User login
Tips for self-care during the COVID-19 crisis
I think it’s fair to say, none of us have seen anything like this before. Yet here we are, and we must lead. We are many weeks into the COVID-19 crisis. We moved our offices home and tried not to miss a beat. Our patients need us more than ever – and in different ways.
Lest we become like the shoemaker’s daughter who has no shoes, let’s make sure we take care of ourselves. The shock waves from this pandemic are going to be massive and long lasting. I am already witnessing massive psychological growth on the part of my patients, and I hope, myself and my family. We must be strong as individuals and as a group of professionals.
Now more than ever, we need to set boundaries. So many are suffering. We must take stock of our own lives. Many of us are extremely fortunate. We have homes, families, and plenty of food. We are doctors performing essential services, and we can do so without risking our lives.
The priority is to make sure you are safe, and keeping your family and loved ones safe. As physicians, we have learned to distance ourselves from illness, but the coronavirus has affected us in disproportionate numbers.
To be physically and mentally strong, we must get enough sleep. This is exhausting for some and energizing for others. It is definitely a marathon not a sprint, so pace yourself. Eat well. This is no time for empty calories, and that goes for alcohol as well.
Create new routines. Exercise at the same time each day or perhaps twice a day. Try to be productive during certain hours, and relax at other times. Eat at similar times each day. We must strive to quickly create a “new normal” as we spend our days at home.
Find safe alternatives to your usual workout routine. Use YouTube and Instagram to help you find ways to stay fit in your own home. Ask friends for tips and consider sharing workout time with them via Zoom or FaceTime. New options are coming on line daily.
Make sure you are getting enough information to stay safe, and follow the advice of experts. Then turn off the news. I offer the same advice for financial worries. Try not to stress too much about finances right now. Most of us are feeling the pain of lost income and lost savings. Many of us have spouses or partners who suddenly found themselves out of work. Most likely, we will have ample ability to recover financially as we move forward and find ourselves with more work than ever.
Meditate. This may be advice you have been telling your patients for years but never found the time to try yourself. You can begin very simply with an app called Headspace or Calm. Google “5-minute meditation” on YouTube or find a meditation of any length you desire. If not now, when?
Reach out to one another. We can all use a caring word, or some humor or advice about how to move our practices online.
You may find your concentration is decreased, so be realistic in your expectations of yourself. I am finding shorter sessions more often are providing more comfort to some patients. Other patients are digging deeper than ever emotionally, and the work is becoming more rewarding.
Make sure you take a break to engage in positive activities. Read a book. Listen to soft music. Dim the lights. Watch the sunset, or be in nature if you can do so safely. Watch a TedTalk. Brush up on a foreign language. Take a deep breath. Journal. Puzzles, games, cooking, magazines, and humor all provide much needed respite from the stress. If you are lucky enough to be with family, try to take advantage of this unique time.
Try to avoid or minimize conflict with others. We need one another now more than ever. If you lose your cool, forgive yourself and make amends.
Even in these most challenging times, we must focus on what we are grateful for. Express gratitude to those around you as it will lift their mood as well. I know I am extremely grateful to be able to continue meaningful work when so many are unable to do so.
The next waves of this virus will be hitting our specialty directly so be strong and be prepared. It is an honor to serve, and we must rise to the occasion.
Dr. Ritvo, a psychiatrist with more than 25 years’ experience, practices in Miami Beach, Fla. She is the author of “Bekindr – The Transformative Power of Kindness” (Hellertown, Pa.: Momosa Publishing, 2018), and is the founder of the Bekindr Global Initiative, a movement aimed at cultivating kindness in the world. Dr. Ritvo also is the cofounder of the Bold Beauty Project, a nonprofit group that pairs women with disabilities with photographers who create art exhibitions to raise awareness.
I think it’s fair to say, none of us have seen anything like this before. Yet here we are, and we must lead. We are many weeks into the COVID-19 crisis. We moved our offices home and tried not to miss a beat. Our patients need us more than ever – and in different ways.
Lest we become like the shoemaker’s daughter who has no shoes, let’s make sure we take care of ourselves. The shock waves from this pandemic are going to be massive and long lasting. I am already witnessing massive psychological growth on the part of my patients, and I hope, myself and my family. We must be strong as individuals and as a group of professionals.
Now more than ever, we need to set boundaries. So many are suffering. We must take stock of our own lives. Many of us are extremely fortunate. We have homes, families, and plenty of food. We are doctors performing essential services, and we can do so without risking our lives.
The priority is to make sure you are safe, and keeping your family and loved ones safe. As physicians, we have learned to distance ourselves from illness, but the coronavirus has affected us in disproportionate numbers.
To be physically and mentally strong, we must get enough sleep. This is exhausting for some and energizing for others. It is definitely a marathon not a sprint, so pace yourself. Eat well. This is no time for empty calories, and that goes for alcohol as well.
Create new routines. Exercise at the same time each day or perhaps twice a day. Try to be productive during certain hours, and relax at other times. Eat at similar times each day. We must strive to quickly create a “new normal” as we spend our days at home.
Find safe alternatives to your usual workout routine. Use YouTube and Instagram to help you find ways to stay fit in your own home. Ask friends for tips and consider sharing workout time with them via Zoom or FaceTime. New options are coming on line daily.
Make sure you are getting enough information to stay safe, and follow the advice of experts. Then turn off the news. I offer the same advice for financial worries. Try not to stress too much about finances right now. Most of us are feeling the pain of lost income and lost savings. Many of us have spouses or partners who suddenly found themselves out of work. Most likely, we will have ample ability to recover financially as we move forward and find ourselves with more work than ever.
Meditate. This may be advice you have been telling your patients for years but never found the time to try yourself. You can begin very simply with an app called Headspace or Calm. Google “5-minute meditation” on YouTube or find a meditation of any length you desire. If not now, when?
Reach out to one another. We can all use a caring word, or some humor or advice about how to move our practices online.
You may find your concentration is decreased, so be realistic in your expectations of yourself. I am finding shorter sessions more often are providing more comfort to some patients. Other patients are digging deeper than ever emotionally, and the work is becoming more rewarding.
Make sure you take a break to engage in positive activities. Read a book. Listen to soft music. Dim the lights. Watch the sunset, or be in nature if you can do so safely. Watch a TedTalk. Brush up on a foreign language. Take a deep breath. Journal. Puzzles, games, cooking, magazines, and humor all provide much needed respite from the stress. If you are lucky enough to be with family, try to take advantage of this unique time.
Try to avoid or minimize conflict with others. We need one another now more than ever. If you lose your cool, forgive yourself and make amends.
Even in these most challenging times, we must focus on what we are grateful for. Express gratitude to those around you as it will lift their mood as well. I know I am extremely grateful to be able to continue meaningful work when so many are unable to do so.
The next waves of this virus will be hitting our specialty directly so be strong and be prepared. It is an honor to serve, and we must rise to the occasion.
Dr. Ritvo, a psychiatrist with more than 25 years’ experience, practices in Miami Beach, Fla. She is the author of “Bekindr – The Transformative Power of Kindness” (Hellertown, Pa.: Momosa Publishing, 2018), and is the founder of the Bekindr Global Initiative, a movement aimed at cultivating kindness in the world. Dr. Ritvo also is the cofounder of the Bold Beauty Project, a nonprofit group that pairs women with disabilities with photographers who create art exhibitions to raise awareness.
I think it’s fair to say, none of us have seen anything like this before. Yet here we are, and we must lead. We are many weeks into the COVID-19 crisis. We moved our offices home and tried not to miss a beat. Our patients need us more than ever – and in different ways.
Lest we become like the shoemaker’s daughter who has no shoes, let’s make sure we take care of ourselves. The shock waves from this pandemic are going to be massive and long lasting. I am already witnessing massive psychological growth on the part of my patients, and I hope, myself and my family. We must be strong as individuals and as a group of professionals.
Now more than ever, we need to set boundaries. So many are suffering. We must take stock of our own lives. Many of us are extremely fortunate. We have homes, families, and plenty of food. We are doctors performing essential services, and we can do so without risking our lives.
The priority is to make sure you are safe, and keeping your family and loved ones safe. As physicians, we have learned to distance ourselves from illness, but the coronavirus has affected us in disproportionate numbers.
To be physically and mentally strong, we must get enough sleep. This is exhausting for some and energizing for others. It is definitely a marathon not a sprint, so pace yourself. Eat well. This is no time for empty calories, and that goes for alcohol as well.
Create new routines. Exercise at the same time each day or perhaps twice a day. Try to be productive during certain hours, and relax at other times. Eat at similar times each day. We must strive to quickly create a “new normal” as we spend our days at home.
Find safe alternatives to your usual workout routine. Use YouTube and Instagram to help you find ways to stay fit in your own home. Ask friends for tips and consider sharing workout time with them via Zoom or FaceTime. New options are coming on line daily.
Make sure you are getting enough information to stay safe, and follow the advice of experts. Then turn off the news. I offer the same advice for financial worries. Try not to stress too much about finances right now. Most of us are feeling the pain of lost income and lost savings. Many of us have spouses or partners who suddenly found themselves out of work. Most likely, we will have ample ability to recover financially as we move forward and find ourselves with more work than ever.
Meditate. This may be advice you have been telling your patients for years but never found the time to try yourself. You can begin very simply with an app called Headspace or Calm. Google “5-minute meditation” on YouTube or find a meditation of any length you desire. If not now, when?
Reach out to one another. We can all use a caring word, or some humor or advice about how to move our practices online.
You may find your concentration is decreased, so be realistic in your expectations of yourself. I am finding shorter sessions more often are providing more comfort to some patients. Other patients are digging deeper than ever emotionally, and the work is becoming more rewarding.
Make sure you take a break to engage in positive activities. Read a book. Listen to soft music. Dim the lights. Watch the sunset, or be in nature if you can do so safely. Watch a TedTalk. Brush up on a foreign language. Take a deep breath. Journal. Puzzles, games, cooking, magazines, and humor all provide much needed respite from the stress. If you are lucky enough to be with family, try to take advantage of this unique time.
Try to avoid or minimize conflict with others. We need one another now more than ever. If you lose your cool, forgive yourself and make amends.
Even in these most challenging times, we must focus on what we are grateful for. Express gratitude to those around you as it will lift their mood as well. I know I am extremely grateful to be able to continue meaningful work when so many are unable to do so.
The next waves of this virus will be hitting our specialty directly so be strong and be prepared. It is an honor to serve, and we must rise to the occasion.
Dr. Ritvo, a psychiatrist with more than 25 years’ experience, practices in Miami Beach, Fla. She is the author of “Bekindr – The Transformative Power of Kindness” (Hellertown, Pa.: Momosa Publishing, 2018), and is the founder of the Bekindr Global Initiative, a movement aimed at cultivating kindness in the world. Dr. Ritvo also is the cofounder of the Bold Beauty Project, a nonprofit group that pairs women with disabilities with photographers who create art exhibitions to raise awareness.
AMA president calls for greater reliance on science in COVID-19 fight
The president of the American Medical Association is calling on politicians and the media to rely on science and evidence to help the public through the COVID-19 pandemic.
“We live in a time when misinformation, falsehoods, and outright lies spread like viruses online, through social media and even, at times, in the media at large,” Patrice A. Harris, MD, said during an April 7 address. “We have witnessed a concerning shift over the last several decades where policy decisions seem to be driven by ideology and politics instead of facts and evidence. The result is a growing mistrust in American institutions, in science, and in the counsel of leading experts whose lives are dedicated to the pursuit of evidence and reason.”
To that end, she called on everyone – from politicians to the general public – to trust the scientific evidence.
Dr. Harris noted that the scientific data on COVID-19 have already yielded important lessons about who is more likely to be affected and how easily the virus can spread. The data also point to the effectiveness of stay-at-home and shelter-in-place orders. “This is our best chance to slow the spread of the virus,” she said, adding that the enhanced emphasis on hand washing and other hygiene practices “may seem ‘simplistic,’ but they are, in fact, based in science and evidence.”
And, as the pandemic continues, Dr. Harris said that now is the time to rely on science. She said the AMA “calls on all elected officials to affirm science, evidence, and fact in their words and actions,” and she urged that the government’s scientific institutions be led by experts who are “protected from political influence.”
It is incumbent upon everyone to actively work to contain and stop the spread of misinformation related to COVID-19, she said. “We must ensure the war is against the virus and not against science,” Dr. Harris said.
The president of the American Medical Association is calling on politicians and the media to rely on science and evidence to help the public through the COVID-19 pandemic.
“We live in a time when misinformation, falsehoods, and outright lies spread like viruses online, through social media and even, at times, in the media at large,” Patrice A. Harris, MD, said during an April 7 address. “We have witnessed a concerning shift over the last several decades where policy decisions seem to be driven by ideology and politics instead of facts and evidence. The result is a growing mistrust in American institutions, in science, and in the counsel of leading experts whose lives are dedicated to the pursuit of evidence and reason.”
To that end, she called on everyone – from politicians to the general public – to trust the scientific evidence.
Dr. Harris noted that the scientific data on COVID-19 have already yielded important lessons about who is more likely to be affected and how easily the virus can spread. The data also point to the effectiveness of stay-at-home and shelter-in-place orders. “This is our best chance to slow the spread of the virus,” she said, adding that the enhanced emphasis on hand washing and other hygiene practices “may seem ‘simplistic,’ but they are, in fact, based in science and evidence.”
And, as the pandemic continues, Dr. Harris said that now is the time to rely on science. She said the AMA “calls on all elected officials to affirm science, evidence, and fact in their words and actions,” and she urged that the government’s scientific institutions be led by experts who are “protected from political influence.”
It is incumbent upon everyone to actively work to contain and stop the spread of misinformation related to COVID-19, she said. “We must ensure the war is against the virus and not against science,” Dr. Harris said.
The president of the American Medical Association is calling on politicians and the media to rely on science and evidence to help the public through the COVID-19 pandemic.
“We live in a time when misinformation, falsehoods, and outright lies spread like viruses online, through social media and even, at times, in the media at large,” Patrice A. Harris, MD, said during an April 7 address. “We have witnessed a concerning shift over the last several decades where policy decisions seem to be driven by ideology and politics instead of facts and evidence. The result is a growing mistrust in American institutions, in science, and in the counsel of leading experts whose lives are dedicated to the pursuit of evidence and reason.”
To that end, she called on everyone – from politicians to the general public – to trust the scientific evidence.
Dr. Harris noted that the scientific data on COVID-19 have already yielded important lessons about who is more likely to be affected and how easily the virus can spread. The data also point to the effectiveness of stay-at-home and shelter-in-place orders. “This is our best chance to slow the spread of the virus,” she said, adding that the enhanced emphasis on hand washing and other hygiene practices “may seem ‘simplistic,’ but they are, in fact, based in science and evidence.”
And, as the pandemic continues, Dr. Harris said that now is the time to rely on science. She said the AMA “calls on all elected officials to affirm science, evidence, and fact in their words and actions,” and she urged that the government’s scientific institutions be led by experts who are “protected from political influence.”
It is incumbent upon everyone to actively work to contain and stop the spread of misinformation related to COVID-19, she said. “We must ensure the war is against the virus and not against science,” Dr. Harris said.
Ergonomics 101 for trainees
To the early trainee, often the goal of performing a colonoscopy is to reach the cecum using whatever technique necessary. Although the recommended amount of colonoscopies for safe independent practice is 140 (with some sources stating more than 500), this only relates to the safety of the patient.1 We receive scant education on how to form good procedural habits to preserve our own safety and efficiency over the course of our career. Here are some tips on how to prevent injury:
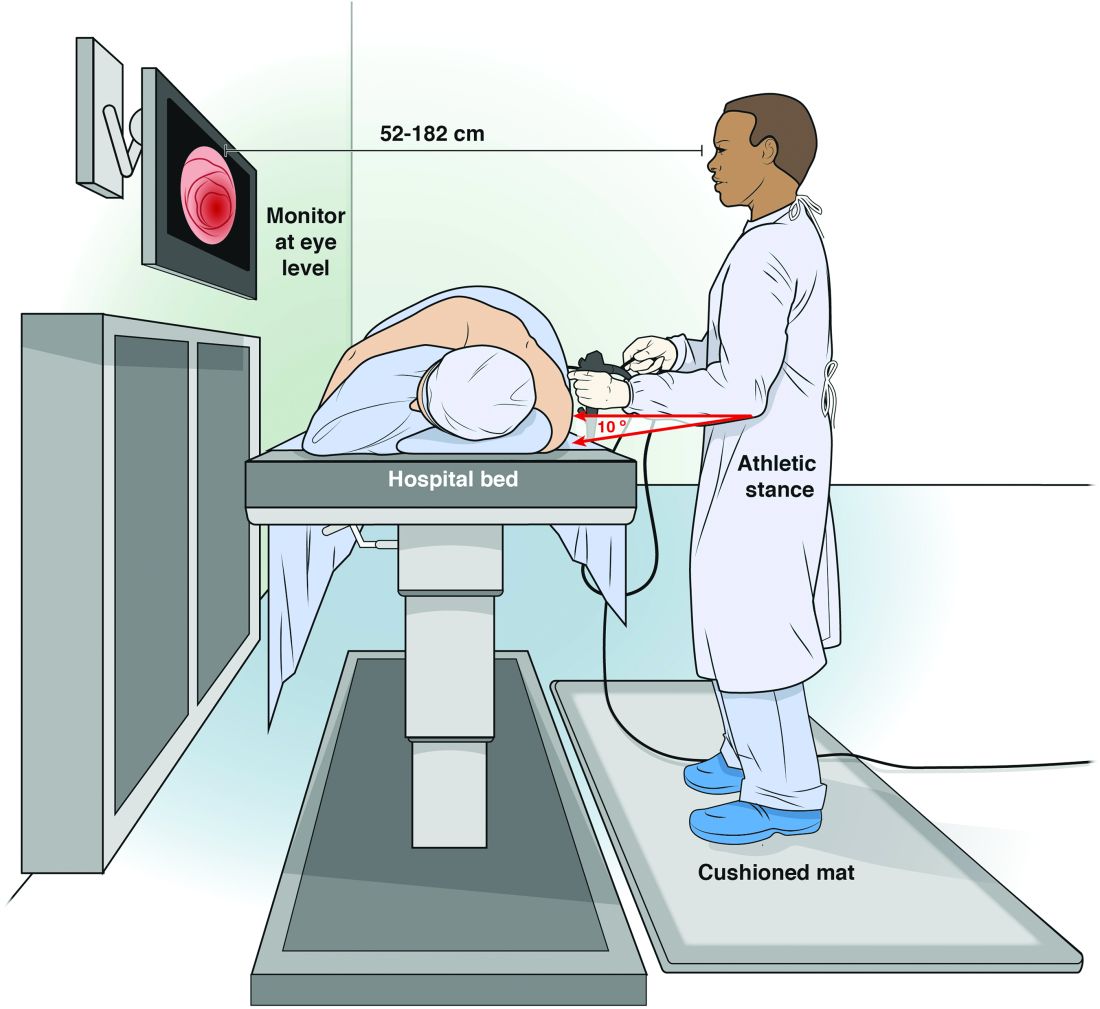
Maintain an appropriate stance. The optimal stance during endoscopy is an athletic stance: chest out, shoulders back to facilitate ease of neck movements, and a slight bend in the knees to facilitate good blood return and distribute weight. Feet should be hip width apart with toes pointed at the endoscopy screen to allow for easy pivoting of the hips and torque of upper body if needed. Ideally, this stance is complemented by the use of proper footwear and a cushioned mat to facilitate weight distribution while standing. An athletic stance facilitates a fluidity for movements from head to toe and an ability to use larger muscles groups to accomplish fine movements.
Handle the endoscope properly. Preserve energy by understanding your equipment and how to manipulate it. Orienting the endoscope directly in front of the endoscopist for upper endoscopy, and at a 45-degree angle for colonoscopy, places the instrument at optimal location to complete the procedure.5 Reviewing how to perform common techniques such as retroflexion, scope reduction, and instrumentation can also facilitate improved ergonomics and adjustment of incorrect techniques at an early stage of endoscopic training. An area of particular concern for most early trainees is the amount of rotational force placed on the right wrist with administration of torque to the endoscope. This is a foreign movement for most endoscopists and requires use of smaller muscle groups of the forearms. We suggest attempting torque with internal and external rotation of the left shoulder to utilize larger muscle groups. We can also combat fatigue during the procedure with the use of microrests intermittently to reduce prolonged muscle contraction. A common way to utilize microrests is by pinning the scope to the patient’s bed with the endoscopist’s hip to provide stability of endoscope and allow removal and relaxation of the right hand. This can be done periodically throughout the procedure to provide the ability to regroup mentally and physically.
Seek feedback. Because it is difficult to focus on ergonomics while performing a diagnostic procedure, utilize your team of observers to facilitate proper form during procedure. This includes your attending gastroenterologists, nurses, and technicians who can observe posture and technique to help detect incorrect positioning early and make corrections. A common practice is to discuss areas of desired improvement before procedures to facilitate a more vigilant observation of areas for improvement.
Assess and adjust often. As early trainees, these endoscopists perform all endoscopies under the direct supervision and often with significant assistance from a supervising gastroenterologist. This can lead to a sharp differential in psychological size; it can be hard to adjust a room to your needs when you have an intimidating and demanding attending physician who has different needs. Despite this disparity, we strongly encourage all trainees to be vigilant about adjusting the room (monitors and beds) to their own needs rather than their attendings’. A great way to head off potential conflict is to discuss the ergonomic positioning of the room before you start endoscopy with your attending, nurse, and technicians so that everyone is in agreement.
Conclusion
We offer this article as a guide for the novice endoscopist to make small changes early to prevent injuries later. Reaching competency with our skills is difficult, and we hope it can be achieved safely with our health in mind.
Dr. Magee, first-year fellow, NCC Gastroenterology; Dr. Singla, associate program director, NCC Gastroenterology, and gastroenterology service, department of internal medicine, Walter Reed National Military Medical Center, Bethesda, Md.
References
1. Spier B et al. Colonoscopy training in gastroenterology fellowships: determining competence. Gastrointest Endosc. 2010 Feb;71(2):319-24G.
2. Malmström EM et al. A slouched body posture decreases arm mobility and changes muscle recruitment in the neck and shoulder region. Eur J Appl Physiol. 2015;115(12):2491-503.
3. Singla M et al. Training the endo-athlete: an update in ergonomics in endoscopy. Clin Gastroenterol Hepatol. 2018 Jul;16(7):1003-6.
4. Bexander CS, et al. Effect of gaze direction on neck muscle activity during cervical rotation. Exp Brain Res. 2005 Dec;167(3):422-32.
5. Soetikno R et al. Holding and manipulating the endoscope: A user’s guide. Techn Gastrointest Endosc. 2019;21:124-32.
To the early trainee, often the goal of performing a colonoscopy is to reach the cecum using whatever technique necessary. Although the recommended amount of colonoscopies for safe independent practice is 140 (with some sources stating more than 500), this only relates to the safety of the patient.1 We receive scant education on how to form good procedural habits to preserve our own safety and efficiency over the course of our career. Here are some tips on how to prevent injury:

Maintain an appropriate stance. The optimal stance during endoscopy is an athletic stance: chest out, shoulders back to facilitate ease of neck movements, and a slight bend in the knees to facilitate good blood return and distribute weight. Feet should be hip width apart with toes pointed at the endoscopy screen to allow for easy pivoting of the hips and torque of upper body if needed. Ideally, this stance is complemented by the use of proper footwear and a cushioned mat to facilitate weight distribution while standing. An athletic stance facilitates a fluidity for movements from head to toe and an ability to use larger muscles groups to accomplish fine movements.
Handle the endoscope properly. Preserve energy by understanding your equipment and how to manipulate it. Orienting the endoscope directly in front of the endoscopist for upper endoscopy, and at a 45-degree angle for colonoscopy, places the instrument at optimal location to complete the procedure.5 Reviewing how to perform common techniques such as retroflexion, scope reduction, and instrumentation can also facilitate improved ergonomics and adjustment of incorrect techniques at an early stage of endoscopic training. An area of particular concern for most early trainees is the amount of rotational force placed on the right wrist with administration of torque to the endoscope. This is a foreign movement for most endoscopists and requires use of smaller muscle groups of the forearms. We suggest attempting torque with internal and external rotation of the left shoulder to utilize larger muscle groups. We can also combat fatigue during the procedure with the use of microrests intermittently to reduce prolonged muscle contraction. A common way to utilize microrests is by pinning the scope to the patient’s bed with the endoscopist’s hip to provide stability of endoscope and allow removal and relaxation of the right hand. This can be done periodically throughout the procedure to provide the ability to regroup mentally and physically.
Seek feedback. Because it is difficult to focus on ergonomics while performing a diagnostic procedure, utilize your team of observers to facilitate proper form during procedure. This includes your attending gastroenterologists, nurses, and technicians who can observe posture and technique to help detect incorrect positioning early and make corrections. A common practice is to discuss areas of desired improvement before procedures to facilitate a more vigilant observation of areas for improvement.
Assess and adjust often. As early trainees, these endoscopists perform all endoscopies under the direct supervision and often with significant assistance from a supervising gastroenterologist. This can lead to a sharp differential in psychological size; it can be hard to adjust a room to your needs when you have an intimidating and demanding attending physician who has different needs. Despite this disparity, we strongly encourage all trainees to be vigilant about adjusting the room (monitors and beds) to their own needs rather than their attendings’. A great way to head off potential conflict is to discuss the ergonomic positioning of the room before you start endoscopy with your attending, nurse, and technicians so that everyone is in agreement.
Conclusion
We offer this article as a guide for the novice endoscopist to make small changes early to prevent injuries later. Reaching competency with our skills is difficult, and we hope it can be achieved safely with our health in mind.
