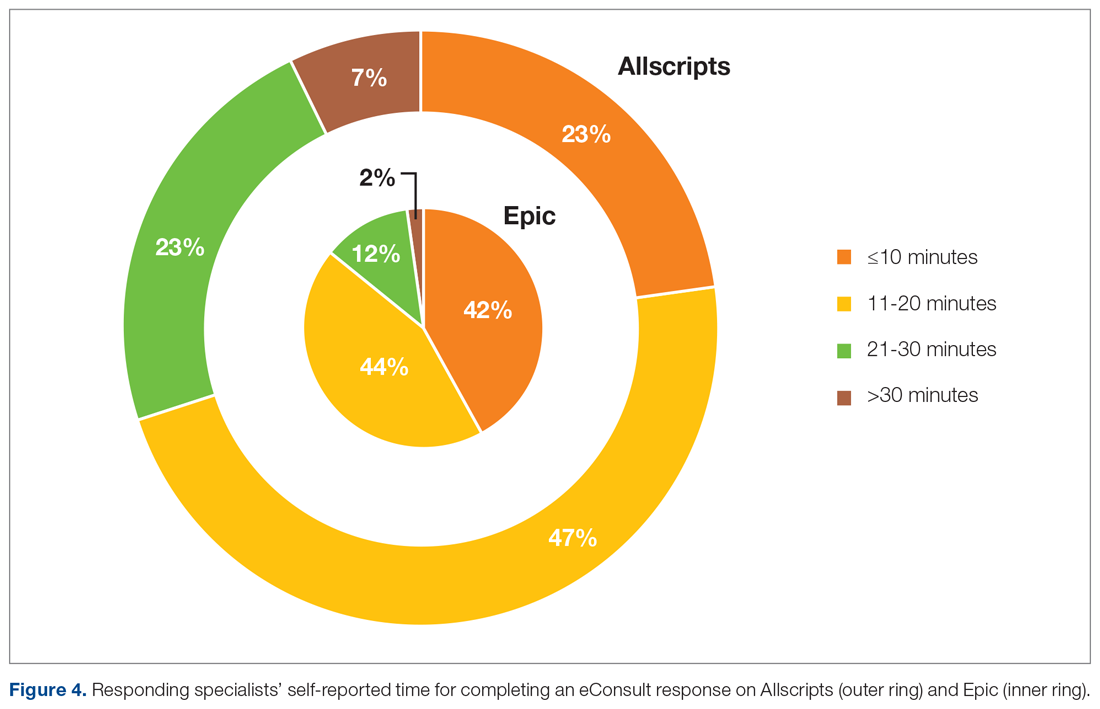
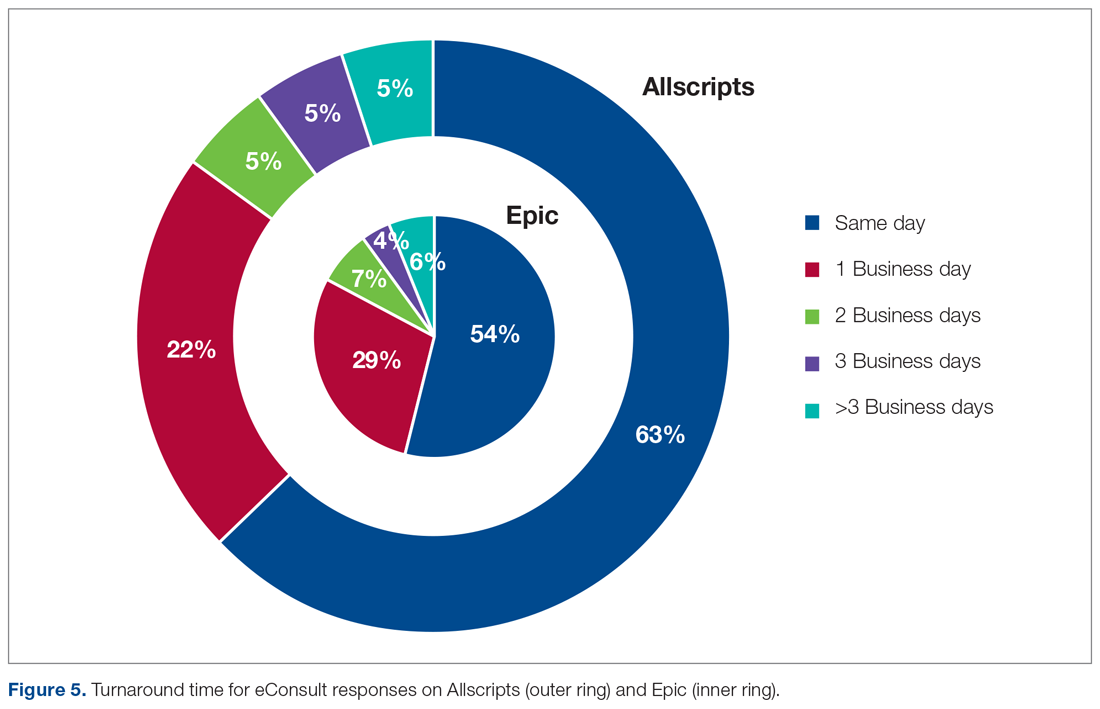
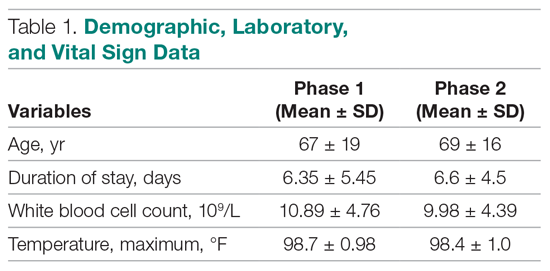
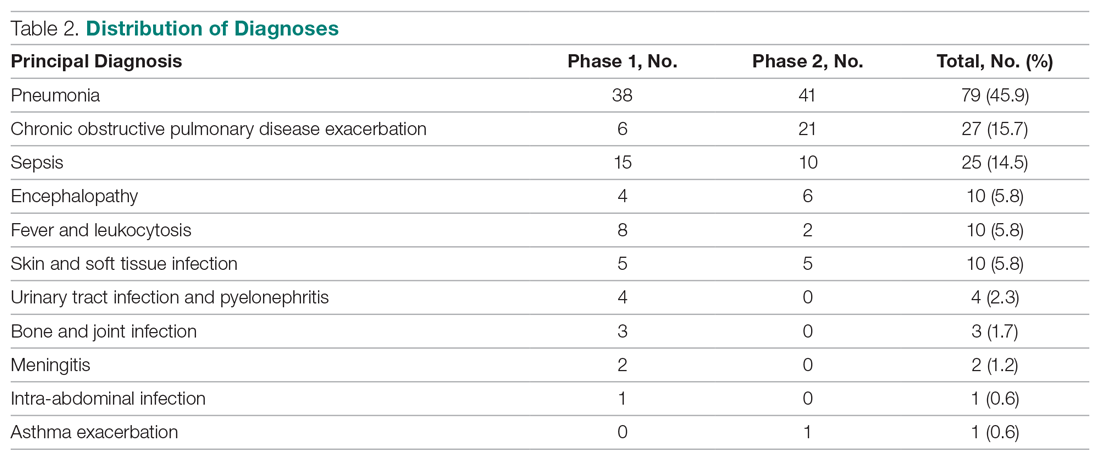
User login
Team-Based Hypertension Management in Outpatient Settings
From Western University of Health Sciences College of Pharmacy, Department of Pharmacy Practice and Administration, Pomona, CA.
Abstract
- Objective: To review the current literature regarding the clinical effectiveness and cost-effectiveness of implementing hypertension team-based care (TBC) interventions in the outpatient setting, and discuss challenges to implementation.
- Methods: A literature review was conducted of meta-analyses, systematic reviews, and randomized controlled trials comparing TBC models to usual care for hypertension management.
- Results: Compared to usual care, TBC models have demonstrated greater blood pressure reductions and improved blood pressure control rates. Evidence was strongest for models involving nurses and pharmacists whose roles included medication management, patient education and counseling, coordination of care and follow-up, population health management, and performance measurement with quality improvement. Although TBC results in an increase in health care costs, the overall long-term benefits support the cost-effectiveness of these models over usual care. The most common barriers to TBC implementation include underutilization of technology, stakeholder engagement, and reimbursement issues.
- Conclusion: Hypertension TBC models have been shown to be clinically effective and cost-effective, but continued research comparing different models is warranted to determine which combination of health professionals and interventions is most impactful and cost-effective in practice. An implementation science approach, in which TBC models unique to each organization’s situation are created, will be useful to identify and overcome challenges and provide a solid foundation for sustainment.
Keywords: blood pressure; pharmacist; nurse; nurse practitioner; cost-effectiveness; team-based care.
Approximately 1 in 3 US adults—or about 100 million people—have high blood pressure, and only about half (48%) have their blood pressure under control.1 Effective blood pressure management has been shown to decrease the incidence of stroke, heart attack, and heart failure.2-4 The American College of Cardiology/American Heart Association (ACC/AHA) 2017 blood pressure guidelines recommended lower thresholds for diagnosing hypertension and initiating antihypertensive medication, and intensified the blood pressure goal to less than 130/80 mm Hg.5 Changing practice standards to more intensive blood pressure goals requires significant adjustments by clinicians and health care systems. In fact, new guideline uptake is often delayed, ignored, or sparsely applied.6 Due to this dramatic change in hypertension practice standards, the ACC/AHA guidelines support interdisciplinary team-based care (TBC) for hypertension management.5,7 Additionally, the Centers for Disease Control and Prevention (CDC) and the Community Preventive Services Task Force (CPSTF) promote TBC to improve blood pressure control in their initiatives to prevent heart disease and stroke.8,9
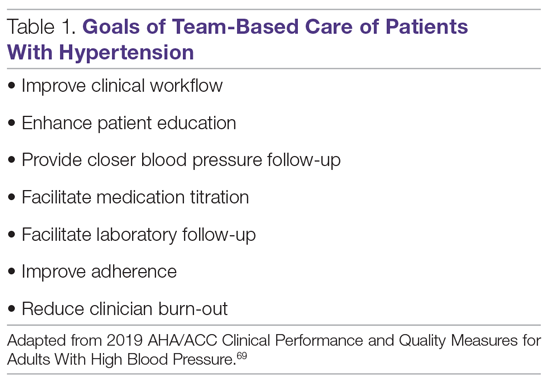
The National Academy of Medicine defines TBC as “the provision of health services to individuals, families, and/or their communities by at least 2 healthcare providers who work collaboratively with patients and their caregivers—to the extent preferred by each patient—to accomplish shared goals within and across settings to achieve coordinated, high-quality care.”10 Specific goals for TBC in hypertension treatment are listed in Table 1, and a checklist of key elements of TBC to consider before implementation are presented in Table 2.
TBC has been shown to have many advantages, including increased access to care due to expanded hours of operation and shorter wait times.11 Team-based models also provide effective and efficient delivery of patient education, behavioral health care, and care coordination.12-14 Patients are more likely to receive high-quality care when multiple providers, each with varied expertise, are on the health care team.11,15 Furthermore, clinicians report improved professional job satisfaction related to their ability to practice in environments where they are encouraged to work at the top of their licenses.16 Consequently, TBC has been accepted as a vital part of the patient-centered medical home (PCMH) model.17-19 Standards set by the National Committee for Quality Assurance (NCQA) include TBC as a requirement health systems must meet in order to achieve the highest level of PCMH recognition. While a team-based approach offers substantial benefits and is recognized as a marker of quality, implementation has presented various challenges, and the sustainability of these models in care settings has been questioned.20
In this article, we review the current literature regarding the clinical effectiveness and cost-effectiveness of implementing hypertension TBC interventions in the outpatient setting. We also discuss the challenges and opportunities of implementing this strategy in health systems and community settings in the United States.
Evidence of Impact and Effectiveness
Various models of hypertension TBC have been shown to increase the proportion of individuals with controlled blood pressure and to lead to a reduction in both systolic (SBP) and diastolic blood pressure (DBP), resulting in a strong recommendation for TBC approaches by the 2017 ACC/AHA blood pressure guidelines.5,21-25 There is great diversity in the types of hypertension treatment models studied, with few utilizing physician specialists and most utilizing nonphysician providers, such as community health workers, physician assistants, nurses, nurse practitioners, dietitians, social workers, and pharmacists.22,26-29 These professionals share duties of hypertension management with primary care physicians to reduce the burden of responsibility for care on any single provider type. TBC is patient-centered, and typically includes interprofessional collaboration, treatment algorithms, adherence counseling, frequent follow-up, home blood pressure monitoring, and patient self-management education.
Numerous studies have supported implementation of TBC in recent years. A systematic review and meta-analysis of 100 trials of hypertension TBC involving 55,920 patients concluded that the most effective blood pressure–lowering strategies use multilevel, multicomponent approaches to address barriers to hypertension control. Nonphysician providers are often involved in measuring blood pressure, ordering and assessing laboratory tests, and titrating medications.30 Compared with usual care, TBC with physician medication titration resulted in reductions in mean SBP and DBP (6.2 mm Hg and 2.7 mm Hg, respectively), while TBC with nonphysician medication titration also resulted in reductions in mean SBP and DBP (7.1 mm Hg and 3.1 mm Hg, respectively). Nurses and pharmacists are specifically mentioned by the 2017 ACC/AHA blood pressure guidelines as essential members of the hypertension treatment team.5 Randomized controlled trials (RCTs) and meta-analyses of TBC involving nurse or pharmacist interventions demonstrated greater reductions in SBP and/or greater attainment of blood pressure goals compared to usual care.21,26,31,32 The literature supports the roles of nurses and pharmacists in hypertension management in all aspects of care, including medication management, patient education and counseling, coordination of care and follow-up, population health management, and performance measurement with quality improvement.33
Nurses
Nurses are commonly part of TBC hypertension management programs. One meta-analysis and systematic review of international RCTs compared nurse, nurse prescriber (United Kingdom), and nurse practitioner interventions for hypertension with usual care. Interventions that included a stepped treatment algorithm and nurse prescribing showed greater reductions in SBP (8.2 mm Hg and 8.9 mm Hg, respectively) compared to usual care.31 Similarly, models that utilized telephone monitoring demonstrated greater achievement of blood pressure targets, while those that involved home monitoring showed significant reductions in blood pressure. Another international meta-analysis and systematic review of 11 nurse-led interventions in hypertensive patients with diabetes demonstrated a 5.8 mm Hg mean decrease in SBP compared to physician-led care. However, nurse-led care was not superior in achievement of study targets.34
A recent meta-analysis and systematic review, performed by Shaw and colleagues, sought to determine whether nurse-led protocols are effective for outpatient management of adults with diabetes, hypertension, and hyperlipidemia. All of the included studies involved a registered nurse who titrated medications by following a protocol, and most were RCTs comparing the nurse protocols to usual care. Overall, mean SBP and DBP decreased by 3.86 mm Hg and 1.56 mm Hg, respectively, while blood glucose and lipid levels were also reduced compared to usual care.24
Limited RCT data have been published since the Shaw et al meta-analysis. A single-blind RCT was performed in an urban community health care center in China among patients with uncontrolled blood pressure (SBP ≥ 140 mm Hg and/or DBP ≥ 90 mm Hg).35 The study group received care via a nurse-led model, which included a delivery design system, decision support, clinical information system, and self-management support, and the control group received usual care. At 12 weeks, patients in the study group had significantly lower blood pressure than control patients, with mean SBP/DBP reduction of 14.37/7.43 mm Hg and 5.10/2.69 mm Hg, respectively (P < 0.01). Improved medication adherence and increased patient satisfaction were other benefits of the nurse-led model.
Nurse case managers (NCM) also play a critical role in hypertension management, coordinating health care services to meet patient health needs. Ogedegbe sought to evaluate the comparative effectiveness of home blood pressure telemonitoring (HBPTM)+NCM versus HBPTM alone on SBP reduction in black and Hispanic stroke survivors.36,37 NCMs evaluated patient profiles, counseled patients on target lifestyle behaviors, and reviewed home blood pressure data. At 6 months, SBP declined by 13.63 mm Hg from baseline in the HBPTM+NCM group and 6.31 mm Hg in the HBPTM alone group (P < 0.0001). At 12 months, SBP in the HBPTM+NCM group declined by 14.76 mm Hg, while blood pressure in the HBPTM alone group declined by 5.53 mm Hg (P < 0.0001).
Pharmacists
Clinical pharmacists are also widely utilized in TBC models for hypertension management. Typical models involve pharmacists entering into collaborative practice agreements with physicians, leading to optimization of medications, avoidance of adverse drug events, and transitional care activities focusing on medication reconciliation and patient education in outpatient settings.30,38 The largest and most recent meta-analysis of pharmacist interventions, conducted in 2014 by Santschi et al,23 combined 2 previous systematic reviews to include a total of 39 RCTs with 14,224 patients.32,39 Pharmacist interventions included patient education, recommendations to physicians, and medication management. Compared with usual care, pharmacist interventions showed greater reductions in SBP (7.6 mm Hg) and DBP (3.9 mm Hg).23
Numerous studies substantiating the impact of pharmacist interventions on clinical outcomes have heavily influenced clinical practice and guideline development. Carter et al conducted a prospective, multi-state, cluster-randomized trial in 32 primary care clinics to evaluate whether clinics randomized to receive the pharmacist-physician collaborative care model (PPCCM) achieved better blood pressure outcomes versus clinics randomized to usual care.25 Investigators enrolled 625 patients with uncontrolled hypertension, 50% of whom had a prior diagnosis of diabetes mellitus or chronic kidney disease. The primary outcome of blood pressure control at 9 months in the intervention clinics compared to the control clinics was 43% and 34%, respectively (P = 0.059). The difference in mean SBP/DBP between the intervention and control clinics for all patients at 9 months was −6.1/−2.9 mm Hg. In a post-hoc analysis of patients with chronic kidney disease and diabetes, the pharmacist-intervention group had a significantly greater mean SBP reduction and higher blood pressure control rates compared to usual care at 9 months.40
A pre-specified secondary analysis from the Carter et al study determined that, in patients from racial minority groups, the mean SBP was 7.3 mm Hg lower in those who received the intervention compared to those in the control group (P = 0.0042).41 In patients with less than 12 years of education, those in the intervention group had a mean SBP 8.1 mm Hg lower than the SBP of those in the control group (P = 0.0001). Similar reductions in blood pressure occurred in patients with low income, Medicaid beneficiaries, or those without insurance. This study demonstrated that pharmacist interventions reduced racial and socioeconomic disparities in blood pressure treatment.
Other studies of pharmacist interventions in underserved populations have yielded positive results. In a retrospective review of uninsured patients, blood pressure control rates in a pharmacist-driven primary care clinic ranked in the 90th percentile of NCQA benchmarks, and was superior to the 2013 reported mean for commercial insurers.42 Similarly, another retrospective cohort study of a PPCCM on time to goal blood pressure in uninsured patients with hypertension showed the median time to blood pressure goal was 36 days in the PPCCM cohort versus 259 days in usual care cohorts (P < 0.001).43 A post-hoc analysis revealed the mean time-in-therapeutic blood pressure range was 46.2% ± 24.3% in the PPCCM group and 24.8% ± 27.4% in the usual care group (P < 0.0001). The blood pressure control rates at 12 months were 89% in the PPCCM group compared with 50% in the usual care group (P < 0.0001).44
Tsuyuki et al conducted the RxACTION study, a multicenter RCT evaluating the effectiveness of enhanced pharmacist care versus usual care in 23 Canadian community pharmacies and outpatient clinics following a 6-month intervention.45 Enhanced pharmacy services included pharmacist assessment of and counseling about cardiovascular disease risk and blood pressure control, review of current antihypertensive medications, and prescribing/titrating drug therapy, as needed, through independent prescriptive authority. Compared to the usual care group (n = 67), the intervention group had a reduction in SBP of 6.6 mm Hg (P = 0.006) and in DBP of 3.2 mm Hg (P = 0.01). This study expanded the pharmacists’ scope of practice, showing evidence for enhancing pharmacist roles on the hypertension care team. Tsuyuki et al also conducted the RxEACH randomized trial, which evaluated community pharmacist cardiovascular risk reduction interventions and showed an improvement in SBP and DBP, with reported results comparable to RxACTION.46
Victor et al conducted the landmark Black Barbershop Study, a cluster RCT involving 319 non-Hispanic black male patients with hypertension from 52 black-owned barbershops.47,48 Barbershops were assigned to 1 of 2 groups. The control group consisted of barbers who encouraged lifestyle modifications and made referrals to primary care providers. The intervention group had pharmacists who met regularly with participants at the barbershops and measured blood pressure, encouraged lifestyle changes, and prescribed drug therapy under collaborative practice agreements with physicians. Both groups demonstrated improvements in blood pressure outcomes, but the intervention group showed greater improvement in SBP and achievement of blood pressure goals compared to the control group. The results in the intervention group proved sustainable over the course of a year, even after the frequency of pharmacists’ visits was reduced. At 6 months, the mean SBP fell by 27.0 mm Hg (to 125.8 mm Hg) in the intervention group, as compared to a 9.3 mm Hg (to 145.4 mm Hg) reduction in the control group (P < 0.001), and blood pressure less than 130/80 mm Hg was achieved among 63.6% of the participants in the intervention group versus 11.7% in the control group (P < 0.001).
This community-level trial brought pharmacists to the barbershop and made them an essential part of the health care team through the endorsement of the barber, who the participants trusted and with whom they had a relationship. Long-standing issues related to distrust of the medical profession by this population were addressed, and trusted community barbershops were utilized as safe spaces for health care delivery. Health care professionals should consider utilizing community locations that other minority populations perceive as social centers and safe places, to reduce health disparities and barriers to care. However, models that bring care to patients need further economic and feasibility evaluations.
Other Health Care Professionals and Future Studies
In addition to models led by nurses and pharmacists, studies have also assessed models of TBC incorporating other health care professionals, including registered dietitians, medical assistants, community health workers, and health coaches (NCT02674464).49,50 Ongoing studies are also looking at the impact of TBC on underserved communities (NCT02674464, NCT03504124). Involving a variety of health care professionals with different communities and populations in TBC studies is warranted to determine the optimal settings in which to utilize different skill sets.
The Impress Study involves nurses who are assessing lifestyle risk and developing an action plan according to a standardized procedure, which may be advantageous given the degree of heterogeneity found in other TBC models.51 There are also studies underway or recently published that compare different components of TBC in order to determine which combination of TBC elements is preferred. Some of these have shown the benefits of using clinical decision-support systems (through a guideline-based treatment protocol) or training programs with ongoing support.52,53 Continued research comparing different TBC models is needed to determine which combination of health professionals and interventions is most impactful in practice.
Cost-Effectiveness
According to the CDC, TBC in hypertension management has proven to be cost-effective.54 Systematic reviews and meta-analyses assessing the cost-effectiveness of TBC in hypertension management have been conducted.26,27,29,55-58 While the general consensus supports this approach as being cost-effective, these determinations are based on studies that are widely heterogeneous. In each of these studies, different types of costs are taken into account when determining cost-effectiveness. The range of costs can be quite wide, depending on how they are calculated, making it difficult to determine the true cost-effectiveness of different TBC models.
Intervention cost is represented by the amount of money spent to implement and maintain the intervention beyond the cost of usual care or the cost without the intervention. For TBC, intervention cost consists of personnel resources such as provider time, patient time, and non-personnel resources, including rent and utilities. Studies show that intervention costs for TBC can range from $35 to $1350 per person per year (mean, $618; median, $428).27,56 One analysis, based on 20 studies comparing TBC to usual care, calculated an intervention cost of $284 per person per year,55 while another study showed an intervention cost of $525 per enrollee per year.56 Intervention cost can vary by the type of provider that is used, the amount of time spent per patient, and the setting where services are provided. Overall, the intervention cost of implementing TBC for hypertension management is consistently higher than the cost of usual care.
Health care cost is another factor to consider. It is the difference in the cost of health care products and services that are utilized in the process of TBC, as compared to care that is provided in the absence of TBC. Health care costs include the costs associated with hospitalizations, outpatient visits, emergency room visits, and medications. One study estimated a median health care cost of hypertension TBC of $65 per person per year.55 Overall, studies evaluating the impact of TBC for hypertension management on health care costs were mixed, with some showing that TBC resulted in an increase in health care cost, and others showing a savings compared to usual care.58 The variability in health care costs was due to the different number of health care components and comorbidities of the patients included in the studies. Also, study duration affected the estimated health care costs of TBC. Most studies did not assess long-term health care cost savings that could be achieved from prolonged blood pressure control.58 When considering both intervention and health care cost, Jacob et al estimated that TBC increased overall net cost by a median value of $329 per person per year.55 While some studies did attribute an overall reduction in health care costs to TBC for hypertension management, on average, team-based models increased health care costs compared to usual care.27,29,55,58,59
However, health care costs do not take into account the long-term reductions in morbidity and mortality or increased quality-adjusted life years (QALY) that result from improved blood pressure control attributed to TBC. In most cost-effectiveness studies, an intervention is considered to be cost-effective if the cost per QALY gained is less than the accepted threshold of $50,000.55 One study estimated that the cost per QALY of TBC in hypertension management is $4763,55,60 while another study estimated a median cost per QALY of $9716 to $13,992.55 A systematic review of 34 international studies estimated the median cost per QALY to be $13,986, ranging from $6683 to $58,610.57 The wide range in cost can be attributed to the variability in interventions, health outcomes used to measure effectiveness, and the settings and countries where the studies were conducted. In another study, a TBC intervention involving pharmacists resulted in a cost per QALY of $26,800.61 The intervention was found to be cost-effective for higher-risk patients, defined as those having diabetes, a smoking history, dyslipidemia, or obesity. For patients who did not have these risk factors, the cost per QALY increased to $43,330.61 Thus, the patient population should be considered before implementing a TBC model. Furthermore, the increased use of technology, allowing for more efficient provision of services and communication between providers, could reduce intervention costs and lead to increased cost efficacy in these models.
The variation in the models used for TBC makes it difficult to draw conclusions on the cost-effectiveness of these interventions. Although it is apparent that TBC in general is cost-effective, more studies are needed comparing different team-based models to determine which specific ones are most cost-effective.
Challenges to Implementation of Team-Based Care
Recognizing and addressing the challenges inherent to a TBC approach is important to the sustainability of such a model within various settings and institutions. Numerous studies conducted on team-based models have identified common challenges that appear to be consistent across multiple settings. These challenges can be categorized as financial, provider-specific, and technology.
Financial Barriers
Although studies have demonstrated the cost-effectiveness of controlling hypertension and preventing serious complications, health systems are still confronted with the challenge of covering the cost for TBC implementation and maintenance.29 The 2 main financial barriers for TBC services are stakeholder engagement and reimbursement for services. According to Kennelty et al, stakeholder engagement is key to the sustainability of the service.27 However, decisions by stakeholders on cost are influenced by many factors, which include available funds, perceived value, and estimates for return on investment. Additionally, interventions must align with the organization’s mission and vision and be feasible to implement, and organizations must have the capacity for administrative support.29 These various financial decisions may greatly influence the sustainability of a TBC model.
The reimbursement challenges for individual providers are an additional barrier to the sustainability of the service. In the United States, most providers are reimbursed via fee-for-service payment plans, but these plans do not reimburse all clinical providers because they are not all recognized as licensed providers.62,63 For example, pharmacists are not recognized by the Centers for Medicare & Medicaid Services as licensed health care providers, which limits their ability to be reimbursed for clinical services provided outside of a traditional dispensing role. Furthermore, state laws determine the services nonphysician providers can offer and how they are recognized for reimbursement by tertiary payers. For instance, pharmacist roles, such as ordering labs and modifying or prescribing medication regimens, vary greatly between states.7,63,64
Financial barriers are a major challenge facing the sustainability of a TBC hypertension service, so including all stakeholders in the decision-making process may improve the organization’s ability to sustain the service.
Provider-Specific Barriers
Notable barriers that are attributed to providers include lack of knowledge, lack of time, lack of initiative to change blood pressure medications, and inability to reach intensive blood pressure goals set in guidelines.29 Studies such as the SPRINT trial have significantly impacted clinical guideline cut-offs for blood pressure, but reaching the intensive blood pressure goals from clinical trials is difficult to emulate in clinical practice.65 In a typical clinical setting, providers may lack the confidence to make adjustments in therapy based on a single blood pressure measurement, and clinical inertia, defined as failure of health care providers to modify therapy when indicated,66 may contribute to the inability to achieve blood pressure goals. Many factors contribute to clinical inertia, including lack of knowledge, time, or clinical protocols on how to modify therapy, causing providers to delay clinical decisions. Implementing site-specific protocols and utilizing hypertension specialist health care professionals in TBC can address the barriers contributing to clinical inertia.
Technology Barriers
A common barrier in a variety of services, but especially prevalent in a TBC service, is access to an electronic health record (EHR) for all providers treating the patient. Some providers who are not directly tied to the same clinical site as the patient’s primary care provider may not have adequate access to the full EHR. For example, pharmacists who are managing hypertension in a TBC model in a community pharmacy may have access only to health information from prescription records. Patient interviews may not provide the pharmacist with adequate information about laboratory results, vitals, and other medical information and history for the patient, making it difficult for the pharmacist to make a proper recommendation for treatment.27 Depending on the setting, communication between providers may be a barrier in achieving optimal outcomes, especially when providers do not have access to a shared medical record.
In addition, patients often lack access to technology used to manage hypertension. Many new technologies exist that aid patients in managing their blood pressure, such as smart phone applications to track blood pressure readings and alarms to remind patients to take their medications. Studies have shown that telemonitoring of blood pressure measurements and management of hypertension, especially in combination with TBC, is effective and reduces costs compared to usual care.67 However, the lack of equal access to the various technologies available may inhibit the success of a TBC hypertension program. Patients may lack access, knowledge, or financial means to utilize the various methods available for managing their hypertension electronically.29
Conclusion
Incorporating nonphysician providers into the health care team for the treatment of hypertension has proven to be more effective than usual care and has been recognized by recent guidelines as a best practice approach to achieving blood pressure goals. Multiple studies have demonstrated that TBC utilizing nurses and pharmacists can improve blood pressure management. While adding members to the team increases health care costs, the long-term benefits of achieving optimal blood pressure goals contribute to the overall cost-effectiveness of TBC strategies over usual care. However, comparisons between different TBC models are warranted to determine which combination of health care professionals and/or interventions is most effective. Cost-analysis estimates are difficult to compare due to widely varied methodology and variance in the models that have been employed. Studies must consider pathways to overcoming reimbursement issues, provider-specific challenges, and technology barriers. Follow-up and monitoring after initiation of drug therapy for hypertension control should include systematic strategies to help improve blood pressure, including use of home blood pressure monitoring, TBC, and telehealth strategies. Future implementation science approaches to hypertension TBC models within specific clinic settings will be useful to identify and overcome challenges and will help to determine the populations who will benefit most, allowing for greater success in sustaining TBC models.
Corresponding author: Shawn R. Smith, PharmD, 309 E. 2nd Street, Pomona, CA 91766; shawnsmith@westernu.edu.
Financial disclosures: None.
1. Fryar CD, Ostchega Y, Hales CM, et al. Hypertension prevalence and control among adults: United States, 2015–2016. NCHS Data Brief. 2017(289):1-8.
2. Ambrosius WT, Sink KM, Foy CG, et al. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: The Systolic Blood Pressure Intervention Trial (SPRINT). Clin Trials. 2014;11:532-546.
3. Lawes CM, Bennett DA, Feigin VL, Rodgers A. Blood pressure and stroke: an overview of published reviews. Stroke. 2017;35:776-785.
4. Zanchetti A, Thomopoulos C, Parati G. Randomized controlled trials of blood pressure lowering in hypertension: A critical reappraisal. Circ Res. 2015;116:1058-1073.
5. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/ AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127-e248.
6. Grol R. Successes and failures in the implementation of evidence-based guidelines for clinical practice. Med Care. 2001;39:II46-II54.
7. Brush JE, Handberg EM, Biga C, et al. 2015 ACC health policy statement on cardiovascular team-based care and the role of advanced practice providers. J Am Coll Cardiol. 2015;65:2118-2136.
8. Centers for Disease Control and Prevention. Best practices for cardiovascular disease prevention programs: a guide to effective health care system interventions and community programs linked to clinical services, promoting team-based care to improve high blood pressure control. www.cdc.gov/dhdsp/pubs/guides/best-practices/team-based-care.htm. Accessed April 30, 2020.
9. Centers for Disease Control and Prevention. Task Force recommends team-based care for improving blood pressure [press release]. May 15, 2012. www.cdc.gov/media/releases/2012/p0515_bp_control.html
10. Mitchell P, Wynia M, Golden R, et al. Core principles & values of effective team-based health care. 2012. Institute of Medicine, Washington, DC.
11. Campbell SM, Hann M, Hacker J, et al. Identifying predictors of high-quality care in English general practice: observational study. BMJ. 2001;323:784-787.
12. Shojania KG, Ranji SR, McDonald KM, et al. Effects of quality improvement strategies for type 2 diabetes on glycemic control: a meta-regression analysis. JAMA. 2006;296:427-440.
13. Walsh JM, McDonald KM, Shojania KG, et al. Quality improvement strategies for hypertension management: a systematic review. Med Care. 2006;44:646-657.
14. Wagner E. The role of patient care teams in chronic disease management. BMJ. 2000;320:560-572.
15. Coleman K, Reid R. Continuous and team-based healing relationships: improving patient care through teams. In: Phillips KE, Weir V, eds. Safety Net Medical Home Initiative Implementation Guide Series. 2nd ed. Seattle, WA: Qualis Health and The MacColl Center for Health Care Innovation at the Group Health Research Institute; 2013.
16. Sinsky CA, Willard-Grace R, Schutzbank AM, et al. In search of joy in practice: a report of 23 high-functioning primary care practices. Ann Fam Med. 2013;11:272-278
17. Howard J, Etz RS, Crocker JB, et al. Maximizing the patient-centered medical home (PCMH) by choosing words wisely. J Am Board Fam Med. 2016;29:248-253.
18. Solberg LI, Crain AL, Tillema JO, et al. Challenges of medical home transformation reported by 118 patient-centered medical home (PCMH) leaders. J Am Board Fam Med. 2014;27:449-457.
19. Crabtree BF, Chase SM, Wise CG, et al. Evaluation of patient centered medical home practice transformation initiatives. Med Care. 2011;49:10-16.
20. Carter BL. Blood pressure control—implementing a team approach. US Cardiol. 2011;8:108-113.
21. Carter BL, Rogers M, Daly J, et al. The potency of team-based care interventions for hypertension: a meta-analysis. Arch Intern Med. 2009;169:1748-1755.
22. Proia KK, Thota AB, Njie GJ, et al. Team-based care and improved blood pressure control: a community guide systematic review. Am J Prev Med. 2014;47:86-99.
23. Santschi V, Chiolero A, Colosimo AL, et al. Improving blood pressure control through pharmacist interventions: a meta-analysis of randomized controlled trials. J Am Heart Assoc. 2014;3:e000718.
24. Shaw RJ, McDuffie JR, Hendrix CC, et al. Effects of nurse-managed protocols in the outpatient management of adults with chronic conditions: a systematic review and meta-analysis. Ann Intern Med. 2014;161:113-121.
25. Carter BL, Coffey CS, Ardery G, et al. Cluster-randomized trial of a physician/pharmacist collaborative model to improve blood pressure control. Circ Cardiovasc Qual Outcomes. 2015;8:235-243
26. Carter BL, Bosworth HB, Green BB. The hypertension team: the role of the pharmacist, nurse and teamwork in hypertension therapy. J Clin Hypertens. 2012;14:51-65.
27. Kennelty KA, Polgreen LA, Carter BL. Team-based care with pharmacists to improve blood pressure: a review of recent literature. Curr Hypertens Rep. 2018;20:1.
28. Brownstein JN, Chowdhury FM, Norris SL, et al. Effectiveness of community health workers in the care of people with hypertension. Am J Prev Med. 2007;32:435-447.
29. Derington CG, King JB, Bryant KB, et al. Cost-effectiveness and challenges of implementing intensive blood pressure goals and team-based care. Curr Hypertens Rep. 2019;21:91.
30. Mills KT, Obst KM, Shen W, et al. Comparative effectiveness of implementation strategies for blood pressure control in hypertensive patients: a systematic review and meta-analysis. Ann Intern Med. 2018;168:110-120.
31. Clark CE, Smith LFP, Taylor RS, et al. Nurse led interventions to improve control of blood pressure in people with hypertension: systematic review and meta-analysis. BMJ. 2010;341:c3995.
32. Santschi V, Chiolero A, Burnand B, et al. Impact of pharmacist care in the management of cardiovascular disease risk factors: a systematic review and meta-analysis of randomized trials. Arch Intern Med. 2011;171:1441-1453.
33. Dennison Himmelfarb CR, Commodore-Mensah Y, Hill MN. Expanding the role of nurses to improve hypertension care and control globally. Ann Glob Health. 2016;82:243-253.
34. Clark CE, Smith LFP, Taylor RS, Campbell JL. Nurse-led interventions used to improve control of high blood pressure in people with diabetes: a systematic review and meta-analysis. DiabetMed. 2011;28:250-261.
35. Zhu X, Wong FKY, Wu CLH. Development and evaluation of a nurse-led hypertension management model: A randomized controlled trial. Int J Nurs Stud. 2018;77:171-178.
36. Spruill TM, Williams O, Teresi JA, et al. Comparative effectiveness of home blood pressure telemonitoring (HBPTM) plus nurse case management versus HBPTM alone among Black and Hispanic stroke survivors: study protocol for a randomized controlled trial. Trials. 2015;16:97.
37. Ogedegbe G. Comparative effectiveness of home BP telemonitoring plus nurse case management (HBPTM+NCM) versus HBPTM alone on systolic BP (SBP) reduction among minority stroke survivors. International Stroke Conference 2020; February 19-21, 2020; Los Angeles, CA. Abstract LB19.
38. Dunn SP, Birtcher KK, Beavers CJ, et al. The role of the clinical pharmacist in the care of patients with cardiovascular disease. J Am Coll Cardiol. 2015;66:2129-2139.
39. Santschi V, Chiolero A, Paradis G et al. Pharmacist interventions to improve cardiovascular disease risk factors in diabetes: a systematic review and meta-analysis of randomized controlled trials. Diabetes Care. 2012;35:2706-2717.
40. Anderegg MD, Gums TH, Uribe L, et al. Pharmacist intervention for blood pressure control in patients with diabetes and/or chronic kidney disease. Pharmacotherapy. 2018;38:309-318.
41. Anderegg MD, Gums TH, Uribe L et al. Physician-pharmacist collaborative management: narrowing the socioeconomic blood pressure gap. Hypertension. 2016;68:1314-1320.
42. Sisson EM, Dixon DL, Kildow DC, et al. Effectiveness of a pharmacist-physician team-based collaboration to improve long-term blood pressure control at an inner-city safety-net clinic. Pharmacotherapy. 2016;36:342-347.
43. Dixon DL, Sisson EM, Parod ED, et al. Pharmacist-physician collaborative care model and time to goal blood pressure in the uninsured population. J Clin Hypertens (Greenwich). 2018;20:88-95.
44. Dixon DL, Parod ED, Sisson EM et al. Impact of a pharmacist-physician collaborative care model on time-in-therapeutic blood pressure range in patients with hypertension. J Am Coll Clin Pharm. 2020;3:404-409.
45. Tsuyuki RT, Houle SK, Charrois TL, et al. Randomized trial of the effect of pharmacist prescribing on improving blood pressure in the community: the Alberta Clinical Trial in Optimizing Hypertension (RxACTION). Circulation. 2015;132:93-100.
