User login
Hypertension goes unmedicated in 40% of adults
Roughly 30% of adults in the United States had hypertension in 2017, and just under 60% of those adults reported using antihypertensive medication, according to the Centers for Disease Control and Prevention.
There is, however, quite a bit of variation from those age-standardized national figures when state-level data are considered.
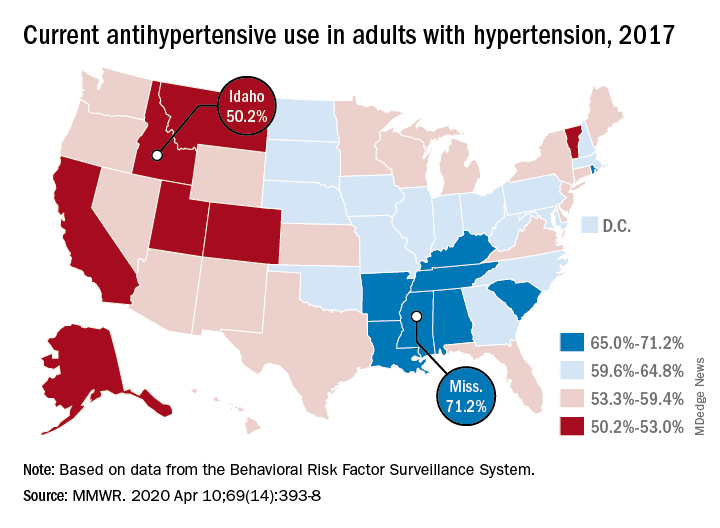
In Alabama and West Virginia, the prevalence of hypertension in 2017 was 38.6%, the highest in the country, with Arkansas (38.5%) and Mississippi (38.2%) not far behind. Meanwhile, Minnesota came in with a lowest-in-the-nation rate of 24.3%, which was nearly matched by Colorado at 24.8%, Claudine M. Samanic, PhD, and associates wrote in the MMWR.
There was also a considerable gap between the states in hypertensive adults’ self-reported use of antihypertensive drugs, which was generally higher in the states with a greater prevalence of disease, they noted.
Adults in Mississippi were the most likely (71.2%) to be taking medication, along with those in Alabama (70.5%) and Arkansas (69.3%). Idaho occupied the other end of the scale with a rate of 50.2%, while Montana and Vermont were slightly better at 51.7%, based on survey data from the Behavioral Risk Factor Surveillance System.
“Prevalence of antihypertensive medication use was higher in older age groups, highest among blacks, and higher among women [64.0%] than men [56.7%]. This overall gender difference has been reported previously, but the reasons are unclear,” wrote Dr. Samanic and associates at the CDC’s National Center for Chronic Disease Prevention and Health Promotion.
The BRFSS data for 2017 are based on based on interviews with 450,016 adults. Respondents were asked, “Have you ever been told by a doctor, nurse, or other health professional that you have high blood pressure?” and were considered to have hypertension if they answered yes.
SOURCE: Samanic CM et al. MMWR. 2020 Apr 10;69(14):393-8.
Roughly 30% of adults in the United States had hypertension in 2017, and just under 60% of those adults reported using antihypertensive medication, according to the Centers for Disease Control and Prevention.

There is, however, quite a bit of variation from those age-standardized national figures when state-level data are considered.
In Alabama and West Virginia, the prevalence of hypertension in 2017 was 38.6%, the highest in the country, with Arkansas (38.5%) and Mississippi (38.2%) not far behind. Meanwhile, Minnesota came in with a lowest-in-the-nation rate of 24.3%, which was nearly matched by Colorado at 24.8%, Claudine M. Samanic, PhD, and associates wrote in the MMWR.
There was also a considerable gap between the states in hypertensive adults’ self-reported use of antihypertensive drugs, which was generally higher in the states with a greater prevalence of disease, they noted.
Adults in Mississippi were the most likely (71.2%) to be taking medication, along with those in Alabama (70.5%) and Arkansas (69.3%). Idaho occupied the other end of the scale with a rate of 50.2%, while Montana and Vermont were slightly better at 51.7%, based on survey data from the Behavioral Risk Factor Surveillance System.
“Prevalence of antihypertensive medication use was higher in older age groups, highest among blacks, and higher among women [64.0%] than men [56.7%]. This overall gender difference has been reported previously, but the reasons are unclear,” wrote Dr. Samanic and associates at the CDC’s National Center for Chronic Disease Prevention and Health Promotion.
The BRFSS data for 2017 are based on based on interviews with 450,016 adults. Respondents were asked, “Have you ever been told by a doctor, nurse, or other health professional that you have high blood pressure?” and were considered to have hypertension if they answered yes.
SOURCE: Samanic CM et al. MMWR. 2020 Apr 10;69(14):393-8.
Roughly 30% of adults in the United States had hypertension in 2017, and just under 60% of those adults reported using antihypertensive medication, according to the Centers for Disease Control and Prevention.

There is, however, quite a bit of variation from those age-standardized national figures when state-level data are considered.
In Alabama and West Virginia, the prevalence of hypertension in 2017 was 38.6%, the highest in the country, with Arkansas (38.5%) and Mississippi (38.2%) not far behind. Meanwhile, Minnesota came in with a lowest-in-the-nation rate of 24.3%, which was nearly matched by Colorado at 24.8%, Claudine M. Samanic, PhD, and associates wrote in the MMWR.
There was also a considerable gap between the states in hypertensive adults’ self-reported use of antihypertensive drugs, which was generally higher in the states with a greater prevalence of disease, they noted.
Adults in Mississippi were the most likely (71.2%) to be taking medication, along with those in Alabama (70.5%) and Arkansas (69.3%). Idaho occupied the other end of the scale with a rate of 50.2%, while Montana and Vermont were slightly better at 51.7%, based on survey data from the Behavioral Risk Factor Surveillance System.
“Prevalence of antihypertensive medication use was higher in older age groups, highest among blacks, and higher among women [64.0%] than men [56.7%]. This overall gender difference has been reported previously, but the reasons are unclear,” wrote Dr. Samanic and associates at the CDC’s National Center for Chronic Disease Prevention and Health Promotion.
The BRFSS data for 2017 are based on based on interviews with 450,016 adults. Respondents were asked, “Have you ever been told by a doctor, nurse, or other health professional that you have high blood pressure?” and were considered to have hypertension if they answered yes.
SOURCE: Samanic CM et al. MMWR. 2020 Apr 10;69(14):393-8.
FROM THE MMWR
AHA updates management when CAD and T2DM coincide
Patients with stable coronary artery disease and type 2 diabetes mellitus could benefit from a “plethora of newly available risk-reduction strategies,” but their “adoption into clinical practice has been slow” and inconsistent, prompting an expert panel organized by the American Heart Association to collate the range of treatment recommendations now applicable to this patient population in a scientific statement released on April 13.
“There are a number of things to consider when treating patients with stable coronary artery disease [CAD] and type 2 diabetes mellitus [T2DM], with new medications and trials and data emerging. It’s difficult to keep up with all of the complexities,” which was why the Association’s Councils on Lifestyle and Cardiometabolic Health and on Clinical Cardiology put together a writing group to summarize and prioritize the range of lifestyle, medical, and interventional options that now require consideration and potential use on patients managed in routine practice, explained Suzanne V. Arnold, MD, chair of the writing group, in an interview.
The new scientific statement (Circulation. 2020 Apr 13; doi: 10.1161/CIR.0000000000000766), aimed primarily at cardiologists but also intended to inform primary care physicians, endocrinologists, and all other clinicians who deal with these patients, pulls together “everything someone needs to think about if they care for patients with CAD and T2DM,” said Deepak L. Bhatt, MD, professor of medicine at Harvard Medical School in Boston and vice chair of the statement-writing panel in an interview. “There is a lot to know,” he added.
The statement covers antithrombotic therapies; blood pressure control, with a discussion of both the appropriate pressure goal and the best drug types used to reach it; lipid management; glycemic control; lifestyle modification; weight management, including the role of bariatric surgery; and approaches to managing stable angina, both medically and with revascularization.
“The goal was to give clinicians a good sense of what new treatments they should consider” for these patients, said Dr. Bhatt, who is also director of interventional cardiovascular programs at Brigham and Women’s Hospital, also in Boston. Because of the tight associations between T2DM and cardiovascular disease in general including CAD, “cardiologists are increasingly involved in managing patients with T2DM,” he noted. The statement gives a comprehensive overview and critical assessment of the management of these patients as of the end of 2019 as a consensus from a panel of 11 experts .
The statement also stressed that “substantial portions of patients with T2DM and CAD, including those after an acute coronary syndrome, do not receive therapies with proven cardiovascular benefit, such as high-intensity statins, dual-antiplatelet therapy, angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers, and glucose-lowering agents with proven cardiovascular benefits.
“These gaps in care highlight a critical opportunity for cardiovascular specialists to assume a more active role in the collaborative care of patients with T2DM and CAD,” the statement said. This includes “encouraging cardiologists to become more active in the selection of glucose-lowering medications” for these patients because it could “really move the needle,” said Dr. Arnold, a cardiologist with Saint Luke’s Health System in Kansas City, Mo. She was referring specifically to broader reliance on both the SGTL2 (sodium-glucose cotransporter 2) inhibitors and the GLP-1 (glucagonlike peptide-1) receptor agonists as top choices for controlling hyperglycemia. Based on recent evidence drugs in these two classes “could be considered first line for patients with T2DM and CAD, and would likely be preferred over metformin,” Dr. Arnold said in an interview. Although the statement identified the SGLT2 inhibitors as “the first drug class [for glycemic control] to show clear benefits on cardiovascular outcomes,” it does not explicitly label the class first-line and it also skirts that designation for the GLP-1 receptor agonist class, while noting that metformin “remains the drug most frequently recommended as first-line therapy in treatment guidelines.”
“I wouldn’t disagree with someone who said that SGLT2 inhibitors and GLP-1 receptor agonists are first line,” but prescribing patterns also depend on familiarity, cost, and access, noted Dr. Bhatt, which can all be issues with agents from these classes compared with metformin, a widely available generic with decades of use. “Metformin is safe and cheap, so we did not want to discount it,” said Dr. Arnold. Dr. Bhatt recently coauthored an editorial that gave an enthusiastic endorsement to using SGLT2 inhibitors in patients with diabetes (Cell Metab. 2019 Nov 5;30[5]:47-9).
Another notable feature of the statement is the potential it assigns to bariatric surgery as a management tool with documented safety and efficacy for improving cardiovascular risk factors. However, the statement also notes that randomized trials “have thus far been inadequately powered to assess cardiovascular events and mortality, although observational studies have consistently shown cardiovascular risk reduction with such procedures.” The statement continues that despite potential cardiovascular benefits “bariatric surgery remains underused among eligible patients,” and said that surgery performed as Roux-en-Y bypass or sleeve gastrectomy “may be another effective tool for cardiovascular risk reduction in the subset of patients with obesity,” particularly patients with a body mass index of at least 35 kg/m2.
“While the percentage of patients who are optimal for bariatric surgery is not known, the most recent NHANES [National Health and Nutrition Examination Study] study showed that less than 0.5% of eligible patients underwent bariatric surgery,” Dr. Arnold noted. Bariatric surgery is “certainly not a recommendation for everyone, or even a majority of patients, but bariatric surgery should be on our radar,” for patients with CAD and T2DM, she said.
Right now, “few cardiologists think about bariatric surgery,” as a treatment option, but study results have shown that “in carefully selected patients treated by skilled surgeons at high-volume centers, patients will do better with bariatric surgery than with best medical therapy for improvements in multiple risk factors, including glycemic control,” Dr. Bhatt said in the interview. “It’s not first-line treatment, but it’s an option to consider,” he added, while also noting that bariatric surgery is most beneficial to patients relatively early in the course of T2DM, when its been in place for just a few years rather than a couple of decades.
The statement also notably included a “first-line” call out for icosapent ethyl (Vascepa), a novel agent approved in December 2019 for routine use in U.S. patients, including those with CAD and T2DM as long as their blood triglyceride level was at least 150 mg/dL. Dr. Bhatt, who led the REDUCE-IT study that was pivotal for proving the safety and efficacy of icosapent ethyl (N Engl J Med. 2019 Jan 3;380[1]:11-22), estimated that anywhere from 15% to as many as half the patients with CAD and T2DM might have a triglyceride level that would allow them to receive icosapent ethyl. One population-based study in Canada of nearly 200,000 people with atherosclerotic cardiovascular disease found a 25% prevalence of the triglyceride level needed to qualify to receive icosapent ethyl under current labeling, he noted (Eur Heart J. 2020 Jan 1;41[1]:86-94). However, the FDA label does not specify that triglycerides be measured when fasting, and a nonfasting level of about 150 mg/dL will likely appear for patients with fasting levels that fall as low as about 100 mg/dL, Dr. Bhatt said. He hoped that future studies will assess the efficacy of icosapent ethyl in patients with even lower triglyceride levels.
Other sections of the statement also recommend that clinicians: Target long-term dual-antiplatelet therapy to CAD and T2DM patients with additional high-risk markers such as prior MI, younger age, and tobacco use; prescribe a low-dose oral anticoagulant along with an antiplatelet drug such as aspirin for secondary-prevention patients; promote a blood pressure target of less than 140/90 mm Hg for all CAD and T2DM patients and apply a goal of less than 130/80 mm Hg in higher-risk patients such as blacks, Asians, and those with cerebrovascular disease; and reassure patients that “despite a modest increase in blood sugars, the risk-benefit ratio is clearly in favor of administering statins to people with T2DM and CAD.”
Dr. Arnold had no disclosures. Dr. Bhatt has been an adviser to Cardax, Cereno Scientific, Medscape Cardiology, PhaseBio; PLx Pharma, and Regado Biosciences, and he has received research funding from numerous companies including Amarin, the company that markets icosapent ethyl.
Patients with stable coronary artery disease and type 2 diabetes mellitus could benefit from a “plethora of newly available risk-reduction strategies,” but their “adoption into clinical practice has been slow” and inconsistent, prompting an expert panel organized by the American Heart Association to collate the range of treatment recommendations now applicable to this patient population in a scientific statement released on April 13.
“There are a number of things to consider when treating patients with stable coronary artery disease [CAD] and type 2 diabetes mellitus [T2DM], with new medications and trials and data emerging. It’s difficult to keep up with all of the complexities,” which was why the Association’s Councils on Lifestyle and Cardiometabolic Health and on Clinical Cardiology put together a writing group to summarize and prioritize the range of lifestyle, medical, and interventional options that now require consideration and potential use on patients managed in routine practice, explained Suzanne V. Arnold, MD, chair of the writing group, in an interview.
The new scientific statement (Circulation. 2020 Apr 13; doi: 10.1161/CIR.0000000000000766), aimed primarily at cardiologists but also intended to inform primary care physicians, endocrinologists, and all other clinicians who deal with these patients, pulls together “everything someone needs to think about if they care for patients with CAD and T2DM,” said Deepak L. Bhatt, MD, professor of medicine at Harvard Medical School in Boston and vice chair of the statement-writing panel in an interview. “There is a lot to know,” he added.
The statement covers antithrombotic therapies; blood pressure control, with a discussion of both the appropriate pressure goal and the best drug types used to reach it; lipid management; glycemic control; lifestyle modification; weight management, including the role of bariatric surgery; and approaches to managing stable angina, both medically and with revascularization.
“The goal was to give clinicians a good sense of what new treatments they should consider” for these patients, said Dr. Bhatt, who is also director of interventional cardiovascular programs at Brigham and Women’s Hospital, also in Boston. Because of the tight associations between T2DM and cardiovascular disease in general including CAD, “cardiologists are increasingly involved in managing patients with T2DM,” he noted. The statement gives a comprehensive overview and critical assessment of the management of these patients as of the end of 2019 as a consensus from a panel of 11 experts .
The statement also stressed that “substantial portions of patients with T2DM and CAD, including those after an acute coronary syndrome, do not receive therapies with proven cardiovascular benefit, such as high-intensity statins, dual-antiplatelet therapy, angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers, and glucose-lowering agents with proven cardiovascular benefits.
“These gaps in care highlight a critical opportunity for cardiovascular specialists to assume a more active role in the collaborative care of patients with T2DM and CAD,” the statement said. This includes “encouraging cardiologists to become more active in the selection of glucose-lowering medications” for these patients because it could “really move the needle,” said Dr. Arnold, a cardiologist with Saint Luke’s Health System in Kansas City, Mo. She was referring specifically to broader reliance on both the SGTL2 (sodium-glucose cotransporter 2) inhibitors and the GLP-1 (glucagonlike peptide-1) receptor agonists as top choices for controlling hyperglycemia. Based on recent evidence drugs in these two classes “could be considered first line for patients with T2DM and CAD, and would likely be preferred over metformin,” Dr. Arnold said in an interview. Although the statement identified the SGLT2 inhibitors as “the first drug class [for glycemic control] to show clear benefits on cardiovascular outcomes,” it does not explicitly label the class first-line and it also skirts that designation for the GLP-1 receptor agonist class, while noting that metformin “remains the drug most frequently recommended as first-line therapy in treatment guidelines.”
“I wouldn’t disagree with someone who said that SGLT2 inhibitors and GLP-1 receptor agonists are first line,” but prescribing patterns also depend on familiarity, cost, and access, noted Dr. Bhatt, which can all be issues with agents from these classes compared with metformin, a widely available generic with decades of use. “Metformin is safe and cheap, so we did not want to discount it,” said Dr. Arnold. Dr. Bhatt recently coauthored an editorial that gave an enthusiastic endorsement to using SGLT2 inhibitors in patients with diabetes (Cell Metab. 2019 Nov 5;30[5]:47-9).
