User login
More RVUs for 3 office hysteroscopy procedures
- Diagnostic hysteroscopy carries .65 more (RVUs) for the office setting.
- Endometrial ablation has 63.25 RVUs for the office setting, but only 9.66 for the hospital setting.
- Essure, a new hysteroscopic sterilization technology, carries 57.91 RVUs in the office setting.
The vastly increased RVU for the latter 2 procedures in the office setting covers the more expensive equipment needed.
Hysteroscopic procedures do not have a professional and technical component in the typical sense. Although you may have additional practice costs such as a dedicated treatment room or special equipment, these may not be accurately reflected in the allowable for the hysteroscopic procedure you perform in the office setting. The current RVU system does not allow for separate payment of a “facility fee”; all practice costs associated with performing the procedure are added into the practice expense portion of the RVU for each procedure. Although all payers bundle the surgical tray into the reimbursement for the procedure, consider negotiating for a “facility fee” that adequately covers your additional expenses, by pointing out that money will be saved when the hysteroscopy is performed in the office.
- Diagnostic hysteroscopy carries .65 more (RVUs) for the office setting.
- Endometrial ablation has 63.25 RVUs for the office setting, but only 9.66 for the hospital setting.
- Essure, a new hysteroscopic sterilization technology, carries 57.91 RVUs in the office setting.
The vastly increased RVU for the latter 2 procedures in the office setting covers the more expensive equipment needed.
Hysteroscopic procedures do not have a professional and technical component in the typical sense. Although you may have additional practice costs such as a dedicated treatment room or special equipment, these may not be accurately reflected in the allowable for the hysteroscopic procedure you perform in the office setting. The current RVU system does not allow for separate payment of a “facility fee”; all practice costs associated with performing the procedure are added into the practice expense portion of the RVU for each procedure. Although all payers bundle the surgical tray into the reimbursement for the procedure, consider negotiating for a “facility fee” that adequately covers your additional expenses, by pointing out that money will be saved when the hysteroscopy is performed in the office.
- Diagnostic hysteroscopy carries .65 more (RVUs) for the office setting.
- Endometrial ablation has 63.25 RVUs for the office setting, but only 9.66 for the hospital setting.
- Essure, a new hysteroscopic sterilization technology, carries 57.91 RVUs in the office setting.
The vastly increased RVU for the latter 2 procedures in the office setting covers the more expensive equipment needed.
Hysteroscopic procedures do not have a professional and technical component in the typical sense. Although you may have additional practice costs such as a dedicated treatment room or special equipment, these may not be accurately reflected in the allowable for the hysteroscopic procedure you perform in the office setting. The current RVU system does not allow for separate payment of a “facility fee”; all practice costs associated with performing the procedure are added into the practice expense portion of the RVU for each procedure. Although all payers bundle the surgical tray into the reimbursement for the procedure, consider negotiating for a “facility fee” that adequately covers your additional expenses, by pointing out that money will be saved when the hysteroscopy is performed in the office.
Strategies for a Safe and Effective Resident Sign‐Out
Modern‐day continuity of patient care in teaching hospitals, once remarkably high because of a cadre of sleep‐deprived residents, is now peppered with breaks, each accompanied by the transfer of patient care responsibility from one resident to another; a process often referred to as a handoff. Such transitions have long been a part of medical practice but have recently received increased attention because of restrictions in the duty hours of house staff. In July 2003 the Accreditation Council for Graduate Medical Education (ACGME) mandated reduced duty hours for all trainees in hopes of improving resident education and well‐being and patient safety.1 In fact, some studies have shown improved resident well‐being2 and fewer medical errors with reductions in duty hours,3, 4 but the growing consensus about the negative consequences of resident fatigue on patient safety has been accompanied by parallel concerns about the potential for information loss with each break in the continuity of care.5, 6
Although the tradeoff of increased discontinuity of care for fewer hours worked is sometimes characterized as an unintended consequence of duty hour regulations, it is in fact predictable and essential. As individuals work fewer hours, discontinuity must necessarily increase (assuming 24‐hour coverage).7 The extent to which this occurs may vary, but the link is consistent. At the University of California, San Francisco (UCSF), for example, we found that compliance with new duty hour requirements for internal medicine resulted in an average of 15 handoffs per patient during a 5‐day hospitalization. Each individual intern was involved in more than 300 handoffs in an average month‐long rotation, an increase of 40% since system changes were introduced to decrease duty hours. We found similar increases at Brigham and Women's Hospital (BWH) and the University of Chicago. Because U.S. teaching hospitals care for more than 6 million patients each year,8 the impact of these handoffs on the quality and efficiency of care is tremendous.
Discontinuity of care is currently managed by sign‐out, or the transfer of patient information from one physician to another. Recognizing the importance of information transfer at these vulnerable transition times for patients, the Joint Commission on Accreditation of Hospital Organizations (JCAHO) issued the 2006 National Patient Safety Goal 2E: Implement a standardized approach to hand off communications, including an opportunity to ask and respond to questions.9 Hospitals have little data to draw on to determine how to comply with this mandate and even less data to guide them in how to achieve its intended goals of improving communication and thus patient safety.
In an effort to better understand sign‐outs and ways to improve this process for house staff on in‐patient services, we reviewed data from the fields of aviation, communications, systems engineering, and human factors research, and we also searched the medical literature using key words pass‐off, handoff, sign‐out, duty hours, work hours, and discontinuity of care and MeSH headings Continuity of Patient Care Internship and Residency/*organization & administration, Personnel Staffing and Scheduling/*organization & administration, and Quality of Health Care. We also searched the websites of the Agency of Healthcare Quality and Research and the National Patient Safety Foundation. On the basis of these reviews, our experiences as hospitalist medical educators organizing resident sign‐out efforts at the University of California, San Francisco, the University of Chicago, and Brigham and Women's Hospital, and our efforts leading national training sessions on sign‐outs at the Society of General Internal Medicine (2004 and 2005), the Society of Hospital Medicine (2004), and the Association of Program Directors in Internal Medicine (2005, 2006), we propose a set of best practices regarding the content and process of sign‐out in an effort to improve communication between residents caring for hospitalized patients, assist programs in building safe and effective sign‐out systems, and improve the quality of patient care.
Effects of Discontinuity on Patient Safety
Research on the effects of discontinuity of care, although limited, suggests it has a negative impact on patient safety. In a study that investigated the institution of code 405 (the regulation that reduced duty hours in New York State), researchers found that the presumed increase in discontinuity with decreased duty hours resulted in delayed test ordering and an increased number of hospital complications.10 Another study found that the number of potentially preventable adverse events doubled when patients were under the care of a physician from a nonprimary team (eg, the cross‐covering intern).11 Studies have also linked resident discontinuity with longer length of stay, increased laboratory testing, and increased medication errors.12, 13
Managing Discontinuity: Sign‐Out as the Means of Information Transfer
In theory, more effective sign‐out systems should mitigate the potential for patient harm, but there is little in the literature describing current effective sign‐out practices or the best ways to design and implement such systems in the health care field. Examining information transfer mechanisms used in fields outside health care can assist in developing these systems.
Information Transfer in Other Industries
Although there is a paucity of data on sign‐out in the medical literature, information transfer has been the subject of substantial research in other industries in which safety depends on effective communication.
Aviation, for example, created systems and processes to improve handoff communication in response to accidents linked to failures in information transfer. One example, the 1977 collision of 2 747s on an airport runway in Tenerife, the Canary Islands, occurred after a garbled transmission from an air traffic controller to the cockpit of one of the aircraft. It was determined that a culture of adherence to a steep hierarchy prevented subordinates from questioning the captain's mistaken certainty that a runway was clear,14 an erroneous belief that was the basis for his decision to continue the aircraft on its course, resulting in its collision with the other airplane.
Subsequently, commercial aviation designed systems that standardized and formalized the process of information transfer and improved teamwork and coordination. These interventions were developed on the basis of detailed observations of cockpit interactions, reviews of communication errors, and focus groups.15 Because of these efforts, today's pilots use standardized checklists to transfer information content, communicate at designated times in specific undistracted environments, and use standard language and read‐backs to enhance understanding.16 The result has been a remarkable decrease in the risk of aviation crashes, one that most experts attribute in large part to these efforts to improve communication.17
Observation of how communication occurs in other high‐risk industries has informed the arena of effective information transfer. For example, direct observation of information transfer at NASA, in nuclear power plants, and in the railway industry identified specific strategies for effective handoffs/sign‐outs such as standardizing the information transferred, ensuring information is up to date, limiting interruptions, and having a structured face‐to‐face verbal interchange.18
Other strategies noted to be effective in diminishing errors are the use of a standardized phonetic alphabet to ensure that information is correctly heard and understood4 and having interactive verbal communication occur at a whiteboard.19
Information Transfer in Health Care
Those in the discipline of nursing have vast experience in the transfer of patient care information. The sign‐out process employed by nurses includes face‐to‐face discussions, typed information, and, most commonly, taped verbal communication.20 Interestingly, this process has not been subject to detailed scrutiny, and there is little information in the literature about best practices in sign‐out. Most articles in the literature on nursing handoffs are ethnographic descriptions of patient care responsibilities,21 on the basis of which, the authors advocate standardization of the information to be transferred, formalization of the channel used to communicate, and attention to increasing a culture of professionalism during sign‐out in order to improve efficiency.20, 22
There is little in the literature on transfer of care among physicians. Improvements in sign‐out have been suggested as part of broad strategies, such as increased training and information technology support,4, 7, 23, 24 and specific strategies have been offered such as managing barriers to communication, including specific types of data when transferring care,25 and involving nurses and senior physicians in sign‐outs.26 Specific outcomes data in this area have focused primarily on the use of computerized systems to improve information transfer. For example, the use of a computerized sign‐out system at Brigham and Women's Hospital (BWH), linked to the hospital's information system to ensure up‐to‐date information on patient demographics, medications, and laboratory values, has resulted in fewer errors,27 as have other similar systems.28 At the University of Washington, use of a similarly linked computerized sign‐out system resulted in fewer patients being missed on rounds and improvement in the quality of sign‐out and continuity of care according to resident self‐reports.29 Unfortunately, fewer than 10% of hospitals have such integrated hospitalwide information systems to support the sign‐out function.30
It has been noted that verbal communication, in concert with advances in technological communication, is important in information transfer in health care,18, 31 especially in emergent or urgent conditions.32 For example, eliminating the phoned‐in report from the lab to the ER and replacing it with delivery by an electronic reporting system lacking verbal communicationresulted in 45% of emergent lab results going unchecked.32 Structured verbal communication tools have been efficacious in improving information transfer outside the formal sign‐outfor example, read‐backs, which reduced errors in the reporting of critical laboratory values,33 and the SBAR (situation, background, assessment, recommendation) tool (designed to frame the transfer of critical information), which improved physician and nurse patient care information transfer in the in‐patient setting of the Kaiser Permanente health system.34
In focus groups and in response to formal and informal surveys, residents at our 3 sites suggested inclusion of the following information, provided in writing and orally, to improve sign‐outs: up‐to‐date administrative information (eg, room number, primary care physician); patient's recent cognitive or cardiopulmonary status; problems the patient had already experienced and treatments previously tried, both successfully and unsuccessfully; patient's code status and discussions on level of care; test results or consultation recommendations that were likely to come back while covering the patient and what to do with the results; and relevant psychosocial information (eg, complex family dynamics).35
The Current Practice of Sign‐Out
In examining sign‐outs at our 3 institutions, we found them to be unstructured and unstandardized. From discussion with faculty participating in national workshops on sign‐out, we found that most sign‐outs are conducted by interns, usually with little or no formal training. Templates, checklists, or other methods to standardize the content of the information transferred were rarely used.
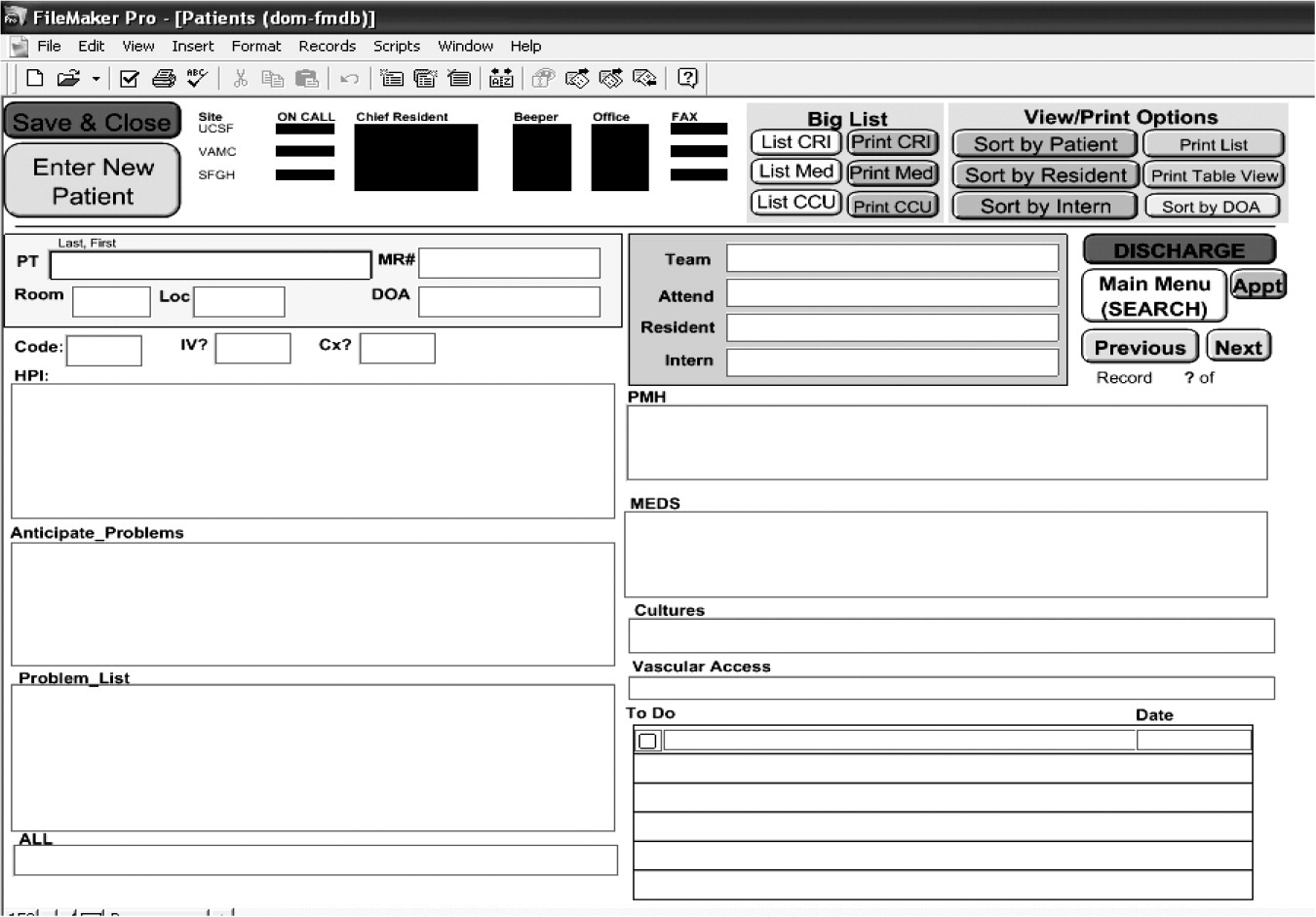
We also noted that the vehicle for written sign‐out is highly variable. At UCSF, different residency training programs used a variety of modalities for written sign‐outs, including index cards, Excel spreadsheets, Word documents, and loose sheets of paper. Recently, the UCSF Department of Medicine designed a simple database (on Filemaker Pro) that allows members of the house staff to update their sign‐out information, share it with other house staff and nurses, and access it at locations throughout the hospital (Fig. 1). Although this database is not yet linked to the hospital information system (planned for 2006), anecdotally resident satisfaction with sign‐out has vastly improved since its implementation. The cost of design and implementation was approximately $10,000. At the University of Chicago, interns used Microsoft Word to create sign‐out sheets containing patient summaries to transfer information. However, during structured interviews, 95% of the interns reported that these sheets were frequently lost or misplaced.7 Although medicine residents at BWH use a computerized system to produce sign‐out sheets, this system did not guarantee complete and structured information. For example, a survey at BWH found that 56% of cross‐covering residents said that when paged about a patient overnight, the relevant information needed to care for that patient was present less than half the time; and 27% of residents reported being paged more than 3 times in the previous 2 weeks about a test result or consultant recommendation that they did not know was pending.36
The process of sign‐out also varied across disciplines and institutions. From our experiences at our sites and at the sites of faculty nationally, we found limited standardization about whether sign‐out was verbal, the data transmitted, and the setting in which it was transmitted. In fact, at UCSF most residents signed out verbally on the fly, wherever and whenever they could find the cross‐coverage intern. At BWH, only 37% of residents said that sign‐out occurred in a quiet place most of the time, and only 52% signed out on every patient both orally and in writing.36 At the University of Chicago, the sign‐out process was characterized by outright failures in communication because of omission of needed information (ie, medications, active or anticipated medical problems, etc.) or by failure‐prone communication (ie, lack of face‐to‐face communication, illegible writing). These failures often led to uncertainty in making patient care decisions, potentially resulting in inefficient or suboptimal care.35
Strategies for Safe and Effective Sign‐Out
Given the current landscape of variability in sign‐outs, the recognition that information lost during sign‐out may result in harm to patients, and evidence of improvements in information transfer in areas outside health care, we aimed to develop mechanisms to improve the sign‐out process for residents working in a hospital setting. These strategies are based on our review of the existing literature supplemented by our experiences at our 3 institutions.
Content of Sign‐Out
The elements of content necessary for safe and effective sign‐out can be divided into 5 broad categories (Table 1), contained in the mnemonic ANTICipate: Administrative information, New clinical information, specific Tasks to be performed, assessment of severity of Illness, and Contingency plans or anticipated problems (Table 1, Fig. 2).
| ✓ Administrative data |
| □ Patient name, age, sex |
| □ Medical record number |
| □ Room number |
| □ Admission date |
| □ Primary inpatient medical team, primary care physician |
| □ Family contact information |
| ✓ New information (clinical update) |
| □ Chief complaint, brief HPI, and diagnosis (or differential diagnosis) |
| □ Updated list of medications with doses, updated allergies |
| □ Updated, brief assessment by system/problem, with dates |
| □ Current baseline status (eg, mental status, cardiopulmonary, vital signs, especially if abnormal but stable) |
| □ Recent procedures and significant events |
| ✓ Tasks (what needs to be done) |
| □ Specific, using if‐then statements |
| □ Prepare cross‐coverage (eg, patient consent for blood transfusion) |
| □ Alert to incoming information (eg, study results, consultant recommendations), and what action, if any, needs to be taken during the cross‐coverage |
| ✓ Illness |
| □ Is the patient sick? |
| ✓ Contingency planning/Code status |
| □ What may go wrong and what to do about it |
| □ What has or has not worked before (eg, responds to 40 mg IV furosemide) |
| □ Difficult family or psychosocial situations |
| □ Code status, especially recent changes or family discussions |

Several general points about this list should be noted. First, the sign‐out content is not meant to replace the chart. The information included reflects the goal of a sign‐out, namely, to provide enough information to allow for a safe transition in patient care. Information we believe is not essential to the sign‐out includes: a complete history and physical exam from the day of admission, a list of tasks already completed, and data necessary only to complete a discharge summary.
Sign‐out must be also be a closed loopthe process of signing in is as important as the process of signing out. This usually entails members of the primary team obtaining information from the cross‐covering physician when they resume care of the patient. The information conveyed in this case is different and includes details on events during cross‐coverage such as: 1) time called to assess patient; 2) reason for call; 3) a brief assessment of the patient, including vital signs; 4) actions taken, for example, medications given and tests ordered; and 5) rationale for those actions. Some of this information may also be included in the chart as an event note (see Fig. 3).
The Vehicle for Sign‐Out
We recommend a computer‐assisted vehicle for patient information transfer. Ideally, this would be linked to the hospital information system to ensure accurate and up‐to‐date information Easy access to the computerized sign‐out is essential (eg, using a hospitalwide computer system, shared hard drive service, intranet, or PDA linked to the computer system), and it should be customizable for the varied needs of different services and departments. The system should have templates to standardize the content of sign‐out, contain robust backup systems, and be HIPAA compliant (ie, restrict access to required health care personnel). However, the perfect should not be the enemy of the good: systems that do not meet these criteria may still help to protect patients by providing legible, predictable, and accessible information.
Sign‐Out Processes
Verbal communication.
Although electronic solutions can facilitate the standardization of written content, face‐to‐face verbal communication adds additional value.19 We recommend that each patient be reviewed separately. Identification of each patient verbally ensures that those engaged in the sign‐out are discussing the same patient. Reiterating the major medical problems gives a snapshot of the patient and frames the sign‐out. The to‐do list, the list of tasks that the cross‐cover resident needs to complete during cross coverage, should be specific and articulated as if, then statements (eg, if the urine output is less than 1 L, then give 40 mg of IV furosemide). The receiver of sign‐out should read back to the person giving the sign‐out each item on the to‐do list (eg, So, I should check the ins and outs at about 10:00 pm, and give 40 of furosemide if the patient is not 1 L negative, right?).
Anticipated problems should also be verbally communicated to promote a dialogue. Points that cannot be adequately transferred in the written sign‐out are particularly important to transmit verbally. Examples include previous code discussions, unusual responses to treatment, and psychosocial and family issues. When delivering verbal sign‐out, it is important to consider the a priori knowledge of the recipient. How much knowledge about a patient is already shared between the outgoing and incoming physicians and the level of experience of the physicians may affect the extent to which information needs be transmitted.37 For instance, 2 experienced physicians who already have been working to cover the same patient will likely have an abbreviated discussion, in contrast to the lengthier sign‐out necessary if the outgoing and incoming physicians are interns, and the incoming intern has no prior knowledge of the patient. Similarly, it is likely the level of detail transmitted will need to be greater during a permanent transfer of patient care (ie, at the end of a resident's rotation) than during a brief, temporary transition (eg, overnight coverage).
The challenges of a busy inpatient service may preclude a complete verbal sign‐out for all patients; we contend, though, it is best to use these practices to the extent possible, especially for patients with treatment plans in flux, those whose status is tenuous, and those who have anticipated changes in status during cross‐coverage. One tool that may be effectively used in signing out such patients is the SBAR tool, according to which a brief description of the situation is given, followed by the background and the physician's specific assessment and complete recommendation.38 For example, a resident signing out might begin by stating, I have 18 patients to sign‐out to you. I'm going to describe 6 active patients in detail. Twelve others are fairly stable, and I will give you basic information about them, and the details are in the written sign‐out. One patient has a plan in flux. The situation is Mr. S. is having trouble breathing, the background is that he has both CHF and COPD, my assessment is that this is more cardiac than pulmonary, and I recommend that you see him first and discuss with the cardiology consultant. Using the tools described here (Table 2), a sign‐out of 15 patients of variable acuity could be verbally signed out in less than 10 minutes.
| ✓ WHO should participate in the sign‐out process? |
| □ Outgoing clinician primarily responsible for patient's care |
| □ Oncoming clinician who will be primarily responsible for patient's care (avoid passing this task to someone else, even if busy) |
| □ Consider supervision by experienced clinicians if early in training |
| ✓ WHAT content needs to be verbally communicated? |
| Use situation briefing model, or SBAR, technique: |
| □ SituationIdentify each patient (name, age, sex, chief complaint) and briefly state any major problems (active and those that may become active during cross‐coverage). |
| □ Backgroundpertinent information relevant to current care (eg, recent vitals and/or baseline exam, labs, test results, etc). |
| □ Assessmentworking diagnosis, response to treatment, anticipated problems during cross‐coverage including anything not adequately described using written form (eg, complex family discussions). |
| □ Recommendationto‐do lists and if/then recommendations. |
| ✓ WHERE should sign‐out occur? |
| □ Designated room or place for sign‐out (eg, avoid patient areas because of HIPPA requirements) |
| □ Proper lighting |
| □ Avoid excessive noise (eg, high‐traffic areas) |
| □ Minimize disruptions (eg, hand over pagers) |
| □ Ensure systems support for sign‐out (eg, computers, printer, paper, etc.) |
| ✓ WHEN is the optimal time for sign‐out? |
| □ Designated time when both parties can be present and pay attention (eg, beware of clinic, other obligations) |
| □ Have enough time for interactive questions at the end (eg, avoid rush at the end of the shift) |
| ✓ HOW should verbal communication be performed? |
| □ Face to face, allowing for questions |
| □ Verbalize data in the same order for each patient at each sign‐out |
| □ Read back all to‐do items |
| □ Adjust length and depth of review according to baseline knowledge of parties involved and type of transition in care |
The Environment and setting.
To improve the setting of sign‐out, we recommend: a designated space that is well lit, quiet, and respects patient confidentiality and a designated time when sign‐out will occur. To limit known distractions and interruptions39, 40 in the hospital, we also recommend the outgoing physician hand off his or her pager to someone else during sign‐out. Also key to an environment conducive to information transfer is ensuring adequate computer support for electronic sign‐out and access to updated clinical information.
Organizational culture and institutional leadership.
The way residents transfer patient care information reflects the culture of the institution. Changing the culture to one in which interactive questioning is valued regardless of position in the hierarchy has been shown to reduce errors in aviation.41 Educating residents on the impact of sign‐outs on patient care is a first step toward improving the culture of sign‐out. Resident commitment to the new sign‐out can be gained by engaging residents in development of the process itself. To cement these changes into the culture, practitioners at all levels should be aware of and support the new system. The role of an institution's leaders in achieving these changes cannot be overlooked. Leaders will need to be creative in order to support sign‐out as described within the obvious constraints of money, time, personnel, and space. Gaining institutional buy‐in can start with heightening the awareness of leaders of the issues surrounding sign‐out, including patient safety, resident efficiency, and the financial impact of discontinuity. Ongoing evaluation of efforts to improve sign‐out is also crucial and can be accomplished with surveys, focus groups, and direct observation. Feeding back the positive impact of the changes to all involved stakeholders will promote confidence in the new systems and pride in their efforts.
CONCLUSIONS
Sign‐outs are a part of the current landscape of academic medical centers as well as hospitals at large. Interns, residents, and consulting fellows, not to mention nurses, physical therapists, and nutritionists, transfer patient care information at each transition point. There are few resources that can assist these caregivers in identifying and implementing the most effective ways to transfer patient care information. Hospitals and other care facilities are now mandated to develop standards and systems to improve sign‐out. On the basis of the limited literature to date and our own experiences, we have proposed standards and best practices to assist hospitals, training programs, and institutional leaders in designing safe and usable sign‐out systems. Effective implementation of the standards must include appropriate allocation of resources, individualization to meet specific needs of each program or institution, intensive training, and ongoing evaluation. Future research should focus on developing valid surrogate measures of continuity of care, conducting rigorous trials to determine the elements of sign‐out that lead to the best patient outcomes, and studying the most effective ways of implementing these improvements. By improving the content and process of sign‐out, we can meet the challenges of the new health care landscape while putting patient safety at the forefront.
- ,,.New requirements for resident duty hours.JAMA.2002;288:1112–1114.
- ,,,,.Effects of limited work hours on surgical training.J Am Coll Surg.2002;195:531–538.
- ,,, et al.Effect of reducing interns' weekly work hours on sleep and attentional failures.N Engl J Med.2004;351:1829–1837.
- ,,, et al.Effect of reducing interns' work hours on serious medical errors in intensive care units.N Engl J Med.2004;351:1838–1848.
- .A precarious exchange.N Engl J Med.2004;351:1822–1824.
- .Awake and informed.N Engl J Med.2004;351:1884.
- . Fumbled handoff: missed communication between teams. Cases and Commentary: Hospital Medicine, Morbidity 269:374–378.
- ,,,,.Does housestaff discontinuity of care increase the risk for preventable adverse events?Ann Intern Med.1994;121:866–872.
- ,,,.Post‐call transfer of resident responsibility: its effect on patient care.J Gen Intern Med.1990;5:501–505.
- ,,,.Effect of a change in house staff work schedule on resource utilization and patient care.Arch Intern Med.1991;151:2065–2070.
- ,.Internal Bleeding: the Truth behind America's Terrifying Epidemic of Medical Mistakes.New York City:Rugged Land, LLC;2004:448.
- ,,.Crew resource management and its applications in medicine. In:Making Health Care Safer: A Critical Analysis of Patient Safety Practices. Evidence Report/Technology Assessment Number 43, AHRQ Publication 01‐E058.Rockville, MD:Agency for Healthcare Research and Quality;2001.
- ,,.System safety and threat and error management: the line operations safety audit (LOSA). In:Jensen RS, ed. Proceedings of the Eleventh International Symposium on Aviation Psychology.Columbus, OH:Ohio State University;2001:1–6.
- ,,.Translating teamwork behaviours from aviation to healthcare: development of behavioural markers for neonatal resuscitation.Qual Saf Health Care.2004;13(Suppl 1):i57–i64.
- ,,,.Handoff strategies in settings with high consequences for failure: lessons for healthcare operations.Intl J Qual Health Care.2004;16:125–132.
- . Available at: http://www.agilemodeling.com/essays/communication.htm. Accessed December 15,2005.
- .Ensuring continuing care: styles and efficiency of the handover process.Aust J Adv Nurs.1998;16:23–27.
- ,.The handover: uncovering the hidden practices of nurses.Intensive Crit Care Nurs.2000;16:373–383.
- .The patient handover: a study of its form, function and efficiency.Nurs Stand.1995;9(52):33–36.
- ,.Residents' suggestions for reducing errors in teaching hospitals.N Engl J Med.2003;348:851–855.
- ,.Is 80 the cost of saving lives? Reduced duty hours, errors, and cost.J Gen Intern Med.2005;20:969–970.
- ,,,.Lost in translation: challenges and opportunities in physician‐to‐physician communication during patient handoffs.Acad Med.2005;80:1094–1099.
- British Medical Association.Safe Handover: Safe Patients: Guidance on Clinical Handover for Clinicians and Managers.London:British Medical Association, Junior Doctors Committee;2004.
- ,,,,.Using a computerized sign‐out program to improve continuity of inpatient care and prevent adverse events.Jt Comm J Qual Improv.1998;24(2):77–87.
- ,,,.Organizing the transfer of patient care information: the development of a computerized resident sign‐out system.Surgery.2004;136:5–13.
- ,,,,.A randomized, controlled trial evaluating the impact of a computerized rounding and sign‐out system on continuity of care and resident work hours.J Am Coll Surg.2005;200:538–545.
- ,,,.Computerized physician order entry in U.S. hospitals: results of a 2002 survey.J Am Med Inform Assoc.2004;11:95–99.
- ,,,,,.The impact of verbal communication on physician prescribing patterns in hospitalized patients with diabetes.Diabetes Educ.2003;29:827–836.
- ,.Use of computer terminals on wards to access emergency test results: a retrospective audit.Br Med J.2001;322:1101–1103.
- ,,,,,.Improving patient safety by repeating (read‐back) telephone reports of critical information.Am J Clin Pathol.2004;121:801–803.
- ,.The human factor: the critical importance of effective teamwork and communication in providing safe care.Qual Saf Health Care.2004;13(Suppl 1):i85–i90.
- ,,,,.Communication failures in patient sign‐out and suggestions for improvement: a critical incident analysis.Qual Saf Health Care.2005;14:401–407.
- ,,.Intern curriculum: the impact of a focused training program on the process and content of signout out patients. Harvard Medical School Education Day, Boston, MA;2004.
- .When conversation is better than computation.J Am Med Inform Assoc.2000;7:277–286.
- SBAR technique for communication: a situational briefing model. Available at: http://www.ihi.org/IHI/Topics/PatientSafety/SafetyGeneral/Tools/SBARTechniqueforCommunicationASituationalBriefingModel.htm. Accessed December2005.
- ,,,,.Impact of reduced duty hours on residents' educational satisfaction at the University of California, San Francisco.Acad Med.2006;81:76–81.
- ,.Communication behaviours in a hospital setting: an observational study.Br Med J.1998;316:673–676.
- ,,.Communication failures: an insidious contributor to medical mishapsAcad Med.2004;79(2):186–194.
Modern‐day continuity of patient care in teaching hospitals, once remarkably high because of a cadre of sleep‐deprived residents, is now peppered with breaks, each accompanied by the transfer of patient care responsibility from one resident to another; a process often referred to as a handoff. Such transitions have long been a part of medical practice but have recently received increased attention because of restrictions in the duty hours of house staff. In July 2003 the Accreditation Council for Graduate Medical Education (ACGME) mandated reduced duty hours for all trainees in hopes of improving resident education and well‐being and patient safety.1 In fact, some studies have shown improved resident well‐being2 and fewer medical errors with reductions in duty hours,3, 4 but the growing consensus about the negative consequences of resident fatigue on patient safety has been accompanied by parallel concerns about the potential for information loss with each break in the continuity of care.5, 6
Although the tradeoff of increased discontinuity of care for fewer hours worked is sometimes characterized as an unintended consequence of duty hour regulations, it is in fact predictable and essential. As individuals work fewer hours, discontinuity must necessarily increase (assuming 24‐hour coverage).7 The extent to which this occurs may vary, but the link is consistent. At the University of California, San Francisco (UCSF), for example, we found that compliance with new duty hour requirements for internal medicine resulted in an average of 15 handoffs per patient during a 5‐day hospitalization. Each individual intern was involved in more than 300 handoffs in an average month‐long rotation, an increase of 40% since system changes were introduced to decrease duty hours. We found similar increases at Brigham and Women's Hospital (BWH) and the University of Chicago. Because U.S. teaching hospitals care for more than 6 million patients each year,8 the impact of these handoffs on the quality and efficiency of care is tremendous.
Discontinuity of care is currently managed by sign‐out, or the transfer of patient information from one physician to another. Recognizing the importance of information transfer at these vulnerable transition times for patients, the Joint Commission on Accreditation of Hospital Organizations (JCAHO) issued the 2006 National Patient Safety Goal 2E: Implement a standardized approach to hand off communications, including an opportunity to ask and respond to questions.9 Hospitals have little data to draw on to determine how to comply with this mandate and even less data to guide them in how to achieve its intended goals of improving communication and thus patient safety.
In an effort to better understand sign‐outs and ways to improve this process for house staff on in‐patient services, we reviewed data from the fields of aviation, communications, systems engineering, and human factors research, and we also searched the medical literature using key words pass‐off, handoff, sign‐out, duty hours, work hours, and discontinuity of care and MeSH headings Continuity of Patient Care Internship and Residency/*organization & administration, Personnel Staffing and Scheduling/*organization & administration, and Quality of Health Care. We also searched the websites of the Agency of Healthcare Quality and Research and the National Patient Safety Foundation. On the basis of these reviews, our experiences as hospitalist medical educators organizing resident sign‐out efforts at the University of California, San Francisco, the University of Chicago, and Brigham and Women's Hospital, and our efforts leading national training sessions on sign‐outs at the Society of General Internal Medicine (2004 and 2005), the Society of Hospital Medicine (2004), and the Association of Program Directors in Internal Medicine (2005, 2006), we propose a set of best practices regarding the content and process of sign‐out in an effort to improve communication between residents caring for hospitalized patients, assist programs in building safe and effective sign‐out systems, and improve the quality of patient care.
Effects of Discontinuity on Patient Safety
Research on the effects of discontinuity of care, although limited, suggests it has a negative impact on patient safety. In a study that investigated the institution of code 405 (the regulation that reduced duty hours in New York State), researchers found that the presumed increase in discontinuity with decreased duty hours resulted in delayed test ordering and an increased number of hospital complications.10 Another study found that the number of potentially preventable adverse events doubled when patients were under the care of a physician from a nonprimary team (eg, the cross‐covering intern).11 Studies have also linked resident discontinuity with longer length of stay, increased laboratory testing, and increased medication errors.12, 13
Managing Discontinuity: Sign‐Out as the Means of Information Transfer
In theory, more effective sign‐out systems should mitigate the potential for patient harm, but there is little in the literature describing current effective sign‐out practices or the best ways to design and implement such systems in the health care field. Examining information transfer mechanisms used in fields outside health care can assist in developing these systems.
Information Transfer in Other Industries
Although there is a paucity of data on sign‐out in the medical literature, information transfer has been the subject of substantial research in other industries in which safety depends on effective communication.
Aviation, for example, created systems and processes to improve handoff communication in response to accidents linked to failures in information transfer. One example, the 1977 collision of 2 747s on an airport runway in Tenerife, the Canary Islands, occurred after a garbled transmission from an air traffic controller to the cockpit of one of the aircraft. It was determined that a culture of adherence to a steep hierarchy prevented subordinates from questioning the captain's mistaken certainty that a runway was clear,14 an erroneous belief that was the basis for his decision to continue the aircraft on its course, resulting in its collision with the other airplane.
Subsequently, commercial aviation designed systems that standardized and formalized the process of information transfer and improved teamwork and coordination. These interventions were developed on the basis of detailed observations of cockpit interactions, reviews of communication errors, and focus groups.15 Because of these efforts, today's pilots use standardized checklists to transfer information content, communicate at designated times in specific undistracted environments, and use standard language and read‐backs to enhance understanding.16 The result has been a remarkable decrease in the risk of aviation crashes, one that most experts attribute in large part to these efforts to improve communication.17
Observation of how communication occurs in other high‐risk industries has informed the arena of effective information transfer. For example, direct observation of information transfer at NASA, in nuclear power plants, and in the railway industry identified specific strategies for effective handoffs/sign‐outs such as standardizing the information transferred, ensuring information is up to date, limiting interruptions, and having a structured face‐to‐face verbal interchange.18
Other strategies noted to be effective in diminishing errors are the use of a standardized phonetic alphabet to ensure that information is correctly heard and understood4 and having interactive verbal communication occur at a whiteboard.19
Information Transfer in Health Care
Those in the discipline of nursing have vast experience in the transfer of patient care information. The sign‐out process employed by nurses includes face‐to‐face discussions, typed information, and, most commonly, taped verbal communication.20 Interestingly, this process has not been subject to detailed scrutiny, and there is little information in the literature about best practices in sign‐out. Most articles in the literature on nursing handoffs are ethnographic descriptions of patient care responsibilities,21 on the basis of which, the authors advocate standardization of the information to be transferred, formalization of the channel used to communicate, and attention to increasing a culture of professionalism during sign‐out in order to improve efficiency.20, 22
There is little in the literature on transfer of care among physicians. Improvements in sign‐out have been suggested as part of broad strategies, such as increased training and information technology support,4, 7, 23, 24 and specific strategies have been offered such as managing barriers to communication, including specific types of data when transferring care,25 and involving nurses and senior physicians in sign‐outs.26 Specific outcomes data in this area have focused primarily on the use of computerized systems to improve information transfer. For example, the use of a computerized sign‐out system at Brigham and Women's Hospital (BWH), linked to the hospital's information system to ensure up‐to‐date information on patient demographics, medications, and laboratory values, has resulted in fewer errors,27 as have other similar systems.28 At the University of Washington, use of a similarly linked computerized sign‐out system resulted in fewer patients being missed on rounds and improvement in the quality of sign‐out and continuity of care according to resident self‐reports.29 Unfortunately, fewer than 10% of hospitals have such integrated hospitalwide information systems to support the sign‐out function.30
It has been noted that verbal communication, in concert with advances in technological communication, is important in information transfer in health care,18, 31 especially in emergent or urgent conditions.32 For example, eliminating the phoned‐in report from the lab to the ER and replacing it with delivery by an electronic reporting system lacking verbal communicationresulted in 45% of emergent lab results going unchecked.32 Structured verbal communication tools have been efficacious in improving information transfer outside the formal sign‐outfor example, read‐backs, which reduced errors in the reporting of critical laboratory values,33 and the SBAR (situation, background, assessment, recommendation) tool (designed to frame the transfer of critical information), which improved physician and nurse patient care information transfer in the in‐patient setting of the Kaiser Permanente health system.34
In focus groups and in response to formal and informal surveys, residents at our 3 sites suggested inclusion of the following information, provided in writing and orally, to improve sign‐outs: up‐to‐date administrative information (eg, room number, primary care physician); patient's recent cognitive or cardiopulmonary status; problems the patient had already experienced and treatments previously tried, both successfully and unsuccessfully; patient's code status and discussions on level of care; test results or consultation recommendations that were likely to come back while covering the patient and what to do with the results; and relevant psychosocial information (eg, complex family dynamics).35
The Current Practice of Sign‐Out
In examining sign‐outs at our 3 institutions, we found them to be unstructured and unstandardized. From discussion with faculty participating in national workshops on sign‐out, we found that most sign‐outs are conducted by interns, usually with little or no formal training. Templates, checklists, or other methods to standardize the content of the information transferred were rarely used.
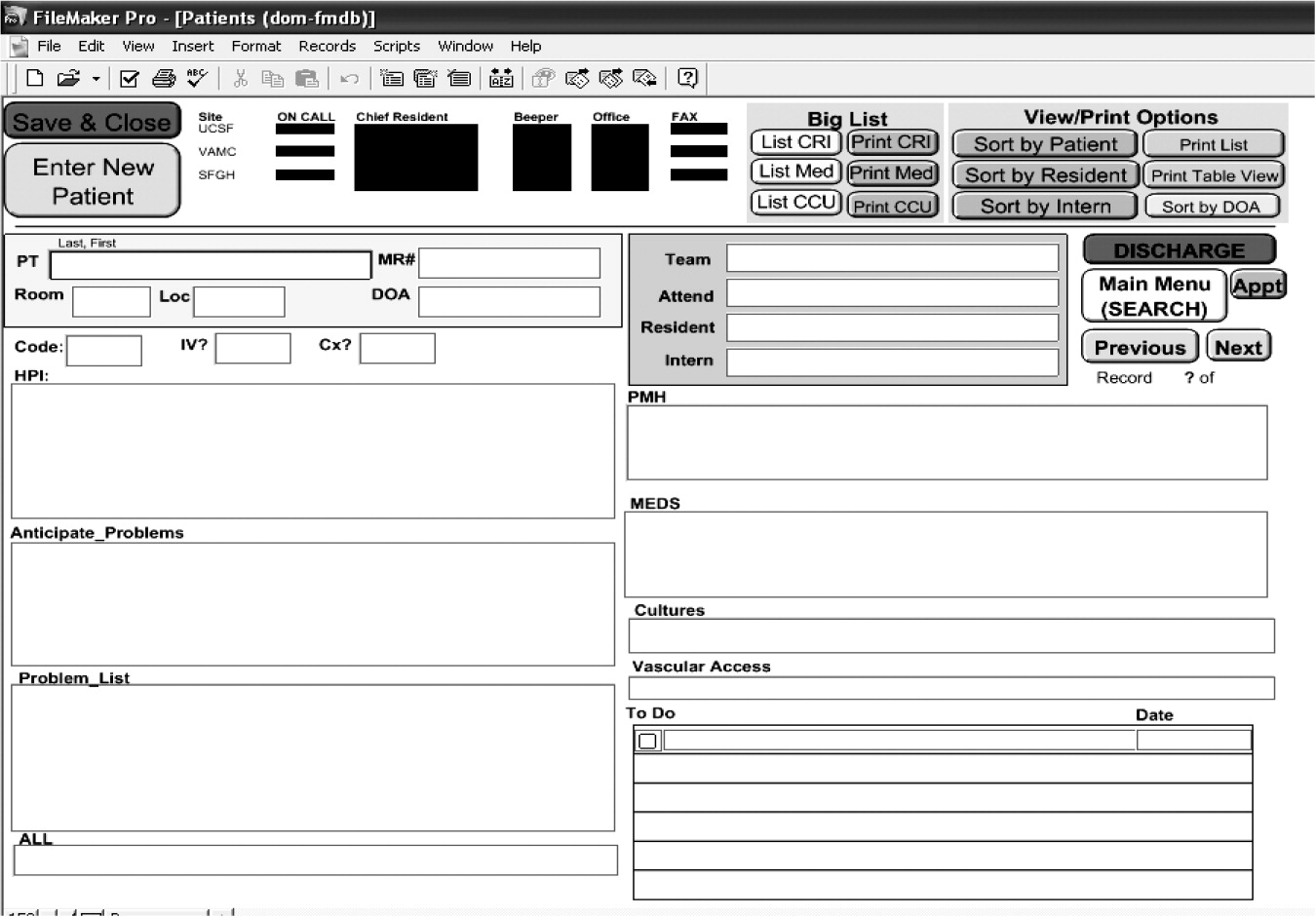
We also noted that the vehicle for written sign‐out is highly variable. At UCSF, different residency training programs used a variety of modalities for written sign‐outs, including index cards, Excel spreadsheets, Word documents, and loose sheets of paper. Recently, the UCSF Department of Medicine designed a simple database (on Filemaker Pro) that allows members of the house staff to update their sign‐out information, share it with other house staff and nurses, and access it at locations throughout the hospital (Fig. 1). Although this database is not yet linked to the hospital information system (planned for 2006), anecdotally resident satisfaction with sign‐out has vastly improved since its implementation. The cost of design and implementation was approximately $10,000. At the University of Chicago, interns used Microsoft Word to create sign‐out sheets containing patient summaries to transfer information. However, during structured interviews, 95% of the interns reported that these sheets were frequently lost or misplaced.7 Although medicine residents at BWH use a computerized system to produce sign‐out sheets, this system did not guarantee complete and structured information. For example, a survey at BWH found that 56% of cross‐covering residents said that when paged about a patient overnight, the relevant information needed to care for that patient was present less than half the time; and 27% of residents reported being paged more than 3 times in the previous 2 weeks about a test result or consultant recommendation that they did not know was pending.36

The process of sign‐out also varied across disciplines and institutions. From our experiences at our sites and at the sites of faculty nationally, we found limited standardization about whether sign‐out was verbal, the data transmitted, and the setting in which it was transmitted. In fact, at UCSF most residents signed out verbally on the fly, wherever and whenever they could find the cross‐coverage intern. At BWH, only 37% of residents said that sign‐out occurred in a quiet place most of the time, and only 52% signed out on every patient both orally and in writing.36 At the University of Chicago, the sign‐out process was characterized by outright failures in communication because of omission of needed information (ie, medications, active or anticipated medical problems, etc.) or by failure‐prone communication (ie, lack of face‐to‐face communication, illegible writing). These failures often led to uncertainty in making patient care decisions, potentially resulting in inefficient or suboptimal care.35
Strategies for Safe and Effective Sign‐Out
Given the current landscape of variability in sign‐outs, the recognition that information lost during sign‐out may result in harm to patients, and evidence of improvements in information transfer in areas outside health care, we aimed to develop mechanisms to improve the sign‐out process for residents working in a hospital setting. These strategies are based on our review of the existing literature supplemented by our experiences at our 3 institutions.
Content of Sign‐Out
The elements of content necessary for safe and effective sign‐out can be divided into 5 broad categories (Table 1), contained in the mnemonic ANTICipate: Administrative information, New clinical information, specific Tasks to be performed, assessment of severity of Illness, and Contingency plans or anticipated problems (Table 1, Fig. 2).
| ✓ Administrative data |
| □ Patient name, age, sex |
| □ Medical record number |
| □ Room number |
| □ Admission date |
| □ Primary inpatient medical team, primary care physician |
| □ Family contact information |
| ✓ New information (clinical update) |
| □ Chief complaint, brief HPI, and diagnosis (or differential diagnosis) |
| □ Updated list of medications with doses, updated allergies |
| □ Updated, brief assessment by system/problem, with dates |
| □ Current baseline status (eg, mental status, cardiopulmonary, vital signs, especially if abnormal but stable) |
| □ Recent procedures and significant events |
| ✓ Tasks (what needs to be done) |
| □ Specific, using if‐then statements |
| □ Prepare cross‐coverage (eg, patient consent for blood transfusion) |
| □ Alert to incoming information (eg, study results, consultant recommendations), and what action, if any, needs to be taken during the cross‐coverage |
| ✓ Illness |
| □ Is the patient sick? |
| ✓ Contingency planning/Code status |
| □ What may go wrong and what to do about it |
| □ What has or has not worked before (eg, responds to 40 mg IV furosemide) |
| □ Difficult family or psychosocial situations |
| □ Code status, especially recent changes or family discussions |

Several general points about this list should be noted. First, the sign‐out content is not meant to replace the chart. The information included reflects the goal of a sign‐out, namely, to provide enough information to allow for a safe transition in patient care. Information we believe is not essential to the sign‐out includes: a complete history and physical exam from the day of admission, a list of tasks already completed, and data necessary only to complete a discharge summary.
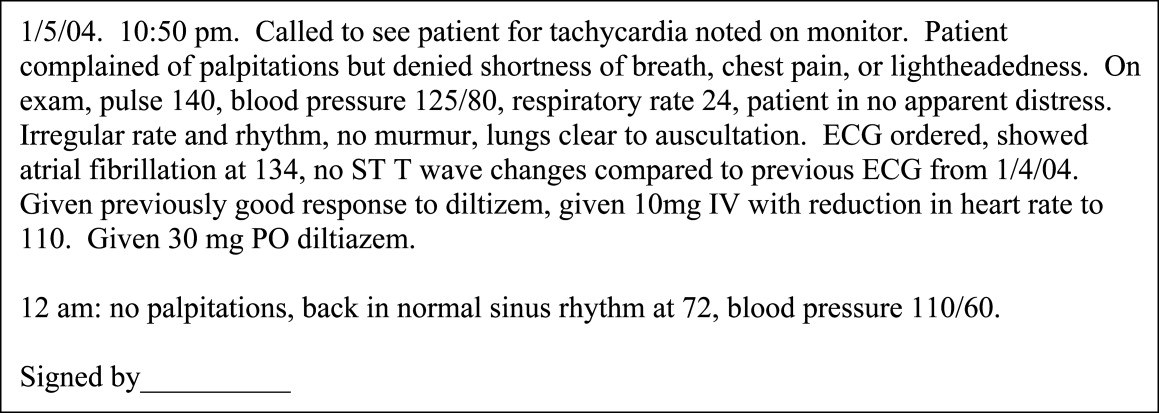
Sign‐out must be also be a closed loopthe process of signing in is as important as the process of signing out. This usually entails members of the primary team obtaining information from the cross‐covering physician when they resume care of the patient. The information conveyed in this case is different and includes details on events during cross‐coverage such as: 1) time called to assess patient; 2) reason for call; 3) a brief assessment of the patient, including vital signs; 4) actions taken, for example, medications given and tests ordered; and 5) rationale for those actions. Some of this information may also be included in the chart as an event note (see Fig. 3).

The Vehicle for Sign‐Out
We recommend a computer‐assisted vehicle for patient information transfer. Ideally, this would be linked to the hospital information system to ensure accurate and up‐to‐date information Easy access to the computerized sign‐out is essential (eg, using a hospitalwide computer system, shared hard drive service, intranet, or PDA linked to the computer system), and it should be customizable for the varied needs of different services and departments. The system should have templates to standardize the content of sign‐out, contain robust backup systems, and be HIPAA compliant (ie, restrict access to required health care personnel). However, the perfect should not be the enemy of the good: systems that do not meet these criteria may still help to protect patients by providing legible, predictable, and accessible information.
Sign‐Out Processes
Verbal communication.
Although electronic solutions can facilitate the standardization of written content, face‐to‐face verbal communication adds additional value.19 We recommend that each patient be reviewed separately. Identification of each patient verbally ensures that those engaged in the sign‐out are discussing the same patient. Reiterating the major medical problems gives a snapshot of the patient and frames the sign‐out. The to‐do list, the list of tasks that the cross‐cover resident needs to complete during cross coverage, should be specific and articulated as if, then statements (eg, if the urine output is less than 1 L, then give 40 mg of IV furosemide). The receiver of sign‐out should read back to the person giving the sign‐out each item on the to‐do list (eg, So, I should check the ins and outs at about 10:00 pm, and give 40 of furosemide if the patient is not 1 L negative, right?).
Anticipated problems should also be verbally communicated to promote a dialogue. Points that cannot be adequately transferred in the written sign‐out are particularly important to transmit verbally. Examples include previous code discussions, unusual responses to treatment, and psychosocial and family issues. When delivering verbal sign‐out, it is important to consider the a priori knowledge of the recipient. How much knowledge about a patient is already shared between the outgoing and incoming physicians and the level of experience of the physicians may affect the extent to which information needs be transmitted.37 For instance, 2 experienced physicians who already have been working to cover the same patient will likely have an abbreviated discussion, in contrast to the lengthier sign‐out necessary if the outgoing and incoming physicians are interns, and the incoming intern has no prior knowledge of the patient. Similarly, it is likely the level of detail transmitted will need to be greater during a permanent transfer of patient care (ie, at the end of a resident's rotation) than during a brief, temporary transition (eg, overnight coverage).
The challenges of a busy inpatient service may preclude a complete verbal sign‐out for all patients; we contend, though, it is best to use these practices to the extent possible, especially for patients with treatment plans in flux, those whose status is tenuous, and those who have anticipated changes in status during cross‐coverage. One tool that may be effectively used in signing out such patients is the SBAR tool, according to which a brief description of the situation is given, followed by the background and the physician's specific assessment and complete recommendation.38 For example, a resident signing out might begin by stating, I have 18 patients to sign‐out to you. I'm going to describe 6 active patients in detail. Twelve others are fairly stable, and I will give you basic information about them, and the details are in the written sign‐out. One patient has a plan in flux. The situation is Mr. S. is having trouble breathing, the background is that he has both CHF and COPD, my assessment is that this is more cardiac than pulmonary, and I recommend that you see him first and discuss with the cardiology consultant. Using the tools described here (Table 2), a sign‐out of 15 patients of variable acuity could be verbally signed out in less than 10 minutes.
| ✓ WHO should participate in the sign‐out process? |
| □ Outgoing clinician primarily responsible for patient's care |
| □ Oncoming clinician who will be primarily responsible for patient's care (avoid passing this task to someone else, even if busy) |
| □ Consider supervision by experienced clinicians if early in training |
| ✓ WHAT content needs to be verbally communicated? |
| Use situation briefing model, or SBAR, technique: |
| □ SituationIdentify each patient (name, age, sex, chief complaint) and briefly state any major problems (active and those that may become active during cross‐coverage). |
| □ Backgroundpertinent information relevant to current care (eg, recent vitals and/or baseline exam, labs, test results, etc). |
| □ Assessmentworking diagnosis, response to treatment, anticipated problems during cross‐coverage including anything not adequately described using written form (eg, complex family discussions). |
| □ Recommendationto‐do lists and if/then recommendations. |
| ✓ WHERE should sign‐out occur? |
| □ Designated room or place for sign‐out (eg, avoid patient areas because of HIPPA requirements) |
| □ Proper lighting |
| □ Avoid excessive noise (eg, high‐traffic areas) |
| □ Minimize disruptions (eg, hand over pagers) |
| □ Ensure systems support for sign‐out (eg, computers, printer, paper, etc.) |
| ✓ WHEN is the optimal time for sign‐out? |
| □ Designated time when both parties can be present and pay attention (eg, beware of clinic, other obligations) |
| □ Have enough time for interactive questions at the end (eg, avoid rush at the end of the shift) |
| ✓ HOW should verbal communication be performed? |
| □ Face to face, allowing for questions |
| □ Verbalize data in the same order for each patient at each sign‐out |
| □ Read back all to‐do items |
| □ Adjust length and depth of review according to baseline knowledge of parties involved and type of transition in care |
The Environment and setting.
To improve the setting of sign‐out, we recommend: a designated space that is well lit, quiet, and respects patient confidentiality and a designated time when sign‐out will occur. To limit known distractions and interruptions39, 40 in the hospital, we also recommend the outgoing physician hand off his or her pager to someone else during sign‐out. Also key to an environment conducive to information transfer is ensuring adequate computer support for electronic sign‐out and access to updated clinical information.
Organizational culture and institutional leadership.
The way residents transfer patient care information reflects the culture of the institution. Changing the culture to one in which interactive questioning is valued regardless of position in the hierarchy has been shown to reduce errors in aviation.41 Educating residents on the impact of sign‐outs on patient care is a first step toward improving the culture of sign‐out. Resident commitment to the new sign‐out can be gained by engaging residents in development of the process itself. To cement these changes into the culture, practitioners at all levels should be aware of and support the new system. The role of an institution's leaders in achieving these changes cannot be overlooked. Leaders will need to be creative in order to support sign‐out as described within the obvious constraints of money, time, personnel, and space. Gaining institutional buy‐in can start with heightening the awareness of leaders of the issues surrounding sign‐out, including patient safety, resident efficiency, and the financial impact of discontinuity. Ongoing evaluation of efforts to improve sign‐out is also crucial and can be accomplished with surveys, focus groups, and direct observation. Feeding back the positive impact of the changes to all involved stakeholders will promote confidence in the new systems and pride in their efforts.
CONCLUSIONS
Sign‐outs are a part of the current landscape of academic medical centers as well as hospitals at large. Interns, residents, and consulting fellows, not to mention nurses, physical therapists, and nutritionists, transfer patient care information at each transition point. There are few resources that can assist these caregivers in identifying and implementing the most effective ways to transfer patient care information. Hospitals and other care facilities are now mandated to develop standards and systems to improve sign‐out. On the basis of the limited literature to date and our own experiences, we have proposed standards and best practices to assist hospitals, training programs, and institutional leaders in designing safe and usable sign‐out systems. Effective implementation of the standards must include appropriate allocation of resources, individualization to meet specific needs of each program or institution, intensive training, and ongoing evaluation. Future research should focus on developing valid surrogate measures of continuity of care, conducting rigorous trials to determine the elements of sign‐out that lead to the best patient outcomes, and studying the most effective ways of implementing these improvements. By improving the content and process of sign‐out, we can meet the challenges of the new health care landscape while putting patient safety at the forefront.
Modern‐day continuity of patient care in teaching hospitals, once remarkably high because of a cadre of sleep‐deprived residents, is now peppered with breaks, each accompanied by the transfer of patient care responsibility from one resident to another; a process often referred to as a handoff. Such transitions have long been a part of medical practice but have recently received increased attention because of restrictions in the duty hours of house staff. In July 2003 the Accreditation Council for Graduate Medical Education (ACGME) mandated reduced duty hours for all trainees in hopes of improving resident education and well‐being and patient safety.1 In fact, some studies have shown improved resident well‐being2 and fewer medical errors with reductions in duty hours,3, 4 but the growing consensus about the negative consequences of resident fatigue on patient safety has been accompanied by parallel concerns about the potential for information loss with each break in the continuity of care.5, 6
Although the tradeoff of increased discontinuity of care for fewer hours worked is sometimes characterized as an unintended consequence of duty hour regulations, it is in fact predictable and essential. As individuals work fewer hours, discontinuity must necessarily increase (assuming 24‐hour coverage).7 The extent to which this occurs may vary, but the link is consistent. At the University of California, San Francisco (UCSF), for example, we found that compliance with new duty hour requirements for internal medicine resulted in an average of 15 handoffs per patient during a 5‐day hospitalization. Each individual intern was involved in more than 300 handoffs in an average month‐long rotation, an increase of 40% since system changes were introduced to decrease duty hours. We found similar increases at Brigham and Women's Hospital (BWH) and the University of Chicago. Because U.S. teaching hospitals care for more than 6 million patients each year,8 the impact of these handoffs on the quality and efficiency of care is tremendous.
Discontinuity of care is currently managed by sign‐out, or the transfer of patient information from one physician to another. Recognizing the importance of information transfer at these vulnerable transition times for patients, the Joint Commission on Accreditation of Hospital Organizations (JCAHO) issued the 2006 National Patient Safety Goal 2E: Implement a standardized approach to hand off communications, including an opportunity to ask and respond to questions.9 Hospitals have little data to draw on to determine how to comply with this mandate and even less data to guide them in how to achieve its intended goals of improving communication and thus patient safety.
In an effort to better understand sign‐outs and ways to improve this process for house staff on in‐patient services, we reviewed data from the fields of aviation, communications, systems engineering, and human factors research, and we also searched the medical literature using key words pass‐off, handoff, sign‐out, duty hours, work hours, and discontinuity of care and MeSH headings Continuity of Patient Care Internship and Residency/*organization & administration, Personnel Staffing and Scheduling/*organization & administration, and Quality of Health Care. We also searched the websites of the Agency of Healthcare Quality and Research and the National Patient Safety Foundation. On the basis of these reviews, our experiences as hospitalist medical educators organizing resident sign‐out efforts at the University of California, San Francisco, the University of Chicago, and Brigham and Women's Hospital, and our efforts leading national training sessions on sign‐outs at the Society of General Internal Medicine (2004 and 2005), the Society of Hospital Medicine (2004), and the Association of Program Directors in Internal Medicine (2005, 2006), we propose a set of best practices regarding the content and process of sign‐out in an effort to improve communication between residents caring for hospitalized patients, assist programs in building safe and effective sign‐out systems, and improve the quality of patient care.
Effects of Discontinuity on Patient Safety
Research on the effects of discontinuity of care, although limited, suggests it has a negative impact on patient safety. In a study that investigated the institution of code 405 (the regulation that reduced duty hours in New York State), researchers found that the presumed increase in discontinuity with decreased duty hours resulted in delayed test ordering and an increased number of hospital complications.10 Another study found that the number of potentially preventable adverse events doubled when patients were under the care of a physician from a nonprimary team (eg, the cross‐covering intern).11 Studies have also linked resident discontinuity with longer length of stay, increased laboratory testing, and increased medication errors.12, 13
Managing Discontinuity: Sign‐Out as the Means of Information Transfer
In theory, more effective sign‐out systems should mitigate the potential for patient harm, but there is little in the literature describing current effective sign‐out practices or the best ways to design and implement such systems in the health care field. Examining information transfer mechanisms used in fields outside health care can assist in developing these systems.
Information Transfer in Other Industries
Although there is a paucity of data on sign‐out in the medical literature, information transfer has been the subject of substantial research in other industries in which safety depends on effective communication.
Aviation, for example, created systems and processes to improve handoff communication in response to accidents linked to failures in information transfer. One example, the 1977 collision of 2 747s on an airport runway in Tenerife, the Canary Islands, occurred after a garbled transmission from an air traffic controller to the cockpit of one of the aircraft. It was determined that a culture of adherence to a steep hierarchy prevented subordinates from questioning the captain's mistaken certainty that a runway was clear,14 an erroneous belief that was the basis for his decision to continue the aircraft on its course, resulting in its collision with the other airplane.
Subsequently, commercial aviation designed systems that standardized and formalized the process of information transfer and improved teamwork and coordination. These interventions were developed on the basis of detailed observations of cockpit interactions, reviews of communication errors, and focus groups.15 Because of these efforts, today's pilots use standardized checklists to transfer information content, communicate at designated times in specific undistracted environments, and use standard language and read‐backs to enhance understanding.16 The result has been a remarkable decrease in the risk of aviation crashes, one that most experts attribute in large part to these efforts to improve communication.17
Observation of how communication occurs in other high‐risk industries has informed the arena of effective information transfer. For example, direct observation of information transfer at NASA, in nuclear power plants, and in the railway industry identified specific strategies for effective handoffs/sign‐outs such as standardizing the information transferred, ensuring information is up to date, limiting interruptions, and having a structured face‐to‐face verbal interchange.18
Other strategies noted to be effective in diminishing errors are the use of a standardized phonetic alphabet to ensure that information is correctly heard and understood4 and having interactive verbal communication occur at a whiteboard.19
Information Transfer in Health Care
Those in the discipline of nursing have vast experience in the transfer of patient care information. The sign‐out process employed by nurses includes face‐to‐face discussions, typed information, and, most commonly, taped verbal communication.20 Interestingly, this process has not been subject to detailed scrutiny, and there is little information in the literature about best practices in sign‐out. Most articles in the literature on nursing handoffs are ethnographic descriptions of patient care responsibilities,21 on the basis of which, the authors advocate standardization of the information to be transferred, formalization of the channel used to communicate, and attention to increasing a culture of professionalism during sign‐out in order to improve efficiency.20, 22
There is little in the literature on transfer of care among physicians. Improvements in sign‐out have been suggested as part of broad strategies, such as increased training and information technology support,4, 7, 23, 24 and specific strategies have been offered such as managing barriers to communication, including specific types of data when transferring care,25 and involving nurses and senior physicians in sign‐outs.26 Specific outcomes data in this area have focused primarily on the use of computerized systems to improve information transfer. For example, the use of a computerized sign‐out system at Brigham and Women's Hospital (BWH), linked to the hospital's information system to ensure up‐to‐date information on patient demographics, medications, and laboratory values, has resulted in fewer errors,27 as have other similar systems.28 At the University of Washington, use of a similarly linked computerized sign‐out system resulted in fewer patients being missed on rounds and improvement in the quality of sign‐out and continuity of care according to resident self‐reports.29 Unfortunately, fewer than 10% of hospitals have such integrated hospitalwide information systems to support the sign‐out function.30
It has been noted that verbal communication, in concert with advances in technological communication, is important in information transfer in health care,18, 31 especially in emergent or urgent conditions.32 For example, eliminating the phoned‐in report from the lab to the ER and replacing it with delivery by an electronic reporting system lacking verbal communicationresulted in 45% of emergent lab results going unchecked.32 Structured verbal communication tools have been efficacious in improving information transfer outside the formal sign‐outfor example, read‐backs, which reduced errors in the reporting of critical laboratory values,33 and the SBAR (situation, background, assessment, recommendation) tool (designed to frame the transfer of critical information), which improved physician and nurse patient care information transfer in the in‐patient setting of the Kaiser Permanente health system.34
In focus groups and in response to formal and informal surveys, residents at our 3 sites suggested inclusion of the following information, provided in writing and orally, to improve sign‐outs: up‐to‐date administrative information (eg, room number, primary care physician); patient's recent cognitive or cardiopulmonary status; problems the patient had already experienced and treatments previously tried, both successfully and unsuccessfully; patient's code status and discussions on level of care; test results or consultation recommendations that were likely to come back while covering the patient and what to do with the results; and relevant psychosocial information (eg, complex family dynamics).35
The Current Practice of Sign‐Out
In examining sign‐outs at our 3 institutions, we found them to be unstructured and unstandardized. From discussion with faculty participating in national workshops on sign‐out, we found that most sign‐outs are conducted by interns, usually with little or no formal training. Templates, checklists, or other methods to standardize the content of the information transferred were rarely used.
We also noted that the vehicle for written sign‐out is highly variable. At UCSF, different residency training programs used a variety of modalities for written sign‐outs, including index cards, Excel spreadsheets, Word documents, and loose sheets of paper. Recently, the UCSF Department of Medicine designed a simple database (on Filemaker Pro) that allows members of the house staff to update their sign‐out information, share it with other house staff and nurses, and access it at locations throughout the hospital (Fig. 1). Although this database is not yet linked to the hospital information system (planned for 2006), anecdotally resident satisfaction with sign‐out has vastly improved since its implementation. The cost of design and implementation was approximately $10,000. At the University of Chicago, interns used Microsoft Word to create sign‐out sheets containing patient summaries to transfer information. However, during structured interviews, 95% of the interns reported that these sheets were frequently lost or misplaced.7 Although medicine residents at BWH use a computerized system to produce sign‐out sheets, this system did not guarantee complete and structured information. For example, a survey at BWH found that 56% of cross‐covering residents said that when paged about a patient overnight, the relevant information needed to care for that patient was present less than half the time; and 27% of residents reported being paged more than 3 times in the previous 2 weeks about a test result or consultant recommendation that they did not know was pending.36

The process of sign‐out also varied across disciplines and institutions. From our experiences at our sites and at the sites of faculty nationally, we found limited standardization about whether sign‐out was verbal, the data transmitted, and the setting in which it was transmitted. In fact, at UCSF most residents signed out verbally on the fly, wherever and whenever they could find the cross‐coverage intern. At BWH, only 37% of residents said that sign‐out occurred in a quiet place most of the time, and only 52% signed out on every patient both orally and in writing.36 At the University of Chicago, the sign‐out process was characterized by outright failures in communication because of omission of needed information (ie, medications, active or anticipated medical problems, etc.) or by failure‐prone communication (ie, lack of face‐to‐face communication, illegible writing). These failures often led to uncertainty in making patient care decisions, potentially resulting in inefficient or suboptimal care.35
Strategies for Safe and Effective Sign‐Out
Given the current landscape of variability in sign‐outs, the recognition that information lost during sign‐out may result in harm to patients, and evidence of improvements in information transfer in areas outside health care, we aimed to develop mechanisms to improve the sign‐out process for residents working in a hospital setting. These strategies are based on our review of the existing literature supplemented by our experiences at our 3 institutions.
Content of Sign‐Out
The elements of content necessary for safe and effective sign‐out can be divided into 5 broad categories (Table 1), contained in the mnemonic ANTICipate: Administrative information, New clinical information, specific Tasks to be performed, assessment of severity of Illness, and Contingency plans or anticipated problems (Table 1, Fig. 2).
| ✓ Administrative data |
| □ Patient name, age, sex |
| □ Medical record number |
| □ Room number |
| □ Admission date |
| □ Primary inpatient medical team, primary care physician |
| □ Family contact information |
| ✓ New information (clinical update) |
| □ Chief complaint, brief HPI, and diagnosis (or differential diagnosis) |
| □ Updated list of medications with doses, updated allergies |
| □ Updated, brief assessment by system/problem, with dates |
| □ Current baseline status (eg, mental status, cardiopulmonary, vital signs, especially if abnormal but stable) |
| □ Recent procedures and significant events |
| ✓ Tasks (what needs to be done) |
| □ Specific, using if‐then statements |
| □ Prepare cross‐coverage (eg, patient consent for blood transfusion) |
| □ Alert to incoming information (eg, study results, consultant recommendations), and what action, if any, needs to be taken during the cross‐coverage |
| ✓ Illness |
| □ Is the patient sick? |
| ✓ Contingency planning/Code status |
| □ What may go wrong and what to do about it |
| □ What has or has not worked before (eg, responds to 40 mg IV furosemide) |
| □ Difficult family or psychosocial situations |
| □ Code status, especially recent changes or family discussions |

Several general points about this list should be noted. First, the sign‐out content is not meant to replace the chart. The information included reflects the goal of a sign‐out, namely, to provide enough information to allow for a safe transition in patient care. Information we believe is not essential to the sign‐out includes: a complete history and physical exam from the day of admission, a list of tasks already completed, and data necessary only to complete a discharge summary.
Sign‐out must be also be a closed loopthe process of signing in is as important as the process of signing out. This usually entails members of the primary team obtaining information from the cross‐covering physician when they resume care of the patient. The information conveyed in this case is different and includes details on events during cross‐coverage such as: 1) time called to assess patient; 2) reason for call; 3) a brief assessment of the patient, including vital signs; 4) actions taken, for example, medications given and tests ordered; and 5) rationale for those actions. Some of this information may also be included in the chart as an event note (see Fig. 3).

