User login
Frequency of Ethical Issues on a Hospitalist Teaching Service at an Urban, Tertiary Care Center
Much has been written about the sources of the hidden curriculum in clerkships and postgraduate medical education.1-3 However, these descriptions do not adequately account for the critical role that hospitalists play in the development of trainees when they encounter ethical challenges on teaching services.4 As a role model, teacher, and the attending of record, a hospitalist’s response to ethical issues in practice can have a pivotal influence on the life and work of trainees, either instilling positive virtues or perpetuating the negative impact of the hidden curriculum.5-8 Understanding the epidemiology of ethical issues arising on academic hospitalist services has important implications for medical education, clinical ethics, and professionalism, as well as for patient care.
METHODS
Study Setting and Design
We conducted a mixed-method observational study at NewYork–Presbyterian–Weill Cornell Medical Center, an 862-bed, tertiary-care, academic institution located in New York, New York. We performed a prospective description of the frequency of all consecutively identified ethical and contextual issues pertinent to clinical decision-making by observing morning rounds with housestaff hospitalist services. Ethical issues were categorized using a comprehensive standardized instrument previously developed and published by the Division of Medical Ethics.9
The Division of Hospital Medicine employs 79 physicians, 30 of whom are dedicated full-time to daytime care on house-staff (or teaching) or physician assistant services. Of these 30 physicians, two (7%) were coinvestigators in this project and were excluded from participation to avoid bias. Between September 2017 and May 2018, the attending physicians of record of all available housestaff services were invited to participate with their teams in our research study on a weekly basis. We observed 10 different Hospital Medicine attending physicians (10/28, 36% of the available physician sample) over 19 sessions. Before rounds, a brief introduction to the nature of the study was provided to each team. It was explicitly stated that the observers were present to identify and document possible ethical issues that may arise while discussing the patients on rounds, and that the purpose of the study was neither an evaluation of the team members or their decisions nor a critique or quality improvement exercise. Observing researchers were not allowed to participate in the discussion of any case.
To avoid potential case duplication, we allowed for a minimum two-week interval before rounding twice on any particular team. To control for interobserver variability, we observed in pairs during these sessions. Discrepancies between observers were resolved by post hoc discussion and application of the definitions of the standardized instrument used to identify and catalog ethical and contextual issues.
Study Variables and Definitions
The following variables were collected in all cases: observation date, name of reviewers, demographic characteristics of the patient (age, gender, race, ethnicity, marital status, religion, preferred language, insurance type, and living situation before the admission), patient’s location during the admission (emergency room, regular nursing floor, step-down unit, or other), and ethical and contextual issues. “Ethical issues” were defined as those situations involving a conflict of values or preferences among different stakeholders, including, but not limited to, providers, patients, and/or families. Explicit definitions of each issue were generated, and additional standard rules for completion were provided.
Statistical Analysis
Results are presented as n (%) or mean ± standard deviation. Percentages were rounded to the closest integer. Interobserver variability between the observers in relation to evaluating the presence or absence of ethical or contextual issues was assessed by the kappa statistic. All P values are two-sided, with statistical significance evaluated at the 0.05 alpha level. A 95% confidence interval (95% CI) for the kappa statistic (ie, for assessing interobserver variability) was calculated to assess the precision of the obtained kappa estimate. All analyses were performed in SAS Version 9.4 (SAS Institute, Inc., Cary, NC) and Stata Version 14.0 (StataCorp, College Station, TX).
RESULTS
General Characteristics of the Study Sample
In total, 270 patients were evaluated from the teaching hospitalist services during the observation period. Ethical issues were identified in 86 of these patients (31.8%). Observer ethicists disagreed in their initial evaluation of 17 cases (6.3%). After review of and adjudication, both observers agreed that nine of these 17 cases (3.3%) should be excluded from the final analysis, as none reached the necessary threshold to be considered as a true ethical issue. Hence, we report the results of 77 patients (28.5%). These cases comprised the Hospitalist group and involved 113 ethical issues (1.48 ± 0.5 ethical issues/case). Only five patients in the Hospitalist group had a formal clinical ethics consult before our observation (5/270 patients [1.9%] vs 77/270 patients [28.5%] with an ethical issue, respectively, P < .001). Although the majority of ethical issues were noted by members of the primary team (84%), 12 of the 77 cases in the Hospitalist group (16%) were identified only by the observing ethicists. The kappa statistic for interobserver variability between the observing ethicists was 0.85 (95% CI = 0.76-0.92). The major demographic characteristics are summarized in Table 1.
Ethical Challenges
The most common ethical issues hospitalists encountered involved discussions about goals of care (including decisions to pursue aggressive treatment versus hospice care, or debates about the team’s ambivalence about the benefits and risks of pursuing investigational chemotherapy), treatment refusals (including the decision to forgo biopsy of a suspected malignancy), or decision-making capacity (Table 2). Less common were issues pertaining to resource allocation (specially related to pressures to discharge patients), pain management (some patients were suspected of drug-seeking behavior), or surrogate decision-making (when alternative decision-makers were suspected to lack decision-making capacity). Discussions about forgoing life-sustaining treatments occurred only in four cases (5%). These involved considerations of withdrawing Bilevel Positive Airway Pressure (BiPAP), artificial nutrition and hydration, and/or stopping antibiotic treatment.
DISCUSSION
Our data are the first prospective description of ethical issues arising on an academic hospitalist teaching service. These results indicate that there is an ethics epidemiology in the routine practice of Hospital Medicine that has heretofore not been characterized. By this, we mean a discreet incidence and prevalence of ethical challenges in Hospital Medicine that is distinct from that which is encountered by clinical ethics consultation (CEC) services. Although most practitioners recognize the utility of a traditional ethics consultation, there is a surprising paucity of data about the sources of ethical conflict encountered by academic hospitalists at the bedside, particularly those addressed without CEC. This suggests that the criteria for requesting a formal ethics consult could be limited and restrictive, which is both undersensitive and overspecific.10 Because of these limitations, viewing traditional ethics consultation as a proxy for ethical issues arising in daily hospitalist practice would lead to an underestimation of the true prevalence, as our data indicate.
More than one-fourth of the patients admitted to hospitalist teaching services pose ethical conflicts. Some of these are addressed on rounds, some are not, and only a handful of these cases will ever be referred to an ethicist. CEC services are made aware of the “tip of the iceberg,” which accounts for a vanishingly small percentage of ethical issues that arise on daily rounds. Some hospitalists may not involve CEC simply because they believe that the services are not helpful. However, the failure to obtain consultation may also reflect an inability to recognize a “problematic situation” and formulate a referral that might benefit from the assistance of an ethics consultation.11
Our study faces several potential limitations. We are presenting a single-center experience that focuses on the perspective of physicians and trainees. Some ethical issues might have been underestimated because the perspectives of patients, families, nurses, social workers, or other ancillary staff were not directly included. Furthermore, since any ethical challenge could have been discussed on any moment other than on morning rounds, our results may underestimate the prevalence of ethical issues arising from the hospital floors. Moreover, medical teams participating in the study could have been subject to the Hawthorne effect and could have tried to identify a greater number of ethical issues on rounds, which would not reflect actual practice.
CONCLUSION
Almost two decades ago, Coulehan and Williams wrote about the positive impact that ethics and humanities could have if these disciplines could be embedded in the daily practice of medicine, which is as follows:
…ethics and humanities curricula are irrelevant unless they can produce a substantive and continuing impact on hospital culture (…) The idea, of course, is to infiltrate the culture by coopting residents and attending physicians(…) If an ethics program can somehow achieve a critical mass of ‘‘value-sensitive’’ clinical faculty, it may begin to influence the institution’s ethos.12
Coulehan and Williams wrote of a need to bring ethics to the bedside. Our data suggest that an ethics epidemiology is deeply embedded in hospitalist services and is waiting to be fully characterized to better inform the care of patients and guide the professional formation and education of students and trainees. Hospitalists frequently confront ethical problems in daily practice that do not come to the attention of the CEC services or the institutional ethics committee. Understanding this emerging epidemiology presents an unrealized opportunity to improve bedside teaching, reinforce normative reasoning, and enhance patient care.
Acknowledgments
The authors want to acknowledge Drs. Augustine I. Choi, Michael G. Stewart, Laura L. Forese, and Anthony Hollenberg for their support of the fellowship in medical ethics and thank Drs. Arthur T. Evans and Monika M. Safford for their guidance.
Disclosures
The authors report no conflicts of interest.
Funding
This work was supported by a Weill Cornell General Internal Medicine Primary Care Innovations Initiative seed grant. Dr. Paul Christos was partially supported by the following grant: Clinical and Translational Science Center at Weill Cornell Medical College (1-UL1-TR002384-01).
1. Doja A, Bould MD, Clarkin C, Eady K, Sutherland S, Writer H. The hidden and informal curriculum across the continuum of training: a cross-sectional qualitative study. Med Teach. 2016;38(4):410-418. doi: 10.3109/0142159X.2015.1073241. PubMed
2. Martimianakis MA, Hafferty FW. Exploring the interstitial space between the ideal and the practised: humanism and the hidden curriculum of system reform. Med Educ. 2016;50(3):278-280. doi: 10.1111/medu.12982. PubMed
3. Lawrence C, Mhlaba T, Stewart KA, Moletsane R, Gaede B, Moshabela M. The hidden curricula of medical education: a scoping review. Acad Med. 2017;93(4):648-656. doi: 10.1097/ACM.0000000000002004. PubMed
4. McCarthy MW, Real de Asua D, Fins JJ. The rise of hospitalists: an opportunity for clinical ethics. J Clin Ethics. 2017;28(4):325-332. PubMed
5. McCarthy M, Fins J. Teaching clinical ethics at the bedside: William Osler and the essential role of the hospitalist. AMA J Ethics. 2017;19(6):528-532. doi: 10.1001/journalofethics.2017.19.6.peer2-1706. PubMed
6. Gabbay E, McCarthy MW, Fins JJ. The care of the ultra-orthodox Jewish patient. J Relig Health. 2017;56(2):545-560. doi: 10.1007/s10943-017-0356-6. PubMed
7. Wachter RM, Goldman L. The emerging role of “hospitalists” in the American health care system. N Engl J Med. 1996;335(7):514-517. doi: 10.1056/NEJM199608153350713. PubMed
8. Hauer KE, Wachter RM, McCulloch CE, Woo GA, Auerbach AD. Effects of hospitalist attending physicians on trainee satisfaction with teaching and with internal medicine rotations. Arch Intern Med. 2004;164(17):1866-1871. doi: 10.1001/archinte.164.17.1866. PubMed
9. Nilson EG, Acres CA, Tamerin NG, Fins JJ. Clinical ethics and the quality initiative: a pilot study for the empirical evaluation of ethics case consultation. Am J Med Qual. 2008;23(5):356-364. doi: 10.1177/1062860608316729. PubMed
10. Hurst SA, Reiter-Theil S, Perrier A, et al. Physicians’ access to ethics support services in four European countries. Health Care Anal. 2007;15(4):321-335. doi: 10.1007/s10728-007-0072-6. PubMed
11. Fins JJ, Bacchetta MD, Miller FG. Clinical pragmatism: a method of moral problem solving. Kennedy Inst Ethics J. 1997;7(2):129-145. doi: 10.1353/ken.1997.0013. PubMed
12. Coulehan J, Williams PC. Vanquishing virtue: the impact of medical education. Acad Med. 2001;76(6):598-605. PubMed
Much has been written about the sources of the hidden curriculum in clerkships and postgraduate medical education.1-3 However, these descriptions do not adequately account for the critical role that hospitalists play in the development of trainees when they encounter ethical challenges on teaching services.4 As a role model, teacher, and the attending of record, a hospitalist’s response to ethical issues in practice can have a pivotal influence on the life and work of trainees, either instilling positive virtues or perpetuating the negative impact of the hidden curriculum.5-8 Understanding the epidemiology of ethical issues arising on academic hospitalist services has important implications for medical education, clinical ethics, and professionalism, as well as for patient care.
METHODS
Study Setting and Design
We conducted a mixed-method observational study at NewYork–Presbyterian–Weill Cornell Medical Center, an 862-bed, tertiary-care, academic institution located in New York, New York. We performed a prospective description of the frequency of all consecutively identified ethical and contextual issues pertinent to clinical decision-making by observing morning rounds with housestaff hospitalist services. Ethical issues were categorized using a comprehensive standardized instrument previously developed and published by the Division of Medical Ethics.9
The Division of Hospital Medicine employs 79 physicians, 30 of whom are dedicated full-time to daytime care on house-staff (or teaching) or physician assistant services. Of these 30 physicians, two (7%) were coinvestigators in this project and were excluded from participation to avoid bias. Between September 2017 and May 2018, the attending physicians of record of all available housestaff services were invited to participate with their teams in our research study on a weekly basis. We observed 10 different Hospital Medicine attending physicians (10/28, 36% of the available physician sample) over 19 sessions. Before rounds, a brief introduction to the nature of the study was provided to each team. It was explicitly stated that the observers were present to identify and document possible ethical issues that may arise while discussing the patients on rounds, and that the purpose of the study was neither an evaluation of the team members or their decisions nor a critique or quality improvement exercise. Observing researchers were not allowed to participate in the discussion of any case.
To avoid potential case duplication, we allowed for a minimum two-week interval before rounding twice on any particular team. To control for interobserver variability, we observed in pairs during these sessions. Discrepancies between observers were resolved by post hoc discussion and application of the definitions of the standardized instrument used to identify and catalog ethical and contextual issues.
Study Variables and Definitions
The following variables were collected in all cases: observation date, name of reviewers, demographic characteristics of the patient (age, gender, race, ethnicity, marital status, religion, preferred language, insurance type, and living situation before the admission), patient’s location during the admission (emergency room, regular nursing floor, step-down unit, or other), and ethical and contextual issues. “Ethical issues” were defined as those situations involving a conflict of values or preferences among different stakeholders, including, but not limited to, providers, patients, and/or families. Explicit definitions of each issue were generated, and additional standard rules for completion were provided.
Statistical Analysis
Results are presented as n (%) or mean ± standard deviation. Percentages were rounded to the closest integer. Interobserver variability between the observers in relation to evaluating the presence or absence of ethical or contextual issues was assessed by the kappa statistic. All P values are two-sided, with statistical significance evaluated at the 0.05 alpha level. A 95% confidence interval (95% CI) for the kappa statistic (ie, for assessing interobserver variability) was calculated to assess the precision of the obtained kappa estimate. All analyses were performed in SAS Version 9.4 (SAS Institute, Inc., Cary, NC) and Stata Version 14.0 (StataCorp, College Station, TX).
RESULTS
General Characteristics of the Study Sample
In total, 270 patients were evaluated from the teaching hospitalist services during the observation period. Ethical issues were identified in 86 of these patients (31.8%). Observer ethicists disagreed in their initial evaluation of 17 cases (6.3%). After review of and adjudication, both observers agreed that nine of these 17 cases (3.3%) should be excluded from the final analysis, as none reached the necessary threshold to be considered as a true ethical issue. Hence, we report the results of 77 patients (28.5%). These cases comprised the Hospitalist group and involved 113 ethical issues (1.48 ± 0.5 ethical issues/case). Only five patients in the Hospitalist group had a formal clinical ethics consult before our observation (5/270 patients [1.9%] vs 77/270 patients [28.5%] with an ethical issue, respectively, P < .001). Although the majority of ethical issues were noted by members of the primary team (84%), 12 of the 77 cases in the Hospitalist group (16%) were identified only by the observing ethicists. The kappa statistic for interobserver variability between the observing ethicists was 0.85 (95% CI = 0.76-0.92). The major demographic characteristics are summarized in Table 1.
Ethical Challenges
The most common ethical issues hospitalists encountered involved discussions about goals of care (including decisions to pursue aggressive treatment versus hospice care, or debates about the team’s ambivalence about the benefits and risks of pursuing investigational chemotherapy), treatment refusals (including the decision to forgo biopsy of a suspected malignancy), or decision-making capacity (Table 2). Less common were issues pertaining to resource allocation (specially related to pressures to discharge patients), pain management (some patients were suspected of drug-seeking behavior), or surrogate decision-making (when alternative decision-makers were suspected to lack decision-making capacity). Discussions about forgoing life-sustaining treatments occurred only in four cases (5%). These involved considerations of withdrawing Bilevel Positive Airway Pressure (BiPAP), artificial nutrition and hydration, and/or stopping antibiotic treatment.
DISCUSSION
Our data are the first prospective description of ethical issues arising on an academic hospitalist teaching service. These results indicate that there is an ethics epidemiology in the routine practice of Hospital Medicine that has heretofore not been characterized. By this, we mean a discreet incidence and prevalence of ethical challenges in Hospital Medicine that is distinct from that which is encountered by clinical ethics consultation (CEC) services. Although most practitioners recognize the utility of a traditional ethics consultation, there is a surprising paucity of data about the sources of ethical conflict encountered by academic hospitalists at the bedside, particularly those addressed without CEC. This suggests that the criteria for requesting a formal ethics consult could be limited and restrictive, which is both undersensitive and overspecific.10 Because of these limitations, viewing traditional ethics consultation as a proxy for ethical issues arising in daily hospitalist practice would lead to an underestimation of the true prevalence, as our data indicate.
More than one-fourth of the patients admitted to hospitalist teaching services pose ethical conflicts. Some of these are addressed on rounds, some are not, and only a handful of these cases will ever be referred to an ethicist. CEC services are made aware of the “tip of the iceberg,” which accounts for a vanishingly small percentage of ethical issues that arise on daily rounds. Some hospitalists may not involve CEC simply because they believe that the services are not helpful. However, the failure to obtain consultation may also reflect an inability to recognize a “problematic situation” and formulate a referral that might benefit from the assistance of an ethics consultation.11
Our study faces several potential limitations. We are presenting a single-center experience that focuses on the perspective of physicians and trainees. Some ethical issues might have been underestimated because the perspectives of patients, families, nurses, social workers, or other ancillary staff were not directly included. Furthermore, since any ethical challenge could have been discussed on any moment other than on morning rounds, our results may underestimate the prevalence of ethical issues arising from the hospital floors. Moreover, medical teams participating in the study could have been subject to the Hawthorne effect and could have tried to identify a greater number of ethical issues on rounds, which would not reflect actual practice.
CONCLUSION
Almost two decades ago, Coulehan and Williams wrote about the positive impact that ethics and humanities could have if these disciplines could be embedded in the daily practice of medicine, which is as follows:
…ethics and humanities curricula are irrelevant unless they can produce a substantive and continuing impact on hospital culture (…) The idea, of course, is to infiltrate the culture by coopting residents and attending physicians(…) If an ethics program can somehow achieve a critical mass of ‘‘value-sensitive’’ clinical faculty, it may begin to influence the institution’s ethos.12
Coulehan and Williams wrote of a need to bring ethics to the bedside. Our data suggest that an ethics epidemiology is deeply embedded in hospitalist services and is waiting to be fully characterized to better inform the care of patients and guide the professional formation and education of students and trainees. Hospitalists frequently confront ethical problems in daily practice that do not come to the attention of the CEC services or the institutional ethics committee. Understanding this emerging epidemiology presents an unrealized opportunity to improve bedside teaching, reinforce normative reasoning, and enhance patient care.
Acknowledgments
The authors want to acknowledge Drs. Augustine I. Choi, Michael G. Stewart, Laura L. Forese, and Anthony Hollenberg for their support of the fellowship in medical ethics and thank Drs. Arthur T. Evans and Monika M. Safford for their guidance.
Disclosures
The authors report no conflicts of interest.
Funding
This work was supported by a Weill Cornell General Internal Medicine Primary Care Innovations Initiative seed grant. Dr. Paul Christos was partially supported by the following grant: Clinical and Translational Science Center at Weill Cornell Medical College (1-UL1-TR002384-01).
Much has been written about the sources of the hidden curriculum in clerkships and postgraduate medical education.1-3 However, these descriptions do not adequately account for the critical role that hospitalists play in the development of trainees when they encounter ethical challenges on teaching services.4 As a role model, teacher, and the attending of record, a hospitalist’s response to ethical issues in practice can have a pivotal influence on the life and work of trainees, either instilling positive virtues or perpetuating the negative impact of the hidden curriculum.5-8 Understanding the epidemiology of ethical issues arising on academic hospitalist services has important implications for medical education, clinical ethics, and professionalism, as well as for patient care.
METHODS
Study Setting and Design
We conducted a mixed-method observational study at NewYork–Presbyterian–Weill Cornell Medical Center, an 862-bed, tertiary-care, academic institution located in New York, New York. We performed a prospective description of the frequency of all consecutively identified ethical and contextual issues pertinent to clinical decision-making by observing morning rounds with housestaff hospitalist services. Ethical issues were categorized using a comprehensive standardized instrument previously developed and published by the Division of Medical Ethics.9
The Division of Hospital Medicine employs 79 physicians, 30 of whom are dedicated full-time to daytime care on house-staff (or teaching) or physician assistant services. Of these 30 physicians, two (7%) were coinvestigators in this project and were excluded from participation to avoid bias. Between September 2017 and May 2018, the attending physicians of record of all available housestaff services were invited to participate with their teams in our research study on a weekly basis. We observed 10 different Hospital Medicine attending physicians (10/28, 36% of the available physician sample) over 19 sessions. Before rounds, a brief introduction to the nature of the study was provided to each team. It was explicitly stated that the observers were present to identify and document possible ethical issues that may arise while discussing the patients on rounds, and that the purpose of the study was neither an evaluation of the team members or their decisions nor a critique or quality improvement exercise. Observing researchers were not allowed to participate in the discussion of any case.
To avoid potential case duplication, we allowed for a minimum two-week interval before rounding twice on any particular team. To control for interobserver variability, we observed in pairs during these sessions. Discrepancies between observers were resolved by post hoc discussion and application of the definitions of the standardized instrument used to identify and catalog ethical and contextual issues.
Study Variables and Definitions
The following variables were collected in all cases: observation date, name of reviewers, demographic characteristics of the patient (age, gender, race, ethnicity, marital status, religion, preferred language, insurance type, and living situation before the admission), patient’s location during the admission (emergency room, regular nursing floor, step-down unit, or other), and ethical and contextual issues. “Ethical issues” were defined as those situations involving a conflict of values or preferences among different stakeholders, including, but not limited to, providers, patients, and/or families. Explicit definitions of each issue were generated, and additional standard rules for completion were provided.
Statistical Analysis
Results are presented as n (%) or mean ± standard deviation. Percentages were rounded to the closest integer. Interobserver variability between the observers in relation to evaluating the presence or absence of ethical or contextual issues was assessed by the kappa statistic. All P values are two-sided, with statistical significance evaluated at the 0.05 alpha level. A 95% confidence interval (95% CI) for the kappa statistic (ie, for assessing interobserver variability) was calculated to assess the precision of the obtained kappa estimate. All analyses were performed in SAS Version 9.4 (SAS Institute, Inc., Cary, NC) and Stata Version 14.0 (StataCorp, College Station, TX).
RESULTS
General Characteristics of the Study Sample
In total, 270 patients were evaluated from the teaching hospitalist services during the observation period. Ethical issues were identified in 86 of these patients (31.8%). Observer ethicists disagreed in their initial evaluation of 17 cases (6.3%). After review of and adjudication, both observers agreed that nine of these 17 cases (3.3%) should be excluded from the final analysis, as none reached the necessary threshold to be considered as a true ethical issue. Hence, we report the results of 77 patients (28.5%). These cases comprised the Hospitalist group and involved 113 ethical issues (1.48 ± 0.5 ethical issues/case). Only five patients in the Hospitalist group had a formal clinical ethics consult before our observation (5/270 patients [1.9%] vs 77/270 patients [28.5%] with an ethical issue, respectively, P < .001). Although the majority of ethical issues were noted by members of the primary team (84%), 12 of the 77 cases in the Hospitalist group (16%) were identified only by the observing ethicists. The kappa statistic for interobserver variability between the observing ethicists was 0.85 (95% CI = 0.76-0.92). The major demographic characteristics are summarized in Table 1.
Ethical Challenges
The most common ethical issues hospitalists encountered involved discussions about goals of care (including decisions to pursue aggressive treatment versus hospice care, or debates about the team’s ambivalence about the benefits and risks of pursuing investigational chemotherapy), treatment refusals (including the decision to forgo biopsy of a suspected malignancy), or decision-making capacity (Table 2). Less common were issues pertaining to resource allocation (specially related to pressures to discharge patients), pain management (some patients were suspected of drug-seeking behavior), or surrogate decision-making (when alternative decision-makers were suspected to lack decision-making capacity). Discussions about forgoing life-sustaining treatments occurred only in four cases (5%). These involved considerations of withdrawing Bilevel Positive Airway Pressure (BiPAP), artificial nutrition and hydration, and/or stopping antibiotic treatment.
DISCUSSION
Our data are the first prospective description of ethical issues arising on an academic hospitalist teaching service. These results indicate that there is an ethics epidemiology in the routine practice of Hospital Medicine that has heretofore not been characterized. By this, we mean a discreet incidence and prevalence of ethical challenges in Hospital Medicine that is distinct from that which is encountered by clinical ethics consultation (CEC) services. Although most practitioners recognize the utility of a traditional ethics consultation, there is a surprising paucity of data about the sources of ethical conflict encountered by academic hospitalists at the bedside, particularly those addressed without CEC. This suggests that the criteria for requesting a formal ethics consult could be limited and restrictive, which is both undersensitive and overspecific.10 Because of these limitations, viewing traditional ethics consultation as a proxy for ethical issues arising in daily hospitalist practice would lead to an underestimation of the true prevalence, as our data indicate.
More than one-fourth of the patients admitted to hospitalist teaching services pose ethical conflicts. Some of these are addressed on rounds, some are not, and only a handful of these cases will ever be referred to an ethicist. CEC services are made aware of the “tip of the iceberg,” which accounts for a vanishingly small percentage of ethical issues that arise on daily rounds. Some hospitalists may not involve CEC simply because they believe that the services are not helpful. However, the failure to obtain consultation may also reflect an inability to recognize a “problematic situation” and formulate a referral that might benefit from the assistance of an ethics consultation.11
Our study faces several potential limitations. We are presenting a single-center experience that focuses on the perspective of physicians and trainees. Some ethical issues might have been underestimated because the perspectives of patients, families, nurses, social workers, or other ancillary staff were not directly included. Furthermore, since any ethical challenge could have been discussed on any moment other than on morning rounds, our results may underestimate the prevalence of ethical issues arising from the hospital floors. Moreover, medical teams participating in the study could have been subject to the Hawthorne effect and could have tried to identify a greater number of ethical issues on rounds, which would not reflect actual practice.
CONCLUSION
Almost two decades ago, Coulehan and Williams wrote about the positive impact that ethics and humanities could have if these disciplines could be embedded in the daily practice of medicine, which is as follows:
…ethics and humanities curricula are irrelevant unless they can produce a substantive and continuing impact on hospital culture (…) The idea, of course, is to infiltrate the culture by coopting residents and attending physicians(…) If an ethics program can somehow achieve a critical mass of ‘‘value-sensitive’’ clinical faculty, it may begin to influence the institution’s ethos.12
Coulehan and Williams wrote of a need to bring ethics to the bedside. Our data suggest that an ethics epidemiology is deeply embedded in hospitalist services and is waiting to be fully characterized to better inform the care of patients and guide the professional formation and education of students and trainees. Hospitalists frequently confront ethical problems in daily practice that do not come to the attention of the CEC services or the institutional ethics committee. Understanding this emerging epidemiology presents an unrealized opportunity to improve bedside teaching, reinforce normative reasoning, and enhance patient care.
Acknowledgments
The authors want to acknowledge Drs. Augustine I. Choi, Michael G. Stewart, Laura L. Forese, and Anthony Hollenberg for their support of the fellowship in medical ethics and thank Drs. Arthur T. Evans and Monika M. Safford for their guidance.
Disclosures
The authors report no conflicts of interest.
Funding
This work was supported by a Weill Cornell General Internal Medicine Primary Care Innovations Initiative seed grant. Dr. Paul Christos was partially supported by the following grant: Clinical and Translational Science Center at Weill Cornell Medical College (1-UL1-TR002384-01).
1. Doja A, Bould MD, Clarkin C, Eady K, Sutherland S, Writer H. The hidden and informal curriculum across the continuum of training: a cross-sectional qualitative study. Med Teach. 2016;38(4):410-418. doi: 10.3109/0142159X.2015.1073241. PubMed
2. Martimianakis MA, Hafferty FW. Exploring the interstitial space between the ideal and the practised: humanism and the hidden curriculum of system reform. Med Educ. 2016;50(3):278-280. doi: 10.1111/medu.12982. PubMed
3. Lawrence C, Mhlaba T, Stewart KA, Moletsane R, Gaede B, Moshabela M. The hidden curricula of medical education: a scoping review. Acad Med. 2017;93(4):648-656. doi: 10.1097/ACM.0000000000002004. PubMed
4. McCarthy MW, Real de Asua D, Fins JJ. The rise of hospitalists: an opportunity for clinical ethics. J Clin Ethics. 2017;28(4):325-332. PubMed
5. McCarthy M, Fins J. Teaching clinical ethics at the bedside: William Osler and the essential role of the hospitalist. AMA J Ethics. 2017;19(6):528-532. doi: 10.1001/journalofethics.2017.19.6.peer2-1706. PubMed
6. Gabbay E, McCarthy MW, Fins JJ. The care of the ultra-orthodox Jewish patient. J Relig Health. 2017;56(2):545-560. doi: 10.1007/s10943-017-0356-6. PubMed
7. Wachter RM, Goldman L. The emerging role of “hospitalists” in the American health care system. N Engl J Med. 1996;335(7):514-517. doi: 10.1056/NEJM199608153350713. PubMed
8. Hauer KE, Wachter RM, McCulloch CE, Woo GA, Auerbach AD. Effects of hospitalist attending physicians on trainee satisfaction with teaching and with internal medicine rotations. Arch Intern Med. 2004;164(17):1866-1871. doi: 10.1001/archinte.164.17.1866. PubMed
9. Nilson EG, Acres CA, Tamerin NG, Fins JJ. Clinical ethics and the quality initiative: a pilot study for the empirical evaluation of ethics case consultation. Am J Med Qual. 2008;23(5):356-364. doi: 10.1177/1062860608316729. PubMed
10. Hurst SA, Reiter-Theil S, Perrier A, et al. Physicians’ access to ethics support services in four European countries. Health Care Anal. 2007;15(4):321-335. doi: 10.1007/s10728-007-0072-6. PubMed
11. Fins JJ, Bacchetta MD, Miller FG. Clinical pragmatism: a method of moral problem solving. Kennedy Inst Ethics J. 1997;7(2):129-145. doi: 10.1353/ken.1997.0013. PubMed
12. Coulehan J, Williams PC. Vanquishing virtue: the impact of medical education. Acad Med. 2001;76(6):598-605. PubMed
1. Doja A, Bould MD, Clarkin C, Eady K, Sutherland S, Writer H. The hidden and informal curriculum across the continuum of training: a cross-sectional qualitative study. Med Teach. 2016;38(4):410-418. doi: 10.3109/0142159X.2015.1073241. PubMed
2. Martimianakis MA, Hafferty FW. Exploring the interstitial space between the ideal and the practised: humanism and the hidden curriculum of system reform. Med Educ. 2016;50(3):278-280. doi: 10.1111/medu.12982. PubMed
3. Lawrence C, Mhlaba T, Stewart KA, Moletsane R, Gaede B, Moshabela M. The hidden curricula of medical education: a scoping review. Acad Med. 2017;93(4):648-656. doi: 10.1097/ACM.0000000000002004. PubMed
4. McCarthy MW, Real de Asua D, Fins JJ. The rise of hospitalists: an opportunity for clinical ethics. J Clin Ethics. 2017;28(4):325-332. PubMed
5. McCarthy M, Fins J. Teaching clinical ethics at the bedside: William Osler and the essential role of the hospitalist. AMA J Ethics. 2017;19(6):528-532. doi: 10.1001/journalofethics.2017.19.6.peer2-1706. PubMed
6. Gabbay E, McCarthy MW, Fins JJ. The care of the ultra-orthodox Jewish patient. J Relig Health. 2017;56(2):545-560. doi: 10.1007/s10943-017-0356-6. PubMed
7. Wachter RM, Goldman L. The emerging role of “hospitalists” in the American health care system. N Engl J Med. 1996;335(7):514-517. doi: 10.1056/NEJM199608153350713. PubMed
8. Hauer KE, Wachter RM, McCulloch CE, Woo GA, Auerbach AD. Effects of hospitalist attending physicians on trainee satisfaction with teaching and with internal medicine rotations. Arch Intern Med. 2004;164(17):1866-1871. doi: 10.1001/archinte.164.17.1866. PubMed
9. Nilson EG, Acres CA, Tamerin NG, Fins JJ. Clinical ethics and the quality initiative: a pilot study for the empirical evaluation of ethics case consultation. Am J Med Qual. 2008;23(5):356-364. doi: 10.1177/1062860608316729. PubMed
10. Hurst SA, Reiter-Theil S, Perrier A, et al. Physicians’ access to ethics support services in four European countries. Health Care Anal. 2007;15(4):321-335. doi: 10.1007/s10728-007-0072-6. PubMed
11. Fins JJ, Bacchetta MD, Miller FG. Clinical pragmatism: a method of moral problem solving. Kennedy Inst Ethics J. 1997;7(2):129-145. doi: 10.1353/ken.1997.0013. PubMed
12. Coulehan J, Williams PC. Vanquishing virtue: the impact of medical education. Acad Med. 2001;76(6):598-605. PubMed
© 2019 Society of Hospital Medicine
Premature death from heart disease hits Asian subgroups hard
Among Asian American subgroups, Asian Indian, Filipino, and Vietnamese populations showed significantly higher premature death rates from ischemic heart disease, compared with other Asian subgroups, based on data from the National Center for Health Statistics for the years 2003 to 2012.
Previous studies have described death rates from cardiovascular disease in Asian subgroups, but premature death in particular has not been well studied, wrote Latha Palaniappan, MD, of the division of primary care and population health at the Stanford (Calif.) University, and her colleagues.
To examine premature mortality from cardiovascular disease in Asian subgroups, the researchers used years of potential life lost (YPLL) to measure premature mortality. “[Years of potential life lost ] compares age at death with average life expectancy to estimate the average time an individual would have lived had he/she not died prematurely from a specific disease,” they explained.
The study population included 354,256 Asian American decedents aged 25 years or older. Of that total, 59,936 died of ischemic heart disease and 28,489 died of cerebrovascular disease.
Overall, Asian men lost 779 years/100,000 people in 2003 and 574 years/100,000 in 2012. However, in 2003, Asian Indian men in particular lost 1,216 years/100,000, more than other Asian male subgroups and non-Hispanic white men.
“Use of race-specific life expectancy revealed greater heterogeneity in YPLL across all Asian subgroups,” the researchers wrote. Similarly, Asian Indian women had the highest years of potential life lost throughout the study period, with a high of 818 years/100,000 people in 2003 and 477 years/100,00 in 2012, compared with 577/100,000 and 426/100,000, respectively, among non-Hispanic white women.
All Asian male subgroups also lost more years of life to cerebrovascular disease, compared with non-Hispanic white men, and women in each Asian subgroup had a higher years of potential life lost, compared with non-Hispanic white women. Filipino men had the highest YPLL values for the period, followed by Vietnamese men, and the patterns were similar for Filipino and Vietnamese women.
Possible explanations for the high rate of premature death from ischemic heart disease in Asian Indians include greater prevalence of risk factors at younger age (including elevated apolipoprotein B100/apolipoprotein A-1 ratios), type 2 diabetes, and cardiometabolic abnormalities in people of normal weight that might go unnoticed in a clinical exam, the researchers said. In the case of cerebrovascular disease, possible risk factors for high years of potential life lost in certain subgroups include hypertension in Filipino populations, limited health literacy about stroke in Vietnamese populations, and high rates of smoking in Vietnamese men.
The study findings were limited by several factors, including the small amount of data on mortality in Asian Americans from census reports, the researchers noted. However, the use of years of potential life lost as a measure of the impact of cardiovascular disease provided a useful model of the impact of cardiovascular disease on life expectancy and total disease burden of cerebrovascular disease on Asian ethnic subgroups, they said.
“Our study also provides evidence that evaluating the Asian population together as one group underestimates the burden of [cerebrovascular disease],” they noted.
The National Institute of Minority Health and Health Disparities Research Project and the National Heart, Lung, and Blood Institute supported the study in part by grants to researchers. The researchers had no financial conflicts to disclose.
SOURCE: Iyer DG et al. J Am Heart Assoc. 2019 Mar 20. doi: 10.1161/JAHA.118.010744.
Among Asian American subgroups, Asian Indian, Filipino, and Vietnamese populations showed significantly higher premature death rates from ischemic heart disease, compared with other Asian subgroups, based on data from the National Center for Health Statistics for the years 2003 to 2012.
Previous studies have described death rates from cardiovascular disease in Asian subgroups, but premature death in particular has not been well studied, wrote Latha Palaniappan, MD, of the division of primary care and population health at the Stanford (Calif.) University, and her colleagues.
To examine premature mortality from cardiovascular disease in Asian subgroups, the researchers used years of potential life lost (YPLL) to measure premature mortality. “[Years of potential life lost ] compares age at death with average life expectancy to estimate the average time an individual would have lived had he/she not died prematurely from a specific disease,” they explained.
The study population included 354,256 Asian American decedents aged 25 years or older. Of that total, 59,936 died of ischemic heart disease and 28,489 died of cerebrovascular disease.
Overall, Asian men lost 779 years/100,000 people in 2003 and 574 years/100,000 in 2012. However, in 2003, Asian Indian men in particular lost 1,216 years/100,000, more than other Asian male subgroups and non-Hispanic white men.
“Use of race-specific life expectancy revealed greater heterogeneity in YPLL across all Asian subgroups,” the researchers wrote. Similarly, Asian Indian women had the highest years of potential life lost throughout the study period, with a high of 818 years/100,000 people in 2003 and 477 years/100,00 in 2012, compared with 577/100,000 and 426/100,000, respectively, among non-Hispanic white women.
All Asian male subgroups also lost more years of life to cerebrovascular disease, compared with non-Hispanic white men, and women in each Asian subgroup had a higher years of potential life lost, compared with non-Hispanic white women. Filipino men had the highest YPLL values for the period, followed by Vietnamese men, and the patterns were similar for Filipino and Vietnamese women.
Possible explanations for the high rate of premature death from ischemic heart disease in Asian Indians include greater prevalence of risk factors at younger age (including elevated apolipoprotein B100/apolipoprotein A-1 ratios), type 2 diabetes, and cardiometabolic abnormalities in people of normal weight that might go unnoticed in a clinical exam, the researchers said. In the case of cerebrovascular disease, possible risk factors for high years of potential life lost in certain subgroups include hypertension in Filipino populations, limited health literacy about stroke in Vietnamese populations, and high rates of smoking in Vietnamese men.
The study findings were limited by several factors, including the small amount of data on mortality in Asian Americans from census reports, the researchers noted. However, the use of years of potential life lost as a measure of the impact of cardiovascular disease provided a useful model of the impact of cardiovascular disease on life expectancy and total disease burden of cerebrovascular disease on Asian ethnic subgroups, they said.
“Our study also provides evidence that evaluating the Asian population together as one group underestimates the burden of [cerebrovascular disease],” they noted.
The National Institute of Minority Health and Health Disparities Research Project and the National Heart, Lung, and Blood Institute supported the study in part by grants to researchers. The researchers had no financial conflicts to disclose.
SOURCE: Iyer DG et al. J Am Heart Assoc. 2019 Mar 20. doi: 10.1161/JAHA.118.010744.
Among Asian American subgroups, Asian Indian, Filipino, and Vietnamese populations showed significantly higher premature death rates from ischemic heart disease, compared with other Asian subgroups, based on data from the National Center for Health Statistics for the years 2003 to 2012.
Previous studies have described death rates from cardiovascular disease in Asian subgroups, but premature death in particular has not been well studied, wrote Latha Palaniappan, MD, of the division of primary care and population health at the Stanford (Calif.) University, and her colleagues.
To examine premature mortality from cardiovascular disease in Asian subgroups, the researchers used years of potential life lost (YPLL) to measure premature mortality. “[Years of potential life lost ] compares age at death with average life expectancy to estimate the average time an individual would have lived had he/she not died prematurely from a specific disease,” they explained.
The study population included 354,256 Asian American decedents aged 25 years or older. Of that total, 59,936 died of ischemic heart disease and 28,489 died of cerebrovascular disease.
Overall, Asian men lost 779 years/100,000 people in 2003 and 574 years/100,000 in 2012. However, in 2003, Asian Indian men in particular lost 1,216 years/100,000, more than other Asian male subgroups and non-Hispanic white men.
“Use of race-specific life expectancy revealed greater heterogeneity in YPLL across all Asian subgroups,” the researchers wrote. Similarly, Asian Indian women had the highest years of potential life lost throughout the study period, with a high of 818 years/100,000 people in 2003 and 477 years/100,00 in 2012, compared with 577/100,000 and 426/100,000, respectively, among non-Hispanic white women.
All Asian male subgroups also lost more years of life to cerebrovascular disease, compared with non-Hispanic white men, and women in each Asian subgroup had a higher years of potential life lost, compared with non-Hispanic white women. Filipino men had the highest YPLL values for the period, followed by Vietnamese men, and the patterns were similar for Filipino and Vietnamese women.
Possible explanations for the high rate of premature death from ischemic heart disease in Asian Indians include greater prevalence of risk factors at younger age (including elevated apolipoprotein B100/apolipoprotein A-1 ratios), type 2 diabetes, and cardiometabolic abnormalities in people of normal weight that might go unnoticed in a clinical exam, the researchers said. In the case of cerebrovascular disease, possible risk factors for high years of potential life lost in certain subgroups include hypertension in Filipino populations, limited health literacy about stroke in Vietnamese populations, and high rates of smoking in Vietnamese men.
The study findings were limited by several factors, including the small amount of data on mortality in Asian Americans from census reports, the researchers noted. However, the use of years of potential life lost as a measure of the impact of cardiovascular disease provided a useful model of the impact of cardiovascular disease on life expectancy and total disease burden of cerebrovascular disease on Asian ethnic subgroups, they said.
“Our study also provides evidence that evaluating the Asian population together as one group underestimates the burden of [cerebrovascular disease],” they noted.
The National Institute of Minority Health and Health Disparities Research Project and the National Heart, Lung, and Blood Institute supported the study in part by grants to researchers. The researchers had no financial conflicts to disclose.
SOURCE: Iyer DG et al. J Am Heart Assoc. 2019 Mar 20. doi: 10.1161/JAHA.118.010744.
FROM THE JOURNAL OF THE AMERICAN HEART ASSOCIATION
Key clinical point: Asian Indian, Filipino, and Vietnamese populations had the greatest loss of life from heart attacks and strokes among Asian population subgroups.
Major finding: Asian Indian men lost an average of 17 years of life to ischemic heart disease.
Study details: The data come from the National Center for Health Statistics Multiple Causes of Death mortality files from 2003 to 2012.
Disclosures: The National Institute of Minority Health and Health Disparities Research Project and the National Heart, Lung, and Blood Institute supported the study in part by grants to researchers. The researchers had no financial conflicts to disclose.
Source: Iyer DG et al. J Am Heart Assoc. 2019 Mar 20. doi: 10.1161/JAHA.118.010744.
Do Hospitals Participating in Accountable Care Organizations Discharge Patients to Higher Quality Nursing Homes?
Accountable care organizations (ACOs) create incentives for more efficient healthcare utilization. For patients being discharged from the hospital, this may mean more efficient use of postacute care (PAC), including discharging patients to higher quality skilled nursing facilities (SNFs) in an effort to limit readmissions and other costly complications. Public reporting of nursing home quality has been associated with improved performance measures, although improvements in preventable hospitalizations have lagged.1 Evidence to date suggests that patients attributed to an ACO are not going to higher quality SNFs,2,3 but these effects may be concentrated in hospitals that participate in ACOs and face stronger incentives to alter their discharge patterns compared with non-ACO hospitals. Therefore, we examined whether hospitals participating in Medicare’s Shared Saving Program (MSSP) increased the use of highly rated SNFs or decreased the use of low-rated SNFs hospital-wide after initiation of their ACO contracts compared with non-ACO hospitals.
METHODS
We used discharge-level data from the 100% MedPAR file for all fee-for-service Medicare beneficiaries discharged from an acute care hospital to an SNF between 2010 and 2013. We measured the SNF quality using Medicare’s Nursing Home Compare star ratings. Our primary outcome was probability of discharge to high-rated (five star) and low-rated (one star) SNFs.
We utilized a difference-in-differences design. Using a linear probability model, we first estimated the change in the probability of discharge to five-star SNFs (compared to all other SNFs) among all beneficiaries discharged from one of the 233 ACO-participating hospitals after the hospital became an ACO provider compared with before and compared withall beneficiaries discharged from one of the 3,081 non-ACO hospitals over the same time period. Individual hospitals were determined to be “ACO-participating” if they were listed on Medicare’s website as being part of an ACO-participating hospital in the MSSP. ACOs joined the MSSP in three waves: April 1, 2012; July 1, 2012; and January 1, 2013, which were also determined based on information on Medicare’s website. We separately estimated the change in probability of discharge to a one-star SNF (compared to all other SNFs) using the same approach. Models were adjusted for beneficiary demographic and clinical characteristics (age, sex, race, dual eligibility, urban ZIP code, diagnosis-related group code, and Elixhauser comorbidities) and market characteristics (the concentration of hospital discharges, SNF discharges, and the number of five-star SNFs, all measured in each hospital referral region).
