User login
Dengue antibodies may reduce Zika infection risk
Previous dengue exposure may confer a protective effect against Zika virus infection, according to a paper published in Science.
In a prospective cohort study, researchers followed 1,453 urban residents in Salvador, Brazil, to assess the impact of the 2015 Zika virus outbreak in the region. Data on dengue immunity was available for 642 of these individuals.
Overall, 73% of the cohort were seropositive for Zika virus. However, the frequency of seropositivity varied significantly by location, from 29% in a valley in the northeastern sector of the study area to 83% in the southeast corner; the authors wrote that this was consistent with some form of acquired immunity “blunting the efficiency of transmission.”
When researchers looked at the relationship between prior immunity to the dengue virus and the risk of Zika infection, they found that each doubling of total IgG titers against dengue NS1 was associated with a 9% reduction in the risk of Zika virus infection.
Individuals in the highest tertile of dengue IgG titers showed a 44% reduction in the odds of Zika seropositivity, compared with individuals with no or low dengue IgG titers, while those in the middle tertile of dengue IgG titer had a 38% reduction.
“These findings provide empirical support for the hypothesis that accumulated immunity drove ZIKV [Zika virus] to local extinction by reducing the efficiency of transmission,” wrote Isabel Rodriguez-Barraquer, MD, PhD, from the department of medicine at the University of California, San Francisco, and her coauthors.
Individuals who were infected with the Zika virus but had high dengue IgG titers were significantly less likely to exhibit fever with viral infection, but had the same risk of developing rash as those with low or no IgG titers.
Researchers also examined the link between a subclass of IgG antibodies that are associated with more recent exposure to dengue virus – within the prior 6 months – and the risk of Zika virus infection. In contrast, they found that the levels of this subclass of antibodies, known as IgG3, were positively associated with an increased risk of Zika virus infection. Each doubling in IgG3 levels was associated with a 23% increase in the odds of being positive for Zika.
“This positive association might reflect an immune profile, in individuals who have experienced a recent DENV [dengue virus] infection, that is associated with having a greater risk of a subsequent ZIKV infection,” the authors wrote. “Alternatively, it is also possible that higher levels of IgG3 are a proxy for frequent DENV exposure and thus greater risk of infection by Aedes aegypti–transmitted viruses.”
The study was supported by Yale University, a number of Brazilian research organizations, the Research Support Foundation for the State of São Paulo, CuraZika Foundation, Wellcome Trust, and the National Institutes of Health. Three authors are listed on a patent application related to the work, and one reported an honoraria from Sanofi-Pasteur.
SOURCE: Rodriguez-Barraquer I et al. Science. 2019;36:607-10.
Previous dengue exposure may confer a protective effect against Zika virus infection, according to a paper published in Science.
In a prospective cohort study, researchers followed 1,453 urban residents in Salvador, Brazil, to assess the impact of the 2015 Zika virus outbreak in the region. Data on dengue immunity was available for 642 of these individuals.
Overall, 73% of the cohort were seropositive for Zika virus. However, the frequency of seropositivity varied significantly by location, from 29% in a valley in the northeastern sector of the study area to 83% in the southeast corner; the authors wrote that this was consistent with some form of acquired immunity “blunting the efficiency of transmission.”
When researchers looked at the relationship between prior immunity to the dengue virus and the risk of Zika infection, they found that each doubling of total IgG titers against dengue NS1 was associated with a 9% reduction in the risk of Zika virus infection.
Individuals in the highest tertile of dengue IgG titers showed a 44% reduction in the odds of Zika seropositivity, compared with individuals with no or low dengue IgG titers, while those in the middle tertile of dengue IgG titer had a 38% reduction.
“These findings provide empirical support for the hypothesis that accumulated immunity drove ZIKV [Zika virus] to local extinction by reducing the efficiency of transmission,” wrote Isabel Rodriguez-Barraquer, MD, PhD, from the department of medicine at the University of California, San Francisco, and her coauthors.
Individuals who were infected with the Zika virus but had high dengue IgG titers were significantly less likely to exhibit fever with viral infection, but had the same risk of developing rash as those with low or no IgG titers.
Researchers also examined the link between a subclass of IgG antibodies that are associated with more recent exposure to dengue virus – within the prior 6 months – and the risk of Zika virus infection. In contrast, they found that the levels of this subclass of antibodies, known as IgG3, were positively associated with an increased risk of Zika virus infection. Each doubling in IgG3 levels was associated with a 23% increase in the odds of being positive for Zika.
“This positive association might reflect an immune profile, in individuals who have experienced a recent DENV [dengue virus] infection, that is associated with having a greater risk of a subsequent ZIKV infection,” the authors wrote. “Alternatively, it is also possible that higher levels of IgG3 are a proxy for frequent DENV exposure and thus greater risk of infection by Aedes aegypti–transmitted viruses.”
The study was supported by Yale University, a number of Brazilian research organizations, the Research Support Foundation for the State of São Paulo, CuraZika Foundation, Wellcome Trust, and the National Institutes of Health. Three authors are listed on a patent application related to the work, and one reported an honoraria from Sanofi-Pasteur.
SOURCE: Rodriguez-Barraquer I et al. Science. 2019;36:607-10.
Previous dengue exposure may confer a protective effect against Zika virus infection, according to a paper published in Science.
In a prospective cohort study, researchers followed 1,453 urban residents in Salvador, Brazil, to assess the impact of the 2015 Zika virus outbreak in the region. Data on dengue immunity was available for 642 of these individuals.
Overall, 73% of the cohort were seropositive for Zika virus. However, the frequency of seropositivity varied significantly by location, from 29% in a valley in the northeastern sector of the study area to 83% in the southeast corner; the authors wrote that this was consistent with some form of acquired immunity “blunting the efficiency of transmission.”
When researchers looked at the relationship between prior immunity to the dengue virus and the risk of Zika infection, they found that each doubling of total IgG titers against dengue NS1 was associated with a 9% reduction in the risk of Zika virus infection.
Individuals in the highest tertile of dengue IgG titers showed a 44% reduction in the odds of Zika seropositivity, compared with individuals with no or low dengue IgG titers, while those in the middle tertile of dengue IgG titer had a 38% reduction.
“These findings provide empirical support for the hypothesis that accumulated immunity drove ZIKV [Zika virus] to local extinction by reducing the efficiency of transmission,” wrote Isabel Rodriguez-Barraquer, MD, PhD, from the department of medicine at the University of California, San Francisco, and her coauthors.
Individuals who were infected with the Zika virus but had high dengue IgG titers were significantly less likely to exhibit fever with viral infection, but had the same risk of developing rash as those with low or no IgG titers.
Researchers also examined the link between a subclass of IgG antibodies that are associated with more recent exposure to dengue virus – within the prior 6 months – and the risk of Zika virus infection. In contrast, they found that the levels of this subclass of antibodies, known as IgG3, were positively associated with an increased risk of Zika virus infection. Each doubling in IgG3 levels was associated with a 23% increase in the odds of being positive for Zika.
“This positive association might reflect an immune profile, in individuals who have experienced a recent DENV [dengue virus] infection, that is associated with having a greater risk of a subsequent ZIKV infection,” the authors wrote. “Alternatively, it is also possible that higher levels of IgG3 are a proxy for frequent DENV exposure and thus greater risk of infection by Aedes aegypti–transmitted viruses.”
The study was supported by Yale University, a number of Brazilian research organizations, the Research Support Foundation for the State of São Paulo, CuraZika Foundation, Wellcome Trust, and the National Institutes of Health. Three authors are listed on a patent application related to the work, and one reported an honoraria from Sanofi-Pasteur.
SOURCE: Rodriguez-Barraquer I et al. Science. 2019;36:607-10.
FROM SCIENCE
Key clinical point: Higher dengue antibody titers are associated with a lower risk of Zika virus infection.
Major finding: The highest tertile of dengue antibody titers was associated with a 44% reduction in the risk of Zika seropositivity.
Study details: A prospective cohort study of 1,453 residents in Salvador, Brazil.
Disclosures: The study was supported by Yale University, a number of Brazilian research organizations, the Research Support Foundation for the State of São Paulo, CuraZika Foundation, Wellcome Trust, and the National Institutes of Health. Three authors are listed on a patent application related to the work, and one reported an honoraria from Sanofi-Pasteur.
Source: Rodriguez-Barraquer I et al. Science. 2019;36:607-10.
Acceptance and commitment therapy reduced IBD stress, depression
Eight weeks of a mindfulness intervention known as acceptance and commitment therapy (ACT) significantly improved stress and depression among patients with inflammatory bowel disease, and these improvements persisted for at least 12 weeks after therapy ended, according to the results of a randomized, controlled trial.
Source: The American Gastroenterological Association
In the intention-to-treat analysis, stress symptoms, as measured by the Depression Anxiety and Stress Scales (DASS-21), improved by 39% at week 8 and by 45% at week 20, reported Brona Wynne, PhD, of University College Dublin together with her associates. These improvements were highly significant compared with baseline and treatment as usual (P = .001 for both comparisons). “Post hoc analyses indicated that baseline stress levels were similar in control and treatment groups,” the researchers wrote in Gastroenterology. “The results of the per protocol analysis were comparable, with a 43% and 49% reduction in stress in the treatment group from baseline to 8 and 20 weeks.”
Multiple studies have documented high levels of stress and psychological dysfunction among patients with Crohn’s disease and ulcerative colitis. Studies of various mindfulness therapy, relaxation, stress management, cognitive-behavioral therapy, and hypnotherapy interventions often failed to collect key clinical data or were underpowered, uncontrolled, and unrandomized. Acceptance and commitment therapy uses mindfulness to identify adverse thoughts and experiences, accept these as part of life, and recommit to “move towards values that have been identified and adopted by the individual,” the investigators wrote. “This can be defined as the ability to contact the present moment more fully as a conscious human being and to change, or persist in, behavior when doing so serves valued ends.”
Their single-center study, which they said was the first to evaluate ACT in IBD patients, included 79 individuals with stable or mildly active Crohn’s disease (38 patients) or ulcerative colitis (41 patients) who were randomly assigned to ACT (37 patients) or control treatment as usual (42 patients). The two comparison groups were demographically and clinically similar. The ACT program involved eight 90-minute, weekly sessions of groups of 14-16 individuals, led by a single psychologist who tailored the course material toward IBD with a focus on lowering stress. An independent psychologist observed each session to assess adherence to protocol.
Not only did ACT meet the primary study endpoint, it also produced a 25% decrease in perceived stress (on a 1-10 scale) by week 8 and a 27% decrease in perceived stress by week 20 (P less than .001 versus treatment as usual). Depression scores in the ACT group also fell by 47% by week 8 and by 45% at week 20 (P = .01 versus treatment as usual). Anxiety levels decreased by 29% at week 8 and by 31% at week 20, but these improvements did not significantly differ from those in the control group (P = .39).
Interestingly, ACT did not significantly improve symptom burden, activities of daily living, disease-related worry, general well-being, C-reactive protein (CRP) levels, fecal calprotectin levels, or scores on the version used of the Clinical Assessment of Depression (CAD) or the short Mayo assessment. Hair cortisol levels showed an association with baseline stress and anxiety, but not with treatment response.
Care programs for IBD increasingly emphasize mental health services despite a lack of robust trials to support these interventions, the investigators noted. Thus, their findings highlight “the need for researchers and clinicians to further develop and optimize the content and delivery of psychological programs for IBD patients.”
Tillotts Pharma and Boston Scientific provided partial funding, but had no other role in the study. The researchers reported having no relevant conflicts of interest.
SOURCE: Wynne B et al. Gastroenterology. 2018 Nov 16. doi: 10.1053/j.gastro.2018.11.030.
Factors that affect stress level and mood symptoms are vast when it comes to living with inflammatory bowel disease (IBD). Comorbid mood symptoms are common in patients with IBD, and psychological interventions are increasingly recommended as part of holistic, multidisciplinary treatment planning. Additionally, patients are open to GI-focused psychology treatments given the recognition that the complexities of living with IBD strongly influence emotional factors.
What must be acknowledged is the importance of long-term adherence to skills learned during the 8 weeks of ACT. Stress and mood symptoms tend to be more prevalent during times of flare. Given the relapsing and remitting nature of IBD, it must be conveyed that patients will need to continue the practice of this mindfulness-based intervention in the long term. Future studies are encouraged to look at longitudinal data assessing the manner in which these patients used their skill set during periods of flare or disease-related stress.
Factors that affect stress level and mood symptoms are vast when it comes to living with inflammatory bowel disease (IBD). Comorbid mood symptoms are common in patients with IBD, and psychological interventions are increasingly recommended as part of holistic, multidisciplinary treatment planning. Additionally, patients are open to GI-focused psychology treatments given the recognition that the complexities of living with IBD strongly influence emotional factors.
What must be acknowledged is the importance of long-term adherence to skills learned during the 8 weeks of ACT. Stress and mood symptoms tend to be more prevalent during times of flare. Given the relapsing and remitting nature of IBD, it must be conveyed that patients will need to continue the practice of this mindfulness-based intervention in the long term. Future studies are encouraged to look at longitudinal data assessing the manner in which these patients used their skill set during periods of flare or disease-related stress.
Factors that affect stress level and mood symptoms are vast when it comes to living with inflammatory bowel disease (IBD). Comorbid mood symptoms are common in patients with IBD, and psychological interventions are increasingly recommended as part of holistic, multidisciplinary treatment planning. Additionally, patients are open to GI-focused psychology treatments given the recognition that the complexities of living with IBD strongly influence emotional factors.
What must be acknowledged is the importance of long-term adherence to skills learned during the 8 weeks of ACT. Stress and mood symptoms tend to be more prevalent during times of flare. Given the relapsing and remitting nature of IBD, it must be conveyed that patients will need to continue the practice of this mindfulness-based intervention in the long term. Future studies are encouraged to look at longitudinal data assessing the manner in which these patients used their skill set during periods of flare or disease-related stress.
Eight weeks of a mindfulness intervention known as acceptance and commitment therapy (ACT) significantly improved stress and depression among patients with inflammatory bowel disease, and these improvements persisted for at least 12 weeks after therapy ended, according to the results of a randomized, controlled trial.
Source: The American Gastroenterological Association
In the intention-to-treat analysis, stress symptoms, as measured by the Depression Anxiety and Stress Scales (DASS-21), improved by 39% at week 8 and by 45% at week 20, reported Brona Wynne, PhD, of University College Dublin together with her associates. These improvements were highly significant compared with baseline and treatment as usual (P = .001 for both comparisons). “Post hoc analyses indicated that baseline stress levels were similar in control and treatment groups,” the researchers wrote in Gastroenterology. “The results of the per protocol analysis were comparable, with a 43% and 49% reduction in stress in the treatment group from baseline to 8 and 20 weeks.”
Multiple studies have documented high levels of stress and psychological dysfunction among patients with Crohn’s disease and ulcerative colitis. Studies of various mindfulness therapy, relaxation, stress management, cognitive-behavioral therapy, and hypnotherapy interventions often failed to collect key clinical data or were underpowered, uncontrolled, and unrandomized. Acceptance and commitment therapy uses mindfulness to identify adverse thoughts and experiences, accept these as part of life, and recommit to “move towards values that have been identified and adopted by the individual,” the investigators wrote. “This can be defined as the ability to contact the present moment more fully as a conscious human being and to change, or persist in, behavior when doing so serves valued ends.”
Their single-center study, which they said was the first to evaluate ACT in IBD patients, included 79 individuals with stable or mildly active Crohn’s disease (38 patients) or ulcerative colitis (41 patients) who were randomly assigned to ACT (37 patients) or control treatment as usual (42 patients). The two comparison groups were demographically and clinically similar. The ACT program involved eight 90-minute, weekly sessions of groups of 14-16 individuals, led by a single psychologist who tailored the course material toward IBD with a focus on lowering stress. An independent psychologist observed each session to assess adherence to protocol.
Not only did ACT meet the primary study endpoint, it also produced a 25% decrease in perceived stress (on a 1-10 scale) by week 8 and a 27% decrease in perceived stress by week 20 (P less than .001 versus treatment as usual). Depression scores in the ACT group also fell by 47% by week 8 and by 45% at week 20 (P = .01 versus treatment as usual). Anxiety levels decreased by 29% at week 8 and by 31% at week 20, but these improvements did not significantly differ from those in the control group (P = .39).
Interestingly, ACT did not significantly improve symptom burden, activities of daily living, disease-related worry, general well-being, C-reactive protein (CRP) levels, fecal calprotectin levels, or scores on the version used of the Clinical Assessment of Depression (CAD) or the short Mayo assessment. Hair cortisol levels showed an association with baseline stress and anxiety, but not with treatment response.
Care programs for IBD increasingly emphasize mental health services despite a lack of robust trials to support these interventions, the investigators noted. Thus, their findings highlight “the need for researchers and clinicians to further develop and optimize the content and delivery of psychological programs for IBD patients.”
Tillotts Pharma and Boston Scientific provided partial funding, but had no other role in the study. The researchers reported having no relevant conflicts of interest.
SOURCE: Wynne B et al. Gastroenterology. 2018 Nov 16. doi: 10.1053/j.gastro.2018.11.030.
Eight weeks of a mindfulness intervention known as acceptance and commitment therapy (ACT) significantly improved stress and depression among patients with inflammatory bowel disease, and these improvements persisted for at least 12 weeks after therapy ended, according to the results of a randomized, controlled trial.
Source: The American Gastroenterological Association
In the intention-to-treat analysis, stress symptoms, as measured by the Depression Anxiety and Stress Scales (DASS-21), improved by 39% at week 8 and by 45% at week 20, reported Brona Wynne, PhD, of University College Dublin together with her associates. These improvements were highly significant compared with baseline and treatment as usual (P = .001 for both comparisons). “Post hoc analyses indicated that baseline stress levels were similar in control and treatment groups,” the researchers wrote in Gastroenterology. “The results of the per protocol analysis were comparable, with a 43% and 49% reduction in stress in the treatment group from baseline to 8 and 20 weeks.”
Multiple studies have documented high levels of stress and psychological dysfunction among patients with Crohn’s disease and ulcerative colitis. Studies of various mindfulness therapy, relaxation, stress management, cognitive-behavioral therapy, and hypnotherapy interventions often failed to collect key clinical data or were underpowered, uncontrolled, and unrandomized. Acceptance and commitment therapy uses mindfulness to identify adverse thoughts and experiences, accept these as part of life, and recommit to “move towards values that have been identified and adopted by the individual,” the investigators wrote. “This can be defined as the ability to contact the present moment more fully as a conscious human being and to change, or persist in, behavior when doing so serves valued ends.”
Their single-center study, which they said was the first to evaluate ACT in IBD patients, included 79 individuals with stable or mildly active Crohn’s disease (38 patients) or ulcerative colitis (41 patients) who were randomly assigned to ACT (37 patients) or control treatment as usual (42 patients). The two comparison groups were demographically and clinically similar. The ACT program involved eight 90-minute, weekly sessions of groups of 14-16 individuals, led by a single psychologist who tailored the course material toward IBD with a focus on lowering stress. An independent psychologist observed each session to assess adherence to protocol.
Not only did ACT meet the primary study endpoint, it also produced a 25% decrease in perceived stress (on a 1-10 scale) by week 8 and a 27% decrease in perceived stress by week 20 (P less than .001 versus treatment as usual). Depression scores in the ACT group also fell by 47% by week 8 and by 45% at week 20 (P = .01 versus treatment as usual). Anxiety levels decreased by 29% at week 8 and by 31% at week 20, but these improvements did not significantly differ from those in the control group (P = .39).
Interestingly, ACT did not significantly improve symptom burden, activities of daily living, disease-related worry, general well-being, C-reactive protein (CRP) levels, fecal calprotectin levels, or scores on the version used of the Clinical Assessment of Depression (CAD) or the short Mayo assessment. Hair cortisol levels showed an association with baseline stress and anxiety, but not with treatment response.
Care programs for IBD increasingly emphasize mental health services despite a lack of robust trials to support these interventions, the investigators noted. Thus, their findings highlight “the need for researchers and clinicians to further develop and optimize the content and delivery of psychological programs for IBD patients.”
Tillotts Pharma and Boston Scientific provided partial funding, but had no other role in the study. The researchers reported having no relevant conflicts of interest.
SOURCE: Wynne B et al. Gastroenterology. 2018 Nov 16. doi: 10.1053/j.gastro.2018.11.030.
FROM GASTROENTEROLOGY
Key clinical point: An 8-week course of acceptance and commitment therapy improved stress and depression in patients with inflammatory bowel disease.
Major finding: Compared with controls, the intervention group experienced significant improvements in stress (P = .001) and depression (P = .01), but not anxiety.
Study details: Randomized controlled trial of 79 patients.
Disclosures: Tillotts Pharma and Boston Scientific provided partial funding but had no other role in the study. The researchers reported having no relevant conflicts of interest.
Source: Wynne B et al. Gastroenterology. 2018 Nov 16. doi: 10.1053/j.gastro.2018.11.030.
Retrospective Analysis of Liraglutide as Add-On Therapy in Type 2 Diabetes Mellitus: Quantifying the Changes in Insulin Requirements
Clinical pharmacists in VA primary care pharmacy clinics can effectively and safely use liraglutide to reduce hemoglobin A1c and insulin requirements in veterans.
Diabetes mellitus (DM) was the third most common medical diagnosis in 2016.1 Uncontrolled DM can lead to cardiovascular disease, nephropathy, neuropathy, and retinopathy. It is estimated that only 52.5% of patients with DM have achieved their goal hemoglobin A1c (HbA1c) level. The 2018 American Diabetes Association (ADA) clinical guidelines lack strong recommendations on sequential therapy for patients who have received a diagnosis of type 2 diabetes mellitus (T2DM) and have been unable to achieve their goal HbA1c level with lifestyle changes and maximum-dose metformin.2 Although those guidelines support treatment intensification with a glucagon-like peptide 1 receptor agonist (GLP-1 RA), prescribing patterns for T2DM most commonly include adding insulin to try to control blood glucose and reduce long-term comorbidities.2,3
Related:
Insulin therapy is known for its ability to effectively lower blood glucose and HbA1c levels but comes with many limitations. Mealtime insulin has the highest risk of hypoglycemia, causes significant weight gain, requires several additional injections per day, and additional monitoring of blood glucose.4,5 The 2018 ADA guidelines state that hypoglycemia is the major limiting factor in the management of insulin-treated T2DM.2
Compared with mealtime insulin, GLP-1 RAs have the benefit of reducing the risk of hypoglycemia, weight gain, and number of daily injections.5 In addition, compared with insulin alone, GLP-1 RAs have the advantage of reducing glycemic variability.6 These advantages are especially attractive in the treatment of geriatric patients. Given its mechanism of action, liraglutide is expected to have an effect on both fasting and postprandial blood glucose. There are no recommendations on how to empirically reduce the dose of insulin when starting liraglutide.7
Background
GLP-1 is an incretin hormone that is secreted in response to meal ingestion. GLP-1 stimulates insulin release, suppresses elevated glucagon levels, and delays gastric emptying. Patients with a DM diagnosis have impaired secretion of GLP-1.8
The GLP-1 RA liraglutide was approved by the FDA in January 2010 as a once-daily injection for patients with uncontrolled T2DM despite lifestyle changes and metformin monotherapy. Because of its intermediate half-life, liraglutide has an effect on both fasting and postprandial blood glucose.7 GLP-1 RAs are associated with reduced hypoglycemic episodes—an association attributable to the mechanism of action and potentially to improved pancreatic α-cell function.3,4 In July 2016, results of the LEADER trial showed that liraglutide therapy had a cardiovascular benefit in high-risk patients.8 In October 2017, liraglutide was FDAapproved for reducing 3-point major adverse cardiac events.7
Xultophy (Novo Nordisk, Plainsboro, NJ) is a fixed-dose medication combining degludec, a long-acting basal insulin analog, with liraglutide. As seen in the DUAL trials, Xultophy was more beneficial in reducing HbA1c levels than each component alone, and minimized hypoglycemic events, weight gain, and complexity of insulin treatment intensification.9-11 Therapy that combines basal insulin and a GLP-1 RA may be more effective than either agent as monotherapy and may have a significant impact on cardiovascular risk because of the synergistic vasodilatory, anti-inflammatory, and antioxidant properties of insulin and GLP-1 RA.6 In addition, combination therapy offers many benefits over traditional basal and bolus insulin regimens. These benefits include fewer daily injections, additional weight reduction resulting from the reduced insulin requirement, and fewer episodes of hypoglycemia. Reported gastrointestinal adverse effects have been transient and were not augmented when a GLP-1 RA was used in combination with basal insulin.11
Methods
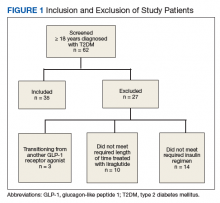
We performed a retrospective chart analysis to quantify the benefit of using liraglutide as an add-on therapy to basal and bolus insulin regimens in veterans treated at VA Boston Healthcare System (VABHS). The analysis evaluated changes in insulin doses and HbA1c levels when liraglutide was added to these regimens. Patients identified for the study had electronic medication orders for concurrent therapy with liraglutide, insulin glargine, and insulin aspart filled through outpatient VABHS campus pharmacies for at least 3 months between January 2010 and December 2016. Sixty-nine patients who were on basal-bolus insulin for T2DM and who were prescribed liraglutide for treatment intensification were screened for inclusion and exclusion criteria. Data were analyzed at baseline and 3 months after liraglutide treatment.
