User login
Practicing solo and feeling grateful – despite COVID-19
I know that the world has gone upside down. It’s a nightmare, and people are filled with fear, and death is everywhere. In my little bubble of a world, however, I’ve been doing well.
I can’t lose my job, because I am my job. I’m a solo practitioner and have been for more than a decade. The restrictions to stay at home have not affected me, because I have a home office. Besides, I’m an introvert and see myself as a bit of a recluse, so the social distancing hasn’t been stressful. Conducting appointments by phone rather than face to face hasn’t undermined my work, since I can do everything that I do in my office over the phone. But I do it now in sweats and at my desk in my bedroom more often than not. I am prepared for a decrease in income as people lose their jobs, but that hasn’t happened yet. There are still people out there who are very motivated to come off their medications holistically. No rest for the wicked, as the saying goes.
On an emotional level, I feel calm because I’m not attached to material things, though I like them when they’re here. My children and friends have remained healthy, so I am grateful for that. I feel grounded in my belief that life goes on one way or another, and I trust in God to direct me wherever I need to go. Socially, I’ve been forced to be less lazy and cook more at home. As a result: less salt, MSG, and greasy food. I’ve spent a lot less on restaurants this past month and am eating less since I have to eat whatever I cook.
Can a person be more pandemic proof? I was joking with a friend about how pandemic-friendly my lifestyle is: spiritually, mentally, emotionally, physically, and socially. Oh, did I forget to mention the year supply of supplements in my office closet? They were for my patients, but those whole food green and red powders may come in handy, just in case.
So, that is how things are going for me. Please don’t hate me for not freaking out. When I read the news, I feel very sad for people who are suffering. I get angry at the politicians who can’t get their egos out of the way. But, I look at the sunshine outside my window, and I feel grateful that, at least in my case, I am not adding to the burden of suffering in the world. Not yet, anyway. I will keep trying to do the little bit that I do to help others for as long as I can.
Dr. Lee specializes in integrative and holistic psychiatry and has a private practice in Gaithersburg, Md. She has no disclosures.
I know that the world has gone upside down. It’s a nightmare, and people are filled with fear, and death is everywhere. In my little bubble of a world, however, I’ve been doing well.
I can’t lose my job, because I am my job. I’m a solo practitioner and have been for more than a decade. The restrictions to stay at home have not affected me, because I have a home office. Besides, I’m an introvert and see myself as a bit of a recluse, so the social distancing hasn’t been stressful. Conducting appointments by phone rather than face to face hasn’t undermined my work, since I can do everything that I do in my office over the phone. But I do it now in sweats and at my desk in my bedroom more often than not. I am prepared for a decrease in income as people lose their jobs, but that hasn’t happened yet. There are still people out there who are very motivated to come off their medications holistically. No rest for the wicked, as the saying goes.
On an emotional level, I feel calm because I’m not attached to material things, though I like them when they’re here. My children and friends have remained healthy, so I am grateful for that. I feel grounded in my belief that life goes on one way or another, and I trust in God to direct me wherever I need to go. Socially, I’ve been forced to be less lazy and cook more at home. As a result: less salt, MSG, and greasy food. I’ve spent a lot less on restaurants this past month and am eating less since I have to eat whatever I cook.
Can a person be more pandemic proof? I was joking with a friend about how pandemic-friendly my lifestyle is: spiritually, mentally, emotionally, physically, and socially. Oh, did I forget to mention the year supply of supplements in my office closet? They were for my patients, but those whole food green and red powders may come in handy, just in case.
So, that is how things are going for me. Please don’t hate me for not freaking out. When I read the news, I feel very sad for people who are suffering. I get angry at the politicians who can’t get their egos out of the way. But, I look at the sunshine outside my window, and I feel grateful that, at least in my case, I am not adding to the burden of suffering in the world. Not yet, anyway. I will keep trying to do the little bit that I do to help others for as long as I can.
Dr. Lee specializes in integrative and holistic psychiatry and has a private practice in Gaithersburg, Md. She has no disclosures.
I know that the world has gone upside down. It’s a nightmare, and people are filled with fear, and death is everywhere. In my little bubble of a world, however, I’ve been doing well.
I can’t lose my job, because I am my job. I’m a solo practitioner and have been for more than a decade. The restrictions to stay at home have not affected me, because I have a home office. Besides, I’m an introvert and see myself as a bit of a recluse, so the social distancing hasn’t been stressful. Conducting appointments by phone rather than face to face hasn’t undermined my work, since I can do everything that I do in my office over the phone. But I do it now in sweats and at my desk in my bedroom more often than not. I am prepared for a decrease in income as people lose their jobs, but that hasn’t happened yet. There are still people out there who are very motivated to come off their medications holistically. No rest for the wicked, as the saying goes.
On an emotional level, I feel calm because I’m not attached to material things, though I like them when they’re here. My children and friends have remained healthy, so I am grateful for that. I feel grounded in my belief that life goes on one way or another, and I trust in God to direct me wherever I need to go. Socially, I’ve been forced to be less lazy and cook more at home. As a result: less salt, MSG, and greasy food. I’ve spent a lot less on restaurants this past month and am eating less since I have to eat whatever I cook.
Can a person be more pandemic proof? I was joking with a friend about how pandemic-friendly my lifestyle is: spiritually, mentally, emotionally, physically, and socially. Oh, did I forget to mention the year supply of supplements in my office closet? They were for my patients, but those whole food green and red powders may come in handy, just in case.
So, that is how things are going for me. Please don’t hate me for not freaking out. When I read the news, I feel very sad for people who are suffering. I get angry at the politicians who can’t get their egos out of the way. But, I look at the sunshine outside my window, and I feel grateful that, at least in my case, I am not adding to the burden of suffering in the world. Not yet, anyway. I will keep trying to do the little bit that I do to help others for as long as I can.
Dr. Lee specializes in integrative and holistic psychiatry and has a private practice in Gaithersburg, Md. She has no disclosures.
Which of the changes that coronavirus has forced upon us will remain?
Eventually this strange Twilight Zone world of coronavirus will end and life will return to normal.
But obviously it won’t be the same, and like everyone else I wonder what will be different.
Telemedicine is one obvious change in my world, though I don’t know how much yet (granted, no one else does, either). I’m seeing a handful of people that way, limited to established patients, where we’re discussing chronic issues or reviewing recent test results.
If I have to see a new patient or an established one with an urgent issue, I’m still willing to meet them at my office (wearing masks and washing hands frequently). In neurology, a lot still depends on a decent exam. It’s pretty hard to check reflexes, sensory modalities, and muscle tone over the phone. If you think a malpractice attorney is going to give you a pass because you missed something by not examining a patient because of coronavirus ... think again.
I’m not sure how the whole telemedicine thing will play out after the dust settles, at least not at my little practice. I’m currently seeing patients by FaceTime and Skype, neither of which is considered HIPAA compliant. The requirement has been waived during the crisis to make sure people can still see doctors, but I don’t see it lasting beyond that. Privacy will always be a central concern in medicine.
When they declare the pandemic over and say I can’t use FaceTime or Skype anymore, that will likely end my use of such. While there are HIPAA-compliant telemedicine services out there, in a small practice I don’t have the time or money to invest in them.
I also wonder how outcomes will change. I suspect the research-minded will be analyzing 2019 vs. 2020 data for years to come, trying to see if a sudden increase in telemedicine led to better or worse clinical outcomes. I’ll be curious to see what they find and how it breaks down by disease and specialty.
How will work change? Right now my staff of three (including me) are all working separately from home, handling phone calls as if it were another office day. In today’s era that’s easy to set up, and we’re used to the drill from when I’m out of town.
Maybe in the future, on lighter days, I’ll do this more often, and have my staff work from home (on typically busy days I’ll still need them to check patients in and out, fax things, file charts, and do all the other things they do to keep the practice running). The marked decrease in air pollution is certainly noticeable and good for all. When the year is over I’d like to see how non-coronavirus respiratory issues changed between 2019 and 2020.
Other businesses will be looking at that, too, with an increase in telecommuting. Why pay for a large office space when a lot can be done over the Internet? It saves rent, gas, and driving time. How it will affect us, as a socially-dependent species, I have no idea.
It’s the same with grocery delivery. While most of us will likely continue to shop at stores, many will stay with the ease of delivery services after this. It may cost more, but it certainly saves time.
There will be social changes, although how long they’ll last is anyone’s guess. Grocery baggers, stockers, and delivery staff, often seen as lower-level occupations, are now considered part of critical infrastructure in keeping people supplied with food and other necessities, as well as preventing fights from breaking out in the toilet paper and hand-sanitizer aisles.
I’d like to think that, in a country divided, the need to work together will help bring people of different opinions together again, but from the way things look I don’t see that happening, which is sad because viruses don’t discriminate, so we shouldn’t either in fighting them.
Like with other challenges that we face, big and little, I can only hope that we’ll learn something from this and have a better world after it’s over. Only time will tell.
Dr. Block has a solo neurology practice in Scottsdale, Ariz. He has no relevant disclosures.
Eventually this strange Twilight Zone world of coronavirus will end and life will return to normal.
But obviously it won’t be the same, and like everyone else I wonder what will be different.
Telemedicine is one obvious change in my world, though I don’t know how much yet (granted, no one else does, either). I’m seeing a handful of people that way, limited to established patients, where we’re discussing chronic issues or reviewing recent test results.
If I have to see a new patient or an established one with an urgent issue, I’m still willing to meet them at my office (wearing masks and washing hands frequently). In neurology, a lot still depends on a decent exam. It’s pretty hard to check reflexes, sensory modalities, and muscle tone over the phone. If you think a malpractice attorney is going to give you a pass because you missed something by not examining a patient because of coronavirus ... think again.
I’m not sure how the whole telemedicine thing will play out after the dust settles, at least not at my little practice. I’m currently seeing patients by FaceTime and Skype, neither of which is considered HIPAA compliant. The requirement has been waived during the crisis to make sure people can still see doctors, but I don’t see it lasting beyond that. Privacy will always be a central concern in medicine.
When they declare the pandemic over and say I can’t use FaceTime or Skype anymore, that will likely end my use of such. While there are HIPAA-compliant telemedicine services out there, in a small practice I don’t have the time or money to invest in them.
I also wonder how outcomes will change. I suspect the research-minded will be analyzing 2019 vs. 2020 data for years to come, trying to see if a sudden increase in telemedicine led to better or worse clinical outcomes. I’ll be curious to see what they find and how it breaks down by disease and specialty.
How will work change? Right now my staff of three (including me) are all working separately from home, handling phone calls as if it were another office day. In today’s era that’s easy to set up, and we’re used to the drill from when I’m out of town.
Maybe in the future, on lighter days, I’ll do this more often, and have my staff work from home (on typically busy days I’ll still need them to check patients in and out, fax things, file charts, and do all the other things they do to keep the practice running). The marked decrease in air pollution is certainly noticeable and good for all. When the year is over I’d like to see how non-coronavirus respiratory issues changed between 2019 and 2020.
Other businesses will be looking at that, too, with an increase in telecommuting. Why pay for a large office space when a lot can be done over the Internet? It saves rent, gas, and driving time. How it will affect us, as a socially-dependent species, I have no idea.
It’s the same with grocery delivery. While most of us will likely continue to shop at stores, many will stay with the ease of delivery services after this. It may cost more, but it certainly saves time.
There will be social changes, although how long they’ll last is anyone’s guess. Grocery baggers, stockers, and delivery staff, often seen as lower-level occupations, are now considered part of critical infrastructure in keeping people supplied with food and other necessities, as well as preventing fights from breaking out in the toilet paper and hand-sanitizer aisles.
I’d like to think that, in a country divided, the need to work together will help bring people of different opinions together again, but from the way things look I don’t see that happening, which is sad because viruses don’t discriminate, so we shouldn’t either in fighting them.
Like with other challenges that we face, big and little, I can only hope that we’ll learn something from this and have a better world after it’s over. Only time will tell.
Dr. Block has a solo neurology practice in Scottsdale, Ariz. He has no relevant disclosures.
Eventually this strange Twilight Zone world of coronavirus will end and life will return to normal.
But obviously it won’t be the same, and like everyone else I wonder what will be different.
Telemedicine is one obvious change in my world, though I don’t know how much yet (granted, no one else does, either). I’m seeing a handful of people that way, limited to established patients, where we’re discussing chronic issues or reviewing recent test results.
If I have to see a new patient or an established one with an urgent issue, I’m still willing to meet them at my office (wearing masks and washing hands frequently). In neurology, a lot still depends on a decent exam. It’s pretty hard to check reflexes, sensory modalities, and muscle tone over the phone. If you think a malpractice attorney is going to give you a pass because you missed something by not examining a patient because of coronavirus ... think again.
I’m not sure how the whole telemedicine thing will play out after the dust settles, at least not at my little practice. I’m currently seeing patients by FaceTime and Skype, neither of which is considered HIPAA compliant. The requirement has been waived during the crisis to make sure people can still see doctors, but I don’t see it lasting beyond that. Privacy will always be a central concern in medicine.
When they declare the pandemic over and say I can’t use FaceTime or Skype anymore, that will likely end my use of such. While there are HIPAA-compliant telemedicine services out there, in a small practice I don’t have the time or money to invest in them.
I also wonder how outcomes will change. I suspect the research-minded will be analyzing 2019 vs. 2020 data for years to come, trying to see if a sudden increase in telemedicine led to better or worse clinical outcomes. I’ll be curious to see what they find and how it breaks down by disease and specialty.
How will work change? Right now my staff of three (including me) are all working separately from home, handling phone calls as if it were another office day. In today’s era that’s easy to set up, and we’re used to the drill from when I’m out of town.
Maybe in the future, on lighter days, I’ll do this more often, and have my staff work from home (on typically busy days I’ll still need them to check patients in and out, fax things, file charts, and do all the other things they do to keep the practice running). The marked decrease in air pollution is certainly noticeable and good for all. When the year is over I’d like to see how non-coronavirus respiratory issues changed between 2019 and 2020.
Other businesses will be looking at that, too, with an increase in telecommuting. Why pay for a large office space when a lot can be done over the Internet? It saves rent, gas, and driving time. How it will affect us, as a socially-dependent species, I have no idea.
It’s the same with grocery delivery. While most of us will likely continue to shop at stores, many will stay with the ease of delivery services after this. It may cost more, but it certainly saves time.
There will be social changes, although how long they’ll last is anyone’s guess. Grocery baggers, stockers, and delivery staff, often seen as lower-level occupations, are now considered part of critical infrastructure in keeping people supplied with food and other necessities, as well as preventing fights from breaking out in the toilet paper and hand-sanitizer aisles.
I’d like to think that, in a country divided, the need to work together will help bring people of different opinions together again, but from the way things look I don’t see that happening, which is sad because viruses don’t discriminate, so we shouldn’t either in fighting them.
Like with other challenges that we face, big and little, I can only hope that we’ll learn something from this and have a better world after it’s over. Only time will tell.
Dr. Block has a solo neurology practice in Scottsdale, Ariz. He has no relevant disclosures.
COVID-19 CRISIS: We must care for ourselves as we care for others
“I do not shrink from this responsibility, I welcome it.” —John F. Kennedy, inaugural address
COVID-19 has changed our world. Social distancing is now the norm and flattening the curve is our motto. Family physicians’ place on the front line of medicine is more important now than it has ever been.
In the Pennsylvania community in which we work, the first person to don protective gear and sample patients for viral testing in a rapidly organized COVID-19 testing site was John Russell, MD, a family physician. When I asked him about his experience, Dr. Russell said, “No one became a fireman to get cats out of trees ... it was to fight fires. As doctors, this is the same idea ... this is a chance to help fight the fires in our community.”
And, of course, it is primary care providers—family physicians, internists, pediatricians, nurse practitioners, physician assistants, and nurses—who day in and day out are putting aside their own fears, while dealing with those of their family, to come to work with a sense of purpose and courage.
The military uses the term “operational tempo” to describe the speed and intensity of actions relative to the speed and intensity of unfolding events in the operational environment. Family physicians are being asked to work at an increased speed in unfamiliar terrain as our environments change by the hour. The challenge is to answer the call—and take care of ourselves—in unprecedented ways. We often use anticipatory guidance with our patients to help prepare them for the challenges they will face. So, too, must we anticipate the things we will need to be attentive to in the coming months in order to sustain the effort that will be required of us.
With this in mind, we would be wise to consider developing plans in 3 domains: physical, mental, and social.
Physical. With gyms closed and restaurants limiting their offerings to take-out, this is an opportune time to create an exercise regimen at home and experiment with healthy meal options. YouTube videos abound for workouts of every length. And of course, you can simply take a daily walk, go for a run, or take a bike ride. Similarly, good choices can be made with take-out and the foods we prepare at home.
Continue to: Mentally...
Mentally we need the discipline to take breaks, delegate when necessary, and use downtime to clear our minds. Need another option? Consider meditation. Google “best meditation apps” and take your pick.
Social distancing doesn’t have to mean emotional isolation; technology allows us to connect with others through messaging and face-to-face video. We need to remember to regularly check in with those we care about; few things in life are as affirming as the connections with those who are close to us: family, co-workers, and patients.
Out of crisis comes opportunity. Should we be quarantined, we can remind ourselves that Sir Isaac Newton, while in quarantine during the bubonic plague, laid the foundation for classical physics, composed theories on light and optics, and penned his first draft of the law of gravity.1
Life carries on, amidst the pandemic. Even though the current focus is on the COVID-19 crisis, our many needs, joys, and challenges as human beings remain. Today, someone will find out she is pregnant; someone else will be diagnosed with cancer, or plan a wedding, or attend the funeral of a loved one. We, as family physicians, have the training to lead with courage and empathy. We have the expertise gained through years of helping patients though diverse physical and emotional challenges.
We will continue to listen to our patients’ stories, diagnose and treat their diseases, and take steps to bring a sense of calm to the chaos around us. We need to be mindful of our own mindset, because we have a choice. As the psychologist Victor Frankl said in 1946, after being liberated from the concentration camps, “Everything can be taken from a man but one thing: the last of the human freedoms—to choose one’s attitude in any given set of circumstances, to choose one’s own way.”2
1. Brockell G. During a pandemic, Isaac Newton had to work from home, too. He used the time wisely. The Washington Post. March 12, 2020. 2. Frankl VE. Man’s Search for Meaning. Boston, MA: Beacon Press; 2006.
“I do not shrink from this responsibility, I welcome it.” —John F. Kennedy, inaugural address
COVID-19 has changed our world. Social distancing is now the norm and flattening the curve is our motto. Family physicians’ place on the front line of medicine is more important now than it has ever been.
In the Pennsylvania community in which we work, the first person to don protective gear and sample patients for viral testing in a rapidly organized COVID-19 testing site was John Russell, MD, a family physician. When I asked him about his experience, Dr. Russell said, “No one became a fireman to get cats out of trees ... it was to fight fires. As doctors, this is the same idea ... this is a chance to help fight the fires in our community.”
And, of course, it is primary care providers—family physicians, internists, pediatricians, nurse practitioners, physician assistants, and nurses—who day in and day out are putting aside their own fears, while dealing with those of their family, to come to work with a sense of purpose and courage.
The military uses the term “operational tempo” to describe the speed and intensity of actions relative to the speed and intensity of unfolding events in the operational environment. Family physicians are being asked to work at an increased speed in unfamiliar terrain as our environments change by the hour. The challenge is to answer the call—and take care of ourselves—in unprecedented ways. We often use anticipatory guidance with our patients to help prepare them for the challenges they will face. So, too, must we anticipate the things we will need to be attentive to in the coming months in order to sustain the effort that will be required of us.
With this in mind, we would be wise to consider developing plans in 3 domains: physical, mental, and social.
Physical. With gyms closed and restaurants limiting their offerings to take-out, this is an opportune time to create an exercise regimen at home and experiment with healthy meal options. YouTube videos abound for workouts of every length. And of course, you can simply take a daily walk, go for a run, or take a bike ride. Similarly, good choices can be made with take-out and the foods we prepare at home.
Continue to: Mentally...
Mentally we need the discipline to take breaks, delegate when necessary, and use downtime to clear our minds. Need another option? Consider meditation. Google “best meditation apps” and take your pick.
Social distancing doesn’t have to mean emotional isolation; technology allows us to connect with others through messaging and face-to-face video. We need to remember to regularly check in with those we care about; few things in life are as affirming as the connections with those who are close to us: family, co-workers, and patients.
Out of crisis comes opportunity. Should we be quarantined, we can remind ourselves that Sir Isaac Newton, while in quarantine during the bubonic plague, laid the foundation for classical physics, composed theories on light and optics, and penned his first draft of the law of gravity.1
Life carries on, amidst the pandemic. Even though the current focus is on the COVID-19 crisis, our many needs, joys, and challenges as human beings remain. Today, someone will find out she is pregnant; someone else will be diagnosed with cancer, or plan a wedding, or attend the funeral of a loved one. We, as family physicians, have the training to lead with courage and empathy. We have the expertise gained through years of helping patients though diverse physical and emotional challenges.
We will continue to listen to our patients’ stories, diagnose and treat their diseases, and take steps to bring a sense of calm to the chaos around us. We need to be mindful of our own mindset, because we have a choice. As the psychologist Victor Frankl said in 1946, after being liberated from the concentration camps, “Everything can be taken from a man but one thing: the last of the human freedoms—to choose one’s attitude in any given set of circumstances, to choose one’s own way.”2
“I do not shrink from this responsibility, I welcome it.” —John F. Kennedy, inaugural address
COVID-19 has changed our world. Social distancing is now the norm and flattening the curve is our motto. Family physicians’ place on the front line of medicine is more important now than it has ever been.
In the Pennsylvania community in which we work, the first person to don protective gear and sample patients for viral testing in a rapidly organized COVID-19 testing site was John Russell, MD, a family physician. When I asked him about his experience, Dr. Russell said, “No one became a fireman to get cats out of trees ... it was to fight fires. As doctors, this is the same idea ... this is a chance to help fight the fires in our community.”
And, of course, it is primary care providers—family physicians, internists, pediatricians, nurse practitioners, physician assistants, and nurses—who day in and day out are putting aside their own fears, while dealing with those of their family, to come to work with a sense of purpose and courage.
The military uses the term “operational tempo” to describe the speed and intensity of actions relative to the speed and intensity of unfolding events in the operational environment. Family physicians are being asked to work at an increased speed in unfamiliar terrain as our environments change by the hour. The challenge is to answer the call—and take care of ourselves—in unprecedented ways. We often use anticipatory guidance with our patients to help prepare them for the challenges they will face. So, too, must we anticipate the things we will need to be attentive to in the coming months in order to sustain the effort that will be required of us.
