User login
Engraftment achieved after conditioning without radiation, alkylating agents
HOUSTON – Use of an alemtuzumab/fludarabine conditioning regimen – without any radiation or alkylating agents – was effective in patients with a systemic regenerative disease who experienced myeloid failure after allogeneic transplantation, according to an investigator.
Of the 20 patients with dyskeratosis congenita who received the regimen, 19 achieved primary engraftment within 42 days of transplant, according to Suneet Agarwal, MD, PhD, of Dana-Farber/Boston Children’s Cancer & Blood Disorders Center and Harvard Medical School, Boston.
However, these findings may have broader implications beyond this rare disease, as this is the first reported series of patients undergoing allogeneic bone marrow transplant to achieve durable myeloid engraftment without receiving alkylating agents or radiation, Dr. Agarwal said at the Transplantation & Cellular Therapy Meetings.
In a late-breaking clinical trial presentation of the results, Dr. Agarwal noted that patients in this study all had telomere disease, defined by clinical syndrome, gene mutation, or short lymphocyte telomere length. That’s because the investigators hypothesized that telomere defects would result in a “replicative disadvantage” in hematopoietic and immune cells, which would favor engraftment.
Dyskeratosis congenita is a prototypic telomere biology disorder caused by mutations that impair telomere maintenance, he said, adding that the disease has high rates of cancer, pulmonary disease, and hepatic disease.
While allogeneic transplants are curative in the disorder, outcomes after transplantation are typically poor, with high rates of bone marrow failure, he added. By eliminating radiation and alkylator exposure, the investigators hoped salvage bone marrow transplant would be more feasible, with lower risks of organ failure and secondary malignancy.
The 20 patients Dr. Agarwal reported on were aged from 30 months to 65 years. They all received alemtuzumab/fludarabine conditioning starting at day 9 before bone marrow graft, along with graft-versus-host disease (GVHD) prophylaxis through the pre- to posttransplant period. Two had matched siblings, while 18 had unrelated donors.
Of those 20 patients, 19 achieved primary engraftment at a median of 22 days after transplant, Dr. Agarwal reported. There was no acute GVHD and four cases of chronic GVHD that resolved with oral or topical steroids.
Of the 20 patients, 18 were alive at a median follow-up of 24 months, which compares favorably with historically reported cases, according to the investigator. There was one disease-related death and one treatment-related death caused by fungal infection at 3 months post transplant, he said.
These results suggest the radiation- and alkylator-free conditioning regimen is “effective,” Dr. Agarwal said, adding that exposure to those modalities is not required for myeloid engraftment in certain clinical settings.
The meeting is held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research. At its meeting, the American Society for Blood and Marrow Transplantation announced a new name for the society: American Society for Transplantation and Cellular Therapy (ASTCT).
Dr. Agarwal reported having no conflicts of interest.
SOURCE: Agarwal S et al. TCT 2019, Abstract LBA2.
HOUSTON – Use of an alemtuzumab/fludarabine conditioning regimen – without any radiation or alkylating agents – was effective in patients with a systemic regenerative disease who experienced myeloid failure after allogeneic transplantation, according to an investigator.
Of the 20 patients with dyskeratosis congenita who received the regimen, 19 achieved primary engraftment within 42 days of transplant, according to Suneet Agarwal, MD, PhD, of Dana-Farber/Boston Children’s Cancer & Blood Disorders Center and Harvard Medical School, Boston.
However, these findings may have broader implications beyond this rare disease, as this is the first reported series of patients undergoing allogeneic bone marrow transplant to achieve durable myeloid engraftment without receiving alkylating agents or radiation, Dr. Agarwal said at the Transplantation & Cellular Therapy Meetings.
In a late-breaking clinical trial presentation of the results, Dr. Agarwal noted that patients in this study all had telomere disease, defined by clinical syndrome, gene mutation, or short lymphocyte telomere length. That’s because the investigators hypothesized that telomere defects would result in a “replicative disadvantage” in hematopoietic and immune cells, which would favor engraftment.
Dyskeratosis congenita is a prototypic telomere biology disorder caused by mutations that impair telomere maintenance, he said, adding that the disease has high rates of cancer, pulmonary disease, and hepatic disease.
While allogeneic transplants are curative in the disorder, outcomes after transplantation are typically poor, with high rates of bone marrow failure, he added. By eliminating radiation and alkylator exposure, the investigators hoped salvage bone marrow transplant would be more feasible, with lower risks of organ failure and secondary malignancy.
The 20 patients Dr. Agarwal reported on were aged from 30 months to 65 years. They all received alemtuzumab/fludarabine conditioning starting at day 9 before bone marrow graft, along with graft-versus-host disease (GVHD) prophylaxis through the pre- to posttransplant period. Two had matched siblings, while 18 had unrelated donors.
Of those 20 patients, 19 achieved primary engraftment at a median of 22 days after transplant, Dr. Agarwal reported. There was no acute GVHD and four cases of chronic GVHD that resolved with oral or topical steroids.
Of the 20 patients, 18 were alive at a median follow-up of 24 months, which compares favorably with historically reported cases, according to the investigator. There was one disease-related death and one treatment-related death caused by fungal infection at 3 months post transplant, he said.
These results suggest the radiation- and alkylator-free conditioning regimen is “effective,” Dr. Agarwal said, adding that exposure to those modalities is not required for myeloid engraftment in certain clinical settings.
The meeting is held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research. At its meeting, the American Society for Blood and Marrow Transplantation announced a new name for the society: American Society for Transplantation and Cellular Therapy (ASTCT).
Dr. Agarwal reported having no conflicts of interest.
SOURCE: Agarwal S et al. TCT 2019, Abstract LBA2.
HOUSTON – Use of an alemtuzumab/fludarabine conditioning regimen – without any radiation or alkylating agents – was effective in patients with a systemic regenerative disease who experienced myeloid failure after allogeneic transplantation, according to an investigator.
Of the 20 patients with dyskeratosis congenita who received the regimen, 19 achieved primary engraftment within 42 days of transplant, according to Suneet Agarwal, MD, PhD, of Dana-Farber/Boston Children’s Cancer & Blood Disorders Center and Harvard Medical School, Boston.
However, these findings may have broader implications beyond this rare disease, as this is the first reported series of patients undergoing allogeneic bone marrow transplant to achieve durable myeloid engraftment without receiving alkylating agents or radiation, Dr. Agarwal said at the Transplantation & Cellular Therapy Meetings.
In a late-breaking clinical trial presentation of the results, Dr. Agarwal noted that patients in this study all had telomere disease, defined by clinical syndrome, gene mutation, or short lymphocyte telomere length. That’s because the investigators hypothesized that telomere defects would result in a “replicative disadvantage” in hematopoietic and immune cells, which would favor engraftment.
Dyskeratosis congenita is a prototypic telomere biology disorder caused by mutations that impair telomere maintenance, he said, adding that the disease has high rates of cancer, pulmonary disease, and hepatic disease.
While allogeneic transplants are curative in the disorder, outcomes after transplantation are typically poor, with high rates of bone marrow failure, he added. By eliminating radiation and alkylator exposure, the investigators hoped salvage bone marrow transplant would be more feasible, with lower risks of organ failure and secondary malignancy.
The 20 patients Dr. Agarwal reported on were aged from 30 months to 65 years. They all received alemtuzumab/fludarabine conditioning starting at day 9 before bone marrow graft, along with graft-versus-host disease (GVHD) prophylaxis through the pre- to posttransplant period. Two had matched siblings, while 18 had unrelated donors.
Of those 20 patients, 19 achieved primary engraftment at a median of 22 days after transplant, Dr. Agarwal reported. There was no acute GVHD and four cases of chronic GVHD that resolved with oral or topical steroids.
Of the 20 patients, 18 were alive at a median follow-up of 24 months, which compares favorably with historically reported cases, according to the investigator. There was one disease-related death and one treatment-related death caused by fungal infection at 3 months post transplant, he said.
These results suggest the radiation- and alkylator-free conditioning regimen is “effective,” Dr. Agarwal said, adding that exposure to those modalities is not required for myeloid engraftment in certain clinical settings.
The meeting is held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research. At its meeting, the American Society for Blood and Marrow Transplantation announced a new name for the society: American Society for Transplantation and Cellular Therapy (ASTCT).
Dr. Agarwal reported having no conflicts of interest.
SOURCE: Agarwal S et al. TCT 2019, Abstract LBA2.
REPORTING FROM TCT 2019
Sore on head
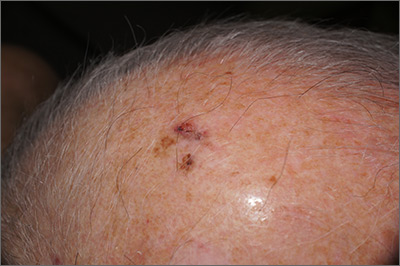
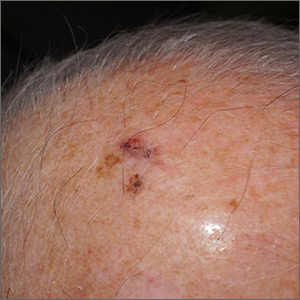
The FP suspected that this was a skin cancer with ulceration and pigmentation. The differential diagnosis included pigmented basal cell carcinoma, melanoma, and squamous cell carcinoma. If this were a melanoma, there would appear to be areas of regression between the islands of pigmentation. The FP explained that a biopsy was needed and suggested a broad shave biopsy because this technique would provide adequate tissue to the pathologist. (See the Watch & Learn video on “Shave biopsy.”)
The FP performed the broad shave biopsy and saw some remaining pigment at the base of the initial cut. He performed a second pass with the DermaBlade and explained this in the pathology requisition. (It is acceptable to send a deeper section of tissue if it appears that there is some remaining tumor below the initial biopsy.)
The biopsy report revealed lentigo maligna melanoma, which was approximately 2 mm thick—much deeper than the clinical appearance suggested. The pathologist combined the depths from the 2 specimens to get an approximate Breslow level. In this case, the most important information was that this was >1 mm in depth, which suggested that a sentinel lymph node biopsy would be needed for prognostic information. The patient was referred to Surgical Oncology for wide excision and sentinel lymph node biopsy.
Fortunately, the nodes were negative for metastasis. The wide excision was closed with a graft that healed well over time. The patient was followed with regular skin exams and careful lymph node exams of the neck and supraclavicular areas. He now wears a hat whenever he is outdoors.
Photo courtesy of Jonathan Karnes, MD and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Squamous cell carcinoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1103-1111.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com
The FP suspected that this was a skin cancer with ulceration and pigmentation. The differential diagnosis included pigmented basal cell carcinoma, melanoma, and squamous cell carcinoma. If this were a melanoma, there would appear to be areas of regression between the islands of pigmentation. The FP explained that a biopsy was needed and suggested a broad shave biopsy because this technique would provide adequate tissue to the pathologist. (See the Watch & Learn video on “Shave biopsy.”)
The FP performed the broad shave biopsy and saw some remaining pigment at the base of the initial cut. He performed a second pass with the DermaBlade and explained this in the pathology requisition. (It is acceptable to send a deeper section of tissue if it appears that there is some remaining tumor below the initial biopsy.)
The biopsy report revealed lentigo maligna melanoma, which was approximately 2 mm thick—much deeper than the clinical appearance suggested. The pathologist combined the depths from the 2 specimens to get an approximate Breslow level. In this case, the most important information was that this was >1 mm in depth, which suggested that a sentinel lymph node biopsy would be needed for prognostic information. The patient was referred to Surgical Oncology for wide excision and sentinel lymph node biopsy.
Fortunately, the nodes were negative for metastasis. The wide excision was closed with a graft that healed well over time. The patient was followed with regular skin exams and careful lymph node exams of the neck and supraclavicular areas. He now wears a hat whenever he is outdoors.
Photo courtesy of Jonathan Karnes, MD and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Squamous cell carcinoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1103-1111.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com
The FP suspected that this was a skin cancer with ulceration and pigmentation. The differential diagnosis included pigmented basal cell carcinoma, melanoma, and squamous cell carcinoma. If this were a melanoma, there would appear to be areas of regression between the islands of pigmentation. The FP explained that a biopsy was needed and suggested a broad shave biopsy because this technique would provide adequate tissue to the pathologist. (See the Watch & Learn video on “Shave biopsy.”)
The FP performed the broad shave biopsy and saw some remaining pigment at the base of the initial cut. He performed a second pass with the DermaBlade and explained this in the pathology requisition. (It is acceptable to send a deeper section of tissue if it appears that there is some remaining tumor below the initial biopsy.)
The biopsy report revealed lentigo maligna melanoma, which was approximately 2 mm thick—much deeper than the clinical appearance suggested. The pathologist combined the depths from the 2 specimens to get an approximate Breslow level. In this case, the most important information was that this was >1 mm in depth, which suggested that a sentinel lymph node biopsy would be needed for prognostic information. The patient was referred to Surgical Oncology for wide excision and sentinel lymph node biopsy.
Fortunately, the nodes were negative for metastasis. The wide excision was closed with a graft that healed well over time. The patient was followed with regular skin exams and careful lymph node exams of the neck and supraclavicular areas. He now wears a hat whenever he is outdoors.
Photo courtesy of Jonathan Karnes, MD and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Squamous cell carcinoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1103-1111.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com
Analysis suggests ‘Burkitt-like lymphoma’ is a misnomer
They found that BLL-11q has a genomic and mutational profile more closely related to that of high grade B-cell lymphoma (HGBCL) or diffuse large B-cell lymphoma (DLBCL) than typical Burkitt lymphoma (BL).
The researchers also found that BLL-11q has clinical, morphologic, and phenotypic features that are “more consistent” with HGBCL or DLBCL than with typical BL.
“These observations support a reconsideration of the ‘Burkitt-like’ term for these tumors,” Blanca Gonzalez-Farre, MD, of Hospital Clínic de Barcelona, and her colleagues wrote in Haematologica.
To reach this conclusion, the researchers performed copy number analysis and sequencing of B-cell lymphoma-related genes in 11 cases of BLL-11q.
The copy number analysis revealed that seven BLL-11q cases had the typical 11q gain/loss pattern, two had an 11q terminal deletion, one had two gains and two losses, and one had an 11q23.3-q25 copy number neutral loss of heterozygosity in addition to gain.
The BLL-11q cases also had frequent gains of 5q21.3-q32 and losses of 6q12.1-q21. However, they lacked the 1q gains observed in MYC-positive BL and alterations typically observed in germinal center B-cell like (GCB) DLBCL, such as gains in 2p16.1 and 7p.
Targeted sequencing of the BLL-11q cases revealed mutations typically observed in germinal center-derived lymphomas, including mutations in BTG2, DDX3X, ETS1, EP300, GNA13, CREBBP, KMT2C, EZH2, ARID1A, KMT2D, HIST1H1D, HIST1H2BC, and TMEM30A.
However, the BLL-11q cases lacked mutations in ID3, TCF3, and CCND3, which are typically observed in BL.
“In addition to the genetic differences, our BLL-11q differed clinically, morphologically, and phenotypically from conventional BL and instead showed features more consistent with HGBCL or DLBCL,” the researchers wrote.
Specifically, the BLL-11q patients were all younger than 40 years, with a median age of 15. Most presented with localized lymphadenopathy. And all had favorable treatment outcomes, remaining alive and free of disease at a median follow-up of 30 months.
All cases had a germinal center phenotype. They did not have the typical cytological features of BL, but they did have a high proliferative index, and some cases had a starry sky pattern.
The researchers said the BLL-11q cases were better classified as HGBCL not otherwise specified (n = 8), DLBCL (n = 2), and atypical BL (n = 1).
Considering these findings together, the team concluded that a more appropriate name for BLL-11q might be “aggressive B-cell lymphoma with 11q aberration.”
This research was supported by Asociación Española Contra el Cáncer and other organizations, as well as the government of Catalonia.
SOURCE: Gonzalez-Farre B et al. Haematologica. 2019 Feb 7. doi: 10.3324/haematol.2018.207928.
They found that BLL-11q has a genomic and mutational profile more closely related to that of high grade B-cell lymphoma (HGBCL) or diffuse large B-cell lymphoma (DLBCL) than typical Burkitt lymphoma (BL).
The researchers also found that BLL-11q has clinical, morphologic, and phenotypic features that are “more consistent” with HGBCL or DLBCL than with typical BL.
“These observations support a reconsideration of the ‘Burkitt-like’ term for these tumors,” Blanca Gonzalez-Farre, MD, of Hospital Clínic de Barcelona, and her colleagues wrote in Haematologica.
To reach this conclusion, the researchers performed copy number analysis and sequencing of B-cell lymphoma-related genes in 11 cases of BLL-11q.
The copy number analysis revealed that seven BLL-11q cases had the typical 11q gain/loss pattern, two had an 11q terminal deletion, one had two gains and two losses, and one had an 11q23.3-q25 copy number neutral loss of heterozygosity in addition to gain.
The BLL-11q cases also had frequent gains of 5q21.3-q32 and losses of 6q12.1-q21. However, they lacked the 1q gains observed in MYC-positive BL and alterations typically observed in germinal center B-cell like (GCB) DLBCL, such as gains in 2p16.1 and 7p.
Targeted sequencing of the BLL-11q cases revealed mutations typically observed in germinal center-derived lymphomas, including mutations in BTG2, DDX3X, ETS1, EP300, GNA13, CREBBP, KMT2C, EZH2, ARID1A, KMT2D, HIST1H1D, HIST1H2BC, and TMEM30A.
However, the BLL-11q cases lacked mutations in ID3, TCF3, and CCND3, which are typically observed in BL.
“In addition to the genetic differences, our BLL-11q differed clinically, morphologically, and phenotypically from conventional BL and instead showed features more consistent with HGBCL or DLBCL,” the researchers wrote.
Specifically, the BLL-11q patients were all younger than 40 years, with a median age of 15. Most presented with localized lymphadenopathy. And all had favorable treatment outcomes, remaining alive and free of disease at a median follow-up of 30 months.
All cases had a germinal center phenotype. They did not have the typical cytological features of BL, but they did have a high proliferative index, and some cases had a starry sky pattern.
The researchers said the BLL-11q cases were better classified as HGBCL not otherwise specified (n = 8), DLBCL (n = 2), and atypical BL (n = 1).
Considering these findings together, the team concluded that a more appropriate name for BLL-11q might be “aggressive B-cell lymphoma with 11q aberration.”
This research was supported by Asociación Española Contra el Cáncer and other organizations, as well as the government of Catalonia.
SOURCE: Gonzalez-Farre B et al. Haematologica. 2019 Feb 7. doi: 10.3324/haematol.2018.207928.
They found that BLL-11q has a genomic and mutational profile more closely related to that of high grade B-cell lymphoma (HGBCL) or diffuse large B-cell lymphoma (DLBCL) than typical Burkitt lymphoma (BL).
The researchers also found that BLL-11q has clinical, morphologic, and phenotypic features that are “more consistent” with HGBCL or DLBCL than with typical BL.
“These observations support a reconsideration of the ‘Burkitt-like’ term for these tumors,” Blanca Gonzalez-Farre, MD, of Hospital Clínic de Barcelona, and her colleagues wrote in Haematologica.
To reach this conclusion, the researchers performed copy number analysis and sequencing of B-cell lymphoma-related genes in 11 cases of BLL-11q.
The copy number analysis revealed that seven BLL-11q cases had the typical 11q gain/loss pattern, two had an 11q terminal deletion, one had two gains and two losses, and one had an 11q23.3-q25 copy number neutral loss of heterozygosity in addition to gain.
The BLL-11q cases also had frequent gains of 5q21.3-q32 and losses of 6q12.1-q21. However, they lacked the 1q gains observed in MYC-positive BL and alterations typically observed in germinal center B-cell like (GCB) DLBCL, such as gains in 2p16.1 and 7p.
Targeted sequencing of the BLL-11q cases revealed mutations typically observed in germinal center-derived lymphomas, including mutations in BTG2, DDX3X, ETS1, EP300, GNA13, CREBBP, KMT2C, EZH2, ARID1A, KMT2D, HIST1H1D, HIST1H2BC, and TMEM30A.
However, the BLL-11q cases lacked mutations in ID3, TCF3, and CCND3, which are typically observed in BL.
“In addition to the genetic differences, our BLL-11q differed clinically, morphologically, and phenotypically from conventional BL and instead showed features more consistent with HGBCL or DLBCL,” the researchers wrote.
Specifically, the BLL-11q patients were all younger than 40 years, with a median age of 15. Most presented with localized lymphadenopathy. And all had favorable treatment outcomes, remaining alive and free of disease at a median follow-up of 30 months.
All cases had a germinal center phenotype. They did not have the typical cytological features of BL, but they did have a high proliferative index, and some cases had a starry sky pattern.
The researchers said the BLL-11q cases were better classified as HGBCL not otherwise specified (n = 8), DLBCL (n = 2), and atypical BL (n = 1).
Considering these findings together, the team concluded that a more appropriate name for BLL-11q might be “aggressive B-cell lymphoma with 11q aberration.”
This research was supported by Asociación Española Contra el Cáncer and other organizations, as well as the government of Catalonia.
SOURCE: Gonzalez-Farre B et al. Haematologica. 2019 Feb 7. doi: 10.3324/haematol.2018.207928.
REPORTING FROM HAEMATOLOGICA
A systematic approach to chronic abnormal uterine bleeding
Menstrual bleeding is considered normal when it occurs regularly (every 21-35 days), lasts 4 to 8 days, and is not associated with heavy bleeding.1 During the first few years after menarche, it is normal for girls to experience irregular menstrual cycles but, by the third year, 60% to 80% of girls have an adult pattern of menstrual bleeding.2
Menstrual flow without normal volume, duration, regularity, or frequency is considered abnormal uterine bleeding (AUB). The condition is considered acute if there is need for immediate intervention. In the absence of the need for immediate intervention, recurrent AUB is classified as chronic.3 Chronic AUB is the focus of this article.
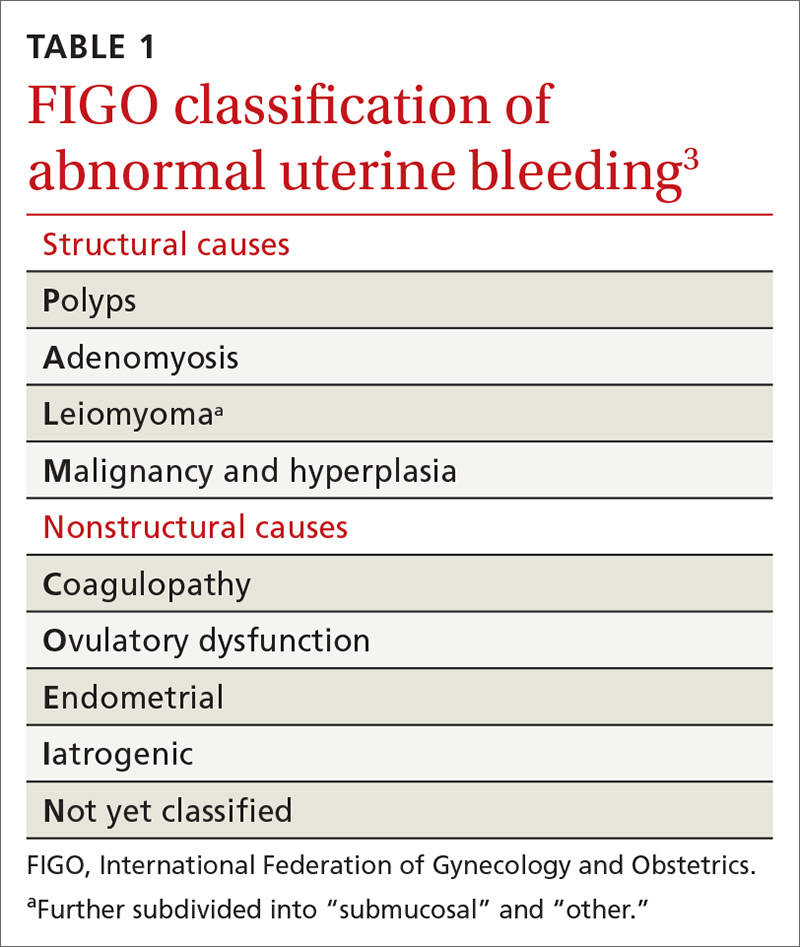
Invaluable tool: The FIGO classification
In 2011, the
- structural causes, recalled by “PALM” (Polyps, Adenomyosis, Leiomyoma, and Malignancy/hyperplasia)
- nonstructural causes, recalled by “COEIN” (Coagulopathy, Ovulatory dysfunction, Endometrial, Iatrogenic, and Not yet classified).