Study Protocol
The inclusion criteria were patients aged ≥ 18 years, T2DM diagnosis, and therapy with insulin glargine and insulin aspart for at least 3 months before treatment intensi fication with liraglutide. Exclusion criteria were diagnosis of type 1 DM. To accurately quantify mean change in number of insulin units used, the study included patients only if they had been prescribed insulin glargine and insulin aspart before starting liraglutide. All other insulin regimens were excluded. To detect the true change that occurs when liraglutide is added to basal-bolus insulin, the study also excluded patients if they had been previously prescribed another GLP-1 RA. Patients with contraindications to liraglutide, insulin aspart, or insulin glargine were excluded as well. In addition, patients were excluded from the exposed arm if they were injecting < 1.2 mg of liraglutide once daily or if they had been on liraglutide for < 3 months.
Study Outcomes
All 35 patients who met the inclusion and exclusion criteria were included in this retrospective chart review. The primary outcome was determined by changes in HbA1c level and number of insulin doses 3 months after treatment with liraglutide. For each patient, a chart review was performed to determine the amount of insulin added or reduced during the study period. Data were collected at baseline and 3 months after initiation of liraglutide.
Statistical Analysis
Statistical analyses were performed with SPSS Version 20.0 (IBM, Armonk, NY). Population characteristics and study outcomes with normal distribution were compared using a paired t test and are reported as means with standard deviations. Nonnormally distributed variables (bolus insulin, HbA1c level) were compared using the nonparametric Wilcoxon rank sum test and are reported as median values with interquartile ranges. Normality was tested with the Shapiro-Wilk test. The primary outcome evaluated was change in number of insulin units used. Secondary outcomes included change in HbA1c level and change in body weight. A Bonferroni correction for multiple comparisons was used to prevent type I error. Significance at the Bonferroni-corrected level of .01 (.05/5 = .01) is indicated.
Results
Patients were included if they were previously on insulin glargine and insulin aspart before starting liraglutide for treatment intensification.
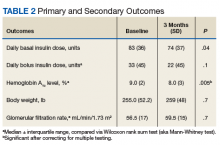
As Table 1 indicates, 100% of patients were male, and mean (SD) age was 65.5 (9.3) years.
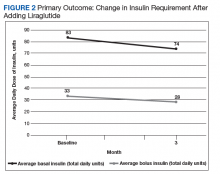
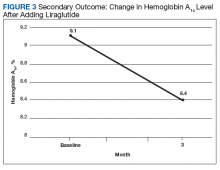
After 3 months of therapy with liraglutide, HbA1c levels were reduced by a mean of 1.0% (P = .005) (Table 2).
Discussion
After 3 months of treatment with liraglutide, patients experienced a significant decrease in HbA1c levels. Insulin doses also decreased, but this finding was not statistically significant after correcting for multiple testing. These results are similar with those in larger studies of the effectiveness of liraglutide and the addition of liraglutide to insulin therapy. 6,8,12,13 Liraglutide has been shown to decrease HbA1c levels, lower rates of progression of kidney failure, decrease weight, and provide cardiovascular benefit.
In a prospective, randomized controlled trial evaluating the effect of adding liraglutide to insulin therapy, 21 of the 37 patients who had T2DM and required more than 100 total units of basal-bolus insulin daily were initiated on liraglutide, and changes in HbA1c level, body weight, and glycemic variability were compared. Results showed statistically significant improvement in all 3 outcomes in the group treated with liraglutide.6 Our findings, in conjunction with those of the larger studies, suggest that many of these results are generalizable to our local veteran population. Importantly, liraglutide was successfully started in pharmacy clinics—an indication that this treatment need not be initiated by an endocrine specialist.
Limitations
Given the lack of gender and racial diversity in this study population, our findings have limited generalizability to other populations. It is possible that, with a larger sample size, these results regarding reduced basal insulin doses would be significant. It has been hypothesized that patients experience fewer episodes of hypoglycemia when insulin doses are reduced, but we were unable to measure the frequency of these episodes. Other study limitations include inability to assess adherence and inability to account for concurrent regimens and/or for lifestyle changes that may have been made during the study period. Further, the study did not collect data on changes made to current DM medication regimens during the study period, and these changes may have influenced outcomes.
Conclusion
Patients who require treatment intensification for insulin-dependent T2DM may benefit from having liraglutide added to their basal-bolus insulin regimen. Liraglutide may prove to be more favorable than bolus insulin when choosing add-on therapy to basal insulin. Benefits include reductions in insulin doses, HbA1c levels, number of daily injections, and body weight. Therefore, we suggest that empirically reducing basal insulin by 10% to 25% and bolus insulin by 25% to 50% will avoid relative hypoglycemia. Prescribers must keep in mind patient-specific factors when adjusting insulin doses, if these doses are adjusted at all. Follow-up of 2 to 4 weeks is recommended for review of home monitoring of glucose for further insulin adjustments.
This study has important clinical implications. First, the finding of a reduction in HbA1c levels supports use of liraglutide therapy for HbA1c reduction in veterans. Second, the number of veterans who were successfully initiated on liraglutide therapy by nonphysician providers indicates that liraglutide can be effectively and safely started in primary care pharmacy clinics, increasing access to the medication.
1. Centers for Medicare & Medicaid Services. ICD-10. https://www.cms.gov/medicare/coding/icd10. Accessed July 26, 2018.
2. American Diabetes Association. Introduction: standards of medical care in diabetes—2018. Diabetes Care. 2018;41(suppl 1):S1-S2.
3. Combination therapy with insulins and GLP-1 receptor agonists. http://www.powerpak.com/course/content/113275. Updated 2018. Accessed July 26, 2018.
4. Carris NW, Taylor JR, Gums JG. Combining a GLP-1 receptor agonist and basal insulin: study evidence and practical considerations. Drugs. 2014;74(18):2141-2152.
5. Young LA, Buse JB, Weaver MA, et al; Monitor Trial Group. Glucose self-monitoring in non-insulin-treated patients with type 2 diabetes in primary care settings: a randomized trial. JAMA Intern Med. 2017;177(7):920-929.
6. Lane W, Weinrib S, Rappaport J, Hale C. The effect of addition of liraglutide to high-dose intensive insulin therapy: a randomized prospective trial. Diabetes Obes Metab. 2014;16(9):827-832.
7. Victoza [package insert]. Plainsboro, NJ: Novo Nordisk Inc; August 2017.
8. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311-322.
9. Buse JB, Vilsbøll T, Thurman J, et al; NN9068-3912 (DUAL-II) Trial Investigators. Contribution of liraglutide in the fixed-ratio combination of insulin degludec and liraglutide (IDegLira). Diabetes Care. 2014;37(11):2926-2933
10. Glough SC, Bode B, Woo V, et al; NN9068-3697 (DUAL I) Trial Investigators. Efficacy and safety of a fixed-ratio combination of insulin degludec and liraglutide (IDegLira) compared with its component given alone: results of a phase 3, open-label, randomized, 26-week, treat-to-target trial in insulin-naïve patients with type 2 diabetes. Lancet Diabetes Endocrinol. 2014;2(11):885-893.
11. Lingvay I, Pérez Manghi F, García-Hernández P, et al; DUAL V Investigators. Effect of insulin glargine up-titration vs insulin degludec/liraglutide on glycated hemoglobin levels in patients with uncontrolled type 2 diabetes: the DUAL V randomized controlled trial. JAMA. 2016;315(9):898-907.
12. Ceriello A, Novials A, Canivell S, et al. Simultaneous GLP-1 and insulin administration acutely enhances their vasodilatory, anti-inflammatory and antioxidant action in type 2 diabetes. Diabetes Care. 2014;37(7):1938-1943.
13. Lind M, Hirsch IB, Tuomilehto J, Dahlqvist S, Torffvit O, Pehrsson NG. Design and methods of a randomised double-blind trial of adding liraglutide to control HbA1c in patients with type 2 diabetes with impaired glycaemic control treated with multiple daily insulin injections (MDI-Liraglutide trial). Prim Care Diabetes. 2015;9(1):15-22.
Clinical pharmacists in VA primary care pharmacy clinics can effectively and safely use liraglutide to reduce hemoglobin A1c and insulin requirements in veterans.
Clinical pharmacists in VA primary care pharmacy clinics can effectively and safely use liraglutide to reduce hemoglobin A1c and insulin requirements in veterans.
Diabetes mellitus (DM) was the third most common medical diagnosis in 2016.1 Uncontrolled DM can lead to cardiovascular disease, nephropathy, neuropathy, and retinopathy. It is estimated that only 52.5% of patients with DM have achieved their goal hemoglobin A1c (HbA1c) level. The 2018 American Diabetes Association (ADA) clinical guidelines lack strong recommendations on sequential therapy for patients who have received a diagnosis of type 2 diabetes mellitus (T2DM) and have been unable to achieve their goal HbA1c level with lifestyle changes and maximum-dose metformin.2 Although those guidelines support treatment intensification with a glucagon-like peptide 1 receptor agonist (GLP-1 RA), prescribing patterns for T2DM most commonly include adding insulin to try to control blood glucose and reduce long-term comorbidities.2,3
Related:
Insulin therapy is known for its ability to effectively lower blood glucose and HbA1c levels but comes with many limitations. Mealtime insulin has the highest risk of hypoglycemia, causes significant weight gain, requires several additional injections per day, and additional monitoring of blood glucose.4,5 The 2018 ADA guidelines state that hypoglycemia is the major limiting factor in the management of insulin-treated T2DM.2
Compared with mealtime insulin, GLP-1 RAs have the benefit of reducing the risk of hypoglycemia, weight gain, and number of daily injections.5 In addition, compared with insulin alone, GLP-1 RAs have the advantage of reducing glycemic variability.6 These advantages are especially attractive in the treatment of geriatric patients. Given its mechanism of action, liraglutide is expected to have an effect on both fasting and postprandial blood glucose. There are no recommendations on how to empirically reduce the dose of insulin when starting liraglutide.7
Background
GLP-1 is an incretin hormone that is secreted in response to meal ingestion. GLP-1 stimulates insulin release, suppresses elevated glucagon levels, and delays gastric emptying. Patients with a DM diagnosis have impaired secretion of GLP-1.8
The GLP-1 RA liraglutide was approved by the FDA in January 2010 as a once-daily injection for patients with uncontrolled T2DM despite lifestyle changes and metformin monotherapy. Because of its intermediate half-life, liraglutide has an effect on both fasting and postprandial blood glucose.7 GLP-1 RAs are associated with reduced hypoglycemic episodes—an association attributable to the mechanism of action and potentially to improved pancreatic α-cell function.3,4 In July 2016, results of the LEADER trial showed that liraglutide therapy had a cardiovascular benefit in high-risk patients.8 In October 2017, liraglutide was FDAapproved for reducing 3-point major adverse cardiac events.7
Xultophy (Novo Nordisk, Plainsboro, NJ) is a fixed-dose medication combining degludec, a long-acting basal insulin analog, with liraglutide. As seen in the DUAL trials, Xultophy was more beneficial in reducing HbA1c levels than each component alone, and minimized hypoglycemic events, weight gain, and complexity of insulin treatment intensification.9-11 Therapy that combines basal insulin and a GLP-1 RA may be more effective than either agent as monotherapy and may have a significant impact on cardiovascular risk because of the synergistic vasodilatory, anti-inflammatory, and antioxidant properties of insulin and GLP-1 RA.6 In addition, combination therapy offers many benefits over traditional basal and bolus insulin regimens. These benefits include fewer daily injections, additional weight reduction resulting from the reduced insulin requirement, and fewer episodes of hypoglycemia. Reported gastrointestinal adverse effects have been transient and were not augmented when a GLP-1 RA was used in combination with basal insulin.11
Methods
We performed a retrospective chart analysis to quantify the benefit of using liraglutide as an add-on therapy to basal and bolus insulin regimens in veterans treated at VA Boston Healthcare System (VABHS). The analysis evaluated changes in insulin doses and HbA1c levels when liraglutide was added to these regimens. Patients identified for the study had electronic medication orders for concurrent therapy with liraglutide, insulin glargine, and insulin aspart filled through outpatient VABHS campus pharmacies for at least 3 months between January 2010 and December 2016. Sixty-nine patients who were on basal-bolus insulin for T2DM and who were prescribed liraglutide for treatment intensification were screened for inclusion and exclusion criteria. Data were analyzed at baseline and 3 months after liraglutide treatment.
Study Protocol
The inclusion criteria were patients aged ≥ 18 years, T2DM diagnosis, and therapy with insulin glargine and insulin aspart for at least 3 months before treatment intensi fication with liraglutide. Exclusion criteria were diagnosis of type 1 DM. To accurately quantify mean change in number of insulin units used, the study included patients only if they had been prescribed insulin glargine and insulin aspart before starting liraglutide. All other insulin regimens were excluded. To detect the true change that occurs when liraglutide is added to basal-bolus insulin, the study also excluded patients if they had been previously prescribed another GLP-1 RA. Patients with contraindications to liraglutide, insulin aspart, or insulin glargine were excluded as well. In addition, patients were excluded from the exposed arm if they were injecting < 1.2 mg of liraglutide once daily or if they had been on liraglutide for < 3 months.
Study Outcomes
All 35 patients who met the inclusion and exclusion criteria were included in this retrospective chart review. The primary outcome was determined by changes in HbA1c level and number of insulin doses 3 months after treatment with liraglutide. For each patient, a chart review was performed to determine the amount of insulin added or reduced during the study period. Data were collected at baseline and 3 months after initiation of liraglutide.
Statistical Analysis
Statistical analyses were performed with SPSS Version 20.0 (IBM, Armonk, NY). Population characteristics and study outcomes with normal distribution were compared using a paired t test and are reported as means with standard deviations. Nonnormally distributed variables (bolus insulin, HbA1c level) were compared using the nonparametric Wilcoxon rank sum test and are reported as median values with interquartile ranges. Normality was tested with the Shapiro-Wilk test. The primary outcome evaluated was change in number of insulin units used. Secondary outcomes included change in HbA1c level and change in body weight. A Bonferroni correction for multiple comparisons was used to prevent type I error. Significance at the Bonferroni-corrected level of .01 (.05/5 = .01) is indicated.
Results
Patients were included if they were previously on insulin glargine and insulin aspart before starting liraglutide for treatment intensification.
As Table 1 indicates, 100% of patients were male, and mean (SD) age was 65.5 (9.3) years.
After 3 months of therapy with liraglutide, HbA1c levels were reduced by a mean of 1.0% (P = .005) (Table 2).
Discussion
After 3 months of treatment with liraglutide, patients experienced a significant decrease in HbA1c levels. Insulin doses also decreased, but this finding was not statistically significant after correcting for multiple testing. These results are similar with those in larger studies of the effectiveness of liraglutide and the addition of liraglutide to insulin therapy. 6,8,12,13 Liraglutide has been shown to decrease HbA1c levels, lower rates of progression of kidney failure, decrease weight, and provide cardiovascular benefit.
In a prospective, randomized controlled trial evaluating the effect of adding liraglutide to insulin therapy, 21 of the 37 patients who had T2DM and required more than 100 total units of basal-bolus insulin daily were initiated on liraglutide, and changes in HbA1c level, body weight, and glycemic variability were compared. Results showed statistically significant improvement in all 3 outcomes in the group treated with liraglutide.6 Our findings, in conjunction with those of the larger studies, suggest that many of these results are generalizable to our local veteran population. Importantly, liraglutide was successfully started in pharmacy clinics—an indication that this treatment need not be initiated by an endocrine specialist.
Limitations
Given the lack of gender and racial diversity in this study population, our findings have limited generalizability to other populations. It is possible that, with a larger sample size, these results regarding reduced basal insulin doses would be significant. It has been hypothesized that patients experience fewer episodes of hypoglycemia when insulin doses are reduced, but we were unable to measure the frequency of these episodes. Other study limitations include inability to assess adherence and inability to account for concurrent regimens and/or for lifestyle changes that may have been made during the study period. Further, the study did not collect data on changes made to current DM medication regimens during the study period, and these changes may have influenced outcomes.
Conclusion
Patients who require treatment intensification for insulin-dependent T2DM may benefit from having liraglutide added to their basal-bolus insulin regimen. Liraglutide may prove to be more favorable than bolus insulin when choosing add-on therapy to basal insulin. Benefits include reductions in insulin doses, HbA1c levels, number of daily injections, and body weight. Therefore, we suggest that empirically reducing basal insulin by 10% to 25% and bolus insulin by 25% to 50% will avoid relative hypoglycemia. Prescribers must keep in mind patient-specific factors when adjusting insulin doses, if these doses are adjusted at all. Follow-up of 2 to 4 weeks is recommended for review of home monitoring of glucose for further insulin adjustments.
This study has important clinical implications. First, the finding of a reduction in HbA1c levels supports use of liraglutide therapy for HbA1c reduction in veterans. Second, the number of veterans who were successfully initiated on liraglutide therapy by nonphysician providers indicates that liraglutide can be effectively and safely started in primary care pharmacy clinics, increasing access to the medication.
Diabetes mellitus (DM) was the third most common medical diagnosis in 2016.1 Uncontrolled DM can lead to cardiovascular disease, nephropathy, neuropathy, and retinopathy. It is estimated that only 52.5% of patients with DM have achieved their goal hemoglobin A1c (HbA1c) level. The 2018 American Diabetes Association (ADA) clinical guidelines lack strong recommendations on sequential therapy for patients who have received a diagnosis of type 2 diabetes mellitus (T2DM) and have been unable to achieve their goal HbA1c level with lifestyle changes and maximum-dose metformin.2 Although those guidelines support treatment intensification with a glucagon-like peptide 1 receptor agonist (GLP-1 RA), prescribing patterns for T2DM most commonly include adding insulin to try to control blood glucose and reduce long-term comorbidities.2,3
Related:
Insulin therapy is known for its ability to effectively lower blood glucose and HbA1c levels but comes with many limitations. Mealtime insulin has the highest risk of hypoglycemia, causes significant weight gain, requires several additional injections per day, and additional monitoring of blood glucose.4,5 The 2018 ADA guidelines state that hypoglycemia is the major limiting factor in the management of insulin-treated T2DM.2
Compared with mealtime insulin, GLP-1 RAs have the benefit of reducing the risk of hypoglycemia, weight gain, and number of daily injections.5 In addition, compared with insulin alone, GLP-1 RAs have the advantage of reducing glycemic variability.6 These advantages are especially attractive in the treatment of geriatric patients. Given its mechanism of action, liraglutide is expected to have an effect on both fasting and postprandial blood glucose. There are no recommendations on how to empirically reduce the dose of insulin when starting liraglutide.7
Background
GLP-1 is an incretin hormone that is secreted in response to meal ingestion. GLP-1 stimulates insulin release, suppresses elevated glucagon levels, and delays gastric emptying. Patients with a DM diagnosis have impaired secretion of GLP-1.8
The GLP-1 RA liraglutide was approved by the FDA in January 2010 as a once-daily injection for patients with uncontrolled T2DM despite lifestyle changes and metformin monotherapy. Because of its intermediate half-life, liraglutide has an effect on both fasting and postprandial blood glucose.7 GLP-1 RAs are associated with reduced hypoglycemic episodes—an association attributable to the mechanism of action and potentially to improved pancreatic α-cell function.3,4 In July 2016, results of the LEADER trial showed that liraglutide therapy had a cardiovascular benefit in high-risk patients.8 In October 2017, liraglutide was FDAapproved for reducing 3-point major adverse cardiac events.7
Xultophy (Novo Nordisk, Plainsboro, NJ) is a fixed-dose medication combining degludec, a long-acting basal insulin analog, with liraglutide. As seen in the DUAL trials, Xultophy was more beneficial in reducing HbA1c levels than each component alone, and minimized hypoglycemic events, weight gain, and complexity of insulin treatment intensification.9-11 Therapy that combines basal insulin and a GLP-1 RA may be more effective than either agent as monotherapy and may have a significant impact on cardiovascular risk because of the synergistic vasodilatory, anti-inflammatory, and antioxidant properties of insulin and GLP-1 RA.6 In addition, combination therapy offers many benefits over traditional basal and bolus insulin regimens. These benefits include fewer daily injections, additional weight reduction resulting from the reduced insulin requirement, and fewer episodes of hypoglycemia. Reported gastrointestinal adverse effects have been transient and were not augmented when a GLP-1 RA was used in combination with basal insulin.11
Methods
We performed a retrospective chart analysis to quantify the benefit of using liraglutide as an add-on therapy to basal and bolus insulin regimens in veterans treated at VA Boston Healthcare System (VABHS). The analysis evaluated changes in insulin doses and HbA1c levels when liraglutide was added to these regimens. Patients identified for the study had electronic medication orders for concurrent therapy with liraglutide, insulin glargine, and insulin aspart filled through outpatient VABHS campus pharmacies for at least 3 months between January 2010 and December 2016. Sixty-nine patients who were on basal-bolus insulin for T2DM and who were prescribed liraglutide for treatment intensification were screened for inclusion and exclusion criteria. Data were analyzed at baseline and 3 months after liraglutide treatment.
Study Protocol
The inclusion criteria were patients aged ≥ 18 years, T2DM diagnosis, and therapy with insulin glargine and insulin aspart for at least 3 months before treatment intensi fication with liraglutide. Exclusion criteria were diagnosis of type 1 DM. To accurately quantify mean change in number of insulin units used, the study included patients only if they had been prescribed insulin glargine and insulin aspart before starting liraglutide. All other insulin regimens were excluded. To detect the true change that occurs when liraglutide is added to basal-bolus insulin, the study also excluded patients if they had been previously prescribed another GLP-1 RA. Patients with contraindications to liraglutide, insulin aspart, or insulin glargine were excluded as well. In addition, patients were excluded from the exposed arm if they were injecting < 1.2 mg of liraglutide once daily or if they had been on liraglutide for < 3 months.
