User login
Combo elicits responses in advanced cervical cancer
regardless of programmed death–ligand 1 (PD-L1) expression, according to preliminary findings from a phase 2 study.
Apatinib, an inhibitor of vascular endothelial growth factor receptor-2, and camrelizumab, an anti-PD-1 monoclonal antibody, produced an objective response rate of 60% in evaluable patients.
Chunyan Lan, MD, of Sun Yat-sen University Cancer Center in Guangzhou, China, and colleagues reported these results in an abstract that had been slated for presentation at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer. The meeting was canceled because of the COVID-19 pandemic. The following data differ somewhat from the abstract.
Dr. Lan and colleagues reported results in 45 patients who had progressed after at least one line of systemic chemotherapy for metastatic, recurrent, or persistent cervical cancer, and had measurable disease. Patients had a median age of 51 years and an Eastern Cooperative Oncology Group performance status of 0-1. They were enrolled at four centers in China between Jan. 21 and Aug. 1, 2019.
Treatment consisted of oral apatinib at a dose of 250 mg once daily and intravenous camrelizumab at a dose of 200 mg every 2 weeks until disease progression, unacceptable toxicity, or consent withdrawal.
As of Jan. 22, 2020, 25 of 42 efficacy-evaluable patients had achieved a response. Two patients had a complete response, 23 had a partial response, and 12 had stable disease.
“We saw responses in patients regardless of PD-L1 expression,” Dr. Lan said. “In our study, 34% were PD-L1 negative, and the response rate is 65% in PD-L1-positive and 50% in PD-L1-negative [patients].”
The median duration of response was not reached, she added.
The median follow-up was 9.2 months, with the last patient enrolled having 6 months of follow-up. At the data cutoff, 19 patients had disease progression, and 8 had died of their disease.
The median overall survival also was not reached, Dr. Lan said, but overall survival at 9 months was 80%. The median progression-free survival was 7.6 months, and the 6-month progression-free survival rate was 58%.
Grade 3 or greater treatment-related adverse events occurred in 68.9% of patients; adverse events occurring in at least 5% of patients included hypertension (22.2%), fatigue (15.6%), anemia (13.3%), and thrombocytopenia (6.7%).
“Nineteen patients were still on treatment at the data cutoff date, and 26 patients discontinued the study,” Dr. Lan said. “The most common reason to discontinue was disease progression, and three patients discontinued the study due to adverse events.”
“These preliminary results are very encouraging,” Dr. Lan said. “As we know, pembrolizumab is approved as the second-line therapy in recurrent cervical cancer [in] PD-L1-positive patients, and the objective response rate with pembrolizumab monotherapy for recurrent cervical cancer is only 17%, as reported in KEYNOTE-028 [J Clin Oncol. 2017 Dec 20;35(36):4035-41].”
Furthermore, apatinib monotherapy has been studied with only modest results.
“But in our study, this combination is really effective in recurrent cervical cancer, and we see a very durable response,” she said, again emphasizing that those responses occurred regardless of PD-L1 expression. “So this is important. ... We think our findings expand the opportunity of patients with recurrent cervical cancer to receive immune therapy.”
Study participants will be followed for 2 years, Dr. Lan noted.
She reported having no disclosures. The study is sponsored by Sun Yat-sen University.
sworcester@mdedge.com
SOURCE: Lan C et al. SGO 2020, Abstract 55.
regardless of programmed death–ligand 1 (PD-L1) expression, according to preliminary findings from a phase 2 study.
Apatinib, an inhibitor of vascular endothelial growth factor receptor-2, and camrelizumab, an anti-PD-1 monoclonal antibody, produced an objective response rate of 60% in evaluable patients.
Chunyan Lan, MD, of Sun Yat-sen University Cancer Center in Guangzhou, China, and colleagues reported these results in an abstract that had been slated for presentation at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer. The meeting was canceled because of the COVID-19 pandemic. The following data differ somewhat from the abstract.
Dr. Lan and colleagues reported results in 45 patients who had progressed after at least one line of systemic chemotherapy for metastatic, recurrent, or persistent cervical cancer, and had measurable disease. Patients had a median age of 51 years and an Eastern Cooperative Oncology Group performance status of 0-1. They were enrolled at four centers in China between Jan. 21 and Aug. 1, 2019.
Treatment consisted of oral apatinib at a dose of 250 mg once daily and intravenous camrelizumab at a dose of 200 mg every 2 weeks until disease progression, unacceptable toxicity, or consent withdrawal.
As of Jan. 22, 2020, 25 of 42 efficacy-evaluable patients had achieved a response. Two patients had a complete response, 23 had a partial response, and 12 had stable disease.
“We saw responses in patients regardless of PD-L1 expression,” Dr. Lan said. “In our study, 34% were PD-L1 negative, and the response rate is 65% in PD-L1-positive and 50% in PD-L1-negative [patients].”
The median duration of response was not reached, she added.
The median follow-up was 9.2 months, with the last patient enrolled having 6 months of follow-up. At the data cutoff, 19 patients had disease progression, and 8 had died of their disease.
The median overall survival also was not reached, Dr. Lan said, but overall survival at 9 months was 80%. The median progression-free survival was 7.6 months, and the 6-month progression-free survival rate was 58%.
Grade 3 or greater treatment-related adverse events occurred in 68.9% of patients; adverse events occurring in at least 5% of patients included hypertension (22.2%), fatigue (15.6%), anemia (13.3%), and thrombocytopenia (6.7%).
“Nineteen patients were still on treatment at the data cutoff date, and 26 patients discontinued the study,” Dr. Lan said. “The most common reason to discontinue was disease progression, and three patients discontinued the study due to adverse events.”
“These preliminary results are very encouraging,” Dr. Lan said. “As we know, pembrolizumab is approved as the second-line therapy in recurrent cervical cancer [in] PD-L1-positive patients, and the objective response rate with pembrolizumab monotherapy for recurrent cervical cancer is only 17%, as reported in KEYNOTE-028 [J Clin Oncol. 2017 Dec 20;35(36):4035-41].”
Furthermore, apatinib monotherapy has been studied with only modest results.
“But in our study, this combination is really effective in recurrent cervical cancer, and we see a very durable response,” she said, again emphasizing that those responses occurred regardless of PD-L1 expression. “So this is important. ... We think our findings expand the opportunity of patients with recurrent cervical cancer to receive immune therapy.”
Study participants will be followed for 2 years, Dr. Lan noted.
She reported having no disclosures. The study is sponsored by Sun Yat-sen University.
sworcester@mdedge.com
SOURCE: Lan C et al. SGO 2020, Abstract 55.
regardless of programmed death–ligand 1 (PD-L1) expression, according to preliminary findings from a phase 2 study.
Apatinib, an inhibitor of vascular endothelial growth factor receptor-2, and camrelizumab, an anti-PD-1 monoclonal antibody, produced an objective response rate of 60% in evaluable patients.
Chunyan Lan, MD, of Sun Yat-sen University Cancer Center in Guangzhou, China, and colleagues reported these results in an abstract that had been slated for presentation at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer. The meeting was canceled because of the COVID-19 pandemic. The following data differ somewhat from the abstract.
Dr. Lan and colleagues reported results in 45 patients who had progressed after at least one line of systemic chemotherapy for metastatic, recurrent, or persistent cervical cancer, and had measurable disease. Patients had a median age of 51 years and an Eastern Cooperative Oncology Group performance status of 0-1. They were enrolled at four centers in China between Jan. 21 and Aug. 1, 2019.
Treatment consisted of oral apatinib at a dose of 250 mg once daily and intravenous camrelizumab at a dose of 200 mg every 2 weeks until disease progression, unacceptable toxicity, or consent withdrawal.
As of Jan. 22, 2020, 25 of 42 efficacy-evaluable patients had achieved a response. Two patients had a complete response, 23 had a partial response, and 12 had stable disease.
“We saw responses in patients regardless of PD-L1 expression,” Dr. Lan said. “In our study, 34% were PD-L1 negative, and the response rate is 65% in PD-L1-positive and 50% in PD-L1-negative [patients].”
The median duration of response was not reached, she added.
The median follow-up was 9.2 months, with the last patient enrolled having 6 months of follow-up. At the data cutoff, 19 patients had disease progression, and 8 had died of their disease.
The median overall survival also was not reached, Dr. Lan said, but overall survival at 9 months was 80%. The median progression-free survival was 7.6 months, and the 6-month progression-free survival rate was 58%.
Grade 3 or greater treatment-related adverse events occurred in 68.9% of patients; adverse events occurring in at least 5% of patients included hypertension (22.2%), fatigue (15.6%), anemia (13.3%), and thrombocytopenia (6.7%).
“Nineteen patients were still on treatment at the data cutoff date, and 26 patients discontinued the study,” Dr. Lan said. “The most common reason to discontinue was disease progression, and three patients discontinued the study due to adverse events.”
“These preliminary results are very encouraging,” Dr. Lan said. “As we know, pembrolizumab is approved as the second-line therapy in recurrent cervical cancer [in] PD-L1-positive patients, and the objective response rate with pembrolizumab monotherapy for recurrent cervical cancer is only 17%, as reported in KEYNOTE-028 [J Clin Oncol. 2017 Dec 20;35(36):4035-41].”
Furthermore, apatinib monotherapy has been studied with only modest results.
“But in our study, this combination is really effective in recurrent cervical cancer, and we see a very durable response,” she said, again emphasizing that those responses occurred regardless of PD-L1 expression. “So this is important. ... We think our findings expand the opportunity of patients with recurrent cervical cancer to receive immune therapy.”
Study participants will be followed for 2 years, Dr. Lan noted.
She reported having no disclosures. The study is sponsored by Sun Yat-sen University.
sworcester@mdedge.com
SOURCE: Lan C et al. SGO 2020, Abstract 55.
FROM SGO 2020
When to suspect calciphylaxis and what to do about it
If the shoe fits a presumptive clinical diagnosis of calciphylaxis, wear it – and don’t assume that ordering imaging studies or histology will make for a better fit or is even necessary.
That was the key message of Karl M. Saardi, MD, during his video presentation at a virtual meeting held by the George Washington University department of dermatology. The virtual meeting included presentations that had been slated for the annual meeting of the American Academy of Dermatology, which was canceled because of the COVID-19 pandemic.
“ said Dr. Saardi, a dermatology resident at Georgetown University in Washington, D.C.
He presented a single-center, retrospective study that underscored the diagnostic challenges posed by calciphylaxis, a condition for which there are no generally accepted clinical, radiographic, or histologic diagnostic criteria.
The rare skin condition is characterized by calcium deposition in small arterioles and capillaries in the skin and subcutaneous tissue. It’s most common in patients with end-stage renal disease who are on dialysis; however, there is also an increasingly recognized nonuremic variant that’s associated with the use of warfarin, chronic steroids, obesity, and possibly with being antiphospholipid antibody positive.
Calciphylaxis is an extremely painful condition – the pain is ischemic in nature – and it’s associated with substantial morbidity as well as a mortality rate that in many series exceeds 50%. Affected individuals typically present with progressive, painful retiform purpura on the legs, belly, buttocks, and other fatty body sites.
Dr. Saardi’s study entailed a retrospective look at the medical records and pathologic reports of 57 patients who underwent skin biopsy for suspected calciphylaxis. Of the 57, 18 had no antecedent imaging studies done during the preceding 3 months; 8 of those 18 (40%), had a confirmatory positive biopsy. A total of 39 patients did have imaging studies, deemed positive for calciphylaxis in 11 cases, which in only 5 of the 11 imaging-positive cases (45%) were subsequently confirmed by positive biopsy.
And finally, of the 28 patients with negative imaging studies, 10 (36%), had a positive biopsy. Those positive biopsy rates, ranging from 36% to 45%, did not differ statistically. Thus, whether an imaging study was positive or negative, or wasn’t even done, made no difference in terms of the ultimate diagnosis.
“You may not need imaging studies, because imaging has often been done before the consultation is requested because people are looking for things like arterial thrombus, cellulitis, [deep vein thrombosis] or something like that,” Dr. Saardi noted. “In our series, the indication was never calciphylaxis, it was always something like pain, infection, swelling, suspected [deep vein thrombosis], things like that.”
The classic signature of calciphylaxis on plain x-ray is net-like calcifications in skin and subcutaneous tissue. In one study, this often-subtle finding was associated with a 830% increased likelihood of a positive biopsy, with a specificity of 90%; however, these x-ray changes were only found in 13 of 29 patients with biopsy-confirmed calciphylaxis.
“It’s really important when you request plain films in these patients to review the images yourself or together with the radiologist because oftentimes the indication for imaging will be very different from what we’re looking for. Radiologists often won’t know to look for this specifically,” Dr. Saardi said.
The classic histopathologic finding is calcification of the small- and medium-sized vessels in the dermis and subcutaneous soft tissue. However, sometimes all that’s present are small intravascular inflammatory thrombi with intimal hyperplasia.
