User login
Automated RA image scoring could be coming
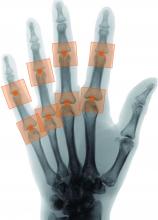
A novel program that aims to automate the Sharp-van der Heijde scoring of radiographs of patients with rheumatoid arthritis has shown good reliability in identifying regions of interest and matching human reader scoring for joint-space narrowing, according to a report given at the annual European Congress of Rheumatology, held online this year because of COVID-19.
First author and presenter Thomas Deimel, MD, and colleagues at the Medical University of Vienna said their program, called autoscoRA, may be a solution to the problem of readers having to make subjective calls on the severity of damage seen on radiographs.
Although the work continues to be validated, Dr. Deimel, a resident at the university, is confident in the system as is. “I think for joint space narrowing, we’re there at the point where this could be used and could be as good as a human reader in terms of reliability,” he said in an interview. To find out, the group plans to compare the variability between autoscoRA and a gold-standard human reader against the variability seen between human readers. If the two measures of variability are similar, it would provide a strong endorsement.
The effort is far from the first to develop an automatic scoring system for RA images, but no fully automated system has emerged as reliable, according to Dr. Deimel. He thinks one main issue for others has been lack of access to a sufficient data set to train systems. It can be difficult to find enough training images because many types of joint damage are comparatively uncommon. The problem is made even worse because images can be hard to interpret: The shapes that the system must decipher can be misleading, especially in positions of tendon insertion or ligament attachment that can resemble damage. Differing angles of view between various training images can also complicate matters.
The autoscoRA program is based on modifications of a form of convolutional neural network called the VGG16 architecture. The team used 2,207 images from 270 patients to train autoscoRA, 1,150 images from 133 patients for validation, and 1,834 images from 237 patients to test it.
The group had access to a high-quality data set of almost 6,000 hand radiographs from their institution, the result of foresight of principal investigator Daniel Aletaha, MD, and his predecessor Josef Smolen, MD. They “thought ahead and started collecting data and had all of it scored,” Dr. Deimel said. The work wasn’t all completed ahead of time, though. Dr. Deimel had to pull images from the hospital’s system sort through them manually.
The group also benefited from close proximity to computer scientists, including coauthor Georg Langs from the Medical University of Vienna’s computational imaging research lab. “We were lucky that we have a computer science department that is very much involved in medical imaging,” Dr. Deimel said.
The trained system successfully identified regions of interest in 96% of joints. It calculated the same score as the human reader in 80.5% of metacarpophalangeal joints and 72.3% of proximal interphalangeal joints. It deviated by more than 1 point from the gold-standard score in just 1.8% of metacarpophalangeal joints and 1.7% of proximal interphalangeal joints.
The researchers aim next to extend the program to bone erosions and also to images of the wrists and feet. They also hope to use scores from the program in clinical trials to measure a treatment’s effect, in registries of routine patient visits where thousands of such images along with clinical data could form the basis of informative observational studies, and in clinical practice, though likely with human oversight.
The study received no outside financial support. Dr. Deimel had no relevant financial disclosures. Mr. Langs reported being cofounder and shareholder of contextflow and receiving grants from Novartis, Siemens Healthineers, and NVIDIA. Dr. Aletaha reported financial relationships with many companies marketing drugs for rheumatoid arthritis.
SOURCE: Deimel T et al. Ann Rheum Dis. 2020 Jun;79(suppl 1):39-40.
A novel program that aims to automate the Sharp-van der Heijde scoring of radiographs of patients with rheumatoid arthritis has shown good reliability in identifying regions of interest and matching human reader scoring for joint-space narrowing, according to a report given at the annual European Congress of Rheumatology, held online this year because of COVID-19.
First author and presenter Thomas Deimel, MD, and colleagues at the Medical University of Vienna said their program, called autoscoRA, may be a solution to the problem of readers having to make subjective calls on the severity of damage seen on radiographs.
Although the work continues to be validated, Dr. Deimel, a resident at the university, is confident in the system as is. “I think for joint space narrowing, we’re there at the point where this could be used and could be as good as a human reader in terms of reliability,” he said in an interview. To find out, the group plans to compare the variability between autoscoRA and a gold-standard human reader against the variability seen between human readers. If the two measures of variability are similar, it would provide a strong endorsement.
The effort is far from the first to develop an automatic scoring system for RA images, but no fully automated system has emerged as reliable, according to Dr. Deimel. He thinks one main issue for others has been lack of access to a sufficient data set to train systems. It can be difficult to find enough training images because many types of joint damage are comparatively uncommon. The problem is made even worse because images can be hard to interpret: The shapes that the system must decipher can be misleading, especially in positions of tendon insertion or ligament attachment that can resemble damage. Differing angles of view between various training images can also complicate matters.
The autoscoRA program is based on modifications of a form of convolutional neural network called the VGG16 architecture. The team used 2,207 images from 270 patients to train autoscoRA, 1,150 images from 133 patients for validation, and 1,834 images from 237 patients to test it.
The group had access to a high-quality data set of almost 6,000 hand radiographs from their institution, the result of foresight of principal investigator Daniel Aletaha, MD, and his predecessor Josef Smolen, MD. They “thought ahead and started collecting data and had all of it scored,” Dr. Deimel said. The work wasn’t all completed ahead of time, though. Dr. Deimel had to pull images from the hospital’s system sort through them manually.
The group also benefited from close proximity to computer scientists, including coauthor Georg Langs from the Medical University of Vienna’s computational imaging research lab. “We were lucky that we have a computer science department that is very much involved in medical imaging,” Dr. Deimel said.
The trained system successfully identified regions of interest in 96% of joints. It calculated the same score as the human reader in 80.5% of metacarpophalangeal joints and 72.3% of proximal interphalangeal joints. It deviated by more than 1 point from the gold-standard score in just 1.8% of metacarpophalangeal joints and 1.7% of proximal interphalangeal joints.
The researchers aim next to extend the program to bone erosions and also to images of the wrists and feet. They also hope to use scores from the program in clinical trials to measure a treatment’s effect, in registries of routine patient visits where thousands of such images along with clinical data could form the basis of informative observational studies, and in clinical practice, though likely with human oversight.
The study received no outside financial support. Dr. Deimel had no relevant financial disclosures. Mr. Langs reported being cofounder and shareholder of contextflow and receiving grants from Novartis, Siemens Healthineers, and NVIDIA. Dr. Aletaha reported financial relationships with many companies marketing drugs for rheumatoid arthritis.
SOURCE: Deimel T et al. Ann Rheum Dis. 2020 Jun;79(suppl 1):39-40.
A novel program that aims to automate the Sharp-van der Heijde scoring of radiographs of patients with rheumatoid arthritis has shown good reliability in identifying regions of interest and matching human reader scoring for joint-space narrowing, according to a report given at the annual European Congress of Rheumatology, held online this year because of COVID-19.
First author and presenter Thomas Deimel, MD, and colleagues at the Medical University of Vienna said their program, called autoscoRA, may be a solution to the problem of readers having to make subjective calls on the severity of damage seen on radiographs.
Although the work continues to be validated, Dr. Deimel, a resident at the university, is confident in the system as is. “I think for joint space narrowing, we’re there at the point where this could be used and could be as good as a human reader in terms of reliability,” he said in an interview. To find out, the group plans to compare the variability between autoscoRA and a gold-standard human reader against the variability seen between human readers. If the two measures of variability are similar, it would provide a strong endorsement.
The effort is far from the first to develop an automatic scoring system for RA images, but no fully automated system has emerged as reliable, according to Dr. Deimel. He thinks one main issue for others has been lack of access to a sufficient data set to train systems. It can be difficult to find enough training images because many types of joint damage are comparatively uncommon. The problem is made even worse because images can be hard to interpret: The shapes that the system must decipher can be misleading, especially in positions of tendon insertion or ligament attachment that can resemble damage. Differing angles of view between various training images can also complicate matters.
The autoscoRA program is based on modifications of a form of convolutional neural network called the VGG16 architecture. The team used 2,207 images from 270 patients to train autoscoRA, 1,150 images from 133 patients for validation, and 1,834 images from 237 patients to test it.
The group had access to a high-quality data set of almost 6,000 hand radiographs from their institution, the result of foresight of principal investigator Daniel Aletaha, MD, and his predecessor Josef Smolen, MD. They “thought ahead and started collecting data and had all of it scored,” Dr. Deimel said. The work wasn’t all completed ahead of time, though. Dr. Deimel had to pull images from the hospital’s system sort through them manually.
The group also benefited from close proximity to computer scientists, including coauthor Georg Langs from the Medical University of Vienna’s computational imaging research lab. “We were lucky that we have a computer science department that is very much involved in medical imaging,” Dr. Deimel said.
The trained system successfully identified regions of interest in 96% of joints. It calculated the same score as the human reader in 80.5% of metacarpophalangeal joints and 72.3% of proximal interphalangeal joints. It deviated by more than 1 point from the gold-standard score in just 1.8% of metacarpophalangeal joints and 1.7% of proximal interphalangeal joints.
The researchers aim next to extend the program to bone erosions and also to images of the wrists and feet. They also hope to use scores from the program in clinical trials to measure a treatment’s effect, in registries of routine patient visits where thousands of such images along with clinical data could form the basis of informative observational studies, and in clinical practice, though likely with human oversight.
The study received no outside financial support. Dr. Deimel had no relevant financial disclosures. Mr. Langs reported being cofounder and shareholder of contextflow and receiving grants from Novartis, Siemens Healthineers, and NVIDIA. Dr. Aletaha reported financial relationships with many companies marketing drugs for rheumatoid arthritis.
SOURCE: Deimel T et al. Ann Rheum Dis. 2020 Jun;79(suppl 1):39-40.
FROM THE EULAR 2020 E-CONGRESS
Ankylosing Spondylitis: The Basics
Potential new biomarker for psychosis severity
ACE levels are lower in individuals with first episode psychosis (FEP) and even lower in those with resistant disease, suggesting the enzyme may be a biomarker of disease severity.
In a longitudinal cohort study, investigators found patients with FEP had significantly reduced ACE levels compared with their healthy peers.
With blood concentrations of the enzyme significantly reduced in those who were treatment-resistant, and results suggest “a possible relationship with disease severity,” noted the researchers, led by investigator Luisa Longo, MD, a resident in psychiatry, University of Bari Aldo Moro in Italy.
Moreover, the finding that lower ACE levels were associated with greater cognitive impairment on neuropsychological tests indicates the enzyme plays a role in “the alteration of neurocognitive abilities” in patients with FEP.
Taken together, the results “highlight ACE as a promising peripheral biomarker to identify patients at risk of treatment resistance to antipsychotics,” the investigators reported.
The findings were presented at the annual congress of the Schizophrenia International Research Society.
Mechanisms “poorly understood”
Previous studies suggest ACE may play a role in neurologic and psychiatric conditions, including schizophrenia, through alterations in function or blood concentrations.
However, the molecular mechanisms underlying disease onset and response to antipsychotics in patients with FEP “remain poorly understood,” the researchers noted. In addition, “despite adequate antipsychotic treatment, 20% of patients have persistent symptoms.”
To determine whether ACE levels are already altered in FEP patients, the investigators examined data on 138 patients with FEP and 115 healthy controls.
After measuring blood concentrations in 122 of the patients and 78 controls, they found that ACE levels were significantly lower in FEP patients (P = 4.1x10-13) after controlling for age, sex, ethnicity, duration of illness, smoking status, and chlorpromazine equivalents.
Cerebrospinal fluid (CSF) levels, which were measured in 19 patients and 18 controls, were also significantly lower in individuals with FEP (P = .01), with a strong correlation observed between blood and CSF levels (P = .0005).
Next, the team used Treatment Response and Resistance in Psychosis criteria to compare ACE levels in 32 treatment-resistant patients and 106 non–treatment-resistant patients. Results showed that ACE blood levels were significantly lower in the treatment-resistant patients (P = .03).
Finally, the association between ACE blood levels and range of clinical and neurocognitive variables were examined across all FEP patients.
The Scale for the Assessment of Negative and Positive Symptoms was administered, along with a battery of tests looking at processing speed, working memory, verbal learning and memory, visual learning and memory, ideational fluency, and executive function. All were combined into a composite score.
While there was no association between ACE blood levels and symptom severity in the patients, there was a significant association between levels and verbal memory (P = .007) and composite cognitive score (P = .04).
Notable finding
In an interview, Thomas W. Sedlak, MD, PhD, assistant professor of psychiatry and behavioral health, Johns Hopkins University, Baltimore, said the finding that ACE levels were associated with lower cognition scores is “notable in that we don’t really have any treatments for cognition.
“Antipsychotic drugs treat more of the famous symptoms of hallucinations and delusions and disordered speech, but they don›t really help cognition a whole lot — and too much medicine might even make that worse,” said Dr. Sedlak, who was not involved with the research.
“So it’s interesting to have another biomarker that might relate a symptom of schizophrenia that we don’t really have a good grip on addressing,” he said.
However, he noted that this study is “preliminary,” has not looked at longitudinal changes in ACE in the same patients, and is one of “many” correlation studies.
“I like to say you can pretty much Google any chemical in the body and somebody has a claim about it and schizophrenia,” Dr. Sedlak said.
Nevertheless, the current analysis is “convergent” with past studies on ACE, including those that have found associations between polymorphisms in the enzyme and schizophrenia, he noted.
Moreover, research has shown that ACE facilitates glutamate transmission, specifically via N-methyl-D-aspartate (NMDA) receptors in the prefrontal cortex, “which is probably the brain region most tied to schizophrenia abnormalities,” Dr. Sedlak said.
No tie to inflammation
The findings do contrast, however, with the increasing body of evidence linking schizophrenia to inflammation.
Dr. Sedlak said that is not surprising, as the more that is learned about schizophrenia, “the less it is likely to be a single homogeneous entity but something more akin to intellectual disability.
“We’re finding that there are different subtypes of schizophrenia, some of which might have more of an inflammatory tie and others which don’t.”
He added that it might be possible over the longer term to cluster patients by ACE levels and other biomarkers and then follow them long term to “see if we can predict outcomes better than purely clinical terms.”
In this way, Dr. Sedlak said he believes that psychiatry may become more like rheumatology, “which I’d say is the area of medicine, at least in terms of making diagnoses, most similar.”
The etiology of conditions such as lupus and rheumatoid arthritis is “not absolutely known” and there is “probably no single cause,” with the diagnosis relying on clinical findings alongside biomarker tests.
“I think psychiatry is, long term, hoping to discover some biomarkers ... that would be used together with the clinical findings to help come to more clinical agreement on how to define cases – and would be predictive long term in terms of the course of illness,” Dr. Sedlak concluded.
The study had no specific funding. The study investigators and Dr. Sedlak have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
ACE levels are lower in individuals with first episode psychosis (FEP) and even lower in those with resistant disease, suggesting the enzyme may be a biomarker of disease severity.
In a longitudinal cohort study, investigators found patients with FEP had significantly reduced ACE levels compared with their healthy peers.
With blood concentrations of the enzyme significantly reduced in those who were treatment-resistant, and results suggest “a possible relationship with disease severity,” noted the researchers, led by investigator Luisa Longo, MD, a resident in psychiatry, University of Bari Aldo Moro in Italy.
Moreover, the finding that lower ACE levels were associated with greater cognitive impairment on neuropsychological tests indicates the enzyme plays a role in “the alteration of neurocognitive abilities” in patients with FEP.
Taken together, the results “highlight ACE as a promising peripheral biomarker to identify patients at risk of treatment resistance to antipsychotics,” the investigators reported.