The PALM–COEIN system also uses descriptive terminology (heavy bleeding, intermenstrual bleeding) to characterize the bleeding pattern.3 The American College of Obstetricians and Gynecologists has adopted this classification system and recommends that such historically used terminology as “dysfunctional uterine bleeding,” “menorrhagia,” and “metrorrhagia” be abandoned.1
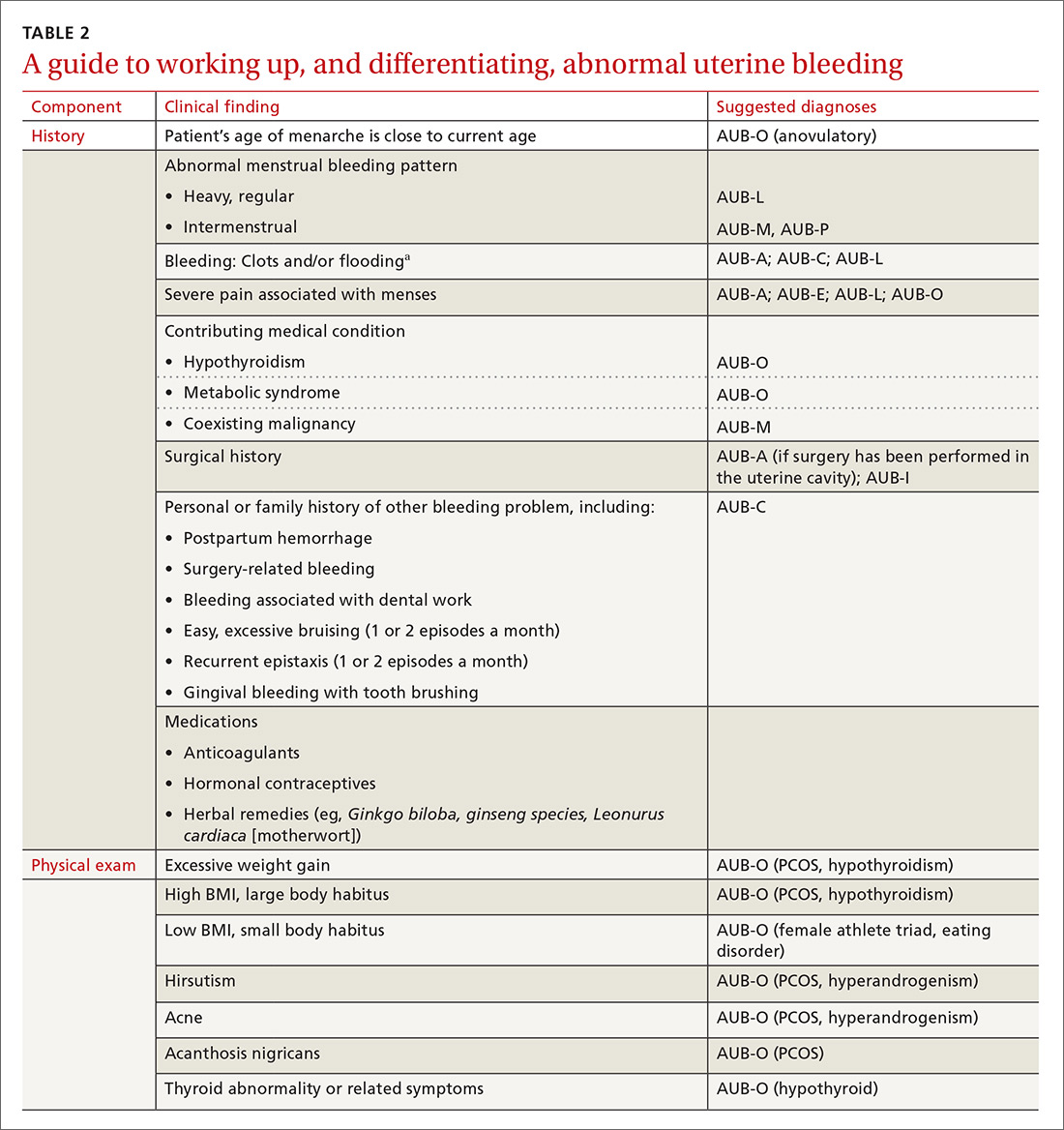
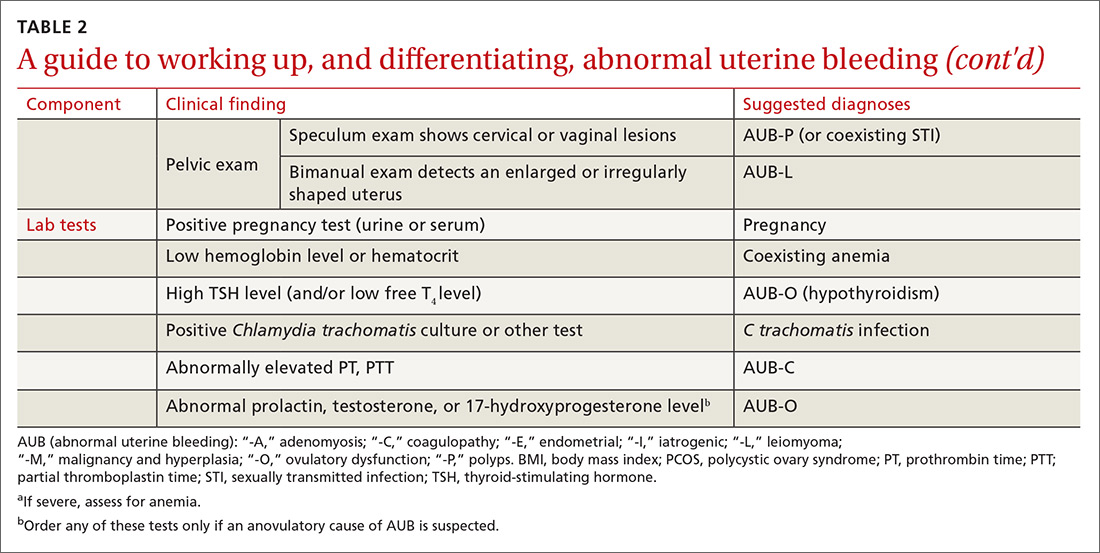
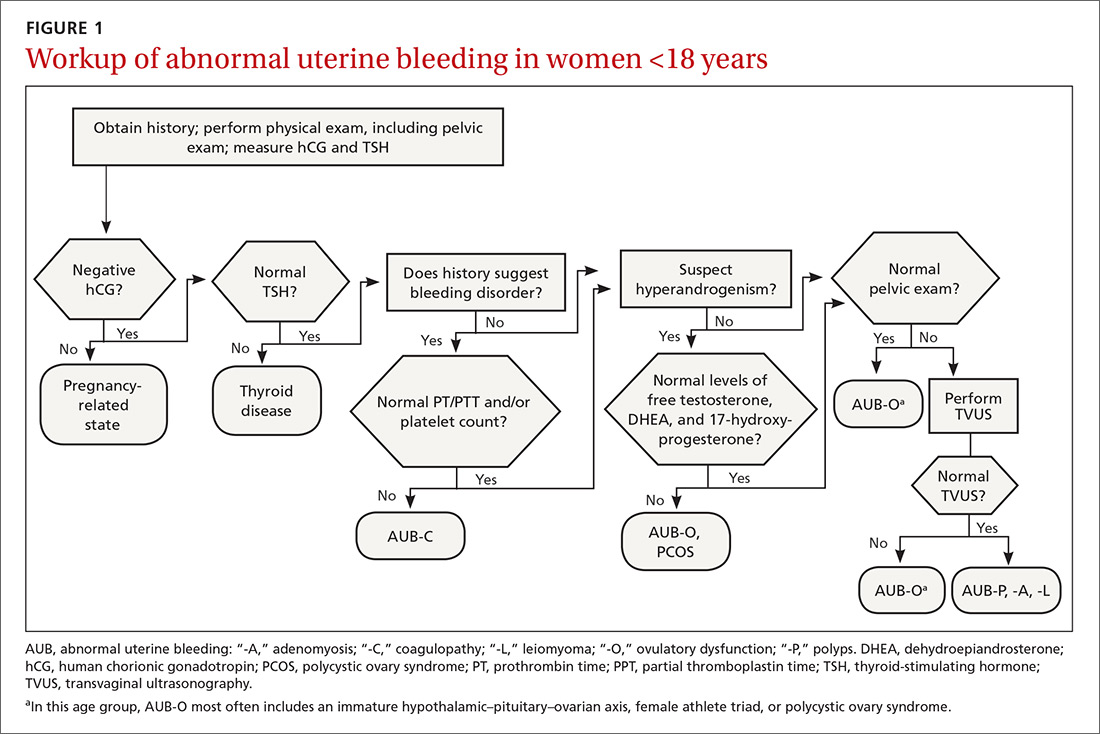
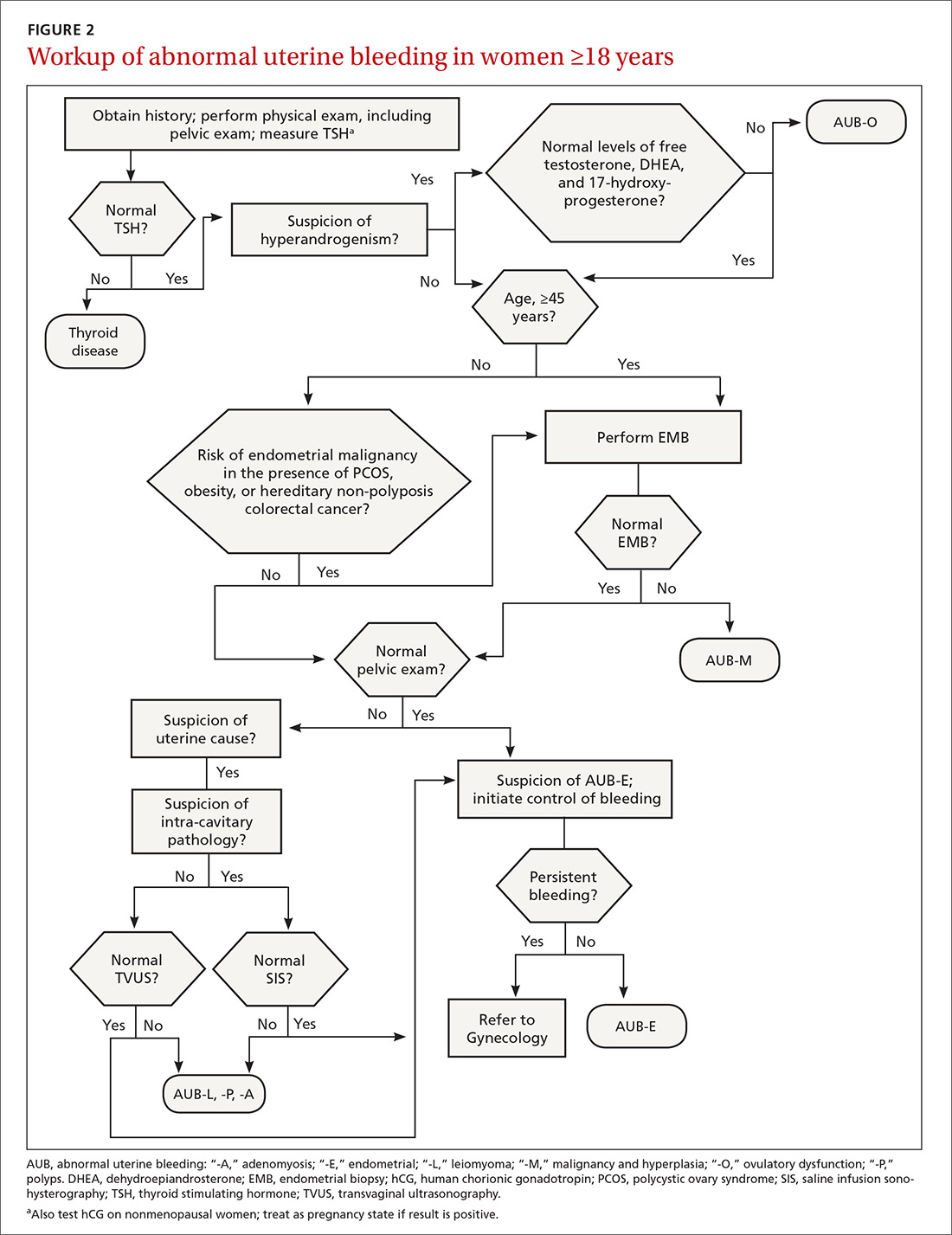
The initial workup of all causes of chronic uterine bleeding begins with a history; physical examination, including pelvic exam; and laboratory testing, including a urine pregnancy test, complete blood count, and a test of thyroid-stimulating hormone (TABLE 2). The need for additional laboratory testing, imaging, or endometrial biopsy depends on the suspected cause of AUB, detailed stepwise in FIGURE 1 (women <18 years) and FIGURE 2 (≥18 years).
We first briefly review the 9 categories of AUB in the PALM–COEIN system; discuss the most common causes in more detail; and review common treatment options (TABLE 3).
CASE 1
Marsha R, a 41-year-old-woman, complains of heavy menstrual bleeding for the past year that has become worse over the past 2 months. Her menstrual cycles have occurred every 28 days and last 10 days; she uses 10 to 12 pads a day.
Continue to: Recently, Ms. R reports...
Recently, Ms. R reports, she has been bleeding continuously for 14 days, with episodes of lighter bleeding followed by heavier bleeding. She also complains of fatigue.
Bimanual examination is notable for an enlarged uterus.
How would you proceed with the workup of this patient, to determine the cause of her bleeding and tailor management accordingly?
Structural AUB: The “PALM” mnemonic
A structural cause of AUB must be considered when you encounter an abnormality on physical exam (TABLE 1).3 In obese women or other patients in whom the physical exam is difficult, historical clues—including postcoital bleeding, intermenstrual bleeding, or pelvic pain or pressure—also suggest a structural abnormality.4
Transvaginal ultrasonography (TVUS) is the initial method of evaluation when a structural abnormality is suspected.1,4 However, although TVUS is excellent at visualizing the myometrium, lesions within the uterine cavity can be missed. If intracavitary pathology, such as submucosal fibroids or endometrial polyps, is suspected, additional imaging with saline infusion sonohysterography (SIS) should be performed. If a cavitary abnormality is confirmed, hysteroscopy is indicated.1 Magnetic resonance imaging (MRI) is reserved for cases in which a uterine cavity abnormality is found on TVUS but cannot be further characterized by SIS or hysteroscopy.1
Continue to: Endometrial biopsy...
Endometrial biopsy (EMB) is indicated as part of the initial evaluation of AUB in all women >45 years and in younger women who have risk factors for endometrial cancer, including polycystic ovary syndrome (PCOS), obesity, and hereditary nonpolyposis colorectal cancer. Such biopsy is necessary in these women whether or not another condition is the cause of the AUB and regardless of findings on TVUS.1-4 Endometrial biopsy should also be performed in women with AUB that persists despite medical management. If office EMB is nondiagnostic, hysteroscopy or SIS can be used to obtain tissue samples for further evaluation.5
Polyps. An endometrial polyp is a benign growth of endometrial tissue that is covered with epithelial cells. Polyps are often diagnosed by EMB or TVUS when these techniques are performed as part of the workup for AUB.6 Endometrial polyps are found more commonly in postmenopausal women, but should be considered as a cause of AUB in premenopausal women, too, especially those with intermenstrual bleeding or postcoital bleeding (or both) that is unresponsive to medical management.7 Risk factors for polyps include older age, obesity, and treatment with tamoxifen.7 The usual treatment for symptomatic endometrial polyps is removal by operative hysteroscopy.7
Adenomyosis. Ectopic endometrial tissue in the myometrium that leads to hypertrophy of the myometrium and uterine enlargement is known as adenomyosis. The disorder is most often diagnosed in women 40 to 50 years of age, who commonly complain of heavy uterine bleeding (40%-60% of cases) and dysmenorrhea (65%).8 Although definitive diagnosis is made histologically at hysterectomy, TVUS and MRI can be useful tools to help narrow the differential diagnosis in women with unexplained AUB.
According to a systematic review,9 the sensitivity and specificity of imaging in the diagnosis of adenomyosis is 72% and 81%, respectively, for TVUS and 77% and 89%, respectively, for MRI. Needle biopsy, performed hysteroscopically or laparoscopically, is less useful because the technique has low sensitivity (reported variously as 8%-56%) in diagnosing adenomyosis.8
Treatment options for adenomyosis are medical management with agents that reduce bleeding (eg, a combination oral contraceptive [OC], nonsteroidal anti-inflammatory drugs [NSAIDs], the antifibrinolytic tranexamic acid, and, when there is no distortion of the uterine cavity, a levonorgestrel intrauterine device [LNG-IUD]); uterine artery embolization; and hysterectomy.8
Continue to: Leiomyoma
Leiomyoma. Uterine fibroids, or leiomyomas, are benign, fibromuscular solid tumors, thought to be hormone-dependent because many regress after menopause. In women of reproductive age, uterine fibroids are the most common cause of structural AUB, with a cumulative incidence of 70% to 80% among women in this age group.3,10 Fibroids are more common in African-American women, women who experienced early menarche, and women who are obese, have PCOS, or had a late first pregnancy.3-10
Many fibroids are asymptomatic, and are found incidentally on sonographic examination performed for other reasons; in one-third of affected patients, the fibroids result in heavy menstrual bleeding.10 Intermenstrual bleeding and postcoital bleeding can occur, but are not common symptoms with fibroids. Consider other causes of AUB, such as endometrial polyps, when these symptoms are present.
Treatment of fibroids is medical or surgical. Medical management is a reasonable first-line option, especially in women who have not completed childbearing and who have small (<3 cm in diameter) fibroids. Options include a combination OC, NSAIDs, tranexamic acid, and, when the uterine cavity is not distorted, an LNG-IUD.4,10,11
For women with larger fibroids, those for whom the aforementioned medical treatments are unsuccessful, and those who are seeking more definitive treatment, uterine artery embolization, myomectomy, or hysterectomy can be considered.
› Uterine artery embolization is performed by an interventional radiologist under local anesthesia and, if necessary, moderate sedation.12 After the procedure, fibroids decrease in size due to avascular necrosis, but the remainder of the myometrium is relatively unaffected because collateral blood supply develops.13,14 Patients might experience abdominal cramping for 2 or 3 days following the procedure, which can be managed with an oral NSAID.12 Approximately 90% of women treated with embolization note improvement in AUB by 3 months after the procedure.15 Uterine artery embolization is not recommended in women who have not completed childbearing.12,16,17
Continue to: Myomectomy
› Myomectomy (removal of the leiomyoma) is the surgical treatment of choice for women who want to maintain fertility. Depending on the size and location of the fibroid(s), myomectomy can be performed as an open surgical procedure, laparoscopically, or hysteroscopically. At the discretion of the surgeon, leuprolide acetate, a gonadotropin-releasing hormone agonist, can be prescribed for 3 months before myomectomy to reduce intraoperative blood loss by decreasing the vascularity of the fibroids.4,18 Reduction in bleeding is reported in 70% to 90% of patients who undergo myomectomy.19
› Hysterectomy, the definitive treatment for uterine fibroids, should be reserved for women who have completed childbearing and who have failed (or have a contraindication to) other treatment options.
Malignancy/hyperplasia. EMB should be performed when endometrial malignancy/hyperplasia is suspected. As noted, endometrial cancer should be considered as a diagnostic possibility in women >45 years, in younger women with risk factors, and in women who have failed to respond to medical treatment for other suspected causes of AUB.5
When hyperplasia without atypia is diagnosed, the LNG-IUD or oral progesterone is an acceptable treatment option; note that fewer women who have an LNG-IUD eventually require hysterectomy, compared to women who take oral hormone therapy for AUB.20 When hyperplasia with atypia is diagnosed, hysterectomy is the treatment of choice. If a woman wishes to maintain fertility, however, oral progesterone therapy can be offered.21
When the diagnosis is cancer, the patient should be referred to a gynecologic oncologist for staging and treatment. Treatment varies depending on stage, but generally requires hysterectomy including bilateral salpingo-oophorectomy, with possible chemotherapy or radiation, or both.22
Continue to: CASE 1
CASE 1
Ms. R undergoes a sonogram that reveals a 4-cm fibroid in the uterine fundus that has not distorted the uterine cavity. Although she has completed childbearing, Ms. R is not interested in a surgical procedure at this time. You recommend insertion of an LNG-IUD; she accepts your advice.
CASE 2
Claire G, 27 years old, with a body mass index of 41,* complains of irregular menses for several months. Her menstrual cycle is irregular, as is the duration of menses and amount of bleeding. She has some mild fatigue without dizziness.
The physical exam is notable for mild hirsutism, without abnormalities on pelvic examination. Lab testing reveals iron-deficiency anemia; a pregnancy test is negative.
The questions that were raised by Ms. R’s case challenge you here, too: What is the appropriate workup of Ms. G’s bleeding? Once the cause is confirmed, how should you treat her?
Nonstructural AUB: The “COEIN” mnemonic
In the absence of abnormalities on a pelvic exam, and after excluding endometrial malignancy/hyperplasia in patients with the aforementioned risk factors, a nonstructural cause of AUB should be considered (TABLE 1).3 In women 20 to 40 years of age, the primary common cause of nonstructural uterine bleeding is ovulatory dysfunction, most often caused by PCOS or anovulatory bleeding.
Continue to: For nonstructual causes of AUB...
For nonstructural causes of AUB, the recommended laboratory workup varies with the suspected diagnosis. In addition, recently pregnant women should have a quantitative assay of β human chorionic gonadotropin to evaluate for trophoblastic disease.5,23
Imaging is not usually recommended when the cause of AUB is suspected to be nonstructural. However, when PCOS is suspected, TVUS can be used to confirm the presence of polycystic ovaries.23
As noted, EMB should be performed when AUB is present in women >45 years, in patients of any age group who fail to respond to medical therapy, and in those at increased risk for endometrial cancer.
Coagulopathy. When heavy bleeding has been present since the onset of menarche, inherited bleeding disorders must be considered, the most common of which is von Willebrand disease, a disorder of platelet adhesion.24 It is estimated that just under 50% of adolescents with abnormal uterine bleeding have a coagulopathy, most often a platelet function disorder.25 Additional clues to the presence of a coagulation disorder include a family history of bleeding disorder, a personal history of bleeding problems associated with surgery, and a history of iron-deficiency anemia.26 Abnormal uterine bleeding might resolve with treatment of the underlying coagulopathy; if it does not, consider consultation with a hematologist before prescribing an NSAID or an OC.
Heavy bleeding in patients taking an anticoagulant falls into the category of coagulopathy-related AUB. No further workup is generally needed for these women.3
Continue to: Ovulatory dysfunction
Ovulatory dysfunction. Abnormal uterine bleeding caused by ovulatory dysfunction is generally due to PCOS or anovulatory bleeding. Other causes, beyond the scope of this discussion, include hypothyroidism, hyperandrogenism, female athlete triad, stress, and hyperprolactinemia.
› Polycystic ovary syndrome. A diagnosis of PCOS is made using any of several recognized criteria. The commonly used Rotterdam 2003 criteria27 require that at least 2 of the following be present to make a diagnosis of PCOS:
- oligo-ovulation or anovulation
- hyperandrogenism
- polycystic ovaries seen on ultrasonography.
In addition, women with PCOS are frequently obese, show signs of insulin resistance (diabetes, prediabetes, acanthosis nigricans), or hyperandrogenism (hirsutism, acne). Even if these latter findings are not present at diagnosis, women with PCOS are at risk for a metabolic disorder. Once a diagnosis of PCOS has been established, therefore, screening tests for diabetes and cardiac risk factors (eg, dyslipidemia) should be performed.28.29
To evaluate for hyperandrogenism, free testosterone should be measured using a high-sensitivity immunoassay in all women in whom PCOS is suspected. Because of a higher prevalence of nonclassical (ie, late-onset) congenital adrenal hyperplasia (CAH) in women of Ashkenazi Jewish (estimated prevalence, 3.7%), Hispanic (1.9%), Slavic (1.6%), and Italian (0.3%) descent, screening for CAH as a possible cause of hyperandrogenism is also recommended, by a test of a morning 17-hydroxyprogesterone level.23,29,30 (Note: The general Caucasian population has an estimated prevalence of nonclassical CAH of 0.1%.30)
Treatment of PCOS should be individualized, based on a patient’s symptoms and comorbidities. For overweight and obese women, weight loss, exercise, and metformin (1500-2000 mg/d) are the mainstays of therapy, and might reduce AUB.29,31 If these measures do not reduce AUB, other options include an OC, an LNG-IUD, and NSAIDs.
Continue to: Information on treating other PCOS-related symptoms...
Information on treating other PCOS-related symptoms (acne, hirsutism) is available from many sources29; these treatments do not typically help the patient’s AUB, however, and are therefore not addressed in this article.
› Anovulatory bleeding. In adolescence, the most common cause of AUB is anovulation resulting from immaturity of the hypothalamic–pituitary–ovarian axis. During anovulatory cycles, the imbalance of estrogen and progesterone creates a fragile endometrium, leading to unpredictable bleeding and irregular cycles. Other less common causes of AUB, such as ovarian or adrenal tumor, should be considered in adolescents who have hirsutism but do not meet the criteria for PCOS.5
When seeing an adolescent for evaluation of AUB, be aware that emotional barriers might be present that make it difficult for her to talk about menses and sexual activity. Be patient and normalize the patient’s symptoms when appropriate. Pelvic exam can be deferred, especially in adolescents who have not yet had vaginal intercourse. When AUB occurs in an adolescent and the cause is thought to be immaturity of the hypothalamic–pituitary–ovarian axis, there is no need for laboratory testing or imaging studies, other than excluding hypothyroidism and pregnancy as the cause.