Study Outcomes
All 35 patients who met the inclusion and exclusion criteria were included in this retrospective chart review. The primary outcome was determined by changes in HbA1c level and number of insulin doses 3 months after treatment with liraglutide. For each patient, a chart review was performed to determine the amount of insulin added or reduced during the study period. Data were collected at baseline and 3 months after initiation of liraglutide.
Statistical Analysis
Statistical analyses were performed with SPSS Version 20.0 (IBM, Armonk, NY). Population characteristics and study outcomes with normal distribution were compared using a paired t test and are reported as means with standard deviations. Nonnormally distributed variables (bolus insulin, HbA1c level) were compared using the nonparametric Wilcoxon rank sum test and are reported as median values with interquartile ranges. Normality was tested with the Shapiro-Wilk test. The primary outcome evaluated was change in number of insulin units used. Secondary outcomes included change in HbA1c level and change in body weight. A Bonferroni correction for multiple comparisons was used to prevent type I error. Significance at the Bonferroni-corrected level of .01 (.05/5 = .01) is indicated.
Results
Patients were included if they were previously on insulin glargine and insulin aspart before starting liraglutide for treatment intensification.
As Table 1 indicates, 100% of patients were male, and mean (SD) age was 65.5 (9.3) years.
After 3 months of therapy with liraglutide, HbA1c levels were reduced by a mean of 1.0% (P = .005) (Table 2).
Discussion
After 3 months of treatment with liraglutide, patients experienced a significant decrease in HbA1c levels. Insulin doses also decreased, but this finding was not statistically significant after correcting for multiple testing. These results are similar with those in larger studies of the effectiveness of liraglutide and the addition of liraglutide to insulin therapy. 6,8,12,13 Liraglutide has been shown to decrease HbA1c levels, lower rates of progression of kidney failure, decrease weight, and provide cardiovascular benefit.
In a prospective, randomized controlled trial evaluating the effect of adding liraglutide to insulin therapy, 21 of the 37 patients who had T2DM and required more than 100 total units of basal-bolus insulin daily were initiated on liraglutide, and changes in HbA1c level, body weight, and glycemic variability were compared. Results showed statistically significant improvement in all 3 outcomes in the group treated with liraglutide.6 Our findings, in conjunction with those of the larger studies, suggest that many of these results are generalizable to our local veteran population. Importantly, liraglutide was successfully started in pharmacy clinics—an indication that this treatment need not be initiated by an endocrine specialist.
Limitations
Given the lack of gender and racial diversity in this study population, our findings have limited generalizability to other populations. It is possible that, with a larger sample size, these results regarding reduced basal insulin doses would be significant. It has been hypothesized that patients experience fewer episodes of hypoglycemia when insulin doses are reduced, but we were unable to measure the frequency of these episodes. Other study limitations include inability to assess adherence and inability to account for concurrent regimens and/or for lifestyle changes that may have been made during the study period. Further, the study did not collect data on changes made to current DM medication regimens during the study period, and these changes may have influenced outcomes.
Conclusion
Patients who require treatment intensification for insulin-dependent T2DM may benefit from having liraglutide added to their basal-bolus insulin regimen. Liraglutide may prove to be more favorable than bolus insulin when choosing add-on therapy to basal insulin. Benefits include reductions in insulin doses, HbA1c levels, number of daily injections, and body weight. Therefore, we suggest that empirically reducing basal insulin by 10% to 25% and bolus insulin by 25% to 50% will avoid relative hypoglycemia. Prescribers must keep in mind patient-specific factors when adjusting insulin doses, if these doses are adjusted at all. Follow-up of 2 to 4 weeks is recommended for review of home monitoring of glucose for further insulin adjustments.
This study has important clinical implications. First, the finding of a reduction in HbA1c levels supports use of liraglutide therapy for HbA1c reduction in veterans. Second, the number of veterans who were successfully initiated on liraglutide therapy by nonphysician providers indicates that liraglutide can be effectively and safely started in primary care pharmacy clinics, increasing access to the medication.
1. Centers for Medicare & Medicaid Services. ICD-10. https://www.cms.gov/medicare/coding/icd10. Accessed July 26, 2018.
2. American Diabetes Association. Introduction: standards of medical care in diabetes—2018. Diabetes Care. 2018;41(suppl 1):S1-S2.
3. Combination therapy with insulins and GLP-1 receptor agonists. http://www.powerpak.com/course/content/113275. Updated 2018. Accessed July 26, 2018.
4. Carris NW, Taylor JR, Gums JG. Combining a GLP-1 receptor agonist and basal insulin: study evidence and practical considerations. Drugs. 2014;74(18):2141-2152.
5. Young LA, Buse JB, Weaver MA, et al; Monitor Trial Group. Glucose self-monitoring in non-insulin-treated patients with type 2 diabetes in primary care settings: a randomized trial. JAMA Intern Med. 2017;177(7):920-929.
6. Lane W, Weinrib S, Rappaport J, Hale C. The effect of addition of liraglutide to high-dose intensive insulin therapy: a randomized prospective trial. Diabetes Obes Metab. 2014;16(9):827-832.
7. Victoza [package insert]. Plainsboro, NJ: Novo Nordisk Inc; August 2017.
8. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311-322.
9. Buse JB, Vilsbøll T, Thurman J, et al; NN9068-3912 (DUAL-II) Trial Investigators. Contribution of liraglutide in the fixed-ratio combination of insulin degludec and liraglutide (IDegLira). Diabetes Care. 2014;37(11):2926-2933
10. Glough SC, Bode B, Woo V, et al; NN9068-3697 (DUAL I) Trial Investigators. Efficacy and safety of a fixed-ratio combination of insulin degludec and liraglutide (IDegLira) compared with its component given alone: results of a phase 3, open-label, randomized, 26-week, treat-to-target trial in insulin-naïve patients with type 2 diabetes. Lancet Diabetes Endocrinol. 2014;2(11):885-893.
11. Lingvay I, Pérez Manghi F, García-Hernández P, et al; DUAL V Investigators. Effect of insulin glargine up-titration vs insulin degludec/liraglutide on glycated hemoglobin levels in patients with uncontrolled type 2 diabetes: the DUAL V randomized controlled trial. JAMA. 2016;315(9):898-907.
12. Ceriello A, Novials A, Canivell S, et al. Simultaneous GLP-1 and insulin administration acutely enhances their vasodilatory, anti-inflammatory and antioxidant action in type 2 diabetes. Diabetes Care. 2014;37(7):1938-1943.
13. Lind M, Hirsch IB, Tuomilehto J, Dahlqvist S, Torffvit O, Pehrsson NG. Design and methods of a randomised double-blind trial of adding liraglutide to control HbA1c in patients with type 2 diabetes with impaired glycaemic control treated with multiple daily insulin injections (MDI-Liraglutide trial). Prim Care Diabetes. 2015;9(1):15-22.
1. Centers for Medicare & Medicaid Services. ICD-10. https://www.cms.gov/medicare/coding/icd10. Accessed July 26, 2018.
2. American Diabetes Association. Introduction: standards of medical care in diabetes—2018. Diabetes Care. 2018;41(suppl 1):S1-S2.
3. Combination therapy with insulins and GLP-1 receptor agonists. http://www.powerpak.com/course/content/113275. Updated 2018. Accessed July 26, 2018.
4. Carris NW, Taylor JR, Gums JG. Combining a GLP-1 receptor agonist and basal insulin: study evidence and practical considerations. Drugs. 2014;74(18):2141-2152.
5. Young LA, Buse JB, Weaver MA, et al; Monitor Trial Group. Glucose self-monitoring in non-insulin-treated patients with type 2 diabetes in primary care settings: a randomized trial. JAMA Intern Med. 2017;177(7):920-929.
6. Lane W, Weinrib S, Rappaport J, Hale C. The effect of addition of liraglutide to high-dose intensive insulin therapy: a randomized prospective trial. Diabetes Obes Metab. 2014;16(9):827-832.
7. Victoza [package insert]. Plainsboro, NJ: Novo Nordisk Inc; August 2017.
8. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311-322.
9. Buse JB, Vilsbøll T, Thurman J, et al; NN9068-3912 (DUAL-II) Trial Investigators. Contribution of liraglutide in the fixed-ratio combination of insulin degludec and liraglutide (IDegLira). Diabetes Care. 2014;37(11):2926-2933
10. Glough SC, Bode B, Woo V, et al; NN9068-3697 (DUAL I) Trial Investigators. Efficacy and safety of a fixed-ratio combination of insulin degludec and liraglutide (IDegLira) compared with its component given alone: results of a phase 3, open-label, randomized, 26-week, treat-to-target trial in insulin-naïve patients with type 2 diabetes. Lancet Diabetes Endocrinol. 2014;2(11):885-893.
11. Lingvay I, Pérez Manghi F, García-Hernández P, et al; DUAL V Investigators. Effect of insulin glargine up-titration vs insulin degludec/liraglutide on glycated hemoglobin levels in patients with uncontrolled type 2 diabetes: the DUAL V randomized controlled trial. JAMA. 2016;315(9):898-907.
12. Ceriello A, Novials A, Canivell S, et al. Simultaneous GLP-1 and insulin administration acutely enhances their vasodilatory, anti-inflammatory and antioxidant action in type 2 diabetes. Diabetes Care. 2014;37(7):1938-1943.
13. Lind M, Hirsch IB, Tuomilehto J, Dahlqvist S, Torffvit O, Pehrsson NG. Design and methods of a randomised double-blind trial of adding liraglutide to control HbA1c in patients with type 2 diabetes with impaired glycaemic control treated with multiple daily insulin injections (MDI-Liraglutide trial). Prim Care Diabetes. 2015;9(1):15-22.
FDA issues warnings to companies selling illegal Alzheimer’s treatments
The Food and Drug Administration has issued warning letters to 12 companies and advisory letters to 5 companies illegally selling more than 58 products claiming to treat Alzheimer’s disease.
The products, many of which are marketed as dietary supplements, are being sold in a variety of forms, including tablets, capsules, and oils. These drugs are either unapproved or mislabeled and claim to prevent, treat, or cure Alzheimer’s disease, as well as a number of other serious diseases and health conditions, in violation of the Federal Food, Drug, and Cosmetic Act.
“Alzheimer’s is a challenging disease that, unfortunately, has no cure. Any products making unproven drug claims could mislead consumers to believe that such therapies exist and keep them from accessing therapies that are known to help support the symptoms of the disease, or worse, as some fraudulent treatments can cause serious or even fatal injuries,” FDA Commissioner Scott Gottlieb, MD, said in a press release.
In an additional statement, Dr. Gottlieb detailed several new strategies for improving the safety and accuracy of dietary supplements, including efforts to more rapidly communicate to the public potential safety issues with dietary supplement products and to establish a flexible regulatory framework that promotes innovation and upholds product safety.
The Food and Drug Administration has issued warning letters to 12 companies and advisory letters to 5 companies illegally selling more than 58 products claiming to treat Alzheimer’s disease.
The products, many of which are marketed as dietary supplements, are being sold in a variety of forms, including tablets, capsules, and oils. These drugs are either unapproved or mislabeled and claim to prevent, treat, or cure Alzheimer’s disease, as well as a number of other serious diseases and health conditions, in violation of the Federal Food, Drug, and Cosmetic Act.
“Alzheimer’s is a challenging disease that, unfortunately, has no cure. Any products making unproven drug claims could mislead consumers to believe that such therapies exist and keep them from accessing therapies that are known to help support the symptoms of the disease, or worse, as some fraudulent treatments can cause serious or even fatal injuries,” FDA Commissioner Scott Gottlieb, MD, said in a press release.
In an additional statement, Dr. Gottlieb detailed several new strategies for improving the safety and accuracy of dietary supplements, including efforts to more rapidly communicate to the public potential safety issues with dietary supplement products and to establish a flexible regulatory framework that promotes innovation and upholds product safety.
The Food and Drug Administration has issued warning letters to 12 companies and advisory letters to 5 companies illegally selling more than 58 products claiming to treat Alzheimer’s disease.
The products, many of which are marketed as dietary supplements, are being sold in a variety of forms, including tablets, capsules, and oils. These drugs are either unapproved or mislabeled and claim to prevent, treat, or cure Alzheimer’s disease, as well as a number of other serious diseases and health conditions, in violation of the Federal Food, Drug, and Cosmetic Act.
“Alzheimer’s is a challenging disease that, unfortunately, has no cure. Any products making unproven drug claims could mislead consumers to believe that such therapies exist and keep them from accessing therapies that are known to help support the symptoms of the disease, or worse, as some fraudulent treatments can cause serious or even fatal injuries,” FDA Commissioner Scott Gottlieb, MD, said in a press release.
In an additional statement, Dr. Gottlieb detailed several new strategies for improving the safety and accuracy of dietary supplements, including efforts to more rapidly communicate to the public potential safety issues with dietary supplement products and to establish a flexible regulatory framework that promotes innovation and upholds product safety.
Miscommunication With Dermatology Patients: Are We Speaking the Same Language?
I was a third-year medical student, dutifully reviewing discharge instructions with a patient and her family. The patient’s adult daughter asked, “What about that diet you put her on?” As they looked at me quizzically, I looked back equally confused, until it clicked: We needed to talk about the word diet. In everyday conversation, diet generally is understood to mean restriction of food to lose weight, which is what the family hoped would be prescribed for their obese family member. I needed to tell them that I was sorry for the misunderstanding. If they overheard us “ordering a diet,” we simply meant providing trays of hospital food.
We become so familiar with the language of our profession that we do not remember it may be foreign to our patients. In dermatology, we are aware that our specialty is full of esoteric jargon and complex concepts that need to be carefully explained to our patients in simpler terms. But since that incident in medical school, I have been interested in the more insidious potential misunderstandings that can arise from words as seemingly simple as diet. There are many examples in dermatology, particularly in the way we prescribe topical therapy and use trade names.
Topical Therapy
Instructions for systemic medications may be as simple as “take 1 pill twice daily.” Prescriptions for topical medications can be written with an equally simple patient signature such as “apply twice daily to affected area,” but the simplicity is deceptive. The direction to “apply” may seem intuitive to the prescriber, but we do not always specify the amount. Sunscreen, for example, is notoriously underapplied when the actual amount of product needed for protection is not demonstrated.1 One study of new dermatology patients given a prescription for a new topical medication found that the majority of patients underdosed.2
Determination of an “affected area,” regardless of whether the site is indicated, can be even less straightforward. In acne treatment, the affected area is the whole region prone to acne breakouts, whereas in psoriasis it may be discrete psoriatic plaques. We may believe our explanations are perfectly clear, but we have all seen patients spot treating their acne or psoriasis patients covering entire territories of normal skin with topical steroids, despite our education. One study of eczema action plans found that there was considerable variability in the way different providers described disease flares that require treatment. For example, redness was only used as a descriptor of an eczema flare in 68.2% of eczema action plans studied.3 Ensuring our patients understand our criteria for skin requiring topical treatment may mean the difference between treatment success and failure and also may help to avoid unnecessary side effects.
Adherence to topical medication regimens is poor, and inadequate patient education is only one factor.4,5 One study found that more than one-third of new prescriptions for topical medications were never even filled.6 However, improving our communication about application of topical drugs is one way we must address the complicated issue of adherence.
Trade Names
In dermatology, we often use trade names to refer to our medications, even if we do not intend to reference the brand name of the drug specifically. We may tell a patient to use Lidex (Medicis Pharmaceutical Corporation) for her hands but then send an escript to her pharmacy for fluocinonide. Trade names are designed to roll off the tongue, in contrast to the unwieldy, clumsily long generic names assigned to many of our medications.
Substituting trade names may facilitate more natural conversation to promote patient understanding in some cases; however, there are pitfalls associated with this habit. First, we may be doing our patients a disservice if we do not clarify when it would be acceptable to substitute with the generic when the medication is available over-the-counter. If we decide to treat with Rogaine (Johnson & Johnson Consumer Inc) but do not suggest the option of purchasing the generic minoxidil, the patient could be unnecessarily overpaying for a brand name by following our instructions.
Conversely, there are scenarios in which the use of a brand name is actually not specific enough. A patient once told me she was using Differin (Galderma Laboratories, LP) as discussed at her prior visit, but she revealed she was washing it off after application. I initially assumed she misunderstood that adapalene was a gel to be applied and left on. After additional questioning, however, it became clear that she purchased the Differin gentle cleanser, a nonmedicated facial wash, rather than the retinoid we had intended for her. I had not considered that Differin would market an entire line of skin care products but now realize we must be cautious using Differin and adapalene interchangeably. Other examples include popular over-the-counter antihistamine brands such as Allegra (Chattem, a Sanofi company) or Benadryl (Johnson & Johnson Consumer Inc) that market multiple products with different active ingredients.
Final Thoughts
The smooth transfer of information between physician and patient is key to a healthy therapeutic relationship. In residency and throughout our careers, we will continue to develop and refine our communication skills to best serve our patients. We should pay particular attention to the unexpected and surprising ways in which we fail to adequately communicate, make note of these patterns, and share with our colleagues so that we can all learn from our collective experiences.
- Schneider, J. The teaspoon rule of applying sunscreen. Arch Dermatol. 2002;138:838-839.
- Storm A, Benfeldt E, Andersen SE, et al. A prospective study of patient adherence to topical treatments: 95% of patients underdose. J Am Acad Dermatol. 2008;59:975-980.
- Stringer T, Yin HS, Gittler J, et al. The readability, suitability, and content features of eczema action plans in the United States. Pediatr Dermatol. 2018;35:800-807.
- Hougeir FG, Cook-Bolden FE, Rodriguez D, et al. Critical considerations on optimizing topical corticosteroid therapy. J Clin Aesthet Dermatol. 2015;8(suppl 1):S2-S14.
- Savary J, Ortonne JP, Aractingi S. The right dose in the right place: an overview of current prescription, instruction and application modalities for topical psoriasis treatments. J Eur Acad Dermatol Venereol. 2005;19:14-17.
- Storm A, Anderson SE, Benfeldt E, et al. One in 3 prescriptions are never redeemed: primary nonadherence in an outpatient clinic. J Am Acad Dermatol. 2008;59:27-33.
I was a third-year medical student, dutifully reviewing discharge instructions with a patient and her family. The patient’s adult daughter asked, “What about that diet you put her on?” As they looked at me quizzically, I looked back equally confused, until it clicked: We needed to talk about the word diet. In everyday conversation, diet generally is understood to mean restriction of food to lose weight, which is what the family hoped would be prescribed for their obese family member. I needed to tell them that I was sorry for the misunderstanding. If they overheard us “ordering a diet,” we simply meant providing trays of hospital food.
We become so familiar with the language of our profession that we do not remember it may be foreign to our patients. In dermatology, we are aware that our specialty is full of esoteric jargon and complex concepts that need to be carefully explained to our patients in simpler terms. But since that incident in medical school, I have been interested in the more insidious potential misunderstandings that can arise from words as seemingly simple as diet. There are many examples in dermatology, particularly in the way we prescribe topical therapy and use trade names.
Topical Therapy
Instructions for systemic medications may be as simple as “take 1 pill twice daily.” Prescriptions for topical medications can be written with an equally simple patient signature such as “apply twice daily to affected area,” but the simplicity is deceptive. The direction to “apply” may seem intuitive to the prescriber, but we do not always specify the amount. Sunscreen, for example, is notoriously underapplied when the actual amount of product needed for protection is not demonstrated.1 One study of new dermatology patients given a prescription for a new topical medication found that the majority of patients underdosed.2
Determination of an “affected area,” regardless of whether the site is indicated, can be even less straightforward. In acne treatment, the affected area is the whole region prone to acne breakouts, whereas in psoriasis it may be discrete psoriatic plaques. We may believe our explanations are perfectly clear, but we have all seen patients spot treating their acne or psoriasis patients covering entire territories of normal skin with topical steroids, despite our education. One study of eczema action plans found that there was considerable variability in the way different providers described disease flares that require treatment. For example, redness was only used as a descriptor of an eczema flare in 68.2% of eczema action plans studied.3 Ensuring our patients understand our criteria for skin requiring topical treatment may mean the difference between treatment success and failure and also may help to avoid unnecessary side effects.
Adherence to topical medication regimens is poor, and inadequate patient education is only one factor.4,5 One study found that more than one-third of new prescriptions for topical medications were never even filled.6 However, improving our communication about application of topical drugs is one way we must address the complicated issue of adherence.
Trade Names
In dermatology, we often use trade names to refer to our medications, even if we do not intend to reference the brand name of the drug specifically. We may tell a patient to use Lidex (Medicis Pharmaceutical Corporation) for her hands but then send an escript to her pharmacy for fluocinonide. Trade names are designed to roll off the tongue, in contrast to the unwieldy, clumsily long generic names assigned to many of our medications.
Substituting trade names may facilitate more natural conversation to promote patient understanding in some cases; however, there are pitfalls associated with this habit. First, we may be doing our patients a disservice if we do not clarify when it would be acceptable to substitute with the generic when the medication is available over-the-counter. If we decide to treat with Rogaine (Johnson & Johnson Consumer Inc) but do not suggest the option of purchasing the generic minoxidil, the patient could be unnecessarily overpaying for a brand name by following our instructions.
Conversely, there are scenarios in which the use of a brand name is actually not specific enough. A patient once told me she was using Differin (Galderma Laboratories, LP) as discussed at her prior visit, but she revealed she was washing it off after application. I initially assumed she misunderstood that adapalene was a gel to be applied and left on. After additional questioning, however, it became clear that she purchased the Differin gentle cleanser, a nonmedicated facial wash, rather than the retinoid we had intended for her. I had not considered that Differin would market an entire line of skin care products but now realize we must be cautious using Differin and adapalene interchangeably. Other examples include popular over-the-counter antihistamine brands such as Allegra (Chattem, a Sanofi company) or Benadryl (Johnson & Johnson Consumer Inc) that market multiple products with different active ingredients.
Final Thoughts
The smooth transfer of information between physician and patient is key to a healthy therapeutic relationship. In residency and throughout our careers, we will continue to develop and refine our communication skills to best serve our patients. We should pay particular attention to the unexpected and surprising ways in which we fail to adequately communicate, make note of these patterns, and share with our colleagues so that we can all learn from our collective experiences.
I was a third-year medical student, dutifully reviewing discharge instructions with a patient and her family. The patient’s adult daughter asked, “What about that diet you put her on?” As they looked at me quizzically, I looked back equally confused, until it clicked: We needed to talk about the word diet. In everyday conversation, diet generally is understood to mean restriction of food to lose weight, which is what the family hoped would be prescribed for their obese family member. I needed to tell them that I was sorry for the misunderstanding. If they overheard us “ordering a diet,” we simply meant providing trays of hospital food.
We become so familiar with the language of our profession that we do not remember it may be foreign to our patients. In dermatology, we are aware that our specialty is full of esoteric jargon and complex concepts that need to be carefully explained to our patients in simpler terms. But since that incident in medical school, I have been interested in the more insidious potential misunderstandings that can arise from words as seemingly simple as diet. There are many examples in dermatology, particularly in the way we prescribe topical therapy and use trade names.
