User login
Cell therapy closes large wounds in epidermolysis bullosa
LONDON –
Of 85 patients with the recessive dystrophic type of EB (RDEB) who were surveyed through the EBCare Registry, 39 had available data from the validated quality of life in EB (QOLEB) questionnaire. Those with the largest wounds (7.5 cm or greater) had an average QOLEB score of 27, compared with 22.5 for those with wounds ranging from 2.5 to 7.5 cm in size, and a score of 14 for those with wounds less than 2.5 cm in size. The maximum score on the 17-item questionnaire is 51, with the higher the number, the greater the impact on quality of life.
“Large wound areas were seen more frequently in chronic open wounds, similar to findings in separate studies,” Emily S. Gorell, DO, a postdoctoral clinical research fellow in dermatology at Stanford (Calif.) University, and associates reported in a poster presentation at the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association.
“Larger wounds correlate with self-reported disease severity and key clinical manifestations,” they said, which includes history of squamous cell carcinoma (P = .04), anemia (P less than.01), osteoporosis (P = .03), and gastrostomy tube use (P = .02).
In total, 28 adults and 37 children and adolescents were surveyed; the majority (59%) were from North America, with the remainder from Europe (26%) or other countries (15%). Just over half of respondents were female (53%), and about 38% of surveys were completed by the individual rather than a parent or care giver (62%).
Dr. Gorell is working with Jean Y. Tang, MD, PhD, professor of dermatology at Stanford. During an oral presentation at the meeting, Dr. Tang observed that wounds could be defined as being recurrent or chronic open wounds. These two types of wounds behave differently, she said, with the latter never fully healing.
Indeed, in the survey, data on 1,226 wounds were collated, with 937 (76%) classified as recurrent – and healing within 12 weeks – but 289 (24%) remaining chronic open wounds, which did not heal for 12 weeks or longer. Some patients have had open wounds for more than 6 years, Dr. Tang noted.
“In our natural history study … you can see that chronic open wounds never reached 100% closure, they hardly ever reached 50% closure,” she said. In contrast, recurrent wounds have a more dynamic nature, healing completely, then reopening time after time. This is important when considering suitable endpoints for clinical trials, she said, as it could make or break some of the novel treatment approaches currently being tested. For instance, the placebo response in phase 3 trials of the topical therapy allantoin might have been high because recurrent wounds were being studied and were more likely to heal with or without active treatment.
“We’ve done a lot of work, it’s been 2 years, and we have benefited tremendously from these data in our negotiations with the FDA [Food and Drug Administration],” Dr. Tang said. “I am glad we did our homework … we were able to convince the FDA that, for a chronic open wound, a meaningful outcome is 50% healing, not 100%.”
Dr. Tang and Dr. Gorell are part of a team looking at gene-corrected autologous cell therapy (EB-101) to help heal large wounds caused by RDEB. The premise is that by replacing the faulty COL7A1 gene in keratinocytes taken from an individual, these skin cells will be able to produce collagen type VII (COL7). After being grown in culture to form epidermal sheets that look like a plastic film, the sheets can then be grafted over an individuals’ wounds.
Dr. Tang noted that the work was the culmination of 17 years’ of hard work by a small group of committed scientists. Preclinical studies started in 2003, when, she said, “the only funding we could get was through the NIH and thankfully some of the patient organizations.”
Initial results with the EB-101 therapy have been promising. Data on the first four subjects included in a seven-patient phase 1/2 study were published 4 years ago (JAMA. 2016;316[17]:1808-17), and the complete data were recently released (JCI Insight. 2019;4. doi: 10.1172/jci.insight.130554). Each trial subject had an EB-101 graft of approximately 35 cm2 (5 cm x 7 cm) transplanted onto three wounds, with three similar wounds used as controls.
At 6 months, 95% of treated wounds had healed by 50% or more, compared with none of the untreated control wounds (P less than .0001). Healing rates at 1 year and 2 years, respectively, were 68% vs. 17% (P = .025) and 71% vs. 17% (P = .019). All grafts were well tolerated and molecular correction was seen to last for up to 2 years in two patients.
EB-101 therapy will be evaluated in a phase 3 study, the VIITAL study, a multicenter, randomized trial involving 10-15 individuals with RDEB; 50% wound healing at 3 months is the trial’s primary endpoint. The trial, funded by Abeona Therapeutics, was given the go ahead by the FDA in December 2019 and has an estimated completion date of March 2021.
Dr. Gorell did not provide a conflict of interest statement. Dr. Tang disclosed receipt of honoraria or consultation fees from Abeona and Menlo Therapeutics and being a stock shareholder in PellePharm and BridgeBio. Dr. Tang also acknowledged receiving research grants from the EB Research Partnership, the Epidermolysis Medical Research Foundation, and the California Institute for Regenerative Medicine.
SOURCES: Gorell ES et al. EB World Congress, poster 29. Tang JYl. EB World Congress, oral presentation.
LONDON –
Of 85 patients with the recessive dystrophic type of EB (RDEB) who were surveyed through the EBCare Registry, 39 had available data from the validated quality of life in EB (QOLEB) questionnaire. Those with the largest wounds (7.5 cm or greater) had an average QOLEB score of 27, compared with 22.5 for those with wounds ranging from 2.5 to 7.5 cm in size, and a score of 14 for those with wounds less than 2.5 cm in size. The maximum score on the 17-item questionnaire is 51, with the higher the number, the greater the impact on quality of life.
“Large wound areas were seen more frequently in chronic open wounds, similar to findings in separate studies,” Emily S. Gorell, DO, a postdoctoral clinical research fellow in dermatology at Stanford (Calif.) University, and associates reported in a poster presentation at the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association.
“Larger wounds correlate with self-reported disease severity and key clinical manifestations,” they said, which includes history of squamous cell carcinoma (P = .04), anemia (P less than.01), osteoporosis (P = .03), and gastrostomy tube use (P = .02).
In total, 28 adults and 37 children and adolescents were surveyed; the majority (59%) were from North America, with the remainder from Europe (26%) or other countries (15%). Just over half of respondents were female (53%), and about 38% of surveys were completed by the individual rather than a parent or care giver (62%).
Dr. Gorell is working with Jean Y. Tang, MD, PhD, professor of dermatology at Stanford. During an oral presentation at the meeting, Dr. Tang observed that wounds could be defined as being recurrent or chronic open wounds. These two types of wounds behave differently, she said, with the latter never fully healing.
Indeed, in the survey, data on 1,226 wounds were collated, with 937 (76%) classified as recurrent – and healing within 12 weeks – but 289 (24%) remaining chronic open wounds, which did not heal for 12 weeks or longer. Some patients have had open wounds for more than 6 years, Dr. Tang noted.
“In our natural history study … you can see that chronic open wounds never reached 100% closure, they hardly ever reached 50% closure,” she said. In contrast, recurrent wounds have a more dynamic nature, healing completely, then reopening time after time. This is important when considering suitable endpoints for clinical trials, she said, as it could make or break some of the novel treatment approaches currently being tested. For instance, the placebo response in phase 3 trials of the topical therapy allantoin might have been high because recurrent wounds were being studied and were more likely to heal with or without active treatment.
“We’ve done a lot of work, it’s been 2 years, and we have benefited tremendously from these data in our negotiations with the FDA [Food and Drug Administration],” Dr. Tang said. “I am glad we did our homework … we were able to convince the FDA that, for a chronic open wound, a meaningful outcome is 50% healing, not 100%.”
Dr. Tang and Dr. Gorell are part of a team looking at gene-corrected autologous cell therapy (EB-101) to help heal large wounds caused by RDEB. The premise is that by replacing the faulty COL7A1 gene in keratinocytes taken from an individual, these skin cells will be able to produce collagen type VII (COL7). After being grown in culture to form epidermal sheets that look like a plastic film, the sheets can then be grafted over an individuals’ wounds.
Dr. Tang noted that the work was the culmination of 17 years’ of hard work by a small group of committed scientists. Preclinical studies started in 2003, when, she said, “the only funding we could get was through the NIH and thankfully some of the patient organizations.”
Initial results with the EB-101 therapy have been promising. Data on the first four subjects included in a seven-patient phase 1/2 study were published 4 years ago (JAMA. 2016;316[17]:1808-17), and the complete data were recently released (JCI Insight. 2019;4. doi: 10.1172/jci.insight.130554). Each trial subject had an EB-101 graft of approximately 35 cm2 (5 cm x 7 cm) transplanted onto three wounds, with three similar wounds used as controls.
At 6 months, 95% of treated wounds had healed by 50% or more, compared with none of the untreated control wounds (P less than .0001). Healing rates at 1 year and 2 years, respectively, were 68% vs. 17% (P = .025) and 71% vs. 17% (P = .019). All grafts were well tolerated and molecular correction was seen to last for up to 2 years in two patients.
EB-101 therapy will be evaluated in a phase 3 study, the VIITAL study, a multicenter, randomized trial involving 10-15 individuals with RDEB; 50% wound healing at 3 months is the trial’s primary endpoint. The trial, funded by Abeona Therapeutics, was given the go ahead by the FDA in December 2019 and has an estimated completion date of March 2021.
Dr. Gorell did not provide a conflict of interest statement. Dr. Tang disclosed receipt of honoraria or consultation fees from Abeona and Menlo Therapeutics and being a stock shareholder in PellePharm and BridgeBio. Dr. Tang also acknowledged receiving research grants from the EB Research Partnership, the Epidermolysis Medical Research Foundation, and the California Institute for Regenerative Medicine.
SOURCES: Gorell ES et al. EB World Congress, poster 29. Tang JYl. EB World Congress, oral presentation.
LONDON –
Of 85 patients with the recessive dystrophic type of EB (RDEB) who were surveyed through the EBCare Registry, 39 had available data from the validated quality of life in EB (QOLEB) questionnaire. Those with the largest wounds (7.5 cm or greater) had an average QOLEB score of 27, compared with 22.5 for those with wounds ranging from 2.5 to 7.5 cm in size, and a score of 14 for those with wounds less than 2.5 cm in size. The maximum score on the 17-item questionnaire is 51, with the higher the number, the greater the impact on quality of life.
“Large wound areas were seen more frequently in chronic open wounds, similar to findings in separate studies,” Emily S. Gorell, DO, a postdoctoral clinical research fellow in dermatology at Stanford (Calif.) University, and associates reported in a poster presentation at the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association.
“Larger wounds correlate with self-reported disease severity and key clinical manifestations,” they said, which includes history of squamous cell carcinoma (P = .04), anemia (P less than.01), osteoporosis (P = .03), and gastrostomy tube use (P = .02).
In total, 28 adults and 37 children and adolescents were surveyed; the majority (59%) were from North America, with the remainder from Europe (26%) or other countries (15%). Just over half of respondents were female (53%), and about 38% of surveys were completed by the individual rather than a parent or care giver (62%).
Dr. Gorell is working with Jean Y. Tang, MD, PhD, professor of dermatology at Stanford. During an oral presentation at the meeting, Dr. Tang observed that wounds could be defined as being recurrent or chronic open wounds. These two types of wounds behave differently, she said, with the latter never fully healing.
Indeed, in the survey, data on 1,226 wounds were collated, with 937 (76%) classified as recurrent – and healing within 12 weeks – but 289 (24%) remaining chronic open wounds, which did not heal for 12 weeks or longer. Some patients have had open wounds for more than 6 years, Dr. Tang noted.
“In our natural history study … you can see that chronic open wounds never reached 100% closure, they hardly ever reached 50% closure,” she said. In contrast, recurrent wounds have a more dynamic nature, healing completely, then reopening time after time. This is important when considering suitable endpoints for clinical trials, she said, as it could make or break some of the novel treatment approaches currently being tested. For instance, the placebo response in phase 3 trials of the topical therapy allantoin might have been high because recurrent wounds were being studied and were more likely to heal with or without active treatment.
“We’ve done a lot of work, it’s been 2 years, and we have benefited tremendously from these data in our negotiations with the FDA [Food and Drug Administration],” Dr. Tang said. “I am glad we did our homework … we were able to convince the FDA that, for a chronic open wound, a meaningful outcome is 50% healing, not 100%.”
Dr. Tang and Dr. Gorell are part of a team looking at gene-corrected autologous cell therapy (EB-101) to help heal large wounds caused by RDEB. The premise is that by replacing the faulty COL7A1 gene in keratinocytes taken from an individual, these skin cells will be able to produce collagen type VII (COL7). After being grown in culture to form epidermal sheets that look like a plastic film, the sheets can then be grafted over an individuals’ wounds.
Dr. Tang noted that the work was the culmination of 17 years’ of hard work by a small group of committed scientists. Preclinical studies started in 2003, when, she said, “the only funding we could get was through the NIH and thankfully some of the patient organizations.”
Initial results with the EB-101 therapy have been promising. Data on the first four subjects included in a seven-patient phase 1/2 study were published 4 years ago (JAMA. 2016;316[17]:1808-17), and the complete data were recently released (JCI Insight. 2019;4. doi: 10.1172/jci.insight.130554). Each trial subject had an EB-101 graft of approximately 35 cm2 (5 cm x 7 cm) transplanted onto three wounds, with three similar wounds used as controls.
At 6 months, 95% of treated wounds had healed by 50% or more, compared with none of the untreated control wounds (P less than .0001). Healing rates at 1 year and 2 years, respectively, were 68% vs. 17% (P = .025) and 71% vs. 17% (P = .019). All grafts were well tolerated and molecular correction was seen to last for up to 2 years in two patients.
EB-101 therapy will be evaluated in a phase 3 study, the VIITAL study, a multicenter, randomized trial involving 10-15 individuals with RDEB; 50% wound healing at 3 months is the trial’s primary endpoint. The trial, funded by Abeona Therapeutics, was given the go ahead by the FDA in December 2019 and has an estimated completion date of March 2021.
Dr. Gorell did not provide a conflict of interest statement. Dr. Tang disclosed receipt of honoraria or consultation fees from Abeona and Menlo Therapeutics and being a stock shareholder in PellePharm and BridgeBio. Dr. Tang also acknowledged receiving research grants from the EB Research Partnership, the Epidermolysis Medical Research Foundation, and the California Institute for Regenerative Medicine.
SOURCES: Gorell ES et al. EB World Congress, poster 29. Tang JYl. EB World Congress, oral presentation.
REPORTING FROM EB WORLD CONGRESS
First protocol on how to use lung ultrasound to triage COVID-19
The first protocol for the use of lung ultrasound to quantitatively and reproducibly assess the degree of lung involvement in patients suspected of having COVID-19 infection has been published by a team of Italian experts with experience using the technology on the front line.
Particularly in Spain and Italy — where the pandemic has struck hardest in Europe — hard-pressed clinicians seeking to quickly understand whether patients with seemingly mild disease could be harboring more serious lung involvement have increasingly relied upon lung ultrasound in the emergency room.
Now Libertario Demi, PhD, head of the ultrasound laboratory, University of Trento, Italy, and colleagues have developed a protocol, published online March 30 in the Journal of Ultrasound Medicine, to standardize practice.
Their research, which builds on previous work by the team, offers broad agreement with industry-led algorithms and emphasizes the use of wireless, handheld ultrasound devices, ideally consisting of a separate probe and tablet, to make sterilization easy.
Firms such as the Butterfly Network, Phillips, Clarius, GE Healthcare, and Siemens are among numerous companies that produce one or more such devices, including some that are completely integrated.
Not Universally Accepted
However, lung ultrasound is not yet universally accepted as a tool for diagnosing pneumonia in the context of COVID-19 and triaging patients.
The National Health Service in England does not even mention lung ultrasound in its radiology decision tool for suspected COVID-19, specifying instead chest X-ray as the first-line diagnostic imaging tool, with CT scanning in equivocal cases.
But Giovanni Volpicelli, MD, University Hospital San Luigi Gonzaga, Turin, Italy, who has previously described his experience to Medscape Medical News, says many patients with COVID-19 in his hospital presented with a negative chest X-ray but were found to have interstitial pneumonia on lung ultrasound.
Moreover, while CT scan remains the gold standard, the risk of nosocomial infection is more easily controlled if patients do not have to be transported to the radiology department but remain in the emergency room and instead undergo lung ultrasound there, he stressed.
Experts Share Experience of Lung Ultrasound in COVID-19
In developing and publishing their protocol, Demi, senior author of the article, and other colleagues from the heavily affected cities of Northern Italy, say their aim is “to share our experience and to propose a standardization with respect to the use of lung ultrasound in the management of COVID-19 patients.”
They reviewed an anonymized database of around 60,000 ultrasound images of confirmed COVID-19 cases and reviewers were blinded to patients’ clinical backgrounds.
For image acquisition, the authors recommend scanning 14 areas in each patient for 10 seconds, making the scans intercostal to cover the widest possible surface area.
They advise the use of a single focal point on the pleural line, which they write, optimizes the beam shape for observing the lung surface.
The authors also urge that the mechanical index (MI) be kept low because high MIs sustained for long periods “may result in damaging the lung.”
They also stress that cosmetic filters and modalities such as harmonic imaging, contrast, doppler, and compounding should be avoided, alongside saturation phenomena.
What Constitutes Intermediate Disease?
Once the images have been taken, they are scored on a 0-3 scale for each of the 14 areas, with no weighting on any individual area.
A score of 0 is given when the pleural line is continuous and regular, with the presence of A-lines, denoting that the lungs are unaffected.
An area is given a score of 3 when the scan shows dense and largely extended white lung tissue, with or without consolidations, indicating severe disease.
At both ends of this spectrum, there is agreement between the Italian protocol and an algorithm developed by the Butterfly Network.
However, the two differ when it comes to scoring intermediate cases. On the Butterfly algorithm, the suggestion is to look for B-lines, caused by fluid and cellular infiltration into the interstitium, and to weigh that against the need for supplementary oxygen.
The Italian team, in contrast, says a score of 1 is given when the pleural line is indented, with vertical areas of white visible below.
A score of 2 is given when the pleural line is broken, with small to large areas of consolidation and associated areas of white below.
Demi told Medscape Medical News that they did not refer to B-lines in their protocol as their visibility depends entirely on the imaging frequency and the probe used.
“This means that scoring on B-lines, people with different machines would give completely different scores for the same patient.”
He continued: “We prefer to refer to horizontal and vertical artifacts, and provide an analysis of the patterns, which is related to the physics of the interactions between the ultrasound waves and lung surface.”
In response, Mike Stone, MD, Legacy Emanuel Medical Center, Portland, Oregon, and director of education at Butterfly, said there appears to be wide variation in lung findings that “may or may not correlate with the severity of symptoms.”
He told Medscape Medical News it is “hard to know exactly if someone with pure B-lines will progress to serious illness or if someone with some subpleural consolidations will do well.”
A Negative Ultrasound Is the Most Useful
Volpicelli believes that, in any case, any patient with an intermediate pattern will require further diagnosis, such as other imaging modalities and blood exams, and the real role of lung ultrasound is in assessing patients at either end of the spectrum.
“In other words, there are situations where lung ultrasound can be considered definitive,” he told Medscape Medical News. “For instance, if I see a patient with mild signs of the disease, just fever, and I perform lung ultrasound and see nothing, lung ultrasound rules out pneumonia.”
“This patient may have COVID-19 of course, but they do not have pneumonia, and they can be treated at home, awaiting the result of the swab test. And this is useful because you can reduce the burden in the emergency department.”
Volpicelli continued: “On the other hand, there are patients with acute respiratory failure in respiratory distress. If the lung ultrasound is normal, you can rule out COVID-19 and you need to use other diagnostic procedures to understand the problem.”
“This is also very important for us because it’s crucial to be able to remove the patient from the isolation area and perform CT scan, chest radiography, and all the other diagnostic tools that we need.”
Are Wireless Machines Needed? Not Necessarily
With regard to the use of wireless technology, the Italian team says that “in the setting of COVID-19, wireless probes and tablets represent the most appropriate ultrasound equipment” because they can “easily be wrapped in single-use plastic covers, reducing the risk of contamination,” and making sterilization easy.
Stone suggests that integrated portable devices, however, are no more likely to cause cross-contamination than separate probes and tablets, as they can fit within a sterile sheath as a single unit.
Volpicelli, for his part, doesn’t like what he sees as undue focus on wireless devices for lung ultrasound in the COVID-19 protocols.
He is concerned that recommending them as the best approach may be sending out the wrong message, which could be very “dangerous” as people may then think they cannot perform this screening with standard ultrasound machines.
For him, the issue of cross contamination with standard lung ultrasound machines is “nonexistent. Cleaning the machine is quite easy and I do it hundreds of times per week.”
He does acknowledge, however, that if the lung ultrasound is performed under certain circumstances, for example when a patient is using a continuous positive airway pressure (CPAP) machine, “the risk of having the machine contaminated is a little bit higher.”
“In these situations...we have a more intensive cleaning procedure to avoid cross-contamination.”
He stressed: “Not all centers have wireless machines, whereas a normal machine is usually in all hospitals.”
“The advantages of using lung ultrasound [in COVID-19] are too great to be limited by something that is not important in my opinion,” he concluded.
Stone is director of education at the Butterfly Network. No other conflicts of interest were declared.
This article first appeared on Medscape.com.
The first protocol for the use of lung ultrasound to quantitatively and reproducibly assess the degree of lung involvement in patients suspected of having COVID-19 infection has been published by a team of Italian experts with experience using the technology on the front line.
Particularly in Spain and Italy — where the pandemic has struck hardest in Europe — hard-pressed clinicians seeking to quickly understand whether patients with seemingly mild disease could be harboring more serious lung involvement have increasingly relied upon lung ultrasound in the emergency room.
Now Libertario Demi, PhD, head of the ultrasound laboratory, University of Trento, Italy, and colleagues have developed a protocol, published online March 30 in the Journal of Ultrasound Medicine, to standardize practice.
Their research, which builds on previous work by the team, offers broad agreement with industry-led algorithms and emphasizes the use of wireless, handheld ultrasound devices, ideally consisting of a separate probe and tablet, to make sterilization easy.
Firms such as the Butterfly Network, Phillips, Clarius, GE Healthcare, and Siemens are among numerous companies that produce one or more such devices, including some that are completely integrated.
Not Universally Accepted
However, lung ultrasound is not yet universally accepted as a tool for diagnosing pneumonia in the context of COVID-19 and triaging patients.
The National Health Service in England does not even mention lung ultrasound in its radiology decision tool for suspected COVID-19, specifying instead chest X-ray as the first-line diagnostic imaging tool, with CT scanning in equivocal cases.
But Giovanni Volpicelli, MD, University Hospital San Luigi Gonzaga, Turin, Italy, who has previously described his experience to Medscape Medical News, says many patients with COVID-19 in his hospital presented with a negative chest X-ray but were found to have interstitial pneumonia on lung ultrasound.
Moreover, while CT scan remains the gold standard, the risk of nosocomial infection is more easily controlled if patients do not have to be transported to the radiology department but remain in the emergency room and instead undergo lung ultrasound there, he stressed.
Experts Share Experience of Lung Ultrasound in COVID-19
In developing and publishing their protocol, Demi, senior author of the article, and other colleagues from the heavily affected cities of Northern Italy, say their aim is “to share our experience and to propose a standardization with respect to the use of lung ultrasound in the management of COVID-19 patients.”
They reviewed an anonymized database of around 60,000 ultrasound images of confirmed COVID-19 cases and reviewers were blinded to patients’ clinical backgrounds.
For image acquisition, the authors recommend scanning 14 areas in each patient for 10 seconds, making the scans intercostal to cover the widest possible surface area.
They advise the use of a single focal point on the pleural line, which they write, optimizes the beam shape for observing the lung surface.
The authors also urge that the mechanical index (MI) be kept low because high MIs sustained for long periods “may result in damaging the lung.”
They also stress that cosmetic filters and modalities such as harmonic imaging, contrast, doppler, and compounding should be avoided, alongside saturation phenomena.
What Constitutes Intermediate Disease?
Once the images have been taken, they are scored on a 0-3 scale for each of the 14 areas, with no weighting on any individual area.
A score of 0 is given when the pleural line is continuous and regular, with the presence of A-lines, denoting that the lungs are unaffected.
An area is given a score of 3 when the scan shows dense and largely extended white lung tissue, with or without consolidations, indicating severe disease.
