User login
EC approves dasatinib plus chemo for kids with Ph+ ALL
The European Commission has approved dasatinib (Sprycel) for use in combination with chemotherapy for the treatment of pediatric patients with newly diagnosed, Philadelphia chromosome–positive (Ph+) acute lymphoblastic leukemia (ALL).
Dasatinib will be available in tablet form and as a powder for oral suspension, Bristol-Myers Squib said in a press release.
The approval was based on an event-free survival rate of 65.5% (95% confidence interval, 57.7-73.7) and an overall survival rate of 91.5% (95% CI, 84.2-95.5) in a phase 2 trial that evaluated the addition of dasatinib to a chemotherapy regimen modeled on a Berlin-Frankfurt-Münster high-risk backbone in pediatric patients with newly diagnosed Ph+ ALL.
Patients treated in the study (n = 106) were all aged younger than 18 years and received dasatinib at a daily dose of 60 mg/m2 on a continuous dosing regimen for up to 24 months, in combination with chemotherapy. About 77 % of patients (n = 82) received tablets exclusively; 23% of patients (n = 24) received the powder for oral suspension at least once.
Hematologic adverse events included grade 3 or 4 febrile neutropenia (75.5%), sepsis (23.6%), and bacteremia (24.5%). Nonhematologic, noninfectious grade 3 or 4 adverse events attributed to dasatinib and reported in more than 10% of patients included elevated ALT (21.7%) and AST (10.4%). Additional grade 3 or 4 adverse events attributed to dasatinib were pleural effusion (3.8%), edema (2.8%), hemorrhage (5.7%), and cardiac failure (0.8%). No events of pulmonary hypertension or pulmonary arterial hypertension were reported, the company said in the press release.
Dasatinib is already approved by the European Commission to treat children with Ph+ chronic myeloid leukemia in the chronic phase, which includes newly diagnosed patients and those with resistance or intolerance to imatinib.
The European Commission has approved dasatinib (Sprycel) for use in combination with chemotherapy for the treatment of pediatric patients with newly diagnosed, Philadelphia chromosome–positive (Ph+) acute lymphoblastic leukemia (ALL).
Dasatinib will be available in tablet form and as a powder for oral suspension, Bristol-Myers Squib said in a press release.
The approval was based on an event-free survival rate of 65.5% (95% confidence interval, 57.7-73.7) and an overall survival rate of 91.5% (95% CI, 84.2-95.5) in a phase 2 trial that evaluated the addition of dasatinib to a chemotherapy regimen modeled on a Berlin-Frankfurt-Münster high-risk backbone in pediatric patients with newly diagnosed Ph+ ALL.
Patients treated in the study (n = 106) were all aged younger than 18 years and received dasatinib at a daily dose of 60 mg/m2 on a continuous dosing regimen for up to 24 months, in combination with chemotherapy. About 77 % of patients (n = 82) received tablets exclusively; 23% of patients (n = 24) received the powder for oral suspension at least once.
Hematologic adverse events included grade 3 or 4 febrile neutropenia (75.5%), sepsis (23.6%), and bacteremia (24.5%). Nonhematologic, noninfectious grade 3 or 4 adverse events attributed to dasatinib and reported in more than 10% of patients included elevated ALT (21.7%) and AST (10.4%). Additional grade 3 or 4 adverse events attributed to dasatinib were pleural effusion (3.8%), edema (2.8%), hemorrhage (5.7%), and cardiac failure (0.8%). No events of pulmonary hypertension or pulmonary arterial hypertension were reported, the company said in the press release.
Dasatinib is already approved by the European Commission to treat children with Ph+ chronic myeloid leukemia in the chronic phase, which includes newly diagnosed patients and those with resistance or intolerance to imatinib.
The European Commission has approved dasatinib (Sprycel) for use in combination with chemotherapy for the treatment of pediatric patients with newly diagnosed, Philadelphia chromosome–positive (Ph+) acute lymphoblastic leukemia (ALL).
Dasatinib will be available in tablet form and as a powder for oral suspension, Bristol-Myers Squib said in a press release.
The approval was based on an event-free survival rate of 65.5% (95% confidence interval, 57.7-73.7) and an overall survival rate of 91.5% (95% CI, 84.2-95.5) in a phase 2 trial that evaluated the addition of dasatinib to a chemotherapy regimen modeled on a Berlin-Frankfurt-Münster high-risk backbone in pediatric patients with newly diagnosed Ph+ ALL.
Patients treated in the study (n = 106) were all aged younger than 18 years and received dasatinib at a daily dose of 60 mg/m2 on a continuous dosing regimen for up to 24 months, in combination with chemotherapy. About 77 % of patients (n = 82) received tablets exclusively; 23% of patients (n = 24) received the powder for oral suspension at least once.
Hematologic adverse events included grade 3 or 4 febrile neutropenia (75.5%), sepsis (23.6%), and bacteremia (24.5%). Nonhematologic, noninfectious grade 3 or 4 adverse events attributed to dasatinib and reported in more than 10% of patients included elevated ALT (21.7%) and AST (10.4%). Additional grade 3 or 4 adverse events attributed to dasatinib were pleural effusion (3.8%), edema (2.8%), hemorrhage (5.7%), and cardiac failure (0.8%). No events of pulmonary hypertension or pulmonary arterial hypertension were reported, the company said in the press release.
Dasatinib is already approved by the European Commission to treat children with Ph+ chronic myeloid leukemia in the chronic phase, which includes newly diagnosed patients and those with resistance or intolerance to imatinib.
Treatment missing for U.S. children with mental illness
according to data from a national survey of parents.

Among the estimated 7.7 million children with a treatable mental illness, 49.4% did not receive needed treatment from a psychiatrist, psychologist, psychiatric nurse, or clinical social worker in the previous 12 months, Daniel G. Whitney, PhD, and Mark D. Peterson, PhD, of the University of Michigan, Ann Arbor, wrote in JAMA Pediatrics.
State-level data from the National Survey of Children’s Health show considerable variation from the national average. North Carolina had the highest prevalence of nontreatment at 72.2% and Washington, D.C., had the lowest rate at 29.5%. The prevalence of at least one mental health disorder was highest in Maine (27.2%) and lowest in Hawaii (7.6%), the investigators reported.
Four states – Alabama, Mississippi, Oklahoma, and Utah – were in the top quartile for both mental health disorder prevalence and prevalence of children with a disorder who did not receive treatment, they noted.
“State-level practices and policies play a role in health care needs and use, which may help to explain the state variability observed in this study. Nevertheless, initiatives that assist systems of care coordination have demonstrated a reduction of mental health–related burdens across multiple domains,” Dr. Whitney and Dr. Peterson wrote.
SOURCE: Whitney DG et al. JAMA Pediatr. 2019 Feb 11. doi: 10.1001/jamapediatrics.2018.5399.
according to data from a national survey of parents.

Among the estimated 7.7 million children with a treatable mental illness, 49.4% did not receive needed treatment from a psychiatrist, psychologist, psychiatric nurse, or clinical social worker in the previous 12 months, Daniel G. Whitney, PhD, and Mark D. Peterson, PhD, of the University of Michigan, Ann Arbor, wrote in JAMA Pediatrics.
State-level data from the National Survey of Children’s Health show considerable variation from the national average. North Carolina had the highest prevalence of nontreatment at 72.2% and Washington, D.C., had the lowest rate at 29.5%. The prevalence of at least one mental health disorder was highest in Maine (27.2%) and lowest in Hawaii (7.6%), the investigators reported.
Four states – Alabama, Mississippi, Oklahoma, and Utah – were in the top quartile for both mental health disorder prevalence and prevalence of children with a disorder who did not receive treatment, they noted.
“State-level practices and policies play a role in health care needs and use, which may help to explain the state variability observed in this study. Nevertheless, initiatives that assist systems of care coordination have demonstrated a reduction of mental health–related burdens across multiple domains,” Dr. Whitney and Dr. Peterson wrote.
SOURCE: Whitney DG et al. JAMA Pediatr. 2019 Feb 11. doi: 10.1001/jamapediatrics.2018.5399.
according to data from a national survey of parents.

Among the estimated 7.7 million children with a treatable mental illness, 49.4% did not receive needed treatment from a psychiatrist, psychologist, psychiatric nurse, or clinical social worker in the previous 12 months, Daniel G. Whitney, PhD, and Mark D. Peterson, PhD, of the University of Michigan, Ann Arbor, wrote in JAMA Pediatrics.
State-level data from the National Survey of Children’s Health show considerable variation from the national average. North Carolina had the highest prevalence of nontreatment at 72.2% and Washington, D.C., had the lowest rate at 29.5%. The prevalence of at least one mental health disorder was highest in Maine (27.2%) and lowest in Hawaii (7.6%), the investigators reported.
Four states – Alabama, Mississippi, Oklahoma, and Utah – were in the top quartile for both mental health disorder prevalence and prevalence of children with a disorder who did not receive treatment, they noted.
“State-level practices and policies play a role in health care needs and use, which may help to explain the state variability observed in this study. Nevertheless, initiatives that assist systems of care coordination have demonstrated a reduction of mental health–related burdens across multiple domains,” Dr. Whitney and Dr. Peterson wrote.
SOURCE: Whitney DG et al. JAMA Pediatr. 2019 Feb 11. doi: 10.1001/jamapediatrics.2018.5399.
FROM JAMA PEDIATRICS
Herpes zoster could pose special threat to younger IBD patients
LAS VEGAS – Herpes zoster infection could pose a special risk for younger patients with inflammatory bowel disease who are on immunosuppressant or biologic therapies, a new study suggests.
About 3% of inflammatory bowel disease (IBD) patients developed herpes zoster (HZ) over a 5-year period at a single center, researchers found, and their average age was 37 years. The mean national age of HZ diagnosis is 59 years, and the latest guidelines from the Centers for Disease Control and Prevention do not recommend that people get vaccinated against HZ, or shingles, until age 50.
“Increased efforts should be made to administer herpes zoster vaccine in all eligible IBD patients, and said gastroenterologist and study coauthor Marie L. Borum, MD, MPH, of George Washington University, Washington. She spoke in an interview before presenting the study findings at the the Crohn’s & Colitis Congress – a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
Dr. Borum and her associates launched the study, published in Inflammatory Bowel Diseases, after noticing an increase in HZ cases among patients with IBD. The authors retrospectively analyzed the medical charts of all patients with IBD who were treated at a single center from 2012 to 2017 (n = 393; 55% female; average age, 44 years). Nearly all had ulcerative colitis (71%) or Crohn’s disease (24%).
Over the 5-year period, 11 patients – 5 with ulcerative colitis, 5 with Crohn’s disease, and 1 patient with unspecified colitis – were diagnosed with HZ. All were taking immunosuppressant or biologic medications, and none had been vaccinated against HZ.
The difference in the average age of diagnosis of the infected patients versus the national mean age (37 years vs. 59 years) was statistically significant (P less than .0001).
The IBD patients with HZ often had postherpetic neuralgia, Dr. Borum said.
Previous studies also have linked IBD to higher rates of HZ. A 2018 retrospective study of veterans found that “the incidence rates of herpes zoster in all age groups and all IBD medication subgroups were substantially higher than that in the oldest group of patients without IBD [older than 60 years]” (Clin Gastroenterol Hepatol. 2018 Dec;16[12]:1919-27).
In 2017, researchers at the University of Wisconsin–Madison received a grant to study immunity to the varicella zoster virus in patients with IBD. A university press release said the results “could support recommendations for universal herpes zoster immunization for all IBD patients above the age of 40.”
Why might IBD boost the risk of HZ? “Individuals with IBD may have an increased risk of developing more episodes of herpes zoster due to immune dysregulation,” Dr. Borum said. “Those on immunosuppressants or biologic therapies have greater risk of more frequent and severe complications. It has been speculated that Janus kinase inhibitors may be associated with an increased risk for developing HZ.”