The findings were presented at the annual congress of the Schizophrenia International Research Society.
Mechanisms “poorly understood”
Previous studies suggest ACE may play a role in neurologic and psychiatric conditions, including schizophrenia, through alterations in function or blood concentrations.
However, the molecular mechanisms underlying disease onset and response to antipsychotics in patients with FEP “remain poorly understood,” the researchers noted. In addition, “despite adequate antipsychotic treatment, 20% of patients have persistent symptoms.”
To determine whether ACE levels are already altered in FEP patients, the investigators examined data on 138 patients with FEP and 115 healthy controls.
After measuring blood concentrations in 122 of the patients and 78 controls, they found that ACE levels were significantly lower in FEP patients (P = 4.1x10-13) after controlling for age, sex, ethnicity, duration of illness, smoking status, and chlorpromazine equivalents.
Cerebrospinal fluid (CSF) levels, which were measured in 19 patients and 18 controls, were also significantly lower in individuals with FEP (P = .01), with a strong correlation observed between blood and CSF levels (P = .0005).
Next, the team used Treatment Response and Resistance in Psychosis criteria to compare ACE levels in 32 treatment-resistant patients and 106 non–treatment-resistant patients. Results showed that ACE blood levels were significantly lower in the treatment-resistant patients (P = .03).
Finally, the association between ACE blood levels and range of clinical and neurocognitive variables were examined across all FEP patients.
The Scale for the Assessment of Negative and Positive Symptoms was administered, along with a battery of tests looking at processing speed, working memory, verbal learning and memory, visual learning and memory, ideational fluency, and executive function. All were combined into a composite score.
While there was no association between ACE blood levels and symptom severity in the patients, there was a significant association between levels and verbal memory (P = .007) and composite cognitive score (P = .04).
Notable finding
In an interview, Thomas W. Sedlak, MD, PhD, assistant professor of psychiatry and behavioral health, Johns Hopkins University, Baltimore, said the finding that ACE levels were associated with lower cognition scores is “notable in that we don’t really have any treatments for cognition.
“Antipsychotic drugs treat more of the famous symptoms of hallucinations and delusions and disordered speech, but they don›t really help cognition a whole lot — and too much medicine might even make that worse,” said Dr. Sedlak, who was not involved with the research.
“So it’s interesting to have another biomarker that might relate a symptom of schizophrenia that we don’t really have a good grip on addressing,” he said.
However, he noted that this study is “preliminary,” has not looked at longitudinal changes in ACE in the same patients, and is one of “many” correlation studies.
“I like to say you can pretty much Google any chemical in the body and somebody has a claim about it and schizophrenia,” Dr. Sedlak said.
Nevertheless, the current analysis is “convergent” with past studies on ACE, including those that have found associations between polymorphisms in the enzyme and schizophrenia, he noted.
Moreover, research has shown that ACE facilitates glutamate transmission, specifically via N-methyl-D-aspartate (NMDA) receptors in the prefrontal cortex, “which is probably the brain region most tied to schizophrenia abnormalities,” Dr. Sedlak said.
No tie to inflammation
The findings do contrast, however, with the increasing body of evidence linking schizophrenia to inflammation.
Dr. Sedlak said that is not surprising, as the more that is learned about schizophrenia, “the less it is likely to be a single homogeneous entity but something more akin to intellectual disability.
“We’re finding that there are different subtypes of schizophrenia, some of which might have more of an inflammatory tie and others which don’t.”
He added that it might be possible over the longer term to cluster patients by ACE levels and other biomarkers and then follow them long term to “see if we can predict outcomes better than purely clinical terms.”
In this way, Dr. Sedlak said he believes that psychiatry may become more like rheumatology, “which I’d say is the area of medicine, at least in terms of making diagnoses, most similar.”
The etiology of conditions such as lupus and rheumatoid arthritis is “not absolutely known” and there is “probably no single cause,” with the diagnosis relying on clinical findings alongside biomarker tests.
“I think psychiatry is, long term, hoping to discover some biomarkers ... that would be used together with the clinical findings to help come to more clinical agreement on how to define cases – and would be predictive long term in terms of the course of illness,” Dr. Sedlak concluded.
The study had no specific funding. The study investigators and Dr. Sedlak have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
ACE levels are lower in individuals with first episode psychosis (FEP) and even lower in those with resistant disease, suggesting the enzyme may be a biomarker of disease severity.
In a longitudinal cohort study, investigators found patients with FEP had significantly reduced ACE levels compared with their healthy peers.
With blood concentrations of the enzyme significantly reduced in those who were treatment-resistant, and results suggest “a possible relationship with disease severity,” noted the researchers, led by investigator Luisa Longo, MD, a resident in psychiatry, University of Bari Aldo Moro in Italy.
Moreover, the finding that lower ACE levels were associated with greater cognitive impairment on neuropsychological tests indicates the enzyme plays a role in “the alteration of neurocognitive abilities” in patients with FEP.
Taken together, the results “highlight ACE as a promising peripheral biomarker to identify patients at risk of treatment resistance to antipsychotics,” the investigators reported.
The findings were presented at the annual congress of the Schizophrenia International Research Society.
Mechanisms “poorly understood”
Previous studies suggest ACE may play a role in neurologic and psychiatric conditions, including schizophrenia, through alterations in function or blood concentrations.
However, the molecular mechanisms underlying disease onset and response to antipsychotics in patients with FEP “remain poorly understood,” the researchers noted. In addition, “despite adequate antipsychotic treatment, 20% of patients have persistent symptoms.”
To determine whether ACE levels are already altered in FEP patients, the investigators examined data on 138 patients with FEP and 115 healthy controls.
After measuring blood concentrations in 122 of the patients and 78 controls, they found that ACE levels were significantly lower in FEP patients (P = 4.1x10-13) after controlling for age, sex, ethnicity, duration of illness, smoking status, and chlorpromazine equivalents.
Cerebrospinal fluid (CSF) levels, which were measured in 19 patients and 18 controls, were also significantly lower in individuals with FEP (P = .01), with a strong correlation observed between blood and CSF levels (P = .0005).
Next, the team used Treatment Response and Resistance in Psychosis criteria to compare ACE levels in 32 treatment-resistant patients and 106 non–treatment-resistant patients. Results showed that ACE blood levels were significantly lower in the treatment-resistant patients (P = .03).
Finally, the association between ACE blood levels and range of clinical and neurocognitive variables were examined across all FEP patients.
The Scale for the Assessment of Negative and Positive Symptoms was administered, along with a battery of tests looking at processing speed, working memory, verbal learning and memory, visual learning and memory, ideational fluency, and executive function. All were combined into a composite score.
While there was no association between ACE blood levels and symptom severity in the patients, there was a significant association between levels and verbal memory (P = .007) and composite cognitive score (P = .04).
Notable finding
In an interview, Thomas W. Sedlak, MD, PhD, assistant professor of psychiatry and behavioral health, Johns Hopkins University, Baltimore, said the finding that ACE levels were associated with lower cognition scores is “notable in that we don’t really have any treatments for cognition.
“Antipsychotic drugs treat more of the famous symptoms of hallucinations and delusions and disordered speech, but they don›t really help cognition a whole lot — and too much medicine might even make that worse,” said Dr. Sedlak, who was not involved with the research.
“So it’s interesting to have another biomarker that might relate a symptom of schizophrenia that we don’t really have a good grip on addressing,” he said.
However, he noted that this study is “preliminary,” has not looked at longitudinal changes in ACE in the same patients, and is one of “many” correlation studies.
“I like to say you can pretty much Google any chemical in the body and somebody has a claim about it and schizophrenia,” Dr. Sedlak said.
Nevertheless, the current analysis is “convergent” with past studies on ACE, including those that have found associations between polymorphisms in the enzyme and schizophrenia, he noted.
Moreover, research has shown that ACE facilitates glutamate transmission, specifically via N-methyl-D-aspartate (NMDA) receptors in the prefrontal cortex, “which is probably the brain region most tied to schizophrenia abnormalities,” Dr. Sedlak said.
No tie to inflammation
The findings do contrast, however, with the increasing body of evidence linking schizophrenia to inflammation.
Dr. Sedlak said that is not surprising, as the more that is learned about schizophrenia, “the less it is likely to be a single homogeneous entity but something more akin to intellectual disability.
“We’re finding that there are different subtypes of schizophrenia, some of which might have more of an inflammatory tie and others which don’t.”
He added that it might be possible over the longer term to cluster patients by ACE levels and other biomarkers and then follow them long term to “see if we can predict outcomes better than purely clinical terms.”
In this way, Dr. Sedlak said he believes that psychiatry may become more like rheumatology, “which I’d say is the area of medicine, at least in terms of making diagnoses, most similar.”
The etiology of conditions such as lupus and rheumatoid arthritis is “not absolutely known” and there is “probably no single cause,” with the diagnosis relying on clinical findings alongside biomarker tests.
“I think psychiatry is, long term, hoping to discover some biomarkers ... that would be used together with the clinical findings to help come to more clinical agreement on how to define cases – and would be predictive long term in terms of the course of illness,” Dr. Sedlak concluded.
The study had no specific funding. The study investigators and Dr. Sedlak have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM SIRS 2020
Tiragolumab plus atezolizumab active in PD-L1+ NSCLC
The combination of the anti-TIGIT antibody tiragolumab with the PD-L1 inhibitor atezolizumab was well tolerated and showed preliminary activity in the phase 1b portion of the study, according to investigator Johanna C. Bendell, MD, of Sarah Cannon Research Institute/Tennessee Oncology in Nashville, Tenn.
Objective responses occurred mainly in chemoimmunotherapy-naive, PD-L1-positive tumors. In an expansion cohort of 13 patients with PD-L1-positive non–small cell lung cancer (NSCLC), the confirmed overall response rate was 46%, with several responses demonstrating durability.
Dr. Bendell reported these results at the AACR virtual meeting II.
While several important research questions remain, the results in the lung cancer expansion cohort were encouraging, particularly in patients who were smokers and previous smokers, said invited discussant Michele Teng, PhD, of QIMR Berghofer Medical Research Institute in Brisbane, Australia.
“Although it was [a] small cohort, the data suggest promising duration of response in some of the patients [n = 4] who have been under study for more than 700 days,” Dr. Teng said.
Based on the preliminary safety and activity seen in this study, the combination of tiragolumab and atezolizumab is being evaluated in a randomized, placebo-controlled phase 2 study, and a phase 3 study is recruiting.
Rationale, design, and safety
TIGIT is a novel inhibitory receptor expressed on multiple immune cells, especially CD8-positive T cells and natural killer cells, Dr. Bendell explained. She added that TIGIT is coexpressed with PD-1 on immune cells.
“Using anti-TIGIT antibodies to prevent TIGIT from binding, and cotargeting TIGIT and PD-L1, may restore antitumor response and enhance anti-PD-L1 effect,” Dr. Bendell said.
The phase 1 study Dr. Bendell presented, known as GO30103, was designed to evaluate tiragolumab as a single agent and in combination with atezolizumab in advanced solid tumors.
There were 24 patients in the phase 1a portion of GO30103, which was intended to determine the preliminary safety, tolerability, and recommended phase 2 dose of tiragolumab. There were 49 patients treated with tiragolumab plus atezolizumab in the phase 1b portion, which was intended to provide data on pharmacokinetics as well as preliminary antitumor activity of the combination.
No dose-limiting toxicities were seen in either cohort. The recommended phase 2 dose of tiragolumab was 600 mg every 3 weeks.
Tiragolumab was well tolerated in the phase 1a and 1b portions of the trial, according to Dr. Bendell.
“Immune-related adverse events were seen, but their incidence was not out of proportion to events seen with atezolizumab alone,” she said.
Treatment-related grade 3-4 adverse events occurred in one patient (4%) in the phase 1a portion of the trial and two patients (4%) in the phase 1b portion. There were no grade 5 adverse events related to treatment.
Efficacy and next steps
No objective responses were seen with tiragolumab monotherapy, although several patients did exhibit tumor reduction.
“We were not really expecting much single-agent activity of the anti-TIGIT drug,” Dr. Bendell said. “There’s some preclinical data that suggests that TIGIT may be more important as a single agent in earlier stages of cancer.”
In contrast, the combination of tiragolumab and atezolizumab resulted in several responses, including one in a patient with PD-L1-positive NSCLC who was previously treated with immunotherapy, according to Dr. Bendell.
The 13-patient expansion cohort of patients with PD-L1-positive NSCLC were treated at the recommended phase 2 dose of tiragolumab and atezolizumab. In these chemoimmunotherapy-naive patients, the overall response rate was 46%. Responses occurred in 6 of 13 patients and included 2 complete responses. Four patients had stable disease, so the disease control rate was 85% (11/13).
Based on that expansion cohort, a randomized, phase 2 study called CITYSCAPE was initiated. Results of CITYSCAPE were recently presented as part of the American Society of Clinical Oncology virtual scientific program.
In that study, tiragolumab plus atezolizumab improved the overall response rate and progression-free survival when compared with placebo plus atezolizumab. More substantial improvement was seen in the subgroup of patients with PD-L1 tumor proportion scores of 50% or greater.
The activity and safety of tiragolumab plus atezolizumab will be confirmed in the ongoing SKYSCRAPER-01 trial (NCT04294810), a phase 3 study of first-line treatment in patients with NSCLC and a PD-L1 tumor proportion score of 50% or greater, according to investigators.
The phase 1 study presented by Dr. Bendell was sponsored by Genentech. Dr. Bendell disclosed relationships with Genentech/Roche, Gilead, Five Prime, Lilly, and other companies.
SOURCE: Bendell JC et al. AACR 2020, Abstract CT302.
The combination of the anti-TIGIT antibody tiragolumab with the PD-L1 inhibitor atezolizumab was well tolerated and showed preliminary activity in the phase 1b portion of the study, according to investigator Johanna C. Bendell, MD, of Sarah Cannon Research Institute/Tennessee Oncology in Nashville, Tenn.
Objective responses occurred mainly in chemoimmunotherapy-naive, PD-L1-positive tumors. In an expansion cohort of 13 patients with PD-L1-positive non–small cell lung cancer (NSCLC), the confirmed overall response rate was 46%, with several responses demonstrating durability.
Dr. Bendell reported these results at the AACR virtual meeting II.
While several important research questions remain, the results in the lung cancer expansion cohort were encouraging, particularly in patients who were smokers and previous smokers, said invited discussant Michele Teng, PhD, of QIMR Berghofer Medical Research Institute in Brisbane, Australia.
“Although it was [a] small cohort, the data suggest promising duration of response in some of the patients [n = 4] who have been under study for more than 700 days,” Dr. Teng said.
Based on the preliminary safety and activity seen in this study, the combination of tiragolumab and atezolizumab is being evaluated in a randomized, placebo-controlled phase 2 study, and a phase 3 study is recruiting.
Rationale, design, and safety
TIGIT is a novel inhibitory receptor expressed on multiple immune cells, especially CD8-positive T cells and natural killer cells, Dr. Bendell explained. She added that TIGIT is coexpressed with PD-1 on immune cells.
“Using anti-TIGIT antibodies to prevent TIGIT from binding, and cotargeting TIGIT and PD-L1, may restore antitumor response and enhance anti-PD-L1 effect,” Dr. Bendell said.
The phase 1 study Dr. Bendell presented, known as GO30103, was designed to evaluate tiragolumab as a single agent and in combination with atezolizumab in advanced solid tumors.
There were 24 patients in the phase 1a portion of GO30103, which was intended to determine the preliminary safety, tolerability, and recommended phase 2 dose of tiragolumab. There were 49 patients treated with tiragolumab plus atezolizumab in the phase 1b portion, which was intended to provide data on pharmacokinetics as well as preliminary antitumor activity of the combination.
