User login
Conflicting Reports About PPE Supply for VA Health Workers
“All VA facilities are equipped with essential items and supplies, and we are continually monitoring the status of those items to ensure a robust supply chain,” US Department of Veterans Affairs (VA) Press Secretary Christina Noel insisted on April 14th. The problem? The Wall Street Journal had just reported that internal VA memos detailing concerns about shortages in personal protective equipment (PPE), including memos saying PPE rationing had begun, were circulating at the highest levels. Top VA officials, including Secretary Robert Wilkie, had been briefed several times on systemwide shortages, the memos indicated.
The department had about 2 weeks’ worth of masks on hand, the Journal said, according to a briefing made to Congress several days earlier.
One April 7, 2020 memo noted that the “United States is experiencing challenges procuring adequate supplies of [facemasks and N95 respirators] to protect Veterans Health Administration (VHA) staff” and suggested limiting access to PPE. “Mask supply levels in VHA do not support providing masks to all other employees not working directly with COVID-19 infected veterans,” the memo noted. The memo also recommended that one mask per day for health care workers involved in “screening program activities” and taking care of “COVID-19 positive patients not undergoing high risk procedures.” Even employees “performing high risk procedures or activities on suspect or confirmed COVID19 patients,” were recommended to “wear an N95 respirator for extended use with multiple COVID-19 patients.”
Secretary Wilkie conceded to the Wall Street Journal that, “[W]e don’t have the supplies that we would have in an optimal situation, we have the supplies that we need as the [CDC] prescribes.”
The VA COVID-19 National Summary reported 5,468 positive cases of COVID-19 and 339 inpatient deaths on April 20. Although the rate of infection for veterans remains low—just .06% of veterans in the VHA system have tested positive—the 6.2% rate of death is higher than the US rate (4.6%) and nearly as high as the global rate. More than 1,600 employees at the VHA have tested positive for COVID-19, according to the VA, and 14 medical center employees had died of complications due to the virus.
The VA now appears to be increasing the number of health workers allotted protective masks. In an April 15 email, Executive in Charge Richard A. Stone, MD, sought to reassure VHA employees. “VA always had a contingency supply of PPE,” Stone explained. “However, when this crisis started to face every healthcare organization in the nation, it became more difficult to project our incoming supply chain. For this reason, and out of an abundance of caution, we implemented austerity measures to ensure that every person working with COVID-19 patients had the equipment they needed.”
According to Stone, the VA is now more confident in its supply chain. Under his direction “all employees in a community living center, spinal cord injury unit or inpatient mental health unit will receive one mask a day to support their duties. We will continue providing N95 masks to those directly in contact with COVID-19-positive patients.”
“Your safety is the most important thing to us – we need to protect you,” Stone insisted. “I give you my word that we are doing everything to help you continue to take care of our Veterans.
“All VA facilities are equipped with essential items and supplies, and we are continually monitoring the status of those items to ensure a robust supply chain,” US Department of Veterans Affairs (VA) Press Secretary Christina Noel insisted on April 14th. The problem? The Wall Street Journal had just reported that internal VA memos detailing concerns about shortages in personal protective equipment (PPE), including memos saying PPE rationing had begun, were circulating at the highest levels. Top VA officials, including Secretary Robert Wilkie, had been briefed several times on systemwide shortages, the memos indicated.
The department had about 2 weeks’ worth of masks on hand, the Journal said, according to a briefing made to Congress several days earlier.
One April 7, 2020 memo noted that the “United States is experiencing challenges procuring adequate supplies of [facemasks and N95 respirators] to protect Veterans Health Administration (VHA) staff” and suggested limiting access to PPE. “Mask supply levels in VHA do not support providing masks to all other employees not working directly with COVID-19 infected veterans,” the memo noted. The memo also recommended that one mask per day for health care workers involved in “screening program activities” and taking care of “COVID-19 positive patients not undergoing high risk procedures.” Even employees “performing high risk procedures or activities on suspect or confirmed COVID19 patients,” were recommended to “wear an N95 respirator for extended use with multiple COVID-19 patients.”
Secretary Wilkie conceded to the Wall Street Journal that, “[W]e don’t have the supplies that we would have in an optimal situation, we have the supplies that we need as the [CDC] prescribes.”
The VA COVID-19 National Summary reported 5,468 positive cases of COVID-19 and 339 inpatient deaths on April 20. Although the rate of infection for veterans remains low—just .06% of veterans in the VHA system have tested positive—the 6.2% rate of death is higher than the US rate (4.6%) and nearly as high as the global rate. More than 1,600 employees at the VHA have tested positive for COVID-19, according to the VA, and 14 medical center employees had died of complications due to the virus.
The VA now appears to be increasing the number of health workers allotted protective masks. In an April 15 email, Executive in Charge Richard A. Stone, MD, sought to reassure VHA employees. “VA always had a contingency supply of PPE,” Stone explained. “However, when this crisis started to face every healthcare organization in the nation, it became more difficult to project our incoming supply chain. For this reason, and out of an abundance of caution, we implemented austerity measures to ensure that every person working with COVID-19 patients had the equipment they needed.”
According to Stone, the VA is now more confident in its supply chain. Under his direction “all employees in a community living center, spinal cord injury unit or inpatient mental health unit will receive one mask a day to support their duties. We will continue providing N95 masks to those directly in contact with COVID-19-positive patients.”
“Your safety is the most important thing to us – we need to protect you,” Stone insisted. “I give you my word that we are doing everything to help you continue to take care of our Veterans.
“All VA facilities are equipped with essential items and supplies, and we are continually monitoring the status of those items to ensure a robust supply chain,” US Department of Veterans Affairs (VA) Press Secretary Christina Noel insisted on April 14th. The problem? The Wall Street Journal had just reported that internal VA memos detailing concerns about shortages in personal protective equipment (PPE), including memos saying PPE rationing had begun, were circulating at the highest levels. Top VA officials, including Secretary Robert Wilkie, had been briefed several times on systemwide shortages, the memos indicated.
The department had about 2 weeks’ worth of masks on hand, the Journal said, according to a briefing made to Congress several days earlier.
One April 7, 2020 memo noted that the “United States is experiencing challenges procuring adequate supplies of [facemasks and N95 respirators] to protect Veterans Health Administration (VHA) staff” and suggested limiting access to PPE. “Mask supply levels in VHA do not support providing masks to all other employees not working directly with COVID-19 infected veterans,” the memo noted. The memo also recommended that one mask per day for health care workers involved in “screening program activities” and taking care of “COVID-19 positive patients not undergoing high risk procedures.” Even employees “performing high risk procedures or activities on suspect or confirmed COVID19 patients,” were recommended to “wear an N95 respirator for extended use with multiple COVID-19 patients.”
Secretary Wilkie conceded to the Wall Street Journal that, “[W]e don’t have the supplies that we would have in an optimal situation, we have the supplies that we need as the [CDC] prescribes.”
The VA COVID-19 National Summary reported 5,468 positive cases of COVID-19 and 339 inpatient deaths on April 20. Although the rate of infection for veterans remains low—just .06% of veterans in the VHA system have tested positive—the 6.2% rate of death is higher than the US rate (4.6%) and nearly as high as the global rate. More than 1,600 employees at the VHA have tested positive for COVID-19, according to the VA, and 14 medical center employees had died of complications due to the virus.
The VA now appears to be increasing the number of health workers allotted protective masks. In an April 15 email, Executive in Charge Richard A. Stone, MD, sought to reassure VHA employees. “VA always had a contingency supply of PPE,” Stone explained. “However, when this crisis started to face every healthcare organization in the nation, it became more difficult to project our incoming supply chain. For this reason, and out of an abundance of caution, we implemented austerity measures to ensure that every person working with COVID-19 patients had the equipment they needed.”
According to Stone, the VA is now more confident in its supply chain. Under his direction “all employees in a community living center, spinal cord injury unit or inpatient mental health unit will receive one mask a day to support their duties. We will continue providing N95 masks to those directly in contact with COVID-19-positive patients.”
“Your safety is the most important thing to us – we need to protect you,” Stone insisted. “I give you my word that we are doing everything to help you continue to take care of our Veterans.
President’s report
As I write, I must admit this message is different than the one I’d envisioned sharing with you weeks ago. I anticipated updating you on meetings and collaborations with sister societies, new educational offerings, and how the Bologna World Congress and Annual Meeting plans were progressing, but activities at CHEST – and our sense of priority – have evolved along with those of our global community.
Pulmonary and critical care providers are now at the forefront of health care. Our patients, and now the greater public, are relying on our efforts and those of our teams. Amid this crisis, there is a renewed appreciation for the work all of you do; and with it, an opportunity for CHEST to lead and help ensure that the profession and our systems emerge stronger.
Back in February, we held the program committee meeting for the Annual Meeting with over 1000 submissions. It is astounding how the program came together over just a few days thanks to the preemptive work done by Chair, Dr. Victor Test, and, Co-Chair, Dr. Christopher Carroll, and all of the curricular groups, program committee members, and staff putting in so much work prior to the face-to-face meeting. Also during February, CHEST leadership held the Forum of International Respiratory Societies’ (FIRS) strategic planning meeting. The main outcome is a plan to engage a lobbyist to represent the worldwide respiratory societies in the WHO in Geneva on universal topics such as air pollution and now, unfortunately, COVID-19. CHEST was represented at the Society of Critical Care Medicine (SCCM) Congress where we heard late-breaking information as the pandemic was beginning to unfold. We met with the Critical Care Societies Collaborative (CCSC), which is composed of representatives from CHEST, SCCM, the American Thoracic Society (ATS), and the American Association of Critical-Care Nurses (AACN). We had an opportunity to meet with the European Society of Intensive Care Medicine (ESICM) and initiate discussions toward future collaboration.
In early March, as COVID-19 began to interfere with in-person meetings, we participated virtually in the NAMDRC meeting, and finalized our commitment to formally joining forces under the umbrella of CHEST to better serve our members in the area of advocacy. To this end, a new standing CHEST committee was founded, consisting of members from the former NAMRDC Board and members from the CHEST Board of Regents and Board of Trustees and chaired by Dr. Neil Freedman and Dr. Jim Lamberti. We look forward to hosting advocacy sessions during our October meeting, and going forward, our Spring Leadership Meeting will be combined with the former NAMDRC meeting to allow our leaders to participate in advocacy efforts. We will continue to publish the Washington Watchline, bringing important news on efforts to enhance access to care and our ability to deliver it effectively. Our spring leadership meetings, board meetings, and committee meetings in early April were held virtually in light of the pandemic.
Since March, CHEST has been heavily immersed in COVID-19 preparation with new plans for alternate methods of educational delivery, new business models, and curtailment of travel on both our home fronts and on the CHEST front. Zoom and like platforms are now my best friend! Our daily vocabulary now includes an abundance of caution, surge, sheltering in place, quarantine, social distancing, flattening the curve, tele-medicine, and don and doff, and we close e-mails, texts, and phone calls with Stay Safe! I established a COVID task force led by Dr. Steve Simpson (CHEST President-Elect) and with representation from the Critical Care, Chest Infections, and Disaster Response and Global Health NetWorks. They have been meeting weekly with the goals of disseminating and distilling COVID-related materials for the busy practitioner with links to the specific article or statement along with the BLUF (Bottom Line Up Front). I’m sure you were able to see and hear some of the reports by Dr. Mangala Narasimhan and others on the front lines in New York, on the CHEST website, 60 Minutes, and CNN. CHEST held a two-part webinar with our Chinese colleagues who shared their COVID experiences with us. These relationships were in part built from the PCCM Fellowship Training program we conducted with Chinese physicians, led by Dr. Darcy Marciniuk and Dr. Chen Wang under the guidance of Dr. Renli Qiao, and with the help of the late Dr. Mark Rosen, Dr. Jack Buckley, and myself. CHEST has posted a webinar on point of care ultrasound testing in the setting of COVID since many units are now using more POCUS instead of standard imaging for the critically ill. We have also posted some of our board review lectures on demand for those who want to brush up on their critical care skills and knowledge.
CHEST, unfortunately, had to reschedule the Bologna meeting due to the tragic situation in Italy and plans to reconvene the meeting June 24-26 of 2021. As of now, CHEST 2020 in Chicago is a go, but, of course, we will monitor that situation carefully. We have extended the deadline for abstracts and case reports to June 1, 2020, given the ongoing crisis. The team is busy planning for standalone and complementary online offerings to ensure seamless delivery of critical education in formats that cater easily to our newly formed habits.