Dr. Borum noted that the study is limited by its size and single-center design. “However, it supports the recommendations that additional research is needed to fully understand the potential impact of HZ on IBD patients.”
The study authors reported no relevant disclosures.
SOURCE: Borum ML et al. Inflamm Bowel Dis. 2019 Feb 7. doi: 10.1093/ibd/izy393.073.
LAS VEGAS – Herpes zoster infection could pose a special risk for younger patients with inflammatory bowel disease who are on immunosuppressant or biologic therapies, a new study suggests.
About 3% of inflammatory bowel disease (IBD) patients developed herpes zoster (HZ) over a 5-year period at a single center, researchers found, and their average age was 37 years. The mean national age of HZ diagnosis is 59 years, and the latest guidelines from the Centers for Disease Control and Prevention do not recommend that people get vaccinated against HZ, or shingles, until age 50.
“Increased efforts should be made to administer herpes zoster vaccine in all eligible IBD patients, and said gastroenterologist and study coauthor Marie L. Borum, MD, MPH, of George Washington University, Washington. She spoke in an interview before presenting the study findings at the the Crohn’s & Colitis Congress – a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
Dr. Borum and her associates launched the study, published in Inflammatory Bowel Diseases, after noticing an increase in HZ cases among patients with IBD. The authors retrospectively analyzed the medical charts of all patients with IBD who were treated at a single center from 2012 to 2017 (n = 393; 55% female; average age, 44 years). Nearly all had ulcerative colitis (71%) or Crohn’s disease (24%).
Over the 5-year period, 11 patients – 5 with ulcerative colitis, 5 with Crohn’s disease, and 1 patient with unspecified colitis – were diagnosed with HZ. All were taking immunosuppressant or biologic medications, and none had been vaccinated against HZ.
The difference in the average age of diagnosis of the infected patients versus the national mean age (37 years vs. 59 years) was statistically significant (P less than .0001).
The IBD patients with HZ often had postherpetic neuralgia, Dr. Borum said.
Previous studies also have linked IBD to higher rates of HZ. A 2018 retrospective study of veterans found that “the incidence rates of herpes zoster in all age groups and all IBD medication subgroups were substantially higher than that in the oldest group of patients without IBD [older than 60 years]” (Clin Gastroenterol Hepatol. 2018 Dec;16[12]:1919-27).
In 2017, researchers at the University of Wisconsin–Madison received a grant to study immunity to the varicella zoster virus in patients with IBD. A university press release said the results “could support recommendations for universal herpes zoster immunization for all IBD patients above the age of 40.”
Why might IBD boost the risk of HZ? “Individuals with IBD may have an increased risk of developing more episodes of herpes zoster due to immune dysregulation,” Dr. Borum said. “Those on immunosuppressants or biologic therapies have greater risk of more frequent and severe complications. It has been speculated that Janus kinase inhibitors may be associated with an increased risk for developing HZ.”
Dr. Borum noted that the study is limited by its size and single-center design. “However, it supports the recommendations that additional research is needed to fully understand the potential impact of HZ on IBD patients.”
The study authors reported no relevant disclosures.
SOURCE: Borum ML et al. Inflamm Bowel Dis. 2019 Feb 7. doi: 10.1093/ibd/izy393.073.
LAS VEGAS – Herpes zoster infection could pose a special risk for younger patients with inflammatory bowel disease who are on immunosuppressant or biologic therapies, a new study suggests.
About 3% of inflammatory bowel disease (IBD) patients developed herpes zoster (HZ) over a 5-year period at a single center, researchers found, and their average age was 37 years. The mean national age of HZ diagnosis is 59 years, and the latest guidelines from the Centers for Disease Control and Prevention do not recommend that people get vaccinated against HZ, or shingles, until age 50.
“Increased efforts should be made to administer herpes zoster vaccine in all eligible IBD patients, and said gastroenterologist and study coauthor Marie L. Borum, MD, MPH, of George Washington University, Washington. She spoke in an interview before presenting the study findings at the the Crohn’s & Colitis Congress – a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
Dr. Borum and her associates launched the study, published in Inflammatory Bowel Diseases, after noticing an increase in HZ cases among patients with IBD. The authors retrospectively analyzed the medical charts of all patients with IBD who were treated at a single center from 2012 to 2017 (n = 393; 55% female; average age, 44 years). Nearly all had ulcerative colitis (71%) or Crohn’s disease (24%).
Over the 5-year period, 11 patients – 5 with ulcerative colitis, 5 with Crohn’s disease, and 1 patient with unspecified colitis – were diagnosed with HZ. All were taking immunosuppressant or biologic medications, and none had been vaccinated against HZ.
The difference in the average age of diagnosis of the infected patients versus the national mean age (37 years vs. 59 years) was statistically significant (P less than .0001).
The IBD patients with HZ often had postherpetic neuralgia, Dr. Borum said.
Previous studies also have linked IBD to higher rates of HZ. A 2018 retrospective study of veterans found that “the incidence rates of herpes zoster in all age groups and all IBD medication subgroups were substantially higher than that in the oldest group of patients without IBD [older than 60 years]” (Clin Gastroenterol Hepatol. 2018 Dec;16[12]:1919-27).
In 2017, researchers at the University of Wisconsin–Madison received a grant to study immunity to the varicella zoster virus in patients with IBD. A university press release said the results “could support recommendations for universal herpes zoster immunization for all IBD patients above the age of 40.”
Why might IBD boost the risk of HZ? “Individuals with IBD may have an increased risk of developing more episodes of herpes zoster due to immune dysregulation,” Dr. Borum said. “Those on immunosuppressants or biologic therapies have greater risk of more frequent and severe complications. It has been speculated that Janus kinase inhibitors may be associated with an increased risk for developing HZ.”
Dr. Borum noted that the study is limited by its size and single-center design. “However, it supports the recommendations that additional research is needed to fully understand the potential impact of HZ on IBD patients.”
The study authors reported no relevant disclosures.
SOURCE: Borum ML et al. Inflamm Bowel Dis. 2019 Feb 7. doi: 10.1093/ibd/izy393.073.
REPORTING FROM THE CROHN’S & COLITIS CONGRESS
Key clinical point: Younger patients with inflammatory bowel disease may face a higher risk of infection with herpes zoster.
Major finding: About 3% of patients with inflammatory bowel disease were diagnosed with herpes zoster infection, and their average age was 37 years.
Study details: A retrospective 5-year chart review of 393 patients with inflammatory bowel disease.
Disclosures: The authors reported no relevant disclosures.
Source: Borum ML et al. Inflamm Bowel Dis. 2019 Feb 7. doi: 10.1093/ibd/izy393.073.
ALA report: Federal and state actions to limit tobacco use fall short
Tobacco use is currently at an all-time low thanks to public and private efforts, but more aggressive action from federal, state, and local governments is needed to protect the public, according to a review of tobacco control trends in the United States.
The American Lung Association (ALA) released “State of Tobacco Control” 2019, its 17th annual state-by-state analysis and list of recommended policy priorities to limit tobacco use. Although the report notes some positive steps taken by the federal and state governments, shortfalls in policy and legislation also are highlighted. The report states, “We know how and are ready to save more lives, but we need our elected officials to do much more. To many, solving America’s tobacco crisis might seem like a complex puzzle with no solution. And yet we have known for years what pieces are needed to reduce the disease and death caused by tobacco use.”
In this report, the federal government and each state are graded on a scale, A through F, for policy actions and laws to limit tobacco use. The grading methodology is based on a detailed point system cataloging the implementation and strength of specific actions and policies to limit tobacco use.
Areas of Impact
The report focused on six areas of public policy that affect exposure to and use of tobacco:
- Smoke-free air: Protecting the public from secondhand smoke should be a priority for policymakers, according the report, but 22 states have no smoke-free workplace laws in place. Laws restricting e-cigarettes in workplaces and public buildings have lagged behind tobacco laws in many states.
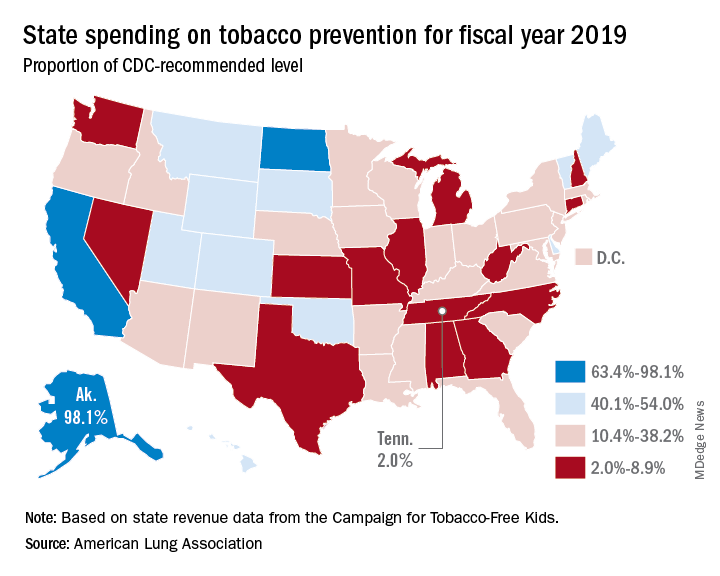
- Tobacco prevention funding: Dedicated funds to prevent tobacco addiction before it starts is a key element of a public health attack on tobacco use, but no U.S. state currently spends what the Centers for Disease Control and Prevention has recommended. Twenty years ago, the Master Settlement Agreement between the tobacco industry and 46 states and the District of Columbia guaranteed ongoing payments to the states to be used for tobacco prevention and control. Although those funds have been collected in the states to the tune of $27 billion since 1998, overall only 2.4% of those funds have been spent for this purpose, and the rest has been budgeted for other purposes.
- Tobacco taxes: Sales taxes on tobacco products have been highly effective in preventing young people from taking up tobacco use, but those taxation rates have remained unchanged in 2018 in all but the District of Columbia and Oklahoma. The tobacco industry spent $22 million in a successful effort to defeat ballot measures to increase sales taxes on tobacco in Montana and South Dakota.
- Tobacco 21: “Increasing the legal age of sale for tobacco products to 21 would decrease tobacco use by 12% and could prevent 223,000 deaths among those born between 2000 and 2019,” the report noted, citing a 2015 report by the Institute of Medicine. So far, the this restriction has been legislated in six states, the District of Columbia, and numerous local governments. The ALA considers increasing the age for tobacco sales to 21 to be a public health priority.
- Helping smokers quit: The addictive qualities of tobacco mean that many smokers struggle unsuccessfully to quit, and medical intervention is needed to help them. The report notes that current law requires that Medicaid expansion health plans and private insurance plans cover comprehensive smoking cessation treatment. However, not all states have the expanded Medicaid program, and many of those with Medicaid expansion don’t offer coverage of all Food and Drug–approved cessation treatments. Despite laws requiring smoking cessation coverage, many private insurance plans still do not include this coverage. The ALA recommends enforcement of the current law with regard to tobacco cessation insurance coverage.
- FDA regulation of tobacco products: The FDA has announced plans to make a major effort to reduce tobacco use in young people, decrease nicotine in cigarettes, and to restrict flavored tobacco products. But these plans fall short of the aggressive action needed to curb the tobacco “epidemic,” according to the report. Delayed action and timid policy have “resulted in tobacco companies becoming more emboldened to devise new and egregious ways to addict youth and sustain addiction among current users.” The ALA report points to the steep rise in e-cigarette use among youth with a 20.8% rise in high school students using these products in 2018, a rise from 11.7% in 2017. This trend is not likely to be reversed by the FDA proposals to date, which rely on voluntary action by the industry to curb youth use, sales restrictions to youth, and restrictions on some flavored tobacco products.