With this in mind, we would be wise to consider developing plans in 3 domains: physical, mental, and social.
Physical. With gyms closed and restaurants limiting their offerings to take-out, this is an opportune time to create an exercise regimen at home and experiment with healthy meal options. YouTube videos abound for workouts of every length. And of course, you can simply take a daily walk, go for a run, or take a bike ride. Similarly, good choices can be made with take-out and the foods we prepare at home.
Continue to: Mentally...
Mentally we need the discipline to take breaks, delegate when necessary, and use downtime to clear our minds. Need another option? Consider meditation. Google “best meditation apps” and take your pick.
Social distancing doesn’t have to mean emotional isolation; technology allows us to connect with others through messaging and face-to-face video. We need to remember to regularly check in with those we care about; few things in life are as affirming as the connections with those who are close to us: family, co-workers, and patients.
Out of crisis comes opportunity. Should we be quarantined, we can remind ourselves that Sir Isaac Newton, while in quarantine during the bubonic plague, laid the foundation for classical physics, composed theories on light and optics, and penned his first draft of the law of gravity.1
Life carries on, amidst the pandemic. Even though the current focus is on the COVID-19 crisis, our many needs, joys, and challenges as human beings remain. Today, someone will find out she is pregnant; someone else will be diagnosed with cancer, or plan a wedding, or attend the funeral of a loved one. We, as family physicians, have the training to lead with courage and empathy. We have the expertise gained through years of helping patients though diverse physical and emotional challenges.
We will continue to listen to our patients’ stories, diagnose and treat their diseases, and take steps to bring a sense of calm to the chaos around us. We need to be mindful of our own mindset, because we have a choice. As the psychologist Victor Frankl said in 1946, after being liberated from the concentration camps, “Everything can be taken from a man but one thing: the last of the human freedoms—to choose one’s attitude in any given set of circumstances, to choose one’s own way.”2
1. Brockell G. During a pandemic, Isaac Newton had to work from home, too. He used the time wisely. The Washington Post. March 12, 2020. 2. Frankl VE. Man’s Search for Meaning. Boston, MA: Beacon Press; 2006.
1. Brockell G. During a pandemic, Isaac Newton had to work from home, too. He used the time wisely. The Washington Post. March 12, 2020. 2. Frankl VE. Man’s Search for Meaning. Boston, MA: Beacon Press; 2006.
Red painful nodules in a hospitalized patient
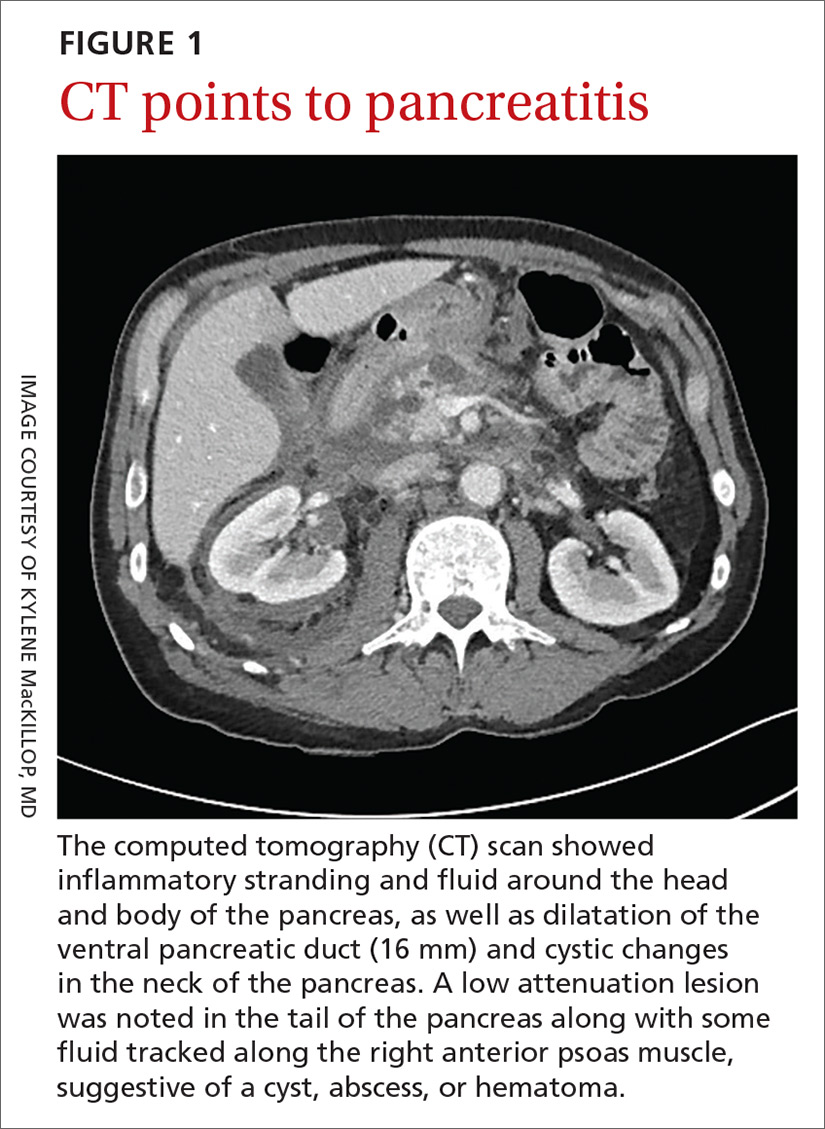
A 58-year-old white man with a history of alcoholism presented to the emergency department with epigastric and right upper quadrant pain radiating to the back, as well as emesis and anorexia. An elevated lipase of 16,609 U/L (reference range, 31–186 U/L) and pathognomonic abdominal computed tomography (CT) findings (FIGURE 1) led to the diagnosis of acute pancreatitis, for which he was admitted.
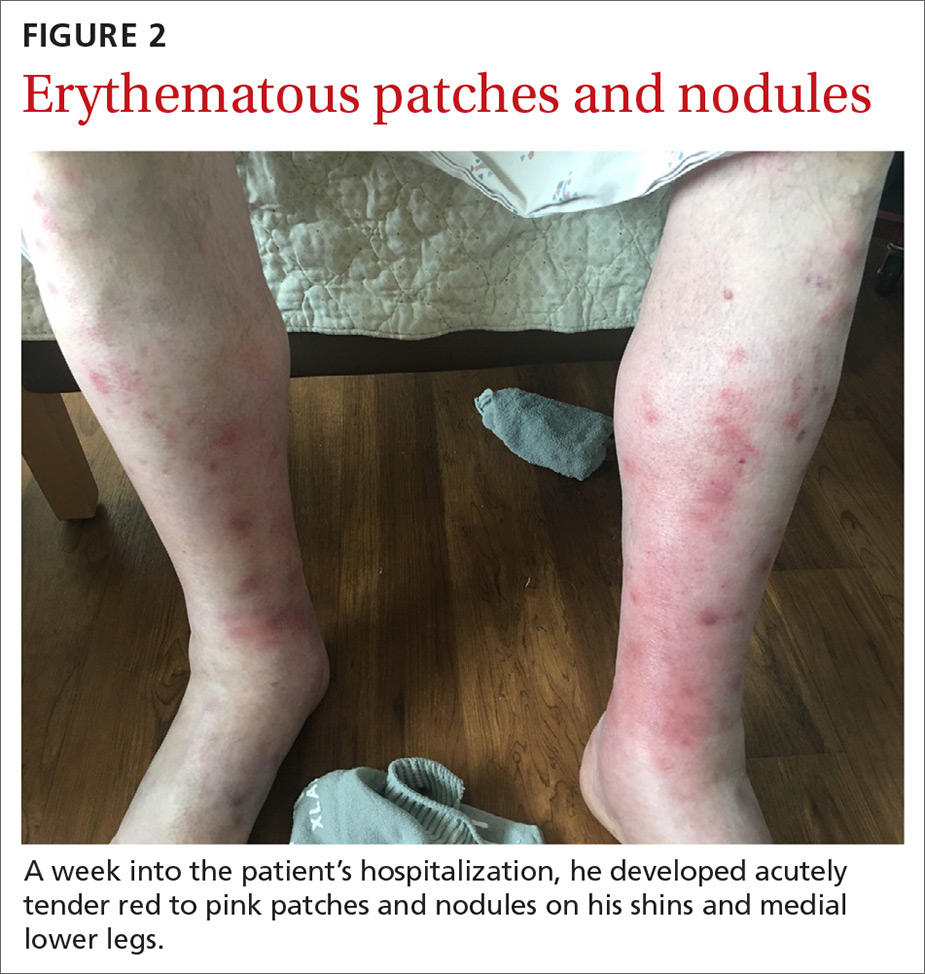
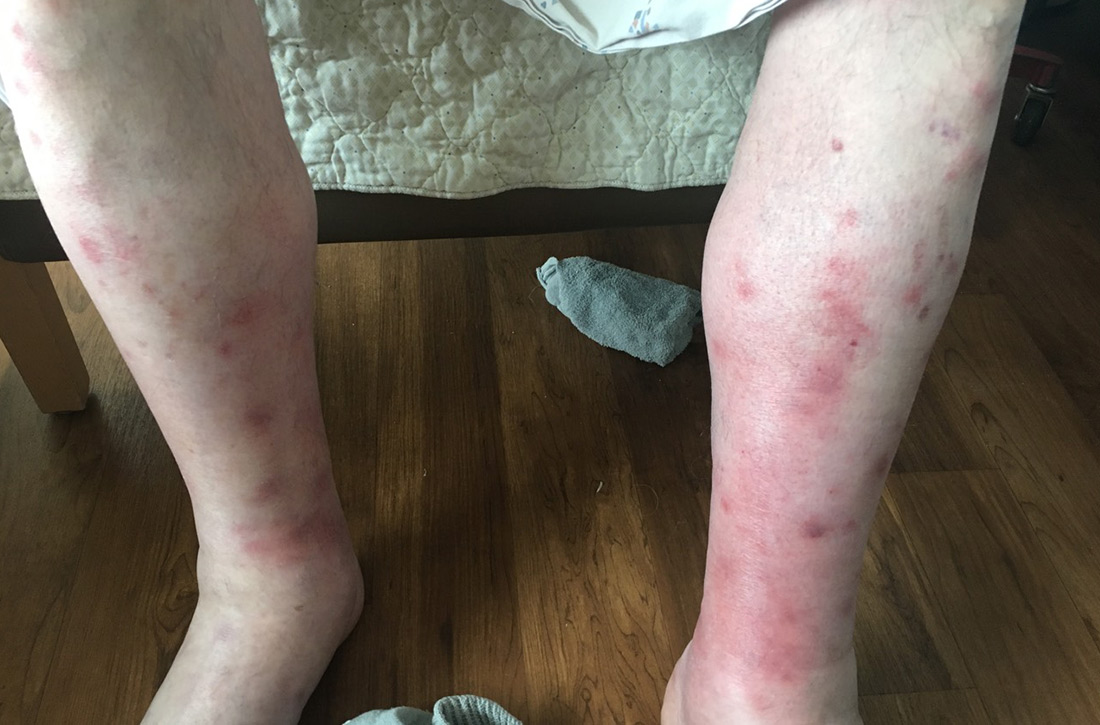
Fluid resuscitation and pain management were implemented, and over 3 days his diet was advanced from NPO to clear fluids to a full diet. On the sixth day of hospitalization, the patient developed increasing abdominal pain and worsening leukocytosis (white blood cell count, 16.6–22 K/mcL [reference range, 4.5–11 K/mcL]). Repeat CT and blood cultures were obtained, and the patient was started on intravenous meropenem 1 g every 8 hours for presumed necrotizing pancreatitis. The next day he developed acutely tender red to pink patches and nodules on his shins and medial lower legs (FIGURE 2).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Pancreatic panniculitis
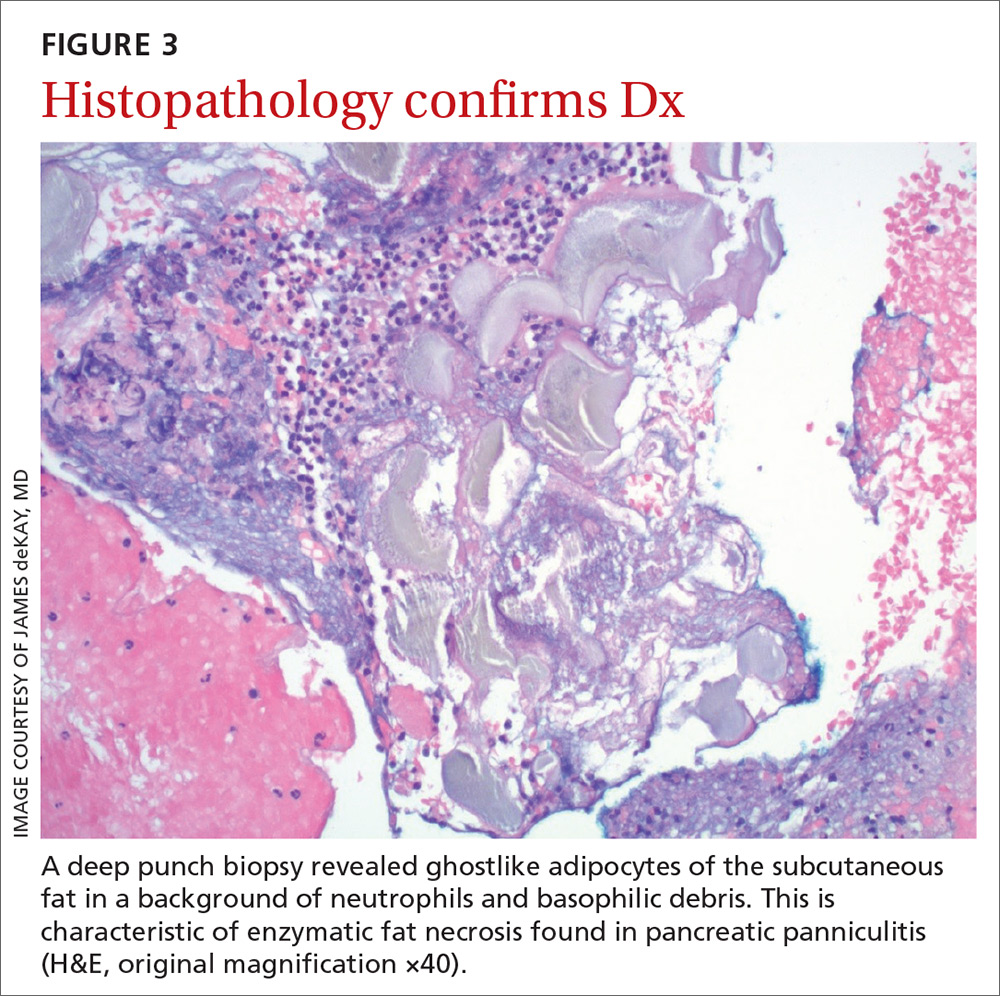
It’s theorized that the systemic release of trypsin from pancreatic cell destruction causes increased capillary permeability and subsequent escape of lipase from the circulation into the subcutaneous fat. This causes fat necrosis, saponification, and inflammation.3,4 Pancreatic panniculitis is demonstrated histologically as hollowed-out adipocytes with granular basophilic cytoplasm and displaced or absent nuclei—aptly named “ghostlike” adipocytes.3-6
Painful, erythematous nodules most commonly present on the distal lower extremities. Nodules may be found over the shins, posterior calves, and periarticular skin. Rarely, nodules may occur on the buttocks, abdomen, or intramedullary bone.7 In severe cases, nodules spontaneously may ulcerate and drain an oily brown, viscous material formed from necrotic adipocytes.1
Timing of the eruption of skin lesions is varied and may even precede abdominal pain. Lesions can involute and regress several weeks after the underlying etiology improves. With pancreatic carcinoma, there is a greater likelihood of persistence, atypical locations of involvement, ulcerations, and recurrences.7
The histologic features of pancreatic panniculitis and the assessment of the subcutaneous fat are paramount in diagnosis. A deep punch biopsy or incisional biopsy is necessary to reliably reach the depth of the subcutaneous tissue. In our patient, a deep punch biopsy from the lateral calf was performed at the suggestion of Dermatology, and histopathology revealed necrosis of fat lobules with calcium soap around necrotic lipocytes, consistent with pancreatic panniculitis (FIGURE 3).
Continue to: Differential was complicated by antibiotic use
Differential was complicated by antibiotic use
The differential diagnosis was broad due to the confounding factors of recent antibiotic use and worsening pancreatitis.
Cellulitis may present as a red patch and is common on the lower legs; it often is associated with skin pathogens including Staphylococcus and Streptococcus. Usually, symptoms are unilateral and associated with warmth to the touch, expanding borders, leukocytosis, and systemic symptoms.
Vasculitis, which is an inflammation of various sized vessels through immunologic or infectious processes, often manifests on the lower legs. The characteristic sign of small vessel vasculitis is nonblanching purpura or petechiae. There often is a preceding illness or medication that triggers immunoglobulin proliferation and off-target inflammation of the vessels. Associated symptoms include pain and pruritus.
Drug eruptions may present as red patches on the skin. Often the patches are scaly and red and have more widespread distribution than the lower legs. A history of exposure is important, but common inciting drugs include nonsteroidal anti-inflammatory drugs that may be used only occasionally and are challenging to elicit in the history. Our patient did have known drug changes (ie, the introduction of meropenem) while hospitalized, but the morphology was not consistent with this diagnosis.
Treatment is directed to underlying disease
Treatment of pancreatic panniculitis primarily is supportive and directed toward treating the underlying pancreatic disease. Depending upon the underlying pancreatic diagnosis, surgical correction of anatomic or ductal anomalies or pseudocysts may lead to resolution of panniculitis.3,7,8
Continue to: In this case
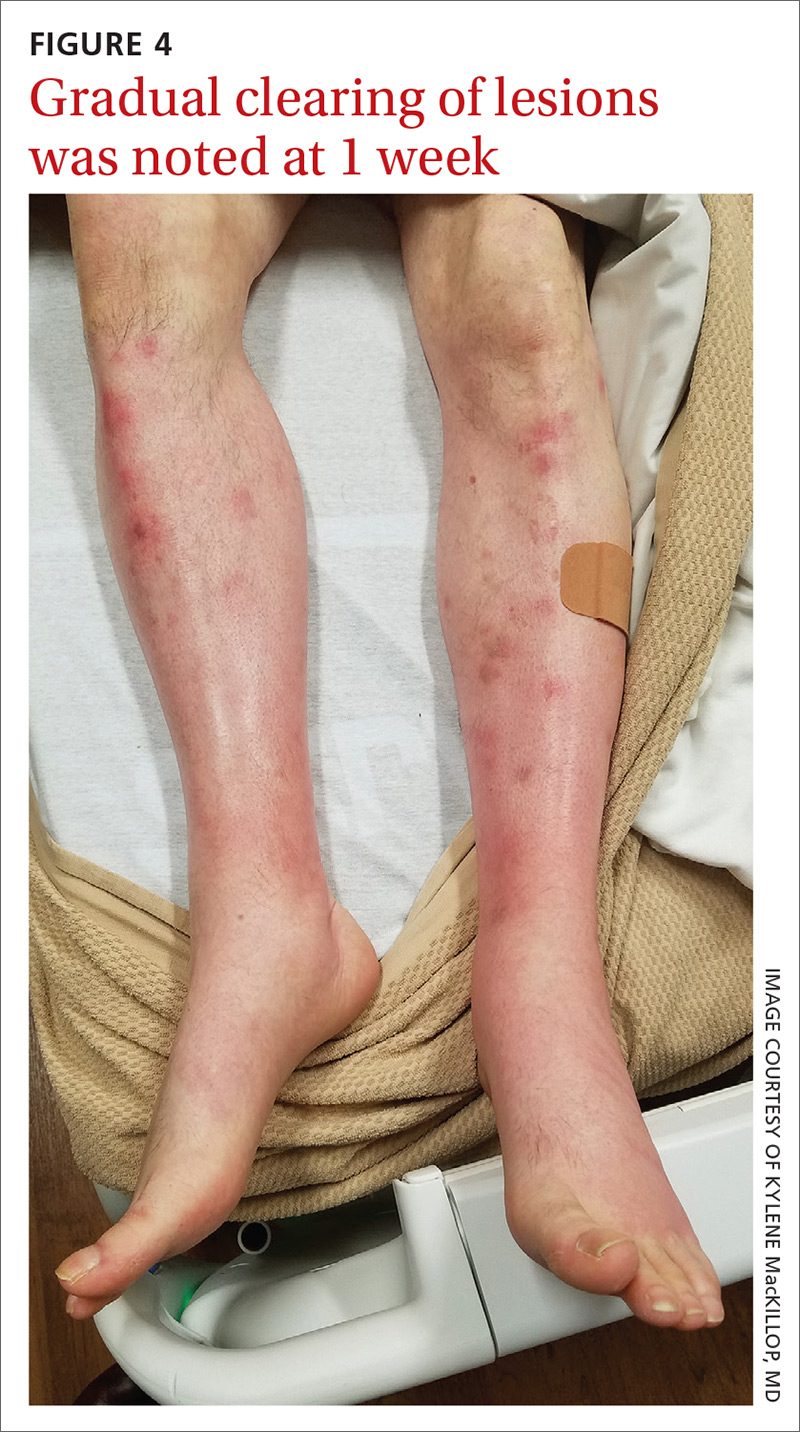
In this case, our patient had already received fluid resuscitation and pain management, and his diet had been advanced. In addition, his antibiotics were changed to exclude drug eruption as a cause. Over the course of a week, our patient saw a reduction in his pain level and an improvement in the appearance of his legs (FIGURE 4).
His pancreatitis, however, continued to persist and resist increases in his diet. He ultimately required transfer to a tertiary care center for consideration of interventional options including stenting. The patient ultimately recovered, after stenting of the main pancreatic duct, and was discharged home.
CORRESPONDENCE
Jonathan Karnes, MD, 6 East Chestnut Street, Augusta, ME 04330; Jonathan.Karnes@mainegeneral.org
1. Madarasingha NP, Satgurunathan K, Fernando R. Pancreatic panniculitis: a rare form of panniculitis. Dermatol Online J. 2009;15:17.