Skin biopsies are not infrequently falsely negative or nondiagnostic. To maximize the utility of the procedure, it’s important to go deep and gather a tissue sample that extends into subcutaneous tissue.
“You need to do a very deep punch or double-punch biopsy,” he said. “Another key is to avoid biopsy if the pretest probability of calciphylaxis is high because a negative biopsy shouldn’t necessarily reassure you or cause you to withhold treatment. And with the concern about pathergy or Koebnerization of the area causing a wound that’s never going to heal, sometimes a biopsy is not needed if the pretest suspicion is high enough.”
Other investigators have shown that the likelihood of an informative biopsy is enhanced by using a calcium stain on the specimen and having an experienced dermatopathologist do the evaluation. Also, the use of a radiographically guided core needle biopsy to ensure that the physician is getting sufficiently deep into subcutaneous fat is now under evaluation.
In addition to plain radiographs, other imaging methods that are sometimes used to evaluate soft-tissue sites for suspected calciphylaxis included CT and ultrasound. Dr. Saardi is particularly intrigued by reports from investigators at Harvard University regarding the utility of nuclear bone scintigraphy; in one study, this form of imaging was positive in 16 of 18 patients with clinically diagnosed calciphylaxis, versus just 1 of 31 controls with end-stage renal disease.
“We’ve started doing this in situations where biopsy is not desirable or feasible at that moment,” he said.
Dr. Saardi reported having no financial conflicts regarding his presentation.
If the shoe fits a presumptive clinical diagnosis of calciphylaxis, wear it – and don’t assume that ordering imaging studies or histology will make for a better fit or is even necessary.
That was the key message of Karl M. Saardi, MD, during his video presentation at a virtual meeting held by the George Washington University department of dermatology. The virtual meeting included presentations that had been slated for the annual meeting of the American Academy of Dermatology, which was canceled because of the COVID-19 pandemic.
“ said Dr. Saardi, a dermatology resident at Georgetown University in Washington, D.C.
He presented a single-center, retrospective study that underscored the diagnostic challenges posed by calciphylaxis, a condition for which there are no generally accepted clinical, radiographic, or histologic diagnostic criteria.
The rare skin condition is characterized by calcium deposition in small arterioles and capillaries in the skin and subcutaneous tissue. It’s most common in patients with end-stage renal disease who are on dialysis; however, there is also an increasingly recognized nonuremic variant that’s associated with the use of warfarin, chronic steroids, obesity, and possibly with being antiphospholipid antibody positive.
Calciphylaxis is an extremely painful condition – the pain is ischemic in nature – and it’s associated with substantial morbidity as well as a mortality rate that in many series exceeds 50%. Affected individuals typically present with progressive, painful retiform purpura on the legs, belly, buttocks, and other fatty body sites.
Dr. Saardi’s study entailed a retrospective look at the medical records and pathologic reports of 57 patients who underwent skin biopsy for suspected calciphylaxis. Of the 57, 18 had no antecedent imaging studies done during the preceding 3 months; 8 of those 18 (40%), had a confirmatory positive biopsy. A total of 39 patients did have imaging studies, deemed positive for calciphylaxis in 11 cases, which in only 5 of the 11 imaging-positive cases (45%) were subsequently confirmed by positive biopsy.
And finally, of the 28 patients with negative imaging studies, 10 (36%), had a positive biopsy. Those positive biopsy rates, ranging from 36% to 45%, did not differ statistically. Thus, whether an imaging study was positive or negative, or wasn’t even done, made no difference in terms of the ultimate diagnosis.
“You may not need imaging studies, because imaging has often been done before the consultation is requested because people are looking for things like arterial thrombus, cellulitis, [deep vein thrombosis] or something like that,” Dr. Saardi noted. “In our series, the indication was never calciphylaxis, it was always something like pain, infection, swelling, suspected [deep vein thrombosis], things like that.”
The classic signature of calciphylaxis on plain x-ray is net-like calcifications in skin and subcutaneous tissue. In one study, this often-subtle finding was associated with a 830% increased likelihood of a positive biopsy, with a specificity of 90%; however, these x-ray changes were only found in 13 of 29 patients with biopsy-confirmed calciphylaxis.
“It’s really important when you request plain films in these patients to review the images yourself or together with the radiologist because oftentimes the indication for imaging will be very different from what we’re looking for. Radiologists often won’t know to look for this specifically,” Dr. Saardi said.
The classic histopathologic finding is calcification of the small- and medium-sized vessels in the dermis and subcutaneous soft tissue. However, sometimes all that’s present are small intravascular inflammatory thrombi with intimal hyperplasia.
Skin biopsies are not infrequently falsely negative or nondiagnostic. To maximize the utility of the procedure, it’s important to go deep and gather a tissue sample that extends into subcutaneous tissue.
“You need to do a very deep punch or double-punch biopsy,” he said. “Another key is to avoid biopsy if the pretest probability of calciphylaxis is high because a negative biopsy shouldn’t necessarily reassure you or cause you to withhold treatment. And with the concern about pathergy or Koebnerization of the area causing a wound that’s never going to heal, sometimes a biopsy is not needed if the pretest suspicion is high enough.”
Other investigators have shown that the likelihood of an informative biopsy is enhanced by using a calcium stain on the specimen and having an experienced dermatopathologist do the evaluation. Also, the use of a radiographically guided core needle biopsy to ensure that the physician is getting sufficiently deep into subcutaneous fat is now under evaluation.
In addition to plain radiographs, other imaging methods that are sometimes used to evaluate soft-tissue sites for suspected calciphylaxis included CT and ultrasound. Dr. Saardi is particularly intrigued by reports from investigators at Harvard University regarding the utility of nuclear bone scintigraphy; in one study, this form of imaging was positive in 16 of 18 patients with clinically diagnosed calciphylaxis, versus just 1 of 31 controls with end-stage renal disease.
“We’ve started doing this in situations where biopsy is not desirable or feasible at that moment,” he said.
Dr. Saardi reported having no financial conflicts regarding his presentation.
If the shoe fits a presumptive clinical diagnosis of calciphylaxis, wear it – and don’t assume that ordering imaging studies or histology will make for a better fit or is even necessary.
That was the key message of Karl M. Saardi, MD, during his video presentation at a virtual meeting held by the George Washington University department of dermatology. The virtual meeting included presentations that had been slated for the annual meeting of the American Academy of Dermatology, which was canceled because of the COVID-19 pandemic.
“ said Dr. Saardi, a dermatology resident at Georgetown University in Washington, D.C.
He presented a single-center, retrospective study that underscored the diagnostic challenges posed by calciphylaxis, a condition for which there are no generally accepted clinical, radiographic, or histologic diagnostic criteria.
The rare skin condition is characterized by calcium deposition in small arterioles and capillaries in the skin and subcutaneous tissue. It’s most common in patients with end-stage renal disease who are on dialysis; however, there is also an increasingly recognized nonuremic variant that’s associated with the use of warfarin, chronic steroids, obesity, and possibly with being antiphospholipid antibody positive.
Calciphylaxis is an extremely painful condition – the pain is ischemic in nature – and it’s associated with substantial morbidity as well as a mortality rate that in many series exceeds 50%. Affected individuals typically present with progressive, painful retiform purpura on the legs, belly, buttocks, and other fatty body sites.
Dr. Saardi’s study entailed a retrospective look at the medical records and pathologic reports of 57 patients who underwent skin biopsy for suspected calciphylaxis. Of the 57, 18 had no antecedent imaging studies done during the preceding 3 months; 8 of those 18 (40%), had a confirmatory positive biopsy. A total of 39 patients did have imaging studies, deemed positive for calciphylaxis in 11 cases, which in only 5 of the 11 imaging-positive cases (45%) were subsequently confirmed by positive biopsy.
And finally, of the 28 patients with negative imaging studies, 10 (36%), had a positive biopsy. Those positive biopsy rates, ranging from 36% to 45%, did not differ statistically. Thus, whether an imaging study was positive or negative, or wasn’t even done, made no difference in terms of the ultimate diagnosis.
“You may not need imaging studies, because imaging has often been done before the consultation is requested because people are looking for things like arterial thrombus, cellulitis, [deep vein thrombosis] or something like that,” Dr. Saardi noted. “In our series, the indication was never calciphylaxis, it was always something like pain, infection, swelling, suspected [deep vein thrombosis], things like that.”
The classic signature of calciphylaxis on plain x-ray is net-like calcifications in skin and subcutaneous tissue. In one study, this often-subtle finding was associated with a 830% increased likelihood of a positive biopsy, with a specificity of 90%; however, these x-ray changes were only found in 13 of 29 patients with biopsy-confirmed calciphylaxis.
“It’s really important when you request plain films in these patients to review the images yourself or together with the radiologist because oftentimes the indication for imaging will be very different from what we’re looking for. Radiologists often won’t know to look for this specifically,” Dr. Saardi said.
The classic histopathologic finding is calcification of the small- and medium-sized vessels in the dermis and subcutaneous soft tissue. However, sometimes all that’s present are small intravascular inflammatory thrombi with intimal hyperplasia.
Skin biopsies are not infrequently falsely negative or nondiagnostic. To maximize the utility of the procedure, it’s important to go deep and gather a tissue sample that extends into subcutaneous tissue.
“You need to do a very deep punch or double-punch biopsy,” he said. “Another key is to avoid biopsy if the pretest probability of calciphylaxis is high because a negative biopsy shouldn’t necessarily reassure you or cause you to withhold treatment. And with the concern about pathergy or Koebnerization of the area causing a wound that’s never going to heal, sometimes a biopsy is not needed if the pretest suspicion is high enough.”
Other investigators have shown that the likelihood of an informative biopsy is enhanced by using a calcium stain on the specimen and having an experienced dermatopathologist do the evaluation. Also, the use of a radiographically guided core needle biopsy to ensure that the physician is getting sufficiently deep into subcutaneous fat is now under evaluation.
In addition to plain radiographs, other imaging methods that are sometimes used to evaluate soft-tissue sites for suspected calciphylaxis included CT and ultrasound. Dr. Saardi is particularly intrigued by reports from investigators at Harvard University regarding the utility of nuclear bone scintigraphy; in one study, this form of imaging was positive in 16 of 18 patients with clinically diagnosed calciphylaxis, versus just 1 of 31 controls with end-stage renal disease.
“We’ve started doing this in situations where biopsy is not desirable or feasible at that moment,” he said.
Dr. Saardi reported having no financial conflicts regarding his presentation.
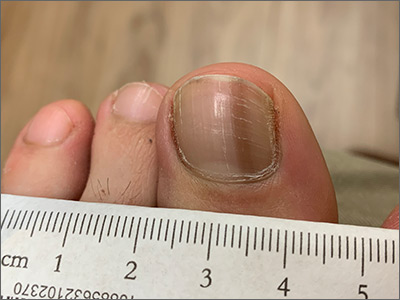
Dark toenail line
This woman’s linear hyperpigmentation is formally known as longitudinal melanonychia.
After the initial diagnosis, it is important to determine if the melanonychia is due to melanoma or a benign process. Many people with darker skin types (Fitzpatrick skin types V or VI) have several of these lines, so it is reassuring when these lines are present on multiple digits. Additional evaluation is warranted, however, when only 1 longitudinal melanonychia is present.
In this case, it was clear that the pigmentation was homogeneous and in a parallel line format. (Dermoscopy can aid in the evaluation of such lesions.) There was no spreading of the pigmentation onto the skin around the nail (Hutchinson sign), which would be suggestive of melanoma. The patient noted that the lesion had been present (without progression) over a 10-year period, which also was consistent with a benign process. A new lesion, rapid change, a triangular pattern wider at the cuticle vs the distal nail, and nail disruption are worrisome features that would have warranted a biopsy.
If a biopsy is called for, be sure to sample the origin of the pigmentation under the cuticle and the nail at the nail matrix. This requires partial or complete nail removal to reach the matrix, and there is a risk of permanent nail disruption. In light of this, it’s important to discriminate which lesions are at higher risk for melanoma.
In this patient, who had a stable lesion and no concerning history or findings, it was likely that she had a benign nevus or lentigo in the nail matrix. She was instructed to watch for any changes and to notify her physician if any occurred, as such changes might require a biopsy.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Singal A, Bisherwal K. Melanonychia: etiology, diagnosis, and treatment. Indian Dermatol Online J. 2020;11:1-11.
This woman’s linear hyperpigmentation is formally known as longitudinal melanonychia.
After the initial diagnosis, it is important to determine if the melanonychia is due to melanoma or a benign process. Many people with darker skin types (Fitzpatrick skin types V or VI) have several of these lines, so it is reassuring when these lines are present on multiple digits. Additional evaluation is warranted, however, when only 1 longitudinal melanonychia is present.
In this case, it was clear that the pigmentation was homogeneous and in a parallel line format. (Dermoscopy can aid in the evaluation of such lesions.) There was no spreading of the pigmentation onto the skin around the nail (Hutchinson sign), which would be suggestive of melanoma. The patient noted that the lesion had been present (without progression) over a 10-year period, which also was consistent with a benign process. A new lesion, rapid change, a triangular pattern wider at the cuticle vs the distal nail, and nail disruption are worrisome features that would have warranted a biopsy.