The report card
Federal government efforts in regulation of tobacco products, taxation, and health insurance coverage of cessation all received an F in this report, while mass media campaigns were given an A.
The states didn’t fare much better. They were graded on prevention and control funding, smoke-free air, taxation, access to cessation services, and minimum age for sales. A total of 19 states received a grade of F in four or five of these areas.
Funding for prevention and control was evaluated as the percentage of the amount recommended by the CDC, adjusted for a variety of state-specific factors such as prevalence of tobacco use, cost and complexity of conducting mass media campaigns, and proportion of the audience below 200% of the federal poverty level. A limitation of this methodology of grading funding is that it doesn’t evaluate effectiveness of the spending or the level of spending in different program categories. The higher spenders on prevention and control were Alaska at 98.1% and California at 74.5% of the CDC recommended level. The lowest spenders were Georgia at 2.8% and Missouri at 3.0%.
All but eight states received an F on minimum age for tobacco sales because most have an age limit 18 instead of the ALA and CDC recommendation of age 21.
Harold Wimmer, the CEO of the American Lung Association, wrote, “Aggressive action by our country’s federal and state policymakers is urgently required. However, ‘State of Tobacco Control’ 2019 has found a disturbing failure by federal and state governments to take action to put in place meaningful and proven-effective policies that would have prevented, and reduced tobacco use during 2018. This failure to act places the lung health and lives of Americans at risk. We have also found that this lack of action has emboldened tobacco companies to be even more brazen in producing and marketing products squarely aimed at kids, such as the JUUL e-cigarettes that look like an easily concealed USB drive, which now dominate the market driven by youth use.”
The full report is available for download at the ALA website.
SOURCE: American Lung Association, “State of Tobacco Control 2019”.
Tobacco use is currently at an all-time low thanks to public and private efforts, but more aggressive action from federal, state, and local governments is needed to protect the public, according to a review of tobacco control trends in the United States.

The American Lung Association (ALA) released “State of Tobacco Control” 2019, its 17th annual state-by-state analysis and list of recommended policy priorities to limit tobacco use. Although the report notes some positive steps taken by the federal and state governments, shortfalls in policy and legislation also are highlighted. The report states, “We know how and are ready to save more lives, but we need our elected officials to do much more. To many, solving America’s tobacco crisis might seem like a complex puzzle with no solution. And yet we have known for years what pieces are needed to reduce the disease and death caused by tobacco use.”
In this report, the federal government and each state are graded on a scale, A through F, for policy actions and laws to limit tobacco use. The grading methodology is based on a detailed point system cataloging the implementation and strength of specific actions and policies to limit tobacco use.
Areas of Impact
The report focused on six areas of public policy that affect exposure to and use of tobacco:
- Smoke-free air: Protecting the public from secondhand smoke should be a priority for policymakers, according the report, but 22 states have no smoke-free workplace laws in place. Laws restricting e-cigarettes in workplaces and public buildings have lagged behind tobacco laws in many states.
- Tobacco prevention funding: Dedicated funds to prevent tobacco addiction before it starts is a key element of a public health attack on tobacco use, but no U.S. state currently spends what the Centers for Disease Control and Prevention has recommended. Twenty years ago, the Master Settlement Agreement between the tobacco industry and 46 states and the District of Columbia guaranteed ongoing payments to the states to be used for tobacco prevention and control. Although those funds have been collected in the states to the tune of $27 billion since 1998, overall only 2.4% of those funds have been spent for this purpose, and the rest has been budgeted for other purposes.
- Tobacco taxes: Sales taxes on tobacco products have been highly effective in preventing young people from taking up tobacco use, but those taxation rates have remained unchanged in 2018 in all but the District of Columbia and Oklahoma. The tobacco industry spent $22 million in a successful effort to defeat ballot measures to increase sales taxes on tobacco in Montana and South Dakota.
- Tobacco 21: “Increasing the legal age of sale for tobacco products to 21 would decrease tobacco use by 12% and could prevent 223,000 deaths among those born between 2000 and 2019,” the report noted, citing a 2015 report by the Institute of Medicine. So far, the this restriction has been legislated in six states, the District of Columbia, and numerous local governments. The ALA considers increasing the age for tobacco sales to 21 to be a public health priority.
- Helping smokers quit: The addictive qualities of tobacco mean that many smokers struggle unsuccessfully to quit, and medical intervention is needed to help them. The report notes that current law requires that Medicaid expansion health plans and private insurance plans cover comprehensive smoking cessation treatment. However, not all states have the expanded Medicaid program, and many of those with Medicaid expansion don’t offer coverage of all Food and Drug–approved cessation treatments. Despite laws requiring smoking cessation coverage, many private insurance plans still do not include this coverage. The ALA recommends enforcement of the current law with regard to tobacco cessation insurance coverage.
- FDA regulation of tobacco products: The FDA has announced plans to make a major effort to reduce tobacco use in young people, decrease nicotine in cigarettes, and to restrict flavored tobacco products. But these plans fall short of the aggressive action needed to curb the tobacco “epidemic,” according to the report. Delayed action and timid policy have “resulted in tobacco companies becoming more emboldened to devise new and egregious ways to addict youth and sustain addiction among current users.” The ALA report points to the steep rise in e-cigarette use among youth with a 20.8% rise in high school students using these products in 2018, a rise from 11.7% in 2017. This trend is not likely to be reversed by the FDA proposals to date, which rely on voluntary action by the industry to curb youth use, sales restrictions to youth, and restrictions on some flavored tobacco products.
The report card
Federal government efforts in regulation of tobacco products, taxation, and health insurance coverage of cessation all received an F in this report, while mass media campaigns were given an A.
The states didn’t fare much better. They were graded on prevention and control funding, smoke-free air, taxation, access to cessation services, and minimum age for sales. A total of 19 states received a grade of F in four or five of these areas.
Funding for prevention and control was evaluated as the percentage of the amount recommended by the CDC, adjusted for a variety of state-specific factors such as prevalence of tobacco use, cost and complexity of conducting mass media campaigns, and proportion of the audience below 200% of the federal poverty level. A limitation of this methodology of grading funding is that it doesn’t evaluate effectiveness of the spending or the level of spending in different program categories. The higher spenders on prevention and control were Alaska at 98.1% and California at 74.5% of the CDC recommended level. The lowest spenders were Georgia at 2.8% and Missouri at 3.0%.
All but eight states received an F on minimum age for tobacco sales because most have an age limit 18 instead of the ALA and CDC recommendation of age 21.
Harold Wimmer, the CEO of the American Lung Association, wrote, “Aggressive action by our country’s federal and state policymakers is urgently required. However, ‘State of Tobacco Control’ 2019 has found a disturbing failure by federal and state governments to take action to put in place meaningful and proven-effective policies that would have prevented, and reduced tobacco use during 2018. This failure to act places the lung health and lives of Americans at risk. We have also found that this lack of action has emboldened tobacco companies to be even more brazen in producing and marketing products squarely aimed at kids, such as the JUUL e-cigarettes that look like an easily concealed USB drive, which now dominate the market driven by youth use.”
The full report is available for download at the ALA website.
SOURCE: American Lung Association, “State of Tobacco Control 2019”.
Tobacco use is currently at an all-time low thanks to public and private efforts, but more aggressive action from federal, state, and local governments is needed to protect the public, according to a review of tobacco control trends in the United States.

The American Lung Association (ALA) released “State of Tobacco Control” 2019, its 17th annual state-by-state analysis and list of recommended policy priorities to limit tobacco use. Although the report notes some positive steps taken by the federal and state governments, shortfalls in policy and legislation also are highlighted. The report states, “We know how and are ready to save more lives, but we need our elected officials to do much more. To many, solving America’s tobacco crisis might seem like a complex puzzle with no solution. And yet we have known for years what pieces are needed to reduce the disease and death caused by tobacco use.”
In this report, the federal government and each state are graded on a scale, A through F, for policy actions and laws to limit tobacco use. The grading methodology is based on a detailed point system cataloging the implementation and strength of specific actions and policies to limit tobacco use.
Areas of Impact
The report focused on six areas of public policy that affect exposure to and use of tobacco:
- Smoke-free air: Protecting the public from secondhand smoke should be a priority for policymakers, according the report, but 22 states have no smoke-free workplace laws in place. Laws restricting e-cigarettes in workplaces and public buildings have lagged behind tobacco laws in many states.
- Tobacco prevention funding: Dedicated funds to prevent tobacco addiction before it starts is a key element of a public health attack on tobacco use, but no U.S. state currently spends what the Centers for Disease Control and Prevention has recommended. Twenty years ago, the Master Settlement Agreement between the tobacco industry and 46 states and the District of Columbia guaranteed ongoing payments to the states to be used for tobacco prevention and control. Although those funds have been collected in the states to the tune of $27 billion since 1998, overall only 2.4% of those funds have been spent for this purpose, and the rest has been budgeted for other purposes.
- Tobacco taxes: Sales taxes on tobacco products have been highly effective in preventing young people from taking up tobacco use, but those taxation rates have remained unchanged in 2018 in all but the District of Columbia and Oklahoma. The tobacco industry spent $22 million in a successful effort to defeat ballot measures to increase sales taxes on tobacco in Montana and South Dakota.
- Tobacco 21: “Increasing the legal age of sale for tobacco products to 21 would decrease tobacco use by 12% and could prevent 223,000 deaths among those born between 2000 and 2019,” the report noted, citing a 2015 report by the Institute of Medicine. So far, the this restriction has been legislated in six states, the District of Columbia, and numerous local governments. The ALA considers increasing the age for tobacco sales to 21 to be a public health priority.
- Helping smokers quit: The addictive qualities of tobacco mean that many smokers struggle unsuccessfully to quit, and medical intervention is needed to help them. The report notes that current law requires that Medicaid expansion health plans and private insurance plans cover comprehensive smoking cessation treatment. However, not all states have the expanded Medicaid program, and many of those with Medicaid expansion don’t offer coverage of all Food and Drug–approved cessation treatments. Despite laws requiring smoking cessation coverage, many private insurance plans still do not include this coverage. The ALA recommends enforcement of the current law with regard to tobacco cessation insurance coverage.
- FDA regulation of tobacco products: The FDA has announced plans to make a major effort to reduce tobacco use in young people, decrease nicotine in cigarettes, and to restrict flavored tobacco products. But these plans fall short of the aggressive action needed to curb the tobacco “epidemic,” according to the report. Delayed action and timid policy have “resulted in tobacco companies becoming more emboldened to devise new and egregious ways to addict youth and sustain addiction among current users.” The ALA report points to the steep rise in e-cigarette use among youth with a 20.8% rise in high school students using these products in 2018, a rise from 11.7% in 2017. This trend is not likely to be reversed by the FDA proposals to date, which rely on voluntary action by the industry to curb youth use, sales restrictions to youth, and restrictions on some flavored tobacco products.
The report card
Federal government efforts in regulation of tobacco products, taxation, and health insurance coverage of cessation all received an F in this report, while mass media campaigns were given an A.
The states didn’t fare much better. They were graded on prevention and control funding, smoke-free air, taxation, access to cessation services, and minimum age for sales. A total of 19 states received a grade of F in four or five of these areas.
Funding for prevention and control was evaluated as the percentage of the amount recommended by the CDC, adjusted for a variety of state-specific factors such as prevalence of tobacco use, cost and complexity of conducting mass media campaigns, and proportion of the audience below 200% of the federal poverty level. A limitation of this methodology of grading funding is that it doesn’t evaluate effectiveness of the spending or the level of spending in different program categories. The higher spenders on prevention and control were Alaska at 98.1% and California at 74.5% of the CDC recommended level. The lowest spenders were Georgia at 2.8% and Missouri at 3.0%.
