User login
Walmart charts new course by steering workers to high-quality imaging centers
Walmart, the nation’s largest private employer, is worried that too many of its workers are having health conditions misdiagnosed, leading to unnecessary surgery and wasted health spending.
The issue crystallized for Walmart officials when they discovered about half of the company’s workers who went to the Mayo Clinic and other specialized hospitals for back surgery in the past few years turned out to not need those operations. They were either misdiagnosed by their doctor or needed only nonsurgical treatment.
A key issue: Their diagnostic imaging, such as CT scans and MRIs, had high error rates, said Lisa Woods, senior director of benefits design for Walmart.
So the company, whose health plans cover 1.1 million U.S. employees and dependents, has recommended that workers use one of 800 imaging centers identified as providing high-quality care. That list was developed for Walmart by Covera Health, a New York City–based health analytics company that uses data to help spot facilities likely to provide accurate imaging for a wide variety of conditions, from cancer to torn knee ligaments.
Although Walmart and other large employers in recent years have been steering workers to medical centers with proven track records for specific procedures such as transplants, the retail giant is believed to be the first to prod workers to use specific imaging providers based on diagnostic accuracy – not price, said employer health experts.
“A quality MRI or CT scan can improve the accuracy of diagnoses early in the care journey, helping create the correct treatment plan with the best opportunity for recovery,” said Ms. Woods. “The goal is to give associates the best chance to get better, and that starts with the right diagnosis.”
Walmart employees are not required to use those 800 centers, but if they don’t use one that is available near them, they will have to pay additional cost sharing. Company officials advise workers that they could have more accurate results if they opt for the specified centers.
Studies show a 3%-5% error rate each workday in a typical radiology practice, but some academic research has found mistakes on advanced images such as CT scans and MRIs can reach up to 30% of diagnoses. Although not every mistake affects patient care, with millions of CT scans and MRIs done each year in the United States, such mistakes can have a significant impact.
“There’s no question that there are a lot of errors that occur,” said Vijay Rao, MD, chair of radiology at the Jefferson Medical College, Philadelphia.
Errors at imaging centers can happen for many reasons, including the radiologist not devoting enough time to reading each image, Dr. Rao said. The average radiologist typically has only seconds to read each image. “It’s just a lot of data that crosses your eye and there is human fatigue, interruptions, and errors are bound to happen,” she added.
Other pitfalls: the technician not positioning the patient correctly in the imaging machine or a radiologist not having sufficient expertise or experience, Dr. Rao said.
Employers and insurers typically do little to help patients identify which radiology practices provide the most accurate results. Instead, employers have been focused on the cost of imaging tests. Some employers or insurers require plan members to use freestanding outpatient centers, rather than those based in hospitals, which tend to be more expensive.
Ms. Woods said Walmart found that deficiencies and variation in imaging services affected employees nationwide. “Unfortunately, it is all over the country. It’s everywhere,” she said.
Walmart’s new imaging strategy is aligned with its efforts over the past decade to direct employees to select hospitals for high-cost health procedures. Since 2013, Walmart has been sending workers and their dependents to select hospitals across the country where it believes they can get better results for spine surgery, heart surgery, joint replacement, weight loss surgery, transplants, and certain cancers.
As part of its “Centers of Excellence” program, the Bentonville, Ark.–based retail giant picks up the tab for the surgeries and all related travel expenses for patients on the company’s health insurance plan, including a caregiver.
Sampling imaging centers’ work
Covera has collected information on thousands of hospital-based and outpatient imaging facilities starting with its previous business work in the workers’ compensation field.
“Our primary interest is understanding which radiologist or radiology practices are achieving the highest level of diagnostic accuracy for their patients,” said Dan Elgort, Covera’s chief data science officer.
Covera has independent radiologists evaluate a sampling of patient care data on imaging centers to determine facilities’ error rates. It uses statistical modeling along with information on each center’s equipment, physicians, and use of industry-accepted patient protocols to determine the facilities’ rates of accuracy.
Covera expects to have about 1,500 imaging centers in the program by year’s end, said CEO Ron Vianu.
There are about 4,000 outpatient imaging centers in the United States, not counting thousands of hospital-based facilities, he estimated.
As a condition for participating in the program, each of the imaging centers has agreed to routinely send a sampling of their patients’ images and reports to Covera.
Mr. Vianu said studies have shown that radiologists frequently offer different diagnoses based on the same image taken during an MRI or CT scan. Among explanations are that some radiologists are better at analyzing certain types of images – like those of the brain or bones – and sometimes radiologists read images from exams they have less experience with, he said.
Mr. Vianu noted that most consumers give little thought to where to get an MRI or CT scan, and usually go where their doctors send them, the closest facility or, increasingly, the one that offers the lowest price. “Most people think of diagnostic imaging as a commodity, and that’s a mistake,” he said.
Dr. Rao applauded the effort by Walmart and Covera to identify imaging facilities likely to provide the most accurate reports. “I am sure centers that are worried about their quality will not be happy, but most quality operations would welcome something like this,” she said.
Few guides for consumers
Consumers have little way to distinguish the quality of care from one imaging center to the next. The American College of Radiology has an accreditation program but does not evaluate diagnostic quality.
“We would love to have more robust ... measurements” than what is currently available, said Geraldine McGinty, MD, chair of the college’s board of chancellors.
Facilities typically conduct peer reviews of their radiologists’ patient reports, but there is no public reporting of such results, she said.
Covera officials said they have worked with Walmart for nearly 2 years to demonstrate they could improve the quality of diagnostic care its employees receive. Part of the process has included reviewing a sample of Walmart employees’ health records to see where changes in imaging services could have caught potential problems.
Covera said the centers in its network were chosen based on quality and price was not a factor.
In an effort to curtail unnecessary tests, Walmart, like many large employers and insurers, requires its insured members to get authorization before getting CT scans and MRIs.
“Walmart is on the leading edge of focusing on quality of diagnostic imaging,” said Suzanne Delbanco, executive director of the Catalyst for Payment Reform, an employer-led health care think tank and advocacy group.
But Mark Stolper, executive vice president of Los Angeles–based RadNet, which owns 335 imaging centers nationally, questions how Covera has enough data to compare facilities. “This would be the first time,” he said, “I have seen or heard of a company trying to narrow a network of imaging centers that is based on quality instead of price.”
Ms. Woods said that, even though the new imaging strategy is not based on financial concerns, it could pay dividends down the road.
“It’s been demonstrated time and time again that high quality ends up being more economical in the long run because inappropriate care is avoided, and patients do better,” she said.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Walmart, the nation’s largest private employer, is worried that too many of its workers are having health conditions misdiagnosed, leading to unnecessary surgery and wasted health spending.
The issue crystallized for Walmart officials when they discovered about half of the company’s workers who went to the Mayo Clinic and other specialized hospitals for back surgery in the past few years turned out to not need those operations. They were either misdiagnosed by their doctor or needed only nonsurgical treatment.
A key issue: Their diagnostic imaging, such as CT scans and MRIs, had high error rates, said Lisa Woods, senior director of benefits design for Walmart.
So the company, whose health plans cover 1.1 million U.S. employees and dependents, has recommended that workers use one of 800 imaging centers identified as providing high-quality care. That list was developed for Walmart by Covera Health, a New York City–based health analytics company that uses data to help spot facilities likely to provide accurate imaging for a wide variety of conditions, from cancer to torn knee ligaments.
Although Walmart and other large employers in recent years have been steering workers to medical centers with proven track records for specific procedures such as transplants, the retail giant is believed to be the first to prod workers to use specific imaging providers based on diagnostic accuracy – not price, said employer health experts.
“A quality MRI or CT scan can improve the accuracy of diagnoses early in the care journey, helping create the correct treatment plan with the best opportunity for recovery,” said Ms. Woods. “The goal is to give associates the best chance to get better, and that starts with the right diagnosis.”
Walmart employees are not required to use those 800 centers, but if they don’t use one that is available near them, they will have to pay additional cost sharing. Company officials advise workers that they could have more accurate results if they opt for the specified centers.
Studies show a 3%-5% error rate each workday in a typical radiology practice, but some academic research has found mistakes on advanced images such as CT scans and MRIs can reach up to 30% of diagnoses. Although not every mistake affects patient care, with millions of CT scans and MRIs done each year in the United States, such mistakes can have a significant impact.
“There’s no question that there are a lot of errors that occur,” said Vijay Rao, MD, chair of radiology at the Jefferson Medical College, Philadelphia.
Errors at imaging centers can happen for many reasons, including the radiologist not devoting enough time to reading each image, Dr. Rao said. The average radiologist typically has only seconds to read each image. “It’s just a lot of data that crosses your eye and there is human fatigue, interruptions, and errors are bound to happen,” she added.
Other pitfalls: the technician not positioning the patient correctly in the imaging machine or a radiologist not having sufficient expertise or experience, Dr. Rao said.
Employers and insurers typically do little to help patients identify which radiology practices provide the most accurate results. Instead, employers have been focused on the cost of imaging tests. Some employers or insurers require plan members to use freestanding outpatient centers, rather than those based in hospitals, which tend to be more expensive.
Ms. Woods said Walmart found that deficiencies and variation in imaging services affected employees nationwide. “Unfortunately, it is all over the country. It’s everywhere,” she said.
Walmart’s new imaging strategy is aligned with its efforts over the past decade to direct employees to select hospitals for high-cost health procedures. Since 2013, Walmart has been sending workers and their dependents to select hospitals across the country where it believes they can get better results for spine surgery, heart surgery, joint replacement, weight loss surgery, transplants, and certain cancers.
As part of its “Centers of Excellence” program, the Bentonville, Ark.–based retail giant picks up the tab for the surgeries and all related travel expenses for patients on the company’s health insurance plan, including a caregiver.
Sampling imaging centers’ work
Covera has collected information on thousands of hospital-based and outpatient imaging facilities starting with its previous business work in the workers’ compensation field.
“Our primary interest is understanding which radiologist or radiology practices are achieving the highest level of diagnostic accuracy for their patients,” said Dan Elgort, Covera’s chief data science officer.
Covera has independent radiologists evaluate a sampling of patient care data on imaging centers to determine facilities’ error rates. It uses statistical modeling along with information on each center’s equipment, physicians, and use of industry-accepted patient protocols to determine the facilities’ rates of accuracy.
Covera expects to have about 1,500 imaging centers in the program by year’s end, said CEO Ron Vianu.
There are about 4,000 outpatient imaging centers in the United States, not counting thousands of hospital-based facilities, he estimated.
As a condition for participating in the program, each of the imaging centers has agreed to routinely send a sampling of their patients’ images and reports to Covera.
Mr. Vianu said studies have shown that radiologists frequently offer different diagnoses based on the same image taken during an MRI or CT scan. Among explanations are that some radiologists are better at analyzing certain types of images – like those of the brain or bones – and sometimes radiologists read images from exams they have less experience with, he said.
Mr. Vianu noted that most consumers give little thought to where to get an MRI or CT scan, and usually go where their doctors send them, the closest facility or, increasingly, the one that offers the lowest price. “Most people think of diagnostic imaging as a commodity, and that’s a mistake,” he said.
Dr. Rao applauded the effort by Walmart and Covera to identify imaging facilities likely to provide the most accurate reports. “I am sure centers that are worried about their quality will not be happy, but most quality operations would welcome something like this,” she said.
Few guides for consumers
Consumers have little way to distinguish the quality of care from one imaging center to the next. The American College of Radiology has an accreditation program but does not evaluate diagnostic quality.
“We would love to have more robust ... measurements” than what is currently available, said Geraldine McGinty, MD, chair of the college’s board of chancellors.
Facilities typically conduct peer reviews of their radiologists’ patient reports, but there is no public reporting of such results, she said.
Covera officials said they have worked with Walmart for nearly 2 years to demonstrate they could improve the quality of diagnostic care its employees receive. Part of the process has included reviewing a sample of Walmart employees’ health records to see where changes in imaging services could have caught potential problems.
Covera said the centers in its network were chosen based on quality and price was not a factor.
In an effort to curtail unnecessary tests, Walmart, like many large employers and insurers, requires its insured members to get authorization before getting CT scans and MRIs.
“Walmart is on the leading edge of focusing on quality of diagnostic imaging,” said Suzanne Delbanco, executive director of the Catalyst for Payment Reform, an employer-led health care think tank and advocacy group.
But Mark Stolper, executive vice president of Los Angeles–based RadNet, which owns 335 imaging centers nationally, questions how Covera has enough data to compare facilities. “This would be the first time,” he said, “I have seen or heard of a company trying to narrow a network of imaging centers that is based on quality instead of price.”
Ms. Woods said that, even though the new imaging strategy is not based on financial concerns, it could pay dividends down the road.
“It’s been demonstrated time and time again that high quality ends up being more economical in the long run because inappropriate care is avoided, and patients do better,” she said.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Walmart, the nation’s largest private employer, is worried that too many of its workers are having health conditions misdiagnosed, leading to unnecessary surgery and wasted health spending.
The issue crystallized for Walmart officials when they discovered about half of the company’s workers who went to the Mayo Clinic and other specialized hospitals for back surgery in the past few years turned out to not need those operations. They were either misdiagnosed by their doctor or needed only nonsurgical treatment.
A key issue: Their diagnostic imaging, such as CT scans and MRIs, had high error rates, said Lisa Woods, senior director of benefits design for Walmart.
So the company, whose health plans cover 1.1 million U.S. employees and dependents, has recommended that workers use one of 800 imaging centers identified as providing high-quality care. That list was developed for Walmart by Covera Health, a New York City–based health analytics company that uses data to help spot facilities likely to provide accurate imaging for a wide variety of conditions, from cancer to torn knee ligaments.
Although Walmart and other large employers in recent years have been steering workers to medical centers with proven track records for specific procedures such as transplants, the retail giant is believed to be the first to prod workers to use specific imaging providers based on diagnostic accuracy – not price, said employer health experts.
“A quality MRI or CT scan can improve the accuracy of diagnoses early in the care journey, helping create the correct treatment plan with the best opportunity for recovery,” said Ms. Woods. “The goal is to give associates the best chance to get better, and that starts with the right diagnosis.”
Walmart employees are not required to use those 800 centers, but if they don’t use one that is available near them, they will have to pay additional cost sharing. Company officials advise workers that they could have more accurate results if they opt for the specified centers.
Studies show a 3%-5% error rate each workday in a typical radiology practice, but some academic research has found mistakes on advanced images such as CT scans and MRIs can reach up to 30% of diagnoses. Although not every mistake affects patient care, with millions of CT scans and MRIs done each year in the United States, such mistakes can have a significant impact.