No dose-limiting toxicities were seen in either cohort. The recommended phase 2 dose of tiragolumab was 600 mg every 3 weeks.
Tiragolumab was well tolerated in the phase 1a and 1b portions of the trial, according to Dr. Bendell.
“Immune-related adverse events were seen, but their incidence was not out of proportion to events seen with atezolizumab alone,” she said.
Treatment-related grade 3-4 adverse events occurred in one patient (4%) in the phase 1a portion of the trial and two patients (4%) in the phase 1b portion. There were no grade 5 adverse events related to treatment.
Efficacy and next steps
No objective responses were seen with tiragolumab monotherapy, although several patients did exhibit tumor reduction.
“We were not really expecting much single-agent activity of the anti-TIGIT drug,” Dr. Bendell said. “There’s some preclinical data that suggests that TIGIT may be more important as a single agent in earlier stages of cancer.”
In contrast, the combination of tiragolumab and atezolizumab resulted in several responses, including one in a patient with PD-L1-positive NSCLC who was previously treated with immunotherapy, according to Dr. Bendell.
The 13-patient expansion cohort of patients with PD-L1-positive NSCLC were treated at the recommended phase 2 dose of tiragolumab and atezolizumab. In these chemoimmunotherapy-naive patients, the overall response rate was 46%. Responses occurred in 6 of 13 patients and included 2 complete responses. Four patients had stable disease, so the disease control rate was 85% (11/13).
Based on that expansion cohort, a randomized, phase 2 study called CITYSCAPE was initiated. Results of CITYSCAPE were recently presented as part of the American Society of Clinical Oncology virtual scientific program.
In that study, tiragolumab plus atezolizumab improved the overall response rate and progression-free survival when compared with placebo plus atezolizumab. More substantial improvement was seen in the subgroup of patients with PD-L1 tumor proportion scores of 50% or greater.
The activity and safety of tiragolumab plus atezolizumab will be confirmed in the ongoing SKYSCRAPER-01 trial (NCT04294810), a phase 3 study of first-line treatment in patients with NSCLC and a PD-L1 tumor proportion score of 50% or greater, according to investigators.
The phase 1 study presented by Dr. Bendell was sponsored by Genentech. Dr. Bendell disclosed relationships with Genentech/Roche, Gilead, Five Prime, Lilly, and other companies.
SOURCE: Bendell JC et al. AACR 2020, Abstract CT302.
The combination of the anti-TIGIT antibody tiragolumab with the PD-L1 inhibitor atezolizumab was well tolerated and showed preliminary activity in the phase 1b portion of the study, according to investigator Johanna C. Bendell, MD, of Sarah Cannon Research Institute/Tennessee Oncology in Nashville, Tenn.
Objective responses occurred mainly in chemoimmunotherapy-naive, PD-L1-positive tumors. In an expansion cohort of 13 patients with PD-L1-positive non–small cell lung cancer (NSCLC), the confirmed overall response rate was 46%, with several responses demonstrating durability.
Dr. Bendell reported these results at the AACR virtual meeting II.
While several important research questions remain, the results in the lung cancer expansion cohort were encouraging, particularly in patients who were smokers and previous smokers, said invited discussant Michele Teng, PhD, of QIMR Berghofer Medical Research Institute in Brisbane, Australia.
“Although it was [a] small cohort, the data suggest promising duration of response in some of the patients [n = 4] who have been under study for more than 700 days,” Dr. Teng said.
Based on the preliminary safety and activity seen in this study, the combination of tiragolumab and atezolizumab is being evaluated in a randomized, placebo-controlled phase 2 study, and a phase 3 study is recruiting.
Rationale, design, and safety
TIGIT is a novel inhibitory receptor expressed on multiple immune cells, especially CD8-positive T cells and natural killer cells, Dr. Bendell explained. She added that TIGIT is coexpressed with PD-1 on immune cells.
“Using anti-TIGIT antibodies to prevent TIGIT from binding, and cotargeting TIGIT and PD-L1, may restore antitumor response and enhance anti-PD-L1 effect,” Dr. Bendell said.
The phase 1 study Dr. Bendell presented, known as GO30103, was designed to evaluate tiragolumab as a single agent and in combination with atezolizumab in advanced solid tumors.
There were 24 patients in the phase 1a portion of GO30103, which was intended to determine the preliminary safety, tolerability, and recommended phase 2 dose of tiragolumab. There were 49 patients treated with tiragolumab plus atezolizumab in the phase 1b portion, which was intended to provide data on pharmacokinetics as well as preliminary antitumor activity of the combination.
No dose-limiting toxicities were seen in either cohort. The recommended phase 2 dose of tiragolumab was 600 mg every 3 weeks.
Tiragolumab was well tolerated in the phase 1a and 1b portions of the trial, according to Dr. Bendell.
“Immune-related adverse events were seen, but their incidence was not out of proportion to events seen with atezolizumab alone,” she said.
Treatment-related grade 3-4 adverse events occurred in one patient (4%) in the phase 1a portion of the trial and two patients (4%) in the phase 1b portion. There were no grade 5 adverse events related to treatment.
Efficacy and next steps
No objective responses were seen with tiragolumab monotherapy, although several patients did exhibit tumor reduction.
“We were not really expecting much single-agent activity of the anti-TIGIT drug,” Dr. Bendell said. “There’s some preclinical data that suggests that TIGIT may be more important as a single agent in earlier stages of cancer.”
In contrast, the combination of tiragolumab and atezolizumab resulted in several responses, including one in a patient with PD-L1-positive NSCLC who was previously treated with immunotherapy, according to Dr. Bendell.
The 13-patient expansion cohort of patients with PD-L1-positive NSCLC were treated at the recommended phase 2 dose of tiragolumab and atezolizumab. In these chemoimmunotherapy-naive patients, the overall response rate was 46%. Responses occurred in 6 of 13 patients and included 2 complete responses. Four patients had stable disease, so the disease control rate was 85% (11/13).
Based on that expansion cohort, a randomized, phase 2 study called CITYSCAPE was initiated. Results of CITYSCAPE were recently presented as part of the American Society of Clinical Oncology virtual scientific program.
In that study, tiragolumab plus atezolizumab improved the overall response rate and progression-free survival when compared with placebo plus atezolizumab. More substantial improvement was seen in the subgroup of patients with PD-L1 tumor proportion scores of 50% or greater.
The activity and safety of tiragolumab plus atezolizumab will be confirmed in the ongoing SKYSCRAPER-01 trial (NCT04294810), a phase 3 study of first-line treatment in patients with NSCLC and a PD-L1 tumor proportion score of 50% or greater, according to investigators.
The phase 1 study presented by Dr. Bendell was sponsored by Genentech. Dr. Bendell disclosed relationships with Genentech/Roche, Gilead, Five Prime, Lilly, and other companies.
SOURCE: Bendell JC et al. AACR 2020, Abstract CT302.
FROM AACR 2020
Treatment developments in obstructive hypertrophic cardiomyopathy (oHCM)
Background: oHCM is characterized by mutations in sarcomeric proteins. Mavacamten is a small-molecule modulator of cardiac myosin, commonly affected in oHCM.
Study design: Open-label, nonrandomized phase 2 trial.
Setting: Five academic medical centers.
Synopsis: A total of 21 patients with oHCM were randomized to cohort A, high-dose mavacamten without additional therapy (beta-blockers, CCBs), or cohort B, low-dose mavacamten plus additional medical therapy. The LVOT gradient at 12 weeks improved in both cohorts: Cohort A had a mean change of –89.5 mm Hg (95% confidence interval, –138.3 to –40.7; P = .008) and cohort B –25.0 mm Hg (95% CI, –47.1 to –3.0, P = .020).
Bottom line: This phase 2 trial provides proof of concept and identified a plasma concentration of mavacamten needed to decrease the LVOT significantly. Phase 3 trials hold significant promise.
Citation: Heitner SB et al. Mavacamten treatment for obstructive hypertrophic cardiomyopathy: A clinical trial. Ann Intern Med. 2019 Apr 30. doi: 10.7326/M18-3016.
Dr. Blount is a hospitalist at the University of Colorado at Denver, Aurora.
Background: oHCM is characterized by mutations in sarcomeric proteins. Mavacamten is a small-molecule modulator of cardiac myosin, commonly affected in oHCM.
Study design: Open-label, nonrandomized phase 2 trial.
Setting: Five academic medical centers.
Synopsis: A total of 21 patients with oHCM were randomized to cohort A, high-dose mavacamten without additional therapy (beta-blockers, CCBs), or cohort B, low-dose mavacamten plus additional medical therapy. The LVOT gradient at 12 weeks improved in both cohorts: Cohort A had a mean change of –89.5 mm Hg (95% confidence interval, –138.3 to –40.7; P = .008) and cohort B –25.0 mm Hg (95% CI, –47.1 to –3.0, P = .020).
Bottom line: This phase 2 trial provides proof of concept and identified a plasma concentration of mavacamten needed to decrease the LVOT significantly. Phase 3 trials hold significant promise.
Citation: Heitner SB et al. Mavacamten treatment for obstructive hypertrophic cardiomyopathy: A clinical trial. Ann Intern Med. 2019 Apr 30. doi: 10.7326/M18-3016.
Dr. Blount is a hospitalist at the University of Colorado at Denver, Aurora.
Background: oHCM is characterized by mutations in sarcomeric proteins. Mavacamten is a small-molecule modulator of cardiac myosin, commonly affected in oHCM.
Study design: Open-label, nonrandomized phase 2 trial.
Setting: Five academic medical centers.
Synopsis: A total of 21 patients with oHCM were randomized to cohort A, high-dose mavacamten without additional therapy (beta-blockers, CCBs), or cohort B, low-dose mavacamten plus additional medical therapy. The LVOT gradient at 12 weeks improved in both cohorts: Cohort A had a mean change of –89.5 mm Hg (95% confidence interval, –138.3 to –40.7; P = .008) and cohort B –25.0 mm Hg (95% CI, –47.1 to –3.0, P = .020).
Bottom line: This phase 2 trial provides proof of concept and identified a plasma concentration of mavacamten needed to decrease the LVOT significantly. Phase 3 trials hold significant promise.
Citation: Heitner SB et al. Mavacamten treatment for obstructive hypertrophic cardiomyopathy: A clinical trial. Ann Intern Med. 2019 Apr 30. doi: 10.7326/M18-3016.
Dr. Blount is a hospitalist at the University of Colorado at Denver, Aurora.
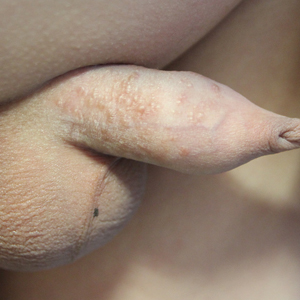
Multiple Yellow-Brown Papules on the Penis
The Diagnosis: Eruptive Syringoma
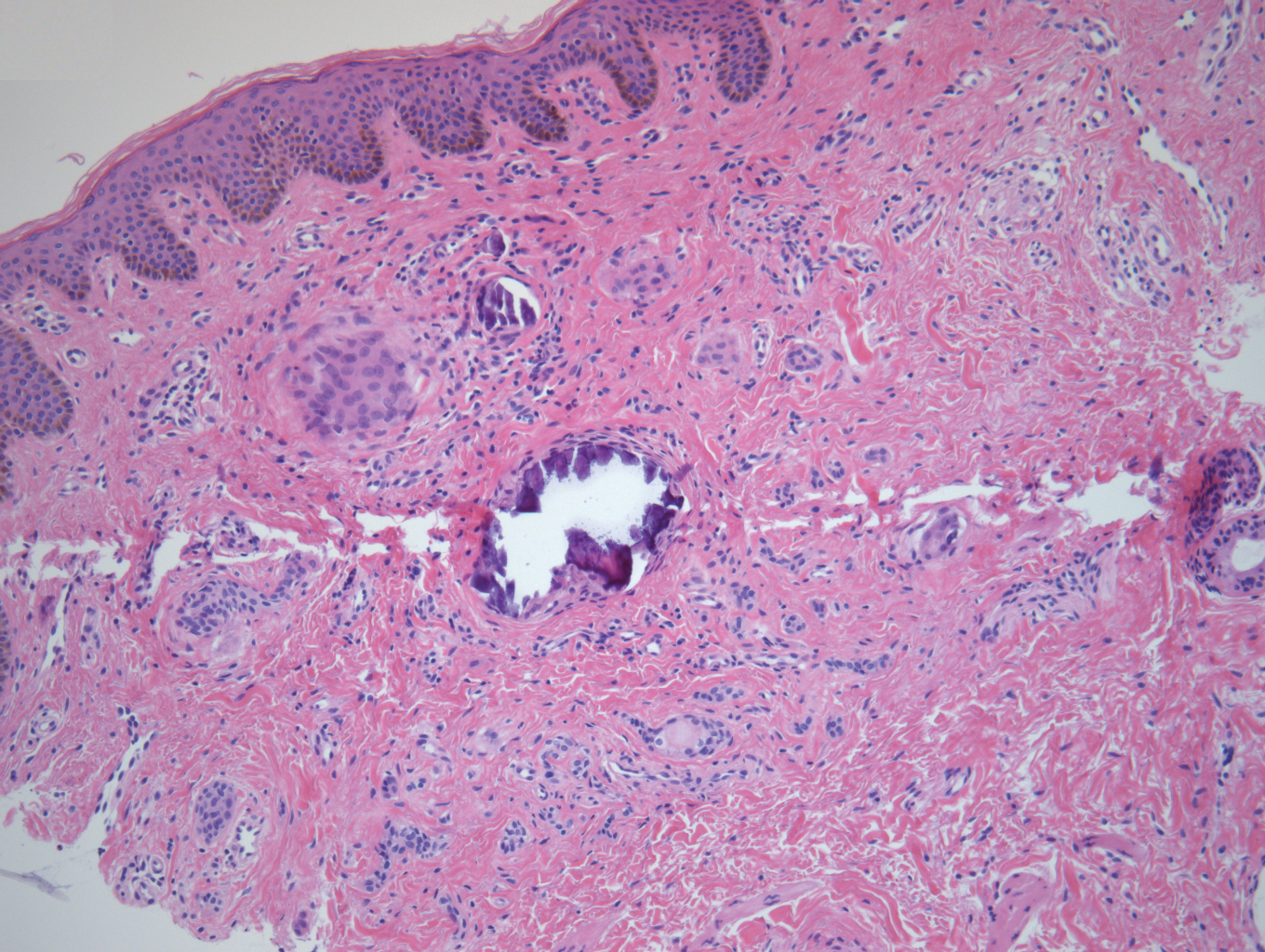
A punch biopsy of a lesion on the penis was performed. Histopathologic examination revealed many tadpole-shaped cords of epithelial cells and small ducts in the dermis (Figure). Based on clinical and histopathological findings, a diagnosis of eruptive syringoma was made. The patient declined treatment.
