User login
Low-risk TAVR loses ground at 2 years in PARTNER 3
Transcatheter aortic valve replacement (TAVR) continued to show superiority over surgical replacement in terms of the primary composite endpoint in low-surgical-risk patients at 2 years of follow-up in the landmark randomized PARTNER 3 trial, but the between-group differences favoring the transcatheter procedure in some key outcomes have narrowed considerably, Michael J. Mack, MD, reported in a video presentation of his research during the joint scientific sessions of the American College of Cardiology and the World Heart Federation, which was presented online this year. ACC organizers chose to present parts of the meeting virtually after COVID-19 concerns caused them to cancel the meeting.
“On the basis of 1-year data, many physicians were counseling patients that TAVR outcomes were better than surgery. Now we see that the outcomes are roughly the same at 2 years,” said Dr. Mack, who is medical director of cardiothoracic surgery and chairman of the Baylor Scott & White The Heart Hospital – Plano (Tex.) Research Center.
PARTNER 3 randomized 1,000 patients with severe symptomatic aortic stenosis with a tricuspid valve and a very low mean Society of Thoracic Surgeons risk score of 1.9% to TAVR with the Sapien 3 valve or surgical aortic valve replacement (SAVR). The 1-year results presented at ACC 2019 caused a huge stir, with the primary composite outcome of death, stroke, or cardiovascular rehospitalization occurring in 8.5% of TAVR patients and 15.6% of the SAVR group, representing a 48% relative risk reduction and a resounding win for TAVR (N Engl J Med. 2019 May 2;380:1695-705). At 2 years, the difference in the composite outcome remained statistically significant, but the gap had closed: 11.5% with TAVR and 17.4% with SAVR for a 37% relative risk reduction.
Moreover, the between-group difference in stroke, which at 1 year was significantly in favor of TAVR at 1.2% versus 3.3%, was no longer significant at 2 years, with rates of 2.4% versus 3.6%. Nor was the difference in mortality significant: 2.4% with TAVR, 3.2% with SAVR.
What was a statistically significant between-group difference at 2 years – and an eye-catching one at that – involved the cumulative incidence of valve thrombosis confirmed by CT or echocardiography: 2.6% in the TAVR arm, compared with 0.7% with SAVR, with most of these unwanted events coming in year 2.
The good news was there was no echocardiographic evidence of deterioration in valve structure or function in either study arm at 2 years. The mean gradients and aortic valve areas remained unchanged in both arms between 1 and 2 years, as did the frequency of mild or moderate paravalvular leak. Prospective follow-up will continue annually out to 10 years.
“I think it’s way too early to expect to see a signal, but I think it’s somewhat comforting at this point that there’s no signal of early structural valve deterioration,” Dr. Mack said.
Discussant Howard C. Hermann, MD, commented: “I guess the biggest concern in looking at the data is the increase in stroke and valve thrombosis, both numerically and relative to SAVR, between years 1 and 2.”
Dr. Mack offered a note of reassurance regarding the valve thrombosis findings: The rates he presented were based upon the now-outdated second Valve Academic Research Consortium (VARC-2) definition, per study protocol. When he and his coinvestigators recalculated the valve thrombosis rates using the contemporary VARC-3 definition of valve deterioration and bioprosthetic valve failure, the incidence was very low and not significantly different in the two study arms, at roughly 1%.
Dr. Hermann, professor of medicine and director of the cardiac catheterization laboratories at the University of Pennsylvania, Philadelphia, had a question: As a clinician taking care of TAVR patients, what clinical or hemodynamic findings should prompt an imaging study looking for valve thrombus or deterioration that might prompt initiating oral anticoagulation?
“If there’s a change in hemodynamics, an increasing valve gradient, if there’s increasing paravalvular leak, or if there’s a change in symptoms, that should prompt an imaging study. Only with confirmation of valve thrombosis on an imaging study should anticoagulation be considered. Oral anticoagulation is not benign: Of the six clinical events associated with valve thrombosis in the study, two were related to anticoagulation,” Dr. Mack replied.
“Regarding whether patients should receive warfarin or a novel anticoagulant, I don’t think we have evidence that there’s benefit to anything other than warfarin at the current time,” he added.
Dr. Mack reported receiving research support from Edwards Lifesciences, the sponsor of PARTNER 3, as well as from Abbott, Gore, and Medtronic.
Transcatheter aortic valve replacement (TAVR) continued to show superiority over surgical replacement in terms of the primary composite endpoint in low-surgical-risk patients at 2 years of follow-up in the landmark randomized PARTNER 3 trial, but the between-group differences favoring the transcatheter procedure in some key outcomes have narrowed considerably, Michael J. Mack, MD, reported in a video presentation of his research during the joint scientific sessions of the American College of Cardiology and the World Heart Federation, which was presented online this year. ACC organizers chose to present parts of the meeting virtually after COVID-19 concerns caused them to cancel the meeting.
“On the basis of 1-year data, many physicians were counseling patients that TAVR outcomes were better than surgery. Now we see that the outcomes are roughly the same at 2 years,” said Dr. Mack, who is medical director of cardiothoracic surgery and chairman of the Baylor Scott & White The Heart Hospital – Plano (Tex.) Research Center.
PARTNER 3 randomized 1,000 patients with severe symptomatic aortic stenosis with a tricuspid valve and a very low mean Society of Thoracic Surgeons risk score of 1.9% to TAVR with the Sapien 3 valve or surgical aortic valve replacement (SAVR). The 1-year results presented at ACC 2019 caused a huge stir, with the primary composite outcome of death, stroke, or cardiovascular rehospitalization occurring in 8.5% of TAVR patients and 15.6% of the SAVR group, representing a 48% relative risk reduction and a resounding win for TAVR (N Engl J Med. 2019 May 2;380:1695-705). At 2 years, the difference in the composite outcome remained statistically significant, but the gap had closed: 11.5% with TAVR and 17.4% with SAVR for a 37% relative risk reduction.
Moreover, the between-group difference in stroke, which at 1 year was significantly in favor of TAVR at 1.2% versus 3.3%, was no longer significant at 2 years, with rates of 2.4% versus 3.6%. Nor was the difference in mortality significant: 2.4% with TAVR, 3.2% with SAVR.
What was a statistically significant between-group difference at 2 years – and an eye-catching one at that – involved the cumulative incidence of valve thrombosis confirmed by CT or echocardiography: 2.6% in the TAVR arm, compared with 0.7% with SAVR, with most of these unwanted events coming in year 2.
The good news was there was no echocardiographic evidence of deterioration in valve structure or function in either study arm at 2 years. The mean gradients and aortic valve areas remained unchanged in both arms between 1 and 2 years, as did the frequency of mild or moderate paravalvular leak. Prospective follow-up will continue annually out to 10 years.
“I think it’s way too early to expect to see a signal, but I think it’s somewhat comforting at this point that there’s no signal of early structural valve deterioration,” Dr. Mack said.
Discussant Howard C. Hermann, MD, commented: “I guess the biggest concern in looking at the data is the increase in stroke and valve thrombosis, both numerically and relative to SAVR, between years 1 and 2.”
Dr. Mack offered a note of reassurance regarding the valve thrombosis findings: The rates he presented were based upon the now-outdated second Valve Academic Research Consortium (VARC-2) definition, per study protocol. When he and his coinvestigators recalculated the valve thrombosis rates using the contemporary VARC-3 definition of valve deterioration and bioprosthetic valve failure, the incidence was very low and not significantly different in the two study arms, at roughly 1%.
Dr. Hermann, professor of medicine and director of the cardiac catheterization laboratories at the University of Pennsylvania, Philadelphia, had a question: As a clinician taking care of TAVR patients, what clinical or hemodynamic findings should prompt an imaging study looking for valve thrombus or deterioration that might prompt initiating oral anticoagulation?
“If there’s a change in hemodynamics, an increasing valve gradient, if there’s increasing paravalvular leak, or if there’s a change in symptoms, that should prompt an imaging study. Only with confirmation of valve thrombosis on an imaging study should anticoagulation be considered. Oral anticoagulation is not benign: Of the six clinical events associated with valve thrombosis in the study, two were related to anticoagulation,” Dr. Mack replied.
“Regarding whether patients should receive warfarin or a novel anticoagulant, I don’t think we have evidence that there’s benefit to anything other than warfarin at the current time,” he added.
Dr. Mack reported receiving research support from Edwards Lifesciences, the sponsor of PARTNER 3, as well as from Abbott, Gore, and Medtronic.
Transcatheter aortic valve replacement (TAVR) continued to show superiority over surgical replacement in terms of the primary composite endpoint in low-surgical-risk patients at 2 years of follow-up in the landmark randomized PARTNER 3 trial, but the between-group differences favoring the transcatheter procedure in some key outcomes have narrowed considerably, Michael J. Mack, MD, reported in a video presentation of his research during the joint scientific sessions of the American College of Cardiology and the World Heart Federation, which was presented online this year. ACC organizers chose to present parts of the meeting virtually after COVID-19 concerns caused them to cancel the meeting.
“On the basis of 1-year data, many physicians were counseling patients that TAVR outcomes were better than surgery. Now we see that the outcomes are roughly the same at 2 years,” said Dr. Mack, who is medical director of cardiothoracic surgery and chairman of the Baylor Scott & White The Heart Hospital – Plano (Tex.) Research Center.
PARTNER 3 randomized 1,000 patients with severe symptomatic aortic stenosis with a tricuspid valve and a very low mean Society of Thoracic Surgeons risk score of 1.9% to TAVR with the Sapien 3 valve or surgical aortic valve replacement (SAVR). The 1-year results presented at ACC 2019 caused a huge stir, with the primary composite outcome of death, stroke, or cardiovascular rehospitalization occurring in 8.5% of TAVR patients and 15.6% of the SAVR group, representing a 48% relative risk reduction and a resounding win for TAVR (N Engl J Med. 2019 May 2;380:1695-705). At 2 years, the difference in the composite outcome remained statistically significant, but the gap had closed: 11.5% with TAVR and 17.4% with SAVR for a 37% relative risk reduction.
Moreover, the between-group difference in stroke, which at 1 year was significantly in favor of TAVR at 1.2% versus 3.3%, was no longer significant at 2 years, with rates of 2.4% versus 3.6%. Nor was the difference in mortality significant: 2.4% with TAVR, 3.2% with SAVR.
What was a statistically significant between-group difference at 2 years – and an eye-catching one at that – involved the cumulative incidence of valve thrombosis confirmed by CT or echocardiography: 2.6% in the TAVR arm, compared with 0.7% with SAVR, with most of these unwanted events coming in year 2.
The good news was there was no echocardiographic evidence of deterioration in valve structure or function in either study arm at 2 years. The mean gradients and aortic valve areas remained unchanged in both arms between 1 and 2 years, as did the frequency of mild or moderate paravalvular leak. Prospective follow-up will continue annually out to 10 years.
“I think it’s way too early to expect to see a signal, but I think it’s somewhat comforting at this point that there’s no signal of early structural valve deterioration,” Dr. Mack said.
Discussant Howard C. Hermann, MD, commented: “I guess the biggest concern in looking at the data is the increase in stroke and valve thrombosis, both numerically and relative to SAVR, between years 1 and 2.”
Dr. Mack offered a note of reassurance regarding the valve thrombosis findings: The rates he presented were based upon the now-outdated second Valve Academic Research Consortium (VARC-2) definition, per study protocol. When he and his coinvestigators recalculated the valve thrombosis rates using the contemporary VARC-3 definition of valve deterioration and bioprosthetic valve failure, the incidence was very low and not significantly different in the two study arms, at roughly 1%.
Dr. Hermann, professor of medicine and director of the cardiac catheterization laboratories at the University of Pennsylvania, Philadelphia, had a question: As a clinician taking care of TAVR patients, what clinical or hemodynamic findings should prompt an imaging study looking for valve thrombus or deterioration that might prompt initiating oral anticoagulation?
“If there’s a change in hemodynamics, an increasing valve gradient, if there’s increasing paravalvular leak, or if there’s a change in symptoms, that should prompt an imaging study. Only with confirmation of valve thrombosis on an imaging study should anticoagulation be considered. Oral anticoagulation is not benign: Of the six clinical events associated with valve thrombosis in the study, two were related to anticoagulation,” Dr. Mack replied.
“Regarding whether patients should receive warfarin or a novel anticoagulant, I don’t think we have evidence that there’s benefit to anything other than warfarin at the current time,” he added.
Dr. Mack reported receiving research support from Edwards Lifesciences, the sponsor of PARTNER 3, as well as from Abbott, Gore, and Medtronic.
FROM ACC 2020
Fellowship Burnout: What can we do to identify those at risk and minimize the impact?
Jeff is a high-performing first-year gastroenterology fellow who started with eagerness and enthusiasm. He seemed to enjoy talking to patients, wrote thorough notes, and often participated during case discussions at morning report. He initiated a quality improvement project and joined a hospital committee. Over the past few months, he has interacted less with his peers in the fellow’s office and stayed late to complete his patient encounters. He now frequently arrives late to work, is unprepared for rounds, and forgets to place important orders. One day, you notice him shuffling through several papers when the attending asks him a question about his patient. Later that day, he snapped at a nurse who paged to ask a question about a patient who just had a colonoscopy. When you ask him how he is doing, he becomes tearful and reports that he is under a lot of stress between work and home and does not feel the work he is doing is meaningful.
Introduction
The above scenario is all too familiar. Gastroenterology training can be a stressful period in an individual’s life. Long hours, steep learning curves for new cognitive and mechanical skill sets, as well as managing personal relationships and responsibilities at home all contribute to the stress of training and finding appropriate work-life balance. These stressors can result in burnout. The last decade has brought about a renewed emphasis on mitigating the impact of occupational burnout and improving trainee lifestyle through interventions such as work-hour restrictions, resiliency training, instruction on the importance of sleep, and team-building activities.
The problem
The World Health Organization (WHO) defines occupational burnout as chronic work-related stress, which may be characterized by feelings of energy depletion, mental distance from one’s job or feelings of negativity toward it, and reduced professional efficacy. Occupational burnout has been identified as an increasing problem both in practicing providers and trainees. Surveys in gastroenterologists show rates of burnout ranging between 37% and 50%,1 with trainees and early-career physicians disproportionately affected.1,2Physicians along the entire training spectrum are more likely to report high emotional exhaustion, high depersonalization, and burnout than a population control sample.2
Several individual factors identified for those at increased risk for burnout include younger age, not being married, and being male.2 Individuals spending less than 20% of their time working on activities they find meaningful and productive were more likely to show evidence of burnout.1
Symptoms of burnout can have a profound impact on trainees’ work performance, personal interactions, and the learning environment as a whole. The Accreditation Council for Graduate Medical Education (ACGME) annual survey of trainees asks them how strongly they agree or disagree on various components of burnout such as how meaningful they find their work, if they have enough time to think and reflect, if they feel emotionally drained at work, and if they feel worn out and weary after work. The intent of these questions is to provide anonymous feedback to training programs to help identify year to year trends and intervene early to prevent occupational burnout from becoming an increasing issue.
The solution
Considerations for any intervention should take several factors into account: the impact it may have on training and the development of a competent physician in their individualized specialty, the sustainability of the intervention, and whether it is something that will be accepted by the invested parties.

One method proposed for preventing burnout during fellowship has been designated as the three R’s: relaxation, reflection, and regrouping.3
- Relax. In order to relax, trainees need ways to decompress. Activities such as exercise and social events can be helpful. Within our own program the fellows have started their own group exercise program, playing wallyball weekly before clinical duties. We also encourage use of vacation days and build comradery by organizing potluck dinners for major holidays, graduation parties at the program director’s house, and an end-of-the-year golf outing in which trainees play against staff followed by a discussion regarding the state of the program. More recently we have added one half-day per a quarter for morale and team building. During this first year, the activities in which trainees have collectively decided to participate include an escape room, a rock-climbing facility, and laser tag. The addition of more team-building days has been well received by our program’s trainees and the simple addition of these team-building days has resulted in the trainees interacting more together outside of work, particularly in the form of group dinners.

Walter Reed National Military Medical Center fellows gathering for wallyball. - Reflect. They describe reflection as a necessary checkpoint which typically occurs every 6 months.3 These “checkpoints” provide an opportunity to provide feedback to the fellow as well as check in on their well-being and receive feedback about the program. We give frequent feedback to fellows in the form of spot, rotational, and mid-/end-of-year feedback. Additionally, we have developed a unique feedback system in which the trainees meet at the end of the year to discuss collective feedback for the staff and the program. This feedback is collated by the chief fellow and given to the program director as anonymous feedback, which is then passed to the individual staff.
- Regroup. Finally, regrouping to form new strategies.3 This regrouping provides an opportunity to improve on areas in which the trainee may have a deficiency and build on their strengths. To facilitate regrouping, we identify a mentor within the department and occasionally in other departments to meet regularly with the trainee. A successful mentor ensures effective regrouping and can help the trainee avoid pitfalls that they may have experienced in similar situations.
Moving forward
Occupational burnout is a systemic problem within the medical field, with trainees disproportionately affected. It is imperative that training programs continue to work toward creating a culture that prevents development of burnout. Along with the ideas presented here, the ACGME has launched AWARE, which is a suite of resources directed specifically at the GME community, with a goal of mitigating stress and preventing burnout. No one approach will be universally applicable but continued awareness and efforts to address this on an individual and programmatic level should be encouraged.
Dr. Ordway is a chief fellow, Dr. Tritsch and Dr. Singla are associate program directors, and Dr. Torres the program director, division of gastroenterology and hepatology, Walter Reed National Military Medical Center, Bethesda, Md.
References
1. Barnes EL et al. Dig Dis Sci. 2019;64(2):302-6.
2. Dyrbye LN et al. Acad Med. 2014;89(3):443-51.
3. Waldo OA. J Am Coll Cardiol. 2015;66(11):1303-6.
Jeff is a high-performing first-year gastroenterology fellow who started with eagerness and enthusiasm. He seemed to enjoy talking to patients, wrote thorough notes, and often participated during case discussions at morning report. He initiated a quality improvement project and joined a hospital committee. Over the past few months, he has interacted less with his peers in the fellow’s office and stayed late to complete his patient encounters. He now frequently arrives late to work, is unprepared for rounds, and forgets to place important orders. One day, you notice him shuffling through several papers when the attending asks him a question about his patient. Later that day, he snapped at a nurse who paged to ask a question about a patient who just had a colonoscopy. When you ask him how he is doing, he becomes tearful and reports that he is under a lot of stress between work and home and does not feel the work he is doing is meaningful.
Introduction
The above scenario is all too familiar. Gastroenterology training can be a stressful period in an individual’s life. Long hours, steep learning curves for new cognitive and mechanical skill sets, as well as managing personal relationships and responsibilities at home all contribute to the stress of training and finding appropriate work-life balance. These stressors can result in burnout. The last decade has brought about a renewed emphasis on mitigating the impact of occupational burnout and improving trainee lifestyle through interventions such as work-hour restrictions, resiliency training, instruction on the importance of sleep, and team-building activities.
The problem
The World Health Organization (WHO) defines occupational burnout as chronic work-related stress, which may be characterized by feelings of energy depletion, mental distance from one’s job or feelings of negativity toward it, and reduced professional efficacy. Occupational burnout has been identified as an increasing problem both in practicing providers and trainees. Surveys in gastroenterologists show rates of burnout ranging between 37% and 50%,1 with trainees and early-career physicians disproportionately affected.1,2Physicians along the entire training spectrum are more likely to report high emotional exhaustion, high depersonalization, and burnout than a population control sample.2
Several individual factors identified for those at increased risk for burnout include younger age, not being married, and being male.2 Individuals spending less than 20% of their time working on activities they find meaningful and productive were more likely to show evidence of burnout.1
Symptoms of burnout can have a profound impact on trainees’ work performance, personal interactions, and the learning environment as a whole. The Accreditation Council for Graduate Medical Education (ACGME) annual survey of trainees asks them how strongly they agree or disagree on various components of burnout such as how meaningful they find their work, if they have enough time to think and reflect, if they feel emotionally drained at work, and if they feel worn out and weary after work. The intent of these questions is to provide anonymous feedback to training programs to help identify year to year trends and intervene early to prevent occupational burnout from becoming an increasing issue.
The solution
Considerations for any intervention should take several factors into account: the impact it may have on training and the development of a competent physician in their individualized specialty, the sustainability of the intervention, and whether it is something that will be accepted by the invested parties.

One method proposed for preventing burnout during fellowship has been designated as the three R’s: relaxation, reflection, and regrouping.3
- Relax. In order to relax, trainees need ways to decompress. Activities such as exercise and social events can be helpful. Within our own program the fellows have started their own group exercise program, playing wallyball weekly before clinical duties. We also encourage use of vacation days and build comradery by organizing potluck dinners for major holidays, graduation parties at the program director’s house, and an end-of-the-year golf outing in which trainees play against staff followed by a discussion regarding the state of the program. More recently we have added one half-day per a quarter for morale and team building. During this first year, the activities in which trainees have collectively decided to participate include an escape room, a rock-climbing facility, and laser tag. The addition of more team-building days has been well received by our program’s trainees and the simple addition of these team-building days has resulted in the trainees interacting more together outside of work, particularly in the form of group dinners.

Walter Reed National Military Medical Center fellows gathering for wallyball. - Reflect. They describe reflection as a necessary checkpoint which typically occurs every 6 months.3 These “checkpoints” provide an opportunity to provide feedback to the fellow as well as check in on their well-being and receive feedback about the program. We give frequent feedback to fellows in the form of spot, rotational, and mid-/end-of-year feedback. Additionally, we have developed a unique feedback system in which the trainees meet at the end of the year to discuss collective feedback for the staff and the program. This feedback is collated by the chief fellow and given to the program director as anonymous feedback, which is then passed to the individual staff.
- Regroup. Finally, regrouping to form new strategies.3 This regrouping provides an opportunity to improve on areas in which the trainee may have a deficiency and build on their strengths. To facilitate regrouping, we identify a mentor within the department and occasionally in other departments to meet regularly with the trainee. A successful mentor ensures effective regrouping and can help the trainee avoid pitfalls that they may have experienced in similar situations.
