User login
Marijuana smoking is an independent risk factor for lung disease in HIV+
Long-term marijuana smoking was associated with lung disease in HIV-infected (HIV+) but not HIV uninfected (HIV–) men who have sex with men (MSM), according to the results of a large, prospective cohort study.
“There were no significant interactions between marijuana and tobacco smoking in any multivariable model tested for HIV+ participants, indicating independent effects of these factors,” wrote David R. Lorenz, PhD, of the Dana-Farber Cancer Institute, Boston, and his colleagues.
These findings are especially important given that the proportion of HIV+ individuals who frequently smoke marijuana is higher than in the general population in the United States, and has increased in recent years, according to the report, published online in EClinicalMedicine.
The study examined 2,704 MSM who met eligibility criteria (1,352 HIV+ and 1,352 HIV− individuals), with a median age of 44 years at baseline and a median follow-up of 10.5 years. A total of 27% of HIV+ participants reported daily or weekly marijuana smoking for 1 year or more during follow-up, compared with 18% of the HIV− participants.
HIV+ participants who smoked marijuana were more likely to report one or more pulmonary diagnoses, versus nonsmoking HIV+ individuals during follow-up (41.0% vs. 30.0% infectious, and 24.8% vs. 19.0% noninfectious), according to the authors. In contrast, there was no association between marijuana smoking and either an infectious or noninfectious pulmonary diagnosis among HIV− participants (24.2% vs. 20.9%, and 14.8% vs. 17.7%, respectively).
For HIV+ individuals, each 10 days/month increase in marijuana smoking in the prior 2-year period was found to be associated with a 6% increased risk of infectious pulmonary diagnosis (hazard risk 1.06 [95% confidence interval 1.00-1.11]; P = .041). Overall, they found that from the 53,000 person-visits in the study, marijuana smoking was associated with increased risk of both infectious and noninfectious pulmonary diagnoses among the 1,352 HIV-infected participants independent of CD4 count, antiretroviral therapy (ART) adherence, and demographic factors as well.
In particular, viral suppression did not seem to interfere with this association between marijuana smoking and infectious pulmonary diagnoses, as it remained significant in models restricted to those person-visits with suppressed HIV viral load (HR 1.41 [1.03-1.91], P = .029).
The authors suggested that HIV-specific factors such as lung immune cell depletion and dysfunction, persistent immune cell activation, systemic inflammation, respiratory microbiome alterations, and oxidative stress, or a combination of these effects, may interact with the alveolar macrophage dysfunction seen in both humans and mouse models exposed to marijuana smoke. Thus, “a potential additive risk of marijuana smoking and HIV disease may explain the increased prevalence of infectious pulmonary diagnoses in our adjusted analyses,” Dr. Lorenz and his colleagues stated.
“These findings suggest that marijuana smoking is a modifiable risk factor that healthcare providers should consider when seeking to prevent or treat lung disease in people infected with HIV, particularly those with other known risk factors including heavy tobacco smoking, and low CD4 T cell count or advanced HIV disease,” they concluded.
The National Institutes of Health funded the study. The authors reported that they had no relevant disclosures.
SOURCE: Lorenz DR et al. EClinicalMedicine. 2019 Jan 24. doi: 10.1016/j.eclinm.2019.01.003.
Long-term marijuana smoking was associated with lung disease in HIV-infected (HIV+) but not HIV uninfected (HIV–) men who have sex with men (MSM), according to the results of a large, prospective cohort study.
“There were no significant interactions between marijuana and tobacco smoking in any multivariable model tested for HIV+ participants, indicating independent effects of these factors,” wrote David R. Lorenz, PhD, of the Dana-Farber Cancer Institute, Boston, and his colleagues.
These findings are especially important given that the proportion of HIV+ individuals who frequently smoke marijuana is higher than in the general population in the United States, and has increased in recent years, according to the report, published online in EClinicalMedicine.
The study examined 2,704 MSM who met eligibility criteria (1,352 HIV+ and 1,352 HIV− individuals), with a median age of 44 years at baseline and a median follow-up of 10.5 years. A total of 27% of HIV+ participants reported daily or weekly marijuana smoking for 1 year or more during follow-up, compared with 18% of the HIV− participants.
HIV+ participants who smoked marijuana were more likely to report one or more pulmonary diagnoses, versus nonsmoking HIV+ individuals during follow-up (41.0% vs. 30.0% infectious, and 24.8% vs. 19.0% noninfectious), according to the authors. In contrast, there was no association between marijuana smoking and either an infectious or noninfectious pulmonary diagnosis among HIV− participants (24.2% vs. 20.9%, and 14.8% vs. 17.7%, respectively).
For HIV+ individuals, each 10 days/month increase in marijuana smoking in the prior 2-year period was found to be associated with a 6% increased risk of infectious pulmonary diagnosis (hazard risk 1.06 [95% confidence interval 1.00-1.11]; P = .041). Overall, they found that from the 53,000 person-visits in the study, marijuana smoking was associated with increased risk of both infectious and noninfectious pulmonary diagnoses among the 1,352 HIV-infected participants independent of CD4 count, antiretroviral therapy (ART) adherence, and demographic factors as well.
In particular, viral suppression did not seem to interfere with this association between marijuana smoking and infectious pulmonary diagnoses, as it remained significant in models restricted to those person-visits with suppressed HIV viral load (HR 1.41 [1.03-1.91], P = .029).
The authors suggested that HIV-specific factors such as lung immune cell depletion and dysfunction, persistent immune cell activation, systemic inflammation, respiratory microbiome alterations, and oxidative stress, or a combination of these effects, may interact with the alveolar macrophage dysfunction seen in both humans and mouse models exposed to marijuana smoke. Thus, “a potential additive risk of marijuana smoking and HIV disease may explain the increased prevalence of infectious pulmonary diagnoses in our adjusted analyses,” Dr. Lorenz and his colleagues stated.
“These findings suggest that marijuana smoking is a modifiable risk factor that healthcare providers should consider when seeking to prevent or treat lung disease in people infected with HIV, particularly those with other known risk factors including heavy tobacco smoking, and low CD4 T cell count or advanced HIV disease,” they concluded.
The National Institutes of Health funded the study. The authors reported that they had no relevant disclosures.
SOURCE: Lorenz DR et al. EClinicalMedicine. 2019 Jan 24. doi: 10.1016/j.eclinm.2019.01.003.
Long-term marijuana smoking was associated with lung disease in HIV-infected (HIV+) but not HIV uninfected (HIV–) men who have sex with men (MSM), according to the results of a large, prospective cohort study.
“There were no significant interactions between marijuana and tobacco smoking in any multivariable model tested for HIV+ participants, indicating independent effects of these factors,” wrote David R. Lorenz, PhD, of the Dana-Farber Cancer Institute, Boston, and his colleagues.
These findings are especially important given that the proportion of HIV+ individuals who frequently smoke marijuana is higher than in the general population in the United States, and has increased in recent years, according to the report, published online in EClinicalMedicine.
The study examined 2,704 MSM who met eligibility criteria (1,352 HIV+ and 1,352 HIV− individuals), with a median age of 44 years at baseline and a median follow-up of 10.5 years. A total of 27% of HIV+ participants reported daily or weekly marijuana smoking for 1 year or more during follow-up, compared with 18% of the HIV− participants.
HIV+ participants who smoked marijuana were more likely to report one or more pulmonary diagnoses, versus nonsmoking HIV+ individuals during follow-up (41.0% vs. 30.0% infectious, and 24.8% vs. 19.0% noninfectious), according to the authors. In contrast, there was no association between marijuana smoking and either an infectious or noninfectious pulmonary diagnosis among HIV− participants (24.2% vs. 20.9%, and 14.8% vs. 17.7%, respectively).
For HIV+ individuals, each 10 days/month increase in marijuana smoking in the prior 2-year period was found to be associated with a 6% increased risk of infectious pulmonary diagnosis (hazard risk 1.06 [95% confidence interval 1.00-1.11]; P = .041). Overall, they found that from the 53,000 person-visits in the study, marijuana smoking was associated with increased risk of both infectious and noninfectious pulmonary diagnoses among the 1,352 HIV-infected participants independent of CD4 count, antiretroviral therapy (ART) adherence, and demographic factors as well.
In particular, viral suppression did not seem to interfere with this association between marijuana smoking and infectious pulmonary diagnoses, as it remained significant in models restricted to those person-visits with suppressed HIV viral load (HR 1.41 [1.03-1.91], P = .029).
The authors suggested that HIV-specific factors such as lung immune cell depletion and dysfunction, persistent immune cell activation, systemic inflammation, respiratory microbiome alterations, and oxidative stress, or a combination of these effects, may interact with the alveolar macrophage dysfunction seen in both humans and mouse models exposed to marijuana smoke. Thus, “a potential additive risk of marijuana smoking and HIV disease may explain the increased prevalence of infectious pulmonary diagnoses in our adjusted analyses,” Dr. Lorenz and his colleagues stated.
“These findings suggest that marijuana smoking is a modifiable risk factor that healthcare providers should consider when seeking to prevent or treat lung disease in people infected with HIV, particularly those with other known risk factors including heavy tobacco smoking, and low CD4 T cell count or advanced HIV disease,” they concluded.
The National Institutes of Health funded the study. The authors reported that they had no relevant disclosures.
SOURCE: Lorenz DR et al. EClinicalMedicine. 2019 Jan 24. doi: 10.1016/j.eclinm.2019.01.003.
FROM ECLINICALMEDICINE
Key clinical point: HIV+ but not HIV– marijuana smokers had an increased rate of pulmonary diagnoses.
Major finding: HIV+ marijuana smokers were more likely to report one or more infectious or noninfectious pulmonary diagnoses, compared with nonsmoking HIV+ individuals (41.0% vs. 30.0%, and 24.8% vs. 19.0%, respectively).
Study details: A prospective cohort study of 1,352 HIV+ vs. 1,352 HIV– men who have sex with men.
Disclosures: The National Institutes of Health funded the study. The authors reported that they had no relevant disclosures.
Source: Lorenz DR et al. EClinicalMedicine. 2019 Jan 24. doi: 10.1016/j.eclinm.2019.01.003.
Supreme Court halts Louisiana abortion law from taking effect
The U.S. Supreme Court has temporarily barred a Louisiana law that would require stricter requirements for physicians who provide abortion care, the first abortion-related decision for the current conservative-leaning high court.
In a Feb. 7, 2019, order, Supreme Court justices stopped the law from moving forward until they can decide whether to accept the case for oral argument. The law in question would require Louisiana physicians who perform abortions to have admitting privileges at a hospital within 30 miles of the clinic where they offer abortion care.
A group of health professionals sued over the Louisiana statute after it was enacted in 2014, arguing that the requirement was unconstitutional because it placed an undue burden on women seeking abortions. A federal court agreed, concluding that the law would leave a significant number of Louisiana women unable to get an abortion. The state appealed to the 5th U.S. Circuit Court of Appeals, which reversed the decision in January 2019. The physician plaintiffs then urged the Supreme Court to stop the law, scheduled to take effect on Feb. 4 while the case continued through the courts. The health professionals argue that no physicians in Louisiana would be available to perform abortions after 17 weeks of pregnancy if the law proceeds and that only one physician in the state would be available to provide an abortion in the earlier stages of pregnancy. Attorneys for the state countered that health providers are overestimating the law’s effect and requested that the measure be allowed to go forward.
In a Feb. 1 order, the Supreme Court provided the plaintiffs a short stay while they reviewed briefs in the case. Then, in a 5-4 decision on Feb. 7, the majority court halted the legal challenge indefinitely until they can decide whether to take up the case.
Four justices – Clarence Thomas, Samuel Alito, Neil Gorsuch, and Brett Kavanaugh – dissented from the majority, writing that they would have allowed Louisiana to enforce the law. Chief Justice John Roberts joined the high court’s four more liberal justices in stopping the law’s enactment.
The plaintiffs’ petition to the Supreme Court is due in April. If the case is accepted, oral arguments would likely be scheduled for fall 2019 or winter 2020, according to court analysts.
The U.S. Supreme Court has temporarily barred a Louisiana law that would require stricter requirements for physicians who provide abortion care, the first abortion-related decision for the current conservative-leaning high court.
In a Feb. 7, 2019, order, Supreme Court justices stopped the law from moving forward until they can decide whether to accept the case for oral argument. The law in question would require Louisiana physicians who perform abortions to have admitting privileges at a hospital within 30 miles of the clinic where they offer abortion care.
A group of health professionals sued over the Louisiana statute after it was enacted in 2014, arguing that the requirement was unconstitutional because it placed an undue burden on women seeking abortions. A federal court agreed, concluding that the law would leave a significant number of Louisiana women unable to get an abortion. The state appealed to the 5th U.S. Circuit Court of Appeals, which reversed the decision in January 2019. The physician plaintiffs then urged the Supreme Court to stop the law, scheduled to take effect on Feb. 4 while the case continued through the courts. The health professionals argue that no physicians in Louisiana would be available to perform abortions after 17 weeks of pregnancy if the law proceeds and that only one physician in the state would be available to provide an abortion in the earlier stages of pregnancy. Attorneys for the state countered that health providers are overestimating the law’s effect and requested that the measure be allowed to go forward.