Oral contraceptives, NSAIDs, tranexamic acid, and the LNG-IUD are all options for treating patients who have anovulatory bleeding4,5 (TABLE 3). An OC has a major advantage for adolescents because it alleviates other complaints related to adolescent hormonal changes, such as acne, and provides contraception when taken on a regular basis.
Alternatively, the LNG-IUD has the benefit of ease of use once inserted, while still providing the added benefit of contraception. In women who have not yet had vaginal intercourse, an intrauterine device might not be the first choice of treatment, however, and should be prescribed only after discussion with the patient. For both OCs and the LNG-IUD, myths surrounding the use of these medications must be addressed with the patient and, if she is a minor, her parents or guardian.32
Continue to: NSAIDS can be effective because...
NSAIDs can be effective because they reduce bleeding by causing vasoconstriction, but they provide the greatest benefit when started before menses, which can be difficult for a patient who has irregular cycles.
Endometrial causes of AUB should be suspected when a patient has heavy menstrual bleeding with regular menstrual cycles and no other causes can be identified. Endometrial dysfunction as the cause of AUB stems from aberrations in the biochemical pathways of endometrial hemostasis and repair, and therefore is difficult to confirm by laboratory analysis or histologic evaluation.3 Medical management focuses on alleviating heavy menstrual bleeding (TABLE 3).
Iatrogenic. The most common type of iatrogenic AUB is unscheduled bleeding, also known as breakthrough bleeding, that occurs during hormonal treatment with an OC or during the first few months after insertion of an LNG-IUD or contraceptive implant.3 In most cases, no specific treatment is required; bleeding resolves upon continued use of the contraceptive.
Not yet classified. This category is difficult to define; it was created for causes of AUB that have not yet been identified and remain unclear. For example, a condition known as chronic endometritis is under study as a possible cause of AUB, but has not been assigned to a PALM–COEIN category.3 As more data become available and understanding of pathophysiologic mechanisms lead to better definitions of disease, this and other poorly understood conditions will be moved to an appropriate category in the FIGO classification system.
CASE 2
Ms. G is given a diagnosis of PCOS, based on her history. You recommend weight loss and exercise; screen her for diabetes and dyslipidemia; and prescribe metformin.
ACKNOWLEDGMENT
Barry D. Weiss, MD, University of Arizona College of Medicine, Department of Family and Community Medicine, Tucson, assisted with the editing of this manuscript.
CORRESPONDENCE
Melody A. Jordahl-Iafrato, MD, Community Hospital East Family Medicine Residency, 10122 East 10th Street, Suite 100, Indianapolis, IN 46229; Mjordahl-iafrato@ecommunity.com.
1. Committee on Practice Bulletins—Gynecology. American College of Obstetricians and Gynecologists Practice Bulletin Number 128, July 2012: Diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206.
2. American College of Obstetricians and Gynecologists Committee Opinion No. 651: Menstruation in girls and adolescents: using the menstrual cycle as a vital sign. Obstet Gynecol. 2015;126:e143-e146.
3. Munro MG, Critchley HO, Broder MS, et al; FIGO Working Group on Menstrual Disorders. FIGO classifcation system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynaecol Obstet. 2011;133:3-13.
4. National Institute for Health and Care Excellence (NICE). Heavy menstrual bleeding: assessment and management [NG88]. www.nice.org.uk/guidance/ng88. Accessed February 28, 2019.
5. Committee on Practice Bulletins—Gynecology. American College of Obstetricians and Gynecologists Practice Bulletin Number 136, July 2013: Management of abnormal uterine bleeding associated with ovulatory dysfunction. Obstet Gynecol. 2013;122:176-185.
6. Hassa H, Tekin B, Senses T, et al. Are the site, diameter, and number of endometrial polyps related with symptomatology? Am J Obstet Gynecol. 2006;194:718-721.
7. Salim S, Won H, Nesbitt-Hawes E, et al. Diagnosis and management of endometrial polyps: a critical review of the literature. J Minim Invasive Gynecol. 2011;18:569-581.
8. Struble J, Reid S, Bedaiwy MA. Adenomyosis: A clinical review of a challenging gynecologic condition. J Minim Invasive Gynecol. 2016;23:164-185.
9. Champaneria R, Abedin P, Daniels J, et al. Ultrasound scan and magnetic resonance imaging for the diagnosis of adenomyosis: systematic review comparing test accuracy. Acta Obstet Gynecol Scand. 2010;89:1374-1384.
10. Bartels CB, Cayton KC, Chuong FS, et al. An evidence-based approach to the medical management of fibroids: a systematic review. Clin Obstet Gynecol. 2016;59:30-52.
11. Lethaby A, Cooke I, Rees MC. Progesterone or progestogen-releasing intrauterine systems for heavy menstrual bleeding. Cochrane Database Syst Rev. 2005;(4):CD002126.
12. Spies JB. Current role of uterine artery embolization in the management of uterine fibroids. Clin Obstet Gynecol. 2016;59:93-102.
13. Gupta JK, Sinha A, Lumsden MA, et al. Uterine artery embolization for symptomatic uterine fibroids. Cochrane Database Syst Rev. 2014;(12):CD005073.
14. Edwards RD, Moss JG, Lumsden MA, et al; Committee of the Randomized Trial of Embolization versus Surgical Treatment for Fibroids. Uterine artery embolization versus surgery for symptomatic uterine fibroids. N Engl J Med. 2007;356:360-370.
15. Pron G, Bennett J, Common A, et al; Ontario Uterine Fibroid Embolization Collaboration Group. The Ontario Uterine Fibroid Embolization Trial. Part 2. Uterine fibroid reduction and symptom relief after uterine artery embolization for fibroids. Fertil Steril. 2003;79:120-127.
16. Torre A, Fauconnier A, Kahn V, et al. Fertility after uterine artery embolization for symptomatic multiple fibroids with no other infertility factors. Eur Radiol. 2017;27:2850-2859.
17. Mara M, Maskova J, Fucikova Z, et al. Midterm clinical and first reproductive results of a randomized controlled trial comparing uterine fibroid embolization and myomectomy. Cardiovasc Intervent Radiol. 2008;31:73-85.
18. Lethaby A, Vollenhoven B, Sowter M. Pre-operative GnRH analogue therapy before hysterectomy or myomectomy for uterine fibroids. Cochrane Database Syst Rev. 2001;(2): CD000547.
19. Capmas P, Levaillant JM, Fernandez H. Surgical techniques and outcome in the management of submucous fibroids. Curr Opin Obstet Gynecol. 2013;25:332-338.
20. Abu Hashim H, Ghayaty E, El Rakhawy M. Levonorgestrel-releasing intrauterine system vs oral progestins for non-atypical endometrial hyperplasia: a systematic review and metaanalysis of randomized trials. Am J Obstet Gynecol. 2015;213:469-478.
21. Reed SD, Voigt LF, Newton KM, et al. Weiss NS. Progestin therapy of complex endometrial hyperplasia with and without atypia. Obstet Gynecol. 2009;113;655-662.
22. Morice P, Leary A, Creutzberg C, et al. Endometrial cancer. Lancet. 2016;387:1094-1108.
23. Goodman NF, Cobin RH, Futterweit W, et al; American Association of Clinical Endocrinologists (AACE); American College of Endocrinology (ACE); Androgen Excess and PCOS Society (AES). American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Excess and PCOS Society disease state clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome—part 1. Endocr Pract. 2015;21:1291-1300.
24. Shankar M, Lee CA, Sabin CA, et al. von Willebrand disease in women with menorrhagia: a systematic review. BJOG. 2004;111:734-740.
25. Seravalli V, Linari S, Peruzzi E, et al. Prevalence of hemostatic disorders in adolescents with abnormal uterine bleeding. J Pediatr Adolesc Gynecol. 2013;26:285-289.
26. Philipp CS, Faiz A, Dowling NF, et al. Development of a screening tool for identifying women with menorrhagia for hemostatic evaluation. Am J Obstet Gynecol. 2008;198:163.e1-e8.
27. The Rotterdam ESHRE/ASRM-sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19:41-47.
28. Goodman NF, Cobin RH, Futterweit W, et al; American Association of Clinical Endocrinologists (AACE); American College of Endocrinology (ACE); Androgen Excess and PCOS Society (AES). American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Excess and PCOS Society disease state clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome—part 2. Endocr Pract. 2015;21:1415-26.
29. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No 194: Polycystic ovary syndrome. Obstet Gynecol. 2018;131:e157-e171.
30. Speiser PW, Dupont B, Rubinstein P, et al. High frequency of nonclassical steroid 21-hydroxylase deficiency. Am J Hum Genet. 1985;37:650-667.
31. Naderpoor N, Shorakae S, de Courten B, et al. Metformin and lifestyle modification in polycystic ovary syndrome: systematic review and meta-analysis. Hum Reprod Update. 2015;21:560-574.
32. Kolman KB, Hadley SK, Jordahl-Iafrato MA. Long-acting reversible contraception: who, what, when, and how. J Fam Pract. 2015;64:479-484.
Menstrual bleeding is considered normal when it occurs regularly (every 21-35 days), lasts 4 to 8 days, and is not associated with heavy bleeding.1 During the first few years after menarche, it is normal for girls to experience irregular menstrual cycles but, by the third year, 60% to 80% of girls have an adult pattern of menstrual bleeding.2
Menstrual flow without normal volume, duration, regularity, or frequency is considered abnormal uterine bleeding (AUB). The condition is considered acute if there is need for immediate intervention. In the absence of the need for immediate intervention, recurrent AUB is classified as chronic.3 Chronic AUB is the focus of this article.
Invaluable tool: The FIGO classification
In 2011, the
- structural causes, recalled by “PALM” (Polyps, Adenomyosis, Leiomyoma, and Malignancy/hyperplasia)
- nonstructural causes, recalled by “COEIN” (Coagulopathy, Ovulatory dysfunction, Endometrial, Iatrogenic, and Not yet classified).
The PALM–COEIN system also uses descriptive terminology (heavy bleeding, intermenstrual bleeding) to characterize the bleeding pattern.3 The American College of Obstetricians and Gynecologists has adopted this classification system and recommends that such historically used terminology as “dysfunctional uterine bleeding,” “menorrhagia,” and “metrorrhagia” be abandoned.1
The initial workup of all causes of chronic uterine bleeding begins with a history; physical examination, including pelvic exam; and laboratory testing, including a urine pregnancy test, complete blood count, and a test of thyroid-stimulating hormone (TABLE 2). The need for additional laboratory testing, imaging, or endometrial biopsy depends on the suspected cause of AUB, detailed stepwise in FIGURE 1 (women <18 years) and FIGURE 2 (≥18 years).
We first briefly review the 9 categories of AUB in the PALM–COEIN system; discuss the most common causes in more detail; and review common treatment options (TABLE 3).
CASE 1
Marsha R, a 41-year-old-woman, complains of heavy menstrual bleeding for the past year that has become worse over the past 2 months. Her menstrual cycles have occurred every 28 days and last 10 days; she uses 10 to 12 pads a day.
Continue to: Recently, Ms. R reports...
Recently, Ms. R reports, she has been bleeding continuously for 14 days, with episodes of lighter bleeding followed by heavier bleeding. She also complains of fatigue.
Bimanual examination is notable for an enlarged uterus.
How would you proceed with the workup of this patient, to determine the cause of her bleeding and tailor management accordingly?
Structural AUB: The “PALM” mnemonic
A structural cause of AUB must be considered when you encounter an abnormality on physical exam (TABLE 1).3 In obese women or other patients in whom the physical exam is difficult, historical clues—including postcoital bleeding, intermenstrual bleeding, or pelvic pain or pressure—also suggest a structural abnormality.4
Transvaginal ultrasonography (TVUS) is the initial method of evaluation when a structural abnormality is suspected.1,4 However, although TVUS is excellent at visualizing the myometrium, lesions within the uterine cavity can be missed. If intracavitary pathology, such as submucosal fibroids or endometrial polyps, is suspected, additional imaging with saline infusion sonohysterography (SIS) should be performed. If a cavitary abnormality is confirmed, hysteroscopy is indicated.1 Magnetic resonance imaging (MRI) is reserved for cases in which a uterine cavity abnormality is found on TVUS but cannot be further characterized by SIS or hysteroscopy.1
Continue to: Endometrial biopsy...
Endometrial biopsy (EMB) is indicated as part of the initial evaluation of AUB in all women >45 years and in younger women who have risk factors for endometrial cancer, including polycystic ovary syndrome (PCOS), obesity, and hereditary nonpolyposis colorectal cancer. Such biopsy is necessary in these women whether or not another condition is the cause of the AUB and regardless of findings on TVUS.1-4 Endometrial biopsy should also be performed in women with AUB that persists despite medical management. If office EMB is nondiagnostic, hysteroscopy or SIS can be used to obtain tissue samples for further evaluation.5
Polyps. An endometrial polyp is a benign growth of endometrial tissue that is covered with epithelial cells. Polyps are often diagnosed by EMB or TVUS when these techniques are performed as part of the workup for AUB.6 Endometrial polyps are found more commonly in postmenopausal women, but should be considered as a cause of AUB in premenopausal women, too, especially those with intermenstrual bleeding or postcoital bleeding (or both) that is unresponsive to medical management.7 Risk factors for polyps include older age, obesity, and treatment with tamoxifen.7 The usual treatment for symptomatic endometrial polyps is removal by operative hysteroscopy.7
Adenomyosis. Ectopic endometrial tissue in the myometrium that leads to hypertrophy of the myometrium and uterine enlargement is known as adenomyosis. The disorder is most often diagnosed in women 40 to 50 years of age, who commonly complain of heavy uterine bleeding (40%-60% of cases) and dysmenorrhea (65%).8 Although definitive diagnosis is made histologically at hysterectomy, TVUS and MRI can be useful tools to help narrow the differential diagnosis in women with unexplained AUB.
According to a systematic review,9 the sensitivity and specificity of imaging in the diagnosis of adenomyosis is 72% and 81%, respectively, for TVUS and 77% and 89%, respectively, for MRI. Needle biopsy, performed hysteroscopically or laparoscopically, is less useful because the technique has low sensitivity (reported variously as 8%-56%) in diagnosing adenomyosis.8
Treatment options for adenomyosis are medical management with agents that reduce bleeding (eg, a combination oral contraceptive [OC], nonsteroidal anti-inflammatory drugs [NSAIDs], the antifibrinolytic tranexamic acid, and, when there is no distortion of the uterine cavity, a levonorgestrel intrauterine device [LNG-IUD]); uterine artery embolization; and hysterectomy.8
Continue to: Leiomyoma
Leiomyoma. Uterine fibroids, or leiomyomas, are benign, fibromuscular solid tumors, thought to be hormone-dependent because many regress after menopause. In women of reproductive age, uterine fibroids are the most common cause of structural AUB, with a cumulative incidence of 70% to 80% among women in this age group.3,10 Fibroids are more common in African-American women, women who experienced early menarche, and women who are obese, have PCOS, or had a late first pregnancy.3-10
Many fibroids are asymptomatic, and are found incidentally on sonographic examination performed for other reasons; in one-third of affected patients, the fibroids result in heavy menstrual bleeding.10 Intermenstrual bleeding and postcoital bleeding can occur, but are not common symptoms with fibroids. Consider other causes of AUB, such as endometrial polyps, when these symptoms are present.
Treatment of fibroids is medical or surgical. Medical management is a reasonable first-line option, especially in women who have not completed childbearing and who have small (<3 cm in diameter) fibroids. Options include a combination OC, NSAIDs, tranexamic acid, and, when the uterine cavity is not distorted, an LNG-IUD.4,10,11
For women with larger fibroids, those for whom the aforementioned medical treatments are unsuccessful, and those who are seeking more definitive treatment, uterine artery embolization, myomectomy, or hysterectomy can be considered.
› Uterine artery embolization is performed by an interventional radiologist under local anesthesia and, if necessary, moderate sedation.12 After the procedure, fibroids decrease in size due to avascular necrosis, but the remainder of the myometrium is relatively unaffected because collateral blood supply develops.13,14 Patients might experience abdominal cramping for 2 or 3 days following the procedure, which can be managed with an oral NSAID.12 Approximately 90% of women treated with embolization note improvement in AUB by 3 months after the procedure.15 Uterine artery embolization is not recommended in women who have not completed childbearing.12,16,17
Continue to: Myomectomy
› Myomectomy (removal of the leiomyoma) is the surgical treatment of choice for women who want to maintain fertility. Depending on the size and location of the fibroid(s), myomectomy can be performed as an open surgical procedure, laparoscopically, or hysteroscopically. At the discretion of the surgeon, leuprolide acetate, a gonadotropin-releasing hormone agonist, can be prescribed for 3 months before myomectomy to reduce intraoperative blood loss by decreasing the vascularity of the fibroids.4,18 Reduction in bleeding is reported in 70% to 90% of patients who undergo myomectomy.19
› Hysterectomy, the definitive treatment for uterine fibroids, should be reserved for women who have completed childbearing and who have failed (or have a contraindication to) other treatment options.
Malignancy/hyperplasia. EMB should be performed when endometrial malignancy/hyperplasia is suspected. As noted, endometrial cancer should be considered as a diagnostic possibility in women >45 years, in younger women with risk factors, and in women who have failed to respond to medical treatment for other suspected causes of AUB.5
When hyperplasia without atypia is diagnosed, the LNG-IUD or oral progesterone is an acceptable treatment option; note that fewer women who have an LNG-IUD eventually require hysterectomy, compared to women who take oral hormone therapy for AUB.20 When hyperplasia with atypia is diagnosed, hysterectomy is the treatment of choice. If a woman wishes to maintain fertility, however, oral progesterone therapy can be offered.21
When the diagnosis is cancer, the patient should be referred to a gynecologic oncologist for staging and treatment. Treatment varies depending on stage, but generally requires hysterectomy including bilateral salpingo-oophorectomy, with possible chemotherapy or radiation, or both.22
Continue to: CASE 1
CASE 1
Ms. R undergoes a sonogram that reveals a 4-cm fibroid in the uterine fundus that has not distorted the uterine cavity. Although she has completed childbearing, Ms. R is not interested in a surgical procedure at this time. You recommend insertion of an LNG-IUD; she accepts your advice.
CASE 2
Claire G, 27 years old, with a body mass index of 41,* complains of irregular menses for several months. Her menstrual cycle is irregular, as is the duration of menses and amount of bleeding. She has some mild fatigue without dizziness.
The physical exam is notable for mild hirsutism, without abnormalities on pelvic examination. Lab testing reveals iron-deficiency anemia; a pregnancy test is negative.
The questions that were raised by Ms. R’s case challenge you here, too: What is the appropriate workup of Ms. G’s bleeding? Once the cause is confirmed, how should you treat her?
Nonstructural AUB: The “COEIN” mnemonic
In the absence of abnormalities on a pelvic exam, and after excluding endometrial malignancy/hyperplasia in patients with the aforementioned risk factors, a nonstructural cause of AUB should be considered (TABLE 1).3 In women 20 to 40 years of age, the primary common cause of nonstructural uterine bleeding is ovulatory dysfunction, most often caused by PCOS or anovulatory bleeding.
Continue to: For nonstructual causes of AUB...
For nonstructural causes of AUB, the recommended laboratory workup varies with the suspected diagnosis. In addition, recently pregnant women should have a quantitative assay of β human chorionic gonadotropin to evaluate for trophoblastic disease.5,23
Imaging is not usually recommended when the cause of AUB is suspected to be nonstructural. However, when PCOS is suspected, TVUS can be used to confirm the presence of polycystic ovaries.23
As noted, EMB should be performed when AUB is present in women >45 years, in patients of any age group who fail to respond to medical therapy, and in those at increased risk for endometrial cancer.
Coagulopathy. When heavy bleeding has been present since the onset of menarche, inherited bleeding disorders must be considered, the most common of which is von Willebrand disease, a disorder of platelet adhesion.24 It is estimated that just under 50% of adolescents with abnormal uterine bleeding have a coagulopathy, most often a platelet function disorder.25 Additional clues to the presence of a coagulation disorder include a family history of bleeding disorder, a personal history of bleeding problems associated with surgery, and a history of iron-deficiency anemia.26 Abnormal uterine bleeding might resolve with treatment of the underlying coagulopathy; if it does not, consider consultation with a hematologist before prescribing an NSAID or an OC.
Heavy bleeding in patients taking an anticoagulant falls into the category of coagulopathy-related AUB. No further workup is generally needed for these women.3
Continue to: Ovulatory dysfunction
Ovulatory dysfunction. Abnormal uterine bleeding caused by ovulatory dysfunction is generally due to PCOS or anovulatory bleeding. Other causes, beyond the scope of this discussion, include hypothyroidism, hyperandrogenism, female athlete triad, stress, and hyperprolactinemia.
› Polycystic ovary syndrome. A diagnosis of PCOS is made using any of several recognized criteria. The commonly used Rotterdam 2003 criteria27 require that at least 2 of the following be present to make a diagnosis of PCOS:
- oligo-ovulation or anovulation
- hyperandrogenism
- polycystic ovaries seen on ultrasonography.
In addition, women with PCOS are frequently obese, show signs of insulin resistance (diabetes, prediabetes, acanthosis nigricans), or hyperandrogenism (hirsutism, acne). Even if these latter findings are not present at diagnosis, women with PCOS are at risk for a metabolic disorder. Once a diagnosis of PCOS has been established, therefore, screening tests for diabetes and cardiac risk factors (eg, dyslipidemia) should be performed.28.29
To evaluate for hyperandrogenism, free testosterone should be measured using a high-sensitivity immunoassay in all women in whom PCOS is suspected. Because of a higher prevalence of nonclassical (ie, late-onset) congenital adrenal hyperplasia (CAH) in women of Ashkenazi Jewish (estimated prevalence, 3.7%), Hispanic (1.9%), Slavic (1.6%), and Italian (0.3%) descent, screening for CAH as a possible cause of hyperandrogenism is also recommended, by a test of a morning 17-hydroxyprogesterone level.23,29,30 (Note: The general Caucasian population has an estimated prevalence of nonclassical CAH of 0.1%.30)
Treatment of PCOS should be individualized, based on a patient’s symptoms and comorbidities. For overweight and obese women, weight loss, exercise, and metformin (1500-2000 mg/d) are the mainstays of therapy, and might reduce AUB.29,31 If these measures do not reduce AUB, other options include an OC, an LNG-IUD, and NSAIDs.
Continue to: Information on treating other PCOS-related symptoms...
Information on treating other PCOS-related symptoms (acne, hirsutism) is available from many sources29; these treatments do not typically help the patient’s AUB, however, and are therefore not addressed in this article.
› Anovulatory bleeding. In adolescence, the most common cause of AUB is anovulation resulting from immaturity of the hypothalamic–pituitary–ovarian axis. During anovulatory cycles, the imbalance of estrogen and progesterone creates a fragile endometrium, leading to unpredictable bleeding and irregular cycles. Other less common causes of AUB, such as ovarian or adrenal tumor, should be considered in adolescents who have hirsutism but do not meet the criteria for PCOS.5
When seeing an adolescent for evaluation of AUB, be aware that emotional barriers might be present that make it difficult for her to talk about menses and sexual activity. Be patient and normalize the patient’s symptoms when appropriate. Pelvic exam can be deferred, especially in adolescents who have not yet had vaginal intercourse. When AUB occurs in an adolescent and the cause is thought to be immaturity of the hypothalamic–pituitary–ovarian axis, there is no need for laboratory testing or imaging studies, other than excluding hypothyroidism and pregnancy as the cause.
Oral contraceptives, NSAIDs, tranexamic acid, and the LNG-IUD are all options for treating patients who have anovulatory bleeding4,5 (TABLE 3). An OC has a major advantage for adolescents because it alleviates other complaints related to adolescent hormonal changes, such as acne, and provides contraception when taken on a regular basis.
Alternatively, the LNG-IUD has the benefit of ease of use once inserted, while still providing the added benefit of contraception. In women who have not yet had vaginal intercourse, an intrauterine device might not be the first choice of treatment, however, and should be prescribed only after discussion with the patient. For both OCs and the LNG-IUD, myths surrounding the use of these medications must be addressed with the patient and, if she is a minor, her parents or guardian.32
Continue to: NSAIDS can be effective because...
NSAIDs can be effective because they reduce bleeding by causing vasoconstriction, but they provide the greatest benefit when started before menses, which can be difficult for a patient who has irregular cycles.
Endometrial causes of AUB should be suspected when a patient has heavy menstrual bleeding with regular menstrual cycles and no other causes can be identified. Endometrial dysfunction as the cause of AUB stems from aberrations in the biochemical pathways of endometrial hemostasis and repair, and therefore is difficult to confirm by laboratory analysis or histologic evaluation.3 Medical management focuses on alleviating heavy menstrual bleeding (TABLE 3).
Iatrogenic. The most common type of iatrogenic AUB is unscheduled bleeding, also known as breakthrough bleeding, that occurs during hormonal treatment with an OC or during the first few months after insertion of an LNG-IUD or contraceptive implant.3 In most cases, no specific treatment is required; bleeding resolves upon continued use of the contraceptive.
Not yet classified. This category is difficult to define; it was created for causes of AUB that have not yet been identified and remain unclear. For example, a condition known as chronic endometritis is under study as a possible cause of AUB, but has not been assigned to a PALM–COEIN category.3 As more data become available and understanding of pathophysiologic mechanisms lead to better definitions of disease, this and other poorly understood conditions will be moved to an appropriate category in the FIGO classification system.