Topical Therapy
Instructions for systemic medications may be as simple as “take 1 pill twice daily.” Prescriptions for topical medications can be written with an equally simple patient signature such as “apply twice daily to affected area,” but the simplicity is deceptive. The direction to “apply” may seem intuitive to the prescriber, but we do not always specify the amount. Sunscreen, for example, is notoriously underapplied when the actual amount of product needed for protection is not demonstrated.1 One study of new dermatology patients given a prescription for a new topical medication found that the majority of patients underdosed.2
Determination of an “affected area,” regardless of whether the site is indicated, can be even less straightforward. In acne treatment, the affected area is the whole region prone to acne breakouts, whereas in psoriasis it may be discrete psoriatic plaques. We may believe our explanations are perfectly clear, but we have all seen patients spot treating their acne or psoriasis patients covering entire territories of normal skin with topical steroids, despite our education. One study of eczema action plans found that there was considerable variability in the way different providers described disease flares that require treatment. For example, redness was only used as a descriptor of an eczema flare in 68.2% of eczema action plans studied.3 Ensuring our patients understand our criteria for skin requiring topical treatment may mean the difference between treatment success and failure and also may help to avoid unnecessary side effects.
Adherence to topical medication regimens is poor, and inadequate patient education is only one factor.4,5 One study found that more than one-third of new prescriptions for topical medications were never even filled.6 However, improving our communication about application of topical drugs is one way we must address the complicated issue of adherence.
Trade Names
In dermatology, we often use trade names to refer to our medications, even if we do not intend to reference the brand name of the drug specifically. We may tell a patient to use Lidex (Medicis Pharmaceutical Corporation) for her hands but then send an escript to her pharmacy for fluocinonide. Trade names are designed to roll off the tongue, in contrast to the unwieldy, clumsily long generic names assigned to many of our medications.
Substituting trade names may facilitate more natural conversation to promote patient understanding in some cases; however, there are pitfalls associated with this habit. First, we may be doing our patients a disservice if we do not clarify when it would be acceptable to substitute with the generic when the medication is available over-the-counter. If we decide to treat with Rogaine (Johnson & Johnson Consumer Inc) but do not suggest the option of purchasing the generic minoxidil, the patient could be unnecessarily overpaying for a brand name by following our instructions.
Conversely, there are scenarios in which the use of a brand name is actually not specific enough. A patient once told me she was using Differin (Galderma Laboratories, LP) as discussed at her prior visit, but she revealed she was washing it off after application. I initially assumed she misunderstood that adapalene was a gel to be applied and left on. After additional questioning, however, it became clear that she purchased the Differin gentle cleanser, a nonmedicated facial wash, rather than the retinoid we had intended for her. I had not considered that Differin would market an entire line of skin care products but now realize we must be cautious using Differin and adapalene interchangeably. Other examples include popular over-the-counter antihistamine brands such as Allegra (Chattem, a Sanofi company) or Benadryl (Johnson & Johnson Consumer Inc) that market multiple products with different active ingredients.
Final Thoughts
The smooth transfer of information between physician and patient is key to a healthy therapeutic relationship. In residency and throughout our careers, we will continue to develop and refine our communication skills to best serve our patients. We should pay particular attention to the unexpected and surprising ways in which we fail to adequately communicate, make note of these patterns, and share with our colleagues so that we can all learn from our collective experiences.
- Schneider, J. The teaspoon rule of applying sunscreen. Arch Dermatol. 2002;138:838-839.
- Storm A, Benfeldt E, Andersen SE, et al. A prospective study of patient adherence to topical treatments: 95% of patients underdose. J Am Acad Dermatol. 2008;59:975-980.
- Stringer T, Yin HS, Gittler J, et al. The readability, suitability, and content features of eczema action plans in the United States. Pediatr Dermatol. 2018;35:800-807.
- Hougeir FG, Cook-Bolden FE, Rodriguez D, et al. Critical considerations on optimizing topical corticosteroid therapy. J Clin Aesthet Dermatol. 2015;8(suppl 1):S2-S14.
- Savary J, Ortonne JP, Aractingi S. The right dose in the right place: an overview of current prescription, instruction and application modalities for topical psoriasis treatments. J Eur Acad Dermatol Venereol. 2005;19:14-17.
- Storm A, Anderson SE, Benfeldt E, et al. One in 3 prescriptions are never redeemed: primary nonadherence in an outpatient clinic. J Am Acad Dermatol. 2008;59:27-33.
- Schneider, J. The teaspoon rule of applying sunscreen. Arch Dermatol. 2002;138:838-839.
- Storm A, Benfeldt E, Andersen SE, et al. A prospective study of patient adherence to topical treatments: 95% of patients underdose. J Am Acad Dermatol. 2008;59:975-980.
- Stringer T, Yin HS, Gittler J, et al. The readability, suitability, and content features of eczema action plans in the United States. Pediatr Dermatol. 2018;35:800-807.
- Hougeir FG, Cook-Bolden FE, Rodriguez D, et al. Critical considerations on optimizing topical corticosteroid therapy. J Clin Aesthet Dermatol. 2015;8(suppl 1):S2-S14.
- Savary J, Ortonne JP, Aractingi S. The right dose in the right place: an overview of current prescription, instruction and application modalities for topical psoriasis treatments. J Eur Acad Dermatol Venereol. 2005;19:14-17.
- Storm A, Anderson SE, Benfeldt E, et al. One in 3 prescriptions are never redeemed: primary nonadherence in an outpatient clinic. J Am Acad Dermatol. 2008;59:27-33.
Resident Pearl
- It is not just the esoteric jargon and complex pathophysiologic concepts in dermatology that can challenge effective communication with our patients. We face potential for misunderstanding even in situations that may seem straightforward. Vigilance in avoiding ambiguity in all our exchanges with patients can help foster therapeutic relationships and optimize patient care.
A 60-year-old white woman presented with a 3-month history of a painful, nonhealing ulceration on her left lateral lower leg
It is a vasculopathy rather than a vasculitis as the former is caused by occlusion of blood vessels and the latter results from inflammation of the vessels. Middle-aged women tend to be affected more frequently. Although the exact cause is unclear, systemic diseases, such as hypercoagulable states, may predispose vessels to develop occlusion. Associated disorders include antiphospholipid syndrome, protein C deficiency, factor V mutation, arteriosclerosis, hyperhomocysteinemia, and hepatitis C.
Typically, lesions begin as painful purpura or reticulated macules on the lower extremities that ulcerate and heal very slowly. Ankles, particularly malleoli, are more frequently affected. When they heal, they form painless white stellate scars typical of atrophie blanche. Surrounding erythema, telangiectasias, and sclerosis may be present; livedo reticularis may be seen as well.
Histologically, the epidermis may be atrophic or necrotic. Hyaline thickening of the blood vessel walls is seen. Thrombi may be present. Direct immunofluorescence of perilesional skin may be positive for complement C3 and immunoglobulin (IgM) in dermal blood vessels.
Livedoid vasculopathy can be difficult to treat. Treatment is aimed at reducing clotting and improving blood flow and includes antiplatelet drugs (low-dose aspirin, dipyridamole), anticoagulants, and vasodilating agents (nifedipine). Pentoxifylline two or three times daily may help by altering blood viscosity. A recent literature search reports success in topical dapsone applied to lesions twice daily under occlusion. Leg elevation and compression stockings help healing. Livedoid vasculopathy may have periods of activity and remission.
The case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/edermatologynews.com. To submit a case for possible publication, send an email to dermnews@mdedge.com
It is a vasculopathy rather than a vasculitis as the former is caused by occlusion of blood vessels and the latter results from inflammation of the vessels. Middle-aged women tend to be affected more frequently. Although the exact cause is unclear, systemic diseases, such as hypercoagulable states, may predispose vessels to develop occlusion. Associated disorders include antiphospholipid syndrome, protein C deficiency, factor V mutation, arteriosclerosis, hyperhomocysteinemia, and hepatitis C.
Typically, lesions begin as painful purpura or reticulated macules on the lower extremities that ulcerate and heal very slowly. Ankles, particularly malleoli, are more frequently affected. When they heal, they form painless white stellate scars typical of atrophie blanche. Surrounding erythema, telangiectasias, and sclerosis may be present; livedo reticularis may be seen as well.
Histologically, the epidermis may be atrophic or necrotic. Hyaline thickening of the blood vessel walls is seen. Thrombi may be present. Direct immunofluorescence of perilesional skin may be positive for complement C3 and immunoglobulin (IgM) in dermal blood vessels.
Livedoid vasculopathy can be difficult to treat. Treatment is aimed at reducing clotting and improving blood flow and includes antiplatelet drugs (low-dose aspirin, dipyridamole), anticoagulants, and vasodilating agents (nifedipine). Pentoxifylline two or three times daily may help by altering blood viscosity. A recent literature search reports success in topical dapsone applied to lesions twice daily under occlusion. Leg elevation and compression stockings help healing. Livedoid vasculopathy may have periods of activity and remission.
The case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/edermatologynews.com. To submit a case for possible publication, send an email to dermnews@mdedge.com
It is a vasculopathy rather than a vasculitis as the former is caused by occlusion of blood vessels and the latter results from inflammation of the vessels. Middle-aged women tend to be affected more frequently. Although the exact cause is unclear, systemic diseases, such as hypercoagulable states, may predispose vessels to develop occlusion. Associated disorders include antiphospholipid syndrome, protein C deficiency, factor V mutation, arteriosclerosis, hyperhomocysteinemia, and hepatitis C.
Typically, lesions begin as painful purpura or reticulated macules on the lower extremities that ulcerate and heal very slowly. Ankles, particularly malleoli, are more frequently affected. When they heal, they form painless white stellate scars typical of atrophie blanche. Surrounding erythema, telangiectasias, and sclerosis may be present; livedo reticularis may be seen as well.
Histologically, the epidermis may be atrophic or necrotic. Hyaline thickening of the blood vessel walls is seen. Thrombi may be present. Direct immunofluorescence of perilesional skin may be positive for complement C3 and immunoglobulin (IgM) in dermal blood vessels.
Livedoid vasculopathy can be difficult to treat. Treatment is aimed at reducing clotting and improving blood flow and includes antiplatelet drugs (low-dose aspirin, dipyridamole), anticoagulants, and vasodilating agents (nifedipine). Pentoxifylline two or three times daily may help by altering blood viscosity. A recent literature search reports success in topical dapsone applied to lesions twice daily under occlusion. Leg elevation and compression stockings help healing. Livedoid vasculopathy may have periods of activity and remission.
The case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/edermatologynews.com. To submit a case for possible publication, send an email to dermnews@mdedge.com
USPSTF recommends counseling for perinatal depression prevention
according to a B recommendation from the U.S. Preventive Services Task Force.
The Task Force determined that counseling interventions are effective in preventing perinatal depression, defined as a major or minor depressive episode during pregnancy or within the first year after delivery. The condition affects an estimated 12% of new mothers in the United States each year, according to lead author Susan J. Curry, PhD, of the University of Iowa, Iowa City, and her colleagues.
The recommendation, published in JAMA, applies to “pregnant persons and persons who are less than 1 year postpartum who do not have a current diagnosis of depression but are at increased risk of developing depression,” according to the authors (JAMA. 2019 Feb 12;321(6):580-7).
Risk factors for development of perinatal depression include:
- Past history of depression.
- Current depressive symptoms (that do not reach a diagnostic threshold).
- History of physical or sexual abuse.
- Unplanned or unwanted pregnancy.
- Stressful life events.
- Lack of social and financial support.
- Intimate partner violence.
- Pregestational or gestational diabetes.
- Complications during pregnancy.
- Adolescent parenthood.
- Low socioeconomic status.
- Lack of social support.
After reviewing the evidence, the USPSTF found a moderate net benefit for counseling interventions, particularly cognitive behavioral therapy and interpersonal therapy, for preventing perinatal depression in women at risk. Counseling sessions reviewed for this recommendation ranged from 4 to 20 meetings (median, 8 meetings).
The USPSTF found inadequate evidence to assess the harms and benefits of other noncounseling interventions, including pharmacologic therapy.
In the evidence review accompanying the recommendations, Elizabeth A. O’Connor, PhD, of Kaiser Permanente, Portland, Ore., and her colleagues analyzed data from 50 studies including 22,385 individuals; 20 of these studies were randomized, controlled trials of counseling interventions (JAMA. 2019 Feb 12;321(6):588-601).
Overall, the likelihood of perinatal depression was significantly lower among women who received counseling, compared with controls, among more than 3,000 women in those studies (pooled risk ratio 0.61). Absolute risk differences for perinatal depression ranged from a 1% increased reduction in controls to a 32% increased reduction among women who received counseling. The effects were strongest for cognitive behavioral therapy and interpersonal therapy as interventions. No adverse events were reported in the counseling intervention studies.
In three studies of health system interventions, the researchers found a benefit for interventions vs. controls, but the difference was not statistically significant.
Trials of most other alternative interventions including infant sleep advice, birth-experience postpartum debriefing, omega-3 fatty acid supplementation, expressive writing, antidepressants, and yoga did not show statistical significance in benefit for reducing perinatal depression.
Only one of three randomized controlled trials of physical activity found a statistically significant group difference.
A trial of nortriptyline to prevent perinatal depression showed no benefit, compared with placebo. A sertraline study of found “a smaller percentage of participants taking sertraline had a depression recurrence, compared with those taking placebo,” the investigators wrote. In these two studies, women who took nortriptyline showed no adverse effects, and those in a trial involving sertraline reported significantly more dizziness and drowsiness compared with placebo patients.
The evidence review was limited by the small number of quality studies, especially studies of alternative interventions. More research is needed; however, the findings support data from similar reviews and support the potential for counseling to prevent perinatal depression, particularly in women at increased risk for perinatal depression, Dr. O’Connor and her associates said.
The USPSTF is supported by the Agency for Healthcare Research and Quality. Coauthor Dr. Michelle L. Henninger reported receiving grants from Pfizer IGLC (Independent Grants for Learning & Change) outside the submitted work. Coauthor Dr. Bradley N. Gaynes reported receiving personal fees from LivaNova and Johnson & Johnson outside the submitted work. The remaining researchers had no financial conflicts to disclose.
SOURCE: Curry SJ et al. JAMA. 2019;321(6):580-7; O’Connor E et al. JAMA. 2019;321(6):588-601.
A proactive approach to prevention and management of perinatal depression as recommended by the USPSTF can potentially improve outcomes for new mothers and their babies, Marlene P. Freeman, MD, wrote in an accompanying editorial. She identified three key challenges to implementing the USPSTF recommendations: identifying women at risk, connecting them to evidence-based treatment, and assessing outcomes after treatment.
The development of screening tools would help clinicians identify women at risk for perinatal depression, Dr. Freeman said. No such tool currently exists, but in the meantime, “women at risk may be identified by targeting those with histories of depression, subthreshold depressive symptoms, and certain sociodemographic factors (i.e., economically disadvantaged, single/young, unplanned pregnancy).”
The counseling interventions shown to be effective in preventing perinatal depression – cognitive behavioral therapy and interpersonal psychotherapy – require education and training outside the time limitations and expertise of many clinicians providing obstetric care, she noted.
“The delivery of effective care on a large scale will require creative solutions,” such as the use of telehealth and smartphone platforms, and the involvement of multidisciplinary teams to care for women with severe illness, Dr. Freeman said. “In addition, a substantial number of reproductive-aged women have serious psychiatric disorders and will be identified as at risk for perinatal depression, although their needs may be more comprehensive,” she wrote. “Women who are identified as at risk for perinatal depression may have psychotic disorders, bipolar spectrum disorders, anxiety disorders, and substance use disorders, and there is comorbidity among psychiatric disorders. Therefore, systematic provisions for referral and treatment for other psychiatric disorders should be considered.” Further research is needed to explore treatment options including pharmacotherapy for women with severe psychiatric disorders.
However, she expressed optimism that the recommendations for screening and counseling for perinatal depression are valuable, and they “may return great dividends in the form of enhanced well-being of mothers and their offspring.”
Dr. Freeman is affiliated with the department of psychiatry at Massachusetts General Hospital, Boston. She commented in an editorial accompanying the article by Curry et al. (JAMA. 2019 Feb 12;321[6]:550-2). Dr. Freeman disclosed relationships with companies including Takeda, JayMac, Sage, Otsuka, Alkermes, Janssen, and Sunovion; she also disclosed serving on an independent data safety and monitoring committee for Janssen (Johnson & Johnson); and editing the GOED (Global Organization for EPA & DHA Omega-3) newsletter.
A proactive approach to prevention and management of perinatal depression as recommended by the USPSTF can potentially improve outcomes for new mothers and their babies, Marlene P. Freeman, MD, wrote in an accompanying editorial. She identified three key challenges to implementing the USPSTF recommendations: identifying women at risk, connecting them to evidence-based treatment, and assessing outcomes after treatment.
The development of screening tools would help clinicians identify women at risk for perinatal depression, Dr. Freeman said. No such tool currently exists, but in the meantime, “women at risk may be identified by targeting those with histories of depression, subthreshold depressive symptoms, and certain sociodemographic factors (i.e., economically disadvantaged, single/young, unplanned pregnancy).”
The counseling interventions shown to be effective in preventing perinatal depression – cognitive behavioral therapy and interpersonal psychotherapy – require education and training outside the time limitations and expertise of many clinicians providing obstetric care, she noted.
“The delivery of effective care on a large scale will require creative solutions,” such as the use of telehealth and smartphone platforms, and the involvement of multidisciplinary teams to care for women with severe illness, Dr. Freeman said. “In addition, a substantial number of reproductive-aged women have serious psychiatric disorders and will be identified as at risk for perinatal depression, although their needs may be more comprehensive,” she wrote. “Women who are identified as at risk for perinatal depression may have psychotic disorders, bipolar spectrum disorders, anxiety disorders, and substance use disorders, and there is comorbidity among psychiatric disorders. Therefore, systematic provisions for referral and treatment for other psychiatric disorders should be considered.” Further research is needed to explore treatment options including pharmacotherapy for women with severe psychiatric disorders.
However, she expressed optimism that the recommendations for screening and counseling for perinatal depression are valuable, and they “may return great dividends in the form of enhanced well-being of mothers and their offspring.”
Dr. Freeman is affiliated with the department of psychiatry at Massachusetts General Hospital, Boston. She commented in an editorial accompanying the article by Curry et al. (JAMA. 2019 Feb 12;321[6]:550-2). Dr. Freeman disclosed relationships with companies including Takeda, JayMac, Sage, Otsuka, Alkermes, Janssen, and Sunovion; she also disclosed serving on an independent data safety and monitoring committee for Janssen (Johnson & Johnson); and editing the GOED (Global Organization for EPA & DHA Omega-3) newsletter.
A proactive approach to prevention and management of perinatal depression as recommended by the USPSTF can potentially improve outcomes for new mothers and their babies, Marlene P. Freeman, MD, wrote in an accompanying editorial. She identified three key challenges to implementing the USPSTF recommendations: identifying women at risk, connecting them to evidence-based treatment, and assessing outcomes after treatment.
The development of screening tools would help clinicians identify women at risk for perinatal depression, Dr. Freeman said. No such tool currently exists, but in the meantime, “women at risk may be identified by targeting those with histories of depression, subthreshold depressive symptoms, and certain sociodemographic factors (i.e., economically disadvantaged, single/young, unplanned pregnancy).”
The counseling interventions shown to be effective in preventing perinatal depression – cognitive behavioral therapy and interpersonal psychotherapy – require education and training outside the time limitations and expertise of many clinicians providing obstetric care, she noted.
“The delivery of effective care on a large scale will require creative solutions,” such as the use of telehealth and smartphone platforms, and the involvement of multidisciplinary teams to care for women with severe illness, Dr. Freeman said. “In addition, a substantial number of reproductive-aged women have serious psychiatric disorders and will be identified as at risk for perinatal depression, although their needs may be more comprehensive,” she wrote. “Women who are identified as at risk for perinatal depression may have psychotic disorders, bipolar spectrum disorders, anxiety disorders, and substance use disorders, and there is comorbidity among psychiatric disorders. Therefore, systematic provisions for referral and treatment for other psychiatric disorders should be considered.” Further research is needed to explore treatment options including pharmacotherapy for women with severe psychiatric disorders.
However, she expressed optimism that the recommendations for screening and counseling for perinatal depression are valuable, and they “may return great dividends in the form of enhanced well-being of mothers and their offspring.”
Dr. Freeman is affiliated with the department of psychiatry at Massachusetts General Hospital, Boston. She commented in an editorial accompanying the article by Curry et al. (JAMA. 2019 Feb 12;321[6]:550-2). Dr. Freeman disclosed relationships with companies including Takeda, JayMac, Sage, Otsuka, Alkermes, Janssen, and Sunovion; she also disclosed serving on an independent data safety and monitoring committee for Janssen (Johnson & Johnson); and editing the GOED (Global Organization for EPA & DHA Omega-3) newsletter.
according to a B recommendation from the U.S. Preventive Services Task Force.
The Task Force determined that counseling interventions are effective in preventing perinatal depression, defined as a major or minor depressive episode during pregnancy or within the first year after delivery. The condition affects an estimated 12% of new mothers in the United States each year, according to lead author Susan J. Curry, PhD, of the University of Iowa, Iowa City, and her colleagues.
The recommendation, published in JAMA, applies to “pregnant persons and persons who are less than 1 year postpartum who do not have a current diagnosis of depression but are at increased risk of developing depression,” according to the authors (JAMA. 2019 Feb 12;321(6):580-7).
Risk factors for development of perinatal depression include:
- Past history of depression.
- Current depressive symptoms (that do not reach a diagnostic threshold).
- History of physical or sexual abuse.
- Unplanned or unwanted pregnancy.
- Stressful life events.
- Lack of social and financial support.
- Intimate partner violence.
- Pregestational or gestational diabetes.
- Complications during pregnancy.
- Adolescent parenthood.
- Low socioeconomic status.
- Lack of social support.
After reviewing the evidence, the USPSTF found a moderate net benefit for counseling interventions, particularly cognitive behavioral therapy and interpersonal therapy, for preventing perinatal depression in women at risk. Counseling sessions reviewed for this recommendation ranged from 4 to 20 meetings (median, 8 meetings).
The USPSTF found inadequate evidence to assess the harms and benefits of other noncounseling interventions, including pharmacologic therapy.
In the evidence review accompanying the recommendations, Elizabeth A. O’Connor, PhD, of Kaiser Permanente, Portland, Ore., and her colleagues analyzed data from 50 studies including 22,385 individuals; 20 of these studies were randomized, controlled trials of counseling interventions (JAMA. 2019 Feb 12;321(6):588-601).
Overall, the likelihood of perinatal depression was significantly lower among women who received counseling, compared with controls, among more than 3,000 women in those studies (pooled risk ratio 0.61). Absolute risk differences for perinatal depression ranged from a 1% increased reduction in controls to a 32% increased reduction among women who received counseling. The effects were strongest for cognitive behavioral therapy and interpersonal therapy as interventions. No adverse events were reported in the counseling intervention studies.
In three studies of health system interventions, the researchers found a benefit for interventions vs. controls, but the difference was not statistically significant.
Trials of most other alternative interventions including infant sleep advice, birth-experience postpartum debriefing, omega-3 fatty acid supplementation, expressive writing, antidepressants, and yoga did not show statistical significance in benefit for reducing perinatal depression.
Only one of three randomized controlled trials of physical activity found a statistically significant group difference.