“There’s no question that there are a lot of errors that occur,” said Vijay Rao, MD, chair of radiology at the Jefferson Medical College, Philadelphia.
Errors at imaging centers can happen for many reasons, including the radiologist not devoting enough time to reading each image, Dr. Rao said. The average radiologist typically has only seconds to read each image. “It’s just a lot of data that crosses your eye and there is human fatigue, interruptions, and errors are bound to happen,” she added.
Other pitfalls: the technician not positioning the patient correctly in the imaging machine or a radiologist not having sufficient expertise or experience, Dr. Rao said.
Employers and insurers typically do little to help patients identify which radiology practices provide the most accurate results. Instead, employers have been focused on the cost of imaging tests. Some employers or insurers require plan members to use freestanding outpatient centers, rather than those based in hospitals, which tend to be more expensive.
Ms. Woods said Walmart found that deficiencies and variation in imaging services affected employees nationwide. “Unfortunately, it is all over the country. It’s everywhere,” she said.
Walmart’s new imaging strategy is aligned with its efforts over the past decade to direct employees to select hospitals for high-cost health procedures. Since 2013, Walmart has been sending workers and their dependents to select hospitals across the country where it believes they can get better results for spine surgery, heart surgery, joint replacement, weight loss surgery, transplants, and certain cancers.
As part of its “Centers of Excellence” program, the Bentonville, Ark.–based retail giant picks up the tab for the surgeries and all related travel expenses for patients on the company’s health insurance plan, including a caregiver.
Sampling imaging centers’ work
Covera has collected information on thousands of hospital-based and outpatient imaging facilities starting with its previous business work in the workers’ compensation field.
“Our primary interest is understanding which radiologist or radiology practices are achieving the highest level of diagnostic accuracy for their patients,” said Dan Elgort, Covera’s chief data science officer.
Covera has independent radiologists evaluate a sampling of patient care data on imaging centers to determine facilities’ error rates. It uses statistical modeling along with information on each center’s equipment, physicians, and use of industry-accepted patient protocols to determine the facilities’ rates of accuracy.
Covera expects to have about 1,500 imaging centers in the program by year’s end, said CEO Ron Vianu.
There are about 4,000 outpatient imaging centers in the United States, not counting thousands of hospital-based facilities, he estimated.
As a condition for participating in the program, each of the imaging centers has agreed to routinely send a sampling of their patients’ images and reports to Covera.
Mr. Vianu said studies have shown that radiologists frequently offer different diagnoses based on the same image taken during an MRI or CT scan. Among explanations are that some radiologists are better at analyzing certain types of images – like those of the brain or bones – and sometimes radiologists read images from exams they have less experience with, he said.
Mr. Vianu noted that most consumers give little thought to where to get an MRI or CT scan, and usually go where their doctors send them, the closest facility or, increasingly, the one that offers the lowest price. “Most people think of diagnostic imaging as a commodity, and that’s a mistake,” he said.
Dr. Rao applauded the effort by Walmart and Covera to identify imaging facilities likely to provide the most accurate reports. “I am sure centers that are worried about their quality will not be happy, but most quality operations would welcome something like this,” she said.
Few guides for consumers
Consumers have little way to distinguish the quality of care from one imaging center to the next. The American College of Radiology has an accreditation program but does not evaluate diagnostic quality.
“We would love to have more robust ... measurements” than what is currently available, said Geraldine McGinty, MD, chair of the college’s board of chancellors.
Facilities typically conduct peer reviews of their radiologists’ patient reports, but there is no public reporting of such results, she said.
Covera officials said they have worked with Walmart for nearly 2 years to demonstrate they could improve the quality of diagnostic care its employees receive. Part of the process has included reviewing a sample of Walmart employees’ health records to see where changes in imaging services could have caught potential problems.
Covera said the centers in its network were chosen based on quality and price was not a factor.
In an effort to curtail unnecessary tests, Walmart, like many large employers and insurers, requires its insured members to get authorization before getting CT scans and MRIs.
“Walmart is on the leading edge of focusing on quality of diagnostic imaging,” said Suzanne Delbanco, executive director of the Catalyst for Payment Reform, an employer-led health care think tank and advocacy group.
But Mark Stolper, executive vice president of Los Angeles–based RadNet, which owns 335 imaging centers nationally, questions how Covera has enough data to compare facilities. “This would be the first time,” he said, “I have seen or heard of a company trying to narrow a network of imaging centers that is based on quality instead of price.”
Ms. Woods said that, even though the new imaging strategy is not based on financial concerns, it could pay dividends down the road.
“It’s been demonstrated time and time again that high quality ends up being more economical in the long run because inappropriate care is avoided, and patients do better,” she said.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Laparoscopy linked to lower surgical infections for hernia surgery
BALTIMORE – A large retrospective study found that even though the laparoscopic group had higher body mass index and rates of other key comorbidities, according to results reported at the annual meeting of the Society of American Gastrointestinal and Endoscopic Surgeons.
“In patients with obesity, even though our laparoscopic umbilical hernia repair [UHR] group had an overall higher BMI; higher rates of diabetes, hypertension, and current smoking status; and longer operative times, they experienced decreased postoperative wound complications, compared to the open-repair group,” said Kristen Williams, MD, of TriHealth in Cincinnati.
The retrospective cohort study evaluated 12,026 adult patients with a BMI of more than 30 kg/m2 in the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) database who had UHR in 2016. Almost four times as many patients had open rather than laparoscopic surgery (9,695 vs. 2,331, respectively)
Dr. Williams noted that two previous studies reported lower wound infection rates after laparoscopic hernia repair in patients with obesity: an analysis of ventral, not just umbilical, hernia repair based on the NSQIP database from 2009-2012 (Am J Surg. 2015;210:1029-30) and a single-institution retrospective chart review from 2003-2009 of patients who had umbilical hernia repair (Am J Surg. 2013;205:231-6). “In our study we wanted to compare the rate of postoperative complications after laparoscopic vs. open umbilical hernia repairs in patients with obesity based on NSQIP review,” she said.
The rate of composite surgical-site infections in the open group was 1.9% vs. 1.1% in the laparoscopic group (P less than .01), Dr. Williams noted. “SSI was statistically significantly higher in the open-repair group, and there was a trend toward higher deep SSI in the open group [0.3% vs. 0.1%; P = .147],” she said. Laparoscopic patients had significantly higher rates of postoperative pneumonia (0.4% vs 0.1%; P = .012), but Dr. Williams noted this was only significant in the non–elective surgery group. Operative times were significantly longer in the laparoscopic repair group (70 vs. 44 minutes). “The literature shows that longer operative times are associated with higher rates of SSI,” Dr. Williams added. “However our laparoscopic group still had lower rates of composite SSI.”
“Logistic regression was utilized and found that morbidity, defined as superficial, deep, and organ-space SSIs, was significantly increased in the open-repair group,” Dr. Williams said.
A higher percentage of patients in the laparoscopic group were women than in the open group (29.4% vs. 24.4%; P less than .001). The laparoscopic group had statistically significant higher average BMI (37.5 vs. 36.1; P less than .001) and higher rates of smoking (18.6% vs. 16.5%; P = .018), diabetes (18.4% vs. 15.8%; P = .002), and hypertension (47.5% vs. 43.8%; P = .001) than the open group.
The study also analyzed outcomes by BMI class. “As BMI class increased, superficial SSI, deep SSI, return-to-OR rates, postoperative pneumonia rates, and composite SSI increased in the open-repair group, indicating that higher BMI is associated with higher rates of complications in the open-repair group,” Dr. Williams said. Likewise, as obesity class increased, so did operative times in both the open and laparoscopic groups, she added.
She also noted that this study reported a significant increase in the proportion of laparoscopic UHRs than did a retrospective cohort study of 2009 and 2010 NSQIP files of UHR (Surg Endosc. 2014;28:741-6), 19.4% in this study versus 10.5% in that one.
Dr. Williams had no financial relationships to disclose.
SOURCE: Williams K et al. SAGES 2019, Abstract S099.
BALTIMORE – A large retrospective study found that even though the laparoscopic group had higher body mass index and rates of other key comorbidities, according to results reported at the annual meeting of the Society of American Gastrointestinal and Endoscopic Surgeons.
“In patients with obesity, even though our laparoscopic umbilical hernia repair [UHR] group had an overall higher BMI; higher rates of diabetes, hypertension, and current smoking status; and longer operative times, they experienced decreased postoperative wound complications, compared to the open-repair group,” said Kristen Williams, MD, of TriHealth in Cincinnati.
The retrospective cohort study evaluated 12,026 adult patients with a BMI of more than 30 kg/m2 in the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) database who had UHR in 2016. Almost four times as many patients had open rather than laparoscopic surgery (9,695 vs. 2,331, respectively)
Dr. Williams noted that two previous studies reported lower wound infection rates after laparoscopic hernia repair in patients with obesity: an analysis of ventral, not just umbilical, hernia repair based on the NSQIP database from 2009-2012 (Am J Surg. 2015;210:1029-30) and a single-institution retrospective chart review from 2003-2009 of patients who had umbilical hernia repair (Am J Surg. 2013;205:231-6). “In our study we wanted to compare the rate of postoperative complications after laparoscopic vs. open umbilical hernia repairs in patients with obesity based on NSQIP review,” she said.
The rate of composite surgical-site infections in the open group was 1.9% vs. 1.1% in the laparoscopic group (P less than .01), Dr. Williams noted. “SSI was statistically significantly higher in the open-repair group, and there was a trend toward higher deep SSI in the open group [0.3% vs. 0.1%; P = .147],” she said. Laparoscopic patients had significantly higher rates of postoperative pneumonia (0.4% vs 0.1%; P = .012), but Dr. Williams noted this was only significant in the non–elective surgery group. Operative times were significantly longer in the laparoscopic repair group (70 vs. 44 minutes). “The literature shows that longer operative times are associated with higher rates of SSI,” Dr. Williams added. “However our laparoscopic group still had lower rates of composite SSI.”
“Logistic regression was utilized and found that morbidity, defined as superficial, deep, and organ-space SSIs, was significantly increased in the open-repair group,” Dr. Williams said.
A higher percentage of patients in the laparoscopic group were women than in the open group (29.4% vs. 24.4%; P less than .001). The laparoscopic group had statistically significant higher average BMI (37.5 vs. 36.1; P less than .001) and higher rates of smoking (18.6% vs. 16.5%; P = .018), diabetes (18.4% vs. 15.8%; P = .002), and hypertension (47.5% vs. 43.8%; P = .001) than the open group.
The study also analyzed outcomes by BMI class. “As BMI class increased, superficial SSI, deep SSI, return-to-OR rates, postoperative pneumonia rates, and composite SSI increased in the open-repair group, indicating that higher BMI is associated with higher rates of complications in the open-repair group,” Dr. Williams said. Likewise, as obesity class increased, so did operative times in both the open and laparoscopic groups, she added.
She also noted that this study reported a significant increase in the proportion of laparoscopic UHRs than did a retrospective cohort study of 2009 and 2010 NSQIP files of UHR (Surg Endosc. 2014;28:741-6), 19.4% in this study versus 10.5% in that one.
Dr. Williams had no financial relationships to disclose.
SOURCE: Williams K et al. SAGES 2019, Abstract S099.
BALTIMORE – A large retrospective study found that even though the laparoscopic group had higher body mass index and rates of other key comorbidities, according to results reported at the annual meeting of the Society of American Gastrointestinal and Endoscopic Surgeons.
“In patients with obesity, even though our laparoscopic umbilical hernia repair [UHR] group had an overall higher BMI; higher rates of diabetes, hypertension, and current smoking status; and longer operative times, they experienced decreased postoperative wound complications, compared to the open-repair group,” said Kristen Williams, MD, of TriHealth in Cincinnati.
The retrospective cohort study evaluated 12,026 adult patients with a BMI of more than 30 kg/m2 in the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) database who had UHR in 2016. Almost four times as many patients had open rather than laparoscopic surgery (9,695 vs. 2,331, respectively)
Dr. Williams noted that two previous studies reported lower wound infection rates after laparoscopic hernia repair in patients with obesity: an analysis of ventral, not just umbilical, hernia repair based on the NSQIP database from 2009-2012 (Am J Surg. 2015;210:1029-30) and a single-institution retrospective chart review from 2003-2009 of patients who had umbilical hernia repair (Am J Surg. 2013;205:231-6). “In our study we wanted to compare the rate of postoperative complications after laparoscopic vs. open umbilical hernia repairs in patients with obesity based on NSQIP review,” she said.
The rate of composite surgical-site infections in the open group was 1.9% vs. 1.1% in the laparoscopic group (P less than .01), Dr. Williams noted. “SSI was statistically significantly higher in the open-repair group, and there was a trend toward higher deep SSI in the open group [0.3% vs. 0.1%; P = .147],” she said. Laparoscopic patients had significantly higher rates of postoperative pneumonia (0.4% vs 0.1%; P = .012), but Dr. Williams noted this was only significant in the non–elective surgery group. Operative times were significantly longer in the laparoscopic repair group (70 vs. 44 minutes). “The literature shows that longer operative times are associated with higher rates of SSI,” Dr. Williams added. “However our laparoscopic group still had lower rates of composite SSI.”
“Logistic regression was utilized and found that morbidity, defined as superficial, deep, and organ-space SSIs, was significantly increased in the open-repair group,” Dr. Williams said.
A higher percentage of patients in the laparoscopic group were women than in the open group (29.4% vs. 24.4%; P less than .001). The laparoscopic group had statistically significant higher average BMI (37.5 vs. 36.1; P less than .001) and higher rates of smoking (18.6% vs. 16.5%; P = .018), diabetes (18.4% vs. 15.8%; P = .002), and hypertension (47.5% vs. 43.8%; P = .001) than the open group.
The study also analyzed outcomes by BMI class. “As BMI class increased, superficial SSI, deep SSI, return-to-OR rates, postoperative pneumonia rates, and composite SSI increased in the open-repair group, indicating that higher BMI is associated with higher rates of complications in the open-repair group,” Dr. Williams said. Likewise, as obesity class increased, so did operative times in both the open and laparoscopic groups, she added.
She also noted that this study reported a significant increase in the proportion of laparoscopic UHRs than did a retrospective cohort study of 2009 and 2010 NSQIP files of UHR (Surg Endosc. 2014;28:741-6), 19.4% in this study versus 10.5% in that one.
Dr. Williams had no financial relationships to disclose.
SOURCE: Williams K et al. SAGES 2019, Abstract S099.
