User login
A Healthy Dose of Superstition
Mr. Smith was once a nice guy.
These days, unfortunately, he’s anything but. The ravages of a neurodegenerative disease have left him demented, impulsive, and agitated.
His family is trying to find placement for him, and in the meantime I’m doing my best to keep his behavior controlled. Like many things in medicine, this is as much art as science. A tablet of this, a capsule of that, increase this slightly, add something for PRN use ... a witch’s brew of modern medicine.
Because of his worsening, his wife was calling us several times a week with updates, not in an annoying way but in an “I need help” way. I began answering the phone myself if I saw her number come up, because it was easier and faster for me to deal with her directly, and I knew she wasn’t calling for fun.
A few months ago I stopped a medication that didn’t seem to be doing much and started a different one.
And then things went quiet. His wife’s calls went from 3-4 a week to none.
This worried me. I mean, maybe the new medicine was working. ... but the sudden silence was deafening.
One week went by, then two ... I did a Google search to make sure he and his wife hadn’t died or been in the news.
Of course, I could have picked up the phone and called his wife, but why tempt fate?
Three weeks ... I was sure my MA, who handles far more calls than I do, had probably noticed this, too.
It would have been easy to mention it, but even with 16 years of school and 5 years of medical training, not to mention 3,000-4,000 years of hard-earned science behind me, there was the old grade school notion of jinxing myself. To say something is to invite trouble.
Four weeks. Finally, his wife called in and reached my MA. The medication had been working, but now was wearing off and the dose needed to be adjusted. So we did that.
Afterward I mentioned the time lapse to my MA, that I’d been afraid of jinxing it by saying something to her, and she told me she’d been thinking the same thing.
Funny when you think about it. We’re both educated people, believers in science, and (I hope) intelligent. We’re living in a (by human standards) technologically advanced time.
Yet, the old superstitions are still there, the idea that we somehow have magical control over time, space, random chance, and the actions of others by not talking about a phone call (or the lack of one).
Surprisingly (or maybe not), this is pretty normal. When on call we never say “quiet,” for fear of enraging the mysterious Call Gods. If needed, we use “the Q word.”
We still try not to walk under ladders, avoid stepping on sidewalk cracks, carry good luck charms, cross fingers, and fight over wishbones.
Superstitions such as saying “bless you” or “gesundheit” when someone sneezes are so ingrained into us that they’re now part of good manners and polite society.
I’ve worked in quite a few hospitals over the years. Not one of them had a room on any floor that ended in 13, always jumping from 12 to 14.
Civilization is roughly 10,000-15,000 years old. We have the internet and can travel to (relatively nearby) space and back. We have probes exploring — and even leaving — our solar system.
But
I’m going to knock on wood now.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Mr. Smith was once a nice guy.
These days, unfortunately, he’s anything but. The ravages of a neurodegenerative disease have left him demented, impulsive, and agitated.
His family is trying to find placement for him, and in the meantime I’m doing my best to keep his behavior controlled. Like many things in medicine, this is as much art as science. A tablet of this, a capsule of that, increase this slightly, add something for PRN use ... a witch’s brew of modern medicine.
Because of his worsening, his wife was calling us several times a week with updates, not in an annoying way but in an “I need help” way. I began answering the phone myself if I saw her number come up, because it was easier and faster for me to deal with her directly, and I knew she wasn’t calling for fun.
A few months ago I stopped a medication that didn’t seem to be doing much and started a different one.
And then things went quiet. His wife’s calls went from 3-4 a week to none.
This worried me. I mean, maybe the new medicine was working. ... but the sudden silence was deafening.
One week went by, then two ... I did a Google search to make sure he and his wife hadn’t died or been in the news.
Of course, I could have picked up the phone and called his wife, but why tempt fate?
Three weeks ... I was sure my MA, who handles far more calls than I do, had probably noticed this, too.
It would have been easy to mention it, but even with 16 years of school and 5 years of medical training, not to mention 3,000-4,000 years of hard-earned science behind me, there was the old grade school notion of jinxing myself. To say something is to invite trouble.
Four weeks. Finally, his wife called in and reached my MA. The medication had been working, but now was wearing off and the dose needed to be adjusted. So we did that.
Afterward I mentioned the time lapse to my MA, that I’d been afraid of jinxing it by saying something to her, and she told me she’d been thinking the same thing.
Funny when you think about it. We’re both educated people, believers in science, and (I hope) intelligent. We’re living in a (by human standards) technologically advanced time.
Yet, the old superstitions are still there, the idea that we somehow have magical control over time, space, random chance, and the actions of others by not talking about a phone call (or the lack of one).
Surprisingly (or maybe not), this is pretty normal. When on call we never say “quiet,” for fear of enraging the mysterious Call Gods. If needed, we use “the Q word.”
We still try not to walk under ladders, avoid stepping on sidewalk cracks, carry good luck charms, cross fingers, and fight over wishbones.
Superstitions such as saying “bless you” or “gesundheit” when someone sneezes are so ingrained into us that they’re now part of good manners and polite society.
I’ve worked in quite a few hospitals over the years. Not one of them had a room on any floor that ended in 13, always jumping from 12 to 14.
Civilization is roughly 10,000-15,000 years old. We have the internet and can travel to (relatively nearby) space and back. We have probes exploring — and even leaving — our solar system.
But
I’m going to knock on wood now.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Mr. Smith was once a nice guy.
These days, unfortunately, he’s anything but. The ravages of a neurodegenerative disease have left him demented, impulsive, and agitated.
His family is trying to find placement for him, and in the meantime I’m doing my best to keep his behavior controlled. Like many things in medicine, this is as much art as science. A tablet of this, a capsule of that, increase this slightly, add something for PRN use ... a witch’s brew of modern medicine.
Because of his worsening, his wife was calling us several times a week with updates, not in an annoying way but in an “I need help” way. I began answering the phone myself if I saw her number come up, because it was easier and faster for me to deal with her directly, and I knew she wasn’t calling for fun.
A few months ago I stopped a medication that didn’t seem to be doing much and started a different one.
And then things went quiet. His wife’s calls went from 3-4 a week to none.
This worried me. I mean, maybe the new medicine was working. ... but the sudden silence was deafening.
One week went by, then two ... I did a Google search to make sure he and his wife hadn’t died or been in the news.
Of course, I could have picked up the phone and called his wife, but why tempt fate?
Three weeks ... I was sure my MA, who handles far more calls than I do, had probably noticed this, too.
It would have been easy to mention it, but even with 16 years of school and 5 years of medical training, not to mention 3,000-4,000 years of hard-earned science behind me, there was the old grade school notion of jinxing myself. To say something is to invite trouble.
Four weeks. Finally, his wife called in and reached my MA. The medication had been working, but now was wearing off and the dose needed to be adjusted. So we did that.
Afterward I mentioned the time lapse to my MA, that I’d been afraid of jinxing it by saying something to her, and she told me she’d been thinking the same thing.
Funny when you think about it. We’re both educated people, believers in science, and (I hope) intelligent. We’re living in a (by human standards) technologically advanced time.
Yet, the old superstitions are still there, the idea that we somehow have magical control over time, space, random chance, and the actions of others by not talking about a phone call (or the lack of one).
Surprisingly (or maybe not), this is pretty normal. When on call we never say “quiet,” for fear of enraging the mysterious Call Gods. If needed, we use “the Q word.”
We still try not to walk under ladders, avoid stepping on sidewalk cracks, carry good luck charms, cross fingers, and fight over wishbones.
Superstitions such as saying “bless you” or “gesundheit” when someone sneezes are so ingrained into us that they’re now part of good manners and polite society.
I’ve worked in quite a few hospitals over the years. Not one of them had a room on any floor that ended in 13, always jumping from 12 to 14.
Civilization is roughly 10,000-15,000 years old. We have the internet and can travel to (relatively nearby) space and back. We have probes exploring — and even leaving — our solar system.
But
I’m going to knock on wood now.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Burnout and Work-Based Well-Being Programs
Since very few of us practice medicine without being either an employer or an employee, we should probably be paying more attention to research in industrial and employee relations, not an area most of us have studied. One of the hot topics for employers in these days of low unemployment is the question of whether to offer free wellness-enhancing programs companywide.
Almost by definition anything “free” has a good public relations aura surrounding it. Recent surveys have shown that a large chunk of the population is feeling stressed. If your boss is offering you a free opportunity to help you feel better about yourself, he/she must understand at least a bit of what you are going through.
From the employer’s standpoint these programs offer the potential for a double win. On one hand, offering a free well-being program is a perk the company can tout as it competes in the tight market for new employees. On the other hand, if the program is effective then the employees will be happier. And we all know that happy workers are more productive and less likely to leave and feed the expensive cycle of hiring and training new workers to replace the unhappy and disgruntled workers who have quit. Even if the employer’s total focus is on the company’s bottom line, offering a wellness program should pay a dividend.
Well ... this may be one of those situations where wishful thinking isn’t going to work. A recent study published in Industrial Relations Journal suggests that these well-being programs, which include employee mental services, may not be living up to their promise. In this large study of nearly 50,00 workers in the United Kingdom, the researcher discovered that workers who had been offered coaching and relaxation classes, internet-based apps, and courses in time management and financial health were “no better off” than their coworkers who had not participated in these programs. In fact, training programs in stress management and resilience appeared to possibly have had a negative effect.
In a New York Times article about this study, the British researcher recommends that employers who are interested in improving their worker’s mental health should turn their attention to “core organizational practices” meaning pay scales, work schedules, and performance reviews.
Not surprisingly, this study has raised some controversy. There are a lot of people invested emotionally and in some cases financially in programs similar to the ones that appeared to be ineffective in this study. Critics argue the study was too short, or too small, or failed to select programs with a proven track record.
Even given these potential flaws, physicians, particularly those who of us who feel they approaching burnout, should take this investigator’s message seriously. Certainly some of us could be doing a better job of building resilience into our lifestyles and may be helped by the kind of well-being programs tested in this study. However, the biggest contribution to the burnout phenomenon is coming from the work environments that are asking too much of even the most resilient among us. This study makes it clear that including work schedules, time-gobbling electronic systems, and short staffing. Trotting out a few feel-good mindfulness programs is not going to do the job.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
Since very few of us practice medicine without being either an employer or an employee, we should probably be paying more attention to research in industrial and employee relations, not an area most of us have studied. One of the hot topics for employers in these days of low unemployment is the question of whether to offer free wellness-enhancing programs companywide.
Almost by definition anything “free” has a good public relations aura surrounding it. Recent surveys have shown that a large chunk of the population is feeling stressed. If your boss is offering you a free opportunity to help you feel better about yourself, he/she must understand at least a bit of what you are going through.
From the employer’s standpoint these programs offer the potential for a double win. On one hand, offering a free well-being program is a perk the company can tout as it competes in the tight market for new employees. On the other hand, if the program is effective then the employees will be happier. And we all know that happy workers are more productive and less likely to leave and feed the expensive cycle of hiring and training new workers to replace the unhappy and disgruntled workers who have quit. Even if the employer’s total focus is on the company’s bottom line, offering a wellness program should pay a dividend.
Well ... this may be one of those situations where wishful thinking isn’t going to work. A recent study published in Industrial Relations Journal suggests that these well-being programs, which include employee mental services, may not be living up to their promise. In this large study of nearly 50,00 workers in the United Kingdom, the researcher discovered that workers who had been offered coaching and relaxation classes, internet-based apps, and courses in time management and financial health were “no better off” than their coworkers who had not participated in these programs. In fact, training programs in stress management and resilience appeared to possibly have had a negative effect.
In a New York Times article about this study, the British researcher recommends that employers who are interested in improving their worker’s mental health should turn their attention to “core organizational practices” meaning pay scales, work schedules, and performance reviews.
Not surprisingly, this study has raised some controversy. There are a lot of people invested emotionally and in some cases financially in programs similar to the ones that appeared to be ineffective in this study. Critics argue the study was too short, or too small, or failed to select programs with a proven track record.
Even given these potential flaws, physicians, particularly those who of us who feel they approaching burnout, should take this investigator’s message seriously. Certainly some of us could be doing a better job of building resilience into our lifestyles and may be helped by the kind of well-being programs tested in this study. However, the biggest contribution to the burnout phenomenon is coming from the work environments that are asking too much of even the most resilient among us. This study makes it clear that including work schedules, time-gobbling electronic systems, and short staffing. Trotting out a few feel-good mindfulness programs is not going to do the job.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
Since very few of us practice medicine without being either an employer or an employee, we should probably be paying more attention to research in industrial and employee relations, not an area most of us have studied. One of the hot topics for employers in these days of low unemployment is the question of whether to offer free wellness-enhancing programs companywide.
Almost by definition anything “free” has a good public relations aura surrounding it. Recent surveys have shown that a large chunk of the population is feeling stressed. If your boss is offering you a free opportunity to help you feel better about yourself, he/she must understand at least a bit of what you are going through.
From the employer’s standpoint these programs offer the potential for a double win. On one hand, offering a free well-being program is a perk the company can tout as it competes in the tight market for new employees. On the other hand, if the program is effective then the employees will be happier. And we all know that happy workers are more productive and less likely to leave and feed the expensive cycle of hiring and training new workers to replace the unhappy and disgruntled workers who have quit. Even if the employer’s total focus is on the company’s bottom line, offering a wellness program should pay a dividend.
Well ... this may be one of those situations where wishful thinking isn’t going to work. A recent study published in Industrial Relations Journal suggests that these well-being programs, which include employee mental services, may not be living up to their promise. In this large study of nearly 50,00 workers in the United Kingdom, the researcher discovered that workers who had been offered coaching and relaxation classes, internet-based apps, and courses in time management and financial health were “no better off” than their coworkers who had not participated in these programs. In fact, training programs in stress management and resilience appeared to possibly have had a negative effect.
In a New York Times article about this study, the British researcher recommends that employers who are interested in improving their worker’s mental health should turn their attention to “core organizational practices” meaning pay scales, work schedules, and performance reviews.
Not surprisingly, this study has raised some controversy. There are a lot of people invested emotionally and in some cases financially in programs similar to the ones that appeared to be ineffective in this study. Critics argue the study was too short, or too small, or failed to select programs with a proven track record.
Even given these potential flaws, physicians, particularly those who of us who feel they approaching burnout, should take this investigator’s message seriously. Certainly some of us could be doing a better job of building resilience into our lifestyles and may be helped by the kind of well-being programs tested in this study. However, the biggest contribution to the burnout phenomenon is coming from the work environments that are asking too much of even the most resilient among us. This study makes it clear that including work schedules, time-gobbling electronic systems, and short staffing. Trotting out a few feel-good mindfulness programs is not going to do the job.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
Why Don’t Physicians Call In Sick?
I began practicing medicine on July 1, 1981. In the 43-plus years since then,
There are several reasons, both good and bad, why this is so: (1) like most physicians, I am a terrible patient; (2) as a solo practitioner, there was (until recently — I’ll get to that in a minute) no one else to see an office full of patients who had waited significant amounts of time for their appointments and in many cases had taken off work themselves to keep them; and (3) there is an unspoken rule against it. Taking sick days is highly frowned upon in the medical world. As a medical student, intern, and resident I was told in so many words not to call in sick, no matter how serious the illness might be.
Apparently, I was not the only doctor-in-training to receive that message. In a survey reported in JAMA Pediatrics several years ago, 95% of the physicians and advanced practice clinicians (APCs) surveyed believed that working while sick put patients at risk — yet 83% reported working sick at least one time over the prior year. They understood the risks, but did it anyway.
There is no question that this practice does put patients’ health at risk. The JAMA study linked numerous reports of outbreaks traceable to symptomatic healthcare workers. Some outbreaks of flu, staph infections, norovirus, and pertussis were shown to originate from a sick physician or supporting staff member. These associations have led to increased morbidity and mortality, as well as excess costs. Those of us who treat immunocompromised patients on a regular basis risk inducing a life-threatening illness by unnecessarily exposing them to pathogens.
The JAMA survey results also confirmed my own observation that many physicians feel boxed in by their institutions or practice situations. “The study illustrates the complex social and logistic factors that cause this behavior,” the authors wrote. “These results may inform efforts to design systems at our hospital to provide support for attending physicians and APCs and help them make the right choice to keep their patients and colleagues safe while caring for themselves.”
What might those efforts look like? For one thing, we can take the obvious and necessary steps to avoid getting sick in the first place, such as staying fit and hydrated, and eating well. We can keep up with routine health visits and measures such as colorectal screening, pap smears, and mammograms, and stay up to date with flu shots and all other essential immunizations.
Next, we can minimize the risk of spreading any illnesses we encounter in the course of our work by practicing the basic infectious disease prevention measures driven home so forcefully by the recent COVID-19 pandemic — washing our hands, using hand sanitizers, and, when appropriate, wearing gloves and masks.
Finally, we can work to overcome this institutional taboo against staying home when we do get sick. Work out a system of mutual coverage for such situations. Two years ago, I merged my solo practice with a local, larger group. I did it for a variety of reasons, but a principal one was to assure that a partner could cover for me if I became ill. Practitioners who choose to remain solo or in small groups should contact colleagues and work out a coverage agreement.
Now, during flu season, it is especially important to resist the temptation to work while sick. The CDC has guidelines for employees specific for the flu, which notes that “persons with the flu are most contagious during the first 3 days of their illness,” and should remain at home until at least 24 hours after their fever subsides (without the use of fever-reducing medications) or after symptoms have improved (at least 4-5 days after they started) — or, if they do not have a fever, after symptoms improve “for at least 4-5 days after the onset of symptoms.”
Of course, we need to remember that COVID-19 is still with us. With the constant evolution of new strains, it is especially important to avoid exposing patients and colleagues to the disease should you become infected. The most recent advice from the CDC includes the recommendation that those who are mildly ill and not moderately or severely immunocompromised should isolate after SARS-CoV-2 infection for at least 5 days after symptom onset (day 0 is the day symptoms appeared, and day 1 is the next full day thereafter) if fever has resolved for at least 24 hours (without taking fever-reducing medications) and other symptoms are improving. In addition, “a high-quality mask should be worn around others at home and in public through day 10.”
We should also extend these rules to our support staff, starting with providing them with adequate sick leave and encouraging them to use it when necessary. Research has found a direct correlation between preventative health care and the number of paid sick leave days a worker gets. In a study of over 3000 US workers, those with 10 paid sick days or more annually accessed preventative care more frequently than those without paid sick days.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at dermnews@mdedge.com.
