User login
Will patient rewards for lower-cost choices impact physicians?
“In the first 12 months of the rewards program, we observed a 2.1% relative reduction in prices across all services eligible for the program,” according to Christopher Whaley, PhD, an associate policy researcher at the RAND Corporation, and his colleagues. “This effect was most evident for MRIs, for which there was a 4.7% reduction in prices.”
The rewards program offered $25-$500 for making lower-cost choices among 131 elective services. Rewards value was based on the provider’s price and service, yielding savings of $2.3 million, or roughly $8 per person across the 269,875 employees and dependents eligible for the rewards program.
Patients who were willing to price-shop chose to save money on imaging tests including ultrasounds, mammograms, and MRIs, Dr. Whaley and his colleagues wrote.
However, initial results showed very little impact in pricing for surgical procedures, including minor (such as breast biopsy), moderate (such as arthroscopy), and major (such as hip and knee replacements), covered by the rewards program.
“There are several potential explanations for this variation across types of services,” the authors wrote. “To receive a reward, patients may need to receive care from a provider different from the one their physician initially recommended. Compared to circumstances where they need an invasive procedure, patients may feel more comfortable asking the provider for a new referral for imaging services.”
An established doctor/patient relationship could have dimmed patient interest in seeking lower cost surgical procedures.
“There is also the complexity of switching their care,” the authors wrote. “For a surgical procedure, switching providers is particularly complex, as it requires identifying a lower-price provider and potentially getting another preoperative visit.”
Quality, while also playing a role in patient choice, is not a factor in the how the rewards program is structured.
“Patients may view imaging services more as commodity services and therefore may be more likely to switch, while patients may be more worried about the quality of lower-price surgeons.”
Building further on that, Dr. Whaley said in an interview that if the program becomes more widely used and successful, it could start to instill more price-shopping for procedures and create levers for pricing wars among local physicians.
“We don’t know if there will be an impact for procedural services in later years,” he said. “On one hand, patients simply may not be willing to price shop for these services. On the other hand, patients may learn about price-shopping for these services or the insurance company might continue to develop the model and try to get patients to shop.”
This, in turn, could potentially affect the dynamic of negotiations between providers and insurance companies for network placement, Dr. Whaley noted.
“It could be a ‘stick’ for insurers to use for negotiations with higher-priced providers. Insurers could say, ‘unless you lower your prices, we’ll pay patients to go to your competitor,’” something he said could ultimately be beneficial to lower-cost providers.
Dr. Whaley also noted that there was a small reduction (0.3 percentage points) in overall health care use among patients using reward-eligible services.
“The intervention population still had to pay their usual out-of-pocket payments, and a patient’s out-of-pocket expense was much higher than the reward amount, on average,” he said. “Therefore, this reduction in utilization may be due to patients’ using the price-shopping tool, becoming more aware of these out-of-pocket liabilities, and deciding to not get care from any provider.”
SOURCE: Whaley C et al. Health Aff (Millwood). 2019 Mar;38(3):440-7.
“In the first 12 months of the rewards program, we observed a 2.1% relative reduction in prices across all services eligible for the program,” according to Christopher Whaley, PhD, an associate policy researcher at the RAND Corporation, and his colleagues. “This effect was most evident for MRIs, for which there was a 4.7% reduction in prices.”
The rewards program offered $25-$500 for making lower-cost choices among 131 elective services. Rewards value was based on the provider’s price and service, yielding savings of $2.3 million, or roughly $8 per person across the 269,875 employees and dependents eligible for the rewards program.
Patients who were willing to price-shop chose to save money on imaging tests including ultrasounds, mammograms, and MRIs, Dr. Whaley and his colleagues wrote.
However, initial results showed very little impact in pricing for surgical procedures, including minor (such as breast biopsy), moderate (such as arthroscopy), and major (such as hip and knee replacements), covered by the rewards program.
“There are several potential explanations for this variation across types of services,” the authors wrote. “To receive a reward, patients may need to receive care from a provider different from the one their physician initially recommended. Compared to circumstances where they need an invasive procedure, patients may feel more comfortable asking the provider for a new referral for imaging services.”
An established doctor/patient relationship could have dimmed patient interest in seeking lower cost surgical procedures.
“There is also the complexity of switching their care,” the authors wrote. “For a surgical procedure, switching providers is particularly complex, as it requires identifying a lower-price provider and potentially getting another preoperative visit.”
Quality, while also playing a role in patient choice, is not a factor in the how the rewards program is structured.
“Patients may view imaging services more as commodity services and therefore may be more likely to switch, while patients may be more worried about the quality of lower-price surgeons.”
Building further on that, Dr. Whaley said in an interview that if the program becomes more widely used and successful, it could start to instill more price-shopping for procedures and create levers for pricing wars among local physicians.
“We don’t know if there will be an impact for procedural services in later years,” he said. “On one hand, patients simply may not be willing to price shop for these services. On the other hand, patients may learn about price-shopping for these services or the insurance company might continue to develop the model and try to get patients to shop.”
This, in turn, could potentially affect the dynamic of negotiations between providers and insurance companies for network placement, Dr. Whaley noted.
“It could be a ‘stick’ for insurers to use for negotiations with higher-priced providers. Insurers could say, ‘unless you lower your prices, we’ll pay patients to go to your competitor,’” something he said could ultimately be beneficial to lower-cost providers.
Dr. Whaley also noted that there was a small reduction (0.3 percentage points) in overall health care use among patients using reward-eligible services.
“The intervention population still had to pay their usual out-of-pocket payments, and a patient’s out-of-pocket expense was much higher than the reward amount, on average,” he said. “Therefore, this reduction in utilization may be due to patients’ using the price-shopping tool, becoming more aware of these out-of-pocket liabilities, and deciding to not get care from any provider.”
SOURCE: Whaley C et al. Health Aff (Millwood). 2019 Mar;38(3):440-7.
“In the first 12 months of the rewards program, we observed a 2.1% relative reduction in prices across all services eligible for the program,” according to Christopher Whaley, PhD, an associate policy researcher at the RAND Corporation, and his colleagues. “This effect was most evident for MRIs, for which there was a 4.7% reduction in prices.”
The rewards program offered $25-$500 for making lower-cost choices among 131 elective services. Rewards value was based on the provider’s price and service, yielding savings of $2.3 million, or roughly $8 per person across the 269,875 employees and dependents eligible for the rewards program.
Patients who were willing to price-shop chose to save money on imaging tests including ultrasounds, mammograms, and MRIs, Dr. Whaley and his colleagues wrote.
However, initial results showed very little impact in pricing for surgical procedures, including minor (such as breast biopsy), moderate (such as arthroscopy), and major (such as hip and knee replacements), covered by the rewards program.
“There are several potential explanations for this variation across types of services,” the authors wrote. “To receive a reward, patients may need to receive care from a provider different from the one their physician initially recommended. Compared to circumstances where they need an invasive procedure, patients may feel more comfortable asking the provider for a new referral for imaging services.”
An established doctor/patient relationship could have dimmed patient interest in seeking lower cost surgical procedures.
“There is also the complexity of switching their care,” the authors wrote. “For a surgical procedure, switching providers is particularly complex, as it requires identifying a lower-price provider and potentially getting another preoperative visit.”
Quality, while also playing a role in patient choice, is not a factor in the how the rewards program is structured.
“Patients may view imaging services more as commodity services and therefore may be more likely to switch, while patients may be more worried about the quality of lower-price surgeons.”
Building further on that, Dr. Whaley said in an interview that if the program becomes more widely used and successful, it could start to instill more price-shopping for procedures and create levers for pricing wars among local physicians.
“We don’t know if there will be an impact for procedural services in later years,” he said. “On one hand, patients simply may not be willing to price shop for these services. On the other hand, patients may learn about price-shopping for these services or the insurance company might continue to develop the model and try to get patients to shop.”
This, in turn, could potentially affect the dynamic of negotiations between providers and insurance companies for network placement, Dr. Whaley noted.
“It could be a ‘stick’ for insurers to use for negotiations with higher-priced providers. Insurers could say, ‘unless you lower your prices, we’ll pay patients to go to your competitor,’” something he said could ultimately be beneficial to lower-cost providers.
Dr. Whaley also noted that there was a small reduction (0.3 percentage points) in overall health care use among patients using reward-eligible services.
“The intervention population still had to pay their usual out-of-pocket payments, and a patient’s out-of-pocket expense was much higher than the reward amount, on average,” he said. “Therefore, this reduction in utilization may be due to patients’ using the price-shopping tool, becoming more aware of these out-of-pocket liabilities, and deciding to not get care from any provider.”
SOURCE: Whaley C et al. Health Aff (Millwood). 2019 Mar;38(3):440-7.
FROM HEALTH AFFAIRS
Radiographic Changes of Osteomyelitis in a Patient With Periungual Lichen Planus
To the Editor:
A 60-year-old woman presented for evaluation of a 1-year history of left hallux nail plate dystrophy and proximal nail fold inflammation. Her medical history included Cushing disease with associated uncontrolled diabetes mellitus (DM) and a remote history of cutaneous lichen planus (LP) that resolved 15 years prior to presentation. She noted improvement during intravenous courses of antibiotics for other infections.
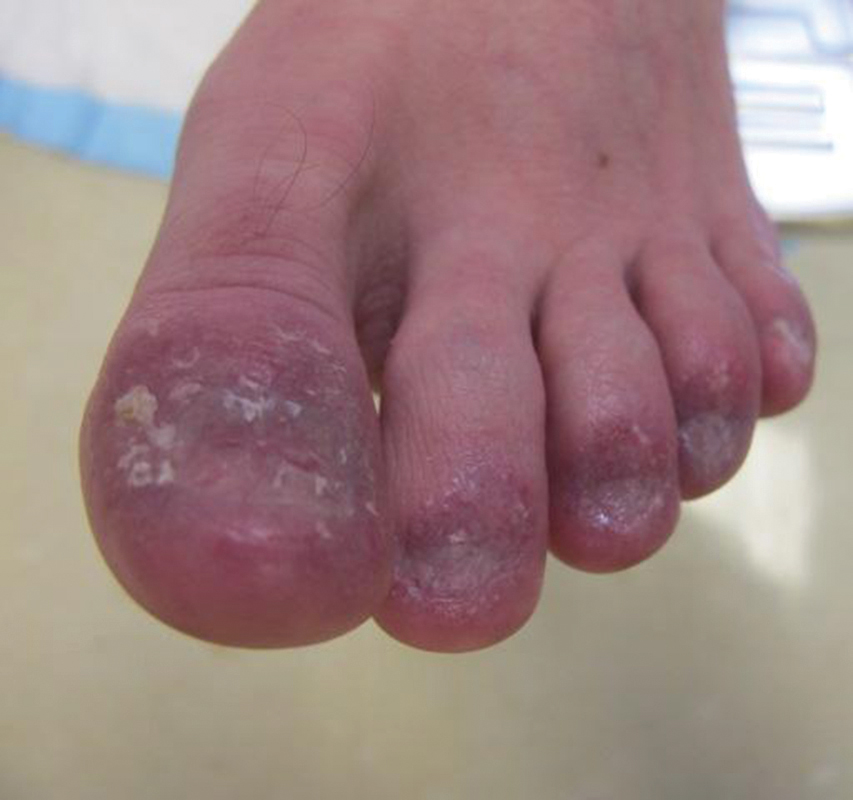
Examination of the left hallux revealed onycholysis, loss of the nail plate, and a yellow fibrinous base alongside erosion, erythema, and edema of the proximal toenail fold (Figure 1). The left second toe pad was markedly tender to palpation with scant exudate expressed from underneath the nail bed. Two biopsies of the hallux were performed. The proximal nail fold specimen revealed mild epidermal hyperplasia, and the nail bed demonstrated a nonspecific ulcer that was negative for acid-fast bacilli and fungi.
