User login
Cutaneous Collagenous Vasculopathy
To the Editor:
Cutaneous microangiopathy describes pathology of the small blood vessels within the dermis.
We report a case of CCV in a 41-year-old woman who presented for evaluation of a rash on the bilateral lower extremities of 7 to 8 months’ duration. The eruption had started on the left ankle and spread over several weeks to the bilateral dorsal feet followed by the ankles and shins. The patient noted associated swelling and a pressure like dysesthesia of the lower legs. She was otherwise in good health, though she had started an oral contraceptive 1 year prior for heavy menstrual bleeding. A review of systems was negative for deep vein thrombosis, pulmonary embolus, and other thromboembolic phenomena, and the patient had no history of hepatic or renal dysfunction, cancer, or heart disease. Her family history was negative for clotting disorders or bleeding diatheses.
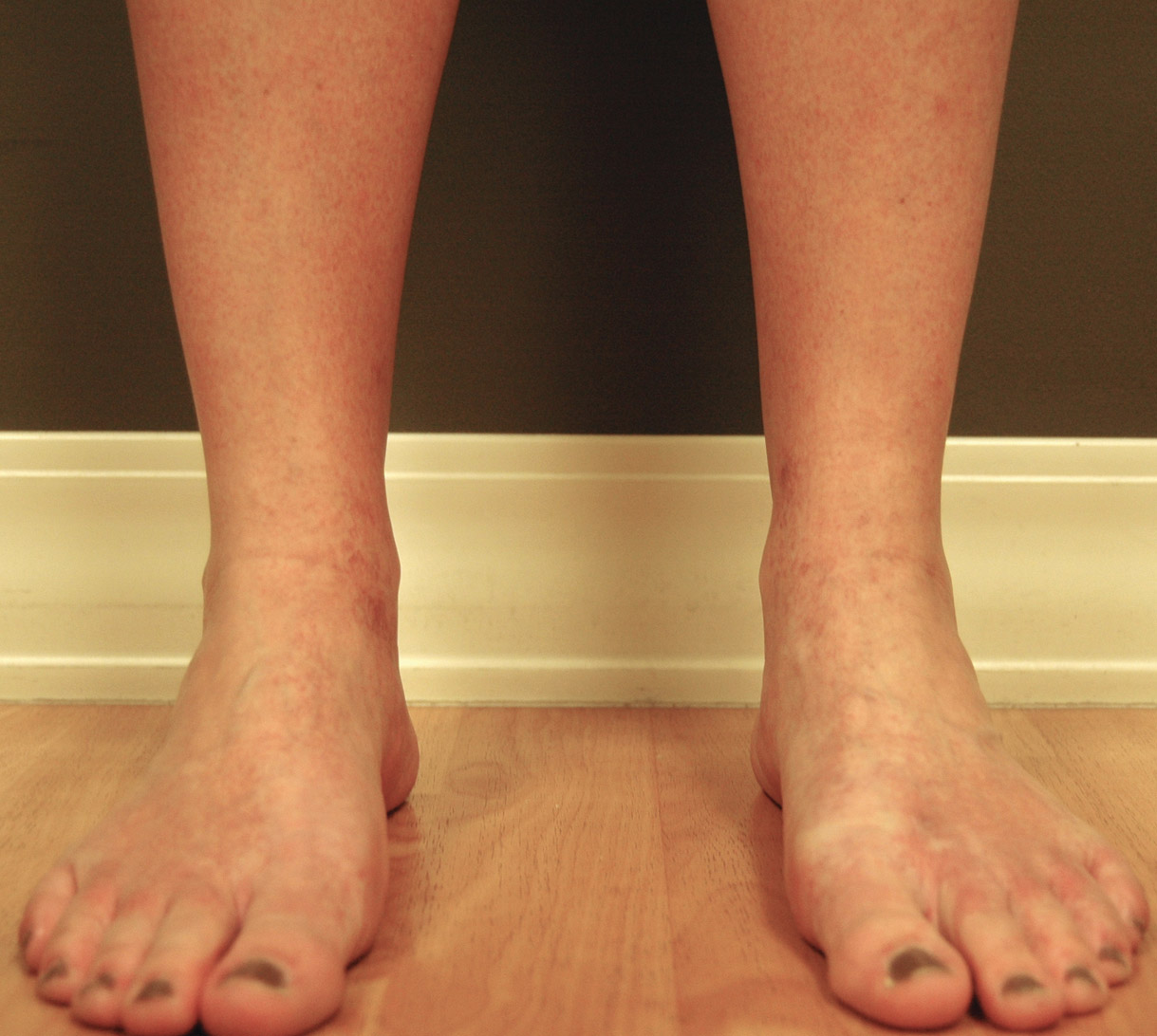
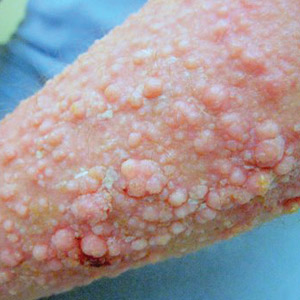
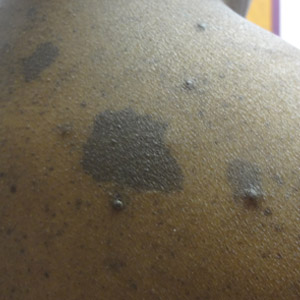
On physical examination, telangiectatic matting was present on the bilateral ankles and dorsal feet with an associated blanchable erythema (Figure 1). The matting extended into a fine, mottled, pretibial telangiectasia associated with Schamberg purpura. She had no pitting edema, and both dorsalis pedis and posterior popliteal pulses were intact and symmetric bilaterally. No popliteal lymphadenopathy or palpable cords were present.
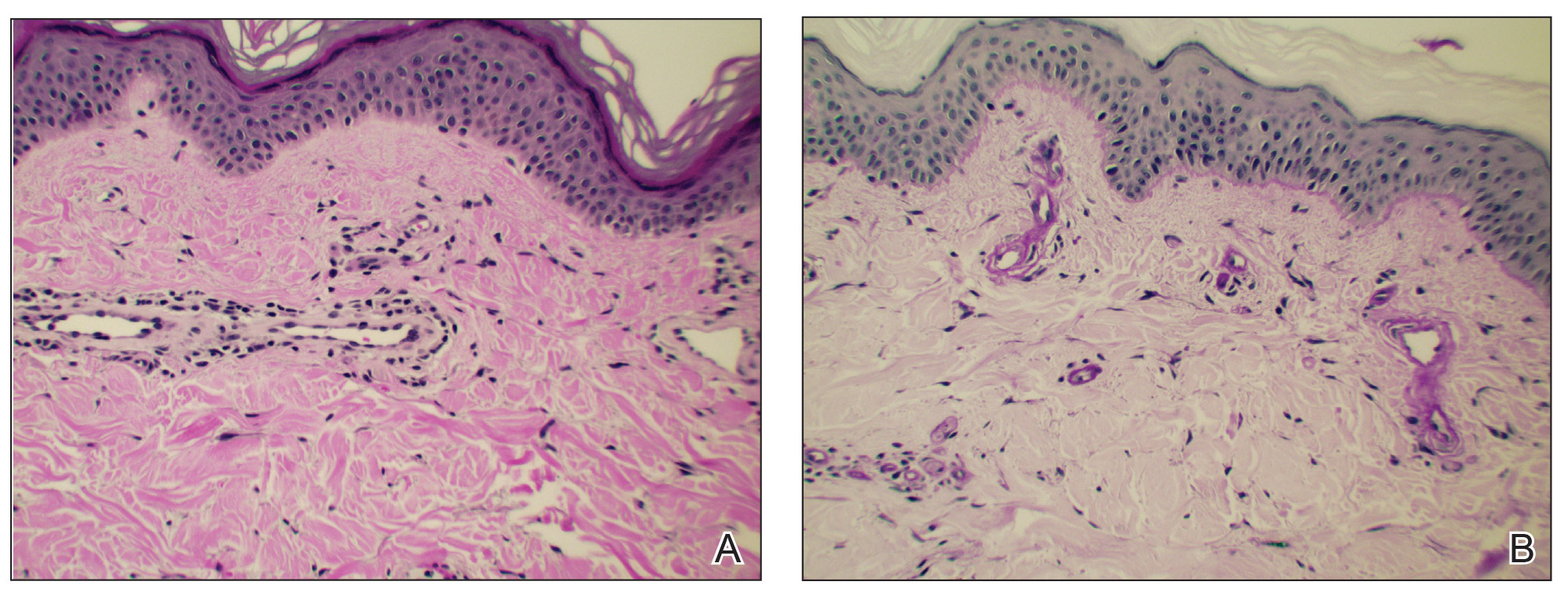
Two punch biopsies taken from the erythematous telangiectatic area on the left foot and metatarsal region demonstrated an unremarkable epidermis without interface change, thickening of the epidermal basement membrane, or single-cell dyskeratosis. There was mild dilatation of blood vessels within the superficial dermis with mild perivascular lymphocytic inflammation and rare extravasated erythrocytes. Leukocytoclastic debris, fibrinoid necrosis of vessel walls, and endothelial cell necrosis were not seen. As is classic in CCV, the vessel walls appeared thickened by eosinophilic hyaline material, which was periodic acid–Schiff positive and diastase resistant (Figure 2). Sclerotic thickening of collagen bundles or absence of periadnexal adipose tissue was not seen. CD34 immunohistochemical staining demonstrated normal retained CD34 interstitial dermal positivity, which excluded morphea. Additionally, direct immunofluorescence testing was negative for IgG, IgA, IgM, C3, fibrin, and C1q. Nodular reduplication of vessels or other changes of stasis were not seen. Fibrin thrombi or neoplastic cells were not identified. The clinical and histopathologic findings were suggestive of CCV.
Prior case reports of CCV have described a similar clinical manifestation with blanching macules that occur symmetrically on the lower extremities and spread cephalically.1-6 A distinction from hereditary hemorrhagic telangiectasia is the noninvolvement of mucous membranes and nails. The etiology of this rare microangiopathy has not been elucidated, though disease concurrence with local trauma, stressful events such as childbirth, and diabetes mellitus has been documented.6 As the body of literature continues to grow, more research regarding the etiology, mechanism, prognosis, and treatment options will enhance our understanding of CCV.
- Bondier L, Tardieu M, Leveque P, et al. Cutaneous collagenous vasculopathy: report of two cases presenting as disseminated telangiectasias and review of the literature. Am J Dermatopathol. 2017;39:682-688.
- Salama S, Rosenthal D. Cutaneous collagenous vasculopathy with generalized telangiectasia: an immunohistochemical and ultrastructural study. J Cutan Pathol. 2000;27:40-48.
- Lloyd BM, Pruden SJ, Lind AC, et al. Cutaneous collagenous vasculopathy: report of the first pediatric case. Pediatr Dermatol. 2011;28:598-599.
- Salama S, Chorneyko K, Belovic B. Cutaneous collagenous vasculopathy associated with intravascular occlusive fibrin thrombi. J Cutan Pathol. 2014;41:386-393.
- Perez A, Wain ME, Robson A, et al. Cutaneous collagenous vasculopathy with generalized telangiectasia in two female patients. J Am Acad Dermatol. 2010;63:882-885.
- Burdick LM, Losher S, Somach SC, et al. Cutaneous collagenous vasculopathy: a rare cutaneous microangiopathy. J Cutan Pathol. 2012;39:741-746.
To the Editor:
Cutaneous microangiopathy describes pathology of the small blood vessels within the dermis.
We report a case of CCV in a 41-year-old woman who presented for evaluation of a rash on the bilateral lower extremities of 7 to 8 months’ duration. The eruption had started on the left ankle and spread over several weeks to the bilateral dorsal feet followed by the ankles and shins. The patient noted associated swelling and a pressure like dysesthesia of the lower legs. She was otherwise in good health, though she had started an oral contraceptive 1 year prior for heavy menstrual bleeding. A review of systems was negative for deep vein thrombosis, pulmonary embolus, and other thromboembolic phenomena, and the patient had no history of hepatic or renal dysfunction, cancer, or heart disease. Her family history was negative for clotting disorders or bleeding diatheses.
On physical examination, telangiectatic matting was present on the bilateral ankles and dorsal feet with an associated blanchable erythema (Figure 1). The matting extended into a fine, mottled, pretibial telangiectasia associated with Schamberg purpura. She had no pitting edema, and both dorsalis pedis and posterior popliteal pulses were intact and symmetric bilaterally. No popliteal lymphadenopathy or palpable cords were present.
Two punch biopsies taken from the erythematous telangiectatic area on the left foot and metatarsal region demonstrated an unremarkable epidermis without interface change, thickening of the epidermal basement membrane, or single-cell dyskeratosis. There was mild dilatation of blood vessels within the superficial dermis with mild perivascular lymphocytic inflammation and rare extravasated erythrocytes. Leukocytoclastic debris, fibrinoid necrosis of vessel walls, and endothelial cell necrosis were not seen. As is classic in CCV, the vessel walls appeared thickened by eosinophilic hyaline material, which was periodic acid–Schiff positive and diastase resistant (Figure 2). Sclerotic thickening of collagen bundles or absence of periadnexal adipose tissue was not seen. CD34 immunohistochemical staining demonstrated normal retained CD34 interstitial dermal positivity, which excluded morphea. Additionally, direct immunofluorescence testing was negative for IgG, IgA, IgM, C3, fibrin, and C1q. Nodular reduplication of vessels or other changes of stasis were not seen. Fibrin thrombi or neoplastic cells were not identified. The clinical and histopathologic findings were suggestive of CCV.
Prior case reports of CCV have described a similar clinical manifestation with blanching macules that occur symmetrically on the lower extremities and spread cephalically.1-6 A distinction from hereditary hemorrhagic telangiectasia is the noninvolvement of mucous membranes and nails. The etiology of this rare microangiopathy has not been elucidated, though disease concurrence with local trauma, stressful events such as childbirth, and diabetes mellitus has been documented.6 As the body of literature continues to grow, more research regarding the etiology, mechanism, prognosis, and treatment options will enhance our understanding of CCV.
To the Editor:
Cutaneous microangiopathy describes pathology of the small blood vessels within the dermis.
We report a case of CCV in a 41-year-old woman who presented for evaluation of a rash on the bilateral lower extremities of 7 to 8 months’ duration. The eruption had started on the left ankle and spread over several weeks to the bilateral dorsal feet followed by the ankles and shins. The patient noted associated swelling and a pressure like dysesthesia of the lower legs. She was otherwise in good health, though she had started an oral contraceptive 1 year prior for heavy menstrual bleeding. A review of systems was negative for deep vein thrombosis, pulmonary embolus, and other thromboembolic phenomena, and the patient had no history of hepatic or renal dysfunction, cancer, or heart disease. Her family history was negative for clotting disorders or bleeding diatheses.
On physical examination, telangiectatic matting was present on the bilateral ankles and dorsal feet with an associated blanchable erythema (Figure 1). The matting extended into a fine, mottled, pretibial telangiectasia associated with Schamberg purpura. She had no pitting edema, and both dorsalis pedis and posterior popliteal pulses were intact and symmetric bilaterally. No popliteal lymphadenopathy or palpable cords were present.
Two punch biopsies taken from the erythematous telangiectatic area on the left foot and metatarsal region demonstrated an unremarkable epidermis without interface change, thickening of the epidermal basement membrane, or single-cell dyskeratosis. There was mild dilatation of blood vessels within the superficial dermis with mild perivascular lymphocytic inflammation and rare extravasated erythrocytes. Leukocytoclastic debris, fibrinoid necrosis of vessel walls, and endothelial cell necrosis were not seen. As is classic in CCV, the vessel walls appeared thickened by eosinophilic hyaline material, which was periodic acid–Schiff positive and diastase resistant (Figure 2). Sclerotic thickening of collagen bundles or absence of periadnexal adipose tissue was not seen. CD34 immunohistochemical staining demonstrated normal retained CD34 interstitial dermal positivity, which excluded morphea. Additionally, direct immunofluorescence testing was negative for IgG, IgA, IgM, C3, fibrin, and C1q. Nodular reduplication of vessels or other changes of stasis were not seen. Fibrin thrombi or neoplastic cells were not identified. The clinical and histopathologic findings were suggestive of CCV.
Prior case reports of CCV have described a similar clinical manifestation with blanching macules that occur symmetrically on the lower extremities and spread cephalically.1-6 A distinction from hereditary hemorrhagic telangiectasia is the noninvolvement of mucous membranes and nails. The etiology of this rare microangiopathy has not been elucidated, though disease concurrence with local trauma, stressful events such as childbirth, and diabetes mellitus has been documented.6 As the body of literature continues to grow, more research regarding the etiology, mechanism, prognosis, and treatment options will enhance our understanding of CCV.
- Bondier L, Tardieu M, Leveque P, et al. Cutaneous collagenous vasculopathy: report of two cases presenting as disseminated telangiectasias and review of the literature. Am J Dermatopathol. 2017;39:682-688.
- Salama S, Rosenthal D. Cutaneous collagenous vasculopathy with generalized telangiectasia: an immunohistochemical and ultrastructural study. J Cutan Pathol. 2000;27:40-48.
- Lloyd BM, Pruden SJ, Lind AC, et al. Cutaneous collagenous vasculopathy: report of the first pediatric case. Pediatr Dermatol. 2011;28:598-599.
- Salama S, Chorneyko K, Belovic B. Cutaneous collagenous vasculopathy associated with intravascular occlusive fibrin thrombi. J Cutan Pathol. 2014;41:386-393.
- Perez A, Wain ME, Robson A, et al. Cutaneous collagenous vasculopathy with generalized telangiectasia in two female patients. J Am Acad Dermatol. 2010;63:882-885.
- Burdick LM, Losher S, Somach SC, et al. Cutaneous collagenous vasculopathy: a rare cutaneous microangiopathy. J Cutan Pathol. 2012;39:741-746.
- Bondier L, Tardieu M, Leveque P, et al. Cutaneous collagenous vasculopathy: report of two cases presenting as disseminated telangiectasias and review of the literature. Am J Dermatopathol. 2017;39:682-688.
- Salama S, Rosenthal D. Cutaneous collagenous vasculopathy with generalized telangiectasia: an immunohistochemical and ultrastructural study. J Cutan Pathol. 2000;27:40-48.
- Lloyd BM, Pruden SJ, Lind AC, et al. Cutaneous collagenous vasculopathy: report of the first pediatric case. Pediatr Dermatol. 2011;28:598-599.
- Salama S, Chorneyko K, Belovic B. Cutaneous collagenous vasculopathy associated with intravascular occlusive fibrin thrombi. J Cutan Pathol. 2014;41:386-393.
- Perez A, Wain ME, Robson A, et al. Cutaneous collagenous vasculopathy with generalized telangiectasia in two female patients. J Am Acad Dermatol. 2010;63:882-885.
- Burdick LM, Losher S, Somach SC, et al. Cutaneous collagenous vasculopathy: a rare cutaneous microangiopathy. J Cutan Pathol. 2012;39:741-746.
Practice Points
- In cutaneous collagenous vasculopathy (CCV), skin biopsy may demonstrate eosinophilic hyaline thickening of superficial dermal blood vessels with mild perivascular lymphocytic inflammation and rare extravasated erythrocytes.
- Lack of mucous membrane and nail involvement differentiates CCV from hereditary hemorrhagic telangiectasia.
Elephantiasis Nostras Verrucosa Secondary to Scleroderma
To the Editor:
Elephantiasis nostras verrucosa (ENV) is a skin disorder caused by marked underlying lymphedema that leads to hyperkeratosis, papillomatosis, and verrucous growths on the epidermis.1 The pathophysiology of ENV relates to noninfectious lymphatic obstruction and lymphatic fibrosis secondary to venous stasis, malignancy, radiation therapy, or trauma.2 We present an unusual case of lymphedema and subsequent ENV limited to the arms and hands in a patient with scleroderma, an autoimmune fibrosing disorder.
A 54-year-old woman with a 5-year history of scleroderma presented to our dermatology clinic for treatment of progressive skin changes including pruritus, tightness, finger ulcerations, and pus exuding from papules on the dorsal arms and hands. She had been experiencing several systemic symptoms including dysphagia and lung involvement, necessitating oxygen therapy and a continuous positive airway pressure device for pulmonary arterial hypertension. A computed tomography scan of the lungs demonstrated an increase in ground-glass infiltrates in the right lower lobe and an air-fluid level in the esophagus. At the time of presentation, she was being treated with bosentan and sildenafil for pulmonary arterial hypertension, in addition to prednisone, venlafaxine, lansoprazole, metoclopramide, levothyroxine, temazepam, aspirin, and oxycodone. In the 2 years prior to presentation, she had been treated with intravenous cyclophosphamide once monthly for 6 months, adalimumab for 1 year, and 1 session of photodynamic therapy to the arms, all without benefit.
Physical examination showed cutaneous signs of scleroderma including marked sclerosis of the skin on the face, hands, V of the neck, proximal arms, and mid and proximal thighs. Excoriated papules with overlying crusting and pustulation were superimposed on the sclerotic skin of the arms (Figure 1).
A superinfection was diagnosed and treated with cephalexin 500 mg 4 times daily for 2 weeks; thereafter, mupirocin cream twice daily was used as needed. She was prescribed fexofenadine 180 mg twice daily and doxepin 20 mg at bedtime for pruritus.
