User login
CDC exhorts more testing and treatment of HIV
Approximately 80% of HIV infections in the United States in 2016 were spread by almost 40% of infected individuals who did not know their status or were not receiving care, according to data from the Centers for Disease Control and Prevention.
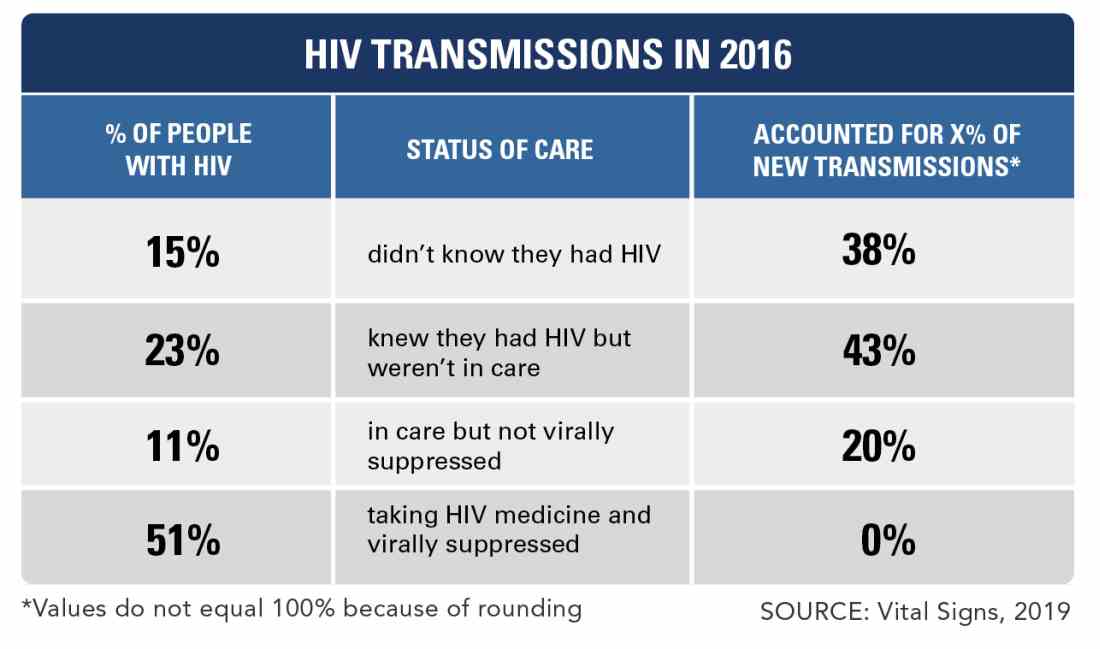
However, leadership at the Department of Health & Human Services has developed a “bold but completely achievable” plan to reduce HIV infections within the next decade, Vice Adm. Jerome M. Adams, MD, the U.S. surgeon general, said in a teleconference to announce the results of a new Vital Signs report on the impact of undiagnosed and untreated HIV.
In the early release Vital Signs from Morbidity and Mortality Weekly Report, Li Zihao, PhD, and his colleagues at the CDC used a model to estimate rates of HIV transmission in 2016 based on data from the National HIV Behavioral Surveillance on needle-sharing behavior and sexual behaviors. The overall transmission rate was 3.5/100,000 person-years. Of these transmissions, 73.0% were from men who have sex with men, 9.7% from intravenous drug users, and 12% from heterosexuals.
The percentages of transmissions for those who were acutely ill with HIV but unaware, not acutely ill but unaware, aware of HIV infection but not treated, receiving care but not virally suppressed, and receiving care and virally suppressed were 4.0%, 33.6%, 42.6%, 19.8%, and 0%, respectively, the researchers said.
The study “emphasizes the impact that HIV resources could have,” by showing the importance of identifying infected individuals early and using the tools now available to treat them before they can transmit the disease, Jonathan Mermin, MD, director of the CDC’s National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, said during the conference.
“Today’s treatment regimens are simpler than those prescribed in the past, sometimes requiring only single-tablet formulations, with fewer side effects; most persons with HIV infection can achieve viral suppression within 6 months of initiating treatment,” the researchers wrote.
In the wake of the findings and at the start of the CDC’s 2019 National HIV Prevention Conference, the CDC proposed a federal initiative, “Ending the HIV Epidemic: A Plan for America.”
The goal is to reduce the incidence of new HIV infections by at least 90% over the next decade, starting with a focus on parts of the country with the highest disease burden, according to the CDC.
“Today’s Vital Signs report illustrates how a goal that once seemed impossible is now within our reach.” Robert R. Redfield, MD, director of the Centers for Disease Control and Prevention, said during the conference. “If we increase access to testing and treatment for people with HIV, we could prevent a lion’s share of infections,” he said.
The plan involves working to identify individuals at risk, treating those who test positive as quickly as possible, and keeping them in care. Updated information on the CDC website provides more details for clinicians on how to have conversations about HIV with patients, the latest information about antiretroviral therapy, and details about prevention for partners including post- and pre-exposure prophylaxis (PEP and PreP), and condoms.
Eugene McCray, MD, director of the CDC’s Division of HIV/AIDS Prevention, emphasized that the CDC recommends HIV testing for all individuals aged 13-64 years at least once in their lives. He added that everyone who tests positive should seek medical care, that everyone with HIV deserves support to combat stigma, and that those at risk should be empowered to take advantage of proven effective prevention strategies.
Clinicians can access the updated CDC page on caring for HIV patients here.
SOURCE: Li Z et al. MMWR Morb Mortal Wkly Rep. 2019 March 18. doi: org/10.15585/mmwr.mm6811e1.
Approximately 80% of HIV infections in the United States in 2016 were spread by almost 40% of infected individuals who did not know their status or were not receiving care, according to data from the Centers for Disease Control and Prevention.

However, leadership at the Department of Health & Human Services has developed a “bold but completely achievable” plan to reduce HIV infections within the next decade, Vice Adm. Jerome M. Adams, MD, the U.S. surgeon general, said in a teleconference to announce the results of a new Vital Signs report on the impact of undiagnosed and untreated HIV.
In the early release Vital Signs from Morbidity and Mortality Weekly Report, Li Zihao, PhD, and his colleagues at the CDC used a model to estimate rates of HIV transmission in 2016 based on data from the National HIV Behavioral Surveillance on needle-sharing behavior and sexual behaviors. The overall transmission rate was 3.5/100,000 person-years. Of these transmissions, 73.0% were from men who have sex with men, 9.7% from intravenous drug users, and 12% from heterosexuals.
The percentages of transmissions for those who were acutely ill with HIV but unaware, not acutely ill but unaware, aware of HIV infection but not treated, receiving care but not virally suppressed, and receiving care and virally suppressed were 4.0%, 33.6%, 42.6%, 19.8%, and 0%, respectively, the researchers said.
The study “emphasizes the impact that HIV resources could have,” by showing the importance of identifying infected individuals early and using the tools now available to treat them before they can transmit the disease, Jonathan Mermin, MD, director of the CDC’s National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, said during the conference.
“Today’s treatment regimens are simpler than those prescribed in the past, sometimes requiring only single-tablet formulations, with fewer side effects; most persons with HIV infection can achieve viral suppression within 6 months of initiating treatment,” the researchers wrote.
In the wake of the findings and at the start of the CDC’s 2019 National HIV Prevention Conference, the CDC proposed a federal initiative, “Ending the HIV Epidemic: A Plan for America.”
The goal is to reduce the incidence of new HIV infections by at least 90% over the next decade, starting with a focus on parts of the country with the highest disease burden, according to the CDC.
“Today’s Vital Signs report illustrates how a goal that once seemed impossible is now within our reach.” Robert R. Redfield, MD, director of the Centers for Disease Control and Prevention, said during the conference. “If we increase access to testing and treatment for people with HIV, we could prevent a lion’s share of infections,” he said.
The plan involves working to identify individuals at risk, treating those who test positive as quickly as possible, and keeping them in care. Updated information on the CDC website provides more details for clinicians on how to have conversations about HIV with patients, the latest information about antiretroviral therapy, and details about prevention for partners including post- and pre-exposure prophylaxis (PEP and PreP), and condoms.
Eugene McCray, MD, director of the CDC’s Division of HIV/AIDS Prevention, emphasized that the CDC recommends HIV testing for all individuals aged 13-64 years at least once in their lives. He added that everyone who tests positive should seek medical care, that everyone with HIV deserves support to combat stigma, and that those at risk should be empowered to take advantage of proven effective prevention strategies.
Clinicians can access the updated CDC page on caring for HIV patients here.
SOURCE: Li Z et al. MMWR Morb Mortal Wkly Rep. 2019 March 18. doi: org/10.15585/mmwr.mm6811e1.
Approximately 80% of HIV infections in the United States in 2016 were spread by almost 40% of infected individuals who did not know their status or were not receiving care, according to data from the Centers for Disease Control and Prevention.

However, leadership at the Department of Health & Human Services has developed a “bold but completely achievable” plan to reduce HIV infections within the next decade, Vice Adm. Jerome M. Adams, MD, the U.S. surgeon general, said in a teleconference to announce the results of a new Vital Signs report on the impact of undiagnosed and untreated HIV.
In the early release Vital Signs from Morbidity and Mortality Weekly Report, Li Zihao, PhD, and his colleagues at the CDC used a model to estimate rates of HIV transmission in 2016 based on data from the National HIV Behavioral Surveillance on needle-sharing behavior and sexual behaviors. The overall transmission rate was 3.5/100,000 person-years. Of these transmissions, 73.0% were from men who have sex with men, 9.7% from intravenous drug users, and 12% from heterosexuals.