A trial of nortriptyline to prevent perinatal depression showed no benefit, compared with placebo. A sertraline study of found “a smaller percentage of participants taking sertraline had a depression recurrence, compared with those taking placebo,” the investigators wrote. In these two studies, women who took nortriptyline showed no adverse effects, and those in a trial involving sertraline reported significantly more dizziness and drowsiness compared with placebo patients.
The evidence review was limited by the small number of quality studies, especially studies of alternative interventions. More research is needed; however, the findings support data from similar reviews and support the potential for counseling to prevent perinatal depression, particularly in women at increased risk for perinatal depression, Dr. O’Connor and her associates said.
The USPSTF is supported by the Agency for Healthcare Research and Quality. Coauthor Dr. Michelle L. Henninger reported receiving grants from Pfizer IGLC (Independent Grants for Learning & Change) outside the submitted work. Coauthor Dr. Bradley N. Gaynes reported receiving personal fees from LivaNova and Johnson & Johnson outside the submitted work. The remaining researchers had no financial conflicts to disclose.
SOURCE: Curry SJ et al. JAMA. 2019;321(6):580-7; O’Connor E et al. JAMA. 2019;321(6):588-601.
according to a B recommendation from the U.S. Preventive Services Task Force.
The Task Force determined that counseling interventions are effective in preventing perinatal depression, defined as a major or minor depressive episode during pregnancy or within the first year after delivery. The condition affects an estimated 12% of new mothers in the United States each year, according to lead author Susan J. Curry, PhD, of the University of Iowa, Iowa City, and her colleagues.
The recommendation, published in JAMA, applies to “pregnant persons and persons who are less than 1 year postpartum who do not have a current diagnosis of depression but are at increased risk of developing depression,” according to the authors (JAMA. 2019 Feb 12;321(6):580-7).
Risk factors for development of perinatal depression include:
- Past history of depression.
- Current depressive symptoms (that do not reach a diagnostic threshold).
- History of physical or sexual abuse.
- Unplanned or unwanted pregnancy.
- Stressful life events.
- Lack of social and financial support.
- Intimate partner violence.
- Pregestational or gestational diabetes.
- Complications during pregnancy.
- Adolescent parenthood.
- Low socioeconomic status.
- Lack of social support.
After reviewing the evidence, the USPSTF found a moderate net benefit for counseling interventions, particularly cognitive behavioral therapy and interpersonal therapy, for preventing perinatal depression in women at risk. Counseling sessions reviewed for this recommendation ranged from 4 to 20 meetings (median, 8 meetings).
The USPSTF found inadequate evidence to assess the harms and benefits of other noncounseling interventions, including pharmacologic therapy.
In the evidence review accompanying the recommendations, Elizabeth A. O’Connor, PhD, of Kaiser Permanente, Portland, Ore., and her colleagues analyzed data from 50 studies including 22,385 individuals; 20 of these studies were randomized, controlled trials of counseling interventions (JAMA. 2019 Feb 12;321(6):588-601).
Overall, the likelihood of perinatal depression was significantly lower among women who received counseling, compared with controls, among more than 3,000 women in those studies (pooled risk ratio 0.61). Absolute risk differences for perinatal depression ranged from a 1% increased reduction in controls to a 32% increased reduction among women who received counseling. The effects were strongest for cognitive behavioral therapy and interpersonal therapy as interventions. No adverse events were reported in the counseling intervention studies.
In three studies of health system interventions, the researchers found a benefit for interventions vs. controls, but the difference was not statistically significant.
Trials of most other alternative interventions including infant sleep advice, birth-experience postpartum debriefing, omega-3 fatty acid supplementation, expressive writing, antidepressants, and yoga did not show statistical significance in benefit for reducing perinatal depression.
Only one of three randomized controlled trials of physical activity found a statistically significant group difference.
A trial of nortriptyline to prevent perinatal depression showed no benefit, compared with placebo. A sertraline study of found “a smaller percentage of participants taking sertraline had a depression recurrence, compared with those taking placebo,” the investigators wrote. In these two studies, women who took nortriptyline showed no adverse effects, and those in a trial involving sertraline reported significantly more dizziness and drowsiness compared with placebo patients.
The evidence review was limited by the small number of quality studies, especially studies of alternative interventions. More research is needed; however, the findings support data from similar reviews and support the potential for counseling to prevent perinatal depression, particularly in women at increased risk for perinatal depression, Dr. O’Connor and her associates said.
The USPSTF is supported by the Agency for Healthcare Research and Quality. Coauthor Dr. Michelle L. Henninger reported receiving grants from Pfizer IGLC (Independent Grants for Learning & Change) outside the submitted work. Coauthor Dr. Bradley N. Gaynes reported receiving personal fees from LivaNova and Johnson & Johnson outside the submitted work. The remaining researchers had no financial conflicts to disclose.
SOURCE: Curry SJ et al. JAMA. 2019;321(6):580-7; O’Connor E et al. JAMA. 2019;321(6):588-601.
FROM JAMA
Evaluation of Interventions by Clinical Pharmacy Specialists in Cardiology at a VA Ambulatory Cardiology Clinic
Integration of CPSs into an ambulatory cardiology clinic may translate to cost avoidance and a reduction in workload burden for other cardiology health care providers.
Health care providers face many challenges in utilizing cardiovascular therapies, such as anticipated shortages in physicians, patients with more complicated conditions, shifting medication regimens, management needs, and increased accountability for quality and performance measures.1 To meet the potential increase in service demand, cardiology practices are embracing cardiovascular team-based care.1 Advanced practice providers, such as advanced practice registered nurses (APRNs), physician assistants (PAs), and clinical pharmacy specialists (CPSs), have education, training, and experience to extend the team’s capability to meet these complex management needs.1
The role of CPSs within a cardiovascular care team includes providing a variety of patient-specific services, such as collaborating with other cardiology providers, to optimize evidence-based pharmacotherapy, preventing medication-related adverse events/errors, improving patient understanding of their medication regimen, and ultimately, improving patient outcomes.2 Health care systems, such as Kaiser Permanente of Colorado, have demonstrated improved clinical outcomes for patients with coronary artery disease (CAD) by implementing a multidisciplinary collaborative cardiac care service, including a clinical pharmacy cardiac risk service, in which CPSs assisted with management of cholesterol-lowering, hypertension, diabetes mellitus (DM), and smoking-cessation therapies, which resulted in a 76% to 89% reduction in all-cause mortality associated with CAD in multiple evaluations.3,4
Pharmacists providing medication therapy management (MTM) services in Minnesota had higher goal attainment for patients with hypertension and hyperlipidemia than did pharmacists who did not provide MTM services.5 MTM services provided by pharmacists led to an improvement in clinical outcomes for patients as well as a reduction in overall health care expenditures compared with that of a control group of patients who did not receive MTM services.5 Furthermore, CPS integration in the heart failure (HF) setting has led to improvements in utilization and optimization of guideline-directed medical therapies, an area in which recent data have suggested deficiencies exist.6-8 A full review of the outcomes associated with CPS involvement in cardiovascular care is beyond the scope of this article; but the recent review by Dunn and colleagues provides more detail.2
With the increasing number of patients with cardiovascular disease,expanding integration of CPSs in the cardiovascular team providing MTM services may reduce the burden of other providers (MD, PA, APRN, etc), thereby increasing access for not only new patients, but also diagnostic and interventional work, while potentially improving clinical and economic outcomes.2 The value of integrating CPSs as members of the cardiovascular care team is recognized in a variety of inpatient and ambulatory practice settings.2-6 However, data are limited on the number and types of interventions made per encounter as direct patient care providers. Expanded granularity regarding the effect of CPSs as active members of the cardiovascular team is an essential component to evaluate the potential benefit of CPS integration into direct patient care.
Methods
The West Palm Beach (WPB) Veteran Affairs Medical Center (VAMC) outpatient cardiology clinic consists of 6 full-time employee (FTE) cardiologists, 4 PAs or APRNs, 10 other cardiology health care staff members (registered/license practical nurses and technicians), and 2 cardiology CPSs providing direct patient care
The cardiology pharmacotherapy clinic is open 20.5 hours per week with 41 appointment slots (30 minutes each), of which 7 appointments are delivered via clinic video telehealth and 34 appointments are traditional face-to-face visits.9 The remaining CPS time is assigned to other clinical care and administrative areas to fit facility need, including oversight of the CPS-run 24-hour ambulatory blood pressure clinic, postgraduate year 2 cardiology pharmacy practice residency program directorship, and other administrative activities for the facility.10
The cardiology CPSs practice under an advanced scope of practice in which they independently manage medications (initiate, modify, discontinue), order diagnostic testing (laboratory, monitoring, imaging, etc) needed for medication management, and create monitoring and treatment plans for patients referred to the cardiology pharmacotherapy clinic by other cardiology providers. The diseases managed within the clinic vary based on patient-specific needs, but may include HF, dyslipidemia, hypertension, anticoagulation, CAD, arrhythmias, cardiovascular risk factor assessment and reduction, and medication reconciliation and teaching. Patients are referred for CPS management directly from facility cardiologist and cardiology clinic PAs and APRNs. Workload and interventions carried out are captured in the Pharmacists Achieve Results with Medications Demonstration (PhARMD) tool and patient care encounter tracking.9
Data Collection
Using local data from workload tracking, the number of CPS encounters was determined from July 6, 2015, to October 1, 2015. Data were collected on the types and volume of interventions made by CPSs in the cardiology pharmacotherapy clinic using the PhARMD tool (Figure).
The PhARMD tool was initially developed and implemented for CPSs in primary care pharmacotherapy clinics and was used to evaluate the types and volume of CPS interventions made in this setting.11 Since this initial evaluation, the tool has been updated, standardized nationally by the Department of Veterans Affairs (VA) Pharmacy Benefits Management Clinical Pharmacy Practice Office, and integrated across numerous VAMCs and associated outpatient clinics. The tool remains embedded within the VA electronic health record (EHR) and allows the capture of specific CPS interventions of several types (ie, both pharmacologic and nonpharmacologic interventions, including adjust dose or frequency; change or discontinue medication; initiate medication; monitor medication; counsel on adherence, contraindications, drug interactions, and drugs not indicated; reconcile medication; and prevent or manage adverse drug events [ADEs]) specific to certain diseases, such as anemia, anticoagulation, HF, type 2 DM (T2DM), hypertension, dyslipidemia, and tobacco cessation.
Given that the interventions captured by the PhARMD tool are based on self-report of the CPS performing the intervention, a quality assurance (QA) measure was taken to audit a random sample of interventions to validate the accuracy of reported data. A Pharmacy Benefits Management PhARMD Project QA report provided the 20% random sample of encounters for each cardiology CPS to be reviewed. This percentage was determined by VAMC Clinical Pharmacy Program Office (CPPO) directives on implementation of the PhARMD tool. During the QA period, the provided sample was reviewed to determine whether the intervention(s) recorded with the PhARMD tool matched the actions documented in the EHR. The QA review was done through a manual chart review by an author not involved in recording the original interventions. Both WPB VAMC cardiology CPSs passed the QA review (> 80% concurrence with tool logged and chart documented interventions as required by VA CPPO directive), with a 90.9% concurrence between the EHR and PhARMD tool documentation.
Statistical Analyses
Data on intervention type and encounter number were evaluated with descriptive statistics. The information was characterized and diagrammed with Excel (Microsoft, Redmond, WA) charts and graphs.
Cost-avoidance calculations were done using previously described methods and are included for exploratory analysis.11,12 Briefly, published estimates of cost avoidance associated with various interventions from the outpatient setting within a VAMC setting were applied as appropriate to the various interventions captured with the PhARMD tool.11,12 These estimates from Lee and colleagues were derived from detailed chart review of interventions made and the potential harm prevented.12 Costs or cost avoidances associated with interventions were calculated from pooled examination of 600 interventions in a VAMC with drug costs before and after the intervention, costs associated with harms prevented by the intervention, as well as the VAMC hourly pharmacist wages associated with making an intervention and processing and filling original vs recommended therapies.
The costs presented represent a “best-case” scenario in which all interventions made are expected to prevent patient harms. The costs related to avoided outcomes, facility overhead, and auxiliary staff cannot be included but highlight the many considerations that must be considered when examining potential cost-avoidance calculations. The estimates and methods at hand were chosen because, to our knowledge, no other consensus model exists that would be more appropriate for use in the situation and health care system at hand. Cost-avoidance estimates were calculated by extrapolating the 88-day study period values to a yearly estimate. All cost estimates were adjusted for inflation using the consumer price index calculator as per convention in previous analyses using the cost-avoidance estimates at hand.11-13
Results
From July 6, 2015, through October 1, 2015, 301 patient encounters occurred, and 529 interventions were documented with the PhARMD tool. The mean number of interventions per encounter was 1.8. Interventions were 65.2% pharmacologic and 34.8% nonpharmacologic. Of pharmacologic interventions, 27.1% were for HF, 12.7% for hypertension, 8.8% for dyslipidemia, 2.8% for anticoagulation, 1.4% for tobacco cessation, 1.1% for T2DM, 0.3% for anemia, and 45.8% for other conditions (Table 1).
The main types of pharmacologic interventions across all diseases were related to adjustments in medication dose or frequency (42.3%) and change or discontinuation of medications (20.0%).
Discussion
Evaluation of the interventions and encounters at the WPB VAMC ambulatory cardiology pharmacotherapy clinic suggests that CPSs are able to contribute to direct patient care independently of interventions performed by other cardiology providers. Specifically, 1.8 interventions per encounter were made by CPSs in this study. In a prior evaluation of CPS interventions recorded with the PhARMD tool in a VAMC primary care setting, 2.3 interventions per encounter were recorded.11 In comparing the present volume of interventions with the volume recorded in the study by Hough and colleagues, the difference in practice setting may account for differences seen.11
The primary care medication management setting would capture a broader array of clinical interventions than would the ambulatory cardiology clinic of the present study, so it is reasonable that more interventions would be captured per encounter in the primary care clinic. The difference in practice settings affecting the character of collected interventions can be seen because most interventions in this study at an ambulatory cardiology clinic were related to HF, whereas in Hough and colleagues 39.2% of the disease-specific interventions were related to DM, and only 2.9% were related to HF.11 The differences inherent in the intervention populations can also be seen by comparing the percentage of interventions related to hypertension and dyslipidemia: 30% and 28% in the study by Hough and colleagues compared with 13% and 9%, respectively, in the present study.11
Comparison of the present evaluation and Hough and colleagues is also hindered by the PhARMD tool used. The PhARMD tool used in the initial evaluation has been modified on a national level to improve the granularity of intervention data collected.
Our cost-avoidance estimate of $433,324.06 per year seems lower than that estimated in the previous evaluation when all applicable interventions were included.11 However, this study had several differences compared with those of previous VAMC studies looking at clinical interventions performed by CPSs. The main differences are the volume and setting in which interventions were being made. For example, in comparison with Hough and colleagues, the studies include different practice settings (primary care vs cardiology specialty clinic) and number of FTEs involved in the study (4.65 vs 1). If the cost avoidance is distributed evenly per FTE in the previous study, the following calculation is observed: $649,551.99 per FTE, which is closer to this study’s estimation. Given that primary care is a broader setting than is ambulatory cardiology, it is not surprising that more types of interventions and the overall volume/absolute number of interventions would be higher. Thus, the lower estimated cost avoidance in our study may be attributed to the lower volume of intervention opportunities availed to the cardiology CPS. Another difference is that detailed types of interventions related to hypertension, DM, dyslipidemia, and HF were not included in Hough and colleagues, whereas our study included all applicable interventions regardless of relation to diseases, which may account for a degree of the variation in intervention breakdown between the 2 studies.11 However, as noted previously, some interventions for these particular diseases may not fully capture the rationale for pharmacotherapy interventions, such as drug dose changes or discontinuations, which may misrepresent the potential cost avoidance associated with them in reality.
Limitations
Of general importance, the PhARMD tool may underestimate the number of interventions made such that multiple interventions for a medical condition may have been completed but only captured as 1 intervention, which may represent a limitation of the tool when multiple interventions are made for the same disease (eg, titration of both β-blocker and angiotensin-converting enzyme inhibitor doses at a single appointment in a patient with HF with reduced left ventricular ejection fraction). Improved clarity about interventions made would require laborious chart review, which was not feasible. The evaluation at hand included a preliminary QA review, adding confidence that overdocumentation was not being done and the values represented at worst an underestimation of actual CPS intervention impact. Because this study was an initial evaluation of interventions made by CPSs in an ambulatory cardiology pharmacotherapy setting, whether these same outcomes would exist in other patient cohorts is unclear. However, these data do provide a foundational understanding of what may be expected from CPS integration into a cardiovascular care team.
These findings may be limited in generalizability to other health care systems and situations in which CPSs are afforded the regulatory opportunity to practice independently within an established scope of practice or collaborative practice agreements. The Veterans Health Administration system has been a leader in integrating CPSs into direct patient care roles and serves as a potential model for application by other groups. This evaluation’s data support continued efforts to create such independent practice environments as they allow for qualified CPSs to practice to their full clinical potential and have the fullest possible effect on cardiovascular outcomes.
Previous studies looking at cost savings in MTM programs have established a substantial return in economic investment with patients being managed by pharmacists.5,14 Given that the interventions made in this study were not tied to attainment of clinical outcomes, a limitation to our study, the cost-avoidance estimates should be interpreted cautiously. However, we know of no such tool that is available to allow accurate capture of clinical event reduction in a single center with consistent CPS involvement in care. A clear opportunity exists regarding design of a model that measures clinical, economic, and humanistic outcomes related to the interventions performed by cardiology CPSs, but developing and deploying such a model may be challenging because guideline-directed medical therapies vary significantly based on many patient-specific issues, and identifying optimal or truly optimized medical therapy is at times a subjective task, especially in a single center. Using the types and volumes of interventions made by CPSs as a surrogate for these higher-level outcomes is still of value in order to understand the effect and role of CPSs in cardiovascular care. At present, the cost-avoidance estimates presented in this evaluation are based on the most appropriate system-specific data at hand, with the realization that actual cost avoidance in practice may vary widely and should be the topic of future research.
Conclusion
As cardiovascular team-based care continues to expand with the support of large organizations, such as the American College of Cardiology Foundation, Heart Failure Society of America, and American College of Clinical Pharmacy Cardiology Practice and Research Network, the need for understanding the effect of CPSs on patient care measures and health care costs becomes more pronounced.2,15 The results of this study demonstrate how integration of CPSs in an ambulatory cardiology clinic may translate to cost avoidance and a reduction in workload burden for cardiology physicians and providers, allowing more availability for diagnostic testing and care.
Interventions made by CPSs functioning as independent providers
1. Brush JE Jr, Handberg EM, Biga C, et al. 2015 ACC health policy statement on cardiovascular team-based care and the role of advanced practice providers. J Am Coll Cardiol. 2015;65(19):2118-2136.
2. Dunn SP, Birtcher KK, Beavers CJ, et al. The role of the clinical pharmacist in the care of patients with cardiovascular disease. J Am Coll Cardiol. 2015;66(19):2129-2139.
3. Sandoff BG, Kuca S, Rasmussen J, Merenich JA. Collaborative cardiac care service: a multidisciplinary approach to caring for patients with coronary artery disease. Perm J. 2008;12(3):4-11.
4. Merenich JA, Olson KL, Delate T, Rasmussen J, Helling DK, Ward DG; Clinical Pharmacy Cardiac Risk Service Study Group. Mortality reduction benefits of a comprehensive cardiac care program for patients with occlusive coronary disease. Pharmacotherapy. 2007;27(10):1370-1378.
5. Isetts BJ, Schondelmeyer SW, Artz MB, et al. Clinical and economic outcomes of medication therapy management services: the Minnesota experience.
6. Martinez AS, Saef J, Paszcuzuk A, Bhatt-Chugani H. Implementation of a pharmacist-managed heart failure medication titration clinic. Am J Health Syst Pharm. 2013;70(12):1070-1076.
7. Roth GA, Poole JE, Zaha R, Zhou W, Skinner J, Morden NE. Use of guideline-directed medications for heart failure before cardioverter-defibrillator implantation. J Am Coll Cardiol. 2016;67(9):1062-1069.
8. Noschese LA, Bergman CL, Brar CK, Kansal MM. The pharmacist’s role in medication optimization for patients with chronic heart failure. Fed Pract. 2017;34(suppl 10):S10-S15.
9. Coakley C, Hough A, Dwyer D, Parra D. Clinical video telehealth in a cardiology pharmacotherapy clinic. Am J Health Syst Pharm. 2013;70(22):1974-1975.
10. Khazan E, Anastasia E, Hough A, Parra D. Pharmacist-managed ambulatory blood pressure monitoring service. Am J Health Syst Pharm. 2017;74(4):190-195.
11. Hough A, Vartan CM, Groppi JA, Reyes S, Beckey NP. Evaluation of clinical pharmacy interventions in a Veterans Affairs medical center primary care clinic. Am J Health Syst Pharm. 2013;70(13):1168-1172.
12. Lee AJ, Boro MS, Knapp KK, Meier JL, Korman NE. Clinical and economic outcomes of pharmacist recommendations in a Veterans Affairs medical center. Am J Health Syst Pharm. 2002;59(21):2070-2077.
13. US Department of Labor. CPI inflation calculator. www.bls.gov/data/inflation_calculator.htm. Accessed January 18, 2019.
14. Perez A, Doloresco F, Hoffman JM, et al. Economic evaluations of clinical pharmacy services: 2001-2005. Pharmacotherapy. 2008;29(1):128.
15. Milfred-LaForest SK, Chow SL, DiDomenico RJ, et al. Clinical pharmacy services in heart failure: an opinion paper from the Heart Failure Society of America and American College of Clinical Pharmacy Cardiology Practice and Research Network. Pharmacotherapy. 2013;33(5):529-548.
Integration of CPSs into an ambulatory cardiology clinic may translate to cost avoidance and a reduction in workload burden for other cardiology health care providers.
Integration of CPSs into an ambulatory cardiology clinic may translate to cost avoidance and a reduction in workload burden for other cardiology health care providers.
Health care providers face many challenges in utilizing cardiovascular therapies, such as anticipated shortages in physicians, patients with more complicated conditions, shifting medication regimens, management needs, and increased accountability for quality and performance measures.1 To meet the potential increase in service demand, cardiology practices are embracing cardiovascular team-based care.1 Advanced practice providers, such as advanced practice registered nurses (APRNs), physician assistants (PAs), and clinical pharmacy specialists (CPSs), have education, training, and experience to extend the team’s capability to meet these complex management needs.1
The role of CPSs within a cardiovascular care team includes providing a variety of patient-specific services, such as collaborating with other cardiology providers, to optimize evidence-based pharmacotherapy, preventing medication-related adverse events/errors, improving patient understanding of their medication regimen, and ultimately, improving patient outcomes.2 Health care systems, such as Kaiser Permanente of Colorado, have demonstrated improved clinical outcomes for patients with coronary artery disease (CAD) by implementing a multidisciplinary collaborative cardiac care service, including a clinical pharmacy cardiac risk service, in which CPSs assisted with management of cholesterol-lowering, hypertension, diabetes mellitus (DM), and smoking-cessation therapies, which resulted in a 76% to 89% reduction in all-cause mortality associated with CAD in multiple evaluations.3,4
Pharmacists providing medication therapy management (MTM) services in Minnesota had higher goal attainment for patients with hypertension and hyperlipidemia than did pharmacists who did not provide MTM services.5 MTM services provided by pharmacists led to an improvement in clinical outcomes for patients as well as a reduction in overall health care expenditures compared with that of a control group of patients who did not receive MTM services.5 Furthermore, CPS integration in the heart failure (HF) setting has led to improvements in utilization and optimization of guideline-directed medical therapies, an area in which recent data have suggested deficiencies exist.6-8 A full review of the outcomes associated with CPS involvement in cardiovascular care is beyond the scope of this article; but the recent review by Dunn and colleagues provides more detail.2
With the increasing number of patients with cardiovascular disease,expanding integration of CPSs in the cardiovascular team providing MTM services may reduce the burden of other providers (MD, PA, APRN, etc), thereby increasing access for not only new patients, but also diagnostic and interventional work, while potentially improving clinical and economic outcomes.2 The value of integrating CPSs as members of the cardiovascular care team is recognized in a variety of inpatient and ambulatory practice settings.2-6 However, data are limited on the number and types of interventions made per encounter as direct patient care providers. Expanded granularity regarding the effect of CPSs as active members of the cardiovascular team is an essential component to evaluate the potential benefit of CPS integration into direct patient care.