Another notable feature of the statement is the potential it assigns to bariatric surgery as a management tool with documented safety and efficacy for improving cardiovascular risk factors. However, the statement also notes that randomized trials “have thus far been inadequately powered to assess cardiovascular events and mortality, although observational studies have consistently shown cardiovascular risk reduction with such procedures.” The statement continues that despite potential cardiovascular benefits “bariatric surgery remains underused among eligible patients,” and said that surgery performed as Roux-en-Y bypass or sleeve gastrectomy “may be another effective tool for cardiovascular risk reduction in the subset of patients with obesity,” particularly patients with a body mass index of at least 35 kg/m2.
“While the percentage of patients who are optimal for bariatric surgery is not known, the most recent NHANES [National Health and Nutrition Examination Study] study showed that less than 0.5% of eligible patients underwent bariatric surgery,” Dr. Arnold noted. Bariatric surgery is “certainly not a recommendation for everyone, or even a majority of patients, but bariatric surgery should be on our radar,” for patients with CAD and T2DM, she said.
Right now, “few cardiologists think about bariatric surgery,” as a treatment option, but study results have shown that “in carefully selected patients treated by skilled surgeons at high-volume centers, patients will do better with bariatric surgery than with best medical therapy for improvements in multiple risk factors, including glycemic control,” Dr. Bhatt said in the interview. “It’s not first-line treatment, but it’s an option to consider,” he added, while also noting that bariatric surgery is most beneficial to patients relatively early in the course of T2DM, when its been in place for just a few years rather than a couple of decades.
The statement also notably included a “first-line” call out for icosapent ethyl (Vascepa), a novel agent approved in December 2019 for routine use in U.S. patients, including those with CAD and T2DM as long as their blood triglyceride level was at least 150 mg/dL. Dr. Bhatt, who led the REDUCE-IT study that was pivotal for proving the safety and efficacy of icosapent ethyl (N Engl J Med. 2019 Jan 3;380[1]:11-22), estimated that anywhere from 15% to as many as half the patients with CAD and T2DM might have a triglyceride level that would allow them to receive icosapent ethyl. One population-based study in Canada of nearly 200,000 people with atherosclerotic cardiovascular disease found a 25% prevalence of the triglyceride level needed to qualify to receive icosapent ethyl under current labeling, he noted (Eur Heart J. 2020 Jan 1;41[1]:86-94). However, the FDA label does not specify that triglycerides be measured when fasting, and a nonfasting level of about 150 mg/dL will likely appear for patients with fasting levels that fall as low as about 100 mg/dL, Dr. Bhatt said. He hoped that future studies will assess the efficacy of icosapent ethyl in patients with even lower triglyceride levels.
Other sections of the statement also recommend that clinicians: Target long-term dual-antiplatelet therapy to CAD and T2DM patients with additional high-risk markers such as prior MI, younger age, and tobacco use; prescribe a low-dose oral anticoagulant along with an antiplatelet drug such as aspirin for secondary-prevention patients; promote a blood pressure target of less than 140/90 mm Hg for all CAD and T2DM patients and apply a goal of less than 130/80 mm Hg in higher-risk patients such as blacks, Asians, and those with cerebrovascular disease; and reassure patients that “despite a modest increase in blood sugars, the risk-benefit ratio is clearly in favor of administering statins to people with T2DM and CAD.”
Dr. Arnold had no disclosures. Dr. Bhatt has been an adviser to Cardax, Cereno Scientific, Medscape Cardiology, PhaseBio; PLx Pharma, and Regado Biosciences, and he has received research funding from numerous companies including Amarin, the company that markets icosapent ethyl.
Patients with stable coronary artery disease and type 2 diabetes mellitus could benefit from a “plethora of newly available risk-reduction strategies,” but their “adoption into clinical practice has been slow” and inconsistent, prompting an expert panel organized by the American Heart Association to collate the range of treatment recommendations now applicable to this patient population in a scientific statement released on April 13.
“There are a number of things to consider when treating patients with stable coronary artery disease [CAD] and type 2 diabetes mellitus [T2DM], with new medications and trials and data emerging. It’s difficult to keep up with all of the complexities,” which was why the Association’s Councils on Lifestyle and Cardiometabolic Health and on Clinical Cardiology put together a writing group to summarize and prioritize the range of lifestyle, medical, and interventional options that now require consideration and potential use on patients managed in routine practice, explained Suzanne V. Arnold, MD, chair of the writing group, in an interview.
The new scientific statement (Circulation. 2020 Apr 13; doi: 10.1161/CIR.0000000000000766), aimed primarily at cardiologists but also intended to inform primary care physicians, endocrinologists, and all other clinicians who deal with these patients, pulls together “everything someone needs to think about if they care for patients with CAD and T2DM,” said Deepak L. Bhatt, MD, professor of medicine at Harvard Medical School in Boston and vice chair of the statement-writing panel in an interview. “There is a lot to know,” he added.
The statement covers antithrombotic therapies; blood pressure control, with a discussion of both the appropriate pressure goal and the best drug types used to reach it; lipid management; glycemic control; lifestyle modification; weight management, including the role of bariatric surgery; and approaches to managing stable angina, both medically and with revascularization.
“The goal was to give clinicians a good sense of what new treatments they should consider” for these patients, said Dr. Bhatt, who is also director of interventional cardiovascular programs at Brigham and Women’s Hospital, also in Boston. Because of the tight associations between T2DM and cardiovascular disease in general including CAD, “cardiologists are increasingly involved in managing patients with T2DM,” he noted. The statement gives a comprehensive overview and critical assessment of the management of these patients as of the end of 2019 as a consensus from a panel of 11 experts .
The statement also stressed that “substantial portions of patients with T2DM and CAD, including those after an acute coronary syndrome, do not receive therapies with proven cardiovascular benefit, such as high-intensity statins, dual-antiplatelet therapy, angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers, and glucose-lowering agents with proven cardiovascular benefits.
“These gaps in care highlight a critical opportunity for cardiovascular specialists to assume a more active role in the collaborative care of patients with T2DM and CAD,” the statement said. This includes “encouraging cardiologists to become more active in the selection of glucose-lowering medications” for these patients because it could “really move the needle,” said Dr. Arnold, a cardiologist with Saint Luke’s Health System in Kansas City, Mo. She was referring specifically to broader reliance on both the SGTL2 (sodium-glucose cotransporter 2) inhibitors and the GLP-1 (glucagonlike peptide-1) receptor agonists as top choices for controlling hyperglycemia. Based on recent evidence drugs in these two classes “could be considered first line for patients with T2DM and CAD, and would likely be preferred over metformin,” Dr. Arnold said in an interview. Although the statement identified the SGLT2 inhibitors as “the first drug class [for glycemic control] to show clear benefits on cardiovascular outcomes,” it does not explicitly label the class first-line and it also skirts that designation for the GLP-1 receptor agonist class, while noting that metformin “remains the drug most frequently recommended as first-line therapy in treatment guidelines.”
“I wouldn’t disagree with someone who said that SGLT2 inhibitors and GLP-1 receptor agonists are first line,” but prescribing patterns also depend on familiarity, cost, and access, noted Dr. Bhatt, which can all be issues with agents from these classes compared with metformin, a widely available generic with decades of use. “Metformin is safe and cheap, so we did not want to discount it,” said Dr. Arnold. Dr. Bhatt recently coauthored an editorial that gave an enthusiastic endorsement to using SGLT2 inhibitors in patients with diabetes (Cell Metab. 2019 Nov 5;30[5]:47-9).
Another notable feature of the statement is the potential it assigns to bariatric surgery as a management tool with documented safety and efficacy for improving cardiovascular risk factors. However, the statement also notes that randomized trials “have thus far been inadequately powered to assess cardiovascular events and mortality, although observational studies have consistently shown cardiovascular risk reduction with such procedures.” The statement continues that despite potential cardiovascular benefits “bariatric surgery remains underused among eligible patients,” and said that surgery performed as Roux-en-Y bypass or sleeve gastrectomy “may be another effective tool for cardiovascular risk reduction in the subset of patients with obesity,” particularly patients with a body mass index of at least 35 kg/m2.
“While the percentage of patients who are optimal for bariatric surgery is not known, the most recent NHANES [National Health and Nutrition Examination Study] study showed that less than 0.5% of eligible patients underwent bariatric surgery,” Dr. Arnold noted. Bariatric surgery is “certainly not a recommendation for everyone, or even a majority of patients, but bariatric surgery should be on our radar,” for patients with CAD and T2DM, she said.
Right now, “few cardiologists think about bariatric surgery,” as a treatment option, but study results have shown that “in carefully selected patients treated by skilled surgeons at high-volume centers, patients will do better with bariatric surgery than with best medical therapy for improvements in multiple risk factors, including glycemic control,” Dr. Bhatt said in the interview. “It’s not first-line treatment, but it’s an option to consider,” he added, while also noting that bariatric surgery is most beneficial to patients relatively early in the course of T2DM, when its been in place for just a few years rather than a couple of decades.
The statement also notably included a “first-line” call out for icosapent ethyl (Vascepa), a novel agent approved in December 2019 for routine use in U.S. patients, including those with CAD and T2DM as long as their blood triglyceride level was at least 150 mg/dL. Dr. Bhatt, who led the REDUCE-IT study that was pivotal for proving the safety and efficacy of icosapent ethyl (N Engl J Med. 2019 Jan 3;380[1]:11-22), estimated that anywhere from 15% to as many as half the patients with CAD and T2DM might have a triglyceride level that would allow them to receive icosapent ethyl. One population-based study in Canada of nearly 200,000 people with atherosclerotic cardiovascular disease found a 25% prevalence of the triglyceride level needed to qualify to receive icosapent ethyl under current labeling, he noted (Eur Heart J. 2020 Jan 1;41[1]:86-94). However, the FDA label does not specify that triglycerides be measured when fasting, and a nonfasting level of about 150 mg/dL will likely appear for patients with fasting levels that fall as low as about 100 mg/dL, Dr. Bhatt said. He hoped that future studies will assess the efficacy of icosapent ethyl in patients with even lower triglyceride levels.
Other sections of the statement also recommend that clinicians: Target long-term dual-antiplatelet therapy to CAD and T2DM patients with additional high-risk markers such as prior MI, younger age, and tobacco use; prescribe a low-dose oral anticoagulant along with an antiplatelet drug such as aspirin for secondary-prevention patients; promote a blood pressure target of less than 140/90 mm Hg for all CAD and T2DM patients and apply a goal of less than 130/80 mm Hg in higher-risk patients such as blacks, Asians, and those with cerebrovascular disease; and reassure patients that “despite a modest increase in blood sugars, the risk-benefit ratio is clearly in favor of administering statins to people with T2DM and CAD.”
Dr. Arnold had no disclosures. Dr. Bhatt has been an adviser to Cardax, Cereno Scientific, Medscape Cardiology, PhaseBio; PLx Pharma, and Regado Biosciences, and he has received research funding from numerous companies including Amarin, the company that markets icosapent ethyl.
FROM CIRCULATION
Pandemic necessitates new strategies to treat migraine
Patients with migraine who are unable to continue preventive procedures such as onabotulinumtoxinA injections during the COVID-19 pandemic may be at risk of worsening migraine, according to an article published March 30 in Headache. To address this scenario, including monoclonal antibodies against calcitonin gene-related peptide (CGRP) or the CGRP receptor, the authors said.
“This is a particularly vulnerable time for individuals with migraine and other disabling headache disorders, with many physical and mental stressors, increased anxiety, and changes in daily routine which may serve as triggering factors for worsening headache,” said lead author Christina L. Szperka, MD, director of the pediatric headache program at Children’s Hospital of Philadelphia, and colleagues.
Acute treatment
The authors described potential treatment regimens based on their experience as headache specialists and the experiences of their colleagues. For acute therapy options, NSAIDs, triptans, and neuroleptics may be used in combination when needed. Medications within the same drug category should not be combined, however, and triptans, dihydroergotamine, and lasmiditan should not be coadministered within 24 hours. Since the 2015 American Headache Society guideline for the acute treatment of migraine, the Food and Drug Administration has approved additional acute migraine medications, including ubrogepant, rimegepant, and lasmiditan, the authors said. The agency also cleared several neuromodulation devices for the acute treatment of migraine.
Although few drugs have been studied as treatments for unusually prolonged severe headaches, headache doctors often recommend NSAIDs before patients seek care at an emergency department or infusion center, the authors said. NSAID options include indomethacin, ketorolac, naproxen, nabumetone, diclofenac, and mefenamic acid. Neuroleptics also may be used. “Long-acting triptan medications can be used as bridge therapies, as is often done in the treatment of menstrually related migraine or in the treatment of medication overuse headache,” they said. “We propose a similar strategy can be trialed as a therapeutic option for refractory or persistent migraine.”
The authors also described the use of antiepileptics and corticosteroids, as well as drugs that may treat specific symptoms, such as difficulty sleeping (hydroxyzine or amitriptyline), neck or muscle pain (tizanidine), and aura with migraine (magnesium). Clinicians should avoid the use of opioids and butalbital, they said.
Preventive treatment
“While the injection of onabotulinumtoxinA is an effective treatment for chronic migraine, the procedure can put the patient and the provider at higher risk of COVID-19 given the close contact encounter,” wrote Dr. Szperka and colleagues. “We believe that other migraine preventive treatments should be utilized first when possible.” Since the publication of a guideline on preventive migraine therapies in 2012, the FDA has approved additional preventive therapies, including the anti-CGRP monoclonal antibodies erenumab‐aooe, galcanezumab‐gnlm, fremanezumab‐vfrm, and eptinezumab‐jjmr. “The first three are intended for self‐injection at home, with detailed instructions available for each product on its website,” they said.
Among angiotensin‐converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), candesartan has evidence of efficacy and tolerability in migraine prevention. Lisinopril has been considered possibly effective. “There has been recent concern in the media about the possibility of these medications interfering with the body’s response to COVID‐19,” the authors said, although this theoretical concern was not based on experimental or clinical data. “For patients in need of a new preventive therapy, the potential for benefit with an ACE/ARB must be weighed against the theoretical increased risk of infection.”
In addition, studies indicate that melatonin may prevent migraine with few side effects and that zonisamide may be effective in patients who have an inadequate response to or experience side effects with topiramate.
Policy changes and telehealth options
Effectively treating patients with migraine during the pandemic requires policy changes, according to the authors. “Migraine preventive prior authorization restrictions need to be lifted for evidence‐based, FDA‐approved therapies; patients need to be able to access these medications quickly and easily. Patients should not be required to fail older medications,” they said. “Similarly, in order to permit the transition of patients from onabotulinumtoxinA to anti‐CGRP [monoclonal antibodies], insurers should remove the prohibition against simultaneous coverage of these drug classes.” Insurers also should loosen restrictions on the off-label use of acute and preventive medication for adolescents, Dr. Szperka and coauthors suggest.
“In the era of COVID‐19, telehealth has become an essential modality for most headache specialists, given the need for providers to take significant precautions for both their patients and themselves, limiting touch or close contact,” they said. Patients with headache may warrant additional screening for COVID-19 as well. “As headache has been reported as an early symptom of COVID‐19, patients with worsening or new onset severe headache should be reviewed for exposure risk and any other symptoms which may be consistent with COVID‐19 infection,” the authors said.
There was no direct funding for the report. Dr. Szperka and a coauthor receive salary support from the National Institutes of Health. Dr. Szperka also has received grant support from Pfizer, and her institution has received compensation for her consulting work for Allergan. Several coauthors disclosed consulting and serving on speakers’ bureaus for and receiving research support from various pharmaceutical companies.
SOURCE: Szperka CL et al. Headache. 2020 Mar 30. doi: 10.1111/head.13810.
Patients with migraine who are unable to continue preventive procedures such as onabotulinumtoxinA injections during the COVID-19 pandemic may be at risk of worsening migraine, according to an article published March 30 in Headache. To address this scenario, including monoclonal antibodies against calcitonin gene-related peptide (CGRP) or the CGRP receptor, the authors said.
“This is a particularly vulnerable time for individuals with migraine and other disabling headache disorders, with many physical and mental stressors, increased anxiety, and changes in daily routine which may serve as triggering factors for worsening headache,” said lead author Christina L. Szperka, MD, director of the pediatric headache program at Children’s Hospital of Philadelphia, and colleagues.
Acute treatment
The authors described potential treatment regimens based on their experience as headache specialists and the experiences of their colleagues. For acute therapy options, NSAIDs, triptans, and neuroleptics may be used in combination when needed. Medications within the same drug category should not be combined, however, and triptans, dihydroergotamine, and lasmiditan should not be coadministered within 24 hours. Since the 2015 American Headache Society guideline for the acute treatment of migraine, the Food and Drug Administration has approved additional acute migraine medications, including ubrogepant, rimegepant, and lasmiditan, the authors said. The agency also cleared several neuromodulation devices for the acute treatment of migraine.
Although few drugs have been studied as treatments for unusually prolonged severe headaches, headache doctors often recommend NSAIDs before patients seek care at an emergency department or infusion center, the authors said. NSAID options include indomethacin, ketorolac, naproxen, nabumetone, diclofenac, and mefenamic acid. Neuroleptics also may be used. “Long-acting triptan medications can be used as bridge therapies, as is often done in the treatment of menstrually related migraine or in the treatment of medication overuse headache,” they said. “We propose a similar strategy can be trialed as a therapeutic option for refractory or persistent migraine.”