I began practicing medicine on July 1, 1981. In the 43-plus years since then,
There are several reasons, both good and bad, why this is so: (1) like most physicians, I am a terrible patient; (2) as a solo practitioner, there was (until recently — I’ll get to that in a minute) no one else to see an office full of patients who had waited significant amounts of time for their appointments and in many cases had taken off work themselves to keep them; and (3) there is an unspoken rule against it. Taking sick days is highly frowned upon in the medical world. As a medical student, intern, and resident I was told in so many words not to call in sick, no matter how serious the illness might be.
Apparently, I was not the only doctor-in-training to receive that message. In a survey reported in JAMA Pediatrics several years ago, 95% of the physicians and advanced practice clinicians (APCs) surveyed believed that working while sick put patients at risk — yet 83% reported working sick at least one time over the prior year. They understood the risks, but did it anyway.
There is no question that this practice does put patients’ health at risk. The JAMA study linked numerous reports of outbreaks traceable to symptomatic healthcare workers. Some outbreaks of flu, staph infections, norovirus, and pertussis were shown to originate from a sick physician or supporting staff member. These associations have led to increased morbidity and mortality, as well as excess costs. Those of us who treat immunocompromised patients on a regular basis risk inducing a life-threatening illness by unnecessarily exposing them to pathogens.
The JAMA survey results also confirmed my own observation that many physicians feel boxed in by their institutions or practice situations. “The study illustrates the complex social and logistic factors that cause this behavior,” the authors wrote. “These results may inform efforts to design systems at our hospital to provide support for attending physicians and APCs and help them make the right choice to keep their patients and colleagues safe while caring for themselves.”
What might those efforts look like? For one thing, we can take the obvious and necessary steps to avoid getting sick in the first place, such as staying fit and hydrated, and eating well. We can keep up with routine health visits and measures such as colorectal screening, pap smears, and mammograms, and stay up to date with flu shots and all other essential immunizations.
Next, we can minimize the risk of spreading any illnesses we encounter in the course of our work by practicing the basic infectious disease prevention measures driven home so forcefully by the recent COVID-19 pandemic — washing our hands, using hand sanitizers, and, when appropriate, wearing gloves and masks.
Finally, we can work to overcome this institutional taboo against staying home when we do get sick. Work out a system of mutual coverage for such situations. Two years ago, I merged my solo practice with a local, larger group. I did it for a variety of reasons, but a principal one was to assure that a partner could cover for me if I became ill. Practitioners who choose to remain solo or in small groups should contact colleagues and work out a coverage agreement.
Now, during flu season, it is especially important to resist the temptation to work while sick. The CDC has guidelines for employees specific for the flu, which notes that “persons with the flu are most contagious during the first 3 days of their illness,” and should remain at home until at least 24 hours after their fever subsides (without the use of fever-reducing medications) or after symptoms have improved (at least 4-5 days after they started) — or, if they do not have a fever, after symptoms improve “for at least 4-5 days after the onset of symptoms.”
Of course, we need to remember that COVID-19 is still with us. With the constant evolution of new strains, it is especially important to avoid exposing patients and colleagues to the disease should you become infected. The most recent advice from the CDC includes the recommendation that those who are mildly ill and not moderately or severely immunocompromised should isolate after SARS-CoV-2 infection for at least 5 days after symptom onset (day 0 is the day symptoms appeared, and day 1 is the next full day thereafter) if fever has resolved for at least 24 hours (without taking fever-reducing medications) and other symptoms are improving. In addition, “a high-quality mask should be worn around others at home and in public through day 10.”
We should also extend these rules to our support staff, starting with providing them with adequate sick leave and encouraging them to use it when necessary. Research has found a direct correlation between preventative health care and the number of paid sick leave days a worker gets. In a study of over 3000 US workers, those with 10 paid sick days or more annually accessed preventative care more frequently than those without paid sick days.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at dermnews@mdedge.com.
I began practicing medicine on July 1, 1981. In the 43-plus years since then,
There are several reasons, both good and bad, why this is so: (1) like most physicians, I am a terrible patient; (2) as a solo practitioner, there was (until recently — I’ll get to that in a minute) no one else to see an office full of patients who had waited significant amounts of time for their appointments and in many cases had taken off work themselves to keep them; and (3) there is an unspoken rule against it. Taking sick days is highly frowned upon in the medical world. As a medical student, intern, and resident I was told in so many words not to call in sick, no matter how serious the illness might be.
Apparently, I was not the only doctor-in-training to receive that message. In a survey reported in JAMA Pediatrics several years ago, 95% of the physicians and advanced practice clinicians (APCs) surveyed believed that working while sick put patients at risk — yet 83% reported working sick at least one time over the prior year. They understood the risks, but did it anyway.
There is no question that this practice does put patients’ health at risk. The JAMA study linked numerous reports of outbreaks traceable to symptomatic healthcare workers. Some outbreaks of flu, staph infections, norovirus, and pertussis were shown to originate from a sick physician or supporting staff member. These associations have led to increased morbidity and mortality, as well as excess costs. Those of us who treat immunocompromised patients on a regular basis risk inducing a life-threatening illness by unnecessarily exposing them to pathogens.
The JAMA survey results also confirmed my own observation that many physicians feel boxed in by their institutions or practice situations. “The study illustrates the complex social and logistic factors that cause this behavior,” the authors wrote. “These results may inform efforts to design systems at our hospital to provide support for attending physicians and APCs and help them make the right choice to keep their patients and colleagues safe while caring for themselves.”
What might those efforts look like? For one thing, we can take the obvious and necessary steps to avoid getting sick in the first place, such as staying fit and hydrated, and eating well. We can keep up with routine health visits and measures such as colorectal screening, pap smears, and mammograms, and stay up to date with flu shots and all other essential immunizations.
Next, we can minimize the risk of spreading any illnesses we encounter in the course of our work by practicing the basic infectious disease prevention measures driven home so forcefully by the recent COVID-19 pandemic — washing our hands, using hand sanitizers, and, when appropriate, wearing gloves and masks.
Finally, we can work to overcome this institutional taboo against staying home when we do get sick. Work out a system of mutual coverage for such situations. Two years ago, I merged my solo practice with a local, larger group. I did it for a variety of reasons, but a principal one was to assure that a partner could cover for me if I became ill. Practitioners who choose to remain solo or in small groups should contact colleagues and work out a coverage agreement.
Now, during flu season, it is especially important to resist the temptation to work while sick. The CDC has guidelines for employees specific for the flu, which notes that “persons with the flu are most contagious during the first 3 days of their illness,” and should remain at home until at least 24 hours after their fever subsides (without the use of fever-reducing medications) or after symptoms have improved (at least 4-5 days after they started) — or, if they do not have a fever, after symptoms improve “for at least 4-5 days after the onset of symptoms.”
Of course, we need to remember that COVID-19 is still with us. With the constant evolution of new strains, it is especially important to avoid exposing patients and colleagues to the disease should you become infected. The most recent advice from the CDC includes the recommendation that those who are mildly ill and not moderately or severely immunocompromised should isolate after SARS-CoV-2 infection for at least 5 days after symptom onset (day 0 is the day symptoms appeared, and day 1 is the next full day thereafter) if fever has resolved for at least 24 hours (without taking fever-reducing medications) and other symptoms are improving. In addition, “a high-quality mask should be worn around others at home and in public through day 10.”
We should also extend these rules to our support staff, starting with providing them with adequate sick leave and encouraging them to use it when necessary. Research has found a direct correlation between preventative health care and the number of paid sick leave days a worker gets. In a study of over 3000 US workers, those with 10 paid sick days or more annually accessed preventative care more frequently than those without paid sick days.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at dermnews@mdedge.com.
How much would you bet on a diagnosis?
“You have psoriasis,” I say all the time. I mean it when I say it, of course. But I don’t always to the same degree. Sometimes I’m trying to say, “You probably have psoriasis.” Other times I mean, “You most definitely have psoriasis.” I rarely use those terms though.
One 36-year-old man with a flaky scalp and scaly elbows wasn’t satisfied with my assessment. His dad has psoriasis. So does his older brother. He was in to see me to find out if he had psoriasis too. “Probably” was what I gave him. He pushed back, “What percent chance?” That’s a good question — must be an engineer. I’m unsure.
With the exception of the poker players, our species is notoriously bad at probabilities. We’re wired to notice the significance of events, but terrible at understanding their likelihood. This is salient in lottery ticket holders and some NFL offensive coordinators who persist despite very long odds of things working out. It’s also reflected in the language we use. Rarely do we say, there’s a sixty percent chance something will happen. Rather, we say, “it’s likely.” There are two problems here. One, we often misjudge the actual probability of something occurring and two, the terms we use are subjective and differences in interpretation can lead to misunderstandings.
Let’s take a look. A 55-year-old man with a chronic eczematous rash on his trunk and extremities is getting worse despite dupilumab. He recently had night sweats. Do you think he has atopic dermatitis or cutaneous T-cell lymphoma? If you had to place a $100 bet, would you change your answer? Immanuel Kant thinks you would. In his “Critique of Pure Reason,” the German philosopher proposes that betting helps clarify the mind, an antidote to brashness. The example Kant uses is of a physician who observes a patient and concludes he has phthisis (tuberculosis), but we really don’t know if the physician is confident. Kant proposes that if he had to bet on his conclusion, then we’d have insight into just how convinced he is of phthisis. So, what’s your bet?
If you’re a bad poker player, then you might bet he has cutaneous T-cell lymphoma. However, not having any additional information, the smart call is atopic dermatitis, which has a base rate 1000-fold higher than CTCL. It is therefore more probable to be eczema even in a case that worsens despite dupilumab or with recent night sweats, both of which could be a result of common variables such as weather and COVID. Failure to account for the base rate is a mistake we physicians sometimes make. Economists rarely do. Try to think like one before answering a likelihood question.
If you think about it, “probably” means something different even to me, depending on the situation. I might say I’ll probably go to Montana this summer and I’ll probably retire at 65. The actual likelihoods might be 95% and 70%. That’s a big difference. What about between probably and likely? Or possibly and maybe? Do they mean the same to you as to the person you’re speaking with? For much of the work we do, precise likelihoods aren’t critical. Yet, it can be important in decision making and in discussing probabilities, such as the risk of hepatitis on terbinafine or of melanoma recurrence after Mohs.
I told my patient “I say about a 70% chance you have psoriasis. I could do a biopsy today to confirm.” He thought for a second and asked, “What is the chance it’s psoriasis if the biopsy shows it?” “Eighty six percent,” I replied.
Seemed like a good bet to me.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on X. Write to him at dermnews@mdedge.com.
“You have psoriasis,” I say all the time. I mean it when I say it, of course. But I don’t always to the same degree. Sometimes I’m trying to say, “You probably have psoriasis.” Other times I mean, “You most definitely have psoriasis.” I rarely use those terms though.
One 36-year-old man with a flaky scalp and scaly elbows wasn’t satisfied with my assessment. His dad has psoriasis. So does his older brother. He was in to see me to find out if he had psoriasis too. “Probably” was what I gave him. He pushed back, “What percent chance?” That’s a good question — must be an engineer. I’m unsure.
With the exception of the poker players, our species is notoriously bad at probabilities. We’re wired to notice the significance of events, but terrible at understanding their likelihood. This is salient in lottery ticket holders and some NFL offensive coordinators who persist despite very long odds of things working out. It’s also reflected in the language we use. Rarely do we say, there’s a sixty percent chance something will happen. Rather, we say, “it’s likely.” There are two problems here. One, we often misjudge the actual probability of something occurring and two, the terms we use are subjective and differences in interpretation can lead to misunderstandings.
Let’s take a look. A 55-year-old man with a chronic eczematous rash on his trunk and extremities is getting worse despite dupilumab. He recently had night sweats. Do you think he has atopic dermatitis or cutaneous T-cell lymphoma? If you had to place a $100 bet, would you change your answer? Immanuel Kant thinks you would. In his “Critique of Pure Reason,” the German philosopher proposes that betting helps clarify the mind, an antidote to brashness. The example Kant uses is of a physician who observes a patient and concludes he has phthisis (tuberculosis), but we really don’t know if the physician is confident. Kant proposes that if he had to bet on his conclusion, then we’d have insight into just how convinced he is of phthisis. So, what’s your bet?
If you’re a bad poker player, then you might bet he has cutaneous T-cell lymphoma. However, not having any additional information, the smart call is atopic dermatitis, which has a base rate 1000-fold higher than CTCL. It is therefore more probable to be eczema even in a case that worsens despite dupilumab or with recent night sweats, both of which could be a result of common variables such as weather and COVID. Failure to account for the base rate is a mistake we physicians sometimes make. Economists rarely do. Try to think like one before answering a likelihood question.
If you think about it, “probably” means something different even to me, depending on the situation. I might say I’ll probably go to Montana this summer and I’ll probably retire at 65. The actual likelihoods might be 95% and 70%. That’s a big difference. What about between probably and likely? Or possibly and maybe? Do they mean the same to you as to the person you’re speaking with? For much of the work we do, precise likelihoods aren’t critical. Yet, it can be important in decision making and in discussing probabilities, such as the risk of hepatitis on terbinafine or of melanoma recurrence after Mohs.
I told my patient “I say about a 70% chance you have psoriasis. I could do a biopsy today to confirm.” He thought for a second and asked, “What is the chance it’s psoriasis if the biopsy shows it?” “Eighty six percent,” I replied.
Seemed like a good bet to me.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on X. Write to him at dermnews@mdedge.com.
“You have psoriasis,” I say all the time. I mean it when I say it, of course. But I don’t always to the same degree. Sometimes I’m trying to say, “You probably have psoriasis.” Other times I mean, “You most definitely have psoriasis.” I rarely use those terms though.
One 36-year-old man with a flaky scalp and scaly elbows wasn’t satisfied with my assessment. His dad has psoriasis. So does his older brother. He was in to see me to find out if he had psoriasis too. “Probably” was what I gave him. He pushed back, “What percent chance?” That’s a good question — must be an engineer. I’m unsure.
With the exception of the poker players, our species is notoriously bad at probabilities. We’re wired to notice the significance of events, but terrible at understanding their likelihood. This is salient in lottery ticket holders and some NFL offensive coordinators who persist despite very long odds of things working out. It’s also reflected in the language we use. Rarely do we say, there’s a sixty percent chance something will happen. Rather, we say, “it’s likely.” There are two problems here. One, we often misjudge the actual probability of something occurring and two, the terms we use are subjective and differences in interpretation can lead to misunderstandings.
Let’s take a look. A 55-year-old man with a chronic eczematous rash on his trunk and extremities is getting worse despite dupilumab. He recently had night sweats. Do you think he has atopic dermatitis or cutaneous T-cell lymphoma? If you had to place a $100 bet, would you change your answer? Immanuel Kant thinks you would. In his “Critique of Pure Reason,” the German philosopher proposes that betting helps clarify the mind, an antidote to brashness. The example Kant uses is of a physician who observes a patient and concludes he has phthisis (tuberculosis), but we really don’t know if the physician is confident. Kant proposes that if he had to bet on his conclusion, then we’d have insight into just how convinced he is of phthisis. So, what’s your bet?
If you’re a bad poker player, then you might bet he has cutaneous T-cell lymphoma. However, not having any additional information, the smart call is atopic dermatitis, which has a base rate 1000-fold higher than CTCL. It is therefore more probable to be eczema even in a case that worsens despite dupilumab or with recent night sweats, both of which could be a result of common variables such as weather and COVID. Failure to account for the base rate is a mistake we physicians sometimes make. Economists rarely do. Try to think like one before answering a likelihood question.
If you think about it, “probably” means something different even to me, depending on the situation. I might say I’ll probably go to Montana this summer and I’ll probably retire at 65. The actual likelihoods might be 95% and 70%. That’s a big difference. What about between probably and likely? Or possibly and maybe? Do they mean the same to you as to the person you’re speaking with? For much of the work we do, precise likelihoods aren’t critical. Yet, it can be important in decision making and in discussing probabilities, such as the risk of hepatitis on terbinafine or of melanoma recurrence after Mohs.
I told my patient “I say about a 70% chance you have psoriasis. I could do a biopsy today to confirm.” He thought for a second and asked, “What is the chance it’s psoriasis if the biopsy shows it?” “Eighty six percent,” I replied.
Seemed like a good bet to me.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on X. Write to him at dermnews@mdedge.com.
Cutting Across the Bias
On a recent rainy afternoon I was speed skimming through the pile of publications sitting on the floor next to my Grampy’s chair. A bright patch of color jumped off the gray background of the printed page forcing me to pause and consider the content.
In the right upper corner was a photograph of an attractive Black woman nursing her baby. Her bare arms suggested she might be slightly overweight. She wore a simple off-white head wrap and smiled broadly as she played with her infant’s fingers. The image was a reproduction of a WIC poster encouraging women to take advantage of the program’s breastfeeding support services. The accompanying article from American Academy of Pediatrics offered ten strategies for achieving breastfeeding equity.
I must admit that I tend to shy away from discussions of equity because I’ve seldom found them very informative. However, the engaging image of this Black woman breastfeeding led me to read beyond the title.
The first of the strategies listed was “Check you biases.” I will certainly admit to having biases. We all have biases and see and interpret the world through lenses ground and tinted by our experiences and the environment we have inhabited. In the case of breastfeeding, I wasn’t sure where my biases lay. Maybe one of mine is reflected in a hesitancy to actively promote exclusive breastfeeding for the first 6 months. I prefer a more nuanced approach adjusted to the unique needs and limitations of each family. But I decided to chase down the Implicit Association Test (IAT) suggested in the article. I couldn’t make that link work, but found a long list of subjects on the Harvard Implicit Association Test website. None dealt with breastfeeding, so I chose the one described as Black/White.
If, like me, you have never had your implicit biases assessed by taking an IAT, you might find it interesting. Probably took me about 15 minutes using my laptop. There are a lot of demographic questions then some rapid-fire exercises in which you must provide your first response to a barrage of photos of faces and words. At times I sensed that the test makers were trying to trick me into making associations that I didn’t want to make by the order in which the exercises were presented. At the end I was told that I was a little slow in associating Black faces with positive words.