RESULTS
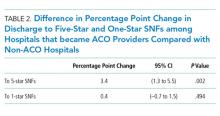
We examined a total of 12,736,287 discharges, 11.8% from ACO-participating hospitals and 88.2% from non-ACO-participating hospitals. ACO-participating hospitals cared for fewer black patients and fewer patients who were dually enrolled in Medicare and Medicaid (Table 1), but these characteristics did not change differentially between the two groups of hospitals over our study period. ACO-participating hospitals were also more likely to discharge patients to five-star SNFs prior to joining an ACO (in 2010-2011). After joining an ACO, the percentage of hospital discharges going to a 5-star SNF increased by 3.4 percentage points on a base of 15.4% (95% confidence interval [CI] 1.3-5.5, P = .002; Table 2) compared with non-ACO-participating hospitals over the same time period. The differential changes did not extend to SNFs rated as three stars and above (change of 0.5 percentage points, 95% CI, 1.3-2.8, P = .600).
The probability of discharge from an ACO hospital to low-quality (one-star) SNFs did not change significantly from its baseline level of 13.5% after joining an ACO compared with non-ACO-participating hospitals (change of 0.4 percentage points, 95% CI, 0.7-1.5, P = .494).
DISCUSSION
Our findings indicate that ACO-participating hospitals were more likely to discharge patients to the highest rated SNFs after they began their ACO contract but did not change the likelihood of discharge to lower rated SNFs in comparison with non-ACO hospitals. Previous research has suggested that patients attributed to a Medicare ACO were not more likely to use high-quality SNFs. However, we examined the effect of hospital participation in an ACO, not individual beneficiaries attributed to an ACO. These contrasting results suggest that hospitals could be instituting hospital-wide changes in discharge patterns once they join an ACO and that hospital-led ACOs could be particularly well positioned to manage postdischarge care relative to physician-led ACOs. One potential limitation of this study is that ACO-participating hospitals may differ in unobservable ways from non-ACO-participating hospitals. However, using hospital fixed effects, w
Disclosures
Dr. Werner reports receiving personal fees from CarePort Health. Dr. Bain reports no conflicts. Mr. Yuan reports no conflicts. Dr. Navathe reports receiving personal fees from Navvis and Company, Navigant Inc., Lynx Medical, Indegene Inc., Sutherland Global Services, and Agathos, Inc.; personal fees and equity from NavaHealth; an honorarium from Elsevier Press, serving on the board of Integrated Services, Inc. without compensation, and grants from Hawaii Medical Service Association, Anthem Public Policy Institute, and Oscar Health, none of which are related to this manuscript.
Funding
This research was funded by R01-HS024266 by the Agency for Healthcare Research and Quality. Rachel Werner was supported in part by K24-AG047908 from the National Institute on Aging.
1. Ryskina KL, Konetzka RT, Werner RM. Association between 5-star nursing home report card ratings and potentially preventable hospitalizations. Inquiry. 2018;55:46958018787323. doi: 10.1177/0046958018787323. PubMed
2. McWilliams JM, Gilstrap LG, Stevenson DG, Chernew ME, Huskamp HA, Grabowski DC. Changes in postacute care in the medicare shared savings program. JAMA Intern Med. 2017;177(4):518-526. doi: 10.1001/jamainternmed.2016.9115. PubMed
3. McWilliams JM, Hatfield LA, Chernew ME, Landon BE, Schwartz AL. Early performance of accountable care organizations in medicare. N Engl J Med. 2016;374(24):2357-2366. doi: 10.1056/NEJMsa1600142. PubMed
Accountable care organizations (ACOs) create incentives for more efficient healthcare utilization. For patients being discharged from the hospital, this may mean more efficient use of postacute care (PAC), including discharging patients to higher quality skilled nursing facilities (SNFs) in an effort to limit readmissions and other costly complications. Public reporting of nursing home quality has been associated with improved performance measures, although improvements in preventable hospitalizations have lagged.1 Evidence to date suggests that patients attributed to an ACO are not going to higher quality SNFs,2,3 but these effects may be concentrated in hospitals that participate in ACOs and face stronger incentives to alter their discharge patterns compared with non-ACO hospitals. Therefore, we examined whether hospitals participating in Medicare’s Shared Saving Program (MSSP) increased the use of highly rated SNFs or decreased the use of low-rated SNFs hospital-wide after initiation of their ACO contracts compared with non-ACO hospitals.
METHODS
We used discharge-level data from the 100% MedPAR file for all fee-for-service Medicare beneficiaries discharged from an acute care hospital to an SNF between 2010 and 2013. We measured the SNF quality using Medicare’s Nursing Home Compare star ratings. Our primary outcome was probability of discharge to high-rated (five star) and low-rated (one star) SNFs.
We utilized a difference-in-differences design. Using a linear probability model, we first estimated the change in the probability of discharge to five-star SNFs (compared to all other SNFs) among all beneficiaries discharged from one of the 233 ACO-participating hospitals after the hospital became an ACO provider compared with before and compared withall beneficiaries discharged from one of the 3,081 non-ACO hospitals over the same time period. Individual hospitals were determined to be “ACO-participating” if they were listed on Medicare’s website as being part of an ACO-participating hospital in the MSSP. ACOs joined the MSSP in three waves: April 1, 2012; July 1, 2012; and January 1, 2013, which were also determined based on information on Medicare’s website. We separately estimated the change in probability of discharge to a one-star SNF (compared to all other SNFs) using the same approach. Models were adjusted for beneficiary demographic and clinical characteristics (age, sex, race, dual eligibility, urban ZIP code, diagnosis-related group code, and Elixhauser comorbidities) and market characteristics (the concentration of hospital discharges, SNF discharges, and the number of five-star SNFs, all measured in each hospital referral region).
RESULTS
We examined a total of 12,736,287 discharges, 11.8% from ACO-participating hospitals and 88.2% from non-ACO-participating hospitals. ACO-participating hospitals cared for fewer black patients and fewer patients who were dually enrolled in Medicare and Medicaid (Table 1), but these characteristics did not change differentially between the two groups of hospitals over our study period. ACO-participating hospitals were also more likely to discharge patients to five-star SNFs prior to joining an ACO (in 2010-2011). After joining an ACO, the percentage of hospital discharges going to a 5-star SNF increased by 3.4 percentage points on a base of 15.4% (95% confidence interval [CI] 1.3-5.5, P = .002; Table 2) compared with non-ACO-participating hospitals over the same time period. The differential changes did not extend to SNFs rated as three stars and above (change of 0.5 percentage points, 95% CI, 1.3-2.8, P = .600).
The probability of discharge from an ACO hospital to low-quality (one-star) SNFs did not change significantly from its baseline level of 13.5% after joining an ACO compared with non-ACO-participating hospitals (change of 0.4 percentage points, 95% CI, 0.7-1.5, P = .494).
DISCUSSION
Our findings indicate that ACO-participating hospitals were more likely to discharge patients to the highest rated SNFs after they began their ACO contract but did not change the likelihood of discharge to lower rated SNFs in comparison with non-ACO hospitals. Previous research has suggested that patients attributed to a Medicare ACO were not more likely to use high-quality SNFs. However, we examined the effect of hospital participation in an ACO, not individual beneficiaries attributed to an ACO. These contrasting results suggest that hospitals could be instituting hospital-wide changes in discharge patterns once they join an ACO and that hospital-led ACOs could be particularly well positioned to manage postdischarge care relative to physician-led ACOs. One potential limitation of this study is that ACO-participating hospitals may differ in unobservable ways from non-ACO-participating hospitals. However, using hospital fixed effects, w
Disclosures
Dr. Werner reports receiving personal fees from CarePort Health. Dr. Bain reports no conflicts. Mr. Yuan reports no conflicts. Dr. Navathe reports receiving personal fees from Navvis and Company, Navigant Inc., Lynx Medical, Indegene Inc., Sutherland Global Services, and Agathos, Inc.; personal fees and equity from NavaHealth; an honorarium from Elsevier Press, serving on the board of Integrated Services, Inc. without compensation, and grants from Hawaii Medical Service Association, Anthem Public Policy Institute, and Oscar Health, none of which are related to this manuscript.
Funding
This research was funded by R01-HS024266 by the Agency for Healthcare Research and Quality. Rachel Werner was supported in part by K24-AG047908 from the National Institute on Aging.
Accountable care organizations (ACOs) create incentives for more efficient healthcare utilization. For patients being discharged from the hospital, this may mean more efficient use of postacute care (PAC), including discharging patients to higher quality skilled nursing facilities (SNFs) in an effort to limit readmissions and other costly complications. Public reporting of nursing home quality has been associated with improved performance measures, although improvements in preventable hospitalizations have lagged.1 Evidence to date suggests that patients attributed to an ACO are not going to higher quality SNFs,2,3 but these effects may be concentrated in hospitals that participate in ACOs and face stronger incentives to alter their discharge patterns compared with non-ACO hospitals. Therefore, we examined whether hospitals participating in Medicare’s Shared Saving Program (MSSP) increased the use of highly rated SNFs or decreased the use of low-rated SNFs hospital-wide after initiation of their ACO contracts compared with non-ACO hospitals.
METHODS
We used discharge-level data from the 100% MedPAR file for all fee-for-service Medicare beneficiaries discharged from an acute care hospital to an SNF between 2010 and 2013. We measured the SNF quality using Medicare’s Nursing Home Compare star ratings. Our primary outcome was probability of discharge to high-rated (five star) and low-rated (one star) SNFs.
We utilized a difference-in-differences design. Using a linear probability model, we first estimated the change in the probability of discharge to five-star SNFs (compared to all other SNFs) among all beneficiaries discharged from one of the 233 ACO-participating hospitals after the hospital became an ACO provider compared with before and compared withall beneficiaries discharged from one of the 3,081 non-ACO hospitals over the same time period. Individual hospitals were determined to be “ACO-participating” if they were listed on Medicare’s website as being part of an ACO-participating hospital in the MSSP. ACOs joined the MSSP in three waves: April 1, 2012; July 1, 2012; and January 1, 2013, which were also determined based on information on Medicare’s website. We separately estimated the change in probability of discharge to a one-star SNF (compared to all other SNFs) using the same approach. Models were adjusted for beneficiary demographic and clinical characteristics (age, sex, race, dual eligibility, urban ZIP code, diagnosis-related group code, and Elixhauser comorbidities) and market characteristics (the concentration of hospital discharges, SNF discharges, and the number of five-star SNFs, all measured in each hospital referral region).
RESULTS
We examined a total of 12,736,287 discharges, 11.8% from ACO-participating hospitals and 88.2% from non-ACO-participating hospitals. ACO-participating hospitals cared for fewer black patients and fewer patients who were dually enrolled in Medicare and Medicaid (Table 1), but these characteristics did not change differentially between the two groups of hospitals over our study period. ACO-participating hospitals were also more likely to discharge patients to five-star SNFs prior to joining an ACO (in 2010-2011). After joining an ACO, the percentage of hospital discharges going to a 5-star SNF increased by 3.4 percentage points on a base of 15.4% (95% confidence interval [CI] 1.3-5.5, P = .002; Table 2) compared with non-ACO-participating hospitals over the same time period. The differential changes did not extend to SNFs rated as three stars and above (change of 0.5 percentage points, 95% CI, 1.3-2.8, P = .600).
The probability of discharge from an ACO hospital to low-quality (one-star) SNFs did not change significantly from its baseline level of 13.5% after joining an ACO compared with non-ACO-participating hospitals (change of 0.4 percentage points, 95% CI, 0.7-1.5, P = .494).
DISCUSSION
Our findings indicate that ACO-participating hospitals were more likely to discharge patients to the highest rated SNFs after they began their ACO contract but did not change the likelihood of discharge to lower rated SNFs in comparison with non-ACO hospitals. Previous research has suggested that patients attributed to a Medicare ACO were not more likely to use high-quality SNFs. However, we examined the effect of hospital participation in an ACO, not individual beneficiaries attributed to an ACO. These contrasting results suggest that hospitals could be instituting hospital-wide changes in discharge patterns once they join an ACO and that hospital-led ACOs could be particularly well positioned to manage postdischarge care relative to physician-led ACOs. One potential limitation of this study is that ACO-participating hospitals may differ in unobservable ways from non-ACO-participating hospitals. However, using hospital fixed effects, w
Disclosures
Dr. Werner reports receiving personal fees from CarePort Health. Dr. Bain reports no conflicts. Mr. Yuan reports no conflicts. Dr. Navathe reports receiving personal fees from Navvis and Company, Navigant Inc., Lynx Medical, Indegene Inc., Sutherland Global Services, and Agathos, Inc.; personal fees and equity from NavaHealth; an honorarium from Elsevier Press, serving on the board of Integrated Services, Inc. without compensation, and grants from Hawaii Medical Service Association, Anthem Public Policy Institute, and Oscar Health, none of which are related to this manuscript.
Funding
This research was funded by R01-HS024266 by the Agency for Healthcare Research and Quality. Rachel Werner was supported in part by K24-AG047908 from the National Institute on Aging.
1. Ryskina KL, Konetzka RT, Werner RM. Association between 5-star nursing home report card ratings and potentially preventable hospitalizations. Inquiry. 2018;55:46958018787323. doi: 10.1177/0046958018787323. PubMed
2. McWilliams JM, Gilstrap LG, Stevenson DG, Chernew ME, Huskamp HA, Grabowski DC. Changes in postacute care in the medicare shared savings program. JAMA Intern Med. 2017;177(4):518-526. doi: 10.1001/jamainternmed.2016.9115. PubMed
3. McWilliams JM, Hatfield LA, Chernew ME, Landon BE, Schwartz AL. Early performance of accountable care organizations in medicare. N Engl J Med. 2016;374(24):2357-2366. doi: 10.1056/NEJMsa1600142. PubMed
1. Ryskina KL, Konetzka RT, Werner RM. Association between 5-star nursing home report card ratings and potentially preventable hospitalizations. Inquiry. 2018;55:46958018787323. doi: 10.1177/0046958018787323. PubMed
2. McWilliams JM, Gilstrap LG, Stevenson DG, Chernew ME, Huskamp HA, Grabowski DC. Changes in postacute care in the medicare shared savings program. JAMA Intern Med. 2017;177(4):518-526. doi: 10.1001/jamainternmed.2016.9115. PubMed
3. McWilliams JM, Hatfield LA, Chernew ME, Landon BE, Schwartz AL. Early performance of accountable care organizations in medicare. N Engl J Med. 2016;374(24):2357-2366. doi: 10.1056/NEJMsa1600142. PubMed
© 2019 Society of Hospital Medicine
Impact of Pharmacist-led Discharge Counseling on Hospital Readmission and Emergency Department Visits: A Systematic Review and Meta-analysis
Transitions of care, such as hospital discharge, represent a moment of patient vulnerability that can contribute to the occurrence of medication errors and, consequently, hospital readmissions and mortality.1 Clinical pharmacists have the potential to optimize the pharmacotherapy, patient safety, and process of care during these transitions, reducing negative outcomes.2,3
Previous studies have shown that pharmacist interventions at hospital discharge, such as medication review, medication reconciliation, and patient counseling, significantly improve medication adherence and reduce adverse drug reactions, hospital readmission rates, and mortality.3-8 A recent systematic review, including nine clinical trials, showed that clinical pharmacy services performed in an inpatient setting significantly enhanced quality, safety, and efficiency of care when compared with usual care.6 Another study referred to pharmacist-led discharge counseling as a cost-effective intervention that may lead to cost savings of 48% in the healthcare setting.9 However, as other studies report no significant impact of pharmacist-led medication counseling at discharge on patient outcomes,9-13 the current benefit or otherwise of such interventions remains uncertain.
Thus, given the inconsistent conclusions about the real effect of pharmacist interventions and the scarcity of systematic reviews regarding patient counseling, we aimed to synthesize the available evidence on the effect of pharmacist-led discharge counseling on healthcare services utilization (ie, hospital readmission and emergency department visit rates) through a systematic review and meta-analysis.
METHODS
This systematic review was conducted following the PRISMA statement and Cochrane recommendations14,15 and was registered in PROSPERO (registration no. CRD42017068444). Screening of titles and abstracts, full-text appraisal, data extraction, and study quality assessment were performed by two reviewers independently, with discrepancies discussed with a third reviewer.
Search and Eligibility Criteria
Systematic searches were conducted in PubMed, Scopus, and DOAJ (Directory of Open Access Journals), without limits for timeframe or language (last updated on November 20, 2018). We performed an additional manual search in the reference lists of the included studies. The following descriptors combined with the Boolean operators “AND” and “OR” were used: “discharge,” “counseling,” and “pharmacist.” The full search str
We included randomized, controlled trials (RCTs) that compared the intervention of pharmacist-led discharge medication counseling versus usual care. Usual care was defined as patients who received the usual treatment in regular practice. The outcomes of interest were the numbers of hospital readmissions and emergency department visits. Patients of any clinical condition, gender, or age were included. The following exclusion criteria were applied: (1) discharge counseling performed by another healthcare professional or a multidisciplinary team, (2) comparison between pharmacist-led discharge counseling and another healthcare professional’s intervention, (3) studies with a control group also receiving discharge counseling by a pharmacist, (4) study designs other than RCTs, (5) studies that reported other pharmacist interventions, but not discharge counseling, (6) counseling not performed at discharge, and (7) studies not reporting the outcomes of interest.
Data Extraction and Quality Assessment
We used a standardized form to collect data on the following general characteristics of the studies: baseline data (author names, year of publication, study design, country, and sample size), methodological aspects, and outcomes of interest (ie, number of hospital readmission or emergency department visits). When outcomes were assessed in different time periods, the last period was considered for the overall analysis.
The methodological quality of the included studies was evaluated using the Cochrane Collaboration’s tool for risk of bias assessment that classifies each study as having a low, unclear, or high risk of bias.14
Data Analysis
Pairwise meta-analyses of the included RCTs were performed using the Comprehens
The betwee
We also conducted sensitivity analyses to test the robustness of the results and to evaluate the effect of individual studies on data heterogeneity. The sensitivity analysis consisted of the hypothetical sequential removal of studies from the meta-analysis. In addition, to verify the influence of small-study effects on the results of a meta-analysis with between-trial heterogeneity (I2 > 0), we compared the results obtained in the random effect model with those obtained from fixed effects models.
When possible, subgroup analyses were performed considering (1) how discharge counseling was delivered (ie, alone or combined with other interventions) and (2) time of evaluation of the outcomes (weeks, months, or years postdischarge). The visual representation of the estimated treatment effect versus the standard error (funnel plots) was also performed to assess the potential role of publication bias.
RESULTS
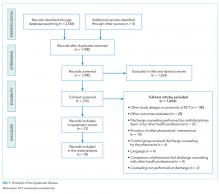
A total of 2,656 records were retrieved from the electronic databases and manual searches. During the screening phase, 276 records were considered for full-text analysis, of which 21 were included in the qualitative analysis20-40 and 18 were suitable for quantitative analyses21,22,24-36,38-40 (Figure 1). The references of excluded studies, with the reasons for exclusion, are mentioned in the Supplemental Material.
The baseline characteristics of the included studies are presented in the Table. A total of 7,244 patients were included in this systematic review, most of them being 60 years or older (81%) and presenting chronic conditions (38.1%) such as cardiovascular and respiratory diseases. The majority of studies were performed in Europe (42.85%), followed by those conducted in the United States of America (28.6%). Overall, studies were classified as high risk of bias (57.14%), because most of them presented two or more domains with unclear risk of bias, especially due to the attrition domain (see Supplemental Material for complete analyses). Given the complexity of pharmacist interventions and the impossibility of blinding participants and personnel, the performance domain of the risk of bias tool was not assessed. Only three studies were considered as low risk of bias for all domains.22,37,40 Analyses on publication bias were performed by visualization of funnel plots and showed overall symmetry in all cases, which demonstrates a relative lack of bias. Few studies contributed to a slight asymmetry in the plots. Additional information is found in the Supplemental Material.
The detailed results for the pharmacist-led discharge medication counseling in each of the 21 included studies are presented in the Supplemental Material. The period of evaluation of the outcomes varied from two weeks (two studies) to one year after discharge (two studies). Only five studies showed statistically significant reductions in the number of hospital readmissions or emergency department visits in the group receiving pharmacist-led discharge counseling.21,24,32,35,36
Readmission Rates
A total of 18 studies evaluating the impact of pharmacist-led discharge counseling on hospital readmission were included in the meta-analysis.21,22,24-36,38-40 The studies by Al-Hashar et al., Bolas et al., and Schnniper et al. were excluded from statistical analyses due to a lack of sufficient data.20,23,27 The results revealed statistical differences between the intervention and usual care (RR = 0.864 [95% CI 0.763-0.997], P = .020; Figure 2). However, the heterogeneity among studies was high (I2 approximately 50%) and the calculation of PI revealed a wider interval, with the loss of the statistical significance (Tau = 0.151; PI 0.542-1.186). Sensitivity analyses with the hypothetical removal of trials showed few reductions in heterogeneity (I2 values ranging from 35.37% to 49.53%) with similar effect size values.
Subgroup analyses considering the time of hospital admission postdischarge (groups for two to three weeks, one month, three months, six months, and one year) did not demonstrate that pharmacist-led counseling reduced the number of hospital readmissions at any time (see Supplemental Material). Again, more than one study contributed to the moderately high heterogeneity in some subgroups (initial I2 values of 49.69% [one month], 69.43% [three months], 50.99% [six months], and 65.55% [one year]). The subgroups of two to three weeks and six months included few studies and caution should be used when interpreting such results (small meta-analysis with wide CIs; I2 value of 0%). Sensitivity analyses did not modify the original results (I2 values ranging from 35.37% to 49.56%).
In the subgroup analyses of how pharmacist interventions were delivered (ie, discharge counseling alone or combined with other interventions), interventions were superior to usual care, but again, few studies were evaluated, and the sensitivity analyses and calculation of PI revealed no true differences between groups. The meta-analysis for discharge counseling alone presented an RR of 0.333 (95% CI 0.129-0.858, P = .023; Supplemental Material), with three studies included (I2 = 48.0%, and Tau = 0.582, PI –11.221-11.880).21,25,35 The meta-analysis of other interventions showed an RR of 0.898 (95% CI 0.813-0.991, P = .033) (I2 = 28.9%; PI 0.690-1.099).22,24-36,38-40 The detailed results of PIs are reported in the Supplemental Material.
Emergency Department Visit Rates
A total of eight studies evaluating the impact of pharmacist-led discharge counseling on emergency department visits were included in the meta-analysis.21,22,24,26,32-34,39 For the study by Farris et al., we used data from the “minimal intervention” branch.26 Although the original results showed differences between intervention and usual care (RR = 0.697 [95% CI 0.535-0.907], P = .007; Figure 3), the meta-analysis presented high heterogeneity with an I2 value of 58.86% (Tau = 0.265; PI 0.027-1.367). Sensitivity analyses with the hypothetical removal of studies did not modify the original results (I2 values ranging from 26.05% to 64.74%).
Subgroup analyses considering time of evaluation of the outcome were possible for studies of one, three, and six months postdischarge (Supplemental Material). No statistical differences were observed for the subgroup of one month (RR = 0.705 [95% CI 0.449-1.106] with the original I2 = 65.5%). Sensitivity analyses showed that the study by Phatak et al. was responsible for the high heterogeneity (results of I2 = 38% after removing this trial),32 without significant changes in the effect sizes. The three-month subgroup included only two studies and presented an RR of 0.763 (95% CI 0.599-0.972, P = .028).21,26 However, sensitivity analysis based on statistical modifications in the model altered the results, and no differences between the intervention and usual care were truly observed (eg, using the inverse variance method, the random model produced an odds ratio of 0.575 [95% CI 0.219-1.512]). Pharmacist-led counseling reduced the number of emergency department visits at six months postdischarge, RR = 0.605 (95% CI 0.459-0.768, P = .001), but only two studies were included in this analysis.33,39
DISCUSSION
The presen
Pharmacist interventions are generally complex, being constituted by several components,41 which are frequently poorly described in the literature and generally inconsistently performed.42-44 These factors can contribute to reduced methodological quality and enhanced heterogeneity, as reported in previous systematic reviews and meta-analyses.8,42,45-47 Moreover, the characteristics of the included patients (eg, different clinical conditions) and the small sample sizes may have increased heterogeneity among trials in our meta-analyses.
Similar to our results, El Hajj et al. were not able to demonstrate significant differences between usual care and pharmacist interventions in the transition of care (eg, medication reconciliation, medication therapy management, discharge medication counseling, motivational interviewing, and postdischarge face-to-face or telephone follow-up) in reducing rates of hospital readmission, visits to emergency units, and mortality, or in improving medication adherence.11 Another systematic review with a meta-analysis also showed that interventions, including discharge counseling, did not reduce the number of hospital readmissions (RR = 0.97 [95% CI 0.89-1.05], P = .470) and visits to emergency units (RR = 0.70 [95% CI 0.59-0.85] P = .001).48 However, both systematic reviews included few RCTs with moderate methodological quality, which may compromise interpretation of the results. In this case, imprecision in estimates and individual study results may be more informative than a meta-analysis.
Ensing et al. highlighted the need for more well-designed RCTs for clinical pharmacy services to provide high-quality information to be included in systematic reviews and meta-analyses.49 This may enable the identification of the true effect of pharmacist interventions in patient care.40 In our systematic review, the high risk of bias in some included studies was attributed especially to the attrition domain, indicating that the outcomes were poorly evaluated or patient losses and withdrawals were not sufficiently described. In addition, most of the studies had an unclear risk of bias, primarily because of poor descriptions of the blindness of the outcome assessors. These pitfalls highlight the need for more rigorous standards for carrying out and reporting RCTs on pharmacist interventions, which should be strictly required by journal editors and reviewers.50Moreover, the standardization of outcomes is also important to allow comparability between studies. Core outcome sets represent agreed sets of outcomes that should be measured and reported by trials in a specific area, as recommend by the COMET Initiative (Core Outcome Measures in Effectiveness Trials).51 Pharmacy practice studies have started defining core outcome sets to be used in future trials,52-54 as recently happened for pharmacist-led discharge counseling.55 It is important to keep in mind the different implications resulting from the use of endpoint outcomes, surrogate outcomes, or process indicators. Although the latter are easily measured but also easily influenced by interventions, endpoint outcomes represent the real impact of the interventions that should be used in economic evaluations.56 Surrogate outcomes are frequently used as a proxy of endpoint outcomes, but precaution is needed when inferring conclusions.57 In our study, we preferred using healthcare services utilization as a measure of intervention success. However, these outcomes could also be affected by other factors not related to medication safety. The use of properly designed RCTs and their synthesis in robust meta-analyses should minimize potential interpretation biases.
Our findings also show the need to better define clinical pharmacy services. A better description of interventions is important to not only allow evidence gathering but also enable the proper replication of complex interventions in practice and to ground further analyses on the economic impact of pharmacist interventions.
Our study has some limitations. Although subgroup and sensitivity analyses were performed, we were not able to reduce the heterogeneity and effect size intervals of some meta-analyses. Caution should be used when interpreting the results from the subgroup meta-analysis, including small numbers of studies (n = 2-4). The absent or minor effects of pharmacist-led interventions on healthcare services utilization found in our study may be due to a real lack of measurable effect of the intervention itself or due to the limited evidence available in the literature. This is related to the small number of primary studies, poor reporting practices, and high heterogeneity between trials. In addition, another limitation that affects our study is the poor measurement of intervention fidelity in primary studies, which precludes an in-depth analysis of the effect of the different intervention components. A better report of intervention fidelity would allow a different sensitive analysis that could differentiate the most successful interventions.
Similar to what happens with other complex interventions by pharmacists, a detailed description of the procedure, together with reporting on a core outcome set, is needed to enhance reproducibility. Future RCTs of clinical pharmacy services that follow standard protocols such as DEPICT58 and CONSORT59 and report in detail how the study and the interventions were performed will contribute to more robust evidence generation.
1
2
3
4
5
6
7
8
9
1
1
1
1
1
1
1
1
1
19
20
21
22
23
24
25
26
27
2
29.
30.
31.
32.
33.
34.
35.
36. S
37. S
38. S
39. T
40. Z
41. W
42. G
43. R
44. S
45. C
46. C
47. S
48. R
49. E
50. R
51. P
52. B
53. M
54. R
55. B
56. P
57. A
58. R
59. M
Transitions of care, such as hospital discharge, represent a moment of patient vulnerability that can contribute to the occurrence of medication errors and, consequently, hospital readmissions and mortality.1 Clinical pharmacists have the potential to optimize the pharmacotherapy, patient safety, and process of care during these transitions, reducing negative outcomes.2,3
Previous studies have shown that pharmacist interventions at hospital discharge, such as medication review, medication reconciliation, and patient counseling, significantly improve medication adherence and reduce adverse drug reactions, hospital readmission rates, and mortality.3-8 A recent systematic review, including nine clinical trials, showed that clinical pharmacy services performed in an inpatient setting significantly enhanced quality, safety, and efficiency of care when compared with usual care.6 Another study referred to pharmacist-led discharge counseling as a cost-effective intervention that may lead to cost savings of 48% in the healthcare setting.9 However, as other studies report no significant impact of pharmacist-led medication counseling at discharge on patient outcomes,9-13 the current benefit or otherwise of such interventions remains uncertain.
Thus, given the inconsistent conclusions about the real effect of pharmacist interventions and the scarcity of systematic reviews regarding patient counseling, we aimed to synthesize the available evidence on the effect of pharmacist-led discharge counseling on healthcare services utilization (ie, hospital readmission and emergency department visit rates) through a systematic review and meta-analysis.
METHODS
This systematic review was conducted following the PRISMA statement and Cochrane recommendations14,15 and was registered in PROSPERO (registration no. CRD42017068444). Screening of titles and abstracts, full-text appraisal, data extraction, and study quality assessment were performed by two reviewers independently, with discrepancies discussed with a third reviewer.
Search and Eligibility Criteria
Systematic searches were conducted in PubMed, Scopus, and DOAJ (Directory of Open Access Journals), without limits for timeframe or language (last updated on November 20, 2018). We performed an additional manual search in the reference lists of the included studies. The following descriptors combined with the Boolean operators “AND” and “OR” were used: “discharge,” “counseling,” and “pharmacist.” The full search str
We included randomized, controlled trials (RCTs) that compared the intervention of pharmacist-led discharge medication counseling versus usual care. Usual care was defined as patients who received the usual treatment in regular practice. The outcomes of interest were the numbers of hospital readmissions and emergency department visits. Patients of any clinical condition, gender, or age were included. The following exclusion criteria were applied: (1) discharge counseling performed by another healthcare professional or a multidisciplinary team, (2) comparison between pharmacist-led discharge counseling and another healthcare professional’s intervention, (3) studies with a control group also receiving discharge counseling by a pharmacist, (4) study designs other than RCTs, (5) studies that reported other pharmacist interventions, but not discharge counseling, (6) counseling not performed at discharge, and (7) studies not reporting the outcomes of interest.
Data Extraction and Quality Assessment
We used a standardized form to collect data on the following general characteristics of the studies: baseline data (author names, year of publication, study design, country, and sample size), methodological aspects, and outcomes of interest (ie, number of hospital readmission or emergency department visits). When outcomes were assessed in different time periods, the last period was considered for the overall analysis.
The methodological quality of the included studies was evaluated using the Cochrane Collaboration’s tool for risk of bias assessment that classifies each study as having a low, unclear, or high risk of bias.14
Data Analysis
Pairwise meta-analyses of the included RCTs were performed using the Comprehens
The betwee
We also conducted sensitivity analyses to test the robustness of the results and to evaluate the effect of individual studies on data heterogeneity. The sensitivity analysis consisted of the hypothetical sequential removal of studies from the meta-analysis. In addition, to verify the influence of small-study effects on the results of a meta-analysis with between-trial heterogeneity (I2 > 0), we compared the results obtained in the random effect model with those obtained from fixed effects models.
When possible, subgroup analyses were performed considering (1) how discharge counseling was delivered (ie, alone or combined with other interventions) and (2) time of evaluation of the outcomes (weeks, months, or years postdischarge). The visual representation of the estimated treatment effect versus the standard error (funnel plots) was also performed to assess the potential role of publication bias.
RESULTS
A total of 2,656 records were retrieved from the electronic databases and manual searches. During the screening phase, 276 records were considered for full-text analysis, of which 21 were included in the qualitative analysis20-40 and 18 were suitable for quantitative analyses21,22,24-36,38-40 (Figure 1). The references of excluded studies, with the reasons for exclusion, are mentioned in the Supplemental Material.
The baseline characteristics of the included studies are presented in the Table. A total of 7,244 patients were included in this systematic review, most of them being 60 years or older (81%) and presenting chronic conditions (38.1%) such as cardiovascular and respiratory diseases. The majority of studies were performed in Europe (42.85%), followed by those conducted in the United States of America (28.6%). Overall, studies were classified as high risk of bias (57.14%), because most of them presented two or more domains with unclear risk of bias, especially due to the attrition domain (see Supplemental Material for complete analyses). Given the complexity of pharmacist interventions and the impossibility of blinding participants and personnel, the performance domain of the risk of bias tool was not assessed. Only three studies were considered as low risk of bias for all domains.22,37,40 Analyses on publication bias were performed by visualization of funnel plots and showed overall symmetry in all cases, which demonstrates a relative lack of bias. Few studies contributed to a slight asymmetry in the plots. Additional information is found in the Supplemental Material.
The detailed results for the pharmacist-led discharge medication counseling in each of the 21 included studies are presented in the Supplemental Material. The period of evaluation of the outcomes varied from two weeks (two studies) to one year after discharge (two studies). Only five studies showed statistically significant reductions in the number of hospital readmissions or emergency department visits in the group receiving pharmacist-led discharge counseling.21,24,32,35,36
Readmission Rates
A total of 18 studies evaluating the impact of pharmacist-led discharge counseling on hospital readmission were included in the meta-analysis.21,22,24-36,38-40 The studies by Al-Hashar et al., Bolas et al., and Schnniper et al. were excluded from statistical analyses due to a lack of sufficient data.20,23,27 The results revealed statistical differences between the intervention and usual care (RR = 0.864 [95% CI 0.763-0.997], P = .020; Figure 2). However, the heterogeneity among studies was high (I2 approximately 50%) and the calculation of PI revealed a wider interval, with the loss of the statistical significance (Tau = 0.151; PI 0.542-1.186). Sensitivity analyses with the hypothetical removal of trials showed few reductions in heterogeneity (I2 values ranging from 35.37% to 49.53%) with similar effect size values.
Subgroup analyses considering the time of hospital admission postdischarge (groups for two to three weeks, one month, three months, six months, and one year) did not demonstrate that pharmacist-led counseling reduced the number of hospital readmissions at any time (see Supplemental Material). Again, more than one study contributed to the moderately high heterogeneity in some subgroups (initial I2 values of 49.69% [one month], 69.43% [three months], 50.99% [six months], and 65.55% [one year]). The subgroups of two to three weeks and six months included few studies and caution should be used when interpreting such results (small meta-analysis with wide CIs; I2 value of 0%). Sensitivity analyses did not modify the original results (I2 values ranging from 35.37% to 49.56%).
In the subgroup analyses of how pharmacist interventions were delivered (ie, discharge counseling alone or combined with other interventions), interventions were superior to usual care, but again, few studies were evaluated, and the sensitivity analyses and calculation of PI revealed no true differences between groups. The meta-analysis for discharge counseling alone presented an RR of 0.333 (95% CI 0.129-0.858, P = .023; Supplemental Material), with three studies included (I2 = 48.0%, and Tau = 0.582, PI –11.221-11.880).21,25,35 The meta-analysis of other interventions showed an RR of 0.898 (95% CI 0.813-0.991, P = .033) (I2 = 28.9%; PI 0.690-1.099).22,24-36,38-40 The detailed results of PIs are reported in the Supplemental Material.
Emergency Department Visit Rates
A total of eight studies evaluating the impact of pharmacist-led discharge counseling on emergency department visits were included in the meta-analysis.21,22,24,26,32-34,39 For the study by Farris et al., we used data from the “minimal intervention” branch.26 Although the original results showed differences between intervention and usual care (RR = 0.697 [95% CI 0.535-0.907], P = .007; Figure 3), the meta-analysis presented high heterogeneity with an I2 value of 58.86% (Tau = 0.265; PI 0.027-1.367). Sensitivity analyses with the hypothetical removal of studies did not modify the original results (I2 values ranging from 26.05% to 64.74%).
Subgroup analyses considering time of evaluation of the outcome were possible for studies of one, three, and six months postdischarge (Supplemental Material). No statistical differences were observed for the subgroup of one month (RR = 0.705 [95% CI 0.449-1.106] with the original I2 = 65.5%). Sensitivity analyses showed that the study by Phatak et al. was responsible for the high heterogeneity (results of I2 = 38% after removing this trial),32 without significant changes in the effect sizes. The three-month subgroup included only two studies and presented an RR of 0.763 (95% CI 0.599-0.972, P = .028).21,26 However, sensitivity analysis based on statistical modifications in the model altered the results, and no differences between the intervention and usual care were truly observed (eg, using the inverse variance method, the random model produced an odds ratio of 0.575 [95% CI 0.219-1.512]). Pharmacist-led counseling reduced the number of emergency department visits at six months postdischarge, RR = 0.605 (95% CI 0.459-0.768, P = .001), but only two studies were included in this analysis.33,39
DISCUSSION
The presen
Pharmacist interventions are generally complex, being constituted by several components,41 which are frequently poorly described in the literature and generally inconsistently performed.42-44 These factors can contribute to reduced methodological quality and enhanced heterogeneity, as reported in previous systematic reviews and meta-analyses.8,42,45-47 Moreover, the characteristics of the included patients (eg, different clinical conditions) and the small sample sizes may have increased heterogeneity among trials in our meta-analyses.
Similar to our results, El Hajj et al. were not able to demonstrate significant differences between usual care and pharmacist interventions in the transition of care (eg, medication reconciliation, medication therapy management, discharge medication counseling, motivational interviewing, and postdischarge face-to-face or telephone follow-up) in reducing rates of hospital readmission, visits to emergency units, and mortality, or in improving medication adherence.11 Another systematic review with a meta-analysis also showed that interventions, including discharge counseling, did not reduce the number of hospital readmissions (RR = 0.97 [95% CI 0.89-1.05], P = .470) and visits to emergency units (RR = 0.70 [95% CI 0.59-0.85] P = .001).48 However, both systematic reviews included few RCTs with moderate methodological quality, which may compromise interpretation of the results. In this case, imprecision in estimates and individual study results may be more informative than a meta-analysis.
Ensing et al. highlighted the need for more well-designed RCTs for clinical pharmacy services to provide high-quality information to be included in systematic reviews and meta-analyses.49 This may enable the identification of the true effect of pharmacist interventions in patient care.40 In our systematic review, the high risk of bias in some included studies was attributed especially to the attrition domain, indicating that the outcomes were poorly evaluated or patient losses and withdrawals were not sufficiently described. In addition, most of the studies had an unclear risk of bias, primarily because of poor descriptions of the blindness of the outcome assessors. These pitfalls highlight the need for more rigorous standards for carrying out and reporting RCTs on pharmacist interventions, which should be strictly required by journal editors and reviewers.50Moreover, the standardization of outcomes is also important to allow comparability between studies. Core outcome sets represent agreed sets of outcomes that should be measured and reported by trials in a specific area, as recommend by the COMET Initiative (Core Outcome Measures in Effectiveness Trials).51 Pharmacy practice studies have started defining core outcome sets to be used in future trials,52-54 as recently happened for pharmacist-led discharge counseling.55 It is important to keep in mind the different implications resulting from the use of endpoint outcomes, surrogate outcomes, or process indicators. Although the latter are easily measured but also easily influenced by interventions, endpoint outcomes represent the real impact of the interventions that should be used in economic evaluations.56 Surrogate outcomes are frequently used as a proxy of endpoint outcomes, but precaution is needed when inferring conclusions.57 In our study, we preferred using healthcare services utilization as a measure of intervention success. However, these outcomes could also be affected by other factors not related to medication safety. The use of properly designed RCTs and their synthesis in robust meta-analyses should minimize potential interpretation biases.
Our findings also show the need to better define clinical pharmacy services. A better description of interventions is important to not only allow evidence gathering but also enable the proper replication of complex interventions in practice and to ground further analyses on the economic impact of pharmacist interventions.
Our study has some limitations. Although subgroup and sensitivity analyses were performed, we were not able to reduce the heterogeneity and effect size intervals of some meta-analyses. Caution should be used when interpreting the results from the subgroup meta-analysis, including small numbers of studies (n = 2-4). The absent or minor effects of pharmacist-led interventions on healthcare services utilization found in our study may be due to a real lack of measurable effect of the intervention itself or due to the limited evidence available in the literature. This is related to the small number of primary studies, poor reporting practices, and high heterogeneity between trials. In addition, another limitation that affects our study is the poor measurement of intervention fidelity in primary studies, which precludes an in-depth analysis of the effect of the different intervention components. A better report of intervention fidelity would allow a different sensitive analysis that could differentiate the most successful interventions.
Similar to what happens with other complex interventions by pharmacists, a detailed description of the procedure, together with reporting on a core outcome set, is needed to enhance reproducibility. Future RCTs of clinical pharmacy services that follow standard protocols such as DEPICT58 and CONSORT59 and report in detail how the study and the interventions were performed will contribute to more robust evidence generation.
Transitions of care, such as hospital discharge, represent a moment of patient vulnerability that can contribute to the occurrence of medication errors and, consequently, hospital readmissions and mortality.1 Clinical pharmacists have the potential to optimize the pharmacotherapy, patient safety, and process of care during these transitions, reducing negative outcomes.2,3
Previous studies have shown that pharmacist interventions at hospital discharge, such as medication review, medication reconciliation, and patient counseling, significantly improve medication adherence and reduce adverse drug reactions, hospital readmission rates, and mortality.3-8 A recent systematic review, including nine clinical trials, showed that clinical pharmacy services performed in an inpatient setting significantly enhanced quality, safety, and efficiency of care when compared with usual care.6 Another study referred to pharmacist-led discharge counseling as a cost-effective intervention that may lead to cost savings of 48% in the healthcare setting.9 However, as other studies report no significant impact of pharmacist-led medication counseling at discharge on patient outcomes,9-13 the current benefit or otherwise of such interventions remains uncertain.
Thus, given the inconsistent conclusions about the real effect of pharmacist interventions and the scarcity of systematic reviews regarding patient counseling, we aimed to synthesize the available evidence on the effect of pharmacist-led discharge counseling on healthcare services utilization (ie, hospital readmission and emergency department visit rates) through a systematic review and meta-analysis.
METHODS
This systematic review was conducted following the PRISMA statement and Cochrane recommendations14,15 and was registered in PROSPERO (registration no. CRD42017068444). Screening of titles and abstracts, full-text appraisal, data extraction, and study quality assessment were performed by two reviewers independently, with discrepancies discussed with a third reviewer.
Search and Eligibility Criteria
Systematic searches were conducted in PubMed, Scopus, and DOAJ (Directory of Open Access Journals), without limits for timeframe or language (last updated on November 20, 2018). We performed an additional manual search in the reference lists of the included studies. The following descriptors combined with the Boolean operators “AND” and “OR” were used: “discharge,” “counseling,” and “pharmacist.” The full search str
We included randomized, controlled trials (RCTs) that compared the intervention of pharmacist-led discharge medication counseling versus usual care. Usual care was defined as patients who received the usual treatment in regular practice. The outcomes of interest were the numbers of hospital readmissions and emergency department visits. Patients of any clinical condition, gender, or age were included. The following exclusion criteria were applied: (1) discharge counseling performed by another healthcare professional or a multidisciplinary team, (2) comparison between pharmacist-led discharge counseling and another healthcare professional’s intervention, (3) studies with a control group also receiving discharge counseling by a pharmacist, (4) study designs other than RCTs, (5) studies that reported other pharmacist interventions, but not discharge counseling, (6) counseling not performed at discharge, and (7) studies not reporting the outcomes of interest.
Data Extraction and Quality Assessment
We used a standardized form to collect data on the following general characteristics of the studies: baseline data (author names, year of publication, study design, country, and sample size), methodological aspects, and outcomes of interest (ie, number of hospital readmission or emergency department visits). When outcomes were assessed in different time periods, the last period was considered for the overall analysis.
The methodological quality of the included studies was evaluated using the Cochrane Collaboration’s tool for risk of bias assessment that classifies each study as having a low, unclear, or high risk of bias.14
Data Analysis
Pairwise meta-analyses of the included RCTs were performed using the Comprehens
The betwee
We also conducted sensitivity analyses to test the robustness of the results and to evaluate the effect of individual studies on data heterogeneity. The sensitivity analysis consisted of the hypothetical sequential removal of studies from the meta-analysis. In addition, to verify the influence of small-study effects on the results of a meta-analysis with between-trial heterogeneity (I2 > 0), we compared the results obtained in the random effect model with those obtained from fixed effects models.
When possible, subgroup analyses were performed considering (1) how discharge counseling was delivered (ie, alone or combined with other interventions) and (2) time of evaluation of the outcomes (weeks, months, or years postdischarge). The visual representation of the estimated treatment effect versus the standard error (funnel plots) was also performed to assess the potential role of publication bias.
RESULTS
A total of 2,656 records were retrieved from the electronic databases and manual searches. During the screening phase, 276 records were considered for full-text analysis, of which 21 were included in the qualitative analysis20-40 and 18 were suitable for quantitative analyses21,22,24-36,38-40 (Figure 1). The references of excluded studies, with the reasons for exclusion, are mentioned in the Supplemental Material.