All but eight states received an F on minimum age for tobacco sales because most have an age limit 18 instead of the ALA and CDC recommendation of age 21.
Harold Wimmer, the CEO of the American Lung Association, wrote, “Aggressive action by our country’s federal and state policymakers is urgently required. However, ‘State of Tobacco Control’ 2019 has found a disturbing failure by federal and state governments to take action to put in place meaningful and proven-effective policies that would have prevented, and reduced tobacco use during 2018. This failure to act places the lung health and lives of Americans at risk. We have also found that this lack of action has emboldened tobacco companies to be even more brazen in producing and marketing products squarely aimed at kids, such as the JUUL e-cigarettes that look like an easily concealed USB drive, which now dominate the market driven by youth use.”
The full report is available for download at the ALA website.
SOURCE: American Lung Association, “State of Tobacco Control 2019”.
February 2019 Highlights
Anthracyclines, bendamustine are options for grade 3A follicular lymphoma
While optimal treatment for grade 3A follicular lymphoma remains in question, either anthracycline-based chemotherapy or bendamustine appear to be preferable to cyclophosphamide, vincristine, and prednisone (CVP), results of a recent analysis suggest.
Time to progression with anthracycline-based chemotherapy was superior to that of CVP in the retrospective, multicenter study.
At the same time, clinical outcomes were comparable between anthracycline-based chemotherapy and bendamustine, according to Nirav N. Shah, MD, of the Medical College of Wisconsin, Milwaukee, and his coinvestigators.
“Both remain appropriate frontline options for this patient population,” Dr. Shah and his colleagues wrote in Clinical Lymphoma, Myeloma & Leukemia.
Frontline therapy for follicular lymphoma has evolved, and recently shifted toward bendamustine-based chemotherapy regimens in light of two large randomized trials, according to the investigators. However, optimal therapy – specifically for grade 3A follicular lymphoma – has been debated for more than 20 years, they added.
“While some approach it as an aggressive malignancy, others treat it as an indolent lymphoma,” they wrote.
Accordingly, Dr. Shah and his colleagues sought to evaluate treatment outcomes with these regimens in 103 advanced stage 3/4 follicular lymphoma patients from six centers seen over a 10-year period.
Of those patients, 65 had received anthracycline-based chemotherapy, 30 received bendamustine, and 8 received CVP. All received either rituximab or ofatumumab in combination with the chemotherapy, and about one-third went on to receive maintenance treatment with one of those two anti-CD20 antibodies.
The proportion of patients not experiencing disease progression at 24 months from the initiation of treatment was significantly different between arms, at 72% for those receiving anthracyclines, 79% for bendamustine, and 50% for CVP (P = .01).
Patients who received CVP had a significantly poorer time-to-progression outcomes versus anthracycline-based chemotherapy, an adjusted analysis showed (hazard ratio, 3.22; 95% confidence interval, 1.26-8.25; P = .01), while by contrast, there was no significant difference between bendamustine and anthracyclines on this endpoint.
Progression-free survival was likewise worse for CVP compared with anthracycline-based chemotherapy, but there was no significant difference in overall survival for either CVP or bendamustine compared with anthracycline-based chemotherapy, the investigators said.
The 5-year overall survival was estimated to be 82% for anthracycline-based chemotherapy, 74% for bendamustine, and 58% for CVP (P = .23).
Optimal treatment of grade 3A follicular lymphoma remains controversial despite these findings, the investigators noted.
“Unfortunately, this specific histology was excluded from pivotal trials comparing anthracycline-based chemotherapy to bendamustine, leaving the question of optimal frontline treatment unanswered in this subset,” they wrote.
The situation could change with a subgroup analysis of GALLIUM, which might provide some prospective data for this histology. Beyond that, it would be helpful to have prospective, randomized studies specifically enrolling grade 3A disease, Dr. Shah and his coauthors wrote.
Dr. Shah reported disclosures related to Exelixis, Oncosec, Geron, Jazz, Kite, Juno, and Lentigen Technology. Coauthors provided disclosures related to Sanofi-Genzyme, Celgene, Takeda, Otsuka, Spectrum, Merck, and Astellas, among others.
SOURCE: Shah NN et al. Clin Lymphoma Myeloma Leuk. 2019 Feb;19(2):95-102.
While optimal treatment for grade 3A follicular lymphoma remains in question, either anthracycline-based chemotherapy or bendamustine appear to be preferable to cyclophosphamide, vincristine, and prednisone (CVP), results of a recent analysis suggest.
Time to progression with anthracycline-based chemotherapy was superior to that of CVP in the retrospective, multicenter study.
At the same time, clinical outcomes were comparable between anthracycline-based chemotherapy and bendamustine, according to Nirav N. Shah, MD, of the Medical College of Wisconsin, Milwaukee, and his coinvestigators.
“Both remain appropriate frontline options for this patient population,” Dr. Shah and his colleagues wrote in Clinical Lymphoma, Myeloma & Leukemia.
Frontline therapy for follicular lymphoma has evolved, and recently shifted toward bendamustine-based chemotherapy regimens in light of two large randomized trials, according to the investigators. However, optimal therapy – specifically for grade 3A follicular lymphoma – has been debated for more than 20 years, they added.
“While some approach it as an aggressive malignancy, others treat it as an indolent lymphoma,” they wrote.
Accordingly, Dr. Shah and his colleagues sought to evaluate treatment outcomes with these regimens in 103 advanced stage 3/4 follicular lymphoma patients from six centers seen over a 10-year period.
Of those patients, 65 had received anthracycline-based chemotherapy, 30 received bendamustine, and 8 received CVP. All received either rituximab or ofatumumab in combination with the chemotherapy, and about one-third went on to receive maintenance treatment with one of those two anti-CD20 antibodies.
The proportion of patients not experiencing disease progression at 24 months from the initiation of treatment was significantly different between arms, at 72% for those receiving anthracyclines, 79% for bendamustine, and 50% for CVP (P = .01).
Patients who received CVP had a significantly poorer time-to-progression outcomes versus anthracycline-based chemotherapy, an adjusted analysis showed (hazard ratio, 3.22; 95% confidence interval, 1.26-8.25; P = .01), while by contrast, there was no significant difference between bendamustine and anthracyclines on this endpoint.
Progression-free survival was likewise worse for CVP compared with anthracycline-based chemotherapy, but there was no significant difference in overall survival for either CVP or bendamustine compared with anthracycline-based chemotherapy, the investigators said.
The 5-year overall survival was estimated to be 82% for anthracycline-based chemotherapy, 74% for bendamustine, and 58% for CVP (P = .23).
Optimal treatment of grade 3A follicular lymphoma remains controversial despite these findings, the investigators noted.
“Unfortunately, this specific histology was excluded from pivotal trials comparing anthracycline-based chemotherapy to bendamustine, leaving the question of optimal frontline treatment unanswered in this subset,” they wrote.
The situation could change with a subgroup analysis of GALLIUM, which might provide some prospective data for this histology. Beyond that, it would be helpful to have prospective, randomized studies specifically enrolling grade 3A disease, Dr. Shah and his coauthors wrote.
Dr. Shah reported disclosures related to Exelixis, Oncosec, Geron, Jazz, Kite, Juno, and Lentigen Technology. Coauthors provided disclosures related to Sanofi-Genzyme, Celgene, Takeda, Otsuka, Spectrum, Merck, and Astellas, among others.
SOURCE: Shah NN et al. Clin Lymphoma Myeloma Leuk. 2019 Feb;19(2):95-102.
While optimal treatment for grade 3A follicular lymphoma remains in question, either anthracycline-based chemotherapy or bendamustine appear to be preferable to cyclophosphamide, vincristine, and prednisone (CVP), results of a recent analysis suggest.
Time to progression with anthracycline-based chemotherapy was superior to that of CVP in the retrospective, multicenter study.
At the same time, clinical outcomes were comparable between anthracycline-based chemotherapy and bendamustine, according to Nirav N. Shah, MD, of the Medical College of Wisconsin, Milwaukee, and his coinvestigators.
“Both remain appropriate frontline options for this patient population,” Dr. Shah and his colleagues wrote in Clinical Lymphoma, Myeloma & Leukemia.
Frontline therapy for follicular lymphoma has evolved, and recently shifted toward bendamustine-based chemotherapy regimens in light of two large randomized trials, according to the investigators. However, optimal therapy – specifically for grade 3A follicular lymphoma – has been debated for more than 20 years, they added.
“While some approach it as an aggressive malignancy, others treat it as an indolent lymphoma,” they wrote.
Accordingly, Dr. Shah and his colleagues sought to evaluate treatment outcomes with these regimens in 103 advanced stage 3/4 follicular lymphoma patients from six centers seen over a 10-year period.
Of those patients, 65 had received anthracycline-based chemotherapy, 30 received bendamustine, and 8 received CVP. All received either rituximab or ofatumumab in combination with the chemotherapy, and about one-third went on to receive maintenance treatment with one of those two anti-CD20 antibodies.
The proportion of patients not experiencing disease progression at 24 months from the initiation of treatment was significantly different between arms, at 72% for those receiving anthracyclines, 79% for bendamustine, and 50% for CVP (P = .01).
Patients who received CVP had a significantly poorer time-to-progression outcomes versus anthracycline-based chemotherapy, an adjusted analysis showed (hazard ratio, 3.22; 95% confidence interval, 1.26-8.25; P = .01), while by contrast, there was no significant difference between bendamustine and anthracyclines on this endpoint.
Progression-free survival was likewise worse for CVP compared with anthracycline-based chemotherapy, but there was no significant difference in overall survival for either CVP or bendamustine compared with anthracycline-based chemotherapy, the investigators said.
The 5-year overall survival was estimated to be 82% for anthracycline-based chemotherapy, 74% for bendamustine, and 58% for CVP (P = .23).
Optimal treatment of grade 3A follicular lymphoma remains controversial despite these findings, the investigators noted.
“Unfortunately, this specific histology was excluded from pivotal trials comparing anthracycline-based chemotherapy to bendamustine, leaving the question of optimal frontline treatment unanswered in this subset,” they wrote.
The situation could change with a subgroup analysis of GALLIUM, which might provide some prospective data for this histology. Beyond that, it would be helpful to have prospective, randomized studies specifically enrolling grade 3A disease, Dr. Shah and his coauthors wrote.
Dr. Shah reported disclosures related to Exelixis, Oncosec, Geron, Jazz, Kite, Juno, and Lentigen Technology. Coauthors provided disclosures related to Sanofi-Genzyme, Celgene, Takeda, Otsuka, Spectrum, Merck, and Astellas, among others.
SOURCE: Shah NN et al. Clin Lymphoma Myeloma Leuk. 2019 Feb;19(2):95-102.
FROM CLINICAL LYMPHOMA, MYELOMA & LEUKEMIA
Key clinical point:
Major finding: Patients who received CVP had a significantly poorer time-to-progression outcome versus anthracycline-based chemotherapy (hazard ratio, 3.22; 95% CI, 1.26-8.25; P = .01), while there was no significant difference between bendamustine and anthracyclines.
Study details: A multicenter analysis including 103 patients with advanced stage grade 3A follicular lymphoma.
Disclosures: The authors reported disclosures related to Exelixis, OncoSec, Geron, Jazz, Kite, Juno, Lentigen Technology, Sanofi-Genzyme, Celgene, Takeda, Otsuka, Spectrum, Merck, and Astellas, among others.
Source: Shah NN et al. Clin Lymphoma Myeloma Leuk. 2019 Feb;19(2):95-102.