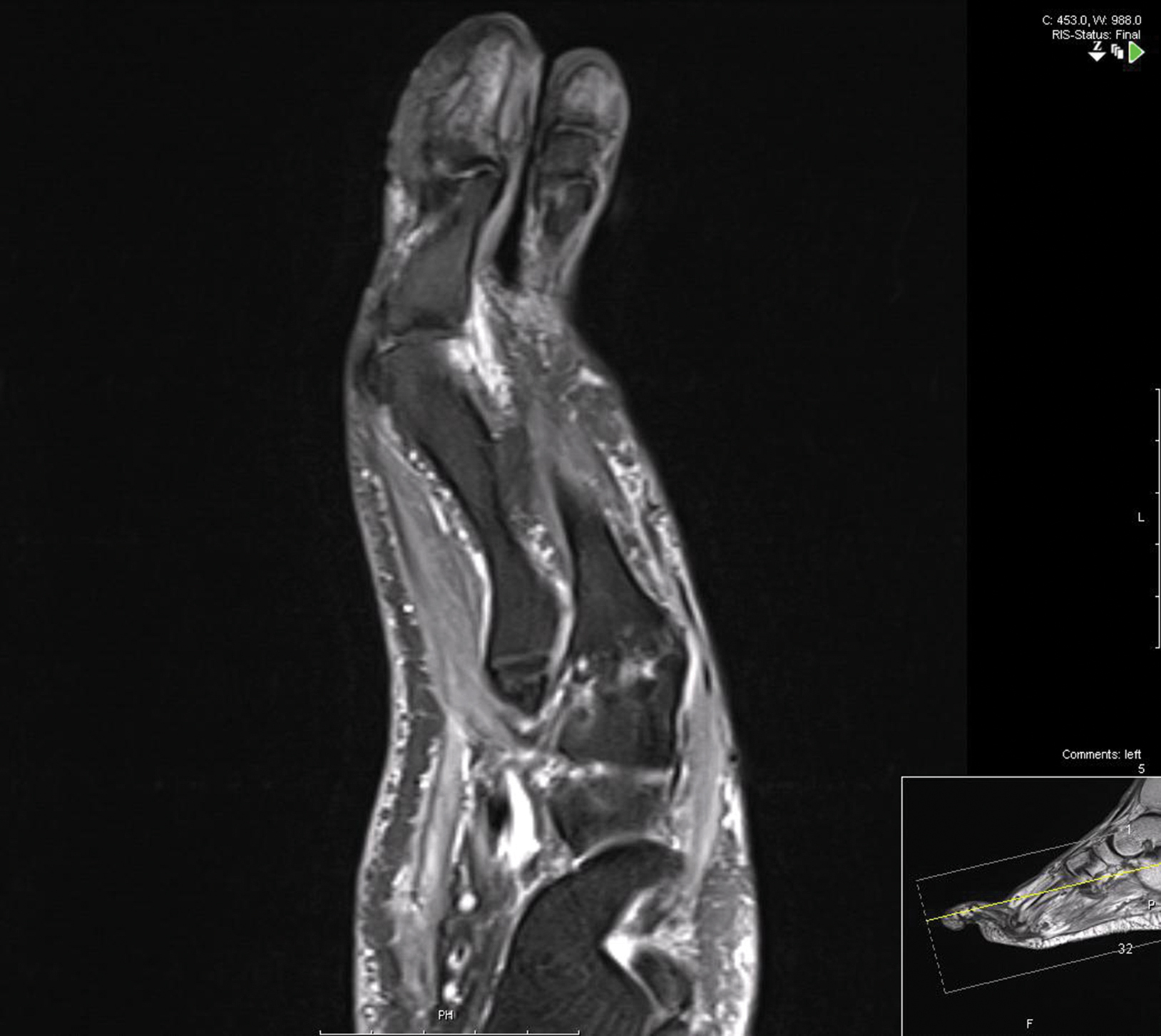
Treatment over 2 months with cephalexin yielded improvement in both erythema and edema. Initial and repeat nail plate cultures grew ampicillin- and penicillin-sensitive Enterococcus faecalis. Magnetic resonance imaging was performed to evaluate for osteomyelitis because of lack of resolution. Results demonstrated osteomyelitis of the distal tuft of the left hallux and the distal phalanx of the second toe (Figure 2). Vascular surgery evaluation revealed no evidence of large vessel arterial insufficiency. She was started on amoxicillin for superficial Enterococcus and ciprofloxacin for underlying enteric bacilli. The persistence of infection was attributed to microvascular disease secondary to the patient's associated DM. Months later, due to suspected worsening of osteomyelitis, she underwent treatment with oral fluconazole to cover potential fungal co-infection and intravenous vancomycin and piperacillin-tazobactam for broad-spectrum antibacterial coverage. She was eventually transitioned to antimicrobial agents including amoxicillin-clavulanate potassium and topical mupirocin with improvement in periungual erythema and edema.
On subsequent dermatologic evaluation after 1 month, she presented with pterygium and loss of all nail plates on the left foot. The nail bed now had a violaceous color and was studded with milia. The clinical findings were suggestive of LP, consistent with her history of LP. In light of these new findings, both topical corticosteroids and retinoids were utilized for treatment without remarkable benefit. The patient declined further management with systemic medications.
We report a case of nail LP associated with underlying radiographic osteomyelitis. Erosive nail LP has been associated with underlying osteomyelitis of the phalanx.1 Our patient developed these manifestations in the setting of Cushing disease, a unique finding given that many report improvement of LP with systemic corticosteroids.2,3 Tacrolimus, a calcineurin inhibitor, has been used in oral or topical formulations for lower extremity ulcers caused by LP as well as nail LP.1,4 Long-term prognosis of nail LP is poor, with high relapse rates and permanent damage to the nail unit.2 It is important to be aware that LP of the nail unit may cause radiographic changes of osteomyelitis that are not infectious in nature.
- Miller S. The effect of tacrolimus on lower extremity ulcers: a case study and review of the literature. Ostomy Wound Manage. 2008;54:36-42.
- Goettmann S, Zaraa I, Moulonguet I. Nail lichen planus: epidemiological, clinical, pathological, therapeutic and prognosis study of 67 cases. Eur Acad Dermatol Venereol. 2012;26:1304-1309.
- Piraccini BM, Saccani E, Starace M, et al. Nail lichen planus: response to treatment and long term follow-up. Eur J Dermatol. 2010;20:489-496.
- Ujiie H, Shibaki A, Akiyama M, et al. Successful treatment of nail lichen planus with topical tacrolimus. Acta Derm Venereol. 2010;90:218-219.
To the Editor:
A 60-year-old woman presented for evaluation of a 1-year history of left hallux nail plate dystrophy and proximal nail fold inflammation. Her medical history included Cushing disease with associated uncontrolled diabetes mellitus (DM) and a remote history of cutaneous lichen planus (LP) that resolved 15 years prior to presentation. She noted improvement during intravenous courses of antibiotics for other infections.
Examination of the left hallux revealed onycholysis, loss of the nail plate, and a yellow fibrinous base alongside erosion, erythema, and edema of the proximal toenail fold (Figure 1). The left second toe pad was markedly tender to palpation with scant exudate expressed from underneath the nail bed. Two biopsies of the hallux were performed. The proximal nail fold specimen revealed mild epidermal hyperplasia, and the nail bed demonstrated a nonspecific ulcer that was negative for acid-fast bacilli and fungi.
Treatment over 2 months with cephalexin yielded improvement in both erythema and edema. Initial and repeat nail plate cultures grew ampicillin- and penicillin-sensitive Enterococcus faecalis. Magnetic resonance imaging was performed to evaluate for osteomyelitis because of lack of resolution. Results demonstrated osteomyelitis of the distal tuft of the left hallux and the distal phalanx of the second toe (Figure 2). Vascular surgery evaluation revealed no evidence of large vessel arterial insufficiency. She was started on amoxicillin for superficial Enterococcus and ciprofloxacin for underlying enteric bacilli. The persistence of infection was attributed to microvascular disease secondary to the patient's associated DM. Months later, due to suspected worsening of osteomyelitis, she underwent treatment with oral fluconazole to cover potential fungal co-infection and intravenous vancomycin and piperacillin-tazobactam for broad-spectrum antibacterial coverage. She was eventually transitioned to antimicrobial agents including amoxicillin-clavulanate potassium and topical mupirocin with improvement in periungual erythema and edema.
On subsequent dermatologic evaluation after 1 month, she presented with pterygium and loss of all nail plates on the left foot. The nail bed now had a violaceous color and was studded with milia. The clinical findings were suggestive of LP, consistent with her history of LP. In light of these new findings, both topical corticosteroids and retinoids were utilized for treatment without remarkable benefit. The patient declined further management with systemic medications.
We report a case of nail LP associated with underlying radiographic osteomyelitis. Erosive nail LP has been associated with underlying osteomyelitis of the phalanx.1 Our patient developed these manifestations in the setting of Cushing disease, a unique finding given that many report improvement of LP with systemic corticosteroids.2,3 Tacrolimus, a calcineurin inhibitor, has been used in oral or topical formulations for lower extremity ulcers caused by LP as well as nail LP.1,4 Long-term prognosis of nail LP is poor, with high relapse rates and permanent damage to the nail unit.2 It is important to be aware that LP of the nail unit may cause radiographic changes of osteomyelitis that are not infectious in nature.
To the Editor:
A 60-year-old woman presented for evaluation of a 1-year history of left hallux nail plate dystrophy and proximal nail fold inflammation. Her medical history included Cushing disease with associated uncontrolled diabetes mellitus (DM) and a remote history of cutaneous lichen planus (LP) that resolved 15 years prior to presentation. She noted improvement during intravenous courses of antibiotics for other infections.
Examination of the left hallux revealed onycholysis, loss of the nail plate, and a yellow fibrinous base alongside erosion, erythema, and edema of the proximal toenail fold (Figure 1). The left second toe pad was markedly tender to palpation with scant exudate expressed from underneath the nail bed. Two biopsies of the hallux were performed. The proximal nail fold specimen revealed mild epidermal hyperplasia, and the nail bed demonstrated a nonspecific ulcer that was negative for acid-fast bacilli and fungi.
Treatment over 2 months with cephalexin yielded improvement in both erythema and edema. Initial and repeat nail plate cultures grew ampicillin- and penicillin-sensitive Enterococcus faecalis. Magnetic resonance imaging was performed to evaluate for osteomyelitis because of lack of resolution. Results demonstrated osteomyelitis of the distal tuft of the left hallux and the distal phalanx of the second toe (Figure 2). Vascular surgery evaluation revealed no evidence of large vessel arterial insufficiency. She was started on amoxicillin for superficial Enterococcus and ciprofloxacin for underlying enteric bacilli. The persistence of infection was attributed to microvascular disease secondary to the patient's associated DM. Months later, due to suspected worsening of osteomyelitis, she underwent treatment with oral fluconazole to cover potential fungal co-infection and intravenous vancomycin and piperacillin-tazobactam for broad-spectrum antibacterial coverage. She was eventually transitioned to antimicrobial agents including amoxicillin-clavulanate potassium and topical mupirocin with improvement in periungual erythema and edema.
On subsequent dermatologic evaluation after 1 month, she presented with pterygium and loss of all nail plates on the left foot. The nail bed now had a violaceous color and was studded with milia. The clinical findings were suggestive of LP, consistent with her history of LP. In light of these new findings, both topical corticosteroids and retinoids were utilized for treatment without remarkable benefit. The patient declined further management with systemic medications.
We report a case of nail LP associated with underlying radiographic osteomyelitis. Erosive nail LP has been associated with underlying osteomyelitis of the phalanx.1 Our patient developed these manifestations in the setting of Cushing disease, a unique finding given that many report improvement of LP with systemic corticosteroids.2,3 Tacrolimus, a calcineurin inhibitor, has been used in oral or topical formulations for lower extremity ulcers caused by LP as well as nail LP.1,4 Long-term prognosis of nail LP is poor, with high relapse rates and permanent damage to the nail unit.2 It is important to be aware that LP of the nail unit may cause radiographic changes of osteomyelitis that are not infectious in nature.
- Miller S. The effect of tacrolimus on lower extremity ulcers: a case study and review of the literature. Ostomy Wound Manage. 2008;54:36-42.
- Goettmann S, Zaraa I, Moulonguet I. Nail lichen planus: epidemiological, clinical, pathological, therapeutic and prognosis study of 67 cases. Eur Acad Dermatol Venereol. 2012;26:1304-1309.
- Piraccini BM, Saccani E, Starace M, et al. Nail lichen planus: response to treatment and long term follow-up. Eur J Dermatol. 2010;20:489-496.
- Ujiie H, Shibaki A, Akiyama M, et al. Successful treatment of nail lichen planus with topical tacrolimus. Acta Derm Venereol. 2010;90:218-219.
