User login
Observational study again suggests lasting impact of COVID-19 on heart
A new study using cardiac magnetic resonance (CMR) imaging to examine the effects of novel coronavirus infection on the heart showed signs suggestive of myocarditis in 4 out of 26 competitive athletes who recovered from asymptomatic or mild cases of COVID-19.
While these and other similar findings are concerning, commentators are saying the results are preliminary and do not indicate widespread CMR screening is appropriate.
Two of the 4 patients showing signs of myocarditis in this series had no symptoms of COVID-19 but tested positive on routine testing. An additional 12 student athletes (46%) showed late gadolinium enhancement (LGE), of whom 8 (30.8%) had LGE without T2 elevation suggestive of prior myocardial injury.
An additional 12 student athletes (46%) showed late gadolinium enhancement (LGE), of whom 8 (31%) had LGE without T2 elevation suggestive of prior myocardial injury.
This finding, said Saurabh Rajpal, MBBS, MD, the study’s lead author, “could suggest prior myocardial injury or it could suggest athletic myocardial adaptation.”
In a research letter published in JAMA Cardiology, Rajpal and colleagues at Ohio State University in Columbus, described the findings of comprehensive CMR examinations in competitive athletes referred to the sport medicine clinic after testing positive for COVID-19 on reverse transcriptase-polymerase chain reaction between June and August 2020.
The university had made the decision in the spring to use CMR imaging as a screening tool for return to play, said Dr. Rajpal. While CMR is being used for research purposes, the American College of Cardiology’s recent “consensus expert opinion” statement on resumption of sport and exercise after COVID-19 infection does not require CMR imaging for resumption of competitive activity (JAMA Cardiol. 2020 May 13. doi:10.1001/jamacardio.2020.2136).
None of the athletes required hospitalization for their illness, and only 27% reported mild symptoms during the short-term infection, including sore throat, shortness of breath, myalgia, and fever.
On the day of CMR imaging, ECG and transthoracic echocardiography were performed, and serum troponin I was measured. There were no diagnostic ST/T wave changes, ventricular function and volumes were normal, and no athletes showed elevated serum troponin levels.
The updated Lake Louise Criteria were used to assess CMR findings consistent with myocarditis.
“I don’t think this is a COVID-specific issue. We have seen myocarditis after other viral infections; it’s just that COVID-19 is the most studied thus far, and with strenuous activity, inflammation in the heart can be risky,” Dr. Rajpal said in an interview. He added that more long-term and larger studies with control populations are needed.
His group is continuing to follow these athletes and has suggested that CMR “may provide an excellent risk-stratification assessment for myocarditis in athletes who have recovered from COVID-19 to guide safe competitive sports participation.”
Significance still unknown
Matthew Martinez, MD, the director of sports cardiology at Atlantic Health – Morristown (N.J.) Medical Center and the Gagnon Cardiovascular Institute, urged caution in making too much of the findings of this small study.
“We know that viruses cause myocardial damage and myocarditis. What we don’t know is how important these findings are. And in terms of risk, would we find the same phenomenon if we did this, say, in flu patients or in other age groups?” Dr. Martinez said in an interview.
“I haven’t seen all the images, but what I’d want to know is are these very subtle findings? Are these overt findings? Is this part of an active individual with symptoms? I need to know a little more data before I can tell if this influences the increased risk of sudden cardiac death that we often associate with myocarditis. I’m not sure how this should influence making decisions with regards to return to play.”
Dr. Martinez, who is the ACC’s chair of Sports and Exercise but was not an author of their recent guidance on return to sport, said that he is not routinely using CMR to assess athletes post-infection, as per the ACC’s recommendations.
“My approach is to evaluate anybody with a history of COVID infection and, first, determine whether it was an important infection with significant symptoms or not. And then, if they’re participating at a high level or are professional athletes, I would suggest an ECG, echo, and troponin. That’s our recommendation for the last several months and is still an appropriate way to evaluate that group.”
“In the presence of an abnormality or ongoing symptoms, I would ask for an MRI at that point,” said Dr. Martinez.
“We just don’t have much data on athletes with no symptoms to use to interpret these CMR findings and the study didn’t offer any controls. We don’t even know if these findings are new findings or old findings that have just been identified now,” he added.
New, updated recommendations from the ACC are coming soon, said Dr. Martinez. “I do not expect them to include CMR as first line.”
Cardiologists concerned about misinformation
This is at least the fourth study showing myocardial damage post-COVID-19 infection and there is concern in the medical community that the media has overstated the risks of heart damage, especially in athletes, and at the same time overstated the benefits of CMR.
In particular, Puntmann et al reported in July a 100-patient study that showed evidence of myocardial inflammation by CMR in 78% of patients recently recovered from a bout of COVID-19 (JAMA Cardiol. 2020 Jul 27; doi:10.1001/jamacardio.2020.3557).
“That paper is completely problematic,” John Mandrola, MD, of Baptists Medical Associates, Louisville, Ky., said in an interview. “It has the same overarching weaknesses [of other studies] that it’s observational and retrospective, but there were also numerical issues. So to me that paper is an interesting observation, but utterly unconvincing and preliminary,” said Dr. Mandrola.
Those limitations didn’t stop the study from garnering media attention, however. The Altmetric score (an attention score that tracks all mentions of an article in the media and on social media) for the Puntmann et al paper is approaching 13,000, including coverage from 276 news outlets and more than 19,000 tweets, putting it in the 99th percentile of all research outputs tracked by Altmetric to date.
To counter this, an “open letter” posted online just days before the Rajpal study published urging professional societies to “offer clear guidance discouraging CMR screening for COVID-19 related heart abnormalities in asymptomatic members of the general public.” The letter was signed by 51 clinicians, researchers, and imaging specialists from around the world.
Dr. Mandrola, one of the signatories, said: “This topic really scares people, and when it gets in the media like this, I think the leaders of these societies need to come out and say something really clear on major news networks letting people know that it’s just way too premature to start doing CMRs on every athlete that’s gotten this virus.”
“I understand that the current guidelines may be clear that CMR is not a first-line test for this indication, but when the media coverage is so extensive and so overblown, I wonder how much impact the guidelines will have in countering this fear that’s in the community,” he added.
Asked to comment on the letter, Dr. Rajpal said he agrees with the signatories that asymptomatic people from general population do not need routine cardiac MRI. “However, competitive athletes are a different story. Testing depends on risk assessment in specific population and competitive athletes as per our protocol will get enhanced cardiac workup including CMR for responsible and safe start of competitive sports. ... In the present scenario, while we get more data including control data, we will continue with our current protocol.”
Dr. Mandrola is Medscape Cardiology’s Chief Cardiology Consultant. MDedge is part of the Medscape Professional Network.
This article first appeared on Medscape.com.
A new study using cardiac magnetic resonance (CMR) imaging to examine the effects of novel coronavirus infection on the heart showed signs suggestive of myocarditis in 4 out of 26 competitive athletes who recovered from asymptomatic or mild cases of COVID-19.
While these and other similar findings are concerning, commentators are saying the results are preliminary and do not indicate widespread CMR screening is appropriate.
Two of the 4 patients showing signs of myocarditis in this series had no symptoms of COVID-19 but tested positive on routine testing. An additional 12 student athletes (46%) showed late gadolinium enhancement (LGE), of whom 8 (30.8%) had LGE without T2 elevation suggestive of prior myocardial injury.
An additional 12 student athletes (46%) showed late gadolinium enhancement (LGE), of whom 8 (31%) had LGE without T2 elevation suggestive of prior myocardial injury.
This finding, said Saurabh Rajpal, MBBS, MD, the study’s lead author, “could suggest prior myocardial injury or it could suggest athletic myocardial adaptation.”
In a research letter published in JAMA Cardiology, Rajpal and colleagues at Ohio State University in Columbus, described the findings of comprehensive CMR examinations in competitive athletes referred to the sport medicine clinic after testing positive for COVID-19 on reverse transcriptase-polymerase chain reaction between June and August 2020.
The university had made the decision in the spring to use CMR imaging as a screening tool for return to play, said Dr. Rajpal. While CMR is being used for research purposes, the American College of Cardiology’s recent “consensus expert opinion” statement on resumption of sport and exercise after COVID-19 infection does not require CMR imaging for resumption of competitive activity (JAMA Cardiol. 2020 May 13. doi:10.1001/jamacardio.2020.2136).
None of the athletes required hospitalization for their illness, and only 27% reported mild symptoms during the short-term infection, including sore throat, shortness of breath, myalgia, and fever.
On the day of CMR imaging, ECG and transthoracic echocardiography were performed, and serum troponin I was measured. There were no diagnostic ST/T wave changes, ventricular function and volumes were normal, and no athletes showed elevated serum troponin levels.
The updated Lake Louise Criteria were used to assess CMR findings consistent with myocarditis.
“I don’t think this is a COVID-specific issue. We have seen myocarditis after other viral infections; it’s just that COVID-19 is the most studied thus far, and with strenuous activity, inflammation in the heart can be risky,” Dr. Rajpal said in an interview. He added that more long-term and larger studies with control populations are needed.
His group is continuing to follow these athletes and has suggested that CMR “may provide an excellent risk-stratification assessment for myocarditis in athletes who have recovered from COVID-19 to guide safe competitive sports participation.”
Significance still unknown
Matthew Martinez, MD, the director of sports cardiology at Atlantic Health – Morristown (N.J.) Medical Center and the Gagnon Cardiovascular Institute, urged caution in making too much of the findings of this small study.
“We know that viruses cause myocardial damage and myocarditis. What we don’t know is how important these findings are. And in terms of risk, would we find the same phenomenon if we did this, say, in flu patients or in other age groups?” Dr. Martinez said in an interview.
“I haven’t seen all the images, but what I’d want to know is are these very subtle findings? Are these overt findings? Is this part of an active individual with symptoms? I need to know a little more data before I can tell if this influences the increased risk of sudden cardiac death that we often associate with myocarditis. I’m not sure how this should influence making decisions with regards to return to play.”
Dr. Martinez, who is the ACC’s chair of Sports and Exercise but was not an author of their recent guidance on return to sport, said that he is not routinely using CMR to assess athletes post-infection, as per the ACC’s recommendations.
“My approach is to evaluate anybody with a history of COVID infection and, first, determine whether it was an important infection with significant symptoms or not. And then, if they’re participating at a high level or are professional athletes, I would suggest an ECG, echo, and troponin. That’s our recommendation for the last several months and is still an appropriate way to evaluate that group.”
“In the presence of an abnormality or ongoing symptoms, I would ask for an MRI at that point,” said Dr. Martinez.
“We just don’t have much data on athletes with no symptoms to use to interpret these CMR findings and the study didn’t offer any controls. We don’t even know if these findings are new findings or old findings that have just been identified now,” he added.
New, updated recommendations from the ACC are coming soon, said Dr. Martinez. “I do not expect them to include CMR as first line.”
Cardiologists concerned about misinformation
This is at least the fourth study showing myocardial damage post-COVID-19 infection and there is concern in the medical community that the media has overstated the risks of heart damage, especially in athletes, and at the same time overstated the benefits of CMR.
In particular, Puntmann et al reported in July a 100-patient study that showed evidence of myocardial inflammation by CMR in 78% of patients recently recovered from a bout of COVID-19 (JAMA Cardiol. 2020 Jul 27; doi:10.1001/jamacardio.2020.3557).
“That paper is completely problematic,” John Mandrola, MD, of Baptists Medical Associates, Louisville, Ky., said in an interview. “It has the same overarching weaknesses [of other studies] that it’s observational and retrospective, but there were also numerical issues. So to me that paper is an interesting observation, but utterly unconvincing and preliminary,” said Dr. Mandrola.
Those limitations didn’t stop the study from garnering media attention, however. The Altmetric score (an attention score that tracks all mentions of an article in the media and on social media) for the Puntmann et al paper is approaching 13,000, including coverage from 276 news outlets and more than 19,000 tweets, putting it in the 99th percentile of all research outputs tracked by Altmetric to date.
To counter this, an “open letter” posted online just days before the Rajpal study published urging professional societies to “offer clear guidance discouraging CMR screening for COVID-19 related heart abnormalities in asymptomatic members of the general public.” The letter was signed by 51 clinicians, researchers, and imaging specialists from around the world.
Dr. Mandrola, one of the signatories, said: “This topic really scares people, and when it gets in the media like this, I think the leaders of these societies need to come out and say something really clear on major news networks letting people know that it’s just way too premature to start doing CMRs on every athlete that’s gotten this virus.”
“I understand that the current guidelines may be clear that CMR is not a first-line test for this indication, but when the media coverage is so extensive and so overblown, I wonder how much impact the guidelines will have in countering this fear that’s in the community,” he added.
Asked to comment on the letter, Dr. Rajpal said he agrees with the signatories that asymptomatic people from general population do not need routine cardiac MRI. “However, competitive athletes are a different story. Testing depends on risk assessment in specific population and competitive athletes as per our protocol will get enhanced cardiac workup including CMR for responsible and safe start of competitive sports. ... In the present scenario, while we get more data including control data, we will continue with our current protocol.”
Dr. Mandrola is Medscape Cardiology’s Chief Cardiology Consultant. MDedge is part of the Medscape Professional Network.
This article first appeared on Medscape.com.
A new study using cardiac magnetic resonance (CMR) imaging to examine the effects of novel coronavirus infection on the heart showed signs suggestive of myocarditis in 4 out of 26 competitive athletes who recovered from asymptomatic or mild cases of COVID-19.
While these and other similar findings are concerning, commentators are saying the results are preliminary and do not indicate widespread CMR screening is appropriate.
Two of the 4 patients showing signs of myocarditis in this series had no symptoms of COVID-19 but tested positive on routine testing. An additional 12 student athletes (46%) showed late gadolinium enhancement (LGE), of whom 8 (30.8%) had LGE without T2 elevation suggestive of prior myocardial injury.
An additional 12 student athletes (46%) showed late gadolinium enhancement (LGE), of whom 8 (31%) had LGE without T2 elevation suggestive of prior myocardial injury.
This finding, said Saurabh Rajpal, MBBS, MD, the study’s lead author, “could suggest prior myocardial injury or it could suggest athletic myocardial adaptation.”
In a research letter published in JAMA Cardiology, Rajpal and colleagues at Ohio State University in Columbus, described the findings of comprehensive CMR examinations in competitive athletes referred to the sport medicine clinic after testing positive for COVID-19 on reverse transcriptase-polymerase chain reaction between June and August 2020.
The university had made the decision in the spring to use CMR imaging as a screening tool for return to play, said Dr. Rajpal. While CMR is being used for research purposes, the American College of Cardiology’s recent “consensus expert opinion” statement on resumption of sport and exercise after COVID-19 infection does not require CMR imaging for resumption of competitive activity (JAMA Cardiol. 2020 May 13. doi:10.1001/jamacardio.2020.2136).
None of the athletes required hospitalization for their illness, and only 27% reported mild symptoms during the short-term infection, including sore throat, shortness of breath, myalgia, and fever.
On the day of CMR imaging, ECG and transthoracic echocardiography were performed, and serum troponin I was measured. There were no diagnostic ST/T wave changes, ventricular function and volumes were normal, and no athletes showed elevated serum troponin levels.
The updated Lake Louise Criteria were used to assess CMR findings consistent with myocarditis.
“I don’t think this is a COVID-specific issue. We have seen myocarditis after other viral infections; it’s just that COVID-19 is the most studied thus far, and with strenuous activity, inflammation in the heart can be risky,” Dr. Rajpal said in an interview. He added that more long-term and larger studies with control populations are needed.
His group is continuing to follow these athletes and has suggested that CMR “may provide an excellent risk-stratification assessment for myocarditis in athletes who have recovered from COVID-19 to guide safe competitive sports participation.”
Significance still unknown
Matthew Martinez, MD, the director of sports cardiology at Atlantic Health – Morristown (N.J.) Medical Center and the Gagnon Cardiovascular Institute, urged caution in making too much of the findings of this small study.
“We know that viruses cause myocardial damage and myocarditis. What we don’t know is how important these findings are. And in terms of risk, would we find the same phenomenon if we did this, say, in flu patients or in other age groups?” Dr. Martinez said in an interview.
“I haven’t seen all the images, but what I’d want to know is are these very subtle findings? Are these overt findings? Is this part of an active individual with symptoms? I need to know a little more data before I can tell if this influences the increased risk of sudden cardiac death that we often associate with myocarditis. I’m not sure how this should influence making decisions with regards to return to play.”
Dr. Martinez, who is the ACC’s chair of Sports and Exercise but was not an author of their recent guidance on return to sport, said that he is not routinely using CMR to assess athletes post-infection, as per the ACC’s recommendations.
“My approach is to evaluate anybody with a history of COVID infection and, first, determine whether it was an important infection with significant symptoms or not. And then, if they’re participating at a high level or are professional athletes, I would suggest an ECG, echo, and troponin. That’s our recommendation for the last several months and is still an appropriate way to evaluate that group.”
“In the presence of an abnormality or ongoing symptoms, I would ask for an MRI at that point,” said Dr. Martinez.
“We just don’t have much data on athletes with no symptoms to use to interpret these CMR findings and the study didn’t offer any controls. We don’t even know if these findings are new findings or old findings that have just been identified now,” he added.
New, updated recommendations from the ACC are coming soon, said Dr. Martinez. “I do not expect them to include CMR as first line.”
Cardiologists concerned about misinformation
This is at least the fourth study showing myocardial damage post-COVID-19 infection and there is concern in the medical community that the media has overstated the risks of heart damage, especially in athletes, and at the same time overstated the benefits of CMR.
In particular, Puntmann et al reported in July a 100-patient study that showed evidence of myocardial inflammation by CMR in 78% of patients recently recovered from a bout of COVID-19 (JAMA Cardiol. 2020 Jul 27; doi:10.1001/jamacardio.2020.3557).
“That paper is completely problematic,” John Mandrola, MD, of Baptists Medical Associates, Louisville, Ky., said in an interview. “It has the same overarching weaknesses [of other studies] that it’s observational and retrospective, but there were also numerical issues. So to me that paper is an interesting observation, but utterly unconvincing and preliminary,” said Dr. Mandrola.
Those limitations didn’t stop the study from garnering media attention, however. The Altmetric score (an attention score that tracks all mentions of an article in the media and on social media) for the Puntmann et al paper is approaching 13,000, including coverage from 276 news outlets and more than 19,000 tweets, putting it in the 99th percentile of all research outputs tracked by Altmetric to date.
To counter this, an “open letter” posted online just days before the Rajpal study published urging professional societies to “offer clear guidance discouraging CMR screening for COVID-19 related heart abnormalities in asymptomatic members of the general public.” The letter was signed by 51 clinicians, researchers, and imaging specialists from around the world.