46. Tsuyuki RT, Al Hamarneh YN, Jones CA, et al. The effectiveness of pharmacist interventions on cardiovascular risk: The Multicenter Randomized Controlled RxEACH trial. J Am Coll Cardiol. 2016;67:2846-2854.
47. Victor RG, Lynch K, Li N, et al. A cluster-randomized trial of blood-pressure reduction in black barbershops. N Engl J Med. 2018;378:1291-1301.
48. Victor RG, Blyler CA, Li N et al. Sustainability of blood pressure reduction in black barbershops. Circulation. 2019;139:10-19.
49. Panattoni L, Hurlimann L, Wilson C, et al. Workflow standardization of a novel team care model to improve chronic care: a quasi-experimental study. BMC Health Serv Res. 2017;17:286.
50. Chang AR, Bonaparte H, Yule C. Randomized controlled trial comparing a self-guided vs. dietitian-led approach using web-based tools to lower blood pressure: study design and rationale. International Stroke Conference 2020; February 19-21, 2020; Los Angeles, CA. Abstract P169.
51. Stephen C, Halcomb E, Mcinnes S, et al. Improving blood pressure control in primary care: The ImPress study. Int J Nurs Stud. 2019;95:28-33.
52. He J, Shi X, Lin M. Comparative effectiveness of implementation strategies on cardiovascular risk factor control in patients with diabetes: The D4C cluster randomized trial. International Stroke Conference 2020; February 19-21, 2020; Los Angeles, CA. Abstract 17.
53. Jafar TH, Gandhi M, de Silva HA, et al. A community-based intervention for managing hypertension in rural South Asia. N Engl J Med. 2020;382:717-726.
54. Centers for Disease Control and Prevention. Promoting team-based care to improve high blood pressure control. www.cdc.gov/dhdsp/pubs/guides/best-practices/team-based-care.htm. Accessed April 30, 2020.
55. Jacob V, Chattopadhyay SK, Thota AB, et al. Economics of team-based care in controlling blood pressure: a community guide systematic review. Am J Prev Med. 2015;49:772-783.
56. Dehmer SP, Baker-Goering MM, Maciosek MV, et al. Modeled health and economic impact of team-based care for hypertension. Am J Prev Med. 2016;50(5 suppl 1):S34-S44.
57. Zhang D, Wang G, Joo H. A systematic review of economic evidence on community hypertension interventions. Am J Prev Med. 2017;53:S121-S130.
58. Community Preventive Services Task Force. Cardiovascular disease: team-based care to improve blood pressure control. 2011. www.thecommunityguide.org/findings/cardiovascular-disease-team-based-care-improve-blood-pressure-control. Accessed April 30, 2020.
59. Kulchaitanaroaj P, Brooks JM, Ardery G et al. Incremental costs associated with physician and pharmacist collaboration to improve blood pressure control. Pharmacotherapy. 2012;32:772-780.
60. Mason JM, Freemantle N, Gibson JM, New JP. Specialist nurse-led clinics to improve control of hypertension and hyperlipidemia in diabetes. Diabetes Care. 2005;28:40-46.
61. Kulchaitanaroaj P, Brooks JM, Chaiyakunapruk N et al. Cost-utility analysis of physician-pharmacist collaborative intervention for treating hypertension compared with usual care. J Hypertens. 2017;35:178-187.
62. Lall D, Engel N, Devadasan N, et al. Models of care for chronic conditions in low/middle-income countries: a ‘best fit’ framework synthesis. BMJ Glob Health. 2018;3:e001077.
63. Bodenheimer T, Chen E, Bennett HD. Confronting the growing burden of chronic disease: can the U.S. health care workforce do the job? Health Aff (Millwood). 2009;28:64-74.
64. Smith M, Bates DW, Bodenheimer T, Cleary PD. Why pharmacists belong in the medical home. Health Aff (Millwood). 2010;29:906-913.
65. Wright JT, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
66. Phillips LS, Branch WT, Cook CB, et al. Clinical inertia. Ann Intern Med. 2001;135:825-834.
67. McManus RJ, Mant J, Franssen M, et al. Efficacy of self-monitored blood pressure, with or without telemonitoring, for titration of antihypertensive medication (TASMINH4): an unmasked randomised controlled trial. Lancet. 2018;391:949-959.
68. Tucker KL, Sheppard JP, Stevens R, et al. Self-monitoring of blood pressure in hypertension: a systematic review and individual patient data meta-analysis. PLoS Med. 2017;14:e1002389.
69. Casey DE, Thomas RJ, Bhalla V, et al. 2019 AHA/ACC clinical performance and quality measures for adults with high blood pressure: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures. J Am Coll Cardiol. 2019;74:2661-2706.
From Western University of Health Sciences College of Pharmacy, Department of Pharmacy Practice and Administration, Pomona, CA.
Abstract
- Objective: To review the current literature regarding the clinical effectiveness and cost-effectiveness of implementing hypertension team-based care (TBC) interventions in the outpatient setting, and discuss challenges to implementation.
- Methods: A literature review was conducted of meta-analyses, systematic reviews, and randomized controlled trials comparing TBC models to usual care for hypertension management.
- Results: Compared to usual care, TBC models have demonstrated greater blood pressure reductions and improved blood pressure control rates. Evidence was strongest for models involving nurses and pharmacists whose roles included medication management, patient education and counseling, coordination of care and follow-up, population health management, and performance measurement with quality improvement. Although TBC results in an increase in health care costs, the overall long-term benefits support the cost-effectiveness of these models over usual care. The most common barriers to TBC implementation include underutilization of technology, stakeholder engagement, and reimbursement issues.
- Conclusion: Hypertension TBC models have been shown to be clinically effective and cost-effective, but continued research comparing different models is warranted to determine which combination of health professionals and interventions is most impactful and cost-effective in practice. An implementation science approach, in which TBC models unique to each organization’s situation are created, will be useful to identify and overcome challenges and provide a solid foundation for sustainment.
Keywords: blood pressure; pharmacist; nurse; nurse practitioner; cost-effectiveness; team-based care.
Approximately 1 in 3 US adults—or about 100 million people—have high blood pressure, and only about half (48%) have their blood pressure under control.1 Effective blood pressure management has been shown to decrease the incidence of stroke, heart attack, and heart failure.2-4 The American College of Cardiology/American Heart Association (ACC/AHA) 2017 blood pressure guidelines recommended lower thresholds for diagnosing hypertension and initiating antihypertensive medication, and intensified the blood pressure goal to less than 130/80 mm Hg.5 Changing practice standards to more intensive blood pressure goals requires significant adjustments by clinicians and health care systems. In fact, new guideline uptake is often delayed, ignored, or sparsely applied.6 Due to this dramatic change in hypertension practice standards, the ACC/AHA guidelines support interdisciplinary team-based care (TBC) for hypertension management.5,7 Additionally, the Centers for Disease Control and Prevention (CDC) and the Community Preventive Services Task Force (CPSTF) promote TBC to improve blood pressure control in their initiatives to prevent heart disease and stroke.8,9
The National Academy of Medicine defines TBC as “the provision of health services to individuals, families, and/or their communities by at least 2 healthcare providers who work collaboratively with patients and their caregivers—to the extent preferred by each patient—to accomplish shared goals within and across settings to achieve coordinated, high-quality care.”10 Specific goals for TBC in hypertension treatment are listed in Table 1, and a checklist of key elements of TBC to consider before implementation are presented in Table 2.
TBC has been shown to have many advantages, including increased access to care due to expanded hours of operation and shorter wait times.11 Team-based models also provide effective and efficient delivery of patient education, behavioral health care, and care coordination.12-14 Patients are more likely to receive high-quality care when multiple providers, each with varied expertise, are on the health care team.11,15 Furthermore, clinicians report improved professional job satisfaction related to their ability to practice in environments where they are encouraged to work at the top of their licenses.16 Consequently, TBC has been accepted as a vital part of the patient-centered medical home (PCMH) model.17-19 Standards set by the National Committee for Quality Assurance (NCQA) include TBC as a requirement health systems must meet in order to achieve the highest level of PCMH recognition. While a team-based approach offers substantial benefits and is recognized as a marker of quality, implementation has presented various challenges, and the sustainability of these models in care settings has been questioned.20
In this article, we review the current literature regarding the clinical effectiveness and cost-effectiveness of implementing hypertension TBC interventions in the outpatient setting. We also discuss the challenges and opportunities of implementing this strategy in health systems and community settings in the United States.
Evidence of Impact and Effectiveness
Various models of hypertension TBC have been shown to increase the proportion of individuals with controlled blood pressure and to lead to a reduction in both systolic (SBP) and diastolic blood pressure (DBP), resulting in a strong recommendation for TBC approaches by the 2017 ACC/AHA blood pressure guidelines.5,21-25 There is great diversity in the types of hypertension treatment models studied, with few utilizing physician specialists and most utilizing nonphysician providers, such as community health workers, physician assistants, nurses, nurse practitioners, dietitians, social workers, and pharmacists.22,26-29 These professionals share duties of hypertension management with primary care physicians to reduce the burden of responsibility for care on any single provider type. TBC is patient-centered, and typically includes interprofessional collaboration, treatment algorithms, adherence counseling, frequent follow-up, home blood pressure monitoring, and patient self-management education.
Numerous studies have supported implementation of TBC in recent years. A systematic review and meta-analysis of 100 trials of hypertension TBC involving 55,920 patients concluded that the most effective blood pressure–lowering strategies use multilevel, multicomponent approaches to address barriers to hypertension control. Nonphysician providers are often involved in measuring blood pressure, ordering and assessing laboratory tests, and titrating medications.30 Compared with usual care, TBC with physician medication titration resulted in reductions in mean SBP and DBP (6.2 mm Hg and 2.7 mm Hg, respectively), while TBC with nonphysician medication titration also resulted in reductions in mean SBP and DBP (7.1 mm Hg and 3.1 mm Hg, respectively). Nurses and pharmacists are specifically mentioned by the 2017 ACC/AHA blood pressure guidelines as essential members of the hypertension treatment team.5 Randomized controlled trials (RCTs) and meta-analyses of TBC involving nurse or pharmacist interventions demonstrated greater reductions in SBP and/or greater attainment of blood pressure goals compared to usual care.21,26,31,32 The literature supports the roles of nurses and pharmacists in hypertension management in all aspects of care, including medication management, patient education and counseling, coordination of care and follow-up, population health management, and performance measurement with quality improvement.33
Nurses
Nurses are commonly part of TBC hypertension management programs. One meta-analysis and systematic review of international RCTs compared nurse, nurse prescriber (United Kingdom), and nurse practitioner interventions for hypertension with usual care. Interventions that included a stepped treatment algorithm and nurse prescribing showed greater reductions in SBP (8.2 mm Hg and 8.9 mm Hg, respectively) compared to usual care.31 Similarly, models that utilized telephone monitoring demonstrated greater achievement of blood pressure targets, while those that involved home monitoring showed significant reductions in blood pressure. Another international meta-analysis and systematic review of 11 nurse-led interventions in hypertensive patients with diabetes demonstrated a 5.8 mm Hg mean decrease in SBP compared to physician-led care. However, nurse-led care was not superior in achievement of study targets.34
A recent meta-analysis and systematic review, performed by Shaw and colleagues, sought to determine whether nurse-led protocols are effective for outpatient management of adults with diabetes, hypertension, and hyperlipidemia. All of the included studies involved a registered nurse who titrated medications by following a protocol, and most were RCTs comparing the nurse protocols to usual care. Overall, mean SBP and DBP decreased by 3.86 mm Hg and 1.56 mm Hg, respectively, while blood glucose and lipid levels were also reduced compared to usual care.24
Limited RCT data have been published since the Shaw et al meta-analysis. A single-blind RCT was performed in an urban community health care center in China among patients with uncontrolled blood pressure (SBP ≥ 140 mm Hg and/or DBP ≥ 90 mm Hg).35 The study group received care via a nurse-led model, which included a delivery design system, decision support, clinical information system, and self-management support, and the control group received usual care. At 12 weeks, patients in the study group had significantly lower blood pressure than control patients, with mean SBP/DBP reduction of 14.37/7.43 mm Hg and 5.10/2.69 mm Hg, respectively (P < 0.01). Improved medication adherence and increased patient satisfaction were other benefits of the nurse-led model.
Nurse case managers (NCM) also play a critical role in hypertension management, coordinating health care services to meet patient health needs. Ogedegbe sought to evaluate the comparative effectiveness of home blood pressure telemonitoring (HBPTM)+NCM versus HBPTM alone on SBP reduction in black and Hispanic stroke survivors.36,37 NCMs evaluated patient profiles, counseled patients on target lifestyle behaviors, and reviewed home blood pressure data. At 6 months, SBP declined by 13.63 mm Hg from baseline in the HBPTM+NCM group and 6.31 mm Hg in the HBPTM alone group (P < 0.0001). At 12 months, SBP in the HBPTM+NCM group declined by 14.76 mm Hg, while blood pressure in the HBPTM alone group declined by 5.53 mm Hg (P < 0.0001).
Pharmacists
Clinical pharmacists are also widely utilized in TBC models for hypertension management. Typical models involve pharmacists entering into collaborative practice agreements with physicians, leading to optimization of medications, avoidance of adverse drug events, and transitional care activities focusing on medication reconciliation and patient education in outpatient settings.30,38 The largest and most recent meta-analysis of pharmacist interventions, conducted in 2014 by Santschi et al,23 combined 2 previous systematic reviews to include a total of 39 RCTs with 14,224 patients.32,39 Pharmacist interventions included patient education, recommendations to physicians, and medication management. Compared with usual care, pharmacist interventions showed greater reductions in SBP (7.6 mm Hg) and DBP (3.9 mm Hg).23
Numerous studies substantiating the impact of pharmacist interventions on clinical outcomes have heavily influenced clinical practice and guideline development. Carter et al conducted a prospective, multi-state, cluster-randomized trial in 32 primary care clinics to evaluate whether clinics randomized to receive the pharmacist-physician collaborative care model (PPCCM) achieved better blood pressure outcomes versus clinics randomized to usual care.25 Investigators enrolled 625 patients with uncontrolled hypertension, 50% of whom had a prior diagnosis of diabetes mellitus or chronic kidney disease. The primary outcome of blood pressure control at 9 months in the intervention clinics compared to the control clinics was 43% and 34%, respectively (P = 0.059). The difference in mean SBP/DBP between the intervention and control clinics for all patients at 9 months was −6.1/−2.9 mm Hg. In a post-hoc analysis of patients with chronic kidney disease and diabetes, the pharmacist-intervention group had a significantly greater mean SBP reduction and higher blood pressure control rates compared to usual care at 9 months.40
A pre-specified secondary analysis from the Carter et al study determined that, in patients from racial minority groups, the mean SBP was 7.3 mm Hg lower in those who received the intervention compared to those in the control group (P = 0.0042).41 In patients with less than 12 years of education, those in the intervention group had a mean SBP 8.1 mm Hg lower than the SBP of those in the control group (P = 0.0001). Similar reductions in blood pressure occurred in patients with low income, Medicaid beneficiaries, or those without insurance. This study demonstrated that pharmacist interventions reduced racial and socioeconomic disparities in blood pressure treatment.
Other studies of pharmacist interventions in underserved populations have yielded positive results. In a retrospective review of uninsured patients, blood pressure control rates in a pharmacist-driven primary care clinic ranked in the 90th percentile of NCQA benchmarks, and was superior to the 2013 reported mean for commercial insurers.42 Similarly, another retrospective cohort study of a PPCCM on time to goal blood pressure in uninsured patients with hypertension showed the median time to blood pressure goal was 36 days in the PPCCM cohort versus 259 days in usual care cohorts (P < 0.001).43 A post-hoc analysis revealed the mean time-in-therapeutic blood pressure range was 46.2% ± 24.3% in the PPCCM group and 24.8% ± 27.4% in the usual care group (P < 0.0001). The blood pressure control rates at 12 months were 89% in the PPCCM group compared with 50% in the usual care group (P < 0.0001).44
Tsuyuki et al conducted the RxACTION study, a multicenter RCT evaluating the effectiveness of enhanced pharmacist care versus usual care in 23 Canadian community pharmacies and outpatient clinics following a 6-month intervention.45 Enhanced pharmacy services included pharmacist assessment of and counseling about cardiovascular disease risk and blood pressure control, review of current antihypertensive medications, and prescribing/titrating drug therapy, as needed, through independent prescriptive authority. Compared to the usual care group (n = 67), the intervention group had a reduction in SBP of 6.6 mm Hg (P = 0.006) and in DBP of 3.2 mm Hg (P = 0.01). This study expanded the pharmacists’ scope of practice, showing evidence for enhancing pharmacist roles on the hypertension care team. Tsuyuki et al also conducted the RxEACH randomized trial, which evaluated community pharmacist cardiovascular risk reduction interventions and showed an improvement in SBP and DBP, with reported results comparable to RxACTION.46
Victor et al conducted the landmark Black Barbershop Study, a cluster RCT involving 319 non-Hispanic black male patients with hypertension from 52 black-owned barbershops.47,48 Barbershops were assigned to 1 of 2 groups. The control group consisted of barbers who encouraged lifestyle modifications and made referrals to primary care providers. The intervention group had pharmacists who met regularly with participants at the barbershops and measured blood pressure, encouraged lifestyle changes, and prescribed drug therapy under collaborative practice agreements with physicians. Both groups demonstrated improvements in blood pressure outcomes, but the intervention group showed greater improvement in SBP and achievement of blood pressure goals compared to the control group. The results in the intervention group proved sustainable over the course of a year, even after the frequency of pharmacists’ visits was reduced. At 6 months, the mean SBP fell by 27.0 mm Hg (to 125.8 mm Hg) in the intervention group, as compared to a 9.3 mm Hg (to 145.4 mm Hg) reduction in the control group (P < 0.001), and blood pressure less than 130/80 mm Hg was achieved among 63.6% of the participants in the intervention group versus 11.7% in the control group (P < 0.001).
This community-level trial brought pharmacists to the barbershop and made them an essential part of the health care team through the endorsement of the barber, who the participants trusted and with whom they had a relationship. Long-standing issues related to distrust of the medical profession by this population were addressed, and trusted community barbershops were utilized as safe spaces for health care delivery. Health care professionals should consider utilizing community locations that other minority populations perceive as social centers and safe places, to reduce health disparities and barriers to care. However, models that bring care to patients need further economic and feasibility evaluations.
Other Health Care Professionals and Future Studies
In addition to models led by nurses and pharmacists, studies have also assessed models of TBC incorporating other health care professionals, including registered dietitians, medical assistants, community health workers, and health coaches (NCT02674464).49,50 Ongoing studies are also looking at the impact of TBC on underserved communities (NCT02674464, NCT03504124). Involving a variety of health care professionals with different communities and populations in TBC studies is warranted to determine the optimal settings in which to utilize different skill sets.
The Impress Study involves nurses who are assessing lifestyle risk and developing an action plan according to a standardized procedure, which may be advantageous given the degree of heterogeneity found in other TBC models.51 There are also studies underway or recently published that compare different components of TBC in order to determine which combination of TBC elements is preferred. Some of these have shown the benefits of using clinical decision-support systems (through a guideline-based treatment protocol) or training programs with ongoing support.52,53 Continued research comparing different TBC models is needed to determine which combination of health professionals and interventions is most impactful in practice.
Cost-Effectiveness
According to the CDC, TBC in hypertension management has proven to be cost-effective.54 Systematic reviews and meta-analyses assessing the cost-effectiveness of TBC in hypertension management have been conducted.26,27,29,55-58 While the general consensus supports this approach as being cost-effective, these determinations are based on studies that are widely heterogeneous. In each of these studies, different types of costs are taken into account when determining cost-effectiveness. The range of costs can be quite wide, depending on how they are calculated, making it difficult to determine the true cost-effectiveness of different TBC models.
Intervention cost is represented by the amount of money spent to implement and maintain the intervention beyond the cost of usual care or the cost without the intervention. For TBC, intervention cost consists of personnel resources such as provider time, patient time, and non-personnel resources, including rent and utilities. Studies show that intervention costs for TBC can range from $35 to $1350 per person per year (mean, $618; median, $428).27,56 One analysis, based on 20 studies comparing TBC to usual care, calculated an intervention cost of $284 per person per year,55 while another study showed an intervention cost of $525 per enrollee per year.56 Intervention cost can vary by the type of provider that is used, the amount of time spent per patient, and the setting where services are provided. Overall, the intervention cost of implementing TBC for hypertension management is consistently higher than the cost of usual care.
Health care cost is another factor to consider. It is the difference in the cost of health care products and services that are utilized in the process of TBC, as compared to care that is provided in the absence of TBC. Health care costs include the costs associated with hospitalizations, outpatient visits, emergency room visits, and medications. One study estimated a median health care cost of hypertension TBC of $65 per person per year.55 Overall, studies evaluating the impact of TBC for hypertension management on health care costs were mixed, with some showing that TBC resulted in an increase in health care cost, and others showing a savings compared to usual care.58 The variability in health care costs was due to the different number of health care components and comorbidities of the patients included in the studies. Also, study duration affected the estimated health care costs of TBC. Most studies did not assess long-term health care cost savings that could be achieved from prolonged blood pressure control.58 When considering both intervention and health care cost, Jacob et al estimated that TBC increased overall net cost by a median value of $329 per person per year.55 While some studies did attribute an overall reduction in health care costs to TBC for hypertension management, on average, team-based models increased health care costs compared to usual care.27,29,55,58,59
However, health care costs do not take into account the long-term reductions in morbidity and mortality or increased quality-adjusted life years (QALY) that result from improved blood pressure control attributed to TBC. In most cost-effectiveness studies, an intervention is considered to be cost-effective if the cost per QALY gained is less than the accepted threshold of $50,000.55 One study estimated that the cost per QALY of TBC in hypertension management is $4763,55,60 while another study estimated a median cost per QALY of $9716 to $13,992.55 A systematic review of 34 international studies estimated the median cost per QALY to be $13,986, ranging from $6683 to $58,610.57 The wide range in cost can be attributed to the variability in interventions, health outcomes used to measure effectiveness, and the settings and countries where the studies were conducted. In another study, a TBC intervention involving pharmacists resulted in a cost per QALY of $26,800.61 The intervention was found to be cost-effective for higher-risk patients, defined as those having diabetes, a smoking history, dyslipidemia, or obesity. For patients who did not have these risk factors, the cost per QALY increased to $43,330.61 Thus, the patient population should be considered before implementing a TBC model. Furthermore, the increased use of technology, allowing for more efficient provision of services and communication between providers, could reduce intervention costs and lead to increased cost efficacy in these models.
The variation in the models used for TBC makes it difficult to draw conclusions on the cost-effectiveness of these interventions. Although it is apparent that TBC in general is cost-effective, more studies are needed comparing different team-based models to determine which specific ones are most cost-effective.
Challenges to Implementation of Team-Based Care
Recognizing and addressing the challenges inherent to a TBC approach is important to the sustainability of such a model within various settings and institutions. Numerous studies conducted on team-based models have identified common challenges that appear to be consistent across multiple settings. These challenges can be categorized as financial, provider-specific, and technology.
Financial Barriers
Although studies have demonstrated the cost-effectiveness of controlling hypertension and preventing serious complications, health systems are still confronted with the challenge of covering the cost for TBC implementation and maintenance.29 The 2 main financial barriers for TBC services are stakeholder engagement and reimbursement for services. According to Kennelty et al, stakeholder engagement is key to the sustainability of the service.27 However, decisions by stakeholders on cost are influenced by many factors, which include available funds, perceived value, and estimates for return on investment. Additionally, interventions must align with the organization’s mission and vision and be feasible to implement, and organizations must have the capacity for administrative support.29 These various financial decisions may greatly influence the sustainability of a TBC model.
The reimbursement challenges for individual providers are an additional barrier to the sustainability of the service. In the United States, most providers are reimbursed via fee-for-service payment plans, but these plans do not reimburse all clinical providers because they are not all recognized as licensed providers.62,63 For example, pharmacists are not recognized by the Centers for Medicare & Medicaid Services as licensed health care providers, which limits their ability to be reimbursed for clinical services provided outside of a traditional dispensing role. Furthermore, state laws determine the services nonphysician providers can offer and how they are recognized for reimbursement by tertiary payers. For instance, pharmacist roles, such as ordering labs and modifying or prescribing medication regimens, vary greatly between states.7,63,64
Financial barriers are a major challenge facing the sustainability of a TBC hypertension service, so including all stakeholders in the decision-making process may improve the organization’s ability to sustain the service.
Provider-Specific Barriers
Notable barriers that are attributed to providers include lack of knowledge, lack of time, lack of initiative to change blood pressure medications, and inability to reach intensive blood pressure goals set in guidelines.29 Studies such as the SPRINT trial have significantly impacted clinical guideline cut-offs for blood pressure, but reaching the intensive blood pressure goals from clinical trials is difficult to emulate in clinical practice.65 In a typical clinical setting, providers may lack the confidence to make adjustments in therapy based on a single blood pressure measurement, and clinical inertia, defined as failure of health care providers to modify therapy when indicated,66 may contribute to the inability to achieve blood pressure goals. Many factors contribute to clinical inertia, including lack of knowledge, time, or clinical protocols on how to modify therapy, causing providers to delay clinical decisions. Implementing site-specific protocols and utilizing hypertension specialist health care professionals in TBC can address the barriers contributing to clinical inertia.
Technology Barriers
A common barrier in a variety of services, but especially prevalent in a TBC service, is access to an electronic health record (EHR) for all providers treating the patient. Some providers who are not directly tied to the same clinical site as the patient’s primary care provider may not have adequate access to the full EHR. For example, pharmacists who are managing hypertension in a TBC model in a community pharmacy may have access only to health information from prescription records. Patient interviews may not provide the pharmacist with adequate information about laboratory results, vitals, and other medical information and history for the patient, making it difficult for the pharmacist to make a proper recommendation for treatment.27 Depending on the setting, communication between providers may be a barrier in achieving optimal outcomes, especially when providers do not have access to a shared medical record.
In addition, patients often lack access to technology used to manage hypertension. Many new technologies exist that aid patients in managing their blood pressure, such as smart phone applications to track blood pressure readings and alarms to remind patients to take their medications. Studies have shown that telemonitoring of blood pressure measurements and management of hypertension, especially in combination with TBC, is effective and reduces costs compared to usual care.67 However, the lack of equal access to the various technologies available may inhibit the success of a TBC hypertension program. Patients may lack access, knowledge, or financial means to utilize the various methods available for managing their hypertension electronically.29
Conclusion
Incorporating nonphysician providers into the health care team for the treatment of hypertension has proven to be more effective than usual care and has been recognized by recent guidelines as a best practice approach to achieving blood pressure goals. Multiple studies have demonstrated that TBC utilizing nurses and pharmacists can improve blood pressure management. While adding members to the team increases health care costs, the long-term benefits of achieving optimal blood pressure goals contribute to the overall cost-effectiveness of TBC strategies over usual care. However, comparisons between different TBC models are warranted to determine which combination of health care professionals and/or interventions is most effective. Cost-analysis estimates are difficult to compare due to widely varied methodology and variance in the models that have been employed. Studies must consider pathways to overcoming reimbursement issues, provider-specific challenges, and technology barriers. Follow-up and monitoring after initiation of drug therapy for hypertension control should include systematic strategies to help improve blood pressure, including use of home blood pressure monitoring, TBC, and telehealth strategies. Future implementation science approaches to hypertension TBC models within specific clinic settings will be useful to identify and overcome challenges and will help to determine the populations who will benefit most, allowing for greater success in sustaining TBC models.
Corresponding author: Shawn R. Smith, PharmD, 309 E. 2nd Street, Pomona, CA 91766; shawnsmith@westernu.edu.
Financial disclosures: None.
From Western University of Health Sciences College of Pharmacy, Department of Pharmacy Practice and Administration, Pomona, CA.
Abstract
- Objective: To review the current literature regarding the clinical effectiveness and cost-effectiveness of implementing hypertension team-based care (TBC) interventions in the outpatient setting, and discuss challenges to implementation.
- Methods: A literature review was conducted of meta-analyses, systematic reviews, and randomized controlled trials comparing TBC models to usual care for hypertension management.
- Results: Compared to usual care, TBC models have demonstrated greater blood pressure reductions and improved blood pressure control rates. Evidence was strongest for models involving nurses and pharmacists whose roles included medication management, patient education and counseling, coordination of care and follow-up, population health management, and performance measurement with quality improvement. Although TBC results in an increase in health care costs, the overall long-term benefits support the cost-effectiveness of these models over usual care. The most common barriers to TBC implementation include underutilization of technology, stakeholder engagement, and reimbursement issues.
- Conclusion: Hypertension TBC models have been shown to be clinically effective and cost-effective, but continued research comparing different models is warranted to determine which combination of health professionals and interventions is most impactful and cost-effective in practice. An implementation science approach, in which TBC models unique to each organization’s situation are created, will be useful to identify and overcome challenges and provide a solid foundation for sustainment.
Keywords: blood pressure; pharmacist; nurse; nurse practitioner; cost-effectiveness; team-based care.
Approximately 1 in 3 US adults—or about 100 million people—have high blood pressure, and only about half (48%) have their blood pressure under control.1 Effective blood pressure management has been shown to decrease the incidence of stroke, heart attack, and heart failure.2-4 The American College of Cardiology/American Heart Association (ACC/AHA) 2017 blood pressure guidelines recommended lower thresholds for diagnosing hypertension and initiating antihypertensive medication, and intensified the blood pressure goal to less than 130/80 mm Hg.5 Changing practice standards to more intensive blood pressure goals requires significant adjustments by clinicians and health care systems. In fact, new guideline uptake is often delayed, ignored, or sparsely applied.6 Due to this dramatic change in hypertension practice standards, the ACC/AHA guidelines support interdisciplinary team-based care (TBC) for hypertension management.5,7 Additionally, the Centers for Disease Control and Prevention (CDC) and the Community Preventive Services Task Force (CPSTF) promote TBC to improve blood pressure control in their initiatives to prevent heart disease and stroke.8,9
The National Academy of Medicine defines TBC as “the provision of health services to individuals, families, and/or their communities by at least 2 healthcare providers who work collaboratively with patients and their caregivers—to the extent preferred by each patient—to accomplish shared goals within and across settings to achieve coordinated, high-quality care.”10 Specific goals for TBC in hypertension treatment are listed in Table 1, and a checklist of key elements of TBC to consider before implementation are presented in Table 2.
TBC has been shown to have many advantages, including increased access to care due to expanded hours of operation and shorter wait times.11 Team-based models also provide effective and efficient delivery of patient education, behavioral health care, and care coordination.12-14 Patients are more likely to receive high-quality care when multiple providers, each with varied expertise, are on the health care team.11,15 Furthermore, clinicians report improved professional job satisfaction related to their ability to practice in environments where they are encouraged to work at the top of their licenses.16 Consequently, TBC has been accepted as a vital part of the patient-centered medical home (PCMH) model.17-19 Standards set by the National Committee for Quality Assurance (NCQA) include TBC as a requirement health systems must meet in order to achieve the highest level of PCMH recognition. While a team-based approach offers substantial benefits and is recognized as a marker of quality, implementation has presented various challenges, and the sustainability of these models in care settings has been questioned.20
In this article, we review the current literature regarding the clinical effectiveness and cost-effectiveness of implementing hypertension TBC interventions in the outpatient setting. We also discuss the challenges and opportunities of implementing this strategy in health systems and community settings in the United States.
Evidence of Impact and Effectiveness
Various models of hypertension TBC have been shown to increase the proportion of individuals with controlled blood pressure and to lead to a reduction in both systolic (SBP) and diastolic blood pressure (DBP), resulting in a strong recommendation for TBC approaches by the 2017 ACC/AHA blood pressure guidelines.5,21-25 There is great diversity in the types of hypertension treatment models studied, with few utilizing physician specialists and most utilizing nonphysician providers, such as community health workers, physician assistants, nurses, nurse practitioners, dietitians, social workers, and pharmacists.22,26-29 These professionals share duties of hypertension management with primary care physicians to reduce the burden of responsibility for care on any single provider type. TBC is patient-centered, and typically includes interprofessional collaboration, treatment algorithms, adherence counseling, frequent follow-up, home blood pressure monitoring, and patient self-management education.
Numerous studies have supported implementation of TBC in recent years. A systematic review and meta-analysis of 100 trials of hypertension TBC involving 55,920 patients concluded that the most effective blood pressure–lowering strategies use multilevel, multicomponent approaches to address barriers to hypertension control. Nonphysician providers are often involved in measuring blood pressure, ordering and assessing laboratory tests, and titrating medications.30 Compared with usual care, TBC with physician medication titration resulted in reductions in mean SBP and DBP (6.2 mm Hg and 2.7 mm Hg, respectively), while TBC with nonphysician medication titration also resulted in reductions in mean SBP and DBP (7.1 mm Hg and 3.1 mm Hg, respectively). Nurses and pharmacists are specifically mentioned by the 2017 ACC/AHA blood pressure guidelines as essential members of the hypertension treatment team.5 Randomized controlled trials (RCTs) and meta-analyses of TBC involving nurse or pharmacist interventions demonstrated greater reductions in SBP and/or greater attainment of blood pressure goals compared to usual care.21,26,31,32 The literature supports the roles of nurses and pharmacists in hypertension management in all aspects of care, including medication management, patient education and counseling, coordination of care and follow-up, population health management, and performance measurement with quality improvement.33
Nurses
Nurses are commonly part of TBC hypertension management programs. One meta-analysis and systematic review of international RCTs compared nurse, nurse prescriber (United Kingdom), and nurse practitioner interventions for hypertension with usual care. Interventions that included a stepped treatment algorithm and nurse prescribing showed greater reductions in SBP (8.2 mm Hg and 8.9 mm Hg, respectively) compared to usual care.31 Similarly, models that utilized telephone monitoring demonstrated greater achievement of blood pressure targets, while those that involved home monitoring showed significant reductions in blood pressure. Another international meta-analysis and systematic review of 11 nurse-led interventions in hypertensive patients with diabetes demonstrated a 5.8 mm Hg mean decrease in SBP compared to physician-led care. However, nurse-led care was not superior in achievement of study targets.34
A recent meta-analysis and systematic review, performed by Shaw and colleagues, sought to determine whether nurse-led protocols are effective for outpatient management of adults with diabetes, hypertension, and hyperlipidemia. All of the included studies involved a registered nurse who titrated medications by following a protocol, and most were RCTs comparing the nurse protocols to usual care. Overall, mean SBP and DBP decreased by 3.86 mm Hg and 1.56 mm Hg, respectively, while blood glucose and lipid levels were also reduced compared to usual care.24
Limited RCT data have been published since the Shaw et al meta-analysis. A single-blind RCT was performed in an urban community health care center in China among patients with uncontrolled blood pressure (SBP ≥ 140 mm Hg and/or DBP ≥ 90 mm Hg).35 The study group received care via a nurse-led model, which included a delivery design system, decision support, clinical information system, and self-management support, and the control group received usual care. At 12 weeks, patients in the study group had significantly lower blood pressure than control patients, with mean SBP/DBP reduction of 14.37/7.43 mm Hg and 5.10/2.69 mm Hg, respectively (P < 0.01). Improved medication adherence and increased patient satisfaction were other benefits of the nurse-led model.
Nurse case managers (NCM) also play a critical role in hypertension management, coordinating health care services to meet patient health needs. Ogedegbe sought to evaluate the comparative effectiveness of home blood pressure telemonitoring (HBPTM)+NCM versus HBPTM alone on SBP reduction in black and Hispanic stroke survivors.36,37 NCMs evaluated patient profiles, counseled patients on target lifestyle behaviors, and reviewed home blood pressure data. At 6 months, SBP declined by 13.63 mm Hg from baseline in the HBPTM+NCM group and 6.31 mm Hg in the HBPTM alone group (P < 0.0001). At 12 months, SBP in the HBPTM+NCM group declined by 14.76 mm Hg, while blood pressure in the HBPTM alone group declined by 5.53 mm Hg (P < 0.0001).
Pharmacists
Clinical pharmacists are also widely utilized in TBC models for hypertension management. Typical models involve pharmacists entering into collaborative practice agreements with physicians, leading to optimization of medications, avoidance of adverse drug events, and transitional care activities focusing on medication reconciliation and patient education in outpatient settings.30,38 The largest and most recent meta-analysis of pharmacist interventions, conducted in 2014 by Santschi et al,23 combined 2 previous systematic reviews to include a total of 39 RCTs with 14,224 patients.32,39 Pharmacist interventions included patient education, recommendations to physicians, and medication management. Compared with usual care, pharmacist interventions showed greater reductions in SBP (7.6 mm Hg) and DBP (3.9 mm Hg).23
Numerous studies substantiating the impact of pharmacist interventions on clinical outcomes have heavily influenced clinical practice and guideline development. Carter et al conducted a prospective, multi-state, cluster-randomized trial in 32 primary care clinics to evaluate whether clinics randomized to receive the pharmacist-physician collaborative care model (PPCCM) achieved better blood pressure outcomes versus clinics randomized to usual care.25 Investigators enrolled 625 patients with uncontrolled hypertension, 50% of whom had a prior diagnosis of diabetes mellitus or chronic kidney disease. The primary outcome of blood pressure control at 9 months in the intervention clinics compared to the control clinics was 43% and 34%, respectively (P = 0.059). The difference in mean SBP/DBP between the intervention and control clinics for all patients at 9 months was −6.1/−2.9 mm Hg. In a post-hoc analysis of patients with chronic kidney disease and diabetes, the pharmacist-intervention group had a significantly greater mean SBP reduction and higher blood pressure control rates compared to usual care at 9 months.40
A pre-specified secondary analysis from the Carter et al study determined that, in patients from racial minority groups, the mean SBP was 7.3 mm Hg lower in those who received the intervention compared to those in the control group (P = 0.0042).41 In patients with less than 12 years of education, those in the intervention group had a mean SBP 8.1 mm Hg lower than the SBP of those in the control group (P = 0.0001). Similar reductions in blood pressure occurred in patients with low income, Medicaid beneficiaries, or those without insurance. This study demonstrated that pharmacist interventions reduced racial and socioeconomic disparities in blood pressure treatment.
Other studies of pharmacist interventions in underserved populations have yielded positive results. In a retrospective review of uninsured patients, blood pressure control rates in a pharmacist-driven primary care clinic ranked in the 90th percentile of NCQA benchmarks, and was superior to the 2013 reported mean for commercial insurers.42 Similarly, another retrospective cohort study of a PPCCM on time to goal blood pressure in uninsured patients with hypertension showed the median time to blood pressure goal was 36 days in the PPCCM cohort versus 259 days in usual care cohorts (P < 0.001).43 A post-hoc analysis revealed the mean time-in-therapeutic blood pressure range was 46.2% ± 24.3% in the PPCCM group and 24.8% ± 27.4% in the usual care group (P < 0.0001). The blood pressure control rates at 12 months were 89% in the PPCCM group compared with 50% in the usual care group (P < 0.0001).44
Tsuyuki et al conducted the RxACTION study, a multicenter RCT evaluating the effectiveness of enhanced pharmacist care versus usual care in 23 Canadian community pharmacies and outpatient clinics following a 6-month intervention.45 Enhanced pharmacy services included pharmacist assessment of and counseling about cardiovascular disease risk and blood pressure control, review of current antihypertensive medications, and prescribing/titrating drug therapy, as needed, through independent prescriptive authority. Compared to the usual care group (n = 67), the intervention group had a reduction in SBP of 6.6 mm Hg (P = 0.006) and in DBP of 3.2 mm Hg (P = 0.01). This study expanded the pharmacists’ scope of practice, showing evidence for enhancing pharmacist roles on the hypertension care team. Tsuyuki et al also conducted the RxEACH randomized trial, which evaluated community pharmacist cardiovascular risk reduction interventions and showed an improvement in SBP and DBP, with reported results comparable to RxACTION.46
Victor et al conducted the landmark Black Barbershop Study, a cluster RCT involving 319 non-Hispanic black male patients with hypertension from 52 black-owned barbershops.47,48 Barbershops were assigned to 1 of 2 groups. The control group consisted of barbers who encouraged lifestyle modifications and made referrals to primary care providers. The intervention group had pharmacists who met regularly with participants at the barbershops and measured blood pressure, encouraged lifestyle changes, and prescribed drug therapy under collaborative practice agreements with physicians. Both groups demonstrated improvements in blood pressure outcomes, but the intervention group showed greater improvement in SBP and achievement of blood pressure goals compared to the control group. The results in the intervention group proved sustainable over the course of a year, even after the frequency of pharmacists’ visits was reduced. At 6 months, the mean SBP fell by 27.0 mm Hg (to 125.8 mm Hg) in the intervention group, as compared to a 9.3 mm Hg (to 145.4 mm Hg) reduction in the control group (P < 0.001), and blood pressure less than 130/80 mm Hg was achieved among 63.6% of the participants in the intervention group versus 11.7% in the control group (P < 0.001).