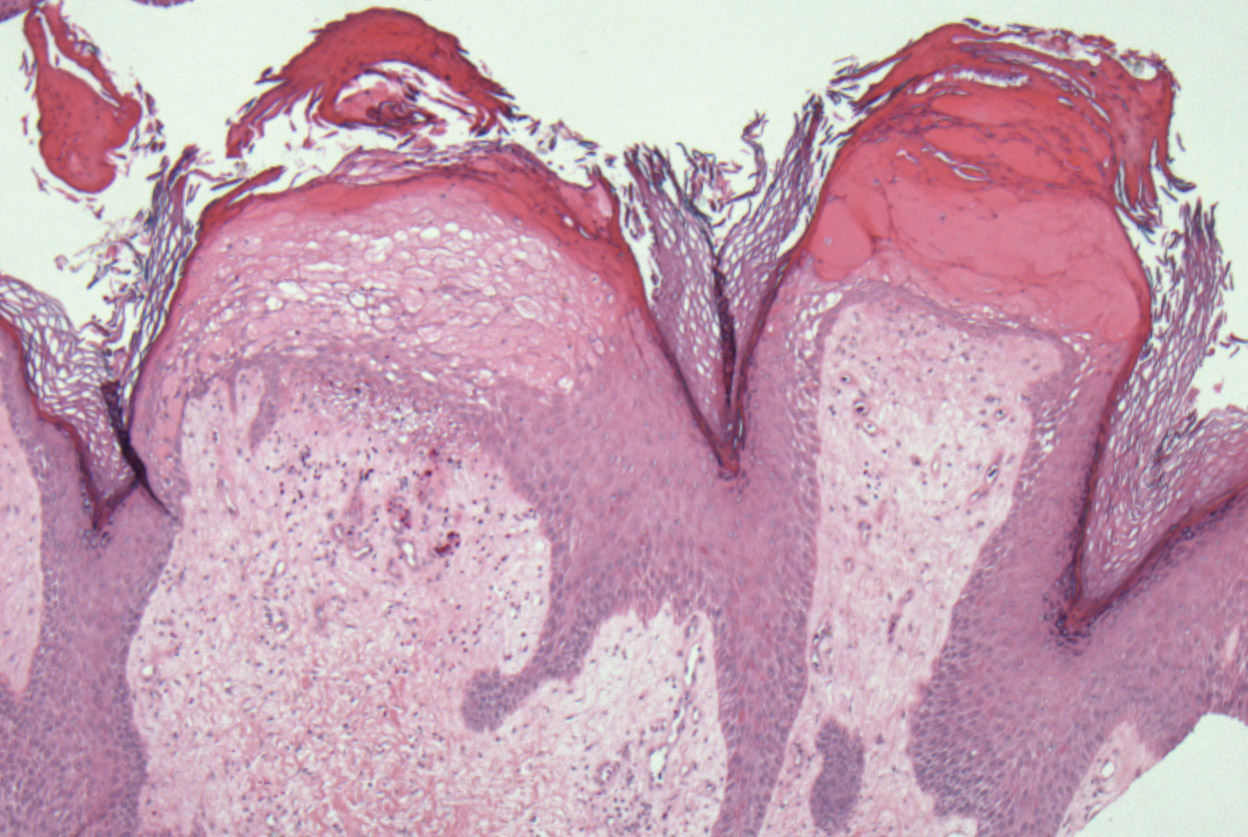
At 3-week follow-up, a trial of narrowband UVB therapy was recommended for control of pruritus. Two weeks later, a modified wet-wrap regimen using clobetasol ointment 0.5% twice daily covered with wet gauze followed by a self-adherent dressing was initiated only on the right arm for comparison purposes. This treatment was not successful. A biopsy taken from the left arm showed lymphedema with perivascular fibroplasia and epidermal hyperplasia consistent with ENV (Figure 2).
Two months after her initial presentation, we instituted treatment with tazarotene gel 0.1% twice daily to the arms as well as a water-based topical emulsion to the finger ulcerations and a healing ointment to the hands. A month later, the patient reported no benefit with tazarotene. She desired more flexibility in her arms and hands; therefore, after a discussion with her rheumatologist, biweekly psoralen plus UVA (PUVA) therapy was initiated. Five months after presentation, methotrexate (MTX) 15 mg once weekly with folic acid 1 mg once daily was added. The PUVA therapy and MTX were stopped 3 months later due to lack of treatment benefit.
The patient was referred to vascular medicine for possible compression therapy. It was determined that her vasculature was intact, but compression therapy was contraindicated due to underlying systemic sclerosis. She was subsequently prescribed mycophenolate mofetil 1000 mg twice daily by her rheumatologist. The options of serial excisions or laser resurfacing were presented, but she declined.
Elephantiasis nostras verrucosa is differentiated from elephantiasis tropica, which is caused by a filarial infection of the lymphatic system. The chronic obstructive lymphedema characteristic of ENV can present as a result of various primary or secondary etiologies including trauma, malignancy, venous stasis, inflammation, or infection.3 In systemic sclerosis, extravascular fibrosis theoretically can lead to lymphatic obstruction and subsequent lymphatic stasis. In turn, the pathophysiology of dermal and subcutaneous fibrosis likely reflects autoantibodies (eg, anticardiolipin antibodies) that can damage lymphatic and nonlymphatic vessels.4,5
As the underlying mechanism of ENV, fibrosis of lymphatic vessels in systemic sclerosis is not well documented. Characteristic features of systemic sclerosis include extensive fibrosis, fibroproliferative vasculopathy, and inflammation, which are all possible mechanisms for the internal lymphatic obstruction resulting in the skin changes observed in ENV.6 It seemed unusual that the fibrotic changes of lymphatic vessels in our patient were extensive enough to cause ENV of the upper extremities; lower extremity involvement is the more common presentation because of the greater likelihood of lymphedema manifesting in the legs and feet. Lower extremity ENV has been reported in association with scleroderma.7,8
Regarding therapeutic options, Boyd et al9 reported a good response in a patient with ENV of the abdomen who was treated with topical tazarotene. Additionally, PUVA and MTX have been reported to be beneficial for the progressive skin changes of systemic sclerosis.10 Mycophenolate mofetil has been used in patients who fail MTX therapy because of its antifibrotic properties without the side-effect profiles of other immunosuppressives, such as imatinib.10,11 In our patient, skin lesions persisted following these varied approaches, and compression therapy was not advised due to the underlying sclerosis.
Because options for medical treatment of severe ENV are limited, surgical debridement of the affected limb often remains the only viable option in advanced cases.12 A PubMed search of articles indexed for MEDLINE using the terms elephantiasis (MeSH terms) or elephantiasis (all fields) and scleroderma, systemic (MeSH terms) or scleroderma (all fields) and systemic (all fields) or systemic scleroderma (all fields) or scleroderma (all fields) or scleroderma, localized (MeSH terms) or scleroderma (all fields) and localized (all fields) or localized scleroderma (all fields) yielded only 1 other case report of lower extremity ENV in a patient with systemic sclerosis who ultimately required bilateral leg amputation.8 When possible, avoiding lymphostasis through compression and control of any underlying infections is important in the treatment and prevention of ENV.3
- Sisto K, Khachemoune A. Elephantiasis nostras verrucosa: a review. Am J Clin Dermatol. 2008;9:141-146.
- Schissel DJ, Hivnor C, Elston DM. Elephantiasis nostras verrucosa. Cutis. 1998;62:77-80.
- Duckworth A, Husain J, DeHeer P. Elephantiasis nostras verrucosa or ‘mossy foot lesions’ in lymphedema praecox. J Am Podiatr Med Assoc. 2008;98:66-69.
- Assous N, Allanore Y, Batteaux F, et al. Prevalence of antiphospholipid antibodies in systemic sclerosis and association with primitive pulmonary arterial hypertension and endothelial injury. Clin Exp Rheumatol. 2005;23:199-204.
- Derrett-Smith EC, Dooley A, Gilbane AJ, et al. Endothelial injury in a transforming growth-factor-dependent mouse model of scleroderma induces pulmonary arterial hypertension. Arthritis Rheum. 2013;65:2928-2939.
- Pattanaik M, Brown M, Postlethwaite A. Vascular involvement in systemic sclerosis (scleroderma). J Inflamm Res. 2011;4:105-125.
- Kerchner K, Fleischer A, Yosipovitch G. Lower extremity lymphedema update: pathophysiology, diagnosis and treatment guidelines. J Am Acad Dermatol. 2008;59:324-331.
- Chatterjee S, Karai L. Elephantiasis nostras verrucosa in a patient with systemic sclerosis. Clin Exp Dermatol. 2009;34:e696-e698.
- Boyd J, Sloan S, Meffert J. Elephantiasis nostrum verrucosa of the abdomen: clinical results with tazarotene. J Drugs Dermatol. 2004;3:446-448.
- Fett, N. Scleroderma: nomenclature, etiology, pathogenesis, prognosis, and treatments: facts and controversies. Clin Dermatol. 2013;31:432-437.
- Moinzadeh P, Krieg T, Hunzelmann N. Imatinib treatment of generalized localized scleroderma (morphea). J Am Acad Dermatol. 2010;63:e102-e104.
- Iwao F, Sato-Matsumura KC, Sawamura D, et al. Elephantiasis nostras verrucosa successfully treated by surgical debridement. Dermatol Surg. 2004;30:939-941.
To the Editor:
Elephantiasis nostras verrucosa (ENV) is a skin disorder caused by marked underlying lymphedema that leads to hyperkeratosis, papillomatosis, and verrucous growths on the epidermis.1 The pathophysiology of ENV relates to noninfectious lymphatic obstruction and lymphatic fibrosis secondary to venous stasis, malignancy, radiation therapy, or trauma.2 We present an unusual case of lymphedema and subsequent ENV limited to the arms and hands in a patient with scleroderma, an autoimmune fibrosing disorder.
A 54-year-old woman with a 5-year history of scleroderma presented to our dermatology clinic for treatment of progressive skin changes including pruritus, tightness, finger ulcerations, and pus exuding from papules on the dorsal arms and hands. She had been experiencing several systemic symptoms including dysphagia and lung involvement, necessitating oxygen therapy and a continuous positive airway pressure device for pulmonary arterial hypertension. A computed tomography scan of the lungs demonstrated an increase in ground-glass infiltrates in the right lower lobe and an air-fluid level in the esophagus. At the time of presentation, she was being treated with bosentan and sildenafil for pulmonary arterial hypertension, in addition to prednisone, venlafaxine, lansoprazole, metoclopramide, levothyroxine, temazepam, aspirin, and oxycodone. In the 2 years prior to presentation, she had been treated with intravenous cyclophosphamide once monthly for 6 months, adalimumab for 1 year, and 1 session of photodynamic therapy to the arms, all without benefit.
Physical examination showed cutaneous signs of scleroderma including marked sclerosis of the skin on the face, hands, V of the neck, proximal arms, and mid and proximal thighs. Excoriated papules with overlying crusting and pustulation were superimposed on the sclerotic skin of the arms (Figure 1).
A superinfection was diagnosed and treated with cephalexin 500 mg 4 times daily for 2 weeks; thereafter, mupirocin cream twice daily was used as needed. She was prescribed fexofenadine 180 mg twice daily and doxepin 20 mg at bedtime for pruritus.
At 3-week follow-up, a trial of narrowband UVB therapy was recommended for control of pruritus. Two weeks later, a modified wet-wrap regimen using clobetasol ointment 0.5% twice daily covered with wet gauze followed by a self-adherent dressing was initiated only on the right arm for comparison purposes. This treatment was not successful. A biopsy taken from the left arm showed lymphedema with perivascular fibroplasia and epidermal hyperplasia consistent with ENV (Figure 2).
Two months after her initial presentation, we instituted treatment with tazarotene gel 0.1% twice daily to the arms as well as a water-based topical emulsion to the finger ulcerations and a healing ointment to the hands. A month later, the patient reported no benefit with tazarotene. She desired more flexibility in her arms and hands; therefore, after a discussion with her rheumatologist, biweekly psoralen plus UVA (PUVA) therapy was initiated. Five months after presentation, methotrexate (MTX) 15 mg once weekly with folic acid 1 mg once daily was added. The PUVA therapy and MTX were stopped 3 months later due to lack of treatment benefit.
The patient was referred to vascular medicine for possible compression therapy. It was determined that her vasculature was intact, but compression therapy was contraindicated due to underlying systemic sclerosis. She was subsequently prescribed mycophenolate mofetil 1000 mg twice daily by her rheumatologist. The options of serial excisions or laser resurfacing were presented, but she declined.
Elephantiasis nostras verrucosa is differentiated from elephantiasis tropica, which is caused by a filarial infection of the lymphatic system. The chronic obstructive lymphedema characteristic of ENV can present as a result of various primary or secondary etiologies including trauma, malignancy, venous stasis, inflammation, or infection.3 In systemic sclerosis, extravascular fibrosis theoretically can lead to lymphatic obstruction and subsequent lymphatic stasis. In turn, the pathophysiology of dermal and subcutaneous fibrosis likely reflects autoantibodies (eg, anticardiolipin antibodies) that can damage lymphatic and nonlymphatic vessels.4,5
As the underlying mechanism of ENV, fibrosis of lymphatic vessels in systemic sclerosis is not well documented. Characteristic features of systemic sclerosis include extensive fibrosis, fibroproliferative vasculopathy, and inflammation, which are all possible mechanisms for the internal lymphatic obstruction resulting in the skin changes observed in ENV.6 It seemed unusual that the fibrotic changes of lymphatic vessels in our patient were extensive enough to cause ENV of the upper extremities; lower extremity involvement is the more common presentation because of the greater likelihood of lymphedema manifesting in the legs and feet. Lower extremity ENV has been reported in association with scleroderma.7,8
Regarding therapeutic options, Boyd et al9 reported a good response in a patient with ENV of the abdomen who was treated with topical tazarotene. Additionally, PUVA and MTX have been reported to be beneficial for the progressive skin changes of systemic sclerosis.10 Mycophenolate mofetil has been used in patients who fail MTX therapy because of its antifibrotic properties without the side-effect profiles of other immunosuppressives, such as imatinib.10,11 In our patient, skin lesions persisted following these varied approaches, and compression therapy was not advised due to the underlying sclerosis.
Because options for medical treatment of severe ENV are limited, surgical debridement of the affected limb often remains the only viable option in advanced cases.12 A PubMed search of articles indexed for MEDLINE using the terms elephantiasis (MeSH terms) or elephantiasis (all fields) and scleroderma, systemic (MeSH terms) or scleroderma (all fields) and systemic (all fields) or systemic scleroderma (all fields) or scleroderma (all fields) or scleroderma, localized (MeSH terms) or scleroderma (all fields) and localized (all fields) or localized scleroderma (all fields) yielded only 1 other case report of lower extremity ENV in a patient with systemic sclerosis who ultimately required bilateral leg amputation.8 When possible, avoiding lymphostasis through compression and control of any underlying infections is important in the treatment and prevention of ENV.3
To the Editor:
Elephantiasis nostras verrucosa (ENV) is a skin disorder caused by marked underlying lymphedema that leads to hyperkeratosis, papillomatosis, and verrucous growths on the epidermis.1 The pathophysiology of ENV relates to noninfectious lymphatic obstruction and lymphatic fibrosis secondary to venous stasis, malignancy, radiation therapy, or trauma.2 We present an unusual case of lymphedema and subsequent ENV limited to the arms and hands in a patient with scleroderma, an autoimmune fibrosing disorder.
A 54-year-old woman with a 5-year history of scleroderma presented to our dermatology clinic for treatment of progressive skin changes including pruritus, tightness, finger ulcerations, and pus exuding from papules on the dorsal arms and hands. She had been experiencing several systemic symptoms including dysphagia and lung involvement, necessitating oxygen therapy and a continuous positive airway pressure device for pulmonary arterial hypertension. A computed tomography scan of the lungs demonstrated an increase in ground-glass infiltrates in the right lower lobe and an air-fluid level in the esophagus. At the time of presentation, she was being treated with bosentan and sildenafil for pulmonary arterial hypertension, in addition to prednisone, venlafaxine, lansoprazole, metoclopramide, levothyroxine, temazepam, aspirin, and oxycodone. In the 2 years prior to presentation, she had been treated with intravenous cyclophosphamide once monthly for 6 months, adalimumab for 1 year, and 1 session of photodynamic therapy to the arms, all without benefit.
Physical examination showed cutaneous signs of scleroderma including marked sclerosis of the skin on the face, hands, V of the neck, proximal arms, and mid and proximal thighs. Excoriated papules with overlying crusting and pustulation were superimposed on the sclerotic skin of the arms (Figure 1).
A superinfection was diagnosed and treated with cephalexin 500 mg 4 times daily for 2 weeks; thereafter, mupirocin cream twice daily was used as needed. She was prescribed fexofenadine 180 mg twice daily and doxepin 20 mg at bedtime for pruritus.
At 3-week follow-up, a trial of narrowband UVB therapy was recommended for control of pruritus. Two weeks later, a modified wet-wrap regimen using clobetasol ointment 0.5% twice daily covered with wet gauze followed by a self-adherent dressing was initiated only on the right arm for comparison purposes. This treatment was not successful. A biopsy taken from the left arm showed lymphedema with perivascular fibroplasia and epidermal hyperplasia consistent with ENV (Figure 2).
Two months after her initial presentation, we instituted treatment with tazarotene gel 0.1% twice daily to the arms as well as a water-based topical emulsion to the finger ulcerations and a healing ointment to the hands. A month later, the patient reported no benefit with tazarotene. She desired more flexibility in her arms and hands; therefore, after a discussion with her rheumatologist, biweekly psoralen plus UVA (PUVA) therapy was initiated. Five months after presentation, methotrexate (MTX) 15 mg once weekly with folic acid 1 mg once daily was added. The PUVA therapy and MTX were stopped 3 months later due to lack of treatment benefit.
The patient was referred to vascular medicine for possible compression therapy. It was determined that her vasculature was intact, but compression therapy was contraindicated due to underlying systemic sclerosis. She was subsequently prescribed mycophenolate mofetil 1000 mg twice daily by her rheumatologist. The options of serial excisions or laser resurfacing were presented, but she declined.
Elephantiasis nostras verrucosa is differentiated from elephantiasis tropica, which is caused by a filarial infection of the lymphatic system. The chronic obstructive lymphedema characteristic of ENV can present as a result of various primary or secondary etiologies including trauma, malignancy, venous stasis, inflammation, or infection.3 In systemic sclerosis, extravascular fibrosis theoretically can lead to lymphatic obstruction and subsequent lymphatic stasis. In turn, the pathophysiology of dermal and subcutaneous fibrosis likely reflects autoantibodies (eg, anticardiolipin antibodies) that can damage lymphatic and nonlymphatic vessels.4,5
As the underlying mechanism of ENV, fibrosis of lymphatic vessels in systemic sclerosis is not well documented. Characteristic features of systemic sclerosis include extensive fibrosis, fibroproliferative vasculopathy, and inflammation, which are all possible mechanisms for the internal lymphatic obstruction resulting in the skin changes observed in ENV.6 It seemed unusual that the fibrotic changes of lymphatic vessels in our patient were extensive enough to cause ENV of the upper extremities; lower extremity involvement is the more common presentation because of the greater likelihood of lymphedema manifesting in the legs and feet. Lower extremity ENV has been reported in association with scleroderma.7,8
Regarding therapeutic options, Boyd et al9 reported a good response in a patient with ENV of the abdomen who was treated with topical tazarotene. Additionally, PUVA and MTX have been reported to be beneficial for the progressive skin changes of systemic sclerosis.10 Mycophenolate mofetil has been used in patients who fail MTX therapy because of its antifibrotic properties without the side-effect profiles of other immunosuppressives, such as imatinib.10,11 In our patient, skin lesions persisted following these varied approaches, and compression therapy was not advised due to the underlying sclerosis.
Because options for medical treatment of severe ENV are limited, surgical debridement of the affected limb often remains the only viable option in advanced cases.12 A PubMed search of articles indexed for MEDLINE using the terms elephantiasis (MeSH terms) or elephantiasis (all fields) and scleroderma, systemic (MeSH terms) or scleroderma (all fields) and systemic (all fields) or systemic scleroderma (all fields) or scleroderma (all fields) or scleroderma, localized (MeSH terms) or scleroderma (all fields) and localized (all fields) or localized scleroderma (all fields) yielded only 1 other case report of lower extremity ENV in a patient with systemic sclerosis who ultimately required bilateral leg amputation.8 When possible, avoiding lymphostasis through compression and control of any underlying infections is important in the treatment and prevention of ENV.3
- Sisto K, Khachemoune A. Elephantiasis nostras verrucosa: a review. Am J Clin Dermatol. 2008;9:141-146.
- Schissel DJ, Hivnor C, Elston DM. Elephantiasis nostras verrucosa. Cutis. 1998;62:77-80.
- Duckworth A, Husain J, DeHeer P. Elephantiasis nostras verrucosa or ‘mossy foot lesions’ in lymphedema praecox. J Am Podiatr Med Assoc. 2008;98:66-69.
- Assous N, Allanore Y, Batteaux F, et al. Prevalence of antiphospholipid antibodies in systemic sclerosis and association with primitive pulmonary arterial hypertension and endothelial injury. Clin Exp Rheumatol. 2005;23:199-204.
- Derrett-Smith EC, Dooley A, Gilbane AJ, et al. Endothelial injury in a transforming growth-factor-dependent mouse model of scleroderma induces pulmonary arterial hypertension. Arthritis Rheum. 2013;65:2928-2939.
- Pattanaik M, Brown M, Postlethwaite A. Vascular involvement in systemic sclerosis (scleroderma). J Inflamm Res. 2011;4:105-125.
- Kerchner K, Fleischer A, Yosipovitch G. Lower extremity lymphedema update: pathophysiology, diagnosis and treatment guidelines. J Am Acad Dermatol. 2008;59:324-331.
- Chatterjee S, Karai L. Elephantiasis nostras verrucosa in a patient with systemic sclerosis. Clin Exp Dermatol. 2009;34:e696-e698.
- Boyd J, Sloan S, Meffert J. Elephantiasis nostrum verrucosa of the abdomen: clinical results with tazarotene. J Drugs Dermatol. 2004;3:446-448.
- Fett, N. Scleroderma: nomenclature, etiology, pathogenesis, prognosis, and treatments: facts and controversies. Clin Dermatol. 2013;31:432-437.
- Moinzadeh P, Krieg T, Hunzelmann N. Imatinib treatment of generalized localized scleroderma (morphea). J Am Acad Dermatol. 2010;63:e102-e104.