In a Feb. 1 order, the Supreme Court provided the plaintiffs a short stay while they reviewed briefs in the case. Then, in a 5-4 decision on Feb. 7, the majority court halted the legal challenge indefinitely until they can decide whether to take up the case.
Four justices – Clarence Thomas, Samuel Alito, Neil Gorsuch, and Brett Kavanaugh – dissented from the majority, writing that they would have allowed Louisiana to enforce the law. Chief Justice John Roberts joined the high court’s four more liberal justices in stopping the law’s enactment.
The plaintiffs’ petition to the Supreme Court is due in April. If the case is accepted, oral arguments would likely be scheduled for fall 2019 or winter 2020, according to court analysts.
The U.S. Supreme Court has temporarily barred a Louisiana law that would require stricter requirements for physicians who provide abortion care, the first abortion-related decision for the current conservative-leaning high court.
In a Feb. 7, 2019, order, Supreme Court justices stopped the law from moving forward until they can decide whether to accept the case for oral argument. The law in question would require Louisiana physicians who perform abortions to have admitting privileges at a hospital within 30 miles of the clinic where they offer abortion care.
A group of health professionals sued over the Louisiana statute after it was enacted in 2014, arguing that the requirement was unconstitutional because it placed an undue burden on women seeking abortions. A federal court agreed, concluding that the law would leave a significant number of Louisiana women unable to get an abortion. The state appealed to the 5th U.S. Circuit Court of Appeals, which reversed the decision in January 2019. The physician plaintiffs then urged the Supreme Court to stop the law, scheduled to take effect on Feb. 4 while the case continued through the courts. The health professionals argue that no physicians in Louisiana would be available to perform abortions after 17 weeks of pregnancy if the law proceeds and that only one physician in the state would be available to provide an abortion in the earlier stages of pregnancy. Attorneys for the state countered that health providers are overestimating the law’s effect and requested that the measure be allowed to go forward.
In a Feb. 1 order, the Supreme Court provided the plaintiffs a short stay while they reviewed briefs in the case. Then, in a 5-4 decision on Feb. 7, the majority court halted the legal challenge indefinitely until they can decide whether to take up the case.
Four justices – Clarence Thomas, Samuel Alito, Neil Gorsuch, and Brett Kavanaugh – dissented from the majority, writing that they would have allowed Louisiana to enforce the law. Chief Justice John Roberts joined the high court’s four more liberal justices in stopping the law’s enactment.
The plaintiffs’ petition to the Supreme Court is due in April. If the case is accepted, oral arguments would likely be scheduled for fall 2019 or winter 2020, according to court analysts.
What is your diagnosis?
It most commonly affects young girls. The pathogenesis of LAHS is thought to involve a sporadic, autosomal dominant mutation that leads to a defect between the hair cuticle and the inner root sheath.1 This defect results in the hair being poorly anchored to the scalp, and therefore easily and painlessly plucked or lost during normal hair care.
The classic presentation of LAHS is that of hair thinning and hair that may be unruly and/or lackluster; the hair rarely, if ever, requires cutting.2 The key feature is the ability to easily and painlessly pluck hairs from the patient’s scalp. The affected area is limited to the scalp, and loss of eyebrows, eyelashes, and body hair should not be seen.
Diagnosis and consideration of the differential
The diagnosis of LAHS can in some cases be made on history and physical exam alone. Patients with LAHS typically will show hair thinning with or without dullness or unruliness. They lack evidence of scalp inflammation, such as erythema, scale, pruritus, and pain. Areas of hair thinning or aberration are typically not well demarcated, and there are typically not areas of complete hair loss. There is no scarring or atrophy of the scalp itself.
Diagnostic tests include the “hair pull test,” as well as trichogram testing. In the “hair pull test” a provider grasps a set of hair at the proximal shaft near the scalp. The traction applied should result in the painless and easy extraction of more than 10% of grasped hairs in a patient with LAHS. Removal of less than 10% of hair is a normal finding, as patients without LAHS typically have about 10% of their scalp hair in the telogen phase at any given time, which would result in removal during the hair pull test.3 In trichography, plucked hairs are examined under magnification, with or without the use of selective dyes. Cinnamaldehyde is a dye that stains citrulline, which is abundant in the inner root sheath, and can be a tool in identifying its presence and/or aberrations.4 A trichogram of the pulled hairs in a patient with LAHS may classically show ruffled appearance of the cuticle, misshapen anagen hair bulbs, and absence of the inner root sheath.5 Examination under magnification also allows providers to better identify telogen versus anagen hairs, which aids in the diagnosis. By carefully considering the patient history, physical exam, and results of additional hair tests, providers can make the diagnosis of LAHS and avoid unnecessary blood work and invasive procedures like scalp biopsies.
The differential diagnosis of hair loss frequently includes alopecia areata. However, in alopecia areata, patients typically have sharply demarcated areas of hair loss, which may involve the eyebrows, eyelids, and body hairs. In alopecia areata, providers may be able to identify the “exclamation point sign” in which the hair shaft thins proximally, leading to the appearance of more pigmented, thicker hairs floating above the scalp.
Telogen effluvium is a condition in which a medical illness or stress, such as systemic illness, surgery, severe emotional distress, childbirth, dietary changes, or another traumatic event, causes a disruption in the natural cycle of hair growth such that the percentage of hairs in the telogen phase increases from about 10% to up to 70%.6 Unlike in LAHS, in which shed hairs are in the anagen phase, the hair that is shed in telogen effluvium is in the telogen phase and will have a different appearance when magnified.
Anagen effluvium, loss of hairs in their growing phase, is typically associated with chemotherapy. The hairs become broken and fractured at the shaft leading to breakage at different points throughout the scalp. Affected areas can include the eyebrows, eyelashes, and body hair. In the absence of a history of administration of a chemotherapy agent (or other drug known to trigger hair loss), the diagnosis of anagen effluvium should not be made.
Patients with trichotillosis (also known as trichotillomania) present with areas of hair loss caused by intentional or subconscious hair pulling. It is considered a psychological condition that can be associated with obsessive compulsive disorder, although the presence of a secondary psychological diagnosis is not required. Providers may see irregular geometric shapes of hair loss, and on close inspection see broken hair shafts of different lengths. Patients most often pull hair from their scalps (over 70% of patients), but also can pull eyelashes, eyebrow hairs, and pubic hairs.7
Treatment
LAHS is self-limited and does not necessitate treatment. However, if patients or parents feel there is significant disease burden, perhaps with poor effects on quality of life or with psychosocial impairment, treatment with minoxidil 5% solution has been studied with some success reported in the literature.1,8,9
Ms. Natsis is a medical student at the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital-San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Ms. Natsis and Dr. Eichenfield had no relevant financial disclosures. Email them at pdnews@mdedge.com.
References
1. Arch Dermatol. 2002;138(4):501-6.
2. Int J Trichology. 2010;2(2):96-100.
3. Pediatric Dermatol. 2016:33(50):507-10.
4. Dermatol Clin. 1986;14:745-51.
5. Arch Dermatol. 2009;145(10):1123-8.
6. J Clin Diagn Res. 2015;9(9):WE01-3.
7. Am J Psychiatry. 2016;173(9):868-74.
8. Australas J Dermatol. 2018;59:e286-e287.
9. Pediatr Dermatol. 2014;31:389-90.
It most commonly affects young girls. The pathogenesis of LAHS is thought to involve a sporadic, autosomal dominant mutation that leads to a defect between the hair cuticle and the inner root sheath.1 This defect results in the hair being poorly anchored to the scalp, and therefore easily and painlessly plucked or lost during normal hair care.
The classic presentation of LAHS is that of hair thinning and hair that may be unruly and/or lackluster; the hair rarely, if ever, requires cutting.2 The key feature is the ability to easily and painlessly pluck hairs from the patient’s scalp. The affected area is limited to the scalp, and loss of eyebrows, eyelashes, and body hair should not be seen.
Diagnosis and consideration of the differential
The diagnosis of LAHS can in some cases be made on history and physical exam alone. Patients with LAHS typically will show hair thinning with or without dullness or unruliness. They lack evidence of scalp inflammation, such as erythema, scale, pruritus, and pain. Areas of hair thinning or aberration are typically not well demarcated, and there are typically not areas of complete hair loss. There is no scarring or atrophy of the scalp itself.
Diagnostic tests include the “hair pull test,” as well as trichogram testing. In the “hair pull test” a provider grasps a set of hair at the proximal shaft near the scalp. The traction applied should result in the painless and easy extraction of more than 10% of grasped hairs in a patient with LAHS. Removal of less than 10% of hair is a normal finding, as patients without LAHS typically have about 10% of their scalp hair in the telogen phase at any given time, which would result in removal during the hair pull test.3 In trichography, plucked hairs are examined under magnification, with or without the use of selective dyes. Cinnamaldehyde is a dye that stains citrulline, which is abundant in the inner root sheath, and can be a tool in identifying its presence and/or aberrations.4 A trichogram of the pulled hairs in a patient with LAHS may classically show ruffled appearance of the cuticle, misshapen anagen hair bulbs, and absence of the inner root sheath.5 Examination under magnification also allows providers to better identify telogen versus anagen hairs, which aids in the diagnosis. By carefully considering the patient history, physical exam, and results of additional hair tests, providers can make the diagnosis of LAHS and avoid unnecessary blood work and invasive procedures like scalp biopsies.
The differential diagnosis of hair loss frequently includes alopecia areata. However, in alopecia areata, patients typically have sharply demarcated areas of hair loss, which may involve the eyebrows, eyelids, and body hairs. In alopecia areata, providers may be able to identify the “exclamation point sign” in which the hair shaft thins proximally, leading to the appearance of more pigmented, thicker hairs floating above the scalp.
Telogen effluvium is a condition in which a medical illness or stress, such as systemic illness, surgery, severe emotional distress, childbirth, dietary changes, or another traumatic event, causes a disruption in the natural cycle of hair growth such that the percentage of hairs in the telogen phase increases from about 10% to up to 70%.6 Unlike in LAHS, in which shed hairs are in the anagen phase, the hair that is shed in telogen effluvium is in the telogen phase and will have a different appearance when magnified.
Anagen effluvium, loss of hairs in their growing phase, is typically associated with chemotherapy. The hairs become broken and fractured at the shaft leading to breakage at different points throughout the scalp. Affected areas can include the eyebrows, eyelashes, and body hair. In the absence of a history of administration of a chemotherapy agent (or other drug known to trigger hair loss), the diagnosis of anagen effluvium should not be made.
Patients with trichotillosis (also known as trichotillomania) present with areas of hair loss caused by intentional or subconscious hair pulling. It is considered a psychological condition that can be associated with obsessive compulsive disorder, although the presence of a secondary psychological diagnosis is not required. Providers may see irregular geometric shapes of hair loss, and on close inspection see broken hair shafts of different lengths. Patients most often pull hair from their scalps (over 70% of patients), but also can pull eyelashes, eyebrow hairs, and pubic hairs.7
Treatment
LAHS is self-limited and does not necessitate treatment. However, if patients or parents feel there is significant disease burden, perhaps with poor effects on quality of life or with psychosocial impairment, treatment with minoxidil 5% solution has been studied with some success reported in the literature.1,8,9
Ms. Natsis is a medical student at the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital-San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Ms. Natsis and Dr. Eichenfield had no relevant financial disclosures. Email them at pdnews@mdedge.com.
References
1. Arch Dermatol. 2002;138(4):501-6.
2. Int J Trichology. 2010;2(2):96-100.
3. Pediatric Dermatol. 2016:33(50):507-10.
4. Dermatol Clin. 1986;14:745-51.
5. Arch Dermatol. 2009;145(10):1123-8.
6. J Clin Diagn Res. 2015;9(9):WE01-3.
7. Am J Psychiatry. 2016;173(9):868-74.
8. Australas J Dermatol. 2018;59:e286-e287.
9. Pediatr Dermatol. 2014;31:389-90.
It most commonly affects young girls. The pathogenesis of LAHS is thought to involve a sporadic, autosomal dominant mutation that leads to a defect between the hair cuticle and the inner root sheath.1 This defect results in the hair being poorly anchored to the scalp, and therefore easily and painlessly plucked or lost during normal hair care.
The classic presentation of LAHS is that of hair thinning and hair that may be unruly and/or lackluster; the hair rarely, if ever, requires cutting.2 The key feature is the ability to easily and painlessly pluck hairs from the patient’s scalp. The affected area is limited to the scalp, and loss of eyebrows, eyelashes, and body hair should not be seen.
Diagnosis and consideration of the differential
The diagnosis of LAHS can in some cases be made on history and physical exam alone. Patients with LAHS typically will show hair thinning with or without dullness or unruliness. They lack evidence of scalp inflammation, such as erythema, scale, pruritus, and pain. Areas of hair thinning or aberration are typically not well demarcated, and there are typically not areas of complete hair loss. There is no scarring or atrophy of the scalp itself.