CHEST staff have been working from home due to the Illinois shelter in place order but continue to work tremendously hard. They are implementing new areas to the website in an effort to improve the user experience by making information easier to find and more timely. In the publishing space, Dr. Peter Mazzone and the journal team have been receiving hundreds of COVID-related publications, which they have been reviewing and expediting for publication where appropriate. There will also be additional podcasts coming from our journal. The guidelines group has been working on shorter expert panel statements in the setting of rapidly changing evidence. And, to keep us all well, there are opportunities to share our personal feelings and experiences with treating those with COVID in video format on the website and across CHEST social media channels. The CHEST and the CHEST Foundation have initiated a new microgrants program and have reached out to over 150 ILD and COPD support groups across the country to offer them the opportunity to apply for a max $2,500 grant. So far, 7seven groups have requested support. These grants go directly to patients and caregivers and provide needed relief through provision of:
1. Groceries
2. Gift cards
3. Medical supplies (including PPE for patients)
4. Technology needed to communicate with their community and HCPs
5. Household supplies, cleaning supplies
In an attempt to assist our colleagues in New York City, a call went out for volunteers at the end of March and has resulted in over 200 volunteers and more than 400 inquiries from our members. Bravo!!! We want to thank our sister societies for joining our efforts during this time to help all of our respective members and, ultimately, those patients stricken with this terrible illness. As I don and doff my COVID gear, I hope you are all safe and well in this time of unprecedented change in our lives. I look forward to my next report in a few months, hopefully on a happier note.
Stay safe!
Stephanie
As I write, I must admit this message is different than the one I’d envisioned sharing with you weeks ago. I anticipated updating you on meetings and collaborations with sister societies, new educational offerings, and how the Bologna World Congress and Annual Meeting plans were progressing, but activities at CHEST – and our sense of priority – have evolved along with those of our global community.
Pulmonary and critical care providers are now at the forefront of health care. Our patients, and now the greater public, are relying on our efforts and those of our teams. Amid this crisis, there is a renewed appreciation for the work all of you do; and with it, an opportunity for CHEST to lead and help ensure that the profession and our systems emerge stronger.
Back in February, we held the program committee meeting for the Annual Meeting with over 1000 submissions. It is astounding how the program came together over just a few days thanks to the preemptive work done by Chair, Dr. Victor Test, and, Co-Chair, Dr. Christopher Carroll, and all of the curricular groups, program committee members, and staff putting in so much work prior to the face-to-face meeting. Also during February, CHEST leadership held the Forum of International Respiratory Societies’ (FIRS) strategic planning meeting. The main outcome is a plan to engage a lobbyist to represent the worldwide respiratory societies in the WHO in Geneva on universal topics such as air pollution and now, unfortunately, COVID-19. CHEST was represented at the Society of Critical Care Medicine (SCCM) Congress where we heard late-breaking information as the pandemic was beginning to unfold. We met with the Critical Care Societies Collaborative (CCSC), which is composed of representatives from CHEST, SCCM, the American Thoracic Society (ATS), and the American Association of Critical-Care Nurses (AACN). We had an opportunity to meet with the European Society of Intensive Care Medicine (ESICM) and initiate discussions toward future collaboration.
In early March, as COVID-19 began to interfere with in-person meetings, we participated virtually in the NAMDRC meeting, and finalized our commitment to formally joining forces under the umbrella of CHEST to better serve our members in the area of advocacy. To this end, a new standing CHEST committee was founded, consisting of members from the former NAMRDC Board and members from the CHEST Board of Regents and Board of Trustees and chaired by Dr. Neil Freedman and Dr. Jim Lamberti. We look forward to hosting advocacy sessions during our October meeting, and going forward, our Spring Leadership Meeting will be combined with the former NAMDRC meeting to allow our leaders to participate in advocacy efforts. We will continue to publish the Washington Watchline, bringing important news on efforts to enhance access to care and our ability to deliver it effectively. Our spring leadership meetings, board meetings, and committee meetings in early April were held virtually in light of the pandemic.
Since March, CHEST has been heavily immersed in COVID-19 preparation with new plans for alternate methods of educational delivery, new business models, and curtailment of travel on both our home fronts and on the CHEST front. Zoom and like platforms are now my best friend! Our daily vocabulary now includes an abundance of caution, surge, sheltering in place, quarantine, social distancing, flattening the curve, tele-medicine, and don and doff, and we close e-mails, texts, and phone calls with Stay Safe! I established a COVID task force led by Dr. Steve Simpson (CHEST President-Elect) and with representation from the Critical Care, Chest Infections, and Disaster Response and Global Health NetWorks. They have been meeting weekly with the goals of disseminating and distilling COVID-related materials for the busy practitioner with links to the specific article or statement along with the BLUF (Bottom Line Up Front). I’m sure you were able to see and hear some of the reports by Dr. Mangala Narasimhan and others on the front lines in New York, on the CHEST website, 60 Minutes, and CNN. CHEST held a two-part webinar with our Chinese colleagues who shared their COVID experiences with us. These relationships were in part built from the PCCM Fellowship Training program we conducted with Chinese physicians, led by Dr. Darcy Marciniuk and Dr. Chen Wang under the guidance of Dr. Renli Qiao, and with the help of the late Dr. Mark Rosen, Dr. Jack Buckley, and myself. CHEST has posted a webinar on point of care ultrasound testing in the setting of COVID since many units are now using more POCUS instead of standard imaging for the critically ill. We have also posted some of our board review lectures on demand for those who want to brush up on their critical care skills and knowledge.
CHEST, unfortunately, had to reschedule the Bologna meeting due to the tragic situation in Italy and plans to reconvene the meeting June 24-26 of 2021. As of now, CHEST 2020 in Chicago is a go, but, of course, we will monitor that situation carefully. We have extended the deadline for abstracts and case reports to June 1, 2020, given the ongoing crisis. The team is busy planning for standalone and complementary online offerings to ensure seamless delivery of critical education in formats that cater easily to our newly formed habits.
CHEST staff have been working from home due to the Illinois shelter in place order but continue to work tremendously hard. They are implementing new areas to the website in an effort to improve the user experience by making information easier to find and more timely. In the publishing space, Dr. Peter Mazzone and the journal team have been receiving hundreds of COVID-related publications, which they have been reviewing and expediting for publication where appropriate. There will also be additional podcasts coming from our journal. The guidelines group has been working on shorter expert panel statements in the setting of rapidly changing evidence. And, to keep us all well, there are opportunities to share our personal feelings and experiences with treating those with COVID in video format on the website and across CHEST social media channels. The CHEST and the CHEST Foundation have initiated a new microgrants program and have reached out to over 150 ILD and COPD support groups across the country to offer them the opportunity to apply for a max $2,500 grant. So far, 7seven groups have requested support. These grants go directly to patients and caregivers and provide needed relief through provision of:
1. Groceries
2. Gift cards
3. Medical supplies (including PPE for patients)
4. Technology needed to communicate with their community and HCPs
5. Household supplies, cleaning supplies
In an attempt to assist our colleagues in New York City, a call went out for volunteers at the end of March and has resulted in over 200 volunteers and more than 400 inquiries from our members. Bravo!!! We want to thank our sister societies for joining our efforts during this time to help all of our respective members and, ultimately, those patients stricken with this terrible illness. As I don and doff my COVID gear, I hope you are all safe and well in this time of unprecedented change in our lives. I look forward to my next report in a few months, hopefully on a happier note.
Stay safe!
Stephanie
As I write, I must admit this message is different than the one I’d envisioned sharing with you weeks ago. I anticipated updating you on meetings and collaborations with sister societies, new educational offerings, and how the Bologna World Congress and Annual Meeting plans were progressing, but activities at CHEST – and our sense of priority – have evolved along with those of our global community.
Pulmonary and critical care providers are now at the forefront of health care. Our patients, and now the greater public, are relying on our efforts and those of our teams. Amid this crisis, there is a renewed appreciation for the work all of you do; and with it, an opportunity for CHEST to lead and help ensure that the profession and our systems emerge stronger.
Back in February, we held the program committee meeting for the Annual Meeting with over 1000 submissions. It is astounding how the program came together over just a few days thanks to the preemptive work done by Chair, Dr. Victor Test, and, Co-Chair, Dr. Christopher Carroll, and all of the curricular groups, program committee members, and staff putting in so much work prior to the face-to-face meeting. Also during February, CHEST leadership held the Forum of International Respiratory Societies’ (FIRS) strategic planning meeting. The main outcome is a plan to engage a lobbyist to represent the worldwide respiratory societies in the WHO in Geneva on universal topics such as air pollution and now, unfortunately, COVID-19. CHEST was represented at the Society of Critical Care Medicine (SCCM) Congress where we heard late-breaking information as the pandemic was beginning to unfold. We met with the Critical Care Societies Collaborative (CCSC), which is composed of representatives from CHEST, SCCM, the American Thoracic Society (ATS), and the American Association of Critical-Care Nurses (AACN). We had an opportunity to meet with the European Society of Intensive Care Medicine (ESICM) and initiate discussions toward future collaboration.
In early March, as COVID-19 began to interfere with in-person meetings, we participated virtually in the NAMDRC meeting, and finalized our commitment to formally joining forces under the umbrella of CHEST to better serve our members in the area of advocacy. To this end, a new standing CHEST committee was founded, consisting of members from the former NAMRDC Board and members from the CHEST Board of Regents and Board of Trustees and chaired by Dr. Neil Freedman and Dr. Jim Lamberti. We look forward to hosting advocacy sessions during our October meeting, and going forward, our Spring Leadership Meeting will be combined with the former NAMDRC meeting to allow our leaders to participate in advocacy efforts. We will continue to publish the Washington Watchline, bringing important news on efforts to enhance access to care and our ability to deliver it effectively. Our spring leadership meetings, board meetings, and committee meetings in early April were held virtually in light of the pandemic.
Since March, CHEST has been heavily immersed in COVID-19 preparation with new plans for alternate methods of educational delivery, new business models, and curtailment of travel on both our home fronts and on the CHEST front. Zoom and like platforms are now my best friend! Our daily vocabulary now includes an abundance of caution, surge, sheltering in place, quarantine, social distancing, flattening the curve, tele-medicine, and don and doff, and we close e-mails, texts, and phone calls with Stay Safe! I established a COVID task force led by Dr. Steve Simpson (CHEST President-Elect) and with representation from the Critical Care, Chest Infections, and Disaster Response and Global Health NetWorks. They have been meeting weekly with the goals of disseminating and distilling COVID-related materials for the busy practitioner with links to the specific article or statement along with the BLUF (Bottom Line Up Front). I’m sure you were able to see and hear some of the reports by Dr. Mangala Narasimhan and others on the front lines in New York, on the CHEST website, 60 Minutes, and CNN. CHEST held a two-part webinar with our Chinese colleagues who shared their COVID experiences with us. These relationships were in part built from the PCCM Fellowship Training program we conducted with Chinese physicians, led by Dr. Darcy Marciniuk and Dr. Chen Wang under the guidance of Dr. Renli Qiao, and with the help of the late Dr. Mark Rosen, Dr. Jack Buckley, and myself. CHEST has posted a webinar on point of care ultrasound testing in the setting of COVID since many units are now using more POCUS instead of standard imaging for the critically ill. We have also posted some of our board review lectures on demand for those who want to brush up on their critical care skills and knowledge.
CHEST, unfortunately, had to reschedule the Bologna meeting due to the tragic situation in Italy and plans to reconvene the meeting June 24-26 of 2021. As of now, CHEST 2020 in Chicago is a go, but, of course, we will monitor that situation carefully. We have extended the deadline for abstracts and case reports to June 1, 2020, given the ongoing crisis. The team is busy planning for standalone and complementary online offerings to ensure seamless delivery of critical education in formats that cater easily to our newly formed habits.
CHEST staff have been working from home due to the Illinois shelter in place order but continue to work tremendously hard. They are implementing new areas to the website in an effort to improve the user experience by making information easier to find and more timely. In the publishing space, Dr. Peter Mazzone and the journal team have been receiving hundreds of COVID-related publications, which they have been reviewing and expediting for publication where appropriate. There will also be additional podcasts coming from our journal. The guidelines group has been working on shorter expert panel statements in the setting of rapidly changing evidence. And, to keep us all well, there are opportunities to share our personal feelings and experiences with treating those with COVID in video format on the website and across CHEST social media channels. The CHEST and the CHEST Foundation have initiated a new microgrants program and have reached out to over 150 ILD and COPD support groups across the country to offer them the opportunity to apply for a max $2,500 grant. So far, 7seven groups have requested support. These grants go directly to patients and caregivers and provide needed relief through provision of:
1. Groceries
2. Gift cards
3. Medical supplies (including PPE for patients)
4. Technology needed to communicate with their community and HCPs
5. Household supplies, cleaning supplies
In an attempt to assist our colleagues in New York City, a call went out for volunteers at the end of March and has resulted in over 200 volunteers and more than 400 inquiries from our members. Bravo!!! We want to thank our sister societies for joining our efforts during this time to help all of our respective members and, ultimately, those patients stricken with this terrible illness. As I don and doff my COVID gear, I hope you are all safe and well in this time of unprecedented change in our lives. I look forward to my next report in a few months, hopefully on a happier note.
Stay safe!
Stephanie
Congress has heard our rally cry
AGA has advocated for provisions to protect our providers and businesses and we’re happy to report that the following provisions are in the third installation of the COVID-19 economic relief legislation.
We’ll continue to push for direct funding for physicians recognizing that many practices and ASCs are struggling.
Small business relief
- Small Business Administration (SBA) loans:
Businesses with 500 employees or less are eligible unless the covered industry’s SBA size standard allows more than 500 employees.
Allows 501(c)(3) non-profits to gain access to the program.
Increases the maximum loan amount to $10 million.