The authors also described the use of antiepileptics and corticosteroids, as well as drugs that may treat specific symptoms, such as difficulty sleeping (hydroxyzine or amitriptyline), neck or muscle pain (tizanidine), and aura with migraine (magnesium). Clinicians should avoid the use of opioids and butalbital, they said.
Preventive treatment
“While the injection of onabotulinumtoxinA is an effective treatment for chronic migraine, the procedure can put the patient and the provider at higher risk of COVID-19 given the close contact encounter,” wrote Dr. Szperka and colleagues. “We believe that other migraine preventive treatments should be utilized first when possible.” Since the publication of a guideline on preventive migraine therapies in 2012, the FDA has approved additional preventive therapies, including the anti-CGRP monoclonal antibodies erenumab‐aooe, galcanezumab‐gnlm, fremanezumab‐vfrm, and eptinezumab‐jjmr. “The first three are intended for self‐injection at home, with detailed instructions available for each product on its website,” they said.
Among angiotensin‐converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), candesartan has evidence of efficacy and tolerability in migraine prevention. Lisinopril has been considered possibly effective. “There has been recent concern in the media about the possibility of these medications interfering with the body’s response to COVID‐19,” the authors said, although this theoretical concern was not based on experimental or clinical data. “For patients in need of a new preventive therapy, the potential for benefit with an ACE/ARB must be weighed against the theoretical increased risk of infection.”
In addition, studies indicate that melatonin may prevent migraine with few side effects and that zonisamide may be effective in patients who have an inadequate response to or experience side effects with topiramate.
Policy changes and telehealth options
Effectively treating patients with migraine during the pandemic requires policy changes, according to the authors. “Migraine preventive prior authorization restrictions need to be lifted for evidence‐based, FDA‐approved therapies; patients need to be able to access these medications quickly and easily. Patients should not be required to fail older medications,” they said. “Similarly, in order to permit the transition of patients from onabotulinumtoxinA to anti‐CGRP [monoclonal antibodies], insurers should remove the prohibition against simultaneous coverage of these drug classes.” Insurers also should loosen restrictions on the off-label use of acute and preventive medication for adolescents, Dr. Szperka and coauthors suggest.
“In the era of COVID‐19, telehealth has become an essential modality for most headache specialists, given the need for providers to take significant precautions for both their patients and themselves, limiting touch or close contact,” they said. Patients with headache may warrant additional screening for COVID-19 as well. “As headache has been reported as an early symptom of COVID‐19, patients with worsening or new onset severe headache should be reviewed for exposure risk and any other symptoms which may be consistent with COVID‐19 infection,” the authors said.
There was no direct funding for the report. Dr. Szperka and a coauthor receive salary support from the National Institutes of Health. Dr. Szperka also has received grant support from Pfizer, and her institution has received compensation for her consulting work for Allergan. Several coauthors disclosed consulting and serving on speakers’ bureaus for and receiving research support from various pharmaceutical companies.
SOURCE: Szperka CL et al. Headache. 2020 Mar 30. doi: 10.1111/head.13810.
Patients with migraine who are unable to continue preventive procedures such as onabotulinumtoxinA injections during the COVID-19 pandemic may be at risk of worsening migraine, according to an article published March 30 in Headache. To address this scenario, including monoclonal antibodies against calcitonin gene-related peptide (CGRP) or the CGRP receptor, the authors said.
“This is a particularly vulnerable time for individuals with migraine and other disabling headache disorders, with many physical and mental stressors, increased anxiety, and changes in daily routine which may serve as triggering factors for worsening headache,” said lead author Christina L. Szperka, MD, director of the pediatric headache program at Children’s Hospital of Philadelphia, and colleagues.
Acute treatment
The authors described potential treatment regimens based on their experience as headache specialists and the experiences of their colleagues. For acute therapy options, NSAIDs, triptans, and neuroleptics may be used in combination when needed. Medications within the same drug category should not be combined, however, and triptans, dihydroergotamine, and lasmiditan should not be coadministered within 24 hours. Since the 2015 American Headache Society guideline for the acute treatment of migraine, the Food and Drug Administration has approved additional acute migraine medications, including ubrogepant, rimegepant, and lasmiditan, the authors said. The agency also cleared several neuromodulation devices for the acute treatment of migraine.
Although few drugs have been studied as treatments for unusually prolonged severe headaches, headache doctors often recommend NSAIDs before patients seek care at an emergency department or infusion center, the authors said. NSAID options include indomethacin, ketorolac, naproxen, nabumetone, diclofenac, and mefenamic acid. Neuroleptics also may be used. “Long-acting triptan medications can be used as bridge therapies, as is often done in the treatment of menstrually related migraine or in the treatment of medication overuse headache,” they said. “We propose a similar strategy can be trialed as a therapeutic option for refractory or persistent migraine.”
The authors also described the use of antiepileptics and corticosteroids, as well as drugs that may treat specific symptoms, such as difficulty sleeping (hydroxyzine or amitriptyline), neck or muscle pain (tizanidine), and aura with migraine (magnesium). Clinicians should avoid the use of opioids and butalbital, they said.
Preventive treatment
“While the injection of onabotulinumtoxinA is an effective treatment for chronic migraine, the procedure can put the patient and the provider at higher risk of COVID-19 given the close contact encounter,” wrote Dr. Szperka and colleagues. “We believe that other migraine preventive treatments should be utilized first when possible.” Since the publication of a guideline on preventive migraine therapies in 2012, the FDA has approved additional preventive therapies, including the anti-CGRP monoclonal antibodies erenumab‐aooe, galcanezumab‐gnlm, fremanezumab‐vfrm, and eptinezumab‐jjmr. “The first three are intended for self‐injection at home, with detailed instructions available for each product on its website,” they said.
Among angiotensin‐converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), candesartan has evidence of efficacy and tolerability in migraine prevention. Lisinopril has been considered possibly effective. “There has been recent concern in the media about the possibility of these medications interfering with the body’s response to COVID‐19,” the authors said, although this theoretical concern was not based on experimental or clinical data. “For patients in need of a new preventive therapy, the potential for benefit with an ACE/ARB must be weighed against the theoretical increased risk of infection.”
In addition, studies indicate that melatonin may prevent migraine with few side effects and that zonisamide may be effective in patients who have an inadequate response to or experience side effects with topiramate.
Policy changes and telehealth options
Effectively treating patients with migraine during the pandemic requires policy changes, according to the authors. “Migraine preventive prior authorization restrictions need to be lifted for evidence‐based, FDA‐approved therapies; patients need to be able to access these medications quickly and easily. Patients should not be required to fail older medications,” they said. “Similarly, in order to permit the transition of patients from onabotulinumtoxinA to anti‐CGRP [monoclonal antibodies], insurers should remove the prohibition against simultaneous coverage of these drug classes.” Insurers also should loosen restrictions on the off-label use of acute and preventive medication for adolescents, Dr. Szperka and coauthors suggest.
“In the era of COVID‐19, telehealth has become an essential modality for most headache specialists, given the need for providers to take significant precautions for both their patients and themselves, limiting touch or close contact,” they said. Patients with headache may warrant additional screening for COVID-19 as well. “As headache has been reported as an early symptom of COVID‐19, patients with worsening or new onset severe headache should be reviewed for exposure risk and any other symptoms which may be consistent with COVID‐19 infection,” the authors said.
There was no direct funding for the report. Dr. Szperka and a coauthor receive salary support from the National Institutes of Health. Dr. Szperka also has received grant support from Pfizer, and her institution has received compensation for her consulting work for Allergan. Several coauthors disclosed consulting and serving on speakers’ bureaus for and receiving research support from various pharmaceutical companies.
SOURCE: Szperka CL et al. Headache. 2020 Mar 30. doi: 10.1111/head.13810.
FROM HEADACHE
‘We’re in great distress here,’ infusion center CMO says
Count Vikram Sengupta, MD, among the slew of health care workers feeling overwhelmed by the impact that COVID-19 is having on the delivery of health care in Manhattan and its surrounding boroughs.
“Nobody in the country is suffering like New York City,” said Dr. Sengupta, chief medical officer of Thrivewell Infusion, which operates three stand-alone infusion centers in the region: a four-chair center in Crown Heights, a 10-chair center in Borough Park, Brooklyn, and an eight-chair center in Manhasset. “We have 30%-50% of all cases in the country. I’ve been reading the news, and some people think this thing is going away. We’re in great distress here. There need to be new strategies moving forward. The whole world has changed. Our whole approach to ambulatory care has changed.”
In early March 2020, when it became clear that New York hospitals would face a tidal wave of citizens infected with COVID-19, Thrivewell began to receive an influx of referrals originating from concerned patients, providers, payers, and even large integrated health care systems, all in an effort to help prevent infectious exposure through infusion in hospital-based settings. “We are trying to accommodate them as swiftly as possible,” said Dr. Sengupta, who was interviewed for this story on April 9. “There’s been a huge uptick from that standpoint. We’ve made sure that we’ve kept our facilities clean by employing standards that have been released by the CDC, as well as by the major academic centers who are dealing with this firsthand, and also with guidance from the National Infusion Center Association.”
He and his colleagues launched a pop-up infusion center in the Bronx to help offload Montefiore Medical Center, “because they’re so overwhelmed with COVID-19 patients that they need help taking care of the autoimmune patients,” Dr. Sengupta said. “That’s the role we’re playing. We’ve made our resources available to these centers in a very flexible way in order to ensure that we do the best thing we can for everybody.”
Thrivewell is also deploying a mobile infusion unit to recovered COVID-19 patients who require an infusion for their autoimmune disease, in order to minimize the risk of contamination and transmission in their stand-alone centers. The RV-sized unit, about the size of a Bloodmobile, is equipped with infusion chairs and staffed by a physician and nurse practitioner. “The objective is continuant care and reduction of cross-contamination, and also, on a broader health care systems level, to ensure that we as ambulatory infusion center providers can offload an overburdened system,” he said.
Dr. Sengupta, who has assisted on COVID-19 inpatient wards at New York University as a volunteer, is also leading a trial of a stem cell-derived therapy developed by Israel-based Pluristem Therapeutics, to treat New York–area patients severely ill from COVID-19 infection. “There are reports from Wuhan, China, in which clinicians are delivering IV mesenchymal stem cells to patients who are on mechanical ventilators, and the patients are getting better,” he said. “I have initiated a study in which we have three cohorts: One is the outpatient setting in which we are trying to treat COVID-19 patients who have hypoxia but have been turned away from overwhelmed EDs and need some therapy. We will be converting one of our infusion centers to conduct this trial. We are also going to be administering this [stem cell-derived therapy] to COVID-19 patients in ICUs, in EDs, and on med-surg floors throughout the city.”
Count Vikram Sengupta, MD, among the slew of health care workers feeling overwhelmed by the impact that COVID-19 is having on the delivery of health care in Manhattan and its surrounding boroughs.
“Nobody in the country is suffering like New York City,” said Dr. Sengupta, chief medical officer of Thrivewell Infusion, which operates three stand-alone infusion centers in the region: a four-chair center in Crown Heights, a 10-chair center in Borough Park, Brooklyn, and an eight-chair center in Manhasset. “We have 30%-50% of all cases in the country. I’ve been reading the news, and some people think this thing is going away. We’re in great distress here. There need to be new strategies moving forward. The whole world has changed. Our whole approach to ambulatory care has changed.”
In early March 2020, when it became clear that New York hospitals would face a tidal wave of citizens infected with COVID-19, Thrivewell began to receive an influx of referrals originating from concerned patients, providers, payers, and even large integrated health care systems, all in an effort to help prevent infectious exposure through infusion in hospital-based settings. “We are trying to accommodate them as swiftly as possible,” said Dr. Sengupta, who was interviewed for this story on April 9. “There’s been a huge uptick from that standpoint. We’ve made sure that we’ve kept our facilities clean by employing standards that have been released by the CDC, as well as by the major academic centers who are dealing with this firsthand, and also with guidance from the National Infusion Center Association.”
He and his colleagues launched a pop-up infusion center in the Bronx to help offload Montefiore Medical Center, “because they’re so overwhelmed with COVID-19 patients that they need help taking care of the autoimmune patients,” Dr. Sengupta said. “That’s the role we’re playing. We’ve made our resources available to these centers in a very flexible way in order to ensure that we do the best thing we can for everybody.”
Thrivewell is also deploying a mobile infusion unit to recovered COVID-19 patients who require an infusion for their autoimmune disease, in order to minimize the risk of contamination and transmission in their stand-alone centers. The RV-sized unit, about the size of a Bloodmobile, is equipped with infusion chairs and staffed by a physician and nurse practitioner. “The objective is continuant care and reduction of cross-contamination, and also, on a broader health care systems level, to ensure that we as ambulatory infusion center providers can offload an overburdened system,” he said.
Dr. Sengupta, who has assisted on COVID-19 inpatient wards at New York University as a volunteer, is also leading a trial of a stem cell-derived therapy developed by Israel-based Pluristem Therapeutics, to treat New York–area patients severely ill from COVID-19 infection. “There are reports from Wuhan, China, in which clinicians are delivering IV mesenchymal stem cells to patients who are on mechanical ventilators, and the patients are getting better,” he said. “I have initiated a study in which we have three cohorts: One is the outpatient setting in which we are trying to treat COVID-19 patients who have hypoxia but have been turned away from overwhelmed EDs and need some therapy. We will be converting one of our infusion centers to conduct this trial. We are also going to be administering this [stem cell-derived therapy] to COVID-19 patients in ICUs, in EDs, and on med-surg floors throughout the city.”
Count Vikram Sengupta, MD, among the slew of health care workers feeling overwhelmed by the impact that COVID-19 is having on the delivery of health care in Manhattan and its surrounding boroughs.
“Nobody in the country is suffering like New York City,” said Dr. Sengupta, chief medical officer of Thrivewell Infusion, which operates three stand-alone infusion centers in the region: a four-chair center in Crown Heights, a 10-chair center in Borough Park, Brooklyn, and an eight-chair center in Manhasset. “We have 30%-50% of all cases in the country. I’ve been reading the news, and some people think this thing is going away. We’re in great distress here. There need to be new strategies moving forward. The whole world has changed. Our whole approach to ambulatory care has changed.”
In early March 2020, when it became clear that New York hospitals would face a tidal wave of citizens infected with COVID-19, Thrivewell began to receive an influx of referrals originating from concerned patients, providers, payers, and even large integrated health care systems, all in an effort to help prevent infectious exposure through infusion in hospital-based settings. “We are trying to accommodate them as swiftly as possible,” said Dr. Sengupta, who was interviewed for this story on April 9. “There’s been a huge uptick from that standpoint. We’ve made sure that we’ve kept our facilities clean by employing standards that have been released by the CDC, as well as by the major academic centers who are dealing with this firsthand, and also with guidance from the National Infusion Center Association.”
He and his colleagues launched a pop-up infusion center in the Bronx to help offload Montefiore Medical Center, “because they’re so overwhelmed with COVID-19 patients that they need help taking care of the autoimmune patients,” Dr. Sengupta said. “That’s the role we’re playing. We’ve made our resources available to these centers in a very flexible way in order to ensure that we do the best thing we can for everybody.”
Thrivewell is also deploying a mobile infusion unit to recovered COVID-19 patients who require an infusion for their autoimmune disease, in order to minimize the risk of contamination and transmission in their stand-alone centers. The RV-sized unit, about the size of a Bloodmobile, is equipped with infusion chairs and staffed by a physician and nurse practitioner. “The objective is continuant care and reduction of cross-contamination, and also, on a broader health care systems level, to ensure that we as ambulatory infusion center providers can offload an overburdened system,” he said.
Dr. Sengupta, who has assisted on COVID-19 inpatient wards at New York University as a volunteer, is also leading a trial of a stem cell-derived therapy developed by Israel-based Pluristem Therapeutics, to treat New York–area patients severely ill from COVID-19 infection. “There are reports from Wuhan, China, in which clinicians are delivering IV mesenchymal stem cells to patients who are on mechanical ventilators, and the patients are getting better,” he said. “I have initiated a study in which we have three cohorts: One is the outpatient setting in which we are trying to treat COVID-19 patients who have hypoxia but have been turned away from overwhelmed EDs and need some therapy. We will be converting one of our infusion centers to conduct this trial. We are also going to be administering this [stem cell-derived therapy] to COVID-19 patients in ICUs, in EDs, and on med-surg floors throughout the city.”
Infusion center directors shuffle treatment services in the era of COVID-19
It’s anything but business as usual for clinicians who oversee office-based infusion centers, as they scramble to maintain services for patients considered to be at heightened risk for severe illness should they become infected with COVID-19.
“For many reasons, the guidance for patients right now is that they stay on their medications,” Max I. Hamburger, MD, a managing partner at Rheumatology Associates of Long Island (N.Y.), said in an interview. “Some have decided to stop the drug, and then they call us up to tell us that they’re flaring. The beginning of a flare is tiredness and other things. Now they’re worried: Are they tired because of the disease, or are they tired because they have COVID-19?”
