User login
Match Day 2019: Another strong year for neurology
according to the National Resident Matching Program (NRMP).
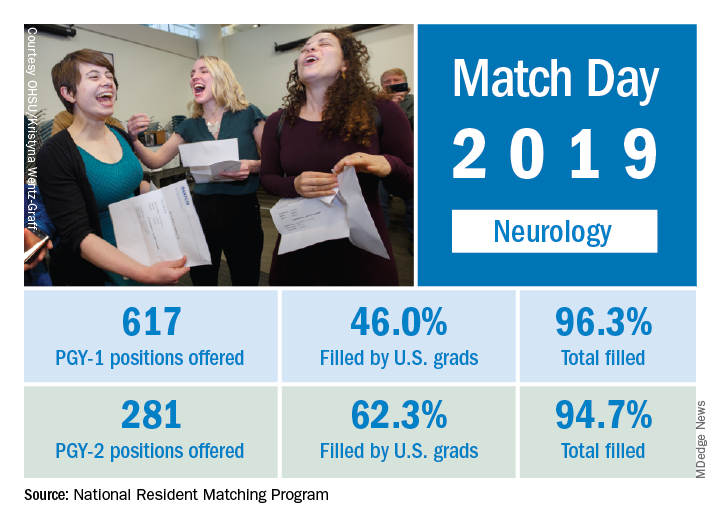
This year, 617 first-year (PGY-1) neurology slots were offered, an increase of 11.8% over the 552 offered in 2018 and well above the 6.5% gain recorded for the Match as whole. The 114 neurology programs participating this year filled 96.3% of those PGY-1 positions, compared with 94.7% for the 52 programs that offered PGY-2 positions, the NRMP reported.
“The results of the Match are closely watched because they can be predictors of future physician workforce supply. There also is significant interest in the competitiveness of specialties, as measured by the percentage of positions filled overall and the percentage filled by senior students in U.S. allopathic medical schools,” the NRMP said.
The proportion of PGY-1 neurology positions filled by U.S. seniors dropped to 46.0% from 50.7% last year, although the number of U.S. seniors filling spots actually went up from 280 in 2018 to 284. The PGY-2 positions saw declines in both cases: The 175 U.S. seniors represented 62.3% of the 2019 spots, compared with the 190 U.S. seniors who filled 66.2% of slots in 2018, the NRMP data show.
The total numbers of applicants (38,376) and positions offered (35,185) were both record highs for the Match, although they were affected, in part, by “increased numbers of osteopathic programs that joined the Main Residency Match as a result of the ongoing transition to a single accreditation system for graduate medical education programs,” the NRMP noted.
according to the National Resident Matching Program (NRMP).

This year, 617 first-year (PGY-1) neurology slots were offered, an increase of 11.8% over the 552 offered in 2018 and well above the 6.5% gain recorded for the Match as whole. The 114 neurology programs participating this year filled 96.3% of those PGY-1 positions, compared with 94.7% for the 52 programs that offered PGY-2 positions, the NRMP reported.
“The results of the Match are closely watched because they can be predictors of future physician workforce supply. There also is significant interest in the competitiveness of specialties, as measured by the percentage of positions filled overall and the percentage filled by senior students in U.S. allopathic medical schools,” the NRMP said.
The proportion of PGY-1 neurology positions filled by U.S. seniors dropped to 46.0% from 50.7% last year, although the number of U.S. seniors filling spots actually went up from 280 in 2018 to 284. The PGY-2 positions saw declines in both cases: The 175 U.S. seniors represented 62.3% of the 2019 spots, compared with the 190 U.S. seniors who filled 66.2% of slots in 2018, the NRMP data show.
The total numbers of applicants (38,376) and positions offered (35,185) were both record highs for the Match, although they were affected, in part, by “increased numbers of osteopathic programs that joined the Main Residency Match as a result of the ongoing transition to a single accreditation system for graduate medical education programs,” the NRMP noted.
according to the National Resident Matching Program (NRMP).

This year, 617 first-year (PGY-1) neurology slots were offered, an increase of 11.8% over the 552 offered in 2018 and well above the 6.5% gain recorded for the Match as whole. The 114 neurology programs participating this year filled 96.3% of those PGY-1 positions, compared with 94.7% for the 52 programs that offered PGY-2 positions, the NRMP reported.
“The results of the Match are closely watched because they can be predictors of future physician workforce supply. There also is significant interest in the competitiveness of specialties, as measured by the percentage of positions filled overall and the percentage filled by senior students in U.S. allopathic medical schools,” the NRMP said.
The proportion of PGY-1 neurology positions filled by U.S. seniors dropped to 46.0% from 50.7% last year, although the number of U.S. seniors filling spots actually went up from 280 in 2018 to 284. The PGY-2 positions saw declines in both cases: The 175 U.S. seniors represented 62.3% of the 2019 spots, compared with the 190 U.S. seniors who filled 66.2% of slots in 2018, the NRMP data show.
The total numbers of applicants (38,376) and positions offered (35,185) were both record highs for the Match, although they were affected, in part, by “increased numbers of osteopathic programs that joined the Main Residency Match as a result of the ongoing transition to a single accreditation system for graduate medical education programs,” the NRMP noted.
Whether diet, vitamins, or supplements can benefit patients with vitiligo remains unclear
WASHINGTON – Many patients with vitiligo are interested in treating their condition with vitamins, supplements, or a modified diet, but research on whether these measures have an impact remains limited, Nada Elbuluk, MD, said at the annual meeting of the American Academy of Dermatology.
, “we need more well designed, controlled studies in the future to know where this belongs in our treatment armamentarium,” said Dr. Elbuluk of the department of dermatology at the University of Southern California, Los Angeles.
During a session at the AAD meeting, Dr. Elbuluk, who is also director of the pigmentary disorders clinic at USC, reviewed the evidence for the use of these adjunctive therapies in patients with vitiligo.
Vitamins
The pathogenesis of vitiligo includes the overproduction of reactive oxygen species and oxidative stress, factors that contribute to melanocyte damage and death. In addition, many patients with vitiligo are deficient in certain vitamins and minerals, the basis of the hypothesis that supplementation could be beneficial, according to Dr. Elbuluk.
Vitamin B12 and folic acid contribute to DNA repair, synthesis, and methylation, and researchers have hypothesized that these vitamins also play a role in melanin synthesis. In a review of the literature, Dr. Elbuluk and her colleagues found four studies that evaluated vitamin B12 and folic acid in vitiligo. In one study, a controlled trial in which patients took B12 and folic acid with and without phototherapy, the investigators observed no significant difference in repigmentation between groups. The other three studies were uncontrolled and thus provide an insufficient understanding of the effect of B12 and folic acid, said Dr. Elbuluk.
Vitamin D is involved in melanocyte and keratinocyte growth and differentiation, and inhibits T cell activation. Data indicate that low vitamin D levels are common in patients with vitiligo and comorbid autoimmune diseases. In one study, patients who received narrow-band UVB had an increase in vitamin D levels that could contribute to photo-induced melanogenesis, and an open-label study indicated that patients who took vitamin D daily (without phototherapy) for 6 months had an increase of repigmentation over time. “Topical vitamin D analogs have also been used in vitiligo treatment with varying success,” Dr. Elbuluk noted.
“I check vitamin D levels on my patients and make sure that they are within normal range. But I think the degree of supplementation and its role in vitiligo needs to be further elucidated,” she said. And because vitamin D is fat soluble, there is a risk of toxicity if a patient takes too much.
Vitamin C, vitamin E, and alpha-lipoic acid have antioxidant properties. In a double-blind, randomized, controlled trial, one group of patients took vitamins C and E and alpha-lipoic acid for 2 months before and during treatment with narrow-band UVB twice per week (Clin Exp Dermatol. 2007 Nov;32[6]:631-6). Another group underwent phototherapy without supplementation. A significantly greater proportion of patients who received the antioxidants obtained more than 75% repigmentation compared with those who did not. In another study, 73% of patients who received oral vitamin E and narrow band UVB phototherapy had marked to excellent repigmentation, compared with 55.6% of those who had phototherapy only (J Clin Pharmacol. 2009 Jul;49[7]:852-5).
The results of these studies support the idea that antioxidants can stabilize disease, reduce oxidative stress, and improve the effect of phototherapy, Dr. Elbuluk said.
Herbal supplements
Several research teams have examined Ginkgo biloba as a possible treatment for vitiligo. This plant is native to China and has antioxidant and anti-inflammatory properties; its most common side effect is gastrointestinal distress. Because it entails a risk of coagulopathy, it may not be appropriate for patients receiving anticoagulant treatment, Dr. Elbuluk pointed out. In a double-blinded, randomized, controlled trial comparing ginkgo biloba alone with placebo in patients with vitiligo, treatment was associated with cessation of active disease in most patients, and more than 40% of patients receiving ginkgo biloba had 75% repigmentation or more.
Polypodium leucotomos, a fern native to Central and South America, protects against UV radiation damage, modulates the immune system, and has anti-inflammatory and antioxidant effects. It has a good safety profile and is well tolerated at a dose of 240 mg/day, she said. It sometimes causes gastrointestinal discomfort or pruritus. Several randomized, controlled trials in patients with vitiligo showed that supplementation with polypodium leucotomos improves repigmentation, particularly in photo-exposed areas, she noted.
Khellin is an extract from the Mediterranean khella plant that is thought to stimulate melanocyte proliferation and melanogenesis. Several studies have examined khellin supplementation in combination with phototherapy. Khellin can be administered orally or topically and appears to be more beneficial than sunlight or phototherapy alone in stabilizing disease or inducing repigmentation. Oral khellin can cause many side effects, including nausea, transaminitis, and hypotension, so researchers have been more interested in using topical khellin as a liposomal vehicle to improve drug delivery, Dr. Elbuluk said.
Minerals
Some patients with vitiligo have deficiencies in zinc and copper. Zinc is an antioxidant that aids wound healing, protects against free radicals, supports melanogenesis, and possibly prevents melanocyte death, but can cause gastrointestinal irritation. Copper, too, is an antioxidant and coenzyme involved in melanogenesis. One study compared topical steroid treatment with and without oral zinc supplementation. Dual treatment was associated with greater repigmentation, but the difference was not statistically significant. No studies have examined copper supplementation, she said.
L-phenylalanine, diet, and green tea
Investigators have proposed that the amino acid L-phenylalanine, a precursor to tyrosine in the pathway of melanin synthesis, might interfere with antibody production against melanocytes. This supplement is administered orally by weight, typically in conjunction with phototherapy or sunlight. Various studies have observed positive outcomes of L-phenylalanine combined with phototherapy or sunlight. L-phenylalanine tends to be safe and has been administered to children with vitiligo.
Many patients with vitiligo “have already tried diets by the time they come to me,” said Dr. Elbuluk. No controlled studies have analyzed the role of diet in the prevention or treatment of vitiligo, but case reports describe gluten-free diets in this population, including one report of a patient with celiac disease whose vitiligo improved after adoption of such a diet. Another case report described a patient without celiac disease who had refractory acrofacial vitiligo, which improved after the adoption of a gluten-free diet. Evidence supports a gluten-free diet for patients with celiac disease, but does not support this challenging diet for people without celiac disease, she pointed out.