If a biopsy is called for, be sure to sample the origin of the pigmentation under the cuticle and the nail at the nail matrix. This requires partial or complete nail removal to reach the matrix, and there is a risk of permanent nail disruption. In light of this, it’s important to discriminate which lesions are at higher risk for melanoma.
In this patient, who had a stable lesion and no concerning history or findings, it was likely that she had a benign nevus or lentigo in the nail matrix. She was instructed to watch for any changes and to notify her physician if any occurred, as such changes might require a biopsy.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
This woman’s linear hyperpigmentation is formally known as longitudinal melanonychia.
After the initial diagnosis, it is important to determine if the melanonychia is due to melanoma or a benign process. Many people with darker skin types (Fitzpatrick skin types V or VI) have several of these lines, so it is reassuring when these lines are present on multiple digits. Additional evaluation is warranted, however, when only 1 longitudinal melanonychia is present.
In this case, it was clear that the pigmentation was homogeneous and in a parallel line format. (Dermoscopy can aid in the evaluation of such lesions.) There was no spreading of the pigmentation onto the skin around the nail (Hutchinson sign), which would be suggestive of melanoma. The patient noted that the lesion had been present (without progression) over a 10-year period, which also was consistent with a benign process. A new lesion, rapid change, a triangular pattern wider at the cuticle vs the distal nail, and nail disruption are worrisome features that would have warranted a biopsy.
If a biopsy is called for, be sure to sample the origin of the pigmentation under the cuticle and the nail at the nail matrix. This requires partial or complete nail removal to reach the matrix, and there is a risk of permanent nail disruption. In light of this, it’s important to discriminate which lesions are at higher risk for melanoma.
In this patient, who had a stable lesion and no concerning history or findings, it was likely that she had a benign nevus or lentigo in the nail matrix. She was instructed to watch for any changes and to notify her physician if any occurred, as such changes might require a biopsy.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Singal A, Bisherwal K. Melanonychia: etiology, diagnosis, and treatment. Indian Dermatol Online J. 2020;11:1-11.
Singal A, Bisherwal K. Melanonychia: etiology, diagnosis, and treatment. Indian Dermatol Online J. 2020;11:1-11.
Cardiology groups push back on hydroxychloroquine, azithromycin for COVID-19
The .
“Hydroxychloroquine and azithromycin have been touted for potential prophylaxis or treatment for COVID-19; both drugs are listed as definite causes of torsade de pointes” and increase in the risk of other arrhythmias and sudden death, the American Heart Association, the American College of Cardiology, and the Heart Rhythm Society said in a joint statement April 8 in Circulation.
The statement came amid ongoing promotion by the Trump administration of hydroxychloroquine, in particular, for COVID-19 despite lack of strong data.
In addition to underlying cardiovascular disease, “seriously ill patients often have comorbidities that can increase risk of serious arrhythmias,” including hypokalemia, hypomagnesemia, fever, and systemic inflammation, the groups said.
They recommended withholding the drugs in patients with baseline QT prolongation (e.g., QTc of at least 500 msec) or with known congenital long QT syndrome; monitoring cardiac rhythm and QT interval and withdrawing hydroxychloroquine and azithromycin if QTc exceeds 500 msec; correcting hypokalemia to levels greater than 4 mEq/L and hypomagnesemia to more than 2 mg/dL; and avoiding other QTc-prolonging agents when possible.
The groups noted that, “in patients critically ill with COVID-19 infection, frequent caregiver contact may need to be minimized, so optimal electrocardiographic interval and rhythm monitoring may not be possible.” There is also a possible compounding arrhythmic effect when hydroxychloroquine and azithromycin are used together, but that has not been studied.
There’s a known risk of torsade de pointes with chloroquine and a possible risk with the antiviral HIV combination drug lopinavir-ritonavir, two other candidates for COVID-19 treatment. Hydroxychloroquine and chloroquine, both antimalarials, might help prevent or treat infection by interfering with angiotensin-converting enzyme 2 receptors, which the COVID-19 virus uses for cell entry, the groups said.
“The urgency of COVID-19 must not diminish the scientific rigor with which we approach COVID-19 treatment. While these medications may work against COVID-19 individually or in combination, we recommend caution with these medications for patients with existing cardiovascular disease,” Robert A. Harrington, MD, AHA president and chair of the department of medicine at Stanford (Calif.) University, emphasized in a press release.
SOURCE: Roden DM et al. Circulation. 2020 Apr 8. doi:10.1161/CIRCULATIONAHA.120.047521.
The .
“Hydroxychloroquine and azithromycin have been touted for potential prophylaxis or treatment for COVID-19; both drugs are listed as definite causes of torsade de pointes” and increase in the risk of other arrhythmias and sudden death, the American Heart Association, the American College of Cardiology, and the Heart Rhythm Society said in a joint statement April 8 in Circulation.
The statement came amid ongoing promotion by the Trump administration of hydroxychloroquine, in particular, for COVID-19 despite lack of strong data.
In addition to underlying cardiovascular disease, “seriously ill patients often have comorbidities that can increase risk of serious arrhythmias,” including hypokalemia, hypomagnesemia, fever, and systemic inflammation, the groups said.
They recommended withholding the drugs in patients with baseline QT prolongation (e.g., QTc of at least 500 msec) or with known congenital long QT syndrome; monitoring cardiac rhythm and QT interval and withdrawing hydroxychloroquine and azithromycin if QTc exceeds 500 msec; correcting hypokalemia to levels greater than 4 mEq/L and hypomagnesemia to more than 2 mg/dL; and avoiding other QTc-prolonging agents when possible.
The groups noted that, “in patients critically ill with COVID-19 infection, frequent caregiver contact may need to be minimized, so optimal electrocardiographic interval and rhythm monitoring may not be possible.” There is also a possible compounding arrhythmic effect when hydroxychloroquine and azithromycin are used together, but that has not been studied.
There’s a known risk of torsade de pointes with chloroquine and a possible risk with the antiviral HIV combination drug lopinavir-ritonavir, two other candidates for COVID-19 treatment. Hydroxychloroquine and chloroquine, both antimalarials, might help prevent or treat infection by interfering with angiotensin-converting enzyme 2 receptors, which the COVID-19 virus uses for cell entry, the groups said.
“The urgency of COVID-19 must not diminish the scientific rigor with which we approach COVID-19 treatment. While these medications may work against COVID-19 individually or in combination, we recommend caution with these medications for patients with existing cardiovascular disease,” Robert A. Harrington, MD, AHA president and chair of the department of medicine at Stanford (Calif.) University, emphasized in a press release.
SOURCE: Roden DM et al. Circulation. 2020 Apr 8. doi:10.1161/CIRCULATIONAHA.120.047521.
The .
“Hydroxychloroquine and azithromycin have been touted for potential prophylaxis or treatment for COVID-19; both drugs are listed as definite causes of torsade de pointes” and increase in the risk of other arrhythmias and sudden death, the American Heart Association, the American College of Cardiology, and the Heart Rhythm Society said in a joint statement April 8 in Circulation.
The statement came amid ongoing promotion by the Trump administration of hydroxychloroquine, in particular, for COVID-19 despite lack of strong data.
In addition to underlying cardiovascular disease, “seriously ill patients often have comorbidities that can increase risk of serious arrhythmias,” including hypokalemia, hypomagnesemia, fever, and systemic inflammation, the groups said.
They recommended withholding the drugs in patients with baseline QT prolongation (e.g., QTc of at least 500 msec) or with known congenital long QT syndrome; monitoring cardiac rhythm and QT interval and withdrawing hydroxychloroquine and azithromycin if QTc exceeds 500 msec; correcting hypokalemia to levels greater than 4 mEq/L and hypomagnesemia to more than 2 mg/dL; and avoiding other QTc-prolonging agents when possible.
The groups noted that, “in patients critically ill with COVID-19 infection, frequent caregiver contact may need to be minimized, so optimal electrocardiographic interval and rhythm monitoring may not be possible.” There is also a possible compounding arrhythmic effect when hydroxychloroquine and azithromycin are used together, but that has not been studied.
There’s a known risk of torsade de pointes with chloroquine and a possible risk with the antiviral HIV combination drug lopinavir-ritonavir, two other candidates for COVID-19 treatment. Hydroxychloroquine and chloroquine, both antimalarials, might help prevent or treat infection by interfering with angiotensin-converting enzyme 2 receptors, which the COVID-19 virus uses for cell entry, the groups said.
“The urgency of COVID-19 must not diminish the scientific rigor with which we approach COVID-19 treatment. While these medications may work against COVID-19 individually or in combination, we recommend caution with these medications for patients with existing cardiovascular disease,” Robert A. Harrington, MD, AHA president and chair of the department of medicine at Stanford (Calif.) University, emphasized in a press release.
SOURCE: Roden DM et al. Circulation. 2020 Apr 8. doi:10.1161/CIRCULATIONAHA.120.047521.
COVID-19: Are acute stroke patients avoiding emergency care?
(EDs).
Stroke specialists in New Orleans, Chicago, Seattle, and elsewhere told Medscape Medical News they are seeing a precipitous drop in the number of acute strokes at their institutions – and not just in the number of milder cases. Doctors on Twitter are sharing similar reports and are using social media to highlight this issue.
Gabriel Vidal, MD, a vascular and interventional neurologist at the Ochsner Medical Center, New Orleans, Louisiana, said there are “definitely” fewer patients with stroke and transient ischemic attack (TIA) seeking care at his facility and others throughout the New Orleans area, which has been hard hit by COVID-19.
“Even in Louisiana, we have a very large 53-hospital telestroke network, and the number of consults has diminished greatly,” Vidal added.
In Chicago, emergency medical service activations for patients with suspected strokes are down about 30%, Shyam Prabhakaran, MD, professor and chair of neurology at the University of Chicago Biological Sciences, Illinois, told Medscape Medical News.
“It appears to be that mild stroke and TIA patients may be more likely to stay at home and seek alternative care rather than come to the ED,” Prabhakaran said. However, “the severe strokes may be less affected and continue to come to emergency departments.”
“Getting the Word Out”
That may not be the whole story in Seattle, Washington, where a stroke specialist at Harborview Medical Center reported a drop in patients across the stroke-severity spectrum.
Some patients with milder strokes no longer come to Harborview for a comprehensive evaluation and workup, but that is only “a partial explanation,” said David Tirschwell, MD, medical director of comprehensive stroke care at the University of Washington (UW) Medicine Stroke Center at Harborview and a professor of neurology at UW.
“The thrombectomies are down also,” he added. “It’s hard to have great numbers in real time, but it’s probably safe to say it’s at least a 50% reduction in the number of admissions.”
As a stroke referral center, his institution is seeing fewer local cases and referrals from outside hospitals. “I think both of those sources for admissions of stroke cases are down,” Tirschwell said.
Recognizing the seriousness of forgoing essential care for acute stroke, neurologists, institutions, and medical groups are taking to social media to potentially save lives.
“Across our @FLStrokeReg we are seeing less patients with #stroke symptoms coming to our hospitals. We need to get the word out that our teams are working hard to safely provide care when needed during #COVID19,” tweeted Ralph Sacco, MD, chief and professor of neurology, University of Miami Miller School of Medicine in South Florida.
Although Florida Stroke Registry data are not publicly available, anecdotal reports suggest that stroke admissions are down among many hospitals, Sacco told Medscape Medical News.
Furthermore, this is not a phenomenon only in the United States. “This has also been reported in other nations hit by COVID-19,” he said.
China is a prime example. There, many stroke centers have shown reduced functioning “because of fear of in-hospital cross infection and lack of experienced stroke care experts,” Jing Zhao, MD, PhD, and colleagues write in an editorial published online March 31 in Stroke.
Preliminary data show that “thrombectomies in Shanghai decreased by 50% in the first month after the Spring Festival compared with the same period in 2019,” write the editorialists, who are from Kings College London and the University of Pennsylvania in Philadelphia.
“Although the control of the COVID-19 is very important, at the same time, the management of stroke must not be neglected,” they add.
“Over 9000 new stroke cases occur each day in China alone. It cannot be right that treatment for one potentially curable disease is euthanized at the expense of another.”
Fear Factor?
The reasons individuals who may have experienced a stroke are avoiding emergency care is unclear at the moment. “I’m not really sure anyone really understands why, quite honestly,” Tirschwell said.
Until survey data or other data emerge, many experts are assuming that fear of COVID-19 is trumping other medical concerns, including emergency treatment of stroke.
“We believe this could represent patients being fearful to come to medical facilities with stroke-like symptoms, given the COVID-19 pandemic,” said Sacco, who is also incoming editor-in-chief of Stroke.
The BBC has been getting the word out in the United Kingdom via social media, with a tweet to “Dial 999 for stroke emergencies despite coronavirus.”