Syringomas are common benign eccrine neoplasms. They present clinically as small flesh-colored to brownish papules symmetrically distributed on the face, neck, trunk, pubic area, arms, and legs.1-3 Classic syringoma occurs more frequently in young adult women.1 Eruptive syringoma is a rare variant, and the age of onset ranges from 3 to 50 years.1-13 Eruptive syringoma is the term for multiple lesions that occur synchronously in any part of the body.1,4,13 The term eruptive is not the opposite of localized and refers to the time of onset of the lesion. There may be both a generalized eruptive syringoma or a localized eruptive syringoma depending on the distribution of the lesions.1 The most common site for syringoma occurrence is the eyelid; penile syringoma is extremely rare. Several cases of penile syringoma have been reported, but eruptive penile syringoma is rare.3,5-10,12,13
Histopathology is essential for the diagnosis of syringoma. Hematoxylin and eosin stain shows multiple small cystic ducts and epithelial cell nests in the dermis. Ductal structures sometimes appear tadpolelike or comma shaped depending on the section.1,2,7,12
The clinical differential diagnosis of syringoma includes sebaceous hyperplasia, verruca plana, molluscum contagiosum, bowenoid papulosis, condyloma acuminatum, lichen planus, lichen nitidus, milia, angiofibroma, epidermal cyst, calcinosis cutis, granuloma annulare, and sarcoidosis.3,8,12
Because syringoma is benign, treatment is not necessary unless there is a cosmetic problem.3,5,7,8,12 There is no satisfactory treatment of eruptive penile syringoma. Treatment options include topical tretinoin and adapalene, oral isotretinoin, cryotherapy, microelectrodesiccation with an epilating needle, dermabrasion, CO2 laser, and surgical excision.2,3,7,8,12
Adult patients with penile syringoma may be concerned about sexually transmitted diseases due to the appearance of the papules. If cosmesis is not an issue, clinicians should reassure the patient after a biopsy that the lesions are benign and self-limiting without recommending treatment.
- Ghanadan A, Khosravi M. Cutaneous syringoma: a clinicopathologic study of 34 new cases and review of the literature. Indian J Dermatol. 2013;58:326.
- Soler-Carrillo J, Estrach T, Mascaro JM. Eruptive syringoma: 27 new cases and review of the literature. J Eur Acad Dermatol Venereol. 2001;15:242-246.
- Baek JO, Jee HJ, Kim TK, et al. Eruptive penile syringomas spreading to the pubic area and lower abdomen. Ann Dermatol. 2013;25:116-118.
- Pruzan DL, Esterly NB, Prose NS. Eruptive syringoma. Arch Dermatol. 1989;125:1119-1120.
- Olson JM, Robles DT, Argenyi ZB, et al. Multiple penile syringomas. J Am Acad Dermatol. 2008;59(2 suppl 1):S46-S47.
- Petersson F, Mjornberg PA, Kazakov DV, et al. Eruptive syringoma of the penis. a report of 2 cases and a review of the literature. Am J Dermatopathol. 2009;31:436-438.
- Huang C, Wang W, Wu B. Multiple brownish papules on the penile shaft. Indian J Dermatol Venereol Leprol. 2011;77:404.
- Cohen PR, Tschen JA, Rapini RP. Penile syringoma: reports and review of patients with syringoma located on the penis. J Clin Aesthet Dermatol. 2013;6:38-42.
- Vaca EE, Mundinger GS, Zelken JA, et al. Surgical excision of multiple penile syringomas with scrotal flap reconstruction. Eplasty. 2014;14:E21.
- Mitkov M, Balagula Y, Taube JM, et al. Plaque-like syringoma with involvement of deep reticular dermis. J Am Acad Dermatol. 2014;71:e206-207.
- Vaca EE, Mundinger GS, Zelken JA, et al. Surgical excision of multiple penile syringomas with scrotal flap reconstruction. Eplasty. 2014;14:e21.
- Dhossche JM, Brodell RT, Al Hmada Y, et al. Skin-colored papules of the penis. Pediatr Dermatol. 2015;32:145-146.
- Todd PS, Gordon SC, Rovner RL, et al. Eruptive penile syringomas in an adolescent: novel approach with serial microexcisions and suture-adhesive repair. Pediatr Dermatol. 2016;33:E57-E60.
The Diagnosis: Eruptive Syringoma
A punch biopsy of a lesion on the penis was performed. Histopathologic examination revealed many tadpole-shaped cords of epithelial cells and small ducts in the dermis (Figure). Based on clinical and histopathological findings, a diagnosis of eruptive syringoma was made. The patient declined treatment.

Syringomas are common benign eccrine neoplasms. They present clinically as small flesh-colored to brownish papules symmetrically distributed on the face, neck, trunk, pubic area, arms, and legs.1-3 Classic syringoma occurs more frequently in young adult women.1 Eruptive syringoma is a rare variant, and the age of onset ranges from 3 to 50 years.1-13 Eruptive syringoma is the term for multiple lesions that occur synchronously in any part of the body.1,4,13 The term eruptive is not the opposite of localized and refers to the time of onset of the lesion. There may be both a generalized eruptive syringoma or a localized eruptive syringoma depending on the distribution of the lesions.1 The most common site for syringoma occurrence is the eyelid; penile syringoma is extremely rare. Several cases of penile syringoma have been reported, but eruptive penile syringoma is rare.3,5-10,12,13
Histopathology is essential for the diagnosis of syringoma. Hematoxylin and eosin stain shows multiple small cystic ducts and epithelial cell nests in the dermis. Ductal structures sometimes appear tadpolelike or comma shaped depending on the section.1,2,7,12
The clinical differential diagnosis of syringoma includes sebaceous hyperplasia, verruca plana, molluscum contagiosum, bowenoid papulosis, condyloma acuminatum, lichen planus, lichen nitidus, milia, angiofibroma, epidermal cyst, calcinosis cutis, granuloma annulare, and sarcoidosis.3,8,12
Because syringoma is benign, treatment is not necessary unless there is a cosmetic problem.3,5,7,8,12 There is no satisfactory treatment of eruptive penile syringoma. Treatment options include topical tretinoin and adapalene, oral isotretinoin, cryotherapy, microelectrodesiccation with an epilating needle, dermabrasion, CO2 laser, and surgical excision.2,3,7,8,12
Adult patients with penile syringoma may be concerned about sexually transmitted diseases due to the appearance of the papules. If cosmesis is not an issue, clinicians should reassure the patient after a biopsy that the lesions are benign and self-limiting without recommending treatment.
The Diagnosis: Eruptive Syringoma
A punch biopsy of a lesion on the penis was performed. Histopathologic examination revealed many tadpole-shaped cords of epithelial cells and small ducts in the dermis (Figure). Based on clinical and histopathological findings, a diagnosis of eruptive syringoma was made. The patient declined treatment.

Syringomas are common benign eccrine neoplasms. They present clinically as small flesh-colored to brownish papules symmetrically distributed on the face, neck, trunk, pubic area, arms, and legs.1-3 Classic syringoma occurs more frequently in young adult women.1 Eruptive syringoma is a rare variant, and the age of onset ranges from 3 to 50 years.1-13 Eruptive syringoma is the term for multiple lesions that occur synchronously in any part of the body.1,4,13 The term eruptive is not the opposite of localized and refers to the time of onset of the lesion. There may be both a generalized eruptive syringoma or a localized eruptive syringoma depending on the distribution of the lesions.1 The most common site for syringoma occurrence is the eyelid; penile syringoma is extremely rare. Several cases of penile syringoma have been reported, but eruptive penile syringoma is rare.3,5-10,12,13
Histopathology is essential for the diagnosis of syringoma. Hematoxylin and eosin stain shows multiple small cystic ducts and epithelial cell nests in the dermis. Ductal structures sometimes appear tadpolelike or comma shaped depending on the section.1,2,7,12
The clinical differential diagnosis of syringoma includes sebaceous hyperplasia, verruca plana, molluscum contagiosum, bowenoid papulosis, condyloma acuminatum, lichen planus, lichen nitidus, milia, angiofibroma, epidermal cyst, calcinosis cutis, granuloma annulare, and sarcoidosis.3,8,12
Because syringoma is benign, treatment is not necessary unless there is a cosmetic problem.3,5,7,8,12 There is no satisfactory treatment of eruptive penile syringoma. Treatment options include topical tretinoin and adapalene, oral isotretinoin, cryotherapy, microelectrodesiccation with an epilating needle, dermabrasion, CO2 laser, and surgical excision.2,3,7,8,12
Adult patients with penile syringoma may be concerned about sexually transmitted diseases due to the appearance of the papules. If cosmesis is not an issue, clinicians should reassure the patient after a biopsy that the lesions are benign and self-limiting without recommending treatment.
- Ghanadan A, Khosravi M. Cutaneous syringoma: a clinicopathologic study of 34 new cases and review of the literature. Indian J Dermatol. 2013;58:326.
- Soler-Carrillo J, Estrach T, Mascaro JM. Eruptive syringoma: 27 new cases and review of the literature. J Eur Acad Dermatol Venereol. 2001;15:242-246.
- Baek JO, Jee HJ, Kim TK, et al. Eruptive penile syringomas spreading to the pubic area and lower abdomen. Ann Dermatol. 2013;25:116-118.
- Pruzan DL, Esterly NB, Prose NS. Eruptive syringoma. Arch Dermatol. 1989;125:1119-1120.
- Olson JM, Robles DT, Argenyi ZB, et al. Multiple penile syringomas. J Am Acad Dermatol. 2008;59(2 suppl 1):S46-S47.
- Petersson F, Mjornberg PA, Kazakov DV, et al. Eruptive syringoma of the penis. a report of 2 cases and a review of the literature. Am J Dermatopathol. 2009;31:436-438.
- Huang C, Wang W, Wu B. Multiple brownish papules on the penile shaft. Indian J Dermatol Venereol Leprol. 2011;77:404.
- Cohen PR, Tschen JA, Rapini RP. Penile syringoma: reports and review of patients with syringoma located on the penis. J Clin Aesthet Dermatol. 2013;6:38-42.
- Vaca EE, Mundinger GS, Zelken JA, et al. Surgical excision of multiple penile syringomas with scrotal flap reconstruction. Eplasty. 2014;14:E21.
- Mitkov M, Balagula Y, Taube JM, et al. Plaque-like syringoma with involvement of deep reticular dermis. J Am Acad Dermatol. 2014;71:e206-207.
- Vaca EE, Mundinger GS, Zelken JA, et al. Surgical excision of multiple penile syringomas with scrotal flap reconstruction. Eplasty. 2014;14:e21.
- Dhossche JM, Brodell RT, Al Hmada Y, et al. Skin-colored papules of the penis. Pediatr Dermatol. 2015;32:145-146.
- Todd PS, Gordon SC, Rovner RL, et al. Eruptive penile syringomas in an adolescent: novel approach with serial microexcisions and suture-adhesive repair. Pediatr Dermatol. 2016;33:E57-E60.
- Ghanadan A, Khosravi M. Cutaneous syringoma: a clinicopathologic study of 34 new cases and review of the literature. Indian J Dermatol. 2013;58:326.
- Soler-Carrillo J, Estrach T, Mascaro JM. Eruptive syringoma: 27 new cases and review of the literature. J Eur Acad Dermatol Venereol. 2001;15:242-246.
- Baek JO, Jee HJ, Kim TK, et al. Eruptive penile syringomas spreading to the pubic area and lower abdomen. Ann Dermatol. 2013;25:116-118.
- Pruzan DL, Esterly NB, Prose NS. Eruptive syringoma. Arch Dermatol. 1989;125:1119-1120.
- Olson JM, Robles DT, Argenyi ZB, et al. Multiple penile syringomas. J Am Acad Dermatol. 2008;59(2 suppl 1):S46-S47.
- Petersson F, Mjornberg PA, Kazakov DV, et al. Eruptive syringoma of the penis. a report of 2 cases and a review of the literature. Am J Dermatopathol. 2009;31:436-438.
- Huang C, Wang W, Wu B. Multiple brownish papules on the penile shaft. Indian J Dermatol Venereol Leprol. 2011;77:404.
- Cohen PR, Tschen JA, Rapini RP. Penile syringoma: reports and review of patients with syringoma located on the penis. J Clin Aesthet Dermatol. 2013;6:38-42.
- Vaca EE, Mundinger GS, Zelken JA, et al. Surgical excision of multiple penile syringomas with scrotal flap reconstruction. Eplasty. 2014;14:E21.
- Mitkov M, Balagula Y, Taube JM, et al. Plaque-like syringoma with involvement of deep reticular dermis. J Am Acad Dermatol. 2014;71:e206-207.
- Vaca EE, Mundinger GS, Zelken JA, et al. Surgical excision of multiple penile syringomas with scrotal flap reconstruction. Eplasty. 2014;14:e21.
- Dhossche JM, Brodell RT, Al Hmada Y, et al. Skin-colored papules of the penis. Pediatr Dermatol. 2015;32:145-146.
- Todd PS, Gordon SC, Rovner RL, et al. Eruptive penile syringomas in an adolescent: novel approach with serial microexcisions and suture-adhesive repair. Pediatr Dermatol. 2016;33:E57-E60.
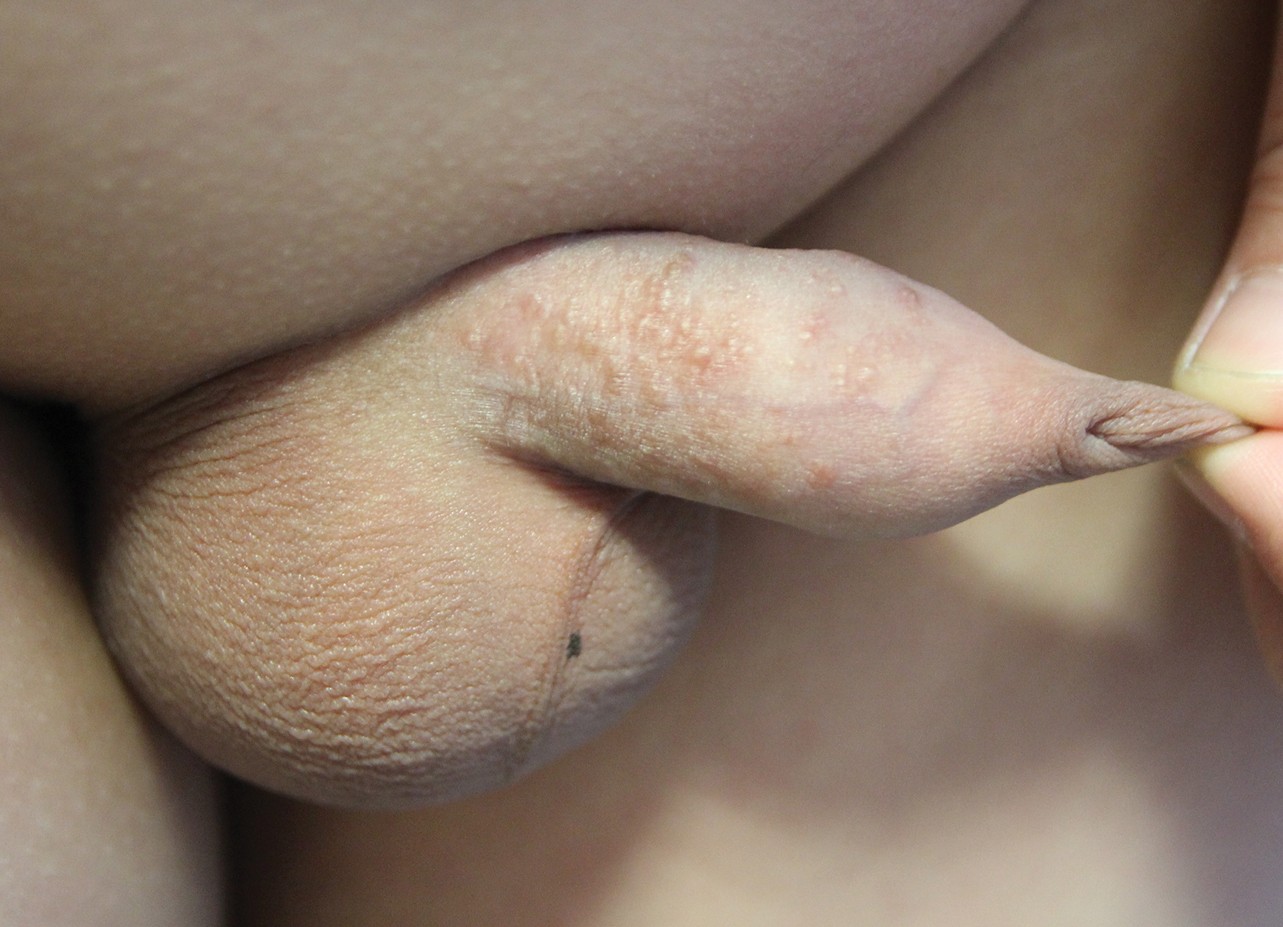
A 12-year-old boy presented with multiple asymptomatic, 0.1-cm, yellow-brown papules on the penile shaft of several years' duration. The lesions appeared suddenly. The patient had no history of trauma, injection, or an underlying disorder.