CASE 2
Ms. G is given a diagnosis of PCOS, based on her history. You recommend weight loss and exercise; screen her for diabetes and dyslipidemia; and prescribe metformin.
ACKNOWLEDGMENT
Barry D. Weiss, MD, University of Arizona College of Medicine, Department of Family and Community Medicine, Tucson, assisted with the editing of this manuscript.
CORRESPONDENCE
Melody A. Jordahl-Iafrato, MD, Community Hospital East Family Medicine Residency, 10122 East 10th Street, Suite 100, Indianapolis, IN 46229; Mjordahl-iafrato@ecommunity.com.
Menstrual bleeding is considered normal when it occurs regularly (every 21-35 days), lasts 4 to 8 days, and is not associated with heavy bleeding.1 During the first few years after menarche, it is normal for girls to experience irregular menstrual cycles but, by the third year, 60% to 80% of girls have an adult pattern of menstrual bleeding.2
Menstrual flow without normal volume, duration, regularity, or frequency is considered abnormal uterine bleeding (AUB). The condition is considered acute if there is need for immediate intervention. In the absence of the need for immediate intervention, recurrent AUB is classified as chronic.3 Chronic AUB is the focus of this article.
Invaluable tool: The FIGO classification
In 2011, the
- structural causes, recalled by “PALM” (Polyps, Adenomyosis, Leiomyoma, and Malignancy/hyperplasia)
- nonstructural causes, recalled by “COEIN” (Coagulopathy, Ovulatory dysfunction, Endometrial, Iatrogenic, and Not yet classified).
The PALM–COEIN system also uses descriptive terminology (heavy bleeding, intermenstrual bleeding) to characterize the bleeding pattern.3 The American College of Obstetricians and Gynecologists has adopted this classification system and recommends that such historically used terminology as “dysfunctional uterine bleeding,” “menorrhagia,” and “metrorrhagia” be abandoned.1
The initial workup of all causes of chronic uterine bleeding begins with a history; physical examination, including pelvic exam; and laboratory testing, including a urine pregnancy test, complete blood count, and a test of thyroid-stimulating hormone (TABLE 2). The need for additional laboratory testing, imaging, or endometrial biopsy depends on the suspected cause of AUB, detailed stepwise in FIGURE 1 (women <18 years) and FIGURE 2 (≥18 years).
We first briefly review the 9 categories of AUB in the PALM–COEIN system; discuss the most common causes in more detail; and review common treatment options (TABLE 3).
CASE 1
Marsha R, a 41-year-old-woman, complains of heavy menstrual bleeding for the past year that has become worse over the past 2 months. Her menstrual cycles have occurred every 28 days and last 10 days; she uses 10 to 12 pads a day.
Continue to: Recently, Ms. R reports...
Recently, Ms. R reports, she has been bleeding continuously for 14 days, with episodes of lighter bleeding followed by heavier bleeding. She also complains of fatigue.
Bimanual examination is notable for an enlarged uterus.
How would you proceed with the workup of this patient, to determine the cause of her bleeding and tailor management accordingly?
Structural AUB: The “PALM” mnemonic
A structural cause of AUB must be considered when you encounter an abnormality on physical exam (TABLE 1).3 In obese women or other patients in whom the physical exam is difficult, historical clues—including postcoital bleeding, intermenstrual bleeding, or pelvic pain or pressure—also suggest a structural abnormality.4
Transvaginal ultrasonography (TVUS) is the initial method of evaluation when a structural abnormality is suspected.1,4 However, although TVUS is excellent at visualizing the myometrium, lesions within the uterine cavity can be missed. If intracavitary pathology, such as submucosal fibroids or endometrial polyps, is suspected, additional imaging with saline infusion sonohysterography (SIS) should be performed. If a cavitary abnormality is confirmed, hysteroscopy is indicated.1 Magnetic resonance imaging (MRI) is reserved for cases in which a uterine cavity abnormality is found on TVUS but cannot be further characterized by SIS or hysteroscopy.1
Continue to: Endometrial biopsy...
Endometrial biopsy (EMB) is indicated as part of the initial evaluation of AUB in all women >45 years and in younger women who have risk factors for endometrial cancer, including polycystic ovary syndrome (PCOS), obesity, and hereditary nonpolyposis colorectal cancer. Such biopsy is necessary in these women whether or not another condition is the cause of the AUB and regardless of findings on TVUS.1-4 Endometrial biopsy should also be performed in women with AUB that persists despite medical management. If office EMB is nondiagnostic, hysteroscopy or SIS can be used to obtain tissue samples for further evaluation.5
Polyps. An endometrial polyp is a benign growth of endometrial tissue that is covered with epithelial cells. Polyps are often diagnosed by EMB or TVUS when these techniques are performed as part of the workup for AUB.6 Endometrial polyps are found more commonly in postmenopausal women, but should be considered as a cause of AUB in premenopausal women, too, especially those with intermenstrual bleeding or postcoital bleeding (or both) that is unresponsive to medical management.7 Risk factors for polyps include older age, obesity, and treatment with tamoxifen.7 The usual treatment for symptomatic endometrial polyps is removal by operative hysteroscopy.7
Adenomyosis. Ectopic endometrial tissue in the myometrium that leads to hypertrophy of the myometrium and uterine enlargement is known as adenomyosis. The disorder is most often diagnosed in women 40 to 50 years of age, who commonly complain of heavy uterine bleeding (40%-60% of cases) and dysmenorrhea (65%).8 Although definitive diagnosis is made histologically at hysterectomy, TVUS and MRI can be useful tools to help narrow the differential diagnosis in women with unexplained AUB.
According to a systematic review,9 the sensitivity and specificity of imaging in the diagnosis of adenomyosis is 72% and 81%, respectively, for TVUS and 77% and 89%, respectively, for MRI. Needle biopsy, performed hysteroscopically or laparoscopically, is less useful because the technique has low sensitivity (reported variously as 8%-56%) in diagnosing adenomyosis.8
Treatment options for adenomyosis are medical management with agents that reduce bleeding (eg, a combination oral contraceptive [OC], nonsteroidal anti-inflammatory drugs [NSAIDs], the antifibrinolytic tranexamic acid, and, when there is no distortion of the uterine cavity, a levonorgestrel intrauterine device [LNG-IUD]); uterine artery embolization; and hysterectomy.8
Continue to: Leiomyoma
Leiomyoma. Uterine fibroids, or leiomyomas, are benign, fibromuscular solid tumors, thought to be hormone-dependent because many regress after menopause. In women of reproductive age, uterine fibroids are the most common cause of structural AUB, with a cumulative incidence of 70% to 80% among women in this age group.3,10 Fibroids are more common in African-American women, women who experienced early menarche, and women who are obese, have PCOS, or had a late first pregnancy.3-10
Many fibroids are asymptomatic, and are found incidentally on sonographic examination performed for other reasons; in one-third of affected patients, the fibroids result in heavy menstrual bleeding.10 Intermenstrual bleeding and postcoital bleeding can occur, but are not common symptoms with fibroids. Consider other causes of AUB, such as endometrial polyps, when these symptoms are present.
Treatment of fibroids is medical or surgical. Medical management is a reasonable first-line option, especially in women who have not completed childbearing and who have small (<3 cm in diameter) fibroids. Options include a combination OC, NSAIDs, tranexamic acid, and, when the uterine cavity is not distorted, an LNG-IUD.4,10,11
For women with larger fibroids, those for whom the aforementioned medical treatments are unsuccessful, and those who are seeking more definitive treatment, uterine artery embolization, myomectomy, or hysterectomy can be considered.
› Uterine artery embolization is performed by an interventional radiologist under local anesthesia and, if necessary, moderate sedation.12 After the procedure, fibroids decrease in size due to avascular necrosis, but the remainder of the myometrium is relatively unaffected because collateral blood supply develops.13,14 Patients might experience abdominal cramping for 2 or 3 days following the procedure, which can be managed with an oral NSAID.12 Approximately 90% of women treated with embolization note improvement in AUB by 3 months after the procedure.15 Uterine artery embolization is not recommended in women who have not completed childbearing.12,16,17
Continue to: Myomectomy
› Myomectomy (removal of the leiomyoma) is the surgical treatment of choice for women who want to maintain fertility. Depending on the size and location of the fibroid(s), myomectomy can be performed as an open surgical procedure, laparoscopically, or hysteroscopically. At the discretion of the surgeon, leuprolide acetate, a gonadotropin-releasing hormone agonist, can be prescribed for 3 months before myomectomy to reduce intraoperative blood loss by decreasing the vascularity of the fibroids.4,18 Reduction in bleeding is reported in 70% to 90% of patients who undergo myomectomy.19
› Hysterectomy, the definitive treatment for uterine fibroids, should be reserved for women who have completed childbearing and who have failed (or have a contraindication to) other treatment options.
Malignancy/hyperplasia. EMB should be performed when endometrial malignancy/hyperplasia is suspected. As noted, endometrial cancer should be considered as a diagnostic possibility in women >45 years, in younger women with risk factors, and in women who have failed to respond to medical treatment for other suspected causes of AUB.5
When hyperplasia without atypia is diagnosed, the LNG-IUD or oral progesterone is an acceptable treatment option; note that fewer women who have an LNG-IUD eventually require hysterectomy, compared to women who take oral hormone therapy for AUB.20 When hyperplasia with atypia is diagnosed, hysterectomy is the treatment of choice. If a woman wishes to maintain fertility, however, oral progesterone therapy can be offered.21
When the diagnosis is cancer, the patient should be referred to a gynecologic oncologist for staging and treatment. Treatment varies depending on stage, but generally requires hysterectomy including bilateral salpingo-oophorectomy, with possible chemotherapy or radiation, or both.22
Continue to: CASE 1
CASE 1
Ms. R undergoes a sonogram that reveals a 4-cm fibroid in the uterine fundus that has not distorted the uterine cavity. Although she has completed childbearing, Ms. R is not interested in a surgical procedure at this time. You recommend insertion of an LNG-IUD; she accepts your advice.
CASE 2
Claire G, 27 years old, with a body mass index of 41,* complains of irregular menses for several months. Her menstrual cycle is irregular, as is the duration of menses and amount of bleeding. She has some mild fatigue without dizziness.
The physical exam is notable for mild hirsutism, without abnormalities on pelvic examination. Lab testing reveals iron-deficiency anemia; a pregnancy test is negative.
The questions that were raised by Ms. R’s case challenge you here, too: What is the appropriate workup of Ms. G’s bleeding? Once the cause is confirmed, how should you treat her?
Nonstructural AUB: The “COEIN” mnemonic
In the absence of abnormalities on a pelvic exam, and after excluding endometrial malignancy/hyperplasia in patients with the aforementioned risk factors, a nonstructural cause of AUB should be considered (TABLE 1).3 In women 20 to 40 years of age, the primary common cause of nonstructural uterine bleeding is ovulatory dysfunction, most often caused by PCOS or anovulatory bleeding.
Continue to: For nonstructual causes of AUB...
For nonstructural causes of AUB, the recommended laboratory workup varies with the suspected diagnosis. In addition, recently pregnant women should have a quantitative assay of β human chorionic gonadotropin to evaluate for trophoblastic disease.5,23
Imaging is not usually recommended when the cause of AUB is suspected to be nonstructural. However, when PCOS is suspected, TVUS can be used to confirm the presence of polycystic ovaries.23
As noted, EMB should be performed when AUB is present in women >45 years, in patients of any age group who fail to respond to medical therapy, and in those at increased risk for endometrial cancer.
Coagulopathy. When heavy bleeding has been present since the onset of menarche, inherited bleeding disorders must be considered, the most common of which is von Willebrand disease, a disorder of platelet adhesion.24 It is estimated that just under 50% of adolescents with abnormal uterine bleeding have a coagulopathy, most often a platelet function disorder.25 Additional clues to the presence of a coagulation disorder include a family history of bleeding disorder, a personal history of bleeding problems associated with surgery, and a history of iron-deficiency anemia.26 Abnormal uterine bleeding might resolve with treatment of the underlying coagulopathy; if it does not, consider consultation with a hematologist before prescribing an NSAID or an OC.
Heavy bleeding in patients taking an anticoagulant falls into the category of coagulopathy-related AUB. No further workup is generally needed for these women.3
Continue to: Ovulatory dysfunction
Ovulatory dysfunction. Abnormal uterine bleeding caused by ovulatory dysfunction is generally due to PCOS or anovulatory bleeding. Other causes, beyond the scope of this discussion, include hypothyroidism, hyperandrogenism, female athlete triad, stress, and hyperprolactinemia.
› Polycystic ovary syndrome. A diagnosis of PCOS is made using any of several recognized criteria. The commonly used Rotterdam 2003 criteria27 require that at least 2 of the following be present to make a diagnosis of PCOS:
- oligo-ovulation or anovulation
- hyperandrogenism
- polycystic ovaries seen on ultrasonography.
In addition, women with PCOS are frequently obese, show signs of insulin resistance (diabetes, prediabetes, acanthosis nigricans), or hyperandrogenism (hirsutism, acne). Even if these latter findings are not present at diagnosis, women with PCOS are at risk for a metabolic disorder. Once a diagnosis of PCOS has been established, therefore, screening tests for diabetes and cardiac risk factors (eg, dyslipidemia) should be performed.28.29
To evaluate for hyperandrogenism, free testosterone should be measured using a high-sensitivity immunoassay in all women in whom PCOS is suspected. Because of a higher prevalence of nonclassical (ie, late-onset) congenital adrenal hyperplasia (CAH) in women of Ashkenazi Jewish (estimated prevalence, 3.7%), Hispanic (1.9%), Slavic (1.6%), and Italian (0.3%) descent, screening for CAH as a possible cause of hyperandrogenism is also recommended, by a test of a morning 17-hydroxyprogesterone level.23,29,30 (Note: The general Caucasian population has an estimated prevalence of nonclassical CAH of 0.1%.30)
Treatment of PCOS should be individualized, based on a patient’s symptoms and comorbidities. For overweight and obese women, weight loss, exercise, and metformin (1500-2000 mg/d) are the mainstays of therapy, and might reduce AUB.29,31 If these measures do not reduce AUB, other options include an OC, an LNG-IUD, and NSAIDs.
Continue to: Information on treating other PCOS-related symptoms...
Information on treating other PCOS-related symptoms (acne, hirsutism) is available from many sources29; these treatments do not typically help the patient’s AUB, however, and are therefore not addressed in this article.
› Anovulatory bleeding. In adolescence, the most common cause of AUB is anovulation resulting from immaturity of the hypothalamic–pituitary–ovarian axis. During anovulatory cycles, the imbalance of estrogen and progesterone creates a fragile endometrium, leading to unpredictable bleeding and irregular cycles. Other less common causes of AUB, such as ovarian or adrenal tumor, should be considered in adolescents who have hirsutism but do not meet the criteria for PCOS.5
When seeing an adolescent for evaluation of AUB, be aware that emotional barriers might be present that make it difficult for her to talk about menses and sexual activity. Be patient and normalize the patient’s symptoms when appropriate. Pelvic exam can be deferred, especially in adolescents who have not yet had vaginal intercourse. When AUB occurs in an adolescent and the cause is thought to be immaturity of the hypothalamic–pituitary–ovarian axis, there is no need for laboratory testing or imaging studies, other than excluding hypothyroidism and pregnancy as the cause.
Oral contraceptives, NSAIDs, tranexamic acid, and the LNG-IUD are all options for treating patients who have anovulatory bleeding4,5 (TABLE 3). An OC has a major advantage for adolescents because it alleviates other complaints related to adolescent hormonal changes, such as acne, and provides contraception when taken on a regular basis.
Alternatively, the LNG-IUD has the benefit of ease of use once inserted, while still providing the added benefit of contraception. In women who have not yet had vaginal intercourse, an intrauterine device might not be the first choice of treatment, however, and should be prescribed only after discussion with the patient. For both OCs and the LNG-IUD, myths surrounding the use of these medications must be addressed with the patient and, if she is a minor, her parents or guardian.32
Continue to: NSAIDS can be effective because...
NSAIDs can be effective because they reduce bleeding by causing vasoconstriction, but they provide the greatest benefit when started before menses, which can be difficult for a patient who has irregular cycles.
Endometrial causes of AUB should be suspected when a patient has heavy menstrual bleeding with regular menstrual cycles and no other causes can be identified. Endometrial dysfunction as the cause of AUB stems from aberrations in the biochemical pathways of endometrial hemostasis and repair, and therefore is difficult to confirm by laboratory analysis or histologic evaluation.3 Medical management focuses on alleviating heavy menstrual bleeding (TABLE 3).
Iatrogenic. The most common type of iatrogenic AUB is unscheduled bleeding, also known as breakthrough bleeding, that occurs during hormonal treatment with an OC or during the first few months after insertion of an LNG-IUD or contraceptive implant.3 In most cases, no specific treatment is required; bleeding resolves upon continued use of the contraceptive.
Not yet classified. This category is difficult to define; it was created for causes of AUB that have not yet been identified and remain unclear. For example, a condition known as chronic endometritis is under study as a possible cause of AUB, but has not been assigned to a PALM–COEIN category.3 As more data become available and understanding of pathophysiologic mechanisms lead to better definitions of disease, this and other poorly understood conditions will be moved to an appropriate category in the FIGO classification system.
CASE 2
Ms. G is given a diagnosis of PCOS, based on her history. You recommend weight loss and exercise; screen her for diabetes and dyslipidemia; and prescribe metformin.
ACKNOWLEDGMENT
Barry D. Weiss, MD, University of Arizona College of Medicine, Department of Family and Community Medicine, Tucson, assisted with the editing of this manuscript.
CORRESPONDENCE
Melody A. Jordahl-Iafrato, MD, Community Hospital East Family Medicine Residency, 10122 East 10th Street, Suite 100, Indianapolis, IN 46229; Mjordahl-iafrato@ecommunity.com.
1. Committee on Practice Bulletins—Gynecology. American College of Obstetricians and Gynecologists Practice Bulletin Number 128, July 2012: Diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206.
2. American College of Obstetricians and Gynecologists Committee Opinion No. 651: Menstruation in girls and adolescents: using the menstrual cycle as a vital sign. Obstet Gynecol. 2015;126:e143-e146.
3. Munro MG, Critchley HO, Broder MS, et al; FIGO Working Group on Menstrual Disorders. FIGO classifcation system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynaecol Obstet. 2011;133:3-13.
4. National Institute for Health and Care Excellence (NICE). Heavy menstrual bleeding: assessment and management [NG88]. www.nice.org.uk/guidance/ng88. Accessed February 28, 2019.
5. Committee on Practice Bulletins—Gynecology. American College of Obstetricians and Gynecologists Practice Bulletin Number 136, July 2013: Management of abnormal uterine bleeding associated with ovulatory dysfunction. Obstet Gynecol. 2013;122:176-185.
6. Hassa H, Tekin B, Senses T, et al. Are the site, diameter, and number of endometrial polyps related with symptomatology? Am J Obstet Gynecol. 2006;194:718-721.
7. Salim S, Won H, Nesbitt-Hawes E, et al. Diagnosis and management of endometrial polyps: a critical review of the literature. J Minim Invasive Gynecol. 2011;18:569-581.
8. Struble J, Reid S, Bedaiwy MA. Adenomyosis: A clinical review of a challenging gynecologic condition. J Minim Invasive Gynecol. 2016;23:164-185.
9. Champaneria R, Abedin P, Daniels J, et al. Ultrasound scan and magnetic resonance imaging for the diagnosis of adenomyosis: systematic review comparing test accuracy. Acta Obstet Gynecol Scand. 2010;89:1374-1384.
10. Bartels CB, Cayton KC, Chuong FS, et al. An evidence-based approach to the medical management of fibroids: a systematic review. Clin Obstet Gynecol. 2016;59:30-52.
11. Lethaby A, Cooke I, Rees MC. Progesterone or progestogen-releasing intrauterine systems for heavy menstrual bleeding. Cochrane Database Syst Rev. 2005;(4):CD002126.
12. Spies JB. Current role of uterine artery embolization in the management of uterine fibroids. Clin Obstet Gynecol. 2016;59:93-102.
13. Gupta JK, Sinha A, Lumsden MA, et al. Uterine artery embolization for symptomatic uterine fibroids. Cochrane Database Syst Rev. 2014;(12):CD005073.
14. Edwards RD, Moss JG, Lumsden MA, et al; Committee of the Randomized Trial of Embolization versus Surgical Treatment for Fibroids. Uterine artery embolization versus surgery for symptomatic uterine fibroids. N Engl J Med. 2007;356:360-370.
15. Pron G, Bennett J, Common A, et al; Ontario Uterine Fibroid Embolization Collaboration Group. The Ontario Uterine Fibroid Embolization Trial. Part 2. Uterine fibroid reduction and symptom relief after uterine artery embolization for fibroids. Fertil Steril. 2003;79:120-127.
16. Torre A, Fauconnier A, Kahn V, et al. Fertility after uterine artery embolization for symptomatic multiple fibroids with no other infertility factors. Eur Radiol. 2017;27:2850-2859.
17. Mara M, Maskova J, Fucikova Z, et al. Midterm clinical and first reproductive results of a randomized controlled trial comparing uterine fibroid embolization and myomectomy. Cardiovasc Intervent Radiol. 2008;31:73-85.
18. Lethaby A, Vollenhoven B, Sowter M. Pre-operative GnRH analogue therapy before hysterectomy or myomectomy for uterine fibroids. Cochrane Database Syst Rev. 2001;(2): CD000547.
19. Capmas P, Levaillant JM, Fernandez H. Surgical techniques and outcome in the management of submucous fibroids. Curr Opin Obstet Gynecol. 2013;25:332-338.
20. Abu Hashim H, Ghayaty E, El Rakhawy M. Levonorgestrel-releasing intrauterine system vs oral progestins for non-atypical endometrial hyperplasia: a systematic review and metaanalysis of randomized trials. Am J Obstet Gynecol. 2015;213:469-478.
21. Reed SD, Voigt LF, Newton KM, et al. Weiss NS. Progestin therapy of complex endometrial hyperplasia with and without atypia. Obstet Gynecol. 2009;113;655-662.
22. Morice P, Leary A, Creutzberg C, et al. Endometrial cancer. Lancet. 2016;387:1094-1108.
23. Goodman NF, Cobin RH, Futterweit W, et al; American Association of Clinical Endocrinologists (AACE); American College of Endocrinology (ACE); Androgen Excess and PCOS Society (AES). American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Excess and PCOS Society disease state clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome—part 1. Endocr Pract. 2015;21:1291-1300.
24. Shankar M, Lee CA, Sabin CA, et al. von Willebrand disease in women with menorrhagia: a systematic review. BJOG. 2004;111:734-740.
25. Seravalli V, Linari S, Peruzzi E, et al. Prevalence of hemostatic disorders in adolescents with abnormal uterine bleeding. J Pediatr Adolesc Gynecol. 2013;26:285-289.
26. Philipp CS, Faiz A, Dowling NF, et al. Development of a screening tool for identifying women with menorrhagia for hemostatic evaluation. Am J Obstet Gynecol. 2008;198:163.e1-e8.
27. The Rotterdam ESHRE/ASRM-sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19:41-47.
28. Goodman NF, Cobin RH, Futterweit W, et al; American Association of Clinical Endocrinologists (AACE); American College of Endocrinology (ACE); Androgen Excess and PCOS Society (AES). American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Excess and PCOS Society disease state clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome—part 2. Endocr Pract. 2015;21:1415-26.
29. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No 194: Polycystic ovary syndrome. Obstet Gynecol. 2018;131:e157-e171.
30. Speiser PW, Dupont B, Rubinstein P, et al. High frequency of nonclassical steroid 21-hydroxylase deficiency. Am J Hum Genet. 1985;37:650-667.
31. Naderpoor N, Shorakae S, de Courten B, et al. Metformin and lifestyle modification in polycystic ovary syndrome: systematic review and meta-analysis. Hum Reprod Update. 2015;21:560-574.
32. Kolman KB, Hadley SK, Jordahl-Iafrato MA. Long-acting reversible contraception: who, what, when, and how. J Fam Pract. 2015;64:479-484.
1. Committee on Practice Bulletins—Gynecology. American College of Obstetricians and Gynecologists Practice Bulletin Number 128, July 2012: Diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206.
2. American College of Obstetricians and Gynecologists Committee Opinion No. 651: Menstruation in girls and adolescents: using the menstrual cycle as a vital sign. Obstet Gynecol. 2015;126:e143-e146.
3. Munro MG, Critchley HO, Broder MS, et al; FIGO Working Group on Menstrual Disorders. FIGO classifcation system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynaecol Obstet. 2011;133:3-13.
4. National Institute for Health and Care Excellence (NICE). Heavy menstrual bleeding: assessment and management [NG88]. www.nice.org.uk/guidance/ng88. Accessed February 28, 2019.
5. Committee on Practice Bulletins—Gynecology. American College of Obstetricians and Gynecologists Practice Bulletin Number 136, July 2013: Management of abnormal uterine bleeding associated with ovulatory dysfunction. Obstet Gynecol. 2013;122:176-185.
6. Hassa H, Tekin B, Senses T, et al. Are the site, diameter, and number of endometrial polyps related with symptomatology? Am J Obstet Gynecol. 2006;194:718-721.