The Vehicle for Sign‐Out
We recommend a computer‐assisted vehicle for patient information transfer. Ideally, this would be linked to the hospital information system to ensure accurate and up‐to‐date information Easy access to the computerized sign‐out is essential (eg, using a hospitalwide computer system, shared hard drive service, intranet, or PDA linked to the computer system), and it should be customizable for the varied needs of different services and departments. The system should have templates to standardize the content of sign‐out, contain robust backup systems, and be HIPAA compliant (ie, restrict access to required health care personnel). However, the perfect should not be the enemy of the good: systems that do not meet these criteria may still help to protect patients by providing legible, predictable, and accessible information.
Sign‐Out Processes
Verbal communication.
Although electronic solutions can facilitate the standardization of written content, face‐to‐face verbal communication adds additional value.19 We recommend that each patient be reviewed separately. Identification of each patient verbally ensures that those engaged in the sign‐out are discussing the same patient. Reiterating the major medical problems gives a snapshot of the patient and frames the sign‐out. The to‐do list, the list of tasks that the cross‐cover resident needs to complete during cross coverage, should be specific and articulated as if, then statements (eg, if the urine output is less than 1 L, then give 40 mg of IV furosemide). The receiver of sign‐out should read back to the person giving the sign‐out each item on the to‐do list (eg, So, I should check the ins and outs at about 10:00 pm, and give 40 of furosemide if the patient is not 1 L negative, right?).
Anticipated problems should also be verbally communicated to promote a dialogue. Points that cannot be adequately transferred in the written sign‐out are particularly important to transmit verbally. Examples include previous code discussions, unusual responses to treatment, and psychosocial and family issues. When delivering verbal sign‐out, it is important to consider the a priori knowledge of the recipient. How much knowledge about a patient is already shared between the outgoing and incoming physicians and the level of experience of the physicians may affect the extent to which information needs be transmitted.37 For instance, 2 experienced physicians who already have been working to cover the same patient will likely have an abbreviated discussion, in contrast to the lengthier sign‐out necessary if the outgoing and incoming physicians are interns, and the incoming intern has no prior knowledge of the patient. Similarly, it is likely the level of detail transmitted will need to be greater during a permanent transfer of patient care (ie, at the end of a resident's rotation) than during a brief, temporary transition (eg, overnight coverage).
The challenges of a busy inpatient service may preclude a complete verbal sign‐out for all patients; we contend, though, it is best to use these practices to the extent possible, especially for patients with treatment plans in flux, those whose status is tenuous, and those who have anticipated changes in status during cross‐coverage. One tool that may be effectively used in signing out such patients is the SBAR tool, according to which a brief description of the situation is given, followed by the background and the physician's specific assessment and complete recommendation.38 For example, a resident signing out might begin by stating, I have 18 patients to sign‐out to you. I'm going to describe 6 active patients in detail. Twelve others are fairly stable, and I will give you basic information about them, and the details are in the written sign‐out. One patient has a plan in flux. The situation is Mr. S. is having trouble breathing, the background is that he has both CHF and COPD, my assessment is that this is more cardiac than pulmonary, and I recommend that you see him first and discuss with the cardiology consultant. Using the tools described here (Table 2), a sign‐out of 15 patients of variable acuity could be verbally signed out in less than 10 minutes.
| ✓ WHO should participate in the sign‐out process? |
| □ Outgoing clinician primarily responsible for patient's care |
| □ Oncoming clinician who will be primarily responsible for patient's care (avoid passing this task to someone else, even if busy) |
| □ Consider supervision by experienced clinicians if early in training |
| ✓ WHAT content needs to be verbally communicated? |
| Use situation briefing model, or SBAR, technique: |
| □ SituationIdentify each patient (name, age, sex, chief complaint) and briefly state any major problems (active and those that may become active during cross‐coverage). |
| □ Backgroundpertinent information relevant to current care (eg, recent vitals and/or baseline exam, labs, test results, etc). |
| □ Assessmentworking diagnosis, response to treatment, anticipated problems during cross‐coverage including anything not adequately described using written form (eg, complex family discussions). |
| □ Recommendationto‐do lists and if/then recommendations. |
| ✓ WHERE should sign‐out occur? |
| □ Designated room or place for sign‐out (eg, avoid patient areas because of HIPPA requirements) |
| □ Proper lighting |
| □ Avoid excessive noise (eg, high‐traffic areas) |
| □ Minimize disruptions (eg, hand over pagers) |
| □ Ensure systems support for sign‐out (eg, computers, printer, paper, etc.) |
| ✓ WHEN is the optimal time for sign‐out? |
| □ Designated time when both parties can be present and pay attention (eg, beware of clinic, other obligations) |
| □ Have enough time for interactive questions at the end (eg, avoid rush at the end of the shift) |
| ✓ HOW should verbal communication be performed? |
| □ Face to face, allowing for questions |
| □ Verbalize data in the same order for each patient at each sign‐out |
| □ Read back all to‐do items |
| □ Adjust length and depth of review according to baseline knowledge of parties involved and type of transition in care |
The Environment and setting.
To improve the setting of sign‐out, we recommend: a designated space that is well lit, quiet, and respects patient confidentiality and a designated time when sign‐out will occur. To limit known distractions and interruptions39, 40 in the hospital, we also recommend the outgoing physician hand off his or her pager to someone else during sign‐out. Also key to an environment conducive to information transfer is ensuring adequate computer support for electronic sign‐out and access to updated clinical information.
Organizational culture and institutional leadership.
The way residents transfer patient care information reflects the culture of the institution. Changing the culture to one in which interactive questioning is valued regardless of position in the hierarchy has been shown to reduce errors in aviation.41 Educating residents on the impact of sign‐outs on patient care is a first step toward improving the culture of sign‐out. Resident commitment to the new sign‐out can be gained by engaging residents in development of the process itself. To cement these changes into the culture, practitioners at all levels should be aware of and support the new system. The role of an institution's leaders in achieving these changes cannot be overlooked. Leaders will need to be creative in order to support sign‐out as described within the obvious constraints of money, time, personnel, and space. Gaining institutional buy‐in can start with heightening the awareness of leaders of the issues surrounding sign‐out, including patient safety, resident efficiency, and the financial impact of discontinuity. Ongoing evaluation of efforts to improve sign‐out is also crucial and can be accomplished with surveys, focus groups, and direct observation. Feeding back the positive impact of the changes to all involved stakeholders will promote confidence in the new systems and pride in their efforts.
CONCLUSIONS
Sign‐outs are a part of the current landscape of academic medical centers as well as hospitals at large. Interns, residents, and consulting fellows, not to mention nurses, physical therapists, and nutritionists, transfer patient care information at each transition point. There are few resources that can assist these caregivers in identifying and implementing the most effective ways to transfer patient care information. Hospitals and other care facilities are now mandated to develop standards and systems to improve sign‐out. On the basis of the limited literature to date and our own experiences, we have proposed standards and best practices to assist hospitals, training programs, and institutional leaders in designing safe and usable sign‐out systems. Effective implementation of the standards must include appropriate allocation of resources, individualization to meet specific needs of each program or institution, intensive training, and ongoing evaluation. Future research should focus on developing valid surrogate measures of continuity of care, conducting rigorous trials to determine the elements of sign‐out that lead to the best patient outcomes, and studying the most effective ways of implementing these improvements. By improving the content and process of sign‐out, we can meet the challenges of the new health care landscape while putting patient safety at the forefront.
- ,,.New requirements for resident duty hours.JAMA.2002;288:1112–1114.
- ,,,,.Effects of limited work hours on surgical training.J Am Coll Surg.2002;195:531–538.
- ,,, et al.Effect of reducing interns' weekly work hours on sleep and attentional failures.N Engl J Med.2004;351:1829–1837.
- ,,, et al.Effect of reducing interns' work hours on serious medical errors in intensive care units.N Engl J Med.2004;351:1838–1848.
- .A precarious exchange.N Engl J Med.2004;351:1822–1824.
- .Awake and informed.N Engl J Med.2004;351:1884.
- . Fumbled handoff: missed communication between teams. Cases and Commentary: Hospital Medicine, Morbidity 269:374–378.
- ,,,,.Does housestaff discontinuity of care increase the risk for preventable adverse events?Ann Intern Med.1994;121:866–872.
- ,,,.Post‐call transfer of resident responsibility: its effect on patient care.J Gen Intern Med.1990;5:501–505.
- ,,,.Effect of a change in house staff work schedule on resource utilization and patient care.Arch Intern Med.1991;151:2065–2070.
- ,.Internal Bleeding: the Truth behind America's Terrifying Epidemic of Medical Mistakes.New York City:Rugged Land, LLC;2004:448.
- ,,.Crew resource management and its applications in medicine. In:Making Health Care Safer: A Critical Analysis of Patient Safety Practices. Evidence Report/Technology Assessment Number 43, AHRQ Publication 01‐E058.Rockville, MD:Agency for Healthcare Research and Quality;2001.
- ,,.System safety and threat and error management: the line operations safety audit (LOSA). In:Jensen RS, ed. Proceedings of the Eleventh International Symposium on Aviation Psychology.Columbus, OH:Ohio State University;2001:1–6.
- ,,.Translating teamwork behaviours from aviation to healthcare: development of behavioural markers for neonatal resuscitation.Qual Saf Health Care.2004;13(Suppl 1):i57–i64.
- ,,,.Handoff strategies in settings with high consequences for failure: lessons for healthcare operations.Intl J Qual Health Care.2004;16:125–132.
- . Available at: http://www.agilemodeling.com/essays/communication.htm. Accessed December 15,2005.
- .Ensuring continuing care: styles and efficiency of the handover process.Aust J Adv Nurs.1998;16:23–27.
- ,.The handover: uncovering the hidden practices of nurses.Intensive Crit Care Nurs.2000;16:373–383.
- .The patient handover: a study of its form, function and efficiency.Nurs Stand.1995;9(52):33–36.
- ,.Residents' suggestions for reducing errors in teaching hospitals.N Engl J Med.2003;348:851–855.
- ,.Is 80 the cost of saving lives? Reduced duty hours, errors, and cost.J Gen Intern Med.2005;20:969–970.
- ,,,.Lost in translation: challenges and opportunities in physician‐to‐physician communication during patient handoffs.Acad Med.2005;80:1094–1099.
- British Medical Association.Safe Handover: Safe Patients: Guidance on Clinical Handover for Clinicians and Managers.London:British Medical Association, Junior Doctors Committee;2004.
- ,,,,.Using a computerized sign‐out program to improve continuity of inpatient care and prevent adverse events.Jt Comm J Qual Improv.1998;24(2):77–87.
- ,,,.Organizing the transfer of patient care information: the development of a computerized resident sign‐out system.Surgery.2004;136:5–13.
- ,,,,.A randomized, controlled trial evaluating the impact of a computerized rounding and sign‐out system on continuity of care and resident work hours.J Am Coll Surg.2005;200:538–545.
- ,,,.Computerized physician order entry in U.S. hospitals: results of a 2002 survey.J Am Med Inform Assoc.2004;11:95–99.
- ,,,,,.The impact of verbal communication on physician prescribing patterns in hospitalized patients with diabetes.Diabetes Educ.2003;29:827–836.
- ,.Use of computer terminals on wards to access emergency test results: a retrospective audit.Br Med J.2001;322:1101–1103.
- ,,,,,.Improving patient safety by repeating (read‐back) telephone reports of critical information.Am J Clin Pathol.2004;121:801–803.
- ,.The human factor: the critical importance of effective teamwork and communication in providing safe care.Qual Saf Health Care.2004;13(Suppl 1):i85–i90.
- ,,,,.Communication failures in patient sign‐out and suggestions for improvement: a critical incident analysis.Qual Saf Health Care.2005;14:401–407.
- ,,.Intern curriculum: the impact of a focused training program on the process and content of signout out patients. Harvard Medical School Education Day, Boston, MA;2004.
- .When conversation is better than computation.J Am Med Inform Assoc.2000;7:277–286.
- SBAR technique for communication: a situational briefing model. Available at: http://www.ihi.org/IHI/Topics/PatientSafety/SafetyGeneral/Tools/SBARTechniqueforCommunicationASituationalBriefingModel.htm. Accessed December2005.
- ,,,,.Impact of reduced duty hours on residents' educational satisfaction at the University of California, San Francisco.Acad Med.2006;81:76–81.
- ,.Communication behaviours in a hospital setting: an observational study.Br Med J.1998;316:673–676.
- ,,.Communication failures: an insidious contributor to medical mishapsAcad Med.2004;79(2):186–194.
- ,,.New requirements for resident duty hours.JAMA.2002;288:1112–1114.
- ,,,,.Effects of limited work hours on surgical training.J Am Coll Surg.2002;195:531–538.
- ,,, et al.Effect of reducing interns' weekly work hours on sleep and attentional failures.N Engl J Med.2004;351:1829–1837.
- ,,, et al.Effect of reducing interns' work hours on serious medical errors in intensive care units.N Engl J Med.2004;351:1838–1848.
- .A precarious exchange.N Engl J Med.2004;351:1822–1824.
- .Awake and informed.N Engl J Med.2004;351:1884.
- . Fumbled handoff: missed communication between teams. Cases and Commentary: Hospital Medicine, Morbidity 269:374–378.
- ,,,,.Does housestaff discontinuity of care increase the risk for preventable adverse events?Ann Intern Med.1994;121:866–872.
- ,,,.Post‐call transfer of resident responsibility: its effect on patient care.J Gen Intern Med.1990;5:501–505.
- ,,,.Effect of a change in house staff work schedule on resource utilization and patient care.Arch Intern Med.1991;151:2065–2070.
- ,.Internal Bleeding: the Truth behind America's Terrifying Epidemic of Medical Mistakes.New York City:Rugged Land, LLC;2004:448.
- ,,.Crew resource management and its applications in medicine. In:Making Health Care Safer: A Critical Analysis of Patient Safety Practices. Evidence Report/Technology Assessment Number 43, AHRQ Publication 01‐E058.Rockville, MD:Agency for Healthcare Research and Quality;2001.
- ,,.System safety and threat and error management: the line operations safety audit (LOSA). In:Jensen RS, ed. Proceedings of the Eleventh International Symposium on Aviation Psychology.Columbus, OH:Ohio State University;2001:1–6.
- ,,.Translating teamwork behaviours from aviation to healthcare: development of behavioural markers for neonatal resuscitation.Qual Saf Health Care.2004;13(Suppl 1):i57–i64.
- ,,,.Handoff strategies in settings with high consequences for failure: lessons for healthcare operations.Intl J Qual Health Care.2004;16:125–132.
- . Available at: http://www.agilemodeling.com/essays/communication.htm. Accessed December 15,2005.
- .Ensuring continuing care: styles and efficiency of the handover process.Aust J Adv Nurs.1998;16:23–27.
- ,.The handover: uncovering the hidden practices of nurses.Intensive Crit Care Nurs.2000;16:373–383.
- .The patient handover: a study of its form, function and efficiency.Nurs Stand.1995;9(52):33–36.
- ,.Residents' suggestions for reducing errors in teaching hospitals.N Engl J Med.2003;348:851–855.
- ,.Is 80 the cost of saving lives? Reduced duty hours, errors, and cost.J Gen Intern Med.2005;20:969–970.
- ,,,.Lost in translation: challenges and opportunities in physician‐to‐physician communication during patient handoffs.Acad Med.2005;80:1094–1099.
- British Medical Association.Safe Handover: Safe Patients: Guidance on Clinical Handover for Clinicians and Managers.London:British Medical Association, Junior Doctors Committee;2004.
- ,,,,.Using a computerized sign‐out program to improve continuity of inpatient care and prevent adverse events.Jt Comm J Qual Improv.1998;24(2):77–87.
- ,,,.Organizing the transfer of patient care information: the development of a computerized resident sign‐out system.Surgery.2004;136:5–13.
- ,,,,.A randomized, controlled trial evaluating the impact of a computerized rounding and sign‐out system on continuity of care and resident work hours.J Am Coll Surg.2005;200:538–545.
- ,,,.Computerized physician order entry in U.S. hospitals: results of a 2002 survey.J Am Med Inform Assoc.2004;11:95–99.
- ,,,,,.The impact of verbal communication on physician prescribing patterns in hospitalized patients with diabetes.Diabetes Educ.2003;29:827–836.
- ,.Use of computer terminals on wards to access emergency test results: a retrospective audit.Br Med J.2001;322:1101–1103.
- ,,,,,.Improving patient safety by repeating (read‐back) telephone reports of critical information.Am J Clin Pathol.2004;121:801–803.
- ,.The human factor: the critical importance of effective teamwork and communication in providing safe care.Qual Saf Health Care.2004;13(Suppl 1):i85–i90.
- ,,,,.Communication failures in patient sign‐out and suggestions for improvement: a critical incident analysis.Qual Saf Health Care.2005;14:401–407.
- ,,.Intern curriculum: the impact of a focused training program on the process and content of signout out patients. Harvard Medical School Education Day, Boston, MA;2004.
- .When conversation is better than computation.J Am Med Inform Assoc.2000;7:277–286.
- SBAR technique for communication: a situational briefing model. Available at: http://www.ihi.org/IHI/Topics/PatientSafety/SafetyGeneral/Tools/SBARTechniqueforCommunicationASituationalBriefingModel.htm. Accessed December2005.
- ,,,,.Impact of reduced duty hours on residents' educational satisfaction at the University of California, San Francisco.Acad Med.2006;81:76–81.
- ,.Communication behaviours in a hospital setting: an observational study.Br Med J.1998;316:673–676.
- ,,.Communication failures: an insidious contributor to medical mishapsAcad Med.2004;79(2):186–194.
Clinical Conundrum
A 45‐year‐old man who immigrated to Canada from Ghana at the age of 33 presented with a 2‐year history of progressive cognitive changes. He had bifrontal headache, right‐sided scalp paresthesias, and a 40‐pound weight loss. He was unable to perform his job as an auto parts worker. His wife noticed short‐ and long‐term memory problems and poor concentration. On physical exam he had no focal neurological findings but his score on the Mini‐Mental Status Exam (MMSE) was 23/30, with deficits in attention and recall.
The first important element of this illness is its chronicity. His symptoms progressed slowly over 2 years. Second, aside from his neurological problems, he is an otherwise healthy young, African‐born male. This clinical picture could be the early presentation of a demyelinating, infiltrative, or vascular illness. If vascular, it is more likely a vasculitis than atherosclerotic disease. Malignancy and infection are definitely in the differential, but at this point, I think they are less likely to be the cause, given the tempo of presentation. I would begin my investigations with basic blood work and a computerized tomography (CT) scan of his brain.
A CT scan of the head with contrast demonstrated an enlarged left lateral ventricle with no evidence of obstruction in the foramen of Munro.
The radiological findings of communicating hydrocephalus with normal parenchyma imply a disease that affects the leptomeningeal space. Given that we are looking at an illness that can change cerebral spinal fluid (CSF) flow rather than primary parenchymal disease, demyelinating and vascular illnesses are less likely etiologies, and infiltrative diseases move up on my list. Malignancy and infectious diseases remain in the differential.
He disappeared to follow up for 1 year, during which he returned to Ghana and experienced progressive neurological deterioration, with incontinence, gait instability, and inability to converse clearly and perform activities of daily living. On his return to Canada, an urgent CT scan and magnetic resonance imaging (MRI) of the brain demonstrated ongoing and unchanged hydrocephalus with aqueductal stenosis. A referral was made to a neurosurgeon for insertion of a ventriculoperitoneal shunt. A routine preoperative chest radiograph demonstrated new bilateral upper‐zone reticulonodular changes.
He had no respiratory symptoms, fevers, or lymphadenopathy. His occupational history revealed no exposure to asbestos, silica, farms, or mines. He had no history of either respiratory or neurological illness in the past and no travel other than to Ghana and Toronto. When he immigrated to Toronto, Canada, 12 years before, he had a normal chest radiograph and negative PPD tuberculin skin test.
Many illnesses produce asymptomatic changes on chest x‐ray. Oslerian principles would suggest that we should think of a single diagnosis to explain both nodular lung disease and more than 3 years of a progressive disease affecting the leptomeninges. It is unlikely that tuberculosis, other fungal diseases, or malignancy would result in the chest and brain pathology over a 3‐year period without other sequelae. Sarcoidosis could cause both chronic leptomeningeal changes and the radiographic lung findings. The next steps in investigating this patient should include measurement of angiotensin‐converting enzyme (ACE) and serum calcium and pulmonary function tests. I would ultimately send him for a pathological biopsy of his lung tissue to confirm noncaseating granuloma and exclude infection and malignancy.
Complete blood count, renal and liver biochemistry, and calcium were normal. An ACE level was elevated at 69 g/L (normal < 40 g/L). A human immunodeficiency virus (HIV‐1 and HIV‐2) test, tuberculin skin test, and syphilis serology were negative. A CT scan of the chest demonstrated bilateral upper‐zone reticulonodular changes and diffuse lymphadenopathy (Fig. 1). Pulmonary function tests (PFTs) demonstrated a forced expiratory volume 1 (FEV1) of 3.4 L (94%), forced vital capacity (FVC) of 4.0 L (83%), an FEV1/FVC of 87%, total lung capacity (TLC) of 92% predicted, and diffusion capacity (DLCO) of 67% predicted. An MRI with gadolinium (Fig. 2) demonstrated hydrocephalus, mild basal leptomeningeal enhancement around the perivascular spaces into the subinsular region, and an increased T2 signal in periventricular white matter.
A bronchoscopy with bronchoalveolar lavage and transbronchial biopsies were performed. Pathology (Fig. 3) demonstrated non‐caseating epitheliod granulomas, with negative special stains for acid‐fast bacilli (AFB) and fungus, and negative fungal and AFB cultures of the bronchial alveolar lavage.
With negative tests for infectious causes such as tuberculosis, I think there is now enough evidence that this patient has sarcoidosis involving the lung and leptomeninges. At this point I would start therapy with steroids.
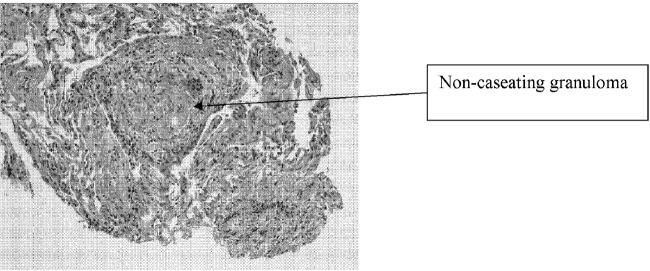
The patient was started on prednisone 40 mg po qd, and his neurological symptoms improved markedly over the course of 1‐2 months, with normalization of his MMSE and a return to cognitive baseline. As his symptoms stabilized with no change in CT imaging, he returned to work, and over the course of 2 years his prednisone dosage was tapered to 10 mg po od. While on prednisone he developed hypertension and hyperglycemia. He continued to have no respiratory symptoms.
He was cognitively at baseline until 20 months later, when he was readmitted to the hospital with a 2‐week history of worsening headache, increased confusion, poor memory, and wandering. His MMSE had deteriorated to 19/30, with deficits again in memory and attention.
First, we can say with reasonable confidence that the diagnosis of sarcoid was correct. His long and sustained response to steroids, plus the absence of the unmasking of an infectious or malignant disease, supports this conclusion. However, he is now exhibiting an apparent relapse that mimics his presentation 3 years earlier. The question is whether he is suffering from a flare of his disease or whether a second illness has occurred. The most obvious second illness is an opportunistic infection after years of steroid use. I would certainly repeat the angiotensin‐converting enzyme and serum calcium tests and repeat the imaging of his lungs and central nervous system. He also warrants a lumbar puncture with CSF culture, stain, and PCR for opportunistic infections. If these studies are inconclusive and do not specifically suggest relapsing sarcoid, I would once again consider biopsy of tissue from either a lung or leptomeninges.
An MRI with gadolinium looked unchanged from the previous one. A lumbar puncture was performed, and his CSF demonstrated 3 WBCs, no RBCs, normal glucose, and elevated protein at 1.17 g/L, and tests for bacteria, TB, fungi, and viruses were all negative. Repeat blood work was unremarkable, and the ACE level was 2 g/L.
A chest radiograph (Fig. 4a) and CT chest (Fig. 4b) showed marked deterioration, with increased diffuse airspace opacities, interstitial nodularity, and small apical bullae. His PFTs showed some deterioration, with FEV1 2.52 L (73%), FVC 3.29 L (73%), FEV1/FVC 76%, TLC 70% predicted, DLCO 72% predicted. However, he still had no respiratory symptoms.
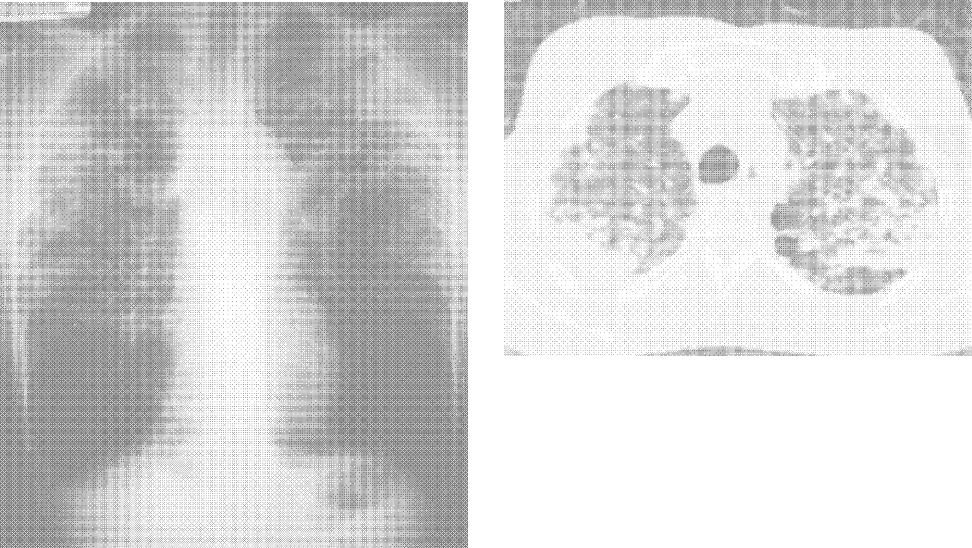
The changes on lumbar puncture are nonspecific. The ACE level is now very low, making sarcoidosis unlikely but not impossible. The chest imaging shows features, specifically interstitial nodularity, consistent with ongoing or relapsing sarcoidosis, but the extensive apical bullae are not characteristic. My best guess is that this patient's illness is not simply relapsing sarcoid but represents superimposed opportunistic infectious disease. I would not reintroduce steroids without pursuing a definitive diagnosis with tissue pathology.
He was placed on prednisone 60 mg po qd and started on trimethoprim‐sulfamethoxazole for Pneumocystis pneumonia (PCP) prophylaxis. He showed modest improvement in his neurological status. A repeat bronchoscopy was not performed. Four months later he was seen by his pulmonologist. He remained without respiratory symptoms and was neurologically unchanged, and a chest radiograph showed no change. He was continued on prednisone 60 mg po qd.
Three weeks later, he was admitted to the hospital with a 2‐week history of anorexia, fatigue, night sweats, right‐sided pleuritic chest pain with productive cough, increasing dyspnea, and no hemoptysis. On admission he was hypoxic with evidence of respiratory distress, and his chest radiograph showed evidence of new right‐sided airspace disease with an associated large right pleural effusion. Initial labs demonstrated a leukocytosis.
I am now very suspicious that this illness is not relapsed sarcoidosis based on his prior clinical response to high‐dose prednisone and that he currently is showing no neurological improvement. His recent clinical deterioration on this very high dose of prednisone makes me think that opportunistic lung infection or disseminated disease is definitely the cause, although the differential is broad. In addition to the typical viral and bacterial causes of community‐acquired pneumonia, this could be caused by unusual bacterial pathogens, tuberculosis, nontuberculous mycobacteria, or fungal diseases including Candida, Aspergillus and dimorphic fungi. I would begin empiric therapy with antibiotics, obtain pleural fluid for examination and culture, and blood cultures.
The patient was treated with a respiratory fluoroquinolone, and blood and sputum cultures were performed. A right thoracentesis removed 300 cc of yellow exudate, with negative gram stain and initial culture. Over the next 24 hours, the patient deteriorated rapidly, with progressive hypoxia and clinical and radiological (Fig. 5) evidence of acute respiratory distress syndrome (ARDS). He required endotracheal intubation with mechanical ventilation.
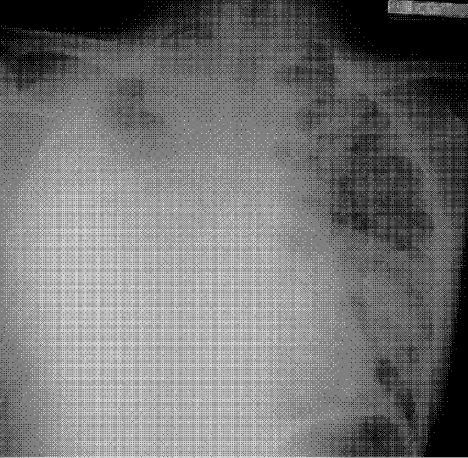
He has a progressive illness not responsive to broad‐spectrum antibiotics, and he has deteriorated. At this point it is imperative that he undergo bronchoscopy and transbronchial biopsy.
Bronchoscopy demonstrated secretions from the right lower lobe. Gram stain from a bronchoalveolar lavage from the right lower lobe was negative, and cultures showed no growth after 24 hours. Immediately after bronchoscopy a third‐generation cephalosporin was empirically added. The next day the patient developed hypotension and was started on norepinephrine. Over the subsequent 48 hours, he developed progressive multiorgan failure. Despite multiple vasopressors, high‐frequency oscillator ventilation, broad‐spectrum antimicrobials, and activated protein C, he died in the intensive care unit. At the time of death, all blood cultures were negative, abdominal CT scans showed no intraabdominal infections, and the BAL performed on admission demonstrated negative gram stain, fungal stain, AFB stain, and PCP and no growth from fungal or bacterial cultures.
I think it is an unavoidable conclusion that this man's progressive systemic inflammatory response syndrome and ultimate multiorgan failure was caused by an opportunistic pathogen that was not antibiotic responsive and not identified from the extensive range of infectious disease studies performed. Despite all the negative studies, there still might be either mycobacterial illness or fungal illness. With negative cultures, Candida or Aspergillus infection is unlikely. Other opportunistic fungi like Blastomyces, Histoplasma, and Cryptococcus are certainly in the differential because these organisms can be notoriously difficult to detect on routine surveying such as bronchoalveolar lavage or lumbar puncture. Blastomyces and Histoplasma are both endemic in the area of Canada where the patient resided. I would also keep the zygomycoses in the differential.
Five days after death, fungal culture was reported demonstrating Blastomyces dermatitidis. Postmortem demonstrated disseminated blastomycosis causing severe bilateral pneumonia (Fig. 6a), empyema of right lung, and involvement of the thyroid, heart, liver, spleen, and kidneys. There was also evidence of active CNS blastomycosis involving the meninges and cerebral cortex and diencephalon (Fig. 6b). As well as active blastomycosis, the leptomeninges demonstrated fibrosis and old granulomas that did not contain an organism.
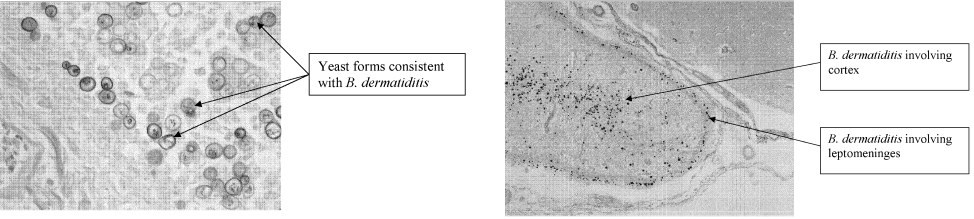
COMMENTARY
This case describes a 45‐year‐old man who presented with chronic cognitive symptoms associated with hydrocephalus. The first step in establishing the diagnosis was made by realizing that a communicating hydrocephalus with no parenchymal CNS disease was highly suggestive of a leptomeningeal process. This narrowed the differential diagnosis to an infiltrative disease affecting the leptomeninges. The next step involved the discovery of an upper‐lobe interstitial lung process, establishing sarcoidosis as the most likely unifying diagnosis. This was confirmed with transbronchial biopsies showing noncaseating granulomas and by the sustained response to treatment with corticosteroids. Unfortunately, after a 2‐year remission, he developed a recurrence of both the neurological and respiratory findings. When his symptoms progressed despite higher doses of corticosteroids, it became apparent that the etiology of his clinical deterioration was not recurrent disease. Instead, the deterioration was caused by disseminated blastomycosis, an opportunistic infection that developed as a result of the immunosuppressants used to treat the sarcoidosis.
With the final diagnosis of blastomycosis, one question about this case becomes: Could it have been blastomycosis and not sarcoid that was responsible for his original neurological presentation? Blastomyces dermatitidis is a thermally dimorphic fungus that causes disease from inhalation of airborne spores found in soil. Areas of North America in which it is endemic include regions bordering the Mississipi and Ohio rivers, as well as the areas bordering the Great Lakes.1 The patient in this case lived in metropolitan Toronto, on Lake Ontario, where blastomycosis is an important yet underreported disease.24 He likely was exposed to blastomyces in Toronto, which in immunocompromised patients may be followed after weeks to months by dissemination to other body sites including the dermis, bones, joints, urogenital system, and, rarely, the central nervous system (CNS) and liver.5 Like sarcoidosis, infection with blastomycosis can produce pathologic evidence of noncaseating granulomatous inflammation. However, as the discussant astutely pointed out, it would be unusual for this patient to have clinically inapparent blastomycosis for almost 2 years while on high‐dose prednisone. The initial diagnosis of sarcoid likely was correct.
CNS disease caused by Blastomyces dermatitidis is quite rare, with only 22 reported cases of meningoencephalitis in the literature.6 As this case demonstrates, CNS blastomycosis is very difficult to diagnose because of the absence of sensitive serologic markers and the difficulty of isolating the organism from blood and cerebrospinal fluid. CSF sampling from lumbar puncture led to its diagnosis in only 2 of the 22 reported cases.7 Furthermore, reliable CSF cultures are usually only obtained via ventricular sampling or tissue biopsy, which itself is limited by the organism's predilection for deep structures of the cerebrum, midbrain, and basal meninges.6 Blastomyces involving the CNS rarely occurs in isolation. In the patient's case, during his neurological deterioration, there was clear radiological evidence of progressive pulmonary pathology despite his being asymptomatic, and as the discussant suggests, pulmonary investigations were warranted.
Pulmonary manifestations of blastomycosis are variable. Acute infections most commonly resemble pneumonia, whereas chronic disease may show reticulonodular changes indistinguishable from sarcoidosis. Severe cases have been shown to progress to respiratory failure with acute respiratory distress syndrome (ARDS).1 The diagnosis is usually established through culture of noninvasive (sensitivity 86%) or bronchoalveolar lavage (sensitivity 92%) specimens.8 However, blastomyces will take between 5 and 30 days to grow in culture.1 In cases where the diagnosis needs to be established quickly, a KOH smear can be done looking for broad‐based budding yeast. Although the yield of this test is lower (0%‐50%), the results can be available within 24 hours.9 As these tests are not always routinely performed, direct communication with the pathologist is recommended if a rapid diagnosis is needed.
The major challenge of this case lay in distinguishing between the recurrence of an old disease and the complications of its treatment. In this case the discussant addresses strategies that might be useful in differentiating recurrent sarcoidosis from an opportunistic infection like blastomycosis. The first issue is the steroid therapy. The exact dose of steroids required to compromise the immune system enough to yield infections is not known. However, in a meta‐analysis of 71 controlled clinical trials performed with steroids, Stuck et. al. were able to show that the occurrence of opportunistic infections depended on both the amount of daily steroid and the cumulative dose.10 Opportunistic infections were unlikely to occur in patients given a mean daily dose of less than 10 mg/day or a cumulative dose of less than 700 mg of prednisone. Although the patient in the present case was only on 10 mg/day of prednisone, his mean daily dose was more than 10 mg/day, and his cumulative dose far exceeded 700 mg. Therefore, an opportunistic infection should have been strongly considered.
The other item used to help distinguish between the 2 diseases was serum angiotensin‐converting enzyme (ACE) level. ACE is an enzyme produced by the epithelial cells of the granulomas in sarcoidosis. ACE alone is inadequate for diagnosis, with a reported sensitivity of 40%‐90%, depending on the population studied and on the definition of normal.1114 Even an ACE level more than twice the normal is not diagnostic for sarcoidosis, with elevated levels found in histoplasmosis, silicosis, tuberculosis, Gaucher's disease, and other disorders.15 Rather than as a diagnostic test, ACE level instead is used to follow disease activity in sarcoidosis, as ACE level often reflects the granuloma burden.1618 The low levels at the initial recurrence suggests the symptoms were not a result of active sarcoid, especially considering that if ACE levels are originally elevated with sarcoidosis, they are almost always elevated again when the disease recurs.14 Normal levels of ACE in sarcoid patients with previously elevated ACE levels should therefore prompt a search for an alternate diagnosis.
This case is an example of the therapy causing a complication that mimics the disease it was intended to cure. When any patient deteriorates while on steroids, the clinician must ask the age‐old question: should more steroids be prescribed or less? As in this case, the answer is not always apparent. Safe decisions in these situations demand awareness of the opportunistic infections endemic to the area and a willingness to perform early invasive procedures (in this case bronchoscopy) to obtain samples to make a definitive diagnosis. By doing so, the devastating chain of events that occurred here hopefully can be avoided.
Acknowledgements
The authors would like to acknowledge Dr. Eleanor Latta and Dr. Serge Jothy, Department of Pathology, St. Michael's Hospital, University of Toronto, for contributing the pathological images.
- ,,.Blastomycosis.Infect Dis Clin North Am.2003;17(1):21,40, vii.
- ,,,,,.Novel cases of blastomycosis acquired in Toronto, Ontario.CMAJ.2000;163:1309–1312.
- ,,,,,.Blastomycosis acquired by three children in Toronto.Can J Infect Dis Med Micro.2002;13(4):259–263.
- ,,, et al.Blastomycosis in Ontario, 1994‐2003.Emerg Infect Dis.2006;12(2):274–279.
- ,,, et al.Epidemiology and clinical spectrum of blastomycosis diagnosed at Manitoba hospitals.Clin Infect Dis.2002;34:1310–1316.
- ,,,.Meningoencephalitis due to Blastomyces dermatitidis: case report and literature review.Mayo Clin Proc.2000;75:403–408.
- ,,,.Chronic blastomycotic meningitis.Am J Med.1981;71:501–505.
- ,.Pulmonary blastomycosis: an appraisal of diagnostic techniques.Chest.2002;121:768–773.
- ,,.Blastomycosis as an etiology of acute lung injury.South Med J.1998;91:861–863.
- ,,.Risk of infectious complications in patients taking glucocorticosteroids.Rev Infect Dis.1989;11:954–963.
- .Elevation of serum angiotensin‐converting‐enzyme (ACE) level in sarcoidosis.Am J Med.1975;59:365–372.
- ,,,.Elevated serum angiotensin I converting enzyme in sarcoidosis.Am Rev Respir Dis.1976;114:525–528.
- ,,.Serum angiotensin‐converting enzyme (SACE) in sarcoidosis and other granulomatous disorders.Lancet.1978;2:1331–1334.
- ,.Serum angiotensin converting enzyme in sarcoidosis: sensitivity and specificity in diagnosis: correlations with disease activity, duration, extra‐thoracic involvement, radiographic type and therapy.Q J Med.1985;55(218):253–270.
- Statement on sarcoidosis.Joint Statement of the American Thoracic Society (ATS), theEuropean Respiratory Society (ERS) and theWorld Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999.Am J Respir Crit Care Med.1999;160:736–755.
- ,,.Value of serial measurement of serum angiotensin converting enzyme in the management of sarcoidosis.Am J Med.1981;70(1):44–50.
- ,,,.Serum angiotensin‐converting enzyme (SACE) activity as an indicator of total body granuloma load and prognosis in sarcoidosis.Sarcoidosis.1987;4(2):142–148.
- ,,.Serum angiotensin converting enzyme in sarcoidosis: clinical significance.Isr J Med Sci.1977;13:1001–1006.
A 45‐year‐old man who immigrated to Canada from Ghana at the age of 33 presented with a 2‐year history of progressive cognitive changes. He had bifrontal headache, right‐sided scalp paresthesias, and a 40‐pound weight loss. He was unable to perform his job as an auto parts worker. His wife noticed short‐ and long‐term memory problems and poor concentration. On physical exam he had no focal neurological findings but his score on the Mini‐Mental Status Exam (MMSE) was 23/30, with deficits in attention and recall.
The first important element of this illness is its chronicity. His symptoms progressed slowly over 2 years. Second, aside from his neurological problems, he is an otherwise healthy young, African‐born male. This clinical picture could be the early presentation of a demyelinating, infiltrative, or vascular illness. If vascular, it is more likely a vasculitis than atherosclerotic disease. Malignancy and infection are definitely in the differential, but at this point, I think they are less likely to be the cause, given the tempo of presentation. I would begin my investigations with basic blood work and a computerized tomography (CT) scan of his brain.
A CT scan of the head with contrast demonstrated an enlarged left lateral ventricle with no evidence of obstruction in the foramen of Munro.
The radiological findings of communicating hydrocephalus with normal parenchyma imply a disease that affects the leptomeningeal space. Given that we are looking at an illness that can change cerebral spinal fluid (CSF) flow rather than primary parenchymal disease, demyelinating and vascular illnesses are less likely etiologies, and infiltrative diseases move up on my list. Malignancy and infectious diseases remain in the differential.
He disappeared to follow up for 1 year, during which he returned to Ghana and experienced progressive neurological deterioration, with incontinence, gait instability, and inability to converse clearly and perform activities of daily living. On his return to Canada, an urgent CT scan and magnetic resonance imaging (MRI) of the brain demonstrated ongoing and unchanged hydrocephalus with aqueductal stenosis. A referral was made to a neurosurgeon for insertion of a ventriculoperitoneal shunt. A routine preoperative chest radiograph demonstrated new bilateral upper‐zone reticulonodular changes.
He had no respiratory symptoms, fevers, or lymphadenopathy. His occupational history revealed no exposure to asbestos, silica, farms, or mines. He had no history of either respiratory or neurological illness in the past and no travel other than to Ghana and Toronto. When he immigrated to Toronto, Canada, 12 years before, he had a normal chest radiograph and negative PPD tuberculin skin test.
Many illnesses produce asymptomatic changes on chest x‐ray. Oslerian principles would suggest that we should think of a single diagnosis to explain both nodular lung disease and more than 3 years of a progressive disease affecting the leptomeninges. It is unlikely that tuberculosis, other fungal diseases, or malignancy would result in the chest and brain pathology over a 3‐year period without other sequelae. Sarcoidosis could cause both chronic leptomeningeal changes and the radiographic lung findings. The next steps in investigating this patient should include measurement of angiotensin‐converting enzyme (ACE) and serum calcium and pulmonary function tests. I would ultimately send him for a pathological biopsy of his lung tissue to confirm noncaseating granuloma and exclude infection and malignancy.
Complete blood count, renal and liver biochemistry, and calcium were normal. An ACE level was elevated at 69 g/L (normal < 40 g/L). A human immunodeficiency virus (HIV‐1 and HIV‐2) test, tuberculin skin test, and syphilis serology were negative. A CT scan of the chest demonstrated bilateral upper‐zone reticulonodular changes and diffuse lymphadenopathy (Fig. 1). Pulmonary function tests (PFTs) demonstrated a forced expiratory volume 1 (FEV1) of 3.4 L (94%), forced vital capacity (FVC) of 4.0 L (83%), an FEV1/FVC of 87%, total lung capacity (TLC) of 92% predicted, and diffusion capacity (DLCO) of 67% predicted. An MRI with gadolinium (Fig. 2) demonstrated hydrocephalus, mild basal leptomeningeal enhancement around the perivascular spaces into the subinsular region, and an increased T2 signal in periventricular white matter.


A bronchoscopy with bronchoalveolar lavage and transbronchial biopsies were performed. Pathology (Fig. 3) demonstrated non‐caseating epitheliod granulomas, with negative special stains for acid‐fast bacilli (AFB) and fungus, and negative fungal and AFB cultures of the bronchial alveolar lavage.
With negative tests for infectious causes such as tuberculosis, I think there is now enough evidence that this patient has sarcoidosis involving the lung and leptomeninges. At this point I would start therapy with steroids.

The patient was started on prednisone 40 mg po qd, and his neurological symptoms improved markedly over the course of 1‐2 months, with normalization of his MMSE and a return to cognitive baseline. As his symptoms stabilized with no change in CT imaging, he returned to work, and over the course of 2 years his prednisone dosage was tapered to 10 mg po od. While on prednisone he developed hypertension and hyperglycemia. He continued to have no respiratory symptoms.
He was cognitively at baseline until 20 months later, when he was readmitted to the hospital with a 2‐week history of worsening headache, increased confusion, poor memory, and wandering. His MMSE had deteriorated to 19/30, with deficits again in memory and attention.
First, we can say with reasonable confidence that the diagnosis of sarcoid was correct. His long and sustained response to steroids, plus the absence of the unmasking of an infectious or malignant disease, supports this conclusion. However, he is now exhibiting an apparent relapse that mimics his presentation 3 years earlier. The question is whether he is suffering from a flare of his disease or whether a second illness has occurred. The most obvious second illness is an opportunistic infection after years of steroid use. I would certainly repeat the angiotensin‐converting enzyme and serum calcium tests and repeat the imaging of his lungs and central nervous system. He also warrants a lumbar puncture with CSF culture, stain, and PCR for opportunistic infections. If these studies are inconclusive and do not specifically suggest relapsing sarcoid, I would once again consider biopsy of tissue from either a lung or leptomeninges.
An MRI with gadolinium looked unchanged from the previous one. A lumbar puncture was performed, and his CSF demonstrated 3 WBCs, no RBCs, normal glucose, and elevated protein at 1.17 g/L, and tests for bacteria, TB, fungi, and viruses were all negative. Repeat blood work was unremarkable, and the ACE level was 2 g/L.
A chest radiograph (Fig. 4a) and CT chest (Fig. 4b) showed marked deterioration, with increased diffuse airspace opacities, interstitial nodularity, and small apical bullae. His PFTs showed some deterioration, with FEV1 2.52 L (73%), FVC 3.29 L (73%), FEV1/FVC 76%, TLC 70% predicted, DLCO 72% predicted. However, he still had no respiratory symptoms.

The changes on lumbar puncture are nonspecific. The ACE level is now very low, making sarcoidosis unlikely but not impossible. The chest imaging shows features, specifically interstitial nodularity, consistent with ongoing or relapsing sarcoidosis, but the extensive apical bullae are not characteristic. My best guess is that this patient's illness is not simply relapsing sarcoid but represents superimposed opportunistic infectious disease. I would not reintroduce steroids without pursuing a definitive diagnosis with tissue pathology.
He was placed on prednisone 60 mg po qd and started on trimethoprim‐sulfamethoxazole for Pneumocystis pneumonia (PCP) prophylaxis. He showed modest improvement in his neurological status. A repeat bronchoscopy was not performed. Four months later he was seen by his pulmonologist. He remained without respiratory symptoms and was neurologically unchanged, and a chest radiograph showed no change. He was continued on prednisone 60 mg po qd.
Three weeks later, he was admitted to the hospital with a 2‐week history of anorexia, fatigue, night sweats, right‐sided pleuritic chest pain with productive cough, increasing dyspnea, and no hemoptysis. On admission he was hypoxic with evidence of respiratory distress, and his chest radiograph showed evidence of new right‐sided airspace disease with an associated large right pleural effusion. Initial labs demonstrated a leukocytosis.
I am now very suspicious that this illness is not relapsed sarcoidosis based on his prior clinical response to high‐dose prednisone and that he currently is showing no neurological improvement. His recent clinical deterioration on this very high dose of prednisone makes me think that opportunistic lung infection or disseminated disease is definitely the cause, although the differential is broad. In addition to the typical viral and bacterial causes of community‐acquired pneumonia, this could be caused by unusual bacterial pathogens, tuberculosis, nontuberculous mycobacteria, or fungal diseases including Candida, Aspergillus and dimorphic fungi. I would begin empiric therapy with antibiotics, obtain pleural fluid for examination and culture, and blood cultures.
The patient was treated with a respiratory fluoroquinolone, and blood and sputum cultures were performed. A right thoracentesis removed 300 cc of yellow exudate, with negative gram stain and initial culture. Over the next 24 hours, the patient deteriorated rapidly, with progressive hypoxia and clinical and radiological (Fig. 5) evidence of acute respiratory distress syndrome (ARDS). He required endotracheal intubation with mechanical ventilation.

He has a progressive illness not responsive to broad‐spectrum antibiotics, and he has deteriorated. At this point it is imperative that he undergo bronchoscopy and transbronchial biopsy.
Bronchoscopy demonstrated secretions from the right lower lobe. Gram stain from a bronchoalveolar lavage from the right lower lobe was negative, and cultures showed no growth after 24 hours. Immediately after bronchoscopy a third‐generation cephalosporin was empirically added. The next day the patient developed hypotension and was started on norepinephrine. Over the subsequent 48 hours, he developed progressive multiorgan failure. Despite multiple vasopressors, high‐frequency oscillator ventilation, broad‐spectrum antimicrobials, and activated protein C, he died in the intensive care unit. At the time of death, all blood cultures were negative, abdominal CT scans showed no intraabdominal infections, and the BAL performed on admission demonstrated negative gram stain, fungal stain, AFB stain, and PCP and no growth from fungal or bacterial cultures.
I think it is an unavoidable conclusion that this man's progressive systemic inflammatory response syndrome and ultimate multiorgan failure was caused by an opportunistic pathogen that was not antibiotic responsive and not identified from the extensive range of infectious disease studies performed. Despite all the negative studies, there still might be either mycobacterial illness or fungal illness. With negative cultures, Candida or Aspergillus infection is unlikely. Other opportunistic fungi like Blastomyces, Histoplasma, and Cryptococcus are certainly in the differential because these organisms can be notoriously difficult to detect on routine surveying such as bronchoalveolar lavage or lumbar puncture. Blastomyces and Histoplasma are both endemic in the area of Canada where the patient resided. I would also keep the zygomycoses in the differential.
Five days after death, fungal culture was reported demonstrating Blastomyces dermatitidis. Postmortem demonstrated disseminated blastomycosis causing severe bilateral pneumonia (Fig. 6a), empyema of right lung, and involvement of the thyroid, heart, liver, spleen, and kidneys. There was also evidence of active CNS blastomycosis involving the meninges and cerebral cortex and diencephalon (Fig. 6b). As well as active blastomycosis, the leptomeninges demonstrated fibrosis and old granulomas that did not contain an organism.

COMMENTARY
This case describes a 45‐year‐old man who presented with chronic cognitive symptoms associated with hydrocephalus. The first step in establishing the diagnosis was made by realizing that a communicating hydrocephalus with no parenchymal CNS disease was highly suggestive of a leptomeningeal process. This narrowed the differential diagnosis to an infiltrative disease affecting the leptomeninges. The next step involved the discovery of an upper‐lobe interstitial lung process, establishing sarcoidosis as the most likely unifying diagnosis. This was confirmed with transbronchial biopsies showing noncaseating granulomas and by the sustained response to treatment with corticosteroids. Unfortunately, after a 2‐year remission, he developed a recurrence of both the neurological and respiratory findings. When his symptoms progressed despite higher doses of corticosteroids, it became apparent that the etiology of his clinical deterioration was not recurrent disease. Instead, the deterioration was caused by disseminated blastomycosis, an opportunistic infection that developed as a result of the immunosuppressants used to treat the sarcoidosis.
With the final diagnosis of blastomycosis, one question about this case becomes: Could it have been blastomycosis and not sarcoid that was responsible for his original neurological presentation? Blastomyces dermatitidis is a thermally dimorphic fungus that causes disease from inhalation of airborne spores found in soil. Areas of North America in which it is endemic include regions bordering the Mississipi and Ohio rivers, as well as the areas bordering the Great Lakes.1 The patient in this case lived in metropolitan Toronto, on Lake Ontario, where blastomycosis is an important yet underreported disease.24 He likely was exposed to blastomyces in Toronto, which in immunocompromised patients may be followed after weeks to months by dissemination to other body sites including the dermis, bones, joints, urogenital system, and, rarely, the central nervous system (CNS) and liver.5 Like sarcoidosis, infection with blastomycosis can produce pathologic evidence of noncaseating granulomatous inflammation. However, as the discussant astutely pointed out, it would be unusual for this patient to have clinically inapparent blastomycosis for almost 2 years while on high‐dose prednisone. The initial diagnosis of sarcoid likely was correct.
CNS disease caused by Blastomyces dermatitidis is quite rare, with only 22 reported cases of meningoencephalitis in the literature.6 As this case demonstrates, CNS blastomycosis is very difficult to diagnose because of the absence of sensitive serologic markers and the difficulty of isolating the organism from blood and cerebrospinal fluid. CSF sampling from lumbar puncture led to its diagnosis in only 2 of the 22 reported cases.7 Furthermore, reliable CSF cultures are usually only obtained via ventricular sampling or tissue biopsy, which itself is limited by the organism's predilection for deep structures of the cerebrum, midbrain, and basal meninges.6 Blastomyces involving the CNS rarely occurs in isolation. In the patient's case, during his neurological deterioration, there was clear radiological evidence of progressive pulmonary pathology despite his being asymptomatic, and as the discussant suggests, pulmonary investigations were warranted.
Pulmonary manifestations of blastomycosis are variable. Acute infections most commonly resemble pneumonia, whereas chronic disease may show reticulonodular changes indistinguishable from sarcoidosis. Severe cases have been shown to progress to respiratory failure with acute respiratory distress syndrome (ARDS).1 The diagnosis is usually established through culture of noninvasive (sensitivity 86%) or bronchoalveolar lavage (sensitivity 92%) specimens.8 However, blastomyces will take between 5 and 30 days to grow in culture.1 In cases where the diagnosis needs to be established quickly, a KOH smear can be done looking for broad‐based budding yeast. Although the yield of this test is lower (0%‐50%), the results can be available within 24 hours.9 As these tests are not always routinely performed, direct communication with the pathologist is recommended if a rapid diagnosis is needed.
The major challenge of this case lay in distinguishing between the recurrence of an old disease and the complications of its treatment. In this case the discussant addresses strategies that might be useful in differentiating recurrent sarcoidosis from an opportunistic infection like blastomycosis. The first issue is the steroid therapy. The exact dose of steroids required to compromise the immune system enough to yield infections is not known. However, in a meta‐analysis of 71 controlled clinical trials performed with steroids, Stuck et. al. were able to show that the occurrence of opportunistic infections depended on both the amount of daily steroid and the cumulative dose.10 Opportunistic infections were unlikely to occur in patients given a mean daily dose of less than 10 mg/day or a cumulative dose of less than 700 mg of prednisone. Although the patient in the present case was only on 10 mg/day of prednisone, his mean daily dose was more than 10 mg/day, and his cumulative dose far exceeded 700 mg. Therefore, an opportunistic infection should have been strongly considered.
The other item used to help distinguish between the 2 diseases was serum angiotensin‐converting enzyme (ACE) level. ACE is an enzyme produced by the epithelial cells of the granulomas in sarcoidosis. ACE alone is inadequate for diagnosis, with a reported sensitivity of 40%‐90%, depending on the population studied and on the definition of normal.1114 Even an ACE level more than twice the normal is not diagnostic for sarcoidosis, with elevated levels found in histoplasmosis, silicosis, tuberculosis, Gaucher's disease, and other disorders.15 Rather than as a diagnostic test, ACE level instead is used to follow disease activity in sarcoidosis, as ACE level often reflects the granuloma burden.1618 The low levels at the initial recurrence suggests the symptoms were not a result of active sarcoid, especially considering that if ACE levels are originally elevated with sarcoidosis, they are almost always elevated again when the disease recurs.14 Normal levels of ACE in sarcoid patients with previously elevated ACE levels should therefore prompt a search for an alternate diagnosis.
This case is an example of the therapy causing a complication that mimics the disease it was intended to cure. When any patient deteriorates while on steroids, the clinician must ask the age‐old question: should more steroids be prescribed or less? As in this case, the answer is not always apparent. Safe decisions in these situations demand awareness of the opportunistic infections endemic to the area and a willingness to perform early invasive procedures (in this case bronchoscopy) to obtain samples to make a definitive diagnosis. By doing so, the devastating chain of events that occurred here hopefully can be avoided.
Acknowledgements
The authors would like to acknowledge Dr. Eleanor Latta and Dr. Serge Jothy, Department of Pathology, St. Michael's Hospital, University of Toronto, for contributing the pathological images.
A 45‐year‐old man who immigrated to Canada from Ghana at the age of 33 presented with a 2‐year history of progressive cognitive changes. He had bifrontal headache, right‐sided scalp paresthesias, and a 40‐pound weight loss. He was unable to perform his job as an auto parts worker. His wife noticed short‐ and long‐term memory problems and poor concentration. On physical exam he had no focal neurological findings but his score on the Mini‐Mental Status Exam (MMSE) was 23/30, with deficits in attention and recall.
The first important element of this illness is its chronicity. His symptoms progressed slowly over 2 years. Second, aside from his neurological problems, he is an otherwise healthy young, African‐born male. This clinical picture could be the early presentation of a demyelinating, infiltrative, or vascular illness. If vascular, it is more likely a vasculitis than atherosclerotic disease. Malignancy and infection are definitely in the differential, but at this point, I think they are less likely to be the cause, given the tempo of presentation. I would begin my investigations with basic blood work and a computerized tomography (CT) scan of his brain.
A CT scan of the head with contrast demonstrated an enlarged left lateral ventricle with no evidence of obstruction in the foramen of Munro.
The radiological findings of communicating hydrocephalus with normal parenchyma imply a disease that affects the leptomeningeal space. Given that we are looking at an illness that can change cerebral spinal fluid (CSF) flow rather than primary parenchymal disease, demyelinating and vascular illnesses are less likely etiologies, and infiltrative diseases move up on my list. Malignancy and infectious diseases remain in the differential.
He disappeared to follow up for 1 year, during which he returned to Ghana and experienced progressive neurological deterioration, with incontinence, gait instability, and inability to converse clearly and perform activities of daily living. On his return to Canada, an urgent CT scan and magnetic resonance imaging (MRI) of the brain demonstrated ongoing and unchanged hydrocephalus with aqueductal stenosis. A referral was made to a neurosurgeon for insertion of a ventriculoperitoneal shunt. A routine preoperative chest radiograph demonstrated new bilateral upper‐zone reticulonodular changes.
He had no respiratory symptoms, fevers, or lymphadenopathy. His occupational history revealed no exposure to asbestos, silica, farms, or mines. He had no history of either respiratory or neurological illness in the past and no travel other than to Ghana and Toronto. When he immigrated to Toronto, Canada, 12 years before, he had a normal chest radiograph and negative PPD tuberculin skin test.
Many illnesses produce asymptomatic changes on chest x‐ray. Oslerian principles would suggest that we should think of a single diagnosis to explain both nodular lung disease and more than 3 years of a progressive disease affecting the leptomeninges. It is unlikely that tuberculosis, other fungal diseases, or malignancy would result in the chest and brain pathology over a 3‐year period without other sequelae. Sarcoidosis could cause both chronic leptomeningeal changes and the radiographic lung findings. The next steps in investigating this patient should include measurement of angiotensin‐converting enzyme (ACE) and serum calcium and pulmonary function tests. I would ultimately send him for a pathological biopsy of his lung tissue to confirm noncaseating granuloma and exclude infection and malignancy.
Complete blood count, renal and liver biochemistry, and calcium were normal. An ACE level was elevated at 69 g/L (normal < 40 g/L). A human immunodeficiency virus (HIV‐1 and HIV‐2) test, tuberculin skin test, and syphilis serology were negative. A CT scan of the chest demonstrated bilateral upper‐zone reticulonodular changes and diffuse lymphadenopathy (Fig. 1). Pulmonary function tests (PFTs) demonstrated a forced expiratory volume 1 (FEV1) of 3.4 L (94%), forced vital capacity (FVC) of 4.0 L (83%), an FEV1/FVC of 87%, total lung capacity (TLC) of 92% predicted, and diffusion capacity (DLCO) of 67% predicted. An MRI with gadolinium (Fig. 2) demonstrated hydrocephalus, mild basal leptomeningeal enhancement around the perivascular spaces into the subinsular region, and an increased T2 signal in periventricular white matter.


