User login
Cochrane/IBD review roundup: Limited evidence keeps verdicts at bay
LAS VEGAS – Cochrane Library reviews of studies into inflammatory bowel disease (IBD) from 2018 revealed limited evidence – so far – to support enteral nutrition therapy (EN) and cannabis in Crohn’s disease (CD) and fecal transplantation in IBD.

But Morris Gordon, MBChB, MMed, PhD, a Cochrane Library researcher who provided a roundup for colleagues, said there’s tremendous opportunity to build upon existing research in these areas.
Dr. Gordon, a pediatrician with a special gastric interest at the University of Central Lancashire in Preston, England, spoke at the Crohn’s & Colitis Congress - a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
In his presentation, Dr. Gordon discussed several Cochrane Library reviews published in 2018 in these topic areas:
Enteral therapy
EN was a hot topic at the Crohn’s & Colitics Congress, which devoted a large panel discussion to the benefits of its use in inducing remission in CD, especially in children.
However, an updated 2018 Cochrane Library systematic review found that “very low quality evidence suggests that corticosteroid therapy may be more effective than EN for induction of clinical remission in adults with active CD. Very low quality evidence also suggests that EN may be more effective than steroids for induction of remission in children with active CD” (Cochrane Database Syst Rev. 2018 Apr 1. doi: 10.1002/14651858.CD000542.pub3).
The review recommended that “EN should be considered in pediatric CD patients or in adult patients who can comply with nasogastric tube feeding or perceive the formulations to be palatable, or when steroid side effects are not tolerated or better avoided.”
Another 2018 Cochrane Library Review concluded that “no firm conclusions regarding the efficacy and safety of enteral nutrition in quiescent CD can be drawn” (Cochrane Database Syst Rev. 2018 Aug 11. doi: 10.1002/14651858.CD005984.pub3).
Dr. Gordon noted that IBD guidelines support EN to induce CD remission in children, and he called for “high quality research” to provide more evidence to support this recommendation.
Cannabis
In regard to cannabis, Dr. Gordon referred to a 2018 Cochrane review that examined three studies that investigated its use in CD and determined “the effects of cannabis and cannabis oil on Crohn’s disease are uncertain” (Cochrane Database Syst Rev. 2018 Nov 8. doi: 10.1002/14651858.CD012853.pub2).
He said future studies should focus on the effect of cannabis on quality of life and pain reduction. “That’s where the research needs to go,” he said.
Another 2018 Cochrane review examined two small studies exploring the use of cannabis in ulcerative colitis and reported similar findings, declaring that “the effects of cannabis and cannabidiol on UC are uncertain” (Cochrane Database Syst Rev. 2018 Nov 8. doi: 10.1002/14651858.CD012954.pub2).
Fecal transplantation
The Cochrane Library also examined research into fecal transplantation for IBD. A 2018 review reported that “fecal microbiota transplantation may increase the proportion of participants achieving clinical remission in UC. However, the number of identified studies was small and the quality of evidence was low. There is uncertainty about the rate of serious adverse events. As a result, no solid conclusions can be drawn at this time” (Cochrane Database Syst Rev. 2018 Nov 13. doi: 10.1002/14651858.CD012774.pub2).
Still, Dr. Gordon said, fecal transplantation is “really promising.”
Another 2018 Cochrane review of IBD research – this one focusing on natalizumab (Tysabri) as a tool for induction of remission of CD – was more conclusive. It examined five trials and found that “high quality data suggest that natalizumab is effective for induction of clinical remission and response in some patients with moderately to severely active CD”(Cochrane Database Syst Rev. 2018 Aug 1. doi: 10.1002/14651858.CD006097.pub3).
However, the review noted that none of the studies was high powered enough to detect rare serious adverse effects such as progressive multifocal leukoencephalopathy (PML). “Due to the association with PML, and the availability of alternative agents that are not associated with PML, natalizumab is not likely to be used in patients who fail currently available medical therapy,” the reviewers wrote. “Further studies of natalizumab are not likely to be done.”
Dr. Gordon reports unrestricted travel grants over the past 3 years from Ferring, Synergy, Tillotts, and BioGaia. He holds a National Institute for Health Research Cochrane IBD Program Grant.
LAS VEGAS – Cochrane Library reviews of studies into inflammatory bowel disease (IBD) from 2018 revealed limited evidence – so far – to support enteral nutrition therapy (EN) and cannabis in Crohn’s disease (CD) and fecal transplantation in IBD.

But Morris Gordon, MBChB, MMed, PhD, a Cochrane Library researcher who provided a roundup for colleagues, said there’s tremendous opportunity to build upon existing research in these areas.
Dr. Gordon, a pediatrician with a special gastric interest at the University of Central Lancashire in Preston, England, spoke at the Crohn’s & Colitis Congress - a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
In his presentation, Dr. Gordon discussed several Cochrane Library reviews published in 2018 in these topic areas:
Enteral therapy
EN was a hot topic at the Crohn’s & Colitics Congress, which devoted a large panel discussion to the benefits of its use in inducing remission in CD, especially in children.
However, an updated 2018 Cochrane Library systematic review found that “very low quality evidence suggests that corticosteroid therapy may be more effective than EN for induction of clinical remission in adults with active CD. Very low quality evidence also suggests that EN may be more effective than steroids for induction of remission in children with active CD” (Cochrane Database Syst Rev. 2018 Apr 1. doi: 10.1002/14651858.CD000542.pub3).
The review recommended that “EN should be considered in pediatric CD patients or in adult patients who can comply with nasogastric tube feeding or perceive the formulations to be palatable, or when steroid side effects are not tolerated or better avoided.”
Another 2018 Cochrane Library Review concluded that “no firm conclusions regarding the efficacy and safety of enteral nutrition in quiescent CD can be drawn” (Cochrane Database Syst Rev. 2018 Aug 11. doi: 10.1002/14651858.CD005984.pub3).
Dr. Gordon noted that IBD guidelines support EN to induce CD remission in children, and he called for “high quality research” to provide more evidence to support this recommendation.
Cannabis
In regard to cannabis, Dr. Gordon referred to a 2018 Cochrane review that examined three studies that investigated its use in CD and determined “the effects of cannabis and cannabis oil on Crohn’s disease are uncertain” (Cochrane Database Syst Rev. 2018 Nov 8. doi: 10.1002/14651858.CD012853.pub2).
He said future studies should focus on the effect of cannabis on quality of life and pain reduction. “That’s where the research needs to go,” he said.
Another 2018 Cochrane review examined two small studies exploring the use of cannabis in ulcerative colitis and reported similar findings, declaring that “the effects of cannabis and cannabidiol on UC are uncertain” (Cochrane Database Syst Rev. 2018 Nov 8. doi: 10.1002/14651858.CD012954.pub2).
Fecal transplantation
The Cochrane Library also examined research into fecal transplantation for IBD. A 2018 review reported that “fecal microbiota transplantation may increase the proportion of participants achieving clinical remission in UC. However, the number of identified studies was small and the quality of evidence was low. There is uncertainty about the rate of serious adverse events. As a result, no solid conclusions can be drawn at this time” (Cochrane Database Syst Rev. 2018 Nov 13. doi: 10.1002/14651858.CD012774.pub2).
Still, Dr. Gordon said, fecal transplantation is “really promising.”
Another 2018 Cochrane review of IBD research – this one focusing on natalizumab (Tysabri) as a tool for induction of remission of CD – was more conclusive. It examined five trials and found that “high quality data suggest that natalizumab is effective for induction of clinical remission and response in some patients with moderately to severely active CD”(Cochrane Database Syst Rev. 2018 Aug 1. doi: 10.1002/14651858.CD006097.pub3).
However, the review noted that none of the studies was high powered enough to detect rare serious adverse effects such as progressive multifocal leukoencephalopathy (PML). “Due to the association with PML, and the availability of alternative agents that are not associated with PML, natalizumab is not likely to be used in patients who fail currently available medical therapy,” the reviewers wrote. “Further studies of natalizumab are not likely to be done.”
Dr. Gordon reports unrestricted travel grants over the past 3 years from Ferring, Synergy, Tillotts, and BioGaia. He holds a National Institute for Health Research Cochrane IBD Program Grant.
LAS VEGAS – Cochrane Library reviews of studies into inflammatory bowel disease (IBD) from 2018 revealed limited evidence – so far – to support enteral nutrition therapy (EN) and cannabis in Crohn’s disease (CD) and fecal transplantation in IBD.

But Morris Gordon, MBChB, MMed, PhD, a Cochrane Library researcher who provided a roundup for colleagues, said there’s tremendous opportunity to build upon existing research in these areas.
Dr. Gordon, a pediatrician with a special gastric interest at the University of Central Lancashire in Preston, England, spoke at the Crohn’s & Colitis Congress - a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
In his presentation, Dr. Gordon discussed several Cochrane Library reviews published in 2018 in these topic areas:
Enteral therapy
EN was a hot topic at the Crohn’s & Colitics Congress, which devoted a large panel discussion to the benefits of its use in inducing remission in CD, especially in children.
However, an updated 2018 Cochrane Library systematic review found that “very low quality evidence suggests that corticosteroid therapy may be more effective than EN for induction of clinical remission in adults with active CD. Very low quality evidence also suggests that EN may be more effective than steroids for induction of remission in children with active CD” (Cochrane Database Syst Rev. 2018 Apr 1. doi: 10.1002/14651858.CD000542.pub3).
The review recommended that “EN should be considered in pediatric CD patients or in adult patients who can comply with nasogastric tube feeding or perceive the formulations to be palatable, or when steroid side effects are not tolerated or better avoided.”
Another 2018 Cochrane Library Review concluded that “no firm conclusions regarding the efficacy and safety of enteral nutrition in quiescent CD can be drawn” (Cochrane Database Syst Rev. 2018 Aug 11. doi: 10.1002/14651858.CD005984.pub3).
Dr. Gordon noted that IBD guidelines support EN to induce CD remission in children, and he called for “high quality research” to provide more evidence to support this recommendation.
Cannabis
In regard to cannabis, Dr. Gordon referred to a 2018 Cochrane review that examined three studies that investigated its use in CD and determined “the effects of cannabis and cannabis oil on Crohn’s disease are uncertain” (Cochrane Database Syst Rev. 2018 Nov 8. doi: 10.1002/14651858.CD012853.pub2).
He said future studies should focus on the effect of cannabis on quality of life and pain reduction. “That’s where the research needs to go,” he said.
Another 2018 Cochrane review examined two small studies exploring the use of cannabis in ulcerative colitis and reported similar findings, declaring that “the effects of cannabis and cannabidiol on UC are uncertain” (Cochrane Database Syst Rev. 2018 Nov 8. doi: 10.1002/14651858.CD012954.pub2).
Fecal transplantation
The Cochrane Library also examined research into fecal transplantation for IBD. A 2018 review reported that “fecal microbiota transplantation may increase the proportion of participants achieving clinical remission in UC. However, the number of identified studies was small and the quality of evidence was low. There is uncertainty about the rate of serious adverse events. As a result, no solid conclusions can be drawn at this time” (Cochrane Database Syst Rev. 2018 Nov 13. doi: 10.1002/14651858.CD012774.pub2).
Still, Dr. Gordon said, fecal transplantation is “really promising.”
Another 2018 Cochrane review of IBD research – this one focusing on natalizumab (Tysabri) as a tool for induction of remission of CD – was more conclusive. It examined five trials and found that “high quality data suggest that natalizumab is effective for induction of clinical remission and response in some patients with moderately to severely active CD”(Cochrane Database Syst Rev. 2018 Aug 1. doi: 10.1002/14651858.CD006097.pub3).
However, the review noted that none of the studies was high powered enough to detect rare serious adverse effects such as progressive multifocal leukoencephalopathy (PML). “Due to the association with PML, and the availability of alternative agents that are not associated with PML, natalizumab is not likely to be used in patients who fail currently available medical therapy,” the reviewers wrote. “Further studies of natalizumab are not likely to be done.”
Dr. Gordon reports unrestricted travel grants over the past 3 years from Ferring, Synergy, Tillotts, and BioGaia. He holds a National Institute for Health Research Cochrane IBD Program Grant.
EXPERT ANALYSIS FROM THE CROHN’S & COLITIS CONGRESS
TNF inhibitor prices rose despite increased drug class competition
according to a new analysis of Medicare claims data and wholesale acquisition costs published online in JAMA Internal Medicine.
A research team led by Alvaro San-Juan-Rodriguez, PharmD, of the University of Pittsburgh said their analysis illustrates “a market failure contributing to the rising costs of prescription drugs.”
Before 2009, etanercept (Enbrel), infliximab (Remicade), and adalimumab (Humira) were the only tumor necrosis factor (TNF) inhibitors approved by the Food and Drug Administration for treating rheumatoid arthritis; infliximab and adalimumab are also approved to treat inflammatory bowel disease. In 2009, subcutaneous golimumab (Simponi) and certolizumab pegol (Cimzia) entered the market, followed by intravenous golimumab (Simponi ARIA) in 2013.
The researchers used an interrupted time series analysis with a linear model that “regressed the annual cost of existing TNF inhibitors against a continuous variable for month, two indicator variables for each period after market entry of new drugs, and the interactions between them.”
Using estimates from this model, the researchers calculated the trends in costs that would have been expected if new anti-TNFs had not entered the market. They examined costs for TNF inhibitors typically reimbursed under Medicare Part D (Enbrel, Humira, Simponi, and Cimzia) and adjusted the data for increases in manufacturer rebates, but “owing to lack of data,” they could not “assess how purchasing prices for drugs typically reimbursed under Medicare Part B [Remicade and Simponi ARIA] changed over time.” All estimates for annual costs of treatment were based on dosing recommendations for a standard 80-kg patient with rheumatoid arthritis.
The annual treatment costs with existing TNF inhibitors increased after the three new agents entered the market. For example, when wholesale acquisition cost data was applied, annual treatment costs with existing TNF inhibitors increased by 144% from April 2009 to December 2016 after new drug entry (from $15.809 to $38,574). However, in the absence of new drugs’ entry, the researchers estimated that annual treatment costs would have increased by 34% (from $15,809 to $21,184).
Medicare annual treatment costs increased by 139% (from $14,901 to $35,613), compared with a 43% increase expected in the absence of new drugs’ entry (from $14,901 to $21,308). Medicare spending increased in parallel with increases in annual treatment costs, but out-of-pocket costs and manufacturer coverage gap discounts remained relatively constant over time.