7. Salim S, Won H, Nesbitt-Hawes E, et al. Diagnosis and management of endometrial polyps: a critical review of the literature. J Minim Invasive Gynecol. 2011;18:569-581.
8. Struble J, Reid S, Bedaiwy MA. Adenomyosis: A clinical review of a challenging gynecologic condition. J Minim Invasive Gynecol. 2016;23:164-185.
9. Champaneria R, Abedin P, Daniels J, et al. Ultrasound scan and magnetic resonance imaging for the diagnosis of adenomyosis: systematic review comparing test accuracy. Acta Obstet Gynecol Scand. 2010;89:1374-1384.
10. Bartels CB, Cayton KC, Chuong FS, et al. An evidence-based approach to the medical management of fibroids: a systematic review. Clin Obstet Gynecol. 2016;59:30-52.
11. Lethaby A, Cooke I, Rees MC. Progesterone or progestogen-releasing intrauterine systems for heavy menstrual bleeding. Cochrane Database Syst Rev. 2005;(4):CD002126.
12. Spies JB. Current role of uterine artery embolization in the management of uterine fibroids. Clin Obstet Gynecol. 2016;59:93-102.
13. Gupta JK, Sinha A, Lumsden MA, et al. Uterine artery embolization for symptomatic uterine fibroids. Cochrane Database Syst Rev. 2014;(12):CD005073.
14. Edwards RD, Moss JG, Lumsden MA, et al; Committee of the Randomized Trial of Embolization versus Surgical Treatment for Fibroids. Uterine artery embolization versus surgery for symptomatic uterine fibroids. N Engl J Med. 2007;356:360-370.
15. Pron G, Bennett J, Common A, et al; Ontario Uterine Fibroid Embolization Collaboration Group. The Ontario Uterine Fibroid Embolization Trial. Part 2. Uterine fibroid reduction and symptom relief after uterine artery embolization for fibroids. Fertil Steril. 2003;79:120-127.
16. Torre A, Fauconnier A, Kahn V, et al. Fertility after uterine artery embolization for symptomatic multiple fibroids with no other infertility factors. Eur Radiol. 2017;27:2850-2859.
17. Mara M, Maskova J, Fucikova Z, et al. Midterm clinical and first reproductive results of a randomized controlled trial comparing uterine fibroid embolization and myomectomy. Cardiovasc Intervent Radiol. 2008;31:73-85.
18. Lethaby A, Vollenhoven B, Sowter M. Pre-operative GnRH analogue therapy before hysterectomy or myomectomy for uterine fibroids. Cochrane Database Syst Rev. 2001;(2): CD000547.
19. Capmas P, Levaillant JM, Fernandez H. Surgical techniques and outcome in the management of submucous fibroids. Curr Opin Obstet Gynecol. 2013;25:332-338.
20. Abu Hashim H, Ghayaty E, El Rakhawy M. Levonorgestrel-releasing intrauterine system vs oral progestins for non-atypical endometrial hyperplasia: a systematic review and metaanalysis of randomized trials. Am J Obstet Gynecol. 2015;213:469-478.
21. Reed SD, Voigt LF, Newton KM, et al. Weiss NS. Progestin therapy of complex endometrial hyperplasia with and without atypia. Obstet Gynecol. 2009;113;655-662.
22. Morice P, Leary A, Creutzberg C, et al. Endometrial cancer. Lancet. 2016;387:1094-1108.
23. Goodman NF, Cobin RH, Futterweit W, et al; American Association of Clinical Endocrinologists (AACE); American College of Endocrinology (ACE); Androgen Excess and PCOS Society (AES). American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Excess and PCOS Society disease state clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome—part 1. Endocr Pract. 2015;21:1291-1300.
24. Shankar M, Lee CA, Sabin CA, et al. von Willebrand disease in women with menorrhagia: a systematic review. BJOG. 2004;111:734-740.
25. Seravalli V, Linari S, Peruzzi E, et al. Prevalence of hemostatic disorders in adolescents with abnormal uterine bleeding. J Pediatr Adolesc Gynecol. 2013;26:285-289.
26. Philipp CS, Faiz A, Dowling NF, et al. Development of a screening tool for identifying women with menorrhagia for hemostatic evaluation. Am J Obstet Gynecol. 2008;198:163.e1-e8.
27. The Rotterdam ESHRE/ASRM-sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19:41-47.
28. Goodman NF, Cobin RH, Futterweit W, et al; American Association of Clinical Endocrinologists (AACE); American College of Endocrinology (ACE); Androgen Excess and PCOS Society (AES). American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Excess and PCOS Society disease state clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome—part 2. Endocr Pract. 2015;21:1415-26.
29. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No 194: Polycystic ovary syndrome. Obstet Gynecol. 2018;131:e157-e171.
30. Speiser PW, Dupont B, Rubinstein P, et al. High frequency of nonclassical steroid 21-hydroxylase deficiency. Am J Hum Genet. 1985;37:650-667.
31. Naderpoor N, Shorakae S, de Courten B, et al. Metformin and lifestyle modification in polycystic ovary syndrome: systematic review and meta-analysis. Hum Reprod Update. 2015;21:560-574.
32. Kolman KB, Hadley SK, Jordahl-Iafrato MA. Long-acting reversible contraception: who, what, when, and how. J Fam Pract. 2015;64:479-484.
PRACTICE RECOMMENDATIONS
› Perform endometrial biopsy on all women who have abnormal uterine bleeding and risk factors for endometrial cancer and on all women ≥45 years, regardless of risk. C
› Initiate a workup for a coagulation disorder in women who are close to the onset of menarche and have a history of heavy menstrual bleeding. C
› Promote lifestyle changes and weight loss as primary treatments for polycystic ovary syndrome. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Severe maternal morbidity increasing in California
The according to a study covering almost 8.3 million births in California.
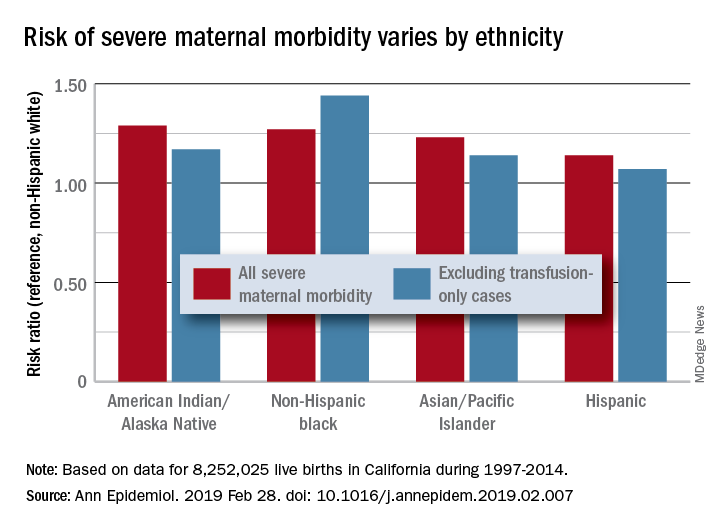
Changes in severe maternal morbidity (SMM) prevalence from 1997 to 2014 were fairly consistent by race/ethnicity, although increases for black (179%), Asian/Pacific Islander (175%), and Hispanic (173%) women were somewhat larger than for whites (163%), Stephanie A. Leonard, PhD, of Stanford (Calif.) University, and her associates reported in Annals of Epidemiology.
Differences between races/ethnicities over the entire study period were seen for SMM with and without transfusion-only cases. Individual-level factors such as cesarean birth, comorbidities, and anemia “contribute to, but do not fully explain, these disparities. Additionally, changes in the characteristics of pregnant women – including increases in comorbidities – have not affected racial/ethnic differences in severe maternal morbidity over time,” the investigators wrote.
The cohort study used data for 8,252,025 live births with birth certificates that were previously linked to delivery discharge records. SMM was measured using the Severe Maternity Morbidity Index. Because “blood transfusion is the only qualifying indicator for approximately half of SMM cases … we also studied a subset of SMM that excluded those cases for which the only indication was a blood transfusion,” they noted.
SOURCE: Leonard SA et al. Ann Epidemiol. 2019 Feb 28. doi: 10.1016/j.annepidem.2019.02.007.
The according to a study covering almost 8.3 million births in California.

Changes in severe maternal morbidity (SMM) prevalence from 1997 to 2014 were fairly consistent by race/ethnicity, although increases for black (179%), Asian/Pacific Islander (175%), and Hispanic (173%) women were somewhat larger than for whites (163%), Stephanie A. Leonard, PhD, of Stanford (Calif.) University, and her associates reported in Annals of Epidemiology.
Differences between races/ethnicities over the entire study period were seen for SMM with and without transfusion-only cases. Individual-level factors such as cesarean birth, comorbidities, and anemia “contribute to, but do not fully explain, these disparities. Additionally, changes in the characteristics of pregnant women – including increases in comorbidities – have not affected racial/ethnic differences in severe maternal morbidity over time,” the investigators wrote.
The cohort study used data for 8,252,025 live births with birth certificates that were previously linked to delivery discharge records. SMM was measured using the Severe Maternity Morbidity Index. Because “blood transfusion is the only qualifying indicator for approximately half of SMM cases … we also studied a subset of SMM that excluded those cases for which the only indication was a blood transfusion,” they noted.
SOURCE: Leonard SA et al. Ann Epidemiol. 2019 Feb 28. doi: 10.1016/j.annepidem.2019.02.007.
The according to a study covering almost 8.3 million births in California.

Changes in severe maternal morbidity (SMM) prevalence from 1997 to 2014 were fairly consistent by race/ethnicity, although increases for black (179%), Asian/Pacific Islander (175%), and Hispanic (173%) women were somewhat larger than for whites (163%), Stephanie A. Leonard, PhD, of Stanford (Calif.) University, and her associates reported in Annals of Epidemiology.
Differences between races/ethnicities over the entire study period were seen for SMM with and without transfusion-only cases. Individual-level factors such as cesarean birth, comorbidities, and anemia “contribute to, but do not fully explain, these disparities. Additionally, changes in the characteristics of pregnant women – including increases in comorbidities – have not affected racial/ethnic differences in severe maternal morbidity over time,” the investigators wrote.
The cohort study used data for 8,252,025 live births with birth certificates that were previously linked to delivery discharge records. SMM was measured using the Severe Maternity Morbidity Index. Because “blood transfusion is the only qualifying indicator for approximately half of SMM cases … we also studied a subset of SMM that excluded those cases for which the only indication was a blood transfusion,” they noted.
SOURCE: Leonard SA et al. Ann Epidemiol. 2019 Feb 28. doi: 10.1016/j.annepidem.2019.02.007.
FROM ANNALS OF EPIDEMIOLOGY
Vitamin C for sepsis? Experts take sides in sharp debate
SAN DIEGO –
“There is evidence supporting benefit, and ample evidence supporting safety,” Michael H. Hooper, MD, who practices in Norfolk, Va., said in a pro-and-con debate over the use of vitamin C in sepsis at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
Dr. Hooper’s debate opponent countered by noting the lack of quality research into vitamin C in sepsis and declared that its time has not yet come. “We need more data to know the safety of this drug,” said Andre Kalil, MD, professor of internal medicine and director of Transplant Infectious Diseases at the University of Nebraska Medical Center, Omaha.
Dr. Hooper was part of a member of a team led by Paul E. Marik, MD, FCCP, of Eastern Virginia Medical School, Norfolk, that made waves in 2017 with a study in Chest suggesting IV vitamin C has tremendous potential as a treatment for sepsis (Chest. 2017 Jun;151[6]:1229-38).
The retrospective study compared two groups of 47 patients with sepsis – a control group and a group that received treatment with intravenous vitamin C, hydrocortisone, and thiamine. Remarkably, the team found that 9% (4 of 47) of those in the treatment group died in the hospital, compared with 40% (19 of 47) in the control group (P less than .001).
The findings make sense, Dr. Hooper said, in light of the fact that “our patients are remarkably deficient” in vitamin C. He pointed to a 2017 study that found nearly 40% of 24 patients with septic shock were deficient in vitamin C – despite getting recommended enteral nutrition, parenteral nutrition or both – compared with 25% of patients who were not septic. The study authors believe the difference is probably due to “increased metabolism due to the enhanced inflammatory response observed in septic shock” (Crit Care. 2017 Dec 11;21[1]:300).
“We’re dealing with a population of patients who need some sort of repletion of this vitamin,” Dr. Hooper said.
Why not try oral administration of vitamin C? “Oral administration at regular doses doesn’t work,” he said. “If you have normal volunteers who are made deficient, then you administer the recommended allowance, it takes days or weeks to return levels to normal.”
Dr. Hooper added that the goal of vitamin C therapy isn’t simply to restore proper levels in plasma. In addition, he said, “we’re trying to restore levels in crucial organs.”
He said the cost of treatment with IV vitamin C is low, and no serious adverse events have been seen in studies of the vitamin’s use in critical care.
In his comments at the debate, Dr. Kalil pointed to several weaknesses in the 2017 study of vitamin C in sepsis. According to him, it had many problems, including a sample size that lacked statistical power and imbalances in the two groups. He raised concerns about the study in a 2017 letter published in Chest titled “Vitamin C Is Not Ready for Prime Time in Sepsis but a Solution Is Close,” noting that the control group was sicker and none of those patients had their vitamin C levels measured (Chest. 2017 Sep;152[3]:676).
He added that “acute renal failure is associated with high doses of vitamin C.”
As of July 2018, several clinical trials into vitamin C, hydrocortisone, and thiamine for the treatment of septic shock were underway or planned, according to a report that described the current randomized, placebo-controlled, multicenter Ascorbic Acid, Corticosteroids, and Thiamine in Sepsis (ACTS) trial in the United States. The report notes that “robust evidence” for this approach is lacking, although “the potential effectiveness of this medication combination is rooted in biologic plausibility and supported by small clinical trials of the various individual components.” (Crit Care. 2018;22:283)
Dr. Hooper is an executive committee member and principal investigator with the Vitamin C, Thiamine And Steroids in Sepsis (VICTAS) study. Dr. Kalil reports no relevant disclosures.
SAN DIEGO –
“There is evidence supporting benefit, and ample evidence supporting safety,” Michael H. Hooper, MD, who practices in Norfolk, Va., said in a pro-and-con debate over the use of vitamin C in sepsis at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
Dr. Hooper’s debate opponent countered by noting the lack of quality research into vitamin C in sepsis and declared that its time has not yet come. “We need more data to know the safety of this drug,” said Andre Kalil, MD, professor of internal medicine and director of Transplant Infectious Diseases at the University of Nebraska Medical Center, Omaha.
Dr. Hooper was part of a member of a team led by Paul E. Marik, MD, FCCP, of Eastern Virginia Medical School, Norfolk, that made waves in 2017 with a study in Chest suggesting IV vitamin C has tremendous potential as a treatment for sepsis (Chest. 2017 Jun;151[6]:1229-38).
The retrospective study compared two groups of 47 patients with sepsis – a control group and a group that received treatment with intravenous vitamin C, hydrocortisone, and thiamine. Remarkably, the team found that 9% (4 of 47) of those in the treatment group died in the hospital, compared with 40% (19 of 47) in the control group (P less than .001).
The findings make sense, Dr. Hooper said, in light of the fact that “our patients are remarkably deficient” in vitamin C. He pointed to a 2017 study that found nearly 40% of 24 patients with septic shock were deficient in vitamin C – despite getting recommended enteral nutrition, parenteral nutrition or both – compared with 25% of patients who were not septic. The study authors believe the difference is probably due to “increased metabolism due to the enhanced inflammatory response observed in septic shock” (Crit Care. 2017 Dec 11;21[1]:300).
“We’re dealing with a population of patients who need some sort of repletion of this vitamin,” Dr. Hooper said.
Why not try oral administration of vitamin C? “Oral administration at regular doses doesn’t work,” he said. “If you have normal volunteers who are made deficient, then you administer the recommended allowance, it takes days or weeks to return levels to normal.”
Dr. Hooper added that the goal of vitamin C therapy isn’t simply to restore proper levels in plasma. In addition, he said, “we’re trying to restore levels in crucial organs.”
He said the cost of treatment with IV vitamin C is low, and no serious adverse events have been seen in studies of the vitamin’s use in critical care.
In his comments at the debate, Dr. Kalil pointed to several weaknesses in the 2017 study of vitamin C in sepsis. According to him, it had many problems, including a sample size that lacked statistical power and imbalances in the two groups. He raised concerns about the study in a 2017 letter published in Chest titled “Vitamin C Is Not Ready for Prime Time in Sepsis but a Solution Is Close,” noting that the control group was sicker and none of those patients had their vitamin C levels measured (Chest. 2017 Sep;152[3]:676).
He added that “acute renal failure is associated with high doses of vitamin C.”
As of July 2018, several clinical trials into vitamin C, hydrocortisone, and thiamine for the treatment of septic shock were underway or planned, according to a report that described the current randomized, placebo-controlled, multicenter Ascorbic Acid, Corticosteroids, and Thiamine in Sepsis (ACTS) trial in the United States. The report notes that “robust evidence” for this approach is lacking, although “the potential effectiveness of this medication combination is rooted in biologic plausibility and supported by small clinical trials of the various individual components.” (Crit Care. 2018;22:283)
Dr. Hooper is an executive committee member and principal investigator with the Vitamin C, Thiamine And Steroids in Sepsis (VICTAS) study. Dr. Kalil reports no relevant disclosures.
SAN DIEGO –
“There is evidence supporting benefit, and ample evidence supporting safety,” Michael H. Hooper, MD, who practices in Norfolk, Va., said in a pro-and-con debate over the use of vitamin C in sepsis at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
Dr. Hooper’s debate opponent countered by noting the lack of quality research into vitamin C in sepsis and declared that its time has not yet come. “We need more data to know the safety of this drug,” said Andre Kalil, MD, professor of internal medicine and director of Transplant Infectious Diseases at the University of Nebraska Medical Center, Omaha.
Dr. Hooper was part of a member of a team led by Paul E. Marik, MD, FCCP, of Eastern Virginia Medical School, Norfolk, that made waves in 2017 with a study in Chest suggesting IV vitamin C has tremendous potential as a treatment for sepsis (Chest. 2017 Jun;151[6]:1229-38).
The retrospective study compared two groups of 47 patients with sepsis – a control group and a group that received treatment with intravenous vitamin C, hydrocortisone, and thiamine. Remarkably, the team found that 9% (4 of 47) of those in the treatment group died in the hospital, compared with 40% (19 of 47) in the control group (P less than .001).
The findings make sense, Dr. Hooper said, in light of the fact that “our patients are remarkably deficient” in vitamin C. He pointed to a 2017 study that found nearly 40% of 24 patients with septic shock were deficient in vitamin C – despite getting recommended enteral nutrition, parenteral nutrition or both – compared with 25% of patients who were not septic. The study authors believe the difference is probably due to “increased metabolism due to the enhanced inflammatory response observed in septic shock” (Crit Care. 2017 Dec 11;21[1]:300).
“We’re dealing with a population of patients who need some sort of repletion of this vitamin,” Dr. Hooper said.
Why not try oral administration of vitamin C? “Oral administration at regular doses doesn’t work,” he said. “If you have normal volunteers who are made deficient, then you administer the recommended allowance, it takes days or weeks to return levels to normal.”
Dr. Hooper added that the goal of vitamin C therapy isn’t simply to restore proper levels in plasma. In addition, he said, “we’re trying to restore levels in crucial organs.”
He said the cost of treatment with IV vitamin C is low, and no serious adverse events have been seen in studies of the vitamin’s use in critical care.
In his comments at the debate, Dr. Kalil pointed to several weaknesses in the 2017 study of vitamin C in sepsis. According to him, it had many problems, including a sample size that lacked statistical power and imbalances in the two groups. He raised concerns about the study in a 2017 letter published in Chest titled “Vitamin C Is Not Ready for Prime Time in Sepsis but a Solution Is Close,” noting that the control group was sicker and none of those patients had their vitamin C levels measured (Chest. 2017 Sep;152[3]:676).
He added that “acute renal failure is associated with high doses of vitamin C.”
As of July 2018, several clinical trials into vitamin C, hydrocortisone, and thiamine for the treatment of septic shock were underway or planned, according to a report that described the current randomized, placebo-controlled, multicenter Ascorbic Acid, Corticosteroids, and Thiamine in Sepsis (ACTS) trial in the United States. The report notes that “robust evidence” for this approach is lacking, although “the potential effectiveness of this medication combination is rooted in biologic plausibility and supported by small clinical trials of the various individual components.” (Crit Care. 2018;22:283)
Dr. Hooper is an executive committee member and principal investigator with the Vitamin C, Thiamine And Steroids in Sepsis (VICTAS) study. Dr. Kalil reports no relevant disclosures.
EXPERT ANALYSIS FROM CCC48
Syphilis rates high in HIV-positive women
SEATTLE – The frequency of syphilis in HIV-infected women is unusually high, prompting concerns about congenital syphilis among newborns. The finding comes from a Centers for Disease Control analysis of the U.S. Center for Aids Research Clinical Network of Integrated Clinical Systems (CNICS) cohort.
“We found significant associations [between syphilis infection] and drug use and hepatitis C infection, which adds to some information from the CDC that what’s driving the epidemic in women is drug use – it’s a very different epidemic potentially in women with HIV than in men with HIV,” said Jodie Dionne-Odom, MD, chief of women’s health services at the University of Alabama, Birmingham’s 1917 Clinic. She described the results of her study at a press conference at the Conference on Retroviruses and Infectious Diseases.
“These predictors are important to understand so that we can come up with interventions to try to reduce the problem. Syphilis screening is relatively easy to do. USPSTF [U.S. Preventive Services Task Force] doesn’t even have a recommendation for that periodicity of screening frequency, so we have a ways to go to define how often we need to be looking, particularly in high-risk groups,” she said.
The likely force driving the increased incidence of syphilis is transactional sex. To explore that possibility, the researchers examined the number of sexual partners and found that more partners were linked to greater likelihood of having syphilis. “So I don’t think it’s the drug use itself – it’s the behavior that comes with the drug use,” Dr. Dionne-Odom said.
The findings shed more light on the interplay between drug use epidemics and disease epidemics. “As we have an increasing opioid epidemic and methamphetamine abuse that we’re all seeing in our clinics, it’s interesting to see this intersection between the drug use problem and the syphilis problem in this country. I think they’re not unrelated,” she said.
She believes the results should have broad implications for screening programs. Women admitted to drug treatment programs should be tested for syphilis. And women who abuse drugs and are pregnant should be screened repeatedly – at entry to care, at their 20-week scan, and at delivery. “We know we have about 6,000 women with HIV who deliver each year, so this is potentially very impactful,” Dr. Dionne-Odom said.
The researchers extracted data from the CNICS cohort, including 4,795 women with records between 2005 and 2016. They defined incident syphilis as a newly positive nontreponemal serologic test or a 300% titer increase, followed by a confirmatory test.
After adjustment, factors associated with syphilis included prior intravenous drug abuse (adjusted odds ratio, 2.3; 95% confidence interval, 1.3-3.9), hepatitis C antibody positivity (aOR, 2.1; 95% CI, 1.3-3.7), as well as black race (aOR, 2.3; 95% CI, 1.4-3.9). There was no association between age and HIV viral load with respect to risk of syphilis.
The frequency appears to be rising, with later entry into the CNICS cohort assorted with a more than doubled risk of syphilis (aOR, 2.3; 95% CI, 1.4-3.9 for 2011-2016, compared with 1994-2004).
The study was funded by the National Institute of Child Health and Human Development. Dr. Dionne-Odom reported no relevant conflicts of interest.
SOURCE: J Dionne-Odom et al. CROI 2019, Abstract 47
SEATTLE – The frequency of syphilis in HIV-infected women is unusually high, prompting concerns about congenital syphilis among newborns. The finding comes from a Centers for Disease Control analysis of the U.S. Center for Aids Research Clinical Network of Integrated Clinical Systems (CNICS) cohort.
“We found significant associations [between syphilis infection] and drug use and hepatitis C infection, which adds to some information from the CDC that what’s driving the epidemic in women is drug use – it’s a very different epidemic potentially in women with HIV than in men with HIV,” said Jodie Dionne-Odom, MD, chief of women’s health services at the University of Alabama, Birmingham’s 1917 Clinic. She described the results of her study at a press conference at the Conference on Retroviruses and Infectious Diseases.
“These predictors are important to understand so that we can come up with interventions to try to reduce the problem. Syphilis screening is relatively easy to do. USPSTF [U.S. Preventive Services Task Force] doesn’t even have a recommendation for that periodicity of screening frequency, so we have a ways to go to define how often we need to be looking, particularly in high-risk groups,” she said.
The likely force driving the increased incidence of syphilis is transactional sex. To explore that possibility, the researchers examined the number of sexual partners and found that more partners were linked to greater likelihood of having syphilis. “So I don’t think it’s the drug use itself – it’s the behavior that comes with the drug use,” Dr. Dionne-Odom said.
The findings shed more light on the interplay between drug use epidemics and disease epidemics. “As we have an increasing opioid epidemic and methamphetamine abuse that we’re all seeing in our clinics, it’s interesting to see this intersection between the drug use problem and the syphilis problem in this country. I think they’re not unrelated,” she said.
