User login
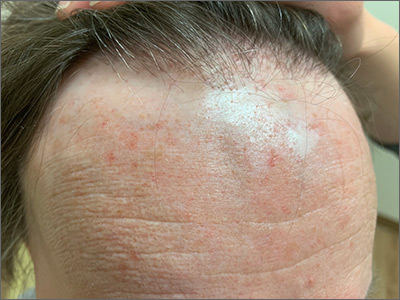
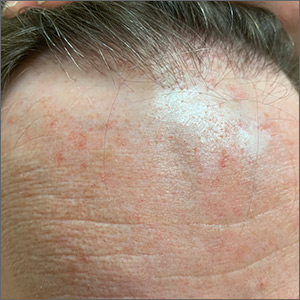
Receding hairline
This patient had frontal fibrosing alopecia (FFA), a subtype of lichen planopilaris (LPP), or follicular lichen planus. LPP causes cicatricial (scarring) alopecia where the follicular epithelium is replaced with connective tissue and the hair follicle is permanently lost. LPP is caused by lymphocytic inflammation that initially presents as perifollicular erythema, with scale and keratotic plugs, and later progresses to scarring. If there is uncertainty in the diagnosis, biopsy can be helpful.
The LPP subtype, FFA, usually occurs in postmenopausal women. It follows a distinctive pattern, as in this patient, where the hair is progressively lost along the frontoparietal hair line (and sometimes the eyebrows). A careful physical examination reveals smooth skin where follicles are lost and there is erythema around the base of the hairs due to active inflammation and keratotic plugging. The specific mechanism of FFA is poorly understood, and hormones may play a role, in addition to the inflammatory response.
The goal of treatment is to arrest the progression of additional hair loss (which usually is permanent). Intralesional steroid injections, which also are used for alopecia areata, are the most common therapy. Triamcinolone 2.5 to 5 mg/mL is injected in the affected dermal layer of the scalp. Oral finasteride (a 5-alpha-reductase inhibitor to decrease androgens) 1 mg/d can be helpful, as can oral hydroxychloroquine 200 mg bid.
Once the inflammation has subsided, treatment can be discontinued. Hair transplantation has been used, but often fails due to the inflammatory scarring process. Our patient noted that her disease process had been stable and declined treatment.
Photo courtesy of Daniel Stulberg, MD, FAAFP. Text courtesy of Rory Aufderheide, MD, and Daniel Stulberg, MD, FAAFP. Drs. Stulberg and Aufderheide are from the Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
To D, Beecker J. Frontal fibrosing alopecia: update and review of challenges and successes. J Cutan Med Surg. 2018;22:182-189.
This patient had frontal fibrosing alopecia (FFA), a subtype of lichen planopilaris (LPP), or follicular lichen planus. LPP causes cicatricial (scarring) alopecia where the follicular epithelium is replaced with connective tissue and the hair follicle is permanently lost. LPP is caused by lymphocytic inflammation that initially presents as perifollicular erythema, with scale and keratotic plugs, and later progresses to scarring. If there is uncertainty in the diagnosis, biopsy can be helpful.
The LPP subtype, FFA, usually occurs in postmenopausal women. It follows a distinctive pattern, as in this patient, where the hair is progressively lost along the frontoparietal hair line (and sometimes the eyebrows). A careful physical examination reveals smooth skin where follicles are lost and there is erythema around the base of the hairs due to active inflammation and keratotic plugging. The specific mechanism of FFA is poorly understood, and hormones may play a role, in addition to the inflammatory response.
The goal of treatment is to arrest the progression of additional hair loss (which usually is permanent). Intralesional steroid injections, which also are used for alopecia areata, are the most common therapy. Triamcinolone 2.5 to 5 mg/mL is injected in the affected dermal layer of the scalp. Oral finasteride (a 5-alpha-reductase inhibitor to decrease androgens) 1 mg/d can be helpful, as can oral hydroxychloroquine 200 mg bid.
Once the inflammation has subsided, treatment can be discontinued. Hair transplantation has been used, but often fails due to the inflammatory scarring process. Our patient noted that her disease process had been stable and declined treatment.
Photo courtesy of Daniel Stulberg, MD, FAAFP. Text courtesy of Rory Aufderheide, MD, and Daniel Stulberg, MD, FAAFP. Drs. Stulberg and Aufderheide are from the Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
This patient had frontal fibrosing alopecia (FFA), a subtype of lichen planopilaris (LPP), or follicular lichen planus. LPP causes cicatricial (scarring) alopecia where the follicular epithelium is replaced with connective tissue and the hair follicle is permanently lost. LPP is caused by lymphocytic inflammation that initially presents as perifollicular erythema, with scale and keratotic plugs, and later progresses to scarring. If there is uncertainty in the diagnosis, biopsy can be helpful.
The LPP subtype, FFA, usually occurs in postmenopausal women. It follows a distinctive pattern, as in this patient, where the hair is progressively lost along the frontoparietal hair line (and sometimes the eyebrows). A careful physical examination reveals smooth skin where follicles are lost and there is erythema around the base of the hairs due to active inflammation and keratotic plugging. The specific mechanism of FFA is poorly understood, and hormones may play a role, in addition to the inflammatory response.
The goal of treatment is to arrest the progression of additional hair loss (which usually is permanent). Intralesional steroid injections, which also are used for alopecia areata, are the most common therapy. Triamcinolone 2.5 to 5 mg/mL is injected in the affected dermal layer of the scalp. Oral finasteride (a 5-alpha-reductase inhibitor to decrease androgens) 1 mg/d can be helpful, as can oral hydroxychloroquine 200 mg bid.
Once the inflammation has subsided, treatment can be discontinued. Hair transplantation has been used, but often fails due to the inflammatory scarring process. Our patient noted that her disease process had been stable and declined treatment.
Photo courtesy of Daniel Stulberg, MD, FAAFP. Text courtesy of Rory Aufderheide, MD, and Daniel Stulberg, MD, FAAFP. Drs. Stulberg and Aufderheide are from the Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
To D, Beecker J. Frontal fibrosing alopecia: update and review of challenges and successes. J Cutan Med Surg. 2018;22:182-189.
To D, Beecker J. Frontal fibrosing alopecia: update and review of challenges and successes. J Cutan Med Surg. 2018;22:182-189.
Breast-conserving surgery deemed okay in high-risk hereditary breast cancers
It’s okay to consider breast-conserving therapy in breast cancer patients with high-risk hereditary genetic mutations, according to guidelines published in the Journal of Clinical Oncology.
The presence of a germline BRCA1 or BRCA2 mutation shouldn’t preclude breast-conserving therapy as long as the patient is otherwise eligible for the procedure, according to the guidelines, which were developed by an expert panel convened by the American Society of Clinical Oncology, American Society for Radiation Oncology, and Society for Surgical Oncology.
Nadine M. Tung, MD, of Beth Israel Deaconess Medical Center in Boston and the rest of the expert panel reviewed evidence from 58 published articles to create the guidelines.
In addition to supporting use of breast-conserving therapy, the guidelines suggest that radiation shouldn’t be withheld because of mutation status, except in patients with TP53 mutations. Furthermore, BRCA1/2 mutation carriers with metastatic HER2-negative disease can receive the poly (ADP-ribose) polymerase (PARP) inhibitors olaparib and talazoparib as an “alternative to chemotherapy” for first-, second-, or third-line therapy.
However, it’s the “license to consider breast-conserving therapy” for high-risk individuals that is one of the most noteworthy points in the guidelines, and the one that may surprise some readers, according to William J. Gradishar, MD, of Northwestern University in Chicago, who was not involved in developing the guidelines.
“We don’t have to be as dogmatic with these patients with respect to local therapies as we were in the past,” Dr. Gradishar said in an interview. “That’s a good thing for patients, but you also have to understand the nuances that go into recommending [breast-conserving surgery] to a patient. Other variables, like the age at which the patient develops breast cancer, family history, etc., all go into it.”
Weighing options for surgery
The guidelines emphasize that, for patients with germline BRCA1/2 mutations, health care providers need to discuss treatment options for the breast cancer at hand. However, patients should also be made aware of their increased risk of contralateral and new ipsilateral breast cancer as compared with noncarriers.
When weighing breast-conserving therapy versus mastectomy in light of contralateral breast cancer risk, the guidelines recommend considering not only age at diagnosis – the strongest predictor of a later contralateral breast cancer – but also family history, comorbidities, life expectancy, ability to undergo MRI, and prognosis from breast or other cancers, such as ovarian cancer.
If a bilateral mastectomy isn’t performed in a BRCA1/2 mutation carrier, an annual mammogram and MRI are warranted thereafter for screening of the remaining breast tissue, according to the guidelines.
The guidelines say breast-conserving therapy should be offered to patients with mutations in moderate-penetrance genes, including PALB2, CHEK2, and ATM. However, there’s not much data regarding the risk of ipsilateral breast cancer after breast-conserving therapy in these patients.
Likewise, there’s limited evidence on contralateral breast cancer risk for patients with mutations in moderate-penetrance genes aside from CHEK2. The guidelines say the risk should be discussed with patients “in the context of shared decision making.”
Nipple-sparing mastectomy is “reasonable” to consider in certain newly diagnosed patients with BRCA1/2 mutations, as well as in newly diagnosed patients with moderate-risk mutations, the guidelines state.
Women with breast cancer and a deleterious BRCA1/2 mutation who are undergoing unilateral mastectomy should be offered contralateral risk-reducing mastectomy. Likewise, women with moderate-risk mutations should be offered contralateral risk-reducing mastectomy, but not solely based on mutation status, according to the guidelines. Data are limited on contralateral breast cancer risk related to those mutations.
Considerations for radiation
Radiation therapy in the context of breast-conserving therapy or mastectomy should not be withheld because of hereditary mutations, except in the case of TP53 mutations, according to the guidelines.
There’s no evidence that radiotherapy increases toxicity or contralateral breast cancer risk for most BRCA1/2 or moderate-penetrance gene mutations. However, the intact breast shouldn’t be irradiated in germline TP53 mutation carriers, the guidelines say, because of the important role that TP53 plays in the ability to repair DNA damage after cellular stress.
“Carriers of a TP53 mutation would be expected to be unable to repair tissue damage from DNA damaging radiotherapy and be at risk for significant [radiotherapy]-associated sequelae,” the guidelines state.
Chemotherapy and PARP inhibitors
For women with metastatic breast cancer harboring germline BRCA1/2 mutations, the guidelines say platinum chemotherapy should be preferred over taxanes for platinum-naive patients.
Provided the breast cancer is HER2 negative, the PARP inhibitors olaparib or talazoparib “should be offered as an alternative to chemotherapy in the first- to third-line settings,” the guidelines state.
The guidelines confirm that PARP inhibitors are a “valid starting point” for treatment of BCRA1/2–associated metastatic breast cancer, Dr. Gradishar said.
“When a patient progresses on a PARP inhibitor, assuming they’re not going on some other investigational drug or clinical trial, they’re going to get chemotherapy,” he said. “So the argument is that, if you have something that’s at least as good or maybe a little bit better and has fewer side effects, why not start with that and then move on to other things?”