The research team noted that if cost trends had not changed after the entry of new products, the costs of Enbrel, Remicade, and Humira in December 2016 would have been 40%-45% lower.
“These increases were born solely by Medicare, while patient out-of-pocket spending remained flat. In addition, these increases were not offset by manufacturer discounts in the Medicare Part D coverage gap. The rising costs of existing products may reflect manufacturers’ opportunism in response to payers’ increased willingness to pay for TNF inhibitors after market entry of new, more expensive agents,” the research team noted.
The study was funded in part by the Myers Family Foundation and one author reported funding from the National Heart, Lung, and Blood Institute.
SOURCE: San-Juan-Rodriguez A et al. JAMA Intern Med. 2019 Feb 18. doi: 10.1001/jamainternmed.2018.7656
according to a new analysis of Medicare claims data and wholesale acquisition costs published online in JAMA Internal Medicine.
A research team led by Alvaro San-Juan-Rodriguez, PharmD, of the University of Pittsburgh said their analysis illustrates “a market failure contributing to the rising costs of prescription drugs.”
Before 2009, etanercept (Enbrel), infliximab (Remicade), and adalimumab (Humira) were the only tumor necrosis factor (TNF) inhibitors approved by the Food and Drug Administration for treating rheumatoid arthritis; infliximab and adalimumab are also approved to treat inflammatory bowel disease. In 2009, subcutaneous golimumab (Simponi) and certolizumab pegol (Cimzia) entered the market, followed by intravenous golimumab (Simponi ARIA) in 2013.
The researchers used an interrupted time series analysis with a linear model that “regressed the annual cost of existing TNF inhibitors against a continuous variable for month, two indicator variables for each period after market entry of new drugs, and the interactions between them.”
Using estimates from this model, the researchers calculated the trends in costs that would have been expected if new anti-TNFs had not entered the market. They examined costs for TNF inhibitors typically reimbursed under Medicare Part D (Enbrel, Humira, Simponi, and Cimzia) and adjusted the data for increases in manufacturer rebates, but “owing to lack of data,” they could not “assess how purchasing prices for drugs typically reimbursed under Medicare Part B [Remicade and Simponi ARIA] changed over time.” All estimates for annual costs of treatment were based on dosing recommendations for a standard 80-kg patient with rheumatoid arthritis.
The annual treatment costs with existing TNF inhibitors increased after the three new agents entered the market. For example, when wholesale acquisition cost data was applied, annual treatment costs with existing TNF inhibitors increased by 144% from April 2009 to December 2016 after new drug entry (from $15.809 to $38,574). However, in the absence of new drugs’ entry, the researchers estimated that annual treatment costs would have increased by 34% (from $15,809 to $21,184).
Medicare annual treatment costs increased by 139% (from $14,901 to $35,613), compared with a 43% increase expected in the absence of new drugs’ entry (from $14,901 to $21,308). Medicare spending increased in parallel with increases in annual treatment costs, but out-of-pocket costs and manufacturer coverage gap discounts remained relatively constant over time.
The research team noted that if cost trends had not changed after the entry of new products, the costs of Enbrel, Remicade, and Humira in December 2016 would have been 40%-45% lower.
“These increases were born solely by Medicare, while patient out-of-pocket spending remained flat. In addition, these increases were not offset by manufacturer discounts in the Medicare Part D coverage gap. The rising costs of existing products may reflect manufacturers’ opportunism in response to payers’ increased willingness to pay for TNF inhibitors after market entry of new, more expensive agents,” the research team noted.
The study was funded in part by the Myers Family Foundation and one author reported funding from the National Heart, Lung, and Blood Institute.
SOURCE: San-Juan-Rodriguez A et al. JAMA Intern Med. 2019 Feb 18. doi: 10.1001/jamainternmed.2018.7656
according to a new analysis of Medicare claims data and wholesale acquisition costs published online in JAMA Internal Medicine.
A research team led by Alvaro San-Juan-Rodriguez, PharmD, of the University of Pittsburgh said their analysis illustrates “a market failure contributing to the rising costs of prescription drugs.”
Before 2009, etanercept (Enbrel), infliximab (Remicade), and adalimumab (Humira) were the only tumor necrosis factor (TNF) inhibitors approved by the Food and Drug Administration for treating rheumatoid arthritis; infliximab and adalimumab are also approved to treat inflammatory bowel disease. In 2009, subcutaneous golimumab (Simponi) and certolizumab pegol (Cimzia) entered the market, followed by intravenous golimumab (Simponi ARIA) in 2013.
The researchers used an interrupted time series analysis with a linear model that “regressed the annual cost of existing TNF inhibitors against a continuous variable for month, two indicator variables for each period after market entry of new drugs, and the interactions between them.”
Using estimates from this model, the researchers calculated the trends in costs that would have been expected if new anti-TNFs had not entered the market. They examined costs for TNF inhibitors typically reimbursed under Medicare Part D (Enbrel, Humira, Simponi, and Cimzia) and adjusted the data for increases in manufacturer rebates, but “owing to lack of data,” they could not “assess how purchasing prices for drugs typically reimbursed under Medicare Part B [Remicade and Simponi ARIA] changed over time.” All estimates for annual costs of treatment were based on dosing recommendations for a standard 80-kg patient with rheumatoid arthritis.
The annual treatment costs with existing TNF inhibitors increased after the three new agents entered the market. For example, when wholesale acquisition cost data was applied, annual treatment costs with existing TNF inhibitors increased by 144% from April 2009 to December 2016 after new drug entry (from $15.809 to $38,574). However, in the absence of new drugs’ entry, the researchers estimated that annual treatment costs would have increased by 34% (from $15,809 to $21,184).
Medicare annual treatment costs increased by 139% (from $14,901 to $35,613), compared with a 43% increase expected in the absence of new drugs’ entry (from $14,901 to $21,308). Medicare spending increased in parallel with increases in annual treatment costs, but out-of-pocket costs and manufacturer coverage gap discounts remained relatively constant over time.
The research team noted that if cost trends had not changed after the entry of new products, the costs of Enbrel, Remicade, and Humira in December 2016 would have been 40%-45% lower.
“These increases were born solely by Medicare, while patient out-of-pocket spending remained flat. In addition, these increases were not offset by manufacturer discounts in the Medicare Part D coverage gap. The rising costs of existing products may reflect manufacturers’ opportunism in response to payers’ increased willingness to pay for TNF inhibitors after market entry of new, more expensive agents,” the research team noted.
The study was funded in part by the Myers Family Foundation and one author reported funding from the National Heart, Lung, and Blood Institute.
SOURCE: San-Juan-Rodriguez A et al. JAMA Intern Med. 2019 Feb 18. doi: 10.1001/jamainternmed.2018.7656
FROM JAMA INTERNAL MEDICINE
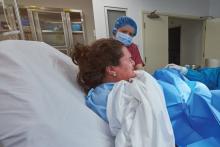
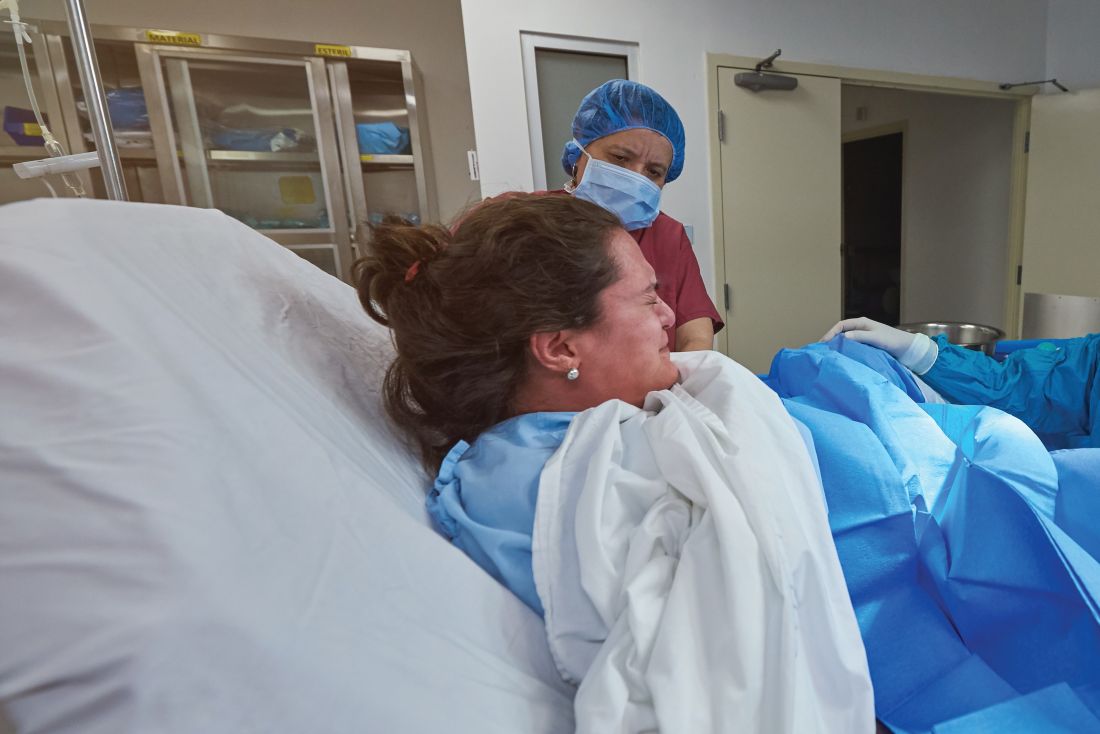
Dental device borrowed from sports world no help in pushing
LAS VEGAS –
In a randomized, controlled trial of 346 nulliparous women, the device, adapted from a design used in athletics, made no difference in the duration of the second stage of labor overall or in passive descent or active pushing time. The findings were presented on behalf of first author Eric Bergh, MD, by Patricia Rekawek, MD, at a fellows abstract session at the meeting sponsored by the Society for Maternal-Fetal Medicine. Dr. Rekawek and Dr. Bergh are maternal-fetal medicine fellows at the Icahn School of Medicine at Mount Sinai, New York.
The dental support device (DSD) was actually used by 128 (74%) of the patients who were randomized to DSD usage. Of these patients, two thirds (n = 85) used the device for all second stage contractions. About one quarter (27%; n = 35) used it less than half the time, and a small minority (6%; n = 8) used it more than half the time but not for all pushing, noted Dr. Rekawek.
Most women (61%) who used the device agreed or strongly agreed that it was helpful. Most also found it comfortable (67%), and would use it again in a future delivery (61%). Having a custom-fit DSD, or spending more time practicing before labor, might help with efficacy in shortening the second stage of labor and merits study, the investigators said.
Reducing time spent in the second stage of labor could benefit both mother and neonate because “a prolonged second stage of labor is associated with multiple maternal and neonatal complications,” including increased risk for chorioamnionitis, neonatal sepsis, low umbilical artery pH, shoulder dystocia, and third- and fourth-degree lacerations, said Dr. Rekawek.
In athletics, a DSD “raises the vertical dimension of the deep bite and increases the size of the oropharynx” by introducing a 3-mm bite plate between the upper and lower rear molars. The DSD mouthpiece integrates the bite plates with a retainer-like band that wraps around the front incisors; speaking and drinking are possible with the DSD in place, said Dr. Rekawek.
She added that athletic evidence suggests that the DSD can lead to increased oxygenation, less head and neck tension, less muscle fatigue, and improved exercise capacity in some sports.
If these benefits translated to the maternal Valsalva maneuver during the second stage of labor, the increased isometric endurance could increase uterine pressure and optimize the expulsive effect of pushing, explained Dr. Rekawek. However, mixed results were seen in previous work that had women using a DSD during the second stage of labor.
The current study was powered to detect a difference of 20% in the duration of the second stage of labor for nulliparous women who were randomized either to use or not use a DSD while pushing. Women and their caregivers learned their random allocation by opening a sealed envelope at the beginning of the second stage of labor.
The study included nulliparous women who were carrying a singleton pregnancy of at least 37 weeks’ gestation and without known fetal anomalies. Enrollment occurred before the second stage of labor, and 173 women participated in each arm of the study.
For those who were assigned to use the DSD, the second stage of labor lasted a median 84 minutes, whereas the median for the control group was 92 minutes (P = .11) in an intention-to-treat analysis. Passive descent and active pushing lasted a median 14 and 50 minutes, respectively, for the DSD group, compared with 13 and 55 minutes for the control group (P = .22 and P = .29, respectively).
Another analysis of the primary outcome measure used Kaplan-Meier curves to compare the curves of the women who remained pregnant in each group across the period of the second stage of labor. There was no significant difference between the groups when the data were examined in this manner (log rank P = .18).
Secondary maternal outcomes included the mode of delivery. Most women (83%-85%) in each group had a normal spontaneous vaginal delivery, with no differences in any mode of delivery (P = .93). Neonatal outcomes also were similar between groups, with no significant differences in sex, birth weight, arterial pH, and neonatal intensive care unit admissions.
The investigators tracked whether patients were admitted in spontaneous labor, for labor induction with active membranes, or with premature rupture of membranes. Also, they looked at the degree of cervical dilation at admission, whether oxytocin or epidural anesthesia were used in labor, and anesthetic administered during the second stage of labor. There were no differences between groups in these variables.
Dr. Rekawek noted that the study benefited from a large sample size and a prospective, randomized design. Blinding lasted only until pushing began. Also, although all patients were taught how to use the device, they didn’t have an opportunity to practice.
Dr. Rekawek presented on behalf of Dr. Bergh because he became a new father the day before the presentation. His wife, said Dr. Rekawek, didn’t use a dental support device.
The authors reported that they have no relevant financial disclosures.
SOURCE: Bergh E et al. Am J Obstet Gynecol. 2019 Jan;220(1):S39, Abstract 49.
LAS VEGAS –
In a randomized, controlled trial of 346 nulliparous women, the device, adapted from a design used in athletics, made no difference in the duration of the second stage of labor overall or in passive descent or active pushing time. The findings were presented on behalf of first author Eric Bergh, MD, by Patricia Rekawek, MD, at a fellows abstract session at the meeting sponsored by the Society for Maternal-Fetal Medicine. Dr. Rekawek and Dr. Bergh are maternal-fetal medicine fellows at the Icahn School of Medicine at Mount Sinai, New York.