The percentages of transmissions for those who were acutely ill with HIV but unaware, not acutely ill but unaware, aware of HIV infection but not treated, receiving care but not virally suppressed, and receiving care and virally suppressed were 4.0%, 33.6%, 42.6%, 19.8%, and 0%, respectively, the researchers said.
The study “emphasizes the impact that HIV resources could have,” by showing the importance of identifying infected individuals early and using the tools now available to treat them before they can transmit the disease, Jonathan Mermin, MD, director of the CDC’s National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, said during the conference.
“Today’s treatment regimens are simpler than those prescribed in the past, sometimes requiring only single-tablet formulations, with fewer side effects; most persons with HIV infection can achieve viral suppression within 6 months of initiating treatment,” the researchers wrote.
In the wake of the findings and at the start of the CDC’s 2019 National HIV Prevention Conference, the CDC proposed a federal initiative, “Ending the HIV Epidemic: A Plan for America.”
The goal is to reduce the incidence of new HIV infections by at least 90% over the next decade, starting with a focus on parts of the country with the highest disease burden, according to the CDC.
“Today’s Vital Signs report illustrates how a goal that once seemed impossible is now within our reach.” Robert R. Redfield, MD, director of the Centers for Disease Control and Prevention, said during the conference. “If we increase access to testing and treatment for people with HIV, we could prevent a lion’s share of infections,” he said.
The plan involves working to identify individuals at risk, treating those who test positive as quickly as possible, and keeping them in care. Updated information on the CDC website provides more details for clinicians on how to have conversations about HIV with patients, the latest information about antiretroviral therapy, and details about prevention for partners including post- and pre-exposure prophylaxis (PEP and PreP), and condoms.
Eugene McCray, MD, director of the CDC’s Division of HIV/AIDS Prevention, emphasized that the CDC recommends HIV testing for all individuals aged 13-64 years at least once in their lives. He added that everyone who tests positive should seek medical care, that everyone with HIV deserves support to combat stigma, and that those at risk should be empowered to take advantage of proven effective prevention strategies.
Clinicians can access the updated CDC page on caring for HIV patients here.
SOURCE: Li Z et al. MMWR Morb Mortal Wkly Rep. 2019 March 18. doi: org/10.15585/mmwr.mm6811e1.
FROM THE CDC
Measles cases confirmed in three more states
Forty more cases and three more states were added to the measles count in the last week, bringing the U.S. total to 268 cases in 15 states so far in 2019, according to the Centers for Disease Control and Prevention.
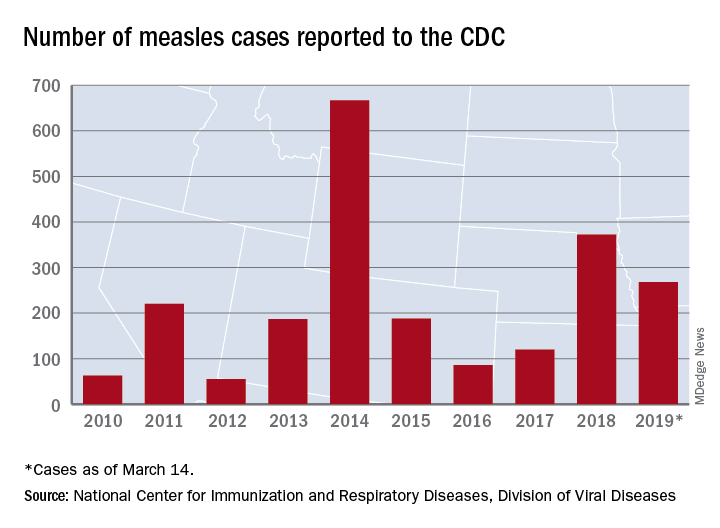
Arizona, Michigan, and Missouri reported their first confirmed cases of the year, joining California (one outbreak), Colorado, Connecticut, Georgia, Illinois (one outbreak), Kentucky, New Hampshire, New Jersey, New York (two outbreaks), Oregon, Texas (one outbreak), and Washington (one outbreak), the CDC reported March 18.
Brooklyn, N.Y., has become the epicenter of measles activity since mid-February, and with 25 of the 40 new cases occurring there, the borough has now led the nation for four consecutive weeks. There have been 157 confirmed cases in Brooklyn and one in Queens since the outbreak began in October of 2018, the New York City Department of Health and Mental Hygiene reported.
Michigan’s first case of measles is travel related and involved an individual visiting from Israel following a stay in New York, the Michigan Department of Health and Human Services and Oakland County Health Division announced March 13.
In Arizona, the state department of health services and the Pima County Public Health Department announced that a 12-month-old infant from Pima County has been diagnosed with measles after traveling to Asia.
A single case of measles, contracted while the person was traveling out of state, has been reported in Jefferson County, Mo., and is being managed by the county health department, according to St. Louis Public Radio.
Forty more cases and three more states were added to the measles count in the last week, bringing the U.S. total to 268 cases in 15 states so far in 2019, according to the Centers for Disease Control and Prevention.

Arizona, Michigan, and Missouri reported their first confirmed cases of the year, joining California (one outbreak), Colorado, Connecticut, Georgia, Illinois (one outbreak), Kentucky, New Hampshire, New Jersey, New York (two outbreaks), Oregon, Texas (one outbreak), and Washington (one outbreak), the CDC reported March 18.
Brooklyn, N.Y., has become the epicenter of measles activity since mid-February, and with 25 of the 40 new cases occurring there, the borough has now led the nation for four consecutive weeks. There have been 157 confirmed cases in Brooklyn and one in Queens since the outbreak began in October of 2018, the New York City Department of Health and Mental Hygiene reported.
Michigan’s first case of measles is travel related and involved an individual visiting from Israel following a stay in New York, the Michigan Department of Health and Human Services and Oakland County Health Division announced March 13.
In Arizona, the state department of health services and the Pima County Public Health Department announced that a 12-month-old infant from Pima County has been diagnosed with measles after traveling to Asia.
A single case of measles, contracted while the person was traveling out of state, has been reported in Jefferson County, Mo., and is being managed by the county health department, according to St. Louis Public Radio.
Forty more cases and three more states were added to the measles count in the last week, bringing the U.S. total to 268 cases in 15 states so far in 2019, according to the Centers for Disease Control and Prevention.

Arizona, Michigan, and Missouri reported their first confirmed cases of the year, joining California (one outbreak), Colorado, Connecticut, Georgia, Illinois (one outbreak), Kentucky, New Hampshire, New Jersey, New York (two outbreaks), Oregon, Texas (one outbreak), and Washington (one outbreak), the CDC reported March 18.
Brooklyn, N.Y., has become the epicenter of measles activity since mid-February, and with 25 of the 40 new cases occurring there, the borough has now led the nation for four consecutive weeks. There have been 157 confirmed cases in Brooklyn and one in Queens since the outbreak began in October of 2018, the New York City Department of Health and Mental Hygiene reported.
Michigan’s first case of measles is travel related and involved an individual visiting from Israel following a stay in New York, the Michigan Department of Health and Human Services and Oakland County Health Division announced March 13.
In Arizona, the state department of health services and the Pima County Public Health Department announced that a 12-month-old infant from Pima County has been diagnosed with measles after traveling to Asia.
A single case of measles, contracted while the person was traveling out of state, has been reported in Jefferson County, Mo., and is being managed by the county health department, according to St. Louis Public Radio.
The New Gastroenterologist seeks its next editor-in-chief
AGA’s cutting-edge, trainee and early-career focused e-newsletter The New Gastroenterologist (TNG) is seeking applications for the position of editor-in-chief (EIC). The role will facilitate the communication of the latest clinical advances among peers and build strong leadership skills managing editorial responsibilities as well as working with reviewers and fellow editors at AGA’s journals.
The term is from Oct. 1, 2019 – Sept. 30, 2022, with a transition period starting July 2019.

About TNG
TNG content covers highly relevant clinical topics, such as endoscopic management of obesity and quality metrics on colonoscopy. Also included in each issue are articles that focus on career pathways, financial and legal matters, perspectives from private practice, and other topics that are relevant to early-career GIs.
Honorarium
The EIC will receive an annual honorarium of $5,000.
Qualifications
• AGA member, between second year of fellowship and five years post-fellowship.
• Experience identifying and promoting newsworthy content that is relevant to the trainee and early-career GI community, as well as excellent judgment that expands the outstanding reputation of TNG and AGA.
• Experience in medical, scientific or news-related publishing is preferred, but not required.
• Familiarity with AGA and its priorities, activities and stances on important issues is ideal, preferably via past volunteer member experience with the association.
• The EIC must be able to devote sufficient time to TNG matters and may not accept editorial appointments to competing publications during their tenure as EIC.
For more information or to apply view the full request for application.
If you have questions, please contact Ryan Farrell, managing editor, The New Gastroenterologist, at rfarrell@gastro.org.
AGA’s cutting-edge, trainee and early-career focused e-newsletter The New Gastroenterologist (TNG) is seeking applications for the position of editor-in-chief (EIC). The role will facilitate the communication of the latest clinical advances among peers and build strong leadership skills managing editorial responsibilities as well as working with reviewers and fellow editors at AGA’s journals.
The term is from Oct. 1, 2019 – Sept. 30, 2022, with a transition period starting July 2019.

About TNG
TNG content covers highly relevant clinical topics, such as endoscopic management of obesity and quality metrics on colonoscopy. Also included in each issue are articles that focus on career pathways, financial and legal matters, perspectives from private practice, and other topics that are relevant to early-career GIs.
Honorarium
The EIC will receive an annual honorarium of $5,000.
Qualifications
• AGA member, between second year of fellowship and five years post-fellowship.