A bronchoscopy with bronchoalveolar lavage and transbronchial biopsies were performed. Pathology (Fig. 3) demonstrated non‐caseating epitheliod granulomas, with negative special stains for acid‐fast bacilli (AFB) and fungus, and negative fungal and AFB cultures of the bronchial alveolar lavage.
With negative tests for infectious causes such as tuberculosis, I think there is now enough evidence that this patient has sarcoidosis involving the lung and leptomeninges. At this point I would start therapy with steroids.

The patient was started on prednisone 40 mg po qd, and his neurological symptoms improved markedly over the course of 1‐2 months, with normalization of his MMSE and a return to cognitive baseline. As his symptoms stabilized with no change in CT imaging, he returned to work, and over the course of 2 years his prednisone dosage was tapered to 10 mg po od. While on prednisone he developed hypertension and hyperglycemia. He continued to have no respiratory symptoms.
He was cognitively at baseline until 20 months later, when he was readmitted to the hospital with a 2‐week history of worsening headache, increased confusion, poor memory, and wandering. His MMSE had deteriorated to 19/30, with deficits again in memory and attention.
First, we can say with reasonable confidence that the diagnosis of sarcoid was correct. His long and sustained response to steroids, plus the absence of the unmasking of an infectious or malignant disease, supports this conclusion. However, he is now exhibiting an apparent relapse that mimics his presentation 3 years earlier. The question is whether he is suffering from a flare of his disease or whether a second illness has occurred. The most obvious second illness is an opportunistic infection after years of steroid use. I would certainly repeat the angiotensin‐converting enzyme and serum calcium tests and repeat the imaging of his lungs and central nervous system. He also warrants a lumbar puncture with CSF culture, stain, and PCR for opportunistic infections. If these studies are inconclusive and do not specifically suggest relapsing sarcoid, I would once again consider biopsy of tissue from either a lung or leptomeninges.
An MRI with gadolinium looked unchanged from the previous one. A lumbar puncture was performed, and his CSF demonstrated 3 WBCs, no RBCs, normal glucose, and elevated protein at 1.17 g/L, and tests for bacteria, TB, fungi, and viruses were all negative. Repeat blood work was unremarkable, and the ACE level was 2 g/L.
A chest radiograph (Fig. 4a) and CT chest (Fig. 4b) showed marked deterioration, with increased diffuse airspace opacities, interstitial nodularity, and small apical bullae. His PFTs showed some deterioration, with FEV1 2.52 L (73%), FVC 3.29 L (73%), FEV1/FVC 76%, TLC 70% predicted, DLCO 72% predicted. However, he still had no respiratory symptoms.

The changes on lumbar puncture are nonspecific. The ACE level is now very low, making sarcoidosis unlikely but not impossible. The chest imaging shows features, specifically interstitial nodularity, consistent with ongoing or relapsing sarcoidosis, but the extensive apical bullae are not characteristic. My best guess is that this patient's illness is not simply relapsing sarcoid but represents superimposed opportunistic infectious disease. I would not reintroduce steroids without pursuing a definitive diagnosis with tissue pathology.
He was placed on prednisone 60 mg po qd and started on trimethoprim‐sulfamethoxazole for Pneumocystis pneumonia (PCP) prophylaxis. He showed modest improvement in his neurological status. A repeat bronchoscopy was not performed. Four months later he was seen by his pulmonologist. He remained without respiratory symptoms and was neurologically unchanged, and a chest radiograph showed no change. He was continued on prednisone 60 mg po qd.
Three weeks later, he was admitted to the hospital with a 2‐week history of anorexia, fatigue, night sweats, right‐sided pleuritic chest pain with productive cough, increasing dyspnea, and no hemoptysis. On admission he was hypoxic with evidence of respiratory distress, and his chest radiograph showed evidence of new right‐sided airspace disease with an associated large right pleural effusion. Initial labs demonstrated a leukocytosis.
I am now very suspicious that this illness is not relapsed sarcoidosis based on his prior clinical response to high‐dose prednisone and that he currently is showing no neurological improvement. His recent clinical deterioration on this very high dose of prednisone makes me think that opportunistic lung infection or disseminated disease is definitely the cause, although the differential is broad. In addition to the typical viral and bacterial causes of community‐acquired pneumonia, this could be caused by unusual bacterial pathogens, tuberculosis, nontuberculous mycobacteria, or fungal diseases including Candida, Aspergillus and dimorphic fungi. I would begin empiric therapy with antibiotics, obtain pleural fluid for examination and culture, and blood cultures.
The patient was treated with a respiratory fluoroquinolone, and blood and sputum cultures were performed. A right thoracentesis removed 300 cc of yellow exudate, with negative gram stain and initial culture. Over the next 24 hours, the patient deteriorated rapidly, with progressive hypoxia and clinical and radiological (Fig. 5) evidence of acute respiratory distress syndrome (ARDS). He required endotracheal intubation with mechanical ventilation.

He has a progressive illness not responsive to broad‐spectrum antibiotics, and he has deteriorated. At this point it is imperative that he undergo bronchoscopy and transbronchial biopsy.
Bronchoscopy demonstrated secretions from the right lower lobe. Gram stain from a bronchoalveolar lavage from the right lower lobe was negative, and cultures showed no growth after 24 hours. Immediately after bronchoscopy a third‐generation cephalosporin was empirically added. The next day the patient developed hypotension and was started on norepinephrine. Over the subsequent 48 hours, he developed progressive multiorgan failure. Despite multiple vasopressors, high‐frequency oscillator ventilation, broad‐spectrum antimicrobials, and activated protein C, he died in the intensive care unit. At the time of death, all blood cultures were negative, abdominal CT scans showed no intraabdominal infections, and the BAL performed on admission demonstrated negative gram stain, fungal stain, AFB stain, and PCP and no growth from fungal or bacterial cultures.
I think it is an unavoidable conclusion that this man's progressive systemic inflammatory response syndrome and ultimate multiorgan failure was caused by an opportunistic pathogen that was not antibiotic responsive and not identified from the extensive range of infectious disease studies performed. Despite all the negative studies, there still might be either mycobacterial illness or fungal illness. With negative cultures, Candida or Aspergillus infection is unlikely. Other opportunistic fungi like Blastomyces, Histoplasma, and Cryptococcus are certainly in the differential because these organisms can be notoriously difficult to detect on routine surveying such as bronchoalveolar lavage or lumbar puncture. Blastomyces and Histoplasma are both endemic in the area of Canada where the patient resided. I would also keep the zygomycoses in the differential.
Five days after death, fungal culture was reported demonstrating Blastomyces dermatitidis. Postmortem demonstrated disseminated blastomycosis causing severe bilateral pneumonia (Fig. 6a), empyema of right lung, and involvement of the thyroid, heart, liver, spleen, and kidneys. There was also evidence of active CNS blastomycosis involving the meninges and cerebral cortex and diencephalon (Fig. 6b). As well as active blastomycosis, the leptomeninges demonstrated fibrosis and old granulomas that did not contain an organism.

COMMENTARY
This case describes a 45‐year‐old man who presented with chronic cognitive symptoms associated with hydrocephalus. The first step in establishing the diagnosis was made by realizing that a communicating hydrocephalus with no parenchymal CNS disease was highly suggestive of a leptomeningeal process. This narrowed the differential diagnosis to an infiltrative disease affecting the leptomeninges. The next step involved the discovery of an upper‐lobe interstitial lung process, establishing sarcoidosis as the most likely unifying diagnosis. This was confirmed with transbronchial biopsies showing noncaseating granulomas and by the sustained response to treatment with corticosteroids. Unfortunately, after a 2‐year remission, he developed a recurrence of both the neurological and respiratory findings. When his symptoms progressed despite higher doses of corticosteroids, it became apparent that the etiology of his clinical deterioration was not recurrent disease. Instead, the deterioration was caused by disseminated blastomycosis, an opportunistic infection that developed as a result of the immunosuppressants used to treat the sarcoidosis.
With the final diagnosis of blastomycosis, one question about this case becomes: Could it have been blastomycosis and not sarcoid that was responsible for his original neurological presentation? Blastomyces dermatitidis is a thermally dimorphic fungus that causes disease from inhalation of airborne spores found in soil. Areas of North America in which it is endemic include regions bordering the Mississipi and Ohio rivers, as well as the areas bordering the Great Lakes.1 The patient in this case lived in metropolitan Toronto, on Lake Ontario, where blastomycosis is an important yet underreported disease.24 He likely was exposed to blastomyces in Toronto, which in immunocompromised patients may be followed after weeks to months by dissemination to other body sites including the dermis, bones, joints, urogenital system, and, rarely, the central nervous system (CNS) and liver.5 Like sarcoidosis, infection with blastomycosis can produce pathologic evidence of noncaseating granulomatous inflammation. However, as the discussant astutely pointed out, it would be unusual for this patient to have clinically inapparent blastomycosis for almost 2 years while on high‐dose prednisone. The initial diagnosis of sarcoid likely was correct.
CNS disease caused by Blastomyces dermatitidis is quite rare, with only 22 reported cases of meningoencephalitis in the literature.6 As this case demonstrates, CNS blastomycosis is very difficult to diagnose because of the absence of sensitive serologic markers and the difficulty of isolating the organism from blood and cerebrospinal fluid. CSF sampling from lumbar puncture led to its diagnosis in only 2 of the 22 reported cases.7 Furthermore, reliable CSF cultures are usually only obtained via ventricular sampling or tissue biopsy, which itself is limited by the organism's predilection for deep structures of the cerebrum, midbrain, and basal meninges.6 Blastomyces involving the CNS rarely occurs in isolation. In the patient's case, during his neurological deterioration, there was clear radiological evidence of progressive pulmonary pathology despite his being asymptomatic, and as the discussant suggests, pulmonary investigations were warranted.
Pulmonary manifestations of blastomycosis are variable. Acute infections most commonly resemble pneumonia, whereas chronic disease may show reticulonodular changes indistinguishable from sarcoidosis. Severe cases have been shown to progress to respiratory failure with acute respiratory distress syndrome (ARDS).1 The diagnosis is usually established through culture of noninvasive (sensitivity 86%) or bronchoalveolar lavage (sensitivity 92%) specimens.8 However, blastomyces will take between 5 and 30 days to grow in culture.1 In cases where the diagnosis needs to be established quickly, a KOH smear can be done looking for broad‐based budding yeast. Although the yield of this test is lower (0%‐50%), the results can be available within 24 hours.9 As these tests are not always routinely performed, direct communication with the pathologist is recommended if a rapid diagnosis is needed.
The major challenge of this case lay in distinguishing between the recurrence of an old disease and the complications of its treatment. In this case the discussant addresses strategies that might be useful in differentiating recurrent sarcoidosis from an opportunistic infection like blastomycosis. The first issue is the steroid therapy. The exact dose of steroids required to compromise the immune system enough to yield infections is not known. However, in a meta‐analysis of 71 controlled clinical trials performed with steroids, Stuck et. al. were able to show that the occurrence of opportunistic infections depended on both the amount of daily steroid and the cumulative dose.10 Opportunistic infections were unlikely to occur in patients given a mean daily dose of less than 10 mg/day or a cumulative dose of less than 700 mg of prednisone. Although the patient in the present case was only on 10 mg/day of prednisone, his mean daily dose was more than 10 mg/day, and his cumulative dose far exceeded 700 mg. Therefore, an opportunistic infection should have been strongly considered.
The other item used to help distinguish between the 2 diseases was serum angiotensin‐converting enzyme (ACE) level. ACE is an enzyme produced by the epithelial cells of the granulomas in sarcoidosis. ACE alone is inadequate for diagnosis, with a reported sensitivity of 40%‐90%, depending on the population studied and on the definition of normal.1114 Even an ACE level more than twice the normal is not diagnostic for sarcoidosis, with elevated levels found in histoplasmosis, silicosis, tuberculosis, Gaucher's disease, and other disorders.15 Rather than as a diagnostic test, ACE level instead is used to follow disease activity in sarcoidosis, as ACE level often reflects the granuloma burden.1618 The low levels at the initial recurrence suggests the symptoms were not a result of active sarcoid, especially considering that if ACE levels are originally elevated with sarcoidosis, they are almost always elevated again when the disease recurs.14 Normal levels of ACE in sarcoid patients with previously elevated ACE levels should therefore prompt a search for an alternate diagnosis.
This case is an example of the therapy causing a complication that mimics the disease it was intended to cure. When any patient deteriorates while on steroids, the clinician must ask the age‐old question: should more steroids be prescribed or less? As in this case, the answer is not always apparent. Safe decisions in these situations demand awareness of the opportunistic infections endemic to the area and a willingness to perform early invasive procedures (in this case bronchoscopy) to obtain samples to make a definitive diagnosis. By doing so, the devastating chain of events that occurred here hopefully can be avoided.
Acknowledgements
The authors would like to acknowledge Dr. Eleanor Latta and Dr. Serge Jothy, Department of Pathology, St. Michael's Hospital, University of Toronto, for contributing the pathological images.
- ,,.Blastomycosis.Infect Dis Clin North Am.2003;17(1):21,40, vii.
- ,,,,,.Novel cases of blastomycosis acquired in Toronto, Ontario.CMAJ.2000;163:1309–1312.
- ,,,,,.Blastomycosis acquired by three children in Toronto.Can J Infect Dis Med Micro.2002;13(4):259–263.
- ,,, et al.Blastomycosis in Ontario, 1994‐2003.Emerg Infect Dis.2006;12(2):274–279.
- ,,, et al.Epidemiology and clinical spectrum of blastomycosis diagnosed at Manitoba hospitals.Clin Infect Dis.2002;34:1310–1316.
- ,,,.Meningoencephalitis due to Blastomyces dermatitidis: case report and literature review.Mayo Clin Proc.2000;75:403–408.
- ,,,.Chronic blastomycotic meningitis.Am J Med.1981;71:501–505.
- ,.Pulmonary blastomycosis: an appraisal of diagnostic techniques.Chest.2002;121:768–773.
- ,,.Blastomycosis as an etiology of acute lung injury.South Med J.1998;91:861–863.
- ,,.Risk of infectious complications in patients taking glucocorticosteroids.Rev Infect Dis.1989;11:954–963.
- .Elevation of serum angiotensin‐converting‐enzyme (ACE) level in sarcoidosis.Am J Med.1975;59:365–372.
- ,,,.Elevated serum angiotensin I converting enzyme in sarcoidosis.Am Rev Respir Dis.1976;114:525–528.
- ,,.Serum angiotensin‐converting enzyme (SACE) in sarcoidosis and other granulomatous disorders.Lancet.1978;2:1331–1334.
- ,.Serum angiotensin converting enzyme in sarcoidosis: sensitivity and specificity in diagnosis: correlations with disease activity, duration, extra‐thoracic involvement, radiographic type and therapy.Q J Med.1985;55(218):253–270.
- Statement on sarcoidosis.Joint Statement of the American Thoracic Society (ATS), theEuropean Respiratory Society (ERS) and theWorld Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999.Am J Respir Crit Care Med.1999;160:736–755.
- ,,.Value of serial measurement of serum angiotensin converting enzyme in the management of sarcoidosis.Am J Med.1981;70(1):44–50.
- ,,,.Serum angiotensin‐converting enzyme (SACE) activity as an indicator of total body granuloma load and prognosis in sarcoidosis.Sarcoidosis.1987;4(2):142–148.
- ,,.Serum angiotensin converting enzyme in sarcoidosis: clinical significance.Isr J Med Sci.1977;13:1001–1006.
- ,,.Blastomycosis.Infect Dis Clin North Am.2003;17(1):21,40, vii.
- ,,,,,.Novel cases of blastomycosis acquired in Toronto, Ontario.CMAJ.2000;163:1309–1312.
- ,,,,,.Blastomycosis acquired by three children in Toronto.Can J Infect Dis Med Micro.2002;13(4):259–263.
- ,,, et al.Blastomycosis in Ontario, 1994‐2003.Emerg Infect Dis.2006;12(2):274–279.
- ,,, et al.Epidemiology and clinical spectrum of blastomycosis diagnosed at Manitoba hospitals.Clin Infect Dis.2002;34:1310–1316.
- ,,,.Meningoencephalitis due to Blastomyces dermatitidis: case report and literature review.Mayo Clin Proc.2000;75:403–408.
- ,,,.Chronic blastomycotic meningitis.Am J Med.1981;71:501–505.
- ,.Pulmonary blastomycosis: an appraisal of diagnostic techniques.Chest.2002;121:768–773.
- ,,.Blastomycosis as an etiology of acute lung injury.South Med J.1998;91:861–863.
- ,,.Risk of infectious complications in patients taking glucocorticosteroids.Rev Infect Dis.1989;11:954–963.
- .Elevation of serum angiotensin‐converting‐enzyme (ACE) level in sarcoidosis.Am J Med.1975;59:365–372.
- ,,,.Elevated serum angiotensin I converting enzyme in sarcoidosis.Am Rev Respir Dis.1976;114:525–528.
- ,,.Serum angiotensin‐converting enzyme (SACE) in sarcoidosis and other granulomatous disorders.Lancet.1978;2:1331–1334.
- ,.Serum angiotensin converting enzyme in sarcoidosis: sensitivity and specificity in diagnosis: correlations with disease activity, duration, extra‐thoracic involvement, radiographic type and therapy.Q J Med.1985;55(218):253–270.
- Statement on sarcoidosis.Joint Statement of the American Thoracic Society (ATS), theEuropean Respiratory Society (ERS) and theWorld Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999.Am J Respir Crit Care Med.1999;160:736–755.
- ,,.Value of serial measurement of serum angiotensin converting enzyme in the management of sarcoidosis.Am J Med.1981;70(1):44–50.
- ,,,.Serum angiotensin‐converting enzyme (SACE) activity as an indicator of total body granuloma load and prognosis in sarcoidosis.Sarcoidosis.1987;4(2):142–148.
- ,,.Serum angiotensin converting enzyme in sarcoidosis: clinical significance.Isr J Med Sci.1977;13:1001–1006.
Handoffs
I was a newly appointed head of a department of medicine. Supervising the care of 44 patients and instructing interns and residents was a new and thrilling experience. Some patients presented complex problems, which satisfied my detective instincts and provided a stimulating intellectual challenge. Many others were less intellectually demanding, but I loved the personal interaction, the ability to change things for the better, and the endless variability.
It amazes me to reflect on how uncritical I was at the time, adopting and following common clinical practices with little questioning. It never really crossed my mind that medicine could be practiced in a different and better way. When I managed after some months to set aside one day a week to continue basic research, I was overjoyed. On that day, I became a scientist, putting each assumption to rigorous testing. At the hospital however, I was much more self‐assured and complacent. It was during a break between experiments at the research institute that I slumped wearily into an armchair in the library and picked up a shabby copy of the Green Journal. Being too tired for anything serious, I started reading what looked like a fairy tale. It was titled In a stew, by Michael LaCombe, whom I knew to be a gifted medical writer.1 Soon I found myself immersed in the story. The princess is seriously sick, and all the court doctors are baffled. She already has had 4 CT scans, 3 MRIs, and dozens of other tests. All the tests were fine, but the princess remains very sick, and the king is terribly worried. Then, somebody remembers an old, forgotten clinician who has been relegated to a small dusty den somewhere in the basement. For his services to be rendered, all he demands is that someone find him his stethoscope and that he be allowed to have a pupil. Using observation, knowledge, and wisdom (but no further tests), he elegantly elicits the relevant history and makes the correct diagnosis, which has eluded all the sophisticated court doctors armed with their batteries of high‐tech tests but with little regard for old‐fashioned clinical methods.
This was good fun, but though I enjoyed it very much, I had no idea that it would remain in my mind and shape my thinking, my practice, and my teaching. Nevertheless, I gradually found myself during rounds reflecting on this story with the patient who had had 2 CT scans done before anyone bothered to listen to him or examine him and with the patient who had been studied for months before a simple fact that should have been noted at once was finally revealed, which led to a single test that was diagnostic and to the patient's recovery.2 Then there was the patient who underwent a procedure, which looked innocent enough, but resulted in an adverse event that cascaded into months of life‐threatening illness.3 Was the procedure really necessary?
One night, a couple of years later, I woke up and instead of going back to sleep, sat in the silent living room, suddenly thinking of our departmental routine and realizing somehow that many things we physicians do may be seriously flawed: taking a superficial history and performing a perfunctory exam; having a light finger on the trigger of test ordering even if imaging and tests may mean little out of the clinical context and often beget more unnecessary testing; skipping significant information only because it is not immediately available but has to be found at another hospital or clinic or by calling the primary physician; disregarding the ubiquitous and influential emotional aspect or the patient's perspective and health literacy, which are essential for shared decisions; and the repeated underuse4 of highly effective medications and especially of proven preventive measures that are not pharmacological and hence not vigorously promoted by the large pharmaceutical companies.
The seed for this heresy was sown by the fable, and it colored my clinical life with a vein of skepticism and self‐criticism. Slowly it also grew into a long‐term commitment to teaching about and research on avoidable pitfalls in patient care.5
Thus, Lacombe's little piece often comes back to me, teaching me that a fairy tale can sometimes be more powerful than a randomized controlled study of 10,000 patients.
- .In a stew.Am J Med.1991;91:276–278.
- ,,,.Asking the right question.Lancet.2003;361:1786.
- .Down the cascade.Br Med J.2004;329:678.
- .The need for perspective in evidence‐based medicine.JAMA.1999;282:2358–2365.
- ,.Pearls and pitfalls in patient care: the need to revive traditional clinical values.Am J Med Sci.2004;327:79–85.
I was a newly appointed head of a department of medicine. Supervising the care of 44 patients and instructing interns and residents was a new and thrilling experience. Some patients presented complex problems, which satisfied my detective instincts and provided a stimulating intellectual challenge. Many others were less intellectually demanding, but I loved the personal interaction, the ability to change things for the better, and the endless variability.
It amazes me to reflect on how uncritical I was at the time, adopting and following common clinical practices with little questioning. It never really crossed my mind that medicine could be practiced in a different and better way. When I managed after some months to set aside one day a week to continue basic research, I was overjoyed. On that day, I became a scientist, putting each assumption to rigorous testing. At the hospital however, I was much more self‐assured and complacent. It was during a break between experiments at the research institute that I slumped wearily into an armchair in the library and picked up a shabby copy of the Green Journal. Being too tired for anything serious, I started reading what looked like a fairy tale. It was titled In a stew, by Michael LaCombe, whom I knew to be a gifted medical writer.1 Soon I found myself immersed in the story. The princess is seriously sick, and all the court doctors are baffled. She already has had 4 CT scans, 3 MRIs, and dozens of other tests. All the tests were fine, but the princess remains very sick, and the king is terribly worried. Then, somebody remembers an old, forgotten clinician who has been relegated to a small dusty den somewhere in the basement. For his services to be rendered, all he demands is that someone find him his stethoscope and that he be allowed to have a pupil. Using observation, knowledge, and wisdom (but no further tests), he elegantly elicits the relevant history and makes the correct diagnosis, which has eluded all the sophisticated court doctors armed with their batteries of high‐tech tests but with little regard for old‐fashioned clinical methods.
This was good fun, but though I enjoyed it very much, I had no idea that it would remain in my mind and shape my thinking, my practice, and my teaching. Nevertheless, I gradually found myself during rounds reflecting on this story with the patient who had had 2 CT scans done before anyone bothered to listen to him or examine him and with the patient who had been studied for months before a simple fact that should have been noted at once was finally revealed, which led to a single test that was diagnostic and to the patient's recovery.2 Then there was the patient who underwent a procedure, which looked innocent enough, but resulted in an adverse event that cascaded into months of life‐threatening illness.3 Was the procedure really necessary?
One night, a couple of years later, I woke up and instead of going back to sleep, sat in the silent living room, suddenly thinking of our departmental routine and realizing somehow that many things we physicians do may be seriously flawed: taking a superficial history and performing a perfunctory exam; having a light finger on the trigger of test ordering even if imaging and tests may mean little out of the clinical context and often beget more unnecessary testing; skipping significant information only because it is not immediately available but has to be found at another hospital or clinic or by calling the primary physician; disregarding the ubiquitous and influential emotional aspect or the patient's perspective and health literacy, which are essential for shared decisions; and the repeated underuse4 of highly effective medications and especially of proven preventive measures that are not pharmacological and hence not vigorously promoted by the large pharmaceutical companies.
The seed for this heresy was sown by the fable, and it colored my clinical life with a vein of skepticism and self‐criticism. Slowly it also grew into a long‐term commitment to teaching about and research on avoidable pitfalls in patient care.5
Thus, Lacombe's little piece often comes back to me, teaching me that a fairy tale can sometimes be more powerful than a randomized controlled study of 10,000 patients.
I was a newly appointed head of a department of medicine. Supervising the care of 44 patients and instructing interns and residents was a new and thrilling experience. Some patients presented complex problems, which satisfied my detective instincts and provided a stimulating intellectual challenge. Many others were less intellectually demanding, but I loved the personal interaction, the ability to change things for the better, and the endless variability.
It amazes me to reflect on how uncritical I was at the time, adopting and following common clinical practices with little questioning. It never really crossed my mind that medicine could be practiced in a different and better way. When I managed after some months to set aside one day a week to continue basic research, I was overjoyed. On that day, I became a scientist, putting each assumption to rigorous testing. At the hospital however, I was much more self‐assured and complacent. It was during a break between experiments at the research institute that I slumped wearily into an armchair in the library and picked up a shabby copy of the Green Journal. Being too tired for anything serious, I started reading what looked like a fairy tale. It was titled In a stew, by Michael LaCombe, whom I knew to be a gifted medical writer.1 Soon I found myself immersed in the story. The princess is seriously sick, and all the court doctors are baffled. She already has had 4 CT scans, 3 MRIs, and dozens of other tests. All the tests were fine, but the princess remains very sick, and the king is terribly worried. Then, somebody remembers an old, forgotten clinician who has been relegated to a small dusty den somewhere in the basement. For his services to be rendered, all he demands is that someone find him his stethoscope and that he be allowed to have a pupil. Using observation, knowledge, and wisdom (but no further tests), he elegantly elicits the relevant history and makes the correct diagnosis, which has eluded all the sophisticated court doctors armed with their batteries of high‐tech tests but with little regard for old‐fashioned clinical methods.
This was good fun, but though I enjoyed it very much, I had no idea that it would remain in my mind and shape my thinking, my practice, and my teaching. Nevertheless, I gradually found myself during rounds reflecting on this story with the patient who had had 2 CT scans done before anyone bothered to listen to him or examine him and with the patient who had been studied for months before a simple fact that should have been noted at once was finally revealed, which led to a single test that was diagnostic and to the patient's recovery.2 Then there was the patient who underwent a procedure, which looked innocent enough, but resulted in an adverse event that cascaded into months of life‐threatening illness.3 Was the procedure really necessary?
One night, a couple of years later, I woke up and instead of going back to sleep, sat in the silent living room, suddenly thinking of our departmental routine and realizing somehow that many things we physicians do may be seriously flawed: taking a superficial history and performing a perfunctory exam; having a light finger on the trigger of test ordering even if imaging and tests may mean little out of the clinical context and often beget more unnecessary testing; skipping significant information only because it is not immediately available but has to be found at another hospital or clinic or by calling the primary physician; disregarding the ubiquitous and influential emotional aspect or the patient's perspective and health literacy, which are essential for shared decisions; and the repeated underuse4 of highly effective medications and especially of proven preventive measures that are not pharmacological and hence not vigorously promoted by the large pharmaceutical companies.
The seed for this heresy was sown by the fable, and it colored my clinical life with a vein of skepticism and self‐criticism. Slowly it also grew into a long‐term commitment to teaching about and research on avoidable pitfalls in patient care.5
Thus, Lacombe's little piece often comes back to me, teaching me that a fairy tale can sometimes be more powerful than a randomized controlled study of 10,000 patients.
- .In a stew.Am J Med.1991;91:276–278.
- ,,,.Asking the right question.Lancet.2003;361:1786.
- .Down the cascade.Br Med J.2004;329:678.
- .The need for perspective in evidence‐based medicine.JAMA.1999;282:2358–2365.
- ,.Pearls and pitfalls in patient care: the need to revive traditional clinical values.Am J Med Sci.2004;327:79–85.
- .In a stew.Am J Med.1991;91:276–278.
- ,,,.Asking the right question.Lancet.2003;361:1786.
- .Down the cascade.Br Med J.2004;329:678.
- .The need for perspective in evidence‐based medicine.JAMA.1999;282:2358–2365.
- ,.Pearls and pitfalls in patient care: the need to revive traditional clinical values.Am J Med Sci.2004;327:79–85.
Physician Attitudes and Use of Computerized Order Entry
It is widely acknowledged that the U.S. health care system is plagued by error and inefficiency and that these factors contribute to as many as 44,000‐98,000 deaths each year in U.S. hospitals. In To Err Is Human: Building a Safer Health System, the Institute of Medicine1 outlined the critical role that information technology can play in improving patient safety and highlighted computerized physician order entry (CPOE) systems for their potential to reduce the frequency of medication errors and to improve the quality of medical care.
Computerized physician order entry systems are specialized software applications that allow physicians to place orders directly into a computer. This process has a number of potential advantages over traditional handwritten ordering, including the ability to structure the ordering process to ensure the completeness of individual orders, to provide clinical decision support through diagnosis‐based order sets, and to automatically check orders for potential drugallergy, drugdrug, and drugfood interactions.2 Finally, entering orders directly into a computer eliminates the problem of transcription‐related errors that stem from the difficulty of interpreting handwriting. In clinical trials, the introduction of CPOE has been shown to reduce the frequency of medication errors, to improve the use of preventive services, and to reduce costs.36 Recognition of the benefits of these systems has not been confined to the medical community. The Leapfrog Organization, a coalition of large businesses in the United States, has chosen CPOE as one of its 3 initial safety leaps and has established a threshold that 70% of medication orders should be entered directly by physicians.7
Although the benefits of CPOE systems are widely recognized, few hospitals have implemented these systems successfully.8, 9 Those that have, have often developed the applications internally, and many have relied on house staff to do most or all of the actual ordering.10 However, most hospitals do not have the expertise for internal development and instead rely on commercially available products. Moreover, most patients hospitalized in the United States are cared for by attending physicians working without the assistance of house staff.11 In light of the importance of successfully implementing CPOE systems in such settings, we assessed the adoption of CPOE by attending physicians at 2 community hospitals where its use was voluntary and examined the characteristics and attitudes associated with use of the system to place orders.
METHODS
Setting and Participants
Baystate Medical Center is a 600‐bed teaching hospital in Springfield, Massachusetts, where approximately 50% of patients are cared for with the assistance of house staff. Franklin Medical Center is a 125‐bed community hospital in rural Greenfield, Massachusetts, and is not a house staff training site. Medical staff membership at the 2 hospitals is largely voluntary. Both institutions share a vendor‐supplied computerized order entry system that was implemented in the early 1990s (E7000, Eclipsys Corporation, Boca Raton, FL). The system provides a structured format for the creation of medication, laboratory, and radiology orders and contains thousands of preconstructed medication order sentences and hundreds of order sets designed to standardize ordering for common diagnoses and procedures. Pharmacists are alerted of potential drugallergy and drugdrug interactions and use clinical judgment about whether to communicate this information to the physician. Although the house staff at Baystate Medical Center is mandated to place orders in the system, attending physicians have no such requirement at either institution. Access to the system is provided though the many fixed workstations located on nursing units, in operating rooms, and in the health sciences library. On a typical medical‐surgical patient care unit most computers are behind the nurses' station, though some are distributed along hallways and in physician charting rooms. No computers are in patient rooms. Although the number varies slightly across units, the average ratio of computers to patient beds is roughly 1 to 1.
Survey
In June 2003 we mailed a 20‐item survey to attending physicians who had been responsible for a minimum of 25 orders during the preceding month at either Baystate or Franklin Medical Center. Orders counted toward this minimum if they had been written, given verbally in person or by phone, or entered directly into the computer by the physician. The survey consisted of 20 questions focused on the topic of computerized order entry. In addition to collecting information about sex and specialty, we asked respondents to describe their use of CPOE during training, their use of computers at home, and, where applicable, their use of computers in their outpatient practices. The survey included questions about how often respondents used the order entry system when caring for hospitalized patients and which features of the system they used. To assess physician attitudes about the order entry process, we asked respondents to consider whether it was faster to place orders directly into the system than it was by handwriting them, whether orders placed in the system were carried out more rapidly, whether placing orders in the system led to fewer medication and other errors, whether order sets were important for the efficient use of the system, whether order sets helped to ensure that important aspects of care did not slip through the cracks, whether the system's user interface supported their work flow, and whether the encouragement of nurses was an important factor in their use of the system. Questions that assessed physician attitudes were presented on a 5‐point Likert scale. Nonrespondents were sent reminder letters along with duplicate surveys twice, approximately 1 and 2 months after the initial mailing. No financial incentive was offered for participation. The study protocol was approved by the Institutional Review Board of Baystate Health System.
Order Entry Rates
Regardless of whether an order is placed directly by a physician into a computer, given verbally, or handwritten, all orders are ultimately entered into the CPOE system. Working with our hospitals' Departments of Information Services, we developed a report that provided physician‐specific information about order entry patterns. For each physician in the study, we determined the total number of orders generated during the month preceding the initial June mailing, as well as the absolute number and percentage of orders of each of the following categories: directly entered, telephone, verbal, and written. Because verbal and telephone orders are required during urgent situations and when physicians give orders from outside the hospital, we calculated and report an adjusted order entry rate as the total number of orders placed directly into the system divided by the sum of the orders entered directly and the number of written orders.
Analysis
Summary statistics for the overall sample were constructed using simple frequencies and proportions for categorical variables and medians and interquartile ranges for continuous variables. We compared characteristics of respondents from the 2 hospitals using chi‐square tests of association for categorical factors and Wilcoxon rank‐sum tests for continuous scale data. We compared the total number of orders placed during the study month and the order entry rates of responders and nonresponders using the Wilcoxon rank‐sum test. We categorized physicians as low (20%), intermediate (21%‐79%), and high (80%) users of the system based on their calculated order entry rate. Responses to each of the attitude questions in the survey were tabulated, and the responses strongly agree and agree were combined for analyses comparing responses. Demographic variables and physician attitudes were tested for associations with order entry rate categories via the Pearson chi‐square for categorical factors, the Mantel‐Haenszel chi‐square for ordered factors, and Kruskal‐Wallis analysis of variance for continuous variables. Initial analyses were stratified by hospital; where no differences in association were found across strata, the data were combined. Statistical tests were performed using SAS version 9.1 (SAS Institute, Cary, NC)
RESULTS
During the study period the target group of physicians placed a total of 135,692 orders, of which 69,654 (51%) were placed directly into the CPOE system, 38,878 (29%) were made using pen and paper, 7,208 (5%) were made verbally, and 19,952 (15%) were placed by telephone. Three hundred and fifty‐six (71%) of the 502 surveys sent out to physicians at the 2 hospitals were returned. Thirteen surveys were excluded from analysis because the respondent was not a physician, and 2 because we were unable to match the survey to system usage data, leaving a total of 341 surveys for analysis. Order entry rates were not computed for an additional 3 physicians who only placed verbal and telephone orders during the study period. Response rates did not differ by clinician specialty (P = .53); compared to those of nonresponders, respondents had a similar median total number of orders (111 vs. 101, P = .67) and a higher median order entry rate (66% vs. 48%, P = .03).
Characteristics of Respondents
Seventy‐two percent of physicians who completed the survey were men; half had graduated from medical school at least 20 years ago, and the median duration of practice at the study institution was 11 years (Table 1). Forty percent practiced internal medicine, 18% were surgeons, and 16% were pediatricians. Thirty‐five percent completed training at an institution that had computerized physician order entry, and 86% cared for patients primarily at Baystate Medical Center. More than half reported they used the system many times each day for patient care, and the features they used most commonly were retrieval of results (95%), placing of orders (78%), and viewing and printing of patient lists (75%). Among those with outpatient practices, 81% used computers in their outpatient practice, and more than half used computers for personal activities at home at least once a day. On average, respondents from Franklin Medical Center had graduated from medical school farther in the past and reported less reliance on the system to carry out all activities other than viewing results.
| Overall n (%) | Baystate n (%) 293 (85.9) | Franklin n (%) 48 (14.1) | Chi square P value | |
|---|---|---|---|---|
| ||||
| Sex | .64 | |||
| Male | 244 (71.6) | 211 (72.0) | 33 (68.8) | |
| Specialty | .24 | |||
| Anesthesia | 23 (6.7) | 23 (7.9) | 0 (0.0) | |
| Internal medicine | 135 (39.6) | 112 (38.2) | 23 (47.9) | |
| Medicine/pediatrics | 13 (3.8) | 6 (2.0) | 7 (14.6) | |
| OB/GYN | 36 (10.6) | 30 (10.2) | 6 (12.5) | |
| Pediatrics | 54 (15.8) | 51 (17.4) | 3 (6.3) | |
| Surgery | 61 (17.9) | 55 (18.8) | 6 (12.5) | |
| Other | 19 (5.6) | 16 (5.5) | 3 (6.3) | |
| Use of CPOE systema | .09 | |||
| Many times a day | 176 (52.2) | 160 (55.0) | 16 (34.8) | |
| At least once a day | 77 (22.9) | 61 (21.0) | 16 (34.8) | |
| A few times a week | 55 (16.3) | 45 (15.5) | 10 (21.7) | |
| Once a week or less | 29 (8.6) | 25 (8.6) | 4 (8.7) | |
| Features useda | ||||
| Viewing and printing patient lists | 254 (75.2) | 212 (72.6) | 42 (91.3) | .01 |
| Looking up results | 320 (94.7) | 277 (94.9) | 43 (93.5) | .70 |
| Viewing current medications | 218 (64.5) | 204 (69.9) | 14 (30.4) | < .01 |
| Placing orders | 263 (77.8) | 244 (83.6) | 19 (41.3) | < .01 |
| Entering discharge summaries | 72 (21.3) | 70 (24.0) | 2 (4.4) | < .01 |
| Use of order setsa | ||||
| Rarely or never | 98 (29.0) | 74 (25.3) | 24 (52.2) | < .01 |
| Minority of patients | 92 (27.2) | 78 (26.7) | 14 (30.4) | |
| Majority of patients | 104 (30.8) | 97 (33.2) | 7 (15.2) | |
| For all or nearly all patients | 44 (13.0) | 43 (14.7) | 1 (2.2) | |
| Percentage of orders placed using order setsa | < .01 | |||
| None | 46 (13.7) | 26 (9.0) | 20 (44.4) | |
| 1%‐25% | 62 (18.5) | 50 (17.2) | 12 (26.7) | |
| 26%‐50% | 29 (8.7) | 23 (7.9) | 6 (13.3) | |
| 51%‐75% | 45 (13.4) | 43 (14.9) | 2 (4.4) | |
| 76%‐99% | 103 (30.8) | 98 (33.8) | 5 (11.1) | |
| All | 50 (14.9) | 50 (17.2) | 0 (0.0) | |
| Use of computer in outpatient practiceab | 243 (81.3) | 206 (80.8) | 37 (84.1) | .60 |
| Personal computer usea | .47 | |||
| At least once a day | 209 (61.7) | 185 (63.4) | 24 (51.1) | |
| Several times a week | 84 (24.8) | 67 (23.0) | 17 (36.2) | |
| A few times a month | 21 (6.2) | 18 (6.2) | 3 (6.4) | |
| Rarely | 25 (7.4) | 22 (7.5) | 3 (6.4) | |
| Training at an institution that had CPOE | 117 (34.7) | 105 (36.1) | 12 (26.1) | 0.19 |
| Use of system to enter orders should be mandatorya | ||||
| Yes | 113 (35.2) | 106 (38.4) | 7 (15.6) | <.01 |
| Median (IQR) | Median (IQR) | Median (IQR) | WilcoxonPvalue | |
| Years since medical school graduationa | 20 (13, 26) | 20 (13, 26) | 24 (17, 28) | .02 |
| Years in practice at study institutiona | 11 (5, 18) | 11 (5, 18) | 13 (7, 19) | .39 |
| Orders directly enteredc | 23 (2, 99) | 27 (5, 108) | 1 (0, 27) | < .01 |
| Orders placed by telephonec | 14 (5, 49) | 12 (3, 38) | 49.5 (16, 123.5) | < .01 |
| Orders placed verballyc | 2 (0, 11) | 3 (0, 13) | 1 (0,3) | < .01 |
| Orders placed in writingc | 21 (4, 73) | 14 (3, 45) | 220 (106.5, 391) | < .01 |
| CPOE ratebc | 66% (3%, 94%) | 76% (19%, 96%) | 0.25% (0%, 17%) | < .01 |
Attitudes Toward Computerized Physician Order Entry
Physicians who completed the survey offered diverse opinions about the impact of computerized order entry on work flow, patient safety, and quality of care. Only 22% believed the system's user interface supported their work flow (Q7), 34% believed it was faster to enter orders directly into the system than to handwrite them (Q1), and 41% believed orders placed into the system were carried out more rapidly (Q2) (Table 2). On the other hand, 63% of respondents believed that placing orders directly into the system led to fewer medication errors (Q3), and 51% stated the system generally reduced medical errors (Q4). Sixty‐nine percent stated order sets were important for efficient use of the system (Q5), and 71% believed order sets served an important decision support role (Q6). Twenty‐six percent stated that the encouragement of nurses was an important factor in their use of the system (Q8). Finally, 35% of attending physicians believed use of the system to place orders should be mandatory.
Characteristics and Attitudes of High, Intermediate, and Low Users
The median order entry rate of respondents was 66%. One hundred and forty‐one (42%) placed at least 80% of their orders directly into the system, whereas 109 (32%) placed no more than 20% of their orders directly in the system (Fig. 1). There was not a significant difference between the low, intermediate, and high use groups in the total number of orders that each physician placed during the study period (Table 3). Sex, years since graduation from medical school, years in practice at the study institution, and use of computers in the outpatient setting were not meaningfully different between the 3 categories of users (Table 3). On the other hand, medical specialty was strongly associated with use of the system, with anesthesiologists, pediatricians, and surgeons the specialties with the largest proportion of high users. Furthermore, physicians who were trained in a CPOE environment and those who reported daily use of computers for personal activities showed the highest levels of adoption. Physicians at Franklin Medical Center showed lower levels of order entry than their counterparts at Baystate.
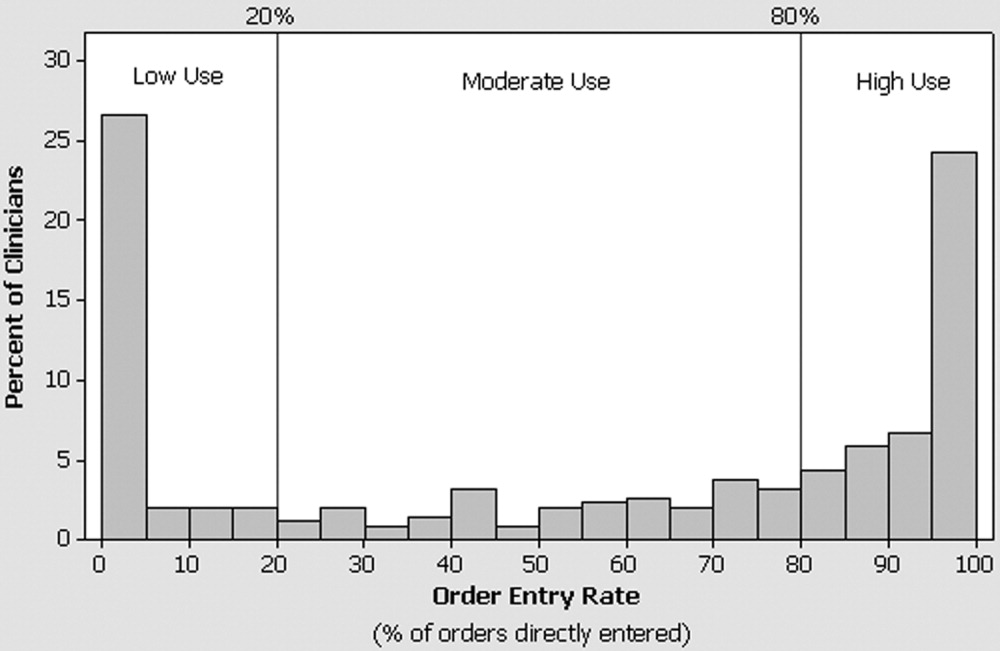
| Low (20%) n (row %) | Intermediate (20%‐79%) n (row %) | High (80%) n (row %) | P value | |
|---|---|---|---|---|
| ||||
| n = 109 | n = 88 | n = 141 | ||
| Hospital | < .01c | |||
| Baystate | 73 (25) | 79 (27) | 138 (48) | |
| Franklin | 36 (75) | 9 (19) | 3 (6) | |
| Sex | .69c | |||
| Female | 28 (29) | 24 (25) | 43 (45) | |
| Male | 81 (33) | 64 (26) | 98 (40) | |
| Specialty | .0001c | |||
| Anesthesia | 8 (35) | 3 (13) | 12 (52) | |
| Internal medicine | 45 (33) | 37 (27) | 53 (39) | |
| Medicine/pediatrics | 6 (46) | 5 (38) | 2 (15) | |
| OB/GYN | 20 (56) | 12 (33) | 4 (11) | |
| Pediatrics | 13 (24) | 9 (17) | 32 (59) | |
| Surgery | 14 (23) | 21 (34) | 26 (43) | |
| Other | 3 (19) | 1 (6) | 12 (75) | |
| Do you use a computer in your outpatient practice?a | ||||
| Yes | 75 (31) | 61 (25) | 105 (44) | .22c |
| No | 20 (36) | 18 (33) | 17 (31) | |
| Level of personal computer useb | .045d | |||
| Rarely | 11 (44) | 8 (32) | 6 (24) | |
| A few times a month | 7 (33) | 4 (19) | 10 (48) | |
| Several times a week | 28 (35) | 25 (31) | 28 (35) | |
| At least once a day | 62 (30) | 50 (24) | 97 (46) | |
| Training at an institution that had CPOE | .037c | |||
| Yes | 30 (26) | 40 (34) | 46 (40) | |
| No | 76 (35) | 48 (22) | 94 (43) | |
| Median (IQR) | Median (IQR) | Median (IQR) | ||
| Years since graduation from medical school | 21 (16, 28) | 18 (14, 25) | 19 (12, 25) | .06e |
| Years in practice at study institution | 12 (5, 19) | 12 (6, 19) | 12 (6, 17) | .84e |
| Total number of orders placed | 112 (45, 306) | 105 (56, 254) | 113 (44, 382) | .92e |
Use of the system was highly associated with physician attitudes toward CPOE, with the views of intermediate and high users consistently different than those of low users (Fig. 2). The associations found held true regardless of hospital: low, intermediate, and high users from Franklin had similar responses to those from Baystate (P > .05 for all questions), and the data from the 2 hospitals therefore were combined for presentation. Although few physicians believed that the user interface of the system supported their work flow, high and intermediate users were 3 times as likely to share this view than were low users (Q7; Fig. 2). Similarly, 19% of low users, 31% of intermediate users, and 45% of high users believed that entering orders into the system was faster than writing orders (Q1). High and intermediate users of the system were more likely than low users to believe that orders entered into the system were carried out more rapidly (Q2) and led to fewer medication (Q3) and nonmedication (Q4) errors. Regardless of their utilization pattern, most physicians believed that order sets played an important role in promoting efficiency and quality.
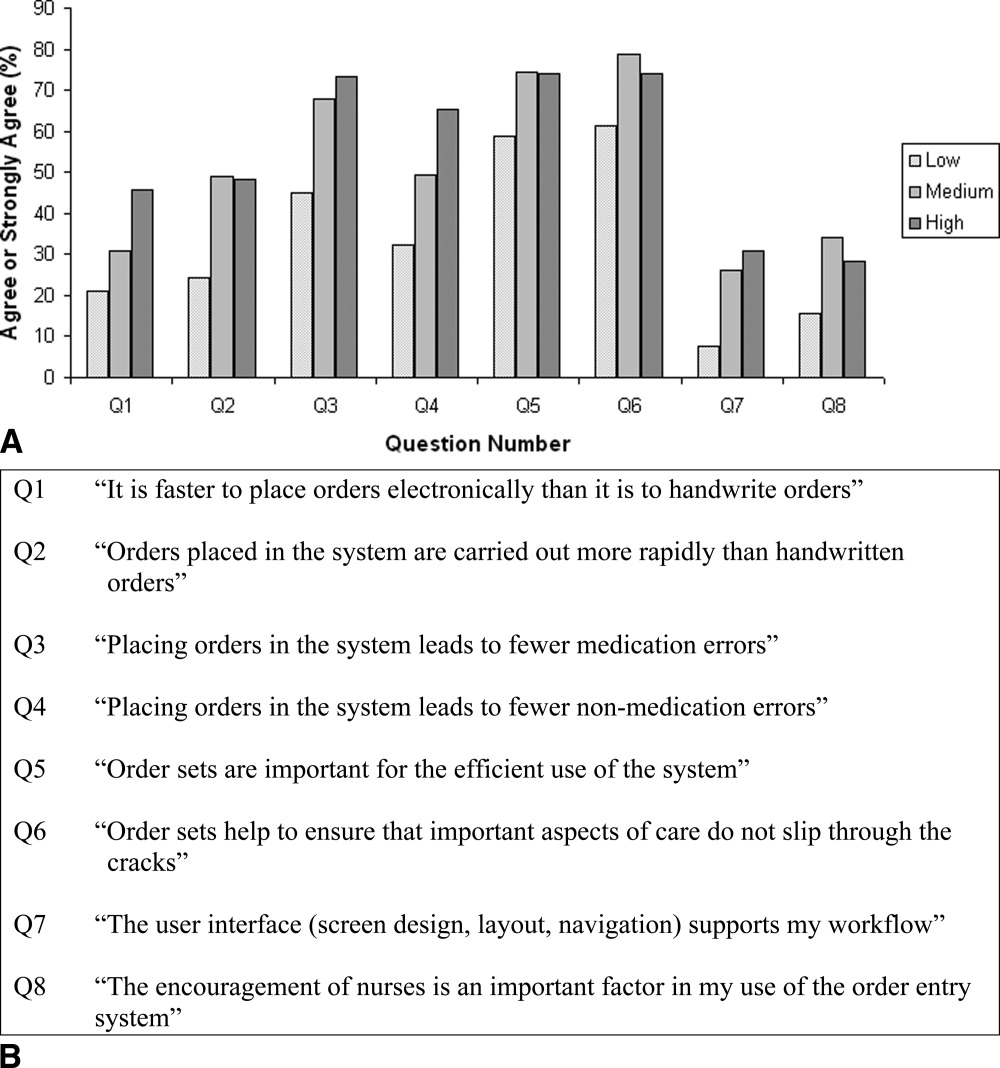
DISCUSSION
In this study of the clinical computing practices of physicians at 2 community hospitals, we observed wide variation in the adoption of CPOE by individual attendings. Although roughly one‐third rarely placed orders directly into the system, 42% had an order entry rate of at least 80%. Contrary to our initial expectation, we found little association between a physician's order entry rate with years in practice, duration of exposure to CPOE, or use of computers in the outpatient setting. On the other hand, we observed marked differences in use of the CPOE system across specialty lines and found that physicians who were exposed to CPOE during training and those who were regular users of computers for personal activities were more likely to embrace this technology. Further, we observed important differences between physicians who used the system to place some or most of their orders and those who did so only rarely in their beliefs and attitudes about the impact and benefits of CPOE. Physicians with higher order entry rates were more likely than their colleagues to believe that placing orders electronically was faster than handwriting and that use of the system led to fewer medical errors. These findings should be encouraging to hospitals hoping to implement CPOE because they suggest that successful adoption of CPOE is not limited to physicians who have just completed their residencies or to hospitals with the capability of designing and building their own systems. On the contrary, we documented that women, older physicians, and those with limited CPOE experience were as likely to be frequent users, especially if they perceived CPOE to be safer than handwriting and if they believed the user interface supported the efficient entering of orders.
On the basis of these results we recommend that in addition to purchasing systems that meet physician work‐flow needs and support the efficient entry of orders, hospital leaders should emphasize the quality and safety benefits of CPOE as part of a comprehensive change management strategy. The differences we observed in order entry rates across specialties may have resulted from several factors, including inherent differences in personality type associated with choice of specialty and in the level of customization of a system reflected in which and how many order sets are included. Such findings suggest that when it comes to CPOE, one size does not fit all, and implementation planning should be carried out at the specialty level. Finally, our observation that physicians who had exposure to CPOE during training were more likely to use the system to place orders suggests that the nation's training institutions will play an important role in fostering universal adoption of this technology.
Several earlier studies have reported on physician experiences with CPOE systems. Murff and Kannry12 surveyed 94 internal medicine house staff to compare experiences with 2 CPOE systems: the Department of Veterans Affairs Computerized Patient Record System (CPRS) and a commercially available product. They found striking differences in user satisfaction with numerous aspects of the systems, however they did not address attitudes toward safety or quality, and because house staff were required to place orders electronically they were unable to correlate responses with actual usage patterns. Weiner et al.13 compared the opinions of internal medicine house staff, attendings, fellows, and nurses about the benefits and challenges of using a computerized provider order entry system. In contrast to the findings from our study, Weiner et al. reported that more than half of physicians believed that provider order entry led to a greater number of errors, and only a minority believed the system increased quality of care overall. Finally, Lee et al.14 surveyed medical and surgical house officers and nurses at a large academic medical center about their satisfaction with a locally developed order entry system. They found that attitudes about the impact of the system on productivity and ease of use were more strongly associated with overall satisfaction than having undergone training or experience with personal computers. These findings are congruous with our own observation that beliefs about the speed with which orders are placed are closely associated with actual use of the system. They reported, as have we, that physicians placed a high value on order sets.
Our study had a number of strengths. First, we were able to offer insight into the attitudes and behaviors of a previously neglected, but critically important groupattending physicians who care for patients at community hospitals without the assistance of house staff. Second, whereas previous studies primarily assessed physician satisfaction with CPOE, we explored how physician attitudes about the impact of CPOE on work flow and on safety were associated with actual ordering habits. Information about ordering was obtained directly from the order entry system and not through self‐report. We conducted the study at 2 hospitals, a large urban community teaching hospital and a smaller rural hospital, and focused on a CPOE system that is in use at many institutions throughout the country, thereby increasing the generalizability of our findings. Although adoption of the system by physicians at the 2 hospitals differed, factors that associated with the use of CPOE to place orders were similar. Finally, we surveyed a large number of physicians, had a high response rate, and found only small differences in the utilization patterns of responders and nonresponders, suggesting that our portrayal of the attitudes of physicians was representative of the views of physicians practicing in our community.
The study had a number of weaknesses. First, we cannot be sure whether preexisting beliefs about the benefits of CPOE directly influenced physicians' use of the system or, conversely, if these attitudes developed in response to experience as users. Nevertheless, it seems practical to suggest that hospitals focus on purchasing systems that support the efficient entering of orders while simultaneously adopting a communication and change management strategy that emphasizes the safety and quality benefits of CPOE more broadly. Second, we did not attempt to validate the opinions expressed by physicians about the usability or safety benefits of the system. That said, the purpose of the study was to determine whether physician attitudes toward these issues was associated with the use of the system to place orders. Whether or not this particular CPOE system actually prevented medication errors, most physicians believed it did, a belief strongly associated with the observed order entry rates. Third, we studied a single CPOE system implemented approximately 10 years ago that does not reflect state‐of‐the‐art user interface design or functionality. Nevertheless, our observation about the importance of the user experience is probably no less relevant today. Fourth, we were unable to ascertain every order given by physicians, as some so‐called MD to RN orders may never have made it into the system. Finally, there is a small risk that some written, telephone, and verbal orders may have been randomly or systematically assigned to incorrect physicians, which would have led us to calculate inaccurate utilization rates.
CONCLUSIONS
In a voluntary community hospital environment the adoption of CPOE by attending physicians varies widely. While placing a premium on the purchase of systems that meet the work‐flow needs of physicians and support the efficient entry of orders, hospital leaders can enhance physician adoption of this technology by communicating the role of CPOE in improving quality and safety.
Acknowledgements
The authors thank Gilad Kuperman, MD, PhD, for his thoughtful comments on an earlier version of the manuscript.
- Kohn LT,Corrigan JM,Donaldson MS, eds.To Err Is Human: Building a Safer Health System.Washington, DC:National Academy Press,2000.
- ,.Computer physician order entry: benefits, costs, and issues.Ann Intern Med.2003;139:31–39.
- ,,, et al.Effect of computerized physician order entry and a team intervention on prevention of serious medication errors.JAMA.1998;280:1311–1316.
- ,,,,,.A computerized reminder system to increase the use of preventive care for hospitalized patients.N Engl J Med.2001;345:965–970.
- ,,,.A randomized trial of “corollary orders” to prevent errors of omission.J Am Med Inform Assoc.1997;4:364–375.
- ,,, et al.A computer‐assisted management program for antibiotics and other antiinfective agents.N Engl J Med.1998;338:232–238.
- The Leapfrog Group. Patient Safety Fact Sheet. Available at: http://www.leapfroggroup.org/FactSheets/LF_FactSheet.pdf. Accessed October 6,2004.
- ,,,.Computerized physician order entry in U.S. hospitals: results of a 2002 survey.J Am Med Inform Assoc.2004;11:95–99.
- ,,.U.S. adoption of computerized physician order entry systems.Health Aff.2005;24:1654–1663.
- ,,.Effects of computerized physician order entry and clinical decision support systems on medication safety: a systematic review.Arch Intern Med.2003;163:1409–1416.
- HCUPnet, Healthcare Cost and Utilization Project. Agency for Healthcare Research and Quality, Rockville, MD. Available at: http://www.ahrq.gov/data/hcup/hcupnet.htm. Accessed October 6,2004.
- ,.Physician satisfaction with two order entry systems.J Am Med Inform Assoc.2001;8:499–509.
- ,,, et al.Contrasting views of physicians and nurses about an inpatient computer‐based provider order‐entry system.J Am Med Inform Assoc.1999;6:234–244.
- ,,,.Implementation of physician order entry: user satisfaction and self‐reported usage patterns.J Am Med Inform Assoc.1996;3:42–55.
It is widely acknowledged that the U.S. health care system is plagued by error and inefficiency and that these factors contribute to as many as 44,000‐98,000 deaths each year in U.S. hospitals. In To Err Is Human: Building a Safer Health System, the Institute of Medicine1 outlined the critical role that information technology can play in improving patient safety and highlighted computerized physician order entry (CPOE) systems for their potential to reduce the frequency of medication errors and to improve the quality of medical care.
Computerized physician order entry systems are specialized software applications that allow physicians to place orders directly into a computer. This process has a number of potential advantages over traditional handwritten ordering, including the ability to structure the ordering process to ensure the completeness of individual orders, to provide clinical decision support through diagnosis‐based order sets, and to automatically check orders for potential drugallergy, drugdrug, and drugfood interactions.2 Finally, entering orders directly into a computer eliminates the problem of transcription‐related errors that stem from the difficulty of interpreting handwriting. In clinical trials, the introduction of CPOE has been shown to reduce the frequency of medication errors, to improve the use of preventive services, and to reduce costs.36 Recognition of the benefits of these systems has not been confined to the medical community. The Leapfrog Organization, a coalition of large businesses in the United States, has chosen CPOE as one of its 3 initial safety leaps and has established a threshold that 70% of medication orders should be entered directly by physicians.7
Although the benefits of CPOE systems are widely recognized, few hospitals have implemented these systems successfully.8, 9 Those that have, have often developed the applications internally, and many have relied on house staff to do most or all of the actual ordering.10 However, most hospitals do not have the expertise for internal development and instead rely on commercially available products. Moreover, most patients hospitalized in the United States are cared for by attending physicians working without the assistance of house staff.11 In light of the importance of successfully implementing CPOE systems in such settings, we assessed the adoption of CPOE by attending physicians at 2 community hospitals where its use was voluntary and examined the characteristics and attitudes associated with use of the system to place orders.
METHODS
Setting and Participants
Baystate Medical Center is a 600‐bed teaching hospital in Springfield, Massachusetts, where approximately 50% of patients are cared for with the assistance of house staff. Franklin Medical Center is a 125‐bed community hospital in rural Greenfield, Massachusetts, and is not a house staff training site. Medical staff membership at the 2 hospitals is largely voluntary. Both institutions share a vendor‐supplied computerized order entry system that was implemented in the early 1990s (E7000, Eclipsys Corporation, Boca Raton, FL). The system provides a structured format for the creation of medication, laboratory, and radiology orders and contains thousands of preconstructed medication order sentences and hundreds of order sets designed to standardize ordering for common diagnoses and procedures. Pharmacists are alerted of potential drugallergy and drugdrug interactions and use clinical judgment about whether to communicate this information to the physician. Although the house staff at Baystate Medical Center is mandated to place orders in the system, attending physicians have no such requirement at either institution. Access to the system is provided though the many fixed workstations located on nursing units, in operating rooms, and in the health sciences library. On a typical medical‐surgical patient care unit most computers are behind the nurses' station, though some are distributed along hallways and in physician charting rooms. No computers are in patient rooms. Although the number varies slightly across units, the average ratio of computers to patient beds is roughly 1 to 1.
Survey
In June 2003 we mailed a 20‐item survey to attending physicians who had been responsible for a minimum of 25 orders during the preceding month at either Baystate or Franklin Medical Center. Orders counted toward this minimum if they had been written, given verbally in person or by phone, or entered directly into the computer by the physician. The survey consisted of 20 questions focused on the topic of computerized order entry. In addition to collecting information about sex and specialty, we asked respondents to describe their use of CPOE during training, their use of computers at home, and, where applicable, their use of computers in their outpatient practices. The survey included questions about how often respondents used the order entry system when caring for hospitalized patients and which features of the system they used. To assess physician attitudes about the order entry process, we asked respondents to consider whether it was faster to place orders directly into the system than it was by handwriting them, whether orders placed in the system were carried out more rapidly, whether placing orders in the system led to fewer medication and other errors, whether order sets were important for the efficient use of the system, whether order sets helped to ensure that important aspects of care did not slip through the cracks, whether the system's user interface supported their work flow, and whether the encouragement of nurses was an important factor in their use of the system. Questions that assessed physician attitudes were presented on a 5‐point Likert scale. Nonrespondents were sent reminder letters along with duplicate surveys twice, approximately 1 and 2 months after the initial mailing. No financial incentive was offered for participation. The study protocol was approved by the Institutional Review Board of Baystate Health System.
Order Entry Rates
Regardless of whether an order is placed directly by a physician into a computer, given verbally, or handwritten, all orders are ultimately entered into the CPOE system. Working with our hospitals' Departments of Information Services, we developed a report that provided physician‐specific information about order entry patterns. For each physician in the study, we determined the total number of orders generated during the month preceding the initial June mailing, as well as the absolute number and percentage of orders of each of the following categories: directly entered, telephone, verbal, and written. Because verbal and telephone orders are required during urgent situations and when physicians give orders from outside the hospital, we calculated and report an adjusted order entry rate as the total number of orders placed directly into the system divided by the sum of the orders entered directly and the number of written orders.
Analysis
Summary statistics for the overall sample were constructed using simple frequencies and proportions for categorical variables and medians and interquartile ranges for continuous variables. We compared characteristics of respondents from the 2 hospitals using chi‐square tests of association for categorical factors and Wilcoxon rank‐sum tests for continuous scale data. We compared the total number of orders placed during the study month and the order entry rates of responders and nonresponders using the Wilcoxon rank‐sum test. We categorized physicians as low (20%), intermediate (21%‐79%), and high (80%) users of the system based on their calculated order entry rate. Responses to each of the attitude questions in the survey were tabulated, and the responses strongly agree and agree were combined for analyses comparing responses. Demographic variables and physician attitudes were tested for associations with order entry rate categories via the Pearson chi‐square for categorical factors, the Mantel‐Haenszel chi‐square for ordered factors, and Kruskal‐Wallis analysis of variance for continuous variables. Initial analyses were stratified by hospital; where no differences in association were found across strata, the data were combined. Statistical tests were performed using SAS version 9.1 (SAS Institute, Cary, NC)
RESULTS
During the study period the target group of physicians placed a total of 135,692 orders, of which 69,654 (51%) were placed directly into the CPOE system, 38,878 (29%) were made using pen and paper, 7,208 (5%) were made verbally, and 19,952 (15%) were placed by telephone. Three hundred and fifty‐six (71%) of the 502 surveys sent out to physicians at the 2 hospitals were returned. Thirteen surveys were excluded from analysis because the respondent was not a physician, and 2 because we were unable to match the survey to system usage data, leaving a total of 341 surveys for analysis. Order entry rates were not computed for an additional 3 physicians who only placed verbal and telephone orders during the study period. Response rates did not differ by clinician specialty (P = .53); compared to those of nonresponders, respondents had a similar median total number of orders (111 vs. 101, P = .67) and a higher median order entry rate (66% vs. 48%, P = .03).
Characteristics of Respondents
Seventy‐two percent of physicians who completed the survey were men; half had graduated from medical school at least 20 years ago, and the median duration of practice at the study institution was 11 years (Table 1). Forty percent practiced internal medicine, 18% were surgeons, and 16% were pediatricians. Thirty‐five percent completed training at an institution that had computerized physician order entry, and 86% cared for patients primarily at Baystate Medical Center. More than half reported they used the system many times each day for patient care, and the features they used most commonly were retrieval of results (95%), placing of orders (78%), and viewing and printing of patient lists (75%). Among those with outpatient practices, 81% used computers in their outpatient practice, and more than half used computers for personal activities at home at least once a day. On average, respondents from Franklin Medical Center had graduated from medical school farther in the past and reported less reliance on the system to carry out all activities other than viewing results.
| Overall n (%) | Baystate n (%) 293 (85.9) | Franklin n (%) 48 (14.1) | Chi square P value | |
|---|---|---|---|---|
| ||||
| Sex | .64 | |||
| Male | 244 (71.6) | 211 (72.0) | 33 (68.8) | |
| Specialty | .24 | |||
| Anesthesia | 23 (6.7) | 23 (7.9) | 0 (0.0) | |
| Internal medicine | 135 (39.6) | 112 (38.2) | 23 (47.9) | |
| Medicine/pediatrics | 13 (3.8) | 6 (2.0) | 7 (14.6) | |
| OB/GYN | 36 (10.6) | 30 (10.2) | 6 (12.5) | |
| Pediatrics | 54 (15.8) | 51 (17.4) | 3 (6.3) | |
| Surgery | 61 (17.9) | 55 (18.8) | 6 (12.5) | |
| Other | 19 (5.6) | 16 (5.5) | 3 (6.3) | |
| Use of CPOE systema | .09 | |||
| Many times a day | 176 (52.2) | 160 (55.0) | 16 (34.8) | |
| At least once a day | 77 (22.9) | 61 (21.0) | 16 (34.8) | |
| A few times a week | 55 (16.3) | 45 (15.5) | 10 (21.7) | |
| Once a week or less | 29 (8.6) | 25 (8.6) | 4 (8.7) | |
| Features useda | ||||
| Viewing and printing patient lists | 254 (75.2) | 212 (72.6) | 42 (91.3) | .01 |
| Looking up results | 320 (94.7) | 277 (94.9) | 43 (93.5) | .70 |
| Viewing current medications | 218 (64.5) | 204 (69.9) | 14 (30.4) | < .01 |
| Placing orders | 263 (77.8) | 244 (83.6) | 19 (41.3) | < .01 |
| Entering discharge summaries | 72 (21.3) | 70 (24.0) | 2 (4.4) | < .01 |
| Use of order setsa | ||||
| Rarely or never | 98 (29.0) | 74 (25.3) | 24 (52.2) | < .01 |
| Minority of patients | 92 (27.2) | 78 (26.7) | 14 (30.4) | |
| Majority of patients | 104 (30.8) | 97 (33.2) | 7 (15.2) | |
| For all or nearly all patients | 44 (13.0) | 43 (14.7) | 1 (2.2) | |
| Percentage of orders placed using order setsa | < .01 | |||
| None | 46 (13.7) | 26 (9.0) | 20 (44.4) | |
| 1%‐25% | 62 (18.5) | 50 (17.2) | 12 (26.7) | |
| 26%‐50% | 29 (8.7) | 23 (7.9) | 6 (13.3) | |
| 51%‐75% | 45 (13.4) | 43 (14.9) | 2 (4.4) | |
| 76%‐99% | 103 (30.8) | 98 (33.8) | 5 (11.1) | |
| All | 50 (14.9) | 50 (17.2) | 0 (0.0) | |
| Use of computer in outpatient practiceab | 243 (81.3) | 206 (80.8) | 37 (84.1) | .60 |
| Personal computer usea | .47 | |||
| At least once a day | 209 (61.7) | 185 (63.4) | 24 (51.1) | |
| Several times a week | 84 (24.8) | 67 (23.0) | 17 (36.2) | |
| A few times a month | 21 (6.2) | 18 (6.2) | 3 (6.4) | |
| Rarely | 25 (7.4) | 22 (7.5) | 3 (6.4) | |
| Training at an institution that had CPOE | 117 (34.7) | 105 (36.1) | 12 (26.1) | 0.19 |
| Use of system to enter orders should be mandatorya | ||||
| Yes | 113 (35.2) | 106 (38.4) | 7 (15.6) | <.01 |
| Median (IQR) | Median (IQR) | Median (IQR) | WilcoxonPvalue | |
| Years since medical school graduationa | 20 (13, 26) | 20 (13, 26) | 24 (17, 28) | .02 |
| Years in practice at study institutiona | 11 (5, 18) | 11 (5, 18) | 13 (7, 19) | .39 |
| Orders directly enteredc | 23 (2, 99) | 27 (5, 108) | 1 (0, 27) | < .01 |
| Orders placed by telephonec | 14 (5, 49) | 12 (3, 38) | 49.5 (16, 123.5) | < .01 |
| Orders placed verballyc | 2 (0, 11) | 3 (0, 13) | 1 (0,3) | < .01 |
| Orders placed in writingc | 21 (4, 73) | 14 (3, 45) | 220 (106.5, 391) | < .01 |
| CPOE ratebc | 66% (3%, 94%) | 76% (19%, 96%) | 0.25% (0%, 17%) | < .01 |
Attitudes Toward Computerized Physician Order Entry
Physicians who completed the survey offered diverse opinions about the impact of computerized order entry on work flow, patient safety, and quality of care. Only 22% believed the system's user interface supported their work flow (Q7), 34% believed it was faster to enter orders directly into the system than to handwrite them (Q1), and 41% believed orders placed into the system were carried out more rapidly (Q2) (Table 2). On the other hand, 63% of respondents believed that placing orders directly into the system led to fewer medication errors (Q3), and 51% stated the system generally reduced medical errors (Q4). Sixty‐nine percent stated order sets were important for efficient use of the system (Q5), and 71% believed order sets served an important decision support role (Q6). Twenty‐six percent stated that the encouragement of nurses was an important factor in their use of the system (Q8). Finally, 35% of attending physicians believed use of the system to place orders should be mandatory.
Characteristics and Attitudes of High, Intermediate, and Low Users
The median order entry rate of respondents was 66%. One hundred and forty‐one (42%) placed at least 80% of their orders directly into the system, whereas 109 (32%) placed no more than 20% of their orders directly in the system (Fig. 1). There was not a significant difference between the low, intermediate, and high use groups in the total number of orders that each physician placed during the study period (Table 3). Sex, years since graduation from medical school, years in practice at the study institution, and use of computers in the outpatient setting were not meaningfully different between the 3 categories of users (Table 3). On the other hand, medical specialty was strongly associated with use of the system, with anesthesiologists, pediatricians, and surgeons the specialties with the largest proportion of high users. Furthermore, physicians who were trained in a CPOE environment and those who reported daily use of computers for personal activities showed the highest levels of adoption. Physicians at Franklin Medical Center showed lower levels of order entry than their counterparts at Baystate.

