I recommend biopsy very early on,” Dr. Shwayder said in this video interview about his presentation entitled “Modern Evaluation of the Endometrium: When to Use Ultrasound, When to Biopsy,” as presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
Conversely, in some cases when biopsy is typically considered the first-line step in evaluation, ultrasound may actually be better, he argued.
“[ACOG] recommends that women over 45 ... should have a biopsy done as their first-line evaluation. I kind of take issue with that a little bit,” said Dr. Shwayder, a professor at the University of Mississippi Medical Center, Jackson, and president and chief executive officer of Shwayder Consulting in Venice, Fla.
Data suggest that a “blind biopsy” could miss up to 18% of cases involving either a submucous myoma or a polyp and that one-third to one-fourth of patients have a structural defect such as a polyp or fibroid that can’t be diagnosed with a biopsy, he explained, noting that sonohysterography is best for preoperative evaluation in such case.
Ultrasound also has utility for evaluating other abnormalities, and it can be a very simple way to evaluate the patient and decide whether they need further evaluation or further treatment, he said.
Dr. Shwayder also discussed evidence for making a choice between biopsy and ultrasound for initial evaluation in postmenopausal women and for assessing women with asymptomatic thickened endometrium.
Dr. Shwayder is a consultant for GE Ultrasound.
NASHVILLE, TENN. – Biopsy isn’t usually the first step in evaluating the endometrium of a reproductive-age woman who presents with abnormal uterine bleeding, but that’s not always the case, according to James M. Shwayder, MD.

“If we have young women come in, generally speaking, we don’t think much about doing biopsies, but there are those patients who really require a biopsy very early on: If they are obese and if they have long histories of oligomenorrhea ... they are at significantly greater risk for either endometrial hyperplasia or cancer, so in those patients I recommend biopsy very early on,” Dr. Shwayder said in this video interview about his presentation entitled “Modern Evaluation of the Endometrium: When to Use Ultrasound, When to Biopsy,” as presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
Conversely, in some cases when biopsy is typically considered the first-line step in evaluation, ultrasound may actually be better, he argued.
“[ACOG] recommends that women over 45 ... should have a biopsy done as their first-line evaluation. I kind of take issue with that a little bit,” said Dr. Shwayder, a professor at the University of Mississippi Medical Center, Jackson, and president and chief executive officer of Shwayder Consulting in Venice, Fla.
Data suggest that a “blind biopsy” could miss up to 18% of cases involving either a submucous myoma or a polyp and that one-third to one-fourth of patients have a structural defect such as a polyp or fibroid that can’t be diagnosed with a biopsy, he explained, noting that sonohysterography is best for preoperative evaluation in such case.
Ultrasound also has utility for evaluating other abnormalities, and it can be a very simple way to evaluate the patient and decide whether they need further evaluation or further treatment, he said.
Dr. Shwayder also discussed evidence for making a choice between biopsy and ultrasound for initial evaluation in postmenopausal women and for assessing women with asymptomatic thickened endometrium.
Dr. Shwayder is a consultant for GE Ultrasound.
NASHVILLE, TENN. – Biopsy isn’t usually the first step in evaluating the endometrium of a reproductive-age woman who presents with abnormal uterine bleeding, but that’s not always the case, according to James M. Shwayder, MD.

“If we have young women come in, generally speaking, we don’t think much about doing biopsies, but there are those patients who really require a biopsy very early on: If they are obese and if they have long histories of oligomenorrhea ... they are at significantly greater risk for either endometrial hyperplasia or cancer, so in those patients I recommend biopsy very early on,” Dr. Shwayder said in this video interview about his presentation entitled “Modern Evaluation of the Endometrium: When to Use Ultrasound, When to Biopsy,” as presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
Conversely, in some cases when biopsy is typically considered the first-line step in evaluation, ultrasound may actually be better, he argued.
“[ACOG] recommends that women over 45 ... should have a biopsy done as their first-line evaluation. I kind of take issue with that a little bit,” said Dr. Shwayder, a professor at the University of Mississippi Medical Center, Jackson, and president and chief executive officer of Shwayder Consulting in Venice, Fla.
Data suggest that a “blind biopsy” could miss up to 18% of cases involving either a submucous myoma or a polyp and that one-third to one-fourth of patients have a structural defect such as a polyp or fibroid that can’t be diagnosed with a biopsy, he explained, noting that sonohysterography is best for preoperative evaluation in such case.
Ultrasound also has utility for evaluating other abnormalities, and it can be a very simple way to evaluate the patient and decide whether they need further evaluation or further treatment, he said.
Dr. Shwayder also discussed evidence for making a choice between biopsy and ultrasound for initial evaluation in postmenopausal women and for assessing women with asymptomatic thickened endometrium.
Dr. Shwayder is a consultant for GE Ultrasound.
EXPERT ANALYSIS FROM ACOG 2019
Immediate postpartum LARC: ‘Agony and ecstasy’
NASHVILLE, TENN. – according to Eve Espey, MD.