I’m not sure what this means. After doing a little internet searching I learned that one of the criticisms of the IAT is that, while it may hint at a bias, it is really more important whether you cut with or across that bias. If I acknowledge that where and how I grew up may have left me with some implicit biases, it is more important that I make a strong and honest effort to act independently of those biases.
In full disclosure I must tell you that there was one Black girl in my high school of a thousand students. I have lived and practiced in Maine for 50 years. At less than 2%, we are sixth from the bottom in Black population among other states. However, in the last 5 or 6 years here in Brunswick we have welcomed a large infusion of asylum seekers who come predominantly from Black African countries.
Skimming through the rest of the article, I found it hard to argue with the remaining nine recommendations for promoting breastfeeding, although most of them we not terribly applicable to small community practices. The photo of the Black woman nursing her baby at the top of the page remains as the primary message. The fact that I was drawn to that image is a testament to several of my biases and another example of a picture being worth far more than a thousand words.
I suspect that I’m not alone in appreciating the uniqueness of that image. Until recently, the standard photos of a mother breastfeeding have used trim White women as their models. I suspect and hope this poster will be effective in encouraging Black women to nurse. I urge you all to hang it in your office as a reminder to you and your staff of your biases and assumptions. Don’t bother to take the Implicit Association Test unless you’re retired and have 15 minutes to burn on a rainy afternoon.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
On a recent rainy afternoon I was speed skimming through the pile of publications sitting on the floor next to my Grampy’s chair. A bright patch of color jumped off the gray background of the printed page forcing me to pause and consider the content.
In the right upper corner was a photograph of an attractive Black woman nursing her baby. Her bare arms suggested she might be slightly overweight. She wore a simple off-white head wrap and smiled broadly as she played with her infant’s fingers. The image was a reproduction of a WIC poster encouraging women to take advantage of the program’s breastfeeding support services. The accompanying article from American Academy of Pediatrics offered ten strategies for achieving breastfeeding equity.
I must admit that I tend to shy away from discussions of equity because I’ve seldom found them very informative. However, the engaging image of this Black woman breastfeeding led me to read beyond the title.
The first of the strategies listed was “Check you biases.” I will certainly admit to having biases. We all have biases and see and interpret the world through lenses ground and tinted by our experiences and the environment we have inhabited. In the case of breastfeeding, I wasn’t sure where my biases lay. Maybe one of mine is reflected in a hesitancy to actively promote exclusive breastfeeding for the first 6 months. I prefer a more nuanced approach adjusted to the unique needs and limitations of each family. But I decided to chase down the Implicit Association Test (IAT) suggested in the article. I couldn’t make that link work, but found a long list of subjects on the Harvard Implicit Association Test website. None dealt with breastfeeding, so I chose the one described as Black/White.
If, like me, you have never had your implicit biases assessed by taking an IAT, you might find it interesting. Probably took me about 15 minutes using my laptop. There are a lot of demographic questions then some rapid-fire exercises in which you must provide your first response to a barrage of photos of faces and words. At times I sensed that the test makers were trying to trick me into making associations that I didn’t want to make by the order in which the exercises were presented. At the end I was told that I was a little slow in associating Black faces with positive words.
I’m not sure what this means. After doing a little internet searching I learned that one of the criticisms of the IAT is that, while it may hint at a bias, it is really more important whether you cut with or across that bias. If I acknowledge that where and how I grew up may have left me with some implicit biases, it is more important that I make a strong and honest effort to act independently of those biases.
In full disclosure I must tell you that there was one Black girl in my high school of a thousand students. I have lived and practiced in Maine for 50 years. At less than 2%, we are sixth from the bottom in Black population among other states. However, in the last 5 or 6 years here in Brunswick we have welcomed a large infusion of asylum seekers who come predominantly from Black African countries.
Skimming through the rest of the article, I found it hard to argue with the remaining nine recommendations for promoting breastfeeding, although most of them we not terribly applicable to small community practices. The photo of the Black woman nursing her baby at the top of the page remains as the primary message. The fact that I was drawn to that image is a testament to several of my biases and another example of a picture being worth far more than a thousand words.
I suspect that I’m not alone in appreciating the uniqueness of that image. Until recently, the standard photos of a mother breastfeeding have used trim White women as their models. I suspect and hope this poster will be effective in encouraging Black women to nurse. I urge you all to hang it in your office as a reminder to you and your staff of your biases and assumptions. Don’t bother to take the Implicit Association Test unless you’re retired and have 15 minutes to burn on a rainy afternoon.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
On a recent rainy afternoon I was speed skimming through the pile of publications sitting on the floor next to my Grampy’s chair. A bright patch of color jumped off the gray background of the printed page forcing me to pause and consider the content.
In the right upper corner was a photograph of an attractive Black woman nursing her baby. Her bare arms suggested she might be slightly overweight. She wore a simple off-white head wrap and smiled broadly as she played with her infant’s fingers. The image was a reproduction of a WIC poster encouraging women to take advantage of the program’s breastfeeding support services. The accompanying article from American Academy of Pediatrics offered ten strategies for achieving breastfeeding equity.
I must admit that I tend to shy away from discussions of equity because I’ve seldom found them very informative. However, the engaging image of this Black woman breastfeeding led me to read beyond the title.
The first of the strategies listed was “Check you biases.” I will certainly admit to having biases. We all have biases and see and interpret the world through lenses ground and tinted by our experiences and the environment we have inhabited. In the case of breastfeeding, I wasn’t sure where my biases lay. Maybe one of mine is reflected in a hesitancy to actively promote exclusive breastfeeding for the first 6 months. I prefer a more nuanced approach adjusted to the unique needs and limitations of each family. But I decided to chase down the Implicit Association Test (IAT) suggested in the article. I couldn’t make that link work, but found a long list of subjects on the Harvard Implicit Association Test website. None dealt with breastfeeding, so I chose the one described as Black/White.
If, like me, you have never had your implicit biases assessed by taking an IAT, you might find it interesting. Probably took me about 15 minutes using my laptop. There are a lot of demographic questions then some rapid-fire exercises in which you must provide your first response to a barrage of photos of faces and words. At times I sensed that the test makers were trying to trick me into making associations that I didn’t want to make by the order in which the exercises were presented. At the end I was told that I was a little slow in associating Black faces with positive words.
I’m not sure what this means. After doing a little internet searching I learned that one of the criticisms of the IAT is that, while it may hint at a bias, it is really more important whether you cut with or across that bias. If I acknowledge that where and how I grew up may have left me with some implicit biases, it is more important that I make a strong and honest effort to act independently of those biases.
In full disclosure I must tell you that there was one Black girl in my high school of a thousand students. I have lived and practiced in Maine for 50 years. At less than 2%, we are sixth from the bottom in Black population among other states. However, in the last 5 or 6 years here in Brunswick we have welcomed a large infusion of asylum seekers who come predominantly from Black African countries.
Skimming through the rest of the article, I found it hard to argue with the remaining nine recommendations for promoting breastfeeding, although most of them we not terribly applicable to small community practices. The photo of the Black woman nursing her baby at the top of the page remains as the primary message. The fact that I was drawn to that image is a testament to several of my biases and another example of a picture being worth far more than a thousand words.
I suspect that I’m not alone in appreciating the uniqueness of that image. Until recently, the standard photos of a mother breastfeeding have used trim White women as their models. I suspect and hope this poster will be effective in encouraging Black women to nurse. I urge you all to hang it in your office as a reminder to you and your staff of your biases and assumptions. Don’t bother to take the Implicit Association Test unless you’re retired and have 15 minutes to burn on a rainy afternoon.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
Rosemary, Part 1
A member of the Lamiaceae family, Salvia rosmarinus (rosemary),* an aromatic plant native to the Mediterranean region and now cultivated globally, has been used for centuries in cuisine and medicine, with several well-established biological activities.1-3 Thought to contribute to preventing hair loss, rosemary oil was also used for hundreds of years in hair rinses in the Mediterranean area.4 In traditional Iranian medicine, rosemary essential oil has been topically applied as an analgesic, anti-inflammatory, and anti-acne remedy.5 Rosemary is known to absorb UV light well and to impart antibacterial and antifungal activity, as well as help maintain skin homeostasis.3 It is also used and under further study for its anti-inflammatory, antioxidant, anti-infective, and anticancer activity.2,6-9 The health benefits of rosemary are typically ascribed to its constituent carnosol/carnosic and ursolic acids.7.
Chemical Constituents
The key chemical components of S. rosmarinus include bitter principle, resin, tannic acid, flavonoids, and volatile oils (made up of borneol, bornyl acetate, camphene, cineol, pinene, and camphor).10 Other important constituents of rosemary oil, in particular, include p-Cymene, linalool, gamma-terpinene, thymol, beta-pinene, alpha-pinene, eucalyptol, and carnosic acid.9 Volatile oils of rosemary have been used in various oils and lotions to treat wounds and with the intention of stimulating hair growth.10
Wound Healing
In a 2022 study in 60 adult male rats, Bulhões and colleagues found that the use of rosemary leaf essential oil-based ointments on skin lesions spurred wound healing, decreased inflammation, and enhanced angiogenesis as well as collagen fiber density.11
Three years earlier, Labib and colleagues studied the wound healing capacity of three chitosan-based topical formulations containing either tea tree essential oil, rosemary essential oil, or a mixture of both oils in an excision wound model in rats.
The combination preparation was found to be the most effective in fostering various stages of wound healing, with significant increases in wound contraction percentage observed in the combination group compared with either group treated using individual essential oils or the untreated animals.12
A 2010 in vivo study by Abu-Al-Basal using BALB/c mice with diabetes revealed that the topical application of rosemary essential oil for three days reduced inflammation, enhanced wound contraction and re-epithelialization, and promoted angiogenesis, granulation tissue regeneration, and collagen deposition.13
Anticancer Activity
Using a 7,12-dimethlybenz(a)anthracene (DMBA)-initiated and croton oil-promoted model in 2006, Sancheti and Goyal determined that rosemary extract administered orally at a dose rate of 500 mg/kg body weight/mouse significantly inhibited two-stage skin tumorigenesis in mice.14 Nearly a decade later, Cattaneo and colleagues determined that a rosemary hydroalcoholic extract displayed antiproliferative effects on the human melanoma A375 cell line.8
The polyphenols carnosic acid and rosmarinic acid are most often cited as the sources of the reputed anticancer effects of rosemary.15
Hair Health
Early in 2023, Begum and colleagues developed a 1% hair lotion including a methanolic extract of the aerial part of S. rosmarinus that they assessed for potential hair growth activity in C57BL/6 mice. Using water as a control and 2% minoxidil hair lotion as standard, the investigators determined that their rosemary hair lotion demonstrated significant hair growth promotion, exceeding that seen in the mice treated with the drug standard.1
In a randomized controlled study in C57BL/6NCrSlc mice a decade earlier, Murata and colleagues evaluated the anti-androgenic activity and hair growth potential imparted by topical rosemary oil compared with finasteride and minoxidil. Rosemary oil leaf extract, with 12-O-methylcarnosic acid as its most active component, robustly suppressed 5alpha-reductase and stimulated hair growth in vivo in both the androgenetic alopecia/testosterone-treated mouse model, as well as the hair growth activating mouse model as compared with minoxidil. Further, the inhibitory activity of rosemary was 82.4% and 94.6% at 200 mcg/mL and 500 mcg/mL, respectively, whereas finasteride demonstrated 81.9% at 250 nM.16
A human study two years later was even more encouraging. Panahi and colleagues conducted a randomized comparative trial with 100 patients to investigate the effects of rosemary oil as opposed to minoxidil 2% for the treatment of androgenetic alopecia over 6 months. By 6 months, significantly greater hair counts were observed in both groups compared with baseline and 3-month readings, but no significant variations between groups. No differences were found in the frequency of dryness, greasiness, or dandruff at any time point or between groups. Scalp itching was significantly greater at the 3- and 6-month points in both groups, particularly in the minoxidil group at both of those time points. The investigators concluded that rosemary oil compared well with minoxidil as androgenetic alopecia therapy.17
Conclusion
Rosemary has been used in traditional medicine for hundreds of years and it has been a common ingredient in cosmetic and cosmeceutical formulations for more than 20 years. Recent findings suggest a broad array of applications in modern medicine, particularly dermatology. The next column will focus on the most recent studies pertaining to the antioxidant and anti-aging activity of this aromatic shrub.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a SaaS company used to generate skin care routines in office and as a ecommerce solution. Write to her at dermnews@mdedge.com.
References
1. Begum A et al. Adv Biomed Res. 2023 Mar 21;12:60.
2. de Oliveira JR et al. J Biomed Sci. 2019 Jan 9;26(1):5.
3. González-Minero FJ et al. Cosmetics. 2020 Oct 3;7(4):77.
4. Dinkins J et al. Int J Dermatol. 2023 Aug;62(8):980-5.
5. Akbari J et al. Pharm Biol. 2015;53(10):1442-7.
6. Allegra A et al. Nutrients. 2020 Jun 10;12(6):1739.
7. de Macedo LM et al. Plants (Basel). 2020 May 21;9(5):651.
8. Cattaneo L et al. PLoS One. 2015 Jul 15;10(7):e0132439.
9. Borges RS et al. J Ethnopharmacol. 2019 Jan 30;229:29-45.
10. Begum A et al. Acta Sci Pol Technol Aliment. 2013 Jan-Mar;12(1):61-73.
11. Bulhões AAVC et al. Acta Cir Bras. 2022 Apr 8;37(1):e370104.
12. Labib RM et al. PLoS One. 2019 Sep 16;14(9):e0219561.
13. Abu-Al-Basal MA. J Ethnopharmacol. 2010 Sep 15;131(2):443-50.
14. Sancheti G and Goyal PK. Phytother Res. 2006 Nov;20(11):981-6.
15. Moore J et al. Nutrients. 2016 Nov 17;8(11):731.
16. Murata K et al. Phytother Res. 2013 Feb;27(2):212-7.
17. Panahi Y et al. Skinmed. 2015 Jan-Feb;13(1):15-21.
*Correction, 2/27: This column was updated with the more recent name for rosemary, Salvia rosmarinus.
A member of the Lamiaceae family, Salvia rosmarinus (rosemary),* an aromatic plant native to the Mediterranean region and now cultivated globally, has been used for centuries in cuisine and medicine, with several well-established biological activities.1-3 Thought to contribute to preventing hair loss, rosemary oil was also used for hundreds of years in hair rinses in the Mediterranean area.4 In traditional Iranian medicine, rosemary essential oil has been topically applied as an analgesic, anti-inflammatory, and anti-acne remedy.5 Rosemary is known to absorb UV light well and to impart antibacterial and antifungal activity, as well as help maintain skin homeostasis.3 It is also used and under further study for its anti-inflammatory, antioxidant, anti-infective, and anticancer activity.2,6-9 The health benefits of rosemary are typically ascribed to its constituent carnosol/carnosic and ursolic acids.7.
Chemical Constituents
The key chemical components of S. rosmarinus include bitter principle, resin, tannic acid, flavonoids, and volatile oils (made up of borneol, bornyl acetate, camphene, cineol, pinene, and camphor).10 Other important constituents of rosemary oil, in particular, include p-Cymene, linalool, gamma-terpinene, thymol, beta-pinene, alpha-pinene, eucalyptol, and carnosic acid.9 Volatile oils of rosemary have been used in various oils and lotions to treat wounds and with the intention of stimulating hair growth.10
Wound Healing
In a 2022 study in 60 adult male rats, Bulhões and colleagues found that the use of rosemary leaf essential oil-based ointments on skin lesions spurred wound healing, decreased inflammation, and enhanced angiogenesis as well as collagen fiber density.11
Three years earlier, Labib and colleagues studied the wound healing capacity of three chitosan-based topical formulations containing either tea tree essential oil, rosemary essential oil, or a mixture of both oils in an excision wound model in rats.
The combination preparation was found to be the most effective in fostering various stages of wound healing, with significant increases in wound contraction percentage observed in the combination group compared with either group treated using individual essential oils or the untreated animals.12
A 2010 in vivo study by Abu-Al-Basal using BALB/c mice with diabetes revealed that the topical application of rosemary essential oil for three days reduced inflammation, enhanced wound contraction and re-epithelialization, and promoted angiogenesis, granulation tissue regeneration, and collagen deposition.13
Anticancer Activity
Using a 7,12-dimethlybenz(a)anthracene (DMBA)-initiated and croton oil-promoted model in 2006, Sancheti and Goyal determined that rosemary extract administered orally at a dose rate of 500 mg/kg body weight/mouse significantly inhibited two-stage skin tumorigenesis in mice.14 Nearly a decade later, Cattaneo and colleagues determined that a rosemary hydroalcoholic extract displayed antiproliferative effects on the human melanoma A375 cell line.8
The polyphenols carnosic acid and rosmarinic acid are most often cited as the sources of the reputed anticancer effects of rosemary.15
Hair Health
Early in 2023, Begum and colleagues developed a 1% hair lotion including a methanolic extract of the aerial part of S. rosmarinus that they assessed for potential hair growth activity in C57BL/6 mice. Using water as a control and 2% minoxidil hair lotion as standard, the investigators determined that their rosemary hair lotion demonstrated significant hair growth promotion, exceeding that seen in the mice treated with the drug standard.1
In a randomized controlled study in C57BL/6NCrSlc mice a decade earlier, Murata and colleagues evaluated the anti-androgenic activity and hair growth potential imparted by topical rosemary oil compared with finasteride and minoxidil. Rosemary oil leaf extract, with 12-O-methylcarnosic acid as its most active component, robustly suppressed 5alpha-reductase and stimulated hair growth in vivo in both the androgenetic alopecia/testosterone-treated mouse model, as well as the hair growth activating mouse model as compared with minoxidil. Further, the inhibitory activity of rosemary was 82.4% and 94.6% at 200 mcg/mL and 500 mcg/mL, respectively, whereas finasteride demonstrated 81.9% at 250 nM.16
A human study two years later was even more encouraging. Panahi and colleagues conducted a randomized comparative trial with 100 patients to investigate the effects of rosemary oil as opposed to minoxidil 2% for the treatment of androgenetic alopecia over 6 months. By 6 months, significantly greater hair counts were observed in both groups compared with baseline and 3-month readings, but no significant variations between groups. No differences were found in the frequency of dryness, greasiness, or dandruff at any time point or between groups. Scalp itching was significantly greater at the 3- and 6-month points in both groups, particularly in the minoxidil group at both of those time points. The investigators concluded that rosemary oil compared well with minoxidil as androgenetic alopecia therapy.17
Conclusion
Rosemary has been used in traditional medicine for hundreds of years and it has been a common ingredient in cosmetic and cosmeceutical formulations for more than 20 years. Recent findings suggest a broad array of applications in modern medicine, particularly dermatology. The next column will focus on the most recent studies pertaining to the antioxidant and anti-aging activity of this aromatic shrub.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a SaaS company used to generate skin care routines in office and as a ecommerce solution. Write to her at dermnews@mdedge.com.