- Miller S. The effect of tacrolimus on lower extremity ulcers: a case study and review of the literature. Ostomy Wound Manage. 2008;54:36-42.
- Goettmann S, Zaraa I, Moulonguet I. Nail lichen planus: epidemiological, clinical, pathological, therapeutic and prognosis study of 67 cases. Eur Acad Dermatol Venereol. 2012;26:1304-1309.
- Piraccini BM, Saccani E, Starace M, et al. Nail lichen planus: response to treatment and long term follow-up. Eur J Dermatol. 2010;20:489-496.
- Ujiie H, Shibaki A, Akiyama M, et al. Successful treatment of nail lichen planus with topical tacrolimus. Acta Derm Venereol. 2010;90:218-219.
Practice Points
- Lichen planus (LP) is an inflammatory mucocutaneous disorder with variable presentations.
- With extensive nail involvement, nail LP may impart radiographic findings suggestive of osteomyelitis.
Concomitant methotrexate boosts pegloticase efficacy in gout patients
MAUI, HAWAII – , Orrin M. Troum, MD, said at the 2019 Rheumatology Winter Clinical Symposium.
He cited what he considers to be a practice-changing, prospective, observational, proof-of-concept study presented by John Botson, MD, at the 2018 annual meeting of the American College of Rheumatology.
Dr. Botson, a rheumatologist at Orthopedic Physicians Alaska, in Anchorage, reported on nine patients with refractory tophaceous gout placed on an 8-mg infusion of pegloticase every 2 weeks as third-line therapy. But 1 month beforehand he put them on oral methotrexate at 15 mg once weekly along with folic acid at 1 mg/day in an effort to prevent the development of treatment-limiting anti-pegloticase antibodies. It’s the same strategy rheumatologists often use when patients with rheumatoid arthritis on a tumor necrosis factor inhibitor begin to develop anti-drug antibodies.
At the time of the ACR meeting, all nine patients had received at least nine infusions, and six had received at least 12 infusions over the course of 6 months. The response rate was 100%, defined as more than 80% of serum uric acid levels being below 6.0 mg/dL. All patients stayed on methotrexate with no dose adjustment. And there were no infusion reactions. In contrast, the response rate in the randomized trials of pegloticase was only 42%, and 26% of pegloticase recipients experienced infusion reactions within 6 months.
“Although this is not [Food and Drug Administration] approved, it makes a lot of sense. From my standpoint, this is something that I’m doing now for my patients starting on pegloticase if there’s no contraindication to using methotrexate,” said Dr. Troum, a rheumatologist at the University of Southern California in Los Angeles.
“I’ve been doing this, too. This really did change my practice,” added his fellow panelist Alvin F. Wells, MD, PhD, director of the Rheumatology and Immunotherapy Center in Franklin, Wisc.
When they asked for a show of hands, only a handful of audience members indicated they are now using methotrexate in conjunction with pegloticase in their tophaceous gout patients.
Dr. Wells said his sole reservation about the practice involves using methotrexate in patients with an elevated creatinine level. What about using azathioprine or corticosteroids instead? he asked.
Dr. Troum replied that he monitors those patients carefully but sticks with the methotrexate because it’s only for a few months, which is the time frame in which patients are especially vulnerable to experiencing loss of response to pegloticase due to development of anti-drug antibodies.
Dr. Botson, who was in the Maui audience, rose to give a study update. With additional follow-up, he said, there has still been no signal of loss of response to pegloticase coadministered with methotrexate.
“A lot of us are starting to feel like immunosuppression, whether it’s with methotrexate or something else, is standard of care now,” according to the rheumatologist.
As to prescribing methotrexate in gout patients with renal insufficiency, he continued, he and his colleagues have given the matter quite a bit of thought.
“You’re talking about using methotrexate for 6 months in most of these cases. A lot of the patients who have really bad tophaceous gout already have renal insufficiency, and in the short term we haven’t really seen any problems with that. We work closely with a nephrologist on those cases. And a lot of nephrologists swear – although I don’t think the data are there – that they actually improve their renal function when we start to treat their tophaceous gout,” Dr. Botson said.
Dr. Troum and Dr. Wells reported serving as consultants to and on speakers bureaus for numerous pharmaceutical companies.
MAUI, HAWAII – , Orrin M. Troum, MD, said at the 2019 Rheumatology Winter Clinical Symposium.
He cited what he considers to be a practice-changing, prospective, observational, proof-of-concept study presented by John Botson, MD, at the 2018 annual meeting of the American College of Rheumatology.
Dr. Botson, a rheumatologist at Orthopedic Physicians Alaska, in Anchorage, reported on nine patients with refractory tophaceous gout placed on an 8-mg infusion of pegloticase every 2 weeks as third-line therapy. But 1 month beforehand he put them on oral methotrexate at 15 mg once weekly along with folic acid at 1 mg/day in an effort to prevent the development of treatment-limiting anti-pegloticase antibodies. It’s the same strategy rheumatologists often use when patients with rheumatoid arthritis on a tumor necrosis factor inhibitor begin to develop anti-drug antibodies.
At the time of the ACR meeting, all nine patients had received at least nine infusions, and six had received at least 12 infusions over the course of 6 months. The response rate was 100%, defined as more than 80% of serum uric acid levels being below 6.0 mg/dL. All patients stayed on methotrexate with no dose adjustment. And there were no infusion reactions. In contrast, the response rate in the randomized trials of pegloticase was only 42%, and 26% of pegloticase recipients experienced infusion reactions within 6 months.
“Although this is not [Food and Drug Administration] approved, it makes a lot of sense. From my standpoint, this is something that I’m doing now for my patients starting on pegloticase if there’s no contraindication to using methotrexate,” said Dr. Troum, a rheumatologist at the University of Southern California in Los Angeles.
“I’ve been doing this, too. This really did change my practice,” added his fellow panelist Alvin F. Wells, MD, PhD, director of the Rheumatology and Immunotherapy Center in Franklin, Wisc.
When they asked for a show of hands, only a handful of audience members indicated they are now using methotrexate in conjunction with pegloticase in their tophaceous gout patients.
Dr. Wells said his sole reservation about the practice involves using methotrexate in patients with an elevated creatinine level. What about using azathioprine or corticosteroids instead? he asked.
Dr. Troum replied that he monitors those patients carefully but sticks with the methotrexate because it’s only for a few months, which is the time frame in which patients are especially vulnerable to experiencing loss of response to pegloticase due to development of anti-drug antibodies.
Dr. Botson, who was in the Maui audience, rose to give a study update. With additional follow-up, he said, there has still been no signal of loss of response to pegloticase coadministered with methotrexate.
“A lot of us are starting to feel like immunosuppression, whether it’s with methotrexate or something else, is standard of care now,” according to the rheumatologist.
As to prescribing methotrexate in gout patients with renal insufficiency, he continued, he and his colleagues have given the matter quite a bit of thought.
“You’re talking about using methotrexate for 6 months in most of these cases. A lot of the patients who have really bad tophaceous gout already have renal insufficiency, and in the short term we haven’t really seen any problems with that. We work closely with a nephrologist on those cases. And a lot of nephrologists swear – although I don’t think the data are there – that they actually improve their renal function when we start to treat their tophaceous gout,” Dr. Botson said.
Dr. Troum and Dr. Wells reported serving as consultants to and on speakers bureaus for numerous pharmaceutical companies.
MAUI, HAWAII – , Orrin M. Troum, MD, said at the 2019 Rheumatology Winter Clinical Symposium.
He cited what he considers to be a practice-changing, prospective, observational, proof-of-concept study presented by John Botson, MD, at the 2018 annual meeting of the American College of Rheumatology.
Dr. Botson, a rheumatologist at Orthopedic Physicians Alaska, in Anchorage, reported on nine patients with refractory tophaceous gout placed on an 8-mg infusion of pegloticase every 2 weeks as third-line therapy. But 1 month beforehand he put them on oral methotrexate at 15 mg once weekly along with folic acid at 1 mg/day in an effort to prevent the development of treatment-limiting anti-pegloticase antibodies. It’s the same strategy rheumatologists often use when patients with rheumatoid arthritis on a tumor necrosis factor inhibitor begin to develop anti-drug antibodies.
At the time of the ACR meeting, all nine patients had received at least nine infusions, and six had received at least 12 infusions over the course of 6 months. The response rate was 100%, defined as more than 80% of serum uric acid levels being below 6.0 mg/dL. All patients stayed on methotrexate with no dose adjustment. And there were no infusion reactions. In contrast, the response rate in the randomized trials of pegloticase was only 42%, and 26% of pegloticase recipients experienced infusion reactions within 6 months.
“Although this is not [Food and Drug Administration] approved, it makes a lot of sense. From my standpoint, this is something that I’m doing now for my patients starting on pegloticase if there’s no contraindication to using methotrexate,” said Dr. Troum, a rheumatologist at the University of Southern California in Los Angeles.
“I’ve been doing this, too. This really did change my practice,” added his fellow panelist Alvin F. Wells, MD, PhD, director of the Rheumatology and Immunotherapy Center in Franklin, Wisc.
When they asked for a show of hands, only a handful of audience members indicated they are now using methotrexate in conjunction with pegloticase in their tophaceous gout patients.
Dr. Wells said his sole reservation about the practice involves using methotrexate in patients with an elevated creatinine level. What about using azathioprine or corticosteroids instead? he asked.
Dr. Troum replied that he monitors those patients carefully but sticks with the methotrexate because it’s only for a few months, which is the time frame in which patients are especially vulnerable to experiencing loss of response to pegloticase due to development of anti-drug antibodies.
Dr. Botson, who was in the Maui audience, rose to give a study update. With additional follow-up, he said, there has still been no signal of loss of response to pegloticase coadministered with methotrexate.
“A lot of us are starting to feel like immunosuppression, whether it’s with methotrexate or something else, is standard of care now,” according to the rheumatologist.
As to prescribing methotrexate in gout patients with renal insufficiency, he continued, he and his colleagues have given the matter quite a bit of thought.
“You’re talking about using methotrexate for 6 months in most of these cases. A lot of the patients who have really bad tophaceous gout already have renal insufficiency, and in the short term we haven’t really seen any problems with that. We work closely with a nephrologist on those cases. And a lot of nephrologists swear – although I don’t think the data are there – that they actually improve their renal function when we start to treat their tophaceous gout,” Dr. Botson said.
Dr. Troum and Dr. Wells reported serving as consultants to and on speakers bureaus for numerous pharmaceutical companies.
REPORTING FROM RWCS 2019
Making an effective referral is surprisingly complex
Referrals actually are a complex procedure that can result in crucial health, developmental, and mental health benefits, yet patients attend referred services at wildly variable rates of 11%-81%, and for mental health and early intervention (EI) less than half the time.1 When surveyed, primary care providers (PCP) say that they want to share in the care of 75% of patients they refer, especially for mental health concerns. Yet after decades of practice, I can count on one hand the number of children I have referred to mental health or EI services for whom I received feedback from the specialist (here meaning agencies or providers outside the office). Lately, if the specialist is using the same EHR, I sometimes discover their note when reviewing the document list, but I was not cc’d. In fact, the most common outcome is that the patient never sees the specialist and we don’t find out until the next visit, often months later when precious time for intervention has passed. Less than 50% of children with a mental health issue that qualifies as a disorder are detected by PCPs, and less than half of those children complete a referral. But there are lots of reasons for that, you say, such as a lack of specialists. But less than half of referrals for toddlers with developmental delays are completed to EI services even when such services are available and free of cost.
What makes referrals so complicated? Lack of referral completion can come from structural factors and interpersonal factors. We and our patients both are frustrated by lack of specialty resources, specialists who do not accept our patient’s insurance (or any insurance), distance, transportation, hours of operation issues, overall life burdens or priorities of families, and of course, cost. We can help with a few of these, either with our own list or ideally with the help of a care coordinator or social worker keeping a list identifying local specialists, payment methods accepted, and perhaps reduced-cost care options or financial assistance. However, the interpersonal issues that can make or break a referral definitely are within our reach.