Green tea includes catechins, which have antioxidant and anti-inflammatory properties. Its main component is epigallocatechin gallate (EGCG), which is thought to modulate T cell mediated responses. In one animal study, administration of EGCG delayed the onset of vitiligo and decreased the area of depigmentation in a mouse model. Although these findings are promising, clinical trials are needed to determine whether EGCG is beneficial in humans with vitiligo, said Dr. Elbuluk.
The literature on diets and supplementation as treatments for vitiligo has several shortcomings, with studies that used heterogeneous methodologies, and many that used nonstandard outcome measures that have not been validated. Sample sizes often are small, and many trials are uncontrolled. “These limitations make it harder to make sense of the data and have take-home conclusions,” Dr. Elbuluk said.
She had no disclosures.
SOURCE: Elbuluk N. AAD 19, Session S002.
WASHINGTON – Many patients with vitiligo are interested in treating their condition with vitamins, supplements, or a modified diet, but research on whether these measures have an impact remains limited, Nada Elbuluk, MD, said at the annual meeting of the American Academy of Dermatology.
, “we need more well designed, controlled studies in the future to know where this belongs in our treatment armamentarium,” said Dr. Elbuluk of the department of dermatology at the University of Southern California, Los Angeles.
During a session at the AAD meeting, Dr. Elbuluk, who is also director of the pigmentary disorders clinic at USC, reviewed the evidence for the use of these adjunctive therapies in patients with vitiligo.
Vitamins
The pathogenesis of vitiligo includes the overproduction of reactive oxygen species and oxidative stress, factors that contribute to melanocyte damage and death. In addition, many patients with vitiligo are deficient in certain vitamins and minerals, the basis of the hypothesis that supplementation could be beneficial, according to Dr. Elbuluk.
Vitamin B12 and folic acid contribute to DNA repair, synthesis, and methylation, and researchers have hypothesized that these vitamins also play a role in melanin synthesis. In a review of the literature, Dr. Elbuluk and her colleagues found four studies that evaluated vitamin B12 and folic acid in vitiligo. In one study, a controlled trial in which patients took B12 and folic acid with and without phototherapy, the investigators observed no significant difference in repigmentation between groups. The other three studies were uncontrolled and thus provide an insufficient understanding of the effect of B12 and folic acid, said Dr. Elbuluk.
Vitamin D is involved in melanocyte and keratinocyte growth and differentiation, and inhibits T cell activation. Data indicate that low vitamin D levels are common in patients with vitiligo and comorbid autoimmune diseases. In one study, patients who received narrow-band UVB had an increase in vitamin D levels that could contribute to photo-induced melanogenesis, and an open-label study indicated that patients who took vitamin D daily (without phototherapy) for 6 months had an increase of repigmentation over time. “Topical vitamin D analogs have also been used in vitiligo treatment with varying success,” Dr. Elbuluk noted.
“I check vitamin D levels on my patients and make sure that they are within normal range. But I think the degree of supplementation and its role in vitiligo needs to be further elucidated,” she said. And because vitamin D is fat soluble, there is a risk of toxicity if a patient takes too much.
Vitamin C, vitamin E, and alpha-lipoic acid have antioxidant properties. In a double-blind, randomized, controlled trial, one group of patients took vitamins C and E and alpha-lipoic acid for 2 months before and during treatment with narrow-band UVB twice per week (Clin Exp Dermatol. 2007 Nov;32[6]:631-6). Another group underwent phototherapy without supplementation. A significantly greater proportion of patients who received the antioxidants obtained more than 75% repigmentation compared with those who did not. In another study, 73% of patients who received oral vitamin E and narrow band UVB phototherapy had marked to excellent repigmentation, compared with 55.6% of those who had phototherapy only (J Clin Pharmacol. 2009 Jul;49[7]:852-5).
The results of these studies support the idea that antioxidants can stabilize disease, reduce oxidative stress, and improve the effect of phototherapy, Dr. Elbuluk said.
Herbal supplements
Several research teams have examined Ginkgo biloba as a possible treatment for vitiligo. This plant is native to China and has antioxidant and anti-inflammatory properties; its most common side effect is gastrointestinal distress. Because it entails a risk of coagulopathy, it may not be appropriate for patients receiving anticoagulant treatment, Dr. Elbuluk pointed out. In a double-blinded, randomized, controlled trial comparing ginkgo biloba alone with placebo in patients with vitiligo, treatment was associated with cessation of active disease in most patients, and more than 40% of patients receiving ginkgo biloba had 75% repigmentation or more.
Polypodium leucotomos, a fern native to Central and South America, protects against UV radiation damage, modulates the immune system, and has anti-inflammatory and antioxidant effects. It has a good safety profile and is well tolerated at a dose of 240 mg/day, she said. It sometimes causes gastrointestinal discomfort or pruritus. Several randomized, controlled trials in patients with vitiligo showed that supplementation with polypodium leucotomos improves repigmentation, particularly in photo-exposed areas, she noted.
Khellin is an extract from the Mediterranean khella plant that is thought to stimulate melanocyte proliferation and melanogenesis. Several studies have examined khellin supplementation in combination with phototherapy. Khellin can be administered orally or topically and appears to be more beneficial than sunlight or phototherapy alone in stabilizing disease or inducing repigmentation. Oral khellin can cause many side effects, including nausea, transaminitis, and hypotension, so researchers have been more interested in using topical khellin as a liposomal vehicle to improve drug delivery, Dr. Elbuluk said.
Minerals
Some patients with vitiligo have deficiencies in zinc and copper. Zinc is an antioxidant that aids wound healing, protects against free radicals, supports melanogenesis, and possibly prevents melanocyte death, but can cause gastrointestinal irritation. Copper, too, is an antioxidant and coenzyme involved in melanogenesis. One study compared topical steroid treatment with and without oral zinc supplementation. Dual treatment was associated with greater repigmentation, but the difference was not statistically significant. No studies have examined copper supplementation, she said.
L-phenylalanine, diet, and green tea
Investigators have proposed that the amino acid L-phenylalanine, a precursor to tyrosine in the pathway of melanin synthesis, might interfere with antibody production against melanocytes. This supplement is administered orally by weight, typically in conjunction with phototherapy or sunlight. Various studies have observed positive outcomes of L-phenylalanine combined with phototherapy or sunlight. L-phenylalanine tends to be safe and has been administered to children with vitiligo.
Many patients with vitiligo “have already tried diets by the time they come to me,” said Dr. Elbuluk. No controlled studies have analyzed the role of diet in the prevention or treatment of vitiligo, but case reports describe gluten-free diets in this population, including one report of a patient with celiac disease whose vitiligo improved after adoption of such a diet. Another case report described a patient without celiac disease who had refractory acrofacial vitiligo, which improved after the adoption of a gluten-free diet. Evidence supports a gluten-free diet for patients with celiac disease, but does not support this challenging diet for people without celiac disease, she pointed out.
Green tea includes catechins, which have antioxidant and anti-inflammatory properties. Its main component is epigallocatechin gallate (EGCG), which is thought to modulate T cell mediated responses. In one animal study, administration of EGCG delayed the onset of vitiligo and decreased the area of depigmentation in a mouse model. Although these findings are promising, clinical trials are needed to determine whether EGCG is beneficial in humans with vitiligo, said Dr. Elbuluk.
The literature on diets and supplementation as treatments for vitiligo has several shortcomings, with studies that used heterogeneous methodologies, and many that used nonstandard outcome measures that have not been validated. Sample sizes often are small, and many trials are uncontrolled. “These limitations make it harder to make sense of the data and have take-home conclusions,” Dr. Elbuluk said.
She had no disclosures.
SOURCE: Elbuluk N. AAD 19, Session S002.
WASHINGTON – Many patients with vitiligo are interested in treating their condition with vitamins, supplements, or a modified diet, but research on whether these measures have an impact remains limited, Nada Elbuluk, MD, said at the annual meeting of the American Academy of Dermatology.
, “we need more well designed, controlled studies in the future to know where this belongs in our treatment armamentarium,” said Dr. Elbuluk of the department of dermatology at the University of Southern California, Los Angeles.
During a session at the AAD meeting, Dr. Elbuluk, who is also director of the pigmentary disorders clinic at USC, reviewed the evidence for the use of these adjunctive therapies in patients with vitiligo.
Vitamins
The pathogenesis of vitiligo includes the overproduction of reactive oxygen species and oxidative stress, factors that contribute to melanocyte damage and death. In addition, many patients with vitiligo are deficient in certain vitamins and minerals, the basis of the hypothesis that supplementation could be beneficial, according to Dr. Elbuluk.
Vitamin B12 and folic acid contribute to DNA repair, synthesis, and methylation, and researchers have hypothesized that these vitamins also play a role in melanin synthesis. In a review of the literature, Dr. Elbuluk and her colleagues found four studies that evaluated vitamin B12 and folic acid in vitiligo. In one study, a controlled trial in which patients took B12 and folic acid with and without phototherapy, the investigators observed no significant difference in repigmentation between groups. The other three studies were uncontrolled and thus provide an insufficient understanding of the effect of B12 and folic acid, said Dr. Elbuluk.
Vitamin D is involved in melanocyte and keratinocyte growth and differentiation, and inhibits T cell activation. Data indicate that low vitamin D levels are common in patients with vitiligo and comorbid autoimmune diseases. In one study, patients who received narrow-band UVB had an increase in vitamin D levels that could contribute to photo-induced melanogenesis, and an open-label study indicated that patients who took vitamin D daily (without phototherapy) for 6 months had an increase of repigmentation over time. “Topical vitamin D analogs have also been used in vitiligo treatment with varying success,” Dr. Elbuluk noted.
“I check vitamin D levels on my patients and make sure that they are within normal range. But I think the degree of supplementation and its role in vitiligo needs to be further elucidated,” she said. And because vitamin D is fat soluble, there is a risk of toxicity if a patient takes too much.
Vitamin C, vitamin E, and alpha-lipoic acid have antioxidant properties. In a double-blind, randomized, controlled trial, one group of patients took vitamins C and E and alpha-lipoic acid for 2 months before and during treatment with narrow-band UVB twice per week (Clin Exp Dermatol. 2007 Nov;32[6]:631-6). Another group underwent phototherapy without supplementation. A significantly greater proportion of patients who received the antioxidants obtained more than 75% repigmentation compared with those who did not. In another study, 73% of patients who received oral vitamin E and narrow band UVB phototherapy had marked to excellent repigmentation, compared with 55.6% of those who had phototherapy only (J Clin Pharmacol. 2009 Jul;49[7]:852-5).
The results of these studies support the idea that antioxidants can stabilize disease, reduce oxidative stress, and improve the effect of phototherapy, Dr. Elbuluk said.