With five office locations located in a region considered to be the epicenter of the COVID-19 pandemic in the United States, Dr. Hamburger and his colleagues are hypervigilant about screening patients for symptoms of the virus before they visit one of the three practice locations that provide infusion services. This starts with an automated phone system that reminds patients of their appointment time. “Part of that robocall now has some questions like, ‘Do you have any symptoms of COVID-19?’ ‘Are you running a fever?’ ‘Do you have any reason to worry about yourself? If so, please call us.’ ” The infusion nurses are also calling the patients in advance of their appointment to check on their status. “When they get to the office location, we ask them again about their general health and check their temperature,” said Dr. Hamburger, who is also founder and executive chairman of United Rheumatology, which is a nationwide rheumatology care management services organization with 650 members in 39 states. “We’re doing everything we can to talk to them about their own state of health and to question them about what I call extended paranoia: like, ‘Who are you living with?’ ‘Who are you hanging out with?’ ‘What are all the six degrees of separation here?’ I want to know what the patient’s husband did last night. I want to know where their kids were over this past week, et cetera. We do everything we can to see if there’s anybody who might have had the slightest [contact with someone who has COVID-19]. Because if I lose my infusion nurse, then I’m up the creek.”
The infusion nurse wears scrubs, a face mask, and latex gloves. She and her staff are using hand sanitizer and cleaning infusion equipment with sanitizing wipes as one might do in a surgical setting. “Every surface is wiped down between patients, and the nurse is changing gloves between patients,” said Dr. Hamburger, who was founding president of the New York State Rheumatology Society before retiring from that post in 2017. “Getting masks has been tough. We’re doing the best we can there. We’re not gloving patients but we’re masking patients.”
As noted in guidance from the American College of Rheumatology and other medical organizations, following the CDC’s recommendation to stay at home during the pandemic has jump-started conversations between physicians and their patients about modifying the time interval between infusions. “If they have been doing well for the last 9 months, we’re having a conversation such as ‘Maybe instead of getting your Orencia every 4 weeks, maybe we’ll push it out to 5 weeks, or maybe we’ll push the Enbrel out to 10 days and the Humira out 3 weeks, et cetera,” Dr. Hamburger said. “One has to be very careful about when you do that, because you don’t want the patient to flare up because it’s hard to get them in, but it is a natural opportunity to look at this. We’re seeing how we can optimize the dose, but I don’t want to send the message that we’re doing this because it changes the patient’s outcome, because there’s zero evidence that it’s a good thing to do in terms of resistance.”
At the infusion centers operated by the Johns Hopkins division of gastroenterology and hepatology, Baltimore, clinicians are not increasing the time interval between infusions for patients at this time. “We’re keeping them as they are, to prevent any flare-ups. Our main goal is to keep patients in remission and out of the hospital,” said Alyssa M. Parian, MD, medical director of the infusion center and associate director of the university’s GI department. “With Remicade specifically, there’s also the risk of developing antibodies if you delay treatment, so we’re basically keeping everyone on track. We’re not recommending a switch from infusions to injectables, and we also are not speeding up infusions, either. Before this pandemic happened, we had already tried to decrease all Remicade infusions from 2 hours to 1 hour for patient satisfaction. The Entyvio is a pretty quick, 30-minute infusion.”
To accommodate patients during this era of physical distancing measures recommended by the Centers for Disease Control and Prevention, Dr. Parian and her infusion nurse manager Elisheva Weiser converted one of their two outpatient GI centers into an infusion-only suite with 12 individual clinic rooms. As soon as patients exit the second-floor elevator, they encounter a workstation prior to entering the office where they are screened for COVID-19 symptoms and their temperature is taken. “If any symptoms or temperature comes back positive, we’re asking them to postpone their treatment and consider COVID testing,” she said.
Instead of one nurse looking after four patients in one room during infusion therapy, now one nurse looks after two patients who are in rooms next to each other. All patients and all staff wear masks while in the center. “We always have physician oversight at our infusion centers,” Dr. Parian said. “We are trying to maintain a ‘COVID-free zone.’ Therefore, no physicians who have served in a hospital ward are allowed in the infusion suite because we don’t want any carriers of COVID-19. Same with the nurses. Additionally, we limit the staff within the suite to only those who are essential and don’t allow anyone to perform telemedicine or urgent clinic visits in this location. Our infusion center staff are on a strict protocol to not come in with any symptoms at all. They are asked to take their temperature before coming in to work.”
She and her colleagues drew from recommendations from the joint GI society message on COVID-19, the Crohn’s and Colitis Foundation, and the International Organization for the Study of Inflammatory Bowel Disease (IOIBD) to inform their approach in serving patients during this unprecedented time. “We went as conservative as possible because these are immunosuppressed patients,” she said. One patient on her panel who receives an infusion every 8 weeks tested positive for COVID-19 between infusions, but was not hospitalized. Dr. Parian said that person will only be treated 14 days after the all symptoms disappear. “That person will wear a mask and will be infused in a separate room,” she said.
In Aventura, Fla., Norman B. Gaylis, MD, and his colleagues at Arthritis & Rheumatic Disease Specialties are looking into shutting down their infusion services during the time period that local public health officials consider to be the peak level of exposure to COVID-19. “We’ve tried to work around that, and bring people in a little early,” said Dr. Gaylis, medical director of rheumatology and infusion services at the practice. “We’ve done our best to mitigate the risk [of exposure] as much as possible.” This includes staggering their caseload by infusing 5 patients at a time, compared with the 15 patients at a time they could treat during prepandemic conditions. “Everyone is at least 20 feet apart,” said Dr. Gaylis, who is a member of the American College of Rheumatology Board of Directors. “While we don’t have the kind of protective garments you might see in an ICU, we still are gowning, gloving, and masking our staff, and trying to practice sterile techniques as much as we can.”
The pandemic has caused him to reflect more broadly on the way he and his colleagues deliver care for patients on infusion therapy. “We see patients who really want their treatment because they feel it’s helpful and beneficial,” he said. “There are also patients who may truly be in remission who could stop [infusion therapy]. We could possibly extend the duration of their therapy, try and push it back.”
Dr. Gaylis emphasized that any discussion about halting infusion therapy requires clinical, serological, and ideally even MRI evidence that the disease is in a dormant state. “You wouldn’t stop treatment in someone who is showing signs in their blood that their disease is still active,” he said. “You’re using all those parameters in that conversation.”
In his clinical opinion, now is not the time to switch patients to self-injectable agents as a perceived matter of convenience. “I don’t really think that’s a good idea because self-injectables are different,” Dr. Gaylis said. “You’re basically switching treatment patterns. The practicality of getting a specialty pharmacy to switch, the insurance companies to cover it, and determine copay for it, is a burden on patients. That’s why I’m against it, because you’re starting a whole new process and problem.”
One patient tested positive for COVID-19 about 3 weeks after an infusion at the facility. “That does lead to a point: Have my staff been tested? We have not had the tests available to us,” Dr. Gaylis said. “One provider had a contact with someone with COVID-19 and stayed home for 2 weeks. That person tested negative. Soon we are going to receive a kit that will allow us to measure IgM and IgG COVID-19 antibodies. Because we’re going to be closed for 2 weeks, measuring us now would be a great way to handle it.”
In rural Western Kentucky, Christopher R. Phillips, MD, and his colleagues at Paducah Rheumatology have arranged for “drive-by” injections for some of their higher-risk patients who require subcutaneous administration of biologic agents. “We have them call us when they’re in the parking lot, and we give them the treatment while they sit in their car,” said Dr. Phillips, who chairs the ACR Insurance Subcommittee and is a member of the ACR COVID-19 Practice and Advocacy Task Force.
For patients who require infusions, they’ve arranged three chairs in the clinic to be at least 6 feet apart, and moved the fourth chair into a separate room. “My infusion nurse knows these patients well; we’re a small community,” he said. “She checks in with them the day before to screen for any symptoms of infection and asks them to call when they get here. A lot of them wait in their car to be brought in. She’ll bring them in, screen for infection symptoms, and check their temperature. She and the receptionist are masked and gloved, and disinfect aggressively between patients. The other thing we are trying to be on top of is making sure that everyone’s insurance coverage is active when they come in, in light of the number of people who have been laid off or had changes in their employment.”
Dr. Phillips has considered increasing the infusion time interval for some patients, but not knowing when current physical distancing guidelines will ease up presents a conundrum. “If I have a patient coming in today, and their next treatment is due in a month, I don’t know how to say that, if we stretch the infusion to 2 months, that things are going to be better,” he said. “For some very well-controlled patients and/or high-risk patients, that is something we’ve done: stretch the interval or skip a treatment. For most patients, our default is to stick with the normal schedule. We feel that, for most patients who have moderate to severe underlying rheumatic disease, the risk of disease flare and subsequent need for steroids may be a larger risk than the treatment itself, though that is an individualized decision.”
To date, Dr. Phillips has not treated a patient who has recovered from COVID-19, but the thought of that scenario gives him pause. “There is some literature suggesting these patients may asymptomatically shed virus for some time after they’ve clinically recovered, but we don’t really know enough about that,” he said. “If I had one of those patients, I’d probably be delaying them for a longer period of time, and I’d be looking for some guidance from the literature on postsymptomatic viral shedding.”
In the meantime, the level of anxiety that many of his patients express during this pandemic is palpable. “They really are between a rock and a hard place,” Dr. Phillips said. “If they come off their effective treatment, they risk flare of a disease that can be life or limb threatening. And yet, because of their disease and their treatment, they’re potentially at increased risk for serious illness if they become infected with COVID-19. We look for ways to try to reassure patients and to comfort them, and work with them to make the best of the situation.”
It’s anything but business as usual for clinicians who oversee office-based infusion centers, as they scramble to maintain services for patients considered to be at heightened risk for severe illness should they become infected with COVID-19.
“For many reasons, the guidance for patients right now is that they stay on their medications,” Max I. Hamburger, MD, a managing partner at Rheumatology Associates of Long Island (N.Y.), said in an interview. “Some have decided to stop the drug, and then they call us up to tell us that they’re flaring. The beginning of a flare is tiredness and other things. Now they’re worried: Are they tired because of the disease, or are they tired because they have COVID-19?”
With five office locations located in a region considered to be the epicenter of the COVID-19 pandemic in the United States, Dr. Hamburger and his colleagues are hypervigilant about screening patients for symptoms of the virus before they visit one of the three practice locations that provide infusion services. This starts with an automated phone system that reminds patients of their appointment time. “Part of that robocall now has some questions like, ‘Do you have any symptoms of COVID-19?’ ‘Are you running a fever?’ ‘Do you have any reason to worry about yourself? If so, please call us.’ ” The infusion nurses are also calling the patients in advance of their appointment to check on their status. “When they get to the office location, we ask them again about their general health and check their temperature,” said Dr. Hamburger, who is also founder and executive chairman of United Rheumatology, which is a nationwide rheumatology care management services organization with 650 members in 39 states. “We’re doing everything we can to talk to them about their own state of health and to question them about what I call extended paranoia: like, ‘Who are you living with?’ ‘Who are you hanging out with?’ ‘What are all the six degrees of separation here?’ I want to know what the patient’s husband did last night. I want to know where their kids were over this past week, et cetera. We do everything we can to see if there’s anybody who might have had the slightest [contact with someone who has COVID-19]. Because if I lose my infusion nurse, then I’m up the creek.”
The infusion nurse wears scrubs, a face mask, and latex gloves. She and her staff are using hand sanitizer and cleaning infusion equipment with sanitizing wipes as one might do in a surgical setting. “Every surface is wiped down between patients, and the nurse is changing gloves between patients,” said Dr. Hamburger, who was founding president of the New York State Rheumatology Society before retiring from that post in 2017. “Getting masks has been tough. We’re doing the best we can there. We’re not gloving patients but we’re masking patients.”
As noted in guidance from the American College of Rheumatology and other medical organizations, following the CDC’s recommendation to stay at home during the pandemic has jump-started conversations between physicians and their patients about modifying the time interval between infusions. “If they have been doing well for the last 9 months, we’re having a conversation such as ‘Maybe instead of getting your Orencia every 4 weeks, maybe we’ll push it out to 5 weeks, or maybe we’ll push the Enbrel out to 10 days and the Humira out 3 weeks, et cetera,” Dr. Hamburger said. “One has to be very careful about when you do that, because you don’t want the patient to flare up because it’s hard to get them in, but it is a natural opportunity to look at this. We’re seeing how we can optimize the dose, but I don’t want to send the message that we’re doing this because it changes the patient’s outcome, because there’s zero evidence that it’s a good thing to do in terms of resistance.”
At the infusion centers operated by the Johns Hopkins division of gastroenterology and hepatology, Baltimore, clinicians are not increasing the time interval between infusions for patients at this time. “We’re keeping them as they are, to prevent any flare-ups. Our main goal is to keep patients in remission and out of the hospital,” said Alyssa M. Parian, MD, medical director of the infusion center and associate director of the university’s GI department. “With Remicade specifically, there’s also the risk of developing antibodies if you delay treatment, so we’re basically keeping everyone on track. We’re not recommending a switch from infusions to injectables, and we also are not speeding up infusions, either. Before this pandemic happened, we had already tried to decrease all Remicade infusions from 2 hours to 1 hour for patient satisfaction. The Entyvio is a pretty quick, 30-minute infusion.”
To accommodate patients during this era of physical distancing measures recommended by the Centers for Disease Control and Prevention, Dr. Parian and her infusion nurse manager Elisheva Weiser converted one of their two outpatient GI centers into an infusion-only suite with 12 individual clinic rooms. As soon as patients exit the second-floor elevator, they encounter a workstation prior to entering the office where they are screened for COVID-19 symptoms and their temperature is taken. “If any symptoms or temperature comes back positive, we’re asking them to postpone their treatment and consider COVID testing,” she said.
Instead of one nurse looking after four patients in one room during infusion therapy, now one nurse looks after two patients who are in rooms next to each other. All patients and all staff wear masks while in the center. “We always have physician oversight at our infusion centers,” Dr. Parian said. “We are trying to maintain a ‘COVID-free zone.’ Therefore, no physicians who have served in a hospital ward are allowed in the infusion suite because we don’t want any carriers of COVID-19. Same with the nurses. Additionally, we limit the staff within the suite to only those who are essential and don’t allow anyone to perform telemedicine or urgent clinic visits in this location. Our infusion center staff are on a strict protocol to not come in with any symptoms at all. They are asked to take their temperature before coming in to work.”
She and her colleagues drew from recommendations from the joint GI society message on COVID-19, the Crohn’s and Colitis Foundation, and the International Organization for the Study of Inflammatory Bowel Disease (IOIBD) to inform their approach in serving patients during this unprecedented time. “We went as conservative as possible because these are immunosuppressed patients,” she said. One patient on her panel who receives an infusion every 8 weeks tested positive for COVID-19 between infusions, but was not hospitalized. Dr. Parian said that person will only be treated 14 days after the all symptoms disappear. “That person will wear a mask and will be infused in a separate room,” she said.
In Aventura, Fla., Norman B. Gaylis, MD, and his colleagues at Arthritis & Rheumatic Disease Specialties are looking into shutting down their infusion services during the time period that local public health officials consider to be the peak level of exposure to COVID-19. “We’ve tried to work around that, and bring people in a little early,” said Dr. Gaylis, medical director of rheumatology and infusion services at the practice. “We’ve done our best to mitigate the risk [of exposure] as much as possible.” This includes staggering their caseload by infusing 5 patients at a time, compared with the 15 patients at a time they could treat during prepandemic conditions. “Everyone is at least 20 feet apart,” said Dr. Gaylis, who is a member of the American College of Rheumatology Board of Directors. “While we don’t have the kind of protective garments you might see in an ICU, we still are gowning, gloving, and masking our staff, and trying to practice sterile techniques as much as we can.”
The pandemic has caused him to reflect more broadly on the way he and his colleagues deliver care for patients on infusion therapy. “We see patients who really want their treatment because they feel it’s helpful and beneficial,” he said. “There are also patients who may truly be in remission who could stop [infusion therapy]. We could possibly extend the duration of their therapy, try and push it back.”
Dr. Gaylis emphasized that any discussion about halting infusion therapy requires clinical, serological, and ideally even MRI evidence that the disease is in a dormant state. “You wouldn’t stop treatment in someone who is showing signs in their blood that their disease is still active,” he said. “You’re using all those parameters in that conversation.”
In his clinical opinion, now is not the time to switch patients to self-injectable agents as a perceived matter of convenience. “I don’t really think that’s a good idea because self-injectables are different,” Dr. Gaylis said. “You’re basically switching treatment patterns. The practicality of getting a specialty pharmacy to switch, the insurance companies to cover it, and determine copay for it, is a burden on patients. That’s why I’m against it, because you’re starting a whole new process and problem.”
One patient tested positive for COVID-19 about 3 weeks after an infusion at the facility. “That does lead to a point: Have my staff been tested? We have not had the tests available to us,” Dr. Gaylis said. “One provider had a contact with someone with COVID-19 and stayed home for 2 weeks. That person tested negative. Soon we are going to receive a kit that will allow us to measure IgM and IgG COVID-19 antibodies. Because we’re going to be closed for 2 weeks, measuring us now would be a great way to handle it.”
In rural Western Kentucky, Christopher R. Phillips, MD, and his colleagues at Paducah Rheumatology have arranged for “drive-by” injections for some of their higher-risk patients who require subcutaneous administration of biologic agents. “We have them call us when they’re in the parking lot, and we give them the treatment while they sit in their car,” said Dr. Phillips, who chairs the ACR Insurance Subcommittee and is a member of the ACR COVID-19 Practice and Advocacy Task Force.