This community-level trial brought pharmacists to the barbershop and made them an essential part of the health care team through the endorsement of the barber, who the participants trusted and with whom they had a relationship. Long-standing issues related to distrust of the medical profession by this population were addressed, and trusted community barbershops were utilized as safe spaces for health care delivery. Health care professionals should consider utilizing community locations that other minority populations perceive as social centers and safe places, to reduce health disparities and barriers to care. However, models that bring care to patients need further economic and feasibility evaluations.
Other Health Care Professionals and Future Studies
In addition to models led by nurses and pharmacists, studies have also assessed models of TBC incorporating other health care professionals, including registered dietitians, medical assistants, community health workers, and health coaches (NCT02674464).49,50 Ongoing studies are also looking at the impact of TBC on underserved communities (NCT02674464, NCT03504124). Involving a variety of health care professionals with different communities and populations in TBC studies is warranted to determine the optimal settings in which to utilize different skill sets.
The Impress Study involves nurses who are assessing lifestyle risk and developing an action plan according to a standardized procedure, which may be advantageous given the degree of heterogeneity found in other TBC models.51 There are also studies underway or recently published that compare different components of TBC in order to determine which combination of TBC elements is preferred. Some of these have shown the benefits of using clinical decision-support systems (through a guideline-based treatment protocol) or training programs with ongoing support.52,53 Continued research comparing different TBC models is needed to determine which combination of health professionals and interventions is most impactful in practice.
Cost-Effectiveness
According to the CDC, TBC in hypertension management has proven to be cost-effective.54 Systematic reviews and meta-analyses assessing the cost-effectiveness of TBC in hypertension management have been conducted.26,27,29,55-58 While the general consensus supports this approach as being cost-effective, these determinations are based on studies that are widely heterogeneous. In each of these studies, different types of costs are taken into account when determining cost-effectiveness. The range of costs can be quite wide, depending on how they are calculated, making it difficult to determine the true cost-effectiveness of different TBC models.
Intervention cost is represented by the amount of money spent to implement and maintain the intervention beyond the cost of usual care or the cost without the intervention. For TBC, intervention cost consists of personnel resources such as provider time, patient time, and non-personnel resources, including rent and utilities. Studies show that intervention costs for TBC can range from $35 to $1350 per person per year (mean, $618; median, $428).27,56 One analysis, based on 20 studies comparing TBC to usual care, calculated an intervention cost of $284 per person per year,55 while another study showed an intervention cost of $525 per enrollee per year.56 Intervention cost can vary by the type of provider that is used, the amount of time spent per patient, and the setting where services are provided. Overall, the intervention cost of implementing TBC for hypertension management is consistently higher than the cost of usual care.
Health care cost is another factor to consider. It is the difference in the cost of health care products and services that are utilized in the process of TBC, as compared to care that is provided in the absence of TBC. Health care costs include the costs associated with hospitalizations, outpatient visits, emergency room visits, and medications. One study estimated a median health care cost of hypertension TBC of $65 per person per year.55 Overall, studies evaluating the impact of TBC for hypertension management on health care costs were mixed, with some showing that TBC resulted in an increase in health care cost, and others showing a savings compared to usual care.58 The variability in health care costs was due to the different number of health care components and comorbidities of the patients included in the studies. Also, study duration affected the estimated health care costs of TBC. Most studies did not assess long-term health care cost savings that could be achieved from prolonged blood pressure control.58 When considering both intervention and health care cost, Jacob et al estimated that TBC increased overall net cost by a median value of $329 per person per year.55 While some studies did attribute an overall reduction in health care costs to TBC for hypertension management, on average, team-based models increased health care costs compared to usual care.27,29,55,58,59
However, health care costs do not take into account the long-term reductions in morbidity and mortality or increased quality-adjusted life years (QALY) that result from improved blood pressure control attributed to TBC. In most cost-effectiveness studies, an intervention is considered to be cost-effective if the cost per QALY gained is less than the accepted threshold of $50,000.55 One study estimated that the cost per QALY of TBC in hypertension management is $4763,55,60 while another study estimated a median cost per QALY of $9716 to $13,992.55 A systematic review of 34 international studies estimated the median cost per QALY to be $13,986, ranging from $6683 to $58,610.57 The wide range in cost can be attributed to the variability in interventions, health outcomes used to measure effectiveness, and the settings and countries where the studies were conducted. In another study, a TBC intervention involving pharmacists resulted in a cost per QALY of $26,800.61 The intervention was found to be cost-effective for higher-risk patients, defined as those having diabetes, a smoking history, dyslipidemia, or obesity. For patients who did not have these risk factors, the cost per QALY increased to $43,330.61 Thus, the patient population should be considered before implementing a TBC model. Furthermore, the increased use of technology, allowing for more efficient provision of services and communication between providers, could reduce intervention costs and lead to increased cost efficacy in these models.
The variation in the models used for TBC makes it difficult to draw conclusions on the cost-effectiveness of these interventions. Although it is apparent that TBC in general is cost-effective, more studies are needed comparing different team-based models to determine which specific ones are most cost-effective.
Challenges to Implementation of Team-Based Care
Recognizing and addressing the challenges inherent to a TBC approach is important to the sustainability of such a model within various settings and institutions. Numerous studies conducted on team-based models have identified common challenges that appear to be consistent across multiple settings. These challenges can be categorized as financial, provider-specific, and technology.
Financial Barriers
Although studies have demonstrated the cost-effectiveness of controlling hypertension and preventing serious complications, health systems are still confronted with the challenge of covering the cost for TBC implementation and maintenance.29 The 2 main financial barriers for TBC services are stakeholder engagement and reimbursement for services. According to Kennelty et al, stakeholder engagement is key to the sustainability of the service.27 However, decisions by stakeholders on cost are influenced by many factors, which include available funds, perceived value, and estimates for return on investment. Additionally, interventions must align with the organization’s mission and vision and be feasible to implement, and organizations must have the capacity for administrative support.29 These various financial decisions may greatly influence the sustainability of a TBC model.
The reimbursement challenges for individual providers are an additional barrier to the sustainability of the service. In the United States, most providers are reimbursed via fee-for-service payment plans, but these plans do not reimburse all clinical providers because they are not all recognized as licensed providers.62,63 For example, pharmacists are not recognized by the Centers for Medicare & Medicaid Services as licensed health care providers, which limits their ability to be reimbursed for clinical services provided outside of a traditional dispensing role. Furthermore, state laws determine the services nonphysician providers can offer and how they are recognized for reimbursement by tertiary payers. For instance, pharmacist roles, such as ordering labs and modifying or prescribing medication regimens, vary greatly between states.7,63,64
Financial barriers are a major challenge facing the sustainability of a TBC hypertension service, so including all stakeholders in the decision-making process may improve the organization’s ability to sustain the service.
Provider-Specific Barriers
Notable barriers that are attributed to providers include lack of knowledge, lack of time, lack of initiative to change blood pressure medications, and inability to reach intensive blood pressure goals set in guidelines.29 Studies such as the SPRINT trial have significantly impacted clinical guideline cut-offs for blood pressure, but reaching the intensive blood pressure goals from clinical trials is difficult to emulate in clinical practice.65 In a typical clinical setting, providers may lack the confidence to make adjustments in therapy based on a single blood pressure measurement, and clinical inertia, defined as failure of health care providers to modify therapy when indicated,66 may contribute to the inability to achieve blood pressure goals. Many factors contribute to clinical inertia, including lack of knowledge, time, or clinical protocols on how to modify therapy, causing providers to delay clinical decisions. Implementing site-specific protocols and utilizing hypertension specialist health care professionals in TBC can address the barriers contributing to clinical inertia.
Technology Barriers
A common barrier in a variety of services, but especially prevalent in a TBC service, is access to an electronic health record (EHR) for all providers treating the patient. Some providers who are not directly tied to the same clinical site as the patient’s primary care provider may not have adequate access to the full EHR. For example, pharmacists who are managing hypertension in a TBC model in a community pharmacy may have access only to health information from prescription records. Patient interviews may not provide the pharmacist with adequate information about laboratory results, vitals, and other medical information and history for the patient, making it difficult for the pharmacist to make a proper recommendation for treatment.27 Depending on the setting, communication between providers may be a barrier in achieving optimal outcomes, especially when providers do not have access to a shared medical record.
In addition, patients often lack access to technology used to manage hypertension. Many new technologies exist that aid patients in managing their blood pressure, such as smart phone applications to track blood pressure readings and alarms to remind patients to take their medications. Studies have shown that telemonitoring of blood pressure measurements and management of hypertension, especially in combination with TBC, is effective and reduces costs compared to usual care.67 However, the lack of equal access to the various technologies available may inhibit the success of a TBC hypertension program. Patients may lack access, knowledge, or financial means to utilize the various methods available for managing their hypertension electronically.29
Conclusion
Incorporating nonphysician providers into the health care team for the treatment of hypertension has proven to be more effective than usual care and has been recognized by recent guidelines as a best practice approach to achieving blood pressure goals. Multiple studies have demonstrated that TBC utilizing nurses and pharmacists can improve blood pressure management. While adding members to the team increases health care costs, the long-term benefits of achieving optimal blood pressure goals contribute to the overall cost-effectiveness of TBC strategies over usual care. However, comparisons between different TBC models are warranted to determine which combination of health care professionals and/or interventions is most effective. Cost-analysis estimates are difficult to compare due to widely varied methodology and variance in the models that have been employed. Studies must consider pathways to overcoming reimbursement issues, provider-specific challenges, and technology barriers. Follow-up and monitoring after initiation of drug therapy for hypertension control should include systematic strategies to help improve blood pressure, including use of home blood pressure monitoring, TBC, and telehealth strategies. Future implementation science approaches to hypertension TBC models within specific clinic settings will be useful to identify and overcome challenges and will help to determine the populations who will benefit most, allowing for greater success in sustaining TBC models.
Corresponding author: Shawn R. Smith, PharmD, 309 E. 2nd Street, Pomona, CA 91766; shawnsmith@westernu.edu.
Financial disclosures: None.
1. Fryar CD, Ostchega Y, Hales CM, et al. Hypertension prevalence and control among adults: United States, 2015–2016. NCHS Data Brief. 2017(289):1-8.
2. Ambrosius WT, Sink KM, Foy CG, et al. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: The Systolic Blood Pressure Intervention Trial (SPRINT). Clin Trials. 2014;11:532-546.
3. Lawes CM, Bennett DA, Feigin VL, Rodgers A. Blood pressure and stroke: an overview of published reviews. Stroke. 2017;35:776-785.
4. Zanchetti A, Thomopoulos C, Parati G. Randomized controlled trials of blood pressure lowering in hypertension: A critical reappraisal. Circ Res. 2015;116:1058-1073.
5. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/ AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127-e248.
6. Grol R. Successes and failures in the implementation of evidence-based guidelines for clinical practice. Med Care. 2001;39:II46-II54.
7. Brush JE, Handberg EM, Biga C, et al. 2015 ACC health policy statement on cardiovascular team-based care and the role of advanced practice providers. J Am Coll Cardiol. 2015;65:2118-2136.
8. Centers for Disease Control and Prevention. Best practices for cardiovascular disease prevention programs: a guide to effective health care system interventions and community programs linked to clinical services, promoting team-based care to improve high blood pressure control. www.cdc.gov/dhdsp/pubs/guides/best-practices/team-based-care.htm. Accessed April 30, 2020.
9. Centers for Disease Control and Prevention. Task Force recommends team-based care for improving blood pressure [press release]. May 15, 2012. www.cdc.gov/media/releases/2012/p0515_bp_control.html
10. Mitchell P, Wynia M, Golden R, et al. Core principles & values of effective team-based health care. 2012. Institute of Medicine, Washington, DC.
11. Campbell SM, Hann M, Hacker J, et al. Identifying predictors of high-quality care in English general practice: observational study. BMJ. 2001;323:784-787.
12. Shojania KG, Ranji SR, McDonald KM, et al. Effects of quality improvement strategies for type 2 diabetes on glycemic control: a meta-regression analysis. JAMA. 2006;296:427-440.
13. Walsh JM, McDonald KM, Shojania KG, et al. Quality improvement strategies for hypertension management: a systematic review. Med Care. 2006;44:646-657.
14. Wagner E. The role of patient care teams in chronic disease management. BMJ. 2000;320:560-572.
15. Coleman K, Reid R. Continuous and team-based healing relationships: improving patient care through teams. In: Phillips KE, Weir V, eds. Safety Net Medical Home Initiative Implementation Guide Series. 2nd ed. Seattle, WA: Qualis Health and The MacColl Center for Health Care Innovation at the Group Health Research Institute; 2013.
16. Sinsky CA, Willard-Grace R, Schutzbank AM, et al. In search of joy in practice: a report of 23 high-functioning primary care practices. Ann Fam Med. 2013;11:272-278
17. Howard J, Etz RS, Crocker JB, et al. Maximizing the patient-centered medical home (PCMH) by choosing words wisely. J Am Board Fam Med. 2016;29:248-253.
18. Solberg LI, Crain AL, Tillema JO, et al. Challenges of medical home transformation reported by 118 patient-centered medical home (PCMH) leaders. J Am Board Fam Med. 2014;27:449-457.
19. Crabtree BF, Chase SM, Wise CG, et al. Evaluation of patient centered medical home practice transformation initiatives. Med Care. 2011;49:10-16.
20. Carter BL. Blood pressure control—implementing a team approach. US Cardiol. 2011;8:108-113.
21. Carter BL, Rogers M, Daly J, et al. The potency of team-based care interventions for hypertension: a meta-analysis. Arch Intern Med. 2009;169:1748-1755.
22. Proia KK, Thota AB, Njie GJ, et al. Team-based care and improved blood pressure control: a community guide systematic review. Am J Prev Med. 2014;47:86-99.
23. Santschi V, Chiolero A, Colosimo AL, et al. Improving blood pressure control through pharmacist interventions: a meta-analysis of randomized controlled trials. J Am Heart Assoc. 2014;3:e000718.
24. Shaw RJ, McDuffie JR, Hendrix CC, et al. Effects of nurse-managed protocols in the outpatient management of adults with chronic conditions: a systematic review and meta-analysis. Ann Intern Med. 2014;161:113-121.
25. Carter BL, Coffey CS, Ardery G, et al. Cluster-randomized trial of a physician/pharmacist collaborative model to improve blood pressure control. Circ Cardiovasc Qual Outcomes. 2015;8:235-243
26. Carter BL, Bosworth HB, Green BB. The hypertension team: the role of the pharmacist, nurse and teamwork in hypertension therapy. J Clin Hypertens. 2012;14:51-65.
27. Kennelty KA, Polgreen LA, Carter BL. Team-based care with pharmacists to improve blood pressure: a review of recent literature. Curr Hypertens Rep. 2018;20:1.
28. Brownstein JN, Chowdhury FM, Norris SL, et al. Effectiveness of community health workers in the care of people with hypertension. Am J Prev Med. 2007;32:435-447.
29. Derington CG, King JB, Bryant KB, et al. Cost-effectiveness and challenges of implementing intensive blood pressure goals and team-based care. Curr Hypertens Rep. 2019;21:91.
30. Mills KT, Obst KM, Shen W, et al. Comparative effectiveness of implementation strategies for blood pressure control in hypertensive patients: a systematic review and meta-analysis. Ann Intern Med. 2018;168:110-120.
31. Clark CE, Smith LFP, Taylor RS, et al. Nurse led interventions to improve control of blood pressure in people with hypertension: systematic review and meta-analysis. BMJ. 2010;341:c3995.
32. Santschi V, Chiolero A, Burnand B, et al. Impact of pharmacist care in the management of cardiovascular disease risk factors: a systematic review and meta-analysis of randomized trials. Arch Intern Med. 2011;171:1441-1453.
33. Dennison Himmelfarb CR, Commodore-Mensah Y, Hill MN. Expanding the role of nurses to improve hypertension care and control globally. Ann Glob Health. 2016;82:243-253.
34. Clark CE, Smith LFP, Taylor RS, Campbell JL. Nurse-led interventions used to improve control of high blood pressure in people with diabetes: a systematic review and meta-analysis. DiabetMed. 2011;28:250-261.
35. Zhu X, Wong FKY, Wu CLH. Development and evaluation of a nurse-led hypertension management model: A randomized controlled trial. Int J Nurs Stud. 2018;77:171-178.
36. Spruill TM, Williams O, Teresi JA, et al. Comparative effectiveness of home blood pressure telemonitoring (HBPTM) plus nurse case management versus HBPTM alone among Black and Hispanic stroke survivors: study protocol for a randomized controlled trial. Trials. 2015;16:97.
37. Ogedegbe G. Comparative effectiveness of home BP telemonitoring plus nurse case management (HBPTM+NCM) versus HBPTM alone on systolic BP (SBP) reduction among minority stroke survivors. International Stroke Conference 2020; February 19-21, 2020; Los Angeles, CA. Abstract LB19.
38. Dunn SP, Birtcher KK, Beavers CJ, et al. The role of the clinical pharmacist in the care of patients with cardiovascular disease. J Am Coll Cardiol. 2015;66:2129-2139.
39. Santschi V, Chiolero A, Paradis G et al. Pharmacist interventions to improve cardiovascular disease risk factors in diabetes: a systematic review and meta-analysis of randomized controlled trials. Diabetes Care. 2012;35:2706-2717.
40. Anderegg MD, Gums TH, Uribe L, et al. Pharmacist intervention for blood pressure control in patients with diabetes and/or chronic kidney disease. Pharmacotherapy. 2018;38:309-318.
41. Anderegg MD, Gums TH, Uribe L et al. Physician-pharmacist collaborative management: narrowing the socioeconomic blood pressure gap. Hypertension. 2016;68:1314-1320.
42. Sisson EM, Dixon DL, Kildow DC, et al. Effectiveness of a pharmacist-physician team-based collaboration to improve long-term blood pressure control at an inner-city safety-net clinic. Pharmacotherapy. 2016;36:342-347.
43. Dixon DL, Sisson EM, Parod ED, et al. Pharmacist-physician collaborative care model and time to goal blood pressure in the uninsured population. J Clin Hypertens (Greenwich). 2018;20:88-95.
44. Dixon DL, Parod ED, Sisson EM et al. Impact of a pharmacist-physician collaborative care model on time-in-therapeutic blood pressure range in patients with hypertension. J Am Coll Clin Pharm. 2020;3:404-409.
45. Tsuyuki RT, Houle SK, Charrois TL, et al. Randomized trial of the effect of pharmacist prescribing on improving blood pressure in the community: the Alberta Clinical Trial in Optimizing Hypertension (RxACTION). Circulation. 2015;132:93-100.
46. Tsuyuki RT, Al Hamarneh YN, Jones CA, et al. The effectiveness of pharmacist interventions on cardiovascular risk: The Multicenter Randomized Controlled RxEACH trial. J Am Coll Cardiol. 2016;67:2846-2854.
47. Victor RG, Lynch K, Li N, et al. A cluster-randomized trial of blood-pressure reduction in black barbershops. N Engl J Med. 2018;378:1291-1301.
48. Victor RG, Blyler CA, Li N et al. Sustainability of blood pressure reduction in black barbershops. Circulation. 2019;139:10-19.
49. Panattoni L, Hurlimann L, Wilson C, et al. Workflow standardization of a novel team care model to improve chronic care: a quasi-experimental study. BMC Health Serv Res. 2017;17:286.
50. Chang AR, Bonaparte H, Yule C. Randomized controlled trial comparing a self-guided vs. dietitian-led approach using web-based tools to lower blood pressure: study design and rationale. International Stroke Conference 2020; February 19-21, 2020; Los Angeles, CA. Abstract P169.
51. Stephen C, Halcomb E, Mcinnes S, et al. Improving blood pressure control in primary care: The ImPress study. Int J Nurs Stud. 2019;95:28-33.
52. He J, Shi X, Lin M. Comparative effectiveness of implementation strategies on cardiovascular risk factor control in patients with diabetes: The D4C cluster randomized trial. International Stroke Conference 2020; February 19-21, 2020; Los Angeles, CA. Abstract 17.
53. Jafar TH, Gandhi M, de Silva HA, et al. A community-based intervention for managing hypertension in rural South Asia. N Engl J Med. 2020;382:717-726.
54. Centers for Disease Control and Prevention. Promoting team-based care to improve high blood pressure control. www.cdc.gov/dhdsp/pubs/guides/best-practices/team-based-care.htm. Accessed April 30, 2020.
55. Jacob V, Chattopadhyay SK, Thota AB, et al. Economics of team-based care in controlling blood pressure: a community guide systematic review. Am J Prev Med. 2015;49:772-783.
56. Dehmer SP, Baker-Goering MM, Maciosek MV, et al. Modeled health and economic impact of team-based care for hypertension. Am J Prev Med. 2016;50(5 suppl 1):S34-S44.
57. Zhang D, Wang G, Joo H. A systematic review of economic evidence on community hypertension interventions. Am J Prev Med. 2017;53:S121-S130.
58. Community Preventive Services Task Force. Cardiovascular disease: team-based care to improve blood pressure control. 2011. www.thecommunityguide.org/findings/cardiovascular-disease-team-based-care-improve-blood-pressure-control. Accessed April 30, 2020.
59. Kulchaitanaroaj P, Brooks JM, Ardery G et al. Incremental costs associated with physician and pharmacist collaboration to improve blood pressure control. Pharmacotherapy. 2012;32:772-780.
60. Mason JM, Freemantle N, Gibson JM, New JP. Specialist nurse-led clinics to improve control of hypertension and hyperlipidemia in diabetes. Diabetes Care. 2005;28:40-46.
61. Kulchaitanaroaj P, Brooks JM, Chaiyakunapruk N et al. Cost-utility analysis of physician-pharmacist collaborative intervention for treating hypertension compared with usual care. J Hypertens. 2017;35:178-187.
62. Lall D, Engel N, Devadasan N, et al. Models of care for chronic conditions in low/middle-income countries: a ‘best fit’ framework synthesis. BMJ Glob Health. 2018;3:e001077.
63. Bodenheimer T, Chen E, Bennett HD. Confronting the growing burden of chronic disease: can the U.S. health care workforce do the job? Health Aff (Millwood). 2009;28:64-74.
64. Smith M, Bates DW, Bodenheimer T, Cleary PD. Why pharmacists belong in the medical home. Health Aff (Millwood). 2010;29:906-913.
65. Wright JT, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
66. Phillips LS, Branch WT, Cook CB, et al. Clinical inertia. Ann Intern Med. 2001;135:825-834.
67. McManus RJ, Mant J, Franssen M, et al. Efficacy of self-monitored blood pressure, with or without telemonitoring, for titration of antihypertensive medication (TASMINH4): an unmasked randomised controlled trial. Lancet. 2018;391:949-959.
68. Tucker KL, Sheppard JP, Stevens R, et al. Self-monitoring of blood pressure in hypertension: a systematic review and individual patient data meta-analysis. PLoS Med. 2017;14:e1002389.
69. Casey DE, Thomas RJ, Bhalla V, et al. 2019 AHA/ACC clinical performance and quality measures for adults with high blood pressure: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures. J Am Coll Cardiol. 2019;74:2661-2706.
1. Fryar CD, Ostchega Y, Hales CM, et al. Hypertension prevalence and control among adults: United States, 2015–2016. NCHS Data Brief. 2017(289):1-8.
2. Ambrosius WT, Sink KM, Foy CG, et al. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: The Systolic Blood Pressure Intervention Trial (SPRINT). Clin Trials. 2014;11:532-546.
3. Lawes CM, Bennett DA, Feigin VL, Rodgers A. Blood pressure and stroke: an overview of published reviews. Stroke. 2017;35:776-785.
4. Zanchetti A, Thomopoulos C, Parati G. Randomized controlled trials of blood pressure lowering in hypertension: A critical reappraisal. Circ Res. 2015;116:1058-1073.
5. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/ AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127-e248.
6. Grol R. Successes and failures in the implementation of evidence-based guidelines for clinical practice. Med Care. 2001;39:II46-II54.
7. Brush JE, Handberg EM, Biga C, et al. 2015 ACC health policy statement on cardiovascular team-based care and the role of advanced practice providers. J Am Coll Cardiol. 2015;65:2118-2136.
8. Centers for Disease Control and Prevention. Best practices for cardiovascular disease prevention programs: a guide to effective health care system interventions and community programs linked to clinical services, promoting team-based care to improve high blood pressure control. www.cdc.gov/dhdsp/pubs/guides/best-practices/team-based-care.htm. Accessed April 30, 2020.
9. Centers for Disease Control and Prevention. Task Force recommends team-based care for improving blood pressure [press release]. May 15, 2012. www.cdc.gov/media/releases/2012/p0515_bp_control.html
10. Mitchell P, Wynia M, Golden R, et al. Core principles & values of effective team-based health care. 2012. Institute of Medicine, Washington, DC.
11. Campbell SM, Hann M, Hacker J, et al. Identifying predictors of high-quality care in English general practice: observational study. BMJ. 2001;323:784-787.
12. Shojania KG, Ranji SR, McDonald KM, et al. Effects of quality improvement strategies for type 2 diabetes on glycemic control: a meta-regression analysis. JAMA. 2006;296:427-440.
13. Walsh JM, McDonald KM, Shojania KG, et al. Quality improvement strategies for hypertension management: a systematic review. Med Care. 2006;44:646-657.
14. Wagner E. The role of patient care teams in chronic disease management. BMJ. 2000;320:560-572.
15. Coleman K, Reid R. Continuous and team-based healing relationships: improving patient care through teams. In: Phillips KE, Weir V, eds. Safety Net Medical Home Initiative Implementation Guide Series. 2nd ed. Seattle, WA: Qualis Health and The MacColl Center for Health Care Innovation at the Group Health Research Institute; 2013.
16. Sinsky CA, Willard-Grace R, Schutzbank AM, et al. In search of joy in practice: a report of 23 high-functioning primary care practices. Ann Fam Med. 2013;11:272-278
17. Howard J, Etz RS, Crocker JB, et al. Maximizing the patient-centered medical home (PCMH) by choosing words wisely. J Am Board Fam Med. 2016;29:248-253.
18. Solberg LI, Crain AL, Tillema JO, et al. Challenges of medical home transformation reported by 118 patient-centered medical home (PCMH) leaders. J Am Board Fam Med. 2014;27:449-457.
19. Crabtree BF, Chase SM, Wise CG, et al. Evaluation of patient centered medical home practice transformation initiatives. Med Care. 2011;49:10-16.
20. Carter BL. Blood pressure control—implementing a team approach. US Cardiol. 2011;8:108-113.
21. Carter BL, Rogers M, Daly J, et al. The potency of team-based care interventions for hypertension: a meta-analysis. Arch Intern Med. 2009;169:1748-1755.
22. Proia KK, Thota AB, Njie GJ, et al. Team-based care and improved blood pressure control: a community guide systematic review. Am J Prev Med. 2014;47:86-99.
23. Santschi V, Chiolero A, Colosimo AL, et al. Improving blood pressure control through pharmacist interventions: a meta-analysis of randomized controlled trials. J Am Heart Assoc. 2014;3:e000718.
24. Shaw RJ, McDuffie JR, Hendrix CC, et al. Effects of nurse-managed protocols in the outpatient management of adults with chronic conditions: a systematic review and meta-analysis. Ann Intern Med. 2014;161:113-121.
25. Carter BL, Coffey CS, Ardery G, et al. Cluster-randomized trial of a physician/pharmacist collaborative model to improve blood pressure control. Circ Cardiovasc Qual Outcomes. 2015;8:235-243
26. Carter BL, Bosworth HB, Green BB. The hypertension team: the role of the pharmacist, nurse and teamwork in hypertension therapy. J Clin Hypertens. 2012;14:51-65.
27. Kennelty KA, Polgreen LA, Carter BL. Team-based care with pharmacists to improve blood pressure: a review of recent literature. Curr Hypertens Rep. 2018;20:1.
28. Brownstein JN, Chowdhury FM, Norris SL, et al. Effectiveness of community health workers in the care of people with hypertension. Am J Prev Med. 2007;32:435-447.
29. Derington CG, King JB, Bryant KB, et al. Cost-effectiveness and challenges of implementing intensive blood pressure goals and team-based care. Curr Hypertens Rep. 2019;21:91.
30. Mills KT, Obst KM, Shen W, et al. Comparative effectiveness of implementation strategies for blood pressure control in hypertensive patients: a systematic review and meta-analysis. Ann Intern Med. 2018;168:110-120.
31. Clark CE, Smith LFP, Taylor RS, et al. Nurse led interventions to improve control of blood pressure in people with hypertension: systematic review and meta-analysis. BMJ. 2010;341:c3995.
32. Santschi V, Chiolero A, Burnand B, et al. Impact of pharmacist care in the management of cardiovascular disease risk factors: a systematic review and meta-analysis of randomized trials. Arch Intern Med. 2011;171:1441-1453.
33. Dennison Himmelfarb CR, Commodore-Mensah Y, Hill MN. Expanding the role of nurses to improve hypertension care and control globally. Ann Glob Health. 2016;82:243-253.
34. Clark CE, Smith LFP, Taylor RS, Campbell JL. Nurse-led interventions used to improve control of high blood pressure in people with diabetes: a systematic review and meta-analysis. DiabetMed. 2011;28:250-261.
35. Zhu X, Wong FKY, Wu CLH. Development and evaluation of a nurse-led hypertension management model: A randomized controlled trial. Int J Nurs Stud. 2018;77:171-178.
36. Spruill TM, Williams O, Teresi JA, et al. Comparative effectiveness of home blood pressure telemonitoring (HBPTM) plus nurse case management versus HBPTM alone among Black and Hispanic stroke survivors: study protocol for a randomized controlled trial. Trials. 2015;16:97.
37. Ogedegbe G. Comparative effectiveness of home BP telemonitoring plus nurse case management (HBPTM+NCM) versus HBPTM alone on systolic BP (SBP) reduction among minority stroke survivors. International Stroke Conference 2020; February 19-21, 2020; Los Angeles, CA. Abstract LB19.
38. Dunn SP, Birtcher KK, Beavers CJ, et al. The role of the clinical pharmacist in the care of patients with cardiovascular disease. J Am Coll Cardiol. 2015;66:2129-2139.
39. Santschi V, Chiolero A, Paradis G et al. Pharmacist interventions to improve cardiovascular disease risk factors in diabetes: a systematic review and meta-analysis of randomized controlled trials. Diabetes Care. 2012;35:2706-2717.
40. Anderegg MD, Gums TH, Uribe L, et al. Pharmacist intervention for blood pressure control in patients with diabetes and/or chronic kidney disease. Pharmacotherapy. 2018;38:309-318.
41. Anderegg MD, Gums TH, Uribe L et al. Physician-pharmacist collaborative management: narrowing the socioeconomic blood pressure gap. Hypertension. 2016;68:1314-1320.
42. Sisson EM, Dixon DL, Kildow DC, et al. Effectiveness of a pharmacist-physician team-based collaboration to improve long-term blood pressure control at an inner-city safety-net clinic. Pharmacotherapy. 2016;36:342-347.
43. Dixon DL, Sisson EM, Parod ED, et al. Pharmacist-physician collaborative care model and time to goal blood pressure in the uninsured population. J Clin Hypertens (Greenwich). 2018;20:88-95.
44. Dixon DL, Parod ED, Sisson EM et al. Impact of a pharmacist-physician collaborative care model on time-in-therapeutic blood pressure range in patients with hypertension. J Am Coll Clin Pharm. 2020;3:404-409.
45. Tsuyuki RT, Houle SK, Charrois TL, et al. Randomized trial of the effect of pharmacist prescribing on improving blood pressure in the community: the Alberta Clinical Trial in Optimizing Hypertension (RxACTION). Circulation. 2015;132:93-100.
46. Tsuyuki RT, Al Hamarneh YN, Jones CA, et al. The effectiveness of pharmacist interventions on cardiovascular risk: The Multicenter Randomized Controlled RxEACH trial. J Am Coll Cardiol. 2016;67:2846-2854.
47. Victor RG, Lynch K, Li N, et al. A cluster-randomized trial of blood-pressure reduction in black barbershops. N Engl J Med. 2018;378:1291-1301.
48. Victor RG, Blyler CA, Li N et al. Sustainability of blood pressure reduction in black barbershops. Circulation. 2019;139:10-19.
49. Panattoni L, Hurlimann L, Wilson C, et al. Workflow standardization of a novel team care model to improve chronic care: a quasi-experimental study. BMC Health Serv Res. 2017;17:286.
50. Chang AR, Bonaparte H, Yule C. Randomized controlled trial comparing a self-guided vs. dietitian-led approach using web-based tools to lower blood pressure: study design and rationale. International Stroke Conference 2020; February 19-21, 2020; Los Angeles, CA. Abstract P169.
51. Stephen C, Halcomb E, Mcinnes S, et al. Improving blood pressure control in primary care: The ImPress study. Int J Nurs Stud. 2019;95:28-33.
52. He J, Shi X, Lin M. Comparative effectiveness of implementation strategies on cardiovascular risk factor control in patients with diabetes: The D4C cluster randomized trial. International Stroke Conference 2020; February 19-21, 2020; Los Angeles, CA. Abstract 17.
53. Jafar TH, Gandhi M, de Silva HA, et al. A community-based intervention for managing hypertension in rural South Asia. N Engl J Med. 2020;382:717-726.
54. Centers for Disease Control and Prevention. Promoting team-based care to improve high blood pressure control. www.cdc.gov/dhdsp/pubs/guides/best-practices/team-based-care.htm. Accessed April 30, 2020.
55. Jacob V, Chattopadhyay SK, Thota AB, et al. Economics of team-based care in controlling blood pressure: a community guide systematic review. Am J Prev Med. 2015;49:772-783.
56. Dehmer SP, Baker-Goering MM, Maciosek MV, et al. Modeled health and economic impact of team-based care for hypertension. Am J Prev Med. 2016;50(5 suppl 1):S34-S44.
57. Zhang D, Wang G, Joo H. A systematic review of economic evidence on community hypertension interventions. Am J Prev Med. 2017;53:S121-S130.
58. Community Preventive Services Task Force. Cardiovascular disease: team-based care to improve blood pressure control. 2011. www.thecommunityguide.org/findings/cardiovascular-disease-team-based-care-improve-blood-pressure-control. Accessed April 30, 2020.
59. Kulchaitanaroaj P, Brooks JM, Ardery G et al. Incremental costs associated with physician and pharmacist collaboration to improve blood pressure control. Pharmacotherapy. 2012;32:772-780.
60. Mason JM, Freemantle N, Gibson JM, New JP. Specialist nurse-led clinics to improve control of hypertension and hyperlipidemia in diabetes. Diabetes Care. 2005;28:40-46.
61. Kulchaitanaroaj P, Brooks JM, Chaiyakunapruk N et al. Cost-utility analysis of physician-pharmacist collaborative intervention for treating hypertension compared with usual care. J Hypertens. 2017;35:178-187.
62. Lall D, Engel N, Devadasan N, et al. Models of care for chronic conditions in low/middle-income countries: a ‘best fit’ framework synthesis. BMJ Glob Health. 2018;3:e001077.
63. Bodenheimer T, Chen E, Bennett HD. Confronting the growing burden of chronic disease: can the U.S. health care workforce do the job? Health Aff (Millwood). 2009;28:64-74.
64. Smith M, Bates DW, Bodenheimer T, Cleary PD. Why pharmacists belong in the medical home. Health Aff (Millwood). 2010;29:906-913.
65. Wright JT, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
66. Phillips LS, Branch WT, Cook CB, et al. Clinical inertia. Ann Intern Med. 2001;135:825-834.
67. McManus RJ, Mant J, Franssen M, et al. Efficacy of self-monitored blood pressure, with or without telemonitoring, for titration of antihypertensive medication (TASMINH4): an unmasked randomised controlled trial. Lancet. 2018;391:949-959.
68. Tucker KL, Sheppard JP, Stevens R, et al. Self-monitoring of blood pressure in hypertension: a systematic review and individual patient data meta-analysis. PLoS Med. 2017;14:e1002389.
69. Casey DE, Thomas RJ, Bhalla V, et al. 2019 AHA/ACC clinical performance and quality measures for adults with high blood pressure: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures. J Am Coll Cardiol. 2019;74:2661-2706.
An eConsults Program to Improve Patient Access to Specialty Care in an Academic Health System
From the Department of Medicine, University of California, Irvine, Orange, CA.
Abstract
Background: Orange County’s residents have difficulty accessing timely, quality, affordable specialty care services. As the county’s only academic health system, the University of California, Irvine (UCI) aimed to improve specialty care access for the communities it serves by implementing an electronic consultations (eConsults) program that allows primary care providers (PCPs) to efficiently receive specialist recommendations on referral problems that do not require an in-person evaluation.
Objective: To implement an eConsults program at the UCI that enhances access to and the delivery of coordinated specialty care for lower-complexity referral problems.
Methods: We developed custom solutions to integrate eConsults into UCI’s 2 electronic health record platforms. The impact of the eConsults program was assessed by continuously evaluating usage and outcomes. Measures used to track usage included the number of submitted eConsult requests per PCP, the number of completed responses per specialty, and the response time for eConsult requests. Outcome measures included the specialist recommendation (eg, in-office visit, consultation avoided) and physician feedback.
Results: Over 4.5 years, more than 1400 successful eConsults have been completed, and the program has expanded to 17 specialties. The average turnaround time for an eConsult response across all specialties was 1 business day. Moreover, more than 50% of the eConsults received specialty responses within the same day of the eConsult request. Most important, about 80% of eConsult requests were addressed without the need for an in-office visit with a specialist.
Conclusion: The enhanced access to and the delivery of coordinated specialty care provided by eConsults resulted in improved efficiency and specialty access, while likely reducing costs and improving patient satisfaction. The improved communication and collaboration among providers with eConsults has also led to overwhelmingly positive feedback from both PCPs and specialists.
Keywords: electronic consultation; access to care; primary care; specialty referral; telehealth.