By contrast, there’s not enough evidence to recommend PARP inhibitors for germline BRCA mutation carriers with nonmetastatic breast cancers, according to the guidelines, and there’s “no robust data” for using PARP inhibitors in patients with breast cancers with mutations in moderate-penetrance genes.
The guideline authors disclosed relationships with AstraZeneca, Myriad Genetics, Pfizer, Lilly, and other companies. Dr. Gradishar has relationships with AstraZeneca, Celltrion, Genentech, MacroGenics, Merck, Pfizer, and Seattle Genetics.
SOURCE: Tung NM et al. J Clin Oncol. 2020 Apr 3;JCO2000299. doi: 10.1200/JCO.20.00299.
It’s okay to consider breast-conserving therapy in breast cancer patients with high-risk hereditary genetic mutations, according to guidelines published in the Journal of Clinical Oncology.
The presence of a germline BRCA1 or BRCA2 mutation shouldn’t preclude breast-conserving therapy as long as the patient is otherwise eligible for the procedure, according to the guidelines, which were developed by an expert panel convened by the American Society of Clinical Oncology, American Society for Radiation Oncology, and Society for Surgical Oncology.
Nadine M. Tung, MD, of Beth Israel Deaconess Medical Center in Boston and the rest of the expert panel reviewed evidence from 58 published articles to create the guidelines.
In addition to supporting use of breast-conserving therapy, the guidelines suggest that radiation shouldn’t be withheld because of mutation status, except in patients with TP53 mutations. Furthermore, BRCA1/2 mutation carriers with metastatic HER2-negative disease can receive the poly (ADP-ribose) polymerase (PARP) inhibitors olaparib and talazoparib as an “alternative to chemotherapy” for first-, second-, or third-line therapy.
However, it’s the “license to consider breast-conserving therapy” for high-risk individuals that is one of the most noteworthy points in the guidelines, and the one that may surprise some readers, according to William J. Gradishar, MD, of Northwestern University in Chicago, who was not involved in developing the guidelines.
“We don’t have to be as dogmatic with these patients with respect to local therapies as we were in the past,” Dr. Gradishar said in an interview. “That’s a good thing for patients, but you also have to understand the nuances that go into recommending [breast-conserving surgery] to a patient. Other variables, like the age at which the patient develops breast cancer, family history, etc., all go into it.”
Weighing options for surgery
The guidelines emphasize that, for patients with germline BRCA1/2 mutations, health care providers need to discuss treatment options for the breast cancer at hand. However, patients should also be made aware of their increased risk of contralateral and new ipsilateral breast cancer as compared with noncarriers.
When weighing breast-conserving therapy versus mastectomy in light of contralateral breast cancer risk, the guidelines recommend considering not only age at diagnosis – the strongest predictor of a later contralateral breast cancer – but also family history, comorbidities, life expectancy, ability to undergo MRI, and prognosis from breast or other cancers, such as ovarian cancer.
If a bilateral mastectomy isn’t performed in a BRCA1/2 mutation carrier, an annual mammogram and MRI are warranted thereafter for screening of the remaining breast tissue, according to the guidelines.
The guidelines say breast-conserving therapy should be offered to patients with mutations in moderate-penetrance genes, including PALB2, CHEK2, and ATM. However, there’s not much data regarding the risk of ipsilateral breast cancer after breast-conserving therapy in these patients.
Likewise, there’s limited evidence on contralateral breast cancer risk for patients with mutations in moderate-penetrance genes aside from CHEK2. The guidelines say the risk should be discussed with patients “in the context of shared decision making.”
Nipple-sparing mastectomy is “reasonable” to consider in certain newly diagnosed patients with BRCA1/2 mutations, as well as in newly diagnosed patients with moderate-risk mutations, the guidelines state.
Women with breast cancer and a deleterious BRCA1/2 mutation who are undergoing unilateral mastectomy should be offered contralateral risk-reducing mastectomy. Likewise, women with moderate-risk mutations should be offered contralateral risk-reducing mastectomy, but not solely based on mutation status, according to the guidelines. Data are limited on contralateral breast cancer risk related to those mutations.
Considerations for radiation
Radiation therapy in the context of breast-conserving therapy or mastectomy should not be withheld because of hereditary mutations, except in the case of TP53 mutations, according to the guidelines.
There’s no evidence that radiotherapy increases toxicity or contralateral breast cancer risk for most BRCA1/2 or moderate-penetrance gene mutations. However, the intact breast shouldn’t be irradiated in germline TP53 mutation carriers, the guidelines say, because of the important role that TP53 plays in the ability to repair DNA damage after cellular stress.
“Carriers of a TP53 mutation would be expected to be unable to repair tissue damage from DNA damaging radiotherapy and be at risk for significant [radiotherapy]-associated sequelae,” the guidelines state.
Chemotherapy and PARP inhibitors
For women with metastatic breast cancer harboring germline BRCA1/2 mutations, the guidelines say platinum chemotherapy should be preferred over taxanes for platinum-naive patients.
Provided the breast cancer is HER2 negative, the PARP inhibitors olaparib or talazoparib “should be offered as an alternative to chemotherapy in the first- to third-line settings,” the guidelines state.
The guidelines confirm that PARP inhibitors are a “valid starting point” for treatment of BCRA1/2–associated metastatic breast cancer, Dr. Gradishar said.
“When a patient progresses on a PARP inhibitor, assuming they’re not going on some other investigational drug or clinical trial, they’re going to get chemotherapy,” he said. “So the argument is that, if you have something that’s at least as good or maybe a little bit better and has fewer side effects, why not start with that and then move on to other things?”
By contrast, there’s not enough evidence to recommend PARP inhibitors for germline BRCA mutation carriers with nonmetastatic breast cancers, according to the guidelines, and there’s “no robust data” for using PARP inhibitors in patients with breast cancers with mutations in moderate-penetrance genes.
The guideline authors disclosed relationships with AstraZeneca, Myriad Genetics, Pfizer, Lilly, and other companies. Dr. Gradishar has relationships with AstraZeneca, Celltrion, Genentech, MacroGenics, Merck, Pfizer, and Seattle Genetics.
SOURCE: Tung NM et al. J Clin Oncol. 2020 Apr 3;JCO2000299. doi: 10.1200/JCO.20.00299.
It’s okay to consider breast-conserving therapy in breast cancer patients with high-risk hereditary genetic mutations, according to guidelines published in the Journal of Clinical Oncology.
The presence of a germline BRCA1 or BRCA2 mutation shouldn’t preclude breast-conserving therapy as long as the patient is otherwise eligible for the procedure, according to the guidelines, which were developed by an expert panel convened by the American Society of Clinical Oncology, American Society for Radiation Oncology, and Society for Surgical Oncology.
Nadine M. Tung, MD, of Beth Israel Deaconess Medical Center in Boston and the rest of the expert panel reviewed evidence from 58 published articles to create the guidelines.
In addition to supporting use of breast-conserving therapy, the guidelines suggest that radiation shouldn’t be withheld because of mutation status, except in patients with TP53 mutations. Furthermore, BRCA1/2 mutation carriers with metastatic HER2-negative disease can receive the poly (ADP-ribose) polymerase (PARP) inhibitors olaparib and talazoparib as an “alternative to chemotherapy” for first-, second-, or third-line therapy.
However, it’s the “license to consider breast-conserving therapy” for high-risk individuals that is one of the most noteworthy points in the guidelines, and the one that may surprise some readers, according to William J. Gradishar, MD, of Northwestern University in Chicago, who was not involved in developing the guidelines.
“We don’t have to be as dogmatic with these patients with respect to local therapies as we were in the past,” Dr. Gradishar said in an interview. “That’s a good thing for patients, but you also have to understand the nuances that go into recommending [breast-conserving surgery] to a patient. Other variables, like the age at which the patient develops breast cancer, family history, etc., all go into it.”
Weighing options for surgery
The guidelines emphasize that, for patients with germline BRCA1/2 mutations, health care providers need to discuss treatment options for the breast cancer at hand. However, patients should also be made aware of their increased risk of contralateral and new ipsilateral breast cancer as compared with noncarriers.
When weighing breast-conserving therapy versus mastectomy in light of contralateral breast cancer risk, the guidelines recommend considering not only age at diagnosis – the strongest predictor of a later contralateral breast cancer – but also family history, comorbidities, life expectancy, ability to undergo MRI, and prognosis from breast or other cancers, such as ovarian cancer.
If a bilateral mastectomy isn’t performed in a BRCA1/2 mutation carrier, an annual mammogram and MRI are warranted thereafter for screening of the remaining breast tissue, according to the guidelines.
The guidelines say breast-conserving therapy should be offered to patients with mutations in moderate-penetrance genes, including PALB2, CHEK2, and ATM. However, there’s not much data regarding the risk of ipsilateral breast cancer after breast-conserving therapy in these patients.
Likewise, there’s limited evidence on contralateral breast cancer risk for patients with mutations in moderate-penetrance genes aside from CHEK2. The guidelines say the risk should be discussed with patients “in the context of shared decision making.”
Nipple-sparing mastectomy is “reasonable” to consider in certain newly diagnosed patients with BRCA1/2 mutations, as well as in newly diagnosed patients with moderate-risk mutations, the guidelines state.
Women with breast cancer and a deleterious BRCA1/2 mutation who are undergoing unilateral mastectomy should be offered contralateral risk-reducing mastectomy. Likewise, women with moderate-risk mutations should be offered contralateral risk-reducing mastectomy, but not solely based on mutation status, according to the guidelines. Data are limited on contralateral breast cancer risk related to those mutations.
Considerations for radiation
Radiation therapy in the context of breast-conserving therapy or mastectomy should not be withheld because of hereditary mutations, except in the case of TP53 mutations, according to the guidelines.
There’s no evidence that radiotherapy increases toxicity or contralateral breast cancer risk for most BRCA1/2 or moderate-penetrance gene mutations. However, the intact breast shouldn’t be irradiated in germline TP53 mutation carriers, the guidelines say, because of the important role that TP53 plays in the ability to repair DNA damage after cellular stress.
“Carriers of a TP53 mutation would be expected to be unable to repair tissue damage from DNA damaging radiotherapy and be at risk for significant [radiotherapy]-associated sequelae,” the guidelines state.
Chemotherapy and PARP inhibitors
For women with metastatic breast cancer harboring germline BRCA1/2 mutations, the guidelines say platinum chemotherapy should be preferred over taxanes for platinum-naive patients.
Provided the breast cancer is HER2 negative, the PARP inhibitors olaparib or talazoparib “should be offered as an alternative to chemotherapy in the first- to third-line settings,” the guidelines state.
The guidelines confirm that PARP inhibitors are a “valid starting point” for treatment of BCRA1/2–associated metastatic breast cancer, Dr. Gradishar said.
“When a patient progresses on a PARP inhibitor, assuming they’re not going on some other investigational drug or clinical trial, they’re going to get chemotherapy,” he said. “So the argument is that, if you have something that’s at least as good or maybe a little bit better and has fewer side effects, why not start with that and then move on to other things?”
By contrast, there’s not enough evidence to recommend PARP inhibitors for germline BRCA mutation carriers with nonmetastatic breast cancers, according to the guidelines, and there’s “no robust data” for using PARP inhibitors in patients with breast cancers with mutations in moderate-penetrance genes.