Moving forward
Occupational burnout is a systemic problem within the medical field, with trainees disproportionately affected. It is imperative that training programs continue to work toward creating a culture that prevents development of burnout. Along with the ideas presented here, the ACGME has launched AWARE, which is a suite of resources directed specifically at the GME community, with a goal of mitigating stress and preventing burnout. No one approach will be universally applicable but continued awareness and efforts to address this on an individual and programmatic level should be encouraged.
Dr. Ordway is a chief fellow, Dr. Tritsch and Dr. Singla are associate program directors, and Dr. Torres the program director, division of gastroenterology and hepatology, Walter Reed National Military Medical Center, Bethesda, Md.
References
1. Barnes EL et al. Dig Dis Sci. 2019;64(2):302-6.
2. Dyrbye LN et al. Acad Med. 2014;89(3):443-51.
3. Waldo OA. J Am Coll Cardiol. 2015;66(11):1303-6.
Jeff is a high-performing first-year gastroenterology fellow who started with eagerness and enthusiasm. He seemed to enjoy talking to patients, wrote thorough notes, and often participated during case discussions at morning report. He initiated a quality improvement project and joined a hospital committee. Over the past few months, he has interacted less with his peers in the fellow’s office and stayed late to complete his patient encounters. He now frequently arrives late to work, is unprepared for rounds, and forgets to place important orders. One day, you notice him shuffling through several papers when the attending asks him a question about his patient. Later that day, he snapped at a nurse who paged to ask a question about a patient who just had a colonoscopy. When you ask him how he is doing, he becomes tearful and reports that he is under a lot of stress between work and home and does not feel the work he is doing is meaningful.
Introduction
The above scenario is all too familiar. Gastroenterology training can be a stressful period in an individual’s life. Long hours, steep learning curves for new cognitive and mechanical skill sets, as well as managing personal relationships and responsibilities at home all contribute to the stress of training and finding appropriate work-life balance. These stressors can result in burnout. The last decade has brought about a renewed emphasis on mitigating the impact of occupational burnout and improving trainee lifestyle through interventions such as work-hour restrictions, resiliency training, instruction on the importance of sleep, and team-building activities.
The problem
The World Health Organization (WHO) defines occupational burnout as chronic work-related stress, which may be characterized by feelings of energy depletion, mental distance from one’s job or feelings of negativity toward it, and reduced professional efficacy. Occupational burnout has been identified as an increasing problem both in practicing providers and trainees. Surveys in gastroenterologists show rates of burnout ranging between 37% and 50%,1 with trainees and early-career physicians disproportionately affected.1,2Physicians along the entire training spectrum are more likely to report high emotional exhaustion, high depersonalization, and burnout than a population control sample.2
Several individual factors identified for those at increased risk for burnout include younger age, not being married, and being male.2 Individuals spending less than 20% of their time working on activities they find meaningful and productive were more likely to show evidence of burnout.1
Symptoms of burnout can have a profound impact on trainees’ work performance, personal interactions, and the learning environment as a whole. The Accreditation Council for Graduate Medical Education (ACGME) annual survey of trainees asks them how strongly they agree or disagree on various components of burnout such as how meaningful they find their work, if they have enough time to think and reflect, if they feel emotionally drained at work, and if they feel worn out and weary after work. The intent of these questions is to provide anonymous feedback to training programs to help identify year to year trends and intervene early to prevent occupational burnout from becoming an increasing issue.
The solution
Considerations for any intervention should take several factors into account: the impact it may have on training and the development of a competent physician in their individualized specialty, the sustainability of the intervention, and whether it is something that will be accepted by the invested parties.

One method proposed for preventing burnout during fellowship has been designated as the three R’s: relaxation, reflection, and regrouping.3
- Relax. In order to relax, trainees need ways to decompress. Activities such as exercise and social events can be helpful. Within our own program the fellows have started their own group exercise program, playing wallyball weekly before clinical duties. We also encourage use of vacation days and build comradery by organizing potluck dinners for major holidays, graduation parties at the program director’s house, and an end-of-the-year golf outing in which trainees play against staff followed by a discussion regarding the state of the program. More recently we have added one half-day per a quarter for morale and team building. During this first year, the activities in which trainees have collectively decided to participate include an escape room, a rock-climbing facility, and laser tag. The addition of more team-building days has been well received by our program’s trainees and the simple addition of these team-building days has resulted in the trainees interacting more together outside of work, particularly in the form of group dinners.

Walter Reed National Military Medical Center fellows gathering for wallyball. - Reflect. They describe reflection as a necessary checkpoint which typically occurs every 6 months.3 These “checkpoints” provide an opportunity to provide feedback to the fellow as well as check in on their well-being and receive feedback about the program. We give frequent feedback to fellows in the form of spot, rotational, and mid-/end-of-year feedback. Additionally, we have developed a unique feedback system in which the trainees meet at the end of the year to discuss collective feedback for the staff and the program. This feedback is collated by the chief fellow and given to the program director as anonymous feedback, which is then passed to the individual staff.
- Regroup. Finally, regrouping to form new strategies.3 This regrouping provides an opportunity to improve on areas in which the trainee may have a deficiency and build on their strengths. To facilitate regrouping, we identify a mentor within the department and occasionally in other departments to meet regularly with the trainee. A successful mentor ensures effective regrouping and can help the trainee avoid pitfalls that they may have experienced in similar situations.
Moving forward
Occupational burnout is a systemic problem within the medical field, with trainees disproportionately affected. It is imperative that training programs continue to work toward creating a culture that prevents development of burnout. Along with the ideas presented here, the ACGME has launched AWARE, which is a suite of resources directed specifically at the GME community, with a goal of mitigating stress and preventing burnout. No one approach will be universally applicable but continued awareness and efforts to address this on an individual and programmatic level should be encouraged.
Dr. Ordway is a chief fellow, Dr. Tritsch and Dr. Singla are associate program directors, and Dr. Torres the program director, division of gastroenterology and hepatology, Walter Reed National Military Medical Center, Bethesda, Md.
References
1. Barnes EL et al. Dig Dis Sci. 2019;64(2):302-6.
2. Dyrbye LN et al. Acad Med. 2014;89(3):443-51.
3. Waldo OA. J Am Coll Cardiol. 2015;66(11):1303-6.
Can drinking more water prevent urinary tract infections?
ILLUSTRATIVE CASE
A 23-year-old nonpregnant woman, whom you treated 3 times in the past year for cystitis, comes to you for follow-up. She wants to know what she can do to prevent another urinary tract infection other than taking prophylactic antibiotics. Should you recommend that this patient increase her daily water intake to prevent recurrent cystitis?
Urinary tract infection (UTI) is the most common bacterial infection encountered in the ambulatory setting. Half of all women report having had at least 1 UTI by the time they are 32 years old.2 Recurrence is also common, with 27% of women having 1 recurrence within 6 months of their first episode.2
Because of growing antimicrobial resistance, the World Health Organization has urged using novel antimicrobial-sparing approaches to infectious diseases.3 Physicians have long recommended behavioral, nonantimicrobial strategies for prevention of recurrent uncomplicated cystitis. Such behavioral recommendations include drinking fluids liberally, urinating after intercourse, not delaying urination, wiping front to back, and avoiding tight-fitting underwear. However, these behavior modification strategies have been studied largely in case-control trials that have yet to find an association between behavior modification and reduced risk of UTI.2 Although unproven as a prevention strategy, increasing daily fluid intake has long been a recommendation because of the belief that it helps to dilute and clear bactiuria.4 This study is the first non–case-control trial to examine the association between increased fluid intake and decreased UTIs.1
STUDY SUMMARY
RCT looks at whether more water leads to fewer UTIs
Hooton and colleagues1 conducted an open-label, randomized controlled trial (RCT) of premenopausal women with recurrent UTIs and low baseline fluid intake and compared increased fluid intake (an additional 1.5 L/d) with no additional fluids. Participants were provided three 500-mL bottles of water per day and were followed for 1 year. Screened women were included if they had 3 or more symptomatic UTIs in the previous year, 1 culture-confirmed UTI, self-reported fluid intake < 1.5 L /d, and were otherwise in good health. Fluid intake was verified by 24-hour urine collection, requiring a volume < 1.2 L and urine osmolality of ≥ 500 mOsm/kg. Exclusion criteria included a history of pyelonephritis within the past year, interstitial cystitis, pregnancy, or current symptoms of UTI.
The primary outcome was frequency of UTI during the study period, defined as 1 urinary symptom and at least 103 CFU/mL uropathogens in a urine culture. Secondary outcomes included the number of antimicrobial agents used, time to first UTI, mean time interval between cystitis episodes, and adverse events.1
A total of 140 participants were randomized with 70 in the water group and 70 in the control group. The mean age of the participants was 35.7 years, and the mean number of reported cystitis episodes was 3.3 in the 12 months prior to the study. By the end of the 12-month study period, mean daily fluid intake had increased by 1.7 L above baseline in the water group. During the 12-month study period, the mean (SD) number of cystitis episodes was 1.7 (95% confidence interval [CI], 1.5-1.8) in the water group compared with 3.2 (95% CI, 3-3.4) in the control group, with a difference in means of 1.5 (95% CI, 1.2-1.8; P < .001).
The mean number of antimicrobial agents used for UTI was 1.9 (95% CI, 1.7-2.2) in the water group and 3.6 (95% CI, 3.3-4) in the control group. The median time to first UTI episode was 148 days in the water group compared with 93.5 days in the control group (hazard ratio [HR] = 0.51; 95% CI, 0.36-0.74; P < .001) and the difference in means for the time interval between UTI episodes was 58.4 days (95% CI, 39.4-77.4; P < .001). No serious adverse events were reported.1
Continue to: WHAT'S NEW
WHAT’S NEW
Proof that increased fluid intake reduces the risk of recurrent UTI
Increasing daily fluid intake is a long-held but previously unproven recommendation. This is the first RCT to show increased daily water intake can reduce the risk of recurrent cystitis in premenopausal patients at high risk for UTI and with low fluid intake. No additional risk of adverse events was found.
CAVEATS
Is there a risk of overhydration?
The study did not address the effect of increasing water intake in women who do not have low-volume fluid intake. Case reports of overhydration emphasize the need to be cautious when making recommendations to hydrate.5 It is not known if physicians should screen for fluid intake at baseline to identify those (with low intake) who would be eligible for this intervention.
CHALLENGES TO IMPLEMENTATION
It’s unclear whether the strategy will work without monitoring
The intervention is both low-risk and low-cost to the patient. However, the intervention was supported by home delivery of water and monthly monitoring interventions that are not typical in normal care. Although the clinical intervention of drinking more fluids (primarily water) appears sound, it is not known whether a physician’s recommendation would result in the same adherence and risk reduction as water delivery and monitoring.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Hooton TM, Vecchio M, Iroz A, et al. Effect of increased daily water intake in premenopausal women with recurrent urinary tract infections: a randomized clinical trial. JAMA Intern Med. 2018;178:1509-1515.
2. Hooton TM. Clinical practice. Uncomplicated urinary tract infection. N Engl J Med. 2012;366:1028-1037.
3. WHO. Antimicrobial resistance: global report on surveillance. April 2014. www.who.int/drugresistance/documents/surveillancereport/en/. Accessed March 23, 2020.
4. Fasugba O, Mitchell BG, McInnes E, et al. Increased fluid intake for the prevention of urinary tract infection in adults and children in all settings: a systematic review. J Hosp Infect. 2020;104:68-77.
5. Lee LC, Noronha M. When plenty is too much: water intoxication in a patient with a simple urinary tract infection. BMJ Case Rep. 2016. doi:10.1136/bcr-2016-216882.
ILLUSTRATIVE CASE
A 23-year-old nonpregnant woman, whom you treated 3 times in the past year for cystitis, comes to you for follow-up. She wants to know what she can do to prevent another urinary tract infection other than taking prophylactic antibiotics. Should you recommend that this patient increase her daily water intake to prevent recurrent cystitis?
Urinary tract infection (UTI) is the most common bacterial infection encountered in the ambulatory setting. Half of all women report having had at least 1 UTI by the time they are 32 years old.2 Recurrence is also common, with 27% of women having 1 recurrence within 6 months of their first episode.2
Because of growing antimicrobial resistance, the World Health Organization has urged using novel antimicrobial-sparing approaches to infectious diseases.3 Physicians have long recommended behavioral, nonantimicrobial strategies for prevention of recurrent uncomplicated cystitis. Such behavioral recommendations include drinking fluids liberally, urinating after intercourse, not delaying urination, wiping front to back, and avoiding tight-fitting underwear. However, these behavior modification strategies have been studied largely in case-control trials that have yet to find an association between behavior modification and reduced risk of UTI.2 Although unproven as a prevention strategy, increasing daily fluid intake has long been a recommendation because of the belief that it helps to dilute and clear bactiuria.4 This study is the first non–case-control trial to examine the association between increased fluid intake and decreased UTIs.1
STUDY SUMMARY
RCT looks at whether more water leads to fewer UTIs
Hooton and colleagues1 conducted an open-label, randomized controlled trial (RCT) of premenopausal women with recurrent UTIs and low baseline fluid intake and compared increased fluid intake (an additional 1.5 L/d) with no additional fluids. Participants were provided three 500-mL bottles of water per day and were followed for 1 year. Screened women were included if they had 3 or more symptomatic UTIs in the previous year, 1 culture-confirmed UTI, self-reported fluid intake < 1.5 L /d, and were otherwise in good health. Fluid intake was verified by 24-hour urine collection, requiring a volume < 1.2 L and urine osmolality of ≥ 500 mOsm/kg. Exclusion criteria included a history of pyelonephritis within the past year, interstitial cystitis, pregnancy, or current symptoms of UTI.
The primary outcome was frequency of UTI during the study period, defined as 1 urinary symptom and at least 103 CFU/mL uropathogens in a urine culture. Secondary outcomes included the number of antimicrobial agents used, time to first UTI, mean time interval between cystitis episodes, and adverse events.1
A total of 140 participants were randomized with 70 in the water group and 70 in the control group. The mean age of the participants was 35.7 years, and the mean number of reported cystitis episodes was 3.3 in the 12 months prior to the study. By the end of the 12-month study period, mean daily fluid intake had increased by 1.7 L above baseline in the water group. During the 12-month study period, the mean (SD) number of cystitis episodes was 1.7 (95% confidence interval [CI], 1.5-1.8) in the water group compared with 3.2 (95% CI, 3-3.4) in the control group, with a difference in means of 1.5 (95% CI, 1.2-1.8; P < .001).
The mean number of antimicrobial agents used for UTI was 1.9 (95% CI, 1.7-2.2) in the water group and 3.6 (95% CI, 3.3-4) in the control group. The median time to first UTI episode was 148 days in the water group compared with 93.5 days in the control group (hazard ratio [HR] = 0.51; 95% CI, 0.36-0.74; P < .001) and the difference in means for the time interval between UTI episodes was 58.4 days (95% CI, 39.4-77.4; P < .001). No serious adverse events were reported.1
Continue to: WHAT'S NEW
WHAT’S NEW
Proof that increased fluid intake reduces the risk of recurrent UTI
Increasing daily fluid intake is a long-held but previously unproven recommendation. This is the first RCT to show increased daily water intake can reduce the risk of recurrent cystitis in premenopausal patients at high risk for UTI and with low fluid intake. No additional risk of adverse events was found.
CAVEATS
Is there a risk of overhydration?
The study did not address the effect of increasing water intake in women who do not have low-volume fluid intake. Case reports of overhydration emphasize the need to be cautious when making recommendations to hydrate.5 It is not known if physicians should screen for fluid intake at baseline to identify those (with low intake) who would be eligible for this intervention.
CHALLENGES TO IMPLEMENTATION
It’s unclear whether the strategy will work without monitoring
The intervention is both low-risk and low-cost to the patient. However, the intervention was supported by home delivery of water and monthly monitoring interventions that are not typical in normal care. Although the clinical intervention of drinking more fluids (primarily water) appears sound, it is not known whether a physician’s recommendation would result in the same adherence and risk reduction as water delivery and monitoring.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 23-year-old nonpregnant woman, whom you treated 3 times in the past year for cystitis, comes to you for follow-up. She wants to know what she can do to prevent another urinary tract infection other than taking prophylactic antibiotics. Should you recommend that this patient increase her daily water intake to prevent recurrent cystitis?
Urinary tract infection (UTI) is the most common bacterial infection encountered in the ambulatory setting. Half of all women report having had at least 1 UTI by the time they are 32 years old.2 Recurrence is also common, with 27% of women having 1 recurrence within 6 months of their first episode.2
Because of growing antimicrobial resistance, the World Health Organization has urged using novel antimicrobial-sparing approaches to infectious diseases.3 Physicians have long recommended behavioral, nonantimicrobial strategies for prevention of recurrent uncomplicated cystitis. Such behavioral recommendations include drinking fluids liberally, urinating after intercourse, not delaying urination, wiping front to back, and avoiding tight-fitting underwear. However, these behavior modification strategies have been studied largely in case-control trials that have yet to find an association between behavior modification and reduced risk of UTI.2 Although unproven as a prevention strategy, increasing daily fluid intake has long been a recommendation because of the belief that it helps to dilute and clear bactiuria.4 This study is the first non–case-control trial to examine the association between increased fluid intake and decreased UTIs.1
STUDY SUMMARY
RCT looks at whether more water leads to fewer UTIs
Hooton and colleagues1 conducted an open-label, randomized controlled trial (RCT) of premenopausal women with recurrent UTIs and low baseline fluid intake and compared increased fluid intake (an additional 1.5 L/d) with no additional fluids. Participants were provided three 500-mL bottles of water per day and were followed for 1 year. Screened women were included if they had 3 or more symptomatic UTIs in the previous year, 1 culture-confirmed UTI, self-reported fluid intake < 1.5 L /d, and were otherwise in good health. Fluid intake was verified by 24-hour urine collection, requiring a volume < 1.2 L and urine osmolality of ≥ 500 mOsm/kg. Exclusion criteria included a history of pyelonephritis within the past year, interstitial cystitis, pregnancy, or current symptoms of UTI.
The primary outcome was frequency of UTI during the study period, defined as 1 urinary symptom and at least 103 CFU/mL uropathogens in a urine culture. Secondary outcomes included the number of antimicrobial agents used, time to first UTI, mean time interval between cystitis episodes, and adverse events.1
A total of 140 participants were randomized with 70 in the water group and 70 in the control group. The mean age of the participants was 35.7 years, and the mean number of reported cystitis episodes was 3.3 in the 12 months prior to the study. By the end of the 12-month study period, mean daily fluid intake had increased by 1.7 L above baseline in the water group. During the 12-month study period, the mean (SD) number of cystitis episodes was 1.7 (95% confidence interval [CI], 1.5-1.8) in the water group compared with 3.2 (95% CI, 3-3.4) in the control group, with a difference in means of 1.5 (95% CI, 1.2-1.8; P < .001).
The mean number of antimicrobial agents used for UTI was 1.9 (95% CI, 1.7-2.2) in the water group and 3.6 (95% CI, 3.3-4) in the control group. The median time to first UTI episode was 148 days in the water group compared with 93.5 days in the control group (hazard ratio [HR] = 0.51; 95% CI, 0.36-0.74; P < .001) and the difference in means for the time interval between UTI episodes was 58.4 days (95% CI, 39.4-77.4; P < .001). No serious adverse events were reported.1
Continue to: WHAT'S NEW
WHAT’S NEW
Proof that increased fluid intake reduces the risk of recurrent UTI
Increasing daily fluid intake is a long-held but previously unproven recommendation. This is the first RCT to show increased daily water intake can reduce the risk of recurrent cystitis in premenopausal patients at high risk for UTI and with low fluid intake. No additional risk of adverse events was found.
CAVEATS
Is there a risk of overhydration?
The study did not address the effect of increasing water intake in women who do not have low-volume fluid intake. Case reports of overhydration emphasize the need to be cautious when making recommendations to hydrate.5 It is not known if physicians should screen for fluid intake at baseline to identify those (with low intake) who would be eligible for this intervention.
CHALLENGES TO IMPLEMENTATION
It’s unclear whether the strategy will work without monitoring
The intervention is both low-risk and low-cost to the patient. However, the intervention was supported by home delivery of water and monthly monitoring interventions that are not typical in normal care. Although the clinical intervention of drinking more fluids (primarily water) appears sound, it is not known whether a physician’s recommendation would result in the same adherence and risk reduction as water delivery and monitoring.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Hooton TM, Vecchio M, Iroz A, et al. Effect of increased daily water intake in premenopausal women with recurrent urinary tract infections: a randomized clinical trial. JAMA Intern Med. 2018;178:1509-1515.
2. Hooton TM. Clinical practice. Uncomplicated urinary tract infection. N Engl J Med. 2012;366:1028-1037.
3. WHO. Antimicrobial resistance: global report on surveillance. April 2014. www.who.int/drugresistance/documents/surveillancereport/en/. Accessed March 23, 2020.
4. Fasugba O, Mitchell BG, McInnes E, et al. Increased fluid intake for the prevention of urinary tract infection in adults and children in all settings: a systematic review. J Hosp Infect. 2020;104:68-77.
5. Lee LC, Noronha M. When plenty is too much: water intoxication in a patient with a simple urinary tract infection. BMJ Case Rep. 2016. doi:10.1136/bcr-2016-216882.
1. Hooton TM, Vecchio M, Iroz A, et al. Effect of increased daily water intake in premenopausal women with recurrent urinary tract infections: a randomized clinical trial. JAMA Intern Med. 2018;178:1509-1515.
2. Hooton TM. Clinical practice. Uncomplicated urinary tract infection. N Engl J Med. 2012;366:1028-1037.
3. WHO. Antimicrobial resistance: global report on surveillance. April 2014. www.who.int/drugresistance/documents/surveillancereport/en/. Accessed March 23, 2020.
4. Fasugba O, Mitchell BG, McInnes E, et al. Increased fluid intake for the prevention of urinary tract infection in adults and children in all settings: a systematic review. J Hosp Infect. 2020;104:68-77.