| Low (20%) n (row %) | Intermediate (20%‐79%) n (row %) | High (80%) n (row %) | P value | |
|---|---|---|---|---|
| ||||
| n = 109 | n = 88 | n = 141 | ||
| Hospital | < .01c | |||
| Baystate | 73 (25) | 79 (27) | 138 (48) | |
| Franklin | 36 (75) | 9 (19) | 3 (6) | |
| Sex | .69c | |||
| Female | 28 (29) | 24 (25) | 43 (45) | |
| Male | 81 (33) | 64 (26) | 98 (40) | |
| Specialty | .0001c | |||
| Anesthesia | 8 (35) | 3 (13) | 12 (52) | |
| Internal medicine | 45 (33) | 37 (27) | 53 (39) | |
| Medicine/pediatrics | 6 (46) | 5 (38) | 2 (15) | |
| OB/GYN | 20 (56) | 12 (33) | 4 (11) | |
| Pediatrics | 13 (24) | 9 (17) | 32 (59) | |
| Surgery | 14 (23) | 21 (34) | 26 (43) | |
| Other | 3 (19) | 1 (6) | 12 (75) | |
| Do you use a computer in your outpatient practice?a | ||||
| Yes | 75 (31) | 61 (25) | 105 (44) | .22c |
| No | 20 (36) | 18 (33) | 17 (31) | |
| Level of personal computer useb | .045d | |||
| Rarely | 11 (44) | 8 (32) | 6 (24) | |
| A few times a month | 7 (33) | 4 (19) | 10 (48) | |
| Several times a week | 28 (35) | 25 (31) | 28 (35) | |
| At least once a day | 62 (30) | 50 (24) | 97 (46) | |
| Training at an institution that had CPOE | .037c | |||
| Yes | 30 (26) | 40 (34) | 46 (40) | |
| No | 76 (35) | 48 (22) | 94 (43) | |
| Median (IQR) | Median (IQR) | Median (IQR) | ||
| Years since graduation from medical school | 21 (16, 28) | 18 (14, 25) | 19 (12, 25) | .06e |
| Years in practice at study institution | 12 (5, 19) | 12 (6, 19) | 12 (6, 17) | .84e |
| Total number of orders placed | 112 (45, 306) | 105 (56, 254) | 113 (44, 382) | .92e |
Use of the system was highly associated with physician attitudes toward CPOE, with the views of intermediate and high users consistently different than those of low users (Fig. 2). The associations found held true regardless of hospital: low, intermediate, and high users from Franklin had similar responses to those from Baystate (P > .05 for all questions), and the data from the 2 hospitals therefore were combined for presentation. Although few physicians believed that the user interface of the system supported their work flow, high and intermediate users were 3 times as likely to share this view than were low users (Q7; Fig. 2). Similarly, 19% of low users, 31% of intermediate users, and 45% of high users believed that entering orders into the system was faster than writing orders (Q1). High and intermediate users of the system were more likely than low users to believe that orders entered into the system were carried out more rapidly (Q2) and led to fewer medication (Q3) and nonmedication (Q4) errors. Regardless of their utilization pattern, most physicians believed that order sets played an important role in promoting efficiency and quality.

DISCUSSION
In this study of the clinical computing practices of physicians at 2 community hospitals, we observed wide variation in the adoption of CPOE by individual attendings. Although roughly one‐third rarely placed orders directly into the system, 42% had an order entry rate of at least 80%. Contrary to our initial expectation, we found little association between a physician's order entry rate with years in practice, duration of exposure to CPOE, or use of computers in the outpatient setting. On the other hand, we observed marked differences in use of the CPOE system across specialty lines and found that physicians who were exposed to CPOE during training and those who were regular users of computers for personal activities were more likely to embrace this technology. Further, we observed important differences between physicians who used the system to place some or most of their orders and those who did so only rarely in their beliefs and attitudes about the impact and benefits of CPOE. Physicians with higher order entry rates were more likely than their colleagues to believe that placing orders electronically was faster than handwriting and that use of the system led to fewer medical errors. These findings should be encouraging to hospitals hoping to implement CPOE because they suggest that successful adoption of CPOE is not limited to physicians who have just completed their residencies or to hospitals with the capability of designing and building their own systems. On the contrary, we documented that women, older physicians, and those with limited CPOE experience were as likely to be frequent users, especially if they perceived CPOE to be safer than handwriting and if they believed the user interface supported the efficient entering of orders.
On the basis of these results we recommend that in addition to purchasing systems that meet physician work‐flow needs and support the efficient entry of orders, hospital leaders should emphasize the quality and safety benefits of CPOE as part of a comprehensive change management strategy. The differences we observed in order entry rates across specialties may have resulted from several factors, including inherent differences in personality type associated with choice of specialty and in the level of customization of a system reflected in which and how many order sets are included. Such findings suggest that when it comes to CPOE, one size does not fit all, and implementation planning should be carried out at the specialty level. Finally, our observation that physicians who had exposure to CPOE during training were more likely to use the system to place orders suggests that the nation's training institutions will play an important role in fostering universal adoption of this technology.
Several earlier studies have reported on physician experiences with CPOE systems. Murff and Kannry12 surveyed 94 internal medicine house staff to compare experiences with 2 CPOE systems: the Department of Veterans Affairs Computerized Patient Record System (CPRS) and a commercially available product. They found striking differences in user satisfaction with numerous aspects of the systems, however they did not address attitudes toward safety or quality, and because house staff were required to place orders electronically they were unable to correlate responses with actual usage patterns. Weiner et al.13 compared the opinions of internal medicine house staff, attendings, fellows, and nurses about the benefits and challenges of using a computerized provider order entry system. In contrast to the findings from our study, Weiner et al. reported that more than half of physicians believed that provider order entry led to a greater number of errors, and only a minority believed the system increased quality of care overall. Finally, Lee et al.14 surveyed medical and surgical house officers and nurses at a large academic medical center about their satisfaction with a locally developed order entry system. They found that attitudes about the impact of the system on productivity and ease of use were more strongly associated with overall satisfaction than having undergone training or experience with personal computers. These findings are congruous with our own observation that beliefs about the speed with which orders are placed are closely associated with actual use of the system. They reported, as have we, that physicians placed a high value on order sets.
Our study had a number of strengths. First, we were able to offer insight into the attitudes and behaviors of a previously neglected, but critically important groupattending physicians who care for patients at community hospitals without the assistance of house staff. Second, whereas previous studies primarily assessed physician satisfaction with CPOE, we explored how physician attitudes about the impact of CPOE on work flow and on safety were associated with actual ordering habits. Information about ordering was obtained directly from the order entry system and not through self‐report. We conducted the study at 2 hospitals, a large urban community teaching hospital and a smaller rural hospital, and focused on a CPOE system that is in use at many institutions throughout the country, thereby increasing the generalizability of our findings. Although adoption of the system by physicians at the 2 hospitals differed, factors that associated with the use of CPOE to place orders were similar. Finally, we surveyed a large number of physicians, had a high response rate, and found only small differences in the utilization patterns of responders and nonresponders, suggesting that our portrayal of the attitudes of physicians was representative of the views of physicians practicing in our community.
The study had a number of weaknesses. First, we cannot be sure whether preexisting beliefs about the benefits of CPOE directly influenced physicians' use of the system or, conversely, if these attitudes developed in response to experience as users. Nevertheless, it seems practical to suggest that hospitals focus on purchasing systems that support the efficient entering of orders while simultaneously adopting a communication and change management strategy that emphasizes the safety and quality benefits of CPOE more broadly. Second, we did not attempt to validate the opinions expressed by physicians about the usability or safety benefits of the system. That said, the purpose of the study was to determine whether physician attitudes toward these issues was associated with the use of the system to place orders. Whether or not this particular CPOE system actually prevented medication errors, most physicians believed it did, a belief strongly associated with the observed order entry rates. Third, we studied a single CPOE system implemented approximately 10 years ago that does not reflect state‐of‐the‐art user interface design or functionality. Nevertheless, our observation about the importance of the user experience is probably no less relevant today. Fourth, we were unable to ascertain every order given by physicians, as some so‐called MD to RN orders may never have made it into the system. Finally, there is a small risk that some written, telephone, and verbal orders may have been randomly or systematically assigned to incorrect physicians, which would have led us to calculate inaccurate utilization rates.
CONCLUSIONS
In a voluntary community hospital environment the adoption of CPOE by attending physicians varies widely. While placing a premium on the purchase of systems that meet the work‐flow needs of physicians and support the efficient entry of orders, hospital leaders can enhance physician adoption of this technology by communicating the role of CPOE in improving quality and safety.
Acknowledgements
The authors thank Gilad Kuperman, MD, PhD, for his thoughtful comments on an earlier version of the manuscript.
It is widely acknowledged that the U.S. health care system is plagued by error and inefficiency and that these factors contribute to as many as 44,000‐98,000 deaths each year in U.S. hospitals. In To Err Is Human: Building a Safer Health System, the Institute of Medicine1 outlined the critical role that information technology can play in improving patient safety and highlighted computerized physician order entry (CPOE) systems for their potential to reduce the frequency of medication errors and to improve the quality of medical care.
Computerized physician order entry systems are specialized software applications that allow physicians to place orders directly into a computer. This process has a number of potential advantages over traditional handwritten ordering, including the ability to structure the ordering process to ensure the completeness of individual orders, to provide clinical decision support through diagnosis‐based order sets, and to automatically check orders for potential drugallergy, drugdrug, and drugfood interactions.2 Finally, entering orders directly into a computer eliminates the problem of transcription‐related errors that stem from the difficulty of interpreting handwriting. In clinical trials, the introduction of CPOE has been shown to reduce the frequency of medication errors, to improve the use of preventive services, and to reduce costs.36 Recognition of the benefits of these systems has not been confined to the medical community. The Leapfrog Organization, a coalition of large businesses in the United States, has chosen CPOE as one of its 3 initial safety leaps and has established a threshold that 70% of medication orders should be entered directly by physicians.7
Although the benefits of CPOE systems are widely recognized, few hospitals have implemented these systems successfully.8, 9 Those that have, have often developed the applications internally, and many have relied on house staff to do most or all of the actual ordering.10 However, most hospitals do not have the expertise for internal development and instead rely on commercially available products. Moreover, most patients hospitalized in the United States are cared for by attending physicians working without the assistance of house staff.11 In light of the importance of successfully implementing CPOE systems in such settings, we assessed the adoption of CPOE by attending physicians at 2 community hospitals where its use was voluntary and examined the characteristics and attitudes associated with use of the system to place orders.
METHODS
Setting and Participants
Baystate Medical Center is a 600‐bed teaching hospital in Springfield, Massachusetts, where approximately 50% of patients are cared for with the assistance of house staff. Franklin Medical Center is a 125‐bed community hospital in rural Greenfield, Massachusetts, and is not a house staff training site. Medical staff membership at the 2 hospitals is largely voluntary. Both institutions share a vendor‐supplied computerized order entry system that was implemented in the early 1990s (E7000, Eclipsys Corporation, Boca Raton, FL). The system provides a structured format for the creation of medication, laboratory, and radiology orders and contains thousands of preconstructed medication order sentences and hundreds of order sets designed to standardize ordering for common diagnoses and procedures. Pharmacists are alerted of potential drugallergy and drugdrug interactions and use clinical judgment about whether to communicate this information to the physician. Although the house staff at Baystate Medical Center is mandated to place orders in the system, attending physicians have no such requirement at either institution. Access to the system is provided though the many fixed workstations located on nursing units, in operating rooms, and in the health sciences library. On a typical medical‐surgical patient care unit most computers are behind the nurses' station, though some are distributed along hallways and in physician charting rooms. No computers are in patient rooms. Although the number varies slightly across units, the average ratio of computers to patient beds is roughly 1 to 1.
Survey
In June 2003 we mailed a 20‐item survey to attending physicians who had been responsible for a minimum of 25 orders during the preceding month at either Baystate or Franklin Medical Center. Orders counted toward this minimum if they had been written, given verbally in person or by phone, or entered directly into the computer by the physician. The survey consisted of 20 questions focused on the topic of computerized order entry. In addition to collecting information about sex and specialty, we asked respondents to describe their use of CPOE during training, their use of computers at home, and, where applicable, their use of computers in their outpatient practices. The survey included questions about how often respondents used the order entry system when caring for hospitalized patients and which features of the system they used. To assess physician attitudes about the order entry process, we asked respondents to consider whether it was faster to place orders directly into the system than it was by handwriting them, whether orders placed in the system were carried out more rapidly, whether placing orders in the system led to fewer medication and other errors, whether order sets were important for the efficient use of the system, whether order sets helped to ensure that important aspects of care did not slip through the cracks, whether the system's user interface supported their work flow, and whether the encouragement of nurses was an important factor in their use of the system. Questions that assessed physician attitudes were presented on a 5‐point Likert scale. Nonrespondents were sent reminder letters along with duplicate surveys twice, approximately 1 and 2 months after the initial mailing. No financial incentive was offered for participation. The study protocol was approved by the Institutional Review Board of Baystate Health System.
Order Entry Rates
Regardless of whether an order is placed directly by a physician into a computer, given verbally, or handwritten, all orders are ultimately entered into the CPOE system. Working with our hospitals' Departments of Information Services, we developed a report that provided physician‐specific information about order entry patterns. For each physician in the study, we determined the total number of orders generated during the month preceding the initial June mailing, as well as the absolute number and percentage of orders of each of the following categories: directly entered, telephone, verbal, and written. Because verbal and telephone orders are required during urgent situations and when physicians give orders from outside the hospital, we calculated and report an adjusted order entry rate as the total number of orders placed directly into the system divided by the sum of the orders entered directly and the number of written orders.
Analysis
Summary statistics for the overall sample were constructed using simple frequencies and proportions for categorical variables and medians and interquartile ranges for continuous variables. We compared characteristics of respondents from the 2 hospitals using chi‐square tests of association for categorical factors and Wilcoxon rank‐sum tests for continuous scale data. We compared the total number of orders placed during the study month and the order entry rates of responders and nonresponders using the Wilcoxon rank‐sum test. We categorized physicians as low (20%), intermediate (21%‐79%), and high (80%) users of the system based on their calculated order entry rate. Responses to each of the attitude questions in the survey were tabulated, and the responses strongly agree and agree were combined for analyses comparing responses. Demographic variables and physician attitudes were tested for associations with order entry rate categories via the Pearson chi‐square for categorical factors, the Mantel‐Haenszel chi‐square for ordered factors, and Kruskal‐Wallis analysis of variance for continuous variables. Initial analyses were stratified by hospital; where no differences in association were found across strata, the data were combined. Statistical tests were performed using SAS version 9.1 (SAS Institute, Cary, NC)
RESULTS
During the study period the target group of physicians placed a total of 135,692 orders, of which 69,654 (51%) were placed directly into the CPOE system, 38,878 (29%) were made using pen and paper, 7,208 (5%) were made verbally, and 19,952 (15%) were placed by telephone. Three hundred and fifty‐six (71%) of the 502 surveys sent out to physicians at the 2 hospitals were returned. Thirteen surveys were excluded from analysis because the respondent was not a physician, and 2 because we were unable to match the survey to system usage data, leaving a total of 341 surveys for analysis. Order entry rates were not computed for an additional 3 physicians who only placed verbal and telephone orders during the study period. Response rates did not differ by clinician specialty (P = .53); compared to those of nonresponders, respondents had a similar median total number of orders (111 vs. 101, P = .67) and a higher median order entry rate (66% vs. 48%, P = .03).
Characteristics of Respondents
Seventy‐two percent of physicians who completed the survey were men; half had graduated from medical school at least 20 years ago, and the median duration of practice at the study institution was 11 years (Table 1). Forty percent practiced internal medicine, 18% were surgeons, and 16% were pediatricians. Thirty‐five percent completed training at an institution that had computerized physician order entry, and 86% cared for patients primarily at Baystate Medical Center. More than half reported they used the system many times each day for patient care, and the features they used most commonly were retrieval of results (95%), placing of orders (78%), and viewing and printing of patient lists (75%). Among those with outpatient practices, 81% used computers in their outpatient practice, and more than half used computers for personal activities at home at least once a day. On average, respondents from Franklin Medical Center had graduated from medical school farther in the past and reported less reliance on the system to carry out all activities other than viewing results.
| Overall n (%) | Baystate n (%) 293 (85.9) | Franklin n (%) 48 (14.1) | Chi square P value | |
|---|---|---|---|---|
| ||||
| Sex | .64 | |||
| Male | 244 (71.6) | 211 (72.0) | 33 (68.8) | |
| Specialty | .24 | |||
| Anesthesia | 23 (6.7) | 23 (7.9) | 0 (0.0) | |
| Internal medicine | 135 (39.6) | 112 (38.2) | 23 (47.9) | |
| Medicine/pediatrics | 13 (3.8) | 6 (2.0) | 7 (14.6) | |
| OB/GYN | 36 (10.6) | 30 (10.2) | 6 (12.5) | |
| Pediatrics | 54 (15.8) | 51 (17.4) | 3 (6.3) | |
| Surgery | 61 (17.9) | 55 (18.8) | 6 (12.5) | |
| Other | 19 (5.6) | 16 (5.5) | 3 (6.3) | |
| Use of CPOE systema | .09 | |||
| Many times a day | 176 (52.2) | 160 (55.0) | 16 (34.8) | |
| At least once a day | 77 (22.9) | 61 (21.0) | 16 (34.8) | |
| A few times a week | 55 (16.3) | 45 (15.5) | 10 (21.7) | |
| Once a week or less | 29 (8.6) | 25 (8.6) | 4 (8.7) | |
| Features useda | ||||
| Viewing and printing patient lists | 254 (75.2) | 212 (72.6) | 42 (91.3) | .01 |
| Looking up results | 320 (94.7) | 277 (94.9) | 43 (93.5) | .70 |
| Viewing current medications | 218 (64.5) | 204 (69.9) | 14 (30.4) | < .01 |
| Placing orders | 263 (77.8) | 244 (83.6) | 19 (41.3) | < .01 |
| Entering discharge summaries | 72 (21.3) | 70 (24.0) | 2 (4.4) | < .01 |
| Use of order setsa | ||||
| Rarely or never | 98 (29.0) | 74 (25.3) | 24 (52.2) | < .01 |
| Minority of patients | 92 (27.2) | 78 (26.7) | 14 (30.4) | |
| Majority of patients | 104 (30.8) | 97 (33.2) | 7 (15.2) | |
| For all or nearly all patients | 44 (13.0) | 43 (14.7) | 1 (2.2) | |
| Percentage of orders placed using order setsa | < .01 | |||
| None | 46 (13.7) | 26 (9.0) | 20 (44.4) | |
| 1%‐25% | 62 (18.5) | 50 (17.2) | 12 (26.7) | |
| 26%‐50% | 29 (8.7) | 23 (7.9) | 6 (13.3) | |
| 51%‐75% | 45 (13.4) | 43 (14.9) | 2 (4.4) | |
| 76%‐99% | 103 (30.8) | 98 (33.8) | 5 (11.1) | |
| All | 50 (14.9) | 50 (17.2) | 0 (0.0) | |
| Use of computer in outpatient practiceab | 243 (81.3) | 206 (80.8) | 37 (84.1) | .60 |
| Personal computer usea | .47 | |||
| At least once a day | 209 (61.7) | 185 (63.4) | 24 (51.1) | |
| Several times a week | 84 (24.8) | 67 (23.0) | 17 (36.2) | |
| A few times a month | 21 (6.2) | 18 (6.2) | 3 (6.4) | |
| Rarely | 25 (7.4) | 22 (7.5) | 3 (6.4) | |
| Training at an institution that had CPOE | 117 (34.7) | 105 (36.1) | 12 (26.1) | 0.19 |
| Use of system to enter orders should be mandatorya | ||||
| Yes | 113 (35.2) | 106 (38.4) | 7 (15.6) | <.01 |
| Median (IQR) | Median (IQR) | Median (IQR) | WilcoxonPvalue | |
| Years since medical school graduationa | 20 (13, 26) | 20 (13, 26) | 24 (17, 28) | .02 |
| Years in practice at study institutiona | 11 (5, 18) | 11 (5, 18) | 13 (7, 19) | .39 |
| Orders directly enteredc | 23 (2, 99) | 27 (5, 108) | 1 (0, 27) | < .01 |
| Orders placed by telephonec | 14 (5, 49) | 12 (3, 38) | 49.5 (16, 123.5) | < .01 |
| Orders placed verballyc | 2 (0, 11) | 3 (0, 13) | 1 (0,3) | < .01 |
| Orders placed in writingc | 21 (4, 73) | 14 (3, 45) | 220 (106.5, 391) | < .01 |
| CPOE ratebc | 66% (3%, 94%) | 76% (19%, 96%) | 0.25% (0%, 17%) | < .01 |
Attitudes Toward Computerized Physician Order Entry
Physicians who completed the survey offered diverse opinions about the impact of computerized order entry on work flow, patient safety, and quality of care. Only 22% believed the system's user interface supported their work flow (Q7), 34% believed it was faster to enter orders directly into the system than to handwrite them (Q1), and 41% believed orders placed into the system were carried out more rapidly (Q2) (Table 2). On the other hand, 63% of respondents believed that placing orders directly into the system led to fewer medication errors (Q3), and 51% stated the system generally reduced medical errors (Q4). Sixty‐nine percent stated order sets were important for efficient use of the system (Q5), and 71% believed order sets served an important decision support role (Q6). Twenty‐six percent stated that the encouragement of nurses was an important factor in their use of the system (Q8). Finally, 35% of attending physicians believed use of the system to place orders should be mandatory.
Characteristics and Attitudes of High, Intermediate, and Low Users
The median order entry rate of respondents was 66%. One hundred and forty‐one (42%) placed at least 80% of their orders directly into the system, whereas 109 (32%) placed no more than 20% of their orders directly in the system (Fig. 1). There was not a significant difference between the low, intermediate, and high use groups in the total number of orders that each physician placed during the study period (Table 3). Sex, years since graduation from medical school, years in practice at the study institution, and use of computers in the outpatient setting were not meaningfully different between the 3 categories of users (Table 3). On the other hand, medical specialty was strongly associated with use of the system, with anesthesiologists, pediatricians, and surgeons the specialties with the largest proportion of high users. Furthermore, physicians who were trained in a CPOE environment and those who reported daily use of computers for personal activities showed the highest levels of adoption. Physicians at Franklin Medical Center showed lower levels of order entry than their counterparts at Baystate.

| Low (20%) n (row %) | Intermediate (20%‐79%) n (row %) | High (80%) n (row %) | P value | |
|---|---|---|---|---|
| ||||
| n = 109 | n = 88 | n = 141 | ||
| Hospital | < .01c | |||
| Baystate | 73 (25) | 79 (27) | 138 (48) | |
| Franklin | 36 (75) | 9 (19) | 3 (6) | |
| Sex | .69c | |||
| Female | 28 (29) | 24 (25) | 43 (45) | |
| Male | 81 (33) | 64 (26) | 98 (40) | |
| Specialty | .0001c | |||
| Anesthesia | 8 (35) | 3 (13) | 12 (52) | |
| Internal medicine | 45 (33) | 37 (27) | 53 (39) | |
| Medicine/pediatrics | 6 (46) | 5 (38) | 2 (15) | |
| OB/GYN | 20 (56) | 12 (33) | 4 (11) | |
| Pediatrics | 13 (24) | 9 (17) | 32 (59) | |
| Surgery | 14 (23) | 21 (34) | 26 (43) | |
| Other | 3 (19) | 1 (6) | 12 (75) | |
| Do you use a computer in your outpatient practice?a | ||||
| Yes | 75 (31) | 61 (25) | 105 (44) | .22c |
| No | 20 (36) | 18 (33) | 17 (31) | |
| Level of personal computer useb | .045d | |||
| Rarely | 11 (44) | 8 (32) | 6 (24) | |
| A few times a month | 7 (33) | 4 (19) | 10 (48) | |
| Several times a week | 28 (35) | 25 (31) | 28 (35) | |
| At least once a day | 62 (30) | 50 (24) | 97 (46) | |
| Training at an institution that had CPOE | .037c | |||
| Yes | 30 (26) | 40 (34) | 46 (40) | |
| No | 76 (35) | 48 (22) | 94 (43) | |
| Median (IQR) | Median (IQR) | Median (IQR) | ||
| Years since graduation from medical school | 21 (16, 28) | 18 (14, 25) | 19 (12, 25) | .06e |
| Years in practice at study institution | 12 (5, 19) | 12 (6, 19) | 12 (6, 17) | .84e |
| Total number of orders placed | 112 (45, 306) | 105 (56, 254) | 113 (44, 382) | .92e |
Use of the system was highly associated with physician attitudes toward CPOE, with the views of intermediate and high users consistently different than those of low users (Fig. 2). The associations found held true regardless of hospital: low, intermediate, and high users from Franklin had similar responses to those from Baystate (P > .05 for all questions), and the data from the 2 hospitals therefore were combined for presentation. Although few physicians believed that the user interface of the system supported their work flow, high and intermediate users were 3 times as likely to share this view than were low users (Q7; Fig. 2). Similarly, 19% of low users, 31% of intermediate users, and 45% of high users believed that entering orders into the system was faster than writing orders (Q1). High and intermediate users of the system were more likely than low users to believe that orders entered into the system were carried out more rapidly (Q2) and led to fewer medication (Q3) and nonmedication (Q4) errors. Regardless of their utilization pattern, most physicians believed that order sets played an important role in promoting efficiency and quality.

DISCUSSION
In this study of the clinical computing practices of physicians at 2 community hospitals, we observed wide variation in the adoption of CPOE by individual attendings. Although roughly one‐third rarely placed orders directly into the system, 42% had an order entry rate of at least 80%. Contrary to our initial expectation, we found little association between a physician's order entry rate with years in practice, duration of exposure to CPOE, or use of computers in the outpatient setting. On the other hand, we observed marked differences in use of the CPOE system across specialty lines and found that physicians who were exposed to CPOE during training and those who were regular users of computers for personal activities were more likely to embrace this technology. Further, we observed important differences between physicians who used the system to place some or most of their orders and those who did so only rarely in their beliefs and attitudes about the impact and benefits of CPOE. Physicians with higher order entry rates were more likely than their colleagues to believe that placing orders electronically was faster than handwriting and that use of the system led to fewer medical errors. These findings should be encouraging to hospitals hoping to implement CPOE because they suggest that successful adoption of CPOE is not limited to physicians who have just completed their residencies or to hospitals with the capability of designing and building their own systems. On the contrary, we documented that women, older physicians, and those with limited CPOE experience were as likely to be frequent users, especially if they perceived CPOE to be safer than handwriting and if they believed the user interface supported the efficient entering of orders.
On the basis of these results we recommend that in addition to purchasing systems that meet physician work‐flow needs and support the efficient entry of orders, hospital leaders should emphasize the quality and safety benefits of CPOE as part of a comprehensive change management strategy. The differences we observed in order entry rates across specialties may have resulted from several factors, including inherent differences in personality type associated with choice of specialty and in the level of customization of a system reflected in which and how many order sets are included. Such findings suggest that when it comes to CPOE, one size does not fit all, and implementation planning should be carried out at the specialty level. Finally, our observation that physicians who had exposure to CPOE during training were more likely to use the system to place orders suggests that the nation's training institutions will play an important role in fostering universal adoption of this technology.
Several earlier studies have reported on physician experiences with CPOE systems. Murff and Kannry12 surveyed 94 internal medicine house staff to compare experiences with 2 CPOE systems: the Department of Veterans Affairs Computerized Patient Record System (CPRS) and a commercially available product. They found striking differences in user satisfaction with numerous aspects of the systems, however they did not address attitudes toward safety or quality, and because house staff were required to place orders electronically they were unable to correlate responses with actual usage patterns. Weiner et al.13 compared the opinions of internal medicine house staff, attendings, fellows, and nurses about the benefits and challenges of using a computerized provider order entry system. In contrast to the findings from our study, Weiner et al. reported that more than half of physicians believed that provider order entry led to a greater number of errors, and only a minority believed the system increased quality of care overall. Finally, Lee et al.14 surveyed medical and surgical house officers and nurses at a large academic medical center about their satisfaction with a locally developed order entry system. They found that attitudes about the impact of the system on productivity and ease of use were more strongly associated with overall satisfaction than having undergone training or experience with personal computers. These findings are congruous with our own observation that beliefs about the speed with which orders are placed are closely associated with actual use of the system. They reported, as have we, that physicians placed a high value on order sets.
Our study had a number of strengths. First, we were able to offer insight into the attitudes and behaviors of a previously neglected, but critically important groupattending physicians who care for patients at community hospitals without the assistance of house staff. Second, whereas previous studies primarily assessed physician satisfaction with CPOE, we explored how physician attitudes about the impact of CPOE on work flow and on safety were associated with actual ordering habits. Information about ordering was obtained directly from the order entry system and not through self‐report. We conducted the study at 2 hospitals, a large urban community teaching hospital and a smaller rural hospital, and focused on a CPOE system that is in use at many institutions throughout the country, thereby increasing the generalizability of our findings. Although adoption of the system by physicians at the 2 hospitals differed, factors that associated with the use of CPOE to place orders were similar. Finally, we surveyed a large number of physicians, had a high response rate, and found only small differences in the utilization patterns of responders and nonresponders, suggesting that our portrayal of the attitudes of physicians was representative of the views of physicians practicing in our community.
The study had a number of weaknesses. First, we cannot be sure whether preexisting beliefs about the benefits of CPOE directly influenced physicians' use of the system or, conversely, if these attitudes developed in response to experience as users. Nevertheless, it seems practical to suggest that hospitals focus on purchasing systems that support the efficient entering of orders while simultaneously adopting a communication and change management strategy that emphasizes the safety and quality benefits of CPOE more broadly. Second, we did not attempt to validate the opinions expressed by physicians about the usability or safety benefits of the system. That said, the purpose of the study was to determine whether physician attitudes toward these issues was associated with the use of the system to place orders. Whether or not this particular CPOE system actually prevented medication errors, most physicians believed it did, a belief strongly associated with the observed order entry rates. Third, we studied a single CPOE system implemented approximately 10 years ago that does not reflect state‐of‐the‐art user interface design or functionality. Nevertheless, our observation about the importance of the user experience is probably no less relevant today. Fourth, we were unable to ascertain every order given by physicians, as some so‐called MD to RN orders may never have made it into the system. Finally, there is a small risk that some written, telephone, and verbal orders may have been randomly or systematically assigned to incorrect physicians, which would have led us to calculate inaccurate utilization rates.
CONCLUSIONS
In a voluntary community hospital environment the adoption of CPOE by attending physicians varies widely. While placing a premium on the purchase of systems that meet the work‐flow needs of physicians and support the efficient entry of orders, hospital leaders can enhance physician adoption of this technology by communicating the role of CPOE in improving quality and safety.
Acknowledgements
The authors thank Gilad Kuperman, MD, PhD, for his thoughtful comments on an earlier version of the manuscript.
- Kohn LT,Corrigan JM,Donaldson MS, eds.To Err Is Human: Building a Safer Health System.Washington, DC:National Academy Press,2000.
- ,.Computer physician order entry: benefits, costs, and issues.Ann Intern Med.2003;139:31–39.
- ,,, et al.Effect of computerized physician order entry and a team intervention on prevention of serious medication errors.JAMA.1998;280:1311–1316.
- ,,,,,.A computerized reminder system to increase the use of preventive care for hospitalized patients.N Engl J Med.2001;345:965–970.
- ,,,.A randomized trial of “corollary orders” to prevent errors of omission.J Am Med Inform Assoc.1997;4:364–375.
- ,,, et al.A computer‐assisted management program for antibiotics and other antiinfective agents.N Engl J Med.1998;338:232–238.
- The Leapfrog Group. Patient Safety Fact Sheet. Available at: http://www.leapfroggroup.org/FactSheets/LF_FactSheet.pdf. Accessed October 6,2004.
- ,,,.Computerized physician order entry in U.S. hospitals: results of a 2002 survey.J Am Med Inform Assoc.2004;11:95–99.
- ,,.U.S. adoption of computerized physician order entry systems.Health Aff.2005;24:1654–1663.
- ,,.Effects of computerized physician order entry and clinical decision support systems on medication safety: a systematic review.Arch Intern Med.2003;163:1409–1416.
- HCUPnet, Healthcare Cost and Utilization Project. Agency for Healthcare Research and Quality, Rockville, MD. Available at: http://www.ahrq.gov/data/hcup/hcupnet.htm. Accessed October 6,2004.
- ,.Physician satisfaction with two order entry systems.J Am Med Inform Assoc.2001;8:499–509.
- ,,, et al.Contrasting views of physicians and nurses about an inpatient computer‐based provider order‐entry system.J Am Med Inform Assoc.1999;6:234–244.
- ,,,.Implementation of physician order entry: user satisfaction and self‐reported usage patterns.J Am Med Inform Assoc.1996;3:42–55.
- Kohn LT,Corrigan JM,Donaldson MS, eds.To Err Is Human: Building a Safer Health System.Washington, DC:National Academy Press,2000.
- ,.Computer physician order entry: benefits, costs, and issues.Ann Intern Med.2003;139:31–39.
- ,,, et al.Effect of computerized physician order entry and a team intervention on prevention of serious medication errors.JAMA.1998;280:1311–1316.
- ,,,,,.A computerized reminder system to increase the use of preventive care for hospitalized patients.N Engl J Med.2001;345:965–970.
- ,,,.A randomized trial of “corollary orders” to prevent errors of omission.J Am Med Inform Assoc.1997;4:364–375.
- ,,, et al.A computer‐assisted management program for antibiotics and other antiinfective agents.N Engl J Med.1998;338:232–238.
- The Leapfrog Group. Patient Safety Fact Sheet. Available at: http://www.leapfroggroup.org/FactSheets/LF_FactSheet.pdf. Accessed October 6,2004.
- ,,,.Computerized physician order entry in U.S. hospitals: results of a 2002 survey.J Am Med Inform Assoc.2004;11:95–99.
- ,,.U.S. adoption of computerized physician order entry systems.Health Aff.2005;24:1654–1663.
- ,,.Effects of computerized physician order entry and clinical decision support systems on medication safety: a systematic review.Arch Intern Med.2003;163:1409–1416.
- HCUPnet, Healthcare Cost and Utilization Project. Agency for Healthcare Research and Quality, Rockville, MD. Available at: http://www.ahrq.gov/data/hcup/hcupnet.htm. Accessed October 6,2004.
- ,.Physician satisfaction with two order entry systems.J Am Med Inform Assoc.2001;8:499–509.
- ,,, et al.Contrasting views of physicians and nurses about an inpatient computer‐based provider order‐entry system.J Am Med Inform Assoc.1999;6:234–244.
- ,,,.Implementation of physician order entry: user satisfaction and self‐reported usage patterns.J Am Med Inform Assoc.1996;3:42–55.
Copyright © 2006 Society of Hospital Medicine
Lipid Management during Stroke Hospitalization
Aortocervicocephalic atherosclerotic disease and coronary artery disease share common risk factors, and patients with one condition are at high risk of harboring or developing the other.1, 2 Over the past decade, several randomized clinical trials of lipid‐lowering medications designed to reduce low‐density lipoprotein cholesterol (LDL‐C) have shown a significant decrease in the risk of coronary events and ischemic stroke among patients who have a history of or are at risk for coronary artery disease, regardless of whether serum cholesterol is elevated.3, 4 Results from more than 3000 stroke patients enrolled in the Heart Protection Study also provide evidence that aggressive lipid‐lowering therapy may prevent recurrent vascular events in individuals who have a total cholesterol level as low as 135 mg/dL and cerebrovascular disease, with or without known coronary artery disease.5
Guidelines from the National Cholesterol Evaluation Program Adult Treatment Panel (ATP) provide target LDL‐C levels for persons with atherosclerotic disease depending on the extent of their vascular risk.6 However, despite the broad dissemination of these guidelines, several published studies of patients with coronary artery disease or dyslipidemia have shown that a large proportion of patients with high vascular risk continue to be underscreened, underdiagnosed, and undertreated for dyslipidemia.79
Few studies have evaluated the quality of cholesterol management among hospitalized patients who have experienced an acute ischemic cerebrovascular event10, 11 So the data are scarce on the management of patients hospitalized for ischemic stroke or transient ischemic attack (TIA) who are, according to ATP criteria, at high risk for future coronary events and on the factors that may govern that management. Systematic reviews have suggested that incorporating a lipid profile during acute stroke presentation could assure baseline assessment and serve as a potential cue for physicians to change their behavior,12 and an American Stroke Association advisory recommends lipid treatment during hospitalization for most patients with ischemic stroke or TIA as it may increase the rate of long‐term use.13
The objectives of this study were to determine the rates of testing for and treatment of dyslipidemia according to national cholesterol guidelines among individuals hospitalized with acute ischemic stroke or TIA and to identify predictors of performance.
METHODS
The California Acute Stroke Prototype Registry (CASPR) is a Centers for Disease Controlsponsored cohort that captured detailed data on patients admitted to 11 hospitals over a 2‐year period. The methods of study have been described elsewhere.14 In brief, CASPR prospectively collected information on acute stroke care at 11 representative hospitals in 5 major population regions of California. Data were collected on diagnostic evaluation, appropriate use of treatment strategies, and disposition on discharge from the hospital. The main goal of CASPR was to pilot‐test a prototype prospective registry of acute stroke and transient ischemic attack to be used as a quality improvement tool. The study population was patients with an admitting or discharge diagnosis of suspected stroke or TIA from November 1, 2002, through January 31, 2003, and from November 1, 2003, through January 31, 2004. The human subjects review board at each participating center approved the study.
For the present analysis, data on all patients with a discharge diagnosis of ischemic stroke or TIA who were admitted during either period were included. We examined the possible association of several variables with 2 primary outcomes: (1) testing lipid profile during hospitalization (as indicated by a documented LDL‐C level) and (2) prescribing lipid‐lowering medication at discharge. In those analyses in which lipid profile testing was the outcome, no variables were considered acceptable reasons for not performing an LDL‐C assessment.
The distribution of LDL‐C levels in this portion of the cohort was determined. Patients were then categorized according to their risk for future coronary events. Patients were classified as at risk for coronary events (ACE) if they either had a documented history of myocardial infarction, coronary artery disease, or diabetes or had undergone carotid endarterectomy or carotid angioplasty/stenting during hospitalization. Criteria for initiating lipid‐lowering therapy were defined according to the ATP III guidelines,6 which were in effect during both CASPR study periods. Continuing the recommendation in ATP II, the ATP III recommendations emphasized that persons with documented coronary artery disease (CAD) receive the most aggressive lipid‐lowering treatment. But this recommendation was expanded to include patients without established CAD, whose coronary risk is equivalent to that of patients with diagnosed CAD.6
As per the ATP III guidelines, CASPR‐ACE patients were considered optimally treated if they were prescribed a lipid‐lowering agent at discharge or if their documented LDL‐C was less than 130 mg/dL. A concurrent history of liver disease, abnormal prothrombin time, life expectancy of less than 1 year, and terminal illness were each considered a valid contraindication to treatment with lipid‐lowering medication. Optimal treatment for non‐ACE patients was defined as receipt of lipid‐lowering medication at discharge or a documented LDL‐C of 160 mg/dL. The rate of optimal treatment of ACE patients was compared to that of non‐ACE patients. The ACE and non‐ACE patients were then further categorized into 1 of 4 groups according to LDL‐C level<100, 100130, 130160, and >160 mg/dLand an assessment for trend of the rate of treatment in each of the 4 categories in the ACE and non‐ACE groups was performed.
Data Analysis
Univariate analyses of potential risk factors with lipid testing and treatment were performed using generalized estimating equations (GEEs) in order to account for both within‐hospital and between‐hospital variance and to acknowledge the impact of clustered observations on confidence intervals. Variables significant at the = .10 level were included in the multivariate models. In the subanalyses of patients with documented LDL‐C tests, GEE models were also used to examine factors associated with having an LDL‐C level below 100 mg/dL. A chi‐square test was used to compare the rate of optimal treatment (as defined above) in the group at risk for coronary disease with that in the group not at risk. The Mantel‐Haenszel chi‐squared test was used to compare trends in treatment rate with increasing LDL‐C level. All analyses were performed using SAS (version 8e, SAS Institute, Cary, NC).
RESULTS
Data were available from the 11 CASPR hospitals for 764 patients diagnosed with either ischemic stroke or TIA. Overall, 53.4% of subjects were women, and the average age at hospitalization of 70.4 ( 15.4) years. In the cohort, 55.3% of the patients were non‐Hispanic white, 9.7% were African American, 13.4% were Hispanic, 13% were Asian, and 8.6% were classified as other. Three hundred and nine individuals (40.5% of the cohort) were classified as at risk for coronary events. Of these, 148 (47.8%) had diabetes only, and 160 (51.8%) had a history of MI, CAD, or both. One patient (0.4%) had undergone angioplasty/stenting during hospitalization but had no history of MI, CAD, or diabetes. Only 4 patients (0.52% of the entire cohort) had undergone a carotid endarterectomy or angioplasty/stenting during hospitalization. Rates of lipid assessment and optimal treatment varied widely between hospitals, but testing and treatment were correlated for each hospital. Overall, however, testing and treatment were correlated (Pearson correlation coefficient = 0.35, P < .0001). On an individual hospital level, the correlation was positive and significant for 6 hospitals, positive but not significant for 2 hospitals, and negative but not significant for 3 hospitals.
Overall, LDL‐C levels were determined in 383 patients (50.1%). The likelihood that a patient would have an LDL‐C test performed during hospitalization varied widely by hospital, ranging from 12% to 88% (P < .0001). Univariate variables significantly associated with documented LDL‐C measurement in the overall cohort at the = .10 level were diagnosis of ischemic stroke (as compared to TIA) and history of dyslipidemia (Table 1). In the CASPR cohort, 53% of the ACE subjects received a lipid profile assessment compared to 48% in the rest of the cohort (P = .14). In multivariate analysis, diagnosis of ischemic stroke and history of dyslipidemia remained significantly associated with documented LDL‐C measurement (Table 1).
| Characteristic | n | With LDL‐C | Univariatea | P value | Adjusteda | P value | |||
|---|---|---|---|---|---|---|---|---|---|
| no. | % | OR | (95% CI) | OR | (95% CI) | ||||
| |||||||||
| Median age | |||||||||
| 73 years | 385 | 210 | (54.5) | Ref | |||||
| > 73 years | 379 | 173 | (45.6) | 0.95 | (0.68, 1.34) | .78 | |||
| Sex | |||||||||
| Female | 408 | 189 | (46.3) | Ref | |||||
| Male | 356 | 194 | (54.5) | 1.05 | (0.84, 1.39) | .53 | |||
| Ethnicity | |||||||||
| Other | 341 | 190 | (56.3) | Ref | |||||
| White | 423 | 193 | (45.6) | 0.88 | (0.60, 1.30) | .53 | |||
| Event type | |||||||||
| TIA | 172 | 62 | (36) | Ref | Ref | ||||
| Ischemic stroke | 592 | 321 | (54) | 1.70 | (1.14, 2.54) | .01 | 1.52 | (1.06, 2.19) | .02 |
| Risk of coronary events | 309 | 165 | (53.4) | 1.14 | (0.78, 1.68) | .50 | |||
| History of:b | |||||||||
| Stroke/TIA | 277 | 122 | (44.0) | 0.85 | (0.58, 1.24) | .39 | |||
| Dyslipidemia | 67 | 32 | (47.8) | 0.94 | (0.47, 1.90) | .86 | |||
| MI | 132 | 63 | (47.7) | 0.84 | (0.65, 1.08) | .17 | |||
| CAD | 158 | 96 | (60.8) | 0.95 | (0.67, 1.34) | .76 | |||
| Smoking | 83 | 31 | (37.3) | 0.67 | (0.40, 1.10) | .12 | |||
| Heart failure | 199 | 109 | (54.8) | 1.13 | (0.74, 1.73) | .58 | |||
| Diabetes | 516 | 259 | (50.2) | 1.09 | (0.83, 1.44) | .54 | |||
| Hypertension | 243 | 140 | (57.6) | 1.45 | (0.98, 2.14) | .07 | 1.41 | (1.01, 1.97) | .05 |
| Atrial fibrillation | 125 | 56 | (44.8) | 0.95 | (0.69, 1.32) | .76 | |||
| Received tPA | |||||||||
| No | 748 | 371 | (49.6) | Ref | |||||
| Yes | 16 | 12 | (75.0) | 2.01 | (0.79, 5.11) | .14 | |||
Lipid‐lowering drugs were prescribed at discharge to 370 patients (48.4%); however, treatment rate varied among hospitals, from a low of 13% of patients to a high of 84% of patients (P < .0001). Univariate factors associated with a higher treatment rate at the = .10 level were diagnosis of ischemic stroke, history of stroke/TIA, history of diabetes, hypertension, history of dyslipidemia, independent ambulation at discharge, and ACE status (Table 2). Patients were less likely to receive lipid‐lowering medication if they had a history of heart failure. Fifty‐nine percent of the CASPR ACE subjects were discharged on lipid‐modifying agents compared to 42% in the rest of the cohort (P = .0006). Multivariate analyses revealed several independent predictors of treatment with lipid‐lowering medication. Diagnosis of ischemic stroke, ACE status, and history of heart failure were negative predictors (less likely to be treated), and history of dyslipidemia was a positive predictor (Table 2). Status as an academic hospital was a hospital characteristic for which a significant association was found. Academic hospitals were significantly more likely to both perform LDL profiles and administer lipid‐lowering medications at discharge than were nonacademic hospitals. This association was found in a logistic regression analysis that did not account for between‐hospital variance. However, when we used GEE analysis, which adjusted for the variance, the difference between academic and nonacademic hospitals was no longer significant.
| Characteristic | n | Use of lipid‐lowering medication | Univariatea | P value | Adjusteda | P value | |||
|---|---|---|---|---|---|---|---|---|---|
| no. | % | OR | (95% CI) | OR | (95% CI) | ||||
| |||||||||
| Median age | |||||||||
| 73 years | 385 | 208 | (54.0) | Ref | |||||
| > 73 years | 379 | 162 | (42.7) | 0.79 | (0.59, 1.06) | .11 | |||
| Sex | |||||||||
| Female | 408 | 184 | (45.1) | Ref | |||||
| Male | 356 | 186 | (52.2) | 1.05 | (0.89, 1.25) | .55 | |||
| Ethnicity | |||||||||
| Other | 341 | 190 | (55.7) | Ref | |||||
| White | 423 | 193 | (45.6) | 0.88 | (0.61, 1.27) | .55 | |||
| Event type | |||||||||
| TIA | 172 | 58 | (34) | Ref | Ref | ||||
| Ischemic stroke | 592 | 312 | (53) | 1.92 | (1.39, 2.65) | < .0001 | 1.95 | (1.33, 2.85) | .0009 |
| At risk, coronary events | 309 | 181 | (58.6) | 1.83 | (1.30, 2.59) | .0006 | 1.49 | (1.06, 2.10) | .02 |
| History of:b | |||||||||
| Stroke/TIA | 277 | 141 | (50.9) | 1.43 | (0.97, 2.12) | .07 | 1.30 | 4(0.87, 2.08) | .18 |
| Dyslipidemia | 243 | 192 | (79.0) | 6.62 | (3.28, 13.36) | < .00 01 | 5.77 | 2.65, 12.54) | < .0001 |
| MI | 67 | 42 | (62.7) | 1.77 | (0.90, 3.45) | .10 | a | ||
| CAD | 132 | 28 | (21.2) | 1.49 | (0.87, 2.54) | .14 | |||
| Smoking | 158 | 89 | (56.3) | 1.00 | (0.74, 1.28) | .86 | |||
| Heart failure | 83 | 28 | (33.7) | 0.60 | (0.41, 0.87) | .007 | 0.40 | 0.26, 0.61) | < .0001 |
| Diabetes | 199 | 119 | (59.8) | 1.67 | (1.26, 2.20) | .007 | a | ||
| Hypertension | 516 | 271 | (52.5)1.82 | (1.45, 2.27) | < .0001 | 1.36 | 7(0.88, 2.212) | .16 | |
| Atrial fibrillation | 125 | 51 | (40.8) | 0.79 | (0.55, 1.12) | 18 | |||
| Received lipid profile | 383 | 253 | (66.1) | 2.77 | (1.75, 4.38) | < .0001 | 2.46 | (1.53, 3.97) | .0002 |
| Received tPA | |||||||||
| No | 748 | 360 | (48.1) | Ref | |||||
| Yes | 16 | 9 | (56.3) | 1.26 | (0.58, 2.71) | .56 | |||
| Ambulatory at discharge | 400 | 206 | (51.5) | 1.36 | (1.05, 1.78) | .02 | 1.33 | (0.96, 1.80) | 0.09 |
Three of the patients with documented LDL‐C levels (0.8%) had documented contraindications to therapy. Among all those who had documented LDL‐C levels, the rate of appropriate treatment with lipid‐lowering medications was high in both the ACE and non‐ACE groups (94.6% and 98.6%, respectively; P = .02). However, because only a small number of patients did not receive optimal treatment, the odds ratio of 0.24 had a fairly wide confidence interval (95% CI = 0.06, 0.91). Although a trend toward a higher rate of treatment with increasing LDL‐C level was seen in both the ACE and non‐ACE groups, this trend was only significant for the group with non‐ACE patients (Figure 1).
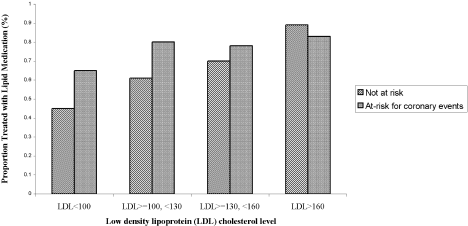
DISCUSSION
We found that only half the patients hospitalized for ischemic stroke or TIA had LDL‐C levels tested while in the hospital, even among those identified by the ATP guidelines as at high risk for future coronary events. Our findings are in accord with those of the Coverdell Project, which evaluated key features of acute stroke care from 4 prototype registries, those in Georgia, Massachusetts, Michigan, and Ohio, finding that fewer than 40% of acute stroke patients had had lipid profiles checked during hospitalization.11 Our study also evaluated predictors for in‐hospital lipid testing and lipid‐lowering treatment during hospitalization for an acute ischemic cerebrovascular event. We found that lipid testing was correlated with treatment during stroke or TIA hospitalization, suggesting that in‐hospital lipid management is related to an overall appreciation of the importance of lipids.
Understanding the factors resulting in such underperformance is critical for improving patient care and outcomes. Lipid assessment and treatment rates varied widely between CASPR hospitals, reflecting dramatic differences in hospital practice. This finding is similar to that noted in a recent study performed in Europe10 and underscores the need to promote a more uniform approach to in‐hospital care of patients with ischemic stroke or TIA. Our study also found that ischemic stroke patients were much more likely to have their lipid level measured and to be discharged on a lipid‐lowering agent than were TIA patients. This may be so because many treating health care professionals perceive TIAs as benign events that carry a more favorable prognosis than do strokes, or it could be that the length of stay for a TIA, often shorter than that for a stroke, limited in‐hospital testing or planning for patient follow‐up.
A high proportion of non‐ACE, lipid‐tested stroke/TIA patients received lipid‐lowering drug treatment, even when their lipid levels were within the treatment range categorized as nonpharmacologic by the national guidelines. This finding could be a result of one of the goals of the primary study.15 In the primary study, the effect of standardized orders implemented during the second observational period were analyzed by comparing them to those in place during the first observational period to see if they had improved the in‐hospital stroke care process. One of the study goals was optimal discharge utilization of a lipid‐lowering agent, defined as prescription of a lipid modifier or an LDL < 100 mg/dL. There was a significant increase in the number of prescriptions for lipid modifiers at discharge after implementing the standardized orders.15 However, as this study has shown, when existing national cholesterol guidelines were strictly applied to all the patients,6 overall there was a suboptimal rate of utilization of lipid modifiers at discharge.
Lipid profile assessment during stroke admission is one of the 10 performance measures in the performance measure set of the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) Stroke Disease‐Specific Care.16 Initiating therapy with lipid‐lowering agents before discharge may help to maintain continuity of care and clarify therapeutic intent, especially when a different physician is responsible for care after discharge from the hospital. Recent studies indicated that in‐hospital initiation of medication following admission for a vascular event tends to improve longer‐term patient adherence to treatment,17, 18 as well as vascular outcomes,19, 20 and is a strategy favored by the American Stroke Association.13, 21
This study had several limitations. Our definitions of dyslipidemia and of adherence to ATP III goals were based on single measurements of LDL‐C, rather than multiple determinations of lipoprotein subfractions. However, we believe that this approach parallels actual clinical practice more closely. Although LDL‐C is the most important of all the components of the lipid profile,6 because lipid subfractions other than LDL‐C were not collected in the CASPR registry, we may have misclassified a few patients. For instance, extremely high trigylceride levels can render LDL‐C levels inaccurate, and as such, not having a documented LDL‐C may not have always indicated that a lipid panel was not performed. It is also conceivable that physicians might actually have been more thorough in measuring LDL‐C, identifying contraindications to lipid‐lowering therapy, or instituting lipid‐lowering therapy than were noted in the hospital charts. However, for quality assurance purposes, what is documented is the only traceable record of what was actually asked for or done. As such, health care professionals are frequently encouraged to keep updated chart notes. This study was an assessment of in‐hospital behavior; the low utilization of lipid‐lowering agents observed may underestimate the final treatment rate, as we did not evaluate the postdischarge rate of therapy. However, recent data suggest in‐hospital prescription patterns are a major predictor of longer‐term care in the community.17, 22 Last, the CASPR investigators did not collect data on the rate of utilization of lipid agents prior to hospitalization or on the mechanisms by which the strokes and TIAs had occurred. Prehospital utilization of lipid agents has previously been revealed to influence the prescribing of lipid‐lowering agents at discharge.10 Knowledge of the mechanisms of the stroke and TIA events would have increased the number of those eligible for lipid treatment, particularly those whose events were to the result of an atherosclerotic mechanism per ATP III's more expansive definition of CHD risk equivalents, which includes carotid and other forms of clinical atherosclerotic disease.6 However, because the results of other studies that evaluated lipid management in all hospitalized stroke patients (regardless of mechanism)11, 23 or in all patients with any form of clinical atherosclerotic disease24 were in accord with those of our study, it would appear unlikely that such information would have made an overwhelming difference to our results.
In conclusion, the results of the present study suggest that considerable improvement is needed in identifying appropriate candidates among those who have had stroke or TIA and treating them with lipid‐lowering agents. Performing lipid testing in individuals hospitalized with ischemic stroke or TIA is important because it may inform the identification of persons for whom treatment should be initiated or modified. Lipid assessment during hospitalization for stroke/TIA and initiation of lipid‐lowering therapy when indicated are major management steps that all patients with ischemic cerebrovascular events should receive.
- ,,, et al.Thrombus formation on atherosclerotic plaques: pathogenesis and clinical consequences.Ann Intern Med.2001;134:224–238.
- ,,, et al.Manifestations of atherosclerosis in various vascular regions. Similarities and differences regarding epidemiology, etiology and prognosis [in German].Med Klin.2002;97:221–228.
- ,,,.Hypolipemic agents for stroke prevention.Clin Exp Hypertens.2002;24:573–594.
- ,,,,,.Differential effects of lipid‐lowering therapies on stroke prevention: a meta‐analysis of randomized trials.Arch Intern Med.2003;163:669–676.
- Heart Protection Study Collaborative Group.Effects of cholesterol‐lowering with simvastatin on stroke and other major vascular events in 20,536 people with cerebrovascular disease or other high‐risk conditions.Lancet.2004;363:757–767.
- Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III).JAMA.2001;285:2486–2497.
- ,,,.The lipid treatment assessment project (L‐TAP): a multicenter survey to evaluate the percentages of dyslipidemic patients receiving lipid‐lowering therapy and achieving low‐density lipoprotein cholesterol goals.Arch Intern Med.2000;160:459–467.
- ,,, et al.Analysis of the degree of undertreatment of hyperlipidemia and congestive heart failure secondary to coronary artery disease.Am J Cardiol.1999;83:1303–1307.
- .Statin therapy after acute myocardial infarction: are we adequately treating high‐risk patients?Curr Atheroscler Rep.2002;4:99–106.
- ,,,.Determination of lipid profiles and use of statins in patients with ischemic stroke or transient ischemic attack.Stroke.2003;34:105–110.
- ,,, et al.Acute stroke care in the US: results from 4 pilot prototypes of the Paul Coverdell National Acute Stroke Registry.Stroke.2005;36:1232–1240.
- ,,.Stroke prevention: narrowing the evidence‐practice gap.Neurology.2000;54:1899–1906.
- Statins after ischemic stroke and transient ischemic attack: an advisory statement from the Stroke Council, American Heart Association and American Stroke Association.Stroke.2004;35:1023.
- California Acute Stroke Pilot Registry (CASPR) Investigators.Prioritizing interventions to improve rates of thrombolysis for ischemic stroke.Neurology.2005;64:654–659.
- California Acute Stroke Pilot Registry (CASPR) Investigators.The impact of standardized stroke orders on adherence to best practices.Neurology.2005;65:360–365.
- JCAHO Stroke Disease‐Specific Care performance measure set. Available at: www.jcaho.org/dscc/dsc/performance+measures/stroke+measure+set.htm. Accessed November 20,2005.
- .The role of in‐hospital initiation of cardiovascular protective therapies to improve treatment rates and clinical outcomes.Rev Cardiovasc Med.2003;4(Suppl 3):S37–S46.
- ,,, et al.In‐hospital initiation of secondary stroke prevention therapies yields high rates of adherence at follow‐up.Stroke.2004;35:2879–2883.
- ,,,.Improved treatment of coronary heart disease by implementation of a Cardiac Hospitalization Atherosclerosis Management Program (CHAMP).Am J Cardiol.2001;87:819–822.
- ,,, et al.Impact of combination evidence‐based medical therapy on mortality in patients with acute coronary syndromes.Circulation.2004;109:745–749.
- American Heart Association Get with the Guidelines Program—Coronary Artery Disease Pilot Test Results. Available at: http://www.americanheart.org/presenter.jhtml?identifier=699. Accessed November 30,2003.
- ,,, et al.In‐hospital initiation of lipid‐lowering therapy after coronary intervention as a predictor of long‐term utilization: a propensity analysis.Arch Intern Med.2003;163:2576–2582.
- University HealthSystem Consortium Ischemic Stroke Clinical Benchmarking Project Clinical Database Analysis—2001. University HealthSystem Consortium Ischemic Stroke Database Report #3.
- ,,.Expanding indications for statins in cerebral ischemia: a quantitative study.Arch Neurol.2005;62:67–72.
Aortocervicocephalic atherosclerotic disease and coronary artery disease share common risk factors, and patients with one condition are at high risk of harboring or developing the other.1, 2 Over the past decade, several randomized clinical trials of lipid‐lowering medications designed to reduce low‐density lipoprotein cholesterol (LDL‐C) have shown a significant decrease in the risk of coronary events and ischemic stroke among patients who have a history of or are at risk for coronary artery disease, regardless of whether serum cholesterol is elevated.3, 4 Results from more than 3000 stroke patients enrolled in the Heart Protection Study also provide evidence that aggressive lipid‐lowering therapy may prevent recurrent vascular events in individuals who have a total cholesterol level as low as 135 mg/dL and cerebrovascular disease, with or without known coronary artery disease.5
Guidelines from the National Cholesterol Evaluation Program Adult Treatment Panel (ATP) provide target LDL‐C levels for persons with atherosclerotic disease depending on the extent of their vascular risk.6 However, despite the broad dissemination of these guidelines, several published studies of patients with coronary artery disease or dyslipidemia have shown that a large proportion of patients with high vascular risk continue to be underscreened, underdiagnosed, and undertreated for dyslipidemia.79
Few studies have evaluated the quality of cholesterol management among hospitalized patients who have experienced an acute ischemic cerebrovascular event10, 11 So the data are scarce on the management of patients hospitalized for ischemic stroke or transient ischemic attack (TIA) who are, according to ATP criteria, at high risk for future coronary events and on the factors that may govern that management. Systematic reviews have suggested that incorporating a lipid profile during acute stroke presentation could assure baseline assessment and serve as a potential cue for physicians to change their behavior,12 and an American Stroke Association advisory recommends lipid treatment during hospitalization for most patients with ischemic stroke or TIA as it may increase the rate of long‐term use.13
The objectives of this study were to determine the rates of testing for and treatment of dyslipidemia according to national cholesterol guidelines among individuals hospitalized with acute ischemic stroke or TIA and to identify predictors of performance.
METHODS
The California Acute Stroke Prototype Registry (CASPR) is a Centers for Disease Controlsponsored cohort that captured detailed data on patients admitted to 11 hospitals over a 2‐year period. The methods of study have been described elsewhere.14 In brief, CASPR prospectively collected information on acute stroke care at 11 representative hospitals in 5 major population regions of California. Data were collected on diagnostic evaluation, appropriate use of treatment strategies, and disposition on discharge from the hospital. The main goal of CASPR was to pilot‐test a prototype prospective registry of acute stroke and transient ischemic attack to be used as a quality improvement tool. The study population was patients with an admitting or discharge diagnosis of suspected stroke or TIA from November 1, 2002, through January 31, 2003, and from November 1, 2003, through January 31, 2004. The human subjects review board at each participating center approved the study.
For the present analysis, data on all patients with a discharge diagnosis of ischemic stroke or TIA who were admitted during either period were included. We examined the possible association of several variables with 2 primary outcomes: (1) testing lipid profile during hospitalization (as indicated by a documented LDL‐C level) and (2) prescribing lipid‐lowering medication at discharge. In those analyses in which lipid profile testing was the outcome, no variables were considered acceptable reasons for not performing an LDL‐C assessment.
The distribution of LDL‐C levels in this portion of the cohort was determined. Patients were then categorized according to their risk for future coronary events. Patients were classified as at risk for coronary events (ACE) if they either had a documented history of myocardial infarction, coronary artery disease, or diabetes or had undergone carotid endarterectomy or carotid angioplasty/stenting during hospitalization. Criteria for initiating lipid‐lowering therapy were defined according to the ATP III guidelines,6 which were in effect during both CASPR study periods. Continuing the recommendation in ATP II, the ATP III recommendations emphasized that persons with documented coronary artery disease (CAD) receive the most aggressive lipid‐lowering treatment. But this recommendation was expanded to include patients without established CAD, whose coronary risk is equivalent to that of patients with diagnosed CAD.6
As per the ATP III guidelines, CASPR‐ACE patients were considered optimally treated if they were prescribed a lipid‐lowering agent at discharge or if their documented LDL‐C was less than 130 mg/dL. A concurrent history of liver disease, abnormal prothrombin time, life expectancy of less than 1 year, and terminal illness were each considered a valid contraindication to treatment with lipid‐lowering medication. Optimal treatment for non‐ACE patients was defined as receipt of lipid‐lowering medication at discharge or a documented LDL‐C of 160 mg/dL. The rate of optimal treatment of ACE patients was compared to that of non‐ACE patients. The ACE and non‐ACE patients were then further categorized into 1 of 4 groups according to LDL‐C level<100, 100130, 130160, and >160 mg/dLand an assessment for trend of the rate of treatment in each of the 4 categories in the ACE and non‐ACE groups was performed.
Data Analysis
Univariate analyses of potential risk factors with lipid testing and treatment were performed using generalized estimating equations (GEEs) in order to account for both within‐hospital and between‐hospital variance and to acknowledge the impact of clustered observations on confidence intervals. Variables significant at the = .10 level were included in the multivariate models. In the subanalyses of patients with documented LDL‐C tests, GEE models were also used to examine factors associated with having an LDL‐C level below 100 mg/dL. A chi‐square test was used to compare the rate of optimal treatment (as defined above) in the group at risk for coronary disease with that in the group not at risk. The Mantel‐Haenszel chi‐squared test was used to compare trends in treatment rate with increasing LDL‐C level. All analyses were performed using SAS (version 8e, SAS Institute, Cary, NC).
RESULTS
Data were available from the 11 CASPR hospitals for 764 patients diagnosed with either ischemic stroke or TIA. Overall, 53.4% of subjects were women, and the average age at hospitalization of 70.4 ( 15.4) years. In the cohort, 55.3% of the patients were non‐Hispanic white, 9.7% were African American, 13.4% were Hispanic, 13% were Asian, and 8.6% were classified as other. Three hundred and nine individuals (40.5% of the cohort) were classified as at risk for coronary events. Of these, 148 (47.8%) had diabetes only, and 160 (51.8%) had a history of MI, CAD, or both. One patient (0.4%) had undergone angioplasty/stenting during hospitalization but had no history of MI, CAD, or diabetes. Only 4 patients (0.52% of the entire cohort) had undergone a carotid endarterectomy or angioplasty/stenting during hospitalization. Rates of lipid assessment and optimal treatment varied widely between hospitals, but testing and treatment were correlated for each hospital. Overall, however, testing and treatment were correlated (Pearson correlation coefficient = 0.35, P < .0001). On an individual hospital level, the correlation was positive and significant for 6 hospitals, positive but not significant for 2 hospitals, and negative but not significant for 3 hospitals.
Overall, LDL‐C levels were determined in 383 patients (50.1%). The likelihood that a patient would have an LDL‐C test performed during hospitalization varied widely by hospital, ranging from 12% to 88% (P < .0001). Univariate variables significantly associated with documented LDL‐C measurement in the overall cohort at the = .10 level were diagnosis of ischemic stroke (as compared to TIA) and history of dyslipidemia (Table 1). In the CASPR cohort, 53% of the ACE subjects received a lipid profile assessment compared to 48% in the rest of the cohort (P = .14). In multivariate analysis, diagnosis of ischemic stroke and history of dyslipidemia remained significantly associated with documented LDL‐C measurement (Table 1).
| Characteristic | n | With LDL‐C | Univariatea | P value | Adjusteda | P value | |||
|---|---|---|---|---|---|---|---|---|---|
| no. | % | OR | (95% CI) | OR | (95% CI) | ||||
| |||||||||
| Median age | |||||||||
| 73 years | 385 | 210 | (54.5) | Ref | |||||
| > 73 years | 379 | 173 | (45.6) | 0.95 | (0.68, 1.34) | .78 | |||
| Sex | |||||||||
| Female | 408 | 189 | (46.3) | Ref | |||||
| Male | 356 | 194 | (54.5) | 1.05 | (0.84, 1.39) | .53 | |||
| Ethnicity | |||||||||
| Other | 341 | 190 | (56.3) | Ref | |||||
| White | 423 | 193 | (45.6) | 0.88 | (0.60, 1.30) | .53 | |||
| Event type | |||||||||
| TIA | 172 | 62 | (36) | Ref | Ref | ||||
| Ischemic stroke | 592 | 321 | (54) | 1.70 | (1.14, 2.54) | .01 | 1.52 | (1.06, 2.19) | .02 |
| Risk of coronary events | 309 | 165 | (53.4) | 1.14 | (0.78, 1.68) | .50 | |||
| History of:b | |||||||||
| Stroke/TIA | 277 | 122 | (44.0) | 0.85 | (0.58, 1.24) | .39 | |||
| Dyslipidemia | 67 | 32 | (47.8) | 0.94 | (0.47, 1.90) | .86 | |||
| MI | 132 | 63 | (47.7) | 0.84 | (0.65, 1.08) | .17 | |||
| CAD | 158 | 96 | (60.8) | 0.95 | (0.67, 1.34) | .76 | |||
| Smoking | 83 | 31 | (37.3) | 0.67 | (0.40, 1.10) | .12 | |||
| Heart failure | 199 | 109 | (54.8) | 1.13 | (0.74, 1.73) | .58 | |||
| Diabetes | 516 | 259 | (50.2) | 1.09 | (0.83, 1.44) | .54 | |||
| Hypertension | 243 | 140 | (57.6) | 1.45 | (0.98, 2.14) | .07 | 1.41 | (1.01, 1.97) | .05 |
| Atrial fibrillation | 125 | 56 | (44.8) | 0.95 | (0.69, 1.32) | .76 | |||
| Received tPA | |||||||||
| No | 748 | 371 | (49.6) | Ref | |||||
| Yes | 16 | 12 | (75.0) | 2.01 | (0.79, 5.11) | .14 | |||
Lipid‐lowering drugs were prescribed at discharge to 370 patients (48.4%); however, treatment rate varied among hospitals, from a low of 13% of patients to a high of 84% of patients (P < .0001). Univariate factors associated with a higher treatment rate at the = .10 level were diagnosis of ischemic stroke, history of stroke/TIA, history of diabetes, hypertension, history of dyslipidemia, independent ambulation at discharge, and ACE status (Table 2). Patients were less likely to receive lipid‐lowering medication if they had a history of heart failure. Fifty‐nine percent of the CASPR ACE subjects were discharged on lipid‐modifying agents compared to 42% in the rest of the cohort (P = .0006). Multivariate analyses revealed several independent predictors of treatment with lipid‐lowering medication. Diagnosis of ischemic stroke, ACE status, and history of heart failure were negative predictors (less likely to be treated), and history of dyslipidemia was a positive predictor (Table 2). Status as an academic hospital was a hospital characteristic for which a significant association was found. Academic hospitals were significantly more likely to both perform LDL profiles and administer lipid‐lowering medications at discharge than were nonacademic hospitals. This association was found in a logistic regression analysis that did not account for between‐hospital variance. However, when we used GEE analysis, which adjusted for the variance, the difference between academic and nonacademic hospitals was no longer significant.
| Characteristic | n | Use of lipid‐lowering medication | Univariatea | P value | Adjusteda | P value | |||
|---|---|---|---|---|---|---|---|---|---|
| no. | % | OR | (95% CI) | OR | (95% CI) | ||||
| |||||||||
| Median age | |||||||||
| 73 years | 385 | 208 | (54.0) | Ref | |||||
| > 73 years | 379 | 162 | (42.7) | 0.79 | (0.59, 1.06) | .11 | |||
| Sex | |||||||||
| Female | 408 | 184 | (45.1) | Ref | |||||
| Male | 356 | 186 | (52.2) | 1.05 | (0.89, 1.25) | .55 | |||
| Ethnicity | |||||||||
| Other | 341 | 190 | (55.7) | Ref | |||||
| White | 423 | 193 | (45.6) | 0.88 | (0.61, 1.27) | .55 | |||
| Event type | |||||||||
| TIA | 172 | 58 | (34) | Ref | Ref | ||||
| Ischemic stroke | 592 | 312 | (53) | 1.92 | (1.39, 2.65) | < .0001 | 1.95 | (1.33, 2.85) | .0009 |
| At risk, coronary events | 309 | 181 | (58.6) | 1.83 | (1.30, 2.59) | .0006 | 1.49 | (1.06, 2.10) | .02 |
| History of:b | |||||||||
| Stroke/TIA | 277 | 141 | (50.9) | 1.43 | (0.97, 2.12) | .07 | 1.30 | 4(0.87, 2.08) | .18 |
| Dyslipidemia | 243 | 192 | (79.0) | 6.62 | (3.28, 13.36) | < .00 01 | 5.77 | 2.65, 12.54) | < .0001 |
| MI | 67 | 42 | (62.7) | 1.77 | (0.90, 3.45) | .10 | a | ||
| CAD | 132 | 28 | (21.2) | 1.49 | (0.87, 2.54) | .14 | |||
| Smoking | 158 | 89 | (56.3) | 1.00 | (0.74, 1.28) | .86 | |||
| Heart failure | 83 | 28 | (33.7) | 0.60 | (0.41, 0.87) | .007 | 0.40 | 0.26, 0.61) | < .0001 |
| Diabetes | 199 | 119 | (59.8) | 1.67 | (1.26, 2.20) | .007 | a | ||
| Hypertension | 516 | 271 | (52.5)1.82 | (1.45, 2.27) | < .0001 | 1.36 | 7(0.88, 2.212) | .16 | |
| Atrial fibrillation | 125 | 51 | (40.8) | 0.79 | (0.55, 1.12) | 18 | |||
| Received lipid profile | 383 | 253 | (66.1) | 2.77 | (1.75, 4.38) | < .0001 | 2.46 | (1.53, 3.97) | .0002 |
| Received tPA | |||||||||
| No | 748 | 360 | (48.1) | Ref | |||||
| Yes | 16 | 9 | (56.3) | 1.26 | (0.58, 2.71) | .56 | |||
| Ambulatory at discharge | 400 | 206 | (51.5) | 1.36 | (1.05, 1.78) | .02 | 1.33 | (0.96, 1.80) | 0.09 |
Three of the patients with documented LDL‐C levels (0.8%) had documented contraindications to therapy. Among all those who had documented LDL‐C levels, the rate of appropriate treatment with lipid‐lowering medications was high in both the ACE and non‐ACE groups (94.6% and 98.6%, respectively; P = .02). However, because only a small number of patients did not receive optimal treatment, the odds ratio of 0.24 had a fairly wide confidence interval (95% CI = 0.06, 0.91). Although a trend toward a higher rate of treatment with increasing LDL‐C level was seen in both the ACE and non‐ACE groups, this trend was only significant for the group with non‐ACE patients (Figure 1).