- Iwao F, Sato-Matsumura KC, Sawamura D, et al. Elephantiasis nostras verrucosa successfully treated by surgical debridement. Dermatol Surg. 2004;30:939-941.
- Sisto K, Khachemoune A. Elephantiasis nostras verrucosa: a review. Am J Clin Dermatol. 2008;9:141-146.
- Schissel DJ, Hivnor C, Elston DM. Elephantiasis nostras verrucosa. Cutis. 1998;62:77-80.
- Duckworth A, Husain J, DeHeer P. Elephantiasis nostras verrucosa or ‘mossy foot lesions’ in lymphedema praecox. J Am Podiatr Med Assoc. 2008;98:66-69.
- Assous N, Allanore Y, Batteaux F, et al. Prevalence of antiphospholipid antibodies in systemic sclerosis and association with primitive pulmonary arterial hypertension and endothelial injury. Clin Exp Rheumatol. 2005;23:199-204.
- Derrett-Smith EC, Dooley A, Gilbane AJ, et al. Endothelial injury in a transforming growth-factor-dependent mouse model of scleroderma induces pulmonary arterial hypertension. Arthritis Rheum. 2013;65:2928-2939.
- Pattanaik M, Brown M, Postlethwaite A. Vascular involvement in systemic sclerosis (scleroderma). J Inflamm Res. 2011;4:105-125.
- Kerchner K, Fleischer A, Yosipovitch G. Lower extremity lymphedema update: pathophysiology, diagnosis and treatment guidelines. J Am Acad Dermatol. 2008;59:324-331.
- Chatterjee S, Karai L. Elephantiasis nostras verrucosa in a patient with systemic sclerosis. Clin Exp Dermatol. 2009;34:e696-e698.
- Boyd J, Sloan S, Meffert J. Elephantiasis nostrum verrucosa of the abdomen: clinical results with tazarotene. J Drugs Dermatol. 2004;3:446-448.
- Fett, N. Scleroderma: nomenclature, etiology, pathogenesis, prognosis, and treatments: facts and controversies. Clin Dermatol. 2013;31:432-437.
- Moinzadeh P, Krieg T, Hunzelmann N. Imatinib treatment of generalized localized scleroderma (morphea). J Am Acad Dermatol. 2010;63:e102-e104.
- Iwao F, Sato-Matsumura KC, Sawamura D, et al. Elephantiasis nostras verrucosa successfully treated by surgical debridement. Dermatol Surg. 2004;30:939-941.
Practice Points
- Scleroderma rarely may lead to elephantiasis nostras verrucosa (ENV) of the upper extremities.
- Avoiding lymphostasis through compression and control of concomitant skin and soft tissue infections is important in the treatment and prevention of ENV.
Neurofibromatosis Type 1 in the Setting of Systemic Lupus Erythematosus
To the Editor:
Patients with concurrent neurofibromatosis type 1 (NF-1) and systemic lupus erythematosus (SLE) rarely have been reported in the literature. Neurofibromatosis type 1 is one of the most common genetic disorders, with a worldwide birth incidence of 1 in 2500 individuals and prevalence of 1 in 4000 individuals.1 The incidence and prevalence of SLE varies widely depending on race and geographic location. Estimated incidence rates for SLE range from 1 to 25 per 100,000 individuals annually in North America, South America, Europe, and Asia.2,3 The reported worldwide prevalence is 20 to 150 cases per 100,000 individuals annually.2,4,5
Given the high prevalence of both conditions, the association between SLE and NF-1 likely is underrecognized; therefore, identifying more patients with concurrent SLE and NF-1 and describing the interplay between the 2 conditions may have important therapeutic implications. We present the case of a middle-aged woman with a history of SLE who had cutaneous lesions characteristic of NF-1 to further the understanding of these concurrent conditions.
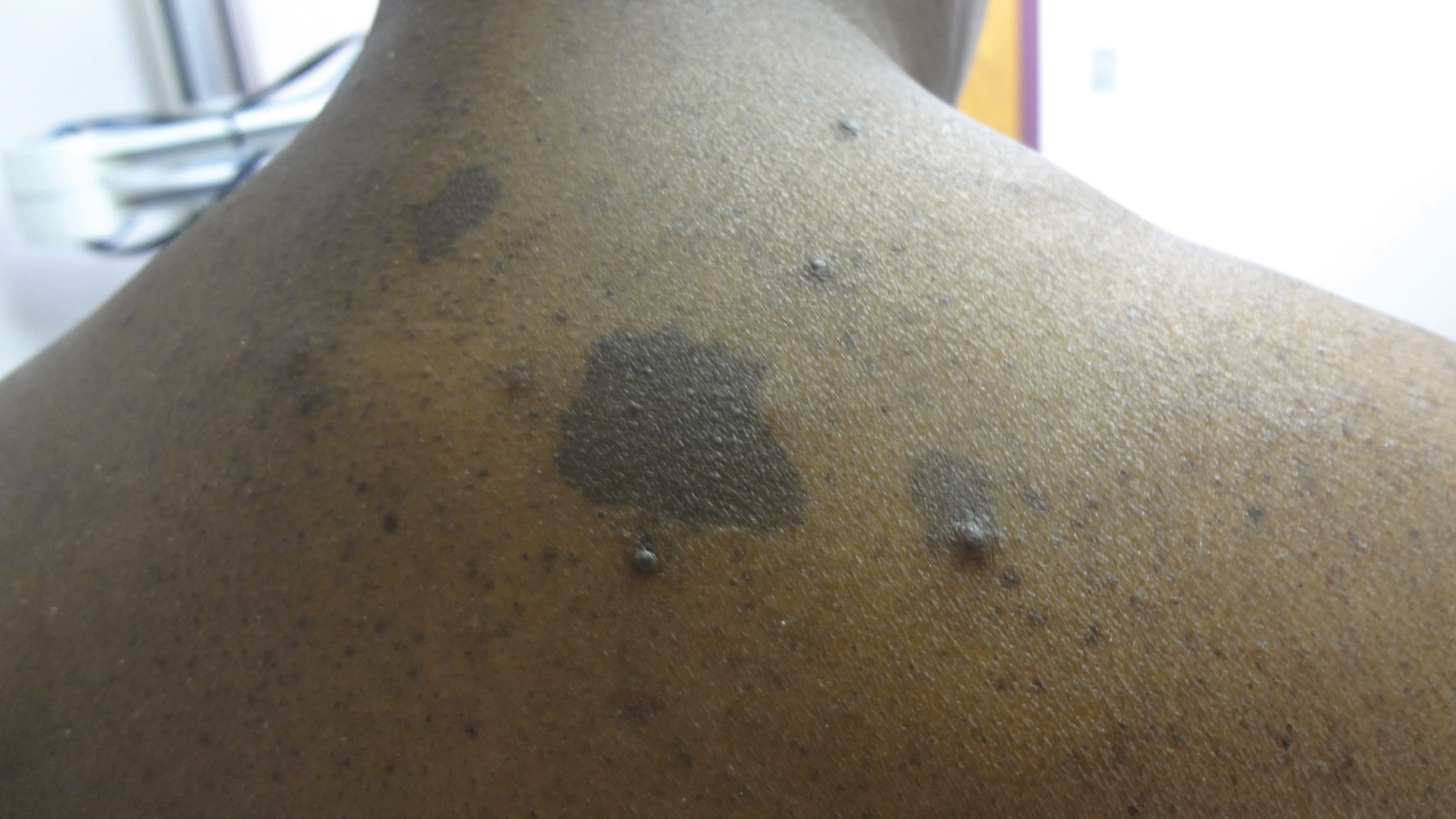
A middle-aged woman presented to our academic dermatology clinic for evaluation and removal of dark spots that had been present diffusely on the trunk and extremities since birth. She reported a history of SLE with lupus nephritis, hypertension, and a nodular goiter following a partial thyroidectomy. She noted that she did not seek treatment for the skin findings sooner because she was more concerned about her other medical conditions; however, because she felt these conditions were now stable, she decided to seek treatment for the “rash.” Physical examination revealed hundreds of café au lait macules and numerous neurofibromas diffusely distributed on the trunk and extremities (Figure 1) as well as bilateral axillary freckling. A clinical diagnosis of NF-1 was made.
When questioned, the patient reported that she may have been diagnosed with NF-1 in the past by another physician, but she did not recall it specifically. The patient was advised that there were no treatments for the café au lait macules. We notified her other physicians of the NF-1 diagnosis so she could be monitored for systemic conditions related to NF-1, including optic gliomas, pheochromocytoma, renal artery stenosis, and internal neurofibromas. We also referred the patient for genetic counseling; of note, the patient reported she had 4 children without any evidence of similar skin lesions or chronic health problems.
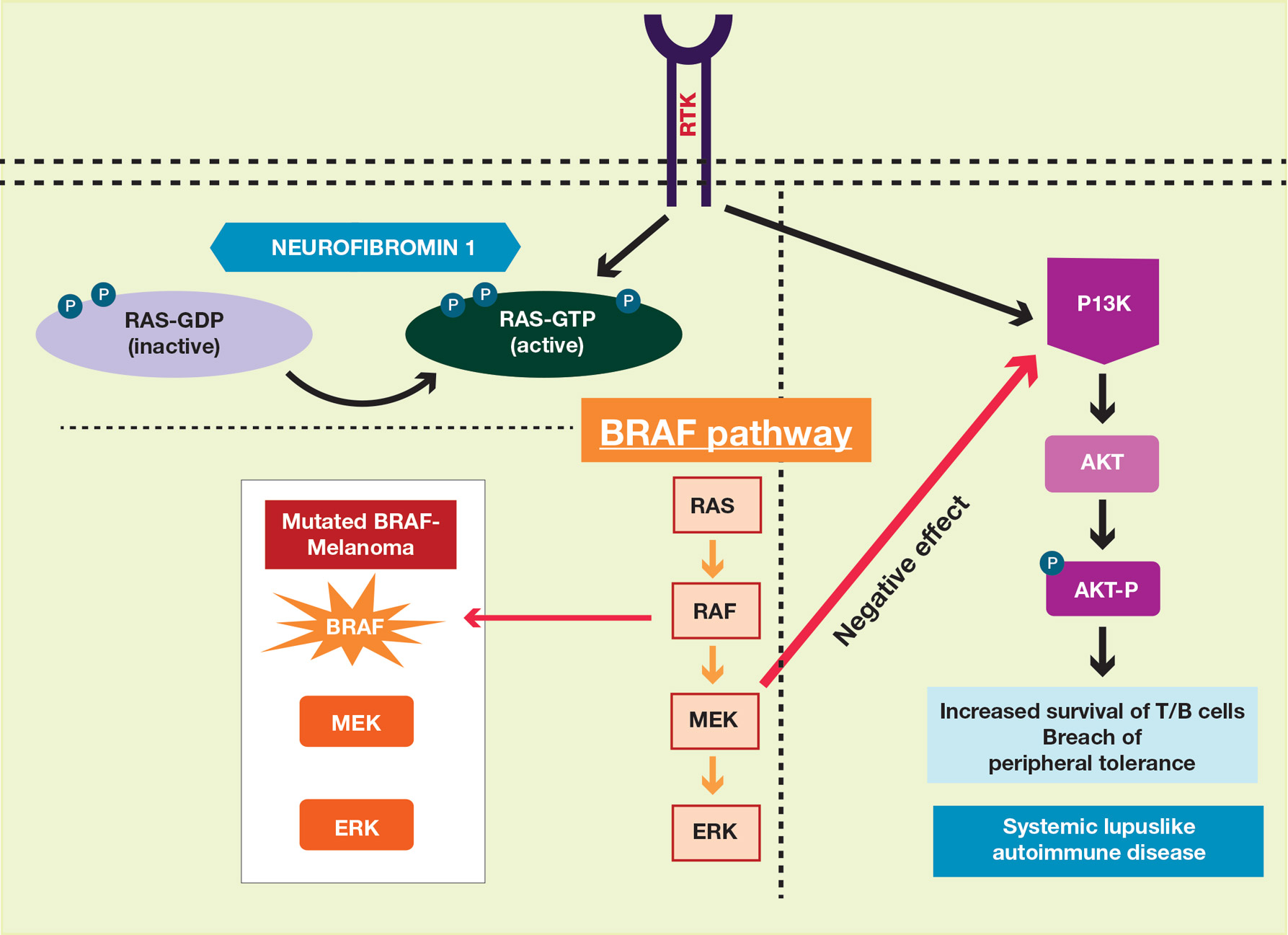
A PubMed search of articles indexed for MEDLINE using the terms systemic lupus and neurofibromatosis yielded 8 cases of patients having both SLE and NF-1 (including our case).6-11 Our patient reported having multiple lesions since birth, decades before the onset and diagnosis of SLE. In 3 other cases, patients were diagnosed with SLE and then presented with neurofibromas, leading to NF-1 diagnosis.In the discussion of those 3 cases, it was proposed that immune system alterations caused by SLE leading to viral illness may have predisposed the patients to the development of tumors and other collagen diseases, or it could be coincidental.6,7 In another case, a patient with NF-1 developed SLE, which was thought to be coincidental.8 Akyuz et al9 described the case of a pediatric patient with NF-1 who subsequently was diagnosed with SLE. The authors suggested that the lack of neurofibromin contributed to the development of SLE, an autoimmune condition. Under normal circumstances, neurofibromin acts as a guanosine triphosphatase–activating protein for RAS in T cells.10 CD8+ T-cell function also is impaired in patients with SLE.9 Additionally, it has been reported that anti–double-stranded DNA antibodies and immune complexes were present in NF-1 patients, even though there were low titers.12 Thus, the authors proposed that the lack of neurofibromin led to dysregulation of the RAS pathway and impairment of T cells, creating an immune milieu that predisposed the patient to development of SLE. Our case gives additional credence to this theory, as our patient had a similar clinical course: the café au lait macules were present since birth and the symptoms of SLE surfaced much later in her late 20s and 30s. Another case by Makino and Tampo10 described a patient with a history of SLE who was later diagnosed with NF-1 based on choroidal findings highly specific for NF-1 but did not have other classic findings of NF-1. The authors mentioned that there might be a potential relationship between these two disorders but did not speculate any theory in particular for their case.10
The interplay between an autoimmune condition such as SLE and NF-1, a condition traditionally thought to be due to a genetic mutation, may have greater clinical and therapeutic implications beyond just these two disorders. Although it is well established that RAS pathway disruption causes NF-1, it has been uncovered that dysfunction in the RAS pathway also can contribute to melanoma oncogenesis.13,14 These insights have led to the development of and approval of targeted drugs designed to inhibit the RAS pathway (eg, vemurafenib, dabrafenib, trametinib).14-17 Melanoma also is considered a “model” tumor for studying the relationship between the immune system and cancer.18
Our case also is instructive in another point: our patient had never sought treatment for her skin lesions, as she said she had other more serious health conditions. Closer evaluation of her skin condition may have led to earlier diagnosis of NF-1, which has important health implications. The average lifespan of patients with NF-1 is 10 to 15 years lower than the general population, with cancer being the leading cause of death.20 Malignant peripheral nerve sheath tumors are the most common malignant tumors observed in such patients.21-23 Other cancers that are associated with NF-1 include rhabdomyosarcomas, gastrointestinal stromal tumors, neuroectodermal tumors, pheochromocytomas, and breast carcinomas.23
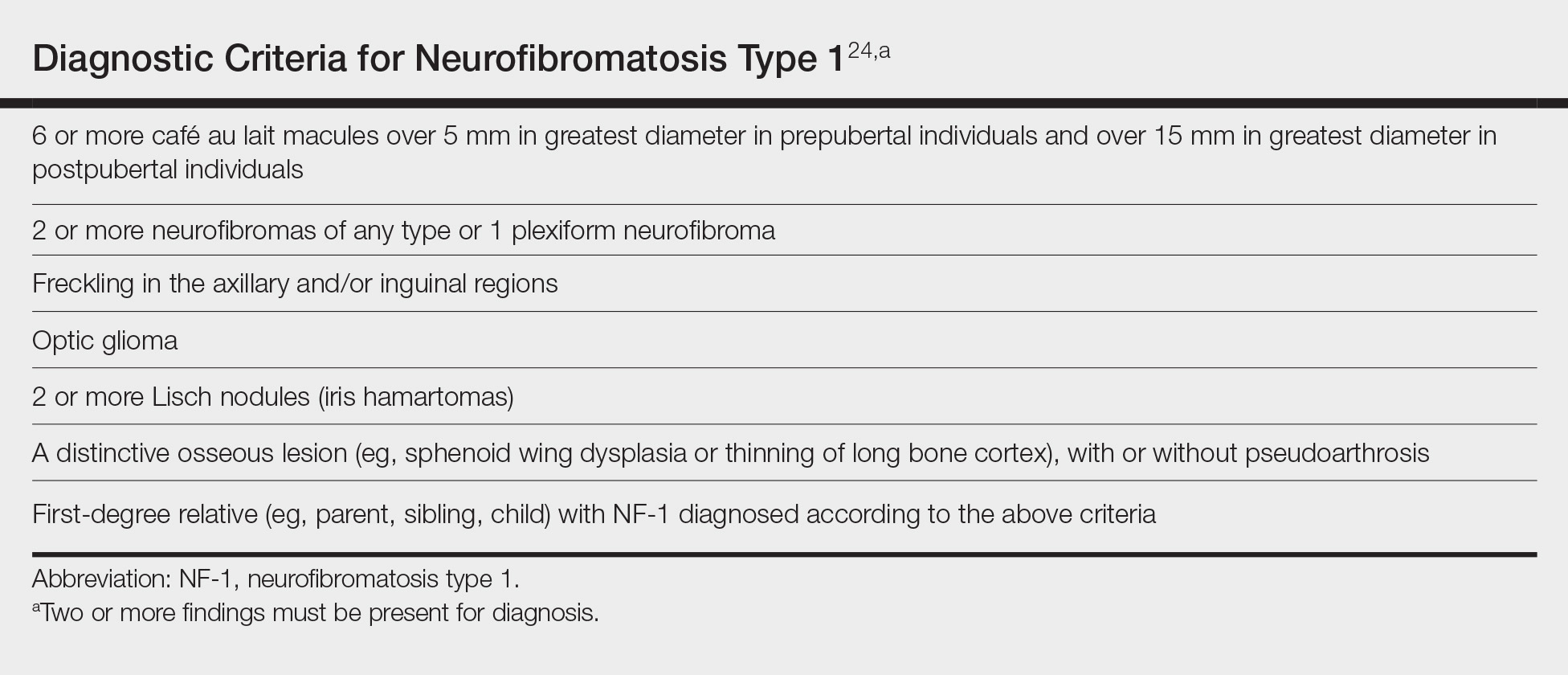
To make a clinical diagnosis of NF-1, a patient must have 2 of 7 cardinal clinical features as defined by the National Institutes of Health (Table).24 In our patient with hundreds of café au lait macules and dozens of neurofibromas, the diagnosis was clear; however, in other patients, the skin findings of NF-1 may not be as prominent. A patient could meet criteria for NF-1 diagnosis with the inconspicuous presentation of 6 café au lait macules and either 1 plexiform neurofibroma or 2 neurofibromas (of any type) on the entire body.
We recommend that patients with SLE undergo skin examinations to look for more subtle presentations of NF-1. Earlier diagnosis will help to initiate close monitoring of the disorder’s associated systemic health risks. In addition, the identification of more patients with both NF-1 and SLE may help shed light on the etiology of both conditions.
- Carey JC, Baty BJ, Johnson JP, et al. The genetic aspects of neurofibromatosis. Ann N Y Acad Sci. 1986;486:45-56.
- Pons-Estel GJ, Alarcón GS, Scofield L, et al. Understanding the epidemiology and progression of systemic lupus erythematosus. Semin Arthritis Rheum. 2010;39:257-268.
- Danchenko N, Satia JA, Anthony MS. Epidemiology of systemic lupus erythematosus: a comparison of worldwide disease burden. Lupus. 2006;15:308-318.
- Lawrence RC, Helmick CG, Arnett FC, et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. 1998;41:778-799.
- Chakravarty EF, Bush TM, Manzi S, et al. Prevalence of adult systemic lupus erythematosus in California and Pennsylvania in 2000: estimates obtained using hospitalization data. Arthritis Rheum. 2007;56:2092-2094.
- Bitnun S, Bassan H. Letter: neurofibromatosis and SLE. N Engl J Med. 1975;292:429-430.
- Riccardi VM. Neurofibromatosis in a patient with systemic lupus erythematosus. Arthritis Rheum. 1983;26:574.
- Corominas H, Guardiola JM, Matas L, et al. Neurofibromatosis and systemic lupus erythematosus. a matter of coincidence? Clin Rhematol. 2003;22:496-497.
- Akyuz SG, Caltik A, Bulbul M, et al. An unusual pediatric case with neurofibromatosis and systemic lupus erythematosus. Rheumatol Int. 2012;32:2345-47.
- Makino S, Tampo H. Rare and unusual choroidal abnormalities in a patient with systemic lupus erythematosus. Case Rep Ophthalmol. 2013;4:81-86.
- Galvan JM, Hofkamp MP. Usefulness of intrapartum magnetic resonance imaging for a parturient with neurofibromatosis type I during induction of labor for preeclampsia. Proc (Bayl Univ Med Cent). 2018;31:92-93.
- Gerosa PL, Vai C, Bizzozer L, et al. Immunological and clinical surveillance in Recklinghausen’s neurofibromatosis (NF1). Panminerva Med. 1993;35:80-85.
- Busca R, Abbe P, Mantoux F, et al. RAS mediates the cAMP-dependent activation of extracellular signal-regulated kinases (ERKs) in melanocytes. EMBO J. 2000;19:2900-2910.
- Sullivan RJ, Flaherty K. MAP kinase signaling and inhibition in melanoma. Oncogene. 2013;32:2373-2379.
- Hennessy BT, Smith DL, Ram PT, et al. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;12:988-1004.
- Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507-2516.
- Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380:358-365.
- Maio M. Melanoma as a model tumour for immuno-oncology. Ann Oncol. 2012;23:(suppl 8):viii10-4.