• Experience identifying and promoting newsworthy content that is relevant to the trainee and early-career GI community, as well as excellent judgment that expands the outstanding reputation of TNG and AGA.
• Experience in medical, scientific or news-related publishing is preferred, but not required.
• Familiarity with AGA and its priorities, activities and stances on important issues is ideal, preferably via past volunteer member experience with the association.
• The EIC must be able to devote sufficient time to TNG matters and may not accept editorial appointments to competing publications during their tenure as EIC.
For more information or to apply view the full request for application.
If you have questions, please contact Ryan Farrell, managing editor, The New Gastroenterologist, at rfarrell@gastro.org.
AGA’s cutting-edge, trainee and early-career focused e-newsletter The New Gastroenterologist (TNG) is seeking applications for the position of editor-in-chief (EIC). The role will facilitate the communication of the latest clinical advances among peers and build strong leadership skills managing editorial responsibilities as well as working with reviewers and fellow editors at AGA’s journals.
The term is from Oct. 1, 2019 – Sept. 30, 2022, with a transition period starting July 2019.

About TNG
TNG content covers highly relevant clinical topics, such as endoscopic management of obesity and quality metrics on colonoscopy. Also included in each issue are articles that focus on career pathways, financial and legal matters, perspectives from private practice, and other topics that are relevant to early-career GIs.
Honorarium
The EIC will receive an annual honorarium of $5,000.
Qualifications
• AGA member, between second year of fellowship and five years post-fellowship.
• Experience identifying and promoting newsworthy content that is relevant to the trainee and early-career GI community, as well as excellent judgment that expands the outstanding reputation of TNG and AGA.
• Experience in medical, scientific or news-related publishing is preferred, but not required.
• Familiarity with AGA and its priorities, activities and stances on important issues is ideal, preferably via past volunteer member experience with the association.
• The EIC must be able to devote sufficient time to TNG matters and may not accept editorial appointments to competing publications during their tenure as EIC.
For more information or to apply view the full request for application.
If you have questions, please contact Ryan Farrell, managing editor, The New Gastroenterologist, at rfarrell@gastro.org.
Survey: Americans support regulation of vaping products
Almost 70% of adults believe that the Food and Drug Administration should raise the legal age to purchase e-cigarettes and tobacco, according to a new survey by NORC at the University of Chicago, a nonpartisan research institution.
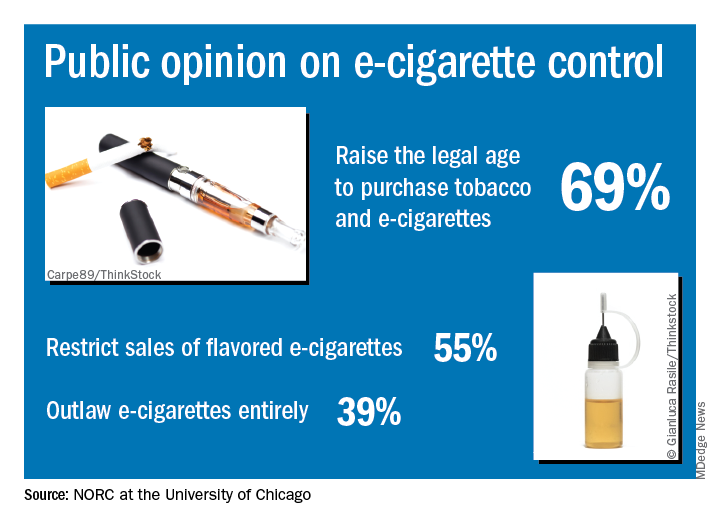
“Americans are particularly concerned about teens becoming newly addicted to e-cigarettes, and they support a range of actions the federal government could take to make vaping products less available, less addictive, and less appealing,” Caroline Pearson, senior vice president at NORC, said in a written statement.
The AmeriSpeak Spotlight on Health Poll, conducted Feb. 14-18, 2019 (margin of error, plus or minus 4.12%), showed that 69% of adults strongly or somewhat support raising the age limit to purchase e-cigarettes and tobacco and 55% support restricting sales of flavored e-cigarettes, NORC reported. Almost 40% of the 1,004 respondents expressed support for a complete ban on e-cigarettes.
Despite FDA efforts under Commissioner Scott Gottlieb, MD, to raise awareness of teen vaping, only 21% of those surveyed correctly responded that e-cigarettes generally contain more nicotine that regular cigarettes. Dr. Gottlieb announced his resignation recently, “but he indicated that the Trump Administration will continue efforts to increase regulation of e-cigarettes,” NORC said.
Almost 70% of adults believe that the Food and Drug Administration should raise the legal age to purchase e-cigarettes and tobacco, according to a new survey by NORC at the University of Chicago, a nonpartisan research institution.

“Americans are particularly concerned about teens becoming newly addicted to e-cigarettes, and they support a range of actions the federal government could take to make vaping products less available, less addictive, and less appealing,” Caroline Pearson, senior vice president at NORC, said in a written statement.
The AmeriSpeak Spotlight on Health Poll, conducted Feb. 14-18, 2019 (margin of error, plus or minus 4.12%), showed that 69% of adults strongly or somewhat support raising the age limit to purchase e-cigarettes and tobacco and 55% support restricting sales of flavored e-cigarettes, NORC reported. Almost 40% of the 1,004 respondents expressed support for a complete ban on e-cigarettes.
Despite FDA efforts under Commissioner Scott Gottlieb, MD, to raise awareness of teen vaping, only 21% of those surveyed correctly responded that e-cigarettes generally contain more nicotine that regular cigarettes. Dr. Gottlieb announced his resignation recently, “but he indicated that the Trump Administration will continue efforts to increase regulation of e-cigarettes,” NORC said.
Almost 70% of adults believe that the Food and Drug Administration should raise the legal age to purchase e-cigarettes and tobacco, according to a new survey by NORC at the University of Chicago, a nonpartisan research institution.

“Americans are particularly concerned about teens becoming newly addicted to e-cigarettes, and they support a range of actions the federal government could take to make vaping products less available, less addictive, and less appealing,” Caroline Pearson, senior vice president at NORC, said in a written statement.
The AmeriSpeak Spotlight on Health Poll, conducted Feb. 14-18, 2019 (margin of error, plus or minus 4.12%), showed that 69% of adults strongly or somewhat support raising the age limit to purchase e-cigarettes and tobacco and 55% support restricting sales of flavored e-cigarettes, NORC reported. Almost 40% of the 1,004 respondents expressed support for a complete ban on e-cigarettes.
Despite FDA efforts under Commissioner Scott Gottlieb, MD, to raise awareness of teen vaping, only 21% of those surveyed correctly responded that e-cigarettes generally contain more nicotine that regular cigarettes. Dr. Gottlieb announced his resignation recently, “but he indicated that the Trump Administration will continue efforts to increase regulation of e-cigarettes,” NORC said.
New data bolster latitude’s association with MS prevalence
DALLAS – Latitude continues to be associated with the prevalence of multiple sclerosis (MS), according to an updated meta-analysis presented at ACTRIMS Forum 2019, held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
After integrating data from 94 new studies, “the latitudinal gradient in MS prevalence ... significantly enhanced in magnitude,” said presenting author Steve Simpson Jr., PhD, and his colleagues. Dr. Simpson is a researcher at the Melbourne School of Population & Global Health at the University of Melbourne and the Menzies Institute for Medical Research at the University of Tasmania.
The researchers’ original study, published in 2011, found a slope of 2.68.
Latitudinal variations in MS prevalence have given credence to the theory that sun exposure and vitamin D may play a role in MS onset. The researchers’ original meta-analysis – based on 650 prevalence estimates from 321 studies – “confirmed the existence of a robust latitudinal gradient,” they said. To examine whether the gradient has changed, the researchers identified relevant studies published between 2010 and 2018.
They included complete, peer-reviewed articles in their analysis and extracted data about the study area, prevalence year, MS diagnostic criteria used, sample size, source population, crude prevalence, and age-specific prevalence. They combined the new prevalence estimates with those from their original study. The estimates were log-transformed and weighted with the inverse of the variance. In addition, the researchers used random-effects meta-regression models that adjusted for prevalence year and method of case ascertainment.
Their literature review identified 126 new studies, 94 of which met inclusion criteria. The new studies yielded 230 additional prevalence points, predominantly in Scandinavia, France, and the Middle East.
“Latitude was consistently and significantly associated with MS prevalence in all analyses, increasing in magnitude on adjustment and persisting on age-standardization,” Dr. Simpson and his colleagues reported.
By region, strong and significant positive gradients continue to exist in Australasia, the United Kingdom/Ireland, and North America. Meanwhile, a significant inverse gradient continues to exist in the Italian region, which the researchers have said relates to the frequency of an MS-related HLA-DRB1 allele there. A negative gradient in the Scandinavian/North Atlantic region in the original meta-analysis, considered potentially related to dietary differences, was “markedly reduced” and no longer statistically significant in the updated meta-analysis.
“While there are potential intra-regional effects contributing to the latitudinal variation in MS prevalence, these results and the relative consistency across the whole of the globe continue to provide indirect evidence in support of the role of sun and vitamin D in MS etiology,” Dr. Simpson and his colleagues concluded.
The researchers had no disclosures.
SOURCE: Simpson S Jr et al. ACTRIMS Forum 2019, Abstract 126.
DALLAS – Latitude continues to be associated with the prevalence of multiple sclerosis (MS), according to an updated meta-analysis presented at ACTRIMS Forum 2019, held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
After integrating data from 94 new studies, “the latitudinal gradient in MS prevalence ... significantly enhanced in magnitude,” said presenting author Steve Simpson Jr., PhD, and his colleagues. Dr. Simpson is a researcher at the Melbourne School of Population & Global Health at the University of Melbourne and the Menzies Institute for Medical Research at the University of Tasmania.