5. Lee LC, Noronha M. When plenty is too much: water intoxication in a patient with a simple urinary tract infection. BMJ Case Rep. 2016. doi:10.1136/bcr-2016-216882.
PRACTICE CHANGER
Advise premenopausal women with recurrent urinary tract infections (UTIs) and low-volume fluid intake to increase their water intake by at least 1.5 liters daily to reduce the frequency of UTIs.1
STRENGTH OF RECOMMENDATION
A: Based on a single, high-quality randomized controlled trial.
Hooton TM, Vecchio M, Iroz A, et al. Effect of increased daily water intake in premenopausal women with recurrent urinary tract infections: a randomized clinical trial. JAMA Intern Med. 2018;178:1509-1515.
Paranoid delusions • ideas of reference • sleep problems • Dx?
THE CASE
A 58-year-old married Asian woman with no apparent psychiatric history presented to the emergency department (ED) in an acute state with ideas of reference, paranoid delusions, and multiple, vague somatic symptoms.
Based on information in the patient’s medical record, there had been suspicion of an underlying psychiatric disorder 6 years earlier. At that time, the patient had presented to her primary care provider (PCP) with vague somatic complaints, including diffuse body pain, dry cough, chills, weakness, facial numbness, and concerns about infections. A physical examination and work-up did not reveal the source of her complaints. Unfortunately, the patient’s complaints increased in number and severity over time.
Her medical records also indicated that she had been assessed for depression severity using the Patient Health Questionnaire-9 (PHQ-9), with scores of 0 (4 years earlier) and 3 (3 years earlier). The scores suggested that she was not suffering from depression.
During this time, the patient also saw a psychiatrist; however, it was unclear whether her symptoms met the criteria for delusional disorder or schizophrenia because she did not exhibit negative symptoms or sensory hallucinations. In addition, the patient was extremely high-functioning in the community—she participated in dance classes and other social events—and had the equivalent of a medical degree from another country. Based on chart review, when she went to the psychiatrist 3 years prior to her current presentation, there were no antipsychotics prescribed.
In the weeks leading up to her current presentation, the patient reported that she was struggling with sleep, sometimes spending days in bed and other times needing unspecified medication obtained overseas to help her sleep. Her husband reported that she had become increasingly withdrawn and stopped attending her dance classes and social events.
The patient believed the government was trying to poison her via radiation and that unknown people were trying to harm her via an online messaging application. Immediately prior to her arrival in the ED, the police were called to pull her away from oncoming traffic because she ran into the road to find the assassins that were stalking her.
During this recent visit to the ED, the patient presented with labile affect, rapid speech, and anxious and angry mood. She complained about darkened spots on her arm (inflicted through radiation by the media), vaginal bleeding, paralysis below the waist (although she was pacing around), and unspecific pain around her belly. Physical examination revealed no obvious signs of head trauma, intact extraocular movements, no coughing or wheezing, regular heart rate and rhythm, a nontender abdomen to palpation, and normal bowel sounds. No focal neurological deficits were appreciated. She had no rashes, bruises, or skin abrasions on her abdomen or upper extremities.
Continue to: The patient tried to...
The patient tried to leave the ED, saying that her third eye could see the radiation. She required medication and 4-point restraints.
Her initial laboratory work-up for heavy metals, Lyme disease, human immunodeficiency virus (HIV), syphilis, delirium, and drug use were all negative. She also underwent head imaging studies that were also found to be negative. Her mental status exam was notable for a tangential thought process, preservation of delusions with loose associations, labile mood, and dysphoric affect. The patient demonstrated limited insight and judgment, although she was fully oriented to person, place, and time, which suggested against delirium at the time of evaluation.
THE DIAGNOSIS
Based on the patient’s current presentation and in light of her medical history, the health care team arrived at a
DISCUSSION
Schizophrenia is a severe, lifelong mental disorder characterized by at least 2 symptoms of delusions, hallucinations, disorganized speech, disorganized or catatonic behavior, or negative symptoms for at least 6 months, with significant social, occupational, and functional deterioration. Current models attribute the neurodevelopmental deregulation of the brain in patients with schizophrenia to dopaminergic hyperactivity and hypofunction of the glutamatergic neurotransmitter system, explaining why its onset is usually in adolescence or young adulthood.1,2 However, 23% of patients present with symptoms after age 40, with 7% of patients being diagnosed between the ages of 51 and 60.3
Late-onset vs early-onset schizophrenia. LOS is often a missed diagnosis because the clinical presentation is different from early-onset schizophrenia (EOS). Although the prodromal symptoms of EOS and LOS are similar and include marked isolation that subsequently progresses to suspiciousness and ideas of reference,4 patients with EOS often also have prodromal negative symptoms. These prodromal negative symptoms associated with EOS may include loss of motivation, social passivity, and disorganized behavior. These symptoms are hypothesized to be caused by dopaminergic dysregulation in the anterior cingulate cortex. EOS is characterized by the patient experiencing more negative symptoms than LOS, which is characterized by the patient experiencing more positive symptoms.
Continue to: Patients with late-onset schizophrenia...
Patients with LOS typically do not exhibit negative symptoms because remodeling and myelination of neuronal circuitry matures by late adulthood, and thus becomes more resistant to impairment of motivational processes in the anterior cingulate gyrus.4,5,6
LOS is characterized by paranoid personality with predominantly positive symptoms, likely due to disruptions in
Other features of LOS include a high female:male ratio and symptomatic improvement with antipsychotics.7,10 Studies show that the LOS ratio of women:men can range from 2.2:1 to 22.5:1, which could be explained by the effect of dopaminergic-modulating estrogen from different sex-specific aging brain patterns.8,11,12 Finally, patients with LOS are less likely to seek care for sensory deficits than their age-equivalent counterparts.8,10 Fortunately, many of the characteristics of LOS predict good prognosis: Patients are usually female, display positive symptoms, have acute onset of symptoms, and are married with social support.10
Diagnosing LOS
LOS can be challenging to diagnose because of its atypical presentation compared with EOS, relative rarity in the population, and its propensity to be confused with progressive Alzheimer disease/dementia, delusional disorder, and major depressive disorder with psychotic features.3,6 Patients with no prior psychiatric history often do not have ready access to psychiatrists and depend on PCPs and other clinicians to identify mental health issues. A careful history, including familial involvement, utilization of the Montreal Cognitive Assessment (MoCA) test, and evaluation of environmental factors, are crucial to arriving at the proper diagnosis.
Continue to: Differential diagnosis
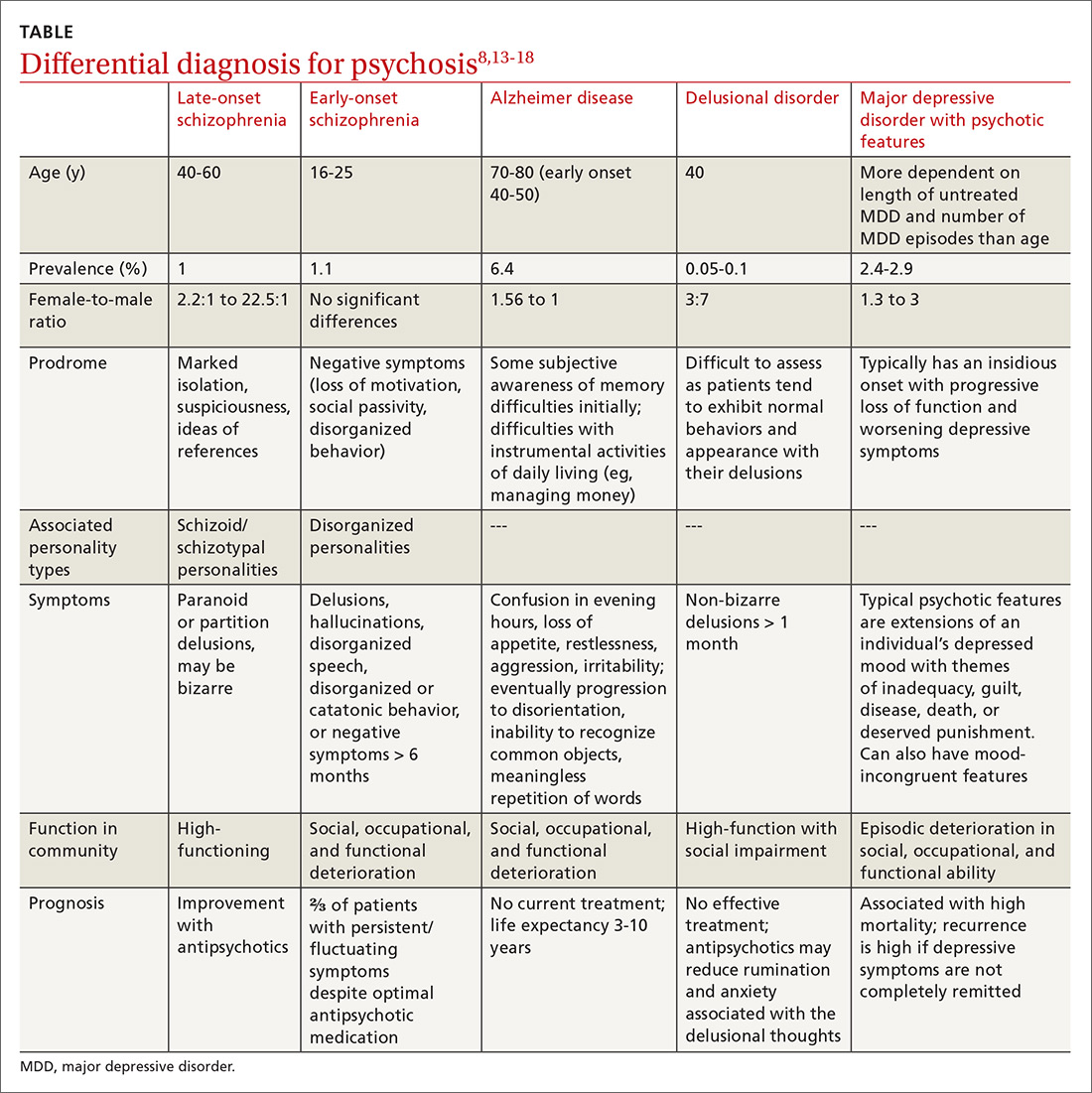
Differential diagnosis. When psychosis appears later in life, it is important to consider a broad differential (TABLE13-18), which includes the following:
Alzheimer disease. LOS can be easily differentiated from psychosis associated with Alzheimer disease or dementia through findings from neuropsychologic assessments and brain imaging. The initial first-line assessment for Alzheimer disease includes determining time course of daily living impairment and memory with follow-up brain imaging. Magnetic resonance imaging of patients with Alzheimer disease shows clear atrophy of the medial temporal lobes and general brain atrophy.19 Other than hypoperfusion in the frontal and temporal area, brain imaging of patients with LOS will not reveal any pathology.1
Delusional disorder and LOS are often more challenging to differentiate because symptoms can overlap, and many of the negative symptoms that would otherwise help clinicians diagnose schizophrenia in a younger population are absent in LOS. The milder symptoms of LOS may also lead clinicians to favor a diagnosis of delusional disorder. However, the following differences can help physicians differentiate between LOS and delusional disorder. Delusional disorder20-22:
- often will include paranoid beliefs, but these beliefs will not be bizarre, and the patient’s daily functioning will not be impaired, whereas patients with schizophrenia would have an increase in isolation and impairment in functioning that tends to be distinct from baseline.
- is more rare than schizophrenia. Delusional disorder has a prevalence of 0.05% to 0.1% compared to 1% for schizophrenia.
Major depressive disorder (MDD) with psychotic features. Major depressive disorder with psychotic features is an important differential to consider in this setting because the treatment intervention can be considerably different. Among patients who have MDD with psychotic features, a significant mood component is present, and treatment typically focuses on optimizing a selective serotonin reuptake inhibitor (SSRI); depending on severity, electroconvulsive therapy (ECT) also may be warranted.19
Continue to: For patients with LOS...
For patients with LOS, optimizing an antipsychotic medication is the typical course of treatment, and ECT would likely have less of an impact than it does with MDD with psychotic features.
Other. Finally, in an acute setting, other differential diagnoses for mental status changes (depending on clinical findings) might include:
- drug/medication use
- delirium
- nutrient deficiencies
- acute head trauma
- chronic subdural hematoma
- syphilis
- Lyme disease
- HIV encephalitis
- heavy metal toxicity.
Treatment involves antipsychotics—especially certain ones
Antipsychotic medications are utilized for the treatment of patients with LOS. A Cochrane review concluded that there are no trial-based evidence guidelines for the treatment of patients with LOS, and that physicians should continue with their current practice and use clinical judgment and prescribing patterns to guide their selection of antipsychotic medications.22,23 Pearlson et al24 found that 76% of patients with schizophrenia achieved at least partial remission and 48% achieved full remission with antipsychotic treatment.
The preferred treatment for patients with schizophrenia is low doses of newer antipsychotics (atypical or second-generation antipsychotics [SGAs]) because they are less likely to cause extrapyramidal symptoms/adverse effects than first-generation antipsychotics. Examples of SGAs include aripiprazole, risperidone, olanzapine, quetiapine, and ziprasidone.
Effective treatment for LOS includes antipsychotics at a quarter to one-half of the usual therapeutic doses. In patients with very late-onset schizophrenia, doses should be started at a tenth of therapeutic dose.1,23 Physicians should titrate up carefully, as needed.
Continue to: As with any significant mental illness...
As with any significant mental illness, to improve clinical outcomes, family support may help patients’ medication adherence and ensure they attend scheduled medical appointments.
Our patient was eventually stabilized on long-acting injectable risperidone, 25 mg, with improvement in symptoms. Unfortunately, she was not convinced that her symptoms were psychiatric in nature and did not continue with her medications as an outpatient.
The patient’s nonadherence to her medication regimen led to 2 more hospitalizations with similar presentations over the following 2 years. On her most recent discharge, she was stabilized on oral olanzapine, 10 mg every night at bedtime, with close outpatient follow-up and family education.
THE TAKEAWAY
The prodromal phase of patients with LOS is similar to patients with EOS and includes withdrawal and isolation from others, making it difficult for physicians to evaluate and treat patients. Patients with LOS predominantly experience positive symptoms that may include delusions and hallucinations. Brain imaging studies can help rule out progressive dementia diseases. A neuropsychological evaluation can assess the patient’s functional level and types of delusions, which helps to differentiate LOS from other late-age psychoses. Treatment with SGAs make for a good prognosis; however, this requires patients to be adherent to treatment.
CORRESPONDENCE
Sandy Chan, MD, Department of Internal Medicine, UMass Memorial Medical Center, 55N Lake Avenue, Worcester, MA 01605; Sandy.Chan@umassmemorial.org
1. Howard R, Rabins P, Seeman M, et al. Late-onset schizophrenia and very-late-onset schizophrenia-like psychosis: an international crisis. Am J Psychiatry 2000;157:172-178.
2. Pickard B. Progress in defining the biological causes of schizophrenia. Expert Rev Mol Med. 2011;13:e25.
3. Jeste D, Symonds L, Harris M, et al. Nondementia nonpraecox dementia praecox? Am J Geriatr Psychiatry. 1997;5:302-317.
4. Gourzis P, Katrivanou A, Beratis S. Symptomatology of the initial prodromal phase of schizophrenia. Schizophr Bull. 2002;28:415-429.
5. Dolan R, Fletcher P, Frith C, et al. Dopaminergic modulation of impaired cognitive activation in the anterior cingulate cortex in schizophrenia. Nature. 1995;378:180-182.
6. Skokou M, Katrivanou A, Andriopoulos I, et al. Active and prodromal phase symptomatology of young-onset and late-onset paranoid schizophrenia. Rev Psiquiatr Salud Ment. 2012;5:150-159.
7. Riecher-Rossler A, Loffler W, Munk-Jorgensen P. What do we really know about late-onset schizophrenia? Eur Arch Psychiatry Clin Neurosci. 1997;247:195-208.
8. Lubman D, Castle D. Late-onset schizophrenia: make the right diagnosis when psychosis emerges after age 60. Current Psychiatry. 2002;1:35-44.
9. Howard R, Castle D, Wessely S, et al. A comparative study of 470 cases of early-onset and late-onset schizophrenia. British Journal of Psychiatry. 1993;163:352-357.
10. Harris M, Jeste D. Late-onset schizophrenia: an overview. Schizophr Bull. 1988;14:39-55.
11. Castle D, Murray R. The epidemiology of late-onset schizophrenia. Schizophr Bull. 1993;19:691-700.
12. Lindamer L, Lohr J, Harris M, Jeste D. Gender, estrogen, and schizophrenia. Psychopharmacol Bull. 1997;33:221-228.
13. Gaudiano BA, Dalrymple KL, Zimmerman M. Prevalence and clinical characteristics of psychotic versus non-psychotic major depression in a general psychiatric outpatient clinic. Depress Anxiety. 2009;26:54-64.
14. Saha S, Chant D, Welham J, et al. A systematic review of the prevalence of schizophrenia. PLoS Med. 2005;2:e141.
15. Gao S, Hendrie H, Hall K. The relationships between age, sex, and the incidence of dementia and Alzheimer Disease. JAMA Psychiatry. 1998;55:809-815.
16. Reitz C, Brayne C, Mayeux R. Epidemiology of Alzheimer Disease. Nature Reviews Neurology. 2011;7:137-152
17. Winokur G. Delusional Disorder (Paranoia). Comprehensive Psychiatry. 1977;18:511-521.
18. Scheltens P, Leys D, Huglo D, et al. Atrophy of medial temporal lobes on MRI in “probable” Alzheimer’s disease and normal ageing: diagnostic value and neuropsychological correlates. J Neurol, Neurosurg Psychiatry. 1992;55:967-972.
19. Copeland J, Dewey M, Scott A, et al. Schizophrenia and delusional disorder in older age: community prevalence, incidence, comorbidity, and outcome. Schizophr Bull. 1998;24:153-161.
20. Kendler K. Demography of paranoid psychosis (delusional disorder): a review and comparison with schizophrenia and affective illness. Arch Gen Psychiatry 1982;39:890-902.
21. McGrath J, Saha S, Chant D, et al. Schizophrenia: a concise overview of incidence, prevalence, and mortality. Epidemiol Rev. 2008;30:67.
22. Essali A, Ali G. Antipsychotic drug treatment for elderly people with late-onset schizophrenia. Cochrane Database Syst Rev. 2012;(2):CD004162.
23. Sweet R, Pollock B. New atypical antipsychotics- experience and utility in the elderly. Drugs Aging. 1998;12:115-127.
24. Pearlson G, Kreger L, Rabins P, et al. A chart review study of late-onset and early-onset schizophrenia. Am J Psychiatry.1989;146:1568-1574.
THE CASE
A 58-year-old married Asian woman with no apparent psychiatric history presented to the emergency department (ED) in an acute state with ideas of reference, paranoid delusions, and multiple, vague somatic symptoms.
Based on information in the patient’s medical record, there had been suspicion of an underlying psychiatric disorder 6 years earlier. At that time, the patient had presented to her primary care provider (PCP) with vague somatic complaints, including diffuse body pain, dry cough, chills, weakness, facial numbness, and concerns about infections. A physical examination and work-up did not reveal the source of her complaints. Unfortunately, the patient’s complaints increased in number and severity over time.
Her medical records also indicated that she had been assessed for depression severity using the Patient Health Questionnaire-9 (PHQ-9), with scores of 0 (4 years earlier) and 3 (3 years earlier). The scores suggested that she was not suffering from depression.
During this time, the patient also saw a psychiatrist; however, it was unclear whether her symptoms met the criteria for delusional disorder or schizophrenia because she did not exhibit negative symptoms or sensory hallucinations. In addition, the patient was extremely high-functioning in the community—she participated in dance classes and other social events—and had the equivalent of a medical degree from another country. Based on chart review, when she went to the psychiatrist 3 years prior to her current presentation, there were no antipsychotics prescribed.
In the weeks leading up to her current presentation, the patient reported that she was struggling with sleep, sometimes spending days in bed and other times needing unspecified medication obtained overseas to help her sleep. Her husband reported that she had become increasingly withdrawn and stopped attending her dance classes and social events.
The patient believed the government was trying to poison her via radiation and that unknown people were trying to harm her via an online messaging application. Immediately prior to her arrival in the ED, the police were called to pull her away from oncoming traffic because she ran into the road to find the assassins that were stalking her.
During this recent visit to the ED, the patient presented with labile affect, rapid speech, and anxious and angry mood. She complained about darkened spots on her arm (inflicted through radiation by the media), vaginal bleeding, paralysis below the waist (although she was pacing around), and unspecific pain around her belly. Physical examination revealed no obvious signs of head trauma, intact extraocular movements, no coughing or wheezing, regular heart rate and rhythm, a nontender abdomen to palpation, and normal bowel sounds. No focal neurological deficits were appreciated. She had no rashes, bruises, or skin abrasions on her abdomen or upper extremities.
Continue to: The patient tried to...
The patient tried to leave the ED, saying that her third eye could see the radiation. She required medication and 4-point restraints.
Her initial laboratory work-up for heavy metals, Lyme disease, human immunodeficiency virus (HIV), syphilis, delirium, and drug use were all negative. She also underwent head imaging studies that were also found to be negative. Her mental status exam was notable for a tangential thought process, preservation of delusions with loose associations, labile mood, and dysphoric affect. The patient demonstrated limited insight and judgment, although she was fully oriented to person, place, and time, which suggested against delirium at the time of evaluation.
THE DIAGNOSIS
Based on the patient’s current presentation and in light of her medical history, the health care team arrived at a
DISCUSSION
Schizophrenia is a severe, lifelong mental disorder characterized by at least 2 symptoms of delusions, hallucinations, disorganized speech, disorganized or catatonic behavior, or negative symptoms for at least 6 months, with significant social, occupational, and functional deterioration. Current models attribute the neurodevelopmental deregulation of the brain in patients with schizophrenia to dopaminergic hyperactivity and hypofunction of the glutamatergic neurotransmitter system, explaining why its onset is usually in adolescence or young adulthood.1,2 However, 23% of patients present with symptoms after age 40, with 7% of patients being diagnosed between the ages of 51 and 60.3
Late-onset vs early-onset schizophrenia. LOS is often a missed diagnosis because the clinical presentation is different from early-onset schizophrenia (EOS). Although the prodromal symptoms of EOS and LOS are similar and include marked isolation that subsequently progresses to suspiciousness and ideas of reference,4 patients with EOS often also have prodromal negative symptoms. These prodromal negative symptoms associated with EOS may include loss of motivation, social passivity, and disorganized behavior. These symptoms are hypothesized to be caused by dopaminergic dysregulation in the anterior cingulate cortex. EOS is characterized by the patient experiencing more negative symptoms than LOS, which is characterized by the patient experiencing more positive symptoms.