Dr. Magee, first-year fellow, NCC Gastroenterology; Dr. Singla, associate program director, NCC Gastroenterology, and gastroenterology service, department of internal medicine, Walter Reed National Military Medical Center, Bethesda, Md.
References
1. Spier B et al. Colonoscopy training in gastroenterology fellowships: determining competence. Gastrointest Endosc. 2010 Feb;71(2):319-24G.
2. Malmström EM et al. A slouched body posture decreases arm mobility and changes muscle recruitment in the neck and shoulder region. Eur J Appl Physiol. 2015;115(12):2491-503.
3. Singla M et al. Training the endo-athlete: an update in ergonomics in endoscopy. Clin Gastroenterol Hepatol. 2018 Jul;16(7):1003-6.
4. Bexander CS, et al. Effect of gaze direction on neck muscle activity during cervical rotation. Exp Brain Res. 2005 Dec;167(3):422-32.
5. Soetikno R et al. Holding and manipulating the endoscope: A user’s guide. Techn Gastrointest Endosc. 2019;21:124-32.
To the early trainee, often the goal of performing a colonoscopy is to reach the cecum using whatever technique necessary. Although the recommended amount of colonoscopies for safe independent practice is 140 (with some sources stating more than 500), this only relates to the safety of the patient.1 We receive scant education on how to form good procedural habits to preserve our own safety and efficiency over the course of our career. Here are some tips on how to prevent injury:

Maintain an appropriate stance. The optimal stance during endoscopy is an athletic stance: chest out, shoulders back to facilitate ease of neck movements, and a slight bend in the knees to facilitate good blood return and distribute weight. Feet should be hip width apart with toes pointed at the endoscopy screen to allow for easy pivoting of the hips and torque of upper body if needed. Ideally, this stance is complemented by the use of proper footwear and a cushioned mat to facilitate weight distribution while standing. An athletic stance facilitates a fluidity for movements from head to toe and an ability to use larger muscles groups to accomplish fine movements.
Handle the endoscope properly. Preserve energy by understanding your equipment and how to manipulate it. Orienting the endoscope directly in front of the endoscopist for upper endoscopy, and at a 45-degree angle for colonoscopy, places the instrument at optimal location to complete the procedure.5 Reviewing how to perform common techniques such as retroflexion, scope reduction, and instrumentation can also facilitate improved ergonomics and adjustment of incorrect techniques at an early stage of endoscopic training. An area of particular concern for most early trainees is the amount of rotational force placed on the right wrist with administration of torque to the endoscope. This is a foreign movement for most endoscopists and requires use of smaller muscle groups of the forearms. We suggest attempting torque with internal and external rotation of the left shoulder to utilize larger muscle groups. We can also combat fatigue during the procedure with the use of microrests intermittently to reduce prolonged muscle contraction. A common way to utilize microrests is by pinning the scope to the patient’s bed with the endoscopist’s hip to provide stability of endoscope and allow removal and relaxation of the right hand. This can be done periodically throughout the procedure to provide the ability to regroup mentally and physically.
Seek feedback. Because it is difficult to focus on ergonomics while performing a diagnostic procedure, utilize your team of observers to facilitate proper form during procedure. This includes your attending gastroenterologists, nurses, and technicians who can observe posture and technique to help detect incorrect positioning early and make corrections. A common practice is to discuss areas of desired improvement before procedures to facilitate a more vigilant observation of areas for improvement.
Assess and adjust often. As early trainees, these endoscopists perform all endoscopies under the direct supervision and often with significant assistance from a supervising gastroenterologist. This can lead to a sharp differential in psychological size; it can be hard to adjust a room to your needs when you have an intimidating and demanding attending physician who has different needs. Despite this disparity, we strongly encourage all trainees to be vigilant about adjusting the room (monitors and beds) to their own needs rather than their attendings’. A great way to head off potential conflict is to discuss the ergonomic positioning of the room before you start endoscopy with your attending, nurse, and technicians so that everyone is in agreement.
Conclusion
We offer this article as a guide for the novice endoscopist to make small changes early to prevent injuries later. Reaching competency with our skills is difficult, and we hope it can be achieved safely with our health in mind.
Dr. Magee, first-year fellow, NCC Gastroenterology; Dr. Singla, associate program director, NCC Gastroenterology, and gastroenterology service, department of internal medicine, Walter Reed National Military Medical Center, Bethesda, Md.
References
1. Spier B et al. Colonoscopy training in gastroenterology fellowships: determining competence. Gastrointest Endosc. 2010 Feb;71(2):319-24G.
2. Malmström EM et al. A slouched body posture decreases arm mobility and changes muscle recruitment in the neck and shoulder region. Eur J Appl Physiol. 2015;115(12):2491-503.
3. Singla M et al. Training the endo-athlete: an update in ergonomics in endoscopy. Clin Gastroenterol Hepatol. 2018 Jul;16(7):1003-6.
4. Bexander CS, et al. Effect of gaze direction on neck muscle activity during cervical rotation. Exp Brain Res. 2005 Dec;167(3):422-32.
5. Soetikno R et al. Holding and manipulating the endoscope: A user’s guide. Techn Gastrointest Endosc. 2019;21:124-32.
Year-long synbiotic regimen fails to improve NAFLD
Synbiotics can alter gut microbiota in patients with nonalcoholic fatty liver disease (NAFLD), but associated liver benefits remain unseen, according to a recent phase II study.
NAFLD patients who received a year-long regimen of fructo-oligosaccharides and Bifidobacterium animalis had no significant changes in liver fat content or fibrosis, compared with those who received placebo, reported lead author Eleonora Scorletti, MD, of the University of Pennsylvania, Philadelphia, and colleagues.
“There is recent growing interest in the role of gut microbiota in NAFLD pathogenesis, and there are several metaorganismal pathways linking altered gut microbiota ... and NAFLD,” the investigators wrote in Gastroenterology.According to the investigators, previous studies have shown that patients with NAFLD may have some characteristic alterations to their microbiota, such as increased Gram-negative bacteria or more abundant Ruminococcus species, the latter of which were associated with worse fibrosis.
“However, there is currently a lack of consistency in these findings due to the marked variance in the population studied, with differing ages, diets, and geographic locations,” the investigators wrote. “Nonetheless, despite these inconsistencies, there is the possibility that manipulation of the gut microbiota to a more favorable profile could provide a beneficial effect on liver disease in patients with NAFLD.”
To evaluate this possibility, the investigators enrolled 104 patients with NAFLD in the United Kingdom. Patients were randomly divided into a placebo (n = 49) and synbiotic group (n = 55), with the latter receiving 4 grams of fructo-oligosaccharides twice per day plus 10 billion colony-forming units of Bifidobacterium animalis subspecies lactis BB-12 on a daily basis. Treatments were given for 10-14 months.
Diagnostics were conducted across all participants at the beginning and end of the study. These included fecal microbiota analysis by 16s ribosomal DNA sequencing, liver fat measurement by proton magnetic resonance spectroscopy, biomarker-based liver fibrosis scoring, and liver stiffness assessment by vibration-controlled transient elastography.
At the end of the study, patients in the synbiotic group had increased abundance of Bifidobacterium and Faecalibacterium species and reduced proportions of Oscillibacter and Alistipes species, compared with baseline. These changes were not observed in the placebo group.
But changes in microbiota had no apparent impact on liver pathology. Although mean liver fat percentages dropped from 32.3% to 28.5% in the synbiotic group (approximately 4%), they also dropped in the placebo group, from 31.3% to 25.2% (approximately 6%), with differences between groups lacking statistical significance. Using multivariate analysis, the investigators linked these liver fat improvements, which occurred in 65% of participants, with weight loss.
“The fact that most patients had an improvement in ... liver fat, regardless of treatment allocation, is consistent with the so-called clinical trial effect, whereby participants benefit from participating in clinical trials,” the investigators wrote.
Similarly to liver fat content, no significant intergroup differences were found for liver fibrosis or stiffness, whereas, again, weight loss was linked with improvements in both disease parameters.
“Our randomized clinical trial suggests that changing the gut microbiota with this synbiotic may occur without clinically significant effects on the liver in NAFLD,” the investigators concluded.
Still, they noted that the failure of one synbiotic regimen does not discount the possibility of microbiota-based NAFLD interventions as a whole.
“Previous studies that have tested the effects of synbiotic treatment in NAFLD have also used a combination of multiple strains of probiotics as a component of the synbiotic treatment,” the investigators wrote. “Therefore, it might be possible that, because the intestine harbors trillions of bacteria, adding 1 single type of bacterium in a synbiotic may not be as effective as adding 3 or 6 different types of bacteria with the potential to influence many more bacterial species.”
The study was supported by the National Institute of Health Research, the Parnell Diabetes Trust, and Chr. Hansen Holding. One author reported funding from Chr. Hansen unrelated to this trial.
SOURCE: Scorletti E et al. Gastro. 2020 Jan 24. doi: 10.1053/j.gastro.2020.01.031.
Synbiotics can alter gut microbiota in patients with nonalcoholic fatty liver disease (NAFLD), but associated liver benefits remain unseen, according to a recent phase II study.
NAFLD patients who received a year-long regimen of fructo-oligosaccharides and Bifidobacterium animalis had no significant changes in liver fat content or fibrosis, compared with those who received placebo, reported lead author Eleonora Scorletti, MD, of the University of Pennsylvania, Philadelphia, and colleagues.
“There is recent growing interest in the role of gut microbiota in NAFLD pathogenesis, and there are several metaorganismal pathways linking altered gut microbiota ... and NAFLD,” the investigators wrote in Gastroenterology.According to the investigators, previous studies have shown that patients with NAFLD may have some characteristic alterations to their microbiota, such as increased Gram-negative bacteria or more abundant Ruminococcus species, the latter of which were associated with worse fibrosis.
“However, there is currently a lack of consistency in these findings due to the marked variance in the population studied, with differing ages, diets, and geographic locations,” the investigators wrote. “Nonetheless, despite these inconsistencies, there is the possibility that manipulation of the gut microbiota to a more favorable profile could provide a beneficial effect on liver disease in patients with NAFLD.”
To evaluate this possibility, the investigators enrolled 104 patients with NAFLD in the United Kingdom. Patients were randomly divided into a placebo (n = 49) and synbiotic group (n = 55), with the latter receiving 4 grams of fructo-oligosaccharides twice per day plus 10 billion colony-forming units of Bifidobacterium animalis subspecies lactis BB-12 on a daily basis. Treatments were given for 10-14 months.
Diagnostics were conducted across all participants at the beginning and end of the study. These included fecal microbiota analysis by 16s ribosomal DNA sequencing, liver fat measurement by proton magnetic resonance spectroscopy, biomarker-based liver fibrosis scoring, and liver stiffness assessment by vibration-controlled transient elastography.
At the end of the study, patients in the synbiotic group had increased abundance of Bifidobacterium and Faecalibacterium species and reduced proportions of Oscillibacter and Alistipes species, compared with baseline. These changes were not observed in the placebo group.
But changes in microbiota had no apparent impact on liver pathology. Although mean liver fat percentages dropped from 32.3% to 28.5% in the synbiotic group (approximately 4%), they also dropped in the placebo group, from 31.3% to 25.2% (approximately 6%), with differences between groups lacking statistical significance. Using multivariate analysis, the investigators linked these liver fat improvements, which occurred in 65% of participants, with weight loss.
“The fact that most patients had an improvement in ... liver fat, regardless of treatment allocation, is consistent with the so-called clinical trial effect, whereby participants benefit from participating in clinical trials,” the investigators wrote.
Similarly to liver fat content, no significant intergroup differences were found for liver fibrosis or stiffness, whereas, again, weight loss was linked with improvements in both disease parameters.
“Our randomized clinical trial suggests that changing the gut microbiota with this synbiotic may occur without clinically significant effects on the liver in NAFLD,” the investigators concluded.
Still, they noted that the failure of one synbiotic regimen does not discount the possibility of microbiota-based NAFLD interventions as a whole.
“Previous studies that have tested the effects of synbiotic treatment in NAFLD have also used a combination of multiple strains of probiotics as a component of the synbiotic treatment,” the investigators wrote. “Therefore, it might be possible that, because the intestine harbors trillions of bacteria, adding 1 single type of bacterium in a synbiotic may not be as effective as adding 3 or 6 different types of bacteria with the potential to influence many more bacterial species.”
The study was supported by the National Institute of Health Research, the Parnell Diabetes Trust, and Chr. Hansen Holding. One author reported funding from Chr. Hansen unrelated to this trial.
SOURCE: Scorletti E et al. Gastro. 2020 Jan 24. doi: 10.1053/j.gastro.2020.01.031.
Synbiotics can alter gut microbiota in patients with nonalcoholic fatty liver disease (NAFLD), but associated liver benefits remain unseen, according to a recent phase II study.
NAFLD patients who received a year-long regimen of fructo-oligosaccharides and Bifidobacterium animalis had no significant changes in liver fat content or fibrosis, compared with those who received placebo, reported lead author Eleonora Scorletti, MD, of the University of Pennsylvania, Philadelphia, and colleagues.
“There is recent growing interest in the role of gut microbiota in NAFLD pathogenesis, and there are several metaorganismal pathways linking altered gut microbiota ... and NAFLD,” the investigators wrote in Gastroenterology.According to the investigators, previous studies have shown that patients with NAFLD may have some characteristic alterations to their microbiota, such as increased Gram-negative bacteria or more abundant Ruminococcus species, the latter of which were associated with worse fibrosis.
“However, there is currently a lack of consistency in these findings due to the marked variance in the population studied, with differing ages, diets, and geographic locations,” the investigators wrote. “Nonetheless, despite these inconsistencies, there is the possibility that manipulation of the gut microbiota to a more favorable profile could provide a beneficial effect on liver disease in patients with NAFLD.”
To evaluate this possibility, the investigators enrolled 104 patients with NAFLD in the United Kingdom. Patients were randomly divided into a placebo (n = 49) and synbiotic group (n = 55), with the latter receiving 4 grams of fructo-oligosaccharides twice per day plus 10 billion colony-forming units of Bifidobacterium animalis subspecies lactis BB-12 on a daily basis. Treatments were given for 10-14 months.
Diagnostics were conducted across all participants at the beginning and end of the study. These included fecal microbiota analysis by 16s ribosomal DNA sequencing, liver fat measurement by proton magnetic resonance spectroscopy, biomarker-based liver fibrosis scoring, and liver stiffness assessment by vibration-controlled transient elastography.
At the end of the study, patients in the synbiotic group had increased abundance of Bifidobacterium and Faecalibacterium species and reduced proportions of Oscillibacter and Alistipes species, compared with baseline. These changes were not observed in the placebo group.
But changes in microbiota had no apparent impact on liver pathology. Although mean liver fat percentages dropped from 32.3% to 28.5% in the synbiotic group (approximately 4%), they also dropped in the placebo group, from 31.3% to 25.2% (approximately 6%), with differences between groups lacking statistical significance. Using multivariate analysis, the investigators linked these liver fat improvements, which occurred in 65% of participants, with weight loss.
“The fact that most patients had an improvement in ... liver fat, regardless of treatment allocation, is consistent with the so-called clinical trial effect, whereby participants benefit from participating in clinical trials,” the investigators wrote.
Similarly to liver fat content, no significant intergroup differences were found for liver fibrosis or stiffness, whereas, again, weight loss was linked with improvements in both disease parameters.
“Our randomized clinical trial suggests that changing the gut microbiota with this synbiotic may occur without clinically significant effects on the liver in NAFLD,” the investigators concluded.
Still, they noted that the failure of one synbiotic regimen does not discount the possibility of microbiota-based NAFLD interventions as a whole.
“Previous studies that have tested the effects of synbiotic treatment in NAFLD have also used a combination of multiple strains of probiotics as a component of the synbiotic treatment,” the investigators wrote. “Therefore, it might be possible that, because the intestine harbors trillions of bacteria, adding 1 single type of bacterium in a synbiotic may not be as effective as adding 3 or 6 different types of bacteria with the potential to influence many more bacterial species.”
The study was supported by the National Institute of Health Research, the Parnell Diabetes Trust, and Chr. Hansen Holding. One author reported funding from Chr. Hansen unrelated to this trial.
SOURCE: Scorletti E et al. Gastro. 2020 Jan 24. doi: 10.1053/j.gastro.2020.01.031.
FROM GASTROENTEROLOGY
Genotyping improves accuracy of pancreatic cancer tumor markers
Stratifying diagnostic cut-off values of tumor markers based on genetic variants may improve detection of pancreatic cancer, according to investigators.
Stratification had the greatest positive impact on accuracy of carbohydrate antigen 19-9 (CA19-9), reported lead author Toshiya Abe, MD, PhD, of Johns Hopkins Hospital, Baltimore, and colleagues.
“Despite the evidence that genetic factors influence tumor marker levels, the potential utility of using a genetic test to improve the interpretation of tumor markers has drawn limited attention,” the investigators wrote in Clinical Gastroenterology and Hepatology.
And improvements are needed, the investigators noted, particularly for early cancer detection in high-risk individuals.
“[T]he toughest hurdle for a pancreatic cancer detection blood test is the detection of stage I disease,” the investigators wrote. “Cancers generally shed biomarkers in proportion to their size, and small stage I pancreatic cancers shed fewer diagnostic biomarkers into the circulation, making diagnosis more difficult.”
Although a 2016 study by Dr. Guopei Luo and colleagues demonstrated that diagnostic accuracy of CA19-9 could be improved via genotyping, tumor marker performance was not characterized by high-specificity cut-off values, which the present study aimed to do.
The control group included 504 high-risk individuals who were prospectively enrolled in the Cancer of the Pancreas Screening (CAPS) studies from 2002 to 2018, while the case group included 245 patients with pancreatic ductal adenocarcinoma (PDAC) who underwent resection at Johns Hopkins from 2010 to 2017.
The control group was randomly divided into discovery and validation sets in order to achieve 99% specificity cut-off values, which were used to measure sensitivity in the case group. According to the investigators, high-specificity cut-off values are necessary for surveillance of asymptomatic high-risk individuals in order to minimize false-positive results.
In all patients, tumor markers and genotype were analyzed. Tumor markers included carcinoembryonic antigen (CEA), CA19-9, and cancer antigen 125 (CA-125). Genotyping included 16 single-nucleotide polymorphisms (SNPs) in 9 genes, including FUT2 and FUT3, which are known to influence levels of CA19-9.
In contrast with previous findings, which identified three relevant subgroups of FUT2/FUT3, the present study found that four distinct subgroups were significantly associated with CA19-9 levels: FUT3-null, FUT3+/-, FUT3+/+, and FUT2-null.
When CA19-9 cut-off levels were stratified by these four subgroups and applied to the 245 patients with pancreatic cancer, the investigators achieved a sensitivity of 60.8%, compared with 52.7% without stratification. The new cut-off values led to reclassification of 28 (11.4%) patients with pancreatic cancer, including 24 who switched from negative to positive, and 4 who switched from positive to negative.
Sensitivity of the SNP-adjusted CA19-9 test was improved to 66.4% when used exclusively in patients with functional FUT3 genes. Conversely, sensitivity was markedly lower, at 36.7%, when the test was used for patients with stage I disease.
While CA19-9 testing was notably improved by SNP-based stratification, results from CEA and CA-125 testing were more modest. Standard CEA testing had a sensitivity of 13.8%, compared with 15.9% when cut-off values were stratified by FUT2 status and ABO blood group. Similarly, modifying CA-125 values based on SNPs in GAL3ST2 raised sensitivity from 15.5% to 17.6%.
Although combining SNP-modified tumor marker results did increase overall sensitivity to as high as 66.1%, this also reduced specificity to as low as 95.4%
Still, Dr. Abe and colleagues suggested that the findings demonstrate proof of concept.
“Our results show that a tumor marker SNP test can improve the diagnostic accuracy of CA19-9 and, to a lesser extent, CEA and CA-125, but further work is needed to improve the diagnostic accuracy of our panel for the detection of early-stage pancreatic cancer,” they concluded.
The investigators also suggested that the technique could have value for surveillance of ovarian cancer; however, again, they emphasized the need for more research.The study was funded by the National Institutes of Health, Susan Wojcicki and Dennis Troper, the Pancreatic Cancer Action Network, and others. The investigators reported no conflicts of interest.
SOURCE: Abe T et al. Clin Gastro Hepatol. 2019 Oct 29. doi: 10.1016/j.cgh.2019.10.036.
Stratifying diagnostic cut-off values of tumor markers based on genetic variants may improve detection of pancreatic cancer, according to investigators.
Stratification had the greatest positive impact on accuracy of carbohydrate antigen 19-9 (CA19-9), reported lead author Toshiya Abe, MD, PhD, of Johns Hopkins Hospital, Baltimore, and colleagues.
“Despite the evidence that genetic factors influence tumor marker levels, the potential utility of using a genetic test to improve the interpretation of tumor markers has drawn limited attention,” the investigators wrote in Clinical Gastroenterology and Hepatology.
And improvements are needed, the investigators noted, particularly for early cancer detection in high-risk individuals.
“[T]he toughest hurdle for a pancreatic cancer detection blood test is the detection of stage I disease,” the investigators wrote. “Cancers generally shed biomarkers in proportion to their size, and small stage I pancreatic cancers shed fewer diagnostic biomarkers into the circulation, making diagnosis more difficult.”
Although a 2016 study by Dr. Guopei Luo and colleagues demonstrated that diagnostic accuracy of CA19-9 could be improved via genotyping, tumor marker performance was not characterized by high-specificity cut-off values, which the present study aimed to do.
The control group included 504 high-risk individuals who were prospectively enrolled in the Cancer of the Pancreas Screening (CAPS) studies from 2002 to 2018, while the case group included 245 patients with pancreatic ductal adenocarcinoma (PDAC) who underwent resection at Johns Hopkins from 2010 to 2017.
The control group was randomly divided into discovery and validation sets in order to achieve 99% specificity cut-off values, which were used to measure sensitivity in the case group. According to the investigators, high-specificity cut-off values are necessary for surveillance of asymptomatic high-risk individuals in order to minimize false-positive results.
In all patients, tumor markers and genotype were analyzed. Tumor markers included carcinoembryonic antigen (CEA), CA19-9, and cancer antigen 125 (CA-125). Genotyping included 16 single-nucleotide polymorphisms (SNPs) in 9 genes, including FUT2 and FUT3, which are known to influence levels of CA19-9.
In contrast with previous findings, which identified three relevant subgroups of FUT2/FUT3, the present study found that four distinct subgroups were significantly associated with CA19-9 levels: FUT3-null, FUT3+/-, FUT3+/+, and FUT2-null.
When CA19-9 cut-off levels were stratified by these four subgroups and applied to the 245 patients with pancreatic cancer, the investigators achieved a sensitivity of 60.8%, compared with 52.7% without stratification. The new cut-off values led to reclassification of 28 (11.4%) patients with pancreatic cancer, including 24 who switched from negative to positive, and 4 who switched from positive to negative.
Sensitivity of the SNP-adjusted CA19-9 test was improved to 66.4% when used exclusively in patients with functional FUT3 genes. Conversely, sensitivity was markedly lower, at 36.7%, when the test was used for patients with stage I disease.
While CA19-9 testing was notably improved by SNP-based stratification, results from CEA and CA-125 testing were more modest. Standard CEA testing had a sensitivity of 13.8%, compared with 15.9% when cut-off values were stratified by FUT2 status and ABO blood group. Similarly, modifying CA-125 values based on SNPs in GAL3ST2 raised sensitivity from 15.5% to 17.6%.
Although combining SNP-modified tumor marker results did increase overall sensitivity to as high as 66.1%, this also reduced specificity to as low as 95.4%
Still, Dr. Abe and colleagues suggested that the findings demonstrate proof of concept.
“Our results show that a tumor marker SNP test can improve the diagnostic accuracy of CA19-9 and, to a lesser extent, CEA and CA-125, but further work is needed to improve the diagnostic accuracy of our panel for the detection of early-stage pancreatic cancer,” they concluded.
The investigators also suggested that the technique could have value for surveillance of ovarian cancer; however, again, they emphasized the need for more research.The study was funded by the National Institutes of Health, Susan Wojcicki and Dennis Troper, the Pancreatic Cancer Action Network, and others. The investigators reported no conflicts of interest.
SOURCE: Abe T et al. Clin Gastro Hepatol. 2019 Oct 29. doi: 10.1016/j.cgh.2019.10.036.
Stratifying diagnostic cut-off values of tumor markers based on genetic variants may improve detection of pancreatic cancer, according to investigators.
Stratification had the greatest positive impact on accuracy of carbohydrate antigen 19-9 (CA19-9), reported lead author Toshiya Abe, MD, PhD, of Johns Hopkins Hospital, Baltimore, and colleagues.
“Despite the evidence that genetic factors influence tumor marker levels, the potential utility of using a genetic test to improve the interpretation of tumor markers has drawn limited attention,” the investigators wrote in Clinical Gastroenterology and Hepatology.
And improvements are needed, the investigators noted, particularly for early cancer detection in high-risk individuals.
“[T]he toughest hurdle for a pancreatic cancer detection blood test is the detection of stage I disease,” the investigators wrote. “Cancers generally shed biomarkers in proportion to their size, and small stage I pancreatic cancers shed fewer diagnostic biomarkers into the circulation, making diagnosis more difficult.”
Although a 2016 study by Dr. Guopei Luo and colleagues demonstrated that diagnostic accuracy of CA19-9 could be improved via genotyping, tumor marker performance was not characterized by high-specificity cut-off values, which the present study aimed to do.
The control group included 504 high-risk individuals who were prospectively enrolled in the Cancer of the Pancreas Screening (CAPS) studies from 2002 to 2018, while the case group included 245 patients with pancreatic ductal adenocarcinoma (PDAC) who underwent resection at Johns Hopkins from 2010 to 2017.