Some of the reasons patients report for not following through on a referral include not feeling that their PCP evaluated the situation adequately through history or that the PCP failed to perform tests, such as screens. Because 27% of referrals are made based on the first phone contact about an issue (a dump?), and most are made at the first visit an issue is considered (two-thirds for mental health referrals), this feeling is unsurprising and likely true.2 Families often do not know what kind of expertise we have to size up a need, especially if discussion about development or mental health have not been a regular part of care before a problem is detected. Parents of children with developmental delays who declined referral felt they were more expert on their child’s development than the PCP. Another reason given for not attending a referral is that the condition being referred for and what to expect from the referral, including logistics, was not clear to the parents of the patient. Low-literacy parents (30% of low-income samples) did not find written materials helpful. Parents referred to EI services, for example, sometimes thought they were being sent to Child Protective Services or feared notification of immigration. PCPs who have more time for visits and/or had a care navigator available to explain the process have more successful referrals (80%), especially if the manager makes the phone contact, which takes a parent on average seven calls to EI. In some cases, the parent does not agree that a consultation is needed. If this had been part of the referral discussion, a shared understanding might have been attained or an intermediate step chosen.
In many practices, language, literacy, and cultural differences are major barriers. Other barriers come from the parent or another family member denying there is an issue, not believing that the intervention being suggested is effective, concern over stigma for diagnoses such as mental illness or autism, not prioritizing therapies we recommend over other potential solutions such as home efforts or herbal medicine, or simple fear. The key here is for us to both give information and nonjudgmentally listen to the parent’s (or child’s) point of view and barriers, showing empathy by echoing their feelings, then using a motivational interviewing approach to weighing pros and cons of taking steps towards a referral. Requesting a “Talk Back” from the parent of what you tried to convey can assure understanding. The “warm hand off” to a smiling colocated professional that is so helpful at overcoming fear has recently been simulated by onsite tele-intake visits, resulting in 80% of patients keeping a visit for mental health care.3
For collaborative and cost-efficient care, we need to provide the specialist with data we have gathered, what questions we want answered, how best to communicate back, and what role we want in subsequent care. Referral completion is three times higher when PCPs schedule the appointment and communicate with the specialist. We need back a timely note or call about their impression, any tests or treatments initiated, and their ideas about sharing care going forward. A structured referral template makes for more satisfactory communication, but the key is actually sending and receiving it! Most PCPs surveyed count on the family to convey information back from a specialist. This respects their ownership of the issue, but what they tell us may be inaccurate, incomplete, and/or miss concerns the specialist may not have wanted to tell the patient, such as rare but serious possibilities being considered or delicate social issues uncovered.
Great discrepancies have been found between the frequency PCPs report providing information to specialists (69%) and what specialists report about frequency of receipt (38%). PCPs report hearing back about 21% of mental health referrals.4 Both may be true if referral information is lost in the system somewhere. Simply faxing the referral form to EI programs (that routinely contact families) rather than just giving families a phone number (33%) increased referral success to 58%! Text reminders also hold promise. Finally, with such low completion rates, tracking referrals made and information back is crucial, yet only 6 of 17 practices in one study did so.5 Apart from intra-EHR referral, newer software-as-a-service systems can transmit consent forms that include permission and information for the specialist to contact the patient and report on kept appointments (such as CHADIS) as well as exchanging results (such as Salesforce) that hold promise for closing the loop.
New interest by health care systems in better referrals is not just caused by care considerations, but for financial reasons. Specialty care costs more than primary care management, and missed specialist appointments are not only missed opportunities but also costly! And one-half of all outpatient visits are for referrals! This may become the best motivator for your practice or system to undertake a quality improvement project to improve this crucial primary care procedure.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She reported no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. Email her at pdnews@mdedge.com
References
1. Acad Pediatr. 2014 May-Jun;14(3):315-23.
2. Hosp Community Psychiatry. 1992 May;43(5):489-93.
3. Pediatrics. 2019 Mar 1;143(3): e20182738.
4. Arch Pediatr Adolesc Med. 2000 May;154(5):499-506.
5. Pediatrics. 2010 Feb 1. doi: 10.1542/peds.2009-0388.
Referrals actually are a complex procedure that can result in crucial health, developmental, and mental health benefits, yet patients attend referred services at wildly variable rates of 11%-81%, and for mental health and early intervention (EI) less than half the time.1 When surveyed, primary care providers (PCP) say that they want to share in the care of 75% of patients they refer, especially for mental health concerns. Yet after decades of practice, I can count on one hand the number of children I have referred to mental health or EI services for whom I received feedback from the specialist (here meaning agencies or providers outside the office). Lately, if the specialist is using the same EHR, I sometimes discover their note when reviewing the document list, but I was not cc’d. In fact, the most common outcome is that the patient never sees the specialist and we don’t find out until the next visit, often months later when precious time for intervention has passed. Less than 50% of children with a mental health issue that qualifies as a disorder are detected by PCPs, and less than half of those children complete a referral. But there are lots of reasons for that, you say, such as a lack of specialists. But less than half of referrals for toddlers with developmental delays are completed to EI services even when such services are available and free of cost.
What makes referrals so complicated? Lack of referral completion can come from structural factors and interpersonal factors. We and our patients both are frustrated by lack of specialty resources, specialists who do not accept our patient’s insurance (or any insurance), distance, transportation, hours of operation issues, overall life burdens or priorities of families, and of course, cost. We can help with a few of these, either with our own list or ideally with the help of a care coordinator or social worker keeping a list identifying local specialists, payment methods accepted, and perhaps reduced-cost care options or financial assistance. However, the interpersonal issues that can make or break a referral definitely are within our reach.
Some of the reasons patients report for not following through on a referral include not feeling that their PCP evaluated the situation adequately through history or that the PCP failed to perform tests, such as screens. Because 27% of referrals are made based on the first phone contact about an issue (a dump?), and most are made at the first visit an issue is considered (two-thirds for mental health referrals), this feeling is unsurprising and likely true.2 Families often do not know what kind of expertise we have to size up a need, especially if discussion about development or mental health have not been a regular part of care before a problem is detected. Parents of children with developmental delays who declined referral felt they were more expert on their child’s development than the PCP. Another reason given for not attending a referral is that the condition being referred for and what to expect from the referral, including logistics, was not clear to the parents of the patient. Low-literacy parents (30% of low-income samples) did not find written materials helpful. Parents referred to EI services, for example, sometimes thought they were being sent to Child Protective Services or feared notification of immigration. PCPs who have more time for visits and/or had a care navigator available to explain the process have more successful referrals (80%), especially if the manager makes the phone contact, which takes a parent on average seven calls to EI. In some cases, the parent does not agree that a consultation is needed. If this had been part of the referral discussion, a shared understanding might have been attained or an intermediate step chosen.
In many practices, language, literacy, and cultural differences are major barriers. Other barriers come from the parent or another family member denying there is an issue, not believing that the intervention being suggested is effective, concern over stigma for diagnoses such as mental illness or autism, not prioritizing therapies we recommend over other potential solutions such as home efforts or herbal medicine, or simple fear. The key here is for us to both give information and nonjudgmentally listen to the parent’s (or child’s) point of view and barriers, showing empathy by echoing their feelings, then using a motivational interviewing approach to weighing pros and cons of taking steps towards a referral. Requesting a “Talk Back” from the parent of what you tried to convey can assure understanding. The “warm hand off” to a smiling colocated professional that is so helpful at overcoming fear has recently been simulated by onsite tele-intake visits, resulting in 80% of patients keeping a visit for mental health care.3
For collaborative and cost-efficient care, we need to provide the specialist with data we have gathered, what questions we want answered, how best to communicate back, and what role we want in subsequent care. Referral completion is three times higher when PCPs schedule the appointment and communicate with the specialist. We need back a timely note or call about their impression, any tests or treatments initiated, and their ideas about sharing care going forward. A structured referral template makes for more satisfactory communication, but the key is actually sending and receiving it! Most PCPs surveyed count on the family to convey information back from a specialist. This respects their ownership of the issue, but what they tell us may be inaccurate, incomplete, and/or miss concerns the specialist may not have wanted to tell the patient, such as rare but serious possibilities being considered or delicate social issues uncovered.
Great discrepancies have been found between the frequency PCPs report providing information to specialists (69%) and what specialists report about frequency of receipt (38%). PCPs report hearing back about 21% of mental health referrals.4 Both may be true if referral information is lost in the system somewhere. Simply faxing the referral form to EI programs (that routinely contact families) rather than just giving families a phone number (33%) increased referral success to 58%! Text reminders also hold promise. Finally, with such low completion rates, tracking referrals made and information back is crucial, yet only 6 of 17 practices in one study did so.5 Apart from intra-EHR referral, newer software-as-a-service systems can transmit consent forms that include permission and information for the specialist to contact the patient and report on kept appointments (such as CHADIS) as well as exchanging results (such as Salesforce) that hold promise for closing the loop.
New interest by health care systems in better referrals is not just caused by care considerations, but for financial reasons. Specialty care costs more than primary care management, and missed specialist appointments are not only missed opportunities but also costly! And one-half of all outpatient visits are for referrals! This may become the best motivator for your practice or system to undertake a quality improvement project to improve this crucial primary care procedure.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She reported no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. Email her at pdnews@mdedge.com
References
1. Acad Pediatr. 2014 May-Jun;14(3):315-23.
2. Hosp Community Psychiatry. 1992 May;43(5):489-93.
3. Pediatrics. 2019 Mar 1;143(3): e20182738.
4. Arch Pediatr Adolesc Med. 2000 May;154(5):499-506.
5. Pediatrics. 2010 Feb 1. doi: 10.1542/peds.2009-0388.
Referrals actually are a complex procedure that can result in crucial health, developmental, and mental health benefits, yet patients attend referred services at wildly variable rates of 11%-81%, and for mental health and early intervention (EI) less than half the time.1 When surveyed, primary care providers (PCP) say that they want to share in the care of 75% of patients they refer, especially for mental health concerns. Yet after decades of practice, I can count on one hand the number of children I have referred to mental health or EI services for whom I received feedback from the specialist (here meaning agencies or providers outside the office). Lately, if the specialist is using the same EHR, I sometimes discover their note when reviewing the document list, but I was not cc’d. In fact, the most common outcome is that the patient never sees the specialist and we don’t find out until the next visit, often months later when precious time for intervention has passed. Less than 50% of children with a mental health issue that qualifies as a disorder are detected by PCPs, and less than half of those children complete a referral. But there are lots of reasons for that, you say, such as a lack of specialists. But less than half of referrals for toddlers with developmental delays are completed to EI services even when such services are available and free of cost.
What makes referrals so complicated? Lack of referral completion can come from structural factors and interpersonal factors. We and our patients both are frustrated by lack of specialty resources, specialists who do not accept our patient’s insurance (or any insurance), distance, transportation, hours of operation issues, overall life burdens or priorities of families, and of course, cost. We can help with a few of these, either with our own list or ideally with the help of a care coordinator or social worker keeping a list identifying local specialists, payment methods accepted, and perhaps reduced-cost care options or financial assistance. However, the interpersonal issues that can make or break a referral definitely are within our reach.
Some of the reasons patients report for not following through on a referral include not feeling that their PCP evaluated the situation adequately through history or that the PCP failed to perform tests, such as screens. Because 27% of referrals are made based on the first phone contact about an issue (a dump?), and most are made at the first visit an issue is considered (two-thirds for mental health referrals), this feeling is unsurprising and likely true.2 Families often do not know what kind of expertise we have to size up a need, especially if discussion about development or mental health have not been a regular part of care before a problem is detected. Parents of children with developmental delays who declined referral felt they were more expert on their child’s development than the PCP. Another reason given for not attending a referral is that the condition being referred for and what to expect from the referral, including logistics, was not clear to the parents of the patient. Low-literacy parents (30% of low-income samples) did not find written materials helpful. Parents referred to EI services, for example, sometimes thought they were being sent to Child Protective Services or feared notification of immigration. PCPs who have more time for visits and/or had a care navigator available to explain the process have more successful referrals (80%), especially if the manager makes the phone contact, which takes a parent on average seven calls to EI. In some cases, the parent does not agree that a consultation is needed. If this had been part of the referral discussion, a shared understanding might have been attained or an intermediate step chosen.