The baseline characteristics of the included studies are presented in the Table. A total of 7,244 patients were included in this systematic review, most of them being 60 years or older (81%) and presenting chronic conditions (38.1%) such as cardiovascular and respiratory diseases. The majority of studies were performed in Europe (42.85%), followed by those conducted in the United States of America (28.6%). Overall, studies were classified as high risk of bias (57.14%), because most of them presented two or more domains with unclear risk of bias, especially due to the attrition domain (see Supplemental Material for complete analyses). Given the complexity of pharmacist interventions and the impossibility of blinding participants and personnel, the performance domain of the risk of bias tool was not assessed. Only three studies were considered as low risk of bias for all domains.22,37,40 Analyses on publication bias were performed by visualization of funnel plots and showed overall symmetry in all cases, which demonstrates a relative lack of bias. Few studies contributed to a slight asymmetry in the plots. Additional information is found in the Supplemental Material.
The detailed results for the pharmacist-led discharge medication counseling in each of the 21 included studies are presented in the Supplemental Material. The period of evaluation of the outcomes varied from two weeks (two studies) to one year after discharge (two studies). Only five studies showed statistically significant reductions in the number of hospital readmissions or emergency department visits in the group receiving pharmacist-led discharge counseling.21,24,32,35,36
Readmission Rates
A total of 18 studies evaluating the impact of pharmacist-led discharge counseling on hospital readmission were included in the meta-analysis.21,22,24-36,38-40 The studies by Al-Hashar et al., Bolas et al., and Schnniper et al. were excluded from statistical analyses due to a lack of sufficient data.20,23,27 The results revealed statistical differences between the intervention and usual care (RR = 0.864 [95% CI 0.763-0.997], P = .020; Figure 2). However, the heterogeneity among studies was high (I2 approximately 50%) and the calculation of PI revealed a wider interval, with the loss of the statistical significance (Tau = 0.151; PI 0.542-1.186). Sensitivity analyses with the hypothetical removal of trials showed few reductions in heterogeneity (I2 values ranging from 35.37% to 49.53%) with similar effect size values.
Subgroup analyses considering the time of hospital admission postdischarge (groups for two to three weeks, one month, three months, six months, and one year) did not demonstrate that pharmacist-led counseling reduced the number of hospital readmissions at any time (see Supplemental Material). Again, more than one study contributed to the moderately high heterogeneity in some subgroups (initial I2 values of 49.69% [one month], 69.43% [three months], 50.99% [six months], and 65.55% [one year]). The subgroups of two to three weeks and six months included few studies and caution should be used when interpreting such results (small meta-analysis with wide CIs; I2 value of 0%). Sensitivity analyses did not modify the original results (I2 values ranging from 35.37% to 49.56%).
In the subgroup analyses of how pharmacist interventions were delivered (ie, discharge counseling alone or combined with other interventions), interventions were superior to usual care, but again, few studies were evaluated, and the sensitivity analyses and calculation of PI revealed no true differences between groups. The meta-analysis for discharge counseling alone presented an RR of 0.333 (95% CI 0.129-0.858, P = .023; Supplemental Material), with three studies included (I2 = 48.0%, and Tau = 0.582, PI –11.221-11.880).21,25,35 The meta-analysis of other interventions showed an RR of 0.898 (95% CI 0.813-0.991, P = .033) (I2 = 28.9%; PI 0.690-1.099).22,24-36,38-40 The detailed results of PIs are reported in the Supplemental Material.
Emergency Department Visit Rates
A total of eight studies evaluating the impact of pharmacist-led discharge counseling on emergency department visits were included in the meta-analysis.21,22,24,26,32-34,39 For the study by Farris et al., we used data from the “minimal intervention” branch.26 Although the original results showed differences between intervention and usual care (RR = 0.697 [95% CI 0.535-0.907], P = .007; Figure 3), the meta-analysis presented high heterogeneity with an I2 value of 58.86% (Tau = 0.265; PI 0.027-1.367). Sensitivity analyses with the hypothetical removal of studies did not modify the original results (I2 values ranging from 26.05% to 64.74%).
Subgroup analyses considering time of evaluation of the outcome were possible for studies of one, three, and six months postdischarge (Supplemental Material). No statistical differences were observed for the subgroup of one month (RR = 0.705 [95% CI 0.449-1.106] with the original I2 = 65.5%). Sensitivity analyses showed that the study by Phatak et al. was responsible for the high heterogeneity (results of I2 = 38% after removing this trial),32 without significant changes in the effect sizes. The three-month subgroup included only two studies and presented an RR of 0.763 (95% CI 0.599-0.972, P = .028).21,26 However, sensitivity analysis based on statistical modifications in the model altered the results, and no differences between the intervention and usual care were truly observed (eg, using the inverse variance method, the random model produced an odds ratio of 0.575 [95% CI 0.219-1.512]). Pharmacist-led counseling reduced the number of emergency department visits at six months postdischarge, RR = 0.605 (95% CI 0.459-0.768, P = .001), but only two studies were included in this analysis.33,39
DISCUSSION
The presen
Pharmacist interventions are generally complex, being constituted by several components,41 which are frequently poorly described in the literature and generally inconsistently performed.42-44 These factors can contribute to reduced methodological quality and enhanced heterogeneity, as reported in previous systematic reviews and meta-analyses.8,42,45-47 Moreover, the characteristics of the included patients (eg, different clinical conditions) and the small sample sizes may have increased heterogeneity among trials in our meta-analyses.
Similar to our results, El Hajj et al. were not able to demonstrate significant differences between usual care and pharmacist interventions in the transition of care (eg, medication reconciliation, medication therapy management, discharge medication counseling, motivational interviewing, and postdischarge face-to-face or telephone follow-up) in reducing rates of hospital readmission, visits to emergency units, and mortality, or in improving medication adherence.11 Another systematic review with a meta-analysis also showed that interventions, including discharge counseling, did not reduce the number of hospital readmissions (RR = 0.97 [95% CI 0.89-1.05], P = .470) and visits to emergency units (RR = 0.70 [95% CI 0.59-0.85] P = .001).48 However, both systematic reviews included few RCTs with moderate methodological quality, which may compromise interpretation of the results. In this case, imprecision in estimates and individual study results may be more informative than a meta-analysis.
Ensing et al. highlighted the need for more well-designed RCTs for clinical pharmacy services to provide high-quality information to be included in systematic reviews and meta-analyses.49 This may enable the identification of the true effect of pharmacist interventions in patient care.40 In our systematic review, the high risk of bias in some included studies was attributed especially to the attrition domain, indicating that the outcomes were poorly evaluated or patient losses and withdrawals were not sufficiently described. In addition, most of the studies had an unclear risk of bias, primarily because of poor descriptions of the blindness of the outcome assessors. These pitfalls highlight the need for more rigorous standards for carrying out and reporting RCTs on pharmacist interventions, which should be strictly required by journal editors and reviewers.50Moreover, the standardization of outcomes is also important to allow comparability between studies. Core outcome sets represent agreed sets of outcomes that should be measured and reported by trials in a specific area, as recommend by the COMET Initiative (Core Outcome Measures in Effectiveness Trials).51 Pharmacy practice studies have started defining core outcome sets to be used in future trials,52-54 as recently happened for pharmacist-led discharge counseling.55 It is important to keep in mind the different implications resulting from the use of endpoint outcomes, surrogate outcomes, or process indicators. Although the latter are easily measured but also easily influenced by interventions, endpoint outcomes represent the real impact of the interventions that should be used in economic evaluations.56 Surrogate outcomes are frequently used as a proxy of endpoint outcomes, but precaution is needed when inferring conclusions.57 In our study, we preferred using healthcare services utilization as a measure of intervention success. However, these outcomes could also be affected by other factors not related to medication safety. The use of properly designed RCTs and their synthesis in robust meta-analyses should minimize potential interpretation biases.
Our findings also show the need to better define clinical pharmacy services. A better description of interventions is important to not only allow evidence gathering but also enable the proper replication of complex interventions in practice and to ground further analyses on the economic impact of pharmacist interventions.
Our study has some limitations. Although subgroup and sensitivity analyses were performed, we were not able to reduce the heterogeneity and effect size intervals of some meta-analyses. Caution should be used when interpreting the results from the subgroup meta-analysis, including small numbers of studies (n = 2-4). The absent or minor effects of pharmacist-led interventions on healthcare services utilization found in our study may be due to a real lack of measurable effect of the intervention itself or due to the limited evidence available in the literature. This is related to the small number of primary studies, poor reporting practices, and high heterogeneity between trials. In addition, another limitation that affects our study is the poor measurement of intervention fidelity in primary studies, which precludes an in-depth analysis of the effect of the different intervention components. A better report of intervention fidelity would allow a different sensitive analysis that could differentiate the most successful interventions.
Similar to what happens with other complex interventions by pharmacists, a detailed description of the procedure, together with reporting on a core outcome set, is needed to enhance reproducibility. Future RCTs of clinical pharmacy services that follow standard protocols such as DEPICT58 and CONSORT59 and report in detail how the study and the interventions were performed will contribute to more robust evidence generation.
1
2
3
4
5
6
7
8
9
1
1
1
1
1
1
1
1
1
19
20
21
22
23
24
25
26
27
2
29.
30.
31.
32.
33.
34.
35.
36. S
37. S
38. S
39. T
40. Z
41. W
42. G
43. R
44. S
45. C
46. C
47. S
48. R
49. E
50. R
51. P
52. B
53. M
54. R
55. B
56. P
57. A
58. R
59. M
1
2
3
4
5
6
7
8
9
1
1
1
1
1
1
1
1
1
19
20
21
22
23
24
25
26
27
2
29.
30.
31.
32.
33.
34.
35.
36. S
37. S
38. S
39. T
40. Z
41. W
42. G
43. R
44. S
45. C
46. C
47. S
48. R
49. E
50. R
51. P
52. B
53. M
54. R
55. B
56. P
57. A
58. R
59. M
© 2020 Society of Hospital Medicine
Hospital at Home and Emergence of the Home Hospitalist
Ms. P., an 86-year-old woman with a history of hypertension, hyperlipidemia, coronary artery disease, and transient ischemic attack, presents to the emergency department with a three-day history of cough, fever, purulent sputum, fatigue, and dyspnea on exertion. Her vital signs are notable for a fever of 39.0°C, blood pressure 136/92, pulse 102, respiratory rate 30, and room air oxygen saturation of 91%. She looks ill. She has a white blood cell count of 16,000, lactate 1.9, and a right lower lobe infiltrate on imaging. The emergency department attending physician presents the case to you for admission, and you accept the patient into your inpatient hospitalist service.
Now, let’s imagine a different future in which you are the attending hospitalist on your institution’s Hospital at Home (HaH) service, where you will provide hospital-level care to Ms. P. in the comfort of her own home. Hospitalists should prepare for this paradigm shift.
WHAT IS HOSPITAL AT HOME?
HaH provides hospital-level care in a patient’s home, for those with qualifying acute illnesses and appropriate degrees of acuity, as a substitute for traditional inpatient care.1 This is achieved by bringing the critical elements of hospital care to the home—physician and nursing care, intravenous medications and fluids, oxygen and respiratory therapies, basic radiography and ultrasound, durable medical equipment, skilled therapies, and more.2
All hospitalists have cared for patients like Ms. P., and she and many patients like her will have a straightforward hospital trajectory: initial evaluation in the emergency department, inpatient care provided by a hospitalist inpatient service, a few days of intravenous antibiotics and other hospital services, and finally, discharge to home.
A SHARED RATIONALE FOR HOSPITAL MEDICINE AND HOSPITAL AT HOME
However, not all patients will experience a smooth, or safe, hospital course. Studies that launched the hospital safety movement also provide the rationale for HaH, namely, that hospitals are often dangerous environments for patients.3
A complementary approach to improving outcomes for patients at high risk of iatrogenic illness such as functional decline, falls, delirium, adverse drug events, and hospital-associated disability syndrome,4-6 is to care for patients outside the traditional inpatient hospital environment. Over the past 20 years, many studies—including dozens of randomized controlled trials and several meta-analyses—have shown better outcomes for patients cared for in HaH: decreased length of stay, decreased incidence of adverse events (including substantially lower six-month mortality), better patient and caregiver care experiences, lower caregiver stress, and lower costs.7-9A recent Center for Medicare and Medicaid Innovation (CMMI) Demonstration conducted at the Mount Sinai Health System found similar results.10
GROWING INTEREST IN HOSPITAL AT HOME AND CHALLENGES TO DISSEMINATION
Interest in HaH has increased markedly over the past few years with increased penetration of Medicare and Medicaid managed care, the development and spread of accountable care organizations (ACOs), and a shift in focus among some health systems towards value-based care, population health, and community-based care. Recently, commercial entities have entered the HaH space and have raised substantial capital to fund development. Despite this growing interest in HaH and substantial evidence of its effectiveness, HaH has not been widely implemented or scaled in the United States.
Widespread dissemination and implementation of HaH has been hampered by several barriers. First, despite growing interest in HaH, the culture of healthcare and health system leadership, for the most part, remains focused on facility-based care.11
Second, while HaH makes financial sense in the managed care arena, given the strong evidence for high-quality, lower-cost care, there is currently no standard payment mechanism for HaH in fee-for-service Medicare or in the commercial insurance space. However, there are indications that this may soon change. In the fall of 2017, a proposal for a bundled payment mechanism for acute HaH care plus 30 days of postacute care was unanimously approved by an Advisory Committee to the Secretary of the Department of Health and Human Services (HHS).12,13 The HHS Secretary recently noted that “the Department of Health and Human Services is keenly interested in ideas for home-based, hospital-level care, and agrees … that this proposal holds promise for testing.”14
Third is the need to create the logistics and supply chain to support HaH. There currently exists a well-established supply chain for providing hospital care. A hospitalist orders a dose of intravenous antibiotic or oxygen, and it is supplied in a timely manner. Similarly, the postacute sector of healthcare has a robust supply chain, though it operates on a somewhat different clock from the acute care setting. However, there is currently no easily replicable supply chain to meet the needs of providing acute care in the home. Each HaH has had to create its own system of logistics with the existing healthcare assets in its local environment. Developing this capacity at scale will require significant capital investment.
There are examples where HaH has scaled. Beginning in 1994, in the state of Victoria, Australia (population 6.3 million), the health authority reimbursed HaH care at the same rates as traditional hospital care. At last report, HaH provided approximately 5% of all hospital bed days of care in Victoria. Providing HaH on this scale helped avoid the need to build a new 500-bed hospital to care for those patients.15 The avoided costs of building new hospital beds (and the ongoing need to fill those beds) represents significant societal return on investment attributable to HaH.
EMERGENCE OF THE HOME HOSPITALIST?
A key element in implementing a HaH program is its physician staff in terms of the types of doctors who provide HaH care, how they are organized, and how they interact with patients. To date, HaH physicians have been predominantly geriatricians, but internists and family medicine physicians, employed as full-time members of a dedicated HaH team, also provide care by physically visiting patients in their homes. The reason for significant involvement of geriatricians in HaH may relate to the fact that geriatric fellowship training includes training in home-based medical care, whereas this is less common in family medicine and internal medicine residency training programs.
In order to provide HaH on a nationwide scale, there will be a need for a larger workforce. There is an opportunity here to leverage existing hospital physician staff, such as hospitalists. In addition, while there is significant value in physicians seeing patients in their homes, more scalable versions of HaH are being developed and implemented that leverage biometrically enhanced telemedicine approaches for a dedicated physician component of care, with in-person visits provided by other members of an interdisciplinary team.
We believe that hospitalists can play a key role as HaH physicians as the HaH model continues to evolve and expand. Hospitalists bring valuable expertise relevant to HaH care delivery, including extensive experience with the triage of acutely ill patients, an understanding of the natural course of acute illness and team-based care, and for some, experience with telemedicine care.
While a hospitalist providing HaH care would leverage many of the competencies of the traditional hospitalist, we suggest that such a provider should receive additional training and clinical experience in home-based medical care to help them better understand the unique aspects of providing care in patients’ homes.16 Such training could include experience in making house calls, which can be a transformational experience in helping physicians improve their skills in dealing with social determinants of health, diagnosing and managing geriatric syndromes, and mobilizing community resources in the care of their patients, as well as managing care transitions. Hospitalists delivering care in HaH may also need to upgrade specific clinical skills commonly addressed by home-based medical care providers: wound care, caregiver-related issues, social and ethical issues specific to home-based care, problems with functional status, psychiatric and cognitive issues, management of gastrostomy tubes and bladder catheters, and dermatologic problems, as well as palliative care and end-of-life symptom management. These skills are slightly different from the usual realm of the typical hospitalists’ wheelhouse. However, it is all learnable.17 Similarly, geriatricians can learn from hospitalists as the HaH model evolves; there are HaH programs in existence today that take care of a sicker tranche of patients than earlier versions of HaH, with continuous telemonitoring of patients and the ability to rapidly deploy providers, labs, imaging, and medications. Going forward, as healthcare organizations begin to develop HaH programs staffed by hospitalists, it is probably wise for hospitalists and geriatricians to collaborate on the optimal physician models for HaH.
There may emerge a new specialty. Ticona and Schulman described a “home intensivist” with competencies including informatics of remote monitoring technology, leadership of multidisciplinary care teams, and the interpersonal skills required for compassionate end-of-life care.18 We prefer the term Home Hospitalist. Home Hospitalists would develop an enhanced understanding of the transitions of care and social determinants of health, and they would gain valuable knowledge about the social and environmental challenges many patients face after discharge from the hospital.
When this vision is realized, there will be enormous benefits to both HaH and Hospital Medicine. HaH could tap into a large and competent workforce to enhance its implementation and dissemination. Hospital Medicine would gain a new pathway for its providers and could develop new collaborative efforts with geriatric, internal, and family medicine.
Disclosures
Dr. Danielsson has nothing to disclose. Dr. Leff reports personal fees from Medically Home, other from Dispatch Health, other from Landmark Health, personal fees from Medibank, personal fees from Apple, personal fees from Health Affairs, other from Honor, personal fees from Institute for Healthcare Improvement, outside the submitted work; and American Academy of Home Care Medicine - member board of directors, voluntary.
Funding
Dr. Leff was supported in this work by a grant from The John A. Hartford Foundation.
1. Leff B, Montalto M. Home hospital-toward a tighter definition. J Am Geriatr Soc. 2004;52(12):2141. doi: 10.1111/j.1532-5415.2004.52579_1.x. PubMed
2. Leff B, Burton L, Mader SL, et al. Hospital at home: feasibility and outcomes of a program to provide hospital-level care at home for acutely ill older patients. Ann Intern Med. 2005;143(11):798-808. doi: 10.7326/0003-4819-143-11-200512060-00008. PubMed
3. Brennan TA, Leape LL, Laird NM, et al. Incidence of adverse events and negligence in hospitalized patients. Results of the Harvard Medical Practice Study I. N Engl J Med. 1991;324(6):370-376. doi: 10.1056/NEJM199102073240604. PubMed
4. Creditor MC. Hazards of hospitalization of the elderly. Ann Intern Med. 1993;118(3):219-223. doi: 10.7326/0003-4819-118-3-199302010-00011. PubMed
5. Covinsky KE, Pierluissi E, Johnston CB. Hospitalization-associated disability: “She was probably able to ambulate, but I’m not sure”. JAMA. 2011;306(16):1782-1793. doi: 10.1001/jama.2011.1556. PubMed
6. Wald HL. The Geometry of Patient Safety: Horizontal and Vertical Approaches to the Hazards of Hospitalization. J Am Geriatr Soc. 2017;65(12):2559-2561. doi: 10.1111/jgs.15049. PubMed
7. Shepperd S, Doll H, Angus RM, et al. Avoiding hospital admission through provision of hospital care at home: a systematic review and meta-analysis of individual patient data. CMAJ. 2009;180(2):175-182. doi: 10.1503/cmaj.081491. PubMed
8. Caplan GA, Sulaiman NS, Mangin N, et al. A meta-analysis of “Hospital in the Home”. Med J Aust. 2012;197:512-519. doi: 10.5694/mja12.10480. PubMed
9. Shepperd S, Iliffe S, Doll HA, et al. Admission avoidance hospital at home. Cochrane Database Syst Rev. 2016;9:CD007491. doi: 10.1002/14651858.CD007491.pub2. PubMed
10. Federman AD, Soones T, DeCherrie LV, Leff B, Siu AL. Association of a bundled hospital-at-home and 30-day postacute transitional care program with clinical outcomes and patient experiences. JAMA Intern Med. 2018;178(8):1033-1040. doi: 10.1001/jamainternmed.2018.2562. PubMed
11. Stein PD, Hull RD, Matta F, Willyerd GL. Modest response in translation to home management of deep venous thrombosis. Am J Med. 2010;123(12):1107-1113. doi: 10.1016/j.amjmed.2010.07.016. PubMed
12. Icahn School of Medicine at Mount Sinai. “HaH-Plus” (Hospital at Home Plus) Provider Focused Payment Model. https://aspe.hhs.gov/system/files/pdf/255906/HaHPlusProviderFocusedPaymentModel.pdf. Accessed November 11, 2018.
13. Physician-F ocused Payment Model Technical Advisory Committee. Report to the Secretary of Health and Human Services. Comments and Recommendation on “HaH-Plus (Hospital at Home Plus) Provider-Focused Payment Model. https://aspe.hhs.gov/system/files/pdf/255906/MtSinaiHAHReportSecretary.pdf. Accessed November 11, 2018.
14. The Secretary of Health and Human Services. Response to the Report to the Secretary of Health and Human Services. Comments and Recommendation on “HaH-Plus (Hospital at Home Plus) Provider-Focused Payment Model. https://downloads.cms.gov/files/cmmi/ptac-hhssecresponse-oct17-may18.pdf. Accessed November 11, 2018.
15. Montalto M. The 500-bed hospital that isn’t there: the Victorian Department of Health review of the Hospital in the Home program. Med J Aust. 2010;193(10);598-601. PubMed
16. Hayashi J, Leff B. Geriatric Home-Based Medical Care. New York, NY: Springer Publishers; 2015. PubMed
17. Reckrey JM, Ornstein KA, Wajnberg A, Kopke MV, DeCherrie LV. Teaching home-based primary care. Home Healthc Now. 2017;35(10):561-565. doi: 10.1097/NHH.0000000000000621. PubMed
18. Ticona L, Schulman KA. Extreme home makeover - the role of intensive home health care. N Engl J Med. 2016;375(18):1707-1709. doi: 10.1056/NEJMp1608301. PubMed
Ms. P., an 86-year-old woman with a history of hypertension, hyperlipidemia, coronary artery disease, and transient ischemic attack, presents to the emergency department with a three-day history of cough, fever, purulent sputum, fatigue, and dyspnea on exertion. Her vital signs are notable for a fever of 39.0°C, blood pressure 136/92, pulse 102, respiratory rate 30, and room air oxygen saturation of 91%. She looks ill. She has a white blood cell count of 16,000, lactate 1.9, and a right lower lobe infiltrate on imaging. The emergency department attending physician presents the case to you for admission, and you accept the patient into your inpatient hospitalist service.
Now, let’s imagine a different future in which you are the attending hospitalist on your institution’s Hospital at Home (HaH) service, where you will provide hospital-level care to Ms. P. in the comfort of her own home. Hospitalists should prepare for this paradigm shift.
WHAT IS HOSPITAL AT HOME?
HaH provides hospital-level care in a patient’s home, for those with qualifying acute illnesses and appropriate degrees of acuity, as a substitute for traditional inpatient care.1 This is achieved by bringing the critical elements of hospital care to the home—physician and nursing care, intravenous medications and fluids, oxygen and respiratory therapies, basic radiography and ultrasound, durable medical equipment, skilled therapies, and more.2
All hospitalists have cared for patients like Ms. P., and she and many patients like her will have a straightforward hospital trajectory: initial evaluation in the emergency department, inpatient care provided by a hospitalist inpatient service, a few days of intravenous antibiotics and other hospital services, and finally, discharge to home.
A SHARED RATIONALE FOR HOSPITAL MEDICINE AND HOSPITAL AT HOME
However, not all patients will experience a smooth, or safe, hospital course. Studies that launched the hospital safety movement also provide the rationale for HaH, namely, that hospitals are often dangerous environments for patients.3
A complementary approach to improving outcomes for patients at high risk of iatrogenic illness such as functional decline, falls, delirium, adverse drug events, and hospital-associated disability syndrome,4-6 is to care for patients outside the traditional inpatient hospital environment. Over the past 20 years, many studies—including dozens of randomized controlled trials and several meta-analyses—have shown better outcomes for patients cared for in HaH: decreased length of stay, decreased incidence of adverse events (including substantially lower six-month mortality), better patient and caregiver care experiences, lower caregiver stress, and lower costs.7-9A recent Center for Medicare and Medicaid Innovation (CMMI) Demonstration conducted at the Mount Sinai Health System found similar results.10
GROWING INTEREST IN HOSPITAL AT HOME AND CHALLENGES TO DISSEMINATION
Interest in HaH has increased markedly over the past few years with increased penetration of Medicare and Medicaid managed care, the development and spread of accountable care organizations (ACOs), and a shift in focus among some health systems towards value-based care, population health, and community-based care. Recently, commercial entities have entered the HaH space and have raised substantial capital to fund development. Despite this growing interest in HaH and substantial evidence of its effectiveness, HaH has not been widely implemented or scaled in the United States.
Widespread dissemination and implementation of HaH has been hampered by several barriers. First, despite growing interest in HaH, the culture of healthcare and health system leadership, for the most part, remains focused on facility-based care.11
Second, while HaH makes financial sense in the managed care arena, given the strong evidence for high-quality, lower-cost care, there is currently no standard payment mechanism for HaH in fee-for-service Medicare or in the commercial insurance space. However, there are indications that this may soon change. In the fall of 2017, a proposal for a bundled payment mechanism for acute HaH care plus 30 days of postacute care was unanimously approved by an Advisory Committee to the Secretary of the Department of Health and Human Services (HHS).12,13 The HHS Secretary recently noted that “the Department of Health and Human Services is keenly interested in ideas for home-based, hospital-level care, and agrees … that this proposal holds promise for testing.”14
Third is the need to create the logistics and supply chain to support HaH. There currently exists a well-established supply chain for providing hospital care. A hospitalist orders a dose of intravenous antibiotic or oxygen, and it is supplied in a timely manner. Similarly, the postacute sector of healthcare has a robust supply chain, though it operates on a somewhat different clock from the acute care setting. However, there is currently no easily replicable supply chain to meet the needs of providing acute care in the home. Each HaH has had to create its own system of logistics with the existing healthcare assets in its local environment. Developing this capacity at scale will require significant capital investment.
There are examples where HaH has scaled. Beginning in 1994, in the state of Victoria, Australia (population 6.3 million), the health authority reimbursed HaH care at the same rates as traditional hospital care. At last report, HaH provided approximately 5% of all hospital bed days of care in Victoria. Providing HaH on this scale helped avoid the need to build a new 500-bed hospital to care for those patients.15 The avoided costs of building new hospital beds (and the ongoing need to fill those beds) represents significant societal return on investment attributable to HaH.
EMERGENCE OF THE HOME HOSPITALIST?
A key element in implementing a HaH program is its physician staff in terms of the types of doctors who provide HaH care, how they are organized, and how they interact with patients. To date, HaH physicians have been predominantly geriatricians, but internists and family medicine physicians, employed as full-time members of a dedicated HaH team, also provide care by physically visiting patients in their homes. The reason for significant involvement of geriatricians in HaH may relate to the fact that geriatric fellowship training includes training in home-based medical care, whereas this is less common in family medicine and internal medicine residency training programs.
In order to provide HaH on a nationwide scale, there will be a need for a larger workforce. There is an opportunity here to leverage existing hospital physician staff, such as hospitalists. In addition, while there is significant value in physicians seeing patients in their homes, more scalable versions of HaH are being developed and implemented that leverage biometrically enhanced telemedicine approaches for a dedicated physician component of care, with in-person visits provided by other members of an interdisciplinary team.
We believe that hospitalists can play a key role as HaH physicians as the HaH model continues to evolve and expand. Hospitalists bring valuable expertise relevant to HaH care delivery, including extensive experience with the triage of acutely ill patients, an understanding of the natural course of acute illness and team-based care, and for some, experience with telemedicine care.
While a hospitalist providing HaH care would leverage many of the competencies of the traditional hospitalist, we suggest that such a provider should receive additional training and clinical experience in home-based medical care to help them better understand the unique aspects of providing care in patients’ homes.16 Such training could include experience in making house calls, which can be a transformational experience in helping physicians improve their skills in dealing with social determinants of health, diagnosing and managing geriatric syndromes, and mobilizing community resources in the care of their patients, as well as managing care transitions. Hospitalists delivering care in HaH may also need to upgrade specific clinical skills commonly addressed by home-based medical care providers: wound care, caregiver-related issues, social and ethical issues specific to home-based care, problems with functional status, psychiatric and cognitive issues, management of gastrostomy tubes and bladder catheters, and dermatologic problems, as well as palliative care and end-of-life symptom management. These skills are slightly different from the usual realm of the typical hospitalists’ wheelhouse. However, it is all learnable.17 Similarly, geriatricians can learn from hospitalists as the HaH model evolves; there are HaH programs in existence today that take care of a sicker tranche of patients than earlier versions of HaH, with continuous telemonitoring of patients and the ability to rapidly deploy providers, labs, imaging, and medications. Going forward, as healthcare organizations begin to develop HaH programs staffed by hospitalists, it is probably wise for hospitalists and geriatricians to collaborate on the optimal physician models for HaH.
There may emerge a new specialty. Ticona and Schulman described a “home intensivist” with competencies including informatics of remote monitoring technology, leadership of multidisciplinary care teams, and the interpersonal skills required for compassionate end-of-life care.18 We prefer the term Home Hospitalist. Home Hospitalists would develop an enhanced understanding of the transitions of care and social determinants of health, and they would gain valuable knowledge about the social and environmental challenges many patients face after discharge from the hospital.
When this vision is realized, there will be enormous benefits to both HaH and Hospital Medicine. HaH could tap into a large and competent workforce to enhance its implementation and dissemination. Hospital Medicine would gain a new pathway for its providers and could develop new collaborative efforts with geriatric, internal, and family medicine.
Disclosures
Dr. Danielsson has nothing to disclose. Dr. Leff reports personal fees from Medically Home, other from Dispatch Health, other from Landmark Health, personal fees from Medibank, personal fees from Apple, personal fees from Health Affairs, other from Honor, personal fees from Institute for Healthcare Improvement, outside the submitted work; and American Academy of Home Care Medicine - member board of directors, voluntary.
Funding
Dr. Leff was supported in this work by a grant from The John A. Hartford Foundation.
Ms. P., an 86-year-old woman with a history of hypertension, hyperlipidemia, coronary artery disease, and transient ischemic attack, presents to the emergency department with a three-day history of cough, fever, purulent sputum, fatigue, and dyspnea on exertion. Her vital signs are notable for a fever of 39.0°C, blood pressure 136/92, pulse 102, respiratory rate 30, and room air oxygen saturation of 91%. She looks ill. She has a white blood cell count of 16,000, lactate 1.9, and a right lower lobe infiltrate on imaging. The emergency department attending physician presents the case to you for admission, and you accept the patient into your inpatient hospitalist service.
Now, let’s imagine a different future in which you are the attending hospitalist on your institution’s Hospital at Home (HaH) service, where you will provide hospital-level care to Ms. P. in the comfort of her own home. Hospitalists should prepare for this paradigm shift.
WHAT IS HOSPITAL AT HOME?
HaH provides hospital-level care in a patient’s home, for those with qualifying acute illnesses and appropriate degrees of acuity, as a substitute for traditional inpatient care.1 This is achieved by bringing the critical elements of hospital care to the home—physician and nursing care, intravenous medications and fluids, oxygen and respiratory therapies, basic radiography and ultrasound, durable medical equipment, skilled therapies, and more.2
All hospitalists have cared for patients like Ms. P., and she and many patients like her will have a straightforward hospital trajectory: initial evaluation in the emergency department, inpatient care provided by a hospitalist inpatient service, a few days of intravenous antibiotics and other hospital services, and finally, discharge to home.
A SHARED RATIONALE FOR HOSPITAL MEDICINE AND HOSPITAL AT HOME
However, not all patients will experience a smooth, or safe, hospital course. Studies that launched the hospital safety movement also provide the rationale for HaH, namely, that hospitals are often dangerous environments for patients.3
A complementary approach to improving outcomes for patients at high risk of iatrogenic illness such as functional decline, falls, delirium, adverse drug events, and hospital-associated disability syndrome,4-6 is to care for patients outside the traditional inpatient hospital environment. Over the past 20 years, many studies—including dozens of randomized controlled trials and several meta-analyses—have shown better outcomes for patients cared for in HaH: decreased length of stay, decreased incidence of adverse events (including substantially lower six-month mortality), better patient and caregiver care experiences, lower caregiver stress, and lower costs.7-9A recent Center for Medicare and Medicaid Innovation (CMMI) Demonstration conducted at the Mount Sinai Health System found similar results.10
GROWING INTEREST IN HOSPITAL AT HOME AND CHALLENGES TO DISSEMINATION
Interest in HaH has increased markedly over the past few years with increased penetration of Medicare and Medicaid managed care, the development and spread of accountable care organizations (ACOs), and a shift in focus among some health systems towards value-based care, population health, and community-based care. Recently, commercial entities have entered the HaH space and have raised substantial capital to fund development. Despite this growing interest in HaH and substantial evidence of its effectiveness, HaH has not been widely implemented or scaled in the United States.
Widespread dissemination and implementation of HaH has been hampered by several barriers. First, despite growing interest in HaH, the culture of healthcare and health system leadership, for the most part, remains focused on facility-based care.11
Second, while HaH makes financial sense in the managed care arena, given the strong evidence for high-quality, lower-cost care, there is currently no standard payment mechanism for HaH in fee-for-service Medicare or in the commercial insurance space. However, there are indications that this may soon change. In the fall of 2017, a proposal for a bundled payment mechanism for acute HaH care plus 30 days of postacute care was unanimously approved by an Advisory Committee to the Secretary of the Department of Health and Human Services (HHS).12,13 The HHS Secretary recently noted that “the Department of Health and Human Services is keenly interested in ideas for home-based, hospital-level care, and agrees … that this proposal holds promise for testing.”14
Third is the need to create the logistics and supply chain to support HaH. There currently exists a well-established supply chain for providing hospital care. A hospitalist orders a dose of intravenous antibiotic or oxygen, and it is supplied in a timely manner. Similarly, the postacute sector of healthcare has a robust supply chain, though it operates on a somewhat different clock from the acute care setting. However, there is currently no easily replicable supply chain to meet the needs of providing acute care in the home. Each HaH has had to create its own system of logistics with the existing healthcare assets in its local environment. Developing this capacity at scale will require significant capital investment.
There are examples where HaH has scaled. Beginning in 1994, in the state of Victoria, Australia (population 6.3 million), the health authority reimbursed HaH care at the same rates as traditional hospital care. At last report, HaH provided approximately 5% of all hospital bed days of care in Victoria. Providing HaH on this scale helped avoid the need to build a new 500-bed hospital to care for those patients.15 The avoided costs of building new hospital beds (and the ongoing need to fill those beds) represents significant societal return on investment attributable to HaH.
EMERGENCE OF THE HOME HOSPITALIST?
A key element in implementing a HaH program is its physician staff in terms of the types of doctors who provide HaH care, how they are organized, and how they interact with patients. To date, HaH physicians have been predominantly geriatricians, but internists and family medicine physicians, employed as full-time members of a dedicated HaH team, also provide care by physically visiting patients in their homes. The reason for significant involvement of geriatricians in HaH may relate to the fact that geriatric fellowship training includes training in home-based medical care, whereas this is less common in family medicine and internal medicine residency training programs.
In order to provide HaH on a nationwide scale, there will be a need for a larger workforce. There is an opportunity here to leverage existing hospital physician staff, such as hospitalists. In addition, while there is significant value in physicians seeing patients in their homes, more scalable versions of HaH are being developed and implemented that leverage biometrically enhanced telemedicine approaches for a dedicated physician component of care, with in-person visits provided by other members of an interdisciplinary team.
We believe that hospitalists can play a key role as HaH physicians as the HaH model continues to evolve and expand. Hospitalists bring valuable expertise relevant to HaH care delivery, including extensive experience with the triage of acutely ill patients, an understanding of the natural course of acute illness and team-based care, and for some, experience with telemedicine care.
While a hospitalist providing HaH care would leverage many of the competencies of the traditional hospitalist, we suggest that such a provider should receive additional training and clinical experience in home-based medical care to help them better understand the unique aspects of providing care in patients’ homes.16 Such training could include experience in making house calls, which can be a transformational experience in helping physicians improve their skills in dealing with social determinants of health, diagnosing and managing geriatric syndromes, and mobilizing community resources in the care of their patients, as well as managing care transitions. Hospitalists delivering care in HaH may also need to upgrade specific clinical skills commonly addressed by home-based medical care providers: wound care, caregiver-related issues, social and ethical issues specific to home-based care, problems with functional status, psychiatric and cognitive issues, management of gastrostomy tubes and bladder catheters, and dermatologic problems, as well as palliative care and end-of-life symptom management. These skills are slightly different from the usual realm of the typical hospitalists’ wheelhouse. However, it is all learnable.17 Similarly, geriatricians can learn from hospitalists as the HaH model evolves; there are HaH programs in existence today that take care of a sicker tranche of patients than earlier versions of HaH, with continuous telemonitoring of patients and the ability to rapidly deploy providers, labs, imaging, and medications. Going forward, as healthcare organizations begin to develop HaH programs staffed by hospitalists, it is probably wise for hospitalists and geriatricians to collaborate on the optimal physician models for HaH.
There may emerge a new specialty. Ticona and Schulman described a “home intensivist” with competencies including informatics of remote monitoring technology, leadership of multidisciplinary care teams, and the interpersonal skills required for compassionate end-of-life care.18 We prefer the term Home Hospitalist. Home Hospitalists would develop an enhanced understanding of the transitions of care and social determinants of health, and they would gain valuable knowledge about the social and environmental challenges many patients face after discharge from the hospital.
When this vision is realized, there will be enormous benefits to both HaH and Hospital Medicine. HaH could tap into a large and competent workforce to enhance its implementation and dissemination. Hospital Medicine would gain a new pathway for its providers and could develop new collaborative efforts with geriatric, internal, and family medicine.
Disclosures
Dr. Danielsson has nothing to disclose. Dr. Leff reports personal fees from Medically Home, other from Dispatch Health, other from Landmark Health, personal fees from Medibank, personal fees from Apple, personal fees from Health Affairs, other from Honor, personal fees from Institute for Healthcare Improvement, outside the submitted work; and American Academy of Home Care Medicine - member board of directors, voluntary.
Funding
Dr. Leff was supported in this work by a grant from The John A. Hartford Foundation.
1. Leff B, Montalto M. Home hospital-toward a tighter definition. J Am Geriatr Soc. 2004;52(12):2141. doi: 10.1111/j.1532-5415.2004.52579_1.x. PubMed
2. Leff B, Burton L, Mader SL, et al. Hospital at home: feasibility and outcomes of a program to provide hospital-level care at home for acutely ill older patients. Ann Intern Med. 2005;143(11):798-808. doi: 10.7326/0003-4819-143-11-200512060-00008. PubMed
3. Brennan TA, Leape LL, Laird NM, et al. Incidence of adverse events and negligence in hospitalized patients. Results of the Harvard Medical Practice Study I. N Engl J Med. 1991;324(6):370-376. doi: 10.1056/NEJM199102073240604. PubMed
4. Creditor MC. Hazards of hospitalization of the elderly. Ann Intern Med. 1993;118(3):219-223. doi: 10.7326/0003-4819-118-3-199302010-00011. PubMed
5. Covinsky KE, Pierluissi E, Johnston CB. Hospitalization-associated disability: “She was probably able to ambulate, but I’m not sure”. JAMA. 2011;306(16):1782-1793. doi: 10.1001/jama.2011.1556. PubMed
6. Wald HL. The Geometry of Patient Safety: Horizontal and Vertical Approaches to the Hazards of Hospitalization. J Am Geriatr Soc. 2017;65(12):2559-2561. doi: 10.1111/jgs.15049. PubMed
7. Shepperd S, Doll H, Angus RM, et al. Avoiding hospital admission through provision of hospital care at home: a systematic review and meta-analysis of individual patient data. CMAJ. 2009;180(2):175-182. doi: 10.1503/cmaj.081491. PubMed
8. Caplan GA, Sulaiman NS, Mangin N, et al. A meta-analysis of “Hospital in the Home”. Med J Aust. 2012;197:512-519. doi: 10.5694/mja12.10480. PubMed
9. Shepperd S, Iliffe S, Doll HA, et al. Admission avoidance hospital at home. Cochrane Database Syst Rev. 2016;9:CD007491. doi: 10.1002/14651858.CD007491.pub2. PubMed
10. Federman AD, Soones T, DeCherrie LV, Leff B, Siu AL. Association of a bundled hospital-at-home and 30-day postacute transitional care program with clinical outcomes and patient experiences. JAMA Intern Med. 2018;178(8):1033-1040. doi: 10.1001/jamainternmed.2018.2562. PubMed
11. Stein PD, Hull RD, Matta F, Willyerd GL. Modest response in translation to home management of deep venous thrombosis. Am J Med. 2010;123(12):1107-1113. doi: 10.1016/j.amjmed.2010.07.016. PubMed
12. Icahn School of Medicine at Mount Sinai. “HaH-Plus” (Hospital at Home Plus) Provider Focused Payment Model. https://aspe.hhs.gov/system/files/pdf/255906/HaHPlusProviderFocusedPaymentModel.pdf. Accessed November 11, 2018.
13. Physician-F ocused Payment Model Technical Advisory Committee. Report to the Secretary of Health and Human Services. Comments and Recommendation on “HaH-Plus (Hospital at Home Plus) Provider-Focused Payment Model. https://aspe.hhs.gov/system/files/pdf/255906/MtSinaiHAHReportSecretary.pdf. Accessed November 11, 2018.
14. The Secretary of Health and Human Services. Response to the Report to the Secretary of Health and Human Services. Comments and Recommendation on “HaH-Plus (Hospital at Home Plus) Provider-Focused Payment Model. https://downloads.cms.gov/files/cmmi/ptac-hhssecresponse-oct17-may18.pdf. Accessed November 11, 2018.
15. Montalto M. The 500-bed hospital that isn’t there: the Victorian Department of Health review of the Hospital in the Home program. Med J Aust. 2010;193(10);598-601. PubMed
16. Hayashi J, Leff B. Geriatric Home-Based Medical Care. New York, NY: Springer Publishers; 2015. PubMed
17. Reckrey JM, Ornstein KA, Wajnberg A, Kopke MV, DeCherrie LV. Teaching home-based primary care. Home Healthc Now. 2017;35(10):561-565. doi: 10.1097/NHH.0000000000000621. PubMed
18. Ticona L, Schulman KA. Extreme home makeover - the role of intensive home health care. N Engl J Med. 2016;375(18):1707-1709. doi: 10.1056/NEJMp1608301. PubMed
1. Leff B, Montalto M. Home hospital-toward a tighter definition. J Am Geriatr Soc. 2004;52(12):2141. doi: 10.1111/j.1532-5415.2004.52579_1.x. PubMed
2. Leff B, Burton L, Mader SL, et al. Hospital at home: feasibility and outcomes of a program to provide hospital-level care at home for acutely ill older patients. Ann Intern Med. 2005;143(11):798-808. doi: 10.7326/0003-4819-143-11-200512060-00008. PubMed
3. Brennan TA, Leape LL, Laird NM, et al. Incidence of adverse events and negligence in hospitalized patients. Results of the Harvard Medical Practice Study I. N Engl J Med. 1991;324(6):370-376. doi: 10.1056/NEJM199102073240604. PubMed
4. Creditor MC. Hazards of hospitalization of the elderly. Ann Intern Med. 1993;118(3):219-223. doi: 10.7326/0003-4819-118-3-199302010-00011. PubMed
5. Covinsky KE, Pierluissi E, Johnston CB. Hospitalization-associated disability: “She was probably able to ambulate, but I’m not sure”. JAMA. 2011;306(16):1782-1793. doi: 10.1001/jama.2011.1556. PubMed
6. Wald HL. The Geometry of Patient Safety: Horizontal and Vertical Approaches to the Hazards of Hospitalization. J Am Geriatr Soc. 2017;65(12):2559-2561. doi: 10.1111/jgs.15049. PubMed
7. Shepperd S, Doll H, Angus RM, et al. Avoiding hospital admission through provision of hospital care at home: a systematic review and meta-analysis of individual patient data. CMAJ. 2009;180(2):175-182. doi: 10.1503/cmaj.081491. PubMed
8. Caplan GA, Sulaiman NS, Mangin N, et al. A meta-analysis of “Hospital in the Home”. Med J Aust. 2012;197:512-519. doi: 10.5694/mja12.10480. PubMed
9. Shepperd S, Iliffe S, Doll HA, et al. Admission avoidance hospital at home. Cochrane Database Syst Rev. 2016;9:CD007491. doi: 10.1002/14651858.CD007491.pub2. PubMed
10. Federman AD, Soones T, DeCherrie LV, Leff B, Siu AL. Association of a bundled hospital-at-home and 30-day postacute transitional care program with clinical outcomes and patient experiences. JAMA Intern Med. 2018;178(8):1033-1040. doi: 10.1001/jamainternmed.2018.2562. PubMed
11. Stein PD, Hull RD, Matta F, Willyerd GL. Modest response in translation to home management of deep venous thrombosis. Am J Med. 2010;123(12):1107-1113. doi: 10.1016/j.amjmed.2010.07.016. PubMed
12. Icahn School of Medicine at Mount Sinai. “HaH-Plus” (Hospital at Home Plus) Provider Focused Payment Model. https://aspe.hhs.gov/system/files/pdf/255906/HaHPlusProviderFocusedPaymentModel.pdf. Accessed November 11, 2018.