She believes the results should have broad implications for screening programs. Women admitted to drug treatment programs should be tested for syphilis. And women who abuse drugs and are pregnant should be screened repeatedly – at entry to care, at their 20-week scan, and at delivery. “We know we have about 6,000 women with HIV who deliver each year, so this is potentially very impactful,” Dr. Dionne-Odom said.
The researchers extracted data from the CNICS cohort, including 4,795 women with records between 2005 and 2016. They defined incident syphilis as a newly positive nontreponemal serologic test or a 300% titer increase, followed by a confirmatory test.
After adjustment, factors associated with syphilis included prior intravenous drug abuse (adjusted odds ratio, 2.3; 95% confidence interval, 1.3-3.9), hepatitis C antibody positivity (aOR, 2.1; 95% CI, 1.3-3.7), as well as black race (aOR, 2.3; 95% CI, 1.4-3.9). There was no association between age and HIV viral load with respect to risk of syphilis.
The frequency appears to be rising, with later entry into the CNICS cohort assorted with a more than doubled risk of syphilis (aOR, 2.3; 95% CI, 1.4-3.9 for 2011-2016, compared with 1994-2004).
The study was funded by the National Institute of Child Health and Human Development. Dr. Dionne-Odom reported no relevant conflicts of interest.
SOURCE: J Dionne-Odom et al. CROI 2019, Abstract 47
SEATTLE – The frequency of syphilis in HIV-infected women is unusually high, prompting concerns about congenital syphilis among newborns. The finding comes from a Centers for Disease Control analysis of the U.S. Center for Aids Research Clinical Network of Integrated Clinical Systems (CNICS) cohort.
“We found significant associations [between syphilis infection] and drug use and hepatitis C infection, which adds to some information from the CDC that what’s driving the epidemic in women is drug use – it’s a very different epidemic potentially in women with HIV than in men with HIV,” said Jodie Dionne-Odom, MD, chief of women’s health services at the University of Alabama, Birmingham’s 1917 Clinic. She described the results of her study at a press conference at the Conference on Retroviruses and Infectious Diseases.
“These predictors are important to understand so that we can come up with interventions to try to reduce the problem. Syphilis screening is relatively easy to do. USPSTF [U.S. Preventive Services Task Force] doesn’t even have a recommendation for that periodicity of screening frequency, so we have a ways to go to define how often we need to be looking, particularly in high-risk groups,” she said.
The likely force driving the increased incidence of syphilis is transactional sex. To explore that possibility, the researchers examined the number of sexual partners and found that more partners were linked to greater likelihood of having syphilis. “So I don’t think it’s the drug use itself – it’s the behavior that comes with the drug use,” Dr. Dionne-Odom said.
The findings shed more light on the interplay between drug use epidemics and disease epidemics. “As we have an increasing opioid epidemic and methamphetamine abuse that we’re all seeing in our clinics, it’s interesting to see this intersection between the drug use problem and the syphilis problem in this country. I think they’re not unrelated,” she said.
She believes the results should have broad implications for screening programs. Women admitted to drug treatment programs should be tested for syphilis. And women who abuse drugs and are pregnant should be screened repeatedly – at entry to care, at their 20-week scan, and at delivery. “We know we have about 6,000 women with HIV who deliver each year, so this is potentially very impactful,” Dr. Dionne-Odom said.
The researchers extracted data from the CNICS cohort, including 4,795 women with records between 2005 and 2016. They defined incident syphilis as a newly positive nontreponemal serologic test or a 300% titer increase, followed by a confirmatory test.
After adjustment, factors associated with syphilis included prior intravenous drug abuse (adjusted odds ratio, 2.3; 95% confidence interval, 1.3-3.9), hepatitis C antibody positivity (aOR, 2.1; 95% CI, 1.3-3.7), as well as black race (aOR, 2.3; 95% CI, 1.4-3.9). There was no association between age and HIV viral load with respect to risk of syphilis.
The frequency appears to be rising, with later entry into the CNICS cohort assorted with a more than doubled risk of syphilis (aOR, 2.3; 95% CI, 1.4-3.9 for 2011-2016, compared with 1994-2004).
The study was funded by the National Institute of Child Health and Human Development. Dr. Dionne-Odom reported no relevant conflicts of interest.
SOURCE: J Dionne-Odom et al. CROI 2019, Abstract 47
REPORTING FROM CROI 2019
Neuraxial analgesia use in labor across the US
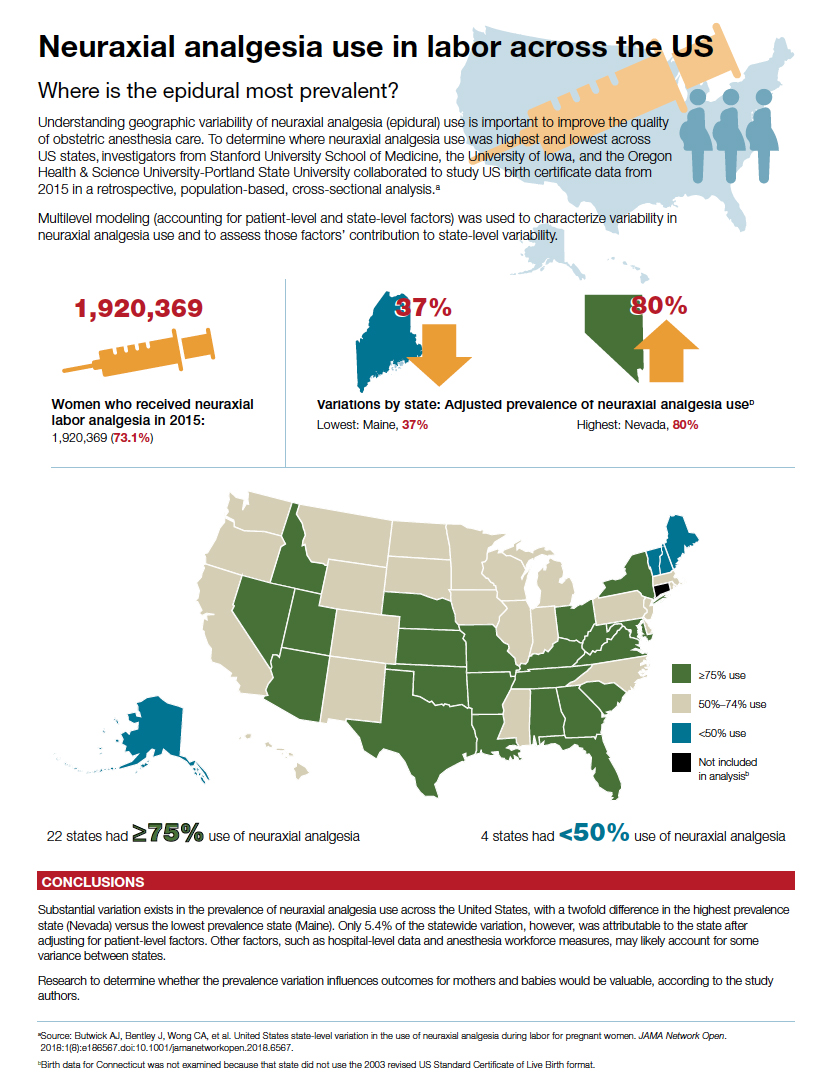


COPD and asthma: Diagnostic accuracy requires spirometry
A study of diagnostic accuracy in the primary care setting showed that among patients receiving inhaled therapies, most had not received an accurate diagnosis of chronic obstructive pulmonary disease (COPD) or asthma according to international guidelines.1,2 Other studies have shown that up to one-third of patients with a diagnosis of asthma3 or COPD4 may not actually have disease based on subsequent lung function testing.
Diagnostic error in medicine leads to numerous lost opportunities including the opportunity to: identify chronic conditions that are the true sources of patients’ symptoms, prevent morbidity and mortality, reduce unnecessary costs to patients and health systems, and deliver high-quality care.5-7 The reasons for diagnostic error in COPD and asthma are multifactorial, stemming from insufficient knowledge of clinical practice guidelines and underutilization of spirometry testing. Spirometry is recommended as part of the workup for suspected COPD and is the preferred test for diagnosing asthma. Spirometry, combined with clinical findings, can help differentiate between these diseases.
In this article, we review the definitions and characteristics of COPD and asthma, address the potential causes for diagnostic error, and explain how current clinical practice guidelines can steer examinations to the right diagnosis, improve clinical management, and contribute to better patient outcomes and quality of life.8,9
COPD and asthma characteristics
COPD. The Global Initiative for Chronic Obstructive Lung Disease (GOLD) defines COPD as a common lung disease characterized by persistent respiratory symptoms and airflow obstruction caused by airway or alveolar abnormalities secondary to significant exposure to noxious particles or gases.10 The most common COPD-risk exposure in the United States is tobacco smoke, chiefly from cigarettes. Risk is also heightened with use of other types of tobacco (pipe, cigar, water pipe), indoor and outdoor air pollution (including second-hand tobacco smoke exposure), and occupational exposures. (Consider testing for alpha-1 antitrypsin deficiency—a known genetic risk factor for COPD—especially when an individual with COPD is younger and has a limited smoking history.)
The most common symptom of COPD is chronic, progressive dyspnea — an increased effort to breathe, with chest heaviness, air hunger, or gasping. About one-third of people with COPD have a chronic cough with sputum production.10 There may be wheezing and chest tightness. Fatigue, weight loss, and anorexia can be seen in severe COPD. Consider this disorder in any individual older than 40 years of age who has dyspnea and chronic cough with sputum production, as well as a history of risk factors. If COPD is suspected, perform spirometry to determine the presence of fixed airflow limitation and confirm the diagnosis.
Asthma is usually characterized by variable airway hyperresponsiveness and chronic inflammation. A typical clinical presentation is an individual with a history of wheezing, shortness of breath, chest tightness, and cough that vary in intensity over time and are coupled with variable expiratory flow limitation. Asthma symptoms are often triggered by allergen or irritant exposure, exercise, weather changes, or viral respiratory infections.2 Symptoms may also be worse at night or first thing in the morning. Once asthma is suspected, document the presence of airflow variability with spirometry to confirm the diagnosis.
[polldaddy:10261486]
Diagnostic error in suspected COPD and asthma
Numerous studies have demonstrated the prevalence of diagnostic error when testing of lung function is neglected.11-14 Using spirometry to confirm a prior clinical diagnosis of COPD, researchers found that:
- 35% to 50% of patients did not have objective evidence of COPD12,13;
- 37% with an asthma-only diagnosis had persistent obstruction, which may indicate COPD or chronic obstructive asthma12; and
- 31% of patients thought to have asthma-COPD overlap did not have a COPD component.12
Continue to: In 2 longitudinal studies...
In 2 longitudinal studies, patients with a diagnosis of asthma were recruited to undergo medication reduction and serial lung function testing. Asthma was excluded in approximately 30% of patients.15,16 Diagnostic error has also been seen in patients hospitalized with exacerbations of COPD and asthma. One study found that only 31% of patients admitted with a diagnosis of COPD exacerbation had undergone a spirometry test prior to hospitalization.17 And of those patients with a diagnosis of COPD who underwent spirometry, 30% had results inconsistent with COPD.17
In another study, 22% of adults hospitalized for COPD or asthma exacerbations had no evidence of obstruction on spirometry at the time of hospitalization.18 This finding refutes a diagnosis of COPD and, in the midst of an exacerbation, challenges an asthma diagnosis as well. Increased awareness of clinical practice guidelines, coupled with the use and accurate interpretation of spirometry are needed for optimal management and treatment of COPD and asthma.
Clinical practice guidelines recommend spirometry for the diagnosis of COPD and asthma and have been issued by GOLD10; the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and the European Respiratory Society19; the Global Initiative for Asthma (GINA)2; and the National Heart, Lung, and Blood Institute.20
When a patient’s symptoms and risk factors suggest COPD, spirometry is needed to show persistent post-bronchodilator airflow obstruction and thereby confirm the diagnosis. However, in the United States, confirmatory spirometry is used only in about one third of patients newly diagnosed with COPD.21,22 Similarly for asthma, in the presence of suggestive symptoms, spirometry is the preferred and most reliable and reproducible test to detect the variable expiratory airflow limitation consistent with this diagnosis.
An alternative to spirometry for the diagnosis of asthma (if needed) is a peak flow meter, a simple tool to measure peak expiratory flow. When compared with spirometry, peak flow measurements are less time consuming, less costly, and not dependent on trained staff to perform.23 However, this option does require that patients perform and document multiple measurements over several days without an objective assessment of their efforts. Unlike spirometry, the peak flow meter has no reference values or reliability and reproducibility standards, and measurements can differ from one peak flow meter to another. Thus, a peak flow meter is less reliable than spirometry for diagnosing asthma. But it can be useful for monitoring asthma control at home and in the clinic setting,24 or for diagnosis if spirometry is unavailable.23
Continue to: Barriers to the use of spirometry...
Barriers to the use of spirometry in the primary care setting exist on several levels. Providers may lack knowledge of clinical practice guidelines that recommend spirometry in the diagnosis of COPD, and they may lack general awareness of the utility of spirometry.25-29 In 2 studies of primary care practices that offered office spirometry, lack of knowledge in conducting and interpreting the test was a barrier to its use.28,30 Primary care physicians also struggle with logistical challenges when clinical visits last just 10 to 15 minutes for patients with multiple comorbidities,27 and maintenance of an office spirometry program may not always be feasible.
Getting to the right diagnosis
Likewise, nonpharmacologic interventions may be misused or go unused when needed if the diagnosis is inaccurate. For patients with COPD, outcomes are improved with pulmonary rehabilitation and supplemental oxygen in the setting of resting hypoxemia, but these resources will not be considered if patients are misdiagnosed as having asthma. A patient with undetected heart failure or obstructive sleep apnea who has been misdiagnosed with COPD or asthma may not receive appropriate diagnostic testing or treatment until asthma or COPD has been ruled out with lung function testing.
Objectively documenting the right diagnosis helps ensure guideline-based management of COPD or asthma. Ruling out these 2 disorders prompts further investigation into other conditions (eg, coronary artery disease, heart failure, gastroesophageal reflux disease, pulmonary hypertension, interstitial lung diseases) that can cause symptoms such as shortness of breath, wheezing, or cough.
The TABLE2,10,34 summarizes some of the more common clinical and spirometric features of COPD and asthma. Onset of COPD usually occurs in those over age 40. Asthma can present in younger individuals, including children. Tobacco use or exposure to noxious substances is more often associated with COPD. Patients with asthma are more likely to have atopy. Symptoms in COPD usually progress with increasing activity or exertion. Symptoms in asthma may vary with certain activities, such as exercise, and with various triggers. These features represent “typical” cases of COPD or asthma, but some patients may have clinical characteristics
Continue to: The utility of spirometry in measuring lung function
The utility of spirometry in measuring lung function. Spirometry is the most reproducible and objective measurement of airflow limitation,10 and it should precede any treatment decisions. This technique—in which the patient performs maximal inhalation followed by forced exhalation—measures airflow over time and determines the lung volume exhaled at any time point. Because this respiratory exercise is patient dependent, a well-trained technician is needed to ensure reproducibility and reliability of results based on technical standards.
Spirometry measures forced vital capacity (FVC) and forced expiratory volume in one second (FEV1), from which the FEV1/FVC ratio is calculated. FVC is the total amount of air from total lung volume that can be exhaled in one breath. FEV1 is the total amount of air exhaled in the first second after initiation of exhalation. Thus, the FEV1/FVC ratio is the percentage of the total amount of air in a single breath that is exhaled in the first second. On average, an individual with normal lungs can exhale approximately 80% of their FVC in the first second, thereby resulting in a FEV1/FVC ratio of 80%.
Spirometry findings with COPD. A post-bronchodilator FEV1/FVC ratio of less than 70% confirms airflow obstruction and is consistent with COPD according to GOLD criteria.10 Post-bronchodilator spirometry is performed after the patient has received a specified dose of an inhaled bronchodilator per lab protocols. In patients with COPD, the FEV1/FVC ratio is persistently low even after administration of a bronchodilator.
Another means of using spirometry to diagnose COPD is referring to age-dependent cutoff values below the lower fifth percentile of the FEV1/FVC ratio (ie, lower limit of normal [LLN]), which differs from the GOLD strategy but is consistent with the American Thoracic Society/European Respiratory Society guidelines.35 Because the FEV1/FVC ratio declines with age, older adults may have a normal post-bronchodilator ratio less than 70%. Admittedly, applying GOLD criteria to older adults could result in overdiagnosis, while using the LLN could lead to underdiagnosis. Although there is no consensus on which method to use, the best approach may be the one that most strongly correlates with pretest probability of disease. In a large Canadian study, the approach that most strongly predicted poor patient outcomes was using a FEV1/FVC based on fixed (70%) and/or LLN criteria, and a low FEV134
Spirometry findings with asthma. According to the American Thoracic Society, a post-bronchodilator response is defined as an increase in FEV1 (or FVC) of 12% if that volume is also ≥200 mL. In patients with suspected asthma, an increase in FEV1 ≥12% and 200 mL is consistent with variable airflow limitation2 and supports the diagnosis. Of note, lung function in patients with asthma may be normal when patients are not symptomatic or when they are receiving therapy. Spirometry is therefore ideally performed before initiating therapy and when maintenance therapy is being considered due to symptoms. If therapy is clinically indicated, a short-acting bronchodilator may be prescribed alone and then held 6 to 8 hours before conducting spirometry. If a trial of a maintenance medication is prescribed before spirometry, consider de-escalation of therapy once the patient is more stable and then perform spirometry to confirm the presence of airflow variability consistent with asthma. (In COPD, there can be a positive bronchodilator response; however, the post-bronchodilator FEV1/FVC ratio remains low.)
Continue to: Don't use in isolation
Don’t use in isolation. Use spirometry to support a clinical suspicion of asthma36 or COPD after a thorough history and physical exam, and not in isolation.
Special consideration: Asthma-COPD overlap syndrome
Some patients have features characteristic of both asthma and COPD and are said to have asthma-COPD overlap syndrome (ACOS). Between 15% and 20% of patients with COPD may in fact have ACOS.36 While there is no specific definition of ACOS, GOLD and GINA describe ACOS as persistent airflow limitation with several features usually associated with asthma and several features usually associated with COPD.2,10,37 ACOS becomes more prevalent with advancing age.
In ACOS, patients with COPD present with increased reversibility or patients with asthma and smoking history develop non-fully reversible airway obstruction at an older age.38 Patients with ACOS have worse lung function, more respiratory symptoms, and lower health-related quality of life than individuals with asthma or COPD alone,39,40 leading to more consumption of medical resources.41 In patients with ACOS, the FEV1/FVC ratio is low and consistent with the diagnosis of COPD. The post-bronchodilator response may be variable, depending on the stage of disease and predominant clinical features. It is still unclear whether ACOS is a separate disease entity, a representation of severe asthma that has morphed into COPD, or not a syndrome but simply 2 separate comorbid disease states.
CORRESPONDENCE
Christina D. Wells, MD, University of Illinois Mile Square Health Center, 1220 S. Wood Street, Chicago, IL 60612; cwells2@uic.edu.
1. Izquierdo JL, Martìn A, de Lucas P, et al. Misdiagnosis of patients receiving inhaled therapies in primary care. Int J Chron Obstruct Pulmon Dis. 2010;5:241-249.
2. Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. 2018. https://ginasthma.org/wp-content/uploads/2018/04/wms-GINA-2018-report-V1.3-002.pdf. Accessed January 11, 2019.
3. Aaron SD, Vandemheen KL, FitzGerald JM. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA. 2017; 317:269-279.
4. Spero K, Bayasi G, Beaudry L, et al. Overdiagnosis of COPD in hospitalized patients. Int J Chron Obstruct Pulmon Dis. 2017;12:2417-2423.
5. Singh H, Graber ML. Improving diagnosis in health care—the next imperative for patient safety. N Engl J Med. 2015;373:2493-2495.
6. Ball JR, Balogh E. Improving diagnosis in health care: highlights of a report from the National Academies of Sciences, Engineering, and Medicine. Ann Intern Med. 2016;164:59-61.
7. Khullar D, Jha AK, Jena AB. Reducing diagnostic errors—why now? N Engl J Med. 2015;373:2491-2493.
8. Lamprecht B, Soriano JB, Studnicka M, et al. Determinants of underdiagnosis of COPD in national and international surveys. Chest. 2015;148:971-985.
9. Yang CL, Simons E, Foty RG, et al. Misdiagnosis of asthma in schoolchildren. Pediatr Pulmonol. 2017;52:293-302.
10. Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. 2019. https://goldcopd.org/wp-content/uploads/2018/11/GOLD-2019-v1.7-FINAL-14Nov2018-WMS.pdf. Accessed January 12, 2019.
11. Tinkelman DG, Price DB, Nordyke RJ, et al. Misdiagnosis of COPD and asthma in primary care patients 40 years of age and over. J Asthma. 2006;43:75-80.
12. Abramson MJ, Schattner RL, Sulaiman ND, et al. Accuracy of asthma and COPD diagnosis in Australian general practice: a mixed methods study. Prim Care Respir J. 2012;21:167-173.
13. Sichletidis L, Chloros D, Spyratos D, et al. The validity of the diagnosis of chronic obstructive pulmonary disease in general practice. Prim Care Respir J. 2007;16:82-88.
14. Marklund B, Tunsäter A, Bengtsson C. How often is the diagnosis bronchial asthma correct? Fam Pract. 1999;16:112-116.
15. Aaron SD, Vandemheen KL, Boulet LP, et al. Overdiagnosis of asthma in obese and nonobese adults. CMAJ. 2008;179:1121-1131.
16. Aaron SD, Vandemheen KL, FitzGerald JM, et al. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA. 2017;317:269-279.
17. Damarla M, Celli BR, Mullerova HX, et al. Discrepancy in the use of confirmatory tests in patients hospitalized with the diagnosis of chronic obstructive pulmonary disease or congestive heart failure. Respir Care. 2006;51:1120-1124.
18. Prieto Centurion V, Huang F, Naureckus ET, et al. Confirmatory spirometry for adults hospitalized with a diagnosis of asthma or chronic obstructive pulmonary disease exacerbation. BMC Pulm Med. 2012;12:73.
19. Qaseem A, Wilt TJ, Weinberger SE, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155:179-191.
20. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007. 120(Suppl):S94-S138.
21. Han MK, Kim MG, Mardon R, et al. Spirometry utilization for COPD: how do we measure up? Chest. 2007;132:403-409.
22. Joo MJ, Lee TA, Weiss KB. Geographic variation of spirometry use in newly diagnosed COPD. Chest. 2008;134:38-45.
23. Thorat YT, Salvi SS, Kodgule RR. Peak flow meter with a questionnaire and mini-spirometer to help detect asthma and COPD in real-life clinical practice: a cross-sectional study. NPJ Prim Care Respir Med. 2017;27:32.
24. Kennedy DT, Chang Z, Small RE. Selection of peak flowmeters in ambulatory asthma patients: a review of the literature. Chest. 1998;114:587-592.
25. Walters JA, Hanson E, Mudge P, et al. Barriers to the use of spirometry in general practice. Aust Fam Physician. 2005;34:201-203.
26. Barr RG, Celli BR, Martinez FJ, et al. Physician and patient perceptions in COPD: the COPD Resource Network Needs Assessment Survey. Am J Med. 2005;118:1415.
27. Caramori G, Bettoncelli G, Tosatto R, et al. Underuse of spirometry by general practitioners for the diagnosis of COPD in Italy. Monaldi Arch Chest Dis. 2005;63:6-12.
28. Kaminsky DA, Marcy TW, Bachand F, et al. Knowledge and use of office spirometry for the detection of chronic obstructive pulmonary disease by primary care physicians. Respir Care. 2005;50:1639-1648.
29. Foster JA, Yawn BP, Maziar A, et al. Enhancing COPD management in primary care settings. MedGenMed. 2007;9:24.
30. Bolton CE, Ionescu AA, Edwards PH, et al. Attaining a correct diagnosis of COPD in general practice. Respir Med. 2005:99:493-500.
31. Drummond MB, Dasenbrook EC, Pitz MW, et al. Inhaled corticosteroids in patients with stable chronic obstructive pulmonary disease: a systematic review and meta-analysis. JAMA. 2008;300:2407-2416.
32. Morales DR. LABA monotherapy in asthma: an avoidable problem. Br J Gen Pract. 2013;63:627-628.
33. Nelson HS, Weiss ST, Bleecker ER, et al. The Salmeterol Multicenter Asthma Research Trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest. 2006;129:15-26.
34. van Dijk W, Tan W, Li P, et al. Clinical relevance of fixed ratio vs lower limit of normal of FEV1/FVC in COPD: patient-reported outcomes from the CanCOLD cohort. Ann Fam Med. 2015;13:41-48.
35. Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319-338.
36. Rogliani P, Ora J, Puxeddu E, et al. Airflow obstruction: is it asthma or is it COPD? Int J Chron Obstruct Pulmon Dis. 2016;11:3007-3013.
37. Global Initiative for Asthma. Diagnosis and initial treatment of asthma, COPD and asthma-COPD overlap syndrome. 2017. https://ginasthma.org/. Accessed January 12, 2019.