Expands allowable uses of loans to include payroll support, such as:
1. Paid sick or medical leave.
2. Employee salaries.
3. Mortgage payments.
Provides a process for loan forgiveness for certain payroll costs as well as mortgage, rent and utility obligations.
- Public Health and Social Services Emergency Fund:
$100 billion for health care services related to the COVID-19.
Reimbursement to eligible health care providers for health care related expenses or lost revenues that are attributable to the pandemic.
- Coronavirus Economic Stabilization Act:
$454 billion for loans, loan guarantees and other investments for companies with losses tied to the pandemic that threaten continued operation.
Medicare provisions
- Suspension of sequestration – Physicians avoid a 2% cut in their Medicare reimbursement.
- Extension of geographic index floor – Increases Medicare payments for providers in nonurban areas.
- Increased Medicare telehealth flexibilities during the emergency period.
- AGA will continue to advocate for audio-only coverage as this issue is still not resolved.
Other key health care provisions
- Liability protections for health care professionals during the emergency response.
- Coverage of preventive services and vaccines.
- $16 billion to replenish the Strategic National Stockpile.
- $1 billion for the Defense Production Act to ensure production of personal protective equipment (PPE).
Correspondence to congressional leadership
- March 25, 2020 – With the American Medical Association, a letter is sent requesting the inclusion of support for physician practices in any economic stimulus package.
- March 24, 2020 – With the Alliance of Specialty Medicine, a letter is sent asking for relief for independent physicians’ offices.
- March 20, 2020 – A joint society letter is sent asking for increased funding for and access to PPE; softened prior authorization, telehealth reimbursement and Medicare reporting requirements; and financial safeguards for health care professionals and practices.
AGA has advocated for provisions to protect our providers and businesses and we’re happy to report that the following provisions are in the third installation of the COVID-19 economic relief legislation.
We’ll continue to push for direct funding for physicians recognizing that many practices and ASCs are struggling.
Small business relief
- Small Business Administration (SBA) loans:
Businesses with 500 employees or less are eligible unless the covered industry’s SBA size standard allows more than 500 employees.
Allows 501(c)(3) non-profits to gain access to the program.
Increases the maximum loan amount to $10 million.
Expands allowable uses of loans to include payroll support, such as:
1. Paid sick or medical leave.
2. Employee salaries.
3. Mortgage payments.
Provides a process for loan forgiveness for certain payroll costs as well as mortgage, rent and utility obligations.
- Public Health and Social Services Emergency Fund:
$100 billion for health care services related to the COVID-19.
Reimbursement to eligible health care providers for health care related expenses or lost revenues that are attributable to the pandemic.
- Coronavirus Economic Stabilization Act:
$454 billion for loans, loan guarantees and other investments for companies with losses tied to the pandemic that threaten continued operation.
Medicare provisions
- Suspension of sequestration – Physicians avoid a 2% cut in their Medicare reimbursement.
- Extension of geographic index floor – Increases Medicare payments for providers in nonurban areas.
- Increased Medicare telehealth flexibilities during the emergency period.
- AGA will continue to advocate for audio-only coverage as this issue is still not resolved.
Other key health care provisions
- Liability protections for health care professionals during the emergency response.
- Coverage of preventive services and vaccines.
- $16 billion to replenish the Strategic National Stockpile.
- $1 billion for the Defense Production Act to ensure production of personal protective equipment (PPE).
Correspondence to congressional leadership
- March 25, 2020 – With the American Medical Association, a letter is sent requesting the inclusion of support for physician practices in any economic stimulus package.
- March 24, 2020 – With the Alliance of Specialty Medicine, a letter is sent asking for relief for independent physicians’ offices.
- March 20, 2020 – A joint society letter is sent asking for increased funding for and access to PPE; softened prior authorization, telehealth reimbursement and Medicare reporting requirements; and financial safeguards for health care professionals and practices.
AGA has advocated for provisions to protect our providers and businesses and we’re happy to report that the following provisions are in the third installation of the COVID-19 economic relief legislation.
We’ll continue to push for direct funding for physicians recognizing that many practices and ASCs are struggling.
Small business relief
- Small Business Administration (SBA) loans:
Businesses with 500 employees or less are eligible unless the covered industry’s SBA size standard allows more than 500 employees.
Allows 501(c)(3) non-profits to gain access to the program.
Increases the maximum loan amount to $10 million.
Expands allowable uses of loans to include payroll support, such as:
1. Paid sick or medical leave.
2. Employee salaries.
3. Mortgage payments.
Provides a process for loan forgiveness for certain payroll costs as well as mortgage, rent and utility obligations.
- Public Health and Social Services Emergency Fund:
$100 billion for health care services related to the COVID-19.
Reimbursement to eligible health care providers for health care related expenses or lost revenues that are attributable to the pandemic.
- Coronavirus Economic Stabilization Act:
$454 billion for loans, loan guarantees and other investments for companies with losses tied to the pandemic that threaten continued operation.
Medicare provisions
- Suspension of sequestration – Physicians avoid a 2% cut in their Medicare reimbursement.
- Extension of geographic index floor – Increases Medicare payments for providers in nonurban areas.
- Increased Medicare telehealth flexibilities during the emergency period.
- AGA will continue to advocate for audio-only coverage as this issue is still not resolved.
Other key health care provisions
- Liability protections for health care professionals during the emergency response.
- Coverage of preventive services and vaccines.
- $16 billion to replenish the Strategic National Stockpile.
- $1 billion for the Defense Production Act to ensure production of personal protective equipment (PPE).
Correspondence to congressional leadership
- March 25, 2020 – With the American Medical Association, a letter is sent requesting the inclusion of support for physician practices in any economic stimulus package.
- March 24, 2020 – With the Alliance of Specialty Medicine, a letter is sent asking for relief for independent physicians’ offices.
- March 20, 2020 – A joint society letter is sent asking for increased funding for and access to PPE; softened prior authorization, telehealth reimbursement and Medicare reporting requirements; and financial safeguards for health care professionals and practices.
From the EVP/CEO: How CHEST is helping to flatten the curve
As you know, the COVID-19 pandemic has caused immense strain on global health systems. With our membership at the epicenter, many of you have experienced firsthand the shortages that result from a surging patient population – lack of personal protective equipment (PPE), access to ventilators, and increasing demand for more qualified health-care workers needed on the front lines to treat and care for patients. As the staff leader of your organization, I feel an immense responsibility to support our community through this crisis.
In recent weeks, CHEST petitioned the federal and local governments on several issues, advocating for tax relief for COVID responders, expansion of liability protections, and the development of a provider relief fund. We will continue to collaborate with other societies and push such efforts. However, we also recognize an obligation to make a more tangible, real-time difference in the circumstances of our membership and the lives of the patients you are working to save.
An opportunity arose when we received a call from Dr. Doreen Addrizzo-Harris, Immediate Past President of the CHEST Foundation and Professor of Medicine at NYU Langone Health. In late March, New York City was seeing an uptick in patients with confirmed COVID infection in critical condition that was escalating by the day. The situation was beginning to resemble the trajectory of hotspots in Wuhan, China and Italy, and it was already taking a toll on health-care teams. Dr. Adrizzo-Harris asked whether there was any way to leverage the strength of the CHEST community to provide help. Already, our headquarters team had received unsolicited offers to travel to areas in need from our members. The question was how could we more proactively identify such willing and able clinicians.
We quickly drew upon our existing CHEST Analytics platform to target physicians outside New York City who might be well-positioned to travel. We harnessed our communication channels to get the word out. The response was immediate, with more than 100 people completing applications to join forces with their colleagues in New York. In the first 10 days of recruitment efforts, we added an additional 250 interested volunteers to the system. The positive response from members showed both the willingness of qualified medical staff to assist on the front lines but also highlighted deficiencies in other registration systems overwhelmed with requests in the face of this pandemic. Finding certified pulmonary and critical care physicians who are willing to step in where they are needed is time- and labor-intensive and detracts from health systems’ ability to focus on care. Watching the projections in other regions, we recognized other areas may soon need this same help.
With this in mind, CHEST approached ATS and our long-time partner PA Consulting to help us address the problem on a national scale. We felt we had the resources to leverage our databases and our analytic tools to create a more efficient process that would put physicians in hospitals where they could do the most good more efficiently. We knew that if we could apply our knowledge and deploy our heroic members, we could develop a solution that could save lives and relieve frontline clinicians. By leveraging the existing CHEST Analytics platform, the team created a solution that can be used by provider institutions, government agencies, and willing clinicians to quickly and effectively provide care where it is needed most. The team has engineered the solution to be scalable nationally and expandable to other critical care specialties (eg, anesthesia, emergency, nursing, respiratory therapy).
The Clinician Matching Network formally launched on April 14, 2020. It provides a two-way input that accepts sign up from individual clinicians and gathers needs and requirements from hospital systems, connecting health-care providers with the systems most in need of the specific support they are equipped to provide. We believe this has the potential to enable us to move ahead of the curve of the crisis.
I am very proud of the teams that lead this effort and have gained a greater appreciation of how CHEST, in partnership with other medical societies, can fully utilize data and analytics toward implementing public health solutions. The design and development of the Clinician Matching Network was accomplished in less than a week, leveraging a methodology that will enable the team to continuously improve and iterate through weekly releases, adding functionality quickly as the pandemic evolves.
In the weeks ahead, communications will be distributed to hospitals and hospital systems to help identify their staffing needs, encourage them to input their needs into the Clinician Matching Network, and expand the clinician-to-hospital matching effort. We aim to increase the number of collaborationg associations to grow the pool of clinicians who can be deployed to areas in need.
Please visit www.chestnet.org/clinician-matching to learn more, sign up to serve, tell us about the needs of your institution, or collaborate toward this cause.
As you know, the COVID-19 pandemic has caused immense strain on global health systems. With our membership at the epicenter, many of you have experienced firsthand the shortages that result from a surging patient population – lack of personal protective equipment (PPE), access to ventilators, and increasing demand for more qualified health-care workers needed on the front lines to treat and care for patients. As the staff leader of your organization, I feel an immense responsibility to support our community through this crisis.
In recent weeks, CHEST petitioned the federal and local governments on several issues, advocating for tax relief for COVID responders, expansion of liability protections, and the development of a provider relief fund. We will continue to collaborate with other societies and push such efforts. However, we also recognize an obligation to make a more tangible, real-time difference in the circumstances of our membership and the lives of the patients you are working to save.
An opportunity arose when we received a call from Dr. Doreen Addrizzo-Harris, Immediate Past President of the CHEST Foundation and Professor of Medicine at NYU Langone Health. In late March, New York City was seeing an uptick in patients with confirmed COVID infection in critical condition that was escalating by the day. The situation was beginning to resemble the trajectory of hotspots in Wuhan, China and Italy, and it was already taking a toll on health-care teams. Dr. Adrizzo-Harris asked whether there was any way to leverage the strength of the CHEST community to provide help. Already, our headquarters team had received unsolicited offers to travel to areas in need from our members. The question was how could we more proactively identify such willing and able clinicians.
We quickly drew upon our existing CHEST Analytics platform to target physicians outside New York City who might be well-positioned to travel. We harnessed our communication channels to get the word out. The response was immediate, with more than 100 people completing applications to join forces with their colleagues in New York. In the first 10 days of recruitment efforts, we added an additional 250 interested volunteers to the system. The positive response from members showed both the willingness of qualified medical staff to assist on the front lines but also highlighted deficiencies in other registration systems overwhelmed with requests in the face of this pandemic. Finding certified pulmonary and critical care physicians who are willing to step in where they are needed is time- and labor-intensive and detracts from health systems’ ability to focus on care. Watching the projections in other regions, we recognized other areas may soon need this same help.
With this in mind, CHEST approached ATS and our long-time partner PA Consulting to help us address the problem on a national scale. We felt we had the resources to leverage our databases and our analytic tools to create a more efficient process that would put physicians in hospitals where they could do the most good more efficiently. We knew that if we could apply our knowledge and deploy our heroic members, we could develop a solution that could save lives and relieve frontline clinicians. By leveraging the existing CHEST Analytics platform, the team created a solution that can be used by provider institutions, government agencies, and willing clinicians to quickly and effectively provide care where it is needed most. The team has engineered the solution to be scalable nationally and expandable to other critical care specialties (eg, anesthesia, emergency, nursing, respiratory therapy).
The Clinician Matching Network formally launched on April 14, 2020. It provides a two-way input that accepts sign up from individual clinicians and gathers needs and requirements from hospital systems, connecting health-care providers with the systems most in need of the specific support they are equipped to provide. We believe this has the potential to enable us to move ahead of the curve of the crisis.
I am very proud of the teams that lead this effort and have gained a greater appreciation of how CHEST, in partnership with other medical societies, can fully utilize data and analytics toward implementing public health solutions. The design and development of the Clinician Matching Network was accomplished in less than a week, leveraging a methodology that will enable the team to continuously improve and iterate through weekly releases, adding functionality quickly as the pandemic evolves.
In the weeks ahead, communications will be distributed to hospitals and hospital systems to help identify their staffing needs, encourage them to input their needs into the Clinician Matching Network, and expand the clinician-to-hospital matching effort. We aim to increase the number of collaborationg associations to grow the pool of clinicians who can be deployed to areas in need.