Diagnostic tests include the “hair pull test,” as well as trichogram testing. In the “hair pull test” a provider grasps a set of hair at the proximal shaft near the scalp. The traction applied should result in the painless and easy extraction of more than 10% of grasped hairs in a patient with LAHS. Removal of less than 10% of hair is a normal finding, as patients without LAHS typically have about 10% of their scalp hair in the telogen phase at any given time, which would result in removal during the hair pull test.3 In trichography, plucked hairs are examined under magnification, with or without the use of selective dyes. Cinnamaldehyde is a dye that stains citrulline, which is abundant in the inner root sheath, and can be a tool in identifying its presence and/or aberrations.4 A trichogram of the pulled hairs in a patient with LAHS may classically show ruffled appearance of the cuticle, misshapen anagen hair bulbs, and absence of the inner root sheath.5 Examination under magnification also allows providers to better identify telogen versus anagen hairs, which aids in the diagnosis. By carefully considering the patient history, physical exam, and results of additional hair tests, providers can make the diagnosis of LAHS and avoid unnecessary blood work and invasive procedures like scalp biopsies.
The differential diagnosis of hair loss frequently includes alopecia areata. However, in alopecia areata, patients typically have sharply demarcated areas of hair loss, which may involve the eyebrows, eyelids, and body hairs. In alopecia areata, providers may be able to identify the “exclamation point sign” in which the hair shaft thins proximally, leading to the appearance of more pigmented, thicker hairs floating above the scalp.
Telogen effluvium is a condition in which a medical illness or stress, such as systemic illness, surgery, severe emotional distress, childbirth, dietary changes, or another traumatic event, causes a disruption in the natural cycle of hair growth such that the percentage of hairs in the telogen phase increases from about 10% to up to 70%.6 Unlike in LAHS, in which shed hairs are in the anagen phase, the hair that is shed in telogen effluvium is in the telogen phase and will have a different appearance when magnified.
Anagen effluvium, loss of hairs in their growing phase, is typically associated with chemotherapy. The hairs become broken and fractured at the shaft leading to breakage at different points throughout the scalp. Affected areas can include the eyebrows, eyelashes, and body hair. In the absence of a history of administration of a chemotherapy agent (or other drug known to trigger hair loss), the diagnosis of anagen effluvium should not be made.
Patients with trichotillosis (also known as trichotillomania) present with areas of hair loss caused by intentional or subconscious hair pulling. It is considered a psychological condition that can be associated with obsessive compulsive disorder, although the presence of a secondary psychological diagnosis is not required. Providers may see irregular geometric shapes of hair loss, and on close inspection see broken hair shafts of different lengths. Patients most often pull hair from their scalps (over 70% of patients), but also can pull eyelashes, eyebrow hairs, and pubic hairs.7
Treatment
LAHS is self-limited and does not necessitate treatment. However, if patients or parents feel there is significant disease burden, perhaps with poor effects on quality of life or with psychosocial impairment, treatment with minoxidil 5% solution has been studied with some success reported in the literature.1,8,9
Ms. Natsis is a medical student at the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital-San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Ms. Natsis and Dr. Eichenfield had no relevant financial disclosures. Email them at pdnews@mdedge.com.
References
1. Arch Dermatol. 2002;138(4):501-6.
2. Int J Trichology. 2010;2(2):96-100.
3. Pediatric Dermatol. 2016:33(50):507-10.
4. Dermatol Clin. 1986;14:745-51.
5. Arch Dermatol. 2009;145(10):1123-8.
6. J Clin Diagn Res. 2015;9(9):WE01-3.
7. Am J Psychiatry. 2016;173(9):868-74.
8. Australas J Dermatol. 2018;59:e286-e287.
9. Pediatr Dermatol. 2014;31:389-90.
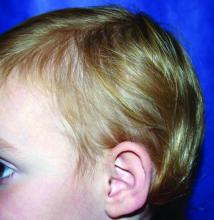
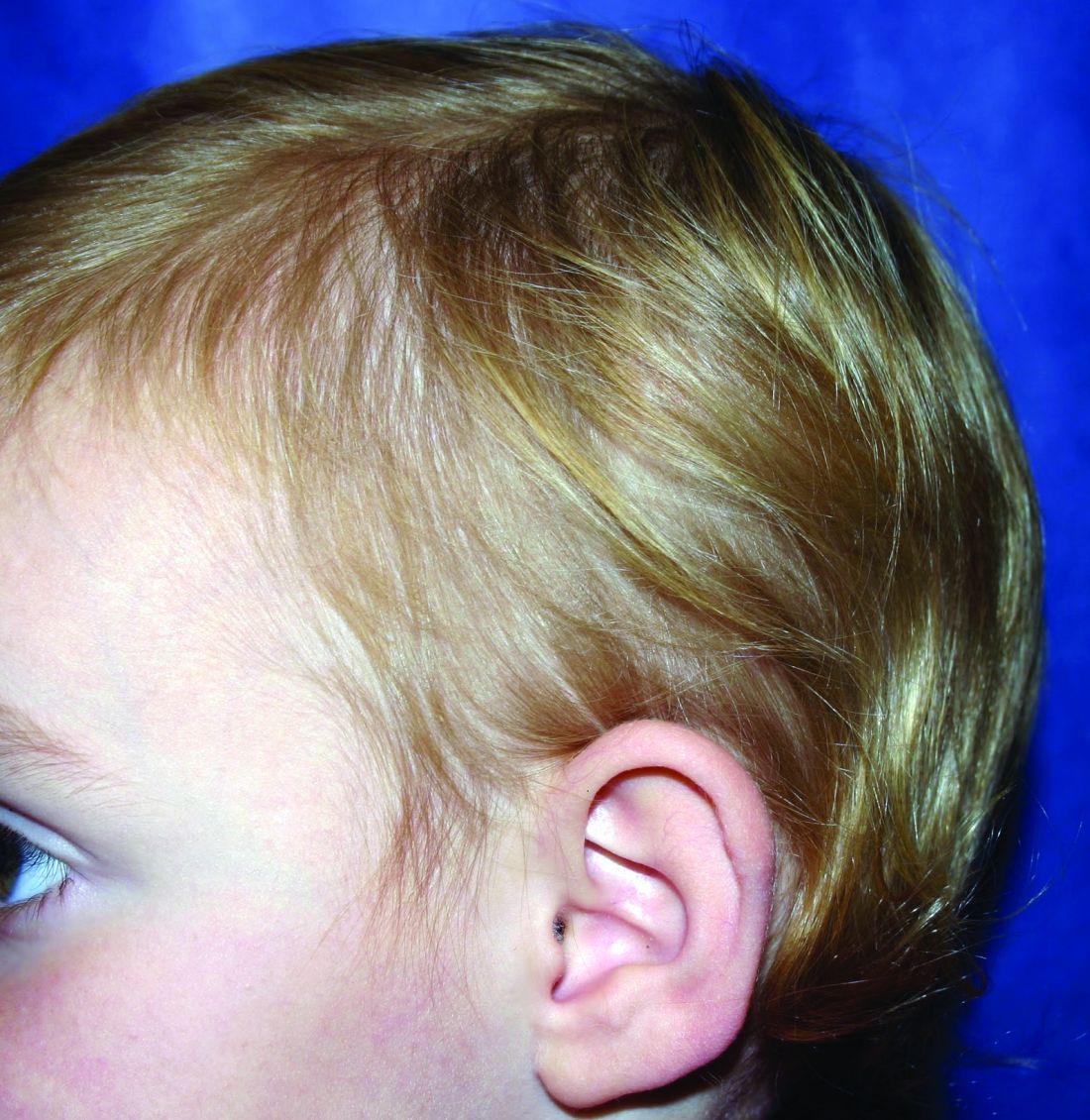
A 5-year-old female is brought to clinic for hair loss. The mother reports that when styling her daughter's hair, she has noticed areas of hair thinning, especially at the temples and at the occiput. The mother denies scale, pruritus, and erythema. The patient reports that her scalp does not hurt. She has never had a haircut because her hair hasn't grown long enough to cut. There is no history of specific bald spots. The patient has no personal history of psoriasis, seborrheic dermatitis, or autoimmune disease. No picking has been noted, and there is no history of compulsive behaviors or anxiety. The patient's mother has a history of Graves disease. The mother reports that the patient's older sister may have had hair thinning when she was younger as well, but no longer has thin hair.
The patient was previously seen by another provider who prescribed hydrocortisone 2.5% ointment, which the mother has been applying nightly without improvement.
The child is otherwise medically well and thriving, with no recent change in activity level and with growth parameters consistently around the 75th percentile for height and weight. On physical exam, the patient has blondish, fine hair with areas of poorly demarcated hair thinning at the left temple and at the occiput. The hair remaining at the occiput is normal in texture. There are no areas of complete hair loss. There is no scale, erythema, or abnormal pigmentation, and no cervical or occipital adenopathy. The patient has intact eyebrows and eyelashes.
Trial supports less aggressive myeloma treatment
For patients with multiple myeloma that remains symptomatic within a year of starting therapy, neither a second autologous stem cell transplant nor more intensive consolidation therapy offered survival benefits superior to those seen with a single first autologous transplant and lenalidomide maintenance, reported investigators in a multicenter U.S. trial.
Among 758 patients with multiple myeloma (MM) who underwent standard induction therapy, followed by melphalan conditioning and autologous hematopoietic cell transplant (AHCT), there were no differences in either progression-free survival (PFS) or overall survival (OS) between the three treatment arms, reported Edward A. Stadtmauer, MD, from the University of Pennsylvania, Philadelphia, and his colleagues.
Patients were randomized to either lenalidomide (Revlimid) maintenance alone; consolidation therapy with four cycles of lenalidomide, bortezomib (Velcade), and dexamethasone (RVD), followed by lenalidomide maintenance; or second transplant followed by lenalidomide maintenance.
“Single AHCT followed by len[alidomide] remains the standard of care. Greater than 80% of patients were alive at 38 months, which highlights excellent contemporary outcomes of patients with MM when treated with a standard approach of a multidrug induction followed by AHCT consolidation and maintenance,” they wrote in the Journal of Clinical Oncology.
The investigators hypothesized that the use of thalidomide analogues and proteasome inhibitors used in first-line therapy, consolidation, and long-term maintenance after high-dose melphalan and AHCT would improve survival, compared with a second AHCT.
To test this idea, they enrolled 758 patients from 54 U.S. centers and randomized them to one of three post-transplant strategies prior to transplant conditioning with high-dose melphalan (200 mg/m2) and AHCT.
Roughly 25% of patients in each treatment arm had high-risk disease, defined as beta-2 microglobulin levels greater than 5.5 mg/L, high-risk cytogenetics, and deletion 13 detected by standard cytogenetics only. The remaining patients in each arm had standard-risk disease.
The patients, who were a median age of 56 years old, had symptomatic multiple myeloma 12 months from the start of therapy without disease progression. They were randomly assigned to either AHCT followed by a second transplant and lenalidomide maintenance (247 patients), single transplant followed by RVD and lenalidomide maintenance (254), or single AHCT plus lenalidomide maintenance (257).
There were no significant differences between the groups in the primary endpoint of PFS at 38 months, with rates of 58.5% for the dual AHCT plus lenalidomide group, 57.8% for AHCT/RVD/lenalidomide, and 53.9% for AHCT/lenalidomide. Respective OS rates also did not differ significantly, at 81.8%, 85.4%, and 83.7%.
Complete response rates at 1 year were 50.5%, 58.4%, and 47.1%, respectively.
The three regimens also were similar in their toxicity profiles and in the risk of second malignancies.
The trial was supported by grants from the National Institutes of Health, research groups, Celgene, and Millennium (Takeda) Pharmaceuticals. Dr. Stadtmauer reported ties to Celgene, Takeda, and other companies. Multiple coauthors reported relationships with industry.
SOURCE: Stadtmauer E et al. J Clin Oncol. 2019 Jan 17. doi: 10.1200/JCO.18.00685.
For patients with multiple myeloma that remains symptomatic within a year of starting therapy, neither a second autologous stem cell transplant nor more intensive consolidation therapy offered survival benefits superior to those seen with a single first autologous transplant and lenalidomide maintenance, reported investigators in a multicenter U.S. trial.
Among 758 patients with multiple myeloma (MM) who underwent standard induction therapy, followed by melphalan conditioning and autologous hematopoietic cell transplant (AHCT), there were no differences in either progression-free survival (PFS) or overall survival (OS) between the three treatment arms, reported Edward A. Stadtmauer, MD, from the University of Pennsylvania, Philadelphia, and his colleagues.
Patients were randomized to either lenalidomide (Revlimid) maintenance alone; consolidation therapy with four cycles of lenalidomide, bortezomib (Velcade), and dexamethasone (RVD), followed by lenalidomide maintenance; or second transplant followed by lenalidomide maintenance.
“Single AHCT followed by len[alidomide] remains the standard of care. Greater than 80% of patients were alive at 38 months, which highlights excellent contemporary outcomes of patients with MM when treated with a standard approach of a multidrug induction followed by AHCT consolidation and maintenance,” they wrote in the Journal of Clinical Oncology.
The investigators hypothesized that the use of thalidomide analogues and proteasome inhibitors used in first-line therapy, consolidation, and long-term maintenance after high-dose melphalan and AHCT would improve survival, compared with a second AHCT.
To test this idea, they enrolled 758 patients from 54 U.S. centers and randomized them to one of three post-transplant strategies prior to transplant conditioning with high-dose melphalan (200 mg/m2) and AHCT.
Roughly 25% of patients in each treatment arm had high-risk disease, defined as beta-2 microglobulin levels greater than 5.5 mg/L, high-risk cytogenetics, and deletion 13 detected by standard cytogenetics only. The remaining patients in each arm had standard-risk disease.