The control group was randomly divided into discovery and validation sets in order to achieve 99% specificity cut-off values, which were used to measure sensitivity in the case group. According to the investigators, high-specificity cut-off values are necessary for surveillance of asymptomatic high-risk individuals in order to minimize false-positive results.
In all patients, tumor markers and genotype were analyzed. Tumor markers included carcinoembryonic antigen (CEA), CA19-9, and cancer antigen 125 (CA-125). Genotyping included 16 single-nucleotide polymorphisms (SNPs) in 9 genes, including FUT2 and FUT3, which are known to influence levels of CA19-9.
In contrast with previous findings, which identified three relevant subgroups of FUT2/FUT3, the present study found that four distinct subgroups were significantly associated with CA19-9 levels: FUT3-null, FUT3+/-, FUT3+/+, and FUT2-null.
When CA19-9 cut-off levels were stratified by these four subgroups and applied to the 245 patients with pancreatic cancer, the investigators achieved a sensitivity of 60.8%, compared with 52.7% without stratification. The new cut-off values led to reclassification of 28 (11.4%) patients with pancreatic cancer, including 24 who switched from negative to positive, and 4 who switched from positive to negative.
Sensitivity of the SNP-adjusted CA19-9 test was improved to 66.4% when used exclusively in patients with functional FUT3 genes. Conversely, sensitivity was markedly lower, at 36.7%, when the test was used for patients with stage I disease.
While CA19-9 testing was notably improved by SNP-based stratification, results from CEA and CA-125 testing were more modest. Standard CEA testing had a sensitivity of 13.8%, compared with 15.9% when cut-off values were stratified by FUT2 status and ABO blood group. Similarly, modifying CA-125 values based on SNPs in GAL3ST2 raised sensitivity from 15.5% to 17.6%.
Although combining SNP-modified tumor marker results did increase overall sensitivity to as high as 66.1%, this also reduced specificity to as low as 95.4%
Still, Dr. Abe and colleagues suggested that the findings demonstrate proof of concept.
“Our results show that a tumor marker SNP test can improve the diagnostic accuracy of CA19-9 and, to a lesser extent, CEA and CA-125, but further work is needed to improve the diagnostic accuracy of our panel for the detection of early-stage pancreatic cancer,” they concluded.
The investigators also suggested that the technique could have value for surveillance of ovarian cancer; however, again, they emphasized the need for more research.The study was funded by the National Institutes of Health, Susan Wojcicki and Dennis Troper, the Pancreatic Cancer Action Network, and others. The investigators reported no conflicts of interest.
SOURCE: Abe T et al. Clin Gastro Hepatol. 2019 Oct 29. doi: 10.1016/j.cgh.2019.10.036.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
The Nonsurgical Sleep Medicine Physician Role in the Development of an Upper Airway Stimulation Program
Obstructive sleep apnea (OSA) is a common disorder in the US and other industrialized countries. The Wisconsin Sleep Cohort Study reported prevalence rates as high as 20% to 30% in men and 10% to 15% in women.1,2 Several studies have shown high prevalence of OSA among veterans. Ancoli-Israel and colleagues reported a OSA rate of 36% in a cohort of elderly patients at a US Department of Veterans Affairs (VA) medical center.3 A study by Kreis and colleagues showed that OSA was present in 27% of patients hospitalized on the medical ward at a VA hospital.4 Incidence of sleep apnea among veterans in the US will likely increase over time as obesity is becoming more prevalent. Rates of obesity have increased from 14% in 2000 to 18% in 2010 among both male and female veterans.5
Untreated OSA is associated with increased risk of coronary artery disease, cerebrovascular accidents, uncontrolled diabetes mellitus, and other complications. Patients with OSA are less productive, have increased health care utilization, and have a higher risk of motor vehicle accidents.6 Continuous positive airway pressure (CPAP) is the main form of treatment of OSA. However, despite the adverse outcomes of untreated sleep apnea, suboptimal CPAP adherence remains a major problem in clinical practice. When adherence is defined as > 4 hours of nightly use, 29% to 83% of patients with OSA have been reported to be nonadherent to treatment.7 Stepnowsky and colleagues estimated that 50% of patients with OSA for whom CPAP was recommended were no longer using it 1 year later.8 CPAP adherence among veterans also has been poor. Wallace and colleagues reported that about one-third of patients with OSA at a VA Miami Healthcare System had mean daily use ≥ 4 hours.9 Typical reasons for poor CPAP adherence include pressure intolerance, mask discomfort, nasal and oropharyngeal dryness and irritation.10 Development and implementation of alternate treatment strategies for OSA is important to reduce disease burden of this widespread and debilitating condition.
Upper airway stimulation (UAS) is a novel therapy for management of OSA that has been gaining popularity and acceptance within the sleep medicine community in the past few years. This treatment option involves implantation of a neurostimulator with a sensing lead and a stimulation lead. The device is similar to a pacemaker and is surgically implanted in chest wall. The sensing lead is placed close to the diaphragm for monitoring of pleural pressure to help assess ventilation. The stimulation lead is placed under the tongue in proximity to the hypoglossal nerve (cranial nerve XII). The neurostimulator delivers electrical pulses to the hypoglossal nerve through the stimulation lead. These stimulating pulses are synchronized with the ventilation detected by the sensing lead. This electrical stimulation results in anterior displacement of the tongue via action of the genioglossus and geniohyoid muscles. Mechanical coupling with the palate also is common and leads to additional airway opening within the oropharynx to prevent apneic episodes. The patient turns on the stimulation through the use of a portable remote control and is turned off in the morning. The patient is able to operate the UAS device by placing the remote control on the skin in proximity of the device. The patient also is able to adjust device voltage within a range set by their physician. The effective voltage range is determined via an overnight sleep study titration performed 1 month after device activation. UAS therapy is not considered first-line treatment for OSA as it requires surgical implantation under general anesthesia; however, it provides an alternative to patients with OSA who are unable to tolerate traditional therapy with CPAP.
The landmark Stimulation Therapy for Apnea Reduction (STAR) trial showed effectiveness of UAS therapy at 12 months postimplantation.11 Follow-up of these participants has proven the sustainability of this effect at 18, 24, 36, and 48 months of therapy.12-15 Inclusion criteria of the study was moderate-to-severe sleep apnea with predominantly obstructive events. Subjects were excluded if there were anatomical abnormalities of the upper airway or if the pattern of airway collapse was not conducive to UAS on sedated endoscopy evaluation. Participants in the trial were predominantly white males, the average age was 54.5 years, and the average body mass index (BMI) was 28.4. The outcomes measured included Functional Outcomes of Sleep Questionnaire, Epworth Sleepiness Scale (ESS), percentage of sleep time with oxygen saturation < 90%, and subjective snoring. All of these objective and subjective markers of sleep improved significantly with UAS therapy at 12 months and were maintained at improved levels at 48 months of therapy.
The adverse effects (AEs) associated with device implantation and subsequent UAS therapy have been infrequent and mostly transient. Out of 126 device implantations, there were 2 participants who had serious AEs due to implantation and required repositioning and fixation of the neurostimulator to resolve discomfort. Other AEs related to the procedure, including sore throat and muscle soreness, were considered nonserious and resolved with supportive care. AEs related to subsequent UAS therapy included temporary tongue weakness and tongue soreness/abrasion. These complications also have either resolved spontaneously or with use of supportive strategies such as a mouth guard. Due to the sustained clinical benefit and acceptable AE profile as demonstrated by the STAR trial, UAS has emerged as a realistic alternative for management of OSA.
Development of a successful program that provides and supports all aspects of UAS, including device implantation and follow-up, necessitates a multispecialty team approach. Ideally surgical and nonsurgical sleep physicians as well as clinical and administrative support staff should be part of this group.
This study is based on the experience of the development of the UAS program at the Clement J. Zablocki VA Medical Center (CJZ VAMC) in Milwaukee. Currently, there are 25 patients who are part of this UAS program. The inclusion and exclusion criteria were adopted from the STAR trial. The patient population is similar to the population in that trial. They are all white males with average age of 57.2 years and BMI of 31.3. The CJZVAMC UAS Program consists of multidisciplinary group of health care professionals. This article describes the role of a nonsurgical sleep medicine physician that was crucial in the development of this UAS program.
Process
Introduction of this novel alternative therapy has sparked much interest among health care providers (HCPs) at CJZVAMC. However, there has been much misunderstanding among patients and HCPs about what this treatment involves and how it is implemented. For example, many patients that called the sleep clinic to set up an evaluation for UAS did not realize that this is a surgical procedure that requires general anesthesia. One of the most important tasks for a nonsurgical sleep physician is to educate patients and HCPs about this therapy. Most of patient education at CJZVAMC has been done during individual clinic appointments; however, setting up group educational classes for patients is a more efficient strategy to deliver this information. Similarly, giving a lecture on UAS at medicine (or another specialty) grand rounds has been effective in the education of HCPs who refer patients to the sleep clinic. If possible, a combined lecture with a surgical colleague could provide a more balanced and complete depiction of UAS and help to answer a broader range of questions for the audience.
Screening
Screening and identification of appropriate candidates is an important first step in the patient pathway in the UAS therapy. Failure of CPAP therapy is a key starting point in this screening process. When patients present to the sleep clinic with difficulty tolerating CPAP therapy, an extensive and thorough troubleshooting process needs to take place to make sure that all CPAP options have been exhausted. This process would typically include trial of various masks, including different mask interfaces. A dedicated appointment with a registered polysomnographic technologist (RPSGT) or another clinic staff member with vast experience in PAP mask fitting is typically part of this effort.
Adjustment of CPAP pressure settings also may be helpful as high PAP pressure may be another obstacle. Patients frequently have trouble tolerating higher pressure settings especially when they are new to this therapy. Pressure restriction to 4-cm to 7-cm water pressure on auto CPAP has been a helpful technique to allow patients to become more comfortable with this therapy. Once patients are able to use PAP at lower pressures, these settings can be titrated up gradually for optimal effectiveness. Other desensitization techniques, such as use during daytime while distracted by other activities (such as watching TV) can be helpful in adjustment to PAP therapy. Addressing problems with nasal congestion can help improve PAP adherence. Finally, patients should be offered opportunities for education about their PAP machine on an ongoing basis. Lack of proficiency with humidifier use is a very common obstacle and frequently leads to PAP nonadherence. Teaching PAP operation should correspond to the patient’s level of education to be effective. PAP therapy remains the first-line treatment strategy for OSA as it is not invasive and highly effective. Nonsurgical sleep medicine physicians are uniquely positioned to implement and troubleshoot this therapy for sleep apnea patients before considering UAS.
As part of the screening process, it can be helpful to conduct routine multidisciplinary meetings to discuss patients who are being evaluated for UAS implantation. These meetings should include the otolaryngologist, nonsurgical sleep medicine physician, as well as additional staff (nurses, respiratory therapists, etc) who are involved in the UAS process. Having a mental health care provider as part of the multidisciplinary team during the screening process also could be a valuable addition as this specialist could evaluate and provide insight into a patient’s emotional status prior to implantation. This is common practice during evaluation for organ transplantation and would help to predict patient’s psychological well-being after this life-changing procedure.16 Having multidisciplinary agreement on patient’s candidacy for UAS therapy could improve long-term success of this treatment. Additionally, these multidisciplinary meetings as part of the UAS program can improve team camaraderie and prevent miscommunications during this therapy.
Drug-Induced Sedated Endoscopy
Patient pathway to neurostimulator implantation involves evaluation of the upper airway using drug-induced sedated endoscopy (DISE). This procedure helps determine whether the patient’s anatomy is appropriate for UAS. DISE also can evaluate the pattern of airway closure during an apneic episode. Anterior-posterior pattern of closure is associated with greater UAS effectiveness compared with concentric pattern of airway closure. DISE is typically performed by the otolaryngologist scheduled to implant the UAS. However, nonsurgical physicians who are part of the patient’s care team can be trained to perform this procedure especially if they have experience in performing endoscopy of the upper airway (such as a pulmonary specialist). This can make the evaluation process more efficient and dramatically improve access to care.
Coordination of Care
In order for the UAS program to be successful, the patient’s care team has to work closely with the device manufacturer throughout the implantation pathway and for ongoing patient care. The device manufacturer can assist with education of HCPs, surgical physicians, clinical support staff, and the patient. However, an even more essential role for industry support is during UAS device activation and subsequent titration of UAS via an overnight in-laboratory sleep study.
After surgical implantation, the UAS device activation can be performed in the nonsurgical sleep clinic and is done about 1 month later. This period allows for tissue healing after the surgery and for the patient to get accustomed to having this new device in their body. This activation can be done with assistance from an industry technician until the HCP is comfortable with this process. The multidisciplinary UAS team could choose to delegate device activation to a technician with specialized relevant training, such as RPSGT or respiratory therapist (RT).
This procedure involves determination of sensory and functional threshold for UAS. Sensory threshold is minimum voltage required for the patient to feel the stimulation. The functional threshold is the minimum voltage required to move the tongue past the lower front teeth during stimulation. After these thresholds are established, a voltage range is set on the device. The voltage at functional threshold is typically set at the lower level of this range, and the maximum level is set at 1 volt higher. Patients are able to adjust voltage within this range and are instructed to increase the voltage gradually (0.1-volt increments) while maintaining levels that are comfortable during sleep.
About a month after device activation, patients undergo another overnight polysomnogram for titration of UAS device. In order to educate and train the institutional RPSGT on how to perform this type of titration, an industry technician is required for the first few overnight titrations. The goal of this study is to establish appropriate voltage to resolve sleep-disordered breathing and insure patient comfort at this setting. Patients typically leave the study with a new voltage range. They are asked to keep effective voltage in mind and make appropriate adjustments to maintain comfortable therapy.
Successful UAS therapy includes multiple steps, such as implantation, activation, and titration. This protocol requires effective coordination of care that includes communication with surgical staff, patients, support staff, and industry liaison. Nonsurgical sleep medicine physicians can play a vital role by helping to coordinate care at the early stages of UAS therapy and facilitate effective communication among various providers involved in this process.
Follow-Up
After completion of the initial therapeutic pathway, patients continue to follow up regularly, monitoring for AEs from UAS therapy and sleep apnea symptoms. Patients can be followed in the nonsurgical sleep clinic after the initial postoperative appointment with the surgeon. Frequency of follow-up depends on the presence and severity of any AEs and residual symptoms of sleep apnea. Even though most AEs related to UAS therapy reported in the STAR trial were nonserious and transient, 2% of participants required surgical revision.3 Therefore, maintaining open channels of communication among the entire UAS patient care team even months and years after surgical implantation is important. The nonsurgical sleep medicine physician who will continue to monitor the patient’s progress may need to consult with the surgical colleague or industry liaison at any point during treatment.
Limitations
This review outlines the UAS therapy pathway and emphasizes the role of the nonsurgical sleep medicine provider. However, the experience describes a UAS program development at a single VA medical center. Since this UAS device and therapy have already been approved by the VA on a national level, we did not face any challenges with authorization and insurance compensation. Therefore, this review does not provide any guidance with these matters. These are certainly common concerns for sleep medicine providers who offer UAS therapy in medical practices outside the VA, and these would hopefully be addressed in the future.
Furthermore, this review is based on the pulmonary sleep medicine provider’s experience and perspective. Therefore, certain aspects of UAS therapy could be better addressed by nonsurgical sleep medicine providers in different fields of expertise. For example, a study by a psychiatrist or psychologist could provide insight into the emotional concerns of patients who are undergoing this novel and life-altering treatment that includes surgical implantation of hardware into the body. A neurologist could explore the long-term effects of recurrent electrical stimulation on the autonomic and somatic nervous system as well as the musculature of the upper airway.
Conclusion
Multidisciplinary perspectives are needed to provide guidance for practitioners and institutions looking to set up and improve established UAS programs. As the long-term outcomes of the STAR trial continue to be published and provide more validation for UAS, this novel therapy will likely continue to gain acceptance as a safe and effective treatment for OSA.11
1. Young T, Palta M, Dempsey J, Peppard PE, Nieto FJ, Hla KM. Burden of sleep apnea: rationale, design, and major findings of the Wisconsin Sleep Cohort Study. WMJ. 2009;108(5):246-249.
2. Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006-1014.
3. Ancoli-Israel S, Kripke DF. Prevalent sleep problems in the aged. Biofeedback Self Regul. 1991;16(4):349-359.
4. Kreis P, Kripke DF, Ancoli-Israel S. Sleep apnea: a prospective study. West J Med. 1983;139(2):171-173.
5. Vimalananda VG, Miller DR, Christiansen CL, Wang W, Tremblay P, Fincke BG. Cardiovascular disease risk factors among women veterans at VA medical facilities. J Gen Intern Med. 2013;28 (suppl 2):S517-S523.
6. Functional and economic impact of sleep loss and sleep-related disorders. In: Colten HR, Altevogt BM, eds. Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem. National Academies Press; 2006:chap 4.
7. Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy. Proc Am Thorac Soc. 2008;5(2):173-178.
8. Stepnowsky C, Moore P. Nasal CPAP treatment for obstructive sleep apnea: developing a new perspective on dosing strategies and compliance. J Psychosom Res. 2003;54:599-605.
9. Wallace DM, Shafazand S, Aloia MS, Wohlgemuth WK. The association of age, insomnia, and self-efficacy with continuous positive airway pressure adherence in black, white, and Hispanic U.S. Veterans. J Clin Sleep Med. 2013;9(9):885-895.
10. Zozula R, Rosen R. Compliance with continuous positive pressure therapy: assessing and improving treatment outcomes. Curr Opin Pulm Med. 2001;7(6):391-398.
11. Strollo PJ Jr, Soose RJ, Maurer JT, et al; STAR Trial Group. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med. 2014;370(2):139-149.
12. Strollo PJ Jr, Gillespie MB, Soose RJ, et al; STAR Trial Group. Upper airway stimulation for obstructive sleep apnea: durability of the treatment effect at 18 months. Sleep. 2015;38(10):1593-1598.
13. Soose RJ, Woodson BT, Gillespie MB, et al; STAR Trial Investigators. Upper airway stimulation for obstructive sleep apnea: self-reported outcomes at 24 months. J Clin Sleep Med. 2016;12(1):43-48.
14. Woodson BT, Soose RJ, Gillespie MB, et al; STAR Trial Investigators. three-year outcomes of cranial nerve stimulation for obstructive sleep apnea: the STAR Trial. Otolaryngol Head Neck Surg. 2016;154(1):181-188.
15. Gillespie MB, Soose RJ, Woodson BT, et al; STAR Trial Investigators. Upper airway stimulation for obstructive sleep apnea: patient-reported outcomes after 48 months of follow-up. Otolaryngol Head Neck Surg. 2017;156(4):765-771.
16. Olbrisch ME, Benedict SM, Ashe K, Levenson JL. Psychological assessment and care of organ transplant patients. J Consult Clin Psychol. 2002;70(3):771-783.
Obstructive sleep apnea (OSA) is a common disorder in the US and other industrialized countries. The Wisconsin Sleep Cohort Study reported prevalence rates as high as 20% to 30% in men and 10% to 15% in women.1,2 Several studies have shown high prevalence of OSA among veterans. Ancoli-Israel and colleagues reported a OSA rate of 36% in a cohort of elderly patients at a US Department of Veterans Affairs (VA) medical center.3 A study by Kreis and colleagues showed that OSA was present in 27% of patients hospitalized on the medical ward at a VA hospital.4 Incidence of sleep apnea among veterans in the US will likely increase over time as obesity is becoming more prevalent. Rates of obesity have increased from 14% in 2000 to 18% in 2010 among both male and female veterans.5
Untreated OSA is associated with increased risk of coronary artery disease, cerebrovascular accidents, uncontrolled diabetes mellitus, and other complications. Patients with OSA are less productive, have increased health care utilization, and have a higher risk of motor vehicle accidents.6 Continuous positive airway pressure (CPAP) is the main form of treatment of OSA. However, despite the adverse outcomes of untreated sleep apnea, suboptimal CPAP adherence remains a major problem in clinical practice. When adherence is defined as > 4 hours of nightly use, 29% to 83% of patients with OSA have been reported to be nonadherent to treatment.7 Stepnowsky and colleagues estimated that 50% of patients with OSA for whom CPAP was recommended were no longer using it 1 year later.8 CPAP adherence among veterans also has been poor. Wallace and colleagues reported that about one-third of patients with OSA at a VA Miami Healthcare System had mean daily use ≥ 4 hours.9 Typical reasons for poor CPAP adherence include pressure intolerance, mask discomfort, nasal and oropharyngeal dryness and irritation.10 Development and implementation of alternate treatment strategies for OSA is important to reduce disease burden of this widespread and debilitating condition.
Upper airway stimulation (UAS) is a novel therapy for management of OSA that has been gaining popularity and acceptance within the sleep medicine community in the past few years. This treatment option involves implantation of a neurostimulator with a sensing lead and a stimulation lead. The device is similar to a pacemaker and is surgically implanted in chest wall. The sensing lead is placed close to the diaphragm for monitoring of pleural pressure to help assess ventilation. The stimulation lead is placed under the tongue in proximity to the hypoglossal nerve (cranial nerve XII). The neurostimulator delivers electrical pulses to the hypoglossal nerve through the stimulation lead. These stimulating pulses are synchronized with the ventilation detected by the sensing lead. This electrical stimulation results in anterior displacement of the tongue via action of the genioglossus and geniohyoid muscles. Mechanical coupling with the palate also is common and leads to additional airway opening within the oropharynx to prevent apneic episodes. The patient turns on the stimulation through the use of a portable remote control and is turned off in the morning. The patient is able to operate the UAS device by placing the remote control on the skin in proximity of the device. The patient also is able to adjust device voltage within a range set by their physician. The effective voltage range is determined via an overnight sleep study titration performed 1 month after device activation. UAS therapy is not considered first-line treatment for OSA as it requires surgical implantation under general anesthesia; however, it provides an alternative to patients with OSA who are unable to tolerate traditional therapy with CPAP.
The landmark Stimulation Therapy for Apnea Reduction (STAR) trial showed effectiveness of UAS therapy at 12 months postimplantation.11 Follow-up of these participants has proven the sustainability of this effect at 18, 24, 36, and 48 months of therapy.12-15 Inclusion criteria of the study was moderate-to-severe sleep apnea with predominantly obstructive events. Subjects were excluded if there were anatomical abnormalities of the upper airway or if the pattern of airway collapse was not conducive to UAS on sedated endoscopy evaluation. Participants in the trial were predominantly white males, the average age was 54.5 years, and the average body mass index (BMI) was 28.4. The outcomes measured included Functional Outcomes of Sleep Questionnaire, Epworth Sleepiness Scale (ESS), percentage of sleep time with oxygen saturation < 90%, and subjective snoring. All of these objective and subjective markers of sleep improved significantly with UAS therapy at 12 months and were maintained at improved levels at 48 months of therapy.
The adverse effects (AEs) associated with device implantation and subsequent UAS therapy have been infrequent and mostly transient. Out of 126 device implantations, there were 2 participants who had serious AEs due to implantation and required repositioning and fixation of the neurostimulator to resolve discomfort. Other AEs related to the procedure, including sore throat and muscle soreness, were considered nonserious and resolved with supportive care. AEs related to subsequent UAS therapy included temporary tongue weakness and tongue soreness/abrasion. These complications also have either resolved spontaneously or with use of supportive strategies such as a mouth guard. Due to the sustained clinical benefit and acceptable AE profile as demonstrated by the STAR trial, UAS has emerged as a realistic alternative for management of OSA.
Development of a successful program that provides and supports all aspects of UAS, including device implantation and follow-up, necessitates a multispecialty team approach. Ideally surgical and nonsurgical sleep physicians as well as clinical and administrative support staff should be part of this group.
This study is based on the experience of the development of the UAS program at the Clement J. Zablocki VA Medical Center (CJZ VAMC) in Milwaukee. Currently, there are 25 patients who are part of this UAS program. The inclusion and exclusion criteria were adopted from the STAR trial. The patient population is similar to the population in that trial. They are all white males with average age of 57.2 years and BMI of 31.3. The CJZVAMC UAS Program consists of multidisciplinary group of health care professionals. This article describes the role of a nonsurgical sleep medicine physician that was crucial in the development of this UAS program.
Process
Introduction of this novel alternative therapy has sparked much interest among health care providers (HCPs) at CJZVAMC. However, there has been much misunderstanding among patients and HCPs about what this treatment involves and how it is implemented. For example, many patients that called the sleep clinic to set up an evaluation for UAS did not realize that this is a surgical procedure that requires general anesthesia. One of the most important tasks for a nonsurgical sleep physician is to educate patients and HCPs about this therapy. Most of patient education at CJZVAMC has been done during individual clinic appointments; however, setting up group educational classes for patients is a more efficient strategy to deliver this information. Similarly, giving a lecture on UAS at medicine (or another specialty) grand rounds has been effective in the education of HCPs who refer patients to the sleep clinic. If possible, a combined lecture with a surgical colleague could provide a more balanced and complete depiction of UAS and help to answer a broader range of questions for the audience.
Screening
Screening and identification of appropriate candidates is an important first step in the patient pathway in the UAS therapy. Failure of CPAP therapy is a key starting point in this screening process. When patients present to the sleep clinic with difficulty tolerating CPAP therapy, an extensive and thorough troubleshooting process needs to take place to make sure that all CPAP options have been exhausted. This process would typically include trial of various masks, including different mask interfaces. A dedicated appointment with a registered polysomnographic technologist (RPSGT) or another clinic staff member with vast experience in PAP mask fitting is typically part of this effort.
