User login
The SHM 2019 Chapter Excellence Awards
The Society of Hospital Medicine is proud to recognize its chapters for their hard work and dedication in 2019 through Chapter Excellence Awards. Each year, chapters strive to demonstrate growth, sustenance, and innovation within their chapter activities, which are then applauded for their successes throughout the subsequent year. In 2019, a new Bronze category was established, for a total of four Status Awards that chapters can earn.
Please join SHM in congratulating the following chapters on their year of success in 2019!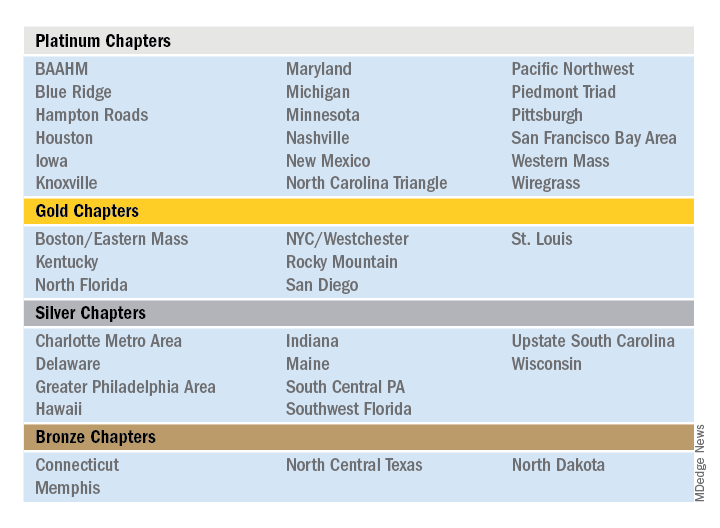
Outstanding Chapter of the Year
The Outstanding Chapter of the Year Award goes to one chapter who exemplifies high performance, going above and beyond the basic chapter requirements. The recipient of the Outstanding Chapter of the Year Award for 2019 is the Wiregrass Chapter of SHM. The chapter has a strong and engaged leadership which includes representation at all levels of the hospital medicine team, including physician hospitalists, advanced care provider hospitalists, practice administrators, nurses, residents, and medical students.
In the last year, the Wiregrass leadership team has organized programs and events to cater to and engage all the chapter’s members. This includes a variety of innovative ideas that catered toward medical education, health care provider well-being, engagement, mentorship, and community involvement.
The SHM Wiregrass Chapter’s biggest accomplishment in 2019 was the creation of an exchange program for physician and advanced practice provider hospitalists between the SHM New Mexico Chapter and the SHM Wiregrass Chapter. This idea first arose at HM19, where the chapter leaders had met during a networking event and debated the role of clinician wellbeing, quality of medical education, and faculty development to individual hospital medicine group (HMG) practice styles.
Clinician well-being is the prerequisite to the triple aim of improving the health of populations, enhancing the patient experience, and reducing the cost of care. Each HMG faces similar challenges but approaches to solving them vary. Professional challenges can affect the well-being of the individual clinicians. Having interinstitutional exchange programs provides a platform to exchange ideas and establish mentors. Also, the quality of medical education is directly linked to the quality of faculty development. Improving the quality of medical education requires a multifaceted approach by highly developed faculty. The complex factors affecting medical education and faculty development are further complicated by geographic location, patient characteristics, and professional growth opportunities. Overcoming these obstacles requires an innovative and collaborative approach. Although faculty exchanges are common in academic medicine, they are not commonly attempted with HMGs.
Hospitalists are responsible for a significant part of inpatient training for residents, medical students, and nurse practitioners/physician assistants (NPs/PAs), but their faculty training can vary based on location. Being a young specialty, only 2 decades old, hospital medicine is still evolving and incorporating NP/PA and physician hospitalists in varied practice models. Each HMG addresses common obstacles differently based on their culture and practice styles. The chapter leaders determined an exchange program would afford the opportunity for visiting faculty members to experience these differences. This emphasized the role and importance of exchanging ideas and contemplated a solution to benefit more practicing hospitalists.
The chapter leaders researched the characteristics of individual academic HMGs and structured a tailored faculty exchange involving physicians and NPs/PAs. During the exchange program planning, the visiting faculty itinerary was tailored to a well-planned agenda for 1 week, with separate tracks for physicians and NPs/PAs giving increased access to their individual peer practice styles. Additionally, the visiting faculty had meetings and discussions with HMG and hospital leadership, to specifically address each visiting faculty institution’s challenges. The overall goal of this exchange program was to promote cross-institutional collaboration, increase engagement, improve medical education through faculty development, and improve the quality of care. The focus of the exchange program was to share ideas and innovation and learn the approaches to unique challenges at each institution. Out of this also came collaboration and mentoring opportunities.
The evaluation process of the exchange involved interviews, a survey, and the establishment of shared QI projects in mutual areas of challenge. The survey provided feedback, lessons learned from the exchange, and areas to be improved. Collaborative QI projects currently underway as a result of the exchange include paging etiquette, quality of sleep for hospitalized patients, and onboarding of NPs/PAs in HMGs.
This innovation addressed faculty development and medical education via clinician well-being. The physician and NP/PA Faculty Exchange was an essential and meaningful innovation that resulted in increased SHM member engagement, cross-institutional collaboration, networking, and mentorship.
Additional projects that the SHM Wiregrass Chapter successfully implemented in 2019 include a “Women in Medicine” event that recognized women physician and advanced practice provider hospitalist leaders, a poster competition that expanded its research, clinical vignettes, and quality categories to include a fourth category of innovation, featuring 75 posters. Additionally, the chapter held a policy meeting with six Alabama state legislators, creating new channels of collaboration between the legislators and the chapter. Lastly, the chapter held a successful community event and launched a mentor program targeting medical students and residents.
Rising Star Chapter
The Rising Star Chapter Award goes to one chapter who has been active for 2 years or less, who in the past 12 months have made improvements to their leadership, stability and growth, and membership. The recipient of the Rising Star Chapter Award for 2019 is the Blue Ridge Chapter of SHM, which has made significant strides to develop since its launch in the fall of 2018. The chapter represents counties in northwest Tennessee, southwest Virginia, and western North Carolina.
The chapter held three meetings in 2019 which were well attended by hospitalists, residents/fellows, administrators, advanced practice providers, and nurses. On average, attendees from five to six different hospitalist groups are represented. The chapter hosted both Dr. Chris Frost, immediate past president of the SHM board of directors, and Dr. Ron Greeno, a past president of the SHM board of directors.
The SHM Blue Ridge Chapter has collaborated with both the ACP Tennessee Chapter and the Healthcare MBA program at Haslam College of Business at the University of Tennessee.
The chapter leadership regularly attends local medical residency programs at noon conferences to attract and recruit young physicians into chapter activities. Overall, the chapter has seen a growth in membership in 2019. The Blue Ridge Chapter is an active, enthusiastic chapter that is rapidly growing and thriving.
Outstanding Membership Recruitment and Retention
The Outstanding Membership Recruitment and Retention Award is a new exemplary award for 2019 that goes to one chapter who has gone above and beyond to implement initiatives to recruit and retain SHM members in their chapter. The recipient of the Outstanding Membership Recruitment and Retention Award for 2019 is the Western Massachusetts Chapter of SHM, which has done outstanding work to recruit and retain the membership. In 2019, the SHM membership in the chapter grew by 24%. The chapter utilized Chapter Development Funds to launch new initiatives to conduct outreach to nonmember hospitalists in the community and invite them to meetings to obtain the SHM experience. Additionally, the chapter encouraged residents to join and get involved by hosting a poster competition.
The Western Massachusetts Chapter focused on being innovative, inclusive, and creative to retain their existing meetings. For example, the chapter hosted a new “Jeopardy Session” event that featured a nontraditional jeopardy game that attracted a large attendance including local residents. Additionally, the chapter insured that all clinical and nonclinical members of the hospital medicine team were included and encouraged to participate in all chapter meetings. Lastly, the chapter launched a local awards program to recognize senior hospitalist and early career hospitalist who contributed to chapter development.
Most Engaged Chapter Leader
The Most Engaged Chapter Leader Award is a new exemplary award for 2019 that goes to one chapter leader or district chair who is either nominated or self-nominated and has demonstrated how they or their nominee has gone above and beyond in the past year to grow and sustain their chapter and/or district and continues to carry out the SHM mission. The recipient of the Most Engaged Chapter Leader Award for 2019 goes to Thérèse Franco, MD, SFHM, president of the Pacific Northwest Chapter.
Dr. Franco has served as the chapter’s president for 2 years and has served on the SHM Chapter Support Committee for 3 years. She has previously participated as a mentor in the glycemic control mentored implementation program, and as chair and cochair of the RIV contest. She continues to review abstracts, volunteer as a judge and offer local education on glycemic control through the Washington State Hospital Association, promoting SHM’s work there. One of Dr. Franco’s core strengths has been effective collaboration with past leaders (such as Rachel Thompson, MD, and Kimberly Bell, MD), future leaders, and other organizations (such as the Washington State Medical Association and the King County Medical Association). Dr. Franco has recruited an outstanding leadership team and new advisory committee for the Pacific Northwest Chapter, resulting a fantastic year of growth, innovation, and development.
The Society of Hospital Medicine is proud to recognize its chapters for their hard work and dedication in 2019 through Chapter Excellence Awards. Each year, chapters strive to demonstrate growth, sustenance, and innovation within their chapter activities, which are then applauded for their successes throughout the subsequent year. In 2019, a new Bronze category was established, for a total of four Status Awards that chapters can earn.
Please join SHM in congratulating the following chapters on their year of success in 2019!
Outstanding Chapter of the Year
The Outstanding Chapter of the Year Award goes to one chapter who exemplifies high performance, going above and beyond the basic chapter requirements. The recipient of the Outstanding Chapter of the Year Award for 2019 is the Wiregrass Chapter of SHM. The chapter has a strong and engaged leadership which includes representation at all levels of the hospital medicine team, including physician hospitalists, advanced care provider hospitalists, practice administrators, nurses, residents, and medical students.
In the last year, the Wiregrass leadership team has organized programs and events to cater to and engage all the chapter’s members. This includes a variety of innovative ideas that catered toward medical education, health care provider well-being, engagement, mentorship, and community involvement.
The SHM Wiregrass Chapter’s biggest accomplishment in 2019 was the creation of an exchange program for physician and advanced practice provider hospitalists between the SHM New Mexico Chapter and the SHM Wiregrass Chapter. This idea first arose at HM19, where the chapter leaders had met during a networking event and debated the role of clinician wellbeing, quality of medical education, and faculty development to individual hospital medicine group (HMG) practice styles.
Clinician well-being is the prerequisite to the triple aim of improving the health of populations, enhancing the patient experience, and reducing the cost of care. Each HMG faces similar challenges but approaches to solving them vary. Professional challenges can affect the well-being of the individual clinicians. Having interinstitutional exchange programs provides a platform to exchange ideas and establish mentors. Also, the quality of medical education is directly linked to the quality of faculty development. Improving the quality of medical education requires a multifaceted approach by highly developed faculty. The complex factors affecting medical education and faculty development are further complicated by geographic location, patient characteristics, and professional growth opportunities. Overcoming these obstacles requires an innovative and collaborative approach. Although faculty exchanges are common in academic medicine, they are not commonly attempted with HMGs.
Hospitalists are responsible for a significant part of inpatient training for residents, medical students, and nurse practitioners/physician assistants (NPs/PAs), but their faculty training can vary based on location. Being a young specialty, only 2 decades old, hospital medicine is still evolving and incorporating NP/PA and physician hospitalists in varied practice models. Each HMG addresses common obstacles differently based on their culture and practice styles. The chapter leaders determined an exchange program would afford the opportunity for visiting faculty members to experience these differences. This emphasized the role and importance of exchanging ideas and contemplated a solution to benefit more practicing hospitalists.
The chapter leaders researched the characteristics of individual academic HMGs and structured a tailored faculty exchange involving physicians and NPs/PAs. During the exchange program planning, the visiting faculty itinerary was tailored to a well-planned agenda for 1 week, with separate tracks for physicians and NPs/PAs giving increased access to their individual peer practice styles. Additionally, the visiting faculty had meetings and discussions with HMG and hospital leadership, to specifically address each visiting faculty institution’s challenges. The overall goal of this exchange program was to promote cross-institutional collaboration, increase engagement, improve medical education through faculty development, and improve the quality of care. The focus of the exchange program was to share ideas and innovation and learn the approaches to unique challenges at each institution. Out of this also came collaboration and mentoring opportunities.
The evaluation process of the exchange involved interviews, a survey, and the establishment of shared QI projects in mutual areas of challenge. The survey provided feedback, lessons learned from the exchange, and areas to be improved. Collaborative QI projects currently underway as a result of the exchange include paging etiquette, quality of sleep for hospitalized patients, and onboarding of NPs/PAs in HMGs.
This innovation addressed faculty development and medical education via clinician well-being. The physician and NP/PA Faculty Exchange was an essential and meaningful innovation that resulted in increased SHM member engagement, cross-institutional collaboration, networking, and mentorship.
Additional projects that the SHM Wiregrass Chapter successfully implemented in 2019 include a “Women in Medicine” event that recognized women physician and advanced practice provider hospitalist leaders, a poster competition that expanded its research, clinical vignettes, and quality categories to include a fourth category of innovation, featuring 75 posters. Additionally, the chapter held a policy meeting with six Alabama state legislators, creating new channels of collaboration between the legislators and the chapter. Lastly, the chapter held a successful community event and launched a mentor program targeting medical students and residents.
Rising Star Chapter
The Rising Star Chapter Award goes to one chapter who has been active for 2 years or less, who in the past 12 months have made improvements to their leadership, stability and growth, and membership. The recipient of the Rising Star Chapter Award for 2019 is the Blue Ridge Chapter of SHM, which has made significant strides to develop since its launch in the fall of 2018. The chapter represents counties in northwest Tennessee, southwest Virginia, and western North Carolina.
The chapter held three meetings in 2019 which were well attended by hospitalists, residents/fellows, administrators, advanced practice providers, and nurses. On average, attendees from five to six different hospitalist groups are represented. The chapter hosted both Dr. Chris Frost, immediate past president of the SHM board of directors, and Dr. Ron Greeno, a past president of the SHM board of directors.
The SHM Blue Ridge Chapter has collaborated with both the ACP Tennessee Chapter and the Healthcare MBA program at Haslam College of Business at the University of Tennessee.
The chapter leadership regularly attends local medical residency programs at noon conferences to attract and recruit young physicians into chapter activities. Overall, the chapter has seen a growth in membership in 2019. The Blue Ridge Chapter is an active, enthusiastic chapter that is rapidly growing and thriving.
Outstanding Membership Recruitment and Retention
The Outstanding Membership Recruitment and Retention Award is a new exemplary award for 2019 that goes to one chapter who has gone above and beyond to implement initiatives to recruit and retain SHM members in their chapter. The recipient of the Outstanding Membership Recruitment and Retention Award for 2019 is the Western Massachusetts Chapter of SHM, which has done outstanding work to recruit and retain the membership. In 2019, the SHM membership in the chapter grew by 24%. The chapter utilized Chapter Development Funds to launch new initiatives to conduct outreach to nonmember hospitalists in the community and invite them to meetings to obtain the SHM experience. Additionally, the chapter encouraged residents to join and get involved by hosting a poster competition.
The Western Massachusetts Chapter focused on being innovative, inclusive, and creative to retain their existing meetings. For example, the chapter hosted a new “Jeopardy Session” event that featured a nontraditional jeopardy game that attracted a large attendance including local residents. Additionally, the chapter insured that all clinical and nonclinical members of the hospital medicine team were included and encouraged to participate in all chapter meetings. Lastly, the chapter launched a local awards program to recognize senior hospitalist and early career hospitalist who contributed to chapter development.
Most Engaged Chapter Leader
The Most Engaged Chapter Leader Award is a new exemplary award for 2019 that goes to one chapter leader or district chair who is either nominated or self-nominated and has demonstrated how they or their nominee has gone above and beyond in the past year to grow and sustain their chapter and/or district and continues to carry out the SHM mission. The recipient of the Most Engaged Chapter Leader Award for 2019 goes to Thérèse Franco, MD, SFHM, president of the Pacific Northwest Chapter.
Dr. Franco has served as the chapter’s president for 2 years and has served on the SHM Chapter Support Committee for 3 years. She has previously participated as a mentor in the glycemic control mentored implementation program, and as chair and cochair of the RIV contest. She continues to review abstracts, volunteer as a judge and offer local education on glycemic control through the Washington State Hospital Association, promoting SHM’s work there. One of Dr. Franco’s core strengths has been effective collaboration with past leaders (such as Rachel Thompson, MD, and Kimberly Bell, MD), future leaders, and other organizations (such as the Washington State Medical Association and the King County Medical Association). Dr. Franco has recruited an outstanding leadership team and new advisory committee for the Pacific Northwest Chapter, resulting a fantastic year of growth, innovation, and development.
The Society of Hospital Medicine is proud to recognize its chapters for their hard work and dedication in 2019 through Chapter Excellence Awards. Each year, chapters strive to demonstrate growth, sustenance, and innovation within their chapter activities, which are then applauded for their successes throughout the subsequent year. In 2019, a new Bronze category was established, for a total of four Status Awards that chapters can earn.
Please join SHM in congratulating the following chapters on their year of success in 2019!
Outstanding Chapter of the Year
The Outstanding Chapter of the Year Award goes to one chapter who exemplifies high performance, going above and beyond the basic chapter requirements. The recipient of the Outstanding Chapter of the Year Award for 2019 is the Wiregrass Chapter of SHM. The chapter has a strong and engaged leadership which includes representation at all levels of the hospital medicine team, including physician hospitalists, advanced care provider hospitalists, practice administrators, nurses, residents, and medical students.
In the last year, the Wiregrass leadership team has organized programs and events to cater to and engage all the chapter’s members. This includes a variety of innovative ideas that catered toward medical education, health care provider well-being, engagement, mentorship, and community involvement.
The SHM Wiregrass Chapter’s biggest accomplishment in 2019 was the creation of an exchange program for physician and advanced practice provider hospitalists between the SHM New Mexico Chapter and the SHM Wiregrass Chapter. This idea first arose at HM19, where the chapter leaders had met during a networking event and debated the role of clinician wellbeing, quality of medical education, and faculty development to individual hospital medicine group (HMG) practice styles.
Clinician well-being is the prerequisite to the triple aim of improving the health of populations, enhancing the patient experience, and reducing the cost of care. Each HMG faces similar challenges but approaches to solving them vary. Professional challenges can affect the well-being of the individual clinicians. Having interinstitutional exchange programs provides a platform to exchange ideas and establish mentors. Also, the quality of medical education is directly linked to the quality of faculty development. Improving the quality of medical education requires a multifaceted approach by highly developed faculty. The complex factors affecting medical education and faculty development are further complicated by geographic location, patient characteristics, and professional growth opportunities. Overcoming these obstacles requires an innovative and collaborative approach. Although faculty exchanges are common in academic medicine, they are not commonly attempted with HMGs.
Hospitalists are responsible for a significant part of inpatient training for residents, medical students, and nurse practitioners/physician assistants (NPs/PAs), but their faculty training can vary based on location. Being a young specialty, only 2 decades old, hospital medicine is still evolving and incorporating NP/PA and physician hospitalists in varied practice models. Each HMG addresses common obstacles differently based on their culture and practice styles. The chapter leaders determined an exchange program would afford the opportunity for visiting faculty members to experience these differences. This emphasized the role and importance of exchanging ideas and contemplated a solution to benefit more practicing hospitalists.
The chapter leaders researched the characteristics of individual academic HMGs and structured a tailored faculty exchange involving physicians and NPs/PAs. During the exchange program planning, the visiting faculty itinerary was tailored to a well-planned agenda for 1 week, with separate tracks for physicians and NPs/PAs giving increased access to their individual peer practice styles. Additionally, the visiting faculty had meetings and discussions with HMG and hospital leadership, to specifically address each visiting faculty institution’s challenges. The overall goal of this exchange program was to promote cross-institutional collaboration, increase engagement, improve medical education through faculty development, and improve the quality of care. The focus of the exchange program was to share ideas and innovation and learn the approaches to unique challenges at each institution. Out of this also came collaboration and mentoring opportunities.
The evaluation process of the exchange involved interviews, a survey, and the establishment of shared QI projects in mutual areas of challenge. The survey provided feedback, lessons learned from the exchange, and areas to be improved. Collaborative QI projects currently underway as a result of the exchange include paging etiquette, quality of sleep for hospitalized patients, and onboarding of NPs/PAs in HMGs.
This innovation addressed faculty development and medical education via clinician well-being. The physician and NP/PA Faculty Exchange was an essential and meaningful innovation that resulted in increased SHM member engagement, cross-institutional collaboration, networking, and mentorship.
Additional projects that the SHM Wiregrass Chapter successfully implemented in 2019 include a “Women in Medicine” event that recognized women physician and advanced practice provider hospitalist leaders, a poster competition that expanded its research, clinical vignettes, and quality categories to include a fourth category of innovation, featuring 75 posters. Additionally, the chapter held a policy meeting with six Alabama state legislators, creating new channels of collaboration between the legislators and the chapter. Lastly, the chapter held a successful community event and launched a mentor program targeting medical students and residents.
Rising Star Chapter
The Rising Star Chapter Award goes to one chapter who has been active for 2 years or less, who in the past 12 months have made improvements to their leadership, stability and growth, and membership. The recipient of the Rising Star Chapter Award for 2019 is the Blue Ridge Chapter of SHM, which has made significant strides to develop since its launch in the fall of 2018. The chapter represents counties in northwest Tennessee, southwest Virginia, and western North Carolina.
The chapter held three meetings in 2019 which were well attended by hospitalists, residents/fellows, administrators, advanced practice providers, and nurses. On average, attendees from five to six different hospitalist groups are represented. The chapter hosted both Dr. Chris Frost, immediate past president of the SHM board of directors, and Dr. Ron Greeno, a past president of the SHM board of directors.
The SHM Blue Ridge Chapter has collaborated with both the ACP Tennessee Chapter and the Healthcare MBA program at Haslam College of Business at the University of Tennessee.
The chapter leadership regularly attends local medical residency programs at noon conferences to attract and recruit young physicians into chapter activities. Overall, the chapter has seen a growth in membership in 2019. The Blue Ridge Chapter is an active, enthusiastic chapter that is rapidly growing and thriving.
Outstanding Membership Recruitment and Retention
The Outstanding Membership Recruitment and Retention Award is a new exemplary award for 2019 that goes to one chapter who has gone above and beyond to implement initiatives to recruit and retain SHM members in their chapter. The recipient of the Outstanding Membership Recruitment and Retention Award for 2019 is the Western Massachusetts Chapter of SHM, which has done outstanding work to recruit and retain the membership. In 2019, the SHM membership in the chapter grew by 24%. The chapter utilized Chapter Development Funds to launch new initiatives to conduct outreach to nonmember hospitalists in the community and invite them to meetings to obtain the SHM experience. Additionally, the chapter encouraged residents to join and get involved by hosting a poster competition.
The Western Massachusetts Chapter focused on being innovative, inclusive, and creative to retain their existing meetings. For example, the chapter hosted a new “Jeopardy Session” event that featured a nontraditional jeopardy game that attracted a large attendance including local residents. Additionally, the chapter insured that all clinical and nonclinical members of the hospital medicine team were included and encouraged to participate in all chapter meetings. Lastly, the chapter launched a local awards program to recognize senior hospitalist and early career hospitalist who contributed to chapter development.
Most Engaged Chapter Leader
The Most Engaged Chapter Leader Award is a new exemplary award for 2019 that goes to one chapter leader or district chair who is either nominated or self-nominated and has demonstrated how they or their nominee has gone above and beyond in the past year to grow and sustain their chapter and/or district and continues to carry out the SHM mission. The recipient of the Most Engaged Chapter Leader Award for 2019 goes to Thérèse Franco, MD, SFHM, president of the Pacific Northwest Chapter.
Dr. Franco has served as the chapter’s president for 2 years and has served on the SHM Chapter Support Committee for 3 years. She has previously participated as a mentor in the glycemic control mentored implementation program, and as chair and cochair of the RIV contest. She continues to review abstracts, volunteer as a judge and offer local education on glycemic control through the Washington State Hospital Association, promoting SHM’s work there. One of Dr. Franco’s core strengths has been effective collaboration with past leaders (such as Rachel Thompson, MD, and Kimberly Bell, MD), future leaders, and other organizations (such as the Washington State Medical Association and the King County Medical Association). Dr. Franco has recruited an outstanding leadership team and new advisory committee for the Pacific Northwest Chapter, resulting a fantastic year of growth, innovation, and development.
New selective S1P modulator FDA-approved for relapsing forms of multiple sclerosis1
Paid content by 
Treatment for relapsing forms of multiple sclerosis (MS) is critical to address the disease’s hallmark relapses and brain lesions.2 There are currently over a dozen approved disease-modifying medications, but no one treatment is right for every patient.2
In March 2020, the U.S. Food and Drug Administration (FDA) approved ZEPOSIA® (ozanimod) 0.92 mg, an oral medication taken once daily for the treatment of adults with relapsing forms of MS, including clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease.1
Patients may start ZEPOSIA as soon as the same day it is prescribed
Binding with high affinity to S1P receptors 1 and 5, ZEPOSIA blocks the capacity of lymphocytes to egress from lymph nodes, reducing the number of lymphocytes in peripheral blood.1 The mechanism by which ZEPOSIA exerts therapeutic effects in MS is unknown, but may involve the reduction of lymphocyte migration into the central nervous system.1
ZEPOSIA is the only S1P receptor modulator that offers relapsing forms of MS patients an initiation with no genetic test and no required label-based first-dose observation.1,3,4 An up-titration scheme should be used to reach the maintenance dosage of ZEPOSIA, as a transient decrease in heart rate and atrioventricular conduction delays may occur.1 Before initiation of treatment with ZEPOSIA, all patients require assessments including a recent complete blood count including lymphocyte count (within six months or after discontinuation of prior MS therapy), an ECG to determine whether preexisting conduction abnormalities are present, a recent liver function test (within six months), and consideration of current and prior medications, including vaccinations.1 For patients with a history of uveitis or macular edema, an ophthalmic assessment is required.1
ZEPOSIA is contraindicated in patients who in the last six months experienced myocardial infarction, unstable angina, stroke, transient ischemic attack (TIA), decompensated heart failure requiring hospitalization, or Class III/IV heart failure; patients who have a presence of Mobitz type II second or third-degree atrioventricular (AV) block, sick sinus syndrome, or sino-atrial, unless the patient has a functioning pacemaker; patients with severe untreated sleep apnea; and patients taking a monoamine oxidase inhibitor.1ZEPOSIA is associated with the following Warnings and Precautions: increased risk of infections, bradyarrhythmia and atrioventricular conduction delays, liver injury, fetal risk, increased blood pressure, respiratory effects, macular edema, posterior reversible encephalopathy syndrome, additive immunosuppressive effects from prior immune-modulating treatments, severe increase in disability after stopping ZEPOSIA, and immune system effects after stopping ZEPOSIA.1 Please see Important Safety Information for additional details. The most common adverse reactions (incidence ≥4%) were upper respiratory infection, hepatic transaminase elevation, orthostatic hypotension, urinary tract infection, back pain, and hypertension.1
“There is no one size fits all treatment for patients with relapsing forms of MS, and doctors and patients are still seeking additional therapies that can address the hallmarks of this devastating disease,” said Mary Beth Harler, M.D., senior vice president, head of immunology and fibrosis development, Bristol Myers Squibb. “ZEPOSIA has demonstrated clinical potential in safety and efficacy and may allow patients to begin treatment the same day it is prescribed.”1
Powerful efficacy demonstrated in clinical trials
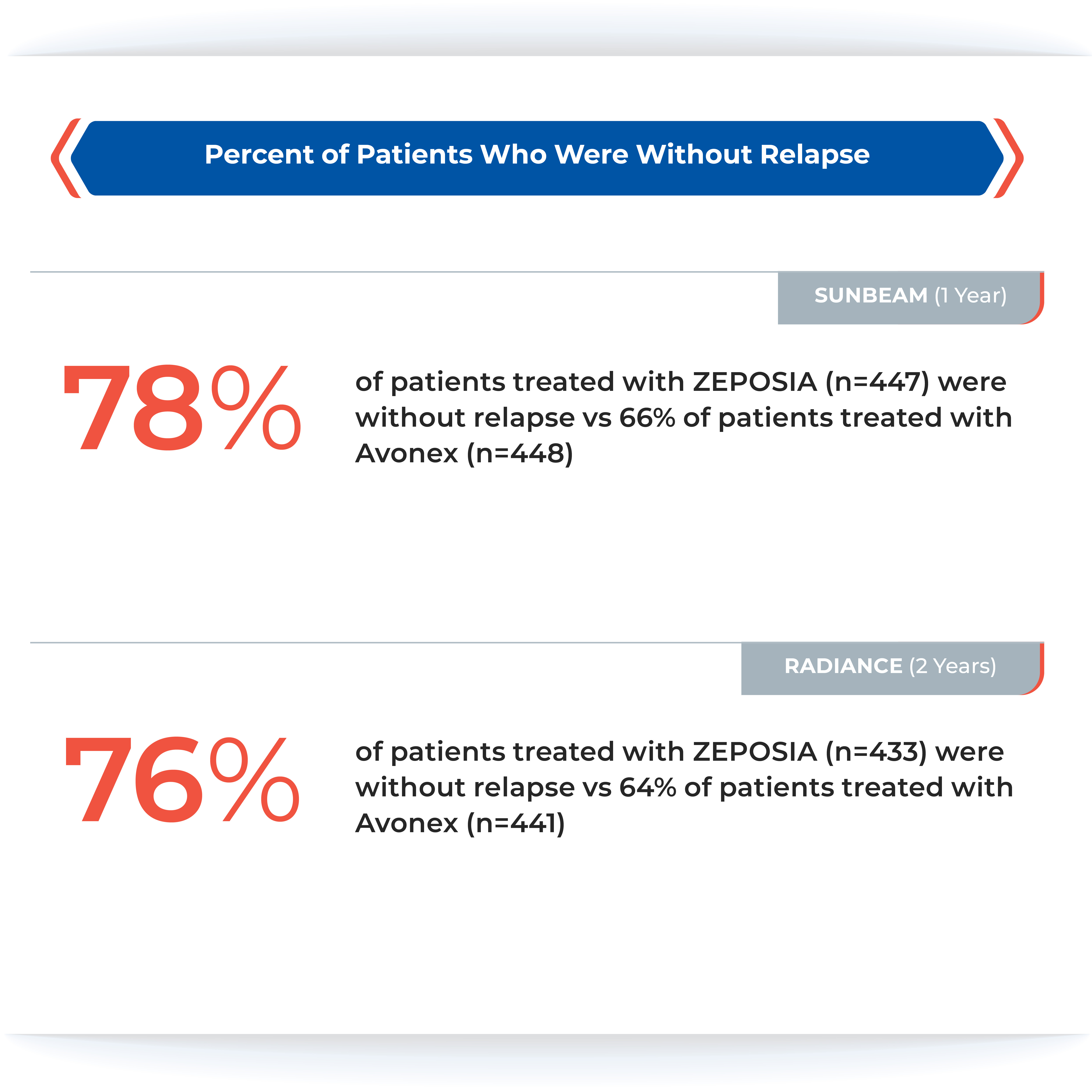
The ZEPOSIA approval was based on data from the largest, pivotal, head-to-head relapsing forms of MS studies with an active comparator to date: the randomized, Phase 3 SUNBEAM™ (safety and efficacy of ZEPOSIA versus interferon beta-1a in relapsing multiple sclerosis) and RADIANCE™ (safety and efficacy of the selective sphingosine 1-phosphate receptor modulator ZEPOSIA in relapsing multiple sclerosis) Part B clinical trials (N=2699).1,5,6,7 In both trials – as compared to AVONEX® (interferon beta-1a) – ZEPOSIA delivered powerful efficacy as measured by the primary endpoint of annualized relapse rate (ARR):
ZEPOSIA demonstrated a relative reduction in ARR versus AVONEX of 48% at one year and 38% at two years (absolute ARR of 0.18 versus 0.35 and 0.17 versus 0.28, respectively).1,5,6