The dental support device (DSD) was actually used by 128 (74%) of the patients who were randomized to DSD usage. Of these patients, two thirds (n = 85) used the device for all second stage contractions. About one quarter (27%; n = 35) used it less than half the time, and a small minority (6%; n = 8) used it more than half the time but not for all pushing, noted Dr. Rekawek.
Most women (61%) who used the device agreed or strongly agreed that it was helpful. Most also found it comfortable (67%), and would use it again in a future delivery (61%). Having a custom-fit DSD, or spending more time practicing before labor, might help with efficacy in shortening the second stage of labor and merits study, the investigators said.
Reducing time spent in the second stage of labor could benefit both mother and neonate because “a prolonged second stage of labor is associated with multiple maternal and neonatal complications,” including increased risk for chorioamnionitis, neonatal sepsis, low umbilical artery pH, shoulder dystocia, and third- and fourth-degree lacerations, said Dr. Rekawek.
In athletics, a DSD “raises the vertical dimension of the deep bite and increases the size of the oropharynx” by introducing a 3-mm bite plate between the upper and lower rear molars. The DSD mouthpiece integrates the bite plates with a retainer-like band that wraps around the front incisors; speaking and drinking are possible with the DSD in place, said Dr. Rekawek.
She added that athletic evidence suggests that the DSD can lead to increased oxygenation, less head and neck tension, less muscle fatigue, and improved exercise capacity in some sports.
If these benefits translated to the maternal Valsalva maneuver during the second stage of labor, the increased isometric endurance could increase uterine pressure and optimize the expulsive effect of pushing, explained Dr. Rekawek. However, mixed results were seen in previous work that had women using a DSD during the second stage of labor.
The current study was powered to detect a difference of 20% in the duration of the second stage of labor for nulliparous women who were randomized either to use or not use a DSD while pushing. Women and their caregivers learned their random allocation by opening a sealed envelope at the beginning of the second stage of labor.
The study included nulliparous women who were carrying a singleton pregnancy of at least 37 weeks’ gestation and without known fetal anomalies. Enrollment occurred before the second stage of labor, and 173 women participated in each arm of the study.
For those who were assigned to use the DSD, the second stage of labor lasted a median 84 minutes, whereas the median for the control group was 92 minutes (P = .11) in an intention-to-treat analysis. Passive descent and active pushing lasted a median 14 and 50 minutes, respectively, for the DSD group, compared with 13 and 55 minutes for the control group (P = .22 and P = .29, respectively).
Another analysis of the primary outcome measure used Kaplan-Meier curves to compare the curves of the women who remained pregnant in each group across the period of the second stage of labor. There was no significant difference between the groups when the data were examined in this manner (log rank P = .18).
Secondary maternal outcomes included the mode of delivery. Most women (83%-85%) in each group had a normal spontaneous vaginal delivery, with no differences in any mode of delivery (P = .93). Neonatal outcomes also were similar between groups, with no significant differences in sex, birth weight, arterial pH, and neonatal intensive care unit admissions.
The investigators tracked whether patients were admitted in spontaneous labor, for labor induction with active membranes, or with premature rupture of membranes. Also, they looked at the degree of cervical dilation at admission, whether oxytocin or epidural anesthesia were used in labor, and anesthetic administered during the second stage of labor. There were no differences between groups in these variables.
Dr. Rekawek noted that the study benefited from a large sample size and a prospective, randomized design. Blinding lasted only until pushing began. Also, although all patients were taught how to use the device, they didn’t have an opportunity to practice.
Dr. Rekawek presented on behalf of Dr. Bergh because he became a new father the day before the presentation. His wife, said Dr. Rekawek, didn’t use a dental support device.
The authors reported that they have no relevant financial disclosures.
SOURCE: Bergh E et al. Am J Obstet Gynecol. 2019 Jan;220(1):S39, Abstract 49.
LAS VEGAS –
In a randomized, controlled trial of 346 nulliparous women, the device, adapted from a design used in athletics, made no difference in the duration of the second stage of labor overall or in passive descent or active pushing time. The findings were presented on behalf of first author Eric Bergh, MD, by Patricia Rekawek, MD, at a fellows abstract session at the meeting sponsored by the Society for Maternal-Fetal Medicine. Dr. Rekawek and Dr. Bergh are maternal-fetal medicine fellows at the Icahn School of Medicine at Mount Sinai, New York.
The dental support device (DSD) was actually used by 128 (74%) of the patients who were randomized to DSD usage. Of these patients, two thirds (n = 85) used the device for all second stage contractions. About one quarter (27%; n = 35) used it less than half the time, and a small minority (6%; n = 8) used it more than half the time but not for all pushing, noted Dr. Rekawek.
Most women (61%) who used the device agreed or strongly agreed that it was helpful. Most also found it comfortable (67%), and would use it again in a future delivery (61%). Having a custom-fit DSD, or spending more time practicing before labor, might help with efficacy in shortening the second stage of labor and merits study, the investigators said.
Reducing time spent in the second stage of labor could benefit both mother and neonate because “a prolonged second stage of labor is associated with multiple maternal and neonatal complications,” including increased risk for chorioamnionitis, neonatal sepsis, low umbilical artery pH, shoulder dystocia, and third- and fourth-degree lacerations, said Dr. Rekawek.
In athletics, a DSD “raises the vertical dimension of the deep bite and increases the size of the oropharynx” by introducing a 3-mm bite plate between the upper and lower rear molars. The DSD mouthpiece integrates the bite plates with a retainer-like band that wraps around the front incisors; speaking and drinking are possible with the DSD in place, said Dr. Rekawek.
She added that athletic evidence suggests that the DSD can lead to increased oxygenation, less head and neck tension, less muscle fatigue, and improved exercise capacity in some sports.
If these benefits translated to the maternal Valsalva maneuver during the second stage of labor, the increased isometric endurance could increase uterine pressure and optimize the expulsive effect of pushing, explained Dr. Rekawek. However, mixed results were seen in previous work that had women using a DSD during the second stage of labor.
The current study was powered to detect a difference of 20% in the duration of the second stage of labor for nulliparous women who were randomized either to use or not use a DSD while pushing. Women and their caregivers learned their random allocation by opening a sealed envelope at the beginning of the second stage of labor.
The study included nulliparous women who were carrying a singleton pregnancy of at least 37 weeks’ gestation and without known fetal anomalies. Enrollment occurred before the second stage of labor, and 173 women participated in each arm of the study.
For those who were assigned to use the DSD, the second stage of labor lasted a median 84 minutes, whereas the median for the control group was 92 minutes (P = .11) in an intention-to-treat analysis. Passive descent and active pushing lasted a median 14 and 50 minutes, respectively, for the DSD group, compared with 13 and 55 minutes for the control group (P = .22 and P = .29, respectively).
Another analysis of the primary outcome measure used Kaplan-Meier curves to compare the curves of the women who remained pregnant in each group across the period of the second stage of labor. There was no significant difference between the groups when the data were examined in this manner (log rank P = .18).
Secondary maternal outcomes included the mode of delivery. Most women (83%-85%) in each group had a normal spontaneous vaginal delivery, with no differences in any mode of delivery (P = .93). Neonatal outcomes also were similar between groups, with no significant differences in sex, birth weight, arterial pH, and neonatal intensive care unit admissions.
The investigators tracked whether patients were admitted in spontaneous labor, for labor induction with active membranes, or with premature rupture of membranes. Also, they looked at the degree of cervical dilation at admission, whether oxytocin or epidural anesthesia were used in labor, and anesthetic administered during the second stage of labor. There were no differences between groups in these variables.
Dr. Rekawek noted that the study benefited from a large sample size and a prospective, randomized design. Blinding lasted only until pushing began. Also, although all patients were taught how to use the device, they didn’t have an opportunity to practice.
Dr. Rekawek presented on behalf of Dr. Bergh because he became a new father the day before the presentation. His wife, said Dr. Rekawek, didn’t use a dental support device.
The authors reported that they have no relevant financial disclosures.
SOURCE: Bergh E et al. Am J Obstet Gynecol. 2019 Jan;220(1):S39, Abstract 49.
REPORTING FROM THE PREGNANCY MEETING
ARAMIS: Darolutamide shines in nonmetastatic CRPC
SAN FRANCISCO – Darolutamide, a novel investigational antiandrogen agent, is efficacious and well tolerated when used to treat nonmetastatic, castration-resistant prostate cancer (nmCRPC), according to results of the phase 3, randomized, controlled ARAMIS trial.
“In men with high-risk M0 CRPC, two next-generation androgen receptor inhibitors, namely, enzalutamide (Xtandi) and apalutamide (Erleada), were recently shown to improve metastasis-free survival, although they were associated with increased cognitive impairment, falls, and other side effects,” commented lead investigator Karim Fizazi, MD, PhD, head of the department of cancer medicine at the Institut Gustave Roussy in Paris. “Enzalutamide and apalutamide are chemically very similar, while darolutamide is structurally distinct. Also, darolutamide does not cross the blood-brain barrier, which may result in less CNS-related side effects.”
ARAMIS enrolled 1,509 men with nmCRPC who had a prostate-specific antigen (PSA) doubling time of 10 months or less and were on and continued androgen deprivation therapy. The men were randomized 2:1 to receive darolutamide or placebo.
With a median follow-up of 17.9 months, median metastasis-free survival was almost 2 years longer with darolutamide, corresponding to a 59% reduction in the risk of distant metastases or death, relative to placebo, according to results reported at the symposium and simultaneously published in the New England Journal of Medicine (2019 Feb 14. doi: 10.1056/NEJMoa1815671). There was also a 29% reduction in risk of death (in an interim analysis), a 35% reduction in risk of pain progression, and 57% reductions each in need for chemotherapy and first symptomatic skeletal events. Meanwhile, the drug had a good safety and tolerability profile, with rates and types of events similar to those seen with placebo.
“We believe that darolutamide should become a new standard of care for men with high-risk nmCRPC,” Dr. Fizazi concluded.
Practice-changing results?
The ARAMIS findings meet some – but not all – of a set of criteria that would support a change in current practice to using darolutamide, according to invited discussant Ian D. Davis, MBBS, PhD, a professor at Monash University, Melbourne.
The first criterion, whether the disease is a condition needing treatment, is likely met, as 69% of patients had a PSA doubling time of 6 months or less, and previous research suggests that this subset, at least, has high risk for bone metastases or death.
A second criterion is whether metastasis-free survival is a meaningful endpoint. “It is according to the FDA [Food and Drug Administration],” Dr. Davis said, noting that they now accept it as a registrable endpoint in nmCRPC if it is supported by positive secondary endpoints. “But are we talking about a sensitivity question here, is M0 a rapidly disappearing condition,” given that new imaging technologies often do reveal metastases in this population? “And is metastasis-free survival truly a surrogate for overall survival? I think those questions are still open, and we need to use our own judgment.”
Darolutamide appears to have acceptable toxicity, a third criterion, but information regarding its impact on subsequent treatment efficacy, a fourth criterion, is still lacking.
The fifth and final criterion, cost-effectiveness, can be assessed using the incremental cost-effectiveness ratio. But because overall survival benefit and price of darolutamide are still unknown, calculations of cost per life-year saved are not yet possible.
“So at the moment, I think it’s unclear whether ARAMIS should change practice,” Dr. Davis concluded. “I would certainly be prepared to change my conclusions on this with more information and further follow-up.”
Study details
The men randomized in ARAMIS had a median PSA doubling time of roughly 4.5 months, Dr. Fizazi reported at the 2019 Genitourinary Cancers Symposium sponsored by the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Urologic Oncology. Only about 5% were receiving a bone-sparing agent.
Median metastasis-free survival was 40.4 months with darolutamide and 18.4 months with placebo (hazard ratio, 0.41; P less than .0001). Benefit was similar across a range of subgroups.
In an interim analysis, median overall survival was not reached in either group, but the 3-year rate was 83% with darolutamide and 73% with placebo (hazard ratio, 0.71; P = .0452). The drug also was superior in terms of progression-free survival (36.8 vs. 14.8 months; HR, 0.38; P less than .0001), time to pain progression (40.3 vs. 25.4 months; HR, 0.65; P less than .0001), time to cytotoxic chemotherapy (not reached vs. 38.2 months; HR, 0.43; P less than .0001), and time to first symptomatic skeletal event (not reached in either group; HR, 0.43; P = .0113).
Patients in the darolutamide and placebo groups had similar rates of treatment discontinuation because of any-grade treatment-emergent adverse events (8.9% vs. 8.7%) and because of grade 3 or 4 treatment-emergent adverse events (3.3% vs. 4.3%). The former had a higher rate of any-grade fatigue/asthenia (15.8% vs. 11.4%), but this difference was no longer evident after adjustment for duration of exposure, according to Dr. Fizazi. Notably, the groups were similar on rates of bone fractures, falls, cognitive disorders, seizures, hypertension, and coronary artery disorders.
Finally, darolutamide was also associated with better health-related quality of life, with men in that group having lower scores for pain interference, pain severity, and urinary symptoms (P less than .01 for all).
Dr. Fizazi reported that he receives honoraria from Astellas Pharma, Janssen, Merck, and Sanofi; that he has a consulting or advisory role with Amgen, Astellas Pharma, AstraZeneca, Bayer, Clovis Oncology, CureVac, ESSA, Janssen Oncology, Orion Pharma, Roche/Genentech, and Sanofi; and that he receives travel, accommodations, and/or expenses from Amgen and Janssen. The trial was sponsored by Bayer.
SOURCE: Fizazi K et al. GUCS 2019, Abstract 140.
SAN FRANCISCO – Darolutamide, a novel investigational antiandrogen agent, is efficacious and well tolerated when used to treat nonmetastatic, castration-resistant prostate cancer (nmCRPC), according to results of the phase 3, randomized, controlled ARAMIS trial.
“In men with high-risk M0 CRPC, two next-generation androgen receptor inhibitors, namely, enzalutamide (Xtandi) and apalutamide (Erleada), were recently shown to improve metastasis-free survival, although they were associated with increased cognitive impairment, falls, and other side effects,” commented lead investigator Karim Fizazi, MD, PhD, head of the department of cancer medicine at the Institut Gustave Roussy in Paris. “Enzalutamide and apalutamide are chemically very similar, while darolutamide is structurally distinct. Also, darolutamide does not cross the blood-brain barrier, which may result in less CNS-related side effects.”
ARAMIS enrolled 1,509 men with nmCRPC who had a prostate-specific antigen (PSA) doubling time of 10 months or less and were on and continued androgen deprivation therapy. The men were randomized 2:1 to receive darolutamide or placebo.