The researchers’ original study, published in 2011, found a slope of 2.68.
Latitudinal variations in MS prevalence have given credence to the theory that sun exposure and vitamin D may play a role in MS onset. The researchers’ original meta-analysis – based on 650 prevalence estimates from 321 studies – “confirmed the existence of a robust latitudinal gradient,” they said. To examine whether the gradient has changed, the researchers identified relevant studies published between 2010 and 2018.
They included complete, peer-reviewed articles in their analysis and extracted data about the study area, prevalence year, MS diagnostic criteria used, sample size, source population, crude prevalence, and age-specific prevalence. They combined the new prevalence estimates with those from their original study. The estimates were log-transformed and weighted with the inverse of the variance. In addition, the researchers used random-effects meta-regression models that adjusted for prevalence year and method of case ascertainment.
Their literature review identified 126 new studies, 94 of which met inclusion criteria. The new studies yielded 230 additional prevalence points, predominantly in Scandinavia, France, and the Middle East.
“Latitude was consistently and significantly associated with MS prevalence in all analyses, increasing in magnitude on adjustment and persisting on age-standardization,” Dr. Simpson and his colleagues reported.
By region, strong and significant positive gradients continue to exist in Australasia, the United Kingdom/Ireland, and North America. Meanwhile, a significant inverse gradient continues to exist in the Italian region, which the researchers have said relates to the frequency of an MS-related HLA-DRB1 allele there. A negative gradient in the Scandinavian/North Atlantic region in the original meta-analysis, considered potentially related to dietary differences, was “markedly reduced” and no longer statistically significant in the updated meta-analysis.
“While there are potential intra-regional effects contributing to the latitudinal variation in MS prevalence, these results and the relative consistency across the whole of the globe continue to provide indirect evidence in support of the role of sun and vitamin D in MS etiology,” Dr. Simpson and his colleagues concluded.
The researchers had no disclosures.
SOURCE: Simpson S Jr et al. ACTRIMS Forum 2019, Abstract 126.
DALLAS – Latitude continues to be associated with the prevalence of multiple sclerosis (MS), according to an updated meta-analysis presented at ACTRIMS Forum 2019, held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
After integrating data from 94 new studies, “the latitudinal gradient in MS prevalence ... significantly enhanced in magnitude,” said presenting author Steve Simpson Jr., PhD, and his colleagues. Dr. Simpson is a researcher at the Melbourne School of Population & Global Health at the University of Melbourne and the Menzies Institute for Medical Research at the University of Tasmania.
The researchers’ original study, published in 2011, found a slope of 2.68.
Latitudinal variations in MS prevalence have given credence to the theory that sun exposure and vitamin D may play a role in MS onset. The researchers’ original meta-analysis – based on 650 prevalence estimates from 321 studies – “confirmed the existence of a robust latitudinal gradient,” they said. To examine whether the gradient has changed, the researchers identified relevant studies published between 2010 and 2018.
They included complete, peer-reviewed articles in their analysis and extracted data about the study area, prevalence year, MS diagnostic criteria used, sample size, source population, crude prevalence, and age-specific prevalence. They combined the new prevalence estimates with those from their original study. The estimates were log-transformed and weighted with the inverse of the variance. In addition, the researchers used random-effects meta-regression models that adjusted for prevalence year and method of case ascertainment.
Their literature review identified 126 new studies, 94 of which met inclusion criteria. The new studies yielded 230 additional prevalence points, predominantly in Scandinavia, France, and the Middle East.
“Latitude was consistently and significantly associated with MS prevalence in all analyses, increasing in magnitude on adjustment and persisting on age-standardization,” Dr. Simpson and his colleagues reported.
By region, strong and significant positive gradients continue to exist in Australasia, the United Kingdom/Ireland, and North America. Meanwhile, a significant inverse gradient continues to exist in the Italian region, which the researchers have said relates to the frequency of an MS-related HLA-DRB1 allele there. A negative gradient in the Scandinavian/North Atlantic region in the original meta-analysis, considered potentially related to dietary differences, was “markedly reduced” and no longer statistically significant in the updated meta-analysis.
“While there are potential intra-regional effects contributing to the latitudinal variation in MS prevalence, these results and the relative consistency across the whole of the globe continue to provide indirect evidence in support of the role of sun and vitamin D in MS etiology,” Dr. Simpson and his colleagues concluded.
The researchers had no disclosures.
SOURCE: Simpson S Jr et al. ACTRIMS Forum 2019, Abstract 126.
REPORTING FROM ACTRIMS FORUM 2019
Key clinical point: Latitude continues to be associated with the prevalence of multiple sclerosis (MS).
Major finding: For every degree of latitude increase, prevalence of MS per 100,000 people increased by 3.64 in a fully adjusted, age-standardized model.
Study details: A meta-analysis of data from more than 400 studies.
Disclosures: The investigators had no disclosures.
Source: Simpson S Jr et al. ACTRIMS Forum 2019, Abstract 126.
Sex differences in MS: It’s the chromosomes, not just the hormones
DALLAS – Hormonal differences are not the only reason that multiple sclerosis (MS) disease progression and severity differ between the sexes, according to Rhonda Voskuhl, MD, who delivered the Kenneth P. Johnson Memorial Lecture at a meeting of the Americas Committee for Treatment and Research in Multiple Sclerosis.
“Sex differences in disease are widely prevalent across immunological and neurological diseases. For example, lupus affects women 9:1 more frequently, rheumatoid arthritis is about 3:1, and MS is 3:1,” said Dr. Voskuhl, director of the MS program and Jack H. Skirball Chair of Multiple Sclerosis Research at the University of California, Los Angeles.
However, although women are more likely to experience these diseases, men are often more severely affected by them, Dr. Voskuhl said. “Sometimes in neurodegenerative diseases like MS, we’re seeing that the men, although they get it less frequently, they do worse. ... So these are actually two very important sex differences in disease, one affecting susceptibility and frequency, and the other affecting how they do over the long run with respect to their progression and severity.”
This clinically apparent observation, known for decades, prompted Dr. Voskuhl and others to parse why sex differences exist in this gamut of diseases.
A novel animal model – the four-core genotype mouse model – has allowed Dr. Voskuhl and others to discern the contributions of hormonal versus chromosomal influences on disease susceptibility and progression. The model separates the sex chromosome complement (XX or XY) from gonadal influences, and it’s been extremely helpful in revealing the surprising influence that sex chromosomes play in MS and similar diseases, said Dr. Voskuhl in an interview.
Dr. Voskuhl is also the president-elect of the Organization for the Study of Sex Differences.
DALLAS – Hormonal differences are not the only reason that multiple sclerosis (MS) disease progression and severity differ between the sexes, according to Rhonda Voskuhl, MD, who delivered the Kenneth P. Johnson Memorial Lecture at a meeting of the Americas Committee for Treatment and Research in Multiple Sclerosis.
“Sex differences in disease are widely prevalent across immunological and neurological diseases. For example, lupus affects women 9:1 more frequently, rheumatoid arthritis is about 3:1, and MS is 3:1,” said Dr. Voskuhl, director of the MS program and Jack H. Skirball Chair of Multiple Sclerosis Research at the University of California, Los Angeles.
However, although women are more likely to experience these diseases, men are often more severely affected by them, Dr. Voskuhl said. “Sometimes in neurodegenerative diseases like MS, we’re seeing that the men, although they get it less frequently, they do worse. ... So these are actually two very important sex differences in disease, one affecting susceptibility and frequency, and the other affecting how they do over the long run with respect to their progression and severity.”
This clinically apparent observation, known for decades, prompted Dr. Voskuhl and others to parse why sex differences exist in this gamut of diseases.
A novel animal model – the four-core genotype mouse model – has allowed Dr. Voskuhl and others to discern the contributions of hormonal versus chromosomal influences on disease susceptibility and progression. The model separates the sex chromosome complement (XX or XY) from gonadal influences, and it’s been extremely helpful in revealing the surprising influence that sex chromosomes play in MS and similar diseases, said Dr. Voskuhl in an interview.
Dr. Voskuhl is also the president-elect of the Organization for the Study of Sex Differences.
DALLAS – Hormonal differences are not the only reason that multiple sclerosis (MS) disease progression and severity differ between the sexes, according to Rhonda Voskuhl, MD, who delivered the Kenneth P. Johnson Memorial Lecture at a meeting of the Americas Committee for Treatment and Research in Multiple Sclerosis.
“Sex differences in disease are widely prevalent across immunological and neurological diseases. For example, lupus affects women 9:1 more frequently, rheumatoid arthritis is about 3:1, and MS is 3:1,” said Dr. Voskuhl, director of the MS program and Jack H. Skirball Chair of Multiple Sclerosis Research at the University of California, Los Angeles.
However, although women are more likely to experience these diseases, men are often more severely affected by them, Dr. Voskuhl said. “Sometimes in neurodegenerative diseases like MS, we’re seeing that the men, although they get it less frequently, they do worse. ... So these are actually two very important sex differences in disease, one affecting susceptibility and frequency, and the other affecting how they do over the long run with respect to their progression and severity.”
This clinically apparent observation, known for decades, prompted Dr. Voskuhl and others to parse why sex differences exist in this gamut of diseases.
A novel animal model – the four-core genotype mouse model – has allowed Dr. Voskuhl and others to discern the contributions of hormonal versus chromosomal influences on disease susceptibility and progression. The model separates the sex chromosome complement (XX or XY) from gonadal influences, and it’s been extremely helpful in revealing the surprising influence that sex chromosomes play in MS and similar diseases, said Dr. Voskuhl in an interview.