References
1. Begum A et al. Adv Biomed Res. 2023 Mar 21;12:60.
2. de Oliveira JR et al. J Biomed Sci. 2019 Jan 9;26(1):5.
3. González-Minero FJ et al. Cosmetics. 2020 Oct 3;7(4):77.
4. Dinkins J et al. Int J Dermatol. 2023 Aug;62(8):980-5.
5. Akbari J et al. Pharm Biol. 2015;53(10):1442-7.
6. Allegra A et al. Nutrients. 2020 Jun 10;12(6):1739.
7. de Macedo LM et al. Plants (Basel). 2020 May 21;9(5):651.
8. Cattaneo L et al. PLoS One. 2015 Jul 15;10(7):e0132439.
9. Borges RS et al. J Ethnopharmacol. 2019 Jan 30;229:29-45.
10. Begum A et al. Acta Sci Pol Technol Aliment. 2013 Jan-Mar;12(1):61-73.
11. Bulhões AAVC et al. Acta Cir Bras. 2022 Apr 8;37(1):e370104.
12. Labib RM et al. PLoS One. 2019 Sep 16;14(9):e0219561.
13. Abu-Al-Basal MA. J Ethnopharmacol. 2010 Sep 15;131(2):443-50.
14. Sancheti G and Goyal PK. Phytother Res. 2006 Nov;20(11):981-6.
15. Moore J et al. Nutrients. 2016 Nov 17;8(11):731.
16. Murata K et al. Phytother Res. 2013 Feb;27(2):212-7.
17. Panahi Y et al. Skinmed. 2015 Jan-Feb;13(1):15-21.
*Correction, 2/27: This column was updated with the more recent name for rosemary, Salvia rosmarinus.
A member of the Lamiaceae family, Salvia rosmarinus (rosemary),* an aromatic plant native to the Mediterranean region and now cultivated globally, has been used for centuries in cuisine and medicine, with several well-established biological activities.1-3 Thought to contribute to preventing hair loss, rosemary oil was also used for hundreds of years in hair rinses in the Mediterranean area.4 In traditional Iranian medicine, rosemary essential oil has been topically applied as an analgesic, anti-inflammatory, and anti-acne remedy.5 Rosemary is known to absorb UV light well and to impart antibacterial and antifungal activity, as well as help maintain skin homeostasis.3 It is also used and under further study for its anti-inflammatory, antioxidant, anti-infective, and anticancer activity.2,6-9 The health benefits of rosemary are typically ascribed to its constituent carnosol/carnosic and ursolic acids.7.
Chemical Constituents
The key chemical components of S. rosmarinus include bitter principle, resin, tannic acid, flavonoids, and volatile oils (made up of borneol, bornyl acetate, camphene, cineol, pinene, and camphor).10 Other important constituents of rosemary oil, in particular, include p-Cymene, linalool, gamma-terpinene, thymol, beta-pinene, alpha-pinene, eucalyptol, and carnosic acid.9 Volatile oils of rosemary have been used in various oils and lotions to treat wounds and with the intention of stimulating hair growth.10
Wound Healing
In a 2022 study in 60 adult male rats, Bulhões and colleagues found that the use of rosemary leaf essential oil-based ointments on skin lesions spurred wound healing, decreased inflammation, and enhanced angiogenesis as well as collagen fiber density.11
Three years earlier, Labib and colleagues studied the wound healing capacity of three chitosan-based topical formulations containing either tea tree essential oil, rosemary essential oil, or a mixture of both oils in an excision wound model in rats.
The combination preparation was found to be the most effective in fostering various stages of wound healing, with significant increases in wound contraction percentage observed in the combination group compared with either group treated using individual essential oils or the untreated animals.12
A 2010 in vivo study by Abu-Al-Basal using BALB/c mice with diabetes revealed that the topical application of rosemary essential oil for three days reduced inflammation, enhanced wound contraction and re-epithelialization, and promoted angiogenesis, granulation tissue regeneration, and collagen deposition.13
Anticancer Activity
Using a 7,12-dimethlybenz(a)anthracene (DMBA)-initiated and croton oil-promoted model in 2006, Sancheti and Goyal determined that rosemary extract administered orally at a dose rate of 500 mg/kg body weight/mouse significantly inhibited two-stage skin tumorigenesis in mice.14 Nearly a decade later, Cattaneo and colleagues determined that a rosemary hydroalcoholic extract displayed antiproliferative effects on the human melanoma A375 cell line.8
The polyphenols carnosic acid and rosmarinic acid are most often cited as the sources of the reputed anticancer effects of rosemary.15
Hair Health
Early in 2023, Begum and colleagues developed a 1% hair lotion including a methanolic extract of the aerial part of S. rosmarinus that they assessed for potential hair growth activity in C57BL/6 mice. Using water as a control and 2% minoxidil hair lotion as standard, the investigators determined that their rosemary hair lotion demonstrated significant hair growth promotion, exceeding that seen in the mice treated with the drug standard.1
In a randomized controlled study in C57BL/6NCrSlc mice a decade earlier, Murata and colleagues evaluated the anti-androgenic activity and hair growth potential imparted by topical rosemary oil compared with finasteride and minoxidil. Rosemary oil leaf extract, with 12-O-methylcarnosic acid as its most active component, robustly suppressed 5alpha-reductase and stimulated hair growth in vivo in both the androgenetic alopecia/testosterone-treated mouse model, as well as the hair growth activating mouse model as compared with minoxidil. Further, the inhibitory activity of rosemary was 82.4% and 94.6% at 200 mcg/mL and 500 mcg/mL, respectively, whereas finasteride demonstrated 81.9% at 250 nM.16
A human study two years later was even more encouraging. Panahi and colleagues conducted a randomized comparative trial with 100 patients to investigate the effects of rosemary oil as opposed to minoxidil 2% for the treatment of androgenetic alopecia over 6 months. By 6 months, significantly greater hair counts were observed in both groups compared with baseline and 3-month readings, but no significant variations between groups. No differences were found in the frequency of dryness, greasiness, or dandruff at any time point or between groups. Scalp itching was significantly greater at the 3- and 6-month points in both groups, particularly in the minoxidil group at both of those time points. The investigators concluded that rosemary oil compared well with minoxidil as androgenetic alopecia therapy.17
Conclusion
Rosemary has been used in traditional medicine for hundreds of years and it has been a common ingredient in cosmetic and cosmeceutical formulations for more than 20 years. Recent findings suggest a broad array of applications in modern medicine, particularly dermatology. The next column will focus on the most recent studies pertaining to the antioxidant and anti-aging activity of this aromatic shrub.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a SaaS company used to generate skin care routines in office and as a ecommerce solution. Write to her at dermnews@mdedge.com.
References
1. Begum A et al. Adv Biomed Res. 2023 Mar 21;12:60.
2. de Oliveira JR et al. J Biomed Sci. 2019 Jan 9;26(1):5.
3. González-Minero FJ et al. Cosmetics. 2020 Oct 3;7(4):77.
4. Dinkins J et al. Int J Dermatol. 2023 Aug;62(8):980-5.
5. Akbari J et al. Pharm Biol. 2015;53(10):1442-7.
6. Allegra A et al. Nutrients. 2020 Jun 10;12(6):1739.
7. de Macedo LM et al. Plants (Basel). 2020 May 21;9(5):651.
8. Cattaneo L et al. PLoS One. 2015 Jul 15;10(7):e0132439.
9. Borges RS et al. J Ethnopharmacol. 2019 Jan 30;229:29-45.
10. Begum A et al. Acta Sci Pol Technol Aliment. 2013 Jan-Mar;12(1):61-73.
11. Bulhões AAVC et al. Acta Cir Bras. 2022 Apr 8;37(1):e370104.
12. Labib RM et al. PLoS One. 2019 Sep 16;14(9):e0219561.
13. Abu-Al-Basal MA. J Ethnopharmacol. 2010 Sep 15;131(2):443-50.
14. Sancheti G and Goyal PK. Phytother Res. 2006 Nov;20(11):981-6.
15. Moore J et al. Nutrients. 2016 Nov 17;8(11):731.
16. Murata K et al. Phytother Res. 2013 Feb;27(2):212-7.
17. Panahi Y et al. Skinmed. 2015 Jan-Feb;13(1):15-21.
*Correction, 2/27: This column was updated with the more recent name for rosemary, Salvia rosmarinus.
‘Stop Teaching’ Children It’s Their Fault They’re Fat
The US Preventive Services Task Force (USPSTF) has published draft recommendations that 6-year-olds with obesity be lectured to about diet and exercise.
Never mind that there are no reproducible or scalable studies demonstrating durable and clinically meaningful benefits of this for adults let alone children. Never mind that children are not household decision-makers on matters of grocery shopping, cooking, or exercise. Never mind the corollary that many children so lectured who fail to see an impact on their weight will perceive that as their own personal failures. And of course, never mind that we’re privileged to be in an era with safe, effective, pharmacotherapeutic options for obesity. No. We must teach children it’s their fault if they’re fat. Because ultimately that’s what many of them will learn.
That’s not to say there’s no room for counseling. But with children as young as 6, that counseling should be delivered exclusively to their parents and caregivers. That counseling should focus as much if not more so on the impact of weight bias and the biological basis of obesity rather than diet and exercise, while explicitly teaching parents the means to discuss nutrition without risking their children feeling worse about themselves, increasing the risk for conflict over changes, or heightening their children’s chance of developing eating disorders or maladaptive relationships with food.
But back to the USPSTF’s actual recommendation for those 6 years old and up. They’re recommending “at least” 26 hours of lectures over a year-long interprofessional intervention. Putting aside the reality that this isn’t scalable time-wise or cost-wise to reach even a fraction of the roughly 15 million US children with obesity, there is also the issue of service provision. Because when it comes to obesity, if the intervention is purely educational, even if you want to believe there is a syllabus out there that would have a dramatic impact, its impact will vary wildly depending on the skill and approach of the service providers. This inconvenient truth is also the one that makes it impossible to meaningfully compare program outcomes even when they share the same content.
The USPSTF’s draft recommendations also explicitly avoid what the American Academy of Pediatrics has rightly embraced: the use where appropriate of medications or surgery. While opponents of the use of pharmacotherapy for childhood obesity tend to point to a lack of long-term data as rationale for its denial, something that the USPSTF has done, again, we have long-term data demonstrating a lack of scalable, clinically meaningful efficacy for service only based programs.
Childhood obesity is a flood and its ongoing current is relentless. Given its tremendous impact, especially at its extremes, on both physical and mental health, this is yet another example of systemic weight bias in action — it’s as if the USPSTF is recommending a swimming lesson–only approach while actively fearmongering, despite an absence of plausible mechanistic risk, about the long-term use of life jackets.
Dr. Freedhoff is associate professor, Department of Family Medicine, University of Ottawa; Medical Director, Bariatric Medical Institute, Ottawa, Ontario, Canada. Dr. Freedhoff has disclosed ties with Bariatric Medical Institute, Constant Health, and Novo Nordisk.
A version of this article appeared on Medscape.com.
The US Preventive Services Task Force (USPSTF) has published draft recommendations that 6-year-olds with obesity be lectured to about diet and exercise.
Never mind that there are no reproducible or scalable studies demonstrating durable and clinically meaningful benefits of this for adults let alone children. Never mind that children are not household decision-makers on matters of grocery shopping, cooking, or exercise. Never mind the corollary that many children so lectured who fail to see an impact on their weight will perceive that as their own personal failures. And of course, never mind that we’re privileged to be in an era with safe, effective, pharmacotherapeutic options for obesity. No. We must teach children it’s their fault if they’re fat. Because ultimately that’s what many of them will learn.
That’s not to say there’s no room for counseling. But with children as young as 6, that counseling should be delivered exclusively to their parents and caregivers. That counseling should focus as much if not more so on the impact of weight bias and the biological basis of obesity rather than diet and exercise, while explicitly teaching parents the means to discuss nutrition without risking their children feeling worse about themselves, increasing the risk for conflict over changes, or heightening their children’s chance of developing eating disorders or maladaptive relationships with food.
But back to the USPSTF’s actual recommendation for those 6 years old and up. They’re recommending “at least” 26 hours of lectures over a year-long interprofessional intervention. Putting aside the reality that this isn’t scalable time-wise or cost-wise to reach even a fraction of the roughly 15 million US children with obesity, there is also the issue of service provision. Because when it comes to obesity, if the intervention is purely educational, even if you want to believe there is a syllabus out there that would have a dramatic impact, its impact will vary wildly depending on the skill and approach of the service providers. This inconvenient truth is also the one that makes it impossible to meaningfully compare program outcomes even when they share the same content.
The USPSTF’s draft recommendations also explicitly avoid what the American Academy of Pediatrics has rightly embraced: the use where appropriate of medications or surgery. While opponents of the use of pharmacotherapy for childhood obesity tend to point to a lack of long-term data as rationale for its denial, something that the USPSTF has done, again, we have long-term data demonstrating a lack of scalable, clinically meaningful efficacy for service only based programs.
Childhood obesity is a flood and its ongoing current is relentless. Given its tremendous impact, especially at its extremes, on both physical and mental health, this is yet another example of systemic weight bias in action — it’s as if the USPSTF is recommending a swimming lesson–only approach while actively fearmongering, despite an absence of plausible mechanistic risk, about the long-term use of life jackets.
Dr. Freedhoff is associate professor, Department of Family Medicine, University of Ottawa; Medical Director, Bariatric Medical Institute, Ottawa, Ontario, Canada. Dr. Freedhoff has disclosed ties with Bariatric Medical Institute, Constant Health, and Novo Nordisk.
A version of this article appeared on Medscape.com.
The US Preventive Services Task Force (USPSTF) has published draft recommendations that 6-year-olds with obesity be lectured to about diet and exercise.
Never mind that there are no reproducible or scalable studies demonstrating durable and clinically meaningful benefits of this for adults let alone children. Never mind that children are not household decision-makers on matters of grocery shopping, cooking, or exercise. Never mind the corollary that many children so lectured who fail to see an impact on their weight will perceive that as their own personal failures. And of course, never mind that we’re privileged to be in an era with safe, effective, pharmacotherapeutic options for obesity. No. We must teach children it’s their fault if they’re fat. Because ultimately that’s what many of them will learn.
That’s not to say there’s no room for counseling. But with children as young as 6, that counseling should be delivered exclusively to their parents and caregivers. That counseling should focus as much if not more so on the impact of weight bias and the biological basis of obesity rather than diet and exercise, while explicitly teaching parents the means to discuss nutrition without risking their children feeling worse about themselves, increasing the risk for conflict over changes, or heightening their children’s chance of developing eating disorders or maladaptive relationships with food.
But back to the USPSTF’s actual recommendation for those 6 years old and up. They’re recommending “at least” 26 hours of lectures over a year-long interprofessional intervention. Putting aside the reality that this isn’t scalable time-wise or cost-wise to reach even a fraction of the roughly 15 million US children with obesity, there is also the issue of service provision. Because when it comes to obesity, if the intervention is purely educational, even if you want to believe there is a syllabus out there that would have a dramatic impact, its impact will vary wildly depending on the skill and approach of the service providers. This inconvenient truth is also the one that makes it impossible to meaningfully compare program outcomes even when they share the same content.
The USPSTF’s draft recommendations also explicitly avoid what the American Academy of Pediatrics has rightly embraced: the use where appropriate of medications or surgery. While opponents of the use of pharmacotherapy for childhood obesity tend to point to a lack of long-term data as rationale for its denial, something that the USPSTF has done, again, we have long-term data demonstrating a lack of scalable, clinically meaningful efficacy for service only based programs.
Childhood obesity is a flood and its ongoing current is relentless. Given its tremendous impact, especially at its extremes, on both physical and mental health, this is yet another example of systemic weight bias in action — it’s as if the USPSTF is recommending a swimming lesson–only approach while actively fearmongering, despite an absence of plausible mechanistic risk, about the long-term use of life jackets.
Dr. Freedhoff is associate professor, Department of Family Medicine, University of Ottawa; Medical Director, Bariatric Medical Institute, Ottawa, Ontario, Canada. Dr. Freedhoff has disclosed ties with Bariatric Medical Institute, Constant Health, and Novo Nordisk.
A version of this article appeared on Medscape.com.
Biosimilar Business Deals Keep Up ‘Musical Chairs’ Game of Formulary Construction
As the saying goes, “The more things change, the more they stay the same.” That is particularly true when it comes to the affordability of drugs for our patients even after the launch of so many Humira biosimilars. And we still have the “musical chairs” game of formulary construction — when the music stops, who knows whether your patient’s drug found a chair to sit on. There seems to be only a few chairs available for the many adalimumab biosimilars playing the game.
Nothing has changed since my testimony before the FDA Arthritis Advisory Committee in July 2016 during the approval hearing of the first Humira biosimilar. Below is a quote from that meeting where I was speaking predominantly about the pharmacy side of drugs.
“I’d like to highlight the term ‘access’ because none of us are really naive enough to believe that just approving a biosimilar gives a patient true, hands-on access to the medication, because even if the biosimilar is offered at a 30% discount, I don’t have any patients that can afford it. This means that access is ultimately controlled by third-party payers.”
My prediction, that approving and launching biosimilars with lower prices would not ensure patient access to the drug unless it is paid for by insurance, is now our reality. Today, a drug with an 85% discount on the price of Humira is still unattainable for patients without a “payer.”
Competition and Lower Prices
Lawmakers and some in the media cry for more competition to lower prices. This is the main reason that there has been such a push to get biosimilars to the market as quickly as possible. It is abundantly clear that competition to get on the formulary is fierce. Placement of a medication on a formulary can make or break a manufacturer’s ability to get a return on the R&D and make a profit on that medication. For a small biotech manufacturer, it can be the difference between “life and death” of the company.