DISCUSSION
We found that only half the patients hospitalized for ischemic stroke or TIA had LDL‐C levels tested while in the hospital, even among those identified by the ATP guidelines as at high risk for future coronary events. Our findings are in accord with those of the Coverdell Project, which evaluated key features of acute stroke care from 4 prototype registries, those in Georgia, Massachusetts, Michigan, and Ohio, finding that fewer than 40% of acute stroke patients had had lipid profiles checked during hospitalization.11 Our study also evaluated predictors for in‐hospital lipid testing and lipid‐lowering treatment during hospitalization for an acute ischemic cerebrovascular event. We found that lipid testing was correlated with treatment during stroke or TIA hospitalization, suggesting that in‐hospital lipid management is related to an overall appreciation of the importance of lipids.
Understanding the factors resulting in such underperformance is critical for improving patient care and outcomes. Lipid assessment and treatment rates varied widely between CASPR hospitals, reflecting dramatic differences in hospital practice. This finding is similar to that noted in a recent study performed in Europe10 and underscores the need to promote a more uniform approach to in‐hospital care of patients with ischemic stroke or TIA. Our study also found that ischemic stroke patients were much more likely to have their lipid level measured and to be discharged on a lipid‐lowering agent than were TIA patients. This may be so because many treating health care professionals perceive TIAs as benign events that carry a more favorable prognosis than do strokes, or it could be that the length of stay for a TIA, often shorter than that for a stroke, limited in‐hospital testing or planning for patient follow‐up.
A high proportion of non‐ACE, lipid‐tested stroke/TIA patients received lipid‐lowering drug treatment, even when their lipid levels were within the treatment range categorized as nonpharmacologic by the national guidelines. This finding could be a result of one of the goals of the primary study.15 In the primary study, the effect of standardized orders implemented during the second observational period were analyzed by comparing them to those in place during the first observational period to see if they had improved the in‐hospital stroke care process. One of the study goals was optimal discharge utilization of a lipid‐lowering agent, defined as prescription of a lipid modifier or an LDL < 100 mg/dL. There was a significant increase in the number of prescriptions for lipid modifiers at discharge after implementing the standardized orders.15 However, as this study has shown, when existing national cholesterol guidelines were strictly applied to all the patients,6 overall there was a suboptimal rate of utilization of lipid modifiers at discharge.
Lipid profile assessment during stroke admission is one of the 10 performance measures in the performance measure set of the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) Stroke Disease‐Specific Care.16 Initiating therapy with lipid‐lowering agents before discharge may help to maintain continuity of care and clarify therapeutic intent, especially when a different physician is responsible for care after discharge from the hospital. Recent studies indicated that in‐hospital initiation of medication following admission for a vascular event tends to improve longer‐term patient adherence to treatment,17, 18 as well as vascular outcomes,19, 20 and is a strategy favored by the American Stroke Association.13, 21
This study had several limitations. Our definitions of dyslipidemia and of adherence to ATP III goals were based on single measurements of LDL‐C, rather than multiple determinations of lipoprotein subfractions. However, we believe that this approach parallels actual clinical practice more closely. Although LDL‐C is the most important of all the components of the lipid profile,6 because lipid subfractions other than LDL‐C were not collected in the CASPR registry, we may have misclassified a few patients. For instance, extremely high trigylceride levels can render LDL‐C levels inaccurate, and as such, not having a documented LDL‐C may not have always indicated that a lipid panel was not performed. It is also conceivable that physicians might actually have been more thorough in measuring LDL‐C, identifying contraindications to lipid‐lowering therapy, or instituting lipid‐lowering therapy than were noted in the hospital charts. However, for quality assurance purposes, what is documented is the only traceable record of what was actually asked for or done. As such, health care professionals are frequently encouraged to keep updated chart notes. This study was an assessment of in‐hospital behavior; the low utilization of lipid‐lowering agents observed may underestimate the final treatment rate, as we did not evaluate the postdischarge rate of therapy. However, recent data suggest in‐hospital prescription patterns are a major predictor of longer‐term care in the community.17, 22 Last, the CASPR investigators did not collect data on the rate of utilization of lipid agents prior to hospitalization or on the mechanisms by which the strokes and TIAs had occurred. Prehospital utilization of lipid agents has previously been revealed to influence the prescribing of lipid‐lowering agents at discharge.10 Knowledge of the mechanisms of the stroke and TIA events would have increased the number of those eligible for lipid treatment, particularly those whose events were to the result of an atherosclerotic mechanism per ATP III's more expansive definition of CHD risk equivalents, which includes carotid and other forms of clinical atherosclerotic disease.6 However, because the results of other studies that evaluated lipid management in all hospitalized stroke patients (regardless of mechanism)11, 23 or in all patients with any form of clinical atherosclerotic disease24 were in accord with those of our study, it would appear unlikely that such information would have made an overwhelming difference to our results.
In conclusion, the results of the present study suggest that considerable improvement is needed in identifying appropriate candidates among those who have had stroke or TIA and treating them with lipid‐lowering agents. Performing lipid testing in individuals hospitalized with ischemic stroke or TIA is important because it may inform the identification of persons for whom treatment should be initiated or modified. Lipid assessment during hospitalization for stroke/TIA and initiation of lipid‐lowering therapy when indicated are major management steps that all patients with ischemic cerebrovascular events should receive.
Aortocervicocephalic atherosclerotic disease and coronary artery disease share common risk factors, and patients with one condition are at high risk of harboring or developing the other.1, 2 Over the past decade, several randomized clinical trials of lipid‐lowering medications designed to reduce low‐density lipoprotein cholesterol (LDL‐C) have shown a significant decrease in the risk of coronary events and ischemic stroke among patients who have a history of or are at risk for coronary artery disease, regardless of whether serum cholesterol is elevated.3, 4 Results from more than 3000 stroke patients enrolled in the Heart Protection Study also provide evidence that aggressive lipid‐lowering therapy may prevent recurrent vascular events in individuals who have a total cholesterol level as low as 135 mg/dL and cerebrovascular disease, with or without known coronary artery disease.5
Guidelines from the National Cholesterol Evaluation Program Adult Treatment Panel (ATP) provide target LDL‐C levels for persons with atherosclerotic disease depending on the extent of their vascular risk.6 However, despite the broad dissemination of these guidelines, several published studies of patients with coronary artery disease or dyslipidemia have shown that a large proportion of patients with high vascular risk continue to be underscreened, underdiagnosed, and undertreated for dyslipidemia.79
Few studies have evaluated the quality of cholesterol management among hospitalized patients who have experienced an acute ischemic cerebrovascular event10, 11 So the data are scarce on the management of patients hospitalized for ischemic stroke or transient ischemic attack (TIA) who are, according to ATP criteria, at high risk for future coronary events and on the factors that may govern that management. Systematic reviews have suggested that incorporating a lipid profile during acute stroke presentation could assure baseline assessment and serve as a potential cue for physicians to change their behavior,12 and an American Stroke Association advisory recommends lipid treatment during hospitalization for most patients with ischemic stroke or TIA as it may increase the rate of long‐term use.13
The objectives of this study were to determine the rates of testing for and treatment of dyslipidemia according to national cholesterol guidelines among individuals hospitalized with acute ischemic stroke or TIA and to identify predictors of performance.
METHODS
The California Acute Stroke Prototype Registry (CASPR) is a Centers for Disease Controlsponsored cohort that captured detailed data on patients admitted to 11 hospitals over a 2‐year period. The methods of study have been described elsewhere.14 In brief, CASPR prospectively collected information on acute stroke care at 11 representative hospitals in 5 major population regions of California. Data were collected on diagnostic evaluation, appropriate use of treatment strategies, and disposition on discharge from the hospital. The main goal of CASPR was to pilot‐test a prototype prospective registry of acute stroke and transient ischemic attack to be used as a quality improvement tool. The study population was patients with an admitting or discharge diagnosis of suspected stroke or TIA from November 1, 2002, through January 31, 2003, and from November 1, 2003, through January 31, 2004. The human subjects review board at each participating center approved the study.
For the present analysis, data on all patients with a discharge diagnosis of ischemic stroke or TIA who were admitted during either period were included. We examined the possible association of several variables with 2 primary outcomes: (1) testing lipid profile during hospitalization (as indicated by a documented LDL‐C level) and (2) prescribing lipid‐lowering medication at discharge. In those analyses in which lipid profile testing was the outcome, no variables were considered acceptable reasons for not performing an LDL‐C assessment.
The distribution of LDL‐C levels in this portion of the cohort was determined. Patients were then categorized according to their risk for future coronary events. Patients were classified as at risk for coronary events (ACE) if they either had a documented history of myocardial infarction, coronary artery disease, or diabetes or had undergone carotid endarterectomy or carotid angioplasty/stenting during hospitalization. Criteria for initiating lipid‐lowering therapy were defined according to the ATP III guidelines,6 which were in effect during both CASPR study periods. Continuing the recommendation in ATP II, the ATP III recommendations emphasized that persons with documented coronary artery disease (CAD) receive the most aggressive lipid‐lowering treatment. But this recommendation was expanded to include patients without established CAD, whose coronary risk is equivalent to that of patients with diagnosed CAD.6
As per the ATP III guidelines, CASPR‐ACE patients were considered optimally treated if they were prescribed a lipid‐lowering agent at discharge or if their documented LDL‐C was less than 130 mg/dL. A concurrent history of liver disease, abnormal prothrombin time, life expectancy of less than 1 year, and terminal illness were each considered a valid contraindication to treatment with lipid‐lowering medication. Optimal treatment for non‐ACE patients was defined as receipt of lipid‐lowering medication at discharge or a documented LDL‐C of 160 mg/dL. The rate of optimal treatment of ACE patients was compared to that of non‐ACE patients. The ACE and non‐ACE patients were then further categorized into 1 of 4 groups according to LDL‐C level<100, 100130, 130160, and >160 mg/dLand an assessment for trend of the rate of treatment in each of the 4 categories in the ACE and non‐ACE groups was performed.
Data Analysis
Univariate analyses of potential risk factors with lipid testing and treatment were performed using generalized estimating equations (GEEs) in order to account for both within‐hospital and between‐hospital variance and to acknowledge the impact of clustered observations on confidence intervals. Variables significant at the = .10 level were included in the multivariate models. In the subanalyses of patients with documented LDL‐C tests, GEE models were also used to examine factors associated with having an LDL‐C level below 100 mg/dL. A chi‐square test was used to compare the rate of optimal treatment (as defined above) in the group at risk for coronary disease with that in the group not at risk. The Mantel‐Haenszel chi‐squared test was used to compare trends in treatment rate with increasing LDL‐C level. All analyses were performed using SAS (version 8e, SAS Institute, Cary, NC).
RESULTS
Data were available from the 11 CASPR hospitals for 764 patients diagnosed with either ischemic stroke or TIA. Overall, 53.4% of subjects were women, and the average age at hospitalization of 70.4 ( 15.4) years. In the cohort, 55.3% of the patients were non‐Hispanic white, 9.7% were African American, 13.4% were Hispanic, 13% were Asian, and 8.6% were classified as other. Three hundred and nine individuals (40.5% of the cohort) were classified as at risk for coronary events. Of these, 148 (47.8%) had diabetes only, and 160 (51.8%) had a history of MI, CAD, or both. One patient (0.4%) had undergone angioplasty/stenting during hospitalization but had no history of MI, CAD, or diabetes. Only 4 patients (0.52% of the entire cohort) had undergone a carotid endarterectomy or angioplasty/stenting during hospitalization. Rates of lipid assessment and optimal treatment varied widely between hospitals, but testing and treatment were correlated for each hospital. Overall, however, testing and treatment were correlated (Pearson correlation coefficient = 0.35, P < .0001). On an individual hospital level, the correlation was positive and significant for 6 hospitals, positive but not significant for 2 hospitals, and negative but not significant for 3 hospitals.
Overall, LDL‐C levels were determined in 383 patients (50.1%). The likelihood that a patient would have an LDL‐C test performed during hospitalization varied widely by hospital, ranging from 12% to 88% (P < .0001). Univariate variables significantly associated with documented LDL‐C measurement in the overall cohort at the = .10 level were diagnosis of ischemic stroke (as compared to TIA) and history of dyslipidemia (Table 1). In the CASPR cohort, 53% of the ACE subjects received a lipid profile assessment compared to 48% in the rest of the cohort (P = .14). In multivariate analysis, diagnosis of ischemic stroke and history of dyslipidemia remained significantly associated with documented LDL‐C measurement (Table 1).
| Characteristic | n | With LDL‐C | Univariatea | P value | Adjusteda | P value | |||
|---|---|---|---|---|---|---|---|---|---|
| no. | % | OR | (95% CI) | OR | (95% CI) | ||||
| |||||||||
| Median age | |||||||||
| 73 years | 385 | 210 | (54.5) | Ref | |||||
| > 73 years | 379 | 173 | (45.6) | 0.95 | (0.68, 1.34) | .78 | |||
| Sex | |||||||||
| Female | 408 | 189 | (46.3) | Ref | |||||
| Male | 356 | 194 | (54.5) | 1.05 | (0.84, 1.39) | .53 | |||
| Ethnicity | |||||||||
| Other | 341 | 190 | (56.3) | Ref | |||||
| White | 423 | 193 | (45.6) | 0.88 | (0.60, 1.30) | .53 | |||
| Event type | |||||||||
| TIA | 172 | 62 | (36) | Ref | Ref | ||||
| Ischemic stroke | 592 | 321 | (54) | 1.70 | (1.14, 2.54) | .01 | 1.52 | (1.06, 2.19) | .02 |
| Risk of coronary events | 309 | 165 | (53.4) | 1.14 | (0.78, 1.68) | .50 | |||
| History of:b | |||||||||
| Stroke/TIA | 277 | 122 | (44.0) | 0.85 | (0.58, 1.24) | .39 | |||
| Dyslipidemia | 67 | 32 | (47.8) | 0.94 | (0.47, 1.90) | .86 | |||
| MI | 132 | 63 | (47.7) | 0.84 | (0.65, 1.08) | .17 | |||
| CAD | 158 | 96 | (60.8) | 0.95 | (0.67, 1.34) | .76 | |||
| Smoking | 83 | 31 | (37.3) | 0.67 | (0.40, 1.10) | .12 | |||
| Heart failure | 199 | 109 | (54.8) | 1.13 | (0.74, 1.73) | .58 | |||
| Diabetes | 516 | 259 | (50.2) | 1.09 | (0.83, 1.44) | .54 | |||
| Hypertension | 243 | 140 | (57.6) | 1.45 | (0.98, 2.14) | .07 | 1.41 | (1.01, 1.97) | .05 |
| Atrial fibrillation | 125 | 56 | (44.8) | 0.95 | (0.69, 1.32) | .76 | |||
| Received tPA | |||||||||
| No | 748 | 371 | (49.6) | Ref | |||||
| Yes | 16 | 12 | (75.0) | 2.01 | (0.79, 5.11) | .14 | |||
Lipid‐lowering drugs were prescribed at discharge to 370 patients (48.4%); however, treatment rate varied among hospitals, from a low of 13% of patients to a high of 84% of patients (P < .0001). Univariate factors associated with a higher treatment rate at the = .10 level were diagnosis of ischemic stroke, history of stroke/TIA, history of diabetes, hypertension, history of dyslipidemia, independent ambulation at discharge, and ACE status (Table 2). Patients were less likely to receive lipid‐lowering medication if they had a history of heart failure. Fifty‐nine percent of the CASPR ACE subjects were discharged on lipid‐modifying agents compared to 42% in the rest of the cohort (P = .0006). Multivariate analyses revealed several independent predictors of treatment with lipid‐lowering medication. Diagnosis of ischemic stroke, ACE status, and history of heart failure were negative predictors (less likely to be treated), and history of dyslipidemia was a positive predictor (Table 2). Status as an academic hospital was a hospital characteristic for which a significant association was found. Academic hospitals were significantly more likely to both perform LDL profiles and administer lipid‐lowering medications at discharge than were nonacademic hospitals. This association was found in a logistic regression analysis that did not account for between‐hospital variance. However, when we used GEE analysis, which adjusted for the variance, the difference between academic and nonacademic hospitals was no longer significant.
| Characteristic | n | Use of lipid‐lowering medication | Univariatea | P value | Adjusteda | P value | |||
|---|---|---|---|---|---|---|---|---|---|
| no. | % | OR | (95% CI) | OR | (95% CI) | ||||
| |||||||||
| Median age | |||||||||
| 73 years | 385 | 208 | (54.0) | Ref | |||||
| > 73 years | 379 | 162 | (42.7) | 0.79 | (0.59, 1.06) | .11 | |||
| Sex | |||||||||
| Female | 408 | 184 | (45.1) | Ref | |||||
| Male | 356 | 186 | (52.2) | 1.05 | (0.89, 1.25) | .55 | |||
| Ethnicity | |||||||||
| Other | 341 | 190 | (55.7) | Ref | |||||
| White | 423 | 193 | (45.6) | 0.88 | (0.61, 1.27) | .55 | |||
| Event type | |||||||||
| TIA | 172 | 58 | (34) | Ref | Ref | ||||
| Ischemic stroke | 592 | 312 | (53) | 1.92 | (1.39, 2.65) | < .0001 | 1.95 | (1.33, 2.85) | .0009 |
| At risk, coronary events | 309 | 181 | (58.6) | 1.83 | (1.30, 2.59) | .0006 | 1.49 | (1.06, 2.10) | .02 |
| History of:b | |||||||||
| Stroke/TIA | 277 | 141 | (50.9) | 1.43 | (0.97, 2.12) | .07 | 1.30 | 4(0.87, 2.08) | .18 |
| Dyslipidemia | 243 | 192 | (79.0) | 6.62 | (3.28, 13.36) | < .00 01 | 5.77 | 2.65, 12.54) | < .0001 |
| MI | 67 | 42 | (62.7) | 1.77 | (0.90, 3.45) | .10 | a | ||
| CAD | 132 | 28 | (21.2) | 1.49 | (0.87, 2.54) | .14 | |||
| Smoking | 158 | 89 | (56.3) | 1.00 | (0.74, 1.28) | .86 | |||
| Heart failure | 83 | 28 | (33.7) | 0.60 | (0.41, 0.87) | .007 | 0.40 | 0.26, 0.61) | < .0001 |
| Diabetes | 199 | 119 | (59.8) | 1.67 | (1.26, 2.20) | .007 | a | ||
| Hypertension | 516 | 271 | (52.5)1.82 | (1.45, 2.27) | < .0001 | 1.36 | 7(0.88, 2.212) | .16 | |
| Atrial fibrillation | 125 | 51 | (40.8) | 0.79 | (0.55, 1.12) | 18 | |||
| Received lipid profile | 383 | 253 | (66.1) | 2.77 | (1.75, 4.38) | < .0001 | 2.46 | (1.53, 3.97) | .0002 |
| Received tPA | |||||||||
| No | 748 | 360 | (48.1) | Ref | |||||
| Yes | 16 | 9 | (56.3) | 1.26 | (0.58, 2.71) | .56 | |||
| Ambulatory at discharge | 400 | 206 | (51.5) | 1.36 | (1.05, 1.78) | .02 | 1.33 | (0.96, 1.80) | 0.09 |
Three of the patients with documented LDL‐C levels (0.8%) had documented contraindications to therapy. Among all those who had documented LDL‐C levels, the rate of appropriate treatment with lipid‐lowering medications was high in both the ACE and non‐ACE groups (94.6% and 98.6%, respectively; P = .02). However, because only a small number of patients did not receive optimal treatment, the odds ratio of 0.24 had a fairly wide confidence interval (95% CI = 0.06, 0.91). Although a trend toward a higher rate of treatment with increasing LDL‐C level was seen in both the ACE and non‐ACE groups, this trend was only significant for the group with non‐ACE patients (Figure 1).