2. Haber RM, Assaad DM. Panniculitis associated with a pancreas divisum. J Am Acad Dermatol. 1986;14(2 pt 2):331-334.
3. Requena L, Sánchez Yus E. Panniculitis. part II. mostly lobular panniculitis. J Am Acad Dermatol. 2001;45:325-361.
4. Rongioletti F, Caputo V. Pancreatic panniculitis. G Ital Dermatol Venereol. 2013;148:419-425.
5. Förström TL, Winkelmann RK. Acute, generalized panniculitis with amylase and lipase in skin. Arch Dermatol. 1975;111:497-502.
6. Hughes SH, Apisarnthanarax P, Mullins F. Subcutaneous fat necrosis associated with pancreatic disease. Arch Dermatol. 1975;111:506-510.
7. Dahl PR, Su WP, Cullimore KC, et al. Pancreatic panniculitis. J Am Acad Dermatol. 1995;33:413-417.
8. Lambiase P, Seery JP, Taylor-Robinson SD, et al. Resolution of panniculitis after placement of pancreatic duct stent in chro nic pancreatitis. Am J Gastroenterol. 1996;91:1835-1837.
A 58-year-old white man with a history of alcoholism presented to the emergency department with epigastric and right upper quadrant pain radiating to the back, as well as emesis and anorexia. An elevated lipase of 16,609 U/L (reference range, 31–186 U/L) and pathognomonic abdominal computed tomography (CT) findings (FIGURE 1) led to the diagnosis of acute pancreatitis, for which he was admitted.
Fluid resuscitation and pain management were implemented, and over 3 days his diet was advanced from NPO to clear fluids to a full diet. On the sixth day of hospitalization, the patient developed increasing abdominal pain and worsening leukocytosis (white blood cell count, 16.6–22 K/mcL [reference range, 4.5–11 K/mcL]). Repeat CT and blood cultures were obtained, and the patient was started on intravenous meropenem 1 g every 8 hours for presumed necrotizing pancreatitis. The next day he developed acutely tender red to pink patches and nodules on his shins and medial lower legs (FIGURE 2).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Pancreatic panniculitis
It’s theorized that the systemic release of trypsin from pancreatic cell destruction causes increased capillary permeability and subsequent escape of lipase from the circulation into the subcutaneous fat. This causes fat necrosis, saponification, and inflammation.3,4 Pancreatic panniculitis is demonstrated histologically as hollowed-out adipocytes with granular basophilic cytoplasm and displaced or absent nuclei—aptly named “ghostlike” adipocytes.3-6
Painful, erythematous nodules most commonly present on the distal lower extremities. Nodules may be found over the shins, posterior calves, and periarticular skin. Rarely, nodules may occur on the buttocks, abdomen, or intramedullary bone.7 In severe cases, nodules spontaneously may ulcerate and drain an oily brown, viscous material formed from necrotic adipocytes.1
Timing of the eruption of skin lesions is varied and may even precede abdominal pain. Lesions can involute and regress several weeks after the underlying etiology improves. With pancreatic carcinoma, there is a greater likelihood of persistence, atypical locations of involvement, ulcerations, and recurrences.7
The histologic features of pancreatic panniculitis and the assessment of the subcutaneous fat are paramount in diagnosis. A deep punch biopsy or incisional biopsy is necessary to reliably reach the depth of the subcutaneous tissue. In our patient, a deep punch biopsy from the lateral calf was performed at the suggestion of Dermatology, and histopathology revealed necrosis of fat lobules with calcium soap around necrotic lipocytes, consistent with pancreatic panniculitis (FIGURE 3).
Continue to: Differential was complicated by antibiotic use
Differential was complicated by antibiotic use
The differential diagnosis was broad due to the confounding factors of recent antibiotic use and worsening pancreatitis.
Cellulitis may present as a red patch and is common on the lower legs; it often is associated with skin pathogens including Staphylococcus and Streptococcus. Usually, symptoms are unilateral and associated with warmth to the touch, expanding borders, leukocytosis, and systemic symptoms.
Vasculitis, which is an inflammation of various sized vessels through immunologic or infectious processes, often manifests on the lower legs. The characteristic sign of small vessel vasculitis is nonblanching purpura or petechiae. There often is a preceding illness or medication that triggers immunoglobulin proliferation and off-target inflammation of the vessels. Associated symptoms include pain and pruritus.
Drug eruptions may present as red patches on the skin. Often the patches are scaly and red and have more widespread distribution than the lower legs. A history of exposure is important, but common inciting drugs include nonsteroidal anti-inflammatory drugs that may be used only occasionally and are challenging to elicit in the history. Our patient did have known drug changes (ie, the introduction of meropenem) while hospitalized, but the morphology was not consistent with this diagnosis.
Treatment is directed to underlying disease
Treatment of pancreatic panniculitis primarily is supportive and directed toward treating the underlying pancreatic disease. Depending upon the underlying pancreatic diagnosis, surgical correction of anatomic or ductal anomalies or pseudocysts may lead to resolution of panniculitis.3,7,8
Continue to: In this case
In this case, our patient had already received fluid resuscitation and pain management, and his diet had been advanced. In addition, his antibiotics were changed to exclude drug eruption as a cause. Over the course of a week, our patient saw a reduction in his pain level and an improvement in the appearance of his legs (FIGURE 4).
His pancreatitis, however, continued to persist and resist increases in his diet. He ultimately required transfer to a tertiary care center for consideration of interventional options including stenting. The patient ultimately recovered, after stenting of the main pancreatic duct, and was discharged home.
CORRESPONDENCE
Jonathan Karnes, MD, 6 East Chestnut Street, Augusta, ME 04330; Jonathan.Karnes@mainegeneral.org
A 58-year-old white man with a history of alcoholism presented to the emergency department with epigastric and right upper quadrant pain radiating to the back, as well as emesis and anorexia. An elevated lipase of 16,609 U/L (reference range, 31–186 U/L) and pathognomonic abdominal computed tomography (CT) findings (FIGURE 1) led to the diagnosis of acute pancreatitis, for which he was admitted.
Fluid resuscitation and pain management were implemented, and over 3 days his diet was advanced from NPO to clear fluids to a full diet. On the sixth day of hospitalization, the patient developed increasing abdominal pain and worsening leukocytosis (white blood cell count, 16.6–22 K/mcL [reference range, 4.5–11 K/mcL]). Repeat CT and blood cultures were obtained, and the patient was started on intravenous meropenem 1 g every 8 hours for presumed necrotizing pancreatitis. The next day he developed acutely tender red to pink patches and nodules on his shins and medial lower legs (FIGURE 2).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Pancreatic panniculitis
It’s theorized that the systemic release of trypsin from pancreatic cell destruction causes increased capillary permeability and subsequent escape of lipase from the circulation into the subcutaneous fat. This causes fat necrosis, saponification, and inflammation.3,4 Pancreatic panniculitis is demonstrated histologically as hollowed-out adipocytes with granular basophilic cytoplasm and displaced or absent nuclei—aptly named “ghostlike” adipocytes.3-6
Painful, erythematous nodules most commonly present on the distal lower extremities. Nodules may be found over the shins, posterior calves, and periarticular skin. Rarely, nodules may occur on the buttocks, abdomen, or intramedullary bone.7 In severe cases, nodules spontaneously may ulcerate and drain an oily brown, viscous material formed from necrotic adipocytes.1
Timing of the eruption of skin lesions is varied and may even precede abdominal pain. Lesions can involute and regress several weeks after the underlying etiology improves. With pancreatic carcinoma, there is a greater likelihood of persistence, atypical locations of involvement, ulcerations, and recurrences.7
The histologic features of pancreatic panniculitis and the assessment of the subcutaneous fat are paramount in diagnosis. A deep punch biopsy or incisional biopsy is necessary to reliably reach the depth of the subcutaneous tissue. In our patient, a deep punch biopsy from the lateral calf was performed at the suggestion of Dermatology, and histopathology revealed necrosis of fat lobules with calcium soap around necrotic lipocytes, consistent with pancreatic panniculitis (FIGURE 3).
Continue to: Differential was complicated by antibiotic use
Differential was complicated by antibiotic use
The differential diagnosis was broad due to the confounding factors of recent antibiotic use and worsening pancreatitis.
Cellulitis may present as a red patch and is common on the lower legs; it often is associated with skin pathogens including Staphylococcus and Streptococcus. Usually, symptoms are unilateral and associated with warmth to the touch, expanding borders, leukocytosis, and systemic symptoms.
Vasculitis, which is an inflammation of various sized vessels through immunologic or infectious processes, often manifests on the lower legs. The characteristic sign of small vessel vasculitis is nonblanching purpura or petechiae. There often is a preceding illness or medication that triggers immunoglobulin proliferation and off-target inflammation of the vessels. Associated symptoms include pain and pruritus.
Drug eruptions may present as red patches on the skin. Often the patches are scaly and red and have more widespread distribution than the lower legs. A history of exposure is important, but common inciting drugs include nonsteroidal anti-inflammatory drugs that may be used only occasionally and are challenging to elicit in the history. Our patient did have known drug changes (ie, the introduction of meropenem) while hospitalized, but the morphology was not consistent with this diagnosis.
Treatment is directed to underlying disease
Treatment of pancreatic panniculitis primarily is supportive and directed toward treating the underlying pancreatic disease. Depending upon the underlying pancreatic diagnosis, surgical correction of anatomic or ductal anomalies or pseudocysts may lead to resolution of panniculitis.3,7,8
Continue to: In this case
In this case, our patient had already received fluid resuscitation and pain management, and his diet had been advanced. In addition, his antibiotics were changed to exclude drug eruption as a cause. Over the course of a week, our patient saw a reduction in his pain level and an improvement in the appearance of his legs (FIGURE 4).
His pancreatitis, however, continued to persist and resist increases in his diet. He ultimately required transfer to a tertiary care center for consideration of interventional options including stenting. The patient ultimately recovered, after stenting of the main pancreatic duct, and was discharged home.
CORRESPONDENCE
Jonathan Karnes, MD, 6 East Chestnut Street, Augusta, ME 04330; Jonathan.Karnes@mainegeneral.org
1. Madarasingha NP, Satgurunathan K, Fernando R. Pancreatic panniculitis: a rare form of panniculitis. Dermatol Online J. 2009;15:17.
2. Haber RM, Assaad DM. Panniculitis associated with a pancreas divisum. J Am Acad Dermatol. 1986;14(2 pt 2):331-334.
3. Requena L, Sánchez Yus E. Panniculitis. part II. mostly lobular panniculitis. J Am Acad Dermatol. 2001;45:325-361.
4. Rongioletti F, Caputo V. Pancreatic panniculitis. G Ital Dermatol Venereol. 2013;148:419-425.
5. Förström TL, Winkelmann RK. Acute, generalized panniculitis with amylase and lipase in skin. Arch Dermatol. 1975;111:497-502.
6. Hughes SH, Apisarnthanarax P, Mullins F. Subcutaneous fat necrosis associated with pancreatic disease. Arch Dermatol. 1975;111:506-510.
7. Dahl PR, Su WP, Cullimore KC, et al. Pancreatic panniculitis. J Am Acad Dermatol. 1995;33:413-417.
8. Lambiase P, Seery JP, Taylor-Robinson SD, et al. Resolution of panniculitis after placement of pancreatic duct stent in chro nic pancreatitis. Am J Gastroenterol. 1996;91:1835-1837.
1. Madarasingha NP, Satgurunathan K, Fernando R. Pancreatic panniculitis: a rare form of panniculitis. Dermatol Online J. 2009;15:17.
2. Haber RM, Assaad DM. Panniculitis associated with a pancreas divisum. J Am Acad Dermatol. 1986;14(2 pt 2):331-334.
3. Requena L, Sánchez Yus E. Panniculitis. part II. mostly lobular panniculitis. J Am Acad Dermatol. 2001;45:325-361.
4. Rongioletti F, Caputo V. Pancreatic panniculitis. G Ital Dermatol Venereol. 2013;148:419-425.
5. Förström TL, Winkelmann RK. Acute, generalized panniculitis with amylase and lipase in skin. Arch Dermatol. 1975;111:497-502.
6. Hughes SH, Apisarnthanarax P, Mullins F. Subcutaneous fat necrosis associated with pancreatic disease. Arch Dermatol. 1975;111:506-510.
7. Dahl PR, Su WP, Cullimore KC, et al. Pancreatic panniculitis. J Am Acad Dermatol. 1995;33:413-417.
8. Lambiase P, Seery JP, Taylor-Robinson SD, et al. Resolution of panniculitis after placement of pancreatic duct stent in chro nic pancreatitis. Am J Gastroenterol. 1996;91:1835-1837.
Aspirin, Yes, for at-risk elderly—but what about the healthy elderly?
ILLUSTRATIVE CASE
A healthy 72-year-old man with well-controlled hypertension on amlodipine 10 mg/d presents to you for an annual exam. He has no history of coronary artery disease or stroke. Should you recommend that he start aspirin for primary prevention of cardiovascular disease?
Cardiovascular disease (CVD) remains the leading cause of death in the United States.2 Aspirin therapy remains the standard of care for secondary prevention of CVD in patients with known coronary artery disease (CAD).3 Aspirin reduces the risk of atherothrombosis by irreversibly inhibiting platelet function. At the same time, it increases the risk of major bleeding, including gastrointestinal bleeds and hemorrhagic strokes. Even though the benefit of aspirin in patients with known CAD is well established, the benefit of aspirin as primary prevention is less certain.
Two recent large randomized controlled trials (RCTs) examined the benefits and risks of aspirin in a variety of patient populations. The ARRIVE trial looked at more than 12,000 patients with a mean age of 63 years with moderate risk of CVD (approximately 15% risk of a cardiovascular event in 10 years) and randomly assigned them to receive aspirin or placebo.4 After an average follow-up period of 5 years, researchers observed that actual cardiovascular event risk was < 10% in both groups, and there was no significant difference in the primary outcome of first cardiovascular event or all-cause mortality. There was, however, a significant increase in bleeding events in the group receiving aspirin.4
The ASCEND trial evaluated aspirin vs placebo in more than 15,000 adult patients with type 2 diabetes mellitus and a low risk of CVD (< 10% risk of cardiovascular event in 5 years). 5 The primary endpoint of the study was first cardiovascular event. The authors found a significantly lower rate of cardiovascular events in the aspirin group, as well as more major bleeding events. Additionally, there was no difference between the aspirin and placebo groups in all-cause mortality after 7 years. The authors concluded that the benefits of aspirin in this group were counterbalanced by the harms.5
Currently, several organizations offer recommendations on aspirin use in people 40 to 70 years of age based on a patient’s risk of bleeding and risk of CVD.6-8 Recommendations regarding aspirin use as primary prevention have been less clear for patients < 40 and > 70 years of age.6
Elderly patients are at higher risk of CVD and bleeding, but until recently, few studies had evaluated elderly populations to assess the benefits vs the risks of aspirin for primary CVD prevention. As of 2016, the US Preventive Services Task Force (USPSTF) stated the evidence was insufficient to assess the balance of the benefits and harms of initiating aspirin use for primary prevention of CVD in patients older than 70 years of age.6 This trial focuses on aspirin use for primary prevention of CVD in healthy elderly adults.
STUDY SUMMARY
Don’t use aspirin as primary prevention of CVD in the elderly
This secondary analysis of a prior double-blind RCT, which found low-dose aspirin did not prolong survival in elderly patients, examined the effect of aspirin on CVD and hemorrhage in 19,114 elderly patients without known CVD.1 The patients were ≥ 70 years of age (≥ 65 years for blacks and Hispanics) with a mean age of 74 years and were from Australia (87%) and the United States (13%). Approximately one-third of the patients were taking a statin, and 14% were taking a nonsteroidal anti-inflammatory drug (NSAID) regularly. Patients were randomized to either aspirin 100 mg/d or matching placebo and were followed for an average of 4.7 years.
Continue to: Outcomes
Outcomes. The outcome of CVD was a composite of fatal coronary heart disease, nonfatal myocardial infarction (MI), fatal or nonfatal ischemic stroke, or hospitalization for heart failure, and the outcome of major adverse cardiovascular event was a composite of fatal cardiovascular disease (excluding death from heart failure), nonfatal MI, or fatal and nonfatal ischemic stroke.
Results. No difference was seen between the aspirin and placebo groups in CVD outcomes (10.7 events per 1000 person-years vs 11.3 events per 1000 person-years, respectively; hazard ratio [HR] = 0.95; 95% confidence interval [CI], 0.83-1.08) or major cardiovascular events (7.8 events per 1000 person-years vs 8.8 events per 1000 person-years, respectively; HR = 0.89; 95% CI, 0.77-1.03). The composite and individual endpoints of fatal cardiovascular disease, heart failure hospitalizations, fatal and nonfatal MI, and ischemic stroke also did not differ significantly between the groups.
The rate of major hemorrhagic events (composite of hemorrhagic stroke, intracranial bleed, or extracranial bleed), however, was higher in the aspirin vs the placebo group (8.6 events per 1000 person-years vs 6.2 events per 1000 person-years, respectively; HR = 1.4; 95% CI, 1.2-1.6; number needed to harm = 98).
WHAT’S NEW
Finding of more harm than good leads to change in ACC/AHA guidelines
Although the most recent USPSTF guidelines state the evidence is insufficient to assess the risks and benefits of aspirin for the primary prevention of cardiovascular disease in this age group, this trial reveals there is a greater risk of hemorrhagic events than there is prevention of cardiovascular outcomes with aspirin use in healthy elderly patients > 70 years of age.6 Because of this trial, the American College of Cardiology (ACC) and the American Heart Association (AHA) have updated their guidelines on the primary prevention of cardiovascular disease to recommend that aspirin not be used routinely in patients > 70 years of age.7
CAVEATS
Potential benefit to people at higher risk?
The rate of cardiovascular disease was lower than expected in this overall healthy population, so it is not known if cardiovascular benefits may outweigh the risk of bleeding in a higher-risk population. The trial also didn’t address the potential harms of deprescribing aspirin. Additionally, although aspirin may not be protective for cardiovascular events and may lead to more bleeding, there may be other benefits to aspirin in this patient population that were not addressed by this study.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
Popular beliefs and wide availability may make tide difficult to change
Patients have been told for years to take a daily aspirin to “protect their heart”; this behavior may be difficult to change. And because aspirin is widely available over the counter, patients may take it without their physician’s knowledge.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. McNeil JJ, Wolfe R, Woods RL, et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med. 2018;379:1509-1518.
2. Murphy SL, Xu JQ, Kochanek KD, et al. Mortality in the United States, 2017. NCHS Data Brief, no. 328. Hyattsville, MD: National Center for Health Statistics. 2018.
3. Smith SC Jr, Benjamin EJ, Bonow RO, et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation. 2011;124:2458-2473.
4. Gaziano JM, Brotons C, Coppolecchia R, et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomised, double-blind, placebo-controlled trial. Lancet. 2018;392:1036-1046.
5. Bowman L, Mafham M, Wallendszus K, et al; ASCEND Study Collaborative Group. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med. 2018;379:1529-1539.
6. Bibbins-Domingo K; U.S. Preventive Services Task Force. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2016;164:836-845.
7. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Amer Coll Cardiol. 2019;74:1376-1414.
8. American Diabetes Association. Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S103-S123.
ILLUSTRATIVE CASE
A healthy 72-year-old man with well-controlled hypertension on amlodipine 10 mg/d presents to you for an annual exam. He has no history of coronary artery disease or stroke. Should you recommend that he start aspirin for primary prevention of cardiovascular disease?
Cardiovascular disease (CVD) remains the leading cause of death in the United States.2 Aspirin therapy remains the standard of care for secondary prevention of CVD in patients with known coronary artery disease (CAD).3 Aspirin reduces the risk of atherothrombosis by irreversibly inhibiting platelet function. At the same time, it increases the risk of major bleeding, including gastrointestinal bleeds and hemorrhagic strokes. Even though the benefit of aspirin in patients with known CAD is well established, the benefit of aspirin as primary prevention is less certain.
Two recent large randomized controlled trials (RCTs) examined the benefits and risks of aspirin in a variety of patient populations. The ARRIVE trial looked at more than 12,000 patients with a mean age of 63 years with moderate risk of CVD (approximately 15% risk of a cardiovascular event in 10 years) and randomly assigned them to receive aspirin or placebo.4 After an average follow-up period of 5 years, researchers observed that actual cardiovascular event risk was < 10% in both groups, and there was no significant difference in the primary outcome of first cardiovascular event or all-cause mortality. There was, however, a significant increase in bleeding events in the group receiving aspirin.4
The ASCEND trial evaluated aspirin vs placebo in more than 15,000 adult patients with type 2 diabetes mellitus and a low risk of CVD (< 10% risk of cardiovascular event in 5 years). 5 The primary endpoint of the study was first cardiovascular event. The authors found a significantly lower rate of cardiovascular events in the aspirin group, as well as more major bleeding events. Additionally, there was no difference between the aspirin and placebo groups in all-cause mortality after 7 years. The authors concluded that the benefits of aspirin in this group were counterbalanced by the harms.5
Currently, several organizations offer recommendations on aspirin use in people 40 to 70 years of age based on a patient’s risk of bleeding and risk of CVD.6-8 Recommendations regarding aspirin use as primary prevention have been less clear for patients < 40 and > 70 years of age.6
Elderly patients are at higher risk of CVD and bleeding, but until recently, few studies had evaluated elderly populations to assess the benefits vs the risks of aspirin for primary CVD prevention. As of 2016, the US Preventive Services Task Force (USPSTF) stated the evidence was insufficient to assess the balance of the benefits and harms of initiating aspirin use for primary prevention of CVD in patients older than 70 years of age.6 This trial focuses on aspirin use for primary prevention of CVD in healthy elderly adults.
STUDY SUMMARY
Don’t use aspirin as primary prevention of CVD in the elderly
This secondary analysis of a prior double-blind RCT, which found low-dose aspirin did not prolong survival in elderly patients, examined the effect of aspirin on CVD and hemorrhage in 19,114 elderly patients without known CVD.1 The patients were ≥ 70 years of age (≥ 65 years for blacks and Hispanics) with a mean age of 74 years and were from Australia (87%) and the United States (13%). Approximately one-third of the patients were taking a statin, and 14% were taking a nonsteroidal anti-inflammatory drug (NSAID) regularly. Patients were randomized to either aspirin 100 mg/d or matching placebo and were followed for an average of 4.7 years.
Continue to: Outcomes
Outcomes. The outcome of CVD was a composite of fatal coronary heart disease, nonfatal myocardial infarction (MI), fatal or nonfatal ischemic stroke, or hospitalization for heart failure, and the outcome of major adverse cardiovascular event was a composite of fatal cardiovascular disease (excluding death from heart failure), nonfatal MI, or fatal and nonfatal ischemic stroke.