In many practices, language, literacy, and cultural differences are major barriers. Other barriers come from the parent or another family member denying there is an issue, not believing that the intervention being suggested is effective, concern over stigma for diagnoses such as mental illness or autism, not prioritizing therapies we recommend over other potential solutions such as home efforts or herbal medicine, or simple fear. The key here is for us to both give information and nonjudgmentally listen to the parent’s (or child’s) point of view and barriers, showing empathy by echoing their feelings, then using a motivational interviewing approach to weighing pros and cons of taking steps towards a referral. Requesting a “Talk Back” from the parent of what you tried to convey can assure understanding. The “warm hand off” to a smiling colocated professional that is so helpful at overcoming fear has recently been simulated by onsite tele-intake visits, resulting in 80% of patients keeping a visit for mental health care.3
For collaborative and cost-efficient care, we need to provide the specialist with data we have gathered, what questions we want answered, how best to communicate back, and what role we want in subsequent care. Referral completion is three times higher when PCPs schedule the appointment and communicate with the specialist. We need back a timely note or call about their impression, any tests or treatments initiated, and their ideas about sharing care going forward. A structured referral template makes for more satisfactory communication, but the key is actually sending and receiving it! Most PCPs surveyed count on the family to convey information back from a specialist. This respects their ownership of the issue, but what they tell us may be inaccurate, incomplete, and/or miss concerns the specialist may not have wanted to tell the patient, such as rare but serious possibilities being considered or delicate social issues uncovered.
Great discrepancies have been found between the frequency PCPs report providing information to specialists (69%) and what specialists report about frequency of receipt (38%). PCPs report hearing back about 21% of mental health referrals.4 Both may be true if referral information is lost in the system somewhere. Simply faxing the referral form to EI programs (that routinely contact families) rather than just giving families a phone number (33%) increased referral success to 58%! Text reminders also hold promise. Finally, with such low completion rates, tracking referrals made and information back is crucial, yet only 6 of 17 practices in one study did so.5 Apart from intra-EHR referral, newer software-as-a-service systems can transmit consent forms that include permission and information for the specialist to contact the patient and report on kept appointments (such as CHADIS) as well as exchanging results (such as Salesforce) that hold promise for closing the loop.
New interest by health care systems in better referrals is not just caused by care considerations, but for financial reasons. Specialty care costs more than primary care management, and missed specialist appointments are not only missed opportunities but also costly! And one-half of all outpatient visits are for referrals! This may become the best motivator for your practice or system to undertake a quality improvement project to improve this crucial primary care procedure.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She reported no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. Email her at pdnews@mdedge.com
References
1. Acad Pediatr. 2014 May-Jun;14(3):315-23.
2. Hosp Community Psychiatry. 1992 May;43(5):489-93.
3. Pediatrics. 2019 Mar 1;143(3): e20182738.
4. Arch Pediatr Adolesc Med. 2000 May;154(5):499-506.
5. Pediatrics. 2010 Feb 1. doi: 10.1542/peds.2009-0388.
Adolescent psychiatric ED visits more than doubled in 4 years
a study found.
Visits by African American youth and Latino youth both increased by a significant amount, yet only a minority of all youth (16%) were seen by mental health professionals during their psychiatric ED visits.
“This study unmistakably reveals that adolescents are a population with urgent mental health needs,” Luther G. Kalb, PhD, of the Johns Hopkins Bloomberg School of Public Health and the Kennedy Krieger Institute in Baltimore, and his colleagues reported in Pediatrics. “Not only were their visits the most acute, but their probability of suicidal attempt and/or self-harm increased as well,” a finding that matches recent national increases in suicidal ideation.
The researchers used the 2011-2015 National Hospital Ambulatory Medical Care Survey to analyze data on psychiatric ED visits among U.S. youth aged 6-24 years. A psychiatric visit was identified based on the patient’s reason for visit and the International Classification of Diseases, ninth revision, codes for mood, behavioral, or substance use disorders; psychosis; or other psychiatric reasons. Suicide attempt or intentional self-harm were identified with reason for visit codes.
Psychiatric ED visits among all youth increased 28%, from 31 to 40 visits per 1,000 U.S. youth, in the period from 2011 to 2015, a finding “heavily driven by 2015, in which the largest increase in visits was observed,” the authors noted.
The biggest jump occurred among adolescents, whose visits increased 54%, and among black and Latino patients, whose visits rose 53% and 91%, respectively. Adolescent suicide-related and self-injury ED visits more than doubled from 2011 to 2015, from 5 to 12 visits per 1,000 U.S. youth. They were the only age group to see an increase in odds of a suicide-related visit over time (odds ratio, 1.27, P less than .01)
“Ultimately, it is unclear if the findings represent a change in identification (by providers) or reporting (by patients or family members) of mental health in the ED, a shift in the epidemiology of psychiatric disorders in the United States, or fluctuations in referral patterns or service-seeking behavior,” Dr. Kalb and his associates reported.
Study limitations included “an inability to confirm diagnostic validity” and some missing data for visit acuity and race/ethnicity, they said.
The research was funded by the National Institute of Health, and in part by the National Institute of Mental Health Intramural Research program. The authors reported no relevant financial disclosures.
SOURCE: Kalb LG et al. Pediatrics. 2019 Mar 18. doi: 10.1542/peds.2018-2192.
Even though the “fast-paced, stimulating environment” of an emergency department is not the ideal setting for children and families to receive care for mental health concerns, it remains a crucial safety net – particularly for lower-income individuals – because so many children lack access to mental health services, Dr. Chun and his associates wrote in an accompanying editorial.
“Some of the factors contributing to deficiencies in ED preparedness include a lack of staff trained in the identification and management of acute mental health problems; safe and quiet spaces within the ED; appropriate milieu for respectful, safe care; policies and procedures for ensuring best practices and consistent care; professional expertise to evaluate children’s mental health problems; and access to appropriate and timely follow-up care.”
Yet rates of youth’s psychiatric visits to the ED continue to climb because of a combination of increasing awareness, decreasing stigma for care seeking, limited mental health services, a shortage of pediatric mental health providers, insufficient specialty services, and true increases in mental health needs.
Solving this problem will require multiple coordinated approaches, but innovative options already are being explored, such as integrating child psychiatry services into EDs and “instituting next-day or other timely mental health evaluations,” they wrote. Other approaches include telepsychiatry; bringing mental health resources into schools and clinics; mental health resource sharing among communities; and mobile crisis units that visit schools, homes, primary care clinics, and low-resourced EDs.
Finally, medical training programs must change to adapt to the increasing need for pediatric mental health needs. “Embracing mental health problems as a routine component of pediatric medicine may be part of the solution to addressing the crisis,” they wrote.
Thomas H. Chun, MD, MPH, and Susan J. Duffy, MD, MPH, are at the Hasbro Children’s Hospital in Providence, R.I., and Jacqueline Grupp-Phelan, MD, MPH, is at the University of California, San Francisco, Benioff Children’s Hospital. These comments are a summary of a commentary accompanying Kalb et al. Pediatrics. 2019 Mar 18. doi: 10.1542/peds.2019-0251. The physicians used no external funding and had no financial disclosures relevant to their commentary.
Even though the “fast-paced, stimulating environment” of an emergency department is not the ideal setting for children and families to receive care for mental health concerns, it remains a crucial safety net – particularly for lower-income individuals – because so many children lack access to mental health services, Dr. Chun and his associates wrote in an accompanying editorial.
“Some of the factors contributing to deficiencies in ED preparedness include a lack of staff trained in the identification and management of acute mental health problems; safe and quiet spaces within the ED; appropriate milieu for respectful, safe care; policies and procedures for ensuring best practices and consistent care; professional expertise to evaluate children’s mental health problems; and access to appropriate and timely follow-up care.”
Yet rates of youth’s psychiatric visits to the ED continue to climb because of a combination of increasing awareness, decreasing stigma for care seeking, limited mental health services, a shortage of pediatric mental health providers, insufficient specialty services, and true increases in mental health needs.
Solving this problem will require multiple coordinated approaches, but innovative options already are being explored, such as integrating child psychiatry services into EDs and “instituting next-day or other timely mental health evaluations,” they wrote. Other approaches include telepsychiatry; bringing mental health resources into schools and clinics; mental health resource sharing among communities; and mobile crisis units that visit schools, homes, primary care clinics, and low-resourced EDs.
Finally, medical training programs must change to adapt to the increasing need for pediatric mental health needs. “Embracing mental health problems as a routine component of pediatric medicine may be part of the solution to addressing the crisis,” they wrote.
Thomas H. Chun, MD, MPH, and Susan J. Duffy, MD, MPH, are at the Hasbro Children’s Hospital in Providence, R.I., and Jacqueline Grupp-Phelan, MD, MPH, is at the University of California, San Francisco, Benioff Children’s Hospital. These comments are a summary of a commentary accompanying Kalb et al. Pediatrics. 2019 Mar 18. doi: 10.1542/peds.2019-0251. The physicians used no external funding and had no financial disclosures relevant to their commentary.
Even though the “fast-paced, stimulating environment” of an emergency department is not the ideal setting for children and families to receive care for mental health concerns, it remains a crucial safety net – particularly for lower-income individuals – because so many children lack access to mental health services, Dr. Chun and his associates wrote in an accompanying editorial.
“Some of the factors contributing to deficiencies in ED preparedness include a lack of staff trained in the identification and management of acute mental health problems; safe and quiet spaces within the ED; appropriate milieu for respectful, safe care; policies and procedures for ensuring best practices and consistent care; professional expertise to evaluate children’s mental health problems; and access to appropriate and timely follow-up care.”
Yet rates of youth’s psychiatric visits to the ED continue to climb because of a combination of increasing awareness, decreasing stigma for care seeking, limited mental health services, a shortage of pediatric mental health providers, insufficient specialty services, and true increases in mental health needs.
Solving this problem will require multiple coordinated approaches, but innovative options already are being explored, such as integrating child psychiatry services into EDs and “instituting next-day or other timely mental health evaluations,” they wrote. Other approaches include telepsychiatry; bringing mental health resources into schools and clinics; mental health resource sharing among communities; and mobile crisis units that visit schools, homes, primary care clinics, and low-resourced EDs.
Finally, medical training programs must change to adapt to the increasing need for pediatric mental health needs. “Embracing mental health problems as a routine component of pediatric medicine may be part of the solution to addressing the crisis,” they wrote.
Thomas H. Chun, MD, MPH, and Susan J. Duffy, MD, MPH, are at the Hasbro Children’s Hospital in Providence, R.I., and Jacqueline Grupp-Phelan, MD, MPH, is at the University of California, San Francisco, Benioff Children’s Hospital. These comments are a summary of a commentary accompanying Kalb et al. Pediatrics. 2019 Mar 18. doi: 10.1542/peds.2019-0251. The physicians used no external funding and had no financial disclosures relevant to their commentary.
a study found.
Visits by African American youth and Latino youth both increased by a significant amount, yet only a minority of all youth (16%) were seen by mental health professionals during their psychiatric ED visits.
“This study unmistakably reveals that adolescents are a population with urgent mental health needs,” Luther G. Kalb, PhD, of the Johns Hopkins Bloomberg School of Public Health and the Kennedy Krieger Institute in Baltimore, and his colleagues reported in Pediatrics. “Not only were their visits the most acute, but their probability of suicidal attempt and/or self-harm increased as well,” a finding that matches recent national increases in suicidal ideation.
The researchers used the 2011-2015 National Hospital Ambulatory Medical Care Survey to analyze data on psychiatric ED visits among U.S. youth aged 6-24 years. A psychiatric visit was identified based on the patient’s reason for visit and the International Classification of Diseases, ninth revision, codes for mood, behavioral, or substance use disorders; psychosis; or other psychiatric reasons. Suicide attempt or intentional self-harm were identified with reason for visit codes.
Psychiatric ED visits among all youth increased 28%, from 31 to 40 visits per 1,000 U.S. youth, in the period from 2011 to 2015, a finding “heavily driven by 2015, in which the largest increase in visits was observed,” the authors noted.
The biggest jump occurred among adolescents, whose visits increased 54%, and among black and Latino patients, whose visits rose 53% and 91%, respectively. Adolescent suicide-related and self-injury ED visits more than doubled from 2011 to 2015, from 5 to 12 visits per 1,000 U.S. youth. They were the only age group to see an increase in odds of a suicide-related visit over time (odds ratio, 1.27, P less than .01)
“Ultimately, it is unclear if the findings represent a change in identification (by providers) or reporting (by patients or family members) of mental health in the ED, a shift in the epidemiology of psychiatric disorders in the United States, or fluctuations in referral patterns or service-seeking behavior,” Dr. Kalb and his associates reported.