DISCUSSION
We found that only half the patients hospitalized for ischemic stroke or TIA had LDL‐C levels tested while in the hospital, even among those identified by the ATP guidelines as at high risk for future coronary events. Our findings are in accord with those of the Coverdell Project, which evaluated key features of acute stroke care from 4 prototype registries, those in Georgia, Massachusetts, Michigan, and Ohio, finding that fewer than 40% of acute stroke patients had had lipid profiles checked during hospitalization.11 Our study also evaluated predictors for in‐hospital lipid testing and lipid‐lowering treatment during hospitalization for an acute ischemic cerebrovascular event. We found that lipid testing was correlated with treatment during stroke or TIA hospitalization, suggesting that in‐hospital lipid management is related to an overall appreciation of the importance of lipids.
Understanding the factors resulting in such underperformance is critical for improving patient care and outcomes. Lipid assessment and treatment rates varied widely between CASPR hospitals, reflecting dramatic differences in hospital practice. This finding is similar to that noted in a recent study performed in Europe10 and underscores the need to promote a more uniform approach to in‐hospital care of patients with ischemic stroke or TIA. Our study also found that ischemic stroke patients were much more likely to have their lipid level measured and to be discharged on a lipid‐lowering agent than were TIA patients. This may be so because many treating health care professionals perceive TIAs as benign events that carry a more favorable prognosis than do strokes, or it could be that the length of stay for a TIA, often shorter than that for a stroke, limited in‐hospital testing or planning for patient follow‐up.
A high proportion of non‐ACE, lipid‐tested stroke/TIA patients received lipid‐lowering drug treatment, even when their lipid levels were within the treatment range categorized as nonpharmacologic by the national guidelines. This finding could be a result of one of the goals of the primary study.15 In the primary study, the effect of standardized orders implemented during the second observational period were analyzed by comparing them to those in place during the first observational period to see if they had improved the in‐hospital stroke care process. One of the study goals was optimal discharge utilization of a lipid‐lowering agent, defined as prescription of a lipid modifier or an LDL < 100 mg/dL. There was a significant increase in the number of prescriptions for lipid modifiers at discharge after implementing the standardized orders.15 However, as this study has shown, when existing national cholesterol guidelines were strictly applied to all the patients,6 overall there was a suboptimal rate of utilization of lipid modifiers at discharge.
Lipid profile assessment during stroke admission is one of the 10 performance measures in the performance measure set of the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) Stroke Disease‐Specific Care.16 Initiating therapy with lipid‐lowering agents before discharge may help to maintain continuity of care and clarify therapeutic intent, especially when a different physician is responsible for care after discharge from the hospital. Recent studies indicated that in‐hospital initiation of medication following admission for a vascular event tends to improve longer‐term patient adherence to treatment,17, 18 as well as vascular outcomes,19, 20 and is a strategy favored by the American Stroke Association.13, 21
This study had several limitations. Our definitions of dyslipidemia and of adherence to ATP III goals were based on single measurements of LDL‐C, rather than multiple determinations of lipoprotein subfractions. However, we believe that this approach parallels actual clinical practice more closely. Although LDL‐C is the most important of all the components of the lipid profile,6 because lipid subfractions other than LDL‐C were not collected in the CASPR registry, we may have misclassified a few patients. For instance, extremely high trigylceride levels can render LDL‐C levels inaccurate, and as such, not having a documented LDL‐C may not have always indicated that a lipid panel was not performed. It is also conceivable that physicians might actually have been more thorough in measuring LDL‐C, identifying contraindications to lipid‐lowering therapy, or instituting lipid‐lowering therapy than were noted in the hospital charts. However, for quality assurance purposes, what is documented is the only traceable record of what was actually asked for or done. As such, health care professionals are frequently encouraged to keep updated chart notes. This study was an assessment of in‐hospital behavior; the low utilization of lipid‐lowering agents observed may underestimate the final treatment rate, as we did not evaluate the postdischarge rate of therapy. However, recent data suggest in‐hospital prescription patterns are a major predictor of longer‐term care in the community.17, 22 Last, the CASPR investigators did not collect data on the rate of utilization of lipid agents prior to hospitalization or on the mechanisms by which the strokes and TIAs had occurred. Prehospital utilization of lipid agents has previously been revealed to influence the prescribing of lipid‐lowering agents at discharge.10 Knowledge of the mechanisms of the stroke and TIA events would have increased the number of those eligible for lipid treatment, particularly those whose events were to the result of an atherosclerotic mechanism per ATP III's more expansive definition of CHD risk equivalents, which includes carotid and other forms of clinical atherosclerotic disease.6 However, because the results of other studies that evaluated lipid management in all hospitalized stroke patients (regardless of mechanism)11, 23 or in all patients with any form of clinical atherosclerotic disease24 were in accord with those of our study, it would appear unlikely that such information would have made an overwhelming difference to our results.
In conclusion, the results of the present study suggest that considerable improvement is needed in identifying appropriate candidates among those who have had stroke or TIA and treating them with lipid‐lowering agents. Performing lipid testing in individuals hospitalized with ischemic stroke or TIA is important because it may inform the identification of persons for whom treatment should be initiated or modified. Lipid assessment during hospitalization for stroke/TIA and initiation of lipid‐lowering therapy when indicated are major management steps that all patients with ischemic cerebrovascular events should receive.
- ,,, et al.Thrombus formation on atherosclerotic plaques: pathogenesis and clinical consequences.Ann Intern Med.2001;134:224–238.
- ,,, et al.Manifestations of atherosclerosis in various vascular regions. Similarities and differences regarding epidemiology, etiology and prognosis [in German].Med Klin.2002;97:221–228.
- ,,,.Hypolipemic agents for stroke prevention.Clin Exp Hypertens.2002;24:573–594.
- ,,,,,.Differential effects of lipid‐lowering therapies on stroke prevention: a meta‐analysis of randomized trials.Arch Intern Med.2003;163:669–676.
- Heart Protection Study Collaborative Group.Effects of cholesterol‐lowering with simvastatin on stroke and other major vascular events in 20,536 people with cerebrovascular disease or other high‐risk conditions.Lancet.2004;363:757–767.
- Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III).JAMA.2001;285:2486–2497.
- ,,,.The lipid treatment assessment project (L‐TAP): a multicenter survey to evaluate the percentages of dyslipidemic patients receiving lipid‐lowering therapy and achieving low‐density lipoprotein cholesterol goals.Arch Intern Med.2000;160:459–467.
- ,,, et al.Analysis of the degree of undertreatment of hyperlipidemia and congestive heart failure secondary to coronary artery disease.Am J Cardiol.1999;83:1303–1307.
- .Statin therapy after acute myocardial infarction: are we adequately treating high‐risk patients?Curr Atheroscler Rep.2002;4:99–106.
- ,,,.Determination of lipid profiles and use of statins in patients with ischemic stroke or transient ischemic attack.Stroke.2003;34:105–110.
- ,,, et al.Acute stroke care in the US: results from 4 pilot prototypes of the Paul Coverdell National Acute Stroke Registry.Stroke.2005;36:1232–1240.
- ,,.Stroke prevention: narrowing the evidence‐practice gap.Neurology.2000;54:1899–1906.
- Statins after ischemic stroke and transient ischemic attack: an advisory statement from the Stroke Council, American Heart Association and American Stroke Association.Stroke.2004;35:1023.
- California Acute Stroke Pilot Registry (CASPR) Investigators.Prioritizing interventions to improve rates of thrombolysis for ischemic stroke.Neurology.2005;64:654–659.
- California Acute Stroke Pilot Registry (CASPR) Investigators.The impact of standardized stroke orders on adherence to best practices.Neurology.2005;65:360–365.
- JCAHO Stroke Disease‐Specific Care performance measure set. Available at: www.jcaho.org/dscc/dsc/performance+measures/stroke+measure+set.htm. Accessed November 20,2005.
- .The role of in‐hospital initiation of cardiovascular protective therapies to improve treatment rates and clinical outcomes.Rev Cardiovasc Med.2003;4(Suppl 3):S37–S46.
- ,,, et al.In‐hospital initiation of secondary stroke prevention therapies yields high rates of adherence at follow‐up.Stroke.2004;35:2879–2883.
- ,,,.Improved treatment of coronary heart disease by implementation of a Cardiac Hospitalization Atherosclerosis Management Program (CHAMP).Am J Cardiol.2001;87:819–822.
- ,,, et al.Impact of combination evidence‐based medical therapy on mortality in patients with acute coronary syndromes.Circulation.2004;109:745–749.
- American Heart Association Get with the Guidelines Program—Coronary Artery Disease Pilot Test Results. Available at: http://www.americanheart.org/presenter.jhtml?identifier=699. Accessed November 30,2003.
- ,,, et al.In‐hospital initiation of lipid‐lowering therapy after coronary intervention as a predictor of long‐term utilization: a propensity analysis.Arch Intern Med.2003;163:2576–2582.
- University HealthSystem Consortium Ischemic Stroke Clinical Benchmarking Project Clinical Database Analysis—2001. University HealthSystem Consortium Ischemic Stroke Database Report #3.
- ,,.Expanding indications for statins in cerebral ischemia: a quantitative study.Arch Neurol.2005;62:67–72.
- ,,, et al.Thrombus formation on atherosclerotic plaques: pathogenesis and clinical consequences.Ann Intern Med.2001;134:224–238.
- ,,, et al.Manifestations of atherosclerosis in various vascular regions. Similarities and differences regarding epidemiology, etiology and prognosis [in German].Med Klin.2002;97:221–228.
- ,,,.Hypolipemic agents for stroke prevention.Clin Exp Hypertens.2002;24:573–594.
- ,,,,,.Differential effects of lipid‐lowering therapies on stroke prevention: a meta‐analysis of randomized trials.Arch Intern Med.2003;163:669–676.
- Heart Protection Study Collaborative Group.Effects of cholesterol‐lowering with simvastatin on stroke and other major vascular events in 20,536 people with cerebrovascular disease or other high‐risk conditions.Lancet.2004;363:757–767.
- Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III).JAMA.2001;285:2486–2497.
- ,,,.The lipid treatment assessment project (L‐TAP): a multicenter survey to evaluate the percentages of dyslipidemic patients receiving lipid‐lowering therapy and achieving low‐density lipoprotein cholesterol goals.Arch Intern Med.2000;160:459–467.
- ,,, et al.Analysis of the degree of undertreatment of hyperlipidemia and congestive heart failure secondary to coronary artery disease.Am J Cardiol.1999;83:1303–1307.
- .Statin therapy after acute myocardial infarction: are we adequately treating high‐risk patients?Curr Atheroscler Rep.2002;4:99–106.
- ,,,.Determination of lipid profiles and use of statins in patients with ischemic stroke or transient ischemic attack.Stroke.2003;34:105–110.
- ,,, et al.Acute stroke care in the US: results from 4 pilot prototypes of the Paul Coverdell National Acute Stroke Registry.Stroke.2005;36:1232–1240.
- ,,.Stroke prevention: narrowing the evidence‐practice gap.Neurology.2000;54:1899–1906.
- Statins after ischemic stroke and transient ischemic attack: an advisory statement from the Stroke Council, American Heart Association and American Stroke Association.Stroke.2004;35:1023.
- California Acute Stroke Pilot Registry (CASPR) Investigators.Prioritizing interventions to improve rates of thrombolysis for ischemic stroke.Neurology.2005;64:654–659.
- California Acute Stroke Pilot Registry (CASPR) Investigators.The impact of standardized stroke orders on adherence to best practices.Neurology.2005;65:360–365.
- JCAHO Stroke Disease‐Specific Care performance measure set. Available at: www.jcaho.org/dscc/dsc/performance+measures/stroke+measure+set.htm. Accessed November 20,2005.
- .The role of in‐hospital initiation of cardiovascular protective therapies to improve treatment rates and clinical outcomes.Rev Cardiovasc Med.2003;4(Suppl 3):S37–S46.
- ,,, et al.In‐hospital initiation of secondary stroke prevention therapies yields high rates of adherence at follow‐up.Stroke.2004;35:2879–2883.
- ,,,.Improved treatment of coronary heart disease by implementation of a Cardiac Hospitalization Atherosclerosis Management Program (CHAMP).Am J Cardiol.2001;87:819–822.
- ,,, et al.Impact of combination evidence‐based medical therapy on mortality in patients with acute coronary syndromes.Circulation.2004;109:745–749.
- American Heart Association Get with the Guidelines Program—Coronary Artery Disease Pilot Test Results. Available at: http://www.americanheart.org/presenter.jhtml?identifier=699. Accessed November 30,2003.
- ,,, et al.In‐hospital initiation of lipid‐lowering therapy after coronary intervention as a predictor of long‐term utilization: a propensity analysis.Arch Intern Med.2003;163:2576–2582.
- University HealthSystem Consortium Ischemic Stroke Clinical Benchmarking Project Clinical Database Analysis—2001. University HealthSystem Consortium Ischemic Stroke Database Report #3.
- ,,.Expanding indications for statins in cerebral ischemia: a quantitative study.Arch Neurol.2005;62:67–72.
Copyright © 2006 Society of Hospital Medicine
AHRQ Overview
In the nearly 10 years since Bob Wachter and Lee Goldman coined the word hospitalist,1 it has been inspiring to see the dramatic growth of this specialty and with it, the growth in membership of the Society of Hospital Medicine.
Over the same period, the health care system has made progress toward ensuring that it provides the safest, highest‐quality health care possible.
In my mind, the two phenomena are related. The Society of Hospital Medicine, along with the hospitalist field more generally, has played a critical role in promoting the use of evidence‐based care, improved teamwork, and health information technology, which can make a significant difference in the care patients receive in the hospital. Similarly, the mission of the Agency for Healthcare Research and Quality (AHRQ) is to improve the quality, safety, efficiency, and effectiveness of health care for all Americans. So, both of our organizations are working to create positive change that will improve the health and health care of all patients.
As a research agency, we support studies, systematic reviews, and evaluations that help to build the foundation of evidence for health care. However, our work goes beyond simply conducting, supporting, and disseminating health services research. At its heart, our mission is helping the health care system translate research into improved practice and policy. We do not see research as an end in itself but rather a vehicle to improve health care and health. We achieve our goals by working with our public‐ and private‐sector partners to translate the research we support and conduct into knowledge and information that can be used immediately to improve health care for all Americans.
Health Information Technology
This commentary features AHRQ's quality‐related initiatives, including promoting the use of health information technology to improve quality and safety, providing the tools to assess health care quality, and expanding training to promote quality improvement in local communities. Many of these tools are ideal for hospitalists to use in their mission to ensure high‐quality care and an efficient and thorough handoff at discharge.
AHRQ is at the leading edge of President Bush's vision of a health care system that harnesses the power of health information technology (IT) to improve quality. AHRQ has invested more than $166 million in more than 100 projects to promote the use of health IT, with a special focus on rural hospitals and communities. These projects will enable providers to improve patient safety and reduce medication errors by eliminating handwritten prescriptions, help to ensure that important information follows patients as they move among health care settings, and reduce duplicative and unnecessary testing.
As part of this investment, AHRQ has awarded multiyear contracts totaling nearly $30 million to Colorado, Delaware, Indiana, Rhode Island, Tennessee, and Utah to help in the development of statewide networks that are secure, ensure privacy, and make information more accessible. Participants in the networks include major purchasers of health care, public and private payers, hospitals, ambulatory care facilities, home health care providers, and long‐term care providers.
In addition, AHRQ created the AHRQ National Resource Center for Health Information Technology (
Effective Health Care Program
However, as we all know, health IT is not a magic bullet or the sole answer to the quality and safety problems facing the American health care system. It is a means to an end. Although health IT has the great potential to deliver evidence to clinicians, patients, and other health care decision makers when they need it, one challenge is to ensure the evidence base is readily available.
To that end, AHRQ's new Effective Health Care Program, authorized under Section 1013 of the Medicare Prescription Drug, Improvement, and Modernization Act (MMA) of 2003, is conducting research with a focus on outcomes, comparative clinical effectiveness, and appropriateness of pharmaceuticals, devices, and health care services. At press time, AHRQ had released two effectiveness reports, Comparative Effectiveness of Management Strategies for Gastroesophageal Reflux Disease2 and Effectiveness of Noninvasive Diagnostic Tests for Breast Abnormalities.3
The AHRQ Effective Health Care Program takes three approaches to research on the comparative effectiveness of different treatments and clinical practices:
-
Review and synthesize knowledge. AHRQ's Evidence‐Based Practice Centers systematically review published and unpublished scientific evidence to develop evidence reports.
-
Promote and generate knowledge. A new AHRQ‐supported research network called DEcIDE (Developing Evidence to Inform Decisions about Effectiveness) conducts accelerated practical studies of new scientific evidence and analytic tools.
-
Compile the findings and translate knowledge. The John M. Eisenberg Clinical Decisions and Communications Science Center compiles the research results into a variety of useful formats for stakeholders.
Interested readers should go to the Effective Health Care Web site,
Patient Safety and Quality
Since 2001, AHRQ has been the leading funder of patient safety research, and I am proud that our $165 million patient safety research program is bearing fruit.
For example, Bob Wachterin his spare timeand his team at the University of California, San Francisco, under contract to AHRQ, developed AHRQ's Patient Safety Network, which can be found at
Readers of JHM also will be interested in AHRQ's Hospital Survey on Patient Safety Culture, which we released in partnership with Premier, Inc., the Department of Defense (DoD), and the American Hospital Association. The survey can be used to evaluate employees' attitudes about patient safety in their facilities or within specific units. It addresses a critical aspect of patient safety improvement: measuring organizational conditions that can lead to adverse events and patient harm. The survey, which is being used in the DoD's medical facilities, is available at
In another quality initiative, AHRQ also has developed a series of software tools that can help hospitals gauge the quality of care they provide. AHRQ's Prevention Quality Indicators allow hospitals to detect potentially avoidable hospital admissions for illnesses that can be effectively treated with high‐quality, community‐based primary care.
Another tool is the Inpatient Quality Indicators, 29 measures that can be used to help hospitals identify potential problem areas and to provide a proxy measure of hospital quality of care. The Patient Safety Indicators can help hospitals enhance their performance by quickly detecting potential medical errors in patients who have undergone medical or surgical care. Staff can then investigate to determine whether the problems detected by the indicators were caused by potentially preventable medical errors or have some other explanation.
Building on its long track record of developing surveys to gauge consumers' experiences in the health care system, AHRQ has developed H‐CAHPS, a survey tool that hospitals, employers, states, and others can use to assess the perceptions of hospital patients about the quality of the care they receive. This information is designed to help patients, their employers, and other purchasers make informed decisions and give hospitals feedback they can use to improve care. The Centers for Medicare & Medicaid Services, in partnership with the nation's major hospital trade groups, is using H‐CAHPS as part of their collaborative Hospital Quality Alliance to develop comparative information about hospitals.
In the future, AHRQ plans to create other surveys, including Ambulatory CAHPS, In‐Center Hemodialysis CAHPS, and Nursing Home CAHPS.
AHRQ also is now working in partnership with the Department of Veterans Affairs to train the third class of state and hospital teams participating in the Patient Safety Improvement Corps. The program was created because states asked us for help in areas such as conducting effective investigations of reports of medical errors and developing interventions and changes in standard clinical practice. When trained, the teams return to their local communities armed with the knowledge to improve patient safety.
National Health Care Quality and Disparities Reports
Finally, in January 2006, AHRQ released the third annual National Healthcare Quality Report (NHQR) and the National Healthcare Disparities Report. These reports provide data on the quality of health care and disparities in the use of health care services associated with patient characteristics, including race, ethnicity, income, education, and area of residence.45 In March 2006, AHRQ released a Web‐based tool called State Snapshots for states to use in measuring health care quality. The State Snapshots provides quick and easy access to the many measures and tables of the 2005 NHQR and also provides trend data that can help in the understanding of the quality of health care in individual states, including strengths, weaknesses, and opportunities for improvement. The reports and the State Snapshots are available on AHRQ's QualityTools Web site at
I hope this article has provided a glimpse into the quality improvement initiatives and activities supported by AHRQ. For ongoing information on these activities, I urge readers to go to AHRQ's Web site and sign up for our electronic newsletter (
AHRQ's aim is to make doing the right thing the easy thing to do for the health care system. We look forward to working with readers of the Journal of Hospital Medicine to achieve that goal.
- ,.The emerging role of “hospitalists” in the American health care system.N Engl J Med.1996;335:514–517.
- ,,,, et al.Comparative Effectiveness of Management Strategies for Gastroesophageal Reflux Disease. Evidence Report/Technology Assessment No. 1. (prepared by Tufts‐New England Medical Center Evidence‐Based Practice Center under Contract No. 290‐02‐0022.);Rockville, MD:Agency for Healthcare Research and Quality,2005.
- ,,,,,.Effectiveness of Noninvasive Diagnostic Tests for Breast Abnormalities. Comparative Effectiveness Review No. 2 (prepared by ECRI Evidence‐Based Practice Center under Contract No. 290‐02‐0019);Rockville, MD:Agency for Healthcare Research and Quality,2006.
- National Healthcare Quality Report,2005.Agency for Healthcare Research and Quality, Rockville, MD. Available at: http://www.ahrq.gov/qual/nhqr05/nhqr05.htm.
- National Healthcare Disparities Report,2005.Agency for Healthcare Research and Quality, Rockville, MD. Available at: http://www.ahrq.gov/qual/nhdr05/nhdr05.htm.
In the nearly 10 years since Bob Wachter and Lee Goldman coined the word hospitalist,1 it has been inspiring to see the dramatic growth of this specialty and with it, the growth in membership of the Society of Hospital Medicine.
Over the same period, the health care system has made progress toward ensuring that it provides the safest, highest‐quality health care possible.
In my mind, the two phenomena are related. The Society of Hospital Medicine, along with the hospitalist field more generally, has played a critical role in promoting the use of evidence‐based care, improved teamwork, and health information technology, which can make a significant difference in the care patients receive in the hospital. Similarly, the mission of the Agency for Healthcare Research and Quality (AHRQ) is to improve the quality, safety, efficiency, and effectiveness of health care for all Americans. So, both of our organizations are working to create positive change that will improve the health and health care of all patients.
As a research agency, we support studies, systematic reviews, and evaluations that help to build the foundation of evidence for health care. However, our work goes beyond simply conducting, supporting, and disseminating health services research. At its heart, our mission is helping the health care system translate research into improved practice and policy. We do not see research as an end in itself but rather a vehicle to improve health care and health. We achieve our goals by working with our public‐ and private‐sector partners to translate the research we support and conduct into knowledge and information that can be used immediately to improve health care for all Americans.
Health Information Technology
This commentary features AHRQ's quality‐related initiatives, including promoting the use of health information technology to improve quality and safety, providing the tools to assess health care quality, and expanding training to promote quality improvement in local communities. Many of these tools are ideal for hospitalists to use in their mission to ensure high‐quality care and an efficient and thorough handoff at discharge.
AHRQ is at the leading edge of President Bush's vision of a health care system that harnesses the power of health information technology (IT) to improve quality. AHRQ has invested more than $166 million in more than 100 projects to promote the use of health IT, with a special focus on rural hospitals and communities. These projects will enable providers to improve patient safety and reduce medication errors by eliminating handwritten prescriptions, help to ensure that important information follows patients as they move among health care settings, and reduce duplicative and unnecessary testing.
As part of this investment, AHRQ has awarded multiyear contracts totaling nearly $30 million to Colorado, Delaware, Indiana, Rhode Island, Tennessee, and Utah to help in the development of statewide networks that are secure, ensure privacy, and make information more accessible. Participants in the networks include major purchasers of health care, public and private payers, hospitals, ambulatory care facilities, home health care providers, and long‐term care providers.
In addition, AHRQ created the AHRQ National Resource Center for Health Information Technology (
Effective Health Care Program
However, as we all know, health IT is not a magic bullet or the sole answer to the quality and safety problems facing the American health care system. It is a means to an end. Although health IT has the great potential to deliver evidence to clinicians, patients, and other health care decision makers when they need it, one challenge is to ensure the evidence base is readily available.
To that end, AHRQ's new Effective Health Care Program, authorized under Section 1013 of the Medicare Prescription Drug, Improvement, and Modernization Act (MMA) of 2003, is conducting research with a focus on outcomes, comparative clinical effectiveness, and appropriateness of pharmaceuticals, devices, and health care services. At press time, AHRQ had released two effectiveness reports, Comparative Effectiveness of Management Strategies for Gastroesophageal Reflux Disease2 and Effectiveness of Noninvasive Diagnostic Tests for Breast Abnormalities.3
The AHRQ Effective Health Care Program takes three approaches to research on the comparative effectiveness of different treatments and clinical practices:
-
Review and synthesize knowledge. AHRQ's Evidence‐Based Practice Centers systematically review published and unpublished scientific evidence to develop evidence reports.
-
Promote and generate knowledge. A new AHRQ‐supported research network called DEcIDE (Developing Evidence to Inform Decisions about Effectiveness) conducts accelerated practical studies of new scientific evidence and analytic tools.
-
Compile the findings and translate knowledge. The John M. Eisenberg Clinical Decisions and Communications Science Center compiles the research results into a variety of useful formats for stakeholders.
Interested readers should go to the Effective Health Care Web site,
Patient Safety and Quality
Since 2001, AHRQ has been the leading funder of patient safety research, and I am proud that our $165 million patient safety research program is bearing fruit.
For example, Bob Wachterin his spare timeand his team at the University of California, San Francisco, under contract to AHRQ, developed AHRQ's Patient Safety Network, which can be found at
Readers of JHM also will be interested in AHRQ's Hospital Survey on Patient Safety Culture, which we released in partnership with Premier, Inc., the Department of Defense (DoD), and the American Hospital Association. The survey can be used to evaluate employees' attitudes about patient safety in their facilities or within specific units. It addresses a critical aspect of patient safety improvement: measuring organizational conditions that can lead to adverse events and patient harm. The survey, which is being used in the DoD's medical facilities, is available at
In another quality initiative, AHRQ also has developed a series of software tools that can help hospitals gauge the quality of care they provide. AHRQ's Prevention Quality Indicators allow hospitals to detect potentially avoidable hospital admissions for illnesses that can be effectively treated with high‐quality, community‐based primary care.
Another tool is the Inpatient Quality Indicators, 29 measures that can be used to help hospitals identify potential problem areas and to provide a proxy measure of hospital quality of care. The Patient Safety Indicators can help hospitals enhance their performance by quickly detecting potential medical errors in patients who have undergone medical or surgical care. Staff can then investigate to determine whether the problems detected by the indicators were caused by potentially preventable medical errors or have some other explanation.
Building on its long track record of developing surveys to gauge consumers' experiences in the health care system, AHRQ has developed H‐CAHPS, a survey tool that hospitals, employers, states, and others can use to assess the perceptions of hospital patients about the quality of the care they receive. This information is designed to help patients, their employers, and other purchasers make informed decisions and give hospitals feedback they can use to improve care. The Centers for Medicare & Medicaid Services, in partnership with the nation's major hospital trade groups, is using H‐CAHPS as part of their collaborative Hospital Quality Alliance to develop comparative information about hospitals.
In the future, AHRQ plans to create other surveys, including Ambulatory CAHPS, In‐Center Hemodialysis CAHPS, and Nursing Home CAHPS.
AHRQ also is now working in partnership with the Department of Veterans Affairs to train the third class of state and hospital teams participating in the Patient Safety Improvement Corps. The program was created because states asked us for help in areas such as conducting effective investigations of reports of medical errors and developing interventions and changes in standard clinical practice. When trained, the teams return to their local communities armed with the knowledge to improve patient safety.
National Health Care Quality and Disparities Reports
Finally, in January 2006, AHRQ released the third annual National Healthcare Quality Report (NHQR) and the National Healthcare Disparities Report. These reports provide data on the quality of health care and disparities in the use of health care services associated with patient characteristics, including race, ethnicity, income, education, and area of residence.45 In March 2006, AHRQ released a Web‐based tool called State Snapshots for states to use in measuring health care quality. The State Snapshots provides quick and easy access to the many measures and tables of the 2005 NHQR and also provides trend data that can help in the understanding of the quality of health care in individual states, including strengths, weaknesses, and opportunities for improvement. The reports and the State Snapshots are available on AHRQ's QualityTools Web site at
I hope this article has provided a glimpse into the quality improvement initiatives and activities supported by AHRQ. For ongoing information on these activities, I urge readers to go to AHRQ's Web site and sign up for our electronic newsletter (
AHRQ's aim is to make doing the right thing the easy thing to do for the health care system. We look forward to working with readers of the Journal of Hospital Medicine to achieve that goal.
In the nearly 10 years since Bob Wachter and Lee Goldman coined the word hospitalist,1 it has been inspiring to see the dramatic growth of this specialty and with it, the growth in membership of the Society of Hospital Medicine.
Over the same period, the health care system has made progress toward ensuring that it provides the safest, highest‐quality health care possible.
In my mind, the two phenomena are related. The Society of Hospital Medicine, along with the hospitalist field more generally, has played a critical role in promoting the use of evidence‐based care, improved teamwork, and health information technology, which can make a significant difference in the care patients receive in the hospital. Similarly, the mission of the Agency for Healthcare Research and Quality (AHRQ) is to improve the quality, safety, efficiency, and effectiveness of health care for all Americans. So, both of our organizations are working to create positive change that will improve the health and health care of all patients.
As a research agency, we support studies, systematic reviews, and evaluations that help to build the foundation of evidence for health care. However, our work goes beyond simply conducting, supporting, and disseminating health services research. At its heart, our mission is helping the health care system translate research into improved practice and policy. We do not see research as an end in itself but rather a vehicle to improve health care and health. We achieve our goals by working with our public‐ and private‐sector partners to translate the research we support and conduct into knowledge and information that can be used immediately to improve health care for all Americans.
Health Information Technology
This commentary features AHRQ's quality‐related initiatives, including promoting the use of health information technology to improve quality and safety, providing the tools to assess health care quality, and expanding training to promote quality improvement in local communities. Many of these tools are ideal for hospitalists to use in their mission to ensure high‐quality care and an efficient and thorough handoff at discharge.
AHRQ is at the leading edge of President Bush's vision of a health care system that harnesses the power of health information technology (IT) to improve quality. AHRQ has invested more than $166 million in more than 100 projects to promote the use of health IT, with a special focus on rural hospitals and communities. These projects will enable providers to improve patient safety and reduce medication errors by eliminating handwritten prescriptions, help to ensure that important information follows patients as they move among health care settings, and reduce duplicative and unnecessary testing.
As part of this investment, AHRQ has awarded multiyear contracts totaling nearly $30 million to Colorado, Delaware, Indiana, Rhode Island, Tennessee, and Utah to help in the development of statewide networks that are secure, ensure privacy, and make information more accessible. Participants in the networks include major purchasers of health care, public and private payers, hospitals, ambulatory care facilities, home health care providers, and long‐term care providers.
In addition, AHRQ created the AHRQ National Resource Center for Health Information Technology (
Effective Health Care Program
However, as we all know, health IT is not a magic bullet or the sole answer to the quality and safety problems facing the American health care system. It is a means to an end. Although health IT has the great potential to deliver evidence to clinicians, patients, and other health care decision makers when they need it, one challenge is to ensure the evidence base is readily available.
To that end, AHRQ's new Effective Health Care Program, authorized under Section 1013 of the Medicare Prescription Drug, Improvement, and Modernization Act (MMA) of 2003, is conducting research with a focus on outcomes, comparative clinical effectiveness, and appropriateness of pharmaceuticals, devices, and health care services. At press time, AHRQ had released two effectiveness reports, Comparative Effectiveness of Management Strategies for Gastroesophageal Reflux Disease2 and Effectiveness of Noninvasive Diagnostic Tests for Breast Abnormalities.3
The AHRQ Effective Health Care Program takes three approaches to research on the comparative effectiveness of different treatments and clinical practices:
-
Review and synthesize knowledge. AHRQ's Evidence‐Based Practice Centers systematically review published and unpublished scientific evidence to develop evidence reports.
-
Promote and generate knowledge. A new AHRQ‐supported research network called DEcIDE (Developing Evidence to Inform Decisions about Effectiveness) conducts accelerated practical studies of new scientific evidence and analytic tools.
-
Compile the findings and translate knowledge. The John M. Eisenberg Clinical Decisions and Communications Science Center compiles the research results into a variety of useful formats for stakeholders.
Interested readers should go to the Effective Health Care Web site,
Patient Safety and Quality
Since 2001, AHRQ has been the leading funder of patient safety research, and I am proud that our $165 million patient safety research program is bearing fruit.
For example, Bob Wachterin his spare timeand his team at the University of California, San Francisco, under contract to AHRQ, developed AHRQ's Patient Safety Network, which can be found at
Readers of JHM also will be interested in AHRQ's Hospital Survey on Patient Safety Culture, which we released in partnership with Premier, Inc., the Department of Defense (DoD), and the American Hospital Association. The survey can be used to evaluate employees' attitudes about patient safety in their facilities or within specific units. It addresses a critical aspect of patient safety improvement: measuring organizational conditions that can lead to adverse events and patient harm. The survey, which is being used in the DoD's medical facilities, is available at
In another quality initiative, AHRQ also has developed a series of software tools that can help hospitals gauge the quality of care they provide. AHRQ's Prevention Quality Indicators allow hospitals to detect potentially avoidable hospital admissions for illnesses that can be effectively treated with high‐quality, community‐based primary care.
Another tool is the Inpatient Quality Indicators, 29 measures that can be used to help hospitals identify potential problem areas and to provide a proxy measure of hospital quality of care. The Patient Safety Indicators can help hospitals enhance their performance by quickly detecting potential medical errors in patients who have undergone medical or surgical care. Staff can then investigate to determine whether the problems detected by the indicators were caused by potentially preventable medical errors or have some other explanation.
Building on its long track record of developing surveys to gauge consumers' experiences in the health care system, AHRQ has developed H‐CAHPS, a survey tool that hospitals, employers, states, and others can use to assess the perceptions of hospital patients about the quality of the care they receive. This information is designed to help patients, their employers, and other purchasers make informed decisions and give hospitals feedback they can use to improve care. The Centers for Medicare & Medicaid Services, in partnership with the nation's major hospital trade groups, is using H‐CAHPS as part of their collaborative Hospital Quality Alliance to develop comparative information about hospitals.
In the future, AHRQ plans to create other surveys, including Ambulatory CAHPS, In‐Center Hemodialysis CAHPS, and Nursing Home CAHPS.
AHRQ also is now working in partnership with the Department of Veterans Affairs to train the third class of state and hospital teams participating in the Patient Safety Improvement Corps. The program was created because states asked us for help in areas such as conducting effective investigations of reports of medical errors and developing interventions and changes in standard clinical practice. When trained, the teams return to their local communities armed with the knowledge to improve patient safety.
National Health Care Quality and Disparities Reports
Finally, in January 2006, AHRQ released the third annual National Healthcare Quality Report (NHQR) and the National Healthcare Disparities Report. These reports provide data on the quality of health care and disparities in the use of health care services associated with patient characteristics, including race, ethnicity, income, education, and area of residence.45 In March 2006, AHRQ released a Web‐based tool called State Snapshots for states to use in measuring health care quality. The State Snapshots provides quick and easy access to the many measures and tables of the 2005 NHQR and also provides trend data that can help in the understanding of the quality of health care in individual states, including strengths, weaknesses, and opportunities for improvement. The reports and the State Snapshots are available on AHRQ's QualityTools Web site at
I hope this article has provided a glimpse into the quality improvement initiatives and activities supported by AHRQ. For ongoing information on these activities, I urge readers to go to AHRQ's Web site and sign up for our electronic newsletter (
AHRQ's aim is to make doing the right thing the easy thing to do for the health care system. We look forward to working with readers of the Journal of Hospital Medicine to achieve that goal.
- ,.The emerging role of “hospitalists” in the American health care system.N Engl J Med.1996;335:514–517.
- ,,,, et al.Comparative Effectiveness of Management Strategies for Gastroesophageal Reflux Disease. Evidence Report/Technology Assessment No. 1. (prepared by Tufts‐New England Medical Center Evidence‐Based Practice Center under Contract No. 290‐02‐0022.);Rockville, MD:Agency for Healthcare Research and Quality,2005.
- ,,,,,.Effectiveness of Noninvasive Diagnostic Tests for Breast Abnormalities. Comparative Effectiveness Review No. 2 (prepared by ECRI Evidence‐Based Practice Center under Contract No. 290‐02‐0019);Rockville, MD:Agency for Healthcare Research and Quality,2006.
- National Healthcare Quality Report,2005.Agency for Healthcare Research and Quality, Rockville, MD. Available at: http://www.ahrq.gov/qual/nhqr05/nhqr05.htm.
- National Healthcare Disparities Report,2005.Agency for Healthcare Research and Quality, Rockville, MD. Available at: http://www.ahrq.gov/qual/nhdr05/nhdr05.htm.
- ,.The emerging role of “hospitalists” in the American health care system.N Engl J Med.1996;335:514–517.
- ,,,, et al.Comparative Effectiveness of Management Strategies for Gastroesophageal Reflux Disease. Evidence Report/Technology Assessment No. 1. (prepared by Tufts‐New England Medical Center Evidence‐Based Practice Center under Contract No. 290‐02‐0022.);Rockville, MD:Agency for Healthcare Research and Quality,2005.
- ,,,,,.Effectiveness of Noninvasive Diagnostic Tests for Breast Abnormalities. Comparative Effectiveness Review No. 2 (prepared by ECRI Evidence‐Based Practice Center under Contract No. 290‐02‐0019);Rockville, MD:Agency for Healthcare Research and Quality,2006.
- National Healthcare Quality Report,2005.Agency for Healthcare Research and Quality, Rockville, MD. Available at: http://www.ahrq.gov/qual/nhqr05/nhqr05.htm.
- National Healthcare Disparities Report,2005.Agency for Healthcare Research and Quality, Rockville, MD. Available at: http://www.ahrq.gov/qual/nhdr05/nhdr05.htm.
Discussing Resuscitation Preferences
Mrs. G is a 58‐year‐old woman with metastatic pancreatic cancer, diagnosed 8 months prior to admission when she presented to her primary care doctor with abdominal pain and weight loss. The cancer was locally advanced and metastatic to the liver at the time of diagnosis, and she underwent first‐line palliative chemotherapy with gemcitabine without objective or symptomatic improvement. She is now admitted to the hospitalist service with intractable abdominal pain, uncontrolled on her outpatient doses of opioids. On the day after admission, her pain is well controlled on morphine via patient‐controlled anesthesia. The hospitalist decides to talk with her about her about resuscitation status. At this point, she is full code.
Dr. S sits down with her and says, There's something I need to talk with you about that I talk with all of my patients about. And that is, if your heart were to stop, and mind you, I'm not expecting this to happen anytime soon, do you want us to do everything possible to bring you back?
Dr. S pauses to catch his breath, and then Mrs. G says, Well, I guess so, if you thought you could bring me back.
He continues, We are not always successful at bringing people back, but let me explain what this would entail: we could do chest compressions, administer shocks to your heart, put a breathing tube down into the back of your throat, hook you up to a machine that helps you breathe
Mrs. G glances up with a confused look. Dr. S says, You know, Mrs. G, I've started this conversation all wrong. I'm going to step out for a few minutes, collect my thoughts, and come back to talk with you again. Is that OK? Mrs. G smiles and says, Of course.
The doctor reenters the room about 20 minutes later, pulls up a chair next to her bed, and in a relaxed tone of voice says, Tell me what you understand about your illness.
Mrs. G says, I understand that I have cancer in my pancreas and liver and that I may not last very long, perhaps months if that. My oncologist offered me more chemotherapy, but I decided against it because it didn't seem that it would offer me much. And the first chemotherapy was really hard.
Dr. S then asks, As you look ahead, what worries you most?
Mrs. G replies, I really worry that I will die in terrible pain. That scares me terribly. I also worry about my husband and how he's going to cope with my worsening condition and with my death, let alone my pain.
Dr. S then says, That sounds really frightening. Tell me more about that. She continues, Well, I really want to spend the time I have left with my husband and familymy children and grandchildrenand I want to have some good time with them. But I keep worrying that it won't be able to be that way. She starts crying. You know, I'm so scared about going home and being in terrible pain again and having my husband and family be terrified
She pauses for a moment. The doctor doesn't say anything.
She starts up again, But I don't want to end up in the hospital again.I don't want to end up on machines like my mother‐in‐law did. She looks up at Dr. S, expecting a response.
Dr. S takes her hand and says, You know, I realize this is not easy to talk about, but it's really important for me to hear about your worries so that I can know how to help. The good news is we have many good methods to control your pain and that we can help you to be comfortable and to remain at home.
Thank you. Thank you so much.
As these conversations illustrate, discussing preferences regarding resuscitation is a challenging and important task for physicians. Understanding patients' wishes at the end of life allows clinicians to provide the type of care patients want, to avoid unwanted interventions, and to promote patient autonomy and dignity. Despite the frequency with which physicians have these conversations, they typically fall short when discussing code status with patients. Clinicians fail to address patients' concerns, monopolize conversations, and commonly misunderstand patients' resuscitation preferences.13 Often these discussions do not occur at all; more than 70% of seriously ill patients have never discussed advance directives with their physician.2, 4 The multicenter SUPPORT study, which demonstrated serious problems in the care of seriously ill hospitalized patients, documented that only 47% of physicians knew when their patients preferred do‐not‐resuscitate status.5
Hospitalists frequently conduct resuscitation discussions. Patients who are admitted to the hospital are usually seriously ill, and hospitalists need to assess rapidly whether an individual patient would want a resuscitation attempt if he or she had a cardiopulmonary arrest in the hospital. They need to build trust and rapport quickly with patients they have never met. Despite this challenge, hospitalists are in a good position to discuss resuscitation preferences.6 Patients may be more willing to discuss these issues in inpatient rather than outpatient settings because their acute deterioration may encourage self‐reflection.6 Furthermore, the time and productivity pressures of office practice often make it difficult for primary care physicians, who often know the patient and family best, to address advance directives. Although studies have documented that patients are interested in talking to their primary physicians about these matters,7, 8 these conversations do not occur with regularity. Preliminary research has raised the possibility that cancer patients may actually prefer to discuss these issues in an inpatient setting with a hospital‐based provider rather than with their oncologists.9 Studies have not addressed the question of whether patients with diseases other than cancer would prefer to discuss these issues with a hospitalist or their outpatient subspecialist.
Given that more than half of all Americans die in hospitals, hospitalists care for many people who are terminally ill and will need to assess preferences for cardiopulmonary resuscitation (CPR) and other treatments. Hospitalists need to be competent and compassionate in their approach to patients and their families. In this articles we review clinician barriers to holding these conversations, offer a variety of approaches to enhance these discussions, and review communication techniques that can be used to improve understanding.
Clinician Barriers to Discussing Resuscitation
Clinicians' own barriers may lead to infrequent and inadequate conversations about resuscitation. Understanding these barriers may allow providers to overcome them and facilitate better and more frequent communication. A discussion of patient barriers is beyond the scope of this article.
Unresolved Feelings about Death and Dying
When discussing resuscitation, or code status, physicians are discussing the possibility of death with a patient. In the first scenario above, the clinician lists the many procedures that could be done if the patient's heart were to stop without using the words die or death. The clinician never explicitly acknowledges that the patient has a serious illness that could lead to her death. Medical culture is focused on cure and on warding off death until the last possible moment. Because clinicians work in this culture, many have unresolved feelings of personal failure that are triggered when treating a dying patient.10 Also, the death of a patient can lead to anxieties about the clinician's own mortality and raise uncomfortable feelings of loss, related to the patient or to memories of deaths of the clinician's loved ones. In an attempt to avoid these feelings, whether conscious or not, physicians may resist talking to patients about death.10
Fear of Taking Away Hope
Clinicians fear that patients will lose hope if they are too honest about prognosis and acknowledge the inevitability of death. This concern may be true for a small minority of patients with advanced terminal illness who are solely focused on continued treatment and in such denial that they never consider the possibility of death. Most patients, however, understand on some level that they are getting sicker and may die, but expect clinicians to initiate discussions about death and dying.8, 11 Clinicians should understand that patients can have hope about many things beyond cure of their illness. For example, they can hope for good control of their symptoms so they can spend meaningful time with family and friends, heal troubled relationships, create a legacy, and say good‐bye. As in other developmental stages throughout life, the process of dying can be a time of emotional and spiritual growth and provide an opportunity to deepen relationships and find greater meaning.12 Despite their fears, physicians are much less likely to take away hope than they think. In fact, they can carry out the important actions of helping patients to refocus on more attainable goals and helping to return hope to what may be perceived by all as a hopeless situation.13, 14
Inadequate Training
There have been many educational interventions in both outpatient and inpatient settings to encourage physicians to discuss advance directives with patients.1521 In most of these studies, clinicians were sent reminders, but did not receive training or feedback to improve their communication skills. Although these interventions have led to modest increases in the number of advance directives, little is known about the quality of the conversations between clinician and patient.
There are acknowledged deficits in undergraduate22 and graduate medical education in discussing preferences and goals of care with patients.23, 24 A national survey of medical education deans showed that two‐thirds believed insufficient time was given to palliative care education including communication skills.25 Reflecting this lack of training, medical students and residents feel unprepared to take care of dying patients.26 In one survey, hospitalists reported that although palliative care was very important to their practice, they had not received enough training in palliative care knowledge and skills.27
Traditional Ethical Frameworks
Another difficulty arises from myths about the ethical perspectives that inform medical decision making and obtaining informed consent. Although these perspectives highlight the importance of patient autonomy and the right of the individual to choose medical treatments, they do not require physicians to describe every possible treatment if, in the clinician's judgment, a particular treatment would not benefit a patient. Physicians do have an obligation to use their medical knowledge and judgment in offering treatments and discussing side effects.28 In an attempt to honor a patient's autonomy when discussing advance directivesand possibly out of fear of coercing patientsclinicians sometimes offer a menu of treatment options without exploring the patient's underlying goals for these treatments. This approach can become meaningless out of context if the patient does not understand the probability that these interventions will work or the interrelatedness of the interventions. For example, when given a list of choices regarding resuscitation, a patient may say, I would like chest compressions and a chest tube, but no shocks please. Such a statement makes little sense clinically. Instead, physicians should have meaningful conversations with their patients in which they describe treatment options in the context of patient goals and values and help patients come to decisions in a shared process.
Practical Concerns
Outpatient providers find that time constraints and the competing demands that occur in caring for patients with multiple chronic health problems make it difficult to discuss advance directives.29 Hospitalists are also subject to productivity pressures and may feel similarly stressed for time. Outpatient providers spend about 5 minutes on each of these conversations,1 and medicine residents in the inpatient setting spend about 10 minutes.30 However, many of the conversations studied were inadequate; thus, it is unknown how long it takes to have an effective conversation. Hospitalists should keep in mind that they need not have these discussions every day with each patient and that having these conversations may end up saving time in the long run if they have a clearer sense of a patient's wishes and goals.
Laying Groundwork for the Discussion
The decision regarding resuscitation should be seen in the context of the patient's goals and values and overall health status. To address code status effectively, it is imperative first to elicit the person's view of his/her illness and then gently correct any misunderstandings. A patient who thinks her/his life will go on indefinitely may feel no need to consider her/his own mortality or alternatives to full resuscitation status. Alternatively, a patient who senses his/her mortality may have already thought about resuscitation and have clear preferences. A key first step in the conversation is to understand a patient's values and goals and comprehension of his/her illness. As in the second discussion above, a clinician can begin a discussion by saying, Tell me what you understand about your illness.
Discussing prognosis with patients can be difficult as physicians struggle with uncertainty. In the most comprehensive study to date of prognostication, physicians overestimated patient survival on average by a factor of 5.31 Nonetheless, there are compelling reasons to discuss prognosis. Failure to do so often results in patients spending their last days in the hospital receiving more aggressive treatments than they might choose if they understood their prognoses.32 Further, patients are denied the opportunity to address issues of life closure, such as spending time with family, thinking about legacy, and settling financial affairs. Physicians also fear they will take away hope with prognostication and believe patients expect greater accuracy than they can provide.33
Physicians can improve their prognostication skills by considering patients' functional status and clinical signs and symptoms and by using validated scales. The Karnofsky Performance Score (KPS) and the Eastern Cooperative Oncology Group (ECOG) Performance Status have been shown to correlate with survival,3440 and the Palliative Prognostic Score (PaP) has been validated in both cancer and noncancer patients.41, 4446 The PaP uses a combination of the KPS, clinical signs and symptoms, and the clinician's clinical prediction of survival. In addition, clinicians can be honest with patients about prognostic uncertainties and give prognoses in ranges, such as days to weeks, or weeks to months.
How to Broach the Subject of Resuscitation
In the first scenario presented in this article, the hospitalist says, There's something I need to talk with you about that I talk with all of my patients about. Although many clinicians begin resuscitation discussions this way, the question is problematic because often it is untrue. Most clinicians do not discuss code status with all patients who are admitted to the hospital. A better option would be to say, When I take care of patients with advanced cancer, I like to talk with them about their wishes regarding resuscitation. Is that all right? Better yet would be to ask a general question such as As you look ahead, what worries you most? or As you look to the future, what do you hope for? These and other useful questions appear in Table 1. These questions allow patients to bring up their concerns, show that the clinician cares about them, and often segue into a discussion of patients' hopes and worries about their own death and dying process. These questions often allow patients to bring up important goals that bear directly on the issue of resuscitation. For example, in the second scenario at the beginning of the article, the patient says she wants to have quality time with her family at home and does not want life‐sustaining technologies. Such a patient may not want resuscitation. When discussing CPR, it can also be helpful to state explicitly that resuscitation is used when a patient has died, rather than to use euphemisms such as, If your heart were to stop. The clinician can ask explicitly, If you were to die, would you want? There are other strategies for introducing the subject of resuscitation if these questions do not work. If a patient seems uncomfortable with the conversation, the clinician can address this discomfort directly by saying, This conversation seems to make you uncomfortable. Other strategies for exploring these issues include inquiring if the person has ever discussed resuscitation with his/her family or another physician, or asking if anyone else in the family has been very sick. Additionally, clinicians can ask questions about surrogate decision making. If you were to get so sick that you were unable to make decisions for yourself, who would you want to make them for you? This can then lead into a discussion of whether the patient has spoken to that person about his/her preferences, and if so, what was the content of that conversation. Another useful question is, Is there any state that would be worse than death for you? This question focuses on outcomes and allows the physician to put the issue of resuscitation into perspective for a patient.
| When I take care of patients with advanced cancer [or heart disease or lung disease, etc.], I like to talk with them about their wishes for care if they were to get very sick and even die suddenly. Is that all right? |
| As you look ahead, what worries you most? |
| As you look to the future, what do you hope for? |
| Has a close friend or family member ever been really sick? |
| If you were to get so sick that you were unable to make decisions for yourself, who would you want to make them for you? |
| Is there any state that would be worse than death for you? |
Knowing the Facts about Cardiopulmonary Resuscitation
In general, about 1 in 7 patients who have undergone CPR in the hospital survive to hospital discharge. Two literature reviews, from 1989 and 1998, of hospitalized patients who underwent CPR in the hospital reported surprisingly similar statistics. Immediate survival in both series was 41%, and survival to hospital discharge was 13%‐14%. Factors that increased survival included arrest due to coronary artery disease or drug overdose/adverse reaction, and location of arrest in the intensive care unit (ICU). Factors that decreased survival included sepsis at time of arrest, cancer, whether localized or metastatic, dementia, acute renal failure, bed‐bound status, and residence in a nursing home. Neither age nor sex was associated with survival.47, 48 In a meta‐analysis of outcome studies of CPR in metastatic cancer patients, none of 117 patients survived to hospital discharge.49
Most people get their information about CPR survival rates from the mass media, which portray CPR as a very successful procedure. For instance, on television, the sole source of information on CPR for most patients, the rate of survival to hospital discharge is 64%, much higher than the 1314% cited in the medical literature.50 Thus, it is no surprise that a patient with metastatic cancer or another life‐limiting illness would assume a positive outcome with CPR.
Knowledge of the facts about CPR survival rates is key when a physician discusses code status with a patient because these data influence patients' decisions. Patients who have a realistic understanding of their chances are less likely to prefer resuscitation.51, 52 Offering patients information about the success rate for CPR in their particular situation is critical in helping them reach a decision consistent with their values and goals. This information can be given quantitatively or qualitatively depending on the clinician assessment of what the patient would prefer.
Avoiding Stark Dichotomies in Resuscitation Discussions
In clinical practice there are 3 ways in which physicians can present resuscitation decisions as black and white and ignore the shades of gray. First, clinicians may present the choice between resuscitation and do not resuscitate (DNR) as a choice between life and death. In the first scenario above, the physician states If your heart were to stop, do you want us to do everything to bring you back? implying he would be able to save the patient's life with resuscitation attempts. When discussing resuscitation, clinicians should avoid language that suggests such a stark dichotomy. The reality is that most patients die despite resuscitation attempts. In fact, a patient is actually choosing between certain death (without resuscitation) and likely death (if resuscitation is attempted). For a patient with a serious, life‐limiting illness, it may be more effective to frame the conversation in terms of how that person envisions the end of life, and not whether death will eventually occur.
Second, clinicians and patients sometimes equate DNR with doing nothing or giving up. Clinicians fail to discuss other treatment options or alternatives, such as continuing ongoing aggressive medical treatments with DNR status or pursuing palliative care. Performing resuscitation is equated with activity and treatment, whereas withholding resuscitation is seen as passivity and giving up. To the patient, this can feel like abandonment, as if the doctor is withholding a treatment and not offering anything in its place. Examples of positive phrases that demonstrate the physician will continue to offer excellent care include: We will continue maximal medical therapy. However, if you die despite these treatments, we will let you die peacefully and won't attempt to revive you; We'll continue the intravenous antibiotics, but we won't plan to move you to the ICU if things worsen53; and We will work hard to treat your pain and other symptoms and to get you home. In addition, hospitalists must ensure, when signing out to physician colleagues, nurses, respiratory therapists, and others, that DNR orders are not overinterpreted to mean no treatment. Although a DNR order states that in the event of a cardiac arrest, no attempt at resuscitation will be made, it should have no bearing on other appropriate and desired interventions, including antibiotics, chemotherapy, and artificial nutrition; treatment for pain and other symptoms; and even monitoring in an ICU. This misunderstanding of DNR status is common among health care providers and has led many to argue that DNR orders should be part of a more comprehensive treatment plan that outlines where the patient's wishes for treatment fall on the spectrum from otherwise aggressive measures to comfort care.54, 55 Physicians who have a clear understanding of a DNR order will be able to reassure a patient that they will continue to receive desired care, but that if the patient dies, no attempt to resuscitate will be made.
Third, it is important to remind patients who choose full resuscitation status that additional decisions will need to be made if resuscitation is successful. Most patients who survive cardiopulmonary arrest end up worse off clinically and spend time in an ICU with life‐support measures in place, such as mechanical ventilation and vasopressors. Even if they survive, there will likely be a period during which they are unable to speak for themselves. This situation puts the burden of decision making on their surrogates or an appointed durable power of attorney for health care (DPOA‐HC). It is important to ask patients ahead of time whether there are conditions under which they would not want ongoing life‐sustaining measures. For example, a person might opt for discontinuation of life‐support measures if the physician and family agreed that there was only a minimal chance of cognitive recovery existed and that ongoing support was only prolonging inevitable death. To clarify the patient's wishes in this situation, you might ask, Are there conditions that would be worse than death? Encouraging the patient to share his/her wishes in this situation with a surrogate or DPOA‐HC will help to ensure those wishes are respected.
Communication Techniques
When discussing advance directives, it is important to give patients the chance to describe their life goals and their values to establish a context for understanding the role of life‐sustaining treatments. One useful method to elicit these goals and values is to ask open‐ended questions, followed by periods of silence so the patient has time to express himself/herself. In the second scenario, above, the physician used open‐ended questions in several instances: Tell me what you understand about your illness and As you look ahead, what worries you most? Tulsky and colleagues documented that medical residents spoke 76% of the time in discussions with patients about code status.30 In an ideal case, this ratio should be reversed or at least be even, allowing patients the time to explain their thoughts. Acknowledging patients' emotions by stating simply, You seem [angry/sad/perplexed], and waiting for an answer can help patients feel they are being understood. Making empathic statements is another powerful communication technique that conveys understanding.56 Examples include, That must be really sad for you, and It must be frightening to be in so much pain. As noted, silence can also be a powerful tool. Clinicians tend to be uncomfortable with silence and so fill the gaps with words. Allowing for silence enables patients to digest what they have heard, encourages them to continue speaking, and shows them the clinician wants to hear what they have to say. When giving information about any medical issues and especially about CPR, it is important to explain concepts in lay terms and to avoid medical jargon.57 Additionally, nonverbal communication techniques such as making eye contact, head nodding, and leaning in toward the patient all help in communicating engagement in the conversation. Having the conversation in a quiet and private place and sitting at the same level as the patient or family is also important. It is always a good idea to check in with patients to assess their understanding. Simply asking, Do you have any questions about what I said? or Does that all make sense? gives patients the opportunity to ask for clarification. Attempting to summarize what a patient has said can also help to clarify misunderstandings. Useful phrases include, Let me see if I've gotten this right or I want to make sure that I understood what you're telling me, followed by the clinician's synopsis of important points discussed.58 A summary of important communication techniques can be found in Table 2.
| Ask open‐ended questions followed by periods of silence |
| Tell me what you understand about your illness. |
| As you look ahead, what worries you most? |
| Acknowledge emotion |
| You seem [angry/sad/perplexed]. |
| Make empathetic statements |
| That must be really hard for you. |
| It must be terrible to be in so much pain. |
| Use nonverbal communication techniques such as eye contact, head nodding, leaning in toward the patient, sitting down, and sitting at patient's level |
| Allow for silence |
| Assess patient's understanding |
| Do you have any questions about what I said? |
| Does that all make sense? |
| Confirm your own understanding |
| Let me see if I've gotten this right. |
| I want to make sure I understand what you've been telling me. |
| Avoid medical jargon |
| Use the I wish statement |
| I wish there were more chemotherapy we could give you that would make a difference. |
| Use the Hope for the best, prepare for the worst statement |
| I think we should hope that the chemotherapy works but prepare for the possibility that it might not. |
There are 2 additional statements that can be very useful when patients and families are struggling with the reality of severe illness and are still hoping for longer life and cure. The first is the I wish statement, in which the clinician allies himself or herself with the patient's or family's wishes by stating, I wish it were different. I wish there were more chemotherapy we could give you that would make a difference.59 Occasionally, when tension is developing because the clinician does not believe an intervention is warranted but the patient desires it nonetheless, the I wish statement can be a powerful way of realigning with the patient. For example, responding to a patient who says, I want chemo to cure my cancer, with the statement There is no chemotherapy to help you can seem antagonistic. In contrast, saying, I wish there were a treatment that would make your cancer go away aligns the clinician with the patient and supports the patient. Another advantage of I wish statements is that they are truethe clinician does wish that there was an effective treatment and would gladly provide it if available. In general, I wish statements are more effective than I'm sorry statements, because the latter can be interpreted as the clinician taking responsibility for the situation. When a provider says, I'm sorry to give you this bad news, the patient may feel the need to say, That's OK. On the other hand, saying, I'm so sorry that your mother is dying, is very human and unlikely to be interpreted as the clinician taking responsibility for the death.
A similar technique is to use the statement Hope for the best, prepare for the worst when speaking with patients and families.60 For example, a physician could say, I really wish your mother could get better, and we should still hope for that; at the same time, we need to prepare for what will happen if she doesn't get better. Once again, this phrase both allows the patient or family to continue hoping things will improve and the clinician to support this hope, while simultaneously beginning the process of planning for the more likely outcome. Over time, the patient and family often move toward accepting that the patient is dying. Finally, trying to help the patient or family maintain hope in the face of illness and death is challenging but important: If your mother can't get better, are there other things you can hope for? Helping to identify tangible and realistic goals, such as being free from pain, seeing an important family member one last time, or getting home can provide hope at a difficult time.
Giving a Recommendation
Most patients with serious illness and their families want help making complex and ethically charged decisions. When clinicians ask patients to make decisions unilaterally, patients often feel anxious, sometimes for weeks.61 Families are often paralyzed when faced with the very difficult decision of whether to withdraw life‐sustaining interventions from a family member with an advanced terminal illness. Even if they understand on an intellectual level that ceasing to provide potentially curative or life‐prolonging therapies is the best choice, they are not yet able to accept this decision on an emotional level and ultimately may feel responsible and then guilty for the patient's death. Physicians need to carry some of the burden of making these difficult decisions. One way to relieve family members of some of this guilt is to recommend a plan of care based on substituted judgment, that is, on what the patient said she or he wanted or what the family thinks the patient would have wanted.6264 In addition, clinicians should use their medical expertise, experience, and understanding of the situation to make recommendations. The patient or family can then accept or reject the physician's advice, which maintains patient autonomy, yet not have to explicitly instruct the clinician to withdraw or limit life‐sustaining interventions.
The preceding discussion and recommendations can guide scenarios like those presented at the beginning of this article. In the second conversation, the clinician had just told the patient that he could help her to achieve her goal of pain control and of returning and staying home.
Dr. S says, I want to make sure I've understood what you've said. To summarize, you've told me how important it is to you to have your pain controlled, to have some good time with your husband and family at home, and not to come back to the hospital. Is that right?
Mrs. G: Yes, that's right.
Dr. S: And how is your pain today?
Mrs. G: So much better. I'm hoping I can go home soon.
Dr. S: That should be possible. In the next day or so, we will be getting you back onto medications that you can take by mouth. But before you go home, we need to figure out how we can support you and your family at home: get you ongoing help with pain control and any other issues that come up and support for your husband and family as well.
Mrs. G: Yes, my family really needs support.
Dr. S: Have you had any experience with hospice before? I'm thinking that that would be the best way to get you the support you and your family need.
Mrs. G: Yes. When my sister died, she had home hospice. They were very good and helpful, especially to her husband, my brother‐in‐law. Yes, I would like that, as I just don't want to come back to the hospital. Hopefully, they can help my husband adjust to things.
Dr. S continues: I think they will help your husband a lot, both before and also after your death. He pauses.
Mrs. G: That's good. I worry about him so much.
Dr. S: As you know, one of the important goals of hospice care is to keep you at home so you don't have to return to the hospital. And when the time comes, to help you die peacefully at home. Mrs. G nods. What this also means is that they would not use CPR, that is, not do chest compressions, when you were actively dying.
Mrs. G: Yes, I want to be able to go home and be at peace. The last thing I would want is someone thumping on my chest as I was dying. She smiles.
Dr. S.: Do you have any other thoughts or questions?
Mrs. G: Well, yes. I'm wondering if we can set up a time to talk with my husband about all of this. I think it would be helpful for him to talk with you. Would that be possible?
Dr. S: I would be happy to talk with your husband. When is he coming in?
They negotiate a time.
Mrs. G: I want to thank you for taking the time to talk with me. I am really grateful.
CONCLUSIONS
The decision about cardiopulmonary resuscitation is part of a larger conversation with a patient about how she or he wants to spend the rest of his/her life. Importantly, the decision should be made in context, rather than in isolation. Given the understanding that develops between physician and patient in the conversation above, it is not necessary to describe all the specific treatments that occur during CPR because the physician has already established that the patient does not want to return to the hospital, and she understands that she has a terminal condition and is dying. Through exploring a patient's goals and values, a clinician can discover a patient's preferences for care generally and come up with a comprehensive plan that addresses the particular individual's medical, social, and emotional needs. For physicians, few interventions are as important or rewarding as relieving patients' suffering and helping them attain their goals during and at the end of life.
- ,,,.Opening the black box: how do physicians communicate about advance directives?Ann Intern Med.1998;129:441–449.
- ,,, et al.Physician understanding of patient resuscitation preferences: insights and clinical implications.J Am Geriatr Soc.2000;48:S44–S51.
- ,,,,.Patient knowledge and physician predictions of treatment preferences after discussion of advance directives.J Gen Intern Med.1998;13:447–454.
- ,,.The value of disease severity in predicting patient readiness to address end‐of‐life issues.Arch Intern Med.2003;163:609–612.
- A controlled trial to improve care for seriously ill hospitalized patients. The study to understand prognoses and preferences for outcomes and risks of treatments (SUPPORT).The SUPPORT Principal Investigators.JAMA.1995;274:1591–1598.
- ,,,,,.Can we talk? Inpatient discussions about advance directives in a community hospital. Attending physicians' attitudes, their inpatients' wishes and reported evidence.Arch Intern Med.1994;154:2299–2308.
- ,,,,.Advance directives for medical care—a case for greater use.N Engl J Med.1991;324:889–895.
- ,,.Patient attitudes to discussing life‐sustaining treatment.Arch Intern Med.1986;146:1613–1615.
- ,.Paradoxes in cancer patients' advance care planning.J Palliat Med.2000;3(1):27–35.
- ,,.The inner life of physicians and care of the seriously ill.JAMA.2001;286:3007–3014.
- ,,.The discussion about advance directives; patient and physician opinions regarding when and how it should be conducted.Arch Intern Med.1995;155:1025–1030.
- .Psychological considerations, growth, and transcendence at the end of life: the art of the possible.JAMA.2001;285:2898–2905.
- .Care of dying patients: beyond symptom management.West J Med.1999;171(4):253–256.
- ,,,.Fostering coping and nurturing hope when discussing the future with terminally ill cancer patients and their caregivers.Cancer.2005;103:1965–1975.
- ,,,,,.Promoting advance directives among elderly primary care patients.J Gen Intern Med.2004;19:944–951.
- ,,,,.Implementing advance directives in the primary care setting.Arch Intern Med.1994;154:2321–2327.
- ,.Will outpatients complete living wills? A comparison of two interventions.J Gen Intern Med.1991;6:41–46.
- ,,, et al.Enhancement of proxy appointments for older persons: physician counselling in ambulatory settings.J Am Geriatr Soc.1996;44(1):37–43.
- ,,,.Strategies to promote the use of advance directives in a residency outpatient practice.J Gen Intern Med.1996;11:657–663.
- ,,,,,.Promoting inpatient directives about life‐sustaining treatments in a community hospital. Results of a 3‐year time‐series intervention trial.Arch Intern Med.1995;155:2317–2323.
- ,,,,.An educational intervention in the surgical ICU to improve ethical decisions.Surgery.1995;118(2):294–299.
- ,,.The status of medical education in end‐of‐life care. A national report.J Gen Intern Med.2003;18:685–695.
- ,.ACGME Requirements for end‐of‐life training in selected residency and fellowship programs: a status report.Acad Med.2002;77(4):299–304.
- ,,,.End‐of‐life care education in internal medicine residency programs: an interinstitutional study.J Palliat Med.2002;5:487–496.
- ,,,,,.End‐of‐life care in the curriculum: a national study of medical education deans.Acad Med.2004;79:760–768.
- ,,,,.Assessing medical students' training in end‐of‐life communication: a survey of interns at one urban teaching hospital.Acad Med.2003;78:530–537.
- ,,,.Hospitalists' perceptions of their residency training needs: results of a national surveyAm J Med.2001;111(3):247–254.
- ,.Legal and ethical myths about informed consent.Arch Intern Med.1996;156:2521–2526.
- ,,.Physician reluctance to discuss advance directives: an empiric investigation of potential barriers.Arch Intern Med.1994;154:2311–2318.
- ,,.How do medical residents discuss resuscitation with patients?J Gen Intern Med.1995;10:436–442.
- ,,,.Extent and determinants of error in doctors' prognoses in terminally ill patients: prospective cohort study.Br Med J.2000;320:469–473.
- .Death Foretold; Prophecy and Prognosis in Medical Care.Chicago:University of Chicago Press,1999.
- ,.Attitude and self‐reported practice regarding prognostication in a national sample of internists.Arch Intern Med.1998;158:2389–2395.
- ,,, et al.Prognostic factors for survival in patients treated in phase I clinical trials.Cancer.1994;74:1965–1973.
- ,,, et al.Prediction of survival of patients terminally ill with cancer. Results of an Italian prospective multicentric studyCancer.1995;75:2613–2622.
- ,,.Evaluation of patients with advanced cancer using Karnovsky performance status.Cancer.1980;45:2220–2224.
- ,,,.The Karnofsky Performance Status Scale. An exam of its reliability and validity in a research setting.Cancer.1984;53:2002–2007.
- .A demographic and prognostic approach to defining the end of life.J Palliat Med.2005;8(suppl 1):s12–s21.
- .Estimating length of survival in end‐stage cancer: a review of the literature.J Pain Symptom Manage.1995;10:548–555.
- ,.Prognostic factors, survival, and advanced cancer.J Palliat Care.1992;1992(8):4.
- ,,.A new palliative prognostic score: a first step for the staging of terminally ill cancer patients.J Pain Symp Management1999;17(4):231–239.
- ,,.Survival prediction in terminal cancer patients: A systematic review of the medical literature.Palliat Med.2000;14:363–374.
- ,,.Clinical symptoms and length of survival in patients with terminal cancer.Arch Intern Med.1988;148:1586–1591.
- ,,.Successful validation of the palliative prognostic score in terminally ill cancer patients.J Pain Symptom Manage.1999;17:240–247.
- ,,.The use of the palliative prognostic score in patients with diagnoses other than cancer.J Pain Symptom Manage.2003;26:883–885.
- ,,.Diagnostic accuracy of the palliative prognostic score in hospitalized patients with advanced cancer.J Clin Oncol.2004;22:4823–4828.
- ,,,.Survival after in‐hospital cardiopulmonary resuscitation: a meta‐analysis.J Gen Intern Med,1998;13(12):805–16.
- .Informing the patient about cardiopulmonary resuscitation: when the risks outweigh the benefits.J Gen Intern Med.1989;4:349–355.
- .Resuscitation of patients with metastatic cancer: Is transient benefit still futile?Arch Intern Med.1991;151:235–239.
- ,,.Cardiopulmonary resuscitation on television—miracles and misinformation.N Engl J Med.1996;334:1578–1582.
- ,,, et al.The influence of the probability of survival on patients' preferences regarding cardiopulmonary resuscitation.N Engl J Med.1994;330:545–549.
- ,,,.Resuscitation decision making in the elderly: the value of outcome data.J Gen Intern Med.1993;8:295–300.
- .Discussing do‐not‐resuscitate status.J Clin Oncol.2001;19:1576–1581.
- .Do‐not‐resuscitate decisions in a community hospital. Incidence, implications, and outcomes.JAMA.1986;256:1164–1169.
- ,,,.A prospective study of the efficacy of the physician order form for life‐sustaining treatment.J Am Geriatr Soc.1998;46:1170–1171.
- ,,, for theACPAE‐o‐LCCP.Discussing palliative care with patients.Ann Intern Med.1999;130:744–749.
- ,,,,.Approaching difficult communication tasks in oncology.CA Cancer J Clin.2005;55(3):164–177.
- ,,, et al.“Let me see if I have this right…”: words that help build empathy.Ann Intern Med.2001;135(3):221–227.
- ,,.“I wish things were different”: expressing wishes in response to loss, futility, and unrealistic hopes.Ann Intern Med.2001;135:551–555.
- ,,.Hope for the best, and prepare for the worst.Ann Intern Med.2003;138:439–443.
- ,,,.When the treatment goal is not cure: are cancer patients equipped to make informed decisions?J Clin Oncol.2002;20:503–513.