FDA approves in-home breast cancer treatment
Advantageous for infusion centers?
The Food and Drug Administration approved a combination of pertuzumab (Perjeta, Genentech/Roche), trastuzumab (Herceptin, Genentech/Roche) and hyaluronidase (Phesgo, Genentech/Roche) that is administered subcutaneously – rather than intravenously – for the treatment of early and metastatic HER2-positive breast cancers.
Phesgo is initially used in combination with chemotherapy at an infusion center but could continue to be administered in a patient’s home by a qualified health care professional once chemotherapy is complete, according to the FDA.
Administration takes approximately 8 minutes for the initial loading dose and approximately 5 minutes for maintenance doses, according to a Genentech press statement. This compares favorably with the 150 minutes needed for the combined loading dose of intravenous pertuzumab and trastuzumab, and the 60-150 minutes for intravenous maintenance infusions, the company said.
“Currently, most patients with HER2-positive breast cancer receive trastuzumab and pertuzumab at infusion centers. With a new administration route, Phesgo offers an outpatient option for patients to receive trastuzumab and pertuzumab,” said Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Oncologic Diseases in the FDA’s Center for Drug Evaluation and Research, in an agency press release.
“The fixed-dose combination of trastuzumab and pertuzumab offers a simpler, faster, and easier treatment experience for patients with HER2-positive breast cancer,” said Antoinette Tan, MD, MHSc, chief of breast medical oncology at Levine Cancer Institute, Charlotte, N.C., in the company statement.
Dr. Tan also said that home administration “can be advantageous for patients and infusion centers.”
However, in April, the Community Oncology Alliance strenuously objected to this type of treatment in a patient’s home, as reported by Medscape Medical News.
The group, which represents U.S. community-based practices, said it “fundamentally opposes home infusion of chemotherapy, cancer immunotherapy, and cancer treatment supportive drugs because of serious patient safety concerns.”
The FDA’s approval was based on the results of the pivotal phase 3 FeDeriCa trial, a noninferiority study in patients with HER2-positive early breast cancer, which demonstrated that the new product had comparable efficacy and safety as intravenous pertuzumab and intravenous trastuzumab.
In terms of efficacy, the subcutaneous product demonstrated noninferior plasma levels of pertuzumab, which was the primary endpoint, when compared with IV administration of pertuzumab.
Safety was comparable between the two approaches, with no new safety signals using the subcutaneous delivery method, including no “meaningful difference” in cardiac toxicity, according to Genentech. However, there were more administration-related reactions with the new product. The most common adverse events in both groups were alopecia, nausea, diarrhea, and anemia.
The new product uses a drug delivery technology (Enhanze, Halozyme Therapeutics) that employs a proprietary enzyme that temporarily degrades hyaluronan, a glycosaminoglycan or chain of natural sugars in the body, to facilitate the dispersion and absorption of injected therapeutic drugs, according to Genentech.
In May, at the European Society for Medical Oncology Breast Cancer Virtual Meeting 2020, investigators of the phase 2 PHranceSCa study reported that “more than 80%” of patients preferred subcutaneous to intravenous administration of pertuzumab and trastuzumab.
This article first appeared on Medscape.com.
Advantageous for infusion centers?
Advantageous for infusion centers?
The Food and Drug Administration approved a combination of pertuzumab (Perjeta, Genentech/Roche), trastuzumab (Herceptin, Genentech/Roche) and hyaluronidase (Phesgo, Genentech/Roche) that is administered subcutaneously – rather than intravenously – for the treatment of early and metastatic HER2-positive breast cancers.
Phesgo is initially used in combination with chemotherapy at an infusion center but could continue to be administered in a patient’s home by a qualified health care professional once chemotherapy is complete, according to the FDA.
Administration takes approximately 8 minutes for the initial loading dose and approximately 5 minutes for maintenance doses, according to a Genentech press statement. This compares favorably with the 150 minutes needed for the combined loading dose of intravenous pertuzumab and trastuzumab, and the 60-150 minutes for intravenous maintenance infusions, the company said.
“Currently, most patients with HER2-positive breast cancer receive trastuzumab and pertuzumab at infusion centers. With a new administration route, Phesgo offers an outpatient option for patients to receive trastuzumab and pertuzumab,” said Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Oncologic Diseases in the FDA’s Center for Drug Evaluation and Research, in an agency press release.
“The fixed-dose combination of trastuzumab and pertuzumab offers a simpler, faster, and easier treatment experience for patients with HER2-positive breast cancer,” said Antoinette Tan, MD, MHSc, chief of breast medical oncology at Levine Cancer Institute, Charlotte, N.C., in the company statement.
Dr. Tan also said that home administration “can be advantageous for patients and infusion centers.”
However, in April, the Community Oncology Alliance strenuously objected to this type of treatment in a patient’s home, as reported by Medscape Medical News.
The group, which represents U.S. community-based practices, said it “fundamentally opposes home infusion of chemotherapy, cancer immunotherapy, and cancer treatment supportive drugs because of serious patient safety concerns.”
The FDA’s approval was based on the results of the pivotal phase 3 FeDeriCa trial, a noninferiority study in patients with HER2-positive early breast cancer, which demonstrated that the new product had comparable efficacy and safety as intravenous pertuzumab and intravenous trastuzumab.
In terms of efficacy, the subcutaneous product demonstrated noninferior plasma levels of pertuzumab, which was the primary endpoint, when compared with IV administration of pertuzumab.
Safety was comparable between the two approaches, with no new safety signals using the subcutaneous delivery method, including no “meaningful difference” in cardiac toxicity, according to Genentech. However, there were more administration-related reactions with the new product. The most common adverse events in both groups were alopecia, nausea, diarrhea, and anemia.
The new product uses a drug delivery technology (Enhanze, Halozyme Therapeutics) that employs a proprietary enzyme that temporarily degrades hyaluronan, a glycosaminoglycan or chain of natural sugars in the body, to facilitate the dispersion and absorption of injected therapeutic drugs, according to Genentech.
In May, at the European Society for Medical Oncology Breast Cancer Virtual Meeting 2020, investigators of the phase 2 PHranceSCa study reported that “more than 80%” of patients preferred subcutaneous to intravenous administration of pertuzumab and trastuzumab.
This article first appeared on Medscape.com.
The Food and Drug Administration approved a combination of pertuzumab (Perjeta, Genentech/Roche), trastuzumab (Herceptin, Genentech/Roche) and hyaluronidase (Phesgo, Genentech/Roche) that is administered subcutaneously – rather than intravenously – for the treatment of early and metastatic HER2-positive breast cancers.
Phesgo is initially used in combination with chemotherapy at an infusion center but could continue to be administered in a patient’s home by a qualified health care professional once chemotherapy is complete, according to the FDA.
Administration takes approximately 8 minutes for the initial loading dose and approximately 5 minutes for maintenance doses, according to a Genentech press statement. This compares favorably with the 150 minutes needed for the combined loading dose of intravenous pertuzumab and trastuzumab, and the 60-150 minutes for intravenous maintenance infusions, the company said.
“Currently, most patients with HER2-positive breast cancer receive trastuzumab and pertuzumab at infusion centers. With a new administration route, Phesgo offers an outpatient option for patients to receive trastuzumab and pertuzumab,” said Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Oncologic Diseases in the FDA’s Center for Drug Evaluation and Research, in an agency press release.
“The fixed-dose combination of trastuzumab and pertuzumab offers a simpler, faster, and easier treatment experience for patients with HER2-positive breast cancer,” said Antoinette Tan, MD, MHSc, chief of breast medical oncology at Levine Cancer Institute, Charlotte, N.C., in the company statement.
Dr. Tan also said that home administration “can be advantageous for patients and infusion centers.”
However, in April, the Community Oncology Alliance strenuously objected to this type of treatment in a patient’s home, as reported by Medscape Medical News.
The group, which represents U.S. community-based practices, said it “fundamentally opposes home infusion of chemotherapy, cancer immunotherapy, and cancer treatment supportive drugs because of serious patient safety concerns.”
The FDA’s approval was based on the results of the pivotal phase 3 FeDeriCa trial, a noninferiority study in patients with HER2-positive early breast cancer, which demonstrated that the new product had comparable efficacy and safety as intravenous pertuzumab and intravenous trastuzumab.
In terms of efficacy, the subcutaneous product demonstrated noninferior plasma levels of pertuzumab, which was the primary endpoint, when compared with IV administration of pertuzumab.
Safety was comparable between the two approaches, with no new safety signals using the subcutaneous delivery method, including no “meaningful difference” in cardiac toxicity, according to Genentech. However, there were more administration-related reactions with the new product. The most common adverse events in both groups were alopecia, nausea, diarrhea, and anemia.
The new product uses a drug delivery technology (Enhanze, Halozyme Therapeutics) that employs a proprietary enzyme that temporarily degrades hyaluronan, a glycosaminoglycan or chain of natural sugars in the body, to facilitate the dispersion and absorption of injected therapeutic drugs, according to Genentech.
In May, at the European Society for Medical Oncology Breast Cancer Virtual Meeting 2020, investigators of the phase 2 PHranceSCa study reported that “more than 80%” of patients preferred subcutaneous to intravenous administration of pertuzumab and trastuzumab.
This article first appeared on Medscape.com.
Visualization tool aids migraine management
The tool is still in the prototype stage, but it could eventually synthesize patient responses to an integrated questionnaire and produce a chart illustrating where the patient stands with respect to a range of modifiable risk factors, including depression, medication overuse, insomnia, and body mass index, among others.
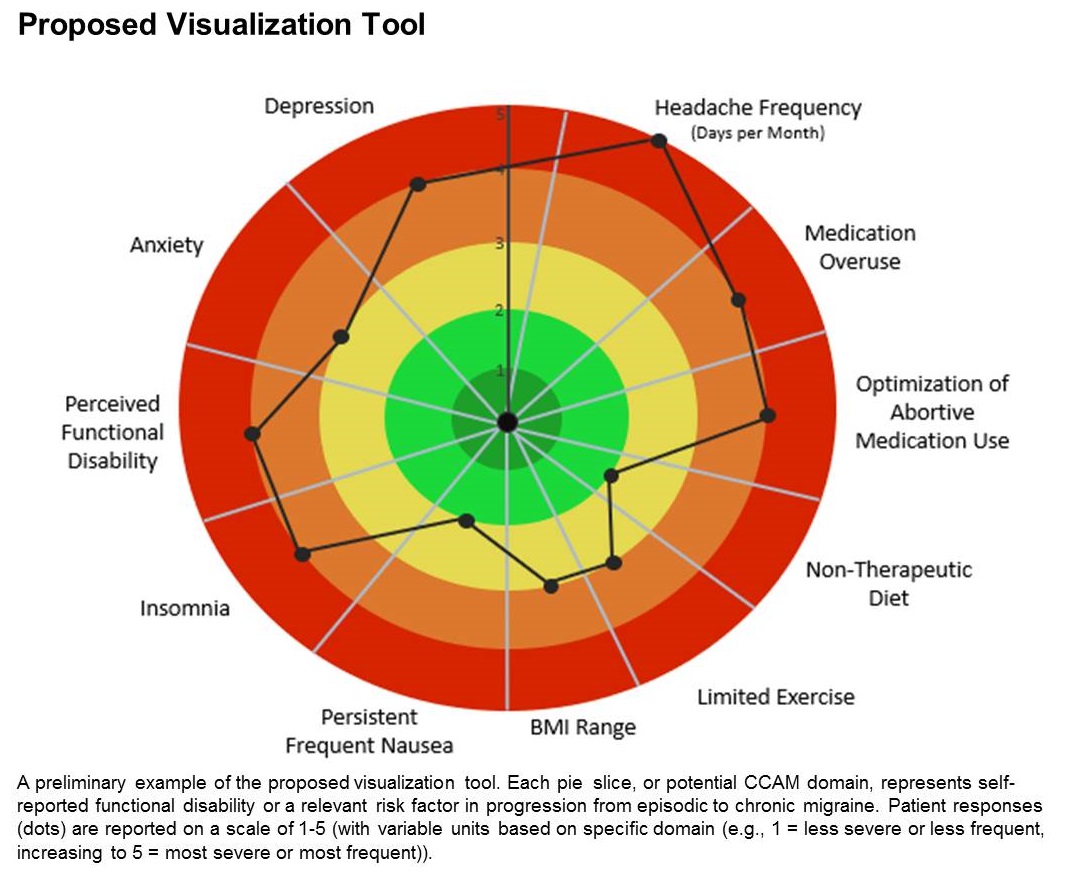
A few such tools exist for other conditions, such as stroke and risk of developing chronic diseases. Existing migraine visualization models focus only on individual risk factors, but they are capable of much more. “Visualization tools can effectively communicate a huge amount of clinical information,” said lead author Ami Cuneo, MD, who is a headache fellow at the University of Washington, Seattle, in an interview. Dr. Cuneo presented a poster describing the concept at the virtual annual meeting of the American Headache Society.
A picture is worth a thousand words
Dr. Cuneo’s background is well suited to the effort: Before entering medicine, she was a documentary producer. “I have a lot of interest in the patient story and history,” she added. She also believes that the tool could improve patient-provider relationships. In rushed sessions, patients may not feel heard. Patients gain a therapeutic benefit from the belief that their provider is listening to them and listening to their story. Visualization tools could promote that if the provider can quickly identify key elements of the patient’s condition. “A lot of headache patients can have a complex picture,” said Dr. Cuneo.
Physicians must see patients in short appointment periods, making it difficult to communicate all of the risk factors and behavioral characteristics that can contribute to risk of progression. “If you have a patient and you’re able to look at a visualization tool quickly and say: ‘Okay, my patient really is having insomnia and sleep issues,’ you can focus the session talking about sleep, cognitive-behavioral therapy for insomnia, and all the things we can help patients with,” said Dr. Cuneo.
The prototype visualization tool uses a color-coded wheel divided into pie slices, each representing a clinical characteristic or modifiable risk factor. In the proposed tool presented in the poster, these included depression, anxiety, functional disability, insomnia, nausea, headache frequency, medication overuse, optimization of abortive medication use, nontherapeutic diet, limited exercise, and body mass index range. The circle also contains colored concentric circles, ranging from red to green, and a small filled circle represents the patient’s status in each category as ranked using the integrated questionnaire. A line connects the circles in each pie, revealing the patient’s overall status.
The visual cue allows both the physician and patient to quickly assess these factors and see them in relationship to one another. Verbally communicating each factor is time consuming and harder for the patient to take in, according to Dr. Cuneo. “The provider can just look at it and see the areas to focus questions on to try to improve care. So it’s a way I’m hopeful that we can help target visits and improve patient-provider communication without extending visit time.”