Study limitations included “an inability to confirm diagnostic validity” and some missing data for visit acuity and race/ethnicity, they said.
The research was funded by the National Institute of Health, and in part by the National Institute of Mental Health Intramural Research program. The authors reported no relevant financial disclosures.
SOURCE: Kalb LG et al. Pediatrics. 2019 Mar 18. doi: 10.1542/peds.2018-2192.
a study found.
Visits by African American youth and Latino youth both increased by a significant amount, yet only a minority of all youth (16%) were seen by mental health professionals during their psychiatric ED visits.
“This study unmistakably reveals that adolescents are a population with urgent mental health needs,” Luther G. Kalb, PhD, of the Johns Hopkins Bloomberg School of Public Health and the Kennedy Krieger Institute in Baltimore, and his colleagues reported in Pediatrics. “Not only were their visits the most acute, but their probability of suicidal attempt and/or self-harm increased as well,” a finding that matches recent national increases in suicidal ideation.
The researchers used the 2011-2015 National Hospital Ambulatory Medical Care Survey to analyze data on psychiatric ED visits among U.S. youth aged 6-24 years. A psychiatric visit was identified based on the patient’s reason for visit and the International Classification of Diseases, ninth revision, codes for mood, behavioral, or substance use disorders; psychosis; or other psychiatric reasons. Suicide attempt or intentional self-harm were identified with reason for visit codes.
Psychiatric ED visits among all youth increased 28%, from 31 to 40 visits per 1,000 U.S. youth, in the period from 2011 to 2015, a finding “heavily driven by 2015, in which the largest increase in visits was observed,” the authors noted.
The biggest jump occurred among adolescents, whose visits increased 54%, and among black and Latino patients, whose visits rose 53% and 91%, respectively. Adolescent suicide-related and self-injury ED visits more than doubled from 2011 to 2015, from 5 to 12 visits per 1,000 U.S. youth. They were the only age group to see an increase in odds of a suicide-related visit over time (odds ratio, 1.27, P less than .01)
“Ultimately, it is unclear if the findings represent a change in identification (by providers) or reporting (by patients or family members) of mental health in the ED, a shift in the epidemiology of psychiatric disorders in the United States, or fluctuations in referral patterns or service-seeking behavior,” Dr. Kalb and his associates reported.
Study limitations included “an inability to confirm diagnostic validity” and some missing data for visit acuity and race/ethnicity, they said.
The research was funded by the National Institute of Health, and in part by the National Institute of Mental Health Intramural Research program. The authors reported no relevant financial disclosures.
SOURCE: Kalb LG et al. Pediatrics. 2019 Mar 18. doi: 10.1542/peds.2018-2192.
FROM PEDIATRICS
Patients at risk of RA may already have abnormal aortic stiffness
according to a study of potential RA patients who underwent cardiac MRI.
“To our knowledge, this is the first study showing subclinical increase in aortic stiffness in at-risk individuals for RA, with values numerically close to those seen in early, treatment-naive RA,” wrote Graham Fent, MBChB, of the University of Leeds (England) and his associates. The study was published in Annals of the Rheumatic Diseases.
Hypothesizing that patients with no systemic inflammation but circulating anti–cyclic citrullinated peptide (CCP) antibodies may already have cardiovascular concerns, Dr. Fent and his colleagues recruited 18 individuals at risk of developing RA and 30 healthy controls. The groups were matched for age and gender and then underwent multiparametric 3.0 Tesla cardiac MRI with late gadolinium enhancement. The at-risk individuals were classified as being at either low (n = 10) or high (n = 8) risk of RA. Over 12 months, five of the at-risk patients progressed to RA.
According to the cardiac MRI findings, aortic distensibility was lower – and thus arterial stiffness was greater – in the at-risk group (3.6 x 10–3 per mm Hg) versus the healthy controls (4.9 x 10–3 per mm Hg). The difference was even more distinct in the high-risk group (3.1 x 10–3 per mm Hg), compared with the low-risk group (4.2 x 10–3 per mm Hg). The group who eventually progressed to RA also showed lower levels of distensibility (3.2 x 10–3 per mm Hg).
The coauthors acknowledged that the major limitation of their study was a lack of control groups. However, they noted that such a pronounced level of aortic stiffness in the high-risk and RA groups should be seen as “implying a particular role of CCP antibodies.”
The study was supported by the U.K. National Institute for Health Research. One author reported being funded by a National Institute for Health Research grant; another reported being funded by a British Heart Foundation Personal Chair.
SOURCE: Fent G et al. Ann Rheum Dis. 2019 Mar 9. doi: 10.1136/annrheumdis-2018-214975.
according to a study of potential RA patients who underwent cardiac MRI.
“To our knowledge, this is the first study showing subclinical increase in aortic stiffness in at-risk individuals for RA, with values numerically close to those seen in early, treatment-naive RA,” wrote Graham Fent, MBChB, of the University of Leeds (England) and his associates. The study was published in Annals of the Rheumatic Diseases.
Hypothesizing that patients with no systemic inflammation but circulating anti–cyclic citrullinated peptide (CCP) antibodies may already have cardiovascular concerns, Dr. Fent and his colleagues recruited 18 individuals at risk of developing RA and 30 healthy controls. The groups were matched for age and gender and then underwent multiparametric 3.0 Tesla cardiac MRI with late gadolinium enhancement. The at-risk individuals were classified as being at either low (n = 10) or high (n = 8) risk of RA. Over 12 months, five of the at-risk patients progressed to RA.
According to the cardiac MRI findings, aortic distensibility was lower – and thus arterial stiffness was greater – in the at-risk group (3.6 x 10–3 per mm Hg) versus the healthy controls (4.9 x 10–3 per mm Hg). The difference was even more distinct in the high-risk group (3.1 x 10–3 per mm Hg), compared with the low-risk group (4.2 x 10–3 per mm Hg). The group who eventually progressed to RA also showed lower levels of distensibility (3.2 x 10–3 per mm Hg).
The coauthors acknowledged that the major limitation of their study was a lack of control groups. However, they noted that such a pronounced level of aortic stiffness in the high-risk and RA groups should be seen as “implying a particular role of CCP antibodies.”
The study was supported by the U.K. National Institute for Health Research. One author reported being funded by a National Institute for Health Research grant; another reported being funded by a British Heart Foundation Personal Chair.
SOURCE: Fent G et al. Ann Rheum Dis. 2019 Mar 9. doi: 10.1136/annrheumdis-2018-214975.
according to a study of potential RA patients who underwent cardiac MRI.
“To our knowledge, this is the first study showing subclinical increase in aortic stiffness in at-risk individuals for RA, with values numerically close to those seen in early, treatment-naive RA,” wrote Graham Fent, MBChB, of the University of Leeds (England) and his associates. The study was published in Annals of the Rheumatic Diseases.
Hypothesizing that patients with no systemic inflammation but circulating anti–cyclic citrullinated peptide (CCP) antibodies may already have cardiovascular concerns, Dr. Fent and his colleagues recruited 18 individuals at risk of developing RA and 30 healthy controls. The groups were matched for age and gender and then underwent multiparametric 3.0 Tesla cardiac MRI with late gadolinium enhancement. The at-risk individuals were classified as being at either low (n = 10) or high (n = 8) risk of RA. Over 12 months, five of the at-risk patients progressed to RA.
According to the cardiac MRI findings, aortic distensibility was lower – and thus arterial stiffness was greater – in the at-risk group (3.6 x 10–3 per mm Hg) versus the healthy controls (4.9 x 10–3 per mm Hg). The difference was even more distinct in the high-risk group (3.1 x 10–3 per mm Hg), compared with the low-risk group (4.2 x 10–3 per mm Hg). The group who eventually progressed to RA also showed lower levels of distensibility (3.2 x 10–3 per mm Hg).
The coauthors acknowledged that the major limitation of their study was a lack of control groups. However, they noted that such a pronounced level of aortic stiffness in the high-risk and RA groups should be seen as “implying a particular role of CCP antibodies.”
The study was supported by the U.K. National Institute for Health Research. One author reported being funded by a National Institute for Health Research grant; another reported being funded by a British Heart Foundation Personal Chair.
SOURCE: Fent G et al. Ann Rheum Dis. 2019 Mar 9. doi: 10.1136/annrheumdis-2018-214975.
FROM ANNALS OF THE RHEUMATIC DISEASES
FDA approves atezolizumab for first-line ES-SCLC treatment
The Food and Drug Administration has approved atezolizumab (Tecentriq), in combination with carboplatin and etoposide, for the first-line treatment of adults with extensive-stage small cell lung cancer (ES-SCLC).
Approval was based on results from the phase 3 IMpower133 study, in which 403 treatment-naive patients with ES-SCLC received atezolizumab at 1,200 mg with carboplatin at 5 mg/mL per minute on day 1 and etoposide 100 mg/m2 on days 1, 2, and 3 of a 21-day cycle for four cycles, followed by atezolizumab at 1,200 mg once every 3 weeks until disease progression or unacceptable toxicity; or received placebo with the same dosage of carboplatin and etoposide for a similar duration.
Overall survival was significantly better in patients who received atezolizumab, compared with placebo (12.3 vs. 10.3 months; hazard ratio, 0.70; 95% confidence interval, 0.54-0.91; P = .0069), as was progression-free survival (5.2 vs. 4.3 months; HR, 0.77; 95% CI, 0.62-0.96; P = .017).
The most common adverse events associated with atezolizumab in the study were fatigue/asthenia, nausea, alopecia, constipation, and decreased appetite.
According to the FDA, the recommended dose is 1,200 mg IV over 60 minutes every 3 weeks. When administered on the same day as chemotherapy, atezolizumab should be given first. If the first infusion is tolerated, all subsequent infusions can be delivered over 30 minutes.
Find the full press release on the FDA website.
The Food and Drug Administration has approved atezolizumab (Tecentriq), in combination with carboplatin and etoposide, for the first-line treatment of adults with extensive-stage small cell lung cancer (ES-SCLC).
Approval was based on results from the phase 3 IMpower133 study, in which 403 treatment-naive patients with ES-SCLC received atezolizumab at 1,200 mg with carboplatin at 5 mg/mL per minute on day 1 and etoposide 100 mg/m2 on days 1, 2, and 3 of a 21-day cycle for four cycles, followed by atezolizumab at 1,200 mg once every 3 weeks until disease progression or unacceptable toxicity; or received placebo with the same dosage of carboplatin and etoposide for a similar duration.
Overall survival was significantly better in patients who received atezolizumab, compared with placebo (12.3 vs. 10.3 months; hazard ratio, 0.70; 95% confidence interval, 0.54-0.91; P = .0069), as was progression-free survival (5.2 vs. 4.3 months; HR, 0.77; 95% CI, 0.62-0.96; P = .017).
The most common adverse events associated with atezolizumab in the study were fatigue/asthenia, nausea, alopecia, constipation, and decreased appetite.
According to the FDA, the recommended dose is 1,200 mg IV over 60 minutes every 3 weeks. When administered on the same day as chemotherapy, atezolizumab should be given first. If the first infusion is tolerated, all subsequent infusions can be delivered over 30 minutes.
Find the full press release on the FDA website.
The Food and Drug Administration has approved atezolizumab (Tecentriq), in combination with carboplatin and etoposide, for the first-line treatment of adults with extensive-stage small cell lung cancer (ES-SCLC).
Approval was based on results from the phase 3 IMpower133 study, in which 403 treatment-naive patients with ES-SCLC received atezolizumab at 1,200 mg with carboplatin at 5 mg/mL per minute on day 1 and etoposide 100 mg/m2 on days 1, 2, and 3 of a 21-day cycle for four cycles, followed by atezolizumab at 1,200 mg once every 3 weeks until disease progression or unacceptable toxicity; or received placebo with the same dosage of carboplatin and etoposide for a similar duration.
Overall survival was significantly better in patients who received atezolizumab, compared with placebo (12.3 vs. 10.3 months; hazard ratio, 0.70; 95% confidence interval, 0.54-0.91; P = .0069), as was progression-free survival (5.2 vs. 4.3 months; HR, 0.77; 95% CI, 0.62-0.96; P = .017).
The most common adverse events associated with atezolizumab in the study were fatigue/asthenia, nausea, alopecia, constipation, and decreased appetite.