The patients, who were a median age of 56 years old, had symptomatic multiple myeloma 12 months from the start of therapy without disease progression. They were randomly assigned to either AHCT followed by a second transplant and lenalidomide maintenance (247 patients), single transplant followed by RVD and lenalidomide maintenance (254), or single AHCT plus lenalidomide maintenance (257).
There were no significant differences between the groups in the primary endpoint of PFS at 38 months, with rates of 58.5% for the dual AHCT plus lenalidomide group, 57.8% for AHCT/RVD/lenalidomide, and 53.9% for AHCT/lenalidomide. Respective OS rates also did not differ significantly, at 81.8%, 85.4%, and 83.7%.
Complete response rates at 1 year were 50.5%, 58.4%, and 47.1%, respectively.
The three regimens also were similar in their toxicity profiles and in the risk of second malignancies.
The trial was supported by grants from the National Institutes of Health, research groups, Celgene, and Millennium (Takeda) Pharmaceuticals. Dr. Stadtmauer reported ties to Celgene, Takeda, and other companies. Multiple coauthors reported relationships with industry.
SOURCE: Stadtmauer E et al. J Clin Oncol. 2019 Jan 17. doi: 10.1200/JCO.18.00685.
For patients with multiple myeloma that remains symptomatic within a year of starting therapy, neither a second autologous stem cell transplant nor more intensive consolidation therapy offered survival benefits superior to those seen with a single first autologous transplant and lenalidomide maintenance, reported investigators in a multicenter U.S. trial.
Among 758 patients with multiple myeloma (MM) who underwent standard induction therapy, followed by melphalan conditioning and autologous hematopoietic cell transplant (AHCT), there were no differences in either progression-free survival (PFS) or overall survival (OS) between the three treatment arms, reported Edward A. Stadtmauer, MD, from the University of Pennsylvania, Philadelphia, and his colleagues.
Patients were randomized to either lenalidomide (Revlimid) maintenance alone; consolidation therapy with four cycles of lenalidomide, bortezomib (Velcade), and dexamethasone (RVD), followed by lenalidomide maintenance; or second transplant followed by lenalidomide maintenance.
“Single AHCT followed by len[alidomide] remains the standard of care. Greater than 80% of patients were alive at 38 months, which highlights excellent contemporary outcomes of patients with MM when treated with a standard approach of a multidrug induction followed by AHCT consolidation and maintenance,” they wrote in the Journal of Clinical Oncology.
The investigators hypothesized that the use of thalidomide analogues and proteasome inhibitors used in first-line therapy, consolidation, and long-term maintenance after high-dose melphalan and AHCT would improve survival, compared with a second AHCT.
To test this idea, they enrolled 758 patients from 54 U.S. centers and randomized them to one of three post-transplant strategies prior to transplant conditioning with high-dose melphalan (200 mg/m2) and AHCT.
Roughly 25% of patients in each treatment arm had high-risk disease, defined as beta-2 microglobulin levels greater than 5.5 mg/L, high-risk cytogenetics, and deletion 13 detected by standard cytogenetics only. The remaining patients in each arm had standard-risk disease.
The patients, who were a median age of 56 years old, had symptomatic multiple myeloma 12 months from the start of therapy without disease progression. They were randomly assigned to either AHCT followed by a second transplant and lenalidomide maintenance (247 patients), single transplant followed by RVD and lenalidomide maintenance (254), or single AHCT plus lenalidomide maintenance (257).
There were no significant differences between the groups in the primary endpoint of PFS at 38 months, with rates of 58.5% for the dual AHCT plus lenalidomide group, 57.8% for AHCT/RVD/lenalidomide, and 53.9% for AHCT/lenalidomide. Respective OS rates also did not differ significantly, at 81.8%, 85.4%, and 83.7%.
Complete response rates at 1 year were 50.5%, 58.4%, and 47.1%, respectively.
The three regimens also were similar in their toxicity profiles and in the risk of second malignancies.
The trial was supported by grants from the National Institutes of Health, research groups, Celgene, and Millennium (Takeda) Pharmaceuticals. Dr. Stadtmauer reported ties to Celgene, Takeda, and other companies. Multiple coauthors reported relationships with industry.
SOURCE: Stadtmauer E et al. J Clin Oncol. 2019 Jan 17. doi: 10.1200/JCO.18.00685.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point:
Major finding: There were no differences in progression-free survival or overall survival among the three trial arms.
Study details: Randomized clinical trial with 758 patients with multiple myeloma.
Disclosures: The trial was supported by grants from the National Institutes of Health, research groups, Celgene, and Millennium (Takeda) Pharmaceuticals. Dr. Stadtmauer reported ties to Celgene, Takeda, and other companies. Multiple coauthors reported relationships with industry.
Source: Stadtmauer E et al. J Clin Oncol. 2019 Jan 17. doi: 10.1200/JCO.18.00685.
Consider hysterectomy in patients with post-irradiated residual cervical cancer
according to a study of Bangladeshi patients with cervical cancer.
“Surgical intervention by either extrafascial or radical hysterectomy should be strongly considered for women with biopsy-confirmed residual disease after chemoradiation,” wrote lead authors Shahana Pervin, MBBS, and Farzana Islam Ruma, MBBS, of the National Institute of Cancer Research and Hospital and of the Railway General Hospital, respectively, in Dhaka, Bangladesh, and their associates. The study was published in the Journal of Global Oncology.
From 2009 to June 2013, this prospective longitudinal study collected data from 40 patients with biopsy-confirmed persistence of cervical cancer. The patients, who were being treated at one of two hospitals in Dhaka, Bangladesh, underwent either radical or extrafascial hysterectomy at least 12 weeks after initial radiation therapy.
At 5 years of follow-up, 36 (90%) had no evidence of disease. Of the 29 women who underwent extrafascial hysterectomy, 4 (14%) developed recurrent disease and 1 died. None of the 11 women who underwent radical hysterectomy had recurrences during the study period; that group, however, did suffer from “intraoperative, postoperative, and long-term complications.”
The investigators acknowledged the study’s several limitations, including a lack of standardized preoperative therapy, incomplete records of radiation dosing, and a limited number of patients. Along the same lines, another larger prospective trial to evaluate the two types of hysterectomy would “help guide what should be the standard of care for salvage therapy,” they wrote.
However, their findings emphasized the need for physicians in limited resource areas to have “a strong index of suspicion” when evaluating patients with locally advanced cervical cancer for residual disease. “Close clinical follow-up is crucial to identify these women in a timely manner,” the investigators added.
The Massachusetts General Hospital Gynecologic Oncology Global Health Fund supported the study. The authors reported no conflicts of interest.
SOURCE: Pervin S et al. J Glob Oncol. 2019 Feb 1. doi: 10.1200/JGO.18.00157.
according to a study of Bangladeshi patients with cervical cancer.
“Surgical intervention by either extrafascial or radical hysterectomy should be strongly considered for women with biopsy-confirmed residual disease after chemoradiation,” wrote lead authors Shahana Pervin, MBBS, and Farzana Islam Ruma, MBBS, of the National Institute of Cancer Research and Hospital and of the Railway General Hospital, respectively, in Dhaka, Bangladesh, and their associates. The study was published in the Journal of Global Oncology.
From 2009 to June 2013, this prospective longitudinal study collected data from 40 patients with biopsy-confirmed persistence of cervical cancer. The patients, who were being treated at one of two hospitals in Dhaka, Bangladesh, underwent either radical or extrafascial hysterectomy at least 12 weeks after initial radiation therapy.
At 5 years of follow-up, 36 (90%) had no evidence of disease. Of the 29 women who underwent extrafascial hysterectomy, 4 (14%) developed recurrent disease and 1 died. None of the 11 women who underwent radical hysterectomy had recurrences during the study period; that group, however, did suffer from “intraoperative, postoperative, and long-term complications.”
The investigators acknowledged the study’s several limitations, including a lack of standardized preoperative therapy, incomplete records of radiation dosing, and a limited number of patients. Along the same lines, another larger prospective trial to evaluate the two types of hysterectomy would “help guide what should be the standard of care for salvage therapy,” they wrote.
However, their findings emphasized the need for physicians in limited resource areas to have “a strong index of suspicion” when evaluating patients with locally advanced cervical cancer for residual disease. “Close clinical follow-up is crucial to identify these women in a timely manner,” the investigators added.
The Massachusetts General Hospital Gynecologic Oncology Global Health Fund supported the study. The authors reported no conflicts of interest.
SOURCE: Pervin S et al. J Glob Oncol. 2019 Feb 1. doi: 10.1200/JGO.18.00157.
according to a study of Bangladeshi patients with cervical cancer.
“Surgical intervention by either extrafascial or radical hysterectomy should be strongly considered for women with biopsy-confirmed residual disease after chemoradiation,” wrote lead authors Shahana Pervin, MBBS, and Farzana Islam Ruma, MBBS, of the National Institute of Cancer Research and Hospital and of the Railway General Hospital, respectively, in Dhaka, Bangladesh, and their associates. The study was published in the Journal of Global Oncology.
From 2009 to June 2013, this prospective longitudinal study collected data from 40 patients with biopsy-confirmed persistence of cervical cancer. The patients, who were being treated at one of two hospitals in Dhaka, Bangladesh, underwent either radical or extrafascial hysterectomy at least 12 weeks after initial radiation therapy.
At 5 years of follow-up, 36 (90%) had no evidence of disease. Of the 29 women who underwent extrafascial hysterectomy, 4 (14%) developed recurrent disease and 1 died. None of the 11 women who underwent radical hysterectomy had recurrences during the study period; that group, however, did suffer from “intraoperative, postoperative, and long-term complications.”
The investigators acknowledged the study’s several limitations, including a lack of standardized preoperative therapy, incomplete records of radiation dosing, and a limited number of patients. Along the same lines, another larger prospective trial to evaluate the two types of hysterectomy would “help guide what should be the standard of care for salvage therapy,” they wrote.
However, their findings emphasized the need for physicians in limited resource areas to have “a strong index of suspicion” when evaluating patients with locally advanced cervical cancer for residual disease. “Close clinical follow-up is crucial to identify these women in a timely manner,” the investigators added.
The Massachusetts General Hospital Gynecologic Oncology Global Health Fund supported the study. The authors reported no conflicts of interest.
SOURCE: Pervin S et al. J Glob Oncol. 2019 Feb 1. doi: 10.1200/JGO.18.00157.
FROM THE JOURNAL OF GLOBAL ONCOLOGY
Key clinical point: Hysterectomy should be strongly considered for women with biopsy-confirmed residual cervical cancer after radiation.
Major finding: At 5 years of follow-up, 90% of the patients who underwent hysterectomy had no evidence of disease.
Study details: A prospective longitudinal study of 40 patients with locally advanced cervical cancer who underwent hysterectomy after radiation at one of two hospitals in Dhaka, Bangladesh.
Disclosures: The Massachusetts General Hospital Gynecologic Oncology Global Health Fund supported the study. The authors reported no conflicts of interest.
Source: Pervin S et al. J Glob Oncol. 2019 Feb 1. doi: 10.1200/JGO.18.00157.
ADT harms likely limited to men with CV comorbidities
WASHINGTON – The cardiovascular effects of androgen deprivation therapy (ADT) for men with advanced prostate cancer are less severe than once feared, but there is evidence to suggest that men with preexisting heart failure or a history of myocardial infarction could be at excess risk for death from cardiovascular causes when they receive ADT, according to a leading prostate cancer expert.
“I think there are concerns about potential cardiovascular harm of ADT, and I think this has reduced ADT use, despite the fact that we know for most men it improves overall survival,” said Paul Nguyen, MD, a radiation oncologist at the Dana-Farber/Brigham and Women’s Cancer Center in Boston.
“In fact, when we looked recently at men with high-risk prostate cancer, this is a group where overall survival is improved by 50% if they get ADT – so it cuts the risk of death in half – but it turns out that nearly a quarter of those patients are not receiving ADT. I think that the concern about cardiovascular harm and the confusion as to where that data stands is a lot of what’s driving that right now,” he said at the American College of Cardiology’s Advancing the Cardiovascular Care of the Oncology Patient meeting.
Randomized trial data
Dr. Nguyen noted that the evidence suggesting that ADT can increase the risk of death from cardiovascular causes came largely from three major studies:
- A 2006 study of 73,196 Medicare enrollees aged 66 or older, which found that ADT with a gonadotropin-releasing hormone (GnRH) agonist was possibly associated with increased risk of incident diabetes and cardiovascular disease (J Clin Oncol. 2006 Sep 20;24[27]:4448-56.).
- A 2007 analysis of data from the Cancer of the Prostate Strategic Urologic Research Endeavor (CAPSURE) database on 3,262 men treated with radical prostatectomy and 1,630 men treated with radiation or cryotherapy for localized prostate cancer, which found that among those 65 and older the 5-year cumulative incidence of cardiovascular death was 5.5% for patients who received ADT, vs. 2% for those who did not (J Natl Cancer Inst. 2007 Oct 17;99[20]:1516-24).
- A 2007 study of 1,372 men in three randomized trials of radiation therapy with or without androgen suppression therapy up to 8 months in duration, which found that men 65 and older who received 6 months of androgen suppression had significantly shorter times to fatal MIs than did men who did not receive the therapy (J Clin Oncol. 2007;25[17]:2420-5).