The World Stroke Campaign is also using Twitter to emphasize the need for urgent stroke care when appropriate:
“Don’t let concerns about COVID19 prevent you from seeking emergency treatment for stroke. If you spot the signs of stroke act FAST. Get emergency medical assistance,” the group urged in a tweet.
Don’t Hesitate
The American Heart Association (AHA) has addressed this troubling trend as well.
“People with serious symptoms shouldn’t ignore them,” Sarah Perlman, MD, associate professor of emergency medicine at the University of Colorado School of Medicine, Denver, states in an article on the AHA website.
Perlman added that some individuals who have signs of stroke and heart disease may hesitate to seek care because of fear that they are adding to an overburdened healthcare staff and system. However, she dismissed those concerns outright.
“If you’re experiencing warning signs of a heart attack or stroke, call 911,” she said. “Clearly, if there’s an emergency, we are available and capable and eager to take care of you.”
This article first appeared on Medscape.com.
(EDs).
Stroke specialists in New Orleans, Chicago, Seattle, and elsewhere told Medscape Medical News they are seeing a precipitous drop in the number of acute strokes at their institutions – and not just in the number of milder cases. Doctors on Twitter are sharing similar reports and are using social media to highlight this issue.
Gabriel Vidal, MD, a vascular and interventional neurologist at the Ochsner Medical Center, New Orleans, Louisiana, said there are “definitely” fewer patients with stroke and transient ischemic attack (TIA) seeking care at his facility and others throughout the New Orleans area, which has been hard hit by COVID-19.
“Even in Louisiana, we have a very large 53-hospital telestroke network, and the number of consults has diminished greatly,” Vidal added.
In Chicago, emergency medical service activations for patients with suspected strokes are down about 30%, Shyam Prabhakaran, MD, professor and chair of neurology at the University of Chicago Biological Sciences, Illinois, told Medscape Medical News.
“It appears to be that mild stroke and TIA patients may be more likely to stay at home and seek alternative care rather than come to the ED,” Prabhakaran said. However, “the severe strokes may be less affected and continue to come to emergency departments.”
“Getting the Word Out”
That may not be the whole story in Seattle, Washington, where a stroke specialist at Harborview Medical Center reported a drop in patients across the stroke-severity spectrum.
Some patients with milder strokes no longer come to Harborview for a comprehensive evaluation and workup, but that is only “a partial explanation,” said David Tirschwell, MD, medical director of comprehensive stroke care at the University of Washington (UW) Medicine Stroke Center at Harborview and a professor of neurology at UW.
“The thrombectomies are down also,” he added. “It’s hard to have great numbers in real time, but it’s probably safe to say it’s at least a 50% reduction in the number of admissions.”
As a stroke referral center, his institution is seeing fewer local cases and referrals from outside hospitals. “I think both of those sources for admissions of stroke cases are down,” Tirschwell said.
Recognizing the seriousness of forgoing essential care for acute stroke, neurologists, institutions, and medical groups are taking to social media to potentially save lives.
“Across our @FLStrokeReg we are seeing less patients with #stroke symptoms coming to our hospitals. We need to get the word out that our teams are working hard to safely provide care when needed during #COVID19,” tweeted Ralph Sacco, MD, chief and professor of neurology, University of Miami Miller School of Medicine in South Florida.
Although Florida Stroke Registry data are not publicly available, anecdotal reports suggest that stroke admissions are down among many hospitals, Sacco told Medscape Medical News.
Furthermore, this is not a phenomenon only in the United States. “This has also been reported in other nations hit by COVID-19,” he said.
China is a prime example. There, many stroke centers have shown reduced functioning “because of fear of in-hospital cross infection and lack of experienced stroke care experts,” Jing Zhao, MD, PhD, and colleagues write in an editorial published online March 31 in Stroke.
Preliminary data show that “thrombectomies in Shanghai decreased by 50% in the first month after the Spring Festival compared with the same period in 2019,” write the editorialists, who are from Kings College London and the University of Pennsylvania in Philadelphia.
“Although the control of the COVID-19 is very important, at the same time, the management of stroke must not be neglected,” they add.
“Over 9000 new stroke cases occur each day in China alone. It cannot be right that treatment for one potentially curable disease is euthanized at the expense of another.”
Fear Factor?
The reasons individuals who may have experienced a stroke are avoiding emergency care is unclear at the moment. “I’m not really sure anyone really understands why, quite honestly,” Tirschwell said.
Until survey data or other data emerge, many experts are assuming that fear of COVID-19 is trumping other medical concerns, including emergency treatment of stroke.
“We believe this could represent patients being fearful to come to medical facilities with stroke-like symptoms, given the COVID-19 pandemic,” said Sacco, who is also incoming editor-in-chief of Stroke.
The BBC has been getting the word out in the United Kingdom via social media, with a tweet to “Dial 999 for stroke emergencies despite coronavirus.”
The World Stroke Campaign is also using Twitter to emphasize the need for urgent stroke care when appropriate:
“Don’t let concerns about COVID19 prevent you from seeking emergency treatment for stroke. If you spot the signs of stroke act FAST. Get emergency medical assistance,” the group urged in a tweet.
Don’t Hesitate
The American Heart Association (AHA) has addressed this troubling trend as well.
“People with serious symptoms shouldn’t ignore them,” Sarah Perlman, MD, associate professor of emergency medicine at the University of Colorado School of Medicine, Denver, states in an article on the AHA website.
Perlman added that some individuals who have signs of stroke and heart disease may hesitate to seek care because of fear that they are adding to an overburdened healthcare staff and system. However, she dismissed those concerns outright.
“If you’re experiencing warning signs of a heart attack or stroke, call 911,” she said. “Clearly, if there’s an emergency, we are available and capable and eager to take care of you.”
This article first appeared on Medscape.com.
(EDs).
Stroke specialists in New Orleans, Chicago, Seattle, and elsewhere told Medscape Medical News they are seeing a precipitous drop in the number of acute strokes at their institutions – and not just in the number of milder cases. Doctors on Twitter are sharing similar reports and are using social media to highlight this issue.
Gabriel Vidal, MD, a vascular and interventional neurologist at the Ochsner Medical Center, New Orleans, Louisiana, said there are “definitely” fewer patients with stroke and transient ischemic attack (TIA) seeking care at his facility and others throughout the New Orleans area, which has been hard hit by COVID-19.
“Even in Louisiana, we have a very large 53-hospital telestroke network, and the number of consults has diminished greatly,” Vidal added.
In Chicago, emergency medical service activations for patients with suspected strokes are down about 30%, Shyam Prabhakaran, MD, professor and chair of neurology at the University of Chicago Biological Sciences, Illinois, told Medscape Medical News.
“It appears to be that mild stroke and TIA patients may be more likely to stay at home and seek alternative care rather than come to the ED,” Prabhakaran said. However, “the severe strokes may be less affected and continue to come to emergency departments.”
“Getting the Word Out”
That may not be the whole story in Seattle, Washington, where a stroke specialist at Harborview Medical Center reported a drop in patients across the stroke-severity spectrum.
Some patients with milder strokes no longer come to Harborview for a comprehensive evaluation and workup, but that is only “a partial explanation,” said David Tirschwell, MD, medical director of comprehensive stroke care at the University of Washington (UW) Medicine Stroke Center at Harborview and a professor of neurology at UW.
“The thrombectomies are down also,” he added. “It’s hard to have great numbers in real time, but it’s probably safe to say it’s at least a 50% reduction in the number of admissions.”
As a stroke referral center, his institution is seeing fewer local cases and referrals from outside hospitals. “I think both of those sources for admissions of stroke cases are down,” Tirschwell said.
Recognizing the seriousness of forgoing essential care for acute stroke, neurologists, institutions, and medical groups are taking to social media to potentially save lives.
“Across our @FLStrokeReg we are seeing less patients with #stroke symptoms coming to our hospitals. We need to get the word out that our teams are working hard to safely provide care when needed during #COVID19,” tweeted Ralph Sacco, MD, chief and professor of neurology, University of Miami Miller School of Medicine in South Florida.
Although Florida Stroke Registry data are not publicly available, anecdotal reports suggest that stroke admissions are down among many hospitals, Sacco told Medscape Medical News.
Furthermore, this is not a phenomenon only in the United States. “This has also been reported in other nations hit by COVID-19,” he said.
China is a prime example. There, many stroke centers have shown reduced functioning “because of fear of in-hospital cross infection and lack of experienced stroke care experts,” Jing Zhao, MD, PhD, and colleagues write in an editorial published online March 31 in Stroke.
Preliminary data show that “thrombectomies in Shanghai decreased by 50% in the first month after the Spring Festival compared with the same period in 2019,” write the editorialists, who are from Kings College London and the University of Pennsylvania in Philadelphia.
“Although the control of the COVID-19 is very important, at the same time, the management of stroke must not be neglected,” they add.
“Over 9000 new stroke cases occur each day in China alone. It cannot be right that treatment for one potentially curable disease is euthanized at the expense of another.”
Fear Factor?
The reasons individuals who may have experienced a stroke are avoiding emergency care is unclear at the moment. “I’m not really sure anyone really understands why, quite honestly,” Tirschwell said.
Until survey data or other data emerge, many experts are assuming that fear of COVID-19 is trumping other medical concerns, including emergency treatment of stroke.
“We believe this could represent patients being fearful to come to medical facilities with stroke-like symptoms, given the COVID-19 pandemic,” said Sacco, who is also incoming editor-in-chief of Stroke.
The BBC has been getting the word out in the United Kingdom via social media, with a tweet to “Dial 999 for stroke emergencies despite coronavirus.”
The World Stroke Campaign is also using Twitter to emphasize the need for urgent stroke care when appropriate:
“Don’t let concerns about COVID19 prevent you from seeking emergency treatment for stroke. If you spot the signs of stroke act FAST. Get emergency medical assistance,” the group urged in a tweet.
Don’t Hesitate
The American Heart Association (AHA) has addressed this troubling trend as well.
“People with serious symptoms shouldn’t ignore them,” Sarah Perlman, MD, associate professor of emergency medicine at the University of Colorado School of Medicine, Denver, states in an article on the AHA website.
Perlman added that some individuals who have signs of stroke and heart disease may hesitate to seek care because of fear that they are adding to an overburdened healthcare staff and system. However, she dismissed those concerns outright.
“If you’re experiencing warning signs of a heart attack or stroke, call 911,” she said. “Clearly, if there’s an emergency, we are available and capable and eager to take care of you.”
This article first appeared on Medscape.com.
COVID-19: Dramatic changes to telepsychiatry rules and regs
In the wake of the coronavirus pandemic,
Under the 1135 emergency waiver, Medicare has expanded telehealth services to include patients across the country – not just in rural areas or under other limited conditions, as was previously the case. In addition, there’s now a waiver to the Ryan Haight Act that allows the prescribing of controlled substances via telemedicine.
Peter Yellowlees, MD, from University of California, Davis, reported that outpatient service at his center was converted to an almost 100% telepsychiatry service from mid- to late March.
He and John Torous, MD, director of digital psychiatry at Beth Israel Deaconess Medical Center, Boston, led a free webinar late last month sponsored by the Substance Abuse and Mental Health Services Administration (SAMHSA).
During the hour-long event, they answered questions and offered tips on changes in licensure, patient safety, new prescribing rules, and equipment needed.
“Clinicians need to be aware of these changes so they can ensure they are reaching as many people as possible and taking advantage of the reduced barriers to offering safe and effective video visits,” Dr. Torous said in an interview.
‘This is huge’
The new 1135 waiver “basically says CMS will pay for any patient on Medicare who is seen by video by any provider who is correctly licensed in any state in this country,” Dr. Yellowlees told webinar attendees.
“You don’t need to be licensed in the state where the patient is if the patient is on Medicare. This opens up a huge number of patients we can now see on video,” he said. “And you can bill at normal Medicare rates for whatever you normally get for your in-person patients.”
Although this temporary rule only applies to Medicare and not to private insurers, or to patients on Medicaid, “these are really big changes. This is huge,” Dr. Torous said.
Previously, the “originating site” rule stated that, for the most part, clinicians had to be licensed in the state where the patient was located and not where the physician was stationed.
Asked about college students receiving mental health care who were in school in the psychiatrist’s area but are now back home in a state where the clinician doesn’t have a license, Dr. Yellowlees said that scenario could be a bit “tricky.”
“Most of those patients probably aren’t on Medicare. Legally, you [usually] can’t see them on video if they have private insurance or Medicaid. So, hopefully you can give them a 3-month supply of medication and then recommend they see a local provider,” he said.
Still, all states have their own rules, Dr. Yellowlees said. He and Dr. Torous noted that the Federation of State Medical Boards has a “very up-to-date” listing of policies at FSMB.org, all of which are organized by state. In addition, the American Psychiatric Association provides a telepsychiatry toolkit on its website.
Ryan Haight Act and prescribing
Physicians are now permitted to prescribe medication to patients assessed via telemedicine.
For those with substance use disorders, the U.S. Drug Enforcement Administration has announced a new waiver for the Ryan Haight Online Pharmacy Consumer Protection Act.