- ,,.Beyond autonomy: Diversifying end‐of‐life decision‐making approaches to serve patients and families.J Am Geriatr Soc.2005;53:1046–1050.
- ,,,.Measuring patients' desire for autonomy: decision making and information‐seeking preferences among medical patients.J Gen Intern Med.1989;4(1):23–30.
- ,.Physician recommendations and patient autonomy: finding a balance between physician power and patient choice.Ann Intern Med.1996;125:763–769.
Mrs. G is a 58‐year‐old woman with metastatic pancreatic cancer, diagnosed 8 months prior to admission when she presented to her primary care doctor with abdominal pain and weight loss. The cancer was locally advanced and metastatic to the liver at the time of diagnosis, and she underwent first‐line palliative chemotherapy with gemcitabine without objective or symptomatic improvement. She is now admitted to the hospitalist service with intractable abdominal pain, uncontrolled on her outpatient doses of opioids. On the day after admission, her pain is well controlled on morphine via patient‐controlled anesthesia. The hospitalist decides to talk with her about her about resuscitation status. At this point, she is full code.
Dr. S sits down with her and says, There's something I need to talk with you about that I talk with all of my patients about. And that is, if your heart were to stop, and mind you, I'm not expecting this to happen anytime soon, do you want us to do everything possible to bring you back?
Dr. S pauses to catch his breath, and then Mrs. G says, Well, I guess so, if you thought you could bring me back.
He continues, We are not always successful at bringing people back, but let me explain what this would entail: we could do chest compressions, administer shocks to your heart, put a breathing tube down into the back of your throat, hook you up to a machine that helps you breathe
Mrs. G glances up with a confused look. Dr. S says, You know, Mrs. G, I've started this conversation all wrong. I'm going to step out for a few minutes, collect my thoughts, and come back to talk with you again. Is that OK? Mrs. G smiles and says, Of course.
The doctor reenters the room about 20 minutes later, pulls up a chair next to her bed, and in a relaxed tone of voice says, Tell me what you understand about your illness.
Mrs. G says, I understand that I have cancer in my pancreas and liver and that I may not last very long, perhaps months if that. My oncologist offered me more chemotherapy, but I decided against it because it didn't seem that it would offer me much. And the first chemotherapy was really hard.
Dr. S then asks, As you look ahead, what worries you most?
Mrs. G replies, I really worry that I will die in terrible pain. That scares me terribly. I also worry about my husband and how he's going to cope with my worsening condition and with my death, let alone my pain.
Dr. S then says, That sounds really frightening. Tell me more about that. She continues, Well, I really want to spend the time I have left with my husband and familymy children and grandchildrenand I want to have some good time with them. But I keep worrying that it won't be able to be that way. She starts crying. You know, I'm so scared about going home and being in terrible pain again and having my husband and family be terrified
She pauses for a moment. The doctor doesn't say anything.
She starts up again, But I don't want to end up in the hospital again.I don't want to end up on machines like my mother‐in‐law did. She looks up at Dr. S, expecting a response.
Dr. S takes her hand and says, You know, I realize this is not easy to talk about, but it's really important for me to hear about your worries so that I can know how to help. The good news is we have many good methods to control your pain and that we can help you to be comfortable and to remain at home.
Thank you. Thank you so much.
As these conversations illustrate, discussing preferences regarding resuscitation is a challenging and important task for physicians. Understanding patients' wishes at the end of life allows clinicians to provide the type of care patients want, to avoid unwanted interventions, and to promote patient autonomy and dignity. Despite the frequency with which physicians have these conversations, they typically fall short when discussing code status with patients. Clinicians fail to address patients' concerns, monopolize conversations, and commonly misunderstand patients' resuscitation preferences.13 Often these discussions do not occur at all; more than 70% of seriously ill patients have never discussed advance directives with their physician.2, 4 The multicenter SUPPORT study, which demonstrated serious problems in the care of seriously ill hospitalized patients, documented that only 47% of physicians knew when their patients preferred do‐not‐resuscitate status.5
Hospitalists frequently conduct resuscitation discussions. Patients who are admitted to the hospital are usually seriously ill, and hospitalists need to assess rapidly whether an individual patient would want a resuscitation attempt if he or she had a cardiopulmonary arrest in the hospital. They need to build trust and rapport quickly with patients they have never met. Despite this challenge, hospitalists are in a good position to discuss resuscitation preferences.6 Patients may be more willing to discuss these issues in inpatient rather than outpatient settings because their acute deterioration may encourage self‐reflection.6 Furthermore, the time and productivity pressures of office practice often make it difficult for primary care physicians, who often know the patient and family best, to address advance directives. Although studies have documented that patients are interested in talking to their primary physicians about these matters,7, 8 these conversations do not occur with regularity. Preliminary research has raised the possibility that cancer patients may actually prefer to discuss these issues in an inpatient setting with a hospital‐based provider rather than with their oncologists.9 Studies have not addressed the question of whether patients with diseases other than cancer would prefer to discuss these issues with a hospitalist or their outpatient subspecialist.
Given that more than half of all Americans die in hospitals, hospitalists care for many people who are terminally ill and will need to assess preferences for cardiopulmonary resuscitation (CPR) and other treatments. Hospitalists need to be competent and compassionate in their approach to patients and their families. In this articles we review clinician barriers to holding these conversations, offer a variety of approaches to enhance these discussions, and review communication techniques that can be used to improve understanding.
Clinician Barriers to Discussing Resuscitation
Clinicians' own barriers may lead to infrequent and inadequate conversations about resuscitation. Understanding these barriers may allow providers to overcome them and facilitate better and more frequent communication. A discussion of patient barriers is beyond the scope of this article.
Unresolved Feelings about Death and Dying
When discussing resuscitation, or code status, physicians are discussing the possibility of death with a patient. In the first scenario above, the clinician lists the many procedures that could be done if the patient's heart were to stop without using the words die or death. The clinician never explicitly acknowledges that the patient has a serious illness that could lead to her death. Medical culture is focused on cure and on warding off death until the last possible moment. Because clinicians work in this culture, many have unresolved feelings of personal failure that are triggered when treating a dying patient.10 Also, the death of a patient can lead to anxieties about the clinician's own mortality and raise uncomfortable feelings of loss, related to the patient or to memories of deaths of the clinician's loved ones. In an attempt to avoid these feelings, whether conscious or not, physicians may resist talking to patients about death.10
Fear of Taking Away Hope
Clinicians fear that patients will lose hope if they are too honest about prognosis and acknowledge the inevitability of death. This concern may be true for a small minority of patients with advanced terminal illness who are solely focused on continued treatment and in such denial that they never consider the possibility of death. Most patients, however, understand on some level that they are getting sicker and may die, but expect clinicians to initiate discussions about death and dying.8, 11 Clinicians should understand that patients can have hope about many things beyond cure of their illness. For example, they can hope for good control of their symptoms so they can spend meaningful time with family and friends, heal troubled relationships, create a legacy, and say good‐bye. As in other developmental stages throughout life, the process of dying can be a time of emotional and spiritual growth and provide an opportunity to deepen relationships and find greater meaning.12 Despite their fears, physicians are much less likely to take away hope than they think. In fact, they can carry out the important actions of helping patients to refocus on more attainable goals and helping to return hope to what may be perceived by all as a hopeless situation.13, 14
Inadequate Training
There have been many educational interventions in both outpatient and inpatient settings to encourage physicians to discuss advance directives with patients.1521 In most of these studies, clinicians were sent reminders, but did not receive training or feedback to improve their communication skills. Although these interventions have led to modest increases in the number of advance directives, little is known about the quality of the conversations between clinician and patient.
There are acknowledged deficits in undergraduate22 and graduate medical education in discussing preferences and goals of care with patients.23, 24 A national survey of medical education deans showed that two‐thirds believed insufficient time was given to palliative care education including communication skills.25 Reflecting this lack of training, medical students and residents feel unprepared to take care of dying patients.26 In one survey, hospitalists reported that although palliative care was very important to their practice, they had not received enough training in palliative care knowledge and skills.27
Traditional Ethical Frameworks
Another difficulty arises from myths about the ethical perspectives that inform medical decision making and obtaining informed consent. Although these perspectives highlight the importance of patient autonomy and the right of the individual to choose medical treatments, they do not require physicians to describe every possible treatment if, in the clinician's judgment, a particular treatment would not benefit a patient. Physicians do have an obligation to use their medical knowledge and judgment in offering treatments and discussing side effects.28 In an attempt to honor a patient's autonomy when discussing advance directivesand possibly out of fear of coercing patientsclinicians sometimes offer a menu of treatment options without exploring the patient's underlying goals for these treatments. This approach can become meaningless out of context if the patient does not understand the probability that these interventions will work or the interrelatedness of the interventions. For example, when given a list of choices regarding resuscitation, a patient may say, I would like chest compressions and a chest tube, but no shocks please. Such a statement makes little sense clinically. Instead, physicians should have meaningful conversations with their patients in which they describe treatment options in the context of patient goals and values and help patients come to decisions in a shared process.
Practical Concerns
Outpatient providers find that time constraints and the competing demands that occur in caring for patients with multiple chronic health problems make it difficult to discuss advance directives.29 Hospitalists are also subject to productivity pressures and may feel similarly stressed for time. Outpatient providers spend about 5 minutes on each of these conversations,1 and medicine residents in the inpatient setting spend about 10 minutes.30 However, many of the conversations studied were inadequate; thus, it is unknown how long it takes to have an effective conversation. Hospitalists should keep in mind that they need not have these discussions every day with each patient and that having these conversations may end up saving time in the long run if they have a clearer sense of a patient's wishes and goals.
Laying Groundwork for the Discussion
The decision regarding resuscitation should be seen in the context of the patient's goals and values and overall health status. To address code status effectively, it is imperative first to elicit the person's view of his/her illness and then gently correct any misunderstandings. A patient who thinks her/his life will go on indefinitely may feel no need to consider her/his own mortality or alternatives to full resuscitation status. Alternatively, a patient who senses his/her mortality may have already thought about resuscitation and have clear preferences. A key first step in the conversation is to understand a patient's values and goals and comprehension of his/her illness. As in the second discussion above, a clinician can begin a discussion by saying, Tell me what you understand about your illness.
Discussing prognosis with patients can be difficult as physicians struggle with uncertainty. In the most comprehensive study to date of prognostication, physicians overestimated patient survival on average by a factor of 5.31 Nonetheless, there are compelling reasons to discuss prognosis. Failure to do so often results in patients spending their last days in the hospital receiving more aggressive treatments than they might choose if they understood their prognoses.32 Further, patients are denied the opportunity to address issues of life closure, such as spending time with family, thinking about legacy, and settling financial affairs. Physicians also fear they will take away hope with prognostication and believe patients expect greater accuracy than they can provide.33
Physicians can improve their prognostication skills by considering patients' functional status and clinical signs and symptoms and by using validated scales. The Karnofsky Performance Score (KPS) and the Eastern Cooperative Oncology Group (ECOG) Performance Status have been shown to correlate with survival,3440 and the Palliative Prognostic Score (PaP) has been validated in both cancer and noncancer patients.41, 4446 The PaP uses a combination of the KPS, clinical signs and symptoms, and the clinician's clinical prediction of survival. In addition, clinicians can be honest with patients about prognostic uncertainties and give prognoses in ranges, such as days to weeks, or weeks to months.
How to Broach the Subject of Resuscitation
In the first scenario presented in this article, the hospitalist says, There's something I need to talk with you about that I talk with all of my patients about. Although many clinicians begin resuscitation discussions this way, the question is problematic because often it is untrue. Most clinicians do not discuss code status with all patients who are admitted to the hospital. A better option would be to say, When I take care of patients with advanced cancer, I like to talk with them about their wishes regarding resuscitation. Is that all right? Better yet would be to ask a general question such as As you look ahead, what worries you most? or As you look to the future, what do you hope for? These and other useful questions appear in Table 1. These questions allow patients to bring up their concerns, show that the clinician cares about them, and often segue into a discussion of patients' hopes and worries about their own death and dying process. These questions often allow patients to bring up important goals that bear directly on the issue of resuscitation. For example, in the second scenario at the beginning of the article, the patient says she wants to have quality time with her family at home and does not want life‐sustaining technologies. Such a patient may not want resuscitation. When discussing CPR, it can also be helpful to state explicitly that resuscitation is used when a patient has died, rather than to use euphemisms such as, If your heart were to stop. The clinician can ask explicitly, If you were to die, would you want? There are other strategies for introducing the subject of resuscitation if these questions do not work. If a patient seems uncomfortable with the conversation, the clinician can address this discomfort directly by saying, This conversation seems to make you uncomfortable. Other strategies for exploring these issues include inquiring if the person has ever discussed resuscitation with his/her family or another physician, or asking if anyone else in the family has been very sick. Additionally, clinicians can ask questions about surrogate decision making. If you were to get so sick that you were unable to make decisions for yourself, who would you want to make them for you? This can then lead into a discussion of whether the patient has spoken to that person about his/her preferences, and if so, what was the content of that conversation. Another useful question is, Is there any state that would be worse than death for you? This question focuses on outcomes and allows the physician to put the issue of resuscitation into perspective for a patient.
| When I take care of patients with advanced cancer [or heart disease or lung disease, etc.], I like to talk with them about their wishes for care if they were to get very sick and even die suddenly. Is that all right? |
| As you look ahead, what worries you most? |
| As you look to the future, what do you hope for? |
| Has a close friend or family member ever been really sick? |
| If you were to get so sick that you were unable to make decisions for yourself, who would you want to make them for you? |
| Is there any state that would be worse than death for you? |
Knowing the Facts about Cardiopulmonary Resuscitation
In general, about 1 in 7 patients who have undergone CPR in the hospital survive to hospital discharge. Two literature reviews, from 1989 and 1998, of hospitalized patients who underwent CPR in the hospital reported surprisingly similar statistics. Immediate survival in both series was 41%, and survival to hospital discharge was 13%‐14%. Factors that increased survival included arrest due to coronary artery disease or drug overdose/adverse reaction, and location of arrest in the intensive care unit (ICU). Factors that decreased survival included sepsis at time of arrest, cancer, whether localized or metastatic, dementia, acute renal failure, bed‐bound status, and residence in a nursing home. Neither age nor sex was associated with survival.47, 48 In a meta‐analysis of outcome studies of CPR in metastatic cancer patients, none of 117 patients survived to hospital discharge.49
Most people get their information about CPR survival rates from the mass media, which portray CPR as a very successful procedure. For instance, on television, the sole source of information on CPR for most patients, the rate of survival to hospital discharge is 64%, much higher than the 1314% cited in the medical literature.50 Thus, it is no surprise that a patient with metastatic cancer or another life‐limiting illness would assume a positive outcome with CPR.
Knowledge of the facts about CPR survival rates is key when a physician discusses code status with a patient because these data influence patients' decisions. Patients who have a realistic understanding of their chances are less likely to prefer resuscitation.51, 52 Offering patients information about the success rate for CPR in their particular situation is critical in helping them reach a decision consistent with their values and goals. This information can be given quantitatively or qualitatively depending on the clinician assessment of what the patient would prefer.
Avoiding Stark Dichotomies in Resuscitation Discussions
In clinical practice there are 3 ways in which physicians can present resuscitation decisions as black and white and ignore the shades of gray. First, clinicians may present the choice between resuscitation and do not resuscitate (DNR) as a choice between life and death. In the first scenario above, the physician states If your heart were to stop, do you want us to do everything to bring you back? implying he would be able to save the patient's life with resuscitation attempts. When discussing resuscitation, clinicians should avoid language that suggests such a stark dichotomy. The reality is that most patients die despite resuscitation attempts. In fact, a patient is actually choosing between certain death (without resuscitation) and likely death (if resuscitation is attempted). For a patient with a serious, life‐limiting illness, it may be more effective to frame the conversation in terms of how that person envisions the end of life, and not whether death will eventually occur.
Second, clinicians and patients sometimes equate DNR with doing nothing or giving up. Clinicians fail to discuss other treatment options or alternatives, such as continuing ongoing aggressive medical treatments with DNR status or pursuing palliative care. Performing resuscitation is equated with activity and treatment, whereas withholding resuscitation is seen as passivity and giving up. To the patient, this can feel like abandonment, as if the doctor is withholding a treatment and not offering anything in its place. Examples of positive phrases that demonstrate the physician will continue to offer excellent care include: We will continue maximal medical therapy. However, if you die despite these treatments, we will let you die peacefully and won't attempt to revive you; We'll continue the intravenous antibiotics, but we won't plan to move you to the ICU if things worsen53; and We will work hard to treat your pain and other symptoms and to get you home. In addition, hospitalists must ensure, when signing out to physician colleagues, nurses, respiratory therapists, and others, that DNR orders are not overinterpreted to mean no treatment. Although a DNR order states that in the event of a cardiac arrest, no attempt at resuscitation will be made, it should have no bearing on other appropriate and desired interventions, including antibiotics, chemotherapy, and artificial nutrition; treatment for pain and other symptoms; and even monitoring in an ICU. This misunderstanding of DNR status is common among health care providers and has led many to argue that DNR orders should be part of a more comprehensive treatment plan that outlines where the patient's wishes for treatment fall on the spectrum from otherwise aggressive measures to comfort care.54, 55 Physicians who have a clear understanding of a DNR order will be able to reassure a patient that they will continue to receive desired care, but that if the patient dies, no attempt to resuscitate will be made.
Third, it is important to remind patients who choose full resuscitation status that additional decisions will need to be made if resuscitation is successful. Most patients who survive cardiopulmonary arrest end up worse off clinically and spend time in an ICU with life‐support measures in place, such as mechanical ventilation and vasopressors. Even if they survive, there will likely be a period during which they are unable to speak for themselves. This situation puts the burden of decision making on their surrogates or an appointed durable power of attorney for health care (DPOA‐HC). It is important to ask patients ahead of time whether there are conditions under which they would not want ongoing life‐sustaining measures. For example, a person might opt for discontinuation of life‐support measures if the physician and family agreed that there was only a minimal chance of cognitive recovery existed and that ongoing support was only prolonging inevitable death. To clarify the patient's wishes in this situation, you might ask, Are there conditions that would be worse than death? Encouraging the patient to share his/her wishes in this situation with a surrogate or DPOA‐HC will help to ensure those wishes are respected.
Communication Techniques
When discussing advance directives, it is important to give patients the chance to describe their life goals and their values to establish a context for understanding the role of life‐sustaining treatments. One useful method to elicit these goals and values is to ask open‐ended questions, followed by periods of silence so the patient has time to express himself/herself. In the second scenario, above, the physician used open‐ended questions in several instances: Tell me what you understand about your illness and As you look ahead, what worries you most? Tulsky and colleagues documented that medical residents spoke 76% of the time in discussions with patients about code status.30 In an ideal case, this ratio should be reversed or at least be even, allowing patients the time to explain their thoughts. Acknowledging patients' emotions by stating simply, You seem [angry/sad/perplexed], and waiting for an answer can help patients feel they are being understood. Making empathic statements is another powerful communication technique that conveys understanding.56 Examples include, That must be really sad for you, and It must be frightening to be in so much pain. As noted, silence can also be a powerful tool. Clinicians tend to be uncomfortable with silence and so fill the gaps with words. Allowing for silence enables patients to digest what they have heard, encourages them to continue speaking, and shows them the clinician wants to hear what they have to say. When giving information about any medical issues and especially about CPR, it is important to explain concepts in lay terms and to avoid medical jargon.57 Additionally, nonverbal communication techniques such as making eye contact, head nodding, and leaning in toward the patient all help in communicating engagement in the conversation. Having the conversation in a quiet and private place and sitting at the same level as the patient or family is also important. It is always a good idea to check in with patients to assess their understanding. Simply asking, Do you have any questions about what I said? or Does that all make sense? gives patients the opportunity to ask for clarification. Attempting to summarize what a patient has said can also help to clarify misunderstandings. Useful phrases include, Let me see if I've gotten this right or I want to make sure that I understood what you're telling me, followed by the clinician's synopsis of important points discussed.58 A summary of important communication techniques can be found in Table 2.
| Ask open‐ended questions followed by periods of silence |
| Tell me what you understand about your illness. |
| As you look ahead, what worries you most? |
| Acknowledge emotion |
| You seem [angry/sad/perplexed]. |
| Make empathetic statements |
| That must be really hard for you. |
| It must be terrible to be in so much pain. |
| Use nonverbal communication techniques such as eye contact, head nodding, leaning in toward the patient, sitting down, and sitting at patient's level |
| Allow for silence |
| Assess patient's understanding |
| Do you have any questions about what I said? |
| Does that all make sense? |
| Confirm your own understanding |
| Let me see if I've gotten this right. |
| I want to make sure I understand what you've been telling me. |
| Avoid medical jargon |
| Use the I wish statement |
| I wish there were more chemotherapy we could give you that would make a difference. |
| Use the Hope for the best, prepare for the worst statement |
| I think we should hope that the chemotherapy works but prepare for the possibility that it might not. |
There are 2 additional statements that can be very useful when patients and families are struggling with the reality of severe illness and are still hoping for longer life and cure. The first is the I wish statement, in which the clinician allies himself or herself with the patient's or family's wishes by stating, I wish it were different. I wish there were more chemotherapy we could give you that would make a difference.59 Occasionally, when tension is developing because the clinician does not believe an intervention is warranted but the patient desires it nonetheless, the I wish statement can be a powerful way of realigning with the patient. For example, responding to a patient who says, I want chemo to cure my cancer, with the statement There is no chemotherapy to help you can seem antagonistic. In contrast, saying, I wish there were a treatment that would make your cancer go away aligns the clinician with the patient and supports the patient. Another advantage of I wish statements is that they are truethe clinician does wish that there was an effective treatment and would gladly provide it if available. In general, I wish statements are more effective than I'm sorry statements, because the latter can be interpreted as the clinician taking responsibility for the situation. When a provider says, I'm sorry to give you this bad news, the patient may feel the need to say, That's OK. On the other hand, saying, I'm so sorry that your mother is dying, is very human and unlikely to be interpreted as the clinician taking responsibility for the death.
A similar technique is to use the statement Hope for the best, prepare for the worst when speaking with patients and families.60 For example, a physician could say, I really wish your mother could get better, and we should still hope for that; at the same time, we need to prepare for what will happen if she doesn't get better. Once again, this phrase both allows the patient or family to continue hoping things will improve and the clinician to support this hope, while simultaneously beginning the process of planning for the more likely outcome. Over time, the patient and family often move toward accepting that the patient is dying. Finally, trying to help the patient or family maintain hope in the face of illness and death is challenging but important: If your mother can't get better, are there other things you can hope for? Helping to identify tangible and realistic goals, such as being free from pain, seeing an important family member one last time, or getting home can provide hope at a difficult time.
Giving a Recommendation
Most patients with serious illness and their families want help making complex and ethically charged decisions. When clinicians ask patients to make decisions unilaterally, patients often feel anxious, sometimes for weeks.61 Families are often paralyzed when faced with the very difficult decision of whether to withdraw life‐sustaining interventions from a family member with an advanced terminal illness. Even if they understand on an intellectual level that ceasing to provide potentially curative or life‐prolonging therapies is the best choice, they are not yet able to accept this decision on an emotional level and ultimately may feel responsible and then guilty for the patient's death. Physicians need to carry some of the burden of making these difficult decisions. One way to relieve family members of some of this guilt is to recommend a plan of care based on substituted judgment, that is, on what the patient said she or he wanted or what the family thinks the patient would have wanted.6264 In addition, clinicians should use their medical expertise, experience, and understanding of the situation to make recommendations. The patient or family can then accept or reject the physician's advice, which maintains patient autonomy, yet not have to explicitly instruct the clinician to withdraw or limit life‐sustaining interventions.
The preceding discussion and recommendations can guide scenarios like those presented at the beginning of this article. In the second conversation, the clinician had just told the patient that he could help her to achieve her goal of pain control and of returning and staying home.
Dr. S says, I want to make sure I've understood what you've said. To summarize, you've told me how important it is to you to have your pain controlled, to have some good time with your husband and family at home, and not to come back to the hospital. Is that right?
Mrs. G: Yes, that's right.
Dr. S: And how is your pain today?
Mrs. G: So much better. I'm hoping I can go home soon.
Dr. S: That should be possible. In the next day or so, we will be getting you back onto medications that you can take by mouth. But before you go home, we need to figure out how we can support you and your family at home: get you ongoing help with pain control and any other issues that come up and support for your husband and family as well.
Mrs. G: Yes, my family really needs support.
Dr. S: Have you had any experience with hospice before? I'm thinking that that would be the best way to get you the support you and your family need.
Mrs. G: Yes. When my sister died, she had home hospice. They were very good and helpful, especially to her husband, my brother‐in‐law. Yes, I would like that, as I just don't want to come back to the hospital. Hopefully, they can help my husband adjust to things.
Dr. S continues: I think they will help your husband a lot, both before and also after your death. He pauses.
Mrs. G: That's good. I worry about him so much.
Dr. S: As you know, one of the important goals of hospice care is to keep you at home so you don't have to return to the hospital. And when the time comes, to help you die peacefully at home. Mrs. G nods. What this also means is that they would not use CPR, that is, not do chest compressions, when you were actively dying.
Mrs. G: Yes, I want to be able to go home and be at peace. The last thing I would want is someone thumping on my chest as I was dying. She smiles.
Dr. S.: Do you have any other thoughts or questions?
Mrs. G: Well, yes. I'm wondering if we can set up a time to talk with my husband about all of this. I think it would be helpful for him to talk with you. Would that be possible?
Dr. S: I would be happy to talk with your husband. When is he coming in?
They negotiate a time.
Mrs. G: I want to thank you for taking the time to talk with me. I am really grateful.
CONCLUSIONS
The decision about cardiopulmonary resuscitation is part of a larger conversation with a patient about how she or he wants to spend the rest of his/her life. Importantly, the decision should be made in context, rather than in isolation. Given the understanding that develops between physician and patient in the conversation above, it is not necessary to describe all the specific treatments that occur during CPR because the physician has already established that the patient does not want to return to the hospital, and she understands that she has a terminal condition and is dying. Through exploring a patient's goals and values, a clinician can discover a patient's preferences for care generally and come up with a comprehensive plan that addresses the particular individual's medical, social, and emotional needs. For physicians, few interventions are as important or rewarding as relieving patients' suffering and helping them attain their goals during and at the end of life.
Mrs. G is a 58‐year‐old woman with metastatic pancreatic cancer, diagnosed 8 months prior to admission when she presented to her primary care doctor with abdominal pain and weight loss. The cancer was locally advanced and metastatic to the liver at the time of diagnosis, and she underwent first‐line palliative chemotherapy with gemcitabine without objective or symptomatic improvement. She is now admitted to the hospitalist service with intractable abdominal pain, uncontrolled on her outpatient doses of opioids. On the day after admission, her pain is well controlled on morphine via patient‐controlled anesthesia. The hospitalist decides to talk with her about her about resuscitation status. At this point, she is full code.
Dr. S sits down with her and says, There's something I need to talk with you about that I talk with all of my patients about. And that is, if your heart were to stop, and mind you, I'm not expecting this to happen anytime soon, do you want us to do everything possible to bring you back?
Dr. S pauses to catch his breath, and then Mrs. G says, Well, I guess so, if you thought you could bring me back.
He continues, We are not always successful at bringing people back, but let me explain what this would entail: we could do chest compressions, administer shocks to your heart, put a breathing tube down into the back of your throat, hook you up to a machine that helps you breathe
Mrs. G glances up with a confused look. Dr. S says, You know, Mrs. G, I've started this conversation all wrong. I'm going to step out for a few minutes, collect my thoughts, and come back to talk with you again. Is that OK? Mrs. G smiles and says, Of course.
The doctor reenters the room about 20 minutes later, pulls up a chair next to her bed, and in a relaxed tone of voice says, Tell me what you understand about your illness.
Mrs. G says, I understand that I have cancer in my pancreas and liver and that I may not last very long, perhaps months if that. My oncologist offered me more chemotherapy, but I decided against it because it didn't seem that it would offer me much. And the first chemotherapy was really hard.
Dr. S then asks, As you look ahead, what worries you most?
Mrs. G replies, I really worry that I will die in terrible pain. That scares me terribly. I also worry about my husband and how he's going to cope with my worsening condition and with my death, let alone my pain.
Dr. S then says, That sounds really frightening. Tell me more about that. She continues, Well, I really want to spend the time I have left with my husband and familymy children and grandchildrenand I want to have some good time with them. But I keep worrying that it won't be able to be that way. She starts crying. You know, I'm so scared about going home and being in terrible pain again and having my husband and family be terrified
She pauses for a moment. The doctor doesn't say anything.
She starts up again, But I don't want to end up in the hospital again.I don't want to end up on machines like my mother‐in‐law did. She looks up at Dr. S, expecting a response.
Dr. S takes her hand and says, You know, I realize this is not easy to talk about, but it's really important for me to hear about your worries so that I can know how to help. The good news is we have many good methods to control your pain and that we can help you to be comfortable and to remain at home.
Thank you. Thank you so much.
As these conversations illustrate, discussing preferences regarding resuscitation is a challenging and important task for physicians. Understanding patients' wishes at the end of life allows clinicians to provide the type of care patients want, to avoid unwanted interventions, and to promote patient autonomy and dignity. Despite the frequency with which physicians have these conversations, they typically fall short when discussing code status with patients. Clinicians fail to address patients' concerns, monopolize conversations, and commonly misunderstand patients' resuscitation preferences.13 Often these discussions do not occur at all; more than 70% of seriously ill patients have never discussed advance directives with their physician.2, 4 The multicenter SUPPORT study, which demonstrated serious problems in the care of seriously ill hospitalized patients, documented that only 47% of physicians knew when their patients preferred do‐not‐resuscitate status.5
Hospitalists frequently conduct resuscitation discussions. Patients who are admitted to the hospital are usually seriously ill, and hospitalists need to assess rapidly whether an individual patient would want a resuscitation attempt if he or she had a cardiopulmonary arrest in the hospital. They need to build trust and rapport quickly with patients they have never met. Despite this challenge, hospitalists are in a good position to discuss resuscitation preferences.6 Patients may be more willing to discuss these issues in inpatient rather than outpatient settings because their acute deterioration may encourage self‐reflection.6 Furthermore, the time and productivity pressures of office practice often make it difficult for primary care physicians, who often know the patient and family best, to address advance directives. Although studies have documented that patients are interested in talking to their primary physicians about these matters,7, 8 these conversations do not occur with regularity. Preliminary research has raised the possibility that cancer patients may actually prefer to discuss these issues in an inpatient setting with a hospital‐based provider rather than with their oncologists.9 Studies have not addressed the question of whether patients with diseases other than cancer would prefer to discuss these issues with a hospitalist or their outpatient subspecialist.
Given that more than half of all Americans die in hospitals, hospitalists care for many people who are terminally ill and will need to assess preferences for cardiopulmonary resuscitation (CPR) and other treatments. Hospitalists need to be competent and compassionate in their approach to patients and their families. In this articles we review clinician barriers to holding these conversations, offer a variety of approaches to enhance these discussions, and review communication techniques that can be used to improve understanding.
Clinician Barriers to Discussing Resuscitation
Clinicians' own barriers may lead to infrequent and inadequate conversations about resuscitation. Understanding these barriers may allow providers to overcome them and facilitate better and more frequent communication. A discussion of patient barriers is beyond the scope of this article.
Unresolved Feelings about Death and Dying
When discussing resuscitation, or code status, physicians are discussing the possibility of death with a patient. In the first scenario above, the clinician lists the many procedures that could be done if the patient's heart were to stop without using the words die or death. The clinician never explicitly acknowledges that the patient has a serious illness that could lead to her death. Medical culture is focused on cure and on warding off death until the last possible moment. Because clinicians work in this culture, many have unresolved feelings of personal failure that are triggered when treating a dying patient.10 Also, the death of a patient can lead to anxieties about the clinician's own mortality and raise uncomfortable feelings of loss, related to the patient or to memories of deaths of the clinician's loved ones. In an attempt to avoid these feelings, whether conscious or not, physicians may resist talking to patients about death.10
Fear of Taking Away Hope
Clinicians fear that patients will lose hope if they are too honest about prognosis and acknowledge the inevitability of death. This concern may be true for a small minority of patients with advanced terminal illness who are solely focused on continued treatment and in such denial that they never consider the possibility of death. Most patients, however, understand on some level that they are getting sicker and may die, but expect clinicians to initiate discussions about death and dying.8, 11 Clinicians should understand that patients can have hope about many things beyond cure of their illness. For example, they can hope for good control of their symptoms so they can spend meaningful time with family and friends, heal troubled relationships, create a legacy, and say good‐bye. As in other developmental stages throughout life, the process of dying can be a time of emotional and spiritual growth and provide an opportunity to deepen relationships and find greater meaning.12 Despite their fears, physicians are much less likely to take away hope than they think. In fact, they can carry out the important actions of helping patients to refocus on more attainable goals and helping to return hope to what may be perceived by all as a hopeless situation.13, 14
Inadequate Training
There have been many educational interventions in both outpatient and inpatient settings to encourage physicians to discuss advance directives with patients.1521 In most of these studies, clinicians were sent reminders, but did not receive training or feedback to improve their communication skills. Although these interventions have led to modest increases in the number of advance directives, little is known about the quality of the conversations between clinician and patient.
There are acknowledged deficits in undergraduate22 and graduate medical education in discussing preferences and goals of care with patients.23, 24 A national survey of medical education deans showed that two‐thirds believed insufficient time was given to palliative care education including communication skills.25 Reflecting this lack of training, medical students and residents feel unprepared to take care of dying patients.26 In one survey, hospitalists reported that although palliative care was very important to their practice, they had not received enough training in palliative care knowledge and skills.27
Traditional Ethical Frameworks
Another difficulty arises from myths about the ethical perspectives that inform medical decision making and obtaining informed consent. Although these perspectives highlight the importance of patient autonomy and the right of the individual to choose medical treatments, they do not require physicians to describe every possible treatment if, in the clinician's judgment, a particular treatment would not benefit a patient. Physicians do have an obligation to use their medical knowledge and judgment in offering treatments and discussing side effects.28 In an attempt to honor a patient's autonomy when discussing advance directivesand possibly out of fear of coercing patientsclinicians sometimes offer a menu of treatment options without exploring the patient's underlying goals for these treatments. This approach can become meaningless out of context if the patient does not understand the probability that these interventions will work or the interrelatedness of the interventions. For example, when given a list of choices regarding resuscitation, a patient may say, I would like chest compressions and a chest tube, but no shocks please. Such a statement makes little sense clinically. Instead, physicians should have meaningful conversations with their patients in which they describe treatment options in the context of patient goals and values and help patients come to decisions in a shared process.
Practical Concerns
Outpatient providers find that time constraints and the competing demands that occur in caring for patients with multiple chronic health problems make it difficult to discuss advance directives.29 Hospitalists are also subject to productivity pressures and may feel similarly stressed for time. Outpatient providers spend about 5 minutes on each of these conversations,1 and medicine residents in the inpatient setting spend about 10 minutes.30 However, many of the conversations studied were inadequate; thus, it is unknown how long it takes to have an effective conversation. Hospitalists should keep in mind that they need not have these discussions every day with each patient and that having these conversations may end up saving time in the long run if they have a clearer sense of a patient's wishes and goals.
Laying Groundwork for the Discussion
The decision regarding resuscitation should be seen in the context of the patient's goals and values and overall health status. To address code status effectively, it is imperative first to elicit the person's view of his/her illness and then gently correct any misunderstandings. A patient who thinks her/his life will go on indefinitely may feel no need to consider her/his own mortality or alternatives to full resuscitation status. Alternatively, a patient who senses his/her mortality may have already thought about resuscitation and have clear preferences. A key first step in the conversation is to understand a patient's values and goals and comprehension of his/her illness. As in the second discussion above, a clinician can begin a discussion by saying, Tell me what you understand about your illness.
Discussing prognosis with patients can be difficult as physicians struggle with uncertainty. In the most comprehensive study to date of prognostication, physicians overestimated patient survival on average by a factor of 5.31 Nonetheless, there are compelling reasons to discuss prognosis. Failure to do so often results in patients spending their last days in the hospital receiving more aggressive treatments than they might choose if they understood their prognoses.32 Further, patients are denied the opportunity to address issues of life closure, such as spending time with family, thinking about legacy, and settling financial affairs. Physicians also fear they will take away hope with prognostication and believe patients expect greater accuracy than they can provide.33
Physicians can improve their prognostication skills by considering patients' functional status and clinical signs and symptoms and by using validated scales. The Karnofsky Performance Score (KPS) and the Eastern Cooperative Oncology Group (ECOG) Performance Status have been shown to correlate with survival,3440 and the Palliative Prognostic Score (PaP) has been validated in both cancer and noncancer patients.41, 4446 The PaP uses a combination of the KPS, clinical signs and symptoms, and the clinician's clinical prediction of survival. In addition, clinicians can be honest with patients about prognostic uncertainties and give prognoses in ranges, such as days to weeks, or weeks to months.
How to Broach the Subject of Resuscitation
In the first scenario presented in this article, the hospitalist says, There's something I need to talk with you about that I talk with all of my patients about. Although many clinicians begin resuscitation discussions this way, the question is problematic because often it is untrue. Most clinicians do not discuss code status with all patients who are admitted to the hospital. A better option would be to say, When I take care of patients with advanced cancer, I like to talk with them about their wishes regarding resuscitation. Is that all right? Better yet would be to ask a general question such as As you look ahead, what worries you most? or As you look to the future, what do you hope for? These and other useful questions appear in Table 1. These questions allow patients to bring up their concerns, show that the clinician cares about them, and often segue into a discussion of patients' hopes and worries about their own death and dying process. These questions often allow patients to bring up important goals that bear directly on the issue of resuscitation. For example, in the second scenario at the beginning of the article, the patient says she wants to have quality time with her family at home and does not want life‐sustaining technologies. Such a patient may not want resuscitation. When discussing CPR, it can also be helpful to state explicitly that resuscitation is used when a patient has died, rather than to use euphemisms such as, If your heart were to stop. The clinician can ask explicitly, If you were to die, would you want? There are other strategies for introducing the subject of resuscitation if these questions do not work. If a patient seems uncomfortable with the conversation, the clinician can address this discomfort directly by saying, This conversation seems to make you uncomfortable. Other strategies for exploring these issues include inquiring if the person has ever discussed resuscitation with his/her family or another physician, or asking if anyone else in the family has been very sick. Additionally, clinicians can ask questions about surrogate decision making. If you were to get so sick that you were unable to make decisions for yourself, who would you want to make them for you? This can then lead into a discussion of whether the patient has spoken to that person about his/her preferences, and if so, what was the content of that conversation. Another useful question is, Is there any state that would be worse than death for you? This question focuses on outcomes and allows the physician to put the issue of resuscitation into perspective for a patient.
| When I take care of patients with advanced cancer [or heart disease or lung disease, etc.], I like to talk with them about their wishes for care if they were to get very sick and even die suddenly. Is that all right? |
| As you look ahead, what worries you most? |
| As you look to the future, what do you hope for? |
| Has a close friend or family member ever been really sick? |
| If you were to get so sick that you were unable to make decisions for yourself, who would you want to make them for you? |
| Is there any state that would be worse than death for you? |
Knowing the Facts about Cardiopulmonary Resuscitation
In general, about 1 in 7 patients who have undergone CPR in the hospital survive to hospital discharge. Two literature reviews, from 1989 and 1998, of hospitalized patients who underwent CPR in the hospital reported surprisingly similar statistics. Immediate survival in both series was 41%, and survival to hospital discharge was 13%‐14%. Factors that increased survival included arrest due to coronary artery disease or drug overdose/adverse reaction, and location of arrest in the intensive care unit (ICU). Factors that decreased survival included sepsis at time of arrest, cancer, whether localized or metastatic, dementia, acute renal failure, bed‐bound status, and residence in a nursing home. Neither age nor sex was associated with survival.47, 48 In a meta‐analysis of outcome studies of CPR in metastatic cancer patients, none of 117 patients survived to hospital discharge.49
Most people get their information about CPR survival rates from the mass media, which portray CPR as a very successful procedure. For instance, on television, the sole source of information on CPR for most patients, the rate of survival to hospital discharge is 64%, much higher than the 1314% cited in the medical literature.50 Thus, it is no surprise that a patient with metastatic cancer or another life‐limiting illness would assume a positive outcome with CPR.
Knowledge of the facts about CPR survival rates is key when a physician discusses code status with a patient because these data influence patients' decisions. Patients who have a realistic understanding of their chances are less likely to prefer resuscitation.51, 52 Offering patients information about the success rate for CPR in their particular situation is critical in helping them reach a decision consistent with their values and goals. This information can be given quantitatively or qualitatively depending on the clinician assessment of what the patient would prefer.
Avoiding Stark Dichotomies in Resuscitation Discussions
In clinical practice there are 3 ways in which physicians can present resuscitation decisions as black and white and ignore the shades of gray. First, clinicians may present the choice between resuscitation and do not resuscitate (DNR) as a choice between life and death. In the first scenario above, the physician states If your heart were to stop, do you want us to do everything to bring you back? implying he would be able to save the patient's life with resuscitation attempts. When discussing resuscitation, clinicians should avoid language that suggests such a stark dichotomy. The reality is that most patients die despite resuscitation attempts. In fact, a patient is actually choosing between certain death (without resuscitation) and likely death (if resuscitation is attempted). For a patient with a serious, life‐limiting illness, it may be more effective to frame the conversation in terms of how that person envisions the end of life, and not whether death will eventually occur.
Second, clinicians and patients sometimes equate DNR with doing nothing or giving up. Clinicians fail to discuss other treatment options or alternatives, such as continuing ongoing aggressive medical treatments with DNR status or pursuing palliative care. Performing resuscitation is equated with activity and treatment, whereas withholding resuscitation is seen as passivity and giving up. To the patient, this can feel like abandonment, as if the doctor is withholding a treatment and not offering anything in its place. Examples of positive phrases that demonstrate the physician will continue to offer excellent care include: We will continue maximal medical therapy. However, if you die despite these treatments, we will let you die peacefully and won't attempt to revive you; We'll continue the intravenous antibiotics, but we won't plan to move you to the ICU if things worsen53; and We will work hard to treat your pain and other symptoms and to get you home. In addition, hospitalists must ensure, when signing out to physician colleagues, nurses, respiratory therapists, and others, that DNR orders are not overinterpreted to mean no treatment. Although a DNR order states that in the event of a cardiac arrest, no attempt at resuscitation will be made, it should have no bearing on other appropriate and desired interventions, including antibiotics, chemotherapy, and artificial nutrition; treatment for pain and other symptoms; and even monitoring in an ICU. This misunderstanding of DNR status is common among health care providers and has led many to argue that DNR orders should be part of a more comprehensive treatment plan that outlines where the patient's wishes for treatment fall on the spectrum from otherwise aggressive measures to comfort care.54, 55 Physicians who have a clear understanding of a DNR order will be able to reassure a patient that they will continue to receive desired care, but that if the patient dies, no attempt to resuscitate will be made.
Third, it is important to remind patients who choose full resuscitation status that additional decisions will need to be made if resuscitation is successful. Most patients who survive cardiopulmonary arrest end up worse off clinically and spend time in an ICU with life‐support measures in place, such as mechanical ventilation and vasopressors. Even if they survive, there will likely be a period during which they are unable to speak for themselves. This situation puts the burden of decision making on their surrogates or an appointed durable power of attorney for health care (DPOA‐HC). It is important to ask patients ahead of time whether there are conditions under which they would not want ongoing life‐sustaining measures. For example, a person might opt for discontinuation of life‐support measures if the physician and family agreed that there was only a minimal chance of cognitive recovery existed and that ongoing support was only prolonging inevitable death. To clarify the patient's wishes in this situation, you might ask, Are there conditions that would be worse than death? Encouraging the patient to share his/her wishes in this situation with a surrogate or DPOA‐HC will help to ensure those wishes are respected.
Communication Techniques
When discussing advance directives, it is important to give patients the chance to describe their life goals and their values to establish a context for understanding the role of life‐sustaining treatments. One useful method to elicit these goals and values is to ask open‐ended questions, followed by periods of silence so the patient has time to express himself/herself. In the second scenario, above, the physician used open‐ended questions in several instances: Tell me what you understand about your illness and As you look ahead, what worries you most? Tulsky and colleagues documented that medical residents spoke 76% of the time in discussions with patients about code status.30 In an ideal case, this ratio should be reversed or at least be even, allowing patients the time to explain their thoughts. Acknowledging patients' emotions by stating simply, You seem [angry/sad/perplexed], and waiting for an answer can help patients feel they are being understood. Making empathic statements is another powerful communication technique that conveys understanding.56 Examples include, That must be really sad for you, and It must be frightening to be in so much pain. As noted, silence can also be a powerful tool. Clinicians tend to be uncomfortable with silence and so fill the gaps with words. Allowing for silence enables patients to digest what they have heard, encourages them to continue speaking, and shows them the clinician wants to hear what they have to say. When giving information about any medical issues and especially about CPR, it is important to explain concepts in lay terms and to avoid medical jargon.57 Additionally, nonverbal communication techniques such as making eye contact, head nodding, and leaning in toward the patient all help in communicating engagement in the conversation. Having the conversation in a quiet and private place and sitting at the same level as the patient or family is also important. It is always a good idea to check in with patients to assess their understanding. Simply asking, Do you have any questions about what I said? or Does that all make sense? gives patients the opportunity to ask for clarification. Attempting to summarize what a patient has said can also help to clarify misunderstandings. Useful phrases include, Let me see if I've gotten this right or I want to make sure that I understood what you're telling me, followed by the clinician's synopsis of important points discussed.58 A summary of important communication techniques can be found in Table 2.
| Ask open‐ended questions followed by periods of silence |
| Tell me what you understand about your illness. |
| As you look ahead, what worries you most? |
| Acknowledge emotion |
| You seem [angry/sad/perplexed]. |
| Make empathetic statements |
| That must be really hard for you. |
| It must be terrible to be in so much pain. |
| Use nonverbal communication techniques such as eye contact, head nodding, leaning in toward the patient, sitting down, and sitting at patient's level |
| Allow for silence |
| Assess patient's understanding |
| Do you have any questions about what I said? |
| Does that all make sense? |
| Confirm your own understanding |
| Let me see if I've gotten this right. |
| I want to make sure I understand what you've been telling me. |
| Avoid medical jargon |
| Use the I wish statement |
| I wish there were more chemotherapy we could give you that would make a difference. |
| Use the Hope for the best, prepare for the worst statement |
| I think we should hope that the chemotherapy works but prepare for the possibility that it might not. |
There are 2 additional statements that can be very useful when patients and families are struggling with the reality of severe illness and are still hoping for longer life and cure. The first is the I wish statement, in which the clinician allies himself or herself with the patient's or family's wishes by stating, I wish it were different. I wish there were more chemotherapy we could give you that would make a difference.59 Occasionally, when tension is developing because the clinician does not believe an intervention is warranted but the patient desires it nonetheless, the I wish statement can be a powerful way of realigning with the patient. For example, responding to a patient who says, I want chemo to cure my cancer, with the statement There is no chemotherapy to help you can seem antagonistic. In contrast, saying, I wish there were a treatment that would make your cancer go away aligns the clinician with the patient and supports the patient. Another advantage of I wish statements is that they are truethe clinician does wish that there was an effective treatment and would gladly provide it if available. In general, I wish statements are more effective than I'm sorry statements, because the latter can be interpreted as the clinician taking responsibility for the situation. When a provider says, I'm sorry to give you this bad news, the patient may feel the need to say, That's OK. On the other hand, saying, I'm so sorry that your mother is dying, is very human and unlikely to be interpreted as the clinician taking responsibility for the death.
A similar technique is to use the statement Hope for the best, prepare for the worst when speaking with patients and families.60 For example, a physician could say, I really wish your mother could get better, and we should still hope for that; at the same time, we need to prepare for what will happen if she doesn't get better. Once again, this phrase both allows the patient or family to continue hoping things will improve and the clinician to support this hope, while simultaneously beginning the process of planning for the more likely outcome. Over time, the patient and family often move toward accepting that the patient is dying. Finally, trying to help the patient or family maintain hope in the face of illness and death is challenging but important: If your mother can't get better, are there other things you can hope for? Helping to identify tangible and realistic goals, such as being free from pain, seeing an important family member one last time, or getting home can provide hope at a difficult time.
Giving a Recommendation
Most patients with serious illness and their families want help making complex and ethically charged decisions. When clinicians ask patients to make decisions unilaterally, patients often feel anxious, sometimes for weeks.61 Families are often paralyzed when faced with the very difficult decision of whether to withdraw life‐sustaining interventions from a family member with an advanced terminal illness. Even if they understand on an intellectual level that ceasing to provide potentially curative or life‐prolonging therapies is the best choice, they are not yet able to accept this decision on an emotional level and ultimately may feel responsible and then guilty for the patient's death. Physicians need to carry some of the burden of making these difficult decisions. One way to relieve family members of some of this guilt is to recommend a plan of care based on substituted judgment, that is, on what the patient said she or he wanted or what the family thinks the patient would have wanted.6264 In addition, clinicians should use their medical expertise, experience, and understanding of the situation to make recommendations. The patient or family can then accept or reject the physician's advice, which maintains patient autonomy, yet not have to explicitly instruct the clinician to withdraw or limit life‐sustaining interventions.
The preceding discussion and recommendations can guide scenarios like those presented at the beginning of this article. In the second conversation, the clinician had just told the patient that he could help her to achieve her goal of pain control and of returning and staying home.
Dr. S says, I want to make sure I've understood what you've said. To summarize, you've told me how important it is to you to have your pain controlled, to have some good time with your husband and family at home, and not to come back to the hospital. Is that right?
Mrs. G: Yes, that's right.
Dr. S: And how is your pain today?
Mrs. G: So much better. I'm hoping I can go home soon.
Dr. S: That should be possible. In the next day or so, we will be getting you back onto medications that you can take by mouth. But before you go home, we need to figure out how we can support you and your family at home: get you ongoing help with pain control and any other issues that come up and support for your husband and family as well.
Mrs. G: Yes, my family really needs support.
Dr. S: Have you had any experience with hospice before? I'm thinking that that would be the best way to get you the support you and your family need.
Mrs. G: Yes. When my sister died, she had home hospice. They were very good and helpful, especially to her husband, my brother‐in‐law. Yes, I would like that, as I just don't want to come back to the hospital. Hopefully, they can help my husband adjust to things.
Dr. S continues: I think they will help your husband a lot, both before and also after your death. He pauses.
Mrs. G: That's good. I worry about him so much.
Dr. S: As you know, one of the important goals of hospice care is to keep you at home so you don't have to return to the hospital. And when the time comes, to help you die peacefully at home. Mrs. G nods. What this also means is that they would not use CPR, that is, not do chest compressions, when you were actively dying.
Mrs. G: Yes, I want to be able to go home and be at peace. The last thing I would want is someone thumping on my chest as I was dying. She smiles.
Dr. S.: Do you have any other thoughts or questions?
Mrs. G: Well, yes. I'm wondering if we can set up a time to talk with my husband about all of this. I think it would be helpful for him to talk with you. Would that be possible?
Dr. S: I would be happy to talk with your husband. When is he coming in?
They negotiate a time.
Mrs. G: I want to thank you for taking the time to talk with me. I am really grateful.
CONCLUSIONS
The decision about cardiopulmonary resuscitation is part of a larger conversation with a patient about how she or he wants to spend the rest of his/her life. Importantly, the decision should be made in context, rather than in isolation. Given the understanding that develops between physician and patient in the conversation above, it is not necessary to describe all the specific treatments that occur during CPR because the physician has already established that the patient does not want to return to the hospital, and she understands that she has a terminal condition and is dying. Through exploring a patient's goals and values, a clinician can discover a patient's preferences for care generally and come up with a comprehensive plan that addresses the particular individual's medical, social, and emotional needs. For physicians, few interventions are as important or rewarding as relieving patients' suffering and helping them attain their goals during and at the end of life.
- ,,,.Opening the black box: how do physicians communicate about advance directives?Ann Intern Med.1998;129:441–449.
- ,,, et al.Physician understanding of patient resuscitation preferences: insights and clinical implications.J Am Geriatr Soc.2000;48:S44–S51.
- ,,,,.Patient knowledge and physician predictions of treatment preferences after discussion of advance directives.J Gen Intern Med.1998;13:447–454.
- ,,.The value of disease severity in predicting patient readiness to address end‐of‐life issues.Arch Intern Med.2003;163:609–612.
- A controlled trial to improve care for seriously ill hospitalized patients. The study to understand prognoses and preferences for outcomes and risks of treatments (SUPPORT).The SUPPORT Principal Investigators.JAMA.1995;274:1591–1598.
- ,,,,,.Can we talk? Inpatient discussions about advance directives in a community hospital. Attending physicians' attitudes, their inpatients' wishes and reported evidence.Arch Intern Med.1994;154:2299–2308.
- ,,,,.Advance directives for medical care—a case for greater use.N Engl J Med.1991;324:889–895.
- ,,.Patient attitudes to discussing life‐sustaining treatment.Arch Intern Med.1986;146:1613–1615.
- ,.Paradoxes in cancer patients' advance care planning.J Palliat Med.2000;3(1):27–35.
- ,,.The inner life of physicians and care of the seriously ill.JAMA.2001;286:3007–3014.
- ,,.The discussion about advance directives; patient and physician opinions regarding when and how it should be conducted.Arch Intern Med.1995;155:1025–1030.
- .Psychological considerations, growth, and transcendence at the end of life: the art of the possible.JAMA.2001;285:2898–2905.
- .Care of dying patients: beyond symptom management.West J Med.1999;171(4):253–256.
- ,,,.Fostering coping and nurturing hope when discussing the future with terminally ill cancer patients and their caregivers.Cancer.2005;103:1965–1975.
- ,,,,,.Promoting advance directives among elderly primary care patients.J Gen Intern Med.2004;19:944–951.
- ,,,,.Implementing advance directives in the primary care setting.Arch Intern Med.1994;154:2321–2327.
- ,.Will outpatients complete living wills? A comparison of two interventions.J Gen Intern Med.1991;6:41–46.
- ,,, et al.Enhancement of proxy appointments for older persons: physician counselling in ambulatory settings.J Am Geriatr Soc.1996;44(1):37–43.
- ,,,.Strategies to promote the use of advance directives in a residency outpatient practice.J Gen Intern Med.1996;11:657–663.
- ,,,,,.Promoting inpatient directives about life‐sustaining treatments in a community hospital. Results of a 3‐year time‐series intervention trial.Arch Intern Med.1995;155:2317–2323.
- ,,,,.An educational intervention in the surgical ICU to improve ethical decisions.Surgery.1995;118(2):294–299.
- ,,.The status of medical education in end‐of‐life care. A national report.J Gen Intern Med.2003;18:685–695.
- ,.ACGME Requirements for end‐of‐life training in selected residency and fellowship programs: a status report.Acad Med.2002;77(4):299–304.
- ,,,.End‐of‐life care education in internal medicine residency programs: an interinstitutional study.J Palliat Med.2002;5:487–496.
- ,,,,,.End‐of‐life care in the curriculum: a national study of medical education deans.Acad Med.2004;79:760–768.
- ,,,,.Assessing medical students' training in end‐of‐life communication: a survey of interns at one urban teaching hospital.Acad Med.2003;78:530–537.
- ,,,.Hospitalists' perceptions of their residency training needs: results of a national surveyAm J Med.2001;111(3):247–254.
- ,.Legal and ethical myths about informed consent.Arch Intern Med.1996;156:2521–2526.
- ,,.Physician reluctance to discuss advance directives: an empiric investigation of potential barriers.Arch Intern Med.1994;154:2311–2318.
- ,,.How do medical residents discuss resuscitation with patients?J Gen Intern Med.1995;10:436–442.
- ,,,.Extent and determinants of error in doctors' prognoses in terminally ill patients: prospective cohort study.Br Med J.2000;320:469–473.
- .Death Foretold; Prophecy and Prognosis in Medical Care.Chicago:University of Chicago Press,1999.
- ,.Attitude and self‐reported practice regarding prognostication in a national sample of internists.Arch Intern Med.1998;158:2389–2395.
- ,,, et al.Prognostic factors for survival in patients treated in phase I clinical trials.Cancer.1994;74:1965–1973.
- ,,, et al.Prediction of survival of patients terminally ill with cancer. Results of an Italian prospective multicentric studyCancer.1995;75:2613–2622.
- ,,.Evaluation of patients with advanced cancer using Karnovsky performance status.Cancer.1980;45:2220–2224.
- ,,,.The Karnofsky Performance Status Scale. An exam of its reliability and validity in a research setting.Cancer.1984;53:2002–2007.
- .A demographic and prognostic approach to defining the end of life.J Palliat Med.2005;8(suppl 1):s12–s21.
- .Estimating length of survival in end‐stage cancer: a review of the literature.J Pain Symptom Manage.1995;10:548–555.
- ,.Prognostic factors, survival, and advanced cancer.J Palliat Care.1992;1992(8):4.
- ,,.A new palliative prognostic score: a first step for the staging of terminally ill cancer patients.J Pain Symp Management1999;17(4):231–239.
- ,,.Survival prediction in terminal cancer patients: A systematic review of the medical literature.Palliat Med.2000;14:363–374.
- ,,.Clinical symptoms and length of survival in patients with terminal cancer.Arch Intern Med.1988;148:1586–1591.
- ,,.Successful validation of the palliative prognostic score in terminally ill cancer patients.J Pain Symptom Manage.1999;17:240–247.
- ,,.The use of the palliative prognostic score in patients with diagnoses other than cancer.J Pain Symptom Manage.2003;26:883–885.
- ,,.Diagnostic accuracy of the palliative prognostic score in hospitalized patients with advanced cancer.J Clin Oncol.2004;22:4823–4828.
- ,,,.Survival after in‐hospital cardiopulmonary resuscitation: a meta‐analysis.J Gen Intern Med,1998;13(12):805–16.
- .Informing the patient about cardiopulmonary resuscitation: when the risks outweigh the benefits.J Gen Intern Med.1989;4:349–355.
- .Resuscitation of patients with metastatic cancer: Is transient benefit still futile?Arch Intern Med.1991;151:235–239.
- ,,.Cardiopulmonary resuscitation on television—miracles and misinformation.N Engl J Med.1996;334:1578–1582.
- ,,, et al.The influence of the probability of survival on patients' preferences regarding cardiopulmonary resuscitation.N Engl J Med.1994;330:545–549.
- ,,,.Resuscitation decision making in the elderly: the value of outcome data.J Gen Intern Med.1993;8:295–300.
- .Discussing do‐not‐resuscitate status.J Clin Oncol.2001;19:1576–1581.
- .Do‐not‐resuscitate decisions in a community hospital. Incidence, implications, and outcomes.JAMA.1986;256:1164–1169.
- ,,,.A prospective study of the efficacy of the physician order form for life‐sustaining treatment.J Am Geriatr Soc.1998;46:1170–1171.
- ,,, for theACPAE‐o‐LCCP.Discussing palliative care with patients.Ann Intern Med.1999;130:744–749.
- ,,,,.Approaching difficult communication tasks in oncology.CA Cancer J Clin.2005;55(3):164–177.
- ,,, et al.“Let me see if I have this right…”: words that help build empathy.Ann Intern Med.2001;135(3):221–227.
- ,,.“I wish things were different”: expressing wishes in response to loss, futility, and unrealistic hopes.Ann Intern Med.2001;135:551–555.
- ,,.Hope for the best, and prepare for the worst.Ann Intern Med.2003;138:439–443.
- ,,,.When the treatment goal is not cure: are cancer patients equipped to make informed decisions?J Clin Oncol.2002;20:503–513.
- ,,.Beyond autonomy: Diversifying end‐of‐life decision‐making approaches to serve patients and families.J Am Geriatr Soc.2005;53:1046–1050.
- ,,,.Measuring patients' desire for autonomy: decision making and information‐seeking preferences among medical patients.J Gen Intern Med.1989;4(1):23–30.
- ,.Physician recommendations and patient autonomy: finding a balance between physician power and patient choice.Ann Intern Med.1996;125:763–769.
- ,,,.Opening the black box: how do physicians communicate about advance directives?Ann Intern Med.1998;129:441–449.
- ,,, et al.Physician understanding of patient resuscitation preferences: insights and clinical implications.J Am Geriatr Soc.2000;48:S44–S51.
- ,,,,.Patient knowledge and physician predictions of treatment preferences after discussion of advance directives.J Gen Intern Med.1998;13:447–454.
- ,,.The value of disease severity in predicting patient readiness to address end‐of‐life issues.Arch Intern Med.2003;163:609–612.
- A controlled trial to improve care for seriously ill hospitalized patients. The study to understand prognoses and preferences for outcomes and risks of treatments (SUPPORT).The SUPPORT Principal Investigators.JAMA.1995;274:1591–1598.
- ,,,,,.Can we talk? Inpatient discussions about advance directives in a community hospital. Attending physicians' attitudes, their inpatients' wishes and reported evidence.Arch Intern Med.1994;154:2299–2308.
- ,,,,.Advance directives for medical care—a case for greater use.N Engl J Med.1991;324:889–895.
- ,,.Patient attitudes to discussing life‐sustaining treatment.Arch Intern Med.1986;146:1613–1615.
- ,.Paradoxes in cancer patients' advance care planning.J Palliat Med.2000;3(1):27–35.
- ,,.The inner life of physicians and care of the seriously ill.JAMA.2001;286:3007–3014.
- ,,.The discussion about advance directives; patient and physician opinions regarding when and how it should be conducted.Arch Intern Med.1995;155:1025–1030.
- .Psychological considerations, growth, and transcendence at the end of life: the art of the possible.JAMA.2001;285:2898–2905.
- .Care of dying patients: beyond symptom management.West J Med.1999;171(4):253–256.
- ,,,.Fostering coping and nurturing hope when discussing the future with terminally ill cancer patients and their caregivers.Cancer.2005;103:1965–1975.
- ,,,,,.Promoting advance directives among elderly primary care patients.J Gen Intern Med.2004;19:944–951.
- ,,,,.Implementing advance directives in the primary care setting.Arch Intern Med.1994;154:2321–2327.
- ,.Will outpatients complete living wills? A comparison of two interventions.J Gen Intern Med.1991;6:41–46.
- ,,, et al.Enhancement of proxy appointments for older persons: physician counselling in ambulatory settings.J Am Geriatr Soc.1996;44(1):37–43.
- ,,,.Strategies to promote the use of advance directives in a residency outpatient practice.J Gen Intern Med.1996;11:657–663.
- ,,,,,.Promoting inpatient directives about life‐sustaining treatments in a community hospital. Results of a 3‐year time‐series intervention trial.Arch Intern Med.1995;155:2317–2323.
- ,,,,.An educational intervention in the surgical ICU to improve ethical decisions.Surgery.1995;118(2):294–299.
- ,,.The status of medical education in end‐of‐life care. A national report.J Gen Intern Med.2003;18:685–695.
- ,.ACGME Requirements for end‐of‐life training in selected residency and fellowship programs: a status report.Acad Med.2002;77(4):299–304.
- ,,,.End‐of‐life care education in internal medicine residency programs: an interinstitutional study.J Palliat Med.2002;5:487–496.
- ,,,,,.End‐of‐life care in the curriculum: a national study of medical education deans.Acad Med.2004;79:760–768.
- ,,,,.Assessing medical students' training in end‐of‐life communication: a survey of interns at one urban teaching hospital.Acad Med.2003;78:530–537.
- ,,,.Hospitalists' perceptions of their residency training needs: results of a national surveyAm J Med.2001;111(3):247–254.
- ,.Legal and ethical myths about informed consent.Arch Intern Med.1996;156:2521–2526.
- ,,.Physician reluctance to discuss advance directives: an empiric investigation of potential barriers.Arch Intern Med.1994;154:2311–2318.
- ,,.How do medical residents discuss resuscitation with patients?J Gen Intern Med.1995;10:436–442.
- ,,,.Extent and determinants of error in doctors' prognoses in terminally ill patients: prospective cohort study.Br Med J.2000;320:469–473.
- .Death Foretold; Prophecy and Prognosis in Medical Care.Chicago:University of Chicago Press,1999.
- ,.Attitude and self‐reported practice regarding prognostication in a national sample of internists.Arch Intern Med.1998;158:2389–2395.
- ,,, et al.Prognostic factors for survival in patients treated in phase I clinical trials.Cancer.1994;74:1965–1973.
- ,,, et al.Prediction of survival of patients terminally ill with cancer. Results of an Italian prospective multicentric studyCancer.1995;75:2613–2622.
- ,,.Evaluation of patients with advanced cancer using Karnovsky performance status.Cancer.1980;45:2220–2224.
- ,,,.The Karnofsky Performance Status Scale. An exam of its reliability and validity in a research setting.Cancer.1984;53:2002–2007.
- .A demographic and prognostic approach to defining the end of life.J Palliat Med.2005;8(suppl 1):s12–s21.
- .Estimating length of survival in end‐stage cancer: a review of the literature.J Pain Symptom Manage.1995;10:548–555.
- ,.Prognostic factors, survival, and advanced cancer.J Palliat Care.1992;1992(8):4.
- ,,.A new palliative prognostic score: a first step for the staging of terminally ill cancer patients.J Pain Symp Management1999;17(4):231–239.
- ,,.Survival prediction in terminal cancer patients: A systematic review of the medical literature.Palliat Med.2000;14:363–374.
- ,,.Clinical symptoms and length of survival in patients with terminal cancer.Arch Intern Med.1988;148:1586–1591.
- ,,.Successful validation of the palliative prognostic score in terminally ill cancer patients.J Pain Symptom Manage.1999;17:240–247.
- ,,.The use of the palliative prognostic score in patients with diagnoses other than cancer.J Pain Symptom Manage.2003;26:883–885.
- ,,.Diagnostic accuracy of the palliative prognostic score in hospitalized patients with advanced cancer.J Clin Oncol.2004;22:4823–4828.
- ,,,.Survival after in‐hospital cardiopulmonary resuscitation: a meta‐analysis.J Gen Intern Med,1998;13(12):805–16.
- .Informing the patient about cardiopulmonary resuscitation: when the risks outweigh the benefits.J Gen Intern Med.1989;4:349–355.
- .Resuscitation of patients with metastatic cancer: Is transient benefit still futile?Arch Intern Med.1991;151:235–239.
- ,,.Cardiopulmonary resuscitation on television—miracles and misinformation.N Engl J Med.1996;334:1578–1582.
- ,,, et al.The influence of the probability of survival on patients' preferences regarding cardiopulmonary resuscitation.N Engl J Med.1994;330:545–549.
- ,,,.Resuscitation decision making in the elderly: the value of outcome data.J Gen Intern Med.1993;8:295–300.
- .Discussing do‐not‐resuscitate status.J Clin Oncol.2001;19:1576–1581.
- .Do‐not‐resuscitate decisions in a community hospital. Incidence, implications, and outcomes.JAMA.1986;256:1164–1169.
- ,,,.A prospective study of the efficacy of the physician order form for life‐sustaining treatment.J Am Geriatr Soc.1998;46:1170–1171.
- ,,, for theACPAE‐o‐LCCP.Discussing palliative care with patients.Ann Intern Med.1999;130:744–749.
- ,,,,.Approaching difficult communication tasks in oncology.CA Cancer J Clin.2005;55(3):164–177.
- ,,, et al.“Let me see if I have this right…”: words that help build empathy.Ann Intern Med.2001;135(3):221–227.
- ,,.“I wish things were different”: expressing wishes in response to loss, futility, and unrealistic hopes.Ann Intern Med.2001;135:551–555.
- ,,.Hope for the best, and prepare for the worst.Ann Intern Med.2003;138:439–443.
- ,,,.When the treatment goal is not cure: are cancer patients equipped to make informed decisions?J Clin Oncol.2002;20:503–513.
- ,,.Beyond autonomy: Diversifying end‐of‐life decision‐making approaches to serve patients and families.J Am Geriatr Soc.2005;53:1046–1050.
- ,,,.Measuring patients' desire for autonomy: decision making and information‐seeking preferences among medical patients.J Gen Intern Med.1989;4(1):23–30.
- ,.Physician recommendations and patient autonomy: finding a balance between physician power and patient choice.Ann Intern Med.1996;125:763–769.
Reflections on Hospitalist Movement
Most people believe the term hospitalist first appeared in the literature in the August 15, 1996, issue of the New England Journal of Medicine (NEJM). That issue carried an article that Lee Goldman and I wrote titled The Emerging Role of Hospitalists in the American Health Care System.1 But the term was actually coined about a year earlier, in an article I wrote for our University of California, San Francisco (UCSF), residents' newsletter, the Medical Residents' Progress Note (MRPN), circulation about 180. In that article, I mused about a new model of care in which separate physicians assumed the role of caring for inpatients in place of patients' primary care doctors. Several peopleboth residents and facultyapproached me soon after the MRPN article was published and said, I read your articleyou should really buff it up and send it to a real journal. (By the way, when you publish a scholarly article, people generally say, I saw your article, rather than I read your article). This prompting led me to polish up the piece, with Lee Goldman's able assistance, and send it to the NEJM.
Although people often introduce me today as the guy who invented hospitalists (to which I typically respond, yeah, just like Al Gore invented the Internet), I did no such thingI merely kept my eyes and ears open, spotted the trend early, and gave it a name that stuck. In the mid‐1990s, the California market was being besieged by managed care, which was seeking new ways to cut hospital utilization and costs. In 1994, the huge Kaiser Permanente system decided to reorganize its hospital care around a cadre of hospital‐based specialists (HBSs), essentially dichotomizing the roles of inpatient and outpatient physicians. (Interestingly, Kaiser's main motivations were to improve outpatient satisfaction by assuring constant availability of primary care physicians and to create a vehicle to promote inpatient quality improvement activities, not necessarily to improve inpatient efficiency.) Around the same time, I read reports in throwaway magazines about Park Nicollet in Minneapolis and the Scripps Clinic in La Jolla, California, doing the same thing. Then one day I heard that a talented young UCSF faculty member was leaving our VA system to take a job as the inpatient manager at a local community teaching hospital. A few weeks later, I took him out to lunchI was intrigued by this new role and wanted to better understand it. As he described it to me over sandwiches, it made all the sense in the world, and the seeds of the MRPNand later NEJMarticle was planted.