At both ends of this spectrum, there is agreement between the Italian protocol and an algorithm developed by the Butterfly Network.
However, the two differ when it comes to scoring intermediate cases. On the Butterfly algorithm, the suggestion is to look for B-lines, caused by fluid and cellular infiltration into the interstitium, and to weigh that against the need for supplementary oxygen.
The Italian team, in contrast, says a score of 1 is given when the pleural line is indented, with vertical areas of white visible below.
A score of 2 is given when the pleural line is broken, with small to large areas of consolidation and associated areas of white below.
Demi told Medscape Medical News that they did not refer to B-lines in their protocol as their visibility depends entirely on the imaging frequency and the probe used.
“This means that scoring on B-lines, people with different machines would give completely different scores for the same patient.”
He continued: “We prefer to refer to horizontal and vertical artifacts, and provide an analysis of the patterns, which is related to the physics of the interactions between the ultrasound waves and lung surface.”
In response, Mike Stone, MD, Legacy Emanuel Medical Center, Portland, Oregon, and director of education at Butterfly, said there appears to be wide variation in lung findings that “may or may not correlate with the severity of symptoms.”
He told Medscape Medical News it is “hard to know exactly if someone with pure B-lines will progress to serious illness or if someone with some subpleural consolidations will do well.”
A Negative Ultrasound Is the Most Useful
Volpicelli believes that, in any case, any patient with an intermediate pattern will require further diagnosis, such as other imaging modalities and blood exams, and the real role of lung ultrasound is in assessing patients at either end of the spectrum.
“In other words, there are situations where lung ultrasound can be considered definitive,” he told Medscape Medical News. “For instance, if I see a patient with mild signs of the disease, just fever, and I perform lung ultrasound and see nothing, lung ultrasound rules out pneumonia.”
“This patient may have COVID-19 of course, but they do not have pneumonia, and they can be treated at home, awaiting the result of the swab test. And this is useful because you can reduce the burden in the emergency department.”
Volpicelli continued: “On the other hand, there are patients with acute respiratory failure in respiratory distress. If the lung ultrasound is normal, you can rule out COVID-19 and you need to use other diagnostic procedures to understand the problem.”
“This is also very important for us because it’s crucial to be able to remove the patient from the isolation area and perform CT scan, chest radiography, and all the other diagnostic tools that we need.”
Are Wireless Machines Needed? Not Necessarily
With regard to the use of wireless technology, the Italian team says that “in the setting of COVID-19, wireless probes and tablets represent the most appropriate ultrasound equipment” because they can “easily be wrapped in single-use plastic covers, reducing the risk of contamination,” and making sterilization easy.
Stone suggests that integrated portable devices, however, are no more likely to cause cross-contamination than separate probes and tablets, as they can fit within a sterile sheath as a single unit.
Volpicelli, for his part, doesn’t like what he sees as undue focus on wireless devices for lung ultrasound in the COVID-19 protocols.
He is concerned that recommending them as the best approach may be sending out the wrong message, which could be very “dangerous” as people may then think they cannot perform this screening with standard ultrasound machines.
For him, the issue of cross contamination with standard lung ultrasound machines is “nonexistent. Cleaning the machine is quite easy and I do it hundreds of times per week.”
He does acknowledge, however, that if the lung ultrasound is performed under certain circumstances, for example when a patient is using a continuous positive airway pressure (CPAP) machine, “the risk of having the machine contaminated is a little bit higher.”
“In these situations...we have a more intensive cleaning procedure to avoid cross-contamination.”
He stressed: “Not all centers have wireless machines, whereas a normal machine is usually in all hospitals.”
“The advantages of using lung ultrasound [in COVID-19] are too great to be limited by something that is not important in my opinion,” he concluded.
Stone is director of education at the Butterfly Network. No other conflicts of interest were declared.
This article first appeared on Medscape.com.
The first protocol for the use of lung ultrasound to quantitatively and reproducibly assess the degree of lung involvement in patients suspected of having COVID-19 infection has been published by a team of Italian experts with experience using the technology on the front line.
Particularly in Spain and Italy — where the pandemic has struck hardest in Europe — hard-pressed clinicians seeking to quickly understand whether patients with seemingly mild disease could be harboring more serious lung involvement have increasingly relied upon lung ultrasound in the emergency room.
Now Libertario Demi, PhD, head of the ultrasound laboratory, University of Trento, Italy, and colleagues have developed a protocol, published online March 30 in the Journal of Ultrasound Medicine, to standardize practice.
Their research, which builds on previous work by the team, offers broad agreement with industry-led algorithms and emphasizes the use of wireless, handheld ultrasound devices, ideally consisting of a separate probe and tablet, to make sterilization easy.
Firms such as the Butterfly Network, Phillips, Clarius, GE Healthcare, and Siemens are among numerous companies that produce one or more such devices, including some that are completely integrated.
Not Universally Accepted
However, lung ultrasound is not yet universally accepted as a tool for diagnosing pneumonia in the context of COVID-19 and triaging patients.
The National Health Service in England does not even mention lung ultrasound in its radiology decision tool for suspected COVID-19, specifying instead chest X-ray as the first-line diagnostic imaging tool, with CT scanning in equivocal cases.
But Giovanni Volpicelli, MD, University Hospital San Luigi Gonzaga, Turin, Italy, who has previously described his experience to Medscape Medical News, says many patients with COVID-19 in his hospital presented with a negative chest X-ray but were found to have interstitial pneumonia on lung ultrasound.
Moreover, while CT scan remains the gold standard, the risk of nosocomial infection is more easily controlled if patients do not have to be transported to the radiology department but remain in the emergency room and instead undergo lung ultrasound there, he stressed.
Experts Share Experience of Lung Ultrasound in COVID-19
In developing and publishing their protocol, Demi, senior author of the article, and other colleagues from the heavily affected cities of Northern Italy, say their aim is “to share our experience and to propose a standardization with respect to the use of lung ultrasound in the management of COVID-19 patients.”
They reviewed an anonymized database of around 60,000 ultrasound images of confirmed COVID-19 cases and reviewers were blinded to patients’ clinical backgrounds.
For image acquisition, the authors recommend scanning 14 areas in each patient for 10 seconds, making the scans intercostal to cover the widest possible surface area.
They advise the use of a single focal point on the pleural line, which they write, optimizes the beam shape for observing the lung surface.
The authors also urge that the mechanical index (MI) be kept low because high MIs sustained for long periods “may result in damaging the lung.”
They also stress that cosmetic filters and modalities such as harmonic imaging, contrast, doppler, and compounding should be avoided, alongside saturation phenomena.
What Constitutes Intermediate Disease?
Once the images have been taken, they are scored on a 0-3 scale for each of the 14 areas, with no weighting on any individual area.
A score of 0 is given when the pleural line is continuous and regular, with the presence of A-lines, denoting that the lungs are unaffected.
An area is given a score of 3 when the scan shows dense and largely extended white lung tissue, with or without consolidations, indicating severe disease.
At both ends of this spectrum, there is agreement between the Italian protocol and an algorithm developed by the Butterfly Network.
However, the two differ when it comes to scoring intermediate cases. On the Butterfly algorithm, the suggestion is to look for B-lines, caused by fluid and cellular infiltration into the interstitium, and to weigh that against the need for supplementary oxygen.
The Italian team, in contrast, says a score of 1 is given when the pleural line is indented, with vertical areas of white visible below.
A score of 2 is given when the pleural line is broken, with small to large areas of consolidation and associated areas of white below.
Demi told Medscape Medical News that they did not refer to B-lines in their protocol as their visibility depends entirely on the imaging frequency and the probe used.
“This means that scoring on B-lines, people with different machines would give completely different scores for the same patient.”
He continued: “We prefer to refer to horizontal and vertical artifacts, and provide an analysis of the patterns, which is related to the physics of the interactions between the ultrasound waves and lung surface.”
In response, Mike Stone, MD, Legacy Emanuel Medical Center, Portland, Oregon, and director of education at Butterfly, said there appears to be wide variation in lung findings that “may or may not correlate with the severity of symptoms.”
He told Medscape Medical News it is “hard to know exactly if someone with pure B-lines will progress to serious illness or if someone with some subpleural consolidations will do well.”
A Negative Ultrasound Is the Most Useful
Volpicelli believes that, in any case, any patient with an intermediate pattern will require further diagnosis, such as other imaging modalities and blood exams, and the real role of lung ultrasound is in assessing patients at either end of the spectrum.
“In other words, there are situations where lung ultrasound can be considered definitive,” he told Medscape Medical News. “For instance, if I see a patient with mild signs of the disease, just fever, and I perform lung ultrasound and see nothing, lung ultrasound rules out pneumonia.”
“This patient may have COVID-19 of course, but they do not have pneumonia, and they can be treated at home, awaiting the result of the swab test. And this is useful because you can reduce the burden in the emergency department.”
Volpicelli continued: “On the other hand, there are patients with acute respiratory failure in respiratory distress. If the lung ultrasound is normal, you can rule out COVID-19 and you need to use other diagnostic procedures to understand the problem.”
“This is also very important for us because it’s crucial to be able to remove the patient from the isolation area and perform CT scan, chest radiography, and all the other diagnostic tools that we need.”
Are Wireless Machines Needed? Not Necessarily
With regard to the use of wireless technology, the Italian team says that “in the setting of COVID-19, wireless probes and tablets represent the most appropriate ultrasound equipment” because they can “easily be wrapped in single-use plastic covers, reducing the risk of contamination,” and making sterilization easy.
Stone suggests that integrated portable devices, however, are no more likely to cause cross-contamination than separate probes and tablets, as they can fit within a sterile sheath as a single unit.
Volpicelli, for his part, doesn’t like what he sees as undue focus on wireless devices for lung ultrasound in the COVID-19 protocols.
He is concerned that recommending them as the best approach may be sending out the wrong message, which could be very “dangerous” as people may then think they cannot perform this screening with standard ultrasound machines.
For him, the issue of cross contamination with standard lung ultrasound machines is “nonexistent. Cleaning the machine is quite easy and I do it hundreds of times per week.”
He does acknowledge, however, that if the lung ultrasound is performed under certain circumstances, for example when a patient is using a continuous positive airway pressure (CPAP) machine, “the risk of having the machine contaminated is a little bit higher.”
“In these situations...we have a more intensive cleaning procedure to avoid cross-contamination.”
He stressed: “Not all centers have wireless machines, whereas a normal machine is usually in all hospitals.”
“The advantages of using lung ultrasound [in COVID-19] are too great to be limited by something that is not important in my opinion,” he concluded.
Stone is director of education at the Butterfly Network. No other conflicts of interest were declared.
This article first appeared on Medscape.com.
Cardiovascular problems already apparent in children with hemophilia A
Negative cardiovascular health indicators were found to be higher in children with hemophilia A, compared with healthy children, according to a small research study.
Biochemical, imaging, and metabolic analyses were performed to compare 17 boys with severe hemophilia A to 23 healthy boys designated as controls.
The myocardial performance index (MPI) was evaluated using tissue Doppler echocardiography. In addition, peripheral and central blood pressure and arterial stiffness were assessed, as were carotid intima–media thicknesses (CIMTs), serum glucose, insulin, insulin resistance, and lipoprotein levels.
Increased MPI is considered an indicator of global deterioration in myocardial functions.
There were no differences between the two groups in terms of age and biochemical parameters, according to the researchers. There were also no significant differences found between the groups in terms of CIMT, peripheral blood pressure, and central systolic blood pressure.
However, the HDL cholesterol levels in the hemophilia group were significantly lower than those in the control group (P < .05). Five of the hemophilia patients had insulin resistance (29.4%), whereas four had low HDL cholesterol levels (23.5%).
The researchers found that the MPI values in the hemophilia group were higher than those in the control group (0.41 vs. 0.34; P = .004). In addition, left ventricle ejection time (ET), which is a predictor of mortality in heart failure and ischemic heart disease, was shorter in the hemophilia group than it was in the control group (266.6 vs. 284.4; P = .014).
As arterial stiffness increases, ejection time decreases owing to the deteriorating myocardial systolic functions, and it has also been reported in the literature that arterial stiffness affects left ventricular systolic functions, according to the authors.
“Arterial stiffness and high [central diastolic blood pressure] developing in patients with severe hemophilia A since their childhood are important risk factors for coronary artery diseases. Predisposition to dyslipidemia and [insulin resistance] noted in the hemophilia group also negatively contributes to this process. Adolescent patients with hemophilia should be monitored for hypertension, obesity, dyslipidemia, and [insulin resistance],” the authors concluded.
The study received no external funding. The authors did not report disclosures.
SOURCE: Özdemir ZC et al. Thrombosis Res. 2020;189:102-7.
Negative cardiovascular health indicators were found to be higher in children with hemophilia A, compared with healthy children, according to a small research study.
Biochemical, imaging, and metabolic analyses were performed to compare 17 boys with severe hemophilia A to 23 healthy boys designated as controls.
The myocardial performance index (MPI) was evaluated using tissue Doppler echocardiography. In addition, peripheral and central blood pressure and arterial stiffness were assessed, as were carotid intima–media thicknesses (CIMTs), serum glucose, insulin, insulin resistance, and lipoprotein levels.
Increased MPI is considered an indicator of global deterioration in myocardial functions.
There were no differences between the two groups in terms of age and biochemical parameters, according to the researchers. There were also no significant differences found between the groups in terms of CIMT, peripheral blood pressure, and central systolic blood pressure.
However, the HDL cholesterol levels in the hemophilia group were significantly lower than those in the control group (P < .05). Five of the hemophilia patients had insulin resistance (29.4%), whereas four had low HDL cholesterol levels (23.5%).
The researchers found that the MPI values in the hemophilia group were higher than those in the control group (0.41 vs. 0.34; P = .004). In addition, left ventricle ejection time (ET), which is a predictor of mortality in heart failure and ischemic heart disease, was shorter in the hemophilia group than it was in the control group (266.6 vs. 284.4; P = .014).
As arterial stiffness increases, ejection time decreases owing to the deteriorating myocardial systolic functions, and it has also been reported in the literature that arterial stiffness affects left ventricular systolic functions, according to the authors.
“Arterial stiffness and high [central diastolic blood pressure] developing in patients with severe hemophilia A since their childhood are important risk factors for coronary artery diseases. Predisposition to dyslipidemia and [insulin resistance] noted in the hemophilia group also negatively contributes to this process. Adolescent patients with hemophilia should be monitored for hypertension, obesity, dyslipidemia, and [insulin resistance],” the authors concluded.
The study received no external funding. The authors did not report disclosures.
SOURCE: Özdemir ZC et al. Thrombosis Res. 2020;189:102-7.
Negative cardiovascular health indicators were found to be higher in children with hemophilia A, compared with healthy children, according to a small research study.
Biochemical, imaging, and metabolic analyses were performed to compare 17 boys with severe hemophilia A to 23 healthy boys designated as controls.
The myocardial performance index (MPI) was evaluated using tissue Doppler echocardiography. In addition, peripheral and central blood pressure and arterial stiffness were assessed, as were carotid intima–media thicknesses (CIMTs), serum glucose, insulin, insulin resistance, and lipoprotein levels.
Increased MPI is considered an indicator of global deterioration in myocardial functions.
There were no differences between the two groups in terms of age and biochemical parameters, according to the researchers. There were also no significant differences found between the groups in terms of CIMT, peripheral blood pressure, and central systolic blood pressure.
However, the HDL cholesterol levels in the hemophilia group were significantly lower than those in the control group (P < .05). Five of the hemophilia patients had insulin resistance (29.4%), whereas four had low HDL cholesterol levels (23.5%).
The researchers found that the MPI values in the hemophilia group were higher than those in the control group (0.41 vs. 0.34; P = .004). In addition, left ventricle ejection time (ET), which is a predictor of mortality in heart failure and ischemic heart disease, was shorter in the hemophilia group than it was in the control group (266.6 vs. 284.4; P = .014).
As arterial stiffness increases, ejection time decreases owing to the deteriorating myocardial systolic functions, and it has also been reported in the literature that arterial stiffness affects left ventricular systolic functions, according to the authors.
“Arterial stiffness and high [central diastolic blood pressure] developing in patients with severe hemophilia A since their childhood are important risk factors for coronary artery diseases. Predisposition to dyslipidemia and [insulin resistance] noted in the hemophilia group also negatively contributes to this process. Adolescent patients with hemophilia should be monitored for hypertension, obesity, dyslipidemia, and [insulin resistance],” the authors concluded.
The study received no external funding. The authors did not report disclosures.
SOURCE: Özdemir ZC et al. Thrombosis Res. 2020;189:102-7.
FROM THROMBOSIS RESEARCH
Crisis counseling, not therapy, is what’s needed in the wake of COVID-19
In the wake of the attacks on the World Trade Center, the public mental health system in the New York City area mounted the largest mental health disaster response in history. I was New York City’s mental health commissioner at the time. We called the initiative Project Liberty and over 3 years obtained $137 million in funding from the Federal Emergency Management Agency (FEMA) to support it.
Through Project Liberty, New York established the Crisis Counseling Assistance and Training Program (CCP). And it didn’t take us long to realize that what affected people need following a disaster is not necessarily psychotherapy, as might be expected, but in fact crisis counseling, or helping impacted individuals and their families regain control of their anxieties and effectively respond to an immediate disaster. This proved true not only after 9/11 but also after other recent disasters, including hurricanes Katrina and Sandy. The mental health system must now step up again to assuage fears and anxieties—both individual and collective—around the rapidly spreading COVID-19 pandemic.
So, what is crisis counseling?
A person’s usual adaptive, problem-solving capabilities are often compromised after a disaster, but they are there, and if accessed, they can help those afflicted with mental symptoms following a crisis to mentally endure. thereby making it a different approach from traditional psychotherapy.
The five key concepts in crisis counseling are:
- It is strength-based, which means its foundation is rooted in the assumption that resilience and competence are innate human qualities.
- Crisis counseling also employs anonymity. Impacted individuals should not be diagnosed or labeled. As a result, there are no resulting medical records.
- The approach is outreach-oriented, in which counselors provide services out in the community rather than in traditional mental health settings. This occurs primarily in homes, community centers, and settings, as well as in disaster shelters.
- It is culturally attuned, whereby all staff appreciate and respect a community’s cultural beliefs, values, and primary language.
- It is aimed at supporting, not replacing, existing community support systems (eg, a crisis counselor supports but does not organize, deliver, or manage community recovery activities).
Crisis counselors are required to be licensed psychologists or have obtained a bachelor’s degree or higher in psychology, human services, or another health-related field. In other words, crisis counseling draws on a broad, though related, group of individuals. Before deployment into a disaster area, an applicant must complete the FEMA Crisis Counseling Assistance and Training, which is offered in the disaster area by the FEMA-funded CCP.
Crisis counselors provide trustworthy and actionable information about the disaster at hand and where to turn for resources and assistance. They assist with emotional support. And they aim to educate individuals, families, and communities about how to be resilient.
Crisis counseling, however, may not suffice for everyone impacted. We know that a person’s severity of response to a crisis is highly associated with the intensity and duration of exposure to the disaster (especially when it is life-threatening) and/or the degree of a person’s serious loss (of a loved one, home, job, health). We also know that previous trauma (eg, from childhood, domestic violence, or forced immigration) also predicts the gravity of the response to a current crisis. Which is why crisis counselors also are taught to identify those experiencing significant and persistent mental health and addiction problems because they need to be assisted, literally, in obtaining professional treatment.
Only in recent years has trauma been a recognized driver of stress, distress, and mental and addictive disorders. Until relatively recently, skill with, and access to, crisis counseling—and trauma-informed care—was rare among New York’s large and talented mental health professional community. Few had been trained in it in graduate school or practiced it because New York had been spared a disaster on par with 9/11. Following the attacks, Project Liberty’s programs served nearly 1.5 million affected individuals of very diverse ages, races, cultural backgrounds, and socioeconomic status. Their levels of “psychological distress,” the term we used and measured, ranged from low to very high.
The coronavirus pandemic now presents us with a tragically similar, catastrophic moment. The human consequences we face—psychologically, economically, and socially—are just beginning. But this time, the need is not just in New York but throughout our country.
We humans are resilient. We can bend the arc of crisis toward the light, to recovering our existing but overwhelmed capabilities. We can achieve this in a variety of ways. We can practice self-care. This isn’t an act of selfishness but is rather like putting on your own oxygen mask before trying to help your friend or loved one do the same. We can stay connected to the people we care about. We can eat well, get sufficient sleep, take a walk.
Identifying and pursuing practical goals is also important, like obtaining food, housing that is safe and reliable, transportation to where you need to go, and drawing upon financial and other resources that are issued in a disaster area. We can practice positive thinking and recall how we’ve mastered our troubles in the past; we can remind ourselves that “this too will pass.” Crises create an unusually opportune time for change and self-discovery. As Churchill said to the British people in the darkest moments of the start of World War II, “Never give up.”
Worthy of its own itemization are spiritual beliefs, faith—that however we think about a higher power (religious or secular), that power is on our side. Faith can comfort and sustain hope, particularly at a time when doubt about ourselves and humanity is triggered by disaster.
Maya Angelou’s words remind us at this moment of disaster: “...let us try to help before we have to offer therapy. That is to say, let’s see if we can’t prevent being ill by trying to offer a love of prevention before illness.”
Dr. Sederer is the former chief medical officer for the New York State Office of Mental Health and an adjunct professor in the Department of Epidemiology at the Columbia University School of Public Health. His latest book is The Addiction Solution: Treating Our Dependence on Opioids and Other Drugs.
This article first appeared on Medscape.com.
In the wake of the attacks on the World Trade Center, the public mental health system in the New York City area mounted the largest mental health disaster response in history. I was New York City’s mental health commissioner at the time. We called the initiative Project Liberty and over 3 years obtained $137 million in funding from the Federal Emergency Management Agency (FEMA) to support it.
Through Project Liberty, New York established the Crisis Counseling Assistance and Training Program (CCP). And it didn’t take us long to realize that what affected people need following a disaster is not necessarily psychotherapy, as might be expected, but in fact crisis counseling, or helping impacted individuals and their families regain control of their anxieties and effectively respond to an immediate disaster. This proved true not only after 9/11 but also after other recent disasters, including hurricanes Katrina and Sandy. The mental health system must now step up again to assuage fears and anxieties—both individual and collective—around the rapidly spreading COVID-19 pandemic.
So, what is crisis counseling?
A person’s usual adaptive, problem-solving capabilities are often compromised after a disaster, but they are there, and if accessed, they can help those afflicted with mental symptoms following a crisis to mentally endure. thereby making it a different approach from traditional psychotherapy.
The five key concepts in crisis counseling are:
- It is strength-based, which means its foundation is rooted in the assumption that resilience and competence are innate human qualities.
- Crisis counseling also employs anonymity. Impacted individuals should not be diagnosed or labeled. As a result, there are no resulting medical records.
- The approach is outreach-oriented, in which counselors provide services out in the community rather than in traditional mental health settings. This occurs primarily in homes, community centers, and settings, as well as in disaster shelters.
- It is culturally attuned, whereby all staff appreciate and respect a community’s cultural beliefs, values, and primary language.
- It is aimed at supporting, not replacing, existing community support systems (eg, a crisis counselor supports but does not organize, deliver, or manage community recovery activities).
Crisis counselors are required to be licensed psychologists or have obtained a bachelor’s degree or higher in psychology, human services, or another health-related field. In other words, crisis counseling draws on a broad, though related, group of individuals. Before deployment into a disaster area, an applicant must complete the FEMA Crisis Counseling Assistance and Training, which is offered in the disaster area by the FEMA-funded CCP.
Crisis counselors provide trustworthy and actionable information about the disaster at hand and where to turn for resources and assistance. They assist with emotional support. And they aim to educate individuals, families, and communities about how to be resilient.
Crisis counseling, however, may not suffice for everyone impacted. We know that a person’s severity of response to a crisis is highly associated with the intensity and duration of exposure to the disaster (especially when it is life-threatening) and/or the degree of a person’s serious loss (of a loved one, home, job, health). We also know that previous trauma (eg, from childhood, domestic violence, or forced immigration) also predicts the gravity of the response to a current crisis. Which is why crisis counselors also are taught to identify those experiencing significant and persistent mental health and addiction problems because they need to be assisted, literally, in obtaining professional treatment.