Dr. Mandrola, one of the signatories, said: “This topic really scares people, and when it gets in the media like this, I think the leaders of these societies need to come out and say something really clear on major news networks letting people know that it’s just way too premature to start doing CMRs on every athlete that’s gotten this virus.”
“I understand that the current guidelines may be clear that CMR is not a first-line test for this indication, but when the media coverage is so extensive and so overblown, I wonder how much impact the guidelines will have in countering this fear that’s in the community,” he added.
Asked to comment on the letter, Dr. Rajpal said he agrees with the signatories that asymptomatic people from general population do not need routine cardiac MRI. “However, competitive athletes are a different story. Testing depends on risk assessment in specific population and competitive athletes as per our protocol will get enhanced cardiac workup including CMR for responsible and safe start of competitive sports. ... In the present scenario, while we get more data including control data, we will continue with our current protocol.”
Dr. Mandrola is Medscape Cardiology’s Chief Cardiology Consultant. MDedge is part of the Medscape Professional Network.
This article first appeared on Medscape.com.
Role of aspirin explored in primary prevention of CVD in systemic rheumatic diseases
Low-dose aspirin may be considered for the primary prevention of cardiovascular disease (CVD) in patients with autoimmune systemic rheumatic diseases who are at particularly high risk because of their individual cardiovascular risk profile, according to authors of a new review article in the journal Rheumatology who acknowledge the controversial nature of the issue, because while significant cardiovascular benefit from aspirin for secondary prevention is well established, it has not been for primary prevention.
Secondary prevention with daily, low-dose aspirin is part of aggressive, comprehensive risk modification in patients who have experienced an MI or stroke or are considered at high risk for CVD. But when it comes to primary prevention of the onset of disease, the authors, led by Serena Fasano, MD, PhD, of the rheumatology unit at the University of Campania, Naples, Italy, acknowledged the contradictory positions of international guidelines and uncertainty over balancing benefit versus harm – including risk of mortality in the context of excess bleeding. They called for “robust data” from high-quality randomized, controlled trials for subgroups of patients with specific rheumatologic diseases in order to better answer the question of aspirin for primary prevention.
“This review is devoted to reporting the present knowledge on the effectiveness of low-dose [aspirin] in primary CV prevention in a number of autoimmune systemic rheumatic diseases, not a systematic review or meta-analysis,” the authors stated. “We are not claiming to have covered more than a selection of the literature for each disease. Available data are not high-quality data and do not provide firm conclusions.”
The authors focused primarily on accelerated, rather than spontaneous, atherosclerosis or buildup of plaque in artery walls, implicated in ischemic heart diseases such as MI and ischemic cerebrovascular diseases such as stroke. They looked at its association with autoimmune rheumatic diseases, primarily systemic lupus erythematosus (SLE) and RA, but also including antiphospholipid syndrome, systemic sclerosis, mixed connective tissue disease, dermatomyositis/polymyositis, primary Sjögren’s syndrome, and systemic vasculitis.
They shared results from a review of 167 patients with SLE consecutively admitted to their tertiary medical center who had not previously experienced a cardiovascular event and who were prescribed low-dose (100 mg) aspirin on their first visit and followed for 8 years. The cardiovascular event-free rate was higher in the aspirin group and no excess bleeding was noted, although this may be attributable to a younger patient population and routine use of proton pump inhibitors. Subsequently, hydroxychloroquine was added to the aspirin treatment and was associated with further reduction in cardiovascular events.
The research group also conducted a retrospective analysis of 746 patients with RA consecutively admitted to four tertiary medical centers who hadn’t experienced a cardiovascular event previously. Incidence of cardiovascular events was significantly lower in aspirin-treated patients.
Individualized aspirin prescribing with cardiologist comanagement
There may be a modest benefit of using low-dose aspirin on a long-term basis, but that benefit needs to be offset by the risk of bleeds, said M. Elaine Husni, MD, MPH, vice chair of rheumatology and director of the Arthritis and Musculoskeletal Center at the Cleveland Clinic. It’s important to remind clinicians of cardiovascular risk, she said. “But the message for rheumatologists is it needs to be prescribed on an individual basis, rather than based on diagnosis of a rheumatic condition – at least until we have better evidence.”
Dr. Husni recommended keeping an open mind regarding individual approaches – for example, low-dose aspirin plus statins. A composite approach to prevention likely is called for, including attention to lifestyle issues such as smoking cessation, exercise, and weight loss. “That kind of complexity in decision-making highlights the need for comanagement with a cardiologist,” she said. “I’m a big believer in comanagement. At my multidisciplinary medical center, I am able to pick up the phone and talk to a cardiologist with whom our group has a relationship.” If physicians don’t have that kind of relationship with a cardiology group, she suggested reaching out to establish one.
The review paper could give some guidance to rheumatologists for use on an individual case, Michael Nurmohamed, MD, PhD, of the Amsterdam Rheumatology and Immunology Center in the Netherlands commented in an interview. “However, firm recommendations cannot be given as proper investigations are still lacking, as acknowledged by the authors. In addition, the review paper itself has some methodological constraints. Although this is a narrative review, the search strategy should have been specified, and a quality assessment of the individual studies is lacking.”
There is no doubt that the CVD burden in RA and other rheumatologic conditions is substantially increased in comparison to the general population, Dr. Nurmohamed said. That has been assessed by several well-designed, prospective, controlled studies. Other relatively frequent inflammatory arthropathies, including ankylosing spondylitis and psoriatic arthritis, also pose cardiovascular risk.
“Aspirin cannot be recommended for primary CVD prevention in inflammatory arthropathies due to the absence of adequate studies. That’s why the EULAR [European League Against Rheumatism] guidelines did not recommend its use,” he said. Currently, a EULAR task force is developing evidence-based guidelines for primary CVD prevention in the diseases discussed by Fasano et al., where the use of aspirin will be reassessed. “As these guidelines will consider the methodological quality of the underlying studies, they will enable a more refined use of aspirin in daily clinical practice.”
Primary prevention of CVD using aspirin is not currently the standard of care in taking care of patients with rheumatologic disease in the Netherlands, Ronald F. van Vollenhoven, MD, PhD, Dr. Nurmohamed’s colleague and director of the Amsterdam Rheumatology and Immunology Center and the chair of the department of rheumatology and clinical immunology at the Amsterdam University Medical Center, said in an interview.
“One reason may be the limited data, as highlighted in the review by Dr. Fasano and colleagues. However, another consideration is the problem of polypharmacy. Rheumatic diseases usually require chronic treatment, sometimes with multiple medications. This makes it even more of a concern to add an additional medication, even a relatively innocuous one such as low-dose aspirin,” he said.
Dr. Husni, Dr. Nurmohamed, and Dr. van Vollenhoven reported having no relevant disclosures. The authors of the review article had no relevant disclosures.
SOURCE: Fasano S et al. Rheumatology. 2020 Aug 25. doi: 10.1093/rheumatology/keaa335.
Low-dose aspirin may be considered for the primary prevention of cardiovascular disease (CVD) in patients with autoimmune systemic rheumatic diseases who are at particularly high risk because of their individual cardiovascular risk profile, according to authors of a new review article in the journal Rheumatology who acknowledge the controversial nature of the issue, because while significant cardiovascular benefit from aspirin for secondary prevention is well established, it has not been for primary prevention.
Secondary prevention with daily, low-dose aspirin is part of aggressive, comprehensive risk modification in patients who have experienced an MI or stroke or are considered at high risk for CVD. But when it comes to primary prevention of the onset of disease, the authors, led by Serena Fasano, MD, PhD, of the rheumatology unit at the University of Campania, Naples, Italy, acknowledged the contradictory positions of international guidelines and uncertainty over balancing benefit versus harm – including risk of mortality in the context of excess bleeding. They called for “robust data” from high-quality randomized, controlled trials for subgroups of patients with specific rheumatologic diseases in order to better answer the question of aspirin for primary prevention.
“This review is devoted to reporting the present knowledge on the effectiveness of low-dose [aspirin] in primary CV prevention in a number of autoimmune systemic rheumatic diseases, not a systematic review or meta-analysis,” the authors stated. “We are not claiming to have covered more than a selection of the literature for each disease. Available data are not high-quality data and do not provide firm conclusions.”
The authors focused primarily on accelerated, rather than spontaneous, atherosclerosis or buildup of plaque in artery walls, implicated in ischemic heart diseases such as MI and ischemic cerebrovascular diseases such as stroke. They looked at its association with autoimmune rheumatic diseases, primarily systemic lupus erythematosus (SLE) and RA, but also including antiphospholipid syndrome, systemic sclerosis, mixed connective tissue disease, dermatomyositis/polymyositis, primary Sjögren’s syndrome, and systemic vasculitis.
They shared results from a review of 167 patients with SLE consecutively admitted to their tertiary medical center who had not previously experienced a cardiovascular event and who were prescribed low-dose (100 mg) aspirin on their first visit and followed for 8 years. The cardiovascular event-free rate was higher in the aspirin group and no excess bleeding was noted, although this may be attributable to a younger patient population and routine use of proton pump inhibitors. Subsequently, hydroxychloroquine was added to the aspirin treatment and was associated with further reduction in cardiovascular events.
The research group also conducted a retrospective analysis of 746 patients with RA consecutively admitted to four tertiary medical centers who hadn’t experienced a cardiovascular event previously. Incidence of cardiovascular events was significantly lower in aspirin-treated patients.
Individualized aspirin prescribing with cardiologist comanagement
There may be a modest benefit of using low-dose aspirin on a long-term basis, but that benefit needs to be offset by the risk of bleeds, said M. Elaine Husni, MD, MPH, vice chair of rheumatology and director of the Arthritis and Musculoskeletal Center at the Cleveland Clinic. It’s important to remind clinicians of cardiovascular risk, she said. “But the message for rheumatologists is it needs to be prescribed on an individual basis, rather than based on diagnosis of a rheumatic condition – at least until we have better evidence.”
Dr. Husni recommended keeping an open mind regarding individual approaches – for example, low-dose aspirin plus statins. A composite approach to prevention likely is called for, including attention to lifestyle issues such as smoking cessation, exercise, and weight loss. “That kind of complexity in decision-making highlights the need for comanagement with a cardiologist,” she said. “I’m a big believer in comanagement. At my multidisciplinary medical center, I am able to pick up the phone and talk to a cardiologist with whom our group has a relationship.” If physicians don’t have that kind of relationship with a cardiology group, she suggested reaching out to establish one.
The review paper could give some guidance to rheumatologists for use on an individual case, Michael Nurmohamed, MD, PhD, of the Amsterdam Rheumatology and Immunology Center in the Netherlands commented in an interview. “However, firm recommendations cannot be given as proper investigations are still lacking, as acknowledged by the authors. In addition, the review paper itself has some methodological constraints. Although this is a narrative review, the search strategy should have been specified, and a quality assessment of the individual studies is lacking.”
There is no doubt that the CVD burden in RA and other rheumatologic conditions is substantially increased in comparison to the general population, Dr. Nurmohamed said. That has been assessed by several well-designed, prospective, controlled studies. Other relatively frequent inflammatory arthropathies, including ankylosing spondylitis and psoriatic arthritis, also pose cardiovascular risk.
“Aspirin cannot be recommended for primary CVD prevention in inflammatory arthropathies due to the absence of adequate studies. That’s why the EULAR [European League Against Rheumatism] guidelines did not recommend its use,” he said. Currently, a EULAR task force is developing evidence-based guidelines for primary CVD prevention in the diseases discussed by Fasano et al., where the use of aspirin will be reassessed. “As these guidelines will consider the methodological quality of the underlying studies, they will enable a more refined use of aspirin in daily clinical practice.”
Primary prevention of CVD using aspirin is not currently the standard of care in taking care of patients with rheumatologic disease in the Netherlands, Ronald F. van Vollenhoven, MD, PhD, Dr. Nurmohamed’s colleague and director of the Amsterdam Rheumatology and Immunology Center and the chair of the department of rheumatology and clinical immunology at the Amsterdam University Medical Center, said in an interview.
“One reason may be the limited data, as highlighted in the review by Dr. Fasano and colleagues. However, another consideration is the problem of polypharmacy. Rheumatic diseases usually require chronic treatment, sometimes with multiple medications. This makes it even more of a concern to add an additional medication, even a relatively innocuous one such as low-dose aspirin,” he said.
Dr. Husni, Dr. Nurmohamed, and Dr. van Vollenhoven reported having no relevant disclosures. The authors of the review article had no relevant disclosures.
SOURCE: Fasano S et al. Rheumatology. 2020 Aug 25. doi: 10.1093/rheumatology/keaa335.
Low-dose aspirin may be considered for the primary prevention of cardiovascular disease (CVD) in patients with autoimmune systemic rheumatic diseases who are at particularly high risk because of their individual cardiovascular risk profile, according to authors of a new review article in the journal Rheumatology who acknowledge the controversial nature of the issue, because while significant cardiovascular benefit from aspirin for secondary prevention is well established, it has not been for primary prevention.
Secondary prevention with daily, low-dose aspirin is part of aggressive, comprehensive risk modification in patients who have experienced an MI or stroke or are considered at high risk for CVD. But when it comes to primary prevention of the onset of disease, the authors, led by Serena Fasano, MD, PhD, of the rheumatology unit at the University of Campania, Naples, Italy, acknowledged the contradictory positions of international guidelines and uncertainty over balancing benefit versus harm – including risk of mortality in the context of excess bleeding. They called for “robust data” from high-quality randomized, controlled trials for subgroups of patients with specific rheumatologic diseases in order to better answer the question of aspirin for primary prevention.
“This review is devoted to reporting the present knowledge on the effectiveness of low-dose [aspirin] in primary CV prevention in a number of autoimmune systemic rheumatic diseases, not a systematic review or meta-analysis,” the authors stated. “We are not claiming to have covered more than a selection of the literature for each disease. Available data are not high-quality data and do not provide firm conclusions.”
The authors focused primarily on accelerated, rather than spontaneous, atherosclerosis or buildup of plaque in artery walls, implicated in ischemic heart diseases such as MI and ischemic cerebrovascular diseases such as stroke. They looked at its association with autoimmune rheumatic diseases, primarily systemic lupus erythematosus (SLE) and RA, but also including antiphospholipid syndrome, systemic sclerosis, mixed connective tissue disease, dermatomyositis/polymyositis, primary Sjögren’s syndrome, and systemic vasculitis.
They shared results from a review of 167 patients with SLE consecutively admitted to their tertiary medical center who had not previously experienced a cardiovascular event and who were prescribed low-dose (100 mg) aspirin on their first visit and followed for 8 years. The cardiovascular event-free rate was higher in the aspirin group and no excess bleeding was noted, although this may be attributable to a younger patient population and routine use of proton pump inhibitors. Subsequently, hydroxychloroquine was added to the aspirin treatment and was associated with further reduction in cardiovascular events.
The research group also conducted a retrospective analysis of 746 patients with RA consecutively admitted to four tertiary medical centers who hadn’t experienced a cardiovascular event previously. Incidence of cardiovascular events was significantly lower in aspirin-treated patients.
Individualized aspirin prescribing with cardiologist comanagement
There may be a modest benefit of using low-dose aspirin on a long-term basis, but that benefit needs to be offset by the risk of bleeds, said M. Elaine Husni, MD, MPH, vice chair of rheumatology and director of the Arthritis and Musculoskeletal Center at the Cleveland Clinic. It’s important to remind clinicians of cardiovascular risk, she said. “But the message for rheumatologists is it needs to be prescribed on an individual basis, rather than based on diagnosis of a rheumatic condition – at least until we have better evidence.”
Dr. Husni recommended keeping an open mind regarding individual approaches – for example, low-dose aspirin plus statins. A composite approach to prevention likely is called for, including attention to lifestyle issues such as smoking cessation, exercise, and weight loss. “That kind of complexity in decision-making highlights the need for comanagement with a cardiologist,” she said. “I’m a big believer in comanagement. At my multidisciplinary medical center, I am able to pick up the phone and talk to a cardiologist with whom our group has a relationship.” If physicians don’t have that kind of relationship with a cardiology group, she suggested reaching out to establish one.
The review paper could give some guidance to rheumatologists for use on an individual case, Michael Nurmohamed, MD, PhD, of the Amsterdam Rheumatology and Immunology Center in the Netherlands commented in an interview. “However, firm recommendations cannot be given as proper investigations are still lacking, as acknowledged by the authors. In addition, the review paper itself has some methodological constraints. Although this is a narrative review, the search strategy should have been specified, and a quality assessment of the individual studies is lacking.”
There is no doubt that the CVD burden in RA and other rheumatologic conditions is substantially increased in comparison to the general population, Dr. Nurmohamed said. That has been assessed by several well-designed, prospective, controlled studies. Other relatively frequent inflammatory arthropathies, including ankylosing spondylitis and psoriatic arthritis, also pose cardiovascular risk.
“Aspirin cannot be recommended for primary CVD prevention in inflammatory arthropathies due to the absence of adequate studies. That’s why the EULAR [European League Against Rheumatism] guidelines did not recommend its use,” he said. Currently, a EULAR task force is developing evidence-based guidelines for primary CVD prevention in the diseases discussed by Fasano et al., where the use of aspirin will be reassessed. “As these guidelines will consider the methodological quality of the underlying studies, they will enable a more refined use of aspirin in daily clinical practice.”
Primary prevention of CVD using aspirin is not currently the standard of care in taking care of patients with rheumatologic disease in the Netherlands, Ronald F. van Vollenhoven, MD, PhD, Dr. Nurmohamed’s colleague and director of the Amsterdam Rheumatology and Immunology Center and the chair of the department of rheumatology and clinical immunology at the Amsterdam University Medical Center, said in an interview.
“One reason may be the limited data, as highlighted in the review by Dr. Fasano and colleagues. However, another consideration is the problem of polypharmacy. Rheumatic diseases usually require chronic treatment, sometimes with multiple medications. This makes it even more of a concern to add an additional medication, even a relatively innocuous one such as low-dose aspirin,” he said.
Dr. Husni, Dr. Nurmohamed, and Dr. van Vollenhoven reported having no relevant disclosures. The authors of the review article had no relevant disclosures.
SOURCE: Fasano S et al. Rheumatology. 2020 Aug 25. doi: 10.1093/rheumatology/keaa335.
FROM RHEUMATOLOGY
What a Slow Boil Got Him
ANSWER
The correct answer is hidradenitis suppurativa (HS; choice “c”).
DISCUSSION
HS (also known as acne inversa) is a relatively common condition first described in 1839 by French physician Frederick Velpeau, who thought it was probably a form of acne. Because HS only affects intertriginous areas (groin, axillae, inframammary, intergluteal, and abdominal folds rich in apocrine glands), during the next 130 years, it was presumed that this disease was solely caused by malfunctioning apocrine glands.