Adjustment of CPAP pressure settings also may be helpful as high PAP pressure may be another obstacle. Patients frequently have trouble tolerating higher pressure settings especially when they are new to this therapy. Pressure restriction to 4-cm to 7-cm water pressure on auto CPAP has been a helpful technique to allow patients to become more comfortable with this therapy. Once patients are able to use PAP at lower pressures, these settings can be titrated up gradually for optimal effectiveness. Other desensitization techniques, such as use during daytime while distracted by other activities (such as watching TV) can be helpful in adjustment to PAP therapy. Addressing problems with nasal congestion can help improve PAP adherence. Finally, patients should be offered opportunities for education about their PAP machine on an ongoing basis. Lack of proficiency with humidifier use is a very common obstacle and frequently leads to PAP nonadherence. Teaching PAP operation should correspond to the patient’s level of education to be effective. PAP therapy remains the first-line treatment strategy for OSA as it is not invasive and highly effective. Nonsurgical sleep medicine physicians are uniquely positioned to implement and troubleshoot this therapy for sleep apnea patients before considering UAS.
As part of the screening process, it can be helpful to conduct routine multidisciplinary meetings to discuss patients who are being evaluated for UAS implantation. These meetings should include the otolaryngologist, nonsurgical sleep medicine physician, as well as additional staff (nurses, respiratory therapists, etc) who are involved in the UAS process. Having a mental health care provider as part of the multidisciplinary team during the screening process also could be a valuable addition as this specialist could evaluate and provide insight into a patient’s emotional status prior to implantation. This is common practice during evaluation for organ transplantation and would help to predict patient’s psychological well-being after this life-changing procedure.16 Having multidisciplinary agreement on patient’s candidacy for UAS therapy could improve long-term success of this treatment. Additionally, these multidisciplinary meetings as part of the UAS program can improve team camaraderie and prevent miscommunications during this therapy.
Drug-Induced Sedated Endoscopy
Patient pathway to neurostimulator implantation involves evaluation of the upper airway using drug-induced sedated endoscopy (DISE). This procedure helps determine whether the patient’s anatomy is appropriate for UAS. DISE also can evaluate the pattern of airway closure during an apneic episode. Anterior-posterior pattern of closure is associated with greater UAS effectiveness compared with concentric pattern of airway closure. DISE is typically performed by the otolaryngologist scheduled to implant the UAS. However, nonsurgical physicians who are part of the patient’s care team can be trained to perform this procedure especially if they have experience in performing endoscopy of the upper airway (such as a pulmonary specialist). This can make the evaluation process more efficient and dramatically improve access to care.
Coordination of Care
In order for the UAS program to be successful, the patient’s care team has to work closely with the device manufacturer throughout the implantation pathway and for ongoing patient care. The device manufacturer can assist with education of HCPs, surgical physicians, clinical support staff, and the patient. However, an even more essential role for industry support is during UAS device activation and subsequent titration of UAS via an overnight in-laboratory sleep study.
After surgical implantation, the UAS device activation can be performed in the nonsurgical sleep clinic and is done about 1 month later. This period allows for tissue healing after the surgery and for the patient to get accustomed to having this new device in their body. This activation can be done with assistance from an industry technician until the HCP is comfortable with this process. The multidisciplinary UAS team could choose to delegate device activation to a technician with specialized relevant training, such as RPSGT or respiratory therapist (RT).
This procedure involves determination of sensory and functional threshold for UAS. Sensory threshold is minimum voltage required for the patient to feel the stimulation. The functional threshold is the minimum voltage required to move the tongue past the lower front teeth during stimulation. After these thresholds are established, a voltage range is set on the device. The voltage at functional threshold is typically set at the lower level of this range, and the maximum level is set at 1 volt higher. Patients are able to adjust voltage within this range and are instructed to increase the voltage gradually (0.1-volt increments) while maintaining levels that are comfortable during sleep.
About a month after device activation, patients undergo another overnight polysomnogram for titration of UAS device. In order to educate and train the institutional RPSGT on how to perform this type of titration, an industry technician is required for the first few overnight titrations. The goal of this study is to establish appropriate voltage to resolve sleep-disordered breathing and insure patient comfort at this setting. Patients typically leave the study with a new voltage range. They are asked to keep effective voltage in mind and make appropriate adjustments to maintain comfortable therapy.
Successful UAS therapy includes multiple steps, such as implantation, activation, and titration. This protocol requires effective coordination of care that includes communication with surgical staff, patients, support staff, and industry liaison. Nonsurgical sleep medicine physicians can play a vital role by helping to coordinate care at the early stages of UAS therapy and facilitate effective communication among various providers involved in this process.
Follow-Up
After completion of the initial therapeutic pathway, patients continue to follow up regularly, monitoring for AEs from UAS therapy and sleep apnea symptoms. Patients can be followed in the nonsurgical sleep clinic after the initial postoperative appointment with the surgeon. Frequency of follow-up depends on the presence and severity of any AEs and residual symptoms of sleep apnea. Even though most AEs related to UAS therapy reported in the STAR trial were nonserious and transient, 2% of participants required surgical revision.3 Therefore, maintaining open channels of communication among the entire UAS patient care team even months and years after surgical implantation is important. The nonsurgical sleep medicine physician who will continue to monitor the patient’s progress may need to consult with the surgical colleague or industry liaison at any point during treatment.
Limitations
This review outlines the UAS therapy pathway and emphasizes the role of the nonsurgical sleep medicine provider. However, the experience describes a UAS program development at a single VA medical center. Since this UAS device and therapy have already been approved by the VA on a national level, we did not face any challenges with authorization and insurance compensation. Therefore, this review does not provide any guidance with these matters. These are certainly common concerns for sleep medicine providers who offer UAS therapy in medical practices outside the VA, and these would hopefully be addressed in the future.
Furthermore, this review is based on the pulmonary sleep medicine provider’s experience and perspective. Therefore, certain aspects of UAS therapy could be better addressed by nonsurgical sleep medicine providers in different fields of expertise. For example, a study by a psychiatrist or psychologist could provide insight into the emotional concerns of patients who are undergoing this novel and life-altering treatment that includes surgical implantation of hardware into the body. A neurologist could explore the long-term effects of recurrent electrical stimulation on the autonomic and somatic nervous system as well as the musculature of the upper airway.
Conclusion
Multidisciplinary perspectives are needed to provide guidance for practitioners and institutions looking to set up and improve established UAS programs. As the long-term outcomes of the STAR trial continue to be published and provide more validation for UAS, this novel therapy will likely continue to gain acceptance as a safe and effective treatment for OSA.11
Obstructive sleep apnea (OSA) is a common disorder in the US and other industrialized countries. The Wisconsin Sleep Cohort Study reported prevalence rates as high as 20% to 30% in men and 10% to 15% in women.1,2 Several studies have shown high prevalence of OSA among veterans. Ancoli-Israel and colleagues reported a OSA rate of 36% in a cohort of elderly patients at a US Department of Veterans Affairs (VA) medical center.3 A study by Kreis and colleagues showed that OSA was present in 27% of patients hospitalized on the medical ward at a VA hospital.4 Incidence of sleep apnea among veterans in the US will likely increase over time as obesity is becoming more prevalent. Rates of obesity have increased from 14% in 2000 to 18% in 2010 among both male and female veterans.5
Untreated OSA is associated with increased risk of coronary artery disease, cerebrovascular accidents, uncontrolled diabetes mellitus, and other complications. Patients with OSA are less productive, have increased health care utilization, and have a higher risk of motor vehicle accidents.6 Continuous positive airway pressure (CPAP) is the main form of treatment of OSA. However, despite the adverse outcomes of untreated sleep apnea, suboptimal CPAP adherence remains a major problem in clinical practice. When adherence is defined as > 4 hours of nightly use, 29% to 83% of patients with OSA have been reported to be nonadherent to treatment.7 Stepnowsky and colleagues estimated that 50% of patients with OSA for whom CPAP was recommended were no longer using it 1 year later.8 CPAP adherence among veterans also has been poor. Wallace and colleagues reported that about one-third of patients with OSA at a VA Miami Healthcare System had mean daily use ≥ 4 hours.9 Typical reasons for poor CPAP adherence include pressure intolerance, mask discomfort, nasal and oropharyngeal dryness and irritation.10 Development and implementation of alternate treatment strategies for OSA is important to reduce disease burden of this widespread and debilitating condition.
Upper airway stimulation (UAS) is a novel therapy for management of OSA that has been gaining popularity and acceptance within the sleep medicine community in the past few years. This treatment option involves implantation of a neurostimulator with a sensing lead and a stimulation lead. The device is similar to a pacemaker and is surgically implanted in chest wall. The sensing lead is placed close to the diaphragm for monitoring of pleural pressure to help assess ventilation. The stimulation lead is placed under the tongue in proximity to the hypoglossal nerve (cranial nerve XII). The neurostimulator delivers electrical pulses to the hypoglossal nerve through the stimulation lead. These stimulating pulses are synchronized with the ventilation detected by the sensing lead. This electrical stimulation results in anterior displacement of the tongue via action of the genioglossus and geniohyoid muscles. Mechanical coupling with the palate also is common and leads to additional airway opening within the oropharynx to prevent apneic episodes. The patient turns on the stimulation through the use of a portable remote control and is turned off in the morning. The patient is able to operate the UAS device by placing the remote control on the skin in proximity of the device. The patient also is able to adjust device voltage within a range set by their physician. The effective voltage range is determined via an overnight sleep study titration performed 1 month after device activation. UAS therapy is not considered first-line treatment for OSA as it requires surgical implantation under general anesthesia; however, it provides an alternative to patients with OSA who are unable to tolerate traditional therapy with CPAP.
The landmark Stimulation Therapy for Apnea Reduction (STAR) trial showed effectiveness of UAS therapy at 12 months postimplantation.11 Follow-up of these participants has proven the sustainability of this effect at 18, 24, 36, and 48 months of therapy.12-15 Inclusion criteria of the study was moderate-to-severe sleep apnea with predominantly obstructive events. Subjects were excluded if there were anatomical abnormalities of the upper airway or if the pattern of airway collapse was not conducive to UAS on sedated endoscopy evaluation. Participants in the trial were predominantly white males, the average age was 54.5 years, and the average body mass index (BMI) was 28.4. The outcomes measured included Functional Outcomes of Sleep Questionnaire, Epworth Sleepiness Scale (ESS), percentage of sleep time with oxygen saturation < 90%, and subjective snoring. All of these objective and subjective markers of sleep improved significantly with UAS therapy at 12 months and were maintained at improved levels at 48 months of therapy.
The adverse effects (AEs) associated with device implantation and subsequent UAS therapy have been infrequent and mostly transient. Out of 126 device implantations, there were 2 participants who had serious AEs due to implantation and required repositioning and fixation of the neurostimulator to resolve discomfort. Other AEs related to the procedure, including sore throat and muscle soreness, were considered nonserious and resolved with supportive care. AEs related to subsequent UAS therapy included temporary tongue weakness and tongue soreness/abrasion. These complications also have either resolved spontaneously or with use of supportive strategies such as a mouth guard. Due to the sustained clinical benefit and acceptable AE profile as demonstrated by the STAR trial, UAS has emerged as a realistic alternative for management of OSA.
Development of a successful program that provides and supports all aspects of UAS, including device implantation and follow-up, necessitates a multispecialty team approach. Ideally surgical and nonsurgical sleep physicians as well as clinical and administrative support staff should be part of this group.
This study is based on the experience of the development of the UAS program at the Clement J. Zablocki VA Medical Center (CJZ VAMC) in Milwaukee. Currently, there are 25 patients who are part of this UAS program. The inclusion and exclusion criteria were adopted from the STAR trial. The patient population is similar to the population in that trial. They are all white males with average age of 57.2 years and BMI of 31.3. The CJZVAMC UAS Program consists of multidisciplinary group of health care professionals. This article describes the role of a nonsurgical sleep medicine physician that was crucial in the development of this UAS program.
Process
Introduction of this novel alternative therapy has sparked much interest among health care providers (HCPs) at CJZVAMC. However, there has been much misunderstanding among patients and HCPs about what this treatment involves and how it is implemented. For example, many patients that called the sleep clinic to set up an evaluation for UAS did not realize that this is a surgical procedure that requires general anesthesia. One of the most important tasks for a nonsurgical sleep physician is to educate patients and HCPs about this therapy. Most of patient education at CJZVAMC has been done during individual clinic appointments; however, setting up group educational classes for patients is a more efficient strategy to deliver this information. Similarly, giving a lecture on UAS at medicine (or another specialty) grand rounds has been effective in the education of HCPs who refer patients to the sleep clinic. If possible, a combined lecture with a surgical colleague could provide a more balanced and complete depiction of UAS and help to answer a broader range of questions for the audience.
Screening
Screening and identification of appropriate candidates is an important first step in the patient pathway in the UAS therapy. Failure of CPAP therapy is a key starting point in this screening process. When patients present to the sleep clinic with difficulty tolerating CPAP therapy, an extensive and thorough troubleshooting process needs to take place to make sure that all CPAP options have been exhausted. This process would typically include trial of various masks, including different mask interfaces. A dedicated appointment with a registered polysomnographic technologist (RPSGT) or another clinic staff member with vast experience in PAP mask fitting is typically part of this effort.
Adjustment of CPAP pressure settings also may be helpful as high PAP pressure may be another obstacle. Patients frequently have trouble tolerating higher pressure settings especially when they are new to this therapy. Pressure restriction to 4-cm to 7-cm water pressure on auto CPAP has been a helpful technique to allow patients to become more comfortable with this therapy. Once patients are able to use PAP at lower pressures, these settings can be titrated up gradually for optimal effectiveness. Other desensitization techniques, such as use during daytime while distracted by other activities (such as watching TV) can be helpful in adjustment to PAP therapy. Addressing problems with nasal congestion can help improve PAP adherence. Finally, patients should be offered opportunities for education about their PAP machine on an ongoing basis. Lack of proficiency with humidifier use is a very common obstacle and frequently leads to PAP nonadherence. Teaching PAP operation should correspond to the patient’s level of education to be effective. PAP therapy remains the first-line treatment strategy for OSA as it is not invasive and highly effective. Nonsurgical sleep medicine physicians are uniquely positioned to implement and troubleshoot this therapy for sleep apnea patients before considering UAS.
As part of the screening process, it can be helpful to conduct routine multidisciplinary meetings to discuss patients who are being evaluated for UAS implantation. These meetings should include the otolaryngologist, nonsurgical sleep medicine physician, as well as additional staff (nurses, respiratory therapists, etc) who are involved in the UAS process. Having a mental health care provider as part of the multidisciplinary team during the screening process also could be a valuable addition as this specialist could evaluate and provide insight into a patient’s emotional status prior to implantation. This is common practice during evaluation for organ transplantation and would help to predict patient’s psychological well-being after this life-changing procedure.16 Having multidisciplinary agreement on patient’s candidacy for UAS therapy could improve long-term success of this treatment. Additionally, these multidisciplinary meetings as part of the UAS program can improve team camaraderie and prevent miscommunications during this therapy.
Drug-Induced Sedated Endoscopy
Patient pathway to neurostimulator implantation involves evaluation of the upper airway using drug-induced sedated endoscopy (DISE). This procedure helps determine whether the patient’s anatomy is appropriate for UAS. DISE also can evaluate the pattern of airway closure during an apneic episode. Anterior-posterior pattern of closure is associated with greater UAS effectiveness compared with concentric pattern of airway closure. DISE is typically performed by the otolaryngologist scheduled to implant the UAS. However, nonsurgical physicians who are part of the patient’s care team can be trained to perform this procedure especially if they have experience in performing endoscopy of the upper airway (such as a pulmonary specialist). This can make the evaluation process more efficient and dramatically improve access to care.
Coordination of Care
In order for the UAS program to be successful, the patient’s care team has to work closely with the device manufacturer throughout the implantation pathway and for ongoing patient care. The device manufacturer can assist with education of HCPs, surgical physicians, clinical support staff, and the patient. However, an even more essential role for industry support is during UAS device activation and subsequent titration of UAS via an overnight in-laboratory sleep study.
After surgical implantation, the UAS device activation can be performed in the nonsurgical sleep clinic and is done about 1 month later. This period allows for tissue healing after the surgery and for the patient to get accustomed to having this new device in their body. This activation can be done with assistance from an industry technician until the HCP is comfortable with this process. The multidisciplinary UAS team could choose to delegate device activation to a technician with specialized relevant training, such as RPSGT or respiratory therapist (RT).
This procedure involves determination of sensory and functional threshold for UAS. Sensory threshold is minimum voltage required for the patient to feel the stimulation. The functional threshold is the minimum voltage required to move the tongue past the lower front teeth during stimulation. After these thresholds are established, a voltage range is set on the device. The voltage at functional threshold is typically set at the lower level of this range, and the maximum level is set at 1 volt higher. Patients are able to adjust voltage within this range and are instructed to increase the voltage gradually (0.1-volt increments) while maintaining levels that are comfortable during sleep.
About a month after device activation, patients undergo another overnight polysomnogram for titration of UAS device. In order to educate and train the institutional RPSGT on how to perform this type of titration, an industry technician is required for the first few overnight titrations. The goal of this study is to establish appropriate voltage to resolve sleep-disordered breathing and insure patient comfort at this setting. Patients typically leave the study with a new voltage range. They are asked to keep effective voltage in mind and make appropriate adjustments to maintain comfortable therapy.
Successful UAS therapy includes multiple steps, such as implantation, activation, and titration. This protocol requires effective coordination of care that includes communication with surgical staff, patients, support staff, and industry liaison. Nonsurgical sleep medicine physicians can play a vital role by helping to coordinate care at the early stages of UAS therapy and facilitate effective communication among various providers involved in this process.
Follow-Up
After completion of the initial therapeutic pathway, patients continue to follow up regularly, monitoring for AEs from UAS therapy and sleep apnea symptoms. Patients can be followed in the nonsurgical sleep clinic after the initial postoperative appointment with the surgeon. Frequency of follow-up depends on the presence and severity of any AEs and residual symptoms of sleep apnea. Even though most AEs related to UAS therapy reported in the STAR trial were nonserious and transient, 2% of participants required surgical revision.3 Therefore, maintaining open channels of communication among the entire UAS patient care team even months and years after surgical implantation is important. The nonsurgical sleep medicine physician who will continue to monitor the patient’s progress may need to consult with the surgical colleague or industry liaison at any point during treatment.
Limitations
This review outlines the UAS therapy pathway and emphasizes the role of the nonsurgical sleep medicine provider. However, the experience describes a UAS program development at a single VA medical center. Since this UAS device and therapy have already been approved by the VA on a national level, we did not face any challenges with authorization and insurance compensation. Therefore, this review does not provide any guidance with these matters. These are certainly common concerns for sleep medicine providers who offer UAS therapy in medical practices outside the VA, and these would hopefully be addressed in the future.
Furthermore, this review is based on the pulmonary sleep medicine provider’s experience and perspective. Therefore, certain aspects of UAS therapy could be better addressed by nonsurgical sleep medicine providers in different fields of expertise. For example, a study by a psychiatrist or psychologist could provide insight into the emotional concerns of patients who are undergoing this novel and life-altering treatment that includes surgical implantation of hardware into the body. A neurologist could explore the long-term effects of recurrent electrical stimulation on the autonomic and somatic nervous system as well as the musculature of the upper airway.
Conclusion
Multidisciplinary perspectives are needed to provide guidance for practitioners and institutions looking to set up and improve established UAS programs. As the long-term outcomes of the STAR trial continue to be published and provide more validation for UAS, this novel therapy will likely continue to gain acceptance as a safe and effective treatment for OSA.11
1. Young T, Palta M, Dempsey J, Peppard PE, Nieto FJ, Hla KM. Burden of sleep apnea: rationale, design, and major findings of the Wisconsin Sleep Cohort Study. WMJ. 2009;108(5):246-249.
2. Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006-1014.
3. Ancoli-Israel S, Kripke DF. Prevalent sleep problems in the aged. Biofeedback Self Regul. 1991;16(4):349-359.
4. Kreis P, Kripke DF, Ancoli-Israel S. Sleep apnea: a prospective study. West J Med. 1983;139(2):171-173.
5. Vimalananda VG, Miller DR, Christiansen CL, Wang W, Tremblay P, Fincke BG. Cardiovascular disease risk factors among women veterans at VA medical facilities. J Gen Intern Med. 2013;28 (suppl 2):S517-S523.
6. Functional and economic impact of sleep loss and sleep-related disorders. In: Colten HR, Altevogt BM, eds. Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem. National Academies Press; 2006:chap 4.
7. Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy. Proc Am Thorac Soc. 2008;5(2):173-178.
8. Stepnowsky C, Moore P. Nasal CPAP treatment for obstructive sleep apnea: developing a new perspective on dosing strategies and compliance. J Psychosom Res. 2003;54:599-605.
9. Wallace DM, Shafazand S, Aloia MS, Wohlgemuth WK. The association of age, insomnia, and self-efficacy with continuous positive airway pressure adherence in black, white, and Hispanic U.S. Veterans. J Clin Sleep Med. 2013;9(9):885-895.
10. Zozula R, Rosen R. Compliance with continuous positive pressure therapy: assessing and improving treatment outcomes. Curr Opin Pulm Med. 2001;7(6):391-398.
11. Strollo PJ Jr, Soose RJ, Maurer JT, et al; STAR Trial Group. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med. 2014;370(2):139-149.
12. Strollo PJ Jr, Gillespie MB, Soose RJ, et al; STAR Trial Group. Upper airway stimulation for obstructive sleep apnea: durability of the treatment effect at 18 months. Sleep. 2015;38(10):1593-1598.
13. Soose RJ, Woodson BT, Gillespie MB, et al; STAR Trial Investigators. Upper airway stimulation for obstructive sleep apnea: self-reported outcomes at 24 months. J Clin Sleep Med. 2016;12(1):43-48.
14. Woodson BT, Soose RJ, Gillespie MB, et al; STAR Trial Investigators. three-year outcomes of cranial nerve stimulation for obstructive sleep apnea: the STAR Trial. Otolaryngol Head Neck Surg. 2016;154(1):181-188.
15. Gillespie MB, Soose RJ, Woodson BT, et al; STAR Trial Investigators. Upper airway stimulation for obstructive sleep apnea: patient-reported outcomes after 48 months of follow-up. Otolaryngol Head Neck Surg. 2017;156(4):765-771.
16. Olbrisch ME, Benedict SM, Ashe K, Levenson JL. Psychological assessment and care of organ transplant patients. J Consult Clin Psychol. 2002;70(3):771-783.
1. Young T, Palta M, Dempsey J, Peppard PE, Nieto FJ, Hla KM. Burden of sleep apnea: rationale, design, and major findings of the Wisconsin Sleep Cohort Study. WMJ. 2009;108(5):246-249.
2. Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006-1014.
3. Ancoli-Israel S, Kripke DF. Prevalent sleep problems in the aged. Biofeedback Self Regul. 1991;16(4):349-359.
4. Kreis P, Kripke DF, Ancoli-Israel S. Sleep apnea: a prospective study. West J Med. 1983;139(2):171-173.
5. Vimalananda VG, Miller DR, Christiansen CL, Wang W, Tremblay P, Fincke BG. Cardiovascular disease risk factors among women veterans at VA medical facilities. J Gen Intern Med. 2013;28 (suppl 2):S517-S523.
6. Functional and economic impact of sleep loss and sleep-related disorders. In: Colten HR, Altevogt BM, eds. Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem. National Academies Press; 2006:chap 4.
7. Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy. Proc Am Thorac Soc. 2008;5(2):173-178.
8. Stepnowsky C, Moore P. Nasal CPAP treatment for obstructive sleep apnea: developing a new perspective on dosing strategies and compliance. J Psychosom Res. 2003;54:599-605.
9. Wallace DM, Shafazand S, Aloia MS, Wohlgemuth WK. The association of age, insomnia, and self-efficacy with continuous positive airway pressure adherence in black, white, and Hispanic U.S. Veterans. J Clin Sleep Med. 2013;9(9):885-895.
10. Zozula R, Rosen R. Compliance with continuous positive pressure therapy: assessing and improving treatment outcomes. Curr Opin Pulm Med. 2001;7(6):391-398.
11. Strollo PJ Jr, Soose RJ, Maurer JT, et al; STAR Trial Group. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med. 2014;370(2):139-149.
12. Strollo PJ Jr, Gillespie MB, Soose RJ, et al; STAR Trial Group. Upper airway stimulation for obstructive sleep apnea: durability of the treatment effect at 18 months. Sleep. 2015;38(10):1593-1598.
13. Soose RJ, Woodson BT, Gillespie MB, et al; STAR Trial Investigators. Upper airway stimulation for obstructive sleep apnea: self-reported outcomes at 24 months. J Clin Sleep Med. 2016;12(1):43-48.
14. Woodson BT, Soose RJ, Gillespie MB, et al; STAR Trial Investigators. three-year outcomes of cranial nerve stimulation for obstructive sleep apnea: the STAR Trial. Otolaryngol Head Neck Surg. 2016;154(1):181-188.
15. Gillespie MB, Soose RJ, Woodson BT, et al; STAR Trial Investigators. Upper airway stimulation for obstructive sleep apnea: patient-reported outcomes after 48 months of follow-up. Otolaryngol Head Neck Surg. 2017;156(4):765-771.
16. Olbrisch ME, Benedict SM, Ashe K, Levenson JL. Psychological assessment and care of organ transplant patients. J Consult Clin Psychol. 2002;70(3):771-783.
Adolescents at risk of nutritional deficiencies after bariatric surgery
In a 5-year prospective study, more than a quarter of the participants who underwent vertical sleeve gastrectomy (VSG) developed two or more nutritional deficiencies, reported lead author Stavra A. Xanthakos, MD, of the Cincinnati Children’s Hospital Medical Center, and colleagues.