Continue to: Patients with late-onset schizophrenia...
Patients with LOS typically do not exhibit negative symptoms because remodeling and myelination of neuronal circuitry matures by late adulthood, and thus becomes more resistant to impairment of motivational processes in the anterior cingulate gyrus.4,5,6
LOS is characterized by paranoid personality with predominantly positive symptoms, likely due to disruptions in
Other features of LOS include a high female:male ratio and symptomatic improvement with antipsychotics.7,10 Studies show that the LOS ratio of women:men can range from 2.2:1 to 22.5:1, which could be explained by the effect of dopaminergic-modulating estrogen from different sex-specific aging brain patterns.8,11,12 Finally, patients with LOS are less likely to seek care for sensory deficits than their age-equivalent counterparts.8,10 Fortunately, many of the characteristics of LOS predict good prognosis: Patients are usually female, display positive symptoms, have acute onset of symptoms, and are married with social support.10
Diagnosing LOS
LOS can be challenging to diagnose because of its atypical presentation compared with EOS, relative rarity in the population, and its propensity to be confused with progressive Alzheimer disease/dementia, delusional disorder, and major depressive disorder with psychotic features.3,6 Patients with no prior psychiatric history often do not have ready access to psychiatrists and depend on PCPs and other clinicians to identify mental health issues. A careful history, including familial involvement, utilization of the Montreal Cognitive Assessment (MoCA) test, and evaluation of environmental factors, are crucial to arriving at the proper diagnosis.
Continue to: Differential diagnosis
Differential diagnosis. When psychosis appears later in life, it is important to consider a broad differential (TABLE13-18), which includes the following:
Alzheimer disease. LOS can be easily differentiated from psychosis associated with Alzheimer disease or dementia through findings from neuropsychologic assessments and brain imaging. The initial first-line assessment for Alzheimer disease includes determining time course of daily living impairment and memory with follow-up brain imaging. Magnetic resonance imaging of patients with Alzheimer disease shows clear atrophy of the medial temporal lobes and general brain atrophy.19 Other than hypoperfusion in the frontal and temporal area, brain imaging of patients with LOS will not reveal any pathology.1
Delusional disorder and LOS are often more challenging to differentiate because symptoms can overlap, and many of the negative symptoms that would otherwise help clinicians diagnose schizophrenia in a younger population are absent in LOS. The milder symptoms of LOS may also lead clinicians to favor a diagnosis of delusional disorder. However, the following differences can help physicians differentiate between LOS and delusional disorder. Delusional disorder20-22:
- often will include paranoid beliefs, but these beliefs will not be bizarre, and the patient’s daily functioning will not be impaired, whereas patients with schizophrenia would have an increase in isolation and impairment in functioning that tends to be distinct from baseline.
- is more rare than schizophrenia. Delusional disorder has a prevalence of 0.05% to 0.1% compared to 1% for schizophrenia.
Major depressive disorder (MDD) with psychotic features. Major depressive disorder with psychotic features is an important differential to consider in this setting because the treatment intervention can be considerably different. Among patients who have MDD with psychotic features, a significant mood component is present, and treatment typically focuses on optimizing a selective serotonin reuptake inhibitor (SSRI); depending on severity, electroconvulsive therapy (ECT) also may be warranted.19
Continue to: For patients with LOS...
For patients with LOS, optimizing an antipsychotic medication is the typical course of treatment, and ECT would likely have less of an impact than it does with MDD with psychotic features.
Other. Finally, in an acute setting, other differential diagnoses for mental status changes (depending on clinical findings) might include:
- drug/medication use
- delirium
- nutrient deficiencies
- acute head trauma
- chronic subdural hematoma
- syphilis
- Lyme disease
- HIV encephalitis
- heavy metal toxicity.
Treatment involves antipsychotics—especially certain ones
Antipsychotic medications are utilized for the treatment of patients with LOS. A Cochrane review concluded that there are no trial-based evidence guidelines for the treatment of patients with LOS, and that physicians should continue with their current practice and use clinical judgment and prescribing patterns to guide their selection of antipsychotic medications.22,23 Pearlson et al24 found that 76% of patients with schizophrenia achieved at least partial remission and 48% achieved full remission with antipsychotic treatment.
The preferred treatment for patients with schizophrenia is low doses of newer antipsychotics (atypical or second-generation antipsychotics [SGAs]) because they are less likely to cause extrapyramidal symptoms/adverse effects than first-generation antipsychotics. Examples of SGAs include aripiprazole, risperidone, olanzapine, quetiapine, and ziprasidone.
Effective treatment for LOS includes antipsychotics at a quarter to one-half of the usual therapeutic doses. In patients with very late-onset schizophrenia, doses should be started at a tenth of therapeutic dose.1,23 Physicians should titrate up carefully, as needed.
Continue to: As with any significant mental illness...
As with any significant mental illness, to improve clinical outcomes, family support may help patients’ medication adherence and ensure they attend scheduled medical appointments.
Our patient was eventually stabilized on long-acting injectable risperidone, 25 mg, with improvement in symptoms. Unfortunately, she was not convinced that her symptoms were psychiatric in nature and did not continue with her medications as an outpatient.
The patient’s nonadherence to her medication regimen led to 2 more hospitalizations with similar presentations over the following 2 years. On her most recent discharge, she was stabilized on oral olanzapine, 10 mg every night at bedtime, with close outpatient follow-up and family education.
THE TAKEAWAY
The prodromal phase of patients with LOS is similar to patients with EOS and includes withdrawal and isolation from others, making it difficult for physicians to evaluate and treat patients. Patients with LOS predominantly experience positive symptoms that may include delusions and hallucinations. Brain imaging studies can help rule out progressive dementia diseases. A neuropsychological evaluation can assess the patient’s functional level and types of delusions, which helps to differentiate LOS from other late-age psychoses. Treatment with SGAs make for a good prognosis; however, this requires patients to be adherent to treatment.
CORRESPONDENCE
Sandy Chan, MD, Department of Internal Medicine, UMass Memorial Medical Center, 55N Lake Avenue, Worcester, MA 01605; Sandy.Chan@umassmemorial.org
THE CASE
A 58-year-old married Asian woman with no apparent psychiatric history presented to the emergency department (ED) in an acute state with ideas of reference, paranoid delusions, and multiple, vague somatic symptoms.
Based on information in the patient’s medical record, there had been suspicion of an underlying psychiatric disorder 6 years earlier. At that time, the patient had presented to her primary care provider (PCP) with vague somatic complaints, including diffuse body pain, dry cough, chills, weakness, facial numbness, and concerns about infections. A physical examination and work-up did not reveal the source of her complaints. Unfortunately, the patient’s complaints increased in number and severity over time.
Her medical records also indicated that she had been assessed for depression severity using the Patient Health Questionnaire-9 (PHQ-9), with scores of 0 (4 years earlier) and 3 (3 years earlier). The scores suggested that she was not suffering from depression.
During this time, the patient also saw a psychiatrist; however, it was unclear whether her symptoms met the criteria for delusional disorder or schizophrenia because she did not exhibit negative symptoms or sensory hallucinations. In addition, the patient was extremely high-functioning in the community—she participated in dance classes and other social events—and had the equivalent of a medical degree from another country. Based on chart review, when she went to the psychiatrist 3 years prior to her current presentation, there were no antipsychotics prescribed.
In the weeks leading up to her current presentation, the patient reported that she was struggling with sleep, sometimes spending days in bed and other times needing unspecified medication obtained overseas to help her sleep. Her husband reported that she had become increasingly withdrawn and stopped attending her dance classes and social events.
The patient believed the government was trying to poison her via radiation and that unknown people were trying to harm her via an online messaging application. Immediately prior to her arrival in the ED, the police were called to pull her away from oncoming traffic because she ran into the road to find the assassins that were stalking her.
During this recent visit to the ED, the patient presented with labile affect, rapid speech, and anxious and angry mood. She complained about darkened spots on her arm (inflicted through radiation by the media), vaginal bleeding, paralysis below the waist (although she was pacing around), and unspecific pain around her belly. Physical examination revealed no obvious signs of head trauma, intact extraocular movements, no coughing or wheezing, regular heart rate and rhythm, a nontender abdomen to palpation, and normal bowel sounds. No focal neurological deficits were appreciated. She had no rashes, bruises, or skin abrasions on her abdomen or upper extremities.
Continue to: The patient tried to...
The patient tried to leave the ED, saying that her third eye could see the radiation. She required medication and 4-point restraints.
Her initial laboratory work-up for heavy metals, Lyme disease, human immunodeficiency virus (HIV), syphilis, delirium, and drug use were all negative. She also underwent head imaging studies that were also found to be negative. Her mental status exam was notable for a tangential thought process, preservation of delusions with loose associations, labile mood, and dysphoric affect. The patient demonstrated limited insight and judgment, although she was fully oriented to person, place, and time, which suggested against delirium at the time of evaluation.
THE DIAGNOSIS
Based on the patient’s current presentation and in light of her medical history, the health care team arrived at a
DISCUSSION
Schizophrenia is a severe, lifelong mental disorder characterized by at least 2 symptoms of delusions, hallucinations, disorganized speech, disorganized or catatonic behavior, or negative symptoms for at least 6 months, with significant social, occupational, and functional deterioration. Current models attribute the neurodevelopmental deregulation of the brain in patients with schizophrenia to dopaminergic hyperactivity and hypofunction of the glutamatergic neurotransmitter system, explaining why its onset is usually in adolescence or young adulthood.1,2 However, 23% of patients present with symptoms after age 40, with 7% of patients being diagnosed between the ages of 51 and 60.3
Late-onset vs early-onset schizophrenia. LOS is often a missed diagnosis because the clinical presentation is different from early-onset schizophrenia (EOS). Although the prodromal symptoms of EOS and LOS are similar and include marked isolation that subsequently progresses to suspiciousness and ideas of reference,4 patients with EOS often also have prodromal negative symptoms. These prodromal negative symptoms associated with EOS may include loss of motivation, social passivity, and disorganized behavior. These symptoms are hypothesized to be caused by dopaminergic dysregulation in the anterior cingulate cortex. EOS is characterized by the patient experiencing more negative symptoms than LOS, which is characterized by the patient experiencing more positive symptoms.
Continue to: Patients with late-onset schizophrenia...
Patients with LOS typically do not exhibit negative symptoms because remodeling and myelination of neuronal circuitry matures by late adulthood, and thus becomes more resistant to impairment of motivational processes in the anterior cingulate gyrus.4,5,6
LOS is characterized by paranoid personality with predominantly positive symptoms, likely due to disruptions in
Other features of LOS include a high female:male ratio and symptomatic improvement with antipsychotics.7,10 Studies show that the LOS ratio of women:men can range from 2.2:1 to 22.5:1, which could be explained by the effect of dopaminergic-modulating estrogen from different sex-specific aging brain patterns.8,11,12 Finally, patients with LOS are less likely to seek care for sensory deficits than their age-equivalent counterparts.8,10 Fortunately, many of the characteristics of LOS predict good prognosis: Patients are usually female, display positive symptoms, have acute onset of symptoms, and are married with social support.10
Diagnosing LOS
LOS can be challenging to diagnose because of its atypical presentation compared with EOS, relative rarity in the population, and its propensity to be confused with progressive Alzheimer disease/dementia, delusional disorder, and major depressive disorder with psychotic features.3,6 Patients with no prior psychiatric history often do not have ready access to psychiatrists and depend on PCPs and other clinicians to identify mental health issues. A careful history, including familial involvement, utilization of the Montreal Cognitive Assessment (MoCA) test, and evaluation of environmental factors, are crucial to arriving at the proper diagnosis.
Continue to: Differential diagnosis
Differential diagnosis. When psychosis appears later in life, it is important to consider a broad differential (TABLE13-18), which includes the following:
Alzheimer disease. LOS can be easily differentiated from psychosis associated with Alzheimer disease or dementia through findings from neuropsychologic assessments and brain imaging. The initial first-line assessment for Alzheimer disease includes determining time course of daily living impairment and memory with follow-up brain imaging. Magnetic resonance imaging of patients with Alzheimer disease shows clear atrophy of the medial temporal lobes and general brain atrophy.19 Other than hypoperfusion in the frontal and temporal area, brain imaging of patients with LOS will not reveal any pathology.1
Delusional disorder and LOS are often more challenging to differentiate because symptoms can overlap, and many of the negative symptoms that would otherwise help clinicians diagnose schizophrenia in a younger population are absent in LOS. The milder symptoms of LOS may also lead clinicians to favor a diagnosis of delusional disorder. However, the following differences can help physicians differentiate between LOS and delusional disorder. Delusional disorder20-22:
- often will include paranoid beliefs, but these beliefs will not be bizarre, and the patient’s daily functioning will not be impaired, whereas patients with schizophrenia would have an increase in isolation and impairment in functioning that tends to be distinct from baseline.
- is more rare than schizophrenia. Delusional disorder has a prevalence of 0.05% to 0.1% compared to 1% for schizophrenia.
Major depressive disorder (MDD) with psychotic features. Major depressive disorder with psychotic features is an important differential to consider in this setting because the treatment intervention can be considerably different. Among patients who have MDD with psychotic features, a significant mood component is present, and treatment typically focuses on optimizing a selective serotonin reuptake inhibitor (SSRI); depending on severity, electroconvulsive therapy (ECT) also may be warranted.19
Continue to: For patients with LOS...
For patients with LOS, optimizing an antipsychotic medication is the typical course of treatment, and ECT would likely have less of an impact than it does with MDD with psychotic features.
Other. Finally, in an acute setting, other differential diagnoses for mental status changes (depending on clinical findings) might include:
- drug/medication use
- delirium
- nutrient deficiencies
- acute head trauma
- chronic subdural hematoma
- syphilis
- Lyme disease
- HIV encephalitis
- heavy metal toxicity.
Treatment involves antipsychotics—especially certain ones
Antipsychotic medications are utilized for the treatment of patients with LOS. A Cochrane review concluded that there are no trial-based evidence guidelines for the treatment of patients with LOS, and that physicians should continue with their current practice and use clinical judgment and prescribing patterns to guide their selection of antipsychotic medications.22,23 Pearlson et al24 found that 76% of patients with schizophrenia achieved at least partial remission and 48% achieved full remission with antipsychotic treatment.
The preferred treatment for patients with schizophrenia is low doses of newer antipsychotics (atypical or second-generation antipsychotics [SGAs]) because they are less likely to cause extrapyramidal symptoms/adverse effects than first-generation antipsychotics. Examples of SGAs include aripiprazole, risperidone, olanzapine, quetiapine, and ziprasidone.
Effective treatment for LOS includes antipsychotics at a quarter to one-half of the usual therapeutic doses. In patients with very late-onset schizophrenia, doses should be started at a tenth of therapeutic dose.1,23 Physicians should titrate up carefully, as needed.
Continue to: As with any significant mental illness...
As with any significant mental illness, to improve clinical outcomes, family support may help patients’ medication adherence and ensure they attend scheduled medical appointments.
Our patient was eventually stabilized on long-acting injectable risperidone, 25 mg, with improvement in symptoms. Unfortunately, she was not convinced that her symptoms were psychiatric in nature and did not continue with her medications as an outpatient.
The patient’s nonadherence to her medication regimen led to 2 more hospitalizations with similar presentations over the following 2 years. On her most recent discharge, she was stabilized on oral olanzapine, 10 mg every night at bedtime, with close outpatient follow-up and family education.
THE TAKEAWAY
The prodromal phase of patients with LOS is similar to patients with EOS and includes withdrawal and isolation from others, making it difficult for physicians to evaluate and treat patients. Patients with LOS predominantly experience positive symptoms that may include delusions and hallucinations. Brain imaging studies can help rule out progressive dementia diseases. A neuropsychological evaluation can assess the patient’s functional level and types of delusions, which helps to differentiate LOS from other late-age psychoses. Treatment with SGAs make for a good prognosis; however, this requires patients to be adherent to treatment.
CORRESPONDENCE
Sandy Chan, MD, Department of Internal Medicine, UMass Memorial Medical Center, 55N Lake Avenue, Worcester, MA 01605; Sandy.Chan@umassmemorial.org
1. Howard R, Rabins P, Seeman M, et al. Late-onset schizophrenia and very-late-onset schizophrenia-like psychosis: an international crisis. Am J Psychiatry 2000;157:172-178.
2. Pickard B. Progress in defining the biological causes of schizophrenia. Expert Rev Mol Med. 2011;13:e25.
3. Jeste D, Symonds L, Harris M, et al. Nondementia nonpraecox dementia praecox? Am J Geriatr Psychiatry. 1997;5:302-317.
4. Gourzis P, Katrivanou A, Beratis S. Symptomatology of the initial prodromal phase of schizophrenia. Schizophr Bull. 2002;28:415-429.
5. Dolan R, Fletcher P, Frith C, et al. Dopaminergic modulation of impaired cognitive activation in the anterior cingulate cortex in schizophrenia. Nature. 1995;378:180-182.
6. Skokou M, Katrivanou A, Andriopoulos I, et al. Active and prodromal phase symptomatology of young-onset and late-onset paranoid schizophrenia. Rev Psiquiatr Salud Ment. 2012;5:150-159.
7. Riecher-Rossler A, Loffler W, Munk-Jorgensen P. What do we really know about late-onset schizophrenia? Eur Arch Psychiatry Clin Neurosci. 1997;247:195-208.
8. Lubman D, Castle D. Late-onset schizophrenia: make the right diagnosis when psychosis emerges after age 60. Current Psychiatry. 2002;1:35-44.
9. Howard R, Castle D, Wessely S, et al. A comparative study of 470 cases of early-onset and late-onset schizophrenia. British Journal of Psychiatry. 1993;163:352-357.
10. Harris M, Jeste D. Late-onset schizophrenia: an overview. Schizophr Bull. 1988;14:39-55.
11. Castle D, Murray R. The epidemiology of late-onset schizophrenia. Schizophr Bull. 1993;19:691-700.
12. Lindamer L, Lohr J, Harris M, Jeste D. Gender, estrogen, and schizophrenia. Psychopharmacol Bull. 1997;33:221-228.
13. Gaudiano BA, Dalrymple KL, Zimmerman M. Prevalence and clinical characteristics of psychotic versus non-psychotic major depression in a general psychiatric outpatient clinic. Depress Anxiety. 2009;26:54-64.
14. Saha S, Chant D, Welham J, et al. A systematic review of the prevalence of schizophrenia. PLoS Med. 2005;2:e141.
15. Gao S, Hendrie H, Hall K. The relationships between age, sex, and the incidence of dementia and Alzheimer Disease. JAMA Psychiatry. 1998;55:809-815.
16. Reitz C, Brayne C, Mayeux R. Epidemiology of Alzheimer Disease. Nature Reviews Neurology. 2011;7:137-152
17. Winokur G. Delusional Disorder (Paranoia). Comprehensive Psychiatry. 1977;18:511-521.
18. Scheltens P, Leys D, Huglo D, et al. Atrophy of medial temporal lobes on MRI in “probable” Alzheimer’s disease and normal ageing: diagnostic value and neuropsychological correlates. J Neurol, Neurosurg Psychiatry. 1992;55:967-972.
19. Copeland J, Dewey M, Scott A, et al. Schizophrenia and delusional disorder in older age: community prevalence, incidence, comorbidity, and outcome. Schizophr Bull. 1998;24:153-161.
20. Kendler K. Demography of paranoid psychosis (delusional disorder): a review and comparison with schizophrenia and affective illness. Arch Gen Psychiatry 1982;39:890-902.
21. McGrath J, Saha S, Chant D, et al. Schizophrenia: a concise overview of incidence, prevalence, and mortality. Epidemiol Rev. 2008;30:67.
22. Essali A, Ali G. Antipsychotic drug treatment for elderly people with late-onset schizophrenia. Cochrane Database Syst Rev. 2012;(2):CD004162.
23. Sweet R, Pollock B. New atypical antipsychotics- experience and utility in the elderly. Drugs Aging. 1998;12:115-127.
24. Pearlson G, Kreger L, Rabins P, et al. A chart review study of late-onset and early-onset schizophrenia. Am J Psychiatry.1989;146:1568-1574.
1. Howard R, Rabins P, Seeman M, et al. Late-onset schizophrenia and very-late-onset schizophrenia-like psychosis: an international crisis. Am J Psychiatry 2000;157:172-178.
2. Pickard B. Progress in defining the biological causes of schizophrenia. Expert Rev Mol Med. 2011;13:e25.
3. Jeste D, Symonds L, Harris M, et al. Nondementia nonpraecox dementia praecox? Am J Geriatr Psychiatry. 1997;5:302-317.
4. Gourzis P, Katrivanou A, Beratis S. Symptomatology of the initial prodromal phase of schizophrenia. Schizophr Bull. 2002;28:415-429.
5. Dolan R, Fletcher P, Frith C, et al. Dopaminergic modulation of impaired cognitive activation in the anterior cingulate cortex in schizophrenia. Nature. 1995;378:180-182.
6. Skokou M, Katrivanou A, Andriopoulos I, et al. Active and prodromal phase symptomatology of young-onset and late-onset paranoid schizophrenia. Rev Psiquiatr Salud Ment. 2012;5:150-159.
7. Riecher-Rossler A, Loffler W, Munk-Jorgensen P. What do we really know about late-onset schizophrenia? Eur Arch Psychiatry Clin Neurosci. 1997;247:195-208.
8. Lubman D, Castle D. Late-onset schizophrenia: make the right diagnosis when psychosis emerges after age 60. Current Psychiatry. 2002;1:35-44.