Though experts disagree about the true etiology of HS, most agree that it is caused by several environmental and genetic factors leading to plugged or malfunctioning hair follicles and apocrine glands that become inflamed, swollen, painful, and purulent. In severe cases, tracts develop between lesions, resulting in the formation of ropy, linear hypertrophic scarring. Early on and in milder cases, comedones can manifest—often in multiples—along with widely scattered cysts. It appears that a case can be made for a genetic predisposition to develop HS because at least one-third of affected patients report a positive family history.
Females (usually postpubescent) are affected at 3 times the rate of males. Hormones, especially androgens, appear to play a role. This assertion is bolstered not only by the age of onset, but also because HS has been observed to improve after pregnancy and after menopause.
Though not causative, smoking and obesity have a strong statistical correlation with HS as exacerbating factors. Many patients with HS find movement from exercise too painful, which may contribute to obesity.
Because HS has a wide range of clinical presentations, the Hurley Staging scale can help clinicians distinguish the condition’s severity by stages I, II, or III. Stage I manifests with scant signs of disease, including comedones and sparsely separated small cysts. Stage II includes numerous painful cysts with tract formation between lesions. Stage III involves symptoms described in the other stages as well as multiple large, painful, draining cysts; widespread erythema; and extensive hypertrophic scarring.
Regarding the differential, the culture would have identified community-acquired methicillin-resistant Staphylococcus aureus (choice “a”) or flesh-eating bacterial infection (choice “d”), but neither would be as chronic as the patient’s disease. Acne conglobata (choice “b”) manifests with chronic dense acne largely confined to the trunk. Interestingly, acne conglobata, HS, dissecting cellulitis of the scalp, and pilonidal cyst are collectively known as the follicular occlusion tetrad.
TREATMENT
Treatment for HS is limited. Adalimumab is the most recent and promising biologic, though its use comes at a high cost ($60,000/y) and with an adverse effect profile that should give any prescriber pause. Also, the best one could hope for in using adalimumab is about a 30% improvement in the patient.
Before using a biologic, other options should at least be considered. Though not entirely satisfactory, there are oral antibiotics (minocycline, trimethoprim/sulfa, or a combination of rifampin and clindamycin) or intralesional steroids. Incision and drainage of individual lesions and isotretinoin therapy can temporarily relieve pain, but they are not effective as long-term therapies. Large-scale surgical extirpation of the affected tissue can be considered in extreme cases, but there can be recurrences and associated morbidity (eg, chronic lymphedema in the affected arm, extreme scarring, and postoperative pain).
ANSWER
The correct answer is hidradenitis suppurativa (HS; choice “c”).
DISCUSSION
HS (also known as acne inversa) is a relatively common condition first described in 1839 by French physician Frederick Velpeau, who thought it was probably a form of acne. Because HS only affects intertriginous areas (groin, axillae, inframammary, intergluteal, and abdominal folds rich in apocrine glands), during the next 130 years, it was presumed that this disease was solely caused by malfunctioning apocrine glands.
Though experts disagree about the true etiology of HS, most agree that it is caused by several environmental and genetic factors leading to plugged or malfunctioning hair follicles and apocrine glands that become inflamed, swollen, painful, and purulent. In severe cases, tracts develop between lesions, resulting in the formation of ropy, linear hypertrophic scarring. Early on and in milder cases, comedones can manifest—often in multiples—along with widely scattered cysts. It appears that a case can be made for a genetic predisposition to develop HS because at least one-third of affected patients report a positive family history.
Females (usually postpubescent) are affected at 3 times the rate of males. Hormones, especially androgens, appear to play a role. This assertion is bolstered not only by the age of onset, but also because HS has been observed to improve after pregnancy and after menopause.
Though not causative, smoking and obesity have a strong statistical correlation with HS as exacerbating factors. Many patients with HS find movement from exercise too painful, which may contribute to obesity.
Because HS has a wide range of clinical presentations, the Hurley Staging scale can help clinicians distinguish the condition’s severity by stages I, II, or III. Stage I manifests with scant signs of disease, including comedones and sparsely separated small cysts. Stage II includes numerous painful cysts with tract formation between lesions. Stage III involves symptoms described in the other stages as well as multiple large, painful, draining cysts; widespread erythema; and extensive hypertrophic scarring.
Regarding the differential, the culture would have identified community-acquired methicillin-resistant Staphylococcus aureus (choice “a”) or flesh-eating bacterial infection (choice “d”), but neither would be as chronic as the patient’s disease. Acne conglobata (choice “b”) manifests with chronic dense acne largely confined to the trunk. Interestingly, acne conglobata, HS, dissecting cellulitis of the scalp, and pilonidal cyst are collectively known as the follicular occlusion tetrad.
TREATMENT
Treatment for HS is limited. Adalimumab is the most recent and promising biologic, though its use comes at a high cost ($60,000/y) and with an adverse effect profile that should give any prescriber pause. Also, the best one could hope for in using adalimumab is about a 30% improvement in the patient.
Before using a biologic, other options should at least be considered. Though not entirely satisfactory, there are oral antibiotics (minocycline, trimethoprim/sulfa, or a combination of rifampin and clindamycin) or intralesional steroids. Incision and drainage of individual lesions and isotretinoin therapy can temporarily relieve pain, but they are not effective as long-term therapies. Large-scale surgical extirpation of the affected tissue can be considered in extreme cases, but there can be recurrences and associated morbidity (eg, chronic lymphedema in the affected arm, extreme scarring, and postoperative pain).
ANSWER
The correct answer is hidradenitis suppurativa (HS; choice “c”).
DISCUSSION
HS (also known as acne inversa) is a relatively common condition first described in 1839 by French physician Frederick Velpeau, who thought it was probably a form of acne. Because HS only affects intertriginous areas (groin, axillae, inframammary, intergluteal, and abdominal folds rich in apocrine glands), during the next 130 years, it was presumed that this disease was solely caused by malfunctioning apocrine glands.
Though experts disagree about the true etiology of HS, most agree that it is caused by several environmental and genetic factors leading to plugged or malfunctioning hair follicles and apocrine glands that become inflamed, swollen, painful, and purulent. In severe cases, tracts develop between lesions, resulting in the formation of ropy, linear hypertrophic scarring. Early on and in milder cases, comedones can manifest—often in multiples—along with widely scattered cysts. It appears that a case can be made for a genetic predisposition to develop HS because at least one-third of affected patients report a positive family history.
Females (usually postpubescent) are affected at 3 times the rate of males. Hormones, especially androgens, appear to play a role. This assertion is bolstered not only by the age of onset, but also because HS has been observed to improve after pregnancy and after menopause.
Though not causative, smoking and obesity have a strong statistical correlation with HS as exacerbating factors. Many patients with HS find movement from exercise too painful, which may contribute to obesity.
Because HS has a wide range of clinical presentations, the Hurley Staging scale can help clinicians distinguish the condition’s severity by stages I, II, or III. Stage I manifests with scant signs of disease, including comedones and sparsely separated small cysts. Stage II includes numerous painful cysts with tract formation between lesions. Stage III involves symptoms described in the other stages as well as multiple large, painful, draining cysts; widespread erythema; and extensive hypertrophic scarring.
Regarding the differential, the culture would have identified community-acquired methicillin-resistant Staphylococcus aureus (choice “a”) or flesh-eating bacterial infection (choice “d”), but neither would be as chronic as the patient’s disease. Acne conglobata (choice “b”) manifests with chronic dense acne largely confined to the trunk. Interestingly, acne conglobata, HS, dissecting cellulitis of the scalp, and pilonidal cyst are collectively known as the follicular occlusion tetrad.
TREATMENT
Treatment for HS is limited. Adalimumab is the most recent and promising biologic, though its use comes at a high cost ($60,000/y) and with an adverse effect profile that should give any prescriber pause. Also, the best one could hope for in using adalimumab is about a 30% improvement in the patient.
Before using a biologic, other options should at least be considered. Though not entirely satisfactory, there are oral antibiotics (minocycline, trimethoprim/sulfa, or a combination of rifampin and clindamycin) or intralesional steroids. Incision and drainage of individual lesions and isotretinoin therapy can temporarily relieve pain, but they are not effective as long-term therapies. Large-scale surgical extirpation of the affected tissue can be considered in extreme cases, but there can be recurrences and associated morbidity (eg, chronic lymphedema in the affected arm, extreme scarring, and postoperative pain).
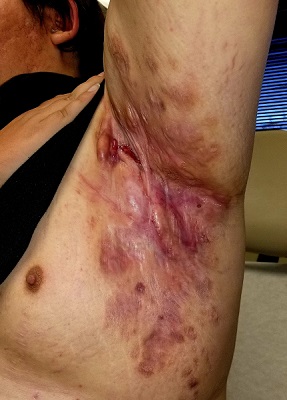
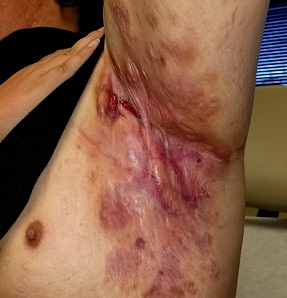
Boils first manifested on this 25-year-old man’s axillae when he was 13 (at the onset of puberty). As he grew older, they appeared in other intertriginous areas, including the groin and between the buttocks. As he became increasingly overweight, the folds under his breasts and abdomen became affected.
Over the years, several primary care providers consistently diagnosed him with either boils or staph infection. They also would reliably admonish him regarding his weight and lack of cleanliness. Treatment included various oral antibiotics that would only calm the condition for a short time. At no point was he referred to dermatology. Family history revealed similar skin problems in his mother and 1 sister.
Recently, his lesions have grown more numerous and painful. They drain pustular fluid, which makes it difficult for him to function in classrooms or at work. Even walking is excruciatingly painful. As a result, he is more sedentary. He also has started smoking.
On examination, the patient is 5 ft 8 in and grossly obese (weight, 300 lb). His affect is decidedly morose. His gait is ponderous and halting. His type 4 skin is swarthy, sweaty, and oily.
All intertriginous areas are similarly affected with large, ropy keloidal and hypertrophic scars, as well as diffuse bright red erythema and edema. There are dozens of single and multiple comedones, along with multiple draining sinuses.
A bacterial culture is collected from the fluid draining from the lesions. The results show a mixed, normal, cutaneous flora—including staph epidermidis—with no predominant organism.
Physician reimbursement 2021: Who are the big winners?
Amid all the chaos and problems caused by COVID-19, one might hope that physicians would get a break on their complicated payment-reporting programs.
But that’s not the case: The government recently released the 2021 proposed rule for the Quality Payment Program (QPP), often referred to by its most popular participation track, the Merit-Based Incentive Payment System (MIPS). The program, which launched in 2017, gets annual updates, and this year is no different.
Some good news has made primary care and some other physicians happy.
The government’s proposal includes significant changes to reimbursement for all physicians.
Specialties that rely heavily on office-based E/M services are delighted at this change. Those include internists, family physicians, neurologists, pulmonologists, dermatologists, and all other specialties that rely heavily on office encounters.
According to the estimates from the Centers for Medicare & Medicaid Services (CMS), endocrinologists and rheumatologists are the big winners, at 17% and 16% projected increases, respectively. The government has been pushing to make this shift in reimbursement from surgeries and procedures to office visits for years. Although some physicians may celebrate the change, others will not.
The reimbursement plan for professional services depends on budget neutrality, meaning that the budget increases need to be counterbalanced by budget declines. Specialties that rely heavily on procedures and surgeries will suffer losses. These corresponding reductions felt by proceduralists and surgeons will counterbalance the good fortune of physicians who rely on office visits for the bulk of their revenue. Radiologists, for example, are projected by CMS to experience a 11% downturn, and cardiac surgeons face a 9% decline.
These consequences are significant. The 2021 shift may be the single biggest transfer of reimbursement in the history of the scale, which was adopted in the early 1990s.
If the change affected only Medicare reimbursement, perhaps it would be less significant. Because the majority of private payers use the government’s scale – the resource-based relative value scale – the impact will reverberate across physicians’ bottom lines. Given the state of many physicians’ finances, driven by the pandemic, this may send some affected physicians into a downward spiral.
The boost to E/M reimbursement – which represents approximately 20% of the overall Medicare payout to physicians each year – puts downward pressure on the professional services conversion factor as well.
For 2021, it is proposed to be $32.2605, representing a decrease of $3.83 from the 2020 conversion factor of $36.0896. The resultant conversion factor – which serves as a multiplier applied to the relative value unit to come up with the payment – effectively reduces payments to physicians across the board by 10.6%. Thus, even those who enjoy the benefits of the new E/M increases will see the potential reimbursement high point cut down.
Before launching into the changes in store for 2021, it’s good to determine whether you are an eligible clinician: You need to have more than $90,000 in Medicare Part B charges per year, see more than 200 Medicare Part B patients per year, and provide 200 or more covered professional services to Part B patients.
The program is voluntary, but there are steep penalties for eligible clinicians who don’t participate. For the 2021 reporting year, a 9% penalty will be imposed on Medicare reimbursement in 2023 in the event of participation failure. You can verify your participation status here; you’ll need your National Provider Identifier to run the search, but it takes only seconds to determine your eligibility.
A 9% penalty is a pretty big hit to your income. With 9% at stake, eligible clinicians need to actively engage in the program. Although there have been changes, the basic four-category system remains the same for the MIPS track, as follows: quality, cost, improvement activities, and promoting interoperability.
The four category weights, used to evaluate performance, are changing in 2021. Cost category weight goes up by 5 percentage points, to be 20% of the clinician’s score, and the quality category goes down by 5 percentage points to contribute 40% to the weight. Promoting interoperability remains 25% of the score, with improvement activities constituting the final 15%.
Other key changes include the following:
- The CMS’s Web interface for submission for quality measures will be shuttered in 2021. Users of this submission method will have to find and use another way to report their quality measures.
- Quality measures will be scored against pre-COVID benchmarks in lieu of comparisons with the 2020 reporting year; 206 quality measures are proposed for 2021, compared with the current list of 219.
- Telehealth will be incorporated in the cost category by updates to the measure specifications for the episode-based and total per capita cost measures.
- A new health information exchange measure is added to the promoting interoperability category, and “incorporating” replaces “reconciling” in the reporting requirement “Support Electronic Referral Loops by Receiving and Incorporating Health Information.”
To avoid the 9% penalty, eligible clinicians must earn 50 points in 2021, up from 45 in the current year. Achieving “exceptional performance” remains at 85 points. This elevated level of engagement allows access to a pot of money Congress set aside for high performers.
Many physicians feel that too much work is required to earn the “paltry” bonuses; even a perfect score of 100 has only resulted in bonuses of 1.88% and 1.68%, respectively, in the past 2 years. That includes the $500 million allocation that Congress set aside; this extra funding to reward exceptional performance is only available for the first 6 years of the law. Although the 2019 scores have been released to participants, CMS has not yet announced the overall national average, but it’s expected to be minimal.
The combination of meager payouts and a diminishing funding mechanism has physicians questioning participation altogether. My recent conversations with physicians who qualify for the program revealed their intention to participate, but only at a level to achieve the minimum threshold of 45 points this year and 50 in 2021. With so little upside, it’s impossible to make a business case to aim for the stars.
Perhaps the biggest change in 2021, however, is that the program is not making the previously planned switch to MIPS Value Pathways (MVPs). MVPs were designed to align the four performance categories around a specialty, medical condition, or patient population.
CMS introduced MVPs by giving an example of diabetes: “Endocrinologist reports same ‘foundation’ of PI [promoting interoperability] and population health measures as all other clinicians but now has a MIPS Value Pathway with measures and activities that focus on diabetes prevention and treatment.” CMS had expected MVPs to launch in 2021 for all program participants; because of the pandemic, CMS announced an extension for at least 1 year. This comes as a relief to physicians who are just trying to keep the lights on given the financial pressures brought on by the pandemic.
MVPs, however, will be incorporated into the MIPS Alternative Payment Model (APM) participation segment. This will affect many physicians because this is the path that accountable care organizations (ACOs) have taken. If you are part of an ACO and you report through it, you’ll see some more changes than your colleagues in 2021.
The good news is that ACOs that participate in MIPS and the Medicare Shared Savings Program will have to report only once to satisfy the requirements for both programs. The construct for this new APM-based program is called the “APM Performance Pathway.” This pathway incorporates six population health–based measures that cross-cut specialties.
CMS is also proposing that telemedicine reimbursement will become permanent. As of now, telemedicine services will only be paid when a public health emergency has been declared. This ability to reimburse physicians for telemedicine would end when the current public health emergency is over. CMS is proposing to extend reimbursement beyond the pandemic, which will benefit all physicians who perform these remote encounters.
The CMS proposal would also make some other requirements easier to achieve. The use of codes 99495 and 99496 – the transitional care management codes – is expanding by reducing several key accompanying-services restrictions. Before the public health emergency, there were constraints related to scope of practice; the proposal would extend the ability of advanced practice providers to order diagnostic tests, even after the public health emergency ends.
Furthermore, the proposal reduces restrictions related to billing for remote physiologic monitoring services and outlines the possibility of a new, higher-paying virtual visit code.
Although the Quality Payment Program will undergo some changes, they are minor. Be aware of the requirement to hit the 50-point mark to avoid the steep penalties, however. Perhaps greater benefit will be achieved through the government’s continued push to refine the reimbursement system. As a result of budget neutrality, however, these changes will boost some physicians while resulting in losses for others.
The government’s proposed changes are not final, and there is a period during which they are accepting comments on the proposal; the final rule will be announced in November.
If you want to wash your hands of this now, apply for the 2020 performance year hardship for the Quality Payment Program. The application is now open and available through December 31, 2020; completing it will release you of any program requirements in 2020 (and avoid that hefty 9% penalty on your 2022 reimbursement).
This way, you won’t have to concern yourself with any of these rules until next year; the government’s extension of this “get out of jail free” card is a welcome relief for physicians who are frustrated by the regulatory burdens despite the pressure exerted by COVID. Spending 15 minutes to complete this form is well worth your time and may eliminate much of your worry.
A version of this article originally appeared on Medscape.com.
Amid all the chaos and problems caused by COVID-19, one might hope that physicians would get a break on their complicated payment-reporting programs.
But that’s not the case: The government recently released the 2021 proposed rule for the Quality Payment Program (QPP), often referred to by its most popular participation track, the Merit-Based Incentive Payment System (MIPS). The program, which launched in 2017, gets annual updates, and this year is no different.
Some good news has made primary care and some other physicians happy.