The waiver states that “practitioners in all areas of the United States may issue prescriptions for all schedule II-V controlled substances” – as long as it’s for a legitimate medical purpose; real-time, two-way interactive communication with patients has been used; and the clinician “is acting in accordance with applicable Federal and State laws.”
“It’s now possible to prescribe all the normal psychiatric drugs but also benzodiazepines, stimulants, and potentially narcotics over telepsychiatry,” even at a first visit via video, Dr. Yellowlees said.
However, he noted at this point the waiver is current for only 60 days. “This isn’t a permanent condition. It could be extended or even shortened at any given time.”
In addition, SAMHSA has relaxed some of its own regulations regarding telehealth and opioid treatment programs. An FAQ section on the organization’s website provides guidance for providing methadone and buprenorphine treatment.
“Some of the previous regulations will probably be put back in place later on, but the new changes are helpful now,” Dr. Yellowlees said.
Simple equipment needed
Regarding equipment, Dr. Yellowlees noted that the most important component is just a laptop, tablet, or smartphone – for the clinician and for the patient.
“You don’t need fancy new technology with a separate camera or microphone,” he said. However, it might be worth investing in a little better system down the line, he added.
Simple platforms that can be used to meet virtually with patients include FaceTime, Google Hangouts, and Skype.
Although some of these (such as FaceTime) are not HIPAA compliant, “that’s okay for now” under the new rules, Dr. Yellowlees said. While the health system/commercial version of Skype is compliant, the normal consumer-downloaded version is not, he noted.
“I would still strongly suggest using HIPAA-compliant video-conferencing programs in the long run,” he added.
Either way, it’s important for various safety practices to be put into place. For example, clinicians should be careful because the consumer version of Skype can show names of patients who were previously spoken with.
A business associate agreement (BAA) is something that HIPAA-compliant video systems will offer and which should be signed. It’s an agreement that “you’ll be, essentially, looking through a tunnel at the persona at the other end, and the company cannot get inside the tunnel and watch you while you’re having your interview,” said Dr. Yellowlees.
“There are multiple videoconferencing systems around that you can use,” he added. “The three major ones are from Zoom, Vidyo, and VSee, but there are probably 40 or 50 more.”
“There are a lot out there, and we’re certainly not endorsing any one of them,” Dr. Torous added.
When evaluating potential programs, Dr. Yellowlees suggested looking at Yelp-style reviews or telemedicine review sites, or talk with colleagues.
“Basically, you want systems that offer high-definition video quality and the ability to ‘lock’ and ‘unlock’ the rooms. And you want it to have an app so mobile devices can use it,” he said.
Phone vs. video
Some patients, especially older ones, may be resistant to the idea of video chats, preferring to talk via telephone instead.
“If you can use video, it’s better to do that if you can, especially when setting up the systems are relatively simple,” Dr. Yellowlees said, adding that it might just be an issue of patients needing help to get started.
However, “for some people, this is a barrier that we have to respect,” Dr. Torous said.
Either way, clinicians should check the American Medical Association’s website for information about coding for both video and phone visits.
Asked whether a clinician needs written consent from patients for conducting telepsychiatry visits, Dr. Yellowlees said it’s important to check state-by-state rules. For example, California allows a verbal consent.
In many cases, “simply jot down a note that consent was given and how” and write down the address where the patient is located at time of visit, such as for their home, he said.
If a patient wants to conduct a telehealth session while in their car, Dr. Yellowlees suggested getting the address of the parking lot. For safety, clinicians also are advised asking for the cell phone number of the patient as well as that of a loved one.
Vital signs
When it comes to checking vital signs, Dr. Yellowlees suggested asking patients to purchase an inexpensive blood pressure (BP) monitor, thermometer, etc, prior to an appointment.
“Ask them to do a BP test on video and show you the readings. For the AIMS [Abnormal Involuntary Movement Scale] test, or to check for tardive dyskinesia, instruct patients to come close to the camera to show movement.”
In addition, most psychiatric rating scales are available online, which patients can fill out before a telehealth visit. The Serious Mental Illness (SMI) Adviser mobile app also includes several of these scales, Dr. Torous noted.
Overall, “there have been dramatic changes in the rules and regulations governing [telepsychiatry] that, for the next 60 days, make it easier to offer telehealth to patients,” Dr. Torous said.
Therefore, all psychiatrists need to “get on board,” as soon as possible, Dr. Yellowlees added.
The webinar was funded in part by a grant from SAMHSA.
A version of this article originally appeared on Medscape.com.
In the wake of the coronavirus pandemic,
Under the 1135 emergency waiver, Medicare has expanded telehealth services to include patients across the country – not just in rural areas or under other limited conditions, as was previously the case. In addition, there’s now a waiver to the Ryan Haight Act that allows the prescribing of controlled substances via telemedicine.
Peter Yellowlees, MD, from University of California, Davis, reported that outpatient service at his center was converted to an almost 100% telepsychiatry service from mid- to late March.
He and John Torous, MD, director of digital psychiatry at Beth Israel Deaconess Medical Center, Boston, led a free webinar late last month sponsored by the Substance Abuse and Mental Health Services Administration (SAMHSA).
During the hour-long event, they answered questions and offered tips on changes in licensure, patient safety, new prescribing rules, and equipment needed.
“Clinicians need to be aware of these changes so they can ensure they are reaching as many people as possible and taking advantage of the reduced barriers to offering safe and effective video visits,” Dr. Torous said in an interview.
‘This is huge’
The new 1135 waiver “basically says CMS will pay for any patient on Medicare who is seen by video by any provider who is correctly licensed in any state in this country,” Dr. Yellowlees told webinar attendees.
“You don’t need to be licensed in the state where the patient is if the patient is on Medicare. This opens up a huge number of patients we can now see on video,” he said. “And you can bill at normal Medicare rates for whatever you normally get for your in-person patients.”
Although this temporary rule only applies to Medicare and not to private insurers, or to patients on Medicaid, “these are really big changes. This is huge,” Dr. Torous said.
Previously, the “originating site” rule stated that, for the most part, clinicians had to be licensed in the state where the patient was located and not where the physician was stationed.
Asked about college students receiving mental health care who were in school in the psychiatrist’s area but are now back home in a state where the clinician doesn’t have a license, Dr. Yellowlees said that scenario could be a bit “tricky.”
“Most of those patients probably aren’t on Medicare. Legally, you [usually] can’t see them on video if they have private insurance or Medicaid. So, hopefully you can give them a 3-month supply of medication and then recommend they see a local provider,” he said.
Still, all states have their own rules, Dr. Yellowlees said. He and Dr. Torous noted that the Federation of State Medical Boards has a “very up-to-date” listing of policies at FSMB.org, all of which are organized by state. In addition, the American Psychiatric Association provides a telepsychiatry toolkit on its website.
Ryan Haight Act and prescribing
Physicians are now permitted to prescribe medication to patients assessed via telemedicine.
For those with substance use disorders, the U.S. Drug Enforcement Administration has announced a new waiver for the Ryan Haight Online Pharmacy Consumer Protection Act.
The waiver states that “practitioners in all areas of the United States may issue prescriptions for all schedule II-V controlled substances” – as long as it’s for a legitimate medical purpose; real-time, two-way interactive communication with patients has been used; and the clinician “is acting in accordance with applicable Federal and State laws.”
“It’s now possible to prescribe all the normal psychiatric drugs but also benzodiazepines, stimulants, and potentially narcotics over telepsychiatry,” even at a first visit via video, Dr. Yellowlees said.
However, he noted at this point the waiver is current for only 60 days. “This isn’t a permanent condition. It could be extended or even shortened at any given time.”
In addition, SAMHSA has relaxed some of its own regulations regarding telehealth and opioid treatment programs. An FAQ section on the organization’s website provides guidance for providing methadone and buprenorphine treatment.
“Some of the previous regulations will probably be put back in place later on, but the new changes are helpful now,” Dr. Yellowlees said.
Simple equipment needed
Regarding equipment, Dr. Yellowlees noted that the most important component is just a laptop, tablet, or smartphone – for the clinician and for the patient.
“You don’t need fancy new technology with a separate camera or microphone,” he said. However, it might be worth investing in a little better system down the line, he added.
Simple platforms that can be used to meet virtually with patients include FaceTime, Google Hangouts, and Skype.
Although some of these (such as FaceTime) are not HIPAA compliant, “that’s okay for now” under the new rules, Dr. Yellowlees said. While the health system/commercial version of Skype is compliant, the normal consumer-downloaded version is not, he noted.
“I would still strongly suggest using HIPAA-compliant video-conferencing programs in the long run,” he added.
Either way, it’s important for various safety practices to be put into place. For example, clinicians should be careful because the consumer version of Skype can show names of patients who were previously spoken with.
A business associate agreement (BAA) is something that HIPAA-compliant video systems will offer and which should be signed. It’s an agreement that “you’ll be, essentially, looking through a tunnel at the persona at the other end, and the company cannot get inside the tunnel and watch you while you’re having your interview,” said Dr. Yellowlees.
“There are multiple videoconferencing systems around that you can use,” he added. “The three major ones are from Zoom, Vidyo, and VSee, but there are probably 40 or 50 more.”
“There are a lot out there, and we’re certainly not endorsing any one of them,” Dr. Torous added.
When evaluating potential programs, Dr. Yellowlees suggested looking at Yelp-style reviews or telemedicine review sites, or talk with colleagues.
“Basically, you want systems that offer high-definition video quality and the ability to ‘lock’ and ‘unlock’ the rooms. And you want it to have an app so mobile devices can use it,” he said.
Phone vs. video
Some patients, especially older ones, may be resistant to the idea of video chats, preferring to talk via telephone instead.
“If you can use video, it’s better to do that if you can, especially when setting up the systems are relatively simple,” Dr. Yellowlees said, adding that it might just be an issue of patients needing help to get started.
However, “for some people, this is a barrier that we have to respect,” Dr. Torous said.
Either way, clinicians should check the American Medical Association’s website for information about coding for both video and phone visits.
Asked whether a clinician needs written consent from patients for conducting telepsychiatry visits, Dr. Yellowlees said it’s important to check state-by-state rules. For example, California allows a verbal consent.
In many cases, “simply jot down a note that consent was given and how” and write down the address where the patient is located at time of visit, such as for their home, he said.
If a patient wants to conduct a telehealth session while in their car, Dr. Yellowlees suggested getting the address of the parking lot. For safety, clinicians also are advised asking for the cell phone number of the patient as well as that of a loved one.
Vital signs
When it comes to checking vital signs, Dr. Yellowlees suggested asking patients to purchase an inexpensive blood pressure (BP) monitor, thermometer, etc, prior to an appointment.
“Ask them to do a BP test on video and show you the readings. For the AIMS [Abnormal Involuntary Movement Scale] test, or to check for tardive dyskinesia, instruct patients to come close to the camera to show movement.”
In addition, most psychiatric rating scales are available online, which patients can fill out before a telehealth visit. The Serious Mental Illness (SMI) Adviser mobile app also includes several of these scales, Dr. Torous noted.
Overall, “there have been dramatic changes in the rules and regulations governing [telepsychiatry] that, for the next 60 days, make it easier to offer telehealth to patients,” Dr. Torous said.
Therefore, all psychiatrists need to “get on board,” as soon as possible, Dr. Yellowlees added.
The webinar was funded in part by a grant from SAMHSA.
A version of this article originally appeared on Medscape.com.
In the wake of the coronavirus pandemic,
Under the 1135 emergency waiver, Medicare has expanded telehealth services to include patients across the country – not just in rural areas or under other limited conditions, as was previously the case. In addition, there’s now a waiver to the Ryan Haight Act that allows the prescribing of controlled substances via telemedicine.
Peter Yellowlees, MD, from University of California, Davis, reported that outpatient service at his center was converted to an almost 100% telepsychiatry service from mid- to late March.
He and John Torous, MD, director of digital psychiatry at Beth Israel Deaconess Medical Center, Boston, led a free webinar late last month sponsored by the Substance Abuse and Mental Health Services Administration (SAMHSA).
During the hour-long event, they answered questions and offered tips on changes in licensure, patient safety, new prescribing rules, and equipment needed.
“Clinicians need to be aware of these changes so they can ensure they are reaching as many people as possible and taking advantage of the reduced barriers to offering safe and effective video visits,” Dr. Torous said in an interview.
‘This is huge’
The new 1135 waiver “basically says CMS will pay for any patient on Medicare who is seen by video by any provider who is correctly licensed in any state in this country,” Dr. Yellowlees told webinar attendees.
“You don’t need to be licensed in the state where the patient is if the patient is on Medicare. This opens up a huge number of patients we can now see on video,” he said. “And you can bill at normal Medicare rates for whatever you normally get for your in-person patients.”
Although this temporary rule only applies to Medicare and not to private insurers, or to patients on Medicaid, “these are really big changes. This is huge,” Dr. Torous said.