- Zmajkovicova K, Jesenberger V, Catalanotti F, et al. MEK1 is required for PTEN membrane recruitment, AKT regulation, and the maintenance of peripheral tolerance. Mol Cell. 2013;50:43-55.
- Patil S, Chamberlain RS. Neoplasms associated with germline and somatic NF1 gene mutations. Oncologist. 2012;17:101-116.
- Carroll SL, Ratner N. How does the Schwann cell lineage form tumors in NF1? Glia. 2008;56:1590-1605.
- Rasmussen SA, Friedman JM. NF1 gene and neurofibromatosis 1. Am J Epidemiol. 2000;151:33-40.
- Yohay K. Neurofibromatosis type 1 and associated malignancies. Curr Neurol Neurosci Rep. 2009;9:247-253.
- Neurofibromatosis. conference statement. National Institutes of Health Consensus Development Conference. Arch Neurol. 1988;45:575-78.
To the Editor:
Patients with concurrent neurofibromatosis type 1 (NF-1) and systemic lupus erythematosus (SLE) rarely have been reported in the literature. Neurofibromatosis type 1 is one of the most common genetic disorders, with a worldwide birth incidence of 1 in 2500 individuals and prevalence of 1 in 4000 individuals.1 The incidence and prevalence of SLE varies widely depending on race and geographic location. Estimated incidence rates for SLE range from 1 to 25 per 100,000 individuals annually in North America, South America, Europe, and Asia.2,3 The reported worldwide prevalence is 20 to 150 cases per 100,000 individuals annually.2,4,5
Given the high prevalence of both conditions, the association between SLE and NF-1 likely is underrecognized; therefore, identifying more patients with concurrent SLE and NF-1 and describing the interplay between the 2 conditions may have important therapeutic implications. We present the case of a middle-aged woman with a history of SLE who had cutaneous lesions characteristic of NF-1 to further the understanding of these concurrent conditions.
A middle-aged woman presented to our academic dermatology clinic for evaluation and removal of dark spots that had been present diffusely on the trunk and extremities since birth. She reported a history of SLE with lupus nephritis, hypertension, and a nodular goiter following a partial thyroidectomy. She noted that she did not seek treatment for the skin findings sooner because she was more concerned about her other medical conditions; however, because she felt these conditions were now stable, she decided to seek treatment for the “rash.” Physical examination revealed hundreds of café au lait macules and numerous neurofibromas diffusely distributed on the trunk and extremities (Figure 1) as well as bilateral axillary freckling. A clinical diagnosis of NF-1 was made.
When questioned, the patient reported that she may have been diagnosed with NF-1 in the past by another physician, but she did not recall it specifically. The patient was advised that there were no treatments for the café au lait macules. We notified her other physicians of the NF-1 diagnosis so she could be monitored for systemic conditions related to NF-1, including optic gliomas, pheochromocytoma, renal artery stenosis, and internal neurofibromas. We also referred the patient for genetic counseling; of note, the patient reported she had 4 children without any evidence of similar skin lesions or chronic health problems.
A PubMed search of articles indexed for MEDLINE using the terms systemic lupus and neurofibromatosis yielded 8 cases of patients having both SLE and NF-1 (including our case).6-11 Our patient reported having multiple lesions since birth, decades before the onset and diagnosis of SLE. In 3 other cases, patients were diagnosed with SLE and then presented with neurofibromas, leading to NF-1 diagnosis.In the discussion of those 3 cases, it was proposed that immune system alterations caused by SLE leading to viral illness may have predisposed the patients to the development of tumors and other collagen diseases, or it could be coincidental.6,7 In another case, a patient with NF-1 developed SLE, which was thought to be coincidental.8 Akyuz et al9 described the case of a pediatric patient with NF-1 who subsequently was diagnosed with SLE. The authors suggested that the lack of neurofibromin contributed to the development of SLE, an autoimmune condition. Under normal circumstances, neurofibromin acts as a guanosine triphosphatase–activating protein for RAS in T cells.10 CD8+ T-cell function also is impaired in patients with SLE.9 Additionally, it has been reported that anti–double-stranded DNA antibodies and immune complexes were present in NF-1 patients, even though there were low titers.12 Thus, the authors proposed that the lack of neurofibromin led to dysregulation of the RAS pathway and impairment of T cells, creating an immune milieu that predisposed the patient to development of SLE. Our case gives additional credence to this theory, as our patient had a similar clinical course: the café au lait macules were present since birth and the symptoms of SLE surfaced much later in her late 20s and 30s. Another case by Makino and Tampo10 described a patient with a history of SLE who was later diagnosed with NF-1 based on choroidal findings highly specific for NF-1 but did not have other classic findings of NF-1. The authors mentioned that there might be a potential relationship between these two disorders but did not speculate any theory in particular for their case.10
The interplay between an autoimmune condition such as SLE and NF-1, a condition traditionally thought to be due to a genetic mutation, may have greater clinical and therapeutic implications beyond just these two disorders. Although it is well established that RAS pathway disruption causes NF-1, it has been uncovered that dysfunction in the RAS pathway also can contribute to melanoma oncogenesis.13,14 These insights have led to the development of and approval of targeted drugs designed to inhibit the RAS pathway (eg, vemurafenib, dabrafenib, trametinib).14-17 Melanoma also is considered a “model” tumor for studying the relationship between the immune system and cancer.18
Our case also is instructive in another point: our patient had never sought treatment for her skin lesions, as she said she had other more serious health conditions. Closer evaluation of her skin condition may have led to earlier diagnosis of NF-1, which has important health implications. The average lifespan of patients with NF-1 is 10 to 15 years lower than the general population, with cancer being the leading cause of death.20 Malignant peripheral nerve sheath tumors are the most common malignant tumors observed in such patients.21-23 Other cancers that are associated with NF-1 include rhabdomyosarcomas, gastrointestinal stromal tumors, neuroectodermal tumors, pheochromocytomas, and breast carcinomas.23
To make a clinical diagnosis of NF-1, a patient must have 2 of 7 cardinal clinical features as defined by the National Institutes of Health (Table).24 In our patient with hundreds of café au lait macules and dozens of neurofibromas, the diagnosis was clear; however, in other patients, the skin findings of NF-1 may not be as prominent. A patient could meet criteria for NF-1 diagnosis with the inconspicuous presentation of 6 café au lait macules and either 1 plexiform neurofibroma or 2 neurofibromas (of any type) on the entire body.
We recommend that patients with SLE undergo skin examinations to look for more subtle presentations of NF-1. Earlier diagnosis will help to initiate close monitoring of the disorder’s associated systemic health risks. In addition, the identification of more patients with both NF-1 and SLE may help shed light on the etiology of both conditions.
To the Editor:
Patients with concurrent neurofibromatosis type 1 (NF-1) and systemic lupus erythematosus (SLE) rarely have been reported in the literature. Neurofibromatosis type 1 is one of the most common genetic disorders, with a worldwide birth incidence of 1 in 2500 individuals and prevalence of 1 in 4000 individuals.1 The incidence and prevalence of SLE varies widely depending on race and geographic location. Estimated incidence rates for SLE range from 1 to 25 per 100,000 individuals annually in North America, South America, Europe, and Asia.2,3 The reported worldwide prevalence is 20 to 150 cases per 100,000 individuals annually.2,4,5
Given the high prevalence of both conditions, the association between SLE and NF-1 likely is underrecognized; therefore, identifying more patients with concurrent SLE and NF-1 and describing the interplay between the 2 conditions may have important therapeutic implications. We present the case of a middle-aged woman with a history of SLE who had cutaneous lesions characteristic of NF-1 to further the understanding of these concurrent conditions.
A middle-aged woman presented to our academic dermatology clinic for evaluation and removal of dark spots that had been present diffusely on the trunk and extremities since birth. She reported a history of SLE with lupus nephritis, hypertension, and a nodular goiter following a partial thyroidectomy. She noted that she did not seek treatment for the skin findings sooner because she was more concerned about her other medical conditions; however, because she felt these conditions were now stable, she decided to seek treatment for the “rash.” Physical examination revealed hundreds of café au lait macules and numerous neurofibromas diffusely distributed on the trunk and extremities (Figure 1) as well as bilateral axillary freckling. A clinical diagnosis of NF-1 was made.
When questioned, the patient reported that she may have been diagnosed with NF-1 in the past by another physician, but she did not recall it specifically. The patient was advised that there were no treatments for the café au lait macules. We notified her other physicians of the NF-1 diagnosis so she could be monitored for systemic conditions related to NF-1, including optic gliomas, pheochromocytoma, renal artery stenosis, and internal neurofibromas. We also referred the patient for genetic counseling; of note, the patient reported she had 4 children without any evidence of similar skin lesions or chronic health problems.
A PubMed search of articles indexed for MEDLINE using the terms systemic lupus and neurofibromatosis yielded 8 cases of patients having both SLE and NF-1 (including our case).6-11 Our patient reported having multiple lesions since birth, decades before the onset and diagnosis of SLE. In 3 other cases, patients were diagnosed with SLE and then presented with neurofibromas, leading to NF-1 diagnosis.In the discussion of those 3 cases, it was proposed that immune system alterations caused by SLE leading to viral illness may have predisposed the patients to the development of tumors and other collagen diseases, or it could be coincidental.6,7 In another case, a patient with NF-1 developed SLE, which was thought to be coincidental.8 Akyuz et al9 described the case of a pediatric patient with NF-1 who subsequently was diagnosed with SLE. The authors suggested that the lack of neurofibromin contributed to the development of SLE, an autoimmune condition. Under normal circumstances, neurofibromin acts as a guanosine triphosphatase–activating protein for RAS in T cells.10 CD8+ T-cell function also is impaired in patients with SLE.9 Additionally, it has been reported that anti–double-stranded DNA antibodies and immune complexes were present in NF-1 patients, even though there were low titers.12 Thus, the authors proposed that the lack of neurofibromin led to dysregulation of the RAS pathway and impairment of T cells, creating an immune milieu that predisposed the patient to development of SLE. Our case gives additional credence to this theory, as our patient had a similar clinical course: the café au lait macules were present since birth and the symptoms of SLE surfaced much later in her late 20s and 30s. Another case by Makino and Tampo10 described a patient with a history of SLE who was later diagnosed with NF-1 based on choroidal findings highly specific for NF-1 but did not have other classic findings of NF-1. The authors mentioned that there might be a potential relationship between these two disorders but did not speculate any theory in particular for their case.10
The interplay between an autoimmune condition such as SLE and NF-1, a condition traditionally thought to be due to a genetic mutation, may have greater clinical and therapeutic implications beyond just these two disorders. Although it is well established that RAS pathway disruption causes NF-1, it has been uncovered that dysfunction in the RAS pathway also can contribute to melanoma oncogenesis.13,14 These insights have led to the development of and approval of targeted drugs designed to inhibit the RAS pathway (eg, vemurafenib, dabrafenib, trametinib).14-17 Melanoma also is considered a “model” tumor for studying the relationship between the immune system and cancer.18
Our case also is instructive in another point: our patient had never sought treatment for her skin lesions, as she said she had other more serious health conditions. Closer evaluation of her skin condition may have led to earlier diagnosis of NF-1, which has important health implications. The average lifespan of patients with NF-1 is 10 to 15 years lower than the general population, with cancer being the leading cause of death.20 Malignant peripheral nerve sheath tumors are the most common malignant tumors observed in such patients.21-23 Other cancers that are associated with NF-1 include rhabdomyosarcomas, gastrointestinal stromal tumors, neuroectodermal tumors, pheochromocytomas, and breast carcinomas.23
To make a clinical diagnosis of NF-1, a patient must have 2 of 7 cardinal clinical features as defined by the National Institutes of Health (Table).24 In our patient with hundreds of café au lait macules and dozens of neurofibromas, the diagnosis was clear; however, in other patients, the skin findings of NF-1 may not be as prominent. A patient could meet criteria for NF-1 diagnosis with the inconspicuous presentation of 6 café au lait macules and either 1 plexiform neurofibroma or 2 neurofibromas (of any type) on the entire body.
We recommend that patients with SLE undergo skin examinations to look for more subtle presentations of NF-1. Earlier diagnosis will help to initiate close monitoring of the disorder’s associated systemic health risks. In addition, the identification of more patients with both NF-1 and SLE may help shed light on the etiology of both conditions.
- Carey JC, Baty BJ, Johnson JP, et al. The genetic aspects of neurofibromatosis. Ann N Y Acad Sci. 1986;486:45-56.
- Pons-Estel GJ, Alarcón GS, Scofield L, et al. Understanding the epidemiology and progression of systemic lupus erythematosus. Semin Arthritis Rheum. 2010;39:257-268.
- Danchenko N, Satia JA, Anthony MS. Epidemiology of systemic lupus erythematosus: a comparison of worldwide disease burden. Lupus. 2006;15:308-318.
- Lawrence RC, Helmick CG, Arnett FC, et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. 1998;41:778-799.
- Chakravarty EF, Bush TM, Manzi S, et al. Prevalence of adult systemic lupus erythematosus in California and Pennsylvania in 2000: estimates obtained using hospitalization data. Arthritis Rheum. 2007;56:2092-2094.
- Bitnun S, Bassan H. Letter: neurofibromatosis and SLE. N Engl J Med. 1975;292:429-430.
- Riccardi VM. Neurofibromatosis in a patient with systemic lupus erythematosus. Arthritis Rheum. 1983;26:574.
- Corominas H, Guardiola JM, Matas L, et al. Neurofibromatosis and systemic lupus erythematosus. a matter of coincidence? Clin Rhematol. 2003;22:496-497.
- Akyuz SG, Caltik A, Bulbul M, et al. An unusual pediatric case with neurofibromatosis and systemic lupus erythematosus. Rheumatol Int. 2012;32:2345-47.
- Makino S, Tampo H. Rare and unusual choroidal abnormalities in a patient with systemic lupus erythematosus. Case Rep Ophthalmol. 2013;4:81-86.
- Galvan JM, Hofkamp MP. Usefulness of intrapartum magnetic resonance imaging for a parturient with neurofibromatosis type I during induction of labor for preeclampsia. Proc (Bayl Univ Med Cent). 2018;31:92-93.
- Gerosa PL, Vai C, Bizzozer L, et al. Immunological and clinical surveillance in Recklinghausen’s neurofibromatosis (NF1). Panminerva Med. 1993;35:80-85.
- Busca R, Abbe P, Mantoux F, et al. RAS mediates the cAMP-dependent activation of extracellular signal-regulated kinases (ERKs) in melanocytes. EMBO J. 2000;19:2900-2910.
- Sullivan RJ, Flaherty K. MAP kinase signaling and inhibition in melanoma. Oncogene. 2013;32:2373-2379.
- Hennessy BT, Smith DL, Ram PT, et al. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;12:988-1004.
- Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507-2516.
- Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380:358-365.
- Maio M. Melanoma as a model tumour for immuno-oncology. Ann Oncol. 2012;23:(suppl 8):viii10-4.
- Zmajkovicova K, Jesenberger V, Catalanotti F, et al. MEK1 is required for PTEN membrane recruitment, AKT regulation, and the maintenance of peripheral tolerance. Mol Cell. 2013;50:43-55.
- Patil S, Chamberlain RS. Neoplasms associated with germline and somatic NF1 gene mutations. Oncologist. 2012;17:101-116.
- Carroll SL, Ratner N. How does the Schwann cell lineage form tumors in NF1? Glia. 2008;56:1590-1605.
- Rasmussen SA, Friedman JM. NF1 gene and neurofibromatosis 1. Am J Epidemiol. 2000;151:33-40.
- Yohay K. Neurofibromatosis type 1 and associated malignancies. Curr Neurol Neurosci Rep. 2009;9:247-253.
- Neurofibromatosis. conference statement. National Institutes of Health Consensus Development Conference. Arch Neurol. 1988;45:575-78.
- Carey JC, Baty BJ, Johnson JP, et al. The genetic aspects of neurofibromatosis. Ann N Y Acad Sci. 1986;486:45-56.
- Pons-Estel GJ, Alarcón GS, Scofield L, et al. Understanding the epidemiology and progression of systemic lupus erythematosus. Semin Arthritis Rheum. 2010;39:257-268.
- Danchenko N, Satia JA, Anthony MS. Epidemiology of systemic lupus erythematosus: a comparison of worldwide disease burden. Lupus. 2006;15:308-318.
- Lawrence RC, Helmick CG, Arnett FC, et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. 1998;41:778-799.
- Chakravarty EF, Bush TM, Manzi S, et al. Prevalence of adult systemic lupus erythematosus in California and Pennsylvania in 2000: estimates obtained using hospitalization data. Arthritis Rheum. 2007;56:2092-2094.
- Bitnun S, Bassan H. Letter: neurofibromatosis and SLE. N Engl J Med. 1975;292:429-430.
- Riccardi VM. Neurofibromatosis in a patient with systemic lupus erythematosus. Arthritis Rheum. 1983;26:574.
- Corominas H, Guardiola JM, Matas L, et al. Neurofibromatosis and systemic lupus erythematosus. a matter of coincidence? Clin Rhematol. 2003;22:496-497.
- Akyuz SG, Caltik A, Bulbul M, et al. An unusual pediatric case with neurofibromatosis and systemic lupus erythematosus. Rheumatol Int. 2012;32:2345-47.
- Makino S, Tampo H. Rare and unusual choroidal abnormalities in a patient with systemic lupus erythematosus. Case Rep Ophthalmol. 2013;4:81-86.
- Galvan JM, Hofkamp MP. Usefulness of intrapartum magnetic resonance imaging for a parturient with neurofibromatosis type I during induction of labor for preeclampsia. Proc (Bayl Univ Med Cent). 2018;31:92-93.
- Gerosa PL, Vai C, Bizzozer L, et al. Immunological and clinical surveillance in Recklinghausen’s neurofibromatosis (NF1). Panminerva Med. 1993;35:80-85.
- Busca R, Abbe P, Mantoux F, et al. RAS mediates the cAMP-dependent activation of extracellular signal-regulated kinases (ERKs) in melanocytes. EMBO J. 2000;19:2900-2910.
- Sullivan RJ, Flaherty K. MAP kinase signaling and inhibition in melanoma. Oncogene. 2013;32:2373-2379.
- Hennessy BT, Smith DL, Ram PT, et al. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;12:988-1004.
- Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507-2516.
- Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380:358-365.
- Maio M. Melanoma as a model tumour for immuno-oncology. Ann Oncol. 2012;23:(suppl 8):viii10-4.
- Zmajkovicova K, Jesenberger V, Catalanotti F, et al. MEK1 is required for PTEN membrane recruitment, AKT regulation, and the maintenance of peripheral tolerance. Mol Cell. 2013;50:43-55.
- Patil S, Chamberlain RS. Neoplasms associated with germline and somatic NF1 gene mutations. Oncologist. 2012;17:101-116.
- Carroll SL, Ratner N. How does the Schwann cell lineage form tumors in NF1? Glia. 2008;56:1590-1605.
- Rasmussen SA, Friedman JM. NF1 gene and neurofibromatosis 1. Am J Epidemiol. 2000;151:33-40.
- Yohay K. Neurofibromatosis type 1 and associated malignancies. Curr Neurol Neurosci Rep. 2009;9:247-253.
- Neurofibromatosis. conference statement. National Institutes of Health Consensus Development Conference. Arch Neurol. 1988;45:575-78.
Practice Points
- Patients with neurofibromatosis type 1 (NF-1) benefit from early diagnosis and long-term follow-up.
- Patients with systemic lupus erythematosus (SLE) may develop different malignancies given the immune dysregulation. We recommend that patients with SLE undergo detailed skin examinations to check for subtle clues for NF-1.
- Similarly, patients with NF-1 can develop SLE later in life.
An unplanned career
A focus on health system transformation
I have to admit that I am not sure I am a legacy in hospital medicine, and the term legacy throws me off a bit. I came to medical school after working at McKinsey & Co. consulting, and I chose pediatrics because of my love of working with children and families, as well as a vague notion that I wanted to work on “system” issues, and therefore, more generalist-type training seemed applicable.
I met Chris Landrigan, MD, MPH, and Vinny Chiang, MD, and learned what a hospitalist was, as an intern in 2002. We had a research elective and I was able to publish a couple of papers in Pediatrics on pediatric hospital medicine with Chris and Raj Srivastava, MD, MPH. In 2004, I went to my first Society of Hospital Medicine meeting and met Larry Wellikson, MD, MHM, and others. From there, I went to the Robert Wood Johnson Clinical Scholars Program, with Ron Keren, MD, MPH, and others, and along with faculty from the Cincinnati Children’s in hospital medicine.
In 2007, I applied for a White House Fellowship and told my wife that I didn’t think there was a chance that I would get it, so we should keep building our new home in Cincinnati. We were both surprised when I was selected. I served Michael Leavitt, the then-Secretary of the Department of Health & Human Services, as his White House fellow during the Bush administration, and then served as his chief medical officer. Exposure to health policy and leadership at that level was career shaping. Cincinnati Children’s was searching for a leader for the conversion of pediatric hospital medicine into a full division in 2009. So I returned to Cincinnati to take on leading pediatric hospital medicine, and a role leading quality measurement and improvement efforts for the entire health system. I loved the work and thought I would remain in that role, and our family would be in Cincinnati for a long time. Best laid plans …
In early 2011, Don Berwick, MD, who was then the administrator of the Centers for Medicare & Medicaid Services called and asked whether I “would come talk with him in D.C.” That talk quickly became a series of interviews, and he offered me the opportunity to be chief medical officer of CMS. He said “this platform is like no other to drive change.” He was right. I have been fortunate to have a few step-change opportunities in my life, and that was one.
On my first day at CMS, I looked around the table of senior executives reporting to me and realized they had more than 200 years of CMS experience. I was a bit scared. Together, we led the implementation of Hospital Value-Based Purchasing, the Compare websites, and numerous quality measurement and improvement programs. Partnership for Patients works on patient safety and was associated with preventing more than 3 million infections and adverse events, over 125,000 lives saved, and more than $26 billion in savings.