The government’s proposal includes significant changes to reimbursement for all physicians.
Specialties that rely heavily on office-based E/M services are delighted at this change. Those include internists, family physicians, neurologists, pulmonologists, dermatologists, and all other specialties that rely heavily on office encounters.
According to the estimates from the Centers for Medicare & Medicaid Services (CMS), endocrinologists and rheumatologists are the big winners, at 17% and 16% projected increases, respectively. The government has been pushing to make this shift in reimbursement from surgeries and procedures to office visits for years. Although some physicians may celebrate the change, others will not.
The reimbursement plan for professional services depends on budget neutrality, meaning that the budget increases need to be counterbalanced by budget declines. Specialties that rely heavily on procedures and surgeries will suffer losses. These corresponding reductions felt by proceduralists and surgeons will counterbalance the good fortune of physicians who rely on office visits for the bulk of their revenue. Radiologists, for example, are projected by CMS to experience a 11% downturn, and cardiac surgeons face a 9% decline.
These consequences are significant. The 2021 shift may be the single biggest transfer of reimbursement in the history of the scale, which was adopted in the early 1990s.
If the change affected only Medicare reimbursement, perhaps it would be less significant. Because the majority of private payers use the government’s scale – the resource-based relative value scale – the impact will reverberate across physicians’ bottom lines. Given the state of many physicians’ finances, driven by the pandemic, this may send some affected physicians into a downward spiral.
The boost to E/M reimbursement – which represents approximately 20% of the overall Medicare payout to physicians each year – puts downward pressure on the professional services conversion factor as well.
For 2021, it is proposed to be $32.2605, representing a decrease of $3.83 from the 2020 conversion factor of $36.0896. The resultant conversion factor – which serves as a multiplier applied to the relative value unit to come up with the payment – effectively reduces payments to physicians across the board by 10.6%. Thus, even those who enjoy the benefits of the new E/M increases will see the potential reimbursement high point cut down.
Before launching into the changes in store for 2021, it’s good to determine whether you are an eligible clinician: You need to have more than $90,000 in Medicare Part B charges per year, see more than 200 Medicare Part B patients per year, and provide 200 or more covered professional services to Part B patients.
The program is voluntary, but there are steep penalties for eligible clinicians who don’t participate. For the 2021 reporting year, a 9% penalty will be imposed on Medicare reimbursement in 2023 in the event of participation failure. You can verify your participation status here; you’ll need your National Provider Identifier to run the search, but it takes only seconds to determine your eligibility.
A 9% penalty is a pretty big hit to your income. With 9% at stake, eligible clinicians need to actively engage in the program. Although there have been changes, the basic four-category system remains the same for the MIPS track, as follows: quality, cost, improvement activities, and promoting interoperability.
The four category weights, used to evaluate performance, are changing in 2021. Cost category weight goes up by 5 percentage points, to be 20% of the clinician’s score, and the quality category goes down by 5 percentage points to contribute 40% to the weight. Promoting interoperability remains 25% of the score, with improvement activities constituting the final 15%.
Other key changes include the following:
- The CMS’s Web interface for submission for quality measures will be shuttered in 2021. Users of this submission method will have to find and use another way to report their quality measures.
- Quality measures will be scored against pre-COVID benchmarks in lieu of comparisons with the 2020 reporting year; 206 quality measures are proposed for 2021, compared with the current list of 219.
- Telehealth will be incorporated in the cost category by updates to the measure specifications for the episode-based and total per capita cost measures.
- A new health information exchange measure is added to the promoting interoperability category, and “incorporating” replaces “reconciling” in the reporting requirement “Support Electronic Referral Loops by Receiving and Incorporating Health Information.”
To avoid the 9% penalty, eligible clinicians must earn 50 points in 2021, up from 45 in the current year. Achieving “exceptional performance” remains at 85 points. This elevated level of engagement allows access to a pot of money Congress set aside for high performers.
Many physicians feel that too much work is required to earn the “paltry” bonuses; even a perfect score of 100 has only resulted in bonuses of 1.88% and 1.68%, respectively, in the past 2 years. That includes the $500 million allocation that Congress set aside; this extra funding to reward exceptional performance is only available for the first 6 years of the law. Although the 2019 scores have been released to participants, CMS has not yet announced the overall national average, but it’s expected to be minimal.
The combination of meager payouts and a diminishing funding mechanism has physicians questioning participation altogether. My recent conversations with physicians who qualify for the program revealed their intention to participate, but only at a level to achieve the minimum threshold of 45 points this year and 50 in 2021. With so little upside, it’s impossible to make a business case to aim for the stars.
Perhaps the biggest change in 2021, however, is that the program is not making the previously planned switch to MIPS Value Pathways (MVPs). MVPs were designed to align the four performance categories around a specialty, medical condition, or patient population.
CMS introduced MVPs by giving an example of diabetes: “Endocrinologist reports same ‘foundation’ of PI [promoting interoperability] and population health measures as all other clinicians but now has a MIPS Value Pathway with measures and activities that focus on diabetes prevention and treatment.” CMS had expected MVPs to launch in 2021 for all program participants; because of the pandemic, CMS announced an extension for at least 1 year. This comes as a relief to physicians who are just trying to keep the lights on given the financial pressures brought on by the pandemic.
MVPs, however, will be incorporated into the MIPS Alternative Payment Model (APM) participation segment. This will affect many physicians because this is the path that accountable care organizations (ACOs) have taken. If you are part of an ACO and you report through it, you’ll see some more changes than your colleagues in 2021.
The good news is that ACOs that participate in MIPS and the Medicare Shared Savings Program will have to report only once to satisfy the requirements for both programs. The construct for this new APM-based program is called the “APM Performance Pathway.” This pathway incorporates six population health–based measures that cross-cut specialties.
CMS is also proposing that telemedicine reimbursement will become permanent. As of now, telemedicine services will only be paid when a public health emergency has been declared. This ability to reimburse physicians for telemedicine would end when the current public health emergency is over. CMS is proposing to extend reimbursement beyond the pandemic, which will benefit all physicians who perform these remote encounters.
The CMS proposal would also make some other requirements easier to achieve. The use of codes 99495 and 99496 – the transitional care management codes – is expanding by reducing several key accompanying-services restrictions. Before the public health emergency, there were constraints related to scope of practice; the proposal would extend the ability of advanced practice providers to order diagnostic tests, even after the public health emergency ends.
Furthermore, the proposal reduces restrictions related to billing for remote physiologic monitoring services and outlines the possibility of a new, higher-paying virtual visit code.
Although the Quality Payment Program will undergo some changes, they are minor. Be aware of the requirement to hit the 50-point mark to avoid the steep penalties, however. Perhaps greater benefit will be achieved through the government’s continued push to refine the reimbursement system. As a result of budget neutrality, however, these changes will boost some physicians while resulting in losses for others.
The government’s proposed changes are not final, and there is a period during which they are accepting comments on the proposal; the final rule will be announced in November.
If you want to wash your hands of this now, apply for the 2020 performance year hardship for the Quality Payment Program. The application is now open and available through December 31, 2020; completing it will release you of any program requirements in 2020 (and avoid that hefty 9% penalty on your 2022 reimbursement).
This way, you won’t have to concern yourself with any of these rules until next year; the government’s extension of this “get out of jail free” card is a welcome relief for physicians who are frustrated by the regulatory burdens despite the pressure exerted by COVID. Spending 15 minutes to complete this form is well worth your time and may eliminate much of your worry.
A version of this article originally appeared on Medscape.com.
Amid all the chaos and problems caused by COVID-19, one might hope that physicians would get a break on their complicated payment-reporting programs.
But that’s not the case: The government recently released the 2021 proposed rule for the Quality Payment Program (QPP), often referred to by its most popular participation track, the Merit-Based Incentive Payment System (MIPS). The program, which launched in 2017, gets annual updates, and this year is no different.
Some good news has made primary care and some other physicians happy.
The government’s proposal includes significant changes to reimbursement for all physicians.
Specialties that rely heavily on office-based E/M services are delighted at this change. Those include internists, family physicians, neurologists, pulmonologists, dermatologists, and all other specialties that rely heavily on office encounters.
According to the estimates from the Centers for Medicare & Medicaid Services (CMS), endocrinologists and rheumatologists are the big winners, at 17% and 16% projected increases, respectively. The government has been pushing to make this shift in reimbursement from surgeries and procedures to office visits for years. Although some physicians may celebrate the change, others will not.
The reimbursement plan for professional services depends on budget neutrality, meaning that the budget increases need to be counterbalanced by budget declines. Specialties that rely heavily on procedures and surgeries will suffer losses. These corresponding reductions felt by proceduralists and surgeons will counterbalance the good fortune of physicians who rely on office visits for the bulk of their revenue. Radiologists, for example, are projected by CMS to experience a 11% downturn, and cardiac surgeons face a 9% decline.
These consequences are significant. The 2021 shift may be the single biggest transfer of reimbursement in the history of the scale, which was adopted in the early 1990s.
If the change affected only Medicare reimbursement, perhaps it would be less significant. Because the majority of private payers use the government’s scale – the resource-based relative value scale – the impact will reverberate across physicians’ bottom lines. Given the state of many physicians’ finances, driven by the pandemic, this may send some affected physicians into a downward spiral.
The boost to E/M reimbursement – which represents approximately 20% of the overall Medicare payout to physicians each year – puts downward pressure on the professional services conversion factor as well.
For 2021, it is proposed to be $32.2605, representing a decrease of $3.83 from the 2020 conversion factor of $36.0896. The resultant conversion factor – which serves as a multiplier applied to the relative value unit to come up with the payment – effectively reduces payments to physicians across the board by 10.6%. Thus, even those who enjoy the benefits of the new E/M increases will see the potential reimbursement high point cut down.
Before launching into the changes in store for 2021, it’s good to determine whether you are an eligible clinician: You need to have more than $90,000 in Medicare Part B charges per year, see more than 200 Medicare Part B patients per year, and provide 200 or more covered professional services to Part B patients.
The program is voluntary, but there are steep penalties for eligible clinicians who don’t participate. For the 2021 reporting year, a 9% penalty will be imposed on Medicare reimbursement in 2023 in the event of participation failure. You can verify your participation status here; you’ll need your National Provider Identifier to run the search, but it takes only seconds to determine your eligibility.
A 9% penalty is a pretty big hit to your income. With 9% at stake, eligible clinicians need to actively engage in the program. Although there have been changes, the basic four-category system remains the same for the MIPS track, as follows: quality, cost, improvement activities, and promoting interoperability.
The four category weights, used to evaluate performance, are changing in 2021. Cost category weight goes up by 5 percentage points, to be 20% of the clinician’s score, and the quality category goes down by 5 percentage points to contribute 40% to the weight. Promoting interoperability remains 25% of the score, with improvement activities constituting the final 15%.
Other key changes include the following:
- The CMS’s Web interface for submission for quality measures will be shuttered in 2021. Users of this submission method will have to find and use another way to report their quality measures.
- Quality measures will be scored against pre-COVID benchmarks in lieu of comparisons with the 2020 reporting year; 206 quality measures are proposed for 2021, compared with the current list of 219.
- Telehealth will be incorporated in the cost category by updates to the measure specifications for the episode-based and total per capita cost measures.
- A new health information exchange measure is added to the promoting interoperability category, and “incorporating” replaces “reconciling” in the reporting requirement “Support Electronic Referral Loops by Receiving and Incorporating Health Information.”
To avoid the 9% penalty, eligible clinicians must earn 50 points in 2021, up from 45 in the current year. Achieving “exceptional performance” remains at 85 points. This elevated level of engagement allows access to a pot of money Congress set aside for high performers.
Many physicians feel that too much work is required to earn the “paltry” bonuses; even a perfect score of 100 has only resulted in bonuses of 1.88% and 1.68%, respectively, in the past 2 years. That includes the $500 million allocation that Congress set aside; this extra funding to reward exceptional performance is only available for the first 6 years of the law. Although the 2019 scores have been released to participants, CMS has not yet announced the overall national average, but it’s expected to be minimal.
The combination of meager payouts and a diminishing funding mechanism has physicians questioning participation altogether. My recent conversations with physicians who qualify for the program revealed their intention to participate, but only at a level to achieve the minimum threshold of 45 points this year and 50 in 2021. With so little upside, it’s impossible to make a business case to aim for the stars.
Perhaps the biggest change in 2021, however, is that the program is not making the previously planned switch to MIPS Value Pathways (MVPs). MVPs were designed to align the four performance categories around a specialty, medical condition, or patient population.
CMS introduced MVPs by giving an example of diabetes: “Endocrinologist reports same ‘foundation’ of PI [promoting interoperability] and population health measures as all other clinicians but now has a MIPS Value Pathway with measures and activities that focus on diabetes prevention and treatment.” CMS had expected MVPs to launch in 2021 for all program participants; because of the pandemic, CMS announced an extension for at least 1 year. This comes as a relief to physicians who are just trying to keep the lights on given the financial pressures brought on by the pandemic.
MVPs, however, will be incorporated into the MIPS Alternative Payment Model (APM) participation segment. This will affect many physicians because this is the path that accountable care organizations (ACOs) have taken. If you are part of an ACO and you report through it, you’ll see some more changes than your colleagues in 2021.
The good news is that ACOs that participate in MIPS and the Medicare Shared Savings Program will have to report only once to satisfy the requirements for both programs. The construct for this new APM-based program is called the “APM Performance Pathway.” This pathway incorporates six population health–based measures that cross-cut specialties.
CMS is also proposing that telemedicine reimbursement will become permanent. As of now, telemedicine services will only be paid when a public health emergency has been declared. This ability to reimburse physicians for telemedicine would end when the current public health emergency is over. CMS is proposing to extend reimbursement beyond the pandemic, which will benefit all physicians who perform these remote encounters.
The CMS proposal would also make some other requirements easier to achieve. The use of codes 99495 and 99496 – the transitional care management codes – is expanding by reducing several key accompanying-services restrictions. Before the public health emergency, there were constraints related to scope of practice; the proposal would extend the ability of advanced practice providers to order diagnostic tests, even after the public health emergency ends.
Furthermore, the proposal reduces restrictions related to billing for remote physiologic monitoring services and outlines the possibility of a new, higher-paying virtual visit code.
Although the Quality Payment Program will undergo some changes, they are minor. Be aware of the requirement to hit the 50-point mark to avoid the steep penalties, however. Perhaps greater benefit will be achieved through the government’s continued push to refine the reimbursement system. As a result of budget neutrality, however, these changes will boost some physicians while resulting in losses for others.
The government’s proposed changes are not final, and there is a period during which they are accepting comments on the proposal; the final rule will be announced in November.
If you want to wash your hands of this now, apply for the 2020 performance year hardship for the Quality Payment Program. The application is now open and available through December 31, 2020; completing it will release you of any program requirements in 2020 (and avoid that hefty 9% penalty on your 2022 reimbursement).
This way, you won’t have to concern yourself with any of these rules until next year; the government’s extension of this “get out of jail free” card is a welcome relief for physicians who are frustrated by the regulatory burdens despite the pressure exerted by COVID. Spending 15 minutes to complete this form is well worth your time and may eliminate much of your worry.
A version of this article originally appeared on Medscape.com.
No benefit with postoperative radiotherapy in stage IIIAN2 NSCLC
In patients with completely resected non–small cell lung cancer and proven N2 disease, postoperative radiotherapy (PORT) provided no significant improvement in disease-free survival (DFS) at 3 years, according to a phase 3 trial.
Investigator Cécile Le Péchoux, MD, a radiation oncologist from Institut Gustave Roussy in Paris, presented the results of the Lung ART trial at the European Society for Medical Oncology Virtual Congress 2020.
The results resolve what ESMO commentator Rafal Dziadziuszko, MD, PhD, of Medical University of Gdansk (Poland), called “perhaps the longest ongoing debate in thoracic oncology,” on whether or not to irradiate patients with mediastinal lymph node involvement after surgery.
Dr. Dziadziuszko noted that some past studies have suggested PORT may provide improved local control and a survival benefit, but other studies have shown no survival benefit and evidence of harm with PORT.
“So here is an academic study with the answer,” Dr. Dziadziuszko said.
Study details
The intention-to-treat population of Lung ART included 252 patients randomized to receive 5.5 weeks of PORT (54 Gy) and 249 patients randomized to the control arm without PORT.
The patients’ median age was 61 years, 66% were men, and adenocarcinoma was the predominant histology (76% of patients in the control arm and 70% in the PORT arm). All patients had received adjuvant or neoadjuvant chemotherapy.
The median follow-up was 4.8 years. The 3-year DFS rate was 47.1% in the PORT arm and 43.8% in the control arm (hazard ratio, 0.85; 95% confidence interval, 0.67-1.07, P = .16). The median DFS was 30.5 months with PORT and 22.8 months in the control group. As for DFS components, the rate for death as a first event was 14.6% in the PORT arm and 5.3% among controls.
The mediastinal relapse rate was higher among controls, at 46.1% versus 25.0% with PORT.
The 3-year overall survival rate was 66.5% among patients in the PORT arm and 68.5% in the control arm.
At least one grade 3-4 toxicity was reported in 23.7% of the PORT group and in 15.0% of controls. The grade 3-4 cardiopulmonary toxicity rate of 10.8% in the PORT arm and 4.9% in the control arm needs to be further explored, Dr. Le Péchoux said.
Applying the results to practice
In response to a question as to which patients might benefit from PORT, Dr. Le Péchoux pointed out that, among patients who have nodes in the inferior part of the mediastinum closer to the heart, PORT might be more toxic.
“For the moment, we did not see anything that would give us a clue,” Dr. Le Péchoux said. “We have a lot to investigate.”
Further analyses looking at patterns of failure, predictive factors of efficacy and toxicity, quality of radiotherapy, and quality of surgery are planned, she added.
Dr. Le Péchoux concluded: “Conformal PORT cannot be recommended as standard of care in all completely resected stage IIIAN2 [non–small cell lung cancer] patients.”
Dr. Dziadziuszko commented further: “For my practice, this is a clear message that routine PORT should not be used in these patients. We are looking for more effective systemic therapies.”
He added that “over 50% 5-year survival in these patients is a great result, and is made possible by modern staging and modern surgery.”
The Lung ART study was sponsored by Gustave Roussy and supported by the French National Cancer Institute, French Health Ministry, and a Cancer Research UK grant. Dr. Le Péchoux and Dr. Dziadziuszko reported no conflicts of interest related to the presentation.
SOURCE: Le Péchoux C et al. ESMO 2020, Abstract LBA3_PR.
In patients with completely resected non–small cell lung cancer and proven N2 disease, postoperative radiotherapy (PORT) provided no significant improvement in disease-free survival (DFS) at 3 years, according to a phase 3 trial.