A key challenge for the project will be choosing and consolidating scales so that the patient isn’t burdened with too many questions in advance of the appointment. The team will draw from existing scales and then create their own and validate it. “The questions will have to be vetted with patients through focus groups, and then the software platform [will have to be developed] so that patients can complete the survey online. Then we have to test it to see if providers and patients feel this is something that’s helpful in the clinical practice,” said Dr. Cuneo.
Will it change behavior?
If successful, the tool would be a welcome addition, according to Andrew Charles, MD, who was asked to comment on the work. “Epidemiological studies have identified these risk factors, but we haven’t had a way of operationalizing a strategy to reduce them systematically, so having some sort of tool that visualizes not just one but multiple risk factors is something I think could be helpful to address those factors more aggressively. The real question would be, if you put it in the hands of practitioners and patients, will they really be able to easily implement it and will it change behavior,” said Dr. Charles, who is a professor of neurology and director of the Goldberg Migraine Program at the University of California, Los Angeles.
The study received no funding. Dr. Cuneo and Dr. Charles have no relevant financial disclosures.
SOURCE; Cuneo A et al. AHS 2020, Abstract 273715.
The tool is still in the prototype stage, but it could eventually synthesize patient responses to an integrated questionnaire and produce a chart illustrating where the patient stands with respect to a range of modifiable risk factors, including depression, medication overuse, insomnia, and body mass index, among others.

A few such tools exist for other conditions, such as stroke and risk of developing chronic diseases. Existing migraine visualization models focus only on individual risk factors, but they are capable of much more. “Visualization tools can effectively communicate a huge amount of clinical information,” said lead author Ami Cuneo, MD, who is a headache fellow at the University of Washington, Seattle, in an interview. Dr. Cuneo presented a poster describing the concept at the virtual annual meeting of the American Headache Society.
A picture is worth a thousand words
Dr. Cuneo’s background is well suited to the effort: Before entering medicine, she was a documentary producer. “I have a lot of interest in the patient story and history,” she added. She also believes that the tool could improve patient-provider relationships. In rushed sessions, patients may not feel heard. Patients gain a therapeutic benefit from the belief that their provider is listening to them and listening to their story. Visualization tools could promote that if the provider can quickly identify key elements of the patient’s condition. “A lot of headache patients can have a complex picture,” said Dr. Cuneo.
Physicians must see patients in short appointment periods, making it difficult to communicate all of the risk factors and behavioral characteristics that can contribute to risk of progression. “If you have a patient and you’re able to look at a visualization tool quickly and say: ‘Okay, my patient really is having insomnia and sleep issues,’ you can focus the session talking about sleep, cognitive-behavioral therapy for insomnia, and all the things we can help patients with,” said Dr. Cuneo.
The prototype visualization tool uses a color-coded wheel divided into pie slices, each representing a clinical characteristic or modifiable risk factor. In the proposed tool presented in the poster, these included depression, anxiety, functional disability, insomnia, nausea, headache frequency, medication overuse, optimization of abortive medication use, nontherapeutic diet, limited exercise, and body mass index range. The circle also contains colored concentric circles, ranging from red to green, and a small filled circle represents the patient’s status in each category as ranked using the integrated questionnaire. A line connects the circles in each pie, revealing the patient’s overall status.
The visual cue allows both the physician and patient to quickly assess these factors and see them in relationship to one another. Verbally communicating each factor is time consuming and harder for the patient to take in, according to Dr. Cuneo. “The provider can just look at it and see the areas to focus questions on to try to improve care. So it’s a way I’m hopeful that we can help target visits and improve patient-provider communication without extending visit time.”
A key challenge for the project will be choosing and consolidating scales so that the patient isn’t burdened with too many questions in advance of the appointment. The team will draw from existing scales and then create their own and validate it. “The questions will have to be vetted with patients through focus groups, and then the software platform [will have to be developed] so that patients can complete the survey online. Then we have to test it to see if providers and patients feel this is something that’s helpful in the clinical practice,” said Dr. Cuneo.
Will it change behavior?
If successful, the tool would be a welcome addition, according to Andrew Charles, MD, who was asked to comment on the work. “Epidemiological studies have identified these risk factors, but we haven’t had a way of operationalizing a strategy to reduce them systematically, so having some sort of tool that visualizes not just one but multiple risk factors is something I think could be helpful to address those factors more aggressively. The real question would be, if you put it in the hands of practitioners and patients, will they really be able to easily implement it and will it change behavior,” said Dr. Charles, who is a professor of neurology and director of the Goldberg Migraine Program at the University of California, Los Angeles.
The study received no funding. Dr. Cuneo and Dr. Charles have no relevant financial disclosures.
SOURCE; Cuneo A et al. AHS 2020, Abstract 273715.
The tool is still in the prototype stage, but it could eventually synthesize patient responses to an integrated questionnaire and produce a chart illustrating where the patient stands with respect to a range of modifiable risk factors, including depression, medication overuse, insomnia, and body mass index, among others.

A few such tools exist for other conditions, such as stroke and risk of developing chronic diseases. Existing migraine visualization models focus only on individual risk factors, but they are capable of much more. “Visualization tools can effectively communicate a huge amount of clinical information,” said lead author Ami Cuneo, MD, who is a headache fellow at the University of Washington, Seattle, in an interview. Dr. Cuneo presented a poster describing the concept at the virtual annual meeting of the American Headache Society.
A picture is worth a thousand words
Dr. Cuneo’s background is well suited to the effort: Before entering medicine, she was a documentary producer. “I have a lot of interest in the patient story and history,” she added. She also believes that the tool could improve patient-provider relationships. In rushed sessions, patients may not feel heard. Patients gain a therapeutic benefit from the belief that their provider is listening to them and listening to their story. Visualization tools could promote that if the provider can quickly identify key elements of the patient’s condition. “A lot of headache patients can have a complex picture,” said Dr. Cuneo.
Physicians must see patients in short appointment periods, making it difficult to communicate all of the risk factors and behavioral characteristics that can contribute to risk of progression. “If you have a patient and you’re able to look at a visualization tool quickly and say: ‘Okay, my patient really is having insomnia and sleep issues,’ you can focus the session talking about sleep, cognitive-behavioral therapy for insomnia, and all the things we can help patients with,” said Dr. Cuneo.
The prototype visualization tool uses a color-coded wheel divided into pie slices, each representing a clinical characteristic or modifiable risk factor. In the proposed tool presented in the poster, these included depression, anxiety, functional disability, insomnia, nausea, headache frequency, medication overuse, optimization of abortive medication use, nontherapeutic diet, limited exercise, and body mass index range. The circle also contains colored concentric circles, ranging from red to green, and a small filled circle represents the patient’s status in each category as ranked using the integrated questionnaire. A line connects the circles in each pie, revealing the patient’s overall status.
The visual cue allows both the physician and patient to quickly assess these factors and see them in relationship to one another. Verbally communicating each factor is time consuming and harder for the patient to take in, according to Dr. Cuneo. “The provider can just look at it and see the areas to focus questions on to try to improve care. So it’s a way I’m hopeful that we can help target visits and improve patient-provider communication without extending visit time.”
A key challenge for the project will be choosing and consolidating scales so that the patient isn’t burdened with too many questions in advance of the appointment. The team will draw from existing scales and then create their own and validate it. “The questions will have to be vetted with patients through focus groups, and then the software platform [will have to be developed] so that patients can complete the survey online. Then we have to test it to see if providers and patients feel this is something that’s helpful in the clinical practice,” said Dr. Cuneo.
Will it change behavior?
If successful, the tool would be a welcome addition, according to Andrew Charles, MD, who was asked to comment on the work. “Epidemiological studies have identified these risk factors, but we haven’t had a way of operationalizing a strategy to reduce them systematically, so having some sort of tool that visualizes not just one but multiple risk factors is something I think could be helpful to address those factors more aggressively. The real question would be, if you put it in the hands of practitioners and patients, will they really be able to easily implement it and will it change behavior,” said Dr. Charles, who is a professor of neurology and director of the Goldberg Migraine Program at the University of California, Los Angeles.
The study received no funding. Dr. Cuneo and Dr. Charles have no relevant financial disclosures.
SOURCE; Cuneo A et al. AHS 2020, Abstract 273715.
FROM AHS 2020
Manage the pandemic with a multidisciplinary coalition
Implement a 6-P framework
The ongoing COVID-19 pandemic, arguably the biggest public health and economic catastrophe of modern times, elevated multiple deficiencies in public health infrastructures across the world, such as a slow or delayed response to suppress and mitigate the virus, an inadequately prepared and protected health care and public health workforce, and decentralized, siloed efforts.1 COVID-19 further highlighted the vulnerabilities of the health care, public health, and economic sectors.2,3 Irrespective of how robust health care systems may have been initially, rapidly spreading and deadly infectious diseases like COVID-19 can quickly derail the system, bringing the workforce and the patients they serve to a breaking point.
Hospital systems in the United States are not only at the crux of the current pandemic but are also well positioned to lead the response to the pandemic. Hospital administrators oversee nearly 33% of national health expenditure that amounts to the hospital-based care in the United States. Additionally, they may have an impact on nearly 30% of the expenditure that is related to physicians, prescriptions, and other facilities.4
The two primary goals underlying our proposed framework to target COVID-19 are based on the World Health Organization recommendations and lessons learned from countries such as South Korea that have successfully implemented these recommendations.5
1. Flatten the curve. According to the WHO and the Centers for Disease Control and Prevention, flattening the curve means that we must do everything that will help us to slow down the rate of infection, so the number of cases do not exceed the capacity of health systems.
2. Establish a standardized, interdisciplinary approach to flattening the curve. Pandemics can have major adverse consequences beyond health outcomes (e.g., economy) that can impact adherence to advisories and introduce multiple unintended consequences (e.g., deferred chronic care, unemployment). Managing the current pandemic and thoughtful consideration of and action regarding its ripple effects is heavily dependent on a standardized, interdisciplinary approach that is monitored, implemented, and evaluated well.
To achieve these two goals, we recommend establishing an interdisciplinary coalition representing multiple sectors. Our 6-P framework described below is intended to guide hospital administrators, to build the coalition, and to achieve these goals.
Structure of the pandemic coalition
A successful coalition invites a collaborative partnership involving senior members of respective disciplines, who would provide valuable, complementary perspectives in the coalition. We recommend hospital administrators take a lead in the formation of such a coalition. While we present the stakeholders and their roles below based on their intended influence and impact on the overall outcome of COVID-19, the basic guiding principles behind our 6-P framework remain true for any large-scale population health intervention.
Although several models for staging the transmission of COVID-19 are available, we adopted a four-stage model followed by the Indian Council for Medical Research.6 Irrespective of the origin of the infection, we believe that the four-stage model can cultivate situational awareness that can help guide the strategic design and systematic implementation of interventions.
Our 6-P framework integrates the four-stage model of COVID-19 transmission to identify action items for each stakeholder group and appropriate strategies selected based on the stages targeted.

1. Policy makers: Policy makers at all levels are critical in establishing policies, orders, and advisories, as well as dedicating resources and infrastructure, to enhance adherence to recommendations and guidelines at the community and population levels.7 They can assist hospitals in workforce expansion across county/state/discipline lines (e.g., accelerate the licensing and credentialing process, authorize graduate medical trainees, nurse practitioners, and other allied health professionals). Policy revisions for data sharing, privacy, communication, liability, and telehealth expansion.82. Providers: The health of the health care workforce itself is at risk because of their frontline services. Their buy-in will be crucial in both the formulation and implementation of evidence- and practice-based guidelines.9 Rapid adoption of telehealth for care continuum, policy revisions for elective procedures, visitor restriction, surge, resurge planning, capacity expansion, effective population health management, and working with employee unions, professional staff organizations are few, but very important action items that need to be implemented.
3. Public health authorities: Representation of public health authorities will be crucial in standardizing data collection, management, and reporting; providing up-to-date guidelines and advisories; developing, implementing, and evaluating short- and long-term public health interventions; and preparing and helping communities throughout the course of the pandemic. They also play a key role in identifying and reducing barriers related to the expansion of testing and contact tracing efforts.
4. Payers: In the United States, the Centers for Medicare & Medicaid Services oversees primary federally funded programs and serves as a point of reference for the American health care system. Having representation from all payer sources is crucial for achieving uniformity and standardization of the care process during the pandemic, with particular priority given to individuals and families who may have recently lost their health insurance because of job loss from COVID-19–related business furloughs, layoffs, and closures. Customer outreach initiatives, revision of patients’ out of pocket responsibilities, rapid claim settlement and denial management services, expansion of telehealth, elimination of prior authorization barriers, rapid credentialing of providers, data sharing, and assisting hospital systems in chronic disease management are examples of time-sensitive initiatives that are vital for population health management.
5. Partners: Establishing partnerships with pharma, health IT, labs, device industries, and other ancillary services is important to facilitate rapid innovation, production, and supply of essential medical devices and resources. These partners directly influence the outcomes of the pandemic and long-term health of the society through expansion of testing capability, contact tracing, leveraging technology for expanding access to COVID-19 and non–COVID-19 care, home monitoring of cases, innovation of treatment and prevention, and data sharing. Partners should consider options such as flexible medication delivery, electronic prescription services, and use of drones in supply chain to deliver test kits, test samples, medication, and blood products.
6. People/patients: Lastly and perhaps most critically, the trust, buy-in, and needs of the overall population are needed to enhance adherence to guidelines and recommendations. Many millions more than those who test positive for COVID-19 have and will continue to experience the crippling adverse economic, social, physical, and mental health effects of stay-at-home advisories, business and school closures, and physical distancing orders. Members of each community need to be heard in voicing their concerns and priorities and providing input on public health interventions to enhance acceptance and adherence (e.g., wear mask/face coverings in public, engage in physical distancing, etc.). Special attention should be given to managing chronic or existing medical problems and seek care when needed (e.g., avoid delaying of medical care).
An interdisciplinary and multipronged approach is necessary to address a complex, widespread, disruptive, and deadly pandemic such as COVID-19. The suggested activities put forth in our table are by no means exhaustive, nor do we expect all coalitions to be able to carry them all out. Our intention is that the 6-P framework encourages cross-sector collaboration to facilitate the design, implementation, evaluation, and scalability of preventive and intervention efforts based on the menu of items we have provided. Each coalition may determine which strategies they are able to prioritize and when within the context of specific national, regional, and local advisories, resulting in a tailored approach for each community or region that is thus better positioned for success.
Dr. Lingisetty is a hospitalist and physician executive at Baptist Health System, Little Rock, Ark. He is cofounder/president of SHM’s Arkansas chapter. Dr. Wang is assistant professor in the department of community health sciences at Boston University and adjunct assistant professor of health policy and management at the Harvard School of Public Health. Dr. Palabindala is the medical director, utilization management and physician advisory services, at the University of Mississippi Medical Center, Jackson. He is an associate professor of medicine and academic hospitalist at the University of Mississippi.
References
1. Powles J, Comim F. Public health infrastructure and knowledge, in Smith R et al. “Global Public Goods for Health.” Oxford: Oxford University Press, 2003.