The guideline authors disclosed relationships with AstraZeneca, Myriad Genetics, Pfizer, Lilly, and other companies. Dr. Gradishar has relationships with AstraZeneca, Celltrion, Genentech, MacroGenics, Merck, Pfizer, and Seattle Genetics.
SOURCE: Tung NM et al. J Clin Oncol. 2020 Apr 3;JCO2000299. doi: 10.1200/JCO.20.00299.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Severe COVID-19 may lower hemoglobin levels
A meta-analysis of four applicable studies found that the hemoglobin value was significantly lower in COVID-19 patients with severe disease, compared with those with milder forms, according to a letter to the editor of Hematology Transfusion and Cell Therapy by Giuseppe Lippi, MD, of the University of Verona (Italy) and colleague.
The four studies comprised 1,210 COVID-19 patients (224 with severe disease; 18.5%). The primary endpoint was defined as a composite of admission to the ICU, need of mechanical ventilation or death. The heterogeneity among the studies was high.
Overall, the hemoglobin value was found to be significantly lower in COVID-19 patients with severe disease than in those with milder forms, yielding a weighted mean difference of −7.1 g/L, with a 95% confidence interval of −8.3 g/L to −5.9 g/L.
“Initial assessment and longitudinal monitoring of hemoglobin values seems advisable in patients with the SARS-CoV-2 infection, whereby a progressive decrease in the hemoglobin concentration may reflect a worse clinical progression,” the authors stated. They also suggested that studies should be “urgently planned to assess whether transfusion support (e.g., with administration of blood or packed red blood cells) may be helpful in this clinical setting to prevent evolution into severe disease and death.”
The authors declared the had no conflicts of interest.
mlesney@mdedge.com
SOURCE: Lippi G et al. Hematol Transfus Cell Ther. 2020 Apr 11; doi:10.1016/j.htct.2020.03.001.
A meta-analysis of four applicable studies found that the hemoglobin value was significantly lower in COVID-19 patients with severe disease, compared with those with milder forms, according to a letter to the editor of Hematology Transfusion and Cell Therapy by Giuseppe Lippi, MD, of the University of Verona (Italy) and colleague.
The four studies comprised 1,210 COVID-19 patients (224 with severe disease; 18.5%). The primary endpoint was defined as a composite of admission to the ICU, need of mechanical ventilation or death. The heterogeneity among the studies was high.
Overall, the hemoglobin value was found to be significantly lower in COVID-19 patients with severe disease than in those with milder forms, yielding a weighted mean difference of −7.1 g/L, with a 95% confidence interval of −8.3 g/L to −5.9 g/L.
“Initial assessment and longitudinal monitoring of hemoglobin values seems advisable in patients with the SARS-CoV-2 infection, whereby a progressive decrease in the hemoglobin concentration may reflect a worse clinical progression,” the authors stated. They also suggested that studies should be “urgently planned to assess whether transfusion support (e.g., with administration of blood or packed red blood cells) may be helpful in this clinical setting to prevent evolution into severe disease and death.”
The authors declared the had no conflicts of interest.
mlesney@mdedge.com
SOURCE: Lippi G et al. Hematol Transfus Cell Ther. 2020 Apr 11; doi:10.1016/j.htct.2020.03.001.
A meta-analysis of four applicable studies found that the hemoglobin value was significantly lower in COVID-19 patients with severe disease, compared with those with milder forms, according to a letter to the editor of Hematology Transfusion and Cell Therapy by Giuseppe Lippi, MD, of the University of Verona (Italy) and colleague.
The four studies comprised 1,210 COVID-19 patients (224 with severe disease; 18.5%). The primary endpoint was defined as a composite of admission to the ICU, need of mechanical ventilation or death. The heterogeneity among the studies was high.
Overall, the hemoglobin value was found to be significantly lower in COVID-19 patients with severe disease than in those with milder forms, yielding a weighted mean difference of −7.1 g/L, with a 95% confidence interval of −8.3 g/L to −5.9 g/L.
“Initial assessment and longitudinal monitoring of hemoglobin values seems advisable in patients with the SARS-CoV-2 infection, whereby a progressive decrease in the hemoglobin concentration may reflect a worse clinical progression,” the authors stated. They also suggested that studies should be “urgently planned to assess whether transfusion support (e.g., with administration of blood or packed red blood cells) may be helpful in this clinical setting to prevent evolution into severe disease and death.”
The authors declared the had no conflicts of interest.
mlesney@mdedge.com
SOURCE: Lippi G et al. Hematol Transfus Cell Ther. 2020 Apr 11; doi:10.1016/j.htct.2020.03.001.
FROM HEMATOLOGY, TRANSFUSION AND CELL THERAPY
Mitomycin approved for low-grade upper tract urothelial cancer
pyelocalyceal
“This is the first approval specifically for patients with low-grade [upper tract urothelial cancer] and provides an option for some patients who may otherwise require a nephroureterectomy,” Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence, said in a statement.
“Due to substantial treatment challenges associated with the complex anatomy of the upper urinary tract, many patients need to be treated with radical surgery – usually complete removal of the affected kidney, ureter, and bladder cuff," Dr. Pazdur added. "Jelmyto gives patients, for the first time, an alternative treatment option for low-grade [upper tract urothelial cancer].”
The FDA’s approval of mitomycin is based on results from the phase 3 OLYMPUS trial (NCT02793128). This ongoing, single-arm trial enrolled 71 patients with treatment-naive or recurrent low-grade noninvasive upper tract urothelial cancer with at least one measurable papillary tumor located above the ureteropelvic junction. Patients with larger tumors were allowed to have prior tumor debulking.
The patients received mitomycin weekly for 6 weeks at the recommended dose of 4 mg/mL, instilled via ureteral catheter or nephrostomy tube, with the total instillation volume based on volumetric measurements using pyelography, not exceeding 15 mL (60 mg mitomycin).
Patients who achieved a complete response at 3 months could receive monthly instillations up to a maximum of 11 additional instillations.
At 3 months, 41 patients (58%) achieved a complete response (CR). At 12 months after CR determination, 19 patients were still in CR, and 7 patients had documented recurrences. The median duration of CR was not reached.
The most common adverse events (occurring in at least 20% of patients) were ureteric obstruction, flank pain, urinary tract infection, hematuria, renal dysfunction, fatigue, nausea, abdominal pain, dysuria, and vomiting. Ureteric obstruction occurred in 58% of patients, and 88% of those patients required ureteral stent placement.
In all, 23% of patients discontinued mitomycin due to adverse events, and 34% had dose interruptions due to adverse events.
The approval of mitomycin was granted to UroGen Pharma. The FDA granted the application priority review, fast track designation, and breakthrough therapy designation.
The full prescribing information for mitomycin is available for download from the FDA website.
pyelocalyceal
“This is the first approval specifically for patients with low-grade [upper tract urothelial cancer] and provides an option for some patients who may otherwise require a nephroureterectomy,” Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence, said in a statement.
“Due to substantial treatment challenges associated with the complex anatomy of the upper urinary tract, many patients need to be treated with radical surgery – usually complete removal of the affected kidney, ureter, and bladder cuff," Dr. Pazdur added. "Jelmyto gives patients, for the first time, an alternative treatment option for low-grade [upper tract urothelial cancer].”
The FDA’s approval of mitomycin is based on results from the phase 3 OLYMPUS trial (NCT02793128). This ongoing, single-arm trial enrolled 71 patients with treatment-naive or recurrent low-grade noninvasive upper tract urothelial cancer with at least one measurable papillary tumor located above the ureteropelvic junction. Patients with larger tumors were allowed to have prior tumor debulking.
The patients received mitomycin weekly for 6 weeks at the recommended dose of 4 mg/mL, instilled via ureteral catheter or nephrostomy tube, with the total instillation volume based on volumetric measurements using pyelography, not exceeding 15 mL (60 mg mitomycin).
Patients who achieved a complete response at 3 months could receive monthly instillations up to a maximum of 11 additional instillations.
At 3 months, 41 patients (58%) achieved a complete response (CR). At 12 months after CR determination, 19 patients were still in CR, and 7 patients had documented recurrences. The median duration of CR was not reached.
The most common adverse events (occurring in at least 20% of patients) were ureteric obstruction, flank pain, urinary tract infection, hematuria, renal dysfunction, fatigue, nausea, abdominal pain, dysuria, and vomiting. Ureteric obstruction occurred in 58% of patients, and 88% of those patients required ureteral stent placement.
In all, 23% of patients discontinued mitomycin due to adverse events, and 34% had dose interruptions due to adverse events.
The approval of mitomycin was granted to UroGen Pharma. The FDA granted the application priority review, fast track designation, and breakthrough therapy designation.
The full prescribing information for mitomycin is available for download from the FDA website.
pyelocalyceal
“This is the first approval specifically for patients with low-grade [upper tract urothelial cancer] and provides an option for some patients who may otherwise require a nephroureterectomy,” Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence, said in a statement.
“Due to substantial treatment challenges associated with the complex anatomy of the upper urinary tract, many patients need to be treated with radical surgery – usually complete removal of the affected kidney, ureter, and bladder cuff," Dr. Pazdur added. "Jelmyto gives patients, for the first time, an alternative treatment option for low-grade [upper tract urothelial cancer].”
The FDA’s approval of mitomycin is based on results from the phase 3 OLYMPUS trial (NCT02793128). This ongoing, single-arm trial enrolled 71 patients with treatment-naive or recurrent low-grade noninvasive upper tract urothelial cancer with at least one measurable papillary tumor located above the ureteropelvic junction. Patients with larger tumors were allowed to have prior tumor debulking.
The patients received mitomycin weekly for 6 weeks at the recommended dose of 4 mg/mL, instilled via ureteral catheter or nephrostomy tube, with the total instillation volume based on volumetric measurements using pyelography, not exceeding 15 mL (60 mg mitomycin).
Patients who achieved a complete response at 3 months could receive monthly instillations up to a maximum of 11 additional instillations.
At 3 months, 41 patients (58%) achieved a complete response (CR). At 12 months after CR determination, 19 patients were still in CR, and 7 patients had documented recurrences. The median duration of CR was not reached.
The most common adverse events (occurring in at least 20% of patients) were ureteric obstruction, flank pain, urinary tract infection, hematuria, renal dysfunction, fatigue, nausea, abdominal pain, dysuria, and vomiting. Ureteric obstruction occurred in 58% of patients, and 88% of those patients required ureteral stent placement.
In all, 23% of patients discontinued mitomycin due to adverse events, and 34% had dose interruptions due to adverse events.
The approval of mitomycin was granted to UroGen Pharma. The FDA granted the application priority review, fast track designation, and breakthrough therapy designation.
The full prescribing information for mitomycin is available for download from the FDA website.
FROM FDA
Survey reveals gender pay discrepancies among gyn-oncs
After controlling for differences between the genders, the male gynecologic oncologists surveyed were 1.28 times more likely than their female counterparts to earn a salary above the median, according to Katherine M. Croft, MD, of the University of Virginia, Charlottesville.
Dr. Croft and colleagues reported findings from the survey in an abstract that was slated for presentation at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer. The meeting was canceled because of the COVID-19 pandemic.