Investigator Cécile Le Péchoux, MD, a radiation oncologist from Institut Gustave Roussy in Paris, presented the results of the Lung ART trial at the European Society for Medical Oncology Virtual Congress 2020.
The results resolve what ESMO commentator Rafal Dziadziuszko, MD, PhD, of Medical University of Gdansk (Poland), called “perhaps the longest ongoing debate in thoracic oncology,” on whether or not to irradiate patients with mediastinal lymph node involvement after surgery.
Dr. Dziadziuszko noted that some past studies have suggested PORT may provide improved local control and a survival benefit, but other studies have shown no survival benefit and evidence of harm with PORT.
“So here is an academic study with the answer,” Dr. Dziadziuszko said.
Study details
The intention-to-treat population of Lung ART included 252 patients randomized to receive 5.5 weeks of PORT (54 Gy) and 249 patients randomized to the control arm without PORT.
The patients’ median age was 61 years, 66% were men, and adenocarcinoma was the predominant histology (76% of patients in the control arm and 70% in the PORT arm). All patients had received adjuvant or neoadjuvant chemotherapy.
The median follow-up was 4.8 years. The 3-year DFS rate was 47.1% in the PORT arm and 43.8% in the control arm (hazard ratio, 0.85; 95% confidence interval, 0.67-1.07, P = .16). The median DFS was 30.5 months with PORT and 22.8 months in the control group. As for DFS components, the rate for death as a first event was 14.6% in the PORT arm and 5.3% among controls.
The mediastinal relapse rate was higher among controls, at 46.1% versus 25.0% with PORT.
The 3-year overall survival rate was 66.5% among patients in the PORT arm and 68.5% in the control arm.
At least one grade 3-4 toxicity was reported in 23.7% of the PORT group and in 15.0% of controls. The grade 3-4 cardiopulmonary toxicity rate of 10.8% in the PORT arm and 4.9% in the control arm needs to be further explored, Dr. Le Péchoux said.
Applying the results to practice
In response to a question as to which patients might benefit from PORT, Dr. Le Péchoux pointed out that, among patients who have nodes in the inferior part of the mediastinum closer to the heart, PORT might be more toxic.
“For the moment, we did not see anything that would give us a clue,” Dr. Le Péchoux said. “We have a lot to investigate.”
Further analyses looking at patterns of failure, predictive factors of efficacy and toxicity, quality of radiotherapy, and quality of surgery are planned, she added.
Dr. Le Péchoux concluded: “Conformal PORT cannot be recommended as standard of care in all completely resected stage IIIAN2 [non–small cell lung cancer] patients.”
Dr. Dziadziuszko commented further: “For my practice, this is a clear message that routine PORT should not be used in these patients. We are looking for more effective systemic therapies.”
He added that “over 50% 5-year survival in these patients is a great result, and is made possible by modern staging and modern surgery.”
The Lung ART study was sponsored by Gustave Roussy and supported by the French National Cancer Institute, French Health Ministry, and a Cancer Research UK grant. Dr. Le Péchoux and Dr. Dziadziuszko reported no conflicts of interest related to the presentation.
SOURCE: Le Péchoux C et al. ESMO 2020, Abstract LBA3_PR.
In patients with completely resected non–small cell lung cancer and proven N2 disease, postoperative radiotherapy (PORT) provided no significant improvement in disease-free survival (DFS) at 3 years, according to a phase 3 trial.
Investigator Cécile Le Péchoux, MD, a radiation oncologist from Institut Gustave Roussy in Paris, presented the results of the Lung ART trial at the European Society for Medical Oncology Virtual Congress 2020.
The results resolve what ESMO commentator Rafal Dziadziuszko, MD, PhD, of Medical University of Gdansk (Poland), called “perhaps the longest ongoing debate in thoracic oncology,” on whether or not to irradiate patients with mediastinal lymph node involvement after surgery.
Dr. Dziadziuszko noted that some past studies have suggested PORT may provide improved local control and a survival benefit, but other studies have shown no survival benefit and evidence of harm with PORT.
“So here is an academic study with the answer,” Dr. Dziadziuszko said.
Study details
The intention-to-treat population of Lung ART included 252 patients randomized to receive 5.5 weeks of PORT (54 Gy) and 249 patients randomized to the control arm without PORT.
The patients’ median age was 61 years, 66% were men, and adenocarcinoma was the predominant histology (76% of patients in the control arm and 70% in the PORT arm). All patients had received adjuvant or neoadjuvant chemotherapy.
The median follow-up was 4.8 years. The 3-year DFS rate was 47.1% in the PORT arm and 43.8% in the control arm (hazard ratio, 0.85; 95% confidence interval, 0.67-1.07, P = .16). The median DFS was 30.5 months with PORT and 22.8 months in the control group. As for DFS components, the rate for death as a first event was 14.6% in the PORT arm and 5.3% among controls.
The mediastinal relapse rate was higher among controls, at 46.1% versus 25.0% with PORT.
The 3-year overall survival rate was 66.5% among patients in the PORT arm and 68.5% in the control arm.
At least one grade 3-4 toxicity was reported in 23.7% of the PORT group and in 15.0% of controls. The grade 3-4 cardiopulmonary toxicity rate of 10.8% in the PORT arm and 4.9% in the control arm needs to be further explored, Dr. Le Péchoux said.
Applying the results to practice
In response to a question as to which patients might benefit from PORT, Dr. Le Péchoux pointed out that, among patients who have nodes in the inferior part of the mediastinum closer to the heart, PORT might be more toxic.
“For the moment, we did not see anything that would give us a clue,” Dr. Le Péchoux said. “We have a lot to investigate.”
Further analyses looking at patterns of failure, predictive factors of efficacy and toxicity, quality of radiotherapy, and quality of surgery are planned, she added.
Dr. Le Péchoux concluded: “Conformal PORT cannot be recommended as standard of care in all completely resected stage IIIAN2 [non–small cell lung cancer] patients.”
Dr. Dziadziuszko commented further: “For my practice, this is a clear message that routine PORT should not be used in these patients. We are looking for more effective systemic therapies.”
He added that “over 50% 5-year survival in these patients is a great result, and is made possible by modern staging and modern surgery.”
The Lung ART study was sponsored by Gustave Roussy and supported by the French National Cancer Institute, French Health Ministry, and a Cancer Research UK grant. Dr. Le Péchoux and Dr. Dziadziuszko reported no conflicts of interest related to the presentation.
SOURCE: Le Péchoux C et al. ESMO 2020, Abstract LBA3_PR.
FROM ESMO 2020
Survey quantifies COVID-19’s impact on oncology
An international survey provides new insights into how COVID-19 has affected, and may continue to affect, the field of oncology.
The survey showed that “COVID-19 has had a major impact on the organization of patient care, on the well-being of caregivers, on continued medical education, and on clinical trial activities in oncology,” stated Guy Jerusalem, MD, PhD, of Centre Hospitalier Universitaire de Liège (Belgium).
Dr. Jerusalem presented these findings at the European Society for Medical Oncology Virtual Congress 2020.
The survey was distributed by 20 oncologists from 10 of the countries most affected by COVID-19. Responses were obtained from 109 oncologists representing centers in 18 countries. The responses were recorded between June 17 and July 14, 2020.
The survey consisted of 95 items intended to evaluate the impact of COVID-19 on the organization of oncologic care. Questions encompassed the capacity and service offered at each center, the magnitude of COVID-19–based care interruptions and the reasons for them, the ensuing challenges faced, interventions implemented, and the estimated harms to patients during the pandemic.
The 109 oncologists surveyed had a median of 20 years of oncology experience. A majority of respondents were men (61.5%), and the median age was 48.5 years.
The respondents had worked predominantly (62.4%) at academic hospitals, with 29.6% at community hospitals. Most respondents worked at general hospitals with an oncology unit (66.1%) rather than a specialized separate cancer center (32.1%).
The most common specialty was breast cancer (60.6%), followed by gastrointestinal cancer (10.1%), urogenital cancer (9.2%), and lung cancer (8.3%).
Impact on treatment
The treatment modalities affected by the pandemic – through cancellations or delays in more than 10% of patients – included surgery (in 34% of centers), chemotherapy (22%), radiotherapy (13.7%), checkpoint inhibitor therapy (9.1%), monoclonal antibodies (9%), and oral targeted therapy (3.7%).
Among oncologists treating breast cancer, cancellations/delays in more than 10% of patients were reported for everolimus (18%), CDK4/6 inhibitors (8.9%), and endocrine therapy (2.2%).
Overall, 34.8% of respondents reported increased use of granulocyte colony–stimulating factor, and 6.4% reported increased use of erythropoietin.
On the other hand, 11.1% of respondents reported a decrease in the use of double immunotherapy, and 21.9% reported decreased use of corticosteroids.
Not only can the immunosuppressive effects of steroid use increase infection risks, Dr. Jerusalem noted, fever suppression can lead to a delayed diagnosis of COVID-19.
“To circumvent potential higher infection risks or greater disease severity, we use lower doses of steroids, but this is not based on studies,” he said.
“Previous exposure to steroids or being on steroids at the time of COVID-19 infection is a detrimental factor for complications and mortality,” commented ESMO President Solange Peters, MD, PhD, of Centre Hospitalier Universitaire Vaudois in Lausanne, Switzerland.
Dr. Peters noted that the observation was based on lung cancer registry findings. Furthermore, because data from smaller outbreaks of other coronavirus infections suggested worse prognosis and increased mortality, steroid use was already feared in the very early days of the COVID-19 pandemic.
Lastly, earlier cessation of palliative treatment was observed in 32.1% of centers, and 64.2% of respondents agreed that undertreatment because of COVID-19 is a major concern.
Dr. Jerusalem noted that the survey data do not explain the early cessation of palliative treatment. “I suspect that many patients died at home rather than alone in institutions because it was the only way they could die with their families around them.”
Telehealth, meetings, and trials
The survey also revealed rationales for the use of teleconsultation, including follow-up (94.5%), oral therapy (92.7%), immunotherapy (57.8%), and chemotherapy (55%).
Most respondents reported more frequent use of virtual meetings for continuing medical education (94%), oncologic team meetings (92%), and tumor boards (82%).
While about 82% of respondents said they were likely to continue the use of telemedicine, 45% said virtual conferences are not an acceptable alternative to live international conferences such as ESMO, Dr. Jerusalem said.
Finally, nearly three-quarters of respondents (72.5%) said all clinical trial activities are or will soon be activated, or never stopped, at their centers. On the other hand, 27.5% of respondents reported that their centers had major protocol violations or deviations, and 37% of respondents said they expect significant reductions in clinical trial activities this year.
Dr. Jerusalem concluded that COVID-19 is having a major, long-term impact on the organization of patient care, caregivers, continued medical education, and clinical trial activities in oncology.
He cautioned that “the risk of a delayed diagnosis of new cancers and economic consequences of COVID-19 on access to health care and cancer treatments have to be carefully evaluated.”
This research was funded by Fondation Léon Fredericq. Dr. Jerusalem disclosed relationships with Novartis, Roche, Lilly, Pfizer, Amgen, Bristol-Myers Squibb, AstraZeneca, Daiichi Sankyo, AbbVie, MedImmune, and Merck. Dr. Peters disclosed relationships with AbbVie, Amgen, AstraZeneca, and many other companies.
SOURCE: Jerusalem G et al. ESMO 2020, Abstract LBA76.
An international survey provides new insights into how COVID-19 has affected, and may continue to affect, the field of oncology.
The survey showed that “COVID-19 has had a major impact on the organization of patient care, on the well-being of caregivers, on continued medical education, and on clinical trial activities in oncology,” stated Guy Jerusalem, MD, PhD, of Centre Hospitalier Universitaire de Liège (Belgium).
Dr. Jerusalem presented these findings at the European Society for Medical Oncology Virtual Congress 2020.
The survey was distributed by 20 oncologists from 10 of the countries most affected by COVID-19. Responses were obtained from 109 oncologists representing centers in 18 countries. The responses were recorded between June 17 and July 14, 2020.
The survey consisted of 95 items intended to evaluate the impact of COVID-19 on the organization of oncologic care. Questions encompassed the capacity and service offered at each center, the magnitude of COVID-19–based care interruptions and the reasons for them, the ensuing challenges faced, interventions implemented, and the estimated harms to patients during the pandemic.
The 109 oncologists surveyed had a median of 20 years of oncology experience. A majority of respondents were men (61.5%), and the median age was 48.5 years.
The respondents had worked predominantly (62.4%) at academic hospitals, with 29.6% at community hospitals. Most respondents worked at general hospitals with an oncology unit (66.1%) rather than a specialized separate cancer center (32.1%).
The most common specialty was breast cancer (60.6%), followed by gastrointestinal cancer (10.1%), urogenital cancer (9.2%), and lung cancer (8.3%).
Impact on treatment
The treatment modalities affected by the pandemic – through cancellations or delays in more than 10% of patients – included surgery (in 34% of centers), chemotherapy (22%), radiotherapy (13.7%), checkpoint inhibitor therapy (9.1%), monoclonal antibodies (9%), and oral targeted therapy (3.7%).
Among oncologists treating breast cancer, cancellations/delays in more than 10% of patients were reported for everolimus (18%), CDK4/6 inhibitors (8.9%), and endocrine therapy (2.2%).
Overall, 34.8% of respondents reported increased use of granulocyte colony–stimulating factor, and 6.4% reported increased use of erythropoietin.
On the other hand, 11.1% of respondents reported a decrease in the use of double immunotherapy, and 21.9% reported decreased use of corticosteroids.
Not only can the immunosuppressive effects of steroid use increase infection risks, Dr. Jerusalem noted, fever suppression can lead to a delayed diagnosis of COVID-19.
“To circumvent potential higher infection risks or greater disease severity, we use lower doses of steroids, but this is not based on studies,” he said.
“Previous exposure to steroids or being on steroids at the time of COVID-19 infection is a detrimental factor for complications and mortality,” commented ESMO President Solange Peters, MD, PhD, of Centre Hospitalier Universitaire Vaudois in Lausanne, Switzerland.
Dr. Peters noted that the observation was based on lung cancer registry findings. Furthermore, because data from smaller outbreaks of other coronavirus infections suggested worse prognosis and increased mortality, steroid use was already feared in the very early days of the COVID-19 pandemic.
Lastly, earlier cessation of palliative treatment was observed in 32.1% of centers, and 64.2% of respondents agreed that undertreatment because of COVID-19 is a major concern.
Dr. Jerusalem noted that the survey data do not explain the early cessation of palliative treatment. “I suspect that many patients died at home rather than alone in institutions because it was the only way they could die with their families around them.”
Telehealth, meetings, and trials
The survey also revealed rationales for the use of teleconsultation, including follow-up (94.5%), oral therapy (92.7%), immunotherapy (57.8%), and chemotherapy (55%).
Most respondents reported more frequent use of virtual meetings for continuing medical education (94%), oncologic team meetings (92%), and tumor boards (82%).
While about 82% of respondents said they were likely to continue the use of telemedicine, 45% said virtual conferences are not an acceptable alternative to live international conferences such as ESMO, Dr. Jerusalem said.
Finally, nearly three-quarters of respondents (72.5%) said all clinical trial activities are or will soon be activated, or never stopped, at their centers. On the other hand, 27.5% of respondents reported that their centers had major protocol violations or deviations, and 37% of respondents said they expect significant reductions in clinical trial activities this year.
Dr. Jerusalem concluded that COVID-19 is having a major, long-term impact on the organization of patient care, caregivers, continued medical education, and clinical trial activities in oncology.
He cautioned that “the risk of a delayed diagnosis of new cancers and economic consequences of COVID-19 on access to health care and cancer treatments have to be carefully evaluated.”
This research was funded by Fondation Léon Fredericq. Dr. Jerusalem disclosed relationships with Novartis, Roche, Lilly, Pfizer, Amgen, Bristol-Myers Squibb, AstraZeneca, Daiichi Sankyo, AbbVie, MedImmune, and Merck. Dr. Peters disclosed relationships with AbbVie, Amgen, AstraZeneca, and many other companies.
SOURCE: Jerusalem G et al. ESMO 2020, Abstract LBA76.
An international survey provides new insights into how COVID-19 has affected, and may continue to affect, the field of oncology.
The survey showed that “COVID-19 has had a major impact on the organization of patient care, on the well-being of caregivers, on continued medical education, and on clinical trial activities in oncology,” stated Guy Jerusalem, MD, PhD, of Centre Hospitalier Universitaire de Liège (Belgium).
Dr. Jerusalem presented these findings at the European Society for Medical Oncology Virtual Congress 2020.
The survey was distributed by 20 oncologists from 10 of the countries most affected by COVID-19. Responses were obtained from 109 oncologists representing centers in 18 countries. The responses were recorded between June 17 and July 14, 2020.
The survey consisted of 95 items intended to evaluate the impact of COVID-19 on the organization of oncologic care. Questions encompassed the capacity and service offered at each center, the magnitude of COVID-19–based care interruptions and the reasons for them, the ensuing challenges faced, interventions implemented, and the estimated harms to patients during the pandemic.
The 109 oncologists surveyed had a median of 20 years of oncology experience. A majority of respondents were men (61.5%), and the median age was 48.5 years.
The respondents had worked predominantly (62.4%) at academic hospitals, with 29.6% at community hospitals. Most respondents worked at general hospitals with an oncology unit (66.1%) rather than a specialized separate cancer center (32.1%).
The most common specialty was breast cancer (60.6%), followed by gastrointestinal cancer (10.1%), urogenital cancer (9.2%), and lung cancer (8.3%).
Impact on treatment
The treatment modalities affected by the pandemic – through cancellations or delays in more than 10% of patients – included surgery (in 34% of centers), chemotherapy (22%), radiotherapy (13.7%), checkpoint inhibitor therapy (9.1%), monoclonal antibodies (9%), and oral targeted therapy (3.7%).
Among oncologists treating breast cancer, cancellations/delays in more than 10% of patients were reported for everolimus (18%), CDK4/6 inhibitors (8.9%), and endocrine therapy (2.2%).
Overall, 34.8% of respondents reported increased use of granulocyte colony–stimulating factor, and 6.4% reported increased use of erythropoietin.
On the other hand, 11.1% of respondents reported a decrease in the use of double immunotherapy, and 21.9% reported decreased use of corticosteroids.
Not only can the immunosuppressive effects of steroid use increase infection risks, Dr. Jerusalem noted, fever suppression can lead to a delayed diagnosis of COVID-19.
“To circumvent potential higher infection risks or greater disease severity, we use lower doses of steroids, but this is not based on studies,” he said.