“Although prevalence of nutritional deficiencies has been estimated largely from adult cohorts, bariatric surgery is an increasingly accepted treatment for severe obesity in youth,” the investigators wrote in Clinical Gastroenterology and Hepatology. “Yet, lower adherence to supplementation and anticipated longer lifespan with altered gastrointestinal physiology may increase risk of adverse nutritional outcomes in these youth.”
Previous research has suggested that teens may be at higher risk for nutritional deficiencies, but these studies were largely retrospective, or when prospective, lacked sufficient long-term follow-up, analysis of comprehensive patient factors, or inclusion of VSG, which is now the predominant technique in the field, the investigators noted.
“Our study is the first to assess comparative nutritional outcomes in adolescents after both VSG and gastric bypass,” they wrote.
The study involved 226 participants aged 13-19 years who underwent either Roux-en-Y gastric bypass (n = 161) or VSG (n = 67) at five tertiary-care centers in the United States during 2007-2012.
Six months after surgery, at 12 months, and on an annual basis thereafter, the investigators gathered clinical data and measured participant serum levels of ferritin; transferrin; albumin; parathyroid hormone; C-reactive protein; and vitamins A, D, B1, B12, and folate. Analyses also included sex, age, ethnicity, race, household demographics, weight, height, comorbidities, and body mass index (BMI).
The majority of participants were female (75%) and white (72%). At baseline, mean BMI and age were 52.7 kg/m2 and 16.5 years, respectively. After 5 years, mean body mass index decreased 23% without a significant difference between procedures.
Generally, nutritional deficiencies occurred earlier and were more common after gastric bypass, although both procedures were ultimately associated with increased risks.
In the gastric bypass group, 59% of participants had two or more nutritional deficiencies at 5 years, and 19% had three more deficiencies, which represented increased rates of fivefold and sixfold, respectively, which the investigators described as “striking.” In the VSG group, 27% of patients had two or more nutritional deficiencies at 5 years; while this fourfold increase was not statistically significant, the investigators suggested that it indicated “a lower, but not negligible, nutritional risk.”
Hypoferritinemia was particularly common in both groups, with rates at year 5 of 71% and 45% among patients who underwent gastric bypass and VSG, respectively.
“Our results now provide critical evidence that VSG does in fact carry significantly lower nutritional risk than Roux-en-Y gastric bypass, but can still worsen iron status,” the investigators wrote.
The investigators also highlighted a nonsignificant increase in the incidence of vitamin B12 deficiency among patients who underwent gastric bypass, with rates increasing from 0.6% at baseline to 11.5% at 5 years.
“Vitamin B12 status likewise worsened disproportionately after [gastric bypass], despite similar trajectories of weight loss after VSG,” the investigators wrote. “This suggests that the differential risk is caused by anatomic and physiological differences between procedures, rather than weight loss alone.”
Beyond surgery type, risk factors for nutritional deficiency included inadequate supplement intake, pregnancy, weight regain, and black race.
“Our findings underscore the importance of long-term nutritional monitoring in adolescents after bariatric surgery and the need to examine impact on health outcomes and quality of life as these youth advance into adulthood, including systematic assessment of anemia and bone health,” the investigators concluded.
The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases and the National Center for Advancing Translational Sciences of the National Institutes of Health. Dr. Courcoulas reported grant support from Allurion.
SOURCE: Xanthakos SA et al. Clin Gastro Hepatol. 2019 Nov 6. doi: 10.1016/j.cgh.2019.10.048.
The prevalence of obesity in adolescents has ballooned to about 20% of children aged 12-19 years. Prevention with diet and exercise remains the cornerstone of obesity policy in the pediatric population. Once patients develop obesity, however, bariatric surgery increasingly is being recommended as a treatment to achieve durable weight loss. Multiple large studies in adults have shown strong evidence of the efficacy of bariatric surgery; comparable data in pediatric patients has been sparse.
The current study by Xanthakos et al. reports on 5-year prospective data from Teen-LABS specifically addressing the nutritional status of adolescents after Roux-en-Y gastric bypass and sleeve gastrectomy. Their data show deficiency only in iron and vitamin B12 levels after gastric bypass. More importantly, vertical sleeve gastrectomy, now the most common procedure, results in decreased risk of nutritional deficiencies compared with gastric bypass. These data add to the reassurance that surgical treatment in the adolescent population is overall safe and should be considered strongly after appropriate counseling.
Wasif M. Abidi, MD, PhD, is an assistant professor of medicine, section of gastroenterology and hepatology, Baylor College of Medicine, Houston. He has received research support from GI Dynamics.
The prevalence of obesity in adolescents has ballooned to about 20% of children aged 12-19 years. Prevention with diet and exercise remains the cornerstone of obesity policy in the pediatric population. Once patients develop obesity, however, bariatric surgery increasingly is being recommended as a treatment to achieve durable weight loss. Multiple large studies in adults have shown strong evidence of the efficacy of bariatric surgery; comparable data in pediatric patients has been sparse.
The current study by Xanthakos et al. reports on 5-year prospective data from Teen-LABS specifically addressing the nutritional status of adolescents after Roux-en-Y gastric bypass and sleeve gastrectomy. Their data show deficiency only in iron and vitamin B12 levels after gastric bypass. More importantly, vertical sleeve gastrectomy, now the most common procedure, results in decreased risk of nutritional deficiencies compared with gastric bypass. These data add to the reassurance that surgical treatment in the adolescent population is overall safe and should be considered strongly after appropriate counseling.
Wasif M. Abidi, MD, PhD, is an assistant professor of medicine, section of gastroenterology and hepatology, Baylor College of Medicine, Houston. He has received research support from GI Dynamics.
The prevalence of obesity in adolescents has ballooned to about 20% of children aged 12-19 years. Prevention with diet and exercise remains the cornerstone of obesity policy in the pediatric population. Once patients develop obesity, however, bariatric surgery increasingly is being recommended as a treatment to achieve durable weight loss. Multiple large studies in adults have shown strong evidence of the efficacy of bariatric surgery; comparable data in pediatric patients has been sparse.
The current study by Xanthakos et al. reports on 5-year prospective data from Teen-LABS specifically addressing the nutritional status of adolescents after Roux-en-Y gastric bypass and sleeve gastrectomy. Their data show deficiency only in iron and vitamin B12 levels after gastric bypass. More importantly, vertical sleeve gastrectomy, now the most common procedure, results in decreased risk of nutritional deficiencies compared with gastric bypass. These data add to the reassurance that surgical treatment in the adolescent population is overall safe and should be considered strongly after appropriate counseling.
Wasif M. Abidi, MD, PhD, is an assistant professor of medicine, section of gastroenterology and hepatology, Baylor College of Medicine, Houston. He has received research support from GI Dynamics.
In a 5-year prospective study, more than a quarter of the participants who underwent vertical sleeve gastrectomy (VSG) developed two or more nutritional deficiencies, reported lead author Stavra A. Xanthakos, MD, of the Cincinnati Children’s Hospital Medical Center, and colleagues.
“Although prevalence of nutritional deficiencies has been estimated largely from adult cohorts, bariatric surgery is an increasingly accepted treatment for severe obesity in youth,” the investigators wrote in Clinical Gastroenterology and Hepatology. “Yet, lower adherence to supplementation and anticipated longer lifespan with altered gastrointestinal physiology may increase risk of adverse nutritional outcomes in these youth.”
Previous research has suggested that teens may be at higher risk for nutritional deficiencies, but these studies were largely retrospective, or when prospective, lacked sufficient long-term follow-up, analysis of comprehensive patient factors, or inclusion of VSG, which is now the predominant technique in the field, the investigators noted.
“Our study is the first to assess comparative nutritional outcomes in adolescents after both VSG and gastric bypass,” they wrote.
The study involved 226 participants aged 13-19 years who underwent either Roux-en-Y gastric bypass (n = 161) or VSG (n = 67) at five tertiary-care centers in the United States during 2007-2012.
Six months after surgery, at 12 months, and on an annual basis thereafter, the investigators gathered clinical data and measured participant serum levels of ferritin; transferrin; albumin; parathyroid hormone; C-reactive protein; and vitamins A, D, B1, B12, and folate. Analyses also included sex, age, ethnicity, race, household demographics, weight, height, comorbidities, and body mass index (BMI).
The majority of participants were female (75%) and white (72%). At baseline, mean BMI and age were 52.7 kg/m2 and 16.5 years, respectively. After 5 years, mean body mass index decreased 23% without a significant difference between procedures.
Generally, nutritional deficiencies occurred earlier and were more common after gastric bypass, although both procedures were ultimately associated with increased risks.
In the gastric bypass group, 59% of participants had two or more nutritional deficiencies at 5 years, and 19% had three more deficiencies, which represented increased rates of fivefold and sixfold, respectively, which the investigators described as “striking.” In the VSG group, 27% of patients had two or more nutritional deficiencies at 5 years; while this fourfold increase was not statistically significant, the investigators suggested that it indicated “a lower, but not negligible, nutritional risk.”
Hypoferritinemia was particularly common in both groups, with rates at year 5 of 71% and 45% among patients who underwent gastric bypass and VSG, respectively.
“Our results now provide critical evidence that VSG does in fact carry significantly lower nutritional risk than Roux-en-Y gastric bypass, but can still worsen iron status,” the investigators wrote.
The investigators also highlighted a nonsignificant increase in the incidence of vitamin B12 deficiency among patients who underwent gastric bypass, with rates increasing from 0.6% at baseline to 11.5% at 5 years.
“Vitamin B12 status likewise worsened disproportionately after [gastric bypass], despite similar trajectories of weight loss after VSG,” the investigators wrote. “This suggests that the differential risk is caused by anatomic and physiological differences between procedures, rather than weight loss alone.”
Beyond surgery type, risk factors for nutritional deficiency included inadequate supplement intake, pregnancy, weight regain, and black race.
“Our findings underscore the importance of long-term nutritional monitoring in adolescents after bariatric surgery and the need to examine impact on health outcomes and quality of life as these youth advance into adulthood, including systematic assessment of anemia and bone health,” the investigators concluded.
The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases and the National Center for Advancing Translational Sciences of the National Institutes of Health. Dr. Courcoulas reported grant support from Allurion.
SOURCE: Xanthakos SA et al. Clin Gastro Hepatol. 2019 Nov 6. doi: 10.1016/j.cgh.2019.10.048.
In a 5-year prospective study, more than a quarter of the participants who underwent vertical sleeve gastrectomy (VSG) developed two or more nutritional deficiencies, reported lead author Stavra A. Xanthakos, MD, of the Cincinnati Children’s Hospital Medical Center, and colleagues.
“Although prevalence of nutritional deficiencies has been estimated largely from adult cohorts, bariatric surgery is an increasingly accepted treatment for severe obesity in youth,” the investigators wrote in Clinical Gastroenterology and Hepatology. “Yet, lower adherence to supplementation and anticipated longer lifespan with altered gastrointestinal physiology may increase risk of adverse nutritional outcomes in these youth.”
Previous research has suggested that teens may be at higher risk for nutritional deficiencies, but these studies were largely retrospective, or when prospective, lacked sufficient long-term follow-up, analysis of comprehensive patient factors, or inclusion of VSG, which is now the predominant technique in the field, the investigators noted.
“Our study is the first to assess comparative nutritional outcomes in adolescents after both VSG and gastric bypass,” they wrote.
The study involved 226 participants aged 13-19 years who underwent either Roux-en-Y gastric bypass (n = 161) or VSG (n = 67) at five tertiary-care centers in the United States during 2007-2012.
Six months after surgery, at 12 months, and on an annual basis thereafter, the investigators gathered clinical data and measured participant serum levels of ferritin; transferrin; albumin; parathyroid hormone; C-reactive protein; and vitamins A, D, B1, B12, and folate. Analyses also included sex, age, ethnicity, race, household demographics, weight, height, comorbidities, and body mass index (BMI).
The majority of participants were female (75%) and white (72%). At baseline, mean BMI and age were 52.7 kg/m2 and 16.5 years, respectively. After 5 years, mean body mass index decreased 23% without a significant difference between procedures.
Generally, nutritional deficiencies occurred earlier and were more common after gastric bypass, although both procedures were ultimately associated with increased risks.
In the gastric bypass group, 59% of participants had two or more nutritional deficiencies at 5 years, and 19% had three more deficiencies, which represented increased rates of fivefold and sixfold, respectively, which the investigators described as “striking.” In the VSG group, 27% of patients had two or more nutritional deficiencies at 5 years; while this fourfold increase was not statistically significant, the investigators suggested that it indicated “a lower, but not negligible, nutritional risk.”
Hypoferritinemia was particularly common in both groups, with rates at year 5 of 71% and 45% among patients who underwent gastric bypass and VSG, respectively.
“Our results now provide critical evidence that VSG does in fact carry significantly lower nutritional risk than Roux-en-Y gastric bypass, but can still worsen iron status,” the investigators wrote.
The investigators also highlighted a nonsignificant increase in the incidence of vitamin B12 deficiency among patients who underwent gastric bypass, with rates increasing from 0.6% at baseline to 11.5% at 5 years.
“Vitamin B12 status likewise worsened disproportionately after [gastric bypass], despite similar trajectories of weight loss after VSG,” the investigators wrote. “This suggests that the differential risk is caused by anatomic and physiological differences between procedures, rather than weight loss alone.”
Beyond surgery type, risk factors for nutritional deficiency included inadequate supplement intake, pregnancy, weight regain, and black race.
“Our findings underscore the importance of long-term nutritional monitoring in adolescents after bariatric surgery and the need to examine impact on health outcomes and quality of life as these youth advance into adulthood, including systematic assessment of anemia and bone health,” the investigators concluded.
The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases and the National Center for Advancing Translational Sciences of the National Institutes of Health. Dr. Courcoulas reported grant support from Allurion.
SOURCE: Xanthakos SA et al. Clin Gastro Hepatol. 2019 Nov 6. doi: 10.1016/j.cgh.2019.10.048.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
NCCN panel: Defer nonurgent skin cancer care during pandemic
Amid the except when metastatic nodes are threatening vital structures or neoadjuvant therapy is not possible or has already failed, the National Comprehensive Cancer Network said in a new document about managing melanoma during the pandemic.
“The NCCN Melanoma Panel does not consider neoadjuvant therapy as a superior option to surgery followed by systemic adjuvant therapy for stage III melanoma, but available data suggest this is a reasonable resource-conserving option during the COVID-19 outbreak,” according to the panel. Surgery should be performed 8-9 weeks after initiation, said the group, an alliance of physicians from 30 U.S. cancer centers.
Echoing pandemic advice from other medical fields, the group’s melanoma recommendations focused on deferring nonurgent care until after the pandemic passes, and in the meantime limiting patient contact with the medical system and preserving hospital resources by, for instance, using telemedicine and opting for treatment regimens that require fewer trips to the clinic.
In a separate document on nonmelanoma skin cancer (NMSC), the group said that, with the exception of Merkel cell carcinoma, excisions for NMSC – including basal and squamous cell carcinoma, dermatofibrosarcoma protuberans, and rare tumors – should also generally be postponed during the pandemic.
The exception is if there is a risk of metastases within 3 months, but “such estimations of risks ... should be weighed against risks of the patient contracting COVID-19 infection or asymptomatically transmitting COVID-19 to health care workers,” the panel said.
Along the same lines, adjuvant therapy after surgical clearance of localized NMSC “should generally not be undertaken given the multiple visits required,” except for more extensive disease.
For primary cutaneous melanoma , “most time-to-treat studies show no adverse patient outcomes following a 90-day treatment delay, even for thicker [cutaneous melanoma],” the group said, so it recommended delaying wide excisions for melanoma in situ, lesions no thicker than 1 mm (T1) so long as the biopsy removed most of the lesion, and invasive melanomas of any depth if the biopsy had clear margins or only peripheral transection of the in situ component. They said sentinel lymph node biopsy can also be delayed for up to 3 months.
Resections for metastatic stage III-IV disease should also be put on hold unless the patient is symptomatic; systemic treatments should instead be continued. However, “given hospital-intensive resources, the use of talimogene laherparepvec for cutaneous/nodal/in-transit metastasis should be cautiously considered and, if possible, deferred until the COVID-19 crisis abates. A single dose of palliative radiation therapy may be useful for larger/symptomatic metastasis, as appropriate,” the group said.
If resection is still a go, the group noted that adjuvant therapy “has not been shown to improve melanoma-specific survival and should be deferred during the COVID-19 pandemic for patients with [a less than] 50% chance of disease relapse.” Dabrafenib/trametinib is the evidence-based choice if adjuvant treatment is opted for, but “alternative BRAF/MEK inhibitor regimens (encorafenib/binimetinib or vemurafenib/cobimetinib) may be substituted if drug supply is limited” by the pandemic, the group said.
For stage IV melanoma, “single-agent anti-PD-1 [programmed cell death 1] is recommended over combination ipilimumab/nivolumab at present” because there’s less inflammation and possible exacerbation of COVID-19, less need for steroids to counter adverse events, and less need for follow up to check for toxicities.
The group said evidence supports that 400 mg pembrolizumab administered intravenously every 6 weeks would likely be as effective as 200 mg intravenously every 3 weeks and would help keep people out of the hospital.
However, for stage IV melanoma with brain metastasis, there’s a strong rate of response to ipilimumab/nivolumab, so it may still be an option. In that case, “a regimen of ipilimumab 1 mg/kg and nivolumab 3 mg/kg every 3 weeks for four infusions, with subsequent consideration for nivolumab monotherapy, is associated with lower rates of immune-mediated toxicity,” compared with standard dosing.
Regarding potential drug shortages, the group noted that encorafenib/binimetinib or vemurafenib/cobimetinib combinations can be substituted for dabrafenib/trametinib for adjuvant therapy, and single-agent BRAF inhibitors can be used in the event of MEK inhibitor shortages.
In hospice, the group said oral temozolomide is the preferred option for palliative chemotherapy since it would limit resource utilization and contact with the medical system.
Amid the except when metastatic nodes are threatening vital structures or neoadjuvant therapy is not possible or has already failed, the National Comprehensive Cancer Network said in a new document about managing melanoma during the pandemic.
“The NCCN Melanoma Panel does not consider neoadjuvant therapy as a superior option to surgery followed by systemic adjuvant therapy for stage III melanoma, but available data suggest this is a reasonable resource-conserving option during the COVID-19 outbreak,” according to the panel. Surgery should be performed 8-9 weeks after initiation, said the group, an alliance of physicians from 30 U.S. cancer centers.
Echoing pandemic advice from other medical fields, the group’s melanoma recommendations focused on deferring nonurgent care until after the pandemic passes, and in the meantime limiting patient contact with the medical system and preserving hospital resources by, for instance, using telemedicine and opting for treatment regimens that require fewer trips to the clinic.
In a separate document on nonmelanoma skin cancer (NMSC), the group said that, with the exception of Merkel cell carcinoma, excisions for NMSC – including basal and squamous cell carcinoma, dermatofibrosarcoma protuberans, and rare tumors – should also generally be postponed during the pandemic.
The exception is if there is a risk of metastases within 3 months, but “such estimations of risks ... should be weighed against risks of the patient contracting COVID-19 infection or asymptomatically transmitting COVID-19 to health care workers,” the panel said.
Along the same lines, adjuvant therapy after surgical clearance of localized NMSC “should generally not be undertaken given the multiple visits required,” except for more extensive disease.
For primary cutaneous melanoma , “most time-to-treat studies show no adverse patient outcomes following a 90-day treatment delay, even for thicker [cutaneous melanoma],” the group said, so it recommended delaying wide excisions for melanoma in situ, lesions no thicker than 1 mm (T1) so long as the biopsy removed most of the lesion, and invasive melanomas of any depth if the biopsy had clear margins or only peripheral transection of the in situ component. They said sentinel lymph node biopsy can also be delayed for up to 3 months.
Resections for metastatic stage III-IV disease should also be put on hold unless the patient is symptomatic; systemic treatments should instead be continued. However, “given hospital-intensive resources, the use of talimogene laherparepvec for cutaneous/nodal/in-transit metastasis should be cautiously considered and, if possible, deferred until the COVID-19 crisis abates. A single dose of palliative radiation therapy may be useful for larger/symptomatic metastasis, as appropriate,” the group said.
If resection is still a go, the group noted that adjuvant therapy “has not been shown to improve melanoma-specific survival and should be deferred during the COVID-19 pandemic for patients with [a less than] 50% chance of disease relapse.” Dabrafenib/trametinib is the evidence-based choice if adjuvant treatment is opted for, but “alternative BRAF/MEK inhibitor regimens (encorafenib/binimetinib or vemurafenib/cobimetinib) may be substituted if drug supply is limited” by the pandemic, the group said.
For stage IV melanoma, “single-agent anti-PD-1 [programmed cell death 1] is recommended over combination ipilimumab/nivolumab at present” because there’s less inflammation and possible exacerbation of COVID-19, less need for steroids to counter adverse events, and less need for follow up to check for toxicities.
The group said evidence supports that 400 mg pembrolizumab administered intravenously every 6 weeks would likely be as effective as 200 mg intravenously every 3 weeks and would help keep people out of the hospital.
However, for stage IV melanoma with brain metastasis, there’s a strong rate of response to ipilimumab/nivolumab, so it may still be an option. In that case, “a regimen of ipilimumab 1 mg/kg and nivolumab 3 mg/kg every 3 weeks for four infusions, with subsequent consideration for nivolumab monotherapy, is associated with lower rates of immune-mediated toxicity,” compared with standard dosing.
Regarding potential drug shortages, the group noted that encorafenib/binimetinib or vemurafenib/cobimetinib combinations can be substituted for dabrafenib/trametinib for adjuvant therapy, and single-agent BRAF inhibitors can be used in the event of MEK inhibitor shortages.
In hospice, the group said oral temozolomide is the preferred option for palliative chemotherapy since it would limit resource utilization and contact with the medical system.
Amid the except when metastatic nodes are threatening vital structures or neoadjuvant therapy is not possible or has already failed, the National Comprehensive Cancer Network said in a new document about managing melanoma during the pandemic.
“The NCCN Melanoma Panel does not consider neoadjuvant therapy as a superior option to surgery followed by systemic adjuvant therapy for stage III melanoma, but available data suggest this is a reasonable resource-conserving option during the COVID-19 outbreak,” according to the panel. Surgery should be performed 8-9 weeks after initiation, said the group, an alliance of physicians from 30 U.S. cancer centers.
Echoing pandemic advice from other medical fields, the group’s melanoma recommendations focused on deferring nonurgent care until after the pandemic passes, and in the meantime limiting patient contact with the medical system and preserving hospital resources by, for instance, using telemedicine and opting for treatment regimens that require fewer trips to the clinic.
In a separate document on nonmelanoma skin cancer (NMSC), the group said that, with the exception of Merkel cell carcinoma, excisions for NMSC – including basal and squamous cell carcinoma, dermatofibrosarcoma protuberans, and rare tumors – should also generally be postponed during the pandemic.
The exception is if there is a risk of metastases within 3 months, but “such estimations of risks ... should be weighed against risks of the patient contracting COVID-19 infection or asymptomatically transmitting COVID-19 to health care workers,” the panel said.
Along the same lines, adjuvant therapy after surgical clearance of localized NMSC “should generally not be undertaken given the multiple visits required,” except for more extensive disease.
For primary cutaneous melanoma , “most time-to-treat studies show no adverse patient outcomes following a 90-day treatment delay, even for thicker [cutaneous melanoma],” the group said, so it recommended delaying wide excisions for melanoma in situ, lesions no thicker than 1 mm (T1) so long as the biopsy removed most of the lesion, and invasive melanomas of any depth if the biopsy had clear margins or only peripheral transection of the in situ component. They said sentinel lymph node biopsy can also be delayed for up to 3 months.
Resections for metastatic stage III-IV disease should also be put on hold unless the patient is symptomatic; systemic treatments should instead be continued. However, “given hospital-intensive resources, the use of talimogene laherparepvec for cutaneous/nodal/in-transit metastasis should be cautiously considered and, if possible, deferred until the COVID-19 crisis abates. A single dose of palliative radiation therapy may be useful for larger/symptomatic metastasis, as appropriate,” the group said.
If resection is still a go, the group noted that adjuvant therapy “has not been shown to improve melanoma-specific survival and should be deferred during the COVID-19 pandemic for patients with [a less than] 50% chance of disease relapse.” Dabrafenib/trametinib is the evidence-based choice if adjuvant treatment is opted for, but “alternative BRAF/MEK inhibitor regimens (encorafenib/binimetinib or vemurafenib/cobimetinib) may be substituted if drug supply is limited” by the pandemic, the group said.
For stage IV melanoma, “single-agent anti-PD-1 [programmed cell death 1] is recommended over combination ipilimumab/nivolumab at present” because there’s less inflammation and possible exacerbation of COVID-19, less need for steroids to counter adverse events, and less need for follow up to check for toxicities.
The group said evidence supports that 400 mg pembrolizumab administered intravenously every 6 weeks would likely be as effective as 200 mg intravenously every 3 weeks and would help keep people out of the hospital.
However, for stage IV melanoma with brain metastasis, there’s a strong rate of response to ipilimumab/nivolumab, so it may still be an option. In that case, “a regimen of ipilimumab 1 mg/kg and nivolumab 3 mg/kg every 3 weeks for four infusions, with subsequent consideration for nivolumab monotherapy, is associated with lower rates of immune-mediated toxicity,” compared with standard dosing.
Regarding potential drug shortages, the group noted that encorafenib/binimetinib or vemurafenib/cobimetinib combinations can be substituted for dabrafenib/trametinib for adjuvant therapy, and single-agent BRAF inhibitors can be used in the event of MEK inhibitor shortages.