In early 2013, I was asked to lead the CMS Innovation Center (CMMI). The goal was to launch new payment and service delivery models to improve quality and lower costs. We launched Accountable Care Organizations, Bundled Payment programs, primary care medical homes, state-based innovation, and so much more. Medicare went from zero dollars in alternative payment models, where providers are accountable for quality and total cost of care, to more than 30% of Medicare payments, representing over $200 billion through agreements with more than 200,000 providers in these alternative payment models. It was the biggest shift in U.S. history in how CMS paid for care. Later, I became principal deputy administrator and acting administrator of CMS, leading an agency that spends over $1 trillion per year, or more than $2.5 billion per day and insures over 130 million Americans. We also improved from being bottom quintile in employee engagement and satisfaction across the federal government to No. 2.
I had assumed that, after working at CMS, I would return to a hospital/health system leadership role. But then, a recruiter called about the CEO role at Blue Cross Blue Shield of North Carolina. It is one of the largest not-for-profit health plans in the country and insures most of the people in North Carolina, many for most of their lives. I met a 75-year-old woman the other day that we have insured every day of her life. I am almost a year into the role and it is a mission-driven organization that drives positive change. I love it so far.
We are going to partner with providers, so that more than half of our payments will be in advanced alternative payment models. No payer in the United States has done that yet. This allows us to innovate and decrease friction in the system (e.g., turn off prior authorization) and be jointly accountable with providers for quality and total cost of care. We insure people through the ACA [Affordable Care Act], commercial, and Medicare markets, and are competing to serve Medicaid as well. We have invested more than $50 million to address social determinants of health across the state. We are making major investments in primary care, and mental and behavioral health. Our goal is to be a Model Blue – or a Model of Health Transformation for our state and nation – and achieve better health outcomes, lower costs, and best-in-class experience for all people we serve. I have learned that no physician leads a health plan of this size, and apparently, no practicing physician has ever led a health plan of this size.
What are some lessons learned over my career? I have had five criteria for all my career decisions: 1) family; 2) impact – better care and outcomes, lower costs, and exceptional experience for populations of patients; 3) people – mentors and colleagues; 4) learning; and 5) joy in work. If someone gives you a chance to lead people in your career as a physician, jump at the chance. We do a relatively poor job of providing this type of opportunity to those early in their careers in medicine, and learning how to manage people and money allows you to progress as a leader and manager.
Don’t listen to the people who say “you must do X before Y” or “you must take this path.” They are usually wrong. Take chances. I applied for many roles for which I was a long shot, and I didn’t always succeed. That’s life and learning. Hospital medicine is a great career. I worked in the hospital on a recent weekend and was able to help families through everything from palliative care decisions and new diagnoses, to recovering from illness. It is an honor to serve and help families in their time of need. Hospitalists have been – and should continue to be – primary drivers of the shift in our health system to value-based care.
As I look back on my career (and I hope I am only halfway done), I could not have predicted more than 90% of it. I was blessed with many opportunities, mentors, and teachers along the way. I try to pass this on by mentoring and teaching others. How did my career happen? I am not sure, but it has been a fun ride! And hopefully I have helped improve the health system some, along the way.
Dr. Conway is president and CEO of Blue Cross and Blue Shield of North Carolina. He is a hospitalist and former deputy administrator for innovation and quality at the Centers for Medicare and Medicaid Services.
A focus on health system transformation
A focus on health system transformation
I have to admit that I am not sure I am a legacy in hospital medicine, and the term legacy throws me off a bit. I came to medical school after working at McKinsey & Co. consulting, and I chose pediatrics because of my love of working with children and families, as well as a vague notion that I wanted to work on “system” issues, and therefore, more generalist-type training seemed applicable.
I met Chris Landrigan, MD, MPH, and Vinny Chiang, MD, and learned what a hospitalist was, as an intern in 2002. We had a research elective and I was able to publish a couple of papers in Pediatrics on pediatric hospital medicine with Chris and Raj Srivastava, MD, MPH. In 2004, I went to my first Society of Hospital Medicine meeting and met Larry Wellikson, MD, MHM, and others. From there, I went to the Robert Wood Johnson Clinical Scholars Program, with Ron Keren, MD, MPH, and others, and along with faculty from the Cincinnati Children’s in hospital medicine.
In 2007, I applied for a White House Fellowship and told my wife that I didn’t think there was a chance that I would get it, so we should keep building our new home in Cincinnati. We were both surprised when I was selected. I served Michael Leavitt, the then-Secretary of the Department of Health & Human Services, as his White House fellow during the Bush administration, and then served as his chief medical officer. Exposure to health policy and leadership at that level was career shaping. Cincinnati Children’s was searching for a leader for the conversion of pediatric hospital medicine into a full division in 2009. So I returned to Cincinnati to take on leading pediatric hospital medicine, and a role leading quality measurement and improvement efforts for the entire health system. I loved the work and thought I would remain in that role, and our family would be in Cincinnati for a long time. Best laid plans …
In early 2011, Don Berwick, MD, who was then the administrator of the Centers for Medicare & Medicaid Services called and asked whether I “would come talk with him in D.C.” That talk quickly became a series of interviews, and he offered me the opportunity to be chief medical officer of CMS. He said “this platform is like no other to drive change.” He was right. I have been fortunate to have a few step-change opportunities in my life, and that was one.
On my first day at CMS, I looked around the table of senior executives reporting to me and realized they had more than 200 years of CMS experience. I was a bit scared. Together, we led the implementation of Hospital Value-Based Purchasing, the Compare websites, and numerous quality measurement and improvement programs. Partnership for Patients works on patient safety and was associated with preventing more than 3 million infections and adverse events, over 125,000 lives saved, and more than $26 billion in savings.
In early 2013, I was asked to lead the CMS Innovation Center (CMMI). The goal was to launch new payment and service delivery models to improve quality and lower costs. We launched Accountable Care Organizations, Bundled Payment programs, primary care medical homes, state-based innovation, and so much more. Medicare went from zero dollars in alternative payment models, where providers are accountable for quality and total cost of care, to more than 30% of Medicare payments, representing over $200 billion through agreements with more than 200,000 providers in these alternative payment models. It was the biggest shift in U.S. history in how CMS paid for care. Later, I became principal deputy administrator and acting administrator of CMS, leading an agency that spends over $1 trillion per year, or more than $2.5 billion per day and insures over 130 million Americans. We also improved from being bottom quintile in employee engagement and satisfaction across the federal government to No. 2.
I had assumed that, after working at CMS, I would return to a hospital/health system leadership role. But then, a recruiter called about the CEO role at Blue Cross Blue Shield of North Carolina. It is one of the largest not-for-profit health plans in the country and insures most of the people in North Carolina, many for most of their lives. I met a 75-year-old woman the other day that we have insured every day of her life. I am almost a year into the role and it is a mission-driven organization that drives positive change. I love it so far.
We are going to partner with providers, so that more than half of our payments will be in advanced alternative payment models. No payer in the United States has done that yet. This allows us to innovate and decrease friction in the system (e.g., turn off prior authorization) and be jointly accountable with providers for quality and total cost of care. We insure people through the ACA [Affordable Care Act], commercial, and Medicare markets, and are competing to serve Medicaid as well. We have invested more than $50 million to address social determinants of health across the state. We are making major investments in primary care, and mental and behavioral health. Our goal is to be a Model Blue – or a Model of Health Transformation for our state and nation – and achieve better health outcomes, lower costs, and best-in-class experience for all people we serve. I have learned that no physician leads a health plan of this size, and apparently, no practicing physician has ever led a health plan of this size.
What are some lessons learned over my career? I have had five criteria for all my career decisions: 1) family; 2) impact – better care and outcomes, lower costs, and exceptional experience for populations of patients; 3) people – mentors and colleagues; 4) learning; and 5) joy in work. If someone gives you a chance to lead people in your career as a physician, jump at the chance. We do a relatively poor job of providing this type of opportunity to those early in their careers in medicine, and learning how to manage people and money allows you to progress as a leader and manager.
Don’t listen to the people who say “you must do X before Y” or “you must take this path.” They are usually wrong. Take chances. I applied for many roles for which I was a long shot, and I didn’t always succeed. That’s life and learning. Hospital medicine is a great career. I worked in the hospital on a recent weekend and was able to help families through everything from palliative care decisions and new diagnoses, to recovering from illness. It is an honor to serve and help families in their time of need. Hospitalists have been – and should continue to be – primary drivers of the shift in our health system to value-based care.
As I look back on my career (and I hope I am only halfway done), I could not have predicted more than 90% of it. I was blessed with many opportunities, mentors, and teachers along the way. I try to pass this on by mentoring and teaching others. How did my career happen? I am not sure, but it has been a fun ride! And hopefully I have helped improve the health system some, along the way.
Dr. Conway is president and CEO of Blue Cross and Blue Shield of North Carolina. He is a hospitalist and former deputy administrator for innovation and quality at the Centers for Medicare and Medicaid Services.
I have to admit that I am not sure I am a legacy in hospital medicine, and the term legacy throws me off a bit. I came to medical school after working at McKinsey & Co. consulting, and I chose pediatrics because of my love of working with children and families, as well as a vague notion that I wanted to work on “system” issues, and therefore, more generalist-type training seemed applicable.
I met Chris Landrigan, MD, MPH, and Vinny Chiang, MD, and learned what a hospitalist was, as an intern in 2002. We had a research elective and I was able to publish a couple of papers in Pediatrics on pediatric hospital medicine with Chris and Raj Srivastava, MD, MPH. In 2004, I went to my first Society of Hospital Medicine meeting and met Larry Wellikson, MD, MHM, and others. From there, I went to the Robert Wood Johnson Clinical Scholars Program, with Ron Keren, MD, MPH, and others, and along with faculty from the Cincinnati Children’s in hospital medicine.
In 2007, I applied for a White House Fellowship and told my wife that I didn’t think there was a chance that I would get it, so we should keep building our new home in Cincinnati. We were both surprised when I was selected. I served Michael Leavitt, the then-Secretary of the Department of Health & Human Services, as his White House fellow during the Bush administration, and then served as his chief medical officer. Exposure to health policy and leadership at that level was career shaping. Cincinnati Children’s was searching for a leader for the conversion of pediatric hospital medicine into a full division in 2009. So I returned to Cincinnati to take on leading pediatric hospital medicine, and a role leading quality measurement and improvement efforts for the entire health system. I loved the work and thought I would remain in that role, and our family would be in Cincinnati for a long time. Best laid plans …
In early 2011, Don Berwick, MD, who was then the administrator of the Centers for Medicare & Medicaid Services called and asked whether I “would come talk with him in D.C.” That talk quickly became a series of interviews, and he offered me the opportunity to be chief medical officer of CMS. He said “this platform is like no other to drive change.” He was right. I have been fortunate to have a few step-change opportunities in my life, and that was one.
On my first day at CMS, I looked around the table of senior executives reporting to me and realized they had more than 200 years of CMS experience. I was a bit scared. Together, we led the implementation of Hospital Value-Based Purchasing, the Compare websites, and numerous quality measurement and improvement programs. Partnership for Patients works on patient safety and was associated with preventing more than 3 million infections and adverse events, over 125,000 lives saved, and more than $26 billion in savings.
In early 2013, I was asked to lead the CMS Innovation Center (CMMI). The goal was to launch new payment and service delivery models to improve quality and lower costs. We launched Accountable Care Organizations, Bundled Payment programs, primary care medical homes, state-based innovation, and so much more. Medicare went from zero dollars in alternative payment models, where providers are accountable for quality and total cost of care, to more than 30% of Medicare payments, representing over $200 billion through agreements with more than 200,000 providers in these alternative payment models. It was the biggest shift in U.S. history in how CMS paid for care. Later, I became principal deputy administrator and acting administrator of CMS, leading an agency that spends over $1 trillion per year, or more than $2.5 billion per day and insures over 130 million Americans. We also improved from being bottom quintile in employee engagement and satisfaction across the federal government to No. 2.
I had assumed that, after working at CMS, I would return to a hospital/health system leadership role. But then, a recruiter called about the CEO role at Blue Cross Blue Shield of North Carolina. It is one of the largest not-for-profit health plans in the country and insures most of the people in North Carolina, many for most of their lives. I met a 75-year-old woman the other day that we have insured every day of her life. I am almost a year into the role and it is a mission-driven organization that drives positive change. I love it so far.
We are going to partner with providers, so that more than half of our payments will be in advanced alternative payment models. No payer in the United States has done that yet. This allows us to innovate and decrease friction in the system (e.g., turn off prior authorization) and be jointly accountable with providers for quality and total cost of care. We insure people through the ACA [Affordable Care Act], commercial, and Medicare markets, and are competing to serve Medicaid as well. We have invested more than $50 million to address social determinants of health across the state. We are making major investments in primary care, and mental and behavioral health. Our goal is to be a Model Blue – or a Model of Health Transformation for our state and nation – and achieve better health outcomes, lower costs, and best-in-class experience for all people we serve. I have learned that no physician leads a health plan of this size, and apparently, no practicing physician has ever led a health plan of this size.
What are some lessons learned over my career? I have had five criteria for all my career decisions: 1) family; 2) impact – better care and outcomes, lower costs, and exceptional experience for populations of patients; 3) people – mentors and colleagues; 4) learning; and 5) joy in work. If someone gives you a chance to lead people in your career as a physician, jump at the chance. We do a relatively poor job of providing this type of opportunity to those early in their careers in medicine, and learning how to manage people and money allows you to progress as a leader and manager.
Don’t listen to the people who say “you must do X before Y” or “you must take this path.” They are usually wrong. Take chances. I applied for many roles for which I was a long shot, and I didn’t always succeed. That’s life and learning. Hospital medicine is a great career. I worked in the hospital on a recent weekend and was able to help families through everything from palliative care decisions and new diagnoses, to recovering from illness. It is an honor to serve and help families in their time of need. Hospitalists have been – and should continue to be – primary drivers of the shift in our health system to value-based care.
As I look back on my career (and I hope I am only halfway done), I could not have predicted more than 90% of it. I was blessed with many opportunities, mentors, and teachers along the way. I try to pass this on by mentoring and teaching others. How did my career happen? I am not sure, but it has been a fun ride! And hopefully I have helped improve the health system some, along the way.
Dr. Conway is president and CEO of Blue Cross and Blue Shield of North Carolina. He is a hospitalist and former deputy administrator for innovation and quality at the Centers for Medicare and Medicaid Services.
ONC aims to help docs, patients with information sharing in proposed rule
The Office of the National Coordinator of Health Information Technology is looking to adopt standardized application programming interfaces (APIs) in an effort to boost interoperability of health data.
The Department of Health & Human Services office posted a proposed rule Feb. 11, 2019, that would, according to an agency press release, “help allow individuals to securely and easily access structured and unstructured EHI [electronic health information] formats using smartphones and other mobile devices.”
“We think our rule is going to help reduce burden and improve care,” Michael Lipinski, director of the Regulatory Affairs Division in the ONC Office of Policy, said in an interview. “It is going to do that through technology. With the APIs, you should be able to get to your information easier and have it readily available. Whether that is from another health care provider or using other health care products through the API to improve care, you will have that ability between the certified API and the information blocking policies to use third party developers and their products.”
The proposed rule also included a requirement that EHRs certified by ONC be able to easily export information contained within the EHR and make the format used to extract and export the data contained within the EHR publicly available.
“Another third party developer can build to that and offer competing services to pull that information out,” Mr. Lipinski said. “That would obviously help if you were choosing to switch [EHRs] if you didn’t like the features you were getting from your EHR. ... That functionality should help if you want to do that.”
The standardizing of APIs to help the delivery of data will go hand in hand with information blocking aspects of the proposed rule, which defines the few exceptions where an activity would not be considered information blocking, such as when engaging in practices will prevent patient harm; engaging in consistent, nondiscriminatory practices to protect patient privacy; and implementing practices to promote the security of health information.
Mr. Lipinski said these changes will help prevent providers from hiding behind HIPAA rules as the excuse to not share patient information, which will help with care coordination. “From a provider’s perspective, this should help them get more access to information, more access in a structured way and then easily get and share that information.”
Ultimately, Mr. Lipinski said, the goal is “to increase competition and lower cost while still improving the quality of care for patients.”
The Office of the National Coordinator of Health Information Technology is looking to adopt standardized application programming interfaces (APIs) in an effort to boost interoperability of health data.
The Department of Health & Human Services office posted a proposed rule Feb. 11, 2019, that would, according to an agency press release, “help allow individuals to securely and easily access structured and unstructured EHI [electronic health information] formats using smartphones and other mobile devices.”
“We think our rule is going to help reduce burden and improve care,” Michael Lipinski, director of the Regulatory Affairs Division in the ONC Office of Policy, said in an interview. “It is going to do that through technology. With the APIs, you should be able to get to your information easier and have it readily available. Whether that is from another health care provider or using other health care products through the API to improve care, you will have that ability between the certified API and the information blocking policies to use third party developers and their products.”
The proposed rule also included a requirement that EHRs certified by ONC be able to easily export information contained within the EHR and make the format used to extract and export the data contained within the EHR publicly available.
“Another third party developer can build to that and offer competing services to pull that information out,” Mr. Lipinski said. “That would obviously help if you were choosing to switch [EHRs] if you didn’t like the features you were getting from your EHR. ... That functionality should help if you want to do that.”
The standardizing of APIs to help the delivery of data will go hand in hand with information blocking aspects of the proposed rule, which defines the few exceptions where an activity would not be considered information blocking, such as when engaging in practices will prevent patient harm; engaging in consistent, nondiscriminatory practices to protect patient privacy; and implementing practices to promote the security of health information.
Mr. Lipinski said these changes will help prevent providers from hiding behind HIPAA rules as the excuse to not share patient information, which will help with care coordination. “From a provider’s perspective, this should help them get more access to information, more access in a structured way and then easily get and share that information.”
Ultimately, Mr. Lipinski said, the goal is “to increase competition and lower cost while still improving the quality of care for patients.”
The Office of the National Coordinator of Health Information Technology is looking to adopt standardized application programming interfaces (APIs) in an effort to boost interoperability of health data.
The Department of Health & Human Services office posted a proposed rule Feb. 11, 2019, that would, according to an agency press release, “help allow individuals to securely and easily access structured and unstructured EHI [electronic health information] formats using smartphones and other mobile devices.”
“We think our rule is going to help reduce burden and improve care,” Michael Lipinski, director of the Regulatory Affairs Division in the ONC Office of Policy, said in an interview. “It is going to do that through technology. With the APIs, you should be able to get to your information easier and have it readily available. Whether that is from another health care provider or using other health care products through the API to improve care, you will have that ability between the certified API and the information blocking policies to use third party developers and their products.”
The proposed rule also included a requirement that EHRs certified by ONC be able to easily export information contained within the EHR and make the format used to extract and export the data contained within the EHR publicly available.
“Another third party developer can build to that and offer competing services to pull that information out,” Mr. Lipinski said. “That would obviously help if you were choosing to switch [EHRs] if you didn’t like the features you were getting from your EHR. ... That functionality should help if you want to do that.”
The standardizing of APIs to help the delivery of data will go hand in hand with information blocking aspects of the proposed rule, which defines the few exceptions where an activity would not be considered information blocking, such as when engaging in practices will prevent patient harm; engaging in consistent, nondiscriminatory practices to protect patient privacy; and implementing practices to promote the security of health information.
Mr. Lipinski said these changes will help prevent providers from hiding behind HIPAA rules as the excuse to not share patient information, which will help with care coordination. “From a provider’s perspective, this should help them get more access to information, more access in a structured way and then easily get and share that information.”
Ultimately, Mr. Lipinski said, the goal is “to increase competition and lower cost while still improving the quality of care for patients.”
Fund projects, not people to address gender bias in research funding
LONDON – Female investigators are less likely to secure research funding than male investigators, not because their proposed project is of lesser scientific merit, but simply because they are women, according to research published in The Lancet.
Women had a 30% lower chance of success in getting funding for a project than did their male counterparts when the caliber of the principal investigator was considered as an explicit part of the grant application process, with an 8.8% probability of getting funded versus 12.7%, respectively. If the application was considered solely on a project basis, however, the gender bias was less (12.1% vs. 12.9%).
The overall success of grant applications was 15.8% in the analysis, which considered almost 24,000 grant applications from more than 7,000 principal investigators submitted to the Canadian Institutes of Health Research (CIHR) between 2011 and 2016.
“I see our study as basically one good thwack in a long game of whack-a-mole,” lead study author Holly O. Witteman, PhD, said during an event to launch a special edition of The Lancet focusing on advancing women in science, medicine, and global health.
Dr. Witteman’s research is one of three original articles included in the thematic issue that brings together female authors and commentators to look at gender equity and what needs to be done to address imbalances. The issue is the result of a call for papers that led to more than 300 submissions from more than 40 countries and, according to an editorial from The Lancet, highlights that gender equity in medicine “is not only a matter of justice and rights, it is crucial for producing the best research and providing the best care to patients.”
That there are discrepancies in research funding awarded to female and male investigators has been known for years, Dr. Witteman, associate professor of family and emergency medicine at Laval University, Quebec City, said at the London press conference. To learn how and why, a “quasiexperimental” approach was used to find out what factors might be influencing the gender gap.
“Women are scored lower for competence compared to men with the same publication record,” she said. It’s not that they publish less or do easier research, or that the quality is lower, they are just viewed less favorably overall throughout their careers. Even when you control for confounding factors, “they still don’t advance as quickly,” she said.
“It had been documented for a while that, overall, women tend to get less grant funding and there hasn’t been any evidence to show either way if maybe women’s grant applications weren’t as good,” Dr. Witteman explained.
In 2014, the CIHR changed the way it funded research projects, creating a “natural experiment.” Two new grant application programs were put in place which largely differed by whether or not an explicit review of the principal investigator and their ability to conduct the research was included.
Adjusting for age and type of research, Dr. Witteman and her coauthors found that there was little difference in the success of women in securing research funding when their grant applications were judged solely on a scientific basis; however, when the focus was placed on the principal investigator, women were disadvantaged.