Orange County’s growing, aging, and diverse population is driving an increased demand for health care.1 But with the county’s high cost of living and worsening shortage of physicians,1-3 many of its residents are struggling to access timely, quality, affordable care. Access to specialty care services is especially frustrating for many patients and their providers, both primary care providers (PCPs) and specialists, due to problems with the referral process. Many patients experience increased wait times for a visit with a specialist due to poor communication between providers, insufficient guidance on the information or diagnostic results needed by specialists, and lack of care coordination.4-6 One promising approach to overcome these challenges is the use of an electronic consultation, or eConsult, in place of a standard in-person referral. An eConsult is an asynchronous, non-face-to-face, provider-to-provider exchange using a secure electronic communication platform. For appropriate referral problems, the patient is able to receive timely access to specialist expertise through electronic referral by their PCP,7-9 and avoid the time and costs associated with a visit to the specialist,10,11 such as travel, missed work, co-pays, and child-care expenses. Clinical questions addressed using an eConsult system subsequently free up office visit appointment slots, improving access for patients requiring in-office evaluation.8,12
Orange County’s only academic health system, the University of California, Irvine (UCI), serves a population of 3.5 million, and its principal priority is providing the communities in the county (which is the sixth largest in United States) and the surrounding region with the highest quality health care possible. Thus, UCI aimed to improve its referral processes and provide timely access to specialty care for its patients by implementing an eConsults program that allows PCPs to efficiently receive specialist recommendations on referral problems that do not require the specialist to evaluate the patient in person. This report describes our experiences with developing and implementing a custom-built eConsults workflow in UCI’s prior electronic health record (EHR) platform, Allscripts, and subsequently transitioning our mature eConsults program to a new EHR system when UCI adopted Epic. UCI is likely the only academic medical center to have experience in successfully implementing eConsults into 2 different EHR systems.
Setting
UCI’s medical center is a 417-bed acute care hospital providing tertiary and quaternary care, ambulatory and specialty medical clinics, behavioral health care, and rehabilitation services. It is located in Orange, CA, and serves a diverse population of 3.5 million persons with broad health care needs. With more than 400 specialty and primary care physicians, UCI offers a full scope of acute and general care services. It is also the primary teaching location for UCI medical and nursing students, medical residents, and fellows, and is home to Orange County’s only adult Level I and pediatric Level II trauma centers and the regional burn center.
eConsults Program
We designed the initial eConsults program within UCI’s Allscripts EHR platform. Our information technology (IT) build team developed unique “documents-based” eConsults workflows that simplified the process of initiating requests directly from the EHR and facilitated rapid responses from participating specialties. The requesting provider’s eConsults interface was user-friendly, and referring providers were able to initiate an eConsult easily by selecting the customized eConsult icon from the Allscripts main toolbar. To ensure that all relevant information is provided to the specialists, condition-specific templates are embedded in the requesting provider’s eConsults workflow that allow PCPs to enter a focused, patient-specific clinical question and provide guidance on recommended patient information (eg, health history, laboratory results, and digital images) that may help the specialist provide an informed response. The eConsult templates were adapted from standardized forms developed by partner University of California Health Systems in an initiative funded by the University of California Center for Health Quality and Innovation.
To facilitate timely responses from specialists, an innovative notification system was created in the responding provider’s eConsults workflow to automatically send an email to participating specialists when a new eConsult is requested. The responding provider’s workflow also includes an option for the specialist to decline the eConsult if the case is deemed too complex to be addressed electronically. For every completed eConsult that does not result in an in-person patient evaluation, the requesting provider and responding specialist each receives a modest reimbursement, which was initially paid by UCI Health System funds.
Implementation
The design and implementation of the eConsults program began in November 2014, and was guided by a steering committee that included the chair of the department of medicine, chief medical information officer, primary care and specialty physician leads, IT build team, and a project manager. Early on, members of this committee engaged UCI leadership to affirm support for the program and obtain the IT resources needed to build the eConsults workflow. Regular steering committee meetings were established to discuss the design of the workflow, adapt the clinical content of the referral templates, and develop a provider reimbursement plan. After completion of the workflow build, the eConsults system was tested to identify failure points and obtain feedback from users. Prior to going live, the eConsults program was publicized by members of the steering committee through meetings with primary care groups and email communications. Committee members also hosted in-person training and orientation sessions with PCPs and participating specialists, and distributed tip sheets summarizing the steps to complete the PCP and specialist eConsult workflows.
The eConsults workflow build, testing, and launch were completed within 5 months (April 2015; Figure 1). eConsults went live in the 3 initial specialties (endocrinology, cardiology, and rheumatology) that were interested in participating in the first wave of the program. UCI’s eConsults service has subsequently expanded to 17 total specialties (allergy, cardiology, dermatology, endocrinology, gastroenterology, geriatrics, gynecology, hematology, hepatology, infectious disease, nephrology, neurology, palliative care, psychiatry, pulmonary, rheumatology, and sports medicine).
Two and half years after the eConsults program was implemented in Allscripts, UCI adopted a new EHR platform, Epic. By this time, the eConsults service had grown into a mature program with greater numbers of PCP users and submitted eConsults (Figure 2). Using our experience with the Allscripts build, our IT team was able to efficiently transition the eConsults service to the new EHR system. In contrast to the “documents-based” eConsult workflows on Allscripts, our IT team utilized an “orders-based” strategy on Epic, which followed a more traditional approach to requesting a consultation. We re-launched the service in Epic within 3 months (February 2018). However, both platforms utilized user-friendly workflows to achieve similar goals, and the program has continued to grow with respect to the number of users and eConsults.
Measurement/Analysis
The impact of the program was assessed by continuously evaluating usage and outcomes. Measures used to track usage included the number of PCP users, the number of submitted eConsult requests per PCP, and the number of requests per specialty. The response time for eConsult requests and the self-reported amount of time spent by specialists on the response were also tracked. Outcome measures included the specialist recommendation (eg, in-office visit, consultation avoided) and physician feedback. Provider satisfaction was primarily obtained by soliciting feedback from individual eConsult users.
Implementation of this eConsults program constituted a quality improvement activity and did not require Institutional Review Board review.
Results
Since the program was launched in April 2015, more than 1400 eConsults have been completed across 17 specialties (Figure 3). There were 654 completed eConsults on the Allscripts platform, and 808 eConsults have been completed using the Epic platform to date. The busiest eConsult specialties were endocrinology (receiving 276, or 19%, of the eConsults requests), hematology (receiving 249 requests, or 17%), infectious disease (receiving 244 requests, or 17% ), and cardiology (receiving 148 requests, or 10%).
The self-reported amount of time specialists spent on the response was different between the 2 EHR systems (Figure 4). On Allscripts, specialists reported that 23% of eConsults took 10 minutes or less to complete, 47% took 11 to 20 minutes, 23% took 21 to 30 minutes, and 7% took more than 30 minutes. On Epic, specialists reported that 42% of eConsults took 10 minutes or less to complete, 44% took 11 to 20 minutes, 12% took 21 to 30 minutes, and 2% took more than 30 minutes. This difference in time spent fielding eConsults likely represents the subtle nuances between Allscripts’ “documents-based” and Epic’s “orders-based” workflows.
As a result of the automated notification system that was introduced early in the eConsults implementation process on Allscripts, the specialty response times were much faster than the expected 3 business days’ turnaround goal instituted by the Center for Health Quality and Innovation initiative, regardless of the EHR platform used. In fact, the average turnaround time for an eConsult response across all specialties was 1 business day, which was similar for both EHR systems (Figure 5). Furthermore, more than 50% of the eConsults on both EHR systems received specialist responses within the same day of the eConsult request (63% on Allscripts, 54% on Epic). There was a small decrease in the percentage of same-day responses when we transitioned to Epic, likely because the functionality of an automated notification email could not be restored in Epic. Regardless, the specialty response times on Epic remained expeditious, likely because the automated notifications on Allscripts instilled good practices for the specialists, and regularly checking for new eConsult requests became an ingrained behavior.
Our most important finding was that approximately 80% of eConsult requests were addressed without the need for an in-office visit with a specialist. This measure was similar for both EHR platforms (83% on Allscripts and 78% on Epic).
Provider feedback has been overwhelmingly positive. PCPs are impressed with the robust educational content of the eConsult responses, since the goal for specialists is to justify their recommendations. Specialists appreciate the convenience and efficiency that eConsults offer, as well as the improved communication and collaboration among physicians. eConsults have been especially beneficial to PCPs at UCI’s Family Health Centers, who are now able to receive subspecialty consultations from UCI specialists despite insurance barriers.
Discussion
Our eConsults program uniquely contrasts with other programs because UCI is likely the only academic medical center to have experience in successfully incorporating eConsults into 2 different EHR systems: initial development of the eConsults workflow in UCI’s existing Allscripts EHR platform, and subsequently transitioning a mature eConsults program to a new EHR system when the institution adopted Epic.
We measured the impact of the eConsults program on access to care by the response time for eConsult requests and the percentage of eConsults that averted an in-office visit with a specialist. We found that the eConsults program at UCI provided our PCPs access to specialist consultations in a timely manner, with much shorter response times than standard in-person referrals. The average turnaround time for an eConsult response we reported is consistent with findings from other studies.12-15 Additionally, our program was able to address about 80% of its eConsults electronically, helping to reduce unnecessary in-person specialist referrals. In the literature, the percentage of eConsults that avoided an in-person specialist visit varies widely.8,12-16
We reported very positive feedback from both PCPs and specialists on UCI’s eConsults service. Similarly, other studies described PCP satisfaction with their respective eConsults programs to be uniformly high,8,9,13,14,17-19 though some reported that the level of satisfaction among specialists was more varied.18-21
Lessons Learned
The successful design and implementation of our eConsults program began with assembling the right clinical champions and technology partners for our steering committee. Establishing regular steering committee meetings helped maintain an appropriate timeline for completion of different aspects of the project. Engaging support from UCI’s leadership also provided us with a dedicated IT team that helped us with the build, training resources, troubleshooting issues, and reporting for the project.
Our experience with implementing the eConsults program on 2 different EHR systems highlighted the importance of creating efficient, user-friendly workflows to foster provider adoption and achieve sustainability. Allscripts’ open platform gave our IT team the ability to create a homegrown solution to implementing an eConsult model that was simple and easy to use. The Epic platform’s interoperability allowed us to leverage our learnings from the Allscripts build to efficiently implement eConsults in Epic.
We also found that providing modest incentive payments or reimbursements to both PCPs and specialists for each completed eConsult helps with both adoption and program sustainability. Initially, credit for the eConsult work was paid by internal UCI Health System funds. Two payers, UC Care (a preferred provider organization plan created just for the University of California) and more recently, the Centers for Medicare & Medicaid Services, have agreed to reimburse for outpatient eConsults. Securing additional payers for reimbursement of the eConsult service will not only ensure the program’s long-term sustainability, but also represents an acknowledgment of the value of eConsults in supporting access to care.
Applicability
Other health care settings that are experiencing issues with specialty care access can successfully implement their own eConsults program by employing strategies similar to those described in this report—assembling the right team, creating user-friendly workflows, and providing incentives. Our advice for successful implementation is to clearly communicate your goals to all involved, including primary care, specialists, leadership, and IT partners, and establish with these stakeholders the appropriate support and resources needed to facilitate the development of the program and overcome any barriers to adoption.
Current Status and Future Directions
Our future plans include continuing to optimize the Epic eConsult backend build and workflows using our experience in Allscripts. We have implemented eConsult workflows for use by graduate medical education trainees and advanced practice providers, with attending supervision. Further work is in progress to optimize these workflows, which will allow for appropriate education and supervision without delaying care. Furthermore, we plan to expand the program to include inpatient-to-inpatient and emergency department-to-inpatient eConsults. We will continue to expand the eConsults program by offering additional specialties, engage providers to encourage ongoing participation, and maximize PCP use by continuing to market the program through regular newsletters and email communications. Finally, the eConsults has served as an effective, important resource in the current era of COVID-19 in several ways: it allows for optimization of specialty input in patient care delivery without subjecting more health care workers to unnecessary exposure; saves on utilization of precious personal protective equipment; and enhances our ability to deal with a potential surge by providing access to specialists remotely and electronically all hours of the day, thus expanding care to the evening and weekend hours.
Acknowledgment: The authors thank our steering committee members (Dr. Ralph Cygan, Dr. Andrew Reikes, Dr. Byron Allen, Dr. George Lawry) and IT build team (Lori Bocchicchio, Meghan van Witsen, Jaymee Zillgitt, Tanya Sickles, Dennis Hoang, Jeanette Lisak-Phillips) for their contributions in the design and implementation of our eConsults program. We also thank additional team members Kurt McArthur and Neaktisia Lee for their assistance with generating reports, and Kathy LaPierre, Jennifer Rios, and Debra Webb Torres for their guidance with compliance and billing issues.
Corresponding author: Alpesh N. Amin, MD, MBA, University of California, Irvine, 101 The City Drive South, Building 26, Room 1000, ZC-4076H, Orange, CA 92868; anamin@uci.edu.
Financial disclosures: None.
1. County of Orange, Health Care Agency, Public Health Services. Orange County Health Profile 2013.
2. Coffman JM, Fix M Ko, M. California physician supply and distribution: headed for a drought? California Health Care Foundation, June 2018.
3. Spetz J, Coffman J, Geyn I. California’s primary care workforce: forecasted supply, demand, and pipeline of trainees, 2016-2030. Healthforce Center at the University of California, San Francisco, August 2017.
4. Gandhi TK, Sittig DF, Franklin M, et al. Communication breakdown in the outpatient referral process. J Gen Intern Med. 2000;15:626-631.
5. McPhee SJ, Lo B, Saika GY, Meltzer R. How good is communication between primary care physicians and subspecialty consultants? Arch Intern Med. 1984;144:1265-1268.
6. Mehrotra A, Forrest CB, Lin CY. Dropping the baton: specialty referrals in the United States. Milbank Q. 2011;89:39-68.
7. Wrenn K, Catschegn S, Cruz M, et al. Analysis of an electronic consultation program at an academic medical centre: Primary care provider questions, specialist responses, and primary care provider actions. J Telemed Telecare. 2017;23: 217-224.
8. Gleason N, Prasad PA, Ackerman S, et al. Adoption and impact of an eConsult system in a fee-for-service setting. Healthc (Amst). 2017;5(1-2):40-45.
9. Stoves J, Connolly J, Cheung CK, et al. Electronic consultation as an alternative to hospital referral for patients with chronic kidney disease: a novel application for networked electronic health records to improve the accessibility and efficiency of healthcare. Qual Saf Health Care. 2010;19: e54.
10. Datta SK, Warshaw EM, Edison KE, et al. Cost and utility analysis of a store-and-forward teledermatology referral system: a randomized clinical trial. JAMA Dermatol. 2015;151:1323-1329.
11. Liddy C, Drosinis P, Deri Armstrong C, et al. What are the cost savings associated with providing access to specialist care through the Champlain BASE eConsult service? A costing evaluation. BMJ Open. 2016;6:e010920.
12. Barnett ML, Yee HF Jr, Mehrotra A, Giboney P. Los Angeles safety-net program eConsult system was rapidly adopted and decreased wait times to see specialists. Health Aff. 2017;36:492-499.
13. Malagrino GD, Chaudhry R, Gardner M, et al. A study of 6,000 electronic specialty consultations for person-centered care at The Mayo Clinic. Int J Person Centered Med. 2012;2:458-466.
14. Keely E, Liddy C, Afkham A. Utilization, benefits, and impact of an e-consultation service across diverse specialties and primary care providers. Telemed J E Health. 2013;19:733-738.
15. Scherpbier-de Haan ND, van Gelder VA, Van Weel C, et al. Initial implementation of a web-based consultation process for patients with chronic kidney disease. Ann Fam Med. 2013;11:151-156.
16. Palen TE, Price D, Shetterly S, Wallace KB. Comparing virtual consults to traditional consults using an electronic health record: an observational case-control study. BMC Med Inform Decis Mak. 2012;12:65.
17. Liddy C, Afkham A, Drosinis P, et al. Impact of and satisfaction with a new eConsult service: a mixed methods study of primary care providers. J Am Board Fam Med. 2015;28:394-403.
18. Angstman KB, Adamson SC, Furst JW, et al. Provider satisfaction with virtual specialist consultations in a family medicine department. Health Care Manag (Frederick). 2009;28:14-18.
19. McAdams M, Cannavo L, Orlander JD. A medical specialty e-consult program in a VA health care system. Fed Pract. 2014; 31:26–31.
20. Keely E, Williams R, Epstein G, et al. Specialist perspectives on Ontario Provincial electronic consultation services. Telemed J E Health. 2019;25:3-10.
21. Kim-Hwang JE, Chen AH, Bell DS, et al. Evaluating electronic referrals for specialty care at a public hospital. J Gen Intern Med. 2010;25:1123-1128.
From the Department of Medicine, University of California, Irvine, Orange, CA.
Abstract
Background: Orange County’s residents have difficulty accessing timely, quality, affordable specialty care services. As the county’s only academic health system, the University of California, Irvine (UCI) aimed to improve specialty care access for the communities it serves by implementing an electronic consultations (eConsults) program that allows primary care providers (PCPs) to efficiently receive specialist recommendations on referral problems that do not require an in-person evaluation.
Objective: To implement an eConsults program at the UCI that enhances access to and the delivery of coordinated specialty care for lower-complexity referral problems.
Methods: We developed custom solutions to integrate eConsults into UCI’s 2 electronic health record platforms. The impact of the eConsults program was assessed by continuously evaluating usage and outcomes. Measures used to track usage included the number of submitted eConsult requests per PCP, the number of completed responses per specialty, and the response time for eConsult requests. Outcome measures included the specialist recommendation (eg, in-office visit, consultation avoided) and physician feedback.
Results: Over 4.5 years, more than 1400 successful eConsults have been completed, and the program has expanded to 17 specialties. The average turnaround time for an eConsult response across all specialties was 1 business day. Moreover, more than 50% of the eConsults received specialty responses within the same day of the eConsult request. Most important, about 80% of eConsult requests were addressed without the need for an in-office visit with a specialist.
Conclusion: The enhanced access to and the delivery of coordinated specialty care provided by eConsults resulted in improved efficiency and specialty access, while likely reducing costs and improving patient satisfaction. The improved communication and collaboration among providers with eConsults has also led to overwhelmingly positive feedback from both PCPs and specialists.
Keywords: electronic consultation; access to care; primary care; specialty referral; telehealth.
Orange County’s growing, aging, and diverse population is driving an increased demand for health care.1 But with the county’s high cost of living and worsening shortage of physicians,1-3 many of its residents are struggling to access timely, quality, affordable care. Access to specialty care services is especially frustrating for many patients and their providers, both primary care providers (PCPs) and specialists, due to problems with the referral process. Many patients experience increased wait times for a visit with a specialist due to poor communication between providers, insufficient guidance on the information or diagnostic results needed by specialists, and lack of care coordination.4-6 One promising approach to overcome these challenges is the use of an electronic consultation, or eConsult, in place of a standard in-person referral. An eConsult is an asynchronous, non-face-to-face, provider-to-provider exchange using a secure electronic communication platform. For appropriate referral problems, the patient is able to receive timely access to specialist expertise through electronic referral by their PCP,7-9 and avoid the time and costs associated with a visit to the specialist,10,11 such as travel, missed work, co-pays, and child-care expenses. Clinical questions addressed using an eConsult system subsequently free up office visit appointment slots, improving access for patients requiring in-office evaluation.8,12
Orange County’s only academic health system, the University of California, Irvine (UCI), serves a population of 3.5 million, and its principal priority is providing the communities in the county (which is the sixth largest in United States) and the surrounding region with the highest quality health care possible. Thus, UCI aimed to improve its referral processes and provide timely access to specialty care for its patients by implementing an eConsults program that allows PCPs to efficiently receive specialist recommendations on referral problems that do not require the specialist to evaluate the patient in person. This report describes our experiences with developing and implementing a custom-built eConsults workflow in UCI’s prior electronic health record (EHR) platform, Allscripts, and subsequently transitioning our mature eConsults program to a new EHR system when UCI adopted Epic. UCI is likely the only academic medical center to have experience in successfully implementing eConsults into 2 different EHR systems.
Setting
UCI’s medical center is a 417-bed acute care hospital providing tertiary and quaternary care, ambulatory and specialty medical clinics, behavioral health care, and rehabilitation services. It is located in Orange, CA, and serves a diverse population of 3.5 million persons with broad health care needs. With more than 400 specialty and primary care physicians, UCI offers a full scope of acute and general care services. It is also the primary teaching location for UCI medical and nursing students, medical residents, and fellows, and is home to Orange County’s only adult Level I and pediatric Level II trauma centers and the regional burn center.
eConsults Program
We designed the initial eConsults program within UCI’s Allscripts EHR platform. Our information technology (IT) build team developed unique “documents-based” eConsults workflows that simplified the process of initiating requests directly from the EHR and facilitated rapid responses from participating specialties. The requesting provider’s eConsults interface was user-friendly, and referring providers were able to initiate an eConsult easily by selecting the customized eConsult icon from the Allscripts main toolbar. To ensure that all relevant information is provided to the specialists, condition-specific templates are embedded in the requesting provider’s eConsults workflow that allow PCPs to enter a focused, patient-specific clinical question and provide guidance on recommended patient information (eg, health history, laboratory results, and digital images) that may help the specialist provide an informed response. The eConsult templates were adapted from standardized forms developed by partner University of California Health Systems in an initiative funded by the University of California Center for Health Quality and Innovation.
To facilitate timely responses from specialists, an innovative notification system was created in the responding provider’s eConsults workflow to automatically send an email to participating specialists when a new eConsult is requested. The responding provider’s workflow also includes an option for the specialist to decline the eConsult if the case is deemed too complex to be addressed electronically. For every completed eConsult that does not result in an in-person patient evaluation, the requesting provider and responding specialist each receives a modest reimbursement, which was initially paid by UCI Health System funds.
Implementation
The design and implementation of the eConsults program began in November 2014, and was guided by a steering committee that included the chair of the department of medicine, chief medical information officer, primary care and specialty physician leads, IT build team, and a project manager. Early on, members of this committee engaged UCI leadership to affirm support for the program and obtain the IT resources needed to build the eConsults workflow. Regular steering committee meetings were established to discuss the design of the workflow, adapt the clinical content of the referral templates, and develop a provider reimbursement plan. After completion of the workflow build, the eConsults system was tested to identify failure points and obtain feedback from users. Prior to going live, the eConsults program was publicized by members of the steering committee through meetings with primary care groups and email communications. Committee members also hosted in-person training and orientation sessions with PCPs and participating specialists, and distributed tip sheets summarizing the steps to complete the PCP and specialist eConsult workflows.
The eConsults workflow build, testing, and launch were completed within 5 months (April 2015; Figure 1). eConsults went live in the 3 initial specialties (endocrinology, cardiology, and rheumatology) that were interested in participating in the first wave of the program. UCI’s eConsults service has subsequently expanded to 17 total specialties (allergy, cardiology, dermatology, endocrinology, gastroenterology, geriatrics, gynecology, hematology, hepatology, infectious disease, nephrology, neurology, palliative care, psychiatry, pulmonary, rheumatology, and sports medicine).
Two and half years after the eConsults program was implemented in Allscripts, UCI adopted a new EHR platform, Epic. By this time, the eConsults service had grown into a mature program with greater numbers of PCP users and submitted eConsults (Figure 2). Using our experience with the Allscripts build, our IT team was able to efficiently transition the eConsults service to the new EHR system. In contrast to the “documents-based” eConsult workflows on Allscripts, our IT team utilized an “orders-based” strategy on Epic, which followed a more traditional approach to requesting a consultation. We re-launched the service in Epic within 3 months (February 2018). However, both platforms utilized user-friendly workflows to achieve similar goals, and the program has continued to grow with respect to the number of users and eConsults.
Measurement/Analysis
The impact of the program was assessed by continuously evaluating usage and outcomes. Measures used to track usage included the number of PCP users, the number of submitted eConsult requests per PCP, and the number of requests per specialty. The response time for eConsult requests and the self-reported amount of time spent by specialists on the response were also tracked. Outcome measures included the specialist recommendation (eg, in-office visit, consultation avoided) and physician feedback. Provider satisfaction was primarily obtained by soliciting feedback from individual eConsult users.
Implementation of this eConsults program constituted a quality improvement activity and did not require Institutional Review Board review.
Results
Since the program was launched in April 2015, more than 1400 eConsults have been completed across 17 specialties (Figure 3). There were 654 completed eConsults on the Allscripts platform, and 808 eConsults have been completed using the Epic platform to date. The busiest eConsult specialties were endocrinology (receiving 276, or 19%, of the eConsults requests), hematology (receiving 249 requests, or 17%), infectious disease (receiving 244 requests, or 17% ), and cardiology (receiving 148 requests, or 10%).
The self-reported amount of time specialists spent on the response was different between the 2 EHR systems (Figure 4). On Allscripts, specialists reported that 23% of eConsults took 10 minutes or less to complete, 47% took 11 to 20 minutes, 23% took 21 to 30 minutes, and 7% took more than 30 minutes. On Epic, specialists reported that 42% of eConsults took 10 minutes or less to complete, 44% took 11 to 20 minutes, 12% took 21 to 30 minutes, and 2% took more than 30 minutes. This difference in time spent fielding eConsults likely represents the subtle nuances between Allscripts’ “documents-based” and Epic’s “orders-based” workflows.
As a result of the automated notification system that was introduced early in the eConsults implementation process on Allscripts, the specialty response times were much faster than the expected 3 business days’ turnaround goal instituted by the Center for Health Quality and Innovation initiative, regardless of the EHR platform used. In fact, the average turnaround time for an eConsult response across all specialties was 1 business day, which was similar for both EHR systems (Figure 5). Furthermore, more than 50% of the eConsults on both EHR systems received specialist responses within the same day of the eConsult request (63% on Allscripts, 54% on Epic). There was a small decrease in the percentage of same-day responses when we transitioned to Epic, likely because the functionality of an automated notification email could not be restored in Epic. Regardless, the specialty response times on Epic remained expeditious, likely because the automated notifications on Allscripts instilled good practices for the specialists, and regularly checking for new eConsult requests became an ingrained behavior.
Our most important finding was that approximately 80% of eConsult requests were addressed without the need for an in-office visit with a specialist. This measure was similar for both EHR platforms (83% on Allscripts and 78% on Epic).
Provider feedback has been overwhelmingly positive. PCPs are impressed with the robust educational content of the eConsult responses, since the goal for specialists is to justify their recommendations. Specialists appreciate the convenience and efficiency that eConsults offer, as well as the improved communication and collaboration among physicians. eConsults have been especially beneficial to PCPs at UCI’s Family Health Centers, who are now able to receive subspecialty consultations from UCI specialists despite insurance barriers.
Discussion
Our eConsults program uniquely contrasts with other programs because UCI is likely the only academic medical center to have experience in successfully incorporating eConsults into 2 different EHR systems: initial development of the eConsults workflow in UCI’s existing Allscripts EHR platform, and subsequently transitioning a mature eConsults program to a new EHR system when the institution adopted Epic.
We measured the impact of the eConsults program on access to care by the response time for eConsult requests and the percentage of eConsults that averted an in-office visit with a specialist. We found that the eConsults program at UCI provided our PCPs access to specialist consultations in a timely manner, with much shorter response times than standard in-person referrals. The average turnaround time for an eConsult response we reported is consistent with findings from other studies.12-15 Additionally, our program was able to address about 80% of its eConsults electronically, helping to reduce unnecessary in-person specialist referrals. In the literature, the percentage of eConsults that avoided an in-person specialist visit varies widely.8,12-16
We reported very positive feedback from both PCPs and specialists on UCI’s eConsults service. Similarly, other studies described PCP satisfaction with their respective eConsults programs to be uniformly high,8,9,13,14,17-19 though some reported that the level of satisfaction among specialists was more varied.18-21
Lessons Learned
The successful design and implementation of our eConsults program began with assembling the right clinical champions and technology partners for our steering committee. Establishing regular steering committee meetings helped maintain an appropriate timeline for completion of different aspects of the project. Engaging support from UCI’s leadership also provided us with a dedicated IT team that helped us with the build, training resources, troubleshooting issues, and reporting for the project.
Our experience with implementing the eConsults program on 2 different EHR systems highlighted the importance of creating efficient, user-friendly workflows to foster provider adoption and achieve sustainability. Allscripts’ open platform gave our IT team the ability to create a homegrown solution to implementing an eConsult model that was simple and easy to use. The Epic platform’s interoperability allowed us to leverage our learnings from the Allscripts build to efficiently implement eConsults in Epic.
We also found that providing modest incentive payments or reimbursements to both PCPs and specialists for each completed eConsult helps with both adoption and program sustainability. Initially, credit for the eConsult work was paid by internal UCI Health System funds. Two payers, UC Care (a preferred provider organization plan created just for the University of California) and more recently, the Centers for Medicare & Medicaid Services, have agreed to reimburse for outpatient eConsults. Securing additional payers for reimbursement of the eConsult service will not only ensure the program’s long-term sustainability, but also represents an acknowledgment of the value of eConsults in supporting access to care.
Applicability
Other health care settings that are experiencing issues with specialty care access can successfully implement their own eConsults program by employing strategies similar to those described in this report—assembling the right team, creating user-friendly workflows, and providing incentives. Our advice for successful implementation is to clearly communicate your goals to all involved, including primary care, specialists, leadership, and IT partners, and establish with these stakeholders the appropriate support and resources needed to facilitate the development of the program and overcome any barriers to adoption.
Current Status and Future Directions
Our future plans include continuing to optimize the Epic eConsult backend build and workflows using our experience in Allscripts. We have implemented eConsult workflows for use by graduate medical education trainees and advanced practice providers, with attending supervision. Further work is in progress to optimize these workflows, which will allow for appropriate education and supervision without delaying care. Furthermore, we plan to expand the program to include inpatient-to-inpatient and emergency department-to-inpatient eConsults. We will continue to expand the eConsults program by offering additional specialties, engage providers to encourage ongoing participation, and maximize PCP use by continuing to market the program through regular newsletters and email communications. Finally, the eConsults has served as an effective, important resource in the current era of COVID-19 in several ways: it allows for optimization of specialty input in patient care delivery without subjecting more health care workers to unnecessary exposure; saves on utilization of precious personal protective equipment; and enhances our ability to deal with a potential surge by providing access to specialists remotely and electronically all hours of the day, thus expanding care to the evening and weekend hours.
Acknowledgment: The authors thank our steering committee members (Dr. Ralph Cygan, Dr. Andrew Reikes, Dr. Byron Allen, Dr. George Lawry) and IT build team (Lori Bocchicchio, Meghan van Witsen, Jaymee Zillgitt, Tanya Sickles, Dennis Hoang, Jeanette Lisak-Phillips) for their contributions in the design and implementation of our eConsults program. We also thank additional team members Kurt McArthur and Neaktisia Lee for their assistance with generating reports, and Kathy LaPierre, Jennifer Rios, and Debra Webb Torres for their guidance with compliance and billing issues.
Corresponding author: Alpesh N. Amin, MD, MBA, University of California, Irvine, 101 The City Drive South, Building 26, Room 1000, ZC-4076H, Orange, CA 92868; anamin@uci.edu.
Financial disclosures: None.
From the Department of Medicine, University of California, Irvine, Orange, CA.
Abstract
Background: Orange County’s residents have difficulty accessing timely, quality, affordable specialty care services. As the county’s only academic health system, the University of California, Irvine (UCI) aimed to improve specialty care access for the communities it serves by implementing an electronic consultations (eConsults) program that allows primary care providers (PCPs) to efficiently receive specialist recommendations on referral problems that do not require an in-person evaluation.
Objective: To implement an eConsults program at the UCI that enhances access to and the delivery of coordinated specialty care for lower-complexity referral problems.
Methods: We developed custom solutions to integrate eConsults into UCI’s 2 electronic health record platforms. The impact of the eConsults program was assessed by continuously evaluating usage and outcomes. Measures used to track usage included the number of submitted eConsult requests per PCP, the number of completed responses per specialty, and the response time for eConsult requests. Outcome measures included the specialist recommendation (eg, in-office visit, consultation avoided) and physician feedback.
Results: Over 4.5 years, more than 1400 successful eConsults have been completed, and the program has expanded to 17 specialties. The average turnaround time for an eConsult response across all specialties was 1 business day. Moreover, more than 50% of the eConsults received specialty responses within the same day of the eConsult request. Most important, about 80% of eConsult requests were addressed without the need for an in-office visit with a specialist.
Conclusion: The enhanced access to and the delivery of coordinated specialty care provided by eConsults resulted in improved efficiency and specialty access, while likely reducing costs and improving patient satisfaction. The improved communication and collaboration among providers with eConsults has also led to overwhelmingly positive feedback from both PCPs and specialists.
Keywords: electronic consultation; access to care; primary care; specialty referral; telehealth.
Orange County’s growing, aging, and diverse population is driving an increased demand for health care.1 But with the county’s high cost of living and worsening shortage of physicians,1-3 many of its residents are struggling to access timely, quality, affordable care. Access to specialty care services is especially frustrating for many patients and their providers, both primary care providers (PCPs) and specialists, due to problems with the referral process. Many patients experience increased wait times for a visit with a specialist due to poor communication between providers, insufficient guidance on the information or diagnostic results needed by specialists, and lack of care coordination.4-6 One promising approach to overcome these challenges is the use of an electronic consultation, or eConsult, in place of a standard in-person referral. An eConsult is an asynchronous, non-face-to-face, provider-to-provider exchange using a secure electronic communication platform. For appropriate referral problems, the patient is able to receive timely access to specialist expertise through electronic referral by their PCP,7-9 and avoid the time and costs associated with a visit to the specialist,10,11 such as travel, missed work, co-pays, and child-care expenses. Clinical questions addressed using an eConsult system subsequently free up office visit appointment slots, improving access for patients requiring in-office evaluation.8,12
Orange County’s only academic health system, the University of California, Irvine (UCI), serves a population of 3.5 million, and its principal priority is providing the communities in the county (which is the sixth largest in United States) and the surrounding region with the highest quality health care possible. Thus, UCI aimed to improve its referral processes and provide timely access to specialty care for its patients by implementing an eConsults program that allows PCPs to efficiently receive specialist recommendations on referral problems that do not require the specialist to evaluate the patient in person. This report describes our experiences with developing and implementing a custom-built eConsults workflow in UCI’s prior electronic health record (EHR) platform, Allscripts, and subsequently transitioning our mature eConsults program to a new EHR system when UCI adopted Epic. UCI is likely the only academic medical center to have experience in successfully implementing eConsults into 2 different EHR systems.
Setting
UCI’s medical center is a 417-bed acute care hospital providing tertiary and quaternary care, ambulatory and specialty medical clinics, behavioral health care, and rehabilitation services. It is located in Orange, CA, and serves a diverse population of 3.5 million persons with broad health care needs. With more than 400 specialty and primary care physicians, UCI offers a full scope of acute and general care services. It is also the primary teaching location for UCI medical and nursing students, medical residents, and fellows, and is home to Orange County’s only adult Level I and pediatric Level II trauma centers and the regional burn center.
eConsults Program
We designed the initial eConsults program within UCI’s Allscripts EHR platform. Our information technology (IT) build team developed unique “documents-based” eConsults workflows that simplified the process of initiating requests directly from the EHR and facilitated rapid responses from participating specialties. The requesting provider’s eConsults interface was user-friendly, and referring providers were able to initiate an eConsult easily by selecting the customized eConsult icon from the Allscripts main toolbar. To ensure that all relevant information is provided to the specialists, condition-specific templates are embedded in the requesting provider’s eConsults workflow that allow PCPs to enter a focused, patient-specific clinical question and provide guidance on recommended patient information (eg, health history, laboratory results, and digital images) that may help the specialist provide an informed response. The eConsult templates were adapted from standardized forms developed by partner University of California Health Systems in an initiative funded by the University of California Center for Health Quality and Innovation.
To facilitate timely responses from specialists, an innovative notification system was created in the responding provider’s eConsults workflow to automatically send an email to participating specialists when a new eConsult is requested. The responding provider’s workflow also includes an option for the specialist to decline the eConsult if the case is deemed too complex to be addressed electronically. For every completed eConsult that does not result in an in-person patient evaluation, the requesting provider and responding specialist each receives a modest reimbursement, which was initially paid by UCI Health System funds.
Implementation
The design and implementation of the eConsults program began in November 2014, and was guided by a steering committee that included the chair of the department of medicine, chief medical information officer, primary care and specialty physician leads, IT build team, and a project manager. Early on, members of this committee engaged UCI leadership to affirm support for the program and obtain the IT resources needed to build the eConsults workflow. Regular steering committee meetings were established to discuss the design of the workflow, adapt the clinical content of the referral templates, and develop a provider reimbursement plan. After completion of the workflow build, the eConsults system was tested to identify failure points and obtain feedback from users. Prior to going live, the eConsults program was publicized by members of the steering committee through meetings with primary care groups and email communications. Committee members also hosted in-person training and orientation sessions with PCPs and participating specialists, and distributed tip sheets summarizing the steps to complete the PCP and specialist eConsult workflows.
The eConsults workflow build, testing, and launch were completed within 5 months (April 2015; Figure 1). eConsults went live in the 3 initial specialties (endocrinology, cardiology, and rheumatology) that were interested in participating in the first wave of the program. UCI’s eConsults service has subsequently expanded to 17 total specialties (allergy, cardiology, dermatology, endocrinology, gastroenterology, geriatrics, gynecology, hematology, hepatology, infectious disease, nephrology, neurology, palliative care, psychiatry, pulmonary, rheumatology, and sports medicine).
Two and half years after the eConsults program was implemented in Allscripts, UCI adopted a new EHR platform, Epic. By this time, the eConsults service had grown into a mature program with greater numbers of PCP users and submitted eConsults (Figure 2). Using our experience with the Allscripts build, our IT team was able to efficiently transition the eConsults service to the new EHR system. In contrast to the “documents-based” eConsult workflows on Allscripts, our IT team utilized an “orders-based” strategy on Epic, which followed a more traditional approach to requesting a consultation. We re-launched the service in Epic within 3 months (February 2018). However, both platforms utilized user-friendly workflows to achieve similar goals, and the program has continued to grow with respect to the number of users and eConsults.
Measurement/Analysis
The impact of the program was assessed by continuously evaluating usage and outcomes. Measures used to track usage included the number of PCP users, the number of submitted eConsult requests per PCP, and the number of requests per specialty. The response time for eConsult requests and the self-reported amount of time spent by specialists on the response were also tracked. Outcome measures included the specialist recommendation (eg, in-office visit, consultation avoided) and physician feedback. Provider satisfaction was primarily obtained by soliciting feedback from individual eConsult users.
Implementation of this eConsults program constituted a quality improvement activity and did not require Institutional Review Board review.
Results
Since the program was launched in April 2015, more than 1400 eConsults have been completed across 17 specialties (Figure 3). There were 654 completed eConsults on the Allscripts platform, and 808 eConsults have been completed using the Epic platform to date. The busiest eConsult specialties were endocrinology (receiving 276, or 19%, of the eConsults requests), hematology (receiving 249 requests, or 17%), infectious disease (receiving 244 requests, or 17% ), and cardiology (receiving 148 requests, or 10%).
The self-reported amount of time specialists spent on the response was different between the 2 EHR systems (Figure 4). On Allscripts, specialists reported that 23% of eConsults took 10 minutes or less to complete, 47% took 11 to 20 minutes, 23% took 21 to 30 minutes, and 7% took more than 30 minutes. On Epic, specialists reported that 42% of eConsults took 10 minutes or less to complete, 44% took 11 to 20 minutes, 12% took 21 to 30 minutes, and 2% took more than 30 minutes. This difference in time spent fielding eConsults likely represents the subtle nuances between Allscripts’ “documents-based” and Epic’s “orders-based” workflows.