In hospice, the group said oral temozolomide is the preferred option for palliative chemotherapy since it would limit resource utilization and contact with the medical system.
Incidence of Chronic Opioid Use in Previously Opioid-Naïve Patients Receiving Opioids for Analgesia in the Intensive Care Unit
Chronic pain is a worldwide cause of impairment. According to data from the 2016 National Health Interview Survey, an estimated 50 million American adults suffer from chronic pain, with 19.6 million adults suffering from high-impact chronic pain.1 This phenomenon is particularly prevalent in the older population. More than 25% of adults aged 65 to 74 years reported that they were often in pain in the past 3 months compared with just 10% of those adults between the ages of 18 and 44 years.2
The economic burdens of chronic pain disorders are well known. In 2010, Gaskin and Richard found that chronic pain has far-reaching consequences for the US economy, ranging from direct health care costs to lost productivity. This study estimated additional health care costs at about $300 billion yearly and lost productivity at $300 billion, bringing total annual costs to about $600 billion. This expense is more than heart disease alone ($309 billion), and cancer and diabetes mellitus ($243 billion and $188 billion respectively) combined.3
Opioid medications are powerful and effective pain-reducing agents that are indicated for short-term acute pain or long-term in the management of chronic, severe cancer-related pain.4 Although efficacious, use of these medications carries with it the inherent risks of abuse, misuse, addiction, and overdose.5 Since 1999, opioid-related overdose deaths have been on the rise. The CDC estimated that > 15,000 deaths were attributable specifically to prescription opioids in 2015.6 The estimates had risen to > 17,000 deaths in 2017, with the number increasing since that time.7 Cumulatively, the CDC estimates that > 200,000 deaths in the US between 1999 and 2017 are attributed to prescription opioid overdose, clearly marking this trend as a growing nationwide epidemic.8
In 2016, Florence and colleagues estimated costs associated with opioid overdose to be just shy of $80 billion in 2013 dollars.9 In October 2017, the US Department of Health and Human Services declared the opioid epidemic a public health emergency and committed $900 million to combating the crisis.10
An abundance of data exist analyzing outpatient prescribing and its impacts on opioid dependence, particularly postoperatively. A study by Brummett and colleagues indicated that the incidence of new persistent opioid use in patients who underwent surgery was 5.9% to 6.5% and did not differ between major and minor surgical procedures. This study concluded that new opioid use could be considered one of the most common complications after elective surgery.11 Similarly, in 2017 Makary and colleagues found that surgeons tend to overprescribe pain medications after procedures; some prescribing as many as 50 to 60 tablets to control pain after simple procedures.12 This is in stark contrast to pain guideline recommendations of no more than 10 tablets for most standard operative procedures.13
Sun and colleagues conducted a retrospective analysis of health care claims data in more than 18 million opioid-naïve patients who did and did not undergo surgery. Seven of the 11 surgical procedures were associated with an increased risk of chronic opioid use. The highest incidence of chronic opioid use in the first postoperative year was for total hip arthroplasty (1.4%, OR 5.10; 95% CI, 1.29-1.53). The study found that the risk factors most associated with chronic opioid use after surgery were male sex, aged > 50 years, and preoperative history of drug abuse, alcohol abuse, or depression, along with benzodiazepine use or antidepressant use.14 In a 2018 cohort study that evaluated predictors associated with transitioning to incident chronic opioid therapy, 4 factors were identified. These included opioid duration of action (adjusted odds ratio [AOR], 12.28; 95% CI, 8.1-06-18.72), the parent opioid compound (eg, tramadol vs codeine; AOR, 7.26; 95% CI, 5.20-10.13), the presence of conditions that are very likely to cause chronic pain (AOR, 5.47; 95% CI, 3.89-7.68), and drug use disorders (AOR, 4.02; 95% CI, 2.53-6.40).15
While there has been research into outpatient risk factors and medical practices that may contribute to chronic opioid use, a relative paucity of data exists on the contribution of hospitalization and inpatient opioid use on patient outcomes. A 2014 Canadian study assessed the impact of opioid use in the intensive care unit (ICU) on opioid use after discharge.16 This study included more than 2,500 patients who were admitted to a Canadian ICU between 2005 and 2008, and then followed after discharge for 48 months to quantify chronic opioid use. Nonopioid users increased from 87.8% in the early post-ICU period to 95.6% at 48 months after discharge. Preadmission chronic opioid use and prolonged hospital length of stay (LOS) were found to be associated with an increased risk of chronic opioid use after discharge.16 To date, there are no published studies that analyze the incidence of opioid-naïve veterans who convert to chronic opioid use after receiving opioids during an acute hospitalization.
In this retrospective analysis, we analyze the incidence of chronic opioid use after administration of opioids in the ICU as well as a variety of risk factors that may influence conversion to chronic opioid use.
Methods
This analysis was a single center, retrospective chart review conducted for patients admitted between July 1, 2017 and December 31, 2017 at the US Department of Veterans Affairs (VA) Michael E. DeBakey VA Medical Center (MEDVAMC) in Houston, Texas. MEDVAMC is a 538-bed academic\teaching hospital serving about 130,000 veterans in Southeast Texas. The hospital has 3 ICUs (medical, cardiovascular, and surgical) and 38 total ICU beds. The study was approved by the Baylor College of Medicine Institutional Review Board and MEDVAMC Research and Development Review Board. Informed consent was not required.
Inclusion criteria consisted of patients aged ≥ 18 years admitted to the ICU in the above-specified time frame, who were administered a continuous infusion of an opioid for at least 12 hours. Patients were excluded if they were not opioid naïve prior to admission, defined as receiving > 30 days of opioids in the prior 12 months. Patients who died during hospital admission, never received an opioid despite having an active order, were hospital-to-hospital transfers, or were still admitted at the time of data collection were excluded from the analysis.
All pertinent data were collected using the VA Computerized Patient Record System (CPRS) and the Critical Care Manager (Picis Clinical Solutions) ICU monitoring application. Critical Care Manager was used to track the time frame, duration, and amounts of opioid infusions administered in the ICU. Patient demographic and preadmission data, including date of birth, age, race, history of substance use/alcohol use disorder (defined as a previous diagnosis) and previous opioid prescriptions within the past year were recorded. For the inpatient admission, the ICU LOS, hospital LOS, primary admission diagnosis, type of opioid medication administered, and total duration and dose of opioid administered were collected. After discharge, opioid medication fill data at 3, 6, and 12 months were collected. This information included names of any outpatient opioids filled, dosage unit, quantity, day supply, and number of refills.
The primary outcome for this study was to determine the incidence of chronic opioid use at 3, 6, and 12 months after discharge, defined as the percentage of patients receiving outpatient opioid prescriptions at each time point. Analyses were conducted to observe the effect of age, race, history of substance use or history of alcohol use (International Classification of Diseases documented diagnosis, 9th edition), ICU type (medical, surgical, or cardiovascular), surgical/nonsurgical admission, ICU LOS, hospital LOS, total time, and amount of opioids administered during admission on risk of conversion to chronic opioid use.
Descriptive statistics were calculated to analyze the incidence of chronic opioid use. Univariate logistic regression analysis, including ORs, 95% CIs, and P values, was conducted to determine the effects of the risk factors noted earlier on chronic opioid use at each time point. A multivariate logistic regression model was performed to assess the effect of multiple independent variables on opioid use at 3, 6, and 12 months. Statistical analysis was performed using StataCorp Stata SE.
Results
During the study period, 330 patients were admitted to the ICU. After applying inclusion/exclusion criteria, 118 patients were included in the final analysis. The most frequent reasons for exclusion from the study were patient death (n = 77), a past history of opioid use (n = 56), and not having received an opioid infusion for at least 12 hours (n = 68). The average age of the patients included was 67 years (Table 1). A total of 14% and 9% of patients, respectively, had a diagnosis of alcohol use disorder or substance use disorder recorded in CPRS. After admission, the most common location for these patients was the surgical ICU (65%). All patients were male. The average hospital LOS was 18.6 days , and the ICU LOS was 8.3 days. The average duration of administration for the opioid (fentanyl) infusion was 63 hours, and the average amount of fentanyl administered to each patient was 57.1 mcg/h.
The incidence of opioid-naïve patients receiving opioids after discharge was 76.3% (n = 90) at 3 months, 19.5% (n = 23) at 6 months and 7.6% (n = 9) at 12 months (Figure). The daily morphine milligram equivalent (MME) of patients prescribed opioids at 3, 6, and 12 months was similar (3 months, 22.7; 6 months, 19.7; 12 months, 20.9). In the univariate regression analysis, several variables were found to be associated with converting to chronic opioid use. Prior history of alcohol use disorder (OR, 0.3; 95% CI, 0.10-0.88; P = .03), ICU type (OR, 3.9; 95% CI, 1.73-8.75; P = .001) and ICU LOS (OR, 0.88; 95% CI, 0.81-0.95; P = .01) had a statistically significant association on opioid use at 3 months. (Table 2). No variables evaluated had a statistically significant effect on chronic opioid use at 6 months, and only age (OR 0.93; 95% CI. 0.87-0.99; P = .02) was statistically significant at 12 months. In the multivariate logistic regression analysis, history of alcohol abuse, admission for surgery, and hospital LOS were significant at 3 months (Table 3).
Discussion
In this single-center analysis conducted at a VA academic hospital of opioid-naïve patients who were administered opioids in the ICU, the incidence of patients subsequently receiving outpatient opioid prescriptions at 12 months after discharge was 7.6%. There also was a decrease in the amount of opioids received by patients (daily MME) after discharge at 3, 6, and 12 months. This trend did not demonstrate the propensity for inpatient opioid use to convert opioid-naïve patients to chronic opioid users.
The most common outpatient opioids prescribed were hydrocodone/acetaminophen, morphine, and tramadol. Logistic regression showed few factors that correlated significantly with opioid use in the long-term (12 month) period. This finding is a deviation from the findings of Yaffe and colleagues who found hospital LOS to be one of the only predictors of long-term opioid use in their population (defined as use at 48 months).16 One important difference between our study and the Yaffe and colleagues study was that they evaluated all patients who were admitted to the ICU, regardless of the exposure to opioids during their inpatient stay. Consequently, this difference may have resulted in the differences in findings.
Strengths and Limitations
Location was a strength of our study, as this analysis was conducted at an integrated health care system that provides comprehensive inpatient and outpatient care. The VA uses a closed electronic health record, which allowed patients to be followed both in the inpatient and outpatient settings to determine which medications were prescribed at each time. In other health care systems this information would have been difficult to follow as patients often fill outpatient prescriptions at community pharmacies not affiliated with the treating hospital. However, any patient not using a VA prescriber for subsequent opioid prescriptions or patients who received opioids through other sources would not have had their continued opioid use captured. These data may be available in the states prescription monitoring program, but it was not available to query for research at this time.
This study also excluded chronic opioid users, which could have been another confounding factor to account for when analyzing the results. However, the primary objective of the study was to determine the impact of opioids prescribed in the ICU on converting previous opioid-naïve patients to chronic users. Finally, a multivariate logistic regression was incorporated to assess for factors that may predispose certain patients to convert to chronic opioid users. This analysis served to extend the applicability of our study by not only analyzing whether receiving opioids in the ICU contributed to chronic opioid use in the long-term, but also which populations may be at greatest risk. This information can be used in the future to target harm-reduction efforts toward high-risk hospitalized patients.
One limitation of this study was that it was conducted as a retrospective, single-center chart review in Houston, Texas. Because this was not a randomized controlled trial, it is difficult to imply any causation between exposure to opioids in the ICU and chronic use. In addition, because this study was conducted at a single site, the results may not be able to be generalized to other populations. VA populations tend to be elderly and predominantly male, as was reflected by the study population. These factors, along with regional variability in patient characteristics, may limit the generalizability of this study to older male patients located in Southeast Texas or other similar populations. Other limitations of this study also included the small sample size, limited period of follow-up obtained, and that other comorbidity information (pain scores during stay, use of nonopioid pain medications, past history of anxiety or depression, or other acute illnesses or surgeries that may have required opioid therapy during admission) was not collected.
This study was only able to review 118 patients for a follow-up duration of 1 year. In the Yaffe and colleagues study, more than 2,500 patients were followed over 4 years, which provided a more long-term overview of the clinical course of these patients and may be another reason for discrepant findings. However, this study did not actually assess the impact on administration of opioids on the development of chronic opioid use.16 Finally, the biggest limitation to this study may be the potential for confounding discharge prescriptions. After discharge, patients’ prescriptions were evaluated from discharge to 3 months, in between 3 and 6 months, and between 6 and 12 months for the presence of an opioid prescription. Due to this methodology, any opioid prescription a patient was discharged with was counted in the 3-month time point. Since many patients included in the study were admitted to the surgical ICU (65%), it was logical that they were discharged with opioids after their procedure. While including the immediate postdischarge prescription data was useful for evaluating the decrease in opioid use and incidence over time, it did cause the 3-month time point to appear overly inflated, potentially signaling that at 3 months after discharge many of these patients were still requiring opioid use.
The Society of Critical Care Medicine still recommends opioids as first-line therapy for non-neuropathic pain in the ICU setting.17 Additionally, postoperative pain can be difficult to manage in the surgical population and is often treated with opioids, though treatment with multimodal pain regimens is becoming more common.18 It is difficult to imagine that a finding that implicates opioid use in the hospital with conversion to chronic opioid use would prompt a cessation in the use of opioid in these settings, especially in the context of analgosedation related to mechanically ventilated patients. However, it would be plausible to use this knowledge to advocate for opioid-sparing therapies and consideration for weaning individuals at high risk for misuse after discharge from opioid-containing sedation or analgesia regimens in a timelier manner.
Though our findings did not show a clinically relevant increase in chronic opioid users, clinicians can still use this information to encourage targeted education and closer monitoring for those patients deemed as high risk at discharge to prevent unnecessary prolonged opioid use. By having more frequent follow-up in pain clinics, switching patients to nonopioid therapies after discharge, and ensuring high-risk patients are discharged with naloxone rescue kits, it would be possible to drastically reduce the number of potential overdoses for patients who previously required opioid therapy in the ICU.
Conclusion
After discharge, 7.6% of previously opioid-naïve patients who were treated with opioids in the ICU were still receiving prescriptions for opioids at 12 months. These findings did not suggest a clinically significant increase in the incidence of chronic opioid use after inpatient administration of opioids. However, these results prompt the need for larger, prospective, multicenter studies to evaluate the effect on hospitalization on converting to chronic opioid use and a deeper evaluation of other potential risk factors that may be present.
1. Dahlhamer J, Lucas J, Zelaya C, et al. Prevalence of chronic pain and high-impact chronic pain among adults—United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(36):1001-1006.
2. Centers for Disease Control and Prevention. QuickStats: percentage of adults aged ≥18 years who often had pain in the past 3 months, by sex and age group. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6217a10.htm. Published May 3, 2103. Accessed February 7, 2020.
3. Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain. 2012;13(8):715-724.
4. Jamison RN, Mao J. Opioid analgesics. Mayo Clin Proc. 2015;90(7):957-68.
5. DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey LM. Pharmacotherapy: A Pathophysiologic Approach, 9e. McGraw Hill Professional; 2014.
6. Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths—United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445-1452.
7. Ahmad FB, Rossen LM, Spencer M, Warner M, Sutton P. Provisional drug overdose death counts. https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm. Reviewed February 12, 2020. Accessed February 18, 2020.
8. National Institute on Drug Abuse. Overdose death rates. https://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates. Revised January 2019. Accessed February 10, 2020.
9. Florence CS, Zhou C, Luo F, Xu L. The economic burden of prescription opioid overdose, abuse, and dependence in the United States, 2013. Med Care. 2016;54(10):901-906.
10. HHS Acting Secretary declares public health emergency to address national opioid crisis [news release]. https://www.hhs.gov/about/news/2017/10/26/hhs-acting-secretary-declares-public-health-emergency-address-national-opioid-crisis.html. Published October 26, 2017. Accessed February 7, 2020.
11. Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg. 2017;152(6):e170504.
12. Makary MA, Overton HN, Wang P. Overprescribing is major contributor to opioid crisis. BMJ. 2017;359:j4792.
13. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65(1):1-49.
14. Sun EC, Darnall BD, Baker LC, Mackey S. Incidence of and risk factors for chronic opioid use among opioid-naive patients in the postoperative period. JAMA Intern Med. 2016;176(9):1286-93.
15. Thornton JD, Dwibedi N, Scott V, et al. Predictors of transitioning to incident chronic opioid therapy among working-age adults in the United States. Am Health Drug Benefits. 2018;11(1):12-21.
16. Yaffe PB, Green RS, Butler MB, Witter T. Is admission to the intensive care unit associated with chronic opioid use? A 4-year follow-up of intensive care unit survivors. J Intensive Care Med. 2017;327(7):429-435.
17. Devlin JW, Skrobik Y, Gélinas C, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46(9):e825-e873.
18. Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17(2):131-157.
Chronic pain is a worldwide cause of impairment. According to data from the 2016 National Health Interview Survey, an estimated 50 million American adults suffer from chronic pain, with 19.6 million adults suffering from high-impact chronic pain.1 This phenomenon is particularly prevalent in the older population. More than 25% of adults aged 65 to 74 years reported that they were often in pain in the past 3 months compared with just 10% of those adults between the ages of 18 and 44 years.2
The economic burdens of chronic pain disorders are well known. In 2010, Gaskin and Richard found that chronic pain has far-reaching consequences for the US economy, ranging from direct health care costs to lost productivity. This study estimated additional health care costs at about $300 billion yearly and lost productivity at $300 billion, bringing total annual costs to about $600 billion. This expense is more than heart disease alone ($309 billion), and cancer and diabetes mellitus ($243 billion and $188 billion respectively) combined.3
Opioid medications are powerful and effective pain-reducing agents that are indicated for short-term acute pain or long-term in the management of chronic, severe cancer-related pain.4 Although efficacious, use of these medications carries with it the inherent risks of abuse, misuse, addiction, and overdose.5 Since 1999, opioid-related overdose deaths have been on the rise. The CDC estimated that > 15,000 deaths were attributable specifically to prescription opioids in 2015.6 The estimates had risen to > 17,000 deaths in 2017, with the number increasing since that time.7 Cumulatively, the CDC estimates that > 200,000 deaths in the US between 1999 and 2017 are attributed to prescription opioid overdose, clearly marking this trend as a growing nationwide epidemic.8
In 2016, Florence and colleagues estimated costs associated with opioid overdose to be just shy of $80 billion in 2013 dollars.9 In October 2017, the US Department of Health and Human Services declared the opioid epidemic a public health emergency and committed $900 million to combating the crisis.10
An abundance of data exist analyzing outpatient prescribing and its impacts on opioid dependence, particularly postoperatively. A study by Brummett and colleagues indicated that the incidence of new persistent opioid use in patients who underwent surgery was 5.9% to 6.5% and did not differ between major and minor surgical procedures. This study concluded that new opioid use could be considered one of the most common complications after elective surgery.11 Similarly, in 2017 Makary and colleagues found that surgeons tend to overprescribe pain medications after procedures; some prescribing as many as 50 to 60 tablets to control pain after simple procedures.12 This is in stark contrast to pain guideline recommendations of no more than 10 tablets for most standard operative procedures.13
Sun and colleagues conducted a retrospective analysis of health care claims data in more than 18 million opioid-naïve patients who did and did not undergo surgery. Seven of the 11 surgical procedures were associated with an increased risk of chronic opioid use. The highest incidence of chronic opioid use in the first postoperative year was for total hip arthroplasty (1.4%, OR 5.10; 95% CI, 1.29-1.53). The study found that the risk factors most associated with chronic opioid use after surgery were male sex, aged > 50 years, and preoperative history of drug abuse, alcohol abuse, or depression, along with benzodiazepine use or antidepressant use.14 In a 2018 cohort study that evaluated predictors associated with transitioning to incident chronic opioid therapy, 4 factors were identified. These included opioid duration of action (adjusted odds ratio [AOR], 12.28; 95% CI, 8.1-06-18.72), the parent opioid compound (eg, tramadol vs codeine; AOR, 7.26; 95% CI, 5.20-10.13), the presence of conditions that are very likely to cause chronic pain (AOR, 5.47; 95% CI, 3.89-7.68), and drug use disorders (AOR, 4.02; 95% CI, 2.53-6.40).15
While there has been research into outpatient risk factors and medical practices that may contribute to chronic opioid use, a relative paucity of data exists on the contribution of hospitalization and inpatient opioid use on patient outcomes. A 2014 Canadian study assessed the impact of opioid use in the intensive care unit (ICU) on opioid use after discharge.16 This study included more than 2,500 patients who were admitted to a Canadian ICU between 2005 and 2008, and then followed after discharge for 48 months to quantify chronic opioid use. Nonopioid users increased from 87.8% in the early post-ICU period to 95.6% at 48 months after discharge. Preadmission chronic opioid use and prolonged hospital length of stay (LOS) were found to be associated with an increased risk of chronic opioid use after discharge.16 To date, there are no published studies that analyze the incidence of opioid-naïve veterans who convert to chronic opioid use after receiving opioids during an acute hospitalization.
In this retrospective analysis, we analyze the incidence of chronic opioid use after administration of opioids in the ICU as well as a variety of risk factors that may influence conversion to chronic opioid use.
Methods
This analysis was a single center, retrospective chart review conducted for patients admitted between July 1, 2017 and December 31, 2017 at the US Department of Veterans Affairs (VA) Michael E. DeBakey VA Medical Center (MEDVAMC) in Houston, Texas. MEDVAMC is a 538-bed academic\teaching hospital serving about 130,000 veterans in Southeast Texas. The hospital has 3 ICUs (medical, cardiovascular, and surgical) and 38 total ICU beds. The study was approved by the Baylor College of Medicine Institutional Review Board and MEDVAMC Research and Development Review Board. Informed consent was not required.
Inclusion criteria consisted of patients aged ≥ 18 years admitted to the ICU in the above-specified time frame, who were administered a continuous infusion of an opioid for at least 12 hours. Patients were excluded if they were not opioid naïve prior to admission, defined as receiving > 30 days of opioids in the prior 12 months. Patients who died during hospital admission, never received an opioid despite having an active order, were hospital-to-hospital transfers, or were still admitted at the time of data collection were excluded from the analysis.
All pertinent data were collected using the VA Computerized Patient Record System (CPRS) and the Critical Care Manager (Picis Clinical Solutions) ICU monitoring application. Critical Care Manager was used to track the time frame, duration, and amounts of opioid infusions administered in the ICU. Patient demographic and preadmission data, including date of birth, age, race, history of substance use/alcohol use disorder (defined as a previous diagnosis) and previous opioid prescriptions within the past year were recorded. For the inpatient admission, the ICU LOS, hospital LOS, primary admission diagnosis, type of opioid medication administered, and total duration and dose of opioid administered were collected. After discharge, opioid medication fill data at 3, 6, and 12 months were collected. This information included names of any outpatient opioids filled, dosage unit, quantity, day supply, and number of refills.
The primary outcome for this study was to determine the incidence of chronic opioid use at 3, 6, and 12 months after discharge, defined as the percentage of patients receiving outpatient opioid prescriptions at each time point. Analyses were conducted to observe the effect of age, race, history of substance use or history of alcohol use (International Classification of Diseases documented diagnosis, 9th edition), ICU type (medical, surgical, or cardiovascular), surgical/nonsurgical admission, ICU LOS, hospital LOS, total time, and amount of opioids administered during admission on risk of conversion to chronic opioid use.
Descriptive statistics were calculated to analyze the incidence of chronic opioid use. Univariate logistic regression analysis, including ORs, 95% CIs, and P values, was conducted to determine the effects of the risk factors noted earlier on chronic opioid use at each time point. A multivariate logistic regression model was performed to assess the effect of multiple independent variables on opioid use at 3, 6, and 12 months. Statistical analysis was performed using StataCorp Stata SE.
Results
During the study period, 330 patients were admitted to the ICU. After applying inclusion/exclusion criteria, 118 patients were included in the final analysis. The most frequent reasons for exclusion from the study were patient death (n = 77), a past history of opioid use (n = 56), and not having received an opioid infusion for at least 12 hours (n = 68). The average age of the patients included was 67 years (Table 1). A total of 14% and 9% of patients, respectively, had a diagnosis of alcohol use disorder or substance use disorder recorded in CPRS. After admission, the most common location for these patients was the surgical ICU (65%). All patients were male. The average hospital LOS was 18.6 days , and the ICU LOS was 8.3 days. The average duration of administration for the opioid (fentanyl) infusion was 63 hours, and the average amount of fentanyl administered to each patient was 57.1 mcg/h.
The incidence of opioid-naïve patients receiving opioids after discharge was 76.3% (n = 90) at 3 months, 19.5% (n = 23) at 6 months and 7.6% (n = 9) at 12 months (Figure). The daily morphine milligram equivalent (MME) of patients prescribed opioids at 3, 6, and 12 months was similar (3 months, 22.7; 6 months, 19.7; 12 months, 20.9). In the univariate regression analysis, several variables were found to be associated with converting to chronic opioid use. Prior history of alcohol use disorder (OR, 0.3; 95% CI, 0.10-0.88; P = .03), ICU type (OR, 3.9; 95% CI, 1.73-8.75; P = .001) and ICU LOS (OR, 0.88; 95% CI, 0.81-0.95; P = .01) had a statistically significant association on opioid use at 3 months. (Table 2). No variables evaluated had a statistically significant effect on chronic opioid use at 6 months, and only age (OR 0.93; 95% CI. 0.87-0.99; P = .02) was statistically significant at 12 months. In the multivariate logistic regression analysis, history of alcohol abuse, admission for surgery, and hospital LOS were significant at 3 months (Table 3).