Only in recent years has trauma been a recognized driver of stress, distress, and mental and addictive disorders. Until relatively recently, skill with, and access to, crisis counseling—and trauma-informed care—was rare among New York’s large and talented mental health professional community. Few had been trained in it in graduate school or practiced it because New York had been spared a disaster on par with 9/11. Following the attacks, Project Liberty’s programs served nearly 1.5 million affected individuals of very diverse ages, races, cultural backgrounds, and socioeconomic status. Their levels of “psychological distress,” the term we used and measured, ranged from low to very high.
The coronavirus pandemic now presents us with a tragically similar, catastrophic moment. The human consequences we face—psychologically, economically, and socially—are just beginning. But this time, the need is not just in New York but throughout our country.
We humans are resilient. We can bend the arc of crisis toward the light, to recovering our existing but overwhelmed capabilities. We can achieve this in a variety of ways. We can practice self-care. This isn’t an act of selfishness but is rather like putting on your own oxygen mask before trying to help your friend or loved one do the same. We can stay connected to the people we care about. We can eat well, get sufficient sleep, take a walk.
Identifying and pursuing practical goals is also important, like obtaining food, housing that is safe and reliable, transportation to where you need to go, and drawing upon financial and other resources that are issued in a disaster area. We can practice positive thinking and recall how we’ve mastered our troubles in the past; we can remind ourselves that “this too will pass.” Crises create an unusually opportune time for change and self-discovery. As Churchill said to the British people in the darkest moments of the start of World War II, “Never give up.”
Worthy of its own itemization are spiritual beliefs, faith—that however we think about a higher power (religious or secular), that power is on our side. Faith can comfort and sustain hope, particularly at a time when doubt about ourselves and humanity is triggered by disaster.
Maya Angelou’s words remind us at this moment of disaster: “...let us try to help before we have to offer therapy. That is to say, let’s see if we can’t prevent being ill by trying to offer a love of prevention before illness.”
Dr. Sederer is the former chief medical officer for the New York State Office of Mental Health and an adjunct professor in the Department of Epidemiology at the Columbia University School of Public Health. His latest book is The Addiction Solution: Treating Our Dependence on Opioids and Other Drugs.
This article first appeared on Medscape.com.
In the wake of the attacks on the World Trade Center, the public mental health system in the New York City area mounted the largest mental health disaster response in history. I was New York City’s mental health commissioner at the time. We called the initiative Project Liberty and over 3 years obtained $137 million in funding from the Federal Emergency Management Agency (FEMA) to support it.
Through Project Liberty, New York established the Crisis Counseling Assistance and Training Program (CCP). And it didn’t take us long to realize that what affected people need following a disaster is not necessarily psychotherapy, as might be expected, but in fact crisis counseling, or helping impacted individuals and their families regain control of their anxieties and effectively respond to an immediate disaster. This proved true not only after 9/11 but also after other recent disasters, including hurricanes Katrina and Sandy. The mental health system must now step up again to assuage fears and anxieties—both individual and collective—around the rapidly spreading COVID-19 pandemic.
So, what is crisis counseling?
A person’s usual adaptive, problem-solving capabilities are often compromised after a disaster, but they are there, and if accessed, they can help those afflicted with mental symptoms following a crisis to mentally endure. thereby making it a different approach from traditional psychotherapy.
The five key concepts in crisis counseling are:
- It is strength-based, which means its foundation is rooted in the assumption that resilience and competence are innate human qualities.
- Crisis counseling also employs anonymity. Impacted individuals should not be diagnosed or labeled. As a result, there are no resulting medical records.
- The approach is outreach-oriented, in which counselors provide services out in the community rather than in traditional mental health settings. This occurs primarily in homes, community centers, and settings, as well as in disaster shelters.
- It is culturally attuned, whereby all staff appreciate and respect a community’s cultural beliefs, values, and primary language.
- It is aimed at supporting, not replacing, existing community support systems (eg, a crisis counselor supports but does not organize, deliver, or manage community recovery activities).
Crisis counselors are required to be licensed psychologists or have obtained a bachelor’s degree or higher in psychology, human services, or another health-related field. In other words, crisis counseling draws on a broad, though related, group of individuals. Before deployment into a disaster area, an applicant must complete the FEMA Crisis Counseling Assistance and Training, which is offered in the disaster area by the FEMA-funded CCP.
Crisis counselors provide trustworthy and actionable information about the disaster at hand and where to turn for resources and assistance. They assist with emotional support. And they aim to educate individuals, families, and communities about how to be resilient.
Crisis counseling, however, may not suffice for everyone impacted. We know that a person’s severity of response to a crisis is highly associated with the intensity and duration of exposure to the disaster (especially when it is life-threatening) and/or the degree of a person’s serious loss (of a loved one, home, job, health). We also know that previous trauma (eg, from childhood, domestic violence, or forced immigration) also predicts the gravity of the response to a current crisis. Which is why crisis counselors also are taught to identify those experiencing significant and persistent mental health and addiction problems because they need to be assisted, literally, in obtaining professional treatment.
Only in recent years has trauma been a recognized driver of stress, distress, and mental and addictive disorders. Until relatively recently, skill with, and access to, crisis counseling—and trauma-informed care—was rare among New York’s large and talented mental health professional community. Few had been trained in it in graduate school or practiced it because New York had been spared a disaster on par with 9/11. Following the attacks, Project Liberty’s programs served nearly 1.5 million affected individuals of very diverse ages, races, cultural backgrounds, and socioeconomic status. Their levels of “psychological distress,” the term we used and measured, ranged from low to very high.
The coronavirus pandemic now presents us with a tragically similar, catastrophic moment. The human consequences we face—psychologically, economically, and socially—are just beginning. But this time, the need is not just in New York but throughout our country.
We humans are resilient. We can bend the arc of crisis toward the light, to recovering our existing but overwhelmed capabilities. We can achieve this in a variety of ways. We can practice self-care. This isn’t an act of selfishness but is rather like putting on your own oxygen mask before trying to help your friend or loved one do the same. We can stay connected to the people we care about. We can eat well, get sufficient sleep, take a walk.
Identifying and pursuing practical goals is also important, like obtaining food, housing that is safe and reliable, transportation to where you need to go, and drawing upon financial and other resources that are issued in a disaster area. We can practice positive thinking and recall how we’ve mastered our troubles in the past; we can remind ourselves that “this too will pass.” Crises create an unusually opportune time for change and self-discovery. As Churchill said to the British people in the darkest moments of the start of World War II, “Never give up.”
Worthy of its own itemization are spiritual beliefs, faith—that however we think about a higher power (religious or secular), that power is on our side. Faith can comfort and sustain hope, particularly at a time when doubt about ourselves and humanity is triggered by disaster.
Maya Angelou’s words remind us at this moment of disaster: “...let us try to help before we have to offer therapy. That is to say, let’s see if we can’t prevent being ill by trying to offer a love of prevention before illness.”
Dr. Sederer is the former chief medical officer for the New York State Office of Mental Health and an adjunct professor in the Department of Epidemiology at the Columbia University School of Public Health. His latest book is The Addiction Solution: Treating Our Dependence on Opioids and Other Drugs.
This article first appeared on Medscape.com.
Breastfeeding reduces invasive ovarian cancer risk
Multiple studies have reported a link between breastfeeding and a reduced risk of ovarian cancer, but other studies have found no such link, and the evidence that the protective effects differ by histologic types has been inconclusive.
“This large study with extensive information on breastfeeding provides epidemiological evidence that breastfeeding, a potentially modifiable factor, may confer significant reduction in ovarian cancer risk, including high-grade serous, the deadliest subtype,” Ana Babic, PhD, of Dana-Farber Cancer Institute and Harvard Medical School, both in Boston, and colleagues reported in JAMA Oncology.
Dr. Babic led the study of a pooled analysis of women from 13 case-control studies participating in the Ovarian Cancer Association Consortium. The study evaluated 9,973 women who had ovarian cancer and 13,843 controls, with a mean age of 57 and 56 years, respectively. The data were collected over 20 years through December 2009. Dr. Babic and colleagues claimed that this is the largest study of breastfeeding and ovarian cancer risk to date.
Besides calculating a lower risk of invasive cancer, the analysis also determined that any breastfeeding was associated with a 28% lower risk of borderline cancers, compared with women who never breastfed. “Among invasive tumors, the association was statistically significant for high-grade serous, endometrioid and clear-cell tumors,” Dr. Babic and colleagues wrote, with 25%, 27% and 22% reduced risk, respectively. The researchers also noted a similar, although not statistically significant, reduced risk for low-grade serous tumors, but no such association for mucinous tumors. For borderline tumors, breastfeeding correlated with a 32% lower risk for mucinous tumors and 23% reduction in risk for serous tumors.
The analysis included five studies with data on exclusive breastfeeding. Women who breastfed exclusively for at least 3 months had a 19% reduced risk of ovarian cancer, compared with women who never breastfed, while women who breastfed albeit not exclusively for 3 months had a 30% reduced risk. The analysis also found an association between longer duration of breastfeeding and reduced risk of invasive ovarian cancer: less than 3 months duration per child was associated with an 18% lower risk, while more than 12 months was associated with a 34% lower risk (P < .001). Other factors that seemed to mitigate risk were older age when breastfeeding and breastfeeding within the previous 10 years.
One of the strengths of the studies is that it separated low-grade and the more common and deadly high-grade serous tumors. While the analysis found similar trends with endometrioid ovarian cancers, it didn’t reach a conclusion about other invasive histotypes because there were fewer cases to evaluate. Because the study population was predominantly white, the researchers acknowledged they could not sufficiently evaluate patterns among blacks, Asian, and other ethnic groups. “The association between breastfeeding and ovarian cancer needs to be investigated in large populations of other races and ethnicities,” Dr. Babic and colleagues added.
Nonetheless, they noted that their results support the World Health Organization recommendations of at least 6 months of exclusive breastfeeding and continued breastfeeding with complementary foods for 2 years or more, even though breastfeeding for less than 3 months is associated with a significant reduction in ovarian cancer risk.
The study is significant because of its “thoughtful approach to addressing potential confounders (parity, age, etc.),” said David Barrington, MD, gynecologic oncology fellow at Ohio State University James Cancer Center in Columbus.
“For general obstetricians and gynecologists, this study provides an additional reason to advocate for breastfeeding,” Dr. Barrington added. “This data should be included in a thorough discussion of the multitudes of benefits breastfeeding provides to both the infant and the mother.”
He added that future studies should evaluate breastfeeding and ovarian cancer risks in a more ethnically diverse population. “Understanding the potential impact of modifiable risk factors for ovarian cancer is paramount to overcoming racial disparities in outcomes,” Dr. Barrington said.
The study was supported by the U.S. National Cancer Institute. Dr. Babic reported grants from the U.S. National Institutes of Health. Some coauthors reported grants from the NIH, the National Health and Medical Research Council of Australia, the Federal Ministry of Education and Research of Germany, the Danish Cancer Society, or the Mermaid I Project. Some coauthors had no disclosures to report. Dr. Barrington has no relevant relationships to disclose.
SOURCE: Babic A et al. JAMA Oncology. 2020. doi: 10.1001/jamaoncol.2020.0421.
Multiple studies have reported a link between breastfeeding and a reduced risk of ovarian cancer, but other studies have found no such link, and the evidence that the protective effects differ by histologic types has been inconclusive.
“This large study with extensive information on breastfeeding provides epidemiological evidence that breastfeeding, a potentially modifiable factor, may confer significant reduction in ovarian cancer risk, including high-grade serous, the deadliest subtype,” Ana Babic, PhD, of Dana-Farber Cancer Institute and Harvard Medical School, both in Boston, and colleagues reported in JAMA Oncology.
Dr. Babic led the study of a pooled analysis of women from 13 case-control studies participating in the Ovarian Cancer Association Consortium. The study evaluated 9,973 women who had ovarian cancer and 13,843 controls, with a mean age of 57 and 56 years, respectively. The data were collected over 20 years through December 2009. Dr. Babic and colleagues claimed that this is the largest study of breastfeeding and ovarian cancer risk to date.
Besides calculating a lower risk of invasive cancer, the analysis also determined that any breastfeeding was associated with a 28% lower risk of borderline cancers, compared with women who never breastfed. “Among invasive tumors, the association was statistically significant for high-grade serous, endometrioid and clear-cell tumors,” Dr. Babic and colleagues wrote, with 25%, 27% and 22% reduced risk, respectively. The researchers also noted a similar, although not statistically significant, reduced risk for low-grade serous tumors, but no such association for mucinous tumors. For borderline tumors, breastfeeding correlated with a 32% lower risk for mucinous tumors and 23% reduction in risk for serous tumors.
The analysis included five studies with data on exclusive breastfeeding. Women who breastfed exclusively for at least 3 months had a 19% reduced risk of ovarian cancer, compared with women who never breastfed, while women who breastfed albeit not exclusively for 3 months had a 30% reduced risk. The analysis also found an association between longer duration of breastfeeding and reduced risk of invasive ovarian cancer: less than 3 months duration per child was associated with an 18% lower risk, while more than 12 months was associated with a 34% lower risk (P < .001). Other factors that seemed to mitigate risk were older age when breastfeeding and breastfeeding within the previous 10 years.
One of the strengths of the studies is that it separated low-grade and the more common and deadly high-grade serous tumors. While the analysis found similar trends with endometrioid ovarian cancers, it didn’t reach a conclusion about other invasive histotypes because there were fewer cases to evaluate. Because the study population was predominantly white, the researchers acknowledged they could not sufficiently evaluate patterns among blacks, Asian, and other ethnic groups. “The association between breastfeeding and ovarian cancer needs to be investigated in large populations of other races and ethnicities,” Dr. Babic and colleagues added.
Nonetheless, they noted that their results support the World Health Organization recommendations of at least 6 months of exclusive breastfeeding and continued breastfeeding with complementary foods for 2 years or more, even though breastfeeding for less than 3 months is associated with a significant reduction in ovarian cancer risk.
The study is significant because of its “thoughtful approach to addressing potential confounders (parity, age, etc.),” said David Barrington, MD, gynecologic oncology fellow at Ohio State University James Cancer Center in Columbus.
“For general obstetricians and gynecologists, this study provides an additional reason to advocate for breastfeeding,” Dr. Barrington added. “This data should be included in a thorough discussion of the multitudes of benefits breastfeeding provides to both the infant and the mother.”
He added that future studies should evaluate breastfeeding and ovarian cancer risks in a more ethnically diverse population. “Understanding the potential impact of modifiable risk factors for ovarian cancer is paramount to overcoming racial disparities in outcomes,” Dr. Barrington said.
The study was supported by the U.S. National Cancer Institute. Dr. Babic reported grants from the U.S. National Institutes of Health. Some coauthors reported grants from the NIH, the National Health and Medical Research Council of Australia, the Federal Ministry of Education and Research of Germany, the Danish Cancer Society, or the Mermaid I Project. Some coauthors had no disclosures to report. Dr. Barrington has no relevant relationships to disclose.
SOURCE: Babic A et al. JAMA Oncology. 2020. doi: 10.1001/jamaoncol.2020.0421.
Multiple studies have reported a link between breastfeeding and a reduced risk of ovarian cancer, but other studies have found no such link, and the evidence that the protective effects differ by histologic types has been inconclusive.
“This large study with extensive information on breastfeeding provides epidemiological evidence that breastfeeding, a potentially modifiable factor, may confer significant reduction in ovarian cancer risk, including high-grade serous, the deadliest subtype,” Ana Babic, PhD, of Dana-Farber Cancer Institute and Harvard Medical School, both in Boston, and colleagues reported in JAMA Oncology.
Dr. Babic led the study of a pooled analysis of women from 13 case-control studies participating in the Ovarian Cancer Association Consortium. The study evaluated 9,973 women who had ovarian cancer and 13,843 controls, with a mean age of 57 and 56 years, respectively. The data were collected over 20 years through December 2009. Dr. Babic and colleagues claimed that this is the largest study of breastfeeding and ovarian cancer risk to date.
Besides calculating a lower risk of invasive cancer, the analysis also determined that any breastfeeding was associated with a 28% lower risk of borderline cancers, compared with women who never breastfed. “Among invasive tumors, the association was statistically significant for high-grade serous, endometrioid and clear-cell tumors,” Dr. Babic and colleagues wrote, with 25%, 27% and 22% reduced risk, respectively. The researchers also noted a similar, although not statistically significant, reduced risk for low-grade serous tumors, but no such association for mucinous tumors. For borderline tumors, breastfeeding correlated with a 32% lower risk for mucinous tumors and 23% reduction in risk for serous tumors.
The analysis included five studies with data on exclusive breastfeeding. Women who breastfed exclusively for at least 3 months had a 19% reduced risk of ovarian cancer, compared with women who never breastfed, while women who breastfed albeit not exclusively for 3 months had a 30% reduced risk. The analysis also found an association between longer duration of breastfeeding and reduced risk of invasive ovarian cancer: less than 3 months duration per child was associated with an 18% lower risk, while more than 12 months was associated with a 34% lower risk (P < .001). Other factors that seemed to mitigate risk were older age when breastfeeding and breastfeeding within the previous 10 years.
One of the strengths of the studies is that it separated low-grade and the more common and deadly high-grade serous tumors. While the analysis found similar trends with endometrioid ovarian cancers, it didn’t reach a conclusion about other invasive histotypes because there were fewer cases to evaluate. Because the study population was predominantly white, the researchers acknowledged they could not sufficiently evaluate patterns among blacks, Asian, and other ethnic groups. “The association between breastfeeding and ovarian cancer needs to be investigated in large populations of other races and ethnicities,” Dr. Babic and colleagues added.
Nonetheless, they noted that their results support the World Health Organization recommendations of at least 6 months of exclusive breastfeeding and continued breastfeeding with complementary foods for 2 years or more, even though breastfeeding for less than 3 months is associated with a significant reduction in ovarian cancer risk.
The study is significant because of its “thoughtful approach to addressing potential confounders (parity, age, etc.),” said David Barrington, MD, gynecologic oncology fellow at Ohio State University James Cancer Center in Columbus.
“For general obstetricians and gynecologists, this study provides an additional reason to advocate for breastfeeding,” Dr. Barrington added. “This data should be included in a thorough discussion of the multitudes of benefits breastfeeding provides to both the infant and the mother.”
He added that future studies should evaluate breastfeeding and ovarian cancer risks in a more ethnically diverse population. “Understanding the potential impact of modifiable risk factors for ovarian cancer is paramount to overcoming racial disparities in outcomes,” Dr. Barrington said.
The study was supported by the U.S. National Cancer Institute. Dr. Babic reported grants from the U.S. National Institutes of Health. Some coauthors reported grants from the NIH, the National Health and Medical Research Council of Australia, the Federal Ministry of Education and Research of Germany, the Danish Cancer Society, or the Mermaid I Project. Some coauthors had no disclosures to report. Dr. Barrington has no relevant relationships to disclose.
SOURCE: Babic A et al. JAMA Oncology. 2020. doi: 10.1001/jamaoncol.2020.0421.
FROM JAMA ONCOLOGY
Early liver transplantation for alcoholic hepatitis
Case
A 45-year-old male was admitted to the hospital with severe alcoholic hepatitis. After several days of supportive care and medical therapy, the patient continued to show clinical decline. The patient is now admitted to the intensive-care unit with a Maddrey’s Discriminant Function score of 45 and a Model for End-Stage Liver Disease score of 38. He has no other significant medical comorbidities. On rounds, the patient’s wife, who is at the bedside, asks the team whether her husband would be a candidate for liver transplantation.
Should this patient be offered liver transplantation? What medical and psychosocial factors should we consider? What ethical principles should we consider?
Medical considerations
With the advent of direct-acting antivirals (DAAs), there has been a decline in the number of liver transplants performed for hepatitis C virus–related cirrhosis.1 Instead, alcohol-related liver disease (ALD) has become the most common indication for liver transplant in the United States.2 The 6-month abstinence requirement was a widespread practice within the transplant community that would exclude any patients who were actively drinking from being considered for liver transplant. However, data are inconclusive whether the 6-month rule serves as a predictor of future drinking or poor outcomes after liver transplant.3,4 Unfortunately, many patients with severe alcoholic hepatitis will not survive long enough to fulfill the 6-month requirement.5
In 2011, Mathurin and colleagues led the pivotal European trial demonstrating the effectiveness of liver transplant as a rescue option for highly selected patients with severe alcoholic hepatitis.5 The selection criteria included patients with severe alcoholic hepatitis unresponsive to medical therapy, first liver-decompensating event, presence of close supportive family members, absence of severe psychiatric disorders, and agreement by patients to adhere to lifelong total alcohol abstinence. The study showed that the 6-month survival rate of patients who received early liver transplant was 77%, compared with 25% among those who did not. The positive outcomes were subsequently replicated at several centers in the United States, and this led to a wider adoption of early liver transplant for severe alcoholic hepatitis.6-8
Psychosocial considerations
At present, we do not have well-validated consensus selection criteria to identify patients with alcoholic hepatitis most suitable for liver transplant. Each transplant center employs its own set of selection criteria with slight variations from the original European trial which prompted a national expert consensus meeting in Dallas in 2019.9 The consortium published a set of guidelines for centers planning to or already performing alcoholic hepatitis transplants. The proposed criteria to determine liver transplant candidacy are the following: 1) patients presenting for the first time with decompensated liver disease who are nonresponders to medical therapy; 2) assessment by a multidisciplinary psychosocial team including a social worker and an additional specialist; 3) no repeated unsuccessful attempts at addiction rehabilitation; 4) lack of other substance use/dependence; 5) insight with a commitment to sobriety; 6) presence of close supportive family members. The goal was to select candidates with the least likelihood of relapse in the hope of preventing poor outcomes after liver transplant. A study by a Johns Hopkins group comparing patients with severe alcoholic hepatitis who underwent careful psychosocial evaluation versus alcoholic cirrhosis with at least 6 months abstinence found that the survival and alcohol relapse rates were similar between the two groups.7
Ethical considerations
Expanding liver transplant indications to include some patients with severe alcoholic hepatitis will uphold the principle of beneficence given clear evidence of a survival benefit. In addition, graft survival rates were comparable with those of patients who underwent liver transplant for other causes.10 However, in an era of persistent organ shortage, it is important to balance justice or fairness to the patient with utilitarian policies that optimize outcomes for all who are in need of liver transplantation.
Justice
Justice means fair and equal distribution of scarce health resources to patients without bias on account of sex, race, wealth, and the nature of a patient’s disease. Based on the principle of justice, a patient with alcoholic hepatitis should be afforded opportunities for liver transplant equal to patients with other etiologies of liver disease.
Opponents of adoption of liver transplant for alcoholic hepatitis often base their reluctance on the following: patients’ failure to gain control of their alcohol use disorder, fears of alcohol relapse, and ultimately perceptions that these patients may be less deserving, compared with patients with other etiologies of liver disease. But, is this fair to the patient?
Alcohol use disorder, in general, is stigmatized and is considered by some to be a self-inflicted condition. As a medical community, we do not withhold life-saving treatment from patients who had inflicted their own injuries. Nevertheless, the stigma against alcoholism is so entrenched in our society that some fear transplanting a patient who is actively drinking would negatively affect the public’s perception of the transplant community and thus diminish the organ donation rate and harm the common good. Interestingly, a public opinion survey actually showed that the majority of respondents were at least neutral about the idea of transplanting patients with alcoholic hepatitis.11
Utility
Utility means achieving the greatest good for the greatest number of patients. The absolute scarcity of available organs imposes a need for a strict allocation decision. A liver that is used for a patient with severe alcoholic hepatitis is an organ not used for another patient suffering chronic liver disease. It is worth noting that about 20% of patients with severe alcoholic hepatitis might recover without a transplant.5 That means about one out of five liver transplants performed for alcoholic hepatitis may have been done in a patient who would have recovered without a transplant. Does this policy optimize the greatest good for everyone who is on the wait list?