Does anyone remember when the first interchangeable biosimilar for the reference insulin glargine product Lantus (insulin glargine-yfgn; Semglee) came to market in 2021? Janet Woodcock, MD, then acting FDA commissioner, called it a “momentous day” and further said, “Today’s approval of the first interchangeable biosimilar product furthers FDA’s longstanding commitment to support a competitive marketplace for biological products and ultimately empowers patients by helping to increase access to safe, effective and high-quality medications at potentially lower cost.” There was a high-priced interchangeable biosimilar and an identical unbranded low-priced interchangeable biosimilar, and the only one that could get formulary placement was the high-priced drug.
Patients pay their cost share on the list price of the drug, and because most pharmacy benefit managers’ (PBMs’) formularies cover only the high-priced biosimilar, patients never share in the savings. So much for the “competitive marketplace” creating lower costs for patients. This is just one of hundreds of examples in which lower-priced drugs are excluded from the formulary. It is unfortunate that the bidding process from manufacturers to PBMs to “win” preferred formulary placement is like an art auction, where the highest bidder wins.
Biosimilars and Formulary Construction
For those of us who have been looking into PBMs for many years, it is no surprise that PBMs’ formulary construction has become a profit center for them. Now, with so many adalimumab biosimilars having entered the market, it has become the Wild West where only those with the most money to fork over to the PBMs get preferred placement. Unfortunately, many of the choices that make money for the PBM cost employers and patients more.
How did we get here? In the 1980s and 90s, the price of medications began to increase to the point that many were not affordable without insurance. And who better to construct the list of drugs that would be covered by insurance (formulary) than the PBMs who were already adjudicating the claims for these drugs. The Federal Trade Commission (FTC) realized the power inherent in constructing this list of medications known as the formulary. So when the manufacturer Merck acquired the PBM Medco in the mid-1990s, the FTC stepped in. The FTC surmised that making the drugs and deciding which ones will be paid for created a “conflict of interest” with anticompetitive ramifications.
So, in 1998, William J. Baer, director of the FTC’s Bureau of Competition, said, “Our investigation into the PBM industry has revealed that Merck’s acquisition of Medco has reduced competition in the market for pharmaceutical products … We have found that Medco has given favorable treatment to Merck drugs. As a result, in some cases, consumers have been denied access to the drugs of competing manufacturers. In addition, the merger has made it possible for Medco to share with Merck sensitive pricing information it gets from Merck’s competitors, which could foster collusion among drug manufacturers.” Wow!
These anticompetitive behaviors and conflicts of interest resulting from the Medco acquisition led the FTC to propose a consent agreement.
The agreement would require Merck-Medco to maintain an “open formulary” — one that includes drugs selected and approved by an independent Pharmacy and Therapeutics Committee regardless of the manufacturer. Medco would have to accept rebates and other price concessions and reflect these in the ranking of the drugs on the formulary. Merck would have to make known the availability of the open formulary to any drug maker with an agreement with Medco.
Let’s hope the FTC of 2024 remembers the stance of the FTC in the 1990s regarding anticompetitive behavior involved in formulary construction.
Conflicts of Interest
But today it is apparent that crafting formularies that pay only for the drugs that make the most money for the PBM is not a conflict of interest. In its policy manual, Cigna directly tells employers and employees that they are collecting and keeping rebates and fees on medical pharmaceuticals, and they are not for the benefit of the employer or the plan.
And now, in August 2023, CVS launched Cordavis, a subsidiary wholly owned by CVS. Cordavis/CVS has partnered with Sandoz, which makes Hyrimoz, an adalimumab biosimilar. There is a high-priced version that is discounted 5% from Humira, a lower-cost unbranded version that is discounted 80% off the list price of Humira, and a co-branded CVS/Sandoz version of Hyrimoz that is lower priced as well.
It isn’t a surprise that CVS’ Standard and Advanced Commercial and Chart formularies are offering only Sandoz adalimumab biosimilar products. While these formularies have excluded Humira, CVS has entered into an agreement with AbbVie to allow Humira on a number of their other formularies. It can be very confusing.
As stated earlier, in the 1990s, the FTC frowned upon manufacturers owning PBMs and allowing them to construct their own formularies. Here we have CVS Health, mothership for the PBM CVS Caremark, owning a company that will be co-producing biosimilars with other manufacturers and then determining which biosimilars are on their formularies. The FTC knew back then that the tendency would be to offer only their own drugs for coverage, thus reducing competition. This is exactly what the CVS-Cordavis-Sandoz partnership has done for their Standard and Advanced Commercial and Chart formularies. It is perhaps anti-competitive but certainly profitable.
Perhaps the FTC should require the same consent agreement that was given to Merck in 1998. CVS Caremark would then have to open their formularies to all competitors of their co-branded, co-produced Sandoz biosimilar.
Summary
It is the same old adage, “The more things change, the more they stay the same.” PBMs are still constructing formularies with biosimilars based on their profitability, with huge differences between gross and net cost. Patients still pay their cost share on the list (gross) price. With the CVS-Cordavis-Sandoz partnership, more vertical integration has led to yet another profit river. Self-funded employers are still getting the wool pulled over their eyes by the big three PBMs who threaten to take away rebates if they don’t choose the preferred formularies. The employers don’t realize that sometimes it is less expensive to choose the lower-priced drugs with no rebates, and that holds true for biosimilars as well.
Let’s hope that the FTC investigates the situation of a PBM partnering with a manufacturer and then choosing only that manufacturer’s drugs for many of their formularies.
We need to continue our advocacy for our patients because the medication that has kept them stable for so long may find itself without a chair the next time the music stops.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s Vice President of Advocacy and Government Affairs and its immediate Past President, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at rhnews@mdedge.com.
As the saying goes, “The more things change, the more they stay the same.” That is particularly true when it comes to the affordability of drugs for our patients even after the launch of so many Humira biosimilars. And we still have the “musical chairs” game of formulary construction — when the music stops, who knows whether your patient’s drug found a chair to sit on. There seems to be only a few chairs available for the many adalimumab biosimilars playing the game.
Nothing has changed since my testimony before the FDA Arthritis Advisory Committee in July 2016 during the approval hearing of the first Humira biosimilar. Below is a quote from that meeting where I was speaking predominantly about the pharmacy side of drugs.
“I’d like to highlight the term ‘access’ because none of us are really naive enough to believe that just approving a biosimilar gives a patient true, hands-on access to the medication, because even if the biosimilar is offered at a 30% discount, I don’t have any patients that can afford it. This means that access is ultimately controlled by third-party payers.”
My prediction, that approving and launching biosimilars with lower prices would not ensure patient access to the drug unless it is paid for by insurance, is now our reality. Today, a drug with an 85% discount on the price of Humira is still unattainable for patients without a “payer.”
Competition and Lower Prices
Lawmakers and some in the media cry for more competition to lower prices. This is the main reason that there has been such a push to get biosimilars to the market as quickly as possible. It is abundantly clear that competition to get on the formulary is fierce. Placement of a medication on a formulary can make or break a manufacturer’s ability to get a return on the R&D and make a profit on that medication. For a small biotech manufacturer, it can be the difference between “life and death” of the company.
Does anyone remember when the first interchangeable biosimilar for the reference insulin glargine product Lantus (insulin glargine-yfgn; Semglee) came to market in 2021? Janet Woodcock, MD, then acting FDA commissioner, called it a “momentous day” and further said, “Today’s approval of the first interchangeable biosimilar product furthers FDA’s longstanding commitment to support a competitive marketplace for biological products and ultimately empowers patients by helping to increase access to safe, effective and high-quality medications at potentially lower cost.” There was a high-priced interchangeable biosimilar and an identical unbranded low-priced interchangeable biosimilar, and the only one that could get formulary placement was the high-priced drug.
Patients pay their cost share on the list price of the drug, and because most pharmacy benefit managers’ (PBMs’) formularies cover only the high-priced biosimilar, patients never share in the savings. So much for the “competitive marketplace” creating lower costs for patients. This is just one of hundreds of examples in which lower-priced drugs are excluded from the formulary. It is unfortunate that the bidding process from manufacturers to PBMs to “win” preferred formulary placement is like an art auction, where the highest bidder wins.
Biosimilars and Formulary Construction
For those of us who have been looking into PBMs for many years, it is no surprise that PBMs’ formulary construction has become a profit center for them. Now, with so many adalimumab biosimilars having entered the market, it has become the Wild West where only those with the most money to fork over to the PBMs get preferred placement. Unfortunately, many of the choices that make money for the PBM cost employers and patients more.
How did we get here? In the 1980s and 90s, the price of medications began to increase to the point that many were not affordable without insurance. And who better to construct the list of drugs that would be covered by insurance (formulary) than the PBMs who were already adjudicating the claims for these drugs. The Federal Trade Commission (FTC) realized the power inherent in constructing this list of medications known as the formulary. So when the manufacturer Merck acquired the PBM Medco in the mid-1990s, the FTC stepped in. The FTC surmised that making the drugs and deciding which ones will be paid for created a “conflict of interest” with anticompetitive ramifications.
So, in 1998, William J. Baer, director of the FTC’s Bureau of Competition, said, “Our investigation into the PBM industry has revealed that Merck’s acquisition of Medco has reduced competition in the market for pharmaceutical products … We have found that Medco has given favorable treatment to Merck drugs. As a result, in some cases, consumers have been denied access to the drugs of competing manufacturers. In addition, the merger has made it possible for Medco to share with Merck sensitive pricing information it gets from Merck’s competitors, which could foster collusion among drug manufacturers.” Wow!
These anticompetitive behaviors and conflicts of interest resulting from the Medco acquisition led the FTC to propose a consent agreement.
The agreement would require Merck-Medco to maintain an “open formulary” — one that includes drugs selected and approved by an independent Pharmacy and Therapeutics Committee regardless of the manufacturer. Medco would have to accept rebates and other price concessions and reflect these in the ranking of the drugs on the formulary. Merck would have to make known the availability of the open formulary to any drug maker with an agreement with Medco.
Let’s hope the FTC of 2024 remembers the stance of the FTC in the 1990s regarding anticompetitive behavior involved in formulary construction.
Conflicts of Interest
But today it is apparent that crafting formularies that pay only for the drugs that make the most money for the PBM is not a conflict of interest. In its policy manual, Cigna directly tells employers and employees that they are collecting and keeping rebates and fees on medical pharmaceuticals, and they are not for the benefit of the employer or the plan.
And now, in August 2023, CVS launched Cordavis, a subsidiary wholly owned by CVS. Cordavis/CVS has partnered with Sandoz, which makes Hyrimoz, an adalimumab biosimilar. There is a high-priced version that is discounted 5% from Humira, a lower-cost unbranded version that is discounted 80% off the list price of Humira, and a co-branded CVS/Sandoz version of Hyrimoz that is lower priced as well.
It isn’t a surprise that CVS’ Standard and Advanced Commercial and Chart formularies are offering only Sandoz adalimumab biosimilar products. While these formularies have excluded Humira, CVS has entered into an agreement with AbbVie to allow Humira on a number of their other formularies. It can be very confusing.
As stated earlier, in the 1990s, the FTC frowned upon manufacturers owning PBMs and allowing them to construct their own formularies. Here we have CVS Health, mothership for the PBM CVS Caremark, owning a company that will be co-producing biosimilars with other manufacturers and then determining which biosimilars are on their formularies. The FTC knew back then that the tendency would be to offer only their own drugs for coverage, thus reducing competition. This is exactly what the CVS-Cordavis-Sandoz partnership has done for their Standard and Advanced Commercial and Chart formularies. It is perhaps anti-competitive but certainly profitable.
Perhaps the FTC should require the same consent agreement that was given to Merck in 1998. CVS Caremark would then have to open their formularies to all competitors of their co-branded, co-produced Sandoz biosimilar.
Summary
It is the same old adage, “The more things change, the more they stay the same.” PBMs are still constructing formularies with biosimilars based on their profitability, with huge differences between gross and net cost. Patients still pay their cost share on the list (gross) price. With the CVS-Cordavis-Sandoz partnership, more vertical integration has led to yet another profit river. Self-funded employers are still getting the wool pulled over their eyes by the big three PBMs who threaten to take away rebates if they don’t choose the preferred formularies. The employers don’t realize that sometimes it is less expensive to choose the lower-priced drugs with no rebates, and that holds true for biosimilars as well.
Let’s hope that the FTC investigates the situation of a PBM partnering with a manufacturer and then choosing only that manufacturer’s drugs for many of their formularies.
We need to continue our advocacy for our patients because the medication that has kept them stable for so long may find itself without a chair the next time the music stops.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s Vice President of Advocacy and Government Affairs and its immediate Past President, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at rhnews@mdedge.com.
As the saying goes, “The more things change, the more they stay the same.” That is particularly true when it comes to the affordability of drugs for our patients even after the launch of so many Humira biosimilars. And we still have the “musical chairs” game of formulary construction — when the music stops, who knows whether your patient’s drug found a chair to sit on. There seems to be only a few chairs available for the many adalimumab biosimilars playing the game.
Nothing has changed since my testimony before the FDA Arthritis Advisory Committee in July 2016 during the approval hearing of the first Humira biosimilar. Below is a quote from that meeting where I was speaking predominantly about the pharmacy side of drugs.
“I’d like to highlight the term ‘access’ because none of us are really naive enough to believe that just approving a biosimilar gives a patient true, hands-on access to the medication, because even if the biosimilar is offered at a 30% discount, I don’t have any patients that can afford it. This means that access is ultimately controlled by third-party payers.”
My prediction, that approving and launching biosimilars with lower prices would not ensure patient access to the drug unless it is paid for by insurance, is now our reality. Today, a drug with an 85% discount on the price of Humira is still unattainable for patients without a “payer.”
Competition and Lower Prices
Lawmakers and some in the media cry for more competition to lower prices. This is the main reason that there has been such a push to get biosimilars to the market as quickly as possible. It is abundantly clear that competition to get on the formulary is fierce. Placement of a medication on a formulary can make or break a manufacturer’s ability to get a return on the R&D and make a profit on that medication. For a small biotech manufacturer, it can be the difference between “life and death” of the company.
Does anyone remember when the first interchangeable biosimilar for the reference insulin glargine product Lantus (insulin glargine-yfgn; Semglee) came to market in 2021? Janet Woodcock, MD, then acting FDA commissioner, called it a “momentous day” and further said, “Today’s approval of the first interchangeable biosimilar product furthers FDA’s longstanding commitment to support a competitive marketplace for biological products and ultimately empowers patients by helping to increase access to safe, effective and high-quality medications at potentially lower cost.” There was a high-priced interchangeable biosimilar and an identical unbranded low-priced interchangeable biosimilar, and the only one that could get formulary placement was the high-priced drug.
Patients pay their cost share on the list price of the drug, and because most pharmacy benefit managers’ (PBMs’) formularies cover only the high-priced biosimilar, patients never share in the savings. So much for the “competitive marketplace” creating lower costs for patients. This is just one of hundreds of examples in which lower-priced drugs are excluded from the formulary. It is unfortunate that the bidding process from manufacturers to PBMs to “win” preferred formulary placement is like an art auction, where the highest bidder wins.
Biosimilars and Formulary Construction
For those of us who have been looking into PBMs for many years, it is no surprise that PBMs’ formulary construction has become a profit center for them. Now, with so many adalimumab biosimilars having entered the market, it has become the Wild West where only those with the most money to fork over to the PBMs get preferred placement. Unfortunately, many of the choices that make money for the PBM cost employers and patients more.
How did we get here? In the 1980s and 90s, the price of medications began to increase to the point that many were not affordable without insurance. And who better to construct the list of drugs that would be covered by insurance (formulary) than the PBMs who were already adjudicating the claims for these drugs. The Federal Trade Commission (FTC) realized the power inherent in constructing this list of medications known as the formulary. So when the manufacturer Merck acquired the PBM Medco in the mid-1990s, the FTC stepped in. The FTC surmised that making the drugs and deciding which ones will be paid for created a “conflict of interest” with anticompetitive ramifications.
So, in 1998, William J. Baer, director of the FTC’s Bureau of Competition, said, “Our investigation into the PBM industry has revealed that Merck’s acquisition of Medco has reduced competition in the market for pharmaceutical products … We have found that Medco has given favorable treatment to Merck drugs. As a result, in some cases, consumers have been denied access to the drugs of competing manufacturers. In addition, the merger has made it possible for Medco to share with Merck sensitive pricing information it gets from Merck’s competitors, which could foster collusion among drug manufacturers.” Wow!
These anticompetitive behaviors and conflicts of interest resulting from the Medco acquisition led the FTC to propose a consent agreement.
The agreement would require Merck-Medco to maintain an “open formulary” — one that includes drugs selected and approved by an independent Pharmacy and Therapeutics Committee regardless of the manufacturer. Medco would have to accept rebates and other price concessions and reflect these in the ranking of the drugs on the formulary. Merck would have to make known the availability of the open formulary to any drug maker with an agreement with Medco.
Let’s hope the FTC of 2024 remembers the stance of the FTC in the 1990s regarding anticompetitive behavior involved in formulary construction.
Conflicts of Interest
But today it is apparent that crafting formularies that pay only for the drugs that make the most money for the PBM is not a conflict of interest. In its policy manual, Cigna directly tells employers and employees that they are collecting and keeping rebates and fees on medical pharmaceuticals, and they are not for the benefit of the employer or the plan.
And now, in August 2023, CVS launched Cordavis, a subsidiary wholly owned by CVS. Cordavis/CVS has partnered with Sandoz, which makes Hyrimoz, an adalimumab biosimilar. There is a high-priced version that is discounted 5% from Humira, a lower-cost unbranded version that is discounted 80% off the list price of Humira, and a co-branded CVS/Sandoz version of Hyrimoz that is lower priced as well.
It isn’t a surprise that CVS’ Standard and Advanced Commercial and Chart formularies are offering only Sandoz adalimumab biosimilar products. While these formularies have excluded Humira, CVS has entered into an agreement with AbbVie to allow Humira on a number of their other formularies. It can be very confusing.
As stated earlier, in the 1990s, the FTC frowned upon manufacturers owning PBMs and allowing them to construct their own formularies. Here we have CVS Health, mothership for the PBM CVS Caremark, owning a company that will be co-producing biosimilars with other manufacturers and then determining which biosimilars are on their formularies. The FTC knew back then that the tendency would be to offer only their own drugs for coverage, thus reducing competition. This is exactly what the CVS-Cordavis-Sandoz partnership has done for their Standard and Advanced Commercial and Chart formularies. It is perhaps anti-competitive but certainly profitable.