With a median follow-up of 17.9 months, median metastasis-free survival was almost 2 years longer with darolutamide, corresponding to a 59% reduction in the risk of distant metastases or death, relative to placebo, according to results reported at the symposium and simultaneously published in the New England Journal of Medicine (2019 Feb 14. doi: 10.1056/NEJMoa1815671). There was also a 29% reduction in risk of death (in an interim analysis), a 35% reduction in risk of pain progression, and 57% reductions each in need for chemotherapy and first symptomatic skeletal events. Meanwhile, the drug had a good safety and tolerability profile, with rates and types of events similar to those seen with placebo.
“We believe that darolutamide should become a new standard of care for men with high-risk nmCRPC,” Dr. Fizazi concluded.
Practice-changing results?
The ARAMIS findings meet some – but not all – of a set of criteria that would support a change in current practice to using darolutamide, according to invited discussant Ian D. Davis, MBBS, PhD, a professor at Monash University, Melbourne.
The first criterion, whether the disease is a condition needing treatment, is likely met, as 69% of patients had a PSA doubling time of 6 months or less, and previous research suggests that this subset, at least, has high risk for bone metastases or death.
A second criterion is whether metastasis-free survival is a meaningful endpoint. “It is according to the FDA [Food and Drug Administration],” Dr. Davis said, noting that they now accept it as a registrable endpoint in nmCRPC if it is supported by positive secondary endpoints. “But are we talking about a sensitivity question here, is M0 a rapidly disappearing condition,” given that new imaging technologies often do reveal metastases in this population? “And is metastasis-free survival truly a surrogate for overall survival? I think those questions are still open, and we need to use our own judgment.”
Darolutamide appears to have acceptable toxicity, a third criterion, but information regarding its impact on subsequent treatment efficacy, a fourth criterion, is still lacking.
The fifth and final criterion, cost-effectiveness, can be assessed using the incremental cost-effectiveness ratio. But because overall survival benefit and price of darolutamide are still unknown, calculations of cost per life-year saved are not yet possible.
“So at the moment, I think it’s unclear whether ARAMIS should change practice,” Dr. Davis concluded. “I would certainly be prepared to change my conclusions on this with more information and further follow-up.”
Study details
The men randomized in ARAMIS had a median PSA doubling time of roughly 4.5 months, Dr. Fizazi reported at the 2019 Genitourinary Cancers Symposium sponsored by the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Urologic Oncology. Only about 5% were receiving a bone-sparing agent.
Median metastasis-free survival was 40.4 months with darolutamide and 18.4 months with placebo (hazard ratio, 0.41; P less than .0001). Benefit was similar across a range of subgroups.
In an interim analysis, median overall survival was not reached in either group, but the 3-year rate was 83% with darolutamide and 73% with placebo (hazard ratio, 0.71; P = .0452). The drug also was superior in terms of progression-free survival (36.8 vs. 14.8 months; HR, 0.38; P less than .0001), time to pain progression (40.3 vs. 25.4 months; HR, 0.65; P less than .0001), time to cytotoxic chemotherapy (not reached vs. 38.2 months; HR, 0.43; P less than .0001), and time to first symptomatic skeletal event (not reached in either group; HR, 0.43; P = .0113).
Patients in the darolutamide and placebo groups had similar rates of treatment discontinuation because of any-grade treatment-emergent adverse events (8.9% vs. 8.7%) and because of grade 3 or 4 treatment-emergent adverse events (3.3% vs. 4.3%). The former had a higher rate of any-grade fatigue/asthenia (15.8% vs. 11.4%), but this difference was no longer evident after adjustment for duration of exposure, according to Dr. Fizazi. Notably, the groups were similar on rates of bone fractures, falls, cognitive disorders, seizures, hypertension, and coronary artery disorders.
Finally, darolutamide was also associated with better health-related quality of life, with men in that group having lower scores for pain interference, pain severity, and urinary symptoms (P less than .01 for all).
Dr. Fizazi reported that he receives honoraria from Astellas Pharma, Janssen, Merck, and Sanofi; that he has a consulting or advisory role with Amgen, Astellas Pharma, AstraZeneca, Bayer, Clovis Oncology, CureVac, ESSA, Janssen Oncology, Orion Pharma, Roche/Genentech, and Sanofi; and that he receives travel, accommodations, and/or expenses from Amgen and Janssen. The trial was sponsored by Bayer.
SOURCE: Fizazi K et al. GUCS 2019, Abstract 140.
SAN FRANCISCO – Darolutamide, a novel investigational antiandrogen agent, is efficacious and well tolerated when used to treat nonmetastatic, castration-resistant prostate cancer (nmCRPC), according to results of the phase 3, randomized, controlled ARAMIS trial.
“In men with high-risk M0 CRPC, two next-generation androgen receptor inhibitors, namely, enzalutamide (Xtandi) and apalutamide (Erleada), were recently shown to improve metastasis-free survival, although they were associated with increased cognitive impairment, falls, and other side effects,” commented lead investigator Karim Fizazi, MD, PhD, head of the department of cancer medicine at the Institut Gustave Roussy in Paris. “Enzalutamide and apalutamide are chemically very similar, while darolutamide is structurally distinct. Also, darolutamide does not cross the blood-brain barrier, which may result in less CNS-related side effects.”
ARAMIS enrolled 1,509 men with nmCRPC who had a prostate-specific antigen (PSA) doubling time of 10 months or less and were on and continued androgen deprivation therapy. The men were randomized 2:1 to receive darolutamide or placebo.
With a median follow-up of 17.9 months, median metastasis-free survival was almost 2 years longer with darolutamide, corresponding to a 59% reduction in the risk of distant metastases or death, relative to placebo, according to results reported at the symposium and simultaneously published in the New England Journal of Medicine (2019 Feb 14. doi: 10.1056/NEJMoa1815671). There was also a 29% reduction in risk of death (in an interim analysis), a 35% reduction in risk of pain progression, and 57% reductions each in need for chemotherapy and first symptomatic skeletal events. Meanwhile, the drug had a good safety and tolerability profile, with rates and types of events similar to those seen with placebo.
“We believe that darolutamide should become a new standard of care for men with high-risk nmCRPC,” Dr. Fizazi concluded.
Practice-changing results?
The ARAMIS findings meet some – but not all – of a set of criteria that would support a change in current practice to using darolutamide, according to invited discussant Ian D. Davis, MBBS, PhD, a professor at Monash University, Melbourne.
The first criterion, whether the disease is a condition needing treatment, is likely met, as 69% of patients had a PSA doubling time of 6 months or less, and previous research suggests that this subset, at least, has high risk for bone metastases or death.
A second criterion is whether metastasis-free survival is a meaningful endpoint. “It is according to the FDA [Food and Drug Administration],” Dr. Davis said, noting that they now accept it as a registrable endpoint in nmCRPC if it is supported by positive secondary endpoints. “But are we talking about a sensitivity question here, is M0 a rapidly disappearing condition,” given that new imaging technologies often do reveal metastases in this population? “And is metastasis-free survival truly a surrogate for overall survival? I think those questions are still open, and we need to use our own judgment.”
Darolutamide appears to have acceptable toxicity, a third criterion, but information regarding its impact on subsequent treatment efficacy, a fourth criterion, is still lacking.
The fifth and final criterion, cost-effectiveness, can be assessed using the incremental cost-effectiveness ratio. But because overall survival benefit and price of darolutamide are still unknown, calculations of cost per life-year saved are not yet possible.
“So at the moment, I think it’s unclear whether ARAMIS should change practice,” Dr. Davis concluded. “I would certainly be prepared to change my conclusions on this with more information and further follow-up.”
Study details
The men randomized in ARAMIS had a median PSA doubling time of roughly 4.5 months, Dr. Fizazi reported at the 2019 Genitourinary Cancers Symposium sponsored by the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Urologic Oncology. Only about 5% were receiving a bone-sparing agent.
Median metastasis-free survival was 40.4 months with darolutamide and 18.4 months with placebo (hazard ratio, 0.41; P less than .0001). Benefit was similar across a range of subgroups.
In an interim analysis, median overall survival was not reached in either group, but the 3-year rate was 83% with darolutamide and 73% with placebo (hazard ratio, 0.71; P = .0452). The drug also was superior in terms of progression-free survival (36.8 vs. 14.8 months; HR, 0.38; P less than .0001), time to pain progression (40.3 vs. 25.4 months; HR, 0.65; P less than .0001), time to cytotoxic chemotherapy (not reached vs. 38.2 months; HR, 0.43; P less than .0001), and time to first symptomatic skeletal event (not reached in either group; HR, 0.43; P = .0113).
Patients in the darolutamide and placebo groups had similar rates of treatment discontinuation because of any-grade treatment-emergent adverse events (8.9% vs. 8.7%) and because of grade 3 or 4 treatment-emergent adverse events (3.3% vs. 4.3%). The former had a higher rate of any-grade fatigue/asthenia (15.8% vs. 11.4%), but this difference was no longer evident after adjustment for duration of exposure, according to Dr. Fizazi. Notably, the groups were similar on rates of bone fractures, falls, cognitive disorders, seizures, hypertension, and coronary artery disorders.
Finally, darolutamide was also associated with better health-related quality of life, with men in that group having lower scores for pain interference, pain severity, and urinary symptoms (P less than .01 for all).
Dr. Fizazi reported that he receives honoraria from Astellas Pharma, Janssen, Merck, and Sanofi; that he has a consulting or advisory role with Amgen, Astellas Pharma, AstraZeneca, Bayer, Clovis Oncology, CureVac, ESSA, Janssen Oncology, Orion Pharma, Roche/Genentech, and Sanofi; and that he receives travel, accommodations, and/or expenses from Amgen and Janssen. The trial was sponsored by Bayer.
SOURCE: Fizazi K et al. GUCS 2019, Abstract 140.
REPORTING FROM GUCS 2019
Diagnostic metal rod, eyeball extramission, fungal foot fetish
Doctor, (fake) doctor, gimme the news
Florida Man strikes again, and this time he’s a faux MD.
Onelio Hipolit-Gonzalez was charged with a felony for impersonating a doctor who promised patients he could easily diagnose their diabetes, cancer, multiple sclerosis, Parkinson’s, and pretty much any other malady … with a metal rod. The “doctor” would have patients hold on to a metal rod connected to a beeping machine (these are the scientific terms, of course), and then he would gravely diagnose them with a variety of ailments that he could cure for the low, low price of $2,000.
If you’re curious how he treated patients, fear not. This intrepid medical professional took the ingenious measure of drawing a patient’s blood and simply injecting it back inside them. Bada-bing, instant cure! Honestly, medical school these days is really overrated. Just learn to properly use a syringe, and you should be good to go. Just make sure not to attempt to treat/con any undercover cops.
Step away from the stinky socks
In another edition of “Humans: What Won’t They Do?” a Chinese man has developed a severe lung infection from prolonged, voluntary inhalation of his sweaty socks.
Take a moment to gag if you need it.
The man reportedly would finish his daily walk home from work with a deep, relaxing session of smelling his socks. Somewhat unsurprisingly, he developed a fungal infection and had to be hospitalized.
His doctor, perhaps in an effort to spare his feelings, conceded that the infection could be attributed to his weakened immune system from looking after his child. Sock-smelling weirdo, or just a good dad? Let’s just hope he doesn’t pass on this … unorthodox hobby to his offspring.
Turn down your eye beams
Do you remember the recent LOTME about a company selling tissues that have already been used by a sick person? Good news! This next item has nothing to do with that.
More good news! Belief in extramission – the ability to emit an invisible energy from the eyes – is down to about 5% among Americans after being greater than 50% at the turn of the century. The bad news? Belief in extramission is about 5% among Americans, according to investigators at Princeton (N.J.) University.
It’s not really a superhero thing, though. The researchers shared an explanation common among the extramission believers: “Light enters the eye, and there is a reflector piece inside the eye. The reflector reflects the light back out and hits the object, allowing the eye to see it.”
Now, we’d like to discount this whole eye-beam business, we really would, but there may be an exception that proves the rule. Ever had a staring contest with a cat? There’s got to be some sort of freaky power going on there.
The world’s worst superpower
Have you ever wondered what it would be like to live in the beginning of a science fiction novel? Well, worry no more – a group of researchers at the Georgia Institute of Technology and Emory University has you covered!
Okay, we’re pretty sure that the nanoparticles they’ve come up with won’t turn you into the Borg, but they will make your urine glow. Specifically, when injected into the bodies of people who’ve recently undergone transplants, they can help identify when an organ is failing.
The particles are tiny (duh), but they’re big enough that they won’t accumulate in normal, native tissue. However, they are small enough that, when a transplanted organ is being attacked by the body, the nanoparticles will end up getting through the kidneys and into urine. The particles are fluorescent and glow under near-infrared light.
The nanoparticles are aimed at replacing biopsies, as they are more predictive and less invasive. Also, who wouldn’t want to claim they’ve been enhanced by nanotechnology? Resistance is futile, after all.

Doctor, (fake) doctor, gimme the news
Florida Man strikes again, and this time he’s a faux MD.
Onelio Hipolit-Gonzalez was charged with a felony for impersonating a doctor who promised patients he could easily diagnose their diabetes, cancer, multiple sclerosis, Parkinson’s, and pretty much any other malady … with a metal rod. The “doctor” would have patients hold on to a metal rod connected to a beeping machine (these are the scientific terms, of course), and then he would gravely diagnose them with a variety of ailments that he could cure for the low, low price of $2,000.
If you’re curious how he treated patients, fear not. This intrepid medical professional took the ingenious measure of drawing a patient’s blood and simply injecting it back inside them. Bada-bing, instant cure! Honestly, medical school these days is really overrated. Just learn to properly use a syringe, and you should be good to go. Just make sure not to attempt to treat/con any undercover cops.
Step away from the stinky socks
In another edition of “Humans: What Won’t They Do?” a Chinese man has developed a severe lung infection from prolonged, voluntary inhalation of his sweaty socks.
Take a moment to gag if you need it.
The man reportedly would finish his daily walk home from work with a deep, relaxing session of smelling his socks. Somewhat unsurprisingly, he developed a fungal infection and had to be hospitalized.
His doctor, perhaps in an effort to spare his feelings, conceded that the infection could be attributed to his weakened immune system from looking after his child. Sock-smelling weirdo, or just a good dad? Let’s just hope he doesn’t pass on this … unorthodox hobby to his offspring.