Of 263 members of the Society of Gynecologic Oncology who responded to the anonymous survey, 41% were women and 59% were men. The median annual salaries were $380,000 and $500,000 respectively.
“Comparing compensation by gender, there was a $120,000 difference in median salary when you compare them on a surface level,” Dr. Croft said. “Combing through the data further, we found that there were few other differences by gender.”
There were no differences between genders with respect to group size, percentage of protected research time, frequency of call, or geographic location. However, men were more likely to be compensated for extra call and were more likely to respond to obstetrical emergencies, and those differences were statistically significant.
Further, female gynecologic oncologists were younger and had been in practice for fewer years. They also were more likely to work in an academic setting and to work with residents.
“For men, the odds of making above the median salary was 1.28 times that of female providers when controlling for these differences” Dr. Croft said.
Significant compensation differences were noted based on practice setting. When these were substratified by gender, only academic or teaching hospitals and teaching hospital/community hybrids had significant pay differences by gender.
Academic or teaching hospitals comprised the largest subgroup, allowing for further analysis.
“Age and years post fellowship were the only significant differences by gender in this group,” Dr. Croft said. “Again, female providers earned less than their male counterparts, with mean compensation of $349,717, compared with $461,054.”
In fact, less than 25% of women in academic practice in this survey made above the median reported salary, Dr. Croft noted. Controlling not only for differences between male and female providers in this group but also for other known factors affecting compensation, the odds of a male provider making greater than the median salary were 1.77 times that of female providers.
Women represent nearly a third of all practicing physicians, but their salaries continue to lag behind those of men, Dr. Croft noted. She added that “this is the first study that has been presented with regards to gynecologic oncology gender salary discrepancies.”
The findings are limited by survey response bias and a potential lack of data that could explain some of the discrepancies. The study was originally designed to look at on-call compensation, so respondents were not queried about academic ranking or specific work responsibilities. Still, Dr. Croft said the findings point to a need for policy reform to ensure equitable compensation.
“My hope is that these data open a dialogue to further explore discrepancies by gender in our field,” she said.
Dr. Croft reported having no disclosures.
sworcester@mdedge.com
SOURCE: Croft K et al. SGO 2020, Abstract 15.
After controlling for differences between the genders, the male gynecologic oncologists surveyed were 1.28 times more likely than their female counterparts to earn a salary above the median, according to Katherine M. Croft, MD, of the University of Virginia, Charlottesville.
Dr. Croft and colleagues reported findings from the survey in an abstract that was slated for presentation at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer. The meeting was canceled because of the COVID-19 pandemic.
Of 263 members of the Society of Gynecologic Oncology who responded to the anonymous survey, 41% were women and 59% were men. The median annual salaries were $380,000 and $500,000 respectively.
“Comparing compensation by gender, there was a $120,000 difference in median salary when you compare them on a surface level,” Dr. Croft said. “Combing through the data further, we found that there were few other differences by gender.”
There were no differences between genders with respect to group size, percentage of protected research time, frequency of call, or geographic location. However, men were more likely to be compensated for extra call and were more likely to respond to obstetrical emergencies, and those differences were statistically significant.
Further, female gynecologic oncologists were younger and had been in practice for fewer years. They also were more likely to work in an academic setting and to work with residents.
“For men, the odds of making above the median salary was 1.28 times that of female providers when controlling for these differences” Dr. Croft said.
Significant compensation differences were noted based on practice setting. When these were substratified by gender, only academic or teaching hospitals and teaching hospital/community hybrids had significant pay differences by gender.
Academic or teaching hospitals comprised the largest subgroup, allowing for further analysis.
“Age and years post fellowship were the only significant differences by gender in this group,” Dr. Croft said. “Again, female providers earned less than their male counterparts, with mean compensation of $349,717, compared with $461,054.”
In fact, less than 25% of women in academic practice in this survey made above the median reported salary, Dr. Croft noted. Controlling not only for differences between male and female providers in this group but also for other known factors affecting compensation, the odds of a male provider making greater than the median salary were 1.77 times that of female providers.
Women represent nearly a third of all practicing physicians, but their salaries continue to lag behind those of men, Dr. Croft noted. She added that “this is the first study that has been presented with regards to gynecologic oncology gender salary discrepancies.”
The findings are limited by survey response bias and a potential lack of data that could explain some of the discrepancies. The study was originally designed to look at on-call compensation, so respondents were not queried about academic ranking or specific work responsibilities. Still, Dr. Croft said the findings point to a need for policy reform to ensure equitable compensation.
“My hope is that these data open a dialogue to further explore discrepancies by gender in our field,” she said.
Dr. Croft reported having no disclosures.
sworcester@mdedge.com
SOURCE: Croft K et al. SGO 2020, Abstract 15.
After controlling for differences between the genders, the male gynecologic oncologists surveyed were 1.28 times more likely than their female counterparts to earn a salary above the median, according to Katherine M. Croft, MD, of the University of Virginia, Charlottesville.
Dr. Croft and colleagues reported findings from the survey in an abstract that was slated for presentation at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer. The meeting was canceled because of the COVID-19 pandemic.
Of 263 members of the Society of Gynecologic Oncology who responded to the anonymous survey, 41% were women and 59% were men. The median annual salaries were $380,000 and $500,000 respectively.
“Comparing compensation by gender, there was a $120,000 difference in median salary when you compare them on a surface level,” Dr. Croft said. “Combing through the data further, we found that there were few other differences by gender.”
There were no differences between genders with respect to group size, percentage of protected research time, frequency of call, or geographic location. However, men were more likely to be compensated for extra call and were more likely to respond to obstetrical emergencies, and those differences were statistically significant.
Further, female gynecologic oncologists were younger and had been in practice for fewer years. They also were more likely to work in an academic setting and to work with residents.
“For men, the odds of making above the median salary was 1.28 times that of female providers when controlling for these differences” Dr. Croft said.
Significant compensation differences were noted based on practice setting. When these were substratified by gender, only academic or teaching hospitals and teaching hospital/community hybrids had significant pay differences by gender.
Academic or teaching hospitals comprised the largest subgroup, allowing for further analysis.
“Age and years post fellowship were the only significant differences by gender in this group,” Dr. Croft said. “Again, female providers earned less than their male counterparts, with mean compensation of $349,717, compared with $461,054.”
In fact, less than 25% of women in academic practice in this survey made above the median reported salary, Dr. Croft noted. Controlling not only for differences between male and female providers in this group but also for other known factors affecting compensation, the odds of a male provider making greater than the median salary were 1.77 times that of female providers.
Women represent nearly a third of all practicing physicians, but their salaries continue to lag behind those of men, Dr. Croft noted. She added that “this is the first study that has been presented with regards to gynecologic oncology gender salary discrepancies.”
The findings are limited by survey response bias and a potential lack of data that could explain some of the discrepancies. The study was originally designed to look at on-call compensation, so respondents were not queried about academic ranking or specific work responsibilities. Still, Dr. Croft said the findings point to a need for policy reform to ensure equitable compensation.
“My hope is that these data open a dialogue to further explore discrepancies by gender in our field,” she said.
Dr. Croft reported having no disclosures.
sworcester@mdedge.com
SOURCE: Croft K et al. SGO 2020, Abstract 15.
FROM SGO 2020
Metformin use linked to improved surgery outcomes
Patients with type 2 diabetes who take metformin may have lower risk-adjusted mortality and readmission rates after surgery than do those who don’t take metformin, findings from a large retrospective cohort study suggest.
Of 10,088 individuals with diabetes who underwent a major surgery requiring hospital admission between January 1, 2010, and January 1, 2016, a total of 5,962 (59%) had received a prescription for metformin in the 180 days before surgery, and 5,460 of those patients were propensity score–matched to controls who did not receive a metformin prescription.
The study participants had a mean age of 67.7 years and underwent surgery requiring general anesthesia and postoperative admission at any of 15 hospitals in a single Pennsylvania health system. In addition to being prescribed metformin within 180 days before surgery, they also had metformin on their list of active medications at their most recent preoperative encounter before the surgery. The were followed until December 18, 2018.
In all, the 90-day and 5-year mortality hazards were reduced by 28% and 26%, respectively, in the metformin prescription recipients, compared with the propensity score–matched controls (hazard ratios, 0.72 and 0.74, respectively), Katherine M. Reitz, MD, and colleagues at the University of Pittsburgh reported in JAMA Surgery.
The readmission hazard – with mortality as a competing risk – was reduced by 16% at 30 days and 14% at 90 days (sub-HRs, 0.84 and 0.86, respectively), the researchers found.
“Hospital readmissions among those with preoperative metformin prescriptions were observed by postdischarge days 30 and 90 (304 [11%] and 538 [20.1%], respectively), whereas among those without prescriptions, 361 readmissions (13%) occurred by day 30 and 614 (23%) by day 90,” they wrote.
The investigators also noted that inflammation was reduced in patients who received a metformin prescription, compared with those who did not (mean preoperative neutrophil to leukocyte ratio, 4.5 vs. 5.0, respectively).
“In the full cohort, multivariable regression analysis similarly demonstrated that metformin was associated with a reduced hazard for both 90-day and 5-year mortality (adjusted HRs, 0.77 and 0.80, respectively) and for 30-day and 90-day readmission (aHR, 0.83 and 0.86), with mortality as a competing risk,” they added.
The findings support those from previous studies showing a decrease in all-cause mortality among diabetes patients taking metformin, said the researchers, noting that those patients had fewer age-related chronic diseases.
“These associations may reflect the anti-aging properties of metformin against the onset of disease or diabetes-associated complications. This study extends these finding by demonstrating that preoperative metformin prescriptions were associated with a reduction in postoperative mortality and readmission, a surrogate for postoperative complications, and with long-term mortality,” they wrote.
The study was limited by a number of factors, such as the potential for residual confounding inherent in retrospective analyses and a lack of adequate power to evaluate the association between metformin use and outcomes for individual surgical procedures. But the authors added that the findings are of note, because adults with comorbidities, such as diabetes, have less physiological reserve and an increased postoperative mortality and readmission rate. The results, therefore, warrant investigation with a prospective randomized clinical trial, they concluded.
In an accompanying editorial, Elizabeth L. George, MD, and Sherry M. Wren, MD, of Stanford (Calif.) University wrote that the study “demonstrates how variables, besides coexisting medical diseases, can affect surgical outcomes.”
“Metformin now joins beta-blockers, statins, and immunonutrition as preoperative agents associated with improved surgical outcomes,” they wrote, adding that future studies should factor in statin use and whether those and other medications should be continued postoperatively because metformin is often held after surgery owing to concerns about contrast agent interactions, whereas statin continuation is recommended.
Future studies of metformin in this setting should exclude patients who are taking statins, or look at possible interactions between the two agents, they said, adding that they would be “interested in seeing a subanalysis of this data set that excludes patients who were prescribed statins.”
“Those data would further solidify the role of metformin as a possible modifiable perioperative factor,” they wrote.
The study was funded by the University of Pittsburgh Medical Center and by grants from the National Heart, Lung, and Blood Institute and the National Institutes of Health. Dr. Reitz reported having no disclosures. Dr. George and Dr. Wren also reported having no disclosures.