Perhaps the FTC should require the same consent agreement that was given to Merck in 1998. CVS Caremark would then have to open their formularies to all competitors of their co-branded, co-produced Sandoz biosimilar.
Summary
It is the same old adage, “The more things change, the more they stay the same.” PBMs are still constructing formularies with biosimilars based on their profitability, with huge differences between gross and net cost. Patients still pay their cost share on the list (gross) price. With the CVS-Cordavis-Sandoz partnership, more vertical integration has led to yet another profit river. Self-funded employers are still getting the wool pulled over their eyes by the big three PBMs who threaten to take away rebates if they don’t choose the preferred formularies. The employers don’t realize that sometimes it is less expensive to choose the lower-priced drugs with no rebates, and that holds true for biosimilars as well.
Let’s hope that the FTC investigates the situation of a PBM partnering with a manufacturer and then choosing only that manufacturer’s drugs for many of their formularies.
We need to continue our advocacy for our patients because the medication that has kept them stable for so long may find itself without a chair the next time the music stops.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s Vice President of Advocacy and Government Affairs and its immediate Past President, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at rhnews@mdedge.com.
Coffee, COVID, and the Universal Antimicrobial
A recent article in Cell & Bioscience suggested that regular coffee consumption can reduce the risk of COVID infections.
The study does make some interesting points about the benefits of coffee’s different polyphenols and antioxidants and their effects on different COVID variants. Most of it is based on lab data, although one section, using serum from coffee versus water drinkers, did find that it was more effective at inhibiting the virions. Caffeinated versus decaffeinated didn’t matter.
I’m not saying coffee doesn’t impair the virus. The data are worth looking at. But the majority of adults in North America, Europe, and pretty much the entire planet drink coffee on a regular basis. A large number of them still caught COVID. Would they have had worse cases if they didn’t drink coffee? Maybe, maybe not.
The problem here is that, as always, preliminary data like this get pushed into mass media, making it sound like “COFFEE CURES COVID!!!” Never mind that that’s not what the article said, but it sure gets clicks and retweets and FaceBook “likes.”
Suddenly fringe groups are claiming the coffee cure was there all along, and hidden from them by the evil government-pharma-medical cartel. Others claim the research is flawed because of this or that. The signal gets drowned out by the noise.
Definitely, food can be a medicine. Look at all the benefits proven of the Mediterranean diet. Coffee may help, especially if we can identify and isolate the specific components that reduce COVID risk. But, as they always say at the end, the study is preliminary and further research is needed.
Once or twice a year, an adult with epilepsy comes in, waving a copy of the ketogenic diet around and upset that I never tried it on them — again proof of the evil government-pharma-medical cartel that I’m in league with. I calm them down and explain the diet in detail. Maybe 50% of them decide to go ahead with it. In 25 years of practice, my record for an otherwise normal adult sticking with it is 5 days.
You don’t have to go too far back to remember Linus Pauling, an absolutely brilliant scientist, but not the best of nutritionists. With two Nobel prizes behind him, he took a stab at medicine in the 1970s, arguing that megadoses of vitamin C worked for the common cold. While it may be good for us, and certainly most people like orange juice, but those claims about the common cold never panned out. In fact, we’re no closer to curing it now than we were then.
It might, but this doesn’t mean the “truth” is being maliciously hidden by an evil cartel. It just means we have (as always) more to learn.
I’ll still drink my single cup of coffee every weekday morning. I’m a creature of habit, and heaven knows I need the caffeine. If it also boosts my immune system, so much the better.
Besides, we still have that universal antimicrobial called chicken soup.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
A recent article in Cell & Bioscience suggested that regular coffee consumption can reduce the risk of COVID infections.
The study does make some interesting points about the benefits of coffee’s different polyphenols and antioxidants and their effects on different COVID variants. Most of it is based on lab data, although one section, using serum from coffee versus water drinkers, did find that it was more effective at inhibiting the virions. Caffeinated versus decaffeinated didn’t matter.
I’m not saying coffee doesn’t impair the virus. The data are worth looking at. But the majority of adults in North America, Europe, and pretty much the entire planet drink coffee on a regular basis. A large number of them still caught COVID. Would they have had worse cases if they didn’t drink coffee? Maybe, maybe not.
The problem here is that, as always, preliminary data like this get pushed into mass media, making it sound like “COFFEE CURES COVID!!!” Never mind that that’s not what the article said, but it sure gets clicks and retweets and FaceBook “likes.”
Suddenly fringe groups are claiming the coffee cure was there all along, and hidden from them by the evil government-pharma-medical cartel. Others claim the research is flawed because of this or that. The signal gets drowned out by the noise.
Definitely, food can be a medicine. Look at all the benefits proven of the Mediterranean diet. Coffee may help, especially if we can identify and isolate the specific components that reduce COVID risk. But, as they always say at the end, the study is preliminary and further research is needed.
Once or twice a year, an adult with epilepsy comes in, waving a copy of the ketogenic diet around and upset that I never tried it on them — again proof of the evil government-pharma-medical cartel that I’m in league with. I calm them down and explain the diet in detail. Maybe 50% of them decide to go ahead with it. In 25 years of practice, my record for an otherwise normal adult sticking with it is 5 days.
You don’t have to go too far back to remember Linus Pauling, an absolutely brilliant scientist, but not the best of nutritionists. With two Nobel prizes behind him, he took a stab at medicine in the 1970s, arguing that megadoses of vitamin C worked for the common cold. While it may be good for us, and certainly most people like orange juice, but those claims about the common cold never panned out. In fact, we’re no closer to curing it now than we were then.
It might, but this doesn’t mean the “truth” is being maliciously hidden by an evil cartel. It just means we have (as always) more to learn.
I’ll still drink my single cup of coffee every weekday morning. I’m a creature of habit, and heaven knows I need the caffeine. If it also boosts my immune system, so much the better.
Besides, we still have that universal antimicrobial called chicken soup.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
A recent article in Cell & Bioscience suggested that regular coffee consumption can reduce the risk of COVID infections.
The study does make some interesting points about the benefits of coffee’s different polyphenols and antioxidants and their effects on different COVID variants. Most of it is based on lab data, although one section, using serum from coffee versus water drinkers, did find that it was more effective at inhibiting the virions. Caffeinated versus decaffeinated didn’t matter.
I’m not saying coffee doesn’t impair the virus. The data are worth looking at. But the majority of adults in North America, Europe, and pretty much the entire planet drink coffee on a regular basis. A large number of them still caught COVID. Would they have had worse cases if they didn’t drink coffee? Maybe, maybe not.
The problem here is that, as always, preliminary data like this get pushed into mass media, making it sound like “COFFEE CURES COVID!!!” Never mind that that’s not what the article said, but it sure gets clicks and retweets and FaceBook “likes.”
Suddenly fringe groups are claiming the coffee cure was there all along, and hidden from them by the evil government-pharma-medical cartel. Others claim the research is flawed because of this or that. The signal gets drowned out by the noise.
Definitely, food can be a medicine. Look at all the benefits proven of the Mediterranean diet. Coffee may help, especially if we can identify and isolate the specific components that reduce COVID risk. But, as they always say at the end, the study is preliminary and further research is needed.
Once or twice a year, an adult with epilepsy comes in, waving a copy of the ketogenic diet around and upset that I never tried it on them — again proof of the evil government-pharma-medical cartel that I’m in league with. I calm them down and explain the diet in detail. Maybe 50% of them decide to go ahead with it. In 25 years of practice, my record for an otherwise normal adult sticking with it is 5 days.
You don’t have to go too far back to remember Linus Pauling, an absolutely brilliant scientist, but not the best of nutritionists. With two Nobel prizes behind him, he took a stab at medicine in the 1970s, arguing that megadoses of vitamin C worked for the common cold. While it may be good for us, and certainly most people like orange juice, but those claims about the common cold never panned out. In fact, we’re no closer to curing it now than we were then.
It might, but this doesn’t mean the “truth” is being maliciously hidden by an evil cartel. It just means we have (as always) more to learn.
I’ll still drink my single cup of coffee every weekday morning. I’m a creature of habit, and heaven knows I need the caffeine. If it also boosts my immune system, so much the better.
Besides, we still have that universal antimicrobial called chicken soup.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Magnesium Sulfate for Fetal Neuroprotection in Preterm Birth
Introduction: The Many Lanes of Research on Magnesium Sulfate
The research that improves human health in the most expedient and most impactful ways is multitiered, with basic or fundamental research, translational research, interventional studies, and retrospective research often occurring simultaneously. There should be no “single lane” of research and one type of research does not preclude the other.
Too often, we fall short in one of these lanes. While we have achieved many moonshots in obstetrics and maternal-fetal medicine, we have tended not to place a high priority on basic research, which can provide a strong understanding of the biology of major diseases and conditions affecting women and their offspring. When conducted with proper commitment and funding, such research can lead to biologically directed therapy.
Within our specialty, research on how we can effectively prevent preterm birth, prematurity, and preeclampsia has taken a long road, with various types of therapies being tried, but none being overwhelmingly effective — with an ongoing need for more basic or fundamental research. Nevertheless, we can benefit and gain great insights from retrospective and interventional studies associated with clinical therapies used to treat premature labor and preeclampsia when these therapies have an unanticipated and important secondary benefit.
This month our Master Class is focused on the neuroprotection of prematurity. Magnesium sulfate is a valuable tool for the treatment of both premature labor and preeclampsia, and more recently, also for neuroprotection of the fetus. Interestingly, this use stemmed from researchers looking retrospectively at outcomes in women who received the compound for other reasons. It took many years for researchers to prove its neuroprotective value through interventional trials, while researchers simultaneously strove to understand on a basic biologic level how magnesium sulfate works to prevent outcomes such as cerebral palsy.
Basic research underway today continues to improve our understanding of its precise mechanisms of action. Combined with other tiers of research — including more interventional studies and more translational research — we can improve its utility for the neuroprotection of prematurity. Alternatively, ongoing research may lead to different, even more effective treatments.
Our guest author is Irina Burd, MD, PhD, Sylvan Freiman, MD Endowed Professor and Chair of the department of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine.* Dr. Burd is also a physician-scientist. She recounts the important story of magnesium sulfate and what is currently known about its biologic plausibility in neuroprotection — including through her own studies – as well as what may be coming in the future.
E. Albert Reece, MD, PhD, MBA, a maternal-fetal medicine specialist, is dean emeritus of the University of Maryland School of Medicine, former university executive vice president; currently the endowed professor and director of the Center for Advanced Research Training and Innovation (CARTI), and senior scientist in the Center for Birth Defects Research. Dr. Reece reported no relevant disclosures. He is the medical editor of this column. Contact him at obnews@mdedge.com.
Magnesium Sulfate for Fetal Neuroprotection in Preterm Birth
Without a doubt, magnesium sulfate (MgSO4) given before anticipated preterm birth reduces the risk of cerebral palsy. It is a valuable tool for fetal neuroprotection at a time when there are no proven alternatives. Yet without the persistent research that occurred over more than 20 years, it may not have won the endorsement of the American College of Obstetrics and Gynecologists in 2010 and worked its way into routine practice.
Its history is worthy of reflection. It took years of observational trials (not all of which showed neuroprotective effects), six randomized controlled trials (none of which met their primary endpoint), three meta-analyses, and a Cochrane Database Systematic Review to arrive at the conclusion that antenatal magnesium sulfate therapy given to women at risk of preterm birth has definitive neuroprotective benefit.
This history also holds lessons for our specialty given the dearth of drugs approved for use in pregnancy and the recent withdrawal from the market of Makena — one of only nine drugs to ever be approved by the Food and Drug Administration for use in pregnancy — after a second trial showed lack of benefit in preventing recurrent preterm birth. The story of MgSO4 tells us it’s acceptable to have major stumbling blocks: At one point, MgSO4 was considered to be not only not helpful, but harmful, causing neonatal death. Further research disproved this initial finding.
Moreover, the MgSO4 story is one that remains unfinished, as my laboratory and other researchers work to better understand its biologic plausibility and to discover additional neuroprotective agents for anticipated preterm birth that may further reduce the risk of cerebral palsy. This leading cause of chronic childhood disability is estimated by the United Cerebral Palsy Foundation to affect approximately 800,000 people in the United States.
Origins and Biologic Plausibility
The MgSO4 story is rooted in the late seventeenth century discovery by physician Nehemiah Grew that the compound was the key component of the then-famous medicinal spring waters in Epsom, England.1 MgSO4 was first used for eclampsia in 1906,2 and was first reported in the American literature for eclampsia in 1925.3 In 1959, its effect as a tocolytic agent was reported.4
More than 30 years later, in 1995, an observational study coauthored by Karin B. Nelson, MD, and Judith K. Grether, PhD of the National Institutes of Health, showed a reduced risk of cerebral palsy in very-low-birth-weight infants (VLBW).5 The report marked a turning point in research interest on neuroprotection for anticipated preterm birth.
The precise molecular mechanisms of action of MgSO4 for neuroprotection are still not well understood. However, research findings from the University of Maryland and other institutions have provided biologic plausibility for its use to prevent cerebral palsy. Our current thinking is that it involves the prevention of periventricular white matter injury and/or the prevention of oxidative stress and a neuronal injury mechanism called excitotoxicity.
Periventricular white matter injury involving injury to preoligodendrocytes before 32 weeks’ gestation is the most prevalent injury seen in cerebral palsy; preoligodendrocytes are precursors of myelinating oligodendrocytes, which constitute a major glial population in the white matter. Our research in a mouse model demonstrated that the intrauterine inflammation frequently associated with preterm birth can lead to neuronal injury as well as white matter damage, and that MgSO4 may ameliorate both.6,7
Excitotoxicity results from excessive stimulation of N-methyl-D-aspartate (NMDA) glutamatergic receptors on preoligodendrocytes and a rush of calcium through the voltage-gated channels. This calcium influx leads to the production of nitric oxide, oxidative stress, and subsequent mitochondrial damage and cell death. As a bivalent ion, MgSO4 sits in the voltage-gated channels of the NMDA receptors and reduces glutamatergic signaling, thus serving as a calcium antagonist and modulating calcium influx (See Figure).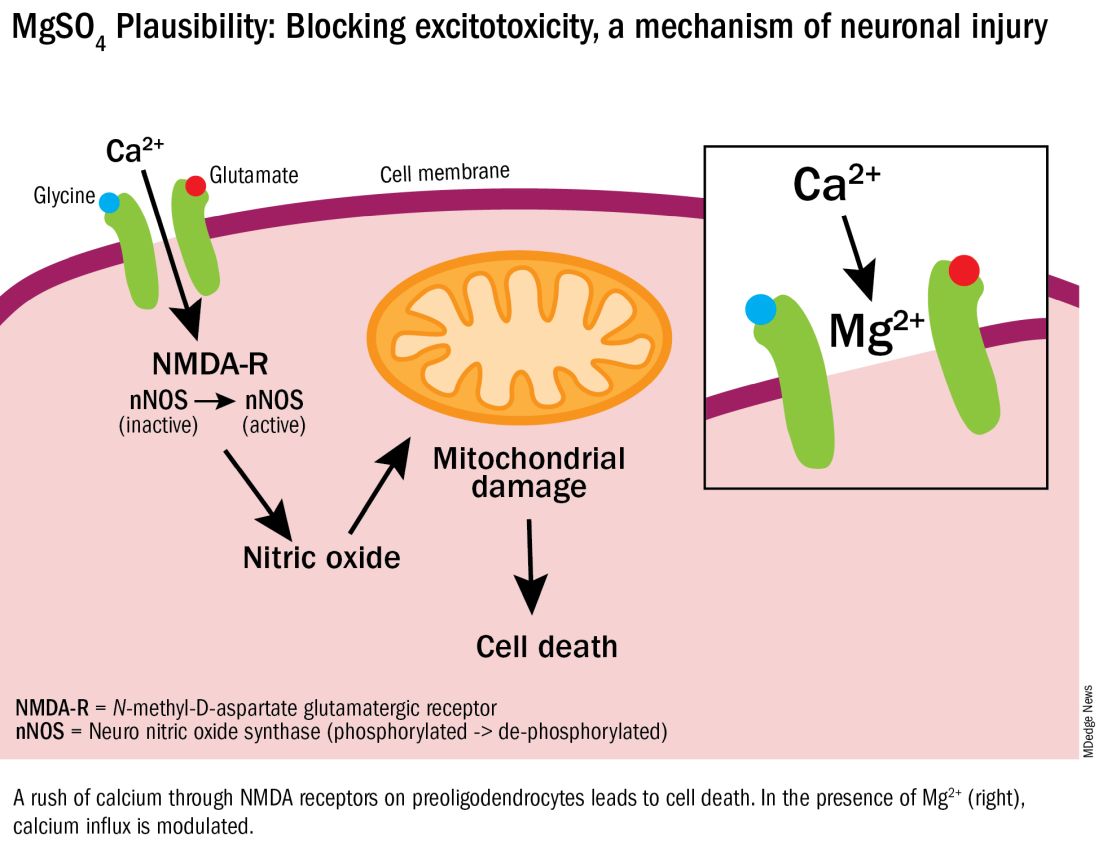
In vitro research in our laboratory has also shown that MgSO4 may dampen inflammatory reactions driven by intrauterine infections, which, like preterm birth, increase the risk of cerebral palsy and adverse neurodevelopmental outcomes.8 MgSO4 appears to do so by blocking the voltage-gated P2X7 receptor in umbilical vein endothelial cells, thus blocking endothelial secretion of the proinflammatory cytokine interleukin (IL)–1beta. Much more research is needed to determine whether MgSO4 could help prevent cerebral palsy through this mechanism.
The Long Route of Research
The 1995 Nelson-Grether study compared VLBW (< 1500 g) infants who survived and developed moderate/severe cerebral palsy within 3 years to randomly selected VLBW controls with respect to whether their mothers had received MgSO4 to prevent seizures in preeclampsia or as a tocolytic agent.5 In a population of more than 155,000 children born between 1983 and 1985, in utero exposure to MgSO4 was reported in 7.1% of 42 VLBW infants with cerebral palsy and 36% of 75 VLBW controls (odds ratio [OR], 0.14; 95% CI, 0.05-0.51). In women without preeclampsia the OR increased to 0.25.