Previously, the “originating site” rule stated that, for the most part, clinicians had to be licensed in the state where the patient was located and not where the physician was stationed.
Asked about college students receiving mental health care who were in school in the psychiatrist’s area but are now back home in a state where the clinician doesn’t have a license, Dr. Yellowlees said that scenario could be a bit “tricky.”
“Most of those patients probably aren’t on Medicare. Legally, you [usually] can’t see them on video if they have private insurance or Medicaid. So, hopefully you can give them a 3-month supply of medication and then recommend they see a local provider,” he said.
Still, all states have their own rules, Dr. Yellowlees said. He and Dr. Torous noted that the Federation of State Medical Boards has a “very up-to-date” listing of policies at FSMB.org, all of which are organized by state. In addition, the American Psychiatric Association provides a telepsychiatry toolkit on its website.
Ryan Haight Act and prescribing
Physicians are now permitted to prescribe medication to patients assessed via telemedicine.
For those with substance use disorders, the U.S. Drug Enforcement Administration has announced a new waiver for the Ryan Haight Online Pharmacy Consumer Protection Act.
The waiver states that “practitioners in all areas of the United States may issue prescriptions for all schedule II-V controlled substances” – as long as it’s for a legitimate medical purpose; real-time, two-way interactive communication with patients has been used; and the clinician “is acting in accordance with applicable Federal and State laws.”
“It’s now possible to prescribe all the normal psychiatric drugs but also benzodiazepines, stimulants, and potentially narcotics over telepsychiatry,” even at a first visit via video, Dr. Yellowlees said.
However, he noted at this point the waiver is current for only 60 days. “This isn’t a permanent condition. It could be extended or even shortened at any given time.”
In addition, SAMHSA has relaxed some of its own regulations regarding telehealth and opioid treatment programs. An FAQ section on the organization’s website provides guidance for providing methadone and buprenorphine treatment.
“Some of the previous regulations will probably be put back in place later on, but the new changes are helpful now,” Dr. Yellowlees said.
Simple equipment needed
Regarding equipment, Dr. Yellowlees noted that the most important component is just a laptop, tablet, or smartphone – for the clinician and for the patient.
“You don’t need fancy new technology with a separate camera or microphone,” he said. However, it might be worth investing in a little better system down the line, he added.
Simple platforms that can be used to meet virtually with patients include FaceTime, Google Hangouts, and Skype.
Although some of these (such as FaceTime) are not HIPAA compliant, “that’s okay for now” under the new rules, Dr. Yellowlees said. While the health system/commercial version of Skype is compliant, the normal consumer-downloaded version is not, he noted.
“I would still strongly suggest using HIPAA-compliant video-conferencing programs in the long run,” he added.
Either way, it’s important for various safety practices to be put into place. For example, clinicians should be careful because the consumer version of Skype can show names of patients who were previously spoken with.
A business associate agreement (BAA) is something that HIPAA-compliant video systems will offer and which should be signed. It’s an agreement that “you’ll be, essentially, looking through a tunnel at the persona at the other end, and the company cannot get inside the tunnel and watch you while you’re having your interview,” said Dr. Yellowlees.
“There are multiple videoconferencing systems around that you can use,” he added. “The three major ones are from Zoom, Vidyo, and VSee, but there are probably 40 or 50 more.”
“There are a lot out there, and we’re certainly not endorsing any one of them,” Dr. Torous added.
When evaluating potential programs, Dr. Yellowlees suggested looking at Yelp-style reviews or telemedicine review sites, or talk with colleagues.
“Basically, you want systems that offer high-definition video quality and the ability to ‘lock’ and ‘unlock’ the rooms. And you want it to have an app so mobile devices can use it,” he said.
Phone vs. video
Some patients, especially older ones, may be resistant to the idea of video chats, preferring to talk via telephone instead.
“If you can use video, it’s better to do that if you can, especially when setting up the systems are relatively simple,” Dr. Yellowlees said, adding that it might just be an issue of patients needing help to get started.
However, “for some people, this is a barrier that we have to respect,” Dr. Torous said.
Either way, clinicians should check the American Medical Association’s website for information about coding for both video and phone visits.
Asked whether a clinician needs written consent from patients for conducting telepsychiatry visits, Dr. Yellowlees said it’s important to check state-by-state rules. For example, California allows a verbal consent.
In many cases, “simply jot down a note that consent was given and how” and write down the address where the patient is located at time of visit, such as for their home, he said.
If a patient wants to conduct a telehealth session while in their car, Dr. Yellowlees suggested getting the address of the parking lot. For safety, clinicians also are advised asking for the cell phone number of the patient as well as that of a loved one.
Vital signs
When it comes to checking vital signs, Dr. Yellowlees suggested asking patients to purchase an inexpensive blood pressure (BP) monitor, thermometer, etc, prior to an appointment.
“Ask them to do a BP test on video and show you the readings. For the AIMS [Abnormal Involuntary Movement Scale] test, or to check for tardive dyskinesia, instruct patients to come close to the camera to show movement.”
In addition, most psychiatric rating scales are available online, which patients can fill out before a telehealth visit. The Serious Mental Illness (SMI) Adviser mobile app also includes several of these scales, Dr. Torous noted.
Overall, “there have been dramatic changes in the rules and regulations governing [telepsychiatry] that, for the next 60 days, make it easier to offer telehealth to patients,” Dr. Torous said.
Therefore, all psychiatrists need to “get on board,” as soon as possible, Dr. Yellowlees added.
The webinar was funded in part by a grant from SAMHSA.
A version of this article originally appeared on Medscape.com.
Frailty indexes fail in sorting elderly MM patients
Despite the perceived benefits of their use in guiding treatment, frailty indexes were not reliable in differentiating elderly multiple myeloma (MM) patients, according to an analysis of a prospective cohort of 40 patients studied at a single institution.
The researchers examined three different models of frailty using data available in the Cancer and Aging Research Group tool to define frailty in their cohort: the international myeloma working group (IMWG) frailty model, the revised myeloma comorbidity index (R-MCI), and the Carolina Frailty Index (CFI).
The researchers found that, for their same population, applying the IMWG frailty index yielded 3 (7.5%) patients categorized as fit, 15 (37.5%) categorized as intermediate fit, and 22 (55%) categorized as frail. The R-MCI yielded 4 (10%) patients categorized as fit, 29 (72.5%) as intermediate, and 7 (17.5%) as frail. When using the CFI, 17 (42.5%) patients were categorized as fit, 8 (20%) were intermediate, and 15 (37.5%) were frail. Of particular note, among 28 patients categorized as frail by at least one of the three indexes, only 3 (11%) patients were categorized as frail by all three models.
The reasons for the differences were discussed by the authors, who pointed out that patients categorized as frail by the IMWG or R-MCI tended to be older than those categorized as frail by CFI, reflecting the fact that the IMWG and R-MCI both include age as a component of frailty, while the CFI does not. In addition, each index incorporates comorbidities into its assessment of frailty in a different way.
For example, falls and depression are incorporated as components of the CFI, reflected in the higher proportion of patients reporting a prior fall and more symptoms of depression in the group categorized as frail by the CFI model than in the IMWG or R-MCI. In the CFI as well, each of the individual instrumental activities of daily living is a component of the model, rather than the summary score, as in the IMWG and R-MCI.
“Our findings highlight the differences in currently available approaches to applying the concept of frailty to older adults with cancer. This problem is not unique to oncology, as there is a continued lack of consensus on defining the concept of frailty in the general geriatric population,” the researchers stated. “Further studies are needed to establish the role of frailty indexes in predicting toxicity of therapy and other outcomes of importance in older adults with multiple myeloma,” they concluded.
The study was funded by the National Cancer Institute and other U.S. government agencies. The authors reported having no conflicts.
SOURCE: Isaacs A et al. J Geriat Onc. 2020;11(2):311-15.
Despite the perceived benefits of their use in guiding treatment, frailty indexes were not reliable in differentiating elderly multiple myeloma (MM) patients, according to an analysis of a prospective cohort of 40 patients studied at a single institution.
The researchers examined three different models of frailty using data available in the Cancer and Aging Research Group tool to define frailty in their cohort: the international myeloma working group (IMWG) frailty model, the revised myeloma comorbidity index (R-MCI), and the Carolina Frailty Index (CFI).
The researchers found that, for their same population, applying the IMWG frailty index yielded 3 (7.5%) patients categorized as fit, 15 (37.5%) categorized as intermediate fit, and 22 (55%) categorized as frail. The R-MCI yielded 4 (10%) patients categorized as fit, 29 (72.5%) as intermediate, and 7 (17.5%) as frail. When using the CFI, 17 (42.5%) patients were categorized as fit, 8 (20%) were intermediate, and 15 (37.5%) were frail. Of particular note, among 28 patients categorized as frail by at least one of the three indexes, only 3 (11%) patients were categorized as frail by all three models.
The reasons for the differences were discussed by the authors, who pointed out that patients categorized as frail by the IMWG or R-MCI tended to be older than those categorized as frail by CFI, reflecting the fact that the IMWG and R-MCI both include age as a component of frailty, while the CFI does not. In addition, each index incorporates comorbidities into its assessment of frailty in a different way.
For example, falls and depression are incorporated as components of the CFI, reflected in the higher proportion of patients reporting a prior fall and more symptoms of depression in the group categorized as frail by the CFI model than in the IMWG or R-MCI. In the CFI as well, each of the individual instrumental activities of daily living is a component of the model, rather than the summary score, as in the IMWG and R-MCI.
“Our findings highlight the differences in currently available approaches to applying the concept of frailty to older adults with cancer. This problem is not unique to oncology, as there is a continued lack of consensus on defining the concept of frailty in the general geriatric population,” the researchers stated. “Further studies are needed to establish the role of frailty indexes in predicting toxicity of therapy and other outcomes of importance in older adults with multiple myeloma,” they concluded.
The study was funded by the National Cancer Institute and other U.S. government agencies. The authors reported having no conflicts.
SOURCE: Isaacs A et al. J Geriat Onc. 2020;11(2):311-15.
Despite the perceived benefits of their use in guiding treatment, frailty indexes were not reliable in differentiating elderly multiple myeloma (MM) patients, according to an analysis of a prospective cohort of 40 patients studied at a single institution.
The researchers examined three different models of frailty using data available in the Cancer and Aging Research Group tool to define frailty in their cohort: the international myeloma working group (IMWG) frailty model, the revised myeloma comorbidity index (R-MCI), and the Carolina Frailty Index (CFI).
The researchers found that, for their same population, applying the IMWG frailty index yielded 3 (7.5%) patients categorized as fit, 15 (37.5%) categorized as intermediate fit, and 22 (55%) categorized as frail. The R-MCI yielded 4 (10%) patients categorized as fit, 29 (72.5%) as intermediate, and 7 (17.5%) as frail. When using the CFI, 17 (42.5%) patients were categorized as fit, 8 (20%) were intermediate, and 15 (37.5%) were frail. Of particular note, among 28 patients categorized as frail by at least one of the three indexes, only 3 (11%) patients were categorized as frail by all three models.
The reasons for the differences were discussed by the authors, who pointed out that patients categorized as frail by the IMWG or R-MCI tended to be older than those categorized as frail by CFI, reflecting the fact that the IMWG and R-MCI both include age as a component of frailty, while the CFI does not. In addition, each index incorporates comorbidities into its assessment of frailty in a different way.
For example, falls and depression are incorporated as components of the CFI, reflected in the higher proportion of patients reporting a prior fall and more symptoms of depression in the group categorized as frail by the CFI model than in the IMWG or R-MCI. In the CFI as well, each of the individual instrumental activities of daily living is a component of the model, rather than the summary score, as in the IMWG and R-MCI.
“Our findings highlight the differences in currently available approaches to applying the concept of frailty to older adults with cancer. This problem is not unique to oncology, as there is a continued lack of consensus on defining the concept of frailty in the general geriatric population,” the researchers stated. “Further studies are needed to establish the role of frailty indexes in predicting toxicity of therapy and other outcomes of importance in older adults with multiple myeloma,” they concluded.
The study was funded by the National Cancer Institute and other U.S. government agencies. The authors reported having no conflicts.
SOURCE: Isaacs A et al. J Geriat Onc. 2020;11(2):311-15.
FROM THE JOURNAL OF GERIATRIC ONCOLOGY
Key clinical point:
Major finding: Although 28 multiple myeloma patients were deemed frail by at least one model, only 3 patients were deemed frail by all three models.
Study details: A total of 40 adults aged 65 years and over with MM were assessed by three frailty indexes.
Disclosures: The study was funded by the National Cancer Institute and other U.S. government agencies. The authors reported having no conflicts.
Source: Isaacs A et al. J Geriat Onc. 2020;11(2):311-5.
FDA approves first generic albuterol inhaler
The Food and Drug Administration has approved the first generic of Proventil HFA (albuterol sulfate) metered-dose inhaler, 90 mcg/inhalation, according to a release from the agency. This inhaler is indicated for prevention of bronchospasm in patients aged 4 years and older. Specifically, these are patients with reversible obstructive airway disease or exercise-induced bronchospasm.