As a result of the automated notification system that was introduced early in the eConsults implementation process on Allscripts, the specialty response times were much faster than the expected 3 business days’ turnaround goal instituted by the Center for Health Quality and Innovation initiative, regardless of the EHR platform used. In fact, the average turnaround time for an eConsult response across all specialties was 1 business day, which was similar for both EHR systems (Figure 5). Furthermore, more than 50% of the eConsults on both EHR systems received specialist responses within the same day of the eConsult request (63% on Allscripts, 54% on Epic). There was a small decrease in the percentage of same-day responses when we transitioned to Epic, likely because the functionality of an automated notification email could not be restored in Epic. Regardless, the specialty response times on Epic remained expeditious, likely because the automated notifications on Allscripts instilled good practices for the specialists, and regularly checking for new eConsult requests became an ingrained behavior.
Our most important finding was that approximately 80% of eConsult requests were addressed without the need for an in-office visit with a specialist. This measure was similar for both EHR platforms (83% on Allscripts and 78% on Epic).
Provider feedback has been overwhelmingly positive. PCPs are impressed with the robust educational content of the eConsult responses, since the goal for specialists is to justify their recommendations. Specialists appreciate the convenience and efficiency that eConsults offer, as well as the improved communication and collaboration among physicians. eConsults have been especially beneficial to PCPs at UCI’s Family Health Centers, who are now able to receive subspecialty consultations from UCI specialists despite insurance barriers.
Discussion
Our eConsults program uniquely contrasts with other programs because UCI is likely the only academic medical center to have experience in successfully incorporating eConsults into 2 different EHR systems: initial development of the eConsults workflow in UCI’s existing Allscripts EHR platform, and subsequently transitioning a mature eConsults program to a new EHR system when the institution adopted Epic.
We measured the impact of the eConsults program on access to care by the response time for eConsult requests and the percentage of eConsults that averted an in-office visit with a specialist. We found that the eConsults program at UCI provided our PCPs access to specialist consultations in a timely manner, with much shorter response times than standard in-person referrals. The average turnaround time for an eConsult response we reported is consistent with findings from other studies.12-15 Additionally, our program was able to address about 80% of its eConsults electronically, helping to reduce unnecessary in-person specialist referrals. In the literature, the percentage of eConsults that avoided an in-person specialist visit varies widely.8,12-16
We reported very positive feedback from both PCPs and specialists on UCI’s eConsults service. Similarly, other studies described PCP satisfaction with their respective eConsults programs to be uniformly high,8,9,13,14,17-19 though some reported that the level of satisfaction among specialists was more varied.18-21
Lessons Learned
The successful design and implementation of our eConsults program began with assembling the right clinical champions and technology partners for our steering committee. Establishing regular steering committee meetings helped maintain an appropriate timeline for completion of different aspects of the project. Engaging support from UCI’s leadership also provided us with a dedicated IT team that helped us with the build, training resources, troubleshooting issues, and reporting for the project.
Our experience with implementing the eConsults program on 2 different EHR systems highlighted the importance of creating efficient, user-friendly workflows to foster provider adoption and achieve sustainability. Allscripts’ open platform gave our IT team the ability to create a homegrown solution to implementing an eConsult model that was simple and easy to use. The Epic platform’s interoperability allowed us to leverage our learnings from the Allscripts build to efficiently implement eConsults in Epic.
We also found that providing modest incentive payments or reimbursements to both PCPs and specialists for each completed eConsult helps with both adoption and program sustainability. Initially, credit for the eConsult work was paid by internal UCI Health System funds. Two payers, UC Care (a preferred provider organization plan created just for the University of California) and more recently, the Centers for Medicare & Medicaid Services, have agreed to reimburse for outpatient eConsults. Securing additional payers for reimbursement of the eConsult service will not only ensure the program’s long-term sustainability, but also represents an acknowledgment of the value of eConsults in supporting access to care.
Applicability
Other health care settings that are experiencing issues with specialty care access can successfully implement their own eConsults program by employing strategies similar to those described in this report—assembling the right team, creating user-friendly workflows, and providing incentives. Our advice for successful implementation is to clearly communicate your goals to all involved, including primary care, specialists, leadership, and IT partners, and establish with these stakeholders the appropriate support and resources needed to facilitate the development of the program and overcome any barriers to adoption.
Current Status and Future Directions
Our future plans include continuing to optimize the Epic eConsult backend build and workflows using our experience in Allscripts. We have implemented eConsult workflows for use by graduate medical education trainees and advanced practice providers, with attending supervision. Further work is in progress to optimize these workflows, which will allow for appropriate education and supervision without delaying care. Furthermore, we plan to expand the program to include inpatient-to-inpatient and emergency department-to-inpatient eConsults. We will continue to expand the eConsults program by offering additional specialties, engage providers to encourage ongoing participation, and maximize PCP use by continuing to market the program through regular newsletters and email communications. Finally, the eConsults has served as an effective, important resource in the current era of COVID-19 in several ways: it allows for optimization of specialty input in patient care delivery without subjecting more health care workers to unnecessary exposure; saves on utilization of precious personal protective equipment; and enhances our ability to deal with a potential surge by providing access to specialists remotely and electronically all hours of the day, thus expanding care to the evening and weekend hours.
Acknowledgment: The authors thank our steering committee members (Dr. Ralph Cygan, Dr. Andrew Reikes, Dr. Byron Allen, Dr. George Lawry) and IT build team (Lori Bocchicchio, Meghan van Witsen, Jaymee Zillgitt, Tanya Sickles, Dennis Hoang, Jeanette Lisak-Phillips) for their contributions in the design and implementation of our eConsults program. We also thank additional team members Kurt McArthur and Neaktisia Lee for their assistance with generating reports, and Kathy LaPierre, Jennifer Rios, and Debra Webb Torres for their guidance with compliance and billing issues.
Corresponding author: Alpesh N. Amin, MD, MBA, University of California, Irvine, 101 The City Drive South, Building 26, Room 1000, ZC-4076H, Orange, CA 92868; anamin@uci.edu.
Financial disclosures: None.
1. County of Orange, Health Care Agency, Public Health Services. Orange County Health Profile 2013.
2. Coffman JM, Fix M Ko, M. California physician supply and distribution: headed for a drought? California Health Care Foundation, June 2018.
3. Spetz J, Coffman J, Geyn I. California’s primary care workforce: forecasted supply, demand, and pipeline of trainees, 2016-2030. Healthforce Center at the University of California, San Francisco, August 2017.
4. Gandhi TK, Sittig DF, Franklin M, et al. Communication breakdown in the outpatient referral process. J Gen Intern Med. 2000;15:626-631.
5. McPhee SJ, Lo B, Saika GY, Meltzer R. How good is communication between primary care physicians and subspecialty consultants? Arch Intern Med. 1984;144:1265-1268.
6. Mehrotra A, Forrest CB, Lin CY. Dropping the baton: specialty referrals in the United States. Milbank Q. 2011;89:39-68.
7. Wrenn K, Catschegn S, Cruz M, et al. Analysis of an electronic consultation program at an academic medical centre: Primary care provider questions, specialist responses, and primary care provider actions. J Telemed Telecare. 2017;23: 217-224.
8. Gleason N, Prasad PA, Ackerman S, et al. Adoption and impact of an eConsult system in a fee-for-service setting. Healthc (Amst). 2017;5(1-2):40-45.
9. Stoves J, Connolly J, Cheung CK, et al. Electronic consultation as an alternative to hospital referral for patients with chronic kidney disease: a novel application for networked electronic health records to improve the accessibility and efficiency of healthcare. Qual Saf Health Care. 2010;19: e54.
10. Datta SK, Warshaw EM, Edison KE, et al. Cost and utility analysis of a store-and-forward teledermatology referral system: a randomized clinical trial. JAMA Dermatol. 2015;151:1323-1329.
11. Liddy C, Drosinis P, Deri Armstrong C, et al. What are the cost savings associated with providing access to specialist care through the Champlain BASE eConsult service? A costing evaluation. BMJ Open. 2016;6:e010920.
12. Barnett ML, Yee HF Jr, Mehrotra A, Giboney P. Los Angeles safety-net program eConsult system was rapidly adopted and decreased wait times to see specialists. Health Aff. 2017;36:492-499.
13. Malagrino GD, Chaudhry R, Gardner M, et al. A study of 6,000 electronic specialty consultations for person-centered care at The Mayo Clinic. Int J Person Centered Med. 2012;2:458-466.
14. Keely E, Liddy C, Afkham A. Utilization, benefits, and impact of an e-consultation service across diverse specialties and primary care providers. Telemed J E Health. 2013;19:733-738.
15. Scherpbier-de Haan ND, van Gelder VA, Van Weel C, et al. Initial implementation of a web-based consultation process for patients with chronic kidney disease. Ann Fam Med. 2013;11:151-156.
16. Palen TE, Price D, Shetterly S, Wallace KB. Comparing virtual consults to traditional consults using an electronic health record: an observational case-control study. BMC Med Inform Decis Mak. 2012;12:65.
17. Liddy C, Afkham A, Drosinis P, et al. Impact of and satisfaction with a new eConsult service: a mixed methods study of primary care providers. J Am Board Fam Med. 2015;28:394-403.
18. Angstman KB, Adamson SC, Furst JW, et al. Provider satisfaction with virtual specialist consultations in a family medicine department. Health Care Manag (Frederick). 2009;28:14-18.
19. McAdams M, Cannavo L, Orlander JD. A medical specialty e-consult program in a VA health care system. Fed Pract. 2014; 31:26–31.
20. Keely E, Williams R, Epstein G, et al. Specialist perspectives on Ontario Provincial electronic consultation services. Telemed J E Health. 2019;25:3-10.
21. Kim-Hwang JE, Chen AH, Bell DS, et al. Evaluating electronic referrals for specialty care at a public hospital. J Gen Intern Med. 2010;25:1123-1128.
1. County of Orange, Health Care Agency, Public Health Services. Orange County Health Profile 2013.
2. Coffman JM, Fix M Ko, M. California physician supply and distribution: headed for a drought? California Health Care Foundation, June 2018.
3. Spetz J, Coffman J, Geyn I. California’s primary care workforce: forecasted supply, demand, and pipeline of trainees, 2016-2030. Healthforce Center at the University of California, San Francisco, August 2017.
4. Gandhi TK, Sittig DF, Franklin M, et al. Communication breakdown in the outpatient referral process. J Gen Intern Med. 2000;15:626-631.
5. McPhee SJ, Lo B, Saika GY, Meltzer R. How good is communication between primary care physicians and subspecialty consultants? Arch Intern Med. 1984;144:1265-1268.
6. Mehrotra A, Forrest CB, Lin CY. Dropping the baton: specialty referrals in the United States. Milbank Q. 2011;89:39-68.
7. Wrenn K, Catschegn S, Cruz M, et al. Analysis of an electronic consultation program at an academic medical centre: Primary care provider questions, specialist responses, and primary care provider actions. J Telemed Telecare. 2017;23: 217-224.
8. Gleason N, Prasad PA, Ackerman S, et al. Adoption and impact of an eConsult system in a fee-for-service setting. Healthc (Amst). 2017;5(1-2):40-45.
9. Stoves J, Connolly J, Cheung CK, et al. Electronic consultation as an alternative to hospital referral for patients with chronic kidney disease: a novel application for networked electronic health records to improve the accessibility and efficiency of healthcare. Qual Saf Health Care. 2010;19: e54.
10. Datta SK, Warshaw EM, Edison KE, et al. Cost and utility analysis of a store-and-forward teledermatology referral system: a randomized clinical trial. JAMA Dermatol. 2015;151:1323-1329.
11. Liddy C, Drosinis P, Deri Armstrong C, et al. What are the cost savings associated with providing access to specialist care through the Champlain BASE eConsult service? A costing evaluation. BMJ Open. 2016;6:e010920.
12. Barnett ML, Yee HF Jr, Mehrotra A, Giboney P. Los Angeles safety-net program eConsult system was rapidly adopted and decreased wait times to see specialists. Health Aff. 2017;36:492-499.
13. Malagrino GD, Chaudhry R, Gardner M, et al. A study of 6,000 electronic specialty consultations for person-centered care at The Mayo Clinic. Int J Person Centered Med. 2012;2:458-466.
14. Keely E, Liddy C, Afkham A. Utilization, benefits, and impact of an e-consultation service across diverse specialties and primary care providers. Telemed J E Health. 2013;19:733-738.
15. Scherpbier-de Haan ND, van Gelder VA, Van Weel C, et al. Initial implementation of a web-based consultation process for patients with chronic kidney disease. Ann Fam Med. 2013;11:151-156.
16. Palen TE, Price D, Shetterly S, Wallace KB. Comparing virtual consults to traditional consults using an electronic health record: an observational case-control study. BMC Med Inform Decis Mak. 2012;12:65.
17. Liddy C, Afkham A, Drosinis P, et al. Impact of and satisfaction with a new eConsult service: a mixed methods study of primary care providers. J Am Board Fam Med. 2015;28:394-403.
18. Angstman KB, Adamson SC, Furst JW, et al. Provider satisfaction with virtual specialist consultations in a family medicine department. Health Care Manag (Frederick). 2009;28:14-18.
19. McAdams M, Cannavo L, Orlander JD. A medical specialty e-consult program in a VA health care system. Fed Pract. 2014; 31:26–31.
20. Keely E, Williams R, Epstein G, et al. Specialist perspectives on Ontario Provincial electronic consultation services. Telemed J E Health. 2019;25:3-10.
21. Kim-Hwang JE, Chen AH, Bell DS, et al. Evaluating electronic referrals for specialty care at a public hospital. J Gen Intern Med. 2010;25:1123-1128.
Procalcitonin-Guided Antibiotic Discontinuation: An Antimicrobial Stewardship Initiative to Assist Providers
From Western Michigan University, Homer Stryker MD School of Medicine, Kalamazoo, MI (Dr. Vaillant and Dr. Kavanaugh), Ferris State University, Grand Rapids, MI (Dr. Mersfelder), and Bronson Methodist Hospital, Kalamazoo, MI (Dr. Maynard).
Abstract
- Background: Procalcitonin has emerged as an important marker of sepsis and lung infections of bacterial origin. The role of procalcitonin in guiding antibiotic stewardship in lower respiratory tract infections and sepsis has been extensively studied, and use of this biomarker has been shown to decrease antibiotic usage in clinical trials. We sought to evaluate the impact of a pharmacist-driven initiative regarding discontinuation of antibiotics utilizing procalcitonin levels at a community teaching hospital.
- Methods: We retrospectively gathered baseline data on adult patients admitted to a community teaching hospital who were 18 years of age and older, under the care of an inpatient service, and had a single procalcitonin level < 0.25 mcg/L obtained during admission. We then prospectively identified an intervention group of similar patients using a web-based, real-time clinical surveillance system. When a low procalcitonin level was identified in the intervention group, the participating clinical pharmacists screened for antibiotic use and the indication(s), determined whether the antibiotic could be discontinued based on the low procalcitonin level and the absence of another indication for antibiotics, and, when appropriate, contacted the patient’s health care provider via telephone to discuss possible antibiotic discontinuation. The total antibiotic treatment duration was compared between the baseline and intervention groups.
- Results: A total of 172 patients were included in this study (86 in each group). The duration of antibiotic use was not significantly different between the baseline (3.14 ± 4.04 days) and the intervention (3.34 ± 2.8 days) groups (P = 0.1083). Other patient demographics did not influence antibiotic duration.
- Conclusion: Our study did not demonstrate a difference in total antibiotic treatment duration with the utilization of procalcitonin and an oral communication intervention made by a clinical pharmacist at a community-based teaching hospital. Outside of clinical trials, and in the absence of an algorithmic approach, procalcitonin has not consistently been shown to aid in the diagnosis and treatment of infectious diseases. It is important to have a comprehensive antimicrobial stewardship program to reduce antibiotic use and effectively use laboratory values.
Keywords: antibiotic use; bacterial infection; biomarkers; procalcitonin.
Procalcitonin is the precursor of the hormone calcitonin, which is normally produced in the parafollicular cells of the thyroid gland under physiological conditions.1 However, procalcitonin is also released in response to a proinflammatory stimulus, especially that of bacterial origin.1 The source of the procalcitonin surge seen during proinflammatory states is not the parafollicular cells of the thyroid, but rather the neuroendocrine cells of the lung and intestine.1 Stimulants of procalcitonin in these scenarios include bacterial endotoxin, tumor necrosis factor, and interleukin-6.1,2 Due to these observations, procalcitonin has emerged as an important marker of sepsis and lung infections of bacterial origin.3
The role of procalcitonin in guiding antibiotic stewardship in lower respiratory tract infections and sepsis has been extensively studied.4,5 Various randomized controlled trials have shown that antibiotic stewardship guided by procalcitonin levels resulted in lower rates of antibiotic initiation and shorter duration of antibiotic use.4-6 Similar results were obtained in prospective studies evaluating its role in patients with chronic obstructive pulmonary disease and sepsis.7,8 Based on these data, protocol-driven procalcitonin-guided antibiotic stewardship appears beneficial.
Many of these studies employed rigorous protocols. Studies of procalcitonin use in a so-called real-world setting, in which the provider can order and use procalcitonin levels without the use of protocols, are limited. The objective of our study was to evaluate the impact of a pharmacist-driven initiative on discontinuing antibiotics, if indicated, utilizing single procalcitonin measurement results of < 0.25 mcg/L at a community teaching hospital.
Methods
Our study utilized a 2-phase approach. The first phase was a retrospective chart review to establish baseline data regarding adult inpatients with a low procalcitonin level; these patients were randomly selected over a 1-year period (2017). Patients were included if they were 18 years of age or older, under the care of an inpatient service, and had a single procalcitonin level < 0.25 mcg/L obtained during their admission. Patients admitted to the intensive care unit were excluded. In the second phase, we prospectively identified similar patients admitted between January and March 2018 using a web-based, real-time clinical surveillance system. When patients with low procalcitonin levels were identified, 2 participating clinical pharmacists screened for antibiotic use and indication. If it was determined that the antibiotic could be discontinued as a result of the low procalcitonin level and no additional indication for antibiotics was present, the pharmacist contacted the patient’s health care provider via telephone to discuss possible antibiotic discontinuation. Data collected before and after the intervention included total antibiotic treatment duration, white blood cell count, maximum temperature, age, and procalcitonin level.
A sample size of 86 was calculated to provide an alpha of 0.05 and a power of 0.8. A nonparametric Wilcoxon 2-sample test was used to test for a difference in duration of antibiotic treatment between the baseline and intervention groups. A nonparametric test was used due to right-skewed data. All patients were included in the group analysis, regardless of antibiotic use, as the procalcitonin level may have been used in the decision to initiate antibiotics, and this is more representative of a real-world application of the test. This allowed for detection of a significant decrease of 2 days in antibiotic duration post intervention, with a 10% margin to compensate for potential missing data. Data from 86 patients obtained prior to the pharmacist intervention acted as a control comparison group. Statistical analysis was performed using SAS 9.4.
Results
A total of 172 patients were included in this study: 86 patients prior to the intervention, and 86 after implementation. Baseline demographics, laboratory values, vitals, and principal diagnoses for both groups are shown in Table 1 and Table 2. The most common indications for procalcitonin measurement were pneumonia (45.9%), chronic obstructive pulmonary disease (15.7%), and sepsis (14.5%). The remaining diagnoses were encephalopathy, fever and leukocytosis, skin and soft tissue infection, urinary tract infection or pyelonephritis, bone and joint infection, meningitis, intra-abdominal infection, and asthma exacerbation.
Antibiotic therapy was initiated in 68% of the patients overall, 59% in the baseline group and 76% in the intervention group. The duration of antibiotic use was not significantly different between the baseline (3.14 ± 4.04 days) and intervention (3.34 ± 2.8 days) groups (P = 0.1083). Furthermore, antibiotic treatment duration did not vary significantly with patient age, white blood cell count, maximum temperature, or procalcitonin level in either group. Although there was no difference in total antibiotic treatment duration, a post-hoc analysis revealed a 0.6-day decrease in the interval between the date of procalcitonin measurement and the stop date of antibiotics in the intervention group. The average time from admission to obtaining a procalcitonin level was 3 days in the baseline group and 2 days in the intervention group.
Discussion
Our study did not demonstrate a difference in total antibiotic treatment duration with procalcitonin measurement and an oral communication intervention made by a clinical pharmacist at a community teaching hospital with a well-established antimicrobial stewardship program. This may be due to several factors. First, the providers did not receive ongoing education regarding the appropriate use or interpretation of procalcitonin. The procalcitonin result in the electronic health record references the risk for progression to severe sepsis and/or septic shock, but does not indicate how to use procalcitonin as an aid in antibiotic decision-making. However, a recent study in patients with lower respiratory tract infections treated by providers who had been educated on the use of procalcitonin failed to find a reduction in total antibiotic use.9 Second, our study included hospital-wide use of procalcitonin, and was not limited to infections for which procalcitonin use has the strongest evidence (eg, upper respiratory tract infections, pneumonia, sepsis). Thus, providers may have been less likely to use protocolized guidelines. Last, we did not limit the data on antibiotic duration to patients with a procalcitonin level obtained within a defined time frame from antibiotic initiation or time of admission, and some patients had procalcitonin levels measured several days into their hospital stay. While this is likely to have skewed the data in favor of longer antibiotic treatment courses, it also represents a more realistic way in which this laboratory test is being used. Our post-hoc finding of earlier discontinuation of antibiotics after procalcitonin measurement suggests that our intervention may have influenced the decision to discontinue antibiotics. Such an effect may be augmented if procalcitonin is measured earlier in a hospital admission.
Previous studies have also failed to show that the use of procalcitonin decreased duration of antibiotics.9,10 In the aforementioned study regarding real-world outcomes in patients with lower respiratory tract infections, antibiotic duration was not reduced, despite provider education.9 A large observational study that evaluated real-world outcomes in intensive care unit patients did not find decreased antibiotic use or improved outcomes with procalcitonin use.10 With these large studies evaluating the 2 most common infectious diseases for which procalcitonin has previously been found to have clinical benefit, it is important for institutions to re-evaluate how procalcitonin is being utilized by providers. Furthermore, institutions should explore ways to optimize procalcitonin use and decrease unnecessary health care costs. Notably, the current community-acquired pneumonia guidelines recommend against routine use of procalcitonin.11
Conclusion
Outside of clinical trials, and in the absence of an algorithmic approach, procalcitonin has not consistently been shown to aid in the diagnosis or treatment of infectious diseases. It is important to have a comprehensive antimicrobial stewardship program that includes an algorithmic protocol to promote appropriate laboratory testing and reduce total antibiotic use. In addition to improved communication with providers, other interventions need to be investigated to effectively use this biomarker or limit its use.
Acknowledgment: The authors thank the Western Michigan University Department of Epidemiology and Biostatistics for their assistance in preparing this article.
Corresponding author: James Vaillant, MD, Western Michigan University, Homer Stryker MD School of Medicine, 1000 Oakland Drive, Kalamazoo, MI, 49008; james.vaillant@med.wmich.edu.
Financial disclosures: None.
1. Maruna P, Nedelníková K, Gürlich R. Physiology and genetics of procalcitonin. Physiol Res. 2000;(49 suppl 1):S57-S61.
2. Becker KL, Snider R, Nylen ES. Procalcitonin in sepsis and systemic inflammation: a harmful biomarker and a therapeutic target. Br J Pharmacol. 2010;159:253-264.
3. Vijayan AL, Vanimaya RS, Saikant R, et al. Procalcitonin: a promising diagnostic marker for sepsis and antibiotic therapy. J Intensive Care. 2017;5:51.
4. Hey J, Thompson-Leduc P, Kirson NY, et al. Procalcitonin guidance in patients with lower respiratory tract infections: A systematic review and meta-analysis. Clin Chem Lab Med. 2018;56:1200-1209.
5. Schuetz P, Wirz Y, Sager R, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2017;10:CD007498.
6. Huang HB, Peng JM, Weng L, et al. Procalcitonin-guided antibiotic therapy in intensive care unit patients: a systematic review and meta-analysis. Ann Intensive Care. 2017;7:114.
7. Stolz D, Christ-Crain M, Bingisser R, et al. Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin-guidance with standard therapy. Chest. 2007;131:9-19.
8. Prkno A, Wacker C, Brunkhorst FM, Schlattmann P. Procalcitonin-guided therapy in intensive care unit patients with severe sepsis and septic shock—a systematic review and meta-analysis. Crit Care. 2013;17:R291.
9. Huang DT, Yealy DM, Filbin MR, et al. Procalcitonin-guided use of antibiotics for lower respiratory tract infections. N Engl J Med. 2018;379:236-249.
10. Chu DC, Mehta AB, Walkey AJ. Practice patterns and outcomes associated with procalcitonin use in critically ill patients with sepsis. Clin Infect Dis. 2017;64:1509-1515.
11. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200:e45-e67.
From Western Michigan University, Homer Stryker MD School of Medicine, Kalamazoo, MI (Dr. Vaillant and Dr. Kavanaugh), Ferris State University, Grand Rapids, MI (Dr. Mersfelder), and Bronson Methodist Hospital, Kalamazoo, MI (Dr. Maynard).
Abstract
- Background: Procalcitonin has emerged as an important marker of sepsis and lung infections of bacterial origin. The role of procalcitonin in guiding antibiotic stewardship in lower respiratory tract infections and sepsis has been extensively studied, and use of this biomarker has been shown to decrease antibiotic usage in clinical trials. We sought to evaluate the impact of a pharmacist-driven initiative regarding discontinuation of antibiotics utilizing procalcitonin levels at a community teaching hospital.
- Methods: We retrospectively gathered baseline data on adult patients admitted to a community teaching hospital who were 18 years of age and older, under the care of an inpatient service, and had a single procalcitonin level < 0.25 mcg/L obtained during admission. We then prospectively identified an intervention group of similar patients using a web-based, real-time clinical surveillance system. When a low procalcitonin level was identified in the intervention group, the participating clinical pharmacists screened for antibiotic use and the indication(s), determined whether the antibiotic could be discontinued based on the low procalcitonin level and the absence of another indication for antibiotics, and, when appropriate, contacted the patient’s health care provider via telephone to discuss possible antibiotic discontinuation. The total antibiotic treatment duration was compared between the baseline and intervention groups.
- Results: A total of 172 patients were included in this study (86 in each group). The duration of antibiotic use was not significantly different between the baseline (3.14 ± 4.04 days) and the intervention (3.34 ± 2.8 days) groups (P = 0.1083). Other patient demographics did not influence antibiotic duration.
- Conclusion: Our study did not demonstrate a difference in total antibiotic treatment duration with the utilization of procalcitonin and an oral communication intervention made by a clinical pharmacist at a community-based teaching hospital. Outside of clinical trials, and in the absence of an algorithmic approach, procalcitonin has not consistently been shown to aid in the diagnosis and treatment of infectious diseases. It is important to have a comprehensive antimicrobial stewardship program to reduce antibiotic use and effectively use laboratory values.
Keywords: antibiotic use; bacterial infection; biomarkers; procalcitonin.
Procalcitonin is the precursor of the hormone calcitonin, which is normally produced in the parafollicular cells of the thyroid gland under physiological conditions.1 However, procalcitonin is also released in response to a proinflammatory stimulus, especially that of bacterial origin.1 The source of the procalcitonin surge seen during proinflammatory states is not the parafollicular cells of the thyroid, but rather the neuroendocrine cells of the lung and intestine.1 Stimulants of procalcitonin in these scenarios include bacterial endotoxin, tumor necrosis factor, and interleukin-6.1,2 Due to these observations, procalcitonin has emerged as an important marker of sepsis and lung infections of bacterial origin.3
The role of procalcitonin in guiding antibiotic stewardship in lower respiratory tract infections and sepsis has been extensively studied.4,5 Various randomized controlled trials have shown that antibiotic stewardship guided by procalcitonin levels resulted in lower rates of antibiotic initiation and shorter duration of antibiotic use.4-6 Similar results were obtained in prospective studies evaluating its role in patients with chronic obstructive pulmonary disease and sepsis.7,8 Based on these data, protocol-driven procalcitonin-guided antibiotic stewardship appears beneficial.
Many of these studies employed rigorous protocols. Studies of procalcitonin use in a so-called real-world setting, in which the provider can order and use procalcitonin levels without the use of protocols, are limited. The objective of our study was to evaluate the impact of a pharmacist-driven initiative on discontinuing antibiotics, if indicated, utilizing single procalcitonin measurement results of < 0.25 mcg/L at a community teaching hospital.
Methods
Our study utilized a 2-phase approach. The first phase was a retrospective chart review to establish baseline data regarding adult inpatients with a low procalcitonin level; these patients were randomly selected over a 1-year period (2017). Patients were included if they were 18 years of age or older, under the care of an inpatient service, and had a single procalcitonin level < 0.25 mcg/L obtained during their admission. Patients admitted to the intensive care unit were excluded. In the second phase, we prospectively identified similar patients admitted between January and March 2018 using a web-based, real-time clinical surveillance system. When patients with low procalcitonin levels were identified, 2 participating clinical pharmacists screened for antibiotic use and indication. If it was determined that the antibiotic could be discontinued as a result of the low procalcitonin level and no additional indication for antibiotics was present, the pharmacist contacted the patient’s health care provider via telephone to discuss possible antibiotic discontinuation. Data collected before and after the intervention included total antibiotic treatment duration, white blood cell count, maximum temperature, age, and procalcitonin level.
A sample size of 86 was calculated to provide an alpha of 0.05 and a power of 0.8. A nonparametric Wilcoxon 2-sample test was used to test for a difference in duration of antibiotic treatment between the baseline and intervention groups. A nonparametric test was used due to right-skewed data. All patients were included in the group analysis, regardless of antibiotic use, as the procalcitonin level may have been used in the decision to initiate antibiotics, and this is more representative of a real-world application of the test. This allowed for detection of a significant decrease of 2 days in antibiotic duration post intervention, with a 10% margin to compensate for potential missing data. Data from 86 patients obtained prior to the pharmacist intervention acted as a control comparison group. Statistical analysis was performed using SAS 9.4.
Results
A total of 172 patients were included in this study: 86 patients prior to the intervention, and 86 after implementation. Baseline demographics, laboratory values, vitals, and principal diagnoses for both groups are shown in Table 1 and Table 2. The most common indications for procalcitonin measurement were pneumonia (45.9%), chronic obstructive pulmonary disease (15.7%), and sepsis (14.5%). The remaining diagnoses were encephalopathy, fever and leukocytosis, skin and soft tissue infection, urinary tract infection or pyelonephritis, bone and joint infection, meningitis, intra-abdominal infection, and asthma exacerbation.
Antibiotic therapy was initiated in 68% of the patients overall, 59% in the baseline group and 76% in the intervention group. The duration of antibiotic use was not significantly different between the baseline (3.14 ± 4.04 days) and intervention (3.34 ± 2.8 days) groups (P = 0.1083). Furthermore, antibiotic treatment duration did not vary significantly with patient age, white blood cell count, maximum temperature, or procalcitonin level in either group. Although there was no difference in total antibiotic treatment duration, a post-hoc analysis revealed a 0.6-day decrease in the interval between the date of procalcitonin measurement and the stop date of antibiotics in the intervention group. The average time from admission to obtaining a procalcitonin level was 3 days in the baseline group and 2 days in the intervention group.
Discussion
Our study did not demonstrate a difference in total antibiotic treatment duration with procalcitonin measurement and an oral communication intervention made by a clinical pharmacist at a community teaching hospital with a well-established antimicrobial stewardship program. This may be due to several factors. First, the providers did not receive ongoing education regarding the appropriate use or interpretation of procalcitonin. The procalcitonin result in the electronic health record references the risk for progression to severe sepsis and/or septic shock, but does not indicate how to use procalcitonin as an aid in antibiotic decision-making. However, a recent study in patients with lower respiratory tract infections treated by providers who had been educated on the use of procalcitonin failed to find a reduction in total antibiotic use.9 Second, our study included hospital-wide use of procalcitonin, and was not limited to infections for which procalcitonin use has the strongest evidence (eg, upper respiratory tract infections, pneumonia, sepsis). Thus, providers may have been less likely to use protocolized guidelines. Last, we did not limit the data on antibiotic duration to patients with a procalcitonin level obtained within a defined time frame from antibiotic initiation or time of admission, and some patients had procalcitonin levels measured several days into their hospital stay. While this is likely to have skewed the data in favor of longer antibiotic treatment courses, it also represents a more realistic way in which this laboratory test is being used. Our post-hoc finding of earlier discontinuation of antibiotics after procalcitonin measurement suggests that our intervention may have influenced the decision to discontinue antibiotics. Such an effect may be augmented if procalcitonin is measured earlier in a hospital admission.
Previous studies have also failed to show that the use of procalcitonin decreased duration of antibiotics.9,10 In the aforementioned study regarding real-world outcomes in patients with lower respiratory tract infections, antibiotic duration was not reduced, despite provider education.9 A large observational study that evaluated real-world outcomes in intensive care unit patients did not find decreased antibiotic use or improved outcomes with procalcitonin use.10 With these large studies evaluating the 2 most common infectious diseases for which procalcitonin has previously been found to have clinical benefit, it is important for institutions to re-evaluate how procalcitonin is being utilized by providers. Furthermore, institutions should explore ways to optimize procalcitonin use and decrease unnecessary health care costs. Notably, the current community-acquired pneumonia guidelines recommend against routine use of procalcitonin.11
Conclusion
Outside of clinical trials, and in the absence of an algorithmic approach, procalcitonin has not consistently been shown to aid in the diagnosis or treatment of infectious diseases. It is important to have a comprehensive antimicrobial stewardship program that includes an algorithmic protocol to promote appropriate laboratory testing and reduce total antibiotic use. In addition to improved communication with providers, other interventions need to be investigated to effectively use this biomarker or limit its use.
Acknowledgment: The authors thank the Western Michigan University Department of Epidemiology and Biostatistics for their assistance in preparing this article.
Corresponding author: James Vaillant, MD, Western Michigan University, Homer Stryker MD School of Medicine, 1000 Oakland Drive, Kalamazoo, MI, 49008; james.vaillant@med.wmich.edu.
Financial disclosures: None.
From Western Michigan University, Homer Stryker MD School of Medicine, Kalamazoo, MI (Dr. Vaillant and Dr. Kavanaugh), Ferris State University, Grand Rapids, MI (Dr. Mersfelder), and Bronson Methodist Hospital, Kalamazoo, MI (Dr. Maynard).
Abstract
- Background: Procalcitonin has emerged as an important marker of sepsis and lung infections of bacterial origin. The role of procalcitonin in guiding antibiotic stewardship in lower respiratory tract infections and sepsis has been extensively studied, and use of this biomarker has been shown to decrease antibiotic usage in clinical trials. We sought to evaluate the impact of a pharmacist-driven initiative regarding discontinuation of antibiotics utilizing procalcitonin levels at a community teaching hospital.
- Methods: We retrospectively gathered baseline data on adult patients admitted to a community teaching hospital who were 18 years of age and older, under the care of an inpatient service, and had a single procalcitonin level < 0.25 mcg/L obtained during admission. We then prospectively identified an intervention group of similar patients using a web-based, real-time clinical surveillance system. When a low procalcitonin level was identified in the intervention group, the participating clinical pharmacists screened for antibiotic use and the indication(s), determined whether the antibiotic could be discontinued based on the low procalcitonin level and the absence of another indication for antibiotics, and, when appropriate, contacted the patient’s health care provider via telephone to discuss possible antibiotic discontinuation. The total antibiotic treatment duration was compared between the baseline and intervention groups.
- Results: A total of 172 patients were included in this study (86 in each group). The duration of antibiotic use was not significantly different between the baseline (3.14 ± 4.04 days) and the intervention (3.34 ± 2.8 days) groups (P = 0.1083). Other patient demographics did not influence antibiotic duration.
- Conclusion: Our study did not demonstrate a difference in total antibiotic treatment duration with the utilization of procalcitonin and an oral communication intervention made by a clinical pharmacist at a community-based teaching hospital. Outside of clinical trials, and in the absence of an algorithmic approach, procalcitonin has not consistently been shown to aid in the diagnosis and treatment of infectious diseases. It is important to have a comprehensive antimicrobial stewardship program to reduce antibiotic use and effectively use laboratory values.
Keywords: antibiotic use; bacterial infection; biomarkers; procalcitonin.
Procalcitonin is the precursor of the hormone calcitonin, which is normally produced in the parafollicular cells of the thyroid gland under physiological conditions.1 However, procalcitonin is also released in response to a proinflammatory stimulus, especially that of bacterial origin.1 The source of the procalcitonin surge seen during proinflammatory states is not the parafollicular cells of the thyroid, but rather the neuroendocrine cells of the lung and intestine.1 Stimulants of procalcitonin in these scenarios include bacterial endotoxin, tumor necrosis factor, and interleukin-6.1,2 Due to these observations, procalcitonin has emerged as an important marker of sepsis and lung infections of bacterial origin.3
The role of procalcitonin in guiding antibiotic stewardship in lower respiratory tract infections and sepsis has been extensively studied.4,5 Various randomized controlled trials have shown that antibiotic stewardship guided by procalcitonin levels resulted in lower rates of antibiotic initiation and shorter duration of antibiotic use.4-6 Similar results were obtained in prospective studies evaluating its role in patients with chronic obstructive pulmonary disease and sepsis.7,8 Based on these data, protocol-driven procalcitonin-guided antibiotic stewardship appears beneficial.
Many of these studies employed rigorous protocols. Studies of procalcitonin use in a so-called real-world setting, in which the provider can order and use procalcitonin levels without the use of protocols, are limited. The objective of our study was to evaluate the impact of a pharmacist-driven initiative on discontinuing antibiotics, if indicated, utilizing single procalcitonin measurement results of < 0.25 mcg/L at a community teaching hospital.
Methods
Our study utilized a 2-phase approach. The first phase was a retrospective chart review to establish baseline data regarding adult inpatients with a low procalcitonin level; these patients were randomly selected over a 1-year period (2017). Patients were included if they were 18 years of age or older, under the care of an inpatient service, and had a single procalcitonin level < 0.25 mcg/L obtained during their admission. Patients admitted to the intensive care unit were excluded. In the second phase, we prospectively identified similar patients admitted between January and March 2018 using a web-based, real-time clinical surveillance system. When patients with low procalcitonin levels were identified, 2 participating clinical pharmacists screened for antibiotic use and indication. If it was determined that the antibiotic could be discontinued as a result of the low procalcitonin level and no additional indication for antibiotics was present, the pharmacist contacted the patient’s health care provider via telephone to discuss possible antibiotic discontinuation. Data collected before and after the intervention included total antibiotic treatment duration, white blood cell count, maximum temperature, age, and procalcitonin level.
A sample size of 86 was calculated to provide an alpha of 0.05 and a power of 0.8. A nonparametric Wilcoxon 2-sample test was used to test for a difference in duration of antibiotic treatment between the baseline and intervention groups. A nonparametric test was used due to right-skewed data. All patients were included in the group analysis, regardless of antibiotic use, as the procalcitonin level may have been used in the decision to initiate antibiotics, and this is more representative of a real-world application of the test. This allowed for detection of a significant decrease of 2 days in antibiotic duration post intervention, with a 10% margin to compensate for potential missing data. Data from 86 patients obtained prior to the pharmacist intervention acted as a control comparison group. Statistical analysis was performed using SAS 9.4.
Results
A total of 172 patients were included in this study: 86 patients prior to the intervention, and 86 after implementation. Baseline demographics, laboratory values, vitals, and principal diagnoses for both groups are shown in Table 1 and Table 2. The most common indications for procalcitonin measurement were pneumonia (45.9%), chronic obstructive pulmonary disease (15.7%), and sepsis (14.5%). The remaining diagnoses were encephalopathy, fever and leukocytosis, skin and soft tissue infection, urinary tract infection or pyelonephritis, bone and joint infection, meningitis, intra-abdominal infection, and asthma exacerbation.