Ibrutinib-MTX-rituximab combo shows promise in CNS lymphoma
The three-drug combination of ibrutinib, high-dose methotrexate (HD-MTX), and rituximab showed positive safety and clinical outcomes in patients with recurrent/refractory primary/secondary CNS lymphoma, according to results from a phase 1b trial.
Ibrutinib has already shown single-agent activity in recurrent/refractory CNS lymphoma, Christian Grommes, MD, of Memorial Sloan Kettering Cancer Center in New York, and his colleagues, wrote in Blood. “The primary objective was to determine the maximum tolerated dose of ibrutinib in combination with HD-MTX alone and ibrutinib in combination with HD-MTX and rituximab.”
With respect to ibrutinib dosing, the initial cohort was started at 560 mg daily, which was increased to 840 mg daily in successive cohorts using a 3+3 design. HD-MTX was administered every 2 weeks at 3.5 g/m2 for a total of eight infusions, or four cycles, with each cycle lasting of 28 days.
After no dose-limiting adverse effects were seen with the ibrutinib-MTX combination, the researchers added rituximab at 500 mg/m2 every 2 weeks, for a total of eight infusions, which completed the induction phase. The three-agent induction therapy was followed by daily ibrutinib monotherapy, which was maintained until discontinuation caused by malignancy progression, intolerable adverse events, or death.
“To minimize the risk of adverse events, we held ibrutinib on days of HD-MTX infusion and resumed 5 days after HD-MTX infusion or after MTX clearance,” they wrote.
After analysis, Dr. Grommes and his colleagues reported that no dose-limiting or grade 5 toxicities were detected. At a median follow-up of 19.7 months, they saw an 80% overall response rate in study patients treated with combination therapy. The median progression free survival for all 15 patients was 9.2 months and the median overall survival was not reached, with 11 of 15 patients alive.
The researchers proposed an 840-mg dose of ibrutinib for future studies.
The most frequent adverse events were lymphopenia, thrombocytopenia, anemia, and transaminase elevations. No fungal infections were seen during the study.
The researchers noted that two key limitations of the study were the nonrandomized design and small sample size. As a result, they reported that the degree of ibrutinib-specific activity in the three-drug combination remains unknown.
The study was supported by grant funding from Pharmacyclics to Memorial Sloan Kettering. The authors reported financial ties to AstraZeneca, Bristol-Myers Squibb, BTH, Kite Pharma, Pfizer, and others.
SOURCE: Grommes C et al. Blood. 2019;133(5):436-45.
The three-drug combination of ibrutinib, high-dose methotrexate (HD-MTX), and rituximab showed positive safety and clinical outcomes in patients with recurrent/refractory primary/secondary CNS lymphoma, according to results from a phase 1b trial.
Ibrutinib has already shown single-agent activity in recurrent/refractory CNS lymphoma, Christian Grommes, MD, of Memorial Sloan Kettering Cancer Center in New York, and his colleagues, wrote in Blood. “The primary objective was to determine the maximum tolerated dose of ibrutinib in combination with HD-MTX alone and ibrutinib in combination with HD-MTX and rituximab.”
With respect to ibrutinib dosing, the initial cohort was started at 560 mg daily, which was increased to 840 mg daily in successive cohorts using a 3+3 design. HD-MTX was administered every 2 weeks at 3.5 g/m2 for a total of eight infusions, or four cycles, with each cycle lasting of 28 days.
After no dose-limiting adverse effects were seen with the ibrutinib-MTX combination, the researchers added rituximab at 500 mg/m2 every 2 weeks, for a total of eight infusions, which completed the induction phase. The three-agent induction therapy was followed by daily ibrutinib monotherapy, which was maintained until discontinuation caused by malignancy progression, intolerable adverse events, or death.
“To minimize the risk of adverse events, we held ibrutinib on days of HD-MTX infusion and resumed 5 days after HD-MTX infusion or after MTX clearance,” they wrote.
After analysis, Dr. Grommes and his colleagues reported that no dose-limiting or grade 5 toxicities were detected. At a median follow-up of 19.7 months, they saw an 80% overall response rate in study patients treated with combination therapy. The median progression free survival for all 15 patients was 9.2 months and the median overall survival was not reached, with 11 of 15 patients alive.
The researchers proposed an 840-mg dose of ibrutinib for future studies.
The most frequent adverse events were lymphopenia, thrombocytopenia, anemia, and transaminase elevations. No fungal infections were seen during the study.
The researchers noted that two key limitations of the study were the nonrandomized design and small sample size. As a result, they reported that the degree of ibrutinib-specific activity in the three-drug combination remains unknown.
The study was supported by grant funding from Pharmacyclics to Memorial Sloan Kettering. The authors reported financial ties to AstraZeneca, Bristol-Myers Squibb, BTH, Kite Pharma, Pfizer, and others.
SOURCE: Grommes C et al. Blood. 2019;133(5):436-45.
The three-drug combination of ibrutinib, high-dose methotrexate (HD-MTX), and rituximab showed positive safety and clinical outcomes in patients with recurrent/refractory primary/secondary CNS lymphoma, according to results from a phase 1b trial.
Ibrutinib has already shown single-agent activity in recurrent/refractory CNS lymphoma, Christian Grommes, MD, of Memorial Sloan Kettering Cancer Center in New York, and his colleagues, wrote in Blood. “The primary objective was to determine the maximum tolerated dose of ibrutinib in combination with HD-MTX alone and ibrutinib in combination with HD-MTX and rituximab.”
With respect to ibrutinib dosing, the initial cohort was started at 560 mg daily, which was increased to 840 mg daily in successive cohorts using a 3+3 design. HD-MTX was administered every 2 weeks at 3.5 g/m2 for a total of eight infusions, or four cycles, with each cycle lasting of 28 days.
After no dose-limiting adverse effects were seen with the ibrutinib-MTX combination, the researchers added rituximab at 500 mg/m2 every 2 weeks, for a total of eight infusions, which completed the induction phase. The three-agent induction therapy was followed by daily ibrutinib monotherapy, which was maintained until discontinuation caused by malignancy progression, intolerable adverse events, or death.
“To minimize the risk of adverse events, we held ibrutinib on days of HD-MTX infusion and resumed 5 days after HD-MTX infusion or after MTX clearance,” they wrote.
After analysis, Dr. Grommes and his colleagues reported that no dose-limiting or grade 5 toxicities were detected. At a median follow-up of 19.7 months, they saw an 80% overall response rate in study patients treated with combination therapy. The median progression free survival for all 15 patients was 9.2 months and the median overall survival was not reached, with 11 of 15 patients alive.
The researchers proposed an 840-mg dose of ibrutinib for future studies.
The most frequent adverse events were lymphopenia, thrombocytopenia, anemia, and transaminase elevations. No fungal infections were seen during the study.
The researchers noted that two key limitations of the study were the nonrandomized design and small sample size. As a result, they reported that the degree of ibrutinib-specific activity in the three-drug combination remains unknown.
The study was supported by grant funding from Pharmacyclics to Memorial Sloan Kettering. The authors reported financial ties to AstraZeneca, Bristol-Myers Squibb, BTH, Kite Pharma, Pfizer, and others.
SOURCE: Grommes C et al. Blood. 2019;133(5):436-45.
FROM BLOOD
Key clinical point:
Major finding: The ibrutinib-based regimen showed an 80% overall response rate; no grade 5 adverse events were reported.
Study details: A phase 1b study of 15 patients with recurrent/refractory CNS lymphoma.
Disclosures: The study was supported by grant funding from Pharmacyclics to Memorial Sloan Kettering. The authors reported financial ties to AstraZeneca, Bristol-Myers Squibb, BTH, Kite Pharma, Pfizer, and others.
Source: Grommes C et al. Blood. 2019;133(5):436-45.
Use three phases of psychiatric disorders in children to guide treatment
BROOKLYN, N.Y. – , John T. Walkup, MD, said at a pediatric psychopharmacology update held by the American Academy of Child and Adolescent Psychiatry.
After the onset of symptoms and over the course of time, those with untreated anxiety disorders are at risk for developing impairment in adaptation and coping, and also the development of maladaptive behaviors like substance abuse and suicidal behavior, said Dr. Walkup, chair of the department of psychiatry at Ann and Robert H. Lurie Children’s Hospital of Chicago.
The focus of his presentation was on the treatment of anxiety disorders in children, but Dr. Walkup said the impact of the three-tier progression is likely relevant to any psychiatric disorder that begins in childhood.
In essence, the scope of problems becomes more complicated over time, and without early treatment, children continue to be symptomatic. But they also develop a lifestyle based on avoidance coping and might engage in maladaptive behaviors, Dr. Walkup said. As a result, the complexity of treatment increases substantially beyond just symptom control.
Providing an example, Dr. Walkup described a child of 7 years of age with separation anxiety. If treated at the time symptoms begin, Dr. Walkup explained, cognitive-behavioral therapy and medication would be expected to be both straightforward and highly effective. If left untreated until age 14, the child might accumulate impairment in independent functioning (due to avoidance coping) at a particularly important time in development.
“In those kids, you can reduce their anxiety burden with acute treatments like [cognitive-behavioral therapy] or meds, but now you also have 7 or 8 years of accumulated impairment due to avoidance coping and parental accommodation,” Dr. Walkup said. “If those kids are going to catch up developmentally, they also need life skill support in addition to symptomatic treatment for their anxiety.”
In the case of any pediatric psychiatric disorder, early treatment has the potential to thwart progression to a more complex and treatment-resistant form, but anxiety is a particularly prominent example. In most children, anxiety is relatively easy to control if caught early but a greater challenge when fears are not contained and the child accumulates ongoing impairment.
The obstacle is that many children are not diagnosed at the time of onset, said Dr. Walkup. The solution, he suggested, is better training of pediatricians and other primary care physicians not only to identify those children but to initiate treatment in uncomplicated cases.
“The person who has that longitudinal relationship with the child is their primary care provider, and this is really the person who is going to do the best job in getting to these kids early and initiating treatment,” Dr. Walkup said.
“We have a program in Chicago where we have trained primary care physicians not only to treat anxiety and depression, but we have specifically focused them on the easiest cases in their caseload, the classic phenotypes,” Dr. Walkup reported. Using a collaborative care model, this approach has been effective in building the confidence of primary care clinicians and in reaching children when symptoms are easier to control.
Importantly, anti-anxiety medication delivered in primary care could be sufficient to help children to manage anxiety effectively when parents cooperate in helping their children manage their fears.
“People suggest that we always start with CBT, but there [are no data] to support that. I think it is a conclusion drawn from the fact that CBT works and medication has side effects,” Dr. Walkup said. He appreciates the evidence that CBT is effective, but he cautioned that this therapy is not available everywhere, and pharmacologic therapies may be as or potentially more effective for some anxiety symptoms like anxiety-related physical symptoms.
Conversely, some have expressed the opinion that drugs might be a better option in late adolescence, when the efficacy of CBT appears to diminish, but Dr. Walkup objected to that characterization as well.
“My sense is that if you treat a 7-year-old for symptoms that have lasted a year it’s very different from treating a 17-year-old who has had symptoms for a decade,” Dr. Walkup said. Referring back to the contention that psychiatric disease in children becomes more complicated with a longer duration, this might explain why “you don’t see as much immediate success” with CBT and medication in the older age groups even if this is an effective treatment tool.
Some psychiatric disorders in children, including anxiety, might resolve with age, but early recognition and treatment should be a goal because of the potential to reduce symptoms and avoidance coping, and improve long-term outcomes, Dr. Walkup reported. Ironically, it might not be just anxiety symptoms, but poor adaptation and coping that might be the most important driver of ongoing impairment.
Dr. Walkup has served as an unpaid adviser to the Anxiety Disorders of Association of America. In addition, he has received royalties from Wolters Kluwer for CME activity on childhood anxiety.
This story was updated 2/11/2019.