“Previous exposure to steroids or being on steroids at the time of COVID-19 infection is a detrimental factor for complications and mortality,” commented ESMO President Solange Peters, MD, PhD, of Centre Hospitalier Universitaire Vaudois in Lausanne, Switzerland.
Dr. Peters noted that the observation was based on lung cancer registry findings. Furthermore, because data from smaller outbreaks of other coronavirus infections suggested worse prognosis and increased mortality, steroid use was already feared in the very early days of the COVID-19 pandemic.
Lastly, earlier cessation of palliative treatment was observed in 32.1% of centers, and 64.2% of respondents agreed that undertreatment because of COVID-19 is a major concern.
Dr. Jerusalem noted that the survey data do not explain the early cessation of palliative treatment. “I suspect that many patients died at home rather than alone in institutions because it was the only way they could die with their families around them.”
Telehealth, meetings, and trials
The survey also revealed rationales for the use of teleconsultation, including follow-up (94.5%), oral therapy (92.7%), immunotherapy (57.8%), and chemotherapy (55%).
Most respondents reported more frequent use of virtual meetings for continuing medical education (94%), oncologic team meetings (92%), and tumor boards (82%).
While about 82% of respondents said they were likely to continue the use of telemedicine, 45% said virtual conferences are not an acceptable alternative to live international conferences such as ESMO, Dr. Jerusalem said.
Finally, nearly three-quarters of respondents (72.5%) said all clinical trial activities are or will soon be activated, or never stopped, at their centers. On the other hand, 27.5% of respondents reported that their centers had major protocol violations or deviations, and 37% of respondents said they expect significant reductions in clinical trial activities this year.
Dr. Jerusalem concluded that COVID-19 is having a major, long-term impact on the organization of patient care, caregivers, continued medical education, and clinical trial activities in oncology.
He cautioned that “the risk of a delayed diagnosis of new cancers and economic consequences of COVID-19 on access to health care and cancer treatments have to be carefully evaluated.”
This research was funded by Fondation Léon Fredericq. Dr. Jerusalem disclosed relationships with Novartis, Roche, Lilly, Pfizer, Amgen, Bristol-Myers Squibb, AstraZeneca, Daiichi Sankyo, AbbVie, MedImmune, and Merck. Dr. Peters disclosed relationships with AbbVie, Amgen, AstraZeneca, and many other companies.
SOURCE: Jerusalem G et al. ESMO 2020, Abstract LBA76.
FROM ESMO 2020
Metformin may delay prostate cancer progression, randomized trial suggests
Adding metformin to standard care for advanced prostate cancer appeared to lengthen time to castration-resistant disease in a small, randomized trial.
“According to our data, metformin potentially prolongs the time to progression … when combined with androgen deprivation therapy,” said investigator Reham ALGhandour, MD, PhD, an assistant lecturer of medical oncology at Mansoura (Egypt) University.
Dr. ALGhandour presented the data at the European Society for Medical Oncology Virtual Congress 2020.
Prior observational studies have indicated that metformin may benefit patients with prostate cancer. A meta-analysis of cohort studies suggested metformin can significantly improve overall, cancer-specific, and recurrence-free survival in prostate cancer patients.
To explore this further, Dr. ALGhandour and colleagues conducted a randomized trial. They enrolled 124 men with high-risk locally advanced or metastatic hormone-sensitive prostate cancer.
The investigators randomized 62 patients to testosterone suppression with or without antiandrogen, and 62 others to a standard regimen plus metformin at 850 mg twice daily.
All patients had an Easter Cooperative Oncology Group performance score of 0-2, were set to receive androgen deprivation therapy long term, and had no prior metformin use. Docetaxel was permitted for metastatic patients, and external beam radiation therapy was used for localized and regional disease.
Results: ‘Dramatic’ but not ‘definitive’
At a median follow-up of 18 months, there was a significant difference in time to castration-resistant prostate cancer. The median time to progression was 29 months with metformin and 20 months with standard therapy alone (P = .01).
Subgroup analyses showed a benefit with metformin in men with N1 disease (P = .001) and men with localized disease/low tumor burden (P = .008).
There was no significant difference in overall survival between the treatment arms (P = .1). The median overall survival was not reached in either arm.
About 4% of metformin patients had grade 2 diarrhea, but adverse events were otherwise comparable between the arms and mostly related to androgen deprivation.
“The authors have got some pretty dramatic findings,” said Noel Clarke, MBBS, a consultant urologist at Salford Royal Hospital and The Christie, Manchester, England, who was a discussant for the study.
Dr. Clarke said the data are “hypothesis generating,” but, because of small numbers, the study “really falls well short of anything that is definitive.”
“We’ll have to wait for bigger studies,” Dr. Clarke said, adding that one arm of the STAMPEDE trial has recruited 2,200 prostate cancer patients to standard of care plus metformin.
“We will report on this trial presently,” he said. “Hopefully, this will add to the body of literature which will determine whether or not metformin is useful with standard of care in this disease.”
Dr. Clarke said the possible benefits of metformin are probably related to energetics in prostate cancer.
“AMPK [AMP-activated protein kinase] is the energy superhighway regulator,” he explained. “[I]t slows down the effects of cell proliferation and energy usage, and it promotes the use of energy storage mechanisms.
“Metformin, because it acts on AMPK to up-regulate it … enhances the effect of AMPK, shutting down the catabolic elements and impeding other elements of prostate cancer migration. So AMPK inhibits epithelial to mesenchymal transition, which is well known as a metastatic mechanism.”
There was no outside funding for this study, and the investigators didn’t report any disclosures. Dr. Clarke disclosed relationships with Janssen, Astellas, Sanofi, and AstraZeneca.
SOURCE: ALGhandour R et al. ESMO 2020, Abstract 617MO.
Adding metformin to standard care for advanced prostate cancer appeared to lengthen time to castration-resistant disease in a small, randomized trial.
“According to our data, metformin potentially prolongs the time to progression … when combined with androgen deprivation therapy,” said investigator Reham ALGhandour, MD, PhD, an assistant lecturer of medical oncology at Mansoura (Egypt) University.
Dr. ALGhandour presented the data at the European Society for Medical Oncology Virtual Congress 2020.
Prior observational studies have indicated that metformin may benefit patients with prostate cancer. A meta-analysis of cohort studies suggested metformin can significantly improve overall, cancer-specific, and recurrence-free survival in prostate cancer patients.
To explore this further, Dr. ALGhandour and colleagues conducted a randomized trial. They enrolled 124 men with high-risk locally advanced or metastatic hormone-sensitive prostate cancer.
The investigators randomized 62 patients to testosterone suppression with or without antiandrogen, and 62 others to a standard regimen plus metformin at 850 mg twice daily.
All patients had an Easter Cooperative Oncology Group performance score of 0-2, were set to receive androgen deprivation therapy long term, and had no prior metformin use. Docetaxel was permitted for metastatic patients, and external beam radiation therapy was used for localized and regional disease.
Results: ‘Dramatic’ but not ‘definitive’
At a median follow-up of 18 months, there was a significant difference in time to castration-resistant prostate cancer. The median time to progression was 29 months with metformin and 20 months with standard therapy alone (P = .01).
Subgroup analyses showed a benefit with metformin in men with N1 disease (P = .001) and men with localized disease/low tumor burden (P = .008).
There was no significant difference in overall survival between the treatment arms (P = .1). The median overall survival was not reached in either arm.
About 4% of metformin patients had grade 2 diarrhea, but adverse events were otherwise comparable between the arms and mostly related to androgen deprivation.
“The authors have got some pretty dramatic findings,” said Noel Clarke, MBBS, a consultant urologist at Salford Royal Hospital and The Christie, Manchester, England, who was a discussant for the study.
Dr. Clarke said the data are “hypothesis generating,” but, because of small numbers, the study “really falls well short of anything that is definitive.”
“We’ll have to wait for bigger studies,” Dr. Clarke said, adding that one arm of the STAMPEDE trial has recruited 2,200 prostate cancer patients to standard of care plus metformin.
“We will report on this trial presently,” he said. “Hopefully, this will add to the body of literature which will determine whether or not metformin is useful with standard of care in this disease.”
Dr. Clarke said the possible benefits of metformin are probably related to energetics in prostate cancer.
“AMPK [AMP-activated protein kinase] is the energy superhighway regulator,” he explained. “[I]t slows down the effects of cell proliferation and energy usage, and it promotes the use of energy storage mechanisms.
“Metformin, because it acts on AMPK to up-regulate it … enhances the effect of AMPK, shutting down the catabolic elements and impeding other elements of prostate cancer migration. So AMPK inhibits epithelial to mesenchymal transition, which is well known as a metastatic mechanism.”
There was no outside funding for this study, and the investigators didn’t report any disclosures. Dr. Clarke disclosed relationships with Janssen, Astellas, Sanofi, and AstraZeneca.
SOURCE: ALGhandour R et al. ESMO 2020, Abstract 617MO.
Adding metformin to standard care for advanced prostate cancer appeared to lengthen time to castration-resistant disease in a small, randomized trial.
“According to our data, metformin potentially prolongs the time to progression … when combined with androgen deprivation therapy,” said investigator Reham ALGhandour, MD, PhD, an assistant lecturer of medical oncology at Mansoura (Egypt) University.
Dr. ALGhandour presented the data at the European Society for Medical Oncology Virtual Congress 2020.
Prior observational studies have indicated that metformin may benefit patients with prostate cancer. A meta-analysis of cohort studies suggested metformin can significantly improve overall, cancer-specific, and recurrence-free survival in prostate cancer patients.
To explore this further, Dr. ALGhandour and colleagues conducted a randomized trial. They enrolled 124 men with high-risk locally advanced or metastatic hormone-sensitive prostate cancer.
The investigators randomized 62 patients to testosterone suppression with or without antiandrogen, and 62 others to a standard regimen plus metformin at 850 mg twice daily.
All patients had an Easter Cooperative Oncology Group performance score of 0-2, were set to receive androgen deprivation therapy long term, and had no prior metformin use. Docetaxel was permitted for metastatic patients, and external beam radiation therapy was used for localized and regional disease.
Results: ‘Dramatic’ but not ‘definitive’
At a median follow-up of 18 months, there was a significant difference in time to castration-resistant prostate cancer. The median time to progression was 29 months with metformin and 20 months with standard therapy alone (P = .01).
Subgroup analyses showed a benefit with metformin in men with N1 disease (P = .001) and men with localized disease/low tumor burden (P = .008).
There was no significant difference in overall survival between the treatment arms (P = .1). The median overall survival was not reached in either arm.
About 4% of metformin patients had grade 2 diarrhea, but adverse events were otherwise comparable between the arms and mostly related to androgen deprivation.
“The authors have got some pretty dramatic findings,” said Noel Clarke, MBBS, a consultant urologist at Salford Royal Hospital and The Christie, Manchester, England, who was a discussant for the study.
Dr. Clarke said the data are “hypothesis generating,” but, because of small numbers, the study “really falls well short of anything that is definitive.”
“We’ll have to wait for bigger studies,” Dr. Clarke said, adding that one arm of the STAMPEDE trial has recruited 2,200 prostate cancer patients to standard of care plus metformin.
“We will report on this trial presently,” he said. “Hopefully, this will add to the body of literature which will determine whether or not metformin is useful with standard of care in this disease.”
Dr. Clarke said the possible benefits of metformin are probably related to energetics in prostate cancer.
“AMPK [AMP-activated protein kinase] is the energy superhighway regulator,” he explained. “[I]t slows down the effects of cell proliferation and energy usage, and it promotes the use of energy storage mechanisms.
“Metformin, because it acts on AMPK to up-regulate it … enhances the effect of AMPK, shutting down the catabolic elements and impeding other elements of prostate cancer migration. So AMPK inhibits epithelial to mesenchymal transition, which is well known as a metastatic mechanism.”
There was no outside funding for this study, and the investigators didn’t report any disclosures. Dr. Clarke disclosed relationships with Janssen, Astellas, Sanofi, and AstraZeneca.
SOURCE: ALGhandour R et al. ESMO 2020, Abstract 617MO.
FROM ESMO 2020
Highlights from the 2020 Scientific Meeting of the Society of Gynecologic Surgeons

- Even in a virtual environment, the Society of Gynecologic Surgeons delivers without a “glitch”
- Transvaginal reconstructive surgery for POP: Innovative approach to graft augmentation in the post-mesh era
- How to build your identity as a physician online
Patrick Culligan, MD
Co-Director, Urogynecology
Valley Hospital System
Ridgewood, New Jersey
Professor, Gynecology & Urology
Weill Cornell Medical College
New York, New York
Jessica Sosa-Stanley, MD
Fellow, Minimally Invasive Gynecologic Surgery
St. Luke’s University Health Network
The Institute for Female Pelvic Medicine
Bethlehem, Pennsylvania
Vincent R. Lucente, MD, MBA
Section Chief, Urogynecology
Chief, Gynecology
Medical Director, Pelvic Health Center
St. Luke’s University Health Network
Partner & Chief Medical Officer
The Institute for Female Pelvic Medicine &
Reconstructive Surgery
Clinical Professor, Obstetrics and Gynecology
Temple University College of Medicine
Bethlehem, Pennsylvania
Michael J. Kennelly, MD
Medical Director, Charlotte Continence Center
Carolinas Medical Center
Director of Urology
Carolinas Rehabilitation Hospital
Co-Director, Women’s Center for Pelvic Health
Clinical Professor, Department of Surgery, Division
of Urology
University of North Carolina, Chapel Hill
Sachin B. Shenoy, MD
Resident
New York-Presbyterian Brooklyn Methodist Hospital
Brooklyn, New York
Brad Bowman, MD
Chief Medical Officer
Healthgrades
Atlanta, Georgia
Peter M. Lotze, MD
Urogynecologist
Women’s Pelvic Restorative Center
Houston, Texas
Heather Schueppert
Chief Marketing Officer
Unified Women’s Healthcare
Boca Raton, Florida

- Even in a virtual environment, the Society of Gynecologic Surgeons delivers without a “glitch”
- Transvaginal reconstructive surgery for POP: Innovative approach to graft augmentation in the post-mesh era
- How to build your identity as a physician online
Patrick Culligan, MD
Co-Director, Urogynecology
Valley Hospital System
Ridgewood, New Jersey
Professor, Gynecology & Urology
Weill Cornell Medical College
New York, New York
Jessica Sosa-Stanley, MD
Fellow, Minimally Invasive Gynecologic Surgery
St. Luke’s University Health Network
The Institute for Female Pelvic Medicine
Bethlehem, Pennsylvania
Vincent R. Lucente, MD, MBA
Section Chief, Urogynecology
Chief, Gynecology
Medical Director, Pelvic Health Center
St. Luke’s University Health Network
Partner & Chief Medical Officer
The Institute for Female Pelvic Medicine &
Reconstructive Surgery
Clinical Professor, Obstetrics and Gynecology
Temple University College of Medicine
Bethlehem, Pennsylvania
Michael J. Kennelly, MD
Medical Director, Charlotte Continence Center
Carolinas Medical Center
Director of Urology
Carolinas Rehabilitation Hospital
Co-Director, Women’s Center for Pelvic Health
Clinical Professor, Department of Surgery, Division
of Urology
University of North Carolina, Chapel Hill
Sachin B. Shenoy, MD
Resident
New York-Presbyterian Brooklyn Methodist Hospital
Brooklyn, New York
Brad Bowman, MD
Chief Medical Officer
Healthgrades
Atlanta, Georgia
Peter M. Lotze, MD
Urogynecologist
Women’s Pelvic Restorative Center
Houston, Texas
Heather Schueppert
Chief Marketing Officer
Unified Women’s Healthcare
Boca Raton, Florida

- Even in a virtual environment, the Society of Gynecologic Surgeons delivers without a “glitch”
- Transvaginal reconstructive surgery for POP: Innovative approach to graft augmentation in the post-mesh era
- How to build your identity as a physician online
Patrick Culligan, MD
Co-Director, Urogynecology
Valley Hospital System
Ridgewood, New Jersey
Professor, Gynecology & Urology
Weill Cornell Medical College
New York, New York
Jessica Sosa-Stanley, MD
Fellow, Minimally Invasive Gynecologic Surgery
St. Luke’s University Health Network
The Institute for Female Pelvic Medicine
Bethlehem, Pennsylvania
Vincent R. Lucente, MD, MBA
Section Chief, Urogynecology
Chief, Gynecology
Medical Director, Pelvic Health Center
St. Luke’s University Health Network
Partner & Chief Medical Officer
The Institute for Female Pelvic Medicine &
Reconstructive Surgery
Clinical Professor, Obstetrics and Gynecology
Temple University College of Medicine
Bethlehem, Pennsylvania
Michael J. Kennelly, MD
Medical Director, Charlotte Continence Center
Carolinas Medical Center
Director of Urology
Carolinas Rehabilitation Hospital
Co-Director, Women’s Center for Pelvic Health
Clinical Professor, Department of Surgery, Division
of Urology
University of North Carolina, Chapel Hill
Sachin B. Shenoy, MD
Resident
New York-Presbyterian Brooklyn Methodist Hospital
Brooklyn, New York
Brad Bowman, MD
Chief Medical Officer
Healthgrades
Atlanta, Georgia
Peter M. Lotze, MD
Urogynecologist
Women’s Pelvic Restorative Center
Houston, Texas
Heather Schueppert
Chief Marketing Officer
Unified Women’s Healthcare
Boca Raton, Florida
ECG promising for predicting major depression, treatment response
Individuals with major depressive disorder (MDD) have an increased heart rate – a finding that may have the potential to identify individuals at risk for the disorder and predict treatment response, early research suggests.
Using the rapid-action of the novel antidepressant ketamine and the latest wearable 24-hour electrocardiogram (ECG) technology, investigators found that heart rate could distinguish MDD patients from healthy individuals.
They also found that patients with MDD with the highest resting heart rate had a greater treatment response. In fact, on average, depressed patients had a heart rate that was roughly 10-15 beats per minute higher than healthy controls.
The innovative design of the proof-of-concept study “allowed us to see that average resting heart rate may change quite suddenly to reflect the change in mood,” lead investigator Carmen Schiweck, PhD, Goethe University, Frankfurt am Main, Germany, said in an interview.
These results could have “exciting implications for treatment selection,” and the researchers plan to assess the potential for heart rate to act as an early warning system for depression, they noted.
The findings were presented at the 33rd European College of Neuropsychopharmacology (ECNP) Congress, which was held online this year because of the COVID-19 pandemic.
Identifying trait markers
There have been recent attempts to assess heart rate or heart rate variability (HRV) in patients with MDD to identify trait markers, which are present regardless of the disease phase, or state markers, which are present only during a depressive phase.