According to the FDA, the recommended dose is 1,200 mg IV over 60 minutes every 3 weeks. When administered on the same day as chemotherapy, atezolizumab should be given first. If the first infusion is tolerated, all subsequent infusions can be delivered over 30 minutes.
Find the full press release on the FDA website.
Socioeconomic status affects scleroderma severity in African Americans
according to findings from an analysis of single-center cohort data over a 10-year period.
Indeed, among patients in the cohort of 402 scleroderma patients at MedStar Georgetown University Hospital in Washington, lower household income was predictive of higher mortality during follow-up, independent of race, according to first author Duncan F. Moore, MD, and his colleagues at the hospital.
Previous studies have demonstrated increased risk for scleroderma in African American patients, who also are more likely than non–African Americans to be diagnosed at a younger age and to have conditions including more diffuse cutaneous disease, more severe restrictive lung disease, more cardiac and renal involvement, and increased mortality, the authors wrote in Arthritis Care & Research.
“We did clearly show that African Americans have worse outcomes and severe pulmonary involvement, but I was surprised that there still was a major contribution of socioeconomic status affecting outcomes for all patients, even though only 10% of our patients were indigent and on medical assistance,” Virginia Steen, MD, senior author of the study and professor of rheumatology at Georgetown University, said in an interview. “I still feel strongly that there are likely genetic issues as to why African Americans have such severe disease. We are eager to learn more from the GRASP [Genome Research in African American Scleroderma Patients] study, which is specifically looking at the genetic issues in African American scleroderma patients,” she said.
Of the 402 scleroderma patients at MedStar Georgetown who were seen during 2006-2016, 202 were African American. A total of 186 African American and 184 non–African American patients in the study met the 2013 American College of Rheumatology/European League Against Rheumatism criteria for systemic sclerosis (SSc). Demographics including gender (87% female) and age (mean of 48 years) were similar between the groups.
Overall, the African American patients showed more severe lung disease, more pulmonary hypertension, and more severe cardiac involvement than did non–African American patients, and autoantibodies were significantly different between the groups.
During follow-up, mortality proved much higher among African Americans at 21%, compared with 11% in non–African Americans (P = .005). However, the unadjusted hazard ratio for death declined from 2.061 (P = .006) to a nonsignificant 1.256 after adjustment for socioeconomic variables.
All socioeconomic measures showed significant differences between the groups. African Americans were more likely to be single and disabled at the initial study visit and to have Medicaid, but they were less likely to be a homemaker, have private insurance, or have a college degree. African Americans’ $74,000 median household income (based on ZIP code) was also a statistically significant $23,000 less than non–African American patients. But the researchers noted that “for every additional $10,000 of household income, independent of race, the hazard of death during follow-up declined by 15.5%.”
Notable differences in antibodies appeared between the groups, with more African American patients having isolated nucleolar ANA, anti-U1RNP antibody, or other positive antinuclear antibodies without SSc-specific antibodies. African American patients also were less likely to have anticentromere or anti-RNA polymerase III antibodies.
The study findings were limited by several factors, including possible bias in the matching process and the use of only index values for socioeconomic variables, the researchers noted.
Regardless of relative socioeconomic and genetic influences, “it is clear that African Americans with scleroderma merit more intensive efforts to facilitate timely diagnosis and access to continued evaluation and suppressive treatment, particularly with respect to cardiopulmonary involvement,” they wrote.
Next steps for research, according to Dr. Steen, include studying clinical subsets of African American patients to try to identify factors to predict outcomes, including the nucleolar pattern ANA, overlap with lupus, history of hypertension, and the relationship with renal crisis.
“We are also looking at whether the African American patients are less responsive to mycophenolate than the non–African American patients. We definitely need to find ways to be more aggressive at identifying and treating African American patients early in their disease,” she added.
The researchers had no financial conflicts to disclose. Dr. Steen serves on the MDedge Rheumatology Editorial Advisory Board.
SOURCE: Moore DF et al. Arthritis Care Res. 2019 March 1. doi: 10.1002/acr.23861.
“Not only do patients who manifest the diffuse cutaneous subset of disease experience a more severe course, but so do affected persons of African American race,” Nadia D. Morgan, MBBS, and Allan C. Gelber, MD, wrote in an accompanying editorial. The effects of socioeconomic status should not be overlooked based on the current study, in which the inclusion of socioeconomic factors eliminated the significance of association between race and mortality among scleroderma patients, they wrote.
“Overall, and in the context of these published reports which underscore the disproportionate and adverse impact of scleroderma among African Americans, and in light of the ongoing efforts of the GRASP study, the current paper by Moore et al. emphasizes the importance of socioeconomic status, and of socioeconomic determinants of health, to account for differences in clinically relevant outcomes,” they wrote.
Dr. Gelber is affiliated with the division of rheumatology at Johns Hopkins University, Baltimore. Dr. Morgan, who was also with Johns Hopkins, died before publication of the editorial. They made no conflict of interest disclosures.
“Not only do patients who manifest the diffuse cutaneous subset of disease experience a more severe course, but so do affected persons of African American race,” Nadia D. Morgan, MBBS, and Allan C. Gelber, MD, wrote in an accompanying editorial. The effects of socioeconomic status should not be overlooked based on the current study, in which the inclusion of socioeconomic factors eliminated the significance of association between race and mortality among scleroderma patients, they wrote.
“Overall, and in the context of these published reports which underscore the disproportionate and adverse impact of scleroderma among African Americans, and in light of the ongoing efforts of the GRASP study, the current paper by Moore et al. emphasizes the importance of socioeconomic status, and of socioeconomic determinants of health, to account for differences in clinically relevant outcomes,” they wrote.
Dr. Gelber is affiliated with the division of rheumatology at Johns Hopkins University, Baltimore. Dr. Morgan, who was also with Johns Hopkins, died before publication of the editorial. They made no conflict of interest disclosures.
“Not only do patients who manifest the diffuse cutaneous subset of disease experience a more severe course, but so do affected persons of African American race,” Nadia D. Morgan, MBBS, and Allan C. Gelber, MD, wrote in an accompanying editorial. The effects of socioeconomic status should not be overlooked based on the current study, in which the inclusion of socioeconomic factors eliminated the significance of association between race and mortality among scleroderma patients, they wrote.
“Overall, and in the context of these published reports which underscore the disproportionate and adverse impact of scleroderma among African Americans, and in light of the ongoing efforts of the GRASP study, the current paper by Moore et al. emphasizes the importance of socioeconomic status, and of socioeconomic determinants of health, to account for differences in clinically relevant outcomes,” they wrote.
Dr. Gelber is affiliated with the division of rheumatology at Johns Hopkins University, Baltimore. Dr. Morgan, who was also with Johns Hopkins, died before publication of the editorial. They made no conflict of interest disclosures.
according to findings from an analysis of single-center cohort data over a 10-year period.
Indeed, among patients in the cohort of 402 scleroderma patients at MedStar Georgetown University Hospital in Washington, lower household income was predictive of higher mortality during follow-up, independent of race, according to first author Duncan F. Moore, MD, and his colleagues at the hospital.
Previous studies have demonstrated increased risk for scleroderma in African American patients, who also are more likely than non–African Americans to be diagnosed at a younger age and to have conditions including more diffuse cutaneous disease, more severe restrictive lung disease, more cardiac and renal involvement, and increased mortality, the authors wrote in Arthritis Care & Research.
“We did clearly show that African Americans have worse outcomes and severe pulmonary involvement, but I was surprised that there still was a major contribution of socioeconomic status affecting outcomes for all patients, even though only 10% of our patients were indigent and on medical assistance,” Virginia Steen, MD, senior author of the study and professor of rheumatology at Georgetown University, said in an interview. “I still feel strongly that there are likely genetic issues as to why African Americans have such severe disease. We are eager to learn more from the GRASP [Genome Research in African American Scleroderma Patients] study, which is specifically looking at the genetic issues in African American scleroderma patients,” she said.
Of the 402 scleroderma patients at MedStar Georgetown who were seen during 2006-2016, 202 were African American. A total of 186 African American and 184 non–African American patients in the study met the 2013 American College of Rheumatology/European League Against Rheumatism criteria for systemic sclerosis (SSc). Demographics including gender (87% female) and age (mean of 48 years) were similar between the groups.
Overall, the African American patients showed more severe lung disease, more pulmonary hypertension, and more severe cardiac involvement than did non–African American patients, and autoantibodies were significantly different between the groups.
During follow-up, mortality proved much higher among African Americans at 21%, compared with 11% in non–African Americans (P = .005). However, the unadjusted hazard ratio for death declined from 2.061 (P = .006) to a nonsignificant 1.256 after adjustment for socioeconomic variables.
All socioeconomic measures showed significant differences between the groups. African Americans were more likely to be single and disabled at the initial study visit and to have Medicaid, but they were less likely to be a homemaker, have private insurance, or have a college degree. African Americans’ $74,000 median household income (based on ZIP code) was also a statistically significant $23,000 less than non–African American patients. But the researchers noted that “for every additional $10,000 of household income, independent of race, the hazard of death during follow-up declined by 15.5%.”
Notable differences in antibodies appeared between the groups, with more African American patients having isolated nucleolar ANA, anti-U1RNP antibody, or other positive antinuclear antibodies without SSc-specific antibodies. African American patients also were less likely to have anticentromere or anti-RNA polymerase III antibodies.
The study findings were limited by several factors, including possible bias in the matching process and the use of only index values for socioeconomic variables, the researchers noted.
Regardless of relative socioeconomic and genetic influences, “it is clear that African Americans with scleroderma merit more intensive efforts to facilitate timely diagnosis and access to continued evaluation and suppressive treatment, particularly with respect to cardiopulmonary involvement,” they wrote.
Next steps for research, according to Dr. Steen, include studying clinical subsets of African American patients to try to identify factors to predict outcomes, including the nucleolar pattern ANA, overlap with lupus, history of hypertension, and the relationship with renal crisis.
“We are also looking at whether the African American patients are less responsive to mycophenolate than the non–African American patients. We definitely need to find ways to be more aggressive at identifying and treating African American patients early in their disease,” she added.
The researchers had no financial conflicts to disclose. Dr. Steen serves on the MDedge Rheumatology Editorial Advisory Board.
SOURCE: Moore DF et al. Arthritis Care Res. 2019 March 1. doi: 10.1002/acr.23861.
according to findings from an analysis of single-center cohort data over a 10-year period.
Indeed, among patients in the cohort of 402 scleroderma patients at MedStar Georgetown University Hospital in Washington, lower household income was predictive of higher mortality during follow-up, independent of race, according to first author Duncan F. Moore, MD, and his colleagues at the hospital.
Previous studies have demonstrated increased risk for scleroderma in African American patients, who also are more likely than non–African Americans to be diagnosed at a younger age and to have conditions including more diffuse cutaneous disease, more severe restrictive lung disease, more cardiac and renal involvement, and increased mortality, the authors wrote in Arthritis Care & Research.
“We did clearly show that African Americans have worse outcomes and severe pulmonary involvement, but I was surprised that there still was a major contribution of socioeconomic status affecting outcomes for all patients, even though only 10% of our patients were indigent and on medical assistance,” Virginia Steen, MD, senior author of the study and professor of rheumatology at Georgetown University, said in an interview. “I still feel strongly that there are likely genetic issues as to why African Americans have such severe disease. We are eager to learn more from the GRASP [Genome Research in African American Scleroderma Patients] study, which is specifically looking at the genetic issues in African American scleroderma patients,” she said.
Of the 402 scleroderma patients at MedStar Georgetown who were seen during 2006-2016, 202 were African American. A total of 186 African American and 184 non–African American patients in the study met the 2013 American College of Rheumatology/European League Against Rheumatism criteria for systemic sclerosis (SSc). Demographics including gender (87% female) and age (mean of 48 years) were similar between the groups.
Overall, the African American patients showed more severe lung disease, more pulmonary hypertension, and more severe cardiac involvement than did non–African American patients, and autoantibodies were significantly different between the groups.
During follow-up, mortality proved much higher among African Americans at 21%, compared with 11% in non–African Americans (P = .005). However, the unadjusted hazard ratio for death declined from 2.061 (P = .006) to a nonsignificant 1.256 after adjustment for socioeconomic variables.