SOURCE: Reitz K et al. JAMA Surg. 2020 Apr 8. doi: 10.1001/jamasurg.2020.0416.
Patients with type 2 diabetes who take metformin may have lower risk-adjusted mortality and readmission rates after surgery than do those who don’t take metformin, findings from a large retrospective cohort study suggest.
Of 10,088 individuals with diabetes who underwent a major surgery requiring hospital admission between January 1, 2010, and January 1, 2016, a total of 5,962 (59%) had received a prescription for metformin in the 180 days before surgery, and 5,460 of those patients were propensity score–matched to controls who did not receive a metformin prescription.
The study participants had a mean age of 67.7 years and underwent surgery requiring general anesthesia and postoperative admission at any of 15 hospitals in a single Pennsylvania health system. In addition to being prescribed metformin within 180 days before surgery, they also had metformin on their list of active medications at their most recent preoperative encounter before the surgery. The were followed until December 18, 2018.
In all, the 90-day and 5-year mortality hazards were reduced by 28% and 26%, respectively, in the metformin prescription recipients, compared with the propensity score–matched controls (hazard ratios, 0.72 and 0.74, respectively), Katherine M. Reitz, MD, and colleagues at the University of Pittsburgh reported in JAMA Surgery.
The readmission hazard – with mortality as a competing risk – was reduced by 16% at 30 days and 14% at 90 days (sub-HRs, 0.84 and 0.86, respectively), the researchers found.
“Hospital readmissions among those with preoperative metformin prescriptions were observed by postdischarge days 30 and 90 (304 [11%] and 538 [20.1%], respectively), whereas among those without prescriptions, 361 readmissions (13%) occurred by day 30 and 614 (23%) by day 90,” they wrote.
The investigators also noted that inflammation was reduced in patients who received a metformin prescription, compared with those who did not (mean preoperative neutrophil to leukocyte ratio, 4.5 vs. 5.0, respectively).
“In the full cohort, multivariable regression analysis similarly demonstrated that metformin was associated with a reduced hazard for both 90-day and 5-year mortality (adjusted HRs, 0.77 and 0.80, respectively) and for 30-day and 90-day readmission (aHR, 0.83 and 0.86), with mortality as a competing risk,” they added.
The findings support those from previous studies showing a decrease in all-cause mortality among diabetes patients taking metformin, said the researchers, noting that those patients had fewer age-related chronic diseases.
“These associations may reflect the anti-aging properties of metformin against the onset of disease or diabetes-associated complications. This study extends these finding by demonstrating that preoperative metformin prescriptions were associated with a reduction in postoperative mortality and readmission, a surrogate for postoperative complications, and with long-term mortality,” they wrote.
The study was limited by a number of factors, such as the potential for residual confounding inherent in retrospective analyses and a lack of adequate power to evaluate the association between metformin use and outcomes for individual surgical procedures. But the authors added that the findings are of note, because adults with comorbidities, such as diabetes, have less physiological reserve and an increased postoperative mortality and readmission rate. The results, therefore, warrant investigation with a prospective randomized clinical trial, they concluded.
In an accompanying editorial, Elizabeth L. George, MD, and Sherry M. Wren, MD, of Stanford (Calif.) University wrote that the study “demonstrates how variables, besides coexisting medical diseases, can affect surgical outcomes.”
“Metformin now joins beta-blockers, statins, and immunonutrition as preoperative agents associated with improved surgical outcomes,” they wrote, adding that future studies should factor in statin use and whether those and other medications should be continued postoperatively because metformin is often held after surgery owing to concerns about contrast agent interactions, whereas statin continuation is recommended.
Future studies of metformin in this setting should exclude patients who are taking statins, or look at possible interactions between the two agents, they said, adding that they would be “interested in seeing a subanalysis of this data set that excludes patients who were prescribed statins.”
“Those data would further solidify the role of metformin as a possible modifiable perioperative factor,” they wrote.
The study was funded by the University of Pittsburgh Medical Center and by grants from the National Heart, Lung, and Blood Institute and the National Institutes of Health. Dr. Reitz reported having no disclosures. Dr. George and Dr. Wren also reported having no disclosures.
SOURCE: Reitz K et al. JAMA Surg. 2020 Apr 8. doi: 10.1001/jamasurg.2020.0416.
Patients with type 2 diabetes who take metformin may have lower risk-adjusted mortality and readmission rates after surgery than do those who don’t take metformin, findings from a large retrospective cohort study suggest.
Of 10,088 individuals with diabetes who underwent a major surgery requiring hospital admission between January 1, 2010, and January 1, 2016, a total of 5,962 (59%) had received a prescription for metformin in the 180 days before surgery, and 5,460 of those patients were propensity score–matched to controls who did not receive a metformin prescription.
The study participants had a mean age of 67.7 years and underwent surgery requiring general anesthesia and postoperative admission at any of 15 hospitals in a single Pennsylvania health system. In addition to being prescribed metformin within 180 days before surgery, they also had metformin on their list of active medications at their most recent preoperative encounter before the surgery. The were followed until December 18, 2018.
In all, the 90-day and 5-year mortality hazards were reduced by 28% and 26%, respectively, in the metformin prescription recipients, compared with the propensity score–matched controls (hazard ratios, 0.72 and 0.74, respectively), Katherine M. Reitz, MD, and colleagues at the University of Pittsburgh reported in JAMA Surgery.
The readmission hazard – with mortality as a competing risk – was reduced by 16% at 30 days and 14% at 90 days (sub-HRs, 0.84 and 0.86, respectively), the researchers found.
“Hospital readmissions among those with preoperative metformin prescriptions were observed by postdischarge days 30 and 90 (304 [11%] and 538 [20.1%], respectively), whereas among those without prescriptions, 361 readmissions (13%) occurred by day 30 and 614 (23%) by day 90,” they wrote.
The investigators also noted that inflammation was reduced in patients who received a metformin prescription, compared with those who did not (mean preoperative neutrophil to leukocyte ratio, 4.5 vs. 5.0, respectively).
“In the full cohort, multivariable regression analysis similarly demonstrated that metformin was associated with a reduced hazard for both 90-day and 5-year mortality (adjusted HRs, 0.77 and 0.80, respectively) and for 30-day and 90-day readmission (aHR, 0.83 and 0.86), with mortality as a competing risk,” they added.
The findings support those from previous studies showing a decrease in all-cause mortality among diabetes patients taking metformin, said the researchers, noting that those patients had fewer age-related chronic diseases.
“These associations may reflect the anti-aging properties of metformin against the onset of disease or diabetes-associated complications. This study extends these finding by demonstrating that preoperative metformin prescriptions were associated with a reduction in postoperative mortality and readmission, a surrogate for postoperative complications, and with long-term mortality,” they wrote.
The study was limited by a number of factors, such as the potential for residual confounding inherent in retrospective analyses and a lack of adequate power to evaluate the association between metformin use and outcomes for individual surgical procedures. But the authors added that the findings are of note, because adults with comorbidities, such as diabetes, have less physiological reserve and an increased postoperative mortality and readmission rate. The results, therefore, warrant investigation with a prospective randomized clinical trial, they concluded.
In an accompanying editorial, Elizabeth L. George, MD, and Sherry M. Wren, MD, of Stanford (Calif.) University wrote that the study “demonstrates how variables, besides coexisting medical diseases, can affect surgical outcomes.”
“Metformin now joins beta-blockers, statins, and immunonutrition as preoperative agents associated with improved surgical outcomes,” they wrote, adding that future studies should factor in statin use and whether those and other medications should be continued postoperatively because metformin is often held after surgery owing to concerns about contrast agent interactions, whereas statin continuation is recommended.
Future studies of metformin in this setting should exclude patients who are taking statins, or look at possible interactions between the two agents, they said, adding that they would be “interested in seeing a subanalysis of this data set that excludes patients who were prescribed statins.”
“Those data would further solidify the role of metformin as a possible modifiable perioperative factor,” they wrote.
The study was funded by the University of Pittsburgh Medical Center and by grants from the National Heart, Lung, and Blood Institute and the National Institutes of Health. Dr. Reitz reported having no disclosures. Dr. George and Dr. Wren also reported having no disclosures.
SOURCE: Reitz K et al. JAMA Surg. 2020 Apr 8. doi: 10.1001/jamasurg.2020.0416.
FROM JAMA SURGERY
COVID-19: When health care personnel become patients
according to the Centers for Disease Control and Prevention.
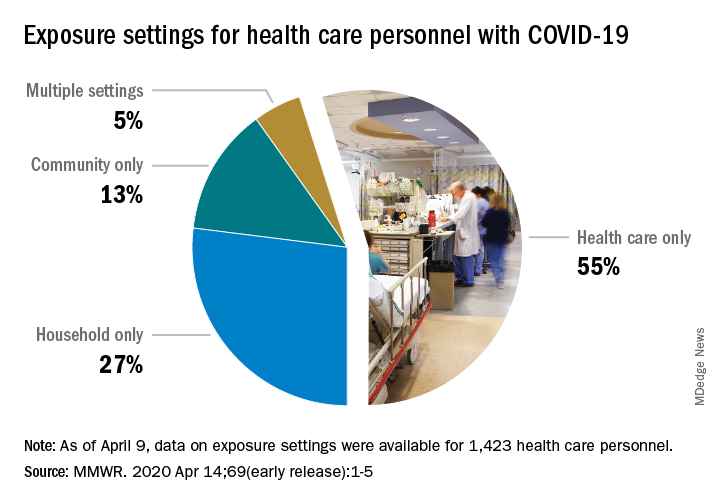
That number, however, is probably an underestimation because health care personnel (HCP) status was available for just over 49,000 of the 315,000 COVID-19 cases reported to the CDC as of April 9. Of the cases with known HCP status, 9,282 (19%) were health care personnel, Matthew J. Stuckey, PhD, and the CDC’s COVID-19 Response Team said.
“The number of cases in HCP reported here must be considered a lower bound because additional cases likely have gone unidentified or unreported,” they said.
The median age of the nearly 9,300 HCP with COVID-19 was 42 years, and the majority (55%) were aged 16-44 years; another 21% were 45-54, 18% were 55-64, and 6% were age 65 and over. The oldest group, however, represented 10 of the 27 known HCP deaths, the investigators reported in the Morbidity and Mortality Weekly Report.
The majority of infected HCP (55%) reported exposure to a COVID-19 patient in the health care setting, but “there were also known exposures in households and in the community, highlighting the potential for exposure in multiple settings, especially as community transmission increases,” the response team said.
Since “contact tracing after recognized occupational exposures likely will fail to identify many HCP at risk for developing COVID-19,” other measures will probably be needed to “reduce the risk for infected HCP transmitting the virus to colleagues and patients,” they added.
HCP with COVID-19 were less likely to be hospitalized (8%-10%) than the overall population (21%-31%), which “might reflect the younger median age … of HCP patients, compared with that of reported COVID-19 patients overall, as well as prioritization of HCP for testing, which might identify less-severe illness,” the investigators suggested.
The prevalence of underlying conditions in HCP patients, 38%, was the same as all patients with COVID-19, and 92% of the HCP patients presented with fever, cough, or shortness of breath. Two-thirds of all HCP reported muscle aches, and 65% reported headache, the CDC response team noted.