Methods
The West Palm Beach (WPB) Veteran Affairs Medical Center (VAMC) outpatient cardiology clinic consists of 6 full-time employee (FTE) cardiologists, 4 PAs or APRNs, 10 other cardiology health care staff members (registered/license practical nurses and technicians), and 2 cardiology CPSs providing direct patient care
The cardiology pharmacotherapy clinic is open 20.5 hours per week with 41 appointment slots (30 minutes each), of which 7 appointments are delivered via clinic video telehealth and 34 appointments are traditional face-to-face visits.9 The remaining CPS time is assigned to other clinical care and administrative areas to fit facility need, including oversight of the CPS-run 24-hour ambulatory blood pressure clinic, postgraduate year 2 cardiology pharmacy practice residency program directorship, and other administrative activities for the facility.10
The cardiology CPSs practice under an advanced scope of practice in which they independently manage medications (initiate, modify, discontinue), order diagnostic testing (laboratory, monitoring, imaging, etc) needed for medication management, and create monitoring and treatment plans for patients referred to the cardiology pharmacotherapy clinic by other cardiology providers. The diseases managed within the clinic vary based on patient-specific needs, but may include HF, dyslipidemia, hypertension, anticoagulation, CAD, arrhythmias, cardiovascular risk factor assessment and reduction, and medication reconciliation and teaching. Patients are referred for CPS management directly from facility cardiologist and cardiology clinic PAs and APRNs. Workload and interventions carried out are captured in the Pharmacists Achieve Results with Medications Demonstration (PhARMD) tool and patient care encounter tracking.9
Data Collection
Using local data from workload tracking, the number of CPS encounters was determined from July 6, 2015, to October 1, 2015. Data were collected on the types and volume of interventions made by CPSs in the cardiology pharmacotherapy clinic using the PhARMD tool (Figure).
The PhARMD tool was initially developed and implemented for CPSs in primary care pharmacotherapy clinics and was used to evaluate the types and volume of CPS interventions made in this setting.11 Since this initial evaluation, the tool has been updated, standardized nationally by the Department of Veterans Affairs (VA) Pharmacy Benefits Management Clinical Pharmacy Practice Office, and integrated across numerous VAMCs and associated outpatient clinics. The tool remains embedded within the VA electronic health record (EHR) and allows the capture of specific CPS interventions of several types (ie, both pharmacologic and nonpharmacologic interventions, including adjust dose or frequency; change or discontinue medication; initiate medication; monitor medication; counsel on adherence, contraindications, drug interactions, and drugs not indicated; reconcile medication; and prevent or manage adverse drug events [ADEs]) specific to certain diseases, such as anemia, anticoagulation, HF, type 2 DM (T2DM), hypertension, dyslipidemia, and tobacco cessation.
Given that the interventions captured by the PhARMD tool are based on self-report of the CPS performing the intervention, a quality assurance (QA) measure was taken to audit a random sample of interventions to validate the accuracy of reported data. A Pharmacy Benefits Management PhARMD Project QA report provided the 20% random sample of encounters for each cardiology CPS to be reviewed. This percentage was determined by VAMC Clinical Pharmacy Program Office (CPPO) directives on implementation of the PhARMD tool. During the QA period, the provided sample was reviewed to determine whether the intervention(s) recorded with the PhARMD tool matched the actions documented in the EHR. The QA review was done through a manual chart review by an author not involved in recording the original interventions. Both WPB VAMC cardiology CPSs passed the QA review (> 80% concurrence with tool logged and chart documented interventions as required by VA CPPO directive), with a 90.9% concurrence between the EHR and PhARMD tool documentation.
Statistical Analyses
Data on intervention type and encounter number were evaluated with descriptive statistics. The information was characterized and diagrammed with Excel (Microsoft, Redmond, WA) charts and graphs.
Cost-avoidance calculations were done using previously described methods and are included for exploratory analysis.11,12 Briefly, published estimates of cost avoidance associated with various interventions from the outpatient setting within a VAMC setting were applied as appropriate to the various interventions captured with the PhARMD tool.11,12 These estimates from Lee and colleagues were derived from detailed chart review of interventions made and the potential harm prevented.12 Costs or cost avoidances associated with interventions were calculated from pooled examination of 600 interventions in a VAMC with drug costs before and after the intervention, costs associated with harms prevented by the intervention, as well as the VAMC hourly pharmacist wages associated with making an intervention and processing and filling original vs recommended therapies.
The costs presented represent a “best-case” scenario in which all interventions made are expected to prevent patient harms. The costs related to avoided outcomes, facility overhead, and auxiliary staff cannot be included but highlight the many considerations that must be considered when examining potential cost-avoidance calculations. The estimates and methods at hand were chosen because, to our knowledge, no other consensus model exists that would be more appropriate for use in the situation and health care system at hand. Cost-avoidance estimates were calculated by extrapolating the 88-day study period values to a yearly estimate. All cost estimates were adjusted for inflation using the consumer price index calculator as per convention in previous analyses using the cost-avoidance estimates at hand.11-13
Results
From July 6, 2015, through October 1, 2015, 301 patient encounters occurred, and 529 interventions were documented with the PhARMD tool. The mean number of interventions per encounter was 1.8. Interventions were 65.2% pharmacologic and 34.8% nonpharmacologic. Of pharmacologic interventions, 27.1% were for HF, 12.7% for hypertension, 8.8% for dyslipidemia, 2.8% for anticoagulation, 1.4% for tobacco cessation, 1.1% for T2DM, 0.3% for anemia, and 45.8% for other conditions (Table 1).
The main types of pharmacologic interventions across all diseases were related to adjustments in medication dose or frequency (42.3%) and change or discontinuation of medications (20.0%).
Discussion
Evaluation of the interventions and encounters at the WPB VAMC ambulatory cardiology pharmacotherapy clinic suggests that CPSs are able to contribute to direct patient care independently of interventions performed by other cardiology providers. Specifically, 1.8 interventions per encounter were made by CPSs in this study. In a prior evaluation of CPS interventions recorded with the PhARMD tool in a VAMC primary care setting, 2.3 interventions per encounter were recorded.11 In comparing the present volume of interventions with the volume recorded in the study by Hough and colleagues, the difference in practice setting may account for differences seen.11
The primary care medication management setting would capture a broader array of clinical interventions than would the ambulatory cardiology clinic of the present study, so it is reasonable that more interventions would be captured per encounter in the primary care clinic. The difference in practice settings affecting the character of collected interventions can be seen because most interventions in this study at an ambulatory cardiology clinic were related to HF, whereas in Hough and colleagues 39.2% of the disease-specific interventions were related to DM, and only 2.9% were related to HF.11 The differences inherent in the intervention populations can also be seen by comparing the percentage of interventions related to hypertension and dyslipidemia: 30% and 28% in the study by Hough and colleagues compared with 13% and 9%, respectively, in the present study.11
Comparison of the present evaluation and Hough and colleagues is also hindered by the PhARMD tool used. The PhARMD tool used in the initial evaluation has been modified on a national level to improve the granularity of intervention data collected.
Our cost-avoidance estimate of $433,324.06 per year seems lower than that estimated in the previous evaluation when all applicable interventions were included.11 However, this study had several differences compared with those of previous VAMC studies looking at clinical interventions performed by CPSs. The main differences are the volume and setting in which interventions were being made. For example, in comparison with Hough and colleagues, the studies include different practice settings (primary care vs cardiology specialty clinic) and number of FTEs involved in the study (4.65 vs 1). If the cost avoidance is distributed evenly per FTE in the previous study, the following calculation is observed: $649,551.99 per FTE, which is closer to this study’s estimation. Given that primary care is a broader setting than is ambulatory cardiology, it is not surprising that more types of interventions and the overall volume/absolute number of interventions would be higher. Thus, the lower estimated cost avoidance in our study may be attributed to the lower volume of intervention opportunities availed to the cardiology CPS. Another difference is that detailed types of interventions related to hypertension, DM, dyslipidemia, and HF were not included in Hough and colleagues, whereas our study included all applicable interventions regardless of relation to diseases, which may account for a degree of the variation in intervention breakdown between the 2 studies.11 However, as noted previously, some interventions for these particular diseases may not fully capture the rationale for pharmacotherapy interventions, such as drug dose changes or discontinuations, which may misrepresent the potential cost avoidance associated with them in reality.
Limitations
Of general importance, the PhARMD tool may underestimate the number of interventions made such that multiple interventions for a medical condition may have been completed but only captured as 1 intervention, which may represent a limitation of the tool when multiple interventions are made for the same disease (eg, titration of both β-blocker and angiotensin-converting enzyme inhibitor doses at a single appointment in a patient with HF with reduced left ventricular ejection fraction). Improved clarity about interventions made would require laborious chart review, which was not feasible. The evaluation at hand included a preliminary QA review, adding confidence that overdocumentation was not being done and the values represented at worst an underestimation of actual CPS intervention impact. Because this study was an initial evaluation of interventions made by CPSs in an ambulatory cardiology pharmacotherapy setting, whether these same outcomes would exist in other patient cohorts is unclear. However, these data do provide a foundational understanding of what may be expected from CPS integration into a cardiovascular care team.
These findings may be limited in generalizability to other health care systems and situations in which CPSs are afforded the regulatory opportunity to practice independently within an established scope of practice or collaborative practice agreements. The Veterans Health Administration system has been a leader in integrating CPSs into direct patient care roles and serves as a potential model for application by other groups. This evaluation’s data support continued efforts to create such independent practice environments as they allow for qualified CPSs to practice to their full clinical potential and have the fullest possible effect on cardiovascular outcomes.
Previous studies looking at cost savings in MTM programs have established a substantial return in economic investment with patients being managed by pharmacists.5,14 Given that the interventions made in this study were not tied to attainment of clinical outcomes, a limitation to our study, the cost-avoidance estimates should be interpreted cautiously. However, we know of no such tool that is available to allow accurate capture of clinical event reduction in a single center with consistent CPS involvement in care. A clear opportunity exists regarding design of a model that measures clinical, economic, and humanistic outcomes related to the interventions performed by cardiology CPSs, but developing and deploying such a model may be challenging because guideline-directed medical therapies vary significantly based on many patient-specific issues, and identifying optimal or truly optimized medical therapy is at times a subjective task, especially in a single center. Using the types and volumes of interventions made by CPSs as a surrogate for these higher-level outcomes is still of value in order to understand the effect and role of CPSs in cardiovascular care. At present, the cost-avoidance estimates presented in this evaluation are based on the most appropriate system-specific data at hand, with the realization that actual cost avoidance in practice may vary widely and should be the topic of future research.
Conclusion
As cardiovascular team-based care continues to expand with the support of large organizations, such as the American College of Cardiology Foundation, Heart Failure Society of America, and American College of Clinical Pharmacy Cardiology Practice and Research Network, the need for understanding the effect of CPSs on patient care measures and health care costs becomes more pronounced.2,15 The results of this study demonstrate how integration of CPSs in an ambulatory cardiology clinic may translate to cost avoidance and a reduction in workload burden for cardiology physicians and providers, allowing more availability for diagnostic testing and care.
Interventions made by CPSs functioning as independent providers
Health care providers face many challenges in utilizing cardiovascular therapies, such as anticipated shortages in physicians, patients with more complicated conditions, shifting medication regimens, management needs, and increased accountability for quality and performance measures.1 To meet the potential increase in service demand, cardiology practices are embracing cardiovascular team-based care.1 Advanced practice providers, such as advanced practice registered nurses (APRNs), physician assistants (PAs), and clinical pharmacy specialists (CPSs), have education, training, and experience to extend the team’s capability to meet these complex management needs.1
The role of CPSs within a cardiovascular care team includes providing a variety of patient-specific services, such as collaborating with other cardiology providers, to optimize evidence-based pharmacotherapy, preventing medication-related adverse events/errors, improving patient understanding of their medication regimen, and ultimately, improving patient outcomes.2 Health care systems, such as Kaiser Permanente of Colorado, have demonstrated improved clinical outcomes for patients with coronary artery disease (CAD) by implementing a multidisciplinary collaborative cardiac care service, including a clinical pharmacy cardiac risk service, in which CPSs assisted with management of cholesterol-lowering, hypertension, diabetes mellitus (DM), and smoking-cessation therapies, which resulted in a 76% to 89% reduction in all-cause mortality associated with CAD in multiple evaluations.3,4
Pharmacists providing medication therapy management (MTM) services in Minnesota had higher goal attainment for patients with hypertension and hyperlipidemia than did pharmacists who did not provide MTM services.5 MTM services provided by pharmacists led to an improvement in clinical outcomes for patients as well as a reduction in overall health care expenditures compared with that of a control group of patients who did not receive MTM services.5 Furthermore, CPS integration in the heart failure (HF) setting has led to improvements in utilization and optimization of guideline-directed medical therapies, an area in which recent data have suggested deficiencies exist.6-8 A full review of the outcomes associated with CPS involvement in cardiovascular care is beyond the scope of this article; but the recent review by Dunn and colleagues provides more detail.2
With the increasing number of patients with cardiovascular disease,expanding integration of CPSs in the cardiovascular team providing MTM services may reduce the burden of other providers (MD, PA, APRN, etc), thereby increasing access for not only new patients, but also diagnostic and interventional work, while potentially improving clinical and economic outcomes.2 The value of integrating CPSs as members of the cardiovascular care team is recognized in a variety of inpatient and ambulatory practice settings.2-6 However, data are limited on the number and types of interventions made per encounter as direct patient care providers. Expanded granularity regarding the effect of CPSs as active members of the cardiovascular team is an essential component to evaluate the potential benefit of CPS integration into direct patient care.
Methods
The West Palm Beach (WPB) Veteran Affairs Medical Center (VAMC) outpatient cardiology clinic consists of 6 full-time employee (FTE) cardiologists, 4 PAs or APRNs, 10 other cardiology health care staff members (registered/license practical nurses and technicians), and 2 cardiology CPSs providing direct patient care
The cardiology pharmacotherapy clinic is open 20.5 hours per week with 41 appointment slots (30 minutes each), of which 7 appointments are delivered via clinic video telehealth and 34 appointments are traditional face-to-face visits.9 The remaining CPS time is assigned to other clinical care and administrative areas to fit facility need, including oversight of the CPS-run 24-hour ambulatory blood pressure clinic, postgraduate year 2 cardiology pharmacy practice residency program directorship, and other administrative activities for the facility.10
The cardiology CPSs practice under an advanced scope of practice in which they independently manage medications (initiate, modify, discontinue), order diagnostic testing (laboratory, monitoring, imaging, etc) needed for medication management, and create monitoring and treatment plans for patients referred to the cardiology pharmacotherapy clinic by other cardiology providers. The diseases managed within the clinic vary based on patient-specific needs, but may include HF, dyslipidemia, hypertension, anticoagulation, CAD, arrhythmias, cardiovascular risk factor assessment and reduction, and medication reconciliation and teaching. Patients are referred for CPS management directly from facility cardiologist and cardiology clinic PAs and APRNs. Workload and interventions carried out are captured in the Pharmacists Achieve Results with Medications Demonstration (PhARMD) tool and patient care encounter tracking.9
Data Collection
Using local data from workload tracking, the number of CPS encounters was determined from July 6, 2015, to October 1, 2015. Data were collected on the types and volume of interventions made by CPSs in the cardiology pharmacotherapy clinic using the PhARMD tool (Figure).
The PhARMD tool was initially developed and implemented for CPSs in primary care pharmacotherapy clinics and was used to evaluate the types and volume of CPS interventions made in this setting.11 Since this initial evaluation, the tool has been updated, standardized nationally by the Department of Veterans Affairs (VA) Pharmacy Benefits Management Clinical Pharmacy Practice Office, and integrated across numerous VAMCs and associated outpatient clinics. The tool remains embedded within the VA electronic health record (EHR) and allows the capture of specific CPS interventions of several types (ie, both pharmacologic and nonpharmacologic interventions, including adjust dose or frequency; change or discontinue medication; initiate medication; monitor medication; counsel on adherence, contraindications, drug interactions, and drugs not indicated; reconcile medication; and prevent or manage adverse drug events [ADEs]) specific to certain diseases, such as anemia, anticoagulation, HF, type 2 DM (T2DM), hypertension, dyslipidemia, and tobacco cessation.
Given that the interventions captured by the PhARMD tool are based on self-report of the CPS performing the intervention, a quality assurance (QA) measure was taken to audit a random sample of interventions to validate the accuracy of reported data. A Pharmacy Benefits Management PhARMD Project QA report provided the 20% random sample of encounters for each cardiology CPS to be reviewed. This percentage was determined by VAMC Clinical Pharmacy Program Office (CPPO) directives on implementation of the PhARMD tool. During the QA period, the provided sample was reviewed to determine whether the intervention(s) recorded with the PhARMD tool matched the actions documented in the EHR. The QA review was done through a manual chart review by an author not involved in recording the original interventions. Both WPB VAMC cardiology CPSs passed the QA review (> 80% concurrence with tool logged and chart documented interventions as required by VA CPPO directive), with a 90.9% concurrence between the EHR and PhARMD tool documentation.
Statistical Analyses
Data on intervention type and encounter number were evaluated with descriptive statistics. The information was characterized and diagrammed with Excel (Microsoft, Redmond, WA) charts and graphs.
Cost-avoidance calculations were done using previously described methods and are included for exploratory analysis.11,12 Briefly, published estimates of cost avoidance associated with various interventions from the outpatient setting within a VAMC setting were applied as appropriate to the various interventions captured with the PhARMD tool.11,12 These estimates from Lee and colleagues were derived from detailed chart review of interventions made and the potential harm prevented.12 Costs or cost avoidances associated with interventions were calculated from pooled examination of 600 interventions in a VAMC with drug costs before and after the intervention, costs associated with harms prevented by the intervention, as well as the VAMC hourly pharmacist wages associated with making an intervention and processing and filling original vs recommended therapies.
The costs presented represent a “best-case” scenario in which all interventions made are expected to prevent patient harms. The costs related to avoided outcomes, facility overhead, and auxiliary staff cannot be included but highlight the many considerations that must be considered when examining potential cost-avoidance calculations. The estimates and methods at hand were chosen because, to our knowledge, no other consensus model exists that would be more appropriate for use in the situation and health care system at hand. Cost-avoidance estimates were calculated by extrapolating the 88-day study period values to a yearly estimate. All cost estimates were adjusted for inflation using the consumer price index calculator as per convention in previous analyses using the cost-avoidance estimates at hand.11-13
Results
From July 6, 2015, through October 1, 2015, 301 patient encounters occurred, and 529 interventions were documented with the PhARMD tool. The mean number of interventions per encounter was 1.8. Interventions were 65.2% pharmacologic and 34.8% nonpharmacologic. Of pharmacologic interventions, 27.1% were for HF, 12.7% for hypertension, 8.8% for dyslipidemia, 2.8% for anticoagulation, 1.4% for tobacco cessation, 1.1% for T2DM, 0.3% for anemia, and 45.8% for other conditions (Table 1).
The main types of pharmacologic interventions across all diseases were related to adjustments in medication dose or frequency (42.3%) and change or discontinuation of medications (20.0%).
Discussion
Evaluation of the interventions and encounters at the WPB VAMC ambulatory cardiology pharmacotherapy clinic suggests that CPSs are able to contribute to direct patient care independently of interventions performed by other cardiology providers. Specifically, 1.8 interventions per encounter were made by CPSs in this study. In a prior evaluation of CPS interventions recorded with the PhARMD tool in a VAMC primary care setting, 2.3 interventions per encounter were recorded.11 In comparing the present volume of interventions with the volume recorded in the study by Hough and colleagues, the difference in practice setting may account for differences seen.11
The primary care medication management setting would capture a broader array of clinical interventions than would the ambulatory cardiology clinic of the present study, so it is reasonable that more interventions would be captured per encounter in the primary care clinic. The difference in practice settings affecting the character of collected interventions can be seen because most interventions in this study at an ambulatory cardiology clinic were related to HF, whereas in Hough and colleagues 39.2% of the disease-specific interventions were related to DM, and only 2.9% were related to HF.11 The differences inherent in the intervention populations can also be seen by comparing the percentage of interventions related to hypertension and dyslipidemia: 30% and 28% in the study by Hough and colleagues compared with 13% and 9%, respectively, in the present study.11
Comparison of the present evaluation and Hough and colleagues is also hindered by the PhARMD tool used. The PhARMD tool used in the initial evaluation has been modified on a national level to improve the granularity of intervention data collected.
Our cost-avoidance estimate of $433,324.06 per year seems lower than that estimated in the previous evaluation when all applicable interventions were included.11 However, this study had several differences compared with those of previous VAMC studies looking at clinical interventions performed by CPSs. The main differences are the volume and setting in which interventions were being made. For example, in comparison with Hough and colleagues, the studies include different practice settings (primary care vs cardiology specialty clinic) and number of FTEs involved in the study (4.65 vs 1). If the cost avoidance is distributed evenly per FTE in the previous study, the following calculation is observed: $649,551.99 per FTE, which is closer to this study’s estimation. Given that primary care is a broader setting than is ambulatory cardiology, it is not surprising that more types of interventions and the overall volume/absolute number of interventions would be higher. Thus, the lower estimated cost avoidance in our study may be attributed to the lower volume of intervention opportunities availed to the cardiology CPS. Another difference is that detailed types of interventions related to hypertension, DM, dyslipidemia, and HF were not included in Hough and colleagues, whereas our study included all applicable interventions regardless of relation to diseases, which may account for a degree of the variation in intervention breakdown between the 2 studies.11 However, as noted previously, some interventions for these particular diseases may not fully capture the rationale for pharmacotherapy interventions, such as drug dose changes or discontinuations, which may misrepresent the potential cost avoidance associated with them in reality.
Limitations
Of general importance, the PhARMD tool may underestimate the number of interventions made such that multiple interventions for a medical condition may have been completed but only captured as 1 intervention, which may represent a limitation of the tool when multiple interventions are made for the same disease (eg, titration of both β-blocker and angiotensin-converting enzyme inhibitor doses at a single appointment in a patient with HF with reduced left ventricular ejection fraction). Improved clarity about interventions made would require laborious chart review, which was not feasible. The evaluation at hand included a preliminary QA review, adding confidence that overdocumentation was not being done and the values represented at worst an underestimation of actual CPS intervention impact. Because this study was an initial evaluation of interventions made by CPSs in an ambulatory cardiology pharmacotherapy setting, whether these same outcomes would exist in other patient cohorts is unclear. However, these data do provide a foundational understanding of what may be expected from CPS integration into a cardiovascular care team.