“The FDA recognizes the increased demand for albuterol products during the novel coronavirus pandemic,” said FDA Commissioner Stephen M. Hahn, MD.
The most common side effects include upper respiratory tract infection, rhinitis, nausea, vomiting, rapid heart rate, tremor, and nervousness.
This approval comes as part of FDA’s efforts to guide industry through the development process of generic products, according to the release. Complex combination products – such as this inhaler, which comprises both medication and a delivery system – can be more challenging to develop than solid oral dosage forms, such as tablets.
The FDA released a draft guidance in March 2020 specific to proposed generic albuterol sulfate metered-dose inhalers, including drug products referencing Proventil HFA. As with other similar guidances, it details the steps companies need to take in developing generics in order to submit complete applications for those products. The full news release regarding this approval is available on the FDA website.
This article was updated 4/8/20.
The Food and Drug Administration has approved the first generic of Proventil HFA (albuterol sulfate) metered-dose inhaler, 90 mcg/inhalation, according to a release from the agency. This inhaler is indicated for prevention of bronchospasm in patients aged 4 years and older. Specifically, these are patients with reversible obstructive airway disease or exercise-induced bronchospasm.
“The FDA recognizes the increased demand for albuterol products during the novel coronavirus pandemic,” said FDA Commissioner Stephen M. Hahn, MD.
The most common side effects include upper respiratory tract infection, rhinitis, nausea, vomiting, rapid heart rate, tremor, and nervousness.
This approval comes as part of FDA’s efforts to guide industry through the development process of generic products, according to the release. Complex combination products – such as this inhaler, which comprises both medication and a delivery system – can be more challenging to develop than solid oral dosage forms, such as tablets.
The FDA released a draft guidance in March 2020 specific to proposed generic albuterol sulfate metered-dose inhalers, including drug products referencing Proventil HFA. As with other similar guidances, it details the steps companies need to take in developing generics in order to submit complete applications for those products. The full news release regarding this approval is available on the FDA website.
This article was updated 4/8/20.
The Food and Drug Administration has approved the first generic of Proventil HFA (albuterol sulfate) metered-dose inhaler, 90 mcg/inhalation, according to a release from the agency. This inhaler is indicated for prevention of bronchospasm in patients aged 4 years and older. Specifically, these are patients with reversible obstructive airway disease or exercise-induced bronchospasm.
“The FDA recognizes the increased demand for albuterol products during the novel coronavirus pandemic,” said FDA Commissioner Stephen M. Hahn, MD.
The most common side effects include upper respiratory tract infection, rhinitis, nausea, vomiting, rapid heart rate, tremor, and nervousness.
This approval comes as part of FDA’s efforts to guide industry through the development process of generic products, according to the release. Complex combination products – such as this inhaler, which comprises both medication and a delivery system – can be more challenging to develop than solid oral dosage forms, such as tablets.
The FDA released a draft guidance in March 2020 specific to proposed generic albuterol sulfate metered-dose inhalers, including drug products referencing Proventil HFA. As with other similar guidances, it details the steps companies need to take in developing generics in order to submit complete applications for those products. The full news release regarding this approval is available on the FDA website.
This article was updated 4/8/20.
ACOG offers guidance on optimizing patient care in the midst of COVID-19
The American College of Obstetricians and Gynecologists (ACOG) posted a useful resource on its website on March 30 for clinicians practicing ambulatory gynecology. The guidance, “COVID-19 FAQs for Obstetrician–Gynecologists, Gynecology” (https://www.acog.org/), is based on expert opinion and is intended to supplement guidance from the Centers for Disease Control and Prevention as well as previously issued ACOG guidance.1
Which patients need to be seen, and when
The ACOG guidance provides examples of patients needing in-person appointments, video or telephone visits, or for whom deferral of a visit until after the COVID-19 outbreak would be appropriate. Highlights include:
In-person appointments
- suspected ectopic pregnancy
- profuse vaginal bleeding
Video or telephone visits
- contraceptive counseling and prescribing
- management of menopausal symptoms
Deferral of a visit until after the COVID-19 outbreak
- routine well-woman visits for average-risk patients.
Cervical screening
With respect to patients with abnormal cervical cancer screening results, ACOG recommends the ASCCP’s guidance that2:
- for patients with low-grade test results, colposcopy/cervical biopsies be deferred up to 6 to 12 months
- for patients with high-grade results, colposcopy/cervical biopsies be performed within 3 months.
Contraception
Regarding contraceptive services, the ACOG guidance suggests that placement of intrauterine devices (IUDs) and contraceptive implants should continue “where possible.” If initiation of long-acting reversible contraception (LARC) is not feasible, the guidance recommends that use of self-administered contraceptives (including subcutaneous injections, oral, transdermal patch, and vaginal ring contraception) be encouraged as a bridge to later initiation of LARC.
The guidance suggests that removal of IUDs and implants be postponed when possible.
Finally, the guidance suggests that patients with an existing IUD or implant who seek removal and replacement of their contraceptives be counseled regarding extended use of these devices.
Individualize your approach
ACOG emphasizes that no single solution applies to all situations and that each practice or clinic should evaluate the individual situation, including the availability of local and regional resources, staffing, and personal protective equipment; the prevalence of COVID-19 in the region; and the type of practice.
A roadmap for care
This guidance from ACOG should help clinicians caring for women during the COVID-19 outbreak to counsel and guide patients in a prudent manner.
- American College of Obstetricians and Gynecologists website. COVID-19 FAQs for obstetrician-gynecologists, gynecology. https://www.acog.org/clinical-information/physician-faqs/covid19-faqs-for-ob-gyns-gynecology. Accessed April 3, 2020.
- ASCCP website. ASCCP interim guidance for timing of diagnostic and treatment procedures for patients with abnormal cervical screening tests. https://www.asccp.org/covid-19. Accessed April 3, 2020.
The American College of Obstetricians and Gynecologists (ACOG) posted a useful resource on its website on March 30 for clinicians practicing ambulatory gynecology. The guidance, “COVID-19 FAQs for Obstetrician–Gynecologists, Gynecology” (https://www.acog.org/), is based on expert opinion and is intended to supplement guidance from the Centers for Disease Control and Prevention as well as previously issued ACOG guidance.1
Which patients need to be seen, and when
The ACOG guidance provides examples of patients needing in-person appointments, video or telephone visits, or for whom deferral of a visit until after the COVID-19 outbreak would be appropriate. Highlights include:
In-person appointments
- suspected ectopic pregnancy
- profuse vaginal bleeding
Video or telephone visits
- contraceptive counseling and prescribing
- management of menopausal symptoms
Deferral of a visit until after the COVID-19 outbreak
- routine well-woman visits for average-risk patients.
Cervical screening
With respect to patients with abnormal cervical cancer screening results, ACOG recommends the ASCCP’s guidance that2:
- for patients with low-grade test results, colposcopy/cervical biopsies be deferred up to 6 to 12 months
- for patients with high-grade results, colposcopy/cervical biopsies be performed within 3 months.
Contraception
Regarding contraceptive services, the ACOG guidance suggests that placement of intrauterine devices (IUDs) and contraceptive implants should continue “where possible.” If initiation of long-acting reversible contraception (LARC) is not feasible, the guidance recommends that use of self-administered contraceptives (including subcutaneous injections, oral, transdermal patch, and vaginal ring contraception) be encouraged as a bridge to later initiation of LARC.
The guidance suggests that removal of IUDs and implants be postponed when possible.
Finally, the guidance suggests that patients with an existing IUD or implant who seek removal and replacement of their contraceptives be counseled regarding extended use of these devices.
Individualize your approach
ACOG emphasizes that no single solution applies to all situations and that each practice or clinic should evaluate the individual situation, including the availability of local and regional resources, staffing, and personal protective equipment; the prevalence of COVID-19 in the region; and the type of practice.
A roadmap for care
This guidance from ACOG should help clinicians caring for women during the COVID-19 outbreak to counsel and guide patients in a prudent manner.
The American College of Obstetricians and Gynecologists (ACOG) posted a useful resource on its website on March 30 for clinicians practicing ambulatory gynecology. The guidance, “COVID-19 FAQs for Obstetrician–Gynecologists, Gynecology” (https://www.acog.org/), is based on expert opinion and is intended to supplement guidance from the Centers for Disease Control and Prevention as well as previously issued ACOG guidance.1
Which patients need to be seen, and when
The ACOG guidance provides examples of patients needing in-person appointments, video or telephone visits, or for whom deferral of a visit until after the COVID-19 outbreak would be appropriate. Highlights include:
In-person appointments
- suspected ectopic pregnancy
- profuse vaginal bleeding
Video or telephone visits
- contraceptive counseling and prescribing
- management of menopausal symptoms
Deferral of a visit until after the COVID-19 outbreak
- routine well-woman visits for average-risk patients.
Cervical screening
With respect to patients with abnormal cervical cancer screening results, ACOG recommends the ASCCP’s guidance that2:
- for patients with low-grade test results, colposcopy/cervical biopsies be deferred up to 6 to 12 months
- for patients with high-grade results, colposcopy/cervical biopsies be performed within 3 months.
Contraception
Regarding contraceptive services, the ACOG guidance suggests that placement of intrauterine devices (IUDs) and contraceptive implants should continue “where possible.” If initiation of long-acting reversible contraception (LARC) is not feasible, the guidance recommends that use of self-administered contraceptives (including subcutaneous injections, oral, transdermal patch, and vaginal ring contraception) be encouraged as a bridge to later initiation of LARC.
The guidance suggests that removal of IUDs and implants be postponed when possible.
Finally, the guidance suggests that patients with an existing IUD or implant who seek removal and replacement of their contraceptives be counseled regarding extended use of these devices.
Individualize your approach
ACOG emphasizes that no single solution applies to all situations and that each practice or clinic should evaluate the individual situation, including the availability of local and regional resources, staffing, and personal protective equipment; the prevalence of COVID-19 in the region; and the type of practice.
A roadmap for care
This guidance from ACOG should help clinicians caring for women during the COVID-19 outbreak to counsel and guide patients in a prudent manner.
- American College of Obstetricians and Gynecologists website. COVID-19 FAQs for obstetrician-gynecologists, gynecology. https://www.acog.org/clinical-information/physician-faqs/covid19-faqs-for-ob-gyns-gynecology. Accessed April 3, 2020.
- ASCCP website. ASCCP interim guidance for timing of diagnostic and treatment procedures for patients with abnormal cervical screening tests. https://www.asccp.org/covid-19. Accessed April 3, 2020.
- American College of Obstetricians and Gynecologists website. COVID-19 FAQs for obstetrician-gynecologists, gynecology. https://www.acog.org/clinical-information/physician-faqs/covid19-faqs-for-ob-gyns-gynecology. Accessed April 3, 2020.
- ASCCP website. ASCCP interim guidance for timing of diagnostic and treatment procedures for patients with abnormal cervical screening tests. https://www.asccp.org/covid-19. Accessed April 3, 2020.
Dupilumab hits the mark for severe AD in younger children
The monoclonal antibody , according to new clinical trial results.
In a cohort of children with severe AD, 33% achieved clear or nearly clear skin after 16 weeks of treatment with every 4-week dosing of the injectable medication, while 30% also achieved that mark when receiving a weight-based dose every 2 weeks. Both groups had results that were significantly better than those receiving placebo, with 11% of these children had clear or nearly clear skin by 16 weeks of dupilumab (Dupixent) therapy (P less than .0001 for both therapy arms versus placebo).
“Dupilumab with a topical corticosteroid showed clinically meaningful and statistically significant improvement in the atopic dermatitis signs and symptoms in children aged 6 to less than 12 years of age with severe atopic dermatitis,” said Amy Paller, MD, the Walter J. Hamlin professor and chair of the department of dermatology at Northwestern University, Chicago, presenting the results at the Revolutionizing Atopic Dermatitis virtual symposium. Portions of the conference, which has been rescheduled to December 2020, in Chicago, were presented virtually because of the COVID-19 pandemic.
The phase 3 trial of subcutaneously injected dupilumab for atopic dermatitis, dubbed LIBERTY AD PEDS, included children aged 6-11 years with severe AD. The study’s primary endpoint was the proportion of patients achieving a score of 0 or 1 (clear or almost clear skin) on the Investigator’s Global Assessment (IGA) scale by study week 16.
For the purposes of reporting results to the European Medicines Agency, the investigators added a coprimary endpoint of patients reaching 75% clearing on the Eczema Area and Severity Index (EASI-75) by week 16.
The randomized, double-blind, placebo-controlled trial enrolled 367 children with IGA scores of 4, denoting severe AD. The EASI score had to be at least 21 and patients had to endorse peak pruritus of at least 4 on a 0-10 numeric rating scale; body surface involvement had to be at least 15%. Patients went through a washout period of any systemic therapies before beginning the trial, which randomized patients 1:1:1 to receive placebo, dupilumab 300 mg every 4 weeks, or dupilumab every 2 weeks with weight-dependent dosing. All participants were also permitted topical corticosteroids.