“It is critical to make every effort to ensure the health and safety of this essential national workforce of approximately 18 million HCP, both at work and in the community,” they wrote.
SOURCE: Stuckey MJ et al. MMWR. Apr 14;69(early release):1-5.
according to the Centers for Disease Control and Prevention.

That number, however, is probably an underestimation because health care personnel (HCP) status was available for just over 49,000 of the 315,000 COVID-19 cases reported to the CDC as of April 9. Of the cases with known HCP status, 9,282 (19%) were health care personnel, Matthew J. Stuckey, PhD, and the CDC’s COVID-19 Response Team said.
“The number of cases in HCP reported here must be considered a lower bound because additional cases likely have gone unidentified or unreported,” they said.
The median age of the nearly 9,300 HCP with COVID-19 was 42 years, and the majority (55%) were aged 16-44 years; another 21% were 45-54, 18% were 55-64, and 6% were age 65 and over. The oldest group, however, represented 10 of the 27 known HCP deaths, the investigators reported in the Morbidity and Mortality Weekly Report.
The majority of infected HCP (55%) reported exposure to a COVID-19 patient in the health care setting, but “there were also known exposures in households and in the community, highlighting the potential for exposure in multiple settings, especially as community transmission increases,” the response team said.
Since “contact tracing after recognized occupational exposures likely will fail to identify many HCP at risk for developing COVID-19,” other measures will probably be needed to “reduce the risk for infected HCP transmitting the virus to colleagues and patients,” they added.
HCP with COVID-19 were less likely to be hospitalized (8%-10%) than the overall population (21%-31%), which “might reflect the younger median age … of HCP patients, compared with that of reported COVID-19 patients overall, as well as prioritization of HCP for testing, which might identify less-severe illness,” the investigators suggested.
The prevalence of underlying conditions in HCP patients, 38%, was the same as all patients with COVID-19, and 92% of the HCP patients presented with fever, cough, or shortness of breath. Two-thirds of all HCP reported muscle aches, and 65% reported headache, the CDC response team noted.
“It is critical to make every effort to ensure the health and safety of this essential national workforce of approximately 18 million HCP, both at work and in the community,” they wrote.
SOURCE: Stuckey MJ et al. MMWR. Apr 14;69(early release):1-5.
according to the Centers for Disease Control and Prevention.

That number, however, is probably an underestimation because health care personnel (HCP) status was available for just over 49,000 of the 315,000 COVID-19 cases reported to the CDC as of April 9. Of the cases with known HCP status, 9,282 (19%) were health care personnel, Matthew J. Stuckey, PhD, and the CDC’s COVID-19 Response Team said.
“The number of cases in HCP reported here must be considered a lower bound because additional cases likely have gone unidentified or unreported,” they said.
The median age of the nearly 9,300 HCP with COVID-19 was 42 years, and the majority (55%) were aged 16-44 years; another 21% were 45-54, 18% were 55-64, and 6% were age 65 and over. The oldest group, however, represented 10 of the 27 known HCP deaths, the investigators reported in the Morbidity and Mortality Weekly Report.
The majority of infected HCP (55%) reported exposure to a COVID-19 patient in the health care setting, but “there were also known exposures in households and in the community, highlighting the potential for exposure in multiple settings, especially as community transmission increases,” the response team said.
Since “contact tracing after recognized occupational exposures likely will fail to identify many HCP at risk for developing COVID-19,” other measures will probably be needed to “reduce the risk for infected HCP transmitting the virus to colleagues and patients,” they added.
HCP with COVID-19 were less likely to be hospitalized (8%-10%) than the overall population (21%-31%), which “might reflect the younger median age … of HCP patients, compared with that of reported COVID-19 patients overall, as well as prioritization of HCP for testing, which might identify less-severe illness,” the investigators suggested.
The prevalence of underlying conditions in HCP patients, 38%, was the same as all patients with COVID-19, and 92% of the HCP patients presented with fever, cough, or shortness of breath. Two-thirds of all HCP reported muscle aches, and 65% reported headache, the CDC response team noted.
“It is critical to make every effort to ensure the health and safety of this essential national workforce of approximately 18 million HCP, both at work and in the community,” they wrote.
SOURCE: Stuckey MJ et al. MMWR. Apr 14;69(early release):1-5.
FROM THE MMWR
The role of FOAM and social networks in COVID-19
“Uncertainty creates weakness. Uncertainty makes one tentative, if not fearful, and tentative steps, even when in the right direction, may not overcome significant obstacles.”1
Recently, I spent my vacation time quarantined reading “The Great Influenza,” which recounts the history of the 1918 pandemic. Despite over a century of scientific and medical progress, the parallels to our current situation are indisputable. Just as in 1918, we are limiting social gatherings, quarantining, wearing face masks, and living with the fear and anxiety of keeping ourselves and our families safe. In 1918, use of aspirin, quinine, and digitalis therapies in a desperate search for relief despite limited evidence mirror the current use of hydroxychloroquine, azithromycin, and lopinavir/ritonavir. While there are many similarities between the two situations, in this pandemic our channels for dissemination of scientific literature are better developed, and online networks are enabling physicians across the globe to communicate their experience and findings in near real time.
During this time of uncertainty, our understanding of COVID-19 evolves daily. Without the advantage of robust randomized, controlled trials and large-scale studies to guide us, we are forced to rely on pattern recognition for surveillance and anecdotal or limited case-based accounts to guide clinical care. Fortunately, free open-access medical education (FOAM) and social networks offer a significant advantage in our ability to collect and disseminate information.
Free open access medical education
The concept of FOAM started in 2012 with the intent of creating a collaborative and constantly evolving community to provide open-access medical education. It encompasses multiple platforms – blogs, podcasts, videos, and social media – and features content experts from across the globe. Since its inception, FOAM has grown in popularity and use, especially within emergency medicine and critical care communities, as an adjunct for asynchronous learning.2,3
In a time where knowledge of COVID-19 is dynamically changing, traditional sources like textbooks, journals, and organizational guidelines often lag behind real-time clinical experience and needs. Additionally, many clinicians are now being tasked with taking care of patient populations and a new critical illness profile with which they are not comfortable. It is challenging to find a well-curated and updated repository of information to answer questions surrounding pathophysiology, critical care, ventilator management, caring for adult patients, and personal protective equipment (PPE). During this rapidly evolving reality, FOAM is becoming the ideal modality for timely and efficient sharing of reviews of current literature, expert discussions, and clinical practice guidelines.
A few self-directed hours on EMCrit’s Internet Book of Critical Care’s COVID-19 chapter reveals a bastion of content regarding diagnosis, pathophysiology, transmission, therapies, and ventilator strategies.4 It includes references to major journals and recommendations from international societies. Websites like EMCrit and REBEL EM are updated daily with podcasts, videos, and blog posts surrounding the latest highly debated topics in COVID-19 management.5 Podcasts like EM:RAP and Peds RAP have made COVID segments discussing important topics like pharmacotherapy, telemedicine, and pregnancy available for free.6,7 Many networks, institutions, and individual physicians have created and posted videos online on critical care topics and refreshers.
Social networks
Online social networks composed of international physicians within Facebook and LinkedIn serve as miniature publishing houses. First-hand accounts of patient presentations and patient care act as case reports. As similar accounts accumulate, they become case series. Patterns emerge and new hypotheses are generated, debated, and critiqued through this informal peer review. Personal accounts of frustration with lack of PPE, fear of exposing loved ones, distress at being separated from family, and grief of witnessing multiple patients die alone are opinion and perspective articles.
These networks offer the space for sharing. Those who have had the experience of caring for the surge of COVID-19 patients offer advice and words of caution to those who have yet to experience it. Protocols from a multitude of institutions on triage, surge, disposition, and end-of-life care are disseminated, serving as templates for those that have not yet developed their own. There is an impressive variety of innovative, do-it-yourself projects surrounding PPE, intubation boxes, and three-dimensionally printed ventilator parts.
Finally, these networks provide emotional support. There are offers to ship additional PPE, videos of cities cheering as clinicians go to work, stories of triumph and recovery, pictures depicting ongoing wellness activities, and the occasional much-needed humorous anecdote or illustration. These networks reinforce the message that our lives continue despite this upheaval, and we are not alone in this struggle.
The end of the passage in The Great Influenza concludes with: “Ultimately a scientist has nothing to believe in but the process of inquiry. To move forcefully and aggressively even while uncertain requires a confidence and strength deeper than physical courage.”
They represent a highly adaptable, evolving, and collaborative global community’s determination to persevere through time of uncertainty together.
Dr. Ren is a pediatric emergency medicine fellow at Children’s National Hospital, Washington. Dr. Simpson is a pediatric emergency medicine attending and medical director of emergency preparedness at the hospital. They reported that they do not have any disclosures or conflicts of interest. Email Dr. Ren and Dr. Simpson at pdnews@mdedge.com.
References
1. “The Great Influenza: The Story of the Deadliest Pandemic in History.” (New York: Penguin Books, 2005, pp. 261-62).
2. Emerg Med J. 2014 Oct;31(e1):e76-7.
3. Acad Med. 2014 Apr;89(4):598-601.
4. “The Internet Book of Critical Care: COVID-19.” EMCrit Project.
5. “Covid-19.” REBEL EM-Emergency Medicine Blog.
6. “EM:RAP COVID-19 Resources.” EM RAP: Emergency Medicine Reviews and Perspectives.
7. “Episodes.” Peds RAP, Hippo Education.
“Uncertainty creates weakness. Uncertainty makes one tentative, if not fearful, and tentative steps, even when in the right direction, may not overcome significant obstacles.”1
Recently, I spent my vacation time quarantined reading “The Great Influenza,” which recounts the history of the 1918 pandemic. Despite over a century of scientific and medical progress, the parallels to our current situation are indisputable. Just as in 1918, we are limiting social gatherings, quarantining, wearing face masks, and living with the fear and anxiety of keeping ourselves and our families safe. In 1918, use of aspirin, quinine, and digitalis therapies in a desperate search for relief despite limited evidence mirror the current use of hydroxychloroquine, azithromycin, and lopinavir/ritonavir. While there are many similarities between the two situations, in this pandemic our channels for dissemination of scientific literature are better developed, and online networks are enabling physicians across the globe to communicate their experience and findings in near real time.
During this time of uncertainty, our understanding of COVID-19 evolves daily. Without the advantage of robust randomized, controlled trials and large-scale studies to guide us, we are forced to rely on pattern recognition for surveillance and anecdotal or limited case-based accounts to guide clinical care. Fortunately, free open-access medical education (FOAM) and social networks offer a significant advantage in our ability to collect and disseminate information.