“I think [the rate] is going to settle out at around 15%-20%, but good cost-effectiveness studies show that, even if it were that high, it is still highly cost effective,” she said during an update on contraceptives at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
Immediate postpartum long-acting reversible contraception (LARC), including an IUD or implant, may reduce rapid-repeat pregnancy, she added, noting, however, that while Medicaid is covering it in many states, “it turns out that payment models are very cumbersome; they actually don’t work very well.”
At the University of New Mexico (UNM) in Albuquerque, where Dr .Espey is a professor and chair of the department of obstetrics and gynecology and director of the family planning fellowship, immediate postpartum LARC is offered to women with Medicaid coverage, and payment is received in about 97% of cases.
It took about 4 years of persistent effort to make that happen, she said, adding that the UNM Hospital still is the only one in the state offering the service, although efforts are underway to help other hospitals “troubleshoot the issues.”
Another challenge is the lack of private insurance coverage for immediate postpartum LARC, she said.
“I was super enthusiastic about this a few years ago, and I remain super enthusiastic about it, but I think it’s going to take another 5 years or so [for better coverage], and honestly I think what we really need is an inpatient LARC CPT code to make this happen.”
In this video interview, Dr. Espey discusses the “agony and ecstasy” of immediate postpartum LARC, summarizing the main points regarding its benefits and challenges as presented during an “EdTalk” she gave at the meeting.
Dr. Espey reported having no relevant financial disclosures.
NASHVILLE, TENN. – according to Eve Espey, MD.
“I think [the rate] is going to settle out at around 15%-20%, but good cost-effectiveness studies show that, even if it were that high, it is still highly cost effective,” she said during an update on contraceptives at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
Immediate postpartum long-acting reversible contraception (LARC), including an IUD or implant, may reduce rapid-repeat pregnancy, she added, noting, however, that while Medicaid is covering it in many states, “it turns out that payment models are very cumbersome; they actually don’t work very well.”
At the University of New Mexico (UNM) in Albuquerque, where Dr .Espey is a professor and chair of the department of obstetrics and gynecology and director of the family planning fellowship, immediate postpartum LARC is offered to women with Medicaid coverage, and payment is received in about 97% of cases.
It took about 4 years of persistent effort to make that happen, she said, adding that the UNM Hospital still is the only one in the state offering the service, although efforts are underway to help other hospitals “troubleshoot the issues.”
Another challenge is the lack of private insurance coverage for immediate postpartum LARC, she said.
“I was super enthusiastic about this a few years ago, and I remain super enthusiastic about it, but I think it’s going to take another 5 years or so [for better coverage], and honestly I think what we really need is an inpatient LARC CPT code to make this happen.”
In this video interview, Dr. Espey discusses the “agony and ecstasy” of immediate postpartum LARC, summarizing the main points regarding its benefits and challenges as presented during an “EdTalk” she gave at the meeting.
Dr. Espey reported having no relevant financial disclosures.
NASHVILLE, TENN. – according to Eve Espey, MD.
“I think [the rate] is going to settle out at around 15%-20%, but good cost-effectiveness studies show that, even if it were that high, it is still highly cost effective,” she said during an update on contraceptives at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
Immediate postpartum long-acting reversible contraception (LARC), including an IUD or implant, may reduce rapid-repeat pregnancy, she added, noting, however, that while Medicaid is covering it in many states, “it turns out that payment models are very cumbersome; they actually don’t work very well.”
At the University of New Mexico (UNM) in Albuquerque, where Dr .Espey is a professor and chair of the department of obstetrics and gynecology and director of the family planning fellowship, immediate postpartum LARC is offered to women with Medicaid coverage, and payment is received in about 97% of cases.
It took about 4 years of persistent effort to make that happen, she said, adding that the UNM Hospital still is the only one in the state offering the service, although efforts are underway to help other hospitals “troubleshoot the issues.”
Another challenge is the lack of private insurance coverage for immediate postpartum LARC, she said.
“I was super enthusiastic about this a few years ago, and I remain super enthusiastic about it, but I think it’s going to take another 5 years or so [for better coverage], and honestly I think what we really need is an inpatient LARC CPT code to make this happen.”
In this video interview, Dr. Espey discusses the “agony and ecstasy” of immediate postpartum LARC, summarizing the main points regarding its benefits and challenges as presented during an “EdTalk” she gave at the meeting.
Dr. Espey reported having no relevant financial disclosures.
EXPERT ANALYSIS FROM ACOG 2019
Add magnesium to treatment of AF with rapid ventricular response
Background: Most large studies of magnesium sulfate for assistance with rate control in AF occurred in the postoperative setting. This study compared rate control in the ED using magnesium sulfate at high (9 g) and low (4.5 g) doses vs. placebo in combination with usual treatment with atrioventricular nodal-blocking agents.
Study design: Double-blind, prospective, randomized, controlled trial.
Setting: Three tertiary Tunisian EDs.
Synopsis: This trial in Tunisian EDs enrolled 450 patients who presented with AF with rapid ventricular response and were divided into three groups: placebo, low-dose magnesium, and high-dose magnesium. Each patient’s trial medication was given as a 100-cc infusion. Patients were then treated with AV nodal-blocking agents at the discretion of the ED physician. The primary outcome was 20% reduction in rate or heart rate of less than 90 beats per minute. Notable exclusion criteria included hypotension, altered consciousness, decompensated heart failure, MI, and renal failure.