REPORTING FROM SAGES 2019
Handling defamatory online reviews
In my last column, I gave you some options for handling those inevitable negative online reviews without violating patient confidentiality or pouring fuel on the fire. Your options in such cases are limited by HIPAA rules (among others) and by the patient’s right to free expression under the First Amendment.
– although you need to carefully consider the situation before going to war with a critic.
Critics have legislative protection in many states, called anti-SLAPP (Strategic Lawsuit Against Public Participation) laws, which allow judges to summarily dismiss lawsuits that they consider retaliatory or intended to intimidate and silence citizens speaking out on issues of public interest – such as health care. Federal courts recently nullified anti-SLAPP laws in Washington and Minnesota as unconstitutional; but as I write this, similar laws remain on the books in 28 other states, plus Washington, D.C., and Guam.
There is also a federal law – the Consumer Review Freedom Act of 2016 – which prohibits any attempt to prevent consumers from giving “honest” reviews about products or services. No law protects demonstrably false statements, of course.
The first thing to do before taking any action is to determine whether that defamatory review is, in fact, defamatory. Defamation is generally defined as the act of making false statements “with malice” – that is, in a deliberate attempt to damage someone’s reputation. The main issue in most defamation cases is whether the statements in question are merely strong opinions, which are protected by the First Amendment; or “assertions of verifiable fact”, which are not.
For example, “Dr. ____’s office does not clean its instruments properly” is a statement that can be proven true or false. Therefore, it is an assertion of fact, not an opinion, and if false, vulnerable to a defamation suit. The only unimpeachable defense in such a suit would be to prove that the assertion is true.
It is worth noting that attempting to disguise assertions of fact as opinions, simply by calling them opinions – for example, “In my opinion, Dr. ____’s office does not clean its instruments properly” – does not make them unverifiable or immunize them from litigation.
Once you have determined that the review fits the legal definition of defamation, the usual first step is to contact the website where the review is posted. Most rating sites are loath to intercede in arguments. (for example, Yelp’s official position: “We don’t typically take sides in factual disputes, and generally allow Yelpers to stand behind their reviews.”) They also have their own legal shield: The U.S. Communications Decency Act, which prohibits lawsuits against websites for publishing reviews, comments, and other third-party content, unless the site itself changes or somehow alters the meaning of the original post.
Even so, websites have their own reputations to protect; they don’t want to be used as venues for acts of defamation, nor be seen as perpetuating false or misleading information, and can sometimes be persuaded to take down really egregious hatchet jobs. It is certainly worth a try – but it may take a lawyer’s letter to get their attention.
If the site won’t remove it, you’ll have to try to persuade the patient to do so. Most attorneys recommend sending a “cease-and-desist” letter, explaining why the review is defamatory and demanding its removal. You should carefully consider the situation before sending such a letter; it may fuel the patient’s anger and trigger additional online attacks.
If a cease-and-desist letter is ineffective, your only further option is to file a lawsuit. Such cases are rare, and success even rarer: Of the 29 health care–related defamation cases that I was able to find in the public record, 19 were summarily dismissed; in 6 of those cases, the plaintiff was ordered to pay the defendant’s court costs. The other 10 were settled on undisclosed terms; only one, apparently, involved a cash payment to the plaintiff.
If you believe that the defamation is causing you real, monetary damage – enough to outweigh the costs of litigation – and you can prove that the allegations against you are false, it might be worth the considerable time, money, and emotional energy that litigation demands to pursue it.
As always, never venture into the litigation jungle without the support and guidance of an experienced attorney.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at dermnews@mdedge.com.
In my last column, I gave you some options for handling those inevitable negative online reviews without violating patient confidentiality or pouring fuel on the fire. Your options in such cases are limited by HIPAA rules (among others) and by the patient’s right to free expression under the First Amendment.
– although you need to carefully consider the situation before going to war with a critic.
Critics have legislative protection in many states, called anti-SLAPP (Strategic Lawsuit Against Public Participation) laws, which allow judges to summarily dismiss lawsuits that they consider retaliatory or intended to intimidate and silence citizens speaking out on issues of public interest – such as health care. Federal courts recently nullified anti-SLAPP laws in Washington and Minnesota as unconstitutional; but as I write this, similar laws remain on the books in 28 other states, plus Washington, D.C., and Guam.
There is also a federal law – the Consumer Review Freedom Act of 2016 – which prohibits any attempt to prevent consumers from giving “honest” reviews about products or services. No law protects demonstrably false statements, of course.
The first thing to do before taking any action is to determine whether that defamatory review is, in fact, defamatory. Defamation is generally defined as the act of making false statements “with malice” – that is, in a deliberate attempt to damage someone’s reputation. The main issue in most defamation cases is whether the statements in question are merely strong opinions, which are protected by the First Amendment; or “assertions of verifiable fact”, which are not.
For example, “Dr. ____’s office does not clean its instruments properly” is a statement that can be proven true or false. Therefore, it is an assertion of fact, not an opinion, and if false, vulnerable to a defamation suit. The only unimpeachable defense in such a suit would be to prove that the assertion is true.
It is worth noting that attempting to disguise assertions of fact as opinions, simply by calling them opinions – for example, “In my opinion, Dr. ____’s office does not clean its instruments properly” – does not make them unverifiable or immunize them from litigation.
Once you have determined that the review fits the legal definition of defamation, the usual first step is to contact the website where the review is posted. Most rating sites are loath to intercede in arguments. (for example, Yelp’s official position: “We don’t typically take sides in factual disputes, and generally allow Yelpers to stand behind their reviews.”) They also have their own legal shield: The U.S. Communications Decency Act, which prohibits lawsuits against websites for publishing reviews, comments, and other third-party content, unless the site itself changes or somehow alters the meaning of the original post.
Even so, websites have their own reputations to protect; they don’t want to be used as venues for acts of defamation, nor be seen as perpetuating false or misleading information, and can sometimes be persuaded to take down really egregious hatchet jobs. It is certainly worth a try – but it may take a lawyer’s letter to get their attention.
If the site won’t remove it, you’ll have to try to persuade the patient to do so. Most attorneys recommend sending a “cease-and-desist” letter, explaining why the review is defamatory and demanding its removal. You should carefully consider the situation before sending such a letter; it may fuel the patient’s anger and trigger additional online attacks.
If a cease-and-desist letter is ineffective, your only further option is to file a lawsuit. Such cases are rare, and success even rarer: Of the 29 health care–related defamation cases that I was able to find in the public record, 19 were summarily dismissed; in 6 of those cases, the plaintiff was ordered to pay the defendant’s court costs. The other 10 were settled on undisclosed terms; only one, apparently, involved a cash payment to the plaintiff.
If you believe that the defamation is causing you real, monetary damage – enough to outweigh the costs of litigation – and you can prove that the allegations against you are false, it might be worth the considerable time, money, and emotional energy that litigation demands to pursue it.
As always, never venture into the litigation jungle without the support and guidance of an experienced attorney.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at dermnews@mdedge.com.
In my last column, I gave you some options for handling those inevitable negative online reviews without violating patient confidentiality or pouring fuel on the fire. Your options in such cases are limited by HIPAA rules (among others) and by the patient’s right to free expression under the First Amendment.
– although you need to carefully consider the situation before going to war with a critic.
Critics have legislative protection in many states, called anti-SLAPP (Strategic Lawsuit Against Public Participation) laws, which allow judges to summarily dismiss lawsuits that they consider retaliatory or intended to intimidate and silence citizens speaking out on issues of public interest – such as health care. Federal courts recently nullified anti-SLAPP laws in Washington and Minnesota as unconstitutional; but as I write this, similar laws remain on the books in 28 other states, plus Washington, D.C., and Guam.
There is also a federal law – the Consumer Review Freedom Act of 2016 – which prohibits any attempt to prevent consumers from giving “honest” reviews about products or services. No law protects demonstrably false statements, of course.
The first thing to do before taking any action is to determine whether that defamatory review is, in fact, defamatory. Defamation is generally defined as the act of making false statements “with malice” – that is, in a deliberate attempt to damage someone’s reputation. The main issue in most defamation cases is whether the statements in question are merely strong opinions, which are protected by the First Amendment; or “assertions of verifiable fact”, which are not.
For example, “Dr. ____’s office does not clean its instruments properly” is a statement that can be proven true or false. Therefore, it is an assertion of fact, not an opinion, and if false, vulnerable to a defamation suit. The only unimpeachable defense in such a suit would be to prove that the assertion is true.
It is worth noting that attempting to disguise assertions of fact as opinions, simply by calling them opinions – for example, “In my opinion, Dr. ____’s office does not clean its instruments properly” – does not make them unverifiable or immunize them from litigation.
Once you have determined that the review fits the legal definition of defamation, the usual first step is to contact the website where the review is posted. Most rating sites are loath to intercede in arguments. (for example, Yelp’s official position: “We don’t typically take sides in factual disputes, and generally allow Yelpers to stand behind their reviews.”) They also have their own legal shield: The U.S. Communications Decency Act, which prohibits lawsuits against websites for publishing reviews, comments, and other third-party content, unless the site itself changes or somehow alters the meaning of the original post.
Even so, websites have their own reputations to protect; they don’t want to be used as venues for acts of defamation, nor be seen as perpetuating false or misleading information, and can sometimes be persuaded to take down really egregious hatchet jobs. It is certainly worth a try – but it may take a lawyer’s letter to get their attention.
If the site won’t remove it, you’ll have to try to persuade the patient to do so. Most attorneys recommend sending a “cease-and-desist” letter, explaining why the review is defamatory and demanding its removal. You should carefully consider the situation before sending such a letter; it may fuel the patient’s anger and trigger additional online attacks.
If a cease-and-desist letter is ineffective, your only further option is to file a lawsuit. Such cases are rare, and success even rarer: Of the 29 health care–related defamation cases that I was able to find in the public record, 19 were summarily dismissed; in 6 of those cases, the plaintiff was ordered to pay the defendant’s court costs. The other 10 were settled on undisclosed terms; only one, apparently, involved a cash payment to the plaintiff.
If you believe that the defamation is causing you real, monetary damage – enough to outweigh the costs of litigation – and you can prove that the allegations against you are false, it might be worth the considerable time, money, and emotional energy that litigation demands to pursue it.
As always, never venture into the litigation jungle without the support and guidance of an experienced attorney.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at dermnews@mdedge.com.
How medical providers can observe LGBT Pride Month
June is Pride Month in the United States. It is a time in which people take a stand against discrimination and violence against lesbian, gay, bisexual, and transgender (LGBT) people and promote dignity, equality, and visibility of this community. During this time, many cities will be holding events ranging from rallies to parades to not only celebrate sexual diversity and gender variance, but also to serve as a reminder of the work that needs to be done to foster equal treatment for LGBT people. As a medical provider, you have the unique role of advancing this cause – from educating your colleagues on the health needs of this population to advocating for policies that protect their health and well-being. If you’re interested in serving the LGBT community as a medical provider, here are some ways you can show this community your commitment to their health and well-being.
Be visible
There will be numerous LGBT Pride events occurring the month of June and even throughout the summer in the United States. They can occur in cities big and small, and they can even be in the city you work in. Visibility matters for LGBT youth. Eight percent of lesbian, gay, and bisexual people report that a health care provider refused to see them because of their sexual orientation and 29% of transgender people report that their health care providers refused to see them because of their gender identity or expression.1 Therefore, LGBT people will expect discrimination everywhere they go.2
Being present at a Pride event signals to the community that you are willing to serve LGBT people. Many Pride events will allow hospitals and clinics to have a table at the event, but keep in mind that many will prioritize organizations that specifically cater to the LGBT community or that are owned and operated by members of the community. Another way to show the community that you will treat LGBT people with dignity and respect is to list your practice in a database for LGBT-friendly providers. The Gay and Lesbian Medical Association keeps a database of LGBT-friendly medical providers, and many Pride events will advertise businesses and organizations that serve the LGBT community. You may want to consider having your clinic or hospital participate in the Human Rights Campaign (HRC) Health Equality Index (HEI). The HEI is a list of best practices for hospitals and clinics to use that affirm and support LGBT health (such as having gender-neutral bathrooms in facilities). Hospitals and clinics that endorse a high amount or all of these practices are listed as committed to the health and well-being of the LGBT community on the HRC website.
Be a part of LGBT Pride
Many LGBT Pride events are supported by local community organizations, most of which are nonprofits. They will need the necessary resources to keep holding these events every year. These resources can include both time and money. Consider donating your time by volunteering at these Pride events. For example, many Pride events hold health screenings, and you can use your skills and knowledge to promote the well-being of the LGBT community. At the same time, make sure that the PRIDE event is created to help serve the community. There is controversy over the commercialization of LGBT Pride events, as some corporate sponsors have been inconsistent in advocating for the LGBT community. Some feel that the commercialization of LGBT Pride ignores the original purpose of the event as a political movement.3 Do some research to make sure that your donation is going to an LGBT Pride event that serves the whole community, not just certain segments of it, and if you feel that it is not, you may consider donating to other LGBT-serving organizations in your community.
Educate yourself
There are many medical providers who have made it their life’s work doing this. Consider learning more about the role medical providers have played in the health and well-being of the LGBT community, which may serve as an inspiration for your work. The list is long, and includes pioneers such as Ben Barres, MD, PhD, a transgender neurobiologist and physician who transitioned from female to male mid-career and was known for his work on interaction between neurons and glial cells in the nervous system, and Rachel Levine, MD, a physician who became the first transgender woman to serve as Physician General, then Secretary of State, of Pennsylvania.
Other providers have tackled health problems that plagued the LGBT community. Joel D. Weisman, DO, was one of the first physicians to identify the AIDS epidemic and became an advocate for AIDS research, treatment, and prevention, whereas Kevin A. Fenton, MD, PhD, a gay black man, was the director for the National Center for HIV/AIDS at the Centers for Disease Control and Prevention; he helped cultivate strategies to combat the HIV epidemic among gay black men.4 Finally, there is Nanette Gartrell, MD, a psychiatrist and researcher who leads the U.S. National Longitudinal Lesbian Family Study. This ongoing, prospective, and influential study was the first to identify that children raised by lesbian mothers had higher levels of social and school/academic competence and significantly lower levels of social problems, rule-breaking behaviors, and aggressive behaviors, compared with children raised by opposite sex parents.5
LGBT Pride is a time to recognize the achievements the LGBT community has made in the last couple of decades, and at the same time, it is a reminder that the work to promote health equity for this community remains unfinished. Health care providers have an important responsibility in fostering this work in a responsible and ethical matter. Many medical providers have dedicated their lives to this movement, and even when the LGBT Pride season is over, their mission will continue.