BROOKLYN, N.Y. – , John T. Walkup, MD, said at a pediatric psychopharmacology update held by the American Academy of Child and Adolescent Psychiatry.
After the onset of symptoms and over the course of time, those with untreated anxiety disorders are at risk for developing impairment in adaptation and coping, and also the development of maladaptive behaviors like substance abuse and suicidal behavior, said Dr. Walkup, chair of the department of psychiatry at Ann and Robert H. Lurie Children’s Hospital of Chicago.
The focus of his presentation was on the treatment of anxiety disorders in children, but Dr. Walkup said the impact of the three-tier progression is likely relevant to any psychiatric disorder that begins in childhood.
In essence, the scope of problems becomes more complicated over time, and without early treatment, children continue to be symptomatic. But they also develop a lifestyle based on avoidance coping and might engage in maladaptive behaviors, Dr. Walkup said. As a result, the complexity of treatment increases substantially beyond just symptom control.
Providing an example, Dr. Walkup described a child of 7 years of age with separation anxiety. If treated at the time symptoms begin, Dr. Walkup explained, cognitive-behavioral therapy and medication would be expected to be both straightforward and highly effective. If left untreated until age 14, the child might accumulate impairment in independent functioning (due to avoidance coping) at a particularly important time in development.
“In those kids, you can reduce their anxiety burden with acute treatments like [cognitive-behavioral therapy] or meds, but now you also have 7 or 8 years of accumulated impairment due to avoidance coping and parental accommodation,” Dr. Walkup said. “If those kids are going to catch up developmentally, they also need life skill support in addition to symptomatic treatment for their anxiety.”
In the case of any pediatric psychiatric disorder, early treatment has the potential to thwart progression to a more complex and treatment-resistant form, but anxiety is a particularly prominent example. In most children, anxiety is relatively easy to control if caught early but a greater challenge when fears are not contained and the child accumulates ongoing impairment.
The obstacle is that many children are not diagnosed at the time of onset, said Dr. Walkup. The solution, he suggested, is better training of pediatricians and other primary care physicians not only to identify those children but to initiate treatment in uncomplicated cases.
“The person who has that longitudinal relationship with the child is their primary care provider, and this is really the person who is going to do the best job in getting to these kids early and initiating treatment,” Dr. Walkup said.
“We have a program in Chicago where we have trained primary care physicians not only to treat anxiety and depression, but we have specifically focused them on the easiest cases in their caseload, the classic phenotypes,” Dr. Walkup reported. Using a collaborative care model, this approach has been effective in building the confidence of primary care clinicians and in reaching children when symptoms are easier to control.
Importantly, anti-anxiety medication delivered in primary care could be sufficient to help children to manage anxiety effectively when parents cooperate in helping their children manage their fears.
“People suggest that we always start with CBT, but there [are no data] to support that. I think it is a conclusion drawn from the fact that CBT works and medication has side effects,” Dr. Walkup said. He appreciates the evidence that CBT is effective, but he cautioned that this therapy is not available everywhere, and pharmacologic therapies may be as or potentially more effective for some anxiety symptoms like anxiety-related physical symptoms.
Conversely, some have expressed the opinion that drugs might be a better option in late adolescence, when the efficacy of CBT appears to diminish, but Dr. Walkup objected to that characterization as well.
“My sense is that if you treat a 7-year-old for symptoms that have lasted a year it’s very different from treating a 17-year-old who has had symptoms for a decade,” Dr. Walkup said. Referring back to the contention that psychiatric disease in children becomes more complicated with a longer duration, this might explain why “you don’t see as much immediate success” with CBT and medication in the older age groups even if this is an effective treatment tool.
Some psychiatric disorders in children, including anxiety, might resolve with age, but early recognition and treatment should be a goal because of the potential to reduce symptoms and avoidance coping, and improve long-term outcomes, Dr. Walkup reported. Ironically, it might not be just anxiety symptoms, but poor adaptation and coping that might be the most important driver of ongoing impairment.
Dr. Walkup has served as an unpaid adviser to the Anxiety Disorders of Association of America. In addition, he has received royalties from Wolters Kluwer for CME activity on childhood anxiety.
This story was updated 2/11/2019.
BROOKLYN, N.Y. – , John T. Walkup, MD, said at a pediatric psychopharmacology update held by the American Academy of Child and Adolescent Psychiatry.
After the onset of symptoms and over the course of time, those with untreated anxiety disorders are at risk for developing impairment in adaptation and coping, and also the development of maladaptive behaviors like substance abuse and suicidal behavior, said Dr. Walkup, chair of the department of psychiatry at Ann and Robert H. Lurie Children’s Hospital of Chicago.
The focus of his presentation was on the treatment of anxiety disorders in children, but Dr. Walkup said the impact of the three-tier progression is likely relevant to any psychiatric disorder that begins in childhood.
In essence, the scope of problems becomes more complicated over time, and without early treatment, children continue to be symptomatic. But they also develop a lifestyle based on avoidance coping and might engage in maladaptive behaviors, Dr. Walkup said. As a result, the complexity of treatment increases substantially beyond just symptom control.
Providing an example, Dr. Walkup described a child of 7 years of age with separation anxiety. If treated at the time symptoms begin, Dr. Walkup explained, cognitive-behavioral therapy and medication would be expected to be both straightforward and highly effective. If left untreated until age 14, the child might accumulate impairment in independent functioning (due to avoidance coping) at a particularly important time in development.
“In those kids, you can reduce their anxiety burden with acute treatments like [cognitive-behavioral therapy] or meds, but now you also have 7 or 8 years of accumulated impairment due to avoidance coping and parental accommodation,” Dr. Walkup said. “If those kids are going to catch up developmentally, they also need life skill support in addition to symptomatic treatment for their anxiety.”
In the case of any pediatric psychiatric disorder, early treatment has the potential to thwart progression to a more complex and treatment-resistant form, but anxiety is a particularly prominent example. In most children, anxiety is relatively easy to control if caught early but a greater challenge when fears are not contained and the child accumulates ongoing impairment.
The obstacle is that many children are not diagnosed at the time of onset, said Dr. Walkup. The solution, he suggested, is better training of pediatricians and other primary care physicians not only to identify those children but to initiate treatment in uncomplicated cases.
“The person who has that longitudinal relationship with the child is their primary care provider, and this is really the person who is going to do the best job in getting to these kids early and initiating treatment,” Dr. Walkup said.
“We have a program in Chicago where we have trained primary care physicians not only to treat anxiety and depression, but we have specifically focused them on the easiest cases in their caseload, the classic phenotypes,” Dr. Walkup reported. Using a collaborative care model, this approach has been effective in building the confidence of primary care clinicians and in reaching children when symptoms are easier to control.
Importantly, anti-anxiety medication delivered in primary care could be sufficient to help children to manage anxiety effectively when parents cooperate in helping their children manage their fears.
“People suggest that we always start with CBT, but there [are no data] to support that. I think it is a conclusion drawn from the fact that CBT works and medication has side effects,” Dr. Walkup said. He appreciates the evidence that CBT is effective, but he cautioned that this therapy is not available everywhere, and pharmacologic therapies may be as or potentially more effective for some anxiety symptoms like anxiety-related physical symptoms.
Conversely, some have expressed the opinion that drugs might be a better option in late adolescence, when the efficacy of CBT appears to diminish, but Dr. Walkup objected to that characterization as well.
“My sense is that if you treat a 7-year-old for symptoms that have lasted a year it’s very different from treating a 17-year-old who has had symptoms for a decade,” Dr. Walkup said. Referring back to the contention that psychiatric disease in children becomes more complicated with a longer duration, this might explain why “you don’t see as much immediate success” with CBT and medication in the older age groups even if this is an effective treatment tool.
Some psychiatric disorders in children, including anxiety, might resolve with age, but early recognition and treatment should be a goal because of the potential to reduce symptoms and avoidance coping, and improve long-term outcomes, Dr. Walkup reported. Ironically, it might not be just anxiety symptoms, but poor adaptation and coping that might be the most important driver of ongoing impairment.
Dr. Walkup has served as an unpaid adviser to the Anxiety Disorders of Association of America. In addition, he has received royalties from Wolters Kluwer for CME activity on childhood anxiety.
This story was updated 2/11/2019.
REPORTING FROM THE PSYCHOPHARMACOLOGY UPDATE INSTITUTE
Daratumumab disappoints in non-Hodgkin lymphoma trial
Daratumumab is safe but ineffective for the treatment of patients with relapsed or refractory non-Hodgkin lymphoma (NHL) and CD38 expression of at least 50%, according to findings from a recent phase 2 trial.
Unfortunately, the study met headwinds early on, when initial screening of 112 patients with available tumor samples showed that only about half (56%) had CD38 expression of at least 50%, reported lead author Giles Salles, MD, PhD, of Claude Bernard University in Lyon, France, and his colleagues. The cutoff was based on preclinical models, suggesting that daratumumab-induced cytotoxicity depends on a high level of CD38 expression.
“Only 36 [patients] were eligible for study enrollment, questioning the generalizability of the study population,” the investigators wrote in Clinical Lymphoma, Myeloma & Leukemia.
Of these 36 patients, 15 had diffuse large B-cell lymphoma (DLBCL), 16 had follicular lymphoma (FL), and 5 had mantle cell lymphoma (MCL). Median CD38 expression was 70%. Patients were given 16 mg/kg of IV daratumumab once a week for two cycles, then every 2 weeks for four cycles, and finally on a monthly basis. Cycles were 28 days long. The primary endpoint was overall response rate. Safety and pharmacokinetics were also evaluated.
Results were generally disappointing, with ORR occurring in two patients (12.5%) with FL and one patient (6.7%) with DLBCL. No patients with MCL responded before the study was terminated. On a more encouraging note, 10 of 16 patients with FL maintained stable disease.
“All 16 patients in the FL cohort had progressed/relapsed on their prior treatment regimen; therefore, the maintenance of stable disease in the FL cohort may suggest some clinical benefit of daratumumab in this subset of NHL,” the investigators wrote.
Pharmacokinetics and safety data were similar to those from multiple myeloma studies of daratumumab; no new safety signals or instances of immunogenicity were encountered. The most common grade 3 or higher treatment-related adverse event was thrombocytopenia, which occurred in 11.1% of patients. Infusion-related reactions occurred in 72.2% of patients, but none were grade 4 and only three reactions were grade 3.
The investigators suggested that daratumumab may still play a role in NHL treatment, but not as a single agent.
“It is possible that daratumumab-based combination therapy would have allowed for more responses to be achieved within the current study,” the investigators wrote. “NHL is an extremely heterogeneous disease and the identification of predictive biomarkers and molecular genetics may provide new personalized therapies.”
The study was funded by Janssen Research & Development; two study authors reported employment by Janssen. Others reported financial ties to Janssen, Celgene, Roche, Gilead, Novartis, Amgen, and others.
SOURCE: Salles G et al. Clin Lymphoma Myeloma Leuk. 2019 Jan 2. doi: 10.1016/j.clml.2018.12.013.
Daratumumab is safe but ineffective for the treatment of patients with relapsed or refractory non-Hodgkin lymphoma (NHL) and CD38 expression of at least 50%, according to findings from a recent phase 2 trial.
Unfortunately, the study met headwinds early on, when initial screening of 112 patients with available tumor samples showed that only about half (56%) had CD38 expression of at least 50%, reported lead author Giles Salles, MD, PhD, of Claude Bernard University in Lyon, France, and his colleagues. The cutoff was based on preclinical models, suggesting that daratumumab-induced cytotoxicity depends on a high level of CD38 expression.
“Only 36 [patients] were eligible for study enrollment, questioning the generalizability of the study population,” the investigators wrote in Clinical Lymphoma, Myeloma & Leukemia.