For patients who require infusions, they’ve arranged three chairs in the clinic to be at least 6 feet apart, and moved the fourth chair into a separate room. “My infusion nurse knows these patients well; we’re a small community,” he said. “She checks in with them the day before to screen for any symptoms of infection and asks them to call when they get here. A lot of them wait in their car to be brought in. She’ll bring them in, screen for infection symptoms, and check their temperature. She and the receptionist are masked and gloved, and disinfect aggressively between patients. The other thing we are trying to be on top of is making sure that everyone’s insurance coverage is active when they come in, in light of the number of people who have been laid off or had changes in their employment.”
Dr. Phillips has considered increasing the infusion time interval for some patients, but not knowing when current physical distancing guidelines will ease up presents a conundrum. “If I have a patient coming in today, and their next treatment is due in a month, I don’t know how to say that, if we stretch the infusion to 2 months, that things are going to be better,” he said. “For some very well-controlled patients and/or high-risk patients, that is something we’ve done: stretch the interval or skip a treatment. For most patients, our default is to stick with the normal schedule. We feel that, for most patients who have moderate to severe underlying rheumatic disease, the risk of disease flare and subsequent need for steroids may be a larger risk than the treatment itself, though that is an individualized decision.”
To date, Dr. Phillips has not treated a patient who has recovered from COVID-19, but the thought of that scenario gives him pause. “There is some literature suggesting these patients may asymptomatically shed virus for some time after they’ve clinically recovered, but we don’t really know enough about that,” he said. “If I had one of those patients, I’d probably be delaying them for a longer period of time, and I’d be looking for some guidance from the literature on postsymptomatic viral shedding.”
In the meantime, the level of anxiety that many of his patients express during this pandemic is palpable. “They really are between a rock and a hard place,” Dr. Phillips said. “If they come off their effective treatment, they risk flare of a disease that can be life or limb threatening. And yet, because of their disease and their treatment, they’re potentially at increased risk for serious illness if they become infected with COVID-19. We look for ways to try to reassure patients and to comfort them, and work with them to make the best of the situation.”
It’s anything but business as usual for clinicians who oversee office-based infusion centers, as they scramble to maintain services for patients considered to be at heightened risk for severe illness should they become infected with COVID-19.
“For many reasons, the guidance for patients right now is that they stay on their medications,” Max I. Hamburger, MD, a managing partner at Rheumatology Associates of Long Island (N.Y.), said in an interview. “Some have decided to stop the drug, and then they call us up to tell us that they’re flaring. The beginning of a flare is tiredness and other things. Now they’re worried: Are they tired because of the disease, or are they tired because they have COVID-19?”
With five office locations located in a region considered to be the epicenter of the COVID-19 pandemic in the United States, Dr. Hamburger and his colleagues are hypervigilant about screening patients for symptoms of the virus before they visit one of the three practice locations that provide infusion services. This starts with an automated phone system that reminds patients of their appointment time. “Part of that robocall now has some questions like, ‘Do you have any symptoms of COVID-19?’ ‘Are you running a fever?’ ‘Do you have any reason to worry about yourself? If so, please call us.’ ” The infusion nurses are also calling the patients in advance of their appointment to check on their status. “When they get to the office location, we ask them again about their general health and check their temperature,” said Dr. Hamburger, who is also founder and executive chairman of United Rheumatology, which is a nationwide rheumatology care management services organization with 650 members in 39 states. “We’re doing everything we can to talk to them about their own state of health and to question them about what I call extended paranoia: like, ‘Who are you living with?’ ‘Who are you hanging out with?’ ‘What are all the six degrees of separation here?’ I want to know what the patient’s husband did last night. I want to know where their kids were over this past week, et cetera. We do everything we can to see if there’s anybody who might have had the slightest [contact with someone who has COVID-19]. Because if I lose my infusion nurse, then I’m up the creek.”
The infusion nurse wears scrubs, a face mask, and latex gloves. She and her staff are using hand sanitizer and cleaning infusion equipment with sanitizing wipes as one might do in a surgical setting. “Every surface is wiped down between patients, and the nurse is changing gloves between patients,” said Dr. Hamburger, who was founding president of the New York State Rheumatology Society before retiring from that post in 2017. “Getting masks has been tough. We’re doing the best we can there. We’re not gloving patients but we’re masking patients.”
As noted in guidance from the American College of Rheumatology and other medical organizations, following the CDC’s recommendation to stay at home during the pandemic has jump-started conversations between physicians and their patients about modifying the time interval between infusions. “If they have been doing well for the last 9 months, we’re having a conversation such as ‘Maybe instead of getting your Orencia every 4 weeks, maybe we’ll push it out to 5 weeks, or maybe we’ll push the Enbrel out to 10 days and the Humira out 3 weeks, et cetera,” Dr. Hamburger said. “One has to be very careful about when you do that, because you don’t want the patient to flare up because it’s hard to get them in, but it is a natural opportunity to look at this. We’re seeing how we can optimize the dose, but I don’t want to send the message that we’re doing this because it changes the patient’s outcome, because there’s zero evidence that it’s a good thing to do in terms of resistance.”
At the infusion centers operated by the Johns Hopkins division of gastroenterology and hepatology, Baltimore, clinicians are not increasing the time interval between infusions for patients at this time. “We’re keeping them as they are, to prevent any flare-ups. Our main goal is to keep patients in remission and out of the hospital,” said Alyssa M. Parian, MD, medical director of the infusion center and associate director of the university’s GI department. “With Remicade specifically, there’s also the risk of developing antibodies if you delay treatment, so we’re basically keeping everyone on track. We’re not recommending a switch from infusions to injectables, and we also are not speeding up infusions, either. Before this pandemic happened, we had already tried to decrease all Remicade infusions from 2 hours to 1 hour for patient satisfaction. The Entyvio is a pretty quick, 30-minute infusion.”
To accommodate patients during this era of physical distancing measures recommended by the Centers for Disease Control and Prevention, Dr. Parian and her infusion nurse manager Elisheva Weiser converted one of their two outpatient GI centers into an infusion-only suite with 12 individual clinic rooms. As soon as patients exit the second-floor elevator, they encounter a workstation prior to entering the office where they are screened for COVID-19 symptoms and their temperature is taken. “If any symptoms or temperature comes back positive, we’re asking them to postpone their treatment and consider COVID testing,” she said.
Instead of one nurse looking after four patients in one room during infusion therapy, now one nurse looks after two patients who are in rooms next to each other. All patients and all staff wear masks while in the center. “We always have physician oversight at our infusion centers,” Dr. Parian said. “We are trying to maintain a ‘COVID-free zone.’ Therefore, no physicians who have served in a hospital ward are allowed in the infusion suite because we don’t want any carriers of COVID-19. Same with the nurses. Additionally, we limit the staff within the suite to only those who are essential and don’t allow anyone to perform telemedicine or urgent clinic visits in this location. Our infusion center staff are on a strict protocol to not come in with any symptoms at all. They are asked to take their temperature before coming in to work.”
She and her colleagues drew from recommendations from the joint GI society message on COVID-19, the Crohn’s and Colitis Foundation, and the International Organization for the Study of Inflammatory Bowel Disease (IOIBD) to inform their approach in serving patients during this unprecedented time. “We went as conservative as possible because these are immunosuppressed patients,” she said. One patient on her panel who receives an infusion every 8 weeks tested positive for COVID-19 between infusions, but was not hospitalized. Dr. Parian said that person will only be treated 14 days after the all symptoms disappear. “That person will wear a mask and will be infused in a separate room,” she said.
In Aventura, Fla., Norman B. Gaylis, MD, and his colleagues at Arthritis & Rheumatic Disease Specialties are looking into shutting down their infusion services during the time period that local public health officials consider to be the peak level of exposure to COVID-19. “We’ve tried to work around that, and bring people in a little early,” said Dr. Gaylis, medical director of rheumatology and infusion services at the practice. “We’ve done our best to mitigate the risk [of exposure] as much as possible.” This includes staggering their caseload by infusing 5 patients at a time, compared with the 15 patients at a time they could treat during prepandemic conditions. “Everyone is at least 20 feet apart,” said Dr. Gaylis, who is a member of the American College of Rheumatology Board of Directors. “While we don’t have the kind of protective garments you might see in an ICU, we still are gowning, gloving, and masking our staff, and trying to practice sterile techniques as much as we can.”
The pandemic has caused him to reflect more broadly on the way he and his colleagues deliver care for patients on infusion therapy. “We see patients who really want their treatment because they feel it’s helpful and beneficial,” he said. “There are also patients who may truly be in remission who could stop [infusion therapy]. We could possibly extend the duration of their therapy, try and push it back.”
Dr. Gaylis emphasized that any discussion about halting infusion therapy requires clinical, serological, and ideally even MRI evidence that the disease is in a dormant state. “You wouldn’t stop treatment in someone who is showing signs in their blood that their disease is still active,” he said. “You’re using all those parameters in that conversation.”
In his clinical opinion, now is not the time to switch patients to self-injectable agents as a perceived matter of convenience. “I don’t really think that’s a good idea because self-injectables are different,” Dr. Gaylis said. “You’re basically switching treatment patterns. The practicality of getting a specialty pharmacy to switch, the insurance companies to cover it, and determine copay for it, is a burden on patients. That’s why I’m against it, because you’re starting a whole new process and problem.”
One patient tested positive for COVID-19 about 3 weeks after an infusion at the facility. “That does lead to a point: Have my staff been tested? We have not had the tests available to us,” Dr. Gaylis said. “One provider had a contact with someone with COVID-19 and stayed home for 2 weeks. That person tested negative. Soon we are going to receive a kit that will allow us to measure IgM and IgG COVID-19 antibodies. Because we’re going to be closed for 2 weeks, measuring us now would be a great way to handle it.”
In rural Western Kentucky, Christopher R. Phillips, MD, and his colleagues at Paducah Rheumatology have arranged for “drive-by” injections for some of their higher-risk patients who require subcutaneous administration of biologic agents. “We have them call us when they’re in the parking lot, and we give them the treatment while they sit in their car,” said Dr. Phillips, who chairs the ACR Insurance Subcommittee and is a member of the ACR COVID-19 Practice and Advocacy Task Force.
For patients who require infusions, they’ve arranged three chairs in the clinic to be at least 6 feet apart, and moved the fourth chair into a separate room. “My infusion nurse knows these patients well; we’re a small community,” he said. “She checks in with them the day before to screen for any symptoms of infection and asks them to call when they get here. A lot of them wait in their car to be brought in. She’ll bring them in, screen for infection symptoms, and check their temperature. She and the receptionist are masked and gloved, and disinfect aggressively between patients. The other thing we are trying to be on top of is making sure that everyone’s insurance coverage is active when they come in, in light of the number of people who have been laid off or had changes in their employment.”
Dr. Phillips has considered increasing the infusion time interval for some patients, but not knowing when current physical distancing guidelines will ease up presents a conundrum. “If I have a patient coming in today, and their next treatment is due in a month, I don’t know how to say that, if we stretch the infusion to 2 months, that things are going to be better,” he said. “For some very well-controlled patients and/or high-risk patients, that is something we’ve done: stretch the interval or skip a treatment. For most patients, our default is to stick with the normal schedule. We feel that, for most patients who have moderate to severe underlying rheumatic disease, the risk of disease flare and subsequent need for steroids may be a larger risk than the treatment itself, though that is an individualized decision.”
To date, Dr. Phillips has not treated a patient who has recovered from COVID-19, but the thought of that scenario gives him pause. “There is some literature suggesting these patients may asymptomatically shed virus for some time after they’ve clinically recovered, but we don’t really know enough about that,” he said. “If I had one of those patients, I’d probably be delaying them for a longer period of time, and I’d be looking for some guidance from the literature on postsymptomatic viral shedding.”
In the meantime, the level of anxiety that many of his patients express during this pandemic is palpable. “They really are between a rock and a hard place,” Dr. Phillips said. “If they come off their effective treatment, they risk flare of a disease that can be life or limb threatening. And yet, because of their disease and their treatment, they’re potentially at increased risk for serious illness if they become infected with COVID-19. We look for ways to try to reassure patients and to comfort them, and work with them to make the best of the situation.”
When to treat, delay, or omit breast cancer therapy in the face of COVID-19
Nothing is business as usual during the COVID-19 pandemic, and that includes breast cancer therapy. That’s why two groups have released guidance documents on treating breast cancer patients during the pandemic.
A guidance on surgery, drug therapy, and radiotherapy was created by the COVID-19 Pandemic Breast Cancer Consortium. This guidance is set to be published in Breast Cancer Research and Treatment and can be downloaded from the American College of Surgeons website.
A group from Memorial Sloan Kettering Cancer Center (MSKCC) created a guidance document on radiotherapy for breast cancer patients, and that guidance was recently published in Advances in Radiation Oncology.
Prioritizing certain patients and treatments
As hospital beds and clinics fill with coronavirus-infected patients, oncologists must balance the need for timely therapy for their patients with the imperative to protect vulnerable, immunosuppressed patients from exposure and keep clinical resources as free as possible.
“As we’re taking care of breast cancer patients during this unprecedented pandemic, what we’re all trying to do is balance the most effective treatments for our patients against the risk of additional exposures, either from other patients [or] from being outside, and considerations about the safety of our staff,” said Steven Isakoff, MD, PhD, of Massachusetts General Hospital Cancer Center in Boston, who is an author of the COVID-19 Pandemic Breast Cancer Consortium guidance.
The consortium’s guidance recommends prioritizing treatment according to patient needs and the disease type and stage. The three basic categories for considering when to treat are:
- Priority A: Patients who have immediately life-threatening conditions, are clinically unstable, or would experience a significant change in prognosis with even a short delay in treatment.
- Priority B: Deferring treatment for a short time (6-12 weeks) would not impact overall outcomes in these patients.
- Priority C: These patients are stable enough that treatment can be delayed for the duration of the COVID-19 pandemic.
“The consortium highly recommends multidisciplinary discussion regarding priority for elective surgery and adjuvant treatments for your breast cancer patients,” the guidance authors wrote. “The COVID-19 pandemic may vary in severity over time, and these recommendations are subject to change with changing COVID-19 pandemic severity.”
For example, depending on local circumstances, the guidance recommends limiting immediate outpatient visits to patients with potentially unstable conditions such as infection or hematoma. Established patients with new problems or patients with a new diagnosis of noninvasive cancer might be managed with telemedicine visits, and patients who are on follow-up with no new issues or who have benign lesions might have their visits safely postponed.
Surgery and drug recommendations
High-priority surgical procedures include operative drainage of a breast abscess in a septic patient and evacuation of expanding hematoma in a hemodynamically unstable patient, according to the consortium guidance.
Other surgical situations are more nuanced. For example, for patients with triple-negative breast cancer (TNBC) or HER2-positive disease, the guidance recommends neoadjuvant chemotherapy or HER2-targeted chemotherapy in some cases. In other cases, institutions may proceed with surgery before chemotherapy, but “these decisions will depend on institutional resources and patient factors,” according to the authors.
The guidance states that chemotherapy and other drug treatments should not be delayed in patients with oncologic emergencies, such as febrile neutropenia, hypercalcemia, intolerable pain, symptomatic pleural effusions, or brain metastases.
In addition, patients with inflammatory breast cancer, TNBC, or HER2-positive breast cancer should receive neoadjuvant/adjuvant chemotherapy. Patients with metastatic disease that is likely to benefit from therapy should start chemotherapy, endocrine therapy, or targeted therapy. And patients who have already started neoadjuvant/adjuvant chemotherapy or oral adjuvant endocrine therapy should continue on these treatments.
Radiation therapy recommendations
The consortium guidance recommends administering radiation to patients with bleeding or painful inoperable locoregional disease, those with symptomatic metastatic disease, and patients who progress on neoadjuvant chemotherapy.
In contrast, older patients (aged 65-70 years) with lower-risk, stage I, hormone receptor–positive, HER2-negative cancers who are on adjuvant endocrine therapy can safely defer or omit radiation without affecting their overall survival, according to the guidance. Patients with ductal carcinoma in situ, especially those with estrogen receptor–positive disease on endocrine therapy, can safely omit radiation.
“There are clearly conditions where radiation might reduce the risk of recurrence but not improve overall survival, where a delay in treatment really will have minimal or no impact,” Dr. Isakoff said.
The MSKCC guidance recommends omitting radiation for some patients with favorable-risk disease and truncating or accelerating regimens using hypofractionation for others who require whole-breast radiation or post-mastectomy treatment.
The MSKCC guidance also contains recommendations for prioritization of patients according to disease state and the urgency of care. It divides cases into high, intermediate, and low priority for breast radiotherapy, as follows:
- Tier 1 (high priority): Patients with inflammatory breast cancer, residual node-positive disease after neoadjuvant chemotherapy, four or more positive nodes (N2), recurrent disease, node-positive TNBC, or extensive lymphovascular invasion.
- Tier 2 (intermediate priority): Patients with estrogen receptor–positive disease with one to three positive nodes (N1a), pathologic stage N0 after neoadjuvant chemotherapy, lymphovascular invasion not otherwise specified, or node-negative TNBC.
- Tier 3 (low priority): Patients with early-stage estrogen receptor-positive breast cancer (especially patients of advanced age), patients with ductal carcinoma in situ, or those who otherwise do not meet the criteria for tiers 1 or 2.
The MSKCC guidance also contains recommended hypofractionated or accelerated radiotherapy regimens for partial and whole-breast irradiation, post-mastectomy treatment, and breast and regional node irradiation, including recommended techniques (for example, 3-D conformal or intensity modulated approaches).