Dr. Witteman said that “this provides robust evidence in support of the idea that women write equally good grant applications but aren’t evaluated as being equally good scientists.”
So how to redress the balance? Dr. Witteman suggested that one way was for funders to collect robust evidence on the success of grant applications and be transparent who is getting funded and how much funding is being awarded. Institutions should invest in and support young investigators, distributing power and flattening traditionally male-led hierarchies. Salaries should be aligned and research support evened out, she said.
Investigators themselves also have a role to play to do the best possible work and try to change the system. “Advocate for others,” she said. That included advocating for others in groups that you may not be part of – which can be easier in some respects than advocating for a group that you are in.
“Funders should evaluate projects, not people,” Jennifer L. Raymond, PhD, and Miriam B. Goodman, PhD, both professors at Stanford (Calif.) University wrote in a comment in The Lancet special issue. They suggested that people-based funding had been gaining popularity but that funders would be better off funding by project to achieve scientific and clinical goals. “Assess the investigator only after double-blind review of the proposed research is complete,” they suggested. “Reduce the assessment of the investigator to a binary judgment of whether or not the investigator has the expertise and resources needed do the proposed research.”
During a panel discussion at The Lancet event, Cassidy R. Sugimoto, PhD, associate professor of informatics at Indiana University in Bloomington and a program director for the Science and Innovation Policy Program at the National Science Foundation (NSF) observed that data on gender equality in research funding were already being collected and will be used to determine how best to adjust funding policies.
“Looking from the 1980s to the present, women make up shy of 20% of the funds given by the National Science Foundation,” Dr. Sugimoto said. “That’s improved over time, and it’s at 28% currently, which is less than their authorship.”
Tammy Clifford, PhD, vice president of research programs at the CIHR observed that data collection was “a critically important step, but of course that’s not the only step,” she said. “We need to look at and analyze the data regularly, and then when you see things that are not on track, you make changes.”
One of the changes the CIHR has made is to train people who are reviewing grant applications on factors that may unconsciously affect their decisions. “There are things to be done, and I don’t think we are quite there yet, but we are committed to continually looking at those data, to making the changes that are required.”
Representing the Wellcome Trust, Ed Whiting, director of policy and chief of staff, said that the funding of projects led by female investigators was moving in the right direction. He noted that there was still a lower rate of applications from women for senior award levels, but that the panels that decide upon the funding were moving toward equal gender representation. The aim was to get to a 50/50 female to male ratio on the panels by 2020, he said; it is was at 46%-52% in 2018.
Dr. Witteman and all other commentators had no financial disclosures.
SOURCE: Witteman HO et al. Lancet. 2019. doi: 10.1016/S0140-6736(18)32611-4
LONDON – Female investigators are less likely to secure research funding than male investigators, not because their proposed project is of lesser scientific merit, but simply because they are women, according to research published in The Lancet.
Women had a 30% lower chance of success in getting funding for a project than did their male counterparts when the caliber of the principal investigator was considered as an explicit part of the grant application process, with an 8.8% probability of getting funded versus 12.7%, respectively. If the application was considered solely on a project basis, however, the gender bias was less (12.1% vs. 12.9%).
The overall success of grant applications was 15.8% in the analysis, which considered almost 24,000 grant applications from more than 7,000 principal investigators submitted to the Canadian Institutes of Health Research (CIHR) between 2011 and 2016.
“I see our study as basically one good thwack in a long game of whack-a-mole,” lead study author Holly O. Witteman, PhD, said during an event to launch a special edition of The Lancet focusing on advancing women in science, medicine, and global health.
Dr. Witteman’s research is one of three original articles included in the thematic issue that brings together female authors and commentators to look at gender equity and what needs to be done to address imbalances. The issue is the result of a call for papers that led to more than 300 submissions from more than 40 countries and, according to an editorial from The Lancet, highlights that gender equity in medicine “is not only a matter of justice and rights, it is crucial for producing the best research and providing the best care to patients.”
That there are discrepancies in research funding awarded to female and male investigators has been known for years, Dr. Witteman, associate professor of family and emergency medicine at Laval University, Quebec City, said at the London press conference. To learn how and why, a “quasiexperimental” approach was used to find out what factors might be influencing the gender gap.
“Women are scored lower for competence compared to men with the same publication record,” she said. It’s not that they publish less or do easier research, or that the quality is lower, they are just viewed less favorably overall throughout their careers. Even when you control for confounding factors, “they still don’t advance as quickly,” she said.
“It had been documented for a while that, overall, women tend to get less grant funding and there hasn’t been any evidence to show either way if maybe women’s grant applications weren’t as good,” Dr. Witteman explained.
In 2014, the CIHR changed the way it funded research projects, creating a “natural experiment.” Two new grant application programs were put in place which largely differed by whether or not an explicit review of the principal investigator and their ability to conduct the research was included.
Adjusting for age and type of research, Dr. Witteman and her coauthors found that there was little difference in the success of women in securing research funding when their grant applications were judged solely on a scientific basis; however, when the focus was placed on the principal investigator, women were disadvantaged.
Dr. Witteman said that “this provides robust evidence in support of the idea that women write equally good grant applications but aren’t evaluated as being equally good scientists.”
So how to redress the balance? Dr. Witteman suggested that one way was for funders to collect robust evidence on the success of grant applications and be transparent who is getting funded and how much funding is being awarded. Institutions should invest in and support young investigators, distributing power and flattening traditionally male-led hierarchies. Salaries should be aligned and research support evened out, she said.
Investigators themselves also have a role to play to do the best possible work and try to change the system. “Advocate for others,” she said. That included advocating for others in groups that you may not be part of – which can be easier in some respects than advocating for a group that you are in.
“Funders should evaluate projects, not people,” Jennifer L. Raymond, PhD, and Miriam B. Goodman, PhD, both professors at Stanford (Calif.) University wrote in a comment in The Lancet special issue. They suggested that people-based funding had been gaining popularity but that funders would be better off funding by project to achieve scientific and clinical goals. “Assess the investigator only after double-blind review of the proposed research is complete,” they suggested. “Reduce the assessment of the investigator to a binary judgment of whether or not the investigator has the expertise and resources needed do the proposed research.”
During a panel discussion at The Lancet event, Cassidy R. Sugimoto, PhD, associate professor of informatics at Indiana University in Bloomington and a program director for the Science and Innovation Policy Program at the National Science Foundation (NSF) observed that data on gender equality in research funding were already being collected and will be used to determine how best to adjust funding policies.
“Looking from the 1980s to the present, women make up shy of 20% of the funds given by the National Science Foundation,” Dr. Sugimoto said. “That’s improved over time, and it’s at 28% currently, which is less than their authorship.”
Tammy Clifford, PhD, vice president of research programs at the CIHR observed that data collection was “a critically important step, but of course that’s not the only step,” she said. “We need to look at and analyze the data regularly, and then when you see things that are not on track, you make changes.”
One of the changes the CIHR has made is to train people who are reviewing grant applications on factors that may unconsciously affect their decisions. “There are things to be done, and I don’t think we are quite there yet, but we are committed to continually looking at those data, to making the changes that are required.”
Representing the Wellcome Trust, Ed Whiting, director of policy and chief of staff, said that the funding of projects led by female investigators was moving in the right direction. He noted that there was still a lower rate of applications from women for senior award levels, but that the panels that decide upon the funding were moving toward equal gender representation. The aim was to get to a 50/50 female to male ratio on the panels by 2020, he said; it is was at 46%-52% in 2018.
Dr. Witteman and all other commentators had no financial disclosures.
SOURCE: Witteman HO et al. Lancet. 2019. doi: 10.1016/S0140-6736(18)32611-4
LONDON – Female investigators are less likely to secure research funding than male investigators, not because their proposed project is of lesser scientific merit, but simply because they are women, according to research published in The Lancet.
Women had a 30% lower chance of success in getting funding for a project than did their male counterparts when the caliber of the principal investigator was considered as an explicit part of the grant application process, with an 8.8% probability of getting funded versus 12.7%, respectively. If the application was considered solely on a project basis, however, the gender bias was less (12.1% vs. 12.9%).
The overall success of grant applications was 15.8% in the analysis, which considered almost 24,000 grant applications from more than 7,000 principal investigators submitted to the Canadian Institutes of Health Research (CIHR) between 2011 and 2016.
“I see our study as basically one good thwack in a long game of whack-a-mole,” lead study author Holly O. Witteman, PhD, said during an event to launch a special edition of The Lancet focusing on advancing women in science, medicine, and global health.
Dr. Witteman’s research is one of three original articles included in the thematic issue that brings together female authors and commentators to look at gender equity and what needs to be done to address imbalances. The issue is the result of a call for papers that led to more than 300 submissions from more than 40 countries and, according to an editorial from The Lancet, highlights that gender equity in medicine “is not only a matter of justice and rights, it is crucial for producing the best research and providing the best care to patients.”
That there are discrepancies in research funding awarded to female and male investigators has been known for years, Dr. Witteman, associate professor of family and emergency medicine at Laval University, Quebec City, said at the London press conference. To learn how and why, a “quasiexperimental” approach was used to find out what factors might be influencing the gender gap.
“Women are scored lower for competence compared to men with the same publication record,” she said. It’s not that they publish less or do easier research, or that the quality is lower, they are just viewed less favorably overall throughout their careers. Even when you control for confounding factors, “they still don’t advance as quickly,” she said.
“It had been documented for a while that, overall, women tend to get less grant funding and there hasn’t been any evidence to show either way if maybe women’s grant applications weren’t as good,” Dr. Witteman explained.
In 2014, the CIHR changed the way it funded research projects, creating a “natural experiment.” Two new grant application programs were put in place which largely differed by whether or not an explicit review of the principal investigator and their ability to conduct the research was included.
Adjusting for age and type of research, Dr. Witteman and her coauthors found that there was little difference in the success of women in securing research funding when their grant applications were judged solely on a scientific basis; however, when the focus was placed on the principal investigator, women were disadvantaged.
Dr. Witteman said that “this provides robust evidence in support of the idea that women write equally good grant applications but aren’t evaluated as being equally good scientists.”
So how to redress the balance? Dr. Witteman suggested that one way was for funders to collect robust evidence on the success of grant applications and be transparent who is getting funded and how much funding is being awarded. Institutions should invest in and support young investigators, distributing power and flattening traditionally male-led hierarchies. Salaries should be aligned and research support evened out, she said.
Investigators themselves also have a role to play to do the best possible work and try to change the system. “Advocate for others,” she said. That included advocating for others in groups that you may not be part of – which can be easier in some respects than advocating for a group that you are in.
“Funders should evaluate projects, not people,” Jennifer L. Raymond, PhD, and Miriam B. Goodman, PhD, both professors at Stanford (Calif.) University wrote in a comment in The Lancet special issue. They suggested that people-based funding had been gaining popularity but that funders would be better off funding by project to achieve scientific and clinical goals. “Assess the investigator only after double-blind review of the proposed research is complete,” they suggested. “Reduce the assessment of the investigator to a binary judgment of whether or not the investigator has the expertise and resources needed do the proposed research.”
During a panel discussion at The Lancet event, Cassidy R. Sugimoto, PhD, associate professor of informatics at Indiana University in Bloomington and a program director for the Science and Innovation Policy Program at the National Science Foundation (NSF) observed that data on gender equality in research funding were already being collected and will be used to determine how best to adjust funding policies.
“Looking from the 1980s to the present, women make up shy of 20% of the funds given by the National Science Foundation,” Dr. Sugimoto said. “That’s improved over time, and it’s at 28% currently, which is less than their authorship.”
Tammy Clifford, PhD, vice president of research programs at the CIHR observed that data collection was “a critically important step, but of course that’s not the only step,” she said. “We need to look at and analyze the data regularly, and then when you see things that are not on track, you make changes.”
One of the changes the CIHR has made is to train people who are reviewing grant applications on factors that may unconsciously affect their decisions. “There are things to be done, and I don’t think we are quite there yet, but we are committed to continually looking at those data, to making the changes that are required.”
Representing the Wellcome Trust, Ed Whiting, director of policy and chief of staff, said that the funding of projects led by female investigators was moving in the right direction. He noted that there was still a lower rate of applications from women for senior award levels, but that the panels that decide upon the funding were moving toward equal gender representation. The aim was to get to a 50/50 female to male ratio on the panels by 2020, he said; it is was at 46%-52% in 2018.
Dr. Witteman and all other commentators had no financial disclosures.
SOURCE: Witteman HO et al. Lancet. 2019. doi: 10.1016/S0140-6736(18)32611-4
FROM A LAUNCH EVENT HELD BY THE LANCET
Key clinical point: Funding bodies should focus on the science of a research project not on who is conducting the research.
Major finding: Between 2011 and 2016, 8.8% of projects proposed by female researchers and 12.7% of those proposed by male researchers were funded.
Study details: Analysis of nearly 24,000 grant applications from more than 7,000 principal investigators submitted to the Canadian Institutes of Health Research during 2011-2016.
Disclosures: The research was unfunded. Dr. Witteman and all other commentators had no financial disclosures.
Source: Witteman HO et al. Lancet. 2019. doi: 10.1016/S0140-6736(18)32611-4.
The psychiatrist of the future
As a psychiatry resident in the early 1980s, Carol A. Bernstein, MD, remembers a teaching setting where young physicians worked long hours, male residents outnumbered female residents, and messages were delivered in the form of handwritten notes.
Today, the learning environment for psychiatry residents is vastly different. Duty-hour restrictions are routine, the gender gap has narrowed, and electronic communication in its many forms, is the norm. Medical advancements give residents a greater ability to treat patients and improve illnesses, said Dr. Bernstein, a clinical psychiatry professor and vice chair for education in psychiatry at New York University. However, residents also face a range of modern challenges, such as higher learning expectations, a more litigious culture, and a practice landscape increasingly reliant on ratings and patient satisfaction scores.
“This is a generation – whether you want to call it the Millennials or the iGen – [who have] been pushed to do more and more,” said Dr. Bernstein, a past president of the American Psychiatric Association. “Medical care has become very complicated, and it is very hard for trainees to get mastery of it.”
At the same time, the digital world that today’s residents are accustomed to has become a double-edged sword in medical education, said Donna M. Sudak, MD, outgoing president of the American Association of Directors of Psychiatric Residency Training. Technology has generated new ways of learning, such as online modules, but also created opportunities for distraction, she said.
“All of us, our learners as well as ourselves, need to figure out the best balance of using technology in order to facilitate learning,” Dr. Sudak said. “The pro is having the world at your fingertips and the ability to work with other people across the country. The con is the temptation to be attached to your screen, rather than truly listening to the person you’re in the room with – as a psychiatrist, that’s even more critical.”
The new faces of psychiatry
Interest in psychiatry has grown steadily over the years. In 2018, 2,739 medical school graduates ranked for a PGY-1 psychiatry residency, up from 1,806 ranked applicants in 2008, according to data from the National Residency Matching Program. Of the 2018 ranked applicants, 1,540 matched to a residency program. Data from the Association of American Medical Colleges (AAMC) show that 47% of psychiatry residency applicants in 2018 were women.
Millennial graduates are choosing psychiatry for a variety of reasons. For Nina Vasan, MD, MBA, the career path meant an opportunity to make a broader impact.
“Mental health is a defining social issue of our time, and in medical school I felt like if I committed my time and energy to improving mental health, I would maximize the impact I make on the world,” said Dr. Vasan, who finished residency at Stanford (Calif.) University in 2018. “I feel even stronger about that today. … I felt drawn to both the fundamental way in which we get to connect with our patients on an individual level and impact their lives, as well as the broader societal-level change that must happen in the coming years that I want to be a part of.”
A sense of social responsibility is a common trait of this generation’s psychiatrists, said Dr. Vasan, who has a private concierge practice in the Silicon Valley.
“We have a global sense of the world and recognize that our role as physicians gives us the unique platform to make an impact at this level,” she said.
Graduates also are attracted to psychiatry because of its focus on the physician-patient connection, particularly as patient time is eroded in other specialties, such as primary care, Dr. Sudak said.
“People who become physicians really want to have relationships with patients, and if you have to see eight people an hour, that’s a tough go,” she said. “Many people are attracted to the capacity to really learn about somebody’s story and make a difference in their life. Psychiatry offers that and then some.”
Working closely with patients to improve their quality of life was a primary motivator for Steven Chan, MD, MBA, who completed his psychiatry residency at the University of California, Davis, in 2016. He currently serves on the addiction treatment services team at the VA Palo Alto Health Care System.
“I additionally pursued a subspecialty in clinical informatics to apply today’s technologies to further improve people’s lives,” he said.
Dr. Chan said he is fortunate to practice in a work environment that is more collaborative with other health professionals than in the past.
“It’s wonderful,” he said. “There’s so much work to be done, and working with others has been rewarding to me. We’re already seeing more psychiatrists take on leadership roles in technology and health care administration, so we’re seeing collaborations with informatics, engineers, and service designers.”
A sea of challenges
Despite the advantages of practicing in modern times, psychiatrists today also face unique challenges, such as an upcoming shortages of physicians.
A 2017 report by the National Council for Behavioral Health estimates that, by 2025, demand might outpace supply by up to 15,600 psychiatrists. An aging population of psychiatrists is part of the problem. Sixty percent of practicing psychiatrists are older than 55, one of the highest volumes of older doctors of all specialties, according to AAMC data.
Physician numbers are improving, but a crisis point looms, especially as more states pass legislation that target the so-called dangerously mentally ill, said Annette L. Hanson, MD, a forensic psychiatrist who is assistant professor of psychiatry at the University of Maryland and at Johns Hopkins University, both in Baltimore.
“The trend seems to be that governments want to provide more involuntary or forced care, which means you’re going to need to have doctors available to provide that care,” Dr. Hanson said in an interview. “We don’t have enough doctors to meet the public policy demand.”
Compounding the problem is the fact that the majority of new psychiatrists pursue community private practices in urban areas, rather than practicing in state hospitals or rural areas, Dr. Hanson added. In addition, some states are passing laws that require state hospitals to admit incompetent criminal defendants within a certain time frame.
“That’s created significant problems where you’re moving someone from an overcrowded, understaffed jail to overcrowded, understaffed hospital,” she said.
The growing use of telepsychiatry might be one answer to the upcoming shortage. A June 2018 letter from the Centers for Medicare & Medicaid Services encouraged more states to use health technology efforts to address the opioid crisis, including through telemedicine and telepsychiatry. Meanwhile, several states have expanded their controlled substance laws to allow remote prescribing through telehealth for the treatment of psychiatric or substance use disorders.
However, licensing issues and reimbursement inconsistencies continue to act as barriers to the practice of telepsychiatry, according to the National Council report.
Some academic institutions are crafting new ways to use technology to meet the demand for mental health care. At Stanford, for example, Dr. Vasan started a lab called Brainstorm, the Stanford Laboratory for Brain Health Innovation and Entrepreneurship, which unites medicine, business, technology, and design to develop tech products for patients. She also chairs Stanford’s Mental Health Technology Hub, a consortium of more than 20 faculty labs addressing the role technology plays in improving mental health.
“We psychiatrists need partners to help increase access to mental health prevention, diagnosis, and treatment,” Dr. Vasan said. “Technology can be that partner.”
Improving diversity is an ongoing challenge for the field, said Dr. Sudak, also professor and vice chair for education in the department of psychiatry at Drexel University in Philadelphia. Of practicing psychiatrists, 42% declare as white, 8% as Asian, 4% as black, and 4% as Hispanic, according to the latest workforce data published by the AAMC. By comparison, 61% of the U.S. population is white, while 18% is Hispanic, 13% is black, and 6% is Asian, according to recent census statistics. By 2044, more than half of all Americans are projected to belong to a minority group.
“In general, we know that more diversity will enhance outcomes of care for our patients,” Dr. Sudak said. “When I talk about workforce, I think about that piece as a significant part of the equation. It’s not just about getting more slots, but it’s about filling those slots with a population of trainees that mirrors the population, rather than mirrors a very small subset.
Training changes
One of the biggest changes affecting residency training today is the decreased length of stay for inpatients, Dr. Hanson said. When she was a resident, the average length of stay was about 3 weeks, compared with 7-10 days now.
“The challenge is sorting out an underlying psychiatric condition from the effects of substances, which is really difficult with that short of a length of stay,” she said. “You lose a longitudinal perspective if you don’t have a chance to observe someone once they’ve been stabilized and the crisis has passed and they’re detoxified from the substances they were using prior to admission.”
The arrival of electronic medical records also has affected the trainee experience by taking time away from the doctor-patient relationship, Dr. Bernstein said. Other technology, such as algorithms used to avoid mistakes, have become both helpful and harmful.
“[Having the technology] is very good, but people have to learn how to think,” Dr. Bernstein said. “There’s a lot of medicine that’s an art, and in psychiatry even more so. You don’t have the blood tests or the imaging tests that other specialties have, and that is both our advantage and our disadvantage.”
In the future, technology will continue to have a central role in residency training, experts said. Already, independent study using technology has become the norm, Dr. Hanson said. When students are in a more structured environment, technology such as cell phones, can act as a distraction, she noted.
“I’ve decided to embrace it and use it,” she said. “My approach is to co-opt the cell phones. Periodically, during a talk, I may put up a website that has a pop quiz on it [in which] students use their cell phones to answer.”
Certainly, efforts to build diversity will be a continued focus for the specialty, said Dr. Sudak. In addition, residency might shift from less inpatient training to more subspecialty rotations for general psychiatry training, she said.
“We will need to teach residents to retain a focus on the patient as a person and use outcomes to help guide treatment,” she said.
Dr. Bernstein would like to see the pendulum swing back on such rigid duty hours, she said, with more emphasis placed on building residents’ confidence in managing complex cases and preparing trainees for overcoming adversity.
Dr. Vasan envisions more integration of psychiatry with neurology and the rest of medicine, more training in business elements, such as managing teams and a practice, as well as education on technological tools for psychiatrists.