13. Physician-F ocused Payment Model Technical Advisory Committee. Report to the Secretary of Health and Human Services. Comments and Recommendation on “HaH-Plus (Hospital at Home Plus) Provider-Focused Payment Model. https://aspe.hhs.gov/system/files/pdf/255906/MtSinaiHAHReportSecretary.pdf. Accessed November 11, 2018.
14. The Secretary of Health and Human Services. Response to the Report to the Secretary of Health and Human Services. Comments and Recommendation on “HaH-Plus (Hospital at Home Plus) Provider-Focused Payment Model. https://downloads.cms.gov/files/cmmi/ptac-hhssecresponse-oct17-may18.pdf. Accessed November 11, 2018.
15. Montalto M. The 500-bed hospital that isn’t there: the Victorian Department of Health review of the Hospital in the Home program. Med J Aust. 2010;193(10);598-601. PubMed
16. Hayashi J, Leff B. Geriatric Home-Based Medical Care. New York, NY: Springer Publishers; 2015. PubMed
17. Reckrey JM, Ornstein KA, Wajnberg A, Kopke MV, DeCherrie LV. Teaching home-based primary care. Home Healthc Now. 2017;35(10):561-565. doi: 10.1097/NHH.0000000000000621. PubMed
18. Ticona L, Schulman KA. Extreme home makeover - the role of intensive home health care. N Engl J Med. 2016;375(18):1707-1709. doi: 10.1056/NEJMp1608301. PubMed
© 2019 Society of Hospital Medicine
An Advanced Practice Provider Clinical Fellowship as a Pipeline to Staffing a Hospitalist Program
There is an increasing utilization of advanced practice providers (APPs) in the delivery of healthcare in the United States.1,2 As of 2016, there were 157, 025 nurse practitioners (NPs) and 102,084 physician assistants (PAs) with a projected growth rate of 6.8% and 4.3%, respectively, which exceeds the physician growth rate of 1.1%.2 This increased growth rate has been attributed to the expectation that APPs can enhance the quality of physician care, relieve physician shortages, and reduce service costs, as APPs are less expensive to hire than physicians.3,4 Hospital medicine is the fastest growing medical field in the United States, and approximately 83% of hospitalist groups around the country utilize APPs; however, the demand for hospitalists continues to exceed the supply, and this has led to increased utilization of APPs in hospital medicine.5-10
APPs receive very limited inpatient training and there is wide variation in their clinical abilities after graduation.11 This is an issue that has become exacerbated in recent years by a change in the training process for PAs. Before 2005, PA programs were typically two to three years long and required the same prerequisite courses as medical schools.11 PA students completed more than 2,000 hours of clinical rotations and then had to pass the Physician Assistant National Certifying Exam before they could practice.12 Traditionally, PA programs typically attracted students with prior healthcare experience.11 In 2005, PA programs began transitioning from bachelor’s degrees to requiring a master’s level degree for completion of the programs. This has shifted the demographics of the students matriculating to younger students with little-to-no prior healthcare experience; moreover, these fresh graduates lack exposure to hospital medicine.11
NPs usually gain clinical experience working as registered nurses (RNs) for two or more years prior to entry into the NP program. NP programs for baccalaureate-prepared RNs vary in length from two to three years.2 There is an acute care focus for NPs in training; however, there is no standardized training or licensure to ensure that hospital medicine competencies are met.13-15 Some studies have shown that a lack of structured support has been found to affect NP role transition negatively during the first year of practice,16 and graduating NPs have indicated that they needed more out of their clinical education in terms of content, clinical experience, and competency testing.17
Hiring new APP graduates as hospitalists requires a longer and more rigorous onboarding process. On‐the‐job training in hospital medicine for new APP graduates can take as long as six to 12 months in order for them to acquire the basic skill set necessary to adequately manage hospitalized patients.15 This extended onboarding is costly because the APPs are receiving a full hospitalist salary, yet they are not functioning at full capacity. Ideally, there should be an intermediary training step between graduation and employment as hospitalist APPs. Studies have shown that APPs are interested in formal postgraduate hospital medicine training, even if it means having a lower stipend during the first year after graduating from their NP or PA program.9,15,18
The growing need for hospitalists, driven by residency work-hour reform, increased age and complexity of patients, and the need to improve the quality of inpatient care while simultaneously reducing waste, has contributed to the increasing utilization of and need for highly qualified APPs in hospital medicine.11,19,20 We established a fellowship to train APPs. The goal of this study was to determine if an APP fellowship is a cost-effective pipeline for filling vacancies within a hospitalist program.
METHODS
Design and Setting
Johns Hopkins Bayview Medical Center (JHBMC) is a 440 bed hospital in Baltimore Maryland. The hospitalist group was started in 1996 with one physician seeing approximately 500 discharges a year. Over the last 20 years, the group has grown and is now its own division with 57 providers, including 42 physicians, 11 APPs, and four APP fellows. The hospitalist division manages ~7,000 discharges a year, which corresponds to approximately 60% of admissions to general medicine. Hospitalist APPs help staff general medicine by working alongside doctors and admitting patients during the day and night. The APPs also staff the pulmonary step down unit with a pulmonary attending and the chemical dependency unit with an internal medicine addiction specialist.
The growth of the division of hospital medicine at JHBMC is a result of increasing volumes and reduced residency duty hours. The increasing full time equivalents (FTEs) resulted in a need for APPs; however, vacancies went unfilled for an average of 35 weeks due to the time it took to post open positions, interview applicants, and hire applicants through the credentialing process. Further, it took as long as 22 to 34 weeks for a new hire to work independently. The APP vacancies and onboarding resulted in increased costs to the division incurred by physician moonlighting to cover open shifts. The hourly physician moonlighting rate at JHBMC is $150. All costs were calculated on the basis of a 40-hour work week. We performed a pre- and postanalysis of outcomes of interest between January 2009 and June 2018. This study was exempt from institutional review board review.
Intervention
In 2014, a one year APP clinical fellowship in hospital medicine was started. The fellows evaluate and manage patients working one-on-one with an experienced hospitalist faculty member. The program consists of 80% clinical experience in the inpatient setting and 20% didactic instruction (Table 1). Up to four fellows are accepted each year and are eligible for hire after training if vacancies exist. The program is cost neutral and was financed by downsizing, through attrition, two physician FTEs. Four APP fellows’ salaries are the equivalent of two entry-level hospitalist physicians’ salaries at JHBMC. The annual salary for an APP fellow is $69,000.
Downsizing by two physician FTEs meant that one less doctor was scheduled every day. The patient load previously seen by that one doctor (10 patients) was absorbed by the MD–APP fellow dyads. Paired with a fellow, each physician sees a higher cap of 13 patients, and it takes six weeks for the fellows to ramp-up to this patient load. When the fellow first starts, the team sees 10 patients. Every two weeks, the pair’s census increases by one patient to the cap of 13. Collectively, the four APP fellow–MD dyads make it possible for four physicians to see an additional 12 patients. The two extra patients absorbed by the service per day results in a net increase in capacity of up to 730 patient encounters a year.
Outcomes and Analysis
Our main outcomes of interest were duration of onboarding and cost incurred by the division to (1) staff the service during a vacancy and (2) onboard new hires. Secondary outcomes included duration of vacancy and total time spent with the group. We collected basic demographic data on participants, including, age, gender, and race. Demographics and outcomes of interest were compared pre- (2009-2013) and post- (2014-2018) initiation of the APP clinical fellowship using the chi-square test, the t-test for normally distributed data, and the Wilcoxon rank-sum for nonnormally distributed data, as appropriate. The normality of the data distribution was tested using the Shapiro-Wilk W test. Two-tailed P values less than .05 were considered to be statistically significant. Results were analyzed using Stata/MP version 13.0 (StataCorp Inc, College Station, Texas).
RESULTS
Twelve fellows have been recruited, and of these, 10 have graduated. Two chose to leave the program prior to completion. Of the 10 fellows that have graduated, six have been hired into our group, one was hired within our facility, and three were hired as hospitalists at other institutions. The median time from APP school graduation to hire was also not different between the two groups (10.5 vs 3.9 months, P = .069). In addition, the total time that the new APP hires spent with the group was nonstatistically significantly different between the two periods (17.9 vs 18.3 months, P = .735). Both the mean duration of onboarding and the cost to the division were significantly reduced after implementation of the program (25.4 vs 11.0 weeks, P = .017 and $361,714 vs $66,000, P = .004; Table 2).
The yearly cost of an APP vacancy and onboarding is incurred by doctor moonlighting costs (at the rate of $150 per hour) to cover open shifts. The mean duration of vacancies and onboarding each year was 34.9 and 25.4 weeks, respectively, before the fellowship. The yearly cost of onboarding, after the establishment of the fellowship, is a maximum of $66,000, derived from physician moonlighting to cover the six-week ramp-up at the very beginning of the fellowship and the five weeks of orientation to the pulmonary and chemical dependency units after the fellowship (Table 3).
DISCUSSION
Our APP clinical fellowship in hospital medicine at JHBMC has produced several benefits. First, the fellowship has become a pipeline for filling APP vacancies within our division. We have been able to hire for four consecutive years from the fellowship. Second, the ready availability of high-functioning and efficient APP hospitalists has cut down on the onboarding time for our new APP hires. Many new APP graduates lack confidence in caring for complex hospitalized patients. Following our 12-month clinical fellowship, our matriculated fellows are able to practice at the top of their license immediately and confidently. Third, the reduced vacancy and shortened onboarding periods have reduced costs to the division. Fourth, the fellowship has created additional teaching avenues for the faculty. The medicine units at JHBMC are comprised of hospitalist and internal medicine residency services. The hospitalists spend the majority of their clinical time in direct patient care; however, they rotate on the residency service for two weeks out of the year. The majority of physicians welcome the chance to teach more, and partnering with an APP fellow provides that opportunity.
As we have developed and grown this program, the one great challenge has been what to do with graduating fellows when we cannot hire them. Fortunately, the market for highly qualified, well trained APPs is strong, and every one of the fellows that we could not hire within our group has been able to find a position either within our facility or outside our institution. To facilitate this process, program directors and recruiters are invited to meet with the fellows toward the end of their fellowship to share employment opportunities with them.
Our study has limitations. First, had the $276,000 from the attrition of two physicians been used to hire nonfellow APPs under the old model, then the costs of the two models would have been similar, but this was simply not possible because the positions could not be filled. Second, this is a single-site experience, and our findings may not be generalizable, particularly those pertaining to remuneration. Third, our study was underpowered to detect small but important differences in characteristics of APPs, especially time from graduation to hire, before and after the implementation of our fellowship. Further research comparing various programs both in structure and outcomes—such as fellows’ readiness for practice, costs, duration of vacancies, and provider satisfaction—are an important next step.
We have developed a pool of applicants within our division to fill vacancies left by turnover from senior NPs and PAs. This program has reduced costs and improved the joy of practice for both doctors and APPs. As the need for highly qualified NPs and PAs in hospital medicine continues to grow, we may see more APP fellowships in hospital medicine in the United States.
Acknowledgments
The authors thank the advanced practice providers who have helped us grow and refine our fellowship.
Disclosures
The authors have nothing to disclose
1. Martsoff G, Nguyen P, Freund D, Poghosyan L. What we know about postgraduate nurse practitioner residency and fellowship programs. J Nurse Pract. 2017;13(7):482-487. doi: 10.1016/j.nurpra.2017.05.013.
2. Auerbach D, Staiger D, Buerhaus P. Growing ranks of advanced practice clinicians-implications for the physician workforce. N Engl J Med. 2018;378(25):2358-2360. doi: 10.1056/NEJMp1801869. PubMed
3. Laurant M, Harmsen M, Wollersheim H, Grol R, Faber M, Sibbald B. The
impact of nonphysician clinicians: do they improve the quality and cost-effectiveness
of health care services? Med Care Res Rev. 2009;66(6 Suppl):36S-89S. doi: 10.1177/1077558709346277. PubMed
4. Auerbach DI. Will the NP workforce grow in the future? New forecasts and
implications for healthcare delivery. Med Care. 2012;50(7):606-610. doi:
10.1097/MLR.0b013e318249d6e7. PubMed
5. Kisuule F, Howell E. Hospital medicine beyond the United States. Int J Gen
Med. 2018;11:65-71. doi: 10.2147/IJGM.S151275. PubMed
6. Wachter RM, Goldman L. Zero to 50, 000-The 20th anniversary of the hospitalist.
N Engl J Med. 2016;375(11):1009-1011. doi: 10.1056/NEJMp1607958. PubMed
7. Conrad, K and Valovska T. The current state of hospital medicine: trends in
compensation, practice patterns, advanced practice providers, malpractice,
and career satisfaction. In: Conrad K, ed. Clinical Approaches to Hospital
Medicine. Cham, Springer; 2017:259-270.
8. Bryant SE. Filling the gaps: preparing nurse practitioners for hospitalist
practice. J Am Assoc Nurse Pract. 2018;30(1):4-9. doi: 10.1097/
JXX.0000000000000008. PubMed
9. Sharma P, Brooks M, Roomiany P, Verma L, Criscione-Schreiber, L. Physician
assistant student training for the inpatient setting: a needs assessment. J Physician
Assist Educ. 2017;28(4):189-195. doi: 10.1097/JPA.0000000000000174. PubMed
10. Society of Hospital Medicine. 2016 State of Hospital Medicine Report. Available
at: https://www.hospitalmedicine.org/about/press-releases/shm-releases-
2016-state-of-hospital-medicine-report/. Accessed July 17, 2018.
11. Will KK, Budavari AI, Wilkens JA, Mishari K, Hartsell ZC. A Hospitalist postgraduate
training program for physician assistants. J Hosp Med. 2010;5(2):94-
8. doi: 10.1002/jhm.619. PubMed
12. Naqvi, S. Is it time for Physician Assistant (PA)/Nurse Practitioner (NP) Hospital
Medicine Residency Training. Available at: http://medicine2.missouri.e.,-
du/jahm/wp-content/uploads/2017/03/Is-it-time-for-PANP-Hospital-Medicine-
Residency-Training-Final.pdf. Accessed July 17, 2018.
13. Scheurer D, Cardin T. The Role of NPs and PAs in Hospital Medicine Programs.
From July, 2017 The Hospitalist. Available at: https://www.the-hospitalist.
org/hospitalist/article/142565/leadership-training/role-nps-and-pashospital-
medicine-programs. Accessed July 17, 2018.
14. Furfari K , Rosenthal L, Tad-y D, Wolfe B, Glasheen J. Nurse practitioners as
inpatinet providers: a hospital medicine fellowship program. J Nurse Pract.
2014;10(6):425-429. doi: 10.1016/j.nurpra.2014.03.022.
15. Taylor D, Broyhill B, Burris A, Wilcox M. A strategic approach for developing
an advanced practice workforce: from postgraduate transition-to-practice
fellowship programs and beyond. Nurs Adm Q. 2017;41(1):11-19. doi:
10.1097/NAQ.0000000000000198. PubMed
16. Barnes H. Exploring the factors that influence nurse practitioners role transition.
J Nurse Pract. 2015;11(2):178-183. doi: 10.1016/j.nurpra.2014.11.004. PubMed
17. Hart MA, Macnee LC. How well are nurse practitioners prepared for practice:
results of a 2004 questionnaire study. J Am Acad Nurse Pract. 2007;19(1):35-
42. doi: 10.1111/j.1745-7599.2006.00191.x PubMed
18. Torok H, Lackner C, Landis R, Wright S. Learning needs of physician assistants
working in hospital medicine. J Hosp Med. 2012;7(3):190-194. doi:
10.1002/jhm.1001. PubMed
19. Kisuule F, Howell E. Hospitalists and their impact on quality, patient safety,
and satisfaction. Obstet Gynecol Clin N Am. 2015;42(3):433-446. doi:
10.1016/j.ogc.2015.05.003. PubMed
20. Ford, W, Britting L. Nonphysician Providers in the hospitalist model: a prescription
for change and a warning about unintended side effects. J Hosp
Med. 2010;5(2):99-102. doi: 10.1002/jhm.556. PubMed
There is an increasing utilization of advanced practice providers (APPs) in the delivery of healthcare in the United States.1,2 As of 2016, there were 157, 025 nurse practitioners (NPs) and 102,084 physician assistants (PAs) with a projected growth rate of 6.8% and 4.3%, respectively, which exceeds the physician growth rate of 1.1%.2 This increased growth rate has been attributed to the expectation that APPs can enhance the quality of physician care, relieve physician shortages, and reduce service costs, as APPs are less expensive to hire than physicians.3,4 Hospital medicine is the fastest growing medical field in the United States, and approximately 83% of hospitalist groups around the country utilize APPs; however, the demand for hospitalists continues to exceed the supply, and this has led to increased utilization of APPs in hospital medicine.5-10
APPs receive very limited inpatient training and there is wide variation in their clinical abilities after graduation.11 This is an issue that has become exacerbated in recent years by a change in the training process for PAs. Before 2005, PA programs were typically two to three years long and required the same prerequisite courses as medical schools.11 PA students completed more than 2,000 hours of clinical rotations and then had to pass the Physician Assistant National Certifying Exam before they could practice.12 Traditionally, PA programs typically attracted students with prior healthcare experience.11 In 2005, PA programs began transitioning from bachelor’s degrees to requiring a master’s level degree for completion of the programs. This has shifted the demographics of the students matriculating to younger students with little-to-no prior healthcare experience; moreover, these fresh graduates lack exposure to hospital medicine.11
NPs usually gain clinical experience working as registered nurses (RNs) for two or more years prior to entry into the NP program. NP programs for baccalaureate-prepared RNs vary in length from two to three years.2 There is an acute care focus for NPs in training; however, there is no standardized training or licensure to ensure that hospital medicine competencies are met.13-15 Some studies have shown that a lack of structured support has been found to affect NP role transition negatively during the first year of practice,16 and graduating NPs have indicated that they needed more out of their clinical education in terms of content, clinical experience, and competency testing.17
Hiring new APP graduates as hospitalists requires a longer and more rigorous onboarding process. On‐the‐job training in hospital medicine for new APP graduates can take as long as six to 12 months in order for them to acquire the basic skill set necessary to adequately manage hospitalized patients.15 This extended onboarding is costly because the APPs are receiving a full hospitalist salary, yet they are not functioning at full capacity. Ideally, there should be an intermediary training step between graduation and employment as hospitalist APPs. Studies have shown that APPs are interested in formal postgraduate hospital medicine training, even if it means having a lower stipend during the first year after graduating from their NP or PA program.9,15,18
The growing need for hospitalists, driven by residency work-hour reform, increased age and complexity of patients, and the need to improve the quality of inpatient care while simultaneously reducing waste, has contributed to the increasing utilization of and need for highly qualified APPs in hospital medicine.11,19,20 We established a fellowship to train APPs. The goal of this study was to determine if an APP fellowship is a cost-effective pipeline for filling vacancies within a hospitalist program.
METHODS
Design and Setting
Johns Hopkins Bayview Medical Center (JHBMC) is a 440 bed hospital in Baltimore Maryland. The hospitalist group was started in 1996 with one physician seeing approximately 500 discharges a year. Over the last 20 years, the group has grown and is now its own division with 57 providers, including 42 physicians, 11 APPs, and four APP fellows. The hospitalist division manages ~7,000 discharges a year, which corresponds to approximately 60% of admissions to general medicine. Hospitalist APPs help staff general medicine by working alongside doctors and admitting patients during the day and night. The APPs also staff the pulmonary step down unit with a pulmonary attending and the chemical dependency unit with an internal medicine addiction specialist.
The growth of the division of hospital medicine at JHBMC is a result of increasing volumes and reduced residency duty hours. The increasing full time equivalents (FTEs) resulted in a need for APPs; however, vacancies went unfilled for an average of 35 weeks due to the time it took to post open positions, interview applicants, and hire applicants through the credentialing process. Further, it took as long as 22 to 34 weeks for a new hire to work independently. The APP vacancies and onboarding resulted in increased costs to the division incurred by physician moonlighting to cover open shifts. The hourly physician moonlighting rate at JHBMC is $150. All costs were calculated on the basis of a 40-hour work week. We performed a pre- and postanalysis of outcomes of interest between January 2009 and June 2018. This study was exempt from institutional review board review.
Intervention
In 2014, a one year APP clinical fellowship in hospital medicine was started. The fellows evaluate and manage patients working one-on-one with an experienced hospitalist faculty member. The program consists of 80% clinical experience in the inpatient setting and 20% didactic instruction (Table 1). Up to four fellows are accepted each year and are eligible for hire after training if vacancies exist. The program is cost neutral and was financed by downsizing, through attrition, two physician FTEs. Four APP fellows’ salaries are the equivalent of two entry-level hospitalist physicians’ salaries at JHBMC. The annual salary for an APP fellow is $69,000.
Downsizing by two physician FTEs meant that one less doctor was scheduled every day. The patient load previously seen by that one doctor (10 patients) was absorbed by the MD–APP fellow dyads. Paired with a fellow, each physician sees a higher cap of 13 patients, and it takes six weeks for the fellows to ramp-up to this patient load. When the fellow first starts, the team sees 10 patients. Every two weeks, the pair’s census increases by one patient to the cap of 13. Collectively, the four APP fellow–MD dyads make it possible for four physicians to see an additional 12 patients. The two extra patients absorbed by the service per day results in a net increase in capacity of up to 730 patient encounters a year.
Outcomes and Analysis
Our main outcomes of interest were duration of onboarding and cost incurred by the division to (1) staff the service during a vacancy and (2) onboard new hires. Secondary outcomes included duration of vacancy and total time spent with the group. We collected basic demographic data on participants, including, age, gender, and race. Demographics and outcomes of interest were compared pre- (2009-2013) and post- (2014-2018) initiation of the APP clinical fellowship using the chi-square test, the t-test for normally distributed data, and the Wilcoxon rank-sum for nonnormally distributed data, as appropriate. The normality of the data distribution was tested using the Shapiro-Wilk W test. Two-tailed P values less than .05 were considered to be statistically significant. Results were analyzed using Stata/MP version 13.0 (StataCorp Inc, College Station, Texas).
RESULTS
Twelve fellows have been recruited, and of these, 10 have graduated. Two chose to leave the program prior to completion. Of the 10 fellows that have graduated, six have been hired into our group, one was hired within our facility, and three were hired as hospitalists at other institutions. The median time from APP school graduation to hire was also not different between the two groups (10.5 vs 3.9 months, P = .069). In addition, the total time that the new APP hires spent with the group was nonstatistically significantly different between the two periods (17.9 vs 18.3 months, P = .735). Both the mean duration of onboarding and the cost to the division were significantly reduced after implementation of the program (25.4 vs 11.0 weeks, P = .017 and $361,714 vs $66,000, P = .004; Table 2).
The yearly cost of an APP vacancy and onboarding is incurred by doctor moonlighting costs (at the rate of $150 per hour) to cover open shifts. The mean duration of vacancies and onboarding each year was 34.9 and 25.4 weeks, respectively, before the fellowship. The yearly cost of onboarding, after the establishment of the fellowship, is a maximum of $66,000, derived from physician moonlighting to cover the six-week ramp-up at the very beginning of the fellowship and the five weeks of orientation to the pulmonary and chemical dependency units after the fellowship (Table 3).
DISCUSSION
Our APP clinical fellowship in hospital medicine at JHBMC has produced several benefits. First, the fellowship has become a pipeline for filling APP vacancies within our division. We have been able to hire for four consecutive years from the fellowship. Second, the ready availability of high-functioning and efficient APP hospitalists has cut down on the onboarding time for our new APP hires. Many new APP graduates lack confidence in caring for complex hospitalized patients. Following our 12-month clinical fellowship, our matriculated fellows are able to practice at the top of their license immediately and confidently. Third, the reduced vacancy and shortened onboarding periods have reduced costs to the division. Fourth, the fellowship has created additional teaching avenues for the faculty. The medicine units at JHBMC are comprised of hospitalist and internal medicine residency services. The hospitalists spend the majority of their clinical time in direct patient care; however, they rotate on the residency service for two weeks out of the year. The majority of physicians welcome the chance to teach more, and partnering with an APP fellow provides that opportunity.
As we have developed and grown this program, the one great challenge has been what to do with graduating fellows when we cannot hire them. Fortunately, the market for highly qualified, well trained APPs is strong, and every one of the fellows that we could not hire within our group has been able to find a position either within our facility or outside our institution. To facilitate this process, program directors and recruiters are invited to meet with the fellows toward the end of their fellowship to share employment opportunities with them.
Our study has limitations. First, had the $276,000 from the attrition of two physicians been used to hire nonfellow APPs under the old model, then the costs of the two models would have been similar, but this was simply not possible because the positions could not be filled. Second, this is a single-site experience, and our findings may not be generalizable, particularly those pertaining to remuneration. Third, our study was underpowered to detect small but important differences in characteristics of APPs, especially time from graduation to hire, before and after the implementation of our fellowship. Further research comparing various programs both in structure and outcomes—such as fellows’ readiness for practice, costs, duration of vacancies, and provider satisfaction—are an important next step.
We have developed a pool of applicants within our division to fill vacancies left by turnover from senior NPs and PAs. This program has reduced costs and improved the joy of practice for both doctors and APPs. As the need for highly qualified NPs and PAs in hospital medicine continues to grow, we may see more APP fellowships in hospital medicine in the United States.
Acknowledgments
The authors thank the advanced practice providers who have helped us grow and refine our fellowship.
Disclosures
The authors have nothing to disclose
There is an increasing utilization of advanced practice providers (APPs) in the delivery of healthcare in the United States.1,2 As of 2016, there were 157, 025 nurse practitioners (NPs) and 102,084 physician assistants (PAs) with a projected growth rate of 6.8% and 4.3%, respectively, which exceeds the physician growth rate of 1.1%.2 This increased growth rate has been attributed to the expectation that APPs can enhance the quality of physician care, relieve physician shortages, and reduce service costs, as APPs are less expensive to hire than physicians.3,4 Hospital medicine is the fastest growing medical field in the United States, and approximately 83% of hospitalist groups around the country utilize APPs; however, the demand for hospitalists continues to exceed the supply, and this has led to increased utilization of APPs in hospital medicine.5-10
APPs receive very limited inpatient training and there is wide variation in their clinical abilities after graduation.11 This is an issue that has become exacerbated in recent years by a change in the training process for PAs. Before 2005, PA programs were typically two to three years long and required the same prerequisite courses as medical schools.11 PA students completed more than 2,000 hours of clinical rotations and then had to pass the Physician Assistant National Certifying Exam before they could practice.12 Traditionally, PA programs typically attracted students with prior healthcare experience.11 In 2005, PA programs began transitioning from bachelor’s degrees to requiring a master’s level degree for completion of the programs. This has shifted the demographics of the students matriculating to younger students with little-to-no prior healthcare experience; moreover, these fresh graduates lack exposure to hospital medicine.11
NPs usually gain clinical experience working as registered nurses (RNs) for two or more years prior to entry into the NP program. NP programs for baccalaureate-prepared RNs vary in length from two to three years.2 There is an acute care focus for NPs in training; however, there is no standardized training or licensure to ensure that hospital medicine competencies are met.13-15 Some studies have shown that a lack of structured support has been found to affect NP role transition negatively during the first year of practice,16 and graduating NPs have indicated that they needed more out of their clinical education in terms of content, clinical experience, and competency testing.17
Hiring new APP graduates as hospitalists requires a longer and more rigorous onboarding process. On‐the‐job training in hospital medicine for new APP graduates can take as long as six to 12 months in order for them to acquire the basic skill set necessary to adequately manage hospitalized patients.15 This extended onboarding is costly because the APPs are receiving a full hospitalist salary, yet they are not functioning at full capacity. Ideally, there should be an intermediary training step between graduation and employment as hospitalist APPs. Studies have shown that APPs are interested in formal postgraduate hospital medicine training, even if it means having a lower stipend during the first year after graduating from their NP or PA program.9,15,18
The growing need for hospitalists, driven by residency work-hour reform, increased age and complexity of patients, and the need to improve the quality of inpatient care while simultaneously reducing waste, has contributed to the increasing utilization of and need for highly qualified APPs in hospital medicine.11,19,20 We established a fellowship to train APPs. The goal of this study was to determine if an APP fellowship is a cost-effective pipeline for filling vacancies within a hospitalist program.
METHODS
Design and Setting
Johns Hopkins Bayview Medical Center (JHBMC) is a 440 bed hospital in Baltimore Maryland. The hospitalist group was started in 1996 with one physician seeing approximately 500 discharges a year. Over the last 20 years, the group has grown and is now its own division with 57 providers, including 42 physicians, 11 APPs, and four APP fellows. The hospitalist division manages ~7,000 discharges a year, which corresponds to approximately 60% of admissions to general medicine. Hospitalist APPs help staff general medicine by working alongside doctors and admitting patients during the day and night. The APPs also staff the pulmonary step down unit with a pulmonary attending and the chemical dependency unit with an internal medicine addiction specialist.
The growth of the division of hospital medicine at JHBMC is a result of increasing volumes and reduced residency duty hours. The increasing full time equivalents (FTEs) resulted in a need for APPs; however, vacancies went unfilled for an average of 35 weeks due to the time it took to post open positions, interview applicants, and hire applicants through the credentialing process. Further, it took as long as 22 to 34 weeks for a new hire to work independently. The APP vacancies and onboarding resulted in increased costs to the division incurred by physician moonlighting to cover open shifts. The hourly physician moonlighting rate at JHBMC is $150. All costs were calculated on the basis of a 40-hour work week. We performed a pre- and postanalysis of outcomes of interest between January 2009 and June 2018. This study was exempt from institutional review board review.
Intervention
In 2014, a one year APP clinical fellowship in hospital medicine was started. The fellows evaluate and manage patients working one-on-one with an experienced hospitalist faculty member. The program consists of 80% clinical experience in the inpatient setting and 20% didactic instruction (Table 1). Up to four fellows are accepted each year and are eligible for hire after training if vacancies exist. The program is cost neutral and was financed by downsizing, through attrition, two physician FTEs. Four APP fellows’ salaries are the equivalent of two entry-level hospitalist physicians’ salaries at JHBMC. The annual salary for an APP fellow is $69,000.
Downsizing by two physician FTEs meant that one less doctor was scheduled every day. The patient load previously seen by that one doctor (10 patients) was absorbed by the MD–APP fellow dyads. Paired with a fellow, each physician sees a higher cap of 13 patients, and it takes six weeks for the fellows to ramp-up to this patient load. When the fellow first starts, the team sees 10 patients. Every two weeks, the pair’s census increases by one patient to the cap of 13. Collectively, the four APP fellow–MD dyads make it possible for four physicians to see an additional 12 patients. The two extra patients absorbed by the service per day results in a net increase in capacity of up to 730 patient encounters a year.
Outcomes and Analysis
Our main outcomes of interest were duration of onboarding and cost incurred by the division to (1) staff the service during a vacancy and (2) onboard new hires. Secondary outcomes included duration of vacancy and total time spent with the group. We collected basic demographic data on participants, including, age, gender, and race. Demographics and outcomes of interest were compared pre- (2009-2013) and post- (2014-2018) initiation of the APP clinical fellowship using the chi-square test, the t-test for normally distributed data, and the Wilcoxon rank-sum for nonnormally distributed data, as appropriate. The normality of the data distribution was tested using the Shapiro-Wilk W test. Two-tailed P values less than .05 were considered to be statistically significant. Results were analyzed using Stata/MP version 13.0 (StataCorp Inc, College Station, Texas).
RESULTS
Twelve fellows have been recruited, and of these, 10 have graduated. Two chose to leave the program prior to completion. Of the 10 fellows that have graduated, six have been hired into our group, one was hired within our facility, and three were hired as hospitalists at other institutions. The median time from APP school graduation to hire was also not different between the two groups (10.5 vs 3.9 months, P = .069). In addition, the total time that the new APP hires spent with the group was nonstatistically significantly different between the two periods (17.9 vs 18.3 months, P = .735). Both the mean duration of onboarding and the cost to the division were significantly reduced after implementation of the program (25.4 vs 11.0 weeks, P = .017 and $361,714 vs $66,000, P = .004; Table 2).
The yearly cost of an APP vacancy and onboarding is incurred by doctor moonlighting costs (at the rate of $150 per hour) to cover open shifts. The mean duration of vacancies and onboarding each year was 34.9 and 25.4 weeks, respectively, before the fellowship. The yearly cost of onboarding, after the establishment of the fellowship, is a maximum of $66,000, derived from physician moonlighting to cover the six-week ramp-up at the very beginning of the fellowship and the five weeks of orientation to the pulmonary and chemical dependency units after the fellowship (Table 3).
DISCUSSION
Our APP clinical fellowship in hospital medicine at JHBMC has produced several benefits. First, the fellowship has become a pipeline for filling APP vacancies within our division. We have been able to hire for four consecutive years from the fellowship. Second, the ready availability of high-functioning and efficient APP hospitalists has cut down on the onboarding time for our new APP hires. Many new APP graduates lack confidence in caring for complex hospitalized patients. Following our 12-month clinical fellowship, our matriculated fellows are able to practice at the top of their license immediately and confidently. Third, the reduced vacancy and shortened onboarding periods have reduced costs to the division. Fourth, the fellowship has created additional teaching avenues for the faculty. The medicine units at JHBMC are comprised of hospitalist and internal medicine residency services. The hospitalists spend the majority of their clinical time in direct patient care; however, they rotate on the residency service for two weeks out of the year. The majority of physicians welcome the chance to teach more, and partnering with an APP fellow provides that opportunity.
As we have developed and grown this program, the one great challenge has been what to do with graduating fellows when we cannot hire them. Fortunately, the market for highly qualified, well trained APPs is strong, and every one of the fellows that we could not hire within our group has been able to find a position either within our facility or outside our institution. To facilitate this process, program directors and recruiters are invited to meet with the fellows toward the end of their fellowship to share employment opportunities with them.
Our study has limitations. First, had the $276,000 from the attrition of two physicians been used to hire nonfellow APPs under the old model, then the costs of the two models would have been similar, but this was simply not possible because the positions could not be filled. Second, this is a single-site experience, and our findings may not be generalizable, particularly those pertaining to remuneration. Third, our study was underpowered to detect small but important differences in characteristics of APPs, especially time from graduation to hire, before and after the implementation of our fellowship. Further research comparing various programs both in structure and outcomes—such as fellows’ readiness for practice, costs, duration of vacancies, and provider satisfaction—are an important next step.
We have developed a pool of applicants within our division to fill vacancies left by turnover from senior NPs and PAs. This program has reduced costs and improved the joy of practice for both doctors and APPs. As the need for highly qualified NPs and PAs in hospital medicine continues to grow, we may see more APP fellowships in hospital medicine in the United States.
Acknowledgments
The authors thank the advanced practice providers who have helped us grow and refine our fellowship.
Disclosures
The authors have nothing to disclose
1. Martsoff G, Nguyen P, Freund D, Poghosyan L. What we know about postgraduate nurse practitioner residency and fellowship programs. J Nurse Pract. 2017;13(7):482-487. doi: 10.1016/j.nurpra.2017.05.013.
2. Auerbach D, Staiger D, Buerhaus P. Growing ranks of advanced practice clinicians-implications for the physician workforce. N Engl J Med. 2018;378(25):2358-2360. doi: 10.1056/NEJMp1801869. PubMed
3. Laurant M, Harmsen M, Wollersheim H, Grol R, Faber M, Sibbald B. The
impact of nonphysician clinicians: do they improve the quality and cost-effectiveness
of health care services? Med Care Res Rev. 2009;66(6 Suppl):36S-89S. doi: 10.1177/1077558709346277. PubMed
4. Auerbach DI. Will the NP workforce grow in the future? New forecasts and
implications for healthcare delivery. Med Care. 2012;50(7):606-610. doi:
10.1097/MLR.0b013e318249d6e7. PubMed
5. Kisuule F, Howell E. Hospital medicine beyond the United States. Int J Gen
Med. 2018;11:65-71. doi: 10.2147/IJGM.S151275. PubMed
6. Wachter RM, Goldman L. Zero to 50, 000-The 20th anniversary of the hospitalist.
N Engl J Med. 2016;375(11):1009-1011. doi: 10.1056/NEJMp1607958. PubMed
7. Conrad, K and Valovska T. The current state of hospital medicine: trends in
compensation, practice patterns, advanced practice providers, malpractice,
and career satisfaction. In: Conrad K, ed. Clinical Approaches to Hospital
Medicine. Cham, Springer; 2017:259-270.
8. Bryant SE. Filling the gaps: preparing nurse practitioners for hospitalist
practice. J Am Assoc Nurse Pract. 2018;30(1):4-9. doi: 10.1097/
JXX.0000000000000008. PubMed
9. Sharma P, Brooks M, Roomiany P, Verma L, Criscione-Schreiber, L. Physician
assistant student training for the inpatient setting: a needs assessment. J Physician
Assist Educ. 2017;28(4):189-195. doi: 10.1097/JPA.0000000000000174. PubMed
10. Society of Hospital Medicine. 2016 State of Hospital Medicine Report. Available
at: https://www.hospitalmedicine.org/about/press-releases/shm-releases-
2016-state-of-hospital-medicine-report/. Accessed July 17, 2018.
11. Will KK, Budavari AI, Wilkens JA, Mishari K, Hartsell ZC. A Hospitalist postgraduate
training program for physician assistants. J Hosp Med. 2010;5(2):94-
8. doi: 10.1002/jhm.619. PubMed
12. Naqvi, S. Is it time for Physician Assistant (PA)/Nurse Practitioner (NP) Hospital
Medicine Residency Training. Available at: http://medicine2.missouri.e.,-
du/jahm/wp-content/uploads/2017/03/Is-it-time-for-PANP-Hospital-Medicine-
Residency-Training-Final.pdf. Accessed July 17, 2018.
13. Scheurer D, Cardin T. The Role of NPs and PAs in Hospital Medicine Programs.
From July, 2017 The Hospitalist. Available at: https://www.the-hospitalist.
org/hospitalist/article/142565/leadership-training/role-nps-and-pashospital-
medicine-programs. Accessed July 17, 2018.
14. Furfari K , Rosenthal L, Tad-y D, Wolfe B, Glasheen J. Nurse practitioners as
inpatinet providers: a hospital medicine fellowship program. J Nurse Pract.
2014;10(6):425-429. doi: 10.1016/j.nurpra.2014.03.022.
15. Taylor D, Broyhill B, Burris A, Wilcox M. A strategic approach for developing
an advanced practice workforce: from postgraduate transition-to-practice
fellowship programs and beyond. Nurs Adm Q. 2017;41(1):11-19. doi:
10.1097/NAQ.0000000000000198. PubMed
16. Barnes H. Exploring the factors that influence nurse practitioners role transition.
J Nurse Pract. 2015;11(2):178-183. doi: 10.1016/j.nurpra.2014.11.004. PubMed
17. Hart MA, Macnee LC. How well are nurse practitioners prepared for practice:
results of a 2004 questionnaire study. J Am Acad Nurse Pract. 2007;19(1):35-
42. doi: 10.1111/j.1745-7599.2006.00191.x PubMed
18. Torok H, Lackner C, Landis R, Wright S. Learning needs of physician assistants
working in hospital medicine. J Hosp Med. 2012;7(3):190-194. doi:
10.1002/jhm.1001. PubMed
19. Kisuule F, Howell E. Hospitalists and their impact on quality, patient safety,
and satisfaction. Obstet Gynecol Clin N Am. 2015;42(3):433-446. doi:
10.1016/j.ogc.2015.05.003. PubMed
20. Ford, W, Britting L. Nonphysician Providers in the hospitalist model: a prescription
for change and a warning about unintended side effects. J Hosp
Med. 2010;5(2):99-102. doi: 10.1002/jhm.556. PubMed
1. Martsoff G, Nguyen P, Freund D, Poghosyan L. What we know about postgraduate nurse practitioner residency and fellowship programs. J Nurse Pract. 2017;13(7):482-487. doi: 10.1016/j.nurpra.2017.05.013.
2. Auerbach D, Staiger D, Buerhaus P. Growing ranks of advanced practice clinicians-implications for the physician workforce. N Engl J Med. 2018;378(25):2358-2360. doi: 10.1056/NEJMp1801869. PubMed
3. Laurant M, Harmsen M, Wollersheim H, Grol R, Faber M, Sibbald B. The
impact of nonphysician clinicians: do they improve the quality and cost-effectiveness
of health care services? Med Care Res Rev. 2009;66(6 Suppl):36S-89S. doi: 10.1177/1077558709346277. PubMed
4. Auerbach DI. Will the NP workforce grow in the future? New forecasts and
implications for healthcare delivery. Med Care. 2012;50(7):606-610. doi:
10.1097/MLR.0b013e318249d6e7. PubMed
5. Kisuule F, Howell E. Hospital medicine beyond the United States. Int J Gen
Med. 2018;11:65-71. doi: 10.2147/IJGM.S151275. PubMed
6. Wachter RM, Goldman L. Zero to 50, 000-The 20th anniversary of the hospitalist.
N Engl J Med. 2016;375(11):1009-1011. doi: 10.1056/NEJMp1607958. PubMed
7. Conrad, K and Valovska T. The current state of hospital medicine: trends in
compensation, practice patterns, advanced practice providers, malpractice,
and career satisfaction. In: Conrad K, ed. Clinical Approaches to Hospital
Medicine. Cham, Springer; 2017:259-270.
8. Bryant SE. Filling the gaps: preparing nurse practitioners for hospitalist
practice. J Am Assoc Nurse Pract. 2018;30(1):4-9. doi: 10.1097/
JXX.0000000000000008. PubMed
9. Sharma P, Brooks M, Roomiany P, Verma L, Criscione-Schreiber, L. Physician
assistant student training for the inpatient setting: a needs assessment. J Physician
Assist Educ. 2017;28(4):189-195. doi: 10.1097/JPA.0000000000000174. PubMed
10. Society of Hospital Medicine. 2016 State of Hospital Medicine Report. Available
at: https://www.hospitalmedicine.org/about/press-releases/shm-releases-
2016-state-of-hospital-medicine-report/. Accessed July 17, 2018.
11. Will KK, Budavari AI, Wilkens JA, Mishari K, Hartsell ZC. A Hospitalist postgraduate
training program for physician assistants. J Hosp Med. 2010;5(2):94-
8. doi: 10.1002/jhm.619. PubMed
12. Naqvi, S. Is it time for Physician Assistant (PA)/Nurse Practitioner (NP) Hospital
Medicine Residency Training. Available at: http://medicine2.missouri.e.,-
du/jahm/wp-content/uploads/2017/03/Is-it-time-for-PANP-Hospital-Medicine-
Residency-Training-Final.pdf. Accessed July 17, 2018.
13. Scheurer D, Cardin T. The Role of NPs and PAs in Hospital Medicine Programs.
From July, 2017 The Hospitalist. Available at: https://www.the-hospitalist.
org/hospitalist/article/142565/leadership-training/role-nps-and-pashospital-
medicine-programs. Accessed July 17, 2018.
14. Furfari K , Rosenthal L, Tad-y D, Wolfe B, Glasheen J. Nurse practitioners as
inpatinet providers: a hospital medicine fellowship program. J Nurse Pract.
2014;10(6):425-429. doi: 10.1016/j.nurpra.2014.03.022.
15. Taylor D, Broyhill B, Burris A, Wilcox M. A strategic approach for developing
an advanced practice workforce: from postgraduate transition-to-practice
fellowship programs and beyond. Nurs Adm Q. 2017;41(1):11-19. doi:
10.1097/NAQ.0000000000000198. PubMed
16. Barnes H. Exploring the factors that influence nurse practitioners role transition.
J Nurse Pract. 2015;11(2):178-183. doi: 10.1016/j.nurpra.2014.11.004. PubMed
17. Hart MA, Macnee LC. How well are nurse practitioners prepared for practice:
results of a 2004 questionnaire study. J Am Acad Nurse Pract. 2007;19(1):35-
42. doi: 10.1111/j.1745-7599.2006.00191.x PubMed
18. Torok H, Lackner C, Landis R, Wright S. Learning needs of physician assistants
working in hospital medicine. J Hosp Med. 2012;7(3):190-194. doi:
10.1002/jhm.1001. PubMed
19. Kisuule F, Howell E. Hospitalists and their impact on quality, patient safety,
and satisfaction. Obstet Gynecol Clin N Am. 2015;42(3):433-446. doi:
10.1016/j.ogc.2015.05.003. PubMed
20. Ford, W, Britting L. Nonphysician Providers in the hospitalist model: a prescription
for change and a warning about unintended side effects. J Hosp
Med. 2010;5(2):99-102. doi: 10.1002/jhm.556. PubMed
© 2019 Society of Hospital Medicine
Things We Do For No Reason: Use of Antipsychotic Medications in Patients with Delirium
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No ReasonTM” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNRTM series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CASE
An 86-year-old woman with mild dementia falls at home while preparing a meal. Her son brings her to the emergency department for excruciating pain in her right hip. X-rays reveal a fractured right femur that requires open reduction and internal fixation. On the first postoperative day, she does not participate in therapy and sleeps most of the day. Overnight, a nurse observes her calmly speaking to a hallucination of a family member in the room and picking at the tape around her peripheral intravenous catheter (PIV) causing the PIV to fall out twice. Her vital signs are temperature 36.7°C, pulse 82 beats per minute, respirations 12 breaths per minute, blood pressure 143/72 mm Hg, and pulse oximetry of 99% on room air. She is hypoactive, distractedly picks at her clothing and PIV, inattentive, and unable to say the day of the week or count months backward. Nursing asks for haloperidol for her delirium.