Herbal supplements
Several research teams have examined Ginkgo biloba as a possible treatment for vitiligo. This plant is native to China and has antioxidant and anti-inflammatory properties; its most common side effect is gastrointestinal distress. Because it entails a risk of coagulopathy, it may not be appropriate for patients receiving anticoagulant treatment, Dr. Elbuluk pointed out. In a double-blinded, randomized, controlled trial comparing ginkgo biloba alone with placebo in patients with vitiligo, treatment was associated with cessation of active disease in most patients, and more than 40% of patients receiving ginkgo biloba had 75% repigmentation or more.
Polypodium leucotomos, a fern native to Central and South America, protects against UV radiation damage, modulates the immune system, and has anti-inflammatory and antioxidant effects. It has a good safety profile and is well tolerated at a dose of 240 mg/day, she said. It sometimes causes gastrointestinal discomfort or pruritus. Several randomized, controlled trials in patients with vitiligo showed that supplementation with polypodium leucotomos improves repigmentation, particularly in photo-exposed areas, she noted.
Khellin is an extract from the Mediterranean khella plant that is thought to stimulate melanocyte proliferation and melanogenesis. Several studies have examined khellin supplementation in combination with phototherapy. Khellin can be administered orally or topically and appears to be more beneficial than sunlight or phototherapy alone in stabilizing disease or inducing repigmentation. Oral khellin can cause many side effects, including nausea, transaminitis, and hypotension, so researchers have been more interested in using topical khellin as a liposomal vehicle to improve drug delivery, Dr. Elbuluk said.
Minerals
Some patients with vitiligo have deficiencies in zinc and copper. Zinc is an antioxidant that aids wound healing, protects against free radicals, supports melanogenesis, and possibly prevents melanocyte death, but can cause gastrointestinal irritation. Copper, too, is an antioxidant and coenzyme involved in melanogenesis. One study compared topical steroid treatment with and without oral zinc supplementation. Dual treatment was associated with greater repigmentation, but the difference was not statistically significant. No studies have examined copper supplementation, she said.
L-phenylalanine, diet, and green tea
Investigators have proposed that the amino acid L-phenylalanine, a precursor to tyrosine in the pathway of melanin synthesis, might interfere with antibody production against melanocytes. This supplement is administered orally by weight, typically in conjunction with phototherapy or sunlight. Various studies have observed positive outcomes of L-phenylalanine combined with phototherapy or sunlight. L-phenylalanine tends to be safe and has been administered to children with vitiligo.
Many patients with vitiligo “have already tried diets by the time they come to me,” said Dr. Elbuluk. No controlled studies have analyzed the role of diet in the prevention or treatment of vitiligo, but case reports describe gluten-free diets in this population, including one report of a patient with celiac disease whose vitiligo improved after adoption of such a diet. Another case report described a patient without celiac disease who had refractory acrofacial vitiligo, which improved after the adoption of a gluten-free diet. Evidence supports a gluten-free diet for patients with celiac disease, but does not support this challenging diet for people without celiac disease, she pointed out.
Green tea includes catechins, which have antioxidant and anti-inflammatory properties. Its main component is epigallocatechin gallate (EGCG), which is thought to modulate T cell mediated responses. In one animal study, administration of EGCG delayed the onset of vitiligo and decreased the area of depigmentation in a mouse model. Although these findings are promising, clinical trials are needed to determine whether EGCG is beneficial in humans with vitiligo, said Dr. Elbuluk.
The literature on diets and supplementation as treatments for vitiligo has several shortcomings, with studies that used heterogeneous methodologies, and many that used nonstandard outcome measures that have not been validated. Sample sizes often are small, and many trials are uncontrolled. “These limitations make it harder to make sense of the data and have take-home conclusions,” Dr. Elbuluk said.
She had no disclosures.
SOURCE: Elbuluk N. AAD 19, Session S002.
REPORTING FROM AAD 19
A new era of TTP treatment
Earlier this year, the Food and Drug Administration approved Cablivi (caplacizumab-yhdp) (Sanofi Genzyme, Cambridge, Mass.) for the treatment of acquired thrombotic thrombocytopenic purpura (TTP), making it the first medication specifically indicated for the treatment of TTP.
The approval of caplacizumab and the clinical trial results that approval is based on are the most promising developments in the treatment of TTP since the introduction of plasma exchange (PE) therapy. However, many questions remain about how to best administer caplacizumab, specifically, which patients should receive it? Should all TTP patients start on caplacizumab therapy or should it be limited to patients with histories of TTP or those slow to respond to standard therapy with PE and immunosuppression?
TTP is a rare thrombotic microangiopathy characterized by thrombocytopenia and microangiopathic hemolytic anemia caused by the inhibition of ADAMTS13, a metalloproteinase, which cleaves large-molecular-weight von Willebrand factor (vWF) multimers. Caplacizumab is a humanized bivalent, variable domain-only immunoglobulin fragment. The drug targets the A1 domain of vWF and inhibits the binding between vWF and the platelet glycoprotein Ib-IX-V receptor, preventing the formation of the microvascular thrombi and platelet loss associated with TTP.
FDA approval of caplacizumab came shortly after the publication of the results of the HERCULES trial in the New England Journal of Medicine by Marie Scully, MD, and her colleagues (N Engl J Med. 2019; 380[4]: 335-46).
HERCULES was an international phase 3, double blinded, placebo-controlled, randomized study designed to evaluate the efficacy and safety of caplacizumab. In total, 145 patients participated in the trial. Caplacizumab or placebo were given in addition to standard therapy of plasma exchange (PE) and immunosuppression. Caplacizumab or placebo were administered as an intravenous loading dose prior to the first PE after randomization and subcutaneously once daily until 30 days after the last PE. All patients received daily PE until 2 days after platelet count normalization.
The primary measure of the study was the time to platelet count response of greater than 150 x 109/L following the cessation of daily PE. Secondary measures included TTP-related death; TTP relapse; major thromboembolic events; proportion of subjects with refractory TTP; normalization of organ damage markers including lactate dehydrogenase, cardiac troponin I, and serum creatinine; and other adverse events.
The authors found that the median time of normalization of the platelet count was shorter in the caplacizumab group, compared with placebo, with the caplacizumab group being 1.55 times more likely to have a normalized platelet count at any given time point in the study. While statistically significant differences were identified in the rate of platelet normalization, the median number of days of PE until normalization was only 2 days less in the caplacizumab group (five treatments) than in the placebo group (seven treatments), which may not be clinically significant for the treating physician.
In fact, the secondary endpoints of the study seem much more clinically promising in the treatment of TTP. The composite rate of TTP-related death, TTP recurrence, or major thromboembolic events during the treatment period was significantly lower in the caplacizumab group (12%) versus placebo (49%). No TTP-related deaths occurred in the caplacizumab group. The caplacizumab group was also statistically less likely to have a TTP exacerbation, defined as disease recurrence within 30 days from the last PE, than the placebo group.
End organ damage serum markers also improved faster in the caplacizumab group, although there was no significant difference between groups. Overall hospitalization (median of 9 vs. 12 days) and ICU stays (median of 3 vs. 5 days) were shorter in the caplacizumab group, compared with the placebo group.
While several relapses, defined as disease recurrence after 30 days from the last PE, occurred in the caplacizumab group, the relapses were only found in patients with ADAMTS13 activity of less than 10% at the end of the treatment period. Mild side effects, such as mucocutaneous bleeding were more frequent in the caplacizumab group. No major bleeding complications were observed.
The HERCULES trial generates more questions about the role of ADAMTS13 activity testing to monitor treatment response and to make therapy decisions. Extremely low ADAMTS13 activity levels at the cessation of therapy may be a sign of treatment inadequacy and may warrant closer follow-up of at-risk patients on caplacizumab.
Sanofi Genzyme estimates that the U.S. list price will be approximately $270,000 for a standard treatment course, according to a news release from the company. Whether payers will add it to formularies remains uncertain, but the high drug cost may be countered by potential savings in the reduction of hospital and ICU days with caplacizumab therapy. Sanofi Genzyme will also have a patient support program for eligible patients.
Caplacizumab has been approved in Europe since August 2018, but is not readily available in the United States. Given the dearth of clinical experience with the drug outside of the TITAN and HERCULES trials, strong recommendations for when and how to initiate therapy remain elusive.
As caplacizumab is further introduced into clinical practice, more studies are needed to identify which patient groups will benefit most from therapy. The current data for caplacizumab shows that it will be used as an adjunct to standard PE therapy, rather than as a replacement. How the drug is used in combination with current TTP treatments – such as corticosteroids, rituximab, bortezomib, vincristine, N-acetylcysteine, and splenectomy – should be evaluated to identify which treatment combinations not only improve platelet counts, but also reduce mortality and morbidity while remaining cost effective.
Dr. Ricci is a staff physician and Apheresis Director at the Taussig Cancer Institute at the Cleveland Clinic. She reported having no conflicts of interest.
Earlier this year, the Food and Drug Administration approved Cablivi (caplacizumab-yhdp) (Sanofi Genzyme, Cambridge, Mass.) for the treatment of acquired thrombotic thrombocytopenic purpura (TTP), making it the first medication specifically indicated for the treatment of TTP.
The approval of caplacizumab and the clinical trial results that approval is based on are the most promising developments in the treatment of TTP since the introduction of plasma exchange (PE) therapy. However, many questions remain about how to best administer caplacizumab, specifically, which patients should receive it? Should all TTP patients start on caplacizumab therapy or should it be limited to patients with histories of TTP or those slow to respond to standard therapy with PE and immunosuppression?
TTP is a rare thrombotic microangiopathy characterized by thrombocytopenia and microangiopathic hemolytic anemia caused by the inhibition of ADAMTS13, a metalloproteinase, which cleaves large-molecular-weight von Willebrand factor (vWF) multimers. Caplacizumab is a humanized bivalent, variable domain-only immunoglobulin fragment. The drug targets the A1 domain of vWF and inhibits the binding between vWF and the platelet glycoprotein Ib-IX-V receptor, preventing the formation of the microvascular thrombi and platelet loss associated with TTP.
FDA approval of caplacizumab came shortly after the publication of the results of the HERCULES trial in the New England Journal of Medicine by Marie Scully, MD, and her colleagues (N Engl J Med. 2019; 380[4]: 335-46).
HERCULES was an international phase 3, double blinded, placebo-controlled, randomized study designed to evaluate the efficacy and safety of caplacizumab. In total, 145 patients participated in the trial. Caplacizumab or placebo were given in addition to standard therapy of plasma exchange (PE) and immunosuppression. Caplacizumab or placebo were administered as an intravenous loading dose prior to the first PE after randomization and subcutaneously once daily until 30 days after the last PE. All patients received daily PE until 2 days after platelet count normalization.
The primary measure of the study was the time to platelet count response of greater than 150 x 109/L following the cessation of daily PE. Secondary measures included TTP-related death; TTP relapse; major thromboembolic events; proportion of subjects with refractory TTP; normalization of organ damage markers including lactate dehydrogenase, cardiac troponin I, and serum creatinine; and other adverse events.