These findings may be limited in generalizability to other health care systems and situations in which CPSs are afforded the regulatory opportunity to practice independently within an established scope of practice or collaborative practice agreements. The Veterans Health Administration system has been a leader in integrating CPSs into direct patient care roles and serves as a potential model for application by other groups. This evaluation’s data support continued efforts to create such independent practice environments as they allow for qualified CPSs to practice to their full clinical potential and have the fullest possible effect on cardiovascular outcomes.
Previous studies looking at cost savings in MTM programs have established a substantial return in economic investment with patients being managed by pharmacists.5,14 Given that the interventions made in this study were not tied to attainment of clinical outcomes, a limitation to our study, the cost-avoidance estimates should be interpreted cautiously. However, we know of no such tool that is available to allow accurate capture of clinical event reduction in a single center with consistent CPS involvement in care. A clear opportunity exists regarding design of a model that measures clinical, economic, and humanistic outcomes related to the interventions performed by cardiology CPSs, but developing and deploying such a model may be challenging because guideline-directed medical therapies vary significantly based on many patient-specific issues, and identifying optimal or truly optimized medical therapy is at times a subjective task, especially in a single center. Using the types and volumes of interventions made by CPSs as a surrogate for these higher-level outcomes is still of value in order to understand the effect and role of CPSs in cardiovascular care. At present, the cost-avoidance estimates presented in this evaluation are based on the most appropriate system-specific data at hand, with the realization that actual cost avoidance in practice may vary widely and should be the topic of future research.
Conclusion
As cardiovascular team-based care continues to expand with the support of large organizations, such as the American College of Cardiology Foundation, Heart Failure Society of America, and American College of Clinical Pharmacy Cardiology Practice and Research Network, the need for understanding the effect of CPSs on patient care measures and health care costs becomes more pronounced.2,15 The results of this study demonstrate how integration of CPSs in an ambulatory cardiology clinic may translate to cost avoidance and a reduction in workload burden for cardiology physicians and providers, allowing more availability for diagnostic testing and care.
Interventions made by CPSs functioning as independent providers
1. Brush JE Jr, Handberg EM, Biga C, et al. 2015 ACC health policy statement on cardiovascular team-based care and the role of advanced practice providers. J Am Coll Cardiol. 2015;65(19):2118-2136.
2. Dunn SP, Birtcher KK, Beavers CJ, et al. The role of the clinical pharmacist in the care of patients with cardiovascular disease. J Am Coll Cardiol. 2015;66(19):2129-2139.
3. Sandoff BG, Kuca S, Rasmussen J, Merenich JA. Collaborative cardiac care service: a multidisciplinary approach to caring for patients with coronary artery disease. Perm J. 2008;12(3):4-11.
4. Merenich JA, Olson KL, Delate T, Rasmussen J, Helling DK, Ward DG; Clinical Pharmacy Cardiac Risk Service Study Group. Mortality reduction benefits of a comprehensive cardiac care program for patients with occlusive coronary disease. Pharmacotherapy. 2007;27(10):1370-1378.
5. Isetts BJ, Schondelmeyer SW, Artz MB, et al. Clinical and economic outcomes of medication therapy management services: the Minnesota experience.
6. Martinez AS, Saef J, Paszcuzuk A, Bhatt-Chugani H. Implementation of a pharmacist-managed heart failure medication titration clinic. Am J Health Syst Pharm. 2013;70(12):1070-1076.
7. Roth GA, Poole JE, Zaha R, Zhou W, Skinner J, Morden NE. Use of guideline-directed medications for heart failure before cardioverter-defibrillator implantation. J Am Coll Cardiol. 2016;67(9):1062-1069.
8. Noschese LA, Bergman CL, Brar CK, Kansal MM. The pharmacist’s role in medication optimization for patients with chronic heart failure. Fed Pract. 2017;34(suppl 10):S10-S15.
9. Coakley C, Hough A, Dwyer D, Parra D. Clinical video telehealth in a cardiology pharmacotherapy clinic. Am J Health Syst Pharm. 2013;70(22):1974-1975.
10. Khazan E, Anastasia E, Hough A, Parra D. Pharmacist-managed ambulatory blood pressure monitoring service. Am J Health Syst Pharm. 2017;74(4):190-195.
11. Hough A, Vartan CM, Groppi JA, Reyes S, Beckey NP. Evaluation of clinical pharmacy interventions in a Veterans Affairs medical center primary care clinic. Am J Health Syst Pharm. 2013;70(13):1168-1172.
12. Lee AJ, Boro MS, Knapp KK, Meier JL, Korman NE. Clinical and economic outcomes of pharmacist recommendations in a Veterans Affairs medical center. Am J Health Syst Pharm. 2002;59(21):2070-2077.
13. US Department of Labor. CPI inflation calculator. www.bls.gov/data/inflation_calculator.htm. Accessed January 18, 2019.
14. Perez A, Doloresco F, Hoffman JM, et al. Economic evaluations of clinical pharmacy services: 2001-2005. Pharmacotherapy. 2008;29(1):128.
15. Milfred-LaForest SK, Chow SL, DiDomenico RJ, et al. Clinical pharmacy services in heart failure: an opinion paper from the Heart Failure Society of America and American College of Clinical Pharmacy Cardiology Practice and Research Network. Pharmacotherapy. 2013;33(5):529-548.
1. Brush JE Jr, Handberg EM, Biga C, et al. 2015 ACC health policy statement on cardiovascular team-based care and the role of advanced practice providers. J Am Coll Cardiol. 2015;65(19):2118-2136.
2. Dunn SP, Birtcher KK, Beavers CJ, et al. The role of the clinical pharmacist in the care of patients with cardiovascular disease. J Am Coll Cardiol. 2015;66(19):2129-2139.
3. Sandoff BG, Kuca S, Rasmussen J, Merenich JA. Collaborative cardiac care service: a multidisciplinary approach to caring for patients with coronary artery disease. Perm J. 2008;12(3):4-11.
4. Merenich JA, Olson KL, Delate T, Rasmussen J, Helling DK, Ward DG; Clinical Pharmacy Cardiac Risk Service Study Group. Mortality reduction benefits of a comprehensive cardiac care program for patients with occlusive coronary disease. Pharmacotherapy. 2007;27(10):1370-1378.
5. Isetts BJ, Schondelmeyer SW, Artz MB, et al. Clinical and economic outcomes of medication therapy management services: the Minnesota experience.
6. Martinez AS, Saef J, Paszcuzuk A, Bhatt-Chugani H. Implementation of a pharmacist-managed heart failure medication titration clinic. Am J Health Syst Pharm. 2013;70(12):1070-1076.
7. Roth GA, Poole JE, Zaha R, Zhou W, Skinner J, Morden NE. Use of guideline-directed medications for heart failure before cardioverter-defibrillator implantation. J Am Coll Cardiol. 2016;67(9):1062-1069.
8. Noschese LA, Bergman CL, Brar CK, Kansal MM. The pharmacist’s role in medication optimization for patients with chronic heart failure. Fed Pract. 2017;34(suppl 10):S10-S15.
9. Coakley C, Hough A, Dwyer D, Parra D. Clinical video telehealth in a cardiology pharmacotherapy clinic. Am J Health Syst Pharm. 2013;70(22):1974-1975.
10. Khazan E, Anastasia E, Hough A, Parra D. Pharmacist-managed ambulatory blood pressure monitoring service. Am J Health Syst Pharm. 2017;74(4):190-195.
11. Hough A, Vartan CM, Groppi JA, Reyes S, Beckey NP. Evaluation of clinical pharmacy interventions in a Veterans Affairs medical center primary care clinic. Am J Health Syst Pharm. 2013;70(13):1168-1172.
12. Lee AJ, Boro MS, Knapp KK, Meier JL, Korman NE. Clinical and economic outcomes of pharmacist recommendations in a Veterans Affairs medical center. Am J Health Syst Pharm. 2002;59(21):2070-2077.
13. US Department of Labor. CPI inflation calculator. www.bls.gov/data/inflation_calculator.htm. Accessed January 18, 2019.
14. Perez A, Doloresco F, Hoffman JM, et al. Economic evaluations of clinical pharmacy services: 2001-2005. Pharmacotherapy. 2008;29(1):128.
15. Milfred-LaForest SK, Chow SL, DiDomenico RJ, et al. Clinical pharmacy services in heart failure: an opinion paper from the Heart Failure Society of America and American College of Clinical Pharmacy Cardiology Practice and Research Network. Pharmacotherapy. 2013;33(5):529-548.
The Best of 2018 Is Also the Worst
I am a doctor, not an engineer.Dr. McCoy, Star Trek “Mirror, Mirror” episode
Last year in my annual wrap-up, I wrote back-to-back editorials (December 2017 and January 2018) on the worst and best of 2017 from a federal health care perspective, emphasizing ethics or the lack thereof. I featured the altruism of federal health care providers (HCPs) responding to natural disasters and the terrible outcome of seemingly banal moral lapses.
This year the best and worst are one and the same, and I am not sure how it could be otherwise: the Department of Veterans Affairs (VA) and Department of Defense (DoD) electronic health record (EHR) contract with Cerner (North Kansas City, MO). Former VA Secretary David Shulkin, MD, announced the deal in 2017 shortly before his departure, and it was signed under then Acting VA Secretary Robert Wilkie in May of 2018.1 But the reason the Cerner contract is the most impactful and momentous ethical event of the year is perhaps not what readers expect. Search engines will efficiently unearth plentiful drama with ethical import about the contract. There were conspiracy charges that the shadow regime improperly engineered the selection.2 The usual Congressional hearings on the VA leadership mismanagement of the EHR culminated in Sen Jon Tester’s (D-MO) martial declaration in a letter to the newly sworn-in VA Chief Information Officer James Paul Gfrerer that “EHR modernization cannot fail.”3
While all this is obviously important, it is not why the annual awards for ethical and unethical behaviors are bestowed on what is essentially an information technology acquisition. The Cerner contract is chosen because of its enormous potential to change the human practice of health care for good or ill; hence, the dual nomination. This column is not about Cerner qua Cerner but about how the EHR has transformed—or deformed—the humanistic aspects of medical practice.
I am old enough to remember the original transition from paper charts to VistA EHR. As an intern with illegible handwriting, I can remember breathing a sigh of relief when the blue screen appeared for the first time. The commands were cumbersome and the code laborious, but it was a technologic marvel to see the clean, organized progress notes and be able to print your medication list or discharge summary. However, it also was the first stuttering waves of a tsunami that would alter medical practice forever. The human cost of the revolution could be seen almost immediately as older clinicians or those who could not type struggled to complete work that with paper and pen would have been easily accomplished.
For many years there was a steady stream of updates to VistA, including the Computerized Patient Record System (CPRS). For a relatively long time in technology terms, VistA and CPRS were the envy of the medical world, which rushed to catch up. Gradually though, VA fell behind; the wizard IT guys could not patch and fix new versions fast enough, and eventually, like all things created, VistA and CPRS became obsolete.4 Attitudes toward this microcosm of the modernization of an aging organization were intense and diverse. Some of us held onto CPRS as though it was a transitional object that we had personalized and became attached to with all its quirks and problems. Others could not wait to get rid of it, believing anything new and streamlined had to be better.
Yet the opposite also is true. EHRs have been, and could be again, incredible time-savers, enabling HCPs to deliver more evidence-based, patient-centered care in a more accurate, integrated, timely, and comprehensive manner. For example, Cerner finally could discover the Holy Grail of VA-DoD interoperability and even—dare we dream—integrate with the community. Yet as science fiction aficionados know, the machine designed to free humankind of drudgery may also end up controlling us.
The other commonplace year-end practice is for ersatz prophets to predict the future. I have no idea whether the Cerner EHR will be good or bad for VA and DoD. According to the insightful critic of medical culture, Atul Gawande, MD, who has examined the practitioner-computer interface, what we must guard against is that it does not replace the practitioner-patient relationship.5 The most common complaint I hear from patients in VA mental health care is: “They never listen to me, they just sit there typing.” Similarly, clinicians complain: “I spend all my time looking at a screen not at a patient.” As an ethicist, I cannot tell you how many times the blight of copy and paste has thwarted or damaged a patient’s care. And the direct correlation between medical computing and burnout has been well documented as all health care systems struggle with a doctor shortage particularly in primary care—arguably where computer fatigue hits hardest.6
What will decide whether EHR modernization will be a positive or negative development for VA and DoD patients? And is there anything we as federal HCPs can do to tip the scales in favor of the what is best for patients and clinicians? The most encouraging step has already been taken: VA and Cerner have set up EHR Councils composed of 60% practicing VA HCPs to provide the clinical perspective and 40% from VA Central Office to encourage synchronization of the top-down and bottom-up processes.7
Many experts have pointed out the inherent tension between how computers and human beings work, which I will simplify as the battle between the 3 S’s and the 3 F’s.5 The optimal operation of EHRs requires systems, structure, stability; to function successfully human beings need flexibility, freedom, and fragmentation. VistA had more than 100 versions according to a report from the Federal News Network (FNN), which is a striking example of the challenge EHR modernization faces in bridging the 2 orientations. As former VA Chief Information Officer Roger Baker told FNN, replacing this approach of EHR tinkering with a locked-down commercial system will require “a culture change that is orders of magnitude bigger than expected.”8
Think of the 2 domains as a Venn diagram. Where the circles overlap is all the things we and patients want and need in health care: empathic listening, strong enduring relationships, accurate diagnosis, accessibility, personalized treatment, continuity of care, mutual respect, patient safety, room to exercise professional judgment, and the data needed to promote shared decision making. Our contribution and duty are to make that inner circle where we all dwell together as wide and full as possible and the overlap between the 2 outer circles as seamless as human imperfection and artificial intelligence permit.
The Gawande article is titled “Why Doctors Hate Their Computers.” Of course, his piece shows that we also love them. None of the proposed liberations from our EHR domination—be they medical scribes or dictation programs—has solved the problem, probably because they are all technologic and just move the slavery downstream. We have come too far, and medicine is too complex, to go back to the age of paper. If we can no longer do the good work of healing and caring without computers, then we have to learn to live with them as our allies not our enemies. After all, even Dr. McCoy had a tricorder.
1. VA Office of Public and Intergovernmental Affairs. Statement by Acting Secretary Robert Wilkie—VA signs contract with Cerner for an electronic health record system. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=4061. Published May 17, 2018. Accessed January 15, 2019.
2. Arnsdorf I. The VA shadow ruler’s signature program is “trending towards red.” https://www.propublica.org/article/va-shadow-rulers-program-is-trending-towards-red. Published November 1, 2018. Accessed January 15, 2019.
3. Murphy K. Senate committee says EHR modernization cannot be allowed to fail. https://ehrintelligence.com/news/senate-committee-says-ehr-modernization-cannot-be-allowed-to-fail. Published January 14, 2019. Accessed January 15, 2019.
4. US Department of Veterans Affairs. A history of the electronic health record. https://www.ehrm.va.gov/about/history. Updated September 28, 2018. Accessed January 16, 2019.
5. Gawande A. Why doctors hate their computers. https://www.newyorker.com/magazine/2018/11/12/why-doctors-hate-their-computers. Published November 12, 2018. Accessed January 16, 2019.
6. Babbott S, Manwell LB, Brown R, et al. Electronic medical records and physician stress in primary care results from the MEMO study. J Am Med Inform Assoc. 2014;21(e1):100-106.
7. US Department of Veterans Affairs. EHRM councils. https://www.ehrm.va.gov/deployment/councils. Updated July 17, 2018. Accessed January 15, 2019.
8. Ogrysko N. In abandoning VistA, VA faces culture change that’s ‘orders of magnitude bigger’ than expected. https://federalnewsnetwork.com/veterans-affairs/2017/06/in-abandoning-vista-va-faces-culture-change-thats-orders-of-magnitude-bigger-than-expected. Published June 26, 2017. Accessed January 16, 2018.
I am a doctor, not an engineer.Dr. McCoy, Star Trek “Mirror, Mirror” episode
Last year in my annual wrap-up, I wrote back-to-back editorials (December 2017 and January 2018) on the worst and best of 2017 from a federal health care perspective, emphasizing ethics or the lack thereof. I featured the altruism of federal health care providers (HCPs) responding to natural disasters and the terrible outcome of seemingly banal moral lapses.
This year the best and worst are one and the same, and I am not sure how it could be otherwise: the Department of Veterans Affairs (VA) and Department of Defense (DoD) electronic health record (EHR) contract with Cerner (North Kansas City, MO). Former VA Secretary David Shulkin, MD, announced the deal in 2017 shortly before his departure, and it was signed under then Acting VA Secretary Robert Wilkie in May of 2018.1 But the reason the Cerner contract is the most impactful and momentous ethical event of the year is perhaps not what readers expect. Search engines will efficiently unearth plentiful drama with ethical import about the contract. There were conspiracy charges that the shadow regime improperly engineered the selection.2 The usual Congressional hearings on the VA leadership mismanagement of the EHR culminated in Sen Jon Tester’s (D-MO) martial declaration in a letter to the newly sworn-in VA Chief Information Officer James Paul Gfrerer that “EHR modernization cannot fail.”3
While all this is obviously important, it is not why the annual awards for ethical and unethical behaviors are bestowed on what is essentially an information technology acquisition. The Cerner contract is chosen because of its enormous potential to change the human practice of health care for good or ill; hence, the dual nomination. This column is not about Cerner qua Cerner but about how the EHR has transformed—or deformed—the humanistic aspects of medical practice.
I am old enough to remember the original transition from paper charts to VistA EHR. As an intern with illegible handwriting, I can remember breathing a sigh of relief when the blue screen appeared for the first time. The commands were cumbersome and the code laborious, but it was a technologic marvel to see the clean, organized progress notes and be able to print your medication list or discharge summary. However, it also was the first stuttering waves of a tsunami that would alter medical practice forever. The human cost of the revolution could be seen almost immediately as older clinicians or those who could not type struggled to complete work that with paper and pen would have been easily accomplished.
For many years there was a steady stream of updates to VistA, including the Computerized Patient Record System (CPRS). For a relatively long time in technology terms, VistA and CPRS were the envy of the medical world, which rushed to catch up. Gradually though, VA fell behind; the wizard IT guys could not patch and fix new versions fast enough, and eventually, like all things created, VistA and CPRS became obsolete.4 Attitudes toward this microcosm of the modernization of an aging organization were intense and diverse. Some of us held onto CPRS as though it was a transitional object that we had personalized and became attached to with all its quirks and problems. Others could not wait to get rid of it, believing anything new and streamlined had to be better.
Yet the opposite also is true. EHRs have been, and could be again, incredible time-savers, enabling HCPs to deliver more evidence-based, patient-centered care in a more accurate, integrated, timely, and comprehensive manner. For example, Cerner finally could discover the Holy Grail of VA-DoD interoperability and even—dare we dream—integrate with the community. Yet as science fiction aficionados know, the machine designed to free humankind of drudgery may also end up controlling us.
The other commonplace year-end practice is for ersatz prophets to predict the future. I have no idea whether the Cerner EHR will be good or bad for VA and DoD. According to the insightful critic of medical culture, Atul Gawande, MD, who has examined the practitioner-computer interface, what we must guard against is that it does not replace the practitioner-patient relationship.5 The most common complaint I hear from patients in VA mental health care is: “They never listen to me, they just sit there typing.” Similarly, clinicians complain: “I spend all my time looking at a screen not at a patient.” As an ethicist, I cannot tell you how many times the blight of copy and paste has thwarted or damaged a patient’s care. And the direct correlation between medical computing and burnout has been well documented as all health care systems struggle with a doctor shortage particularly in primary care—arguably where computer fatigue hits hardest.6
What will decide whether EHR modernization will be a positive or negative development for VA and DoD patients? And is there anything we as federal HCPs can do to tip the scales in favor of the what is best for patients and clinicians? The most encouraging step has already been taken: VA and Cerner have set up EHR Councils composed of 60% practicing VA HCPs to provide the clinical perspective and 40% from VA Central Office to encourage synchronization of the top-down and bottom-up processes.7
Many experts have pointed out the inherent tension between how computers and human beings work, which I will simplify as the battle between the 3 S’s and the 3 F’s.5 The optimal operation of EHRs requires systems, structure, stability; to function successfully human beings need flexibility, freedom, and fragmentation. VistA had more than 100 versions according to a report from the Federal News Network (FNN), which is a striking example of the challenge EHR modernization faces in bridging the 2 orientations. As former VA Chief Information Officer Roger Baker told FNN, replacing this approach of EHR tinkering with a locked-down commercial system will require “a culture change that is orders of magnitude bigger than expected.”8
Think of the 2 domains as a Venn diagram. Where the circles overlap is all the things we and patients want and need in health care: empathic listening, strong enduring relationships, accurate diagnosis, accessibility, personalized treatment, continuity of care, mutual respect, patient safety, room to exercise professional judgment, and the data needed to promote shared decision making. Our contribution and duty are to make that inner circle where we all dwell together as wide and full as possible and the overlap between the 2 outer circles as seamless as human imperfection and artificial intelligence permit.
The Gawande article is titled “Why Doctors Hate Their Computers.” Of course, his piece shows that we also love them. None of the proposed liberations from our EHR domination—be they medical scribes or dictation programs—has solved the problem, probably because they are all technologic and just move the slavery downstream. We have come too far, and medicine is too complex, to go back to the age of paper. If we can no longer do the good work of healing and caring without computers, then we have to learn to live with them as our allies not our enemies. After all, even Dr. McCoy had a tricorder.
I am a doctor, not an engineer.Dr. McCoy, Star Trek “Mirror, Mirror” episode
Last year in my annual wrap-up, I wrote back-to-back editorials (December 2017 and January 2018) on the worst and best of 2017 from a federal health care perspective, emphasizing ethics or the lack thereof. I featured the altruism of federal health care providers (HCPs) responding to natural disasters and the terrible outcome of seemingly banal moral lapses.
This year the best and worst are one and the same, and I am not sure how it could be otherwise: the Department of Veterans Affairs (VA) and Department of Defense (DoD) electronic health record (EHR) contract with Cerner (North Kansas City, MO). Former VA Secretary David Shulkin, MD, announced the deal in 2017 shortly before his departure, and it was signed under then Acting VA Secretary Robert Wilkie in May of 2018.1 But the reason the Cerner contract is the most impactful and momentous ethical event of the year is perhaps not what readers expect. Search engines will efficiently unearth plentiful drama with ethical import about the contract. There were conspiracy charges that the shadow regime improperly engineered the selection.2 The usual Congressional hearings on the VA leadership mismanagement of the EHR culminated in Sen Jon Tester’s (D-MO) martial declaration in a letter to the newly sworn-in VA Chief Information Officer James Paul Gfrerer that “EHR modernization cannot fail.”3
While all this is obviously important, it is not why the annual awards for ethical and unethical behaviors are bestowed on what is essentially an information technology acquisition. The Cerner contract is chosen because of its enormous potential to change the human practice of health care for good or ill; hence, the dual nomination. This column is not about Cerner qua Cerner but about how the EHR has transformed—or deformed—the humanistic aspects of medical practice.
I am old enough to remember the original transition from paper charts to VistA EHR. As an intern with illegible handwriting, I can remember breathing a sigh of relief when the blue screen appeared for the first time. The commands were cumbersome and the code laborious, but it was a technologic marvel to see the clean, organized progress notes and be able to print your medication list or discharge summary. However, it also was the first stuttering waves of a tsunami that would alter medical practice forever. The human cost of the revolution could be seen almost immediately as older clinicians or those who could not type struggled to complete work that with paper and pen would have been easily accomplished.