9. Howard R, Castle D, Wessely S, et al. A comparative study of 470 cases of early-onset and late-onset schizophrenia. British Journal of Psychiatry. 1993;163:352-357.
10. Harris M, Jeste D. Late-onset schizophrenia: an overview. Schizophr Bull. 1988;14:39-55.
11. Castle D, Murray R. The epidemiology of late-onset schizophrenia. Schizophr Bull. 1993;19:691-700.
12. Lindamer L, Lohr J, Harris M, Jeste D. Gender, estrogen, and schizophrenia. Psychopharmacol Bull. 1997;33:221-228.
13. Gaudiano BA, Dalrymple KL, Zimmerman M. Prevalence and clinical characteristics of psychotic versus non-psychotic major depression in a general psychiatric outpatient clinic. Depress Anxiety. 2009;26:54-64.
14. Saha S, Chant D, Welham J, et al. A systematic review of the prevalence of schizophrenia. PLoS Med. 2005;2:e141.
15. Gao S, Hendrie H, Hall K. The relationships between age, sex, and the incidence of dementia and Alzheimer Disease. JAMA Psychiatry. 1998;55:809-815.
16. Reitz C, Brayne C, Mayeux R. Epidemiology of Alzheimer Disease. Nature Reviews Neurology. 2011;7:137-152
17. Winokur G. Delusional Disorder (Paranoia). Comprehensive Psychiatry. 1977;18:511-521.
18. Scheltens P, Leys D, Huglo D, et al. Atrophy of medial temporal lobes on MRI in “probable” Alzheimer’s disease and normal ageing: diagnostic value and neuropsychological correlates. J Neurol, Neurosurg Psychiatry. 1992;55:967-972.
19. Copeland J, Dewey M, Scott A, et al. Schizophrenia and delusional disorder in older age: community prevalence, incidence, comorbidity, and outcome. Schizophr Bull. 1998;24:153-161.
20. Kendler K. Demography of paranoid psychosis (delusional disorder): a review and comparison with schizophrenia and affective illness. Arch Gen Psychiatry 1982;39:890-902.
21. McGrath J, Saha S, Chant D, et al. Schizophrenia: a concise overview of incidence, prevalence, and mortality. Epidemiol Rev. 2008;30:67.
22. Essali A, Ali G. Antipsychotic drug treatment for elderly people with late-onset schizophrenia. Cochrane Database Syst Rev. 2012;(2):CD004162.
23. Sweet R, Pollock B. New atypical antipsychotics- experience and utility in the elderly. Drugs Aging. 1998;12:115-127.
24. Pearlson G, Kreger L, Rabins P, et al. A chart review study of late-onset and early-onset schizophrenia. Am J Psychiatry.1989;146:1568-1574.
Home-based chemo skyrockets at one U.S. center
Major organization opposes concept
In the fall of 2019, the University of Pennsylvania in Philadelphia started a pilot program of home-based chemotherapy for two treatment regimens (one via infusion and one via injection). Six months later, the Cancer Care at Home program had treated 40 patients.
The uptake within the university’s large regional health system was acceptable but not rapid, admitted Amy Laughlin, MD, a hematology-oncology fellow involved with the program.
Then COVID-19 arrived, along with related travel restrictions.
Suddenly, in a 5-week period (March to April 7), 175 patients had been treated – a 300% increase from the first half year. Program staff jumped from 12 to 80 employees. The list of chemotherapies delivered went from two to seven, with more coming.
“We’re not the pilot anymore – we’re the standard of care,” Laughlin told Medscape Medical News.
“The impact [on patients] is amazing,” she said. “As long as you are selecting the right patients and right therapy, it is feasible and even preferable for a lot of patients.”
For example, patients with hormone-positive breast cancer who receive leuprolide (to shut down the ovaries and suppress estrogen production) ordinarily would have to visit a Penn facility for an injection every month, potentially for years. Now, a nurse can meet patients at home (or before the COVID-19 pandemic, even at their place of work) and administer the injection, saving the patient travel time and associated costs.
This home-based chemotherapy service does not appear to be offered elsewhere in the United States, and a major oncology organization – the Community Oncology Alliance – is opposed to the practice because of patient safety concerns.
The service is not offered at a sample of cancer centers queried by Medscape Medical News, including the Dana-Farber Cancer Institute in Boston, the Moffitt Cancer Center in Tampa, the Huntsman Cancer Institute in Salt Lake City, Utah, and Moores Cancer Center, the University of California, San Diego.
Opposition because of safety concerns
On April 9, the Community Oncology Alliance (COA) issued a statement saying it “fundamentally opposes home infusion of chemotherapy, cancer immunotherapy, and cancer treatment supportive drugs because of serious patient safety concerns.”
The COA warned that “many of the side effects caused by cancer treatment can have a rapid, unpredictable onset that places patients in incredible jeopardy and can even be life-threatening.”
In contrast, in a recent communication related to COVID-19, the National Comprehensive Cancer Network tacitly endorsed the concept, stating that a number of chemotherapies may potentially be administered at home, but it did not include guidelines for doing so.
The American Society of Clinical Oncology said that chemotherapy at home is “an issue [we] are monitoring closely,” according to a spokesperson.
What’s involved
Criteria for home-based chemotherapy at Penn include use of anticancer therapies that a patient has previously tolerated and low toxicity (that can be readily managed in the home setting). In addition, patients must be capable of following a med chart.
The chemotherapy is reconstituted at a Penn facility in a Philadelphia suburb. A courier then delivers the drug to the patient’s home, where it is administered by an oncology-trained nurse. Drugs must be stable for at least a few hours to qualify for the program.
The Penn program started with two regimens: EPOCH (etoposide, vincristine, doxorubicin, cyclophosphamide, and prednisone) for lymphoma, and leuprolide acetate injections for either breast or prostate cancer.
The two treatments are polar opposites in terms of complexity, common usage, and time required, which was intended, said Laughlin.
Time to deliver the chemo varies from a matter of minutes with leuprolide to more than 2 hours for rituximab, a lymphoma drug that may be added to EPOCH.
The current list of at-home chemo agents in the Penn program also includes bortezomib, lanreotide, zoledronic acid, and denosumab. Soon to come are rituximab and pembrolizumab for lung cancer and head and neck cancer.
Already practiced in some European countries
Home-based chemotherapy dates from at least the 1980s in the medical literature and is practiced in some European countries.
A 2018 randomized study of adjuvant treatment with capecitabine and oxaliplatin for stage II/III colon cancer in Denmark, where home-based care has been practiced for the past 2 years and is growing in use, concluded that “it might be a valuable alternative to treatment at an outpatient clinic.”
However, in the study, there was no difference in quality of life between the home and outpatient settings, which is somewhat surprising, inasmuch as a major appeal to receiving chemotherapy at home is that it is less disruptive compared to receiving it in a hospital or clinic, which requires travel.
Also, chemo at home “may be resource intensive” and have a “lower throughput of patients due to transportation time,” cautioned the Danish investigators, who were from Herlev and Gentofte Hospital.
A 2015 review called home chemo “a safe and patient‐centered alternative to hospital‐ and outpatient‐based service.” Jenna Evans, PhD, McMaster University, Toronto, Canada, and lead author of that review, says there are two major barriers to infusion chemotherapy in homes.
One is inadequate resources in the community, such as oncology-trained nurses to deliver treatment, and the other is perceptions of safety and quality, including among healthcare providers.
COVID-19 might prompt more chemo at home, said Evans, a health policy expert, in an email to Medscape Medical News. “It is not unusual for change of this type and scale to require a seismic event to become more mainstream,” she argued.
Reimbursement for home-based chemo is usually the same as for chemo in a free-standing infusion suite, says Cassandra Redmond, PharmD, MBA, director of pharmacy, Penn Home Infusion Therapy.
Private insurers and Medicare cover a subset of infused medications at home, but coverage is limited. “The opportunity now is to expand these initiatives ... to include other cancer therapies,” she said about coverage.
This article first appeared on Medscape.com.
Major organization opposes concept
Major organization opposes concept
In the fall of 2019, the University of Pennsylvania in Philadelphia started a pilot program of home-based chemotherapy for two treatment regimens (one via infusion and one via injection). Six months later, the Cancer Care at Home program had treated 40 patients.
The uptake within the university’s large regional health system was acceptable but not rapid, admitted Amy Laughlin, MD, a hematology-oncology fellow involved with the program.
Then COVID-19 arrived, along with related travel restrictions.
Suddenly, in a 5-week period (March to April 7), 175 patients had been treated – a 300% increase from the first half year. Program staff jumped from 12 to 80 employees. The list of chemotherapies delivered went from two to seven, with more coming.
“We’re not the pilot anymore – we’re the standard of care,” Laughlin told Medscape Medical News.
“The impact [on patients] is amazing,” she said. “As long as you are selecting the right patients and right therapy, it is feasible and even preferable for a lot of patients.”
For example, patients with hormone-positive breast cancer who receive leuprolide (to shut down the ovaries and suppress estrogen production) ordinarily would have to visit a Penn facility for an injection every month, potentially for years. Now, a nurse can meet patients at home (or before the COVID-19 pandemic, even at their place of work) and administer the injection, saving the patient travel time and associated costs.
This home-based chemotherapy service does not appear to be offered elsewhere in the United States, and a major oncology organization – the Community Oncology Alliance – is opposed to the practice because of patient safety concerns.
The service is not offered at a sample of cancer centers queried by Medscape Medical News, including the Dana-Farber Cancer Institute in Boston, the Moffitt Cancer Center in Tampa, the Huntsman Cancer Institute in Salt Lake City, Utah, and Moores Cancer Center, the University of California, San Diego.
Opposition because of safety concerns
On April 9, the Community Oncology Alliance (COA) issued a statement saying it “fundamentally opposes home infusion of chemotherapy, cancer immunotherapy, and cancer treatment supportive drugs because of serious patient safety concerns.”
The COA warned that “many of the side effects caused by cancer treatment can have a rapid, unpredictable onset that places patients in incredible jeopardy and can even be life-threatening.”
In contrast, in a recent communication related to COVID-19, the National Comprehensive Cancer Network tacitly endorsed the concept, stating that a number of chemotherapies may potentially be administered at home, but it did not include guidelines for doing so.
The American Society of Clinical Oncology said that chemotherapy at home is “an issue [we] are monitoring closely,” according to a spokesperson.
What’s involved
Criteria for home-based chemotherapy at Penn include use of anticancer therapies that a patient has previously tolerated and low toxicity (that can be readily managed in the home setting). In addition, patients must be capable of following a med chart.
The chemotherapy is reconstituted at a Penn facility in a Philadelphia suburb. A courier then delivers the drug to the patient’s home, where it is administered by an oncology-trained nurse. Drugs must be stable for at least a few hours to qualify for the program.
The Penn program started with two regimens: EPOCH (etoposide, vincristine, doxorubicin, cyclophosphamide, and prednisone) for lymphoma, and leuprolide acetate injections for either breast or prostate cancer.
The two treatments are polar opposites in terms of complexity, common usage, and time required, which was intended, said Laughlin.
Time to deliver the chemo varies from a matter of minutes with leuprolide to more than 2 hours for rituximab, a lymphoma drug that may be added to EPOCH.
The current list of at-home chemo agents in the Penn program also includes bortezomib, lanreotide, zoledronic acid, and denosumab. Soon to come are rituximab and pembrolizumab for lung cancer and head and neck cancer.
Already practiced in some European countries
Home-based chemotherapy dates from at least the 1980s in the medical literature and is practiced in some European countries.
A 2018 randomized study of adjuvant treatment with capecitabine and oxaliplatin for stage II/III colon cancer in Denmark, where home-based care has been practiced for the past 2 years and is growing in use, concluded that “it might be a valuable alternative to treatment at an outpatient clinic.”
However, in the study, there was no difference in quality of life between the home and outpatient settings, which is somewhat surprising, inasmuch as a major appeal to receiving chemotherapy at home is that it is less disruptive compared to receiving it in a hospital or clinic, which requires travel.
Also, chemo at home “may be resource intensive” and have a “lower throughput of patients due to transportation time,” cautioned the Danish investigators, who were from Herlev and Gentofte Hospital.
A 2015 review called home chemo “a safe and patient‐centered alternative to hospital‐ and outpatient‐based service.” Jenna Evans, PhD, McMaster University, Toronto, Canada, and lead author of that review, says there are two major barriers to infusion chemotherapy in homes.
One is inadequate resources in the community, such as oncology-trained nurses to deliver treatment, and the other is perceptions of safety and quality, including among healthcare providers.
COVID-19 might prompt more chemo at home, said Evans, a health policy expert, in an email to Medscape Medical News. “It is not unusual for change of this type and scale to require a seismic event to become more mainstream,” she argued.
Reimbursement for home-based chemo is usually the same as for chemo in a free-standing infusion suite, says Cassandra Redmond, PharmD, MBA, director of pharmacy, Penn Home Infusion Therapy.
Private insurers and Medicare cover a subset of infused medications at home, but coverage is limited. “The opportunity now is to expand these initiatives ... to include other cancer therapies,” she said about coverage.
This article first appeared on Medscape.com.
In the fall of 2019, the University of Pennsylvania in Philadelphia started a pilot program of home-based chemotherapy for two treatment regimens (one via infusion and one via injection). Six months later, the Cancer Care at Home program had treated 40 patients.
The uptake within the university’s large regional health system was acceptable but not rapid, admitted Amy Laughlin, MD, a hematology-oncology fellow involved with the program.
Then COVID-19 arrived, along with related travel restrictions.
Suddenly, in a 5-week period (March to April 7), 175 patients had been treated – a 300% increase from the first half year. Program staff jumped from 12 to 80 employees. The list of chemotherapies delivered went from two to seven, with more coming.
“We’re not the pilot anymore – we’re the standard of care,” Laughlin told Medscape Medical News.
“The impact [on patients] is amazing,” she said. “As long as you are selecting the right patients and right therapy, it is feasible and even preferable for a lot of patients.”
For example, patients with hormone-positive breast cancer who receive leuprolide (to shut down the ovaries and suppress estrogen production) ordinarily would have to visit a Penn facility for an injection every month, potentially for years. Now, a nurse can meet patients at home (or before the COVID-19 pandemic, even at their place of work) and administer the injection, saving the patient travel time and associated costs.
This home-based chemotherapy service does not appear to be offered elsewhere in the United States, and a major oncology organization – the Community Oncology Alliance – is opposed to the practice because of patient safety concerns.
The service is not offered at a sample of cancer centers queried by Medscape Medical News, including the Dana-Farber Cancer Institute in Boston, the Moffitt Cancer Center in Tampa, the Huntsman Cancer Institute in Salt Lake City, Utah, and Moores Cancer Center, the University of California, San Diego.
Opposition because of safety concerns
On April 9, the Community Oncology Alliance (COA) issued a statement saying it “fundamentally opposes home infusion of chemotherapy, cancer immunotherapy, and cancer treatment supportive drugs because of serious patient safety concerns.”
The COA warned that “many of the side effects caused by cancer treatment can have a rapid, unpredictable onset that places patients in incredible jeopardy and can even be life-threatening.”
In contrast, in a recent communication related to COVID-19, the National Comprehensive Cancer Network tacitly endorsed the concept, stating that a number of chemotherapies may potentially be administered at home, but it did not include guidelines for doing so.
The American Society of Clinical Oncology said that chemotherapy at home is “an issue [we] are monitoring closely,” according to a spokesperson.
What’s involved
Criteria for home-based chemotherapy at Penn include use of anticancer therapies that a patient has previously tolerated and low toxicity (that can be readily managed in the home setting). In addition, patients must be capable of following a med chart.
The chemotherapy is reconstituted at a Penn facility in a Philadelphia suburb. A courier then delivers the drug to the patient’s home, where it is administered by an oncology-trained nurse. Drugs must be stable for at least a few hours to qualify for the program.
The Penn program started with two regimens: EPOCH (etoposide, vincristine, doxorubicin, cyclophosphamide, and prednisone) for lymphoma, and leuprolide acetate injections for either breast or prostate cancer.
The two treatments are polar opposites in terms of complexity, common usage, and time required, which was intended, said Laughlin.
Time to deliver the chemo varies from a matter of minutes with leuprolide to more than 2 hours for rituximab, a lymphoma drug that may be added to EPOCH.
The current list of at-home chemo agents in the Penn program also includes bortezomib, lanreotide, zoledronic acid, and denosumab. Soon to come are rituximab and pembrolizumab for lung cancer and head and neck cancer.
Already practiced in some European countries
Home-based chemotherapy dates from at least the 1980s in the medical literature and is practiced in some European countries.
A 2018 randomized study of adjuvant treatment with capecitabine and oxaliplatin for stage II/III colon cancer in Denmark, where home-based care has been practiced for the past 2 years and is growing in use, concluded that “it might be a valuable alternative to treatment at an outpatient clinic.”
However, in the study, there was no difference in quality of life between the home and outpatient settings, which is somewhat surprising, inasmuch as a major appeal to receiving chemotherapy at home is that it is less disruptive compared to receiving it in a hospital or clinic, which requires travel.
Also, chemo at home “may be resource intensive” and have a “lower throughput of patients due to transportation time,” cautioned the Danish investigators, who were from Herlev and Gentofte Hospital.
A 2015 review called home chemo “a safe and patient‐centered alternative to hospital‐ and outpatient‐based service.” Jenna Evans, PhD, McMaster University, Toronto, Canada, and lead author of that review, says there are two major barriers to infusion chemotherapy in homes.
One is inadequate resources in the community, such as oncology-trained nurses to deliver treatment, and the other is perceptions of safety and quality, including among healthcare providers.
COVID-19 might prompt more chemo at home, said Evans, a health policy expert, in an email to Medscape Medical News. “It is not unusual for change of this type and scale to require a seismic event to become more mainstream,” she argued.
Reimbursement for home-based chemo is usually the same as for chemo in a free-standing infusion suite, says Cassandra Redmond, PharmD, MBA, director of pharmacy, Penn Home Infusion Therapy.
Private insurers and Medicare cover a subset of infused medications at home, but coverage is limited. “The opportunity now is to expand these initiatives ... to include other cancer therapies,” she said about coverage.
This article first appeared on Medscape.com.
AGA CPU: Screening and surveillance for hepatocellular carcinoma in patients with NAFLD
Physicians should consider liver cancer screening for all patients with nonalcoholic fatty liver disease (NAFLD) and cirrhosis, according to a new clinical practice update from the American Gastroenterological Association.
Screening “should be offered for patients with cirrhosis of varying etiologies when the risk of hepatocellular carcinoma is approximately at least 1.5% per year, as has been noted with NAFLD cirrhosis,” wrote Rohit Loomba, MD, of the University of California, San Diego, and associates. Although patients with noncirrhotic NAFLD also can develop hepatocellular carcinoma, “[a]t this point, we believe that [the benefit of screening] is restricted to patients with compensated cirrhosis or those with decompensated cirrhosis listed for liver transplantation,” they wrote in Gastroenterology.
Liver cancer in NAFLD often goes undetected until it is advanced enough that patients are not candidates for curative therapy. Current guidelines provide limited recommendations on which patients with NAFLD to monitor for hepatocellular carcinoma, how best to do so, and how often. To fill this gap, Dr. Loomba and associates reviewed and cited 79 published papers and developed eight suggestions for clinical practice.
Patients with NAFLD and stage 0-2 fibrosis are at “extremely low” risk for hepatocellular carcinoma and should not be routinely screened, the practice update stated. Advanced fibrosis is a clear risk factor but can be challenging to detect in NAFLD – imaging is often insensitive, and screening biopsy tends to be infeasible. Hence, the experts suggest considering liver cancer screening if patients with NAFLD show evidence of advanced fibrosis or cirrhosis on at least two noninvasive tests of distinct modalities (that is, the two tests should not both be point-of-care, specialized blood tests or noninvasive imaging). To improve specificity, the recommended cut-point thresholds for cirrhosis are 16.1 kPa for vibration-controlled transient elastography and 5 kPa for magnetic resonance elastography.
Screening ultrasound accurately detects hepatocellular carcinoma in patients with cirrhosis who have a good acoustic window. However, ultrasound quality is operator dependent, and it can be difficult even for experienced users to detect mass lesions in overweight or obese patients. Thus, it is important always to document parenchymal heterogeneity, beam attenuation, and whether the entire liver was visualized. If ultrasound quality is inadequate, patients should be screened every 6 months with CT or MRI, with or without alpha-fetoprotein, according to the practice update.
The authors advised clinicians to counsel all patients with NAFLD and cirrhosis to avoid alcohol and tobacco. “Irrespective of NAFLD, the bulk of epidemiological data support alcohol drinking as a major risk for hepatocellular carcinoma,” they note. Likewise, pooled studies indicate that current smokers are at about 50%-85% greater risk of liver cancer than never smokers. The experts add that “[al]though specific data do not exist, we believe that e-cigarettes may turn out to be equally harmful and patients be counseled to abstain from those as well.”
They also recommended optimally managing dyslipidemia and diabetes among patients with NAFLD who are at risk for hepatocellular carcinoma. Statins are safe for patients with NAFLD and dyslipidemia and may lower hepatocellular carcinoma risk, although more research is needed, according to the experts. For now, they support “the notion that the benefits of statin therapy among patients with dyslipidemia and NAFLD significantly outweigh the risk and should be utilized routinely.” Type 2 diabetes mellitus clearly heightens the risk of hepatocellular carcinoma, which metformin appears to reduce among patients with NAFLD, cirrhosis, and type 2 diabetes. Glucagonlike peptide–1 receptor agonists and some thiazolidinediones also appear to attenuate liver steatosis, inflammation, degeneration, and fibrosis, but it remains unclear if these effects ultimately lower cancer risk.
It is unclear if obesity directly contributes to hepatocellular carcinoma among patients with NAFLD, but obesity is an “important risk factor” for NAFLD itself, and “weight-loss interventions are strongly recommended to improve NAFLD-related outcomes,” the experts wrote. Pending further studies on whether weight loss reduces liver cancer risk in patients with NAFLD, they called for lifestyle modifications, pharmacotherapy, or bariatric surgery or bariatric endoscopy procedures to optimally manage obesity in patients with NAFLD who are at risk for liver cancer.