The authors found that the median time of normalization of the platelet count was shorter in the caplacizumab group, compared with placebo, with the caplacizumab group being 1.55 times more likely to have a normalized platelet count at any given time point in the study. While statistically significant differences were identified in the rate of platelet normalization, the median number of days of PE until normalization was only 2 days less in the caplacizumab group (five treatments) than in the placebo group (seven treatments), which may not be clinically significant for the treating physician.
In fact, the secondary endpoints of the study seem much more clinically promising in the treatment of TTP. The composite rate of TTP-related death, TTP recurrence, or major thromboembolic events during the treatment period was significantly lower in the caplacizumab group (12%) versus placebo (49%). No TTP-related deaths occurred in the caplacizumab group. The caplacizumab group was also statistically less likely to have a TTP exacerbation, defined as disease recurrence within 30 days from the last PE, than the placebo group.
End organ damage serum markers also improved faster in the caplacizumab group, although there was no significant difference between groups. Overall hospitalization (median of 9 vs. 12 days) and ICU stays (median of 3 vs. 5 days) were shorter in the caplacizumab group, compared with the placebo group.
While several relapses, defined as disease recurrence after 30 days from the last PE, occurred in the caplacizumab group, the relapses were only found in patients with ADAMTS13 activity of less than 10% at the end of the treatment period. Mild side effects, such as mucocutaneous bleeding were more frequent in the caplacizumab group. No major bleeding complications were observed.
The HERCULES trial generates more questions about the role of ADAMTS13 activity testing to monitor treatment response and to make therapy decisions. Extremely low ADAMTS13 activity levels at the cessation of therapy may be a sign of treatment inadequacy and may warrant closer follow-up of at-risk patients on caplacizumab.
Sanofi Genzyme estimates that the U.S. list price will be approximately $270,000 for a standard treatment course, according to a news release from the company. Whether payers will add it to formularies remains uncertain, but the high drug cost may be countered by potential savings in the reduction of hospital and ICU days with caplacizumab therapy. Sanofi Genzyme will also have a patient support program for eligible patients.
Caplacizumab has been approved in Europe since August 2018, but is not readily available in the United States. Given the dearth of clinical experience with the drug outside of the TITAN and HERCULES trials, strong recommendations for when and how to initiate therapy remain elusive.
As caplacizumab is further introduced into clinical practice, more studies are needed to identify which patient groups will benefit most from therapy. The current data for caplacizumab shows that it will be used as an adjunct to standard PE therapy, rather than as a replacement. How the drug is used in combination with current TTP treatments – such as corticosteroids, rituximab, bortezomib, vincristine, N-acetylcysteine, and splenectomy – should be evaluated to identify which treatment combinations not only improve platelet counts, but also reduce mortality and morbidity while remaining cost effective.
Dr. Ricci is a staff physician and Apheresis Director at the Taussig Cancer Institute at the Cleveland Clinic. She reported having no conflicts of interest.
Earlier this year, the Food and Drug Administration approved Cablivi (caplacizumab-yhdp) (Sanofi Genzyme, Cambridge, Mass.) for the treatment of acquired thrombotic thrombocytopenic purpura (TTP), making it the first medication specifically indicated for the treatment of TTP.
The approval of caplacizumab and the clinical trial results that approval is based on are the most promising developments in the treatment of TTP since the introduction of plasma exchange (PE) therapy. However, many questions remain about how to best administer caplacizumab, specifically, which patients should receive it? Should all TTP patients start on caplacizumab therapy or should it be limited to patients with histories of TTP or those slow to respond to standard therapy with PE and immunosuppression?
TTP is a rare thrombotic microangiopathy characterized by thrombocytopenia and microangiopathic hemolytic anemia caused by the inhibition of ADAMTS13, a metalloproteinase, which cleaves large-molecular-weight von Willebrand factor (vWF) multimers. Caplacizumab is a humanized bivalent, variable domain-only immunoglobulin fragment. The drug targets the A1 domain of vWF and inhibits the binding between vWF and the platelet glycoprotein Ib-IX-V receptor, preventing the formation of the microvascular thrombi and platelet loss associated with TTP.
FDA approval of caplacizumab came shortly after the publication of the results of the HERCULES trial in the New England Journal of Medicine by Marie Scully, MD, and her colleagues (N Engl J Med. 2019; 380[4]: 335-46).
HERCULES was an international phase 3, double blinded, placebo-controlled, randomized study designed to evaluate the efficacy and safety of caplacizumab. In total, 145 patients participated in the trial. Caplacizumab or placebo were given in addition to standard therapy of plasma exchange (PE) and immunosuppression. Caplacizumab or placebo were administered as an intravenous loading dose prior to the first PE after randomization and subcutaneously once daily until 30 days after the last PE. All patients received daily PE until 2 days after platelet count normalization.
The primary measure of the study was the time to platelet count response of greater than 150 x 109/L following the cessation of daily PE. Secondary measures included TTP-related death; TTP relapse; major thromboembolic events; proportion of subjects with refractory TTP; normalization of organ damage markers including lactate dehydrogenase, cardiac troponin I, and serum creatinine; and other adverse events.
The authors found that the median time of normalization of the platelet count was shorter in the caplacizumab group, compared with placebo, with the caplacizumab group being 1.55 times more likely to have a normalized platelet count at any given time point in the study. While statistically significant differences were identified in the rate of platelet normalization, the median number of days of PE until normalization was only 2 days less in the caplacizumab group (five treatments) than in the placebo group (seven treatments), which may not be clinically significant for the treating physician.
In fact, the secondary endpoints of the study seem much more clinically promising in the treatment of TTP. The composite rate of TTP-related death, TTP recurrence, or major thromboembolic events during the treatment period was significantly lower in the caplacizumab group (12%) versus placebo (49%). No TTP-related deaths occurred in the caplacizumab group. The caplacizumab group was also statistically less likely to have a TTP exacerbation, defined as disease recurrence within 30 days from the last PE, than the placebo group.
End organ damage serum markers also improved faster in the caplacizumab group, although there was no significant difference between groups. Overall hospitalization (median of 9 vs. 12 days) and ICU stays (median of 3 vs. 5 days) were shorter in the caplacizumab group, compared with the placebo group.
While several relapses, defined as disease recurrence after 30 days from the last PE, occurred in the caplacizumab group, the relapses were only found in patients with ADAMTS13 activity of less than 10% at the end of the treatment period. Mild side effects, such as mucocutaneous bleeding were more frequent in the caplacizumab group. No major bleeding complications were observed.
The HERCULES trial generates more questions about the role of ADAMTS13 activity testing to monitor treatment response and to make therapy decisions. Extremely low ADAMTS13 activity levels at the cessation of therapy may be a sign of treatment inadequacy and may warrant closer follow-up of at-risk patients on caplacizumab.
Sanofi Genzyme estimates that the U.S. list price will be approximately $270,000 for a standard treatment course, according to a news release from the company. Whether payers will add it to formularies remains uncertain, but the high drug cost may be countered by potential savings in the reduction of hospital and ICU days with caplacizumab therapy. Sanofi Genzyme will also have a patient support program for eligible patients.
Caplacizumab has been approved in Europe since August 2018, but is not readily available in the United States. Given the dearth of clinical experience with the drug outside of the TITAN and HERCULES trials, strong recommendations for when and how to initiate therapy remain elusive.
As caplacizumab is further introduced into clinical practice, more studies are needed to identify which patient groups will benefit most from therapy. The current data for caplacizumab shows that it will be used as an adjunct to standard PE therapy, rather than as a replacement. How the drug is used in combination with current TTP treatments – such as corticosteroids, rituximab, bortezomib, vincristine, N-acetylcysteine, and splenectomy – should be evaluated to identify which treatment combinations not only improve platelet counts, but also reduce mortality and morbidity while remaining cost effective.
Dr. Ricci is a staff physician and Apheresis Director at the Taussig Cancer Institute at the Cleveland Clinic. She reported having no conflicts of interest.
Match Day 2019
For the seventh consecutive year overall match numbers are up, some specialties are concerned about post-residency workforce numbers, and social media offers congratulations and support.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
For the seventh consecutive year overall match numbers are up, some specialties are concerned about post-residency workforce numbers, and social media offers congratulations and support.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
For the seventh consecutive year overall match numbers are up, some specialties are concerned about post-residency workforce numbers, and social media offers congratulations and support.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
CREOLE: Amlodipine may be preferable for lowering blood pressure in black patients
Amlodipine plus either hydrochlorothiazide or perindopril effectively reduced blood pressure better than perindopril and hydrochlorothiazide in black African patients with hypertension, based on data presented at the annual meeting of the American College of Cardiology.
“(A) long-acting dihydropyridine calcium-channel blocker (in this case, amlodipine) may be critical to more efficacious blood-pressure lowering among black patients as part of the two-drug combinations used (in Africa),” wrote lead author Dike B. Ojji, PhD, of the University of Abuja in Gwagwalada, Abuja, Nigeria, and his coauthors. “These results contrast with recommendations for black patients in the most recent U.S. guidelines” which recommend either a calcium channel blocker or a diuretic in combination with a different drug class.
The study was published simultaneously in the New England Journal of Medicine.
In the CREOLE study, Dr. Ojji and his colleagues enrolled 728 black patients, mean age 51 years and 63% of them women, in a randomized, single-blind, three-group trial across six countries in sub-Saharan Africa. All patients had uncontrolled hypertension and were assigned to one of three treatment groups: amlodipine plus hydrochlorothiazide (n = 244), amlodipine plus perindopril (n = 243), and perindopril plus hydrochlorothiazide (n = 241). Patients underwent 24-hour ambulatory blood-pressure measuring at baseline and at 6 months.
Of the 621 patients who completed the trial, those in the two groups receiving amlodipine had a larger mean reduction in systolic blood pressure after 6 months than the group receiving perindopril plus hydrochlorothiazide. Compared with the perindopril plus hydrochlorothiazide group, the amlodipine plus hydrochlorothiazide group had an additional -3.14 mm Hg reduction in systolic blood pressure from baseline (95% confidence interval, -5.90 to -0.38, P = 0.03) while the amlodipine plus perindopril group had an additional -3.00 mm Hg reduction (95% CI, -5.81 to -0.20, P = 0.04). The difference between the amlodipine plus hydrochlorothiazide group and the amlodipine plus perindopril group was -0.14 mm Hg (95% CI, -2.90 to 2.61, P = 0.92).
The limitations of the study included using nonmatching trial drugs and not adjusting the P values for the three comparisons of the primary end point, the authors wrote. It’s uncertain whether the findings can be extrapolated to black patients with diabetes or to those living outside of sub-Saharan Africa.
The study was sponsored by a grant from the GlaxoSmithKline Africa Noncommunicable Disease Open Lab. Trial drugs were donated by Aspen Pharmacare. Two authors reported receiving grants and personal fees from numerous pharmaceutical companies.