Moss and Siegler argued that it was not the alcoholism that made patients with alcoholic liver disease less deserving of liver transplant, but rather their failure to seek treatment for alcoholism that made their claim for liver transplant weaker, compared with those who developed cirrhosis through no fault of their own.12 This argument is problematic. For example, patients with nonalcoholic steatohepatitis (NASH) are often compared with patients with alcoholic liver disease when it comes to modifiable behaviors that affect their health, whether it is through weight loss or abstinence, respectively. Yet, there is very little argument for lower priority for NASH patients who failed to lose weight. Secondly, alcohol use disorder is a psychosocially complex disease that requires a multidisciplinary treatment approach. Substance abuse rehabilitation is not readily available to most patients and could single out vulnerable patients from lower socioeconomic classes who are at higher risk for developing alcohol use disorder. Imposing a strict abstinence period regardless of a patient’s medical need is punitive and does not treat the underlying disease. Instead of focusing on disease causality, we ought to advocate for medical treatment of the underlying disease.
Conclusions
Liver transplant effectively functions as a zero-sum game. Efforts to save individual patients with severe alcoholic hepatitis can result in trade-offs to other patients on the wait list. Balancing the ethical principles of utility and justice is challenging. A strict 6-month rule, while convenient, does not strike the balance. The decision to transplant a patient with alcoholic hepatitis should be made on a case-by-case basis. As stewards of donor organs, transplant centers have a duty to carefully evaluate a potential candidate based on medical needs and recipient outcome without the influence of bias. We feel that, when considering liver transplant in patients with severe alcoholic hepatitis, the principle of justice or fairness to the patient is the overriding ethical principle. Provided the patient meets medical and psychosocial criteria that available evidence suggests would result in long-term survival post transplantation, we would support listing for liver transplantation.
References
1. Goldberg D et al. Gastroenterology, 2017;152(5):1090-9.e1.
2. Cholankeril G, Ahmed A. Clin Gastroenterol Hepatol. 2018;16(8):1356-8.
3. Neuberger J et al. J Hepatol. 2002;36(1):130-7.
4. DiMartini A, et al. Clin Liver Dis. 2011;15(4):727-51.
5. Mathurin P et al. N Engl J Med, 2011;365(19):1790-800.
6. Im GY et al. Am J Transplant. 2016;16(3):841-9.
7. Lee BP et al. Ann Surg. 2017;265(1):20-9.
8. Lee BP et al. Gastroenterology. 2018. 155(2):422-30.e1.
9. Asrani SK et al. Liver Transpl. 2020;26(1):127-40.
10. Singal AK et al. Transplantation. 2013;95(5):755-60.
11. Stroh G et al. Am J Transplant. 2015;15(6):1598-604.
12. Moss AH, Siegler M. JAMA. 1991;265(10):1295-8.
Dr. Wang is a gastroenterology fellow in the division of gastroenterology, hepatology, and nutrition, department of internal medicine, University of Chicago Medicine; Dr. Aronsohn is associate professor in the division of gastroenterology, hepatology, and nutrition, department of internal medicine, University of Chicago Medicine.
Case
A 45-year-old male was admitted to the hospital with severe alcoholic hepatitis. After several days of supportive care and medical therapy, the patient continued to show clinical decline. The patient is now admitted to the intensive-care unit with a Maddrey’s Discriminant Function score of 45 and a Model for End-Stage Liver Disease score of 38. He has no other significant medical comorbidities. On rounds, the patient’s wife, who is at the bedside, asks the team whether her husband would be a candidate for liver transplantation.
Should this patient be offered liver transplantation? What medical and psychosocial factors should we consider? What ethical principles should we consider?
Medical considerations
With the advent of direct-acting antivirals (DAAs), there has been a decline in the number of liver transplants performed for hepatitis C virus–related cirrhosis.1 Instead, alcohol-related liver disease (ALD) has become the most common indication for liver transplant in the United States.2 The 6-month abstinence requirement was a widespread practice within the transplant community that would exclude any patients who were actively drinking from being considered for liver transplant. However, data are inconclusive whether the 6-month rule serves as a predictor of future drinking or poor outcomes after liver transplant.3,4 Unfortunately, many patients with severe alcoholic hepatitis will not survive long enough to fulfill the 6-month requirement.5
In 2011, Mathurin and colleagues led the pivotal European trial demonstrating the effectiveness of liver transplant as a rescue option for highly selected patients with severe alcoholic hepatitis.5 The selection criteria included patients with severe alcoholic hepatitis unresponsive to medical therapy, first liver-decompensating event, presence of close supportive family members, absence of severe psychiatric disorders, and agreement by patients to adhere to lifelong total alcohol abstinence. The study showed that the 6-month survival rate of patients who received early liver transplant was 77%, compared with 25% among those who did not. The positive outcomes were subsequently replicated at several centers in the United States, and this led to a wider adoption of early liver transplant for severe alcoholic hepatitis.6-8
Psychosocial considerations
At present, we do not have well-validated consensus selection criteria to identify patients with alcoholic hepatitis most suitable for liver transplant. Each transplant center employs its own set of selection criteria with slight variations from the original European trial which prompted a national expert consensus meeting in Dallas in 2019.9 The consortium published a set of guidelines for centers planning to or already performing alcoholic hepatitis transplants. The proposed criteria to determine liver transplant candidacy are the following: 1) patients presenting for the first time with decompensated liver disease who are nonresponders to medical therapy; 2) assessment by a multidisciplinary psychosocial team including a social worker and an additional specialist; 3) no repeated unsuccessful attempts at addiction rehabilitation; 4) lack of other substance use/dependence; 5) insight with a commitment to sobriety; 6) presence of close supportive family members. The goal was to select candidates with the least likelihood of relapse in the hope of preventing poor outcomes after liver transplant. A study by a Johns Hopkins group comparing patients with severe alcoholic hepatitis who underwent careful psychosocial evaluation versus alcoholic cirrhosis with at least 6 months abstinence found that the survival and alcohol relapse rates were similar between the two groups.7
Ethical considerations
Expanding liver transplant indications to include some patients with severe alcoholic hepatitis will uphold the principle of beneficence given clear evidence of a survival benefit. In addition, graft survival rates were comparable with those of patients who underwent liver transplant for other causes.10 However, in an era of persistent organ shortage, it is important to balance justice or fairness to the patient with utilitarian policies that optimize outcomes for all who are in need of liver transplantation.
Justice
Justice means fair and equal distribution of scarce health resources to patients without bias on account of sex, race, wealth, and the nature of a patient’s disease. Based on the principle of justice, a patient with alcoholic hepatitis should be afforded opportunities for liver transplant equal to patients with other etiologies of liver disease.
Opponents of adoption of liver transplant for alcoholic hepatitis often base their reluctance on the following: patients’ failure to gain control of their alcohol use disorder, fears of alcohol relapse, and ultimately perceptions that these patients may be less deserving, compared with patients with other etiologies of liver disease. But, is this fair to the patient?
Alcohol use disorder, in general, is stigmatized and is considered by some to be a self-inflicted condition. As a medical community, we do not withhold life-saving treatment from patients who had inflicted their own injuries. Nevertheless, the stigma against alcoholism is so entrenched in our society that some fear transplanting a patient who is actively drinking would negatively affect the public’s perception of the transplant community and thus diminish the organ donation rate and harm the common good. Interestingly, a public opinion survey actually showed that the majority of respondents were at least neutral about the idea of transplanting patients with alcoholic hepatitis.11
Utility
Utility means achieving the greatest good for the greatest number of patients. The absolute scarcity of available organs imposes a need for a strict allocation decision. A liver that is used for a patient with severe alcoholic hepatitis is an organ not used for another patient suffering chronic liver disease. It is worth noting that about 20% of patients with severe alcoholic hepatitis might recover without a transplant.5 That means about one out of five liver transplants performed for alcoholic hepatitis may have been done in a patient who would have recovered without a transplant. Does this policy optimize the greatest good for everyone who is on the wait list?
Moss and Siegler argued that it was not the alcoholism that made patients with alcoholic liver disease less deserving of liver transplant, but rather their failure to seek treatment for alcoholism that made their claim for liver transplant weaker, compared with those who developed cirrhosis through no fault of their own.12 This argument is problematic. For example, patients with nonalcoholic steatohepatitis (NASH) are often compared with patients with alcoholic liver disease when it comes to modifiable behaviors that affect their health, whether it is through weight loss or abstinence, respectively. Yet, there is very little argument for lower priority for NASH patients who failed to lose weight. Secondly, alcohol use disorder is a psychosocially complex disease that requires a multidisciplinary treatment approach. Substance abuse rehabilitation is not readily available to most patients and could single out vulnerable patients from lower socioeconomic classes who are at higher risk for developing alcohol use disorder. Imposing a strict abstinence period regardless of a patient’s medical need is punitive and does not treat the underlying disease. Instead of focusing on disease causality, we ought to advocate for medical treatment of the underlying disease.
Conclusions
Liver transplant effectively functions as a zero-sum game. Efforts to save individual patients with severe alcoholic hepatitis can result in trade-offs to other patients on the wait list. Balancing the ethical principles of utility and justice is challenging. A strict 6-month rule, while convenient, does not strike the balance. The decision to transplant a patient with alcoholic hepatitis should be made on a case-by-case basis. As stewards of donor organs, transplant centers have a duty to carefully evaluate a potential candidate based on medical needs and recipient outcome without the influence of bias. We feel that, when considering liver transplant in patients with severe alcoholic hepatitis, the principle of justice or fairness to the patient is the overriding ethical principle. Provided the patient meets medical and psychosocial criteria that available evidence suggests would result in long-term survival post transplantation, we would support listing for liver transplantation.
References
1. Goldberg D et al. Gastroenterology, 2017;152(5):1090-9.e1.
2. Cholankeril G, Ahmed A. Clin Gastroenterol Hepatol. 2018;16(8):1356-8.
3. Neuberger J et al. J Hepatol. 2002;36(1):130-7.
4. DiMartini A, et al. Clin Liver Dis. 2011;15(4):727-51.
5. Mathurin P et al. N Engl J Med, 2011;365(19):1790-800.
6. Im GY et al. Am J Transplant. 2016;16(3):841-9.
7. Lee BP et al. Ann Surg. 2017;265(1):20-9.
8. Lee BP et al. Gastroenterology. 2018. 155(2):422-30.e1.
9. Asrani SK et al. Liver Transpl. 2020;26(1):127-40.
10. Singal AK et al. Transplantation. 2013;95(5):755-60.
11. Stroh G et al. Am J Transplant. 2015;15(6):1598-604.
12. Moss AH, Siegler M. JAMA. 1991;265(10):1295-8.
Dr. Wang is a gastroenterology fellow in the division of gastroenterology, hepatology, and nutrition, department of internal medicine, University of Chicago Medicine; Dr. Aronsohn is associate professor in the division of gastroenterology, hepatology, and nutrition, department of internal medicine, University of Chicago Medicine.
Case
A 45-year-old male was admitted to the hospital with severe alcoholic hepatitis. After several days of supportive care and medical therapy, the patient continued to show clinical decline. The patient is now admitted to the intensive-care unit with a Maddrey’s Discriminant Function score of 45 and a Model for End-Stage Liver Disease score of 38. He has no other significant medical comorbidities. On rounds, the patient’s wife, who is at the bedside, asks the team whether her husband would be a candidate for liver transplantation.
Should this patient be offered liver transplantation? What medical and psychosocial factors should we consider? What ethical principles should we consider?
Medical considerations
With the advent of direct-acting antivirals (DAAs), there has been a decline in the number of liver transplants performed for hepatitis C virus–related cirrhosis.1 Instead, alcohol-related liver disease (ALD) has become the most common indication for liver transplant in the United States.2 The 6-month abstinence requirement was a widespread practice within the transplant community that would exclude any patients who were actively drinking from being considered for liver transplant. However, data are inconclusive whether the 6-month rule serves as a predictor of future drinking or poor outcomes after liver transplant.3,4 Unfortunately, many patients with severe alcoholic hepatitis will not survive long enough to fulfill the 6-month requirement.5
In 2011, Mathurin and colleagues led the pivotal European trial demonstrating the effectiveness of liver transplant as a rescue option for highly selected patients with severe alcoholic hepatitis.5 The selection criteria included patients with severe alcoholic hepatitis unresponsive to medical therapy, first liver-decompensating event, presence of close supportive family members, absence of severe psychiatric disorders, and agreement by patients to adhere to lifelong total alcohol abstinence. The study showed that the 6-month survival rate of patients who received early liver transplant was 77%, compared with 25% among those who did not. The positive outcomes were subsequently replicated at several centers in the United States, and this led to a wider adoption of early liver transplant for severe alcoholic hepatitis.6-8
Psychosocial considerations
At present, we do not have well-validated consensus selection criteria to identify patients with alcoholic hepatitis most suitable for liver transplant. Each transplant center employs its own set of selection criteria with slight variations from the original European trial which prompted a national expert consensus meeting in Dallas in 2019.9 The consortium published a set of guidelines for centers planning to or already performing alcoholic hepatitis transplants. The proposed criteria to determine liver transplant candidacy are the following: 1) patients presenting for the first time with decompensated liver disease who are nonresponders to medical therapy; 2) assessment by a multidisciplinary psychosocial team including a social worker and an additional specialist; 3) no repeated unsuccessful attempts at addiction rehabilitation; 4) lack of other substance use/dependence; 5) insight with a commitment to sobriety; 6) presence of close supportive family members. The goal was to select candidates with the least likelihood of relapse in the hope of preventing poor outcomes after liver transplant. A study by a Johns Hopkins group comparing patients with severe alcoholic hepatitis who underwent careful psychosocial evaluation versus alcoholic cirrhosis with at least 6 months abstinence found that the survival and alcohol relapse rates were similar between the two groups.7
Ethical considerations
Expanding liver transplant indications to include some patients with severe alcoholic hepatitis will uphold the principle of beneficence given clear evidence of a survival benefit. In addition, graft survival rates were comparable with those of patients who underwent liver transplant for other causes.10 However, in an era of persistent organ shortage, it is important to balance justice or fairness to the patient with utilitarian policies that optimize outcomes for all who are in need of liver transplantation.
Justice
Justice means fair and equal distribution of scarce health resources to patients without bias on account of sex, race, wealth, and the nature of a patient’s disease. Based on the principle of justice, a patient with alcoholic hepatitis should be afforded opportunities for liver transplant equal to patients with other etiologies of liver disease.
Opponents of adoption of liver transplant for alcoholic hepatitis often base their reluctance on the following: patients’ failure to gain control of their alcohol use disorder, fears of alcohol relapse, and ultimately perceptions that these patients may be less deserving, compared with patients with other etiologies of liver disease. But, is this fair to the patient?
Alcohol use disorder, in general, is stigmatized and is considered by some to be a self-inflicted condition. As a medical community, we do not withhold life-saving treatment from patients who had inflicted their own injuries. Nevertheless, the stigma against alcoholism is so entrenched in our society that some fear transplanting a patient who is actively drinking would negatively affect the public’s perception of the transplant community and thus diminish the organ donation rate and harm the common good. Interestingly, a public opinion survey actually showed that the majority of respondents were at least neutral about the idea of transplanting patients with alcoholic hepatitis.11
Utility
Utility means achieving the greatest good for the greatest number of patients. The absolute scarcity of available organs imposes a need for a strict allocation decision. A liver that is used for a patient with severe alcoholic hepatitis is an organ not used for another patient suffering chronic liver disease. It is worth noting that about 20% of patients with severe alcoholic hepatitis might recover without a transplant.5 That means about one out of five liver transplants performed for alcoholic hepatitis may have been done in a patient who would have recovered without a transplant. Does this policy optimize the greatest good for everyone who is on the wait list?
Moss and Siegler argued that it was not the alcoholism that made patients with alcoholic liver disease less deserving of liver transplant, but rather their failure to seek treatment for alcoholism that made their claim for liver transplant weaker, compared with those who developed cirrhosis through no fault of their own.12 This argument is problematic. For example, patients with nonalcoholic steatohepatitis (NASH) are often compared with patients with alcoholic liver disease when it comes to modifiable behaviors that affect their health, whether it is through weight loss or abstinence, respectively. Yet, there is very little argument for lower priority for NASH patients who failed to lose weight. Secondly, alcohol use disorder is a psychosocially complex disease that requires a multidisciplinary treatment approach. Substance abuse rehabilitation is not readily available to most patients and could single out vulnerable patients from lower socioeconomic classes who are at higher risk for developing alcohol use disorder. Imposing a strict abstinence period regardless of a patient’s medical need is punitive and does not treat the underlying disease. Instead of focusing on disease causality, we ought to advocate for medical treatment of the underlying disease.
Conclusions
Liver transplant effectively functions as a zero-sum game. Efforts to save individual patients with severe alcoholic hepatitis can result in trade-offs to other patients on the wait list. Balancing the ethical principles of utility and justice is challenging. A strict 6-month rule, while convenient, does not strike the balance. The decision to transplant a patient with alcoholic hepatitis should be made on a case-by-case basis. As stewards of donor organs, transplant centers have a duty to carefully evaluate a potential candidate based on medical needs and recipient outcome without the influence of bias. We feel that, when considering liver transplant in patients with severe alcoholic hepatitis, the principle of justice or fairness to the patient is the overriding ethical principle. Provided the patient meets medical and psychosocial criteria that available evidence suggests would result in long-term survival post transplantation, we would support listing for liver transplantation.
References
1. Goldberg D et al. Gastroenterology, 2017;152(5):1090-9.e1.
2. Cholankeril G, Ahmed A. Clin Gastroenterol Hepatol. 2018;16(8):1356-8.
3. Neuberger J et al. J Hepatol. 2002;36(1):130-7.
4. DiMartini A, et al. Clin Liver Dis. 2011;15(4):727-51.
5. Mathurin P et al. N Engl J Med, 2011;365(19):1790-800.
6. Im GY et al. Am J Transplant. 2016;16(3):841-9.
7. Lee BP et al. Ann Surg. 2017;265(1):20-9.
8. Lee BP et al. Gastroenterology. 2018. 155(2):422-30.e1.
9. Asrani SK et al. Liver Transpl. 2020;26(1):127-40.
10. Singal AK et al. Transplantation. 2013;95(5):755-60.
11. Stroh G et al. Am J Transplant. 2015;15(6):1598-604.
12. Moss AH, Siegler M. JAMA. 1991;265(10):1295-8.
Dr. Wang is a gastroenterology fellow in the division of gastroenterology, hepatology, and nutrition, department of internal medicine, University of Chicago Medicine; Dr. Aronsohn is associate professor in the division of gastroenterology, hepatology, and nutrition, department of internal medicine, University of Chicago Medicine.
Concerns for clinicians over 65 grow in the face of COVID-19
When Judith Salerno, MD, heard that New York was calling for volunteer clinicians to assist with the COVID-19 response, she didn’t hesitate to sign up.
Although Dr. Salerno, 68, has held administrative, research, and policy roles for 25 years, she has kept her medical license active and always found ways to squeeze some clinical work into her busy schedule.
“I have what I could consider ‘rusty’ clinical skills, but pretty good clinical judgment,” said Dr. Salerno, president of the New York Academy of Medicine. “I thought in this situation that I could resurrect and hone those skills, even if it was just taking care of routine patients and working on a team, there was a lot of good I can do.”
Dr. Salerno is among 80,000 health care professionals who have volunteered to work temporarily in New York during the COVID-19 pandemic as of March 31, 2020, according to New York state officials. In mid-March, New York Governor Andrew Cuomo (D) issued a plea for retired physicians and nurses to help the state by signing up for on-call work. Other states have made similar appeals for retired health care professionals to return to medicine in an effort to relieve overwhelmed hospital staffs and aid capacity if health care workers become ill. Such redeployments, however, are raising concerns about exposing senior physicians to a virus that causes more severe illness in individuals aged over 65 years and kills them at a higher rate.
At the same time, a significant portion of the current health care workforce is aged 55 years and older, placing them at higher risk for serious illness, hospitalization, and death from COVID-19, said Douglas O. Staiger, PhD, a researcher and economics professor at Dartmouth College, Hanover, N.H. Dr. Staiger recently coauthored a viewpoint in JAMA called “Older clinicians and the surge in novel coronavirus disease 2019,” which outlines the risks and mortality rates from the novel coronavirus among patients aged 55 years and older.
Among the 1.2 million practicing physicians in the United States, about 20% are aged 55-64 years and an estimated 9% are 65 years or older, according to the paper. Of the nation’s nearly 2 million registered nurses employed in hospitals, about 19% are aged 55-64 years, and an estimated 3% are aged 65 years or older.
“In some metro areas, this proportion is even higher,” Dr. Staiger said in an interview. “Hospitals and other health care providers should consider ways of utilizing older clinicians’ skills and experience in a way that minimizes their risk of exposure to COVID-19, such as transferring them from jobs interacting with patients to more supervisory, administrative, or telehealth roles. This is increasingly important as retired physicians and nurses are being asked to return to the workforce.”
Protecting staff, screening volunteers
Hematologist-oncologist David H. Henry, MD, said his eight-physician group practice at Pennsylvania Hospital, Philadelphia, has already taken steps to protect him from COVID exposure.
At the request of his younger colleagues, Dr. Henry, 69, said he is no longer seeing patients in the hospital where there is increased exposure risk to the virus. He and the staff also limit their time in the office to 2-3 days a week and practice telemedicine the rest of the week, Dr. Henry said in an interview.
“Whether you’re a person trying to stay at home because you’re quote ‘nonessential,’ or you’re a health care worker and you have to keep seeing patients to some extent, the less we’re face to face with others the better,” said Dr. Henry, who hosts the Blood & Cancer podcast for MDedge News. “There’s an extreme and a middle ground. If they told me just to stay home that wouldn’t help anybody. If they said, ‘business as usual,’ that would be wrong. This is a middle strategy, which is reasonable, rational, and will help dial this dangerous time down as fast as possible.”
On a recent weekend when Dr. Henry would normally have been on call in the hospital, he took phone calls for his colleagues at home while they saw patients in the hospital. This included calls with patients who had questions and consultation calls with other physicians.
“They are helping me and I am helping them,” Dr. Henry said. “Taking those calls makes it easier for my partners to see all those patients. We all want to help and be there, within reason. You want to step up an do your job, but you want to be safe.”
Peter D. Quinn, DMD, MD, chief executive physician of the Penn Medicine Medical Group, said safeguarding the health of its workforce is a top priority as Penn Medicine works to fight the COVID-19 pandemic.
“This includes ensuring that all employees adhere to Centers for Disease Control and Penn Medicine infection prevention guidance as they continue their normal clinical work,” Dr. Quinn said in an interview. “Though age alone is not a criterion to remove frontline staff from direct clinical care during the COVID-19 outbreak, certain conditions such as cardiac or lung disease may be, and clinicians who have concerns are urged to speak with their leadership about options to fill clinical or support roles remotely.”
Meanwhile, for states calling on retired health professionals to assist during the pandemic, thorough screenings that identify high-risk volunteers are essential to protect vulnerable clinicians, said Nathaniel Hibbs, DO, president of the Colorado chapter of the American College of Emergency Physicians.
After Colorado issued a statewide request for retired clinicians to help, Dr. Hibbs became concerned that the state’s website initially included only a basic set of questions for interested volunteers.
“It didn’t have screening questions for prior health problems, comorbidities, or things like high blood pressure, heart disease, lung disease – the high-risk factors that we associate with bad outcomes if people get infected with COVID,” Dr. Hibbs said in an interview.
To address this, Dr. Hibbs and associates recently provided recommendations to the state about its screening process that advised collecting more health information from volunteers and considering lower-risk assignments for high-risk individuals. State officials indicated they would strongly consider the recommendations, Dr. Hibbs said.
The Colorado Department of Public Health & Environment did not respond to messages seeking comment. Officials at the New York State Department of Health declined to be interviewed for this article but confirmed that they are reviewing the age and background of all volunteers, and individual hospitals will also review each volunteer to find suitable jobs.
The American Medical Association on March 30 issued guidance for retired physicians about rejoining the workforce to help with the COVID response. The guidance outlines license considerations, contribution options, professional liability considerations, and questions to ask volunteer coordinators.
“Throughout the COVID-19 pandemic, many physicians over the age of 65 will provide care to patients,” AMA President Patrice A. Harris, MD, said in a statement. “Whether ‘senior’ physicians should be on the front line of patient care at this time is a complex issue that must balance several factors against the benefit these physicians can provide. As with all people in high-risk age groups, careful consideration must be given to the health and safety of retired physicians and their immediate family members, especially those with chronic medical conditions.”