Of these 36 patients, 15 had diffuse large B-cell lymphoma (DLBCL), 16 had follicular lymphoma (FL), and 5 had mantle cell lymphoma (MCL). Median CD38 expression was 70%. Patients were given 16 mg/kg of IV daratumumab once a week for two cycles, then every 2 weeks for four cycles, and finally on a monthly basis. Cycles were 28 days long. The primary endpoint was overall response rate. Safety and pharmacokinetics were also evaluated.
Results were generally disappointing, with ORR occurring in two patients (12.5%) with FL and one patient (6.7%) with DLBCL. No patients with MCL responded before the study was terminated. On a more encouraging note, 10 of 16 patients with FL maintained stable disease.
“All 16 patients in the FL cohort had progressed/relapsed on their prior treatment regimen; therefore, the maintenance of stable disease in the FL cohort may suggest some clinical benefit of daratumumab in this subset of NHL,” the investigators wrote.
Pharmacokinetics and safety data were similar to those from multiple myeloma studies of daratumumab; no new safety signals or instances of immunogenicity were encountered. The most common grade 3 or higher treatment-related adverse event was thrombocytopenia, which occurred in 11.1% of patients. Infusion-related reactions occurred in 72.2% of patients, but none were grade 4 and only three reactions were grade 3.
The investigators suggested that daratumumab may still play a role in NHL treatment, but not as a single agent.
“It is possible that daratumumab-based combination therapy would have allowed for more responses to be achieved within the current study,” the investigators wrote. “NHL is an extremely heterogeneous disease and the identification of predictive biomarkers and molecular genetics may provide new personalized therapies.”
The study was funded by Janssen Research & Development; two study authors reported employment by Janssen. Others reported financial ties to Janssen, Celgene, Roche, Gilead, Novartis, Amgen, and others.
SOURCE: Salles G et al. Clin Lymphoma Myeloma Leuk. 2019 Jan 2. doi: 10.1016/j.clml.2018.12.013.
Daratumumab is safe but ineffective for the treatment of patients with relapsed or refractory non-Hodgkin lymphoma (NHL) and CD38 expression of at least 50%, according to findings from a recent phase 2 trial.
Unfortunately, the study met headwinds early on, when initial screening of 112 patients with available tumor samples showed that only about half (56%) had CD38 expression of at least 50%, reported lead author Giles Salles, MD, PhD, of Claude Bernard University in Lyon, France, and his colleagues. The cutoff was based on preclinical models, suggesting that daratumumab-induced cytotoxicity depends on a high level of CD38 expression.
“Only 36 [patients] were eligible for study enrollment, questioning the generalizability of the study population,” the investigators wrote in Clinical Lymphoma, Myeloma & Leukemia.
Of these 36 patients, 15 had diffuse large B-cell lymphoma (DLBCL), 16 had follicular lymphoma (FL), and 5 had mantle cell lymphoma (MCL). Median CD38 expression was 70%. Patients were given 16 mg/kg of IV daratumumab once a week for two cycles, then every 2 weeks for four cycles, and finally on a monthly basis. Cycles were 28 days long. The primary endpoint was overall response rate. Safety and pharmacokinetics were also evaluated.
Results were generally disappointing, with ORR occurring in two patients (12.5%) with FL and one patient (6.7%) with DLBCL. No patients with MCL responded before the study was terminated. On a more encouraging note, 10 of 16 patients with FL maintained stable disease.
“All 16 patients in the FL cohort had progressed/relapsed on their prior treatment regimen; therefore, the maintenance of stable disease in the FL cohort may suggest some clinical benefit of daratumumab in this subset of NHL,” the investigators wrote.
Pharmacokinetics and safety data were similar to those from multiple myeloma studies of daratumumab; no new safety signals or instances of immunogenicity were encountered. The most common grade 3 or higher treatment-related adverse event was thrombocytopenia, which occurred in 11.1% of patients. Infusion-related reactions occurred in 72.2% of patients, but none were grade 4 and only three reactions were grade 3.
The investigators suggested that daratumumab may still play a role in NHL treatment, but not as a single agent.
“It is possible that daratumumab-based combination therapy would have allowed for more responses to be achieved within the current study,” the investigators wrote. “NHL is an extremely heterogeneous disease and the identification of predictive biomarkers and molecular genetics may provide new personalized therapies.”
The study was funded by Janssen Research & Development; two study authors reported employment by Janssen. Others reported financial ties to Janssen, Celgene, Roche, Gilead, Novartis, Amgen, and others.
SOURCE: Salles G et al. Clin Lymphoma Myeloma Leuk. 2019 Jan 2. doi: 10.1016/j.clml.2018.12.013.
FROM CLINICAL LYMPHOMA, MYELOMA & LEUKEMIA
Key clinical point:
Major finding: The overall response rate was 12.5% for patients with follicular lymphoma and 6.7% for diffuse large B-cell lymphoma (DLBCL). There were no responders in the mantle cell lymphoma cohort.
Study details: An open-label, phase 2 trial involving 15 patients with diffuse large B-cell lymphoma, 16 patients with follicular lymphoma, and 5 patients with mantle cell lymphoma.
Disclosures: The study was funded by Janssen Research & Development; two study authors reported employment by Janssen. Others reported financial ties to Janssen, Celgene, Roche, Gilead, Novartis, Amgen, and others.
Source: Salles G et al. Clin Lymphoma Myeloma Leuk. 2019 Jan 2. doi: 10.1016/j.clml.2018.12.013.
Pancreatic cancer expression signature is linked to chemoresistance
SAN FRANCISCO – , according to new data from the COMPASS trial reported at the 2019 GI Cancers Symposium.
“We now have two standard chemotherapy regimens that we use in the first-line setting, modified FOLFIRINOX (mFOLFIRINOX) and gemcitabine–nab-paclitaxel, but we lack any real biomarker strategies on which to choose for treatments, and many of our clinical trials in pancreas cancer have failed,” noted senior investigator Jennifer J. Knox, MD, FRCPC, codirector of the McCain Centre for Pancreatic Cancer, Princess Margaret Cancer Centre, Toronto.
In the past year, genomic profiling studies using different platforms have yielded similar results, suggesting that about 30% of patients with pancreatic cancer have actionable mutations that could help guide treatment choices.
The COMPASS trial prospectively recruited patients with advanced pancreatic ductal adenocarcinoma prior to first-line chemotherapy for tumor whole-genome sequencing and RNA sequencing. The trial previously established feasibility, with sequencing successfully completed in more than 90% of patients and availability of whole-genome results before the first disease assessment CT scan at 8 weeks (Clin Cancer Res. 2018;24[6]:1344-54).
New outcomes data for the 150 patients in the intention-to-treat population showed that overall survival was almost 4 months longer for those having a classic modified Moffitt RNA expression signature compared with those having a basal-like one. Similarly, it was more than 2 months longer for those having high versus low expression of the transcription factor GATA6.
In addition, among the subset of patients given mFOLFIRINOX, those having the classic signature were two-thirds less likely to die than were those having the basal-like signature.
“The RNA signature and GATA6 seem to discriminate two prognostic groups in advanced pancreas cancer. The basal-like cohort, or GATA6-low, may be particularly resistant to mFOLFIRINOX,” Dr. Knox said. “GATA6, and perhaps other markers, need to be validated as predictive biomarkers so that we can discover and use more effective therapies earlier on for our patients.
“COMPASS provides a very rich discovery set for other hypotheses and collaborators,” she said, noting that the investigators are adding trial data to the Enhanced Pancreatic Cancer Profiling for Individualized Care (EPPIC) project and to the Accelerate Research in Genomic Oncology (ARGO) project of the International Cancer Genome Consortium.
Rationally based treatment decisions
The COMPASS trial “will show us ... how we should move forward,” said invited discussant Heinz-Josef Lenz, MD, associate director for adult oncology and coleader of the Gastrointestinal Cancers Program, USC Norris Comprehensive Cancer Center, Los Angeles. “We don’t necessarily have the answer today to what the best treatment options are, but this is the first step to understand and develop rationally based treatment decisions for these molecularly characterized groups,” he said.
Whole-genome sequencing has been critically important for finding mutations in single genes, the proverbial needles in the haystack, he maintained. But in the future, the emphasis will likely be on combinations of mutations and other alterations.
Here, RNA is attractive in that it provides information about not only single genes, but also fusions, amplifications, and signatures, as well as treatment-induced changes. “We know that genetic makeup of tumors undergoes significant dynamic changes under treatment pressure. So if we have a gene expression signature associated with sensitivity to chemotherapy, will this change over time if there is progression of disease?” Dr. Lenz said. “That will be the challenge in the future, to better understand the dynamics in order to then recommend second- and third-line treatments.”
Some issues must be overcome to use RNA sequencing in routine clinical care, he acknowledged. For example, this sequencing requires fresh frozen tumor tissue, and it must be largely free of any normal tissue.
“There is no doubt that comprehensive molecular characterization with DNA and RNA will lead to better treatment options and identifying patients who benefit most from very unique treatments,” Dr. Lenz concluded. “The key is, you need to do the testing in order to find and identify the most successful treatments for our patients.”
Study details
Most of the 150 COMPASS patients had metastatic disease (87%), and they were about evenly split between receiving mFOLFIRINOX versus gemcitabine plus nab-paclitaxel as first-line chemotherapy, Dr. Knox reported at the symposium, sponsored by the American Gastroenterological Association, the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
Genomic profiling revealed that roughly 40% overall had potentially actionable variants: alterations in the presence of KRAS wild type (8%), homologous recombination deficiency involving BRCA (6%), and others (25%) such as activating PIK3CA mutations or HER2 amplification.
In findings that Dr. Knox called “quite striking,” among patients given mFOLFIRINOX, median progression-free survival was 7.17 months for those with the RNA classic expression signature versus 2.50 months for those with the RNA basal-like signature (hazard ratio, 0.17; P less than .0001). In contrast, among patients given gemcitabine plus nab-paclitaxel, there was no significant difference by signature (5.65 vs. 4.93 months; P = .69).
GATA6 expression was strongly related to signature type, with classic tumors having high expression and basal-like tumors having low expression (P less than .0001).
Overall survival was similarly better for patients with classic versus basal-like tumors (8.8 vs. 5.2 months; HR, 0.53; P = .006) and for patients with GATA6 high- versus low-expression tumors (8.2 vs. 5.8 months; HR, 0.66; P = .08).
In the whole cohort, overall survival differed across subsets according to RNA signature and type of chemotherapy received (P = .0134). When analysis was restricted to patients given mFOLFIRINOX, median overall survival was 10.1 months with the classic signature but just 5.3 months with the basal-like signature (HR, 0.33; P = .0005).
A multivariate analysis among all chemotherapy-treated patients confirmed the survival benefit of classic signature over basal-like signature (HR, 0.55; P = .03).
Dr. Knox disclosed that she has a consulting or advisory role with Lilly, Merck, and Bristol-Myers Squibb; that she receives honoraria from Novartis; and that she receives research funding from AstraZeneca and Merck. The study is sponsored by University Health Network, Toronto.
SOURCE: O’Kane GM et al. GI Cancers Symposium, Abstract 188.
SAN FRANCISCO – , according to new data from the COMPASS trial reported at the 2019 GI Cancers Symposium.
“We now have two standard chemotherapy regimens that we use in the first-line setting, modified FOLFIRINOX (mFOLFIRINOX) and gemcitabine–nab-paclitaxel, but we lack any real biomarker strategies on which to choose for treatments, and many of our clinical trials in pancreas cancer have failed,” noted senior investigator Jennifer J. Knox, MD, FRCPC, codirector of the McCain Centre for Pancreatic Cancer, Princess Margaret Cancer Centre, Toronto.