Dr. Montano is an assistant professor of pediatrics at the University of Pittsburgh and an adolescent medicine physician at Children’s Hospital of Pittsburgh of UPMC. Email him at pdnews@mdedge.com.
References
1. “Discrimination Prevents LGBTQ People from Accessing Health Care,” Center for American Progress, Jan. 18, 2018.
2. Psychol Bull. 2003 Sep;129(5): 674-97.
3. “How LGBTQ Pride Month became a branded holiday,” Vox, Jun 25, 2018.
4. “Dr. Kevin Fenton stepping down after 8 years,” The Georgia Voice, Dec 7, 2012.
5. Pediatrics. 2010;126(1):28-36.
June is Pride Month in the United States. It is a time in which people take a stand against discrimination and violence against lesbian, gay, bisexual, and transgender (LGBT) people and promote dignity, equality, and visibility of this community. During this time, many cities will be holding events ranging from rallies to parades to not only celebrate sexual diversity and gender variance, but also to serve as a reminder of the work that needs to be done to foster equal treatment for LGBT people. As a medical provider, you have the unique role of advancing this cause – from educating your colleagues on the health needs of this population to advocating for policies that protect their health and well-being. If you’re interested in serving the LGBT community as a medical provider, here are some ways you can show this community your commitment to their health and well-being.
Be visible
There will be numerous LGBT Pride events occurring the month of June and even throughout the summer in the United States. They can occur in cities big and small, and they can even be in the city you work in. Visibility matters for LGBT youth. Eight percent of lesbian, gay, and bisexual people report that a health care provider refused to see them because of their sexual orientation and 29% of transgender people report that their health care providers refused to see them because of their gender identity or expression.1 Therefore, LGBT people will expect discrimination everywhere they go.2
Being present at a Pride event signals to the community that you are willing to serve LGBT people. Many Pride events will allow hospitals and clinics to have a table at the event, but keep in mind that many will prioritize organizations that specifically cater to the LGBT community or that are owned and operated by members of the community. Another way to show the community that you will treat LGBT people with dignity and respect is to list your practice in a database for LGBT-friendly providers. The Gay and Lesbian Medical Association keeps a database of LGBT-friendly medical providers, and many Pride events will advertise businesses and organizations that serve the LGBT community. You may want to consider having your clinic or hospital participate in the Human Rights Campaign (HRC) Health Equality Index (HEI). The HEI is a list of best practices for hospitals and clinics to use that affirm and support LGBT health (such as having gender-neutral bathrooms in facilities). Hospitals and clinics that endorse a high amount or all of these practices are listed as committed to the health and well-being of the LGBT community on the HRC website.
Be a part of LGBT Pride
Many LGBT Pride events are supported by local community organizations, most of which are nonprofits. They will need the necessary resources to keep holding these events every year. These resources can include both time and money. Consider donating your time by volunteering at these Pride events. For example, many Pride events hold health screenings, and you can use your skills and knowledge to promote the well-being of the LGBT community. At the same time, make sure that the PRIDE event is created to help serve the community. There is controversy over the commercialization of LGBT Pride events, as some corporate sponsors have been inconsistent in advocating for the LGBT community. Some feel that the commercialization of LGBT Pride ignores the original purpose of the event as a political movement.3 Do some research to make sure that your donation is going to an LGBT Pride event that serves the whole community, not just certain segments of it, and if you feel that it is not, you may consider donating to other LGBT-serving organizations in your community.
Educate yourself
There are many medical providers who have made it their life’s work doing this. Consider learning more about the role medical providers have played in the health and well-being of the LGBT community, which may serve as an inspiration for your work. The list is long, and includes pioneers such as Ben Barres, MD, PhD, a transgender neurobiologist and physician who transitioned from female to male mid-career and was known for his work on interaction between neurons and glial cells in the nervous system, and Rachel Levine, MD, a physician who became the first transgender woman to serve as Physician General, then Secretary of State, of Pennsylvania.
Other providers have tackled health problems that plagued the LGBT community. Joel D. Weisman, DO, was one of the first physicians to identify the AIDS epidemic and became an advocate for AIDS research, treatment, and prevention, whereas Kevin A. Fenton, MD, PhD, a gay black man, was the director for the National Center for HIV/AIDS at the Centers for Disease Control and Prevention; he helped cultivate strategies to combat the HIV epidemic among gay black men.4 Finally, there is Nanette Gartrell, MD, a psychiatrist and researcher who leads the U.S. National Longitudinal Lesbian Family Study. This ongoing, prospective, and influential study was the first to identify that children raised by lesbian mothers had higher levels of social and school/academic competence and significantly lower levels of social problems, rule-breaking behaviors, and aggressive behaviors, compared with children raised by opposite sex parents.5
LGBT Pride is a time to recognize the achievements the LGBT community has made in the last couple of decades, and at the same time, it is a reminder that the work to promote health equity for this community remains unfinished. Health care providers have an important responsibility in fostering this work in a responsible and ethical matter. Many medical providers have dedicated their lives to this movement, and even when the LGBT Pride season is over, their mission will continue.
Dr. Montano is an assistant professor of pediatrics at the University of Pittsburgh and an adolescent medicine physician at Children’s Hospital of Pittsburgh of UPMC. Email him at pdnews@mdedge.com.
References
1. “Discrimination Prevents LGBTQ People from Accessing Health Care,” Center for American Progress, Jan. 18, 2018.
2. Psychol Bull. 2003 Sep;129(5): 674-97.
3. “How LGBTQ Pride Month became a branded holiday,” Vox, Jun 25, 2018.
4. “Dr. Kevin Fenton stepping down after 8 years,” The Georgia Voice, Dec 7, 2012.
5. Pediatrics. 2010;126(1):28-36.
June is Pride Month in the United States. It is a time in which people take a stand against discrimination and violence against lesbian, gay, bisexual, and transgender (LGBT) people and promote dignity, equality, and visibility of this community. During this time, many cities will be holding events ranging from rallies to parades to not only celebrate sexual diversity and gender variance, but also to serve as a reminder of the work that needs to be done to foster equal treatment for LGBT people. As a medical provider, you have the unique role of advancing this cause – from educating your colleagues on the health needs of this population to advocating for policies that protect their health and well-being. If you’re interested in serving the LGBT community as a medical provider, here are some ways you can show this community your commitment to their health and well-being.
Be visible
There will be numerous LGBT Pride events occurring the month of June and even throughout the summer in the United States. They can occur in cities big and small, and they can even be in the city you work in. Visibility matters for LGBT youth. Eight percent of lesbian, gay, and bisexual people report that a health care provider refused to see them because of their sexual orientation and 29% of transgender people report that their health care providers refused to see them because of their gender identity or expression.1 Therefore, LGBT people will expect discrimination everywhere they go.2
Being present at a Pride event signals to the community that you are willing to serve LGBT people. Many Pride events will allow hospitals and clinics to have a table at the event, but keep in mind that many will prioritize organizations that specifically cater to the LGBT community or that are owned and operated by members of the community. Another way to show the community that you will treat LGBT people with dignity and respect is to list your practice in a database for LGBT-friendly providers. The Gay and Lesbian Medical Association keeps a database of LGBT-friendly medical providers, and many Pride events will advertise businesses and organizations that serve the LGBT community. You may want to consider having your clinic or hospital participate in the Human Rights Campaign (HRC) Health Equality Index (HEI). The HEI is a list of best practices for hospitals and clinics to use that affirm and support LGBT health (such as having gender-neutral bathrooms in facilities). Hospitals and clinics that endorse a high amount or all of these practices are listed as committed to the health and well-being of the LGBT community on the HRC website.
Be a part of LGBT Pride
Many LGBT Pride events are supported by local community organizations, most of which are nonprofits. They will need the necessary resources to keep holding these events every year. These resources can include both time and money. Consider donating your time by volunteering at these Pride events. For example, many Pride events hold health screenings, and you can use your skills and knowledge to promote the well-being of the LGBT community. At the same time, make sure that the PRIDE event is created to help serve the community. There is controversy over the commercialization of LGBT Pride events, as some corporate sponsors have been inconsistent in advocating for the LGBT community. Some feel that the commercialization of LGBT Pride ignores the original purpose of the event as a political movement.3 Do some research to make sure that your donation is going to an LGBT Pride event that serves the whole community, not just certain segments of it, and if you feel that it is not, you may consider donating to other LGBT-serving organizations in your community.
Educate yourself
There are many medical providers who have made it their life’s work doing this. Consider learning more about the role medical providers have played in the health and well-being of the LGBT community, which may serve as an inspiration for your work. The list is long, and includes pioneers such as Ben Barres, MD, PhD, a transgender neurobiologist and physician who transitioned from female to male mid-career and was known for his work on interaction between neurons and glial cells in the nervous system, and Rachel Levine, MD, a physician who became the first transgender woman to serve as Physician General, then Secretary of State, of Pennsylvania.
Other providers have tackled health problems that plagued the LGBT community. Joel D. Weisman, DO, was one of the first physicians to identify the AIDS epidemic and became an advocate for AIDS research, treatment, and prevention, whereas Kevin A. Fenton, MD, PhD, a gay black man, was the director for the National Center for HIV/AIDS at the Centers for Disease Control and Prevention; he helped cultivate strategies to combat the HIV epidemic among gay black men.4 Finally, there is Nanette Gartrell, MD, a psychiatrist and researcher who leads the U.S. National Longitudinal Lesbian Family Study. This ongoing, prospective, and influential study was the first to identify that children raised by lesbian mothers had higher levels of social and school/academic competence and significantly lower levels of social problems, rule-breaking behaviors, and aggressive behaviors, compared with children raised by opposite sex parents.5
LGBT Pride is a time to recognize the achievements the LGBT community has made in the last couple of decades, and at the same time, it is a reminder that the work to promote health equity for this community remains unfinished. Health care providers have an important responsibility in fostering this work in a responsible and ethical matter. Many medical providers have dedicated their lives to this movement, and even when the LGBT Pride season is over, their mission will continue.
Dr. Montano is an assistant professor of pediatrics at the University of Pittsburgh and an adolescent medicine physician at Children’s Hospital of Pittsburgh of UPMC. Email him at pdnews@mdedge.com.
References
1. “Discrimination Prevents LGBTQ People from Accessing Health Care,” Center for American Progress, Jan. 18, 2018.
2. Psychol Bull. 2003 Sep;129(5): 674-97.
3. “How LGBTQ Pride Month became a branded holiday,” Vox, Jun 25, 2018.
4. “Dr. Kevin Fenton stepping down after 8 years,” The Georgia Voice, Dec 7, 2012.
5. Pediatrics. 2010;126(1):28-36.
Study identifies predictors of bariatric surgery attrition
BALTIMORE – Even in a public health system like Canada’s, almost and researchers have identified patient characteristics that could be predictive of dropout risk that would potentially have implications in a nonuniversal system, such as that of the United States, according to a study of almost 18,000 patients reported at the annual meeting Society of American Gastrointestinal and Endoscopic Surgeons.
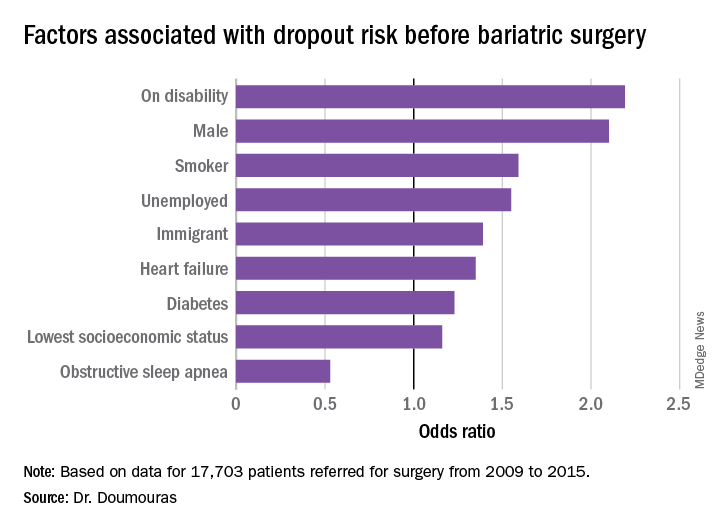
“Even in a universal health care system, clear disparities exist among patient populations having bariatric surgery,” said Aristithes Doumouras, MD, of McMaster University in Hamilton, Ont. “Extensive work-ups and long wait times can have an impact on the delivery of bariatric care.”
Dr. Doumouras reported on results of a retrospective, population-based study of 17,703 patients referred for surgery during 2009-2015 in the Ontario Bariatric Network, a province-wide network of 11 hospitals credentialed to perform bariatric surgery. The study found that 23.2% of patients referred for bariatric surgery did not go through with it and that overall average wait times between referral and the operation were just short of a year – 362.2 days to be precise.
The goal of the study was to identify any factors associated with attrition, Dr. Doumouras said.
“Predictors of interest included patient demographics – age, sex, income quintile, immigration status, employment status, smoking status – and comorbidities, such as diabetes, heart failure, hypertension, sleep apnea, and renal disease,” he said. “The study also evaluated health services factors, such as overall wait time to bariatric surgery, presence of centers of excellence, and health care utilization.”
The study found that demographics with more than twice the odds of attrition were male gender and presence of a disability (P less than .01). Smokers were 60% more likely to drop out (P less than .01), he said. “To receive bariatric surgery in Ontario, smokers must go through a smoking cessation program.”
Unemployed individuals and immigrants also had higher rates of attrition, at 55% and 39%, respectively, and were more likely to not go through with the operation (P less than .01). Health factors associated with attrition, but to a lesser extent, were diabetes (odds ratio, 1.23) and heart failure (OR, 1.35; P less than .01).
“Low socioeconomic status actually had a very low impact in our system on attrition after adjustment for other demographic factors such as disability and unemployment,” Dr. Doumouras said, noting a 16% greater risk of attrition in this group (P = .02).
“Interestingly,” he noted, “there was one factor associated with less dropout – obstructive sleep apnea – probably because people hate using the CPAP machines every single night.” People with OSA were 47% less likely to drop out than were people without the disease (P less than .001).
When asked if the findings would be applicable in the United States, Dr. Doumouras said they would to an extent.
“I think we can say confidently that they would apply to most universal health care systems,” he said. “In nonuniversal health care systems, the interplay between insurance status, socioeconomic status, and the like makes it more of a complex relationship, but if you were to take any kind of health care system, even in the United States, you would probably see very similar trends in terms of who can get bariatric surgery.”
He added, “I think also the length of work-up matters. Only a 3- or 4-week work-up probably affects attrition as well. These are relatively universal things.”
Dr. Doumouras has no financial relationships to disclose.