I have always had an abiding interest in the notion of valuea fundamental belief that our system is inexorably becoming one in which health care choices and competition will be based on demonstrable quality, safety, the patient's experience, and cost rather than on tradition, impression, and proximity. As I began thinking about hospital care, it seemed likely this new modeldichotomizing the roles of inpatient and outpatient doctors such that the former could be constantly available and become an expert in inpatient clinical care and hospital microsystemswould provide more value than the traditional structure, both in community settings (replacing the single primary care doctor managing both inpatients and outpatients) and the academic setting (replacing the traditional one‐month‐a‐year ward attending).
At the time I was thinking all this through, a new chairman of our department of medicine arrived from Harvard. Lee Goldman, who virtually invented the field of clinical epidemiology, came to UCSF with a powerful vision that matched mineto transform training and clinical care to improve both value and education. Lee had been a resident at UCSF 20 years earlier and returned in 1995 to an inpatient service whose structure and culture had barely changed over a generation. Lee (who, to my great chagrin, recently left UCSF to become Columbia's medical school dean, and who does not have the term good enough in his vocabulary) sat down with me and articulated his vision for a new type of academic inpatient model, led by faculty who cared for inpatients and taught trainees hospital medicine for a living. This was entirely in sync with my thoughts, and so we set out to build it.
Reaction to both the New England Journal of Medicine article and our vision for an academic hospitalist service was swift and negative. One letter to the NEJM said it all:
Patients ill enough to be in the hospital are those who need their regular physicians the most. This is especially true if the patients have incurable diseases, in the context of which the usual buzzwords of efficiency and outcomes have little meaning. It is sad, but the most important part of medicine, the relationship between the doctor and the patient, is being forgotten. It is especially sad that physicians are beginning to think like MBAs.2
Our response to this and the other letters emphasized the need for evidence:
Our description of the emerging role of hospitalists is based not on an assertion that the hospitalist model is the only way to provide in‐hospital care, but rather on irrefutable evidence that both teaching and non‐teaching hospitals are adopting the model. We do not believe the debate about hospitalists is served by anecdotal claims about greater satisfaction among patients and providers. We recommend that the shape of our health care system be guided by measuring clinical outcomes, costs, and satisfaction rather than by following passion or tradition.3
My father, a retired businessman living in Florida, brought the controversy to an even finer point a year later. I met this guy playing tennis today, he told me on the phone one day. And he's heard of you! I listened for the heartwarming sounds of fatherly pride, but none were forthcoming. He hates you, he added.
Our attempts to build an academic hospitalist program generated other concerns. Many faculty enjoyed serving as ward attendings and worried about being kicked off the wards (although many privately told me that they knew their time was up and were grateful for a way to exit with dignity.) One world‐famous faculty member bolted out of his seat during the Q&A period after my medical grand rounds at his institution in 1997. How will the house staff learn anything if we don't allow them to learn from their mistakes? he huffed. (I told him that I was flying cross‐country the next day, and I'll be really pissed off if my pilot is there to learn from his mistakes.) Our residents also worried terribly about losing their autonomy, having these bright young attendings breathing down our backs. Everyone worried about where the resources to pay for the program would come from.
At UCSF, our strategy was to reassure everyone that we would be measuring the impact of the new model in terms of cost, quality, patient satisfaction, and education. By making clear that the results of this research would guide further change (and that we were willing to end the experiment if it turned out negatively), the faculty and house staff largely suspended their disbelief for the first year. That study4 would demonstrate impressive cost savings with no adverse impact on quality and patient satisfaction and a hint of improved resident satisfaction (later proven more conclusively5), allowing us to expand the program over time and to make the argument for ongoing medical center support of the new model.
Just as Lee Goldman's arrival at UCSF in 1995 was a remarkable and crucial bit of serendipity, my partnership with Dr. Win Whitcomb and Dr. John Nelson was every bit as important for the growth of the movement nationally. John, at that time a young internist in Gainesville, Florida, had been practicing as a hospitalist (though it wasn't called that) since completing his internal medicine residency in the later 1980s. He had hooked up with Win, another young internist who had left a private practice job to begin a hospital‐based practice at Mercy Medical Center in Springfield, Massachusetts. Together the two of them had begun to network with the handful of physicians around the country who were practicing in this new model. But they needed a larger megaphone, both to let other hospitalists know about each other, and to make hospitals and systems more aware of this new model of care.
John tells the story of pulling the August 15, 1996, issue of the NEJM out of his mailbox, seeing my article, and literally running into to his house to tell his wife that his practice had finally been discovered. John's thoughtful exuberance is one of the reasons for the growth of our field, and he did something that is uniquely Johncalling the author of an article that piqued his interest to discuss its contents, something he'd been doing for years. At that point, Lee Goldman was an internationally known leader in internal medicine; as chair of a major academic department, he had several layers of administrative assistants running interference when he received cold calls. I, on the other hand, ran a sleepy medical service and had little to do other than to answer calls and to respond to this new thing called e‐mail. John didn't know that; in his experience, first authors of articles in major journals were nearly always too busy to answer calls from country docs like him. So he tried Lee Goldman first but failed to get through. Win, on the other hand, decided to call me and had no problem getting through immediately. We hit it off like we'd been buddies for decades, sharing our instinctive recognition that that we were at the cutting edge of a new specialty. In what, in retrospect, seems like an extraordinary amount of hubris, we essentially divided up the world, asking the question: what does an emerging specialty need in order to be successful? I'm reminded of one of my favorite parts of the brilliant dialogue by Mel Brooks and Carl Reiner, The 2000 Year Old Man. Brooks, playing the title part, describes his relationship with Joan of Arc (What a cutie, he gushes) to Reiner (playing the interviewer), and how Joan's mission got in the way of their ardor. She used to say to me, Ive got to save France,' says the 2000 Year Old Man. I said, Look, Ive got to wash up, you save France, I'll see you later' That was usWin and John agreed to focus on building a new professional society and on networking with community‐based hospitalists, while I emphasized the academic side of things: organizing meetings, developing training programs, publishing a textbook, and launching a research agenda.
The first national gathering of hospitalists was astonishing. In the spring of 1997, I hosted what I thought would be a small hospital medicine CME meeting at a Holiday Inn in San Francisco in a seedy part of town. I expected about 50 people to attend and was shocked to see 3 times that (plus several homeless people who wandered into the sessions). Most remarkably, at the end of day 1, following 8 hours of clinical lectures, Win, John, and I asked the attendees if anybody wanted to stay a while and discuss the possibility of forming a new society. To our amazement, virtually everybody stayedmore than 100 people! Would anybody be willing to contribute some money to get this started? asked John, expecting nothing. And people began passing $20 bills up to the front of the room. That was the moment we knew we were onto something very bigthe atmosphere was electric, the enthusiasm easily palpable.
We initially called the new society the National Association of Inpatient Physicians (NAIP), as the name hospitalist was still very controversial, and many thought it would not have legsthe term inpatient physician was believed to be more inclusive. NAIP rapidly reached a crucial turning point. Our few hundred dollars in dues collections and ad revenues lived in Win's shoebox in Massachusetts (and later in a checking account opened by Ron Angus in Texas), and Win, John, and I were keeping databases of hospitalists on our computers and the backs of napkins. It was clear we needed to either create a full‐fledged infrastructure or partner with an organization that could help us. I approached Hal Sox, now the editor of the Annals of Internal Medicine but at that time president of the American College of Physicians (and an old fellowship mentor of mine), about the possibility of NAIP establishing a formal relationship with the ACP. Hal was reluctant at first, noting many ACP members were pretty strongly against the idea of hospitalists. In one of many acts of brinksmanship, I told him we would need to look for other partners if ACP did not get over its ambivalence and embrace our new field. To his credit and to the credit particularly of Dr. Walt McDonald, ACP's executive director at the time, both recognized the potential growth of this new field and worked through the internal politics to offer us an affiliation. However, we found their initial offerto become the Section on Hospital Medicine within the ACPunattractive. Wanting to be a full‐fledged independent organization that enjoyed a relationship with the College, we proposed a relationship that would link us and allow ACP to support our infrastructure, but that allowed us to retain independent decision making, governance, and budget. John, in his charming Southern drawl, described our position to an early gathering of about 100 hospitalists at a NAIP meeting in San Diego. Their offer would have them up here, and we'd be down there, he said, his hands depicting an obvious hierarchy, with us on the bottom. But we insisted on being equal partners, he said, with his hands on the same plane. I turned to Win, sitting next to me in the audience, and whispered something like, Yeah, equalexcept for the small fact that they have 120,000 members and we have 87. Nevertheless, they agreed, and our relationship has been incredibly positive for hospitalists, and I believe for the ACP as well.
The rest, as they say, is history. The society, renamed the Society of Hospital Medicine in April 2003, has thrived under the leadership of a strong series of boards, a wonderful staff, and a charismatic and highly effective CEO, Dr. Larry Wellikson. We successfully navigated the many early challenges and took advantage of key opportunities. In this regard, I consider our 3 most important decisions and actions to be: 1) creating a body of research that demonstrated, in an evidence‐based way, that the theoretical promise of the field was real6 (it was this research that led hospitals to embrace the field more vigorously and that justified the crucial support that most hospitals provide their hospitalist programs); 2) vigorously pushing back against managed care‐based hospitalist models that had begun to force primary care physicians to hand their patients off to hospitalists against their will (NAIP's first policy pronouncement was to come out strongly against such mandatory models, which seemed counterintuitive to some but which markedly decreased our vulnerability to being tagged as a cost‐cutting vehicle of managed care); and 3) linking ourselves as strongly as possible with the growing quality and safety movements. When the IOM reports on medical errors7 and later quality8 were published, we immediately saw in the new agendas a tremendous opportunity to brand hospitalists as indispensable leaders of quality and safety in hospitalsanother key rationale for hospitalists' value proposition and another reason for hospitals and policymakers to support the young field.
Looking back at the 1996 New England Journal of Medicine article, I am struck by both the number of things I got right (even a blind squirrel) and the number that I did not anticipate or got wrong. Lee and I thought that many hospitalists would be subspecialists who would focus on hospital medicine for only part of their work. This was true early on, but the field has evolved to be more of a generalist endeavor (although recently there have emerged laborists, neurology hospitalists, and even surgical hospitalists). I probably could have anticipated the growth of the field in pediatrics, but it certainly was not on my radar screen until years later.9 I did not count on the work hours of house staff being regulated; even if I had, I'm not sure I would have fully recognized how the need to create nonteaching services would turbo‐charge the growth of the hospitalist field in teaching hospitals. The one mild disappointment: I anticipated stronger evidence by now of the field's salutary impact on safety and quality. The effort to study and hopefully demonstrate such improvements should be a major focus for the next 510 years. Finally, although I thought the field would grow rapidly, I did not anticipate that a decade later there would be 15,000 hospitalists nationally or 24 in my group at UCSF. I also did not guess that an April 2006 Medline search of hospitalist would find 561 articles or that a Google search of hospitalist would yield 689,000 entries (hell, there was no Google to search in 1996!).
As I reflect back on the last decade, I am humbled by the remarkable work I have seen from hospitalists around the country and grateful for the wonderful friendships I have enjoyed with my colleagues in our new field. I am even more convinced of the fundamental accuracy of my underlying premise: the U.S. health care system will increasingly embrace models, strategies, and providers who can demonstrably improve the value of care. I have no doubt thatcollectivelyAmerican hospitalists have saved tens of thousands of lives, prevented tens of thousands of errors, orchestrated tens of thousands of good deaths, comforted tens of thousands of families, and saved billions of dollars. It is an ongoing legacy that gives me considerable pride and joy.
- ,.The emerging role of “hospitalists” in the American health care system.N Engl J Med.1996;335:514–517. http://content.nejm.org/cgi/content/full/335/7/514
- .The role of “hospitalists” in the health care system.N Engl J Med.1996;336:444–446.
- ,.The role of “hospitalists” in the health care system.N Engl J Med.1996;336:444–446.
- ,,,,.Reorganizing an academic medical service: Impact on cost, quality, patient satisfaction, and education.JAMA.1998;279:1560–1565.
- ,,,,.Effects of hospitalist attendings on trainee satisfaction with teaching and with internal medicine rotations.Arch Intern Med.2004;164:1866–1871.
- .The hospitalist movement 5 years later.J Am Med Assoc2002;282:487–494.
- ,,.To Err Is Human: Building a Safer Health System.Washington, DC:Committee on Quality of Health Care in America, Institute of Medicine.National Academy Press;2000.
- Committee on Quality of Health Care in America, IOM.Crossing the Quality Chasm: A New Health System for the 21st Century.Washington, DC:National Academy Press;2001.
- ,.The hospitalist movement and its implications for the care of hospitalized children.Pediatrics.1999;103:473–77.
Most people believe the term hospitalist first appeared in the literature in the August 15, 1996, issue of the New England Journal of Medicine (NEJM). That issue carried an article that Lee Goldman and I wrote titled The Emerging Role of Hospitalists in the American Health Care System.1 But the term was actually coined about a year earlier, in an article I wrote for our University of California, San Francisco (UCSF), residents' newsletter, the Medical Residents' Progress Note (MRPN), circulation about 180. In that article, I mused about a new model of care in which separate physicians assumed the role of caring for inpatients in place of patients' primary care doctors. Several peopleboth residents and facultyapproached me soon after the MRPN article was published and said, I read your articleyou should really buff it up and send it to a real journal. (By the way, when you publish a scholarly article, people generally say, I saw your article, rather than I read your article). This prompting led me to polish up the piece, with Lee Goldman's able assistance, and send it to the NEJM.
Although people often introduce me today as the guy who invented hospitalists (to which I typically respond, yeah, just like Al Gore invented the Internet), I did no such thingI merely kept my eyes and ears open, spotted the trend early, and gave it a name that stuck. In the mid‐1990s, the California market was being besieged by managed care, which was seeking new ways to cut hospital utilization and costs. In 1994, the huge Kaiser Permanente system decided to reorganize its hospital care around a cadre of hospital‐based specialists (HBSs), essentially dichotomizing the roles of inpatient and outpatient physicians. (Interestingly, Kaiser's main motivations were to improve outpatient satisfaction by assuring constant availability of primary care physicians and to create a vehicle to promote inpatient quality improvement activities, not necessarily to improve inpatient efficiency.) Around the same time, I read reports in throwaway magazines about Park Nicollet in Minneapolis and the Scripps Clinic in La Jolla, California, doing the same thing. Then one day I heard that a talented young UCSF faculty member was leaving our VA system to take a job as the inpatient manager at a local community teaching hospital. A few weeks later, I took him out to lunchI was intrigued by this new role and wanted to better understand it. As he described it to me over sandwiches, it made all the sense in the world, and the seeds of the MRPNand later NEJMarticle was planted.
I have always had an abiding interest in the notion of valuea fundamental belief that our system is inexorably becoming one in which health care choices and competition will be based on demonstrable quality, safety, the patient's experience, and cost rather than on tradition, impression, and proximity. As I began thinking about hospital care, it seemed likely this new modeldichotomizing the roles of inpatient and outpatient doctors such that the former could be constantly available and become an expert in inpatient clinical care and hospital microsystemswould provide more value than the traditional structure, both in community settings (replacing the single primary care doctor managing both inpatients and outpatients) and the academic setting (replacing the traditional one‐month‐a‐year ward attending).
At the time I was thinking all this through, a new chairman of our department of medicine arrived from Harvard. Lee Goldman, who virtually invented the field of clinical epidemiology, came to UCSF with a powerful vision that matched mineto transform training and clinical care to improve both value and education. Lee had been a resident at UCSF 20 years earlier and returned in 1995 to an inpatient service whose structure and culture had barely changed over a generation. Lee (who, to my great chagrin, recently left UCSF to become Columbia's medical school dean, and who does not have the term good enough in his vocabulary) sat down with me and articulated his vision for a new type of academic inpatient model, led by faculty who cared for inpatients and taught trainees hospital medicine for a living. This was entirely in sync with my thoughts, and so we set out to build it.
Reaction to both the New England Journal of Medicine article and our vision for an academic hospitalist service was swift and negative. One letter to the NEJM said it all:
Patients ill enough to be in the hospital are those who need their regular physicians the most. This is especially true if the patients have incurable diseases, in the context of which the usual buzzwords of efficiency and outcomes have little meaning. It is sad, but the most important part of medicine, the relationship between the doctor and the patient, is being forgotten. It is especially sad that physicians are beginning to think like MBAs.2
Our response to this and the other letters emphasized the need for evidence:
Our description of the emerging role of hospitalists is based not on an assertion that the hospitalist model is the only way to provide in‐hospital care, but rather on irrefutable evidence that both teaching and non‐teaching hospitals are adopting the model. We do not believe the debate about hospitalists is served by anecdotal claims about greater satisfaction among patients and providers. We recommend that the shape of our health care system be guided by measuring clinical outcomes, costs, and satisfaction rather than by following passion or tradition.3
My father, a retired businessman living in Florida, brought the controversy to an even finer point a year later. I met this guy playing tennis today, he told me on the phone one day. And he's heard of you! I listened for the heartwarming sounds of fatherly pride, but none were forthcoming. He hates you, he added.
Our attempts to build an academic hospitalist program generated other concerns. Many faculty enjoyed serving as ward attendings and worried about being kicked off the wards (although many privately told me that they knew their time was up and were grateful for a way to exit with dignity.) One world‐famous faculty member bolted out of his seat during the Q&A period after my medical grand rounds at his institution in 1997. How will the house staff learn anything if we don't allow them to learn from their mistakes? he huffed. (I told him that I was flying cross‐country the next day, and I'll be really pissed off if my pilot is there to learn from his mistakes.) Our residents also worried terribly about losing their autonomy, having these bright young attendings breathing down our backs. Everyone worried about where the resources to pay for the program would come from.
At UCSF, our strategy was to reassure everyone that we would be measuring the impact of the new model in terms of cost, quality, patient satisfaction, and education. By making clear that the results of this research would guide further change (and that we were willing to end the experiment if it turned out negatively), the faculty and house staff largely suspended their disbelief for the first year. That study4 would demonstrate impressive cost savings with no adverse impact on quality and patient satisfaction and a hint of improved resident satisfaction (later proven more conclusively5), allowing us to expand the program over time and to make the argument for ongoing medical center support of the new model.
Just as Lee Goldman's arrival at UCSF in 1995 was a remarkable and crucial bit of serendipity, my partnership with Dr. Win Whitcomb and Dr. John Nelson was every bit as important for the growth of the movement nationally. John, at that time a young internist in Gainesville, Florida, had been practicing as a hospitalist (though it wasn't called that) since completing his internal medicine residency in the later 1980s. He had hooked up with Win, another young internist who had left a private practice job to begin a hospital‐based practice at Mercy Medical Center in Springfield, Massachusetts. Together the two of them had begun to network with the handful of physicians around the country who were practicing in this new model. But they needed a larger megaphone, both to let other hospitalists know about each other, and to make hospitals and systems more aware of this new model of care.
John tells the story of pulling the August 15, 1996, issue of the NEJM out of his mailbox, seeing my article, and literally running into to his house to tell his wife that his practice had finally been discovered. John's thoughtful exuberance is one of the reasons for the growth of our field, and he did something that is uniquely Johncalling the author of an article that piqued his interest to discuss its contents, something he'd been doing for years. At that point, Lee Goldman was an internationally known leader in internal medicine; as chair of a major academic department, he had several layers of administrative assistants running interference when he received cold calls. I, on the other hand, ran a sleepy medical service and had little to do other than to answer calls and to respond to this new thing called e‐mail. John didn't know that; in his experience, first authors of articles in major journals were nearly always too busy to answer calls from country docs like him. So he tried Lee Goldman first but failed to get through. Win, on the other hand, decided to call me and had no problem getting through immediately. We hit it off like we'd been buddies for decades, sharing our instinctive recognition that that we were at the cutting edge of a new specialty. In what, in retrospect, seems like an extraordinary amount of hubris, we essentially divided up the world, asking the question: what does an emerging specialty need in order to be successful? I'm reminded of one of my favorite parts of the brilliant dialogue by Mel Brooks and Carl Reiner, The 2000 Year Old Man. Brooks, playing the title part, describes his relationship with Joan of Arc (What a cutie, he gushes) to Reiner (playing the interviewer), and how Joan's mission got in the way of their ardor. She used to say to me, Ive got to save France,' says the 2000 Year Old Man. I said, Look, Ive got to wash up, you save France, I'll see you later' That was usWin and John agreed to focus on building a new professional society and on networking with community‐based hospitalists, while I emphasized the academic side of things: organizing meetings, developing training programs, publishing a textbook, and launching a research agenda.
The first national gathering of hospitalists was astonishing. In the spring of 1997, I hosted what I thought would be a small hospital medicine CME meeting at a Holiday Inn in San Francisco in a seedy part of town. I expected about 50 people to attend and was shocked to see 3 times that (plus several homeless people who wandered into the sessions). Most remarkably, at the end of day 1, following 8 hours of clinical lectures, Win, John, and I asked the attendees if anybody wanted to stay a while and discuss the possibility of forming a new society. To our amazement, virtually everybody stayedmore than 100 people! Would anybody be willing to contribute some money to get this started? asked John, expecting nothing. And people began passing $20 bills up to the front of the room. That was the moment we knew we were onto something very bigthe atmosphere was electric, the enthusiasm easily palpable.
We initially called the new society the National Association of Inpatient Physicians (NAIP), as the name hospitalist was still very controversial, and many thought it would not have legsthe term inpatient physician was believed to be more inclusive. NAIP rapidly reached a crucial turning point. Our few hundred dollars in dues collections and ad revenues lived in Win's shoebox in Massachusetts (and later in a checking account opened by Ron Angus in Texas), and Win, John, and I were keeping databases of hospitalists on our computers and the backs of napkins. It was clear we needed to either create a full‐fledged infrastructure or partner with an organization that could help us. I approached Hal Sox, now the editor of the Annals of Internal Medicine but at that time president of the American College of Physicians (and an old fellowship mentor of mine), about the possibility of NAIP establishing a formal relationship with the ACP. Hal was reluctant at first, noting many ACP members were pretty strongly against the idea of hospitalists. In one of many acts of brinksmanship, I told him we would need to look for other partners if ACP did not get over its ambivalence and embrace our new field. To his credit and to the credit particularly of Dr. Walt McDonald, ACP's executive director at the time, both recognized the potential growth of this new field and worked through the internal politics to offer us an affiliation. However, we found their initial offerto become the Section on Hospital Medicine within the ACPunattractive. Wanting to be a full‐fledged independent organization that enjoyed a relationship with the College, we proposed a relationship that would link us and allow ACP to support our infrastructure, but that allowed us to retain independent decision making, governance, and budget. John, in his charming Southern drawl, described our position to an early gathering of about 100 hospitalists at a NAIP meeting in San Diego. Their offer would have them up here, and we'd be down there, he said, his hands depicting an obvious hierarchy, with us on the bottom. But we insisted on being equal partners, he said, with his hands on the same plane. I turned to Win, sitting next to me in the audience, and whispered something like, Yeah, equalexcept for the small fact that they have 120,000 members and we have 87. Nevertheless, they agreed, and our relationship has been incredibly positive for hospitalists, and I believe for the ACP as well.
The rest, as they say, is history. The society, renamed the Society of Hospital Medicine in April 2003, has thrived under the leadership of a strong series of boards, a wonderful staff, and a charismatic and highly effective CEO, Dr. Larry Wellikson. We successfully navigated the many early challenges and took advantage of key opportunities. In this regard, I consider our 3 most important decisions and actions to be: 1) creating a body of research that demonstrated, in an evidence‐based way, that the theoretical promise of the field was real6 (it was this research that led hospitals to embrace the field more vigorously and that justified the crucial support that most hospitals provide their hospitalist programs); 2) vigorously pushing back against managed care‐based hospitalist models that had begun to force primary care physicians to hand their patients off to hospitalists against their will (NAIP's first policy pronouncement was to come out strongly against such mandatory models, which seemed counterintuitive to some but which markedly decreased our vulnerability to being tagged as a cost‐cutting vehicle of managed care); and 3) linking ourselves as strongly as possible with the growing quality and safety movements. When the IOM reports on medical errors7 and later quality8 were published, we immediately saw in the new agendas a tremendous opportunity to brand hospitalists as indispensable leaders of quality and safety in hospitalsanother key rationale for hospitalists' value proposition and another reason for hospitals and policymakers to support the young field.
Looking back at the 1996 New England Journal of Medicine article, I am struck by both the number of things I got right (even a blind squirrel) and the number that I did not anticipate or got wrong. Lee and I thought that many hospitalists would be subspecialists who would focus on hospital medicine for only part of their work. This was true early on, but the field has evolved to be more of a generalist endeavor (although recently there have emerged laborists, neurology hospitalists, and even surgical hospitalists). I probably could have anticipated the growth of the field in pediatrics, but it certainly was not on my radar screen until years later.9 I did not count on the work hours of house staff being regulated; even if I had, I'm not sure I would have fully recognized how the need to create nonteaching services would turbo‐charge the growth of the hospitalist field in teaching hospitals. The one mild disappointment: I anticipated stronger evidence by now of the field's salutary impact on safety and quality. The effort to study and hopefully demonstrate such improvements should be a major focus for the next 510 years. Finally, although I thought the field would grow rapidly, I did not anticipate that a decade later there would be 15,000 hospitalists nationally or 24 in my group at UCSF. I also did not guess that an April 2006 Medline search of hospitalist would find 561 articles or that a Google search of hospitalist would yield 689,000 entries (hell, there was no Google to search in 1996!).
As I reflect back on the last decade, I am humbled by the remarkable work I have seen from hospitalists around the country and grateful for the wonderful friendships I have enjoyed with my colleagues in our new field. I am even more convinced of the fundamental accuracy of my underlying premise: the U.S. health care system will increasingly embrace models, strategies, and providers who can demonstrably improve the value of care. I have no doubt thatcollectivelyAmerican hospitalists have saved tens of thousands of lives, prevented tens of thousands of errors, orchestrated tens of thousands of good deaths, comforted tens of thousands of families, and saved billions of dollars. It is an ongoing legacy that gives me considerable pride and joy.
Most people believe the term hospitalist first appeared in the literature in the August 15, 1996, issue of the New England Journal of Medicine (NEJM). That issue carried an article that Lee Goldman and I wrote titled The Emerging Role of Hospitalists in the American Health Care System.1 But the term was actually coined about a year earlier, in an article I wrote for our University of California, San Francisco (UCSF), residents' newsletter, the Medical Residents' Progress Note (MRPN), circulation about 180. In that article, I mused about a new model of care in which separate physicians assumed the role of caring for inpatients in place of patients' primary care doctors. Several peopleboth residents and facultyapproached me soon after the MRPN article was published and said, I read your articleyou should really buff it up and send it to a real journal. (By the way, when you publish a scholarly article, people generally say, I saw your article, rather than I read your article). This prompting led me to polish up the piece, with Lee Goldman's able assistance, and send it to the NEJM.
Although people often introduce me today as the guy who invented hospitalists (to which I typically respond, yeah, just like Al Gore invented the Internet), I did no such thingI merely kept my eyes and ears open, spotted the trend early, and gave it a name that stuck. In the mid‐1990s, the California market was being besieged by managed care, which was seeking new ways to cut hospital utilization and costs. In 1994, the huge Kaiser Permanente system decided to reorganize its hospital care around a cadre of hospital‐based specialists (HBSs), essentially dichotomizing the roles of inpatient and outpatient physicians. (Interestingly, Kaiser's main motivations were to improve outpatient satisfaction by assuring constant availability of primary care physicians and to create a vehicle to promote inpatient quality improvement activities, not necessarily to improve inpatient efficiency.) Around the same time, I read reports in throwaway magazines about Park Nicollet in Minneapolis and the Scripps Clinic in La Jolla, California, doing the same thing. Then one day I heard that a talented young UCSF faculty member was leaving our VA system to take a job as the inpatient manager at a local community teaching hospital. A few weeks later, I took him out to lunchI was intrigued by this new role and wanted to better understand it. As he described it to me over sandwiches, it made all the sense in the world, and the seeds of the MRPNand later NEJMarticle was planted.
I have always had an abiding interest in the notion of valuea fundamental belief that our system is inexorably becoming one in which health care choices and competition will be based on demonstrable quality, safety, the patient's experience, and cost rather than on tradition, impression, and proximity. As I began thinking about hospital care, it seemed likely this new modeldichotomizing the roles of inpatient and outpatient doctors such that the former could be constantly available and become an expert in inpatient clinical care and hospital microsystemswould provide more value than the traditional structure, both in community settings (replacing the single primary care doctor managing both inpatients and outpatients) and the academic setting (replacing the traditional one‐month‐a‐year ward attending).
At the time I was thinking all this through, a new chairman of our department of medicine arrived from Harvard. Lee Goldman, who virtually invented the field of clinical epidemiology, came to UCSF with a powerful vision that matched mineto transform training and clinical care to improve both value and education. Lee had been a resident at UCSF 20 years earlier and returned in 1995 to an inpatient service whose structure and culture had barely changed over a generation. Lee (who, to my great chagrin, recently left UCSF to become Columbia's medical school dean, and who does not have the term good enough in his vocabulary) sat down with me and articulated his vision for a new type of academic inpatient model, led by faculty who cared for inpatients and taught trainees hospital medicine for a living. This was entirely in sync with my thoughts, and so we set out to build it.
Reaction to both the New England Journal of Medicine article and our vision for an academic hospitalist service was swift and negative. One letter to the NEJM said it all:
Patients ill enough to be in the hospital are those who need their regular physicians the most. This is especially true if the patients have incurable diseases, in the context of which the usual buzzwords of efficiency and outcomes have little meaning. It is sad, but the most important part of medicine, the relationship between the doctor and the patient, is being forgotten. It is especially sad that physicians are beginning to think like MBAs.2
Our response to this and the other letters emphasized the need for evidence:
Our description of the emerging role of hospitalists is based not on an assertion that the hospitalist model is the only way to provide in‐hospital care, but rather on irrefutable evidence that both teaching and non‐teaching hospitals are adopting the model. We do not believe the debate about hospitalists is served by anecdotal claims about greater satisfaction among patients and providers. We recommend that the shape of our health care system be guided by measuring clinical outcomes, costs, and satisfaction rather than by following passion or tradition.3
My father, a retired businessman living in Florida, brought the controversy to an even finer point a year later. I met this guy playing tennis today, he told me on the phone one day. And he's heard of you! I listened for the heartwarming sounds of fatherly pride, but none were forthcoming. He hates you, he added.
Our attempts to build an academic hospitalist program generated other concerns. Many faculty enjoyed serving as ward attendings and worried about being kicked off the wards (although many privately told me that they knew their time was up and were grateful for a way to exit with dignity.) One world‐famous faculty member bolted out of his seat during the Q&A period after my medical grand rounds at his institution in 1997. How will the house staff learn anything if we don't allow them to learn from their mistakes? he huffed. (I told him that I was flying cross‐country the next day, and I'll be really pissed off if my pilot is there to learn from his mistakes.) Our residents also worried terribly about losing their autonomy, having these bright young attendings breathing down our backs. Everyone worried about where the resources to pay for the program would come from.
At UCSF, our strategy was to reassure everyone that we would be measuring the impact of the new model in terms of cost, quality, patient satisfaction, and education. By making clear that the results of this research would guide further change (and that we were willing to end the experiment if it turned out negatively), the faculty and house staff largely suspended their disbelief for the first year. That study4 would demonstrate impressive cost savings with no adverse impact on quality and patient satisfaction and a hint of improved resident satisfaction (later proven more conclusively5), allowing us to expand the program over time and to make the argument for ongoing medical center support of the new model.
Just as Lee Goldman's arrival at UCSF in 1995 was a remarkable and crucial bit of serendipity, my partnership with Dr. Win Whitcomb and Dr. John Nelson was every bit as important for the growth of the movement nationally. John, at that time a young internist in Gainesville, Florida, had been practicing as a hospitalist (though it wasn't called that) since completing his internal medicine residency in the later 1980s. He had hooked up with Win, another young internist who had left a private practice job to begin a hospital‐based practice at Mercy Medical Center in Springfield, Massachusetts. Together the two of them had begun to network with the handful of physicians around the country who were practicing in this new model. But they needed a larger megaphone, both to let other hospitalists know about each other, and to make hospitals and systems more aware of this new model of care.
John tells the story of pulling the August 15, 1996, issue of the NEJM out of his mailbox, seeing my article, and literally running into to his house to tell his wife that his practice had finally been discovered. John's thoughtful exuberance is one of the reasons for the growth of our field, and he did something that is uniquely Johncalling the author of an article that piqued his interest to discuss its contents, something he'd been doing for years. At that point, Lee Goldman was an internationally known leader in internal medicine; as chair of a major academic department, he had several layers of administrative assistants running interference when he received cold calls. I, on the other hand, ran a sleepy medical service and had little to do other than to answer calls and to respond to this new thing called e‐mail. John didn't know that; in his experience, first authors of articles in major journals were nearly always too busy to answer calls from country docs like him. So he tried Lee Goldman first but failed to get through. Win, on the other hand, decided to call me and had no problem getting through immediately. We hit it off like we'd been buddies for decades, sharing our instinctive recognition that that we were at the cutting edge of a new specialty. In what, in retrospect, seems like an extraordinary amount of hubris, we essentially divided up the world, asking the question: what does an emerging specialty need in order to be successful? I'm reminded of one of my favorite parts of the brilliant dialogue by Mel Brooks and Carl Reiner, The 2000 Year Old Man. Brooks, playing the title part, describes his relationship with Joan of Arc (What a cutie, he gushes) to Reiner (playing the interviewer), and how Joan's mission got in the way of their ardor. She used to say to me, Ive got to save France,' says the 2000 Year Old Man. I said, Look, Ive got to wash up, you save France, I'll see you later' That was usWin and John agreed to focus on building a new professional society and on networking with community‐based hospitalists, while I emphasized the academic side of things: organizing meetings, developing training programs, publishing a textbook, and launching a research agenda.
The first national gathering of hospitalists was astonishing. In the spring of 1997, I hosted what I thought would be a small hospital medicine CME meeting at a Holiday Inn in San Francisco in a seedy part of town. I expected about 50 people to attend and was shocked to see 3 times that (plus several homeless people who wandered into the sessions). Most remarkably, at the end of day 1, following 8 hours of clinical lectures, Win, John, and I asked the attendees if anybody wanted to stay a while and discuss the possibility of forming a new society. To our amazement, virtually everybody stayedmore than 100 people! Would anybody be willing to contribute some money to get this started? asked John, expecting nothing. And people began passing $20 bills up to the front of the room. That was the moment we knew we were onto something very bigthe atmosphere was electric, the enthusiasm easily palpable.
We initially called the new society the National Association of Inpatient Physicians (NAIP), as the name hospitalist was still very controversial, and many thought it would not have legsthe term inpatient physician was believed to be more inclusive. NAIP rapidly reached a crucial turning point. Our few hundred dollars in dues collections and ad revenues lived in Win's shoebox in Massachusetts (and later in a checking account opened by Ron Angus in Texas), and Win, John, and I were keeping databases of hospitalists on our computers and the backs of napkins. It was clear we needed to either create a full‐fledged infrastructure or partner with an organization that could help us. I approached Hal Sox, now the editor of the Annals of Internal Medicine but at that time president of the American College of Physicians (and an old fellowship mentor of mine), about the possibility of NAIP establishing a formal relationship with the ACP. Hal was reluctant at first, noting many ACP members were pretty strongly against the idea of hospitalists. In one of many acts of brinksmanship, I told him we would need to look for other partners if ACP did not get over its ambivalence and embrace our new field. To his credit and to the credit particularly of Dr. Walt McDonald, ACP's executive director at the time, both recognized the potential growth of this new field and worked through the internal politics to offer us an affiliation. However, we found their initial offerto become the Section on Hospital Medicine within the ACPunattractive. Wanting to be a full‐fledged independent organization that enjoyed a relationship with the College, we proposed a relationship that would link us and allow ACP to support our infrastructure, but that allowed us to retain independent decision making, governance, and budget. John, in his charming Southern drawl, described our position to an early gathering of about 100 hospitalists at a NAIP meeting in San Diego. Their offer would have them up here, and we'd be down there, he said, his hands depicting an obvious hierarchy, with us on the bottom. But we insisted on being equal partners, he said, with his hands on the same plane. I turned to Win, sitting next to me in the audience, and whispered something like, Yeah, equalexcept for the small fact that they have 120,000 members and we have 87. Nevertheless, they agreed, and our relationship has been incredibly positive for hospitalists, and I believe for the ACP as well.
The rest, as they say, is history. The society, renamed the Society of Hospital Medicine in April 2003, has thrived under the leadership of a strong series of boards, a wonderful staff, and a charismatic and highly effective CEO, Dr. Larry Wellikson. We successfully navigated the many early challenges and took advantage of key opportunities. In this regard, I consider our 3 most important decisions and actions to be: 1) creating a body of research that demonstrated, in an evidence‐based way, that the theoretical promise of the field was real6 (it was this research that led hospitals to embrace the field more vigorously and that justified the crucial support that most hospitals provide their hospitalist programs); 2) vigorously pushing back against managed care‐based hospitalist models that had begun to force primary care physicians to hand their patients off to hospitalists against their will (NAIP's first policy pronouncement was to come out strongly against such mandatory models, which seemed counterintuitive to some but which markedly decreased our vulnerability to being tagged as a cost‐cutting vehicle of managed care); and 3) linking ourselves as strongly as possible with the growing quality and safety movements. When the IOM reports on medical errors7 and later quality8 were published, we immediately saw in the new agendas a tremendous opportunity to brand hospitalists as indispensable leaders of quality and safety in hospitalsanother key rationale for hospitalists' value proposition and another reason for hospitals and policymakers to support the young field.
Looking back at the 1996 New England Journal of Medicine article, I am struck by both the number of things I got right (even a blind squirrel) and the number that I did not anticipate or got wrong. Lee and I thought that many hospitalists would be subspecialists who would focus on hospital medicine for only part of their work. This was true early on, but the field has evolved to be more of a generalist endeavor (although recently there have emerged laborists, neurology hospitalists, and even surgical hospitalists). I probably could have anticipated the growth of the field in pediatrics, but it certainly was not on my radar screen until years later.9 I did not count on the work hours of house staff being regulated; even if I had, I'm not sure I would have fully recognized how the need to create nonteaching services would turbo‐charge the growth of the hospitalist field in teaching hospitals. The one mild disappointment: I anticipated stronger evidence by now of the field's salutary impact on safety and quality. The effort to study and hopefully demonstrate such improvements should be a major focus for the next 510 years. Finally, although I thought the field would grow rapidly, I did not anticipate that a decade later there would be 15,000 hospitalists nationally or 24 in my group at UCSF. I also did not guess that an April 2006 Medline search of hospitalist would find 561 articles or that a Google search of hospitalist would yield 689,000 entries (hell, there was no Google to search in 1996!).
As I reflect back on the last decade, I am humbled by the remarkable work I have seen from hospitalists around the country and grateful for the wonderful friendships I have enjoyed with my colleagues in our new field. I am even more convinced of the fundamental accuracy of my underlying premise: the U.S. health care system will increasingly embrace models, strategies, and providers who can demonstrably improve the value of care. I have no doubt thatcollectivelyAmerican hospitalists have saved tens of thousands of lives, prevented tens of thousands of errors, orchestrated tens of thousands of good deaths, comforted tens of thousands of families, and saved billions of dollars. It is an ongoing legacy that gives me considerable pride and joy.
- ,.The emerging role of “hospitalists” in the American health care system.N Engl J Med.1996;335:514–517. http://content.nejm.org/cgi/content/full/335/7/514
- .The role of “hospitalists” in the health care system.N Engl J Med.1996;336:444–446.
- ,.The role of “hospitalists” in the health care system.N Engl J Med.1996;336:444–446.
- ,,,,.Reorganizing an academic medical service: Impact on cost, quality, patient satisfaction, and education.JAMA.1998;279:1560–1565.
- ,,,,.Effects of hospitalist attendings on trainee satisfaction with teaching and with internal medicine rotations.Arch Intern Med.2004;164:1866–1871.
- .The hospitalist movement 5 years later.J Am Med Assoc2002;282:487–494.
- ,,.To Err Is Human: Building a Safer Health System.Washington, DC:Committee on Quality of Health Care in America, Institute of Medicine.National Academy Press;2000.
- Committee on Quality of Health Care in America, IOM.Crossing the Quality Chasm: A New Health System for the 21st Century.Washington, DC:National Academy Press;2001.
- ,.The hospitalist movement and its implications for the care of hospitalized children.Pediatrics.1999;103:473–77.
- ,.The emerging role of “hospitalists” in the American health care system.N Engl J Med.1996;335:514–517. http://content.nejm.org/cgi/content/full/335/7/514
- .The role of “hospitalists” in the health care system.N Engl J Med.1996;336:444–446.
- ,.The role of “hospitalists” in the health care system.N Engl J Med.1996;336:444–446.
- ,,,,.Reorganizing an academic medical service: Impact on cost, quality, patient satisfaction, and education.JAMA.1998;279:1560–1565.
- ,,,,.Effects of hospitalist attendings on trainee satisfaction with teaching and with internal medicine rotations.Arch Intern Med.2004;164:1866–1871.
- .The hospitalist movement 5 years later.J Am Med Assoc2002;282:487–494.
- ,,.To Err Is Human: Building a Safer Health System.Washington, DC:Committee on Quality of Health Care in America, Institute of Medicine.National Academy Press;2000.
- Committee on Quality of Health Care in America, IOM.Crossing the Quality Chasm: A New Health System for the 21st Century.Washington, DC:National Academy Press;2001.
- ,.The hospitalist movement and its implications for the care of hospitalized children.Pediatrics.1999;103:473–77.
Universal acceptance of computerized physician order entry: What would it take?
Self‐check‐in kiosks started to appear in airports in the late 1990s, and within a few years, they seem to have become ubiquitous in the airline industry. Today, almost 70% of business travelers use them, and other sectors of the travel industry are beginning to experiment with the technology.1 Compared to this innovation in the airline industry, adoption of computerized physician order entry (CPOE) in U.S. hospitals, first pioneered in the early 1970s,2, 3 has taken a much more leisurely pace. Despite numerous studies documenting its benefits,47 promotion by prominent national patient safety advocacy groups such as LeapFrog,8 and numerous guides on best adoption practices.912 fewer than 10% of U.S. hospitals have fully adopted this technology.13 Moreover, as Lindenauer et al.14 pointed out, most hospitals that have successfully implemented CPOE are academic medical centers that rely on house staff to enter orders. With notable exceptions,3 adoption of CPOE in community hospitals where attending physicians write most orders remains anemic.
Although an increasing number of scholarly articles has documented the reasons for this slow rate of adoption even in hospitals that have the resources to invest in this technology, much of that research is based on expert opinion and case studies.11, 1519 In this context, Lindenauer et al.14 should be commended for using empirical evidence to delineate the predictors of adoption. Lindenauer et al. found that physicians who trained in hospitals with CPOE were more likely to be frequent users of CPOE in their new environment. Although the analysis did not account for possible confounding such as employment status of the physician, this result does confirm the conventional wisdom that physicians‐in‐training are more malleable and that residency is an important opportunity to expose physicians to safety technologies. If this finding is borne out by further research, it would bode well for the adoption of CPOE, as many physicians are trained in academic institutions, which are more likely to have CPOE,20 and almost all physicians spend part of their training in a VA hospital, which has uniformly adopted CPOE. Similarly, Lindenauer et al. found that physicians who use computers for personal purposes are more likely to be frequent users of CPOE. Given the increasingly ubiquitous use of computers in all spheres of life, time is on the side of increasing acceptance of CPOE.
However, a closer examination of the data presented by Lindenauer et al. raises several concerns. First, the substantial number of infrequent users across all demographic subgroups and clinical disciplines, even among users who were exposed to CPOE during training or those who used computers regularly for personal purposes, highlights the absence of shortcuts to the universal acceptance of CPOE. Second, whereas 63% of surveyed physicians believed that CPOE would reduce the incidence of medication errors and 71% believed that CPOE would prevent aspects of care from slipping through the cracks, only 42% of the surveyed physicians were frequent users of CPOE. This implies that even when physicians believe in the safety and quality benefits of CPOE, that belief alone may not be sufficient to convince all of them to adopt this technology wholeheartedly; other factors such as speed, ease of use, and training are likely important prerequisites. Third, although 66% of orders placed in person at the 2 study hospitals were entered through CPOE, acceptance of this technology, as measured by Lindenauer et al, was moderate at both institutions. This suggests that even when organizations have reached the 70% threshold set by Leapfrog as the proportion of orders placed in CPOE that qualifies as full implementation, they may continue to face resistance to full acceptance of the technology.
Compared to their academic counterparts, community hospitals face additional hurdles as they implement CPOE. Not only does their smaller size make it difficult to achieve economies of scale, they are also at a disadvantage because of the relationship the community hospital has with its physicians. Unlike physicians‐in‐training in academic medical centers, physicians in community hospitals function as largely autonomous agents over whom the hospital administration has little control. Although these physicians and their hospitals share the common goals of patient safety and quality, the financial incentives for the adoption of CPOE are often misaligned. For example, a recent cost benefit analysis21 showed the enormous potential for hospitals to cut costs if physicians fully adopt a CPOE system with rich decision support features. However, those savings typically accrue to the hospital, not to the physicians who use the system. Assuming the typical learning curve that accompanies the use of any new technology, physicians in community hospitals may have little incentive to invest the time to learn to use the system efficiently.
So what can be done to overcome these seemingly formidable barriers to full adoption of CPOE? Emerging research, which has so far largely focused on CPOE implementation at academic hospitals, suggests there is no silver bullet. Instead, it has taught us how the complex interplay among vendor capability, organizational behavior, clinician work flow, and implementation strategy determines the success or failure of adoption.11, 17, 18, 22 Although physician characteristics will play a role in determining whether an individual adopts this technology, local factors such as the presence of champions, governance model for the project, support for staff throughout the process, and relationship between administration and physicians are likely important determinants of success at both academic and community hospitals. In addition, organizations that embark on CPOE implementation need to understand the enormity of the task at hand and must devote not only sufficient financial but also human capital over time.11, 18 In the words of a chief medical information officer, Implementing CPOE should not be thought of as an event, but a long‐term commitment.
Beyond following proposed best practices for the implementation of CPOE, community hospitals may need to adopt additional strategies to address their unique challenges. Given the misalignment of incentives for physicians' use of CPOE, leadership in community hospitals must be particularly skilled at articulating the benefits of CPOE to physicians. These benefits include not only decreased professional liability from improved patient safety and better quality of care, but also fewer pharmacy callbacks, remote access, and rapid ordering through order sets. Hospitals may also want to elicit support from physicians early by empowering them to create order sets for their disciplines. Mechanisms for hospitals and physicians to engage in mutual cost‐sharing arrangements may provide addition opportunities for hospitals to entice physicians to adopt the technology. Finally, and of particular interest to the readership of this journal, as hospitalists become more prevalent and take care of an increasing proportion of hospitalized patients,23 they are often ideal candidates to lead the implementation of CPOE in community hospitals. Because hospitalists spend most of their time in the hospital, they are often in the best position to get fully trained on CPOE, to define their own order sets, and to redesign care processes in order to take full advantage of CPOE capabilities. In addition, as many hospitalists are directly employed or supported by the hospital, their goals for quality, safety, and efficiency are usually better aligned with those of the hospital.
The stakes involved in implementing CPOE are high. Hospitals invest enormous sums of money in these systems, and many will not have the financial or political capital to attempt a second implementation after an initial failure. In addition, as recent research has pointed out,24 inappropriate implementation strategies may lead to delays in essential care and direct patient harm. In many ways, the complex task of implementing CPOE is not unlike other endeavors in patient care, where optimal outcomes require sound knowledge and reliable processes and where disaster can strike for lack of attention to detail or common sense. If Hippocrates were alive today, he might have this to say about CPOE implementation: Life is short, the art long, opportunity fleeting, experience treacherous, judgment difficult.
- Travel self‐serve kiosks here to stay.Adelman Group. Available at: http://www.adelmantravel.com/index_news_past.asp?Date=031406. Accessed March 14,2006.
- ,.Computer‐based physician order entry: the state of the art.J Am Med Inform Assoc.1994;1:108–123.
- ,,,.Final report on evaluation of the implementation of a medical information system in a general community hospital.Battelle Laboratories NTIS PB.1975;248:340.
- ,,, et al.Effect of computerized physician order entry and a team intervention on prevention of serious medication errors.JAMA.1998;280:1311–1316.
- ,,,,,.Effects of computerized physician order entry in prescribing practices.Arch Intern Med.2000;160:2741–2747.
- ,,,,,.A computerized reminder system to increase the use of preventive care for hospitalized patients [see comments].N Eng J M.2001;345:965–970.
- ,,,.A randomized trial of “corollary orders” to prevent errors of omission.J Am Med Inform Assoc.1997;4:364–75.
- The Leapfrog Group for Patient Safety: Rewarding Higher Standards.2001. Available at: www.leapfroggroup.org.
- ,,,,,.Understanding hospital readiness for computerized physician order entry.Jt Comm J Qual Saf.2003;29:336–344.
- ,,,,.Antecedents of the people and organizational aspects of medical informatics: review of the literature.J Am Med Informatics Assoc.1997;4:79–93.
- ,,.A consensus statement on considerations for a successful CPOE implementation.J Am Med Informatics Assoc.2003;10:229–234.
- AHA Guide to Computerized Physician Order‐Entry Systems.American Hospital Association:Chicago;2000.
- ,,,.Computerized physician order entry in US hospitals: results of a 2002 survey.J Am Med Inform Assoc.2004;11:95–99.
- ,,, et al.Physician characteristics, attitudes, and use of computerized order entry.J Hosp Med.2006;1:.
- ,.Computerized physician order entry systems in hospitals: mandates and incentives.Health Aff,2002;21(4):180–188.
- ,,.The use of computers for clinical care: a case series of advanced U.S. sites.J Am Med Inform Assoc.2003;10:94–107.
- ,,.Some unintended consequences of information technology in health care: the nature of patient care information system‐related errors.J Am Med Inform Assoc.2003;21:104–112.
- ,,,,,.Overcoming the barriers to implementing computerized physician order entry systems in US hospitals: perspectives from senior management.Health Aff.2004;23(4):184–190.
- ,,.Understanding Implementation: The case of a computerized physician order entry system in a large Dutch university medical cneter.J Am Med Inform Assoc.2004;11:207–216.
- ,,.U.S. adoption of computerized physician order entry systems.Health Aff.2005;24:1654–1663.
- ,,, et al.Return on investment for a computerized physician order entry system.J Am Med Inform Assoc.2006;13:261–266.
- ,,,.A diffusion of innovations model of physician order entry.AMIA Annu Symp Proc.2001;2001:22–26.
- ,,,.The status of hospital medicine groups in the United States.J Hosp Med.2006;1:75–80.
- ,,, et al.Unexpected increased mortality after implementation of a commercially sold computerized physician order entry system.Pediatrics.2005;116:1506–1512.
Self‐check‐in kiosks started to appear in airports in the late 1990s, and within a few years, they seem to have become ubiquitous in the airline industry. Today, almost 70% of business travelers use them, and other sectors of the travel industry are beginning to experiment with the technology.1 Compared to this innovation in the airline industry, adoption of computerized physician order entry (CPOE) in U.S. hospitals, first pioneered in the early 1970s,2, 3 has taken a much more leisurely pace. Despite numerous studies documenting its benefits,47 promotion by prominent national patient safety advocacy groups such as LeapFrog,8 and numerous guides on best adoption practices.912 fewer than 10% of U.S. hospitals have fully adopted this technology.13 Moreover, as Lindenauer et al.14 pointed out, most hospitals that have successfully implemented CPOE are academic medical centers that rely on house staff to enter orders. With notable exceptions,3 adoption of CPOE in community hospitals where attending physicians write most orders remains anemic.
Although an increasing number of scholarly articles has documented the reasons for this slow rate of adoption even in hospitals that have the resources to invest in this technology, much of that research is based on expert opinion and case studies.11, 1519 In this context, Lindenauer et al.14 should be commended for using empirical evidence to delineate the predictors of adoption. Lindenauer et al. found that physicians who trained in hospitals with CPOE were more likely to be frequent users of CPOE in their new environment. Although the analysis did not account for possible confounding such as employment status of the physician, this result does confirm the conventional wisdom that physicians‐in‐training are more malleable and that residency is an important opportunity to expose physicians to safety technologies. If this finding is borne out by further research, it would bode well for the adoption of CPOE, as many physicians are trained in academic institutions, which are more likely to have CPOE,20 and almost all physicians spend part of their training in a VA hospital, which has uniformly adopted CPOE. Similarly, Lindenauer et al. found that physicians who use computers for personal purposes are more likely to be frequent users of CPOE. Given the increasingly ubiquitous use of computers in all spheres of life, time is on the side of increasing acceptance of CPOE.
However, a closer examination of the data presented by Lindenauer et al. raises several concerns. First, the substantial number of infrequent users across all demographic subgroups and clinical disciplines, even among users who were exposed to CPOE during training or those who used computers regularly for personal purposes, highlights the absence of shortcuts to the universal acceptance of CPOE. Second, whereas 63% of surveyed physicians believed that CPOE would reduce the incidence of medication errors and 71% believed that CPOE would prevent aspects of care from slipping through the cracks, only 42% of the surveyed physicians were frequent users of CPOE. This implies that even when physicians believe in the safety and quality benefits of CPOE, that belief alone may not be sufficient to convince all of them to adopt this technology wholeheartedly; other factors such as speed, ease of use, and training are likely important prerequisites. Third, although 66% of orders placed in person at the 2 study hospitals were entered through CPOE, acceptance of this technology, as measured by Lindenauer et al, was moderate at both institutions. This suggests that even when organizations have reached the 70% threshold set by Leapfrog as the proportion of orders placed in CPOE that qualifies as full implementation, they may continue to face resistance to full acceptance of the technology.
Compared to their academic counterparts, community hospitals face additional hurdles as they implement CPOE. Not only does their smaller size make it difficult to achieve economies of scale, they are also at a disadvantage because of the relationship the community hospital has with its physicians. Unlike physicians‐in‐training in academic medical centers, physicians in community hospitals function as largely autonomous agents over whom the hospital administration has little control. Although these physicians and their hospitals share the common goals of patient safety and quality, the financial incentives for the adoption of CPOE are often misaligned. For example, a recent cost benefit analysis21 showed the enormous potential for hospitals to cut costs if physicians fully adopt a CPOE system with rich decision support features. However, those savings typically accrue to the hospital, not to the physicians who use the system. Assuming the typical learning curve that accompanies the use of any new technology, physicians in community hospitals may have little incentive to invest the time to learn to use the system efficiently.
So what can be done to overcome these seemingly formidable barriers to full adoption of CPOE? Emerging research, which has so far largely focused on CPOE implementation at academic hospitals, suggests there is no silver bullet. Instead, it has taught us how the complex interplay among vendor capability, organizational behavior, clinician work flow, and implementation strategy determines the success or failure of adoption.11, 17, 18, 22 Although physician characteristics will play a role in determining whether an individual adopts this technology, local factors such as the presence of champions, governance model for the project, support for staff throughout the process, and relationship between administration and physicians are likely important determinants of success at both academic and community hospitals. In addition, organizations that embark on CPOE implementation need to understand the enormity of the task at hand and must devote not only sufficient financial but also human capital over time.11, 18 In the words of a chief medical information officer, Implementing CPOE should not be thought of as an event, but a long‐term commitment.
Beyond following proposed best practices for the implementation of CPOE, community hospitals may need to adopt additional strategies to address their unique challenges. Given the misalignment of incentives for physicians' use of CPOE, leadership in community hospitals must be particularly skilled at articulating the benefits of CPOE to physicians. These benefits include not only decreased professional liability from improved patient safety and better quality of care, but also fewer pharmacy callbacks, remote access, and rapid ordering through order sets. Hospitals may also want to elicit support from physicians early by empowering them to create order sets for their disciplines. Mechanisms for hospitals and physicians to engage in mutual cost‐sharing arrangements may provide addition opportunities for hospitals to entice physicians to adopt the technology. Finally, and of particular interest to the readership of this journal, as hospitalists become more prevalent and take care of an increasing proportion of hospitalized patients,23 they are often ideal candidates to lead the implementation of CPOE in community hospitals. Because hospitalists spend most of their time in the hospital, they are often in the best position to get fully trained on CPOE, to define their own order sets, and to redesign care processes in order to take full advantage of CPOE capabilities. In addition, as many hospitalists are directly employed or supported by the hospital, their goals for quality, safety, and efficiency are usually better aligned with those of the hospital.
The stakes involved in implementing CPOE are high. Hospitals invest enormous sums of money in these systems, and many will not have the financial or political capital to attempt a second implementation after an initial failure. In addition, as recent research has pointed out,24 inappropriate implementation strategies may lead to delays in essential care and direct patient harm. In many ways, the complex task of implementing CPOE is not unlike other endeavors in patient care, where optimal outcomes require sound knowledge and reliable processes and where disaster can strike for lack of attention to detail or common sense. If Hippocrates were alive today, he might have this to say about CPOE implementation: Life is short, the art long, opportunity fleeting, experience treacherous, judgment difficult.
Self‐check‐in kiosks started to appear in airports in the late 1990s, and within a few years, they seem to have become ubiquitous in the airline industry. Today, almost 70% of business travelers use them, and other sectors of the travel industry are beginning to experiment with the technology.1 Compared to this innovation in the airline industry, adoption of computerized physician order entry (CPOE) in U.S. hospitals, first pioneered in the early 1970s,2, 3 has taken a much more leisurely pace. Despite numerous studies documenting its benefits,47 promotion by prominent national patient safety advocacy groups such as LeapFrog,8 and numerous guides on best adoption practices.912 fewer than 10% of U.S. hospitals have fully adopted this technology.13 Moreover, as Lindenauer et al.14 pointed out, most hospitals that have successfully implemented CPOE are academic medical centers that rely on house staff to enter orders. With notable exceptions,3 adoption of CPOE in community hospitals where attending physicians write most orders remains anemic.
Although an increasing number of scholarly articles has documented the reasons for this slow rate of adoption even in hospitals that have the resources to invest in this technology, much of that research is based on expert opinion and case studies.11, 1519 In this context, Lindenauer et al.14 should be commended for using empirical evidence to delineate the predictors of adoption. Lindenauer et al. found that physicians who trained in hospitals with CPOE were more likely to be frequent users of CPOE in their new environment. Although the analysis did not account for possible confounding such as employment status of the physician, this result does confirm the conventional wisdom that physicians‐in‐training are more malleable and that residency is an important opportunity to expose physicians to safety technologies. If this finding is borne out by further research, it would bode well for the adoption of CPOE, as many physicians are trained in academic institutions, which are more likely to have CPOE,20 and almost all physicians spend part of their training in a VA hospital, which has uniformly adopted CPOE. Similarly, Lindenauer et al. found that physicians who use computers for personal purposes are more likely to be frequent users of CPOE. Given the increasingly ubiquitous use of computers in all spheres of life, time is on the side of increasing acceptance of CPOE.
However, a closer examination of the data presented by Lindenauer et al. raises several concerns. First, the substantial number of infrequent users across all demographic subgroups and clinical disciplines, even among users who were exposed to CPOE during training or those who used computers regularly for personal purposes, highlights the absence of shortcuts to the universal acceptance of CPOE. Second, whereas 63% of surveyed physicians believed that CPOE would reduce the incidence of medication errors and 71% believed that CPOE would prevent aspects of care from slipping through the cracks, only 42% of the surveyed physicians were frequent users of CPOE. This implies that even when physicians believe in the safety and quality benefits of CPOE, that belief alone may not be sufficient to convince all of them to adopt this technology wholeheartedly; other factors such as speed, ease of use, and training are likely important prerequisites. Third, although 66% of orders placed in person at the 2 study hospitals were entered through CPOE, acceptance of this technology, as measured by Lindenauer et al, was moderate at both institutions. This suggests that even when organizations have reached the 70% threshold set by Leapfrog as the proportion of orders placed in CPOE that qualifies as full implementation, they may continue to face resistance to full acceptance of the technology.
Compared to their academic counterparts, community hospitals face additional hurdles as they implement CPOE. Not only does their smaller size make it difficult to achieve economies of scale, they are also at a disadvantage because of the relationship the community hospital has with its physicians. Unlike physicians‐in‐training in academic medical centers, physicians in community hospitals function as largely autonomous agents over whom the hospital administration has little control. Although these physicians and their hospitals share the common goals of patient safety and quality, the financial incentives for the adoption of CPOE are often misaligned. For example, a recent cost benefit analysis21 showed the enormous potential for hospitals to cut costs if physicians fully adopt a CPOE system with rich decision support features. However, those savings typically accrue to the hospital, not to the physicians who use the system. Assuming the typical learning curve that accompanies the use of any new technology, physicians in community hospitals may have little incentive to invest the time to learn to use the system efficiently.
So what can be done to overcome these seemingly formidable barriers to full adoption of CPOE? Emerging research, which has so far largely focused on CPOE implementation at academic hospitals, suggests there is no silver bullet. Instead, it has taught us how the complex interplay among vendor capability, organizational behavior, clinician work flow, and implementation strategy determines the success or failure of adoption.11, 17, 18, 22 Although physician characteristics will play a role in determining whether an individual adopts this technology, local factors such as the presence of champions, governance model for the project, support for staff throughout the process, and relationship between administration and physicians are likely important determinants of success at both academic and community hospitals. In addition, organizations that embark on CPOE implementation need to understand the enormity of the task at hand and must devote not only sufficient financial but also human capital over time.11, 18 In the words of a chief medical information officer, Implementing CPOE should not be thought of as an event, but a long‐term commitment.
Beyond following proposed best practices for the implementation of CPOE, community hospitals may need to adopt additional strategies to address their unique challenges. Given the misalignment of incentives for physicians' use of CPOE, leadership in community hospitals must be particularly skilled at articulating the benefits of CPOE to physicians. These benefits include not only decreased professional liability from improved patient safety and better quality of care, but also fewer pharmacy callbacks, remote access, and rapid ordering through order sets. Hospitals may also want to elicit support from physicians early by empowering them to create order sets for their disciplines. Mechanisms for hospitals and physicians to engage in mutual cost‐sharing arrangements may provide addition opportunities for hospitals to entice physicians to adopt the technology. Finally, and of particular interest to the readership of this journal, as hospitalists become more prevalent and take care of an increasing proportion of hospitalized patients,23 they are often ideal candidates to lead the implementation of CPOE in community hospitals. Because hospitalists spend most of their time in the hospital, they are often in the best position to get fully trained on CPOE, to define their own order sets, and to redesign care processes in order to take full advantage of CPOE capabilities. In addition, as many hospitalists are directly employed or supported by the hospital, their goals for quality, safety, and efficiency are usually better aligned with those of the hospital.
The stakes involved in implementing CPOE are high. Hospitals invest enormous sums of money in these systems, and many will not have the financial or political capital to attempt a second implementation after an initial failure. In addition, as recent research has pointed out,24 inappropriate implementation strategies may lead to delays in essential care and direct patient harm. In many ways, the complex task of implementing CPOE is not unlike other endeavors in patient care, where optimal outcomes require sound knowledge and reliable processes and where disaster can strike for lack of attention to detail or common sense. If Hippocrates were alive today, he might have this to say about CPOE implementation: Life is short, the art long, opportunity fleeting, experience treacherous, judgment difficult.
- Travel self‐serve kiosks here to stay.Adelman Group. Available at: http://www.adelmantravel.com/index_news_past.asp?Date=031406. Accessed March 14,2006.
- ,.Computer‐based physician order entry: the state of the art.J Am Med Inform Assoc.1994;1:108–123.
- ,,,.Final report on evaluation of the implementation of a medical information system in a general community hospital.Battelle Laboratories NTIS PB.1975;248:340.
- ,,, et al.Effect of computerized physician order entry and a team intervention on prevention of serious medication errors.JAMA.1998;280:1311–1316.
- ,,,,,.Effects of computerized physician order entry in prescribing practices.Arch Intern Med.2000;160:2741–2747.
- ,,,,,.A computerized reminder system to increase the use of preventive care for hospitalized patients [see comments].N Eng J M.2001;345:965–970.
- ,,,.A randomized trial of “corollary orders” to prevent errors of omission.J Am Med Inform Assoc.1997;4:364–75.
- The Leapfrog Group for Patient Safety: Rewarding Higher Standards.2001. Available at: www.leapfroggroup.org.
- ,,,,,.Understanding hospital readiness for computerized physician order entry.Jt Comm J Qual Saf.2003;29:336–344.
- ,,,,.Antecedents of the people and organizational aspects of medical informatics: review of the literature.J Am Med Informatics Assoc.1997;4:79–93.
- ,,.A consensus statement on considerations for a successful CPOE implementation.J Am Med Informatics Assoc.2003;10:229–234.
- AHA Guide to Computerized Physician Order‐Entry Systems.American Hospital Association:Chicago;2000.
- ,,,.Computerized physician order entry in US hospitals: results of a 2002 survey.J Am Med Inform Assoc.2004;11:95–99.
- ,,, et al.Physician characteristics, attitudes, and use of computerized order entry.J Hosp Med.2006;1:.
- ,.Computerized physician order entry systems in hospitals: mandates and incentives.Health Aff,2002;21(4):180–188.
- ,,.The use of computers for clinical care: a case series of advanced U.S. sites.J Am Med Inform Assoc.2003;10:94–107.
- ,,.Some unintended consequences of information technology in health care: the nature of patient care information system‐related errors.J Am Med Inform Assoc.2003;21:104–112.
- ,,,,,.Overcoming the barriers to implementing computerized physician order entry systems in US hospitals: perspectives from senior management.Health Aff.2004;23(4):184–190.
- ,,.Understanding Implementation: The case of a computerized physician order entry system in a large Dutch university medical cneter.J Am Med Inform Assoc.2004;11:207–216.
- ,,.U.S. adoption of computerized physician order entry systems.Health Aff.2005;24:1654–1663.
- ,,, et al.Return on investment for a computerized physician order entry system.J Am Med Inform Assoc.2006;13:261–266.
- ,,,.A diffusion of innovations model of physician order entry.AMIA Annu Symp Proc.2001;2001:22–26.
- ,,,.The status of hospital medicine groups in the United States.J Hosp Med.2006;1:75–80.
- ,,, et al.Unexpected increased mortality after implementation of a commercially sold computerized physician order entry system.Pediatrics.2005;116:1506–1512.
- Travel self‐serve kiosks here to stay.Adelman Group. Available at: http://www.adelmantravel.com/index_news_past.asp?Date=031406. Accessed March 14,2006.
- ,.Computer‐based physician order entry: the state of the art.J Am Med Inform Assoc.1994;1:108–123.
- ,,,.Final report on evaluation of the implementation of a medical information system in a general community hospital.Battelle Laboratories NTIS PB.1975;248:340.
- ,,, et al.Effect of computerized physician order entry and a team intervention on prevention of serious medication errors.JAMA.1998;280:1311–1316.
- ,,,,,.Effects of computerized physician order entry in prescribing practices.Arch Intern Med.2000;160:2741–2747.
- ,,,,,.A computerized reminder system to increase the use of preventive care for hospitalized patients [see comments].N Eng J M.2001;345:965–970.
- ,,,.A randomized trial of “corollary orders” to prevent errors of omission.J Am Med Inform Assoc.1997;4:364–75.
- The Leapfrog Group for Patient Safety: Rewarding Higher Standards.2001. Available at: www.leapfroggroup.org.
- ,,,,,.Understanding hospital readiness for computerized physician order entry.Jt Comm J Qual Saf.2003;29:336–344.
- ,,,,.Antecedents of the people and organizational aspects of medical informatics: review of the literature.J Am Med Informatics Assoc.1997;4:79–93.
- ,,.A consensus statement on considerations for a successful CPOE implementation.J Am Med Informatics Assoc.2003;10:229–234.
- AHA Guide to Computerized Physician Order‐Entry Systems.American Hospital Association:Chicago;2000.
- ,,,.Computerized physician order entry in US hospitals: results of a 2002 survey.J Am Med Inform Assoc.2004;11:95–99.
- ,,, et al.Physician characteristics, attitudes, and use of computerized order entry.J Hosp Med.2006;1:.
- ,.Computerized physician order entry systems in hospitals: mandates and incentives.Health Aff,2002;21(4):180–188.
- ,,.The use of computers for clinical care: a case series of advanced U.S. sites.J Am Med Inform Assoc.2003;10:94–107.
- ,,.Some unintended consequences of information technology in health care: the nature of patient care information system‐related errors.J Am Med Inform Assoc.2003;21:104–112.
- ,,,,,.Overcoming the barriers to implementing computerized physician order entry systems in US hospitals: perspectives from senior management.Health Aff.2004;23(4):184–190.
- ,,.Understanding Implementation: The case of a computerized physician order entry system in a large Dutch university medical cneter.J Am Med Inform Assoc.2004;11:207–216.
- ,,.U.S. adoption of computerized physician order entry systems.Health Aff.2005;24:1654–1663.
- ,,, et al.Return on investment for a computerized physician order entry system.J Am Med Inform Assoc.2006;13:261–266.
- ,,,.A diffusion of innovations model of physician order entry.AMIA Annu Symp Proc.2001;2001:22–26.
- ,,,.The status of hospital medicine groups in the United States.J Hosp Med.2006;1:75–80.
- ,,, et al.Unexpected increased mortality after implementation of a commercially sold computerized physician order entry system.Pediatrics.2005;116:1506–1512.