Tapping talent, sharing knowledge
When Barbara L. Schuster, MD, 69, filled out paperwork to join the Georgia Medical Reserve Corps, she answered a range of questions, including inquiries about her age, specialty, licensing, and whether she had any major medical conditions.
“They sent out instructions that said, if you are over the age of 60, we really don’t want you to be doing inpatient or ambulatory with active patients,” said Dr. Schuster, a retired medical school dean in the Athens, Ga., area. “Unless they get to a point where it’s going to be you or nobody, I think that they try to protect us for both our sake and also theirs.”
Dr. Schuster opted for telehealth or administrative duties, but has not yet been called upon to help. The Athens area has not seen high numbers of COVID-19 patients, compared with other parts of the country, and there have not been many volunteer opportunities for physicians thus far, she said. In the meantime, Dr. Schuster has found other ways to give her time, such as answering questions from community members on both COVID-19 and non–COVID-19 topics, and offering guidance to medical students.
“I’ve spent an increasing number of hours on Zoom, Skype, or FaceTime meeting with them to talk about various issues,” Dr. Schuster said.
As hospitals and organizations ramp up pandemic preparation, now is the time to consider roles for older clinicians and how they can best contribute, said Peter I. Buerhaus, PhD, RN, a nurse and director of the Center for Interdisciplinary Health Workforce Studies at Montana State University, Bozeman, Mont. Dr. Buerhaus was the first author of the recent JAMA viewpoint “Older clinicians and the surge in novel coronavirus 2019.”
“It’s important for hospitals that are anticipating a surge of critically ill patients to assess their workforce’s capability, including the proportion of older clinicians,” he said. “Is there something organizations can do differently to lessen older physicians’ and nurses’ direct patient contact and reduce their risk of infection?”
Dr. Buerhaus’ JAMA piece offers a range of ideas and assignments for older clinicians during the pandemic, including consulting with younger staff, advising on resources, assisting with clinical and organizational problem solving, aiding clinicians and managers with challenging decisions, consulting with patient families, advising managers and executives, being public spokespersons, and working with public and community health organizations.
“Older clinicians are at increased risk of becoming seriously ill if infected, but yet they’re also the ones who perhaps some of the best minds and experiences to help organizations combat the pandemic,” Dr. Buerhaus said. “These clinicians have great backgrounds and skills and 20, 30, 40 years of experience to draw on, including dealing with prior medical emergencies. I would hope that organizations, if they can, use the time before becoming a hotspot as an opportunity where the younger workforce could be teamed up with some of the older clinicians and learn as much as possible. It’s a great opportunity to share this wealth of knowledge with the workforce that will carry on after the pandemic.”
Since responding to New York’s call for volunteers, Dr. Salerno has been assigned to a palliative care inpatient team at a Manhattan hospital where she is working with large numbers of ICU patients and their families.
“My experience as a geriatrician helps me in talking with anxious and concerned families, especially when they are unable to see or communicate with their critically ill loved ones,” she said.
Before she was assigned the post, Dr. Salerno said she heard concerns from her adult children, who would prefer their mom take on a volunteer telehealth role. At the time, Dr. Salerno said she was not opposed to a telehealth assignment, but stressed to her family that she would go where she was needed.
“I’m healthy enough to run an organization, work long hours, long weeks; I have the stamina. The only thing working against me is age,” she said. “To say I’m not concerned is not honest. Of course I’m concerned. Am I afraid? No. I’m hoping that we can all be kept safe.”
When Judith Salerno, MD, heard that New York was calling for volunteer clinicians to assist with the COVID-19 response, she didn’t hesitate to sign up.
Although Dr. Salerno, 68, has held administrative, research, and policy roles for 25 years, she has kept her medical license active and always found ways to squeeze some clinical work into her busy schedule.
“I have what I could consider ‘rusty’ clinical skills, but pretty good clinical judgment,” said Dr. Salerno, president of the New York Academy of Medicine. “I thought in this situation that I could resurrect and hone those skills, even if it was just taking care of routine patients and working on a team, there was a lot of good I can do.”
Dr. Salerno is among 80,000 health care professionals who have volunteered to work temporarily in New York during the COVID-19 pandemic as of March 31, 2020, according to New York state officials. In mid-March, New York Governor Andrew Cuomo (D) issued a plea for retired physicians and nurses to help the state by signing up for on-call work. Other states have made similar appeals for retired health care professionals to return to medicine in an effort to relieve overwhelmed hospital staffs and aid capacity if health care workers become ill. Such redeployments, however, are raising concerns about exposing senior physicians to a virus that causes more severe illness in individuals aged over 65 years and kills them at a higher rate.
At the same time, a significant portion of the current health care workforce is aged 55 years and older, placing them at higher risk for serious illness, hospitalization, and death from COVID-19, said Douglas O. Staiger, PhD, a researcher and economics professor at Dartmouth College, Hanover, N.H. Dr. Staiger recently coauthored a viewpoint in JAMA called “Older clinicians and the surge in novel coronavirus disease 2019,” which outlines the risks and mortality rates from the novel coronavirus among patients aged 55 years and older.
Among the 1.2 million practicing physicians in the United States, about 20% are aged 55-64 years and an estimated 9% are 65 years or older, according to the paper. Of the nation’s nearly 2 million registered nurses employed in hospitals, about 19% are aged 55-64 years, and an estimated 3% are aged 65 years or older.
“In some metro areas, this proportion is even higher,” Dr. Staiger said in an interview. “Hospitals and other health care providers should consider ways of utilizing older clinicians’ skills and experience in a way that minimizes their risk of exposure to COVID-19, such as transferring them from jobs interacting with patients to more supervisory, administrative, or telehealth roles. This is increasingly important as retired physicians and nurses are being asked to return to the workforce.”
Protecting staff, screening volunteers
Hematologist-oncologist David H. Henry, MD, said his eight-physician group practice at Pennsylvania Hospital, Philadelphia, has already taken steps to protect him from COVID exposure.
At the request of his younger colleagues, Dr. Henry, 69, said he is no longer seeing patients in the hospital where there is increased exposure risk to the virus. He and the staff also limit their time in the office to 2-3 days a week and practice telemedicine the rest of the week, Dr. Henry said in an interview.
“Whether you’re a person trying to stay at home because you’re quote ‘nonessential,’ or you’re a health care worker and you have to keep seeing patients to some extent, the less we’re face to face with others the better,” said Dr. Henry, who hosts the Blood & Cancer podcast for MDedge News. “There’s an extreme and a middle ground. If they told me just to stay home that wouldn’t help anybody. If they said, ‘business as usual,’ that would be wrong. This is a middle strategy, which is reasonable, rational, and will help dial this dangerous time down as fast as possible.”
On a recent weekend when Dr. Henry would normally have been on call in the hospital, he took phone calls for his colleagues at home while they saw patients in the hospital. This included calls with patients who had questions and consultation calls with other physicians.
“They are helping me and I am helping them,” Dr. Henry said. “Taking those calls makes it easier for my partners to see all those patients. We all want to help and be there, within reason. You want to step up an do your job, but you want to be safe.”
Peter D. Quinn, DMD, MD, chief executive physician of the Penn Medicine Medical Group, said safeguarding the health of its workforce is a top priority as Penn Medicine works to fight the COVID-19 pandemic.
“This includes ensuring that all employees adhere to Centers for Disease Control and Penn Medicine infection prevention guidance as they continue their normal clinical work,” Dr. Quinn said in an interview. “Though age alone is not a criterion to remove frontline staff from direct clinical care during the COVID-19 outbreak, certain conditions such as cardiac or lung disease may be, and clinicians who have concerns are urged to speak with their leadership about options to fill clinical or support roles remotely.”
Meanwhile, for states calling on retired health professionals to assist during the pandemic, thorough screenings that identify high-risk volunteers are essential to protect vulnerable clinicians, said Nathaniel Hibbs, DO, president of the Colorado chapter of the American College of Emergency Physicians.
After Colorado issued a statewide request for retired clinicians to help, Dr. Hibbs became concerned that the state’s website initially included only a basic set of questions for interested volunteers.
“It didn’t have screening questions for prior health problems, comorbidities, or things like high blood pressure, heart disease, lung disease – the high-risk factors that we associate with bad outcomes if people get infected with COVID,” Dr. Hibbs said in an interview.
To address this, Dr. Hibbs and associates recently provided recommendations to the state about its screening process that advised collecting more health information from volunteers and considering lower-risk assignments for high-risk individuals. State officials indicated they would strongly consider the recommendations, Dr. Hibbs said.
The Colorado Department of Public Health & Environment did not respond to messages seeking comment. Officials at the New York State Department of Health declined to be interviewed for this article but confirmed that they are reviewing the age and background of all volunteers, and individual hospitals will also review each volunteer to find suitable jobs.
The American Medical Association on March 30 issued guidance for retired physicians about rejoining the workforce to help with the COVID response. The guidance outlines license considerations, contribution options, professional liability considerations, and questions to ask volunteer coordinators.
“Throughout the COVID-19 pandemic, many physicians over the age of 65 will provide care to patients,” AMA President Patrice A. Harris, MD, said in a statement. “Whether ‘senior’ physicians should be on the front line of patient care at this time is a complex issue that must balance several factors against the benefit these physicians can provide. As with all people in high-risk age groups, careful consideration must be given to the health and safety of retired physicians and their immediate family members, especially those with chronic medical conditions.”
Tapping talent, sharing knowledge
When Barbara L. Schuster, MD, 69, filled out paperwork to join the Georgia Medical Reserve Corps, she answered a range of questions, including inquiries about her age, specialty, licensing, and whether she had any major medical conditions.
“They sent out instructions that said, if you are over the age of 60, we really don’t want you to be doing inpatient or ambulatory with active patients,” said Dr. Schuster, a retired medical school dean in the Athens, Ga., area. “Unless they get to a point where it’s going to be you or nobody, I think that they try to protect us for both our sake and also theirs.”
Dr. Schuster opted for telehealth or administrative duties, but has not yet been called upon to help. The Athens area has not seen high numbers of COVID-19 patients, compared with other parts of the country, and there have not been many volunteer opportunities for physicians thus far, she said. In the meantime, Dr. Schuster has found other ways to give her time, such as answering questions from community members on both COVID-19 and non–COVID-19 topics, and offering guidance to medical students.
“I’ve spent an increasing number of hours on Zoom, Skype, or FaceTime meeting with them to talk about various issues,” Dr. Schuster said.
As hospitals and organizations ramp up pandemic preparation, now is the time to consider roles for older clinicians and how they can best contribute, said Peter I. Buerhaus, PhD, RN, a nurse and director of the Center for Interdisciplinary Health Workforce Studies at Montana State University, Bozeman, Mont. Dr. Buerhaus was the first author of the recent JAMA viewpoint “Older clinicians and the surge in novel coronavirus 2019.”
“It’s important for hospitals that are anticipating a surge of critically ill patients to assess their workforce’s capability, including the proportion of older clinicians,” he said. “Is there something organizations can do differently to lessen older physicians’ and nurses’ direct patient contact and reduce their risk of infection?”
Dr. Buerhaus’ JAMA piece offers a range of ideas and assignments for older clinicians during the pandemic, including consulting with younger staff, advising on resources, assisting with clinical and organizational problem solving, aiding clinicians and managers with challenging decisions, consulting with patient families, advising managers and executives, being public spokespersons, and working with public and community health organizations.
“Older clinicians are at increased risk of becoming seriously ill if infected, but yet they’re also the ones who perhaps some of the best minds and experiences to help organizations combat the pandemic,” Dr. Buerhaus said. “These clinicians have great backgrounds and skills and 20, 30, 40 years of experience to draw on, including dealing with prior medical emergencies. I would hope that organizations, if they can, use the time before becoming a hotspot as an opportunity where the younger workforce could be teamed up with some of the older clinicians and learn as much as possible. It’s a great opportunity to share this wealth of knowledge with the workforce that will carry on after the pandemic.”
Since responding to New York’s call for volunteers, Dr. Salerno has been assigned to a palliative care inpatient team at a Manhattan hospital where she is working with large numbers of ICU patients and their families.
“My experience as a geriatrician helps me in talking with anxious and concerned families, especially when they are unable to see or communicate with their critically ill loved ones,” she said.
Before she was assigned the post, Dr. Salerno said she heard concerns from her adult children, who would prefer their mom take on a volunteer telehealth role. At the time, Dr. Salerno said she was not opposed to a telehealth assignment, but stressed to her family that she would go where she was needed.
“I’m healthy enough to run an organization, work long hours, long weeks; I have the stamina. The only thing working against me is age,” she said. “To say I’m not concerned is not honest. Of course I’m concerned. Am I afraid? No. I’m hoping that we can all be kept safe.”
When Judith Salerno, MD, heard that New York was calling for volunteer clinicians to assist with the COVID-19 response, she didn’t hesitate to sign up.
Although Dr. Salerno, 68, has held administrative, research, and policy roles for 25 years, she has kept her medical license active and always found ways to squeeze some clinical work into her busy schedule.
“I have what I could consider ‘rusty’ clinical skills, but pretty good clinical judgment,” said Dr. Salerno, president of the New York Academy of Medicine. “I thought in this situation that I could resurrect and hone those skills, even if it was just taking care of routine patients and working on a team, there was a lot of good I can do.”
Dr. Salerno is among 80,000 health care professionals who have volunteered to work temporarily in New York during the COVID-19 pandemic as of March 31, 2020, according to New York state officials. In mid-March, New York Governor Andrew Cuomo (D) issued a plea for retired physicians and nurses to help the state by signing up for on-call work. Other states have made similar appeals for retired health care professionals to return to medicine in an effort to relieve overwhelmed hospital staffs and aid capacity if health care workers become ill. Such redeployments, however, are raising concerns about exposing senior physicians to a virus that causes more severe illness in individuals aged over 65 years and kills them at a higher rate.
At the same time, a significant portion of the current health care workforce is aged 55 years and older, placing them at higher risk for serious illness, hospitalization, and death from COVID-19, said Douglas O. Staiger, PhD, a researcher and economics professor at Dartmouth College, Hanover, N.H. Dr. Staiger recently coauthored a viewpoint in JAMA called “Older clinicians and the surge in novel coronavirus disease 2019,” which outlines the risks and mortality rates from the novel coronavirus among patients aged 55 years and older.
Among the 1.2 million practicing physicians in the United States, about 20% are aged 55-64 years and an estimated 9% are 65 years or older, according to the paper. Of the nation’s nearly 2 million registered nurses employed in hospitals, about 19% are aged 55-64 years, and an estimated 3% are aged 65 years or older.
“In some metro areas, this proportion is even higher,” Dr. Staiger said in an interview. “Hospitals and other health care providers should consider ways of utilizing older clinicians’ skills and experience in a way that minimizes their risk of exposure to COVID-19, such as transferring them from jobs interacting with patients to more supervisory, administrative, or telehealth roles. This is increasingly important as retired physicians and nurses are being asked to return to the workforce.”
Protecting staff, screening volunteers
Hematologist-oncologist David H. Henry, MD, said his eight-physician group practice at Pennsylvania Hospital, Philadelphia, has already taken steps to protect him from COVID exposure.
At the request of his younger colleagues, Dr. Henry, 69, said he is no longer seeing patients in the hospital where there is increased exposure risk to the virus. He and the staff also limit their time in the office to 2-3 days a week and practice telemedicine the rest of the week, Dr. Henry said in an interview.
“Whether you’re a person trying to stay at home because you’re quote ‘nonessential,’ or you’re a health care worker and you have to keep seeing patients to some extent, the less we’re face to face with others the better,” said Dr. Henry, who hosts the Blood & Cancer podcast for MDedge News. “There’s an extreme and a middle ground. If they told me just to stay home that wouldn’t help anybody. If they said, ‘business as usual,’ that would be wrong. This is a middle strategy, which is reasonable, rational, and will help dial this dangerous time down as fast as possible.”
On a recent weekend when Dr. Henry would normally have been on call in the hospital, he took phone calls for his colleagues at home while they saw patients in the hospital. This included calls with patients who had questions and consultation calls with other physicians.
“They are helping me and I am helping them,” Dr. Henry said. “Taking those calls makes it easier for my partners to see all those patients. We all want to help and be there, within reason. You want to step up an do your job, but you want to be safe.”
Peter D. Quinn, DMD, MD, chief executive physician of the Penn Medicine Medical Group, said safeguarding the health of its workforce is a top priority as Penn Medicine works to fight the COVID-19 pandemic.
“This includes ensuring that all employees adhere to Centers for Disease Control and Penn Medicine infection prevention guidance as they continue their normal clinical work,” Dr. Quinn said in an interview. “Though age alone is not a criterion to remove frontline staff from direct clinical care during the COVID-19 outbreak, certain conditions such as cardiac or lung disease may be, and clinicians who have concerns are urged to speak with their leadership about options to fill clinical or support roles remotely.”
Meanwhile, for states calling on retired health professionals to assist during the pandemic, thorough screenings that identify high-risk volunteers are essential to protect vulnerable clinicians, said Nathaniel Hibbs, DO, president of the Colorado chapter of the American College of Emergency Physicians.
After Colorado issued a statewide request for retired clinicians to help, Dr. Hibbs became concerned that the state’s website initially included only a basic set of questions for interested volunteers.
“It didn’t have screening questions for prior health problems, comorbidities, or things like high blood pressure, heart disease, lung disease – the high-risk factors that we associate with bad outcomes if people get infected with COVID,” Dr. Hibbs said in an interview.
To address this, Dr. Hibbs and associates recently provided recommendations to the state about its screening process that advised collecting more health information from volunteers and considering lower-risk assignments for high-risk individuals. State officials indicated they would strongly consider the recommendations, Dr. Hibbs said.
The Colorado Department of Public Health & Environment did not respond to messages seeking comment. Officials at the New York State Department of Health declined to be interviewed for this article but confirmed that they are reviewing the age and background of all volunteers, and individual hospitals will also review each volunteer to find suitable jobs.
The American Medical Association on March 30 issued guidance for retired physicians about rejoining the workforce to help with the COVID response. The guidance outlines license considerations, contribution options, professional liability considerations, and questions to ask volunteer coordinators.
“Throughout the COVID-19 pandemic, many physicians over the age of 65 will provide care to patients,” AMA President Patrice A. Harris, MD, said in a statement. “Whether ‘senior’ physicians should be on the front line of patient care at this time is a complex issue that must balance several factors against the benefit these physicians can provide. As with all people in high-risk age groups, careful consideration must be given to the health and safety of retired physicians and their immediate family members, especially those with chronic medical conditions.”
Tapping talent, sharing knowledge
When Barbara L. Schuster, MD, 69, filled out paperwork to join the Georgia Medical Reserve Corps, she answered a range of questions, including inquiries about her age, specialty, licensing, and whether she had any major medical conditions.
“They sent out instructions that said, if you are over the age of 60, we really don’t want you to be doing inpatient or ambulatory with active patients,” said Dr. Schuster, a retired medical school dean in the Athens, Ga., area. “Unless they get to a point where it’s going to be you or nobody, I think that they try to protect us for both our sake and also theirs.”
Dr. Schuster opted for telehealth or administrative duties, but has not yet been called upon to help. The Athens area has not seen high numbers of COVID-19 patients, compared with other parts of the country, and there have not been many volunteer opportunities for physicians thus far, she said. In the meantime, Dr. Schuster has found other ways to give her time, such as answering questions from community members on both COVID-19 and non–COVID-19 topics, and offering guidance to medical students.
“I’ve spent an increasing number of hours on Zoom, Skype, or FaceTime meeting with them to talk about various issues,” Dr. Schuster said.
As hospitals and organizations ramp up pandemic preparation, now is the time to consider roles for older clinicians and how they can best contribute, said Peter I. Buerhaus, PhD, RN, a nurse and director of the Center for Interdisciplinary Health Workforce Studies at Montana State University, Bozeman, Mont. Dr. Buerhaus was the first author of the recent JAMA viewpoint “Older clinicians and the surge in novel coronavirus 2019.”
“It’s important for hospitals that are anticipating a surge of critically ill patients to assess their workforce’s capability, including the proportion of older clinicians,” he said. “Is there something organizations can do differently to lessen older physicians’ and nurses’ direct patient contact and reduce their risk of infection?”
Dr. Buerhaus’ JAMA piece offers a range of ideas and assignments for older clinicians during the pandemic, including consulting with younger staff, advising on resources, assisting with clinical and organizational problem solving, aiding clinicians and managers with challenging decisions, consulting with patient families, advising managers and executives, being public spokespersons, and working with public and community health organizations.
“Older clinicians are at increased risk of becoming seriously ill if infected, but yet they’re also the ones who perhaps some of the best minds and experiences to help organizations combat the pandemic,” Dr. Buerhaus said. “These clinicians have great backgrounds and skills and 20, 30, 40 years of experience to draw on, including dealing with prior medical emergencies. I would hope that organizations, if they can, use the time before becoming a hotspot as an opportunity where the younger workforce could be teamed up with some of the older clinicians and learn as much as possible. It’s a great opportunity to share this wealth of knowledge with the workforce that will carry on after the pandemic.”
Since responding to New York’s call for volunteers, Dr. Salerno has been assigned to a palliative care inpatient team at a Manhattan hospital where she is working with large numbers of ICU patients and their families.
“My experience as a geriatrician helps me in talking with anxious and concerned families, especially when they are unable to see or communicate with their critically ill loved ones,” she said.
Before she was assigned the post, Dr. Salerno said she heard concerns from her adult children, who would prefer their mom take on a volunteer telehealth role. At the time, Dr. Salerno said she was not opposed to a telehealth assignment, but stressed to her family that she would go where she was needed.
“I’m healthy enough to run an organization, work long hours, long weeks; I have the stamina. The only thing working against me is age,” she said. “To say I’m not concerned is not honest. Of course I’m concerned. Am I afraid? No. I’m hoping that we can all be kept safe.”
Finding the Value in Personal Protective Equipment for Hospitalized Patients During a Pandemic and Beyond
Due to the pandemic spread of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the cause of COVID-19 infection, there is significant disruption to the global supply of PPE.2 Order volumes of PPE have increased, prices have surged, and distributors are experiencing challenges meeting order demands.3 With decreased overseas exports, suppliers have placed hospitals on PPE allocations, and many hospitals’ orders for PPE have been only partially filled.3,4 Unless hospitals have established stockpiles, most only have supplies for 3-7 days of routine use, leaving them vulnerable to exhausting PPE supplies. At the onset of the pandemic, 86% of United States hospitals reported concerns about their PPE supply.4
The potential for PPE shortages has led both the Centers for Disease Control and Prevention (CDC) and the World Health Organization to call for the rational and appropriate use of PPE in order to conserve supplies.2,3 By the time COVID-19 was declared a pandemic, 54% of hospitals had imposed PPE conservation protocols,4 with more expected to follow in the weeks and months to come. Innovative protocols have been conceptualized and used to conserve PPE in hospitals (Table).
Yet these conservation protocols often fail to identify missed opportunities to improve the value of PPE that already exist in hospital care. By defining the value of inpatient PPE, hospitals can identify opportunities for value improvement. Changes implemented now will maximize PPE value and preserve supply during this pandemic and beyond.
THE VALUE OF PPE
In order to conserve PPE supply, hospitals might consider limiting PPE to cases in which clear evidence exists to support its use. However, evidence for PPE use can be challenging to interpret because the impact of preventing nosocomial infections (an outcome that did not occur) is inherently problematic to measure. This makes assessing the value of PPE in preventing nosocomial transmission in specific situations difficult.
The basis of using PPE is its effectiveness in controlling outbreaks.1 A meta-analysis of 6 case-control studies from the SARS outbreak of 2003, which disproportionately infected healthcare workers, suggested that handwashing and PPE were effective in preventing disease transmission. Handwashing alone reduced transmission by 55%, wearing gloves by 57%, and wearing facemasks by 68%; the cumulative effect of handwashing, masks, gloves, and gowns reduced transmission by 91%.5 A cohort study of healthcare workers exposed to H1N1 influenza A in 2009 found that use of a facemask or an N95 respirator was associated with negative viral serology suggesting noninfected status.6 With respiratory syncytial virus (RSV) outbreaks, a narrative synthesis of 4 studies examining transmission also suggested gowns, facemasks, and eye protection are effective, with eye protection perhaps more effective than gowns and masks.7 Yet these studies’ conclusions are limited by study design differences and small sample sizes.