Antibiotic therapy was initiated in 68% of the patients overall, 59% in the baseline group and 76% in the intervention group. The duration of antibiotic use was not significantly different between the baseline (3.14 ± 4.04 days) and intervention (3.34 ± 2.8 days) groups (P = 0.1083). Furthermore, antibiotic treatment duration did not vary significantly with patient age, white blood cell count, maximum temperature, or procalcitonin level in either group. Although there was no difference in total antibiotic treatment duration, a post-hoc analysis revealed a 0.6-day decrease in the interval between the date of procalcitonin measurement and the stop date of antibiotics in the intervention group. The average time from admission to obtaining a procalcitonin level was 3 days in the baseline group and 2 days in the intervention group.
Discussion
Our study did not demonstrate a difference in total antibiotic treatment duration with procalcitonin measurement and an oral communication intervention made by a clinical pharmacist at a community teaching hospital with a well-established antimicrobial stewardship program. This may be due to several factors. First, the providers did not receive ongoing education regarding the appropriate use or interpretation of procalcitonin. The procalcitonin result in the electronic health record references the risk for progression to severe sepsis and/or septic shock, but does not indicate how to use procalcitonin as an aid in antibiotic decision-making. However, a recent study in patients with lower respiratory tract infections treated by providers who had been educated on the use of procalcitonin failed to find a reduction in total antibiotic use.9 Second, our study included hospital-wide use of procalcitonin, and was not limited to infections for which procalcitonin use has the strongest evidence (eg, upper respiratory tract infections, pneumonia, sepsis). Thus, providers may have been less likely to use protocolized guidelines. Last, we did not limit the data on antibiotic duration to patients with a procalcitonin level obtained within a defined time frame from antibiotic initiation or time of admission, and some patients had procalcitonin levels measured several days into their hospital stay. While this is likely to have skewed the data in favor of longer antibiotic treatment courses, it also represents a more realistic way in which this laboratory test is being used. Our post-hoc finding of earlier discontinuation of antibiotics after procalcitonin measurement suggests that our intervention may have influenced the decision to discontinue antibiotics. Such an effect may be augmented if procalcitonin is measured earlier in a hospital admission.
Previous studies have also failed to show that the use of procalcitonin decreased duration of antibiotics.9,10 In the aforementioned study regarding real-world outcomes in patients with lower respiratory tract infections, antibiotic duration was not reduced, despite provider education.9 A large observational study that evaluated real-world outcomes in intensive care unit patients did not find decreased antibiotic use or improved outcomes with procalcitonin use.10 With these large studies evaluating the 2 most common infectious diseases for which procalcitonin has previously been found to have clinical benefit, it is important for institutions to re-evaluate how procalcitonin is being utilized by providers. Furthermore, institutions should explore ways to optimize procalcitonin use and decrease unnecessary health care costs. Notably, the current community-acquired pneumonia guidelines recommend against routine use of procalcitonin.11
Conclusion
Outside of clinical trials, and in the absence of an algorithmic approach, procalcitonin has not consistently been shown to aid in the diagnosis or treatment of infectious diseases. It is important to have a comprehensive antimicrobial stewardship program that includes an algorithmic protocol to promote appropriate laboratory testing and reduce total antibiotic use. In addition to improved communication with providers, other interventions need to be investigated to effectively use this biomarker or limit its use.
Acknowledgment: The authors thank the Western Michigan University Department of Epidemiology and Biostatistics for their assistance in preparing this article.
Corresponding author: James Vaillant, MD, Western Michigan University, Homer Stryker MD School of Medicine, 1000 Oakland Drive, Kalamazoo, MI, 49008; james.vaillant@med.wmich.edu.
Financial disclosures: None.
1. Maruna P, Nedelníková K, Gürlich R. Physiology and genetics of procalcitonin. Physiol Res. 2000;(49 suppl 1):S57-S61.
2. Becker KL, Snider R, Nylen ES. Procalcitonin in sepsis and systemic inflammation: a harmful biomarker and a therapeutic target. Br J Pharmacol. 2010;159:253-264.
3. Vijayan AL, Vanimaya RS, Saikant R, et al. Procalcitonin: a promising diagnostic marker for sepsis and antibiotic therapy. J Intensive Care. 2017;5:51.
4. Hey J, Thompson-Leduc P, Kirson NY, et al. Procalcitonin guidance in patients with lower respiratory tract infections: A systematic review and meta-analysis. Clin Chem Lab Med. 2018;56:1200-1209.
5. Schuetz P, Wirz Y, Sager R, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2017;10:CD007498.
6. Huang HB, Peng JM, Weng L, et al. Procalcitonin-guided antibiotic therapy in intensive care unit patients: a systematic review and meta-analysis. Ann Intensive Care. 2017;7:114.
7. Stolz D, Christ-Crain M, Bingisser R, et al. Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin-guidance with standard therapy. Chest. 2007;131:9-19.
8. Prkno A, Wacker C, Brunkhorst FM, Schlattmann P. Procalcitonin-guided therapy in intensive care unit patients with severe sepsis and septic shock—a systematic review and meta-analysis. Crit Care. 2013;17:R291.
9. Huang DT, Yealy DM, Filbin MR, et al. Procalcitonin-guided use of antibiotics for lower respiratory tract infections. N Engl J Med. 2018;379:236-249.
10. Chu DC, Mehta AB, Walkey AJ. Practice patterns and outcomes associated with procalcitonin use in critically ill patients with sepsis. Clin Infect Dis. 2017;64:1509-1515.
11. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200:e45-e67.
1. Maruna P, Nedelníková K, Gürlich R. Physiology and genetics of procalcitonin. Physiol Res. 2000;(49 suppl 1):S57-S61.
2. Becker KL, Snider R, Nylen ES. Procalcitonin in sepsis and systemic inflammation: a harmful biomarker and a therapeutic target. Br J Pharmacol. 2010;159:253-264.
3. Vijayan AL, Vanimaya RS, Saikant R, et al. Procalcitonin: a promising diagnostic marker for sepsis and antibiotic therapy. J Intensive Care. 2017;5:51.
4. Hey J, Thompson-Leduc P, Kirson NY, et al. Procalcitonin guidance in patients with lower respiratory tract infections: A systematic review and meta-analysis. Clin Chem Lab Med. 2018;56:1200-1209.
5. Schuetz P, Wirz Y, Sager R, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2017;10:CD007498.
6. Huang HB, Peng JM, Weng L, et al. Procalcitonin-guided antibiotic therapy in intensive care unit patients: a systematic review and meta-analysis. Ann Intensive Care. 2017;7:114.
7. Stolz D, Christ-Crain M, Bingisser R, et al. Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin-guidance with standard therapy. Chest. 2007;131:9-19.
8. Prkno A, Wacker C, Brunkhorst FM, Schlattmann P. Procalcitonin-guided therapy in intensive care unit patients with severe sepsis and septic shock—a systematic review and meta-analysis. Crit Care. 2013;17:R291.
9. Huang DT, Yealy DM, Filbin MR, et al. Procalcitonin-guided use of antibiotics for lower respiratory tract infections. N Engl J Med. 2018;379:236-249.
10. Chu DC, Mehta AB, Walkey AJ. Practice patterns and outcomes associated with procalcitonin use in critically ill patients with sepsis. Clin Infect Dis. 2017;64:1509-1515.
11. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200:e45-e67.
Timing of Surgery in Patients With Asymptomatic Severe Aortic Stenosis
Study Overview
Objective. To determine the timing of surgical intervention in asymptomatic patients with severe aortic stenosis.
Design. Open-label, multicenter, randomized controlled study.
Setting and participants. A total of 145 asymptomatic patients with very severe aortic stenosis were randomly assigned to early surgery or conservative care.
Main outcome measures. The primary endpoint was a composite of operative mortality or death from a cardiovascular cause during follow-up. The major secondary endpoint was death from any cause during follow-up.
Main results. The primary endpoint occurred in 1 of 73 patients (1%) in the early surgery group and 11 of 72 patients (15%) in the conservative care group (hazard ratio [HR], 0.09; 95% confidence interval [CI], 0.01-0.67, P = 0.003). The secondary endpoint occurred in 7% of patients in the early surgery group and 21% of patients in the conservative care group (HR, 0.33; 95% CI, 0.12-0.90).
Conclusion. Among asymptomatic patients with very severe aortic stenosis, the incidence of the composite of operative mortality or death from cardiovascular causes during follow-up was significantly lower among those who underwent early valve replacement surgery compared to those who received conservative care.
Commentary
Aortic stenosis is a progressive disease that can lead to angina, heart failure, and death.1A higher mortality rate is reported in patients with symptomatic aortic stenosis, as compared to patients with asymptomatic disease, and current guidelines require symptoms to be present in order to proceed with aortic valve replacement.2 Management of asymptomatic patients is often determined by the treating physician, with treatment decisions based on multiple factors, such as left ventricular function, stress test results, and the local level of expertise for surgery.2
In this context, the RECOVERY investigators report the findings of their well-designed randomized controlled study assessing patients with asymptomatic severe aortic stenosis, which was defined as aortic valve area ≤ 0.75 cm2 and either transvalvular velocity > 4.5 m/s or a mean gradient ≥ 50 mm Hg. Compared to patients who received conservative care, patients who underwent early valve surgery had a significantly lower rate of a composite of operative mortality or death from any cardiovascular causes during follow-up. Notably, the number needed to treat to prevent 1 death from cardiovascular causes within 4 years was 20.
The strengths of this trial include complete long-term follow-up (> 4 years) and low cross-over rates. Furthermore, as the study targeted a previously understudied population, there were a number of interesting observations, in addition to the primary endpoint. First, the risk of sudden death was high in patients who received conservative care, 4% at 4 years and 14% at 8 years, a finding contrary to the common belief that asymptomatic patients are at lower risk of sudden cardiac death. Second, 74% of patients assigned to initial conservative care required aortic valve replacement during the follow-up period. Furthermore, when the patients assigned to conservative care required surgery, it was often performed emergently (17%), which could have contributed to the higher mortality in this group of patients. Finally, hospitalization for heart failure was more common in patients randomized to conservative care compared to patients with early surgery. These findings will help physicians conduct detailed, informed discussions with their patients regarding the risks/benefits of early surgery versus conservative management.
There are a few limitations of the RECOVERY trial to consider. First, this study investigated the effect of surgical aortic valve replacement; whether its findings can be extended to transcatheter aortic valve replacement (TAVR) requires further investigation. Patients who were enrolled in this study were younger and had fewer comorbidities than typical patients referred for TAVR. Second, all patients included in this study had the most severe form of aortic stenosis (valve area ≤ 0.75 cm2 with either a peak velocity of ≥ 4.5 m/s or mean gradient ≥ 50 mm Hg). Finally, the study was performed in highly experienced centers, as evidenced by a very low (0%) mortality rate after aortic valve replacement. Therefore, the finding may not be applicable to centers that have less experience with aortic valve replacement surgery.
Applications for Clinical Practice
The findings of the RECOVERY trial strongly suggest a mortality benefit of early surgery compared to conservative management in patients with asymptomatic severe aortic stenosis.
–Taishi Hirai, MD
1. Otto CM, Prendergast B. Aortic-valve stenosis--from patients at risk to severe valve obstruction. N Engl J Med. 2014;371:744-756.
2. Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e1159-e1195.
Study Overview
Objective. To determine the timing of surgical intervention in asymptomatic patients with severe aortic stenosis.
Design. Open-label, multicenter, randomized controlled study.
Setting and participants. A total of 145 asymptomatic patients with very severe aortic stenosis were randomly assigned to early surgery or conservative care.
Main outcome measures. The primary endpoint was a composite of operative mortality or death from a cardiovascular cause during follow-up. The major secondary endpoint was death from any cause during follow-up.
Main results. The primary endpoint occurred in 1 of 73 patients (1%) in the early surgery group and 11 of 72 patients (15%) in the conservative care group (hazard ratio [HR], 0.09; 95% confidence interval [CI], 0.01-0.67, P = 0.003). The secondary endpoint occurred in 7% of patients in the early surgery group and 21% of patients in the conservative care group (HR, 0.33; 95% CI, 0.12-0.90).
Conclusion. Among asymptomatic patients with very severe aortic stenosis, the incidence of the composite of operative mortality or death from cardiovascular causes during follow-up was significantly lower among those who underwent early valve replacement surgery compared to those who received conservative care.
Commentary
Aortic stenosis is a progressive disease that can lead to angina, heart failure, and death.1A higher mortality rate is reported in patients with symptomatic aortic stenosis, as compared to patients with asymptomatic disease, and current guidelines require symptoms to be present in order to proceed with aortic valve replacement.2 Management of asymptomatic patients is often determined by the treating physician, with treatment decisions based on multiple factors, such as left ventricular function, stress test results, and the local level of expertise for surgery.2
In this context, the RECOVERY investigators report the findings of their well-designed randomized controlled study assessing patients with asymptomatic severe aortic stenosis, which was defined as aortic valve area ≤ 0.75 cm2 and either transvalvular velocity > 4.5 m/s or a mean gradient ≥ 50 mm Hg. Compared to patients who received conservative care, patients who underwent early valve surgery had a significantly lower rate of a composite of operative mortality or death from any cardiovascular causes during follow-up. Notably, the number needed to treat to prevent 1 death from cardiovascular causes within 4 years was 20.
The strengths of this trial include complete long-term follow-up (> 4 years) and low cross-over rates. Furthermore, as the study targeted a previously understudied population, there were a number of interesting observations, in addition to the primary endpoint. First, the risk of sudden death was high in patients who received conservative care, 4% at 4 years and 14% at 8 years, a finding contrary to the common belief that asymptomatic patients are at lower risk of sudden cardiac death. Second, 74% of patients assigned to initial conservative care required aortic valve replacement during the follow-up period. Furthermore, when the patients assigned to conservative care required surgery, it was often performed emergently (17%), which could have contributed to the higher mortality in this group of patients. Finally, hospitalization for heart failure was more common in patients randomized to conservative care compared to patients with early surgery. These findings will help physicians conduct detailed, informed discussions with their patients regarding the risks/benefits of early surgery versus conservative management.
There are a few limitations of the RECOVERY trial to consider. First, this study investigated the effect of surgical aortic valve replacement; whether its findings can be extended to transcatheter aortic valve replacement (TAVR) requires further investigation. Patients who were enrolled in this study were younger and had fewer comorbidities than typical patients referred for TAVR. Second, all patients included in this study had the most severe form of aortic stenosis (valve area ≤ 0.75 cm2 with either a peak velocity of ≥ 4.5 m/s or mean gradient ≥ 50 mm Hg). Finally, the study was performed in highly experienced centers, as evidenced by a very low (0%) mortality rate after aortic valve replacement. Therefore, the finding may not be applicable to centers that have less experience with aortic valve replacement surgery.
Applications for Clinical Practice
The findings of the RECOVERY trial strongly suggest a mortality benefit of early surgery compared to conservative management in patients with asymptomatic severe aortic stenosis.
–Taishi Hirai, MD
Study Overview
Objective. To determine the timing of surgical intervention in asymptomatic patients with severe aortic stenosis.
Design. Open-label, multicenter, randomized controlled study.
Setting and participants. A total of 145 asymptomatic patients with very severe aortic stenosis were randomly assigned to early surgery or conservative care.
Main outcome measures. The primary endpoint was a composite of operative mortality or death from a cardiovascular cause during follow-up. The major secondary endpoint was death from any cause during follow-up.
Main results. The primary endpoint occurred in 1 of 73 patients (1%) in the early surgery group and 11 of 72 patients (15%) in the conservative care group (hazard ratio [HR], 0.09; 95% confidence interval [CI], 0.01-0.67, P = 0.003). The secondary endpoint occurred in 7% of patients in the early surgery group and 21% of patients in the conservative care group (HR, 0.33; 95% CI, 0.12-0.90).
Conclusion. Among asymptomatic patients with very severe aortic stenosis, the incidence of the composite of operative mortality or death from cardiovascular causes during follow-up was significantly lower among those who underwent early valve replacement surgery compared to those who received conservative care.
Commentary
Aortic stenosis is a progressive disease that can lead to angina, heart failure, and death.1A higher mortality rate is reported in patients with symptomatic aortic stenosis, as compared to patients with asymptomatic disease, and current guidelines require symptoms to be present in order to proceed with aortic valve replacement.2 Management of asymptomatic patients is often determined by the treating physician, with treatment decisions based on multiple factors, such as left ventricular function, stress test results, and the local level of expertise for surgery.2
In this context, the RECOVERY investigators report the findings of their well-designed randomized controlled study assessing patients with asymptomatic severe aortic stenosis, which was defined as aortic valve area ≤ 0.75 cm2 and either transvalvular velocity > 4.5 m/s or a mean gradient ≥ 50 mm Hg. Compared to patients who received conservative care, patients who underwent early valve surgery had a significantly lower rate of a composite of operative mortality or death from any cardiovascular causes during follow-up. Notably, the number needed to treat to prevent 1 death from cardiovascular causes within 4 years was 20.
The strengths of this trial include complete long-term follow-up (> 4 years) and low cross-over rates. Furthermore, as the study targeted a previously understudied population, there were a number of interesting observations, in addition to the primary endpoint. First, the risk of sudden death was high in patients who received conservative care, 4% at 4 years and 14% at 8 years, a finding contrary to the common belief that asymptomatic patients are at lower risk of sudden cardiac death. Second, 74% of patients assigned to initial conservative care required aortic valve replacement during the follow-up period. Furthermore, when the patients assigned to conservative care required surgery, it was often performed emergently (17%), which could have contributed to the higher mortality in this group of patients. Finally, hospitalization for heart failure was more common in patients randomized to conservative care compared to patients with early surgery. These findings will help physicians conduct detailed, informed discussions with their patients regarding the risks/benefits of early surgery versus conservative management.
There are a few limitations of the RECOVERY trial to consider. First, this study investigated the effect of surgical aortic valve replacement; whether its findings can be extended to transcatheter aortic valve replacement (TAVR) requires further investigation. Patients who were enrolled in this study were younger and had fewer comorbidities than typical patients referred for TAVR. Second, all patients included in this study had the most severe form of aortic stenosis (valve area ≤ 0.75 cm2 with either a peak velocity of ≥ 4.5 m/s or mean gradient ≥ 50 mm Hg). Finally, the study was performed in highly experienced centers, as evidenced by a very low (0%) mortality rate after aortic valve replacement. Therefore, the finding may not be applicable to centers that have less experience with aortic valve replacement surgery.
Applications for Clinical Practice
The findings of the RECOVERY trial strongly suggest a mortality benefit of early surgery compared to conservative management in patients with asymptomatic severe aortic stenosis.
–Taishi Hirai, MD
1. Otto CM, Prendergast B. Aortic-valve stenosis--from patients at risk to severe valve obstruction. N Engl J Med. 2014;371:744-756.
2. Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e1159-e1195.
1. Otto CM, Prendergast B. Aortic-valve stenosis--from patients at risk to severe valve obstruction. N Engl J Med. 2014;371:744-756.
2. Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e1159-e1195.
How Does Telemedicine Compare to Conventional Follow-Up After General Surgery?
Study Overview
Objective. To compare the impact of conventional versus telemedicine follow-up of general surgery patients in outpatient clinics.
Design. Prospective randomized clinical trial.
Setting and participants. Participants were recruited from Hospital Germans Trias i Pujol, a tertiary care university hospital located in the outskirts of Barcelona (Catalonia, Spain). To be included in this study, participants had to have been treated in the general surgery department, have basic computer knowledge (ability to use e-mail or a social network), have a computer with webcam, and be 18 to 75 years of age, or they had to have a partner who met these criteria. Exclusion criteria included any disability making telemedicine follow-up impossible (eg, blindness, deafness, or mental disability; proctologic treatment; difficulty describing and/or showing complications in the surgical area; and clinical complications before discharge more severe than Clavien Dindo II), as well as withdrawal of consent. Patients who met the criteria and had just been discharged from the hospital were offered the opportunity to enroll by the surgeon in charge. Patients who agreed to participate provided informed consent and were assigned using a computerized block randomization list (allocation ratio 1:1).
Intervention. Time to visit was generally between 2 and 4 weeks after discharge (the interval to the follow-up visit was determined at the discretion of the treating surgeon, but always followed the usual schedule). To conduct the telemedicine follow-up through a video call, a medical cloud-based program fulfilling all European Union security and privacy policies was used. Four surgeons were assigned to perform the telemedicine visits and were trained on how to use the program before the study started. Visit format was the same in both groups: clinical and wound condition were assessed and pathology was discussed (the one difference was that physical exploration was not performed in the telemedicine group).
Main outcome measures. The primary outcome was the feasibility of telemedicine follow-up, and this was measured as the percentage of participants who completed follow-up in their corresponding group by the date scheduled at hospital discharge. Secondary outcomes included a comparison of clinical results and patient satisfaction. To assess the clinical results, extra visits to an outpatient clinic and/or the emergency department during the first 30 days after the follow-up visit were collected.
To evaluate patient satisfaction, a questionnaire was sent via email to the participants after the visit and, if they did not respond, a telephone survey was carried out (if there was no contact after 2 telephone calls, the participants was considered a missing value). The questionnaire was informed by the United Kingdom National Health Service outpatients questionnaire and the Telehealth Usability Questionnaire. It included 27 general questions asked of participants in both groups, plus 8 specific questions for participants in the conventional follow-up group and 14 specific questions for participants in the telemedicine group. To summarize all the included fields in the questionnaires (time to visit and visit length, comfort, tests and procedures performed before and during the visit, transport, waiting time, privacy, dealings with staff, platform usability, telemedicine, and satisfaction), participants were asked to provide a global satisfaction score on a scale from 1 to 5.
Analysis. To compare the groups in terms of proportion of outcomes, a chi-square test was used to analyze categorical variables. To compare medians between the groups, ordinal variables were analyzed using the Mann-Whitney U test. Statistical significance was set at P < 0.05.
Main results. Two-hundred patients were randomly allocated to 1 of the 2 groups, with 100 patients in each group. The groups did not differ significantly based on age (P = 0.836), gender (P = 0.393), or American Society of Anesthesiologists (ASA) score (P = 0.232). Time to visit did not differ significantly between the groups (P = 0.169), and while visits were generally shorter in the telemedicine group, the difference was not significant (P = 0.153). Diagnoses and treatments did not differ significantly between the groups (P = 0.853 and P = 0.461, respectively).
The primary outcome (follow-up feasibility) was achieved in 90% of the conventional follow-up group and in 74% of the telemedicine group (P = 0.003). Of the 10 patients in the conventional follow-up group who did not complete the follow-up, 8 did not attend the visit on the scheduled day and 2 were hospitalized for reasons not related to the study. In the telemedicine group, the 2 main reasons for failure to follow-up were technical difficulties (n = 10) and requests by patients to attend a conventional visit after being allocated to the telemedicine group (n = 10). Among the remaining 6 patients in the telemedicine group who did not attend a visit, 3 visited the outpatient clinic because of a known surgical wound infection before the visit, 2 did not respond to the video call and could not be contacted by other means, and 1 had other face-to-face visits scheduled in different departments of the hospital the same day as the telemedicine appointment.
There were no statistically significant differences in the clinical results of the 164 patients meeting the primary endpoint (P = 0.832). Twelve of the 90 (13.3%) patients in the conventional group attended extra visits after the follow-up, while 9 of the 74 patients (12.1%) in the telemedicine group (P = 0.823) attended extra visits after follow-up. The median global patient satisfaction score was 5 in both the conventional group (range, 2-5) and the telemedicine group (range, 1-5), with no statistically significant differences (P = 0.099). When patients in the telemedicine group were asked if they would accept the use of telemedicine as part of their medical treatment on an ongoing basis, they rated the proposition with a median score of 5 (range, 1-5).
Conclusion. Telemedicine is a feasible and acceptable complementary service to facilitate postoperative management in selected general surgery patients. This option produces good satisfaction rates and maintains clinical outcomes.
Commentary
In recent years, telemedicine has gained increased popularity in both medicine and surgery, affording surgeons greater opportunities for patient care, mentoring, collaboration, and teaching, without the limits of geographic boundaries. Telemedicine can be broadly described as a health care service utilizing telecommunication technologies for the purpose of communicating with and diagnosing and treating patients remotely.1-4 To date, literature on telemedicine in surgical care has been limited.
In their systematic review, published in 2018, Asiri et al identified 24 studies published between 1998 and 2018, which included 3 randomized controlled trials, 3 pilot studies, 4 retrospective studies, and 14 prospective observational studies. In these studies, telemedicine protocols were used for preoperative assessment, diagnostic purposes, or consultation with another surgical department (10 studies); postoperative wound assessment (9 studies); and follow-up in place of conventional clinic visits (5 studies).3 In a 2017 systematic review of telemedicine for post-discharge surgical care, Gunter et al identified 21 studies, which included 3 randomized controlled trials, 6 pilot or feasibility studies, 4 retrospective record reviews, 2 case series, and 6 surveys.4 In these studies, telemedicine protocols were used for scheduled follow-up (10 studies), routine and ongoing monitoring (5 studies), or management of issues that arose after surgery (2 studies). These 2 reviews found telemedicine to be feasible, useful, and acceptable for postoperative evaluation and follow-up among both providers and patients.
Additional benefits noted in these studies included savings in patient travel, time, and cost. Perspectives on savings to the health system were mixed—while clinic time slots may open as a result of follow-up visits being done via telemedicine (resulting in potential improvements in access to surgical services and decreased wait times), there are still significant direct costs for purchasing necessary equipment and for educating and training providers on the use of the equipment. Other published reviews have discussed in greater detail the application, benefits, limitations, and barriers to telemedicine and provided insight from the perspectives of patients, providers, and health care systems.1,2
Because studies on the use of telemedicine are limited, particularly in general surgery, and few of these studies have used a randomized clinical trial design, the present study is an important contribution to the literature. The authors found a significant difference between groups in terms of percentage of completed follow-up visits—90% of conventional follow-up group participants completed their visit versus 74% of telemedicine group participants. However, these differences were primarily attributed to technical difficulties experienced by telemedicine group participants, as well requests to have a conventional follow-up visit. In addition, telemedicine capabilities were limited to video calls via computers and webcams, and it is likely that successful completion of the follow-up visit would have been higher in the telemedicine group had the use of video calls via tablets or smartphones been an option. Perhaps more important, no significant differences were found in clinical outcomes (extra visits within 30 days after the follow-up visit) or patient satisfaction.
A key strength of this study is the use of a randomized clinical trial design to evaluate telemedicine as an alternative method for conducting patient visits following general surgery. Inclusion and exclusion criteria did not impose strict limitations on potential participants. Also, the authors evaluated differences in time to visit, length of visit, clinical results, and patient satisfaction between groups, in addition to the primary measure of completion of the follow-up visit.
This study has important limitations that should be noted as well, particularly related to the study design, some of which are acknowledged by the authors. Because this study was implemented in only 1 hospital, specifically, a tertiary care university hospital on the outskirts of an urban European city, the generalizability of the findings is limited. Also, the likelihood of selection bias is high, as enrollment was not offered to all patients who were discharged from the hospital and met inclusion criteria (limited by patient workload). The comparison of clinical results was limited, as the selected measure focused only on extra visits to an outpatient clinic and/or the emergency department during the first 30 days after the follow-up visit. This chosen measure does not account for less severe clinical results that did not require an additional visit, and does not represent a nuanced comparison of specific clinical indicators. In addition, this measure does not account for clinical complications that may have occurred beyond the 30-day period. Recall bias also was likely, given that the patient satisfaction questionnaire was delivered via email to patients at a later time after the follow-up visit, instead of being administered immediately after the visit. Last, group differences at baseline were assessed based only on age, gender, and ASA score, which does not preclude potential differences related to other factors, such as race/ethnicity, household income, comorbidities, insurance, and zip code. Future research with a similar objective would benefit from a randomized clinical trial design that recruits a wider diversity of patients across different clinic settings and incorporates more nuanced measures of primary and secondary outcomes.
Applications for Clinical Practice
With the ongoing COVID-19 pandemic, the integration of telemedicine capabilities into hospital systems is becoming more widespread and is proceeding at an accelerated pace. This study provides evidence that telemedicine is a feasible and acceptable complementary service to facilitate postoperative management in selected general surgery patients. Assuming that the needed technology and appropriate program training are available, telemedicine should be offered to patients, especially to maximize savings in terms of travel, time, and cost. However, the option for conventional (in-person) follow-up should remain, particularly in cases where there may be barriers to successful follow-up visits via telemedicine, including limited digital literacy, lack of access to necessary equipment, language/communication barriers, complex follow-up treatment, and difficulties in describing or showing complications in the surgical area.
–Katrina F. Mateo, PhD, MPH
1. Williams AM, Bhatti UF, Alam HB, Nikolian VC. The role of telemedicine in postoperative care. mHealth. 2018 May;4:11-11.
2. Huang EY, Knight S, Guetter CR et al. Telemedicine and telementoring in the surgical specialties: A narrative review. Am J Surg. 2019;218:760-766.
3. Asiri A, AlBishi S, AlMadani W, et al. The use of telemedicine in surgical care: A systematic review. Acta Informatica Medica. 2018;26:201-206.
4. Gunter RL, Chouinard S, Fernandes-Taylor S, et al. Current use of telemedicine for post-discharge surgical care: a systematic review. J Am College Surg. 2016;222:915-927.
Study Overview
Objective. To compare the impact of conventional versus telemedicine follow-up of general surgery patients in outpatient clinics.
Design. Prospective randomized clinical trial.
Setting and participants. Participants were recruited from Hospital Germans Trias i Pujol, a tertiary care university hospital located in the outskirts of Barcelona (Catalonia, Spain). To be included in this study, participants had to have been treated in the general surgery department, have basic computer knowledge (ability to use e-mail or a social network), have a computer with webcam, and be 18 to 75 years of age, or they had to have a partner who met these criteria. Exclusion criteria included any disability making telemedicine follow-up impossible (eg, blindness, deafness, or mental disability; proctologic treatment; difficulty describing and/or showing complications in the surgical area; and clinical complications before discharge more severe than Clavien Dindo II), as well as withdrawal of consent. Patients who met the criteria and had just been discharged from the hospital were offered the opportunity to enroll by the surgeon in charge. Patients who agreed to participate provided informed consent and were assigned using a computerized block randomization list (allocation ratio 1:1).
Intervention. Time to visit was generally between 2 and 4 weeks after discharge (the interval to the follow-up visit was determined at the discretion of the treating surgeon, but always followed the usual schedule). To conduct the telemedicine follow-up through a video call, a medical cloud-based program fulfilling all European Union security and privacy policies was used. Four surgeons were assigned to perform the telemedicine visits and were trained on how to use the program before the study started. Visit format was the same in both groups: clinical and wound condition were assessed and pathology was discussed (the one difference was that physical exploration was not performed in the telemedicine group).
Main outcome measures. The primary outcome was the feasibility of telemedicine follow-up, and this was measured as the percentage of participants who completed follow-up in their corresponding group by the date scheduled at hospital discharge. Secondary outcomes included a comparison of clinical results and patient satisfaction. To assess the clinical results, extra visits to an outpatient clinic and/or the emergency department during the first 30 days after the follow-up visit were collected.
To evaluate patient satisfaction, a questionnaire was sent via email to the participants after the visit and, if they did not respond, a telephone survey was carried out (if there was no contact after 2 telephone calls, the participants was considered a missing value). The questionnaire was informed by the United Kingdom National Health Service outpatients questionnaire and the Telehealth Usability Questionnaire. It included 27 general questions asked of participants in both groups, plus 8 specific questions for participants in the conventional follow-up group and 14 specific questions for participants in the telemedicine group. To summarize all the included fields in the questionnaires (time to visit and visit length, comfort, tests and procedures performed before and during the visit, transport, waiting time, privacy, dealings with staff, platform usability, telemedicine, and satisfaction), participants were asked to provide a global satisfaction score on a scale from 1 to 5.
Analysis. To compare the groups in terms of proportion of outcomes, a chi-square test was used to analyze categorical variables. To compare medians between the groups, ordinal variables were analyzed using the Mann-Whitney U test. Statistical significance was set at P < 0.05.
Main results. Two-hundred patients were randomly allocated to 1 of the 2 groups, with 100 patients in each group. The groups did not differ significantly based on age (P = 0.836), gender (P = 0.393), or American Society of Anesthesiologists (ASA) score (P = 0.232). Time to visit did not differ significantly between the groups (P = 0.169), and while visits were generally shorter in the telemedicine group, the difference was not significant (P = 0.153). Diagnoses and treatments did not differ significantly between the groups (P = 0.853 and P = 0.461, respectively).
The primary outcome (follow-up feasibility) was achieved in 90% of the conventional follow-up group and in 74% of the telemedicine group (P = 0.003). Of the 10 patients in the conventional follow-up group who did not complete the follow-up, 8 did not attend the visit on the scheduled day and 2 were hospitalized for reasons not related to the study. In the telemedicine group, the 2 main reasons for failure to follow-up were technical difficulties (n = 10) and requests by patients to attend a conventional visit after being allocated to the telemedicine group (n = 10). Among the remaining 6 patients in the telemedicine group who did not attend a visit, 3 visited the outpatient clinic because of a known surgical wound infection before the visit, 2 did not respond to the video call and could not be contacted by other means, and 1 had other face-to-face visits scheduled in different departments of the hospital the same day as the telemedicine appointment.
There were no statistically significant differences in the clinical results of the 164 patients meeting the primary endpoint (P = 0.832). Twelve of the 90 (13.3%) patients in the conventional group attended extra visits after the follow-up, while 9 of the 74 patients (12.1%) in the telemedicine group (P = 0.823) attended extra visits after follow-up. The median global patient satisfaction score was 5 in both the conventional group (range, 2-5) and the telemedicine group (range, 1-5), with no statistically significant differences (P = 0.099). When patients in the telemedicine group were asked if they would accept the use of telemedicine as part of their medical treatment on an ongoing basis, they rated the proposition with a median score of 5 (range, 1-5).
Conclusion. Telemedicine is a feasible and acceptable complementary service to facilitate postoperative management in selected general surgery patients. This option produces good satisfaction rates and maintains clinical outcomes.
Commentary
In recent years, telemedicine has gained increased popularity in both medicine and surgery, affording surgeons greater opportunities for patient care, mentoring, collaboration, and teaching, without the limits of geographic boundaries. Telemedicine can be broadly described as a health care service utilizing telecommunication technologies for the purpose of communicating with and diagnosing and treating patients remotely.1-4 To date, literature on telemedicine in surgical care has been limited.
In their systematic review, published in 2018, Asiri et al identified 24 studies published between 1998 and 2018, which included 3 randomized controlled trials, 3 pilot studies, 4 retrospective studies, and 14 prospective observational studies. In these studies, telemedicine protocols were used for preoperative assessment, diagnostic purposes, or consultation with another surgical department (10 studies); postoperative wound assessment (9 studies); and follow-up in place of conventional clinic visits (5 studies).3 In a 2017 systematic review of telemedicine for post-discharge surgical care, Gunter et al identified 21 studies, which included 3 randomized controlled trials, 6 pilot or feasibility studies, 4 retrospective record reviews, 2 case series, and 6 surveys.4 In these studies, telemedicine protocols were used for scheduled follow-up (10 studies), routine and ongoing monitoring (5 studies), or management of issues that arose after surgery (2 studies). These 2 reviews found telemedicine to be feasible, useful, and acceptable for postoperative evaluation and follow-up among both providers and patients.
Additional benefits noted in these studies included savings in patient travel, time, and cost. Perspectives on savings to the health system were mixed—while clinic time slots may open as a result of follow-up visits being done via telemedicine (resulting in potential improvements in access to surgical services and decreased wait times), there are still significant direct costs for purchasing necessary equipment and for educating and training providers on the use of the equipment. Other published reviews have discussed in greater detail the application, benefits, limitations, and barriers to telemedicine and provided insight from the perspectives of patients, providers, and health care systems.1,2
Because studies on the use of telemedicine are limited, particularly in general surgery, and few of these studies have used a randomized clinical trial design, the present study is an important contribution to the literature. The authors found a significant difference between groups in terms of percentage of completed follow-up visits—90% of conventional follow-up group participants completed their visit versus 74% of telemedicine group participants. However, these differences were primarily attributed to technical difficulties experienced by telemedicine group participants, as well requests to have a conventional follow-up visit. In addition, telemedicine capabilities were limited to video calls via computers and webcams, and it is likely that successful completion of the follow-up visit would have been higher in the telemedicine group had the use of video calls via tablets or smartphones been an option. Perhaps more important, no significant differences were found in clinical outcomes (extra visits within 30 days after the follow-up visit) or patient satisfaction.
A key strength of this study is the use of a randomized clinical trial design to evaluate telemedicine as an alternative method for conducting patient visits following general surgery. Inclusion and exclusion criteria did not impose strict limitations on potential participants. Also, the authors evaluated differences in time to visit, length of visit, clinical results, and patient satisfaction between groups, in addition to the primary measure of completion of the follow-up visit.
This study has important limitations that should be noted as well, particularly related to the study design, some of which are acknowledged by the authors. Because this study was implemented in only 1 hospital, specifically, a tertiary care university hospital on the outskirts of an urban European city, the generalizability of the findings is limited. Also, the likelihood of selection bias is high, as enrollment was not offered to all patients who were discharged from the hospital and met inclusion criteria (limited by patient workload). The comparison of clinical results was limited, as the selected measure focused only on extra visits to an outpatient clinic and/or the emergency department during the first 30 days after the follow-up visit. This chosen measure does not account for less severe clinical results that did not require an additional visit, and does not represent a nuanced comparison of specific clinical indicators. In addition, this measure does not account for clinical complications that may have occurred beyond the 30-day period. Recall bias also was likely, given that the patient satisfaction questionnaire was delivered via email to patients at a later time after the follow-up visit, instead of being administered immediately after the visit. Last, group differences at baseline were assessed based only on age, gender, and ASA score, which does not preclude potential differences related to other factors, such as race/ethnicity, household income, comorbidities, insurance, and zip code. Future research with a similar objective would benefit from a randomized clinical trial design that recruits a wider diversity of patients across different clinic settings and incorporates more nuanced measures of primary and secondary outcomes.
Applications for Clinical Practice
With the ongoing COVID-19 pandemic, the integration of telemedicine capabilities into hospital systems is becoming more widespread and is proceeding at an accelerated pace. This study provides evidence that telemedicine is a feasible and acceptable complementary service to facilitate postoperative management in selected general surgery patients. Assuming that the needed technology and appropriate program training are available, telemedicine should be offered to patients, especially to maximize savings in terms of travel, time, and cost. However, the option for conventional (in-person) follow-up should remain, particularly in cases where there may be barriers to successful follow-up visits via telemedicine, including limited digital literacy, lack of access to necessary equipment, language/communication barriers, complex follow-up treatment, and difficulties in describing or showing complications in the surgical area.
–Katrina F. Mateo, PhD, MPH
Study Overview
Objective. To compare the impact of conventional versus telemedicine follow-up of general surgery patients in outpatient clinics.
Design. Prospective randomized clinical trial.
Setting and participants. Participants were recruited from Hospital Germans Trias i Pujol, a tertiary care university hospital located in the outskirts of Barcelona (Catalonia, Spain). To be included in this study, participants had to have been treated in the general surgery department, have basic computer knowledge (ability to use e-mail or a social network), have a computer with webcam, and be 18 to 75 years of age, or they had to have a partner who met these criteria. Exclusion criteria included any disability making telemedicine follow-up impossible (eg, blindness, deafness, or mental disability; proctologic treatment; difficulty describing and/or showing complications in the surgical area; and clinical complications before discharge more severe than Clavien Dindo II), as well as withdrawal of consent. Patients who met the criteria and had just been discharged from the hospital were offered the opportunity to enroll by the surgeon in charge. Patients who agreed to participate provided informed consent and were assigned using a computerized block randomization list (allocation ratio 1:1).