38. Barrecheguren M, Esquinas C, Miravitlles M. The asthma-chronic obstructive pulmonary disease overlap syndrome (ACOS): opportunities and challenges. Curr Opin Pulm Med. 2015;21:74-79.
39. Kauppi P, Kupiainen H, Lindqvist A, et al. Overlap syndrome of asthma and COPD predicts low quality of life. J Asthma. 2011;48:279-285.
40. Mannino DM, Gagnon RC, Petty TL, et al. Obstructive lung disease and low lung function in adults in the United States: data from the National Health and Nutrition Examination Survey, 1988-1994. Arch Intern Med. 2000;160:1683-1689.
41. Shaya FT, Dongyi D, Akazawa MO, et al. Burden of concomitant asthma and COPD in a Medicaid population. Chest. 2008;134:14-19.
A study of diagnostic accuracy in the primary care setting showed that among patients receiving inhaled therapies, most had not received an accurate diagnosis of chronic obstructive pulmonary disease (COPD) or asthma according to international guidelines.1,2 Other studies have shown that up to one-third of patients with a diagnosis of asthma3 or COPD4 may not actually have disease based on subsequent lung function testing.
Diagnostic error in medicine leads to numerous lost opportunities including the opportunity to: identify chronic conditions that are the true sources of patients’ symptoms, prevent morbidity and mortality, reduce unnecessary costs to patients and health systems, and deliver high-quality care.5-7 The reasons for diagnostic error in COPD and asthma are multifactorial, stemming from insufficient knowledge of clinical practice guidelines and underutilization of spirometry testing. Spirometry is recommended as part of the workup for suspected COPD and is the preferred test for diagnosing asthma. Spirometry, combined with clinical findings, can help differentiate between these diseases.
In this article, we review the definitions and characteristics of COPD and asthma, address the potential causes for diagnostic error, and explain how current clinical practice guidelines can steer examinations to the right diagnosis, improve clinical management, and contribute to better patient outcomes and quality of life.8,9
COPD and asthma characteristics
COPD. The Global Initiative for Chronic Obstructive Lung Disease (GOLD) defines COPD as a common lung disease characterized by persistent respiratory symptoms and airflow obstruction caused by airway or alveolar abnormalities secondary to significant exposure to noxious particles or gases.10 The most common COPD-risk exposure in the United States is tobacco smoke, chiefly from cigarettes. Risk is also heightened with use of other types of tobacco (pipe, cigar, water pipe), indoor and outdoor air pollution (including second-hand tobacco smoke exposure), and occupational exposures. (Consider testing for alpha-1 antitrypsin deficiency—a known genetic risk factor for COPD—especially when an individual with COPD is younger and has a limited smoking history.)
The most common symptom of COPD is chronic, progressive dyspnea — an increased effort to breathe, with chest heaviness, air hunger, or gasping. About one-third of people with COPD have a chronic cough with sputum production.10 There may be wheezing and chest tightness. Fatigue, weight loss, and anorexia can be seen in severe COPD. Consider this disorder in any individual older than 40 years of age who has dyspnea and chronic cough with sputum production, as well as a history of risk factors. If COPD is suspected, perform spirometry to determine the presence of fixed airflow limitation and confirm the diagnosis.
Asthma is usually characterized by variable airway hyperresponsiveness and chronic inflammation. A typical clinical presentation is an individual with a history of wheezing, shortness of breath, chest tightness, and cough that vary in intensity over time and are coupled with variable expiratory flow limitation. Asthma symptoms are often triggered by allergen or irritant exposure, exercise, weather changes, or viral respiratory infections.2 Symptoms may also be worse at night or first thing in the morning. Once asthma is suspected, document the presence of airflow variability with spirometry to confirm the diagnosis.
[polldaddy:10261486]
Diagnostic error in suspected COPD and asthma
Numerous studies have demonstrated the prevalence of diagnostic error when testing of lung function is neglected.11-14 Using spirometry to confirm a prior clinical diagnosis of COPD, researchers found that:
- 35% to 50% of patients did not have objective evidence of COPD12,13;
- 37% with an asthma-only diagnosis had persistent obstruction, which may indicate COPD or chronic obstructive asthma12; and
- 31% of patients thought to have asthma-COPD overlap did not have a COPD component.12
Continue to: In 2 longitudinal studies...
In 2 longitudinal studies, patients with a diagnosis of asthma were recruited to undergo medication reduction and serial lung function testing. Asthma was excluded in approximately 30% of patients.15,16 Diagnostic error has also been seen in patients hospitalized with exacerbations of COPD and asthma. One study found that only 31% of patients admitted with a diagnosis of COPD exacerbation had undergone a spirometry test prior to hospitalization.17 And of those patients with a diagnosis of COPD who underwent spirometry, 30% had results inconsistent with COPD.17
In another study, 22% of adults hospitalized for COPD or asthma exacerbations had no evidence of obstruction on spirometry at the time of hospitalization.18 This finding refutes a diagnosis of COPD and, in the midst of an exacerbation, challenges an asthma diagnosis as well. Increased awareness of clinical practice guidelines, coupled with the use and accurate interpretation of spirometry are needed for optimal management and treatment of COPD and asthma.
Clinical practice guidelines recommend spirometry for the diagnosis of COPD and asthma and have been issued by GOLD10; the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and the European Respiratory Society19; the Global Initiative for Asthma (GINA)2; and the National Heart, Lung, and Blood Institute.20
When a patient’s symptoms and risk factors suggest COPD, spirometry is needed to show persistent post-bronchodilator airflow obstruction and thereby confirm the diagnosis. However, in the United States, confirmatory spirometry is used only in about one third of patients newly diagnosed with COPD.21,22 Similarly for asthma, in the presence of suggestive symptoms, spirometry is the preferred and most reliable and reproducible test to detect the variable expiratory airflow limitation consistent with this diagnosis.
An alternative to spirometry for the diagnosis of asthma (if needed) is a peak flow meter, a simple tool to measure peak expiratory flow. When compared with spirometry, peak flow measurements are less time consuming, less costly, and not dependent on trained staff to perform.23 However, this option does require that patients perform and document multiple measurements over several days without an objective assessment of their efforts. Unlike spirometry, the peak flow meter has no reference values or reliability and reproducibility standards, and measurements can differ from one peak flow meter to another. Thus, a peak flow meter is less reliable than spirometry for diagnosing asthma. But it can be useful for monitoring asthma control at home and in the clinic setting,24 or for diagnosis if spirometry is unavailable.23
Continue to: Barriers to the use of spirometry...
Barriers to the use of spirometry in the primary care setting exist on several levels. Providers may lack knowledge of clinical practice guidelines that recommend spirometry in the diagnosis of COPD, and they may lack general awareness of the utility of spirometry.25-29 In 2 studies of primary care practices that offered office spirometry, lack of knowledge in conducting and interpreting the test was a barrier to its use.28,30 Primary care physicians also struggle with logistical challenges when clinical visits last just 10 to 15 minutes for patients with multiple comorbidities,27 and maintenance of an office spirometry program may not always be feasible.
Getting to the right diagnosis
Likewise, nonpharmacologic interventions may be misused or go unused when needed if the diagnosis is inaccurate. For patients with COPD, outcomes are improved with pulmonary rehabilitation and supplemental oxygen in the setting of resting hypoxemia, but these resources will not be considered if patients are misdiagnosed as having asthma. A patient with undetected heart failure or obstructive sleep apnea who has been misdiagnosed with COPD or asthma may not receive appropriate diagnostic testing or treatment until asthma or COPD has been ruled out with lung function testing.
Objectively documenting the right diagnosis helps ensure guideline-based management of COPD or asthma. Ruling out these 2 disorders prompts further investigation into other conditions (eg, coronary artery disease, heart failure, gastroesophageal reflux disease, pulmonary hypertension, interstitial lung diseases) that can cause symptoms such as shortness of breath, wheezing, or cough.
The TABLE2,10,34 summarizes some of the more common clinical and spirometric features of COPD and asthma. Onset of COPD usually occurs in those over age 40. Asthma can present in younger individuals, including children. Tobacco use or exposure to noxious substances is more often associated with COPD. Patients with asthma are more likely to have atopy. Symptoms in COPD usually progress with increasing activity or exertion. Symptoms in asthma may vary with certain activities, such as exercise, and with various triggers. These features represent “typical” cases of COPD or asthma, but some patients may have clinical characteristics
Continue to: The utility of spirometry in measuring lung function
The utility of spirometry in measuring lung function. Spirometry is the most reproducible and objective measurement of airflow limitation,10 and it should precede any treatment decisions. This technique—in which the patient performs maximal inhalation followed by forced exhalation—measures airflow over time and determines the lung volume exhaled at any time point. Because this respiratory exercise is patient dependent, a well-trained technician is needed to ensure reproducibility and reliability of results based on technical standards.
Spirometry measures forced vital capacity (FVC) and forced expiratory volume in one second (FEV1), from which the FEV1/FVC ratio is calculated. FVC is the total amount of air from total lung volume that can be exhaled in one breath. FEV1 is the total amount of air exhaled in the first second after initiation of exhalation. Thus, the FEV1/FVC ratio is the percentage of the total amount of air in a single breath that is exhaled in the first second. On average, an individual with normal lungs can exhale approximately 80% of their FVC in the first second, thereby resulting in a FEV1/FVC ratio of 80%.
Spirometry findings with COPD. A post-bronchodilator FEV1/FVC ratio of less than 70% confirms airflow obstruction and is consistent with COPD according to GOLD criteria.10 Post-bronchodilator spirometry is performed after the patient has received a specified dose of an inhaled bronchodilator per lab protocols. In patients with COPD, the FEV1/FVC ratio is persistently low even after administration of a bronchodilator.
Another means of using spirometry to diagnose COPD is referring to age-dependent cutoff values below the lower fifth percentile of the FEV1/FVC ratio (ie, lower limit of normal [LLN]), which differs from the GOLD strategy but is consistent with the American Thoracic Society/European Respiratory Society guidelines.35 Because the FEV1/FVC ratio declines with age, older adults may have a normal post-bronchodilator ratio less than 70%. Admittedly, applying GOLD criteria to older adults could result in overdiagnosis, while using the LLN could lead to underdiagnosis. Although there is no consensus on which method to use, the best approach may be the one that most strongly correlates with pretest probability of disease. In a large Canadian study, the approach that most strongly predicted poor patient outcomes was using a FEV1/FVC based on fixed (70%) and/or LLN criteria, and a low FEV134
Spirometry findings with asthma. According to the American Thoracic Society, a post-bronchodilator response is defined as an increase in FEV1 (or FVC) of 12% if that volume is also ≥200 mL. In patients with suspected asthma, an increase in FEV1 ≥12% and 200 mL is consistent with variable airflow limitation2 and supports the diagnosis. Of note, lung function in patients with asthma may be normal when patients are not symptomatic or when they are receiving therapy. Spirometry is therefore ideally performed before initiating therapy and when maintenance therapy is being considered due to symptoms. If therapy is clinically indicated, a short-acting bronchodilator may be prescribed alone and then held 6 to 8 hours before conducting spirometry. If a trial of a maintenance medication is prescribed before spirometry, consider de-escalation of therapy once the patient is more stable and then perform spirometry to confirm the presence of airflow variability consistent with asthma. (In COPD, there can be a positive bronchodilator response; however, the post-bronchodilator FEV1/FVC ratio remains low.)
Continue to: Don't use in isolation
Don’t use in isolation. Use spirometry to support a clinical suspicion of asthma36 or COPD after a thorough history and physical exam, and not in isolation.
Special consideration: Asthma-COPD overlap syndrome
Some patients have features characteristic of both asthma and COPD and are said to have asthma-COPD overlap syndrome (ACOS). Between 15% and 20% of patients with COPD may in fact have ACOS.36 While there is no specific definition of ACOS, GOLD and GINA describe ACOS as persistent airflow limitation with several features usually associated with asthma and several features usually associated with COPD.2,10,37 ACOS becomes more prevalent with advancing age.
In ACOS, patients with COPD present with increased reversibility or patients with asthma and smoking history develop non-fully reversible airway obstruction at an older age.38 Patients with ACOS have worse lung function, more respiratory symptoms, and lower health-related quality of life than individuals with asthma or COPD alone,39,40 leading to more consumption of medical resources.41 In patients with ACOS, the FEV1/FVC ratio is low and consistent with the diagnosis of COPD. The post-bronchodilator response may be variable, depending on the stage of disease and predominant clinical features. It is still unclear whether ACOS is a separate disease entity, a representation of severe asthma that has morphed into COPD, or not a syndrome but simply 2 separate comorbid disease states.
CORRESPONDENCE
Christina D. Wells, MD, University of Illinois Mile Square Health Center, 1220 S. Wood Street, Chicago, IL 60612; cwells2@uic.edu.
A study of diagnostic accuracy in the primary care setting showed that among patients receiving inhaled therapies, most had not received an accurate diagnosis of chronic obstructive pulmonary disease (COPD) or asthma according to international guidelines.1,2 Other studies have shown that up to one-third of patients with a diagnosis of asthma3 or COPD4 may not actually have disease based on subsequent lung function testing.
Diagnostic error in medicine leads to numerous lost opportunities including the opportunity to: identify chronic conditions that are the true sources of patients’ symptoms, prevent morbidity and mortality, reduce unnecessary costs to patients and health systems, and deliver high-quality care.5-7 The reasons for diagnostic error in COPD and asthma are multifactorial, stemming from insufficient knowledge of clinical practice guidelines and underutilization of spirometry testing. Spirometry is recommended as part of the workup for suspected COPD and is the preferred test for diagnosing asthma. Spirometry, combined with clinical findings, can help differentiate between these diseases.
In this article, we review the definitions and characteristics of COPD and asthma, address the potential causes for diagnostic error, and explain how current clinical practice guidelines can steer examinations to the right diagnosis, improve clinical management, and contribute to better patient outcomes and quality of life.8,9
COPD and asthma characteristics
COPD. The Global Initiative for Chronic Obstructive Lung Disease (GOLD) defines COPD as a common lung disease characterized by persistent respiratory symptoms and airflow obstruction caused by airway or alveolar abnormalities secondary to significant exposure to noxious particles or gases.10 The most common COPD-risk exposure in the United States is tobacco smoke, chiefly from cigarettes. Risk is also heightened with use of other types of tobacco (pipe, cigar, water pipe), indoor and outdoor air pollution (including second-hand tobacco smoke exposure), and occupational exposures. (Consider testing for alpha-1 antitrypsin deficiency—a known genetic risk factor for COPD—especially when an individual with COPD is younger and has a limited smoking history.)
The most common symptom of COPD is chronic, progressive dyspnea — an increased effort to breathe, with chest heaviness, air hunger, or gasping. About one-third of people with COPD have a chronic cough with sputum production.10 There may be wheezing and chest tightness. Fatigue, weight loss, and anorexia can be seen in severe COPD. Consider this disorder in any individual older than 40 years of age who has dyspnea and chronic cough with sputum production, as well as a history of risk factors. If COPD is suspected, perform spirometry to determine the presence of fixed airflow limitation and confirm the diagnosis.
Asthma is usually characterized by variable airway hyperresponsiveness and chronic inflammation. A typical clinical presentation is an individual with a history of wheezing, shortness of breath, chest tightness, and cough that vary in intensity over time and are coupled with variable expiratory flow limitation. Asthma symptoms are often triggered by allergen or irritant exposure, exercise, weather changes, or viral respiratory infections.2 Symptoms may also be worse at night or first thing in the morning. Once asthma is suspected, document the presence of airflow variability with spirometry to confirm the diagnosis.
[polldaddy:10261486]
Diagnostic error in suspected COPD and asthma
Numerous studies have demonstrated the prevalence of diagnostic error when testing of lung function is neglected.11-14 Using spirometry to confirm a prior clinical diagnosis of COPD, researchers found that:
- 35% to 50% of patients did not have objective evidence of COPD12,13;
- 37% with an asthma-only diagnosis had persistent obstruction, which may indicate COPD or chronic obstructive asthma12; and
- 31% of patients thought to have asthma-COPD overlap did not have a COPD component.12
Continue to: In 2 longitudinal studies...
In 2 longitudinal studies, patients with a diagnosis of asthma were recruited to undergo medication reduction and serial lung function testing. Asthma was excluded in approximately 30% of patients.15,16 Diagnostic error has also been seen in patients hospitalized with exacerbations of COPD and asthma. One study found that only 31% of patients admitted with a diagnosis of COPD exacerbation had undergone a spirometry test prior to hospitalization.17 And of those patients with a diagnosis of COPD who underwent spirometry, 30% had results inconsistent with COPD.17
In another study, 22% of adults hospitalized for COPD or asthma exacerbations had no evidence of obstruction on spirometry at the time of hospitalization.18 This finding refutes a diagnosis of COPD and, in the midst of an exacerbation, challenges an asthma diagnosis as well. Increased awareness of clinical practice guidelines, coupled with the use and accurate interpretation of spirometry are needed for optimal management and treatment of COPD and asthma.
Clinical practice guidelines recommend spirometry for the diagnosis of COPD and asthma and have been issued by GOLD10; the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and the European Respiratory Society19; the Global Initiative for Asthma (GINA)2; and the National Heart, Lung, and Blood Institute.20
When a patient’s symptoms and risk factors suggest COPD, spirometry is needed to show persistent post-bronchodilator airflow obstruction and thereby confirm the diagnosis. However, in the United States, confirmatory spirometry is used only in about one third of patients newly diagnosed with COPD.21,22 Similarly for asthma, in the presence of suggestive symptoms, spirometry is the preferred and most reliable and reproducible test to detect the variable expiratory airflow limitation consistent with this diagnosis.
An alternative to spirometry for the diagnosis of asthma (if needed) is a peak flow meter, a simple tool to measure peak expiratory flow. When compared with spirometry, peak flow measurements are less time consuming, less costly, and not dependent on trained staff to perform.23 However, this option does require that patients perform and document multiple measurements over several days without an objective assessment of their efforts. Unlike spirometry, the peak flow meter has no reference values or reliability and reproducibility standards, and measurements can differ from one peak flow meter to another. Thus, a peak flow meter is less reliable than spirometry for diagnosing asthma. But it can be useful for monitoring asthma control at home and in the clinic setting,24 or for diagnosis if spirometry is unavailable.23
Continue to: Barriers to the use of spirometry...
Barriers to the use of spirometry in the primary care setting exist on several levels. Providers may lack knowledge of clinical practice guidelines that recommend spirometry in the diagnosis of COPD, and they may lack general awareness of the utility of spirometry.25-29 In 2 studies of primary care practices that offered office spirometry, lack of knowledge in conducting and interpreting the test was a barrier to its use.28,30 Primary care physicians also struggle with logistical challenges when clinical visits last just 10 to 15 minutes for patients with multiple comorbidities,27 and maintenance of an office spirometry program may not always be feasible.
Getting to the right diagnosis
Likewise, nonpharmacologic interventions may be misused or go unused when needed if the diagnosis is inaccurate. For patients with COPD, outcomes are improved with pulmonary rehabilitation and supplemental oxygen in the setting of resting hypoxemia, but these resources will not be considered if patients are misdiagnosed as having asthma. A patient with undetected heart failure or obstructive sleep apnea who has been misdiagnosed with COPD or asthma may not receive appropriate diagnostic testing or treatment until asthma or COPD has been ruled out with lung function testing.
Objectively documenting the right diagnosis helps ensure guideline-based management of COPD or asthma. Ruling out these 2 disorders prompts further investigation into other conditions (eg, coronary artery disease, heart failure, gastroesophageal reflux disease, pulmonary hypertension, interstitial lung diseases) that can cause symptoms such as shortness of breath, wheezing, or cough.
The TABLE2,10,34 summarizes some of the more common clinical and spirometric features of COPD and asthma. Onset of COPD usually occurs in those over age 40. Asthma can present in younger individuals, including children. Tobacco use or exposure to noxious substances is more often associated with COPD. Patients with asthma are more likely to have atopy. Symptoms in COPD usually progress with increasing activity or exertion. Symptoms in asthma may vary with certain activities, such as exercise, and with various triggers. These features represent “typical” cases of COPD or asthma, but some patients may have clinical characteristics
Continue to: The utility of spirometry in measuring lung function
The utility of spirometry in measuring lung function. Spirometry is the most reproducible and objective measurement of airflow limitation,10 and it should precede any treatment decisions. This technique—in which the patient performs maximal inhalation followed by forced exhalation—measures airflow over time and determines the lung volume exhaled at any time point. Because this respiratory exercise is patient dependent, a well-trained technician is needed to ensure reproducibility and reliability of results based on technical standards.
Spirometry measures forced vital capacity (FVC) and forced expiratory volume in one second (FEV1), from which the FEV1/FVC ratio is calculated. FVC is the total amount of air from total lung volume that can be exhaled in one breath. FEV1 is the total amount of air exhaled in the first second after initiation of exhalation. Thus, the FEV1/FVC ratio is the percentage of the total amount of air in a single breath that is exhaled in the first second. On average, an individual with normal lungs can exhale approximately 80% of their FVC in the first second, thereby resulting in a FEV1/FVC ratio of 80%.
Spirometry findings with COPD. A post-bronchodilator FEV1/FVC ratio of less than 70% confirms airflow obstruction and is consistent with COPD according to GOLD criteria.10 Post-bronchodilator spirometry is performed after the patient has received a specified dose of an inhaled bronchodilator per lab protocols. In patients with COPD, the FEV1/FVC ratio is persistently low even after administration of a bronchodilator.
Another means of using spirometry to diagnose COPD is referring to age-dependent cutoff values below the lower fifth percentile of the FEV1/FVC ratio (ie, lower limit of normal [LLN]), which differs from the GOLD strategy but is consistent with the American Thoracic Society/European Respiratory Society guidelines.35 Because the FEV1/FVC ratio declines with age, older adults may have a normal post-bronchodilator ratio less than 70%. Admittedly, applying GOLD criteria to older adults could result in overdiagnosis, while using the LLN could lead to underdiagnosis. Although there is no consensus on which method to use, the best approach may be the one that most strongly correlates with pretest probability of disease. In a large Canadian study, the approach that most strongly predicted poor patient outcomes was using a FEV1/FVC based on fixed (70%) and/or LLN criteria, and a low FEV134
Spirometry findings with asthma. According to the American Thoracic Society, a post-bronchodilator response is defined as an increase in FEV1 (or FVC) of 12% if that volume is also ≥200 mL. In patients with suspected asthma, an increase in FEV1 ≥12% and 200 mL is consistent with variable airflow limitation2 and supports the diagnosis. Of note, lung function in patients with asthma may be normal when patients are not symptomatic or when they are receiving therapy. Spirometry is therefore ideally performed before initiating therapy and when maintenance therapy is being considered due to symptoms. If therapy is clinically indicated, a short-acting bronchodilator may be prescribed alone and then held 6 to 8 hours before conducting spirometry. If a trial of a maintenance medication is prescribed before spirometry, consider de-escalation of therapy once the patient is more stable and then perform spirometry to confirm the presence of airflow variability consistent with asthma. (In COPD, there can be a positive bronchodilator response; however, the post-bronchodilator FEV1/FVC ratio remains low.)
Continue to: Don't use in isolation
Don’t use in isolation. Use spirometry to support a clinical suspicion of asthma36 or COPD after a thorough history and physical exam, and not in isolation.
Special consideration: Asthma-COPD overlap syndrome
Some patients have features characteristic of both asthma and COPD and are said to have asthma-COPD overlap syndrome (ACOS). Between 15% and 20% of patients with COPD may in fact have ACOS.36 While there is no specific definition of ACOS, GOLD and GINA describe ACOS as persistent airflow limitation with several features usually associated with asthma and several features usually associated with COPD.2,10,37 ACOS becomes more prevalent with advancing age.
In ACOS, patients with COPD present with increased reversibility or patients with asthma and smoking history develop non-fully reversible airway obstruction at an older age.38 Patients with ACOS have worse lung function, more respiratory symptoms, and lower health-related quality of life than individuals with asthma or COPD alone,39,40 leading to more consumption of medical resources.41 In patients with ACOS, the FEV1/FVC ratio is low and consistent with the diagnosis of COPD. The post-bronchodilator response may be variable, depending on the stage of disease and predominant clinical features. It is still unclear whether ACOS is a separate disease entity, a representation of severe asthma that has morphed into COPD, or not a syndrome but simply 2 separate comorbid disease states.
CORRESPONDENCE
Christina D. Wells, MD, University of Illinois Mile Square Health Center, 1220 S. Wood Street, Chicago, IL 60612; cwells2@uic.edu.
1. Izquierdo JL, Martìn A, de Lucas P, et al. Misdiagnosis of patients receiving inhaled therapies in primary care. Int J Chron Obstruct Pulmon Dis. 2010;5:241-249.
2. Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. 2018. https://ginasthma.org/wp-content/uploads/2018/04/wms-GINA-2018-report-V1.3-002.pdf. Accessed January 11, 2019.
3. Aaron SD, Vandemheen KL, FitzGerald JM. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA. 2017; 317:269-279.
4. Spero K, Bayasi G, Beaudry L, et al. Overdiagnosis of COPD in hospitalized patients. Int J Chron Obstruct Pulmon Dis. 2017;12:2417-2423.
5. Singh H, Graber ML. Improving diagnosis in health care—the next imperative for patient safety. N Engl J Med. 2015;373:2493-2495.