The evidence supporting PPE use for routine hospital conditions is more challenging to interpret. One pediatric study of seasonal respiratory viruses showed that adding droplet precautions to an existing policy of contact precautions alone decreased nosocomial infections for most viruses evaluated.8 Yet this study, like many of PPE use, is limited by sample size and possible misclassification of exposure and outcome biases. Because PPE is always utilized in conjunction with other preventive measures, isolating the impact of PPE is challenging, let alone isolating the individual effects of PPE components. In the absence of strong empirical evidence, hospitals must rely on the inherent rationale of PPE use for patient and healthcare worker safety in assessing its value.
In order to protect patients from disease transmission during a pandemic, hospitals might also reconsider whether to use PPE in cases in which evidence is absent, such as routine prevention for colonized but noninfected patients. However, evidence of the possible patient harms of PPE are emerging. Healthcare providers spend less time with isolated patients9,10 and document fewer vital signs.11 Patients in PPE may experience delays in admission12 and discharge,13 and have higher rates of falls, pressure ulcers, and medication errors.14,15 They may also experience higher rates of anxiety and depression.16 Yet no evidence suggests PPE use for noninfected patients prevents transmission to patients or to healthcare workers. Using PPE when it is not indicated deemphasizes the value of other preventative precautions (eg, handwashing), unnecessarily depletes PPE supply, and may create patient harm without added benefit. High-value PPE, both during a pandemic and beyond, is defined by a system designed so that healthcare workers use PPE when they need it, and do not use PPE when not indicated.
ORDERING PPE IN A COMPLEX HEALTHCARE ENVIRONMENT
While all hospitalized patients are admitted using standard precautions, decisions surrounding PPE can be nuanced for even experienced clinicians. Although the CDC does provide guidance for PPE use based on symptoms that correlate with potential for transmission (eg, patients with cough should be placed in at least droplet precautions),1 guidelines must rely on provider evaluation and interpretation. For instance, three etiologies of cough—pneumococcal pneumonia, RSV bronchiolitis, and pulmonary tuberculosis—would all require different PPE. The clinician must weigh the probabilities of each pathogen and assess the harm of not protecting against certain pathogens in his or her decision.
Amidst the stress and cognitive burdens placed on clinicians, accuracy in PPE decisions is easily deprioritized. Clinicians may not completely consider patient-specific indications for PPE, implications for patients and staff, and supply shortages. Although the CDC and many hospitals have PPE initiation and discontinuation criteria, clinicians may favor educated guesswork and reliance on past experience when guidelines are poorly accessible or poorly searchable. Such individual, nonstandardized decisions likely lead to variability in practice patterns, inaccuracies in PPE decisions, and ultimately waste of PPE resources.
WHERE OUR HOSPITAL USES PPE IN A LOW-VALUE WAY
At our institution, the inconveniences, cognitive burden, and perceived benefit of routine PPE interventions have created a system in which PPE is regularly overused. On our hospital medicine wards, we found that PPE was both over-ordered upon admission (eg, contact/droplet precautions ordered for influenza when droplet precautions only would have sufficed) and unnecessarily continued even after children met discontinuation criteria.
On discharge review from our general pediatric ward in 2019, 18% of children discharged with PPE orders no longer met criteria for PPE. Two conditions—community-acquired bacterial pneumonia and skin and soft-tissue infections—accounted for 47% of discharges with unnecessary PPE orders. At an estimated cost of $0.13-$0.53 for droplet precautions per use, $0.69 for contact precautions, and $0.82-$1.22 for both, the absolute cost of continuing PPE without indication could be as high as $61/day per patient when estimating 50 uses per day. This direct cost represents healthcare spending without added value when PPE are not necessary. Furthermore, the additional emotional cost to the patient and family in their hospitalization experience, the cost of clinician time donning and doffing, the environmental cost of PPE waste, and the cost to the limited PPE supply are not considered in these calculations.
During a pandemic characterized by PPE shortages nationwide, allowing missed opportunities for PPE discontinuation to persist is not only wasteful, but inattentive to public health.
OPPORTUNITIES FOR HOSPITALS TO MAXIMIZE THE VALUE OF PPE
For individual clinicians, opportunities exist to improve PPE usage in daily patient care. Clinicians should not overlook PPE decisions; instead they should make it a practice to review PPE orders daily during rounds as they would lab orders. Clinicians and nursing staff should work together to identify PPE discontinuation opportunities, leveraging the electronic medical record when possible. For the benefit of patients and families, clinicians and bedside staff should recognize and assist in managing patient expectations of PPE.
Hospitals should work to make PPE references easily accessible and interpretable by frontline clinicians. To minimize variability of use, PPE ordering for routine conditions should be standardized and streamlined, including discontinuation criteria. Hospitals should invest in behavioral health programs to support patients with conditions necessitating PPE and develop policies to ensure ancillary services are equally available to all patients. To alleviate concerns about limited clinician time spent with isolated patients, hospitals should assign clinician workloads while accounting for the known increased time needed to care for patients with PPE.
For hospitals with extreme supply shortages, conservation might include decreased use of PPE for conditions in which its use is controversial (eg, patients colonized with methicillin-resistant Staphylococcus aureus or multidrug resistant organisms) as has been trialed in institutions prior to this pandemic.17,18 Such PPE policy changes might occur in addition to, or in conjunction with, the conservation strategies suggested by other institutions (Table).
Healthcare systems should continually reassess the value of PPE for their hospitals and make changes accordingly. In the midst of difficulties directly demonstrating PPE’s value, hospitals must rely on the inherent rationale of PPE use in assessing value decisions weighed against harms while balancing healthcare worker protection regulations. Decisions should always occur while continuing other sensible infection-control procedures, such as handwashing and environmental hygiene measures.
To effect maximal change, healthcare systems should invest in redesigning PPE ordering systems at the highest level. This should include harnessing existing technologies to streamline PPE ordering decisions to meet clinicians’ cognitive needs. Decision support and auditing technologies could automate and monitor PPE orders efficiently. Likely to be most effective, an investment in creating and maintaining centralized PPE expert management teams to assess, order, and discontinue PPE would minimize individual ordering variation, minimize cost, and maximize value to patients, staff, and hospitals.
CONCLUSION
In this pandemic, we have the opportunity to rethink how we understand and use PPE in hospitalized patients. It is vitally important now more than ever to consciously conserve the limited supply of PPE resources. As we seek to increase healthcare value while limiting overuse and waste, PPE is a prime target for value improvement efforts as the effective but also burdensome tool that it is. Hospitalists are well-positioned to lead the redesign of how, when, and why PPE is used and to create a more optimized, lasting system that provides maximal value to patients, families, and healthcare workers during this current crisis and beyond.
1. Siegel JD, Rhinehart E, Jackson M, Chiarello L. 2007 Guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am J Infect Control. 2007;35(10 Suppl 2):S65-164. https://doi.org/10.1016/j.ajic.2007.10.007.
2. World Health Organization. Shortage of personal protective equipment endangering health workers worldwide. https://www.who.int/news-room/detail/03-03-2020-shortage-of-personal-protective-equipment-endangering-health-workers-worldwide. Accessed March 12, 2020.
3. Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19): healthcare supply of personal protective equipment. https://www.cdc.gov/coronavirus/2019-ncov/hcp/healthcare-supply-ppe.html. Accessed March 12, 2020.
4. Premier Inc. Premier Inc survey finds 86 percent of health systems are concerned about personal protective equipment shortages due to coronavirus. https://www.premierinc.com/newsroom/press-releases/premier-inc-survey-finds-86-percent-of-health-systems-are-concerned-about-personal-protective-equipment-shortages-due-to-coronavirus. Accessed March 22, 2020.
5. Jefferson T, Foxlee R, Del Mar C, et al. Physical interventions to interrupt or reduce the spread of respiratory viruses: systematic review. BMJ. 2008;336(7635):77-80. https://doi.org/10.1136/bmj.39393.510347.BE.
6. Jaeger JL, Patel M, Dharan N, et al. Transmission of 2009 pandemic influenza A (H1N1) virus among healthcare personnel-Southern California, 2009. Infect Control Hosp Epidemiol. 2011;32(12):1149-1157. https://doi.org/10.1086/662709.
7. French CE, McKenzie BC, Coope C, et al. Risk of nosocomial respiratory syncytial virus infection and effectiveness of control measures to prevent transmission events: a systematic review. Influenza Other Respir Viruses. 2016;10(4):268-290. https://doi.org/10.1111/irv.12379.
8. Rubin LG, Kohn N, Nullet S, Hill M. Reduction in rate of nosocomial respiratory virus infections in a children’s hospital associated with enhanced isolation precautions. Infect Control Hosp Epidemiol. 2018;39(2):152-156. https://doi.org/10.1017/ice.2017.282.
9. Dashiell-Earp CN, Bell DS, Ang AO, Uslan DZ. Do physicians spend less time with patients in contact isolation?: A time-motion study of internal medicine interns. JAMA Intern Med. 2014;174(5):814-815. https://doi.org/10.1001/jamainternmed.2014.537.
10. Saint S, Higgins LA, Nallamothu BK, Chenoweth C. Do physicians examine patients in contact isolation less frequently? A brief report. Am J Infect Control. 2003;31(6):354-356. https://doi.org/10.1016/S0196-6553(02)48250-8.
11. Kirkland KB, Weinstein JM. Adverse effects of contact isolation. Lancet. 1999(354):1177-1178. https://doi.org/10.1016/S0140-6736(99)04196-3.
12. McLemore A, Bearman G, Edmond MB. Effect of contact precautions on wait time from emergency room disposition to inpatient admission. Infect Control Hosp Epidemiol. 2011;32(3):298-299. https://doi.org/10.1086/658913.
13. Tran K, Bell C, Stall N, et al. The effect of hospital isolation precautions on patient outcomes and cost of care: a multi-site, retrospective, propensity score-matched cohort study. J Gen Intern Med. 2017;32(3):262-268. https://doi.org/10.1007/s11606-016-3862-4.
14. Karki S, Leder K, Cheng AC. Patients under contact precautions have an increased risk of injuries and medication errors: a retrospective cohort study. Infect Control Hosp Epidemiol. 2013;34(10):1118-1120. https://doi.org/10.1086/673153.
15. Stelfox HT, Bates DW, Redelmeier DA. Safety of patients isolated for infection control. JAMA. 2003;290(14):1899-1905. https://doi.org/10.1001/jama.290.14.1899.
16. Catalano G, Houston SH, Catalano MC, et al. Anxiety and depression in hospitalized patients in resistant organism isolation. South Med J. 2003;96(2):141-145. https://doi.org/10.1097/01.SMJ.0000050683.36014.2E.
17. Young K, Doernberg SB, Snedecor RF, Mallin E. Things we do for no reason: contact precautions for MRSA and VRE. J Hosp Med. 2019;14(3):178-180. https://doi.org/10.12788/jhm.3126.
18. Bearman G, Abbas S, Masroor N, et al. Impact of discontinuing contact precautions for methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus: an interrupted time series analysis. Infect Control Hosp Epidemiol. 2018;39(6):676-682. https://doi.org/10.1017/ice.2018.57.
Due to the pandemic spread of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the cause of COVID-19 infection, there is significant disruption to the global supply of PPE.2 Order volumes of PPE have increased, prices have surged, and distributors are experiencing challenges meeting order demands.3 With decreased overseas exports, suppliers have placed hospitals on PPE allocations, and many hospitals’ orders for PPE have been only partially filled.3,4 Unless hospitals have established stockpiles, most only have supplies for 3-7 days of routine use, leaving them vulnerable to exhausting PPE supplies. At the onset of the pandemic, 86% of United States hospitals reported concerns about their PPE supply.4
The potential for PPE shortages has led both the Centers for Disease Control and Prevention (CDC) and the World Health Organization to call for the rational and appropriate use of PPE in order to conserve supplies.2,3 By the time COVID-19 was declared a pandemic, 54% of hospitals had imposed PPE conservation protocols,4 with more expected to follow in the weeks and months to come. Innovative protocols have been conceptualized and used to conserve PPE in hospitals (Table).
Yet these conservation protocols often fail to identify missed opportunities to improve the value of PPE that already exist in hospital care. By defining the value of inpatient PPE, hospitals can identify opportunities for value improvement. Changes implemented now will maximize PPE value and preserve supply during this pandemic and beyond.
THE VALUE OF PPE
In order to conserve PPE supply, hospitals might consider limiting PPE to cases in which clear evidence exists to support its use. However, evidence for PPE use can be challenging to interpret because the impact of preventing nosocomial infections (an outcome that did not occur) is inherently problematic to measure. This makes assessing the value of PPE in preventing nosocomial transmission in specific situations difficult.
The basis of using PPE is its effectiveness in controlling outbreaks.1 A meta-analysis of 6 case-control studies from the SARS outbreak of 2003, which disproportionately infected healthcare workers, suggested that handwashing and PPE were effective in preventing disease transmission. Handwashing alone reduced transmission by 55%, wearing gloves by 57%, and wearing facemasks by 68%; the cumulative effect of handwashing, masks, gloves, and gowns reduced transmission by 91%.5 A cohort study of healthcare workers exposed to H1N1 influenza A in 2009 found that use of a facemask or an N95 respirator was associated with negative viral serology suggesting noninfected status.6 With respiratory syncytial virus (RSV) outbreaks, a narrative synthesis of 4 studies examining transmission also suggested gowns, facemasks, and eye protection are effective, with eye protection perhaps more effective than gowns and masks.7 Yet these studies’ conclusions are limited by study design differences and small sample sizes.
The evidence supporting PPE use for routine hospital conditions is more challenging to interpret. One pediatric study of seasonal respiratory viruses showed that adding droplet precautions to an existing policy of contact precautions alone decreased nosocomial infections for most viruses evaluated.8 Yet this study, like many of PPE use, is limited by sample size and possible misclassification of exposure and outcome biases. Because PPE is always utilized in conjunction with other preventive measures, isolating the impact of PPE is challenging, let alone isolating the individual effects of PPE components. In the absence of strong empirical evidence, hospitals must rely on the inherent rationale of PPE use for patient and healthcare worker safety in assessing its value.
In order to protect patients from disease transmission during a pandemic, hospitals might also reconsider whether to use PPE in cases in which evidence is absent, such as routine prevention for colonized but noninfected patients. However, evidence of the possible patient harms of PPE are emerging. Healthcare providers spend less time with isolated patients9,10 and document fewer vital signs.11 Patients in PPE may experience delays in admission12 and discharge,13 and have higher rates of falls, pressure ulcers, and medication errors.14,15 They may also experience higher rates of anxiety and depression.16 Yet no evidence suggests PPE use for noninfected patients prevents transmission to patients or to healthcare workers. Using PPE when it is not indicated deemphasizes the value of other preventative precautions (eg, handwashing), unnecessarily depletes PPE supply, and may create patient harm without added benefit. High-value PPE, both during a pandemic and beyond, is defined by a system designed so that healthcare workers use PPE when they need it, and do not use PPE when not indicated.
ORDERING PPE IN A COMPLEX HEALTHCARE ENVIRONMENT
While all hospitalized patients are admitted using standard precautions, decisions surrounding PPE can be nuanced for even experienced clinicians. Although the CDC does provide guidance for PPE use based on symptoms that correlate with potential for transmission (eg, patients with cough should be placed in at least droplet precautions),1 guidelines must rely on provider evaluation and interpretation. For instance, three etiologies of cough—pneumococcal pneumonia, RSV bronchiolitis, and pulmonary tuberculosis—would all require different PPE. The clinician must weigh the probabilities of each pathogen and assess the harm of not protecting against certain pathogens in his or her decision.
Amidst the stress and cognitive burdens placed on clinicians, accuracy in PPE decisions is easily deprioritized. Clinicians may not completely consider patient-specific indications for PPE, implications for patients and staff, and supply shortages. Although the CDC and many hospitals have PPE initiation and discontinuation criteria, clinicians may favor educated guesswork and reliance on past experience when guidelines are poorly accessible or poorly searchable. Such individual, nonstandardized decisions likely lead to variability in practice patterns, inaccuracies in PPE decisions, and ultimately waste of PPE resources.
WHERE OUR HOSPITAL USES PPE IN A LOW-VALUE WAY
At our institution, the inconveniences, cognitive burden, and perceived benefit of routine PPE interventions have created a system in which PPE is regularly overused. On our hospital medicine wards, we found that PPE was both over-ordered upon admission (eg, contact/droplet precautions ordered for influenza when droplet precautions only would have sufficed) and unnecessarily continued even after children met discontinuation criteria.
On discharge review from our general pediatric ward in 2019, 18% of children discharged with PPE orders no longer met criteria for PPE. Two conditions—community-acquired bacterial pneumonia and skin and soft-tissue infections—accounted for 47% of discharges with unnecessary PPE orders. At an estimated cost of $0.13-$0.53 for droplet precautions per use, $0.69 for contact precautions, and $0.82-$1.22 for both, the absolute cost of continuing PPE without indication could be as high as $61/day per patient when estimating 50 uses per day. This direct cost represents healthcare spending without added value when PPE are not necessary. Furthermore, the additional emotional cost to the patient and family in their hospitalization experience, the cost of clinician time donning and doffing, the environmental cost of PPE waste, and the cost to the limited PPE supply are not considered in these calculations.
During a pandemic characterized by PPE shortages nationwide, allowing missed opportunities for PPE discontinuation to persist is not only wasteful, but inattentive to public health.
OPPORTUNITIES FOR HOSPITALS TO MAXIMIZE THE VALUE OF PPE
For individual clinicians, opportunities exist to improve PPE usage in daily patient care. Clinicians should not overlook PPE decisions; instead they should make it a practice to review PPE orders daily during rounds as they would lab orders. Clinicians and nursing staff should work together to identify PPE discontinuation opportunities, leveraging the electronic medical record when possible. For the benefit of patients and families, clinicians and bedside staff should recognize and assist in managing patient expectations of PPE.
Hospitals should work to make PPE references easily accessible and interpretable by frontline clinicians. To minimize variability of use, PPE ordering for routine conditions should be standardized and streamlined, including discontinuation criteria. Hospitals should invest in behavioral health programs to support patients with conditions necessitating PPE and develop policies to ensure ancillary services are equally available to all patients. To alleviate concerns about limited clinician time spent with isolated patients, hospitals should assign clinician workloads while accounting for the known increased time needed to care for patients with PPE.
For hospitals with extreme supply shortages, conservation might include decreased use of PPE for conditions in which its use is controversial (eg, patients colonized with methicillin-resistant Staphylococcus aureus or multidrug resistant organisms) as has been trialed in institutions prior to this pandemic.17,18 Such PPE policy changes might occur in addition to, or in conjunction with, the conservation strategies suggested by other institutions (Table).
Healthcare systems should continually reassess the value of PPE for their hospitals and make changes accordingly. In the midst of difficulties directly demonstrating PPE’s value, hospitals must rely on the inherent rationale of PPE use in assessing value decisions weighed against harms while balancing healthcare worker protection regulations. Decisions should always occur while continuing other sensible infection-control procedures, such as handwashing and environmental hygiene measures.
To effect maximal change, healthcare systems should invest in redesigning PPE ordering systems at the highest level. This should include harnessing existing technologies to streamline PPE ordering decisions to meet clinicians’ cognitive needs. Decision support and auditing technologies could automate and monitor PPE orders efficiently. Likely to be most effective, an investment in creating and maintaining centralized PPE expert management teams to assess, order, and discontinue PPE would minimize individual ordering variation, minimize cost, and maximize value to patients, staff, and hospitals.
CONCLUSION
In this pandemic, we have the opportunity to rethink how we understand and use PPE in hospitalized patients. It is vitally important now more than ever to consciously conserve the limited supply of PPE resources. As we seek to increase healthcare value while limiting overuse and waste, PPE is a prime target for value improvement efforts as the effective but also burdensome tool that it is. Hospitalists are well-positioned to lead the redesign of how, when, and why PPE is used and to create a more optimized, lasting system that provides maximal value to patients, families, and healthcare workers during this current crisis and beyond.
Due to the pandemic spread of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the cause of COVID-19 infection, there is significant disruption to the global supply of PPE.2 Order volumes of PPE have increased, prices have surged, and distributors are experiencing challenges meeting order demands.3 With decreased overseas exports, suppliers have placed hospitals on PPE allocations, and many hospitals’ orders for PPE have been only partially filled.3,4 Unless hospitals have established stockpiles, most only have supplies for 3-7 days of routine use, leaving them vulnerable to exhausting PPE supplies. At the onset of the pandemic, 86% of United States hospitals reported concerns about their PPE supply.4
The potential for PPE shortages has led both the Centers for Disease Control and Prevention (CDC) and the World Health Organization to call for the rational and appropriate use of PPE in order to conserve supplies.2,3 By the time COVID-19 was declared a pandemic, 54% of hospitals had imposed PPE conservation protocols,4 with more expected to follow in the weeks and months to come. Innovative protocols have been conceptualized and used to conserve PPE in hospitals (Table).
Yet these conservation protocols often fail to identify missed opportunities to improve the value of PPE that already exist in hospital care. By defining the value of inpatient PPE, hospitals can identify opportunities for value improvement. Changes implemented now will maximize PPE value and preserve supply during this pandemic and beyond.
THE VALUE OF PPE
In order to conserve PPE supply, hospitals might consider limiting PPE to cases in which clear evidence exists to support its use. However, evidence for PPE use can be challenging to interpret because the impact of preventing nosocomial infections (an outcome that did not occur) is inherently problematic to measure. This makes assessing the value of PPE in preventing nosocomial transmission in specific situations difficult.
The basis of using PPE is its effectiveness in controlling outbreaks.1 A meta-analysis of 6 case-control studies from the SARS outbreak of 2003, which disproportionately infected healthcare workers, suggested that handwashing and PPE were effective in preventing disease transmission. Handwashing alone reduced transmission by 55%, wearing gloves by 57%, and wearing facemasks by 68%; the cumulative effect of handwashing, masks, gloves, and gowns reduced transmission by 91%.5 A cohort study of healthcare workers exposed to H1N1 influenza A in 2009 found that use of a facemask or an N95 respirator was associated with negative viral serology suggesting noninfected status.6 With respiratory syncytial virus (RSV) outbreaks, a narrative synthesis of 4 studies examining transmission also suggested gowns, facemasks, and eye protection are effective, with eye protection perhaps more effective than gowns and masks.7 Yet these studies’ conclusions are limited by study design differences and small sample sizes.
The evidence supporting PPE use for routine hospital conditions is more challenging to interpret. One pediatric study of seasonal respiratory viruses showed that adding droplet precautions to an existing policy of contact precautions alone decreased nosocomial infections for most viruses evaluated.8 Yet this study, like many of PPE use, is limited by sample size and possible misclassification of exposure and outcome biases. Because PPE is always utilized in conjunction with other preventive measures, isolating the impact of PPE is challenging, let alone isolating the individual effects of PPE components. In the absence of strong empirical evidence, hospitals must rely on the inherent rationale of PPE use for patient and healthcare worker safety in assessing its value.
In order to protect patients from disease transmission during a pandemic, hospitals might also reconsider whether to use PPE in cases in which evidence is absent, such as routine prevention for colonized but noninfected patients. However, evidence of the possible patient harms of PPE are emerging. Healthcare providers spend less time with isolated patients9,10 and document fewer vital signs.11 Patients in PPE may experience delays in admission12 and discharge,13 and have higher rates of falls, pressure ulcers, and medication errors.14,15 They may also experience higher rates of anxiety and depression.16 Yet no evidence suggests PPE use for noninfected patients prevents transmission to patients or to healthcare workers. Using PPE when it is not indicated deemphasizes the value of other preventative precautions (eg, handwashing), unnecessarily depletes PPE supply, and may create patient harm without added benefit. High-value PPE, both during a pandemic and beyond, is defined by a system designed so that healthcare workers use PPE when they need it, and do not use PPE when not indicated.