The authors of the MSKCC guidance disclosed relationships with eContour, Volastra Therapeutics, Sanofi, the Prostate Cancer Foundation, and Cancer Research UK. The authors of the COVID-19 Pandemic Breast Cancer Consortium guidance did not disclose any conflicts and said there was no funding source for the guidance.
SOURCES: Braunstein LZ et al. Adv Radiat Oncol. 2020 Apr 1. doi:10.1016/j.adro.2020.03.013; Dietz JR et al. 2020 Apr. Recommendations for prioritization, treatment and triage of breast cancer patients during the COVID-19 pandemic. Accepted for publication in Breast Cancer Research and Treatment.
Nothing is business as usual during the COVID-19 pandemic, and that includes breast cancer therapy. That’s why two groups have released guidance documents on treating breast cancer patients during the pandemic.
A guidance on surgery, drug therapy, and radiotherapy was created by the COVID-19 Pandemic Breast Cancer Consortium. This guidance is set to be published in Breast Cancer Research and Treatment and can be downloaded from the American College of Surgeons website.
A group from Memorial Sloan Kettering Cancer Center (MSKCC) created a guidance document on radiotherapy for breast cancer patients, and that guidance was recently published in Advances in Radiation Oncology.
Prioritizing certain patients and treatments
As hospital beds and clinics fill with coronavirus-infected patients, oncologists must balance the need for timely therapy for their patients with the imperative to protect vulnerable, immunosuppressed patients from exposure and keep clinical resources as free as possible.
“As we’re taking care of breast cancer patients during this unprecedented pandemic, what we’re all trying to do is balance the most effective treatments for our patients against the risk of additional exposures, either from other patients [or] from being outside, and considerations about the safety of our staff,” said Steven Isakoff, MD, PhD, of Massachusetts General Hospital Cancer Center in Boston, who is an author of the COVID-19 Pandemic Breast Cancer Consortium guidance.
The consortium’s guidance recommends prioritizing treatment according to patient needs and the disease type and stage. The three basic categories for considering when to treat are:
- Priority A: Patients who have immediately life-threatening conditions, are clinically unstable, or would experience a significant change in prognosis with even a short delay in treatment.
- Priority B: Deferring treatment for a short time (6-12 weeks) would not impact overall outcomes in these patients.
- Priority C: These patients are stable enough that treatment can be delayed for the duration of the COVID-19 pandemic.
“The consortium highly recommends multidisciplinary discussion regarding priority for elective surgery and adjuvant treatments for your breast cancer patients,” the guidance authors wrote. “The COVID-19 pandemic may vary in severity over time, and these recommendations are subject to change with changing COVID-19 pandemic severity.”
For example, depending on local circumstances, the guidance recommends limiting immediate outpatient visits to patients with potentially unstable conditions such as infection or hematoma. Established patients with new problems or patients with a new diagnosis of noninvasive cancer might be managed with telemedicine visits, and patients who are on follow-up with no new issues or who have benign lesions might have their visits safely postponed.
Surgery and drug recommendations
High-priority surgical procedures include operative drainage of a breast abscess in a septic patient and evacuation of expanding hematoma in a hemodynamically unstable patient, according to the consortium guidance.
Other surgical situations are more nuanced. For example, for patients with triple-negative breast cancer (TNBC) or HER2-positive disease, the guidance recommends neoadjuvant chemotherapy or HER2-targeted chemotherapy in some cases. In other cases, institutions may proceed with surgery before chemotherapy, but “these decisions will depend on institutional resources and patient factors,” according to the authors.
The guidance states that chemotherapy and other drug treatments should not be delayed in patients with oncologic emergencies, such as febrile neutropenia, hypercalcemia, intolerable pain, symptomatic pleural effusions, or brain metastases.
In addition, patients with inflammatory breast cancer, TNBC, or HER2-positive breast cancer should receive neoadjuvant/adjuvant chemotherapy. Patients with metastatic disease that is likely to benefit from therapy should start chemotherapy, endocrine therapy, or targeted therapy. And patients who have already started neoadjuvant/adjuvant chemotherapy or oral adjuvant endocrine therapy should continue on these treatments.
Radiation therapy recommendations
The consortium guidance recommends administering radiation to patients with bleeding or painful inoperable locoregional disease, those with symptomatic metastatic disease, and patients who progress on neoadjuvant chemotherapy.
In contrast, older patients (aged 65-70 years) with lower-risk, stage I, hormone receptor–positive, HER2-negative cancers who are on adjuvant endocrine therapy can safely defer or omit radiation without affecting their overall survival, according to the guidance. Patients with ductal carcinoma in situ, especially those with estrogen receptor–positive disease on endocrine therapy, can safely omit radiation.
“There are clearly conditions where radiation might reduce the risk of recurrence but not improve overall survival, where a delay in treatment really will have minimal or no impact,” Dr. Isakoff said.
The MSKCC guidance recommends omitting radiation for some patients with favorable-risk disease and truncating or accelerating regimens using hypofractionation for others who require whole-breast radiation or post-mastectomy treatment.
The MSKCC guidance also contains recommendations for prioritization of patients according to disease state and the urgency of care. It divides cases into high, intermediate, and low priority for breast radiotherapy, as follows:
- Tier 1 (high priority): Patients with inflammatory breast cancer, residual node-positive disease after neoadjuvant chemotherapy, four or more positive nodes (N2), recurrent disease, node-positive TNBC, or extensive lymphovascular invasion.
- Tier 2 (intermediate priority): Patients with estrogen receptor–positive disease with one to three positive nodes (N1a), pathologic stage N0 after neoadjuvant chemotherapy, lymphovascular invasion not otherwise specified, or node-negative TNBC.
- Tier 3 (low priority): Patients with early-stage estrogen receptor-positive breast cancer (especially patients of advanced age), patients with ductal carcinoma in situ, or those who otherwise do not meet the criteria for tiers 1 or 2.
The MSKCC guidance also contains recommended hypofractionated or accelerated radiotherapy regimens for partial and whole-breast irradiation, post-mastectomy treatment, and breast and regional node irradiation, including recommended techniques (for example, 3-D conformal or intensity modulated approaches).
The authors of the MSKCC guidance disclosed relationships with eContour, Volastra Therapeutics, Sanofi, the Prostate Cancer Foundation, and Cancer Research UK. The authors of the COVID-19 Pandemic Breast Cancer Consortium guidance did not disclose any conflicts and said there was no funding source for the guidance.
SOURCES: Braunstein LZ et al. Adv Radiat Oncol. 2020 Apr 1. doi:10.1016/j.adro.2020.03.013; Dietz JR et al. 2020 Apr. Recommendations for prioritization, treatment and triage of breast cancer patients during the COVID-19 pandemic. Accepted for publication in Breast Cancer Research and Treatment.
Nothing is business as usual during the COVID-19 pandemic, and that includes breast cancer therapy. That’s why two groups have released guidance documents on treating breast cancer patients during the pandemic.
A guidance on surgery, drug therapy, and radiotherapy was created by the COVID-19 Pandemic Breast Cancer Consortium. This guidance is set to be published in Breast Cancer Research and Treatment and can be downloaded from the American College of Surgeons website.
A group from Memorial Sloan Kettering Cancer Center (MSKCC) created a guidance document on radiotherapy for breast cancer patients, and that guidance was recently published in Advances in Radiation Oncology.
Prioritizing certain patients and treatments
As hospital beds and clinics fill with coronavirus-infected patients, oncologists must balance the need for timely therapy for their patients with the imperative to protect vulnerable, immunosuppressed patients from exposure and keep clinical resources as free as possible.
“As we’re taking care of breast cancer patients during this unprecedented pandemic, what we’re all trying to do is balance the most effective treatments for our patients against the risk of additional exposures, either from other patients [or] from being outside, and considerations about the safety of our staff,” said Steven Isakoff, MD, PhD, of Massachusetts General Hospital Cancer Center in Boston, who is an author of the COVID-19 Pandemic Breast Cancer Consortium guidance.
The consortium’s guidance recommends prioritizing treatment according to patient needs and the disease type and stage. The three basic categories for considering when to treat are:
- Priority A: Patients who have immediately life-threatening conditions, are clinically unstable, or would experience a significant change in prognosis with even a short delay in treatment.
- Priority B: Deferring treatment for a short time (6-12 weeks) would not impact overall outcomes in these patients.
- Priority C: These patients are stable enough that treatment can be delayed for the duration of the COVID-19 pandemic.
“The consortium highly recommends multidisciplinary discussion regarding priority for elective surgery and adjuvant treatments for your breast cancer patients,” the guidance authors wrote. “The COVID-19 pandemic may vary in severity over time, and these recommendations are subject to change with changing COVID-19 pandemic severity.”
For example, depending on local circumstances, the guidance recommends limiting immediate outpatient visits to patients with potentially unstable conditions such as infection or hematoma. Established patients with new problems or patients with a new diagnosis of noninvasive cancer might be managed with telemedicine visits, and patients who are on follow-up with no new issues or who have benign lesions might have their visits safely postponed.
Surgery and drug recommendations
High-priority surgical procedures include operative drainage of a breast abscess in a septic patient and evacuation of expanding hematoma in a hemodynamically unstable patient, according to the consortium guidance.
Other surgical situations are more nuanced. For example, for patients with triple-negative breast cancer (TNBC) or HER2-positive disease, the guidance recommends neoadjuvant chemotherapy or HER2-targeted chemotherapy in some cases. In other cases, institutions may proceed with surgery before chemotherapy, but “these decisions will depend on institutional resources and patient factors,” according to the authors.
The guidance states that chemotherapy and other drug treatments should not be delayed in patients with oncologic emergencies, such as febrile neutropenia, hypercalcemia, intolerable pain, symptomatic pleural effusions, or brain metastases.
In addition, patients with inflammatory breast cancer, TNBC, or HER2-positive breast cancer should receive neoadjuvant/adjuvant chemotherapy. Patients with metastatic disease that is likely to benefit from therapy should start chemotherapy, endocrine therapy, or targeted therapy. And patients who have already started neoadjuvant/adjuvant chemotherapy or oral adjuvant endocrine therapy should continue on these treatments.
Radiation therapy recommendations
The consortium guidance recommends administering radiation to patients with bleeding or painful inoperable locoregional disease, those with symptomatic metastatic disease, and patients who progress on neoadjuvant chemotherapy.
In contrast, older patients (aged 65-70 years) with lower-risk, stage I, hormone receptor–positive, HER2-negative cancers who are on adjuvant endocrine therapy can safely defer or omit radiation without affecting their overall survival, according to the guidance. Patients with ductal carcinoma in situ, especially those with estrogen receptor–positive disease on endocrine therapy, can safely omit radiation.
“There are clearly conditions where radiation might reduce the risk of recurrence but not improve overall survival, where a delay in treatment really will have minimal or no impact,” Dr. Isakoff said.
The MSKCC guidance recommends omitting radiation for some patients with favorable-risk disease and truncating or accelerating regimens using hypofractionation for others who require whole-breast radiation or post-mastectomy treatment.
The MSKCC guidance also contains recommendations for prioritization of patients according to disease state and the urgency of care. It divides cases into high, intermediate, and low priority for breast radiotherapy, as follows:
- Tier 1 (high priority): Patients with inflammatory breast cancer, residual node-positive disease after neoadjuvant chemotherapy, four or more positive nodes (N2), recurrent disease, node-positive TNBC, or extensive lymphovascular invasion.
- Tier 2 (intermediate priority): Patients with estrogen receptor–positive disease with one to three positive nodes (N1a), pathologic stage N0 after neoadjuvant chemotherapy, lymphovascular invasion not otherwise specified, or node-negative TNBC.
- Tier 3 (low priority): Patients with early-stage estrogen receptor-positive breast cancer (especially patients of advanced age), patients with ductal carcinoma in situ, or those who otherwise do not meet the criteria for tiers 1 or 2.
The MSKCC guidance also contains recommended hypofractionated or accelerated radiotherapy regimens for partial and whole-breast irradiation, post-mastectomy treatment, and breast and regional node irradiation, including recommended techniques (for example, 3-D conformal or intensity modulated approaches).
The authors of the MSKCC guidance disclosed relationships with eContour, Volastra Therapeutics, Sanofi, the Prostate Cancer Foundation, and Cancer Research UK. The authors of the COVID-19 Pandemic Breast Cancer Consortium guidance did not disclose any conflicts and said there was no funding source for the guidance.
SOURCES: Braunstein LZ et al. Adv Radiat Oncol. 2020 Apr 1. doi:10.1016/j.adro.2020.03.013; Dietz JR et al. 2020 Apr. Recommendations for prioritization, treatment and triage of breast cancer patients during the COVID-19 pandemic. Accepted for publication in Breast Cancer Research and Treatment.
Biotin may benefit patients with IBD
Patients with inflammatory bowel disease (IBD) may benefit from biotin supplementation, according to a preclinical study.
In mice, biotin supplementation delayed onset of colitis, minimized pathology, and accelerated healing, reported lead author Jonathan Skupsky, MD, of the University of California, Irvine, and colleagues.
“Biotin deficiency often is overlooked in the setting of IBD and there have been several reports of biotin deficiency in patients with IBD,” the investigators wrote in Cellular and Molecular Gastroenterology and Hepatology.
In addition to these clinical reports, Dr. Skupsky and colleagues were motivated by their previous research, which showed that, in mice, knockout of the sodium-dependent multivitamin transporter (SMVT) for intestinal biotin uptake led to intestinal inflammation and dysplasia, thereby adding evidence that IBD and biotin could be linked.
In the present study, the investigators first compared mice fed a biotin-deficient diet with those fed a biotin-rich diet. Mice lacking biotin developed alopecia and weight loss within 7 weeks, and over time, stool that was soft and bloody. At week 14, mice fed the biotin-deficient diet had intestinal inflammation, based on elevated fecal calprotectin levels. In contrast, mice fed a biotin-rich diet had no gastrointestinal pathology.
“Although no mouse model entirely recapitulates patients with IBD, this model reproduces many of the findings including weight loss, bloody diarrhea, increased fecal calprotectin, altered crypt architecture, and infiltration of neutrophils and lymphocytes to the mucosa and submucosa,” the investigators wrote.
After this experiment, another group of mice were given drinking water with 3% dextran sodium sulfate (DSS), which induced severe colitis within 7 days. The distal colons of these mice had reduced expression of SMVT, the biotin transporter. This finding was also observed in biopsy samples from patients with ulcerative colitis, suggesting a shared pathway.
“This raises the possibility that [the biotin transport pathway] could be a target for therapy,” the investigators wrote.
Next the investigators tested the effect of prophylactic biotin supplementation in mice receiving 1.5% DSS in drinking water. Compared with mice that went without biotin, those that received a week of supplementation before DSS challenge had delayed, milder colitis, with histologic findings and fecal calprotectin levels that approximated those of healthy controls.
In a similar experiment, two groups of mice were given DSS for 7 days, then water or water plus biotin. Those in the biotin group recovered faster and more completely, again with clinical and histologic findings that was close to controls.
According to the investigators, these findings suggest that biotin may be able to protect against development of colitis and speed healing during early remission.
Further experiments dove deeper into cellular processes and molecular mechanisms, ultimately revealing that biotin supplementation reduced activation of NF-kappaB, which led to decreased intestinal permeability and inflammatory cytokines.
“The specific mechanism(s) linking biotin and NF-kappaB is unclear but could be mediated via the different cellular pathways that are affected by biotin availability,” the investigators wrote.
They noted that IBD is a complex condition, which can make it difficult to accurately model the disease; however, they also suggested that the findings are compelling enough to prompt further investigation in human patients because biotin could be a convenient therapeutic add-on.
“We are optimistic that the data presented here will serve as the foundation for future clinical studies to determine if biotin supplementation should be used as adjunct therapy in IBD,” the investigators wrote. “Biotin is available over the counter, is affordable, and it has minimal side effects, making it an ideal therapeutic if clinical trials can show similar efficacy to what we have seen in this preclinical model.
The study was funded by the Veteran’s Administration and the National Institutes of Health. The investigators reported no conflicts of interest.
SOURCE: Skupsky J et al. Cell Mol Gastroenterol Hepatol. 2019 Nov 28. doi: 10.1016/j.jcmgh.2019.11.011.
Nutrient deficiency is commonly observed in patients with inflammatory bowel disease (IBD). In fact, over half of IBD patients show deficiency in micronutrients (essential vitamins and minerals). Similarly, there are also reports of the potential negative effect of nutrient deprivation on intestinal epithelium, which could ultimately contribute to IBD. However, to date there is limited evidence supporting the notion of nutrient deficiency as a cause or an effect of IBD.
This study by Skupsky et al. highlights the role of this essential vitamin biotin in IBD pathogenesis and its potential use as a therapeutic modality in colitis. Specifically, the authors first described how biotin deficiency could lead to a colitis-like phenotype in mice and then demonstrated that deficiency of biotin was observed in a mouse model of colitis. Further, it was also shown that biotin supplementation during colitis in mice was capable of alleviating inflammation. The authors also alluded to the potential loss of the biotin transporter, a sodium-dependent multivitamin transporter (SMVT), (which was found to be down-regulated in mice with colitis, as well as in IBD patients) as one of the causative factors for biotin deficiency in IBD. However, to date, biotin deficiency has not been conclusively established in IBD patients and further systematic and well-powered studies are needed.
Since micronutrients have emerged as safe and relatively less explored agents for beneficial effects in IBD, it may be worthwhile to initiate clinical studies to examine the potential beneficial role of biotin supplementation in IBD patients.