Intervention. Time to visit was generally between 2 and 4 weeks after discharge (the interval to the follow-up visit was determined at the discretion of the treating surgeon, but always followed the usual schedule). To conduct the telemedicine follow-up through a video call, a medical cloud-based program fulfilling all European Union security and privacy policies was used. Four surgeons were assigned to perform the telemedicine visits and were trained on how to use the program before the study started. Visit format was the same in both groups: clinical and wound condition were assessed and pathology was discussed (the one difference was that physical exploration was not performed in the telemedicine group).
Main outcome measures. The primary outcome was the feasibility of telemedicine follow-up, and this was measured as the percentage of participants who completed follow-up in their corresponding group by the date scheduled at hospital discharge. Secondary outcomes included a comparison of clinical results and patient satisfaction. To assess the clinical results, extra visits to an outpatient clinic and/or the emergency department during the first 30 days after the follow-up visit were collected.
To evaluate patient satisfaction, a questionnaire was sent via email to the participants after the visit and, if they did not respond, a telephone survey was carried out (if there was no contact after 2 telephone calls, the participants was considered a missing value). The questionnaire was informed by the United Kingdom National Health Service outpatients questionnaire and the Telehealth Usability Questionnaire. It included 27 general questions asked of participants in both groups, plus 8 specific questions for participants in the conventional follow-up group and 14 specific questions for participants in the telemedicine group. To summarize all the included fields in the questionnaires (time to visit and visit length, comfort, tests and procedures performed before and during the visit, transport, waiting time, privacy, dealings with staff, platform usability, telemedicine, and satisfaction), participants were asked to provide a global satisfaction score on a scale from 1 to 5.
Analysis. To compare the groups in terms of proportion of outcomes, a chi-square test was used to analyze categorical variables. To compare medians between the groups, ordinal variables were analyzed using the Mann-Whitney U test. Statistical significance was set at P < 0.05.
Main results. Two-hundred patients were randomly allocated to 1 of the 2 groups, with 100 patients in each group. The groups did not differ significantly based on age (P = 0.836), gender (P = 0.393), or American Society of Anesthesiologists (ASA) score (P = 0.232). Time to visit did not differ significantly between the groups (P = 0.169), and while visits were generally shorter in the telemedicine group, the difference was not significant (P = 0.153). Diagnoses and treatments did not differ significantly between the groups (P = 0.853 and P = 0.461, respectively).
The primary outcome (follow-up feasibility) was achieved in 90% of the conventional follow-up group and in 74% of the telemedicine group (P = 0.003). Of the 10 patients in the conventional follow-up group who did not complete the follow-up, 8 did not attend the visit on the scheduled day and 2 were hospitalized for reasons not related to the study. In the telemedicine group, the 2 main reasons for failure to follow-up were technical difficulties (n = 10) and requests by patients to attend a conventional visit after being allocated to the telemedicine group (n = 10). Among the remaining 6 patients in the telemedicine group who did not attend a visit, 3 visited the outpatient clinic because of a known surgical wound infection before the visit, 2 did not respond to the video call and could not be contacted by other means, and 1 had other face-to-face visits scheduled in different departments of the hospital the same day as the telemedicine appointment.
There were no statistically significant differences in the clinical results of the 164 patients meeting the primary endpoint (P = 0.832). Twelve of the 90 (13.3%) patients in the conventional group attended extra visits after the follow-up, while 9 of the 74 patients (12.1%) in the telemedicine group (P = 0.823) attended extra visits after follow-up. The median global patient satisfaction score was 5 in both the conventional group (range, 2-5) and the telemedicine group (range, 1-5), with no statistically significant differences (P = 0.099). When patients in the telemedicine group were asked if they would accept the use of telemedicine as part of their medical treatment on an ongoing basis, they rated the proposition with a median score of 5 (range, 1-5).
Conclusion. Telemedicine is a feasible and acceptable complementary service to facilitate postoperative management in selected general surgery patients. This option produces good satisfaction rates and maintains clinical outcomes.
Commentary
In recent years, telemedicine has gained increased popularity in both medicine and surgery, affording surgeons greater opportunities for patient care, mentoring, collaboration, and teaching, without the limits of geographic boundaries. Telemedicine can be broadly described as a health care service utilizing telecommunication technologies for the purpose of communicating with and diagnosing and treating patients remotely.1-4 To date, literature on telemedicine in surgical care has been limited.
In their systematic review, published in 2018, Asiri et al identified 24 studies published between 1998 and 2018, which included 3 randomized controlled trials, 3 pilot studies, 4 retrospective studies, and 14 prospective observational studies. In these studies, telemedicine protocols were used for preoperative assessment, diagnostic purposes, or consultation with another surgical department (10 studies); postoperative wound assessment (9 studies); and follow-up in place of conventional clinic visits (5 studies).3 In a 2017 systematic review of telemedicine for post-discharge surgical care, Gunter et al identified 21 studies, which included 3 randomized controlled trials, 6 pilot or feasibility studies, 4 retrospective record reviews, 2 case series, and 6 surveys.4 In these studies, telemedicine protocols were used for scheduled follow-up (10 studies), routine and ongoing monitoring (5 studies), or management of issues that arose after surgery (2 studies). These 2 reviews found telemedicine to be feasible, useful, and acceptable for postoperative evaluation and follow-up among both providers and patients.
Additional benefits noted in these studies included savings in patient travel, time, and cost. Perspectives on savings to the health system were mixed—while clinic time slots may open as a result of follow-up visits being done via telemedicine (resulting in potential improvements in access to surgical services and decreased wait times), there are still significant direct costs for purchasing necessary equipment and for educating and training providers on the use of the equipment. Other published reviews have discussed in greater detail the application, benefits, limitations, and barriers to telemedicine and provided insight from the perspectives of patients, providers, and health care systems.1,2
Because studies on the use of telemedicine are limited, particularly in general surgery, and few of these studies have used a randomized clinical trial design, the present study is an important contribution to the literature. The authors found a significant difference between groups in terms of percentage of completed follow-up visits—90% of conventional follow-up group participants completed their visit versus 74% of telemedicine group participants. However, these differences were primarily attributed to technical difficulties experienced by telemedicine group participants, as well requests to have a conventional follow-up visit. In addition, telemedicine capabilities were limited to video calls via computers and webcams, and it is likely that successful completion of the follow-up visit would have been higher in the telemedicine group had the use of video calls via tablets or smartphones been an option. Perhaps more important, no significant differences were found in clinical outcomes (extra visits within 30 days after the follow-up visit) or patient satisfaction.
A key strength of this study is the use of a randomized clinical trial design to evaluate telemedicine as an alternative method for conducting patient visits following general surgery. Inclusion and exclusion criteria did not impose strict limitations on potential participants. Also, the authors evaluated differences in time to visit, length of visit, clinical results, and patient satisfaction between groups, in addition to the primary measure of completion of the follow-up visit.
This study has important limitations that should be noted as well, particularly related to the study design, some of which are acknowledged by the authors. Because this study was implemented in only 1 hospital, specifically, a tertiary care university hospital on the outskirts of an urban European city, the generalizability of the findings is limited. Also, the likelihood of selection bias is high, as enrollment was not offered to all patients who were discharged from the hospital and met inclusion criteria (limited by patient workload). The comparison of clinical results was limited, as the selected measure focused only on extra visits to an outpatient clinic and/or the emergency department during the first 30 days after the follow-up visit. This chosen measure does not account for less severe clinical results that did not require an additional visit, and does not represent a nuanced comparison of specific clinical indicators. In addition, this measure does not account for clinical complications that may have occurred beyond the 30-day period. Recall bias also was likely, given that the patient satisfaction questionnaire was delivered via email to patients at a later time after the follow-up visit, instead of being administered immediately after the visit. Last, group differences at baseline were assessed based only on age, gender, and ASA score, which does not preclude potential differences related to other factors, such as race/ethnicity, household income, comorbidities, insurance, and zip code. Future research with a similar objective would benefit from a randomized clinical trial design that recruits a wider diversity of patients across different clinic settings and incorporates more nuanced measures of primary and secondary outcomes.
Applications for Clinical Practice
With the ongoing COVID-19 pandemic, the integration of telemedicine capabilities into hospital systems is becoming more widespread and is proceeding at an accelerated pace. This study provides evidence that telemedicine is a feasible and acceptable complementary service to facilitate postoperative management in selected general surgery patients. Assuming that the needed technology and appropriate program training are available, telemedicine should be offered to patients, especially to maximize savings in terms of travel, time, and cost. However, the option for conventional (in-person) follow-up should remain, particularly in cases where there may be barriers to successful follow-up visits via telemedicine, including limited digital literacy, lack of access to necessary equipment, language/communication barriers, complex follow-up treatment, and difficulties in describing or showing complications in the surgical area.
–Katrina F. Mateo, PhD, MPH
1. Williams AM, Bhatti UF, Alam HB, Nikolian VC. The role of telemedicine in postoperative care. mHealth. 2018 May;4:11-11.
2. Huang EY, Knight S, Guetter CR et al. Telemedicine and telementoring in the surgical specialties: A narrative review. Am J Surg. 2019;218:760-766.
3. Asiri A, AlBishi S, AlMadani W, et al. The use of telemedicine in surgical care: A systematic review. Acta Informatica Medica. 2018;26:201-206.
4. Gunter RL, Chouinard S, Fernandes-Taylor S, et al. Current use of telemedicine for post-discharge surgical care: a systematic review. J Am College Surg. 2016;222:915-927.
1. Williams AM, Bhatti UF, Alam HB, Nikolian VC. The role of telemedicine in postoperative care. mHealth. 2018 May;4:11-11.
2. Huang EY, Knight S, Guetter CR et al. Telemedicine and telementoring in the surgical specialties: A narrative review. Am J Surg. 2019;218:760-766.
3. Asiri A, AlBishi S, AlMadani W, et al. The use of telemedicine in surgical care: A systematic review. Acta Informatica Medica. 2018;26:201-206.
4. Gunter RL, Chouinard S, Fernandes-Taylor S, et al. Current use of telemedicine for post-discharge surgical care: a systematic review. J Am College Surg. 2016;222:915-927.
Today’s top news highlights: ACE inhibitors in COVID patients, fewer AMI admissions, and more
Here are the stories our MDedge editors across specialties think you need to know about today:
Are ACE inhibitors protective in COVID-19?
Older patients with COVID-19 had a lower risk of developing severe illness if they were taking ACE inhibitors, according to a large observational U.S. study. ACE inhibitor use was associated with an almost 40% lower risk for COVID-19 hospitalization for older people enrolled in Medicare Advantage plans. Senior investigator Harlan M. Krumholz, MD, said that while he and his associates think this finding is worthy of further study, “We don’t believe this is enough info to change practice.” The study was published on the MedRxiv preprint server and has not yet been peer reviewed.
READ MORE.
AMI: Admissions drop, deaths rise
In Italy, sharp nationwide decreases in hospitalizations for acute myocardial infarctions (AMIs) during the height of COVID-19 were offset by higher mortality for patients who did present. The study counted AMIs at 54 hospitals nationwide for the week of March 12-19, 2020, and compared that with an equivalent week in 2019 – 319 vs. 618 AMIs, respectively, representing a 48% reduction in hospitalizations. Mortality for ST-segment elevation MI cases more than tripled to 14% during the outbreak, compared with 4% in 2019. “The concern is fewer MIs most likely means people are dying at home or presenting later as this study suggests,” commented Martha Gulati, MD, chief of cardiology at the University of Arizona, Phoenix, who was not involved with the study.
READ MORE.
Prenatal, postpartum screening for depression falls short
Health care providers fail to ask one in five prenatal patients and one in eight postpartum patients about depression, according to the Centers for Disease Control and Prevention. Researchers analyzed self-reported data on postpartum depressive symptoms collected in 2018 by the Pregnancy Risk Assessment Monitoring System. Mental health conditions play a role in approximately 9% of pregnancy-related deaths and not asking about depression represents “missed opportunities to potentially identify and treat women with depression,” said coauthor Jean Y. Ko, PhD, from the division of reproductive health at the National Center for Chronic Disease Prevention and Health Promotion.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
Are ACE inhibitors protective in COVID-19?
Older patients with COVID-19 had a lower risk of developing severe illness if they were taking ACE inhibitors, according to a large observational U.S. study. ACE inhibitor use was associated with an almost 40% lower risk for COVID-19 hospitalization for older people enrolled in Medicare Advantage plans. Senior investigator Harlan M. Krumholz, MD, said that while he and his associates think this finding is worthy of further study, “We don’t believe this is enough info to change practice.” The study was published on the MedRxiv preprint server and has not yet been peer reviewed.
READ MORE.
AMI: Admissions drop, deaths rise
In Italy, sharp nationwide decreases in hospitalizations for acute myocardial infarctions (AMIs) during the height of COVID-19 were offset by higher mortality for patients who did present. The study counted AMIs at 54 hospitals nationwide for the week of March 12-19, 2020, and compared that with an equivalent week in 2019 – 319 vs. 618 AMIs, respectively, representing a 48% reduction in hospitalizations. Mortality for ST-segment elevation MI cases more than tripled to 14% during the outbreak, compared with 4% in 2019. “The concern is fewer MIs most likely means people are dying at home or presenting later as this study suggests,” commented Martha Gulati, MD, chief of cardiology at the University of Arizona, Phoenix, who was not involved with the study.
READ MORE.
Prenatal, postpartum screening for depression falls short
Health care providers fail to ask one in five prenatal patients and one in eight postpartum patients about depression, according to the Centers for Disease Control and Prevention. Researchers analyzed self-reported data on postpartum depressive symptoms collected in 2018 by the Pregnancy Risk Assessment Monitoring System. Mental health conditions play a role in approximately 9% of pregnancy-related deaths and not asking about depression represents “missed opportunities to potentially identify and treat women with depression,” said coauthor Jean Y. Ko, PhD, from the division of reproductive health at the National Center for Chronic Disease Prevention and Health Promotion.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
Are ACE inhibitors protective in COVID-19?
Older patients with COVID-19 had a lower risk of developing severe illness if they were taking ACE inhibitors, according to a large observational U.S. study. ACE inhibitor use was associated with an almost 40% lower risk for COVID-19 hospitalization for older people enrolled in Medicare Advantage plans. Senior investigator Harlan M. Krumholz, MD, said that while he and his associates think this finding is worthy of further study, “We don’t believe this is enough info to change practice.” The study was published on the MedRxiv preprint server and has not yet been peer reviewed.
READ MORE.
AMI: Admissions drop, deaths rise
In Italy, sharp nationwide decreases in hospitalizations for acute myocardial infarctions (AMIs) during the height of COVID-19 were offset by higher mortality for patients who did present. The study counted AMIs at 54 hospitals nationwide for the week of March 12-19, 2020, and compared that with an equivalent week in 2019 – 319 vs. 618 AMIs, respectively, representing a 48% reduction in hospitalizations. Mortality for ST-segment elevation MI cases more than tripled to 14% during the outbreak, compared with 4% in 2019. “The concern is fewer MIs most likely means people are dying at home or presenting later as this study suggests,” commented Martha Gulati, MD, chief of cardiology at the University of Arizona, Phoenix, who was not involved with the study.
READ MORE.
Prenatal, postpartum screening for depression falls short
Health care providers fail to ask one in five prenatal patients and one in eight postpartum patients about depression, according to the Centers for Disease Control and Prevention. Researchers analyzed self-reported data on postpartum depressive symptoms collected in 2018 by the Pregnancy Risk Assessment Monitoring System. Mental health conditions play a role in approximately 9% of pregnancy-related deaths and not asking about depression represents “missed opportunities to potentially identify and treat women with depression,” said coauthor Jean Y. Ko, PhD, from the division of reproductive health at the National Center for Chronic Disease Prevention and Health Promotion.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Out of the pipeline: Remdesivir
Although the US Food and Drug Administration (FDA) has granted emergency use authorization of remdesivir (Gilead Sciences, Inc., Foster City, California) to treat COVID-19, the disease caused by SARS-CoV-2, the drug is considered an investigational agent, not yet formally approved by the FDA and whose efficacy and safety has not yet been fully characterized. Remdesivir has been shown to be effective in reducing the time to recovery of people with COVID-19 disease. It has not been tested in a large controlled clinical trial of pregnant women with COVID-19; however, remdesivir has been given to pregnant women infected with COVID-19 in a compassionate use protocol. For pregnant women, the drug should only be used if the potential benefit justifies the potential risk to the mother and fetus.1
Pharmacology. Remdesivir is a nucleoside RNA polymerase inhibitor. It has a molecular formula of
C27H35N6O8P and a molecular weight of 602.6 g/mol.1
Mechanism of action. From FDA’s fact sheet: “Remdesivir is an adenosine nucleotide prodrug that distributes into cells where it is metabolized to form the pharmacologically active nucleoside triphosphate metabolite. Metabolism of remdesivir to remdesivir triphosphate has been demonstrated in multiple cell types. Remdesivir triphosphate acts as an analog of adenosine triphosphate (ATP) and competes with the natural ATP substrate for incorporation into nascent RNA chains by the SARS-CoV-2 RNA-dependent RNA polymerase, which results in chain termination during replication of the viral RNA. Remdesivir triphosphate is a weak inhibitor of mammalian DNA and RNA polymerases with low potential for mitochondrial toxicity.”1
Treatment protocols
Remdesivir is authorized for treatment of hospitalized patients with severe COVID-19 disease, defined as patients with an oxygen saturation ≤ 94% on room air or requiring supplemental oxygen or requiring mechanical ventilation or requiring extracorporeal membrane oxygenation (ECMO). The optimal dose and duration of treatment of COVID-19 with remdesivir is unknown.1
Prior to initiating treatment, the estimated glomerular filtration rate should be documented to be ≥ 30 mL/min. An excipient used in the remdesivir formulation—sulfobutylether-β-cylcodextrin sodium salt—is renally cleared and accumulates in patients with decreased renal function.
Baseline liver function tests should be performed prior to treatment and daily during the course of treatment. Remdesivir should not be initiated in patients with an alanine aminotransferase (ALT) level ≥ 5 times the upper limit of normal at baseline. Remdesivir should be discontinued in patients who develop an ALT level ≥ 5 times the upper limit of normal or in patients who develop elevated ALT levels and have increased bilirubin, alkaline phosphatase, or international normalized ratio.1
In one open-label study (GS-US-540-5773), remdesivir treatment was discontinued due to an adverse event in 5% of patients on a 5-day regimen and in 10% of patients on a 10-day regimen.1
Under the emergency use authorization, two treatment protocols have been proposed depending on the clinical severity of the COVID-19 infection1:
- Protocol 1: For people with COVID-19 requiring mechanical ventilation and/or ECMO, the duration of therapy is 10 days, beginning with a loading dose of remdesivir 200 mg infused intravenously for 30 to 120 minutes on day 1 followed by a once-daily dose of 100 mg for 9 days.
- Protocol 2: For people with COVID-19 disease not requiring mechanical ventilation and/or ECMO, the duration of therapy is 5 days, beginning with a loading dose of remdesivir 200 mg infused intravenously for 30 to 120 minutes on day 1 followed by a once-daily dose of 100 mg for 4 days. If the patient does not show clinical improvement, treatment may be extended for an additional 5 days.
Continue to: Randomized placebo-controlled trial results...
Randomized placebo-controlled trial results
The Adaptive COVID-19 Treatment Trial (ACTT), sponsored by the National Institute of Allergy and Infectious Diseases, is a randomized, double-blind, placebo-controlled trial conducted by Gilead Sciences. The study began in February and evaluated up to 10 days of remdesivir treatment—200 mg IV once daily for 1 day followed by 100 mg IV once daily for 9 days in hospitalized adult patients with COVID-19. Patients were enrolled in a 1:1 manner to remdesivir or placebo, and time to recovery within 28 days after randomization was the trial’s endpoint. According to preliminary analysis of 606 recovered patients, recovery took a median of 11 days in the remdesivir group and 15 days in the placebo group (hazard ratio, 1.31; 95% confidence interval (CI), 1.12‒1.54; P<.001). Mortality rates were 8.0% and 11.6% in the remdesivir and placebo groups, respectively (P=.059).1
5 vs 10 days of remdesivir treatment
The Gilead Sciences‒sponsored study GS-US-540-5773 was a randomized, open-label multicenter trial of patients with severe COVID-19. A total of 197 adult patients received 10-day remdesivir treatment (200 mg IV once daily for 1 day followed by 100 mg IV once daily for 9 days). An additional 200 adult patients received 5-day remdesivir treatment (200 mg IV once daily followed by 100 mg IV for 4 days). Both groups also received standard of care. Results suggested that patients receiving 10 days of remdesivir had similar improvement in clinical status compared with those receiving a 5-day treatment course (10-to-5 day odds ratio, 0.76; 95% CI, 0.51‒1.13] on day 14).1 Improvement in clinical status was defined as an improvement of 2 or more points from baseline on a predefined 7-point scale that ranged from hospital discharge to increasing levels of oxygen support to death. Clinical recovery was achieved if patients ceased the need for oxygen support or were discharged.1
The time to clinical improvement for 50% of patients was similar in each treatment group (10 days in the 5-day group versus 11 days in the 10-day group). By day 14, observed clinical improvement rates were 65% and 54% in the 5- and 10-day treatment groups, respectively. Clinical recovery rates were 70% and 59% in the 5- and 10-day treatment groups and mortality rates were 8% and 11%.1
Adverse events
The use of remdesivir is contraindicated in patients who are hypersensitive to the drug. Its infusion may cause hypotension, nausea, vomiting, diaphoresis, and shivering. If signs of a clinically significant infusion reaction are observed the infusion should be discontinued. As noted above, elevation in ALT levels occurs with remdesivir treatment.1
Reporting serious adverse events. If a serious and unexpected adverse event occurs and appears to be associated with the use of remdesivir, the prescribing health care provider and/or the provider’s designee should complete and submit a MedWatch form to the FDA using one of the following methods1:
- Complete and submit the report online: www.fda.gov/medwatch/report.htm
- Return form FDA 3500 (available at http://www.fda.gov/downloads/AboutFDA/ReportsManualsForms/Forms/UCM163919.pdf) to the FDA by mail (MedWatch, 5600 Fishers Lane, Rockville, MD 20852-9787) or fax (1-800-FDA-0178)
- Gilead requests that all FDA MedWatch forms also be returned to Gilead Pharmacovigilance and Epidemiology: fax: 1-650-522-5477 726; e-mail: Safety_fc@gilead.com
Continue to: Drug interactions...
Drug interactions
Remdesivir has not been evaluated for drug-drug interactions in humans. The clinical relevance of in vitro drug interactions also has not been established. According to the FDA, remdesivir is a substrate for the drug metabolizing enzymes CYP2C8, CYP2D6, and CYP3A4, and is a substrate for organic anion transporting polypeptides 1B1 (OAPT1B1) and P-glycoprotein (P-gp) transporters. In vitro, remdesivir inhibits CYP3A4, OATP1B1, OATP1B3, BSEP, MRP4, and NTCP.1
Pregnancy risk summary
Remdesivir has not been studied adequately in pregnant women and only should be used during pregnancy if the potential benefit of the drug justifies the potential risk to both mother and fetus.
Nonclinical animal studies that included systemic exposure of the predominant circulating metabolite of remdesivir in pregnant rats and rabbits (at 4 times the recommended dose of human exposure) demonstrated no adverse effect on embryofetal development.1
Breastfeeding
The only information regarding breastfeeding and remdesivir comes from animal studies. The drug and its metabolites were detected in the plasma of nursing rat pups whose mothers given intravenous remdesivir daily from gestation day 6 to lactation day 20. Measured on lactation day 10, remdesivir exposure in the pups was about 1% that of maternal exposure.1
“Because of the potential for viral transmission to SARS-CoV-2-negative infants and adverse reactions from the drug in breastfeeding infants, the developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for remdesivir and any potential adverse effects on the breastfed child from remdesivir or from the underlying maternal condition.”1
- US Food and Drug Administration. Fact Sheet for Health Care Providers Emergency Use Authorization (UA) of Remdesivir (GS-5734)TM. https://www.fda.gov/media/137566/download. Accessed May 19, 2020.
Although the US Food and Drug Administration (FDA) has granted emergency use authorization of remdesivir (Gilead Sciences, Inc., Foster City, California) to treat COVID-19, the disease caused by SARS-CoV-2, the drug is considered an investigational agent, not yet formally approved by the FDA and whose efficacy and safety has not yet been fully characterized. Remdesivir has been shown to be effective in reducing the time to recovery of people with COVID-19 disease. It has not been tested in a large controlled clinical trial of pregnant women with COVID-19; however, remdesivir has been given to pregnant women infected with COVID-19 in a compassionate use protocol. For pregnant women, the drug should only be used if the potential benefit justifies the potential risk to the mother and fetus.1
Pharmacology. Remdesivir is a nucleoside RNA polymerase inhibitor. It has a molecular formula of
C27H35N6O8P and a molecular weight of 602.6 g/mol.1
Mechanism of action. From FDA’s fact sheet: “Remdesivir is an adenosine nucleotide prodrug that distributes into cells where it is metabolized to form the pharmacologically active nucleoside triphosphate metabolite. Metabolism of remdesivir to remdesivir triphosphate has been demonstrated in multiple cell types. Remdesivir triphosphate acts as an analog of adenosine triphosphate (ATP) and competes with the natural ATP substrate for incorporation into nascent RNA chains by the SARS-CoV-2 RNA-dependent RNA polymerase, which results in chain termination during replication of the viral RNA. Remdesivir triphosphate is a weak inhibitor of mammalian DNA and RNA polymerases with low potential for mitochondrial toxicity.”1
Treatment protocols
Remdesivir is authorized for treatment of hospitalized patients with severe COVID-19 disease, defined as patients with an oxygen saturation ≤ 94% on room air or requiring supplemental oxygen or requiring mechanical ventilation or requiring extracorporeal membrane oxygenation (ECMO). The optimal dose and duration of treatment of COVID-19 with remdesivir is unknown.1
Prior to initiating treatment, the estimated glomerular filtration rate should be documented to be ≥ 30 mL/min. An excipient used in the remdesivir formulation—sulfobutylether-β-cylcodextrin sodium salt—is renally cleared and accumulates in patients with decreased renal function.
Baseline liver function tests should be performed prior to treatment and daily during the course of treatment. Remdesivir should not be initiated in patients with an alanine aminotransferase (ALT) level ≥ 5 times the upper limit of normal at baseline. Remdesivir should be discontinued in patients who develop an ALT level ≥ 5 times the upper limit of normal or in patients who develop elevated ALT levels and have increased bilirubin, alkaline phosphatase, or international normalized ratio.1
In one open-label study (GS-US-540-5773), remdesivir treatment was discontinued due to an adverse event in 5% of patients on a 5-day regimen and in 10% of patients on a 10-day regimen.1
Under the emergency use authorization, two treatment protocols have been proposed depending on the clinical severity of the COVID-19 infection1:
- Protocol 1: For people with COVID-19 requiring mechanical ventilation and/or ECMO, the duration of therapy is 10 days, beginning with a loading dose of remdesivir 200 mg infused intravenously for 30 to 120 minutes on day 1 followed by a once-daily dose of 100 mg for 9 days.
- Protocol 2: For people with COVID-19 disease not requiring mechanical ventilation and/or ECMO, the duration of therapy is 5 days, beginning with a loading dose of remdesivir 200 mg infused intravenously for 30 to 120 minutes on day 1 followed by a once-daily dose of 100 mg for 4 days. If the patient does not show clinical improvement, treatment may be extended for an additional 5 days.
Continue to: Randomized placebo-controlled trial results...
Randomized placebo-controlled trial results
The Adaptive COVID-19 Treatment Trial (ACTT), sponsored by the National Institute of Allergy and Infectious Diseases, is a randomized, double-blind, placebo-controlled trial conducted by Gilead Sciences. The study began in February and evaluated up to 10 days of remdesivir treatment—200 mg IV once daily for 1 day followed by 100 mg IV once daily for 9 days in hospitalized adult patients with COVID-19. Patients were enrolled in a 1:1 manner to remdesivir or placebo, and time to recovery within 28 days after randomization was the trial’s endpoint. According to preliminary analysis of 606 recovered patients, recovery took a median of 11 days in the remdesivir group and 15 days in the placebo group (hazard ratio, 1.31; 95% confidence interval (CI), 1.12‒1.54; P<.001). Mortality rates were 8.0% and 11.6% in the remdesivir and placebo groups, respectively (P=.059).1

5 vs 10 days of remdesivir treatment
The Gilead Sciences‒sponsored study GS-US-540-5773 was a randomized, open-label multicenter trial of patients with severe COVID-19. A total of 197 adult patients received 10-day remdesivir treatment (200 mg IV once daily for 1 day followed by 100 mg IV once daily for 9 days). An additional 200 adult patients received 5-day remdesivir treatment (200 mg IV once daily followed by 100 mg IV for 4 days). Both groups also received standard of care. Results suggested that patients receiving 10 days of remdesivir had similar improvement in clinical status compared with those receiving a 5-day treatment course (10-to-5 day odds ratio, 0.76; 95% CI, 0.51‒1.13] on day 14).1 Improvement in clinical status was defined as an improvement of 2 or more points from baseline on a predefined 7-point scale that ranged from hospital discharge to increasing levels of oxygen support to death. Clinical recovery was achieved if patients ceased the need for oxygen support or were discharged.1
The time to clinical improvement for 50% of patients was similar in each treatment group (10 days in the 5-day group versus 11 days in the 10-day group). By day 14, observed clinical improvement rates were 65% and 54% in the 5- and 10-day treatment groups, respectively. Clinical recovery rates were 70% and 59% in the 5- and 10-day treatment groups and mortality rates were 8% and 11%.1
Adverse events
The use of remdesivir is contraindicated in patients who are hypersensitive to the drug. Its infusion may cause hypotension, nausea, vomiting, diaphoresis, and shivering. If signs of a clinically significant infusion reaction are observed the infusion should be discontinued. As noted above, elevation in ALT levels occurs with remdesivir treatment.1
Reporting serious adverse events. If a serious and unexpected adverse event occurs and appears to be associated with the use of remdesivir, the prescribing health care provider and/or the provider’s designee should complete and submit a MedWatch form to the FDA using one of the following methods1:
- Complete and submit the report online: www.fda.gov/medwatch/report.htm
- Return form FDA 3500 (available at http://www.fda.gov/downloads/AboutFDA/ReportsManualsForms/Forms/UCM163919.pdf) to the FDA by mail (MedWatch, 5600 Fishers Lane, Rockville, MD 20852-9787) or fax (1-800-FDA-0178)
- Gilead requests that all FDA MedWatch forms also be returned to Gilead Pharmacovigilance and Epidemiology: fax: 1-650-522-5477 726; e-mail: Safety_fc@gilead.com
Continue to: Drug interactions...
Drug interactions
Remdesivir has not been evaluated for drug-drug interactions in humans. The clinical relevance of in vitro drug interactions also has not been established. According to the FDA, remdesivir is a substrate for the drug metabolizing enzymes CYP2C8, CYP2D6, and CYP3A4, and is a substrate for organic anion transporting polypeptides 1B1 (OAPT1B1) and P-glycoprotein (P-gp) transporters. In vitro, remdesivir inhibits CYP3A4, OATP1B1, OATP1B3, BSEP, MRP4, and NTCP.1
Pregnancy risk summary
Remdesivir has not been studied adequately in pregnant women and only should be used during pregnancy if the potential benefit of the drug justifies the potential risk to both mother and fetus.
Nonclinical animal studies that included systemic exposure of the predominant circulating metabolite of remdesivir in pregnant rats and rabbits (at 4 times the recommended dose of human exposure) demonstrated no adverse effect on embryofetal development.1
Breastfeeding
The only information regarding breastfeeding and remdesivir comes from animal studies. The drug and its metabolites were detected in the plasma of nursing rat pups whose mothers given intravenous remdesivir daily from gestation day 6 to lactation day 20. Measured on lactation day 10, remdesivir exposure in the pups was about 1% that of maternal exposure.1
“Because of the potential for viral transmission to SARS-CoV-2-negative infants and adverse reactions from the drug in breastfeeding infants, the developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for remdesivir and any potential adverse effects on the breastfed child from remdesivir or from the underlying maternal condition.”1
Although the US Food and Drug Administration (FDA) has granted emergency use authorization of remdesivir (Gilead Sciences, Inc., Foster City, California) to treat COVID-19, the disease caused by SARS-CoV-2, the drug is considered an investigational agent, not yet formally approved by the FDA and whose efficacy and safety has not yet been fully characterized. Remdesivir has been shown to be effective in reducing the time to recovery of people with COVID-19 disease. It has not been tested in a large controlled clinical trial of pregnant women with COVID-19; however, remdesivir has been given to pregnant women infected with COVID-19 in a compassionate use protocol. For pregnant women, the drug should only be used if the potential benefit justifies the potential risk to the mother and fetus.1
Pharmacology. Remdesivir is a nucleoside RNA polymerase inhibitor. It has a molecular formula of
C27H35N6O8P and a molecular weight of 602.6 g/mol.1
Mechanism of action. From FDA’s fact sheet: “Remdesivir is an adenosine nucleotide prodrug that distributes into cells where it is metabolized to form the pharmacologically active nucleoside triphosphate metabolite. Metabolism of remdesivir to remdesivir triphosphate has been demonstrated in multiple cell types. Remdesivir triphosphate acts as an analog of adenosine triphosphate (ATP) and competes with the natural ATP substrate for incorporation into nascent RNA chains by the SARS-CoV-2 RNA-dependent RNA polymerase, which results in chain termination during replication of the viral RNA. Remdesivir triphosphate is a weak inhibitor of mammalian DNA and RNA polymerases with low potential for mitochondrial toxicity.”1
Treatment protocols
Remdesivir is authorized for treatment of hospitalized patients with severe COVID-19 disease, defined as patients with an oxygen saturation ≤ 94% on room air or requiring supplemental oxygen or requiring mechanical ventilation or requiring extracorporeal membrane oxygenation (ECMO). The optimal dose and duration of treatment of COVID-19 with remdesivir is unknown.1
Prior to initiating treatment, the estimated glomerular filtration rate should be documented to be ≥ 30 mL/min. An excipient used in the remdesivir formulation—sulfobutylether-β-cylcodextrin sodium salt—is renally cleared and accumulates in patients with decreased renal function.
Baseline liver function tests should be performed prior to treatment and daily during the course of treatment. Remdesivir should not be initiated in patients with an alanine aminotransferase (ALT) level ≥ 5 times the upper limit of normal at baseline. Remdesivir should be discontinued in patients who develop an ALT level ≥ 5 times the upper limit of normal or in patients who develop elevated ALT levels and have increased bilirubin, alkaline phosphatase, or international normalized ratio.1
In one open-label study (GS-US-540-5773), remdesivir treatment was discontinued due to an adverse event in 5% of patients on a 5-day regimen and in 10% of patients on a 10-day regimen.1
Under the emergency use authorization, two treatment protocols have been proposed depending on the clinical severity of the COVID-19 infection1:
- Protocol 1: For people with COVID-19 requiring mechanical ventilation and/or ECMO, the duration of therapy is 10 days, beginning with a loading dose of remdesivir 200 mg infused intravenously for 30 to 120 minutes on day 1 followed by a once-daily dose of 100 mg for 9 days.
- Protocol 2: For people with COVID-19 disease not requiring mechanical ventilation and/or ECMO, the duration of therapy is 5 days, beginning with a loading dose of remdesivir 200 mg infused intravenously for 30 to 120 minutes on day 1 followed by a once-daily dose of 100 mg for 4 days. If the patient does not show clinical improvement, treatment may be extended for an additional 5 days.
Continue to: Randomized placebo-controlled trial results...
Randomized placebo-controlled trial results
The Adaptive COVID-19 Treatment Trial (ACTT), sponsored by the National Institute of Allergy and Infectious Diseases, is a randomized, double-blind, placebo-controlled trial conducted by Gilead Sciences. The study began in February and evaluated up to 10 days of remdesivir treatment—200 mg IV once daily for 1 day followed by 100 mg IV once daily for 9 days in hospitalized adult patients with COVID-19. Patients were enrolled in a 1:1 manner to remdesivir or placebo, and time to recovery within 28 days after randomization was the trial’s endpoint. According to preliminary analysis of 606 recovered patients, recovery took a median of 11 days in the remdesivir group and 15 days in the placebo group (hazard ratio, 1.31; 95% confidence interval (CI), 1.12‒1.54; P<.001). Mortality rates were 8.0% and 11.6% in the remdesivir and placebo groups, respectively (P=.059).1

5 vs 10 days of remdesivir treatment
The Gilead Sciences‒sponsored study GS-US-540-5773 was a randomized, open-label multicenter trial of patients with severe COVID-19. A total of 197 adult patients received 10-day remdesivir treatment (200 mg IV once daily for 1 day followed by 100 mg IV once daily for 9 days). An additional 200 adult patients received 5-day remdesivir treatment (200 mg IV once daily followed by 100 mg IV for 4 days). Both groups also received standard of care. Results suggested that patients receiving 10 days of remdesivir had similar improvement in clinical status compared with those receiving a 5-day treatment course (10-to-5 day odds ratio, 0.76; 95% CI, 0.51‒1.13] on day 14).1 Improvement in clinical status was defined as an improvement of 2 or more points from baseline on a predefined 7-point scale that ranged from hospital discharge to increasing levels of oxygen support to death. Clinical recovery was achieved if patients ceased the need for oxygen support or were discharged.1
The time to clinical improvement for 50% of patients was similar in each treatment group (10 days in the 5-day group versus 11 days in the 10-day group). By day 14, observed clinical improvement rates were 65% and 54% in the 5- and 10-day treatment groups, respectively. Clinical recovery rates were 70% and 59% in the 5- and 10-day treatment groups and mortality rates were 8% and 11%.1
Adverse events
The use of remdesivir is contraindicated in patients who are hypersensitive to the drug. Its infusion may cause hypotension, nausea, vomiting, diaphoresis, and shivering. If signs of a clinically significant infusion reaction are observed the infusion should be discontinued. As noted above, elevation in ALT levels occurs with remdesivir treatment.1
Reporting serious adverse events. If a serious and unexpected adverse event occurs and appears to be associated with the use of remdesivir, the prescribing health care provider and/or the provider’s designee should complete and submit a MedWatch form to the FDA using one of the following methods1:
- Complete and submit the report online: www.fda.gov/medwatch/report.htm
- Return form FDA 3500 (available at http://www.fda.gov/downloads/AboutFDA/ReportsManualsForms/Forms/UCM163919.pdf) to the FDA by mail (MedWatch, 5600 Fishers Lane, Rockville, MD 20852-9787) or fax (1-800-FDA-0178)
- Gilead requests that all FDA MedWatch forms also be returned to Gilead Pharmacovigilance and Epidemiology: fax: 1-650-522-5477 726; e-mail: Safety_fc@gilead.com
Continue to: Drug interactions...
Drug interactions
Remdesivir has not been evaluated for drug-drug interactions in humans. The clinical relevance of in vitro drug interactions also has not been established. According to the FDA, remdesivir is a substrate for the drug metabolizing enzymes CYP2C8, CYP2D6, and CYP3A4, and is a substrate for organic anion transporting polypeptides 1B1 (OAPT1B1) and P-glycoprotein (P-gp) transporters. In vitro, remdesivir inhibits CYP3A4, OATP1B1, OATP1B3, BSEP, MRP4, and NTCP.1
Pregnancy risk summary
Remdesivir has not been studied adequately in pregnant women and only should be used during pregnancy if the potential benefit of the drug justifies the potential risk to both mother and fetus.