The majority of patients in both groups experienced zero relapses.1
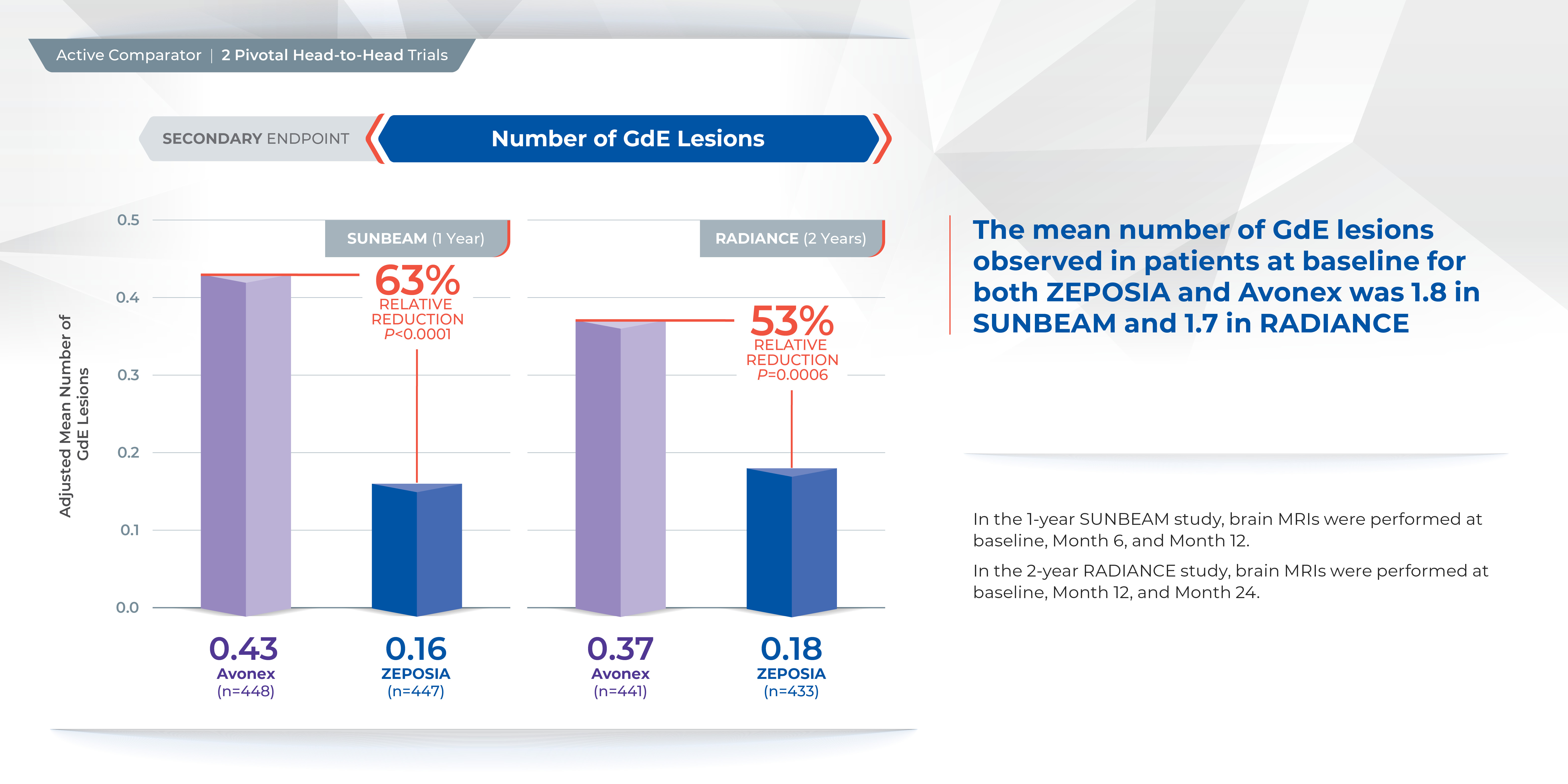
Proven superiority in reducing brain lesions
Treatment with ZEPOSIA reduced the number of T1-weighted gadolinium-enhanced (GdE) brain lesions more than AVONEX at one year (0.16 vs 0.43), a relative reduction of 63%, and at two years (0.18 vs 0.37), a relative reduction of 53%.1
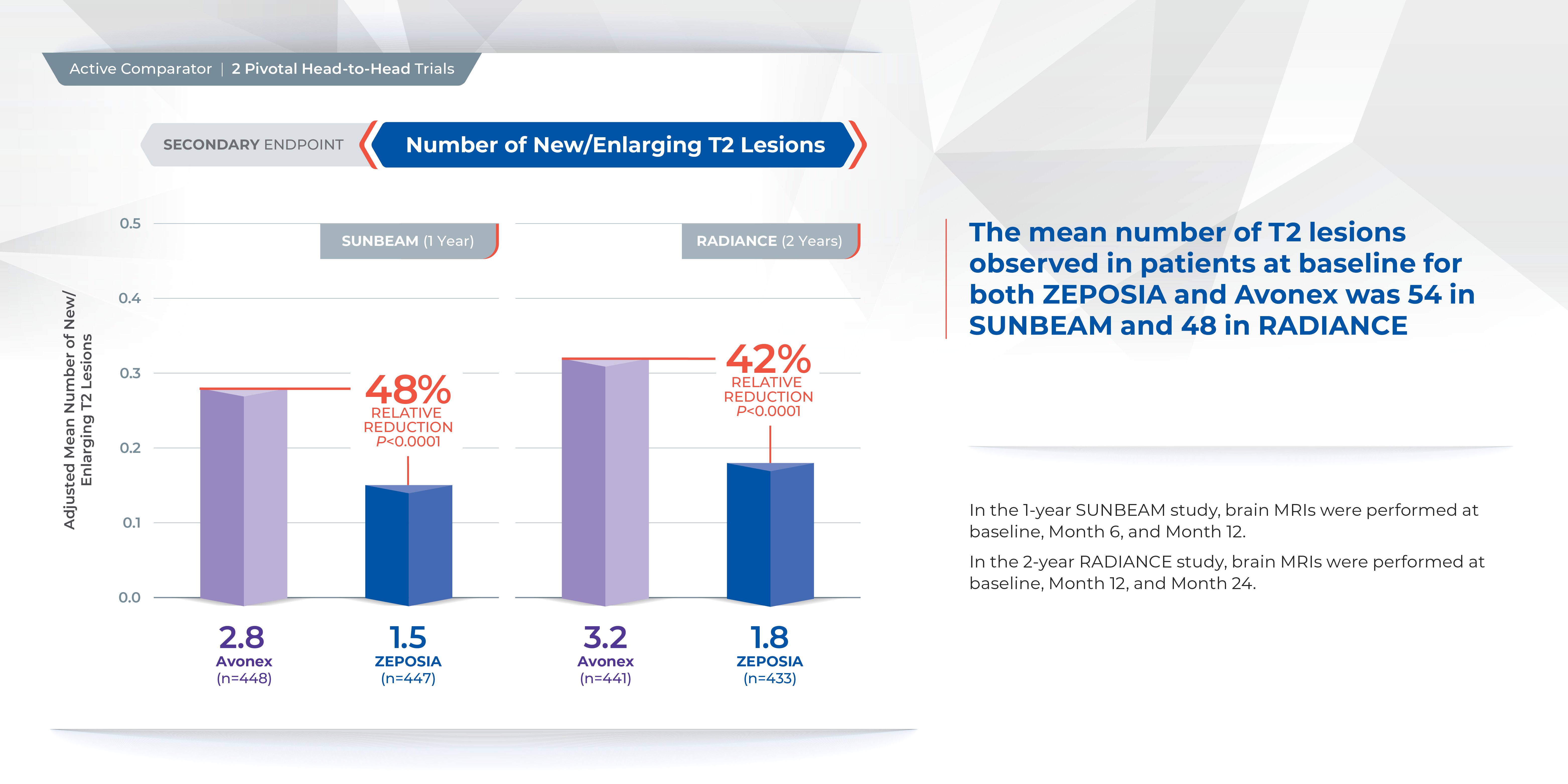
In addition, the number of new or enlarging T2 lesions was reduced at one year (1.47 vs. 2.84), a relative reduction of 48%, as well as at two years (1.84 versus 3.18), a relative reduction of 42%.1
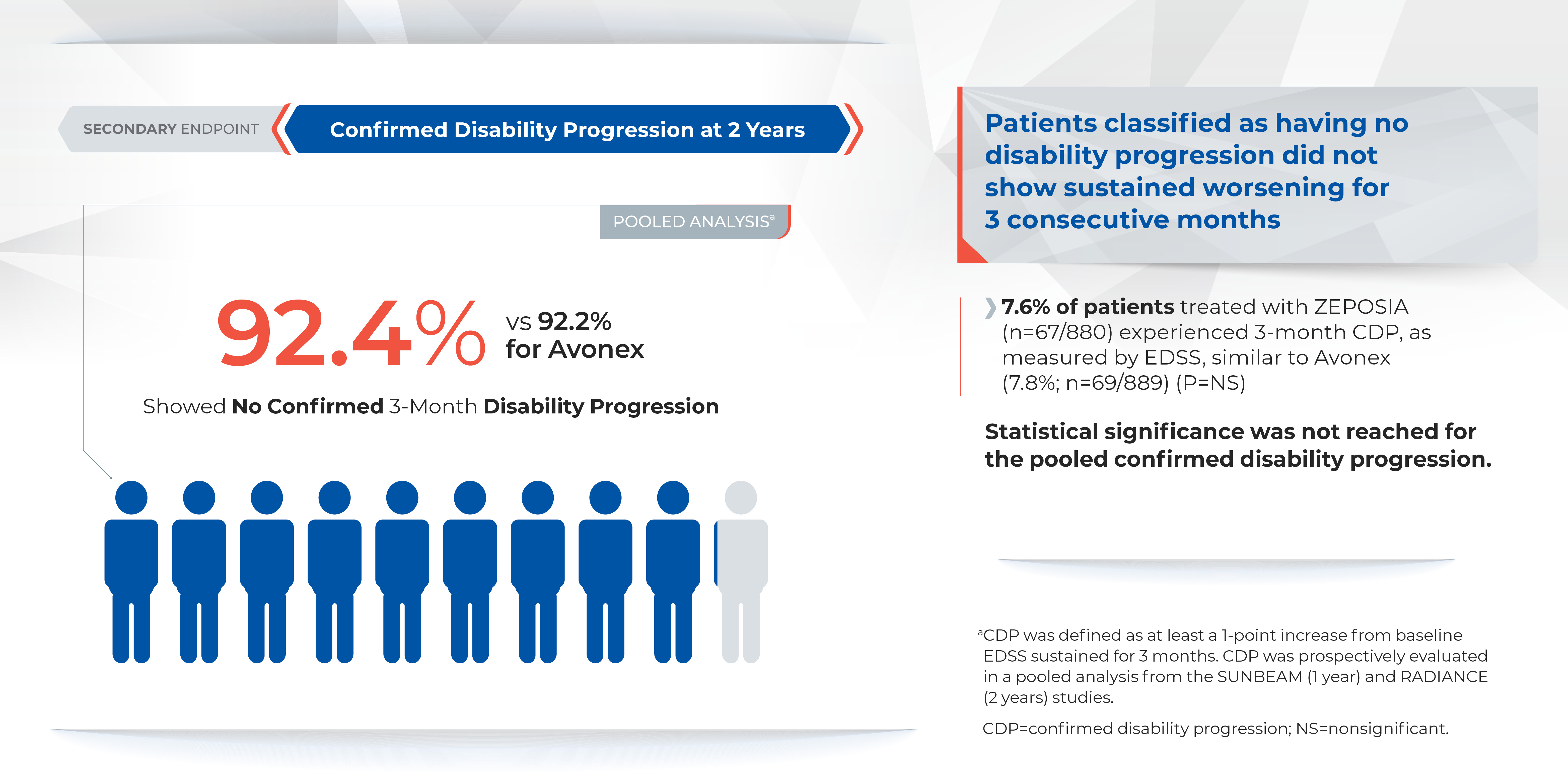
There was no statistically significant difference in the three-month and six-month confirmed disability progression between ZEPOSIA- and AVONEX- treated patients over two years.1
Comparable safety to AVONEX in overall incidence of adverse events
The overall incidence of adverse reactions experienced by patients for ZEPOSIA vs. AVONEX at one year were 59.8% and 75.5%, respectively, and at two years were 74.7% and 83.0%, respectively.1 ZEPOSIA demonstrated consistently low discontinuation rates due to adverse events versus AVONEX (<3%); at one year discontinuation rates due to adverse events for AVONEX were 3.6% and for ZEPOSIA were 2.9%, at two years discontinuation rates due to adverse events for AVONEX were 4.1% and for ZEPOSIA were 3.0%1 Overall discontinuation rates were <10% versus AVONEX across both trials. At one year discontinuation rates for AVONEX were 8% and for ZEPOSIA were 6%, at two years discontinuation rates for AVONEX were 15% and for ZEPOSIA were 10%.1
ZEPOSIA is contraindicated in patients who in the last six months experienced myocardial infarction, unstable angina, stroke, transient ischemic attack (TIA), decompensated heart failure requiring hospitalization, or Class III/IV heart failure; patients who have a presence of Mobitz type II second or third-degree atrioventricular (AV) block, sick sinus syndrome, or sino-atrial, unless the patient has a functioning pacemaker; patients with severe untreated sleep apnea; and patients taking a monoamine oxidase inhibitor.1 ZEPOSIA is associated with the following Warnings and Precautions: increased risk of infections, bradyarrhythmia and atrioventricular conduction delays, liver injury, fetal risk, increased blood pressure, respiratory effects, macular edema, posterior reversible encephalopathy syndrome, additive immunosuppressive effects from prior immune-modulating treatments, severe increase in disability after stopping ZEPOSIA, and immune system effects after stopping ZEPOSIA.1 Please see Important Safety Information for additional details.1 The most common adverse reactions (incidence ≥4%) were upper respiratory infection, hepatic transaminase elevation, orthostatic hypotension, urinary tract infection, back pain, and hypertension.1
Please visit www.ZEPOSIAHCP.com to learn more.
Indication
ZEPOSIA is indicated for the treatment of relapsing forms of multiple sclerosis (MS), to include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, in adults.
IMPORTANT SAFETY INFORMATION1
Contraindications:
- Patients who in the last 6 months, experienced myocardial infarction, unstable angina, stroke, transient ischemic attack (TIA), decompensated heart failure requiring hospitalization, or Class III/IV heart failure or have a presence of Mobitz type II second or third-degree atrioventricular (AV) block, sick sinus syndrome, or sino-atrial, unless the patient has a functioning pacemaker
- Patients with severe untreated sleep apnea
- Patients taking a monoamine oxidase (MAO) inhibitor
Infections: ZEPOSIA may increase the susceptibility to infections. Life-threatening and rare fatal infections have occurred in patients receiving ZEPOSIA. Obtain a recent (i.e., within 6 months or after discontinuation of prior MS therapy) complete blood count (CBC) including lymphocyte count before initiation of ZEPOSIA. Delay initiation of ZEPOSIA in patients with an active infection until the infection is resolved. Consider interruption of treatment with ZEPOSIA if a patient develops a serious infection. Continue monitoring for infections up to 3 months after discontinuing ZEPOSIA
- Herpes zoster was reported as an adverse reaction in ZEPOSIA-treated patients. Herpes simplex encephalitis and varicella zoster meningitis have been reported with sphingosine 1-phosphate (S1P) receptor modulators. Patients without a healthcare professional-confirmed history of varicella (chickenpox), or without documentation of a full course of vaccination against varicella zoster virus (VZV), should be tested for antibodies to VZV before initiating ZEPOSIA. A full course of vaccination for antibody-negative patients with varicella vaccine is recommended prior to commencing treatment with ZEPOSIA
- Cases of fatal cryptococcal meningitis (CM) were reported in patients treated with another S1P receptor modulator. If CM is suspected, ZEPOSIA should be suspended until cryptococcal infection has been excluded. If CM is diagnosed, appropriate treatment should be initiated.
- Progressive Multifocal Leukoencephalopathy (PML) is an opportunistic viral infection of the brain that typically occurs in patients who are immunocompromised, and that usually leads to death or severe disability. No cases of PML were identified in active-controlled MS clinical trials with ZEPOSIA. PML has been reported in patients treated with S1P receptor modulators and other MS therapies and has been associated with some risk factors. If PML is suspected, withhold ZEPOSIA and perform an appropriate diagnostic evaluation. If confirmed, treatment with ZEPOSIA should be discontinued
- In clinical studies, patients who received ZEPOSIA were not to receive concomitant treatment with antineoplastic, non-corticosteroid immunosuppressive, or immune-modulating therapies used for treatment of MS. Concomitant use of ZEPOSIA with any of these therapies would be expected to increase the risk of immunosuppression. When switching to ZEPOSIA from immunosuppressive medications, consider the duration of their effects and their mode of action to avoid unintended additive immunosuppressive effects
- Use of live attenuated vaccines should be avoided during and for 3 months after treatment with ZEPOSIA. If live attenuated vaccine immunizations are required, administer at least 1 month prior to initiation of ZEPOSIA
Bradyarrhythmia and Atrioventricular Conduction Delays: Since initiation of ZEPOSIA may result in a transient decrease in heart rate and atrioventricular conduction delays, dose titration is recommended to help reduce cardiac effects. Initiation of ZEPOSIA without dose escalation may result in greater decreases in heart rate. If treatment with ZEPOSIA is considered, advice from a cardiologist should be sought for those individuals:
- with significant QT prolongation
- with arrhythmias requiring treatment with Class 1a or III anti-arrhythmic drugs
- with ischemic heart disease, heart failure, history of cardiac arrest or myocardial infarction, cerebrovascular disease, and uncontrolled hypertension
- with a history of Mobitz type II second-degree or higher AV block, sick-sinus syndrome, or sinoatrial heart block
Liver Injury: Elevations of aminotransferases may occur in patients receiving ZEPOSIA. Obtain liver function tests, if not recently available (i.e., within 6 months), before initiation of ZEPOSIA. Patients who develop symptoms suggestive of hepatic dysfunction should have hepatic enzymes checked and ZEPOSIA should be discontinued if significant liver injury is confirmed. Caution should be exercised when using ZEPOSIA in patients with history of significant liver disease
Fetal Risk: There are no adequate and well-controlled studies in pregnant women. Based on animal studies, ZEPOSIA may cause fetal harm. Women of childbearing potential should use effective contraception to avoid pregnancy during treatment and for 3 months after stopping ZEPOSIA
Increased Blood Pressure: Increase in systolic pressure was observed after about 3 months of treatment and persisted throughout treatment. Blood pressure should be monitored during treatment and managed appropriately. Certain foods that may contain very high amounts of tyramine could cause severe hypertension in patients taking ZEPOSIA. Patients should be advised to avoid foods containing a very large amount of tyramine while taking ZEPOSIA
Respiratory Effects: ZEPOSIA may cause a decline in pulmonary function. Spirometric evaluation of respiratory function should be performed during therapy, if clinically indicated
Macular edema: S1P modulators have been associated with an increased risk of macular edema. Patients with a history of uveitis or diabetes mellitus are at increased risk. Patients with a history of these conditions should have an ophthalmic evaluation of the fundus, including the macula, prior to treatment initiation and regular follow-up examinations. An ophthalmic evaluation is recommended in all patients at any time if there is a change in vision. Continued use of ZEPOSIA in patients with macular edema has not been evaluated; potential benefits and risks for the individual patient should be considered if deciding whether ZEPOSIA should be discontinued
Posterior Reversible Encephalopathy Syndrome (PRES): Rare cases of PRES have been reported in patients receiving a S1P receptor modulator. If a ZEPOSIA-treated patient develops unexpected neurological or psychiatric symptoms or any symptom/sign suggestive of an increase in intracranial pressure, a complete physical and neurological examination should be conducted. Symptoms of PRES are usually reversible but may evolve into ischemic stroke or cerebral hemorrhage. Delay in diagnosis and treatment may lead to permanent neurological sequelae. If PRES is suspected, treatment with ZEPOSIA should be discontinued
Unintended Additive Immunosuppressive Effects From Prior Immunosuppressive or Immune-Modulating Drugs: When switching from drugs with prolonged immune effects, the half-life and mode of action of these drugs must be considered to avoid unintended additive immunosuppressive effects while at the same time minimizing risk of disease reactivation. Initiating treatment with ZEPOSIA after treatment with alemtuzumab is not recommended
Severe Increase in Disability After Stopping ZEPOSIA: Severe exacerbation of disease, including disease rebound, has been rarely reported after discontinuation of a S1P receptor modulator. The possibility of severe exacerbation of disease should be considered after stopping ZEPOSIA treatment so patients should be monitored upon discontinuation
Immune System Effects After Stopping ZEPOSIA: After discontinuing ZEPOSIA, the median time for lymphocyte counts to return to the normal range was 30 days with approximately 90% of patients in the normal range within 3 months. Use of immunosuppressants within this period may lead to an additive effect on the immune system, therefore caution should be applied when initiating other drugs 4 weeks after the last dose of ZEPOSIA
Most common Adverse Reactions (≥ 4%): upper respiratory infection, hepatic transaminase elevation, orthostatic hypotension, urinary tract infection, back pain, and hypertension.
For additional safety information, please see the full Prescribing Information and Medication Guide.
1. ZEPOSIA (ozanimod) capsules for oral use. Celgene Corporation. Full prescribing information. 3/2020.
2. National Multiple Sclerosis Society. Treating RMSS. www.nationalmssociety.org/What-is-MS/Types-of-MS/Relapsing-remitting-MS/Treatment. Accessed on April 24, 2020.
3. GILENYA (fingolimod) capsules for oral use. Novartis Pharmaceuticals Corporation. Full prescribing information. 8/2019.
4. MAYZENT (siponimod) tablets for oral use. Novartis Pharmaceuticals Corporation. Full prescribing information. 3/2019.
5. Cohen, JA, Comi, G, Selmaj, KW, et al. Safety and efficacy of ozanimod versus interferon beta-1a in relapsing multiple sclerosis (RADIANCE): a multicenter, randomized, 24-month, phase 3 trial. The Lancet: Neurology. DOI: 10.1016/S1474-4422(19)30238-8.
6. Comi, G, Kappos, L, Selmaj, KW, et at. Safety and efficacy of ozanimod versus interferon beta-1a in relapsing multiple sclerosis (SUNBEAM): a multicenter, randomized, minimum 12-month, phase 3 trial. The Lancet: Neurology. DOI: 10.1016/S1474-4422(19)30239-X.
7. McCann Agency. Pivotal Trials for MS Therapies: ZEPOSIA –2 head-to-head with Active Comparator Based on PIs. March 2020.
ZEPOSIA® is a trademark of Bristol-Myers Squibb Company.
© 2020 Bristol-Myers Squibb Company. All Rights Reserved.
US-ZEP-20-1095 10/20
Paid content by 