Please visit www.chestnet.org/clinician-matching to learn more, sign up to serve, tell us about the needs of your institution, or collaborate toward this cause.
As you know, the COVID-19 pandemic has caused immense strain on global health systems. With our membership at the epicenter, many of you have experienced firsthand the shortages that result from a surging patient population – lack of personal protective equipment (PPE), access to ventilators, and increasing demand for more qualified health-care workers needed on the front lines to treat and care for patients. As the staff leader of your organization, I feel an immense responsibility to support our community through this crisis.
In recent weeks, CHEST petitioned the federal and local governments on several issues, advocating for tax relief for COVID responders, expansion of liability protections, and the development of a provider relief fund. We will continue to collaborate with other societies and push such efforts. However, we also recognize an obligation to make a more tangible, real-time difference in the circumstances of our membership and the lives of the patients you are working to save.
An opportunity arose when we received a call from Dr. Doreen Addrizzo-Harris, Immediate Past President of the CHEST Foundation and Professor of Medicine at NYU Langone Health. In late March, New York City was seeing an uptick in patients with confirmed COVID infection in critical condition that was escalating by the day. The situation was beginning to resemble the trajectory of hotspots in Wuhan, China and Italy, and it was already taking a toll on health-care teams. Dr. Adrizzo-Harris asked whether there was any way to leverage the strength of the CHEST community to provide help. Already, our headquarters team had received unsolicited offers to travel to areas in need from our members. The question was how could we more proactively identify such willing and able clinicians.
We quickly drew upon our existing CHEST Analytics platform to target physicians outside New York City who might be well-positioned to travel. We harnessed our communication channels to get the word out. The response was immediate, with more than 100 people completing applications to join forces with their colleagues in New York. In the first 10 days of recruitment efforts, we added an additional 250 interested volunteers to the system. The positive response from members showed both the willingness of qualified medical staff to assist on the front lines but also highlighted deficiencies in other registration systems overwhelmed with requests in the face of this pandemic. Finding certified pulmonary and critical care physicians who are willing to step in where they are needed is time- and labor-intensive and detracts from health systems’ ability to focus on care. Watching the projections in other regions, we recognized other areas may soon need this same help.
With this in mind, CHEST approached ATS and our long-time partner PA Consulting to help us address the problem on a national scale. We felt we had the resources to leverage our databases and our analytic tools to create a more efficient process that would put physicians in hospitals where they could do the most good more efficiently. We knew that if we could apply our knowledge and deploy our heroic members, we could develop a solution that could save lives and relieve frontline clinicians. By leveraging the existing CHEST Analytics platform, the team created a solution that can be used by provider institutions, government agencies, and willing clinicians to quickly and effectively provide care where it is needed most. The team has engineered the solution to be scalable nationally and expandable to other critical care specialties (eg, anesthesia, emergency, nursing, respiratory therapy).
The Clinician Matching Network formally launched on April 14, 2020. It provides a two-way input that accepts sign up from individual clinicians and gathers needs and requirements from hospital systems, connecting health-care providers with the systems most in need of the specific support they are equipped to provide. We believe this has the potential to enable us to move ahead of the curve of the crisis.
I am very proud of the teams that lead this effort and have gained a greater appreciation of how CHEST, in partnership with other medical societies, can fully utilize data and analytics toward implementing public health solutions. The design and development of the Clinician Matching Network was accomplished in less than a week, leveraging a methodology that will enable the team to continuously improve and iterate through weekly releases, adding functionality quickly as the pandemic evolves.
In the weeks ahead, communications will be distributed to hospitals and hospital systems to help identify their staffing needs, encourage them to input their needs into the Clinician Matching Network, and expand the clinician-to-hospital matching effort. We aim to increase the number of collaborationg associations to grow the pool of clinicians who can be deployed to areas in need.
Please visit www.chestnet.org/clinician-matching to learn more, sign up to serve, tell us about the needs of your institution, or collaborate toward this cause.
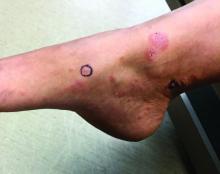
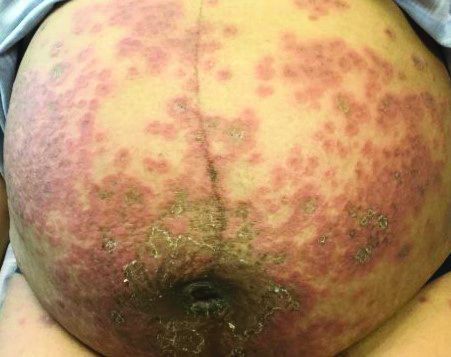
Itchy, vesicular rash
Pemphigoid gestationis
It typically presents with the abrupt onset of very pruritic urticarial plaques and papules, which start around the umbilicus and then spread to involve the trunk and extremities. The papules and plaques evolve to generalized tense blisters, which typically spare the face, palms, soles, and mucous membranes. Half of affected patients may present in an atypical distribution involving the extremities, palms, or soles. Patients may be at an increased risk for the development of Graves disease.
The cause of pemphigoid gestationis is a factor known as “herpes gestationis factor” that induces C3 deposition along the dermal-epidermal junction. As in bullous pemphigoid, patients with pemphigoid gestationis have antibodies to a transmembrane hemidesmosomal protein called BPAG2/BP180/collagen XVII.
Three-quarters of patients worsen at the time of delivery and up to 10% of newborns will have bullous lesions secondary to placental transfer of antibodies. In most cases, lesions will spontaneously resolve over a few weeks following delivery. Recurrence with future pregnancies is common, with severity increasing with each pregnancy. Recurrence with menstruation and with the use of oral contraceptives can also occur. Although there is no increase in maternal mortality, onset in the first or second trimester and presence of blisters is associated with decreased gestational age of baby at delivery and lower-birth-weight infants. There is no increase in fetal mortality.
Histopathology reveals a subepidermal vesicle and perivascular infiltrate consisting of lymphocytes and eosinophils. Diagnosis can be confirmed with direct immunofluorescence showing C3 in a linear band along the basement membrane zone. IgG may be present as well. Complement added indirect immunofluorescence reveals circulating anti–basement zone IgG, which allows differentiation from pruritic urticarial papules and plaques of pregnancy.
Treatment for localized disease includes class I topical steroids and oral antihistamines. More severe cases require systemic corticosteroid treatment. Systemic steroids may cause lower-birth-weight infants.
This case and the photos were submitted by Dr. Hanson of Associated Skin Care Specialists in Eden Prairie, Minn. The case was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
Pemphigoid gestationis
It typically presents with the abrupt onset of very pruritic urticarial plaques and papules, which start around the umbilicus and then spread to involve the trunk and extremities. The papules and plaques evolve to generalized tense blisters, which typically spare the face, palms, soles, and mucous membranes. Half of affected patients may present in an atypical distribution involving the extremities, palms, or soles. Patients may be at an increased risk for the development of Graves disease.
The cause of pemphigoid gestationis is a factor known as “herpes gestationis factor” that induces C3 deposition along the dermal-epidermal junction. As in bullous pemphigoid, patients with pemphigoid gestationis have antibodies to a transmembrane hemidesmosomal protein called BPAG2/BP180/collagen XVII.
Three-quarters of patients worsen at the time of delivery and up to 10% of newborns will have bullous lesions secondary to placental transfer of antibodies. In most cases, lesions will spontaneously resolve over a few weeks following delivery. Recurrence with future pregnancies is common, with severity increasing with each pregnancy. Recurrence with menstruation and with the use of oral contraceptives can also occur. Although there is no increase in maternal mortality, onset in the first or second trimester and presence of blisters is associated with decreased gestational age of baby at delivery and lower-birth-weight infants. There is no increase in fetal mortality.
Histopathology reveals a subepidermal vesicle and perivascular infiltrate consisting of lymphocytes and eosinophils. Diagnosis can be confirmed with direct immunofluorescence showing C3 in a linear band along the basement membrane zone. IgG may be present as well. Complement added indirect immunofluorescence reveals circulating anti–basement zone IgG, which allows differentiation from pruritic urticarial papules and plaques of pregnancy.
Treatment for localized disease includes class I topical steroids and oral antihistamines. More severe cases require systemic corticosteroid treatment. Systemic steroids may cause lower-birth-weight infants.
This case and the photos were submitted by Dr. Hanson of Associated Skin Care Specialists in Eden Prairie, Minn. The case was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
Pemphigoid gestationis
It typically presents with the abrupt onset of very pruritic urticarial plaques and papules, which start around the umbilicus and then spread to involve the trunk and extremities. The papules and plaques evolve to generalized tense blisters, which typically spare the face, palms, soles, and mucous membranes. Half of affected patients may present in an atypical distribution involving the extremities, palms, or soles. Patients may be at an increased risk for the development of Graves disease.
The cause of pemphigoid gestationis is a factor known as “herpes gestationis factor” that induces C3 deposition along the dermal-epidermal junction. As in bullous pemphigoid, patients with pemphigoid gestationis have antibodies to a transmembrane hemidesmosomal protein called BPAG2/BP180/collagen XVII.
Three-quarters of patients worsen at the time of delivery and up to 10% of newborns will have bullous lesions secondary to placental transfer of antibodies. In most cases, lesions will spontaneously resolve over a few weeks following delivery. Recurrence with future pregnancies is common, with severity increasing with each pregnancy. Recurrence with menstruation and with the use of oral contraceptives can also occur. Although there is no increase in maternal mortality, onset in the first or second trimester and presence of blisters is associated with decreased gestational age of baby at delivery and lower-birth-weight infants. There is no increase in fetal mortality.
Histopathology reveals a subepidermal vesicle and perivascular infiltrate consisting of lymphocytes and eosinophils. Diagnosis can be confirmed with direct immunofluorescence showing C3 in a linear band along the basement membrane zone. IgG may be present as well. Complement added indirect immunofluorescence reveals circulating anti–basement zone IgG, which allows differentiation from pruritic urticarial papules and plaques of pregnancy.
Treatment for localized disease includes class I topical steroids and oral antihistamines. More severe cases require systemic corticosteroid treatment. Systemic steroids may cause lower-birth-weight infants.
This case and the photos were submitted by Dr. Hanson of Associated Skin Care Specialists in Eden Prairie, Minn. The case was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
Almost half of med practices furloughing staff, one-fifth have layoffs
Clinicians all over the country already likely know this, but a survey by the Medical Group Management Association (MGMA) made it official: 97% of physician practices have experienced negative financial effects directly or indirectly related to COVID-19.
The survey, which was conducted April 7-8, also shows that 55% of practices have seen a decrease in revenue and 60% have experienced a decline in patient volume since the beginning of the COVID-19 crisis.
A significant number of medical practices have also been forced to lay off or furlough staff in response to the COVID-19 crisis, the MGMA said. Many practices that have not yet laid off or furloughed employees will consider doing so if the conditions persist over the next 30 days.
Through April 8, 22% of survey respondents reported they had laid off staff. In the same period, 48% had furloughed employees. The survey projects that, by May 8, if the COVID-19 situation hasn’t improved, 36% of practices will have laid off staff members and 60% will have furloughed them.
The survey received 724 applicable responses, the MGMA said. Approximately 75% of respondents are part of independent medical practices and employ fewer than 50 full-time-equivalent physicians. But the respondents belong to practices of all sizes and specialties.
The bare numbers only scratch the surface of the pain that many groups and owners of physician practices are feeling.
“Not only has 70% of our revenue disappeared, but our physicians are still working every day, exposing themselves to risks, taking care of patients, and taking care of their employees by continuing to pay them while they have taken over a 50% pay cut,” said a representative of an independent anesthesiology practice in Alabama in the MGMA press release.
“All doctors and administrative staff have deferred their salaries during this period,” a representative from a small independent practice in Mississippi that specializes in pain management said in the press release. “We have laid off most of our staff except five people.”
Employed groups tend to be in better financial shape than independent practices because they have the resources of large health care systems behind them. Some hospitals have laid off employees, however, and some of the cuts are starting to hit outpatient clinics.
Elective procedures down
In an interview conducted before the survey was released, Halee Fischer-Wright, MD, president and CEO of MGMA, said in an interview that single-specialty groups that perform elective procedures have seen “dramatic decreases in volume.” The Trump administration and at least two dozen states have asked hospitals to halt those procedures during this phase of the crisis, according to multiple media reports.
Some groups with multiple offices, Dr. Fischer-Wright noted, are deciding whether to staff them all because of their decreased volume and their concern about staff exposure to the coronavirus.
“We see them condensing down and delegating sick and well offices,” she said. “The benefit is that it allows them to be efficient with their staff use and also to place their limited PPE [personal protective equipment] supplies in the right office.”
Noting that there are costs involved in laying off staff and that practices want to retain good people if possible, Dr. Fischer-Wright advised practices to furlough employees rather than lay them off if they can.
A version of this article originally appeared on Medscape.com.
Clinicians all over the country already likely know this, but a survey by the Medical Group Management Association (MGMA) made it official: 97% of physician practices have experienced negative financial effects directly or indirectly related to COVID-19.