SOURCE: Ojji DB et al. NEJM. 2019 Mar 18. doi: 10.1056/NEJMoa1901113.
Amlodipine plus either hydrochlorothiazide or perindopril effectively reduced blood pressure better than perindopril and hydrochlorothiazide in black African patients with hypertension, based on data presented at the annual meeting of the American College of Cardiology.
“(A) long-acting dihydropyridine calcium-channel blocker (in this case, amlodipine) may be critical to more efficacious blood-pressure lowering among black patients as part of the two-drug combinations used (in Africa),” wrote lead author Dike B. Ojji, PhD, of the University of Abuja in Gwagwalada, Abuja, Nigeria, and his coauthors. “These results contrast with recommendations for black patients in the most recent U.S. guidelines” which recommend either a calcium channel blocker or a diuretic in combination with a different drug class.
The study was published simultaneously in the New England Journal of Medicine.
In the CREOLE study, Dr. Ojji and his colleagues enrolled 728 black patients, mean age 51 years and 63% of them women, in a randomized, single-blind, three-group trial across six countries in sub-Saharan Africa. All patients had uncontrolled hypertension and were assigned to one of three treatment groups: amlodipine plus hydrochlorothiazide (n = 244), amlodipine plus perindopril (n = 243), and perindopril plus hydrochlorothiazide (n = 241). Patients underwent 24-hour ambulatory blood-pressure measuring at baseline and at 6 months.
Of the 621 patients who completed the trial, those in the two groups receiving amlodipine had a larger mean reduction in systolic blood pressure after 6 months than the group receiving perindopril plus hydrochlorothiazide. Compared with the perindopril plus hydrochlorothiazide group, the amlodipine plus hydrochlorothiazide group had an additional -3.14 mm Hg reduction in systolic blood pressure from baseline (95% confidence interval, -5.90 to -0.38, P = 0.03) while the amlodipine plus perindopril group had an additional -3.00 mm Hg reduction (95% CI, -5.81 to -0.20, P = 0.04). The difference between the amlodipine plus hydrochlorothiazide group and the amlodipine plus perindopril group was -0.14 mm Hg (95% CI, -2.90 to 2.61, P = 0.92).
The limitations of the study included using nonmatching trial drugs and not adjusting the P values for the three comparisons of the primary end point, the authors wrote. It’s uncertain whether the findings can be extrapolated to black patients with diabetes or to those living outside of sub-Saharan Africa.
The study was sponsored by a grant from the GlaxoSmithKline Africa Noncommunicable Disease Open Lab. Trial drugs were donated by Aspen Pharmacare. Two authors reported receiving grants and personal fees from numerous pharmaceutical companies.
SOURCE: Ojji DB et al. NEJM. 2019 Mar 18. doi: 10.1056/NEJMoa1901113.
Amlodipine plus either hydrochlorothiazide or perindopril effectively reduced blood pressure better than perindopril and hydrochlorothiazide in black African patients with hypertension, based on data presented at the annual meeting of the American College of Cardiology.
“(A) long-acting dihydropyridine calcium-channel blocker (in this case, amlodipine) may be critical to more efficacious blood-pressure lowering among black patients as part of the two-drug combinations used (in Africa),” wrote lead author Dike B. Ojji, PhD, of the University of Abuja in Gwagwalada, Abuja, Nigeria, and his coauthors. “These results contrast with recommendations for black patients in the most recent U.S. guidelines” which recommend either a calcium channel blocker or a diuretic in combination with a different drug class.
The study was published simultaneously in the New England Journal of Medicine.
In the CREOLE study, Dr. Ojji and his colleagues enrolled 728 black patients, mean age 51 years and 63% of them women, in a randomized, single-blind, three-group trial across six countries in sub-Saharan Africa. All patients had uncontrolled hypertension and were assigned to one of three treatment groups: amlodipine plus hydrochlorothiazide (n = 244), amlodipine plus perindopril (n = 243), and perindopril plus hydrochlorothiazide (n = 241). Patients underwent 24-hour ambulatory blood-pressure measuring at baseline and at 6 months.
Of the 621 patients who completed the trial, those in the two groups receiving amlodipine had a larger mean reduction in systolic blood pressure after 6 months than the group receiving perindopril plus hydrochlorothiazide. Compared with the perindopril plus hydrochlorothiazide group, the amlodipine plus hydrochlorothiazide group had an additional -3.14 mm Hg reduction in systolic blood pressure from baseline (95% confidence interval, -5.90 to -0.38, P = 0.03) while the amlodipine plus perindopril group had an additional -3.00 mm Hg reduction (95% CI, -5.81 to -0.20, P = 0.04). The difference between the amlodipine plus hydrochlorothiazide group and the amlodipine plus perindopril group was -0.14 mm Hg (95% CI, -2.90 to 2.61, P = 0.92).
The limitations of the study included using nonmatching trial drugs and not adjusting the P values for the three comparisons of the primary end point, the authors wrote. It’s uncertain whether the findings can be extrapolated to black patients with diabetes or to those living outside of sub-Saharan Africa.
The study was sponsored by a grant from the GlaxoSmithKline Africa Noncommunicable Disease Open Lab. Trial drugs were donated by Aspen Pharmacare. Two authors reported receiving grants and personal fees from numerous pharmaceutical companies.
SOURCE: Ojji DB et al. NEJM. 2019 Mar 18. doi: 10.1056/NEJMoa1901113.
FROM ACC 2019
VEText 1 Year Later—Still Growing
Nearly 6 million veterans get health care scheduling reminders via VEText, an interactive mobile program launched a year ago. More than 70 million text messages later, how is VEText doing? Apparently, well. The VA says an “overwhelming majority” of veterans like the enhanced access. Only 4% have opted out.
The VA has worked to improve the user experience along the way. The latest enhancement allows the VA to send facility and clinic location in the unsecured text message appointment reminders. One user, James Preston, interviewed for a VA article, says at the Loma Linda VA Medical Center veterans still had to use the information desk to find out exactly where they needed to go. “Now it’s almost perfect,” he says, “because it provides all the necessary information.” Deanna Callahan, innovation specialist and National Program Manager for VEText, says before VEText, the VA was relying on phone calls, robocalls, and mail. “We wanted to modernize our efforts to not only bring text message appointment reminders but go above and beyond and positively affect the No Show rate.” It worked—the No Show rate has dropped from 13.7% to 11.7% in the year the program has been active.
The system automatically enrolls veterans based on phone information already on file, but they can opt out by replying STOP to a reminder. Accidentally opting out is easily reversed by replying START to a previous reminder. Reminders are sent for clinical appointments at local medical centers and outpatient clinics, but not for Lab, Community Care, Research, Telephone Clinics, or Home-based Primary Care. (The reminders are additional—they do not replace letters, postcards, or automated phone call reminders.) VEText itself does not cost the veteran anything, but text messaging rates may apply, depending on individual cell phone plans. For more information, go to www.va.gov/HEALTH/vetext_faqs.asp.
Nearly 6 million veterans get health care scheduling reminders via VEText, an interactive mobile program launched a year ago. More than 70 million text messages later, how is VEText doing? Apparently, well. The VA says an “overwhelming majority” of veterans like the enhanced access. Only 4% have opted out.
The VA has worked to improve the user experience along the way. The latest enhancement allows the VA to send facility and clinic location in the unsecured text message appointment reminders. One user, James Preston, interviewed for a VA article, says at the Loma Linda VA Medical Center veterans still had to use the information desk to find out exactly where they needed to go. “Now it’s almost perfect,” he says, “because it provides all the necessary information.” Deanna Callahan, innovation specialist and National Program Manager for VEText, says before VEText, the VA was relying on phone calls, robocalls, and mail. “We wanted to modernize our efforts to not only bring text message appointment reminders but go above and beyond and positively affect the No Show rate.” It worked—the No Show rate has dropped from 13.7% to 11.7% in the year the program has been active.
The system automatically enrolls veterans based on phone information already on file, but they can opt out by replying STOP to a reminder. Accidentally opting out is easily reversed by replying START to a previous reminder. Reminders are sent for clinical appointments at local medical centers and outpatient clinics, but not for Lab, Community Care, Research, Telephone Clinics, or Home-based Primary Care. (The reminders are additional—they do not replace letters, postcards, or automated phone call reminders.) VEText itself does not cost the veteran anything, but text messaging rates may apply, depending on individual cell phone plans. For more information, go to www.va.gov/HEALTH/vetext_faqs.asp.
Nearly 6 million veterans get health care scheduling reminders via VEText, an interactive mobile program launched a year ago. More than 70 million text messages later, how is VEText doing? Apparently, well. The VA says an “overwhelming majority” of veterans like the enhanced access. Only 4% have opted out.
The VA has worked to improve the user experience along the way. The latest enhancement allows the VA to send facility and clinic location in the unsecured text message appointment reminders. One user, James Preston, interviewed for a VA article, says at the Loma Linda VA Medical Center veterans still had to use the information desk to find out exactly where they needed to go. “Now it’s almost perfect,” he says, “because it provides all the necessary information.” Deanna Callahan, innovation specialist and National Program Manager for VEText, says before VEText, the VA was relying on phone calls, robocalls, and mail. “We wanted to modernize our efforts to not only bring text message appointment reminders but go above and beyond and positively affect the No Show rate.” It worked—the No Show rate has dropped from 13.7% to 11.7% in the year the program has been active.
The system automatically enrolls veterans based on phone information already on file, but they can opt out by replying STOP to a reminder. Accidentally opting out is easily reversed by replying START to a previous reminder. Reminders are sent for clinical appointments at local medical centers and outpatient clinics, but not for Lab, Community Care, Research, Telephone Clinics, or Home-based Primary Care. (The reminders are additional—they do not replace letters, postcards, or automated phone call reminders.) VEText itself does not cost the veteran anything, but text messaging rates may apply, depending on individual cell phone plans. For more information, go to www.va.gov/HEALTH/vetext_faqs.asp.
DDNA19: Cardiac Complications in Liver Disease Patients

Dr. Marc Klapholz of Rutgers University, Newark, N.J., explains the latest developments in portopulmonary arterial hypertension, hepatopulmonary syndrome, and cirrhotic cardiomyopathy, as well as the emerging field and association between non-alcoholic fatty liver disease and cardiovascular disease.

Dr. Marc Klapholz of Rutgers University, Newark, N.J., explains the latest developments in portopulmonary arterial hypertension, hepatopulmonary syndrome, and cirrhotic cardiomyopathy, as well as the emerging field and association between non-alcoholic fatty liver disease and cardiovascular disease.

Dr. Marc Klapholz of Rutgers University, Newark, N.J., explains the latest developments in portopulmonary arterial hypertension, hepatopulmonary syndrome, and cirrhotic cardiomyopathy, as well as the emerging field and association between non-alcoholic fatty liver disease and cardiovascular disease.
AT DIGESTIVE DISEASES: NEW ADVANCES
ACC, AHA release first cardiovascular disease primary prevention guideline
that takes into account each person’s social determinants of health. The guideline substantially dialed down prior recommendations on aspirin for primary prevention by calling for no use in people older than 70 years and infrequent use in those 40-70 years old.