These studies, combined with observational data, led to a 2010 consensus statement from the American Heart Association, American Cancer Society, and American Urological Association, with endorsement from the American Society for Radiation Oncology, which stated that “there may be a relation between ADT and cardiovascular events and death.”
Also in 2010, the Food and Drug Administration required new labeling on GnRH agonists warning of “increased risk of diabetes and certain cardiovascular diseases (heart attack, sudden cardiac death, stroke).”
Not unanimous
Two other large randomized studies (J Clin Oncol. 2008 Feb 1;26[4]:585-91 and J Clin Oncol. 2009 Jan 1;27[1]:92-9) and two retrospective studies (J Clin Oncol. 2009 Jul 20;27[21]:3452-8 and J Clin Oncol. 2011 Sep 10;29[26]3510-16) found no excess risk of cardiovascular disease from ADT, Dr. Nguyen said, prompting him and his colleagues to see whether they could get a better estimate of the actual risk.
They did so through a 2011 meta-analysis (JAMA. 2011;306[21]:2359-66) of data on 4,141 patients from eight randomized trials. They found that among patients with unfavorable-risk prostate cancer, ADT was not associated with an increased risk of cardiovascular death, but was associated with lower risks for both prostate-specific and all-cause mortality.
Subpopulations may still be at risk
Dr. Nguyen said that the principal finding of the meta-analysis, while reassuring, “doesn’t let ADT off the hook for metabolic events, diabetes which we know happens, and the possibility of nonfatal cardiac events.”
He noted that while ADT was not associated with cardiovascular disease in clinical trials, observational studies showed significantly increased risk for fatal or non-fatal MI.
One possible explanation for the difference is that observational studies included nonfatal MI, while randomized trials looked only at cardiovascular deaths. It’s also possible that ADT causes harm primarily in men with preexisting comorbidities, who are often excluded from or underrepresented in clinical trials.
Evidence from a 2009 study (JAMA. 2009 Aug 26;302[8]:866-73) showed that among men with clinical stage T1 to T3 noninvasive, nonmetastatic prostate cancer, neoadjuvant hormonal therapy with both a luteinizing hormone-releasing hormone (LHRH) agonist and a nonsteroidal antiandrogen was associated with increased risk for all-cause mortality for those with a history of coronary artery disease–induced heart failure, but not for men with either no comorbidities or only a single comorbidity such as hypertension, hypercholesterolemia, or diabetes.
Clinical considerations
The decision to treat men with prostate cancer with ADT is therefore a balancing act, Dr. Nguyen said.
“As the risk of prostate cancer death goes up, the benefit of ADT goes up. However, as the comorbidity level goes up, the potential cardiovascular harm of ADT goes up,” he said.
For patients at the extreme ends of each continuum, such as a patient with high-risk prostate cancer and no cardiovascular comorbidities or a patient with low-risk cancer but multiple CV risk factors, the decision to give or withhold ADT is relatively simple, he said.
But for patients in between, such as a man with intermediate-risk cancer and one risk factor or a man with high risk disease with multiple comorbidities, the decision is far more complex.
“This where I think the dialogue with the cardiologist really needs to come into this decision,” he said.
Evidence to support the decision comes from retrospective studies suggesting that even men with high-risk prostate cancer have poorer overall survival with ADT if they have a history of heart failure or MI.
For patients with low-risk cancer and diabetes, ADT is associated with worse overall survival, but ADT does not cause additional harm to men with intermediate- to high-risk prostate cancer who have concomitant diabetes, Dr. Nguyen said.
“My view is that ADT has not been shown to increase cardiovascular death in randomized trials, so I think that for the vast majority of patients it probably does not increase cardiovascular deaths. But I think there could very well be a vulnerable 5% of patients who might have an excess risk of cardiovascular death, and I think we have to be careful, but we still have to balance it out against their risks for prostate cancer death,” he said.
Dr. Nguyen reported consulting fees/honoraria from Astellas, Augmenix, Blue Earth Diagnostics, Cota, Dendreon, Ferring Pharmaceuticals, GenomeDx, Janssen, and Nanobiotix.
WASHINGTON – The cardiovascular effects of androgen deprivation therapy (ADT) for men with advanced prostate cancer are less severe than once feared, but there is evidence to suggest that men with preexisting heart failure or a history of myocardial infarction could be at excess risk for death from cardiovascular causes when they receive ADT, according to a leading prostate cancer expert.
“I think there are concerns about potential cardiovascular harm of ADT, and I think this has reduced ADT use, despite the fact that we know for most men it improves overall survival,” said Paul Nguyen, MD, a radiation oncologist at the Dana-Farber/Brigham and Women’s Cancer Center in Boston.
“In fact, when we looked recently at men with high-risk prostate cancer, this is a group where overall survival is improved by 50% if they get ADT – so it cuts the risk of death in half – but it turns out that nearly a quarter of those patients are not receiving ADT. I think that the concern about cardiovascular harm and the confusion as to where that data stands is a lot of what’s driving that right now,” he said at the American College of Cardiology’s Advancing the Cardiovascular Care of the Oncology Patient meeting.
Randomized trial data
Dr. Nguyen noted that the evidence suggesting that ADT can increase the risk of death from cardiovascular causes came largely from three major studies:
- A 2006 study of 73,196 Medicare enrollees aged 66 or older, which found that ADT with a gonadotropin-releasing hormone (GnRH) agonist was possibly associated with increased risk of incident diabetes and cardiovascular disease (J Clin Oncol. 2006 Sep 20;24[27]:4448-56.).
- A 2007 analysis of data from the Cancer of the Prostate Strategic Urologic Research Endeavor (CAPSURE) database on 3,262 men treated with radical prostatectomy and 1,630 men treated with radiation or cryotherapy for localized prostate cancer, which found that among those 65 and older the 5-year cumulative incidence of cardiovascular death was 5.5% for patients who received ADT, vs. 2% for those who did not (J Natl Cancer Inst. 2007 Oct 17;99[20]:1516-24).
- A 2007 study of 1,372 men in three randomized trials of radiation therapy with or without androgen suppression therapy up to 8 months in duration, which found that men 65 and older who received 6 months of androgen suppression had significantly shorter times to fatal MIs than did men who did not receive the therapy (J Clin Oncol. 2007;25[17]:2420-5).
These studies, combined with observational data, led to a 2010 consensus statement from the American Heart Association, American Cancer Society, and American Urological Association, with endorsement from the American Society for Radiation Oncology, which stated that “there may be a relation between ADT and cardiovascular events and death.”
Also in 2010, the Food and Drug Administration required new labeling on GnRH agonists warning of “increased risk of diabetes and certain cardiovascular diseases (heart attack, sudden cardiac death, stroke).”
Not unanimous
Two other large randomized studies (J Clin Oncol. 2008 Feb 1;26[4]:585-91 and J Clin Oncol. 2009 Jan 1;27[1]:92-9) and two retrospective studies (J Clin Oncol. 2009 Jul 20;27[21]:3452-8 and J Clin Oncol. 2011 Sep 10;29[26]3510-16) found no excess risk of cardiovascular disease from ADT, Dr. Nguyen said, prompting him and his colleagues to see whether they could get a better estimate of the actual risk.
They did so through a 2011 meta-analysis (JAMA. 2011;306[21]:2359-66) of data on 4,141 patients from eight randomized trials. They found that among patients with unfavorable-risk prostate cancer, ADT was not associated with an increased risk of cardiovascular death, but was associated with lower risks for both prostate-specific and all-cause mortality.
Subpopulations may still be at risk
Dr. Nguyen said that the principal finding of the meta-analysis, while reassuring, “doesn’t let ADT off the hook for metabolic events, diabetes which we know happens, and the possibility of nonfatal cardiac events.”
He noted that while ADT was not associated with cardiovascular disease in clinical trials, observational studies showed significantly increased risk for fatal or non-fatal MI.
One possible explanation for the difference is that observational studies included nonfatal MI, while randomized trials looked only at cardiovascular deaths. It’s also possible that ADT causes harm primarily in men with preexisting comorbidities, who are often excluded from or underrepresented in clinical trials.
Evidence from a 2009 study (JAMA. 2009 Aug 26;302[8]:866-73) showed that among men with clinical stage T1 to T3 noninvasive, nonmetastatic prostate cancer, neoadjuvant hormonal therapy with both a luteinizing hormone-releasing hormone (LHRH) agonist and a nonsteroidal antiandrogen was associated with increased risk for all-cause mortality for those with a history of coronary artery disease–induced heart failure, but not for men with either no comorbidities or only a single comorbidity such as hypertension, hypercholesterolemia, or diabetes.
Clinical considerations
The decision to treat men with prostate cancer with ADT is therefore a balancing act, Dr. Nguyen said.
“As the risk of prostate cancer death goes up, the benefit of ADT goes up. However, as the comorbidity level goes up, the potential cardiovascular harm of ADT goes up,” he said.
For patients at the extreme ends of each continuum, such as a patient with high-risk prostate cancer and no cardiovascular comorbidities or a patient with low-risk cancer but multiple CV risk factors, the decision to give or withhold ADT is relatively simple, he said.
But for patients in between, such as a man with intermediate-risk cancer and one risk factor or a man with high risk disease with multiple comorbidities, the decision is far more complex.
“This where I think the dialogue with the cardiologist really needs to come into this decision,” he said.
Evidence to support the decision comes from retrospective studies suggesting that even men with high-risk prostate cancer have poorer overall survival with ADT if they have a history of heart failure or MI.
For patients with low-risk cancer and diabetes, ADT is associated with worse overall survival, but ADT does not cause additional harm to men with intermediate- to high-risk prostate cancer who have concomitant diabetes, Dr. Nguyen said.
“My view is that ADT has not been shown to increase cardiovascular death in randomized trials, so I think that for the vast majority of patients it probably does not increase cardiovascular deaths. But I think there could very well be a vulnerable 5% of patients who might have an excess risk of cardiovascular death, and I think we have to be careful, but we still have to balance it out against their risks for prostate cancer death,” he said.
Dr. Nguyen reported consulting fees/honoraria from Astellas, Augmenix, Blue Earth Diagnostics, Cota, Dendreon, Ferring Pharmaceuticals, GenomeDx, Janssen, and Nanobiotix.
WASHINGTON – The cardiovascular effects of androgen deprivation therapy (ADT) for men with advanced prostate cancer are less severe than once feared, but there is evidence to suggest that men with preexisting heart failure or a history of myocardial infarction could be at excess risk for death from cardiovascular causes when they receive ADT, according to a leading prostate cancer expert.
“I think there are concerns about potential cardiovascular harm of ADT, and I think this has reduced ADT use, despite the fact that we know for most men it improves overall survival,” said Paul Nguyen, MD, a radiation oncologist at the Dana-Farber/Brigham and Women’s Cancer Center in Boston.
“In fact, when we looked recently at men with high-risk prostate cancer, this is a group where overall survival is improved by 50% if they get ADT – so it cuts the risk of death in half – but it turns out that nearly a quarter of those patients are not receiving ADT. I think that the concern about cardiovascular harm and the confusion as to where that data stands is a lot of what’s driving that right now,” he said at the American College of Cardiology’s Advancing the Cardiovascular Care of the Oncology Patient meeting.
Randomized trial data
Dr. Nguyen noted that the evidence suggesting that ADT can increase the risk of death from cardiovascular causes came largely from three major studies:
- A 2006 study of 73,196 Medicare enrollees aged 66 or older, which found that ADT with a gonadotropin-releasing hormone (GnRH) agonist was possibly associated with increased risk of incident diabetes and cardiovascular disease (J Clin Oncol. 2006 Sep 20;24[27]:4448-56.).
- A 2007 analysis of data from the Cancer of the Prostate Strategic Urologic Research Endeavor (CAPSURE) database on 3,262 men treated with radical prostatectomy and 1,630 men treated with radiation or cryotherapy for localized prostate cancer, which found that among those 65 and older the 5-year cumulative incidence of cardiovascular death was 5.5% for patients who received ADT, vs. 2% for those who did not (J Natl Cancer Inst. 2007 Oct 17;99[20]:1516-24).
- A 2007 study of 1,372 men in three randomized trials of radiation therapy with or without androgen suppression therapy up to 8 months in duration, which found that men 65 and older who received 6 months of androgen suppression had significantly shorter times to fatal MIs than did men who did not receive the therapy (J Clin Oncol. 2007;25[17]:2420-5).
These studies, combined with observational data, led to a 2010 consensus statement from the American Heart Association, American Cancer Society, and American Urological Association, with endorsement from the American Society for Radiation Oncology, which stated that “there may be a relation between ADT and cardiovascular events and death.”
Also in 2010, the Food and Drug Administration required new labeling on GnRH agonists warning of “increased risk of diabetes and certain cardiovascular diseases (heart attack, sudden cardiac death, stroke).”
Not unanimous
Two other large randomized studies (J Clin Oncol. 2008 Feb 1;26[4]:585-91 and J Clin Oncol. 2009 Jan 1;27[1]:92-9) and two retrospective studies (J Clin Oncol. 2009 Jul 20;27[21]:3452-8 and J Clin Oncol. 2011 Sep 10;29[26]3510-16) found no excess risk of cardiovascular disease from ADT, Dr. Nguyen said, prompting him and his colleagues to see whether they could get a better estimate of the actual risk.