Rate control was achieved at 4 hours in 64% of patients with low-dose magnesium, 59% with high-dose magnesium, and 43% with placebo. At 24 hours, reduction in rate was controlled for 97% of patients on the low dose, 94% on the high dose, and 83% on placebo. Adverse events were mostly flushing, which occurred more frequently with the high dose than the low dose. Major limitations of the study included a lack of statistical assessment regarding baseline similarity between the two groups and that generalizability was limited by a preference for digoxin as the AV nodal agent.
Bottom line: This trial demonstrated that 4.5 g of magnesium sulfate was a useful addition to AV nodal blockers in achieving faster rate control for atrial fibrillation with rapid ventricular response in selected ED patients.
Citation: Bouida W et al. Low-dose magnesium sulfate versus high dose in the early management of rapid atrial fibrillation: Randomized controlled double blind study. Acad Emerg Med. 2018 Jul 19. doi: 10.1111/acem.13522.
Dr. Scott is an assistant professor in the division of hospital medicine, University of New Mexico.
Background: Most large studies of magnesium sulfate for assistance with rate control in AF occurred in the postoperative setting. This study compared rate control in the ED using magnesium sulfate at high (9 g) and low (4.5 g) doses vs. placebo in combination with usual treatment with atrioventricular nodal-blocking agents.
Study design: Double-blind, prospective, randomized, controlled trial.
Setting: Three tertiary Tunisian EDs.
Synopsis: This trial in Tunisian EDs enrolled 450 patients who presented with AF with rapid ventricular response and were divided into three groups: placebo, low-dose magnesium, and high-dose magnesium. Each patient’s trial medication was given as a 100-cc infusion. Patients were then treated with AV nodal-blocking agents at the discretion of the ED physician. The primary outcome was 20% reduction in rate or heart rate of less than 90 beats per minute. Notable exclusion criteria included hypotension, altered consciousness, decompensated heart failure, MI, and renal failure.
Rate control was achieved at 4 hours in 64% of patients with low-dose magnesium, 59% with high-dose magnesium, and 43% with placebo. At 24 hours, reduction in rate was controlled for 97% of patients on the low dose, 94% on the high dose, and 83% on placebo. Adverse events were mostly flushing, which occurred more frequently with the high dose than the low dose. Major limitations of the study included a lack of statistical assessment regarding baseline similarity between the two groups and that generalizability was limited by a preference for digoxin as the AV nodal agent.
Bottom line: This trial demonstrated that 4.5 g of magnesium sulfate was a useful addition to AV nodal blockers in achieving faster rate control for atrial fibrillation with rapid ventricular response in selected ED patients.
Citation: Bouida W et al. Low-dose magnesium sulfate versus high dose in the early management of rapid atrial fibrillation: Randomized controlled double blind study. Acad Emerg Med. 2018 Jul 19. doi: 10.1111/acem.13522.
Dr. Scott is an assistant professor in the division of hospital medicine, University of New Mexico.
Background: Most large studies of magnesium sulfate for assistance with rate control in AF occurred in the postoperative setting. This study compared rate control in the ED using magnesium sulfate at high (9 g) and low (4.5 g) doses vs. placebo in combination with usual treatment with atrioventricular nodal-blocking agents.
Study design: Double-blind, prospective, randomized, controlled trial.
Setting: Three tertiary Tunisian EDs.
Synopsis: This trial in Tunisian EDs enrolled 450 patients who presented with AF with rapid ventricular response and were divided into three groups: placebo, low-dose magnesium, and high-dose magnesium. Each patient’s trial medication was given as a 100-cc infusion. Patients were then treated with AV nodal-blocking agents at the discretion of the ED physician. The primary outcome was 20% reduction in rate or heart rate of less than 90 beats per minute. Notable exclusion criteria included hypotension, altered consciousness, decompensated heart failure, MI, and renal failure.
Rate control was achieved at 4 hours in 64% of patients with low-dose magnesium, 59% with high-dose magnesium, and 43% with placebo. At 24 hours, reduction in rate was controlled for 97% of patients on the low dose, 94% on the high dose, and 83% on placebo. Adverse events were mostly flushing, which occurred more frequently with the high dose than the low dose. Major limitations of the study included a lack of statistical assessment regarding baseline similarity between the two groups and that generalizability was limited by a preference for digoxin as the AV nodal agent.
Bottom line: This trial demonstrated that 4.5 g of magnesium sulfate was a useful addition to AV nodal blockers in achieving faster rate control for atrial fibrillation with rapid ventricular response in selected ED patients.
Citation: Bouida W et al. Low-dose magnesium sulfate versus high dose in the early management of rapid atrial fibrillation: Randomized controlled double blind study. Acad Emerg Med. 2018 Jul 19. doi: 10.1111/acem.13522.
Dr. Scott is an assistant professor in the division of hospital medicine, University of New Mexico.