Dr. Voskuhl is also the president-elect of the Organization for the Study of Sex Differences.
REPORTING FROM ACTRIMS FORUM 2019
Venetoclax and obinutuzumab induces deep responses in CLL
The combination of venetoclax and obinutuzumab provided high response rates and deep remissions regardless of cytogenetic risk factors in patients with chronic lymphocytic leukemia, according to recently reported results of a phase 1b study.
The regimen elicited high rates of undetectable minimal residual disease in peripheral blood and had an acceptable safety profile with manageable toxicities in the study reported in Blood, which included patients with previously untreated or relapsed/refractory chronic lymphocytic leukemia (CLL).
“The deep remission rates we observed with venetoclax-obinutuzumab have not been reported with previously available CLL treatments, including FCR [fludarabine, cyclophosphamide, and rituximab], which is currently considered the most efficacious regimen with limited-duration therapy,” wrote the investigators, led by Ian W. Flinn, MD, PhD, of Sarah Cannon Research Institute/Tennessee Oncology, Nashville.
Venetoclax-obinutuzumab combinations are meanwhile being tested in other studies – including the phase 3 CLL13 and CLL14 studies – which have enrolled previously untreated fit or unfit CLL patients, respectively.
“If the primary endpoints of these large-scale trials are met, venetoclax-obinutuzumab may become a new standard treatment option in [first-line] CLL, irrespective of clinical fitness,” Dr. Flinn and his colleagues wrote in their report.
The present phase 1b, dose-escalation study enrolled 32 patients who were previously untreated (median age, 63 years) and 46 patients who were relapsed or refractory to previous treatments (median age, 61 years).
Doses of venetoclax were escalated from 100 mg to 400 mg to determine its maximum tolerated dose when combined with obinutuzumab, the investigators wrote. Some patients received venetoclax first, while others received obinutuzumab first, for a total of 1 year of treatment.
The study confirmed favorable risk-benefit treatment used a dose of 400 mg venetoclax plus the standard dose of obinutuzumab, according to the researchers.
The overall best response rate was 95% for relapsed/refractory patients, including a 37% rate of complete response or complete response with incomplete marrow recovery. In previously untreated patients, the overall best response rate was 100%, including a 78% rate of complete responses by those criteria.
Undetectable minimal residual disease was observed in 64% of relapsed/refractory patients and 91% of previously untreated patients at 3 months after the last obinutuzumab dose, the investigators reported.
There were no dose-limiting toxicities in the study, no clinical tumor lysis syndrome, and no differences between the two schedules (venetoclax first or obinutuzumab first) in terms of adverse events, the investigators wrote.
Neutropenia was the most common serious (grade 3-4) adverse event, occurring in 58% of relapsed/refractory patients and 53% of patients treated in the first line. Grade 3-4 infections were seen in 29% and 13% of the relapsed/refractory and previously untreated patients, respectively.
There were no fatal infections among previously untreated patients, while three relapsed/refractory patients (7%) had fatal adverse events, including one case of acute respiratory failure in a patient with suspected Richter’s transformation, pneumonia in a patient with metastatic squamous cell lung carcinoma, and another case of pneumonia occurring about 3 months after the last dose of venetoclax.
Genentech and AbbVie provided financial support for the study. Dr. Flinn reported receiving research funding for his institution from Genentech, AbbVie, and several other companies.
SOURCE: Flinn IW et al. Blood. 2019 Mar 12. doi: 10.1182/blood-2019-01-896290.
The combination of venetoclax and obinutuzumab provided high response rates and deep remissions regardless of cytogenetic risk factors in patients with chronic lymphocytic leukemia, according to recently reported results of a phase 1b study.
The regimen elicited high rates of undetectable minimal residual disease in peripheral blood and had an acceptable safety profile with manageable toxicities in the study reported in Blood, which included patients with previously untreated or relapsed/refractory chronic lymphocytic leukemia (CLL).
“The deep remission rates we observed with venetoclax-obinutuzumab have not been reported with previously available CLL treatments, including FCR [fludarabine, cyclophosphamide, and rituximab], which is currently considered the most efficacious regimen with limited-duration therapy,” wrote the investigators, led by Ian W. Flinn, MD, PhD, of Sarah Cannon Research Institute/Tennessee Oncology, Nashville.
Venetoclax-obinutuzumab combinations are meanwhile being tested in other studies – including the phase 3 CLL13 and CLL14 studies – which have enrolled previously untreated fit or unfit CLL patients, respectively.
“If the primary endpoints of these large-scale trials are met, venetoclax-obinutuzumab may become a new standard treatment option in [first-line] CLL, irrespective of clinical fitness,” Dr. Flinn and his colleagues wrote in their report.
The present phase 1b, dose-escalation study enrolled 32 patients who were previously untreated (median age, 63 years) and 46 patients who were relapsed or refractory to previous treatments (median age, 61 years).
Doses of venetoclax were escalated from 100 mg to 400 mg to determine its maximum tolerated dose when combined with obinutuzumab, the investigators wrote. Some patients received venetoclax first, while others received obinutuzumab first, for a total of 1 year of treatment.
The study confirmed favorable risk-benefit treatment used a dose of 400 mg venetoclax plus the standard dose of obinutuzumab, according to the researchers.
The overall best response rate was 95% for relapsed/refractory patients, including a 37% rate of complete response or complete response with incomplete marrow recovery. In previously untreated patients, the overall best response rate was 100%, including a 78% rate of complete responses by those criteria.
Undetectable minimal residual disease was observed in 64% of relapsed/refractory patients and 91% of previously untreated patients at 3 months after the last obinutuzumab dose, the investigators reported.
There were no dose-limiting toxicities in the study, no clinical tumor lysis syndrome, and no differences between the two schedules (venetoclax first or obinutuzumab first) in terms of adverse events, the investigators wrote.
Neutropenia was the most common serious (grade 3-4) adverse event, occurring in 58% of relapsed/refractory patients and 53% of patients treated in the first line. Grade 3-4 infections were seen in 29% and 13% of the relapsed/refractory and previously untreated patients, respectively.
There were no fatal infections among previously untreated patients, while three relapsed/refractory patients (7%) had fatal adverse events, including one case of acute respiratory failure in a patient with suspected Richter’s transformation, pneumonia in a patient with metastatic squamous cell lung carcinoma, and another case of pneumonia occurring about 3 months after the last dose of venetoclax.
Genentech and AbbVie provided financial support for the study. Dr. Flinn reported receiving research funding for his institution from Genentech, AbbVie, and several other companies.
SOURCE: Flinn IW et al. Blood. 2019 Mar 12. doi: 10.1182/blood-2019-01-896290.
The combination of venetoclax and obinutuzumab provided high response rates and deep remissions regardless of cytogenetic risk factors in patients with chronic lymphocytic leukemia, according to recently reported results of a phase 1b study.
The regimen elicited high rates of undetectable minimal residual disease in peripheral blood and had an acceptable safety profile with manageable toxicities in the study reported in Blood, which included patients with previously untreated or relapsed/refractory chronic lymphocytic leukemia (CLL).
“The deep remission rates we observed with venetoclax-obinutuzumab have not been reported with previously available CLL treatments, including FCR [fludarabine, cyclophosphamide, and rituximab], which is currently considered the most efficacious regimen with limited-duration therapy,” wrote the investigators, led by Ian W. Flinn, MD, PhD, of Sarah Cannon Research Institute/Tennessee Oncology, Nashville.
Venetoclax-obinutuzumab combinations are meanwhile being tested in other studies – including the phase 3 CLL13 and CLL14 studies – which have enrolled previously untreated fit or unfit CLL patients, respectively.
“If the primary endpoints of these large-scale trials are met, venetoclax-obinutuzumab may become a new standard treatment option in [first-line] CLL, irrespective of clinical fitness,” Dr. Flinn and his colleagues wrote in their report.
The present phase 1b, dose-escalation study enrolled 32 patients who were previously untreated (median age, 63 years) and 46 patients who were relapsed or refractory to previous treatments (median age, 61 years).
Doses of venetoclax were escalated from 100 mg to 400 mg to determine its maximum tolerated dose when combined with obinutuzumab, the investigators wrote. Some patients received venetoclax first, while others received obinutuzumab first, for a total of 1 year of treatment.
The study confirmed favorable risk-benefit treatment used a dose of 400 mg venetoclax plus the standard dose of obinutuzumab, according to the researchers.
The overall best response rate was 95% for relapsed/refractory patients, including a 37% rate of complete response or complete response with incomplete marrow recovery. In previously untreated patients, the overall best response rate was 100%, including a 78% rate of complete responses by those criteria.
Undetectable minimal residual disease was observed in 64% of relapsed/refractory patients and 91% of previously untreated patients at 3 months after the last obinutuzumab dose, the investigators reported.
There were no dose-limiting toxicities in the study, no clinical tumor lysis syndrome, and no differences between the two schedules (venetoclax first or obinutuzumab first) in terms of adverse events, the investigators wrote.
Neutropenia was the most common serious (grade 3-4) adverse event, occurring in 58% of relapsed/refractory patients and 53% of patients treated in the first line. Grade 3-4 infections were seen in 29% and 13% of the relapsed/refractory and previously untreated patients, respectively.
There were no fatal infections among previously untreated patients, while three relapsed/refractory patients (7%) had fatal adverse events, including one case of acute respiratory failure in a patient with suspected Richter’s transformation, pneumonia in a patient with metastatic squamous cell lung carcinoma, and another case of pneumonia occurring about 3 months after the last dose of venetoclax.