The American College of Cardiology and the American Heart Association released their 2019 guideline on the primary prevention of cardiovascular disease on March 17, during the annual meeting of the American College of Cardiology (J Amer Coll Cardiol. 2019 March 17;doi: 10.1016/j.jacc.2019.03.010.). The guideline is a “one-stop shop” that pulls together existing recommendations from the two organizations and combines it with some new recommendations that address issues such as aspirin prophylaxis, and the social setting of each person, said Donna K. Arnett, Ph.D., professor of epidemiology at the University of Kentucky, dean of the university’s College of Public Health, and co-chair of the guideline writing panel.
“We made the social determinants of health front and center. With many people, clinicians don’t ask whether they have access to healthy foods or a way to get to the pharmacy. Asking about these issues is step one,” toward helping people address their social situation, Dr. Arnett said while introducing the new guideline in a press briefing. The guideline recommends that clinicians assess the social determinants for each person treated for cardiovascular disease prevention using a screening tool developed by the U.S. Centers for Medicare & Medicaid Services and made available by the National Academy of Medicine (NAM Perspectives. 2017; doi:10.31478/201705b).
“No other guideline has highlighted the social determinants of health,” noted Erin D. Michos, MD, associate director of preventive cardiology at Johns Hopkins Medicine in Baltimore, and a member of the guideline-writing panel. Other overarching themes of the guideline are its emphasis on the need for a team of clinicians to deliver all the disparate and time-consuming facets of care needed for comprehensive primary prevention of cardiovascular disease, and its call for a healthy lifestyle throughout life as foundations for prevention, Dr. Michos said in an interview.
With 48 recommendations, the guideline also deals with prevention issues such as a healthy diet and body mass, appropriate control of diabetes, smoking cessation, and control of blood pressure and cholesterol (see chart). The writing committee took the cholesterol and blood pressure recommendations directly from recent guidelines from the ACC and AHA in 2017 (blood pressure:J Amer Coll Cardiol. 2018 May;71[19]:e177-e248) and 2018 (cholesterol:Circulation. 2018 Nov 10;doi: 10.1161/CIR.0000000000000625).
The other major, new recommendations in the guideline deal with aspirin use for primary prevention, which recently underwent a shake up with publication of results from several studies that showed less cardiovascular benefit and more potential bleeding harm from routine aspirin prophylaxis than previously appreciated. Among the most notable of these reports, which led to a class III recommendation – do not use – for aspirin in people more than 70 years old came from the ASPREE (Aspirin in Reducing Events in the Elderly) study (New Engl J Med. 2018 Oct 18;379[16]:1519-28). For those 40-70 years old, the recommendation is class IIb, worded as “might be considered for select adults.”

“Generally no, occasionally yes,” is aspirin appropriate for people in this age group, notably those at high risk for cardiovascular disease and also at low risk for bleeding, explained Amit Khera, MD, a guideline-panel member, and professor of medicine and director of preventive cardiology at the University of Texas Southwestern Medical Center in Dallas.
As a guideline for primary prevention, a prime target audience is primary care physicians, who would need to be instrumental in applying the guideline. But the guideline recommendations released by the ACC and AHA for blood pressure management in 2017 were not accepted by U.S. groups that represent primary care physicians, the American College of Physicians, and the American Academy of Family Physicians.
John J. Warner, MD, an interventional cardiologist, executive vice president for health system affairs at UT Southwestern, and president of the AHA when the blood pressure guideline came out said that the ACC and AHA “learned some lessons” from the blood pressure experience. The societies responded this time around by “trying to view the document through as many lenses as possible” during the peer review process, Dr. Warner said during the press conference.
“I don’t think the new guideline will be seen as anything except positive,” commented Martha Gulati, MD, professor of medicine and chief of cardiology at the University of Arizona in Phoenix. Collecting all the cardiovascular disease recommendations for primary prevention in one document “helps clinicians access the information easily and helps patients see the big picture,” said Dr. Gulati, who was not involved in the guideline’s writing or review.
She especially applauded the recommendations to assess each person’s social determinants of health, the team-care approach, and the recommendations dealing with diet and other aspects of a healthy lifestyle. “This was a perfect time” to bring together the existing blood pressure and cholesterol guidelines, the new guidance on aspirin use, and the other recommendation in a single document, she said in an interview.
Dr. Arnett, Dr. Michos, Dr. Khera, Dr. Warner, and Dr. Gulati had no disclosures.
mzoler@mdedge.com
On Twitter @mitchelzoler
SOURCE: Arnett DK et al. J Amer Coll Cardiol. 2019 March 17;doi: 10.1016/j.jacc.2019.03.010.
that takes into account each person’s social determinants of health. The guideline substantially dialed down prior recommendations on aspirin for primary prevention by calling for no use in people older than 70 years and infrequent use in those 40-70 years old.

The American College of Cardiology and the American Heart Association released their 2019 guideline on the primary prevention of cardiovascular disease on March 17, during the annual meeting of the American College of Cardiology (J Amer Coll Cardiol. 2019 March 17;doi: 10.1016/j.jacc.2019.03.010.). The guideline is a “one-stop shop” that pulls together existing recommendations from the two organizations and combines it with some new recommendations that address issues such as aspirin prophylaxis, and the social setting of each person, said Donna K. Arnett, Ph.D., professor of epidemiology at the University of Kentucky, dean of the university’s College of Public Health, and co-chair of the guideline writing panel.
“We made the social determinants of health front and center. With many people, clinicians don’t ask whether they have access to healthy foods or a way to get to the pharmacy. Asking about these issues is step one,” toward helping people address their social situation, Dr. Arnett said while introducing the new guideline in a press briefing. The guideline recommends that clinicians assess the social determinants for each person treated for cardiovascular disease prevention using a screening tool developed by the U.S. Centers for Medicare & Medicaid Services and made available by the National Academy of Medicine (NAM Perspectives. 2017; doi:10.31478/201705b).
“No other guideline has highlighted the social determinants of health,” noted Erin D. Michos, MD, associate director of preventive cardiology at Johns Hopkins Medicine in Baltimore, and a member of the guideline-writing panel. Other overarching themes of the guideline are its emphasis on the need for a team of clinicians to deliver all the disparate and time-consuming facets of care needed for comprehensive primary prevention of cardiovascular disease, and its call for a healthy lifestyle throughout life as foundations for prevention, Dr. Michos said in an interview.
With 48 recommendations, the guideline also deals with prevention issues such as a healthy diet and body mass, appropriate control of diabetes, smoking cessation, and control of blood pressure and cholesterol (see chart). The writing committee took the cholesterol and blood pressure recommendations directly from recent guidelines from the ACC and AHA in 2017 (blood pressure:J Amer Coll Cardiol. 2018 May;71[19]:e177-e248) and 2018 (cholesterol:Circulation. 2018 Nov 10;doi: 10.1161/CIR.0000000000000625).
The other major, new recommendations in the guideline deal with aspirin use for primary prevention, which recently underwent a shake up with publication of results from several studies that showed less cardiovascular benefit and more potential bleeding harm from routine aspirin prophylaxis than previously appreciated. Among the most notable of these reports, which led to a class III recommendation – do not use – for aspirin in people more than 70 years old came from the ASPREE (Aspirin in Reducing Events in the Elderly) study (New Engl J Med. 2018 Oct 18;379[16]:1519-28). For those 40-70 years old, the recommendation is class IIb, worded as “might be considered for select adults.”

“Generally no, occasionally yes,” is aspirin appropriate for people in this age group, notably those at high risk for cardiovascular disease and also at low risk for bleeding, explained Amit Khera, MD, a guideline-panel member, and professor of medicine and director of preventive cardiology at the University of Texas Southwestern Medical Center in Dallas.
As a guideline for primary prevention, a prime target audience is primary care physicians, who would need to be instrumental in applying the guideline. But the guideline recommendations released by the ACC and AHA for blood pressure management in 2017 were not accepted by U.S. groups that represent primary care physicians, the American College of Physicians, and the American Academy of Family Physicians.
John J. Warner, MD, an interventional cardiologist, executive vice president for health system affairs at UT Southwestern, and president of the AHA when the blood pressure guideline came out said that the ACC and AHA “learned some lessons” from the blood pressure experience. The societies responded this time around by “trying to view the document through as many lenses as possible” during the peer review process, Dr. Warner said during the press conference.
“I don’t think the new guideline will be seen as anything except positive,” commented Martha Gulati, MD, professor of medicine and chief of cardiology at the University of Arizona in Phoenix. Collecting all the cardiovascular disease recommendations for primary prevention in one document “helps clinicians access the information easily and helps patients see the big picture,” said Dr. Gulati, who was not involved in the guideline’s writing or review.
She especially applauded the recommendations to assess each person’s social determinants of health, the team-care approach, and the recommendations dealing with diet and other aspects of a healthy lifestyle. “This was a perfect time” to bring together the existing blood pressure and cholesterol guidelines, the new guidance on aspirin use, and the other recommendation in a single document, she said in an interview.
Dr. Arnett, Dr. Michos, Dr. Khera, Dr. Warner, and Dr. Gulati had no disclosures.
mzoler@mdedge.com
On Twitter @mitchelzoler
SOURCE: Arnett DK et al. J Amer Coll Cardiol. 2019 March 17;doi: 10.1016/j.jacc.2019.03.010.
that takes into account each person’s social determinants of health. The guideline substantially dialed down prior recommendations on aspirin for primary prevention by calling for no use in people older than 70 years and infrequent use in those 40-70 years old.

The American College of Cardiology and the American Heart Association released their 2019 guideline on the primary prevention of cardiovascular disease on March 17, during the annual meeting of the American College of Cardiology (J Amer Coll Cardiol. 2019 March 17;doi: 10.1016/j.jacc.2019.03.010.). The guideline is a “one-stop shop” that pulls together existing recommendations from the two organizations and combines it with some new recommendations that address issues such as aspirin prophylaxis, and the social setting of each person, said Donna K. Arnett, Ph.D., professor of epidemiology at the University of Kentucky, dean of the university’s College of Public Health, and co-chair of the guideline writing panel.
“We made the social determinants of health front and center. With many people, clinicians don’t ask whether they have access to healthy foods or a way to get to the pharmacy. Asking about these issues is step one,” toward helping people address their social situation, Dr. Arnett said while introducing the new guideline in a press briefing. The guideline recommends that clinicians assess the social determinants for each person treated for cardiovascular disease prevention using a screening tool developed by the U.S. Centers for Medicare & Medicaid Services and made available by the National Academy of Medicine (NAM Perspectives. 2017; doi:10.31478/201705b).
“No other guideline has highlighted the social determinants of health,” noted Erin D. Michos, MD, associate director of preventive cardiology at Johns Hopkins Medicine in Baltimore, and a member of the guideline-writing panel. Other overarching themes of the guideline are its emphasis on the need for a team of clinicians to deliver all the disparate and time-consuming facets of care needed for comprehensive primary prevention of cardiovascular disease, and its call for a healthy lifestyle throughout life as foundations for prevention, Dr. Michos said in an interview.