WHY YOU MIGHT THINK ANTIPSYCHOTICS FOR DELIRIUM ARE HELPFUL
Delirium is an acute change in cognition characterized by inattention typically associated with disorganized thinking and/or alteration in consciousness.1 Delirium occurs in almost 25% of hospitalized patients, and clinicians have a limited pharmacologic armamentarium to treat it, given the absence of benefit for acetylcholinesterase inhibitors and concern that benzodiazepine medications cause/exacerbate delirium.2-4 Another treatment option is antipsychotic medications which block dopamine since dopamine excess is a key element in the neurotransmitter pathophysiology of delirium.5 A small 2005 trial of haloperidol prophylaxis in hip fracture patients found that haloperidol reduced the overall severity and duration of delirium.6 Based in part on this trial, a 2007 Cochrane Systematic Review concluded that antipsychotics “may reduce severity and duration of delirium episodes and shorten length of hospital stay in hip surgery.”7 Another study in 2010 demonstrated a 55% faster decline in total Delirium Rating Scale-Revised 98 (DRS-R-98) scores in patients on a general/medical-surgical floor receiving quetiapine treatment compared to those who received placebo.8
Studies show that 10%-30% of patients receive antipsychotics at some point during their hospitalization, usually for delirium.9,10 Variability in antipsychotic prescribing patterns not explained by patient characteristics suggests the local culture may influence antipsychotic prescribing practices when evidence from randomized controlled trials is sparse or conflicting.10
WHY ANTIPSYCHOTIC MEDICATIONS ARE NOT HELPFUL IN PATIENTS WITH DELIRIUM
While few studies have demonstrated positive effects of antipsychotics in delirium treatment, the overall evidence is not persuasive. The results of some studies have not been reproduced while only the positive effects rather than the adverse side effects of antipsychotic medications were highlighted in other articles. For instance, the 2005 hip fracture delirium prophylaxis trial found there was no difference in the incidence of delirium in patients on postoperative day one.7 Furthermore, the 2010 quetiapine study was underpowered for the primary outcome of lower DRS-R-98 scores. Importantly, there was no significant difference in severity of delirium between treatment (quetiapine) and placebo groups on days one, three, or 10.10 These studies show that antipsychotics were neither effective at preventing delirium or in reducing its severity compared to placebo. In 2016, a systemic review in the Journal of the American Geriatric Society included both of the above studies in addition to 17 other studies to assess the efficacy of antipsychotics in preventing and treating delirium. This analysis concluded that antipsychotics did not change the length of delirium or length of stay.11 In addition, the absence of convincing evidence of antipsychotics benefits in postoperative delirium has led the American Geriatrics Society to recommend: “The prescribing practitioner should not prescribe antipsychotic… medications for the treatment of older adults with postoperative delirium who are not agitated and threatening substantial harm to self or others.”12
There is a paucity of data speaking directly to whether antipsychotics reduce patient distress. A recent randomized controlled study compared haloperidol, risperidone, and placebo for delirium treatment in palliative care and hospice patients. With treatment, the patients in the antipsychotic arms demonstrated slightly more severe delirium and a significantly higher incidence of extrapyramidal symptoms (EPS) than the patients receiving placebo.13
Side effects such as EPS, aspiration pneumonia, and arrhythmia are concerns when using antipsychotics for delirium treatment.14 A systematic review and meta-analysis found the difference in EPS incidence between patients treated for delirium with antipsychotics versus no intervention ranged from no difference to over 10%.11 In addition to EPS, patients receiving antipsychotics in a cohort study were at increased risk for aspiration pneumonia compared to patients who did not receive antipsychotics (adjusted odds ratio = 1.5, 95% CI, 1.2-1.9).15 These serious side effects led the Food and Drug Administration (FDA) to issue a black box warning for antipsychotic treatment in dementia-related psychosis. Most importantly, the FDA warns that there is an increased risk of death.16
WHAT YOU SHOULD DO INSTEAD OF USING ANTIPSYCHOTICS
In the first line management of delirium, hospitalists should address underlying modifiable contributions to the condition with attention to medications, pain, electrolytes, ischemia, infection, alcohol withdrawal, and reducing invasive lines. For example, two studies demonstrated a decrease in delirium severity and duration of palliative care in patients by treating delirium triggers, such as dehydration, electrolyte abnormalities, or infection, rather than using antipsychotics.13,17 Furthermore, hospitalists should review the medication list carefully and look for opportunities to deprescribe sedative/hypnotics and anticholinergics.
In addition, hospitalists should implement the core elements of the nursing delirium protocol from the Hospital Elder Life Program (http://www.hospitalelderlifeprogram.org/). The program focuses on orientation, hydration, mobility, sensory aids, and an environment conducive to sleep.18 When not representing an acute threat to the patient or staff, hospitalists should manage transient agitation from blood draws or vital sign checks by having staff members deescalate and re-approach the intervention later. While multicomponent nonpharmacologic interventions have more robust evidence for prevention of delirium than for treatment, they are low risk and still recommended for the patient with established delirium.19,20
A delirious patient picking at PIVs should prompt clinicians to re-evaluate the need for continued PIV access. If still necessary, experience suggests that PIVs can be protected with a combination of well-taped gauze extending from wrist to shoulder with any attached tubing exiting out of reach behind the shoulder. Also “beneficial distraction” with a task or “activity vest” that consists of an apron with zips, ties, and buttons designed to provide harmless objects can occupy the patient’s hands.
WHEN IT IS HELPFUL TO USE ANTIPSYCHOTICS FOR DELIRIUM
The literature does not provide clear evidence for when the use of antipsychotics is warranted. Antipsychotics may have a role for patients who are having severe psychotic symptoms posing an acute safety risk. In those situations, the American Geriatrics Society recommends using the “lowest effective dose for the shortest possible duration to treat patients who are severely agitated or distressed, and are threatening substantial harm to self and/or others…only if behavioral interventions have failed or are not possible.”12 In those patients who are having an acute myocardial infarction, consider atypical antipsychotics since haloperidol carries a small increased risk of mortality in that patient population.21
RECOMMENDATIONS
- Address underlying modifiable contributions to the delirium paying attention to medications, pain, electrolytes, ischemia, infection, alcohol withdrawal, and reducing invasive lines. Deprescribe sedative/hypnotic and anticholinergic medications.
- After addressing modifiable risk factors, attempt behavioral interventions for continuous problematic behaviors or symptoms of delirium.
- Reserve antipsychotics for cases where the patient poses an immediate danger of self-harm or harm to others. Treat for the shortest possible duration with the lowest effective dose of antipsychotic.
CONCLUSION
Returning to our case presentation, the hospitalist should not prescribe antipsychotic medications since there is no immediate risk of harm and antipsychotics do not treat hypoactive delirium. Delirium is a complex condition requiring a review of multifactorial causes. The hospitalist should investigate and address modifiable contributions. Furthermore, the hospitalist can make the PIV less accessible to deter the patient’s efforts to remove it and offer a distracting activity. Resolution of delirium, in all its forms, is still best achieved by treating the underlying etiology. The use of antipsychotics for treatment of patients with delirium in the absence of severe agitation
Do you think this is a low-value practice? Is this truly a “Thing We Do for No ReasonTM?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No ReasonTM” topics by emailing TWDFNR@hospitalmedicine.org.
Disclosures
Dr. Pahwa has received compensation for Expert Testimony, royalties from Aquifer, and owns stock/stock options in Pfizer and Aetna outside the submitted work. Dr. Qureshi and Dr. Cumbler have nothing to disclose.
1. American Psychiatric Association. DSM-5 Task Force. Diagnostic and statistical manual of mental disorders: DSM-5. 5th ed. Washington, D.C.: American Psychiatric Association; 2013.
2. Lonergan E, Luxenberg J, Areosa Sastre A. Benzodiazepines for delirium. Cochrane Database Syst Rev. 2009;(4):CD006379. https://doi.org/10.1002/14651858.CD006379.pub2.
3. Overshott R, Karim S, Burns A. Cholinesterase inhibitors for delirium. Cochrane Database Syst Rev. 2008;(1):CD005317. https://doi.org/10.1002/14651858.CD005317.pub2.
4. Zaal IJ, Devlin JW, Hazelbag M, et al. Benzodiazepine-associated delirium in critically ill adults. Intensive Care Med. 2015;41(12):2130-2137. https://doi.org/10.1007/s00134-015-4063-z.
5. Maldonado JR. Neuropathogenesis of delirium: review of current etiologic theories and common pathways. Am J Geriatr Psychiatry. 2013;21(12):1190-1222. https://doi.org/10.1016/j.jagp.2013.09.005.
6. Kalisvaart KJ, de Jonghe JF, Bogaards MJ, et al. Haloperidol prophylaxis for elderly hip-surgery patients at risk for delirium: a randomized placebo-controlled study. J Am Geriatr Soc. 2005;53(10):1658-1666. https://doi.org/10.1111/j.1532-5415.2005.53503.x
7. Siddiqi N, Stockdale R, Britton AM, Holmes J. Interventions for preventing delirium in hospitalized patients. Cochrane Database Syst Rev. 2007;(2):CD005563. https://doi.org/10.1002/14651858.CD005563.pub2
8. Tahir TA, Eeles E, Karapareddy V, et al. A randomized controlled trial of quetiapine versus placebo in the treatment of delirium. J Psychosom Res. 2010;69(5):485-490. https://doi.org/10.1016/j.jpsychores.2010.05.006.
9. Loh KP, Ramdass S, Garb JL, Brennan MJ, Lindenauer PK, Lagu T. From hospital to community: use of antipsychotics in hospitalized elders. J Hosp Med. 2014;9(12):802-804. https://doi.org/10.1002/jhm.2277.
10. Herzig SJ, Rothberg MB, Guess JR, Gurwitz JH, Marcantonio ER. Antipsychotic medication utilization in nonpsychiatric hospitalizations. J Hosp Med. 2016;11(8):543-549. https://doi.org/10.1002/jhm.2596.
11. Neufeld KJ, Yue J, Robinson TN, Inouye SK, Needham DM. Antipsychotic medication for prevention and treatment of delirium in hospitalized adults: a systematic review and meta-analysis. J Am Geriatr Soc. 2016;64(4):705-714. https://doi.org/10.1111/jgs.14076.
12. American Geriatrics Society Expert Panel on Postoperative Delirium in Older A. American Geriatrics Society abstracted clinical practice guideline for postoperative delirium in older adults. J Am Geriatr Soc. 2015;63(1):142-150. https://doi.org/10.1111/jgs.13281
13. Agar MR, Lawlor PG, Quinn S, et al. Efficacy of oral risperidone, haloperidol, or placebo for symptoms of delirium among patients in palliative care: a randomized clinical trial. JAMA Intern Med. 2017;177(1):34-42. https://doi.org/10.1001/jamainternmed.2016.7491.
14. Hatta K, Kishi Y, Wada K, et al. Antipsychotics for delirium in the general hospital setting in consecutive 2453 inpatients: a prospective observational study. Int J Geriatr Psychiatry. 2014;29(3):253-262. https://doi.org/10.1002/gps.3999.
15. Herzig SJ, LaSalvia MT, Naidus E, et al. Antipsychotics and the risk of aspiration pneumonia in individuals hospitalized for nonpsychiatric conditions: a cohort study. J Am Geriatr Soc. 2017;65(12):2580-2586. https://doi.org/10.1111/jgs.15066.
16. Jeste DV, Blazer D, Casey D, et al. ACNP White Paper: update on use of antipsychotic drugs in elderly persons with dementia. Neuropsychopharmacology. 2008;33(5):957-970. https://doi.org/10.1038/sj.npp.1301492
17. Hui D, Frisbee-Hume S, Wilson A, et al. Effect of lorazepam with haloperidol vs haloperidol alone on agitated delirium in patients with advanced cancer receiving palliative care: a randomized clinical trial. JAMA. 2017;318(11):1047-1056. https://doi.org/10.1001/jama.2017.11468.
18. Inouye SK, Bogardus ST, Jr., Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340(9):669-676. https://doi.org/10.1056/NEJM199903043400901
19. Milisen K, Lemiengre J, Braes T, Foreman MD. Multicomponent intervention strategies for managing delirium in hospitalized older people: systematic review. J Adv Nurs. 2005;52(1):79-90. https://doi.org/10.1111/j.1365-2648.2005.03557.x
20. Marcantonio ER. Delirium in hospitalized older adults. N Engl J Med. 2017;377(15):1456-1466. https://doi.org/10.1056/NEJMcp1605501.
21. Park Y, Bateman BT, Kim DH, et al. Use of haloperidol versus atypical antipsychotics and risk of in-hospital death in patients with acute myocardial infarction: cohort study. BMJ. 2018;360. https://doi.org/10.1136/bmj.k1218.
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No ReasonTM” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNRTM series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CASE
An 86-year-old woman with mild dementia falls at home while preparing a meal. Her son brings her to the emergency department for excruciating pain in her right hip. X-rays reveal a fractured right femur that requires open reduction and internal fixation. On the first postoperative day, she does not participate in therapy and sleeps most of the day. Overnight, a nurse observes her calmly speaking to a hallucination of a family member in the room and picking at the tape around her peripheral intravenous catheter (PIV) causing the PIV to fall out twice. Her vital signs are temperature 36.7°C, pulse 82 beats per minute, respirations 12 breaths per minute, blood pressure 143/72 mm Hg, and pulse oximetry of 99% on room air. She is hypoactive, distractedly picks at her clothing and PIV, inattentive, and unable to say the day of the week or count months backward. Nursing asks for haloperidol for her delirium.
WHY YOU MIGHT THINK ANTIPSYCHOTICS FOR DELIRIUM ARE HELPFUL
Delirium is an acute change in cognition characterized by inattention typically associated with disorganized thinking and/or alteration in consciousness.1 Delirium occurs in almost 25% of hospitalized patients, and clinicians have a limited pharmacologic armamentarium to treat it, given the absence of benefit for acetylcholinesterase inhibitors and concern that benzodiazepine medications cause/exacerbate delirium.2-4 Another treatment option is antipsychotic medications which block dopamine since dopamine excess is a key element in the neurotransmitter pathophysiology of delirium.5 A small 2005 trial of haloperidol prophylaxis in hip fracture patients found that haloperidol reduced the overall severity and duration of delirium.6 Based in part on this trial, a 2007 Cochrane Systematic Review concluded that antipsychotics “may reduce severity and duration of delirium episodes and shorten length of hospital stay in hip surgery.”7 Another study in 2010 demonstrated a 55% faster decline in total Delirium Rating Scale-Revised 98 (DRS-R-98) scores in patients on a general/medical-surgical floor receiving quetiapine treatment compared to those who received placebo.8
Studies show that 10%-30% of patients receive antipsychotics at some point during their hospitalization, usually for delirium.9,10 Variability in antipsychotic prescribing patterns not explained by patient characteristics suggests the local culture may influence antipsychotic prescribing practices when evidence from randomized controlled trials is sparse or conflicting.10
WHY ANTIPSYCHOTIC MEDICATIONS ARE NOT HELPFUL IN PATIENTS WITH DELIRIUM
While few studies have demonstrated positive effects of antipsychotics in delirium treatment, the overall evidence is not persuasive. The results of some studies have not been reproduced while only the positive effects rather than the adverse side effects of antipsychotic medications were highlighted in other articles. For instance, the 2005 hip fracture delirium prophylaxis trial found there was no difference in the incidence of delirium in patients on postoperative day one.7 Furthermore, the 2010 quetiapine study was underpowered for the primary outcome of lower DRS-R-98 scores. Importantly, there was no significant difference in severity of delirium between treatment (quetiapine) and placebo groups on days one, three, or 10.10 These studies show that antipsychotics were neither effective at preventing delirium or in reducing its severity compared to placebo. In 2016, a systemic review in the Journal of the American Geriatric Society included both of the above studies in addition to 17 other studies to assess the efficacy of antipsychotics in preventing and treating delirium. This analysis concluded that antipsychotics did not change the length of delirium or length of stay.11 In addition, the absence of convincing evidence of antipsychotics benefits in postoperative delirium has led the American Geriatrics Society to recommend: “The prescribing practitioner should not prescribe antipsychotic… medications for the treatment of older adults with postoperative delirium who are not agitated and threatening substantial harm to self or others.”12
There is a paucity of data speaking directly to whether antipsychotics reduce patient distress. A recent randomized controlled study compared haloperidol, risperidone, and placebo for delirium treatment in palliative care and hospice patients. With treatment, the patients in the antipsychotic arms demonstrated slightly more severe delirium and a significantly higher incidence of extrapyramidal symptoms (EPS) than the patients receiving placebo.13
Side effects such as EPS, aspiration pneumonia, and arrhythmia are concerns when using antipsychotics for delirium treatment.14 A systematic review and meta-analysis found the difference in EPS incidence between patients treated for delirium with antipsychotics versus no intervention ranged from no difference to over 10%.11 In addition to EPS, patients receiving antipsychotics in a cohort study were at increased risk for aspiration pneumonia compared to patients who did not receive antipsychotics (adjusted odds ratio = 1.5, 95% CI, 1.2-1.9).15 These serious side effects led the Food and Drug Administration (FDA) to issue a black box warning for antipsychotic treatment in dementia-related psychosis. Most importantly, the FDA warns that there is an increased risk of death.16
WHAT YOU SHOULD DO INSTEAD OF USING ANTIPSYCHOTICS
In the first line management of delirium, hospitalists should address underlying modifiable contributions to the condition with attention to medications, pain, electrolytes, ischemia, infection, alcohol withdrawal, and reducing invasive lines. For example, two studies demonstrated a decrease in delirium severity and duration of palliative care in patients by treating delirium triggers, such as dehydration, electrolyte abnormalities, or infection, rather than using antipsychotics.13,17 Furthermore, hospitalists should review the medication list carefully and look for opportunities to deprescribe sedative/hypnotics and anticholinergics.
In addition, hospitalists should implement the core elements of the nursing delirium protocol from the Hospital Elder Life Program (http://www.hospitalelderlifeprogram.org/). The program focuses on orientation, hydration, mobility, sensory aids, and an environment conducive to sleep.18 When not representing an acute threat to the patient or staff, hospitalists should manage transient agitation from blood draws or vital sign checks by having staff members deescalate and re-approach the intervention later. While multicomponent nonpharmacologic interventions have more robust evidence for prevention of delirium than for treatment, they are low risk and still recommended for the patient with established delirium.19,20
A delirious patient picking at PIVs should prompt clinicians to re-evaluate the need for continued PIV access. If still necessary, experience suggests that PIVs can be protected with a combination of well-taped gauze extending from wrist to shoulder with any attached tubing exiting out of reach behind the shoulder. Also “beneficial distraction” with a task or “activity vest” that consists of an apron with zips, ties, and buttons designed to provide harmless objects can occupy the patient’s hands.
WHEN IT IS HELPFUL TO USE ANTIPSYCHOTICS FOR DELIRIUM
The literature does not provide clear evidence for when the use of antipsychotics is warranted. Antipsychotics may have a role for patients who are having severe psychotic symptoms posing an acute safety risk. In those situations, the American Geriatrics Society recommends using the “lowest effective dose for the shortest possible duration to treat patients who are severely agitated or distressed, and are threatening substantial harm to self and/or others…only if behavioral interventions have failed or are not possible.”12 In those patients who are having an acute myocardial infarction, consider atypical antipsychotics since haloperidol carries a small increased risk of mortality in that patient population.21
RECOMMENDATIONS
- Address underlying modifiable contributions to the delirium paying attention to medications, pain, electrolytes, ischemia, infection, alcohol withdrawal, and reducing invasive lines. Deprescribe sedative/hypnotic and anticholinergic medications.
- After addressing modifiable risk factors, attempt behavioral interventions for continuous problematic behaviors or symptoms of delirium.
- Reserve antipsychotics for cases where the patient poses an immediate danger of self-harm or harm to others. Treat for the shortest possible duration with the lowest effective dose of antipsychotic.
CONCLUSION
Returning to our case presentation, the hospitalist should not prescribe antipsychotic medications since there is no immediate risk of harm and antipsychotics do not treat hypoactive delirium. Delirium is a complex condition requiring a review of multifactorial causes. The hospitalist should investigate and address modifiable contributions. Furthermore, the hospitalist can make the PIV less accessible to deter the patient’s efforts to remove it and offer a distracting activity. Resolution of delirium, in all its forms, is still best achieved by treating the underlying etiology. The use of antipsychotics for treatment of patients with delirium in the absence of severe agitation
Do you think this is a low-value practice? Is this truly a “Thing We Do for No ReasonTM?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No ReasonTM” topics by emailing TWDFNR@hospitalmedicine.org.
Disclosures
Dr. Pahwa has received compensation for Expert Testimony, royalties from Aquifer, and owns stock/stock options in Pfizer and Aetna outside the submitted work. Dr. Qureshi and Dr. Cumbler have nothing to disclose.
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No ReasonTM” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNRTM series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CASE
An 86-year-old woman with mild dementia falls at home while preparing a meal. Her son brings her to the emergency department for excruciating pain in her right hip. X-rays reveal a fractured right femur that requires open reduction and internal fixation. On the first postoperative day, she does not participate in therapy and sleeps most of the day. Overnight, a nurse observes her calmly speaking to a hallucination of a family member in the room and picking at the tape around her peripheral intravenous catheter (PIV) causing the PIV to fall out twice. Her vital signs are temperature 36.7°C, pulse 82 beats per minute, respirations 12 breaths per minute, blood pressure 143/72 mm Hg, and pulse oximetry of 99% on room air. She is hypoactive, distractedly picks at her clothing and PIV, inattentive, and unable to say the day of the week or count months backward. Nursing asks for haloperidol for her delirium.
WHY YOU MIGHT THINK ANTIPSYCHOTICS FOR DELIRIUM ARE HELPFUL
Delirium is an acute change in cognition characterized by inattention typically associated with disorganized thinking and/or alteration in consciousness.1 Delirium occurs in almost 25% of hospitalized patients, and clinicians have a limited pharmacologic armamentarium to treat it, given the absence of benefit for acetylcholinesterase inhibitors and concern that benzodiazepine medications cause/exacerbate delirium.2-4 Another treatment option is antipsychotic medications which block dopamine since dopamine excess is a key element in the neurotransmitter pathophysiology of delirium.5 A small 2005 trial of haloperidol prophylaxis in hip fracture patients found that haloperidol reduced the overall severity and duration of delirium.6 Based in part on this trial, a 2007 Cochrane Systematic Review concluded that antipsychotics “may reduce severity and duration of delirium episodes and shorten length of hospital stay in hip surgery.”7 Another study in 2010 demonstrated a 55% faster decline in total Delirium Rating Scale-Revised 98 (DRS-R-98) scores in patients on a general/medical-surgical floor receiving quetiapine treatment compared to those who received placebo.8
Studies show that 10%-30% of patients receive antipsychotics at some point during their hospitalization, usually for delirium.9,10 Variability in antipsychotic prescribing patterns not explained by patient characteristics suggests the local culture may influence antipsychotic prescribing practices when evidence from randomized controlled trials is sparse or conflicting.10
WHY ANTIPSYCHOTIC MEDICATIONS ARE NOT HELPFUL IN PATIENTS WITH DELIRIUM
While few studies have demonstrated positive effects of antipsychotics in delirium treatment, the overall evidence is not persuasive. The results of some studies have not been reproduced while only the positive effects rather than the adverse side effects of antipsychotic medications were highlighted in other articles. For instance, the 2005 hip fracture delirium prophylaxis trial found there was no difference in the incidence of delirium in patients on postoperative day one.7 Furthermore, the 2010 quetiapine study was underpowered for the primary outcome of lower DRS-R-98 scores. Importantly, there was no significant difference in severity of delirium between treatment (quetiapine) and placebo groups on days one, three, or 10.10 These studies show that antipsychotics were neither effective at preventing delirium or in reducing its severity compared to placebo. In 2016, a systemic review in the Journal of the American Geriatric Society included both of the above studies in addition to 17 other studies to assess the efficacy of antipsychotics in preventing and treating delirium. This analysis concluded that antipsychotics did not change the length of delirium or length of stay.11 In addition, the absence of convincing evidence of antipsychotics benefits in postoperative delirium has led the American Geriatrics Society to recommend: “The prescribing practitioner should not prescribe antipsychotic… medications for the treatment of older adults with postoperative delirium who are not agitated and threatening substantial harm to self or others.”12
There is a paucity of data speaking directly to whether antipsychotics reduce patient distress. A recent randomized controlled study compared haloperidol, risperidone, and placebo for delirium treatment in palliative care and hospice patients. With treatment, the patients in the antipsychotic arms demonstrated slightly more severe delirium and a significantly higher incidence of extrapyramidal symptoms (EPS) than the patients receiving placebo.13
Side effects such as EPS, aspiration pneumonia, and arrhythmia are concerns when using antipsychotics for delirium treatment.14 A systematic review and meta-analysis found the difference in EPS incidence between patients treated for delirium with antipsychotics versus no intervention ranged from no difference to over 10%.11 In addition to EPS, patients receiving antipsychotics in a cohort study were at increased risk for aspiration pneumonia compared to patients who did not receive antipsychotics (adjusted odds ratio = 1.5, 95% CI, 1.2-1.9).15 These serious side effects led the Food and Drug Administration (FDA) to issue a black box warning for antipsychotic treatment in dementia-related psychosis. Most importantly, the FDA warns that there is an increased risk of death.16
WHAT YOU SHOULD DO INSTEAD OF USING ANTIPSYCHOTICS
In the first line management of delirium, hospitalists should address underlying modifiable contributions to the condition with attention to medications, pain, electrolytes, ischemia, infection, alcohol withdrawal, and reducing invasive lines. For example, two studies demonstrated a decrease in delirium severity and duration of palliative care in patients by treating delirium triggers, such as dehydration, electrolyte abnormalities, or infection, rather than using antipsychotics.13,17 Furthermore, hospitalists should review the medication list carefully and look for opportunities to deprescribe sedative/hypnotics and anticholinergics.
In addition, hospitalists should implement the core elements of the nursing delirium protocol from the Hospital Elder Life Program (http://www.hospitalelderlifeprogram.org/). The program focuses on orientation, hydration, mobility, sensory aids, and an environment conducive to sleep.18 When not representing an acute threat to the patient or staff, hospitalists should manage transient agitation from blood draws or vital sign checks by having staff members deescalate and re-approach the intervention later. While multicomponent nonpharmacologic interventions have more robust evidence for prevention of delirium than for treatment, they are low risk and still recommended for the patient with established delirium.19,20
A delirious patient picking at PIVs should prompt clinicians to re-evaluate the need for continued PIV access. If still necessary, experience suggests that PIVs can be protected with a combination of well-taped gauze extending from wrist to shoulder with any attached tubing exiting out of reach behind the shoulder. Also “beneficial distraction” with a task or “activity vest” that consists of an apron with zips, ties, and buttons designed to provide harmless objects can occupy the patient’s hands.
WHEN IT IS HELPFUL TO USE ANTIPSYCHOTICS FOR DELIRIUM
The literature does not provide clear evidence for when the use of antipsychotics is warranted. Antipsychotics may have a role for patients who are having severe psychotic symptoms posing an acute safety risk. In those situations, the American Geriatrics Society recommends using the “lowest effective dose for the shortest possible duration to treat patients who are severely agitated or distressed, and are threatening substantial harm to self and/or others…only if behavioral interventions have failed or are not possible.”12 In those patients who are having an acute myocardial infarction, consider atypical antipsychotics since haloperidol carries a small increased risk of mortality in that patient population.21
RECOMMENDATIONS
- Address underlying modifiable contributions to the delirium paying attention to medications, pain, electrolytes, ischemia, infection, alcohol withdrawal, and reducing invasive lines. Deprescribe sedative/hypnotic and anticholinergic medications.
- After addressing modifiable risk factors, attempt behavioral interventions for continuous problematic behaviors or symptoms of delirium.
- Reserve antipsychotics for cases where the patient poses an immediate danger of self-harm or harm to others. Treat for the shortest possible duration with the lowest effective dose of antipsychotic.
CONCLUSION
Returning to our case presentation, the hospitalist should not prescribe antipsychotic medications since there is no immediate risk of harm and antipsychotics do not treat hypoactive delirium. Delirium is a complex condition requiring a review of multifactorial causes. The hospitalist should investigate and address modifiable contributions. Furthermore, the hospitalist can make the PIV less accessible to deter the patient’s efforts to remove it and offer a distracting activity. Resolution of delirium, in all its forms, is still best achieved by treating the underlying etiology. The use of antipsychotics for treatment of patients with delirium in the absence of severe agitation
Do you think this is a low-value practice? Is this truly a “Thing We Do for No ReasonTM?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No ReasonTM” topics by emailing TWDFNR@hospitalmedicine.org.
Disclosures
Dr. Pahwa has received compensation for Expert Testimony, royalties from Aquifer, and owns stock/stock options in Pfizer and Aetna outside the submitted work. Dr. Qureshi and Dr. Cumbler have nothing to disclose.
1. American Psychiatric Association. DSM-5 Task Force. Diagnostic and statistical manual of mental disorders: DSM-5. 5th ed. Washington, D.C.: American Psychiatric Association; 2013.
2. Lonergan E, Luxenberg J, Areosa Sastre A. Benzodiazepines for delirium. Cochrane Database Syst Rev. 2009;(4):CD006379. https://doi.org/10.1002/14651858.CD006379.pub2.
3. Overshott R, Karim S, Burns A. Cholinesterase inhibitors for delirium. Cochrane Database Syst Rev. 2008;(1):CD005317. https://doi.org/10.1002/14651858.CD005317.pub2.
4. Zaal IJ, Devlin JW, Hazelbag M, et al. Benzodiazepine-associated delirium in critically ill adults. Intensive Care Med. 2015;41(12):2130-2137. https://doi.org/10.1007/s00134-015-4063-z.
5. Maldonado JR. Neuropathogenesis of delirium: review of current etiologic theories and common pathways. Am J Geriatr Psychiatry. 2013;21(12):1190-1222. https://doi.org/10.1016/j.jagp.2013.09.005.
6. Kalisvaart KJ, de Jonghe JF, Bogaards MJ, et al. Haloperidol prophylaxis for elderly hip-surgery patients at risk for delirium: a randomized placebo-controlled study. J Am Geriatr Soc. 2005;53(10):1658-1666. https://doi.org/10.1111/j.1532-5415.2005.53503.x
7. Siddiqi N, Stockdale R, Britton AM, Holmes J. Interventions for preventing delirium in hospitalized patients. Cochrane Database Syst Rev. 2007;(2):CD005563. https://doi.org/10.1002/14651858.CD005563.pub2
8. Tahir TA, Eeles E, Karapareddy V, et al. A randomized controlled trial of quetiapine versus placebo in the treatment of delirium. J Psychosom Res. 2010;69(5):485-490. https://doi.org/10.1016/j.jpsychores.2010.05.006.
9. Loh KP, Ramdass S, Garb JL, Brennan MJ, Lindenauer PK, Lagu T. From hospital to community: use of antipsychotics in hospitalized elders. J Hosp Med. 2014;9(12):802-804. https://doi.org/10.1002/jhm.2277.
10. Herzig SJ, Rothberg MB, Guess JR, Gurwitz JH, Marcantonio ER. Antipsychotic medication utilization in nonpsychiatric hospitalizations. J Hosp Med. 2016;11(8):543-549. https://doi.org/10.1002/jhm.2596.
11. Neufeld KJ, Yue J, Robinson TN, Inouye SK, Needham DM. Antipsychotic medication for prevention and treatment of delirium in hospitalized adults: a systematic review and meta-analysis. J Am Geriatr Soc. 2016;64(4):705-714. https://doi.org/10.1111/jgs.14076.
12. American Geriatrics Society Expert Panel on Postoperative Delirium in Older A. American Geriatrics Society abstracted clinical practice guideline for postoperative delirium in older adults. J Am Geriatr Soc. 2015;63(1):142-150. https://doi.org/10.1111/jgs.13281
13. Agar MR, Lawlor PG, Quinn S, et al. Efficacy of oral risperidone, haloperidol, or placebo for symptoms of delirium among patients in palliative care: a randomized clinical trial. JAMA Intern Med. 2017;177(1):34-42. https://doi.org/10.1001/jamainternmed.2016.7491.
14. Hatta K, Kishi Y, Wada K, et al. Antipsychotics for delirium in the general hospital setting in consecutive 2453 inpatients: a prospective observational study. Int J Geriatr Psychiatry. 2014;29(3):253-262. https://doi.org/10.1002/gps.3999.
15. Herzig SJ, LaSalvia MT, Naidus E, et al. Antipsychotics and the risk of aspiration pneumonia in individuals hospitalized for nonpsychiatric conditions: a cohort study. J Am Geriatr Soc. 2017;65(12):2580-2586. https://doi.org/10.1111/jgs.15066.
16. Jeste DV, Blazer D, Casey D, et al. ACNP White Paper: update on use of antipsychotic drugs in elderly persons with dementia. Neuropsychopharmacology. 2008;33(5):957-970. https://doi.org/10.1038/sj.npp.1301492
17. Hui D, Frisbee-Hume S, Wilson A, et al. Effect of lorazepam with haloperidol vs haloperidol alone on agitated delirium in patients with advanced cancer receiving palliative care: a randomized clinical trial. JAMA. 2017;318(11):1047-1056. https://doi.org/10.1001/jama.2017.11468.
18. Inouye SK, Bogardus ST, Jr., Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340(9):669-676. https://doi.org/10.1056/NEJM199903043400901
19. Milisen K, Lemiengre J, Braes T, Foreman MD. Multicomponent intervention strategies for managing delirium in hospitalized older people: systematic review. J Adv Nurs. 2005;52(1):79-90. https://doi.org/10.1111/j.1365-2648.2005.03557.x
20. Marcantonio ER. Delirium in hospitalized older adults. N Engl J Med. 2017;377(15):1456-1466. https://doi.org/10.1056/NEJMcp1605501.
21. Park Y, Bateman BT, Kim DH, et al. Use of haloperidol versus atypical antipsychotics and risk of in-hospital death in patients with acute myocardial infarction: cohort study. BMJ. 2018;360. https://doi.org/10.1136/bmj.k1218.
1. American Psychiatric Association. DSM-5 Task Force. Diagnostic and statistical manual of mental disorders: DSM-5. 5th ed. Washington, D.C.: American Psychiatric Association; 2013.
2. Lonergan E, Luxenberg J, Areosa Sastre A. Benzodiazepines for delirium. Cochrane Database Syst Rev. 2009;(4):CD006379. https://doi.org/10.1002/14651858.CD006379.pub2.
3. Overshott R, Karim S, Burns A. Cholinesterase inhibitors for delirium. Cochrane Database Syst Rev. 2008;(1):CD005317. https://doi.org/10.1002/14651858.CD005317.pub2.
4. Zaal IJ, Devlin JW, Hazelbag M, et al. Benzodiazepine-associated delirium in critically ill adults. Intensive Care Med. 2015;41(12):2130-2137. https://doi.org/10.1007/s00134-015-4063-z.
5. Maldonado JR. Neuropathogenesis of delirium: review of current etiologic theories and common pathways. Am J Geriatr Psychiatry. 2013;21(12):1190-1222. https://doi.org/10.1016/j.jagp.2013.09.005.
6. Kalisvaart KJ, de Jonghe JF, Bogaards MJ, et al. Haloperidol prophylaxis for elderly hip-surgery patients at risk for delirium: a randomized placebo-controlled study. J Am Geriatr Soc. 2005;53(10):1658-1666. https://doi.org/10.1111/j.1532-5415.2005.53503.x
7. Siddiqi N, Stockdale R, Britton AM, Holmes J. Interventions for preventing delirium in hospitalized patients. Cochrane Database Syst Rev. 2007;(2):CD005563. https://doi.org/10.1002/14651858.CD005563.pub2
8. Tahir TA, Eeles E, Karapareddy V, et al. A randomized controlled trial of quetiapine versus placebo in the treatment of delirium. J Psychosom Res. 2010;69(5):485-490. https://doi.org/10.1016/j.jpsychores.2010.05.006.
9. Loh KP, Ramdass S, Garb JL, Brennan MJ, Lindenauer PK, Lagu T. From hospital to community: use of antipsychotics in hospitalized elders. J Hosp Med. 2014;9(12):802-804. https://doi.org/10.1002/jhm.2277.
10. Herzig SJ, Rothberg MB, Guess JR, Gurwitz JH, Marcantonio ER. Antipsychotic medication utilization in nonpsychiatric hospitalizations. J Hosp Med. 2016;11(8):543-549. https://doi.org/10.1002/jhm.2596.
11. Neufeld KJ, Yue J, Robinson TN, Inouye SK, Needham DM. Antipsychotic medication for prevention and treatment of delirium in hospitalized adults: a systematic review and meta-analysis. J Am Geriatr Soc. 2016;64(4):705-714. https://doi.org/10.1111/jgs.14076.
12. American Geriatrics Society Expert Panel on Postoperative Delirium in Older A. American Geriatrics Society abstracted clinical practice guideline for postoperative delirium in older adults. J Am Geriatr Soc. 2015;63(1):142-150. https://doi.org/10.1111/jgs.13281
13. Agar MR, Lawlor PG, Quinn S, et al. Efficacy of oral risperidone, haloperidol, or placebo for symptoms of delirium among patients in palliative care: a randomized clinical trial. JAMA Intern Med. 2017;177(1):34-42. https://doi.org/10.1001/jamainternmed.2016.7491.
14. Hatta K, Kishi Y, Wada K, et al. Antipsychotics for delirium in the general hospital setting in consecutive 2453 inpatients: a prospective observational study. Int J Geriatr Psychiatry. 2014;29(3):253-262. https://doi.org/10.1002/gps.3999.
15. Herzig SJ, LaSalvia MT, Naidus E, et al. Antipsychotics and the risk of aspiration pneumonia in individuals hospitalized for nonpsychiatric conditions: a cohort study. J Am Geriatr Soc. 2017;65(12):2580-2586. https://doi.org/10.1111/jgs.15066.
16. Jeste DV, Blazer D, Casey D, et al. ACNP White Paper: update on use of antipsychotic drugs in elderly persons with dementia. Neuropsychopharmacology. 2008;33(5):957-970. https://doi.org/10.1038/sj.npp.1301492
17. Hui D, Frisbee-Hume S, Wilson A, et al. Effect of lorazepam with haloperidol vs haloperidol alone on agitated delirium in patients with advanced cancer receiving palliative care: a randomized clinical trial. JAMA. 2017;318(11):1047-1056. https://doi.org/10.1001/jama.2017.11468.
18. Inouye SK, Bogardus ST, Jr., Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340(9):669-676. https://doi.org/10.1056/NEJM199903043400901
19. Milisen K, Lemiengre J, Braes T, Foreman MD. Multicomponent intervention strategies for managing delirium in hospitalized older people: systematic review. J Adv Nurs. 2005;52(1):79-90. https://doi.org/10.1111/j.1365-2648.2005.03557.x
20. Marcantonio ER. Delirium in hospitalized older adults. N Engl J Med. 2017;377(15):1456-1466. https://doi.org/10.1056/NEJMcp1605501.
21. Park Y, Bateman BT, Kim DH, et al. Use of haloperidol versus atypical antipsychotics and risk of in-hospital death in patients with acute myocardial infarction: cohort study. BMJ. 2018;360. https://doi.org/10.1136/bmj.k1218.
Amit K Pahwa, MD, FAAP; E-mail: apahwa1@jhmi.edu; Telephone: 410-502-1934.
© 2019 Society of Hospital Medicine
Things We Do for No Reason: Neuroimaging for Hospitalized Patients with Delirium
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 67-year-old woman with a history of hypertension and osteoarthritis was hospitalized for fever, flank pain, and dysuria with pyuria on urinalysis. She was diagnosed with acute pyelonephritis and started ceftriaxone, ondansetron for nausea, and oxycodone for pain. On hospital day two, she developed acute confusion that waxed and waned in severity throughout the day. On examination, she appeared mildly agitated, inattentive, and was noted to pick at her linens and garment. She was oriented to person only and had a nonfocal neurologic examination. Her nurse reported no recent falls or trauma. As part of the patient’s evaluation, her attending physician ordered a head computed tomography (CT) scan.
BACKGROUND
Delirium is commonly diagnosed in hospitalized patients. It has a prevalence of 29%-64% and is associated with longer lengths of stay, higher mortality, and costs of over $164 billion per year in the United States.1 While a number of practice guidelines have been created to help guide delirium diagnosis and management, there is not a clear consensus on when neuroimaging should be performed during the evaluation.2-4 It should also be noted that numerous guidelines for delirium management exist, with variable quality and a heavy reliance on expert opinion.5 Perhaps due to this lack of consensus, neuroimaging is performed in 33% to 67% of hospitalized patients with delirium.6,7
WHY YOU MAY THINK NEUROIMAGING IS HELPFUL IN EVALUATING UNDIFFERENTIATED HOSPITALIZED PATIENTS WITH DELIRIUM
Delirium is known to be associated with intracranial processes. For example, delirium occurs in 13% to 48% of patients with acute stroke8 and conversely 7% of patients with new confusion evaluated in emergency departments or inpatient settings were found to have an acute stroke.9 The inclusion of neuroimaging as part of a delirium evaluation is supported in certain circumstances, such as in patients with recent falls, focal neurologic signs (including papilledema), systemic anticoagulation,2 or increased risk of intracranial processes such as metastatic malignancy.4
WHY NEUROIMAGING IS NOT HELPFUL IN EVALUATING UNDIFFERENTIATED HOSPITALIZED PATIENTS WITH DELIRIUM
A number of studies have evaluated the diagnostic yield of neuroimaging in hospitalized patients with delirium (Table).6,7,10,11 Two studies included patients with delirium that developed after hospitalization10,11 and two included patients with delirium at admission.6,7
Theisen-Toupal et al. conducted a retrospective study of 220 hospitalized general medical patients who underwent head CT scans for an indication of delirium, altered mental status, confusion, encephalopathy, somnolence or unresponsiveness.10 Patients were excluded if they had a history of falls, head trauma, or new neurologic deficits in the preceding two weeks or if the admitting diagnosis was stroke or cerebral hemorrhage. Additionally, the authors limited patients to those who developed delirium 24 hours or more after admission. There were 6/220 (2.7%) patients identified with an acute intracranial process. Of these six patients, three were receiving anticoagulation. An additional 4/220 (1.8%) head CT scans were identified as equivocal, prompting further neuroimaging, which ultimately showed chronic findings.
Vijayakrishnan et al. performed a retrospective review of 400 hospitalized patients who underwent inpatient CT scans, then limited to those with new delirium.11 They identified 36 patients, of which four (11%) had acute findings on CT: one case each of acute hemorrhage, subdural hematoma, brain metastases, and septic emboli. The authors state “all the four patients had preimaging clinical symptoms and signs, which warranted imaging as per guidelines suggested by the British Geriatrics Society and the Australian and New Zealand Society for Geriatric Medicine,” though they do not provide further details. The strength of this paper is that it isolated patients who developed delirium while hospitalized; however, conclusions were limited by the small sample size.
Lai et al.’s case-control study evaluated 300 consecutive patients admitted to a delirium unit over 18 months.6 Of these 300 patients, 200 (67%) had CT performed; 29/200 (14.5%) had intracranial findings on CT that explained their delirium, including 13 ischemic strokes, seven subdural hemorrhages, nine intracerebral hemorrhages, and three additional ischemic strokes that evolved on follow-up imaging but were not present on the initial scans. The authors performed univariate and multivariate analyses to identify risk factors for an intracranial cause of delirium. Only 3/29 patients with a positive scan did not have one of three main risk factors the authors identified: a fall in the preceding two weeks, new neurologic findings, or sudden deterioration of consciousness. It should be noted that authors did not define “deterioration of consciousness” and that all patients had confusion on admission to the unit, rather than developing during hospitalization.
Hijazi et al. conducted a retrospective cohort study over a 20-month period of 1,653 patients with delirium at the time of admission or during their hospitalization. Patients with delirium due to drug or medication withdrawal or “psychiatric reasons” were excluded. Overall, 538 (32.5%) patients underwent CT, MRI or both, and 78 (14.5%) patients had a positive finding on neuroimaging. This study’s 14.5 % overall yield matches that of Lai et al. Unfortunately, the study included all patients with delirium and did not report the rates of fall, neurologic deficits, and/or use of anticoagulation among those with positive neuroimaging. This limits the generalizability of the findings to a cohort of patients without intracranial pathology risk factors.