Free open access medical education
The concept of FOAM started in 2012 with the intent of creating a collaborative and constantly evolving community to provide open-access medical education. It encompasses multiple platforms – blogs, podcasts, videos, and social media – and features content experts from across the globe. Since its inception, FOAM has grown in popularity and use, especially within emergency medicine and critical care communities, as an adjunct for asynchronous learning.2,3
In a time where knowledge of COVID-19 is dynamically changing, traditional sources like textbooks, journals, and organizational guidelines often lag behind real-time clinical experience and needs. Additionally, many clinicians are now being tasked with taking care of patient populations and a new critical illness profile with which they are not comfortable. It is challenging to find a well-curated and updated repository of information to answer questions surrounding pathophysiology, critical care, ventilator management, caring for adult patients, and personal protective equipment (PPE). During this rapidly evolving reality, FOAM is becoming the ideal modality for timely and efficient sharing of reviews of current literature, expert discussions, and clinical practice guidelines.
A few self-directed hours on EMCrit’s Internet Book of Critical Care’s COVID-19 chapter reveals a bastion of content regarding diagnosis, pathophysiology, transmission, therapies, and ventilator strategies.4 It includes references to major journals and recommendations from international societies. Websites like EMCrit and REBEL EM are updated daily with podcasts, videos, and blog posts surrounding the latest highly debated topics in COVID-19 management.5 Podcasts like EM:RAP and Peds RAP have made COVID segments discussing important topics like pharmacotherapy, telemedicine, and pregnancy available for free.6,7 Many networks, institutions, and individual physicians have created and posted videos online on critical care topics and refreshers.
Social networks
Online social networks composed of international physicians within Facebook and LinkedIn serve as miniature publishing houses. First-hand accounts of patient presentations and patient care act as case reports. As similar accounts accumulate, they become case series. Patterns emerge and new hypotheses are generated, debated, and critiqued through this informal peer review. Personal accounts of frustration with lack of PPE, fear of exposing loved ones, distress at being separated from family, and grief of witnessing multiple patients die alone are opinion and perspective articles.
These networks offer the space for sharing. Those who have had the experience of caring for the surge of COVID-19 patients offer advice and words of caution to those who have yet to experience it. Protocols from a multitude of institutions on triage, surge, disposition, and end-of-life care are disseminated, serving as templates for those that have not yet developed their own. There is an impressive variety of innovative, do-it-yourself projects surrounding PPE, intubation boxes, and three-dimensionally printed ventilator parts.
Finally, these networks provide emotional support. There are offers to ship additional PPE, videos of cities cheering as clinicians go to work, stories of triumph and recovery, pictures depicting ongoing wellness activities, and the occasional much-needed humorous anecdote or illustration. These networks reinforce the message that our lives continue despite this upheaval, and we are not alone in this struggle.
The end of the passage in The Great Influenza concludes with: “Ultimately a scientist has nothing to believe in but the process of inquiry. To move forcefully and aggressively even while uncertain requires a confidence and strength deeper than physical courage.”
They represent a highly adaptable, evolving, and collaborative global community’s determination to persevere through time of uncertainty together.
Dr. Ren is a pediatric emergency medicine fellow at Children’s National Hospital, Washington. Dr. Simpson is a pediatric emergency medicine attending and medical director of emergency preparedness at the hospital. They reported that they do not have any disclosures or conflicts of interest. Email Dr. Ren and Dr. Simpson at pdnews@mdedge.com.
References
1. “The Great Influenza: The Story of the Deadliest Pandemic in History.” (New York: Penguin Books, 2005, pp. 261-62).
2. Emerg Med J. 2014 Oct;31(e1):e76-7.
3. Acad Med. 2014 Apr;89(4):598-601.
4. “The Internet Book of Critical Care: COVID-19.” EMCrit Project.
5. “Covid-19.” REBEL EM-Emergency Medicine Blog.
6. “EM:RAP COVID-19 Resources.” EM RAP: Emergency Medicine Reviews and Perspectives.
7. “Episodes.” Peds RAP, Hippo Education.
“Uncertainty creates weakness. Uncertainty makes one tentative, if not fearful, and tentative steps, even when in the right direction, may not overcome significant obstacles.”1
Recently, I spent my vacation time quarantined reading “The Great Influenza,” which recounts the history of the 1918 pandemic. Despite over a century of scientific and medical progress, the parallels to our current situation are indisputable. Just as in 1918, we are limiting social gatherings, quarantining, wearing face masks, and living with the fear and anxiety of keeping ourselves and our families safe. In 1918, use of aspirin, quinine, and digitalis therapies in a desperate search for relief despite limited evidence mirror the current use of hydroxychloroquine, azithromycin, and lopinavir/ritonavir. While there are many similarities between the two situations, in this pandemic our channels for dissemination of scientific literature are better developed, and online networks are enabling physicians across the globe to communicate their experience and findings in near real time.
During this time of uncertainty, our understanding of COVID-19 evolves daily. Without the advantage of robust randomized, controlled trials and large-scale studies to guide us, we are forced to rely on pattern recognition for surveillance and anecdotal or limited case-based accounts to guide clinical care. Fortunately, free open-access medical education (FOAM) and social networks offer a significant advantage in our ability to collect and disseminate information.
Free open access medical education
The concept of FOAM started in 2012 with the intent of creating a collaborative and constantly evolving community to provide open-access medical education. It encompasses multiple platforms – blogs, podcasts, videos, and social media – and features content experts from across the globe. Since its inception, FOAM has grown in popularity and use, especially within emergency medicine and critical care communities, as an adjunct for asynchronous learning.2,3
In a time where knowledge of COVID-19 is dynamically changing, traditional sources like textbooks, journals, and organizational guidelines often lag behind real-time clinical experience and needs. Additionally, many clinicians are now being tasked with taking care of patient populations and a new critical illness profile with which they are not comfortable. It is challenging to find a well-curated and updated repository of information to answer questions surrounding pathophysiology, critical care, ventilator management, caring for adult patients, and personal protective equipment (PPE). During this rapidly evolving reality, FOAM is becoming the ideal modality for timely and efficient sharing of reviews of current literature, expert discussions, and clinical practice guidelines.
A few self-directed hours on EMCrit’s Internet Book of Critical Care’s COVID-19 chapter reveals a bastion of content regarding diagnosis, pathophysiology, transmission, therapies, and ventilator strategies.4 It includes references to major journals and recommendations from international societies. Websites like EMCrit and REBEL EM are updated daily with podcasts, videos, and blog posts surrounding the latest highly debated topics in COVID-19 management.5 Podcasts like EM:RAP and Peds RAP have made COVID segments discussing important topics like pharmacotherapy, telemedicine, and pregnancy available for free.6,7 Many networks, institutions, and individual physicians have created and posted videos online on critical care topics and refreshers.
Social networks
Online social networks composed of international physicians within Facebook and LinkedIn serve as miniature publishing houses. First-hand accounts of patient presentations and patient care act as case reports. As similar accounts accumulate, they become case series. Patterns emerge and new hypotheses are generated, debated, and critiqued through this informal peer review. Personal accounts of frustration with lack of PPE, fear of exposing loved ones, distress at being separated from family, and grief of witnessing multiple patients die alone are opinion and perspective articles.
These networks offer the space for sharing. Those who have had the experience of caring for the surge of COVID-19 patients offer advice and words of caution to those who have yet to experience it. Protocols from a multitude of institutions on triage, surge, disposition, and end-of-life care are disseminated, serving as templates for those that have not yet developed their own. There is an impressive variety of innovative, do-it-yourself projects surrounding PPE, intubation boxes, and three-dimensionally printed ventilator parts.
Finally, these networks provide emotional support. There are offers to ship additional PPE, videos of cities cheering as clinicians go to work, stories of triumph and recovery, pictures depicting ongoing wellness activities, and the occasional much-needed humorous anecdote or illustration. These networks reinforce the message that our lives continue despite this upheaval, and we are not alone in this struggle.
The end of the passage in The Great Influenza concludes with: “Ultimately a scientist has nothing to believe in but the process of inquiry. To move forcefully and aggressively even while uncertain requires a confidence and strength deeper than physical courage.”
They represent a highly adaptable, evolving, and collaborative global community’s determination to persevere through time of uncertainty together.
Dr. Ren is a pediatric emergency medicine fellow at Children’s National Hospital, Washington. Dr. Simpson is a pediatric emergency medicine attending and medical director of emergency preparedness at the hospital. They reported that they do not have any disclosures or conflicts of interest. Email Dr. Ren and Dr. Simpson at pdnews@mdedge.com.
References
1. “The Great Influenza: The Story of the Deadliest Pandemic in History.” (New York: Penguin Books, 2005, pp. 261-62).
2. Emerg Med J. 2014 Oct;31(e1):e76-7.
3. Acad Med. 2014 Apr;89(4):598-601.
4. “The Internet Book of Critical Care: COVID-19.” EMCrit Project.
5. “Covid-19.” REBEL EM-Emergency Medicine Blog.
6. “EM:RAP COVID-19 Resources.” EM RAP: Emergency Medicine Reviews and Perspectives.
7. “Episodes.” Peds RAP, Hippo Education.
FDA approves emergency use of saliva test to detect COVID-19
As the race to develop rapid testing for COVID-19 expands, the Food and Drug Administration has granted emergency approval for an approach that uses saliva as the primary test biomaterial.
According to a document provided to the FDA, the Rutgers Clinical Genomics Laboratory TaqPath SARS-CoV-2 Assay is intended for the qualitative detection of nucleic acid from SARS-CoV-2 in oropharyngeal (throat) swab, nasopharyngeal swab, anterior nasal swab, mid-turbinate nasal swab from individuals suspected of COVID-19 by their health care clinicians. To expand on this assay, Rutgers University–based RUCDR Infinite Biologics developed a saliva collection method in partnership with Spectrum Solutions and Accurate Diagnostic Labs.
The document states that Samples are transported for RNA extraction and are tested within 48 hours of collection. In saliva samples obtained from 60 patients evaluated by the researchers, all were in agreement with the presence of COVID-19.
“If shown to be as accurate as nasopharyngeal and oropharyngeal samples, saliva as a biomatrix offers the advantage of not generating aerosols or creating as many respiratory droplets during specimen procurement, therefore decreasing the risk of transmission to the health care worker doing the testing,” said Matthew P. Cheng, MDCM, of the division of infectious diseases at McGill University Health Centre, Montreal, who was not involved in development of the test but who has written about diagnostic testing for the virus.
“Also, it may be easy enough for patients to do saliva self-collection at home. However, it is important to note that SARS-CoV-2 tests on saliva have not yet undergone the more rigorous evaluation of full FDA authorization, and saliva is not a preferred specimen type of the FDA nor the [Centers for Disease Control and Prevention] for respiratory virus testing.”
In a prepared statement, Andrew I. Brooks, PhD, chief operating officer at RUCDR Infinite Biologics, said the saliva collection method enables clinicians to preserve personal protective equipment for use in patient care instead of testing. “We can significantly increase the number of people tested each and every day as self-collection of saliva is quicker and more scalable than swab collections,” he said. “All of this combined will have a tremendous impact on testing in New Jersey and across the United States.”
The tests are currently available to the RWJBarnabas Health network, based in West Orange, N.J., which has partnered with Rutgers University.
As the race to develop rapid testing for COVID-19 expands, the Food and Drug Administration has granted emergency approval for an approach that uses saliva as the primary test biomaterial.