All socioeconomic measures showed significant differences between the groups. African Americans were more likely to be single and disabled at the initial study visit and to have Medicaid, but they were less likely to be a homemaker, have private insurance, or have a college degree. African Americans’ $74,000 median household income (based on ZIP code) was also a statistically significant $23,000 less than non–African American patients. But the researchers noted that “for every additional $10,000 of household income, independent of race, the hazard of death during follow-up declined by 15.5%.”
Notable differences in antibodies appeared between the groups, with more African American patients having isolated nucleolar ANA, anti-U1RNP antibody, or other positive antinuclear antibodies without SSc-specific antibodies. African American patients also were less likely to have anticentromere or anti-RNA polymerase III antibodies.
The study findings were limited by several factors, including possible bias in the matching process and the use of only index values for socioeconomic variables, the researchers noted.
Regardless of relative socioeconomic and genetic influences, “it is clear that African Americans with scleroderma merit more intensive efforts to facilitate timely diagnosis and access to continued evaluation and suppressive treatment, particularly with respect to cardiopulmonary involvement,” they wrote.
Next steps for research, according to Dr. Steen, include studying clinical subsets of African American patients to try to identify factors to predict outcomes, including the nucleolar pattern ANA, overlap with lupus, history of hypertension, and the relationship with renal crisis.
“We are also looking at whether the African American patients are less responsive to mycophenolate than the non–African American patients. We definitely need to find ways to be more aggressive at identifying and treating African American patients early in their disease,” she added.
The researchers had no financial conflicts to disclose. Dr. Steen serves on the MDedge Rheumatology Editorial Advisory Board.
SOURCE: Moore DF et al. Arthritis Care Res. 2019 March 1. doi: 10.1002/acr.23861.
FROM ARTHRITIS CARE & RESEARCH
Will inpatient albumin help in decompensated cirrhosis?
PHILADELPHIA – , according to Vijay Shah, MD, chair of the division of gastroenterology and hepatology at Mayo Clinic, Rochester, Minn.
“These are interesting studies, but I don’t think we’re ready yet to use this broadly,” Dr. Shah said at the meeting jointly provided by Rutgers and Global Academy for Medical Education.
Current guidelines do describe the use of albumin for large-volume paracentesis and other specific inpatient situations; however, extrapolating its long-term use has been explored in two major studies that recently came out with contradictory findings.
In the ANSWER trial, as reported in the Lancet, investigators at 33 centers randomized patients with cirrhosis and uncomplicated ascites to either standard medical treatment with or without human albumin, at 40 g twice weekly for 2 weeks, followed by 40 g weekly for up to 18 months.
Those investigators found that long-term albumin prolonged overall survival, with a 38% reduction in the mortality hazard ratio, with similar rates of serious, nonliver adverse events, leading them to conclude that this intervention may act as a disease-modifying treatment in decompensated cirrhosis patients.
By contrast, however, a recent randomized, placebo-controlled trial reported in the Journal of Hepatology showed that albumin plus midodrine, an alpha-adrenergic vasoconstrictor, did not improve survival among patients with decompensated cirrhosis on the liver transplant waiting list, at least at the doses administered (midodrine 15-30 mg/day and albumin 40 g every 15 days for a year).
While this particular combination of albumin plus midodrine did decrease renin and aldosterone levels, the intervention did not prevent complications or improve survival, investigators said at the time. Complication rates were 37% and 43% for treatment and placebo, respectively (P = .402), with low rates of death in both groups and no significant difference in mortality at 1 year (P = .527).
Dr. Shah said the discrepant results may be attributable to specific differences in study design or enrollment.
“It’s just hand waving, but it may be related to the dose of albumin, or may be related to the types of patients – the second study was in patients who are waiting for liver transplantation,” he told attendees. “But I don’t think that there’s currently enough evidence to use albumin in your patients in the outpatient setting.”
There is a third study, recently published in the American Journal of Gastroenterology, looking at data for a large end-stage liver disease cohort with hyponatremia. Investigators observed a higher rate of hyponatremia resolution and improved 30-day survival in those who had received albumin (total mean amount, 225 g) versus those who had not.
Considering all of this evidence taken together, Dr. Shah said he would not favor using outpatient albumin at this point – though he advised attendees to watch for a currently recruiting phase 3 randomized study, known as PRECIOSA, which is evaluating long-term administration of human albumin 20% injectable solution, dosed by body weight, in patients with decompensated cirrhosis and ascites.
Dr. Shah indicated that he is a consultant for Afimmune, Durect Corporation, Enterome, GRI Bio, Merck Research Laboratories, Novartis Pharma, and Vital Therapeutics. Global Academy and this news organization are owned by the same company.
PHILADELPHIA – , according to Vijay Shah, MD, chair of the division of gastroenterology and hepatology at Mayo Clinic, Rochester, Minn.
“These are interesting studies, but I don’t think we’re ready yet to use this broadly,” Dr. Shah said at the meeting jointly provided by Rutgers and Global Academy for Medical Education.
Current guidelines do describe the use of albumin for large-volume paracentesis and other specific inpatient situations; however, extrapolating its long-term use has been explored in two major studies that recently came out with contradictory findings.
In the ANSWER trial, as reported in the Lancet, investigators at 33 centers randomized patients with cirrhosis and uncomplicated ascites to either standard medical treatment with or without human albumin, at 40 g twice weekly for 2 weeks, followed by 40 g weekly for up to 18 months.
Those investigators found that long-term albumin prolonged overall survival, with a 38% reduction in the mortality hazard ratio, with similar rates of serious, nonliver adverse events, leading them to conclude that this intervention may act as a disease-modifying treatment in decompensated cirrhosis patients.
By contrast, however, a recent randomized, placebo-controlled trial reported in the Journal of Hepatology showed that albumin plus midodrine, an alpha-adrenergic vasoconstrictor, did not improve survival among patients with decompensated cirrhosis on the liver transplant waiting list, at least at the doses administered (midodrine 15-30 mg/day and albumin 40 g every 15 days for a year).
While this particular combination of albumin plus midodrine did decrease renin and aldosterone levels, the intervention did not prevent complications or improve survival, investigators said at the time. Complication rates were 37% and 43% for treatment and placebo, respectively (P = .402), with low rates of death in both groups and no significant difference in mortality at 1 year (P = .527).
Dr. Shah said the discrepant results may be attributable to specific differences in study design or enrollment.
“It’s just hand waving, but it may be related to the dose of albumin, or may be related to the types of patients – the second study was in patients who are waiting for liver transplantation,” he told attendees. “But I don’t think that there’s currently enough evidence to use albumin in your patients in the outpatient setting.”
There is a third study, recently published in the American Journal of Gastroenterology, looking at data for a large end-stage liver disease cohort with hyponatremia. Investigators observed a higher rate of hyponatremia resolution and improved 30-day survival in those who had received albumin (total mean amount, 225 g) versus those who had not.
Considering all of this evidence taken together, Dr. Shah said he would not favor using outpatient albumin at this point – though he advised attendees to watch for a currently recruiting phase 3 randomized study, known as PRECIOSA, which is evaluating long-term administration of human albumin 20% injectable solution, dosed by body weight, in patients with decompensated cirrhosis and ascites.
Dr. Shah indicated that he is a consultant for Afimmune, Durect Corporation, Enterome, GRI Bio, Merck Research Laboratories, Novartis Pharma, and Vital Therapeutics. Global Academy and this news organization are owned by the same company.
PHILADELPHIA – , according to Vijay Shah, MD, chair of the division of gastroenterology and hepatology at Mayo Clinic, Rochester, Minn.
“These are interesting studies, but I don’t think we’re ready yet to use this broadly,” Dr. Shah said at the meeting jointly provided by Rutgers and Global Academy for Medical Education.
Current guidelines do describe the use of albumin for large-volume paracentesis and other specific inpatient situations; however, extrapolating its long-term use has been explored in two major studies that recently came out with contradictory findings.
In the ANSWER trial, as reported in the Lancet, investigators at 33 centers randomized patients with cirrhosis and uncomplicated ascites to either standard medical treatment with or without human albumin, at 40 g twice weekly for 2 weeks, followed by 40 g weekly for up to 18 months.
Those investigators found that long-term albumin prolonged overall survival, with a 38% reduction in the mortality hazard ratio, with similar rates of serious, nonliver adverse events, leading them to conclude that this intervention may act as a disease-modifying treatment in decompensated cirrhosis patients.
By contrast, however, a recent randomized, placebo-controlled trial reported in the Journal of Hepatology showed that albumin plus midodrine, an alpha-adrenergic vasoconstrictor, did not improve survival among patients with decompensated cirrhosis on the liver transplant waiting list, at least at the doses administered (midodrine 15-30 mg/day and albumin 40 g every 15 days for a year).
While this particular combination of albumin plus midodrine did decrease renin and aldosterone levels, the intervention did not prevent complications or improve survival, investigators said at the time. Complication rates were 37% and 43% for treatment and placebo, respectively (P = .402), with low rates of death in both groups and no significant difference in mortality at 1 year (P = .527).
Dr. Shah said the discrepant results may be attributable to specific differences in study design or enrollment.
“It’s just hand waving, but it may be related to the dose of albumin, or may be related to the types of patients – the second study was in patients who are waiting for liver transplantation,” he told attendees. “But I don’t think that there’s currently enough evidence to use albumin in your patients in the outpatient setting.”
There is a third study, recently published in the American Journal of Gastroenterology, looking at data for a large end-stage liver disease cohort with hyponatremia. Investigators observed a higher rate of hyponatremia resolution and improved 30-day survival in those who had received albumin (total mean amount, 225 g) versus those who had not.
Considering all of this evidence taken together, Dr. Shah said he would not favor using outpatient albumin at this point – though he advised attendees to watch for a currently recruiting phase 3 randomized study, known as PRECIOSA, which is evaluating long-term administration of human albumin 20% injectable solution, dosed by body weight, in patients with decompensated cirrhosis and ascites.
Dr. Shah indicated that he is a consultant for Afimmune, Durect Corporation, Enterome, GRI Bio, Merck Research Laboratories, Novartis Pharma, and Vital Therapeutics. Global Academy and this news organization are owned by the same company.
REPORTING FROM DIGESTIVE DISEASES: NEW ADVANCES
Resistant hypertension hits SLE patients hard
at a tertiary care center.
A patient with resistant hypertension either has blood pressure remaining above 140/90 mm Hg while taking three antihypertensive medications or requires the use of four or more antihypertensives to attain blood pressure control. Resistant hypertension, which was more likely to occur among blacks and patients with lower renal function, hypercholesterolemia, and increased inflammatory markers, increased the risk of death nearly threefold (hazard ratio, 2.91; P = .0005) when compared with those who didn’t have this condition.
The results of this analysis were published March 15 in Arthritis Care & Research (doi: 10.1002/acr.23880). We covered this study at the 2018 annual meeting of the American College of Rheumatology in Chicago before it was published in the journal. Read our previous story at the link above.
at a tertiary care center.
A patient with resistant hypertension either has blood pressure remaining above 140/90 mm Hg while taking three antihypertensive medications or requires the use of four or more antihypertensives to attain blood pressure control. Resistant hypertension, which was more likely to occur among blacks and patients with lower renal function, hypercholesterolemia, and increased inflammatory markers, increased the risk of death nearly threefold (hazard ratio, 2.91; P = .0005) when compared with those who didn’t have this condition.
The results of this analysis were published March 15 in Arthritis Care & Research (doi: 10.1002/acr.23880). We covered this study at the 2018 annual meeting of the American College of Rheumatology in Chicago before it was published in the journal. Read our previous story at the link above.
at a tertiary care center.
A patient with resistant hypertension either has blood pressure remaining above 140/90 mm Hg while taking three antihypertensive medications or requires the use of four or more antihypertensives to attain blood pressure control. Resistant hypertension, which was more likely to occur among blacks and patients with lower renal function, hypercholesterolemia, and increased inflammatory markers, increased the risk of death nearly threefold (hazard ratio, 2.91; P = .0005) when compared with those who didn’t have this condition.
The results of this analysis were published March 15 in Arthritis Care & Research (doi: 10.1002/acr.23880). We covered this study at the 2018 annual meeting of the American College of Rheumatology in Chicago before it was published in the journal. Read our previous story at the link above.
FROM ARTHRITIS CARE & RESEARCH