The survey, which was conducted April 7-8, also shows that 55% of practices have seen a decrease in revenue and 60% have experienced a decline in patient volume since the beginning of the COVID-19 crisis.
A significant number of medical practices have also been forced to lay off or furlough staff in response to the COVID-19 crisis, the MGMA said. Many practices that have not yet laid off or furloughed employees will consider doing so if the conditions persist over the next 30 days.
Through April 8, 22% of survey respondents reported they had laid off staff. In the same period, 48% had furloughed employees. The survey projects that, by May 8, if the COVID-19 situation hasn’t improved, 36% of practices will have laid off staff members and 60% will have furloughed them.
The survey received 724 applicable responses, the MGMA said. Approximately 75% of respondents are part of independent medical practices and employ fewer than 50 full-time-equivalent physicians. But the respondents belong to practices of all sizes and specialties.
The bare numbers only scratch the surface of the pain that many groups and owners of physician practices are feeling.
“Not only has 70% of our revenue disappeared, but our physicians are still working every day, exposing themselves to risks, taking care of patients, and taking care of their employees by continuing to pay them while they have taken over a 50% pay cut,” said a representative of an independent anesthesiology practice in Alabama in the MGMA press release.
“All doctors and administrative staff have deferred their salaries during this period,” a representative from a small independent practice in Mississippi that specializes in pain management said in the press release. “We have laid off most of our staff except five people.”
Employed groups tend to be in better financial shape than independent practices because they have the resources of large health care systems behind them. Some hospitals have laid off employees, however, and some of the cuts are starting to hit outpatient clinics.
Elective procedures down
In an interview conducted before the survey was released, Halee Fischer-Wright, MD, president and CEO of MGMA, said in an interview that single-specialty groups that perform elective procedures have seen “dramatic decreases in volume.” The Trump administration and at least two dozen states have asked hospitals to halt those procedures during this phase of the crisis, according to multiple media reports.
Some groups with multiple offices, Dr. Fischer-Wright noted, are deciding whether to staff them all because of their decreased volume and their concern about staff exposure to the coronavirus.
“We see them condensing down and delegating sick and well offices,” she said. “The benefit is that it allows them to be efficient with their staff use and also to place their limited PPE [personal protective equipment] supplies in the right office.”
Noting that there are costs involved in laying off staff and that practices want to retain good people if possible, Dr. Fischer-Wright advised practices to furlough employees rather than lay them off if they can.
A version of this article originally appeared on Medscape.com.
Clinicians all over the country already likely know this, but a survey by the Medical Group Management Association (MGMA) made it official: 97% of physician practices have experienced negative financial effects directly or indirectly related to COVID-19.
The survey, which was conducted April 7-8, also shows that 55% of practices have seen a decrease in revenue and 60% have experienced a decline in patient volume since the beginning of the COVID-19 crisis.
A significant number of medical practices have also been forced to lay off or furlough staff in response to the COVID-19 crisis, the MGMA said. Many practices that have not yet laid off or furloughed employees will consider doing so if the conditions persist over the next 30 days.
Through April 8, 22% of survey respondents reported they had laid off staff. In the same period, 48% had furloughed employees. The survey projects that, by May 8, if the COVID-19 situation hasn’t improved, 36% of practices will have laid off staff members and 60% will have furloughed them.
The survey received 724 applicable responses, the MGMA said. Approximately 75% of respondents are part of independent medical practices and employ fewer than 50 full-time-equivalent physicians. But the respondents belong to practices of all sizes and specialties.
The bare numbers only scratch the surface of the pain that many groups and owners of physician practices are feeling.
“Not only has 70% of our revenue disappeared, but our physicians are still working every day, exposing themselves to risks, taking care of patients, and taking care of their employees by continuing to pay them while they have taken over a 50% pay cut,” said a representative of an independent anesthesiology practice in Alabama in the MGMA press release.
“All doctors and administrative staff have deferred their salaries during this period,” a representative from a small independent practice in Mississippi that specializes in pain management said in the press release. “We have laid off most of our staff except five people.”
Employed groups tend to be in better financial shape than independent practices because they have the resources of large health care systems behind them. Some hospitals have laid off employees, however, and some of the cuts are starting to hit outpatient clinics.
Elective procedures down
In an interview conducted before the survey was released, Halee Fischer-Wright, MD, president and CEO of MGMA, said in an interview that single-specialty groups that perform elective procedures have seen “dramatic decreases in volume.” The Trump administration and at least two dozen states have asked hospitals to halt those procedures during this phase of the crisis, according to multiple media reports.
Some groups with multiple offices, Dr. Fischer-Wright noted, are deciding whether to staff them all because of their decreased volume and their concern about staff exposure to the coronavirus.
“We see them condensing down and delegating sick and well offices,” she said. “The benefit is that it allows them to be efficient with their staff use and also to place their limited PPE [personal protective equipment] supplies in the right office.”
Noting that there are costs involved in laying off staff and that practices want to retain good people if possible, Dr. Fischer-Wright advised practices to furlough employees rather than lay them off if they can.
A version of this article originally appeared on Medscape.com.
FDA approves first targeted drug for bile duct cancer
The U.S. Food and Drug Administration (FDA) has granted accelerated approval of a new targeted therapy for use in some patients with cholangiocarcinoma, a rare cancer of the bile ducts.
The product is pemigatinib (Pemazyre, Incyte), an oral kinase inhibitor.
It was approved specifically for use in patients with advanced cholangiocarcinoma who have received prior treatment and who have tumors that have a fusion or other rearrangement of the fibroblast growth factor receptor 2 (FGFR2) gene.
These FGFR2 genetic abnormalities are found in about 9% to 14% of patients with cholangiocarcinoma, notes the FDA.
At diagnosis, a majority of patients with cholangiocarcinoma have advanced disease, meaning that the disease is no longer treatable with surgery, the agency also notes. Until now, a combination of chemotherapy drugs has been the standard initial treatment.
Now the subgroup of patients with FGFR2 tumors have the option of a targeted therapy.
“Although cholangiocarcinoma is considered a rare disease, it has been on the rise over the past three decades,” commented Ghassan Abou-Alfa, MD, of Memorial Sloan Kettering Cancer Center, New York City, in a press release. “It is encouraging to have a new targeted treatment option for patients who historically have had limited options after first-line chemotherapy or surgery, in which relapse rates remain high.”
The accelerated approval was based on the overall response rate (ORR) and duration of response in an open-label clinical trial that involved 107 patients (the FIGHT-202 study).
As a condition of the accelerated approval, the manufacturer will complete and submit the results of a randomized trial demonstrating an improvement in progression-free survival or overall survival, the FDA noted.
Results from open-label clinical trial
The FIGHT-202 study enrolled 107 patients with locally advanced or metastatic cholangiocarcinoma who had received prior treatment and who had tumors with an FGFR2 fusion or rearrangement.
All patients received pemigatinib once a day for 14 days, followed by 7 days off, in 21-day cycles until the disease progressed or the patient experienced an unreasonable level of side effects. Patients underwent scanning every 8 weeks to assess ORR.
The ORR was 36% (38 of 107 patients), which included 2.8% of patients with a complete response and 33% with partial response.
Among the 38 patients who had a response, 24 patients (63%) had a response that lasted 6 months or longer, and 7 patients (18%) had a response that lasted 12 months or longer.
“With pemigatinib, we considered the observed efficacy results to be clinically meaningful and the overall risk to benefit assessment for patients with tumors harboring FGFR2 gene fusions and other rearrangements to be favorable, particularly when we considered that these patients have no other good options following first-line treatment with chemotherapy,” commented Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Oncologic Diseases in the FDA’s Center for Drug Evaluation and Research.
The most common adverse reactions, which occurred in 20% or more of patients who received pemigatinib, were electrolyte disorders (hyperphosphatemia and hypophosphatemia), alopecia, diarrhea, nail toxicity, fatigue, dysgeusia, nausea, constipation, stomatitis, dry eye, dry mouth, decreased appetite, vomiting, joint pain, abdominal pain, back pain, and dry skin. Ocular toxicity was seen rarely, the agency notes.
This article first appeared on Medscape.com.
The U.S. Food and Drug Administration (FDA) has granted accelerated approval of a new targeted therapy for use in some patients with cholangiocarcinoma, a rare cancer of the bile ducts.
The product is pemigatinib (Pemazyre, Incyte), an oral kinase inhibitor.
It was approved specifically for use in patients with advanced cholangiocarcinoma who have received prior treatment and who have tumors that have a fusion or other rearrangement of the fibroblast growth factor receptor 2 (FGFR2) gene.
These FGFR2 genetic abnormalities are found in about 9% to 14% of patients with cholangiocarcinoma, notes the FDA.
At diagnosis, a majority of patients with cholangiocarcinoma have advanced disease, meaning that the disease is no longer treatable with surgery, the agency also notes. Until now, a combination of chemotherapy drugs has been the standard initial treatment.
Now the subgroup of patients with FGFR2 tumors have the option of a targeted therapy.
“Although cholangiocarcinoma is considered a rare disease, it has been on the rise over the past three decades,” commented Ghassan Abou-Alfa, MD, of Memorial Sloan Kettering Cancer Center, New York City, in a press release. “It is encouraging to have a new targeted treatment option for patients who historically have had limited options after first-line chemotherapy or surgery, in which relapse rates remain high.”
The accelerated approval was based on the overall response rate (ORR) and duration of response in an open-label clinical trial that involved 107 patients (the FIGHT-202 study).
As a condition of the accelerated approval, the manufacturer will complete and submit the results of a randomized trial demonstrating an improvement in progression-free survival or overall survival, the FDA noted.
Results from open-label clinical trial
The FIGHT-202 study enrolled 107 patients with locally advanced or metastatic cholangiocarcinoma who had received prior treatment and who had tumors with an FGFR2 fusion or rearrangement.
All patients received pemigatinib once a day for 14 days, followed by 7 days off, in 21-day cycles until the disease progressed or the patient experienced an unreasonable level of side effects. Patients underwent scanning every 8 weeks to assess ORR.
The ORR was 36% (38 of 107 patients), which included 2.8% of patients with a complete response and 33% with partial response.
Among the 38 patients who had a response, 24 patients (63%) had a response that lasted 6 months or longer, and 7 patients (18%) had a response that lasted 12 months or longer.
“With pemigatinib, we considered the observed efficacy results to be clinically meaningful and the overall risk to benefit assessment for patients with tumors harboring FGFR2 gene fusions and other rearrangements to be favorable, particularly when we considered that these patients have no other good options following first-line treatment with chemotherapy,” commented Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Oncologic Diseases in the FDA’s Center for Drug Evaluation and Research.
The most common adverse reactions, which occurred in 20% or more of patients who received pemigatinib, were electrolyte disorders (hyperphosphatemia and hypophosphatemia), alopecia, diarrhea, nail toxicity, fatigue, dysgeusia, nausea, constipation, stomatitis, dry eye, dry mouth, decreased appetite, vomiting, joint pain, abdominal pain, back pain, and dry skin. Ocular toxicity was seen rarely, the agency notes.
This article first appeared on Medscape.com.
The U.S. Food and Drug Administration (FDA) has granted accelerated approval of a new targeted therapy for use in some patients with cholangiocarcinoma, a rare cancer of the bile ducts.
The product is pemigatinib (Pemazyre, Incyte), an oral kinase inhibitor.
It was approved specifically for use in patients with advanced cholangiocarcinoma who have received prior treatment and who have tumors that have a fusion or other rearrangement of the fibroblast growth factor receptor 2 (FGFR2) gene.
These FGFR2 genetic abnormalities are found in about 9% to 14% of patients with cholangiocarcinoma, notes the FDA.
At diagnosis, a majority of patients with cholangiocarcinoma have advanced disease, meaning that the disease is no longer treatable with surgery, the agency also notes. Until now, a combination of chemotherapy drugs has been the standard initial treatment.
Now the subgroup of patients with FGFR2 tumors have the option of a targeted therapy.
“Although cholangiocarcinoma is considered a rare disease, it has been on the rise over the past three decades,” commented Ghassan Abou-Alfa, MD, of Memorial Sloan Kettering Cancer Center, New York City, in a press release. “It is encouraging to have a new targeted treatment option for patients who historically have had limited options after first-line chemotherapy or surgery, in which relapse rates remain high.”
The accelerated approval was based on the overall response rate (ORR) and duration of response in an open-label clinical trial that involved 107 patients (the FIGHT-202 study).
As a condition of the accelerated approval, the manufacturer will complete and submit the results of a randomized trial demonstrating an improvement in progression-free survival or overall survival, the FDA noted.
Results from open-label clinical trial
The FIGHT-202 study enrolled 107 patients with locally advanced or metastatic cholangiocarcinoma who had received prior treatment and who had tumors with an FGFR2 fusion or rearrangement.
All patients received pemigatinib once a day for 14 days, followed by 7 days off, in 21-day cycles until the disease progressed or the patient experienced an unreasonable level of side effects. Patients underwent scanning every 8 weeks to assess ORR.
The ORR was 36% (38 of 107 patients), which included 2.8% of patients with a complete response and 33% with partial response.
Among the 38 patients who had a response, 24 patients (63%) had a response that lasted 6 months or longer, and 7 patients (18%) had a response that lasted 12 months or longer.