SOURCE: Doumouras A et al. SAGES 2019, Abstract S118.
BALTIMORE – Even in a public health system like Canada’s, almost and researchers have identified patient characteristics that could be predictive of dropout risk that would potentially have implications in a nonuniversal system, such as that of the United States, according to a study of almost 18,000 patients reported at the annual meeting Society of American Gastrointestinal and Endoscopic Surgeons.

“Even in a universal health care system, clear disparities exist among patient populations having bariatric surgery,” said Aristithes Doumouras, MD, of McMaster University in Hamilton, Ont. “Extensive work-ups and long wait times can have an impact on the delivery of bariatric care.”
Dr. Doumouras reported on results of a retrospective, population-based study of 17,703 patients referred for surgery during 2009-2015 in the Ontario Bariatric Network, a province-wide network of 11 hospitals credentialed to perform bariatric surgery. The study found that 23.2% of patients referred for bariatric surgery did not go through with it and that overall average wait times between referral and the operation were just short of a year – 362.2 days to be precise.
The goal of the study was to identify any factors associated with attrition, Dr. Doumouras said.
“Predictors of interest included patient demographics – age, sex, income quintile, immigration status, employment status, smoking status – and comorbidities, such as diabetes, heart failure, hypertension, sleep apnea, and renal disease,” he said. “The study also evaluated health services factors, such as overall wait time to bariatric surgery, presence of centers of excellence, and health care utilization.”
The study found that demographics with more than twice the odds of attrition were male gender and presence of a disability (P less than .01). Smokers were 60% more likely to drop out (P less than .01), he said. “To receive bariatric surgery in Ontario, smokers must go through a smoking cessation program.”
Unemployed individuals and immigrants also had higher rates of attrition, at 55% and 39%, respectively, and were more likely to not go through with the operation (P less than .01). Health factors associated with attrition, but to a lesser extent, were diabetes (odds ratio, 1.23) and heart failure (OR, 1.35; P less than .01).
“Low socioeconomic status actually had a very low impact in our system on attrition after adjustment for other demographic factors such as disability and unemployment,” Dr. Doumouras said, noting a 16% greater risk of attrition in this group (P = .02).
“Interestingly,” he noted, “there was one factor associated with less dropout – obstructive sleep apnea – probably because people hate using the CPAP machines every single night.” People with OSA were 47% less likely to drop out than were people without the disease (P less than .001).
When asked if the findings would be applicable in the United States, Dr. Doumouras said they would to an extent.
“I think we can say confidently that they would apply to most universal health care systems,” he said. “In nonuniversal health care systems, the interplay between insurance status, socioeconomic status, and the like makes it more of a complex relationship, but if you were to take any kind of health care system, even in the United States, you would probably see very similar trends in terms of who can get bariatric surgery.”
He added, “I think also the length of work-up matters. Only a 3- or 4-week work-up probably affects attrition as well. These are relatively universal things.”
Dr. Doumouras has no financial relationships to disclose.
SOURCE: Doumouras A et al. SAGES 2019, Abstract S118.
BALTIMORE – Even in a public health system like Canada’s, almost and researchers have identified patient characteristics that could be predictive of dropout risk that would potentially have implications in a nonuniversal system, such as that of the United States, according to a study of almost 18,000 patients reported at the annual meeting Society of American Gastrointestinal and Endoscopic Surgeons.

“Even in a universal health care system, clear disparities exist among patient populations having bariatric surgery,” said Aristithes Doumouras, MD, of McMaster University in Hamilton, Ont. “Extensive work-ups and long wait times can have an impact on the delivery of bariatric care.”
Dr. Doumouras reported on results of a retrospective, population-based study of 17,703 patients referred for surgery during 2009-2015 in the Ontario Bariatric Network, a province-wide network of 11 hospitals credentialed to perform bariatric surgery. The study found that 23.2% of patients referred for bariatric surgery did not go through with it and that overall average wait times between referral and the operation were just short of a year – 362.2 days to be precise.
The goal of the study was to identify any factors associated with attrition, Dr. Doumouras said.
“Predictors of interest included patient demographics – age, sex, income quintile, immigration status, employment status, smoking status – and comorbidities, such as diabetes, heart failure, hypertension, sleep apnea, and renal disease,” he said. “The study also evaluated health services factors, such as overall wait time to bariatric surgery, presence of centers of excellence, and health care utilization.”
The study found that demographics with more than twice the odds of attrition were male gender and presence of a disability (P less than .01). Smokers were 60% more likely to drop out (P less than .01), he said. “To receive bariatric surgery in Ontario, smokers must go through a smoking cessation program.”
Unemployed individuals and immigrants also had higher rates of attrition, at 55% and 39%, respectively, and were more likely to not go through with the operation (P less than .01). Health factors associated with attrition, but to a lesser extent, were diabetes (odds ratio, 1.23) and heart failure (OR, 1.35; P less than .01).
“Low socioeconomic status actually had a very low impact in our system on attrition after adjustment for other demographic factors such as disability and unemployment,” Dr. Doumouras said, noting a 16% greater risk of attrition in this group (P = .02).
“Interestingly,” he noted, “there was one factor associated with less dropout – obstructive sleep apnea – probably because people hate using the CPAP machines every single night.” People with OSA were 47% less likely to drop out than were people without the disease (P less than .001).
When asked if the findings would be applicable in the United States, Dr. Doumouras said they would to an extent.
“I think we can say confidently that they would apply to most universal health care systems,” he said. “In nonuniversal health care systems, the interplay between insurance status, socioeconomic status, and the like makes it more of a complex relationship, but if you were to take any kind of health care system, even in the United States, you would probably see very similar trends in terms of who can get bariatric surgery.”
He added, “I think also the length of work-up matters. Only a 3- or 4-week work-up probably affects attrition as well. These are relatively universal things.”
Dr. Doumouras has no financial relationships to disclose.
SOURCE: Doumouras A et al. SAGES 2019, Abstract S118.
REPORTING FROM SAGES 2019
Aspirin shows little benefit for primary prevention of vascular disease in diabetes
Background: Multiple large, randomized, controlled trials and meta-analyses that used aspirin as primary prevention for vascular events showed decreased vascular events, but a significant counterbalanced risk of bleeding. Since diabetes carries a higher risk of vascular events, this study examines aspirin for primary prevention of vascular events in diabetic patients.
Study design: Large, randomized, controlled trial.
Setting: British registry-based study.
Synopsis: This is a 9-year randomized, controlled trial that included 15,480 British patients with diabetes without known vascular disease who were randomized to receive a 100-mg aspirin daily or placebo. Participants in each group were closely matched patients with diabetes who were recruited using registry data and were aged 40 years and older with no alternative strong indication for aspirin.
Overall, aspirin provided no difference in mortality but showed an absolute 1.3% decrease in first vascular events or revascularization procedures with an absolute 1.1% increase in first occurrence of major bleeding event. Approximately 60% of the bleeding events were gastrointestinal or “other” urinary/nose bleeding, and there was no statistically significant increase in intracranial hemorrhage, hemorrhagic stroke, or vision-threatening eye bleeding. Vascular events were defined as transient ischemic attack (TIA), nonfatal MI, nonfatal ischemic stroke, or vascular death excluding intracranial hemorrhage. The major limitation of this study is that it had a composite of endpoints of different clinical significance. Furthermore, TIA as a major vascular event was added after the study began to increase statistical power, and when it is excluded, the difference for vascular events is not statistically significant.
Bottom line: Aspirin when used in primary prevention of vascular events in diabetes provides no improvement in mortality, and the benefit of prevention of vascular events must be weighed against the risks of bleeding.
Citation: The ASCEND Study Collaborative Group. Effects of aspirin for primary prevention in diabetes. N Eng J Med. 2018 Oct 18;379(16):1529-39.
Dr. Scott is an assistant professor in the division of hospital medicine, University of New Mexico.
Background: Multiple large, randomized, controlled trials and meta-analyses that used aspirin as primary prevention for vascular events showed decreased vascular events, but a significant counterbalanced risk of bleeding. Since diabetes carries a higher risk of vascular events, this study examines aspirin for primary prevention of vascular events in diabetic patients.
Study design: Large, randomized, controlled trial.
Setting: British registry-based study.
Synopsis: This is a 9-year randomized, controlled trial that included 15,480 British patients with diabetes without known vascular disease who were randomized to receive a 100-mg aspirin daily or placebo. Participants in each group were closely matched patients with diabetes who were recruited using registry data and were aged 40 years and older with no alternative strong indication for aspirin.
Overall, aspirin provided no difference in mortality but showed an absolute 1.3% decrease in first vascular events or revascularization procedures with an absolute 1.1% increase in first occurrence of major bleeding event. Approximately 60% of the bleeding events were gastrointestinal or “other” urinary/nose bleeding, and there was no statistically significant increase in intracranial hemorrhage, hemorrhagic stroke, or vision-threatening eye bleeding. Vascular events were defined as transient ischemic attack (TIA), nonfatal MI, nonfatal ischemic stroke, or vascular death excluding intracranial hemorrhage. The major limitation of this study is that it had a composite of endpoints of different clinical significance. Furthermore, TIA as a major vascular event was added after the study began to increase statistical power, and when it is excluded, the difference for vascular events is not statistically significant.
Bottom line: Aspirin when used in primary prevention of vascular events in diabetes provides no improvement in mortality, and the benefit of prevention of vascular events must be weighed against the risks of bleeding.
Citation: The ASCEND Study Collaborative Group. Effects of aspirin for primary prevention in diabetes. N Eng J Med. 2018 Oct 18;379(16):1529-39.
Dr. Scott is an assistant professor in the division of hospital medicine, University of New Mexico.
Background: Multiple large, randomized, controlled trials and meta-analyses that used aspirin as primary prevention for vascular events showed decreased vascular events, but a significant counterbalanced risk of bleeding. Since diabetes carries a higher risk of vascular events, this study examines aspirin for primary prevention of vascular events in diabetic patients.
Study design: Large, randomized, controlled trial.
Setting: British registry-based study.
Synopsis: This is a 9-year randomized, controlled trial that included 15,480 British patients with diabetes without known vascular disease who were randomized to receive a 100-mg aspirin daily or placebo. Participants in each group were closely matched patients with diabetes who were recruited using registry data and were aged 40 years and older with no alternative strong indication for aspirin.
Overall, aspirin provided no difference in mortality but showed an absolute 1.3% decrease in first vascular events or revascularization procedures with an absolute 1.1% increase in first occurrence of major bleeding event. Approximately 60% of the bleeding events were gastrointestinal or “other” urinary/nose bleeding, and there was no statistically significant increase in intracranial hemorrhage, hemorrhagic stroke, or vision-threatening eye bleeding. Vascular events were defined as transient ischemic attack (TIA), nonfatal MI, nonfatal ischemic stroke, or vascular death excluding intracranial hemorrhage. The major limitation of this study is that it had a composite of endpoints of different clinical significance. Furthermore, TIA as a major vascular event was added after the study began to increase statistical power, and when it is excluded, the difference for vascular events is not statistically significant.
Bottom line: Aspirin when used in primary prevention of vascular events in diabetes provides no improvement in mortality, and the benefit of prevention of vascular events must be weighed against the risks of bleeding.
Citation: The ASCEND Study Collaborative Group. Effects of aspirin for primary prevention in diabetes. N Eng J Med. 2018 Oct 18;379(16):1529-39.
Dr. Scott is an assistant professor in the division of hospital medicine, University of New Mexico.
Whatever “my last doctor” did, I don’t take the bait
In car repair, there’s a mysterious bogeyman known as “the last guy.”
“The last guy put it in wrong.”
“The last guy didn’t use the right part.”
“I have no idea what the last guy was thinking.”
In medicine, there’s “my last doctor.”
“My last doctor ordered the wrong test.”
“The medication, from my last doctor, almost killed me.”
“My last doctor didn’t know what he was doing.”
I don’t say anything, I just listen. Most of the time I’m not convinced the other doctor did anything wrong, and even if I were, I’d stay silent. Every doctor makes mistakes. It’s inevitable in any job.
Sometimes the patients mention this in passing, at other times they seem to be hoping for a response from me. I don’t give them one. Bashing other doctors is common enough as it is, and I’m not going to join in. My job is to do my best to help them, which is what the last doctor was trying to do, too.
The fact is that you can’t make everyone happy. Outside competency and human errors, there are too many variables in human relationships – the chemistry between people – to know what went wrong. Some patients have legitimate grievances, others may just be nitpicking and looking for trouble. It’s not my role to address it. If the patients came here for that, they’re at the wrong place. Most of the time, I happen to know their previous physicians, and think they’re decent neurologists.
The problem with these types of things is that it propagates. Even if I do everything right, and try my best, there’s a good chance that, in a few months, I’ll be “my last doctor.” I can only hope the next doctor feels the same way about me as I do about the last one.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
In car repair, there’s a mysterious bogeyman known as “the last guy.”
“The last guy put it in wrong.”
“The last guy didn’t use the right part.”
“I have no idea what the last guy was thinking.”
In medicine, there’s “my last doctor.”
“My last doctor ordered the wrong test.”
“The medication, from my last doctor, almost killed me.”
“My last doctor didn’t know what he was doing.”
I don’t say anything, I just listen. Most of the time I’m not convinced the other doctor did anything wrong, and even if I were, I’d stay silent. Every doctor makes mistakes. It’s inevitable in any job.
Sometimes the patients mention this in passing, at other times they seem to be hoping for a response from me. I don’t give them one. Bashing other doctors is common enough as it is, and I’m not going to join in. My job is to do my best to help them, which is what the last doctor was trying to do, too.
The fact is that you can’t make everyone happy. Outside competency and human errors, there are too many variables in human relationships – the chemistry between people – to know what went wrong. Some patients have legitimate grievances, others may just be nitpicking and looking for trouble. It’s not my role to address it. If the patients came here for that, they’re at the wrong place. Most of the time, I happen to know their previous physicians, and think they’re decent neurologists.
The problem with these types of things is that it propagates. Even if I do everything right, and try my best, there’s a good chance that, in a few months, I’ll be “my last doctor.” I can only hope the next doctor feels the same way about me as I do about the last one.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
In car repair, there’s a mysterious bogeyman known as “the last guy.”
“The last guy put it in wrong.”
“The last guy didn’t use the right part.”
“I have no idea what the last guy was thinking.”
In medicine, there’s “my last doctor.”
“My last doctor ordered the wrong test.”
“The medication, from my last doctor, almost killed me.”
“My last doctor didn’t know what he was doing.”