However, heart rate and HRV are “highly variable” over a 24-hour cycle, a fact that has been ignored by recent classification efforts, the researchers noted. Moreover, most commonly used antidepressants have a long onset of action, which makes studying their impact on the heart rate challenging.
The researchers’ goal was to determine whether heart rate and HRV could be used as trait markers to distinguish MDD patients from healthy individuals and, through the use of ketamine, whether they can also act as state markers for depression.
For the study, 16 treatment-resistant patients with MDD and 16 age- and sex-matched healthy controls wore a portable ECG device for 4 consecutive days and 3 nights. Heart rate and HRV data were subsequently averaged to obtain a 24-hour ECG.
Participants then received a single infusion of intravenous ketamine for 40 minutes. After waiting for 1 hour, the patients resumed ECG recording for an additional 4 days, with changes in mood assessed using the Hamilton Rating Scale for Depression (HAM-D).
Results showed that, compared with the control group, patients with MDD had a significantly higher 24-hour heart rate (P < .001) and a significantly lower HRV, as measured by the root mean square of successive differences (P < .001).
The investigators also found a reduction in heart rate amplitude, which indicates “significant blunting of circadian rhythm variation throughout the day and less recovery at night.”
Ninety percent accuracy
Harmonic and binary regression showed that heart rate was able to identify those with MDD versus those in the control group, particularly using nighttime readings, with 90.6% accuracy. The data correctly identified 14 (87.5%) patients and 15 (93.8%) members of the control group.
Following treatment, heart rate decreased significantly among the MDD group (P < .001), but there was no significant change in HRV (P = .295).
There was a significant positive association between baseline heart rate and response to treatment on the HAM-D (r = .55; P = .046), which suggested better outcomes in patients with a higher heart rate.
Interestingly, while heart rate was positively correlated with depression severity before treatment (r = .59; P = .03), this relationship disappeared following treatment (r = –0.04; P = .90), suggesting heart rate changes were not linked to depression states.
While heart rate levels may be useful as a trait marker and, potentially, for predicting response to antidepressant treatment, they did not show potential as a state marker, the investigators noted.
They suggested that, while the results need to be confirmed in longitudinal studies, approval of a ketamine nasal spray “may open up new avenues to conceive treatment paradigms, as explored in this study.”
However, “this is a small proof-of-concept study,” the investigators acknowledged. They also point out that only six of the patients with MDD had a reduction in HAM-D scores of at least 30% in response to treatment.
Future research plans
Dr. Schiweck said in an interview that her team was able to identify differences in heart rate and HRV in MDD patients that were not observed in other studies because portable at-home devices allowed them to monitor heart rate continuously over days.
The use of ketamine may also have been advantageous because the Netherlands Study of Depression and Anxiety (NESDA), which was published in 2010, clearly showed that “traditional antidepressants,” in particular tricyclic antidepressants, have a strong influence on heart rate and HRV, Dr. Schiweck said.
because most of the recent studies “just assess patients who are remitted and patients who are currently depressed, but it’s a cross-sectional study design,” said Schiweck.
“If we can follow up the same patients over time then we might really know if it is possible to use heart rate as a state marker for depression,” she added. “That’s what we tried to do with ketamine, but our study was very, very small.”
She noted that the investigators would also like to assess individuals who are “very stressed” and may show some depressive symptoms but don’t yet have a diagnosis of depression.
Mind-body link
Commenting on the findings, Brenda W.J.H. Penninx, MD, PhD, professor of psychiatric epidemiology at the VU University Medical Center in Amsterdam, said the concept of higher heart rate and lower HRV in depression “is indicative of more sympathetic drive and less parasympathetic drive of the autonomic nervous system.”
“That fits with the overall thought that depression is a state with more continuous exposure to stress overactivation of the body, which can be reflected in the [hypothalamic-pituitary-adrenal] axis, leading to higher levels of cortisol stress hormone. But it can also be reflected in the parasympathetic and sympathetic activation of the autonomic nervous system,” she said.
Dr. Penninx, who was also the principal investigator of the NESDA study, was not involved with the current research.
She noted that the NESDA study showed that, when patients with depressive disorder are compared with a healthy controls group, they have a higher heart rate and lower HRV. “But if we then divide people into medicated and nonmedicated people ... then we see that these deviations are only seen in people using medication,” she added.
“Our findings indicate that at least the use of antidepressants is having quite a large impact on autonomic nervous system dysregulation,” Dr. Penninx said. The difference with the current study, she pointed out, is that “it examines the problem over a completely different time scale.”
Although this offers advantages, the current study did not have the large patient numbers that were included in the NESDA study and “they were not clearly able to distinguish the effect of disease from medication,” noted Dr. Penninx.
In addition, this is not an easy area to investigate because there are multiple factors that can mitigate results, including the psychiatric state of the patient, use of medications for both mental illness and cardiometabolic disease, and a patient’s age and gender.
Still, the study clearly illustrates the importance of the interplay between mental health and somatic health and that there is “a very clear indication that we don’t need to separate those,” Dr. Penninx said.
The study was funded by a TGO-IWT Grant from Belgium. The study authors and Dr. Penninx have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Individuals with major depressive disorder (MDD) have an increased heart rate – a finding that may have the potential to identify individuals at risk for the disorder and predict treatment response, early research suggests.
Using the rapid-action of the novel antidepressant ketamine and the latest wearable 24-hour electrocardiogram (ECG) technology, investigators found that heart rate could distinguish MDD patients from healthy individuals.
They also found that patients with MDD with the highest resting heart rate had a greater treatment response. In fact, on average, depressed patients had a heart rate that was roughly 10-15 beats per minute higher than healthy controls.
The innovative design of the proof-of-concept study “allowed us to see that average resting heart rate may change quite suddenly to reflect the change in mood,” lead investigator Carmen Schiweck, PhD, Goethe University, Frankfurt am Main, Germany, said in an interview.
These results could have “exciting implications for treatment selection,” and the researchers plan to assess the potential for heart rate to act as an early warning system for depression, they noted.
The findings were presented at the 33rd European College of Neuropsychopharmacology (ECNP) Congress, which was held online this year because of the COVID-19 pandemic.
Identifying trait markers
There have been recent attempts to assess heart rate or heart rate variability (HRV) in patients with MDD to identify trait markers, which are present regardless of the disease phase, or state markers, which are present only during a depressive phase.
However, heart rate and HRV are “highly variable” over a 24-hour cycle, a fact that has been ignored by recent classification efforts, the researchers noted. Moreover, most commonly used antidepressants have a long onset of action, which makes studying their impact on the heart rate challenging.
The researchers’ goal was to determine whether heart rate and HRV could be used as trait markers to distinguish MDD patients from healthy individuals and, through the use of ketamine, whether they can also act as state markers for depression.
For the study, 16 treatment-resistant patients with MDD and 16 age- and sex-matched healthy controls wore a portable ECG device for 4 consecutive days and 3 nights. Heart rate and HRV data were subsequently averaged to obtain a 24-hour ECG.
Participants then received a single infusion of intravenous ketamine for 40 minutes. After waiting for 1 hour, the patients resumed ECG recording for an additional 4 days, with changes in mood assessed using the Hamilton Rating Scale for Depression (HAM-D).
Results showed that, compared with the control group, patients with MDD had a significantly higher 24-hour heart rate (P < .001) and a significantly lower HRV, as measured by the root mean square of successive differences (P < .001).
The investigators also found a reduction in heart rate amplitude, which indicates “significant blunting of circadian rhythm variation throughout the day and less recovery at night.”
Ninety percent accuracy
Harmonic and binary regression showed that heart rate was able to identify those with MDD versus those in the control group, particularly using nighttime readings, with 90.6% accuracy. The data correctly identified 14 (87.5%) patients and 15 (93.8%) members of the control group.
Following treatment, heart rate decreased significantly among the MDD group (P < .001), but there was no significant change in HRV (P = .295).
There was a significant positive association between baseline heart rate and response to treatment on the HAM-D (r = .55; P = .046), which suggested better outcomes in patients with a higher heart rate.
Interestingly, while heart rate was positively correlated with depression severity before treatment (r = .59; P = .03), this relationship disappeared following treatment (r = –0.04; P = .90), suggesting heart rate changes were not linked to depression states.
While heart rate levels may be useful as a trait marker and, potentially, for predicting response to antidepressant treatment, they did not show potential as a state marker, the investigators noted.
They suggested that, while the results need to be confirmed in longitudinal studies, approval of a ketamine nasal spray “may open up new avenues to conceive treatment paradigms, as explored in this study.”
However, “this is a small proof-of-concept study,” the investigators acknowledged. They also point out that only six of the patients with MDD had a reduction in HAM-D scores of at least 30% in response to treatment.
Future research plans
Dr. Schiweck said in an interview that her team was able to identify differences in heart rate and HRV in MDD patients that were not observed in other studies because portable at-home devices allowed them to monitor heart rate continuously over days.
The use of ketamine may also have been advantageous because the Netherlands Study of Depression and Anxiety (NESDA), which was published in 2010, clearly showed that “traditional antidepressants,” in particular tricyclic antidepressants, have a strong influence on heart rate and HRV, Dr. Schiweck said.
because most of the recent studies “just assess patients who are remitted and patients who are currently depressed, but it’s a cross-sectional study design,” said Schiweck.
“If we can follow up the same patients over time then we might really know if it is possible to use heart rate as a state marker for depression,” she added. “That’s what we tried to do with ketamine, but our study was very, very small.”
She noted that the investigators would also like to assess individuals who are “very stressed” and may show some depressive symptoms but don’t yet have a diagnosis of depression.
Mind-body link
Commenting on the findings, Brenda W.J.H. Penninx, MD, PhD, professor of psychiatric epidemiology at the VU University Medical Center in Amsterdam, said the concept of higher heart rate and lower HRV in depression “is indicative of more sympathetic drive and less parasympathetic drive of the autonomic nervous system.”
“That fits with the overall thought that depression is a state with more continuous exposure to stress overactivation of the body, which can be reflected in the [hypothalamic-pituitary-adrenal] axis, leading to higher levels of cortisol stress hormone. But it can also be reflected in the parasympathetic and sympathetic activation of the autonomic nervous system,” she said.
Dr. Penninx, who was also the principal investigator of the NESDA study, was not involved with the current research.
She noted that the NESDA study showed that, when patients with depressive disorder are compared with a healthy controls group, they have a higher heart rate and lower HRV. “But if we then divide people into medicated and nonmedicated people ... then we see that these deviations are only seen in people using medication,” she added.
“Our findings indicate that at least the use of antidepressants is having quite a large impact on autonomic nervous system dysregulation,” Dr. Penninx said. The difference with the current study, she pointed out, is that “it examines the problem over a completely different time scale.”
Although this offers advantages, the current study did not have the large patient numbers that were included in the NESDA study and “they were not clearly able to distinguish the effect of disease from medication,” noted Dr. Penninx.
In addition, this is not an easy area to investigate because there are multiple factors that can mitigate results, including the psychiatric state of the patient, use of medications for both mental illness and cardiometabolic disease, and a patient’s age and gender.
Still, the study clearly illustrates the importance of the interplay between mental health and somatic health and that there is “a very clear indication that we don’t need to separate those,” Dr. Penninx said.
The study was funded by a TGO-IWT Grant from Belgium. The study authors and Dr. Penninx have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Individuals with major depressive disorder (MDD) have an increased heart rate – a finding that may have the potential to identify individuals at risk for the disorder and predict treatment response, early research suggests.
Using the rapid-action of the novel antidepressant ketamine and the latest wearable 24-hour electrocardiogram (ECG) technology, investigators found that heart rate could distinguish MDD patients from healthy individuals.
They also found that patients with MDD with the highest resting heart rate had a greater treatment response. In fact, on average, depressed patients had a heart rate that was roughly 10-15 beats per minute higher than healthy controls.
The innovative design of the proof-of-concept study “allowed us to see that average resting heart rate may change quite suddenly to reflect the change in mood,” lead investigator Carmen Schiweck, PhD, Goethe University, Frankfurt am Main, Germany, said in an interview.
These results could have “exciting implications for treatment selection,” and the researchers plan to assess the potential for heart rate to act as an early warning system for depression, they noted.
The findings were presented at the 33rd European College of Neuropsychopharmacology (ECNP) Congress, which was held online this year because of the COVID-19 pandemic.
Identifying trait markers
There have been recent attempts to assess heart rate or heart rate variability (HRV) in patients with MDD to identify trait markers, which are present regardless of the disease phase, or state markers, which are present only during a depressive phase.
However, heart rate and HRV are “highly variable” over a 24-hour cycle, a fact that has been ignored by recent classification efforts, the researchers noted. Moreover, most commonly used antidepressants have a long onset of action, which makes studying their impact on the heart rate challenging.
The researchers’ goal was to determine whether heart rate and HRV could be used as trait markers to distinguish MDD patients from healthy individuals and, through the use of ketamine, whether they can also act as state markers for depression.
For the study, 16 treatment-resistant patients with MDD and 16 age- and sex-matched healthy controls wore a portable ECG device for 4 consecutive days and 3 nights. Heart rate and HRV data were subsequently averaged to obtain a 24-hour ECG.
Participants then received a single infusion of intravenous ketamine for 40 minutes. After waiting for 1 hour, the patients resumed ECG recording for an additional 4 days, with changes in mood assessed using the Hamilton Rating Scale for Depression (HAM-D).
Results showed that, compared with the control group, patients with MDD had a significantly higher 24-hour heart rate (P < .001) and a significantly lower HRV, as measured by the root mean square of successive differences (P < .001).
The investigators also found a reduction in heart rate amplitude, which indicates “significant blunting of circadian rhythm variation throughout the day and less recovery at night.”
Ninety percent accuracy
Harmonic and binary regression showed that heart rate was able to identify those with MDD versus those in the control group, particularly using nighttime readings, with 90.6% accuracy. The data correctly identified 14 (87.5%) patients and 15 (93.8%) members of the control group.
Following treatment, heart rate decreased significantly among the MDD group (P < .001), but there was no significant change in HRV (P = .295).
There was a significant positive association between baseline heart rate and response to treatment on the HAM-D (r = .55; P = .046), which suggested better outcomes in patients with a higher heart rate.
Interestingly, while heart rate was positively correlated with depression severity before treatment (r = .59; P = .03), this relationship disappeared following treatment (r = –0.04; P = .90), suggesting heart rate changes were not linked to depression states.
While heart rate levels may be useful as a trait marker and, potentially, for predicting response to antidepressant treatment, they did not show potential as a state marker, the investigators noted.
They suggested that, while the results need to be confirmed in longitudinal studies, approval of a ketamine nasal spray “may open up new avenues to conceive treatment paradigms, as explored in this study.”
However, “this is a small proof-of-concept study,” the investigators acknowledged. They also point out that only six of the patients with MDD had a reduction in HAM-D scores of at least 30% in response to treatment.
Future research plans
Dr. Schiweck said in an interview that her team was able to identify differences in heart rate and HRV in MDD patients that were not observed in other studies because portable at-home devices allowed them to monitor heart rate continuously over days.
The use of ketamine may also have been advantageous because the Netherlands Study of Depression and Anxiety (NESDA), which was published in 2010, clearly showed that “traditional antidepressants,” in particular tricyclic antidepressants, have a strong influence on heart rate and HRV, Dr. Schiweck said.
because most of the recent studies “just assess patients who are remitted and patients who are currently depressed, but it’s a cross-sectional study design,” said Schiweck.
“If we can follow up the same patients over time then we might really know if it is possible to use heart rate as a state marker for depression,” she added. “That’s what we tried to do with ketamine, but our study was very, very small.”
She noted that the investigators would also like to assess individuals who are “very stressed” and may show some depressive symptoms but don’t yet have a diagnosis of depression.
Mind-body link
Commenting on the findings, Brenda W.J.H. Penninx, MD, PhD, professor of psychiatric epidemiology at the VU University Medical Center in Amsterdam, said the concept of higher heart rate and lower HRV in depression “is indicative of more sympathetic drive and less parasympathetic drive of the autonomic nervous system.”
“That fits with the overall thought that depression is a state with more continuous exposure to stress overactivation of the body, which can be reflected in the [hypothalamic-pituitary-adrenal] axis, leading to higher levels of cortisol stress hormone. But it can also be reflected in the parasympathetic and sympathetic activation of the autonomic nervous system,” she said.
Dr. Penninx, who was also the principal investigator of the NESDA study, was not involved with the current research.
She noted that the NESDA study showed that, when patients with depressive disorder are compared with a healthy controls group, they have a higher heart rate and lower HRV. “But if we then divide people into medicated and nonmedicated people ... then we see that these deviations are only seen in people using medication,” she added.
“Our findings indicate that at least the use of antidepressants is having quite a large impact on autonomic nervous system dysregulation,” Dr. Penninx said. The difference with the current study, she pointed out, is that “it examines the problem over a completely different time scale.”
Although this offers advantages, the current study did not have the large patient numbers that were included in the NESDA study and “they were not clearly able to distinguish the effect of disease from medication,” noted Dr. Penninx.
In addition, this is not an easy area to investigate because there are multiple factors that can mitigate results, including the psychiatric state of the patient, use of medications for both mental illness and cardiometabolic disease, and a patient’s age and gender.
Still, the study clearly illustrates the importance of the interplay between mental health and somatic health and that there is “a very clear indication that we don’t need to separate those,” Dr. Penninx said.
The study was funded by a TGO-IWT Grant from Belgium. The study authors and Dr. Penninx have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
AML maintenance: It’s now a thing
Maintenance is a important component of therapy for acute lymphoblastic leukemia, but it’s still a relatively new concept in the treat of patients with acute myeloid leukemia (AML).
The topic of AML maintenance “has become quite hot actually, recently, after languishing for years behind ALL, a disease where maintenance is absolutely critical to overall survival; we haven’t had that much to talk about it in AML until recently,” said Gail J. Roboz, MD, from Weill Cornell Medicine and The New York Presbyterian Hospital, both in New York.
Dr. Roboz discussed her approach to AML maintenance during the virtual American Society of Hematology Meeting on Hematologic Malignancies.
The current AML treatment paradigm starts with remission induction via intensive or less-intensive therapies, followed by consolidation with chemotherapy or with autologous or allogeneic stem cell transplantation (SCT), with maintenance considered as a possibility for some patients.
“Strictly speaking, maintenance is the idea of keeping someone in remission, but we’ve gotten used to it that maintenance is postremission therapy that is different from what you had in your induction,” she noted. “That said, the exact nature of maintenance, while it most traditionally refers to an ongoing lower-intensity therapy, is a little bit complicated these days of what exactly constitutes maintenance.”
Current AML treatments generally fail to completely eliminate leukemic cells, so nearly all patients have remissions with minimal residual disease (MRD).