Treatment for relapsing forms of multiple sclerosis (MS) is critical to address the disease’s hallmark relapses and brain lesions.2 There are currently over a dozen approved disease-modifying medications, but no one treatment is right for every patient.2
In March 2020, the U.S. Food and Drug Administration (FDA) approved ZEPOSIA® (ozanimod) 0.92 mg, an oral medication taken once daily for the treatment of adults with relapsing forms of MS, including clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease.1
Patients may start ZEPOSIA as soon as the same day it is prescribed
Binding with high affinity to S1P receptors 1 and 5, ZEPOSIA blocks the capacity of lymphocytes to egress from lymph nodes, reducing the number of lymphocytes in peripheral blood.1 The mechanism by which ZEPOSIA exerts therapeutic effects in MS is unknown, but may involve the reduction of lymphocyte migration into the central nervous system.1
ZEPOSIA is the only S1P receptor modulator that offers relapsing forms of MS patients an initiation with no genetic test and no required label-based first-dose observation.1,3,4 An up-titration scheme should be used to reach the maintenance dosage of ZEPOSIA, as a transient decrease in heart rate and atrioventricular conduction delays may occur.1 Before initiation of treatment with ZEPOSIA, all patients require assessments including a recent complete blood count including lymphocyte count (within six months or after discontinuation of prior MS therapy), an ECG to determine whether preexisting conduction abnormalities are present, a recent liver function test (within six months), and consideration of current and prior medications, including vaccinations.1 For patients with a history of uveitis or macular edema, an ophthalmic assessment is required.1
ZEPOSIA is contraindicated in patients who in the last six months experienced myocardial infarction, unstable angina, stroke, transient ischemic attack (TIA), decompensated heart failure requiring hospitalization, or Class III/IV heart failure; patients who have a presence of Mobitz type II second or third-degree atrioventricular (AV) block, sick sinus syndrome, or sino-atrial, unless the patient has a functioning pacemaker; patients with severe untreated sleep apnea; and patients taking a monoamine oxidase inhibitor.1ZEPOSIA is associated with the following Warnings and Precautions: increased risk of infections, bradyarrhythmia and atrioventricular conduction delays, liver injury, fetal risk, increased blood pressure, respiratory effects, macular edema, posterior reversible encephalopathy syndrome, additive immunosuppressive effects from prior immune-modulating treatments, severe increase in disability after stopping ZEPOSIA, and immune system effects after stopping ZEPOSIA.1 Please see Important Safety Information for additional details. The most common adverse reactions (incidence ≥4%) were upper respiratory infection, hepatic transaminase elevation, orthostatic hypotension, urinary tract infection, back pain, and hypertension.1
“There is no one size fits all treatment for patients with relapsing forms of MS, and doctors and patients are still seeking additional therapies that can address the hallmarks of this devastating disease,” said Mary Beth Harler, M.D., senior vice president, head of immunology and fibrosis development, Bristol Myers Squibb. “ZEPOSIA has demonstrated clinical potential in safety and efficacy and may allow patients to begin treatment the same day it is prescribed.”1
Powerful efficacy demonstrated in clinical trials
The ZEPOSIA approval was based on data from the largest, pivotal, head-to-head relapsing forms of MS studies with an active comparator to date: the randomized, Phase 3 SUNBEAM™ (safety and efficacy of ZEPOSIA versus interferon beta-1a in relapsing multiple sclerosis) and RADIANCE™ (safety and efficacy of the selective sphingosine 1-phosphate receptor modulator ZEPOSIA in relapsing multiple sclerosis) Part B clinical trials (N=2699).1,5,6,7 In both trials – as compared to AVONEX® (interferon beta-1a) – ZEPOSIA delivered powerful efficacy as measured by the primary endpoint of annualized relapse rate (ARR):
ZEPOSIA demonstrated a relative reduction in ARR versus AVONEX of 48% at one year and 38% at two years (absolute ARR of 0.18 versus 0.35 and 0.17 versus 0.28, respectively).1,5,6

The majority of patients in both groups experienced zero relapses.1

Proven superiority in reducing brain lesions
Treatment with ZEPOSIA reduced the number of T1-weighted gadolinium-enhanced (GdE) brain lesions more than AVONEX at one year (0.16 vs 0.43), a relative reduction of 63%, and at two years (0.18 vs 0.37), a relative reduction of 53%.1

In addition, the number of new or enlarging T2 lesions was reduced at one year (1.47 vs. 2.84), a relative reduction of 48%, as well as at two years (1.84 versus 3.18), a relative reduction of 42%.1

There was no statistically significant difference in the three-month and six-month confirmed disability progression between ZEPOSIA- and AVONEX- treated patients over two years.1

Comparable safety to AVONEX in overall incidence of adverse events
The overall incidence of adverse reactions experienced by patients for ZEPOSIA vs. AVONEX at one year were 59.8% and 75.5%, respectively, and at two years were 74.7% and 83.0%, respectively.1 ZEPOSIA demonstrated consistently low discontinuation rates due to adverse events versus AVONEX (<3%); at one year discontinuation rates due to adverse events for AVONEX were 3.6% and for ZEPOSIA were 2.9%, at two years discontinuation rates due to adverse events for AVONEX were 4.1% and for ZEPOSIA were 3.0%1 Overall discontinuation rates were <10% versus AVONEX across both trials. At one year discontinuation rates for AVONEX were 8% and for ZEPOSIA were 6%, at two years discontinuation rates for AVONEX were 15% and for ZEPOSIA were 10%.1
ZEPOSIA is contraindicated in patients who in the last six months experienced myocardial infarction, unstable angina, stroke, transient ischemic attack (TIA), decompensated heart failure requiring hospitalization, or Class III/IV heart failure; patients who have a presence of Mobitz type II second or third-degree atrioventricular (AV) block, sick sinus syndrome, or sino-atrial, unless the patient has a functioning pacemaker; patients with severe untreated sleep apnea; and patients taking a monoamine oxidase inhibitor.1 ZEPOSIA is associated with the following Warnings and Precautions: increased risk of infections, bradyarrhythmia and atrioventricular conduction delays, liver injury, fetal risk, increased blood pressure, respiratory effects, macular edema, posterior reversible encephalopathy syndrome, additive immunosuppressive effects from prior immune-modulating treatments, severe increase in disability after stopping ZEPOSIA, and immune system effects after stopping ZEPOSIA.1 Please see Important Safety Information for additional details.1 The most common adverse reactions (incidence ≥4%) were upper respiratory infection, hepatic transaminase elevation, orthostatic hypotension, urinary tract infection, back pain, and hypertension.1
Please visit www.ZEPOSIAHCP.com to learn more.
Indication
ZEPOSIA is indicated for the treatment of relapsing forms of multiple sclerosis (MS), to include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, in adults.
IMPORTANT SAFETY INFORMATION1
Contraindications:
- Patients who in the last 6 months, experienced myocardial infarction, unstable angina, stroke, transient ischemic attack (TIA), decompensated heart failure requiring hospitalization, or Class III/IV heart failure or have a presence of Mobitz type II second or third-degree atrioventricular (AV) block, sick sinus syndrome, or sino-atrial, unless the patient has a functioning pacemaker
- Patients with severe untreated sleep apnea
- Patients taking a monoamine oxidase (MAO) inhibitor
Infections: ZEPOSIA may increase the susceptibility to infections. Life-threatening and rare fatal infections have occurred in patients receiving ZEPOSIA. Obtain a recent (i.e., within 6 months or after discontinuation of prior MS therapy) complete blood count (CBC) including lymphocyte count before initiation of ZEPOSIA. Delay initiation of ZEPOSIA in patients with an active infection until the infection is resolved. Consider interruption of treatment with ZEPOSIA if a patient develops a serious infection. Continue monitoring for infections up to 3 months after discontinuing ZEPOSIA
- Herpes zoster was reported as an adverse reaction in ZEPOSIA-treated patients. Herpes simplex encephalitis and varicella zoster meningitis have been reported with sphingosine 1-phosphate (S1P) receptor modulators. Patients without a healthcare professional-confirmed history of varicella (chickenpox), or without documentation of a full course of vaccination against varicella zoster virus (VZV), should be tested for antibodies to VZV before initiating ZEPOSIA. A full course of vaccination for antibody-negative patients with varicella vaccine is recommended prior to commencing treatment with ZEPOSIA
- Cases of fatal cryptococcal meningitis (CM) were reported in patients treated with another S1P receptor modulator. If CM is suspected, ZEPOSIA should be suspended until cryptococcal infection has been excluded. If CM is diagnosed, appropriate treatment should be initiated.
- Progressive Multifocal Leukoencephalopathy (PML) is an opportunistic viral infection of the brain that typically occurs in patients who are immunocompromised, and that usually leads to death or severe disability. No cases of PML were identified in active-controlled MS clinical trials with ZEPOSIA. PML has been reported in patients treated with S1P receptor modulators and other MS therapies and has been associated with some risk factors. If PML is suspected, withhold ZEPOSIA and perform an appropriate diagnostic evaluation. If confirmed, treatment with ZEPOSIA should be discontinued
- In clinical studies, patients who received ZEPOSIA were not to receive concomitant treatment with antineoplastic, non-corticosteroid immunosuppressive, or immune-modulating therapies used for treatment of MS. Concomitant use of ZEPOSIA with any of these therapies would be expected to increase the risk of immunosuppression. When switching to ZEPOSIA from immunosuppressive medications, consider the duration of their effects and their mode of action to avoid unintended additive immunosuppressive effects
- Use of live attenuated vaccines should be avoided during and for 3 months after treatment with ZEPOSIA. If live attenuated vaccine immunizations are required, administer at least 1 month prior to initiation of ZEPOSIA
Bradyarrhythmia and Atrioventricular Conduction Delays: Since initiation of ZEPOSIA may result in a transient decrease in heart rate and atrioventricular conduction delays, dose titration is recommended to help reduce cardiac effects. Initiation of ZEPOSIA without dose escalation may result in greater decreases in heart rate. If treatment with ZEPOSIA is considered, advice from a cardiologist should be sought for those individuals:
- with significant QT prolongation
- with arrhythmias requiring treatment with Class 1a or III anti-arrhythmic drugs
- with ischemic heart disease, heart failure, history of cardiac arrest or myocardial infarction, cerebrovascular disease, and uncontrolled hypertension
- with a history of Mobitz type II second-degree or higher AV block, sick-sinus syndrome, or sinoatrial heart block
Liver Injury: Elevations of aminotransferases may occur in patients receiving ZEPOSIA. Obtain liver function tests, if not recently available (i.e., within 6 months), before initiation of ZEPOSIA. Patients who develop symptoms suggestive of hepatic dysfunction should have hepatic enzymes checked and ZEPOSIA should be discontinued if significant liver injury is confirmed. Caution should be exercised when using ZEPOSIA in patients with history of significant liver disease
Fetal Risk: There are no adequate and well-controlled studies in pregnant women. Based on animal studies, ZEPOSIA may cause fetal harm. Women of childbearing potential should use effective contraception to avoid pregnancy during treatment and for 3 months after stopping ZEPOSIA
Increased Blood Pressure: Increase in systolic pressure was observed after about 3 months of treatment and persisted throughout treatment. Blood pressure should be monitored during treatment and managed appropriately. Certain foods that may contain very high amounts of tyramine could cause severe hypertension in patients taking ZEPOSIA. Patients should be advised to avoid foods containing a very large amount of tyramine while taking ZEPOSIA
Respiratory Effects: ZEPOSIA may cause a decline in pulmonary function. Spirometric evaluation of respiratory function should be performed during therapy, if clinically indicated
Macular edema: S1P modulators have been associated with an increased risk of macular edema. Patients with a history of uveitis or diabetes mellitus are at increased risk. Patients with a history of these conditions should have an ophthalmic evaluation of the fundus, including the macula, prior to treatment initiation and regular follow-up examinations. An ophthalmic evaluation is recommended in all patients at any time if there is a change in vision. Continued use of ZEPOSIA in patients with macular edema has not been evaluated; potential benefits and risks for the individual patient should be considered if deciding whether ZEPOSIA should be discontinued
Posterior Reversible Encephalopathy Syndrome (PRES): Rare cases of PRES have been reported in patients receiving a S1P receptor modulator. If a ZEPOSIA-treated patient develops unexpected neurological or psychiatric symptoms or any symptom/sign suggestive of an increase in intracranial pressure, a complete physical and neurological examination should be conducted. Symptoms of PRES are usually reversible but may evolve into ischemic stroke or cerebral hemorrhage. Delay in diagnosis and treatment may lead to permanent neurological sequelae. If PRES is suspected, treatment with ZEPOSIA should be discontinued
Unintended Additive Immunosuppressive Effects From Prior Immunosuppressive or Immune-Modulating Drugs: When switching from drugs with prolonged immune effects, the half-life and mode of action of these drugs must be considered to avoid unintended additive immunosuppressive effects while at the same time minimizing risk of disease reactivation. Initiating treatment with ZEPOSIA after treatment with alemtuzumab is not recommended
Severe Increase in Disability After Stopping ZEPOSIA: Severe exacerbation of disease, including disease rebound, has been rarely reported after discontinuation of a S1P receptor modulator. The possibility of severe exacerbation of disease should be considered after stopping ZEPOSIA treatment so patients should be monitored upon discontinuation
Immune System Effects After Stopping ZEPOSIA: After discontinuing ZEPOSIA, the median time for lymphocyte counts to return to the normal range was 30 days with approximately 90% of patients in the normal range within 3 months. Use of immunosuppressants within this period may lead to an additive effect on the immune system, therefore caution should be applied when initiating other drugs 4 weeks after the last dose of ZEPOSIA
Most common Adverse Reactions (≥ 4%): upper respiratory infection, hepatic transaminase elevation, orthostatic hypotension, urinary tract infection, back pain, and hypertension.
For additional safety information, please see the full Prescribing Information and Medication Guide.
1. ZEPOSIA (ozanimod) capsules for oral use. Celgene Corporation. Full prescribing information. 3/2020.
2. National Multiple Sclerosis Society. Treating RMSS. www.nationalmssociety.org/What-is-MS/Types-of-MS/Relapsing-remitting-MS/Treatment. Accessed on April 24, 2020.
3. GILENYA (fingolimod) capsules for oral use. Novartis Pharmaceuticals Corporation. Full prescribing information. 8/2019.
4. MAYZENT (siponimod) tablets for oral use. Novartis Pharmaceuticals Corporation. Full prescribing information. 3/2019.
5. Cohen, JA, Comi, G, Selmaj, KW, et al. Safety and efficacy of ozanimod versus interferon beta-1a in relapsing multiple sclerosis (RADIANCE): a multicenter, randomized, 24-month, phase 3 trial. The Lancet: Neurology. DOI: 10.1016/S1474-4422(19)30238-8.
6. Comi, G, Kappos, L, Selmaj, KW, et at. Safety and efficacy of ozanimod versus interferon beta-1a in relapsing multiple sclerosis (SUNBEAM): a multicenter, randomized, minimum 12-month, phase 3 trial. The Lancet: Neurology. DOI: 10.1016/S1474-4422(19)30239-X.
7. McCann Agency. Pivotal Trials for MS Therapies: ZEPOSIA –2 head-to-head with Active Comparator Based on PIs. March 2020.
ZEPOSIA® is a trademark of Bristol-Myers Squibb Company.
© 2020 Bristol-Myers Squibb Company. All Rights Reserved.
US-ZEP-20-1095 10/20
Paid content by 

Treatment for relapsing forms of multiple sclerosis (MS) is critical to address the disease’s hallmark relapses and brain lesions.2 There are currently over a dozen approved disease-modifying medications, but no one treatment is right for every patient.2
In March 2020, the U.S. Food and Drug Administration (FDA) approved ZEPOSIA® (ozanimod) 0.92 mg, an oral medication taken once daily for the treatment of adults with relapsing forms of MS, including clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease.1
Patients may start ZEPOSIA as soon as the same day it is prescribed
Binding with high affinity to S1P receptors 1 and 5, ZEPOSIA blocks the capacity of lymphocytes to egress from lymph nodes, reducing the number of lymphocytes in peripheral blood.1 The mechanism by which ZEPOSIA exerts therapeutic effects in MS is unknown, but may involve the reduction of lymphocyte migration into the central nervous system.1
ZEPOSIA is the only S1P receptor modulator that offers relapsing forms of MS patients an initiation with no genetic test and no required label-based first-dose observation.1,3,4 An up-titration scheme should be used to reach the maintenance dosage of ZEPOSIA, as a transient decrease in heart rate and atrioventricular conduction delays may occur.1 Before initiation of treatment with ZEPOSIA, all patients require assessments including a recent complete blood count including lymphocyte count (within six months or after discontinuation of prior MS therapy), an ECG to determine whether preexisting conduction abnormalities are present, a recent liver function test (within six months), and consideration of current and prior medications, including vaccinations.1 For patients with a history of uveitis or macular edema, an ophthalmic assessment is required.1
ZEPOSIA is contraindicated in patients who in the last six months experienced myocardial infarction, unstable angina, stroke, transient ischemic attack (TIA), decompensated heart failure requiring hospitalization, or Class III/IV heart failure; patients who have a presence of Mobitz type II second or third-degree atrioventricular (AV) block, sick sinus syndrome, or sino-atrial, unless the patient has a functioning pacemaker; patients with severe untreated sleep apnea; and patients taking a monoamine oxidase inhibitor.1ZEPOSIA is associated with the following Warnings and Precautions: increased risk of infections, bradyarrhythmia and atrioventricular conduction delays, liver injury, fetal risk, increased blood pressure, respiratory effects, macular edema, posterior reversible encephalopathy syndrome, additive immunosuppressive effects from prior immune-modulating treatments, severe increase in disability after stopping ZEPOSIA, and immune system effects after stopping ZEPOSIA.1 Please see Important Safety Information for additional details. The most common adverse reactions (incidence ≥4%) were upper respiratory infection, hepatic transaminase elevation, orthostatic hypotension, urinary tract infection, back pain, and hypertension.1
“There is no one size fits all treatment for patients with relapsing forms of MS, and doctors and patients are still seeking additional therapies that can address the hallmarks of this devastating disease,” said Mary Beth Harler, M.D., senior vice president, head of immunology and fibrosis development, Bristol Myers Squibb. “ZEPOSIA has demonstrated clinical potential in safety and efficacy and may allow patients to begin treatment the same day it is prescribed.”1
Powerful efficacy demonstrated in clinical trials
The ZEPOSIA approval was based on data from the largest, pivotal, head-to-head relapsing forms of MS studies with an active comparator to date: the randomized, Phase 3 SUNBEAM™ (safety and efficacy of ZEPOSIA versus interferon beta-1a in relapsing multiple sclerosis) and RADIANCE™ (safety and efficacy of the selective sphingosine 1-phosphate receptor modulator ZEPOSIA in relapsing multiple sclerosis) Part B clinical trials (N=2699).1,5,6,7 In both trials – as compared to AVONEX® (interferon beta-1a) – ZEPOSIA delivered powerful efficacy as measured by the primary endpoint of annualized relapse rate (ARR):
ZEPOSIA demonstrated a relative reduction in ARR versus AVONEX of 48% at one year and 38% at two years (absolute ARR of 0.18 versus 0.35 and 0.17 versus 0.28, respectively).1,5,6

The majority of patients in both groups experienced zero relapses.1

Proven superiority in reducing brain lesions
Treatment with ZEPOSIA reduced the number of T1-weighted gadolinium-enhanced (GdE) brain lesions more than AVONEX at one year (0.16 vs 0.43), a relative reduction of 63%, and at two years (0.18 vs 0.37), a relative reduction of 53%.1

In addition, the number of new or enlarging T2 lesions was reduced at one year (1.47 vs. 2.84), a relative reduction of 48%, as well as at two years (1.84 versus 3.18), a relative reduction of 42%.1

There was no statistically significant difference in the three-month and six-month confirmed disability progression between ZEPOSIA- and AVONEX- treated patients over two years.1