Results. No difference was seen between the aspirin and placebo groups in CVD outcomes (10.7 events per 1000 person-years vs 11.3 events per 1000 person-years, respectively; hazard ratio [HR] = 0.95; 95% confidence interval [CI], 0.83-1.08) or major cardiovascular events (7.8 events per 1000 person-years vs 8.8 events per 1000 person-years, respectively; HR = 0.89; 95% CI, 0.77-1.03). The composite and individual endpoints of fatal cardiovascular disease, heart failure hospitalizations, fatal and nonfatal MI, and ischemic stroke also did not differ significantly between the groups.
The rate of major hemorrhagic events (composite of hemorrhagic stroke, intracranial bleed, or extracranial bleed), however, was higher in the aspirin vs the placebo group (8.6 events per 1000 person-years vs 6.2 events per 1000 person-years, respectively; HR = 1.4; 95% CI, 1.2-1.6; number needed to harm = 98).
WHAT’S NEW
Finding of more harm than good leads to change in ACC/AHA guidelines
Although the most recent USPSTF guidelines state the evidence is insufficient to assess the risks and benefits of aspirin for the primary prevention of cardiovascular disease in this age group, this trial reveals there is a greater risk of hemorrhagic events than there is prevention of cardiovascular outcomes with aspirin use in healthy elderly patients > 70 years of age.6 Because of this trial, the American College of Cardiology (ACC) and the American Heart Association (AHA) have updated their guidelines on the primary prevention of cardiovascular disease to recommend that aspirin not be used routinely in patients > 70 years of age.7
CAVEATS
Potential benefit to people at higher risk?
The rate of cardiovascular disease was lower than expected in this overall healthy population, so it is not known if cardiovascular benefits may outweigh the risk of bleeding in a higher-risk population. The trial also didn’t address the potential harms of deprescribing aspirin. Additionally, although aspirin may not be protective for cardiovascular events and may lead to more bleeding, there may be other benefits to aspirin in this patient population that were not addressed by this study.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
Popular beliefs and wide availability may make tide difficult to change
Patients have been told for years to take a daily aspirin to “protect their heart”; this behavior may be difficult to change. And because aspirin is widely available over the counter, patients may take it without their physician’s knowledge.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A healthy 72-year-old man with well-controlled hypertension on amlodipine 10 mg/d presents to you for an annual exam. He has no history of coronary artery disease or stroke. Should you recommend that he start aspirin for primary prevention of cardiovascular disease?
Cardiovascular disease (CVD) remains the leading cause of death in the United States.2 Aspirin therapy remains the standard of care for secondary prevention of CVD in patients with known coronary artery disease (CAD).3 Aspirin reduces the risk of atherothrombosis by irreversibly inhibiting platelet function. At the same time, it increases the risk of major bleeding, including gastrointestinal bleeds and hemorrhagic strokes. Even though the benefit of aspirin in patients with known CAD is well established, the benefit of aspirin as primary prevention is less certain.
Two recent large randomized controlled trials (RCTs) examined the benefits and risks of aspirin in a variety of patient populations. The ARRIVE trial looked at more than 12,000 patients with a mean age of 63 years with moderate risk of CVD (approximately 15% risk of a cardiovascular event in 10 years) and randomly assigned them to receive aspirin or placebo.4 After an average follow-up period of 5 years, researchers observed that actual cardiovascular event risk was < 10% in both groups, and there was no significant difference in the primary outcome of first cardiovascular event or all-cause mortality. There was, however, a significant increase in bleeding events in the group receiving aspirin.4
The ASCEND trial evaluated aspirin vs placebo in more than 15,000 adult patients with type 2 diabetes mellitus and a low risk of CVD (< 10% risk of cardiovascular event in 5 years). 5 The primary endpoint of the study was first cardiovascular event. The authors found a significantly lower rate of cardiovascular events in the aspirin group, as well as more major bleeding events. Additionally, there was no difference between the aspirin and placebo groups in all-cause mortality after 7 years. The authors concluded that the benefits of aspirin in this group were counterbalanced by the harms.5
Currently, several organizations offer recommendations on aspirin use in people 40 to 70 years of age based on a patient’s risk of bleeding and risk of CVD.6-8 Recommendations regarding aspirin use as primary prevention have been less clear for patients < 40 and > 70 years of age.6
Elderly patients are at higher risk of CVD and bleeding, but until recently, few studies had evaluated elderly populations to assess the benefits vs the risks of aspirin for primary CVD prevention. As of 2016, the US Preventive Services Task Force (USPSTF) stated the evidence was insufficient to assess the balance of the benefits and harms of initiating aspirin use for primary prevention of CVD in patients older than 70 years of age.6 This trial focuses on aspirin use for primary prevention of CVD in healthy elderly adults.
STUDY SUMMARY
Don’t use aspirin as primary prevention of CVD in the elderly
This secondary analysis of a prior double-blind RCT, which found low-dose aspirin did not prolong survival in elderly patients, examined the effect of aspirin on CVD and hemorrhage in 19,114 elderly patients without known CVD.1 The patients were ≥ 70 years of age (≥ 65 years for blacks and Hispanics) with a mean age of 74 years and were from Australia (87%) and the United States (13%). Approximately one-third of the patients were taking a statin, and 14% were taking a nonsteroidal anti-inflammatory drug (NSAID) regularly. Patients were randomized to either aspirin 100 mg/d or matching placebo and were followed for an average of 4.7 years.
Continue to: Outcomes
Outcomes. The outcome of CVD was a composite of fatal coronary heart disease, nonfatal myocardial infarction (MI), fatal or nonfatal ischemic stroke, or hospitalization for heart failure, and the outcome of major adverse cardiovascular event was a composite of fatal cardiovascular disease (excluding death from heart failure), nonfatal MI, or fatal and nonfatal ischemic stroke.
Results. No difference was seen between the aspirin and placebo groups in CVD outcomes (10.7 events per 1000 person-years vs 11.3 events per 1000 person-years, respectively; hazard ratio [HR] = 0.95; 95% confidence interval [CI], 0.83-1.08) or major cardiovascular events (7.8 events per 1000 person-years vs 8.8 events per 1000 person-years, respectively; HR = 0.89; 95% CI, 0.77-1.03). The composite and individual endpoints of fatal cardiovascular disease, heart failure hospitalizations, fatal and nonfatal MI, and ischemic stroke also did not differ significantly between the groups.
The rate of major hemorrhagic events (composite of hemorrhagic stroke, intracranial bleed, or extracranial bleed), however, was higher in the aspirin vs the placebo group (8.6 events per 1000 person-years vs 6.2 events per 1000 person-years, respectively; HR = 1.4; 95% CI, 1.2-1.6; number needed to harm = 98).
WHAT’S NEW
Finding of more harm than good leads to change in ACC/AHA guidelines
Although the most recent USPSTF guidelines state the evidence is insufficient to assess the risks and benefits of aspirin for the primary prevention of cardiovascular disease in this age group, this trial reveals there is a greater risk of hemorrhagic events than there is prevention of cardiovascular outcomes with aspirin use in healthy elderly patients > 70 years of age.6 Because of this trial, the American College of Cardiology (ACC) and the American Heart Association (AHA) have updated their guidelines on the primary prevention of cardiovascular disease to recommend that aspirin not be used routinely in patients > 70 years of age.7
CAVEATS
Potential benefit to people at higher risk?
The rate of cardiovascular disease was lower than expected in this overall healthy population, so it is not known if cardiovascular benefits may outweigh the risk of bleeding in a higher-risk population. The trial also didn’t address the potential harms of deprescribing aspirin. Additionally, although aspirin may not be protective for cardiovascular events and may lead to more bleeding, there may be other benefits to aspirin in this patient population that were not addressed by this study.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
Popular beliefs and wide availability may make tide difficult to change
Patients have been told for years to take a daily aspirin to “protect their heart”; this behavior may be difficult to change. And because aspirin is widely available over the counter, patients may take it without their physician’s knowledge.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. McNeil JJ, Wolfe R, Woods RL, et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med. 2018;379:1509-1518.
2. Murphy SL, Xu JQ, Kochanek KD, et al. Mortality in the United States, 2017. NCHS Data Brief, no. 328. Hyattsville, MD: National Center for Health Statistics. 2018.
3. Smith SC Jr, Benjamin EJ, Bonow RO, et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation. 2011;124:2458-2473.
4. Gaziano JM, Brotons C, Coppolecchia R, et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomised, double-blind, placebo-controlled trial. Lancet. 2018;392:1036-1046.
5. Bowman L, Mafham M, Wallendszus K, et al; ASCEND Study Collaborative Group. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med. 2018;379:1529-1539.
6. Bibbins-Domingo K; U.S. Preventive Services Task Force. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2016;164:836-845.
7. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Amer Coll Cardiol. 2019;74:1376-1414.
8. American Diabetes Association. Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S103-S123.
1. McNeil JJ, Wolfe R, Woods RL, et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med. 2018;379:1509-1518.
2. Murphy SL, Xu JQ, Kochanek KD, et al. Mortality in the United States, 2017. NCHS Data Brief, no. 328. Hyattsville, MD: National Center for Health Statistics. 2018.
3. Smith SC Jr, Benjamin EJ, Bonow RO, et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation. 2011;124:2458-2473.
4. Gaziano JM, Brotons C, Coppolecchia R, et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomised, double-blind, placebo-controlled trial. Lancet. 2018;392:1036-1046.
5. Bowman L, Mafham M, Wallendszus K, et al; ASCEND Study Collaborative Group. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med. 2018;379:1529-1539.
6. Bibbins-Domingo K; U.S. Preventive Services Task Force. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2016;164:836-845.
7. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Amer Coll Cardiol. 2019;74:1376-1414.
8. American Diabetes Association. Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S103-S123.
PRACTICE CHANGER
Do not prescribe aspirin for primary prevention of cardiovascular disease in your elderly patients. Aspirin does not improve cardiovascular outcomes and it significantly increases the risk of bleeding events.
STRENGTH OF RECOMMENDATION
B: Based on a single randomized controlled trial.
McNeil JJ, Wolfe R, Woods RL, et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med. 2018;379:1509-1518.1
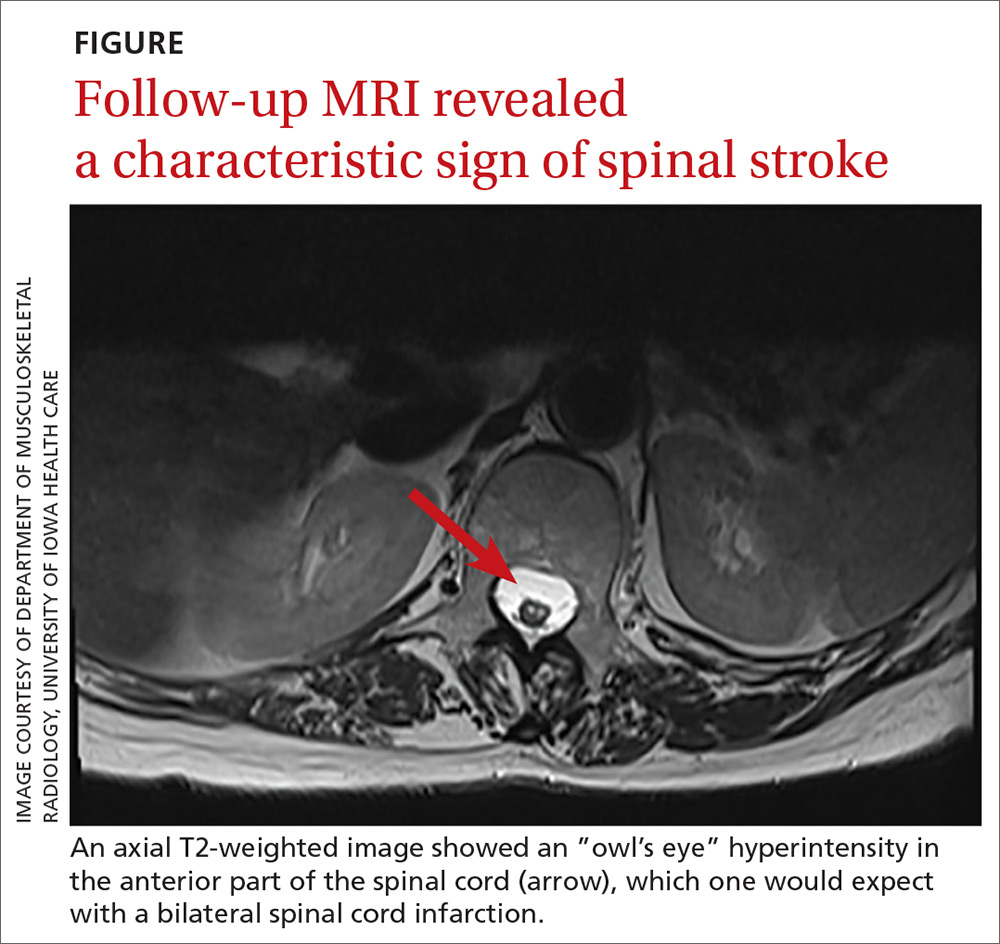
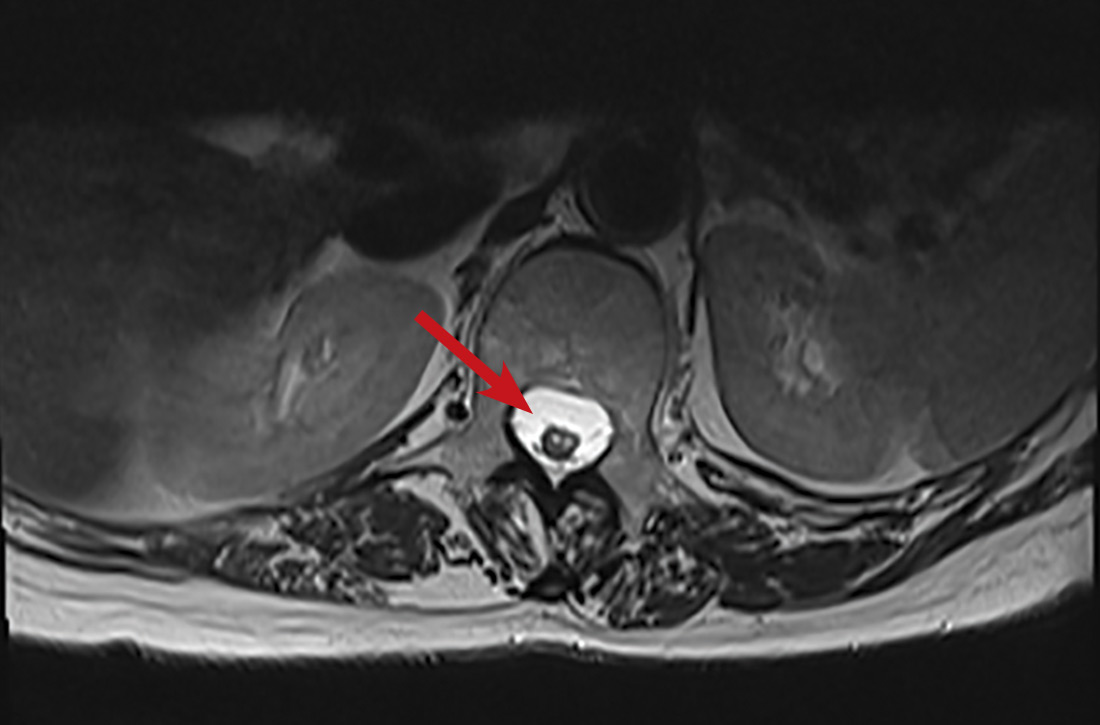
Sharp lower back pain • left-side paraspinal tenderness • anterior thigh sensory loss • Dx?
THE CASE
A 64-year-old woman with a history of late-onset type 1 diabetes mellitus, Hashimoto thyroiditis, and scoliosis presented to the sports medicine clinic with acute-onset, sharp, nonradiating right lower back pain that began when she bent forward to apply lotion. At presentation, she denied fever, chills, numbness, tingling, aggravation of pain with movement, weakness, and incontinence. Her neuromuscular examination was unremarkable except for left-side paraspinal tenderness. She was prescribed cyclobenzaprine for symptomatic relief.
Two days later, she was seen for worsening pain. Her physical exam was unchanged. She was prescribed tramadol and advised to start physical therapy gradually. As the day progressed, however, she developed anterior thigh sensory loss, which gradually extended distally.
The following day, she was brought to the emergency department with severe left-side weakness without urinary incontinence. Her mental status and cranial nerve exams were normal. On examination, strength of the iliopsoas and quadriceps was 1/5 bilaterally, and of the peroneal tendon and gastrocnemius, 3/5 bilaterally. Reflexes of triceps, biceps, knee, and Achilles tendon were symmetric and 3+ with bilateral clonus of the ankle. The Babinski sign was positive bilaterally. The patient had diminished pain sensation bilaterally, extending down from the T11 dermatome (left more than right side) with diminished vibration sensation at the left ankle. Her perianal sensation, bilateral temperature sensation, and cerebellar examination were normal.
Magnetic resonance imaging (MRI) without contrast of the lumbar spine demonstrated ischemia findings corresponding to T12-L1. Degenerative changes from L1-S1 were noted, with multiple osteophytes impinging on the neural foramina without cord compression.
THE DIAGNOSIS
The initial presentation was consistent with mechanical low back pain with signs of anterior spinal artery infarction and medial lemniscus pathway involvement 48 hours after initial presentation. Spinal cord infarction occurs more commonly in women and in the young than does cerebral infarction,1 with better reemployment rates.1,2 Similar to other strokes, long-term prognosis is primarily determined by the initial severity of motor impairment, which is linked to long-term immobility and need for bladder catheterization.3
Neurogenic pain developing years after spinal cord infarction is most often observed in anterior spinal artery infarction4 without functional limitations.
Initial treatment. Our patient was started on aspirin 325 mg/d and clopidogrel 75 mg/d. Her mean arterial blood pressure was maintained above 80 mm Hg. Computed tomography angiography of the abdomen and pelvis was negative for aortic dissection. Lumbar puncture for cerebrospinal fluid analysis was unremarkable. Results of antineutrophil cytoplasmic antibody testing, antinuclear antibody testing, a hepatitis panel, and an antiphospholipid panel were all negative. The patient was started on IV steroids with a plan for gradual tapering. The neurosurgical team agreed with medical management.
Continue to: DISCUSSION
DISCUSSION
Possible etiologies for acute spinal cord infarction include spinal cord ischemia from compression of the vessels, fibrocartilaginous embolism, and arterial thrombosis or atherosclerosis, especially in patients with diabetes.5
The majority (86%) of spinal strokes are due to spontaneous occlusion of the vessels with no identifiable cause; much less frequently (9% of cases), hemorrhage is the causative factor.1 A retrospective study demonstrated that 10 of 27 patients with spinal stroke had an anterior spinal infarct. Of those 10 patients, 6 reported a mechanical triggering movement (similar to this case), indicating potential compression of the radicular arteries due to said movement.4
Fibrocartilaginous embolism (FCE) is worth considering as a possible cause, because it accounts for 5.5% of all cases of acute spinal cord infarction.3 FCE is thought to arise after a precipitating event such as minor trauma, heavy lifting, physical exertion, or Valsalva maneuver causing embolization of the fragments of nucleus pulposus to the arterial system. In a case series of 8 patients, 2 had possible FCE with precipitating events occurring within the prior 24 hours. This was also demonstrated in another case series6 in which 7 of 9 patients had precipitating events.
Although FCE can only definitively be diagnosed postmortem, the researchers6 proposed clinical criteria for its diagnosis in living patients, based on 40 postmortem and 11 suspected antemortem cases of FCE. These criteria include a rapid evolution of symptoms consistent with vascular etiology, with or without preceding minor trauma or Valsalva maneuver; MRI changes consistent with ischemic myelopathy, with or without evidence of disc herniation; and no more than 2 vascular risk factors.
Our patient had no trauma (although there was a triggering movement), no signs of disc herniation, and 2 risk factors (> 60 years and diabetes mellitus). Also, a neurologically symptom-free interval between the painful movement and the onset of neurologic manifestations in our case parallels the clinical picture of FCE.
Continue to: The role of factor V Leiden (FVL) mutation
The role of factor V Leiden (FVL) mutation in arterial thrombosis is questionable. Previous reports demonstrate a risk for venous thrombosis 7 to 10 times higher with heterozygous FVL mutation and 100 times higher with homozygous mutation, with a less established role in arterial thrombosis.7 A retrospective Turkish study compared the incidence of FVL mutation in patients with arterial thrombosis vs healthy subjects; incidence was significantly higher in female patients than female controls (37.5% vs. 2%).7 A meta-analysis of published studies showed an association between arterial ischemic events and FVL mutation to be modest, with an odds ratio of 1.21 (95% CI, 0.99-1.49).8
In contrast, a 3.4-year longitudinal health study of patients ages 65 and older found no significant difference in the occurrence of myocardial infarction, transient ischemic attack, stroke, or angina for more than 5000 patients with heterozygous FVL mutation compared to fewer than 500 controls.9 The case patient’s clinical course did not fit a thrombotic clinical picture.
Evaluating for “red flags” is crucial in any case of low back pain to exclude serious pathologies. Red flag symptoms include signs of myelopathy, signs of infection, history of trauma with focal tenderness to palpation, and steroid or anticoagulant use (to rule out medication adverse effects).10 Our patient lacked these classical signs, but she did have subjective pain out of proportion to the clinical exam findings.
Of note: The above red flags for low back pain are all based on expert opinion,11 and the positive predictive value of a red flag is always low because of the low prevalence of serious spinal pathologies.12
Striking a proper balance. This case emphasizes the necessity to keep uncommon causes—such as nontraumatic spinal stroke, which has a prevalence of about 5% to 8% of all acute myelopathies—in the differential diagnosis.3
Continue to: We recommend watchful...
We recommend watchful waiting coupled with communication with the patient regarding monitoring for changes in symptoms over time.11 Any changes in symptoms concerning for underlying spinal cord injury indicate necessity for transfer to a tertiary care center (if possible), along with immediate evaluation with imaging—including computed tomography angiography of the abdomen to rule out aortic dissection (1%-2% of all spinal cord infarcts), followed by a specialist consultation based on the findings.3
Our patient
Our patient was discharged to rehabilitation on hospital Day 5, after progressive return of lower extremity strength. At the 2-month follow-up visit, she demonstrated grade 4+ strength throughout her lower extremities bilaterally. Weakness was predominant at the hip flexors and ankle dorsiflexors, which was consistent with her status at discharge. She had burning pain in the distribution of the L1 dermatome that responded to ibuprofen.