They did so through a 2011 meta-analysis (JAMA. 2011;306[21]:2359-66) of data on 4,141 patients from eight randomized trials. They found that among patients with unfavorable-risk prostate cancer, ADT was not associated with an increased risk of cardiovascular death, but was associated with lower risks for both prostate-specific and all-cause mortality.
Subpopulations may still be at risk
Dr. Nguyen said that the principal finding of the meta-analysis, while reassuring, “doesn’t let ADT off the hook for metabolic events, diabetes which we know happens, and the possibility of nonfatal cardiac events.”
He noted that while ADT was not associated with cardiovascular disease in clinical trials, observational studies showed significantly increased risk for fatal or non-fatal MI.
One possible explanation for the difference is that observational studies included nonfatal MI, while randomized trials looked only at cardiovascular deaths. It’s also possible that ADT causes harm primarily in men with preexisting comorbidities, who are often excluded from or underrepresented in clinical trials.
Evidence from a 2009 study (JAMA. 2009 Aug 26;302[8]:866-73) showed that among men with clinical stage T1 to T3 noninvasive, nonmetastatic prostate cancer, neoadjuvant hormonal therapy with both a luteinizing hormone-releasing hormone (LHRH) agonist and a nonsteroidal antiandrogen was associated with increased risk for all-cause mortality for those with a history of coronary artery disease–induced heart failure, but not for men with either no comorbidities or only a single comorbidity such as hypertension, hypercholesterolemia, or diabetes.
Clinical considerations
The decision to treat men with prostate cancer with ADT is therefore a balancing act, Dr. Nguyen said.
“As the risk of prostate cancer death goes up, the benefit of ADT goes up. However, as the comorbidity level goes up, the potential cardiovascular harm of ADT goes up,” he said.
For patients at the extreme ends of each continuum, such as a patient with high-risk prostate cancer and no cardiovascular comorbidities or a patient with low-risk cancer but multiple CV risk factors, the decision to give or withhold ADT is relatively simple, he said.
But for patients in between, such as a man with intermediate-risk cancer and one risk factor or a man with high risk disease with multiple comorbidities, the decision is far more complex.
“This where I think the dialogue with the cardiologist really needs to come into this decision,” he said.
Evidence to support the decision comes from retrospective studies suggesting that even men with high-risk prostate cancer have poorer overall survival with ADT if they have a history of heart failure or MI.
For patients with low-risk cancer and diabetes, ADT is associated with worse overall survival, but ADT does not cause additional harm to men with intermediate- to high-risk prostate cancer who have concomitant diabetes, Dr. Nguyen said.
“My view is that ADT has not been shown to increase cardiovascular death in randomized trials, so I think that for the vast majority of patients it probably does not increase cardiovascular deaths. But I think there could very well be a vulnerable 5% of patients who might have an excess risk of cardiovascular death, and I think we have to be careful, but we still have to balance it out against their risks for prostate cancer death,” he said.
Dr. Nguyen reported consulting fees/honoraria from Astellas, Augmenix, Blue Earth Diagnostics, Cota, Dendreon, Ferring Pharmaceuticals, GenomeDx, Janssen, and Nanobiotix.
REPORTING FROM ACC CARDIO-ONCOLOGY
Key clinical point: Risk of cardiovascular death should be weighed against proven ADT benefits.
Major finding: ADT-related cardiovascular events appear limited to men with comorbid cardiovascular disease.
Study details: Review of clinical data on the cardiovascular consequences of ADT.
Disclosures: Dr. Nguyen reported consulting fees/honoraria from Astellas, Augmenix, Blue Earth Diagnostics, Cota, Dendreon, Ferring Pharmaceuticals, GenomeDx, Janssen, and Nanobiotix.
Adolescence does not rule out bullous pemphigoid
Although there are only 14 cases in the literature, it should still be kept in mind, wrote investigators led by Aikaterini Patsatsi, MD, PhD, of Aristotle University, Thessaloniki, Greece, and senior author Victoria Werth, MD, of the University of Pennsylvania, Philadelphia.
The good news is that the course of adolescent bullous pemphigoid “seems favorable, with long remission after disease control,” the investigators reported in Pediatric Dermatology.
Bullous pemphigoid (BP) is the most common autoimmune blistering disease in the elderly, but is rare in children, with the majority of pediatric cases occurring in early childhood. Even so, BP is still possible in adolescents, and should be worked up with “salt‐split skin [testing] in all cases, and the detection of circulating anti-BP180 and anti‐BP230 autoantibodies by ELISA [enzyme-linked immunosorbent assay] tests, not routinely done for this diagnosis,” the investigators wrote.
BP hasn’t been well characterized in teenagers, so Dr. Patsatsi and her associates searched Medline for “bullous pemphigoid in childhood and adolescence,” “childhood bullous pemphigoid,” “juvenile bullous pemphigoid,” and “autoimmune blistering and autoimmune bullous diseases in childhood.”
It turned out that “all authors agree that the management plan should be the least aggressive possible” with “the addition of immunomodulating agents such as dapsone, azathioprine, mycophenolate mofetil, or doxycycline/niacinamide,” although systemic steroids were used in 13 of the 14 cases, the investigators wrote.
They found nine cases in children aged 10‐13 years (six in girls, two in boys, and one case with no sex identified), with the first case reported in 1970. Five had mucosal involvement. One case was diagnosed as localized BP of the perineum. The children were treated with systemic prednisone (eight of nine), in combination with dapsone (two of nine), azathioprine (two of nine), and erythromycin/nicotinamide (one of nine). Three relapsed; there was no report of what was done for them or how they fared.
“The clinical features of BP in this age range include a pruritic generalized bullous eruption, similar to ... adult BP, with frequent involvement of the oral mucosa,” Dr. Patsatsi and her associates wrote.
The team also found five cases in children aged 14‐17 years (three girls, two boys), with the first reported in 1994. None had mucosal involvement. Treatment included systemic prednisone (five of five), in combination with dapsone (three of five), azathioprine (two of five), doxycycline/nicotinamide (one of five), and mycophenolate mofetil (one of five). Two cases relapsed; subsequent treatment and outcomes weren’t reported.
The clinical features again were similar to those seen in adults, “with disseminated tense blisters and erosions,” the investigators noted.
Only one case was reported in adolescents aged 18-21 years, though it was excluded from the review because it overlapped with pemphigus vulgaris.
No funding and no relevant financial disclosures were reported for the work.
SOURCE: Patsatsi A et al. Pediatr Dermatol. 2018 Dec 19. doi: 10.1111/pde.13717.
Although there are only 14 cases in the literature, it should still be kept in mind, wrote investigators led by Aikaterini Patsatsi, MD, PhD, of Aristotle University, Thessaloniki, Greece, and senior author Victoria Werth, MD, of the University of Pennsylvania, Philadelphia.
The good news is that the course of adolescent bullous pemphigoid “seems favorable, with long remission after disease control,” the investigators reported in Pediatric Dermatology.
Bullous pemphigoid (BP) is the most common autoimmune blistering disease in the elderly, but is rare in children, with the majority of pediatric cases occurring in early childhood. Even so, BP is still possible in adolescents, and should be worked up with “salt‐split skin [testing] in all cases, and the detection of circulating anti-BP180 and anti‐BP230 autoantibodies by ELISA [enzyme-linked immunosorbent assay] tests, not routinely done for this diagnosis,” the investigators wrote.
BP hasn’t been well characterized in teenagers, so Dr. Patsatsi and her associates searched Medline for “bullous pemphigoid in childhood and adolescence,” “childhood bullous pemphigoid,” “juvenile bullous pemphigoid,” and “autoimmune blistering and autoimmune bullous diseases in childhood.”
It turned out that “all authors agree that the management plan should be the least aggressive possible” with “the addition of immunomodulating agents such as dapsone, azathioprine, mycophenolate mofetil, or doxycycline/niacinamide,” although systemic steroids were used in 13 of the 14 cases, the investigators wrote.
They found nine cases in children aged 10‐13 years (six in girls, two in boys, and one case with no sex identified), with the first case reported in 1970. Five had mucosal involvement. One case was diagnosed as localized BP of the perineum. The children were treated with systemic prednisone (eight of nine), in combination with dapsone (two of nine), azathioprine (two of nine), and erythromycin/nicotinamide (one of nine). Three relapsed; there was no report of what was done for them or how they fared.
“The clinical features of BP in this age range include a pruritic generalized bullous eruption, similar to ... adult BP, with frequent involvement of the oral mucosa,” Dr. Patsatsi and her associates wrote.
The team also found five cases in children aged 14‐17 years (three girls, two boys), with the first reported in 1994. None had mucosal involvement. Treatment included systemic prednisone (five of five), in combination with dapsone (three of five), azathioprine (two of five), doxycycline/nicotinamide (one of five), and mycophenolate mofetil (one of five). Two cases relapsed; subsequent treatment and outcomes weren’t reported.
The clinical features again were similar to those seen in adults, “with disseminated tense blisters and erosions,” the investigators noted.
Only one case was reported in adolescents aged 18-21 years, though it was excluded from the review because it overlapped with pemphigus vulgaris.
No funding and no relevant financial disclosures were reported for the work.
SOURCE: Patsatsi A et al. Pediatr Dermatol. 2018 Dec 19. doi: 10.1111/pde.13717.
Although there are only 14 cases in the literature, it should still be kept in mind, wrote investigators led by Aikaterini Patsatsi, MD, PhD, of Aristotle University, Thessaloniki, Greece, and senior author Victoria Werth, MD, of the University of Pennsylvania, Philadelphia.
The good news is that the course of adolescent bullous pemphigoid “seems favorable, with long remission after disease control,” the investigators reported in Pediatric Dermatology.
Bullous pemphigoid (BP) is the most common autoimmune blistering disease in the elderly, but is rare in children, with the majority of pediatric cases occurring in early childhood. Even so, BP is still possible in adolescents, and should be worked up with “salt‐split skin [testing] in all cases, and the detection of circulating anti-BP180 and anti‐BP230 autoantibodies by ELISA [enzyme-linked immunosorbent assay] tests, not routinely done for this diagnosis,” the investigators wrote.
BP hasn’t been well characterized in teenagers, so Dr. Patsatsi and her associates searched Medline for “bullous pemphigoid in childhood and adolescence,” “childhood bullous pemphigoid,” “juvenile bullous pemphigoid,” and “autoimmune blistering and autoimmune bullous diseases in childhood.”
It turned out that “all authors agree that the management plan should be the least aggressive possible” with “the addition of immunomodulating agents such as dapsone, azathioprine, mycophenolate mofetil, or doxycycline/niacinamide,” although systemic steroids were used in 13 of the 14 cases, the investigators wrote.
They found nine cases in children aged 10‐13 years (six in girls, two in boys, and one case with no sex identified), with the first case reported in 1970. Five had mucosal involvement. One case was diagnosed as localized BP of the perineum. The children were treated with systemic prednisone (eight of nine), in combination with dapsone (two of nine), azathioprine (two of nine), and erythromycin/nicotinamide (one of nine). Three relapsed; there was no report of what was done for them or how they fared.
“The clinical features of BP in this age range include a pruritic generalized bullous eruption, similar to ... adult BP, with frequent involvement of the oral mucosa,” Dr. Patsatsi and her associates wrote.
The team also found five cases in children aged 14‐17 years (three girls, two boys), with the first reported in 1994. None had mucosal involvement. Treatment included systemic prednisone (five of five), in combination with dapsone (three of five), azathioprine (two of five), doxycycline/nicotinamide (one of five), and mycophenolate mofetil (one of five). Two cases relapsed; subsequent treatment and outcomes weren’t reported.
The clinical features again were similar to those seen in adults, “with disseminated tense blisters and erosions,” the investigators noted.
Only one case was reported in adolescents aged 18-21 years, though it was excluded from the review because it overlapped with pemphigus vulgaris.
No funding and no relevant financial disclosures were reported for the work.
SOURCE: Patsatsi A et al. Pediatr Dermatol. 2018 Dec 19. doi: 10.1111/pde.13717.
FROM PEDIATRIC DERMATOLOGY
Key clinical point: The course of adolescent bullous pemphigoid appears favorable, with long remission after the disease is controlled.
Major finding: The investigators found nine cases in children aged 10‐13 years, and five cases in children aged 14‐17 years.
Study details: A search in Medline detected 14 adolescents with a diagnosis of bullous pemphigoid.
Disclosures: No funding and no relevant financial disclosures were reported for the work.
Source: Patsatsi A et al. Pediatr Dermatol. 2018 Dec 19. doi: 10.1111/pde.13717.
Checkpoint inhibitors ‘viable treatment option’ in HIV-infected individuals
Immune checkpoint inhibitors are safe and effective in HIV-infected patients with advanced cancers, according to authors of a recently published systematic review.
The treatment was well tolerated and associated with a 9% rate of grade 3 or higher immune-related adverse events, according to results of the review of 73 patient cases.
There were no adverse impacts on HIV load or CD4 cell count detected in the patients, according to researchers Michael R. Cook, MD, and Chul Kim, MD, MPH, of Georgetown University, Washington.
Antitumor activity of the checkpoint inhibitors in lung cancer patients was comparable to what has been seen in previous randomized clinical trials that excluded HIV-infected individuals, Dr. Cook and Dr. Kim reported in JAMA Oncology.