The reported yield of neuroimaging for hospitalized patients with delirium ranged from 2.7% to 14.5% across studies. However, in studies taking into account specific patient risk factors; the reported yields in patients without focal neurologic findings, new decline in mental status, systemic anticoagulation, or recent falls were 0%,11 1.4%,10 and 1.5%.6 While a rate of 1.5% may appear high for a serious outcome such as stroke or intracranial bleeding, it is comparable to rates reported for missed major cardiac events in clinical algorithms for evaluating chest pain.12 It should also be noted that neuroimaging is imperfect for acute stroke, and thus the positive or negative predictive value may be poor in the setting of low prevalence. For example, for detection of any acute stroke, the sensitivity/specificity of MRI and CT are 83%/97% and 26%/98% respectively.13
Neuroimaging is expensive and has risks. The average charge for a head CT is approximately $1,400 at academic institutions.14 Moreover, computed tomography exposes patients to significant radiation and up to 2% of malignancies in the United States may be attributable to prior tomography exposure.15 Additionally, there are non-negligible rates of incidental findings during neuroimaging, 1% for CT16 and 2.7%-13.7% for MRI,17,18 which may result in further evaluation or treatment that causes significant patient anxiety. Obtaining neuroimaging on delirious patients can be time consuming and labor intensive, which could delay care to other patients. Additionally, sedating medications are often administered to agitated patients prior to imaging, which risk worsening delirium. Ordering neuroimaging for all patients with acute delirium, therefore, exposes the large majority to unnecessary costs and potential harms.
WHEN NEUROIMAGING TO EVALUATE DELIRIUM IN HOSPITALIZED PATIENTS COULD BE REASONABLE
The diagnostic yield of head CT in the evaluation of delirium is significantly higher in patients with specific risk factors. Lai et al. found adjusted odds ratios for abnormal CT of 18.2 in patients with new focal deficits, 5.6 with a fall in the preceding two weeks and 4.6 in patients with deterioration in consciousness. Patients with systemic anticoagulation had higher unadjusted, (OR 2.4) though not adjusted odds of having an abnormal CT.6 Thiesen-Toupal et al. excluded patients with recent falls or neurologic deficits but reported that three out of six delirious patients with abnormal neuroimaging were anticoagulated.10 Vijayakrishnan et al. found that all four delirious patients with intracranial findings met guideline criteria for neuroimaging.11 Thus, current recommendations for neuroimaging in delirious patients with falls, focal neurologic deficits, or systemic anticoagulation are appropriate. In situations when a provider lacks an accurate history and is unable to determine if risk factors are present (for example a confused patient found sitting on the floor next to the bed), it may also be reasonable to consider neuroimaging.
Data are limited, but some authors advocate for neuroimaging in cases of delirium that do not improve with treatment.6 Additionally, it may be reasonable to consider neuroimaging in delirium patients with predispositions to embolic or metastatic intracranial processes such as endovascular infections and certain malignancies.4
WHAT YOU SHOULD DO INSTEAD OF NEUROIMAGING TO EVALUATE DELIRIUM IN HOSPITALIZED PATIENTS
Hospitalized patients with acute confusion should be assessed for delirium with a validated instrument such as the Confusion Assessment Method (CAM).19,20 The original CAM included several components: acute change in mental status with a fluctuating course and inattention, plus either disorganized thinking and/or altered level of consciousness. Multiple delirium assessment tools have been created and validated, all of which include inattention as a required feature. A recent hospital-based study using a two item bedside test asking the patient to name the day of the week and list the months of the year backwards detected delirium with a sensitivity of 93% and specificity of 64%.21 Once the diagnosis of delirium is established, evaluation should begin with a careful history and physical examination focused on the identification of risk factors such as physical restraints, indwelling urinary catheters, and drugs known to precipitate delirium, particularly those with withdrawal potential, anticholinergic properties, and sedative-hypnotic agents.22-24 Delirium may be the first harbinger of serious medical illness and specific testing should be guided by clinical suspicion. In general, a thorough physical examination should look for focal neurologic deficits, hypoxia, signs of infection, and other inflammatory or painful processes that could precipitate delirium.25 Targeted laboratory evaluation may include a basic metabolic panel to identify electrolyte (including calcium) and metabolic derangements, complete blood count, and urinalysis if infection is suspected.
RECOMMENDATIONS
- Use a validated instrument such as CAM to evaluate hospitalized patients who develop altered mental status.
- Delirious patients should undergo a thorough history including a review of medications, physical exam, and targeted laboratory testing aimed at identifying common risk factors and precipitants of delirium that should be addressed.
- Perform neuroimaging if there is a history of fall or head trauma in the preceding two weeks, any new focal abnormalities on neurologic exam or if the patient is receiving systemic anticoagulation.
- It may be reasonable to consider neuroimaging for patients with an atypical course of delirium, such as a sudden decline in the level of consciousness, persistence despite addressing identified factors, or if there is a high degree of suspicion for embolic or metastatic processes.
CONCLUSIONS
Performing neuroimaging in undifferentiated patients who develop delirium while hospitalized has a low diagnostic yield, is costly, and is potentially harmful. Neuroimaging should be reserved for those with identified risk factors for intracranial pathology. For the patient described in the initial vignette with no risk factors for intracranial cause, neuroimaging would be unlikely to contribute to her care. To change provider beliefs and behaviors regarding neuroimaging, prospective studies evaluating guideline implementation are needed. However, based on the current evidence, neuroimaging should be reserved for those with identified risk factors.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by e-mailing TWDFNR@hospitalmedicine.org.
Disclosures
The authors have no conflicts of interest relevant to this article to disclose.
1. Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922. doi: 10.1016/S0140-6736(13)60688-1. PubMed
2. Clinical practice guidelines for the management of delirium in older people. 2006; https://www2.health.vic.gov.au/Api/downloadmedia/%7BDAA8404B-FEE7-4BDA-8A1E-A32494783B7F%7D. Accessed June 4, 2018.
3. Delirium: prevention, diagnosis and management. NICE Guidance 2010; https://www.nice.org.uk/guidance/cg103. Accessed June 4, 2018.
4. Michaud L, Bula C, Berney A, et al. Delirium: guidelines for general hospitals. J Psychosom Res. 2007;62(3):371-383. doi: 10.1016/j.jpsychores.2006.10.004. PubMed
5. Bush SH, Marchington KL, Agar M, Davis DH, Sikora L, Tsang TW. Quality of clinical practice guidelines in delirium: a systematic appraisal. BMJ Open. 2017;7(3):e013809. doi: 10.1136/bmjopen-2016-013809. PubMed
6. Lai MM, Wong Tin Niam DM. Intracranial cause of delirium: computed tomography yield and predictive factors. Intern Med J. 2012;42(4):422-427. doi: 10.1111/j.1445-5994.2010.02400.x. PubMed
7. Hijazi Z, Lange P, Watson R, Maier AB. The use of cerebral imaging for investigating delirium aetiology. Eur J Intern Med. 2018;52:35-39. doi: 10.1016/j.ejim.2018.01.024. PubMed
8. Oldenbeuving AW, de Kort PL, Jansen BP, Roks G, Kappelle LJ. Delirium in acute stroke: a review. Int J Stroke. 2007;2(4):270-275. doi: 10.1111/j.1747-4949.2007.00163.x. PubMed
9. Benbadis SR, Sila CA, Cristea RL. Mental status changes and stroke. J Stroke Cerebrovasc Dis. 1994;4(4):216-219. doi: 10.1016/S1052-3057(10)80093-X. PubMed
10. Theisen-Toupal J, Breu AC, Mattison ML, Arnaout R. Diagnostic yield of head computed tomography for the hospitalized medical patient with delirium. J Hosp Med. 2014;9(8):497-501. doi: 10.1002/jhm.2198. PubMed
11. Vijayakrishnan R, Ramasubramanian A, Dhand S. Utility of head CT scan for acute inpatient delirium. Hosp Top. 2015;93(1):9-12. doi: 10.1080/00185868.2015.1012928. PubMed
12. Backus BE, Six AJ, Kelder JC, et al. A prospective validation of the HEART score for chest pain patients at the emergency department. Int J Cardiol. 2013;168(3):2153-2158. doi: 10.1016/j.ijcard.2013.01.255. PubMed
13. Chalela JA, Kidwell CS, Nentwich LM, et al. Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison. Lancet. 2007;369(9558):293-298. doi: 10.1016/S0140-6736(07)60151-2. PubMed
14. Paul AB, Oklu R, Saini S, Prabhakar AM. How much is that head CT? Price transparency and variability in radiology. J Am Coll Radiol. 2015;12(5):453-457. doi: 10.1016/j.jacr.2014.12.016. PubMed
15. Brenner DJ, Hall EJ. Computed tomography--an increasing source of radiation exposure. N Engl J Med. 2007;357(22):2277-2284. doi: 10.1056/NEJMra072149. PubMed
16. Eskandary H, Sabbagh M, Khajehpour F, Eskandari M. Incidental findings in brain computed tomography scans of 3000 head trauma patients. Surg Neurol. 2005;63(6):550-553; discussion 553. doi: 10.1016/j.surneu.2004.07.049. PubMed
17. Vernooij MW, Ikram MA, Tanghe HL, et al. Incidental findings on brain MRI in the general population. N Engl J Med. 2007;357(18):1821-1828. doi: 10.1056/NEJMoa070972. PubMed
18. Morris Z, Whiteley WN, Longstreth WT, Jr., et al. Incidental findings on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2009;339:b3016. doi: 10.1136/bmj.b3016. PubMed
19. Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941-948. doi: 10.7326/0003-4819-113-12-941. PubMed
20. Wei LA, Fearing MA, Sternberg EJ, Inouye SK. The Confusion Assessment Method: a systematic review of current usage. J Am Geriatr Soc. 2008;56(5):823-830. doi: 10.1111/j.1532-5415.2008.01674.x. PubMed
21. Fick DM, Inouye SK, Guess J, et al. Preliminary development of an ultrabrief two-item bedside test for delirium. J Hosp Med. 2015;10(10):645-650. doi: 10.1002/jhm.2418. PubMed
22. Tune L, Carr S, Hoag E, Cooper T. Anticholinergic effects of drugs commonly prescribed for the elderly: potential means for assessing the risk of delirium. Am J Psychiatry. 1992;149(10):1393-1394. doi: 10.1176/ajp.149.10.1393. PubMed
23. Inouye SK, Schlesinger MJ, Lydon TJ. Delirium: a symptom of how hospital care is failing older persons and a window to improve the quality of hospital care. Am J Med. 1999;106(5):565-573. doi: 0.1016/S0002-9343(99)00070-4. PubMed
24. Marcantonio ER. Delirium in hospitalized older adults. N Engl J Med. 2017;377(15):1456-1466. doi: 10.1056/NEJMc1714932. PubMed
25. Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA. 1996;275(11):852-857. doi: 10.1001/jama.1996.03530350034031. PubMed
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 67-year-old woman with a history of hypertension and osteoarthritis was hospitalized for fever, flank pain, and dysuria with pyuria on urinalysis. She was diagnosed with acute pyelonephritis and started ceftriaxone, ondansetron for nausea, and oxycodone for pain. On hospital day two, she developed acute confusion that waxed and waned in severity throughout the day. On examination, she appeared mildly agitated, inattentive, and was noted to pick at her linens and garment. She was oriented to person only and had a nonfocal neurologic examination. Her nurse reported no recent falls or trauma. As part of the patient’s evaluation, her attending physician ordered a head computed tomography (CT) scan.
BACKGROUND
Delirium is commonly diagnosed in hospitalized patients. It has a prevalence of 29%-64% and is associated with longer lengths of stay, higher mortality, and costs of over $164 billion per year in the United States.1 While a number of practice guidelines have been created to help guide delirium diagnosis and management, there is not a clear consensus on when neuroimaging should be performed during the evaluation.2-4 It should also be noted that numerous guidelines for delirium management exist, with variable quality and a heavy reliance on expert opinion.5 Perhaps due to this lack of consensus, neuroimaging is performed in 33% to 67% of hospitalized patients with delirium.6,7
WHY YOU MAY THINK NEUROIMAGING IS HELPFUL IN EVALUATING UNDIFFERENTIATED HOSPITALIZED PATIENTS WITH DELIRIUM
Delirium is known to be associated with intracranial processes. For example, delirium occurs in 13% to 48% of patients with acute stroke8 and conversely 7% of patients with new confusion evaluated in emergency departments or inpatient settings were found to have an acute stroke.9 The inclusion of neuroimaging as part of a delirium evaluation is supported in certain circumstances, such as in patients with recent falls, focal neurologic signs (including papilledema), systemic anticoagulation,2 or increased risk of intracranial processes such as metastatic malignancy.4
WHY NEUROIMAGING IS NOT HELPFUL IN EVALUATING UNDIFFERENTIATED HOSPITALIZED PATIENTS WITH DELIRIUM
A number of studies have evaluated the diagnostic yield of neuroimaging in hospitalized patients with delirium (Table).6,7,10,11 Two studies included patients with delirium that developed after hospitalization10,11 and two included patients with delirium at admission.6,7
Theisen-Toupal et al. conducted a retrospective study of 220 hospitalized general medical patients who underwent head CT scans for an indication of delirium, altered mental status, confusion, encephalopathy, somnolence or unresponsiveness.10 Patients were excluded if they had a history of falls, head trauma, or new neurologic deficits in the preceding two weeks or if the admitting diagnosis was stroke or cerebral hemorrhage. Additionally, the authors limited patients to those who developed delirium 24 hours or more after admission. There were 6/220 (2.7%) patients identified with an acute intracranial process. Of these six patients, three were receiving anticoagulation. An additional 4/220 (1.8%) head CT scans were identified as equivocal, prompting further neuroimaging, which ultimately showed chronic findings.
Vijayakrishnan et al. performed a retrospective review of 400 hospitalized patients who underwent inpatient CT scans, then limited to those with new delirium.11 They identified 36 patients, of which four (11%) had acute findings on CT: one case each of acute hemorrhage, subdural hematoma, brain metastases, and septic emboli. The authors state “all the four patients had preimaging clinical symptoms and signs, which warranted imaging as per guidelines suggested by the British Geriatrics Society and the Australian and New Zealand Society for Geriatric Medicine,” though they do not provide further details. The strength of this paper is that it isolated patients who developed delirium while hospitalized; however, conclusions were limited by the small sample size.
Lai et al.’s case-control study evaluated 300 consecutive patients admitted to a delirium unit over 18 months.6 Of these 300 patients, 200 (67%) had CT performed; 29/200 (14.5%) had intracranial findings on CT that explained their delirium, including 13 ischemic strokes, seven subdural hemorrhages, nine intracerebral hemorrhages, and three additional ischemic strokes that evolved on follow-up imaging but were not present on the initial scans. The authors performed univariate and multivariate analyses to identify risk factors for an intracranial cause of delirium. Only 3/29 patients with a positive scan did not have one of three main risk factors the authors identified: a fall in the preceding two weeks, new neurologic findings, or sudden deterioration of consciousness. It should be noted that authors did not define “deterioration of consciousness” and that all patients had confusion on admission to the unit, rather than developing during hospitalization.
Hijazi et al. conducted a retrospective cohort study over a 20-month period of 1,653 patients with delirium at the time of admission or during their hospitalization. Patients with delirium due to drug or medication withdrawal or “psychiatric reasons” were excluded. Overall, 538 (32.5%) patients underwent CT, MRI or both, and 78 (14.5%) patients had a positive finding on neuroimaging. This study’s 14.5 % overall yield matches that of Lai et al. Unfortunately, the study included all patients with delirium and did not report the rates of fall, neurologic deficits, and/or use of anticoagulation among those with positive neuroimaging. This limits the generalizability of the findings to a cohort of patients without intracranial pathology risk factors.
The reported yield of neuroimaging for hospitalized patients with delirium ranged from 2.7% to 14.5% across studies. However, in studies taking into account specific patient risk factors; the reported yields in patients without focal neurologic findings, new decline in mental status, systemic anticoagulation, or recent falls were 0%,11 1.4%,10 and 1.5%.6 While a rate of 1.5% may appear high for a serious outcome such as stroke or intracranial bleeding, it is comparable to rates reported for missed major cardiac events in clinical algorithms for evaluating chest pain.12 It should also be noted that neuroimaging is imperfect for acute stroke, and thus the positive or negative predictive value may be poor in the setting of low prevalence. For example, for detection of any acute stroke, the sensitivity/specificity of MRI and CT are 83%/97% and 26%/98% respectively.13
Neuroimaging is expensive and has risks. The average charge for a head CT is approximately $1,400 at academic institutions.14 Moreover, computed tomography exposes patients to significant radiation and up to 2% of malignancies in the United States may be attributable to prior tomography exposure.15 Additionally, there are non-negligible rates of incidental findings during neuroimaging, 1% for CT16 and 2.7%-13.7% for MRI,17,18 which may result in further evaluation or treatment that causes significant patient anxiety. Obtaining neuroimaging on delirious patients can be time consuming and labor intensive, which could delay care to other patients. Additionally, sedating medications are often administered to agitated patients prior to imaging, which risk worsening delirium. Ordering neuroimaging for all patients with acute delirium, therefore, exposes the large majority to unnecessary costs and potential harms.
WHEN NEUROIMAGING TO EVALUATE DELIRIUM IN HOSPITALIZED PATIENTS COULD BE REASONABLE
The diagnostic yield of head CT in the evaluation of delirium is significantly higher in patients with specific risk factors. Lai et al. found adjusted odds ratios for abnormal CT of 18.2 in patients with new focal deficits, 5.6 with a fall in the preceding two weeks and 4.6 in patients with deterioration in consciousness. Patients with systemic anticoagulation had higher unadjusted, (OR 2.4) though not adjusted odds of having an abnormal CT.6 Thiesen-Toupal et al. excluded patients with recent falls or neurologic deficits but reported that three out of six delirious patients with abnormal neuroimaging were anticoagulated.10 Vijayakrishnan et al. found that all four delirious patients with intracranial findings met guideline criteria for neuroimaging.11 Thus, current recommendations for neuroimaging in delirious patients with falls, focal neurologic deficits, or systemic anticoagulation are appropriate. In situations when a provider lacks an accurate history and is unable to determine if risk factors are present (for example a confused patient found sitting on the floor next to the bed), it may also be reasonable to consider neuroimaging.
Data are limited, but some authors advocate for neuroimaging in cases of delirium that do not improve with treatment.6 Additionally, it may be reasonable to consider neuroimaging in delirium patients with predispositions to embolic or metastatic intracranial processes such as endovascular infections and certain malignancies.4
WHAT YOU SHOULD DO INSTEAD OF NEUROIMAGING TO EVALUATE DELIRIUM IN HOSPITALIZED PATIENTS
Hospitalized patients with acute confusion should be assessed for delirium with a validated instrument such as the Confusion Assessment Method (CAM).19,20 The original CAM included several components: acute change in mental status with a fluctuating course and inattention, plus either disorganized thinking and/or altered level of consciousness. Multiple delirium assessment tools have been created and validated, all of which include inattention as a required feature. A recent hospital-based study using a two item bedside test asking the patient to name the day of the week and list the months of the year backwards detected delirium with a sensitivity of 93% and specificity of 64%.21 Once the diagnosis of delirium is established, evaluation should begin with a careful history and physical examination focused on the identification of risk factors such as physical restraints, indwelling urinary catheters, and drugs known to precipitate delirium, particularly those with withdrawal potential, anticholinergic properties, and sedative-hypnotic agents.22-24 Delirium may be the first harbinger of serious medical illness and specific testing should be guided by clinical suspicion. In general, a thorough physical examination should look for focal neurologic deficits, hypoxia, signs of infection, and other inflammatory or painful processes that could precipitate delirium.25 Targeted laboratory evaluation may include a basic metabolic panel to identify electrolyte (including calcium) and metabolic derangements, complete blood count, and urinalysis if infection is suspected.
RECOMMENDATIONS
- Use a validated instrument such as CAM to evaluate hospitalized patients who develop altered mental status.
- Delirious patients should undergo a thorough history including a review of medications, physical exam, and targeted laboratory testing aimed at identifying common risk factors and precipitants of delirium that should be addressed.
- Perform neuroimaging if there is a history of fall or head trauma in the preceding two weeks, any new focal abnormalities on neurologic exam or if the patient is receiving systemic anticoagulation.
- It may be reasonable to consider neuroimaging for patients with an atypical course of delirium, such as a sudden decline in the level of consciousness, persistence despite addressing identified factors, or if there is a high degree of suspicion for embolic or metastatic processes.
CONCLUSIONS
Performing neuroimaging in undifferentiated patients who develop delirium while hospitalized has a low diagnostic yield, is costly, and is potentially harmful. Neuroimaging should be reserved for those with identified risk factors for intracranial pathology. For the patient described in the initial vignette with no risk factors for intracranial cause, neuroimaging would be unlikely to contribute to her care. To change provider beliefs and behaviors regarding neuroimaging, prospective studies evaluating guideline implementation are needed. However, based on the current evidence, neuroimaging should be reserved for those with identified risk factors.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by e-mailing TWDFNR@hospitalmedicine.org.
Disclosures
The authors have no conflicts of interest relevant to this article to disclose.
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 67-year-old woman with a history of hypertension and osteoarthritis was hospitalized for fever, flank pain, and dysuria with pyuria on urinalysis. She was diagnosed with acute pyelonephritis and started ceftriaxone, ondansetron for nausea, and oxycodone for pain. On hospital day two, she developed acute confusion that waxed and waned in severity throughout the day. On examination, she appeared mildly agitated, inattentive, and was noted to pick at her linens and garment. She was oriented to person only and had a nonfocal neurologic examination. Her nurse reported no recent falls or trauma. As part of the patient’s evaluation, her attending physician ordered a head computed tomography (CT) scan.
BACKGROUND
Delirium is commonly diagnosed in hospitalized patients. It has a prevalence of 29%-64% and is associated with longer lengths of stay, higher mortality, and costs of over $164 billion per year in the United States.1 While a number of practice guidelines have been created to help guide delirium diagnosis and management, there is not a clear consensus on when neuroimaging should be performed during the evaluation.2-4 It should also be noted that numerous guidelines for delirium management exist, with variable quality and a heavy reliance on expert opinion.5 Perhaps due to this lack of consensus, neuroimaging is performed in 33% to 67% of hospitalized patients with delirium.6,7
WHY YOU MAY THINK NEUROIMAGING IS HELPFUL IN EVALUATING UNDIFFERENTIATED HOSPITALIZED PATIENTS WITH DELIRIUM
Delirium is known to be associated with intracranial processes. For example, delirium occurs in 13% to 48% of patients with acute stroke8 and conversely 7% of patients with new confusion evaluated in emergency departments or inpatient settings were found to have an acute stroke.9 The inclusion of neuroimaging as part of a delirium evaluation is supported in certain circumstances, such as in patients with recent falls, focal neurologic signs (including papilledema), systemic anticoagulation,2 or increased risk of intracranial processes such as metastatic malignancy.4
WHY NEUROIMAGING IS NOT HELPFUL IN EVALUATING UNDIFFERENTIATED HOSPITALIZED PATIENTS WITH DELIRIUM
A number of studies have evaluated the diagnostic yield of neuroimaging in hospitalized patients with delirium (Table).6,7,10,11 Two studies included patients with delirium that developed after hospitalization10,11 and two included patients with delirium at admission.6,7
Theisen-Toupal et al. conducted a retrospective study of 220 hospitalized general medical patients who underwent head CT scans for an indication of delirium, altered mental status, confusion, encephalopathy, somnolence or unresponsiveness.10 Patients were excluded if they had a history of falls, head trauma, or new neurologic deficits in the preceding two weeks or if the admitting diagnosis was stroke or cerebral hemorrhage. Additionally, the authors limited patients to those who developed delirium 24 hours or more after admission. There were 6/220 (2.7%) patients identified with an acute intracranial process. Of these six patients, three were receiving anticoagulation. An additional 4/220 (1.8%) head CT scans were identified as equivocal, prompting further neuroimaging, which ultimately showed chronic findings.
Vijayakrishnan et al. performed a retrospective review of 400 hospitalized patients who underwent inpatient CT scans, then limited to those with new delirium.11 They identified 36 patients, of which four (11%) had acute findings on CT: one case each of acute hemorrhage, subdural hematoma, brain metastases, and septic emboli. The authors state “all the four patients had preimaging clinical symptoms and signs, which warranted imaging as per guidelines suggested by the British Geriatrics Society and the Australian and New Zealand Society for Geriatric Medicine,” though they do not provide further details. The strength of this paper is that it isolated patients who developed delirium while hospitalized; however, conclusions were limited by the small sample size.
Lai et al.’s case-control study evaluated 300 consecutive patients admitted to a delirium unit over 18 months.6 Of these 300 patients, 200 (67%) had CT performed; 29/200 (14.5%) had intracranial findings on CT that explained their delirium, including 13 ischemic strokes, seven subdural hemorrhages, nine intracerebral hemorrhages, and three additional ischemic strokes that evolved on follow-up imaging but were not present on the initial scans. The authors performed univariate and multivariate analyses to identify risk factors for an intracranial cause of delirium. Only 3/29 patients with a positive scan did not have one of three main risk factors the authors identified: a fall in the preceding two weeks, new neurologic findings, or sudden deterioration of consciousness. It should be noted that authors did not define “deterioration of consciousness” and that all patients had confusion on admission to the unit, rather than developing during hospitalization.
Hijazi et al. conducted a retrospective cohort study over a 20-month period of 1,653 patients with delirium at the time of admission or during their hospitalization. Patients with delirium due to drug or medication withdrawal or “psychiatric reasons” were excluded. Overall, 538 (32.5%) patients underwent CT, MRI or both, and 78 (14.5%) patients had a positive finding on neuroimaging. This study’s 14.5 % overall yield matches that of Lai et al. Unfortunately, the study included all patients with delirium and did not report the rates of fall, neurologic deficits, and/or use of anticoagulation among those with positive neuroimaging. This limits the generalizability of the findings to a cohort of patients without intracranial pathology risk factors.
The reported yield of neuroimaging for hospitalized patients with delirium ranged from 2.7% to 14.5% across studies. However, in studies taking into account specific patient risk factors; the reported yields in patients without focal neurologic findings, new decline in mental status, systemic anticoagulation, or recent falls were 0%,11 1.4%,10 and 1.5%.6 While a rate of 1.5% may appear high for a serious outcome such as stroke or intracranial bleeding, it is comparable to rates reported for missed major cardiac events in clinical algorithms for evaluating chest pain.12 It should also be noted that neuroimaging is imperfect for acute stroke, and thus the positive or negative predictive value may be poor in the setting of low prevalence. For example, for detection of any acute stroke, the sensitivity/specificity of MRI and CT are 83%/97% and 26%/98% respectively.13
Neuroimaging is expensive and has risks. The average charge for a head CT is approximately $1,400 at academic institutions.14 Moreover, computed tomography exposes patients to significant radiation and up to 2% of malignancies in the United States may be attributable to prior tomography exposure.15 Additionally, there are non-negligible rates of incidental findings during neuroimaging, 1% for CT16 and 2.7%-13.7% for MRI,17,18 which may result in further evaluation or treatment that causes significant patient anxiety. Obtaining neuroimaging on delirious patients can be time consuming and labor intensive, which could delay care to other patients. Additionally, sedating medications are often administered to agitated patients prior to imaging, which risk worsening delirium. Ordering neuroimaging for all patients with acute delirium, therefore, exposes the large majority to unnecessary costs and potential harms.
WHEN NEUROIMAGING TO EVALUATE DELIRIUM IN HOSPITALIZED PATIENTS COULD BE REASONABLE
The diagnostic yield of head CT in the evaluation of delirium is significantly higher in patients with specific risk factors. Lai et al. found adjusted odds ratios for abnormal CT of 18.2 in patients with new focal deficits, 5.6 with a fall in the preceding two weeks and 4.6 in patients with deterioration in consciousness. Patients with systemic anticoagulation had higher unadjusted, (OR 2.4) though not adjusted odds of having an abnormal CT.6 Thiesen-Toupal et al. excluded patients with recent falls or neurologic deficits but reported that three out of six delirious patients with abnormal neuroimaging were anticoagulated.10 Vijayakrishnan et al. found that all four delirious patients with intracranial findings met guideline criteria for neuroimaging.11 Thus, current recommendations for neuroimaging in delirious patients with falls, focal neurologic deficits, or systemic anticoagulation are appropriate. In situations when a provider lacks an accurate history and is unable to determine if risk factors are present (for example a confused patient found sitting on the floor next to the bed), it may also be reasonable to consider neuroimaging.
Data are limited, but some authors advocate for neuroimaging in cases of delirium that do not improve with treatment.6 Additionally, it may be reasonable to consider neuroimaging in delirium patients with predispositions to embolic or metastatic intracranial processes such as endovascular infections and certain malignancies.4
WHAT YOU SHOULD DO INSTEAD OF NEUROIMAGING TO EVALUATE DELIRIUM IN HOSPITALIZED PATIENTS
Hospitalized patients with acute confusion should be assessed for delirium with a validated instrument such as the Confusion Assessment Method (CAM).19,20 The original CAM included several components: acute change in mental status with a fluctuating course and inattention, plus either disorganized thinking and/or altered level of consciousness. Multiple delirium assessment tools have been created and validated, all of which include inattention as a required feature. A recent hospital-based study using a two item bedside test asking the patient to name the day of the week and list the months of the year backwards detected delirium with a sensitivity of 93% and specificity of 64%.21 Once the diagnosis of delirium is established, evaluation should begin with a careful history and physical examination focused on the identification of risk factors such as physical restraints, indwelling urinary catheters, and drugs known to precipitate delirium, particularly those with withdrawal potential, anticholinergic properties, and sedative-hypnotic agents.22-24 Delirium may be the first harbinger of serious medical illness and specific testing should be guided by clinical suspicion. In general, a thorough physical examination should look for focal neurologic deficits, hypoxia, signs of infection, and other inflammatory or painful processes that could precipitate delirium.25 Targeted laboratory evaluation may include a basic metabolic panel to identify electrolyte (including calcium) and metabolic derangements, complete blood count, and urinalysis if infection is suspected.
RECOMMENDATIONS
- Use a validated instrument such as CAM to evaluate hospitalized patients who develop altered mental status.
- Delirious patients should undergo a thorough history including a review of medications, physical exam, and targeted laboratory testing aimed at identifying common risk factors and precipitants of delirium that should be addressed.
- Perform neuroimaging if there is a history of fall or head trauma in the preceding two weeks, any new focal abnormalities on neurologic exam or if the patient is receiving systemic anticoagulation.
- It may be reasonable to consider neuroimaging for patients with an atypical course of delirium, such as a sudden decline in the level of consciousness, persistence despite addressing identified factors, or if there is a high degree of suspicion for embolic or metastatic processes.
CONCLUSIONS
Performing neuroimaging in undifferentiated patients who develop delirium while hospitalized has a low diagnostic yield, is costly, and is potentially harmful. Neuroimaging should be reserved for those with identified risk factors for intracranial pathology. For the patient described in the initial vignette with no risk factors for intracranial cause, neuroimaging would be unlikely to contribute to her care. To change provider beliefs and behaviors regarding neuroimaging, prospective studies evaluating guideline implementation are needed. However, based on the current evidence, neuroimaging should be reserved for those with identified risk factors.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by e-mailing TWDFNR@hospitalmedicine.org.
Disclosures
The authors have no conflicts of interest relevant to this article to disclose.
1. Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922. doi: 10.1016/S0140-6736(13)60688-1. PubMed
2. Clinical practice guidelines for the management of delirium in older people. 2006; https://www2.health.vic.gov.au/Api/downloadmedia/%7BDAA8404B-FEE7-4BDA-8A1E-A32494783B7F%7D. Accessed June 4, 2018.
3. Delirium: prevention, diagnosis and management. NICE Guidance 2010; https://www.nice.org.uk/guidance/cg103. Accessed June 4, 2018.
4. Michaud L, Bula C, Berney A, et al. Delirium: guidelines for general hospitals. J Psychosom Res. 2007;62(3):371-383. doi: 10.1016/j.jpsychores.2006.10.004. PubMed
5. Bush SH, Marchington KL, Agar M, Davis DH, Sikora L, Tsang TW. Quality of clinical practice guidelines in delirium: a systematic appraisal. BMJ Open. 2017;7(3):e013809. doi: 10.1136/bmjopen-2016-013809. PubMed
6. Lai MM, Wong Tin Niam DM. Intracranial cause of delirium: computed tomography yield and predictive factors. Intern Med J. 2012;42(4):422-427. doi: 10.1111/j.1445-5994.2010.02400.x. PubMed
7. Hijazi Z, Lange P, Watson R, Maier AB. The use of cerebral imaging for investigating delirium aetiology. Eur J Intern Med. 2018;52:35-39. doi: 10.1016/j.ejim.2018.01.024. PubMed
8. Oldenbeuving AW, de Kort PL, Jansen BP, Roks G, Kappelle LJ. Delirium in acute stroke: a review. Int J Stroke. 2007;2(4):270-275. doi: 10.1111/j.1747-4949.2007.00163.x. PubMed
9. Benbadis SR, Sila CA, Cristea RL. Mental status changes and stroke. J Stroke Cerebrovasc Dis. 1994;4(4):216-219. doi: 10.1016/S1052-3057(10)80093-X. PubMed
10. Theisen-Toupal J, Breu AC, Mattison ML, Arnaout R. Diagnostic yield of head computed tomography for the hospitalized medical patient with delirium. J Hosp Med. 2014;9(8):497-501. doi: 10.1002/jhm.2198. PubMed
11. Vijayakrishnan R, Ramasubramanian A, Dhand S. Utility of head CT scan for acute inpatient delirium. Hosp Top. 2015;93(1):9-12. doi: 10.1080/00185868.2015.1012928. PubMed
12. Backus BE, Six AJ, Kelder JC, et al. A prospective validation of the HEART score for chest pain patients at the emergency department. Int J Cardiol. 2013;168(3):2153-2158. doi: 10.1016/j.ijcard.2013.01.255. PubMed
13. Chalela JA, Kidwell CS, Nentwich LM, et al. Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison. Lancet. 2007;369(9558):293-298. doi: 10.1016/S0140-6736(07)60151-2. PubMed
14. Paul AB, Oklu R, Saini S, Prabhakar AM. How much is that head CT? Price transparency and variability in radiology. J Am Coll Radiol. 2015;12(5):453-457. doi: 10.1016/j.jacr.2014.12.016. PubMed
15. Brenner DJ, Hall EJ. Computed tomography--an increasing source of radiation exposure. N Engl J Med. 2007;357(22):2277-2284. doi: 10.1056/NEJMra072149. PubMed
16. Eskandary H, Sabbagh M, Khajehpour F, Eskandari M. Incidental findings in brain computed tomography scans of 3000 head trauma patients. Surg Neurol. 2005;63(6):550-553; discussion 553. doi: 10.1016/j.surneu.2004.07.049. PubMed
17. Vernooij MW, Ikram MA, Tanghe HL, et al. Incidental findings on brain MRI in the general population. N Engl J Med. 2007;357(18):1821-1828. doi: 10.1056/NEJMoa070972. PubMed
18. Morris Z, Whiteley WN, Longstreth WT, Jr., et al. Incidental findings on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2009;339:b3016. doi: 10.1136/bmj.b3016. PubMed
19. Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941-948. doi: 10.7326/0003-4819-113-12-941. PubMed
20. Wei LA, Fearing MA, Sternberg EJ, Inouye SK. The Confusion Assessment Method: a systematic review of current usage. J Am Geriatr Soc. 2008;56(5):823-830. doi: 10.1111/j.1532-5415.2008.01674.x. PubMed
21. Fick DM, Inouye SK, Guess J, et al. Preliminary development of an ultrabrief two-item bedside test for delirium. J Hosp Med. 2015;10(10):645-650. doi: 10.1002/jhm.2418. PubMed
22. Tune L, Carr S, Hoag E, Cooper T. Anticholinergic effects of drugs commonly prescribed for the elderly: potential means for assessing the risk of delirium. Am J Psychiatry. 1992;149(10):1393-1394. doi: 10.1176/ajp.149.10.1393. PubMed
23. Inouye SK, Schlesinger MJ, Lydon TJ. Delirium: a symptom of how hospital care is failing older persons and a window to improve the quality of hospital care. Am J Med. 1999;106(5):565-573. doi: 0.1016/S0002-9343(99)00070-4. PubMed
24. Marcantonio ER. Delirium in hospitalized older adults. N Engl J Med. 2017;377(15):1456-1466. doi: 10.1056/NEJMc1714932. PubMed
25. Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA. 1996;275(11):852-857. doi: 10.1001/jama.1996.03530350034031. PubMed
1. Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922. doi: 10.1016/S0140-6736(13)60688-1. PubMed
2. Clinical practice guidelines for the management of delirium in older people. 2006; https://www2.health.vic.gov.au/Api/downloadmedia/%7BDAA8404B-FEE7-4BDA-8A1E-A32494783B7F%7D. Accessed June 4, 2018.
3. Delirium: prevention, diagnosis and management. NICE Guidance 2010; https://www.nice.org.uk/guidance/cg103. Accessed June 4, 2018.
4. Michaud L, Bula C, Berney A, et al. Delirium: guidelines for general hospitals. J Psychosom Res. 2007;62(3):371-383. doi: 10.1016/j.jpsychores.2006.10.004. PubMed
5. Bush SH, Marchington KL, Agar M, Davis DH, Sikora L, Tsang TW. Quality of clinical practice guidelines in delirium: a systematic appraisal. BMJ Open. 2017;7(3):e013809. doi: 10.1136/bmjopen-2016-013809. PubMed
6. Lai MM, Wong Tin Niam DM. Intracranial cause of delirium: computed tomography yield and predictive factors. Intern Med J. 2012;42(4):422-427. doi: 10.1111/j.1445-5994.2010.02400.x. PubMed
7. Hijazi Z, Lange P, Watson R, Maier AB. The use of cerebral imaging for investigating delirium aetiology. Eur J Intern Med. 2018;52:35-39. doi: 10.1016/j.ejim.2018.01.024. PubMed
8. Oldenbeuving AW, de Kort PL, Jansen BP, Roks G, Kappelle LJ. Delirium in acute stroke: a review. Int J Stroke. 2007;2(4):270-275. doi: 10.1111/j.1747-4949.2007.00163.x. PubMed
9. Benbadis SR, Sila CA, Cristea RL. Mental status changes and stroke. J Stroke Cerebrovasc Dis. 1994;4(4):216-219. doi: 10.1016/S1052-3057(10)80093-X. PubMed
10. Theisen-Toupal J, Breu AC, Mattison ML, Arnaout R. Diagnostic yield of head computed tomography for the hospitalized medical patient with delirium. J Hosp Med. 2014;9(8):497-501. doi: 10.1002/jhm.2198. PubMed
11. Vijayakrishnan R, Ramasubramanian A, Dhand S. Utility of head CT scan for acute inpatient delirium. Hosp Top. 2015;93(1):9-12. doi: 10.1080/00185868.2015.1012928. PubMed
12. Backus BE, Six AJ, Kelder JC, et al. A prospective validation of the HEART score for chest pain patients at the emergency department. Int J Cardiol. 2013;168(3):2153-2158. doi: 10.1016/j.ijcard.2013.01.255. PubMed
13. Chalela JA, Kidwell CS, Nentwich LM, et al. Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison. Lancet. 2007;369(9558):293-298. doi: 10.1016/S0140-6736(07)60151-2. PubMed
14. Paul AB, Oklu R, Saini S, Prabhakar AM. How much is that head CT? Price transparency and variability in radiology. J Am Coll Radiol. 2015;12(5):453-457. doi: 10.1016/j.jacr.2014.12.016. PubMed
15. Brenner DJ, Hall EJ. Computed tomography--an increasing source of radiation exposure. N Engl J Med. 2007;357(22):2277-2284. doi: 10.1056/NEJMra072149. PubMed
16. Eskandary H, Sabbagh M, Khajehpour F, Eskandari M. Incidental findings in brain computed tomography scans of 3000 head trauma patients. Surg Neurol. 2005;63(6):550-553; discussion 553. doi: 10.1016/j.surneu.2004.07.049. PubMed
17. Vernooij MW, Ikram MA, Tanghe HL, et al. Incidental findings on brain MRI in the general population. N Engl J Med. 2007;357(18):1821-1828. doi: 10.1056/NEJMoa070972. PubMed
18. Morris Z, Whiteley WN, Longstreth WT, Jr., et al. Incidental findings on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2009;339:b3016. doi: 10.1136/bmj.b3016. PubMed
19. Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941-948. doi: 10.7326/0003-4819-113-12-941. PubMed
20. Wei LA, Fearing MA, Sternberg EJ, Inouye SK. The Confusion Assessment Method: a systematic review of current usage. J Am Geriatr Soc. 2008;56(5):823-830. doi: 10.1111/j.1532-5415.2008.01674.x. PubMed
21. Fick DM, Inouye SK, Guess J, et al. Preliminary development of an ultrabrief two-item bedside test for delirium. J Hosp Med. 2015;10(10):645-650. doi: 10.1002/jhm.2418. PubMed
22. Tune L, Carr S, Hoag E, Cooper T. Anticholinergic effects of drugs commonly prescribed for the elderly: potential means for assessing the risk of delirium. Am J Psychiatry. 1992;149(10):1393-1394. doi: 10.1176/ajp.149.10.1393. PubMed
23. Inouye SK, Schlesinger MJ, Lydon TJ. Delirium: a symptom of how hospital care is failing older persons and a window to improve the quality of hospital care. Am J Med. 1999;106(5):565-573. doi: 0.1016/S0002-9343(99)00070-4. PubMed
24. Marcantonio ER. Delirium in hospitalized older adults. N Engl J Med. 2017;377(15):1456-1466. doi: 10.1056/NEJMc1714932. PubMed
25. Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA. 1996;275(11):852-857. doi: 10.1001/jama.1996.03530350034031. PubMed
© 2019 Society of Hospital Medicine
Every Nook and Cranny
A 46-year-old man presented to the emergency room in the postmonsoon month of September with a seven-day history of high fevers as well as a four-day history of a dry cough, dyspnea, and progressive rash. The patient reported no chest pain, hemoptysis, chest tightness, palpitations, wheezing, orthopnea, paroxysmal nocturnal dyspnea, or leg swelling. He lived and sought healthcare in Delhi, India.
Fever followed by a progressive but as yet uncharacterized rash and pulmonary symptoms in a middle-aged man suggests a host of possibilities. While it is tempting to ascribe his symptoms to an infectious process, especially a “tropical” infection based on his residence in Delhi, the location may simply represent a red herring. Potential infections can be divided into those endemic to the Indian subcontinent, and those encountered more globally. The former include diseases such as measles and dengue, while the latter include entities such as Mycoplasma pneumonia, varicella, and acute human immunodeficiency virus (HIV) infection. Noninfectious categories of diseases that should be considered include drug reactions and rheumatologic processes. Several rheumatologic diseases, including granulomatosis with polyangiitis, eosinophilic granulomatosis with polyangiitis, and systemic lupus erythematosus (SLE) may present with fever, rash, and pulmonary symptomatology.
A history of the patient’s exposures, both environmental and pharmaceutical, should be obtained. More information regarding his immunization history, rash characteristics (distribution and nature of the lesions), and other salient exam findings such as organomegaly and joint abnormalities will be helpful.
Fever reached a maximum of 103° Fahrenheit and was associated with chills but not rigors. There were several fever spikes daily, relieved completely with antipyretics. The patient’s dyspnea was predominantly noted on exertion, nonpleuritic, not temporally related to cough, and progressively worsening over three days. The skin lesions were first noticed on his trunk and were described as reddish, flat, and pinpoint size. However, the rash spread to the face and extremities sparing the palms and soles. There was no bleeding, nausea, vomiting, abdominal pain, change in bowel habits, dysuria, headache, photophobia, neck stiffness, or joint pain.
The patient reported no significant past medical history, took no medications, and had no recent travel outside of Delhi, India in the past year. He was married and monogamous. He had no pets nor did he report any contact with animals. He did not use tobacco, alcohol, or illicit substances. He did not remember being bitten by an insect. He worked as a software engineer. There was no history of similar illness in the patient’s family or at his workplace. He had no history of recent blood transfusion or immunization (including MMR and Tdap).
Several noninfectious and inflammatory conditions can explain his symptoms. Eosinophilic granulomatosis with polyangiitis is considerably less likely in the absence of asthma, and vasculitic processes, in general, are less likely given the nongravity dependent nature of the rash. SLE and sarcoidosis are possible causes of a systemic inflammatory illness presenting acutely with fever, rash, and pulmonary symptoms.
The patient’s expanded history makes several infections less likely. Although much of the presentation is consistent with measles, the initial appearance of the truncal rash is atypical, and there is no mention of coryza or conjunctivitis. Likewise, the description of the exanthem is not suggestive of varicella, and dengue and chikungunya are much less likely in the absence of a headache and arthralgias. Other infections including leptospirosis and scrub typhus are possible, and both might be contracted in greater Delhi. Typhoid is another infectious syndrome endemic to the Indian subcontinent that should be considered. The presence of rash involving the face and extremities would be highly atypical, however; and the presence of dyspnea and the absence of a headache argue against typhoid. Acute HIV infection and Mycoplasma pneumonia remain possible diagnoses. Toxic shock syndrome is possible, but a faster and fulminant course would be expected.
On physical examination, the temperature was 103° Fahrenheit, heart rate was 120 beats per minute and regular, respiratory rate was 24 breaths per minute, blood pressure was 100/60 mm Hg, and resting oxygen saturation was 93% while breathing ambient air. He appeared uncomfortable. Jugular venous pulse was elevated at 10 cm H2O. Mild icterus was present, but there was neither conjunctival congestion nor subconjunctival hemorrhage. S1 and S2 heart sounds were loud, but there were no murmurs. Chest auscultation revealed bilateral basal coarse crackles. The abdominal right upper quadrant was mildly tender to palpation, and the liver edge was palpable 2 cm below the subcostal margin. There was neither splenomegaly nor peripheral lymphadenopathy. Kernig and Brudzinski signs were negative, and there were no focal neurological deficits. A generalized, nonpalpable, maculopapular and petechial rash was present on the face, extremities, and trunk.