The authors disclosed funding from the National Institute of Environmental Health Sciences, the National Center for Advancing Translational Sciences, the National Institute of Diabetes and Digestive and Kidney Diseases, the Cancer Prevention & Research Institute of Texas, and the Center for Gastrointestinal Development, Infection and Injury. Dr. Loomba disclosed ties to Intercept Pharmaceuticals, Bird Rock Bio, Celgene, Enanta Pharmaceuticals, and a number of other companies. Two coauthors disclosed ties to Allergan, AbbVie, Conatus Pharmaceuticals, Genfit, Gilead, and Intercept. The remaining coauthor reported having no conflicts of interest.
SOURCE: Loomba R et al. Gastroenterology. 2020 Jan 29. doi: 10.1053/j.gastro.2019.12.053.
Physicians should consider liver cancer screening for all patients with nonalcoholic fatty liver disease (NAFLD) and cirrhosis, according to a new clinical practice update from the American Gastroenterological Association.
Screening “should be offered for patients with cirrhosis of varying etiologies when the risk of hepatocellular carcinoma is approximately at least 1.5% per year, as has been noted with NAFLD cirrhosis,” wrote Rohit Loomba, MD, of the University of California, San Diego, and associates. Although patients with noncirrhotic NAFLD also can develop hepatocellular carcinoma, “[a]t this point, we believe that [the benefit of screening] is restricted to patients with compensated cirrhosis or those with decompensated cirrhosis listed for liver transplantation,” they wrote in Gastroenterology.
Liver cancer in NAFLD often goes undetected until it is advanced enough that patients are not candidates for curative therapy. Current guidelines provide limited recommendations on which patients with NAFLD to monitor for hepatocellular carcinoma, how best to do so, and how often. To fill this gap, Dr. Loomba and associates reviewed and cited 79 published papers and developed eight suggestions for clinical practice.
Patients with NAFLD and stage 0-2 fibrosis are at “extremely low” risk for hepatocellular carcinoma and should not be routinely screened, the practice update stated. Advanced fibrosis is a clear risk factor but can be challenging to detect in NAFLD – imaging is often insensitive, and screening biopsy tends to be infeasible. Hence, the experts suggest considering liver cancer screening if patients with NAFLD show evidence of advanced fibrosis or cirrhosis on at least two noninvasive tests of distinct modalities (that is, the two tests should not both be point-of-care, specialized blood tests or noninvasive imaging). To improve specificity, the recommended cut-point thresholds for cirrhosis are 16.1 kPa for vibration-controlled transient elastography and 5 kPa for magnetic resonance elastography.
Screening ultrasound accurately detects hepatocellular carcinoma in patients with cirrhosis who have a good acoustic window. However, ultrasound quality is operator dependent, and it can be difficult even for experienced users to detect mass lesions in overweight or obese patients. Thus, it is important always to document parenchymal heterogeneity, beam attenuation, and whether the entire liver was visualized. If ultrasound quality is inadequate, patients should be screened every 6 months with CT or MRI, with or without alpha-fetoprotein, according to the practice update.
The authors advised clinicians to counsel all patients with NAFLD and cirrhosis to avoid alcohol and tobacco. “Irrespective of NAFLD, the bulk of epidemiological data support alcohol drinking as a major risk for hepatocellular carcinoma,” they note. Likewise, pooled studies indicate that current smokers are at about 50%-85% greater risk of liver cancer than never smokers. The experts add that “[al]though specific data do not exist, we believe that e-cigarettes may turn out to be equally harmful and patients be counseled to abstain from those as well.”
They also recommended optimally managing dyslipidemia and diabetes among patients with NAFLD who are at risk for hepatocellular carcinoma. Statins are safe for patients with NAFLD and dyslipidemia and may lower hepatocellular carcinoma risk, although more research is needed, according to the experts. For now, they support “the notion that the benefits of statin therapy among patients with dyslipidemia and NAFLD significantly outweigh the risk and should be utilized routinely.” Type 2 diabetes mellitus clearly heightens the risk of hepatocellular carcinoma, which metformin appears to reduce among patients with NAFLD, cirrhosis, and type 2 diabetes. Glucagonlike peptide–1 receptor agonists and some thiazolidinediones also appear to attenuate liver steatosis, inflammation, degeneration, and fibrosis, but it remains unclear if these effects ultimately lower cancer risk.
It is unclear if obesity directly contributes to hepatocellular carcinoma among patients with NAFLD, but obesity is an “important risk factor” for NAFLD itself, and “weight-loss interventions are strongly recommended to improve NAFLD-related outcomes,” the experts wrote. Pending further studies on whether weight loss reduces liver cancer risk in patients with NAFLD, they called for lifestyle modifications, pharmacotherapy, or bariatric surgery or bariatric endoscopy procedures to optimally manage obesity in patients with NAFLD who are at risk for liver cancer.
The authors disclosed funding from the National Institute of Environmental Health Sciences, the National Center for Advancing Translational Sciences, the National Institute of Diabetes and Digestive and Kidney Diseases, the Cancer Prevention & Research Institute of Texas, and the Center for Gastrointestinal Development, Infection and Injury. Dr. Loomba disclosed ties to Intercept Pharmaceuticals, Bird Rock Bio, Celgene, Enanta Pharmaceuticals, and a number of other companies. Two coauthors disclosed ties to Allergan, AbbVie, Conatus Pharmaceuticals, Genfit, Gilead, and Intercept. The remaining coauthor reported having no conflicts of interest.
SOURCE: Loomba R et al. Gastroenterology. 2020 Jan 29. doi: 10.1053/j.gastro.2019.12.053.
Physicians should consider liver cancer screening for all patients with nonalcoholic fatty liver disease (NAFLD) and cirrhosis, according to a new clinical practice update from the American Gastroenterological Association.
Screening “should be offered for patients with cirrhosis of varying etiologies when the risk of hepatocellular carcinoma is approximately at least 1.5% per year, as has been noted with NAFLD cirrhosis,” wrote Rohit Loomba, MD, of the University of California, San Diego, and associates. Although patients with noncirrhotic NAFLD also can develop hepatocellular carcinoma, “[a]t this point, we believe that [the benefit of screening] is restricted to patients with compensated cirrhosis or those with decompensated cirrhosis listed for liver transplantation,” they wrote in Gastroenterology.
Liver cancer in NAFLD often goes undetected until it is advanced enough that patients are not candidates for curative therapy. Current guidelines provide limited recommendations on which patients with NAFLD to monitor for hepatocellular carcinoma, how best to do so, and how often. To fill this gap, Dr. Loomba and associates reviewed and cited 79 published papers and developed eight suggestions for clinical practice.
Patients with NAFLD and stage 0-2 fibrosis are at “extremely low” risk for hepatocellular carcinoma and should not be routinely screened, the practice update stated. Advanced fibrosis is a clear risk factor but can be challenging to detect in NAFLD – imaging is often insensitive, and screening biopsy tends to be infeasible. Hence, the experts suggest considering liver cancer screening if patients with NAFLD show evidence of advanced fibrosis or cirrhosis on at least two noninvasive tests of distinct modalities (that is, the two tests should not both be point-of-care, specialized blood tests or noninvasive imaging). To improve specificity, the recommended cut-point thresholds for cirrhosis are 16.1 kPa for vibration-controlled transient elastography and 5 kPa for magnetic resonance elastography.
Screening ultrasound accurately detects hepatocellular carcinoma in patients with cirrhosis who have a good acoustic window. However, ultrasound quality is operator dependent, and it can be difficult even for experienced users to detect mass lesions in overweight or obese patients. Thus, it is important always to document parenchymal heterogeneity, beam attenuation, and whether the entire liver was visualized. If ultrasound quality is inadequate, patients should be screened every 6 months with CT or MRI, with or without alpha-fetoprotein, according to the practice update.
The authors advised clinicians to counsel all patients with NAFLD and cirrhosis to avoid alcohol and tobacco. “Irrespective of NAFLD, the bulk of epidemiological data support alcohol drinking as a major risk for hepatocellular carcinoma,” they note. Likewise, pooled studies indicate that current smokers are at about 50%-85% greater risk of liver cancer than never smokers. The experts add that “[al]though specific data do not exist, we believe that e-cigarettes may turn out to be equally harmful and patients be counseled to abstain from those as well.”
They also recommended optimally managing dyslipidemia and diabetes among patients with NAFLD who are at risk for hepatocellular carcinoma. Statins are safe for patients with NAFLD and dyslipidemia and may lower hepatocellular carcinoma risk, although more research is needed, according to the experts. For now, they support “the notion that the benefits of statin therapy among patients with dyslipidemia and NAFLD significantly outweigh the risk and should be utilized routinely.” Type 2 diabetes mellitus clearly heightens the risk of hepatocellular carcinoma, which metformin appears to reduce among patients with NAFLD, cirrhosis, and type 2 diabetes. Glucagonlike peptide–1 receptor agonists and some thiazolidinediones also appear to attenuate liver steatosis, inflammation, degeneration, and fibrosis, but it remains unclear if these effects ultimately lower cancer risk.
It is unclear if obesity directly contributes to hepatocellular carcinoma among patients with NAFLD, but obesity is an “important risk factor” for NAFLD itself, and “weight-loss interventions are strongly recommended to improve NAFLD-related outcomes,” the experts wrote. Pending further studies on whether weight loss reduces liver cancer risk in patients with NAFLD, they called for lifestyle modifications, pharmacotherapy, or bariatric surgery or bariatric endoscopy procedures to optimally manage obesity in patients with NAFLD who are at risk for liver cancer.
The authors disclosed funding from the National Institute of Environmental Health Sciences, the National Center for Advancing Translational Sciences, the National Institute of Diabetes and Digestive and Kidney Diseases, the Cancer Prevention & Research Institute of Texas, and the Center for Gastrointestinal Development, Infection and Injury. Dr. Loomba disclosed ties to Intercept Pharmaceuticals, Bird Rock Bio, Celgene, Enanta Pharmaceuticals, and a number of other companies. Two coauthors disclosed ties to Allergan, AbbVie, Conatus Pharmaceuticals, Genfit, Gilead, and Intercept. The remaining coauthor reported having no conflicts of interest.
SOURCE: Loomba R et al. Gastroenterology. 2020 Jan 29. doi: 10.1053/j.gastro.2019.12.053.
FROM GASTROENTEROLOGY
The 7 strategies of highly effective people facing the COVID-19 pandemic
A few weeks ago, I saw more than 60 responses to a post on Nextdoor.com entitled, “Toilet paper strategies?”
Asking for help is a great coping mechanism when one is struggling to find a strategy, even if it’s for toilet paper. What other kinds of coping strategies can help us through this historic and unprecedented time?
The late Stephen R. Covey, PhD, wrote about the coping strategies of highly effective people in his book, “The 7 Habits of Highly Effective People.”1 For, no matter how smart, perfect, or careful you may be, life will never be trouble free. When trouble comes, it’s important to have coping strategies that help you navigate through choppy waters. Whether you are a practitioner trying to help your patients or someone who wants to maximize their personal resilience during a worldwide pandemic, here are my conceptualizations of the seven top strategies highly effective people use when facing challenges.
Strategy #1: Begin with the end in mind
In 2007, this strategy helped me not only survive but thrive when I battled for my right to practice as a holistic psychiatrist against the Maryland Board of Physicians.2 From the first moment when I read the letter from the board, to the last when I read the administrative law judge’s dismissal, I turned to this strategy to help me cope with unrelenting stress.
I imagined myself remembering being the kind of person I wanted to be, wrote that script for myself, and created those memories for my future self. I wanted to remember myself as being brave, calm, strong, and grounded, so I behaved each day as if I were all of those things.
As Dr. Covey wrote, “ ‘Begin with the end in mind’ is based on the principle that all things are created twice. There’s a mental or first creation, and a physical or second creation to all things.” Imagine who you would like to remember yourself being a year or two down the road. Do you want to remember yourself showing good judgment and being positive and compassionate during this pandemic? Then, follow the script you’ve created in your mind and be that person now, knowing that you are forming memories for your future self. Your future self will look back at who you are right now with appreciation and satisfaction. Of course, this is a habit that you can apply to your entire life.
Strategy #2: Be proactive
Between the event and the outcome is you. You are the interpreter and transformer of the event, with the freedom to apply your will and intention on the event. Whether it is living through a pandemic or dealing with misplaced keys, every day you are revealing your nature through how you deal with life. To be proactive is different from being reactive. Within each of us there is a will, the drive, to rise above our difficult environments.
Dr. Covey wrote, “the ability to subordinate an impulse to a value is the essence of the proactive person.” A woman shared with me that she created an Excel spreadsheet with some of the things she plans to do with her free time while she stays in her NYC apartment. She doesn’t want to slip into a passive state and waste her time. That’s being proactive.
Strategy #3: Set proper priorities
Or, as Dr. Covey would say, “Put first things first.” During a pandemic, when the world seems to be precariously tilting at an angle, it’s easy to cling to outdated standards, expectations, and behavioral patterns. Doing so heightens our sense of regret, fear, and scarcity. Valuing gratitude will empower you to deal with financial loss differently because you can still remain grateful despite uncontrollable losses. We can choose “to have or to be” as psychoanalyst, Erich Fromm, PhD, would say.3 If your happiness is measured by how much money you have, then it would make sense that, when the amount shrinks, so does your happiness. However, if your happiness is a side effect of who you are, you will remain a mountain before the winds and tides of circumstance.
Strategy #4: Create a win/win mentality
This state of mind is built on character. Dr. Covey separates character into three categories: integrity, maturity, and abundance mentality. A lack of character resulted in the hoarding of toilet paper in many communities and the cry for help from Nextdoor.com. I noticed that, in the 60+ responses that included advice about using bidets, old towels, and even leaves, no one offered to share a bag of toilet paper. That’s because people experienced the fear of scarcity, in turn, causing the scarcity they feared.
During a pandemic, a highly effective person or company thinks beyond themselves to create a win/win scenario. At a grocery store in my neighborhood, a man stands at its entrance with a bottle of disinfectant spray in one hand for the shoppers and a sign on the sidewalk with guidelines for purchasing products to avoid hoarding. He tells you where the wipes are for the carts as you enter the store. People line up 6 feet apart, waiting to enter, to limit the number of shoppers inside the store, facilitating proper physical distancing. Instead of maximizing profits at the expense of everyone’s health and safety, the process is a win/win for everyone, from shoppers to employees.
Strategy #5: Develop empathy and understanding
Seeking to first understand and then be understood is one of the most powerful tools of effective people. In my holistic practice, every patient comes in with their own unique needs that evolve and transform over time. I must remain open, or I fail to deliver appropriately.
Learning to listen and then to clearly communicate ideas is essential to effective health care. During this time, it is critical that health care providers and political leaders first listen/understand and then communicate clearly to serve everyone in the best way possible.
In our brains, the frontal lobes (the adult in the room) manages our amygdala (the child in the room) when we get enough sleep, meditate, spend time in nature, exercise, and eat healthy food.4 Stress can interfere with the frontal lobe’s ability to maintain empathy, inhibit unhealthy impulses, and delay gratification. During the pandemic, we can help to shift from the stress response, or “fight-or-flight” response, driven by the sympathetic nervous system to a “rest-and-digest” response driven by the parasympathetic system through coherent breathing, taking slow, deep, relaxed breaths (6 seconds on inhalation and 6 seconds on exhalation). The vagus nerve connected to our diaphragm will help the heart return to a healthy rhythm.5
Strategy #6: Synergize and integrate
All of life is interdependent, each part no more or less important than any other. Is oxygen more important than hydrogen? Is H2O different from the oxygen and hydrogen atoms that make it?
During a pandemic, it’s important for us to appreciate each other’s contributions and work synergistically for the good of the whole. Our survival depends on valuing each other and our planet. This perspective informs the practice of physical distancing and staying home to minimize the spread of the virus and its impact on the health care system, regardless of whether an individual belongs in the high-risk group or not.
Many high-achieving people train in extremely competitive settings in which survival depends on individual performance rather than mutual cooperation. This training process encourages a disregard for others. Good leaders, however, understand that cooperation and mutual respect are essential to personal well-being.
Strategy #7: Practice self-care
There are five aspects of our lives that depend on our self-care: spiritual, mental, emotional, physical, and social. Unfortunately, many kind-hearted people are kinder to others than to themselves. There is really only one person who can truly take care of you properly, and that is yourself. In Seattle, where many suffered early in the pandemic, holistic psychiatrist David Kopacz, MD, is reminding people to nurture themselves in his post, “Nurture Yourself During the Pandemic: Try New Recipes!”6 Indeed, that is what many must do since eating out is not an option now. If you find yourself stuck at home with more time on your hands, take the opportunity to care for yourself. Ask yourself what you really need during this time, and make the effort to provide it to yourself.
After the pandemic is over, will you have grown from the experiences and become a better person from it? Despite our current circumstances, we can continue to grow as individuals and as a community, armed with strategies that can benefit all of us.
References
1. Covey SR. The 7 Habits of Highly Effective People. New York: Simon & Schuster; 1989.
2. Lee AW. Townsend Letter. 2009 Jun;311:22-3.
3. Fromm E. To Have or To Be? New York: Continuum International Publishing; 2005.
4. Rushlau K. Integrative Healthcare Symposium. 2020 Feb 21.
5. Gerbarg PL. Mind Body Practices for Post-Traumatic Stress Disorder. Presentation at Integrative Medicine for Mental Health Conference. 2016 Sep.
6. Kopacz D. Nurture Yourself During the Pandemic: Try New Recipes! Being Fully Human. 2020 Mar 22.
Dr. Lee specializes in integrative and holistic psychiatry and has a private practice in Gaithersburg, Md. She has no disclosures.
A few weeks ago, I saw more than 60 responses to a post on Nextdoor.com entitled, “Toilet paper strategies?”
Asking for help is a great coping mechanism when one is struggling to find a strategy, even if it’s for toilet paper. What other kinds of coping strategies can help us through this historic and unprecedented time?
The late Stephen R. Covey, PhD, wrote about the coping strategies of highly effective people in his book, “The 7 Habits of Highly Effective People.”1 For, no matter how smart, perfect, or careful you may be, life will never be trouble free. When trouble comes, it’s important to have coping strategies that help you navigate through choppy waters. Whether you are a practitioner trying to help your patients or someone who wants to maximize their personal resilience during a worldwide pandemic, here are my conceptualizations of the seven top strategies highly effective people use when facing challenges.
Strategy #1: Begin with the end in mind
In 2007, this strategy helped me not only survive but thrive when I battled for my right to practice as a holistic psychiatrist against the Maryland Board of Physicians.2 From the first moment when I read the letter from the board, to the last when I read the administrative law judge’s dismissal, I turned to this strategy to help me cope with unrelenting stress.
I imagined myself remembering being the kind of person I wanted to be, wrote that script for myself, and created those memories for my future self. I wanted to remember myself as being brave, calm, strong, and grounded, so I behaved each day as if I were all of those things.
As Dr. Covey wrote, “ ‘Begin with the end in mind’ is based on the principle that all things are created twice. There’s a mental or first creation, and a physical or second creation to all things.” Imagine who you would like to remember yourself being a year or two down the road. Do you want to remember yourself showing good judgment and being positive and compassionate during this pandemic? Then, follow the script you’ve created in your mind and be that person now, knowing that you are forming memories for your future self. Your future self will look back at who you are right now with appreciation and satisfaction. Of course, this is a habit that you can apply to your entire life.
Strategy #2: Be proactive
Between the event and the outcome is you. You are the interpreter and transformer of the event, with the freedom to apply your will and intention on the event. Whether it is living through a pandemic or dealing with misplaced keys, every day you are revealing your nature through how you deal with life. To be proactive is different from being reactive. Within each of us there is a will, the drive, to rise above our difficult environments.
Dr. Covey wrote, “the ability to subordinate an impulse to a value is the essence of the proactive person.” A woman shared with me that she created an Excel spreadsheet with some of the things she plans to do with her free time while she stays in her NYC apartment. She doesn’t want to slip into a passive state and waste her time. That’s being proactive.
Strategy #3: Set proper priorities
Or, as Dr. Covey would say, “Put first things first.” During a pandemic, when the world seems to be precariously tilting at an angle, it’s easy to cling to outdated standards, expectations, and behavioral patterns. Doing so heightens our sense of regret, fear, and scarcity. Valuing gratitude will empower you to deal with financial loss differently because you can still remain grateful despite uncontrollable losses. We can choose “to have or to be” as psychoanalyst, Erich Fromm, PhD, would say.3 If your happiness is measured by how much money you have, then it would make sense that, when the amount shrinks, so does your happiness. However, if your happiness is a side effect of who you are, you will remain a mountain before the winds and tides of circumstance.
Strategy #4: Create a win/win mentality
This state of mind is built on character. Dr. Covey separates character into three categories: integrity, maturity, and abundance mentality. A lack of character resulted in the hoarding of toilet paper in many communities and the cry for help from Nextdoor.com. I noticed that, in the 60+ responses that included advice about using bidets, old towels, and even leaves, no one offered to share a bag of toilet paper. That’s because people experienced the fear of scarcity, in turn, causing the scarcity they feared.
During a pandemic, a highly effective person or company thinks beyond themselves to create a win/win scenario. At a grocery store in my neighborhood, a man stands at its entrance with a bottle of disinfectant spray in one hand for the shoppers and a sign on the sidewalk with guidelines for purchasing products to avoid hoarding. He tells you where the wipes are for the carts as you enter the store. People line up 6 feet apart, waiting to enter, to limit the number of shoppers inside the store, facilitating proper physical distancing. Instead of maximizing profits at the expense of everyone’s health and safety, the process is a win/win for everyone, from shoppers to employees.
Strategy #5: Develop empathy and understanding
Seeking to first understand and then be understood is one of the most powerful tools of effective people. In my holistic practice, every patient comes in with their own unique needs that evolve and transform over time. I must remain open, or I fail to deliver appropriately.
Learning to listen and then to clearly communicate ideas is essential to effective health care. During this time, it is critical that health care providers and political leaders first listen/understand and then communicate clearly to serve everyone in the best way possible.