Turn down your eye beams
Do you remember the recent LOTME about a company selling tissues that have already been used by a sick person? Good news! This next item has nothing to do with that.
More good news! Belief in extramission – the ability to emit an invisible energy from the eyes – is down to about 5% among Americans after being greater than 50% at the turn of the century. The bad news? Belief in extramission is about 5% among Americans, according to investigators at Princeton (N.J.) University.
It’s not really a superhero thing, though. The researchers shared an explanation common among the extramission believers: “Light enters the eye, and there is a reflector piece inside the eye. The reflector reflects the light back out and hits the object, allowing the eye to see it.”
Now, we’d like to discount this whole eye-beam business, we really would, but there may be an exception that proves the rule. Ever had a staring contest with a cat? There’s got to be some sort of freaky power going on there.
The world’s worst superpower
Have you ever wondered what it would be like to live in the beginning of a science fiction novel? Well, worry no more – a group of researchers at the Georgia Institute of Technology and Emory University has you covered!
Okay, we’re pretty sure that the nanoparticles they’ve come up with won’t turn you into the Borg, but they will make your urine glow. Specifically, when injected into the bodies of people who’ve recently undergone transplants, they can help identify when an organ is failing.
The particles are tiny (duh), but they’re big enough that they won’t accumulate in normal, native tissue. However, they are small enough that, when a transplanted organ is being attacked by the body, the nanoparticles will end up getting through the kidneys and into urine. The particles are fluorescent and glow under near-infrared light.
The nanoparticles are aimed at replacing biopsies, as they are more predictive and less invasive. Also, who wouldn’t want to claim they’ve been enhanced by nanotechnology? Resistance is futile, after all.

Doctor, (fake) doctor, gimme the news
Florida Man strikes again, and this time he’s a faux MD.
Onelio Hipolit-Gonzalez was charged with a felony for impersonating a doctor who promised patients he could easily diagnose their diabetes, cancer, multiple sclerosis, Parkinson’s, and pretty much any other malady … with a metal rod. The “doctor” would have patients hold on to a metal rod connected to a beeping machine (these are the scientific terms, of course), and then he would gravely diagnose them with a variety of ailments that he could cure for the low, low price of $2,000.
If you’re curious how he treated patients, fear not. This intrepid medical professional took the ingenious measure of drawing a patient’s blood and simply injecting it back inside them. Bada-bing, instant cure! Honestly, medical school these days is really overrated. Just learn to properly use a syringe, and you should be good to go. Just make sure not to attempt to treat/con any undercover cops.
Step away from the stinky socks
In another edition of “Humans: What Won’t They Do?” a Chinese man has developed a severe lung infection from prolonged, voluntary inhalation of his sweaty socks.
Take a moment to gag if you need it.
The man reportedly would finish his daily walk home from work with a deep, relaxing session of smelling his socks. Somewhat unsurprisingly, he developed a fungal infection and had to be hospitalized.
His doctor, perhaps in an effort to spare his feelings, conceded that the infection could be attributed to his weakened immune system from looking after his child. Sock-smelling weirdo, or just a good dad? Let’s just hope he doesn’t pass on this … unorthodox hobby to his offspring.
Turn down your eye beams
Do you remember the recent LOTME about a company selling tissues that have already been used by a sick person? Good news! This next item has nothing to do with that.
More good news! Belief in extramission – the ability to emit an invisible energy from the eyes – is down to about 5% among Americans after being greater than 50% at the turn of the century. The bad news? Belief in extramission is about 5% among Americans, according to investigators at Princeton (N.J.) University.
It’s not really a superhero thing, though. The researchers shared an explanation common among the extramission believers: “Light enters the eye, and there is a reflector piece inside the eye. The reflector reflects the light back out and hits the object, allowing the eye to see it.”
Now, we’d like to discount this whole eye-beam business, we really would, but there may be an exception that proves the rule. Ever had a staring contest with a cat? There’s got to be some sort of freaky power going on there.
The world’s worst superpower
Have you ever wondered what it would be like to live in the beginning of a science fiction novel? Well, worry no more – a group of researchers at the Georgia Institute of Technology and Emory University has you covered!
Okay, we’re pretty sure that the nanoparticles they’ve come up with won’t turn you into the Borg, but they will make your urine glow. Specifically, when injected into the bodies of people who’ve recently undergone transplants, they can help identify when an organ is failing.
The particles are tiny (duh), but they’re big enough that they won’t accumulate in normal, native tissue. However, they are small enough that, when a transplanted organ is being attacked by the body, the nanoparticles will end up getting through the kidneys and into urine. The particles are fluorescent and glow under near-infrared light.
The nanoparticles are aimed at replacing biopsies, as they are more predictive and less invasive. Also, who wouldn’t want to claim they’ve been enhanced by nanotechnology? Resistance is futile, after all.

Checklists, colleagues key when psychiatric patient overdoses
BONITA SPRINGS, FLA. — Specialized checklists and colleague support prove crucial to psychiatrists when one of their patients in treatment for substance use disorder dies from an overdose, an expert said at the Annual Meeting of the American Academy of Addiction Psychiatry.
Much of the knowledge about how psychiatrists are affected by overdose deaths, and what can help them handle them better, is drawn from the literature on patient suicide – both types of death are sudden and unexpected, and both involve stigma and can isolate the patients’ families and providers, said Amy Yule, MD, medical director of the Addiction Recovery Management Service at Massachusetts General Hospital in Boston.
“To our knowledge, the provider’s experience after an overdose has not been studied, and [there are] no practice guidelines to guide providers after an overdose death,” she said.
The overdose death of a patient is a particularly difficult matter because psychiatrists struggle with the emotional toll at the same time that they are dealing with fairly urgent details, including some with important legal implications, Dr. Yule said.
“Literature on the provider experience after suicide death indicates that providers are highly impacted by a patient’s suicide,” she said.
A key question is whether to contact the patient’s family. And generally, the answer should be yes.
“It’s really important to offer the option to meet with family members since these families may feel very isolated stigma as they grieve,” Dr. Yule said. What’s more, when families are not contacted by the physician, they might turn to litigation to try to seek information to help them understand their loss, she said.
In a survey of therapists whose patients died by suicide, 73% said they made contact with patient families and, in most instances, the family was not critical and expressed gratitude.
She emphasized the importance of knowing whether a patient’s family knew of the treatment. Because privacy laws extend after a patient’s death, providers cannot disclose treatment to families who did not already know, she said.
Also, she said, “communication with families should be focused on addressing the family members’ feelings and not the clinical details of the case.”
Most states have “apology statutes” that prevent expressions of sympathy – such as, “I’m sorry for your loss” – to be used as admission of liability, but providers should check the laws in their own states, she said.
If you have a colleague whose patient has overdosed or lost their lives to suicide, certain approaches are better than others, Dr. Yule said.
“It’s helpful when colleagues share their own experience with the suicide of a patient or patient who has overdosed and died,” she said. “What’s not helpful is the premature reassurance that the clinician has done nothing wrong. We may feel in these instances that we want to provide that premature reassurance, but it’s important not to do that because it doesn’t help providers resolve their grief.”
For solo providers, it’s especially important to be part of a physician network because they might otherwise not have the same support that those in larger organizations have, she said.
Beyond the grieving process, logistical details also need tending to, she said. The malpractice insurance carrier should be notified, even when there was no sign of a contentious interaction with the family. And, in her organization, the staff run down a checklist that includes not only calling the family and sending a condolence card, notifying staff promptly, and documenting the death, but also easily overlooked details like canceling future appointments in the scheduling system.
“You really don’t want a phone call going to the patient’s family with an appointment reminder after the patient is deceased,” Dr. Yule said. “These are the little details that you may not remember when you’re acutely grieving a patient’s death. And that’s why we feel it’s important to have a list.”
Dr. Yule reported no relevant disclosures.
BONITA SPRINGS, FLA. — Specialized checklists and colleague support prove crucial to psychiatrists when one of their patients in treatment for substance use disorder dies from an overdose, an expert said at the Annual Meeting of the American Academy of Addiction Psychiatry.
Much of the knowledge about how psychiatrists are affected by overdose deaths, and what can help them handle them better, is drawn from the literature on patient suicide – both types of death are sudden and unexpected, and both involve stigma and can isolate the patients’ families and providers, said Amy Yule, MD, medical director of the Addiction Recovery Management Service at Massachusetts General Hospital in Boston.
“To our knowledge, the provider’s experience after an overdose has not been studied, and [there are] no practice guidelines to guide providers after an overdose death,” she said.
The overdose death of a patient is a particularly difficult matter because psychiatrists struggle with the emotional toll at the same time that they are dealing with fairly urgent details, including some with important legal implications, Dr. Yule said.
“Literature on the provider experience after suicide death indicates that providers are highly impacted by a patient’s suicide,” she said.
A key question is whether to contact the patient’s family. And generally, the answer should be yes.
“It’s really important to offer the option to meet with family members since these families may feel very isolated stigma as they grieve,” Dr. Yule said. What’s more, when families are not contacted by the physician, they might turn to litigation to try to seek information to help them understand their loss, she said.
In a survey of therapists whose patients died by suicide, 73% said they made contact with patient families and, in most instances, the family was not critical and expressed gratitude.
She emphasized the importance of knowing whether a patient’s family knew of the treatment. Because privacy laws extend after a patient’s death, providers cannot disclose treatment to families who did not already know, she said.
Also, she said, “communication with families should be focused on addressing the family members’ feelings and not the clinical details of the case.”
Most states have “apology statutes” that prevent expressions of sympathy – such as, “I’m sorry for your loss” – to be used as admission of liability, but providers should check the laws in their own states, she said.
If you have a colleague whose patient has overdosed or lost their lives to suicide, certain approaches are better than others, Dr. Yule said.
“It’s helpful when colleagues share their own experience with the suicide of a patient or patient who has overdosed and died,” she said. “What’s not helpful is the premature reassurance that the clinician has done nothing wrong. We may feel in these instances that we want to provide that premature reassurance, but it’s important not to do that because it doesn’t help providers resolve their grief.”
For solo providers, it’s especially important to be part of a physician network because they might otherwise not have the same support that those in larger organizations have, she said.
Beyond the grieving process, logistical details also need tending to, she said. The malpractice insurance carrier should be notified, even when there was no sign of a contentious interaction with the family. And, in her organization, the staff run down a checklist that includes not only calling the family and sending a condolence card, notifying staff promptly, and documenting the death, but also easily overlooked details like canceling future appointments in the scheduling system.
“You really don’t want a phone call going to the patient’s family with an appointment reminder after the patient is deceased,” Dr. Yule said. “These are the little details that you may not remember when you’re acutely grieving a patient’s death. And that’s why we feel it’s important to have a list.”
Dr. Yule reported no relevant disclosures.
BONITA SPRINGS, FLA. — Specialized checklists and colleague support prove crucial to psychiatrists when one of their patients in treatment for substance use disorder dies from an overdose, an expert said at the Annual Meeting of the American Academy of Addiction Psychiatry.
Much of the knowledge about how psychiatrists are affected by overdose deaths, and what can help them handle them better, is drawn from the literature on patient suicide – both types of death are sudden and unexpected, and both involve stigma and can isolate the patients’ families and providers, said Amy Yule, MD, medical director of the Addiction Recovery Management Service at Massachusetts General Hospital in Boston.
“To our knowledge, the provider’s experience after an overdose has not been studied, and [there are] no practice guidelines to guide providers after an overdose death,” she said.
The overdose death of a patient is a particularly difficult matter because psychiatrists struggle with the emotional toll at the same time that they are dealing with fairly urgent details, including some with important legal implications, Dr. Yule said.
“Literature on the provider experience after suicide death indicates that providers are highly impacted by a patient’s suicide,” she said.
A key question is whether to contact the patient’s family. And generally, the answer should be yes.
“It’s really important to offer the option to meet with family members since these families may feel very isolated stigma as they grieve,” Dr. Yule said. What’s more, when families are not contacted by the physician, they might turn to litigation to try to seek information to help them understand their loss, she said.
In a survey of therapists whose patients died by suicide, 73% said they made contact with patient families and, in most instances, the family was not critical and expressed gratitude.
She emphasized the importance of knowing whether a patient’s family knew of the treatment. Because privacy laws extend after a patient’s death, providers cannot disclose treatment to families who did not already know, she said.
Also, she said, “communication with families should be focused on addressing the family members’ feelings and not the clinical details of the case.”
Most states have “apology statutes” that prevent expressions of sympathy – such as, “I’m sorry for your loss” – to be used as admission of liability, but providers should check the laws in their own states, she said.
If you have a colleague whose patient has overdosed or lost their lives to suicide, certain approaches are better than others, Dr. Yule said.
“It’s helpful when colleagues share their own experience with the suicide of a patient or patient who has overdosed and died,” she said. “What’s not helpful is the premature reassurance that the clinician has done nothing wrong. We may feel in these instances that we want to provide that premature reassurance, but it’s important not to do that because it doesn’t help providers resolve their grief.”
For solo providers, it’s especially important to be part of a physician network because they might otherwise not have the same support that those in larger organizations have, she said.
Beyond the grieving process, logistical details also need tending to, she said. The malpractice insurance carrier should be notified, even when there was no sign of a contentious interaction with the family. And, in her organization, the staff run down a checklist that includes not only calling the family and sending a condolence card, notifying staff promptly, and documenting the death, but also easily overlooked details like canceling future appointments in the scheduling system.
“You really don’t want a phone call going to the patient’s family with an appointment reminder after the patient is deceased,” Dr. Yule said. “These are the little details that you may not remember when you’re acutely grieving a patient’s death. And that’s why we feel it’s important to have a list.”
Dr. Yule reported no relevant disclosures.
EXPERT ANALYSIS FROM AAAP 2018
What’s new with adalimumab? Plenty
WAIKOLOA, HAWAII – A flurry of recent impressive , identifies a simple biomarker predictive of the likelihood of a favorable PASI 75 response, and highlights a disconnect in psoriatic arthritis (PsA) patients between clinical response as reflected in disease activity and radiographic progression of joint disease, according to Kristina C. Duffin, MD.