Comparable safety to AVONEX in overall incidence of adverse events
The overall incidence of adverse reactions experienced by patients for ZEPOSIA vs. AVONEX at one year were 59.8% and 75.5%, respectively, and at two years were 74.7% and 83.0%, respectively.1 ZEPOSIA demonstrated consistently low discontinuation rates due to adverse events versus AVONEX (<3%); at one year discontinuation rates due to adverse events for AVONEX were 3.6% and for ZEPOSIA were 2.9%, at two years discontinuation rates due to adverse events for AVONEX were 4.1% and for ZEPOSIA were 3.0%1 Overall discontinuation rates were <10% versus AVONEX across both trials. At one year discontinuation rates for AVONEX were 8% and for ZEPOSIA were 6%, at two years discontinuation rates for AVONEX were 15% and for ZEPOSIA were 10%.1
ZEPOSIA is contraindicated in patients who in the last six months experienced myocardial infarction, unstable angina, stroke, transient ischemic attack (TIA), decompensated heart failure requiring hospitalization, or Class III/IV heart failure; patients who have a presence of Mobitz type II second or third-degree atrioventricular (AV) block, sick sinus syndrome, or sino-atrial, unless the patient has a functioning pacemaker; patients with severe untreated sleep apnea; and patients taking a monoamine oxidase inhibitor.1 ZEPOSIA is associated with the following Warnings and Precautions: increased risk of infections, bradyarrhythmia and atrioventricular conduction delays, liver injury, fetal risk, increased blood pressure, respiratory effects, macular edema, posterior reversible encephalopathy syndrome, additive immunosuppressive effects from prior immune-modulating treatments, severe increase in disability after stopping ZEPOSIA, and immune system effects after stopping ZEPOSIA.1 Please see Important Safety Information for additional details.1 The most common adverse reactions (incidence ≥4%) were upper respiratory infection, hepatic transaminase elevation, orthostatic hypotension, urinary tract infection, back pain, and hypertension.1
Please visit www.ZEPOSIAHCP.com to learn more.
Indication
ZEPOSIA is indicated for the treatment of relapsing forms of multiple sclerosis (MS), to include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, in adults.
IMPORTANT SAFETY INFORMATION1
Contraindications:
- Patients who in the last 6 months, experienced myocardial infarction, unstable angina, stroke, transient ischemic attack (TIA), decompensated heart failure requiring hospitalization, or Class III/IV heart failure or have a presence of Mobitz type II second or third-degree atrioventricular (AV) block, sick sinus syndrome, or sino-atrial, unless the patient has a functioning pacemaker
- Patients with severe untreated sleep apnea
- Patients taking a monoamine oxidase (MAO) inhibitor
Infections: ZEPOSIA may increase the susceptibility to infections. Life-threatening and rare fatal infections have occurred in patients receiving ZEPOSIA. Obtain a recent (i.e., within 6 months or after discontinuation of prior MS therapy) complete blood count (CBC) including lymphocyte count before initiation of ZEPOSIA. Delay initiation of ZEPOSIA in patients with an active infection until the infection is resolved. Consider interruption of treatment with ZEPOSIA if a patient develops a serious infection. Continue monitoring for infections up to 3 months after discontinuing ZEPOSIA
- Herpes zoster was reported as an adverse reaction in ZEPOSIA-treated patients. Herpes simplex encephalitis and varicella zoster meningitis have been reported with sphingosine 1-phosphate (S1P) receptor modulators. Patients without a healthcare professional-confirmed history of varicella (chickenpox), or without documentation of a full course of vaccination against varicella zoster virus (VZV), should be tested for antibodies to VZV before initiating ZEPOSIA. A full course of vaccination for antibody-negative patients with varicella vaccine is recommended prior to commencing treatment with ZEPOSIA
- Cases of fatal cryptococcal meningitis (CM) were reported in patients treated with another S1P receptor modulator. If CM is suspected, ZEPOSIA should be suspended until cryptococcal infection has been excluded. If CM is diagnosed, appropriate treatment should be initiated.
- Progressive Multifocal Leukoencephalopathy (PML) is an opportunistic viral infection of the brain that typically occurs in patients who are immunocompromised, and that usually leads to death or severe disability. No cases of PML were identified in active-controlled MS clinical trials with ZEPOSIA. PML has been reported in patients treated with S1P receptor modulators and other MS therapies and has been associated with some risk factors. If PML is suspected, withhold ZEPOSIA and perform an appropriate diagnostic evaluation. If confirmed, treatment with ZEPOSIA should be discontinued
- In clinical studies, patients who received ZEPOSIA were not to receive concomitant treatment with antineoplastic, non-corticosteroid immunosuppressive, or immune-modulating therapies used for treatment of MS. Concomitant use of ZEPOSIA with any of these therapies would be expected to increase the risk of immunosuppression. When switching to ZEPOSIA from immunosuppressive medications, consider the duration of their effects and their mode of action to avoid unintended additive immunosuppressive effects
- Use of live attenuated vaccines should be avoided during and for 3 months after treatment with ZEPOSIA. If live attenuated vaccine immunizations are required, administer at least 1 month prior to initiation of ZEPOSIA
Bradyarrhythmia and Atrioventricular Conduction Delays: Since initiation of ZEPOSIA may result in a transient decrease in heart rate and atrioventricular conduction delays, dose titration is recommended to help reduce cardiac effects. Initiation of ZEPOSIA without dose escalation may result in greater decreases in heart rate. If treatment with ZEPOSIA is considered, advice from a cardiologist should be sought for those individuals:
- with significant QT prolongation
- with arrhythmias requiring treatment with Class 1a or III anti-arrhythmic drugs
- with ischemic heart disease, heart failure, history of cardiac arrest or myocardial infarction, cerebrovascular disease, and uncontrolled hypertension
- with a history of Mobitz type II second-degree or higher AV block, sick-sinus syndrome, or sinoatrial heart block
Liver Injury: Elevations of aminotransferases may occur in patients receiving ZEPOSIA. Obtain liver function tests, if not recently available (i.e., within 6 months), before initiation of ZEPOSIA. Patients who develop symptoms suggestive of hepatic dysfunction should have hepatic enzymes checked and ZEPOSIA should be discontinued if significant liver injury is confirmed. Caution should be exercised when using ZEPOSIA in patients with history of significant liver disease
Fetal Risk: There are no adequate and well-controlled studies in pregnant women. Based on animal studies, ZEPOSIA may cause fetal harm. Women of childbearing potential should use effective contraception to avoid pregnancy during treatment and for 3 months after stopping ZEPOSIA
Increased Blood Pressure: Increase in systolic pressure was observed after about 3 months of treatment and persisted throughout treatment. Blood pressure should be monitored during treatment and managed appropriately. Certain foods that may contain very high amounts of tyramine could cause severe hypertension in patients taking ZEPOSIA. Patients should be advised to avoid foods containing a very large amount of tyramine while taking ZEPOSIA
Respiratory Effects: ZEPOSIA may cause a decline in pulmonary function. Spirometric evaluation of respiratory function should be performed during therapy, if clinically indicated
Macular edema: S1P modulators have been associated with an increased risk of macular edema. Patients with a history of uveitis or diabetes mellitus are at increased risk. Patients with a history of these conditions should have an ophthalmic evaluation of the fundus, including the macula, prior to treatment initiation and regular follow-up examinations. An ophthalmic evaluation is recommended in all patients at any time if there is a change in vision. Continued use of ZEPOSIA in patients with macular edema has not been evaluated; potential benefits and risks for the individual patient should be considered if deciding whether ZEPOSIA should be discontinued
Posterior Reversible Encephalopathy Syndrome (PRES): Rare cases of PRES have been reported in patients receiving a S1P receptor modulator. If a ZEPOSIA-treated patient develops unexpected neurological or psychiatric symptoms or any symptom/sign suggestive of an increase in intracranial pressure, a complete physical and neurological examination should be conducted. Symptoms of PRES are usually reversible but may evolve into ischemic stroke or cerebral hemorrhage. Delay in diagnosis and treatment may lead to permanent neurological sequelae. If PRES is suspected, treatment with ZEPOSIA should be discontinued
Unintended Additive Immunosuppressive Effects From Prior Immunosuppressive or Immune-Modulating Drugs: When switching from drugs with prolonged immune effects, the half-life and mode of action of these drugs must be considered to avoid unintended additive immunosuppressive effects while at the same time minimizing risk of disease reactivation. Initiating treatment with ZEPOSIA after treatment with alemtuzumab is not recommended
Severe Increase in Disability After Stopping ZEPOSIA: Severe exacerbation of disease, including disease rebound, has been rarely reported after discontinuation of a S1P receptor modulator. The possibility of severe exacerbation of disease should be considered after stopping ZEPOSIA treatment so patients should be monitored upon discontinuation
Immune System Effects After Stopping ZEPOSIA: After discontinuing ZEPOSIA, the median time for lymphocyte counts to return to the normal range was 30 days with approximately 90% of patients in the normal range within 3 months. Use of immunosuppressants within this period may lead to an additive effect on the immune system, therefore caution should be applied when initiating other drugs 4 weeks after the last dose of ZEPOSIA
Most common Adverse Reactions (≥ 4%): upper respiratory infection, hepatic transaminase elevation, orthostatic hypotension, urinary tract infection, back pain, and hypertension.
For additional safety information, please see the full Prescribing Information and Medication Guide.
1. ZEPOSIA (ozanimod) capsules for oral use. Celgene Corporation. Full prescribing information. 3/2020.
2. National Multiple Sclerosis Society. Treating RMSS. www.nationalmssociety.org/What-is-MS/Types-of-MS/Relapsing-remitting-MS/Treatment. Accessed on April 24, 2020.
3. GILENYA (fingolimod) capsules for oral use. Novartis Pharmaceuticals Corporation. Full prescribing information. 8/2019.
4. MAYZENT (siponimod) tablets for oral use. Novartis Pharmaceuticals Corporation. Full prescribing information. 3/2019.
5. Cohen, JA, Comi, G, Selmaj, KW, et al. Safety and efficacy of ozanimod versus interferon beta-1a in relapsing multiple sclerosis (RADIANCE): a multicenter, randomized, 24-month, phase 3 trial. The Lancet: Neurology. DOI: 10.1016/S1474-4422(19)30238-8.
6. Comi, G, Kappos, L, Selmaj, KW, et at. Safety and efficacy of ozanimod versus interferon beta-1a in relapsing multiple sclerosis (SUNBEAM): a multicenter, randomized, minimum 12-month, phase 3 trial. The Lancet: Neurology. DOI: 10.1016/S1474-4422(19)30239-X.
7. McCann Agency. Pivotal Trials for MS Therapies: ZEPOSIA –2 head-to-head with Active Comparator Based on PIs. March 2020.
ZEPOSIA® is a trademark of Bristol-Myers Squibb Company.
© 2020 Bristol-Myers Squibb Company. All Rights Reserved.
US-ZEP-20-1095 10/20
Tumor molecular profiling may help identify ‘exceptional responders’
Virtually all oncologists, at one time or another, have treated a patient who defied the odds and achieved an unexpectedly long-lasting response. These “exceptional responders” are patients who experience a unique response to therapies that have largely failed to be effective for others with similar cancers.
Genetic and molecular mechanisms may partly account for these responses and may offer clues about why the treatment works for only a few and not for others. To delve more deeply into that area of research, the National Cancer Institute (NCI) began the Exceptional Responders Initiative (ERI) with the goal of identifying potential biological processes that may be responsible, at least in part, for these unusual responses.
NCI researchers have now successfully completed a pilot study that analyzed tumor specimens from more than 100 cases, and the study has affirmed the feasibility of this approach.
Of these cases, six were identified as involving potentially clinically actionable germline mutations.
The findings were published online ahead of print in the Journal of the National Cancer Institute.
“Clearly, the analysis and validation of these results will prove critical to determining the success of this approach,” write James M. Ford, MD, and Beverly S. Mitchell, MD, both of Stanford University School of Medicine, California, in an accompanying editorial. “Ultimately, prospective studies of tumors from exceptional responders, particularly to novel, genomically-targeted agents, may provide a powerful approach to cancer treatment discoveries.”
A special case
Molecular profiling technology, including next-generation sequencing, has significantly changed the landscape of the development of cancer therapies, and clinical trials in early drug development are increasingly selecting patients on the basis of molecular alterations.
The ERI grew out of several meetings held by the NCI in 2013 and 2014. It was built on the ability to profile archived tumor material, explained study author S. Percy Ivy, PhD, associate chief of the Investigational Drug Branch in the Division of Cancer Treatment and Diagnosis of the NCI. “This made it possible to collect cases from participating clinicians from all over the country.
“Published cases included patients treated with a targeted therapy but not treated with knowledge of their tumor’s genomics, who then later turned out to have genomic changes that made their tumor exquisitely sensitive to inhibition of a driving pathway,” she said. “There have been published cases as well as cases in the experience of practicing oncologists that seem to do much better than expected.
“We wondered if we could find molecular reasons why tumors respond not only to targeted therapies but also to standard chemotherapy,” said Percy. “If so, we could refine our choice of therapy to patients who are most likely to respond to it.”’
On its website, the NCI writes that there was a particular case that triggered the interest in going ahead with this initiative. Mutations in the TSC1 and NF2 genes, which result in a loss of gene function, were detected in a patient with metastatic bladder cancer. In a clinical trial, the patient was treated with everolimus (Afinitor, Novartis), an inhibitor of the mammalian target of rapamycin (mTORC1), and achieved a complete response with a duration of more than 2 years.
In a separate analysis, researchers sequenced tumors from 96 other individuals with high-grade bladder cancer and identified five TSC1 gene mutations. Tumors were sequenced from 13 patients with bladder cancer who had received everolimus. Results showed that 3 of 4 patients with TSC1 gene mutations experienced some degree of tumor shrinkage after treatment; 8 of 9 patients who did not have the mutation experienced disease progression.
The NCI notes that in “subsequent workshops and discussions, it became obvious that all clinicians have seen a few exceptional responders.”
Testing for feasibility
The aim of the current study was to assess the feasibility and potential usefulness of sequencing DNA and RNA from clinical tumor specimens from patients who had experienced unusually profound or durable responses to systemic therapy.
Its main feasibility goal was to identify at least 100 cases involving exceptional responders whose cases could be analyzed in less than 3 years.
An exceptional patient was defined as one who had experienced a complete response to one or more drugs in which complete responses were seen in fewer than 10% of patients who received similar treatment; or a partial response lasting at least 6 months in which such a response is seen in fewer than 10% of patients who receive similar treatment; or a complete or partial response of a duration that is three times the median response duration represented in the literature for the treatment.
Studying exceptional responders presents many challenges, the first being to define what an exceptional response is and what it is not, explained Ivy. “This definition relies on the existence of data that a particular therapy will produce particular responses in groups of patients with similar tumors, as defined by organ of origin,” she said.
Other challenges include obtaining tumor tissue and all the relevant clinical data, such as the number of prior treatments and the patient’s response, as well as any known molecular characteristics (eg, HER2/NEU amplification, estrogen-receptor expression, germline mutations). “We also do not have data on other exposures, such as smoking or chemical exposure,” she said. “In addition, when patients are not on clinical trial, the data are not uniformly obtained ― such as that scans may not be performed at particular intervals.”
Importantly, the molecular tools used to analyze tumors were not available in the past, so many trials did not collect tumor tissue for subsequent research. “Even now, we are learning that there are characteristics beyond DNA and RNA that are potentially important to the ability of a tumor to respond, such as the immune system or epigenetic changes,” she said.
From August 2014 to July 2017, a total of 520 cases were proposed by clinicians as possibly involving exceptional responders, and 222 cases met the criteria.
Analyzable tissue was available for 117 patients. Most of the responders (n = 80, 68.4%) had been treated with combination chemotherapy regimens; 34 patients (29.0%) had received one or more antiangiogenesis agents. In addition, six patients had an exceptional response following treatment with immune checkpoint inhibitors. The final analysis included 109 cases.
One exceptional responder was a woman with metastatic squamous lung cancer that was treated with paclitaxel and carboplatin. The patient achieved a 41-month complete response (expected rate, <10%). Another patient with esophageal adenocarcinoma who was treated with docetaxel and cisplatin experienced a partial response that lasted 128 months (reported median response duration, 24 months). After the patient’s tumor recurred, he experienced for the second time a response to concurrent chemoradiation with the same drug regimen.
Overall, potentially clinically relevant germline mutations were identified in six tumors. Pathogenic BRCA1 or BRCA2 mutations were found in two breast cancer patients, one patient with non–small cell lung cancer, and one patient with rectal cancer. A breast cancer patient had a pathogenic BRCA1 germline mutation, and another had a likely germline mutation in CHEK2. A patient with poorly differentiated lung cancer and a history of breast cancer had a PALB2 mutation.
Future steps
Molecular mechanisms are important, but other factors could also play a role in eliciting a response. One is the presence of comorbidities, which was not assessed in the study. Ivy noted that comorbidities could be very important to responses, along with medications that the patient is using for different types of ailments. In addition, the use of complementary and alternative medicines may also have an impact.
“As the field matures, we hope that others will collect these and other characteristics, so that all the data could be used to develop hypotheses about molecular and other factors that can better predict response or resistance,” she said.
The results from this pilot study demonstrated feasibility. Ivy noted that “additional collaboration in similar studies would be welcome, as would methods to use data from various sources to improve our ability to correlate patient characteristics, tumor characteristics and response.
“We envision a larger national and international effort to collect more exceptional responder cases, including more from patients treated with targeted therapies,” she added. “The NCI has been meeting with an interest group that focuses on ER cases in the UK, France, Italy, Canada, and Australia, and this collaborative effort is maturing, albeit slowly.”
The project has been funded in whole or in part with federal funds from the NCI and NIH. Ivy has disclosed no relevant financial relationships. Several coauthors report relationships with industry. The editorialists have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Virtually all oncologists, at one time or another, have treated a patient who defied the odds and achieved an unexpectedly long-lasting response. These “exceptional responders” are patients who experience a unique response to therapies that have largely failed to be effective for others with similar cancers.
Genetic and molecular mechanisms may partly account for these responses and may offer clues about why the treatment works for only a few and not for others. To delve more deeply into that area of research, the National Cancer Institute (NCI) began the Exceptional Responders Initiative (ERI) with the goal of identifying potential biological processes that may be responsible, at least in part, for these unusual responses.
NCI researchers have now successfully completed a pilot study that analyzed tumor specimens from more than 100 cases, and the study has affirmed the feasibility of this approach.
Of these cases, six were identified as involving potentially clinically actionable germline mutations.
The findings were published online ahead of print in the Journal of the National Cancer Institute.
“Clearly, the analysis and validation of these results will prove critical to determining the success of this approach,” write James M. Ford, MD, and Beverly S. Mitchell, MD, both of Stanford University School of Medicine, California, in an accompanying editorial. “Ultimately, prospective studies of tumors from exceptional responders, particularly to novel, genomically-targeted agents, may provide a powerful approach to cancer treatment discoveries.”
A special case
Molecular profiling technology, including next-generation sequencing, has significantly changed the landscape of the development of cancer therapies, and clinical trials in early drug development are increasingly selecting patients on the basis of molecular alterations.
The ERI grew out of several meetings held by the NCI in 2013 and 2014. It was built on the ability to profile archived tumor material, explained study author S. Percy Ivy, PhD, associate chief of the Investigational Drug Branch in the Division of Cancer Treatment and Diagnosis of the NCI. “This made it possible to collect cases from participating clinicians from all over the country.
“Published cases included patients treated with a targeted therapy but not treated with knowledge of their tumor’s genomics, who then later turned out to have genomic changes that made their tumor exquisitely sensitive to inhibition of a driving pathway,” she said. “There have been published cases as well as cases in the experience of practicing oncologists that seem to do much better than expected.
“We wondered if we could find molecular reasons why tumors respond not only to targeted therapies but also to standard chemotherapy,” said Percy. “If so, we could refine our choice of therapy to patients who are most likely to respond to it.”’
On its website, the NCI writes that there was a particular case that triggered the interest in going ahead with this initiative. Mutations in the TSC1 and NF2 genes, which result in a loss of gene function, were detected in a patient with metastatic bladder cancer. In a clinical trial, the patient was treated with everolimus (Afinitor, Novartis), an inhibitor of the mammalian target of rapamycin (mTORC1), and achieved a complete response with a duration of more than 2 years.
In a separate analysis, researchers sequenced tumors from 96 other individuals with high-grade bladder cancer and identified five TSC1 gene mutations. Tumors were sequenced from 13 patients with bladder cancer who had received everolimus. Results showed that 3 of 4 patients with TSC1 gene mutations experienced some degree of tumor shrinkage after treatment; 8 of 9 patients who did not have the mutation experienced disease progression.
The NCI notes that in “subsequent workshops and discussions, it became obvious that all clinicians have seen a few exceptional responders.”
Testing for feasibility
The aim of the current study was to assess the feasibility and potential usefulness of sequencing DNA and RNA from clinical tumor specimens from patients who had experienced unusually profound or durable responses to systemic therapy.
Its main feasibility goal was to identify at least 100 cases involving exceptional responders whose cases could be analyzed in less than 3 years.
An exceptional patient was defined as one who had experienced a complete response to one or more drugs in which complete responses were seen in fewer than 10% of patients who received similar treatment; or a partial response lasting at least 6 months in which such a response is seen in fewer than 10% of patients who receive similar treatment; or a complete or partial response of a duration that is three times the median response duration represented in the literature for the treatment.
Studying exceptional responders presents many challenges, the first being to define what an exceptional response is and what it is not, explained Ivy. “This definition relies on the existence of data that a particular therapy will produce particular responses in groups of patients with similar tumors, as defined by organ of origin,” she said.
Other challenges include obtaining tumor tissue and all the relevant clinical data, such as the number of prior treatments and the patient’s response, as well as any known molecular characteristics (eg, HER2/NEU amplification, estrogen-receptor expression, germline mutations). “We also do not have data on other exposures, such as smoking or chemical exposure,” she said. “In addition, when patients are not on clinical trial, the data are not uniformly obtained ― such as that scans may not be performed at particular intervals.”
Importantly, the molecular tools used to analyze tumors were not available in the past, so many trials did not collect tumor tissue for subsequent research. “Even now, we are learning that there are characteristics beyond DNA and RNA that are potentially important to the ability of a tumor to respond, such as the immune system or epigenetic changes,” she said.
From August 2014 to July 2017, a total of 520 cases were proposed by clinicians as possibly involving exceptional responders, and 222 cases met the criteria.
Analyzable tissue was available for 117 patients. Most of the responders (n = 80, 68.4%) had been treated with combination chemotherapy regimens; 34 patients (29.0%) had received one or more antiangiogenesis agents. In addition, six patients had an exceptional response following treatment with immune checkpoint inhibitors. The final analysis included 109 cases.
One exceptional responder was a woman with metastatic squamous lung cancer that was treated with paclitaxel and carboplatin. The patient achieved a 41-month complete response (expected rate, <10%). Another patient with esophageal adenocarcinoma who was treated with docetaxel and cisplatin experienced a partial response that lasted 128 months (reported median response duration, 24 months). After the patient’s tumor recurred, he experienced for the second time a response to concurrent chemoradiation with the same drug regimen.
Overall, potentially clinically relevant germline mutations were identified in six tumors. Pathogenic BRCA1 or BRCA2 mutations were found in two breast cancer patients, one patient with non–small cell lung cancer, and one patient with rectal cancer. A breast cancer patient had a pathogenic BRCA1 germline mutation, and another had a likely germline mutation in CHEK2. A patient with poorly differentiated lung cancer and a history of breast cancer had a PALB2 mutation.
Future steps
Molecular mechanisms are important, but other factors could also play a role in eliciting a response. One is the presence of comorbidities, which was not assessed in the study. Ivy noted that comorbidities could be very important to responses, along with medications that the patient is using for different types of ailments. In addition, the use of complementary and alternative medicines may also have an impact.
“As the field matures, we hope that others will collect these and other characteristics, so that all the data could be used to develop hypotheses about molecular and other factors that can better predict response or resistance,” she said.
The results from this pilot study demonstrated feasibility. Ivy noted that “additional collaboration in similar studies would be welcome, as would methods to use data from various sources to improve our ability to correlate patient characteristics, tumor characteristics and response.
“We envision a larger national and international effort to collect more exceptional responder cases, including more from patients treated with targeted therapies,” she added. “The NCI has been meeting with an interest group that focuses on ER cases in the UK, France, Italy, Canada, and Australia, and this collaborative effort is maturing, albeit slowly.”
The project has been funded in whole or in part with federal funds from the NCI and NIH. Ivy has disclosed no relevant financial relationships. Several coauthors report relationships with industry. The editorialists have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Virtually all oncologists, at one time or another, have treated a patient who defied the odds and achieved an unexpectedly long-lasting response. These “exceptional responders” are patients who experience a unique response to therapies that have largely failed to be effective for others with similar cancers.
Genetic and molecular mechanisms may partly account for these responses and may offer clues about why the treatment works for only a few and not for others. To delve more deeply into that area of research, the National Cancer Institute (NCI) began the Exceptional Responders Initiative (ERI) with the goal of identifying potential biological processes that may be responsible, at least in part, for these unusual responses.
NCI researchers have now successfully completed a pilot study that analyzed tumor specimens from more than 100 cases, and the study has affirmed the feasibility of this approach.
Of these cases, six were identified as involving potentially clinically actionable germline mutations.
The findings were published online ahead of print in the Journal of the National Cancer Institute.
“Clearly, the analysis and validation of these results will prove critical to determining the success of this approach,” write James M. Ford, MD, and Beverly S. Mitchell, MD, both of Stanford University School of Medicine, California, in an accompanying editorial. “Ultimately, prospective studies of tumors from exceptional responders, particularly to novel, genomically-targeted agents, may provide a powerful approach to cancer treatment discoveries.”
A special case
Molecular profiling technology, including next-generation sequencing, has significantly changed the landscape of the development of cancer therapies, and clinical trials in early drug development are increasingly selecting patients on the basis of molecular alterations.
The ERI grew out of several meetings held by the NCI in 2013 and 2014. It was built on the ability to profile archived tumor material, explained study author S. Percy Ivy, PhD, associate chief of the Investigational Drug Branch in the Division of Cancer Treatment and Diagnosis of the NCI. “This made it possible to collect cases from participating clinicians from all over the country.
“Published cases included patients treated with a targeted therapy but not treated with knowledge of their tumor’s genomics, who then later turned out to have genomic changes that made their tumor exquisitely sensitive to inhibition of a driving pathway,” she said. “There have been published cases as well as cases in the experience of practicing oncologists that seem to do much better than expected.
“We wondered if we could find molecular reasons why tumors respond not only to targeted therapies but also to standard chemotherapy,” said Percy. “If so, we could refine our choice of therapy to patients who are most likely to respond to it.”’
On its website, the NCI writes that there was a particular case that triggered the interest in going ahead with this initiative. Mutations in the TSC1 and NF2 genes, which result in a loss of gene function, were detected in a patient with metastatic bladder cancer. In a clinical trial, the patient was treated with everolimus (Afinitor, Novartis), an inhibitor of the mammalian target of rapamycin (mTORC1), and achieved a complete response with a duration of more than 2 years.