Hypercoagulability work-up was positive for heterozygous FVL mutation without any previous history of venous thromboembolic disease. She was continued on aspirin 325 mg/d, as per American College of Chest Physicians antithrombotic guidelines.13
One year later, our patient underwent a follow-up MRI of the thoracic spine, which showed an “owl’s eye” hyperintensity in the anterior cord (FIGURE), a sign that’s often seen in bilateral spinal cord infarction
THE TAKEAWAY
Spinal stroke is rare, but a missed diagnosis and lack of treatment can result in long-term morbidity. Therefore, it is prudent to consider this diagnosis in the differential—especially when the patient’s subjective back pain is out of proportion to the clinical examination findings.
CORRESPONDENCE
Srikanth Nithyanandam, MBBS, MS, University of Kentucky Family and Community Medicine, 2195 Harrodsburg Road, Suite 125, Lexington, KY 40504-3504; sri.nisi89@uky.edu.
1. Romi F, Naess H. Spinal cord infarction in clinical neurology: a review of characteristics and long-term prognosis in comparison to cerebral infarction. Eur Neurol. 2016;76:95-98.
2. Hanson SR, Romi F, Rekand T, et al. Long-term outcome after spinal cord infarctions. Acta Neurol Scand. 2015;131:253-257.
3. Rigney L, Cappelen-Smith C, Sebire D, et al. Nontraumatic spinal cord ischaemic syndrome. J Clin Neurosci. 2015;22:1544-1549.
4. Novy J, Carruzzo A, Maeder P, Bogousslavsky J. Spinal cord ischemia: clinical and imaging patterns, pathogenesis, and outcomes in 27 patients. Arch Neurol. 2006;63:1113-1120.
5. Goldstein LB, Adams R, Alberts MJ, et al; American Heart Association; American Stroke Association Stroke Council. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council: cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2006;113:e873-e923.
6. Mateen FJ, Monrad PA, Hunderfund AN, et al. Clinically suspected fibrocartilaginous embolism: clinical characteristics, treatments, and outcomes. Eur J Neurol. 2011;18:218-225.
7. Ozmen F, Ozmen MM, Ozalp N, et al. The prevalence of factor V (G1691A), MTHFR (C677T) and PT (G20210A) gene mutations in arterial thrombosis. Ulus Travma Acil Cerrahi Derg. 2009;15:113-119.
8. Kim RJ, Becker RC. Association between factor V Leiden, prothrombin G20210A, and methylenetetrahydrofolate reductase C677T mutations and events of the arterial circulatory system: a meta-analysis of published studies. Am Heart J. 2003;146:948-957.
9. Cushman M, Rosendaal FR, Psaty BM, et al. Factor V Leiden is not a risk factor for arterial vascular disease in the elderly: results from the Cardiovascular Health Study. Thromb Haemost. 1998;79:912-915.
10. Strudwick K, McPhee M, Bell A, et al. Review article: best practice management of low back pain in the emergency department (part 1 of the musculoskeletal injuries rapid review series). Emerg Med Australas. 2018;30:18-35.
11. Cook CE, George SZ, Reiman MP. Red flag screening for low back pain: nothing to see here, move along: a narrative review. Br J Sports Med. 2018;52:493-496.
12. Grunau GL, Darlow B, Flynn T, et al. Red flags or red herrings? Redefining the role of red flags in low back pain to reduce overimaging. Br J Sports Med. 2018;52:488-489.
13. Lansberg MG, O’Donnell MJ, Khatri P, et al. Antithrombotic and thrombolytic therapy for ischemic stroke: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e601S-e636S.
14. Pikija S, Mutzenbach JS, Kunz AB, et al. Delayed hospital presentation and neuroimaging in non-surgical spinal cord infarction. Front Neurol. 2017;8:143.
THE CASE
A 64-year-old woman with a history of late-onset type 1 diabetes mellitus, Hashimoto thyroiditis, and scoliosis presented to the sports medicine clinic with acute-onset, sharp, nonradiating right lower back pain that began when she bent forward to apply lotion. At presentation, she denied fever, chills, numbness, tingling, aggravation of pain with movement, weakness, and incontinence. Her neuromuscular examination was unremarkable except for left-side paraspinal tenderness. She was prescribed cyclobenzaprine for symptomatic relief.
Two days later, she was seen for worsening pain. Her physical exam was unchanged. She was prescribed tramadol and advised to start physical therapy gradually. As the day progressed, however, she developed anterior thigh sensory loss, which gradually extended distally.
The following day, she was brought to the emergency department with severe left-side weakness without urinary incontinence. Her mental status and cranial nerve exams were normal. On examination, strength of the iliopsoas and quadriceps was 1/5 bilaterally, and of the peroneal tendon and gastrocnemius, 3/5 bilaterally. Reflexes of triceps, biceps, knee, and Achilles tendon were symmetric and 3+ with bilateral clonus of the ankle. The Babinski sign was positive bilaterally. The patient had diminished pain sensation bilaterally, extending down from the T11 dermatome (left more than right side) with diminished vibration sensation at the left ankle. Her perianal sensation, bilateral temperature sensation, and cerebellar examination were normal.
Magnetic resonance imaging (MRI) without contrast of the lumbar spine demonstrated ischemia findings corresponding to T12-L1. Degenerative changes from L1-S1 were noted, with multiple osteophytes impinging on the neural foramina without cord compression.
THE DIAGNOSIS
The initial presentation was consistent with mechanical low back pain with signs of anterior spinal artery infarction and medial lemniscus pathway involvement 48 hours after initial presentation. Spinal cord infarction occurs more commonly in women and in the young than does cerebral infarction,1 with better reemployment rates.1,2 Similar to other strokes, long-term prognosis is primarily determined by the initial severity of motor impairment, which is linked to long-term immobility and need for bladder catheterization.3
Neurogenic pain developing years after spinal cord infarction is most often observed in anterior spinal artery infarction4 without functional limitations.
Initial treatment. Our patient was started on aspirin 325 mg/d and clopidogrel 75 mg/d. Her mean arterial blood pressure was maintained above 80 mm Hg. Computed tomography angiography of the abdomen and pelvis was negative for aortic dissection. Lumbar puncture for cerebrospinal fluid analysis was unremarkable. Results of antineutrophil cytoplasmic antibody testing, antinuclear antibody testing, a hepatitis panel, and an antiphospholipid panel were all negative. The patient was started on IV steroids with a plan for gradual tapering. The neurosurgical team agreed with medical management.
Continue to: DISCUSSION
DISCUSSION
Possible etiologies for acute spinal cord infarction include spinal cord ischemia from compression of the vessels, fibrocartilaginous embolism, and arterial thrombosis or atherosclerosis, especially in patients with diabetes.5
The majority (86%) of spinal strokes are due to spontaneous occlusion of the vessels with no identifiable cause; much less frequently (9% of cases), hemorrhage is the causative factor.1 A retrospective study demonstrated that 10 of 27 patients with spinal stroke had an anterior spinal infarct. Of those 10 patients, 6 reported a mechanical triggering movement (similar to this case), indicating potential compression of the radicular arteries due to said movement.4
Fibrocartilaginous embolism (FCE) is worth considering as a possible cause, because it accounts for 5.5% of all cases of acute spinal cord infarction.3 FCE is thought to arise after a precipitating event such as minor trauma, heavy lifting, physical exertion, or Valsalva maneuver causing embolization of the fragments of nucleus pulposus to the arterial system. In a case series of 8 patients, 2 had possible FCE with precipitating events occurring within the prior 24 hours. This was also demonstrated in another case series6 in which 7 of 9 patients had precipitating events.
Although FCE can only definitively be diagnosed postmortem, the researchers6 proposed clinical criteria for its diagnosis in living patients, based on 40 postmortem and 11 suspected antemortem cases of FCE. These criteria include a rapid evolution of symptoms consistent with vascular etiology, with or without preceding minor trauma or Valsalva maneuver; MRI changes consistent with ischemic myelopathy, with or without evidence of disc herniation; and no more than 2 vascular risk factors.
Our patient had no trauma (although there was a triggering movement), no signs of disc herniation, and 2 risk factors (> 60 years and diabetes mellitus). Also, a neurologically symptom-free interval between the painful movement and the onset of neurologic manifestations in our case parallels the clinical picture of FCE.
Continue to: The role of factor V Leiden (FVL) mutation
The role of factor V Leiden (FVL) mutation in arterial thrombosis is questionable. Previous reports demonstrate a risk for venous thrombosis 7 to 10 times higher with heterozygous FVL mutation and 100 times higher with homozygous mutation, with a less established role in arterial thrombosis.7 A retrospective Turkish study compared the incidence of FVL mutation in patients with arterial thrombosis vs healthy subjects; incidence was significantly higher in female patients than female controls (37.5% vs. 2%).7 A meta-analysis of published studies showed an association between arterial ischemic events and FVL mutation to be modest, with an odds ratio of 1.21 (95% CI, 0.99-1.49).8
In contrast, a 3.4-year longitudinal health study of patients ages 65 and older found no significant difference in the occurrence of myocardial infarction, transient ischemic attack, stroke, or angina for more than 5000 patients with heterozygous FVL mutation compared to fewer than 500 controls.9 The case patient’s clinical course did not fit a thrombotic clinical picture.
Evaluating for “red flags” is crucial in any case of low back pain to exclude serious pathologies. Red flag symptoms include signs of myelopathy, signs of infection, history of trauma with focal tenderness to palpation, and steroid or anticoagulant use (to rule out medication adverse effects).10 Our patient lacked these classical signs, but she did have subjective pain out of proportion to the clinical exam findings.
Of note: The above red flags for low back pain are all based on expert opinion,11 and the positive predictive value of a red flag is always low because of the low prevalence of serious spinal pathologies.12
Striking a proper balance. This case emphasizes the necessity to keep uncommon causes—such as nontraumatic spinal stroke, which has a prevalence of about 5% to 8% of all acute myelopathies—in the differential diagnosis.3
Continue to: We recommend watchful...
We recommend watchful waiting coupled with communication with the patient regarding monitoring for changes in symptoms over time.11 Any changes in symptoms concerning for underlying spinal cord injury indicate necessity for transfer to a tertiary care center (if possible), along with immediate evaluation with imaging—including computed tomography angiography of the abdomen to rule out aortic dissection (1%-2% of all spinal cord infarcts), followed by a specialist consultation based on the findings.3
Our patient
Our patient was discharged to rehabilitation on hospital Day 5, after progressive return of lower extremity strength. At the 2-month follow-up visit, she demonstrated grade 4+ strength throughout her lower extremities bilaterally. Weakness was predominant at the hip flexors and ankle dorsiflexors, which was consistent with her status at discharge. She had burning pain in the distribution of the L1 dermatome that responded to ibuprofen.
Hypercoagulability work-up was positive for heterozygous FVL mutation without any previous history of venous thromboembolic disease. She was continued on aspirin 325 mg/d, as per American College of Chest Physicians antithrombotic guidelines.13
One year later, our patient underwent a follow-up MRI of the thoracic spine, which showed an “owl’s eye” hyperintensity in the anterior cord (FIGURE), a sign that’s often seen in bilateral spinal cord infarction
THE TAKEAWAY
Spinal stroke is rare, but a missed diagnosis and lack of treatment can result in long-term morbidity. Therefore, it is prudent to consider this diagnosis in the differential—especially when the patient’s subjective back pain is out of proportion to the clinical examination findings.
CORRESPONDENCE
Srikanth Nithyanandam, MBBS, MS, University of Kentucky Family and Community Medicine, 2195 Harrodsburg Road, Suite 125, Lexington, KY 40504-3504; sri.nisi89@uky.edu.
THE CASE
A 64-year-old woman with a history of late-onset type 1 diabetes mellitus, Hashimoto thyroiditis, and scoliosis presented to the sports medicine clinic with acute-onset, sharp, nonradiating right lower back pain that began when she bent forward to apply lotion. At presentation, she denied fever, chills, numbness, tingling, aggravation of pain with movement, weakness, and incontinence. Her neuromuscular examination was unremarkable except for left-side paraspinal tenderness. She was prescribed cyclobenzaprine for symptomatic relief.
Two days later, she was seen for worsening pain. Her physical exam was unchanged. She was prescribed tramadol and advised to start physical therapy gradually. As the day progressed, however, she developed anterior thigh sensory loss, which gradually extended distally.
The following day, she was brought to the emergency department with severe left-side weakness without urinary incontinence. Her mental status and cranial nerve exams were normal. On examination, strength of the iliopsoas and quadriceps was 1/5 bilaterally, and of the peroneal tendon and gastrocnemius, 3/5 bilaterally. Reflexes of triceps, biceps, knee, and Achilles tendon were symmetric and 3+ with bilateral clonus of the ankle. The Babinski sign was positive bilaterally. The patient had diminished pain sensation bilaterally, extending down from the T11 dermatome (left more than right side) with diminished vibration sensation at the left ankle. Her perianal sensation, bilateral temperature sensation, and cerebellar examination were normal.
Magnetic resonance imaging (MRI) without contrast of the lumbar spine demonstrated ischemia findings corresponding to T12-L1. Degenerative changes from L1-S1 were noted, with multiple osteophytes impinging on the neural foramina without cord compression.
THE DIAGNOSIS
The initial presentation was consistent with mechanical low back pain with signs of anterior spinal artery infarction and medial lemniscus pathway involvement 48 hours after initial presentation. Spinal cord infarction occurs more commonly in women and in the young than does cerebral infarction,1 with better reemployment rates.1,2 Similar to other strokes, long-term prognosis is primarily determined by the initial severity of motor impairment, which is linked to long-term immobility and need for bladder catheterization.3
Neurogenic pain developing years after spinal cord infarction is most often observed in anterior spinal artery infarction4 without functional limitations.
Initial treatment. Our patient was started on aspirin 325 mg/d and clopidogrel 75 mg/d. Her mean arterial blood pressure was maintained above 80 mm Hg. Computed tomography angiography of the abdomen and pelvis was negative for aortic dissection. Lumbar puncture for cerebrospinal fluid analysis was unremarkable. Results of antineutrophil cytoplasmic antibody testing, antinuclear antibody testing, a hepatitis panel, and an antiphospholipid panel were all negative. The patient was started on IV steroids with a plan for gradual tapering. The neurosurgical team agreed with medical management.
Continue to: DISCUSSION
DISCUSSION
Possible etiologies for acute spinal cord infarction include spinal cord ischemia from compression of the vessels, fibrocartilaginous embolism, and arterial thrombosis or atherosclerosis, especially in patients with diabetes.5
The majority (86%) of spinal strokes are due to spontaneous occlusion of the vessels with no identifiable cause; much less frequently (9% of cases), hemorrhage is the causative factor.1 A retrospective study demonstrated that 10 of 27 patients with spinal stroke had an anterior spinal infarct. Of those 10 patients, 6 reported a mechanical triggering movement (similar to this case), indicating potential compression of the radicular arteries due to said movement.4
Fibrocartilaginous embolism (FCE) is worth considering as a possible cause, because it accounts for 5.5% of all cases of acute spinal cord infarction.3 FCE is thought to arise after a precipitating event such as minor trauma, heavy lifting, physical exertion, or Valsalva maneuver causing embolization of the fragments of nucleus pulposus to the arterial system. In a case series of 8 patients, 2 had possible FCE with precipitating events occurring within the prior 24 hours. This was also demonstrated in another case series6 in which 7 of 9 patients had precipitating events.
Although FCE can only definitively be diagnosed postmortem, the researchers6 proposed clinical criteria for its diagnosis in living patients, based on 40 postmortem and 11 suspected antemortem cases of FCE. These criteria include a rapid evolution of symptoms consistent with vascular etiology, with or without preceding minor trauma or Valsalva maneuver; MRI changes consistent with ischemic myelopathy, with or without evidence of disc herniation; and no more than 2 vascular risk factors.
Our patient had no trauma (although there was a triggering movement), no signs of disc herniation, and 2 risk factors (> 60 years and diabetes mellitus). Also, a neurologically symptom-free interval between the painful movement and the onset of neurologic manifestations in our case parallels the clinical picture of FCE.
Continue to: The role of factor V Leiden (FVL) mutation
The role of factor V Leiden (FVL) mutation in arterial thrombosis is questionable. Previous reports demonstrate a risk for venous thrombosis 7 to 10 times higher with heterozygous FVL mutation and 100 times higher with homozygous mutation, with a less established role in arterial thrombosis.7 A retrospective Turkish study compared the incidence of FVL mutation in patients with arterial thrombosis vs healthy subjects; incidence was significantly higher in female patients than female controls (37.5% vs. 2%).7 A meta-analysis of published studies showed an association between arterial ischemic events and FVL mutation to be modest, with an odds ratio of 1.21 (95% CI, 0.99-1.49).8
In contrast, a 3.4-year longitudinal health study of patients ages 65 and older found no significant difference in the occurrence of myocardial infarction, transient ischemic attack, stroke, or angina for more than 5000 patients with heterozygous FVL mutation compared to fewer than 500 controls.9 The case patient’s clinical course did not fit a thrombotic clinical picture.
Evaluating for “red flags” is crucial in any case of low back pain to exclude serious pathologies. Red flag symptoms include signs of myelopathy, signs of infection, history of trauma with focal tenderness to palpation, and steroid or anticoagulant use (to rule out medication adverse effects).10 Our patient lacked these classical signs, but she did have subjective pain out of proportion to the clinical exam findings.
Of note: The above red flags for low back pain are all based on expert opinion,11 and the positive predictive value of a red flag is always low because of the low prevalence of serious spinal pathologies.12
Striking a proper balance. This case emphasizes the necessity to keep uncommon causes—such as nontraumatic spinal stroke, which has a prevalence of about 5% to 8% of all acute myelopathies—in the differential diagnosis.3
Continue to: We recommend watchful...
We recommend watchful waiting coupled with communication with the patient regarding monitoring for changes in symptoms over time.11 Any changes in symptoms concerning for underlying spinal cord injury indicate necessity for transfer to a tertiary care center (if possible), along with immediate evaluation with imaging—including computed tomography angiography of the abdomen to rule out aortic dissection (1%-2% of all spinal cord infarcts), followed by a specialist consultation based on the findings.3
Our patient
Our patient was discharged to rehabilitation on hospital Day 5, after progressive return of lower extremity strength. At the 2-month follow-up visit, she demonstrated grade 4+ strength throughout her lower extremities bilaterally. Weakness was predominant at the hip flexors and ankle dorsiflexors, which was consistent with her status at discharge. She had burning pain in the distribution of the L1 dermatome that responded to ibuprofen.
Hypercoagulability work-up was positive for heterozygous FVL mutation without any previous history of venous thromboembolic disease. She was continued on aspirin 325 mg/d, as per American College of Chest Physicians antithrombotic guidelines.13
One year later, our patient underwent a follow-up MRI of the thoracic spine, which showed an “owl’s eye” hyperintensity in the anterior cord (FIGURE), a sign that’s often seen in bilateral spinal cord infarction
THE TAKEAWAY
Spinal stroke is rare, but a missed diagnosis and lack of treatment can result in long-term morbidity. Therefore, it is prudent to consider this diagnosis in the differential—especially when the patient’s subjective back pain is out of proportion to the clinical examination findings.
CORRESPONDENCE
Srikanth Nithyanandam, MBBS, MS, University of Kentucky Family and Community Medicine, 2195 Harrodsburg Road, Suite 125, Lexington, KY 40504-3504; sri.nisi89@uky.edu.
1. Romi F, Naess H. Spinal cord infarction in clinical neurology: a review of characteristics and long-term prognosis in comparison to cerebral infarction. Eur Neurol. 2016;76:95-98.
2. Hanson SR, Romi F, Rekand T, et al. Long-term outcome after spinal cord infarctions. Acta Neurol Scand. 2015;131:253-257.
3. Rigney L, Cappelen-Smith C, Sebire D, et al. Nontraumatic spinal cord ischaemic syndrome. J Clin Neurosci. 2015;22:1544-1549.
4. Novy J, Carruzzo A, Maeder P, Bogousslavsky J. Spinal cord ischemia: clinical and imaging patterns, pathogenesis, and outcomes in 27 patients. Arch Neurol. 2006;63:1113-1120.
5. Goldstein LB, Adams R, Alberts MJ, et al; American Heart Association; American Stroke Association Stroke Council. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council: cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2006;113:e873-e923.
6. Mateen FJ, Monrad PA, Hunderfund AN, et al. Clinically suspected fibrocartilaginous embolism: clinical characteristics, treatments, and outcomes. Eur J Neurol. 2011;18:218-225.
7. Ozmen F, Ozmen MM, Ozalp N, et al. The prevalence of factor V (G1691A), MTHFR (C677T) and PT (G20210A) gene mutations in arterial thrombosis. Ulus Travma Acil Cerrahi Derg. 2009;15:113-119.
8. Kim RJ, Becker RC. Association between factor V Leiden, prothrombin G20210A, and methylenetetrahydrofolate reductase C677T mutations and events of the arterial circulatory system: a meta-analysis of published studies. Am Heart J. 2003;146:948-957.
9. Cushman M, Rosendaal FR, Psaty BM, et al. Factor V Leiden is not a risk factor for arterial vascular disease in the elderly: results from the Cardiovascular Health Study. Thromb Haemost. 1998;79:912-915.
10. Strudwick K, McPhee M, Bell A, et al. Review article: best practice management of low back pain in the emergency department (part 1 of the musculoskeletal injuries rapid review series). Emerg Med Australas. 2018;30:18-35.
11. Cook CE, George SZ, Reiman MP. Red flag screening for low back pain: nothing to see here, move along: a narrative review. Br J Sports Med. 2018;52:493-496.
12. Grunau GL, Darlow B, Flynn T, et al. Red flags or red herrings? Redefining the role of red flags in low back pain to reduce overimaging. Br J Sports Med. 2018;52:488-489.
13. Lansberg MG, O’Donnell MJ, Khatri P, et al. Antithrombotic and thrombolytic therapy for ischemic stroke: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e601S-e636S.
14. Pikija S, Mutzenbach JS, Kunz AB, et al. Delayed hospital presentation and neuroimaging in non-surgical spinal cord infarction. Front Neurol. 2017;8:143.
1. Romi F, Naess H. Spinal cord infarction in clinical neurology: a review of characteristics and long-term prognosis in comparison to cerebral infarction. Eur Neurol. 2016;76:95-98.