Nonclinical animal studies that included systemic exposure of the predominant circulating metabolite of remdesivir in pregnant rats and rabbits (at 4 times the recommended dose of human exposure) demonstrated no adverse effect on embryofetal development.1
Breastfeeding
The only information regarding breastfeeding and remdesivir comes from animal studies. The drug and its metabolites were detected in the plasma of nursing rat pups whose mothers given intravenous remdesivir daily from gestation day 6 to lactation day 20. Measured on lactation day 10, remdesivir exposure in the pups was about 1% that of maternal exposure.1
“Because of the potential for viral transmission to SARS-CoV-2-negative infants and adverse reactions from the drug in breastfeeding infants, the developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for remdesivir and any potential adverse effects on the breastfed child from remdesivir or from the underlying maternal condition.”1
- US Food and Drug Administration. Fact Sheet for Health Care Providers Emergency Use Authorization (UA) of Remdesivir (GS-5734)TM. https://www.fda.gov/media/137566/download. Accessed May 19, 2020.
- US Food and Drug Administration. Fact Sheet for Health Care Providers Emergency Use Authorization (UA) of Remdesivir (GS-5734)TM. https://www.fda.gov/media/137566/download. Accessed May 19, 2020.
The ‘Three Rs’ of email effectiveness
Resist, Reorganize, and Respond
PING – you look down at your phone and the words “URGENT – Meeting Today” stare back at you. The elevator door opens, and you step inside – 1 minute, the seemingly perfect amount of time for a quick inbox check.
As a hospitalist, chances are you have experienced this scenario, likely more than once. Email has become a double-edged sword, both a valuable communication tool and a source of stress and frustration.1 A 2012 McKinsey analysis found that the average professional spends 28% of the day reading and answering emails.2 Smartphone technology with email alerts and push notifications constantly diverts hospitalists’ attention away from important and nonurgent responsibilities such as manuscript writing, family time, and personal well-being.3
How can we break this cycle of compulsive connectivity? To keep email from controlling your life, we suggest the “Three Rs” (Resist, Reorganize, and Respond) of email effectiveness.
RESIST
The first key to take control of your inbox is to resist the urge to impulsively check and respond to emails. Consider these three solutions to bolster your ability to resist.
- Disable email push notifications. This will reduce the urge to continuously refresh your inbox on the wards.4 Excessively checking email can waste as much as 21 minutes per day.2
- Set an email budget.5 Schedule one to two appointments each day to handle email.6 Consider blocking 30 minutes after rounds and 30 minutes at the end of each day to address emails.
- Correspond at a computer. Limit email correspondence to your laptop or desktop. Access to a full keyboard and larger screen will maximize the efficiency of each email appointment.
REORGANIZE
After implementing these strategies to resist email temptations, reorganize your inbox with the following two-pronged approach.
- Focus your inbox: There are many options for reducing the volume of emails that flood your inbox. Try collaborative tools like Google Docs, Dropbox, Doodle polls, and Slack to shift communication away from email onto platforms optimized to your project’s specific needs. Additionally, email management tools like SaneBox and OtherInbox triage less important messages directly to folders, leaving only must-read-now messages in your inbox.2 Lastly, activate spam filters and unsubscribe from mailing lists to eliminate email clutter.
- Commit to concise filing and finding: Archiving emails into a complex array of folders wastes as much as 14 minutes each day. Instead, limit your filing system to two folders: “Action” for email requiring further action and “Reading” for messages to reference at a later date.2 Activating “Communication View” on Microsoft Outlook allows rapid review of messages that share the same subject heading.
RESPOND
Finally, once your inbox is reorganized, use the Four Ds for Decision Making model to optimize the way you respond to email.6 When you sit down for an email appointment, use the Four Ds, detailed below to avoid reading the same message repeatedly without taking action.
- Delete: Quickly delete any emails that do not directly require your attention or follow-up. Many emails can be immediately deleted without further thought.
- Do: If a task or response to an email will take less than 2 minutes, do it immediately. It will take at least the same amount to retrieve and reread an email as it will to handle it in real time.7 Often, this can be accomplished with a quick phone call or email reply.
- Defer: If an email response will take more than 2 minutes, use a system to take action at a later time. Move actionable items from your inbox to a to-do list or calendar appointment and file appropriate emails into the Action or Reading folders, detailed above. This method allows completion of important tasks in a timely manner outside of your fixed email budget. Delaying an email reply can also be advantageous by letting a problem mature, given that some of these issues will resolve without your specific intervention.
- Delegate: This can be difficult for many hospitalists who are accustomed to finishing each task themselves. If someone else can do the task as good as or better than you can, it is wise to delegate whenever possible.
Over the next few weeks, challenge yourself to resist email temptations, reorganize your inbox, and methodically respond to emails. This practice will help structure your day, maximize your efficiency, manage colleagues’ expectations, and create new time windows throughout your on-service weeks.
Dr. Nelson is a hospitalist at Ochsner Medical Center in New Orleans. Dr. Esquivel is a hospitalist and assistant professor at Weill Cornell Medicine, New York. Dr. Hall is a med-peds hospitalist and assistant professor at the University of Kentucky, Lexington.
References
1. MacKinnon R. How you manage your emails may be bad for your health. Science Daily. https://www.sciencedaily.com/releases/2016/01/160104081249.htm. Published Jan 4, 2016.
2. Plummer M. How to spend way less time on email every day. Harvard Business Review. https://hbr.org/2019/01/how-to-spend-way-less-time-on-email-every-day. 2019 Jan 22.
3. Covey SR. The 7 Habits of Highly Effective People: Powerful Lessons in Personal Change. New York: Free Press, 2004.
4. Ericson C. 5 Ways to Take Control of Your Email Inbox. Forbes. https://www.forbes.com/sites/learnvest/2014/03/17/5-ways-to-take-control-of-your-email-inbox/#3711f5946342. 2014 Mar 17.
5. Limit the time you spend on email. Harvard Business Review. https://hbr.org/2014/02/limit-the-time-you-spend-on-email. 2014 Feb 6.
6. McGhee S. Empty your inbox: 4 ways to take control of your email. Internet and Telephone Blog. https://www.itllc.net/it-support-ma/empty-your-inbox-4-ways-to-take-control-of-your-email/.
7. Allen D. Getting Things Done: The Art of Stress-Free Productivity. New York: Penguin Books, 2015.
Resist, Reorganize, and Respond
Resist, Reorganize, and Respond
PING – you look down at your phone and the words “URGENT – Meeting Today” stare back at you. The elevator door opens, and you step inside – 1 minute, the seemingly perfect amount of time for a quick inbox check.
As a hospitalist, chances are you have experienced this scenario, likely more than once. Email has become a double-edged sword, both a valuable communication tool and a source of stress and frustration.1 A 2012 McKinsey analysis found that the average professional spends 28% of the day reading and answering emails.2 Smartphone technology with email alerts and push notifications constantly diverts hospitalists’ attention away from important and nonurgent responsibilities such as manuscript writing, family time, and personal well-being.3
How can we break this cycle of compulsive connectivity? To keep email from controlling your life, we suggest the “Three Rs” (Resist, Reorganize, and Respond) of email effectiveness.
RESIST
The first key to take control of your inbox is to resist the urge to impulsively check and respond to emails. Consider these three solutions to bolster your ability to resist.
- Disable email push notifications. This will reduce the urge to continuously refresh your inbox on the wards.4 Excessively checking email can waste as much as 21 minutes per day.2
- Set an email budget.5 Schedule one to two appointments each day to handle email.6 Consider blocking 30 minutes after rounds and 30 minutes at the end of each day to address emails.
- Correspond at a computer. Limit email correspondence to your laptop or desktop. Access to a full keyboard and larger screen will maximize the efficiency of each email appointment.
REORGANIZE
After implementing these strategies to resist email temptations, reorganize your inbox with the following two-pronged approach.
- Focus your inbox: There are many options for reducing the volume of emails that flood your inbox. Try collaborative tools like Google Docs, Dropbox, Doodle polls, and Slack to shift communication away from email onto platforms optimized to your project’s specific needs. Additionally, email management tools like SaneBox and OtherInbox triage less important messages directly to folders, leaving only must-read-now messages in your inbox.2 Lastly, activate spam filters and unsubscribe from mailing lists to eliminate email clutter.
- Commit to concise filing and finding: Archiving emails into a complex array of folders wastes as much as 14 minutes each day. Instead, limit your filing system to two folders: “Action” for email requiring further action and “Reading” for messages to reference at a later date.2 Activating “Communication View” on Microsoft Outlook allows rapid review of messages that share the same subject heading.
RESPOND
Finally, once your inbox is reorganized, use the Four Ds for Decision Making model to optimize the way you respond to email.6 When you sit down for an email appointment, use the Four Ds, detailed below to avoid reading the same message repeatedly without taking action.
- Delete: Quickly delete any emails that do not directly require your attention or follow-up. Many emails can be immediately deleted without further thought.
- Do: If a task or response to an email will take less than 2 minutes, do it immediately. It will take at least the same amount to retrieve and reread an email as it will to handle it in real time.7 Often, this can be accomplished with a quick phone call or email reply.
- Defer: If an email response will take more than 2 minutes, use a system to take action at a later time. Move actionable items from your inbox to a to-do list or calendar appointment and file appropriate emails into the Action or Reading folders, detailed above. This method allows completion of important tasks in a timely manner outside of your fixed email budget. Delaying an email reply can also be advantageous by letting a problem mature, given that some of these issues will resolve without your specific intervention.
- Delegate: This can be difficult for many hospitalists who are accustomed to finishing each task themselves. If someone else can do the task as good as or better than you can, it is wise to delegate whenever possible.
Over the next few weeks, challenge yourself to resist email temptations, reorganize your inbox, and methodically respond to emails. This practice will help structure your day, maximize your efficiency, manage colleagues’ expectations, and create new time windows throughout your on-service weeks.
Dr. Nelson is a hospitalist at Ochsner Medical Center in New Orleans. Dr. Esquivel is a hospitalist and assistant professor at Weill Cornell Medicine, New York. Dr. Hall is a med-peds hospitalist and assistant professor at the University of Kentucky, Lexington.
References
1. MacKinnon R. How you manage your emails may be bad for your health. Science Daily. https://www.sciencedaily.com/releases/2016/01/160104081249.htm. Published Jan 4, 2016.
2. Plummer M. How to spend way less time on email every day. Harvard Business Review. https://hbr.org/2019/01/how-to-spend-way-less-time-on-email-every-day. 2019 Jan 22.
3. Covey SR. The 7 Habits of Highly Effective People: Powerful Lessons in Personal Change. New York: Free Press, 2004.
4. Ericson C. 5 Ways to Take Control of Your Email Inbox. Forbes. https://www.forbes.com/sites/learnvest/2014/03/17/5-ways-to-take-control-of-your-email-inbox/#3711f5946342. 2014 Mar 17.
5. Limit the time you spend on email. Harvard Business Review. https://hbr.org/2014/02/limit-the-time-you-spend-on-email. 2014 Feb 6.
6. McGhee S. Empty your inbox: 4 ways to take control of your email. Internet and Telephone Blog. https://www.itllc.net/it-support-ma/empty-your-inbox-4-ways-to-take-control-of-your-email/.
7. Allen D. Getting Things Done: The Art of Stress-Free Productivity. New York: Penguin Books, 2015.
PING – you look down at your phone and the words “URGENT – Meeting Today” stare back at you. The elevator door opens, and you step inside – 1 minute, the seemingly perfect amount of time for a quick inbox check.
As a hospitalist, chances are you have experienced this scenario, likely more than once. Email has become a double-edged sword, both a valuable communication tool and a source of stress and frustration.1 A 2012 McKinsey analysis found that the average professional spends 28% of the day reading and answering emails.2 Smartphone technology with email alerts and push notifications constantly diverts hospitalists’ attention away from important and nonurgent responsibilities such as manuscript writing, family time, and personal well-being.3
How can we break this cycle of compulsive connectivity? To keep email from controlling your life, we suggest the “Three Rs” (Resist, Reorganize, and Respond) of email effectiveness.
RESIST
The first key to take control of your inbox is to resist the urge to impulsively check and respond to emails. Consider these three solutions to bolster your ability to resist.
- Disable email push notifications. This will reduce the urge to continuously refresh your inbox on the wards.4 Excessively checking email can waste as much as 21 minutes per day.2
- Set an email budget.5 Schedule one to two appointments each day to handle email.6 Consider blocking 30 minutes after rounds and 30 minutes at the end of each day to address emails.
- Correspond at a computer. Limit email correspondence to your laptop or desktop. Access to a full keyboard and larger screen will maximize the efficiency of each email appointment.
REORGANIZE
After implementing these strategies to resist email temptations, reorganize your inbox with the following two-pronged approach.
- Focus your inbox: There are many options for reducing the volume of emails that flood your inbox. Try collaborative tools like Google Docs, Dropbox, Doodle polls, and Slack to shift communication away from email onto platforms optimized to your project’s specific needs. Additionally, email management tools like SaneBox and OtherInbox triage less important messages directly to folders, leaving only must-read-now messages in your inbox.2 Lastly, activate spam filters and unsubscribe from mailing lists to eliminate email clutter.
- Commit to concise filing and finding: Archiving emails into a complex array of folders wastes as much as 14 minutes each day. Instead, limit your filing system to two folders: “Action” for email requiring further action and “Reading” for messages to reference at a later date.2 Activating “Communication View” on Microsoft Outlook allows rapid review of messages that share the same subject heading.
RESPOND
Finally, once your inbox is reorganized, use the Four Ds for Decision Making model to optimize the way you respond to email.6 When you sit down for an email appointment, use the Four Ds, detailed below to avoid reading the same message repeatedly without taking action.
- Delete: Quickly delete any emails that do not directly require your attention or follow-up. Many emails can be immediately deleted without further thought.
- Do: If a task or response to an email will take less than 2 minutes, do it immediately. It will take at least the same amount to retrieve and reread an email as it will to handle it in real time.7 Often, this can be accomplished with a quick phone call or email reply.
- Defer: If an email response will take more than 2 minutes, use a system to take action at a later time. Move actionable items from your inbox to a to-do list or calendar appointment and file appropriate emails into the Action or Reading folders, detailed above. This method allows completion of important tasks in a timely manner outside of your fixed email budget. Delaying an email reply can also be advantageous by letting a problem mature, given that some of these issues will resolve without your specific intervention.
- Delegate: This can be difficult for many hospitalists who are accustomed to finishing each task themselves. If someone else can do the task as good as or better than you can, it is wise to delegate whenever possible.
Over the next few weeks, challenge yourself to resist email temptations, reorganize your inbox, and methodically respond to emails. This practice will help structure your day, maximize your efficiency, manage colleagues’ expectations, and create new time windows throughout your on-service weeks.
Dr. Nelson is a hospitalist at Ochsner Medical Center in New Orleans. Dr. Esquivel is a hospitalist and assistant professor at Weill Cornell Medicine, New York. Dr. Hall is a med-peds hospitalist and assistant professor at the University of Kentucky, Lexington.
References
1. MacKinnon R. How you manage your emails may be bad for your health. Science Daily. https://www.sciencedaily.com/releases/2016/01/160104081249.htm. Published Jan 4, 2016.
2. Plummer M. How to spend way less time on email every day. Harvard Business Review. https://hbr.org/2019/01/how-to-spend-way-less-time-on-email-every-day. 2019 Jan 22.
3. Covey SR. The 7 Habits of Highly Effective People: Powerful Lessons in Personal Change. New York: Free Press, 2004.
4. Ericson C. 5 Ways to Take Control of Your Email Inbox. Forbes. https://www.forbes.com/sites/learnvest/2014/03/17/5-ways-to-take-control-of-your-email-inbox/#3711f5946342. 2014 Mar 17.
5. Limit the time you spend on email. Harvard Business Review. https://hbr.org/2014/02/limit-the-time-you-spend-on-email. 2014 Feb 6.
6. McGhee S. Empty your inbox: 4 ways to take control of your email. Internet and Telephone Blog. https://www.itllc.net/it-support-ma/empty-your-inbox-4-ways-to-take-control-of-your-email/.
7. Allen D. Getting Things Done: The Art of Stress-Free Productivity. New York: Penguin Books, 2015.
Internists least likely to choose their specialty again, survey shows
Internists spent an average of 18.5 hours per week on paperwork, according to the Medscape Internist Compensation Report 2020. That number was surpassed only by intensivists, who spent 19.1 hours on such tasks.
Although that number was up $8,000 from last year, it was still less than half that of the top-earning specialists.
The top four specialties in terms of pay were the same this year as they were last year and ranked in the same order: orthopedists made the most, at $511,000, followed by plastic surgeons ($479,000), otolaryngologists ($455,000), and cardiologists ($438,000).
However, internists ranked in the middle of all physicians as to feeling fairly compensated. Just more than half (52%) reported they were fairly compensated, compared with 67% of oncologists, emergency medicine physicians, and radiologists, who were at the top of the ranking, and 44% of nephrologists, who were on the low end.
Also, just as last year, male internists earned 23% more than their female colleagues, which is a slightly smaller pay gap than the 31% gap seen overall.
COVID-19 reversing income gains
However, the compensation picture is changing for all physicians. This report reflects data gathered between Oct. 4, 2019, and Feb. 10, 2020. Since that time, the COVID-19 crisis has reversed income gains for physicians overall. A study from the Medical Group Management Association (MGMA) indicates that more than half of medical practices reported a drop in revenue by early April of 55% and a drop in patient volume of 60%.
The MGMA noted, “Practices are struggling to stay afloat – and many fear that this is only the beginning.”
Specialty choice may vary
In the Medscape survey, internists were the physicians least likely to say they would choose their specialty again. Only 66% said they would choose it again, compared with the most enthusiastic specialists: orthopedists (97%), oncologists (96%), and ophthalmologists and dermatologists (both at 95%).
However, three-fourths of internists (75%) said they would choose medicine again, which was a larger proportion than that reported by family physicians (74%), neurologists (73%), and plastic surgeons (72%).
This year’s Medscape survey is the first to ask about incentive bonuses. More than half of all physicians (56%) reported receiving one. Bonuses for internists ranked near the bottom, at an average of $27,000. Orthopedists averaged $96,000 bonuses, and family physicians received the least, at an average of $24,000.
Most internists (63%) said their bonus had no effect on the number of hours worked, which was similar to physicians in other specialties.
In good news, internists lost less money on claims that were denied or that required resubmission than most of their colleagues in other specialties. By comparison, internists reported losing 15% on such claims, and plastic surgeons lost almost twice that percentage (28%).
The survey authors noted, “One study found that, on average, 63% of denied claims are recoverable, but healthcare professionals spend about $118 per claim on appeals.”
Relationships with patients most rewarding
When asked about the most rewarding part of their job, internists ranked “gratitude/relationships with patients” at the top. In this survey, internists spent about the same amount of time with patients that all physicians spent with patients on average, 37.9 hours per week.
“Making good money at a job I like” was the fourth-biggest driver of satisfaction (only 11% said that was the most rewarding part), behind “being very good at what I do/finding answers, diagnoses” and “knowing that I’m making the world a better place.”
Some questions on the survey pertained to the use of advanced practice providers. More than half of internists (54%) reported their practice included nurse practitioners (NPs), and 36% included physician assistants (PAs); 37% employed neither.
Half of the internists who employed NPs and PAs said they had no effect on profitability, 44% said they increased it, and 6% said they decreased it. Physicians overall were split (47% each) on whether NPs and PAs increased profitability or had no effect on it.
A version of this article originally appeared on Medscape.com.
Internists spent an average of 18.5 hours per week on paperwork, according to the Medscape Internist Compensation Report 2020. That number was surpassed only by intensivists, who spent 19.1 hours on such tasks.
Although that number was up $8,000 from last year, it was still less than half that of the top-earning specialists.
The top four specialties in terms of pay were the same this year as they were last year and ranked in the same order: orthopedists made the most, at $511,000, followed by plastic surgeons ($479,000), otolaryngologists ($455,000), and cardiologists ($438,000).
However, internists ranked in the middle of all physicians as to feeling fairly compensated. Just more than half (52%) reported they were fairly compensated, compared with 67% of oncologists, emergency medicine physicians, and radiologists, who were at the top of the ranking, and 44% of nephrologists, who were on the low end.
Also, just as last year, male internists earned 23% more than their female colleagues, which is a slightly smaller pay gap than the 31% gap seen overall.
COVID-19 reversing income gains
However, the compensation picture is changing for all physicians. This report reflects data gathered between Oct. 4, 2019, and Feb. 10, 2020. Since that time, the COVID-19 crisis has reversed income gains for physicians overall. A study from the Medical Group Management Association (MGMA) indicates that more than half of medical practices reported a drop in revenue by early April of 55% and a drop in patient volume of 60%.
The MGMA noted, “Practices are struggling to stay afloat – and many fear that this is only the beginning.”
Specialty choice may vary
In the Medscape survey, internists were the physicians least likely to say they would choose their specialty again. Only 66% said they would choose it again, compared with the most enthusiastic specialists: orthopedists (97%), oncologists (96%), and ophthalmologists and dermatologists (both at 95%).
However, three-fourths of internists (75%) said they would choose medicine again, which was a larger proportion than that reported by family physicians (74%), neurologists (73%), and plastic surgeons (72%).
This year’s Medscape survey is the first to ask about incentive bonuses. More than half of all physicians (56%) reported receiving one. Bonuses for internists ranked near the bottom, at an average of $27,000. Orthopedists averaged $96,000 bonuses, and family physicians received the least, at an average of $24,000.
Most internists (63%) said their bonus had no effect on the number of hours worked, which was similar to physicians in other specialties.
In good news, internists lost less money on claims that were denied or that required resubmission than most of their colleagues in other specialties. By comparison, internists reported losing 15% on such claims, and plastic surgeons lost almost twice that percentage (28%).
The survey authors noted, “One study found that, on average, 63% of denied claims are recoverable, but healthcare professionals spend about $118 per claim on appeals.”
Relationships with patients most rewarding
When asked about the most rewarding part of their job, internists ranked “gratitude/relationships with patients” at the top. In this survey, internists spent about the same amount of time with patients that all physicians spent with patients on average, 37.9 hours per week.
“Making good money at a job I like” was the fourth-biggest driver of satisfaction (only 11% said that was the most rewarding part), behind “being very good at what I do/finding answers, diagnoses” and “knowing that I’m making the world a better place.”
Some questions on the survey pertained to the use of advanced practice providers. More than half of internists (54%) reported their practice included nurse practitioners (NPs), and 36% included physician assistants (PAs); 37% employed neither.
Half of the internists who employed NPs and PAs said they had no effect on profitability, 44% said they increased it, and 6% said they decreased it. Physicians overall were split (47% each) on whether NPs and PAs increased profitability or had no effect on it.
A version of this article originally appeared on Medscape.com.
Internists spent an average of 18.5 hours per week on paperwork, according to the Medscape Internist Compensation Report 2020. That number was surpassed only by intensivists, who spent 19.1 hours on such tasks.
Although that number was up $8,000 from last year, it was still less than half that of the top-earning specialists.
The top four specialties in terms of pay were the same this year as they were last year and ranked in the same order: orthopedists made the most, at $511,000, followed by plastic surgeons ($479,000), otolaryngologists ($455,000), and cardiologists ($438,000).
However, internists ranked in the middle of all physicians as to feeling fairly compensated. Just more than half (52%) reported they were fairly compensated, compared with 67% of oncologists, emergency medicine physicians, and radiologists, who were at the top of the ranking, and 44% of nephrologists, who were on the low end.
Also, just as last year, male internists earned 23% more than their female colleagues, which is a slightly smaller pay gap than the 31% gap seen overall.
COVID-19 reversing income gains
However, the compensation picture is changing for all physicians. This report reflects data gathered between Oct. 4, 2019, and Feb. 10, 2020. Since that time, the COVID-19 crisis has reversed income gains for physicians overall. A study from the Medical Group Management Association (MGMA) indicates that more than half of medical practices reported a drop in revenue by early April of 55% and a drop in patient volume of 60%.
The MGMA noted, “Practices are struggling to stay afloat – and many fear that this is only the beginning.”
Specialty choice may vary
In the Medscape survey, internists were the physicians least likely to say they would choose their specialty again. Only 66% said they would choose it again, compared with the most enthusiastic specialists: orthopedists (97%), oncologists (96%), and ophthalmologists and dermatologists (both at 95%).
However, three-fourths of internists (75%) said they would choose medicine again, which was a larger proportion than that reported by family physicians (74%), neurologists (73%), and plastic surgeons (72%).
This year’s Medscape survey is the first to ask about incentive bonuses. More than half of all physicians (56%) reported receiving one. Bonuses for internists ranked near the bottom, at an average of $27,000. Orthopedists averaged $96,000 bonuses, and family physicians received the least, at an average of $24,000.
Most internists (63%) said their bonus had no effect on the number of hours worked, which was similar to physicians in other specialties.
In good news, internists lost less money on claims that were denied or that required resubmission than most of their colleagues in other specialties. By comparison, internists reported losing 15% on such claims, and plastic surgeons lost almost twice that percentage (28%).
The survey authors noted, “One study found that, on average, 63% of denied claims are recoverable, but healthcare professionals spend about $118 per claim on appeals.”
Relationships with patients most rewarding
When asked about the most rewarding part of their job, internists ranked “gratitude/relationships with patients” at the top. In this survey, internists spent about the same amount of time with patients that all physicians spent with patients on average, 37.9 hours per week.
“Making good money at a job I like” was the fourth-biggest driver of satisfaction (only 11% said that was the most rewarding part), behind “being very good at what I do/finding answers, diagnoses” and “knowing that I’m making the world a better place.”
Some questions on the survey pertained to the use of advanced practice providers. More than half of internists (54%) reported their practice included nurse practitioners (NPs), and 36% included physician assistants (PAs); 37% employed neither.
Half of the internists who employed NPs and PAs said they had no effect on profitability, 44% said they increased it, and 6% said they decreased it. Physicians overall were split (47% each) on whether NPs and PAs increased profitability or had no effect on it.
A version of this article originally appeared on Medscape.com.
Is anemia due to folate deficiency a myth?
A 46-year-old man who lives in Tacoma, Wash., is seen for fatigue. He has a no significant past medical history. He is not taking any medications. His physical exam is unremarkable. His hemoglobin is 12 gm/dL, hematocrit is 37 gm/dL, mean corpuscular volume (MCV) is 103 fL, and thyroid-stimulating hormone level is 1.2 mU/L.
What workup do you recommend?
A) B12, folate testing
B) Alcohol history, B12, folate testing
C) Alcohol history, B12 testing
I would choose doing a careful alcohol history and vitamin B12 testing.
Dr. Seppä and colleagues looked at all outpatients who had a blood count done over an 8-month period.1 A total of 9,527 blood counts were ordered, and 287 (3%) had macrocytosis.1 Further workup was done for 113 of the patients. The most common cause found for macrocytosis was alcohol abuse, in 74 (65%) of the patients (80% of the men and 36% of the women). In several studies, vitamin B12 deficiency was the cause of macrocytosis in 5%-7% of patients.2,3
In 1978, a study by Davidson and Hamilton looked at 200 consecutive patients with MCVs over 100, and were able to find a cause in 80%.4 Sixteen of these patients had a low B12 level and 10 had a low folate level.
In 1998, the Food and Drug Administration required folic acid fortification of enriched grain products in the United States to help decrease the risk of neural tube defects. Similar fortification efforts were undertaken in Canada. Since 1998, anemia due to folate deficiency has essentially disappeared in individuals who have access to fortified grain products.
Joelson and colleagues looked at data on folate testing from the year prior to fortification of the grain supply (1997) and after (2004).5 They found that, in 1997, 4.8% of tests had a folate level less than 160 ng/mL compared with only 0.6% of tests in 2004.
When a more stringent cutoff for deficiency was used (94 ng/mL) 0.98% of tests were below that level in 1997, and 0.09% in 2004. The mean RBC folate level in 1997 was 420 ng/mL and rose to 697 ng/mL in 2004. Of the patients who did have low folate levels, only a minority had elevated MCVs.
Shojania et al. looked at folate testing in Canada after widespread fortification had started.6 They found that 0.5% of 2,154 serum folate levels were low and 0.7% of 560 red blood cell folate levels were low. Folate deficiency was not the cause of anemia in any of the patients with low folate levels.
Theisen-Toupal and colleagues did a retrospective study looking at folate testing over an 11-year period after fortification.7 The researchers examined the results of 84,187 assessments of folate levels. Forty-seven (0.056%) of the tests found patients with folate deficiency, 166 (0.197%), found patients with low-normal folate levels, 57,411 (68.195%) of tests yielded normal results, and 26,563 (31.552%) of tests found high folate levels. The opinion of the authors was that folate testing should be severely reduced or eliminated. Furthermore, the American Society for Clinical Pathology, as part of the Choosing Wisely campaign, states: “Do not order red blood cell folate levels at all.”8
So what does this all mean? We have been taught to have a reflex response to the evaluation of macrocytosis to test for B12 and folate. Neither of these are particularly common causes of macrocytosis, and in countries where there is grain fortification, folate deficiency is exceedingly uncommon, and should not be tested for early in any diagnostic process.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at imnews@mdedge.com.
References
1. Seppä K et al. Evaluation of macrocytosis by general practitioners. J Stud Alcohol. 1996 Jan;57(1):97-100.
2. Seppä K et al. Blood count and hematologic morphology in nonanemic macrocytosis: Differences between alcohol abuse and pernicious anemia. Alcohol. 1993 Sep-Oct;10(5):343-7.
3. Wymer A, Becker DM. Recognition and evaluation of red blood cell macrocytosis in the primary care setting. J Gen Intern Med. 1990 May-Jun;5(3):192-7.
4. Davidson RJ, Hamilton PJ. High mean red cell volume: Its incidence and significance in routine haematology. J Clin Pathol. 1978 May;31[5]:493-8.
5. Joelson DW, Fiebig EW. Diminished need for folate measurements among indigent populations in the post folic acid supplementation era. Arch Pathol Lab Med. 2007 Mar;131(3):477-80.
6. Shojania AM, von Kuster K. Ordering folate assays is no longer justified for investigation of anemias, in folic acid fortified countries. BMC Res Notes. 2010 Jan 25;3:22. doi: 10.1186/1756-0500-3-22.
7. Theisen-Toupal et al. Low yield of outpatient serum folate testing. JAMA Intern Med. 2014 Oct. doi: 10.1001/jamainternmed.2014.3593.
8. Choosing Wisely: American Society for Clinical Pathology, Oct. 19, 2017. Recommendation.
A 46-year-old man who lives in Tacoma, Wash., is seen for fatigue. He has a no significant past medical history. He is not taking any medications. His physical exam is unremarkable. His hemoglobin is 12 gm/dL, hematocrit is 37 gm/dL, mean corpuscular volume (MCV) is 103 fL, and thyroid-stimulating hormone level is 1.2 mU/L.
What workup do you recommend?
A) B12, folate testing
B) Alcohol history, B12, folate testing
C) Alcohol history, B12 testing
I would choose doing a careful alcohol history and vitamin B12 testing.
Dr. Seppä and colleagues looked at all outpatients who had a blood count done over an 8-month period.1 A total of 9,527 blood counts were ordered, and 287 (3%) had macrocytosis.1 Further workup was done for 113 of the patients. The most common cause found for macrocytosis was alcohol abuse, in 74 (65%) of the patients (80% of the men and 36% of the women). In several studies, vitamin B12 deficiency was the cause of macrocytosis in 5%-7% of patients.2,3
In 1978, a study by Davidson and Hamilton looked at 200 consecutive patients with MCVs over 100, and were able to find a cause in 80%.4 Sixteen of these patients had a low B12 level and 10 had a low folate level.
In 1998, the Food and Drug Administration required folic acid fortification of enriched grain products in the United States to help decrease the risk of neural tube defects. Similar fortification efforts were undertaken in Canada. Since 1998, anemia due to folate deficiency has essentially disappeared in individuals who have access to fortified grain products.
Joelson and colleagues looked at data on folate testing from the year prior to fortification of the grain supply (1997) and after (2004).5 They found that, in 1997, 4.8% of tests had a folate level less than 160 ng/mL compared with only 0.6% of tests in 2004.
When a more stringent cutoff for deficiency was used (94 ng/mL) 0.98% of tests were below that level in 1997, and 0.09% in 2004. The mean RBC folate level in 1997 was 420 ng/mL and rose to 697 ng/mL in 2004. Of the patients who did have low folate levels, only a minority had elevated MCVs.
Shojania et al. looked at folate testing in Canada after widespread fortification had started.6 They found that 0.5% of 2,154 serum folate levels were low and 0.7% of 560 red blood cell folate levels were low. Folate deficiency was not the cause of anemia in any of the patients with low folate levels.
Theisen-Toupal and colleagues did a retrospective study looking at folate testing over an 11-year period after fortification.7 The researchers examined the results of 84,187 assessments of folate levels. Forty-seven (0.056%) of the tests found patients with folate deficiency, 166 (0.197%), found patients with low-normal folate levels, 57,411 (68.195%) of tests yielded normal results, and 26,563 (31.552%) of tests found high folate levels. The opinion of the authors was that folate testing should be severely reduced or eliminated. Furthermore, the American Society for Clinical Pathology, as part of the Choosing Wisely campaign, states: “Do not order red blood cell folate levels at all.”8
So what does this all mean? We have been taught to have a reflex response to the evaluation of macrocytosis to test for B12 and folate. Neither of these are particularly common causes of macrocytosis, and in countries where there is grain fortification, folate deficiency is exceedingly uncommon, and should not be tested for early in any diagnostic process.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at imnews@mdedge.com.
References
1. Seppä K et al. Evaluation of macrocytosis by general practitioners. J Stud Alcohol. 1996 Jan;57(1):97-100.
2. Seppä K et al. Blood count and hematologic morphology in nonanemic macrocytosis: Differences between alcohol abuse and pernicious anemia. Alcohol. 1993 Sep-Oct;10(5):343-7.
3. Wymer A, Becker DM. Recognition and evaluation of red blood cell macrocytosis in the primary care setting. J Gen Intern Med. 1990 May-Jun;5(3):192-7.
4. Davidson RJ, Hamilton PJ. High mean red cell volume: Its incidence and significance in routine haematology. J Clin Pathol. 1978 May;31[5]:493-8.
5. Joelson DW, Fiebig EW. Diminished need for folate measurements among indigent populations in the post folic acid supplementation era. Arch Pathol Lab Med. 2007 Mar;131(3):477-80.
6. Shojania AM, von Kuster K. Ordering folate assays is no longer justified for investigation of anemias, in folic acid fortified countries. BMC Res Notes. 2010 Jan 25;3:22. doi: 10.1186/1756-0500-3-22.
7. Theisen-Toupal et al. Low yield of outpatient serum folate testing. JAMA Intern Med. 2014 Oct. doi: 10.1001/jamainternmed.2014.3593.
8. Choosing Wisely: American Society for Clinical Pathology, Oct. 19, 2017. Recommendation.
A 46-year-old man who lives in Tacoma, Wash., is seen for fatigue. He has a no significant past medical history. He is not taking any medications. His physical exam is unremarkable. His hemoglobin is 12 gm/dL, hematocrit is 37 gm/dL, mean corpuscular volume (MCV) is 103 fL, and thyroid-stimulating hormone level is 1.2 mU/L.
What workup do you recommend?
A) B12, folate testing
B) Alcohol history, B12, folate testing
C) Alcohol history, B12 testing
I would choose doing a careful alcohol history and vitamin B12 testing.
Dr. Seppä and colleagues looked at all outpatients who had a blood count done over an 8-month period.1 A total of 9,527 blood counts were ordered, and 287 (3%) had macrocytosis.1 Further workup was done for 113 of the patients. The most common cause found for macrocytosis was alcohol abuse, in 74 (65%) of the patients (80% of the men and 36% of the women). In several studies, vitamin B12 deficiency was the cause of macrocytosis in 5%-7% of patients.2,3
In 1978, a study by Davidson and Hamilton looked at 200 consecutive patients with MCVs over 100, and were able to find a cause in 80%.4 Sixteen of these patients had a low B12 level and 10 had a low folate level.
In 1998, the Food and Drug Administration required folic acid fortification of enriched grain products in the United States to help decrease the risk of neural tube defects. Similar fortification efforts were undertaken in Canada. Since 1998, anemia due to folate deficiency has essentially disappeared in individuals who have access to fortified grain products.
Joelson and colleagues looked at data on folate testing from the year prior to fortification of the grain supply (1997) and after (2004).5 They found that, in 1997, 4.8% of tests had a folate level less than 160 ng/mL compared with only 0.6% of tests in 2004.
When a more stringent cutoff for deficiency was used (94 ng/mL) 0.98% of tests were below that level in 1997, and 0.09% in 2004. The mean RBC folate level in 1997 was 420 ng/mL and rose to 697 ng/mL in 2004. Of the patients who did have low folate levels, only a minority had elevated MCVs.
Shojania et al. looked at folate testing in Canada after widespread fortification had started.6 They found that 0.5% of 2,154 serum folate levels were low and 0.7% of 560 red blood cell folate levels were low. Folate deficiency was not the cause of anemia in any of the patients with low folate levels.
Theisen-Toupal and colleagues did a retrospective study looking at folate testing over an 11-year period after fortification.7 The researchers examined the results of 84,187 assessments of folate levels. Forty-seven (0.056%) of the tests found patients with folate deficiency, 166 (0.197%), found patients with low-normal folate levels, 57,411 (68.195%) of tests yielded normal results, and 26,563 (31.552%) of tests found high folate levels. The opinion of the authors was that folate testing should be severely reduced or eliminated. Furthermore, the American Society for Clinical Pathology, as part of the Choosing Wisely campaign, states: “Do not order red blood cell folate levels at all.”8
So what does this all mean? We have been taught to have a reflex response to the evaluation of macrocytosis to test for B12 and folate. Neither of these are particularly common causes of macrocytosis, and in countries where there is grain fortification, folate deficiency is exceedingly uncommon, and should not be tested for early in any diagnostic process.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at imnews@mdedge.com.
References
1. Seppä K et al. Evaluation of macrocytosis by general practitioners. J Stud Alcohol. 1996 Jan;57(1):97-100.
2. Seppä K et al. Blood count and hematologic morphology in nonanemic macrocytosis: Differences between alcohol abuse and pernicious anemia. Alcohol. 1993 Sep-Oct;10(5):343-7.
3. Wymer A, Becker DM. Recognition and evaluation of red blood cell macrocytosis in the primary care setting. J Gen Intern Med. 1990 May-Jun;5(3):192-7.
4. Davidson RJ, Hamilton PJ. High mean red cell volume: Its incidence and significance in routine haematology. J Clin Pathol. 1978 May;31[5]:493-8.
5. Joelson DW, Fiebig EW. Diminished need for folate measurements among indigent populations in the post folic acid supplementation era. Arch Pathol Lab Med. 2007 Mar;131(3):477-80.
6. Shojania AM, von Kuster K. Ordering folate assays is no longer justified for investigation of anemias, in folic acid fortified countries. BMC Res Notes. 2010 Jan 25;3:22. doi: 10.1186/1756-0500-3-22.
7. Theisen-Toupal et al. Low yield of outpatient serum folate testing. JAMA Intern Med. 2014 Oct. doi: 10.1001/jamainternmed.2014.3593.
8. Choosing Wisely: American Society for Clinical Pathology, Oct. 19, 2017. Recommendation.