ORDERING PPE IN A COMPLEX HEALTHCARE ENVIRONMENT
While all hospitalized patients are admitted using standard precautions, decisions surrounding PPE can be nuanced for even experienced clinicians. Although the CDC does provide guidance for PPE use based on symptoms that correlate with potential for transmission (eg, patients with cough should be placed in at least droplet precautions),1 guidelines must rely on provider evaluation and interpretation. For instance, three etiologies of cough—pneumococcal pneumonia, RSV bronchiolitis, and pulmonary tuberculosis—would all require different PPE. The clinician must weigh the probabilities of each pathogen and assess the harm of not protecting against certain pathogens in his or her decision.
Amidst the stress and cognitive burdens placed on clinicians, accuracy in PPE decisions is easily deprioritized. Clinicians may not completely consider patient-specific indications for PPE, implications for patients and staff, and supply shortages. Although the CDC and many hospitals have PPE initiation and discontinuation criteria, clinicians may favor educated guesswork and reliance on past experience when guidelines are poorly accessible or poorly searchable. Such individual, nonstandardized decisions likely lead to variability in practice patterns, inaccuracies in PPE decisions, and ultimately waste of PPE resources.
WHERE OUR HOSPITAL USES PPE IN A LOW-VALUE WAY
At our institution, the inconveniences, cognitive burden, and perceived benefit of routine PPE interventions have created a system in which PPE is regularly overused. On our hospital medicine wards, we found that PPE was both over-ordered upon admission (eg, contact/droplet precautions ordered for influenza when droplet precautions only would have sufficed) and unnecessarily continued even after children met discontinuation criteria.
On discharge review from our general pediatric ward in 2019, 18% of children discharged with PPE orders no longer met criteria for PPE. Two conditions—community-acquired bacterial pneumonia and skin and soft-tissue infections—accounted for 47% of discharges with unnecessary PPE orders. At an estimated cost of $0.13-$0.53 for droplet precautions per use, $0.69 for contact precautions, and $0.82-$1.22 for both, the absolute cost of continuing PPE without indication could be as high as $61/day per patient when estimating 50 uses per day. This direct cost represents healthcare spending without added value when PPE are not necessary. Furthermore, the additional emotional cost to the patient and family in their hospitalization experience, the cost of clinician time donning and doffing, the environmental cost of PPE waste, and the cost to the limited PPE supply are not considered in these calculations.
During a pandemic characterized by PPE shortages nationwide, allowing missed opportunities for PPE discontinuation to persist is not only wasteful, but inattentive to public health.
OPPORTUNITIES FOR HOSPITALS TO MAXIMIZE THE VALUE OF PPE
For individual clinicians, opportunities exist to improve PPE usage in daily patient care. Clinicians should not overlook PPE decisions; instead they should make it a practice to review PPE orders daily during rounds as they would lab orders. Clinicians and nursing staff should work together to identify PPE discontinuation opportunities, leveraging the electronic medical record when possible. For the benefit of patients and families, clinicians and bedside staff should recognize and assist in managing patient expectations of PPE.
Hospitals should work to make PPE references easily accessible and interpretable by frontline clinicians. To minimize variability of use, PPE ordering for routine conditions should be standardized and streamlined, including discontinuation criteria. Hospitals should invest in behavioral health programs to support patients with conditions necessitating PPE and develop policies to ensure ancillary services are equally available to all patients. To alleviate concerns about limited clinician time spent with isolated patients, hospitals should assign clinician workloads while accounting for the known increased time needed to care for patients with PPE.
For hospitals with extreme supply shortages, conservation might include decreased use of PPE for conditions in which its use is controversial (eg, patients colonized with methicillin-resistant Staphylococcus aureus or multidrug resistant organisms) as has been trialed in institutions prior to this pandemic.17,18 Such PPE policy changes might occur in addition to, or in conjunction with, the conservation strategies suggested by other institutions (Table).
Healthcare systems should continually reassess the value of PPE for their hospitals and make changes accordingly. In the midst of difficulties directly demonstrating PPE’s value, hospitals must rely on the inherent rationale of PPE use in assessing value decisions weighed against harms while balancing healthcare worker protection regulations. Decisions should always occur while continuing other sensible infection-control procedures, such as handwashing and environmental hygiene measures.
To effect maximal change, healthcare systems should invest in redesigning PPE ordering systems at the highest level. This should include harnessing existing technologies to streamline PPE ordering decisions to meet clinicians’ cognitive needs. Decision support and auditing technologies could automate and monitor PPE orders efficiently. Likely to be most effective, an investment in creating and maintaining centralized PPE expert management teams to assess, order, and discontinue PPE would minimize individual ordering variation, minimize cost, and maximize value to patients, staff, and hospitals.
CONCLUSION
In this pandemic, we have the opportunity to rethink how we understand and use PPE in hospitalized patients. It is vitally important now more than ever to consciously conserve the limited supply of PPE resources. As we seek to increase healthcare value while limiting overuse and waste, PPE is a prime target for value improvement efforts as the effective but also burdensome tool that it is. Hospitalists are well-positioned to lead the redesign of how, when, and why PPE is used and to create a more optimized, lasting system that provides maximal value to patients, families, and healthcare workers during this current crisis and beyond.
1. Siegel JD, Rhinehart E, Jackson M, Chiarello L. 2007 Guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am J Infect Control. 2007;35(10 Suppl 2):S65-164. https://doi.org/10.1016/j.ajic.2007.10.007.
2. World Health Organization. Shortage of personal protective equipment endangering health workers worldwide. https://www.who.int/news-room/detail/03-03-2020-shortage-of-personal-protective-equipment-endangering-health-workers-worldwide. Accessed March 12, 2020.
3. Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19): healthcare supply of personal protective equipment. https://www.cdc.gov/coronavirus/2019-ncov/hcp/healthcare-supply-ppe.html. Accessed March 12, 2020.
4. Premier Inc. Premier Inc survey finds 86 percent of health systems are concerned about personal protective equipment shortages due to coronavirus. https://www.premierinc.com/newsroom/press-releases/premier-inc-survey-finds-86-percent-of-health-systems-are-concerned-about-personal-protective-equipment-shortages-due-to-coronavirus. Accessed March 22, 2020.
5. Jefferson T, Foxlee R, Del Mar C, et al. Physical interventions to interrupt or reduce the spread of respiratory viruses: systematic review. BMJ. 2008;336(7635):77-80. https://doi.org/10.1136/bmj.39393.510347.BE.
6. Jaeger JL, Patel M, Dharan N, et al. Transmission of 2009 pandemic influenza A (H1N1) virus among healthcare personnel-Southern California, 2009. Infect Control Hosp Epidemiol. 2011;32(12):1149-1157. https://doi.org/10.1086/662709.
7. French CE, McKenzie BC, Coope C, et al. Risk of nosocomial respiratory syncytial virus infection and effectiveness of control measures to prevent transmission events: a systematic review. Influenza Other Respir Viruses. 2016;10(4):268-290. https://doi.org/10.1111/irv.12379.
8. Rubin LG, Kohn N, Nullet S, Hill M. Reduction in rate of nosocomial respiratory virus infections in a children’s hospital associated with enhanced isolation precautions. Infect Control Hosp Epidemiol. 2018;39(2):152-156. https://doi.org/10.1017/ice.2017.282.
9. Dashiell-Earp CN, Bell DS, Ang AO, Uslan DZ. Do physicians spend less time with patients in contact isolation?: A time-motion study of internal medicine interns. JAMA Intern Med. 2014;174(5):814-815. https://doi.org/10.1001/jamainternmed.2014.537.
10. Saint S, Higgins LA, Nallamothu BK, Chenoweth C. Do physicians examine patients in contact isolation less frequently? A brief report. Am J Infect Control. 2003;31(6):354-356. https://doi.org/10.1016/S0196-6553(02)48250-8.
11. Kirkland KB, Weinstein JM. Adverse effects of contact isolation. Lancet. 1999(354):1177-1178. https://doi.org/10.1016/S0140-6736(99)04196-3.
12. McLemore A, Bearman G, Edmond MB. Effect of contact precautions on wait time from emergency room disposition to inpatient admission. Infect Control Hosp Epidemiol. 2011;32(3):298-299. https://doi.org/10.1086/658913.
13. Tran K, Bell C, Stall N, et al. The effect of hospital isolation precautions on patient outcomes and cost of care: a multi-site, retrospective, propensity score-matched cohort study. J Gen Intern Med. 2017;32(3):262-268. https://doi.org/10.1007/s11606-016-3862-4.
14. Karki S, Leder K, Cheng AC. Patients under contact precautions have an increased risk of injuries and medication errors: a retrospective cohort study. Infect Control Hosp Epidemiol. 2013;34(10):1118-1120. https://doi.org/10.1086/673153.
15. Stelfox HT, Bates DW, Redelmeier DA. Safety of patients isolated for infection control. JAMA. 2003;290(14):1899-1905. https://doi.org/10.1001/jama.290.14.1899.
16. Catalano G, Houston SH, Catalano MC, et al. Anxiety and depression in hospitalized patients in resistant organism isolation. South Med J. 2003;96(2):141-145. https://doi.org/10.1097/01.SMJ.0000050683.36014.2E.
17. Young K, Doernberg SB, Snedecor RF, Mallin E. Things we do for no reason: contact precautions for MRSA and VRE. J Hosp Med. 2019;14(3):178-180. https://doi.org/10.12788/jhm.3126.
18. Bearman G, Abbas S, Masroor N, et al. Impact of discontinuing contact precautions for methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus: an interrupted time series analysis. Infect Control Hosp Epidemiol. 2018;39(6):676-682. https://doi.org/10.1017/ice.2018.57.
1. Siegel JD, Rhinehart E, Jackson M, Chiarello L. 2007 Guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am J Infect Control. 2007;35(10 Suppl 2):S65-164. https://doi.org/10.1016/j.ajic.2007.10.007.
2. World Health Organization. Shortage of personal protective equipment endangering health workers worldwide. https://www.who.int/news-room/detail/03-03-2020-shortage-of-personal-protective-equipment-endangering-health-workers-worldwide. Accessed March 12, 2020.
3. Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19): healthcare supply of personal protective equipment. https://www.cdc.gov/coronavirus/2019-ncov/hcp/healthcare-supply-ppe.html. Accessed March 12, 2020.
4. Premier Inc. Premier Inc survey finds 86 percent of health systems are concerned about personal protective equipment shortages due to coronavirus. https://www.premierinc.com/newsroom/press-releases/premier-inc-survey-finds-86-percent-of-health-systems-are-concerned-about-personal-protective-equipment-shortages-due-to-coronavirus. Accessed March 22, 2020.
5. Jefferson T, Foxlee R, Del Mar C, et al. Physical interventions to interrupt or reduce the spread of respiratory viruses: systematic review. BMJ. 2008;336(7635):77-80. https://doi.org/10.1136/bmj.39393.510347.BE.
6. Jaeger JL, Patel M, Dharan N, et al. Transmission of 2009 pandemic influenza A (H1N1) virus among healthcare personnel-Southern California, 2009. Infect Control Hosp Epidemiol. 2011;32(12):1149-1157. https://doi.org/10.1086/662709.
7. French CE, McKenzie BC, Coope C, et al. Risk of nosocomial respiratory syncytial virus infection and effectiveness of control measures to prevent transmission events: a systematic review. Influenza Other Respir Viruses. 2016;10(4):268-290. https://doi.org/10.1111/irv.12379.
8. Rubin LG, Kohn N, Nullet S, Hill M. Reduction in rate of nosocomial respiratory virus infections in a children’s hospital associated with enhanced isolation precautions. Infect Control Hosp Epidemiol. 2018;39(2):152-156. https://doi.org/10.1017/ice.2017.282.
9. Dashiell-Earp CN, Bell DS, Ang AO, Uslan DZ. Do physicians spend less time with patients in contact isolation?: A time-motion study of internal medicine interns. JAMA Intern Med. 2014;174(5):814-815. https://doi.org/10.1001/jamainternmed.2014.537.
10. Saint S, Higgins LA, Nallamothu BK, Chenoweth C. Do physicians examine patients in contact isolation less frequently? A brief report. Am J Infect Control. 2003;31(6):354-356. https://doi.org/10.1016/S0196-6553(02)48250-8.
11. Kirkland KB, Weinstein JM. Adverse effects of contact isolation. Lancet. 1999(354):1177-1178. https://doi.org/10.1016/S0140-6736(99)04196-3.
12. McLemore A, Bearman G, Edmond MB. Effect of contact precautions on wait time from emergency room disposition to inpatient admission. Infect Control Hosp Epidemiol. 2011;32(3):298-299. https://doi.org/10.1086/658913.
13. Tran K, Bell C, Stall N, et al. The effect of hospital isolation precautions on patient outcomes and cost of care: a multi-site, retrospective, propensity score-matched cohort study. J Gen Intern Med. 2017;32(3):262-268. https://doi.org/10.1007/s11606-016-3862-4.
14. Karki S, Leder K, Cheng AC. Patients under contact precautions have an increased risk of injuries and medication errors: a retrospective cohort study. Infect Control Hosp Epidemiol. 2013;34(10):1118-1120. https://doi.org/10.1086/673153.
15. Stelfox HT, Bates DW, Redelmeier DA. Safety of patients isolated for infection control. JAMA. 2003;290(14):1899-1905. https://doi.org/10.1001/jama.290.14.1899.
16. Catalano G, Houston SH, Catalano MC, et al. Anxiety and depression in hospitalized patients in resistant organism isolation. South Med J. 2003;96(2):141-145. https://doi.org/10.1097/01.SMJ.0000050683.36014.2E.
17. Young K, Doernberg SB, Snedecor RF, Mallin E. Things we do for no reason: contact precautions for MRSA and VRE. J Hosp Med. 2019;14(3):178-180. https://doi.org/10.12788/jhm.3126.
18. Bearman G, Abbas S, Masroor N, et al. Impact of discontinuing contact precautions for methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus: an interrupted time series analysis. Infect Control Hosp Epidemiol. 2018;39(6):676-682. https://doi.org/10.1017/ice.2018.57.
© 2020 Society of Hospital Medicine
Managing pediatric heme/onc departments during the pandemic
Given the possibility that children with hematologic malignancies may have increased susceptibility to coronavirus disease 2019 (COVID-19), clinicians from China and the United States have proposed a plan for preventing and managing outbreaks in hospitals’ pediatric hematology and oncology departments.
The plan is focused primarily on infection prevention and control strategies, Yulei He, MD, of Chengdu (China) Women’s and Children’s Central Hospital and colleagues explained in an article published in The Lancet Haematology.
The authors noted that close contact with COVID-19 patients is thought to be the main route of transmission, and a retrospective study indicated that 41.3% of initial COVID-19 cases were caused by hospital-related transmission.
“Children with hematological malignancies might have increased susceptibility to infection with SARS-CoV-2 because of immunodeficiency; therefore, procedures are needed to avoid hospital-related transmission and infection for these patients,” the authors wrote.
Preventing the spread of infection
Dr. He and colleagues advised that medical staff be kept up-to-date with the latest information about COVID-19 and perform assessments regularly to identify cases in their departments.
The authors also recommended establishing a COVID-19 expert committee – consisting of infectious disease physicians, hematologists, oncologists, radiologists, pharmacists, and hospital infection control staff – to make medical decisions in multidisciplinary consultation meetings. In addition, the authors recommended regional management strategies be adopted to minimize cross infection within the hospital. Specifically, the authors proposed creating the following four zones:
1. A surveillance and screening zone for patients potentially infected with SARS-CoV-2
2. A suspected-case quarantine zone where patients thought to have COVID-19 are isolated in single rooms
3. A confirmed-case quarantine zone where patients are treated for COVID-19
4. A hematology/oncology ward for treating non–COVID-19 patients with malignancies.
Dr. He and colleagues also stressed the importance of providing personal protective equipment for all zones, along with instructions for proper use and disposal. The authors recommended developing and following specific protocols for outpatient visits in the hematology/oncology ward, and providing COVID-19 prevention and control information to families and health care workers.
Managing cancer treatment
For patients with acute leukemias who have induction chemotherapy planned, Dr. He and colleagues argued that scheduled chemotherapy should not be interrupted unless COVID-19 is suspected or diagnosed. The authors said treatment should not be delayed more than 7 days during induction, consolidation, or the intermediate phase of chemotherapy because the virus has an incubation period of 2-7 days. This will allow a short period of observation to screen for potential infection.
The authors recommended that patients with lymphoma and solid tumors first undergo COVID-19 screening and then receive treatment in hematology/oncology wards “according to their chemotherapy schedule, and without delay, until they are in complete remission.”
“If the patient is in complete remission, we recommend a treatment delay of no more than 7 days to allow a short period of observation to screen for COVID-19,” the authors added.
Maintenance chemotherapy should not be delayed for more than 14 days, Dr. He and colleagues wrote. “This increase in the maximum delay before chemotherapy strikes a balance between the potential risk of SARS-CoV-2 infection and tumor recurrence, since pediatric patients in this phase of treatment have a reduced risk of tumor recurrence,” the authors added.
Caring for patients with COVID-19
For inpatients diagnosed with COVID-19, Dr. He and colleagues recommended the following:
- Prioritize COVID-19 treatment for children with primary disease remission.
- For children not in remission, prioritize treatment for critical patients.
- Isolated patients should be treated for COVID-19, and their chemotherapy should be temporarily suspended or reduced in intensity..
Dr. He and colleagues noted that, by following these recommendations for infection prevention, they had no cases of COVID-19 among children in their hematology/oncology departments. However, the authors said the recommendations “could fail to some extent” based on “differences in medical resources, health care settings, and the policy of the specific government.”
The authors said their recommendations should be updated continuously as new information and clinical evidence emerges.
Dr. He and colleagues reported having no conflicts of interest.
SOURCE: He Y et al. Lancet Haematol. doi: 10/1016/s2352-3026(20)30104-6.
Given the possibility that children with hematologic malignancies may have increased susceptibility to coronavirus disease 2019 (COVID-19), clinicians from China and the United States have proposed a plan for preventing and managing outbreaks in hospitals’ pediatric hematology and oncology departments.
The plan is focused primarily on infection prevention and control strategies, Yulei He, MD, of Chengdu (China) Women’s and Children’s Central Hospital and colleagues explained in an article published in The Lancet Haematology.
The authors noted that close contact with COVID-19 patients is thought to be the main route of transmission, and a retrospective study indicated that 41.3% of initial COVID-19 cases were caused by hospital-related transmission.
“Children with hematological malignancies might have increased susceptibility to infection with SARS-CoV-2 because of immunodeficiency; therefore, procedures are needed to avoid hospital-related transmission and infection for these patients,” the authors wrote.
Preventing the spread of infection
Dr. He and colleagues advised that medical staff be kept up-to-date with the latest information about COVID-19 and perform assessments regularly to identify cases in their departments.
The authors also recommended establishing a COVID-19 expert committee – consisting of infectious disease physicians, hematologists, oncologists, radiologists, pharmacists, and hospital infection control staff – to make medical decisions in multidisciplinary consultation meetings. In addition, the authors recommended regional management strategies be adopted to minimize cross infection within the hospital. Specifically, the authors proposed creating the following four zones:
1. A surveillance and screening zone for patients potentially infected with SARS-CoV-2
2. A suspected-case quarantine zone where patients thought to have COVID-19 are isolated in single rooms
3. A confirmed-case quarantine zone where patients are treated for COVID-19
4. A hematology/oncology ward for treating non–COVID-19 patients with malignancies.
Dr. He and colleagues also stressed the importance of providing personal protective equipment for all zones, along with instructions for proper use and disposal. The authors recommended developing and following specific protocols for outpatient visits in the hematology/oncology ward, and providing COVID-19 prevention and control information to families and health care workers.
Managing cancer treatment
For patients with acute leukemias who have induction chemotherapy planned, Dr. He and colleagues argued that scheduled chemotherapy should not be interrupted unless COVID-19 is suspected or diagnosed. The authors said treatment should not be delayed more than 7 days during induction, consolidation, or the intermediate phase of chemotherapy because the virus has an incubation period of 2-7 days. This will allow a short period of observation to screen for potential infection.
The authors recommended that patients with lymphoma and solid tumors first undergo COVID-19 screening and then receive treatment in hematology/oncology wards “according to their chemotherapy schedule, and without delay, until they are in complete remission.”
“If the patient is in complete remission, we recommend a treatment delay of no more than 7 days to allow a short period of observation to screen for COVID-19,” the authors added.
Maintenance chemotherapy should not be delayed for more than 14 days, Dr. He and colleagues wrote. “This increase in the maximum delay before chemotherapy strikes a balance between the potential risk of SARS-CoV-2 infection and tumor recurrence, since pediatric patients in this phase of treatment have a reduced risk of tumor recurrence,” the authors added.
Caring for patients with COVID-19
For inpatients diagnosed with COVID-19, Dr. He and colleagues recommended the following:
- Prioritize COVID-19 treatment for children with primary disease remission.
- For children not in remission, prioritize treatment for critical patients.
- Isolated patients should be treated for COVID-19, and their chemotherapy should be temporarily suspended or reduced in intensity..
Dr. He and colleagues noted that, by following these recommendations for infection prevention, they had no cases of COVID-19 among children in their hematology/oncology departments. However, the authors said the recommendations “could fail to some extent” based on “differences in medical resources, health care settings, and the policy of the specific government.”
The authors said their recommendations should be updated continuously as new information and clinical evidence emerges.
Dr. He and colleagues reported having no conflicts of interest.
SOURCE: He Y et al. Lancet Haematol. doi: 10/1016/s2352-3026(20)30104-6.
Given the possibility that children with hematologic malignancies may have increased susceptibility to coronavirus disease 2019 (COVID-19), clinicians from China and the United States have proposed a plan for preventing and managing outbreaks in hospitals’ pediatric hematology and oncology departments.
The plan is focused primarily on infection prevention and control strategies, Yulei He, MD, of Chengdu (China) Women’s and Children’s Central Hospital and colleagues explained in an article published in The Lancet Haematology.
The authors noted that close contact with COVID-19 patients is thought to be the main route of transmission, and a retrospective study indicated that 41.3% of initial COVID-19 cases were caused by hospital-related transmission.
“Children with hematological malignancies might have increased susceptibility to infection with SARS-CoV-2 because of immunodeficiency; therefore, procedures are needed to avoid hospital-related transmission and infection for these patients,” the authors wrote.
Preventing the spread of infection
Dr. He and colleagues advised that medical staff be kept up-to-date with the latest information about COVID-19 and perform assessments regularly to identify cases in their departments.
The authors also recommended establishing a COVID-19 expert committee – consisting of infectious disease physicians, hematologists, oncologists, radiologists, pharmacists, and hospital infection control staff – to make medical decisions in multidisciplinary consultation meetings. In addition, the authors recommended regional management strategies be adopted to minimize cross infection within the hospital. Specifically, the authors proposed creating the following four zones:
1. A surveillance and screening zone for patients potentially infected with SARS-CoV-2
2. A suspected-case quarantine zone where patients thought to have COVID-19 are isolated in single rooms
3. A confirmed-case quarantine zone where patients are treated for COVID-19
4. A hematology/oncology ward for treating non–COVID-19 patients with malignancies.
Dr. He and colleagues also stressed the importance of providing personal protective equipment for all zones, along with instructions for proper use and disposal. The authors recommended developing and following specific protocols for outpatient visits in the hematology/oncology ward, and providing COVID-19 prevention and control information to families and health care workers.
Managing cancer treatment
For patients with acute leukemias who have induction chemotherapy planned, Dr. He and colleagues argued that scheduled chemotherapy should not be interrupted unless COVID-19 is suspected or diagnosed. The authors said treatment should not be delayed more than 7 days during induction, consolidation, or the intermediate phase of chemotherapy because the virus has an incubation period of 2-7 days. This will allow a short period of observation to screen for potential infection.
The authors recommended that patients with lymphoma and solid tumors first undergo COVID-19 screening and then receive treatment in hematology/oncology wards “according to their chemotherapy schedule, and without delay, until they are in complete remission.”
“If the patient is in complete remission, we recommend a treatment delay of no more than 7 days to allow a short period of observation to screen for COVID-19,” the authors added.