Genentech and AbbVie provided financial support for the study. Dr. Flinn reported receiving research funding for his institution from Genentech, AbbVie, and several other companies.
SOURCE: Flinn IW et al. Blood. 2019 Mar 12. doi: 10.1182/blood-2019-01-896290.
FROM BLOOD
Breast cancer survivors offer realistic strategies for easing cost burden
A qualitative study representing the patient perspective provides insight on reducing economic burden after breast cancer, including specific recommendations for changes to insurance, supportive services, financial assistance, and protective policies.
As part of a 6-month observational study conducted in 2015, Lorraine T. Dean, ScD, of Johns Hopkins Schools of Public Health and Medicine, Baltimore, and her associates, interviewed 40 women diagnosed with invasive stage I-III breast cancer who had completed active cancer treatment. All patients, who reported having more than one lymph node removed resided in Pennsylvania or New Jersey. The mean age of the women was 64 years.
Of those interviewed, 53% were white; 42.5% were black. More than half of participants (53%) were college graduates or had received a graduate degree. Annual income for 58% of the patients ranged from $30,000 to $70,000; 11% earned under $30,000. All participants included in the study were insured, including 82.5% who had private insurance. The patients had been diagnosed a mean of 12 years prior. Breast cancer–related lymphedema was reported in 60% of patients, Dr. Dean and her associates reported in a report published in Cancer.
Among the 40 participants, 27 made recommendations for easing economic burden, including nine key recommendations across four significant areas: insurance, supportive services and care, financial assistance, and protective policies. These findings are consistent with previous studies that examined patient recommendations, but they address additional areas where cost-saving services and policies could be offered or improved upon, the investigators noted.
Insurance-related recommendations included offering more complementary and integrative treatments as well as helping patients understand what insurance plans cover and how to adjust to changes under new insurance plans. Providing high-quality plans with low copays, premiums, and deductibles that cover required as well as elective cancer-related services, and covering lymphedema-related materials and treatments also were flagged as important.
Supportive service recommendations included addressing psychosocial costs through expansion of support groups and buddy services, offering extended home health services following cancer treatment, and providing domestic assistance with household chores, child care, and transportation.
Financial assistance that broadens financial aid and social services eligibility to those not classified as being in poverty was considered important.
Protective policy recommendations focused on expanding employment and medical leave policies concerning the amount of time offered off from work.
Patient recommendations offer just one viewpoint concerning potential challenges to the overall system, but “their thoughts on how it can be improved add value to decision-making processes,” noted Dr. Dean and her associates.
They were careful to acknowledge the benefits of the Patient Protection and Affordable Care Act, but they noted that it does not include provisions to address the adverse treatment effects of conditions such as cancer. While some states already have successfully passed legislation requiring private insurance carriers to cover lymphedema treatment, similar legislation should be adopted at a national level through joint efforts of Congress and the Department of Labor, they advised.
Any such efforts to make sweeping changes within the insurance industry would take considerable effort on the part of patients, providers, insurers, and state and federal policy makers, as well as the pharmaceutical industry. Yet, such “top-down and bottom-up strategies that involve all parties are warranted,” they urged.
Several important limitations of the study are worth noting. All participants were from the East Coast, had insurance coverage, and reported an overall low level of economic burden. Responses may have differed had the study been conducted in other regions of the country. The study was voluntary, so it is important to consider that patients with greater financial challenges may not have had time to enroll and participate, which suggests that the level of economic burden affecting this population actually could be understated.
SOURCE: Dean LT et al. Cancer 2019 Mar 6. doi: 10.1002/cncr.32012.
A qualitative study representing the patient perspective provides insight on reducing economic burden after breast cancer, including specific recommendations for changes to insurance, supportive services, financial assistance, and protective policies.
As part of a 6-month observational study conducted in 2015, Lorraine T. Dean, ScD, of Johns Hopkins Schools of Public Health and Medicine, Baltimore, and her associates, interviewed 40 women diagnosed with invasive stage I-III breast cancer who had completed active cancer treatment. All patients, who reported having more than one lymph node removed resided in Pennsylvania or New Jersey. The mean age of the women was 64 years.
Of those interviewed, 53% were white; 42.5% were black. More than half of participants (53%) were college graduates or had received a graduate degree. Annual income for 58% of the patients ranged from $30,000 to $70,000; 11% earned under $30,000. All participants included in the study were insured, including 82.5% who had private insurance. The patients had been diagnosed a mean of 12 years prior. Breast cancer–related lymphedema was reported in 60% of patients, Dr. Dean and her associates reported in a report published in Cancer.
Among the 40 participants, 27 made recommendations for easing economic burden, including nine key recommendations across four significant areas: insurance, supportive services and care, financial assistance, and protective policies. These findings are consistent with previous studies that examined patient recommendations, but they address additional areas where cost-saving services and policies could be offered or improved upon, the investigators noted.
Insurance-related recommendations included offering more complementary and integrative treatments as well as helping patients understand what insurance plans cover and how to adjust to changes under new insurance plans. Providing high-quality plans with low copays, premiums, and deductibles that cover required as well as elective cancer-related services, and covering lymphedema-related materials and treatments also were flagged as important.
Supportive service recommendations included addressing psychosocial costs through expansion of support groups and buddy services, offering extended home health services following cancer treatment, and providing domestic assistance with household chores, child care, and transportation.
Financial assistance that broadens financial aid and social services eligibility to those not classified as being in poverty was considered important.
Protective policy recommendations focused on expanding employment and medical leave policies concerning the amount of time offered off from work.
Patient recommendations offer just one viewpoint concerning potential challenges to the overall system, but “their thoughts on how it can be improved add value to decision-making processes,” noted Dr. Dean and her associates.
They were careful to acknowledge the benefits of the Patient Protection and Affordable Care Act, but they noted that it does not include provisions to address the adverse treatment effects of conditions such as cancer. While some states already have successfully passed legislation requiring private insurance carriers to cover lymphedema treatment, similar legislation should be adopted at a national level through joint efforts of Congress and the Department of Labor, they advised.
Any such efforts to make sweeping changes within the insurance industry would take considerable effort on the part of patients, providers, insurers, and state and federal policy makers, as well as the pharmaceutical industry. Yet, such “top-down and bottom-up strategies that involve all parties are warranted,” they urged.
Several important limitations of the study are worth noting. All participants were from the East Coast, had insurance coverage, and reported an overall low level of economic burden. Responses may have differed had the study been conducted in other regions of the country. The study was voluntary, so it is important to consider that patients with greater financial challenges may not have had time to enroll and participate, which suggests that the level of economic burden affecting this population actually could be understated.
SOURCE: Dean LT et al. Cancer 2019 Mar 6. doi: 10.1002/cncr.32012.
A qualitative study representing the patient perspective provides insight on reducing economic burden after breast cancer, including specific recommendations for changes to insurance, supportive services, financial assistance, and protective policies.
As part of a 6-month observational study conducted in 2015, Lorraine T. Dean, ScD, of Johns Hopkins Schools of Public Health and Medicine, Baltimore, and her associates, interviewed 40 women diagnosed with invasive stage I-III breast cancer who had completed active cancer treatment. All patients, who reported having more than one lymph node removed resided in Pennsylvania or New Jersey. The mean age of the women was 64 years.
Of those interviewed, 53% were white; 42.5% were black. More than half of participants (53%) were college graduates or had received a graduate degree. Annual income for 58% of the patients ranged from $30,000 to $70,000; 11% earned under $30,000. All participants included in the study were insured, including 82.5% who had private insurance. The patients had been diagnosed a mean of 12 years prior. Breast cancer–related lymphedema was reported in 60% of patients, Dr. Dean and her associates reported in a report published in Cancer.
Among the 40 participants, 27 made recommendations for easing economic burden, including nine key recommendations across four significant areas: insurance, supportive services and care, financial assistance, and protective policies. These findings are consistent with previous studies that examined patient recommendations, but they address additional areas where cost-saving services and policies could be offered or improved upon, the investigators noted.
Insurance-related recommendations included offering more complementary and integrative treatments as well as helping patients understand what insurance plans cover and how to adjust to changes under new insurance plans. Providing high-quality plans with low copays, premiums, and deductibles that cover required as well as elective cancer-related services, and covering lymphedema-related materials and treatments also were flagged as important.
Supportive service recommendations included addressing psychosocial costs through expansion of support groups and buddy services, offering extended home health services following cancer treatment, and providing domestic assistance with household chores, child care, and transportation.
Financial assistance that broadens financial aid and social services eligibility to those not classified as being in poverty was considered important.
Protective policy recommendations focused on expanding employment and medical leave policies concerning the amount of time offered off from work.
Patient recommendations offer just one viewpoint concerning potential challenges to the overall system, but “their thoughts on how it can be improved add value to decision-making processes,” noted Dr. Dean and her associates.
They were careful to acknowledge the benefits of the Patient Protection and Affordable Care Act, but they noted that it does not include provisions to address the adverse treatment effects of conditions such as cancer. While some states already have successfully passed legislation requiring private insurance carriers to cover lymphedema treatment, similar legislation should be adopted at a national level through joint efforts of Congress and the Department of Labor, they advised.
Any such efforts to make sweeping changes within the insurance industry would take considerable effort on the part of patients, providers, insurers, and state and federal policy makers, as well as the pharmaceutical industry. Yet, such “top-down and bottom-up strategies that involve all parties are warranted,” they urged.
Several important limitations of the study are worth noting. All participants were from the East Coast, had insurance coverage, and reported an overall low level of economic burden. Responses may have differed had the study been conducted in other regions of the country. The study was voluntary, so it is important to consider that patients with greater financial challenges may not have had time to enroll and participate, which suggests that the level of economic burden affecting this population actually could be understated.