2. Lombardi P, Petroni G. Virus outbreak pushes Italy’s health care system to the brink. Wall Street Journal. 2020 Mar 12. https://www.wsj.com/articles/virus-outbreak-pushes-italys-healthcare-system-to-the-brink-11583968769
3. Davies, R. How coronavirus is affecting the global economy. The Guardian. 2020 Feb 5. https://www.theguardian.com/world/2020/feb/05/coronavirus-global-economy
4. National Center for Health Statistics. FastStats. 2017. https://www.cdc.gov/nchs/fastats/health-expenditures.htm.
5. World Health Organization. Country & Technical Guidance–Coronavirus disease (COVID-19). https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance
6. Indian Council of Medical Research. Stages of transmission of COVID-19. https://main.icmr.nic.in/content/covid-19
7. Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19) – Prevention & treatment. 2020 Apr 24. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/prevention.html
8. Ostriker R. Cutbacks for some doctors and nurses as they battle on the front line. Boston Globe. 2020 Mar 27. https://www.bostonglobe.com/2020/03/27/metro/coronavirus-rages-doctors-hit-with-cuts-compensation/
9. Centers for Medicare & Medicaid Services. News alert. 2020 Mar 26. https://www.cms.gov/newsroom/press-releases/cms-news-alert-march-26-2020
Implement a 6-P framework
Implement a 6-P framework
The ongoing COVID-19 pandemic, arguably the biggest public health and economic catastrophe of modern times, elevated multiple deficiencies in public health infrastructures across the world, such as a slow or delayed response to suppress and mitigate the virus, an inadequately prepared and protected health care and public health workforce, and decentralized, siloed efforts.1 COVID-19 further highlighted the vulnerabilities of the health care, public health, and economic sectors.2,3 Irrespective of how robust health care systems may have been initially, rapidly spreading and deadly infectious diseases like COVID-19 can quickly derail the system, bringing the workforce and the patients they serve to a breaking point.
Hospital systems in the United States are not only at the crux of the current pandemic but are also well positioned to lead the response to the pandemic. Hospital administrators oversee nearly 33% of national health expenditure that amounts to the hospital-based care in the United States. Additionally, they may have an impact on nearly 30% of the expenditure that is related to physicians, prescriptions, and other facilities.4
The two primary goals underlying our proposed framework to target COVID-19 are based on the World Health Organization recommendations and lessons learned from countries such as South Korea that have successfully implemented these recommendations.5
1. Flatten the curve. According to the WHO and the Centers for Disease Control and Prevention, flattening the curve means that we must do everything that will help us to slow down the rate of infection, so the number of cases do not exceed the capacity of health systems.
2. Establish a standardized, interdisciplinary approach to flattening the curve. Pandemics can have major adverse consequences beyond health outcomes (e.g., economy) that can impact adherence to advisories and introduce multiple unintended consequences (e.g., deferred chronic care, unemployment). Managing the current pandemic and thoughtful consideration of and action regarding its ripple effects is heavily dependent on a standardized, interdisciplinary approach that is monitored, implemented, and evaluated well.
To achieve these two goals, we recommend establishing an interdisciplinary coalition representing multiple sectors. Our 6-P framework described below is intended to guide hospital administrators, to build the coalition, and to achieve these goals.
Structure of the pandemic coalition
A successful coalition invites a collaborative partnership involving senior members of respective disciplines, who would provide valuable, complementary perspectives in the coalition. We recommend hospital administrators take a lead in the formation of such a coalition. While we present the stakeholders and their roles below based on their intended influence and impact on the overall outcome of COVID-19, the basic guiding principles behind our 6-P framework remain true for any large-scale population health intervention.
Although several models for staging the transmission of COVID-19 are available, we adopted a four-stage model followed by the Indian Council for Medical Research.6 Irrespective of the origin of the infection, we believe that the four-stage model can cultivate situational awareness that can help guide the strategic design and systematic implementation of interventions.
Our 6-P framework integrates the four-stage model of COVID-19 transmission to identify action items for each stakeholder group and appropriate strategies selected based on the stages targeted.

1. Policy makers: Policy makers at all levels are critical in establishing policies, orders, and advisories, as well as dedicating resources and infrastructure, to enhance adherence to recommendations and guidelines at the community and population levels.7 They can assist hospitals in workforce expansion across county/state/discipline lines (e.g., accelerate the licensing and credentialing process, authorize graduate medical trainees, nurse practitioners, and other allied health professionals). Policy revisions for data sharing, privacy, communication, liability, and telehealth expansion.82. Providers: The health of the health care workforce itself is at risk because of their frontline services. Their buy-in will be crucial in both the formulation and implementation of evidence- and practice-based guidelines.9 Rapid adoption of telehealth for care continuum, policy revisions for elective procedures, visitor restriction, surge, resurge planning, capacity expansion, effective population health management, and working with employee unions, professional staff organizations are few, but very important action items that need to be implemented.
3. Public health authorities: Representation of public health authorities will be crucial in standardizing data collection, management, and reporting; providing up-to-date guidelines and advisories; developing, implementing, and evaluating short- and long-term public health interventions; and preparing and helping communities throughout the course of the pandemic. They also play a key role in identifying and reducing barriers related to the expansion of testing and contact tracing efforts.
4. Payers: In the United States, the Centers for Medicare & Medicaid Services oversees primary federally funded programs and serves as a point of reference for the American health care system. Having representation from all payer sources is crucial for achieving uniformity and standardization of the care process during the pandemic, with particular priority given to individuals and families who may have recently lost their health insurance because of job loss from COVID-19–related business furloughs, layoffs, and closures. Customer outreach initiatives, revision of patients’ out of pocket responsibilities, rapid claim settlement and denial management services, expansion of telehealth, elimination of prior authorization barriers, rapid credentialing of providers, data sharing, and assisting hospital systems in chronic disease management are examples of time-sensitive initiatives that are vital for population health management.
5. Partners: Establishing partnerships with pharma, health IT, labs, device industries, and other ancillary services is important to facilitate rapid innovation, production, and supply of essential medical devices and resources. These partners directly influence the outcomes of the pandemic and long-term health of the society through expansion of testing capability, contact tracing, leveraging technology for expanding access to COVID-19 and non–COVID-19 care, home monitoring of cases, innovation of treatment and prevention, and data sharing. Partners should consider options such as flexible medication delivery, electronic prescription services, and use of drones in supply chain to deliver test kits, test samples, medication, and blood products.
6. People/patients: Lastly and perhaps most critically, the trust, buy-in, and needs of the overall population are needed to enhance adherence to guidelines and recommendations. Many millions more than those who test positive for COVID-19 have and will continue to experience the crippling adverse economic, social, physical, and mental health effects of stay-at-home advisories, business and school closures, and physical distancing orders. Members of each community need to be heard in voicing their concerns and priorities and providing input on public health interventions to enhance acceptance and adherence (e.g., wear mask/face coverings in public, engage in physical distancing, etc.). Special attention should be given to managing chronic or existing medical problems and seek care when needed (e.g., avoid delaying of medical care).
An interdisciplinary and multipronged approach is necessary to address a complex, widespread, disruptive, and deadly pandemic such as COVID-19. The suggested activities put forth in our table are by no means exhaustive, nor do we expect all coalitions to be able to carry them all out. Our intention is that the 6-P framework encourages cross-sector collaboration to facilitate the design, implementation, evaluation, and scalability of preventive and intervention efforts based on the menu of items we have provided. Each coalition may determine which strategies they are able to prioritize and when within the context of specific national, regional, and local advisories, resulting in a tailored approach for each community or region that is thus better positioned for success.
Dr. Lingisetty is a hospitalist and physician executive at Baptist Health System, Little Rock, Ark. He is cofounder/president of SHM’s Arkansas chapter. Dr. Wang is assistant professor in the department of community health sciences at Boston University and adjunct assistant professor of health policy and management at the Harvard School of Public Health. Dr. Palabindala is the medical director, utilization management and physician advisory services, at the University of Mississippi Medical Center, Jackson. He is an associate professor of medicine and academic hospitalist at the University of Mississippi.
References
1. Powles J, Comim F. Public health infrastructure and knowledge, in Smith R et al. “Global Public Goods for Health.” Oxford: Oxford University Press, 2003.
2. Lombardi P, Petroni G. Virus outbreak pushes Italy’s health care system to the brink. Wall Street Journal. 2020 Mar 12. https://www.wsj.com/articles/virus-outbreak-pushes-italys-healthcare-system-to-the-brink-11583968769
3. Davies, R. How coronavirus is affecting the global economy. The Guardian. 2020 Feb 5. https://www.theguardian.com/world/2020/feb/05/coronavirus-global-economy
4. National Center for Health Statistics. FastStats. 2017. https://www.cdc.gov/nchs/fastats/health-expenditures.htm.
5. World Health Organization. Country & Technical Guidance–Coronavirus disease (COVID-19). https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance
6. Indian Council of Medical Research. Stages of transmission of COVID-19. https://main.icmr.nic.in/content/covid-19
7. Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19) – Prevention & treatment. 2020 Apr 24. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/prevention.html
8. Ostriker R. Cutbacks for some doctors and nurses as they battle on the front line. Boston Globe. 2020 Mar 27. https://www.bostonglobe.com/2020/03/27/metro/coronavirus-rages-doctors-hit-with-cuts-compensation/
9. Centers for Medicare & Medicaid Services. News alert. 2020 Mar 26. https://www.cms.gov/newsroom/press-releases/cms-news-alert-march-26-2020
The ongoing COVID-19 pandemic, arguably the biggest public health and economic catastrophe of modern times, elevated multiple deficiencies in public health infrastructures across the world, such as a slow or delayed response to suppress and mitigate the virus, an inadequately prepared and protected health care and public health workforce, and decentralized, siloed efforts.1 COVID-19 further highlighted the vulnerabilities of the health care, public health, and economic sectors.2,3 Irrespective of how robust health care systems may have been initially, rapidly spreading and deadly infectious diseases like COVID-19 can quickly derail the system, bringing the workforce and the patients they serve to a breaking point.
Hospital systems in the United States are not only at the crux of the current pandemic but are also well positioned to lead the response to the pandemic. Hospital administrators oversee nearly 33% of national health expenditure that amounts to the hospital-based care in the United States. Additionally, they may have an impact on nearly 30% of the expenditure that is related to physicians, prescriptions, and other facilities.4
The two primary goals underlying our proposed framework to target COVID-19 are based on the World Health Organization recommendations and lessons learned from countries such as South Korea that have successfully implemented these recommendations.5
1. Flatten the curve. According to the WHO and the Centers for Disease Control and Prevention, flattening the curve means that we must do everything that will help us to slow down the rate of infection, so the number of cases do not exceed the capacity of health systems.
2. Establish a standardized, interdisciplinary approach to flattening the curve. Pandemics can have major adverse consequences beyond health outcomes (e.g., economy) that can impact adherence to advisories and introduce multiple unintended consequences (e.g., deferred chronic care, unemployment). Managing the current pandemic and thoughtful consideration of and action regarding its ripple effects is heavily dependent on a standardized, interdisciplinary approach that is monitored, implemented, and evaluated well.
To achieve these two goals, we recommend establishing an interdisciplinary coalition representing multiple sectors. Our 6-P framework described below is intended to guide hospital administrators, to build the coalition, and to achieve these goals.
Structure of the pandemic coalition
A successful coalition invites a collaborative partnership involving senior members of respective disciplines, who would provide valuable, complementary perspectives in the coalition. We recommend hospital administrators take a lead in the formation of such a coalition. While we present the stakeholders and their roles below based on their intended influence and impact on the overall outcome of COVID-19, the basic guiding principles behind our 6-P framework remain true for any large-scale population health intervention.
Although several models for staging the transmission of COVID-19 are available, we adopted a four-stage model followed by the Indian Council for Medical Research.6 Irrespective of the origin of the infection, we believe that the four-stage model can cultivate situational awareness that can help guide the strategic design and systematic implementation of interventions.
Our 6-P framework integrates the four-stage model of COVID-19 transmission to identify action items for each stakeholder group and appropriate strategies selected based on the stages targeted.

1. Policy makers: Policy makers at all levels are critical in establishing policies, orders, and advisories, as well as dedicating resources and infrastructure, to enhance adherence to recommendations and guidelines at the community and population levels.7 They can assist hospitals in workforce expansion across county/state/discipline lines (e.g., accelerate the licensing and credentialing process, authorize graduate medical trainees, nurse practitioners, and other allied health professionals). Policy revisions for data sharing, privacy, communication, liability, and telehealth expansion.82. Providers: The health of the health care workforce itself is at risk because of their frontline services. Their buy-in will be crucial in both the formulation and implementation of evidence- and practice-based guidelines.9 Rapid adoption of telehealth for care continuum, policy revisions for elective procedures, visitor restriction, surge, resurge planning, capacity expansion, effective population health management, and working with employee unions, professional staff organizations are few, but very important action items that need to be implemented.
3. Public health authorities: Representation of public health authorities will be crucial in standardizing data collection, management, and reporting; providing up-to-date guidelines and advisories; developing, implementing, and evaluating short- and long-term public health interventions; and preparing and helping communities throughout the course of the pandemic. They also play a key role in identifying and reducing barriers related to the expansion of testing and contact tracing efforts.
4. Payers: In the United States, the Centers for Medicare & Medicaid Services oversees primary federally funded programs and serves as a point of reference for the American health care system. Having representation from all payer sources is crucial for achieving uniformity and standardization of the care process during the pandemic, with particular priority given to individuals and families who may have recently lost their health insurance because of job loss from COVID-19–related business furloughs, layoffs, and closures. Customer outreach initiatives, revision of patients’ out of pocket responsibilities, rapid claim settlement and denial management services, expansion of telehealth, elimination of prior authorization barriers, rapid credentialing of providers, data sharing, and assisting hospital systems in chronic disease management are examples of time-sensitive initiatives that are vital for population health management.
5. Partners: Establishing partnerships with pharma, health IT, labs, device industries, and other ancillary services is important to facilitate rapid innovation, production, and supply of essential medical devices and resources. These partners directly influence the outcomes of the pandemic and long-term health of the society through expansion of testing capability, contact tracing, leveraging technology for expanding access to COVID-19 and non–COVID-19 care, home monitoring of cases, innovation of treatment and prevention, and data sharing. Partners should consider options such as flexible medication delivery, electronic prescription services, and use of drones in supply chain to deliver test kits, test samples, medication, and blood products.
6. People/patients: Lastly and perhaps most critically, the trust, buy-in, and needs of the overall population are needed to enhance adherence to guidelines and recommendations. Many millions more than those who test positive for COVID-19 have and will continue to experience the crippling adverse economic, social, physical, and mental health effects of stay-at-home advisories, business and school closures, and physical distancing orders. Members of each community need to be heard in voicing their concerns and priorities and providing input on public health interventions to enhance acceptance and adherence (e.g., wear mask/face coverings in public, engage in physical distancing, etc.). Special attention should be given to managing chronic or existing medical problems and seek care when needed (e.g., avoid delaying of medical care).
An interdisciplinary and multipronged approach is necessary to address a complex, widespread, disruptive, and deadly pandemic such as COVID-19. The suggested activities put forth in our table are by no means exhaustive, nor do we expect all coalitions to be able to carry them all out. Our intention is that the 6-P framework encourages cross-sector collaboration to facilitate the design, implementation, evaluation, and scalability of preventive and intervention efforts based on the menu of items we have provided. Each coalition may determine which strategies they are able to prioritize and when within the context of specific national, regional, and local advisories, resulting in a tailored approach for each community or region that is thus better positioned for success.
Dr. Lingisetty is a hospitalist and physician executive at Baptist Health System, Little Rock, Ark. He is cofounder/president of SHM’s Arkansas chapter. Dr. Wang is assistant professor in the department of community health sciences at Boston University and adjunct assistant professor of health policy and management at the Harvard School of Public Health. Dr. Palabindala is the medical director, utilization management and physician advisory services, at the University of Mississippi Medical Center, Jackson. He is an associate professor of medicine and academic hospitalist at the University of Mississippi.