In a separate analysis, researchers sequenced tumors from 96 other individuals with high-grade bladder cancer and identified five TSC1 gene mutations. Tumors were sequenced from 13 patients with bladder cancer who had received everolimus. Results showed that 3 of 4 patients with TSC1 gene mutations experienced some degree of tumor shrinkage after treatment; 8 of 9 patients who did not have the mutation experienced disease progression.
The NCI notes that in “subsequent workshops and discussions, it became obvious that all clinicians have seen a few exceptional responders.”
Testing for feasibility
The aim of the current study was to assess the feasibility and potential usefulness of sequencing DNA and RNA from clinical tumor specimens from patients who had experienced unusually profound or durable responses to systemic therapy.
Its main feasibility goal was to identify at least 100 cases involving exceptional responders whose cases could be analyzed in less than 3 years.
An exceptional patient was defined as one who had experienced a complete response to one or more drugs in which complete responses were seen in fewer than 10% of patients who received similar treatment; or a partial response lasting at least 6 months in which such a response is seen in fewer than 10% of patients who receive similar treatment; or a complete or partial response of a duration that is three times the median response duration represented in the literature for the treatment.
Studying exceptional responders presents many challenges, the first being to define what an exceptional response is and what it is not, explained Ivy. “This definition relies on the existence of data that a particular therapy will produce particular responses in groups of patients with similar tumors, as defined by organ of origin,” she said.
Other challenges include obtaining tumor tissue and all the relevant clinical data, such as the number of prior treatments and the patient’s response, as well as any known molecular characteristics (eg, HER2/NEU amplification, estrogen-receptor expression, germline mutations). “We also do not have data on other exposures, such as smoking or chemical exposure,” she said. “In addition, when patients are not on clinical trial, the data are not uniformly obtained ― such as that scans may not be performed at particular intervals.”
Importantly, the molecular tools used to analyze tumors were not available in the past, so many trials did not collect tumor tissue for subsequent research. “Even now, we are learning that there are characteristics beyond DNA and RNA that are potentially important to the ability of a tumor to respond, such as the immune system or epigenetic changes,” she said.
From August 2014 to July 2017, a total of 520 cases were proposed by clinicians as possibly involving exceptional responders, and 222 cases met the criteria.
Analyzable tissue was available for 117 patients. Most of the responders (n = 80, 68.4%) had been treated with combination chemotherapy regimens; 34 patients (29.0%) had received one or more antiangiogenesis agents. In addition, six patients had an exceptional response following treatment with immune checkpoint inhibitors. The final analysis included 109 cases.
One exceptional responder was a woman with metastatic squamous lung cancer that was treated with paclitaxel and carboplatin. The patient achieved a 41-month complete response (expected rate, <10%). Another patient with esophageal adenocarcinoma who was treated with docetaxel and cisplatin experienced a partial response that lasted 128 months (reported median response duration, 24 months). After the patient’s tumor recurred, he experienced for the second time a response to concurrent chemoradiation with the same drug regimen.
Overall, potentially clinically relevant germline mutations were identified in six tumors. Pathogenic BRCA1 or BRCA2 mutations were found in two breast cancer patients, one patient with non–small cell lung cancer, and one patient with rectal cancer. A breast cancer patient had a pathogenic BRCA1 germline mutation, and another had a likely germline mutation in CHEK2. A patient with poorly differentiated lung cancer and a history of breast cancer had a PALB2 mutation.
Future steps
Molecular mechanisms are important, but other factors could also play a role in eliciting a response. One is the presence of comorbidities, which was not assessed in the study. Ivy noted that comorbidities could be very important to responses, along with medications that the patient is using for different types of ailments. In addition, the use of complementary and alternative medicines may also have an impact.
“As the field matures, we hope that others will collect these and other characteristics, so that all the data could be used to develop hypotheses about molecular and other factors that can better predict response or resistance,” she said.
The results from this pilot study demonstrated feasibility. Ivy noted that “additional collaboration in similar studies would be welcome, as would methods to use data from various sources to improve our ability to correlate patient characteristics, tumor characteristics and response.
“We envision a larger national and international effort to collect more exceptional responder cases, including more from patients treated with targeted therapies,” she added. “The NCI has been meeting with an interest group that focuses on ER cases in the UK, France, Italy, Canada, and Australia, and this collaborative effort is maturing, albeit slowly.”
The project has been funded in whole or in part with federal funds from the NCI and NIH. Ivy has disclosed no relevant financial relationships. Several coauthors report relationships with industry. The editorialists have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Large COVID-19 dataset: Kidney injury in >35% of those in hospital
As a new report shows that over a third of U.S. patients hospitalized with COVID-19 developed acute kidney injury (AKI), and nearly 15% of these patients needed dialysis, experts in the field are calling for more robust research into multiple aspects of this increasingly important issue.
Among 5,449 patients admitted to 13 Northwell Health New York–based hospitals between March and April 2020, 36.6% (1,993) developed AKI.
– the rate of kidney injury was 89.7% among ventilated patients, compared with 21.7% among other patients.
AKI in COVID-19 was also linked to a poor prognosis: 35% of those who developed AKI had died at the time of publication.
The study includes the largest defined cohort of hospitalized COVID-19 patients to date with a focus on AKI, says Jamie S. Hirsch, MD, of Northwell Health in Great Neck, N.Y., and colleagues in their article published online in Kidney International.
The findings track with those of a study of New York hospitals published online in The Lancet. In that dataset, just under a third (31%) of critically ill patients developed severe kidney damage and needed dialysis.
Both of these studies help solidify the experiences of clinicians on the ground, with many U.S. hospitals in the early phases of the pandemic underestimating the problem of AKI and having to scramble to find enough dialysis machines and dialysate solution to treat the most severely affected patients.
“We hope to learn more about the COVID-19–related AKI in the coming weeks, and that by sharing what we have learned from our patients, other doctors and their patients can benefit,” said senior author of the new study, Kenar D. Jhaveri, MD, associated chief of nephrology at Hofstra/Northwell.
The new report also comes as scientists from the National Institute of Diabetes and Digestive and Kidney Diseases highlighted the importance of AKI as a sequela of COVID-19 in an editorial published in Diabetes Care.
They, too, said that it is vitally important to better understand what is happening, as more and more hospitals will face COVID-19 patients with this complication.
“The natural history and heterogeneity of the kidney disease caused by COVID-19 need to be unraveled,” one of the authors, Robert A. Star, MD, director of the division of kidney, urologic, and hematologic diseases at NIDDK, said in an interview.
Such research is key because “low kidney function is an exclusion criterion in current studies” examining antiviral medications in COVID-19, he said. “Clinical trials are needed to test therapeutic interventions to prevent or treat COVID-19–induced AKI.”
Extremely ill patients develop AKI as their condition deteriorates
Identifying risk factors for the development of AKI in COVID-19 will be critical in helping shed more light on diagnostic and predictive biomarkers, Dr. Star said.
Dr. Hirsch and colleagues said that extremely ill patients often develop kidney failure as their condition deteriorates, and this happens quickly. Indeed, the clearest risk factors for the development of AKI were “the need for ventilator support or vasopressor drug treatment.”
Other independent predictors of AKI were older age, black race, diabetes, hypertension, and cardiovascular disease.
Of those on mechanical ventilation overall in the more than 5,000-patient study, almost a quarter (23.2%) developed AKI and needed renal replacement therapy, which consisted of either intermittent or continuous hemodialysis.
Dr. Star and associates wrote that these numbers are important because of the knock-on effects.
“Hemodialysis in critically ill infected patients is associated with significant clotting complications and mortality as well as increased infection risk to staff,” they pointed out.
Dr. Star said that “the incidence rate of AKI reported in this study is higher than what had been previously reported by others in the United States and China and may reflect differences in population demographics, severity of illness, prevalence of comorbidities, socioeconomic factors, patient volume overwhelming hospital capacity, or other factors not yet determined.
“It may be caused by dehydration (volume depletion), heart failure, the inflammatory response to the virus (cytokine storm), respiratory failure, clotting of blood vessels (hypercoagulation), muscle tissue breakdown (rhabdomyolysis), and/or a direct viral infection of the kidney,” he said.
Renal biopsies from patients with AKI may help shed some light
The editorialists went on to say that findings from kidney biopsies of COVID-19 patients with AKI may help shed some light on this condition.
“While difficult to perform, kidney biopsies from patients with early AKI could help us understand the underlying pathophysiologies at the cellular and molecular level and begin to target specific treatments to specific subgroups of patients,” they wrote.
The authors noted that, as part of funding opportunities provided by the National Institutes of Health for COVID-19 research, the NIDDK has published a Notice of Special Interest outlining the most urgent areas in need of research, with one of the focuses being on the kidney.
“As the research community emerges from the crisis situation, there should be renewed efforts for multidisciplinary research to conduct integrated basic, translational, and clinical studies aimed at greatly increasing the knowledge base to understand how both the current COVID-19 threat and future health threats affect both healthy people and people with chronic diseases and conditions,” the editorials noted.
The authors of the Diabetes Care editorial have reported no relevant financial relationships. Dr. Jhaveri has reported being a consultant for Astex Pharmaceuticals.
A version of this article originally appeared on Medscape.com.
As a new report shows that over a third of U.S. patients hospitalized with COVID-19 developed acute kidney injury (AKI), and nearly 15% of these patients needed dialysis, experts in the field are calling for more robust research into multiple aspects of this increasingly important issue.
Among 5,449 patients admitted to 13 Northwell Health New York–based hospitals between March and April 2020, 36.6% (1,993) developed AKI.
– the rate of kidney injury was 89.7% among ventilated patients, compared with 21.7% among other patients.
AKI in COVID-19 was also linked to a poor prognosis: 35% of those who developed AKI had died at the time of publication.
The study includes the largest defined cohort of hospitalized COVID-19 patients to date with a focus on AKI, says Jamie S. Hirsch, MD, of Northwell Health in Great Neck, N.Y., and colleagues in their article published online in Kidney International.
The findings track with those of a study of New York hospitals published online in The Lancet. In that dataset, just under a third (31%) of critically ill patients developed severe kidney damage and needed dialysis.
Both of these studies help solidify the experiences of clinicians on the ground, with many U.S. hospitals in the early phases of the pandemic underestimating the problem of AKI and having to scramble to find enough dialysis machines and dialysate solution to treat the most severely affected patients.
“We hope to learn more about the COVID-19–related AKI in the coming weeks, and that by sharing what we have learned from our patients, other doctors and their patients can benefit,” said senior author of the new study, Kenar D. Jhaveri, MD, associated chief of nephrology at Hofstra/Northwell.
The new report also comes as scientists from the National Institute of Diabetes and Digestive and Kidney Diseases highlighted the importance of AKI as a sequela of COVID-19 in an editorial published in Diabetes Care.
They, too, said that it is vitally important to better understand what is happening, as more and more hospitals will face COVID-19 patients with this complication.
“The natural history and heterogeneity of the kidney disease caused by COVID-19 need to be unraveled,” one of the authors, Robert A. Star, MD, director of the division of kidney, urologic, and hematologic diseases at NIDDK, said in an interview.
Such research is key because “low kidney function is an exclusion criterion in current studies” examining antiviral medications in COVID-19, he said. “Clinical trials are needed to test therapeutic interventions to prevent or treat COVID-19–induced AKI.”
Extremely ill patients develop AKI as their condition deteriorates
Identifying risk factors for the development of AKI in COVID-19 will be critical in helping shed more light on diagnostic and predictive biomarkers, Dr. Star said.
Dr. Hirsch and colleagues said that extremely ill patients often develop kidney failure as their condition deteriorates, and this happens quickly. Indeed, the clearest risk factors for the development of AKI were “the need for ventilator support or vasopressor drug treatment.”
Other independent predictors of AKI were older age, black race, diabetes, hypertension, and cardiovascular disease.
Of those on mechanical ventilation overall in the more than 5,000-patient study, almost a quarter (23.2%) developed AKI and needed renal replacement therapy, which consisted of either intermittent or continuous hemodialysis.
Dr. Star and associates wrote that these numbers are important because of the knock-on effects.
“Hemodialysis in critically ill infected patients is associated with significant clotting complications and mortality as well as increased infection risk to staff,” they pointed out.
Dr. Star said that “the incidence rate of AKI reported in this study is higher than what had been previously reported by others in the United States and China and may reflect differences in population demographics, severity of illness, prevalence of comorbidities, socioeconomic factors, patient volume overwhelming hospital capacity, or other factors not yet determined.
“It may be caused by dehydration (volume depletion), heart failure, the inflammatory response to the virus (cytokine storm), respiratory failure, clotting of blood vessels (hypercoagulation), muscle tissue breakdown (rhabdomyolysis), and/or a direct viral infection of the kidney,” he said.
Renal biopsies from patients with AKI may help shed some light
The editorialists went on to say that findings from kidney biopsies of COVID-19 patients with AKI may help shed some light on this condition.
“While difficult to perform, kidney biopsies from patients with early AKI could help us understand the underlying pathophysiologies at the cellular and molecular level and begin to target specific treatments to specific subgroups of patients,” they wrote.
The authors noted that, as part of funding opportunities provided by the National Institutes of Health for COVID-19 research, the NIDDK has published a Notice of Special Interest outlining the most urgent areas in need of research, with one of the focuses being on the kidney.
“As the research community emerges from the crisis situation, there should be renewed efforts for multidisciplinary research to conduct integrated basic, translational, and clinical studies aimed at greatly increasing the knowledge base to understand how both the current COVID-19 threat and future health threats affect both healthy people and people with chronic diseases and conditions,” the editorials noted.
The authors of the Diabetes Care editorial have reported no relevant financial relationships. Dr. Jhaveri has reported being a consultant for Astex Pharmaceuticals.
A version of this article originally appeared on Medscape.com.
As a new report shows that over a third of U.S. patients hospitalized with COVID-19 developed acute kidney injury (AKI), and nearly 15% of these patients needed dialysis, experts in the field are calling for more robust research into multiple aspects of this increasingly important issue.
Among 5,449 patients admitted to 13 Northwell Health New York–based hospitals between March and April 2020, 36.6% (1,993) developed AKI.
– the rate of kidney injury was 89.7% among ventilated patients, compared with 21.7% among other patients.
AKI in COVID-19 was also linked to a poor prognosis: 35% of those who developed AKI had died at the time of publication.
The study includes the largest defined cohort of hospitalized COVID-19 patients to date with a focus on AKI, says Jamie S. Hirsch, MD, of Northwell Health in Great Neck, N.Y., and colleagues in their article published online in Kidney International.
The findings track with those of a study of New York hospitals published online in The Lancet. In that dataset, just under a third (31%) of critically ill patients developed severe kidney damage and needed dialysis.
Both of these studies help solidify the experiences of clinicians on the ground, with many U.S. hospitals in the early phases of the pandemic underestimating the problem of AKI and having to scramble to find enough dialysis machines and dialysate solution to treat the most severely affected patients.
“We hope to learn more about the COVID-19–related AKI in the coming weeks, and that by sharing what we have learned from our patients, other doctors and their patients can benefit,” said senior author of the new study, Kenar D. Jhaveri, MD, associated chief of nephrology at Hofstra/Northwell.
The new report also comes as scientists from the National Institute of Diabetes and Digestive and Kidney Diseases highlighted the importance of AKI as a sequela of COVID-19 in an editorial published in Diabetes Care.
They, too, said that it is vitally important to better understand what is happening, as more and more hospitals will face COVID-19 patients with this complication.
“The natural history and heterogeneity of the kidney disease caused by COVID-19 need to be unraveled,” one of the authors, Robert A. Star, MD, director of the division of kidney, urologic, and hematologic diseases at NIDDK, said in an interview.
Such research is key because “low kidney function is an exclusion criterion in current studies” examining antiviral medications in COVID-19, he said. “Clinical trials are needed to test therapeutic interventions to prevent or treat COVID-19–induced AKI.”
Extremely ill patients develop AKI as their condition deteriorates
Identifying risk factors for the development of AKI in COVID-19 will be critical in helping shed more light on diagnostic and predictive biomarkers, Dr. Star said.
Dr. Hirsch and colleagues said that extremely ill patients often develop kidney failure as their condition deteriorates, and this happens quickly. Indeed, the clearest risk factors for the development of AKI were “the need for ventilator support or vasopressor drug treatment.”
Other independent predictors of AKI were older age, black race, diabetes, hypertension, and cardiovascular disease.
Of those on mechanical ventilation overall in the more than 5,000-patient study, almost a quarter (23.2%) developed AKI and needed renal replacement therapy, which consisted of either intermittent or continuous hemodialysis.
Dr. Star and associates wrote that these numbers are important because of the knock-on effects.
“Hemodialysis in critically ill infected patients is associated with significant clotting complications and mortality as well as increased infection risk to staff,” they pointed out.
Dr. Star said that “the incidence rate of AKI reported in this study is higher than what had been previously reported by others in the United States and China and may reflect differences in population demographics, severity of illness, prevalence of comorbidities, socioeconomic factors, patient volume overwhelming hospital capacity, or other factors not yet determined.
“It may be caused by dehydration (volume depletion), heart failure, the inflammatory response to the virus (cytokine storm), respiratory failure, clotting of blood vessels (hypercoagulation), muscle tissue breakdown (rhabdomyolysis), and/or a direct viral infection of the kidney,” he said.
Renal biopsies from patients with AKI may help shed some light
The editorialists went on to say that findings from kidney biopsies of COVID-19 patients with AKI may help shed some light on this condition.
“While difficult to perform, kidney biopsies from patients with early AKI could help us understand the underlying pathophysiologies at the cellular and molecular level and begin to target specific treatments to specific subgroups of patients,” they wrote.
The authors noted that, as part of funding opportunities provided by the National Institutes of Health for COVID-19 research, the NIDDK has published a Notice of Special Interest outlining the most urgent areas in need of research, with one of the focuses being on the kidney.
“As the research community emerges from the crisis situation, there should be renewed efforts for multidisciplinary research to conduct integrated basic, translational, and clinical studies aimed at greatly increasing the knowledge base to understand how both the current COVID-19 threat and future health threats affect both healthy people and people with chronic diseases and conditions,” the editorials noted.
The authors of the Diabetes Care editorial have reported no relevant financial relationships. Dr. Jhaveri has reported being a consultant for Astex Pharmaceuticals.
A version of this article originally appeared on Medscape.com.
AAN publishes ethical guidance on patient care during the pandemic
The document, which was published online May 15 in Neurology, reviews adaptations to the inpatient and outpatient settings and addresses the need to develop protocols for the allocation of scarce medical resources. The guidance is the product of a joint committee of the AAN, the American Neurological Association, the Child Neurology Society, and the Neurocritical Care Society Ethics Committee.
“Now is one of the most challenging times of our careers as neurologists,” said James C. Stevens, MD, president of the AAN, in a press release. “Clinics and hospitals are adapting to caring for the most ill, managing scarce resources, and trying to protect people without the disease. As neurologists, we must continue to adapt our daily practice, continue to care for our most ill neurology patients, and help contribute to the care of those afflicted with COVID-19.”
The role of telehealth
The authors recommended that ordinary appointments be held using telehealth, which, they say, already has become part of patient care. Telehealth enables neurologists to continue providing care while reducing the risk of exposure to and spread of SARS-CoV-2. The disadvantages of telehealth are that it limits physical examinations and behavioral health examinations, the authors acknowledged. “Each clinician should decide, in concert with his or her patient, if an in-person evaluation warrants the risk of an encounter,” according to the guidance.
Neurologists also should advise their patients that their neurologic condition could affect their relative risk of hospitalization and death resulting from COVID-19. Patients with multiple sclerosis or myasthenia gravis, for example, may be receiving corticosteroids or immunomodulatory therapies that make them more vulnerable to COVID-19 infection. “Even if desired services are available, neurologists and their patients ought to consider whether their care plans can safely be delayed in order to mitigate risk,” wrote the authors. Neurologists must try to maintain the customary standard of care, however, for patients with neurologic disease severe enough to warrant hospitalization, such as stroke or epilepsy.
The potential need for triage
Resources such as ventilators and ICU beds are limited, and health care facilities have had to triage them during the pandemic. Patients with a neurologic disease that decreases their likelihood of survival from a respiratory illness may not be offered these resources. Neurologists should discuss with patients and decision makers the ways in which reduced resources might affect patient care. Neurologists must “be aware of the burden of disease in their local community and how healthcare leaders plan on coping with a surge,” according to the guidance.
Advance directives, which should be a standard part of clinical care, take on increased importance during the pandemic. Patients who have not completed advance care planning documents should be encouraged to do so, according to the authors. These documents include patients’ preferences for “do not attempt resuscitation” status. Nevertheless, “we must assure patients with chronic illness that diminished resources in this healthcare crisis will not restrict their access to comfort and palliative care,” the document states.
Scarce resource allocation protocols
In the event that a surge in patients overwhelms a hospital’s contingencies and forces it to operate in crisis mode, it should have a scarce resource allocation protocol in place.
“This will surely be the most challenging aspect of patient care during this pandemic public health emergency,” wrote the authors. To ensure transparency and to mitigate the emotional effect of these decisions on patients and clinicians, scarce resource allocation protocols should be developed by teams that include intensivists, clinical ethicists, and nursing representatives who are not directly involved in the care of the critically ill patients. The goal of these protocols is to maximize the number of lives saved. They generally include an initial patient assessment followed by regular reevaluations to determine whether patients using scarce resources are benefiting less than other patients who need the same resources. The protocols should consider not only patients with COVID-19 infection, but also patients with stroke, traumatic injury, influenza, and heart failure who may need the same resources. Race, gender, ethnicity, socioeconomics, and perceived social worth should not influence care decisions, according to the guidance. Validated mortality prediction scales, such as the Glasgow Outcome Scale, can contribute to care decisions. Obtaining community input into these protocols will ensure trust in the health care system.
“If the situation necessitates hard decisions, we need to be fair, objective, transparent, and adamantly preserve our professional integrity,” wrote the authors. “Through it all, we owe it to our patients and families, as well as ourselves, to maintain our own health and wellness.”
The guidance was developed without funding, and the authors reported no relevant disclosures.
SOURCE: Rubin MA et al. Neurology. 2020 May 15. doi: 10.1212/WNL.0000000000009744.
The document, which was published online May 15 in Neurology, reviews adaptations to the inpatient and outpatient settings and addresses the need to develop protocols for the allocation of scarce medical resources. The guidance is the product of a joint committee of the AAN, the American Neurological Association, the Child Neurology Society, and the Neurocritical Care Society Ethics Committee.
“Now is one of the most challenging times of our careers as neurologists,” said James C. Stevens, MD, president of the AAN, in a press release. “Clinics and hospitals are adapting to caring for the most ill, managing scarce resources, and trying to protect people without the disease. As neurologists, we must continue to adapt our daily practice, continue to care for our most ill neurology patients, and help contribute to the care of those afflicted with COVID-19.”
The role of telehealth
The authors recommended that ordinary appointments be held using telehealth, which, they say, already has become part of patient care. Telehealth enables neurologists to continue providing care while reducing the risk of exposure to and spread of SARS-CoV-2. The disadvantages of telehealth are that it limits physical examinations and behavioral health examinations, the authors acknowledged. “Each clinician should decide, in concert with his or her patient, if an in-person evaluation warrants the risk of an encounter,” according to the guidance.
Neurologists also should advise their patients that their neurologic condition could affect their relative risk of hospitalization and death resulting from COVID-19. Patients with multiple sclerosis or myasthenia gravis, for example, may be receiving corticosteroids or immunomodulatory therapies that make them more vulnerable to COVID-19 infection. “Even if desired services are available, neurologists and their patients ought to consider whether their care plans can safely be delayed in order to mitigate risk,” wrote the authors. Neurologists must try to maintain the customary standard of care, however, for patients with neurologic disease severe enough to warrant hospitalization, such as stroke or epilepsy.
The potential need for triage
Resources such as ventilators and ICU beds are limited, and health care facilities have had to triage them during the pandemic. Patients with a neurologic disease that decreases their likelihood of survival from a respiratory illness may not be offered these resources. Neurologists should discuss with patients and decision makers the ways in which reduced resources might affect patient care. Neurologists must “be aware of the burden of disease in their local community and how healthcare leaders plan on coping with a surge,” according to the guidance.
Advance directives, which should be a standard part of clinical care, take on increased importance during the pandemic. Patients who have not completed advance care planning documents should be encouraged to do so, according to the authors. These documents include patients’ preferences for “do not attempt resuscitation” status. Nevertheless, “we must assure patients with chronic illness that diminished resources in this healthcare crisis will not restrict their access to comfort and palliative care,” the document states.
Scarce resource allocation protocols
In the event that a surge in patients overwhelms a hospital’s contingencies and forces it to operate in crisis mode, it should have a scarce resource allocation protocol in place.
“This will surely be the most challenging aspect of patient care during this pandemic public health emergency,” wrote the authors. To ensure transparency and to mitigate the emotional effect of these decisions on patients and clinicians, scarce resource allocation protocols should be developed by teams that include intensivists, clinical ethicists, and nursing representatives who are not directly involved in the care of the critically ill patients. The goal of these protocols is to maximize the number of lives saved. They generally include an initial patient assessment followed by regular reevaluations to determine whether patients using scarce resources are benefiting less than other patients who need the same resources. The protocols should consider not only patients with COVID-19 infection, but also patients with stroke, traumatic injury, influenza, and heart failure who may need the same resources. Race, gender, ethnicity, socioeconomics, and perceived social worth should not influence care decisions, according to the guidance. Validated mortality prediction scales, such as the Glasgow Outcome Scale, can contribute to care decisions. Obtaining community input into these protocols will ensure trust in the health care system.
“If the situation necessitates hard decisions, we need to be fair, objective, transparent, and adamantly preserve our professional integrity,” wrote the authors. “Through it all, we owe it to our patients and families, as well as ourselves, to maintain our own health and wellness.”
The guidance was developed without funding, and the authors reported no relevant disclosures.
SOURCE: Rubin MA et al. Neurology. 2020 May 15. doi: 10.1212/WNL.0000000000009744.
The document, which was published online May 15 in Neurology, reviews adaptations to the inpatient and outpatient settings and addresses the need to develop protocols for the allocation of scarce medical resources. The guidance is the product of a joint committee of the AAN, the American Neurological Association, the Child Neurology Society, and the Neurocritical Care Society Ethics Committee.