“With pemigatinib, we considered the observed efficacy results to be clinically meaningful and the overall risk to benefit assessment for patients with tumors harboring FGFR2 gene fusions and other rearrangements to be favorable, particularly when we considered that these patients have no other good options following first-line treatment with chemotherapy,” commented Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Oncologic Diseases in the FDA’s Center for Drug Evaluation and Research.
The most common adverse reactions, which occurred in 20% or more of patients who received pemigatinib, were electrolyte disorders (hyperphosphatemia and hypophosphatemia), alopecia, diarrhea, nail toxicity, fatigue, dysgeusia, nausea, constipation, stomatitis, dry eye, dry mouth, decreased appetite, vomiting, joint pain, abdominal pain, back pain, and dry skin. Ocular toxicity was seen rarely, the agency notes.
This article first appeared on Medscape.com.
FDA approves first new breast cancer drug with international group
The U.S. Food and Drug Administration has approved the oral therapy tucatinib (Tukysa, Seattle Genetics) for the treatment of advanced HER2-positive breast cancer. This is the first new drug approved under Project Orbis, an international collaboration.
Tucatinib, which is a small-molecule tyrosine kinase inhibitor, is approved in combination with trastuzumab and capecitabine to treat patients who have received one or more prior treatments for advanced disease.
The FDA collaborated with the regulatory authorities of Australia, Canada, Singapore, and Switzerland on this review. However, only the FDA has approved tucatinib; the application is still under review at the other agencies.
While working with Project Orbis in 2019, the FDA granted an accelerated, conditional approval to a drug combination that included previously approved agents.
“The FDA’s Project Orbis provides a framework for concurrent submission and review of oncology drug applications among the FDA’s international collaborators,” said Richard Pazdur, MD, acting director of the Office of Oncologic Diseases in the FDA’s Center for Drug Evaluation and Research, in a statement.
Collaboration among regulators may allow patients with cancer to receive earlier access to products in other countries where there may be significant delays in regulatory submissions, according to the FDA.
The new drug is a “valuable addition” to the roster of treatments for advanced HER2-positive breast cancer, said study investigator Eric Winer, MD, Dana-Farber Cancer Institute, Boston, Massachusetts, in a company press statement.
“With highly significant and clinically important results for overall and progression-free survival, the addition of [tucatinib] to trastuzumab and capecitabine has the potential to become a standard of care for people with HER2-positive metastatic breast cancer after having received one or more previous anti-HER2 therapies in the metastatic setting,” he said.
The new approval is based on safety and efficacy results from the phase 2 HER2CLIMB trial that enrolled 612 patients with HER2-positive unresectable locally advanced or metastatic breast cancer who had previously received, either separately or in combination, trastuzumab, pertuzumab, and ado-trastuzumab emtansine.
Nearly half (48%) of patients in the study had brain metastases at the start of the trial. The primary outcome measure was progression-free survival (PFS). All patients received trastuzumab and capecitabine and were randomly assigned to either tucatinib or placebo.
Median PFS in the tucatinib patient group was 7.8 months, compared with 5.6 months in the placebo group. The PFS results in the subgroup of patients with brain metastases were nearly the same.
Median overall survival in the tucatinib patient group was 21.9 months versus 17.4 months in the placebo group.
The new drug is a rare success in the treatment of breast cancer brain metastases, said Jawad Fares, MD, of Northwestern University, Chicago, Illinois, who spoke to Medscape Medical News when the phase 3 trial data were first presented at the 2019 San Antonio Breast Cancer Symposium.
“Outcomes in the field have been pretty dismal,” summarized Fares, who was not involved in the study.
The results of the HER2CLIMB study, which was funded by Seattle Genetics, were published in the New England Journal of Medicine last year.
According to the FDA, common side effects with tucatinib were diarrhea, palmar-plantar erythrodysesthesia syndrome, nausea, fatigue, hepatotoxicity, vomiting, stomatitis, decreased appetite, abdominal pain, headache, anemia, and rash.
Tucatinib can cause serious side effects, including diarrhea associated with dehydration, acute kidney injury, and death. Health care professionals should start antidiarrheals as clinically indicated if diarrhea occurs and should interrupt treatment or reduce the dosage. Tucatinib can also cause severe hepatotoxicity; patients should be monitored with liver tests.
This article first appeared on Medscape.com.
The U.S. Food and Drug Administration has approved the oral therapy tucatinib (Tukysa, Seattle Genetics) for the treatment of advanced HER2-positive breast cancer. This is the first new drug approved under Project Orbis, an international collaboration.
Tucatinib, which is a small-molecule tyrosine kinase inhibitor, is approved in combination with trastuzumab and capecitabine to treat patients who have received one or more prior treatments for advanced disease.
The FDA collaborated with the regulatory authorities of Australia, Canada, Singapore, and Switzerland on this review. However, only the FDA has approved tucatinib; the application is still under review at the other agencies.
While working with Project Orbis in 2019, the FDA granted an accelerated, conditional approval to a drug combination that included previously approved agents.
“The FDA’s Project Orbis provides a framework for concurrent submission and review of oncology drug applications among the FDA’s international collaborators,” said Richard Pazdur, MD, acting director of the Office of Oncologic Diseases in the FDA’s Center for Drug Evaluation and Research, in a statement.
Collaboration among regulators may allow patients with cancer to receive earlier access to products in other countries where there may be significant delays in regulatory submissions, according to the FDA.
The new drug is a “valuable addition” to the roster of treatments for advanced HER2-positive breast cancer, said study investigator Eric Winer, MD, Dana-Farber Cancer Institute, Boston, Massachusetts, in a company press statement.
“With highly significant and clinically important results for overall and progression-free survival, the addition of [tucatinib] to trastuzumab and capecitabine has the potential to become a standard of care for people with HER2-positive metastatic breast cancer after having received one or more previous anti-HER2 therapies in the metastatic setting,” he said.
The new approval is based on safety and efficacy results from the phase 2 HER2CLIMB trial that enrolled 612 patients with HER2-positive unresectable locally advanced or metastatic breast cancer who had previously received, either separately or in combination, trastuzumab, pertuzumab, and ado-trastuzumab emtansine.
Nearly half (48%) of patients in the study had brain metastases at the start of the trial. The primary outcome measure was progression-free survival (PFS). All patients received trastuzumab and capecitabine and were randomly assigned to either tucatinib or placebo.
Median PFS in the tucatinib patient group was 7.8 months, compared with 5.6 months in the placebo group. The PFS results in the subgroup of patients with brain metastases were nearly the same.
Median overall survival in the tucatinib patient group was 21.9 months versus 17.4 months in the placebo group.
The new drug is a rare success in the treatment of breast cancer brain metastases, said Jawad Fares, MD, of Northwestern University, Chicago, Illinois, who spoke to Medscape Medical News when the phase 3 trial data were first presented at the 2019 San Antonio Breast Cancer Symposium.
“Outcomes in the field have been pretty dismal,” summarized Fares, who was not involved in the study.
The results of the HER2CLIMB study, which was funded by Seattle Genetics, were published in the New England Journal of Medicine last year.
According to the FDA, common side effects with tucatinib were diarrhea, palmar-plantar erythrodysesthesia syndrome, nausea, fatigue, hepatotoxicity, vomiting, stomatitis, decreased appetite, abdominal pain, headache, anemia, and rash.
Tucatinib can cause serious side effects, including diarrhea associated with dehydration, acute kidney injury, and death. Health care professionals should start antidiarrheals as clinically indicated if diarrhea occurs and should interrupt treatment or reduce the dosage. Tucatinib can also cause severe hepatotoxicity; patients should be monitored with liver tests.
This article first appeared on Medscape.com.
The U.S. Food and Drug Administration has approved the oral therapy tucatinib (Tukysa, Seattle Genetics) for the treatment of advanced HER2-positive breast cancer. This is the first new drug approved under Project Orbis, an international collaboration.
Tucatinib, which is a small-molecule tyrosine kinase inhibitor, is approved in combination with trastuzumab and capecitabine to treat patients who have received one or more prior treatments for advanced disease.
The FDA collaborated with the regulatory authorities of Australia, Canada, Singapore, and Switzerland on this review. However, only the FDA has approved tucatinib; the application is still under review at the other agencies.
While working with Project Orbis in 2019, the FDA granted an accelerated, conditional approval to a drug combination that included previously approved agents.
“The FDA’s Project Orbis provides a framework for concurrent submission and review of oncology drug applications among the FDA’s international collaborators,” said Richard Pazdur, MD, acting director of the Office of Oncologic Diseases in the FDA’s Center for Drug Evaluation and Research, in a statement.
Collaboration among regulators may allow patients with cancer to receive earlier access to products in other countries where there may be significant delays in regulatory submissions, according to the FDA.
The new drug is a “valuable addition” to the roster of treatments for advanced HER2-positive breast cancer, said study investigator Eric Winer, MD, Dana-Farber Cancer Institute, Boston, Massachusetts, in a company press statement.
“With highly significant and clinically important results for overall and progression-free survival, the addition of [tucatinib] to trastuzumab and capecitabine has the potential to become a standard of care for people with HER2-positive metastatic breast cancer after having received one or more previous anti-HER2 therapies in the metastatic setting,” he said.
The new approval is based on safety and efficacy results from the phase 2 HER2CLIMB trial that enrolled 612 patients with HER2-positive unresectable locally advanced or metastatic breast cancer who had previously received, either separately or in combination, trastuzumab, pertuzumab, and ado-trastuzumab emtansine.
Nearly half (48%) of patients in the study had brain metastases at the start of the trial. The primary outcome measure was progression-free survival (PFS). All patients received trastuzumab and capecitabine and were randomly assigned to either tucatinib or placebo.
Median PFS in the tucatinib patient group was 7.8 months, compared with 5.6 months in the placebo group. The PFS results in the subgroup of patients with brain metastases were nearly the same.
Median overall survival in the tucatinib patient group was 21.9 months versus 17.4 months in the placebo group.
The new drug is a rare success in the treatment of breast cancer brain metastases, said Jawad Fares, MD, of Northwestern University, Chicago, Illinois, who spoke to Medscape Medical News when the phase 3 trial data were first presented at the 2019 San Antonio Breast Cancer Symposium.
“Outcomes in the field have been pretty dismal,” summarized Fares, who was not involved in the study.
The results of the HER2CLIMB study, which was funded by Seattle Genetics, were published in the New England Journal of Medicine last year.
According to the FDA, common side effects with tucatinib were diarrhea, palmar-plantar erythrodysesthesia syndrome, nausea, fatigue, hepatotoxicity, vomiting, stomatitis, decreased appetite, abdominal pain, headache, anemia, and rash.
Tucatinib can cause serious side effects, including diarrhea associated with dehydration, acute kidney injury, and death. Health care professionals should start antidiarrheals as clinically indicated if diarrhea occurs and should interrupt treatment or reduce the dosage. Tucatinib can also cause severe hepatotoxicity; patients should be monitored with liver tests.
This article first appeared on Medscape.com.
Differentiating hypersensitivity reactions to monoclonal antibodies
MAUI, HAWAII – Desensitization is a powerful and effective tool in patients with certain types of hypersensitivity reactions to therapeutic monoclonal antibodies, but it’s best considered a last resort reserved for individuals with no options left other than the offending biologic, Anna Postolova, MD, said at the 2020 Rheumatology Winter Clinical Symposium.
Why so selective? Desensitization is considered a high-risk intervention. It’s typically done as an inpatient procedure involving an overnight hospital stay followed by an elaborate 12-step protocol involving administration of small quantities of the culprit biologic in ascending concentrations over a 5- to 6-hour period.
Moreover, for an intravenous agent, such as infliximab (Remicade), desensitization has to be repeated prior to giving every dose of the biologic. So it makes sense to skip desensitization and simply switch to an alternative tumor necrosis factor inhibitor or a different class of biologic unless experience has shown that the culprit monoclonal antibody is the only one that works for that patient. It’s known, for example, that infliximab has no crossreactivity with adalimumab (Humira), explained Dr. Postolova, a dual rheumatologist and allergist/immunologist at Stanford (Calif.) University.
Defining type and severity of the hypersensitivity reaction
Dr. Postolova favors the hypersensitivity reaction classification system developed by Mariana Castells, MD, PhD, and coworkers at Brigham and Women’s Hospital, Boston.
They divide the field into immediate and delayed hypersensitivity reactions. Immediate hypersensitivity reactions arise rapidly, between minutes and a few hours. They can be categorized as infusion reactions, cytokine-release reactions, and IgE-mediated reactions. Phenotypically, infusion reactions and cytokine-release reactions are typically characterized by various combinations of chills, fever, flushing, hypertension, tachycardia, nausea, vomiting, syncope, and shortness of breath.
IgE-mediated reactions can also involve flushing and shortness of breath, and in addition itch, urticaria, and hypotension. These are anaphylactic reactions. Neither hypertension nor fever is part of the anaphylactic picture; those findings point instead to an infusion reaction or cytokine-release reaction.
Most allergists grade reaction severity on a 1-3 scale. Grade 1 reactions are considered mild and involve symptoms limited to the skin, such as flushing, or a single other organ system.