“Based on the results of the present systematic review, and in the absence of definitive prospective data suggesting an unfavorable risk-to-benefit ratio, immune checkpoint inhibitor therapy may be considered as a viable treatment option for HIV-infected patients with advanced cancer,” they said.
There are preclinical data suggesting that immune checkpoint modulation could improve function of HIV-specific T cells, the investigators added.
“Prospective trials of immune checkpoint inhibitors are necessary to elucidate the antiviral efficacy of immune checkpoint inhibitor therapy in patients with HIV infection and cancer,” they said.
Several such trials are underway to evaluate the role of the pembrolizumab, nivolumab, nivolumab plus ipilimumab, and durvalumab in HIV-infected patients with advanced-stage cancers, according to the review authors.
In the present systematic review, Dr. Cook and Dr. Kim conducted a literature search and reviewed presentations from major annual medical conferences.
Of the 73 HIV-infected patients they identified, most had non–small cell lung cancer (34.2%), melanoma (21.9%), or Kaposi sarcoma (12.3%), while the rest had anal cancer, head and neck cancer, or other malignancies. Most patients had received either nivolumab (39.7%) or pembrolizumab (35.6%).
There were “no concerning findings” among these patients with regard to immune-mediated toxicities or changes in HIV-related parameters.
Six of 70 patients had immune-related adverse events of grade 3 or greater.
Thirty-four patients had documented HIV loads before and after receiving an immune checkpoint inhibitor. Of those, 28 had undetectable HIV loads at baseline, and all but 2 (7%) maintained undetectable loads in the posttreatment evaluation.
Of the remaining six with detectable HIV loads before treatment, five had a decrease in viral load, to the point that four had undetectable HIV viral load in the posttreatment evaluation, the investigators reported.
The overall response rate was 30% for the lung cancer patients, 27% for melanoma, and 63% for Kaposi sarcoma.
In the non–small cell lung cancer subset, response rates were 26% for those who had received previous systemic treatment, and 50% for those who had not, which was similar to findings from major checkpoint inhibitor trials that excluded HIV-infected individuals, the investigators said.
The American Society of Clinical Oncology Conquer Cancer Foundation and Georgetown University supported the study. Dr. Kim reported disclosures related to CARIS Life Science and AstraZeneca.
SOURCE: Cook MR and Kim C. JAMA Oncol. 2019 Feb 7. doi: 10.1001/jamaoncol.2018.6737.
Immune checkpoint inhibitors are safe and effective in HIV-infected patients with advanced cancers, according to authors of a recently published systematic review.
The treatment was well tolerated and associated with a 9% rate of grade 3 or higher immune-related adverse events, according to results of the review of 73 patient cases.
There were no adverse impacts on HIV load or CD4 cell count detected in the patients, according to researchers Michael R. Cook, MD, and Chul Kim, MD, MPH, of Georgetown University, Washington.
Antitumor activity of the checkpoint inhibitors in lung cancer patients was comparable to what has been seen in previous randomized clinical trials that excluded HIV-infected individuals, Dr. Cook and Dr. Kim reported in JAMA Oncology.
“Based on the results of the present systematic review, and in the absence of definitive prospective data suggesting an unfavorable risk-to-benefit ratio, immune checkpoint inhibitor therapy may be considered as a viable treatment option for HIV-infected patients with advanced cancer,” they said.
There are preclinical data suggesting that immune checkpoint modulation could improve function of HIV-specific T cells, the investigators added.
“Prospective trials of immune checkpoint inhibitors are necessary to elucidate the antiviral efficacy of immune checkpoint inhibitor therapy in patients with HIV infection and cancer,” they said.
Several such trials are underway to evaluate the role of the pembrolizumab, nivolumab, nivolumab plus ipilimumab, and durvalumab in HIV-infected patients with advanced-stage cancers, according to the review authors.
In the present systematic review, Dr. Cook and Dr. Kim conducted a literature search and reviewed presentations from major annual medical conferences.
Of the 73 HIV-infected patients they identified, most had non–small cell lung cancer (34.2%), melanoma (21.9%), or Kaposi sarcoma (12.3%), while the rest had anal cancer, head and neck cancer, or other malignancies. Most patients had received either nivolumab (39.7%) or pembrolizumab (35.6%).
There were “no concerning findings” among these patients with regard to immune-mediated toxicities or changes in HIV-related parameters.
Six of 70 patients had immune-related adverse events of grade 3 or greater.
Thirty-four patients had documented HIV loads before and after receiving an immune checkpoint inhibitor. Of those, 28 had undetectable HIV loads at baseline, and all but 2 (7%) maintained undetectable loads in the posttreatment evaluation.
Of the remaining six with detectable HIV loads before treatment, five had a decrease in viral load, to the point that four had undetectable HIV viral load in the posttreatment evaluation, the investigators reported.
The overall response rate was 30% for the lung cancer patients, 27% for melanoma, and 63% for Kaposi sarcoma.
In the non–small cell lung cancer subset, response rates were 26% for those who had received previous systemic treatment, and 50% for those who had not, which was similar to findings from major checkpoint inhibitor trials that excluded HIV-infected individuals, the investigators said.
The American Society of Clinical Oncology Conquer Cancer Foundation and Georgetown University supported the study. Dr. Kim reported disclosures related to CARIS Life Science and AstraZeneca.
SOURCE: Cook MR and Kim C. JAMA Oncol. 2019 Feb 7. doi: 10.1001/jamaoncol.2018.6737.
Immune checkpoint inhibitors are safe and effective in HIV-infected patients with advanced cancers, according to authors of a recently published systematic review.
The treatment was well tolerated and associated with a 9% rate of grade 3 or higher immune-related adverse events, according to results of the review of 73 patient cases.
There were no adverse impacts on HIV load or CD4 cell count detected in the patients, according to researchers Michael R. Cook, MD, and Chul Kim, MD, MPH, of Georgetown University, Washington.
Antitumor activity of the checkpoint inhibitors in lung cancer patients was comparable to what has been seen in previous randomized clinical trials that excluded HIV-infected individuals, Dr. Cook and Dr. Kim reported in JAMA Oncology.
“Based on the results of the present systematic review, and in the absence of definitive prospective data suggesting an unfavorable risk-to-benefit ratio, immune checkpoint inhibitor therapy may be considered as a viable treatment option for HIV-infected patients with advanced cancer,” they said.
There are preclinical data suggesting that immune checkpoint modulation could improve function of HIV-specific T cells, the investigators added.
“Prospective trials of immune checkpoint inhibitors are necessary to elucidate the antiviral efficacy of immune checkpoint inhibitor therapy in patients with HIV infection and cancer,” they said.
Several such trials are underway to evaluate the role of the pembrolizumab, nivolumab, nivolumab plus ipilimumab, and durvalumab in HIV-infected patients with advanced-stage cancers, according to the review authors.
In the present systematic review, Dr. Cook and Dr. Kim conducted a literature search and reviewed presentations from major annual medical conferences.
Of the 73 HIV-infected patients they identified, most had non–small cell lung cancer (34.2%), melanoma (21.9%), or Kaposi sarcoma (12.3%), while the rest had anal cancer, head and neck cancer, or other malignancies. Most patients had received either nivolumab (39.7%) or pembrolizumab (35.6%).
There were “no concerning findings” among these patients with regard to immune-mediated toxicities or changes in HIV-related parameters.
Six of 70 patients had immune-related adverse events of grade 3 or greater.
Thirty-four patients had documented HIV loads before and after receiving an immune checkpoint inhibitor. Of those, 28 had undetectable HIV loads at baseline, and all but 2 (7%) maintained undetectable loads in the posttreatment evaluation.
Of the remaining six with detectable HIV loads before treatment, five had a decrease in viral load, to the point that four had undetectable HIV viral load in the posttreatment evaluation, the investigators reported.
The overall response rate was 30% for the lung cancer patients, 27% for melanoma, and 63% for Kaposi sarcoma.
In the non–small cell lung cancer subset, response rates were 26% for those who had received previous systemic treatment, and 50% for those who had not, which was similar to findings from major checkpoint inhibitor trials that excluded HIV-infected individuals, the investigators said.
The American Society of Clinical Oncology Conquer Cancer Foundation and Georgetown University supported the study. Dr. Kim reported disclosures related to CARIS Life Science and AstraZeneca.
SOURCE: Cook MR and Kim C. JAMA Oncol. 2019 Feb 7. doi: 10.1001/jamaoncol.2018.6737.
FROM JAMA ONCOLOGY
Key clinical point: Immune checkpoint inhibitors are a viable treatment option for HIV-infected patients, according to data supporting their safety and efficacy in this patient population.
Major finding: The treatment was well tolerated, with an 8.6% rate of grade 3 or greater immune-related adverse events, and no impact on HIV-related parameters.
Study details: A systematic review of 73 patients with HIV infection who had received treatment with a checkpoint inhibitor.
Disclosures: The American Society of Clinical Oncology Conquer Cancer Foundation and Georgetown University supported the study. One study author reported disclosures related to CARIS Life Science and AstraZeneca.
Source: Cook MR and Kim C. JAMA Oncol. 2019 Feb 7. doi: 10.1001/jamaoncol.2018.6737.
Combo appears to overcome aggressive L-NN-MCL
Some patients with aggressive leukemic nonnodal mantle cell lymphoma (L-NN-MCL) respond very well to combination therapy with rituximab and ibrutinib, according to two case reports.
Both patients, who had aggressive L-NN-MCL and P53 abnormalities, remain free of disease 18 months after treatment with rituximab/ibrutinib and autologous stem cell transplantation (ASCT), reported Shahram Mori, MD, PhD, of the Florida Hospital Cancer Institute in Orlando, and his colleagues.
The findings suggest that P53 gene status in L-NN-MCL may have a significant impact on prognosis and treatment planning. There are currently no guidelines for risk stratifying L-NN-MCL patients.
“Although the recognition of L-NN-MCL is important to avoid overtreatment, there appears to be a subset of patients who either have a more aggressive form or disease that has transformed to a more aggressive form who present with symptomatic disease and/or cytopenias,” the investigators wrote in Clinical Lymphoma, Myeloma & Leukemia.
The investigators described two such cases in their report. Both patients had leukocytosis with various other blood cell derangements and splenomegaly without lymphadenopathy.
The first patient was a 53-year-old African American man with L-NN-MCL and a number of genetic aberrations, including loss of the P53 gene. After two cycles of rituximab with bendamustine proved ineffective, he was switched to rituxan with cyclophosphamide, vincristine, adriamycin, and dexamethasone with high-dose methotrexate and cytarabine. This regimen was also ineffective and his white blood cell count kept rising.
His story changed for the better when the patient was switched to ibrutinib 560 mg daily and rituximab 375 mg/m2 monthly. Within 2 months of starting therapy, his blood abnormalities normalized, and bone marrow biopsy at the end of treatment revealed complete remission without evidence of minimal residual disease. The patient remains in complete remission 18 months after ASCT.
The second patient was a 49-year-old Hispanic man with L-NN-MCL. He had missense mutations in TP53 and KMT2A (MLL), a frameshift mutation in BCOR, and a t(11;14) translocation. Ibrutinib/rituximab was started immediately. After 1 month, his blood levels began to normalize. After five cycles, bone marrow biopsy showed complete remission with no evidence of minimal residual disease. Like the first patient, the second patient remains in complete remission 18 months after ASCT.
“To our knowledge, these are the first two cases of L-NN-MCL with P53 gene mutations/alterations that were successfully treated with a combination of rituximab and ibrutinib,” the investigators wrote. “Our two cases confirm the previous studies by Chapman-Fredricks et al, who also noted P53 gene mutation or deletion is associated with the aggressive course.”
The researchers reported having no financial disclosures.
SOURCE: Mori S et al. Clin Lymphoma Myeloma Leuk. 2019 Feb;19(2):e93-7.
Some patients with aggressive leukemic nonnodal mantle cell lymphoma (L-NN-MCL) respond very well to combination therapy with rituximab and ibrutinib, according to two case reports.
Both patients, who had aggressive L-NN-MCL and P53 abnormalities, remain free of disease 18 months after treatment with rituximab/ibrutinib and autologous stem cell transplantation (ASCT), reported Shahram Mori, MD, PhD, of the Florida Hospital Cancer Institute in Orlando, and his colleagues.
The findings suggest that P53 gene status in L-NN-MCL may have a significant impact on prognosis and treatment planning. There are currently no guidelines for risk stratifying L-NN-MCL patients.
“Although the recognition of L-NN-MCL is important to avoid overtreatment, there appears to be a subset of patients who either have a more aggressive form or disease that has transformed to a more aggressive form who present with symptomatic disease and/or cytopenias,” the investigators wrote in Clinical Lymphoma, Myeloma & Leukemia.
The investigators described two such cases in their report. Both patients had leukocytosis with various other blood cell derangements and splenomegaly without lymphadenopathy.
The first patient was a 53-year-old African American man with L-NN-MCL and a number of genetic aberrations, including loss of the P53 gene. After two cycles of rituximab with bendamustine proved ineffective, he was switched to rituxan with cyclophosphamide, vincristine, adriamycin, and dexamethasone with high-dose methotrexate and cytarabine. This regimen was also ineffective and his white blood cell count kept rising.
His story changed for the better when the patient was switched to ibrutinib 560 mg daily and rituximab 375 mg/m2 monthly. Within 2 months of starting therapy, his blood abnormalities normalized, and bone marrow biopsy at the end of treatment revealed complete remission without evidence of minimal residual disease. The patient remains in complete remission 18 months after ASCT.