SOURCE: Dean LT et al. Cancer 2019 Mar 6. doi: 10.1002/cncr.32012.
FROM CANCER
Genetic signature helps identify those at risk of MS
DALLAS – in a precision medicine–focused session at the meeting of the Americas Committee on Treatment and Research in Multiple Sclerosis.
“MS remains a diagnosis of exclusion ... But we’re now beginning to understand a lot more about the earliest stages of the disease, and we’re constantly redefining the disease in terms of when it starts, and what it consists of,” said Dr. De Jager, professor of neurology and chief of neuroimmunology at Columbia University, New York, in an interview.
For example, physicians are now starting to treat asymptomatic individuals with radiologically isolated syndrome, he said. “Is that part of the disease? Well, a lot of us think so, and we’re currently doing the studies to see whether treating them has an impact on long-term disability.”
“In this effort to redefine this disease and when it starts, these molecular and cellular studies are becoming very important,” Dr. De Jager said. Both individuals in the general population and high-risk individuals, such as family members of people with MS, will benefit from these research approaches, he said.
Right now, it’s hard to know who could benefit most from future preventive therapies, or who should have the most rigorous surveillance.
Dr. De Jager pointed to a presentation by his collaborator, Nikolaos Patsopoulos, MD, PhD, of Brigham and Women’s Hospital, Boston, who reported on the activities of the International MS Genetics Consortium. The consortium has collected and is nearing publication of data from more than 45,000 people with MS and 65,000 control participants to identify the genetic architecture of MS onset.
“We’re going to be reporting that there are over 234 genetic variations” that contribute to the onset of MS, Dr. De Jager said. “There are more to be found, but that’s a large number,” he said. The data point toward a genetic fingerprint that’s close to lupus, type 1 diabetes, and other inflammatory diseases. This shared genetic architecture means that there’s overlapping susceptibility for many diseases in this spectrum.
DALLAS – in a precision medicine–focused session at the meeting of the Americas Committee on Treatment and Research in Multiple Sclerosis.
“MS remains a diagnosis of exclusion ... But we’re now beginning to understand a lot more about the earliest stages of the disease, and we’re constantly redefining the disease in terms of when it starts, and what it consists of,” said Dr. De Jager, professor of neurology and chief of neuroimmunology at Columbia University, New York, in an interview.
For example, physicians are now starting to treat asymptomatic individuals with radiologically isolated syndrome, he said. “Is that part of the disease? Well, a lot of us think so, and we’re currently doing the studies to see whether treating them has an impact on long-term disability.”
“In this effort to redefine this disease and when it starts, these molecular and cellular studies are becoming very important,” Dr. De Jager said. Both individuals in the general population and high-risk individuals, such as family members of people with MS, will benefit from these research approaches, he said.
Right now, it’s hard to know who could benefit most from future preventive therapies, or who should have the most rigorous surveillance.
Dr. De Jager pointed to a presentation by his collaborator, Nikolaos Patsopoulos, MD, PhD, of Brigham and Women’s Hospital, Boston, who reported on the activities of the International MS Genetics Consortium. The consortium has collected and is nearing publication of data from more than 45,000 people with MS and 65,000 control participants to identify the genetic architecture of MS onset.
“We’re going to be reporting that there are over 234 genetic variations” that contribute to the onset of MS, Dr. De Jager said. “There are more to be found, but that’s a large number,” he said. The data point toward a genetic fingerprint that’s close to lupus, type 1 diabetes, and other inflammatory diseases. This shared genetic architecture means that there’s overlapping susceptibility for many diseases in this spectrum.
DALLAS – in a precision medicine–focused session at the meeting of the Americas Committee on Treatment and Research in Multiple Sclerosis.
“MS remains a diagnosis of exclusion ... But we’re now beginning to understand a lot more about the earliest stages of the disease, and we’re constantly redefining the disease in terms of when it starts, and what it consists of,” said Dr. De Jager, professor of neurology and chief of neuroimmunology at Columbia University, New York, in an interview.
For example, physicians are now starting to treat asymptomatic individuals with radiologically isolated syndrome, he said. “Is that part of the disease? Well, a lot of us think so, and we’re currently doing the studies to see whether treating them has an impact on long-term disability.”
“In this effort to redefine this disease and when it starts, these molecular and cellular studies are becoming very important,” Dr. De Jager said. Both individuals in the general population and high-risk individuals, such as family members of people with MS, will benefit from these research approaches, he said.
Right now, it’s hard to know who could benefit most from future preventive therapies, or who should have the most rigorous surveillance.
Dr. De Jager pointed to a presentation by his collaborator, Nikolaos Patsopoulos, MD, PhD, of Brigham and Women’s Hospital, Boston, who reported on the activities of the International MS Genetics Consortium. The consortium has collected and is nearing publication of data from more than 45,000 people with MS and 65,000 control participants to identify the genetic architecture of MS onset.
“We’re going to be reporting that there are over 234 genetic variations” that contribute to the onset of MS, Dr. De Jager said. “There are more to be found, but that’s a large number,” he said. The data point toward a genetic fingerprint that’s close to lupus, type 1 diabetes, and other inflammatory diseases. This shared genetic architecture means that there’s overlapping susceptibility for many diseases in this spectrum.
REPORTING FROM ACTRIMS FORUM 2019
Andexanet alfa effectively reverses factor Xa inhibition
HONOLULU – according to a study presented at the International Stroke Conference sponsored by the American Heart Association. The medication is associated with a low rate of mortality resulting from intracerebral hemorrhage (ICH), compared with the general population of patients with ICH receiving anticoagulation.
Factor Xa inhibitors such as apixaban and rivaroxaban effectively prevent thromboembolic events but may cause or exacerbate acute major bleeding. Andexanet alfa, a modified, recombinant, inactive form of human factor Xa, was developed and approved as a reversal agent for factor Xa inhibitors. In a 2015 study, andexanet rapidly and safely reversed anti–factor Xa activity in large cohorts of patients without bleeding.
A single-cohort study
Truman John Milling Jr., MD, an emergency medicine physician at Dell Seton Medical Center at the University of Texas in Austin, and his colleagues conducted the Andexanet Alfa, a Novel Antidote to the Anticoagulation Effects of Factor Xa Inhibitors (ANNEXA-4) study to evaluate the drug’s safety and efficacy in patients with acute major bleeding associated with treatment with a factor Xa inhibitor. For participants to be eligible, their bleeding had to be life threatening with signs of hemodynamic compromise, be associated with a decrease in hemoglobin level of at least 2 g/dL, or occur in a critical organ such as the brain. An independent academic committee determined whether patients met these criteria.
The trial’s primary efficacy outcomes were change from baseline in anti–factor Xa activity and the percentage of patients with excellent or good hemostatic efficacy at 12 hours. The primary safety endpoints were death, thrombotic events, and the development of neutralizing antibodies to andexanet or to native factor X and factor Xa. The efficacy population included patients with major bleeding and baseline anti–factor Xa activity of at least 75 ng/mL. The safety population included all patients who received a dose of andexanet. The independent committee adjudicated the efficacy and safety outcomes.
Hemostasis was sustained for 12 hours
The investigators enrolled 352 participants into the study, all of whom received andexanet and were followed for at least 30 days or until death. The population’s mean age was 77 years. “These were older and sicker patients with a significant amount of comorbid disease,” said Dr. Milling. The primary indication for anticoagulation was atrial fibrillation in 80% of patients. The primary site of bleeding was intracranial in 64% of patients and gastrointestinal in 26% of patients. The remaining 10% of patients had bleeding affecting other areas (such as pericardial or intramuscular bleeding).
The investigators included 254 patients in the efficacy population. At the end of the administration of the andexanet bolus, the median value for anti–factor Xa activity decreased by 92% among participants receiving apixaban, 92% among participants receiving rivaroxaban, and 75% among patients receiving enoxaparin. Among patients receiving apixaban, the median value for anti–factor Xa activity was decreased by 32% at 4 hours, 34% at 8 hours, and 38% at 12 hours. Among patients receiving rivaroxaban, the median value for anti–factor Xa activity was decreased by 42% at 4 hours, 48% at 8 hours, and 62% at 12 hours.
Dr. Milling and his colleagues assessed hemostatic efficacy in 249 patients. Of this group, 82% achieved good or excellent hemostasis. Among participants with good or excellent hemostasis, 84% had excellent results, and 16% had good results. Subanalysis by factor Xa inhibitor, type of bleed, age, and dose of andexanet did not alter the findings significantly.
To determine whether hemostasis had been sustained sufficiently to prevent clinical deterioration, the investigators examined 71 patients with ICH and a single-compartment bleed. From 1 hour to 12 hours, one patient’s outcome changed from excellent/good to poor/none, and one patient’s outcome changed from excellent to good. For the majority of these patients, however, good hemostasis was sustained from 1 to 12 hours.
The rate of thromboembolic events was 9.7%, which is in the expected range for this population, said Dr. Milling. These events were distributed evenly among the 4 weeks of the study. Stroke and deep vein thrombosis accounted for most of these events, and pulmonary emboli and heart attacks occurred as well. “Once we restarted oral anticoagulation ... there were no more thrombotic events,” said Dr. Milling. No patient developed neutralizing antibodies to factor X or factor Xa, nor did any patient develop neutralizing antibodies to andexanet.
The overall mortality rate was 13.9%. The rate of mortality resulting from ICH was 15%, and the rate of mortality resulting from gastrointestinal bleeding was 11%. These results are impressive, considering that patients had received anticoagulants, said Dr. Milling.