Pradeep K. Dudeja, PhD, is professor of physiology and director, divisional scholarly activities, division of gastroenterology and hepatology, department of medicine, University of Illinois at Chicago, as well as senior research career scientist, Jesse Brown VA Medical Center, Chicago.
Nutrient deficiency is commonly observed in patients with inflammatory bowel disease (IBD). In fact, over half of IBD patients show deficiency in micronutrients (essential vitamins and minerals). Similarly, there are also reports of the potential negative effect of nutrient deprivation on intestinal epithelium, which could ultimately contribute to IBD. However, to date there is limited evidence supporting the notion of nutrient deficiency as a cause or an effect of IBD.
This study by Skupsky et al. highlights the role of this essential vitamin biotin in IBD pathogenesis and its potential use as a therapeutic modality in colitis. Specifically, the authors first described how biotin deficiency could lead to a colitis-like phenotype in mice and then demonstrated that deficiency of biotin was observed in a mouse model of colitis. Further, it was also shown that biotin supplementation during colitis in mice was capable of alleviating inflammation. The authors also alluded to the potential loss of the biotin transporter, a sodium-dependent multivitamin transporter (SMVT), (which was found to be down-regulated in mice with colitis, as well as in IBD patients) as one of the causative factors for biotin deficiency in IBD. However, to date, biotin deficiency has not been conclusively established in IBD patients and further systematic and well-powered studies are needed.
Since micronutrients have emerged as safe and relatively less explored agents for beneficial effects in IBD, it may be worthwhile to initiate clinical studies to examine the potential beneficial role of biotin supplementation in IBD patients.
Pradeep K. Dudeja, PhD, is professor of physiology and director, divisional scholarly activities, division of gastroenterology and hepatology, department of medicine, University of Illinois at Chicago, as well as senior research career scientist, Jesse Brown VA Medical Center, Chicago.
Nutrient deficiency is commonly observed in patients with inflammatory bowel disease (IBD). In fact, over half of IBD patients show deficiency in micronutrients (essential vitamins and minerals). Similarly, there are also reports of the potential negative effect of nutrient deprivation on intestinal epithelium, which could ultimately contribute to IBD. However, to date there is limited evidence supporting the notion of nutrient deficiency as a cause or an effect of IBD.
This study by Skupsky et al. highlights the role of this essential vitamin biotin in IBD pathogenesis and its potential use as a therapeutic modality in colitis. Specifically, the authors first described how biotin deficiency could lead to a colitis-like phenotype in mice and then demonstrated that deficiency of biotin was observed in a mouse model of colitis. Further, it was also shown that biotin supplementation during colitis in mice was capable of alleviating inflammation. The authors also alluded to the potential loss of the biotin transporter, a sodium-dependent multivitamin transporter (SMVT), (which was found to be down-regulated in mice with colitis, as well as in IBD patients) as one of the causative factors for biotin deficiency in IBD. However, to date, biotin deficiency has not been conclusively established in IBD patients and further systematic and well-powered studies are needed.
Since micronutrients have emerged as safe and relatively less explored agents for beneficial effects in IBD, it may be worthwhile to initiate clinical studies to examine the potential beneficial role of biotin supplementation in IBD patients.
Pradeep K. Dudeja, PhD, is professor of physiology and director, divisional scholarly activities, division of gastroenterology and hepatology, department of medicine, University of Illinois at Chicago, as well as senior research career scientist, Jesse Brown VA Medical Center, Chicago.
Patients with inflammatory bowel disease (IBD) may benefit from biotin supplementation, according to a preclinical study.
In mice, biotin supplementation delayed onset of colitis, minimized pathology, and accelerated healing, reported lead author Jonathan Skupsky, MD, of the University of California, Irvine, and colleagues.
“Biotin deficiency often is overlooked in the setting of IBD and there have been several reports of biotin deficiency in patients with IBD,” the investigators wrote in Cellular and Molecular Gastroenterology and Hepatology.
In addition to these clinical reports, Dr. Skupsky and colleagues were motivated by their previous research, which showed that, in mice, knockout of the sodium-dependent multivitamin transporter (SMVT) for intestinal biotin uptake led to intestinal inflammation and dysplasia, thereby adding evidence that IBD and biotin could be linked.
In the present study, the investigators first compared mice fed a biotin-deficient diet with those fed a biotin-rich diet. Mice lacking biotin developed alopecia and weight loss within 7 weeks, and over time, stool that was soft and bloody. At week 14, mice fed the biotin-deficient diet had intestinal inflammation, based on elevated fecal calprotectin levels. In contrast, mice fed a biotin-rich diet had no gastrointestinal pathology.
“Although no mouse model entirely recapitulates patients with IBD, this model reproduces many of the findings including weight loss, bloody diarrhea, increased fecal calprotectin, altered crypt architecture, and infiltration of neutrophils and lymphocytes to the mucosa and submucosa,” the investigators wrote.
After this experiment, another group of mice were given drinking water with 3% dextran sodium sulfate (DSS), which induced severe colitis within 7 days. The distal colons of these mice had reduced expression of SMVT, the biotin transporter. This finding was also observed in biopsy samples from patients with ulcerative colitis, suggesting a shared pathway.
“This raises the possibility that [the biotin transport pathway] could be a target for therapy,” the investigators wrote.
Next the investigators tested the effect of prophylactic biotin supplementation in mice receiving 1.5% DSS in drinking water. Compared with mice that went without biotin, those that received a week of supplementation before DSS challenge had delayed, milder colitis, with histologic findings and fecal calprotectin levels that approximated those of healthy controls.
In a similar experiment, two groups of mice were given DSS for 7 days, then water or water plus biotin. Those in the biotin group recovered faster and more completely, again with clinical and histologic findings that was close to controls.
According to the investigators, these findings suggest that biotin may be able to protect against development of colitis and speed healing during early remission.
Further experiments dove deeper into cellular processes and molecular mechanisms, ultimately revealing that biotin supplementation reduced activation of NF-kappaB, which led to decreased intestinal permeability and inflammatory cytokines.
“The specific mechanism(s) linking biotin and NF-kappaB is unclear but could be mediated via the different cellular pathways that are affected by biotin availability,” the investigators wrote.
They noted that IBD is a complex condition, which can make it difficult to accurately model the disease; however, they also suggested that the findings are compelling enough to prompt further investigation in human patients because biotin could be a convenient therapeutic add-on.
“We are optimistic that the data presented here will serve as the foundation for future clinical studies to determine if biotin supplementation should be used as adjunct therapy in IBD,” the investigators wrote. “Biotin is available over the counter, is affordable, and it has minimal side effects, making it an ideal therapeutic if clinical trials can show similar efficacy to what we have seen in this preclinical model.
The study was funded by the Veteran’s Administration and the National Institutes of Health. The investigators reported no conflicts of interest.
SOURCE: Skupsky J et al. Cell Mol Gastroenterol Hepatol. 2019 Nov 28. doi: 10.1016/j.jcmgh.2019.11.011.
Patients with inflammatory bowel disease (IBD) may benefit from biotin supplementation, according to a preclinical study.
In mice, biotin supplementation delayed onset of colitis, minimized pathology, and accelerated healing, reported lead author Jonathan Skupsky, MD, of the University of California, Irvine, and colleagues.
“Biotin deficiency often is overlooked in the setting of IBD and there have been several reports of biotin deficiency in patients with IBD,” the investigators wrote in Cellular and Molecular Gastroenterology and Hepatology.
In addition to these clinical reports, Dr. Skupsky and colleagues were motivated by their previous research, which showed that, in mice, knockout of the sodium-dependent multivitamin transporter (SMVT) for intestinal biotin uptake led to intestinal inflammation and dysplasia, thereby adding evidence that IBD and biotin could be linked.
In the present study, the investigators first compared mice fed a biotin-deficient diet with those fed a biotin-rich diet. Mice lacking biotin developed alopecia and weight loss within 7 weeks, and over time, stool that was soft and bloody. At week 14, mice fed the biotin-deficient diet had intestinal inflammation, based on elevated fecal calprotectin levels. In contrast, mice fed a biotin-rich diet had no gastrointestinal pathology.
“Although no mouse model entirely recapitulates patients with IBD, this model reproduces many of the findings including weight loss, bloody diarrhea, increased fecal calprotectin, altered crypt architecture, and infiltration of neutrophils and lymphocytes to the mucosa and submucosa,” the investigators wrote.
After this experiment, another group of mice were given drinking water with 3% dextran sodium sulfate (DSS), which induced severe colitis within 7 days. The distal colons of these mice had reduced expression of SMVT, the biotin transporter. This finding was also observed in biopsy samples from patients with ulcerative colitis, suggesting a shared pathway.
“This raises the possibility that [the biotin transport pathway] could be a target for therapy,” the investigators wrote.
Next the investigators tested the effect of prophylactic biotin supplementation in mice receiving 1.5% DSS in drinking water. Compared with mice that went without biotin, those that received a week of supplementation before DSS challenge had delayed, milder colitis, with histologic findings and fecal calprotectin levels that approximated those of healthy controls.
In a similar experiment, two groups of mice were given DSS for 7 days, then water or water plus biotin. Those in the biotin group recovered faster and more completely, again with clinical and histologic findings that was close to controls.
According to the investigators, these findings suggest that biotin may be able to protect against development of colitis and speed healing during early remission.
Further experiments dove deeper into cellular processes and molecular mechanisms, ultimately revealing that biotin supplementation reduced activation of NF-kappaB, which led to decreased intestinal permeability and inflammatory cytokines.
“The specific mechanism(s) linking biotin and NF-kappaB is unclear but could be mediated via the different cellular pathways that are affected by biotin availability,” the investigators wrote.
They noted that IBD is a complex condition, which can make it difficult to accurately model the disease; however, they also suggested that the findings are compelling enough to prompt further investigation in human patients because biotin could be a convenient therapeutic add-on.
“We are optimistic that the data presented here will serve as the foundation for future clinical studies to determine if biotin supplementation should be used as adjunct therapy in IBD,” the investigators wrote. “Biotin is available over the counter, is affordable, and it has minimal side effects, making it an ideal therapeutic if clinical trials can show similar efficacy to what we have seen in this preclinical model.
The study was funded by the Veteran’s Administration and the National Institutes of Health. The investigators reported no conflicts of interest.
SOURCE: Skupsky J et al. Cell Mol Gastroenterol Hepatol. 2019 Nov 28. doi: 10.1016/j.jcmgh.2019.11.011.
FROM CELLULAR AND MOLECULAR GASTROENTEROLOGY AND HEPATOLOGY
CDC issues new return-to-work guidelines
The Centers for Disease Control and Prevention is releasing new guidance on return-to-work rules for critical workers exposed to a COVID-19 case, or a suspected case, replacing previous guidance to stay home for 14 days.
“One of the most important things we can do is keep our critical workforce working,” CDC Director Robert Redfield said at a White House briefing on April 8. “In certain circumstances they can go back to work,” he said.
Neither Redfield nor the other governmental officials specified what counts as an essential worker, although it has generally referred to food-service and health care workers.
They must take their temperature before work, wear a facial mask at all times and practice social distancing when at work, the new guidance says. They cannot share headsets or other objects used near the face.
Employers must take the worker’s temperature and assess each one for symptoms before work starts, sending a worker home if he or she is sick. Employers must increase the cleaning of frequently used surfaces, increase air exchange in the building and test the use of face masks to be sure they do not interfere with workflow.
Pressed on whether he would reopen the country at the end of the 30-day Stop the Spread effort on April 30 — since one model has revised the U.S. death toll down from 100,000-240,000 to 61,000 — President Donald Trump said meetings will take place soon to discuss the decision and that he will ‘’rely very heavily” on health experts.
“We know now for sure that the mitigation we have been doing is having a positive effect,” said Anthony Fauci, MD, a coronavirus task force member and director of the National Institute of Allergy and Infectious Diseases.
This article first appeared on WebMD.
The Centers for Disease Control and Prevention is releasing new guidance on return-to-work rules for critical workers exposed to a COVID-19 case, or a suspected case, replacing previous guidance to stay home for 14 days.
“One of the most important things we can do is keep our critical workforce working,” CDC Director Robert Redfield said at a White House briefing on April 8. “In certain circumstances they can go back to work,” he said.
Neither Redfield nor the other governmental officials specified what counts as an essential worker, although it has generally referred to food-service and health care workers.
They must take their temperature before work, wear a facial mask at all times and practice social distancing when at work, the new guidance says. They cannot share headsets or other objects used near the face.
Employers must take the worker’s temperature and assess each one for symptoms before work starts, sending a worker home if he or she is sick. Employers must increase the cleaning of frequently used surfaces, increase air exchange in the building and test the use of face masks to be sure they do not interfere with workflow.
Pressed on whether he would reopen the country at the end of the 30-day Stop the Spread effort on April 30 — since one model has revised the U.S. death toll down from 100,000-240,000 to 61,000 — President Donald Trump said meetings will take place soon to discuss the decision and that he will ‘’rely very heavily” on health experts.
“We know now for sure that the mitigation we have been doing is having a positive effect,” said Anthony Fauci, MD, a coronavirus task force member and director of the National Institute of Allergy and Infectious Diseases.
This article first appeared on WebMD.
The Centers for Disease Control and Prevention is releasing new guidance on return-to-work rules for critical workers exposed to a COVID-19 case, or a suspected case, replacing previous guidance to stay home for 14 days.
“One of the most important things we can do is keep our critical workforce working,” CDC Director Robert Redfield said at a White House briefing on April 8. “In certain circumstances they can go back to work,” he said.
Neither Redfield nor the other governmental officials specified what counts as an essential worker, although it has generally referred to food-service and health care workers.
They must take their temperature before work, wear a facial mask at all times and practice social distancing when at work, the new guidance says. They cannot share headsets or other objects used near the face.
Employers must take the worker’s temperature and assess each one for symptoms before work starts, sending a worker home if he or she is sick. Employers must increase the cleaning of frequently used surfaces, increase air exchange in the building and test the use of face masks to be sure they do not interfere with workflow.
Pressed on whether he would reopen the country at the end of the 30-day Stop the Spread effort on April 30 — since one model has revised the U.S. death toll down from 100,000-240,000 to 61,000 — President Donald Trump said meetings will take place soon to discuss the decision and that he will ‘’rely very heavily” on health experts.
“We know now for sure that the mitigation we have been doing is having a positive effect,” said Anthony Fauci, MD, a coronavirus task force member and director of the National Institute of Allergy and Infectious Diseases.
This article first appeared on WebMD.
May 2020 – ICYMI
Gastroenterology
February 2020
Gastric electrical stimulation reduces refractory vomiting in a randomized crossover trial. Philippe Ducrotte et al. 2020 Feb;158(3):506-14.e2. doi: 10.1053/j.gastro.2019.10.018
Efficacy and safety of vedolizumab subcutaneous formulation in a randomized trial of patients with ulcerative colitis. William J. Sandborn et al. 2020 Feb;158(3)562-72.e12. doi: 10.1053/j.gastro.2019.08.027
AGA Clinical Practice Guidelines on management of gastric intestinal metaplasia. Samir Gupta et al. 2020 Feb;158(3):693-702. doi: 10.1053/j.gastro.2019.12.003
March 2020
Approaches and challenges to management of pediatric and adult patients with eosinophilic esophagitis. Ikuo Hirano, Glenn T. Furuta. 2020 Mar;158(4):840-51. doi: 10.1053/j.gastro.2019.09.052
Uptake of colorectal cancer screening by physicians is associated with greater uptake by their patients. Owen Litwin et al. 2020 Mar;158(4):905-14. doi: 10.1053/j.gastro.2019.10.027
Recommendations for follow-up after colonoscopy and polypectomy: A consensus update by the US Multi-Society Task Force on Colorectal Cancer. Samir Gupta et al. 2020 Mar;158(4):1131-53.e5. doi: 10.1053/j.gastro.2019.10.026
Differences in fecal microbiomes and metabolomes of people with vs without irritable bowel syndrome and bile acid malabsorption. Ian B. Jeffery et al. 2020 Mar;158(4):1016-28.e8. doi: 10.1053/j.gastro.2019.11.301
April 2020
How to set up a successful motility lab. Rena Yadlapati et al. 2020 April;158(5):1202-10. doi: 10.1053/j.gastro.2020.01.030
Mechanisms, evaluation, and management of chronic constipation. Adil E. Bharucha, Brian E. Lacy. 2020 April;158(5):1232-49.e3. doi: 10.1053/j.gastro.2019.12.034.