Discussion
In this single-center analysis conducted at a VA academic hospital of opioid-naïve patients who were administered opioids in the ICU, the incidence of patients subsequently receiving outpatient opioid prescriptions at 12 months after discharge was 7.6%. There also was a decrease in the amount of opioids received by patients (daily MME) after discharge at 3, 6, and 12 months. This trend did not demonstrate the propensity for inpatient opioid use to convert opioid-naïve patients to chronic opioid users.
The most common outpatient opioids prescribed were hydrocodone/acetaminophen, morphine, and tramadol. Logistic regression showed few factors that correlated significantly with opioid use in the long-term (12 month) period. This finding is a deviation from the findings of Yaffe and colleagues who found hospital LOS to be one of the only predictors of long-term opioid use in their population (defined as use at 48 months).16 One important difference between our study and the Yaffe and colleagues study was that they evaluated all patients who were admitted to the ICU, regardless of the exposure to opioids during their inpatient stay. Consequently, this difference may have resulted in the differences in findings.
Strengths and Limitations
Location was a strength of our study, as this analysis was conducted at an integrated health care system that provides comprehensive inpatient and outpatient care. The VA uses a closed electronic health record, which allowed patients to be followed both in the inpatient and outpatient settings to determine which medications were prescribed at each time. In other health care systems this information would have been difficult to follow as patients often fill outpatient prescriptions at community pharmacies not affiliated with the treating hospital. However, any patient not using a VA prescriber for subsequent opioid prescriptions or patients who received opioids through other sources would not have had their continued opioid use captured. These data may be available in the states prescription monitoring program, but it was not available to query for research at this time.
This study also excluded chronic opioid users, which could have been another confounding factor to account for when analyzing the results. However, the primary objective of the study was to determine the impact of opioids prescribed in the ICU on converting previous opioid-naïve patients to chronic users. Finally, a multivariate logistic regression was incorporated to assess for factors that may predispose certain patients to convert to chronic opioid users. This analysis served to extend the applicability of our study by not only analyzing whether receiving opioids in the ICU contributed to chronic opioid use in the long-term, but also which populations may be at greatest risk. This information can be used in the future to target harm-reduction efforts toward high-risk hospitalized patients.
One limitation of this study was that it was conducted as a retrospective, single-center chart review in Houston, Texas. Because this was not a randomized controlled trial, it is difficult to imply any causation between exposure to opioids in the ICU and chronic use. In addition, because this study was conducted at a single site, the results may not be able to be generalized to other populations. VA populations tend to be elderly and predominantly male, as was reflected by the study population. These factors, along with regional variability in patient characteristics, may limit the generalizability of this study to older male patients located in Southeast Texas or other similar populations. Other limitations of this study also included the small sample size, limited period of follow-up obtained, and that other comorbidity information (pain scores during stay, use of nonopioid pain medications, past history of anxiety or depression, or other acute illnesses or surgeries that may have required opioid therapy during admission) was not collected.
This study was only able to review 118 patients for a follow-up duration of 1 year. In the Yaffe and colleagues study, more than 2,500 patients were followed over 4 years, which provided a more long-term overview of the clinical course of these patients and may be another reason for discrepant findings. However, this study did not actually assess the impact on administration of opioids on the development of chronic opioid use.16 Finally, the biggest limitation to this study may be the potential for confounding discharge prescriptions. After discharge, patients’ prescriptions were evaluated from discharge to 3 months, in between 3 and 6 months, and between 6 and 12 months for the presence of an opioid prescription. Due to this methodology, any opioid prescription a patient was discharged with was counted in the 3-month time point. Since many patients included in the study were admitted to the surgical ICU (65%), it was logical that they were discharged with opioids after their procedure. While including the immediate postdischarge prescription data was useful for evaluating the decrease in opioid use and incidence over time, it did cause the 3-month time point to appear overly inflated, potentially signaling that at 3 months after discharge many of these patients were still requiring opioid use.
The Society of Critical Care Medicine still recommends opioids as first-line therapy for non-neuropathic pain in the ICU setting.17 Additionally, postoperative pain can be difficult to manage in the surgical population and is often treated with opioids, though treatment with multimodal pain regimens is becoming more common.18 It is difficult to imagine that a finding that implicates opioid use in the hospital with conversion to chronic opioid use would prompt a cessation in the use of opioid in these settings, especially in the context of analgosedation related to mechanically ventilated patients. However, it would be plausible to use this knowledge to advocate for opioid-sparing therapies and consideration for weaning individuals at high risk for misuse after discharge from opioid-containing sedation or analgesia regimens in a timelier manner.
Though our findings did not show a clinically relevant increase in chronic opioid users, clinicians can still use this information to encourage targeted education and closer monitoring for those patients deemed as high risk at discharge to prevent unnecessary prolonged opioid use. By having more frequent follow-up in pain clinics, switching patients to nonopioid therapies after discharge, and ensuring high-risk patients are discharged with naloxone rescue kits, it would be possible to drastically reduce the number of potential overdoses for patients who previously required opioid therapy in the ICU.
Conclusion
After discharge, 7.6% of previously opioid-naïve patients who were treated with opioids in the ICU were still receiving prescriptions for opioids at 12 months. These findings did not suggest a clinically significant increase in the incidence of chronic opioid use after inpatient administration of opioids. However, these results prompt the need for larger, prospective, multicenter studies to evaluate the effect on hospitalization on converting to chronic opioid use and a deeper evaluation of other potential risk factors that may be present.
Chronic pain is a worldwide cause of impairment. According to data from the 2016 National Health Interview Survey, an estimated 50 million American adults suffer from chronic pain, with 19.6 million adults suffering from high-impact chronic pain.1 This phenomenon is particularly prevalent in the older population. More than 25% of adults aged 65 to 74 years reported that they were often in pain in the past 3 months compared with just 10% of those adults between the ages of 18 and 44 years.2
The economic burdens of chronic pain disorders are well known. In 2010, Gaskin and Richard found that chronic pain has far-reaching consequences for the US economy, ranging from direct health care costs to lost productivity. This study estimated additional health care costs at about $300 billion yearly and lost productivity at $300 billion, bringing total annual costs to about $600 billion. This expense is more than heart disease alone ($309 billion), and cancer and diabetes mellitus ($243 billion and $188 billion respectively) combined.3
Opioid medications are powerful and effective pain-reducing agents that are indicated for short-term acute pain or long-term in the management of chronic, severe cancer-related pain.4 Although efficacious, use of these medications carries with it the inherent risks of abuse, misuse, addiction, and overdose.5 Since 1999, opioid-related overdose deaths have been on the rise. The CDC estimated that > 15,000 deaths were attributable specifically to prescription opioids in 2015.6 The estimates had risen to > 17,000 deaths in 2017, with the number increasing since that time.7 Cumulatively, the CDC estimates that > 200,000 deaths in the US between 1999 and 2017 are attributed to prescription opioid overdose, clearly marking this trend as a growing nationwide epidemic.8
In 2016, Florence and colleagues estimated costs associated with opioid overdose to be just shy of $80 billion in 2013 dollars.9 In October 2017, the US Department of Health and Human Services declared the opioid epidemic a public health emergency and committed $900 million to combating the crisis.10
An abundance of data exist analyzing outpatient prescribing and its impacts on opioid dependence, particularly postoperatively. A study by Brummett and colleagues indicated that the incidence of new persistent opioid use in patients who underwent surgery was 5.9% to 6.5% and did not differ between major and minor surgical procedures. This study concluded that new opioid use could be considered one of the most common complications after elective surgery.11 Similarly, in 2017 Makary and colleagues found that surgeons tend to overprescribe pain medications after procedures; some prescribing as many as 50 to 60 tablets to control pain after simple procedures.12 This is in stark contrast to pain guideline recommendations of no more than 10 tablets for most standard operative procedures.13
Sun and colleagues conducted a retrospective analysis of health care claims data in more than 18 million opioid-naïve patients who did and did not undergo surgery. Seven of the 11 surgical procedures were associated with an increased risk of chronic opioid use. The highest incidence of chronic opioid use in the first postoperative year was for total hip arthroplasty (1.4%, OR 5.10; 95% CI, 1.29-1.53). The study found that the risk factors most associated with chronic opioid use after surgery were male sex, aged > 50 years, and preoperative history of drug abuse, alcohol abuse, or depression, along with benzodiazepine use or antidepressant use.14 In a 2018 cohort study that evaluated predictors associated with transitioning to incident chronic opioid therapy, 4 factors were identified. These included opioid duration of action (adjusted odds ratio [AOR], 12.28; 95% CI, 8.1-06-18.72), the parent opioid compound (eg, tramadol vs codeine; AOR, 7.26; 95% CI, 5.20-10.13), the presence of conditions that are very likely to cause chronic pain (AOR, 5.47; 95% CI, 3.89-7.68), and drug use disorders (AOR, 4.02; 95% CI, 2.53-6.40).15
While there has been research into outpatient risk factors and medical practices that may contribute to chronic opioid use, a relative paucity of data exists on the contribution of hospitalization and inpatient opioid use on patient outcomes. A 2014 Canadian study assessed the impact of opioid use in the intensive care unit (ICU) on opioid use after discharge.16 This study included more than 2,500 patients who were admitted to a Canadian ICU between 2005 and 2008, and then followed after discharge for 48 months to quantify chronic opioid use. Nonopioid users increased from 87.8% in the early post-ICU period to 95.6% at 48 months after discharge. Preadmission chronic opioid use and prolonged hospital length of stay (LOS) were found to be associated with an increased risk of chronic opioid use after discharge.16 To date, there are no published studies that analyze the incidence of opioid-naïve veterans who convert to chronic opioid use after receiving opioids during an acute hospitalization.
In this retrospective analysis, we analyze the incidence of chronic opioid use after administration of opioids in the ICU as well as a variety of risk factors that may influence conversion to chronic opioid use.
Methods
This analysis was a single center, retrospective chart review conducted for patients admitted between July 1, 2017 and December 31, 2017 at the US Department of Veterans Affairs (VA) Michael E. DeBakey VA Medical Center (MEDVAMC) in Houston, Texas. MEDVAMC is a 538-bed academic\teaching hospital serving about 130,000 veterans in Southeast Texas. The hospital has 3 ICUs (medical, cardiovascular, and surgical) and 38 total ICU beds. The study was approved by the Baylor College of Medicine Institutional Review Board and MEDVAMC Research and Development Review Board. Informed consent was not required.
Inclusion criteria consisted of patients aged ≥ 18 years admitted to the ICU in the above-specified time frame, who were administered a continuous infusion of an opioid for at least 12 hours. Patients were excluded if they were not opioid naïve prior to admission, defined as receiving > 30 days of opioids in the prior 12 months. Patients who died during hospital admission, never received an opioid despite having an active order, were hospital-to-hospital transfers, or were still admitted at the time of data collection were excluded from the analysis.
All pertinent data were collected using the VA Computerized Patient Record System (CPRS) and the Critical Care Manager (Picis Clinical Solutions) ICU monitoring application. Critical Care Manager was used to track the time frame, duration, and amounts of opioid infusions administered in the ICU. Patient demographic and preadmission data, including date of birth, age, race, history of substance use/alcohol use disorder (defined as a previous diagnosis) and previous opioid prescriptions within the past year were recorded. For the inpatient admission, the ICU LOS, hospital LOS, primary admission diagnosis, type of opioid medication administered, and total duration and dose of opioid administered were collected. After discharge, opioid medication fill data at 3, 6, and 12 months were collected. This information included names of any outpatient opioids filled, dosage unit, quantity, day supply, and number of refills.
The primary outcome for this study was to determine the incidence of chronic opioid use at 3, 6, and 12 months after discharge, defined as the percentage of patients receiving outpatient opioid prescriptions at each time point. Analyses were conducted to observe the effect of age, race, history of substance use or history of alcohol use (International Classification of Diseases documented diagnosis, 9th edition), ICU type (medical, surgical, or cardiovascular), surgical/nonsurgical admission, ICU LOS, hospital LOS, total time, and amount of opioids administered during admission on risk of conversion to chronic opioid use.
Descriptive statistics were calculated to analyze the incidence of chronic opioid use. Univariate logistic regression analysis, including ORs, 95% CIs, and P values, was conducted to determine the effects of the risk factors noted earlier on chronic opioid use at each time point. A multivariate logistic regression model was performed to assess the effect of multiple independent variables on opioid use at 3, 6, and 12 months. Statistical analysis was performed using StataCorp Stata SE.
Results
During the study period, 330 patients were admitted to the ICU. After applying inclusion/exclusion criteria, 118 patients were included in the final analysis. The most frequent reasons for exclusion from the study were patient death (n = 77), a past history of opioid use (n = 56), and not having received an opioid infusion for at least 12 hours (n = 68). The average age of the patients included was 67 years (Table 1). A total of 14% and 9% of patients, respectively, had a diagnosis of alcohol use disorder or substance use disorder recorded in CPRS. After admission, the most common location for these patients was the surgical ICU (65%). All patients were male. The average hospital LOS was 18.6 days , and the ICU LOS was 8.3 days. The average duration of administration for the opioid (fentanyl) infusion was 63 hours, and the average amount of fentanyl administered to each patient was 57.1 mcg/h.
The incidence of opioid-naïve patients receiving opioids after discharge was 76.3% (n = 90) at 3 months, 19.5% (n = 23) at 6 months and 7.6% (n = 9) at 12 months (Figure). The daily morphine milligram equivalent (MME) of patients prescribed opioids at 3, 6, and 12 months was similar (3 months, 22.7; 6 months, 19.7; 12 months, 20.9). In the univariate regression analysis, several variables were found to be associated with converting to chronic opioid use. Prior history of alcohol use disorder (OR, 0.3; 95% CI, 0.10-0.88; P = .03), ICU type (OR, 3.9; 95% CI, 1.73-8.75; P = .001) and ICU LOS (OR, 0.88; 95% CI, 0.81-0.95; P = .01) had a statistically significant association on opioid use at 3 months. (Table 2). No variables evaluated had a statistically significant effect on chronic opioid use at 6 months, and only age (OR 0.93; 95% CI. 0.87-0.99; P = .02) was statistically significant at 12 months. In the multivariate logistic regression analysis, history of alcohol abuse, admission for surgery, and hospital LOS were significant at 3 months (Table 3).
Discussion
In this single-center analysis conducted at a VA academic hospital of opioid-naïve patients who were administered opioids in the ICU, the incidence of patients subsequently receiving outpatient opioid prescriptions at 12 months after discharge was 7.6%. There also was a decrease in the amount of opioids received by patients (daily MME) after discharge at 3, 6, and 12 months. This trend did not demonstrate the propensity for inpatient opioid use to convert opioid-naïve patients to chronic opioid users.
The most common outpatient opioids prescribed were hydrocodone/acetaminophen, morphine, and tramadol. Logistic regression showed few factors that correlated significantly with opioid use in the long-term (12 month) period. This finding is a deviation from the findings of Yaffe and colleagues who found hospital LOS to be one of the only predictors of long-term opioid use in their population (defined as use at 48 months).16 One important difference between our study and the Yaffe and colleagues study was that they evaluated all patients who were admitted to the ICU, regardless of the exposure to opioids during their inpatient stay. Consequently, this difference may have resulted in the differences in findings.
Strengths and Limitations
Location was a strength of our study, as this analysis was conducted at an integrated health care system that provides comprehensive inpatient and outpatient care. The VA uses a closed electronic health record, which allowed patients to be followed both in the inpatient and outpatient settings to determine which medications were prescribed at each time. In other health care systems this information would have been difficult to follow as patients often fill outpatient prescriptions at community pharmacies not affiliated with the treating hospital. However, any patient not using a VA prescriber for subsequent opioid prescriptions or patients who received opioids through other sources would not have had their continued opioid use captured. These data may be available in the states prescription monitoring program, but it was not available to query for research at this time.
This study also excluded chronic opioid users, which could have been another confounding factor to account for when analyzing the results. However, the primary objective of the study was to determine the impact of opioids prescribed in the ICU on converting previous opioid-naïve patients to chronic users. Finally, a multivariate logistic regression was incorporated to assess for factors that may predispose certain patients to convert to chronic opioid users. This analysis served to extend the applicability of our study by not only analyzing whether receiving opioids in the ICU contributed to chronic opioid use in the long-term, but also which populations may be at greatest risk. This information can be used in the future to target harm-reduction efforts toward high-risk hospitalized patients.
One limitation of this study was that it was conducted as a retrospective, single-center chart review in Houston, Texas. Because this was not a randomized controlled trial, it is difficult to imply any causation between exposure to opioids in the ICU and chronic use. In addition, because this study was conducted at a single site, the results may not be able to be generalized to other populations. VA populations tend to be elderly and predominantly male, as was reflected by the study population. These factors, along with regional variability in patient characteristics, may limit the generalizability of this study to older male patients located in Southeast Texas or other similar populations. Other limitations of this study also included the small sample size, limited period of follow-up obtained, and that other comorbidity information (pain scores during stay, use of nonopioid pain medications, past history of anxiety or depression, or other acute illnesses or surgeries that may have required opioid therapy during admission) was not collected.
This study was only able to review 118 patients for a follow-up duration of 1 year. In the Yaffe and colleagues study, more than 2,500 patients were followed over 4 years, which provided a more long-term overview of the clinical course of these patients and may be another reason for discrepant findings. However, this study did not actually assess the impact on administration of opioids on the development of chronic opioid use.16 Finally, the biggest limitation to this study may be the potential for confounding discharge prescriptions. After discharge, patients’ prescriptions were evaluated from discharge to 3 months, in between 3 and 6 months, and between 6 and 12 months for the presence of an opioid prescription. Due to this methodology, any opioid prescription a patient was discharged with was counted in the 3-month time point. Since many patients included in the study were admitted to the surgical ICU (65%), it was logical that they were discharged with opioids after their procedure. While including the immediate postdischarge prescription data was useful for evaluating the decrease in opioid use and incidence over time, it did cause the 3-month time point to appear overly inflated, potentially signaling that at 3 months after discharge many of these patients were still requiring opioid use.
The Society of Critical Care Medicine still recommends opioids as first-line therapy for non-neuropathic pain in the ICU setting.17 Additionally, postoperative pain can be difficult to manage in the surgical population and is often treated with opioids, though treatment with multimodal pain regimens is becoming more common.18 It is difficult to imagine that a finding that implicates opioid use in the hospital with conversion to chronic opioid use would prompt a cessation in the use of opioid in these settings, especially in the context of analgosedation related to mechanically ventilated patients. However, it would be plausible to use this knowledge to advocate for opioid-sparing therapies and consideration for weaning individuals at high risk for misuse after discharge from opioid-containing sedation or analgesia regimens in a timelier manner.
Though our findings did not show a clinically relevant increase in chronic opioid users, clinicians can still use this information to encourage targeted education and closer monitoring for those patients deemed as high risk at discharge to prevent unnecessary prolonged opioid use. By having more frequent follow-up in pain clinics, switching patients to nonopioid therapies after discharge, and ensuring high-risk patients are discharged with naloxone rescue kits, it would be possible to drastically reduce the number of potential overdoses for patients who previously required opioid therapy in the ICU.
Conclusion
After discharge, 7.6% of previously opioid-naïve patients who were treated with opioids in the ICU were still receiving prescriptions for opioids at 12 months. These findings did not suggest a clinically significant increase in the incidence of chronic opioid use after inpatient administration of opioids. However, these results prompt the need for larger, prospective, multicenter studies to evaluate the effect on hospitalization on converting to chronic opioid use and a deeper evaluation of other potential risk factors that may be present.
1. Dahlhamer J, Lucas J, Zelaya C, et al. Prevalence of chronic pain and high-impact chronic pain among adults—United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(36):1001-1006.
2. Centers for Disease Control and Prevention. QuickStats: percentage of adults aged ≥18 years who often had pain in the past 3 months, by sex and age group. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6217a10.htm. Published May 3, 2103. Accessed February 7, 2020.
3. Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain. 2012;13(8):715-724.
4. Jamison RN, Mao J. Opioid analgesics. Mayo Clin Proc. 2015;90(7):957-68.
5. DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey LM. Pharmacotherapy: A Pathophysiologic Approach, 9e. McGraw Hill Professional; 2014.
6. Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths—United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445-1452.
7. Ahmad FB, Rossen LM, Spencer M, Warner M, Sutton P. Provisional drug overdose death counts. https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm. Reviewed February 12, 2020. Accessed February 18, 2020.
8. National Institute on Drug Abuse. Overdose death rates. https://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates. Revised January 2019. Accessed February 10, 2020.
9. Florence CS, Zhou C, Luo F, Xu L. The economic burden of prescription opioid overdose, abuse, and dependence in the United States, 2013. Med Care. 2016;54(10):901-906.
10. HHS Acting Secretary declares public health emergency to address national opioid crisis [news release]. https://www.hhs.gov/about/news/2017/10/26/hhs-acting-secretary-declares-public-health-emergency-address-national-opioid-crisis.html. Published October 26, 2017. Accessed February 7, 2020.
11. Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg. 2017;152(6):e170504.
12. Makary MA, Overton HN, Wang P. Overprescribing is major contributor to opioid crisis. BMJ. 2017;359:j4792.
13. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65(1):1-49.
14. Sun EC, Darnall BD, Baker LC, Mackey S. Incidence of and risk factors for chronic opioid use among opioid-naive patients in the postoperative period. JAMA Intern Med. 2016;176(9):1286-93.
15. Thornton JD, Dwibedi N, Scott V, et al. Predictors of transitioning to incident chronic opioid therapy among working-age adults in the United States. Am Health Drug Benefits. 2018;11(1):12-21.
16. Yaffe PB, Green RS, Butler MB, Witter T. Is admission to the intensive care unit associated with chronic opioid use? A 4-year follow-up of intensive care unit survivors. J Intensive Care Med. 2017;327(7):429-435.
17. Devlin JW, Skrobik Y, Gélinas C, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46(9):e825-e873.
18. Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17(2):131-157.
1. Dahlhamer J, Lucas J, Zelaya C, et al. Prevalence of chronic pain and high-impact chronic pain among adults—United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(36):1001-1006.
2. Centers for Disease Control and Prevention. QuickStats: percentage of adults aged ≥18 years who often had pain in the past 3 months, by sex and age group. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6217a10.htm. Published May 3, 2103. Accessed February 7, 2020.
3. Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain. 2012;13(8):715-724.
4. Jamison RN, Mao J. Opioid analgesics. Mayo Clin Proc. 2015;90(7):957-68.
5. DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey LM. Pharmacotherapy: A Pathophysiologic Approach, 9e. McGraw Hill Professional; 2014.
6. Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths—United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445-1452.
7. Ahmad FB, Rossen LM, Spencer M, Warner M, Sutton P. Provisional drug overdose death counts. https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm. Reviewed February 12, 2020. Accessed February 18, 2020.
8. National Institute on Drug Abuse. Overdose death rates. https://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates. Revised January 2019. Accessed February 10, 2020.
9. Florence CS, Zhou C, Luo F, Xu L. The economic burden of prescription opioid overdose, abuse, and dependence in the United States, 2013. Med Care. 2016;54(10):901-906.
10. HHS Acting Secretary declares public health emergency to address national opioid crisis [news release]. https://www.hhs.gov/about/news/2017/10/26/hhs-acting-secretary-declares-public-health-emergency-address-national-opioid-crisis.html. Published October 26, 2017. Accessed February 7, 2020.
11. Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg. 2017;152(6):e170504.
12. Makary MA, Overton HN, Wang P. Overprescribing is major contributor to opioid crisis. BMJ. 2017;359:j4792.
13. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65(1):1-49.
14. Sun EC, Darnall BD, Baker LC, Mackey S. Incidence of and risk factors for chronic opioid use among opioid-naive patients in the postoperative period. JAMA Intern Med. 2016;176(9):1286-93.
15. Thornton JD, Dwibedi N, Scott V, et al. Predictors of transitioning to incident chronic opioid therapy among working-age adults in the United States. Am Health Drug Benefits. 2018;11(1):12-21.
16. Yaffe PB, Green RS, Butler MB, Witter T. Is admission to the intensive care unit associated with chronic opioid use? A 4-year follow-up of intensive care unit survivors. J Intensive Care Med. 2017;327(7):429-435.
17. Devlin JW, Skrobik Y, Gélinas C, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46(9):e825-e873.
18. Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17(2):131-157.
All Hands on Deck: Learning to “Un-specialize” in the COVID-19 Pandemic
Specialization, as detailed in Adam Smith’s 1776 landmark treatise, Wealth of Nations,1 has been an enduring trend in labor and economics for centuries. Mirroring evolution in other sectors of the economy, the healthcare workforce has become ever more specialized.2 General practitioners and family doctors have ceded ground to a bevy of specialists and subspecialists ranging from pediatric endocrinologists to otolaryngology-neurotologists. Given the growth in medical knowledge over the past century, this specialization seems both necessary and good. This same specialization that serves us in good times, though, leaves us woefully underprepared for an epidemic that will require large numbers of hospitalists/generalists and intensivists, such as the current coronavirus disease 2019 (COVID-19) pandemic.
A bit on terminology before we proceed. For purposes of this paper we define generalists as physicians trained in Internal Medicine, Family Medicine, Pediatrics, or Med/Peds who provide primary hospital care to adults and children. While some may argue that hospitalists are specialists in inpatient care, we would like to focus on hospitalists as generalists who focus on inpatient care and what we have in common with the broader community of generalists. We include as generalists anyone, irrespective of clinical training, who chooses broad primary patient responsibility over the narrower consultative role. There is always a specialist in our midst who knows more about a particular disease or condition; as generalists, most of us appreciate and welcome that expertise.