“That being said, if my patient is having a reaction with bronchospasm, I consider that a moderate, grade 2 reaction, and I stop the infusion. There’s only so much you can do for bronchospasm. It’s a very serious reaction,” Dr. Postolova observed.
Grade 2 reactions ordinarily involve two or more organ systems, but without hypotension or cyanosis. Grade 3 reactions are severe anaphylactic reactions with cardiovascular and/or neurologic compromise.
Delayed hypersensitivity reactions are of two types: serum sickness–like reactions and type IV cell-mediated mucocutaneous reactions.
Type IV reactions can range from a mild maculopapular rash to erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis, and DRESS (drug reaction with eosinophilia and systemic symptoms). Onset of type IV reactions can occur after 12 hours up to several weeks after exposure.
Serum sickness–like reactions typically begin 5-7 days after the infusion. These reactions are marked by evidence of immune overactivation: fever, arthralgia, arthritis, malaise, purpura, skin rash, and even renal failure.
Management of reactions
A patient with a grade 3 reaction who needs to continue using the culprit monoclonal antibody should be referred to an allergist for skin testing in an effort to identify an IgE-mediated reaction.
The timing of the referral for skin testing is important: The allergist wants to test roughly 4-6 weeks after the hypersensitivity reaction. Test too early and the results will be uninformative because the patient will still be anergic. On the other hand, after 7-8 weeks the patient will have lost the allergy. So there is a sweet spot.
If the patient is skin test positive – with the caveat that skin testing in this setting is not well validated – then the allergist will suggest desensitization, usually as an inpatient.
In contrast, infusion reactions can be handled in the rheumatologist’s infusion center. They are self-limited upon repeat exposure with premedication using antihistamines, NSAIDs, oral or injectable steroids, and perhaps montelukast (Singulair).
If a patient initially thought to have an infusion reaction continues to experience reactions even after the biologic is being delivered more slowly and under the protection of premedication, it’s time to consider the possibility that what’s really going on is a cytokine-release reaction or an IgE-mediated reaction. Diagnostic skin testing is in order.
For a skin test–negative patient with a suspected cytokine-release reaction, the allergist may propose a therapeutic challenge. This is reserved for patients who the allergist believe will not experience an immediate reaction, and unlike desensitization it’s not an intervention intended to induce drug tolerance. The challenge involves giving 10% of a full dose of the biologic, waiting in the allergist’s office for 30-60 minutes, then giving the other 90% of the medication, followed by an hour of in-office observation.
The solution to severe type IV delayed hypersensitivity reactions is strict medication avoidance, not desensitization, according to Dr. Postolova.
Top offending monoclonal antibodies
Infliximab and rituximab (Rituxan) are the most common culprits when it comes to immediate hypersensitivity reactions. About 10% of infliximab-treated patients develop these reactions. Although the reaction can occur with the first dose, the peak incidence is with the seventh infusion. Patients with anti-infliximab IgG antibodies are at 140%-300% increased risk; however, concomitant disease-modifying antirheumatic drug therapy lessens that risk.
Infusion reactions or cytokine-release reactions occur upon the first infusion of rituximab in about 25% of treated rheumatology patients and in a higher proportion of cancer patients. Most of these reactions are mild and don’t recur when the biologic is administered more slowly and with premedication. Severe recurrent reactions upon subsequent exposure are much more likely to be an IgE-mediated hypersensitivity reaction.
“Stop the medication, send the patient to your local allergist for skin testing, and they’ll use a desensitization protocol if rituximab is the best drug for your patient,” Dr. Postolova advised.
She reported having no financial conflicts regarding her presentation.
MAUI, HAWAII – Desensitization is a powerful and effective tool in patients with certain types of hypersensitivity reactions to therapeutic monoclonal antibodies, but it’s best considered a last resort reserved for individuals with no options left other than the offending biologic, Anna Postolova, MD, said at the 2020 Rheumatology Winter Clinical Symposium.
Why so selective? Desensitization is considered a high-risk intervention. It’s typically done as an inpatient procedure involving an overnight hospital stay followed by an elaborate 12-step protocol involving administration of small quantities of the culprit biologic in ascending concentrations over a 5- to 6-hour period.
Moreover, for an intravenous agent, such as infliximab (Remicade), desensitization has to be repeated prior to giving every dose of the biologic. So it makes sense to skip desensitization and simply switch to an alternative tumor necrosis factor inhibitor or a different class of biologic unless experience has shown that the culprit monoclonal antibody is the only one that works for that patient. It’s known, for example, that infliximab has no crossreactivity with adalimumab (Humira), explained Dr. Postolova, a dual rheumatologist and allergist/immunologist at Stanford (Calif.) University.
Defining type and severity of the hypersensitivity reaction
Dr. Postolova favors the hypersensitivity reaction classification system developed by Mariana Castells, MD, PhD, and coworkers at Brigham and Women’s Hospital, Boston.
They divide the field into immediate and delayed hypersensitivity reactions. Immediate hypersensitivity reactions arise rapidly, between minutes and a few hours. They can be categorized as infusion reactions, cytokine-release reactions, and IgE-mediated reactions. Phenotypically, infusion reactions and cytokine-release reactions are typically characterized by various combinations of chills, fever, flushing, hypertension, tachycardia, nausea, vomiting, syncope, and shortness of breath.
IgE-mediated reactions can also involve flushing and shortness of breath, and in addition itch, urticaria, and hypotension. These are anaphylactic reactions. Neither hypertension nor fever is part of the anaphylactic picture; those findings point instead to an infusion reaction or cytokine-release reaction.
Most allergists grade reaction severity on a 1-3 scale. Grade 1 reactions are considered mild and involve symptoms limited to the skin, such as flushing, or a single other organ system.
“That being said, if my patient is having a reaction with bronchospasm, I consider that a moderate, grade 2 reaction, and I stop the infusion. There’s only so much you can do for bronchospasm. It’s a very serious reaction,” Dr. Postolova observed.
Grade 2 reactions ordinarily involve two or more organ systems, but without hypotension or cyanosis. Grade 3 reactions are severe anaphylactic reactions with cardiovascular and/or neurologic compromise.
Delayed hypersensitivity reactions are of two types: serum sickness–like reactions and type IV cell-mediated mucocutaneous reactions.
Type IV reactions can range from a mild maculopapular rash to erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis, and DRESS (drug reaction with eosinophilia and systemic symptoms). Onset of type IV reactions can occur after 12 hours up to several weeks after exposure.
Serum sickness–like reactions typically begin 5-7 days after the infusion. These reactions are marked by evidence of immune overactivation: fever, arthralgia, arthritis, malaise, purpura, skin rash, and even renal failure.
Management of reactions
A patient with a grade 3 reaction who needs to continue using the culprit monoclonal antibody should be referred to an allergist for skin testing in an effort to identify an IgE-mediated reaction.
The timing of the referral for skin testing is important: The allergist wants to test roughly 4-6 weeks after the hypersensitivity reaction. Test too early and the results will be uninformative because the patient will still be anergic. On the other hand, after 7-8 weeks the patient will have lost the allergy. So there is a sweet spot.
If the patient is skin test positive – with the caveat that skin testing in this setting is not well validated – then the allergist will suggest desensitization, usually as an inpatient.
In contrast, infusion reactions can be handled in the rheumatologist’s infusion center. They are self-limited upon repeat exposure with premedication using antihistamines, NSAIDs, oral or injectable steroids, and perhaps montelukast (Singulair).
If a patient initially thought to have an infusion reaction continues to experience reactions even after the biologic is being delivered more slowly and under the protection of premedication, it’s time to consider the possibility that what’s really going on is a cytokine-release reaction or an IgE-mediated reaction. Diagnostic skin testing is in order.
For a skin test–negative patient with a suspected cytokine-release reaction, the allergist may propose a therapeutic challenge. This is reserved for patients who the allergist believe will not experience an immediate reaction, and unlike desensitization it’s not an intervention intended to induce drug tolerance. The challenge involves giving 10% of a full dose of the biologic, waiting in the allergist’s office for 30-60 minutes, then giving the other 90% of the medication, followed by an hour of in-office observation.
The solution to severe type IV delayed hypersensitivity reactions is strict medication avoidance, not desensitization, according to Dr. Postolova.
Top offending monoclonal antibodies
Infliximab and rituximab (Rituxan) are the most common culprits when it comes to immediate hypersensitivity reactions. About 10% of infliximab-treated patients develop these reactions. Although the reaction can occur with the first dose, the peak incidence is with the seventh infusion. Patients with anti-infliximab IgG antibodies are at 140%-300% increased risk; however, concomitant disease-modifying antirheumatic drug therapy lessens that risk.
Infusion reactions or cytokine-release reactions occur upon the first infusion of rituximab in about 25% of treated rheumatology patients and in a higher proportion of cancer patients. Most of these reactions are mild and don’t recur when the biologic is administered more slowly and with premedication. Severe recurrent reactions upon subsequent exposure are much more likely to be an IgE-mediated hypersensitivity reaction.
“Stop the medication, send the patient to your local allergist for skin testing, and they’ll use a desensitization protocol if rituximab is the best drug for your patient,” Dr. Postolova advised.
She reported having no financial conflicts regarding her presentation.
MAUI, HAWAII – Desensitization is a powerful and effective tool in patients with certain types of hypersensitivity reactions to therapeutic monoclonal antibodies, but it’s best considered a last resort reserved for individuals with no options left other than the offending biologic, Anna Postolova, MD, said at the 2020 Rheumatology Winter Clinical Symposium.
Why so selective? Desensitization is considered a high-risk intervention. It’s typically done as an inpatient procedure involving an overnight hospital stay followed by an elaborate 12-step protocol involving administration of small quantities of the culprit biologic in ascending concentrations over a 5- to 6-hour period.
Moreover, for an intravenous agent, such as infliximab (Remicade), desensitization has to be repeated prior to giving every dose of the biologic. So it makes sense to skip desensitization and simply switch to an alternative tumor necrosis factor inhibitor or a different class of biologic unless experience has shown that the culprit monoclonal antibody is the only one that works for that patient. It’s known, for example, that infliximab has no crossreactivity with adalimumab (Humira), explained Dr. Postolova, a dual rheumatologist and allergist/immunologist at Stanford (Calif.) University.
Defining type and severity of the hypersensitivity reaction
Dr. Postolova favors the hypersensitivity reaction classification system developed by Mariana Castells, MD, PhD, and coworkers at Brigham and Women’s Hospital, Boston.
They divide the field into immediate and delayed hypersensitivity reactions. Immediate hypersensitivity reactions arise rapidly, between minutes and a few hours. They can be categorized as infusion reactions, cytokine-release reactions, and IgE-mediated reactions. Phenotypically, infusion reactions and cytokine-release reactions are typically characterized by various combinations of chills, fever, flushing, hypertension, tachycardia, nausea, vomiting, syncope, and shortness of breath.
IgE-mediated reactions can also involve flushing and shortness of breath, and in addition itch, urticaria, and hypotension. These are anaphylactic reactions. Neither hypertension nor fever is part of the anaphylactic picture; those findings point instead to an infusion reaction or cytokine-release reaction.
Most allergists grade reaction severity on a 1-3 scale. Grade 1 reactions are considered mild and involve symptoms limited to the skin, such as flushing, or a single other organ system.
“That being said, if my patient is having a reaction with bronchospasm, I consider that a moderate, grade 2 reaction, and I stop the infusion. There’s only so much you can do for bronchospasm. It’s a very serious reaction,” Dr. Postolova observed.
Grade 2 reactions ordinarily involve two or more organ systems, but without hypotension or cyanosis. Grade 3 reactions are severe anaphylactic reactions with cardiovascular and/or neurologic compromise.
Delayed hypersensitivity reactions are of two types: serum sickness–like reactions and type IV cell-mediated mucocutaneous reactions.
Type IV reactions can range from a mild maculopapular rash to erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis, and DRESS (drug reaction with eosinophilia and systemic symptoms). Onset of type IV reactions can occur after 12 hours up to several weeks after exposure.
Serum sickness–like reactions typically begin 5-7 days after the infusion. These reactions are marked by evidence of immune overactivation: fever, arthralgia, arthritis, malaise, purpura, skin rash, and even renal failure.
Management of reactions
A patient with a grade 3 reaction who needs to continue using the culprit monoclonal antibody should be referred to an allergist for skin testing in an effort to identify an IgE-mediated reaction.
The timing of the referral for skin testing is important: The allergist wants to test roughly 4-6 weeks after the hypersensitivity reaction. Test too early and the results will be uninformative because the patient will still be anergic. On the other hand, after 7-8 weeks the patient will have lost the allergy. So there is a sweet spot.
If the patient is skin test positive – with the caveat that skin testing in this setting is not well validated – then the allergist will suggest desensitization, usually as an inpatient.
In contrast, infusion reactions can be handled in the rheumatologist’s infusion center. They are self-limited upon repeat exposure with premedication using antihistamines, NSAIDs, oral or injectable steroids, and perhaps montelukast (Singulair).
If a patient initially thought to have an infusion reaction continues to experience reactions even after the biologic is being delivered more slowly and under the protection of premedication, it’s time to consider the possibility that what’s really going on is a cytokine-release reaction or an IgE-mediated reaction. Diagnostic skin testing is in order.