References
1. Powles J, Comim F. Public health infrastructure and knowledge, in Smith R et al. “Global Public Goods for Health.” Oxford: Oxford University Press, 2003.
2. Lombardi P, Petroni G. Virus outbreak pushes Italy’s health care system to the brink. Wall Street Journal. 2020 Mar 12. https://www.wsj.com/articles/virus-outbreak-pushes-italys-healthcare-system-to-the-brink-11583968769
3. Davies, R. How coronavirus is affecting the global economy. The Guardian. 2020 Feb 5. https://www.theguardian.com/world/2020/feb/05/coronavirus-global-economy
4. National Center for Health Statistics. FastStats. 2017. https://www.cdc.gov/nchs/fastats/health-expenditures.htm.
5. World Health Organization. Country & Technical Guidance–Coronavirus disease (COVID-19). https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance
6. Indian Council of Medical Research. Stages of transmission of COVID-19. https://main.icmr.nic.in/content/covid-19
7. Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19) – Prevention & treatment. 2020 Apr 24. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/prevention.html
8. Ostriker R. Cutbacks for some doctors and nurses as they battle on the front line. Boston Globe. 2020 Mar 27. https://www.bostonglobe.com/2020/03/27/metro/coronavirus-rages-doctors-hit-with-cuts-compensation/
9. Centers for Medicare & Medicaid Services. News alert. 2020 Mar 26. https://www.cms.gov/newsroom/press-releases/cms-news-alert-march-26-2020
Subclinical hypothyroidism appears common in women with miscarriage
according to a prospective observational study published in the Journal of Clinical Endocrinology & Metabolism.
Whether asymptomatic patients should be screened for mild subclinical hypothyroidism (SCH) or thyroid peroxidase antibodies (TPOAb) remains an open question, however. “In the absence of evidence of benefit with LT4 [levothyroxine] treatment and possible suggestion of harm ... we pose the question of whether screening should be performed at all in asymptomatic individuals,” wrote Rima K. Dhillon-Smith, MBChB, PhD, of the University of Birmingham (England), and colleagues. “Large randomized trials are needed to establish if preconception LT4 treatment of mild SCH with or without TPOAb positivity is beneficial. If treatment is found to be beneficial, this study presents the prevalence of thyroid disorders that can be expected.”
Subclinical hypothyroidism may represent an early stage of thyroid dysfunction. The condition has been associated with subfertility, miscarriage, preterm birth, preeclampsia, and perinatal mortality. Thyroid peroxidase antibodies also have been associated with adverse pregnancy outcomes, and their presence increases the risk of subclinical and overt thyroid disease in pregnancy. “There is international agreement on the treatment of overt thyroid disease,” the researchers wrote. “However, the treatment strategies for SCH or TPOAb preconception and antenatally are debated.”
The Thyroid Antibodies and Levothyroxine (TABLET) trial, to which the present study was linked, “found no improvement in live birth or any secondary pregnancy or neonatal outcomes in euthyroid women with TPOAb taking 50 mcg of LT4, compared with placebo.”
To examine various thyroid-stimulating hormone (TSH) cutoff levels for diagnosing subclinical thyroid disease in preconception asymptomatic women with a history of miscarriage or subfertility, Dr. Dhillon-Smith and colleagues conducted a prospective, observational cohort study at 49 hospitals in the United Kingdom. The study included more than 19,200 patients between November 2011 and January 2016. Participants were aged 16-41 years, had a history of miscarriage or subfertility, and were actively trying to get pregnant.
Using accepted reference ranges, the investigators identified undiagnosed overt hypothyroidism in 0.2%, overt hyperthyroidism in 0.3%, severe SCH (TSH greater than 10 mIU/L) in 0.2%, and SCH (TSH greater than 4.5 mIU/L) in 2.4%. “Lowering the upper limit of TSH to 2.5 mIU/L, as is the recommendation by international societies for ‘high-risk’ women,” such as those with recurrent pregnancy loss or those undergoing assisted reproductive technology, “would class 16%-20% of women as subclinically hypothyroid,” the authors reported.
The prevalence of TPOAb was 9.5%, and the presence of these antibodies “was the factor associated most significantly with any degree of thyroid dysfunction, after adjustment for confounders,” Dr. Dhillon-Smith and colleagues wrote. Multiple regression analyses found that the likelihood of subclinical hypothyroidism (TSH greater than 4.5 mIU/L) was increased for participants with a body mass index of 35 kg/m2 or greater (adjusted odds ratio, 1.71) and Asian ethnicity (aOR, 1.76).
The U.K. rates of thyroid dysfunction appear to be lower than rates in the United States, and it is unclear why higher body mass index and Asian ethnicity were independently associated with higher TSH concentrations, commented Mark P. Trolice, MD, director of Fertility CARE: The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
Women with a history of three or more miscarriages or subfertility were not more likely to be TPOAb positive, compared with women with one or two previous miscarriages, which “underscores the evidence that a recurrent pregnancy loss evaluation yields similar diagnostic findings at two versus three or more losses,” Dr. Trolice said.
According to the American Society for Reproductive Medicine, it is reasonable to test infertile women trying to conceive and to treat SCH with levothyroxine to maintain a TSH within the normal range. Women who have TSH greater than 2.5 mIU/L and/or are TPOAb positive can be considered for treatment with levothyroxine.
The Endocrine Society recommends levothyroxine treatment in women with SCH, especially if they are TPOAb positive.
An American Thyroid Association guideline recommends that subclinically hypothyroid women undergoing in vitro fertilization or intracytoplasmic sperm injection be treated with levothyroxine. A 2019 Cochrane Database review, however, found that low-quality evidence precludes clear conclusions.
“While thyroid autoimmunity has been associated with increased miscarriage, preterm births, and lower live birth rates, the confusion lies in which preconception women to test, when to obtain testing, and how to manage nonovert thyroid disease,” said Dr. Trolice, who is a member of the Ob.Gyn. News editorial advisory board.
The observational study was linked to the TABLET trial, which was funded by the National Institute for Health Research. The researchers had no relevant conflicts of interest.
SOURCE: Dhillon-Smith RK et al. J Clin Endocrinol Metab. 2020 Jun 17. doi: 10.1210/clinem/dgaa302.
according to a prospective observational study published in the Journal of Clinical Endocrinology & Metabolism.
Whether asymptomatic patients should be screened for mild subclinical hypothyroidism (SCH) or thyroid peroxidase antibodies (TPOAb) remains an open question, however. “In the absence of evidence of benefit with LT4 [levothyroxine] treatment and possible suggestion of harm ... we pose the question of whether screening should be performed at all in asymptomatic individuals,” wrote Rima K. Dhillon-Smith, MBChB, PhD, of the University of Birmingham (England), and colleagues. “Large randomized trials are needed to establish if preconception LT4 treatment of mild SCH with or without TPOAb positivity is beneficial. If treatment is found to be beneficial, this study presents the prevalence of thyroid disorders that can be expected.”
Subclinical hypothyroidism may represent an early stage of thyroid dysfunction. The condition has been associated with subfertility, miscarriage, preterm birth, preeclampsia, and perinatal mortality. Thyroid peroxidase antibodies also have been associated with adverse pregnancy outcomes, and their presence increases the risk of subclinical and overt thyroid disease in pregnancy. “There is international agreement on the treatment of overt thyroid disease,” the researchers wrote. “However, the treatment strategies for SCH or TPOAb preconception and antenatally are debated.”
The Thyroid Antibodies and Levothyroxine (TABLET) trial, to which the present study was linked, “found no improvement in live birth or any secondary pregnancy or neonatal outcomes in euthyroid women with TPOAb taking 50 mcg of LT4, compared with placebo.”
To examine various thyroid-stimulating hormone (TSH) cutoff levels for diagnosing subclinical thyroid disease in preconception asymptomatic women with a history of miscarriage or subfertility, Dr. Dhillon-Smith and colleagues conducted a prospective, observational cohort study at 49 hospitals in the United Kingdom. The study included more than 19,200 patients between November 2011 and January 2016. Participants were aged 16-41 years, had a history of miscarriage or subfertility, and were actively trying to get pregnant.
Using accepted reference ranges, the investigators identified undiagnosed overt hypothyroidism in 0.2%, overt hyperthyroidism in 0.3%, severe SCH (TSH greater than 10 mIU/L) in 0.2%, and SCH (TSH greater than 4.5 mIU/L) in 2.4%. “Lowering the upper limit of TSH to 2.5 mIU/L, as is the recommendation by international societies for ‘high-risk’ women,” such as those with recurrent pregnancy loss or those undergoing assisted reproductive technology, “would class 16%-20% of women as subclinically hypothyroid,” the authors reported.
The prevalence of TPOAb was 9.5%, and the presence of these antibodies “was the factor associated most significantly with any degree of thyroid dysfunction, after adjustment for confounders,” Dr. Dhillon-Smith and colleagues wrote. Multiple regression analyses found that the likelihood of subclinical hypothyroidism (TSH greater than 4.5 mIU/L) was increased for participants with a body mass index of 35 kg/m2 or greater (adjusted odds ratio, 1.71) and Asian ethnicity (aOR, 1.76).
The U.K. rates of thyroid dysfunction appear to be lower than rates in the United States, and it is unclear why higher body mass index and Asian ethnicity were independently associated with higher TSH concentrations, commented Mark P. Trolice, MD, director of Fertility CARE: The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
Women with a history of three or more miscarriages or subfertility were not more likely to be TPOAb positive, compared with women with one or two previous miscarriages, which “underscores the evidence that a recurrent pregnancy loss evaluation yields similar diagnostic findings at two versus three or more losses,” Dr. Trolice said.
According to the American Society for Reproductive Medicine, it is reasonable to test infertile women trying to conceive and to treat SCH with levothyroxine to maintain a TSH within the normal range. Women who have TSH greater than 2.5 mIU/L and/or are TPOAb positive can be considered for treatment with levothyroxine.
The Endocrine Society recommends levothyroxine treatment in women with SCH, especially if they are TPOAb positive.
An American Thyroid Association guideline recommends that subclinically hypothyroid women undergoing in vitro fertilization or intracytoplasmic sperm injection be treated with levothyroxine. A 2019 Cochrane Database review, however, found that low-quality evidence precludes clear conclusions.
“While thyroid autoimmunity has been associated with increased miscarriage, preterm births, and lower live birth rates, the confusion lies in which preconception women to test, when to obtain testing, and how to manage nonovert thyroid disease,” said Dr. Trolice, who is a member of the Ob.Gyn. News editorial advisory board.
The observational study was linked to the TABLET trial, which was funded by the National Institute for Health Research. The researchers had no relevant conflicts of interest.
SOURCE: Dhillon-Smith RK et al. J Clin Endocrinol Metab. 2020 Jun 17. doi: 10.1210/clinem/dgaa302.
according to a prospective observational study published in the Journal of Clinical Endocrinology & Metabolism.
Whether asymptomatic patients should be screened for mild subclinical hypothyroidism (SCH) or thyroid peroxidase antibodies (TPOAb) remains an open question, however. “In the absence of evidence of benefit with LT4 [levothyroxine] treatment and possible suggestion of harm ... we pose the question of whether screening should be performed at all in asymptomatic individuals,” wrote Rima K. Dhillon-Smith, MBChB, PhD, of the University of Birmingham (England), and colleagues. “Large randomized trials are needed to establish if preconception LT4 treatment of mild SCH with or without TPOAb positivity is beneficial. If treatment is found to be beneficial, this study presents the prevalence of thyroid disorders that can be expected.”
Subclinical hypothyroidism may represent an early stage of thyroid dysfunction. The condition has been associated with subfertility, miscarriage, preterm birth, preeclampsia, and perinatal mortality. Thyroid peroxidase antibodies also have been associated with adverse pregnancy outcomes, and their presence increases the risk of subclinical and overt thyroid disease in pregnancy. “There is international agreement on the treatment of overt thyroid disease,” the researchers wrote. “However, the treatment strategies for SCH or TPOAb preconception and antenatally are debated.”
The Thyroid Antibodies and Levothyroxine (TABLET) trial, to which the present study was linked, “found no improvement in live birth or any secondary pregnancy or neonatal outcomes in euthyroid women with TPOAb taking 50 mcg of LT4, compared with placebo.”
To examine various thyroid-stimulating hormone (TSH) cutoff levels for diagnosing subclinical thyroid disease in preconception asymptomatic women with a history of miscarriage or subfertility, Dr. Dhillon-Smith and colleagues conducted a prospective, observational cohort study at 49 hospitals in the United Kingdom. The study included more than 19,200 patients between November 2011 and January 2016. Participants were aged 16-41 years, had a history of miscarriage or subfertility, and were actively trying to get pregnant.
Using accepted reference ranges, the investigators identified undiagnosed overt hypothyroidism in 0.2%, overt hyperthyroidism in 0.3%, severe SCH (TSH greater than 10 mIU/L) in 0.2%, and SCH (TSH greater than 4.5 mIU/L) in 2.4%. “Lowering the upper limit of TSH to 2.5 mIU/L, as is the recommendation by international societies for ‘high-risk’ women,” such as those with recurrent pregnancy loss or those undergoing assisted reproductive technology, “would class 16%-20% of women as subclinically hypothyroid,” the authors reported.
The prevalence of TPOAb was 9.5%, and the presence of these antibodies “was the factor associated most significantly with any degree of thyroid dysfunction, after adjustment for confounders,” Dr. Dhillon-Smith and colleagues wrote. Multiple regression analyses found that the likelihood of subclinical hypothyroidism (TSH greater than 4.5 mIU/L) was increased for participants with a body mass index of 35 kg/m2 or greater (adjusted odds ratio, 1.71) and Asian ethnicity (aOR, 1.76).
The U.K. rates of thyroid dysfunction appear to be lower than rates in the United States, and it is unclear why higher body mass index and Asian ethnicity were independently associated with higher TSH concentrations, commented Mark P. Trolice, MD, director of Fertility CARE: The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
Women with a history of three or more miscarriages or subfertility were not more likely to be TPOAb positive, compared with women with one or two previous miscarriages, which “underscores the evidence that a recurrent pregnancy loss evaluation yields similar diagnostic findings at two versus three or more losses,” Dr. Trolice said.
According to the American Society for Reproductive Medicine, it is reasonable to test infertile women trying to conceive and to treat SCH with levothyroxine to maintain a TSH within the normal range. Women who have TSH greater than 2.5 mIU/L and/or are TPOAb positive can be considered for treatment with levothyroxine.
The Endocrine Society recommends levothyroxine treatment in women with SCH, especially if they are TPOAb positive.
An American Thyroid Association guideline recommends that subclinically hypothyroid women undergoing in vitro fertilization or intracytoplasmic sperm injection be treated with levothyroxine. A 2019 Cochrane Database review, however, found that low-quality evidence precludes clear conclusions.
“While thyroid autoimmunity has been associated with increased miscarriage, preterm births, and lower live birth rates, the confusion lies in which preconception women to test, when to obtain testing, and how to manage nonovert thyroid disease,” said Dr. Trolice, who is a member of the Ob.Gyn. News editorial advisory board.
The observational study was linked to the TABLET trial, which was funded by the National Institute for Health Research. The researchers had no relevant conflicts of interest.
SOURCE: Dhillon-Smith RK et al. J Clin Endocrinol Metab. 2020 Jun 17. doi: 10.1210/clinem/dgaa302.
FROM THE JOURNAL OF CLINICAL ENDOCRINOLOGY & METABOLISM