Also, a new citrate-free version of adalimumab (Humira) is available. It requires a new prescription, and an additional prior authorization is mandated by some insurers. But this is a welcome innovation for patients bothered by significant burning and stinging with their injections of classic adalimumab, Dr. Duffin, cochair of the department of dermatology at the University of Utah, Salt Lake City, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
New long-term safety data
Adalimumab is a market leader in biologic therapy for psoriasis. But the long-term experience with biologics in dermatology is still relatively limited, so the recent publication of two large studies providing encouraging evidence of the long-term safety of adalimumab is noteworthy.
Craig L. Leonardi, MD, of Saint Louis University, St. Louis, Mo., was first author of an analysis of long-term safety data from 18 clinical trials in adults with moderate to severe plaque psoriasis. The key takeaway, in Dr. Duffin’s view, was that the rate of adverse events, including serious infections and malignancies other than nonmelanoma skin cancer, remained stable over time out to 240 weeks of follow-up in patients on continuous treatment, with no new safety signals emerging (Br J Dermatol. 2019 Jan;180[1]:76-85).
However, randomized clinical trials often paint an overly rosy safety picture because of their strict inclusion and exclusion criteria.
“We single out patients for clinical trials because they’re especially healthy. That doesn’t happen in real-world registries,” she noted.
That’s why a systematic review of adalimumab’s safety performance in 10 real-world registries of adalimumab-treated psoriasis patients is particularly informative. The registries included in the systematic review, led by Bruce E. Strober, MD, PhD, professor of dermatology at the University of Connecticut, Farmington, didn’t all measure the same outcomes. But the three registries that documented major adverse cardiovascular events showed rates of less than 0.1 to less than 1 per 100 patient-years. Rates of malignancies other than nonmelanoma skin cancer were consistently in the 0.3-0.6 events per 100 patient-years range, similar to what has been reported in studies of other systemic psoriasis therapies, biologic as well as nonbiologic (J Eur Acad Dermatol Venereol. 2018 Dec;32[12]:2126-33).
Overall infection rates reported in the real-world registries ranged from 7.7 to 14.7 events per 100 patient-years, which is actually considerably lower than in the clinical trials. Rates of serious infections ranged from less than one up to two events per 100 patient-years, with the most common ones being cellulitis and pneumonia, consistent with the randomized trial experience.
Predicting response to adalimumab
A prospective, multicenter, observational cohort study of 544 psoriasis patients on adalimumab monotherapy conducted by U.K. investigators concluded that a patient’s serum drug level is the single most important predictor of treatment response. A cut point of 3.2 mcg/mL, which is considered the minimal effective circulating drug level, was associated with a 65% probability of a 75% improvement in Psoriasis Area and Severity Index from baseline, or PASI 75 response. The higher the serum drug level, the greater the likelihood of a PASI 75 response, up to a serum level of 7 mcg/mL, which was associated with an 81% probability of achieving PASI 75. Beyond 7 mcg/mL, however, the relationship with treatment response plateaued. Importantly, drug levels measured early on – at 1-12 weeks into therapy – were predictive of response 6 months later. So were steady-state levels (J Invest Dermatol. 2019 Jan;139[1]:115-23).
This is clinically useful information, Dr. Duffin observed.
“I’m hoping we’re going to see more real-world use of checking drug levels,” she said.
Indeed, even though the approved dosing of adalimumab for psoriasis is 40 mg by subcutaneous injection every 2 weeks, the new American Academy of Dermatology/National Psoriasis Foundation joint guidelines for treatment of psoriasis with biologics declare that “a maintenance dose of adalimumab at 40 mg/week is recommended for better disease control in some patients” (J Am Acad Dermatol. 2019 Feb 7. doi: 10.1016/j.jaad.2018.11.057. [Epub ahead of print]).
The new guidelines provide support for dermatologists who decide weekly therapy is best for a given patient, and adalimumab drug levels could prove useful in identifying the patient subgroup likely to benefit.
Dr. Duffin is often consulted by other physicians as to whether they should check for neutralizing antibodies in patients who appear to be losing therapeutic efficacy on a given biologic. She’s not a fan of the practice.
“There are commercial assays out there, but it’s very hard to interpret them because we don’t really know if they’re truly measuring neutralizing antibodies. And the cost is not insignificant; it can be hundreds of dollars,” she noted.
She believes a straightforward measurement of the serum biologic level is a better strategy.
“It makes sense: This is an indirect way of determining if there’s been neutralization of the drug, rather than trying to check the antibody that’s doing it, which is fraught with problems,” Dr. Duffin said.
Radiographic progression and clinical PsA activity on adalimumab don’t always correlate
A post hoc analysis of the randomized, double-blind, placebo-controlled ADEPT trial in PsA patients demonstrated that inhibition of radiographic progression as measured by change in modified total Sharp score from baseline through 24 weeks of adalimumab therapy was greater than expected based upon control of clinical disease activity (Rheumatology [Oxford]. 2019 Jan 3. doi: 10.1093/rheumatology/key417. [Epub ahead of print]).
One implication of the disconnect between radiographic progression and clinical disease documented in this study is that a dermatologist shouldn’t be too quick to change from adalimumab to another biologic just because a patient with PsA reports continued but bearable joint pain. And the converse is also true.
“I think that we as dermatologists probably shouldn’t be reassured when a patient says, ‘My joints feel great!” That’s because you may not necessarily be able to predict lack of progression in Sharp score based upon clinical response,” Dr. Duffin cautioned. “I think you should still have a rheumatologist check in with the patient and do x-rays periodically. The rheumatologist I work with does that, usually about on a yearly basis.”
Another key finding in the ADEPT analysis was that concomitant methotrexate had no added effect in terms of preventing joint destruction. This underscores the prescience of the first-ever collaborative American College of Rheumatology/National Psoriasis Foundation guidelines for the treatment of PsA (Arthritis Care Res (Hoboken). 2019 Jan;71[1]:2-29).
The new guidelines recommend that, in a psoriasis patient with confirmed PsA, the first-line treatment is a tumor necrosis factor (TNF) inhibitor. Agents from this class are preferred over other biologics because they are backed by a larger body of data regarding inhibition of joint disease progression. If the patient fails on the first TNF inhibitor prescribed, second-line therapy is another TNF inhibitor. So is third-line therapy.
Adalimumab citrate free
Not only does this new iteration of adalimumab do away with citrate as a buffer because it can cause pain and burning, it also utilizes a thinner 29-gauge needle rather than the standard 27-gauge. And the needle cover isn’t made with natural rubber latex. Also, both the pen and prefilled syringe contain half the volume of liquid, compared with the classic version of the biologic, so it’s 40 mg of drug in 0.4 mL rather than in 0.8 mL.
The packaging of adalimumab citrate free is different. It comes in a blue box to distinguish the product from the classic version.
Dr. Duffin reported receiving research grants from and serving as a consultant to AbbVie, which markets adalimumab, as well as close to a dozen other pharmaceutical companies.
The SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – A flurry of recent impressive , identifies a simple biomarker predictive of the likelihood of a favorable PASI 75 response, and highlights a disconnect in psoriatic arthritis (PsA) patients between clinical response as reflected in disease activity and radiographic progression of joint disease, according to Kristina C. Duffin, MD.
Also, a new citrate-free version of adalimumab (Humira) is available. It requires a new prescription, and an additional prior authorization is mandated by some insurers. But this is a welcome innovation for patients bothered by significant burning and stinging with their injections of classic adalimumab, Dr. Duffin, cochair of the department of dermatology at the University of Utah, Salt Lake City, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
New long-term safety data
Adalimumab is a market leader in biologic therapy for psoriasis. But the long-term experience with biologics in dermatology is still relatively limited, so the recent publication of two large studies providing encouraging evidence of the long-term safety of adalimumab is noteworthy.
Craig L. Leonardi, MD, of Saint Louis University, St. Louis, Mo., was first author of an analysis of long-term safety data from 18 clinical trials in adults with moderate to severe plaque psoriasis. The key takeaway, in Dr. Duffin’s view, was that the rate of adverse events, including serious infections and malignancies other than nonmelanoma skin cancer, remained stable over time out to 240 weeks of follow-up in patients on continuous treatment, with no new safety signals emerging (Br J Dermatol. 2019 Jan;180[1]:76-85).
However, randomized clinical trials often paint an overly rosy safety picture because of their strict inclusion and exclusion criteria.
“We single out patients for clinical trials because they’re especially healthy. That doesn’t happen in real-world registries,” she noted.
That’s why a systematic review of adalimumab’s safety performance in 10 real-world registries of adalimumab-treated psoriasis patients is particularly informative. The registries included in the systematic review, led by Bruce E. Strober, MD, PhD, professor of dermatology at the University of Connecticut, Farmington, didn’t all measure the same outcomes. But the three registries that documented major adverse cardiovascular events showed rates of less than 0.1 to less than 1 per 100 patient-years. Rates of malignancies other than nonmelanoma skin cancer were consistently in the 0.3-0.6 events per 100 patient-years range, similar to what has been reported in studies of other systemic psoriasis therapies, biologic as well as nonbiologic (J Eur Acad Dermatol Venereol. 2018 Dec;32[12]:2126-33).
Overall infection rates reported in the real-world registries ranged from 7.7 to 14.7 events per 100 patient-years, which is actually considerably lower than in the clinical trials. Rates of serious infections ranged from less than one up to two events per 100 patient-years, with the most common ones being cellulitis and pneumonia, consistent with the randomized trial experience.
Predicting response to adalimumab
A prospective, multicenter, observational cohort study of 544 psoriasis patients on adalimumab monotherapy conducted by U.K. investigators concluded that a patient’s serum drug level is the single most important predictor of treatment response. A cut point of 3.2 mcg/mL, which is considered the minimal effective circulating drug level, was associated with a 65% probability of a 75% improvement in Psoriasis Area and Severity Index from baseline, or PASI 75 response. The higher the serum drug level, the greater the likelihood of a PASI 75 response, up to a serum level of 7 mcg/mL, which was associated with an 81% probability of achieving PASI 75. Beyond 7 mcg/mL, however, the relationship with treatment response plateaued. Importantly, drug levels measured early on – at 1-12 weeks into therapy – were predictive of response 6 months later. So were steady-state levels (J Invest Dermatol. 2019 Jan;139[1]:115-23).
This is clinically useful information, Dr. Duffin observed.
“I’m hoping we’re going to see more real-world use of checking drug levels,” she said.
Indeed, even though the approved dosing of adalimumab for psoriasis is 40 mg by subcutaneous injection every 2 weeks, the new American Academy of Dermatology/National Psoriasis Foundation joint guidelines for treatment of psoriasis with biologics declare that “a maintenance dose of adalimumab at 40 mg/week is recommended for better disease control in some patients” (J Am Acad Dermatol. 2019 Feb 7. doi: 10.1016/j.jaad.2018.11.057. [Epub ahead of print]).
The new guidelines provide support for dermatologists who decide weekly therapy is best for a given patient, and adalimumab drug levels could prove useful in identifying the patient subgroup likely to benefit.
Dr. Duffin is often consulted by other physicians as to whether they should check for neutralizing antibodies in patients who appear to be losing therapeutic efficacy on a given biologic. She’s not a fan of the practice.
“There are commercial assays out there, but it’s very hard to interpret them because we don’t really know if they’re truly measuring neutralizing antibodies. And the cost is not insignificant; it can be hundreds of dollars,” she noted.
She believes a straightforward measurement of the serum biologic level is a better strategy.
“It makes sense: This is an indirect way of determining if there’s been neutralization of the drug, rather than trying to check the antibody that’s doing it, which is fraught with problems,” Dr. Duffin said.
Radiographic progression and clinical PsA activity on adalimumab don’t always correlate
A post hoc analysis of the randomized, double-blind, placebo-controlled ADEPT trial in PsA patients demonstrated that inhibition of radiographic progression as measured by change in modified total Sharp score from baseline through 24 weeks of adalimumab therapy was greater than expected based upon control of clinical disease activity (Rheumatology [Oxford]. 2019 Jan 3. doi: 10.1093/rheumatology/key417. [Epub ahead of print]).
One implication of the disconnect between radiographic progression and clinical disease documented in this study is that a dermatologist shouldn’t be too quick to change from adalimumab to another biologic just because a patient with PsA reports continued but bearable joint pain. And the converse is also true.
“I think that we as dermatologists probably shouldn’t be reassured when a patient says, ‘My joints feel great!” That’s because you may not necessarily be able to predict lack of progression in Sharp score based upon clinical response,” Dr. Duffin cautioned. “I think you should still have a rheumatologist check in with the patient and do x-rays periodically. The rheumatologist I work with does that, usually about on a yearly basis.”
Another key finding in the ADEPT analysis was that concomitant methotrexate had no added effect in terms of preventing joint destruction. This underscores the prescience of the first-ever collaborative American College of Rheumatology/National Psoriasis Foundation guidelines for the treatment of PsA (Arthritis Care Res (Hoboken). 2019 Jan;71[1]:2-29).
The new guidelines recommend that, in a psoriasis patient with confirmed PsA, the first-line treatment is a tumor necrosis factor (TNF) inhibitor. Agents from this class are preferred over other biologics because they are backed by a larger body of data regarding inhibition of joint disease progression. If the patient fails on the first TNF inhibitor prescribed, second-line therapy is another TNF inhibitor. So is third-line therapy.
Adalimumab citrate free
Not only does this new iteration of adalimumab do away with citrate as a buffer because it can cause pain and burning, it also utilizes a thinner 29-gauge needle rather than the standard 27-gauge. And the needle cover isn’t made with natural rubber latex. Also, both the pen and prefilled syringe contain half the volume of liquid, compared with the classic version of the biologic, so it’s 40 mg of drug in 0.4 mL rather than in 0.8 mL.
The packaging of adalimumab citrate free is different. It comes in a blue box to distinguish the product from the classic version.
Dr. Duffin reported receiving research grants from and serving as a consultant to AbbVie, which markets adalimumab, as well as close to a dozen other pharmaceutical companies.
The SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – A flurry of recent impressive , identifies a simple biomarker predictive of the likelihood of a favorable PASI 75 response, and highlights a disconnect in psoriatic arthritis (PsA) patients between clinical response as reflected in disease activity and radiographic progression of joint disease, according to Kristina C. Duffin, MD.
Also, a new citrate-free version of adalimumab (Humira) is available. It requires a new prescription, and an additional prior authorization is mandated by some insurers. But this is a welcome innovation for patients bothered by significant burning and stinging with their injections of classic adalimumab, Dr. Duffin, cochair of the department of dermatology at the University of Utah, Salt Lake City, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
New long-term safety data
Adalimumab is a market leader in biologic therapy for psoriasis. But the long-term experience with biologics in dermatology is still relatively limited, so the recent publication of two large studies providing encouraging evidence of the long-term safety of adalimumab is noteworthy.