Patients were an average of aged 8 years, about half were female, and about two-thirds were white. Most participants had developed AD within their first year of life. Patients were about evenly divided between weighing over and under 30 kg, which was the cutoff for 100 mcg versus 200 mcg dupilumab for the every-2-week dosing group.
Over 90% of patients had other atopic comorbidities, and the mean EASI score was about 38 with average weekly peak pruritus averaging 7.8 on the numeric rating scale.
“When we’re talking about how severe this population is, it’s interesting to note that about 30 to 35% were all that had been previously treated with either systemic steroids or some systemic nonsteroidal immunosuppressants,” Dr. Paller pointed out. “I think that reflects the fact that so many of these very severely affected children are not put on a systemic therapy, but are still staying on topical therapies to try to control their disease.”
Looking at the proportion of patients reaching EASI-75, both dosing strategies for dupilumab out-performed placebo, with 70% of the every 4-week group and 67% of the every 2-week group reaching EASI-75 at 16 weeks, compared with 27% of those on placebo (P less than .0001 for both active arms). “These differences were seen very early on; by 2 weeks already, we can see that we’re starting to see a difference in both of these arms,” noted Dr. Paller, adding that the difference was statistically significant by 4 weeks into the study.
The overall group of dupilumab participants saw their EASI scores drop by about 80%, while those taking placebo saw a 49% drop in EASI scores.
For the group of participants weighing less than 30 kg, the every 4-week strategy resulted in better clearing as measured by both IGA and EASI-75. This effect wasn’t seen for heavier patients. Trough dupilumab concentrations at 16 weeks were higher for lighter patients with every 4-week dosing and for heavier patients with the biweekly strategy, noted Dr. Paller.
In terms of itch, 60% to 68% of participants receiving dupilumab had a drop of at least 3 points in peak pruritus on the numeric rating scale, compared with 21% of those receiving placebo (P less than .001), while about half of the dupilumab groups and 12% of the placebo group saw pruritus improvements of 4 points or more (P less than .001). Pruritus improved early in the active arms of the study, becoming statistically significant at the 2 to 4 week range.
Treatment-emergent adverse events were numerically higher in patients in the placebo group, including infections and adjudicated skin infections. Conjunctivitis occurred more frequently in the dupilumab group, as did injection-site reactions.
“Overall, dupilumab was well tolerated, and data were consistent with the known dupilumab safety profile observed in adults and adolescents,” Dr. Paller said.
Dupilumab has been approved by the Food and Drug Administration to treat moderate to severe AD in those aged 12 years and older whose disease can’t be adequately controlled with topical prescription medications, or when those treatments are not advisable.
The fully human monoclonal antibody blocks a shared receptor component for interleukin-4 and interleukin-13, which contribute to inflammation in AD, as well as asthma, chronic rhinosinusitis with nasal polyps, and eosinophilic esophagitis.
Dr. Paller reported receiving support from multiple pharmaceutical companies including Sanofi and Regeneron Pharmaceuticals, which sponsored the study.
The monoclonal antibody , according to new clinical trial results.
In a cohort of children with severe AD, 33% achieved clear or nearly clear skin after 16 weeks of treatment with every 4-week dosing of the injectable medication, while 30% also achieved that mark when receiving a weight-based dose every 2 weeks. Both groups had results that were significantly better than those receiving placebo, with 11% of these children had clear or nearly clear skin by 16 weeks of dupilumab (Dupixent) therapy (P less than .0001 for both therapy arms versus placebo).
“Dupilumab with a topical corticosteroid showed clinically meaningful and statistically significant improvement in the atopic dermatitis signs and symptoms in children aged 6 to less than 12 years of age with severe atopic dermatitis,” said Amy Paller, MD, the Walter J. Hamlin professor and chair of the department of dermatology at Northwestern University, Chicago, presenting the results at the Revolutionizing Atopic Dermatitis virtual symposium. Portions of the conference, which has been rescheduled to December 2020, in Chicago, were presented virtually because of the COVID-19 pandemic.
The phase 3 trial of subcutaneously injected dupilumab for atopic dermatitis, dubbed LIBERTY AD PEDS, included children aged 6-11 years with severe AD. The study’s primary endpoint was the proportion of patients achieving a score of 0 or 1 (clear or almost clear skin) on the Investigator’s Global Assessment (IGA) scale by study week 16.
For the purposes of reporting results to the European Medicines Agency, the investigators added a coprimary endpoint of patients reaching 75% clearing on the Eczema Area and Severity Index (EASI-75) by week 16.
The randomized, double-blind, placebo-controlled trial enrolled 367 children with IGA scores of 4, denoting severe AD. The EASI score had to be at least 21 and patients had to endorse peak pruritus of at least 4 on a 0-10 numeric rating scale; body surface involvement had to be at least 15%. Patients went through a washout period of any systemic therapies before beginning the trial, which randomized patients 1:1:1 to receive placebo, dupilumab 300 mg every 4 weeks, or dupilumab every 2 weeks with weight-dependent dosing. All participants were also permitted topical corticosteroids.
Patients were an average of aged 8 years, about half were female, and about two-thirds were white. Most participants had developed AD within their first year of life. Patients were about evenly divided between weighing over and under 30 kg, which was the cutoff for 100 mcg versus 200 mcg dupilumab for the every-2-week dosing group.
Over 90% of patients had other atopic comorbidities, and the mean EASI score was about 38 with average weekly peak pruritus averaging 7.8 on the numeric rating scale.
“When we’re talking about how severe this population is, it’s interesting to note that about 30 to 35% were all that had been previously treated with either systemic steroids or some systemic nonsteroidal immunosuppressants,” Dr. Paller pointed out. “I think that reflects the fact that so many of these very severely affected children are not put on a systemic therapy, but are still staying on topical therapies to try to control their disease.”
Looking at the proportion of patients reaching EASI-75, both dosing strategies for dupilumab out-performed placebo, with 70% of the every 4-week group and 67% of the every 2-week group reaching EASI-75 at 16 weeks, compared with 27% of those on placebo (P less than .0001 for both active arms). “These differences were seen very early on; by 2 weeks already, we can see that we’re starting to see a difference in both of these arms,” noted Dr. Paller, adding that the difference was statistically significant by 4 weeks into the study.
The overall group of dupilumab participants saw their EASI scores drop by about 80%, while those taking placebo saw a 49% drop in EASI scores.
For the group of participants weighing less than 30 kg, the every 4-week strategy resulted in better clearing as measured by both IGA and EASI-75. This effect wasn’t seen for heavier patients. Trough dupilumab concentrations at 16 weeks were higher for lighter patients with every 4-week dosing and for heavier patients with the biweekly strategy, noted Dr. Paller.
In terms of itch, 60% to 68% of participants receiving dupilumab had a drop of at least 3 points in peak pruritus on the numeric rating scale, compared with 21% of those receiving placebo (P less than .001), while about half of the dupilumab groups and 12% of the placebo group saw pruritus improvements of 4 points or more (P less than .001). Pruritus improved early in the active arms of the study, becoming statistically significant at the 2 to 4 week range.
Treatment-emergent adverse events were numerically higher in patients in the placebo group, including infections and adjudicated skin infections. Conjunctivitis occurred more frequently in the dupilumab group, as did injection-site reactions.
“Overall, dupilumab was well tolerated, and data were consistent with the known dupilumab safety profile observed in adults and adolescents,” Dr. Paller said.
Dupilumab has been approved by the Food and Drug Administration to treat moderate to severe AD in those aged 12 years and older whose disease can’t be adequately controlled with topical prescription medications, or when those treatments are not advisable.
The fully human monoclonal antibody blocks a shared receptor component for interleukin-4 and interleukin-13, which contribute to inflammation in AD, as well as asthma, chronic rhinosinusitis with nasal polyps, and eosinophilic esophagitis.
Dr. Paller reported receiving support from multiple pharmaceutical companies including Sanofi and Regeneron Pharmaceuticals, which sponsored the study.
The monoclonal antibody , according to new clinical trial results.
In a cohort of children with severe AD, 33% achieved clear or nearly clear skin after 16 weeks of treatment with every 4-week dosing of the injectable medication, while 30% also achieved that mark when receiving a weight-based dose every 2 weeks. Both groups had results that were significantly better than those receiving placebo, with 11% of these children had clear or nearly clear skin by 16 weeks of dupilumab (Dupixent) therapy (P less than .0001 for both therapy arms versus placebo).
“Dupilumab with a topical corticosteroid showed clinically meaningful and statistically significant improvement in the atopic dermatitis signs and symptoms in children aged 6 to less than 12 years of age with severe atopic dermatitis,” said Amy Paller, MD, the Walter J. Hamlin professor and chair of the department of dermatology at Northwestern University, Chicago, presenting the results at the Revolutionizing Atopic Dermatitis virtual symposium. Portions of the conference, which has been rescheduled to December 2020, in Chicago, were presented virtually because of the COVID-19 pandemic.
The phase 3 trial of subcutaneously injected dupilumab for atopic dermatitis, dubbed LIBERTY AD PEDS, included children aged 6-11 years with severe AD. The study’s primary endpoint was the proportion of patients achieving a score of 0 or 1 (clear or almost clear skin) on the Investigator’s Global Assessment (IGA) scale by study week 16.
For the purposes of reporting results to the European Medicines Agency, the investigators added a coprimary endpoint of patients reaching 75% clearing on the Eczema Area and Severity Index (EASI-75) by week 16.
The randomized, double-blind, placebo-controlled trial enrolled 367 children with IGA scores of 4, denoting severe AD. The EASI score had to be at least 21 and patients had to endorse peak pruritus of at least 4 on a 0-10 numeric rating scale; body surface involvement had to be at least 15%. Patients went through a washout period of any systemic therapies before beginning the trial, which randomized patients 1:1:1 to receive placebo, dupilumab 300 mg every 4 weeks, or dupilumab every 2 weeks with weight-dependent dosing. All participants were also permitted topical corticosteroids.
Patients were an average of aged 8 years, about half were female, and about two-thirds were white. Most participants had developed AD within their first year of life. Patients were about evenly divided between weighing over and under 30 kg, which was the cutoff for 100 mcg versus 200 mcg dupilumab for the every-2-week dosing group.
Over 90% of patients had other atopic comorbidities, and the mean EASI score was about 38 with average weekly peak pruritus averaging 7.8 on the numeric rating scale.
“When we’re talking about how severe this population is, it’s interesting to note that about 30 to 35% were all that had been previously treated with either systemic steroids or some systemic nonsteroidal immunosuppressants,” Dr. Paller pointed out. “I think that reflects the fact that so many of these very severely affected children are not put on a systemic therapy, but are still staying on topical therapies to try to control their disease.”
Looking at the proportion of patients reaching EASI-75, both dosing strategies for dupilumab out-performed placebo, with 70% of the every 4-week group and 67% of the every 2-week group reaching EASI-75 at 16 weeks, compared with 27% of those on placebo (P less than .0001 for both active arms). “These differences were seen very early on; by 2 weeks already, we can see that we’re starting to see a difference in both of these arms,” noted Dr. Paller, adding that the difference was statistically significant by 4 weeks into the study.
The overall group of dupilumab participants saw their EASI scores drop by about 80%, while those taking placebo saw a 49% drop in EASI scores.
For the group of participants weighing less than 30 kg, the every 4-week strategy resulted in better clearing as measured by both IGA and EASI-75. This effect wasn’t seen for heavier patients. Trough dupilumab concentrations at 16 weeks were higher for lighter patients with every 4-week dosing and for heavier patients with the biweekly strategy, noted Dr. Paller.
In terms of itch, 60% to 68% of participants receiving dupilumab had a drop of at least 3 points in peak pruritus on the numeric rating scale, compared with 21% of those receiving placebo (P less than .001), while about half of the dupilumab groups and 12% of the placebo group saw pruritus improvements of 4 points or more (P less than .001). Pruritus improved early in the active arms of the study, becoming statistically significant at the 2 to 4 week range.
Treatment-emergent adverse events were numerically higher in patients in the placebo group, including infections and adjudicated skin infections. Conjunctivitis occurred more frequently in the dupilumab group, as did injection-site reactions.
“Overall, dupilumab was well tolerated, and data were consistent with the known dupilumab safety profile observed in adults and adolescents,” Dr. Paller said.
Dupilumab has been approved by the Food and Drug Administration to treat moderate to severe AD in those aged 12 years and older whose disease can’t be adequately controlled with topical prescription medications, or when those treatments are not advisable.
The fully human monoclonal antibody blocks a shared receptor component for interleukin-4 and interleukin-13, which contribute to inflammation in AD, as well as asthma, chronic rhinosinusitis with nasal polyps, and eosinophilic esophagitis.
Dr. Paller reported receiving support from multiple pharmaceutical companies including Sanofi and Regeneron Pharmaceuticals, which sponsored the study.
FROM REVOLUTIONIZING AD 2020