2. Hanson SR, Romi F, Rekand T, et al. Long-term outcome after spinal cord infarctions. Acta Neurol Scand. 2015;131:253-257.
3. Rigney L, Cappelen-Smith C, Sebire D, et al. Nontraumatic spinal cord ischaemic syndrome. J Clin Neurosci. 2015;22:1544-1549.
4. Novy J, Carruzzo A, Maeder P, Bogousslavsky J. Spinal cord ischemia: clinical and imaging patterns, pathogenesis, and outcomes in 27 patients. Arch Neurol. 2006;63:1113-1120.
5. Goldstein LB, Adams R, Alberts MJ, et al; American Heart Association; American Stroke Association Stroke Council. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council: cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2006;113:e873-e923.
6. Mateen FJ, Monrad PA, Hunderfund AN, et al. Clinically suspected fibrocartilaginous embolism: clinical characteristics, treatments, and outcomes. Eur J Neurol. 2011;18:218-225.
7. Ozmen F, Ozmen MM, Ozalp N, et al. The prevalence of factor V (G1691A), MTHFR (C677T) and PT (G20210A) gene mutations in arterial thrombosis. Ulus Travma Acil Cerrahi Derg. 2009;15:113-119.
8. Kim RJ, Becker RC. Association between factor V Leiden, prothrombin G20210A, and methylenetetrahydrofolate reductase C677T mutations and events of the arterial circulatory system: a meta-analysis of published studies. Am Heart J. 2003;146:948-957.
9. Cushman M, Rosendaal FR, Psaty BM, et al. Factor V Leiden is not a risk factor for arterial vascular disease in the elderly: results from the Cardiovascular Health Study. Thromb Haemost. 1998;79:912-915.
10. Strudwick K, McPhee M, Bell A, et al. Review article: best practice management of low back pain in the emergency department (part 1 of the musculoskeletal injuries rapid review series). Emerg Med Australas. 2018;30:18-35.
11. Cook CE, George SZ, Reiman MP. Red flag screening for low back pain: nothing to see here, move along: a narrative review. Br J Sports Med. 2018;52:493-496.
12. Grunau GL, Darlow B, Flynn T, et al. Red flags or red herrings? Redefining the role of red flags in low back pain to reduce overimaging. Br J Sports Med. 2018;52:488-489.
13. Lansberg MG, O’Donnell MJ, Khatri P, et al. Antithrombotic and thrombolytic therapy for ischemic stroke: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e601S-e636S.
14. Pikija S, Mutzenbach JS, Kunz AB, et al. Delayed hospital presentation and neuroimaging in non-surgical spinal cord infarction. Front Neurol. 2017;8:143.
Is protocol-driven COVID-19 respiratory therapy doing more harm than good?
Physicians in the COVID-19 trenches are beginning to question whether standard respiratory therapy protocols for acute respiratory distress syndrome (ARDS) are the best approach for treating patients with COVID-19 pneumonia.
At issue is the standard use of ventilators for a virus whose presentation has not followed the standard for ARDS, but is looking more like high-altitude pulmonary edema (HAPE) in some patients.
In a letter to the editor published in the American Journal of Respiratory and Critical Care Medicine on March 30, and in an editorial accepted for publication in Intensive Care Medicine, Luciano Gattinoni, MD, of the Medical University of Göttingen in Germany and colleagues make the case that protocol-driven ventilator use for patients with COVID-19 could be doing more harm than good.
Dr. Gattinoni noted that COVID-19 patients in ICUs in northern Italy had an atypical ARDS presentation with severe hypoxemia and well-preserved lung gas volume. He and colleagues suggested that instead of high positive end-expiratory pressure (PEEP), physicians should consider the lowest possible PEEP and gentle ventilation–practicing patience to “buy time with minimum additional damage.”
Similar observations were made by Cameron Kyle-Sidell, MD, a critical care physician working in New York City, who has been speaking out about this issue on Twitter and who shared his own experiences in this video interview with WebMD chief medical officer John Whyte, MD.
The bottom line, as Dr. Kyle-Sidell and Dr. Gattinoni agree, is that protocol-driven ventilator use may be causing lung injury in COVID-19 patients.
Consider disease phenotype
In the editorial, Dr. Gattinoni and colleagues explained further that ventilator settings should be based on physiological findings – with different respiratory treatment based on disease phenotype rather than using standard protocols.
‘“This, of course, is a conceptual model, but based on the observations we have this far, I don’t know of any model which is better,” he said in an interview.
Anecdotal evidence has increasingly demonstrated that this proposed physiological approach is associated with much lower mortality rates among COVID-19 patients, he said.
While not willing to name the hospitals at this time, he said that one center in Europe has had a 0% mortality rate among COVID-19 patients in the ICU when using this approach, compared with a 60% mortality rate at a nearby hospital using a protocol-driven approach.
In his editorial, Dr. Gattinoni disputed the recently published recommendation from the Surviving Sepsis Campaign that “mechanically ventilated patients with COVID-19 should be managed similarly to other patients with acute respiratory failure in the ICU.”
“Yet, COVID-19 pneumonia, despite falling in most of the circumstances under the Berlin definition of ARDS, is a specific disease, whose distinctive features are severe hypoxemia often associated with near normal respiratory system compliance,” Dr. Gattinoni and colleagues wrote, noting that this was true for more than half of the 150 patients he and his colleagues had assessed, and that several other colleagues in northern Italy reported similar findings. “This remarkable combination is almost never seen in severe ARDS.”
Dr. Gattinoni and colleagues hypothesized that COVID-19 patterns at patient presentation depend on interaction between three sets of factors: 1) disease severity, host response, physiological reserve and comorbidities; 2) ventilatory responsiveness of the patient to hypoxemia; and 3) time elapsed between disease onset and hospitalization.
They identified two primary phenotypes based on the interaction of these factors: Type L, characterized by low elastance, low ventilator perfusion ratio, low lung weight, and low recruitability; and Type H, characterized by high elastance, high right-to-left shunt, high lung weight, and high recruitability.
“Given this conceptual model, it follows that the respiratory treatment offered to Type L and Type H patients must be different,” Dr. Gattinoni said.
Patients may transition between phenotypes as their disease evolves. “If you start with the wrong protocol, at the end they become similar,” he said.
Rather, it is important to identify the phenotype at presentation to understand the pathophysiology and treat accordingly, he advised.
The phenotypes are best identified by CT scan, but signs implicit in each of the phenotypes, including respiratory system elastance and recruitability, can be used as surrogates if CT is unavailable, he noted.
“This is a kind of disease in which you don’t have to follow the protocol – you have to follow the physiology,” he said. “Unfortunately, many, many doctors around the world cannot think outside the protocol.”
In his interview with Dr. Whyte, Dr. Kyle-Sidell stressed that doctors must begin to consider other approaches. “We are desperate now, in the sense that everything we are doing does not seem to be working,” Dr. Kyle-Sidell said, noting that the first step toward improving outcomes is admitting that “this is something new.”
“I think it all starts from there, and I think we have the kind of scientific technology and the human capital in this country to solve this or at least have a very good shot at it,” he said.
Proposed treatment model
Dr. Gattinoni and his colleagues offered a proposed treatment model based on their conceptualization:
- Reverse hypoxemia through an increase in FiO2 to a level at which the Type L patient responds well, particularly for Type L patients who are not experiencing dyspnea.
- In Type L patients with dyspnea, try noninvasive options such as high-flow nasal cannula, continuous positive airway pressure, or noninvasive ventilation, and be sure to measure inspiratory esophageal pressure using esophageal manometry or surrogate measures. In intubated patients, determine P0.1 and P occlusion. High PEEP may decrease pleural pressure swings “and stop the vicious cycle that exacerbates lung injury,” but may be associated with high failure rates and delayed intubation.
- Intubate as soon as possible for esophageal pressure swings that increase from 5-10 cm H2O to above 15 cm H2O, which marks a transition from Type L to Type H phenotype and represents the level at which lung injury risk increases.
- For intubated and deeply sedated Type L patients who are hypercapnic, ventilate with volumes greater than 6 mL/kg up to 8-9 mL/kg as this high compliance results in tolerable strain without risk of ventilator-associated lung injury. Prone positioning should be used only as a rescue maneuver. Reduce PEEP to 8-10 cm H2O, given that the recruitability is low and the risk of hemodynamic failure increases at higher levels. Early intubation may avert the transition to Type H phenotype.
- Treat Type H phenotype like severe ARDS, including with higher PEEP if compatible with hemodynamics, and with prone positioning and extracorporeal support.
Dr. Gattinoni reported having no financial disclosures.
sworcester@mdedge.com
Physicians in the COVID-19 trenches are beginning to question whether standard respiratory therapy protocols for acute respiratory distress syndrome (ARDS) are the best approach for treating patients with COVID-19 pneumonia.
At issue is the standard use of ventilators for a virus whose presentation has not followed the standard for ARDS, but is looking more like high-altitude pulmonary edema (HAPE) in some patients.
In a letter to the editor published in the American Journal of Respiratory and Critical Care Medicine on March 30, and in an editorial accepted for publication in Intensive Care Medicine, Luciano Gattinoni, MD, of the Medical University of Göttingen in Germany and colleagues make the case that protocol-driven ventilator use for patients with COVID-19 could be doing more harm than good.
Dr. Gattinoni noted that COVID-19 patients in ICUs in northern Italy had an atypical ARDS presentation with severe hypoxemia and well-preserved lung gas volume. He and colleagues suggested that instead of high positive end-expiratory pressure (PEEP), physicians should consider the lowest possible PEEP and gentle ventilation–practicing patience to “buy time with minimum additional damage.”
Similar observations were made by Cameron Kyle-Sidell, MD, a critical care physician working in New York City, who has been speaking out about this issue on Twitter and who shared his own experiences in this video interview with WebMD chief medical officer John Whyte, MD.
The bottom line, as Dr. Kyle-Sidell and Dr. Gattinoni agree, is that protocol-driven ventilator use may be causing lung injury in COVID-19 patients.
Consider disease phenotype
In the editorial, Dr. Gattinoni and colleagues explained further that ventilator settings should be based on physiological findings – with different respiratory treatment based on disease phenotype rather than using standard protocols.
‘“This, of course, is a conceptual model, but based on the observations we have this far, I don’t know of any model which is better,” he said in an interview.
Anecdotal evidence has increasingly demonstrated that this proposed physiological approach is associated with much lower mortality rates among COVID-19 patients, he said.
While not willing to name the hospitals at this time, he said that one center in Europe has had a 0% mortality rate among COVID-19 patients in the ICU when using this approach, compared with a 60% mortality rate at a nearby hospital using a protocol-driven approach.
In his editorial, Dr. Gattinoni disputed the recently published recommendation from the Surviving Sepsis Campaign that “mechanically ventilated patients with COVID-19 should be managed similarly to other patients with acute respiratory failure in the ICU.”
“Yet, COVID-19 pneumonia, despite falling in most of the circumstances under the Berlin definition of ARDS, is a specific disease, whose distinctive features are severe hypoxemia often associated with near normal respiratory system compliance,” Dr. Gattinoni and colleagues wrote, noting that this was true for more than half of the 150 patients he and his colleagues had assessed, and that several other colleagues in northern Italy reported similar findings. “This remarkable combination is almost never seen in severe ARDS.”
Dr. Gattinoni and colleagues hypothesized that COVID-19 patterns at patient presentation depend on interaction between three sets of factors: 1) disease severity, host response, physiological reserve and comorbidities; 2) ventilatory responsiveness of the patient to hypoxemia; and 3) time elapsed between disease onset and hospitalization.
They identified two primary phenotypes based on the interaction of these factors: Type L, characterized by low elastance, low ventilator perfusion ratio, low lung weight, and low recruitability; and Type H, characterized by high elastance, high right-to-left shunt, high lung weight, and high recruitability.
“Given this conceptual model, it follows that the respiratory treatment offered to Type L and Type H patients must be different,” Dr. Gattinoni said.
Patients may transition between phenotypes as their disease evolves. “If you start with the wrong protocol, at the end they become similar,” he said.
Rather, it is important to identify the phenotype at presentation to understand the pathophysiology and treat accordingly, he advised.
The phenotypes are best identified by CT scan, but signs implicit in each of the phenotypes, including respiratory system elastance and recruitability, can be used as surrogates if CT is unavailable, he noted.
“This is a kind of disease in which you don’t have to follow the protocol – you have to follow the physiology,” he said. “Unfortunately, many, many doctors around the world cannot think outside the protocol.”
In his interview with Dr. Whyte, Dr. Kyle-Sidell stressed that doctors must begin to consider other approaches. “We are desperate now, in the sense that everything we are doing does not seem to be working,” Dr. Kyle-Sidell said, noting that the first step toward improving outcomes is admitting that “this is something new.”
“I think it all starts from there, and I think we have the kind of scientific technology and the human capital in this country to solve this or at least have a very good shot at it,” he said.
Proposed treatment model
Dr. Gattinoni and his colleagues offered a proposed treatment model based on their conceptualization:
- Reverse hypoxemia through an increase in FiO2 to a level at which the Type L patient responds well, particularly for Type L patients who are not experiencing dyspnea.
- In Type L patients with dyspnea, try noninvasive options such as high-flow nasal cannula, continuous positive airway pressure, or noninvasive ventilation, and be sure to measure inspiratory esophageal pressure using esophageal manometry or surrogate measures. In intubated patients, determine P0.1 and P occlusion. High PEEP may decrease pleural pressure swings “and stop the vicious cycle that exacerbates lung injury,” but may be associated with high failure rates and delayed intubation.
- Intubate as soon as possible for esophageal pressure swings that increase from 5-10 cm H2O to above 15 cm H2O, which marks a transition from Type L to Type H phenotype and represents the level at which lung injury risk increases.
- For intubated and deeply sedated Type L patients who are hypercapnic, ventilate with volumes greater than 6 mL/kg up to 8-9 mL/kg as this high compliance results in tolerable strain without risk of ventilator-associated lung injury. Prone positioning should be used only as a rescue maneuver. Reduce PEEP to 8-10 cm H2O, given that the recruitability is low and the risk of hemodynamic failure increases at higher levels. Early intubation may avert the transition to Type H phenotype.
- Treat Type H phenotype like severe ARDS, including with higher PEEP if compatible with hemodynamics, and with prone positioning and extracorporeal support.
Dr. Gattinoni reported having no financial disclosures.
sworcester@mdedge.com
Physicians in the COVID-19 trenches are beginning to question whether standard respiratory therapy protocols for acute respiratory distress syndrome (ARDS) are the best approach for treating patients with COVID-19 pneumonia.
At issue is the standard use of ventilators for a virus whose presentation has not followed the standard for ARDS, but is looking more like high-altitude pulmonary edema (HAPE) in some patients.
In a letter to the editor published in the American Journal of Respiratory and Critical Care Medicine on March 30, and in an editorial accepted for publication in Intensive Care Medicine, Luciano Gattinoni, MD, of the Medical University of Göttingen in Germany and colleagues make the case that protocol-driven ventilator use for patients with COVID-19 could be doing more harm than good.
Dr. Gattinoni noted that COVID-19 patients in ICUs in northern Italy had an atypical ARDS presentation with severe hypoxemia and well-preserved lung gas volume. He and colleagues suggested that instead of high positive end-expiratory pressure (PEEP), physicians should consider the lowest possible PEEP and gentle ventilation–practicing patience to “buy time with minimum additional damage.”
Similar observations were made by Cameron Kyle-Sidell, MD, a critical care physician working in New York City, who has been speaking out about this issue on Twitter and who shared his own experiences in this video interview with WebMD chief medical officer John Whyte, MD.
The bottom line, as Dr. Kyle-Sidell and Dr. Gattinoni agree, is that protocol-driven ventilator use may be causing lung injury in COVID-19 patients.
Consider disease phenotype
In the editorial, Dr. Gattinoni and colleagues explained further that ventilator settings should be based on physiological findings – with different respiratory treatment based on disease phenotype rather than using standard protocols.
‘“This, of course, is a conceptual model, but based on the observations we have this far, I don’t know of any model which is better,” he said in an interview.
Anecdotal evidence has increasingly demonstrated that this proposed physiological approach is associated with much lower mortality rates among COVID-19 patients, he said.
While not willing to name the hospitals at this time, he said that one center in Europe has had a 0% mortality rate among COVID-19 patients in the ICU when using this approach, compared with a 60% mortality rate at a nearby hospital using a protocol-driven approach.
In his editorial, Dr. Gattinoni disputed the recently published recommendation from the Surviving Sepsis Campaign that “mechanically ventilated patients with COVID-19 should be managed similarly to other patients with acute respiratory failure in the ICU.”
“Yet, COVID-19 pneumonia, despite falling in most of the circumstances under the Berlin definition of ARDS, is a specific disease, whose distinctive features are severe hypoxemia often associated with near normal respiratory system compliance,” Dr. Gattinoni and colleagues wrote, noting that this was true for more than half of the 150 patients he and his colleagues had assessed, and that several other colleagues in northern Italy reported similar findings. “This remarkable combination is almost never seen in severe ARDS.”
Dr. Gattinoni and colleagues hypothesized that COVID-19 patterns at patient presentation depend on interaction between three sets of factors: 1) disease severity, host response, physiological reserve and comorbidities; 2) ventilatory responsiveness of the patient to hypoxemia; and 3) time elapsed between disease onset and hospitalization.
They identified two primary phenotypes based on the interaction of these factors: Type L, characterized by low elastance, low ventilator perfusion ratio, low lung weight, and low recruitability; and Type H, characterized by high elastance, high right-to-left shunt, high lung weight, and high recruitability.
“Given this conceptual model, it follows that the respiratory treatment offered to Type L and Type H patients must be different,” Dr. Gattinoni said.
Patients may transition between phenotypes as their disease evolves. “If you start with the wrong protocol, at the end they become similar,” he said.
Rather, it is important to identify the phenotype at presentation to understand the pathophysiology and treat accordingly, he advised.
The phenotypes are best identified by CT scan, but signs implicit in each of the phenotypes, including respiratory system elastance and recruitability, can be used as surrogates if CT is unavailable, he noted.
“This is a kind of disease in which you don’t have to follow the protocol – you have to follow the physiology,” he said. “Unfortunately, many, many doctors around the world cannot think outside the protocol.”
In his interview with Dr. Whyte, Dr. Kyle-Sidell stressed that doctors must begin to consider other approaches. “We are desperate now, in the sense that everything we are doing does not seem to be working,” Dr. Kyle-Sidell said, noting that the first step toward improving outcomes is admitting that “this is something new.”
“I think it all starts from there, and I think we have the kind of scientific technology and the human capital in this country to solve this or at least have a very good shot at it,” he said.
Proposed treatment model
Dr. Gattinoni and his colleagues offered a proposed treatment model based on their conceptualization:
- Reverse hypoxemia through an increase in FiO2 to a level at which the Type L patient responds well, particularly for Type L patients who are not experiencing dyspnea.
- In Type L patients with dyspnea, try noninvasive options such as high-flow nasal cannula, continuous positive airway pressure, or noninvasive ventilation, and be sure to measure inspiratory esophageal pressure using esophageal manometry or surrogate measures. In intubated patients, determine P0.1 and P occlusion. High PEEP may decrease pleural pressure swings “and stop the vicious cycle that exacerbates lung injury,” but may be associated with high failure rates and delayed intubation.
- Intubate as soon as possible for esophageal pressure swings that increase from 5-10 cm H2O to above 15 cm H2O, which marks a transition from Type L to Type H phenotype and represents the level at which lung injury risk increases.
- For intubated and deeply sedated Type L patients who are hypercapnic, ventilate with volumes greater than 6 mL/kg up to 8-9 mL/kg as this high compliance results in tolerable strain without risk of ventilator-associated lung injury. Prone positioning should be used only as a rescue maneuver. Reduce PEEP to 8-10 cm H2O, given that the recruitability is low and the risk of hemodynamic failure increases at higher levels. Early intubation may avert the transition to Type H phenotype.
- Treat Type H phenotype like severe ARDS, including with higher PEEP if compatible with hemodynamics, and with prone positioning and extracorporeal support.
Dr. Gattinoni reported having no financial disclosures.
sworcester@mdedge.com
Evaluating and managing the patient with nipple discharge
CASE Young woman with discharge from one nipple
A 26-year-old African American woman presents with a 10-month history of left nipple discharge. The patient describes the discharge as spontaneous, colored dark brown to yellow, and occurring from a single opening in the nipple. The discharge is associated with left breast pain and fullness, without a palpable lump. The patient has no family or personal history of breast cancer.
Nipple discharge is the third most common breast-related symptom (after palpable masses and breast pain), with an estimated prevalence of 5% to 8% among premenopausal women.1 While most causes of nipple discharge reflect benign issues, approximately 5% to 12% of breast cancers have nipple discharge as the only symptom.2 Not surprisingly, nipple discharge creates anxiety for both patients and clinicians.
In this article, we—a breast imaging radiologist, gynecologist, and breast surgeon—outline key steps for evaluating and managing patients with nipple discharge.
Two types of nipple discharge
Nipple discharge can be characterized as physiologic or pathologic. The distinction is based on the patient’s history in conjunction with the clinical breast exam.
Physiologic nipple discharge often is bilateral, nonspontaneous, and white, yellow, green, or brown (TABLE).3 It often is due to nipple stimulation, and the patient can elicit discharge by manually manipulating the breast. Usually, multiple ducts are involved. Galactorrhea refers specifically to milky discharge and occurs most commonly during pregnancy or lactation.2 Galactorrhea that is not associated with pregnancy or lactation often is related to elevated prolactin or thyroid-stimulating hormone levels or to medications. One study reported that no cancers were found when discharge was nonspontaneous and colored or milky.4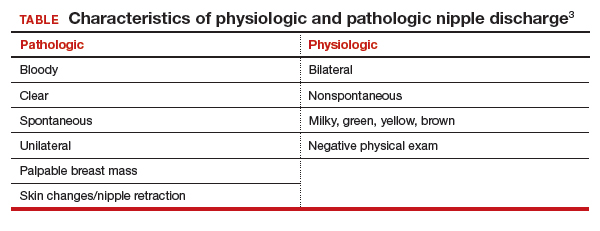
Pathologic nipple discharge is defined as a spontaneous, bloody, clear, or single-duct discharge. A palpable mass in the same breast automatically increases the suspicion of the discharge, regardless of its color or spontaneity.2 The most common cause of pathologic nipple discharge is an intraductal papilloma, a benign epithelial tumor, which accounts for approximately 57% of cases.5
Although the risk of malignancy is low for all patients with nipple discharge, increasing age is associated with increased risk of breast cancer. One study demonstrated that among women aged 40 to 60 years presenting with nipple discharge, the prevalence of invasive cancer is 10%, and the percentage jumps to 32% among women older than 60.6
Breast exam. For any patient with nonlactational nipple discharge, we recommend a thorough breast examination. Deep palpation of all quadrants of the symptomatic breast, especially near the nipple areolar complex, should elicit nipple discharge without any direct squeezing of the nipple. If the patient’s history and physical exam are consistent with physiologic discharge, no further workup is needed. Reassure the patient and recommend appropriate breast cancer screening. Encourage the patient to decrease stimulation or manual manipulation of the nipples if the discharge bothers her.