In our brains, the frontal lobes (the adult in the room) manages our amygdala (the child in the room) when we get enough sleep, meditate, spend time in nature, exercise, and eat healthy food.4 Stress can interfere with the frontal lobe’s ability to maintain empathy, inhibit unhealthy impulses, and delay gratification. During the pandemic, we can help to shift from the stress response, or “fight-or-flight” response, driven by the sympathetic nervous system to a “rest-and-digest” response driven by the parasympathetic system through coherent breathing, taking slow, deep, relaxed breaths (6 seconds on inhalation and 6 seconds on exhalation). The vagus nerve connected to our diaphragm will help the heart return to a healthy rhythm.5
Strategy #6: Synergize and integrate
All of life is interdependent, each part no more or less important than any other. Is oxygen more important than hydrogen? Is H2O different from the oxygen and hydrogen atoms that make it?
During a pandemic, it’s important for us to appreciate each other’s contributions and work synergistically for the good of the whole. Our survival depends on valuing each other and our planet. This perspective informs the practice of physical distancing and staying home to minimize the spread of the virus and its impact on the health care system, regardless of whether an individual belongs in the high-risk group or not.
Many high-achieving people train in extremely competitive settings in which survival depends on individual performance rather than mutual cooperation. This training process encourages a disregard for others. Good leaders, however, understand that cooperation and mutual respect are essential to personal well-being.
Strategy #7: Practice self-care
There are five aspects of our lives that depend on our self-care: spiritual, mental, emotional, physical, and social. Unfortunately, many kind-hearted people are kinder to others than to themselves. There is really only one person who can truly take care of you properly, and that is yourself. In Seattle, where many suffered early in the pandemic, holistic psychiatrist David Kopacz, MD, is reminding people to nurture themselves in his post, “Nurture Yourself During the Pandemic: Try New Recipes!”6 Indeed, that is what many must do since eating out is not an option now. If you find yourself stuck at home with more time on your hands, take the opportunity to care for yourself. Ask yourself what you really need during this time, and make the effort to provide it to yourself.
After the pandemic is over, will you have grown from the experiences and become a better person from it? Despite our current circumstances, we can continue to grow as individuals and as a community, armed with strategies that can benefit all of us.
References
1. Covey SR. The 7 Habits of Highly Effective People. New York: Simon & Schuster; 1989.
2. Lee AW. Townsend Letter. 2009 Jun;311:22-3.
3. Fromm E. To Have or To Be? New York: Continuum International Publishing; 2005.
4. Rushlau K. Integrative Healthcare Symposium. 2020 Feb 21.
5. Gerbarg PL. Mind Body Practices for Post-Traumatic Stress Disorder. Presentation at Integrative Medicine for Mental Health Conference. 2016 Sep.
6. Kopacz D. Nurture Yourself During the Pandemic: Try New Recipes! Being Fully Human. 2020 Mar 22.
Dr. Lee specializes in integrative and holistic psychiatry and has a private practice in Gaithersburg, Md. She has no disclosures.
A few weeks ago, I saw more than 60 responses to a post on Nextdoor.com entitled, “Toilet paper strategies?”
Asking for help is a great coping mechanism when one is struggling to find a strategy, even if it’s for toilet paper. What other kinds of coping strategies can help us through this historic and unprecedented time?
The late Stephen R. Covey, PhD, wrote about the coping strategies of highly effective people in his book, “The 7 Habits of Highly Effective People.”1 For, no matter how smart, perfect, or careful you may be, life will never be trouble free. When trouble comes, it’s important to have coping strategies that help you navigate through choppy waters. Whether you are a practitioner trying to help your patients or someone who wants to maximize their personal resilience during a worldwide pandemic, here are my conceptualizations of the seven top strategies highly effective people use when facing challenges.
Strategy #1: Begin with the end in mind
In 2007, this strategy helped me not only survive but thrive when I battled for my right to practice as a holistic psychiatrist against the Maryland Board of Physicians.2 From the first moment when I read the letter from the board, to the last when I read the administrative law judge’s dismissal, I turned to this strategy to help me cope with unrelenting stress.
I imagined myself remembering being the kind of person I wanted to be, wrote that script for myself, and created those memories for my future self. I wanted to remember myself as being brave, calm, strong, and grounded, so I behaved each day as if I were all of those things.
As Dr. Covey wrote, “ ‘Begin with the end in mind’ is based on the principle that all things are created twice. There’s a mental or first creation, and a physical or second creation to all things.” Imagine who you would like to remember yourself being a year or two down the road. Do you want to remember yourself showing good judgment and being positive and compassionate during this pandemic? Then, follow the script you’ve created in your mind and be that person now, knowing that you are forming memories for your future self. Your future self will look back at who you are right now with appreciation and satisfaction. Of course, this is a habit that you can apply to your entire life.
Strategy #2: Be proactive
Between the event and the outcome is you. You are the interpreter and transformer of the event, with the freedom to apply your will and intention on the event. Whether it is living through a pandemic or dealing with misplaced keys, every day you are revealing your nature through how you deal with life. To be proactive is different from being reactive. Within each of us there is a will, the drive, to rise above our difficult environments.
Dr. Covey wrote, “the ability to subordinate an impulse to a value is the essence of the proactive person.” A woman shared with me that she created an Excel spreadsheet with some of the things she plans to do with her free time while she stays in her NYC apartment. She doesn’t want to slip into a passive state and waste her time. That’s being proactive.
Strategy #3: Set proper priorities
Or, as Dr. Covey would say, “Put first things first.” During a pandemic, when the world seems to be precariously tilting at an angle, it’s easy to cling to outdated standards, expectations, and behavioral patterns. Doing so heightens our sense of regret, fear, and scarcity. Valuing gratitude will empower you to deal with financial loss differently because you can still remain grateful despite uncontrollable losses. We can choose “to have or to be” as psychoanalyst, Erich Fromm, PhD, would say.3 If your happiness is measured by how much money you have, then it would make sense that, when the amount shrinks, so does your happiness. However, if your happiness is a side effect of who you are, you will remain a mountain before the winds and tides of circumstance.
Strategy #4: Create a win/win mentality
This state of mind is built on character. Dr. Covey separates character into three categories: integrity, maturity, and abundance mentality. A lack of character resulted in the hoarding of toilet paper in many communities and the cry for help from Nextdoor.com. I noticed that, in the 60+ responses that included advice about using bidets, old towels, and even leaves, no one offered to share a bag of toilet paper. That’s because people experienced the fear of scarcity, in turn, causing the scarcity they feared.
During a pandemic, a highly effective person or company thinks beyond themselves to create a win/win scenario. At a grocery store in my neighborhood, a man stands at its entrance with a bottle of disinfectant spray in one hand for the shoppers and a sign on the sidewalk with guidelines for purchasing products to avoid hoarding. He tells you where the wipes are for the carts as you enter the store. People line up 6 feet apart, waiting to enter, to limit the number of shoppers inside the store, facilitating proper physical distancing. Instead of maximizing profits at the expense of everyone’s health and safety, the process is a win/win for everyone, from shoppers to employees.
Strategy #5: Develop empathy and understanding
Seeking to first understand and then be understood is one of the most powerful tools of effective people. In my holistic practice, every patient comes in with their own unique needs that evolve and transform over time. I must remain open, or I fail to deliver appropriately.
Learning to listen and then to clearly communicate ideas is essential to effective health care. During this time, it is critical that health care providers and political leaders first listen/understand and then communicate clearly to serve everyone in the best way possible.
In our brains, the frontal lobes (the adult in the room) manages our amygdala (the child in the room) when we get enough sleep, meditate, spend time in nature, exercise, and eat healthy food.4 Stress can interfere with the frontal lobe’s ability to maintain empathy, inhibit unhealthy impulses, and delay gratification. During the pandemic, we can help to shift from the stress response, or “fight-or-flight” response, driven by the sympathetic nervous system to a “rest-and-digest” response driven by the parasympathetic system through coherent breathing, taking slow, deep, relaxed breaths (6 seconds on inhalation and 6 seconds on exhalation). The vagus nerve connected to our diaphragm will help the heart return to a healthy rhythm.5
Strategy #6: Synergize and integrate
All of life is interdependent, each part no more or less important than any other. Is oxygen more important than hydrogen? Is H2O different from the oxygen and hydrogen atoms that make it?
During a pandemic, it’s important for us to appreciate each other’s contributions and work synergistically for the good of the whole. Our survival depends on valuing each other and our planet. This perspective informs the practice of physical distancing and staying home to minimize the spread of the virus and its impact on the health care system, regardless of whether an individual belongs in the high-risk group or not.
Many high-achieving people train in extremely competitive settings in which survival depends on individual performance rather than mutual cooperation. This training process encourages a disregard for others. Good leaders, however, understand that cooperation and mutual respect are essential to personal well-being.
Strategy #7: Practice self-care
There are five aspects of our lives that depend on our self-care: spiritual, mental, emotional, physical, and social. Unfortunately, many kind-hearted people are kinder to others than to themselves. There is really only one person who can truly take care of you properly, and that is yourself. In Seattle, where many suffered early in the pandemic, holistic psychiatrist David Kopacz, MD, is reminding people to nurture themselves in his post, “Nurture Yourself During the Pandemic: Try New Recipes!”6 Indeed, that is what many must do since eating out is not an option now. If you find yourself stuck at home with more time on your hands, take the opportunity to care for yourself. Ask yourself what you really need during this time, and make the effort to provide it to yourself.
After the pandemic is over, will you have grown from the experiences and become a better person from it? Despite our current circumstances, we can continue to grow as individuals and as a community, armed with strategies that can benefit all of us.
References
1. Covey SR. The 7 Habits of Highly Effective People. New York: Simon & Schuster; 1989.
2. Lee AW. Townsend Letter. 2009 Jun;311:22-3.
3. Fromm E. To Have or To Be? New York: Continuum International Publishing; 2005.
4. Rushlau K. Integrative Healthcare Symposium. 2020 Feb 21.
5. Gerbarg PL. Mind Body Practices for Post-Traumatic Stress Disorder. Presentation at Integrative Medicine for Mental Health Conference. 2016 Sep.
6. Kopacz D. Nurture Yourself During the Pandemic: Try New Recipes! Being Fully Human. 2020 Mar 22.
Dr. Lee specializes in integrative and holistic psychiatry and has a private practice in Gaithersburg, Md. She has no disclosures.
Learning to live with COVID-19: Postpandemic life will be reflected in how effectively we leverage this crisis
While often compared with the Spanish influenza contagion of 1918, the current COVID-19 pandemic is arguably unprecedented in scale and scope, global reach, and the rate at which it has spread across the world.
Unprecedented times
The United States now has the greatest burden of COVID-19 disease worldwide.1 Although Boston has thus far been spared the full force of the disease’s impact, it is likely only a matter of time before it reaches here. To prepare for the imminent surge, we at Tufts Medical Center defined 4 short-term strategic imperatives to help guide our COVID-19 preparedness. Having a single unified strategy across our organization has helped to maintain focus and consistency in the messaging amidst all of the uncertainty. Our focus areas are outlined below.
1 Flatten the curve
This term refers to the use of “social distancing” and community isolation measures to keep the number of disease cases at a manageable level. COVID-19 is spread almost exclusively through contact with contaminated respiratory droplets. While several categories of risk have been described, the US Centers for Disease Control and Prevention (CDC) defines disease “exposure” as face-to-face contact within 6 feet of an infected individual for more than 15 minutes without wearing a mask.2 Intervening at all 3 of these touchpoints effectively reduces transmission. Interventions include limiting in-person meetings, increasing the space between individuals (both providers and patients), and routinely using personal protective equipment (PPE).
Another effective strategy is to divide frontline providers into smaller units or teams to limit cross-contamination: the inpatient team versus the outpatient team, the day team versus the night team, the “on” team versus the “off” team. If the infection lays one team low, other providers can step in until they recover and return to work.
Visitor policies should be developed and strictly implemented. Many institutions do allow one support person in labor and delivery (L&D) regardless of the patient’s COVID-19 status, although that person should not be symptomatic or COVID-19 positive. Whether to test all patients and support persons for COVID-19 on arrival at L&D remains controversial.3 At a minimum, these individuals should be screened for symptoms. Although it was a major focus of initial preventative efforts, taking a travel and exposure history is no longer informative as the virus is now endemic and community spread is common.
Initial preventative efforts focused also on high-risk patients, but routine use of PPE for all encounters clearly is more effective because of the high rate of asymptomatic shedding. The virus can survive suspended in the air for up to 2 hours following an aerosol-generating procedure (AGP) and on surfaces for several hours or even days. Practices such as regular handwashing, cleaning of exposed work surfaces, and avoiding face touching should by now be part of our everyday routine.
Institutions throughout the United States have established inpatient COVID-19 units—so-called “dirty” units—with mixed success. As the pandemic spreads and the number of patients with asymptomatic shedding increases, it is harder to determine who is and who is not infected. Cross-contamination has rendered this approach largely ineffective. Whether this will change with the introduction of rapid point-of-care testing remains to be seen.
Continue to: 2 Preserve PPE...
2 Preserve PPE
PPE use is effective in reducing transmission. This includes tier 1 PPE with or without enhanced droplet precaution (surgical mask, eye protection, gloves, yellow gown) and tier 2 PPE (tier 1 plus N95 respirators or powered air-purifying respirators [PAPR]). Given the acute PPE shortage in many parts of the country, appropriate use of PPE is critical to maintain an adequate supply. For example, tier 2 PPE is required only in the setting of an AGP. This includes intubation and, in our determination, the second stage of labor for COVID-19–positive patients and patients under investigation (PUIs); we do not employ tier 2 PPE for all patients in the second stage of labor, although some hospitals endorse this practice.
Creative solutions to the impending PPE shortage abound, such as the use of 3D printers to make face shields and novel techniques to sterilize and reuse N95 respirators.
3 Create capacity
In the absence of effective treatment for COVID-19 and with a vaccine still many months away, supportive care is critical. The pulmonary sequelae with cytokine storm and acute hypoxemia can come on quickly, require urgent mechanical ventilatory support, and take several weeks to resolve.
Our ability to create inpatient capacity to accommodate ill patients, monitor them closely, and intubate early will likely be the most critical driver of the case fatality rate. This requires deferring outpatient visits (or doing them via telemedicine), expanding intensive care unit capabilities (especially ventilator beds), and canceling elective surgeries. What constitutes “elective surgery” is not always clear. Our institution, for example, regards abortion services as essential and not elective, but this is not the case throughout the United States.
Creating capacity also refers to staffing. Where necessary, providers should be retrained and redeployed. This may require emergency credentialing of providers in areas outside their usual clinical practice and permission may be needed from the Accreditation Council for Graduate Medical Education to engage trainees outside their usual duty hours.
4 Support and protect your workforce
Everyone is anxious, and people convey their anxiety in different ways. I have found it helpful to acknowledge those feelings and provide a forum for staff to express and share their anxieties. That said, hospitals are not a democracy. While staff members should be encouraged to ask questions and voice their opinions, everyone is expected to follow protocol regarding patient care.
Celebrating small successes and finding creative ways to alleviate the stress and inject humor can help. Most institutions are using electronic conferencing platforms (such as Zoom or Microsoft Teams) to stay in touch and to continue education initiatives through interactive didactic sessions, grand rounds, morbidity and mortality conferences, and e-journal clubs. These are also a great platform for social events, such as w(h)ine and book clubs and virtual karaoke.
Since many ObGyn providers are women, the closure of day-care centers and schools is particularly challenging. Share best practices among your staff on how to address this problem, such as alternating on-call shifts or matching providers needing day care with ‘furloughed’ college students who are looking to keep busy and make a little money.
Continue to: Avoid overcommunicating...
Avoid overcommunicating
Clear, concise, and timely communication is key. This can be challenging given the rapidly evolving science of COVID-19 and the daily barrage of information from both reliable and unreliable sources. Setting up regular online meetings with your faculty 2 or 3 times per week can keep people informed, promote engagement, and boost morale.
If an urgent e-mail announcement is needed, keep the message focused. Highlight only updated information and changes to existing policies and guidelines. And consider adding a brief anecdote to illustrate the staff’s creativity and resilience: a “best catch” story, for example, or a staff member who started a “commit to sit” program (spending time in the room with patients who want company but are not able to have their family in attendance).
Look to the future
COVID-19 will pass. Herd immunity will inevitably develop. The question is how quickly and at what cost. Children delivered today are being born into a society already profoundly altered by COVID-19. Some have started to call them Generation C.
Exactly what life will look like at the back end of this pandemic depends on how effectively we leverage this crisis. There are numerous opportunities to change the way we think about health care and educate the next generation of providers. These include increasing the use of telehealth and remote education, redesigning our traditional prenatal care paradigms, and reinforcing the importance of preventive medicine. This is an opportunity to put the “health” back into “health care.”
Look after yourself
Amid all the chaos and uncertainty, do not forget to take care of yourself and your family. Be calm, be kind, and be flexible. Stay safe.
- Kommenda N, Gutierrez P, Adolphe J. Coronavirus world map: which countries have the most cases and deaths? The Guardian. April 1, 2020. https://www.theguardian.com/world/2020/mar/31/coronavirus-mapped-which-countries-have-the-most-cases-and-deaths. Accessed April 1, 2020.
- Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19).Interim US guidance for risk assessment and public health management of healthcare personnel with potential exposure in a healthcare setting to patients with coronavirus disease (COVID-19). https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-risk-assesment-hcp.html. Accessed April 1, 2020.
- Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19). Evaluating and testing persons for coronavirus disease 2020 (COVID-19). https://www.cdc.gov/coronavirus/2019-nCoV/hcp/clinical-criteria.html. Accessed April 1, 2020.
While often compared with the Spanish influenza contagion of 1918, the current COVID-19 pandemic is arguably unprecedented in scale and scope, global reach, and the rate at which it has spread across the world.
Unprecedented times
The United States now has the greatest burden of COVID-19 disease worldwide.1 Although Boston has thus far been spared the full force of the disease’s impact, it is likely only a matter of time before it reaches here. To prepare for the imminent surge, we at Tufts Medical Center defined 4 short-term strategic imperatives to help guide our COVID-19 preparedness. Having a single unified strategy across our organization has helped to maintain focus and consistency in the messaging amidst all of the uncertainty. Our focus areas are outlined below.
1 Flatten the curve
This term refers to the use of “social distancing” and community isolation measures to keep the number of disease cases at a manageable level. COVID-19 is spread almost exclusively through contact with contaminated respiratory droplets. While several categories of risk have been described, the US Centers for Disease Control and Prevention (CDC) defines disease “exposure” as face-to-face contact within 6 feet of an infected individual for more than 15 minutes without wearing a mask.2 Intervening at all 3 of these touchpoints effectively reduces transmission. Interventions include limiting in-person meetings, increasing the space between individuals (both providers and patients), and routinely using personal protective equipment (PPE).
Another effective strategy is to divide frontline providers into smaller units or teams to limit cross-contamination: the inpatient team versus the outpatient team, the day team versus the night team, the “on” team versus the “off” team. If the infection lays one team low, other providers can step in until they recover and return to work.
Visitor policies should be developed and strictly implemented. Many institutions do allow one support person in labor and delivery (L&D) regardless of the patient’s COVID-19 status, although that person should not be symptomatic or COVID-19 positive. Whether to test all patients and support persons for COVID-19 on arrival at L&D remains controversial.3 At a minimum, these individuals should be screened for symptoms. Although it was a major focus of initial preventative efforts, taking a travel and exposure history is no longer informative as the virus is now endemic and community spread is common.
Initial preventative efforts focused also on high-risk patients, but routine use of PPE for all encounters clearly is more effective because of the high rate of asymptomatic shedding. The virus can survive suspended in the air for up to 2 hours following an aerosol-generating procedure (AGP) and on surfaces for several hours or even days. Practices such as regular handwashing, cleaning of exposed work surfaces, and avoiding face touching should by now be part of our everyday routine.
Institutions throughout the United States have established inpatient COVID-19 units—so-called “dirty” units—with mixed success. As the pandemic spreads and the number of patients with asymptomatic shedding increases, it is harder to determine who is and who is not infected. Cross-contamination has rendered this approach largely ineffective. Whether this will change with the introduction of rapid point-of-care testing remains to be seen.
Continue to: 2 Preserve PPE...
2 Preserve PPE
PPE use is effective in reducing transmission. This includes tier 1 PPE with or without enhanced droplet precaution (surgical mask, eye protection, gloves, yellow gown) and tier 2 PPE (tier 1 plus N95 respirators or powered air-purifying respirators [PAPR]). Given the acute PPE shortage in many parts of the country, appropriate use of PPE is critical to maintain an adequate supply. For example, tier 2 PPE is required only in the setting of an AGP. This includes intubation and, in our determination, the second stage of labor for COVID-19–positive patients and patients under investigation (PUIs); we do not employ tier 2 PPE for all patients in the second stage of labor, although some hospitals endorse this practice.
Creative solutions to the impending PPE shortage abound, such as the use of 3D printers to make face shields and novel techniques to sterilize and reuse N95 respirators.
3 Create capacity
In the absence of effective treatment for COVID-19 and with a vaccine still many months away, supportive care is critical. The pulmonary sequelae with cytokine storm and acute hypoxemia can come on quickly, require urgent mechanical ventilatory support, and take several weeks to resolve.
Our ability to create inpatient capacity to accommodate ill patients, monitor them closely, and intubate early will likely be the most critical driver of the case fatality rate. This requires deferring outpatient visits (or doing them via telemedicine), expanding intensive care unit capabilities (especially ventilator beds), and canceling elective surgeries. What constitutes “elective surgery” is not always clear. Our institution, for example, regards abortion services as essential and not elective, but this is not the case throughout the United States.
Creating capacity also refers to staffing. Where necessary, providers should be retrained and redeployed. This may require emergency credentialing of providers in areas outside their usual clinical practice and permission may be needed from the Accreditation Council for Graduate Medical Education to engage trainees outside their usual duty hours.
4 Support and protect your workforce
Everyone is anxious, and people convey their anxiety in different ways. I have found it helpful to acknowledge those feelings and provide a forum for staff to express and share their anxieties. That said, hospitals are not a democracy. While staff members should be encouraged to ask questions and voice their opinions, everyone is expected to follow protocol regarding patient care.