Sometimes it takes a pandemic like COVID-19 to highlight a tremendous blind spot in our healthcare system that, in retrospect, seems hard to have missed. What do we do when we need more generalists and have only a surplus of specialists, many of whom were involuntarily “furloughed” by canceled elective procedures and postponed clinics? How do we “un-specialize” our specialist workforce?
We will discuss some of the most pressing problems facing hospitals working to ensure adequate staffing for general inpatient units caused by the simultaneous reductions in physician availability (because of illness and/or quarantine) and markedly increased admissions of undifferentiated COVID-19–related illnesses. We will assume that hospitals have already activated all providers practicing in areas most similar to hospital medicine, including generalists who have mixed inpatient/outpatient practices, subspecialists with significant inpatient clinical roles, fellows, and advanced practice providers (APPs) with inpatient experience. The Accreditation Council for Graduate Medical Education released guidance around the roles of physician trainees during the pandemic.3 Despite these measures, though, further workforce augmentation will be vital. To that end, several challenges to clinical staffing are enumerated below, accompanied by strategies to address them.
CLINICAL STAFFING CHALLENGES
1. Clinicians eager to help, but out of practice in the inpatient setting: As hospitals across the country work to develop physician staffing contingency plans for scenarios in which general inpatient volumes increase by 50%-300% while 33%-50% of hospitalists either become infected or require quarantine, many hospitals are looking to bolster their physician depth. We have been extremely gratified by the tremendous response from the broader physician communities in which we work. We have encountered retired physicians who have volunteered to come back to work despite being at higher risk of severe COVID-19 complications and physician-scientists offering to step back into clinical roles. We have found outstanding subspecialists asking to work under the tutelage of experienced hospitalists; these specialists recognize how, despite years of clinical experience, they would need significant supervision to function in the inpatient setting. The humility and self-awareness of these volunteers has been phenomenal.
Retraining researchers, subspecialists, and retirees as hospitalists requires purposeful onboarding to target key educational goals. This onboarding should stress COVID-19–specific medical management, training in infection prevention and control, and hospital-specific workflow processes (eg, shift length, sign-over). Onboarding must also include access and orientation to electronic health records, training around inpatient documentation requirements, and billing practices. Non–COVID-19 healthcare will continue; hospitals and clinical leaders will need to determine whether certain specialists should focus on COVID-19 care alone and leave others to continue with speciality practice still needed. Ready access to hospital medicine and medical subspecialty consultation will be pivotal in supervising providers asked to step into hospitalist roles.
The onboarding process we describe might best be viewed through the lens of focused professional practice evaluation (FPPE). Required by the Joint Commission, FPPE is a process for the medical staff of a facility to evaluate privilege-specific competence by clinicians and is used for any new clinical privileges and when there may be question as to a current practitioner’s capabilities. The usual FPPE process includes reassessment of provider practice, typically at 3 to 6 months. Doing so may be challenging given overall workforce stress and the timing of clinical demand—eg, time for medical record review will be limited. Consideration of a “preceptorship” with an experienced hospitalist providing verbal oversight for providers with emergency privileges may be very appropriate. Indeed the Joint Commission recently published guidance around FPPE during the COVID-19 epidemic with the suggestion that mentorship and direct observation are reasonable ways to ensure quality.4
Concerns around scope of practice and medicolegal liability must be rapidly addressed by professional practice organizations, state medical boards, and medical malpractice insurers to protect frontline providers, nurses, and pharmacists. In particular, Joint Commission FPPE process requirements may need to be relaxed to respond to a surge in clinical demand. Contingency and crisis standards of care permit doing so. We welcome the introduction of processes to expedite provider licensure in many hard-hit states.
2. Clinicians who should not help because of medical comorbidities or age: Individuals with certain significant comorbidities (eg, inflammatory conditions treated with immunosuppressants, pulmonary disease, cancer with active treatment) or meeting certain age criteria should be discouraged from clinical work because the dangers of illness for them and of transmission of illness are high. Judgment and a version of mutual informed consent will be needed to address fewer clear scenarios, such as whether a 35-year-old physician who requires a steroid inhaler for asthma or a 64-year-old physician who is otherwise healthy have higher risk. It is our opinion that all physicians should contribute to the care of patients with documented or suspected COVID-19 unless they meet institutionally defined exclusion criteria. We should recognize that physicians who are unable to provide direct care to patients with COVID-19 infection may have significant remorse and feelings that they are letting down their colleagues and the oath they have taken. As the COVID -19 pandemic continues, we are quickly learning that physicians who have contraindications to providing care to patients with active COVID-19 infection can still contribute in numerous mission-critical ways. This may include virtual (telehealth) visits, preceptorship via telehealth of providers completing FPPE in hospital medicine practice, postdischarge follow-up of patients who are no longer infectious, and other care-coordination activities, such as triaging direct admission calls.
3. Clinicians who should be able to help but are fearful: All efforts must be undertaken to protect healthcare workers from acquiring COVID-19. Nevertheless, there are models predicting that ultimately the vast majority of the world’s population will be exposed, including healthcare workers.5,6 In our personal experience as hospitalists and leaders, the vast majority (95%-plus) of our hospitalists have not only continued to do their job but taken on additional responsibilities and clinical work despite the risk. We are hesitant to co-opt words like courage and bravery that we typically would reserve for people in far more hazardous lines of work than physicians, but in the current setting perhaps courage is the correct term. In quiet conversation, many are vaguely unnerved and some significantly so, but they set their angst aside and get to work. The same can be said for the numerous subspecialists, surgeons, nurses, and others who have volunteered to help.
Alternatively, as leaders, we must manage an extremely small minority of faculty who request to not care for patients with COVID-19 despite no clear contraindication. These situations are nuanced and fraught with difficulty for leaders. As physicians we have moral and ethical obligations to society.7 We also have contractual obligations to our employers. Finally, we have a professional duty to our colleagues. When such cases arise, as leaders we should try to understand the perspective of the physician making the request. It is also important to remember that as leaders we are obliged to be fair and equitable to all faculty; granting exceptions to some who ask to avoid COVID-19-related work, but not to others, is difficult to justify. Moreover, granting exceptions can undermine faith in leadership and inevitably sow discord. We suggest setting clear mutual expectations of engagement and not granting unwarranted exceptions.
CONCLUSION
In this time of a global pandemic, we face a looming shortage of hospital generalists, which calls for immediate and purposeful workforce expansion facilitated by learning to “un-specialize” certain providers. We propose utilizing the framework of FPPE to educate and support those joining hospital medicine teams. Hospitalists are innovators and health systems science leaders. Let’s draw on that strength now to rise to the challenge of COVID-19.
1. Smith A. An Inquiry into the Nature and Causes of the Wealth of Nations. Chicago, Illinois: University of Chicago Press; 1976.
2. Cram P, Ettinger WH, Jr. Generalists or specialists--who does it better? Physician Exec. 1998;24(1):40-45.
3. Accreditation Council for Graduate Medical Education. ACGME Response to Pandemic Crisis. https://acgme.org/COVID-19. Accessed April 1, 2020.
4. The Joint Commission. Emergency Management—Meeting FPPE and OPPE Requirements During the COVID-19 Emergency. https://www.jointcommission.org/standards/standard-faqs/hospital-and-hospital-clinics/medical-staff-ms/000002291/. Accessed April 1, 2020.
5. Petropoulos F, Makridakis S. Forecasting the novel coronavirus COVID-19. PLoS One. 2020;15(3):e0231236. https://doi.org/10.1371/journal.pone.0231236.eCollection 2020.
6. Ioannidis JPA. Coronavirus disease 2019: the harms of exaggerated information and non-evidence-based measures. Eur J Clin Invest. 2020;e13222. https://doi.org/10.1111/eci.13222.
7. Antommaria M. Conflicting duties and reciprocal obligations during a pandemic. J Hosp Med. 2020;15(5):xx-xx. https://doi.org/10.12788/jhm.3425.
Specialization, as detailed in Adam Smith’s 1776 landmark treatise, Wealth of Nations,1 has been an enduring trend in labor and economics for centuries. Mirroring evolution in other sectors of the economy, the healthcare workforce has become ever more specialized.2 General practitioners and family doctors have ceded ground to a bevy of specialists and subspecialists ranging from pediatric endocrinologists to otolaryngology-neurotologists. Given the growth in medical knowledge over the past century, this specialization seems both necessary and good. This same specialization that serves us in good times, though, leaves us woefully underprepared for an epidemic that will require large numbers of hospitalists/generalists and intensivists, such as the current coronavirus disease 2019 (COVID-19) pandemic.
A bit on terminology before we proceed. For purposes of this paper we define generalists as physicians trained in Internal Medicine, Family Medicine, Pediatrics, or Med/Peds who provide primary hospital care to adults and children. While some may argue that hospitalists are specialists in inpatient care, we would like to focus on hospitalists as generalists who focus on inpatient care and what we have in common with the broader community of generalists. We include as generalists anyone, irrespective of clinical training, who chooses broad primary patient responsibility over the narrower consultative role. There is always a specialist in our midst who knows more about a particular disease or condition; as generalists, most of us appreciate and welcome that expertise.
Sometimes it takes a pandemic like COVID-19 to highlight a tremendous blind spot in our healthcare system that, in retrospect, seems hard to have missed. What do we do when we need more generalists and have only a surplus of specialists, many of whom were involuntarily “furloughed” by canceled elective procedures and postponed clinics? How do we “un-specialize” our specialist workforce?
We will discuss some of the most pressing problems facing hospitals working to ensure adequate staffing for general inpatient units caused by the simultaneous reductions in physician availability (because of illness and/or quarantine) and markedly increased admissions of undifferentiated COVID-19–related illnesses. We will assume that hospitals have already activated all providers practicing in areas most similar to hospital medicine, including generalists who have mixed inpatient/outpatient practices, subspecialists with significant inpatient clinical roles, fellows, and advanced practice providers (APPs) with inpatient experience. The Accreditation Council for Graduate Medical Education released guidance around the roles of physician trainees during the pandemic.3 Despite these measures, though, further workforce augmentation will be vital. To that end, several challenges to clinical staffing are enumerated below, accompanied by strategies to address them.
CLINICAL STAFFING CHALLENGES
1. Clinicians eager to help, but out of practice in the inpatient setting: As hospitals across the country work to develop physician staffing contingency plans for scenarios in which general inpatient volumes increase by 50%-300% while 33%-50% of hospitalists either become infected or require quarantine, many hospitals are looking to bolster their physician depth. We have been extremely gratified by the tremendous response from the broader physician communities in which we work. We have encountered retired physicians who have volunteered to come back to work despite being at higher risk of severe COVID-19 complications and physician-scientists offering to step back into clinical roles. We have found outstanding subspecialists asking to work under the tutelage of experienced hospitalists; these specialists recognize how, despite years of clinical experience, they would need significant supervision to function in the inpatient setting. The humility and self-awareness of these volunteers has been phenomenal.
Retraining researchers, subspecialists, and retirees as hospitalists requires purposeful onboarding to target key educational goals. This onboarding should stress COVID-19–specific medical management, training in infection prevention and control, and hospital-specific workflow processes (eg, shift length, sign-over). Onboarding must also include access and orientation to electronic health records, training around inpatient documentation requirements, and billing practices. Non–COVID-19 healthcare will continue; hospitals and clinical leaders will need to determine whether certain specialists should focus on COVID-19 care alone and leave others to continue with speciality practice still needed. Ready access to hospital medicine and medical subspecialty consultation will be pivotal in supervising providers asked to step into hospitalist roles.
The onboarding process we describe might best be viewed through the lens of focused professional practice evaluation (FPPE). Required by the Joint Commission, FPPE is a process for the medical staff of a facility to evaluate privilege-specific competence by clinicians and is used for any new clinical privileges and when there may be question as to a current practitioner’s capabilities. The usual FPPE process includes reassessment of provider practice, typically at 3 to 6 months. Doing so may be challenging given overall workforce stress and the timing of clinical demand—eg, time for medical record review will be limited. Consideration of a “preceptorship” with an experienced hospitalist providing verbal oversight for providers with emergency privileges may be very appropriate. Indeed the Joint Commission recently published guidance around FPPE during the COVID-19 epidemic with the suggestion that mentorship and direct observation are reasonable ways to ensure quality.4
Concerns around scope of practice and medicolegal liability must be rapidly addressed by professional practice organizations, state medical boards, and medical malpractice insurers to protect frontline providers, nurses, and pharmacists. In particular, Joint Commission FPPE process requirements may need to be relaxed to respond to a surge in clinical demand. Contingency and crisis standards of care permit doing so. We welcome the introduction of processes to expedite provider licensure in many hard-hit states.
2. Clinicians who should not help because of medical comorbidities or age: Individuals with certain significant comorbidities (eg, inflammatory conditions treated with immunosuppressants, pulmonary disease, cancer with active treatment) or meeting certain age criteria should be discouraged from clinical work because the dangers of illness for them and of transmission of illness are high. Judgment and a version of mutual informed consent will be needed to address fewer clear scenarios, such as whether a 35-year-old physician who requires a steroid inhaler for asthma or a 64-year-old physician who is otherwise healthy have higher risk. It is our opinion that all physicians should contribute to the care of patients with documented or suspected COVID-19 unless they meet institutionally defined exclusion criteria. We should recognize that physicians who are unable to provide direct care to patients with COVID-19 infection may have significant remorse and feelings that they are letting down their colleagues and the oath they have taken. As the COVID -19 pandemic continues, we are quickly learning that physicians who have contraindications to providing care to patients with active COVID-19 infection can still contribute in numerous mission-critical ways. This may include virtual (telehealth) visits, preceptorship via telehealth of providers completing FPPE in hospital medicine practice, postdischarge follow-up of patients who are no longer infectious, and other care-coordination activities, such as triaging direct admission calls.
3. Clinicians who should be able to help but are fearful: All efforts must be undertaken to protect healthcare workers from acquiring COVID-19. Nevertheless, there are models predicting that ultimately the vast majority of the world’s population will be exposed, including healthcare workers.5,6 In our personal experience as hospitalists and leaders, the vast majority (95%-plus) of our hospitalists have not only continued to do their job but taken on additional responsibilities and clinical work despite the risk. We are hesitant to co-opt words like courage and bravery that we typically would reserve for people in far more hazardous lines of work than physicians, but in the current setting perhaps courage is the correct term. In quiet conversation, many are vaguely unnerved and some significantly so, but they set their angst aside and get to work. The same can be said for the numerous subspecialists, surgeons, nurses, and others who have volunteered to help.
Alternatively, as leaders, we must manage an extremely small minority of faculty who request to not care for patients with COVID-19 despite no clear contraindication. These situations are nuanced and fraught with difficulty for leaders. As physicians we have moral and ethical obligations to society.7 We also have contractual obligations to our employers. Finally, we have a professional duty to our colleagues. When such cases arise, as leaders we should try to understand the perspective of the physician making the request. It is also important to remember that as leaders we are obliged to be fair and equitable to all faculty; granting exceptions to some who ask to avoid COVID-19-related work, but not to others, is difficult to justify. Moreover, granting exceptions can undermine faith in leadership and inevitably sow discord. We suggest setting clear mutual expectations of engagement and not granting unwarranted exceptions.
CONCLUSION
In this time of a global pandemic, we face a looming shortage of hospital generalists, which calls for immediate and purposeful workforce expansion facilitated by learning to “un-specialize” certain providers. We propose utilizing the framework of FPPE to educate and support those joining hospital medicine teams. Hospitalists are innovators and health systems science leaders. Let’s draw on that strength now to rise to the challenge of COVID-19.
Specialization, as detailed in Adam Smith’s 1776 landmark treatise, Wealth of Nations,1 has been an enduring trend in labor and economics for centuries. Mirroring evolution in other sectors of the economy, the healthcare workforce has become ever more specialized.2 General practitioners and family doctors have ceded ground to a bevy of specialists and subspecialists ranging from pediatric endocrinologists to otolaryngology-neurotologists. Given the growth in medical knowledge over the past century, this specialization seems both necessary and good. This same specialization that serves us in good times, though, leaves us woefully underprepared for an epidemic that will require large numbers of hospitalists/generalists and intensivists, such as the current coronavirus disease 2019 (COVID-19) pandemic.
A bit on terminology before we proceed. For purposes of this paper we define generalists as physicians trained in Internal Medicine, Family Medicine, Pediatrics, or Med/Peds who provide primary hospital care to adults and children. While some may argue that hospitalists are specialists in inpatient care, we would like to focus on hospitalists as generalists who focus on inpatient care and what we have in common with the broader community of generalists. We include as generalists anyone, irrespective of clinical training, who chooses broad primary patient responsibility over the narrower consultative role. There is always a specialist in our midst who knows more about a particular disease or condition; as generalists, most of us appreciate and welcome that expertise.
Sometimes it takes a pandemic like COVID-19 to highlight a tremendous blind spot in our healthcare system that, in retrospect, seems hard to have missed. What do we do when we need more generalists and have only a surplus of specialists, many of whom were involuntarily “furloughed” by canceled elective procedures and postponed clinics? How do we “un-specialize” our specialist workforce?
We will discuss some of the most pressing problems facing hospitals working to ensure adequate staffing for general inpatient units caused by the simultaneous reductions in physician availability (because of illness and/or quarantine) and markedly increased admissions of undifferentiated COVID-19–related illnesses. We will assume that hospitals have already activated all providers practicing in areas most similar to hospital medicine, including generalists who have mixed inpatient/outpatient practices, subspecialists with significant inpatient clinical roles, fellows, and advanced practice providers (APPs) with inpatient experience. The Accreditation Council for Graduate Medical Education released guidance around the roles of physician trainees during the pandemic.3 Despite these measures, though, further workforce augmentation will be vital. To that end, several challenges to clinical staffing are enumerated below, accompanied by strategies to address them.
CLINICAL STAFFING CHALLENGES
1. Clinicians eager to help, but out of practice in the inpatient setting: As hospitals across the country work to develop physician staffing contingency plans for scenarios in which general inpatient volumes increase by 50%-300% while 33%-50% of hospitalists either become infected or require quarantine, many hospitals are looking to bolster their physician depth. We have been extremely gratified by the tremendous response from the broader physician communities in which we work. We have encountered retired physicians who have volunteered to come back to work despite being at higher risk of severe COVID-19 complications and physician-scientists offering to step back into clinical roles. We have found outstanding subspecialists asking to work under the tutelage of experienced hospitalists; these specialists recognize how, despite years of clinical experience, they would need significant supervision to function in the inpatient setting. The humility and self-awareness of these volunteers has been phenomenal.
Retraining researchers, subspecialists, and retirees as hospitalists requires purposeful onboarding to target key educational goals. This onboarding should stress COVID-19–specific medical management, training in infection prevention and control, and hospital-specific workflow processes (eg, shift length, sign-over). Onboarding must also include access and orientation to electronic health records, training around inpatient documentation requirements, and billing practices. Non–COVID-19 healthcare will continue; hospitals and clinical leaders will need to determine whether certain specialists should focus on COVID-19 care alone and leave others to continue with speciality practice still needed. Ready access to hospital medicine and medical subspecialty consultation will be pivotal in supervising providers asked to step into hospitalist roles.
The onboarding process we describe might best be viewed through the lens of focused professional practice evaluation (FPPE). Required by the Joint Commission, FPPE is a process for the medical staff of a facility to evaluate privilege-specific competence by clinicians and is used for any new clinical privileges and when there may be question as to a current practitioner’s capabilities. The usual FPPE process includes reassessment of provider practice, typically at 3 to 6 months. Doing so may be challenging given overall workforce stress and the timing of clinical demand—eg, time for medical record review will be limited. Consideration of a “preceptorship” with an experienced hospitalist providing verbal oversight for providers with emergency privileges may be very appropriate. Indeed the Joint Commission recently published guidance around FPPE during the COVID-19 epidemic with the suggestion that mentorship and direct observation are reasonable ways to ensure quality.4
Concerns around scope of practice and medicolegal liability must be rapidly addressed by professional practice organizations, state medical boards, and medical malpractice insurers to protect frontline providers, nurses, and pharmacists. In particular, Joint Commission FPPE process requirements may need to be relaxed to respond to a surge in clinical demand. Contingency and crisis standards of care permit doing so. We welcome the introduction of processes to expedite provider licensure in many hard-hit states.
2. Clinicians who should not help because of medical comorbidities or age: Individuals with certain significant comorbidities (eg, inflammatory conditions treated with immunosuppressants, pulmonary disease, cancer with active treatment) or meeting certain age criteria should be discouraged from clinical work because the dangers of illness for them and of transmission of illness are high. Judgment and a version of mutual informed consent will be needed to address fewer clear scenarios, such as whether a 35-year-old physician who requires a steroid inhaler for asthma or a 64-year-old physician who is otherwise healthy have higher risk. It is our opinion that all physicians should contribute to the care of patients with documented or suspected COVID-19 unless they meet institutionally defined exclusion criteria. We should recognize that physicians who are unable to provide direct care to patients with COVID-19 infection may have significant remorse and feelings that they are letting down their colleagues and the oath they have taken. As the COVID -19 pandemic continues, we are quickly learning that physicians who have contraindications to providing care to patients with active COVID-19 infection can still contribute in numerous mission-critical ways. This may include virtual (telehealth) visits, preceptorship via telehealth of providers completing FPPE in hospital medicine practice, postdischarge follow-up of patients who are no longer infectious, and other care-coordination activities, such as triaging direct admission calls.
3. Clinicians who should be able to help but are fearful: All efforts must be undertaken to protect healthcare workers from acquiring COVID-19. Nevertheless, there are models predicting that ultimately the vast majority of the world’s population will be exposed, including healthcare workers.5,6 In our personal experience as hospitalists and leaders, the vast majority (95%-plus) of our hospitalists have not only continued to do their job but taken on additional responsibilities and clinical work despite the risk. We are hesitant to co-opt words like courage and bravery that we typically would reserve for people in far more hazardous lines of work than physicians, but in the current setting perhaps courage is the correct term. In quiet conversation, many are vaguely unnerved and some significantly so, but they set their angst aside and get to work. The same can be said for the numerous subspecialists, surgeons, nurses, and others who have volunteered to help.
Alternatively, as leaders, we must manage an extremely small minority of faculty who request to not care for patients with COVID-19 despite no clear contraindication. These situations are nuanced and fraught with difficulty for leaders. As physicians we have moral and ethical obligations to society.7 We also have contractual obligations to our employers. Finally, we have a professional duty to our colleagues. When such cases arise, as leaders we should try to understand the perspective of the physician making the request. It is also important to remember that as leaders we are obliged to be fair and equitable to all faculty; granting exceptions to some who ask to avoid COVID-19-related work, but not to others, is difficult to justify. Moreover, granting exceptions can undermine faith in leadership and inevitably sow discord. We suggest setting clear mutual expectations of engagement and not granting unwarranted exceptions.
CONCLUSION
In this time of a global pandemic, we face a looming shortage of hospital generalists, which calls for immediate and purposeful workforce expansion facilitated by learning to “un-specialize” certain providers. We propose utilizing the framework of FPPE to educate and support those joining hospital medicine teams. Hospitalists are innovators and health systems science leaders. Let’s draw on that strength now to rise to the challenge of COVID-19.
1. Smith A. An Inquiry into the Nature and Causes of the Wealth of Nations. Chicago, Illinois: University of Chicago Press; 1976.
2. Cram P, Ettinger WH, Jr. Generalists or specialists--who does it better? Physician Exec. 1998;24(1):40-45.
3. Accreditation Council for Graduate Medical Education. ACGME Response to Pandemic Crisis. https://acgme.org/COVID-19. Accessed April 1, 2020.
4. The Joint Commission. Emergency Management—Meeting FPPE and OPPE Requirements During the COVID-19 Emergency. https://www.jointcommission.org/standards/standard-faqs/hospital-and-hospital-clinics/medical-staff-ms/000002291/. Accessed April 1, 2020.
5. Petropoulos F, Makridakis S. Forecasting the novel coronavirus COVID-19. PLoS One. 2020;15(3):e0231236. https://doi.org/10.1371/journal.pone.0231236.eCollection 2020.
6. Ioannidis JPA. Coronavirus disease 2019: the harms of exaggerated information and non-evidence-based measures. Eur J Clin Invest. 2020;e13222. https://doi.org/10.1111/eci.13222.
7. Antommaria M. Conflicting duties and reciprocal obligations during a pandemic. J Hosp Med. 2020;15(5):xx-xx. https://doi.org/10.12788/jhm.3425.
1. Smith A. An Inquiry into the Nature and Causes of the Wealth of Nations. Chicago, Illinois: University of Chicago Press; 1976.
2. Cram P, Ettinger WH, Jr. Generalists or specialists--who does it better? Physician Exec. 1998;24(1):40-45.
3. Accreditation Council for Graduate Medical Education. ACGME Response to Pandemic Crisis. https://acgme.org/COVID-19. Accessed April 1, 2020.
4. The Joint Commission. Emergency Management—Meeting FPPE and OPPE Requirements During the COVID-19 Emergency. https://www.jointcommission.org/standards/standard-faqs/hospital-and-hospital-clinics/medical-staff-ms/000002291/. Accessed April 1, 2020.
5. Petropoulos F, Makridakis S. Forecasting the novel coronavirus COVID-19. PLoS One. 2020;15(3):e0231236. https://doi.org/10.1371/journal.pone.0231236.eCollection 2020.
6. Ioannidis JPA. Coronavirus disease 2019: the harms of exaggerated information and non-evidence-based measures. Eur J Clin Invest. 2020;e13222. https://doi.org/10.1111/eci.13222.
7. Antommaria M. Conflicting duties and reciprocal obligations during a pandemic. J Hosp Med. 2020;15(5):xx-xx. https://doi.org/10.12788/jhm.3425.
© 2020 Society of Hospital Medicine