“Now is one of the most challenging times of our careers as neurologists,” said James C. Stevens, MD, president of the AAN, in a press release. “Clinics and hospitals are adapting to caring for the most ill, managing scarce resources, and trying to protect people without the disease. As neurologists, we must continue to adapt our daily practice, continue to care for our most ill neurology patients, and help contribute to the care of those afflicted with COVID-19.”
The role of telehealth
The authors recommended that ordinary appointments be held using telehealth, which, they say, already has become part of patient care. Telehealth enables neurologists to continue providing care while reducing the risk of exposure to and spread of SARS-CoV-2. The disadvantages of telehealth are that it limits physical examinations and behavioral health examinations, the authors acknowledged. “Each clinician should decide, in concert with his or her patient, if an in-person evaluation warrants the risk of an encounter,” according to the guidance.
Neurologists also should advise their patients that their neurologic condition could affect their relative risk of hospitalization and death resulting from COVID-19. Patients with multiple sclerosis or myasthenia gravis, for example, may be receiving corticosteroids or immunomodulatory therapies that make them more vulnerable to COVID-19 infection. “Even if desired services are available, neurologists and their patients ought to consider whether their care plans can safely be delayed in order to mitigate risk,” wrote the authors. Neurologists must try to maintain the customary standard of care, however, for patients with neurologic disease severe enough to warrant hospitalization, such as stroke or epilepsy.
The potential need for triage
Resources such as ventilators and ICU beds are limited, and health care facilities have had to triage them during the pandemic. Patients with a neurologic disease that decreases their likelihood of survival from a respiratory illness may not be offered these resources. Neurologists should discuss with patients and decision makers the ways in which reduced resources might affect patient care. Neurologists must “be aware of the burden of disease in their local community and how healthcare leaders plan on coping with a surge,” according to the guidance.
Advance directives, which should be a standard part of clinical care, take on increased importance during the pandemic. Patients who have not completed advance care planning documents should be encouraged to do so, according to the authors. These documents include patients’ preferences for “do not attempt resuscitation” status. Nevertheless, “we must assure patients with chronic illness that diminished resources in this healthcare crisis will not restrict their access to comfort and palliative care,” the document states.
Scarce resource allocation protocols
In the event that a surge in patients overwhelms a hospital’s contingencies and forces it to operate in crisis mode, it should have a scarce resource allocation protocol in place.
“This will surely be the most challenging aspect of patient care during this pandemic public health emergency,” wrote the authors. To ensure transparency and to mitigate the emotional effect of these decisions on patients and clinicians, scarce resource allocation protocols should be developed by teams that include intensivists, clinical ethicists, and nursing representatives who are not directly involved in the care of the critically ill patients. The goal of these protocols is to maximize the number of lives saved. They generally include an initial patient assessment followed by regular reevaluations to determine whether patients using scarce resources are benefiting less than other patients who need the same resources. The protocols should consider not only patients with COVID-19 infection, but also patients with stroke, traumatic injury, influenza, and heart failure who may need the same resources. Race, gender, ethnicity, socioeconomics, and perceived social worth should not influence care decisions, according to the guidance. Validated mortality prediction scales, such as the Glasgow Outcome Scale, can contribute to care decisions. Obtaining community input into these protocols will ensure trust in the health care system.
“If the situation necessitates hard decisions, we need to be fair, objective, transparent, and adamantly preserve our professional integrity,” wrote the authors. “Through it all, we owe it to our patients and families, as well as ourselves, to maintain our own health and wellness.”
The guidance was developed without funding, and the authors reported no relevant disclosures.
SOURCE: Rubin MA et al. Neurology. 2020 May 15. doi: 10.1212/WNL.0000000000009744.
FROM NEUROLOGY
Advanced prostate cancers still rising in U.S.
The incidence of advanced prostate cancers in the United States “persistently” increased annually for 5 years after the United States Preventive Services Task Force (USPSTF) controversially advised in 2012 against prostate-specific antigen (PSA) screening in men of all ages, new research indicates.
But a biostatistician not involved with the study said the USPSTF’s recommendation is not wholly to blame because “you need 5 to 7 years of lag time at a minimum to influence PSA screening,” and suggested that other factors were at play.
In the new study, Ahmedin Jemal, DVM, PhD, of the American Cancer Society, and colleagues report that for the period 2012–2016 there were yearly statistically significant upticks in the incidence of regional-stage disease (by an absolute 11% per year) and in distant-stage disease (by an absolute 5% per year).
At the same time, there were annual drops in the incidence of localized prostate cancers in men 50 years or older.
The new study is the first to report data out to the end of 2016.
The two trends — the increase in advanced cancers and decrease in early-stage cancers — have been occurring for 10 years, more or less, but with a steady, sharp rise in advanced disease starting in 2010 to 2012, the findings show.
“These data illustrate the trade-off between higher screening rates and more early-stage disease diagnoses (possibly overdiagnosis and overtreatment) and lower screening rates and more late-stage (possibly fatal) disease,” the authors comment.
The study was published online May 20 in the Journal of the National Cancer Institute.
Several previous studies have reported incidence pattern changes following the USPSTF recommendations against PSA screening for men aged 75 or older in 2008 and all men in 2012, but the data went no further than 2015.
“We saw hints of these changes in the past few years and now we have further confirmation,” said Ahmad Shabsigh, MD, urologic oncologist at the Ohio State University Comprehensive Cancer Center, who was asked for independent comment.
“What is a surprise is that it’s every year,” Shabsigh told Medscape Medical News, referring to the advanced cancer incidence increases.
“To see it so clearly in this study is sad,” Shabsigh added.
The study period started in 2005, but did not cover the years after 2018, when USPSTF recommendations changed again and advised that screening be “individualized” for men 55 to 69, and that men 70 and over should be excluded.
US cancer registry data, which are the source of the current study, are not yet available to assess the impact of this most recent change.
End in sight?
There has been a decline in the proportion of men undergoing PSA tests in the US in recent years, the study authors point out.
Routine PSA testing rates among men aged 50 and older declined from 40.6% in 2008 to 38.3% in 2010, and dropped to 31.5% in 2013, a percentage which held again in 2015, per national self-reported survey data.
The study authors say the cause of the rise in advanced cancers is uncertain because of the descriptive nature of their research.
But Andrew Vickers, PhD, a biostatistician at the Memorial Sloan Kettering Cancer Center in New York City, said the rise in advanced cancers and the drop in early-stage cancers reported in the study are “suggestive of a causal relationship” and a “screening effect.”
Vickers argues that there were “a whole bunch of trends that came together in the late [2000s] to influence [PSA] screening.”
For example, two landmark randomized clinical trials of PSA screening first reported “unfavorable” results in 2009, which is during the period covered in the current study, and dampened enthusiasm for screening.
The European Randomized Study of Screening for Prostate Cancer (ERSPC) and the US-based Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial reported little or no effect on mortality, the primary outcome of the trials.
Medscape Medical News asked Vickers to speculate on how long the incidence of advanced prostate cancers will continue to rise in the United States.
“It would surprise me if we had bottomed out [and reached peak increases in advanced cancers] or if we had much longer to go,” he said. “My prediction is that if nothing were to change we will probably see some further increases in [the incidence of] advanced disease.”
What needs to change? Vickers ticked off a list of “golden rules.”
- First, physicians need to get consent for all PSA tests.
- Second, PSA tests should not be administered to older men “who won’t benefit,” such as men 75 years and older with comorbidities such as heart disease.
- Third, PSA testing should be restricted to younger men.
- Fourth, clinicians need to be more restrictive about biopsy. “It used to be if you had a high PSA, you would get a biopsy,” he said, adding that this approach yielded a lot invasive testing in men with low-grade disease. By using additional tests such as the 4Kscore or Prostate Health Index or MRI, clinicians can limit biopsies to men with greater likelihood of a high-grade cancer. Vickers acknowledged conflict of interest on this point, as he is a patent holder of the 4Kscore.
- Fifth, don’t treat men who are very unlikely to benefit, especially men with Gleason grade 6 disease. Use active surveillance for these men, he said. “Using our existing knowledge, I believe we can completely transform the harm-to-benefit ratio of PSA screening. We would drastically reduce overdiagnosis and overtreatment,” he stated.
Additionally, Vickers believes that urologists need to educate local internists and general practitioners and acknowledge that screening and subsequent treatment were “done wrong for a long time.” At the same time, urologists should make it clear that patients will not be biopsied “unless there is a really good reason to believe that they have a high risk of high-grade disease.”
Vickers concluded: “We can reduce the harm and maintain the benefit of screening.”
The study was supported by the American Cancer Society. Jemal and Shabsigh have disclosed no relevant financial relationships. Vickers declared that he is a patent holder of the 4Kscore.
This article first appeared on Medscape.com.
The incidence of advanced prostate cancers in the United States “persistently” increased annually for 5 years after the United States Preventive Services Task Force (USPSTF) controversially advised in 2012 against prostate-specific antigen (PSA) screening in men of all ages, new research indicates.
But a biostatistician not involved with the study said the USPSTF’s recommendation is not wholly to blame because “you need 5 to 7 years of lag time at a minimum to influence PSA screening,” and suggested that other factors were at play.
In the new study, Ahmedin Jemal, DVM, PhD, of the American Cancer Society, and colleagues report that for the period 2012–2016 there were yearly statistically significant upticks in the incidence of regional-stage disease (by an absolute 11% per year) and in distant-stage disease (by an absolute 5% per year).
At the same time, there were annual drops in the incidence of localized prostate cancers in men 50 years or older.
The new study is the first to report data out to the end of 2016.
The two trends — the increase in advanced cancers and decrease in early-stage cancers — have been occurring for 10 years, more or less, but with a steady, sharp rise in advanced disease starting in 2010 to 2012, the findings show.
“These data illustrate the trade-off between higher screening rates and more early-stage disease diagnoses (possibly overdiagnosis and overtreatment) and lower screening rates and more late-stage (possibly fatal) disease,” the authors comment.
The study was published online May 20 in the Journal of the National Cancer Institute.
Several previous studies have reported incidence pattern changes following the USPSTF recommendations against PSA screening for men aged 75 or older in 2008 and all men in 2012, but the data went no further than 2015.
“We saw hints of these changes in the past few years and now we have further confirmation,” said Ahmad Shabsigh, MD, urologic oncologist at the Ohio State University Comprehensive Cancer Center, who was asked for independent comment.
“What is a surprise is that it’s every year,” Shabsigh told Medscape Medical News, referring to the advanced cancer incidence increases.
“To see it so clearly in this study is sad,” Shabsigh added.
The study period started in 2005, but did not cover the years after 2018, when USPSTF recommendations changed again and advised that screening be “individualized” for men 55 to 69, and that men 70 and over should be excluded.
US cancer registry data, which are the source of the current study, are not yet available to assess the impact of this most recent change.
End in sight?
There has been a decline in the proportion of men undergoing PSA tests in the US in recent years, the study authors point out.
Routine PSA testing rates among men aged 50 and older declined from 40.6% in 2008 to 38.3% in 2010, and dropped to 31.5% in 2013, a percentage which held again in 2015, per national self-reported survey data.
The study authors say the cause of the rise in advanced cancers is uncertain because of the descriptive nature of their research.
But Andrew Vickers, PhD, a biostatistician at the Memorial Sloan Kettering Cancer Center in New York City, said the rise in advanced cancers and the drop in early-stage cancers reported in the study are “suggestive of a causal relationship” and a “screening effect.”
Vickers argues that there were “a whole bunch of trends that came together in the late [2000s] to influence [PSA] screening.”
For example, two landmark randomized clinical trials of PSA screening first reported “unfavorable” results in 2009, which is during the period covered in the current study, and dampened enthusiasm for screening.
The European Randomized Study of Screening for Prostate Cancer (ERSPC) and the US-based Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial reported little or no effect on mortality, the primary outcome of the trials.
Medscape Medical News asked Vickers to speculate on how long the incidence of advanced prostate cancers will continue to rise in the United States.
“It would surprise me if we had bottomed out [and reached peak increases in advanced cancers] or if we had much longer to go,” he said. “My prediction is that if nothing were to change we will probably see some further increases in [the incidence of] advanced disease.”
What needs to change? Vickers ticked off a list of “golden rules.”
- First, physicians need to get consent for all PSA tests.
- Second, PSA tests should not be administered to older men “who won’t benefit,” such as men 75 years and older with comorbidities such as heart disease.
- Third, PSA testing should be restricted to younger men.
- Fourth, clinicians need to be more restrictive about biopsy. “It used to be if you had a high PSA, you would get a biopsy,” he said, adding that this approach yielded a lot invasive testing in men with low-grade disease. By using additional tests such as the 4Kscore or Prostate Health Index or MRI, clinicians can limit biopsies to men with greater likelihood of a high-grade cancer. Vickers acknowledged conflict of interest on this point, as he is a patent holder of the 4Kscore.
- Fifth, don’t treat men who are very unlikely to benefit, especially men with Gleason grade 6 disease. Use active surveillance for these men, he said. “Using our existing knowledge, I believe we can completely transform the harm-to-benefit ratio of PSA screening. We would drastically reduce overdiagnosis and overtreatment,” he stated.
Additionally, Vickers believes that urologists need to educate local internists and general practitioners and acknowledge that screening and subsequent treatment were “done wrong for a long time.” At the same time, urologists should make it clear that patients will not be biopsied “unless there is a really good reason to believe that they have a high risk of high-grade disease.”
Vickers concluded: “We can reduce the harm and maintain the benefit of screening.”
The study was supported by the American Cancer Society. Jemal and Shabsigh have disclosed no relevant financial relationships. Vickers declared that he is a patent holder of the 4Kscore.
This article first appeared on Medscape.com.
The incidence of advanced prostate cancers in the United States “persistently” increased annually for 5 years after the United States Preventive Services Task Force (USPSTF) controversially advised in 2012 against prostate-specific antigen (PSA) screening in men of all ages, new research indicates.
But a biostatistician not involved with the study said the USPSTF’s recommendation is not wholly to blame because “you need 5 to 7 years of lag time at a minimum to influence PSA screening,” and suggested that other factors were at play.
In the new study, Ahmedin Jemal, DVM, PhD, of the American Cancer Society, and colleagues report that for the period 2012–2016 there were yearly statistically significant upticks in the incidence of regional-stage disease (by an absolute 11% per year) and in distant-stage disease (by an absolute 5% per year).
At the same time, there were annual drops in the incidence of localized prostate cancers in men 50 years or older.
The new study is the first to report data out to the end of 2016.
The two trends — the increase in advanced cancers and decrease in early-stage cancers — have been occurring for 10 years, more or less, but with a steady, sharp rise in advanced disease starting in 2010 to 2012, the findings show.
“These data illustrate the trade-off between higher screening rates and more early-stage disease diagnoses (possibly overdiagnosis and overtreatment) and lower screening rates and more late-stage (possibly fatal) disease,” the authors comment.
The study was published online May 20 in the Journal of the National Cancer Institute.
Several previous studies have reported incidence pattern changes following the USPSTF recommendations against PSA screening for men aged 75 or older in 2008 and all men in 2012, but the data went no further than 2015.
“We saw hints of these changes in the past few years and now we have further confirmation,” said Ahmad Shabsigh, MD, urologic oncologist at the Ohio State University Comprehensive Cancer Center, who was asked for independent comment.
“What is a surprise is that it’s every year,” Shabsigh told Medscape Medical News, referring to the advanced cancer incidence increases.
“To see it so clearly in this study is sad,” Shabsigh added.
The study period started in 2005, but did not cover the years after 2018, when USPSTF recommendations changed again and advised that screening be “individualized” for men 55 to 69, and that men 70 and over should be excluded.
US cancer registry data, which are the source of the current study, are not yet available to assess the impact of this most recent change.
End in sight?
There has been a decline in the proportion of men undergoing PSA tests in the US in recent years, the study authors point out.
Routine PSA testing rates among men aged 50 and older declined from 40.6% in 2008 to 38.3% in 2010, and dropped to 31.5% in 2013, a percentage which held again in 2015, per national self-reported survey data.
The study authors say the cause of the rise in advanced cancers is uncertain because of the descriptive nature of their research.
But Andrew Vickers, PhD, a biostatistician at the Memorial Sloan Kettering Cancer Center in New York City, said the rise in advanced cancers and the drop in early-stage cancers reported in the study are “suggestive of a causal relationship” and a “screening effect.”
Vickers argues that there were “a whole bunch of trends that came together in the late [2000s] to influence [PSA] screening.”
For example, two landmark randomized clinical trials of PSA screening first reported “unfavorable” results in 2009, which is during the period covered in the current study, and dampened enthusiasm for screening.
The European Randomized Study of Screening for Prostate Cancer (ERSPC) and the US-based Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial reported little or no effect on mortality, the primary outcome of the trials.
Medscape Medical News asked Vickers to speculate on how long the incidence of advanced prostate cancers will continue to rise in the United States.
“It would surprise me if we had bottomed out [and reached peak increases in advanced cancers] or if we had much longer to go,” he said. “My prediction is that if nothing were to change we will probably see some further increases in [the incidence of] advanced disease.”
What needs to change? Vickers ticked off a list of “golden rules.”
- First, physicians need to get consent for all PSA tests.
- Second, PSA tests should not be administered to older men “who won’t benefit,” such as men 75 years and older with comorbidities such as heart disease.
- Third, PSA testing should be restricted to younger men.
- Fourth, clinicians need to be more restrictive about biopsy. “It used to be if you had a high PSA, you would get a biopsy,” he said, adding that this approach yielded a lot invasive testing in men with low-grade disease. By using additional tests such as the 4Kscore or Prostate Health Index or MRI, clinicians can limit biopsies to men with greater likelihood of a high-grade cancer. Vickers acknowledged conflict of interest on this point, as he is a patent holder of the 4Kscore.
- Fifth, don’t treat men who are very unlikely to benefit, especially men with Gleason grade 6 disease. Use active surveillance for these men, he said. “Using our existing knowledge, I believe we can completely transform the harm-to-benefit ratio of PSA screening. We would drastically reduce overdiagnosis and overtreatment,” he stated.
Additionally, Vickers believes that urologists need to educate local internists and general practitioners and acknowledge that screening and subsequent treatment were “done wrong for a long time.” At the same time, urologists should make it clear that patients will not be biopsied “unless there is a really good reason to believe that they have a high risk of high-grade disease.”
Vickers concluded: “We can reduce the harm and maintain the benefit of screening.”
The study was supported by the American Cancer Society. Jemal and Shabsigh have disclosed no relevant financial relationships. Vickers declared that he is a patent holder of the 4Kscore.
This article first appeared on Medscape.com.
Testicular sperm may improve IVF outcomes in some cases
and rates of clinical pregnancy and live birth, a retrospective observational study has found.
The findings were released ahead of the study’s scheduled presentation at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. ACOG canceled the meeting and released abstracts for press coverage.
The study, which won the college’s Donald F. Richardson Memorial Prize Research Paper award, evaluated 112 nonazoospermic couples with an average of 2.3 failed in vitro fertilization (IVF) cycles (range of 1-8). The couples, patients at Shade Grove Fertility in Washington, underwent 157 total intracytoplasmic sperm injection (ICSI) cycles (133 using fresh testicular sperm and 24 using frozen/thawed sperm) and had a total of 101 embryo transfers.
Use of ICSI with testicular sperm compared with prior cycles using ejaculated sperm significantly improved blastocyst development (65% vs. 33%, P < .001), blastocyst conversion rates (67% vs. 35%, P < .001) and the number of embryos available for vitrification (1.6 vs. 0.7, P < .001). Fertilization rates were similar (70% vs. 58%). The clinical pregnancy and live birth rates in couples who used testicular sperm were 44% and 32%, respectively.
The findings suggest improved embryo development and pregnancy rates, and offer more evidence “that this might be something we can offer patients who’ve had multiple failures and no other reason as to why,” M. Blake Evans, DO, clinical fellow in reproductive endocrinology and infertility at the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Rockville, Md., said in a interview. “It looks like there is promise, and we need more research to be conducted.”
The integration of the use of testicular sperm at Shady Grove, a private practice fertility center, and the newly completed analysis of outcomes, were driven by studies “showing that testicular sperm has a low DNA fragmentation index and suggesting that it [offers a] better chance of successful IVF outcomes in patients who have had prior failures,” he said.
Almost all of the men who had ICSC using testicular sperm – 105 of the 112 – had a sperm DNA fragmentation (SDF) assessment of their ejaculate sperm. The mean SDF was 32% and of these 105 men, 66 had an SDF greater than 25% (mean of 49%), a value considered abnormal. The outcomes for patients with elevated SDF did not differ significantly from the overall cohort, Dr. Evans and coinvestigators reported in their abstract.
Dr. Evans said that it’s too early to draw any conclusions about the utility of SDF testing, and that the investigators plan to start prospectively evaluating whether levels of sperm DNA damage as reflected in SDF testing correlate with IVF outcomes.
“Right now the evidence is so conflicting as to whether [SDF testing offers] information that all IVF patients or infertility patients should be receiving,” he said. “Is the reason that testicular sperm works better because there’s lower DNA fragmentation? We think so. … But now that we see [that it] appears the outcomes are better [using testicular sperm], we need to take it a step further and look prospectively at the impact of DNA fragmentation, comparing all the outcomes with normal and abnormal DNA [levels].”
Mark P. Trolice, MD, director of Fertility CARE: The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando, said in an interview that while “there is increasing evidence – and rather clear evidence – that testicular sperm has less DNA damage,” there has been controversy over available outcomes data, most of which have come from small, retrospective studies. Dr. Trolice was not involved in this study presented at ACOG.
In the case of “very poor outcomes with use of ejaculated sperm and a high SDF index, there seems to be support for the use of testicular sperm on the next IVF cycle,” he said. “But there’s also evidence to support that there’s no significant difference in the outcomes of IUI [intrauterine insemination] or IVF based on the SDF index. So this [study] really took a tremendous leap of faith.”
Dr. Trolice said he looks forward to more research – ideally prospective, randomized studies of men with high SDF levels who proceed with assisted reproductive technologies using ejaculated or testicular sperm.
The research was supported by the division of intramural research at the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Dr. Evans did not report any relevant financial disclosures. One of his coinvestigators. Micah J. Hill, DO, disclosed having served on the advisory board of Ohana Biosciences. Dr. Trolice reported that he has no relevant financial disclosures. He is a member of the Ob.Gyn. News editorial advisory board.
The abstract was first presented by coauthor Lt. Allison A. Eubanks, MD, of Walter Reed National Military Medical Center, at the ACOG Armed Forces District Annual District Meeting in September 2019.
and rates of clinical pregnancy and live birth, a retrospective observational study has found.
The findings were released ahead of the study’s scheduled presentation at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. ACOG canceled the meeting and released abstracts for press coverage.
The study, which won the college’s Donald F. Richardson Memorial Prize Research Paper award, evaluated 112 nonazoospermic couples with an average of 2.3 failed in vitro fertilization (IVF) cycles (range of 1-8). The couples, patients at Shade Grove Fertility in Washington, underwent 157 total intracytoplasmic sperm injection (ICSI) cycles (133 using fresh testicular sperm and 24 using frozen/thawed sperm) and had a total of 101 embryo transfers.
Use of ICSI with testicular sperm compared with prior cycles using ejaculated sperm significantly improved blastocyst development (65% vs. 33%, P < .001), blastocyst conversion rates (67% vs. 35%, P < .001) and the number of embryos available for vitrification (1.6 vs. 0.7, P < .001). Fertilization rates were similar (70% vs. 58%). The clinical pregnancy and live birth rates in couples who used testicular sperm were 44% and 32%, respectively.
The findings suggest improved embryo development and pregnancy rates, and offer more evidence “that this might be something we can offer patients who’ve had multiple failures and no other reason as to why,” M. Blake Evans, DO, clinical fellow in reproductive endocrinology and infertility at the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Rockville, Md., said in a interview. “It looks like there is promise, and we need more research to be conducted.”
The integration of the use of testicular sperm at Shady Grove, a private practice fertility center, and the newly completed analysis of outcomes, were driven by studies “showing that testicular sperm has a low DNA fragmentation index and suggesting that it [offers a] better chance of successful IVF outcomes in patients who have had prior failures,” he said.
Almost all of the men who had ICSC using testicular sperm – 105 of the 112 – had a sperm DNA fragmentation (SDF) assessment of their ejaculate sperm. The mean SDF was 32% and of these 105 men, 66 had an SDF greater than 25% (mean of 49%), a value considered abnormal. The outcomes for patients with elevated SDF did not differ significantly from the overall cohort, Dr. Evans and coinvestigators reported in their abstract.
Dr. Evans said that it’s too early to draw any conclusions about the utility of SDF testing, and that the investigators plan to start prospectively evaluating whether levels of sperm DNA damage as reflected in SDF testing correlate with IVF outcomes.
“Right now the evidence is so conflicting as to whether [SDF testing offers] information that all IVF patients or infertility patients should be receiving,” he said. “Is the reason that testicular sperm works better because there’s lower DNA fragmentation? We think so. … But now that we see [that it] appears the outcomes are better [using testicular sperm], we need to take it a step further and look prospectively at the impact of DNA fragmentation, comparing all the outcomes with normal and abnormal DNA [levels].”
Mark P. Trolice, MD, director of Fertility CARE: The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando, said in an interview that while “there is increasing evidence – and rather clear evidence – that testicular sperm has less DNA damage,” there has been controversy over available outcomes data, most of which have come from small, retrospective studies. Dr. Trolice was not involved in this study presented at ACOG.
In the case of “very poor outcomes with use of ejaculated sperm and a high SDF index, there seems to be support for the use of testicular sperm on the next IVF cycle,” he said. “But there’s also evidence to support that there’s no significant difference in the outcomes of IUI [intrauterine insemination] or IVF based on the SDF index. So this [study] really took a tremendous leap of faith.”
Dr. Trolice said he looks forward to more research – ideally prospective, randomized studies of men with high SDF levels who proceed with assisted reproductive technologies using ejaculated or testicular sperm.
The research was supported by the division of intramural research at the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Dr. Evans did not report any relevant financial disclosures. One of his coinvestigators. Micah J. Hill, DO, disclosed having served on the advisory board of Ohana Biosciences. Dr. Trolice reported that he has no relevant financial disclosures. He is a member of the Ob.Gyn. News editorial advisory board.
The abstract was first presented by coauthor Lt. Allison A. Eubanks, MD, of Walter Reed National Military Medical Center, at the ACOG Armed Forces District Annual District Meeting in September 2019.
and rates of clinical pregnancy and live birth, a retrospective observational study has found.
The findings were released ahead of the study’s scheduled presentation at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. ACOG canceled the meeting and released abstracts for press coverage.