6. Ball JR, Balogh E. Improving diagnosis in health care: highlights of a report from the National Academies of Sciences, Engineering, and Medicine. Ann Intern Med. 2016;164:59-61.
7. Khullar D, Jha AK, Jena AB. Reducing diagnostic errors—why now? N Engl J Med. 2015;373:2491-2493.
8. Lamprecht B, Soriano JB, Studnicka M, et al. Determinants of underdiagnosis of COPD in national and international surveys. Chest. 2015;148:971-985.
9. Yang CL, Simons E, Foty RG, et al. Misdiagnosis of asthma in schoolchildren. Pediatr Pulmonol. 2017;52:293-302.
10. Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. 2019. https://goldcopd.org/wp-content/uploads/2018/11/GOLD-2019-v1.7-FINAL-14Nov2018-WMS.pdf. Accessed January 12, 2019.
11. Tinkelman DG, Price DB, Nordyke RJ, et al. Misdiagnosis of COPD and asthma in primary care patients 40 years of age and over. J Asthma. 2006;43:75-80.
12. Abramson MJ, Schattner RL, Sulaiman ND, et al. Accuracy of asthma and COPD diagnosis in Australian general practice: a mixed methods study. Prim Care Respir J. 2012;21:167-173.
13. Sichletidis L, Chloros D, Spyratos D, et al. The validity of the diagnosis of chronic obstructive pulmonary disease in general practice. Prim Care Respir J. 2007;16:82-88.
14. Marklund B, Tunsäter A, Bengtsson C. How often is the diagnosis bronchial asthma correct? Fam Pract. 1999;16:112-116.
15. Aaron SD, Vandemheen KL, Boulet LP, et al. Overdiagnosis of asthma in obese and nonobese adults. CMAJ. 2008;179:1121-1131.
16. Aaron SD, Vandemheen KL, FitzGerald JM, et al. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA. 2017;317:269-279.
17. Damarla M, Celli BR, Mullerova HX, et al. Discrepancy in the use of confirmatory tests in patients hospitalized with the diagnosis of chronic obstructive pulmonary disease or congestive heart failure. Respir Care. 2006;51:1120-1124.
18. Prieto Centurion V, Huang F, Naureckus ET, et al. Confirmatory spirometry for adults hospitalized with a diagnosis of asthma or chronic obstructive pulmonary disease exacerbation. BMC Pulm Med. 2012;12:73.
19. Qaseem A, Wilt TJ, Weinberger SE, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155:179-191.
20. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007. 120(Suppl):S94-S138.
21. Han MK, Kim MG, Mardon R, et al. Spirometry utilization for COPD: how do we measure up? Chest. 2007;132:403-409.
22. Joo MJ, Lee TA, Weiss KB. Geographic variation of spirometry use in newly diagnosed COPD. Chest. 2008;134:38-45.
23. Thorat YT, Salvi SS, Kodgule RR. Peak flow meter with a questionnaire and mini-spirometer to help detect asthma and COPD in real-life clinical practice: a cross-sectional study. NPJ Prim Care Respir Med. 2017;27:32.
24. Kennedy DT, Chang Z, Small RE. Selection of peak flowmeters in ambulatory asthma patients: a review of the literature. Chest. 1998;114:587-592.
25. Walters JA, Hanson E, Mudge P, et al. Barriers to the use of spirometry in general practice. Aust Fam Physician. 2005;34:201-203.
26. Barr RG, Celli BR, Martinez FJ, et al. Physician and patient perceptions in COPD: the COPD Resource Network Needs Assessment Survey. Am J Med. 2005;118:1415.
27. Caramori G, Bettoncelli G, Tosatto R, et al. Underuse of spirometry by general practitioners for the diagnosis of COPD in Italy. Monaldi Arch Chest Dis. 2005;63:6-12.
28. Kaminsky DA, Marcy TW, Bachand F, et al. Knowledge and use of office spirometry for the detection of chronic obstructive pulmonary disease by primary care physicians. Respir Care. 2005;50:1639-1648.
29. Foster JA, Yawn BP, Maziar A, et al. Enhancing COPD management in primary care settings. MedGenMed. 2007;9:24.
30. Bolton CE, Ionescu AA, Edwards PH, et al. Attaining a correct diagnosis of COPD in general practice. Respir Med. 2005:99:493-500.
31. Drummond MB, Dasenbrook EC, Pitz MW, et al. Inhaled corticosteroids in patients with stable chronic obstructive pulmonary disease: a systematic review and meta-analysis. JAMA. 2008;300:2407-2416.
32. Morales DR. LABA monotherapy in asthma: an avoidable problem. Br J Gen Pract. 2013;63:627-628.
33. Nelson HS, Weiss ST, Bleecker ER, et al. The Salmeterol Multicenter Asthma Research Trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest. 2006;129:15-26.
34. van Dijk W, Tan W, Li P, et al. Clinical relevance of fixed ratio vs lower limit of normal of FEV1/FVC in COPD: patient-reported outcomes from the CanCOLD cohort. Ann Fam Med. 2015;13:41-48.
35. Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319-338.
36. Rogliani P, Ora J, Puxeddu E, et al. Airflow obstruction: is it asthma or is it COPD? Int J Chron Obstruct Pulmon Dis. 2016;11:3007-3013.
37. Global Initiative for Asthma. Diagnosis and initial treatment of asthma, COPD and asthma-COPD overlap syndrome. 2017. https://ginasthma.org/. Accessed January 12, 2019.
38. Barrecheguren M, Esquinas C, Miravitlles M. The asthma-chronic obstructive pulmonary disease overlap syndrome (ACOS): opportunities and challenges. Curr Opin Pulm Med. 2015;21:74-79.
39. Kauppi P, Kupiainen H, Lindqvist A, et al. Overlap syndrome of asthma and COPD predicts low quality of life. J Asthma. 2011;48:279-285.
40. Mannino DM, Gagnon RC, Petty TL, et al. Obstructive lung disease and low lung function in adults in the United States: data from the National Health and Nutrition Examination Survey, 1988-1994. Arch Intern Med. 2000;160:1683-1689.
41. Shaya FT, Dongyi D, Akazawa MO, et al. Burden of concomitant asthma and COPD in a Medicaid population. Chest. 2008;134:14-19.
1. Izquierdo JL, Martìn A, de Lucas P, et al. Misdiagnosis of patients receiving inhaled therapies in primary care. Int J Chron Obstruct Pulmon Dis. 2010;5:241-249.
2. Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. 2018. https://ginasthma.org/wp-content/uploads/2018/04/wms-GINA-2018-report-V1.3-002.pdf. Accessed January 11, 2019.
3. Aaron SD, Vandemheen KL, FitzGerald JM. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA. 2017; 317:269-279.
4. Spero K, Bayasi G, Beaudry L, et al. Overdiagnosis of COPD in hospitalized patients. Int J Chron Obstruct Pulmon Dis. 2017;12:2417-2423.
5. Singh H, Graber ML. Improving diagnosis in health care—the next imperative for patient safety. N Engl J Med. 2015;373:2493-2495.
6. Ball JR, Balogh E. Improving diagnosis in health care: highlights of a report from the National Academies of Sciences, Engineering, and Medicine. Ann Intern Med. 2016;164:59-61.
7. Khullar D, Jha AK, Jena AB. Reducing diagnostic errors—why now? N Engl J Med. 2015;373:2491-2493.
8. Lamprecht B, Soriano JB, Studnicka M, et al. Determinants of underdiagnosis of COPD in national and international surveys. Chest. 2015;148:971-985.
9. Yang CL, Simons E, Foty RG, et al. Misdiagnosis of asthma in schoolchildren. Pediatr Pulmonol. 2017;52:293-302.
10. Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. 2019. https://goldcopd.org/wp-content/uploads/2018/11/GOLD-2019-v1.7-FINAL-14Nov2018-WMS.pdf. Accessed January 12, 2019.
11. Tinkelman DG, Price DB, Nordyke RJ, et al. Misdiagnosis of COPD and asthma in primary care patients 40 years of age and over. J Asthma. 2006;43:75-80.
12. Abramson MJ, Schattner RL, Sulaiman ND, et al. Accuracy of asthma and COPD diagnosis in Australian general practice: a mixed methods study. Prim Care Respir J. 2012;21:167-173.
13. Sichletidis L, Chloros D, Spyratos D, et al. The validity of the diagnosis of chronic obstructive pulmonary disease in general practice. Prim Care Respir J. 2007;16:82-88.
14. Marklund B, Tunsäter A, Bengtsson C. How often is the diagnosis bronchial asthma correct? Fam Pract. 1999;16:112-116.
15. Aaron SD, Vandemheen KL, Boulet LP, et al. Overdiagnosis of asthma in obese and nonobese adults. CMAJ. 2008;179:1121-1131.
16. Aaron SD, Vandemheen KL, FitzGerald JM, et al. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA. 2017;317:269-279.
17. Damarla M, Celli BR, Mullerova HX, et al. Discrepancy in the use of confirmatory tests in patients hospitalized with the diagnosis of chronic obstructive pulmonary disease or congestive heart failure. Respir Care. 2006;51:1120-1124.
18. Prieto Centurion V, Huang F, Naureckus ET, et al. Confirmatory spirometry for adults hospitalized with a diagnosis of asthma or chronic obstructive pulmonary disease exacerbation. BMC Pulm Med. 2012;12:73.
19. Qaseem A, Wilt TJ, Weinberger SE, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155:179-191.
20. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007. 120(Suppl):S94-S138.
21. Han MK, Kim MG, Mardon R, et al. Spirometry utilization for COPD: how do we measure up? Chest. 2007;132:403-409.
22. Joo MJ, Lee TA, Weiss KB. Geographic variation of spirometry use in newly diagnosed COPD. Chest. 2008;134:38-45.
23. Thorat YT, Salvi SS, Kodgule RR. Peak flow meter with a questionnaire and mini-spirometer to help detect asthma and COPD in real-life clinical practice: a cross-sectional study. NPJ Prim Care Respir Med. 2017;27:32.
24. Kennedy DT, Chang Z, Small RE. Selection of peak flowmeters in ambulatory asthma patients: a review of the literature. Chest. 1998;114:587-592.
25. Walters JA, Hanson E, Mudge P, et al. Barriers to the use of spirometry in general practice. Aust Fam Physician. 2005;34:201-203.
26. Barr RG, Celli BR, Martinez FJ, et al. Physician and patient perceptions in COPD: the COPD Resource Network Needs Assessment Survey. Am J Med. 2005;118:1415.
27. Caramori G, Bettoncelli G, Tosatto R, et al. Underuse of spirometry by general practitioners for the diagnosis of COPD in Italy. Monaldi Arch Chest Dis. 2005;63:6-12.
28. Kaminsky DA, Marcy TW, Bachand F, et al. Knowledge and use of office spirometry for the detection of chronic obstructive pulmonary disease by primary care physicians. Respir Care. 2005;50:1639-1648.
29. Foster JA, Yawn BP, Maziar A, et al. Enhancing COPD management in primary care settings. MedGenMed. 2007;9:24.
30. Bolton CE, Ionescu AA, Edwards PH, et al. Attaining a correct diagnosis of COPD in general practice. Respir Med. 2005:99:493-500.
31. Drummond MB, Dasenbrook EC, Pitz MW, et al. Inhaled corticosteroids in patients with stable chronic obstructive pulmonary disease: a systematic review and meta-analysis. JAMA. 2008;300:2407-2416.
32. Morales DR. LABA monotherapy in asthma: an avoidable problem. Br J Gen Pract. 2013;63:627-628.
33. Nelson HS, Weiss ST, Bleecker ER, et al. The Salmeterol Multicenter Asthma Research Trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest. 2006;129:15-26.
34. van Dijk W, Tan W, Li P, et al. Clinical relevance of fixed ratio vs lower limit of normal of FEV1/FVC in COPD: patient-reported outcomes from the CanCOLD cohort. Ann Fam Med. 2015;13:41-48.
35. Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319-338.
36. Rogliani P, Ora J, Puxeddu E, et al. Airflow obstruction: is it asthma or is it COPD? Int J Chron Obstruct Pulmon Dis. 2016;11:3007-3013.
37. Global Initiative for Asthma. Diagnosis and initial treatment of asthma, COPD and asthma-COPD overlap syndrome. 2017. https://ginasthma.org/. Accessed January 12, 2019.
38. Barrecheguren M, Esquinas C, Miravitlles M. The asthma-chronic obstructive pulmonary disease overlap syndrome (ACOS): opportunities and challenges. Curr Opin Pulm Med. 2015;21:74-79.
39. Kauppi P, Kupiainen H, Lindqvist A, et al. Overlap syndrome of asthma and COPD predicts low quality of life. J Asthma. 2011;48:279-285.
40. Mannino DM, Gagnon RC, Petty TL, et al. Obstructive lung disease and low lung function in adults in the United States: data from the National Health and Nutrition Examination Survey, 1988-1994. Arch Intern Med. 2000;160:1683-1689.
41. Shaya FT, Dongyi D, Akazawa MO, et al. Burden of concomitant asthma and COPD in a Medicaid population. Chest. 2008;134:14-19.
PRACTICE RECOMMENDATIONS
› Perform spirometry in all patients with symptoms and risk factors suggestive of chronic obstructive pulmonary disease (COPD) or asthma. B
› Consider having a patient use a peak flow meter to support a diagnosis of asthma if spirometry is unavailable. B
› Consider the possibility of a diagnostic error if COPD or asthma is unresponsive to treatment and the initial diagnosis was made without spirometry. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
KD025 produces durable responses in patients with cGVHD
HOUSTON – The according to a speaker at the Transplantation & Cellular Therapy Meetings.
In an ongoing phase 2 trial (NCT02841995), KD025 produced an overall response rate of 59%. Responses occurred in all affected organ systems, and 72% of responders experienced an improvement in Lee Symptom Scale (LSS) score.
The median duration of response was 28 weeks, but durability data are still maturing, according to Madan Jagasia, MBBS, of Vanderbilt University in Nashville, Tenn.
Dr. Jagasia and his colleagues evaluated KD025 in 54 adults who had persistent, active cGVHD after at least 2 months of steroid therapy. Sixty-seven percent of patients had received at least two prior lines of therapy, and 48% had involvement in four or more organs.
KD025 was given at three doses: 200 mg once daily (cohort 1), 200 mg twice daily (cohort 2), and 400 mg once daily (cohort 3). Patients also received glucocorticoid therapy, with or without calcineurin inhibitor therapy.
Cohort 1 included 17 patients who had a median age of 50 years (range, 20-63). They received KD025 for a median of 37 weeks, and six patients were still receiving KD025 at last follow-up.
Cohort 2 included 16 patients who had a median age of 55 years (range, 30-75). They received KD025 for a median of 33 weeks, and three patients were still receiving KD025 at last follow-up.
Cohort 3 included 21 patients who had a median age of 46 years (range, 25-75). They received KD025 for a median of 27 weeks, and 11 patients were still receiving KD025 at last follow-up.
The ORR was 59% (32/54) across the study, 65% (11/17) in cohort 1, 63% (10/16) in cohort 2, and 52% (11/21) in cohort 3. Three patients in cohort 3 didn’t reach the first response assessment, so the ORR in response-evaluable patients was 61% (11/18).
The ORR was 58% in patients who had received two or more prior lines of therapy, 55% in patients with severe cGVHD, and 62% in patients who had four or more organs involved.
“Responses were observed across all affected organ systems,” Dr. Jagasia said. “CRs [complete responses] were seen in all organs except the lung, and there were two partial responses observed in the lung.”
The median duration of response was 28 weeks. Eighty-two percent of responders in cohort 1, 50% of responders in cohort 2, and 36% of responders in cohort 3 had responses lasting 20 weeks or more.
“Keep in mind, the median duration of follow-up in cohort 3 is still short, and the durability data will continue to mature,” Dr. Jagasia said.
Most responders (72%) had at least a 7-point reduction in LSS score. Considering responders and nonresponders together, 65% of patients in cohort 1, 56% in cohort 2, and 52% in cohort 3 had an improvement in LSS score.
In all, 69% of patients stopped or reduced their use of steroids or other immunosuppressants. Seven patients completely discontinued steroids.
Forty-four percent of patients (n = 24) had a treatment-related adverse event (AE), but none of these AEs were considered serious. Four percent of patients (n = 2) had a related AE that led to treatment discontinuation, and 15% (n = 8) had grade 3/4 related AEs.
“The AEs were, overall, consistent with those expected in chronic graft-versus-host disease patients receiving corticosteroids,” Dr. Jagasia said. “Most important to note, there was no apparent increased risk of infection. Specifically, there was no CMV [cytomegalovirus] infection.”
The most common AEs were upper respiratory tract infection, fatigue, nausea, AST/ALT increase, and diarrhea. The most common grade 3/4 AEs were gamma-glutamyltransferase increase, hyperglycemia, anemia, and dyspnea.
There were three on-study deaths, all considered unrelated to KD025. One patient died of leukemia relapse, one died of lung infection, and one died of cardiac arrest.
Dr. Jagasia presented these results at the Transplantation & Cellular Therapy Meetings, held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research. At the meeting, the American Society for Blood and Marrow Transplantation announced a new name for the society: the American Society for Transplantation and Cellular Therapy (ASTCT).
Dr. Jagasia reported relationships with Kadmon Holdings, Mallinckrodt, and Janssen. The trial was sponsored by Kadmon Holdings.
SOURCE: Jagasia M et al. TCT 2019, Abstract 36.
HOUSTON – The according to a speaker at the Transplantation & Cellular Therapy Meetings.
In an ongoing phase 2 trial (NCT02841995), KD025 produced an overall response rate of 59%. Responses occurred in all affected organ systems, and 72% of responders experienced an improvement in Lee Symptom Scale (LSS) score.
The median duration of response was 28 weeks, but durability data are still maturing, according to Madan Jagasia, MBBS, of Vanderbilt University in Nashville, Tenn.
Dr. Jagasia and his colleagues evaluated KD025 in 54 adults who had persistent, active cGVHD after at least 2 months of steroid therapy. Sixty-seven percent of patients had received at least two prior lines of therapy, and 48% had involvement in four or more organs.
KD025 was given at three doses: 200 mg once daily (cohort 1), 200 mg twice daily (cohort 2), and 400 mg once daily (cohort 3). Patients also received glucocorticoid therapy, with or without calcineurin inhibitor therapy.
Cohort 1 included 17 patients who had a median age of 50 years (range, 20-63). They received KD025 for a median of 37 weeks, and six patients were still receiving KD025 at last follow-up.
Cohort 2 included 16 patients who had a median age of 55 years (range, 30-75). They received KD025 for a median of 33 weeks, and three patients were still receiving KD025 at last follow-up.
Cohort 3 included 21 patients who had a median age of 46 years (range, 25-75). They received KD025 for a median of 27 weeks, and 11 patients were still receiving KD025 at last follow-up.
The ORR was 59% (32/54) across the study, 65% (11/17) in cohort 1, 63% (10/16) in cohort 2, and 52% (11/21) in cohort 3. Three patients in cohort 3 didn’t reach the first response assessment, so the ORR in response-evaluable patients was 61% (11/18).
The ORR was 58% in patients who had received two or more prior lines of therapy, 55% in patients with severe cGVHD, and 62% in patients who had four or more organs involved.
“Responses were observed across all affected organ systems,” Dr. Jagasia said. “CRs [complete responses] were seen in all organs except the lung, and there were two partial responses observed in the lung.”
The median duration of response was 28 weeks. Eighty-two percent of responders in cohort 1, 50% of responders in cohort 2, and 36% of responders in cohort 3 had responses lasting 20 weeks or more.
“Keep in mind, the median duration of follow-up in cohort 3 is still short, and the durability data will continue to mature,” Dr. Jagasia said.
Most responders (72%) had at least a 7-point reduction in LSS score. Considering responders and nonresponders together, 65% of patients in cohort 1, 56% in cohort 2, and 52% in cohort 3 had an improvement in LSS score.
In all, 69% of patients stopped or reduced their use of steroids or other immunosuppressants. Seven patients completely discontinued steroids.
Forty-four percent of patients (n = 24) had a treatment-related adverse event (AE), but none of these AEs were considered serious. Four percent of patients (n = 2) had a related AE that led to treatment discontinuation, and 15% (n = 8) had grade 3/4 related AEs.
“The AEs were, overall, consistent with those expected in chronic graft-versus-host disease patients receiving corticosteroids,” Dr. Jagasia said. “Most important to note, there was no apparent increased risk of infection. Specifically, there was no CMV [cytomegalovirus] infection.”
The most common AEs were upper respiratory tract infection, fatigue, nausea, AST/ALT increase, and diarrhea. The most common grade 3/4 AEs were gamma-glutamyltransferase increase, hyperglycemia, anemia, and dyspnea.
There were three on-study deaths, all considered unrelated to KD025. One patient died of leukemia relapse, one died of lung infection, and one died of cardiac arrest.
Dr. Jagasia presented these results at the Transplantation & Cellular Therapy Meetings, held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research. At the meeting, the American Society for Blood and Marrow Transplantation announced a new name for the society: the American Society for Transplantation and Cellular Therapy (ASTCT).
Dr. Jagasia reported relationships with Kadmon Holdings, Mallinckrodt, and Janssen. The trial was sponsored by Kadmon Holdings.
SOURCE: Jagasia M et al. TCT 2019, Abstract 36.
HOUSTON – The according to a speaker at the Transplantation & Cellular Therapy Meetings.
In an ongoing phase 2 trial (NCT02841995), KD025 produced an overall response rate of 59%. Responses occurred in all affected organ systems, and 72% of responders experienced an improvement in Lee Symptom Scale (LSS) score.
The median duration of response was 28 weeks, but durability data are still maturing, according to Madan Jagasia, MBBS, of Vanderbilt University in Nashville, Tenn.
Dr. Jagasia and his colleagues evaluated KD025 in 54 adults who had persistent, active cGVHD after at least 2 months of steroid therapy. Sixty-seven percent of patients had received at least two prior lines of therapy, and 48% had involvement in four or more organs.
KD025 was given at three doses: 200 mg once daily (cohort 1), 200 mg twice daily (cohort 2), and 400 mg once daily (cohort 3). Patients also received glucocorticoid therapy, with or without calcineurin inhibitor therapy.
Cohort 1 included 17 patients who had a median age of 50 years (range, 20-63). They received KD025 for a median of 37 weeks, and six patients were still receiving KD025 at last follow-up.
Cohort 2 included 16 patients who had a median age of 55 years (range, 30-75). They received KD025 for a median of 33 weeks, and three patients were still receiving KD025 at last follow-up.
Cohort 3 included 21 patients who had a median age of 46 years (range, 25-75). They received KD025 for a median of 27 weeks, and 11 patients were still receiving KD025 at last follow-up.
The ORR was 59% (32/54) across the study, 65% (11/17) in cohort 1, 63% (10/16) in cohort 2, and 52% (11/21) in cohort 3. Three patients in cohort 3 didn’t reach the first response assessment, so the ORR in response-evaluable patients was 61% (11/18).
The ORR was 58% in patients who had received two or more prior lines of therapy, 55% in patients with severe cGVHD, and 62% in patients who had four or more organs involved.
“Responses were observed across all affected organ systems,” Dr. Jagasia said. “CRs [complete responses] were seen in all organs except the lung, and there were two partial responses observed in the lung.”
The median duration of response was 28 weeks. Eighty-two percent of responders in cohort 1, 50% of responders in cohort 2, and 36% of responders in cohort 3 had responses lasting 20 weeks or more.
“Keep in mind, the median duration of follow-up in cohort 3 is still short, and the durability data will continue to mature,” Dr. Jagasia said.
Most responders (72%) had at least a 7-point reduction in LSS score. Considering responders and nonresponders together, 65% of patients in cohort 1, 56% in cohort 2, and 52% in cohort 3 had an improvement in LSS score.
In all, 69% of patients stopped or reduced their use of steroids or other immunosuppressants. Seven patients completely discontinued steroids.
Forty-four percent of patients (n = 24) had a treatment-related adverse event (AE), but none of these AEs were considered serious. Four percent of patients (n = 2) had a related AE that led to treatment discontinuation, and 15% (n = 8) had grade 3/4 related AEs.
“The AEs were, overall, consistent with those expected in chronic graft-versus-host disease patients receiving corticosteroids,” Dr. Jagasia said. “Most important to note, there was no apparent increased risk of infection. Specifically, there was no CMV [cytomegalovirus] infection.”
The most common AEs were upper respiratory tract infection, fatigue, nausea, AST/ALT increase, and diarrhea. The most common grade 3/4 AEs were gamma-glutamyltransferase increase, hyperglycemia, anemia, and dyspnea.
There were three on-study deaths, all considered unrelated to KD025. One patient died of leukemia relapse, one died of lung infection, and one died of cardiac arrest.
Dr. Jagasia presented these results at the Transplantation & Cellular Therapy Meetings, held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research. At the meeting, the American Society for Blood and Marrow Transplantation announced a new name for the society: the American Society for Transplantation and Cellular Therapy (ASTCT).
Dr. Jagasia reported relationships with Kadmon Holdings, Mallinckrodt, and Janssen. The trial was sponsored by Kadmon Holdings.
SOURCE: Jagasia M et al. TCT 2019, Abstract 36.
REPORTING FROM TCT 2019