According to a document provided to the FDA, the Rutgers Clinical Genomics Laboratory TaqPath SARS-CoV-2 Assay is intended for the qualitative detection of nucleic acid from SARS-CoV-2 in oropharyngeal (throat) swab, nasopharyngeal swab, anterior nasal swab, mid-turbinate nasal swab from individuals suspected of COVID-19 by their health care clinicians. To expand on this assay, Rutgers University–based RUCDR Infinite Biologics developed a saliva collection method in partnership with Spectrum Solutions and Accurate Diagnostic Labs.
The document states that Samples are transported for RNA extraction and are tested within 48 hours of collection. In saliva samples obtained from 60 patients evaluated by the researchers, all were in agreement with the presence of COVID-19.
“If shown to be as accurate as nasopharyngeal and oropharyngeal samples, saliva as a biomatrix offers the advantage of not generating aerosols or creating as many respiratory droplets during specimen procurement, therefore decreasing the risk of transmission to the health care worker doing the testing,” said Matthew P. Cheng, MDCM, of the division of infectious diseases at McGill University Health Centre, Montreal, who was not involved in development of the test but who has written about diagnostic testing for the virus.
“Also, it may be easy enough for patients to do saliva self-collection at home. However, it is important to note that SARS-CoV-2 tests on saliva have not yet undergone the more rigorous evaluation of full FDA authorization, and saliva is not a preferred specimen type of the FDA nor the [Centers for Disease Control and Prevention] for respiratory virus testing.”
In a prepared statement, Andrew I. Brooks, PhD, chief operating officer at RUCDR Infinite Biologics, said the saliva collection method enables clinicians to preserve personal protective equipment for use in patient care instead of testing. “We can significantly increase the number of people tested each and every day as self-collection of saliva is quicker and more scalable than swab collections,” he said. “All of this combined will have a tremendous impact on testing in New Jersey and across the United States.”
The tests are currently available to the RWJBarnabas Health network, based in West Orange, N.J., which has partnered with Rutgers University.
As the race to develop rapid testing for COVID-19 expands, the Food and Drug Administration has granted emergency approval for an approach that uses saliva as the primary test biomaterial.
According to a document provided to the FDA, the Rutgers Clinical Genomics Laboratory TaqPath SARS-CoV-2 Assay is intended for the qualitative detection of nucleic acid from SARS-CoV-2 in oropharyngeal (throat) swab, nasopharyngeal swab, anterior nasal swab, mid-turbinate nasal swab from individuals suspected of COVID-19 by their health care clinicians. To expand on this assay, Rutgers University–based RUCDR Infinite Biologics developed a saliva collection method in partnership with Spectrum Solutions and Accurate Diagnostic Labs.
The document states that Samples are transported for RNA extraction and are tested within 48 hours of collection. In saliva samples obtained from 60 patients evaluated by the researchers, all were in agreement with the presence of COVID-19.
“If shown to be as accurate as nasopharyngeal and oropharyngeal samples, saliva as a biomatrix offers the advantage of not generating aerosols or creating as many respiratory droplets during specimen procurement, therefore decreasing the risk of transmission to the health care worker doing the testing,” said Matthew P. Cheng, MDCM, of the division of infectious diseases at McGill University Health Centre, Montreal, who was not involved in development of the test but who has written about diagnostic testing for the virus.
“Also, it may be easy enough for patients to do saliva self-collection at home. However, it is important to note that SARS-CoV-2 tests on saliva have not yet undergone the more rigorous evaluation of full FDA authorization, and saliva is not a preferred specimen type of the FDA nor the [Centers for Disease Control and Prevention] for respiratory virus testing.”
In a prepared statement, Andrew I. Brooks, PhD, chief operating officer at RUCDR Infinite Biologics, said the saliva collection method enables clinicians to preserve personal protective equipment for use in patient care instead of testing. “We can significantly increase the number of people tested each and every day as self-collection of saliva is quicker and more scalable than swab collections,” he said. “All of this combined will have a tremendous impact on testing in New Jersey and across the United States.”
The tests are currently available to the RWJBarnabas Health network, based in West Orange, N.J., which has partnered with Rutgers University.
Learning about the curve
Empty shelves that once cradled toilet paper rolls; lines of shoppers, some with masks; waiting 6 feet or at least a shopping cart length apart to get into grocery stores; hazmat-suited workers loading body bags into makeshift mortuaries ... These are the images we have come to associate with the COVID-19 pandemic. But then there also are the graphs and charts, none of them bearing good news. Some are difficult to interpret because they may be missing a key ingredient, such as a scale. Day to day fluctuations in the timeliness of the data points can make valid comparisons impossible. In most cases, it is too early to look at the graphs and hope for the big picture. Whether you are concerned about the stock market or the number of new cases of the virus in your county, you are hoping to see some graphic depiction of a favorable trend.
We have suddenly learned about the urgency of a process called “flattening the curve.” Are we doing as good a job of flattening as we could be? Are we doing better than France or Spain? Or are we heading toward an Italianesque apocalypse? Who is going to tell us when the flattening is for real and not just a 2- or 3-day statistical aberration?
The curves we are obsessed with today are those showing us new cases and new deaths. But And we won’t be seeing this curve in four-color graphics on the front page of our newspapers. It is the learning curve, and we want it to be as steep as we can make it without any hint of flattening in the foreseeable future.
We need to learn more about corona-like viruses. Why are some of us more vulnerable? We need to learn more about contagion. Does the 6-foot guideline make any sense? How long are viral particles floating in the air capable of initiating disease? What about air flow and dilution? Can we build a cruise ship or airplane that will be less of a health hazard?
More importantly, we need to learn to be better prepared. Even before the pandemic there have been shortages in intravenous solutions and drugs of critical importance to common diseases. Can we learn how to create reliable and affordable supply chains that allow researchers and developers to make a reasonable profit? Can we relearn to value science? Can we learn to invest more heavily in epidemiology and make it a specialty that attracts our best thinkers and communicators? Then can we elect officials who will share our trust in their recommendations?
Can we do a better job of resolving the tension between those who believe in a strong federal government and those who believe in local autonomy because we are seeing every day that this is an issue of survival, not just coexistence? Can we learn that the globalization that has allowed this viral spread can also be leveraged to beat it into submission?
Over the last half century there has been an unfortunate flattening of the learning curve. Ironically we have seen exponential growth among hi-tech industries that have forced us to keep abreast of new developments. But along with this has been a growing skepticism about value of scientific investigation. It is time we climbed back on that steep learning curve. The view gets better the higher we climb.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at pdnews@mdedge.com.
Empty shelves that once cradled toilet paper rolls; lines of shoppers, some with masks; waiting 6 feet or at least a shopping cart length apart to get into grocery stores; hazmat-suited workers loading body bags into makeshift mortuaries ... These are the images we have come to associate with the COVID-19 pandemic. But then there also are the graphs and charts, none of them bearing good news. Some are difficult to interpret because they may be missing a key ingredient, such as a scale. Day to day fluctuations in the timeliness of the data points can make valid comparisons impossible. In most cases, it is too early to look at the graphs and hope for the big picture. Whether you are concerned about the stock market or the number of new cases of the virus in your county, you are hoping to see some graphic depiction of a favorable trend.
We have suddenly learned about the urgency of a process called “flattening the curve.” Are we doing as good a job of flattening as we could be? Are we doing better than France or Spain? Or are we heading toward an Italianesque apocalypse? Who is going to tell us when the flattening is for real and not just a 2- or 3-day statistical aberration?
The curves we are obsessed with today are those showing us new cases and new deaths. But And we won’t be seeing this curve in four-color graphics on the front page of our newspapers. It is the learning curve, and we want it to be as steep as we can make it without any hint of flattening in the foreseeable future.
We need to learn more about corona-like viruses. Why are some of us more vulnerable? We need to learn more about contagion. Does the 6-foot guideline make any sense? How long are viral particles floating in the air capable of initiating disease? What about air flow and dilution? Can we build a cruise ship or airplane that will be less of a health hazard?
More importantly, we need to learn to be better prepared. Even before the pandemic there have been shortages in intravenous solutions and drugs of critical importance to common diseases. Can we learn how to create reliable and affordable supply chains that allow researchers and developers to make a reasonable profit? Can we relearn to value science? Can we learn to invest more heavily in epidemiology and make it a specialty that attracts our best thinkers and communicators? Then can we elect officials who will share our trust in their recommendations?
Can we do a better job of resolving the tension between those who believe in a strong federal government and those who believe in local autonomy because we are seeing every day that this is an issue of survival, not just coexistence? Can we learn that the globalization that has allowed this viral spread can also be leveraged to beat it into submission?
Over the last half century there has been an unfortunate flattening of the learning curve. Ironically we have seen exponential growth among hi-tech industries that have forced us to keep abreast of new developments. But along with this has been a growing skepticism about value of scientific investigation. It is time we climbed back on that steep learning curve. The view gets better the higher we climb.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at pdnews@mdedge.com.
Empty shelves that once cradled toilet paper rolls; lines of shoppers, some with masks; waiting 6 feet or at least a shopping cart length apart to get into grocery stores; hazmat-suited workers loading body bags into makeshift mortuaries ... These are the images we have come to associate with the COVID-19 pandemic. But then there also are the graphs and charts, none of them bearing good news. Some are difficult to interpret because they may be missing a key ingredient, such as a scale. Day to day fluctuations in the timeliness of the data points can make valid comparisons impossible. In most cases, it is too early to look at the graphs and hope for the big picture. Whether you are concerned about the stock market or the number of new cases of the virus in your county, you are hoping to see some graphic depiction of a favorable trend.
We have suddenly learned about the urgency of a process called “flattening the curve.” Are we doing as good a job of flattening as we could be? Are we doing better than France or Spain? Or are we heading toward an Italianesque apocalypse? Who is going to tell us when the flattening is for real and not just a 2- or 3-day statistical aberration?
The curves we are obsessed with today are those showing us new cases and new deaths. But And we won’t be seeing this curve in four-color graphics on the front page of our newspapers. It is the learning curve, and we want it to be as steep as we can make it without any hint of flattening in the foreseeable future.
We need to learn more about corona-like viruses. Why are some of us more vulnerable? We need to learn more about contagion. Does the 6-foot guideline make any sense? How long are viral particles floating in the air capable of initiating disease? What about air flow and dilution? Can we build a cruise ship or airplane that will be less of a health hazard?
More importantly, we need to learn to be better prepared. Even before the pandemic there have been shortages in intravenous solutions and drugs of critical importance to common diseases. Can we learn how to create reliable and affordable supply chains that allow researchers and developers to make a reasonable profit? Can we relearn to value science? Can we learn to invest more heavily in epidemiology and make it a specialty that attracts our best thinkers and communicators? Then can we elect officials who will share our trust in their recommendations?
Can we do a better job of resolving the tension between those who believe in a strong federal government and those who believe in local autonomy because we are seeing every day that this is an issue of survival, not just coexistence? Can we learn that the globalization that has allowed this viral spread can also be leveraged to beat it into submission?
Over the last half century there has been an unfortunate flattening of the learning curve. Ironically we have seen exponential growth among hi-tech industries that have forced us to keep abreast of new developments. But along with this has been a growing skepticism about value of scientific investigation. It is time we climbed back on that steep learning curve. The view gets better the higher we climb.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at pdnews@mdedge.com.