“You have a heterogeneous mix of leukemia stem cells, progenitors, blast cells, and you are in remission but there are still leftovers, and those leftovers result in disease relapse, and the goal of postremission therapy to basically target and hopefully eradicate the leftovers,” Dr. Roboz said.
Focusing on the postremission period is vital because most patients with AML will die within 1 year after disease relapse.
Many options, none great
National Comprehensive Cancer Network AML guidelines recommend a variety of approaches to postremission therapy for patients younger than 60 years with AML, including, depending on risk, either histone deacetylase inhibitors, gemtuzumab ozogamicin (Mylotarg), chemotherapy, and/or SCT, but none of these options, strictly speaking, is called maintenance, she noted.
For patients 60 years and older, “there’s also a likelihood of proceeding with hypomethylating [HMA]-based therapy in such a way that, if they’re responding to initial treatment, they get ongoing therapy with whatever HMA or hypomethylating-based regimen they’re responding to. So is that called maintenance? Is that called ongoing therapy? Continuing therapy? It’s a subject of some controversy,” she added.
For patients younger than 60, hematopoietic SCT has been the ultimate form of maintenance, and increasingly allogeneic SCT is being employed in the United States for patients older than 60 years, including those 70 years and older.
“That has been a good thing, because we’ve been able to offer more patients potentially curative therapy, but the problem is that allo transplant is not a free lunch either, and there are significant risks of nonrelapse mortality, especially for patients going into the transplant with other comorbidities,” Dr. Roboz said.
The majority of older patients may not be cured with transplant because of the use of reduced-intensity conditioning regimens with the result of extended disease-free survival but eventual relapse from residual disease.
“The question is, if you’re an older patient and you can’t get an ablative transplant and you do have residual disease, what’s the likelihood of actually being cured at 2 years, and do you really want to go through the headaches of having a transplant?” she said.
Nontransplant options
In the RATIFY trial, patients 60 years and younger with newly diagnosed AML with activating FLT3-mutations were randomized to induction chemotherapy with daunorubicin and cytarabine, consolidation with an histone deacetylase inhibitor, and then maintenance for up to 12 cycles with either midostaurin or placebo.
Although the trial met its primary endpoint of an improvement in overall survival with midostaurin (4-year overall survival, 51.4% vs. 44.3% with placebo), there is still uncertainty as to whether the observed survival benefit was caused by midostaurin or by something else, Dr. Roboz said.
“That said, it certainly has become common to use FLT3 inhibitors as postremission strategy for patients, with consolidation, with allo transplant, after allo transplant – wherever you can get the inhibitor in there,” she said.
The isocitrate dehydrogenase inhibitors ivosidenib (for IDH1) and enasidenib (IDH2) have also been tried in a postremission maintenance-like setting, but have thus far not been demonstrated in clinical trials to be effective in this setting, she added.
Gemtuzumab ozogamicin, an anti-CD33 antibody conjugated to calicheamicin, was approved in 2000 for adults 60 years and older with CD33+ AML in first remission, and in 2017 for adults with newly diagnosed CD33+ AML, as well as adults and children 2 years and older with relapsed or refractory CD33+ AML, but there are no data to show whether this antibody-drug conjugate could have benefit in a maintenance setting.
As previously reported, the combination of the BCL-2 inhibitor venetoclax (Venclexta) with azacitidine was associated with a significant improvement in overall survival, compared with azacitidine alone, in the VIALE-A trial.
“The question now is, in these studies most of the time patients are given a combination of aza and venetoclax that actually continues until they progress; is that called maintenance if you’re getting ongoing cycles? Not sure what to call it, but this is quite myelosuppressive, and there are many, many postremission modifications in dose and schedule that could take up a whole separate lecture,” Dr. Roboz commented.
She added, however, that the combination is effective across nearly all subgroups, and may be more generally applicable for maintenance-style therapy in older patients with AML.
Survival benefit
Dr. Roboz was a coinvestigator for the QUAZAR AML-001 trial (NCT01757535), results of which were reported at the 2019 ASH annual meeting. It was the first trial to show that a maintenance therapy with CC-486, an oral formulation of azacitidine, can improve overall survival in patients with AML in remission.
Among 472 patients with poor-risk AML in first complete remission who were not eligible for transplantation, median relapse-free survival was 10.2 months with CC-486 vs. 4.8 months with placebo plus best supportive care, and median overall survival with CC-486 was 24.7 months vs. 14.8 months, an absolute difference of 9.9 months.
This oral formulation of azacitidine was approved by U.S. Food and Drug Administration on Sept. 1, 2020 for use in adult patients with AML in complete remission or complete remission with incomplete blood count recovery following intensive induction chemotherapy and are not able to complete intensive curative therapy. It is sold under the trade name Onureg.
“This is likely to become now the standard of care for AML patients, for the group that was shown to benefit in the clinical trial,” Dr. Roboz said.
The drug was effective at prolonging relapse-free survival regardless of sex, age, remission status (complete remission or complete remission with incomplete blood count recovery) at randomization, cytogenetic risk category, or MRD status.
“We were very gratified to see that there was no reduction in health-related quality of life, which meant that the agent was tolerable, it could be continued for multiple cycles, and there are actually patients, including one of mine, who are many years out with ongoing therapy,” she said.
No funding source for the presentation was reported. Dr. Roboz disclosed consultancy/advisory board activity for multiple pharmaceutical companies; data safety and monitoring committee activity for MEI Pharma, Helsinn, and Takeda; and research funding from Cellectis.
Maintenance is a important component of therapy for acute lymphoblastic leukemia, but it’s still a relatively new concept in the treat of patients with acute myeloid leukemia (AML).
The topic of AML maintenance “has become quite hot actually, recently, after languishing for years behind ALL, a disease where maintenance is absolutely critical to overall survival; we haven’t had that much to talk about it in AML until recently,” said Gail J. Roboz, MD, from Weill Cornell Medicine and The New York Presbyterian Hospital, both in New York.
Dr. Roboz discussed her approach to AML maintenance during the virtual American Society of Hematology Meeting on Hematologic Malignancies.
The current AML treatment paradigm starts with remission induction via intensive or less-intensive therapies, followed by consolidation with chemotherapy or with autologous or allogeneic stem cell transplantation (SCT), with maintenance considered as a possibility for some patients.
“Strictly speaking, maintenance is the idea of keeping someone in remission, but we’ve gotten used to it that maintenance is postremission therapy that is different from what you had in your induction,” she noted. “That said, the exact nature of maintenance, while it most traditionally refers to an ongoing lower-intensity therapy, is a little bit complicated these days of what exactly constitutes maintenance.”
Current AML treatments generally fail to completely eliminate leukemic cells, so nearly all patients have remissions with minimal residual disease (MRD).
“You have a heterogeneous mix of leukemia stem cells, progenitors, blast cells, and you are in remission but there are still leftovers, and those leftovers result in disease relapse, and the goal of postremission therapy to basically target and hopefully eradicate the leftovers,” Dr. Roboz said.
Focusing on the postremission period is vital because most patients with AML will die within 1 year after disease relapse.
Many options, none great
National Comprehensive Cancer Network AML guidelines recommend a variety of approaches to postremission therapy for patients younger than 60 years with AML, including, depending on risk, either histone deacetylase inhibitors, gemtuzumab ozogamicin (Mylotarg), chemotherapy, and/or SCT, but none of these options, strictly speaking, is called maintenance, she noted.
For patients 60 years and older, “there’s also a likelihood of proceeding with hypomethylating [HMA]-based therapy in such a way that, if they’re responding to initial treatment, they get ongoing therapy with whatever HMA or hypomethylating-based regimen they’re responding to. So is that called maintenance? Is that called ongoing therapy? Continuing therapy? It’s a subject of some controversy,” she added.
For patients younger than 60, hematopoietic SCT has been the ultimate form of maintenance, and increasingly allogeneic SCT is being employed in the United States for patients older than 60 years, including those 70 years and older.
“That has been a good thing, because we’ve been able to offer more patients potentially curative therapy, but the problem is that allo transplant is not a free lunch either, and there are significant risks of nonrelapse mortality, especially for patients going into the transplant with other comorbidities,” Dr. Roboz said.
The majority of older patients may not be cured with transplant because of the use of reduced-intensity conditioning regimens with the result of extended disease-free survival but eventual relapse from residual disease.
“The question is, if you’re an older patient and you can’t get an ablative transplant and you do have residual disease, what’s the likelihood of actually being cured at 2 years, and do you really want to go through the headaches of having a transplant?” she said.
Nontransplant options
In the RATIFY trial, patients 60 years and younger with newly diagnosed AML with activating FLT3-mutations were randomized to induction chemotherapy with daunorubicin and cytarabine, consolidation with an histone deacetylase inhibitor, and then maintenance for up to 12 cycles with either midostaurin or placebo.
Although the trial met its primary endpoint of an improvement in overall survival with midostaurin (4-year overall survival, 51.4% vs. 44.3% with placebo), there is still uncertainty as to whether the observed survival benefit was caused by midostaurin or by something else, Dr. Roboz said.
“That said, it certainly has become common to use FLT3 inhibitors as postremission strategy for patients, with consolidation, with allo transplant, after allo transplant – wherever you can get the inhibitor in there,” she said.
The isocitrate dehydrogenase inhibitors ivosidenib (for IDH1) and enasidenib (IDH2) have also been tried in a postremission maintenance-like setting, but have thus far not been demonstrated in clinical trials to be effective in this setting, she added.
Gemtuzumab ozogamicin, an anti-CD33 antibody conjugated to calicheamicin, was approved in 2000 for adults 60 years and older with CD33+ AML in first remission, and in 2017 for adults with newly diagnosed CD33+ AML, as well as adults and children 2 years and older with relapsed or refractory CD33+ AML, but there are no data to show whether this antibody-drug conjugate could have benefit in a maintenance setting.
As previously reported, the combination of the BCL-2 inhibitor venetoclax (Venclexta) with azacitidine was associated with a significant improvement in overall survival, compared with azacitidine alone, in the VIALE-A trial.
“The question now is, in these studies most of the time patients are given a combination of aza and venetoclax that actually continues until they progress; is that called maintenance if you’re getting ongoing cycles? Not sure what to call it, but this is quite myelosuppressive, and there are many, many postremission modifications in dose and schedule that could take up a whole separate lecture,” Dr. Roboz commented.
She added, however, that the combination is effective across nearly all subgroups, and may be more generally applicable for maintenance-style therapy in older patients with AML.
Survival benefit
Dr. Roboz was a coinvestigator for the QUAZAR AML-001 trial (NCT01757535), results of which were reported at the 2019 ASH annual meeting. It was the first trial to show that a maintenance therapy with CC-486, an oral formulation of azacitidine, can improve overall survival in patients with AML in remission.
Among 472 patients with poor-risk AML in first complete remission who were not eligible for transplantation, median relapse-free survival was 10.2 months with CC-486 vs. 4.8 months with placebo plus best supportive care, and median overall survival with CC-486 was 24.7 months vs. 14.8 months, an absolute difference of 9.9 months.
This oral formulation of azacitidine was approved by U.S. Food and Drug Administration on Sept. 1, 2020 for use in adult patients with AML in complete remission or complete remission with incomplete blood count recovery following intensive induction chemotherapy and are not able to complete intensive curative therapy. It is sold under the trade name Onureg.
“This is likely to become now the standard of care for AML patients, for the group that was shown to benefit in the clinical trial,” Dr. Roboz said.
The drug was effective at prolonging relapse-free survival regardless of sex, age, remission status (complete remission or complete remission with incomplete blood count recovery) at randomization, cytogenetic risk category, or MRD status.
“We were very gratified to see that there was no reduction in health-related quality of life, which meant that the agent was tolerable, it could be continued for multiple cycles, and there are actually patients, including one of mine, who are many years out with ongoing therapy,” she said.
No funding source for the presentation was reported. Dr. Roboz disclosed consultancy/advisory board activity for multiple pharmaceutical companies; data safety and monitoring committee activity for MEI Pharma, Helsinn, and Takeda; and research funding from Cellectis.
Maintenance is a important component of therapy for acute lymphoblastic leukemia, but it’s still a relatively new concept in the treat of patients with acute myeloid leukemia (AML).
The topic of AML maintenance “has become quite hot actually, recently, after languishing for years behind ALL, a disease where maintenance is absolutely critical to overall survival; we haven’t had that much to talk about it in AML until recently,” said Gail J. Roboz, MD, from Weill Cornell Medicine and The New York Presbyterian Hospital, both in New York.
Dr. Roboz discussed her approach to AML maintenance during the virtual American Society of Hematology Meeting on Hematologic Malignancies.
The current AML treatment paradigm starts with remission induction via intensive or less-intensive therapies, followed by consolidation with chemotherapy or with autologous or allogeneic stem cell transplantation (SCT), with maintenance considered as a possibility for some patients.
“Strictly speaking, maintenance is the idea of keeping someone in remission, but we’ve gotten used to it that maintenance is postremission therapy that is different from what you had in your induction,” she noted. “That said, the exact nature of maintenance, while it most traditionally refers to an ongoing lower-intensity therapy, is a little bit complicated these days of what exactly constitutes maintenance.”
Current AML treatments generally fail to completely eliminate leukemic cells, so nearly all patients have remissions with minimal residual disease (MRD).
“You have a heterogeneous mix of leukemia stem cells, progenitors, blast cells, and you are in remission but there are still leftovers, and those leftovers result in disease relapse, and the goal of postremission therapy to basically target and hopefully eradicate the leftovers,” Dr. Roboz said.
Focusing on the postremission period is vital because most patients with AML will die within 1 year after disease relapse.
Many options, none great
National Comprehensive Cancer Network AML guidelines recommend a variety of approaches to postremission therapy for patients younger than 60 years with AML, including, depending on risk, either histone deacetylase inhibitors, gemtuzumab ozogamicin (Mylotarg), chemotherapy, and/or SCT, but none of these options, strictly speaking, is called maintenance, she noted.
For patients 60 years and older, “there’s also a likelihood of proceeding with hypomethylating [HMA]-based therapy in such a way that, if they’re responding to initial treatment, they get ongoing therapy with whatever HMA or hypomethylating-based regimen they’re responding to. So is that called maintenance? Is that called ongoing therapy? Continuing therapy? It’s a subject of some controversy,” she added.
For patients younger than 60, hematopoietic SCT has been the ultimate form of maintenance, and increasingly allogeneic SCT is being employed in the United States for patients older than 60 years, including those 70 years and older.
“That has been a good thing, because we’ve been able to offer more patients potentially curative therapy, but the problem is that allo transplant is not a free lunch either, and there are significant risks of nonrelapse mortality, especially for patients going into the transplant with other comorbidities,” Dr. Roboz said.
The majority of older patients may not be cured with transplant because of the use of reduced-intensity conditioning regimens with the result of extended disease-free survival but eventual relapse from residual disease.
“The question is, if you’re an older patient and you can’t get an ablative transplant and you do have residual disease, what’s the likelihood of actually being cured at 2 years, and do you really want to go through the headaches of having a transplant?” she said.
Nontransplant options
In the RATIFY trial, patients 60 years and younger with newly diagnosed AML with activating FLT3-mutations were randomized to induction chemotherapy with daunorubicin and cytarabine, consolidation with an histone deacetylase inhibitor, and then maintenance for up to 12 cycles with either midostaurin or placebo.
Although the trial met its primary endpoint of an improvement in overall survival with midostaurin (4-year overall survival, 51.4% vs. 44.3% with placebo), there is still uncertainty as to whether the observed survival benefit was caused by midostaurin or by something else, Dr. Roboz said.
“That said, it certainly has become common to use FLT3 inhibitors as postremission strategy for patients, with consolidation, with allo transplant, after allo transplant – wherever you can get the inhibitor in there,” she said.
The isocitrate dehydrogenase inhibitors ivosidenib (for IDH1) and enasidenib (IDH2) have also been tried in a postremission maintenance-like setting, but have thus far not been demonstrated in clinical trials to be effective in this setting, she added.
Gemtuzumab ozogamicin, an anti-CD33 antibody conjugated to calicheamicin, was approved in 2000 for adults 60 years and older with CD33+ AML in first remission, and in 2017 for adults with newly diagnosed CD33+ AML, as well as adults and children 2 years and older with relapsed or refractory CD33+ AML, but there are no data to show whether this antibody-drug conjugate could have benefit in a maintenance setting.
As previously reported, the combination of the BCL-2 inhibitor venetoclax (Venclexta) with azacitidine was associated with a significant improvement in overall survival, compared with azacitidine alone, in the VIALE-A trial.
“The question now is, in these studies most of the time patients are given a combination of aza and venetoclax that actually continues until they progress; is that called maintenance if you’re getting ongoing cycles? Not sure what to call it, but this is quite myelosuppressive, and there are many, many postremission modifications in dose and schedule that could take up a whole separate lecture,” Dr. Roboz commented.
She added, however, that the combination is effective across nearly all subgroups, and may be more generally applicable for maintenance-style therapy in older patients with AML.
Survival benefit
Dr. Roboz was a coinvestigator for the QUAZAR AML-001 trial (NCT01757535), results of which were reported at the 2019 ASH annual meeting. It was the first trial to show that a maintenance therapy with CC-486, an oral formulation of azacitidine, can improve overall survival in patients with AML in remission.
Among 472 patients with poor-risk AML in first complete remission who were not eligible for transplantation, median relapse-free survival was 10.2 months with CC-486 vs. 4.8 months with placebo plus best supportive care, and median overall survival with CC-486 was 24.7 months vs. 14.8 months, an absolute difference of 9.9 months.
This oral formulation of azacitidine was approved by U.S. Food and Drug Administration on Sept. 1, 2020 for use in adult patients with AML in complete remission or complete remission with incomplete blood count recovery following intensive induction chemotherapy and are not able to complete intensive curative therapy. It is sold under the trade name Onureg.
“This is likely to become now the standard of care for AML patients, for the group that was shown to benefit in the clinical trial,” Dr. Roboz said.
The drug was effective at prolonging relapse-free survival regardless of sex, age, remission status (complete remission or complete remission with incomplete blood count recovery) at randomization, cytogenetic risk category, or MRD status.
“We were very gratified to see that there was no reduction in health-related quality of life, which meant that the agent was tolerable, it could be continued for multiple cycles, and there are actually patients, including one of mine, who are many years out with ongoing therapy,” she said.
No funding source for the presentation was reported. Dr. Roboz disclosed consultancy/advisory board activity for multiple pharmaceutical companies; data safety and monitoring committee activity for MEI Pharma, Helsinn, and Takeda; and research funding from Cellectis.
FROM ASH HEMATOLOGIC MALIGNANCIES 2020