Craig L. Leonardi, MD, of Saint Louis University, St. Louis, Mo., was first author of an analysis of long-term safety data from 18 clinical trials in adults with moderate to severe plaque psoriasis. The key takeaway, in Dr. Duffin’s view, was that the rate of adverse events, including serious infections and malignancies other than nonmelanoma skin cancer, remained stable over time out to 240 weeks of follow-up in patients on continuous treatment, with no new safety signals emerging (Br J Dermatol. 2019 Jan;180[1]:76-85).
However, randomized clinical trials often paint an overly rosy safety picture because of their strict inclusion and exclusion criteria.
“We single out patients for clinical trials because they’re especially healthy. That doesn’t happen in real-world registries,” she noted.
That’s why a systematic review of adalimumab’s safety performance in 10 real-world registries of adalimumab-treated psoriasis patients is particularly informative. The registries included in the systematic review, led by Bruce E. Strober, MD, PhD, professor of dermatology at the University of Connecticut, Farmington, didn’t all measure the same outcomes. But the three registries that documented major adverse cardiovascular events showed rates of less than 0.1 to less than 1 per 100 patient-years. Rates of malignancies other than nonmelanoma skin cancer were consistently in the 0.3-0.6 events per 100 patient-years range, similar to what has been reported in studies of other systemic psoriasis therapies, biologic as well as nonbiologic (J Eur Acad Dermatol Venereol. 2018 Dec;32[12]:2126-33).
Overall infection rates reported in the real-world registries ranged from 7.7 to 14.7 events per 100 patient-years, which is actually considerably lower than in the clinical trials. Rates of serious infections ranged from less than one up to two events per 100 patient-years, with the most common ones being cellulitis and pneumonia, consistent with the randomized trial experience.
Predicting response to adalimumab
A prospective, multicenter, observational cohort study of 544 psoriasis patients on adalimumab monotherapy conducted by U.K. investigators concluded that a patient’s serum drug level is the single most important predictor of treatment response. A cut point of 3.2 mcg/mL, which is considered the minimal effective circulating drug level, was associated with a 65% probability of a 75% improvement in Psoriasis Area and Severity Index from baseline, or PASI 75 response. The higher the serum drug level, the greater the likelihood of a PASI 75 response, up to a serum level of 7 mcg/mL, which was associated with an 81% probability of achieving PASI 75. Beyond 7 mcg/mL, however, the relationship with treatment response plateaued. Importantly, drug levels measured early on – at 1-12 weeks into therapy – were predictive of response 6 months later. So were steady-state levels (J Invest Dermatol. 2019 Jan;139[1]:115-23).
This is clinically useful information, Dr. Duffin observed.
“I’m hoping we’re going to see more real-world use of checking drug levels,” she said.
Indeed, even though the approved dosing of adalimumab for psoriasis is 40 mg by subcutaneous injection every 2 weeks, the new American Academy of Dermatology/National Psoriasis Foundation joint guidelines for treatment of psoriasis with biologics declare that “a maintenance dose of adalimumab at 40 mg/week is recommended for better disease control in some patients” (J Am Acad Dermatol. 2019 Feb 7. doi: 10.1016/j.jaad.2018.11.057. [Epub ahead of print]).
The new guidelines provide support for dermatologists who decide weekly therapy is best for a given patient, and adalimumab drug levels could prove useful in identifying the patient subgroup likely to benefit.
Dr. Duffin is often consulted by other physicians as to whether they should check for neutralizing antibodies in patients who appear to be losing therapeutic efficacy on a given biologic. She’s not a fan of the practice.
“There are commercial assays out there, but it’s very hard to interpret them because we don’t really know if they’re truly measuring neutralizing antibodies. And the cost is not insignificant; it can be hundreds of dollars,” she noted.
She believes a straightforward measurement of the serum biologic level is a better strategy.
“It makes sense: This is an indirect way of determining if there’s been neutralization of the drug, rather than trying to check the antibody that’s doing it, which is fraught with problems,” Dr. Duffin said.
Radiographic progression and clinical PsA activity on adalimumab don’t always correlate
A post hoc analysis of the randomized, double-blind, placebo-controlled ADEPT trial in PsA patients demonstrated that inhibition of radiographic progression as measured by change in modified total Sharp score from baseline through 24 weeks of adalimumab therapy was greater than expected based upon control of clinical disease activity (Rheumatology [Oxford]. 2019 Jan 3. doi: 10.1093/rheumatology/key417. [Epub ahead of print]).
One implication of the disconnect between radiographic progression and clinical disease documented in this study is that a dermatologist shouldn’t be too quick to change from adalimumab to another biologic just because a patient with PsA reports continued but bearable joint pain. And the converse is also true.
“I think that we as dermatologists probably shouldn’t be reassured when a patient says, ‘My joints feel great!” That’s because you may not necessarily be able to predict lack of progression in Sharp score based upon clinical response,” Dr. Duffin cautioned. “I think you should still have a rheumatologist check in with the patient and do x-rays periodically. The rheumatologist I work with does that, usually about on a yearly basis.”
Another key finding in the ADEPT analysis was that concomitant methotrexate had no added effect in terms of preventing joint destruction. This underscores the prescience of the first-ever collaborative American College of Rheumatology/National Psoriasis Foundation guidelines for the treatment of PsA (Arthritis Care Res (Hoboken). 2019 Jan;71[1]:2-29).
The new guidelines recommend that, in a psoriasis patient with confirmed PsA, the first-line treatment is a tumor necrosis factor (TNF) inhibitor. Agents from this class are preferred over other biologics because they are backed by a larger body of data regarding inhibition of joint disease progression. If the patient fails on the first TNF inhibitor prescribed, second-line therapy is another TNF inhibitor. So is third-line therapy.
Adalimumab citrate free
Not only does this new iteration of adalimumab do away with citrate as a buffer because it can cause pain and burning, it also utilizes a thinner 29-gauge needle rather than the standard 27-gauge. And the needle cover isn’t made with natural rubber latex. Also, both the pen and prefilled syringe contain half the volume of liquid, compared with the classic version of the biologic, so it’s 40 mg of drug in 0.4 mL rather than in 0.8 mL.
The packaging of adalimumab citrate free is different. It comes in a blue box to distinguish the product from the classic version.
Dr. Duffin reported receiving research grants from and serving as a consultant to AbbVie, which markets adalimumab, as well as close to a dozen other pharmaceutical companies.
The SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
REPORTING FROM SDEF HAWAII DERMATOLOGY SEMINAR
Not all AF maze operations are aMAZE-ing
SNOWMASS, COLO. – The term “maze procedure” for surgical ablation of atrial fibrillation is bandied about rather loosely these days, but as far as Hartzell V. Schaff, MD, is concerned, the operation of choice remains the classic cut-and-sew maze III procedure developed by James L. Cox, MD, while at Washington University, St. Louis.
“The classic Cox maze III, the cut-and-sew maze, is the best procedure for getting rid of atrial fibrillation and is in my view the gold standard. Some people argue that transmurality isn’t important, but it can occur because of gap lesions,” he said at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
Unlike modifications of the Cox maze III – such as the mini maze or the maze IV, which utilizes radiofrequency energy or cryoablation to create scars in an effort to achieve pulmonary vein isolation – the maze III cannot be done as a minimally invasive procedure. After all, it requires making incisions in both atria, along with aortic cross-clamping and cardiopulmonary bypass. But it has a significantly higher long-term rate of freedom from recurrent atrial fibrillation (AF) than the other operations. And crucially, it enables the surgeon to readily obliterate the left atrial appendage.
“The most important thing when you do any surgical procedure for atrial fibrillation, I think, is getting rid of the left atrial appendage. When you do cut-and-sew maze, that’s done 100% of the time,” explained Dr. Schaff, professor of surgery at the Mayo Clinic in Rochester, Minn.
“We really have a lot of work left to do as surgeons in improving the outcome of surgery for atrial fibrillation. One of the things we as surgeons don’t do well is getting rid of the left atrial appendage. This ought to be done in every patient that has surgical ablation for atrial fibrillation,” according to the cardiothoracic surgeon.
And yet, he continued, in a series of nearly 87,000 patients with AF who underwent nonemergent cardiac surgery in the Society of Thoracic Surgeons database, 48.0% of whom underwent surgical ablation for AF, only 63.9% of those who had standalone ablation for lone AF got their left atrial appendage dealt with, compared with 86%-89% of those who underwent concomitant cardiac surgery, such as mitral valve repair or replacement (Ann Thorac Surg. 2017 Aug;104[2]:493-500).
“That’s awful, really. And the reason for that low left atrial appendage obliteration rate is this: For many of those patients who had surgery for lone atrial fibrillation, the surgeons were trying to do minimally invasive surgery, where they do pulmonary vein isolation on the right side, so they don’t have access to the left atrial appendage,” Dr. Schaff said.
“In the past,” he recalled, “we would ligate the left atrial appendage. Nowadays because of echocardiographic studies that show there’s persistent patency in a sizable percentage of patients, we amputate the left atrial appendage in almost all of the patients.”
The terminology surrounding surgical ablation for AF, in his view, has become rather confusing. “Most of you, when you refer a patient for surgical ablation for AF, the surgeons will just say they do a maze procedure,” Dr. Schaff cautioned. “Somehow, all of that [maze IV, mini maze] today is lumped together as a classic maze procedure, but it’s really not. We have different lesion sets and energy sources.”
And different outcomes as well. In a series of 1,189 adults who underwent surgical ablation for AF at the Mayo Clinic, of whom 44% had a biatrial cut-and-sew maze while the rest had surgical cryotherapy, radiofrequency ablation, or a combination of the two, the rate of freedom from AF 1 year post surgery was 85% with the cut-and-sew maze versus 71% with the alternatives. At 5 years or more, the rates were 78% and 52%, respectively. In a multivariate analysis, freedom from AF was independently associated with preoperative paroxysmal rather than permanent AF, performance of the classic maze III procedure, concomitant treatment of associated mitral valve disease, and younger age.
Moreover, rates of the major early postoperative complications – stroke, bleeding, and renal failure – were similar in the cut-and-sew maze III and other groups.
“So a lesser procedure doesn’t necessarily mean fewer complications,” Dr. Schaff noted.
One of the criticisms levied against the maze III is that it’s too much surgery for AF. But it’s actually relatively inexpensive because the disposables – suture, needles, scalpel – are those used in the commonly performed concomitant cardiac surgical procedures. “The Cox maze III does take extra time, but with experience it’s not much extra time,” he asserted.
Indeed, in a series of 452 Mayo Clinic maze III patients, the cross-clamp and cardiopulmonary bypass times were 52 and 73 minutes, respectively, for those undergoing an isolated maze III, compared with 73 and 86 minutes for patients whose maze III was done in conjunction with other procedures, most commonly mitral valve repair or replacement.
An underrecognized group of patients who benefit from a standalone cut-and-sew maze are those with tachycardia-induced cardiomyopathy marked by AF or atrial flutter, rapid uncontrolled ventricular response, a decreased left ventricular ejection fraction, and no associated valvular or congenital heart disease. In a series of 37 such patients identified and treated with a maze III operation at the Mayo Clinic, their average preoperative left ventricular ejection fraction of 43% improved to about 55% at discharge, a benefit sustained at last follow-up a median of 63 months later. The outcome was particularly impressive in the 11 patients with a severely depressed left ventricular ejection fraction averaging 31% preoperatively, which jumped to 53% at discharge (Ann Thorac Surg. 2006 Aug;82[2]:494-500).
“Their ejection fraction goes up when you control the tachycardia-induced cardiomyopathy,” he observed. “So reduced left ventricular ejection fraction may be an indication for surgery rather than a contraindication.”
Dr. Schaff emphasized that it’s important for cardiologists and surgeons not to overpromise what surgical ablation of AF can accomplish. The only randomized trial of surgical ablation of AF versus no ablation during mitral valve surgery, sponsored by the National Institutes of Health and Canadian Institutes of Health Research and carried out by the Cardiothoracic Surgical Trials Network, showed no significant between-group differences at 1 year in any of numerous quality of life measures, nor was there a survival benefit for ablation (N Engl J Med. 2015 Apr 9;372(15):1399-409).
“We must point out that there’s no indication that controlling atrial fibrillation has anything to do with improving survival. It has to do with symptomatic benefit and perhaps reducing risk of stroke,” he said.
Dr. Schaff reported having no financial conflicts regarding his presentation.
SNOWMASS, COLO. – The term “maze procedure” for surgical ablation of atrial fibrillation is bandied about rather loosely these days, but as far as Hartzell V. Schaff, MD, is concerned, the operation of choice remains the classic cut-and-sew maze III procedure developed by James L. Cox, MD, while at Washington University, St. Louis.
“The classic Cox maze III, the cut-and-sew maze, is the best procedure for getting rid of atrial fibrillation and is in my view the gold standard. Some people argue that transmurality isn’t important, but it can occur because of gap lesions,” he said at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
Unlike modifications of the Cox maze III – such as the mini maze or the maze IV, which utilizes radiofrequency energy or cryoablation to create scars in an effort to achieve pulmonary vein isolation – the maze III cannot be done as a minimally invasive procedure. After all, it requires making incisions in both atria, along with aortic cross-clamping and cardiopulmonary bypass. But it has a significantly higher long-term rate of freedom from recurrent atrial fibrillation (AF) than the other operations. And crucially, it enables the surgeon to readily obliterate the left atrial appendage.
“The most important thing when you do any surgical procedure for atrial fibrillation, I think, is getting rid of the left atrial appendage. When you do cut-and-sew maze, that’s done 100% of the time,” explained Dr. Schaff, professor of surgery at the Mayo Clinic in Rochester, Minn.
“We really have a lot of work left to do as surgeons in improving the outcome of surgery for atrial fibrillation. One of the things we as surgeons don’t do well is getting rid of the left atrial appendage. This ought to be done in every patient that has surgical ablation for atrial fibrillation,” according to the cardiothoracic surgeon.
And yet, he continued, in a series of nearly 87,000 patients with AF who underwent nonemergent cardiac surgery in the Society of Thoracic Surgeons database, 48.0% of whom underwent surgical ablation for AF, only 63.9% of those who had standalone ablation for lone AF got their left atrial appendage dealt with, compared with 86%-89% of those who underwent concomitant cardiac surgery, such as mitral valve repair or replacement (Ann Thorac Surg. 2017 Aug;104[2]:493-500).