The second patient was a 49-year-old Hispanic man with L-NN-MCL. He had missense mutations in TP53 and KMT2A (MLL), a frameshift mutation in BCOR, and a t(11;14) translocation. Ibrutinib/rituximab was started immediately. After 1 month, his blood levels began to normalize. After five cycles, bone marrow biopsy showed complete remission with no evidence of minimal residual disease. Like the first patient, the second patient remains in complete remission 18 months after ASCT.
“To our knowledge, these are the first two cases of L-NN-MCL with P53 gene mutations/alterations that were successfully treated with a combination of rituximab and ibrutinib,” the investigators wrote. “Our two cases confirm the previous studies by Chapman-Fredricks et al, who also noted P53 gene mutation or deletion is associated with the aggressive course.”
The researchers reported having no financial disclosures.
SOURCE: Mori S et al. Clin Lymphoma Myeloma Leuk. 2019 Feb;19(2):e93-7.
Some patients with aggressive leukemic nonnodal mantle cell lymphoma (L-NN-MCL) respond very well to combination therapy with rituximab and ibrutinib, according to two case reports.
Both patients, who had aggressive L-NN-MCL and P53 abnormalities, remain free of disease 18 months after treatment with rituximab/ibrutinib and autologous stem cell transplantation (ASCT), reported Shahram Mori, MD, PhD, of the Florida Hospital Cancer Institute in Orlando, and his colleagues.
The findings suggest that P53 gene status in L-NN-MCL may have a significant impact on prognosis and treatment planning. There are currently no guidelines for risk stratifying L-NN-MCL patients.
“Although the recognition of L-NN-MCL is important to avoid overtreatment, there appears to be a subset of patients who either have a more aggressive form or disease that has transformed to a more aggressive form who present with symptomatic disease and/or cytopenias,” the investigators wrote in Clinical Lymphoma, Myeloma & Leukemia.
The investigators described two such cases in their report. Both patients had leukocytosis with various other blood cell derangements and splenomegaly without lymphadenopathy.
The first patient was a 53-year-old African American man with L-NN-MCL and a number of genetic aberrations, including loss of the P53 gene. After two cycles of rituximab with bendamustine proved ineffective, he was switched to rituxan with cyclophosphamide, vincristine, adriamycin, and dexamethasone with high-dose methotrexate and cytarabine. This regimen was also ineffective and his white blood cell count kept rising.
His story changed for the better when the patient was switched to ibrutinib 560 mg daily and rituximab 375 mg/m2 monthly. Within 2 months of starting therapy, his blood abnormalities normalized, and bone marrow biopsy at the end of treatment revealed complete remission without evidence of minimal residual disease. The patient remains in complete remission 18 months after ASCT.
The second patient was a 49-year-old Hispanic man with L-NN-MCL. He had missense mutations in TP53 and KMT2A (MLL), a frameshift mutation in BCOR, and a t(11;14) translocation. Ibrutinib/rituximab was started immediately. After 1 month, his blood levels began to normalize. After five cycles, bone marrow biopsy showed complete remission with no evidence of minimal residual disease. Like the first patient, the second patient remains in complete remission 18 months after ASCT.
“To our knowledge, these are the first two cases of L-NN-MCL with P53 gene mutations/alterations that were successfully treated with a combination of rituximab and ibrutinib,” the investigators wrote. “Our two cases confirm the previous studies by Chapman-Fredricks et al, who also noted P53 gene mutation or deletion is associated with the aggressive course.”
The researchers reported having no financial disclosures.
SOURCE: Mori S et al. Clin Lymphoma Myeloma Leuk. 2019 Feb;19(2):e93-7.
FROM CLINICAL LYMPHOMA, MYELOMA & LEUKEMIA
Key clinical point:
Major finding: Two patients with aggressive L-NN-MCL and P53 abnormalities who were treated with rituximab/ibrutinib and autologous stem cell transplantation remain free of disease 18 months later.
Study details: Two case reports.
Disclosures: The authors reported having no financial disclosures.
Source: Mori S et al. Clin Lymphoma Myeloma Leuk. 2019 Feb;19(2):e93-7.
Socioeconomic status, race found to impact CPAP compliance
SAN DIEGO –
“CPAP is the gold standard treatment for OSA [obstructive sleep apnea] and is very effective, especially for those with severe disease,” researchers led by Philip S. LoSavio, MD, wrote in an abstract presented at the Triological Society’s Combined Sections Meeting. “However, CPAP is a significant challenge for patients for various reasons, with reports of only 46%-80% of OSA patients using CPAP for more than 4 consecutive hours on two out of three nights.”
In an effort to identify and define different factors associated with CPAP compliance, Dr. LoSavio and his colleagues collected data on 578 patients with OSA on CPAP who were treated at Rush University Medical Center, Chicago. The mean patient age was 58 years, 52% were female, 43% were African American, 40% were white, their mean body mass index was 36.91 kg/m2, and their mean apnea-hypopnea index was 37.25 events per hour. The researchers recorded CPAP use at office visits via CPAP module or card, and patients were considered CPAP compliant if their machines logged 4 consecutive hours of use for 70% or more of nights. During the office visits, patients completed a questionnaire asking if they were suffering from different otolaryngology-related diseases, including sinus headaches, gastroesophageal reflex, and enlarged tonsils. Dr. LoSavio, who heads the section of sleep surgery in the department of otorhinolaryngology at Rush University Medical Center, and his colleagues performed logistic regression to ascertain the effects of race and socioeconomic status on CPAP compliance while adjusting for OSA severity. They also analyzed the adjusted association of median income and self-reported symptoms of sinus headaches, GERD, and enlarged tonsils, on CPAP compliance.
They found that African American patients were less compliant with CPAP, compared with their white counterparts (OR 0.42; P less than .01). In addition, patients with mild OSA were less likely to be compliant compared with those who had severe disease (OR 0.57; P less than .03). Self-reported symptoms of sinus headaches, GERD, and enlarged tonsils were associated with significantly lower levels of compliance, while higher median income was positively associated with higher levels of compliance. When the researchers grouped incomes based on the 2018 federal tax classification brackets, they observed a significant association between compliance and median income (P less than .001), with a likelihood ratio of 20.4.
“Previous studies have shown that with increases in OSA disease severity, defined by higher [apnea-hypopnea index], comes increases in CPAP compliance, while other studies have alluded to the fact that lower socioeconomic status can affect CPAP compliance,” Dr. LoSavio and his associates wrote in their abstract. “A novel aspect of our study hoped to shed light on different otolaryngology-related diseases and how they might affect compliance. The patients with comorbid GERD, sinus headaches, and enlarged tonsils were less CPAP compliant in our study. These conditions are relatively easily treated and could therefore provide an avenue to increase CPAP compliance if addressed.” They acknowledged certain limitations of the study, including its single-center design and the self-reported nature of the patient questionnaire.
The researchers reported having no financial disclosures. The meeting was jointly sponsored by the Triological Society and the American College of Surgeons.
SOURCE: LoSavio P et al. Triological CSM 2019, Abstracts.
SAN DIEGO –
“CPAP is the gold standard treatment for OSA [obstructive sleep apnea] and is very effective, especially for those with severe disease,” researchers led by Philip S. LoSavio, MD, wrote in an abstract presented at the Triological Society’s Combined Sections Meeting. “However, CPAP is a significant challenge for patients for various reasons, with reports of only 46%-80% of OSA patients using CPAP for more than 4 consecutive hours on two out of three nights.”
In an effort to identify and define different factors associated with CPAP compliance, Dr. LoSavio and his colleagues collected data on 578 patients with OSA on CPAP who were treated at Rush University Medical Center, Chicago. The mean patient age was 58 years, 52% were female, 43% were African American, 40% were white, their mean body mass index was 36.91 kg/m2, and their mean apnea-hypopnea index was 37.25 events per hour. The researchers recorded CPAP use at office visits via CPAP module or card, and patients were considered CPAP compliant if their machines logged 4 consecutive hours of use for 70% or more of nights. During the office visits, patients completed a questionnaire asking if they were suffering from different otolaryngology-related diseases, including sinus headaches, gastroesophageal reflex, and enlarged tonsils. Dr. LoSavio, who heads the section of sleep surgery in the department of otorhinolaryngology at Rush University Medical Center, and his colleagues performed logistic regression to ascertain the effects of race and socioeconomic status on CPAP compliance while adjusting for OSA severity. They also analyzed the adjusted association of median income and self-reported symptoms of sinus headaches, GERD, and enlarged tonsils, on CPAP compliance.
They found that African American patients were less compliant with CPAP, compared with their white counterparts (OR 0.42; P less than .01). In addition, patients with mild OSA were less likely to be compliant compared with those who had severe disease (OR 0.57; P less than .03). Self-reported symptoms of sinus headaches, GERD, and enlarged tonsils were associated with significantly lower levels of compliance, while higher median income was positively associated with higher levels of compliance. When the researchers grouped incomes based on the 2018 federal tax classification brackets, they observed a significant association between compliance and median income (P less than .001), with a likelihood ratio of 20.4.
“Previous studies have shown that with increases in OSA disease severity, defined by higher [apnea-hypopnea index], comes increases in CPAP compliance, while other studies have alluded to the fact that lower socioeconomic status can affect CPAP compliance,” Dr. LoSavio and his associates wrote in their abstract. “A novel aspect of our study hoped to shed light on different otolaryngology-related diseases and how they might affect compliance. The patients with comorbid GERD, sinus headaches, and enlarged tonsils were less CPAP compliant in our study. These conditions are relatively easily treated and could therefore provide an avenue to increase CPAP compliance if addressed.” They acknowledged certain limitations of the study, including its single-center design and the self-reported nature of the patient questionnaire.
The researchers reported having no financial disclosures. The meeting was jointly sponsored by the Triological Society and the American College of Surgeons.
SOURCE: LoSavio P et al. Triological CSM 2019, Abstracts.
SAN DIEGO –
“CPAP is the gold standard treatment for OSA [obstructive sleep apnea] and is very effective, especially for those with severe disease,” researchers led by Philip S. LoSavio, MD, wrote in an abstract presented at the Triological Society’s Combined Sections Meeting. “However, CPAP is a significant challenge for patients for various reasons, with reports of only 46%-80% of OSA patients using CPAP for more than 4 consecutive hours on two out of three nights.”
In an effort to identify and define different factors associated with CPAP compliance, Dr. LoSavio and his colleagues collected data on 578 patients with OSA on CPAP who were treated at Rush University Medical Center, Chicago. The mean patient age was 58 years, 52% were female, 43% were African American, 40% were white, their mean body mass index was 36.91 kg/m2, and their mean apnea-hypopnea index was 37.25 events per hour. The researchers recorded CPAP use at office visits via CPAP module or card, and patients were considered CPAP compliant if their machines logged 4 consecutive hours of use for 70% or more of nights. During the office visits, patients completed a questionnaire asking if they were suffering from different otolaryngology-related diseases, including sinus headaches, gastroesophageal reflex, and enlarged tonsils. Dr. LoSavio, who heads the section of sleep surgery in the department of otorhinolaryngology at Rush University Medical Center, and his colleagues performed logistic regression to ascertain the effects of race and socioeconomic status on CPAP compliance while adjusting for OSA severity. They also analyzed the adjusted association of median income and self-reported symptoms of sinus headaches, GERD, and enlarged tonsils, on CPAP compliance.
They found that African American patients were less compliant with CPAP, compared with their white counterparts (OR 0.42; P less than .01). In addition, patients with mild OSA were less likely to be compliant compared with those who had severe disease (OR 0.57; P less than .03). Self-reported symptoms of sinus headaches, GERD, and enlarged tonsils were associated with significantly lower levels of compliance, while higher median income was positively associated with higher levels of compliance. When the researchers grouped incomes based on the 2018 federal tax classification brackets, they observed a significant association between compliance and median income (P less than .001), with a likelihood ratio of 20.4.
“Previous studies have shown that with increases in OSA disease severity, defined by higher [apnea-hypopnea index], comes increases in CPAP compliance, while other studies have alluded to the fact that lower socioeconomic status can affect CPAP compliance,” Dr. LoSavio and his associates wrote in their abstract. “A novel aspect of our study hoped to shed light on different otolaryngology-related diseases and how they might affect compliance. The patients with comorbid GERD, sinus headaches, and enlarged tonsils were less CPAP compliant in our study. These conditions are relatively easily treated and could therefore provide an avenue to increase CPAP compliance if addressed.” They acknowledged certain limitations of the study, including its single-center design and the self-reported nature of the patient questionnaire.
The researchers reported having no financial disclosures. The meeting was jointly sponsored by the Triological Society and the American College of Surgeons.
SOURCE: LoSavio P et al. Triological CSM 2019, Abstracts.
REPORTING FROM THE TRIOLOGICAL CSM
Key clinical point: Compliance with continuous positive airway pressure is affected by patient socioeconomic status and race.
Major finding: African American patients were less compliant with CPAP, compared with their white counterparts (OR 0.42; P less than .01).
Study details: A retrospective study of 578 obstructive sleep apnea patients on CPAP.
Disclosures: The researchers reported having no financial disclosures.
Source: LoSavio P et al. Triological CSM 2019, Abstracts.