The study, which won the college’s Donald F. Richardson Memorial Prize Research Paper award, evaluated 112 nonazoospermic couples with an average of 2.3 failed in vitro fertilization (IVF) cycles (range of 1-8). The couples, patients at Shade Grove Fertility in Washington, underwent 157 total intracytoplasmic sperm injection (ICSI) cycles (133 using fresh testicular sperm and 24 using frozen/thawed sperm) and had a total of 101 embryo transfers.
Use of ICSI with testicular sperm compared with prior cycles using ejaculated sperm significantly improved blastocyst development (65% vs. 33%, P < .001), blastocyst conversion rates (67% vs. 35%, P < .001) and the number of embryos available for vitrification (1.6 vs. 0.7, P < .001). Fertilization rates were similar (70% vs. 58%). The clinical pregnancy and live birth rates in couples who used testicular sperm were 44% and 32%, respectively.
The findings suggest improved embryo development and pregnancy rates, and offer more evidence “that this might be something we can offer patients who’ve had multiple failures and no other reason as to why,” M. Blake Evans, DO, clinical fellow in reproductive endocrinology and infertility at the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Rockville, Md., said in a interview. “It looks like there is promise, and we need more research to be conducted.”
The integration of the use of testicular sperm at Shady Grove, a private practice fertility center, and the newly completed analysis of outcomes, were driven by studies “showing that testicular sperm has a low DNA fragmentation index and suggesting that it [offers a] better chance of successful IVF outcomes in patients who have had prior failures,” he said.
Almost all of the men who had ICSC using testicular sperm – 105 of the 112 – had a sperm DNA fragmentation (SDF) assessment of their ejaculate sperm. The mean SDF was 32% and of these 105 men, 66 had an SDF greater than 25% (mean of 49%), a value considered abnormal. The outcomes for patients with elevated SDF did not differ significantly from the overall cohort, Dr. Evans and coinvestigators reported in their abstract.
Dr. Evans said that it’s too early to draw any conclusions about the utility of SDF testing, and that the investigators plan to start prospectively evaluating whether levels of sperm DNA damage as reflected in SDF testing correlate with IVF outcomes.
“Right now the evidence is so conflicting as to whether [SDF testing offers] information that all IVF patients or infertility patients should be receiving,” he said. “Is the reason that testicular sperm works better because there’s lower DNA fragmentation? We think so. … But now that we see [that it] appears the outcomes are better [using testicular sperm], we need to take it a step further and look prospectively at the impact of DNA fragmentation, comparing all the outcomes with normal and abnormal DNA [levels].”
Mark P. Trolice, MD, director of Fertility CARE: The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando, said in an interview that while “there is increasing evidence – and rather clear evidence – that testicular sperm has less DNA damage,” there has been controversy over available outcomes data, most of which have come from small, retrospective studies. Dr. Trolice was not involved in this study presented at ACOG.
In the case of “very poor outcomes with use of ejaculated sperm and a high SDF index, there seems to be support for the use of testicular sperm on the next IVF cycle,” he said. “But there’s also evidence to support that there’s no significant difference in the outcomes of IUI [intrauterine insemination] or IVF based on the SDF index. So this [study] really took a tremendous leap of faith.”
Dr. Trolice said he looks forward to more research – ideally prospective, randomized studies of men with high SDF levels who proceed with assisted reproductive technologies using ejaculated or testicular sperm.
The research was supported by the division of intramural research at the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Dr. Evans did not report any relevant financial disclosures. One of his coinvestigators. Micah J. Hill, DO, disclosed having served on the advisory board of Ohana Biosciences. Dr. Trolice reported that he has no relevant financial disclosures. He is a member of the Ob.Gyn. News editorial advisory board.
The abstract was first presented by coauthor Lt. Allison A. Eubanks, MD, of Walter Reed National Military Medical Center, at the ACOG Armed Forces District Annual District Meeting in September 2019.
FROM ACOG 2020
Inflammatory back pain likely underrecognized in primary care
according to a review of 239 charts there by rheumatologists and rheumatology fellows.
In more than two-thirds of cases, the reviewers were unable to determine if patients had inflammatory back pain or not based on what was documented. When symptoms relevant to inflammation – such as improvement with movement – were documented, it wasn’t clear if providers were actually trying to solicit a history of inflammation or if they simply recorded what patients volunteered.
Spondyloarthritis was listed in the differential of just five charts (2%), and only eight (3.3%) documented considering a rheumatology referral.
It raises the possibility that, in at least some cases, an opportunity to diagnose and treat spondyloarthritis early was missed. It’s a known problem in the literature; previous studies report a delay of 2-10 years before ankylosing spondylitis diagnosis.
“In our primary care practice, there appears to be poor awareness of inflammatory back pain [that] could lead to diagnostic delay,” said senior investigator and rheumatologist Steven Vlad, MD, PhD, an assistant professor at Tufts. Primary care providers are usually the first to see back pain patients, but they “did not seem to be screening for” inflammation, he said.
Dr. Vlad presented the study results at the virtual annual meeting of the Spondyloarthritis Research and Treatment Network. The meeting was held online this year because of the COVID-19 pandemic.
The findings suggest that a reminder to check for inflammation might be in order. Dr. Vlad and his colleagues have since held educational sessions, and plan to do more, with the idea of repeating the study in a year or 2 to see if the sessions made a difference.
“People take away what they learn as residents. We probably need to focus on resident education if we really want to make a dent in this,” he said.
The generalizability of the single-center results is unclear, and it’s possible at least in some cases that providers asked the right questions but did not document them in the chart. Even so, the issue “deserves future study in other populations,” Dr. Vlad said.
The subjects all had a diagnostic code for low back pain and were seen by Tuft’s primary care at least twice 3 or more months apart, which indicated chronic pain. Chart reviews included clinical notes, labs, imaging studies, and consultation reports. “We looked for specific documentation that primary care physicians had been asking questions related to inflammatory back pain,” Dr. Vlad explained.
Overall, 128 charts (53.6%) documented some feature of inflammatory low back pain. Insidious onset was the most common, but morning stiffness, a cardinal sign, was the least common, noted in only five charts (2%). About 30% of the subjects had a lumbar spine x-ray, which was the most common imaging study, followed by lumbar spine MRI. Only a handful had imaging of the sacroiliac joints.
In 111 charts (46.4%), there was no documentation that primary care providers had looked for inflammatory features or asked questions about them.
Patients were seen from Jan. 2016 to May 2018. The average age in the study was 37.6 years, and two-thirds of the subjects were women.
Funding source and disclosures weren’t reported.
according to a review of 239 charts there by rheumatologists and rheumatology fellows.
In more than two-thirds of cases, the reviewers were unable to determine if patients had inflammatory back pain or not based on what was documented. When symptoms relevant to inflammation – such as improvement with movement – were documented, it wasn’t clear if providers were actually trying to solicit a history of inflammation or if they simply recorded what patients volunteered.
Spondyloarthritis was listed in the differential of just five charts (2%), and only eight (3.3%) documented considering a rheumatology referral.
It raises the possibility that, in at least some cases, an opportunity to diagnose and treat spondyloarthritis early was missed. It’s a known problem in the literature; previous studies report a delay of 2-10 years before ankylosing spondylitis diagnosis.
“In our primary care practice, there appears to be poor awareness of inflammatory back pain [that] could lead to diagnostic delay,” said senior investigator and rheumatologist Steven Vlad, MD, PhD, an assistant professor at Tufts. Primary care providers are usually the first to see back pain patients, but they “did not seem to be screening for” inflammation, he said.
Dr. Vlad presented the study results at the virtual annual meeting of the Spondyloarthritis Research and Treatment Network. The meeting was held online this year because of the COVID-19 pandemic.
The findings suggest that a reminder to check for inflammation might be in order. Dr. Vlad and his colleagues have since held educational sessions, and plan to do more, with the idea of repeating the study in a year or 2 to see if the sessions made a difference.
“People take away what they learn as residents. We probably need to focus on resident education if we really want to make a dent in this,” he said.
The generalizability of the single-center results is unclear, and it’s possible at least in some cases that providers asked the right questions but did not document them in the chart. Even so, the issue “deserves future study in other populations,” Dr. Vlad said.
The subjects all had a diagnostic code for low back pain and were seen by Tuft’s primary care at least twice 3 or more months apart, which indicated chronic pain. Chart reviews included clinical notes, labs, imaging studies, and consultation reports. “We looked for specific documentation that primary care physicians had been asking questions related to inflammatory back pain,” Dr. Vlad explained.
Overall, 128 charts (53.6%) documented some feature of inflammatory low back pain. Insidious onset was the most common, but morning stiffness, a cardinal sign, was the least common, noted in only five charts (2%). About 30% of the subjects had a lumbar spine x-ray, which was the most common imaging study, followed by lumbar spine MRI. Only a handful had imaging of the sacroiliac joints.
In 111 charts (46.4%), there was no documentation that primary care providers had looked for inflammatory features or asked questions about them.
Patients were seen from Jan. 2016 to May 2018. The average age in the study was 37.6 years, and two-thirds of the subjects were women.
Funding source and disclosures weren’t reported.
according to a review of 239 charts there by rheumatologists and rheumatology fellows.
In more than two-thirds of cases, the reviewers were unable to determine if patients had inflammatory back pain or not based on what was documented. When symptoms relevant to inflammation – such as improvement with movement – were documented, it wasn’t clear if providers were actually trying to solicit a history of inflammation or if they simply recorded what patients volunteered.
Spondyloarthritis was listed in the differential of just five charts (2%), and only eight (3.3%) documented considering a rheumatology referral.
It raises the possibility that, in at least some cases, an opportunity to diagnose and treat spondyloarthritis early was missed. It’s a known problem in the literature; previous studies report a delay of 2-10 years before ankylosing spondylitis diagnosis.
“In our primary care practice, there appears to be poor awareness of inflammatory back pain [that] could lead to diagnostic delay,” said senior investigator and rheumatologist Steven Vlad, MD, PhD, an assistant professor at Tufts. Primary care providers are usually the first to see back pain patients, but they “did not seem to be screening for” inflammation, he said.
Dr. Vlad presented the study results at the virtual annual meeting of the Spondyloarthritis Research and Treatment Network. The meeting was held online this year because of the COVID-19 pandemic.
The findings suggest that a reminder to check for inflammation might be in order. Dr. Vlad and his colleagues have since held educational sessions, and plan to do more, with the idea of repeating the study in a year or 2 to see if the sessions made a difference.
“People take away what they learn as residents. We probably need to focus on resident education if we really want to make a dent in this,” he said.
The generalizability of the single-center results is unclear, and it’s possible at least in some cases that providers asked the right questions but did not document them in the chart. Even so, the issue “deserves future study in other populations,” Dr. Vlad said.
The subjects all had a diagnostic code for low back pain and were seen by Tuft’s primary care at least twice 3 or more months apart, which indicated chronic pain. Chart reviews included clinical notes, labs, imaging studies, and consultation reports. “We looked for specific documentation that primary care physicians had been asking questions related to inflammatory back pain,” Dr. Vlad explained.
Overall, 128 charts (53.6%) documented some feature of inflammatory low back pain. Insidious onset was the most common, but morning stiffness, a cardinal sign, was the least common, noted in only five charts (2%). About 30% of the subjects had a lumbar spine x-ray, which was the most common imaging study, followed by lumbar spine MRI. Only a handful had imaging of the sacroiliac joints.
In 111 charts (46.4%), there was no documentation that primary care providers had looked for inflammatory features or asked questions about them.
Patients were seen from Jan. 2016 to May 2018. The average age in the study was 37.6 years, and two-thirds of the subjects were women.
Funding source and disclosures weren’t reported.
REPORTING FROM SPARTAN 2020
COVID-19 exacerbating challenges for Latino patients
Disproportionate burden of pandemic complicates mental health care
Pamela Montano, MD, recalls the recent case of a patient with bipolar II disorder who was improving after treatment with medication and therapy when her life was upended by the COVID-19 pandemic.
The patient, who is Puerto Rican, lost two cousins to the virus, two of her brothers fell ill, and her sister became sick with coronavirus, said Dr. Montano, director of the Latino Bicultural Clinic at Gouverneur Health in New York. The patient was then left to care for her sister’s toddlers along with the patient’s own children, one of whom has special needs.
“After this happened, it increased her anxiety,” Dr. Montano said in an interview. “She’s not sleeping, and she started having panic attacks. My main concern was how to help her cope.”
Across the country, clinicians who treat mental illness and behavioral disorders in Latino patients are facing similar experiences and challenges associated with COVID-19 and the ensuing pandemic response. Current data suggest a disproportionate burden of illness and death from the novel coronavirus among racial and ethnic groups, particularly black and Hispanic patients. The disparities are likely attributable to economic and social conditions more common among such populations, compared with non-Hispanic whites, in addition to isolation from resources, according to the Centers for Disease Control and Prevention.
A recent New York City Department of Health study based on data that were available in late April found that deaths from COVID-19 were substantially higher for black and Hispanic/Latino patients than for white and Asian patients. The death rate per 100,000 population was 209.4 for blacks, 195.3 for Hispanics/Latinos, 107.7 for whites, and 90.8 for Asians.
“The COVID pandemic has highlighted the structural inequities that affect the Latino population [both] immigrant and nonimmigrant,” said Dr. Montano, a board member of the American Society of Hispanic Psychiatry and the officer of infrastructure and advocacy for the Hispanic Caucus of the American Psychiatric Association. “This includes income inequality, poor nutrition, history of trauma and discrimination, employment issues, quality education, access to technology, and overall access to appropriate cultural linguistic health care.”
Navigating challenges
For mental health professionals treating Latino patients, COVID-19 and the pandemic response have generated a range of treatment obstacles.
The transition to telehealth for example, has not been easy for some patients, said Jacqueline Posada, MD, consultation-liaison psychiatry fellow at the Inova Fairfax Hospital–George Washington University program in Falls Church, Va., and an APA Substance Abuse and Mental Health Services Administration minority fellow. Some patients lack Internet services, others forget virtual visits, and some do not have working phones, she said.
“I’ve had to be very flexible,” she said in an interview. “Ideally, I’d love to see everybody via video chat, but a lot of people either don’t have a stable Internet connection or Internet, so I meet the patient where they are. Whatever they have available, that’s what I’m going to use. If they don’t answer on the first call, I will call again at least three to five times in the first 15 minutes to make sure I’m giving them an opportunity to pick up the phone.”
In addition, Dr. Posada has encountered disconnected phones when calling patients for appointments. In such cases, Dr. Posada contacts the patient’s primary care physician to relay medication recommendations in case the patient resurfaces at the clinic.
In other instances, patients are not familiar with video technology, or they must travel to a friend or neighbor’s house to access the technology, said Hector Colón-Rivera, MD, an addiction psychiatrist and medical director of the Asociación Puertorriqueños en Marcha Behavioral Health Program, a nonprofit organization based in the Philadelphia area. Telehealth visits frequently include appearances by children, family members, barking dogs, and other distractions, said Dr. Colón-Rivera, president of the APA Hispanic Caucus.
“We’re seeing things that we didn’t used to see when they came to our office – for good or for bad,” said Dr. Colón-Rivera, an attending telemedicine physician at the University of Pittsburgh Medical Center. “It could be a good chance to meet our patient in a different way. Of course, it creates different stressors. If you have five kids on top of you and you’re the only one at home, it’s hard to do therapy.”
Psychiatrists are also seeing prior health conditions in patients exacerbated by COVID-19 fears and new health problems arising from the current pandemic environment. Dr. Posada recalls a patient whom she successfully treated for premenstrual dysphoric disorder who recently descended into severe clinical depression. The patient, from Colombia, was attending school in the United States on a student visa and supporting herself through child care jobs.
“So much of her depression was based on her social circumstance,” Dr. Posada said. “She had lost her job, her sister had lost her job so they were scraping by on her sister’s husband’s income, and the thing that brought her joy, which was going to school and studying so she could make a different life for herself than what her parents had in Colombia, also seemed like it was out of reach.”
Dr. Colón-Rivera recently received a call from a hospital where one of his patients was admitted after becoming delusional and psychotic. The patient was correctly taking medication prescribed by Dr. Colón-Rivera, but her diabetes had become uncontrolled because she was unable to reach her primary care doctor and couldn’t access the pharmacy. Her blood sugar level became elevated, leading to the delusions.
“A patient that was perfectly stable now is unstable,” he said. “Her diet has not been good enough through the pandemic, exacerbating her diabetes. She was admitted to the hospital for delirium.
Compounding of traumas
For many Latino patients, the adverse impacts of the pandemic comes on top of multiple prior traumas, such as violence exposures, discrimination, and economic issues, said Lisa Fortuna, MD, MPH, MDiv, chief of psychiatry and vice chair at Zuckerberg San Francisco General Hospital. A 2017 analysis found that nearly four in five Latino youth face at least one traumatic childhood experience, like poverty or abuse, and that about 29% of Latino youth experience four or more of these traumas.
Immigrants in particular, may have faced trauma in their home country and/or immigration trauma, Dr. Fortuna added. A 2013 study on immigrant Latino adolescents for example, found that 29% of foreign-born adolescents and 34% of foreign-born parents experienced trauma during the migration process (Int Migr Rev. 2013 Dec;47(4):10).
“All of these things are cumulative,” Dr. Fortuna said. “Then when you’re hit with a pandemic, all of the disparities that you already have and all the stress that you already have are compounded. This is for the kids, too, who have been exposed to a lot of stressors and now maybe have family members that have been ill or have died. All of these things definitely put people at risk for increased depression [and] the worsening of any preexisting posttraumatic stress disorder. We’ve seen this in previous disasters, and I expect that’s what we’re going to see more of with the COVID-19 pandemic.”
At the same time, a central cultural value of many Latinos is family unity, Dr. Montano said, a foundation that is now being strained by social distancing and severed connections.
“This has separated many families,” she said. “There has been a lot of loneliness and grief.”
Mistrust and fear toward the government, public agencies, and even the health system itself act as further hurdles for some Latinos in the face of COVID-19. In areas with large immigrant populations such as San Francisco, Dr. Fortuna noted, it’s not uncommon for undocumented patients to avoid accessing medical care and social services, or visiting emergency departments for needed care for fear of drawing attention to themselves or possible detainment.
“The fact that so many people showed up at our hospital so ill and ended up in the ICU – that could be a combination of factors. Because the population has high rates of diabetes and hypertension, that might have put people at increased risk for severe illness,” she said. “But some people may have been holding out for care because they wanted to avoid being in places out of fear of immigration scrutiny.”
Overcoming language barriers
Compounding the challenging pandemic landscape for Latino patients is the fact that many state resources about COVID-19 have not been translated to Spanish, Dr. Colón-Rivera said. He was troubled recently when he went to several state websites and found limited to no information in Spanish about the coronavirus. Some data about COVID-19 from the federal government were not translated to Spanish until officials received pushback, he added. Even now, press releases and other information disseminated by the federal government about the virus appear to be translated by an automated service – and lack sense and context.
The state agencies in Pennsylvania have been alerted to the absence of Spanish information, but change has been slow, he noted.
“In Philadelphia, 23% speaks a language other than English,” he said. “So we missed a lot of critical information that could have helped to avoid spreading the illness and access support.”
Dr. Fortuna said that California has done better with providing COVID-19–related information in Spanish, compared with some other states, but misinformation about the virus and lingering myths have still been a problem among the Latino community. The University of California, San Francisco, recently launched a Latino Task Force resource website for the Latino community that includes information in English, Spanish, and Yucatec Maya about COVID-19, health and wellness tips, and resources for various assistance needs.
The concerning lack of COVID-19 information translated to Spanish led Dr. Montano to start a Facebook page in Spanish about mental health tips and guidance for managing COVID-19–related issues. She and her team of clinicians share information, videos, relaxation exercises, and community resources on the page, among other posts. “There is also general info and recommendations about COVID-19 that I think can be useful for the community,” she said. “The idea is that patients, the general community, and providers can have share information, hope messages, and ask questions in Spanish.”
Feeling ‘helpless’
A central part of caring for Latino patients during the COVID-19 crisis has been referring them to outside agencies and social services, psychiatrists say. But finding the right resources amid a pandemic and ensuring that patients connect with the correct aid has been an uphill battle.
“We sometimes feel like our hands are tied,” Dr. Colón-Rivera said. “Sometimes, we need to call a place to bring food. Some of the state agencies and nonprofits don’t have delivery systems, so the patient has to go pick up for food or medication. Some of our patients don’t want to go outside. Some do not have cars.”
As a clinician, it can be easy to feel helpless when trying to navigate new challenges posed by the pandemic in addition to other longstanding barriers, Dr. Posada said.
“Already, mental health disorders are so influenced by social situations like poverty, job insecurity, or family issues, and now it just seems those obstacles are even more insurmountable,” she said. “At the end of the day, I can feel like: ‘Did I make a difference?’ That’s a big struggle.”
Dr. Montano’s team, which includes psychiatrists, psychologists, and social workers, have come to rely on virtual debriefings to vent, express frustrations, and support one another, she said. She also recently joined a virtual mind-body skills group as a participant.
“I recognize the importance of getting additional support and ways to alleviate burnout,” she said. “We need to take care of ourselves or we won’t be able to help others.”
Focusing on resilience during the current crisis can be beneficial for both patients and providers in coping and drawing strength, Dr. Posada said.
“When it comes to fostering resilience during times of hardship, I think it’s most helpful to reflect on what skills or attributes have helped during past crises and apply those now – whether it’s turning to comfort from close relationships, looking to religion and spirituality, practicing self-care like rest or exercise, or really tapping into one’s purpose and reason for practicing psychiatry and being a physician,” she said. “The same advice goes for clinicians: We’ve all been through hard times in the past, it’s part of the human condition and we’ve also witnessed a lot of suffering in our patients, so now is the time to practice those skills that have gotten us through hard times in the past.”
Learning lessons from COVID-19
Despite the challenges with moving to telehealth, Dr. Fortuna said the tool has proved beneficial overall for mental health care. For Dr. Fortuna’s team for example, telehealth by phone has decreased the no-show rate, compared with clinic visits, and improved care access.
“We need to figure out how to maintain that,” she said. “If we can build ways for equity and access to Internet, especially equipment, I think that’s going to help.”
In addition, more data are needed about the ways in which COVID-19 is affecting Latino patients, Dr. Colón-Rivera said. Mortality statistics have been published, but information is needed about the rates of infection and manifestation of illness.
Most importantly, the COVID-19 crisis has emphasized the critical need to address and improve the underlying inequity issues among Latino patients, psychiatrists say.
“We really need to think about how there can be partnerships, in terms of community-based Latino business and leaders, multisector resources, trying to think about how we can improve conditions both work and safety for Latinos,” Dr. Fortuna said. “How can schools get support in integrating mental health and support for families, especially now after COVID-19? And really looking at some of these underlying inequities that are the underpinnings of why people were at risk for the disproportionate effects of the COVID-19 pandemic.”
Disproportionate burden of pandemic complicates mental health care
Disproportionate burden of pandemic complicates mental health care
Pamela Montano, MD, recalls the recent case of a patient with bipolar II disorder who was improving after treatment with medication and therapy when her life was upended by the COVID-19 pandemic.
The patient, who is Puerto Rican, lost two cousins to the virus, two of her brothers fell ill, and her sister became sick with coronavirus, said Dr. Montano, director of the Latino Bicultural Clinic at Gouverneur Health in New York. The patient was then left to care for her sister’s toddlers along with the patient’s own children, one of whom has special needs.
“After this happened, it increased her anxiety,” Dr. Montano said in an interview. “She’s not sleeping, and she started having panic attacks. My main concern was how to help her cope.”
Across the country, clinicians who treat mental illness and behavioral disorders in Latino patients are facing similar experiences and challenges associated with COVID-19 and the ensuing pandemic response. Current data suggest a disproportionate burden of illness and death from the novel coronavirus among racial and ethnic groups, particularly black and Hispanic patients. The disparities are likely attributable to economic and social conditions more common among such populations, compared with non-Hispanic whites, in addition to isolation from resources, according to the Centers for Disease Control and Prevention.
A recent New York City Department of Health study based on data that were available in late April found that deaths from COVID-19 were substantially higher for black and Hispanic/Latino patients than for white and Asian patients. The death rate per 100,000 population was 209.4 for blacks, 195.3 for Hispanics/Latinos, 107.7 for whites, and 90.8 for Asians.
“The COVID pandemic has highlighted the structural inequities that affect the Latino population [both] immigrant and nonimmigrant,” said Dr. Montano, a board member of the American Society of Hispanic Psychiatry and the officer of infrastructure and advocacy for the Hispanic Caucus of the American Psychiatric Association. “This includes income inequality, poor nutrition, history of trauma and discrimination, employment issues, quality education, access to technology, and overall access to appropriate cultural linguistic health care.”
Navigating challenges
For mental health professionals treating Latino patients, COVID-19 and the pandemic response have generated a range of treatment obstacles.
The transition to telehealth for example, has not been easy for some patients, said Jacqueline Posada, MD, consultation-liaison psychiatry fellow at the Inova Fairfax Hospital–George Washington University program in Falls Church, Va., and an APA Substance Abuse and Mental Health Services Administration minority fellow. Some patients lack Internet services, others forget virtual visits, and some do not have working phones, she said.
“I’ve had to be very flexible,” she said in an interview. “Ideally, I’d love to see everybody via video chat, but a lot of people either don’t have a stable Internet connection or Internet, so I meet the patient where they are. Whatever they have available, that’s what I’m going to use. If they don’t answer on the first call, I will call again at least three to five times in the first 15 minutes to make sure I’m giving them an opportunity to pick up the phone.”
In addition, Dr. Posada has encountered disconnected phones when calling patients for appointments. In such cases, Dr. Posada contacts the patient’s primary care physician to relay medication recommendations in case the patient resurfaces at the clinic.
In other instances, patients are not familiar with video technology, or they must travel to a friend or neighbor’s house to access the technology, said Hector Colón-Rivera, MD, an addiction psychiatrist and medical director of the Asociación Puertorriqueños en Marcha Behavioral Health Program, a nonprofit organization based in the Philadelphia area. Telehealth visits frequently include appearances by children, family members, barking dogs, and other distractions, said Dr. Colón-Rivera, president of the APA Hispanic Caucus.
“We’re seeing things that we didn’t used to see when they came to our office – for good or for bad,” said Dr. Colón-Rivera, an attending telemedicine physician at the University of Pittsburgh Medical Center. “It could be a good chance to meet our patient in a different way. Of course, it creates different stressors. If you have five kids on top of you and you’re the only one at home, it’s hard to do therapy.”
Psychiatrists are also seeing prior health conditions in patients exacerbated by COVID-19 fears and new health problems arising from the current pandemic environment. Dr. Posada recalls a patient whom she successfully treated for premenstrual dysphoric disorder who recently descended into severe clinical depression. The patient, from Colombia, was attending school in the United States on a student visa and supporting herself through child care jobs.