The patient’s presentation must now incorporate the additional findings of bibasilar chest crackles, maculopapular/petechial rash, icterus, modest hypoxia, and hepatomegaly. Some of the noninfectious entities already mentioned (SLE and sarcoidosis) remain possible explanations. Hemophagocytic lymphohistiocytosis (HLH) may also explain most of the patient’s presenting signs and symptoms, and several other infectious diseases account for his presentation. Scrub typhus (or a more uncommon rickettsia disease, Indian tick typhus), leptospirosis, and perhaps infective endocarditis seem most likely to provide a unifying diagnosis for the symptoms mentioned above. Leptospirosis presents in a minority of instances as a severe illness known as Weil disease, characterized by several of this patient’s findings including icterus, kidney injury, and pulmonary symptoms. However, the rash is relatively uncommon in leptospirosis and when present, is usually more localized. The patient’s rash as described is not typically expected in infective endocarditis, although high-grade Staphylococcus aureus bacteremia will occasionally present with a diffuse rash that may be confused with that of meningococcemia. The etiology of the patient’s elevated jugular venous pressure is not readily apparent, with the cardiac examination making acute valvular insufficiency much less likely. Myocarditis, however, is possible in the setting of several of the diseases listed above, including leptospirosis, scrub typhus, SLE, and dengue.
In addition to basic laboratory studies and a chest radiograph, multiple sets of blood cultures should be obtained, along with a transthoracic echocardiogram and a ferritin level. The evidence to support leptospirosis and scrub typhus is strong enough to justify empiric use of doxycycline once the blood cultures are obtained, especially given the difficulty in definitively diagnosing these diseases in a timely fashion.
Laboratory analysis revealed a total leukocyte count of 13,600/uL (85% neutrophils), hemoglobin 10 g/dL, and platelet count 35,000/uL. Absolute eosinophil count was 136/uL. Serum chemistry showed sodium of 145 meq/L, potassium 4.1 meq/L, blood urea nitrogen 80 mg/dL, creatinine 1.6 mg/dL, aspartate transaminase (AST) 44 U/L (normal, 0-40), alanine transaminase (ALT) 81 U/L (normal, 0-40), direct bilirubin 3 mg/dL, and indirect bilirubin 3 mg/dL. Lactate dehydrogenase, alkaline phosphatase, albumin, and coagulation studies were normal. Erythrocyte sedimentation rate (ESR) was 42 mm (normal, 0-25) and highly sensitive C-reactive protein was 42 mg/L (normal, 0-10). Arterial blood gas on ambient air revealed a pH of 7.52, PaCO2 24 mm Hg, PaO2 55 mm Hg, and bicarbonate 20 meq/L. Urinalysis was normal. Blood cultures were obtained. Electrocardiogram (ECG) showed regular narrow complex tachycardia with incomplete left bundle branch block. Old ECGs were not available for comparison. Chest radiograph showed bilateral air space opacities with evidence of vein cephalization. Abdominal and pelvis ultrasonography showed pericholecystic fluid and mild hepatomegaly, but no free fluid, pleural effusion, or evidence of cholecystitis. Point of care immunochromatographic rapid malarial antigen detection test (detects Plasmodium falciparum, Plasmodium vivax, Plasmodium malaria, and Plasmodium ovale) was negative.
Most of the findings described are commonly observed in both scrub typhus and leptospirosis, including cytopenias, parenchymal infiltrates, hepatomegaly, elevated transaminases and bilirubin, cardiac involvement, fever, and rash. The rash described is more consistent with scrub typhus than with leptospirosis. The absence of a headache and joint findings argue modestly against these diagnoses. Likewise, HLH provides an adequate explanation for most of the patient’s symptoms, signs, and test results. These include fever, lung involvement, rash, hepatomegaly, elevated bilirubin, and cytopenias; however, leukocytosis and cardiac involvement are less characteristic. SLE also provides a satisfactory explanation for much of the symptoms, although the rash characteristics, normal urinalysis, and leukocytosis make this diagnosis less likely.
Additional testing that should be performed includes serum antinuclear antibody (ANA) and ferritin, since the latter may be markedly elevated in the setting of HLH. Bone marrow aspirate and biopsy should be performed looking specifically for evidence of hemophagocytosis. Finally, a transthoracic echocardiogram (TTE) should be performed to assess evidence of myocardial dysfunction as it may alter the therapeutic approach, although the results will be unlikely to differentiate between the preceding considerations.
Troponin I was negative, but N-terminal probrain natriuretic peptide was elevated at 20,000 pg/mL (normal, 0-900). D-dimer was negative. TTE showed left ventricular ejection fraction (LVEF) of 35% with global left ventricular hypokinesis. On three separate examinations, the peripheral blood smear did not show malarial parasites, atypical lymphocytes, or schistocytes. Three sets of blood cultures, testing for bacteria and fungi, were sterile. A throat culture was sterile. Widal test, as well as Leptospira and Mycoplasma serologies, were negative. Serology for Legionella pneumophila was positive, but the urinary antigen testing was negative. Antibodies to HIV 1 and 2 and anti-hepatitis C virus (HCV) antibody were negative. Dengue IgM ELISA (qualitative) returned positive.
Despite the absence of arthralgias, myalgias, headache, and retro-orbital pain, a positive dengue IgM ELISA supports acute dengue infection, provided the patient did not experience an unexplained febrile illness in the previous months. Most of his presentation may be explained by dengue, including fever, rash, liver abnormalities, myocardial dysfunction, and thrombocytopenia. The bilateral airspace opacities seen on chest radiograph also fit reasonably provided these actually reflect pulmonary edema. Leukocytosis (as opposed to leukopenia) is highly unexpected in dengue, but its presence could be an outlier.
If dengue does indeed explain the entire presentation, defervescence should have occurred by the time the blood cultures and serologic studies returned. Also, by that time, the patient would be expected to demonstrate evidence of improvement, barring the appearance of the serious complications of dengue hemorrhagic fever/dengue shock syndrome. Should fever persist and signs of recovery fail to materialize, the possibility of a superimposed process will need to be considered. Of note, the sensitivity of Leptospira serology early in the course of illness is low, and leptospirosis is thus not yet excluded.
A presumptive diagnosis of severe dengue fever was made, based on evidence of pulmonary edema and sepsis. The patient was managed conservatively with oral fluid restriction, low dose of diuretics, and supplemental oxygenation. The patient was also given levofloxacin for possible legionellosis. Despite these therapies, the patient had no improvement in 24 hours. His tachypnea increased, and his measured PaO2 to FIO2 (P:F) ratio decreased to 230 from 285 on admission. This prompted the initiation of BiPAP at 10 cm H2O inspiration PAP and 5 cm H2O expiration PAP. However, he did not tolerate BiPAP, and his P:F ratio decreased to below 200.
The patient was transferred to the intensive care unit and underwent elective intubation with mechanical ventilation. Axial and coronal computed tomography of the thorax (Figure 1A and 1B, respectively) showed extensive ground-glass opacities and consolidation sparing the nondependent portions of the lungs. On physical inspection, a round, well-defined, painless black lesion surrounded by erythema was noticed in the right axilla (Figure 2). The rest of the examination findings were unchanged.
The discovery of eschar in the axilla provides a “pivot point” in determining the cause of the patient’s illness. This finding appears to point, with high specificity, toward rickettsia as the explanation of the patient’s disease, and this is most likely to be scrub typhus. The report of a positive dengue IgM may represent concurrent infection or may simply reflect a recent infection in an area that is highly endemic for dengue. Although most of the patient’s clinical presentation could be attributed to dengue, multiple features including the leukocytosis, myocarditis, and elevated bilirubin are more likely to be seen in scrub typhus. In any event, dengue cannot satisfactorily explain the eschar.
No mention has been made to the initiation of doxycycline thus far; this agent needs to be started promptly. Polymerase chain reaction (PCR) testing for scrub typhus should be ordered if available; if not, acute and convalescent serology may be obtained.
Given the finding of axillary eschar, the patient was diagnosed with scrub typhus. Doxycycline 100 mg by nasogastric tube twice a day was initiated. The patient began to show marked symptomatic improvement. His P:F ratio improved, and he was successfully weaned off and extubated after 24 hours. Postextubation, he was kept on BiPAP for 12 hours. He was transferred out of the ICU and monitored for 72 hours. With therapy, his cytopenias, liver and renal function, and ECG normalized. Indirect immunofluorescence assay for scrub typhus returned positive at a dilution of > 1:512. PCR assay targeting the 56 kDa region of Orientia tsutsugamushi was also positive. Repeated TTE showed an LVEF of 65%. He was subsequently discharged with oral doxycycline and a plan to complete a course of 14 days on an outpatient basis. The final diagnosis was scrub typhus with myocarditis leading to acutely decompensated heart failure with reduced ejection fraction.
DISCUSSION
Scrub typhus is a mite-borne tropical infection caused by the gram-negative intracellular parasite Orientia tsutsugamushi from the Rickettsiaceae family that is known to occur in certain parts of Asia and Australia. Although this entity is well known in the Sub Himalayan belt and southern part of India, very few cases have been described in Delhi, the capital state in North India. Scrub typhus, like most other tropical infections, is found most often during the postmonsoon season.1,2
Patients with scrub typhus present with fever in addition to a variety of nonspecific symptoms and findings. These often manifest within 10 days of being bitten by a mite. Malaise, headache, myalgias, lymphadenopathy, and maculopapular or petechial rash are common. If present, the rash manifests on the 3rd to 5th day of fever.3 Disseminated vasculitis due to scrub typhus can frequently result in multiorgan system involvement. Pulmonary involvement often leads to acute respiratory distress syndrome (ARDS) with an incidence of 8%-10%.1,4 Acute kidney injury, mostly mild and nonoliguric, has been reported in up to 2/3 cases.4-6 The cardiac myocyte is a known target cell affected by scrub typhus, and therefore patients commonly present with myocarditis.7 Liver involvement in scrub typhus is evident through elevated liver enzymes and can occur without other clinical evidence of the illness.4,6,8,9 As in dengue, patients often develop thrombocytopenia, but normal hemoglobin in scrub typhus differentiates it from dengue.6,8
Given the nonspecific presentation, it can be challenging to diagnose and treat scrub typhus. The gold standard for diagnosis is the detection of IgM antibodies to Orientia tsutsugamushi using an indirect immunofluorescence assay (IFA). For patients from endemic regions, it may be necessary to show a four-fold increase in titers two weeks apart to distinguish from background immunity. Presence of the characteristic eschar, as discussed below, is highly suggestive of scrub typhus. The treatment of choice is doxycycline or azithromycin for seven days.10,11 Early initiation of doxycycline when considering either scrub typhus or leptospirosis is appropriate and may be life-saving.
Medical decision making is fraught with uncertainty, and physicians must use their experience, evidence base, and cognitive heuristics wisely to care for patients effectively. For this patient, the region of Delhi experiences massive outbreaks of dengue every year during the time the patient presented to the hospital, whereas rickettsia infections are relatively uncommon. The clinical presentation was conceivably consistent with either dengue or scrub typhus, though somewhat more suggestive of the latter. Once the serological diagnosis of recent or concomitant dengue was obtained, however, scrub typhus was considered even less. The team called upon Occam’s razor or the heuristic that the simplest and most unifying explanation for any given problem is the one most likely to be correct and that other, less satisfactory explanations (in this case, scrub typhus) are “shaven off.” The patient was managed conservatively for dengue. Only when his condition worsened did the team recognize this conflicting information without dismissing it, consider alternative possibilities, and reexamined the patient.
An eschar can be an important clue in the diagnosis of scrub typhus, though it is not often obvious. The presence of this necrotic skin lesion with black crust is highly suggestive of scrub typhus, and in the right clinical context, it is virtually diagnostic. However, it is uncommon (9.5%-45%) in most of the studies from the Indian subcontinent (ie, high specificity but low sensitivity).1,12 An eschar is often found in obscure locations such as the axillae or groin, areas that may easily be missed or overlooked. Eschars may be seen in a variety of other infectious diseases, including rickettsia pox, Rocky Mountain spotted fever, other members of the spotted fever group, tularemia, and cutaneous anthrax. Given this patient’s lack of improvement, repeated examination revealed an eschar in the right axilla, a finding that was either missed or still evolving at the time of presentation.
This case illustrates the challenges in interpreting the significance of multiple positive serological tests in the context of an undifferentiated clinical syndrome. Possible reasons for a positive dengue serology could have been persistent antibodies from a previous infection, recent asymptomatic infection, concurrent infection, or cross-reactivity with flaviviruses such as West Nile Virus or Japanese Encephalitis.13 The patient also had positive IgM antibodies against Legionella pneumophila, but the urinary antigen was negative. In view of a negative antigen test, low specificity of the serologic test, low incidence of legionellosis in the Indian subcontinent, and absence of therapeutic response to a trial of fluoroquinolones, the diagnosis of legionellosis was considered unlikely in this patient.
With rapid advancements in technology, the importance of history taking and physical examination is at risk of being overshadowed. Approximately 80% of correct diagnoses in medicine can arrive through history and physical examination alone.14,15 In this case, Occam’s razor combined with multiple serological tests was relied on to create the likely list of diagnoses. However, recognition of the limitations of these heuristics and tests proved critical. The life-saving diagnosis was only made when the clinicians returned to basics, looked in every nook and cranny, and found the eschar on physical examination.
KEY TEACHING POINTS
- In patients living in endemic areas who present with an acute febrile illness, the differential diagnosis should include “tropical” infections such as dengue, chikungunya, enteric fever, leptospirosis, malaria, and scrub typhus.
- Serology is commonly employed for diagnosis of tropical infections, which may be misleading. These tests can be falsely positive from past asymptomatic infection or cross reactivity between antibodies, or falsely negative, as in the first few days of infection.
- Presence of eschar is a very useful clue in the diagnosis of scrub typhus, but this finding can be missed since it is often found in obscure locations. A thorough clinical history and physical examination are paramount.
Disclosures
The authors do not report any conflict of interest.
1. Gupta N, Chaudhry R, Kabra SK, et al. In search of scrub typhus: a prospective analysis of clinical and epidemiological profile of patients from a tertiary care hospital in New Delhi. Adv Infect Dis. 2015;5(4):140. doi: 10.4236/aid.2015.54017.
2. Kamarasu K, Malathi M, Rajagopal V, Subramani K, Jagadeeshramasamy D, Mathai E. Serological evidence for the wide distribution of spotted fevers & typhus fever in Tamil Nadu. Indian J Med Res. 2007;126(2):128-130. PubMed
3. Mahajan SK. Scrub typhus. J Assoc Physicians India. 2005;53:954-958. PubMed
4. Mahajan SK, Rolain JM, Kashyap R, et al. Scrub typhus in the Himalayas. Emerg Infect Dis. 2006;12(10):1590-1592. doi: 10.3201/eid1210.051697. PubMed
5. Attur RP, Kuppasamy S, Bairy M, et al. Acute kidney injury in scrub typhus. Clin Exp Nephrol. 2013;17(5):725-729. doi: 10.1007/s10157-012-0753-9. PubMed
6. Varghese GM, Trowbridge P, Janardhanan J, et al. Clinical profile and improving mortality trend of scrub typhus in South India. Int J Infect Dis. 2014;23:39-43. doi: 10.1016/j.ijid.2014.02.009. PubMed
7. Taylor AJ, Paris DH, Newton PN. A systematic review of mortality from untreated scrub typhus (Orientia tsutsugamushi). PLoS Negl Trop Dis. 2015;9(8):e0003971. doi.org/10.1371/journal.pntd.0003971 PubMed
8. Chrispal A, Boorugu H, Gopinath KG, et al. Scrub typhus: an unrecognized threat in South India-clinical profile and predictors of mortality. Trop Doct. 2010;40(3):129-133. doi: 10.1258/td.2010.090452. PubMed
9. Mathai E, Rolain JM, Verghese GM, et al. Outbreak of scrub typhus in southern India during the cooler months. Ann N Y Acad Sci. 2003;990:359-364. doi: 10.1111/j.1749-6632.2003.tb07391.x PubMed
10. Gupta N, Chaudhry R, Kabra SK, et al. Comparative evaluation of serological and molecular methods for the diagnosis of scrub typhus in Indian settings. Jpn J Infect Dis. 2017;70(2):221-222. doi: 10.7883/yoken.JJID.2016.139. PubMed
11. Rahi M, Gupte MD, Bhargava A, Varghese GM, Arora R. DHR-ICMR Guidelines for diagnosis & management of Rickettsial diseases in India. Indian J Med Res. 2015;141(4):417-422. doi: 10.4103/0971-5916.159279. PubMed
12. Sharma A, Mahajan S, Gupta ML, Kanga A, Sharma V. Investigation of an outbreak of scrub typhus in the Himalayan region of India. Jpn J Infect Dis. 2005;58(4):208-210. PubMed
13. Gupta N, Chaudhry R, Mirdha B, et al. Scrub typhus and leptospirosis: the fallacy of diagnosing with IgM enzyme-linked immunosorbent assay. J Microb Biochem Technol. 2016;8:71-75. doi: 10.4172/1948-5948.1000265.
14. Peterson MC, Holbrook JH, Von Hales D, Smith NL, Staker LV. Contributions of the history, physical examination, and laboratory investigation in making medical diagnoses. West J Med. 1992;156(2):163-165. doi: 10.1097/00006254-199210000-00013 PubMed
15. Roshan M, Rao AP. A study on relative contributions of the history, physical examination and investigations in making a medical diagnosis. J Assoc Physicians India. 2000;48(8):771-775. PubMed
A 46-year-old man presented to the emergency room in the postmonsoon month of September with a seven-day history of high fevers as well as a four-day history of a dry cough, dyspnea, and progressive rash. The patient reported no chest pain, hemoptysis, chest tightness, palpitations, wheezing, orthopnea, paroxysmal nocturnal dyspnea, or leg swelling. He lived and sought healthcare in Delhi, India.
Fever followed by a progressive but as yet uncharacterized rash and pulmonary symptoms in a middle-aged man suggests a host of possibilities. While it is tempting to ascribe his symptoms to an infectious process, especially a “tropical” infection based on his residence in Delhi, the location may simply represent a red herring. Potential infections can be divided into those endemic to the Indian subcontinent, and those encountered more globally. The former include diseases such as measles and dengue, while the latter include entities such as Mycoplasma pneumonia, varicella, and acute human immunodeficiency virus (HIV) infection. Noninfectious categories of diseases that should be considered include drug reactions and rheumatologic processes. Several rheumatologic diseases, including granulomatosis with polyangiitis, eosinophilic granulomatosis with polyangiitis, and systemic lupus erythematosus (SLE) may present with fever, rash, and pulmonary symptomatology.
A history of the patient’s exposures, both environmental and pharmaceutical, should be obtained. More information regarding his immunization history, rash characteristics (distribution and nature of the lesions), and other salient exam findings such as organomegaly and joint abnormalities will be helpful.
Fever reached a maximum of 103° Fahrenheit and was associated with chills but not rigors. There were several fever spikes daily, relieved completely with antipyretics. The patient’s dyspnea was predominantly noted on exertion, nonpleuritic, not temporally related to cough, and progressively worsening over three days. The skin lesions were first noticed on his trunk and were described as reddish, flat, and pinpoint size. However, the rash spread to the face and extremities sparing the palms and soles. There was no bleeding, nausea, vomiting, abdominal pain, change in bowel habits, dysuria, headache, photophobia, neck stiffness, or joint pain.
The patient reported no significant past medical history, took no medications, and had no recent travel outside of Delhi, India in the past year. He was married and monogamous. He had no pets nor did he report any contact with animals. He did not use tobacco, alcohol, or illicit substances. He did not remember being bitten by an insect. He worked as a software engineer. There was no history of similar illness in the patient’s family or at his workplace. He had no history of recent blood transfusion or immunization (including MMR and Tdap).
Several noninfectious and inflammatory conditions can explain his symptoms. Eosinophilic granulomatosis with polyangiitis is considerably less likely in the absence of asthma, and vasculitic processes, in general, are less likely given the nongravity dependent nature of the rash. SLE and sarcoidosis are possible causes of a systemic inflammatory illness presenting acutely with fever, rash, and pulmonary symptoms.
The patient’s expanded history makes several infections less likely. Although much of the presentation is consistent with measles, the initial appearance of the truncal rash is atypical, and there is no mention of coryza or conjunctivitis. Likewise, the description of the exanthem is not suggestive of varicella, and dengue and chikungunya are much less likely in the absence of a headache and arthralgias. Other infections including leptospirosis and scrub typhus are possible, and both might be contracted in greater Delhi. Typhoid is another infectious syndrome endemic to the Indian subcontinent that should be considered. The presence of rash involving the face and extremities would be highly atypical, however; and the presence of dyspnea and the absence of a headache argue against typhoid. Acute HIV infection and Mycoplasma pneumonia remain possible diagnoses. Toxic shock syndrome is possible, but a faster and fulminant course would be expected.
On physical examination, the temperature was 103° Fahrenheit, heart rate was 120 beats per minute and regular, respiratory rate was 24 breaths per minute, blood pressure was 100/60 mm Hg, and resting oxygen saturation was 93% while breathing ambient air. He appeared uncomfortable. Jugular venous pulse was elevated at 10 cm H2O. Mild icterus was present, but there was neither conjunctival congestion nor subconjunctival hemorrhage. S1 and S2 heart sounds were loud, but there were no murmurs. Chest auscultation revealed bilateral basal coarse crackles. The abdominal right upper quadrant was mildly tender to palpation, and the liver edge was palpable 2 cm below the subcostal margin. There was neither splenomegaly nor peripheral lymphadenopathy. Kernig and Brudzinski signs were negative, and there were no focal neurological deficits. A generalized, nonpalpable, maculopapular and petechial rash was present on the face, extremities, and trunk.
The patient’s presentation must now incorporate the additional findings of bibasilar chest crackles, maculopapular/petechial rash, icterus, modest hypoxia, and hepatomegaly. Some of the noninfectious entities already mentioned (SLE and sarcoidosis) remain possible explanations. Hemophagocytic lymphohistiocytosis (HLH) may also explain most of the patient’s presenting signs and symptoms, and several other infectious diseases account for his presentation. Scrub typhus (or a more uncommon rickettsia disease, Indian tick typhus), leptospirosis, and perhaps infective endocarditis seem most likely to provide a unifying diagnosis for the symptoms mentioned above. Leptospirosis presents in a minority of instances as a severe illness known as Weil disease, characterized by several of this patient’s findings including icterus, kidney injury, and pulmonary symptoms. However, the rash is relatively uncommon in leptospirosis and when present, is usually more localized. The patient’s rash as described is not typically expected in infective endocarditis, although high-grade Staphylococcus aureus bacteremia will occasionally present with a diffuse rash that may be confused with that of meningococcemia. The etiology of the patient’s elevated jugular venous pressure is not readily apparent, with the cardiac examination making acute valvular insufficiency much less likely. Myocarditis, however, is possible in the setting of several of the diseases listed above, including leptospirosis, scrub typhus, SLE, and dengue.
In addition to basic laboratory studies and a chest radiograph, multiple sets of blood cultures should be obtained, along with a transthoracic echocardiogram and a ferritin level. The evidence to support leptospirosis and scrub typhus is strong enough to justify empiric use of doxycycline once the blood cultures are obtained, especially given the difficulty in definitively diagnosing these diseases in a timely fashion.
Laboratory analysis revealed a total leukocyte count of 13,600/uL (85% neutrophils), hemoglobin 10 g/dL, and platelet count 35,000/uL. Absolute eosinophil count was 136/uL. Serum chemistry showed sodium of 145 meq/L, potassium 4.1 meq/L, blood urea nitrogen 80 mg/dL, creatinine 1.6 mg/dL, aspartate transaminase (AST) 44 U/L (normal, 0-40), alanine transaminase (ALT) 81 U/L (normal, 0-40), direct bilirubin 3 mg/dL, and indirect bilirubin 3 mg/dL. Lactate dehydrogenase, alkaline phosphatase, albumin, and coagulation studies were normal. Erythrocyte sedimentation rate (ESR) was 42 mm (normal, 0-25) and highly sensitive C-reactive protein was 42 mg/L (normal, 0-10). Arterial blood gas on ambient air revealed a pH of 7.52, PaCO2 24 mm Hg, PaO2 55 mm Hg, and bicarbonate 20 meq/L. Urinalysis was normal. Blood cultures were obtained. Electrocardiogram (ECG) showed regular narrow complex tachycardia with incomplete left bundle branch block. Old ECGs were not available for comparison. Chest radiograph showed bilateral air space opacities with evidence of vein cephalization. Abdominal and pelvis ultrasonography showed pericholecystic fluid and mild hepatomegaly, but no free fluid, pleural effusion, or evidence of cholecystitis. Point of care immunochromatographic rapid malarial antigen detection test (detects Plasmodium falciparum, Plasmodium vivax, Plasmodium malaria, and Plasmodium ovale) was negative.
Most of the findings described are commonly observed in both scrub typhus and leptospirosis, including cytopenias, parenchymal infiltrates, hepatomegaly, elevated transaminases and bilirubin, cardiac involvement, fever, and rash. The rash described is more consistent with scrub typhus than with leptospirosis. The absence of a headache and joint findings argue modestly against these diagnoses. Likewise, HLH provides an adequate explanation for most of the patient’s symptoms, signs, and test results. These include fever, lung involvement, rash, hepatomegaly, elevated bilirubin, and cytopenias; however, leukocytosis and cardiac involvement are less characteristic. SLE also provides a satisfactory explanation for much of the symptoms, although the rash characteristics, normal urinalysis, and leukocytosis make this diagnosis less likely.
Additional testing that should be performed includes serum antinuclear antibody (ANA) and ferritin, since the latter may be markedly elevated in the setting of HLH. Bone marrow aspirate and biopsy should be performed looking specifically for evidence of hemophagocytosis. Finally, a transthoracic echocardiogram (TTE) should be performed to assess evidence of myocardial dysfunction as it may alter the therapeutic approach, although the results will be unlikely to differentiate between the preceding considerations.
Troponin I was negative, but N-terminal probrain natriuretic peptide was elevated at 20,000 pg/mL (normal, 0-900). D-dimer was negative. TTE showed left ventricular ejection fraction (LVEF) of 35% with global left ventricular hypokinesis. On three separate examinations, the peripheral blood smear did not show malarial parasites, atypical lymphocytes, or schistocytes. Three sets of blood cultures, testing for bacteria and fungi, were sterile. A throat culture was sterile. Widal test, as well as Leptospira and Mycoplasma serologies, were negative. Serology for Legionella pneumophila was positive, but the urinary antigen testing was negative. Antibodies to HIV 1 and 2 and anti-hepatitis C virus (HCV) antibody were negative. Dengue IgM ELISA (qualitative) returned positive.
Despite the absence of arthralgias, myalgias, headache, and retro-orbital pain, a positive dengue IgM ELISA supports acute dengue infection, provided the patient did not experience an unexplained febrile illness in the previous months. Most of his presentation may be explained by dengue, including fever, rash, liver abnormalities, myocardial dysfunction, and thrombocytopenia. The bilateral airspace opacities seen on chest radiograph also fit reasonably provided these actually reflect pulmonary edema. Leukocytosis (as opposed to leukopenia) is highly unexpected in dengue, but its presence could be an outlier.
If dengue does indeed explain the entire presentation, defervescence should have occurred by the time the blood cultures and serologic studies returned. Also, by that time, the patient would be expected to demonstrate evidence of improvement, barring the appearance of the serious complications of dengue hemorrhagic fever/dengue shock syndrome. Should fever persist and signs of recovery fail to materialize, the possibility of a superimposed process will need to be considered. Of note, the sensitivity of Leptospira serology early in the course of illness is low, and leptospirosis is thus not yet excluded.
A presumptive diagnosis of severe dengue fever was made, based on evidence of pulmonary edema and sepsis. The patient was managed conservatively with oral fluid restriction, low dose of diuretics, and supplemental oxygenation. The patient was also given levofloxacin for possible legionellosis. Despite these therapies, the patient had no improvement in 24 hours. His tachypnea increased, and his measured PaO2 to FIO2 (P:F) ratio decreased to 230 from 285 on admission. This prompted the initiation of BiPAP at 10 cm H2O inspiration PAP and 5 cm H2O expiration PAP. However, he did not tolerate BiPAP, and his P:F ratio decreased to below 200.
The patient was transferred to the intensive care unit and underwent elective intubation with mechanical ventilation. Axial and coronal computed tomography of the thorax (Figure 1A and 1B, respectively) showed extensive ground-glass opacities and consolidation sparing the nondependent portions of the lungs. On physical inspection, a round, well-defined, painless black lesion surrounded by erythema was noticed in the right axilla (Figure 2). The rest of the examination findings were unchanged.
The discovery of eschar in the axilla provides a “pivot point” in determining the cause of the patient’s illness. This finding appears to point, with high specificity, toward rickettsia as the explanation of the patient’s disease, and this is most likely to be scrub typhus. The report of a positive dengue IgM may represent concurrent infection or may simply reflect a recent infection in an area that is highly endemic for dengue. Although most of the patient’s clinical presentation could be attributed to dengue, multiple features including the leukocytosis, myocarditis, and elevated bilirubin are more likely to be seen in scrub typhus. In any event, dengue cannot satisfactorily explain the eschar.
No mention has been made to the initiation of doxycycline thus far; this agent needs to be started promptly. Polymerase chain reaction (PCR) testing for scrub typhus should be ordered if available; if not, acute and convalescent serology may be obtained.
Given the finding of axillary eschar, the patient was diagnosed with scrub typhus. Doxycycline 100 mg by nasogastric tube twice a day was initiated. The patient began to show marked symptomatic improvement. His P:F ratio improved, and he was successfully weaned off and extubated after 24 hours. Postextubation, he was kept on BiPAP for 12 hours. He was transferred out of the ICU and monitored for 72 hours. With therapy, his cytopenias, liver and renal function, and ECG normalized. Indirect immunofluorescence assay for scrub typhus returned positive at a dilution of > 1:512. PCR assay targeting the 56 kDa region of Orientia tsutsugamushi was also positive. Repeated TTE showed an LVEF of 65%. He was subsequently discharged with oral doxycycline and a plan to complete a course of 14 days on an outpatient basis. The final diagnosis was scrub typhus with myocarditis leading to acutely decompensated heart failure with reduced ejection fraction.
DISCUSSION
Scrub typhus is a mite-borne tropical infection caused by the gram-negative intracellular parasite Orientia tsutsugamushi from the Rickettsiaceae family that is known to occur in certain parts of Asia and Australia. Although this entity is well known in the Sub Himalayan belt and southern part of India, very few cases have been described in Delhi, the capital state in North India. Scrub typhus, like most other tropical infections, is found most often during the postmonsoon season.1,2
Patients with scrub typhus present with fever in addition to a variety of nonspecific symptoms and findings. These often manifest within 10 days of being bitten by a mite. Malaise, headache, myalgias, lymphadenopathy, and maculopapular or petechial rash are common. If present, the rash manifests on the 3rd to 5th day of fever.3 Disseminated vasculitis due to scrub typhus can frequently result in multiorgan system involvement. Pulmonary involvement often leads to acute respiratory distress syndrome (ARDS) with an incidence of 8%-10%.1,4 Acute kidney injury, mostly mild and nonoliguric, has been reported in up to 2/3 cases.4-6 The cardiac myocyte is a known target cell affected by scrub typhus, and therefore patients commonly present with myocarditis.7 Liver involvement in scrub typhus is evident through elevated liver enzymes and can occur without other clinical evidence of the illness.4,6,8,9 As in dengue, patients often develop thrombocytopenia, but normal hemoglobin in scrub typhus differentiates it from dengue.6,8
Given the nonspecific presentation, it can be challenging to diagnose and treat scrub typhus. The gold standard for diagnosis is the detection of IgM antibodies to Orientia tsutsugamushi using an indirect immunofluorescence assay (IFA). For patients from endemic regions, it may be necessary to show a four-fold increase in titers two weeks apart to distinguish from background immunity. Presence of the characteristic eschar, as discussed below, is highly suggestive of scrub typhus. The treatment of choice is doxycycline or azithromycin for seven days.10,11 Early initiation of doxycycline when considering either scrub typhus or leptospirosis is appropriate and may be life-saving.
Medical decision making is fraught with uncertainty, and physicians must use their experience, evidence base, and cognitive heuristics wisely to care for patients effectively. For this patient, the region of Delhi experiences massive outbreaks of dengue every year during the time the patient presented to the hospital, whereas rickettsia infections are relatively uncommon. The clinical presentation was conceivably consistent with either dengue or scrub typhus, though somewhat more suggestive of the latter. Once the serological diagnosis of recent or concomitant dengue was obtained, however, scrub typhus was considered even less. The team called upon Occam’s razor or the heuristic that the simplest and most unifying explanation for any given problem is the one most likely to be correct and that other, less satisfactory explanations (in this case, scrub typhus) are “shaven off.” The patient was managed conservatively for dengue. Only when his condition worsened did the team recognize this conflicting information without dismissing it, consider alternative possibilities, and reexamined the patient.
An eschar can be an important clue in the diagnosis of scrub typhus, though it is not often obvious. The presence of this necrotic skin lesion with black crust is highly suggestive of scrub typhus, and in the right clinical context, it is virtually diagnostic. However, it is uncommon (9.5%-45%) in most of the studies from the Indian subcontinent (ie, high specificity but low sensitivity).1,12 An eschar is often found in obscure locations such as the axillae or groin, areas that may easily be missed or overlooked. Eschars may be seen in a variety of other infectious diseases, including rickettsia pox, Rocky Mountain spotted fever, other members of the spotted fever group, tularemia, and cutaneous anthrax. Given this patient’s lack of improvement, repeated examination revealed an eschar in the right axilla, a finding that was either missed or still evolving at the time of presentation.
This case illustrates the challenges in interpreting the significance of multiple positive serological tests in the context of an undifferentiated clinical syndrome. Possible reasons for a positive dengue serology could have been persistent antibodies from a previous infection, recent asymptomatic infection, concurrent infection, or cross-reactivity with flaviviruses such as West Nile Virus or Japanese Encephalitis.13 The patient also had positive IgM antibodies against Legionella pneumophila, but the urinary antigen was negative. In view of a negative antigen test, low specificity of the serologic test, low incidence of legionellosis in the Indian subcontinent, and absence of therapeutic response to a trial of fluoroquinolones, the diagnosis of legionellosis was considered unlikely in this patient.
With rapid advancements in technology, the importance of history taking and physical examination is at risk of being overshadowed. Approximately 80% of correct diagnoses in medicine can arrive through history and physical examination alone.14,15 In this case, Occam’s razor combined with multiple serological tests was relied on to create the likely list of diagnoses. However, recognition of the limitations of these heuristics and tests proved critical. The life-saving diagnosis was only made when the clinicians returned to basics, looked in every nook and cranny, and found the eschar on physical examination.
KEY TEACHING POINTS
- In patients living in endemic areas who present with an acute febrile illness, the differential diagnosis should include “tropical” infections such as dengue, chikungunya, enteric fever, leptospirosis, malaria, and scrub typhus.
- Serology is commonly employed for diagnosis of tropical infections, which may be misleading. These tests can be falsely positive from past asymptomatic infection or cross reactivity between antibodies, or falsely negative, as in the first few days of infection.
- Presence of eschar is a very useful clue in the diagnosis of scrub typhus, but this finding can be missed since it is often found in obscure locations. A thorough clinical history and physical examination are paramount.
Disclosures
The authors do not report any conflict of interest.
A 46-year-old man presented to the emergency room in the postmonsoon month of September with a seven-day history of high fevers as well as a four-day history of a dry cough, dyspnea, and progressive rash. The patient reported no chest pain, hemoptysis, chest tightness, palpitations, wheezing, orthopnea, paroxysmal nocturnal dyspnea, or leg swelling. He lived and sought healthcare in Delhi, India.
Fever followed by a progressive but as yet uncharacterized rash and pulmonary symptoms in a middle-aged man suggests a host of possibilities. While it is tempting to ascribe his symptoms to an infectious process, especially a “tropical” infection based on his residence in Delhi, the location may simply represent a red herring. Potential infections can be divided into those endemic to the Indian subcontinent, and those encountered more globally. The former include diseases such as measles and dengue, while the latter include entities such as Mycoplasma pneumonia, varicella, and acute human immunodeficiency virus (HIV) infection. Noninfectious categories of diseases that should be considered include drug reactions and rheumatologic processes. Several rheumatologic diseases, including granulomatosis with polyangiitis, eosinophilic granulomatosis with polyangiitis, and systemic lupus erythematosus (SLE) may present with fever, rash, and pulmonary symptomatology.
A history of the patient’s exposures, both environmental and pharmaceutical, should be obtained. More information regarding his immunization history, rash characteristics (distribution and nature of the lesions), and other salient exam findings such as organomegaly and joint abnormalities will be helpful.
Fever reached a maximum of 103° Fahrenheit and was associated with chills but not rigors. There were several fever spikes daily, relieved completely with antipyretics. The patient’s dyspnea was predominantly noted on exertion, nonpleuritic, not temporally related to cough, and progressively worsening over three days. The skin lesions were first noticed on his trunk and were described as reddish, flat, and pinpoint size. However, the rash spread to the face and extremities sparing the palms and soles. There was no bleeding, nausea, vomiting, abdominal pain, change in bowel habits, dysuria, headache, photophobia, neck stiffness, or joint pain.
The patient reported no significant past medical history, took no medications, and had no recent travel outside of Delhi, India in the past year. He was married and monogamous. He had no pets nor did he report any contact with animals. He did not use tobacco, alcohol, or illicit substances. He did not remember being bitten by an insect. He worked as a software engineer. There was no history of similar illness in the patient’s family or at his workplace. He had no history of recent blood transfusion or immunization (including MMR and Tdap).
Several noninfectious and inflammatory conditions can explain his symptoms. Eosinophilic granulomatosis with polyangiitis is considerably less likely in the absence of asthma, and vasculitic processes, in general, are less likely given the nongravity dependent nature of the rash. SLE and sarcoidosis are possible causes of a systemic inflammatory illness presenting acutely with fever, rash, and pulmonary symptoms.
The patient’s expanded history makes several infections less likely. Although much of the presentation is consistent with measles, the initial appearance of the truncal rash is atypical, and there is no mention of coryza or conjunctivitis. Likewise, the description of the exanthem is not suggestive of varicella, and dengue and chikungunya are much less likely in the absence of a headache and arthralgias. Other infections including leptospirosis and scrub typhus are possible, and both might be contracted in greater Delhi. Typhoid is another infectious syndrome endemic to the Indian subcontinent that should be considered. The presence of rash involving the face and extremities would be highly atypical, however; and the presence of dyspnea and the absence of a headache argue against typhoid. Acute HIV infection and Mycoplasma pneumonia remain possible diagnoses. Toxic shock syndrome is possible, but a faster and fulminant course would be expected.
On physical examination, the temperature was 103° Fahrenheit, heart rate was 120 beats per minute and regular, respiratory rate was 24 breaths per minute, blood pressure was 100/60 mm Hg, and resting oxygen saturation was 93% while breathing ambient air. He appeared uncomfortable. Jugular venous pulse was elevated at 10 cm H2O. Mild icterus was present, but there was neither conjunctival congestion nor subconjunctival hemorrhage. S1 and S2 heart sounds were loud, but there were no murmurs. Chest auscultation revealed bilateral basal coarse crackles. The abdominal right upper quadrant was mildly tender to palpation, and the liver edge was palpable 2 cm below the subcostal margin. There was neither splenomegaly nor peripheral lymphadenopathy. Kernig and Brudzinski signs were negative, and there were no focal neurological deficits. A generalized, nonpalpable, maculopapular and petechial rash was present on the face, extremities, and trunk.
The patient’s presentation must now incorporate the additional findings of bibasilar chest crackles, maculopapular/petechial rash, icterus, modest hypoxia, and hepatomegaly. Some of the noninfectious entities already mentioned (SLE and sarcoidosis) remain possible explanations. Hemophagocytic lymphohistiocytosis (HLH) may also explain most of the patient’s presenting signs and symptoms, and several other infectious diseases account for his presentation. Scrub typhus (or a more uncommon rickettsia disease, Indian tick typhus), leptospirosis, and perhaps infective endocarditis seem most likely to provide a unifying diagnosis for the symptoms mentioned above. Leptospirosis presents in a minority of instances as a severe illness known as Weil disease, characterized by several of this patient’s findings including icterus, kidney injury, and pulmonary symptoms. However, the rash is relatively uncommon in leptospirosis and when present, is usually more localized. The patient’s rash as described is not typically expected in infective endocarditis, although high-grade Staphylococcus aureus bacteremia will occasionally present with a diffuse rash that may be confused with that of meningococcemia. The etiology of the patient’s elevated jugular venous pressure is not readily apparent, with the cardiac examination making acute valvular insufficiency much less likely. Myocarditis, however, is possible in the setting of several of the diseases listed above, including leptospirosis, scrub typhus, SLE, and dengue.
In addition to basic laboratory studies and a chest radiograph, multiple sets of blood cultures should be obtained, along with a transthoracic echocardiogram and a ferritin level. The evidence to support leptospirosis and scrub typhus is strong enough to justify empiric use of doxycycline once the blood cultures are obtained, especially given the difficulty in definitively diagnosing these diseases in a timely fashion.
Laboratory analysis revealed a total leukocyte count of 13,600/uL (85% neutrophils), hemoglobin 10 g/dL, and platelet count 35,000/uL. Absolute eosinophil count was 136/uL. Serum chemistry showed sodium of 145 meq/L, potassium 4.1 meq/L, blood urea nitrogen 80 mg/dL, creatinine 1.6 mg/dL, aspartate transaminase (AST) 44 U/L (normal, 0-40), alanine transaminase (ALT) 81 U/L (normal, 0-40), direct bilirubin 3 mg/dL, and indirect bilirubin 3 mg/dL. Lactate dehydrogenase, alkaline phosphatase, albumin, and coagulation studies were normal. Erythrocyte sedimentation rate (ESR) was 42 mm (normal, 0-25) and highly sensitive C-reactive protein was 42 mg/L (normal, 0-10). Arterial blood gas on ambient air revealed a pH of 7.52, PaCO2 24 mm Hg, PaO2 55 mm Hg, and bicarbonate 20 meq/L. Urinalysis was normal. Blood cultures were obtained. Electrocardiogram (ECG) showed regular narrow complex tachycardia with incomplete left bundle branch block. Old ECGs were not available for comparison. Chest radiograph showed bilateral air space opacities with evidence of vein cephalization. Abdominal and pelvis ultrasonography showed pericholecystic fluid and mild hepatomegaly, but no free fluid, pleural effusion, or evidence of cholecystitis. Point of care immunochromatographic rapid malarial antigen detection test (detects Plasmodium falciparum, Plasmodium vivax, Plasmodium malaria, and Plasmodium ovale) was negative.
Most of the findings described are commonly observed in both scrub typhus and leptospirosis, including cytopenias, parenchymal infiltrates, hepatomegaly, elevated transaminases and bilirubin, cardiac involvement, fever, and rash. The rash described is more consistent with scrub typhus than with leptospirosis. The absence of a headache and joint findings argue modestly against these diagnoses. Likewise, HLH provides an adequate explanation for most of the patient’s symptoms, signs, and test results. These include fever, lung involvement, rash, hepatomegaly, elevated bilirubin, and cytopenias; however, leukocytosis and cardiac involvement are less characteristic. SLE also provides a satisfactory explanation for much of the symptoms, although the rash characteristics, normal urinalysis, and leukocytosis make this diagnosis less likely.
Additional testing that should be performed includes serum antinuclear antibody (ANA) and ferritin, since the latter may be markedly elevated in the setting of HLH. Bone marrow aspirate and biopsy should be performed looking specifically for evidence of hemophagocytosis. Finally, a transthoracic echocardiogram (TTE) should be performed to assess evidence of myocardial dysfunction as it may alter the therapeutic approach, although the results will be unlikely to differentiate between the preceding considerations.
Troponin I was negative, but N-terminal probrain natriuretic peptide was elevated at 20,000 pg/mL (normal, 0-900). D-dimer was negative. TTE showed left ventricular ejection fraction (LVEF) of 35% with global left ventricular hypokinesis. On three separate examinations, the peripheral blood smear did not show malarial parasites, atypical lymphocytes, or schistocytes. Three sets of blood cultures, testing for bacteria and fungi, were sterile. A throat culture was sterile. Widal test, as well as Leptospira and Mycoplasma serologies, were negative. Serology for Legionella pneumophila was positive, but the urinary antigen testing was negative. Antibodies to HIV 1 and 2 and anti-hepatitis C virus (HCV) antibody were negative. Dengue IgM ELISA (qualitative) returned positive.
Despite the absence of arthralgias, myalgias, headache, and retro-orbital pain, a positive dengue IgM ELISA supports acute dengue infection, provided the patient did not experience an unexplained febrile illness in the previous months. Most of his presentation may be explained by dengue, including fever, rash, liver abnormalities, myocardial dysfunction, and thrombocytopenia. The bilateral airspace opacities seen on chest radiograph also fit reasonably provided these actually reflect pulmonary edema. Leukocytosis (as opposed to leukopenia) is highly unexpected in dengue, but its presence could be an outlier.
If dengue does indeed explain the entire presentation, defervescence should have occurred by the time the blood cultures and serologic studies returned. Also, by that time, the patient would be expected to demonstrate evidence of improvement, barring the appearance of the serious complications of dengue hemorrhagic fever/dengue shock syndrome. Should fever persist and signs of recovery fail to materialize, the possibility of a superimposed process will need to be considered. Of note, the sensitivity of Leptospira serology early in the course of illness is low, and leptospirosis is thus not yet excluded.