I don’t say anything, I just listen. Most of the time I’m not convinced the other doctor did anything wrong, and even if I were, I’d stay silent. Every doctor makes mistakes. It’s inevitable in any job.
Sometimes the patients mention this in passing, at other times they seem to be hoping for a response from me. I don’t give them one. Bashing other doctors is common enough as it is, and I’m not going to join in. My job is to do my best to help them, which is what the last doctor was trying to do, too.
The fact is that you can’t make everyone happy. Outside competency and human errors, there are too many variables in human relationships – the chemistry between people – to know what went wrong. Some patients have legitimate grievances, others may just be nitpicking and looking for trouble. It’s not my role to address it. If the patients came here for that, they’re at the wrong place. Most of the time, I happen to know their previous physicians, and think they’re decent neurologists.
The problem with these types of things is that it propagates. Even if I do everything right, and try my best, there’s a good chance that, in a few months, I’ll be “my last doctor.” I can only hope the next doctor feels the same way about me as I do about the last one.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Maternal immunization protects against serious RSV infection in infancy
LJUBLJANA, SLOVENIA – Passive protection of infants from severe respiratory syncytial virus lower respiratory tract infection during the first 6 months of life has convincingly been achieved through maternal immunization using a novel nanoparticle vaccine in the landmark PREPARE trial.
“I think it’s important for everyone, especially people like myself who’ve been working on maternal immunization for about 20 years, to realize that this is a historic study,” Flor M. Munoz, MD, declared in reporting the study results at the annual meeting of the European Society for Paediatric Infectious Diseases.
“We have here for the first time a phase-3, global, randomized, placebo-controlled, observer-blinded clinical trial looking at an experimental vaccine in pregnant women for the protection of infants from a disease for which we really don’t have other potential solutions quite yet, and in a period of high vulnerability,” said Dr. Munoz, a pediatric infectious disease specialist at Baylor College of Medicine, Houston.
Indeed, respiratory syncytial virus (RSV) is the No. 2 cause of mortality worldwide during the first year of life. Moreover, most cases of severe RSV lower respiratory tract infection occur in otherwise healthy infants aged less than 5 months, when active immunization presents daunting challenges.
“While certainly mortality is uncommon in high-income countries, we do see significant hospitalization there due to severe RSV lower respiratory tract infection in the first year of life, sometimes more than other common diseases, like influenza,” she noted.
PREPARE included 4,636 women with low-risk pregnancies who were randomized 2:1 to a single intramuscular injection of the investigational RSV vaccine or placebo during gestational weeks 28-36, with efficacy assessed through the first 180 days of life. The study took place at 87 sites in 11 countries during 4 years worth of RSV seasons. Roughly half of participants were South African, one-quarter were in the United States, and the rest were drawn from nine other low-, middle-, or high-income countries in the Northern and Southern Hemispheres. The median gestational age at vaccination was 32 weeks.
The primary efficacy endpoint specified by the Food and Drug Administration – but not other regulatory agencies – was the placebo-subtracted rate of RSV lower respiratory tract infection as defined by RSV detected by reverse transcription polymerase chain reaction, along with at least one clinical manifestation of lower respiratory tract infection, oxygen saturation below 95%, and/or tachypnea. The risk of this outcome was reduced by 39% during the first 90 days of life and by 27% through 180 days in infants in the maternal immunization group, a difference which didn’t achieve statistical significance.
However, prespecified major secondary endpoints arguably of greater clinical relevance were consistently positive. Notably, when levels of transplacentally transferred neutralizing antibodies against RSV A and B were highest, with events occurring in 57 of 2,765 evaluable infants in the active treatment arm and in 53 of 1,430 controls. Similarly, there was a 40% reduction through day 180. Moreover, rates of another key secondary endpoint – RSV lower respiratory tract infection plus severe hypoxemia with an oxygen saturation below 92% – were reduced by 48% and 42% through days 90 and 180, respectively. Thus, the vaccine’s protective effect was greatest against the most severe outcomes of RSV infection in infancy, according to Dr. Munoz.
No safety signals related to this immunization strategy were seen during 1 year of follow-up of infants and 6 months for the mothers. Side effects were essentially limited to mild, self-limited injection site reactions, with zero impact on pregnancy and delivery.
An intriguing finding in an exploratory analysis was that the vaccine appeared to have ancillary benefits beyond prevention of medically significant RSV disease in the young infants. For example, the rate of all lower respiratory tract infections with severe hypoxemia – with no requirement for demonstration of RSV infection – was reduced by 46% during the first 90 days of life in the immunized group. Similarly, the rate of all-cause lower respiratory tract infection resulting in hospitalization was reduced by 28%.
“This is actually quite interesting, because these are unexpected benefits in terms of all-cause effects,” the pediatrician commented, adding that she and her coinvestigators are delving into this phenomenon in order to gain better understanding.
Additional analyses of the recently completed PREPARE study are ongoing but already have yielded some important findings. For example, women immunized before 33 weeks’ gestation had significantly greater transplacental antibody transfer than those immunized later in pregnancy, with resultant markedly greater vaccine efficacy in their offspring as well: A placebo-subtracted 70% reduction in RSV lower respiratory tract infection with severe hypoxemia through 90 days, compared with a 44% reduction associated with immunization at gestational week 33 or later. And when the interval between immunization and delivery was at least 30 days, the risk of this endpoint was reduced by 65%; in contrast, there was no significant difference between vaccine and placebo groups when time from immunization to delivery was less than 30 days.
Also noteworthy was that maternal immunization afforded no infant protection in the United States. This unanticipated finding is still under investigation, although suspicion centers around the fact that RSV seasons were generally milder there, and American women were vaccinated at a later gestational age, with a corresponding shorter interval to delivery.
The novel recombinant nanoparticle vaccine tested in PREPARE contains a nearly full-length RSV fusion protein produced in insect cells. The nanoparticles express both prefusion epitopes and epitopes common to pre- and postfusion conformations. Aluminum phosphate is employed as the adjuvant.
Novavax’s stock price has been kicked to the curb since the company earlier reported that a large phase 3 trial of the vaccine failed to meet its primary endpoint for prevention of RSV lower respiratory tract infection in older adults. Now the vaccine’s failure to meet its prespecified FDA-mandated primary endpoint in the maternal immunization study will doubtless spawn further financially dismissive headlines in the business press as well.
But pediatricians are famously advocates for children, and PREPARE received a warm welcome from the pediatric infectious disease community, regardless of investor response. Indeed, PREPARE was the only clinical trial deemed of sufficient import to be featured in the opening plenary session of ESPID 2019.
Ulrich Heininger, MD, professor of pediatrics at the University of Basel (Switzerland), who cochaired the session, jointly sponsored by ESPID and the Pediatric Infectious Diseases Society, declared, “These findings, I think, are a great step forward.”
Dr. Munoz reported receiving research grants from Janssen, the National Institutes of Health, the Centers for Disease Control and Prevention, and Novavax, which sponsored the PREPARE trial, assisted by an $89 million grant from the Bill and Melinda Gates Foundation.
LJUBLJANA, SLOVENIA – Passive protection of infants from severe respiratory syncytial virus lower respiratory tract infection during the first 6 months of life has convincingly been achieved through maternal immunization using a novel nanoparticle vaccine in the landmark PREPARE trial.
“I think it’s important for everyone, especially people like myself who’ve been working on maternal immunization for about 20 years, to realize that this is a historic study,” Flor M. Munoz, MD, declared in reporting the study results at the annual meeting of the European Society for Paediatric Infectious Diseases.
“We have here for the first time a phase-3, global, randomized, placebo-controlled, observer-blinded clinical trial looking at an experimental vaccine in pregnant women for the protection of infants from a disease for which we really don’t have other potential solutions quite yet, and in a period of high vulnerability,” said Dr. Munoz, a pediatric infectious disease specialist at Baylor College of Medicine, Houston.
Indeed, respiratory syncytial virus (RSV) is the No. 2 cause of mortality worldwide during the first year of life. Moreover, most cases of severe RSV lower respiratory tract infection occur in otherwise healthy infants aged less than 5 months, when active immunization presents daunting challenges.
“While certainly mortality is uncommon in high-income countries, we do see significant hospitalization there due to severe RSV lower respiratory tract infection in the first year of life, sometimes more than other common diseases, like influenza,” she noted.
PREPARE included 4,636 women with low-risk pregnancies who were randomized 2:1 to a single intramuscular injection of the investigational RSV vaccine or placebo during gestational weeks 28-36, with efficacy assessed through the first 180 days of life. The study took place at 87 sites in 11 countries during 4 years worth of RSV seasons. Roughly half of participants were South African, one-quarter were in the United States, and the rest were drawn from nine other low-, middle-, or high-income countries in the Northern and Southern Hemispheres. The median gestational age at vaccination was 32 weeks.
The primary efficacy endpoint specified by the Food and Drug Administration – but not other regulatory agencies – was the placebo-subtracted rate of RSV lower respiratory tract infection as defined by RSV detected by reverse transcription polymerase chain reaction, along with at least one clinical manifestation of lower respiratory tract infection, oxygen saturation below 95%, and/or tachypnea. The risk of this outcome was reduced by 39% during the first 90 days of life and by 27% through 180 days in infants in the maternal immunization group, a difference which didn’t achieve statistical significance.
However, prespecified major secondary endpoints arguably of greater clinical relevance were consistently positive. Notably, when levels of transplacentally transferred neutralizing antibodies against RSV A and B were highest, with events occurring in 57 of 2,765 evaluable infants in the active treatment arm and in 53 of 1,430 controls. Similarly, there was a 40% reduction through day 180. Moreover, rates of another key secondary endpoint – RSV lower respiratory tract infection plus severe hypoxemia with an oxygen saturation below 92% – were reduced by 48% and 42% through days 90 and 180, respectively. Thus, the vaccine’s protective effect was greatest against the most severe outcomes of RSV infection in infancy, according to Dr. Munoz.
No safety signals related to this immunization strategy were seen during 1 year of follow-up of infants and 6 months for the mothers. Side effects were essentially limited to mild, self-limited injection site reactions, with zero impact on pregnancy and delivery.
An intriguing finding in an exploratory analysis was that the vaccine appeared to have ancillary benefits beyond prevention of medically significant RSV disease in the young infants. For example, the rate of all lower respiratory tract infections with severe hypoxemia – with no requirement for demonstration of RSV infection – was reduced by 46% during the first 90 days of life in the immunized group. Similarly, the rate of all-cause lower respiratory tract infection resulting in hospitalization was reduced by 28%.
“This is actually quite interesting, because these are unexpected benefits in terms of all-cause effects,” the pediatrician commented, adding that she and her coinvestigators are delving into this phenomenon in order to gain better understanding.
Additional analyses of the recently completed PREPARE study are ongoing but already have yielded some important findings. For example, women immunized before 33 weeks’ gestation had significantly greater transplacental antibody transfer than those immunized later in pregnancy, with resultant markedly greater vaccine efficacy in their offspring as well: A placebo-subtracted 70% reduction in RSV lower respiratory tract infection with severe hypoxemia through 90 days, compared with a 44% reduction associated with immunization at gestational week 33 or later. And when the interval between immunization and delivery was at least 30 days, the risk of this endpoint was reduced by 65%; in contrast, there was no significant difference between vaccine and placebo groups when time from immunization to delivery was less than 30 days.
Also noteworthy was that maternal immunization afforded no infant protection in the United States. This unanticipated finding is still under investigation, although suspicion centers around the fact that RSV seasons were generally milder there, and American women were vaccinated at a later gestational age, with a corresponding shorter interval to delivery.
The novel recombinant nanoparticle vaccine tested in PREPARE contains a nearly full-length RSV fusion protein produced in insect cells. The nanoparticles express both prefusion epitopes and epitopes common to pre- and postfusion conformations. Aluminum phosphate is employed as the adjuvant.
Novavax’s stock price has been kicked to the curb since the company earlier reported that a large phase 3 trial of the vaccine failed to meet its primary endpoint for prevention of RSV lower respiratory tract infection in older adults. Now the vaccine’s failure to meet its prespecified FDA-mandated primary endpoint in the maternal immunization study will doubtless spawn further financially dismissive headlines in the business press as well.
But pediatricians are famously advocates for children, and PREPARE received a warm welcome from the pediatric infectious disease community, regardless of investor response. Indeed, PREPARE was the only clinical trial deemed of sufficient import to be featured in the opening plenary session of ESPID 2019.
Ulrich Heininger, MD, professor of pediatrics at the University of Basel (Switzerland), who cochaired the session, jointly sponsored by ESPID and the Pediatric Infectious Diseases Society, declared, “These findings, I think, are a great step forward.”
Dr. Munoz reported receiving research grants from Janssen, the National Institutes of Health, the Centers for Disease Control and Prevention, and Novavax, which sponsored the PREPARE trial, assisted by an $89 million grant from the Bill and Melinda Gates Foundation.
LJUBLJANA, SLOVENIA – Passive protection of infants from severe respiratory syncytial virus lower respiratory tract infection during the first 6 months of life has convincingly been achieved through maternal immunization using a novel nanoparticle vaccine in the landmark PREPARE trial.
“I think it’s important for everyone, especially people like myself who’ve been working on maternal immunization for about 20 years, to realize that this is a historic study,” Flor M. Munoz, MD, declared in reporting the study results at the annual meeting of the European Society for Paediatric Infectious Diseases.
“We have here for the first time a phase-3, global, randomized, placebo-controlled, observer-blinded clinical trial looking at an experimental vaccine in pregnant women for the protection of infants from a disease for which we really don’t have other potential solutions quite yet, and in a period of high vulnerability,” said Dr. Munoz, a pediatric infectious disease specialist at Baylor College of Medicine, Houston.
Indeed, respiratory syncytial virus (RSV) is the No. 2 cause of mortality worldwide during the first year of life. Moreover, most cases of severe RSV lower respiratory tract infection occur in otherwise healthy infants aged less than 5 months, when active immunization presents daunting challenges.
“While certainly mortality is uncommon in high-income countries, we do see significant hospitalization there due to severe RSV lower respiratory tract infection in the first year of life, sometimes more than other common diseases, like influenza,” she noted.
PREPARE included 4,636 women with low-risk pregnancies who were randomized 2:1 to a single intramuscular injection of the investigational RSV vaccine or placebo during gestational weeks 28-36, with efficacy assessed through the first 180 days of life. The study took place at 87 sites in 11 countries during 4 years worth of RSV seasons. Roughly half of participants were South African, one-quarter were in the United States, and the rest were drawn from nine other low-, middle-, or high-income countries in the Northern and Southern Hemispheres. The median gestational age at vaccination was 32 weeks.