Portola Pharmaceuticals, the maker of andexanet alfa, funded the study. Dr. Milling reported receiving funding and honoraria from the Population Health Research Institute at McMasters University, Janssen, CSL Behring, and Octapharma. He also received a small research payment from Portola Pharmaceuticals. Several of the investigators reported receiving funding from Portola Pharmaceuticals.
SOURCE: Milling TJ et al. ISC 2019, Abstract LB7.
HONOLULU – according to a study presented at the International Stroke Conference sponsored by the American Heart Association. The medication is associated with a low rate of mortality resulting from intracerebral hemorrhage (ICH), compared with the general population of patients with ICH receiving anticoagulation.
Factor Xa inhibitors such as apixaban and rivaroxaban effectively prevent thromboembolic events but may cause or exacerbate acute major bleeding. Andexanet alfa, a modified, recombinant, inactive form of human factor Xa, was developed and approved as a reversal agent for factor Xa inhibitors. In a 2015 study, andexanet rapidly and safely reversed anti–factor Xa activity in large cohorts of patients without bleeding.
A single-cohort study
Truman John Milling Jr., MD, an emergency medicine physician at Dell Seton Medical Center at the University of Texas in Austin, and his colleagues conducted the Andexanet Alfa, a Novel Antidote to the Anticoagulation Effects of Factor Xa Inhibitors (ANNEXA-4) study to evaluate the drug’s safety and efficacy in patients with acute major bleeding associated with treatment with a factor Xa inhibitor. For participants to be eligible, their bleeding had to be life threatening with signs of hemodynamic compromise, be associated with a decrease in hemoglobin level of at least 2 g/dL, or occur in a critical organ such as the brain. An independent academic committee determined whether patients met these criteria.
The trial’s primary efficacy outcomes were change from baseline in anti–factor Xa activity and the percentage of patients with excellent or good hemostatic efficacy at 12 hours. The primary safety endpoints were death, thrombotic events, and the development of neutralizing antibodies to andexanet or to native factor X and factor Xa. The efficacy population included patients with major bleeding and baseline anti–factor Xa activity of at least 75 ng/mL. The safety population included all patients who received a dose of andexanet. The independent committee adjudicated the efficacy and safety outcomes.
Hemostasis was sustained for 12 hours
The investigators enrolled 352 participants into the study, all of whom received andexanet and were followed for at least 30 days or until death. The population’s mean age was 77 years. “These were older and sicker patients with a significant amount of comorbid disease,” said Dr. Milling. The primary indication for anticoagulation was atrial fibrillation in 80% of patients. The primary site of bleeding was intracranial in 64% of patients and gastrointestinal in 26% of patients. The remaining 10% of patients had bleeding affecting other areas (such as pericardial or intramuscular bleeding).
The investigators included 254 patients in the efficacy population. At the end of the administration of the andexanet bolus, the median value for anti–factor Xa activity decreased by 92% among participants receiving apixaban, 92% among participants receiving rivaroxaban, and 75% among patients receiving enoxaparin. Among patients receiving apixaban, the median value for anti–factor Xa activity was decreased by 32% at 4 hours, 34% at 8 hours, and 38% at 12 hours. Among patients receiving rivaroxaban, the median value for anti–factor Xa activity was decreased by 42% at 4 hours, 48% at 8 hours, and 62% at 12 hours.
Dr. Milling and his colleagues assessed hemostatic efficacy in 249 patients. Of this group, 82% achieved good or excellent hemostasis. Among participants with good or excellent hemostasis, 84% had excellent results, and 16% had good results. Subanalysis by factor Xa inhibitor, type of bleed, age, and dose of andexanet did not alter the findings significantly.
To determine whether hemostasis had been sustained sufficiently to prevent clinical deterioration, the investigators examined 71 patients with ICH and a single-compartment bleed. From 1 hour to 12 hours, one patient’s outcome changed from excellent/good to poor/none, and one patient’s outcome changed from excellent to good. For the majority of these patients, however, good hemostasis was sustained from 1 to 12 hours.
The rate of thromboembolic events was 9.7%, which is in the expected range for this population, said Dr. Milling. These events were distributed evenly among the 4 weeks of the study. Stroke and deep vein thrombosis accounted for most of these events, and pulmonary emboli and heart attacks occurred as well. “Once we restarted oral anticoagulation ... there were no more thrombotic events,” said Dr. Milling. No patient developed neutralizing antibodies to factor X or factor Xa, nor did any patient develop neutralizing antibodies to andexanet.
The overall mortality rate was 13.9%. The rate of mortality resulting from ICH was 15%, and the rate of mortality resulting from gastrointestinal bleeding was 11%. These results are impressive, considering that patients had received anticoagulants, said Dr. Milling.
Portola Pharmaceuticals, the maker of andexanet alfa, funded the study. Dr. Milling reported receiving funding and honoraria from the Population Health Research Institute at McMasters University, Janssen, CSL Behring, and Octapharma. He also received a small research payment from Portola Pharmaceuticals. Several of the investigators reported receiving funding from Portola Pharmaceuticals.
SOURCE: Milling TJ et al. ISC 2019, Abstract LB7.
HONOLULU – according to a study presented at the International Stroke Conference sponsored by the American Heart Association. The medication is associated with a low rate of mortality resulting from intracerebral hemorrhage (ICH), compared with the general population of patients with ICH receiving anticoagulation.
Factor Xa inhibitors such as apixaban and rivaroxaban effectively prevent thromboembolic events but may cause or exacerbate acute major bleeding. Andexanet alfa, a modified, recombinant, inactive form of human factor Xa, was developed and approved as a reversal agent for factor Xa inhibitors. In a 2015 study, andexanet rapidly and safely reversed anti–factor Xa activity in large cohorts of patients without bleeding.
A single-cohort study
Truman John Milling Jr., MD, an emergency medicine physician at Dell Seton Medical Center at the University of Texas in Austin, and his colleagues conducted the Andexanet Alfa, a Novel Antidote to the Anticoagulation Effects of Factor Xa Inhibitors (ANNEXA-4) study to evaluate the drug’s safety and efficacy in patients with acute major bleeding associated with treatment with a factor Xa inhibitor. For participants to be eligible, their bleeding had to be life threatening with signs of hemodynamic compromise, be associated with a decrease in hemoglobin level of at least 2 g/dL, or occur in a critical organ such as the brain. An independent academic committee determined whether patients met these criteria.
The trial’s primary efficacy outcomes were change from baseline in anti–factor Xa activity and the percentage of patients with excellent or good hemostatic efficacy at 12 hours. The primary safety endpoints were death, thrombotic events, and the development of neutralizing antibodies to andexanet or to native factor X and factor Xa. The efficacy population included patients with major bleeding and baseline anti–factor Xa activity of at least 75 ng/mL. The safety population included all patients who received a dose of andexanet. The independent committee adjudicated the efficacy and safety outcomes.
Hemostasis was sustained for 12 hours
The investigators enrolled 352 participants into the study, all of whom received andexanet and were followed for at least 30 days or until death. The population’s mean age was 77 years. “These were older and sicker patients with a significant amount of comorbid disease,” said Dr. Milling. The primary indication for anticoagulation was atrial fibrillation in 80% of patients. The primary site of bleeding was intracranial in 64% of patients and gastrointestinal in 26% of patients. The remaining 10% of patients had bleeding affecting other areas (such as pericardial or intramuscular bleeding).
The investigators included 254 patients in the efficacy population. At the end of the administration of the andexanet bolus, the median value for anti–factor Xa activity decreased by 92% among participants receiving apixaban, 92% among participants receiving rivaroxaban, and 75% among patients receiving enoxaparin. Among patients receiving apixaban, the median value for anti–factor Xa activity was decreased by 32% at 4 hours, 34% at 8 hours, and 38% at 12 hours. Among patients receiving rivaroxaban, the median value for anti–factor Xa activity was decreased by 42% at 4 hours, 48% at 8 hours, and 62% at 12 hours.
Dr. Milling and his colleagues assessed hemostatic efficacy in 249 patients. Of this group, 82% achieved good or excellent hemostasis. Among participants with good or excellent hemostasis, 84% had excellent results, and 16% had good results. Subanalysis by factor Xa inhibitor, type of bleed, age, and dose of andexanet did not alter the findings significantly.
To determine whether hemostasis had been sustained sufficiently to prevent clinical deterioration, the investigators examined 71 patients with ICH and a single-compartment bleed. From 1 hour to 12 hours, one patient’s outcome changed from excellent/good to poor/none, and one patient’s outcome changed from excellent to good. For the majority of these patients, however, good hemostasis was sustained from 1 to 12 hours.
The rate of thromboembolic events was 9.7%, which is in the expected range for this population, said Dr. Milling. These events were distributed evenly among the 4 weeks of the study. Stroke and deep vein thrombosis accounted for most of these events, and pulmonary emboli and heart attacks occurred as well. “Once we restarted oral anticoagulation ... there were no more thrombotic events,” said Dr. Milling. No patient developed neutralizing antibodies to factor X or factor Xa, nor did any patient develop neutralizing antibodies to andexanet.
The overall mortality rate was 13.9%. The rate of mortality resulting from ICH was 15%, and the rate of mortality resulting from gastrointestinal bleeding was 11%. These results are impressive, considering that patients had received anticoagulants, said Dr. Milling.
Portola Pharmaceuticals, the maker of andexanet alfa, funded the study. Dr. Milling reported receiving funding and honoraria from the Population Health Research Institute at McMasters University, Janssen, CSL Behring, and Octapharma. He also received a small research payment from Portola Pharmaceuticals. Several of the investigators reported receiving funding from Portola Pharmaceuticals.
SOURCE: Milling TJ et al. ISC 2019, Abstract LB7.
REPORTING FROM ISC 2019