“So much of her depression was based on her social circumstance,” Dr. Posada said. “She had lost her job, her sister had lost her job so they were scraping by on her sister’s husband’s income, and the thing that brought her joy, which was going to school and studying so she could make a different life for herself than what her parents had in Colombia, also seemed like it was out of reach.”
Dr. Colón-Rivera recently received a call from a hospital where one of his patients was admitted after becoming delusional and psychotic. The patient was correctly taking medication prescribed by Dr. Colón-Rivera, but her diabetes had become uncontrolled because she was unable to reach her primary care doctor and couldn’t access the pharmacy. Her blood sugar level became elevated, leading to the delusions.
“A patient that was perfectly stable now is unstable,” he said. “Her diet has not been good enough through the pandemic, exacerbating her diabetes. She was admitted to the hospital for delirium.
Compounding of traumas
For many Latino patients, the adverse impacts of the pandemic comes on top of multiple prior traumas, such as violence exposures, discrimination, and economic issues, said Lisa Fortuna, MD, MPH, MDiv, chief of psychiatry and vice chair at Zuckerberg San Francisco General Hospital. A 2017 analysis found that nearly four in five Latino youth face at least one traumatic childhood experience, like poverty or abuse, and that about 29% of Latino youth experience four or more of these traumas.
Immigrants in particular, may have faced trauma in their home country and/or immigration trauma, Dr. Fortuna added. A 2013 study on immigrant Latino adolescents for example, found that 29% of foreign-born adolescents and 34% of foreign-born parents experienced trauma during the migration process (Int Migr Rev. 2013 Dec;47(4):10).
“All of these things are cumulative,” Dr. Fortuna said. “Then when you’re hit with a pandemic, all of the disparities that you already have and all the stress that you already have are compounded. This is for the kids, too, who have been exposed to a lot of stressors and now maybe have family members that have been ill or have died. All of these things definitely put people at risk for increased depression [and] the worsening of any preexisting posttraumatic stress disorder. We’ve seen this in previous disasters, and I expect that’s what we’re going to see more of with the COVID-19 pandemic.”
At the same time, a central cultural value of many Latinos is family unity, Dr. Montano said, a foundation that is now being strained by social distancing and severed connections.
“This has separated many families,” she said. “There has been a lot of loneliness and grief.”
Mistrust and fear toward the government, public agencies, and even the health system itself act as further hurdles for some Latinos in the face of COVID-19. In areas with large immigrant populations such as San Francisco, Dr. Fortuna noted, it’s not uncommon for undocumented patients to avoid accessing medical care and social services, or visiting emergency departments for needed care for fear of drawing attention to themselves or possible detainment.
“The fact that so many people showed up at our hospital so ill and ended up in the ICU – that could be a combination of factors. Because the population has high rates of diabetes and hypertension, that might have put people at increased risk for severe illness,” she said. “But some people may have been holding out for care because they wanted to avoid being in places out of fear of immigration scrutiny.”
Overcoming language barriers
Compounding the challenging pandemic landscape for Latino patients is the fact that many state resources about COVID-19 have not been translated to Spanish, Dr. Colón-Rivera said. He was troubled recently when he went to several state websites and found limited to no information in Spanish about the coronavirus. Some data about COVID-19 from the federal government were not translated to Spanish until officials received pushback, he added. Even now, press releases and other information disseminated by the federal government about the virus appear to be translated by an automated service – and lack sense and context.
The state agencies in Pennsylvania have been alerted to the absence of Spanish information, but change has been slow, he noted.
“In Philadelphia, 23% speaks a language other than English,” he said. “So we missed a lot of critical information that could have helped to avoid spreading the illness and access support.”
Dr. Fortuna said that California has done better with providing COVID-19–related information in Spanish, compared with some other states, but misinformation about the virus and lingering myths have still been a problem among the Latino community. The University of California, San Francisco, recently launched a Latino Task Force resource website for the Latino community that includes information in English, Spanish, and Yucatec Maya about COVID-19, health and wellness tips, and resources for various assistance needs.
The concerning lack of COVID-19 information translated to Spanish led Dr. Montano to start a Facebook page in Spanish about mental health tips and guidance for managing COVID-19–related issues. She and her team of clinicians share information, videos, relaxation exercises, and community resources on the page, among other posts. “There is also general info and recommendations about COVID-19 that I think can be useful for the community,” she said. “The idea is that patients, the general community, and providers can have share information, hope messages, and ask questions in Spanish.”
Feeling ‘helpless’
A central part of caring for Latino patients during the COVID-19 crisis has been referring them to outside agencies and social services, psychiatrists say. But finding the right resources amid a pandemic and ensuring that patients connect with the correct aid has been an uphill battle.
“We sometimes feel like our hands are tied,” Dr. Colón-Rivera said. “Sometimes, we need to call a place to bring food. Some of the state agencies and nonprofits don’t have delivery systems, so the patient has to go pick up for food or medication. Some of our patients don’t want to go outside. Some do not have cars.”
As a clinician, it can be easy to feel helpless when trying to navigate new challenges posed by the pandemic in addition to other longstanding barriers, Dr. Posada said.
“Already, mental health disorders are so influenced by social situations like poverty, job insecurity, or family issues, and now it just seems those obstacles are even more insurmountable,” she said. “At the end of the day, I can feel like: ‘Did I make a difference?’ That’s a big struggle.”
Dr. Montano’s team, which includes psychiatrists, psychologists, and social workers, have come to rely on virtual debriefings to vent, express frustrations, and support one another, she said. She also recently joined a virtual mind-body skills group as a participant.
“I recognize the importance of getting additional support and ways to alleviate burnout,” she said. “We need to take care of ourselves or we won’t be able to help others.”
Focusing on resilience during the current crisis can be beneficial for both patients and providers in coping and drawing strength, Dr. Posada said.
“When it comes to fostering resilience during times of hardship, I think it’s most helpful to reflect on what skills or attributes have helped during past crises and apply those now – whether it’s turning to comfort from close relationships, looking to religion and spirituality, practicing self-care like rest or exercise, or really tapping into one’s purpose and reason for practicing psychiatry and being a physician,” she said. “The same advice goes for clinicians: We’ve all been through hard times in the past, it’s part of the human condition and we’ve also witnessed a lot of suffering in our patients, so now is the time to practice those skills that have gotten us through hard times in the past.”
Learning lessons from COVID-19
Despite the challenges with moving to telehealth, Dr. Fortuna said the tool has proved beneficial overall for mental health care. For Dr. Fortuna’s team for example, telehealth by phone has decreased the no-show rate, compared with clinic visits, and improved care access.
“We need to figure out how to maintain that,” she said. “If we can build ways for equity and access to Internet, especially equipment, I think that’s going to help.”
In addition, more data are needed about the ways in which COVID-19 is affecting Latino patients, Dr. Colón-Rivera said. Mortality statistics have been published, but information is needed about the rates of infection and manifestation of illness.
Most importantly, the COVID-19 crisis has emphasized the critical need to address and improve the underlying inequity issues among Latino patients, psychiatrists say.
“We really need to think about how there can be partnerships, in terms of community-based Latino business and leaders, multisector resources, trying to think about how we can improve conditions both work and safety for Latinos,” Dr. Fortuna said. “How can schools get support in integrating mental health and support for families, especially now after COVID-19? And really looking at some of these underlying inequities that are the underpinnings of why people were at risk for the disproportionate effects of the COVID-19 pandemic.”
Pamela Montano, MD, recalls the recent case of a patient with bipolar II disorder who was improving after treatment with medication and therapy when her life was upended by the COVID-19 pandemic.
The patient, who is Puerto Rican, lost two cousins to the virus, two of her brothers fell ill, and her sister became sick with coronavirus, said Dr. Montano, director of the Latino Bicultural Clinic at Gouverneur Health in New York. The patient was then left to care for her sister’s toddlers along with the patient’s own children, one of whom has special needs.
“After this happened, it increased her anxiety,” Dr. Montano said in an interview. “She’s not sleeping, and she started having panic attacks. My main concern was how to help her cope.”
Across the country, clinicians who treat mental illness and behavioral disorders in Latino patients are facing similar experiences and challenges associated with COVID-19 and the ensuing pandemic response. Current data suggest a disproportionate burden of illness and death from the novel coronavirus among racial and ethnic groups, particularly black and Hispanic patients. The disparities are likely attributable to economic and social conditions more common among such populations, compared with non-Hispanic whites, in addition to isolation from resources, according to the Centers for Disease Control and Prevention.
A recent New York City Department of Health study based on data that were available in late April found that deaths from COVID-19 were substantially higher for black and Hispanic/Latino patients than for white and Asian patients. The death rate per 100,000 population was 209.4 for blacks, 195.3 for Hispanics/Latinos, 107.7 for whites, and 90.8 for Asians.
“The COVID pandemic has highlighted the structural inequities that affect the Latino population [both] immigrant and nonimmigrant,” said Dr. Montano, a board member of the American Society of Hispanic Psychiatry and the officer of infrastructure and advocacy for the Hispanic Caucus of the American Psychiatric Association. “This includes income inequality, poor nutrition, history of trauma and discrimination, employment issues, quality education, access to technology, and overall access to appropriate cultural linguistic health care.”
Navigating challenges
For mental health professionals treating Latino patients, COVID-19 and the pandemic response have generated a range of treatment obstacles.
The transition to telehealth for example, has not been easy for some patients, said Jacqueline Posada, MD, consultation-liaison psychiatry fellow at the Inova Fairfax Hospital–George Washington University program in Falls Church, Va., and an APA Substance Abuse and Mental Health Services Administration minority fellow. Some patients lack Internet services, others forget virtual visits, and some do not have working phones, she said.
“I’ve had to be very flexible,” she said in an interview. “Ideally, I’d love to see everybody via video chat, but a lot of people either don’t have a stable Internet connection or Internet, so I meet the patient where they are. Whatever they have available, that’s what I’m going to use. If they don’t answer on the first call, I will call again at least three to five times in the first 15 minutes to make sure I’m giving them an opportunity to pick up the phone.”
In addition, Dr. Posada has encountered disconnected phones when calling patients for appointments. In such cases, Dr. Posada contacts the patient’s primary care physician to relay medication recommendations in case the patient resurfaces at the clinic.
In other instances, patients are not familiar with video technology, or they must travel to a friend or neighbor’s house to access the technology, said Hector Colón-Rivera, MD, an addiction psychiatrist and medical director of the Asociación Puertorriqueños en Marcha Behavioral Health Program, a nonprofit organization based in the Philadelphia area. Telehealth visits frequently include appearances by children, family members, barking dogs, and other distractions, said Dr. Colón-Rivera, president of the APA Hispanic Caucus.
“We’re seeing things that we didn’t used to see when they came to our office – for good or for bad,” said Dr. Colón-Rivera, an attending telemedicine physician at the University of Pittsburgh Medical Center. “It could be a good chance to meet our patient in a different way. Of course, it creates different stressors. If you have five kids on top of you and you’re the only one at home, it’s hard to do therapy.”
Psychiatrists are also seeing prior health conditions in patients exacerbated by COVID-19 fears and new health problems arising from the current pandemic environment. Dr. Posada recalls a patient whom she successfully treated for premenstrual dysphoric disorder who recently descended into severe clinical depression. The patient, from Colombia, was attending school in the United States on a student visa and supporting herself through child care jobs.
“So much of her depression was based on her social circumstance,” Dr. Posada said. “She had lost her job, her sister had lost her job so they were scraping by on her sister’s husband’s income, and the thing that brought her joy, which was going to school and studying so she could make a different life for herself than what her parents had in Colombia, also seemed like it was out of reach.”
Dr. Colón-Rivera recently received a call from a hospital where one of his patients was admitted after becoming delusional and psychotic. The patient was correctly taking medication prescribed by Dr. Colón-Rivera, but her diabetes had become uncontrolled because she was unable to reach her primary care doctor and couldn’t access the pharmacy. Her blood sugar level became elevated, leading to the delusions.
“A patient that was perfectly stable now is unstable,” he said. “Her diet has not been good enough through the pandemic, exacerbating her diabetes. She was admitted to the hospital for delirium.
Compounding of traumas
For many Latino patients, the adverse impacts of the pandemic comes on top of multiple prior traumas, such as violence exposures, discrimination, and economic issues, said Lisa Fortuna, MD, MPH, MDiv, chief of psychiatry and vice chair at Zuckerberg San Francisco General Hospital. A 2017 analysis found that nearly four in five Latino youth face at least one traumatic childhood experience, like poverty or abuse, and that about 29% of Latino youth experience four or more of these traumas.
Immigrants in particular, may have faced trauma in their home country and/or immigration trauma, Dr. Fortuna added. A 2013 study on immigrant Latino adolescents for example, found that 29% of foreign-born adolescents and 34% of foreign-born parents experienced trauma during the migration process (Int Migr Rev. 2013 Dec;47(4):10).
“All of these things are cumulative,” Dr. Fortuna said. “Then when you’re hit with a pandemic, all of the disparities that you already have and all the stress that you already have are compounded. This is for the kids, too, who have been exposed to a lot of stressors and now maybe have family members that have been ill or have died. All of these things definitely put people at risk for increased depression [and] the worsening of any preexisting posttraumatic stress disorder. We’ve seen this in previous disasters, and I expect that’s what we’re going to see more of with the COVID-19 pandemic.”
At the same time, a central cultural value of many Latinos is family unity, Dr. Montano said, a foundation that is now being strained by social distancing and severed connections.
“This has separated many families,” she said. “There has been a lot of loneliness and grief.”
Mistrust and fear toward the government, public agencies, and even the health system itself act as further hurdles for some Latinos in the face of COVID-19. In areas with large immigrant populations such as San Francisco, Dr. Fortuna noted, it’s not uncommon for undocumented patients to avoid accessing medical care and social services, or visiting emergency departments for needed care for fear of drawing attention to themselves or possible detainment.
“The fact that so many people showed up at our hospital so ill and ended up in the ICU – that could be a combination of factors. Because the population has high rates of diabetes and hypertension, that might have put people at increased risk for severe illness,” she said. “But some people may have been holding out for care because they wanted to avoid being in places out of fear of immigration scrutiny.”
Overcoming language barriers
Compounding the challenging pandemic landscape for Latino patients is the fact that many state resources about COVID-19 have not been translated to Spanish, Dr. Colón-Rivera said. He was troubled recently when he went to several state websites and found limited to no information in Spanish about the coronavirus. Some data about COVID-19 from the federal government were not translated to Spanish until officials received pushback, he added. Even now, press releases and other information disseminated by the federal government about the virus appear to be translated by an automated service – and lack sense and context.
The state agencies in Pennsylvania have been alerted to the absence of Spanish information, but change has been slow, he noted.
“In Philadelphia, 23% speaks a language other than English,” he said. “So we missed a lot of critical information that could have helped to avoid spreading the illness and access support.”
Dr. Fortuna said that California has done better with providing COVID-19–related information in Spanish, compared with some other states, but misinformation about the virus and lingering myths have still been a problem among the Latino community. The University of California, San Francisco, recently launched a Latino Task Force resource website for the Latino community that includes information in English, Spanish, and Yucatec Maya about COVID-19, health and wellness tips, and resources for various assistance needs.
The concerning lack of COVID-19 information translated to Spanish led Dr. Montano to start a Facebook page in Spanish about mental health tips and guidance for managing COVID-19–related issues. She and her team of clinicians share information, videos, relaxation exercises, and community resources on the page, among other posts. “There is also general info and recommendations about COVID-19 that I think can be useful for the community,” she said. “The idea is that patients, the general community, and providers can have share information, hope messages, and ask questions in Spanish.”
Feeling ‘helpless’
A central part of caring for Latino patients during the COVID-19 crisis has been referring them to outside agencies and social services, psychiatrists say. But finding the right resources amid a pandemic and ensuring that patients connect with the correct aid has been an uphill battle.
“We sometimes feel like our hands are tied,” Dr. Colón-Rivera said. “Sometimes, we need to call a place to bring food. Some of the state agencies and nonprofits don’t have delivery systems, so the patient has to go pick up for food or medication. Some of our patients don’t want to go outside. Some do not have cars.”
As a clinician, it can be easy to feel helpless when trying to navigate new challenges posed by the pandemic in addition to other longstanding barriers, Dr. Posada said.
“Already, mental health disorders are so influenced by social situations like poverty, job insecurity, or family issues, and now it just seems those obstacles are even more insurmountable,” she said. “At the end of the day, I can feel like: ‘Did I make a difference?’ That’s a big struggle.”
Dr. Montano’s team, which includes psychiatrists, psychologists, and social workers, have come to rely on virtual debriefings to vent, express frustrations, and support one another, she said. She also recently joined a virtual mind-body skills group as a participant.
“I recognize the importance of getting additional support and ways to alleviate burnout,” she said. “We need to take care of ourselves or we won’t be able to help others.”
Focusing on resilience during the current crisis can be beneficial for both patients and providers in coping and drawing strength, Dr. Posada said.
“When it comes to fostering resilience during times of hardship, I think it’s most helpful to reflect on what skills or attributes have helped during past crises and apply those now – whether it’s turning to comfort from close relationships, looking to religion and spirituality, practicing self-care like rest or exercise, or really tapping into one’s purpose and reason for practicing psychiatry and being a physician,” she said. “The same advice goes for clinicians: We’ve all been through hard times in the past, it’s part of the human condition and we’ve also witnessed a lot of suffering in our patients, so now is the time to practice those skills that have gotten us through hard times in the past.”
Learning lessons from COVID-19
Despite the challenges with moving to telehealth, Dr. Fortuna said the tool has proved beneficial overall for mental health care. For Dr. Fortuna’s team for example, telehealth by phone has decreased the no-show rate, compared with clinic visits, and improved care access.
“We need to figure out how to maintain that,” she said. “If we can build ways for equity and access to Internet, especially equipment, I think that’s going to help.”
In addition, more data are needed about the ways in which COVID-19 is affecting Latino patients, Dr. Colón-Rivera said. Mortality statistics have been published, but information is needed about the rates of infection and manifestation of illness.
Most importantly, the COVID-19 crisis has emphasized the critical need to address and improve the underlying inequity issues among Latino patients, psychiatrists say.
“We really need to think about how there can be partnerships, in terms of community-based Latino business and leaders, multisector resources, trying to think about how we can improve conditions both work and safety for Latinos,” Dr. Fortuna said. “How can schools get support in integrating mental health and support for families, especially now after COVID-19? And really looking at some of these underlying inequities that are the underpinnings of why people were at risk for the disproportionate effects of the COVID-19 pandemic.”
Race and location appear to play a role in the incidence of CLL and DLBCL
Exposure to carcinogens has been implicated in the development of non-Hodgkin lymphoma (NHL), suggesting that an examination of the environment on a population-based level might provide some insights. On that basis, researchers performed a study that found that living in an urban vs. rural area was associated with an increased risk of developing non-Hodgkin lymphoma (NHL) among diverse, urban populations.
The study, published online in Clinical Lymphoma, Myeloma & Leukemia, found an increased incidence of diffuse large B-cell lymphoma (DLBCL) in urban vs. rural Hispanics, and a similar increased incidence of chronic lymphocytic leukemia (CLL) in non-metropolitan urban non-Hispanic blacks.
A total of 482,096 adults aged 20 years and older with incident NHL were reported to 21 Surveillance, Epidemiology,and End Results (SEER) population-based registries for the period 2000 to 2016. Deanna Blansky of the Albert Einstein College of Medicine, Bronx, N.Y., and her colleagues compared patients by NHL subtype and urban-rural status, using rural-urban continuum codes from the U.S. Department of Agriculture.
The researchers found 136,197 DLBCL, 70,882 follicular lymphoma (FL), and 120,319 CLL cases of patients aged ≥ 20 years. The DLBCL patients comprised 73.6% non-Hispanic white, 11.8% Hispanic, and 7.3% non-Hispanic black, with a similar distribution observed for FL and CLL. Patients were adjusted for age, sex, and family poverty.
The study showed that, overall, there was a higher DLBCL incidence rate in metropolitan urban areas, compared with rural areas overall (incidence rate ratio [IRR] = 1.20, 95% confidence interval [CI] 1.11-1.30). Most pronounced was an increased DLBCL incidence among Hispanics in urban areas, compared with rural areas (rural IRR = 1.00; non-metropolitan urban IRR = 1.32, 95% CI 1.16-1.51; metropolitan urban = 1.55, 95% CI 1.36-1.76).
In contrast, metropolitan urban areas had a lower overall incidence of CLL than rural areas (8.4 vs. 9.7 per 100,000; IRR = .87; 95% CI .86-.89).
However, increased CLL incidence rates were found to be associated with non-metropolitan urban areas, compared with rural areas (IRR = 1.19; 95% CI 1.10-1.28), particularly among non-Hispanic Blacks (IRR = 1.49, 95% CI 1.27-1.72).
Unlike DLBCL and CLL, there were no differences observed in FL incidence rates by urban-rural status after adjusting for age, sex, and family poverty rates, the researchers reported.
“Overall, our findings suggest that factors related to urban status may be associated with DLBCL and CLL pathogenesis. Our results may help provide epidemiological clues to understanding the racial disparities seen among hematological malignancies, particularly regarding the risk of DLBCL in Hispanics and CLL in non-Hispanic Blacks,” the researchers concluded.
The study was sponsored by the U.S. National Institutes of Health. The researchers did not report conflict information.
SOURCE: Blansky D et al. Clin Lymphoma Myeloma Leuk. 2020 May 15; doi.org/10.1016/j.clml.2020.05.010.
Exposure to carcinogens has been implicated in the development of non-Hodgkin lymphoma (NHL), suggesting that an examination of the environment on a population-based level might provide some insights. On that basis, researchers performed a study that found that living in an urban vs. rural area was associated with an increased risk of developing non-Hodgkin lymphoma (NHL) among diverse, urban populations.
The study, published online in Clinical Lymphoma, Myeloma & Leukemia, found an increased incidence of diffuse large B-cell lymphoma (DLBCL) in urban vs. rural Hispanics, and a similar increased incidence of chronic lymphocytic leukemia (CLL) in non-metropolitan urban non-Hispanic blacks.
A total of 482,096 adults aged 20 years and older with incident NHL were reported to 21 Surveillance, Epidemiology,and End Results (SEER) population-based registries for the period 2000 to 2016. Deanna Blansky of the Albert Einstein College of Medicine, Bronx, N.Y., and her colleagues compared patients by NHL subtype and urban-rural status, using rural-urban continuum codes from the U.S. Department of Agriculture.
The researchers found 136,197 DLBCL, 70,882 follicular lymphoma (FL), and 120,319 CLL cases of patients aged ≥ 20 years. The DLBCL patients comprised 73.6% non-Hispanic white, 11.8% Hispanic, and 7.3% non-Hispanic black, with a similar distribution observed for FL and CLL. Patients were adjusted for age, sex, and family poverty.
The study showed that, overall, there was a higher DLBCL incidence rate in metropolitan urban areas, compared with rural areas overall (incidence rate ratio [IRR] = 1.20, 95% confidence interval [CI] 1.11-1.30). Most pronounced was an increased DLBCL incidence among Hispanics in urban areas, compared with rural areas (rural IRR = 1.00; non-metropolitan urban IRR = 1.32, 95% CI 1.16-1.51; metropolitan urban = 1.55, 95% CI 1.36-1.76).
In contrast, metropolitan urban areas had a lower overall incidence of CLL than rural areas (8.4 vs. 9.7 per 100,000; IRR = .87; 95% CI .86-.89).
However, increased CLL incidence rates were found to be associated with non-metropolitan urban areas, compared with rural areas (IRR = 1.19; 95% CI 1.10-1.28), particularly among non-Hispanic Blacks (IRR = 1.49, 95% CI 1.27-1.72).
Unlike DLBCL and CLL, there were no differences observed in FL incidence rates by urban-rural status after adjusting for age, sex, and family poverty rates, the researchers reported.
“Overall, our findings suggest that factors related to urban status may be associated with DLBCL and CLL pathogenesis. Our results may help provide epidemiological clues to understanding the racial disparities seen among hematological malignancies, particularly regarding the risk of DLBCL in Hispanics and CLL in non-Hispanic Blacks,” the researchers concluded.
The study was sponsored by the U.S. National Institutes of Health. The researchers did not report conflict information.
SOURCE: Blansky D et al. Clin Lymphoma Myeloma Leuk. 2020 May 15; doi.org/10.1016/j.clml.2020.05.010.
Exposure to carcinogens has been implicated in the development of non-Hodgkin lymphoma (NHL), suggesting that an examination of the environment on a population-based level might provide some insights. On that basis, researchers performed a study that found that living in an urban vs. rural area was associated with an increased risk of developing non-Hodgkin lymphoma (NHL) among diverse, urban populations.
The study, published online in Clinical Lymphoma, Myeloma & Leukemia, found an increased incidence of diffuse large B-cell lymphoma (DLBCL) in urban vs. rural Hispanics, and a similar increased incidence of chronic lymphocytic leukemia (CLL) in non-metropolitan urban non-Hispanic blacks.
A total of 482,096 adults aged 20 years and older with incident NHL were reported to 21 Surveillance, Epidemiology,and End Results (SEER) population-based registries for the period 2000 to 2016. Deanna Blansky of the Albert Einstein College of Medicine, Bronx, N.Y., and her colleagues compared patients by NHL subtype and urban-rural status, using rural-urban continuum codes from the U.S. Department of Agriculture.
The researchers found 136,197 DLBCL, 70,882 follicular lymphoma (FL), and 120,319 CLL cases of patients aged ≥ 20 years. The DLBCL patients comprised 73.6% non-Hispanic white, 11.8% Hispanic, and 7.3% non-Hispanic black, with a similar distribution observed for FL and CLL. Patients were adjusted for age, sex, and family poverty.
The study showed that, overall, there was a higher DLBCL incidence rate in metropolitan urban areas, compared with rural areas overall (incidence rate ratio [IRR] = 1.20, 95% confidence interval [CI] 1.11-1.30). Most pronounced was an increased DLBCL incidence among Hispanics in urban areas, compared with rural areas (rural IRR = 1.00; non-metropolitan urban IRR = 1.32, 95% CI 1.16-1.51; metropolitan urban = 1.55, 95% CI 1.36-1.76).
In contrast, metropolitan urban areas had a lower overall incidence of CLL than rural areas (8.4 vs. 9.7 per 100,000; IRR = .87; 95% CI .86-.89).
However, increased CLL incidence rates were found to be associated with non-metropolitan urban areas, compared with rural areas (IRR = 1.19; 95% CI 1.10-1.28), particularly among non-Hispanic Blacks (IRR = 1.49, 95% CI 1.27-1.72).
Unlike DLBCL and CLL, there were no differences observed in FL incidence rates by urban-rural status after adjusting for age, sex, and family poverty rates, the researchers reported.
“Overall, our findings suggest that factors related to urban status may be associated with DLBCL and CLL pathogenesis. Our results may help provide epidemiological clues to understanding the racial disparities seen among hematological malignancies, particularly regarding the risk of DLBCL in Hispanics and CLL in non-Hispanic Blacks,” the researchers concluded.
The study was sponsored by the U.S. National Institutes of Health. The researchers did not report conflict information.
SOURCE: Blansky D et al. Clin Lymphoma Myeloma Leuk. 2020 May 15; doi.org/10.1016/j.clml.2020.05.010.
FROM CLINICAL LYMPHOMA, MYELOMA & LEUKEMIA




![“The COVID pandemic has highlighted the structural inequities that affect the Latino population [both] immigrant and non-immigrant,” said Dr. Pamela Montano.](https://cdn.mdedge.com/files/s3fs-public/styles/medium/public/Montano_Pamela_NY_web.jpg?itok=9aJ-niUG)



![“The COVID pandemic has highlighted the structural inequities that affect the Latino population [both] immigrant and non-immigrant,” said Dr. Pamela Montano.](https://cdn.mdedge.com/files/s3fs-public/Montano_Pamela_NY_web.jpg)