For a skin test–negative patient with a suspected cytokine-release reaction, the allergist may propose a therapeutic challenge. This is reserved for patients who the allergist believe will not experience an immediate reaction, and unlike desensitization it’s not an intervention intended to induce drug tolerance. The challenge involves giving 10% of a full dose of the biologic, waiting in the allergist’s office for 30-60 minutes, then giving the other 90% of the medication, followed by an hour of in-office observation.
The solution to severe type IV delayed hypersensitivity reactions is strict medication avoidance, not desensitization, according to Dr. Postolova.
Top offending monoclonal antibodies
Infliximab and rituximab (Rituxan) are the most common culprits when it comes to immediate hypersensitivity reactions. About 10% of infliximab-treated patients develop these reactions. Although the reaction can occur with the first dose, the peak incidence is with the seventh infusion. Patients with anti-infliximab IgG antibodies are at 140%-300% increased risk; however, concomitant disease-modifying antirheumatic drug therapy lessens that risk.
Infusion reactions or cytokine-release reactions occur upon the first infusion of rituximab in about 25% of treated rheumatology patients and in a higher proportion of cancer patients. Most of these reactions are mild and don’t recur when the biologic is administered more slowly and with premedication. Severe recurrent reactions upon subsequent exposure are much more likely to be an IgE-mediated hypersensitivity reaction.
“Stop the medication, send the patient to your local allergist for skin testing, and they’ll use a desensitization protocol if rituximab is the best drug for your patient,” Dr. Postolova advised.
She reported having no financial conflicts regarding her presentation.
REPORTING FROM RWCS 2020
Proximal fractures linked to higher mortality
Bone fracture in older adults is associated with greater mortality risk, but the location of the break may be a key factor, according to a new study of outcomes in a Danish database.
Over the follow-up period, those with proximal fractures – breaks in the hip, femur, pelvis, rib, clavicle, and humerus – were more likely to be hospitalized and to die, compared with their matched controls, than were those were with distal fractures in regions like the ankle, forearm, hand, or foot, where the mortality was similar to the matched controls.
“Compared with someone with similar comorbidities without a proximal fracture, there seemed to be an increased hospitalization rate for things like diabetes, heart disease, and lung disease, and then for some of those hospitalizations, there seemed to be an increased mortality, compared with people who hadn’t fractured who were hospitalized,” said Jacqueline Center, MBBS, PhD, of the Garvan Institute of Medical Research, Sydney, in an interview. The study abstract was released online by the Endocrine Society. It had been slated for presentation during ENDO 2020, the society's annual meeting, which was canceled because of the COVID-19 pandemic.*
The study included 212,498 women and 95,372 men aged over 50 years who had a fragility fracture between 2001 and 2014. The researchers excluded high-trauma fractures. They matched each fracture patient with four nonfracture patients, based on sex, age, and comorbidity status. There were 30,677 deaths among women over 384,995 person-years of follow-up, and 19,519 deaths in men over 163,482 person-years of follow-up. Women were a mean age of 72 at the time of fracture, while men were a mean age of 75.
The researchers found that proximal fractures were associated with increased risk of mortality, compared with nonfractured controls, with hazard ratios ranging between 1.5 and 4.0. Distal fractures were not associated with any increased mortality risk.
Comorbidities were common in the study population, with 75% of men and 60% of women having at least one. The risk of mortality increased with increasing numbers of comorbidities in each fracture type, but only proximal fractures were associated with an independent increase in mortality risk over and above comorbidity status.
In the 2 years following fracture, compared with matched controls, proximal fractures were associated with a greater risk of major hospital admission for conditions like cardiovascular disease, cancer, stroke, diabetes, pneumonia, and pulmonary disease. There was no significant difference between controls and those with distal fractures in hospital admission rate. The 2-year mortality risk was higher among subjects with proximal fractures, compared with patients in the no-fracture control group, regardless of whether they were admitted to the hospital, but there was no significant difference in those with distal fractures.
The differing clinical trajectories between those with proximal and distal fractures is a key finding, according to Dr. Center. The cause still isn’t clear, but she suspects that, in those patients who do badly, the fractures are either a signal that something is happening with existing comorbidities of the underlying frailty or that it may exacerbate them. Comorbidity independently and additively contributes to mortality, so that someone with a hip fracture and no comorbidities might have a similar mortality risk as someone with an upper-arm fracture and a couple of comorbidities. “I think it tells us that the person has to be treated as a whole. We need to treat the fracture to treat the underlying osteoporosis, but we also need to look closely at the person with the fracture and treat their comorbidities as well, because they seem to be more vulnerable,” Dr. Center said.
Although patients and clinicians are attuned to the concerns over hip fractures, other fractures should also be noted, according to Nelson Watts, MD, who is director of osteoporosis and bone-health services at Mercy Health in Cincinnati and was not involved in the research. “I think the message for clinicians and patients is that all of these [proximal] fractures need to be taken seriously. The good news is that that we have medications that can cut the risk of further fractures by 50%-70%,” he said in an interview.
Dr. Center has been on an advisory board for Amgen. Dr. Watts has been a speaker for Amgen and Radius and has conducted numerous clinical trials of osteoporosis drugs.
In addition to a series of news conferences, the Endocrine Society is also planning to host ENDO Online 2020 during June 8-22, which will feature on-demand and live programming for clinicians and researchers.
SOURCE: Center J et al. ENDO 2020, Abstract OR13-03.
Correction, 4/21/20: An earlier version of this article misstated when the interview with Dr. Center took place.
Bone fracture in older adults is associated with greater mortality risk, but the location of the break may be a key factor, according to a new study of outcomes in a Danish database.
Over the follow-up period, those with proximal fractures – breaks in the hip, femur, pelvis, rib, clavicle, and humerus – were more likely to be hospitalized and to die, compared with their matched controls, than were those were with distal fractures in regions like the ankle, forearm, hand, or foot, where the mortality was similar to the matched controls.
“Compared with someone with similar comorbidities without a proximal fracture, there seemed to be an increased hospitalization rate for things like diabetes, heart disease, and lung disease, and then for some of those hospitalizations, there seemed to be an increased mortality, compared with people who hadn’t fractured who were hospitalized,” said Jacqueline Center, MBBS, PhD, of the Garvan Institute of Medical Research, Sydney, in an interview. The study abstract was released online by the Endocrine Society. It had been slated for presentation during ENDO 2020, the society's annual meeting, which was canceled because of the COVID-19 pandemic.*
The study included 212,498 women and 95,372 men aged over 50 years who had a fragility fracture between 2001 and 2014. The researchers excluded high-trauma fractures. They matched each fracture patient with four nonfracture patients, based on sex, age, and comorbidity status. There were 30,677 deaths among women over 384,995 person-years of follow-up, and 19,519 deaths in men over 163,482 person-years of follow-up. Women were a mean age of 72 at the time of fracture, while men were a mean age of 75.
The researchers found that proximal fractures were associated with increased risk of mortality, compared with nonfractured controls, with hazard ratios ranging between 1.5 and 4.0. Distal fractures were not associated with any increased mortality risk.
Comorbidities were common in the study population, with 75% of men and 60% of women having at least one. The risk of mortality increased with increasing numbers of comorbidities in each fracture type, but only proximal fractures were associated with an independent increase in mortality risk over and above comorbidity status.
In the 2 years following fracture, compared with matched controls, proximal fractures were associated with a greater risk of major hospital admission for conditions like cardiovascular disease, cancer, stroke, diabetes, pneumonia, and pulmonary disease. There was no significant difference between controls and those with distal fractures in hospital admission rate. The 2-year mortality risk was higher among subjects with proximal fractures, compared with patients in the no-fracture control group, regardless of whether they were admitted to the hospital, but there was no significant difference in those with distal fractures.
The differing clinical trajectories between those with proximal and distal fractures is a key finding, according to Dr. Center. The cause still isn’t clear, but she suspects that, in those patients who do badly, the fractures are either a signal that something is happening with existing comorbidities of the underlying frailty or that it may exacerbate them. Comorbidity independently and additively contributes to mortality, so that someone with a hip fracture and no comorbidities might have a similar mortality risk as someone with an upper-arm fracture and a couple of comorbidities. “I think it tells us that the person has to be treated as a whole. We need to treat the fracture to treat the underlying osteoporosis, but we also need to look closely at the person with the fracture and treat their comorbidities as well, because they seem to be more vulnerable,” Dr. Center said.
Although patients and clinicians are attuned to the concerns over hip fractures, other fractures should also be noted, according to Nelson Watts, MD, who is director of osteoporosis and bone-health services at Mercy Health in Cincinnati and was not involved in the research. “I think the message for clinicians and patients is that all of these [proximal] fractures need to be taken seriously. The good news is that that we have medications that can cut the risk of further fractures by 50%-70%,” he said in an interview.
Dr. Center has been on an advisory board for Amgen. Dr. Watts has been a speaker for Amgen and Radius and has conducted numerous clinical trials of osteoporosis drugs.
In addition to a series of news conferences, the Endocrine Society is also planning to host ENDO Online 2020 during June 8-22, which will feature on-demand and live programming for clinicians and researchers.
SOURCE: Center J et al. ENDO 2020, Abstract OR13-03.
Correction, 4/21/20: An earlier version of this article misstated when the interview with Dr. Center took place.
Bone fracture in older adults is associated with greater mortality risk, but the location of the break may be a key factor, according to a new study of outcomes in a Danish database.
Over the follow-up period, those with proximal fractures – breaks in the hip, femur, pelvis, rib, clavicle, and humerus – were more likely to be hospitalized and to die, compared with their matched controls, than were those were with distal fractures in regions like the ankle, forearm, hand, or foot, where the mortality was similar to the matched controls.
“Compared with someone with similar comorbidities without a proximal fracture, there seemed to be an increased hospitalization rate for things like diabetes, heart disease, and lung disease, and then for some of those hospitalizations, there seemed to be an increased mortality, compared with people who hadn’t fractured who were hospitalized,” said Jacqueline Center, MBBS, PhD, of the Garvan Institute of Medical Research, Sydney, in an interview. The study abstract was released online by the Endocrine Society. It had been slated for presentation during ENDO 2020, the society's annual meeting, which was canceled because of the COVID-19 pandemic.*
The study included 212,498 women and 95,372 men aged over 50 years who had a fragility fracture between 2001 and 2014. The researchers excluded high-trauma fractures. They matched each fracture patient with four nonfracture patients, based on sex, age, and comorbidity status. There were 30,677 deaths among women over 384,995 person-years of follow-up, and 19,519 deaths in men over 163,482 person-years of follow-up. Women were a mean age of 72 at the time of fracture, while men were a mean age of 75.
The researchers found that proximal fractures were associated with increased risk of mortality, compared with nonfractured controls, with hazard ratios ranging between 1.5 and 4.0. Distal fractures were not associated with any increased mortality risk.
Comorbidities were common in the study population, with 75% of men and 60% of women having at least one. The risk of mortality increased with increasing numbers of comorbidities in each fracture type, but only proximal fractures were associated with an independent increase in mortality risk over and above comorbidity status.
In the 2 years following fracture, compared with matched controls, proximal fractures were associated with a greater risk of major hospital admission for conditions like cardiovascular disease, cancer, stroke, diabetes, pneumonia, and pulmonary disease. There was no significant difference between controls and those with distal fractures in hospital admission rate. The 2-year mortality risk was higher among subjects with proximal fractures, compared with patients in the no-fracture control group, regardless of whether they were admitted to the hospital, but there was no significant difference in those with distal fractures.
The differing clinical trajectories between those with proximal and distal fractures is a key finding, according to Dr. Center. The cause still isn’t clear, but she suspects that, in those patients who do badly, the fractures are either a signal that something is happening with existing comorbidities of the underlying frailty or that it may exacerbate them. Comorbidity independently and additively contributes to mortality, so that someone with a hip fracture and no comorbidities might have a similar mortality risk as someone with an upper-arm fracture and a couple of comorbidities. “I think it tells us that the person has to be treated as a whole. We need to treat the fracture to treat the underlying osteoporosis, but we also need to look closely at the person with the fracture and treat their comorbidities as well, because they seem to be more vulnerable,” Dr. Center said.
Although patients and clinicians are attuned to the concerns over hip fractures, other fractures should also be noted, according to Nelson Watts, MD, who is director of osteoporosis and bone-health services at Mercy Health in Cincinnati and was not involved in the research. “I think the message for clinicians and patients is that all of these [proximal] fractures need to be taken seriously. The good news is that that we have medications that can cut the risk of further fractures by 50%-70%,” he said in an interview.
Dr. Center has been on an advisory board for Amgen. Dr. Watts has been a speaker for Amgen and Radius and has conducted numerous clinical trials of osteoporosis drugs.
In addition to a series of news conferences, the Endocrine Society is also planning to host ENDO Online 2020 during June 8-22, which will feature on-demand and live programming for clinicians and researchers.
SOURCE: Center J et al. ENDO 2020, Abstract OR13-03.
Correction, 4/21/20: An earlier version of this article misstated when the interview with Dr. Center took place.
FROM ENDO 2020