The primary efficacy endpoint specified by the Food and Drug Administration – but not other regulatory agencies – was the placebo-subtracted rate of RSV lower respiratory tract infection as defined by RSV detected by reverse transcription polymerase chain reaction, along with at least one clinical manifestation of lower respiratory tract infection, oxygen saturation below 95%, and/or tachypnea. The risk of this outcome was reduced by 39% during the first 90 days of life and by 27% through 180 days in infants in the maternal immunization group, a difference which didn’t achieve statistical significance.
However, prespecified major secondary endpoints arguably of greater clinical relevance were consistently positive. Notably, when levels of transplacentally transferred neutralizing antibodies against RSV A and B were highest, with events occurring in 57 of 2,765 evaluable infants in the active treatment arm and in 53 of 1,430 controls. Similarly, there was a 40% reduction through day 180. Moreover, rates of another key secondary endpoint – RSV lower respiratory tract infection plus severe hypoxemia with an oxygen saturation below 92% – were reduced by 48% and 42% through days 90 and 180, respectively. Thus, the vaccine’s protective effect was greatest against the most severe outcomes of RSV infection in infancy, according to Dr. Munoz.
No safety signals related to this immunization strategy were seen during 1 year of follow-up of infants and 6 months for the mothers. Side effects were essentially limited to mild, self-limited injection site reactions, with zero impact on pregnancy and delivery.
An intriguing finding in an exploratory analysis was that the vaccine appeared to have ancillary benefits beyond prevention of medically significant RSV disease in the young infants. For example, the rate of all lower respiratory tract infections with severe hypoxemia – with no requirement for demonstration of RSV infection – was reduced by 46% during the first 90 days of life in the immunized group. Similarly, the rate of all-cause lower respiratory tract infection resulting in hospitalization was reduced by 28%.
“This is actually quite interesting, because these are unexpected benefits in terms of all-cause effects,” the pediatrician commented, adding that she and her coinvestigators are delving into this phenomenon in order to gain better understanding.
Additional analyses of the recently completed PREPARE study are ongoing but already have yielded some important findings. For example, women immunized before 33 weeks’ gestation had significantly greater transplacental antibody transfer than those immunized later in pregnancy, with resultant markedly greater vaccine efficacy in their offspring as well: A placebo-subtracted 70% reduction in RSV lower respiratory tract infection with severe hypoxemia through 90 days, compared with a 44% reduction associated with immunization at gestational week 33 or later. And when the interval between immunization and delivery was at least 30 days, the risk of this endpoint was reduced by 65%; in contrast, there was no significant difference between vaccine and placebo groups when time from immunization to delivery was less than 30 days.
Also noteworthy was that maternal immunization afforded no infant protection in the United States. This unanticipated finding is still under investigation, although suspicion centers around the fact that RSV seasons were generally milder there, and American women were vaccinated at a later gestational age, with a corresponding shorter interval to delivery.
The novel recombinant nanoparticle vaccine tested in PREPARE contains a nearly full-length RSV fusion protein produced in insect cells. The nanoparticles express both prefusion epitopes and epitopes common to pre- and postfusion conformations. Aluminum phosphate is employed as the adjuvant.
Novavax’s stock price has been kicked to the curb since the company earlier reported that a large phase 3 trial of the vaccine failed to meet its primary endpoint for prevention of RSV lower respiratory tract infection in older adults. Now the vaccine’s failure to meet its prespecified FDA-mandated primary endpoint in the maternal immunization study will doubtless spawn further financially dismissive headlines in the business press as well.
But pediatricians are famously advocates for children, and PREPARE received a warm welcome from the pediatric infectious disease community, regardless of investor response. Indeed, PREPARE was the only clinical trial deemed of sufficient import to be featured in the opening plenary session of ESPID 2019.
Ulrich Heininger, MD, professor of pediatrics at the University of Basel (Switzerland), who cochaired the session, jointly sponsored by ESPID and the Pediatric Infectious Diseases Society, declared, “These findings, I think, are a great step forward.”
Dr. Munoz reported receiving research grants from Janssen, the National Institutes of Health, the Centers for Disease Control and Prevention, and Novavax, which sponsored the PREPARE trial, assisted by an $89 million grant from the Bill and Melinda Gates Foundation.
REPORTING FROM ESPID 2019
Is it measles? – Diagnosis and management for the pediatric provider
The mother of an 8-month-old calls your office and is hysterical. Her daughter has had cough for a few days with high fevers and now has developed a full body rash. She is worried about measles and is on her way to your office.
We are in the middle of a measles epidemic, there’s no denying it. Measles was declared eliminated in 2000, but reported cases in the United States have been on the rise, and are now at the highest number since 2014. Five months into 2019, there have been 839 reported cases as of May 13). Measles outbreaks (defined by the Centers for Disease Control and Prevention as three or more cases) have been reported in California, Georgia, Maryland, Michigan, New Jersey, New York, and Pennsylvania. When vaccination rates fall, it is easy for measles to spread. The virus is highly contagious in nonimmune people, because of its airborne spread and its persistence in the environment for hours.
First – is it really measles?
It can be difficult to distinguish the maculopapular rash of measles from similar rashes that occur with more benign viral illnesses. Adding to the challenge, the last major measles outbreak in the United States was over 2 decades ago, and many practicing pediatricians have never seen a single case. So, what clinical features can help distinguish measles from other febrile illnesses?
The prodromal phase of measles lasts approximately 2-4 days and children have high fevers (103°-105° F), anorexia, and malaise. Conjunctivitis, coryza, and cough develop during this phase, and precede any rash. Koplik spots appear during the prodromal phase, but are not seen in all cases. These spots are 1- to 3-mm blue-white lesions on an erythematous base on the buccal mucosa, classically opposite the first molar. The spots often slough once the rash appears. The rash appears 2-4 days after the onset of fever, and is initially maculopapular and blanching. The first lesions appear on the face and neck, and the rash spreads cranial to caudal, typically sparing palms and soles. After days 3-4, the rash will no longer blanch. High fevers persist for 2-4 more days with rash, ongoing respiratory symptoms, conjunctivitis, and pharyngitis. Note that the fever will persist even with development of the rash, unlike in roseola.
It is not only important to diagnosis measles from a public health standpoint, but also because measles can have severe complications, especially in infants and children under 5 years. During the 1989-1991 outbreak, the mortality rate was 2.2 deaths per 1,000 cases (J Infect Dis. 2004 May 1. doi: 10.1086/377694).
Six percent of patients develop pneumonia, which in infants and toddlers can lead to respiratory distress or failure requiring hospitalization. Pneumonia is responsible for 60% of measles deaths, according to the CDC “Pink Book,” Epidemiology and Prevention of Vaccine-Preventable Diseases, chapter 13 on measles, 13th Ed., 2015. Ocular complications include keratitis and corneal ulceration. Measles also can cause serious neurologic complications. Encephalitis, seen in 1 per 1,000 cases, usually arises several days after the rash and may present with seizure or encephalopathy. Acute disseminated encephalomyelitis (ADEM), an inflammatory demyelinating disease of the central nervous system, occurs in approximately 1 per 1,000 cases, typically presents during the recovery phase (1-2 weeks after rash), and can have long-term sequelae. Subacute sclerosing panencephalitis (SSPE) is a progressive and fatal neurodegenerative disorder, and presents 7-10 years after measles infection.
Should you transfer the patient to a hospital?
Unless there is a medical need for the child to be admitted, sending a patient with potential measles to the hospital is not necessary, and can cause exposure to a large group of medical personnel, and patients who cannot be vaccinated (such as infants, immunocompromised patients, and pregnant women). However, if there is concern for complications such as seizures, encephalitis, or pneumonia, then transfer is indicated. Call the accepting hospital in advance so the staff can prepare for the patient. During transfer, place a standard face mask on the patient and instruct the patient not to remove it.
For hospitals accepting a suspected measles case, meet the patient outside of the facility and ensure that the patient is wearing a standard face mask. All staff interacting with the patient should practice contact and airborne precautions (N95 respirator mask). Take the patient directly to an isolation room with negative airflow. Caution pregnant staff that they should not have contact with the patient.
Which diagnostic tests should you use?
Diagnosis can be made based on serum antibody tests (measles IgM and IgG), throat or urine viral cultures, and nasopharyngeal and throat specimen polymerase chain reaction (PCR) testing. The CDC recommends obtaining a serum sample for measles IgM testing and a throat swab for PCR in all suspected cases, but local health departments vary in their specific testing recommendations. Familiarize yourself with the tests recommended by your local department of health, and where they prefer testing on outpatients to be done. Confirmed measles should be reported to your department of health.
What are considerations for community pediatric offices?
Update families in emails to call ahead if they suspect measles. This way the office can prepare a room for the family, and have the family immediately brought back without exposing staff and other families in the waiting area. It may be more prudent to examine these children at the end of the clinic day as the virus can persist for up to 2 hours on fomites and in the air. Therefore, all waiting areas and shared air spaces (including those with shared air ducts) should be cleared for 2 hours after the patient leaves.
When should you provide prophylaxis after exposure?
A patient with suspected measles does not require immediate vaccination. If it is measles, it is already too late to vaccinate. If measles is ruled out, the child should follow the standard measles vaccination guidelines.
Individuals are contagious from 4 days before to 4 days after the rash appears.
If measles is confirmed, all people who are unvaccinated or undervaccinated and were exposed to the confirmed case during the contagious period should be vaccinated within 72 hours of exposure. Infants 6 months or older may safely receive the MMR vaccine. However, infants vaccinated with MMR before their first birthday must be vaccinated again at age 12-15 months (greater than 28 days after prior vaccine) and at 4-6 years. Immunoglobulin prophylaxis should be given intramuscularly in exposed infants ages birth to less than 6 months, and in those ages 6-12 months who present beyond the 72-hour window. Unvaccinated or undervaccinated, exposed individuals at high risk for complications from measles (immunocompromised, pregnant) also should receive immunoglobulin.
What should you tell traveling families?
Several countries have large, ongoing measles outbreaks, including Israel, Ukraine, and the Philippines. Before international travel, infants 6-11 months should receive one dose of MMR vaccine, and children 12 months and older need two doses separated by at least 28 days. For unvaccinated or undervaccinated children, consider advising families to hold off travel to high-risk countries, or understand the indications to vaccinate a child upon return.
Dr. Angelica DesPain is a pediatric emergency medicine fellow at Children’s National Medical Center in Washington. She said she has no relevant financial disclosures. Dr. Emily Willner is a pediatric emergency medicine attending at Children’s National Medical Center, and an assistant professor of pediatrics and emergency medicine at George Washington University, Washington. She has no relevant financial disclosures.
The mother of an 8-month-old calls your office and is hysterical. Her daughter has had cough for a few days with high fevers and now has developed a full body rash. She is worried about measles and is on her way to your office.
We are in the middle of a measles epidemic, there’s no denying it. Measles was declared eliminated in 2000, but reported cases in the United States have been on the rise, and are now at the highest number since 2014. Five months into 2019, there have been 839 reported cases as of May 13). Measles outbreaks (defined by the Centers for Disease Control and Prevention as three or more cases) have been reported in California, Georgia, Maryland, Michigan, New Jersey, New York, and Pennsylvania. When vaccination rates fall, it is easy for measles to spread. The virus is highly contagious in nonimmune people, because of its airborne spread and its persistence in the environment for hours.
First – is it really measles?
It can be difficult to distinguish the maculopapular rash of measles from similar rashes that occur with more benign viral illnesses. Adding to the challenge, the last major measles outbreak in the United States was over 2 decades ago, and many practicing pediatricians have never seen a single case. So, what clinical features can help distinguish measles from other febrile illnesses?
The prodromal phase of measles lasts approximately 2-4 days and children have high fevers (103°-105° F), anorexia, and malaise. Conjunctivitis, coryza, and cough develop during this phase, and precede any rash. Koplik spots appear during the prodromal phase, but are not seen in all cases. These spots are 1- to 3-mm blue-white lesions on an erythematous base on the buccal mucosa, classically opposite the first molar. The spots often slough once the rash appears. The rash appears 2-4 days after the onset of fever, and is initially maculopapular and blanching. The first lesions appear on the face and neck, and the rash spreads cranial to caudal, typically sparing palms and soles. After days 3-4, the rash will no longer blanch. High fevers persist for 2-4 more days with rash, ongoing respiratory symptoms, conjunctivitis, and pharyngitis. Note that the fever will persist even with development of the rash, unlike in roseola.
It is not only important to diagnosis measles from a public health standpoint, but also because measles can have severe complications, especially in infants and children under 5 years. During the 1989-1991 outbreak, the mortality rate was 2.2 deaths per 1,000 cases (J Infect Dis. 2004 May 1. doi: 10.1086/377694).
Six percent of patients develop pneumonia, which in infants and toddlers can lead to respiratory distress or failure requiring hospitalization. Pneumonia is responsible for 60% of measles deaths, according to the CDC “Pink Book,” Epidemiology and Prevention of Vaccine-Preventable Diseases, chapter 13 on measles, 13th Ed., 2015. Ocular complications include keratitis and corneal ulceration. Measles also can cause serious neurologic complications. Encephalitis, seen in 1 per 1,000 cases, usually arises several days after the rash and may present with seizure or encephalopathy. Acute disseminated encephalomyelitis (ADEM), an inflammatory demyelinating disease of the central nervous system, occurs in approximately 1 per 1,000 cases, typically presents during the recovery phase (1-2 weeks after rash), and can have long-term sequelae. Subacute sclerosing panencephalitis (SSPE) is a progressive and fatal neurodegenerative disorder, and presents 7-10 years after measles infection.
Should you transfer the patient to a hospital?
Unless there is a medical need for the child to be admitted, sending a patient with potential measles to the hospital is not necessary, and can cause exposure to a large group of medical personnel, and patients who cannot be vaccinated (such as infants, immunocompromised patients, and pregnant women). However, if there is concern for complications such as seizures, encephalitis, or pneumonia, then transfer is indicated. Call the accepting hospital in advance so the staff can prepare for the patient. During transfer, place a standard face mask on the patient and instruct the patient not to remove it.
For hospitals accepting a suspected measles case, meet the patient outside of the facility and ensure that the patient is wearing a standard face mask. All staff interacting with the patient should practice contact and airborne precautions (N95 respirator mask). Take the patient directly to an isolation room with negative airflow. Caution pregnant staff that they should not have contact with the patient.
Which diagnostic tests should you use?