Continue to: CASE Continued: Workup...
CASE Continued: Workup
On physical exam, the patient’s breasts are noted to be cup size DDD and asymmetric, with the left breast larger than the right; there is no contour deformity. There is no skin or nipple retraction, skin rash, swelling, or nipple changes bilaterally. No dominant masses are appreciated bilaterally. Manual compression elicits no nipple discharge.
Although the discharge is nonbloody, its spontaneity, unilaterality, and single-duct/orifice origin suggest a pathologic cause. The patient is referred for breast imaging.
Imaging workup for pathologic discharge
The American College of Radiology (ACR) Appropriateness Criteria is a useful tool that provides an evidence-based, easy-to-use algorithm for breast imaging in the patient with pathologic nipple discharge (FIGURE 1).6 The algorithm is categorized by patient age, with diagnostic mammography recommended for women aged 30 and older.6 Diagnostic mammography is recommended if the patient has not had a mammogram study in the last 6 months.6 For patients with no prior mammograms, we recommend bilateral diagnostic mammography to compare symmetry of the breasts.
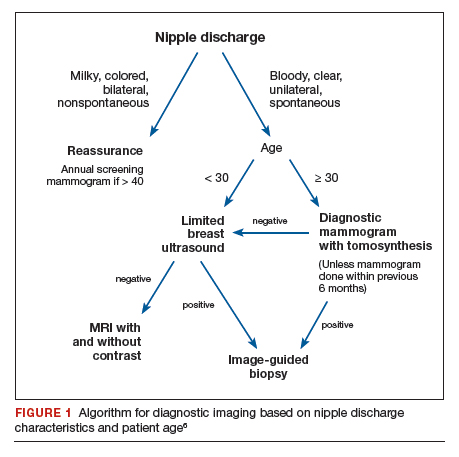
Currently, no studies show that digital breast tomosynthesis (3-D mammography) has a benefit compared with standard 2-D mammography in women with pathologic nipple discharge.6 Given the increased sensitivity of digital breast tomosynthesis for cancer detection, however, in our practice it is standard to use tomosynthesis in the diagnostic evaluation of most patients.
Mammography
On mammography, ductal carcinoma in situ (DCIS) usually presents as calcifications. Both the morphology and distribution of calcifications are used to characterize them as suspicious or, typically, benign. DCIS usually presents as fine pleomorphic or fine linear branching calcifications in a segmental or linear distribution. In patients with pathologic nipple discharge and no other symptoms, the radiologist must closely examine the retroareolar region of the breast to assess for faint calcifications. Magnification views also can be performed to better characterize calcifications.
The sensitivity of mammography for nipple discharge varies in the literature, ranging from approximately 15% to 68%, with a specificity range of 38% to 98%.6 This results in a relatively low positive predictive value but a high negative predictive value of 90%.7 Mammographic sensitivity largely is limited by increased breast density. As more data emerge on the utility of digital breast tomosynthesis in dense breasts, mammographic sensitivity for nipple discharge will likely increase.
Ultrasonography
As an adjunct to mammography, the ACR Appropriateness Criteria recommends targeted (or “limited”) ultrasonography of the retroareolar region of the affected breast for patients aged 30 and older. Ultrasonography is useful to assess for intraductal masses and architectural distortion, and it has higher sensitivity but lower specificity than mammography. The sensitivity of ultrasonography for detecting breast cancer in patients presenting with nipple discharge is reported to be 56% to 80%.6 Ultrasonography can identify lesions not visible mammographically in 63% to 69% of cases.8 Although DCIS usually presents as calcifications, it also can present as an intraductal mass on ultrasonography.
The ACR recommends targeted ultrasonography for patients with nipple discharge and a negative mammogram, or to evaluate a suspicious mammographic abnormality such as architectural distortion, focal asymmetry, or a mass.6 For patient comfort, ultrasonography is the preferred modality for image-guided biopsy.
For women younger than 30 years, targeted ultrasonography is the initial imaging study of choice, according to the ACR criteria.6 Women younger than 30 years with pathologic nipple discharge have a very low risk of breast cancer and tend to have higher breast density, making mammography less useful. Although the radiation dose from mammography is negligible given technological improvements and dose-reduction techniques, ultrasonography remains the preferred initial imaging modality in young women, not only for nipple discharge but also for palpable lumps and focal breast pain.
Mammography is used as an adjunct to ultrasonography in women younger than 30 years when a suspicious abnormality is detected on ultrasonography, such as an intraductal mass or architectural distortion. In these cases, mammography can be used to assess for extent of disease or to visualize suspicious calcifications not well seen on ultrasonography.
For practical purposes regarding which imaging study to order for a patient, it is most efficient to order both a diagnostic mammogram (with tomosynthesis, if possible) and a targeted ultrasound scan of the affected breast. Even if both orders are not needed, having them available increases efficiency for both the radiologist and the ordering physician.
Continue to: CASE Continued: Imaging findings...
CASE Continued: Imaging findings
Given her age, the patient initially undergoes targeted ultrasonography. The grayscale image (FIGURE 2) demonstrates multiple mildly dilated ducts (white arrows) with surrounding hyperechogenicity of the fat (red arrows), indicating soft tissue edema. No intraductal mass is imaged. Given that the ultrasonography findings are not completely negative and are equivocal for malignancy, bilateral diagnostic mammography (FIGURE 3, left breast only) is performed. Standard full-field craniocaudal (FIGURE 3A) and mediolateral oblique (FIGURE 3B) mammographic views demonstrate a heterogeneously dense breast with a few calcifications in the retroareolar left breast (red ovals). No associated mass or architectural distortions are noted. The mammographic and sonographic findings do not reveal a definitive biopsy target.

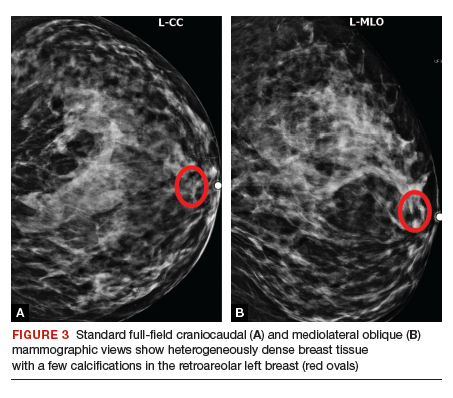
Ductography
When a suspicious abnormality is visualized on either mammography or ultrasonography, the standard of care is to perform an image-guided biopsy of the abnormality. When the standard workup is negative or equivocal, the standard of care historically was to perform ductography.
Ductography is an invasive procedure that involves cannulating the suspicious duct with a small catheter and injecting radiopaque dye into the duct under mammographic guidance. While the sensitivity of ductography is higher than that of both mammography and ultrasonography, its specificity is lower than that of either modality.
Most cases of pathologic discharge are spontaneous and are not reproducible on the day of the procedure. If the procedural radiologist cannot visualize the duct that is producing the discharge, the procedure cannot be performed. Although most patients tolerate the procedure well, ductography produces patient discomfort from cannulation of the duct and injection of contrast.
Magnetic resonance imaging
Dynamic contrast-enhanced magnetic resonance imaging (MRI) is the most sensitive imaging study for evaluating pathologic nipple discharge, and it has largely replaced ductography as an adjunct to mammography and ultrasonography. MRI’s sensitivity for detecting breast cancer ranges from 93% to 100%.6 In addition, MRI allows visualization of the entire breast and areas peripheral to the field of view of a standard ductogram or ultrasound scan.9
Clinicians commonly ask, “Why not skip the mammogram and ultrasound scan and go straight to MRI, since it is so much more sensitive?” Breast MRI has several limitations, including relatively low specificity, cost, use of intravenous contrast, and patient discomfort (that is, claustrophobia, prone positioning). MRI should be utilized for pathologic discharge only when the mammogram and/or targeted ultrasound scans are negative or equivocal.
CASE Continued: Additional imaging
A contrast-enhanced MRI of the breasts (FIGURE 4) demonstrates a large area of non-mass enhancement (red oval) in the left breast, which involves most of the upper breast extending from the nipple to the posterior breast tissue; it measures approximately 7.3 x 14 x 9.1 cm in transverse, anteroposterior, and craniocaudal dimensions, respectively. There is no evidence of left pectoralis muscle involvement. An MRI-directed second look left breast ultrasonography (FIGURE 5) is performed, revealing a small irregular mass in the left breast 1 o’clock position, 10 to 11 cm from the nipple (red arrow). This area had not been imaged in the prior ultrasound scan due to its posterior location far from the nipple. Ultrasound-guided core needle biopsy is performed; moderately differentiated invasive ductal carcinoma (IDC) with high-grade DCIS is found.

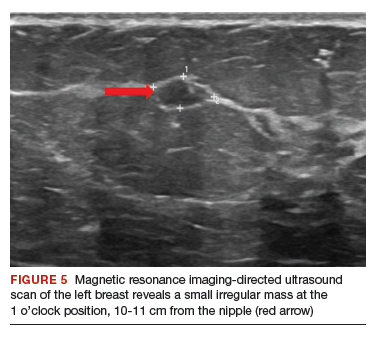
Continue to: When to refer for surgery...
When to refer for surgery
No surgical evaluation or intervention is needed for physiologic nipple discharge. As mentioned previously, reassure the patient and recommend appropriate breast cancer screening. In the setting of pathologic discharge, however, referral to a breast surgeon may be indicated after appropriate imaging workup has been done.
Since abnormal imaging almost always results in a recommendation for image-guided biopsy, typically the biopsy is performed prior to the surgical consultation. Once the pathology report from the biopsy is available, the radiologist makes a radiologic-pathologic concordance statement and recommends surgical consultation. This process allows the surgeon to have all the necessary information at the initial visit.
However, in the setting of pathologic nipple discharge with normal breast imaging, the surgeon and patient may opt for close observation or surgery for definitive diagnosis. Surgical options include single-duct excision when nipple discharge is localized to one duct or central duct excision when nipple discharge cannot be localized to one duct.
CASE Continued: Follow-up
The patient was referred to a breast surgeon. Given the extent of disease in the left breast, breast conservation was not possible. The patient underwent left breast simple mastectomy with sentinel lymph node biopsy and prophylactic right simple mastectomy. Final pathology results revealed stage IA IDC with DCIS. Sentinel lymph nodes were negative for malignancy. The patient underwent adjuvant left chest wall radiation, endocrine therapy with tamoxifen, and implant reconstruction. After 2 years of follow-up, she is disease free.
In summary
Nipple discharge can be classified as physiologic or pathologic. For pathologic discharge, a thorough physical examination should be performed with subsequent imaging evaluation. First-line tools, based on patient age, include diagnostic mammography and targeted ultrasonography. Contrast-enhanced MRI is then recommended for negative or equivocal cases. All patients with pathologic nipple discharge should be referred to a breast surgeon following appropriate imaging evaluation. ●
- Alcock C, Layer GT. Predicting occult malignancy in nipple discharge. ANZ J Surg. 2010;80:646-649.
- Patel BK, Falcon S, Drukteinis J. Management of nipple discharge and the associated imaging findings. Am J Med. 2015;128:353-360.
- Mazzarello S, Arnaout A. Five things to know about nipple discharge. CMAJ. 2015;187:599.
- Goksel HA, Yagmurdur MC, Demirhan B, et al. Management strategies for patients with nipple discharge. Langenbecks Arch Surg. 2005;390:52-58.
- Vargas HI, Vargas MP, Eldrageely K, et al. Outcomes of clinical and surgical assessment of women with pathological nipple discharge. Am Surg. 2006;72:124-128.
- Expert Panel on Breast Imaging; Lee S, Tikha S, Moy L, et al. American College of Radiology Appropriateness Criteria: Evaluation of nipple discharge. https://acsearch.acr.org /docs/3099312/Narrative/. Accessed February 2, 2020.
- Cabioglu N, Hunt KK, Singletary SE, et al. Surgical decision making and factors determining a diagnosis of breast carcinoma in women presenting with nipple discharge. J Am Coll Surg. 2003;196:354-364.
- Morrogh M, Park A, Elkin EB, et al. Lessons learned from 416 cases of nipple discharge of the breast. Am J Surg. 2010;200:73-80.
- Morrogh M, Morris EA, Liberman L, et al. The predictive value of ductography and magnetic resonance imaging in the management of nipple discharge. Ann Surg Oncol. 2007;14:3369-3377.
CASE Young woman with discharge from one nipple
A 26-year-old African American woman presents with a 10-month history of left nipple discharge. The patient describes the discharge as spontaneous, colored dark brown to yellow, and occurring from a single opening in the nipple. The discharge is associated with left breast pain and fullness, without a palpable lump. The patient has no family or personal history of breast cancer.
Nipple discharge is the third most common breast-related symptom (after palpable masses and breast pain), with an estimated prevalence of 5% to 8% among premenopausal women.1 While most causes of nipple discharge reflect benign issues, approximately 5% to 12% of breast cancers have nipple discharge as the only symptom.2 Not surprisingly, nipple discharge creates anxiety for both patients and clinicians.
In this article, we—a breast imaging radiologist, gynecologist, and breast surgeon—outline key steps for evaluating and managing patients with nipple discharge.
Two types of nipple discharge
Nipple discharge can be characterized as physiologic or pathologic. The distinction is based on the patient’s history in conjunction with the clinical breast exam.
Physiologic nipple discharge often is bilateral, nonspontaneous, and white, yellow, green, or brown (TABLE).3 It often is due to nipple stimulation, and the patient can elicit discharge by manually manipulating the breast. Usually, multiple ducts are involved. Galactorrhea refers specifically to milky discharge and occurs most commonly during pregnancy or lactation.2 Galactorrhea that is not associated with pregnancy or lactation often is related to elevated prolactin or thyroid-stimulating hormone levels or to medications. One study reported that no cancers were found when discharge was nonspontaneous and colored or milky.4
Pathologic nipple discharge is defined as a spontaneous, bloody, clear, or single-duct discharge. A palpable mass in the same breast automatically increases the suspicion of the discharge, regardless of its color or spontaneity.2 The most common cause of pathologic nipple discharge is an intraductal papilloma, a benign epithelial tumor, which accounts for approximately 57% of cases.5
Although the risk of malignancy is low for all patients with nipple discharge, increasing age is associated with increased risk of breast cancer. One study demonstrated that among women aged 40 to 60 years presenting with nipple discharge, the prevalence of invasive cancer is 10%, and the percentage jumps to 32% among women older than 60.6
Breast exam. For any patient with nonlactational nipple discharge, we recommend a thorough breast examination. Deep palpation of all quadrants of the symptomatic breast, especially near the nipple areolar complex, should elicit nipple discharge without any direct squeezing of the nipple. If the patient’s history and physical exam are consistent with physiologic discharge, no further workup is needed. Reassure the patient and recommend appropriate breast cancer screening. Encourage the patient to decrease stimulation or manual manipulation of the nipples if the discharge bothers her.
Continue to: CASE Continued: Workup...
CASE Continued: Workup
On physical exam, the patient’s breasts are noted to be cup size DDD and asymmetric, with the left breast larger than the right; there is no contour deformity. There is no skin or nipple retraction, skin rash, swelling, or nipple changes bilaterally. No dominant masses are appreciated bilaterally. Manual compression elicits no nipple discharge.
Although the discharge is nonbloody, its spontaneity, unilaterality, and single-duct/orifice origin suggest a pathologic cause. The patient is referred for breast imaging.
Imaging workup for pathologic discharge
The American College of Radiology (ACR) Appropriateness Criteria is a useful tool that provides an evidence-based, easy-to-use algorithm for breast imaging in the patient with pathologic nipple discharge (FIGURE 1).6 The algorithm is categorized by patient age, with diagnostic mammography recommended for women aged 30 and older.6 Diagnostic mammography is recommended if the patient has not had a mammogram study in the last 6 months.6 For patients with no prior mammograms, we recommend bilateral diagnostic mammography to compare symmetry of the breasts.

Currently, no studies show that digital breast tomosynthesis (3-D mammography) has a benefit compared with standard 2-D mammography in women with pathologic nipple discharge.6 Given the increased sensitivity of digital breast tomosynthesis for cancer detection, however, in our practice it is standard to use tomosynthesis in the diagnostic evaluation of most patients.
Mammography
On mammography, ductal carcinoma in situ (DCIS) usually presents as calcifications. Both the morphology and distribution of calcifications are used to characterize them as suspicious or, typically, benign. DCIS usually presents as fine pleomorphic or fine linear branching calcifications in a segmental or linear distribution. In patients with pathologic nipple discharge and no other symptoms, the radiologist must closely examine the retroareolar region of the breast to assess for faint calcifications. Magnification views also can be performed to better characterize calcifications.
The sensitivity of mammography for nipple discharge varies in the literature, ranging from approximately 15% to 68%, with a specificity range of 38% to 98%.6 This results in a relatively low positive predictive value but a high negative predictive value of 90%.7 Mammographic sensitivity largely is limited by increased breast density. As more data emerge on the utility of digital breast tomosynthesis in dense breasts, mammographic sensitivity for nipple discharge will likely increase.
Ultrasonography
As an adjunct to mammography, the ACR Appropriateness Criteria recommends targeted (or “limited”) ultrasonography of the retroareolar region of the affected breast for patients aged 30 and older. Ultrasonography is useful to assess for intraductal masses and architectural distortion, and it has higher sensitivity but lower specificity than mammography. The sensitivity of ultrasonography for detecting breast cancer in patients presenting with nipple discharge is reported to be 56% to 80%.6 Ultrasonography can identify lesions not visible mammographically in 63% to 69% of cases.8 Although DCIS usually presents as calcifications, it also can present as an intraductal mass on ultrasonography.
The ACR recommends targeted ultrasonography for patients with nipple discharge and a negative mammogram, or to evaluate a suspicious mammographic abnormality such as architectural distortion, focal asymmetry, or a mass.6 For patient comfort, ultrasonography is the preferred modality for image-guided biopsy.
For women younger than 30 years, targeted ultrasonography is the initial imaging study of choice, according to the ACR criteria.6 Women younger than 30 years with pathologic nipple discharge have a very low risk of breast cancer and tend to have higher breast density, making mammography less useful. Although the radiation dose from mammography is negligible given technological improvements and dose-reduction techniques, ultrasonography remains the preferred initial imaging modality in young women, not only for nipple discharge but also for palpable lumps and focal breast pain.
Mammography is used as an adjunct to ultrasonography in women younger than 30 years when a suspicious abnormality is detected on ultrasonography, such as an intraductal mass or architectural distortion. In these cases, mammography can be used to assess for extent of disease or to visualize suspicious calcifications not well seen on ultrasonography.
For practical purposes regarding which imaging study to order for a patient, it is most efficient to order both a diagnostic mammogram (with tomosynthesis, if possible) and a targeted ultrasound scan of the affected breast. Even if both orders are not needed, having them available increases efficiency for both the radiologist and the ordering physician.
Continue to: CASE Continued: Imaging findings...
CASE Continued: Imaging findings
Given her age, the patient initially undergoes targeted ultrasonography. The grayscale image (FIGURE 2) demonstrates multiple mildly dilated ducts (white arrows) with surrounding hyperechogenicity of the fat (red arrows), indicating soft tissue edema. No intraductal mass is imaged. Given that the ultrasonography findings are not completely negative and are equivocal for malignancy, bilateral diagnostic mammography (FIGURE 3, left breast only) is performed. Standard full-field craniocaudal (FIGURE 3A) and mediolateral oblique (FIGURE 3B) mammographic views demonstrate a heterogeneously dense breast with a few calcifications in the retroareolar left breast (red ovals). No associated mass or architectural distortions are noted. The mammographic and sonographic findings do not reveal a definitive biopsy target.


Ductography
When a suspicious abnormality is visualized on either mammography or ultrasonography, the standard of care is to perform an image-guided biopsy of the abnormality. When the standard workup is negative or equivocal, the standard of care historically was to perform ductography.
Ductography is an invasive procedure that involves cannulating the suspicious duct with a small catheter and injecting radiopaque dye into the duct under mammographic guidance. While the sensitivity of ductography is higher than that of both mammography and ultrasonography, its specificity is lower than that of either modality.
Most cases of pathologic discharge are spontaneous and are not reproducible on the day of the procedure. If the procedural radiologist cannot visualize the duct that is producing the discharge, the procedure cannot be performed. Although most patients tolerate the procedure well, ductography produces patient discomfort from cannulation of the duct and injection of contrast.
Magnetic resonance imaging
Dynamic contrast-enhanced magnetic resonance imaging (MRI) is the most sensitive imaging study for evaluating pathologic nipple discharge, and it has largely replaced ductography as an adjunct to mammography and ultrasonography. MRI’s sensitivity for detecting breast cancer ranges from 93% to 100%.6 In addition, MRI allows visualization of the entire breast and areas peripheral to the field of view of a standard ductogram or ultrasound scan.9
Clinicians commonly ask, “Why not skip the mammogram and ultrasound scan and go straight to MRI, since it is so much more sensitive?” Breast MRI has several limitations, including relatively low specificity, cost, use of intravenous contrast, and patient discomfort (that is, claustrophobia, prone positioning). MRI should be utilized for pathologic discharge only when the mammogram and/or targeted ultrasound scans are negative or equivocal.
CASE Continued: Additional imaging
A contrast-enhanced MRI of the breasts (FIGURE 4) demonstrates a large area of non-mass enhancement (red oval) in the left breast, which involves most of the upper breast extending from the nipple to the posterior breast tissue; it measures approximately 7.3 x 14 x 9.1 cm in transverse, anteroposterior, and craniocaudal dimensions, respectively. There is no evidence of left pectoralis muscle involvement. An MRI-directed second look left breast ultrasonography (FIGURE 5) is performed, revealing a small irregular mass in the left breast 1 o’clock position, 10 to 11 cm from the nipple (red arrow). This area had not been imaged in the prior ultrasound scan due to its posterior location far from the nipple. Ultrasound-guided core needle biopsy is performed; moderately differentiated invasive ductal carcinoma (IDC) with high-grade DCIS is found.