Maintenance chemotherapy should not be delayed for more than 14 days, Dr. He and colleagues wrote. “This increase in the maximum delay before chemotherapy strikes a balance between the potential risk of SARS-CoV-2 infection and tumor recurrence, since pediatric patients in this phase of treatment have a reduced risk of tumor recurrence,” the authors added.
Caring for patients with COVID-19
For inpatients diagnosed with COVID-19, Dr. He and colleagues recommended the following:
- Prioritize COVID-19 treatment for children with primary disease remission.
- For children not in remission, prioritize treatment for critical patients.
- Isolated patients should be treated for COVID-19, and their chemotherapy should be temporarily suspended or reduced in intensity..
Dr. He and colleagues noted that, by following these recommendations for infection prevention, they had no cases of COVID-19 among children in their hematology/oncology departments. However, the authors said the recommendations “could fail to some extent” based on “differences in medical resources, health care settings, and the policy of the specific government.”
The authors said their recommendations should be updated continuously as new information and clinical evidence emerges.
Dr. He and colleagues reported having no conflicts of interest.
SOURCE: He Y et al. Lancet Haematol. doi: 10/1016/s2352-3026(20)30104-6.
FROM THE LANCET HAEMATOLOGY
COVID-19 and surge capacity in U.S. hospitals
Background
As of April 2020, the United States is faced with the early stages of the coronavirus disease 2019 (COVID-19) pandemic. Experts predict up to 60% of the population will become infected with a fatality rate of 1% and a hospitalization rate of approximately 20%. Efforts to suppress viral spread have been unsuccessful as cases are reported in all 50 states, and fatalities are rising. Currently many American hospitals are ill-prepared for a significant increase in their census of critically ill and contagious patients, i.e., hospitals lack adequate surge capacity to safely handle a nationwide outbreak of COVID-19. As seen in other nations such as Italy, China, and Iran, this leads to rationing of life-saving health care and potentially preventable morbidity and mortality.
Introduction
Hospitals will be unable to provide the current standard of care to patients as the rate of infection with coronavirus disease 2019 (COVID-19) escalates. As of April 9, the World Health Organization has confirmed 1,539,118 cases and 89,998 deaths globally; and the Centers for Disease Control and Prevention has confirmed 435,941 cases and 14,865 deaths in the United States.1,2 Experts predict up to 60% of the population will eventually become infected with a fatality rate of about 1% and a hospitalization rate of approximately 20%.3,4
In the United States, with a population of 300 million people, this represents up to 180 million infected, 36 million requiring hospitalization, 11 million requiring intensive care, and 2 million fatalities over the duration of the pandemic. On March 13, President Donald Trump declared a state of national emergency, authorizing $50 billion dollars in emergency health care spending as well as asking every hospital in the country to immediately activate its emergency response plan. The use of isolation and quarantine may space out casualties over time, however high rates and volumes of hospitalizations are still expected.4,5
As the influx of patients afflicted with COVID-19 grows, needs will outstrip hospital resources forcing clinicians to ration beds and supplies. In Italy, China, and Iran, physicians are already faced with these difficult decisions. Antonio Pesenti, head of the Italian Lombardy regional crisis response unit, characterized the change in health care delivery: “We’re now being forced to set up intensive care treatment in corridors, in operating theaters, in recovery rooms. We’ve emptied entire hospital sections to make space for seriously sick people.”6
Surge capacity
Surge capacity is a hospital’s ability to adequately care for a significant influx of patients.7 Since 2011, the American College of Emergency Physicians has published guidelines calling for hospitals to have a surge capacity accounting for infectious disease outbreaks, and demands on supplies, personnel, and physical space.7 Even prior to the development of COVID-19, many hospitals faced emergency department crowding and strains on hospital capacity.8 The Organization for Economic Co-operation and Development (OECD) estimates hospital beds per 1,000 inhabitants at 2.77 for the USA, 3.18 for Italy, 4.34 for China, and 13.05 for Japan.9 Before COVID-19 many American hospitals had an insufficient number of beds. Now, in the initial phase of the pandemic, it is even more important to optimize surge capacity across the American health care system.
Requirements for COVID-19 preparation
To prepare for the increased number of seriously and critically ill patients, individual hospitals and regions must perform a needs assessment. The fundamental disease process of COVID-19 is a contagious viral pneumonia; treatment hinges on four major categories of intervention: spatial isolation (including physical space, beds, partitions, droplet precautions, food, water, and sanitation), oxygenation (including wall and portable oxygen, nasal canulae, and masks), mechanical ventilation (including ventilator machines, tubing, anesthetics, and reliable electrical power) and personnel (including physicians, nurses, technicians, and adequate personal protective equipment).10 In special circumstances and where available, extra corporeal membrane oxygenation may be considered.10 The necessary interventions are summarized in Table 1. 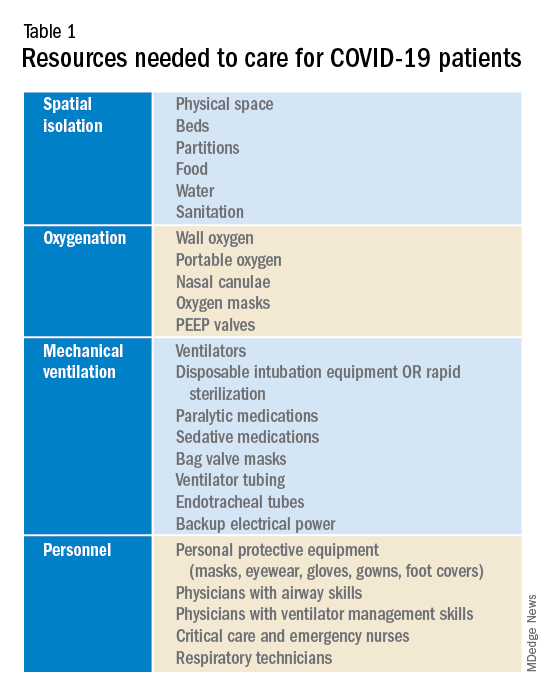
Emergency, critical care, nursing, and medical leadership should consider what sort of space, personnel, and supplies will be needed to care for a large volume of patients with contagious viral pneumonia at the same time as other hospital patients. Attention should also be given to potential need for morgue expansion. Hospitals must be proactive in procuring supplies and preparing for demands on beds and physical space. Specifically, logistics coordinators should start stockpiling ventilators, oxygen, respiratory equipment, and personal protective equipment. Reallocating supplies from other regions of the hospital such as operating rooms and ambulatory surgery centers may be considered. These resources, particularly ventilators and ventilator supplies, are already in disturbingly limited supply, and they are likely to be single most important limiting factor for survival rates. To prevent regional shortages, stockpiling efforts should ideally be aided by state and federal governments. The production and acquisition of ventilators should be immediately and significantly increased.
Hospitals must additionally prepare for demands for physical space and beds. Techniques to maximize space and bed availability (see Table 2) include discharging patients who do not require hospitalization, and canceling elective procedures and admissions. Additional methods would be to utilize unconventional preexisting spaces such as hallways, operating rooms, recovery rooms, hallways, closed hospital wards, basements, lobbies, cafeterias, and parking lots. Administrators should also consider establishing field hospitals or field wards, such as tents in open spaces and nearby roads. Medical care performed in unconventional environments will need to account for electricity, temperature control, oxygen delivery, and sanitation.
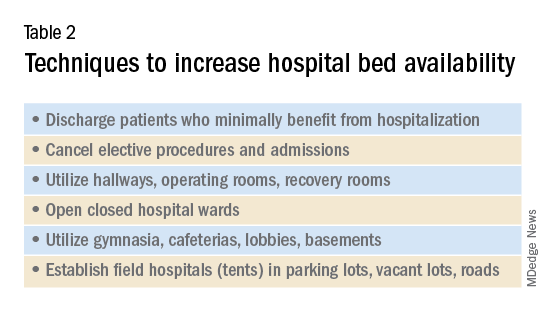
Conclusion
To minimize unnecessary loss of life and suffering, hospitals must expand their surge capacities in preparation for the predictable rise in demand for health care resources related to COVID-19. Numerous hospitals, particularly those that serve low-income and underserved communities, operate with a narrow financial margin.11 Independently preparing for the surge capacity needed to face COVID-19 may be infeasible for several hospitals. As a result, many health care systems will rely on government aid during this period for financial and material support. To maximize preparedness and response, hospitals should ask for and receive aid from the Federal Emergency Management Agency (FEMA), American Red Cross, state governments, and the military; these resources should be mobilized now.
Dr. Blumenberg, Dr. Noble, and Dr. Hendrickson are based in the department of emergency medicine & toxicology, Oregon Health and Science University, Portland.
References
1. Coronavirus disease 2019 (COVID-19) situation report – 60. 2020 Mar 19.
2. Coronavirus disease 2019 (COVID-19) Cases in the U.S. CDC. 2020 Apr 8.
3. Li Q et al. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med. 2020 Jan. doi: 10.1056/NEJMoa2001316.
4. Anderson RM et al. How will country-based mitigation measures influence the course of the COVID-19 epidemic? Lancet. 2020 Mar. doi: 10.1016/S0140-6736(20)30567-5.
5. Fraser C et al. Factors that make an infectious disease outbreak controllable. Proc Natl Acad Sci U S A. 2004;101(16):6146-51. doi: 10.1073/pnas.0307506101.
6. Mackenzie J and Balmer C. Italy locks down millions as its coronavirus deaths jump. Reuters. 2020 Mar 9.
7. Health care system surge capacity recognition, preparedness, and response. Ann Emerg Med. 2012;59(3):240-1. doi: 10.1016/j.annemergmed.2011.11.030.
8. Pitts SR et al. A cross-sectional study of emergency department boarding practices in the United States. Acad Emerg Med. 2014;21(5):497-503. doi: 10.1111/acem.12375.
9. Health at a Glance 2019. OECD; 2019. doi: 10.1787/4dd50c09-en.
10. Murthy S et al. Care for critically ill patients with COVID-19. JAMA. 2020 Mar. doi: 10.1001/jama.2020.3633.
11. Ly DP et al. The association between hospital margins, quality of care, and closure or other change in operating status. J Gen Intern Med. 2011;26(11):1291-6. doi: 10.1007/s11606-011-1815-5.
Background
As of April 2020, the United States is faced with the early stages of the coronavirus disease 2019 (COVID-19) pandemic. Experts predict up to 60% of the population will become infected with a fatality rate of 1% and a hospitalization rate of approximately 20%. Efforts to suppress viral spread have been unsuccessful as cases are reported in all 50 states, and fatalities are rising. Currently many American hospitals are ill-prepared for a significant increase in their census of critically ill and contagious patients, i.e., hospitals lack adequate surge capacity to safely handle a nationwide outbreak of COVID-19. As seen in other nations such as Italy, China, and Iran, this leads to rationing of life-saving health care and potentially preventable morbidity and mortality.
Introduction
Hospitals will be unable to provide the current standard of care to patients as the rate of infection with coronavirus disease 2019 (COVID-19) escalates. As of April 9, the World Health Organization has confirmed 1,539,118 cases and 89,998 deaths globally; and the Centers for Disease Control and Prevention has confirmed 435,941 cases and 14,865 deaths in the United States.1,2 Experts predict up to 60% of the population will eventually become infected with a fatality rate of about 1% and a hospitalization rate of approximately 20%.3,4
In the United States, with a population of 300 million people, this represents up to 180 million infected, 36 million requiring hospitalization, 11 million requiring intensive care, and 2 million fatalities over the duration of the pandemic. On March 13, President Donald Trump declared a state of national emergency, authorizing $50 billion dollars in emergency health care spending as well as asking every hospital in the country to immediately activate its emergency response plan. The use of isolation and quarantine may space out casualties over time, however high rates and volumes of hospitalizations are still expected.4,5
As the influx of patients afflicted with COVID-19 grows, needs will outstrip hospital resources forcing clinicians to ration beds and supplies. In Italy, China, and Iran, physicians are already faced with these difficult decisions. Antonio Pesenti, head of the Italian Lombardy regional crisis response unit, characterized the change in health care delivery: “We’re now being forced to set up intensive care treatment in corridors, in operating theaters, in recovery rooms. We’ve emptied entire hospital sections to make space for seriously sick people.”6
Surge capacity
Surge capacity is a hospital’s ability to adequately care for a significant influx of patients.7 Since 2011, the American College of Emergency Physicians has published guidelines calling for hospitals to have a surge capacity accounting for infectious disease outbreaks, and demands on supplies, personnel, and physical space.7 Even prior to the development of COVID-19, many hospitals faced emergency department crowding and strains on hospital capacity.8 The Organization for Economic Co-operation and Development (OECD) estimates hospital beds per 1,000 inhabitants at 2.77 for the USA, 3.18 for Italy, 4.34 for China, and 13.05 for Japan.9 Before COVID-19 many American hospitals had an insufficient number of beds. Now, in the initial phase of the pandemic, it is even more important to optimize surge capacity across the American health care system.
Requirements for COVID-19 preparation
To prepare for the increased number of seriously and critically ill patients, individual hospitals and regions must perform a needs assessment. The fundamental disease process of COVID-19 is a contagious viral pneumonia; treatment hinges on four major categories of intervention: spatial isolation (including physical space, beds, partitions, droplet precautions, food, water, and sanitation), oxygenation (including wall and portable oxygen, nasal canulae, and masks), mechanical ventilation (including ventilator machines, tubing, anesthetics, and reliable electrical power) and personnel (including physicians, nurses, technicians, and adequate personal protective equipment).10 In special circumstances and where available, extra corporeal membrane oxygenation may be considered.10 The necessary interventions are summarized in Table 1. 
Emergency, critical care, nursing, and medical leadership should consider what sort of space, personnel, and supplies will be needed to care for a large volume of patients with contagious viral pneumonia at the same time as other hospital patients. Attention should also be given to potential need for morgue expansion. Hospitals must be proactive in procuring supplies and preparing for demands on beds and physical space. Specifically, logistics coordinators should start stockpiling ventilators, oxygen, respiratory equipment, and personal protective equipment. Reallocating supplies from other regions of the hospital such as operating rooms and ambulatory surgery centers may be considered. These resources, particularly ventilators and ventilator supplies, are already in disturbingly limited supply, and they are likely to be single most important limiting factor for survival rates. To prevent regional shortages, stockpiling efforts should ideally be aided by state and federal governments. The production and acquisition of ventilators should be immediately and significantly increased.
Hospitals must additionally prepare for demands for physical space and beds. Techniques to maximize space and bed availability (see Table 2) include discharging patients who do not require hospitalization, and canceling elective procedures and admissions. Additional methods would be to utilize unconventional preexisting spaces such as hallways, operating rooms, recovery rooms, hallways, closed hospital wards, basements, lobbies, cafeterias, and parking lots. Administrators should also consider establishing field hospitals or field wards, such as tents in open spaces and nearby roads. Medical care performed in unconventional environments will need to account for electricity, temperature control, oxygen delivery, and sanitation.

Conclusion
To minimize unnecessary loss of life and suffering, hospitals must expand their surge capacities in preparation for the predictable rise in demand for health care resources related to COVID-19. Numerous hospitals, particularly those that serve low-income and underserved communities, operate with a narrow financial margin.11 Independently preparing for the surge capacity needed to face COVID-19 may be infeasible for several hospitals. As a result, many health care systems will rely on government aid during this period for financial and material support. To maximize preparedness and response, hospitals should ask for and receive aid from the Federal Emergency Management Agency (FEMA), American Red Cross, state governments, and the military; these resources should be mobilized now.
Dr. Blumenberg, Dr. Noble, and Dr. Hendrickson are based in the department of emergency medicine & toxicology, Oregon Health and Science University, Portland.
References
1. Coronavirus disease 2019 (COVID-19) situation report – 60. 2020 Mar 19.
2. Coronavirus disease 2019 (COVID-19) Cases in the U.S. CDC. 2020 Apr 8.
3. Li Q et al. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med. 2020 Jan. doi: 10.1056/NEJMoa2001316.
4. Anderson RM et al. How will country-based mitigation measures influence the course of the COVID-19 epidemic? Lancet. 2020 Mar. doi: 10.1016/S0140-6736(20)30567-5.
5. Fraser C et al. Factors that make an infectious disease outbreak controllable. Proc Natl Acad Sci U S A. 2004;101(16):6146-51. doi: 10.1073/pnas.0307506101.
6. Mackenzie J and Balmer C. Italy locks down millions as its coronavirus deaths jump. Reuters. 2020 Mar 9.
7. Health care system surge capacity recognition, preparedness, and response. Ann Emerg Med. 2012;59(3):240-1. doi: 10.1016/j.annemergmed.2011.11.030.
8. Pitts SR et al. A cross-sectional study of emergency department boarding practices in the United States. Acad Emerg Med. 2014;21(5):497-503. doi: 10.1111/acem.12375.
9. Health at a Glance 2019. OECD; 2019. doi: 10.1787/4dd50c09-en.
10. Murthy S et al. Care for critically ill patients with COVID-19. JAMA. 2020 Mar. doi: 10.1001/jama.2020.3633.
11. Ly DP et al. The association between hospital margins, quality of care, and closure or other change in operating status. J Gen Intern Med. 2011;26(11):1291-6. doi: 10.1007/s11606-011-1815-5.
Background
As of April 2020, the United States is faced with the early stages of the coronavirus disease 2019 (COVID-19) pandemic. Experts predict up to 60% of the population will become infected with a fatality rate of 1% and a hospitalization rate of approximately 20%. Efforts to suppress viral spread have been unsuccessful as cases are reported in all 50 states, and fatalities are rising. Currently many American hospitals are ill-prepared for a significant increase in their census of critically ill and contagious patients, i.e., hospitals lack adequate surge capacity to safely handle a nationwide outbreak of COVID-19. As seen in other nations such as Italy, China, and Iran, this leads to rationing of life-saving health care and potentially preventable morbidity and mortality.
Introduction
Hospitals will be unable to provide the current standard of care to patients as the rate of infection with coronavirus disease 2019 (COVID-19) escalates. As of April 9, the World Health Organization has confirmed 1,539,118 cases and 89,998 deaths globally; and the Centers for Disease Control and Prevention has confirmed 435,941 cases and 14,865 deaths in the United States.1,2 Experts predict up to 60% of the population will eventually become infected with a fatality rate of about 1% and a hospitalization rate of approximately 20%.3,4
In the United States, with a population of 300 million people, this represents up to 180 million infected, 36 million requiring hospitalization, 11 million requiring intensive care, and 2 million fatalities over the duration of the pandemic. On March 13, President Donald Trump declared a state of national emergency, authorizing $50 billion dollars in emergency health care spending as well as asking every hospital in the country to immediately activate its emergency response plan. The use of isolation and quarantine may space out casualties over time, however high rates and volumes of hospitalizations are still expected.4,5
As the influx of patients afflicted with COVID-19 grows, needs will outstrip hospital resources forcing clinicians to ration beds and supplies. In Italy, China, and Iran, physicians are already faced with these difficult decisions. Antonio Pesenti, head of the Italian Lombardy regional crisis response unit, characterized the change in health care delivery: “We’re now being forced to set up intensive care treatment in corridors, in operating theaters, in recovery rooms. We’ve emptied entire hospital sections to make space for seriously sick people.”6
Surge capacity
Surge capacity is a hospital’s ability to adequately care for a significant influx of patients.7 Since 2011, the American College of Emergency Physicians has published guidelines calling for hospitals to have a surge capacity accounting for infectious disease outbreaks, and demands on supplies, personnel, and physical space.7 Even prior to the development of COVID-19, many hospitals faced emergency department crowding and strains on hospital capacity.8 The Organization for Economic Co-operation and Development (OECD) estimates hospital beds per 1,000 inhabitants at 2.77 for the USA, 3.18 for Italy, 4.34 for China, and 13.05 for Japan.9 Before COVID-19 many American hospitals had an insufficient number of beds. Now, in the initial phase of the pandemic, it is even more important to optimize surge capacity across the American health care system.
Requirements for COVID-19 preparation
To prepare for the increased number of seriously and critically ill patients, individual hospitals and regions must perform a needs assessment. The fundamental disease process of COVID-19 is a contagious viral pneumonia; treatment hinges on four major categories of intervention: spatial isolation (including physical space, beds, partitions, droplet precautions, food, water, and sanitation), oxygenation (including wall and portable oxygen, nasal canulae, and masks), mechanical ventilation (including ventilator machines, tubing, anesthetics, and reliable electrical power) and personnel (including physicians, nurses, technicians, and adequate personal protective equipment).10 In special circumstances and where available, extra corporeal membrane oxygenation may be considered.10 The necessary interventions are summarized in Table 1. 
Emergency, critical care, nursing, and medical leadership should consider what sort of space, personnel, and supplies will be needed to care for a large volume of patients with contagious viral pneumonia at the same time as other hospital patients. Attention should also be given to potential need for morgue expansion. Hospitals must be proactive in procuring supplies and preparing for demands on beds and physical space. Specifically, logistics coordinators should start stockpiling ventilators, oxygen, respiratory equipment, and personal protective equipment. Reallocating supplies from other regions of the hospital such as operating rooms and ambulatory surgery centers may be considered. These resources, particularly ventilators and ventilator supplies, are already in disturbingly limited supply, and they are likely to be single most important limiting factor for survival rates. To prevent regional shortages, stockpiling efforts should ideally be aided by state and federal governments. The production and acquisition of ventilators should be immediately and significantly increased.
Hospitals must additionally prepare for demands for physical space and beds. Techniques to maximize space and bed availability (see Table 2) include discharging patients who do not require hospitalization, and canceling elective procedures and admissions. Additional methods would be to utilize unconventional preexisting spaces such as hallways, operating rooms, recovery rooms, hallways, closed hospital wards, basements, lobbies, cafeterias, and parking lots. Administrators should also consider establishing field hospitals or field wards, such as tents in open spaces and nearby roads. Medical care performed in unconventional environments will need to account for electricity, temperature control, oxygen delivery, and sanitation.

Conclusion
To minimize unnecessary loss of life and suffering, hospitals must expand their surge capacities in preparation for the predictable rise in demand for health care resources related to COVID-19. Numerous hospitals, particularly those that serve low-income and underserved communities, operate with a narrow financial margin.11 Independently preparing for the surge capacity needed to face COVID-19 may be infeasible for several hospitals. As a result, many health care systems will rely on government aid during this period for financial and material support. To maximize preparedness and response, hospitals should ask for and receive aid from the Federal Emergency Management Agency (FEMA), American Red Cross, state governments, and the military; these resources should be mobilized now.
Dr. Blumenberg, Dr. Noble, and Dr. Hendrickson are based in the department of emergency medicine & toxicology, Oregon Health and Science University, Portland.
References
1. Coronavirus disease 2019 (COVID-19) situation report – 60. 2020 Mar 19.
2. Coronavirus disease 2019 (COVID-19) Cases in the U.S. CDC. 2020 Apr 8.
3. Li Q et al. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med. 2020 Jan. doi: 10.1056/NEJMoa2001316.
4. Anderson RM et al. How will country-based mitigation measures influence the course of the COVID-19 epidemic? Lancet. 2020 Mar. doi: 10.1016/S0140-6736(20)30567-5.
5. Fraser C et al. Factors that make an infectious disease outbreak controllable. Proc Natl Acad Sci U S A. 2004;101(16):6146-51. doi: 10.1073/pnas.0307506101.
6. Mackenzie J and Balmer C. Italy locks down millions as its coronavirus deaths jump. Reuters. 2020 Mar 9.
7. Health care system surge capacity recognition, preparedness, and response. Ann Emerg Med. 2012;59(3):240-1. doi: 10.1016/j.annemergmed.2011.11.030.
8. Pitts SR et al. A cross-sectional study of emergency department boarding practices in the United States. Acad Emerg Med. 2014;21(5):497-503. doi: 10.1111/acem.12375.
9. Health at a Glance 2019. OECD; 2019. doi: 10.1787/4dd50c09-en.
10. Murthy S et al. Care for critically ill patients with COVID-19. JAMA. 2020 Mar. doi: 10.1001/jama.2020.3633.
11. Ly DP et al. The association between hospital margins, quality of care, and closure or other change in operating status. J Gen Intern Med. 2011;26(11):1291-6. doi: 10.1007/s11606-011-1815-5.






