Diagnosis can be made based on serum antibody tests (measles IgM and IgG), throat or urine viral cultures, and nasopharyngeal and throat specimen polymerase chain reaction (PCR) testing. The CDC recommends obtaining a serum sample for measles IgM testing and a throat swab for PCR in all suspected cases, but local health departments vary in their specific testing recommendations. Familiarize yourself with the tests recommended by your local department of health, and where they prefer testing on outpatients to be done. Confirmed measles should be reported to your department of health.
What are considerations for community pediatric offices?
Update families in emails to call ahead if they suspect measles. This way the office can prepare a room for the family, and have the family immediately brought back without exposing staff and other families in the waiting area. It may be more prudent to examine these children at the end of the clinic day as the virus can persist for up to 2 hours on fomites and in the air. Therefore, all waiting areas and shared air spaces (including those with shared air ducts) should be cleared for 2 hours after the patient leaves.
When should you provide prophylaxis after exposure?
A patient with suspected measles does not require immediate vaccination. If it is measles, it is already too late to vaccinate. If measles is ruled out, the child should follow the standard measles vaccination guidelines.
Individuals are contagious from 4 days before to 4 days after the rash appears.
If measles is confirmed, all people who are unvaccinated or undervaccinated and were exposed to the confirmed case during the contagious period should be vaccinated within 72 hours of exposure. Infants 6 months or older may safely receive the MMR vaccine. However, infants vaccinated with MMR before their first birthday must be vaccinated again at age 12-15 months (greater than 28 days after prior vaccine) and at 4-6 years. Immunoglobulin prophylaxis should be given intramuscularly in exposed infants ages birth to less than 6 months, and in those ages 6-12 months who present beyond the 72-hour window. Unvaccinated or undervaccinated, exposed individuals at high risk for complications from measles (immunocompromised, pregnant) also should receive immunoglobulin.
What should you tell traveling families?
Several countries have large, ongoing measles outbreaks, including Israel, Ukraine, and the Philippines. Before international travel, infants 6-11 months should receive one dose of MMR vaccine, and children 12 months and older need two doses separated by at least 28 days. For unvaccinated or undervaccinated children, consider advising families to hold off travel to high-risk countries, or understand the indications to vaccinate a child upon return.
Dr. Angelica DesPain is a pediatric emergency medicine fellow at Children’s National Medical Center in Washington. She said she has no relevant financial disclosures. Dr. Emily Willner is a pediatric emergency medicine attending at Children’s National Medical Center, and an assistant professor of pediatrics and emergency medicine at George Washington University, Washington. She has no relevant financial disclosures.
The mother of an 8-month-old calls your office and is hysterical. Her daughter has had cough for a few days with high fevers and now has developed a full body rash. She is worried about measles and is on her way to your office.
We are in the middle of a measles epidemic, there’s no denying it. Measles was declared eliminated in 2000, but reported cases in the United States have been on the rise, and are now at the highest number since 2014. Five months into 2019, there have been 839 reported cases as of May 13). Measles outbreaks (defined by the Centers for Disease Control and Prevention as three or more cases) have been reported in California, Georgia, Maryland, Michigan, New Jersey, New York, and Pennsylvania. When vaccination rates fall, it is easy for measles to spread. The virus is highly contagious in nonimmune people, because of its airborne spread and its persistence in the environment for hours.
First – is it really measles?
It can be difficult to distinguish the maculopapular rash of measles from similar rashes that occur with more benign viral illnesses. Adding to the challenge, the last major measles outbreak in the United States was over 2 decades ago, and many practicing pediatricians have never seen a single case. So, what clinical features can help distinguish measles from other febrile illnesses?
The prodromal phase of measles lasts approximately 2-4 days and children have high fevers (103°-105° F), anorexia, and malaise. Conjunctivitis, coryza, and cough develop during this phase, and precede any rash. Koplik spots appear during the prodromal phase, but are not seen in all cases. These spots are 1- to 3-mm blue-white lesions on an erythematous base on the buccal mucosa, classically opposite the first molar. The spots often slough once the rash appears. The rash appears 2-4 days after the onset of fever, and is initially maculopapular and blanching. The first lesions appear on the face and neck, and the rash spreads cranial to caudal, typically sparing palms and soles. After days 3-4, the rash will no longer blanch. High fevers persist for 2-4 more days with rash, ongoing respiratory symptoms, conjunctivitis, and pharyngitis. Note that the fever will persist even with development of the rash, unlike in roseola.
It is not only important to diagnosis measles from a public health standpoint, but also because measles can have severe complications, especially in infants and children under 5 years. During the 1989-1991 outbreak, the mortality rate was 2.2 deaths per 1,000 cases (J Infect Dis. 2004 May 1. doi: 10.1086/377694).
Six percent of patients develop pneumonia, which in infants and toddlers can lead to respiratory distress or failure requiring hospitalization. Pneumonia is responsible for 60% of measles deaths, according to the CDC “Pink Book,” Epidemiology and Prevention of Vaccine-Preventable Diseases, chapter 13 on measles, 13th Ed., 2015. Ocular complications include keratitis and corneal ulceration. Measles also can cause serious neurologic complications. Encephalitis, seen in 1 per 1,000 cases, usually arises several days after the rash and may present with seizure or encephalopathy. Acute disseminated encephalomyelitis (ADEM), an inflammatory demyelinating disease of the central nervous system, occurs in approximately 1 per 1,000 cases, typically presents during the recovery phase (1-2 weeks after rash), and can have long-term sequelae. Subacute sclerosing panencephalitis (SSPE) is a progressive and fatal neurodegenerative disorder, and presents 7-10 years after measles infection.
Should you transfer the patient to a hospital?
Unless there is a medical need for the child to be admitted, sending a patient with potential measles to the hospital is not necessary, and can cause exposure to a large group of medical personnel, and patients who cannot be vaccinated (such as infants, immunocompromised patients, and pregnant women). However, if there is concern for complications such as seizures, encephalitis, or pneumonia, then transfer is indicated. Call the accepting hospital in advance so the staff can prepare for the patient. During transfer, place a standard face mask on the patient and instruct the patient not to remove it.
For hospitals accepting a suspected measles case, meet the patient outside of the facility and ensure that the patient is wearing a standard face mask. All staff interacting with the patient should practice contact and airborne precautions (N95 respirator mask). Take the patient directly to an isolation room with negative airflow. Caution pregnant staff that they should not have contact with the patient.
Which diagnostic tests should you use?
Diagnosis can be made based on serum antibody tests (measles IgM and IgG), throat or urine viral cultures, and nasopharyngeal and throat specimen polymerase chain reaction (PCR) testing. The CDC recommends obtaining a serum sample for measles IgM testing and a throat swab for PCR in all suspected cases, but local health departments vary in their specific testing recommendations. Familiarize yourself with the tests recommended by your local department of health, and where they prefer testing on outpatients to be done. Confirmed measles should be reported to your department of health.
What are considerations for community pediatric offices?
Update families in emails to call ahead if they suspect measles. This way the office can prepare a room for the family, and have the family immediately brought back without exposing staff and other families in the waiting area. It may be more prudent to examine these children at the end of the clinic day as the virus can persist for up to 2 hours on fomites and in the air. Therefore, all waiting areas and shared air spaces (including those with shared air ducts) should be cleared for 2 hours after the patient leaves.
When should you provide prophylaxis after exposure?
A patient with suspected measles does not require immediate vaccination. If it is measles, it is already too late to vaccinate. If measles is ruled out, the child should follow the standard measles vaccination guidelines.
Individuals are contagious from 4 days before to 4 days after the rash appears.
If measles is confirmed, all people who are unvaccinated or undervaccinated and were exposed to the confirmed case during the contagious period should be vaccinated within 72 hours of exposure. Infants 6 months or older may safely receive the MMR vaccine. However, infants vaccinated with MMR before their first birthday must be vaccinated again at age 12-15 months (greater than 28 days after prior vaccine) and at 4-6 years. Immunoglobulin prophylaxis should be given intramuscularly in exposed infants ages birth to less than 6 months, and in those ages 6-12 months who present beyond the 72-hour window. Unvaccinated or undervaccinated, exposed individuals at high risk for complications from measles (immunocompromised, pregnant) also should receive immunoglobulin.
What should you tell traveling families?
Several countries have large, ongoing measles outbreaks, including Israel, Ukraine, and the Philippines. Before international travel, infants 6-11 months should receive one dose of MMR vaccine, and children 12 months and older need two doses separated by at least 28 days. For unvaccinated or undervaccinated children, consider advising families to hold off travel to high-risk countries, or understand the indications to vaccinate a child upon return.
Dr. Angelica DesPain is a pediatric emergency medicine fellow at Children’s National Medical Center in Washington. She said she has no relevant financial disclosures. Dr. Emily Willner is a pediatric emergency medicine attending at Children’s National Medical Center, and an assistant professor of pediatrics and emergency medicine at George Washington University, Washington. She has no relevant financial disclosures.
Younger patients with NSCLC tend to live longer
Younger patients with non–small cell lung cancer (NSCLC) may have better survival, despite higher rates of brain metastasis and driver mutations, according to results from a retrospective analysis.
“We carried out a comprehensive analysis of patient clinicopathologic features and clinical outcomes in both young (age ≤ 50 years) and older (age > 60 years) patients with NSCLC,” wrote Anna May Suidan of Tel Aviv University, and colleagues. The findings were published in the Journal of Global Oncology.
The researchers reviewed medical records of patients who were diagnosed with lung cancer at a large cancer treatment facility in Israel from 2010 to 2015. Patients were categorized into two groups according to age at cancer diagnosis, which was established based on tumor pathology.
Various clinical data were collected, including demographic information, history of malignancy, smoking history, histologic subtype, and survival data.
In all, 62 patients were included in the younger cohort (median age, 44.5 years) and 124 patients in the older cohort (median age, 68.0 years).
After analysis, the researchers found that younger patients had a higher incidence of brain metastasis (39% vs. 25%, respectively; P = .04), and increased rates of EGFR mutations (23% vs. 18%, respectively; P = .4) and ALK translocations (13% vs. 2%, respectively; P = .002) versus older patients.
“Our cohort, which was [composed] of white patients, demonstrated that younger patients harbored more targetable driver mutations compared with older patients (34% vs. 18%; P = .01),” the researchers wrote.
In addition, among those with a driver mutation, younger patients showed a trend toward better survival (median survival, 33 vs. 25 months, respectively; P = .4).
Two key limitations of the study were the small sample size and retrospective design.
“[These results] highlight the importance of genetic background assessments and considering lung cancer as a possible diagnosis in young symptomatic patients in clinical settings,” the researchers concluded.
No funding sources were reported. The authors reported financial affiliations with Astra Zeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Novartis, Roche, Teva Pharmaceuticals, and several others.
SOURCE: Suidan AM et al. J Glob Oncol. 2019 May 8. doi: 10.1200/JGO.18.00216.
Younger patients with non–small cell lung cancer (NSCLC) may have better survival, despite higher rates of brain metastasis and driver mutations, according to results from a retrospective analysis.
“We carried out a comprehensive analysis of patient clinicopathologic features and clinical outcomes in both young (age ≤ 50 years) and older (age > 60 years) patients with NSCLC,” wrote Anna May Suidan of Tel Aviv University, and colleagues. The findings were published in the Journal of Global Oncology.
The researchers reviewed medical records of patients who were diagnosed with lung cancer at a large cancer treatment facility in Israel from 2010 to 2015. Patients were categorized into two groups according to age at cancer diagnosis, which was established based on tumor pathology.
Various clinical data were collected, including demographic information, history of malignancy, smoking history, histologic subtype, and survival data.
In all, 62 patients were included in the younger cohort (median age, 44.5 years) and 124 patients in the older cohort (median age, 68.0 years).
After analysis, the researchers found that younger patients had a higher incidence of brain metastasis (39% vs. 25%, respectively; P = .04), and increased rates of EGFR mutations (23% vs. 18%, respectively; P = .4) and ALK translocations (13% vs. 2%, respectively; P = .002) versus older patients.
“Our cohort, which was [composed] of white patients, demonstrated that younger patients harbored more targetable driver mutations compared with older patients (34% vs. 18%; P = .01),” the researchers wrote.
In addition, among those with a driver mutation, younger patients showed a trend toward better survival (median survival, 33 vs. 25 months, respectively; P = .4).
Two key limitations of the study were the small sample size and retrospective design.
“[These results] highlight the importance of genetic background assessments and considering lung cancer as a possible diagnosis in young symptomatic patients in clinical settings,” the researchers concluded.
No funding sources were reported. The authors reported financial affiliations with Astra Zeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Novartis, Roche, Teva Pharmaceuticals, and several others.
SOURCE: Suidan AM et al. J Glob Oncol. 2019 May 8. doi: 10.1200/JGO.18.00216.
Younger patients with non–small cell lung cancer (NSCLC) may have better survival, despite higher rates of brain metastasis and driver mutations, according to results from a retrospective analysis.
“We carried out a comprehensive analysis of patient clinicopathologic features and clinical outcomes in both young (age ≤ 50 years) and older (age > 60 years) patients with NSCLC,” wrote Anna May Suidan of Tel Aviv University, and colleagues. The findings were published in the Journal of Global Oncology.
The researchers reviewed medical records of patients who were diagnosed with lung cancer at a large cancer treatment facility in Israel from 2010 to 2015. Patients were categorized into two groups according to age at cancer diagnosis, which was established based on tumor pathology.
Various clinical data were collected, including demographic information, history of malignancy, smoking history, histologic subtype, and survival data.
In all, 62 patients were included in the younger cohort (median age, 44.5 years) and 124 patients in the older cohort (median age, 68.0 years).
After analysis, the researchers found that younger patients had a higher incidence of brain metastasis (39% vs. 25%, respectively; P = .04), and increased rates of EGFR mutations (23% vs. 18%, respectively; P = .4) and ALK translocations (13% vs. 2%, respectively; P = .002) versus older patients.
“Our cohort, which was [composed] of white patients, demonstrated that younger patients harbored more targetable driver mutations compared with older patients (34% vs. 18%; P = .01),” the researchers wrote.
In addition, among those with a driver mutation, younger patients showed a trend toward better survival (median survival, 33 vs. 25 months, respectively; P = .4).
Two key limitations of the study were the small sample size and retrospective design.
“[These results] highlight the importance of genetic background assessments and considering lung cancer as a possible diagnosis in young symptomatic patients in clinical settings,” the researchers concluded.
No funding sources were reported. The authors reported financial affiliations with Astra Zeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Novartis, Roche, Teva Pharmaceuticals, and several others.
SOURCE: Suidan AM et al. J Glob Oncol. 2019 May 8. doi: 10.1200/JGO.18.00216.
FROM THE JOURNAL OF GLOBAL ONCOLOGY
