For many years there was a steady stream of updates to VistA, including the Computerized Patient Record System (CPRS). For a relatively long time in technology terms, VistA and CPRS were the envy of the medical world, which rushed to catch up. Gradually though, VA fell behind; the wizard IT guys could not patch and fix new versions fast enough, and eventually, like all things created, VistA and CPRS became obsolete.4 Attitudes toward this microcosm of the modernization of an aging organization were intense and diverse. Some of us held onto CPRS as though it was a transitional object that we had personalized and became attached to with all its quirks and problems. Others could not wait to get rid of it, believing anything new and streamlined had to be better.
Yet the opposite also is true. EHRs have been, and could be again, incredible time-savers, enabling HCPs to deliver more evidence-based, patient-centered care in a more accurate, integrated, timely, and comprehensive manner. For example, Cerner finally could discover the Holy Grail of VA-DoD interoperability and even—dare we dream—integrate with the community. Yet as science fiction aficionados know, the machine designed to free humankind of drudgery may also end up controlling us.
The other commonplace year-end practice is for ersatz prophets to predict the future. I have no idea whether the Cerner EHR will be good or bad for VA and DoD. According to the insightful critic of medical culture, Atul Gawande, MD, who has examined the practitioner-computer interface, what we must guard against is that it does not replace the practitioner-patient relationship.5 The most common complaint I hear from patients in VA mental health care is: “They never listen to me, they just sit there typing.” Similarly, clinicians complain: “I spend all my time looking at a screen not at a patient.” As an ethicist, I cannot tell you how many times the blight of copy and paste has thwarted or damaged a patient’s care. And the direct correlation between medical computing and burnout has been well documented as all health care systems struggle with a doctor shortage particularly in primary care—arguably where computer fatigue hits hardest.6
What will decide whether EHR modernization will be a positive or negative development for VA and DoD patients? And is there anything we as federal HCPs can do to tip the scales in favor of the what is best for patients and clinicians? The most encouraging step has already been taken: VA and Cerner have set up EHR Councils composed of 60% practicing VA HCPs to provide the clinical perspective and 40% from VA Central Office to encourage synchronization of the top-down and bottom-up processes.7
Many experts have pointed out the inherent tension between how computers and human beings work, which I will simplify as the battle between the 3 S’s and the 3 F’s.5 The optimal operation of EHRs requires systems, structure, stability; to function successfully human beings need flexibility, freedom, and fragmentation. VistA had more than 100 versions according to a report from the Federal News Network (FNN), which is a striking example of the challenge EHR modernization faces in bridging the 2 orientations. As former VA Chief Information Officer Roger Baker told FNN, replacing this approach of EHR tinkering with a locked-down commercial system will require “a culture change that is orders of magnitude bigger than expected.”8
Think of the 2 domains as a Venn diagram. Where the circles overlap is all the things we and patients want and need in health care: empathic listening, strong enduring relationships, accurate diagnosis, accessibility, personalized treatment, continuity of care, mutual respect, patient safety, room to exercise professional judgment, and the data needed to promote shared decision making. Our contribution and duty are to make that inner circle where we all dwell together as wide and full as possible and the overlap between the 2 outer circles as seamless as human imperfection and artificial intelligence permit.
The Gawande article is titled “Why Doctors Hate Their Computers.” Of course, his piece shows that we also love them. None of the proposed liberations from our EHR domination—be they medical scribes or dictation programs—has solved the problem, probably because they are all technologic and just move the slavery downstream. We have come too far, and medicine is too complex, to go back to the age of paper. If we can no longer do the good work of healing and caring without computers, then we have to learn to live with them as our allies not our enemies. After all, even Dr. McCoy had a tricorder.
1. VA Office of Public and Intergovernmental Affairs. Statement by Acting Secretary Robert Wilkie—VA signs contract with Cerner for an electronic health record system. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=4061. Published May 17, 2018. Accessed January 15, 2019.
2. Arnsdorf I. The VA shadow ruler’s signature program is “trending towards red.” https://www.propublica.org/article/va-shadow-rulers-program-is-trending-towards-red. Published November 1, 2018. Accessed January 15, 2019.
3. Murphy K. Senate committee says EHR modernization cannot be allowed to fail. https://ehrintelligence.com/news/senate-committee-says-ehr-modernization-cannot-be-allowed-to-fail. Published January 14, 2019. Accessed January 15, 2019.
4. US Department of Veterans Affairs. A history of the electronic health record. https://www.ehrm.va.gov/about/history. Updated September 28, 2018. Accessed January 16, 2019.
5. Gawande A. Why doctors hate their computers. https://www.newyorker.com/magazine/2018/11/12/why-doctors-hate-their-computers. Published November 12, 2018. Accessed January 16, 2019.
6. Babbott S, Manwell LB, Brown R, et al. Electronic medical records and physician stress in primary care results from the MEMO study. J Am Med Inform Assoc. 2014;21(e1):100-106.
7. US Department of Veterans Affairs. EHRM councils. https://www.ehrm.va.gov/deployment/councils. Updated July 17, 2018. Accessed January 15, 2019.
8. Ogrysko N. In abandoning VistA, VA faces culture change that’s ‘orders of magnitude bigger’ than expected. https://federalnewsnetwork.com/veterans-affairs/2017/06/in-abandoning-vista-va-faces-culture-change-thats-orders-of-magnitude-bigger-than-expected. Published June 26, 2017. Accessed January 16, 2018.
1. VA Office of Public and Intergovernmental Affairs. Statement by Acting Secretary Robert Wilkie—VA signs contract with Cerner for an electronic health record system. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=4061. Published May 17, 2018. Accessed January 15, 2019.
2. Arnsdorf I. The VA shadow ruler’s signature program is “trending towards red.” https://www.propublica.org/article/va-shadow-rulers-program-is-trending-towards-red. Published November 1, 2018. Accessed January 15, 2019.
3. Murphy K. Senate committee says EHR modernization cannot be allowed to fail. https://ehrintelligence.com/news/senate-committee-says-ehr-modernization-cannot-be-allowed-to-fail. Published January 14, 2019. Accessed January 15, 2019.
4. US Department of Veterans Affairs. A history of the electronic health record. https://www.ehrm.va.gov/about/history. Updated September 28, 2018. Accessed January 16, 2019.
5. Gawande A. Why doctors hate their computers. https://www.newyorker.com/magazine/2018/11/12/why-doctors-hate-their-computers. Published November 12, 2018. Accessed January 16, 2019.
6. Babbott S, Manwell LB, Brown R, et al. Electronic medical records and physician stress in primary care results from the MEMO study. J Am Med Inform Assoc. 2014;21(e1):100-106.
7. US Department of Veterans Affairs. EHRM councils. https://www.ehrm.va.gov/deployment/councils. Updated July 17, 2018. Accessed January 15, 2019.
8. Ogrysko N. In abandoning VistA, VA faces culture change that’s ‘orders of magnitude bigger’ than expected. https://federalnewsnetwork.com/veterans-affairs/2017/06/in-abandoning-vista-va-faces-culture-change-thats-orders-of-magnitude-bigger-than-expected. Published June 26, 2017. Accessed January 16, 2018.
Breast cancer recurrence lower, survival better with dose-intensified regimens
Dose-intense adjuvant chemotherapy is associated with significant if modest improvements in recurrence-free, breast cancer–specific, and overall survival among women with early breast cancer, results of a meta-analysis of data on individual patients showed.
Among more than 37,000 patients treated in 26 clinical trials with a median follow-up of 7.4 years, there was a 14% reduction in relative risk and 3.4% reduction in absolute 10-year risk of breast cancer recurrence for women who were treated either with accelerated-schedule or sequential chemotherapy, reported members of the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG).
There were no differences in deaths from cardiovascular disease, acute myeloid leukemia, or other cancers between patients treated with dose-intense regimens or schedules and those treated with standard chemotherapy, although patients on dose-intense regimens had higher incidence of grade 3 or 4 anemia, and more did not complete the prescribed courses compared with standard chemotherapy, the investigators noted.
“The balance of benefit versus toxicity, therefore, appears to favor more dose-intense chemotherapy. A further advantage of 2-weekly versus 3-weekly chemotherapy – but not of sequential versus concurrent chemotherapy – is treatment is completed sooner,” they wrote in The Lancet.
The investigators examined individual patient data for 26 of 33 trials comparing either 2-weekly chemotherapy with 3-weekly therapy, or sequential vs. concurrent anthracycline and taxane-based chemotherapy.
The trials comprised a total cohort of 37,297 women randomized, most of whom were younger than 70 years at the time of diagnose and had node-positive disease.
The 10 year-risk for breast cancer recurrence, one of two primary endpoints, was 28% with dose intensification vs. 31.4% with standard dosing, translating into a first-event rate ratio (RR) for recurrence of 0.86 (P less than .0001).
Ten-year breast-cancer mortality, the other primary endpoint, was 18.9% among patients treated with dose-intensified regimens or schedules, compared with 21.3% for patients treated under standard protocols.
All-cause mortality was lower with dose intensification (22.1% vs. 24.8%, P less than .0001), and death without recurrence was also slightly but significantly lower (4.1% vs. 4.6%, respectively, P = .034).
The reductions in recurrence rates were similar among trials comparing 2-week vs. 3-week chemotherapy cycles, sequential vs. concurrent schedules, and both strategies together.
“The proportional reductions in recurrence with dose-intense chemotherapy were similar and highly significant [P less than .0001) in estrogen receptor (ER)-positive and ER-negative disease and did not differ significantly by other patient or tumor characteristics,” the investigators wrote.
“The present findings are of limited relevance to the question of which women with early breast cancer should be offered chemotherapy, although they do indicate that chemotherapy can reduce breast cancer mortality rates by 40% rather than a third. The absolute gain from this proportional reduction in recurrence depends chiefly on what the risk of distant recurrence would be without chemotherapy, which varies greatly from one woman to another, and is the subject of much ongoing research,” the investigators wrote.
“The findings are, however, directly relevant to selection of what regimen to use, and they show that, if chemotherapy is to be given, a dose-intense regimen should at least be considered,” they wrote.
The meta-analysis was funded by Cancer Research UK and the Medical Research Council. All authors reported having no relevant disclosures.
SOURCE: EBCTCG. The Lancet. 2019 Feb 7. doi: 10.1016/S0140-6736(18)33137-4.
Although these results are meaningful, several limitations should be recognized as we translate these findings into practice. First, the benefits of dose intensification have not been established in the era of targeted therapy. Given that these studies enrolled women from 1985 to 2011, HER2 status was known for only 50% of tumors. Of those tested, 16% (n = 2,994) were HER2 positive. Use of trastuzumab was not reported but was probably uncommon since adjuvant trastuzumab was not approved until 2006. The remaining 18,625 patients did not have HER2 testing; thus, no HER2-directed therapy would have been given. Therefore, the majority of patients with HER2- positive breast cancer did not receive targeted therapy.
Although the authors report that women with HER2-positive and HER2-negative disease benefit similarly from dose intensification, it is impossible to know whether dose intensification benefits trastuzumab-treated patients or those who receive more than one HER2-targeted therapy (pertuzumab, neratinib, or trastuzumab-emtansine). Similarly, if other targeted therapies such as CDK4/6 inhibitors and PARP inhibitors show significant benefit in the curative setting for high-risk estrogen receptor (ER)-positive or BRCA-mutated breast cancer, prospective studies will be required to establish whether dose-intensive chemotherapy is better than standard chemotherapy in those settings.
Second, it is premature to conclude that patients older than 70 years or those with node-negative disease benefit from dose intensification, given the small number of patients in those groups and the fact that no significant benefit was observed for these patients. Moreover, gene-expression profiling was not used in these studies; thus, the benefit, if any, of a dose-intense approach for women with lymph-node-negative, high-risk, ER-positive disease is impossible to know. Finally, the use of dose intensification has not been studied in non-anthracycline, taxane-based regimens, which are being increasingly evaluated and used in women with node-negative, ER-positive disease.
With these caveats in mind, the results of this meta-analysis are undoubtedly clinically important. In modern practice, if anthracycline-based chemotherapy is warranted, these data provide convincing evidence that a dose-intense approach should be considered.
Sara A Hurvitz, MD, is from the David Geffen School of Medicine at UCLA, Santa Monica, Calif. Her remarks are excerpted from an editorial accompanying the study. She reports institutional research funding and fees for abstract and manuscript writing from several pharmaceutical companies outside of the submitted work, and travel reimbursement from Lilly outside of the submitted work.
Although these results are meaningful, several limitations should be recognized as we translate these findings into practice. First, the benefits of dose intensification have not been established in the era of targeted therapy. Given that these studies enrolled women from 1985 to 2011, HER2 status was known for only 50% of tumors. Of those tested, 16% (n = 2,994) were HER2 positive. Use of trastuzumab was not reported but was probably uncommon since adjuvant trastuzumab was not approved until 2006. The remaining 18,625 patients did not have HER2 testing; thus, no HER2-directed therapy would have been given. Therefore, the majority of patients with HER2- positive breast cancer did not receive targeted therapy.
Although the authors report that women with HER2-positive and HER2-negative disease benefit similarly from dose intensification, it is impossible to know whether dose intensification benefits trastuzumab-treated patients or those who receive more than one HER2-targeted therapy (pertuzumab, neratinib, or trastuzumab-emtansine). Similarly, if other targeted therapies such as CDK4/6 inhibitors and PARP inhibitors show significant benefit in the curative setting for high-risk estrogen receptor (ER)-positive or BRCA-mutated breast cancer, prospective studies will be required to establish whether dose-intensive chemotherapy is better than standard chemotherapy in those settings.
Second, it is premature to conclude that patients older than 70 years or those with node-negative disease benefit from dose intensification, given the small number of patients in those groups and the fact that no significant benefit was observed for these patients. Moreover, gene-expression profiling was not used in these studies; thus, the benefit, if any, of a dose-intense approach for women with lymph-node-negative, high-risk, ER-positive disease is impossible to know. Finally, the use of dose intensification has not been studied in non-anthracycline, taxane-based regimens, which are being increasingly evaluated and used in women with node-negative, ER-positive disease.
With these caveats in mind, the results of this meta-analysis are undoubtedly clinically important. In modern practice, if anthracycline-based chemotherapy is warranted, these data provide convincing evidence that a dose-intense approach should be considered.
Sara A Hurvitz, MD, is from the David Geffen School of Medicine at UCLA, Santa Monica, Calif. Her remarks are excerpted from an editorial accompanying the study. She reports institutional research funding and fees for abstract and manuscript writing from several pharmaceutical companies outside of the submitted work, and travel reimbursement from Lilly outside of the submitted work.
Although these results are meaningful, several limitations should be recognized as we translate these findings into practice. First, the benefits of dose intensification have not been established in the era of targeted therapy. Given that these studies enrolled women from 1985 to 2011, HER2 status was known for only 50% of tumors. Of those tested, 16% (n = 2,994) were HER2 positive. Use of trastuzumab was not reported but was probably uncommon since adjuvant trastuzumab was not approved until 2006. The remaining 18,625 patients did not have HER2 testing; thus, no HER2-directed therapy would have been given. Therefore, the majority of patients with HER2- positive breast cancer did not receive targeted therapy.
Although the authors report that women with HER2-positive and HER2-negative disease benefit similarly from dose intensification, it is impossible to know whether dose intensification benefits trastuzumab-treated patients or those who receive more than one HER2-targeted therapy (pertuzumab, neratinib, or trastuzumab-emtansine). Similarly, if other targeted therapies such as CDK4/6 inhibitors and PARP inhibitors show significant benefit in the curative setting for high-risk estrogen receptor (ER)-positive or BRCA-mutated breast cancer, prospective studies will be required to establish whether dose-intensive chemotherapy is better than standard chemotherapy in those settings.
Second, it is premature to conclude that patients older than 70 years or those with node-negative disease benefit from dose intensification, given the small number of patients in those groups and the fact that no significant benefit was observed for these patients. Moreover, gene-expression profiling was not used in these studies; thus, the benefit, if any, of a dose-intense approach for women with lymph-node-negative, high-risk, ER-positive disease is impossible to know. Finally, the use of dose intensification has not been studied in non-anthracycline, taxane-based regimens, which are being increasingly evaluated and used in women with node-negative, ER-positive disease.
With these caveats in mind, the results of this meta-analysis are undoubtedly clinically important. In modern practice, if anthracycline-based chemotherapy is warranted, these data provide convincing evidence that a dose-intense approach should be considered.
Sara A Hurvitz, MD, is from the David Geffen School of Medicine at UCLA, Santa Monica, Calif. Her remarks are excerpted from an editorial accompanying the study. She reports institutional research funding and fees for abstract and manuscript writing from several pharmaceutical companies outside of the submitted work, and travel reimbursement from Lilly outside of the submitted work.
Dose-intense adjuvant chemotherapy is associated with significant if modest improvements in recurrence-free, breast cancer–specific, and overall survival among women with early breast cancer, results of a meta-analysis of data on individual patients showed.
Among more than 37,000 patients treated in 26 clinical trials with a median follow-up of 7.4 years, there was a 14% reduction in relative risk and 3.4% reduction in absolute 10-year risk of breast cancer recurrence for women who were treated either with accelerated-schedule or sequential chemotherapy, reported members of the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG).
There were no differences in deaths from cardiovascular disease, acute myeloid leukemia, or other cancers between patients treated with dose-intense regimens or schedules and those treated with standard chemotherapy, although patients on dose-intense regimens had higher incidence of grade 3 or 4 anemia, and more did not complete the prescribed courses compared with standard chemotherapy, the investigators noted.
“The balance of benefit versus toxicity, therefore, appears to favor more dose-intense chemotherapy. A further advantage of 2-weekly versus 3-weekly chemotherapy – but not of sequential versus concurrent chemotherapy – is treatment is completed sooner,” they wrote in The Lancet.
The investigators examined individual patient data for 26 of 33 trials comparing either 2-weekly chemotherapy with 3-weekly therapy, or sequential vs. concurrent anthracycline and taxane-based chemotherapy.
The trials comprised a total cohort of 37,297 women randomized, most of whom were younger than 70 years at the time of diagnose and had node-positive disease.
The 10 year-risk for breast cancer recurrence, one of two primary endpoints, was 28% with dose intensification vs. 31.4% with standard dosing, translating into a first-event rate ratio (RR) for recurrence of 0.86 (P less than .0001).
Ten-year breast-cancer mortality, the other primary endpoint, was 18.9% among patients treated with dose-intensified regimens or schedules, compared with 21.3% for patients treated under standard protocols.
All-cause mortality was lower with dose intensification (22.1% vs. 24.8%, P less than .0001), and death without recurrence was also slightly but significantly lower (4.1% vs. 4.6%, respectively, P = .034).
The reductions in recurrence rates were similar among trials comparing 2-week vs. 3-week chemotherapy cycles, sequential vs. concurrent schedules, and both strategies together.
“The proportional reductions in recurrence with dose-intense chemotherapy were similar and highly significant [P less than .0001) in estrogen receptor (ER)-positive and ER-negative disease and did not differ significantly by other patient or tumor characteristics,” the investigators wrote.
“The present findings are of limited relevance to the question of which women with early breast cancer should be offered chemotherapy, although they do indicate that chemotherapy can reduce breast cancer mortality rates by 40% rather than a third. The absolute gain from this proportional reduction in recurrence depends chiefly on what the risk of distant recurrence would be without chemotherapy, which varies greatly from one woman to another, and is the subject of much ongoing research,” the investigators wrote.
“The findings are, however, directly relevant to selection of what regimen to use, and they show that, if chemotherapy is to be given, a dose-intense regimen should at least be considered,” they wrote.
The meta-analysis was funded by Cancer Research UK and the Medical Research Council. All authors reported having no relevant disclosures.
SOURCE: EBCTCG. The Lancet. 2019 Feb 7. doi: 10.1016/S0140-6736(18)33137-4.
Dose-intense adjuvant chemotherapy is associated with significant if modest improvements in recurrence-free, breast cancer–specific, and overall survival among women with early breast cancer, results of a meta-analysis of data on individual patients showed.
Among more than 37,000 patients treated in 26 clinical trials with a median follow-up of 7.4 years, there was a 14% reduction in relative risk and 3.4% reduction in absolute 10-year risk of breast cancer recurrence for women who were treated either with accelerated-schedule or sequential chemotherapy, reported members of the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG).
There were no differences in deaths from cardiovascular disease, acute myeloid leukemia, or other cancers between patients treated with dose-intense regimens or schedules and those treated with standard chemotherapy, although patients on dose-intense regimens had higher incidence of grade 3 or 4 anemia, and more did not complete the prescribed courses compared with standard chemotherapy, the investigators noted.
“The balance of benefit versus toxicity, therefore, appears to favor more dose-intense chemotherapy. A further advantage of 2-weekly versus 3-weekly chemotherapy – but not of sequential versus concurrent chemotherapy – is treatment is completed sooner,” they wrote in The Lancet.
The investigators examined individual patient data for 26 of 33 trials comparing either 2-weekly chemotherapy with 3-weekly therapy, or sequential vs. concurrent anthracycline and taxane-based chemotherapy.
The trials comprised a total cohort of 37,297 women randomized, most of whom were younger than 70 years at the time of diagnose and had node-positive disease.
The 10 year-risk for breast cancer recurrence, one of two primary endpoints, was 28% with dose intensification vs. 31.4% with standard dosing, translating into a first-event rate ratio (RR) for recurrence of 0.86 (P less than .0001).
Ten-year breast-cancer mortality, the other primary endpoint, was 18.9% among patients treated with dose-intensified regimens or schedules, compared with 21.3% for patients treated under standard protocols.
All-cause mortality was lower with dose intensification (22.1% vs. 24.8%, P less than .0001), and death without recurrence was also slightly but significantly lower (4.1% vs. 4.6%, respectively, P = .034).
The reductions in recurrence rates were similar among trials comparing 2-week vs. 3-week chemotherapy cycles, sequential vs. concurrent schedules, and both strategies together.
“The proportional reductions in recurrence with dose-intense chemotherapy were similar and highly significant [P less than .0001) in estrogen receptor (ER)-positive and ER-negative disease and did not differ significantly by other patient or tumor characteristics,” the investigators wrote.
“The present findings are of limited relevance to the question of which women with early breast cancer should be offered chemotherapy, although they do indicate that chemotherapy can reduce breast cancer mortality rates by 40% rather than a third. The absolute gain from this proportional reduction in recurrence depends chiefly on what the risk of distant recurrence would be without chemotherapy, which varies greatly from one woman to another, and is the subject of much ongoing research,” the investigators wrote.
“The findings are, however, directly relevant to selection of what regimen to use, and they show that, if chemotherapy is to be given, a dose-intense regimen should at least be considered,” they wrote.
The meta-analysis was funded by Cancer Research UK and the Medical Research Council. All authors reported having no relevant disclosures.
SOURCE: EBCTCG. The Lancet. 2019 Feb 7. doi: 10.1016/S0140-6736(18)33137-4.
FROM THE LANCET
Key clinical point: Consider dose-intensification or sequential therapy for patients undergoing chemotherapy.
Major finding: Ten-year recurrence rates were 28% with dose intensification vs. 31.4% for standard dosing.
Study details: Meta-analysis of individual data on 37,298 women enrolled in 26 randomized trials.
Disclosures: The meta-analysis was funded by Cancer Research UK and the Medical Research Council. All authors reported having no relevant disclosures.
Source: EBCTCG. The Lancet. 2019 Feb 7. doi: 10.1016/S0140-6736(18)33137-4.