This motivating study had been preceded by several observational studies showing that infants born to women with preeclampsia who received MgSO4 had significantly lower risks of developing intraventricular hemorrhage (IVH) and germinal matrix hemorrhage (GMH). In one of these studies, published in 1992, Karl C. Kuban, MD, and coauthors reported that “maternal receipt of magnesium sulfate was associated with diminished risk of GMH-IVH even in those babies born to mothers who apparently did not have preeclampsia.”9
In the several years following the 1995 Nelson-Grether study, several other case-control/observational studies were reported, with conflicting conclusions, and investigators around the world began designing and conducting needed randomized controlled trials.
The six published randomized controlled trials looking at MgSO4 and neuroprotection varied in their inclusion and exclusion criteria, their recruitment and enrollment style, the gestational ages for MgSO4 administration, loading and maintenance doses, how cerebral palsy or neuroprotection was assessed, and other factors (See Table for RCT characteristics and main outcomes).10-14 One of the trials aimed primarily at evaluating the efficacy of MgSO4 for preventing preeclampsia.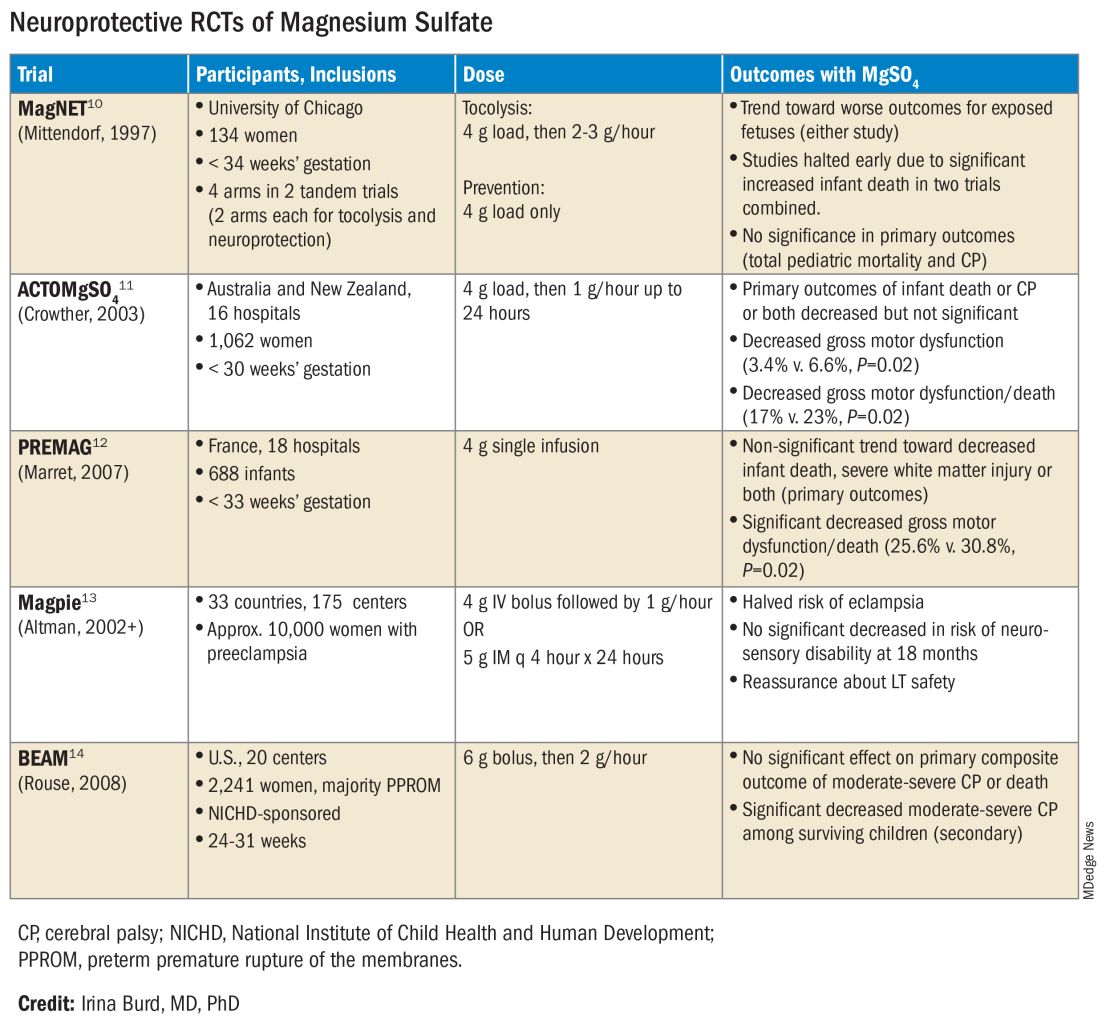
Again, none of the randomized controlled trials demonstrated statistical significance for their primary outcomes or concluded that there was a significant neuroprotective effect for cerebral palsy. Rather, most suggested benefit through secondary analyses. Moreover, as mentioned earlier, research that proceeded after the first published randomized controlled trial — the Magnesium and Neurologic Endpoints (MAGnet) trial — was suspended early when an interim analysis showed a significantly increased risk of mortality in MgSO4-exposed fetuses. All told, it wasn’t until researchers obtained unpublished data and conducted meta-analyses and systematic reviews that a significant effect of MgSO4 on cerebral palsy could be seen.
The three systematic reviews and the Cochrane review, each of which used slightly different methodologies, were published in rapid succession in 2009. One review calculated a relative risk of cerebral palsy of 0.71 (95% CI, 0.55-0.91) — and a relative risk for the combined outcome of death and cerebral palsy at 0.85 (95% CI, 0.74-0.98) — when women at risk of preterm birth were given MgSO4.15 The number needed to treat (NNT) to prevent one case of cerebral palsy was 63, investigators determined, and the NNT to prevent one case of cerebral palsy or infant death was 44.
Another review estimated the NNT for prevention of one case of cerebral palsy at 52 when MgSO4 is given at less than 34 weeks’ gestation, and similarly concluded that MgSO4 is associated with a significantly “reduced risk of moderate/severe CP and substantial gross motor dysfunction without any statistically significant effect on the risk of total pediatric mortality.”16
A third review, from the National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units Network (MFMU), estimated an NNT of 46 to prevent one case of cerebral palsy in infants exposed to MgSO4 before 30 weeks, and an NNT of 56 when exposure occurs before 32-34 weeks.17
The Cochrane Review, meanwhile, reported a relative reduction in the risk of cerebral palsy of 0.68 (95% CI, 0.54-0.87) when antenatal MgSO4 is given at less than 37 weeks’ gestation, as well as a significant reduction in the rate of substantial gross motor dysfunction (RR, 0.61; 95% CI, 0.44-0.85).18 The NNT to avoid one case of cerebral palsy, researchers reported, was 63.
Moving Forward
The NNTs calculated in these reviews — ranging from 44 to 63 — are convincing, and are comparable with evidence-based medicine data for prevention of other common diseases.19 For instance, the NNT for a life saved when aspirin is given immediately after a heart attack is 42. Statins given for 5 years in people with known heart disease have an NNT of 83 to save one life, an NNT of 39 to prevent one nonfatal heart attack, and an NNT of 125 to prevent one stroke. For oral anticoagulants used in nonvalvular atrial fibrillation for primary stroke prevention, the NNTs to prevent one stroke, and one death, are 22 and 42, respectively.19
In its 2010 Committee Opinion on Magnesium Sulfate Before Anticipated Preterm Birth for Neuroprotection (reaffirmed in 2020), the American College of Obstetricians and Gynecologists left it to institutions to develop their own guidelines “regarding inclusion criteria, treatment regimens, concurrent tocolysis, and monitoring in accordance with one of the larger trials.”20
Not surprisingly, most if not all hospitals have chosen a higher dose of MgSO4 administered up to 31 weeks’ gestation in keeping with the protocols employed in the NICHD-sponsored BEAM trial (See Table).
The hope moving forward is to expand treatment options for neuroprotection in cases of imminent preterm birth. Researchers have been assessing the ability of melatonin to provide neuroprotection in cases of growth restriction and neonatal asphyxia. Melatonin has anti-inflammatory and antioxidant properties and is known to mediate neuronal generation and synaptic plasticity.21
N-acetyl-L-cysteine is another potential neuroprotective agent. It acts as an antioxidant, a precursor to glutathione, and a modulator of the glutamate system and has been studied as a neuroprotective agent in cases of maternal chorioamnionitis.21 Both melatonin and N-acetyl-L-cysteine are regarded as safe in pregnancy, but much more clinical study is needed to prove their neuroprotective potential when given shortly before birth or earlier.
Dr. Burd is the Sylvan Freiman, MD Endowed Professor and Chair of the department of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, Baltimore. She has no conflicts of interest.
References
1. Clio Med. 1984;19(1-2):1-21.
2. Medicinsk Rev. (Bergen) 1906;32:264-272.
3. Am J Obstet Gynecol. 1996;174(4):1390-1391.
4. Am J Obstet Gynecol. 1959;78(1):27-32.
5. Pediatrics. 1995;95(2):263-269.
6. Am J Obstet Gynecol. 2009;201(3):279.e1-279.e8.
7. Am J Obstet Gynecol. 2010;202(3):292.e1-292.e9.
8. Pediatr Res. 2020;87(3):463-471.
9. J Child Neurol. 1992;7(1):70-76.
10. Lancet. 1997;350:1517-1518.
11. JAMA. 2003;290:2669-2676.
12. BJOG. 2007;114(3):310-318.
13. Lancet. 2002;359(9321):1877-1890.
14. N Engl J Med. 2008;359:895-905.
15. Obstet Gynecol. 2009;113(6):1327-1333.
16. Am J Obstet Gynecol. 2009;200(6):595-609.
17. Obstet Gynecol 2009;114:354-364.
18. Cochrane Database Syst Rev. 2009 Jan 21:(1):CD004661.
19. www.thennt.com.
20. Obstet Gynecol. 2010;115:669-671.
21. Front Synaptic Neurosci. 2012;13:680899.
*This story was corrected on June 10, 2024.
Introduction: The Many Lanes of Research on Magnesium Sulfate
The research that improves human health in the most expedient and most impactful ways is multitiered, with basic or fundamental research, translational research, interventional studies, and retrospective research often occurring simultaneously. There should be no “single lane” of research and one type of research does not preclude the other.
Too often, we fall short in one of these lanes. While we have achieved many moonshots in obstetrics and maternal-fetal medicine, we have tended not to place a high priority on basic research, which can provide a strong understanding of the biology of major diseases and conditions affecting women and their offspring. When conducted with proper commitment and funding, such research can lead to biologically directed therapy.
Within our specialty, research on how we can effectively prevent preterm birth, prematurity, and preeclampsia has taken a long road, with various types of therapies being tried, but none being overwhelmingly effective — with an ongoing need for more basic or fundamental research. Nevertheless, we can benefit and gain great insights from retrospective and interventional studies associated with clinical therapies used to treat premature labor and preeclampsia when these therapies have an unanticipated and important secondary benefit.
This month our Master Class is focused on the neuroprotection of prematurity. Magnesium sulfate is a valuable tool for the treatment of both premature labor and preeclampsia, and more recently, also for neuroprotection of the fetus. Interestingly, this use stemmed from researchers looking retrospectively at outcomes in women who received the compound for other reasons. It took many years for researchers to prove its neuroprotective value through interventional trials, while researchers simultaneously strove to understand on a basic biologic level how magnesium sulfate works to prevent outcomes such as cerebral palsy.
Basic research underway today continues to improve our understanding of its precise mechanisms of action. Combined with other tiers of research — including more interventional studies and more translational research — we can improve its utility for the neuroprotection of prematurity. Alternatively, ongoing research may lead to different, even more effective treatments.
Our guest author is Irina Burd, MD, PhD, Sylvan Freiman, MD Endowed Professor and Chair of the department of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine.* Dr. Burd is also a physician-scientist. She recounts the important story of magnesium sulfate and what is currently known about its biologic plausibility in neuroprotection — including through her own studies – as well as what may be coming in the future.
E. Albert Reece, MD, PhD, MBA, a maternal-fetal medicine specialist, is dean emeritus of the University of Maryland School of Medicine, former university executive vice president; currently the endowed professor and director of the Center for Advanced Research Training and Innovation (CARTI), and senior scientist in the Center for Birth Defects Research. Dr. Reece reported no relevant disclosures. He is the medical editor of this column. Contact him at obnews@mdedge.com.
Magnesium Sulfate for Fetal Neuroprotection in Preterm Birth
Without a doubt, magnesium sulfate (MgSO4) given before anticipated preterm birth reduces the risk of cerebral palsy. It is a valuable tool for fetal neuroprotection at a time when there are no proven alternatives. Yet without the persistent research that occurred over more than 20 years, it may not have won the endorsement of the American College of Obstetrics and Gynecologists in 2010 and worked its way into routine practice.
Its history is worthy of reflection. It took years of observational trials (not all of which showed neuroprotective effects), six randomized controlled trials (none of which met their primary endpoint), three meta-analyses, and a Cochrane Database Systematic Review to arrive at the conclusion that antenatal magnesium sulfate therapy given to women at risk of preterm birth has definitive neuroprotective benefit.
This history also holds lessons for our specialty given the dearth of drugs approved for use in pregnancy and the recent withdrawal from the market of Makena — one of only nine drugs to ever be approved by the Food and Drug Administration for use in pregnancy — after a second trial showed lack of benefit in preventing recurrent preterm birth. The story of MgSO4 tells us it’s acceptable to have major stumbling blocks: At one point, MgSO4 was considered to be not only not helpful, but harmful, causing neonatal death. Further research disproved this initial finding.
Moreover, the MgSO4 story is one that remains unfinished, as my laboratory and other researchers work to better understand its biologic plausibility and to discover additional neuroprotective agents for anticipated preterm birth that may further reduce the risk of cerebral palsy. This leading cause of chronic childhood disability is estimated by the United Cerebral Palsy Foundation to affect approximately 800,000 people in the United States.
Origins and Biologic Plausibility
The MgSO4 story is rooted in the late seventeenth century discovery by physician Nehemiah Grew that the compound was the key component of the then-famous medicinal spring waters in Epsom, England.1 MgSO4 was first used for eclampsia in 1906,2 and was first reported in the American literature for eclampsia in 1925.3 In 1959, its effect as a tocolytic agent was reported.4
More than 30 years later, in 1995, an observational study coauthored by Karin B. Nelson, MD, and Judith K. Grether, PhD of the National Institutes of Health, showed a reduced risk of cerebral palsy in very-low-birth-weight infants (VLBW).5 The report marked a turning point in research interest on neuroprotection for anticipated preterm birth.
The precise molecular mechanisms of action of MgSO4 for neuroprotection are still not well understood. However, research findings from the University of Maryland and other institutions have provided biologic plausibility for its use to prevent cerebral palsy. Our current thinking is that it involves the prevention of periventricular white matter injury and/or the prevention of oxidative stress and a neuronal injury mechanism called excitotoxicity.
Periventricular white matter injury involving injury to preoligodendrocytes before 32 weeks’ gestation is the most prevalent injury seen in cerebral palsy; preoligodendrocytes are precursors of myelinating oligodendrocytes, which constitute a major glial population in the white matter. Our research in a mouse model demonstrated that the intrauterine inflammation frequently associated with preterm birth can lead to neuronal injury as well as white matter damage, and that MgSO4 may ameliorate both.6,7
Excitotoxicity results from excessive stimulation of N-methyl-D-aspartate (NMDA) glutamatergic receptors on preoligodendrocytes and a rush of calcium through the voltage-gated channels. This calcium influx leads to the production of nitric oxide, oxidative stress, and subsequent mitochondrial damage and cell death. As a bivalent ion, MgSO4 sits in the voltage-gated channels of the NMDA receptors and reduces glutamatergic signaling, thus serving as a calcium antagonist and modulating calcium influx (See Figure).
In vitro research in our laboratory has also shown that MgSO4 may dampen inflammatory reactions driven by intrauterine infections, which, like preterm birth, increase the risk of cerebral palsy and adverse neurodevelopmental outcomes.8 MgSO4 appears to do so by blocking the voltage-gated P2X7 receptor in umbilical vein endothelial cells, thus blocking endothelial secretion of the proinflammatory cytokine interleukin (IL)–1beta. Much more research is needed to determine whether MgSO4 could help prevent cerebral palsy through this mechanism.
The Long Route of Research
The 1995 Nelson-Grether study compared VLBW (< 1500 g) infants who survived and developed moderate/severe cerebral palsy within 3 years to randomly selected VLBW controls with respect to whether their mothers had received MgSO4 to prevent seizures in preeclampsia or as a tocolytic agent.5 In a population of more than 155,000 children born between 1983 and 1985, in utero exposure to MgSO4 was reported in 7.1% of 42 VLBW infants with cerebral palsy and 36% of 75 VLBW controls (odds ratio [OR], 0.14; 95% CI, 0.05-0.51). In women without preeclampsia the OR increased to 0.25.
This motivating study had been preceded by several observational studies showing that infants born to women with preeclampsia who received MgSO4 had significantly lower risks of developing intraventricular hemorrhage (IVH) and germinal matrix hemorrhage (GMH). In one of these studies, published in 1992, Karl C. Kuban, MD, and coauthors reported that “maternal receipt of magnesium sulfate was associated with diminished risk of GMH-IVH even in those babies born to mothers who apparently did not have preeclampsia.”9
In the several years following the 1995 Nelson-Grether study, several other case-control/observational studies were reported, with conflicting conclusions, and investigators around the world began designing and conducting needed randomized controlled trials.
The six published randomized controlled trials looking at MgSO4 and neuroprotection varied in their inclusion and exclusion criteria, their recruitment and enrollment style, the gestational ages for MgSO4 administration, loading and maintenance doses, how cerebral palsy or neuroprotection was assessed, and other factors (See Table for RCT characteristics and main outcomes).10-14 One of the trials aimed primarily at evaluating the efficacy of MgSO4 for preventing preeclampsia.
Again, none of the randomized controlled trials demonstrated statistical significance for their primary outcomes or concluded that there was a significant neuroprotective effect for cerebral palsy. Rather, most suggested benefit through secondary analyses. Moreover, as mentioned earlier, research that proceeded after the first published randomized controlled trial — the Magnesium and Neurologic Endpoints (MAGnet) trial — was suspended early when an interim analysis showed a significantly increased risk of mortality in MgSO4-exposed fetuses. All told, it wasn’t until researchers obtained unpublished data and conducted meta-analyses and systematic reviews that a significant effect of MgSO4 on cerebral palsy could be seen.