With 48 recommendations, the guideline also deals with prevention issues such as a healthy diet and body mass, appropriate control of diabetes, smoking cessation, and control of blood pressure and cholesterol (see chart). The writing committee took the cholesterol and blood pressure recommendations directly from recent guidelines from the ACC and AHA in 2017 (blood pressure:J Amer Coll Cardiol. 2018 May;71[19]:e177-e248) and 2018 (cholesterol:Circulation. 2018 Nov 10;doi: 10.1161/CIR.0000000000000625).
The other major, new recommendations in the guideline deal with aspirin use for primary prevention, which recently underwent a shake up with publication of results from several studies that showed less cardiovascular benefit and more potential bleeding harm from routine aspirin prophylaxis than previously appreciated. Among the most notable of these reports, which led to a class III recommendation – do not use – for aspirin in people more than 70 years old came from the ASPREE (Aspirin in Reducing Events in the Elderly) study (New Engl J Med. 2018 Oct 18;379[16]:1519-28). For those 40-70 years old, the recommendation is class IIb, worded as “might be considered for select adults.”

“Generally no, occasionally yes,” is aspirin appropriate for people in this age group, notably those at high risk for cardiovascular disease and also at low risk for bleeding, explained Amit Khera, MD, a guideline-panel member, and professor of medicine and director of preventive cardiology at the University of Texas Southwestern Medical Center in Dallas.
As a guideline for primary prevention, a prime target audience is primary care physicians, who would need to be instrumental in applying the guideline. But the guideline recommendations released by the ACC and AHA for blood pressure management in 2017 were not accepted by U.S. groups that represent primary care physicians, the American College of Physicians, and the American Academy of Family Physicians.
John J. Warner, MD, an interventional cardiologist, executive vice president for health system affairs at UT Southwestern, and president of the AHA when the blood pressure guideline came out said that the ACC and AHA “learned some lessons” from the blood pressure experience. The societies responded this time around by “trying to view the document through as many lenses as possible” during the peer review process, Dr. Warner said during the press conference.
“I don’t think the new guideline will be seen as anything except positive,” commented Martha Gulati, MD, professor of medicine and chief of cardiology at the University of Arizona in Phoenix. Collecting all the cardiovascular disease recommendations for primary prevention in one document “helps clinicians access the information easily and helps patients see the big picture,” said Dr. Gulati, who was not involved in the guideline’s writing or review.
She especially applauded the recommendations to assess each person’s social determinants of health, the team-care approach, and the recommendations dealing with diet and other aspects of a healthy lifestyle. “This was a perfect time” to bring together the existing blood pressure and cholesterol guidelines, the new guidance on aspirin use, and the other recommendation in a single document, she said in an interview.
Dr. Arnett, Dr. Michos, Dr. Khera, Dr. Warner, and Dr. Gulati had no disclosures.
mzoler@mdedge.com
On Twitter @mitchelzoler
SOURCE: Arnett DK et al. J Amer Coll Cardiol. 2019 March 17;doi: 10.1016/j.jacc.2019.03.010.
REPORTING FROM ACC 2019
Antibiotic-eluting envelope reduces CIED infections
An absorbable, antibiotic-eluting envelope around cardiac implantable electronic devices could significantly reduce the incidence of infection, according to a presentation at the annual meeting of the American College of Cardiology.
The WRAP-IT trial, which was simultaneously published online March 17 in the New England Journal of Medicine, involved 6,983 patients undergoing cardiac implantable electronic device (CIED) implantation, replacement, revision, or upgrade. Patients were randomized either to receive the TYRX Absorbable Antibacterial Envelope or not.
After a mean follow-up of 20.7 months, there was a significant 40% lower rate of major infections in the envelope group compared to the control group, which met the efficacy objective of the study. Researchers saw 30 major infections in 25 patients in the envelope group; in the control group, there were 45 major infections in 42 patients (P = 0.04). The trial excluded patients at high risk of systemic infection due to other sources and patients with existing infection.
“CIED infection is a rare but serious event, and its management requires prolonged hospitalization, which involves device and lead extraction with adjunctive antibiotic therapy,” wrote Dr. Khaldoun G. Tarakji, from The Cleveland Clinic, and co-authors. “Despite proper management of CIED infection, both short- and long-term mortality remains high.”
One previous randomized study had shown that intravenous administration of antibiotics during CIED procedures can reduce the risk of infection, while a different study failed to find a benefit. The vast majority of patients in this study (98.7%) received periprocedural antibiotics, 74.5% received pocket wash and 29.6% received post-procedural antibiotics. These strategies were not controlled, but there is no clear evidence that any particular strategy influenced the infection rate, the authors wrote.
Patients in the envelope group experienced numerically fewer pocket infections but more endocarditis or bacteremia compared to those in the control group, a finding that the authors could not explain.
The most common pathogen responsible was staphylococcus, but data on antibiotic susceptibility was not collected. The authors stressed that this limited their ability to assess the risk of antibiotic resistance developing.
In this study, the researchers noted that the reduction in the risk of infection was greater among individuals who were implanted with higher-power devices, compared to those implanted with low-power devices or an initial cardiac resynchronization therapy device.
However, they said, the rate of infections was generally lower among those receiving low-power devices.
There was no increase in complications related to use of the envelope. The rate of complications occurring within 12 months of the procedure and relating to the CIED procedure or envelope was 6% in the envelope group and 6.9% in the control group.
When major infections were excluded, the rate of complications in each group was 5.7% and 5/9% respectively. There was also no significant difference in mortality rates between the two groups (17.4% and 17.8% respectively).
The authors wrote that while use of the envelope can require a slightly larger CIED dissection pocket, this was not associated with increased procedural time or complications. The envelope was successfully implanted in 99.7% of procedure attempts.
“There were fewer system revisions in the envelope group than in the control group and no complications due to allergy to the envelope mesh, polymer, or antibiotics,” they wrote.
The study was supported by Medtronic, the maker of the TYRX Absorbable Antibacterial Envelope. Twenty-four authors declared institutional funding or research grants from Medtronic, thirteen declared fees, consultancies and other support from private industry outside the submitted work. Three authors were employees of Medtronic.
SOURCE: Tarakji K et al. NEJM, 2019, March 17. DOI: 10.1056/NEJMoa1901111
An absorbable, antibiotic-eluting envelope around cardiac implantable electronic devices could significantly reduce the incidence of infection, according to a presentation at the annual meeting of the American College of Cardiology.
The WRAP-IT trial, which was simultaneously published online March 17 in the New England Journal of Medicine, involved 6,983 patients undergoing cardiac implantable electronic device (CIED) implantation, replacement, revision, or upgrade. Patients were randomized either to receive the TYRX Absorbable Antibacterial Envelope or not.
After a mean follow-up of 20.7 months, there was a significant 40% lower rate of major infections in the envelope group compared to the control group, which met the efficacy objective of the study. Researchers saw 30 major infections in 25 patients in the envelope group; in the control group, there were 45 major infections in 42 patients (P = 0.04). The trial excluded patients at high risk of systemic infection due to other sources and patients with existing infection.
“CIED infection is a rare but serious event, and its management requires prolonged hospitalization, which involves device and lead extraction with adjunctive antibiotic therapy,” wrote Dr. Khaldoun G. Tarakji, from The Cleveland Clinic, and co-authors. “Despite proper management of CIED infection, both short- and long-term mortality remains high.”
One previous randomized study had shown that intravenous administration of antibiotics during CIED procedures can reduce the risk of infection, while a different study failed to find a benefit. The vast majority of patients in this study (98.7%) received periprocedural antibiotics, 74.5% received pocket wash and 29.6% received post-procedural antibiotics. These strategies were not controlled, but there is no clear evidence that any particular strategy influenced the infection rate, the authors wrote.
Patients in the envelope group experienced numerically fewer pocket infections but more endocarditis or bacteremia compared to those in the control group, a finding that the authors could not explain.
The most common pathogen responsible was staphylococcus, but data on antibiotic susceptibility was not collected. The authors stressed that this limited their ability to assess the risk of antibiotic resistance developing.
In this study, the researchers noted that the reduction in the risk of infection was greater among individuals who were implanted with higher-power devices, compared to those implanted with low-power devices or an initial cardiac resynchronization therapy device.
However, they said, the rate of infections was generally lower among those receiving low-power devices.
There was no increase in complications related to use of the envelope. The rate of complications occurring within 12 months of the procedure and relating to the CIED procedure or envelope was 6% in the envelope group and 6.9% in the control group.
When major infections were excluded, the rate of complications in each group was 5.7% and 5/9% respectively. There was also no significant difference in mortality rates between the two groups (17.4% and 17.8% respectively).
The authors wrote that while use of the envelope can require a slightly larger CIED dissection pocket, this was not associated with increased procedural time or complications. The envelope was successfully implanted in 99.7% of procedure attempts.
“There were fewer system revisions in the envelope group than in the control group and no complications due to allergy to the envelope mesh, polymer, or antibiotics,” they wrote.
The study was supported by Medtronic, the maker of the TYRX Absorbable Antibacterial Envelope. Twenty-four authors declared institutional funding or research grants from Medtronic, thirteen declared fees, consultancies and other support from private industry outside the submitted work. Three authors were employees of Medtronic.
SOURCE: Tarakji K et al. NEJM, 2019, March 17. DOI: 10.1056/NEJMoa1901111
An absorbable, antibiotic-eluting envelope around cardiac implantable electronic devices could significantly reduce the incidence of infection, according to a presentation at the annual meeting of the American College of Cardiology.
The WRAP-IT trial, which was simultaneously published online March 17 in the New England Journal of Medicine, involved 6,983 patients undergoing cardiac implantable electronic device (CIED) implantation, replacement, revision, or upgrade. Patients were randomized either to receive the TYRX Absorbable Antibacterial Envelope or not.
After a mean follow-up of 20.7 months, there was a significant 40% lower rate of major infections in the envelope group compared to the control group, which met the efficacy objective of the study. Researchers saw 30 major infections in 25 patients in the envelope group; in the control group, there were 45 major infections in 42 patients (P = 0.04). The trial excluded patients at high risk of systemic infection due to other sources and patients with existing infection.
“CIED infection is a rare but serious event, and its management requires prolonged hospitalization, which involves device and lead extraction with adjunctive antibiotic therapy,” wrote Dr. Khaldoun G. Tarakji, from The Cleveland Clinic, and co-authors. “Despite proper management of CIED infection, both short- and long-term mortality remains high.”
One previous randomized study had shown that intravenous administration of antibiotics during CIED procedures can reduce the risk of infection, while a different study failed to find a benefit. The vast majority of patients in this study (98.7%) received periprocedural antibiotics, 74.5% received pocket wash and 29.6% received post-procedural antibiotics. These strategies were not controlled, but there is no clear evidence that any particular strategy influenced the infection rate, the authors wrote.