From a broader perspective, Dr. Vasan hopes that the stigma around mental health will continue to improve and that society at large becomes more supportive of the work of psychiatrists.
“In some ways it seems like we have come far in openly discussing and understanding mental illness, as well as the fact that having these diseases does not need to hold anyone back from realizing their potential,” she said. “But not far enough. The public’s understanding of the scope of the problem and the urgency and value for addressing mental health has increased tremendously.
“Our colleagues in other fields of medicine, employers, politicians, educators ... they all value, seem to value psychiatry more, and I hope this continues to grow.”
As a psychiatry resident in the early 1980s, Carol A. Bernstein, MD, remembers a teaching setting where young physicians worked long hours, male residents outnumbered female residents, and messages were delivered in the form of handwritten notes.
Today, the learning environment for psychiatry residents is vastly different. Duty-hour restrictions are routine, the gender gap has narrowed, and electronic communication in its many forms, is the norm. Medical advancements give residents a greater ability to treat patients and improve illnesses, said Dr. Bernstein, a clinical psychiatry professor and vice chair for education in psychiatry at New York University. However, residents also face a range of modern challenges, such as higher learning expectations, a more litigious culture, and a practice landscape increasingly reliant on ratings and patient satisfaction scores.
“This is a generation – whether you want to call it the Millennials or the iGen – [who have] been pushed to do more and more,” said Dr. Bernstein, a past president of the American Psychiatric Association. “Medical care has become very complicated, and it is very hard for trainees to get mastery of it.”
At the same time, the digital world that today’s residents are accustomed to has become a double-edged sword in medical education, said Donna M. Sudak, MD, outgoing president of the American Association of Directors of Psychiatric Residency Training. Technology has generated new ways of learning, such as online modules, but also created opportunities for distraction, she said.
“All of us, our learners as well as ourselves, need to figure out the best balance of using technology in order to facilitate learning,” Dr. Sudak said. “The pro is having the world at your fingertips and the ability to work with other people across the country. The con is the temptation to be attached to your screen, rather than truly listening to the person you’re in the room with – as a psychiatrist, that’s even more critical.”
The new faces of psychiatry
Interest in psychiatry has grown steadily over the years. In 2018, 2,739 medical school graduates ranked for a PGY-1 psychiatry residency, up from 1,806 ranked applicants in 2008, according to data from the National Residency Matching Program. Of the 2018 ranked applicants, 1,540 matched to a residency program. Data from the Association of American Medical Colleges (AAMC) show that 47% of psychiatry residency applicants in 2018 were women.
Millennial graduates are choosing psychiatry for a variety of reasons. For Nina Vasan, MD, MBA, the career path meant an opportunity to make a broader impact.
“Mental health is a defining social issue of our time, and in medical school I felt like if I committed my time and energy to improving mental health, I would maximize the impact I make on the world,” said Dr. Vasan, who finished residency at Stanford (Calif.) University in 2018. “I feel even stronger about that today. … I felt drawn to both the fundamental way in which we get to connect with our patients on an individual level and impact their lives, as well as the broader societal-level change that must happen in the coming years that I want to be a part of.”
A sense of social responsibility is a common trait of this generation’s psychiatrists, said Dr. Vasan, who has a private concierge practice in the Silicon Valley.
“We have a global sense of the world and recognize that our role as physicians gives us the unique platform to make an impact at this level,” she said.
Graduates also are attracted to psychiatry because of its focus on the physician-patient connection, particularly as patient time is eroded in other specialties, such as primary care, Dr. Sudak said.
“People who become physicians really want to have relationships with patients, and if you have to see eight people an hour, that’s a tough go,” she said. “Many people are attracted to the capacity to really learn about somebody’s story and make a difference in their life. Psychiatry offers that and then some.”
Working closely with patients to improve their quality of life was a primary motivator for Steven Chan, MD, MBA, who completed his psychiatry residency at the University of California, Davis, in 2016. He currently serves on the addiction treatment services team at the VA Palo Alto Health Care System.
“I additionally pursued a subspecialty in clinical informatics to apply today’s technologies to further improve people’s lives,” he said.
Dr. Chan said he is fortunate to practice in a work environment that is more collaborative with other health professionals than in the past.
“It’s wonderful,” he said. “There’s so much work to be done, and working with others has been rewarding to me. We’re already seeing more psychiatrists take on leadership roles in technology and health care administration, so we’re seeing collaborations with informatics, engineers, and service designers.”
A sea of challenges
Despite the advantages of practicing in modern times, psychiatrists today also face unique challenges, such as an upcoming shortages of physicians.
A 2017 report by the National Council for Behavioral Health estimates that, by 2025, demand might outpace supply by up to 15,600 psychiatrists. An aging population of psychiatrists is part of the problem. Sixty percent of practicing psychiatrists are older than 55, one of the highest volumes of older doctors of all specialties, according to AAMC data.
Physician numbers are improving, but a crisis point looms, especially as more states pass legislation that target the so-called dangerously mentally ill, said Annette L. Hanson, MD, a forensic psychiatrist who is assistant professor of psychiatry at the University of Maryland and at Johns Hopkins University, both in Baltimore.
“The trend seems to be that governments want to provide more involuntary or forced care, which means you’re going to need to have doctors available to provide that care,” Dr. Hanson said in an interview. “We don’t have enough doctors to meet the public policy demand.”
Compounding the problem is the fact that the majority of new psychiatrists pursue community private practices in urban areas, rather than practicing in state hospitals or rural areas, Dr. Hanson added. In addition, some states are passing laws that require state hospitals to admit incompetent criminal defendants within a certain time frame.
“That’s created significant problems where you’re moving someone from an overcrowded, understaffed jail to overcrowded, understaffed hospital,” she said.
The growing use of telepsychiatry might be one answer to the upcoming shortage. A June 2018 letter from the Centers for Medicare & Medicaid Services encouraged more states to use health technology efforts to address the opioid crisis, including through telemedicine and telepsychiatry. Meanwhile, several states have expanded their controlled substance laws to allow remote prescribing through telehealth for the treatment of psychiatric or substance use disorders.
However, licensing issues and reimbursement inconsistencies continue to act as barriers to the practice of telepsychiatry, according to the National Council report.
Some academic institutions are crafting new ways to use technology to meet the demand for mental health care. At Stanford, for example, Dr. Vasan started a lab called Brainstorm, the Stanford Laboratory for Brain Health Innovation and Entrepreneurship, which unites medicine, business, technology, and design to develop tech products for patients. She also chairs Stanford’s Mental Health Technology Hub, a consortium of more than 20 faculty labs addressing the role technology plays in improving mental health.
“We psychiatrists need partners to help increase access to mental health prevention, diagnosis, and treatment,” Dr. Vasan said. “Technology can be that partner.”
Improving diversity is an ongoing challenge for the field, said Dr. Sudak, also professor and vice chair for education in the department of psychiatry at Drexel University in Philadelphia. Of practicing psychiatrists, 42% declare as white, 8% as Asian, 4% as black, and 4% as Hispanic, according to the latest workforce data published by the AAMC. By comparison, 61% of the U.S. population is white, while 18% is Hispanic, 13% is black, and 6% is Asian, according to recent census statistics. By 2044, more than half of all Americans are projected to belong to a minority group.
“In general, we know that more diversity will enhance outcomes of care for our patients,” Dr. Sudak said. “When I talk about workforce, I think about that piece as a significant part of the equation. It’s not just about getting more slots, but it’s about filling those slots with a population of trainees that mirrors the population, rather than mirrors a very small subset.
Training changes
One of the biggest changes affecting residency training today is the decreased length of stay for inpatients, Dr. Hanson said. When she was a resident, the average length of stay was about 3 weeks, compared with 7-10 days now.
“The challenge is sorting out an underlying psychiatric condition from the effects of substances, which is really difficult with that short of a length of stay,” she said. “You lose a longitudinal perspective if you don’t have a chance to observe someone once they’ve been stabilized and the crisis has passed and they’re detoxified from the substances they were using prior to admission.”
The arrival of electronic medical records also has affected the trainee experience by taking time away from the doctor-patient relationship, Dr. Bernstein said. Other technology, such as algorithms used to avoid mistakes, have become both helpful and harmful.
“[Having the technology] is very good, but people have to learn how to think,” Dr. Bernstein said. “There’s a lot of medicine that’s an art, and in psychiatry even more so. You don’t have the blood tests or the imaging tests that other specialties have, and that is both our advantage and our disadvantage.”
In the future, technology will continue to have a central role in residency training, experts said. Already, independent study using technology has become the norm, Dr. Hanson said. When students are in a more structured environment, technology such as cell phones, can act as a distraction, she noted.
“I’ve decided to embrace it and use it,” she said. “My approach is to co-opt the cell phones. Periodically, during a talk, I may put up a website that has a pop quiz on it [in which] students use their cell phones to answer.”
Certainly, efforts to build diversity will be a continued focus for the specialty, said Dr. Sudak. In addition, residency might shift from less inpatient training to more subspecialty rotations for general psychiatry training, she said.
“We will need to teach residents to retain a focus on the patient as a person and use outcomes to help guide treatment,” she said.
Dr. Bernstein would like to see the pendulum swing back on such rigid duty hours, she said, with more emphasis placed on building residents’ confidence in managing complex cases and preparing trainees for overcoming adversity.
Dr. Vasan envisions more integration of psychiatry with neurology and the rest of medicine, more training in business elements, such as managing teams and a practice, as well as education on technological tools for psychiatrists.
From a broader perspective, Dr. Vasan hopes that the stigma around mental health will continue to improve and that society at large becomes more supportive of the work of psychiatrists.
“In some ways it seems like we have come far in openly discussing and understanding mental illness, as well as the fact that having these diseases does not need to hold anyone back from realizing their potential,” she said. “But not far enough. The public’s understanding of the scope of the problem and the urgency and value for addressing mental health has increased tremendously.
“Our colleagues in other fields of medicine, employers, politicians, educators ... they all value, seem to value psychiatry more, and I hope this continues to grow.”
As a psychiatry resident in the early 1980s, Carol A. Bernstein, MD, remembers a teaching setting where young physicians worked long hours, male residents outnumbered female residents, and messages were delivered in the form of handwritten notes.
Today, the learning environment for psychiatry residents is vastly different. Duty-hour restrictions are routine, the gender gap has narrowed, and electronic communication in its many forms, is the norm. Medical advancements give residents a greater ability to treat patients and improve illnesses, said Dr. Bernstein, a clinical psychiatry professor and vice chair for education in psychiatry at New York University. However, residents also face a range of modern challenges, such as higher learning expectations, a more litigious culture, and a practice landscape increasingly reliant on ratings and patient satisfaction scores.
“This is a generation – whether you want to call it the Millennials or the iGen – [who have] been pushed to do more and more,” said Dr. Bernstein, a past president of the American Psychiatric Association. “Medical care has become very complicated, and it is very hard for trainees to get mastery of it.”
At the same time, the digital world that today’s residents are accustomed to has become a double-edged sword in medical education, said Donna M. Sudak, MD, outgoing president of the American Association of Directors of Psychiatric Residency Training. Technology has generated new ways of learning, such as online modules, but also created opportunities for distraction, she said.
“All of us, our learners as well as ourselves, need to figure out the best balance of using technology in order to facilitate learning,” Dr. Sudak said. “The pro is having the world at your fingertips and the ability to work with other people across the country. The con is the temptation to be attached to your screen, rather than truly listening to the person you’re in the room with – as a psychiatrist, that’s even more critical.”
The new faces of psychiatry
Interest in psychiatry has grown steadily over the years. In 2018, 2,739 medical school graduates ranked for a PGY-1 psychiatry residency, up from 1,806 ranked applicants in 2008, according to data from the National Residency Matching Program. Of the 2018 ranked applicants, 1,540 matched to a residency program. Data from the Association of American Medical Colleges (AAMC) show that 47% of psychiatry residency applicants in 2018 were women.
Millennial graduates are choosing psychiatry for a variety of reasons. For Nina Vasan, MD, MBA, the career path meant an opportunity to make a broader impact.
“Mental health is a defining social issue of our time, and in medical school I felt like if I committed my time and energy to improving mental health, I would maximize the impact I make on the world,” said Dr. Vasan, who finished residency at Stanford (Calif.) University in 2018. “I feel even stronger about that today. … I felt drawn to both the fundamental way in which we get to connect with our patients on an individual level and impact their lives, as well as the broader societal-level change that must happen in the coming years that I want to be a part of.”
A sense of social responsibility is a common trait of this generation’s psychiatrists, said Dr. Vasan, who has a private concierge practice in the Silicon Valley.
“We have a global sense of the world and recognize that our role as physicians gives us the unique platform to make an impact at this level,” she said.
Graduates also are attracted to psychiatry because of its focus on the physician-patient connection, particularly as patient time is eroded in other specialties, such as primary care, Dr. Sudak said.
“People who become physicians really want to have relationships with patients, and if you have to see eight people an hour, that’s a tough go,” she said. “Many people are attracted to the capacity to really learn about somebody’s story and make a difference in their life. Psychiatry offers that and then some.”
Working closely with patients to improve their quality of life was a primary motivator for Steven Chan, MD, MBA, who completed his psychiatry residency at the University of California, Davis, in 2016. He currently serves on the addiction treatment services team at the VA Palo Alto Health Care System.
“I additionally pursued a subspecialty in clinical informatics to apply today’s technologies to further improve people’s lives,” he said.
Dr. Chan said he is fortunate to practice in a work environment that is more collaborative with other health professionals than in the past.
“It’s wonderful,” he said. “There’s so much work to be done, and working with others has been rewarding to me. We’re already seeing more psychiatrists take on leadership roles in technology and health care administration, so we’re seeing collaborations with informatics, engineers, and service designers.”
A sea of challenges
Despite the advantages of practicing in modern times, psychiatrists today also face unique challenges, such as an upcoming shortages of physicians.
A 2017 report by the National Council for Behavioral Health estimates that, by 2025, demand might outpace supply by up to 15,600 psychiatrists. An aging population of psychiatrists is part of the problem. Sixty percent of practicing psychiatrists are older than 55, one of the highest volumes of older doctors of all specialties, according to AAMC data.
Physician numbers are improving, but a crisis point looms, especially as more states pass legislation that target the so-called dangerously mentally ill, said Annette L. Hanson, MD, a forensic psychiatrist who is assistant professor of psychiatry at the University of Maryland and at Johns Hopkins University, both in Baltimore.
“The trend seems to be that governments want to provide more involuntary or forced care, which means you’re going to need to have doctors available to provide that care,” Dr. Hanson said in an interview. “We don’t have enough doctors to meet the public policy demand.”
Compounding the problem is the fact that the majority of new psychiatrists pursue community private practices in urban areas, rather than practicing in state hospitals or rural areas, Dr. Hanson added. In addition, some states are passing laws that require state hospitals to admit incompetent criminal defendants within a certain time frame.
“That’s created significant problems where you’re moving someone from an overcrowded, understaffed jail to overcrowded, understaffed hospital,” she said.
The growing use of telepsychiatry might be one answer to the upcoming shortage. A June 2018 letter from the Centers for Medicare & Medicaid Services encouraged more states to use health technology efforts to address the opioid crisis, including through telemedicine and telepsychiatry. Meanwhile, several states have expanded their controlled substance laws to allow remote prescribing through telehealth for the treatment of psychiatric or substance use disorders.
However, licensing issues and reimbursement inconsistencies continue to act as barriers to the practice of telepsychiatry, according to the National Council report.
Some academic institutions are crafting new ways to use technology to meet the demand for mental health care. At Stanford, for example, Dr. Vasan started a lab called Brainstorm, the Stanford Laboratory for Brain Health Innovation and Entrepreneurship, which unites medicine, business, technology, and design to develop tech products for patients. She also chairs Stanford’s Mental Health Technology Hub, a consortium of more than 20 faculty labs addressing the role technology plays in improving mental health.
“We psychiatrists need partners to help increase access to mental health prevention, diagnosis, and treatment,” Dr. Vasan said. “Technology can be that partner.”
Improving diversity is an ongoing challenge for the field, said Dr. Sudak, also professor and vice chair for education in the department of psychiatry at Drexel University in Philadelphia. Of practicing psychiatrists, 42% declare as white, 8% as Asian, 4% as black, and 4% as Hispanic, according to the latest workforce data published by the AAMC. By comparison, 61% of the U.S. population is white, while 18% is Hispanic, 13% is black, and 6% is Asian, according to recent census statistics. By 2044, more than half of all Americans are projected to belong to a minority group.
“In general, we know that more diversity will enhance outcomes of care for our patients,” Dr. Sudak said. “When I talk about workforce, I think about that piece as a significant part of the equation. It’s not just about getting more slots, but it’s about filling those slots with a population of trainees that mirrors the population, rather than mirrors a very small subset.
Training changes
One of the biggest changes affecting residency training today is the decreased length of stay for inpatients, Dr. Hanson said. When she was a resident, the average length of stay was about 3 weeks, compared with 7-10 days now.
“The challenge is sorting out an underlying psychiatric condition from the effects of substances, which is really difficult with that short of a length of stay,” she said. “You lose a longitudinal perspective if you don’t have a chance to observe someone once they’ve been stabilized and the crisis has passed and they’re detoxified from the substances they were using prior to admission.”
The arrival of electronic medical records also has affected the trainee experience by taking time away from the doctor-patient relationship, Dr. Bernstein said. Other technology, such as algorithms used to avoid mistakes, have become both helpful and harmful.
“[Having the technology] is very good, but people have to learn how to think,” Dr. Bernstein said. “There’s a lot of medicine that’s an art, and in psychiatry even more so. You don’t have the blood tests or the imaging tests that other specialties have, and that is both our advantage and our disadvantage.”
In the future, technology will continue to have a central role in residency training, experts said. Already, independent study using technology has become the norm, Dr. Hanson said. When students are in a more structured environment, technology such as cell phones, can act as a distraction, she noted.
“I’ve decided to embrace it and use it,” she said. “My approach is to co-opt the cell phones. Periodically, during a talk, I may put up a website that has a pop quiz on it [in which] students use their cell phones to answer.”
Certainly, efforts to build diversity will be a continued focus for the specialty, said Dr. Sudak. In addition, residency might shift from less inpatient training to more subspecialty rotations for general psychiatry training, she said.
“We will need to teach residents to retain a focus on the patient as a person and use outcomes to help guide treatment,” she said.
Dr. Bernstein would like to see the pendulum swing back on such rigid duty hours, she said, with more emphasis placed on building residents’ confidence in managing complex cases and preparing trainees for overcoming adversity.
Dr. Vasan envisions more integration of psychiatry with neurology and the rest of medicine, more training in business elements, such as managing teams and a practice, as well as education on technological tools for psychiatrists.
From a broader perspective, Dr. Vasan hopes that the stigma around mental health will continue to improve and that society at large becomes more supportive of the work of psychiatrists.
“In some ways it seems like we have come far in openly discussing and understanding mental illness, as well as the fact that having these diseases does not need to hold anyone back from realizing their potential,” she said. “But not far enough. The public’s understanding of the scope of the problem and the urgency and value for addressing mental health has increased tremendously.
“Our colleagues in other fields of medicine, employers, politicians, educators ... they all value, seem to value psychiatry more, and I hope this continues to grow.”
Getting a good night’s sleep
For most things, the harder you work at it, the more successful you’ll be. Except when it comes to sleep. Nothing frightens sleep away faster than an all-out effort to find it. And yet, it should be the easiest of all health habits to cultivate. Sleep should be a hardwired, physiologic, default condition (sort of like eating and sex, all are which are evolutionary imperatives). And yet, lack of sleep is a common and grave problem even in our safe and comfortable modern environment.
Lack of sleep depletes your willpower, making it less likely you’ll actually go to the gym or be able to resist that bear claw pastry calling you back to the break room. Poor sleep impairs your ability to lose and keep off weight. It can lead to mistakes of inattention – a problem if you’re flying a plane or screening for melanoma.
As a recovering insomniac, I’ve scouted out the territory for you and have taken a few notes as a Baedeker on your journey to better sleep. Tracking sleep is easy; most any fitness tracker or smart watch outfitted with the right app will do the work for you. I’ve used my Apple Watch and Pillow for years. (I’ve no conflict of interest). I’ve found that the quality score it provides each night is interesting, but not all that important. Using pad and paper you could just as easily quantify your sleep: How many hours were you in bed, asleep, and how did you feel the next day.
Here is something important I learned about myself: I don’t need 8 hours. You might not either. Most articles say that we adults need 7-8 hours of sleep. I wasted a lot of effort trying to keep it above the 7-hour mark. Then I realized that even on nights when I got 6-7, I felt fine the next day! Don’t assume you need 8 hours. It could be 6 or it could be 9. It might in fact change depending on how you slept recently, what is happening in your life, or which season it is. If you feel alert and well rested, then you’ve likely found all the sleep you need.
Let’s assume you aren’t well rested. Now what? Like most of good health, a behavioral approach is needed to get you on the right path. You’ve likely heard that bright, particularly blue, light is harmful to falling asleep. Good news! Most devices will let you filter blue light out if you must continue that “Better Call Saul” binge. Better options: Leave your tablet in the living room and plug in your phone on the opposite side of the room (with a short cord). Invest instead in a book light and actual books. There is something about the patina of paper that can encourage sleep to come find you.
Keep the room comfortably cool. What’s important here is the temperature drop. That is, going from warm to cool. This is why a warm shower or bath before getting into bed can help you. Your temperature will drop, a signal for sleep.
So now you’re asleep. But wait, you say you’re awake again and it’s 3:00 a.m.? This is sleep maintenance insomnia. You lie there, patiently waiting, like anticipating your waiter’s return when you’re eating in Rome – ah, you could be there all night. Nothing you do seems to bring sleep back around. The best advice is to try to retrain yourself that when you are up, you’re up, and when in bed, you’re asleep. You can try getting up, moving to a different room. Try meditation or reading. Wait until you feel the urge to sleep sneak back on you, then head back to bed. Although sometimes difficult, you might consider riding it out. If you can’t fall back, then get on with your day (although I don’t recommend sending emails at 3:45 a.m., it freaks people out, I’ve learned). The following night, you will likely be sleep deprived and might find you can fall asleep easier and for longer.