Incidence of venous thromboembolism in patients with newly diagnosed pancreatic cancer and factors associated with outcomes. Corinne Frere et al. 2020 April;158(5):1346-58.e4. doi: 10.1053/j.gastro.2019.12.009
Clinical Gastroenterology and Hepatology
February 2020
Increased incidence and mortality of gastric cancer in immigrant populations from high to low regions of incidence: A systematic review and meta-analysis. Baldeep S. Pabla et al. 2020 Feb;18(2):347-59.e5. doi: 10.1016/j.cgh.2019.05.032
Risk of gastrointestinal bleeding increases with combinations of antithrombotic agents and patient age. Neena S. Abraham et al. 2020 Feb;18(2):337-46.e19. doi: 10.1016/j.cgh.2019.05.017
Alcohol rehabilitation within 30 days of hospital discharge is associated with reduced readmission, relapse, and death in patients with alcoholic hepatitis. Thoetchai (Bee) Peeraphatdit et al. 2020 Feb;18(2):477-85.e5. doi: 10.1016/j.cgh.2019.04.048
March 2020
Telemedicine in gastroenterology: A value-added service for patients. Theresa Lee, Lawrence Kim. 2020 Mar;18(3):530-3. doi: 10.1016/j.cgh.2019.12.005
Best practices in teaching endoscopy based on a Delphi survey of gastroenterology program directors and experts in endoscopy education. Navin L. Kumar et al. 2020 Mar;18(3):574-9.e1. doi: 10.1016/j.cgh.2019.05.023
Consumption of fish and long-chain n-3 polyunsaturated fatty acids is associated with reduced risk of colorectal cancer in a large European cohort. Elom K. Aglago et al. 2020 Mar;18(3):654-66.e6. doi: 10.1016/j.cgh.2019.06.031
April 2020
Low incidence of aerodigestive cancers in patients with negative results from colonoscopies, regardless of findings from multitarget stool DNA tests. Barry M. Berger et al. 2020 April;18(4):864-71. doi: 10.1016/j.cgh.2019.07.057
Lifetime economic burden of Crohn’s disease and ulcerative colitis by age at diagnosis. Gary R. Lichtenstein et al. 2020 April;18(4):889-97.e10. doi: 10.1016/j.cgh.2019.07.022
Clinical and Molecular Gastroenterology and Hepatology
Etiopathogenetic mechanisms in diverticular disease of the colon. Michael Camilleri et al. 2020;9(1):15-32. doi: 10.1016/j.jcmgh.2019.07.007
Gastroenterology
February 2020
Gastric electrical stimulation reduces refractory vomiting in a randomized crossover trial. Philippe Ducrotte et al. 2020 Feb;158(3):506-14.e2. doi: 10.1053/j.gastro.2019.10.018
Efficacy and safety of vedolizumab subcutaneous formulation in a randomized trial of patients with ulcerative colitis. William J. Sandborn et al. 2020 Feb;158(3)562-72.e12. doi: 10.1053/j.gastro.2019.08.027
AGA Clinical Practice Guidelines on management of gastric intestinal metaplasia. Samir Gupta et al. 2020 Feb;158(3):693-702. doi: 10.1053/j.gastro.2019.12.003
March 2020
Approaches and challenges to management of pediatric and adult patients with eosinophilic esophagitis. Ikuo Hirano, Glenn T. Furuta. 2020 Mar;158(4):840-51. doi: 10.1053/j.gastro.2019.09.052
Uptake of colorectal cancer screening by physicians is associated with greater uptake by their patients. Owen Litwin et al. 2020 Mar;158(4):905-14. doi: 10.1053/j.gastro.2019.10.027
Recommendations for follow-up after colonoscopy and polypectomy: A consensus update by the US Multi-Society Task Force on Colorectal Cancer. Samir Gupta et al. 2020 Mar;158(4):1131-53.e5. doi: 10.1053/j.gastro.2019.10.026
Differences in fecal microbiomes and metabolomes of people with vs without irritable bowel syndrome and bile acid malabsorption. Ian B. Jeffery et al. 2020 Mar;158(4):1016-28.e8. doi: 10.1053/j.gastro.2019.11.301
April 2020
How to set up a successful motility lab. Rena Yadlapati et al. 2020 April;158(5):1202-10. doi: 10.1053/j.gastro.2020.01.030
Mechanisms, evaluation, and management of chronic constipation. Adil E. Bharucha, Brian E. Lacy. 2020 April;158(5):1232-49.e3. doi: 10.1053/j.gastro.2019.12.034.
Incidence of venous thromboembolism in patients with newly diagnosed pancreatic cancer and factors associated with outcomes. Corinne Frere et al. 2020 April;158(5):1346-58.e4. doi: 10.1053/j.gastro.2019.12.009
Clinical Gastroenterology and Hepatology
February 2020
Increased incidence and mortality of gastric cancer in immigrant populations from high to low regions of incidence: A systematic review and meta-analysis. Baldeep S. Pabla et al. 2020 Feb;18(2):347-59.e5. doi: 10.1016/j.cgh.2019.05.032
Risk of gastrointestinal bleeding increases with combinations of antithrombotic agents and patient age. Neena S. Abraham et al. 2020 Feb;18(2):337-46.e19. doi: 10.1016/j.cgh.2019.05.017
Alcohol rehabilitation within 30 days of hospital discharge is associated with reduced readmission, relapse, and death in patients with alcoholic hepatitis. Thoetchai (Bee) Peeraphatdit et al. 2020 Feb;18(2):477-85.e5. doi: 10.1016/j.cgh.2019.04.048
March 2020
Telemedicine in gastroenterology: A value-added service for patients. Theresa Lee, Lawrence Kim. 2020 Mar;18(3):530-3. doi: 10.1016/j.cgh.2019.12.005
Best practices in teaching endoscopy based on a Delphi survey of gastroenterology program directors and experts in endoscopy education. Navin L. Kumar et al. 2020 Mar;18(3):574-9.e1. doi: 10.1016/j.cgh.2019.05.023
Consumption of fish and long-chain n-3 polyunsaturated fatty acids is associated with reduced risk of colorectal cancer in a large European cohort. Elom K. Aglago et al. 2020 Mar;18(3):654-66.e6. doi: 10.1016/j.cgh.2019.06.031
April 2020
Low incidence of aerodigestive cancers in patients with negative results from colonoscopies, regardless of findings from multitarget stool DNA tests. Barry M. Berger et al. 2020 April;18(4):864-71. doi: 10.1016/j.cgh.2019.07.057
Lifetime economic burden of Crohn’s disease and ulcerative colitis by age at diagnosis. Gary R. Lichtenstein et al. 2020 April;18(4):889-97.e10. doi: 10.1016/j.cgh.2019.07.022
Clinical and Molecular Gastroenterology and Hepatology
Etiopathogenetic mechanisms in diverticular disease of the colon. Michael Camilleri et al. 2020;9(1):15-32. doi: 10.1016/j.jcmgh.2019.07.007
Gastroenterology
February 2020
Gastric electrical stimulation reduces refractory vomiting in a randomized crossover trial. Philippe Ducrotte et al. 2020 Feb;158(3):506-14.e2. doi: 10.1053/j.gastro.2019.10.018
Efficacy and safety of vedolizumab subcutaneous formulation in a randomized trial of patients with ulcerative colitis. William J. Sandborn et al. 2020 Feb;158(3)562-72.e12. doi: 10.1053/j.gastro.2019.08.027
AGA Clinical Practice Guidelines on management of gastric intestinal metaplasia. Samir Gupta et al. 2020 Feb;158(3):693-702. doi: 10.1053/j.gastro.2019.12.003
March 2020
Approaches and challenges to management of pediatric and adult patients with eosinophilic esophagitis. Ikuo Hirano, Glenn T. Furuta. 2020 Mar;158(4):840-51. doi: 10.1053/j.gastro.2019.09.052
Uptake of colorectal cancer screening by physicians is associated with greater uptake by their patients. Owen Litwin et al. 2020 Mar;158(4):905-14. doi: 10.1053/j.gastro.2019.10.027
Recommendations for follow-up after colonoscopy and polypectomy: A consensus update by the US Multi-Society Task Force on Colorectal Cancer. Samir Gupta et al. 2020 Mar;158(4):1131-53.e5. doi: 10.1053/j.gastro.2019.10.026
Differences in fecal microbiomes and metabolomes of people with vs without irritable bowel syndrome and bile acid malabsorption. Ian B. Jeffery et al. 2020 Mar;158(4):1016-28.e8. doi: 10.1053/j.gastro.2019.11.301
April 2020
How to set up a successful motility lab. Rena Yadlapati et al. 2020 April;158(5):1202-10. doi: 10.1053/j.gastro.2020.01.030
Mechanisms, evaluation, and management of chronic constipation. Adil E. Bharucha, Brian E. Lacy. 2020 April;158(5):1232-49.e3. doi: 10.1053/j.gastro.2019.12.034.
Incidence of venous thromboembolism in patients with newly diagnosed pancreatic cancer and factors associated with outcomes. Corinne Frere et al. 2020 April;158(5):1346-58.e4. doi: 10.1053/j.gastro.2019.12.009
Clinical Gastroenterology and Hepatology
February 2020
Increased incidence and mortality of gastric cancer in immigrant populations from high to low regions of incidence: A systematic review and meta-analysis. Baldeep S. Pabla et al. 2020 Feb;18(2):347-59.e5. doi: 10.1016/j.cgh.2019.05.032
Risk of gastrointestinal bleeding increases with combinations of antithrombotic agents and patient age. Neena S. Abraham et al. 2020 Feb;18(2):337-46.e19. doi: 10.1016/j.cgh.2019.05.017
Alcohol rehabilitation within 30 days of hospital discharge is associated with reduced readmission, relapse, and death in patients with alcoholic hepatitis. Thoetchai (Bee) Peeraphatdit et al. 2020 Feb;18(2):477-85.e5. doi: 10.1016/j.cgh.2019.04.048
March 2020
Telemedicine in gastroenterology: A value-added service for patients. Theresa Lee, Lawrence Kim. 2020 Mar;18(3):530-3. doi: 10.1016/j.cgh.2019.12.005
Best practices in teaching endoscopy based on a Delphi survey of gastroenterology program directors and experts in endoscopy education. Navin L. Kumar et al. 2020 Mar;18(3):574-9.e1. doi: 10.1016/j.cgh.2019.05.023
Consumption of fish and long-chain n-3 polyunsaturated fatty acids is associated with reduced risk of colorectal cancer in a large European cohort. Elom K. Aglago et al. 2020 Mar;18(3):654-66.e6. doi: 10.1016/j.cgh.2019.06.031
April 2020
Low incidence of aerodigestive cancers in patients with negative results from colonoscopies, regardless of findings from multitarget stool DNA tests. Barry M. Berger et al. 2020 April;18(4):864-71. doi: 10.1016/j.cgh.2019.07.057
Lifetime economic burden of Crohn’s disease and ulcerative colitis by age at diagnosis. Gary R. Lichtenstein et al. 2020 April;18(4):889-97.e10. doi: 10.1016/j.cgh.2019.07.022
Clinical and Molecular Gastroenterology and Hepatology
Etiopathogenetic mechanisms in diverticular disease of the colon. Michael Camilleri et al. 2020;9(1):15-32. doi: 10.1016/j.jcmgh.2019.07.007
With mild or stable lupus, few patients flare during, after pregnancy
Approximately 26% of women with inactive or mild lupus at conception experienced flares at some point during pregnancy, based on data from 384 patients.
Active systemic lupus erythematosus (SLE) is a known predictor of poor pregnancy outcomes, including preterm birth, growth restriction, and fetal loss, but predictors of flares during and after pregnancy in women with SLE have not been well studied, wrote Julia Davis-Porada, MD, of the Hospital for Special Surgery, New York, and her colleagues.
In a study published in Arthritis Research & Therapy, the investigators reviewed data from the PROMISSE (Predictors of Pregnancy Outcome: Biomarkers in Antiphospholipid Antibody Syndrome and Systemic Lupus Erythematosus) study, a prospective study of pregnant women aged 18-45 years. The women were enrolled at less than 12 weeks’ gestation, and participants had a baseline hematocrit greater than 26%. Participants met criteria for inactive or mild/stable disease at the time of conception.
Overall, 20.8% of patients experienced at least one mild or moderate flare and 6.25% had one or more severe flares during pregnancy. Mild to moderate flares and severe flares occurred postpartum (2-6 months after the end of pregnancy) in 22.7% and 1.7% of patients, respectively.
Patients who were younger and those who had lower C4 at baseline and higher Physician Global Assessment scores at baseline were significantly more likely to have at least one flare during pregnancy (P = .003, P = .024, P = .0005, respectively).
In the analysis of postpartum flares, the incidence rates for mild to moderate and severe flares were 0.8 and 0.06 per person-year, respectively. “In contrast to the findings observed for flares that occurred during pregnancy, baseline patient characteristics were not correlated with postpartum flares,” the researchers wrote.
No medications were associated with flares during or after pregnancy.
The study findings were limited by several factors, including the exclusion of SLE patients with current nephritis and those who needed high-dose prednisone; the potential for missed flares; and the lack of postpartum data for approximately 10% of patients, the researchers noted. Also, “since many patients presented to this study only after conception, we have no data to review disease activity prior to pregnancy to determine whether pregnancy per se increased the risk for flare,” they said.
However, the results were strengthened by the large, multiethnic population and prospective study design, and support physicians in reassuring patients with SLE that pregnancy and postpartum flares are unlikely if they plan pregnancy during a time of mild or inactive disease, they concluded.
The study was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The researchers had no financial conflicts to disclose.
SOURCE: Davis-Porada J et al. Arthritis Res Ther. 2020 Mar 19. doi: 10.1186/s13075-020-2139-9.
Approximately 26% of women with inactive or mild lupus at conception experienced flares at some point during pregnancy, based on data from 384 patients.
Active systemic lupus erythematosus (SLE) is a known predictor of poor pregnancy outcomes, including preterm birth, growth restriction, and fetal loss, but predictors of flares during and after pregnancy in women with SLE have not been well studied, wrote Julia Davis-Porada, MD, of the Hospital for Special Surgery, New York, and her colleagues.
In a study published in Arthritis Research & Therapy, the investigators reviewed data from the PROMISSE (Predictors of Pregnancy Outcome: Biomarkers in Antiphospholipid Antibody Syndrome and Systemic Lupus Erythematosus) study, a prospective study of pregnant women aged 18-45 years. The women were enrolled at less than 12 weeks’ gestation, and participants had a baseline hematocrit greater than 26%. Participants met criteria for inactive or mild/stable disease at the time of conception.
Overall, 20.8% of patients experienced at least one mild or moderate flare and 6.25% had one or more severe flares during pregnancy. Mild to moderate flares and severe flares occurred postpartum (2-6 months after the end of pregnancy) in 22.7% and 1.7% of patients, respectively.
Patients who were younger and those who had lower C4 at baseline and higher Physician Global Assessment scores at baseline were significantly more likely to have at least one flare during pregnancy (P = .003, P = .024, P = .0005, respectively).
In the analysis of postpartum flares, the incidence rates for mild to moderate and severe flares were 0.8 and 0.06 per person-year, respectively. “In contrast to the findings observed for flares that occurred during pregnancy, baseline patient characteristics were not correlated with postpartum flares,” the researchers wrote.
No medications were associated with flares during or after pregnancy.
The study findings were limited by several factors, including the exclusion of SLE patients with current nephritis and those who needed high-dose prednisone; the potential for missed flares; and the lack of postpartum data for approximately 10% of patients, the researchers noted. Also, “since many patients presented to this study only after conception, we have no data to review disease activity prior to pregnancy to determine whether pregnancy per se increased the risk for flare,” they said.
However, the results were strengthened by the large, multiethnic population and prospective study design, and support physicians in reassuring patients with SLE that pregnancy and postpartum flares are unlikely if they plan pregnancy during a time of mild or inactive disease, they concluded.
The study was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The researchers had no financial conflicts to disclose.
SOURCE: Davis-Porada J et al. Arthritis Res Ther. 2020 Mar 19. doi: 10.1186/s13075-020-2139-9.
Approximately 26% of women with inactive or mild lupus at conception experienced flares at some point during pregnancy, based on data from 384 patients.
Active systemic lupus erythematosus (SLE) is a known predictor of poor pregnancy outcomes, including preterm birth, growth restriction, and fetal loss, but predictors of flares during and after pregnancy in women with SLE have not been well studied, wrote Julia Davis-Porada, MD, of the Hospital for Special Surgery, New York, and her colleagues.
In a study published in Arthritis Research & Therapy, the investigators reviewed data from the PROMISSE (Predictors of Pregnancy Outcome: Biomarkers in Antiphospholipid Antibody Syndrome and Systemic Lupus Erythematosus) study, a prospective study of pregnant women aged 18-45 years. The women were enrolled at less than 12 weeks’ gestation, and participants had a baseline hematocrit greater than 26%. Participants met criteria for inactive or mild/stable disease at the time of conception.
Overall, 20.8% of patients experienced at least one mild or moderate flare and 6.25% had one or more severe flares during pregnancy. Mild to moderate flares and severe flares occurred postpartum (2-6 months after the end of pregnancy) in 22.7% and 1.7% of patients, respectively.
Patients who were younger and those who had lower C4 at baseline and higher Physician Global Assessment scores at baseline were significantly more likely to have at least one flare during pregnancy (P = .003, P = .024, P = .0005, respectively).
In the analysis of postpartum flares, the incidence rates for mild to moderate and severe flares were 0.8 and 0.06 per person-year, respectively. “In contrast to the findings observed for flares that occurred during pregnancy, baseline patient characteristics were not correlated with postpartum flares,” the researchers wrote.
No medications were associated with flares during or after pregnancy.
The study findings were limited by several factors, including the exclusion of SLE patients with current nephritis and those who needed high-dose prednisone; the potential for missed flares; and the lack of postpartum data for approximately 10% of patients, the researchers noted. Also, “since many patients presented to this study only after conception, we have no data to review disease activity prior to pregnancy to determine whether pregnancy per se increased the risk for flare,” they said.
However, the results were strengthened by the large, multiethnic population and prospective study design, and support physicians in reassuring patients with SLE that pregnancy and postpartum flares are unlikely if they plan pregnancy during a time of mild or inactive disease, they concluded.
The study was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The researchers had no financial conflicts to disclose.
SOURCE: Davis-Porada J et al. Arthritis Res Ther. 2020 Mar 19. doi: 10.1186/s13075-020-2139-9.
FROM ARTHRITIS RESEARCH & THERAPY