“That’s awful, really. And the reason for that low left atrial appendage obliteration rate is this: For many of those patients who had surgery for lone atrial fibrillation, the surgeons were trying to do minimally invasive surgery, where they do pulmonary vein isolation on the right side, so they don’t have access to the left atrial appendage,” Dr. Schaff said.
“In the past,” he recalled, “we would ligate the left atrial appendage. Nowadays because of echocardiographic studies that show there’s persistent patency in a sizable percentage of patients, we amputate the left atrial appendage in almost all of the patients.”
The terminology surrounding surgical ablation for AF, in his view, has become rather confusing. “Most of you, when you refer a patient for surgical ablation for AF, the surgeons will just say they do a maze procedure,” Dr. Schaff cautioned. “Somehow, all of that [maze IV, mini maze] today is lumped together as a classic maze procedure, but it’s really not. We have different lesion sets and energy sources.”
And different outcomes as well. In a series of 1,189 adults who underwent surgical ablation for AF at the Mayo Clinic, of whom 44% had a biatrial cut-and-sew maze while the rest had surgical cryotherapy, radiofrequency ablation, or a combination of the two, the rate of freedom from AF 1 year post surgery was 85% with the cut-and-sew maze versus 71% with the alternatives. At 5 years or more, the rates were 78% and 52%, respectively. In a multivariate analysis, freedom from AF was independently associated with preoperative paroxysmal rather than permanent AF, performance of the classic maze III procedure, concomitant treatment of associated mitral valve disease, and younger age.
Moreover, rates of the major early postoperative complications – stroke, bleeding, and renal failure – were similar in the cut-and-sew maze III and other groups.
“So a lesser procedure doesn’t necessarily mean fewer complications,” Dr. Schaff noted.
One of the criticisms levied against the maze III is that it’s too much surgery for AF. But it’s actually relatively inexpensive because the disposables – suture, needles, scalpel – are those used in the commonly performed concomitant cardiac surgical procedures. “The Cox maze III does take extra time, but with experience it’s not much extra time,” he asserted.
Indeed, in a series of 452 Mayo Clinic maze III patients, the cross-clamp and cardiopulmonary bypass times were 52 and 73 minutes, respectively, for those undergoing an isolated maze III, compared with 73 and 86 minutes for patients whose maze III was done in conjunction with other procedures, most commonly mitral valve repair or replacement.
An underrecognized group of patients who benefit from a standalone cut-and-sew maze are those with tachycardia-induced cardiomyopathy marked by AF or atrial flutter, rapid uncontrolled ventricular response, a decreased left ventricular ejection fraction, and no associated valvular or congenital heart disease. In a series of 37 such patients identified and treated with a maze III operation at the Mayo Clinic, their average preoperative left ventricular ejection fraction of 43% improved to about 55% at discharge, a benefit sustained at last follow-up a median of 63 months later. The outcome was particularly impressive in the 11 patients with a severely depressed left ventricular ejection fraction averaging 31% preoperatively, which jumped to 53% at discharge (Ann Thorac Surg. 2006 Aug;82[2]:494-500).
“Their ejection fraction goes up when you control the tachycardia-induced cardiomyopathy,” he observed. “So reduced left ventricular ejection fraction may be an indication for surgery rather than a contraindication.”
Dr. Schaff emphasized that it’s important for cardiologists and surgeons not to overpromise what surgical ablation of AF can accomplish. The only randomized trial of surgical ablation of AF versus no ablation during mitral valve surgery, sponsored by the National Institutes of Health and Canadian Institutes of Health Research and carried out by the Cardiothoracic Surgical Trials Network, showed no significant between-group differences at 1 year in any of numerous quality of life measures, nor was there a survival benefit for ablation (N Engl J Med. 2015 Apr 9;372(15):1399-409).
“We must point out that there’s no indication that controlling atrial fibrillation has anything to do with improving survival. It has to do with symptomatic benefit and perhaps reducing risk of stroke,” he said.
Dr. Schaff reported having no financial conflicts regarding his presentation.
SNOWMASS, COLO. – The term “maze procedure” for surgical ablation of atrial fibrillation is bandied about rather loosely these days, but as far as Hartzell V. Schaff, MD, is concerned, the operation of choice remains the classic cut-and-sew maze III procedure developed by James L. Cox, MD, while at Washington University, St. Louis.
“The classic Cox maze III, the cut-and-sew maze, is the best procedure for getting rid of atrial fibrillation and is in my view the gold standard. Some people argue that transmurality isn’t important, but it can occur because of gap lesions,” he said at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
Unlike modifications of the Cox maze III – such as the mini maze or the maze IV, which utilizes radiofrequency energy or cryoablation to create scars in an effort to achieve pulmonary vein isolation – the maze III cannot be done as a minimally invasive procedure. After all, it requires making incisions in both atria, along with aortic cross-clamping and cardiopulmonary bypass. But it has a significantly higher long-term rate of freedom from recurrent atrial fibrillation (AF) than the other operations. And crucially, it enables the surgeon to readily obliterate the left atrial appendage.
“The most important thing when you do any surgical procedure for atrial fibrillation, I think, is getting rid of the left atrial appendage. When you do cut-and-sew maze, that’s done 100% of the time,” explained Dr. Schaff, professor of surgery at the Mayo Clinic in Rochester, Minn.
“We really have a lot of work left to do as surgeons in improving the outcome of surgery for atrial fibrillation. One of the things we as surgeons don’t do well is getting rid of the left atrial appendage. This ought to be done in every patient that has surgical ablation for atrial fibrillation,” according to the cardiothoracic surgeon.
And yet, he continued, in a series of nearly 87,000 patients with AF who underwent nonemergent cardiac surgery in the Society of Thoracic Surgeons database, 48.0% of whom underwent surgical ablation for AF, only 63.9% of those who had standalone ablation for lone AF got their left atrial appendage dealt with, compared with 86%-89% of those who underwent concomitant cardiac surgery, such as mitral valve repair or replacement (Ann Thorac Surg. 2017 Aug;104[2]:493-500).
“That’s awful, really. And the reason for that low left atrial appendage obliteration rate is this: For many of those patients who had surgery for lone atrial fibrillation, the surgeons were trying to do minimally invasive surgery, where they do pulmonary vein isolation on the right side, so they don’t have access to the left atrial appendage,” Dr. Schaff said.
“In the past,” he recalled, “we would ligate the left atrial appendage. Nowadays because of echocardiographic studies that show there’s persistent patency in a sizable percentage of patients, we amputate the left atrial appendage in almost all of the patients.”
The terminology surrounding surgical ablation for AF, in his view, has become rather confusing. “Most of you, when you refer a patient for surgical ablation for AF, the surgeons will just say they do a maze procedure,” Dr. Schaff cautioned. “Somehow, all of that [maze IV, mini maze] today is lumped together as a classic maze procedure, but it’s really not. We have different lesion sets and energy sources.”
And different outcomes as well. In a series of 1,189 adults who underwent surgical ablation for AF at the Mayo Clinic, of whom 44% had a biatrial cut-and-sew maze while the rest had surgical cryotherapy, radiofrequency ablation, or a combination of the two, the rate of freedom from AF 1 year post surgery was 85% with the cut-and-sew maze versus 71% with the alternatives. At 5 years or more, the rates were 78% and 52%, respectively. In a multivariate analysis, freedom from AF was independently associated with preoperative paroxysmal rather than permanent AF, performance of the classic maze III procedure, concomitant treatment of associated mitral valve disease, and younger age.
Moreover, rates of the major early postoperative complications – stroke, bleeding, and renal failure – were similar in the cut-and-sew maze III and other groups.
“So a lesser procedure doesn’t necessarily mean fewer complications,” Dr. Schaff noted.
One of the criticisms levied against the maze III is that it’s too much surgery for AF. But it’s actually relatively inexpensive because the disposables – suture, needles, scalpel – are those used in the commonly performed concomitant cardiac surgical procedures. “The Cox maze III does take extra time, but with experience it’s not much extra time,” he asserted.
Indeed, in a series of 452 Mayo Clinic maze III patients, the cross-clamp and cardiopulmonary bypass times were 52 and 73 minutes, respectively, for those undergoing an isolated maze III, compared with 73 and 86 minutes for patients whose maze III was done in conjunction with other procedures, most commonly mitral valve repair or replacement.
An underrecognized group of patients who benefit from a standalone cut-and-sew maze are those with tachycardia-induced cardiomyopathy marked by AF or atrial flutter, rapid uncontrolled ventricular response, a decreased left ventricular ejection fraction, and no associated valvular or congenital heart disease. In a series of 37 such patients identified and treated with a maze III operation at the Mayo Clinic, their average preoperative left ventricular ejection fraction of 43% improved to about 55% at discharge, a benefit sustained at last follow-up a median of 63 months later. The outcome was particularly impressive in the 11 patients with a severely depressed left ventricular ejection fraction averaging 31% preoperatively, which jumped to 53% at discharge (Ann Thorac Surg. 2006 Aug;82[2]:494-500).
“Their ejection fraction goes up when you control the tachycardia-induced cardiomyopathy,” he observed. “So reduced left ventricular ejection fraction may be an indication for surgery rather than a contraindication.”
Dr. Schaff emphasized that it’s important for cardiologists and surgeons not to overpromise what surgical ablation of AF can accomplish. The only randomized trial of surgical ablation of AF versus no ablation during mitral valve surgery, sponsored by the National Institutes of Health and Canadian Institutes of Health Research and carried out by the Cardiothoracic Surgical Trials Network, showed no significant between-group differences at 1 year in any of numerous quality of life measures, nor was there a survival benefit for ablation (N Engl J Med. 2015 Apr 9;372(15):1399-409).
“We must point out that there’s no indication that controlling atrial fibrillation has anything to do with improving survival. It has to do with symptomatic benefit and perhaps reducing risk of stroke,” he said.
Dr. Schaff reported having no financial conflicts regarding his presentation.
REPORTING FROM ACC SNOWMASS 2019
Migraine Update: A Review of CGRP-targeted Therapies
This supplement to Neurology Reviews features an interview with Stewart J. Tepper, MD, who reviews promising data on monoclonal antibodies that target calcitonin gene-related peptide (CGRP) and discusses how the introduction of these treatments is benefitting patients with migraine.
"These medications can manifest very different levels of efficacy, safety, and tolerability than we've ever seen before. What I'm seeing in practice can be jaw-dropping." - Stewart J. Tepper, MD
This supplement to Neurology Reviews features an interview with Stewart J. Tepper, MD, who reviews promising data on monoclonal antibodies that target calcitonin gene-related peptide (CGRP) and discusses how the introduction of these treatments is benefitting patients with migraine.
"These medications can manifest very different levels of efficacy, safety, and tolerability than we've ever seen before. What I'm seeing in practice can be jaw-dropping." - Stewart J. Tepper, MD
This supplement to Neurology Reviews features an interview with Stewart J. Tepper, MD, who reviews promising data on monoclonal antibodies that target calcitonin gene-related peptide (CGRP) and discusses how the introduction of these treatments is benefitting patients with migraine.
"These medications can manifest very different levels of efficacy, safety, and tolerability than we've ever seen before. What I'm seeing in practice can be jaw-dropping." - Stewart J. Tepper, MD
Parental Migraine Linked with Offspring Migraine
Parental migraine is associated with offspring migraine, with a stronger association for maternal migraine, according to a recent study. Therefore, this may indicate maternal-specific transmission. Researchers utilized data from the HUNT Study, a large, population-based cohort study. Using a cross-sectional design, the sample consisted of 13,731 parents and 8970 offspring. Logistic regression was used to calculate odds ratios with 95% confidence intervals for active migraine and non-migrainous headache in offspring, given active maternal or paternal headache. They found:
There was a significant association between maternal migraine and offspring migraine (odds ratio 2.76).
A weaker association was found between paternal migraine and offspring migraine (odds ratio 1.67).
For non-migrainous headache, there was a significant association between mothers and offspring (odds ratio 1.25), but not between fathers and offspring.
Børte S, Zwart J-A, Stendland SØ, Hagen K, Winsvold BS. Parental migraine in relation to migraine in offspring: Family linkage analyses from the HUNT Study. [Published online ahead of print February 2, 2019]. Cephalalgia. doi:10.1177%2F0333102419828989.
Parental migraine is associated with offspring migraine, with a stronger association for maternal migraine, according to a recent study. Therefore, this may indicate maternal-specific transmission. Researchers utilized data from the HUNT Study, a large, population-based cohort study. Using a cross-sectional design, the sample consisted of 13,731 parents and 8970 offspring. Logistic regression was used to calculate odds ratios with 95% confidence intervals for active migraine and non-migrainous headache in offspring, given active maternal or paternal headache. They found:
There was a significant association between maternal migraine and offspring migraine (odds ratio 2.76).
A weaker association was found between paternal migraine and offspring migraine (odds ratio 1.67).
For non-migrainous headache, there was a significant association between mothers and offspring (odds ratio 1.25), but not between fathers and offspring.
Børte S, Zwart J-A, Stendland SØ, Hagen K, Winsvold BS. Parental migraine in relation to migraine in offspring: Family linkage analyses from the HUNT Study. [Published online ahead of print February 2, 2019]. Cephalalgia. doi:10.1177%2F0333102419828989.
Parental migraine is associated with offspring migraine, with a stronger association for maternal migraine, according to a recent study. Therefore, this may indicate maternal-specific transmission. Researchers utilized data from the HUNT Study, a large, population-based cohort study. Using a cross-sectional design, the sample consisted of 13,731 parents and 8970 offspring. Logistic regression was used to calculate odds ratios with 95% confidence intervals for active migraine and non-migrainous headache in offspring, given active maternal or paternal headache. They found:
There was a significant association between maternal migraine and offspring migraine (odds ratio 2.76).
A weaker association was found between paternal migraine and offspring migraine (odds ratio 1.67).
For non-migrainous headache, there was a significant association between mothers and offspring (odds ratio 1.25), but not between fathers and offspring.
Børte S, Zwart J-A, Stendland SØ, Hagen K, Winsvold BS. Parental migraine in relation to migraine in offspring: Family linkage analyses from the HUNT Study. [Published online ahead of print February 2, 2019]. Cephalalgia. doi:10.1177%2F0333102419828989.