The three systematic reviews and the Cochrane review, each of which used slightly different methodologies, were published in rapid succession in 2009. One review calculated a relative risk of cerebral palsy of 0.71 (95% CI, 0.55-0.91) — and a relative risk for the combined outcome of death and cerebral palsy at 0.85 (95% CI, 0.74-0.98) — when women at risk of preterm birth were given MgSO4.15 The number needed to treat (NNT) to prevent one case of cerebral palsy was 63, investigators determined, and the NNT to prevent one case of cerebral palsy or infant death was 44.
Another review estimated the NNT for prevention of one case of cerebral palsy at 52 when MgSO4 is given at less than 34 weeks’ gestation, and similarly concluded that MgSO4 is associated with a significantly “reduced risk of moderate/severe CP and substantial gross motor dysfunction without any statistically significant effect on the risk of total pediatric mortality.”16
A third review, from the National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units Network (MFMU), estimated an NNT of 46 to prevent one case of cerebral palsy in infants exposed to MgSO4 before 30 weeks, and an NNT of 56 when exposure occurs before 32-34 weeks.17
The Cochrane Review, meanwhile, reported a relative reduction in the risk of cerebral palsy of 0.68 (95% CI, 0.54-0.87) when antenatal MgSO4 is given at less than 37 weeks’ gestation, as well as a significant reduction in the rate of substantial gross motor dysfunction (RR, 0.61; 95% CI, 0.44-0.85).18 The NNT to avoid one case of cerebral palsy, researchers reported, was 63.
Moving Forward
The NNTs calculated in these reviews — ranging from 44 to 63 — are convincing, and are comparable with evidence-based medicine data for prevention of other common diseases.19 For instance, the NNT for a life saved when aspirin is given immediately after a heart attack is 42. Statins given for 5 years in people with known heart disease have an NNT of 83 to save one life, an NNT of 39 to prevent one nonfatal heart attack, and an NNT of 125 to prevent one stroke. For oral anticoagulants used in nonvalvular atrial fibrillation for primary stroke prevention, the NNTs to prevent one stroke, and one death, are 22 and 42, respectively.19
In its 2010 Committee Opinion on Magnesium Sulfate Before Anticipated Preterm Birth for Neuroprotection (reaffirmed in 2020), the American College of Obstetricians and Gynecologists left it to institutions to develop their own guidelines “regarding inclusion criteria, treatment regimens, concurrent tocolysis, and monitoring in accordance with one of the larger trials.”20
Not surprisingly, most if not all hospitals have chosen a higher dose of MgSO4 administered up to 31 weeks’ gestation in keeping with the protocols employed in the NICHD-sponsored BEAM trial (See Table).
The hope moving forward is to expand treatment options for neuroprotection in cases of imminent preterm birth. Researchers have been assessing the ability of melatonin to provide neuroprotection in cases of growth restriction and neonatal asphyxia. Melatonin has anti-inflammatory and antioxidant properties and is known to mediate neuronal generation and synaptic plasticity.21
N-acetyl-L-cysteine is another potential neuroprotective agent. It acts as an antioxidant, a precursor to glutathione, and a modulator of the glutamate system and has been studied as a neuroprotective agent in cases of maternal chorioamnionitis.21 Both melatonin and N-acetyl-L-cysteine are regarded as safe in pregnancy, but much more clinical study is needed to prove their neuroprotective potential when given shortly before birth or earlier.
Dr. Burd is the Sylvan Freiman, MD Endowed Professor and Chair of the department of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, Baltimore. She has no conflicts of interest.
References
1. Clio Med. 1984;19(1-2):1-21.
2. Medicinsk Rev. (Bergen) 1906;32:264-272.
3. Am J Obstet Gynecol. 1996;174(4):1390-1391.
4. Am J Obstet Gynecol. 1959;78(1):27-32.
5. Pediatrics. 1995;95(2):263-269.
6. Am J Obstet Gynecol. 2009;201(3):279.e1-279.e8.
7. Am J Obstet Gynecol. 2010;202(3):292.e1-292.e9.
8. Pediatr Res. 2020;87(3):463-471.
9. J Child Neurol. 1992;7(1):70-76.
10. Lancet. 1997;350:1517-1518.
11. JAMA. 2003;290:2669-2676.
12. BJOG. 2007;114(3):310-318.
13. Lancet. 2002;359(9321):1877-1890.
14. N Engl J Med. 2008;359:895-905.
15. Obstet Gynecol. 2009;113(6):1327-1333.
16. Am J Obstet Gynecol. 2009;200(6):595-609.
17. Obstet Gynecol 2009;114:354-364.
18. Cochrane Database Syst Rev. 2009 Jan 21:(1):CD004661.
19. www.thennt.com.
20. Obstet Gynecol. 2010;115:669-671.
21. Front Synaptic Neurosci. 2012;13:680899.
*This story was corrected on June 10, 2024.
Introduction: The Many Lanes of Research on Magnesium Sulfate
The research that improves human health in the most expedient and most impactful ways is multitiered, with basic or fundamental research, translational research, interventional studies, and retrospective research often occurring simultaneously. There should be no “single lane” of research and one type of research does not preclude the other.
Too often, we fall short in one of these lanes. While we have achieved many moonshots in obstetrics and maternal-fetal medicine, we have tended not to place a high priority on basic research, which can provide a strong understanding of the biology of major diseases and conditions affecting women and their offspring. When conducted with proper commitment and funding, such research can lead to biologically directed therapy.
Within our specialty, research on how we can effectively prevent preterm birth, prematurity, and preeclampsia has taken a long road, with various types of therapies being tried, but none being overwhelmingly effective — with an ongoing need for more basic or fundamental research. Nevertheless, we can benefit and gain great insights from retrospective and interventional studies associated with clinical therapies used to treat premature labor and preeclampsia when these therapies have an unanticipated and important secondary benefit.
This month our Master Class is focused on the neuroprotection of prematurity. Magnesium sulfate is a valuable tool for the treatment of both premature labor and preeclampsia, and more recently, also for neuroprotection of the fetus. Interestingly, this use stemmed from researchers looking retrospectively at outcomes in women who received the compound for other reasons. It took many years for researchers to prove its neuroprotective value through interventional trials, while researchers simultaneously strove to understand on a basic biologic level how magnesium sulfate works to prevent outcomes such as cerebral palsy.
Basic research underway today continues to improve our understanding of its precise mechanisms of action. Combined with other tiers of research — including more interventional studies and more translational research — we can improve its utility for the neuroprotection of prematurity. Alternatively, ongoing research may lead to different, even more effective treatments.
Our guest author is Irina Burd, MD, PhD, Sylvan Freiman, MD Endowed Professor and Chair of the department of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine.* Dr. Burd is also a physician-scientist. She recounts the important story of magnesium sulfate and what is currently known about its biologic plausibility in neuroprotection — including through her own studies – as well as what may be coming in the future.
E. Albert Reece, MD, PhD, MBA, a maternal-fetal medicine specialist, is dean emeritus of the University of Maryland School of Medicine, former university executive vice president; currently the endowed professor and director of the Center for Advanced Research Training and Innovation (CARTI), and senior scientist in the Center for Birth Defects Research. Dr. Reece reported no relevant disclosures. He is the medical editor of this column. Contact him at obnews@mdedge.com.
Magnesium Sulfate for Fetal Neuroprotection in Preterm Birth
Without a doubt, magnesium sulfate (MgSO4) given before anticipated preterm birth reduces the risk of cerebral palsy. It is a valuable tool for fetal neuroprotection at a time when there are no proven alternatives. Yet without the persistent research that occurred over more than 20 years, it may not have won the endorsement of the American College of Obstetrics and Gynecologists in 2010 and worked its way into routine practice.
Its history is worthy of reflection. It took years of observational trials (not all of which showed neuroprotective effects), six randomized controlled trials (none of which met their primary endpoint), three meta-analyses, and a Cochrane Database Systematic Review to arrive at the conclusion that antenatal magnesium sulfate therapy given to women at risk of preterm birth has definitive neuroprotective benefit.
This history also holds lessons for our specialty given the dearth of drugs approved for use in pregnancy and the recent withdrawal from the market of Makena — one of only nine drugs to ever be approved by the Food and Drug Administration for use in pregnancy — after a second trial showed lack of benefit in preventing recurrent preterm birth. The story of MgSO4 tells us it’s acceptable to have major stumbling blocks: At one point, MgSO4 was considered to be not only not helpful, but harmful, causing neonatal death. Further research disproved this initial finding.
Moreover, the MgSO4 story is one that remains unfinished, as my laboratory and other researchers work to better understand its biologic plausibility and to discover additional neuroprotective agents for anticipated preterm birth that may further reduce the risk of cerebral palsy. This leading cause of chronic childhood disability is estimated by the United Cerebral Palsy Foundation to affect approximately 800,000 people in the United States.
Origins and Biologic Plausibility
The MgSO4 story is rooted in the late seventeenth century discovery by physician Nehemiah Grew that the compound was the key component of the then-famous medicinal spring waters in Epsom, England.1 MgSO4 was first used for eclampsia in 1906,2 and was first reported in the American literature for eclampsia in 1925.3 In 1959, its effect as a tocolytic agent was reported.4
More than 30 years later, in 1995, an observational study coauthored by Karin B. Nelson, MD, and Judith K. Grether, PhD of the National Institutes of Health, showed a reduced risk of cerebral palsy in very-low-birth-weight infants (VLBW).5 The report marked a turning point in research interest on neuroprotection for anticipated preterm birth.
The precise molecular mechanisms of action of MgSO4 for neuroprotection are still not well understood. However, research findings from the University of Maryland and other institutions have provided biologic plausibility for its use to prevent cerebral palsy. Our current thinking is that it involves the prevention of periventricular white matter injury and/or the prevention of oxidative stress and a neuronal injury mechanism called excitotoxicity.
Periventricular white matter injury involving injury to preoligodendrocytes before 32 weeks’ gestation is the most prevalent injury seen in cerebral palsy; preoligodendrocytes are precursors of myelinating oligodendrocytes, which constitute a major glial population in the white matter. Our research in a mouse model demonstrated that the intrauterine inflammation frequently associated with preterm birth can lead to neuronal injury as well as white matter damage, and that MgSO4 may ameliorate both.6,7
Excitotoxicity results from excessive stimulation of N-methyl-D-aspartate (NMDA) glutamatergic receptors on preoligodendrocytes and a rush of calcium through the voltage-gated channels. This calcium influx leads to the production of nitric oxide, oxidative stress, and subsequent mitochondrial damage and cell death. As a bivalent ion, MgSO4 sits in the voltage-gated channels of the NMDA receptors and reduces glutamatergic signaling, thus serving as a calcium antagonist and modulating calcium influx (See Figure).
In vitro research in our laboratory has also shown that MgSO4 may dampen inflammatory reactions driven by intrauterine infections, which, like preterm birth, increase the risk of cerebral palsy and adverse neurodevelopmental outcomes.8 MgSO4 appears to do so by blocking the voltage-gated P2X7 receptor in umbilical vein endothelial cells, thus blocking endothelial secretion of the proinflammatory cytokine interleukin (IL)–1beta. Much more research is needed to determine whether MgSO4 could help prevent cerebral palsy through this mechanism.
The Long Route of Research
The 1995 Nelson-Grether study compared VLBW (< 1500 g) infants who survived and developed moderate/severe cerebral palsy within 3 years to randomly selected VLBW controls with respect to whether their mothers had received MgSO4 to prevent seizures in preeclampsia or as a tocolytic agent.5 In a population of more than 155,000 children born between 1983 and 1985, in utero exposure to MgSO4 was reported in 7.1% of 42 VLBW infants with cerebral palsy and 36% of 75 VLBW controls (odds ratio [OR], 0.14; 95% CI, 0.05-0.51). In women without preeclampsia the OR increased to 0.25.
This motivating study had been preceded by several observational studies showing that infants born to women with preeclampsia who received MgSO4 had significantly lower risks of developing intraventricular hemorrhage (IVH) and germinal matrix hemorrhage (GMH). In one of these studies, published in 1992, Karl C. Kuban, MD, and coauthors reported that “maternal receipt of magnesium sulfate was associated with diminished risk of GMH-IVH even in those babies born to mothers who apparently did not have preeclampsia.”9
In the several years following the 1995 Nelson-Grether study, several other case-control/observational studies were reported, with conflicting conclusions, and investigators around the world began designing and conducting needed randomized controlled trials.
The six published randomized controlled trials looking at MgSO4 and neuroprotection varied in their inclusion and exclusion criteria, their recruitment and enrollment style, the gestational ages for MgSO4 administration, loading and maintenance doses, how cerebral palsy or neuroprotection was assessed, and other factors (See Table for RCT characteristics and main outcomes).10-14 One of the trials aimed primarily at evaluating the efficacy of MgSO4 for preventing preeclampsia.
Again, none of the randomized controlled trials demonstrated statistical significance for their primary outcomes or concluded that there was a significant neuroprotective effect for cerebral palsy. Rather, most suggested benefit through secondary analyses. Moreover, as mentioned earlier, research that proceeded after the first published randomized controlled trial — the Magnesium and Neurologic Endpoints (MAGnet) trial — was suspended early when an interim analysis showed a significantly increased risk of mortality in MgSO4-exposed fetuses. All told, it wasn’t until researchers obtained unpublished data and conducted meta-analyses and systematic reviews that a significant effect of MgSO4 on cerebral palsy could be seen.
The three systematic reviews and the Cochrane review, each of which used slightly different methodologies, were published in rapid succession in 2009. One review calculated a relative risk of cerebral palsy of 0.71 (95% CI, 0.55-0.91) — and a relative risk for the combined outcome of death and cerebral palsy at 0.85 (95% CI, 0.74-0.98) — when women at risk of preterm birth were given MgSO4.15 The number needed to treat (NNT) to prevent one case of cerebral palsy was 63, investigators determined, and the NNT to prevent one case of cerebral palsy or infant death was 44.
Another review estimated the NNT for prevention of one case of cerebral palsy at 52 when MgSO4 is given at less than 34 weeks’ gestation, and similarly concluded that MgSO4 is associated with a significantly “reduced risk of moderate/severe CP and substantial gross motor dysfunction without any statistically significant effect on the risk of total pediatric mortality.”16
A third review, from the National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units Network (MFMU), estimated an NNT of 46 to prevent one case of cerebral palsy in infants exposed to MgSO4 before 30 weeks, and an NNT of 56 when exposure occurs before 32-34 weeks.17
The Cochrane Review, meanwhile, reported a relative reduction in the risk of cerebral palsy of 0.68 (95% CI, 0.54-0.87) when antenatal MgSO4 is given at less than 37 weeks’ gestation, as well as a significant reduction in the rate of substantial gross motor dysfunction (RR, 0.61; 95% CI, 0.44-0.85).18 The NNT to avoid one case of cerebral palsy, researchers reported, was 63.
Moving Forward
The NNTs calculated in these reviews — ranging from 44 to 63 — are convincing, and are comparable with evidence-based medicine data for prevention of other common diseases.19 For instance, the NNT for a life saved when aspirin is given immediately after a heart attack is 42. Statins given for 5 years in people with known heart disease have an NNT of 83 to save one life, an NNT of 39 to prevent one nonfatal heart attack, and an NNT of 125 to prevent one stroke. For oral anticoagulants used in nonvalvular atrial fibrillation for primary stroke prevention, the NNTs to prevent one stroke, and one death, are 22 and 42, respectively.19
In its 2010 Committee Opinion on Magnesium Sulfate Before Anticipated Preterm Birth for Neuroprotection (reaffirmed in 2020), the American College of Obstetricians and Gynecologists left it to institutions to develop their own guidelines “regarding inclusion criteria, treatment regimens, concurrent tocolysis, and monitoring in accordance with one of the larger trials.”20
Not surprisingly, most if not all hospitals have chosen a higher dose of MgSO4 administered up to 31 weeks’ gestation in keeping with the protocols employed in the NICHD-sponsored BEAM trial (See Table).
The hope moving forward is to expand treatment options for neuroprotection in cases of imminent preterm birth. Researchers have been assessing the ability of melatonin to provide neuroprotection in cases of growth restriction and neonatal asphyxia. Melatonin has anti-inflammatory and antioxidant properties and is known to mediate neuronal generation and synaptic plasticity.21
N-acetyl-L-cysteine is another potential neuroprotective agent. It acts as an antioxidant, a precursor to glutathione, and a modulator of the glutamate system and has been studied as a neuroprotective agent in cases of maternal chorioamnionitis.21 Both melatonin and N-acetyl-L-cysteine are regarded as safe in pregnancy, but much more clinical study is needed to prove their neuroprotective potential when given shortly before birth or earlier.
Dr. Burd is the Sylvan Freiman, MD Endowed Professor and Chair of the department of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, Baltimore. She has no conflicts of interest.
References
1. Clio Med. 1984;19(1-2):1-21.
2. Medicinsk Rev. (Bergen) 1906;32:264-272.
3. Am J Obstet Gynecol. 1996;174(4):1390-1391.
4. Am J Obstet Gynecol. 1959;78(1):27-32.
5. Pediatrics. 1995;95(2):263-269.
6. Am J Obstet Gynecol. 2009;201(3):279.e1-279.e8.
7. Am J Obstet Gynecol. 2010;202(3):292.e1-292.e9.
8. Pediatr Res. 2020;87(3):463-471.
9. J Child Neurol. 1992;7(1):70-76.
10. Lancet. 1997;350:1517-1518.
11. JAMA. 2003;290:2669-2676.
12. BJOG. 2007;114(3):310-318.
13. Lancet. 2002;359(9321):1877-1890.
14. N Engl J Med. 2008;359:895-905.
15. Obstet Gynecol. 2009;113(6):1327-1333.
16. Am J Obstet Gynecol. 2009;200(6):595-609.
17. Obstet Gynecol 2009;114:354-364.
18. Cochrane Database Syst Rev. 2009 Jan 21:(1):CD004661.
19. www.thennt.com.
20. Obstet Gynecol. 2010;115:669-671.
21. Front Synaptic Neurosci. 2012;13:680899.
*This story was corrected on June 10, 2024.

