A presumptive diagnosis of severe dengue fever was made, based on evidence of pulmonary edema and sepsis. The patient was managed conservatively with oral fluid restriction, low dose of diuretics, and supplemental oxygenation. The patient was also given levofloxacin for possible legionellosis. Despite these therapies, the patient had no improvement in 24 hours. His tachypnea increased, and his measured PaO2 to FIO2 (P:F) ratio decreased to 230 from 285 on admission. This prompted the initiation of BiPAP at 10 cm H2O inspiration PAP and 5 cm H2O expiration PAP. However, he did not tolerate BiPAP, and his P:F ratio decreased to below 200.
The patient was transferred to the intensive care unit and underwent elective intubation with mechanical ventilation. Axial and coronal computed tomography of the thorax (Figure 1A and 1B, respectively) showed extensive ground-glass opacities and consolidation sparing the nondependent portions of the lungs. On physical inspection, a round, well-defined, painless black lesion surrounded by erythema was noticed in the right axilla (Figure 2). The rest of the examination findings were unchanged.
The discovery of eschar in the axilla provides a “pivot point” in determining the cause of the patient’s illness. This finding appears to point, with high specificity, toward rickettsia as the explanation of the patient’s disease, and this is most likely to be scrub typhus. The report of a positive dengue IgM may represent concurrent infection or may simply reflect a recent infection in an area that is highly endemic for dengue. Although most of the patient’s clinical presentation could be attributed to dengue, multiple features including the leukocytosis, myocarditis, and elevated bilirubin are more likely to be seen in scrub typhus. In any event, dengue cannot satisfactorily explain the eschar.
No mention has been made to the initiation of doxycycline thus far; this agent needs to be started promptly. Polymerase chain reaction (PCR) testing for scrub typhus should be ordered if available; if not, acute and convalescent serology may be obtained.
Given the finding of axillary eschar, the patient was diagnosed with scrub typhus. Doxycycline 100 mg by nasogastric tube twice a day was initiated. The patient began to show marked symptomatic improvement. His P:F ratio improved, and he was successfully weaned off and extubated after 24 hours. Postextubation, he was kept on BiPAP for 12 hours. He was transferred out of the ICU and monitored for 72 hours. With therapy, his cytopenias, liver and renal function, and ECG normalized. Indirect immunofluorescence assay for scrub typhus returned positive at a dilution of > 1:512. PCR assay targeting the 56 kDa region of Orientia tsutsugamushi was also positive. Repeated TTE showed an LVEF of 65%. He was subsequently discharged with oral doxycycline and a plan to complete a course of 14 days on an outpatient basis. The final diagnosis was scrub typhus with myocarditis leading to acutely decompensated heart failure with reduced ejection fraction.
DISCUSSION
Scrub typhus is a mite-borne tropical infection caused by the gram-negative intracellular parasite Orientia tsutsugamushi from the Rickettsiaceae family that is known to occur in certain parts of Asia and Australia. Although this entity is well known in the Sub Himalayan belt and southern part of India, very few cases have been described in Delhi, the capital state in North India. Scrub typhus, like most other tropical infections, is found most often during the postmonsoon season.1,2
Patients with scrub typhus present with fever in addition to a variety of nonspecific symptoms and findings. These often manifest within 10 days of being bitten by a mite. Malaise, headache, myalgias, lymphadenopathy, and maculopapular or petechial rash are common. If present, the rash manifests on the 3rd to 5th day of fever.3 Disseminated vasculitis due to scrub typhus can frequently result in multiorgan system involvement. Pulmonary involvement often leads to acute respiratory distress syndrome (ARDS) with an incidence of 8%-10%.1,4 Acute kidney injury, mostly mild and nonoliguric, has been reported in up to 2/3 cases.4-6 The cardiac myocyte is a known target cell affected by scrub typhus, and therefore patients commonly present with myocarditis.7 Liver involvement in scrub typhus is evident through elevated liver enzymes and can occur without other clinical evidence of the illness.4,6,8,9 As in dengue, patients often develop thrombocytopenia, but normal hemoglobin in scrub typhus differentiates it from dengue.6,8
Given the nonspecific presentation, it can be challenging to diagnose and treat scrub typhus. The gold standard for diagnosis is the detection of IgM antibodies to Orientia tsutsugamushi using an indirect immunofluorescence assay (IFA). For patients from endemic regions, it may be necessary to show a four-fold increase in titers two weeks apart to distinguish from background immunity. Presence of the characteristic eschar, as discussed below, is highly suggestive of scrub typhus. The treatment of choice is doxycycline or azithromycin for seven days.10,11 Early initiation of doxycycline when considering either scrub typhus or leptospirosis is appropriate and may be life-saving.
Medical decision making is fraught with uncertainty, and physicians must use their experience, evidence base, and cognitive heuristics wisely to care for patients effectively. For this patient, the region of Delhi experiences massive outbreaks of dengue every year during the time the patient presented to the hospital, whereas rickettsia infections are relatively uncommon. The clinical presentation was conceivably consistent with either dengue or scrub typhus, though somewhat more suggestive of the latter. Once the serological diagnosis of recent or concomitant dengue was obtained, however, scrub typhus was considered even less. The team called upon Occam’s razor or the heuristic that the simplest and most unifying explanation for any given problem is the one most likely to be correct and that other, less satisfactory explanations (in this case, scrub typhus) are “shaven off.” The patient was managed conservatively for dengue. Only when his condition worsened did the team recognize this conflicting information without dismissing it, consider alternative possibilities, and reexamined the patient.
An eschar can be an important clue in the diagnosis of scrub typhus, though it is not often obvious. The presence of this necrotic skin lesion with black crust is highly suggestive of scrub typhus, and in the right clinical context, it is virtually diagnostic. However, it is uncommon (9.5%-45%) in most of the studies from the Indian subcontinent (ie, high specificity but low sensitivity).1,12 An eschar is often found in obscure locations such as the axillae or groin, areas that may easily be missed or overlooked. Eschars may be seen in a variety of other infectious diseases, including rickettsia pox, Rocky Mountain spotted fever, other members of the spotted fever group, tularemia, and cutaneous anthrax. Given this patient’s lack of improvement, repeated examination revealed an eschar in the right axilla, a finding that was either missed or still evolving at the time of presentation.
This case illustrates the challenges in interpreting the significance of multiple positive serological tests in the context of an undifferentiated clinical syndrome. Possible reasons for a positive dengue serology could have been persistent antibodies from a previous infection, recent asymptomatic infection, concurrent infection, or cross-reactivity with flaviviruses such as West Nile Virus or Japanese Encephalitis.13 The patient also had positive IgM antibodies against Legionella pneumophila, but the urinary antigen was negative. In view of a negative antigen test, low specificity of the serologic test, low incidence of legionellosis in the Indian subcontinent, and absence of therapeutic response to a trial of fluoroquinolones, the diagnosis of legionellosis was considered unlikely in this patient.
With rapid advancements in technology, the importance of history taking and physical examination is at risk of being overshadowed. Approximately 80% of correct diagnoses in medicine can arrive through history and physical examination alone.14,15 In this case, Occam’s razor combined with multiple serological tests was relied on to create the likely list of diagnoses. However, recognition of the limitations of these heuristics and tests proved critical. The life-saving diagnosis was only made when the clinicians returned to basics, looked in every nook and cranny, and found the eschar on physical examination.
KEY TEACHING POINTS
- In patients living in endemic areas who present with an acute febrile illness, the differential diagnosis should include “tropical” infections such as dengue, chikungunya, enteric fever, leptospirosis, malaria, and scrub typhus.
- Serology is commonly employed for diagnosis of tropical infections, which may be misleading. These tests can be falsely positive from past asymptomatic infection or cross reactivity between antibodies, or falsely negative, as in the first few days of infection.
- Presence of eschar is a very useful clue in the diagnosis of scrub typhus, but this finding can be missed since it is often found in obscure locations. A thorough clinical history and physical examination are paramount.
Disclosures
The authors do not report any conflict of interest.
1. Gupta N, Chaudhry R, Kabra SK, et al. In search of scrub typhus: a prospective analysis of clinical and epidemiological profile of patients from a tertiary care hospital in New Delhi. Adv Infect Dis. 2015;5(4):140. doi: 10.4236/aid.2015.54017.
2. Kamarasu K, Malathi M, Rajagopal V, Subramani K, Jagadeeshramasamy D, Mathai E. Serological evidence for the wide distribution of spotted fevers & typhus fever in Tamil Nadu. Indian J Med Res. 2007;126(2):128-130. PubMed
3. Mahajan SK. Scrub typhus. J Assoc Physicians India. 2005;53:954-958. PubMed
4. Mahajan SK, Rolain JM, Kashyap R, et al. Scrub typhus in the Himalayas. Emerg Infect Dis. 2006;12(10):1590-1592. doi: 10.3201/eid1210.051697. PubMed
5. Attur RP, Kuppasamy S, Bairy M, et al. Acute kidney injury in scrub typhus. Clin Exp Nephrol. 2013;17(5):725-729. doi: 10.1007/s10157-012-0753-9. PubMed
6. Varghese GM, Trowbridge P, Janardhanan J, et al. Clinical profile and improving mortality trend of scrub typhus in South India. Int J Infect Dis. 2014;23:39-43. doi: 10.1016/j.ijid.2014.02.009. PubMed
7. Taylor AJ, Paris DH, Newton PN. A systematic review of mortality from untreated scrub typhus (Orientia tsutsugamushi). PLoS Negl Trop Dis. 2015;9(8):e0003971. doi.org/10.1371/journal.pntd.0003971 PubMed
8. Chrispal A, Boorugu H, Gopinath KG, et al. Scrub typhus: an unrecognized threat in South India-clinical profile and predictors of mortality. Trop Doct. 2010;40(3):129-133. doi: 10.1258/td.2010.090452. PubMed
9. Mathai E, Rolain JM, Verghese GM, et al. Outbreak of scrub typhus in southern India during the cooler months. Ann N Y Acad Sci. 2003;990:359-364. doi: 10.1111/j.1749-6632.2003.tb07391.x PubMed
10. Gupta N, Chaudhry R, Kabra SK, et al. Comparative evaluation of serological and molecular methods for the diagnosis of scrub typhus in Indian settings. Jpn J Infect Dis. 2017;70(2):221-222. doi: 10.7883/yoken.JJID.2016.139. PubMed
11. Rahi M, Gupte MD, Bhargava A, Varghese GM, Arora R. DHR-ICMR Guidelines for diagnosis & management of Rickettsial diseases in India. Indian J Med Res. 2015;141(4):417-422. doi: 10.4103/0971-5916.159279. PubMed
12. Sharma A, Mahajan S, Gupta ML, Kanga A, Sharma V. Investigation of an outbreak of scrub typhus in the Himalayan region of India. Jpn J Infect Dis. 2005;58(4):208-210. PubMed
13. Gupta N, Chaudhry R, Mirdha B, et al. Scrub typhus and leptospirosis: the fallacy of diagnosing with IgM enzyme-linked immunosorbent assay. J Microb Biochem Technol. 2016;8:71-75. doi: 10.4172/1948-5948.1000265.
14. Peterson MC, Holbrook JH, Von Hales D, Smith NL, Staker LV. Contributions of the history, physical examination, and laboratory investigation in making medical diagnoses. West J Med. 1992;156(2):163-165. doi: 10.1097/00006254-199210000-00013 PubMed
15. Roshan M, Rao AP. A study on relative contributions of the history, physical examination and investigations in making a medical diagnosis. J Assoc Physicians India. 2000;48(8):771-775. PubMed
1. Gupta N, Chaudhry R, Kabra SK, et al. In search of scrub typhus: a prospective analysis of clinical and epidemiological profile of patients from a tertiary care hospital in New Delhi. Adv Infect Dis. 2015;5(4):140. doi: 10.4236/aid.2015.54017.
2. Kamarasu K, Malathi M, Rajagopal V, Subramani K, Jagadeeshramasamy D, Mathai E. Serological evidence for the wide distribution of spotted fevers & typhus fever in Tamil Nadu. Indian J Med Res. 2007;126(2):128-130. PubMed
3. Mahajan SK. Scrub typhus. J Assoc Physicians India. 2005;53:954-958. PubMed
4. Mahajan SK, Rolain JM, Kashyap R, et al. Scrub typhus in the Himalayas. Emerg Infect Dis. 2006;12(10):1590-1592. doi: 10.3201/eid1210.051697. PubMed
5. Attur RP, Kuppasamy S, Bairy M, et al. Acute kidney injury in scrub typhus. Clin Exp Nephrol. 2013;17(5):725-729. doi: 10.1007/s10157-012-0753-9. PubMed
6. Varghese GM, Trowbridge P, Janardhanan J, et al. Clinical profile and improving mortality trend of scrub typhus in South India. Int J Infect Dis. 2014;23:39-43. doi: 10.1016/j.ijid.2014.02.009. PubMed
7. Taylor AJ, Paris DH, Newton PN. A systematic review of mortality from untreated scrub typhus (Orientia tsutsugamushi). PLoS Negl Trop Dis. 2015;9(8):e0003971. doi.org/10.1371/journal.pntd.0003971 PubMed
8. Chrispal A, Boorugu H, Gopinath KG, et al. Scrub typhus: an unrecognized threat in South India-clinical profile and predictors of mortality. Trop Doct. 2010;40(3):129-133. doi: 10.1258/td.2010.090452. PubMed
9. Mathai E, Rolain JM, Verghese GM, et al. Outbreak of scrub typhus in southern India during the cooler months. Ann N Y Acad Sci. 2003;990:359-364. doi: 10.1111/j.1749-6632.2003.tb07391.x PubMed
10. Gupta N, Chaudhry R, Kabra SK, et al. Comparative evaluation of serological and molecular methods for the diagnosis of scrub typhus in Indian settings. Jpn J Infect Dis. 2017;70(2):221-222. doi: 10.7883/yoken.JJID.2016.139. PubMed
11. Rahi M, Gupte MD, Bhargava A, Varghese GM, Arora R. DHR-ICMR Guidelines for diagnosis & management of Rickettsial diseases in India. Indian J Med Res. 2015;141(4):417-422. doi: 10.4103/0971-5916.159279. PubMed
12. Sharma A, Mahajan S, Gupta ML, Kanga A, Sharma V. Investigation of an outbreak of scrub typhus in the Himalayan region of India. Jpn J Infect Dis. 2005;58(4):208-210. PubMed
13. Gupta N, Chaudhry R, Mirdha B, et al. Scrub typhus and leptospirosis: the fallacy of diagnosing with IgM enzyme-linked immunosorbent assay. J Microb Biochem Technol. 2016;8:71-75. doi: 10.4172/1948-5948.1000265.
14. Peterson MC, Holbrook JH, Von Hales D, Smith NL, Staker LV. Contributions of the history, physical examination, and laboratory investigation in making medical diagnoses. West J Med. 1992;156(2):163-165. doi: 10.1097/00006254-199210000-00013 PubMed
15. Roshan M, Rao AP. A study on relative contributions of the history, physical examination and investigations in making a medical diagnosis. J Assoc Physicians India. 2000;48(8):771-775. PubMed
© 2019 Society of Hospital Medicine
Condom Catheters versus Indwelling Urethral Catheters in Men: A Prospective, Observational Study
Millions of patients use urinary collection devices. For men, both indwelling and condom-style urinary catheters (known as “external catheters”) are commonly used. National infection prevention guidelines recommend condom catheters as a preferred alternative to indwelling catheters for patients without urinary retention1,2 to reduce the risk of catheter-associated urinary tract infection (UTI). Unfortunately, little outcome data comparing condom catheters with indwelling urethral catheters exists. We therefore assessed the incidence of infectious and noninfectious complications in condom catheter and indwelling urethral catheter users.
PATIENTS AND METHODS
Study Overview
As part of a larger prospective, observational study,3 we compared complications in patients who received a condom catheter during hospitalization with those in patients who received an indwelling urethral catheter. Hospitalized patients with either a condom catheter or indwelling urethral catheter were identified at two Veterans Affairs (VA) medical centers and followed for 30 days after initial catheter placement. Patient-reported data were collected during in-person patient interviews at baseline (within three days of catheter placement), and by in-person or phone interviews at 14 days and 30 days postplacement (Supplementary Appendix A and B). Questions were primarily closed-ended, except for a final question inviting open comments. Information about the catheter and any reported complications was also collected from electronic medical record documentation for each patient. Institutional review board approval was received from both participating study sites.
Data Collection and Inclusion Criteria
Hospitalized patients who had a condom or indwelling urethral catheter placed were eligible to participate if they met the following criteria: (1) were hospitalized on an acute care unit; (2) had a new condom catheter or indwelling urethral catheter placed during this hospital stay that was not present on admission; (3) had a device in place for three days or less; (4) were at least 18 years old; and (5) were able to speak English. Patients were excluded if they: (1) did not have the capacity to give consent or participate in the interview/assessment process; (2) refused to provide written informed consent to participate; or (3) had previously participated in this project.
As the larger study was focused on indwelling urethral catheter users, participants with a condom catheter were recruited from only one facility, while those with an indwelling urethral catheter were recruited from both hospitals. Indwelling catheter patients that had a possible contraindication to condom catheter use (such as urinary retention or perioperative use for a surgical procedure) were excluded to make the groups comparable. Any indication for condom catheterization was permitted.
Information about catheter-related complications was collected from two sources: directly from patients and through medical record review. Patients were interviewed at baseline and approximately 14 days and 30 days after catheter placement. The follow-up assessments asked patients about their symptoms and experience over the previous two weeks. We also conducted a medical record review covering the 30 days after initial catheter placement.
Study Measures
Data Analysis
The primary outcome was the percentage of patients who experienced a complication related to a urinary catheter during the 30 days after the catheter was initially placed. Comparisons by group—condom versus indwelling catheter—were conducted using chi-square tests (Fisher’s exact test when necessary) for categorical variables and the Student’s t-test for continuous variables. All analyses were performed using SAS (Cary, North Carolina). All statistical tests were two-sided with alpha set to .05.
RESULTS
Of the 76 patients invited to participate after having a condom catheter placed, 49 consented (64.5%). Of those, 36 had sufficient data for inclusion in this analysis. The comparison group consisted of 44 patients with an indwelling urethral catheter. There were no statistically significant differences between the two groups in terms of age, race, or ethnicity (Table 1). There were statistically significant differences in patient-reported reasons for catheter placement, but these were due to the exclusion criteria used for indwelling urethral catheter patients.
Both patient-reported and clinician-reported (ie, recorded in the patient’s medical record) outcomes are described in Table 2. In total, 80.6% of condom catheter users reported experiencing at least one catheter-related complication during the month after initial catheter placement compared with 88.6% of indwelling catheter users (P = .32). A similar number of condom catheter patients and indwelling urethral catheter patients experienced an infectious complication according to both self-report data (8.3% condom, 6.8% indwelling; P = .99) and medical record review (11.1% condom, 6.8% indwelling; P = .69).
At least one noninfectious complication was identified in 77.8% of condom catheter patients (28 of 36) and 88.6% of indwelling urethral catheter patients (39 of 44) using combined self-report and medical record review data (P = .19); most of these were based on self-reported data. Significantly fewer condom catheter patients reported complications during placement (eg, pain, discomfort, bleeding, or other trauma) compared with those with indwelling catheters (13.9% vs 43.2%, P < .001). Pain, discomfort, bleeding, or other trauma during catheter removal were commonly reported by both condom catheter and indwelling urethral catheter patients (40.9% vs 42.1%, respectively; P = .99).
Patient-reported noninfectious complications were often not documented in the medical record: 75.0% of condom catheter patients and 86.4% of indwelling catheter patients reported complications, in comparison with the 25.0% of condom catheter patients and 27.3% of indwelling urethral catheter patients with noninfectious complications identified during medical record review.
DISCUSSION
Our study revealed three important findings. First, noninfectious complications greatly outnumbered infectious complications, regardless of the device type. Second, condom catheter users reported significantly less pain related to placement of their device compared with the indwelling urethral catheter group. Finally, many patients reported complications that were not documented in the medical record.
The only randomized trial comparing these devices enrolled 75 men hospitalized at a single VA medical center and found that using a condom catheter rather than an indwelling catheter in patients without urinary retention lowered the composite endpoint of bacteriuria, symptomatic UTI, or death.4 Additionally, patients in this trial reported that the condom catheter was significantly more comfortable (90% vs 58%; P = .02) and less painful (5% vs 36%; P = .02) than the indwelling catheter,4 supporting a previous study in hospitalized male Veterans.5
Importantly, we included patient-reported complications that may be of concern to patients but inconsistently documented in the medical record. Pain associated with removal of both condom catheters and indwelling urethral catheters was reported in over 40% in both groups but was not documented in the medical record. One patient with a condom catheter described removal this way: “It got stuck on my hair, so was hard to get off…” Condom catheters also posed some issues with staying in place as has been previously described.6 As one condom catheter user said: “When I was laying down it was okay, but every time I moved around…it would slide off.”
Recent efforts to reduce catheter-associated UTI,7-9 which have focused on reducing the use of indwelling urethral catheters,10,11 have been relatively successful. Clinical policy makers should consider similar efforts to address the noninfectious harms of both catheter types. Such efforts could include further decreasing any type of catheter use along with improved training of those placing such devices.12 Substantial improvement will require a systematic approach to surveilling noninfectious complications of both types of urinary catheters.
Our study has several limitations. First, we conducted the study at two VA hospitals; therefore, the results may not be generalizable to a non-VA population. Second, we only included 80 patients because we recruited a limited number of condom catheter users.
Limitations notwithstanding, we provide comparison data between condom and indwelling urethral catheters. Condom catheter users reported significantly less pain related to initial placement of their device compared with those using an indwelling urethral catheter. For both devices, patients experienced noninfectious complications much more commonly than infectious ones, underscoring the need to systematically address such complications, perhaps through a surveillance system that includes the patient’s perspective. The patient’s voice is important and necessary in view of the apparent underreporting of noninfectious harms in the medical record.
A cknowledgments
Disclaimer
The funding sources played no role in the design, conducting, or evaluation of this study. The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the official position of the Department of Veterans Affairs.
1. Gould CV, Umscheid CA, Agarwal RK, Kuntz G, Pegues DA, Healthcare Infection Control Practices Advisory Committee. Guideline for prevention of catheter-associated urinary tract infections 2009. Infect Control Hosp Epidemiol. 2010;31(4):319-326. doi: 10.1086/651091.
2. Lo E, Nicolle LE, Coffin SE, et al. Strategies to prevent catheter-associated urinary tract infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(5):464-479. doi: 10.1086/675718.
3. Saint S, Trautner BW, Fowler KE, et al. A multicenter study of patient-reported infectious and noninfectious complications associated with indwelling urethral catheters. JAMA Intern Med. 2018. doi:10.1001/jamainternmed.2018.2417.
4. Saint S, Kaufman SR, Rogers MA, Baker PD, Ossenkop K, Lipsky BA. Condom versus indwelling urinary catheters: a randomized trial. J Am Geriatr Soc. 2006;54(7):1055-1061. doi: 10.1111/j.1532-5415.2006.00785.x.
5. Saint S, Lipsky BA, Baker PD, McDonald LL, Ossenkop K. Urinary catheters: what type do men and their nurses prefer? J Am Geriatr Soc. 1999;47(12):1453-1457. doi: 10.1111/j.1532-5415.1999.tb01567.x.
6. Smart C. Male urinary incontinence and the urinary sheath. Br J Nurs. 2014;23(9):S20, S22-S25. doi: 10.12968/bjon.2014.23.Sup9.S20.
7. Saint S, Greene MT, Kowalski CP, Watson SR, Hofer TP, Krein SL. Preventing catheter-associated urinary tract infection in the United States: a national comparative study. JAMA Intern Med. 2013;173(10):874-879. doi: 10.1001/jamainternmed.2013.101.
8. Saint S, Greene MT, Krein SL, et al. A program to prevent catheter-associated urinary tract infection in acute care. N Engl J Med. 2016;374(22):2111-2119. doi: 10.1056/NEJMoa1504906.
9. Saint S, Fowler KE, Sermak K, et al. Introducing the No preventable harms campaign: creating the safest health care system in the world, starting with catheter-associated urinary tract infection prevention. Am J Infect Control. 2015;43(3):254-259. doi: 10.1016/j.ajic.2014.11.016.
10. Fakih MG, Watson SR, Greene MT, et al. Reducing inappropriate urinary catheter use: a statewide effort. Arch Intern Med. 2012;172(3):255-260. doi: 10.1001/archinternmed.2011.627.
11. Krein SL, Kowalski CP, Harrod M, Forman J, Saint S. Barriers to reducing urinary catheter use: a qualitative assessment of a statewide initiative. JAMA Intern Med. 2013;173(10):881-886. doi: 10.1001/jamainternmed.2013.105.
12. Manojlovich M, Saint S, Meddings J, et al. Indwelling urinary catheter insertion practices in the emergency department: an observational study. Infect Control Hosp Epidemiol. 2016;37(1):117-119. doi: 10.1017/ice.2015.238.
13. Meddings JA, Reichert H, Rogers MA, Saint S, Stephansky J, McMahon LF. Effect of nonpayment for hospital-acquired, catheter-associated urinary tract infection: a statewide analysis. Ann Intern Med. 2012;157(5):305-312. doi: 10.7326/0003-4819-157-5-201209040-00003.
Millions of patients use urinary collection devices. For men, both indwelling and condom-style urinary catheters (known as “external catheters”) are commonly used. National infection prevention guidelines recommend condom catheters as a preferred alternative to indwelling catheters for patients without urinary retention1,2 to reduce the risk of catheter-associated urinary tract infection (UTI). Unfortunately, little outcome data comparing condom catheters with indwelling urethral catheters exists. We therefore assessed the incidence of infectious and noninfectious complications in condom catheter and indwelling urethral catheter users.
PATIENTS AND METHODS
Study Overview
As part of a larger prospective, observational study,3 we compared complications in patients who received a condom catheter during hospitalization with those in patients who received an indwelling urethral catheter. Hospitalized patients with either a condom catheter or indwelling urethral catheter were identified at two Veterans Affairs (VA) medical centers and followed for 30 days after initial catheter placement. Patient-reported data were collected during in-person patient interviews at baseline (within three days of catheter placement), and by in-person or phone interviews at 14 days and 30 days postplacement (Supplementary Appendix A and B). Questions were primarily closed-ended, except for a final question inviting open comments. Information about the catheter and any reported complications was also collected from electronic medical record documentation for each patient. Institutional review board approval was received from both participating study sites.
Data Collection and Inclusion Criteria
Hospitalized patients who had a condom or indwelling urethral catheter placed were eligible to participate if they met the following criteria: (1) were hospitalized on an acute care unit; (2) had a new condom catheter or indwelling urethral catheter placed during this hospital stay that was not present on admission; (3) had a device in place for three days or less; (4) were at least 18 years old; and (5) were able to speak English. Patients were excluded if they: (1) did not have the capacity to give consent or participate in the interview/assessment process; (2) refused to provide written informed consent to participate; or (3) had previously participated in this project.
As the larger study was focused on indwelling urethral catheter users, participants with a condom catheter were recruited from only one facility, while those with an indwelling urethral catheter were recruited from both hospitals. Indwelling catheter patients that had a possible contraindication to condom catheter use (such as urinary retention or perioperative use for a surgical procedure) were excluded to make the groups comparable. Any indication for condom catheterization was permitted.
Information about catheter-related complications was collected from two sources: directly from patients and through medical record review. Patients were interviewed at baseline and approximately 14 days and 30 days after catheter placement. The follow-up assessments asked patients about their symptoms and experience over the previous two weeks. We also conducted a medical record review covering the 30 days after initial catheter placement.
Study Measures
Data Analysis
The primary outcome was the percentage of patients who experienced a complication related to a urinary catheter during the 30 days after the catheter was initially placed. Comparisons by group—condom versus indwelling catheter—were conducted using chi-square tests (Fisher’s exact test when necessary) for categorical variables and the Student’s t-test for continuous variables. All analyses were performed using SAS (Cary, North Carolina). All statistical tests were two-sided with alpha set to .05.
RESULTS
Of the 76 patients invited to participate after having a condom catheter placed, 49 consented (64.5%). Of those, 36 had sufficient data for inclusion in this analysis. The comparison group consisted of 44 patients with an indwelling urethral catheter. There were no statistically significant differences between the two groups in terms of age, race, or ethnicity (Table 1). There were statistically significant differences in patient-reported reasons for catheter placement, but these were due to the exclusion criteria used for indwelling urethral catheter patients.
Both patient-reported and clinician-reported (ie, recorded in the patient’s medical record) outcomes are described in Table 2. In total, 80.6% of condom catheter users reported experiencing at least one catheter-related complication during the month after initial catheter placement compared with 88.6% of indwelling catheter users (P = .32). A similar number of condom catheter patients and indwelling urethral catheter patients experienced an infectious complication according to both self-report data (8.3% condom, 6.8% indwelling; P = .99) and medical record review (11.1% condom, 6.8% indwelling; P = .69).
At least one noninfectious complication was identified in 77.8% of condom catheter patients (28 of 36) and 88.6% of indwelling urethral catheter patients (39 of 44) using combined self-report and medical record review data (P = .19); most of these were based on self-reported data. Significantly fewer condom catheter patients reported complications during placement (eg, pain, discomfort, bleeding, or other trauma) compared with those with indwelling catheters (13.9% vs 43.2%, P < .001). Pain, discomfort, bleeding, or other trauma during catheter removal were commonly reported by both condom catheter and indwelling urethral catheter patients (40.9% vs 42.1%, respectively; P = .99).
Patient-reported noninfectious complications were often not documented in the medical record: 75.0% of condom catheter patients and 86.4% of indwelling catheter patients reported complications, in comparison with the 25.0% of condom catheter patients and 27.3% of indwelling urethral catheter patients with noninfectious complications identified during medical record review.
DISCUSSION
Our study revealed three important findings. First, noninfectious complications greatly outnumbered infectious complications, regardless of the device type. Second, condom catheter users reported significantly less pain related to placement of their device compared with the indwelling urethral catheter group. Finally, many patients reported complications that were not documented in the medical record.
The only randomized trial comparing these devices enrolled 75 men hospitalized at a single VA medical center and found that using a condom catheter rather than an indwelling catheter in patients without urinary retention lowered the composite endpoint of bacteriuria, symptomatic UTI, or death.4 Additionally, patients in this trial reported that the condom catheter was significantly more comfortable (90% vs 58%; P = .02) and less painful (5% vs 36%; P = .02) than the indwelling catheter,4 supporting a previous study in hospitalized male Veterans.5
Importantly, we included patient-reported complications that may be of concern to patients but inconsistently documented in the medical record. Pain associated with removal of both condom catheters and indwelling urethral catheters was reported in over 40% in both groups but was not documented in the medical record. One patient with a condom catheter described removal this way: “It got stuck on my hair, so was hard to get off…” Condom catheters also posed some issues with staying in place as has been previously described.6 As one condom catheter user said: “When I was laying down it was okay, but every time I moved around…it would slide off.”
Recent efforts to reduce catheter-associated UTI,7-9 which have focused on reducing the use of indwelling urethral catheters,10,11 have been relatively successful. Clinical policy makers should consider similar efforts to address the noninfectious harms of both catheter types. Such efforts could include further decreasing any type of catheter use along with improved training of those placing such devices.12 Substantial improvement will require a systematic approach to surveilling noninfectious complications of both types of urinary catheters.
Our study has several limitations. First, we conducted the study at two VA hospitals; therefore, the results may not be generalizable to a non-VA population. Second, we only included 80 patients because we recruited a limited number of condom catheter users.
Limitations notwithstanding, we provide comparison data between condom and indwelling urethral catheters. Condom catheter users reported significantly less pain related to initial placement of their device compared with those using an indwelling urethral catheter. For both devices, patients experienced noninfectious complications much more commonly than infectious ones, underscoring the need to systematically address such complications, perhaps through a surveillance system that includes the patient’s perspective. The patient’s voice is important and necessary in view of the apparent underreporting of noninfectious harms in the medical record.
A cknowledgments
Disclaimer
The funding sources played no role in the design, conducting, or evaluation of this study. The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the official position of the Department of Veterans Affairs.
Millions of patients use urinary collection devices. For men, both indwelling and condom-style urinary catheters (known as “external catheters”) are commonly used. National infection prevention guidelines recommend condom catheters as a preferred alternative to indwelling catheters for patients without urinary retention1,2 to reduce the risk of catheter-associated urinary tract infection (UTI). Unfortunately, little outcome data comparing condom catheters with indwelling urethral catheters exists. We therefore assessed the incidence of infectious and noninfectious complications in condom catheter and indwelling urethral catheter users.
PATIENTS AND METHODS
Study Overview
As part of a larger prospective, observational study,3 we compared complications in patients who received a condom catheter during hospitalization with those in patients who received an indwelling urethral catheter. Hospitalized patients with either a condom catheter or indwelling urethral catheter were identified at two Veterans Affairs (VA) medical centers and followed for 30 days after initial catheter placement. Patient-reported data were collected during in-person patient interviews at baseline (within three days of catheter placement), and by in-person or phone interviews at 14 days and 30 days postplacement (Supplementary Appendix A and B). Questions were primarily closed-ended, except for a final question inviting open comments. Information about the catheter and any reported complications was also collected from electronic medical record documentation for each patient. Institutional review board approval was received from both participating study sites.
Data Collection and Inclusion Criteria
Hospitalized patients who had a condom or indwelling urethral catheter placed were eligible to participate if they met the following criteria: (1) were hospitalized on an acute care unit; (2) had a new condom catheter or indwelling urethral catheter placed during this hospital stay that was not present on admission; (3) had a device in place for three days or less; (4) were at least 18 years old; and (5) were able to speak English. Patients were excluded if they: (1) did not have the capacity to give consent or participate in the interview/assessment process; (2) refused to provide written informed consent to participate; or (3) had previously participated in this project.
As the larger study was focused on indwelling urethral catheter users, participants with a condom catheter were recruited from only one facility, while those with an indwelling urethral catheter were recruited from both hospitals. Indwelling catheter patients that had a possible contraindication to condom catheter use (such as urinary retention or perioperative use for a surgical procedure) were excluded to make the groups comparable. Any indication for condom catheterization was permitted.
Information about catheter-related complications was collected from two sources: directly from patients and through medical record review. Patients were interviewed at baseline and approximately 14 days and 30 days after catheter placement. The follow-up assessments asked patients about their symptoms and experience over the previous two weeks. We also conducted a medical record review covering the 30 days after initial catheter placement.
Study Measures
Data Analysis
The primary outcome was the percentage of patients who experienced a complication related to a urinary catheter during the 30 days after the catheter was initially placed. Comparisons by group—condom versus indwelling catheter—were conducted using chi-square tests (Fisher’s exact test when necessary) for categorical variables and the Student’s t-test for continuous variables. All analyses were performed using SAS (Cary, North Carolina). All statistical tests were two-sided with alpha set to .05.
RESULTS
Of the 76 patients invited to participate after having a condom catheter placed, 49 consented (64.5%). Of those, 36 had sufficient data for inclusion in this analysis. The comparison group consisted of 44 patients with an indwelling urethral catheter. There were no statistically significant differences between the two groups in terms of age, race, or ethnicity (Table 1). There were statistically significant differences in patient-reported reasons for catheter placement, but these were due to the exclusion criteria used for indwelling urethral catheter patients.
Both patient-reported and clinician-reported (ie, recorded in the patient’s medical record) outcomes are described in Table 2. In total, 80.6% of condom catheter users reported experiencing at least one catheter-related complication during the month after initial catheter placement compared with 88.6% of indwelling catheter users (P = .32). A similar number of condom catheter patients and indwelling urethral catheter patients experienced an infectious complication according to both self-report data (8.3% condom, 6.8% indwelling; P = .99) and medical record review (11.1% condom, 6.8% indwelling; P = .69).
At least one noninfectious complication was identified in 77.8% of condom catheter patients (28 of 36) and 88.6% of indwelling urethral catheter patients (39 of 44) using combined self-report and medical record review data (P = .19); most of these were based on self-reported data. Significantly fewer condom catheter patients reported complications during placement (eg, pain, discomfort, bleeding, or other trauma) compared with those with indwelling catheters (13.9% vs 43.2%, P < .001). Pain, discomfort, bleeding, or other trauma during catheter removal were commonly reported by both condom catheter and indwelling urethral catheter patients (40.9% vs 42.1%, respectively; P = .99).
Patient-reported noninfectious complications were often not documented in the medical record: 75.0% of condom catheter patients and 86.4% of indwelling catheter patients reported complications, in comparison with the 25.0% of condom catheter patients and 27.3% of indwelling urethral catheter patients with noninfectious complications identified during medical record review.
DISCUSSION
Our study revealed three important findings. First, noninfectious complications greatly outnumbered infectious complications, regardless of the device type. Second, condom catheter users reported significantly less pain related to placement of their device compared with the indwelling urethral catheter group. Finally, many patients reported complications that were not documented in the medical record.
The only randomized trial comparing these devices enrolled 75 men hospitalized at a single VA medical center and found that using a condom catheter rather than an indwelling catheter in patients without urinary retention lowered the composite endpoint of bacteriuria, symptomatic UTI, or death.4 Additionally, patients in this trial reported that the condom catheter was significantly more comfortable (90% vs 58%; P = .02) and less painful (5% vs 36%; P = .02) than the indwelling catheter,4 supporting a previous study in hospitalized male Veterans.5
Importantly, we included patient-reported complications that may be of concern to patients but inconsistently documented in the medical record. Pain associated with removal of both condom catheters and indwelling urethral catheters was reported in over 40% in both groups but was not documented in the medical record. One patient with a condom catheter described removal this way: “It got stuck on my hair, so was hard to get off…” Condom catheters also posed some issues with staying in place as has been previously described.6 As one condom catheter user said: “When I was laying down it was okay, but every time I moved around…it would slide off.”
Recent efforts to reduce catheter-associated UTI,7-9 which have focused on reducing the use of indwelling urethral catheters,10,11 have been relatively successful. Clinical policy makers should consider similar efforts to address the noninfectious harms of both catheter types. Such efforts could include further decreasing any type of catheter use along with improved training of those placing such devices.12 Substantial improvement will require a systematic approach to surveilling noninfectious complications of both types of urinary catheters.
Our study has several limitations. First, we conducted the study at two VA hospitals; therefore, the results may not be generalizable to a non-VA population. Second, we only included 80 patients because we recruited a limited number of condom catheter users.
Limitations notwithstanding, we provide comparison data between condom and indwelling urethral catheters. Condom catheter users reported significantly less pain related to initial placement of their device compared with those using an indwelling urethral catheter. For both devices, patients experienced noninfectious complications much more commonly than infectious ones, underscoring the need to systematically address such complications, perhaps through a surveillance system that includes the patient’s perspective. The patient’s voice is important and necessary in view of the apparent underreporting of noninfectious harms in the medical record.
A cknowledgments
Disclaimer
The funding sources played no role in the design, conducting, or evaluation of this study. The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the official position of the Department of Veterans Affairs.
1. Gould CV, Umscheid CA, Agarwal RK, Kuntz G, Pegues DA, Healthcare Infection Control Practices Advisory Committee. Guideline for prevention of catheter-associated urinary tract infections 2009. Infect Control Hosp Epidemiol. 2010;31(4):319-326. doi: 10.1086/651091.
2. Lo E, Nicolle LE, Coffin SE, et al. Strategies to prevent catheter-associated urinary tract infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(5):464-479. doi: 10.1086/675718.
3. Saint S, Trautner BW, Fowler KE, et al. A multicenter study of patient-reported infectious and noninfectious complications associated with indwelling urethral catheters. JAMA Intern Med. 2018. doi:10.1001/jamainternmed.2018.2417.
4. Saint S, Kaufman SR, Rogers MA, Baker PD, Ossenkop K, Lipsky BA. Condom versus indwelling urinary catheters: a randomized trial. J Am Geriatr Soc. 2006;54(7):1055-1061. doi: 10.1111/j.1532-5415.2006.00785.x.
5. Saint S, Lipsky BA, Baker PD, McDonald LL, Ossenkop K. Urinary catheters: what type do men and their nurses prefer? J Am Geriatr Soc. 1999;47(12):1453-1457. doi: 10.1111/j.1532-5415.1999.tb01567.x.
6. Smart C. Male urinary incontinence and the urinary sheath. Br J Nurs. 2014;23(9):S20, S22-S25. doi: 10.12968/bjon.2014.23.Sup9.S20.
7. Saint S, Greene MT, Kowalski CP, Watson SR, Hofer TP, Krein SL. Preventing catheter-associated urinary tract infection in the United States: a national comparative study. JAMA Intern Med. 2013;173(10):874-879. doi: 10.1001/jamainternmed.2013.101.
8. Saint S, Greene MT, Krein SL, et al. A program to prevent catheter-associated urinary tract infection in acute care. N Engl J Med. 2016;374(22):2111-2119. doi: 10.1056/NEJMoa1504906.
9. Saint S, Fowler KE, Sermak K, et al. Introducing the No preventable harms campaign: creating the safest health care system in the world, starting with catheter-associated urinary tract infection prevention. Am J Infect Control. 2015;43(3):254-259. doi: 10.1016/j.ajic.2014.11.016.
10. Fakih MG, Watson SR, Greene MT, et al. Reducing inappropriate urinary catheter use: a statewide effort. Arch Intern Med. 2012;172(3):255-260. doi: 10.1001/archinternmed.2011.627.
11. Krein SL, Kowalski CP, Harrod M, Forman J, Saint S. Barriers to reducing urinary catheter use: a qualitative assessment of a statewide initiative. JAMA Intern Med. 2013;173(10):881-886. doi: 10.1001/jamainternmed.2013.105.
12. Manojlovich M, Saint S, Meddings J, et al. Indwelling urinary catheter insertion practices in the emergency department: an observational study. Infect Control Hosp Epidemiol. 2016;37(1):117-119. doi: 10.1017/ice.2015.238.
13. Meddings JA, Reichert H, Rogers MA, Saint S, Stephansky J, McMahon LF. Effect of nonpayment for hospital-acquired, catheter-associated urinary tract infection: a statewide analysis. Ann Intern Med. 2012;157(5):305-312. doi: 10.7326/0003-4819-157-5-201209040-00003.
1. Gould CV, Umscheid CA, Agarwal RK, Kuntz G, Pegues DA, Healthcare Infection Control Practices Advisory Committee. Guideline for prevention of catheter-associated urinary tract infections 2009. Infect Control Hosp Epidemiol. 2010;31(4):319-326. doi: 10.1086/651091.
2. Lo E, Nicolle LE, Coffin SE, et al. Strategies to prevent catheter-associated urinary tract infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(5):464-479. doi: 10.1086/675718.
3. Saint S, Trautner BW, Fowler KE, et al. A multicenter study of patient-reported infectious and noninfectious complications associated with indwelling urethral catheters. JAMA Intern Med. 2018. doi:10.1001/jamainternmed.2018.2417.
4. Saint S, Kaufman SR, Rogers MA, Baker PD, Ossenkop K, Lipsky BA. Condom versus indwelling urinary catheters: a randomized trial. J Am Geriatr Soc. 2006;54(7):1055-1061. doi: 10.1111/j.1532-5415.2006.00785.x.
5. Saint S, Lipsky BA, Baker PD, McDonald LL, Ossenkop K. Urinary catheters: what type do men and their nurses prefer? J Am Geriatr Soc. 1999;47(12):1453-1457. doi: 10.1111/j.1532-5415.1999.tb01567.x.
6. Smart C. Male urinary incontinence and the urinary sheath. Br J Nurs. 2014;23(9):S20, S22-S25. doi: 10.12968/bjon.2014.23.Sup9.S20.
7. Saint S, Greene MT, Kowalski CP, Watson SR, Hofer TP, Krein SL. Preventing catheter-associated urinary tract infection in the United States: a national comparative study. JAMA Intern Med. 2013;173(10):874-879. doi: 10.1001/jamainternmed.2013.101.
8. Saint S, Greene MT, Krein SL, et al. A program to prevent catheter-associated urinary tract infection in acute care. N Engl J Med. 2016;374(22):2111-2119. doi: 10.1056/NEJMoa1504906.
9. Saint S, Fowler KE, Sermak K, et al. Introducing the No preventable harms campaign: creating the safest health care system in the world, starting with catheter-associated urinary tract infection prevention. Am J Infect Control. 2015;43(3):254-259. doi: 10.1016/j.ajic.2014.11.016.
10. Fakih MG, Watson SR, Greene MT, et al. Reducing inappropriate urinary catheter use: a statewide effort. Arch Intern Med. 2012;172(3):255-260. doi: 10.1001/archinternmed.2011.627.
11. Krein SL, Kowalski CP, Harrod M, Forman J, Saint S. Barriers to reducing urinary catheter use: a qualitative assessment of a statewide initiative. JAMA Intern Med. 2013;173(10):881-886. doi: 10.1001/jamainternmed.2013.105.
12. Manojlovich M, Saint S, Meddings J, et al. Indwelling urinary catheter insertion practices in the emergency department: an observational study. Infect Control Hosp Epidemiol. 2016;37(1):117-119. doi: 10.1017/ice.2015.238.
13. Meddings JA, Reichert H, Rogers MA, Saint S, Stephansky J, McMahon LF. Effect of nonpayment for hospital-acquired, catheter-associated urinary tract infection: a statewide analysis. Ann Intern Med. 2012;157(5):305-312. doi: 10.7326/0003-4819-157-5-201209040-00003.
© 2019 Society of Hospital Medicine