Celebrating small successes and finding creative ways to alleviate the stress and inject humor can help. Most institutions are using electronic conferencing platforms (such as Zoom or Microsoft Teams) to stay in touch and to continue education initiatives through interactive didactic sessions, grand rounds, morbidity and mortality conferences, and e-journal clubs. These are also a great platform for social events, such as w(h)ine and book clubs and virtual karaoke.
Since many ObGyn providers are women, the closure of day-care centers and schools is particularly challenging. Share best practices among your staff on how to address this problem, such as alternating on-call shifts or matching providers needing day care with ‘furloughed’ college students who are looking to keep busy and make a little money.
Continue to: Avoid overcommunicating...
Avoid overcommunicating
Clear, concise, and timely communication is key. This can be challenging given the rapidly evolving science of COVID-19 and the daily barrage of information from both reliable and unreliable sources. Setting up regular online meetings with your faculty 2 or 3 times per week can keep people informed, promote engagement, and boost morale.
If an urgent e-mail announcement is needed, keep the message focused. Highlight only updated information and changes to existing policies and guidelines. And consider adding a brief anecdote to illustrate the staff’s creativity and resilience: a “best catch” story, for example, or a staff member who started a “commit to sit” program (spending time in the room with patients who want company but are not able to have their family in attendance).
Look to the future
COVID-19 will pass. Herd immunity will inevitably develop. The question is how quickly and at what cost. Children delivered today are being born into a society already profoundly altered by COVID-19. Some have started to call them Generation C.
Exactly what life will look like at the back end of this pandemic depends on how effectively we leverage this crisis. There are numerous opportunities to change the way we think about health care and educate the next generation of providers. These include increasing the use of telehealth and remote education, redesigning our traditional prenatal care paradigms, and reinforcing the importance of preventive medicine. This is an opportunity to put the “health” back into “health care.”
Look after yourself
Amid all the chaos and uncertainty, do not forget to take care of yourself and your family. Be calm, be kind, and be flexible. Stay safe.
While often compared with the Spanish influenza contagion of 1918, the current COVID-19 pandemic is arguably unprecedented in scale and scope, global reach, and the rate at which it has spread across the world.
Unprecedented times
The United States now has the greatest burden of COVID-19 disease worldwide.1 Although Boston has thus far been spared the full force of the disease’s impact, it is likely only a matter of time before it reaches here. To prepare for the imminent surge, we at Tufts Medical Center defined 4 short-term strategic imperatives to help guide our COVID-19 preparedness. Having a single unified strategy across our organization has helped to maintain focus and consistency in the messaging amidst all of the uncertainty. Our focus areas are outlined below.
1 Flatten the curve
This term refers to the use of “social distancing” and community isolation measures to keep the number of disease cases at a manageable level. COVID-19 is spread almost exclusively through contact with contaminated respiratory droplets. While several categories of risk have been described, the US Centers for Disease Control and Prevention (CDC) defines disease “exposure” as face-to-face contact within 6 feet of an infected individual for more than 15 minutes without wearing a mask.2 Intervening at all 3 of these touchpoints effectively reduces transmission. Interventions include limiting in-person meetings, increasing the space between individuals (both providers and patients), and routinely using personal protective equipment (PPE).
Another effective strategy is to divide frontline providers into smaller units or teams to limit cross-contamination: the inpatient team versus the outpatient team, the day team versus the night team, the “on” team versus the “off” team. If the infection lays one team low, other providers can step in until they recover and return to work.
Visitor policies should be developed and strictly implemented. Many institutions do allow one support person in labor and delivery (L&D) regardless of the patient’s COVID-19 status, although that person should not be symptomatic or COVID-19 positive. Whether to test all patients and support persons for COVID-19 on arrival at L&D remains controversial.3 At a minimum, these individuals should be screened for symptoms. Although it was a major focus of initial preventative efforts, taking a travel and exposure history is no longer informative as the virus is now endemic and community spread is common.
Initial preventative efforts focused also on high-risk patients, but routine use of PPE for all encounters clearly is more effective because of the high rate of asymptomatic shedding. The virus can survive suspended in the air for up to 2 hours following an aerosol-generating procedure (AGP) and on surfaces for several hours or even days. Practices such as regular handwashing, cleaning of exposed work surfaces, and avoiding face touching should by now be part of our everyday routine.
Institutions throughout the United States have established inpatient COVID-19 units—so-called “dirty” units—with mixed success. As the pandemic spreads and the number of patients with asymptomatic shedding increases, it is harder to determine who is and who is not infected. Cross-contamination has rendered this approach largely ineffective. Whether this will change with the introduction of rapid point-of-care testing remains to be seen.
Continue to: 2 Preserve PPE...
2 Preserve PPE
PPE use is effective in reducing transmission. This includes tier 1 PPE with or without enhanced droplet precaution (surgical mask, eye protection, gloves, yellow gown) and tier 2 PPE (tier 1 plus N95 respirators or powered air-purifying respirators [PAPR]). Given the acute PPE shortage in many parts of the country, appropriate use of PPE is critical to maintain an adequate supply. For example, tier 2 PPE is required only in the setting of an AGP. This includes intubation and, in our determination, the second stage of labor for COVID-19–positive patients and patients under investigation (PUIs); we do not employ tier 2 PPE for all patients in the second stage of labor, although some hospitals endorse this practice.
Creative solutions to the impending PPE shortage abound, such as the use of 3D printers to make face shields and novel techniques to sterilize and reuse N95 respirators.
3 Create capacity
In the absence of effective treatment for COVID-19 and with a vaccine still many months away, supportive care is critical. The pulmonary sequelae with cytokine storm and acute hypoxemia can come on quickly, require urgent mechanical ventilatory support, and take several weeks to resolve.
Our ability to create inpatient capacity to accommodate ill patients, monitor them closely, and intubate early will likely be the most critical driver of the case fatality rate. This requires deferring outpatient visits (or doing them via telemedicine), expanding intensive care unit capabilities (especially ventilator beds), and canceling elective surgeries. What constitutes “elective surgery” is not always clear. Our institution, for example, regards abortion services as essential and not elective, but this is not the case throughout the United States.
Creating capacity also refers to staffing. Where necessary, providers should be retrained and redeployed. This may require emergency credentialing of providers in areas outside their usual clinical practice and permission may be needed from the Accreditation Council for Graduate Medical Education to engage trainees outside their usual duty hours.
4 Support and protect your workforce
Everyone is anxious, and people convey their anxiety in different ways. I have found it helpful to acknowledge those feelings and provide a forum for staff to express and share their anxieties. That said, hospitals are not a democracy. While staff members should be encouraged to ask questions and voice their opinions, everyone is expected to follow protocol regarding patient care.
Celebrating small successes and finding creative ways to alleviate the stress and inject humor can help. Most institutions are using electronic conferencing platforms (such as Zoom or Microsoft Teams) to stay in touch and to continue education initiatives through interactive didactic sessions, grand rounds, morbidity and mortality conferences, and e-journal clubs. These are also a great platform for social events, such as w(h)ine and book clubs and virtual karaoke.
Since many ObGyn providers are women, the closure of day-care centers and schools is particularly challenging. Share best practices among your staff on how to address this problem, such as alternating on-call shifts or matching providers needing day care with ‘furloughed’ college students who are looking to keep busy and make a little money.
Continue to: Avoid overcommunicating...
Avoid overcommunicating
Clear, concise, and timely communication is key. This can be challenging given the rapidly evolving science of COVID-19 and the daily barrage of information from both reliable and unreliable sources. Setting up regular online meetings with your faculty 2 or 3 times per week can keep people informed, promote engagement, and boost morale.
If an urgent e-mail announcement is needed, keep the message focused. Highlight only updated information and changes to existing policies and guidelines. And consider adding a brief anecdote to illustrate the staff’s creativity and resilience: a “best catch” story, for example, or a staff member who started a “commit to sit” program (spending time in the room with patients who want company but are not able to have their family in attendance).
Look to the future
COVID-19 will pass. Herd immunity will inevitably develop. The question is how quickly and at what cost. Children delivered today are being born into a society already profoundly altered by COVID-19. Some have started to call them Generation C.
Exactly what life will look like at the back end of this pandemic depends on how effectively we leverage this crisis. There are numerous opportunities to change the way we think about health care and educate the next generation of providers. These include increasing the use of telehealth and remote education, redesigning our traditional prenatal care paradigms, and reinforcing the importance of preventive medicine. This is an opportunity to put the “health” back into “health care.”
Look after yourself
Amid all the chaos and uncertainty, do not forget to take care of yourself and your family. Be calm, be kind, and be flexible. Stay safe.
- Kommenda N, Gutierrez P, Adolphe J. Coronavirus world map: which countries have the most cases and deaths? The Guardian. April 1, 2020. https://www.theguardian.com/world/2020/mar/31/coronavirus-mapped-which-countries-have-the-most-cases-and-deaths. Accessed April 1, 2020.
- Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19).Interim US guidance for risk assessment and public health management of healthcare personnel with potential exposure in a healthcare setting to patients with coronavirus disease (COVID-19). https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-risk-assesment-hcp.html. Accessed April 1, 2020.
- Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19). Evaluating and testing persons for coronavirus disease 2020 (COVID-19). https://www.cdc.gov/coronavirus/2019-nCoV/hcp/clinical-criteria.html. Accessed April 1, 2020.
- Kommenda N, Gutierrez P, Adolphe J. Coronavirus world map: which countries have the most cases and deaths? The Guardian. April 1, 2020. https://www.theguardian.com/world/2020/mar/31/coronavirus-mapped-which-countries-have-the-most-cases-and-deaths. Accessed April 1, 2020.
- Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19).Interim US guidance for risk assessment and public health management of healthcare personnel with potential exposure in a healthcare setting to patients with coronavirus disease (COVID-19). https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-risk-assesment-hcp.html. Accessed April 1, 2020.
- Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19). Evaluating and testing persons for coronavirus disease 2020 (COVID-19). https://www.cdc.gov/coronavirus/2019-nCoV/hcp/clinical-criteria.html. Accessed April 1, 2020.
American Lung Association announces $25 million initiative to end COVID-19
The goals of the COVID-19 Action Initiative will be to expand the ALA’s respiratory research program, improve public health measures, and create an advanced network to prevent future respiratory virus pandemics. In cooperation with the public and private sectors, the initiative will promote research collaboration and develop new vaccines, diagnostic tests, and therapies. The initiative will take advantage of the ALA’s current research network and will also fund respiratory virus research. It also will fund education and advocacy to support public health measures against current and future respiratory viruses.
The COVID-19 Action Initiative will fund respiratory virus research through three main activities. It will expand COVID-19 research within the current clinical trials of the Airways Clinical Research Center (ACRC) Network. Second, it will fund coronavirus awards and grants for preventive research, vaccines, antivirals, and efforts to promote preparedness for future outbreaks. Third, it will provide ACRC pilot grants to evaluate the effect of COVID-19 on patients with chronic lung disease.
“More than 36 million people in the U.S. suffer from lung disease, which places them at higher risk for experiencing complications of COVID-19, making it even more critical that we urgently work on reducing its impact,” said Harold Wimmer, president and CEO of the ALA, in a press release.
The ALA has $8 million available and earmarked for the initiative. The association plans to raise additional funds during the next 3 years by reaching out to corporate partners, public health entities, and individuals. “With the help of our staff and volunteers, and with the support and donations of generous Americans, we can stand together and face the challenges to lung health of today and tomorrow,” said Mr. Wimmer in a press briefing.
The goals of the COVID-19 Action Initiative will be to expand the ALA’s respiratory research program, improve public health measures, and create an advanced network to prevent future respiratory virus pandemics. In cooperation with the public and private sectors, the initiative will promote research collaboration and develop new vaccines, diagnostic tests, and therapies. The initiative will take advantage of the ALA’s current research network and will also fund respiratory virus research. It also will fund education and advocacy to support public health measures against current and future respiratory viruses.
The COVID-19 Action Initiative will fund respiratory virus research through three main activities. It will expand COVID-19 research within the current clinical trials of the Airways Clinical Research Center (ACRC) Network. Second, it will fund coronavirus awards and grants for preventive research, vaccines, antivirals, and efforts to promote preparedness for future outbreaks. Third, it will provide ACRC pilot grants to evaluate the effect of COVID-19 on patients with chronic lung disease.
“More than 36 million people in the U.S. suffer from lung disease, which places them at higher risk for experiencing complications of COVID-19, making it even more critical that we urgently work on reducing its impact,” said Harold Wimmer, president and CEO of the ALA, in a press release.
The ALA has $8 million available and earmarked for the initiative. The association plans to raise additional funds during the next 3 years by reaching out to corporate partners, public health entities, and individuals. “With the help of our staff and volunteers, and with the support and donations of generous Americans, we can stand together and face the challenges to lung health of today and tomorrow,” said Mr. Wimmer in a press briefing.
The goals of the COVID-19 Action Initiative will be to expand the ALA’s respiratory research program, improve public health measures, and create an advanced network to prevent future respiratory virus pandemics. In cooperation with the public and private sectors, the initiative will promote research collaboration and develop new vaccines, diagnostic tests, and therapies. The initiative will take advantage of the ALA’s current research network and will also fund respiratory virus research. It also will fund education and advocacy to support public health measures against current and future respiratory viruses.
The COVID-19 Action Initiative will fund respiratory virus research through three main activities. It will expand COVID-19 research within the current clinical trials of the Airways Clinical Research Center (ACRC) Network. Second, it will fund coronavirus awards and grants for preventive research, vaccines, antivirals, and efforts to promote preparedness for future outbreaks. Third, it will provide ACRC pilot grants to evaluate the effect of COVID-19 on patients with chronic lung disease.
“More than 36 million people in the U.S. suffer from lung disease, which places them at higher risk for experiencing complications of COVID-19, making it even more critical that we urgently work on reducing its impact,” said Harold Wimmer, president and CEO of the ALA, in a press release.
The ALA has $8 million available and earmarked for the initiative. The association plans to raise additional funds during the next 3 years by reaching out to corporate partners, public health entities, and individuals. “With the help of our staff and volunteers, and with the support and donations of generous Americans, we can stand together and face the challenges to lung health of today and tomorrow,” said Mr. Wimmer in a press briefing.
CDC: Screen nearly all adults, including pregnant women, for HCV
In the latest issue of the Morbidity and Mortality Weekly Report, the Centers for Disease Control and Prevention recommended hepatitis C virus screening for all adults and all pregnant women – during each of their pregnancies – in areas where prevalence of the infection is 0.1% or greater.
That’s essentially the entire United States; there’s no state with a statewide adult prevalence below 0.1%, and “few settings are known to exist” otherwise, the CDC noted (MMWR Recomm Rep. 2020 Apr 10;69(2):1-17).
The agency encouraged providers to consult state or local health departments or the CDC directly to determine local HCV prevalence. “As a general guide ... approximately 59% of anti-HCV positive persons are HCV RNA positive,” indicating active infection, the agency noted.
The advice was an expansion from the CDC’s last universal screening recommendation in 2012, which was limited to people born from 1945 to 1965; the incidence of acute infections has climbed since then and is highest now among younger people, so the guideline needed to be revisited, explained authors led by Sarah Schillie, MD, of the CDC’s Division of Viral Hepatitis, Atlanta.
The U.S. Preventive Services Task Force also recently recommended universal adult screening after previously limiting it to baby boomers.
As for pregnancy, the CDC’s past advice was to screen pregnant women with known risk factors, but that needed to be revisited as well. For one thing, the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America have since recommended testing all pregnant women.
But also, the CDC said, it’s an opportune time for screening because “many women only have access to health care during pregnancy and the immediate postpartum period,” when treatment, if needed, can be started. Plus, HCV status is important for management decisions, such as using amniocentesis in positive women instead of chorionic villus sampling.
The rest of CDC’s 2012 recommendations stand, including screening all people with risk factors and repeating screening while they persist. Also, “any person who requests hepatitis C testing should receive it, regardless of disclosure of risk,” because people might be reluctant to report things like IV drug use, the authors said.
Screening in the guidelines means an HCV antibody test, followed by a nucleic acid test to check for active infection. The CDC encouraged automatic reflex testing, meaning immediately checking antibody positive samples for HCV RNA. RNA in the blood indicates active, replicating virus.
The new recommendations penciled out in modeling, with an incremental cost-effectiveness ratio (ICER) for universal adult screening of approximately $36,000 per quality-adjusted life year (QALY) gained, and an ICER of approximately $15,000 per QALY gained for pregnancy screening, where HCV prevalence is 0.1%; the 0.1% cost/benefit cutpoint was one of the reasons it was chosen as the prevalence threshold. An ICER under $50,000 is the conservative benchmark for cost-effectiveness, the authors noted.
There was no external funding, and the authors had no disclosures.
SOURCE: Schillie S et al. MMWR Recomm Rep. 2020 Apr 10;69[2]:1-17).
In the latest issue of the Morbidity and Mortality Weekly Report, the Centers for Disease Control and Prevention recommended hepatitis C virus screening for all adults and all pregnant women – during each of their pregnancies – in areas where prevalence of the infection is 0.1% or greater.
That’s essentially the entire United States; there’s no state with a statewide adult prevalence below 0.1%, and “few settings are known to exist” otherwise, the CDC noted (MMWR Recomm Rep. 2020 Apr 10;69(2):1-17).
The agency encouraged providers to consult state or local health departments or the CDC directly to determine local HCV prevalence. “As a general guide ... approximately 59% of anti-HCV positive persons are HCV RNA positive,” indicating active infection, the agency noted.
The advice was an expansion from the CDC’s last universal screening recommendation in 2012, which was limited to people born from 1945 to 1965; the incidence of acute infections has climbed since then and is highest now among younger people, so the guideline needed to be revisited, explained authors led by Sarah Schillie, MD, of the CDC’s Division of Viral Hepatitis, Atlanta.
The U.S. Preventive Services Task Force also recently recommended universal adult screening after previously limiting it to baby boomers.
As for pregnancy, the CDC’s past advice was to screen pregnant women with known risk factors, but that needed to be revisited as well. For one thing, the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America have since recommended testing all pregnant women.
But also, the CDC said, it’s an opportune time for screening because “many women only have access to health care during pregnancy and the immediate postpartum period,” when treatment, if needed, can be started. Plus, HCV status is important for management decisions, such as using amniocentesis in positive women instead of chorionic villus sampling.
The rest of CDC’s 2012 recommendations stand, including screening all people with risk factors and repeating screening while they persist. Also, “any person who requests hepatitis C testing should receive it, regardless of disclosure of risk,” because people might be reluctant to report things like IV drug use, the authors said.
Screening in the guidelines means an HCV antibody test, followed by a nucleic acid test to check for active infection. The CDC encouraged automatic reflex testing, meaning immediately checking antibody positive samples for HCV RNA. RNA in the blood indicates active, replicating virus.
The new recommendations penciled out in modeling, with an incremental cost-effectiveness ratio (ICER) for universal adult screening of approximately $36,000 per quality-adjusted life year (QALY) gained, and an ICER of approximately $15,000 per QALY gained for pregnancy screening, where HCV prevalence is 0.1%; the 0.1% cost/benefit cutpoint was one of the reasons it was chosen as the prevalence threshold. An ICER under $50,000 is the conservative benchmark for cost-effectiveness, the authors noted.
There was no external funding, and the authors had no disclosures.
SOURCE: Schillie S et al. MMWR Recomm Rep. 2020 Apr 10;69[2]:1-17).
In the latest issue of the Morbidity and Mortality Weekly Report, the Centers for Disease Control and Prevention recommended hepatitis C virus screening for all adults and all pregnant women – during each of their pregnancies – in areas where prevalence of the infection is 0.1% or greater.
That’s essentially the entire United States; there’s no state with a statewide adult prevalence below 0.1%, and “few settings are known to exist” otherwise, the CDC noted (MMWR Recomm Rep. 2020 Apr 10;69(2):1-17).
The agency encouraged providers to consult state or local health departments or the CDC directly to determine local HCV prevalence. “As a general guide ... approximately 59% of anti-HCV positive persons are HCV RNA positive,” indicating active infection, the agency noted.
The advice was an expansion from the CDC’s last universal screening recommendation in 2012, which was limited to people born from 1945 to 1965; the incidence of acute infections has climbed since then and is highest now among younger people, so the guideline needed to be revisited, explained authors led by Sarah Schillie, MD, of the CDC’s Division of Viral Hepatitis, Atlanta.
The U.S. Preventive Services Task Force also recently recommended universal adult screening after previously limiting it to baby boomers.
As for pregnancy, the CDC’s past advice was to screen pregnant women with known risk factors, but that needed to be revisited as well. For one thing, the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America have since recommended testing all pregnant women.
But also, the CDC said, it’s an opportune time for screening because “many women only have access to health care during pregnancy and the immediate postpartum period,” when treatment, if needed, can be started. Plus, HCV status is important for management decisions, such as using amniocentesis in positive women instead of chorionic villus sampling.
The rest of CDC’s 2012 recommendations stand, including screening all people with risk factors and repeating screening while they persist. Also, “any person who requests hepatitis C testing should receive it, regardless of disclosure of risk,” because people might be reluctant to report things like IV drug use, the authors said.
Screening in the guidelines means an HCV antibody test, followed by a nucleic acid test to check for active infection. The CDC encouraged automatic reflex testing, meaning immediately checking antibody positive samples for HCV RNA. RNA in the blood indicates active, replicating virus.
The new recommendations penciled out in modeling, with an incremental cost-effectiveness ratio (ICER) for universal adult screening of approximately $36,000 per quality-adjusted life year (QALY) gained, and an ICER of approximately $15,000 per QALY gained for pregnancy screening, where HCV prevalence is 0.1%; the 0.1% cost/benefit cutpoint was one of the reasons it was chosen as the prevalence threshold. An ICER under $50,000 is the conservative benchmark for cost-effectiveness, the authors noted.
There was no external funding, and the authors had no disclosures.
SOURCE: Schillie S et al. MMWR Recomm Rep. 2020 Apr 10;69[2]:1-17).