Patients in the envelope group experienced numerically fewer pocket infections but more endocarditis or bacteremia compared to those in the control group, a finding that the authors could not explain.
The most common pathogen responsible was staphylococcus, but data on antibiotic susceptibility was not collected. The authors stressed that this limited their ability to assess the risk of antibiotic resistance developing.
In this study, the researchers noted that the reduction in the risk of infection was greater among individuals who were implanted with higher-power devices, compared to those implanted with low-power devices or an initial cardiac resynchronization therapy device.
However, they said, the rate of infections was generally lower among those receiving low-power devices.
There was no increase in complications related to use of the envelope. The rate of complications occurring within 12 months of the procedure and relating to the CIED procedure or envelope was 6% in the envelope group and 6.9% in the control group.
When major infections were excluded, the rate of complications in each group was 5.7% and 5/9% respectively. There was also no significant difference in mortality rates between the two groups (17.4% and 17.8% respectively).
The authors wrote that while use of the envelope can require a slightly larger CIED dissection pocket, this was not associated with increased procedural time or complications. The envelope was successfully implanted in 99.7% of procedure attempts.
“There were fewer system revisions in the envelope group than in the control group and no complications due to allergy to the envelope mesh, polymer, or antibiotics,” they wrote.
The study was supported by Medtronic, the maker of the TYRX Absorbable Antibacterial Envelope. Twenty-four authors declared institutional funding or research grants from Medtronic, thirteen declared fees, consultancies and other support from private industry outside the submitted work. Three authors were employees of Medtronic.
SOURCE: Tarakji K et al. NEJM, 2019, March 17. DOI: 10.1056/NEJMoa1901111
FROM ACC 19
Financial toxicity may be common in gynecologic cancer patients
HONOLULU – Financial toxicity may be common among gynecologic cancer patients starting a new line of treatment, based on the results of a survey presented at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer.
More than half of the 121 patients surveyed reported mild, moderate, or severe financial toxicity, Margaret Liang, MD, of University of Alabama at Birmingham, said in presenting the findings.
Younger age and lower income were both risk factors for financial toxicity, and having insurance did not protect patients from financial toxicity. Dr. Liang noted that insurance coverage “was not protective for financial toxicity, as the majority of those who screened positive for financial toxicity were insured.” Specifically, 89% of patients with financial toxicity and 98% of patients without it were insured (P = .07).
“This finding supports financial toxicity screening regardless of insurance status and may favor universal screening,” Dr. Liang said.
She and her colleagues conducted the survey of 121 gynecologic cancer patients who had started a new line of systemic therapy in the previous 8 weeks.
The patients’ mean age was 59 years (range, 33-80), and 58% were starting their first line of systemic therapy. Half of the patients had a household income below $40,000, and about one-third of the patients were employed. Most had private (74%) or public (20%) insurance, and 7% of patients were uninsured.
To assess financial toxicity, the researchers used the Comprehensive Score for Financial Toxicity (COST). A score of less than 26 was used as a threshold of financial toxicity. The severity of financial toxicity was graded on a scale of 1 to 4, with a lower score indicating worse toxicity.
Patients were most concerned about having enough money to cover the cost of treatment. Patients reported the lowest mean score—0.97—in response to the statement, “I know that I have enough money in savings, retirement, or assets to cover the costs of my treatment.”
Patients were least concerned about losing their job or income. They reported the highest mean score—3.29—in response to the statement, “I am concerned about keeping my job and income, including working at home.” However, as Dr. Liang pointed out, only one-third of patients were employed at baseline.
In all, 54% of patients reported financial toxicity—37% mild, 16% moderate, and 1% severe.
Dr. Liang and her colleagues found that younger age and lower income were associated with an increased risk for financial toxicity. The mean age was 57 years in patients with financial toxicity and 62 years in patients without it (P = .02). Household incomes were below $40,000 in 63% of patients with financial toxicity and 34% of those without it (P less than .01).
The researchers also found that patients with financial toxicity were significantly more likely to say their cancer diagnosis resulted in lost wages, borrowed money, altered spending habits, the need to sacrifice other things, and not paying bills on time (P less than .01 for all).
On the other hand, patients with financial toxicity were not significantly more likely to become unemployed, file for bankruptcy, sell their house, or get a second job due to their cancer diagnosis.
However, it’s important to note that these data were collected within 8 weeks of patients starting their new line of therapy. The final survey results will include follow-up at 3 months and 6 months.
Dr. Liang said the fact that more than half of patients reported financial toxicity within 8 weeks of starting a new line of therapy suggests early interventions are needed to prevent or reduce financial toxicity. This may include counseling patients on anticipated costs of care, screening for financial toxicity, and linking patients to available financial resources.
Dr. Liang had no financial disclosures.
jensmith@mdedge.com
SOURCE: Liang M et al. SGO 2019. Abstract 8.
HONOLULU – Financial toxicity may be common among gynecologic cancer patients starting a new line of treatment, based on the results of a survey presented at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer.
More than half of the 121 patients surveyed reported mild, moderate, or severe financial toxicity, Margaret Liang, MD, of University of Alabama at Birmingham, said in presenting the findings.
Younger age and lower income were both risk factors for financial toxicity, and having insurance did not protect patients from financial toxicity. Dr. Liang noted that insurance coverage “was not protective for financial toxicity, as the majority of those who screened positive for financial toxicity were insured.” Specifically, 89% of patients with financial toxicity and 98% of patients without it were insured (P = .07).
“This finding supports financial toxicity screening regardless of insurance status and may favor universal screening,” Dr. Liang said.
She and her colleagues conducted the survey of 121 gynecologic cancer patients who had started a new line of systemic therapy in the previous 8 weeks.
The patients’ mean age was 59 years (range, 33-80), and 58% were starting their first line of systemic therapy. Half of the patients had a household income below $40,000, and about one-third of the patients were employed. Most had private (74%) or public (20%) insurance, and 7% of patients were uninsured.
To assess financial toxicity, the researchers used the Comprehensive Score for Financial Toxicity (COST). A score of less than 26 was used as a threshold of financial toxicity. The severity of financial toxicity was graded on a scale of 1 to 4, with a lower score indicating worse toxicity.
Patients were most concerned about having enough money to cover the cost of treatment. Patients reported the lowest mean score—0.97—in response to the statement, “I know that I have enough money in savings, retirement, or assets to cover the costs of my treatment.”
Patients were least concerned about losing their job or income. They reported the highest mean score—3.29—in response to the statement, “I am concerned about keeping my job and income, including working at home.” However, as Dr. Liang pointed out, only one-third of patients were employed at baseline.
In all, 54% of patients reported financial toxicity—37% mild, 16% moderate, and 1% severe.
Dr. Liang and her colleagues found that younger age and lower income were associated with an increased risk for financial toxicity. The mean age was 57 years in patients with financial toxicity and 62 years in patients without it (P = .02). Household incomes were below $40,000 in 63% of patients with financial toxicity and 34% of those without it (P less than .01).
The researchers also found that patients with financial toxicity were significantly more likely to say their cancer diagnosis resulted in lost wages, borrowed money, altered spending habits, the need to sacrifice other things, and not paying bills on time (P less than .01 for all).
On the other hand, patients with financial toxicity were not significantly more likely to become unemployed, file for bankruptcy, sell their house, or get a second job due to their cancer diagnosis.
However, it’s important to note that these data were collected within 8 weeks of patients starting their new line of therapy. The final survey results will include follow-up at 3 months and 6 months.
Dr. Liang said the fact that more than half of patients reported financial toxicity within 8 weeks of starting a new line of therapy suggests early interventions are needed to prevent or reduce financial toxicity. This may include counseling patients on anticipated costs of care, screening for financial toxicity, and linking patients to available financial resources.
Dr. Liang had no financial disclosures.
jensmith@mdedge.com
SOURCE: Liang M et al. SGO 2019. Abstract 8.
HONOLULU – Financial toxicity may be common among gynecologic cancer patients starting a new line of treatment, based on the results of a survey presented at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer.
More than half of the 121 patients surveyed reported mild, moderate, or severe financial toxicity, Margaret Liang, MD, of University of Alabama at Birmingham, said in presenting the findings.
Younger age and lower income were both risk factors for financial toxicity, and having insurance did not protect patients from financial toxicity. Dr. Liang noted that insurance coverage “was not protective for financial toxicity, as the majority of those who screened positive for financial toxicity were insured.” Specifically, 89% of patients with financial toxicity and 98% of patients without it were insured (P = .07).
“This finding supports financial toxicity screening regardless of insurance status and may favor universal screening,” Dr. Liang said.
She and her colleagues conducted the survey of 121 gynecologic cancer patients who had started a new line of systemic therapy in the previous 8 weeks.
The patients’ mean age was 59 years (range, 33-80), and 58% were starting their first line of systemic therapy. Half of the patients had a household income below $40,000, and about one-third of the patients were employed. Most had private (74%) or public (20%) insurance, and 7% of patients were uninsured.
To assess financial toxicity, the researchers used the Comprehensive Score for Financial Toxicity (COST). A score of less than 26 was used as a threshold of financial toxicity. The severity of financial toxicity was graded on a scale of 1 to 4, with a lower score indicating worse toxicity.
Patients were most concerned about having enough money to cover the cost of treatment. Patients reported the lowest mean score—0.97—in response to the statement, “I know that I have enough money in savings, retirement, or assets to cover the costs of my treatment.”
Patients were least concerned about losing their job or income. They reported the highest mean score—3.29—in response to the statement, “I am concerned about keeping my job and income, including working at home.” However, as Dr. Liang pointed out, only one-third of patients were employed at baseline.
In all, 54% of patients reported financial toxicity—37% mild, 16% moderate, and 1% severe.
Dr. Liang and her colleagues found that younger age and lower income were associated with an increased risk for financial toxicity. The mean age was 57 years in patients with financial toxicity and 62 years in patients without it (P = .02). Household incomes were below $40,000 in 63% of patients with financial toxicity and 34% of those without it (P less than .01).
The researchers also found that patients with financial toxicity were significantly more likely to say their cancer diagnosis resulted in lost wages, borrowed money, altered spending habits, the need to sacrifice other things, and not paying bills on time (P less than .01 for all).
On the other hand, patients with financial toxicity were not significantly more likely to become unemployed, file for bankruptcy, sell their house, or get a second job due to their cancer diagnosis.
However, it’s important to note that these data were collected within 8 weeks of patients starting their new line of therapy. The final survey results will include follow-up at 3 months and 6 months.
Dr. Liang said the fact that more than half of patients reported financial toxicity within 8 weeks of starting a new line of therapy suggests early interventions are needed to prevent or reduce financial toxicity. This may include counseling patients on anticipated costs of care, screening for financial toxicity, and linking patients to available financial resources.
Dr. Liang had no financial disclosures.
jensmith@mdedge.com
SOURCE: Liang M et al. SGO 2019. Abstract 8.
REPORTING FROM SGO 2019