In the past year, genomic profiling studies using different platforms have yielded similar results, suggesting that about 30% of patients with pancreatic cancer have actionable mutations that could help guide treatment choices.
The COMPASS trial prospectively recruited patients with advanced pancreatic ductal adenocarcinoma prior to first-line chemotherapy for tumor whole-genome sequencing and RNA sequencing. The trial previously established feasibility, with sequencing successfully completed in more than 90% of patients and availability of whole-genome results before the first disease assessment CT scan at 8 weeks (Clin Cancer Res. 2018;24[6]:1344-54).
New outcomes data for the 150 patients in the intention-to-treat population showed that overall survival was almost 4 months longer for those having a classic modified Moffitt RNA expression signature compared with those having a basal-like one. Similarly, it was more than 2 months longer for those having high versus low expression of the transcription factor GATA6.
In addition, among the subset of patients given mFOLFIRINOX, those having the classic signature were two-thirds less likely to die than were those having the basal-like signature.
“The RNA signature and GATA6 seem to discriminate two prognostic groups in advanced pancreas cancer. The basal-like cohort, or GATA6-low, may be particularly resistant to mFOLFIRINOX,” Dr. Knox said. “GATA6, and perhaps other markers, need to be validated as predictive biomarkers so that we can discover and use more effective therapies earlier on for our patients.
“COMPASS provides a very rich discovery set for other hypotheses and collaborators,” she said, noting that the investigators are adding trial data to the Enhanced Pancreatic Cancer Profiling for Individualized Care (EPPIC) project and to the Accelerate Research in Genomic Oncology (ARGO) project of the International Cancer Genome Consortium.
Rationally based treatment decisions
The COMPASS trial “will show us ... how we should move forward,” said invited discussant Heinz-Josef Lenz, MD, associate director for adult oncology and coleader of the Gastrointestinal Cancers Program, USC Norris Comprehensive Cancer Center, Los Angeles. “We don’t necessarily have the answer today to what the best treatment options are, but this is the first step to understand and develop rationally based treatment decisions for these molecularly characterized groups,” he said.
Whole-genome sequencing has been critically important for finding mutations in single genes, the proverbial needles in the haystack, he maintained. But in the future, the emphasis will likely be on combinations of mutations and other alterations.
Here, RNA is attractive in that it provides information about not only single genes, but also fusions, amplifications, and signatures, as well as treatment-induced changes. “We know that genetic makeup of tumors undergoes significant dynamic changes under treatment pressure. So if we have a gene expression signature associated with sensitivity to chemotherapy, will this change over time if there is progression of disease?” Dr. Lenz said. “That will be the challenge in the future, to better understand the dynamics in order to then recommend second- and third-line treatments.”
Some issues must be overcome to use RNA sequencing in routine clinical care, he acknowledged. For example, this sequencing requires fresh frozen tumor tissue, and it must be largely free of any normal tissue.
“There is no doubt that comprehensive molecular characterization with DNA and RNA will lead to better treatment options and identifying patients who benefit most from very unique treatments,” Dr. Lenz concluded. “The key is, you need to do the testing in order to find and identify the most successful treatments for our patients.”
Study details
Most of the 150 COMPASS patients had metastatic disease (87%), and they were about evenly split between receiving mFOLFIRINOX versus gemcitabine plus nab-paclitaxel as first-line chemotherapy, Dr. Knox reported at the symposium, sponsored by the American Gastroenterological Association, the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
Genomic profiling revealed that roughly 40% overall had potentially actionable variants: alterations in the presence of KRAS wild type (8%), homologous recombination deficiency involving BRCA (6%), and others (25%) such as activating PIK3CA mutations or HER2 amplification.
In findings that Dr. Knox called “quite striking,” among patients given mFOLFIRINOX, median progression-free survival was 7.17 months for those with the RNA classic expression signature versus 2.50 months for those with the RNA basal-like signature (hazard ratio, 0.17; P less than .0001). In contrast, among patients given gemcitabine plus nab-paclitaxel, there was no significant difference by signature (5.65 vs. 4.93 months; P = .69).
GATA6 expression was strongly related to signature type, with classic tumors having high expression and basal-like tumors having low expression (P less than .0001).
Overall survival was similarly better for patients with classic versus basal-like tumors (8.8 vs. 5.2 months; HR, 0.53; P = .006) and for patients with GATA6 high- versus low-expression tumors (8.2 vs. 5.8 months; HR, 0.66; P = .08).
In the whole cohort, overall survival differed across subsets according to RNA signature and type of chemotherapy received (P = .0134). When analysis was restricted to patients given mFOLFIRINOX, median overall survival was 10.1 months with the classic signature but just 5.3 months with the basal-like signature (HR, 0.33; P = .0005).
A multivariate analysis among all chemotherapy-treated patients confirmed the survival benefit of classic signature over basal-like signature (HR, 0.55; P = .03).
Dr. Knox disclosed that she has a consulting or advisory role with Lilly, Merck, and Bristol-Myers Squibb; that she receives honoraria from Novartis; and that she receives research funding from AstraZeneca and Merck. The study is sponsored by University Health Network, Toronto.
SOURCE: O’Kane GM et al. GI Cancers Symposium, Abstract 188.
SAN FRANCISCO – , according to new data from the COMPASS trial reported at the 2019 GI Cancers Symposium.
“We now have two standard chemotherapy regimens that we use in the first-line setting, modified FOLFIRINOX (mFOLFIRINOX) and gemcitabine–nab-paclitaxel, but we lack any real biomarker strategies on which to choose for treatments, and many of our clinical trials in pancreas cancer have failed,” noted senior investigator Jennifer J. Knox, MD, FRCPC, codirector of the McCain Centre for Pancreatic Cancer, Princess Margaret Cancer Centre, Toronto.
In the past year, genomic profiling studies using different platforms have yielded similar results, suggesting that about 30% of patients with pancreatic cancer have actionable mutations that could help guide treatment choices.
The COMPASS trial prospectively recruited patients with advanced pancreatic ductal adenocarcinoma prior to first-line chemotherapy for tumor whole-genome sequencing and RNA sequencing. The trial previously established feasibility, with sequencing successfully completed in more than 90% of patients and availability of whole-genome results before the first disease assessment CT scan at 8 weeks (Clin Cancer Res. 2018;24[6]:1344-54).
New outcomes data for the 150 patients in the intention-to-treat population showed that overall survival was almost 4 months longer for those having a classic modified Moffitt RNA expression signature compared with those having a basal-like one. Similarly, it was more than 2 months longer for those having high versus low expression of the transcription factor GATA6.
In addition, among the subset of patients given mFOLFIRINOX, those having the classic signature were two-thirds less likely to die than were those having the basal-like signature.
“The RNA signature and GATA6 seem to discriminate two prognostic groups in advanced pancreas cancer. The basal-like cohort, or GATA6-low, may be particularly resistant to mFOLFIRINOX,” Dr. Knox said. “GATA6, and perhaps other markers, need to be validated as predictive biomarkers so that we can discover and use more effective therapies earlier on for our patients.
“COMPASS provides a very rich discovery set for other hypotheses and collaborators,” she said, noting that the investigators are adding trial data to the Enhanced Pancreatic Cancer Profiling for Individualized Care (EPPIC) project and to the Accelerate Research in Genomic Oncology (ARGO) project of the International Cancer Genome Consortium.
Rationally based treatment decisions
The COMPASS trial “will show us ... how we should move forward,” said invited discussant Heinz-Josef Lenz, MD, associate director for adult oncology and coleader of the Gastrointestinal Cancers Program, USC Norris Comprehensive Cancer Center, Los Angeles. “We don’t necessarily have the answer today to what the best treatment options are, but this is the first step to understand and develop rationally based treatment decisions for these molecularly characterized groups,” he said.
Whole-genome sequencing has been critically important for finding mutations in single genes, the proverbial needles in the haystack, he maintained. But in the future, the emphasis will likely be on combinations of mutations and other alterations.
Here, RNA is attractive in that it provides information about not only single genes, but also fusions, amplifications, and signatures, as well as treatment-induced changes. “We know that genetic makeup of tumors undergoes significant dynamic changes under treatment pressure. So if we have a gene expression signature associated with sensitivity to chemotherapy, will this change over time if there is progression of disease?” Dr. Lenz said. “That will be the challenge in the future, to better understand the dynamics in order to then recommend second- and third-line treatments.”
Some issues must be overcome to use RNA sequencing in routine clinical care, he acknowledged. For example, this sequencing requires fresh frozen tumor tissue, and it must be largely free of any normal tissue.
“There is no doubt that comprehensive molecular characterization with DNA and RNA will lead to better treatment options and identifying patients who benefit most from very unique treatments,” Dr. Lenz concluded. “The key is, you need to do the testing in order to find and identify the most successful treatments for our patients.”
Study details
Most of the 150 COMPASS patients had metastatic disease (87%), and they were about evenly split between receiving mFOLFIRINOX versus gemcitabine plus nab-paclitaxel as first-line chemotherapy, Dr. Knox reported at the symposium, sponsored by the American Gastroenterological Association, the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
Genomic profiling revealed that roughly 40% overall had potentially actionable variants: alterations in the presence of KRAS wild type (8%), homologous recombination deficiency involving BRCA (6%), and others (25%) such as activating PIK3CA mutations or HER2 amplification.
In findings that Dr. Knox called “quite striking,” among patients given mFOLFIRINOX, median progression-free survival was 7.17 months for those with the RNA classic expression signature versus 2.50 months for those with the RNA basal-like signature (hazard ratio, 0.17; P less than .0001). In contrast, among patients given gemcitabine plus nab-paclitaxel, there was no significant difference by signature (5.65 vs. 4.93 months; P = .69).
GATA6 expression was strongly related to signature type, with classic tumors having high expression and basal-like tumors having low expression (P less than .0001).
Overall survival was similarly better for patients with classic versus basal-like tumors (8.8 vs. 5.2 months; HR, 0.53; P = .006) and for patients with GATA6 high- versus low-expression tumors (8.2 vs. 5.8 months; HR, 0.66; P = .08).
In the whole cohort, overall survival differed across subsets according to RNA signature and type of chemotherapy received (P = .0134). When analysis was restricted to patients given mFOLFIRINOX, median overall survival was 10.1 months with the classic signature but just 5.3 months with the basal-like signature (HR, 0.33; P = .0005).
A multivariate analysis among all chemotherapy-treated patients confirmed the survival benefit of classic signature over basal-like signature (HR, 0.55; P = .03).
Dr. Knox disclosed that she has a consulting or advisory role with Lilly, Merck, and Bristol-Myers Squibb; that she receives honoraria from Novartis; and that she receives research funding from AstraZeneca and Merck. The study is sponsored by University Health Network, Toronto.
SOURCE: O’Kane GM et al. GI Cancers Symposium, Abstract 188.
REPORTING FROM THE 2019 GI CANCERS SYMPOSIUM
Key clinical point: Molecular features of pancreatic cancer may help estimate prognosis and predict response to chemotherapy.
Major finding: Compared with peers whose tumors had a classic RNA expression signature, patients whose tumors had a basal-like RNA expression signature had poorer overall survival when given mFOLFIRINOX (hazard ratio, 0.33; P = .0005).
Study details: Prospective trial with whole-genome sequencing and RNA sequencing of tumors in 150 patients with advanced pancreatic ductal adenocarcinoma before start of first-line therapy (COMPASS trial).
Disclosures: Dr. Knox disclosed that she has a consulting or advisory role with Lilly, Merck, and Bristol-Myers Squibb; that she receives honoraria from Novartis; and that she receives research funding from AstraZeneca and Merck. The study is sponsored by University Health Network, Toronto.
Source: O’Kane GM et al. GI Cancers Symposium, Abstract 188.