Be forgiving. Unlike your diet or exercise, sleep isn’t as much in your control. You can work a little harder in spin, or double your effort to keep to your plant/keto diet. But for sleep, you must just be patient. It will come. When it is good and ready.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at dermnews@mdedge.com.
For most things, the harder you work at it, the more successful you’ll be. Except when it comes to sleep. Nothing frightens sleep away faster than an all-out effort to find it. And yet, it should be the easiest of all health habits to cultivate. Sleep should be a hardwired, physiologic, default condition (sort of like eating and sex, all are which are evolutionary imperatives). And yet, lack of sleep is a common and grave problem even in our safe and comfortable modern environment.
Lack of sleep depletes your willpower, making it less likely you’ll actually go to the gym or be able to resist that bear claw pastry calling you back to the break room. Poor sleep impairs your ability to lose and keep off weight. It can lead to mistakes of inattention – a problem if you’re flying a plane or screening for melanoma.
As a recovering insomniac, I’ve scouted out the territory for you and have taken a few notes as a Baedeker on your journey to better sleep. Tracking sleep is easy; most any fitness tracker or smart watch outfitted with the right app will do the work for you. I’ve used my Apple Watch and Pillow for years. (I’ve no conflict of interest). I’ve found that the quality score it provides each night is interesting, but not all that important. Using pad and paper you could just as easily quantify your sleep: How many hours were you in bed, asleep, and how did you feel the next day.
Here is something important I learned about myself: I don’t need 8 hours. You might not either. Most articles say that we adults need 7-8 hours of sleep. I wasted a lot of effort trying to keep it above the 7-hour mark. Then I realized that even on nights when I got 6-7, I felt fine the next day! Don’t assume you need 8 hours. It could be 6 or it could be 9. It might in fact change depending on how you slept recently, what is happening in your life, or which season it is. If you feel alert and well rested, then you’ve likely found all the sleep you need.
Let’s assume you aren’t well rested. Now what? Like most of good health, a behavioral approach is needed to get you on the right path. You’ve likely heard that bright, particularly blue, light is harmful to falling asleep. Good news! Most devices will let you filter blue light out if you must continue that “Better Call Saul” binge. Better options: Leave your tablet in the living room and plug in your phone on the opposite side of the room (with a short cord). Invest instead in a book light and actual books. There is something about the patina of paper that can encourage sleep to come find you.
Keep the room comfortably cool. What’s important here is the temperature drop. That is, going from warm to cool. This is why a warm shower or bath before getting into bed can help you. Your temperature will drop, a signal for sleep.
So now you’re asleep. But wait, you say you’re awake again and it’s 3:00 a.m.? This is sleep maintenance insomnia. You lie there, patiently waiting, like anticipating your waiter’s return when you’re eating in Rome – ah, you could be there all night. Nothing you do seems to bring sleep back around. The best advice is to try to retrain yourself that when you are up, you’re up, and when in bed, you’re asleep. You can try getting up, moving to a different room. Try meditation or reading. Wait until you feel the urge to sleep sneak back on you, then head back to bed. Although sometimes difficult, you might consider riding it out. If you can’t fall back, then get on with your day (although I don’t recommend sending emails at 3:45 a.m., it freaks people out, I’ve learned). The following night, you will likely be sleep deprived and might find you can fall asleep easier and for longer.
Be forgiving. Unlike your diet or exercise, sleep isn’t as much in your control. You can work a little harder in spin, or double your effort to keep to your plant/keto diet. But for sleep, you must just be patient. It will come. When it is good and ready.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at dermnews@mdedge.com.
For most things, the harder you work at it, the more successful you’ll be. Except when it comes to sleep. Nothing frightens sleep away faster than an all-out effort to find it. And yet, it should be the easiest of all health habits to cultivate. Sleep should be a hardwired, physiologic, default condition (sort of like eating and sex, all are which are evolutionary imperatives). And yet, lack of sleep is a common and grave problem even in our safe and comfortable modern environment.
Lack of sleep depletes your willpower, making it less likely you’ll actually go to the gym or be able to resist that bear claw pastry calling you back to the break room. Poor sleep impairs your ability to lose and keep off weight. It can lead to mistakes of inattention – a problem if you’re flying a plane or screening for melanoma.
As a recovering insomniac, I’ve scouted out the territory for you and have taken a few notes as a Baedeker on your journey to better sleep. Tracking sleep is easy; most any fitness tracker or smart watch outfitted with the right app will do the work for you. I’ve used my Apple Watch and Pillow for years. (I’ve no conflict of interest). I’ve found that the quality score it provides each night is interesting, but not all that important. Using pad and paper you could just as easily quantify your sleep: How many hours were you in bed, asleep, and how did you feel the next day.
Here is something important I learned about myself: I don’t need 8 hours. You might not either. Most articles say that we adults need 7-8 hours of sleep. I wasted a lot of effort trying to keep it above the 7-hour mark. Then I realized that even on nights when I got 6-7, I felt fine the next day! Don’t assume you need 8 hours. It could be 6 or it could be 9. It might in fact change depending on how you slept recently, what is happening in your life, or which season it is. If you feel alert and well rested, then you’ve likely found all the sleep you need.
Let’s assume you aren’t well rested. Now what? Like most of good health, a behavioral approach is needed to get you on the right path. You’ve likely heard that bright, particularly blue, light is harmful to falling asleep. Good news! Most devices will let you filter blue light out if you must continue that “Better Call Saul” binge. Better options: Leave your tablet in the living room and plug in your phone on the opposite side of the room (with a short cord). Invest instead in a book light and actual books. There is something about the patina of paper that can encourage sleep to come find you.
Keep the room comfortably cool. What’s important here is the temperature drop. That is, going from warm to cool. This is why a warm shower or bath before getting into bed can help you. Your temperature will drop, a signal for sleep.
So now you’re asleep. But wait, you say you’re awake again and it’s 3:00 a.m.? This is sleep maintenance insomnia. You lie there, patiently waiting, like anticipating your waiter’s return when you’re eating in Rome – ah, you could be there all night. Nothing you do seems to bring sleep back around. The best advice is to try to retrain yourself that when you are up, you’re up, and when in bed, you’re asleep. You can try getting up, moving to a different room. Try meditation or reading. Wait until you feel the urge to sleep sneak back on you, then head back to bed. Although sometimes difficult, you might consider riding it out. If you can’t fall back, then get on with your day (although I don’t recommend sending emails at 3:45 a.m., it freaks people out, I’ve learned). The following night, you will likely be sleep deprived and might find you can fall asleep easier and for longer.
Be forgiving. Unlike your diet or exercise, sleep isn’t as much in your control. You can work a little harder in spin, or double your effort to keep to your plant/keto diet. But for sleep, you must just be patient. It will come. When it is good and ready.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at dermnews@mdedge.com.
Inhibitor risk nears zero after 75 days in previously untreated hemophilia A
PRAGUE—For previously untreated patients (PUPs) with severe hemophilia A, the risk of developing factor VIII (FVIII) alloantibodies (inhibitors) becomes negligible after 75 exposure days, according to a recent study involving more than 1,000 infants.
This finding answers a long-standing and important question in the management of hemophilia A, reported lead author H. Marijke van den Berg, MD, PhD, of University Medical Centre in Utrecht, The Netherlands.
Inhibitor development is the biggest safety concern facing infants with severe hemophilia A because it affects 25%-35% of the patient population, but no previous studies have adequately described the associated risk profile, she noted.
“Most studies until now collected data until about 50 [exposure days] and not that far beyond,” Dr. van den Berg said at the annual congress of the European Association for Haemophilia and Allied Disorders. “So we were interested to see the serum plateau in our large cohort.”
Such a plateau would represent the time point at which risk of inhibitor development approaches zero.
Dr. van den Berg and her colleagues followed 1,038 PUPs with severe hemophilia A from first exposure to FVIII onward. Data were from drawn from the PedNet Registry. From the initial group, 943 patients (91%) were followed until 50 exposure days, and 899 (87%) were followed until 75 exposure days.
Inhibitor development was defined by a minimum of two positive inhibitor titers. In addition to determining the point in time of inhibitor development, the investigators performed a survival analysis for inhibitor incidence and reported median ages at first exposure and at exposure day 75.
The results showed that 298 out of 300 instances of inhibitor development occurred within 75 exposure days, and no inhibitors developed between exposure day 75 and 150. The final two instances occurred at exposure day 249 and 262, both with a low titer.
Median age at first exposure was 1.1 years, compared with 2.3 years at exposure day 75.
These findings suggest that risk of inhibitors is “near zero” after 75 days and that risk is approaching zero just 1 year after first exposure to FVIII, she said.
The results from this study could affect the design of future clinical trials for PUPs.
“Our recommendation will be to continue frequent [inhibitor] testing until 75 exposure days,” Dr. van den Berg said.
The time frame involved is very short, so close monitoring should be feasible for investigators, she noted.
Dr. van den Berg said that additional data, including Kaplan-Meier curves, would “hopefully” be published in a journal soon.
Dr. van den Berg reported having no relevant financial disclosures.
SOURCE: van den Berg HM et al. EAHAD 2019, Abstract OR05.
PRAGUE—For previously untreated patients (PUPs) with severe hemophilia A, the risk of developing factor VIII (FVIII) alloantibodies (inhibitors) becomes negligible after 75 exposure days, according to a recent study involving more than 1,000 infants.
This finding answers a long-standing and important question in the management of hemophilia A, reported lead author H. Marijke van den Berg, MD, PhD, of University Medical Centre in Utrecht, The Netherlands.
Inhibitor development is the biggest safety concern facing infants with severe hemophilia A because it affects 25%-35% of the patient population, but no previous studies have adequately described the associated risk profile, she noted.
“Most studies until now collected data until about 50 [exposure days] and not that far beyond,” Dr. van den Berg said at the annual congress of the European Association for Haemophilia and Allied Disorders. “So we were interested to see the serum plateau in our large cohort.”
Such a plateau would represent the time point at which risk of inhibitor development approaches zero.
Dr. van den Berg and her colleagues followed 1,038 PUPs with severe hemophilia A from first exposure to FVIII onward. Data were from drawn from the PedNet Registry. From the initial group, 943 patients (91%) were followed until 50 exposure days, and 899 (87%) were followed until 75 exposure days.
Inhibitor development was defined by a minimum of two positive inhibitor titers. In addition to determining the point in time of inhibitor development, the investigators performed a survival analysis for inhibitor incidence and reported median ages at first exposure and at exposure day 75.
The results showed that 298 out of 300 instances of inhibitor development occurred within 75 exposure days, and no inhibitors developed between exposure day 75 and 150. The final two instances occurred at exposure day 249 and 262, both with a low titer.
Median age at first exposure was 1.1 years, compared with 2.3 years at exposure day 75.
These findings suggest that risk of inhibitors is “near zero” after 75 days and that risk is approaching zero just 1 year after first exposure to FVIII, she said.
The results from this study could affect the design of future clinical trials for PUPs.
“Our recommendation will be to continue frequent [inhibitor] testing until 75 exposure days,” Dr. van den Berg said.
The time frame involved is very short, so close monitoring should be feasible for investigators, she noted.
Dr. van den Berg said that additional data, including Kaplan-Meier curves, would “hopefully” be published in a journal soon.
Dr. van den Berg reported having no relevant financial disclosures.
SOURCE: van den Berg HM et al. EAHAD 2019, Abstract OR05.
PRAGUE—For previously untreated patients (PUPs) with severe hemophilia A, the risk of developing factor VIII (FVIII) alloantibodies (inhibitors) becomes negligible after 75 exposure days, according to a recent study involving more than 1,000 infants.
This finding answers a long-standing and important question in the management of hemophilia A, reported lead author H. Marijke van den Berg, MD, PhD, of University Medical Centre in Utrecht, The Netherlands.
Inhibitor development is the biggest safety concern facing infants with severe hemophilia A because it affects 25%-35% of the patient population, but no previous studies have adequately described the associated risk profile, she noted.
“Most studies until now collected data until about 50 [exposure days] and not that far beyond,” Dr. van den Berg said at the annual congress of the European Association for Haemophilia and Allied Disorders. “So we were interested to see the serum plateau in our large cohort.”
Such a plateau would represent the time point at which risk of inhibitor development approaches zero.
Dr. van den Berg and her colleagues followed 1,038 PUPs with severe hemophilia A from first exposure to FVIII onward. Data were from drawn from the PedNet Registry. From the initial group, 943 patients (91%) were followed until 50 exposure days, and 899 (87%) were followed until 75 exposure days.
Inhibitor development was defined by a minimum of two positive inhibitor titers. In addition to determining the point in time of inhibitor development, the investigators performed a survival analysis for inhibitor incidence and reported median ages at first exposure and at exposure day 75.
The results showed that 298 out of 300 instances of inhibitor development occurred within 75 exposure days, and no inhibitors developed between exposure day 75 and 150. The final two instances occurred at exposure day 249 and 262, both with a low titer.
Median age at first exposure was 1.1 years, compared with 2.3 years at exposure day 75.
These findings suggest that risk of inhibitors is “near zero” after 75 days and that risk is approaching zero just 1 year after first exposure to FVIII, she said.
The results from this study could affect the design of future clinical trials for PUPs.
“Our recommendation will be to continue frequent [inhibitor] testing until 75 exposure days,” Dr. van den Berg said.
The time frame involved is very short, so close monitoring should be feasible for investigators, she noted.
Dr. van den Berg said that additional data, including Kaplan-Meier curves, would “hopefully” be published in a journal soon.
Dr. van den Berg reported having no relevant financial disclosures.
SOURCE: van den Berg HM et al. EAHAD 2019, Abstract OR05.
REPORTING FROM EAHAD 2019
Key clinical point: Major finding: Less than 1% of infants with severe hemophilia A developed inhibitors after 75 exposure days.
Study details: An observational study involving 1,038 previously untreated patients with severe hemophilia A, of which 899 (87%) were followed until 75 exposure days.
Disclosures: Dr. van den Berg reported having no relevant financial disclosures.
Source: van den Berg HM et al. EAHAD 2019, Abstract OR05.
Hemophilia intracranial hemorrhage rates declining
PRAGUE – Despite improvements over the past 60 years, intracranial hemorrhage (ICH) remains a significant complication in hemophilia, occurring most frequently among patients with severe forms of the disease, according to a large-scale meta-analysis involving 56 studies and nearly 80,000 patients.
The consequences of a single incident of ICH can be irreparable and life-changing, so clinician knowledge of incidence rates and risk factors is essential, said lead author Anne-Fleur Zwagemaker, a PhD candidate from Amsterdam University Medical Center.
“Intracranial hemorrhage is one of the most severe and fearful complications in hemophilia,” Ms. Zwagemaker said in a presentation at the annual congress of the European Association for Haemophilia and Allied Disorders. “Our aim was to give more precise estimates of ICH numbers and risk factors in hemophilia.”
The review is notable for its scale and quality. After eliminating studies with fewer than 50 patients or other insufficiencies, the investigators were left with 56 studies conducted between 1960 and 2018, involving 79,818 patients with hemophilia. With a mean observation period of 12 years, the data encompassed almost 1 million person-years of data.
Across all studies, 1,508 ICH events were reported. Incidence and mortality rates were 400 and 80 per 100,000 person-years, respectively.
To optimize accuracy, the investigators further restricted studies to those with a sample size of at least 365 patients, leading to a pooled incidence rate of 3.8%. Studies with relevant data showed that about half of the cases of ICH (48%) were spontaneous. Regarding most common bleed locations, about two-thirds were either subdural (30%) or intracerebral (32%).
Pooled incidence rates of ICH have decreased steadily over time, from 7%-8% during the 1960-1979 time period, to 5%-6% from 1980-1999, and most recently to about 3%.
Mortality rates during the same time periods decreased in a similar fashion, from 300, to 100, to 75 deaths per 100,000 person-years.
Additional analysis revealed an expected relationship between disease severity and likelihood of ICH. Mild cases of hemophilia had an ICH incidence rate of 0.9%, moderate cases had a rate of 1.3%, and severe cases topped the scale at 4.5%, entailing an incidence rate ratio of 2.7 between severe and nonsevere patients.
“I think our data show that in hemophilia, ICH is still a very important and frequent complication,” Ms. Zwagemaker said. “Luckily, we also see a decline in numbers, but I think it’s still very important that we identify those at risk in hemophilia and that we acknowledge it’s still a very important problem.”
Dr. Zwagemaker reported having no relevant financial disclosures.
SOURCE: Zwagemaker AF et al. EAHAD 2019, Abstract OR08.
PRAGUE – Despite improvements over the past 60 years, intracranial hemorrhage (ICH) remains a significant complication in hemophilia, occurring most frequently among patients with severe forms of the disease, according to a large-scale meta-analysis involving 56 studies and nearly 80,000 patients.
The consequences of a single incident of ICH can be irreparable and life-changing, so clinician knowledge of incidence rates and risk factors is essential, said lead author Anne-Fleur Zwagemaker, a PhD candidate from Amsterdam University Medical Center.
“Intracranial hemorrhage is one of the most severe and fearful complications in hemophilia,” Ms. Zwagemaker said in a presentation at the annual congress of the European Association for Haemophilia and Allied Disorders. “Our aim was to give more precise estimates of ICH numbers and risk factors in hemophilia.”
The review is notable for its scale and quality. After eliminating studies with fewer than 50 patients or other insufficiencies, the investigators were left with 56 studies conducted between 1960 and 2018, involving 79,818 patients with hemophilia. With a mean observation period of 12 years, the data encompassed almost 1 million person-years of data.
Across all studies, 1,508 ICH events were reported. Incidence and mortality rates were 400 and 80 per 100,000 person-years, respectively.
To optimize accuracy, the investigators further restricted studies to those with a sample size of at least 365 patients, leading to a pooled incidence rate of 3.8%. Studies with relevant data showed that about half of the cases of ICH (48%) were spontaneous. Regarding most common bleed locations, about two-thirds were either subdural (30%) or intracerebral (32%).
Pooled incidence rates of ICH have decreased steadily over time, from 7%-8% during the 1960-1979 time period, to 5%-6% from 1980-1999, and most recently to about 3%.
Mortality rates during the same time periods decreased in a similar fashion, from 300, to 100, to 75 deaths per 100,000 person-years.
Additional analysis revealed an expected relationship between disease severity and likelihood of ICH. Mild cases of hemophilia had an ICH incidence rate of 0.9%, moderate cases had a rate of 1.3%, and severe cases topped the scale at 4.5%, entailing an incidence rate ratio of 2.7 between severe and nonsevere patients.
“I think our data show that in hemophilia, ICH is still a very important and frequent complication,” Ms. Zwagemaker said. “Luckily, we also see a decline in numbers, but I think it’s still very important that we identify those at risk in hemophilia and that we acknowledge it’s still a very important problem.”
Dr. Zwagemaker reported having no relevant financial disclosures.
SOURCE: Zwagemaker AF et al. EAHAD 2019, Abstract OR08.
PRAGUE – Despite improvements over the past 60 years, intracranial hemorrhage (ICH) remains a significant complication in hemophilia, occurring most frequently among patients with severe forms of the disease, according to a large-scale meta-analysis involving 56 studies and nearly 80,000 patients.
The consequences of a single incident of ICH can be irreparable and life-changing, so clinician knowledge of incidence rates and risk factors is essential, said lead author Anne-Fleur Zwagemaker, a PhD candidate from Amsterdam University Medical Center.
“Intracranial hemorrhage is one of the most severe and fearful complications in hemophilia,” Ms. Zwagemaker said in a presentation at the annual congress of the European Association for Haemophilia and Allied Disorders. “Our aim was to give more precise estimates of ICH numbers and risk factors in hemophilia.”
The review is notable for its scale and quality. After eliminating studies with fewer than 50 patients or other insufficiencies, the investigators were left with 56 studies conducted between 1960 and 2018, involving 79,818 patients with hemophilia. With a mean observation period of 12 years, the data encompassed almost 1 million person-years of data.
Across all studies, 1,508 ICH events were reported. Incidence and mortality rates were 400 and 80 per 100,000 person-years, respectively.
To optimize accuracy, the investigators further restricted studies to those with a sample size of at least 365 patients, leading to a pooled incidence rate of 3.8%. Studies with relevant data showed that about half of the cases of ICH (48%) were spontaneous. Regarding most common bleed locations, about two-thirds were either subdural (30%) or intracerebral (32%).
Pooled incidence rates of ICH have decreased steadily over time, from 7%-8% during the 1960-1979 time period, to 5%-6% from 1980-1999, and most recently to about 3%.
Mortality rates during the same time periods decreased in a similar fashion, from 300, to 100, to 75 deaths per 100,000 person-years.
Additional analysis revealed an expected relationship between disease severity and likelihood of ICH. Mild cases of hemophilia had an ICH incidence rate of 0.9%, moderate cases had a rate of 1.3%, and severe cases topped the scale at 4.5%, entailing an incidence rate ratio of 2.7 between severe and nonsevere patients.
“I think our data show that in hemophilia, ICH is still a very important and frequent complication,” Ms. Zwagemaker said. “Luckily, we also see a decline in numbers, but I think it’s still very important that we identify those at risk in hemophilia and that we acknowledge it’s still a very important problem.”
Dr. Zwagemaker reported having no relevant financial disclosures.
SOURCE: Zwagemaker AF et al. EAHAD 2019, Abstract OR08.
REPORTING FROM EAHAD 2019
Key clinical point:
Major finding: The incidence rate of ICH was approximately 7%-8% from 1960 to 1979, compared with approximately 3% from 2000 to 2018.
Study details: A review of 56 studies conducted between 1960 and 2018, involving 79,818 patients with hemophilia.
Disclosures: Dr. Zwagemaker reported having no relevant financial disclosures.
Source: Zwagemaker AF et al. EAHAD 2019, Abstract OR08.