Continue to: When to refer for surgery...
When to refer for surgery
No surgical evaluation or intervention is needed for physiologic nipple discharge. As mentioned previously, reassure the patient and recommend appropriate breast cancer screening. In the setting of pathologic discharge, however, referral to a breast surgeon may be indicated after appropriate imaging workup has been done.
Since abnormal imaging almost always results in a recommendation for image-guided biopsy, typically the biopsy is performed prior to the surgical consultation. Once the pathology report from the biopsy is available, the radiologist makes a radiologic-pathologic concordance statement and recommends surgical consultation. This process allows the surgeon to have all the necessary information at the initial visit.
However, in the setting of pathologic nipple discharge with normal breast imaging, the surgeon and patient may opt for close observation or surgery for definitive diagnosis. Surgical options include single-duct excision when nipple discharge is localized to one duct or central duct excision when nipple discharge cannot be localized to one duct.
CASE Continued: Follow-up
The patient was referred to a breast surgeon. Given the extent of disease in the left breast, breast conservation was not possible. The patient underwent left breast simple mastectomy with sentinel lymph node biopsy and prophylactic right simple mastectomy. Final pathology results revealed stage IA IDC with DCIS. Sentinel lymph nodes were negative for malignancy. The patient underwent adjuvant left chest wall radiation, endocrine therapy with tamoxifen, and implant reconstruction. After 2 years of follow-up, she is disease free.
In summary
Nipple discharge can be classified as physiologic or pathologic. For pathologic discharge, a thorough physical examination should be performed with subsequent imaging evaluation. First-line tools, based on patient age, include diagnostic mammography and targeted ultrasonography. Contrast-enhanced MRI is then recommended for negative or equivocal cases. All patients with pathologic nipple discharge should be referred to a breast surgeon following appropriate imaging evaluation. ●
CASE Young woman with discharge from one nipple
A 26-year-old African American woman presents with a 10-month history of left nipple discharge. The patient describes the discharge as spontaneous, colored dark brown to yellow, and occurring from a single opening in the nipple. The discharge is associated with left breast pain and fullness, without a palpable lump. The patient has no family or personal history of breast cancer.
Nipple discharge is the third most common breast-related symptom (after palpable masses and breast pain), with an estimated prevalence of 5% to 8% among premenopausal women.1 While most causes of nipple discharge reflect benign issues, approximately 5% to 12% of breast cancers have nipple discharge as the only symptom.2 Not surprisingly, nipple discharge creates anxiety for both patients and clinicians.
In this article, we—a breast imaging radiologist, gynecologist, and breast surgeon—outline key steps for evaluating and managing patients with nipple discharge.
Two types of nipple discharge
Nipple discharge can be characterized as physiologic or pathologic. The distinction is based on the patient’s history in conjunction with the clinical breast exam.
Physiologic nipple discharge often is bilateral, nonspontaneous, and white, yellow, green, or brown (TABLE).3 It often is due to nipple stimulation, and the patient can elicit discharge by manually manipulating the breast. Usually, multiple ducts are involved. Galactorrhea refers specifically to milky discharge and occurs most commonly during pregnancy or lactation.2 Galactorrhea that is not associated with pregnancy or lactation often is related to elevated prolactin or thyroid-stimulating hormone levels or to medications. One study reported that no cancers were found when discharge was nonspontaneous and colored or milky.4
Pathologic nipple discharge is defined as a spontaneous, bloody, clear, or single-duct discharge. A palpable mass in the same breast automatically increases the suspicion of the discharge, regardless of its color or spontaneity.2 The most common cause of pathologic nipple discharge is an intraductal papilloma, a benign epithelial tumor, which accounts for approximately 57% of cases.5
Although the risk of malignancy is low for all patients with nipple discharge, increasing age is associated with increased risk of breast cancer. One study demonstrated that among women aged 40 to 60 years presenting with nipple discharge, the prevalence of invasive cancer is 10%, and the percentage jumps to 32% among women older than 60.6
Breast exam. For any patient with nonlactational nipple discharge, we recommend a thorough breast examination. Deep palpation of all quadrants of the symptomatic breast, especially near the nipple areolar complex, should elicit nipple discharge without any direct squeezing of the nipple. If the patient’s history and physical exam are consistent with physiologic discharge, no further workup is needed. Reassure the patient and recommend appropriate breast cancer screening. Encourage the patient to decrease stimulation or manual manipulation of the nipples if the discharge bothers her.
Continue to: CASE Continued: Workup...
CASE Continued: Workup
On physical exam, the patient’s breasts are noted to be cup size DDD and asymmetric, with the left breast larger than the right; there is no contour deformity. There is no skin or nipple retraction, skin rash, swelling, or nipple changes bilaterally. No dominant masses are appreciated bilaterally. Manual compression elicits no nipple discharge.
Although the discharge is nonbloody, its spontaneity, unilaterality, and single-duct/orifice origin suggest a pathologic cause. The patient is referred for breast imaging.
Imaging workup for pathologic discharge
The American College of Radiology (ACR) Appropriateness Criteria is a useful tool that provides an evidence-based, easy-to-use algorithm for breast imaging in the patient with pathologic nipple discharge (FIGURE 1).6 The algorithm is categorized by patient age, with diagnostic mammography recommended for women aged 30 and older.6 Diagnostic mammography is recommended if the patient has not had a mammogram study in the last 6 months.6 For patients with no prior mammograms, we recommend bilateral diagnostic mammography to compare symmetry of the breasts.

Currently, no studies show that digital breast tomosynthesis (3-D mammography) has a benefit compared with standard 2-D mammography in women with pathologic nipple discharge.6 Given the increased sensitivity of digital breast tomosynthesis for cancer detection, however, in our practice it is standard to use tomosynthesis in the diagnostic evaluation of most patients.
Mammography
On mammography, ductal carcinoma in situ (DCIS) usually presents as calcifications. Both the morphology and distribution of calcifications are used to characterize them as suspicious or, typically, benign. DCIS usually presents as fine pleomorphic or fine linear branching calcifications in a segmental or linear distribution. In patients with pathologic nipple discharge and no other symptoms, the radiologist must closely examine the retroareolar region of the breast to assess for faint calcifications. Magnification views also can be performed to better characterize calcifications.
The sensitivity of mammography for nipple discharge varies in the literature, ranging from approximately 15% to 68%, with a specificity range of 38% to 98%.6 This results in a relatively low positive predictive value but a high negative predictive value of 90%.7 Mammographic sensitivity largely is limited by increased breast density. As more data emerge on the utility of digital breast tomosynthesis in dense breasts, mammographic sensitivity for nipple discharge will likely increase.
Ultrasonography
As an adjunct to mammography, the ACR Appropriateness Criteria recommends targeted (or “limited”) ultrasonography of the retroareolar region of the affected breast for patients aged 30 and older. Ultrasonography is useful to assess for intraductal masses and architectural distortion, and it has higher sensitivity but lower specificity than mammography. The sensitivity of ultrasonography for detecting breast cancer in patients presenting with nipple discharge is reported to be 56% to 80%.6 Ultrasonography can identify lesions not visible mammographically in 63% to 69% of cases.8 Although DCIS usually presents as calcifications, it also can present as an intraductal mass on ultrasonography.
The ACR recommends targeted ultrasonography for patients with nipple discharge and a negative mammogram, or to evaluate a suspicious mammographic abnormality such as architectural distortion, focal asymmetry, or a mass.6 For patient comfort, ultrasonography is the preferred modality for image-guided biopsy.
For women younger than 30 years, targeted ultrasonography is the initial imaging study of choice, according to the ACR criteria.6 Women younger than 30 years with pathologic nipple discharge have a very low risk of breast cancer and tend to have higher breast density, making mammography less useful. Although the radiation dose from mammography is negligible given technological improvements and dose-reduction techniques, ultrasonography remains the preferred initial imaging modality in young women, not only for nipple discharge but also for palpable lumps and focal breast pain.
Mammography is used as an adjunct to ultrasonography in women younger than 30 years when a suspicious abnormality is detected on ultrasonography, such as an intraductal mass or architectural distortion. In these cases, mammography can be used to assess for extent of disease or to visualize suspicious calcifications not well seen on ultrasonography.
For practical purposes regarding which imaging study to order for a patient, it is most efficient to order both a diagnostic mammogram (with tomosynthesis, if possible) and a targeted ultrasound scan of the affected breast. Even if both orders are not needed, having them available increases efficiency for both the radiologist and the ordering physician.
Continue to: CASE Continued: Imaging findings...
CASE Continued: Imaging findings
Given her age, the patient initially undergoes targeted ultrasonography. The grayscale image (FIGURE 2) demonstrates multiple mildly dilated ducts (white arrows) with surrounding hyperechogenicity of the fat (red arrows), indicating soft tissue edema. No intraductal mass is imaged. Given that the ultrasonography findings are not completely negative and are equivocal for malignancy, bilateral diagnostic mammography (FIGURE 3, left breast only) is performed. Standard full-field craniocaudal (FIGURE 3A) and mediolateral oblique (FIGURE 3B) mammographic views demonstrate a heterogeneously dense breast with a few calcifications in the retroareolar left breast (red ovals). No associated mass or architectural distortions are noted. The mammographic and sonographic findings do not reveal a definitive biopsy target.


Ductography
When a suspicious abnormality is visualized on either mammography or ultrasonography, the standard of care is to perform an image-guided biopsy of the abnormality. When the standard workup is negative or equivocal, the standard of care historically was to perform ductography.
Ductography is an invasive procedure that involves cannulating the suspicious duct with a small catheter and injecting radiopaque dye into the duct under mammographic guidance. While the sensitivity of ductography is higher than that of both mammography and ultrasonography, its specificity is lower than that of either modality.
Most cases of pathologic discharge are spontaneous and are not reproducible on the day of the procedure. If the procedural radiologist cannot visualize the duct that is producing the discharge, the procedure cannot be performed. Although most patients tolerate the procedure well, ductography produces patient discomfort from cannulation of the duct and injection of contrast.
Magnetic resonance imaging
Dynamic contrast-enhanced magnetic resonance imaging (MRI) is the most sensitive imaging study for evaluating pathologic nipple discharge, and it has largely replaced ductography as an adjunct to mammography and ultrasonography. MRI’s sensitivity for detecting breast cancer ranges from 93% to 100%.6 In addition, MRI allows visualization of the entire breast and areas peripheral to the field of view of a standard ductogram or ultrasound scan.9
Clinicians commonly ask, “Why not skip the mammogram and ultrasound scan and go straight to MRI, since it is so much more sensitive?” Breast MRI has several limitations, including relatively low specificity, cost, use of intravenous contrast, and patient discomfort (that is, claustrophobia, prone positioning). MRI should be utilized for pathologic discharge only when the mammogram and/or targeted ultrasound scans are negative or equivocal.
CASE Continued: Additional imaging
A contrast-enhanced MRI of the breasts (FIGURE 4) demonstrates a large area of non-mass enhancement (red oval) in the left breast, which involves most of the upper breast extending from the nipple to the posterior breast tissue; it measures approximately 7.3 x 14 x 9.1 cm in transverse, anteroposterior, and craniocaudal dimensions, respectively. There is no evidence of left pectoralis muscle involvement. An MRI-directed second look left breast ultrasonography (FIGURE 5) is performed, revealing a small irregular mass in the left breast 1 o’clock position, 10 to 11 cm from the nipple (red arrow). This area had not been imaged in the prior ultrasound scan due to its posterior location far from the nipple. Ultrasound-guided core needle biopsy is performed; moderately differentiated invasive ductal carcinoma (IDC) with high-grade DCIS is found.


Continue to: When to refer for surgery...
When to refer for surgery
No surgical evaluation or intervention is needed for physiologic nipple discharge. As mentioned previously, reassure the patient and recommend appropriate breast cancer screening. In the setting of pathologic discharge, however, referral to a breast surgeon may be indicated after appropriate imaging workup has been done.
Since abnormal imaging almost always results in a recommendation for image-guided biopsy, typically the biopsy is performed prior to the surgical consultation. Once the pathology report from the biopsy is available, the radiologist makes a radiologic-pathologic concordance statement and recommends surgical consultation. This process allows the surgeon to have all the necessary information at the initial visit.
However, in the setting of pathologic nipple discharge with normal breast imaging, the surgeon and patient may opt for close observation or surgery for definitive diagnosis. Surgical options include single-duct excision when nipple discharge is localized to one duct or central duct excision when nipple discharge cannot be localized to one duct.
CASE Continued: Follow-up
The patient was referred to a breast surgeon. Given the extent of disease in the left breast, breast conservation was not possible. The patient underwent left breast simple mastectomy with sentinel lymph node biopsy and prophylactic right simple mastectomy. Final pathology results revealed stage IA IDC with DCIS. Sentinel lymph nodes were negative for malignancy. The patient underwent adjuvant left chest wall radiation, endocrine therapy with tamoxifen, and implant reconstruction. After 2 years of follow-up, she is disease free.
In summary
Nipple discharge can be classified as physiologic or pathologic. For pathologic discharge, a thorough physical examination should be performed with subsequent imaging evaluation. First-line tools, based on patient age, include diagnostic mammography and targeted ultrasonography. Contrast-enhanced MRI is then recommended for negative or equivocal cases. All patients with pathologic nipple discharge should be referred to a breast surgeon following appropriate imaging evaluation. ●
- Alcock C, Layer GT. Predicting occult malignancy in nipple discharge. ANZ J Surg. 2010;80:646-649.
- Patel BK, Falcon S, Drukteinis J. Management of nipple discharge and the associated imaging findings. Am J Med. 2015;128:353-360.
- Mazzarello S, Arnaout A. Five things to know about nipple discharge. CMAJ. 2015;187:599.
- Goksel HA, Yagmurdur MC, Demirhan B, et al. Management strategies for patients with nipple discharge. Langenbecks Arch Surg. 2005;390:52-58.
- Vargas HI, Vargas MP, Eldrageely K, et al. Outcomes of clinical and surgical assessment of women with pathological nipple discharge. Am Surg. 2006;72:124-128.
- Expert Panel on Breast Imaging; Lee S, Tikha S, Moy L, et al. American College of Radiology Appropriateness Criteria: Evaluation of nipple discharge. https://acsearch.acr.org /docs/3099312/Narrative/. Accessed February 2, 2020.
- Cabioglu N, Hunt KK, Singletary SE, et al. Surgical decision making and factors determining a diagnosis of breast carcinoma in women presenting with nipple discharge. J Am Coll Surg. 2003;196:354-364.
- Morrogh M, Park A, Elkin EB, et al. Lessons learned from 416 cases of nipple discharge of the breast. Am J Surg. 2010;200:73-80.
- Morrogh M, Morris EA, Liberman L, et al. The predictive value of ductography and magnetic resonance imaging in the management of nipple discharge. Ann Surg Oncol. 2007;14:3369-3377.
- Alcock C, Layer GT. Predicting occult malignancy in nipple discharge. ANZ J Surg. 2010;80:646-649.
- Patel BK, Falcon S, Drukteinis J. Management of nipple discharge and the associated imaging findings. Am J Med. 2015;128:353-360.
- Mazzarello S, Arnaout A. Five things to know about nipple discharge. CMAJ. 2015;187:599.
- Goksel HA, Yagmurdur MC, Demirhan B, et al. Management strategies for patients with nipple discharge. Langenbecks Arch Surg. 2005;390:52-58.
- Vargas HI, Vargas MP, Eldrageely K, et al. Outcomes of clinical and surgical assessment of women with pathological nipple discharge. Am Surg. 2006;72:124-128.
- Expert Panel on Breast Imaging; Lee S, Tikha S, Moy L, et al. American College of Radiology Appropriateness Criteria: Evaluation of nipple discharge. https://acsearch.acr.org /docs/3099312/Narrative/. Accessed February 2, 2020.
- Cabioglu N, Hunt KK, Singletary SE, et al. Surgical decision making and factors determining a diagnosis of breast carcinoma in women presenting with nipple discharge. J Am Coll Surg. 2003;196:354-364.
- Morrogh M, Park A, Elkin EB, et al. Lessons learned from 416 cases of nipple discharge of the breast. Am J Surg. 2010;200:73-80.
- Morrogh M, Morris EA, Liberman L, et al. The predictive value of ductography and magnetic resonance imaging in the management of nipple discharge. Ann Surg Oncol. 2007;14:3369-3377.
2020 Update on prenatal phenotyping
As prenatal genetic testing and imaging have advanced, the diagnosis of genetic disorders has moved from the postnatal to the prenatal time frame. This has largely been facilitated by the increasing use of exome sequencing (ES) in the prenatal setting. Two landmark trials published in January 2019 highlighted the overall diagnostic yields of prenatal ES as 8.5% and 10% in fetuses with normal karyotype and microarray.1,2
Although this is a huge step forward in prenatal diagnosis, ES is currently a manually curated, labor-intensive task. The process involves reviewing thousands of sequence variants for any given sample and prioritizing each variant based on bioinformatic data, prediction models, literature review, and specific patient characteristics. The patient characteristics, or phenotypic information, are critically important in prioritizing candidate variants.
To date, prenatal ES has been limited by the use of inconsistent terminology and the lack of well-understood prenatal phenotypes. In this Update, we highlight how recently published work draws attention to these critical gaps in prenatal diagnosis.
Standardizing phenotyping language in the prenatal setting
Tomar S, Sethi R, Lai PS. Specific phenotype semantics facilitate gene prioritization in clinical exome sequencing. Eur J Hum Genet. 2019;27:1389-1397.
Clinical ES in pediatric and adult populations is enhanced by the use of standardized vocabulary to describe disorders. Standardized language ensures that identified variants are filtered correctly and in a systematic fashion based on the patient characteristics that are provided. One commonly used platform is the Human Phenotype Ontology (HPO).
Tomar and colleagues assessed the impact of HPO-based clinical information on the performance of a gene prioritization tool.3 Gene prioritization (or simulation) tools are used for interpretation of ES data to help analysts efficiently sort through the thousands of variants in an individual’s genetic sequence. The performance, or accuracy, of a prioritization tool can be assessed by looking at the location of the disease-causing gene in the suggested gene list.
Continue to: Cohort of diagnosed patients and gene prioritization...
Cohort of diagnosed patients and gene prioritization
In this experimental model, Tomar and colleagues included 50 cases with neuromuscular disorders; all had available sequencing data, fully described phenotypes, and known causal genes. The authors varied the level of available clinical information in the HPO terms used for simulated variant analysis. Using 3 web-based gene prioritization tools on the 50 cases, they varied the HPO input to include a random selection of 10%, 30%, and 50% of HPO terms derived from deep phenotyping.
The 3 prioritization tools ranked input genes based on gene-phenotype associations that were derived from gene-phenotype databases. The authors then assessed the quality of the candidate gene lists by the location of the known causative gene on the generated rank lists. They repeated this analysis 4 times with different randomly selected HPO terms.
Inclusion of more HPO terms allowed for more accurate diagnoses in rare disorders
The authors found that the phenotype input for ES matters. When only 10% and 30% of the HPO terms were used to create a candidate gene list, the causative gene was less likely to be in the top portions of gene lists than when 50% or 100% of the available HPO terms were used.
For well-characterized disorders, use of the top 10% HPO terms performed as well as when all available HPO terms were used. For previously undescribed disease-gene associations, identification of the disease gene suffered with more limited HPO term availability.
What this study contributes
This study was a simulation of previously sequenced patients with neuromuscular disorders. It examined a small sample size for a narrow spectrum of disease. However, it clearly illustrated the principle that completeness of phenotypic information for ES pipelines is relevant for interpretation.
The quantity and quality of phenotype input into ES matters for assessing genetic variants. HPO terms have been developed to represent prenatal sonographic findings, and these have been extended to include gestational age of onset in some cases. Providing as much data as possible about the prenatal phenotype through accepted uniform vocabulary (such as HPO) will increase the likelihood that a prenatal diagnosis can be made.
Detailed description of prenatal findings is essential to diagnosis
Aarabi M, Sniezek O, Jiang H, et al. Importance of complete phenotyping in prenatal whole exome sequencing. Hum Genet. 2018;137:175-181.
In a retrospective cohort study, Aarabi and colleagues evaluated the diagnostic utility and limitations of ES in prenatal cases with structural birth defects.4
A case series study
The investigators included 20 pregnancies with structural birth defects that were referred to their center for prenatal diagnosis between 12 and 20 weeks’ gestation. All pregnancies had normal karyotype and microarray analyses prior to enrollment.
ES was performed on trio samples, which included fetal and parental DNA samples (extracted from peripheral blood). Reports provided by the commercial laboratories were normal for all cases and included no pathogenic or likely pathogenic variants. The laboratory provided the investigators with the FASTQ (genetic sequence) files for reanalysis, which was performed using both prenatal and postnatal detailed phenotypic information.
Continue to: Use of postnatal information facilitated diagnoses...
Use of postnatal information facilitated diagnoses
Reanalysis of ES data using detailed postnatal findings revealed a possible diagnosis in 20% of cases. Each case in which a diagnosis was made, detailed below, highlights an important limitation in our current ability to make prenatal diagnoses.
Case 1. A fetus was diagnosed prenatally with arthrogryposis, plagiocephaly, and club feet. After birth, the infant also was found to have generalized muscle weakness, elevated creatine phosphokinase, and congenital hip dislocation.
Reanalysis of the ES data revealed compound heterozygous missense variants in the nebulin gene (NEB). Although classified as variants of uncertain significance (VUS), these are consistent with the phenotype, the authors argued, and with the diagnosis of autosomal recessive nemaline myopathy 2.
Case 2. Prenatal diagnosis was made of a right limb anomaly, tetralogy of Fallot, intrauterine growth restriction, ambiguous genitalia, and dextrocardia. Postnatal evaluation revealed absent pulmonary valve syndrome, right arm dysplasia, pectus carinatum deformity, and failure to thrive.
In this case, ES with the postnatal information revealed a VUS in the NOTCH1 gene, which has been associated with Adams-Oliver syndrome. Although by strict criteria this variant is also of uncertain significance, Adams-Oliver syndrome is characterized, in part, by transverse limb defects and congenital heart disease, as was found in the proband.
Case 3. Prenatal ultrasonography revealed microcephaly and absence of the septum pellucidum. Postnatal magnetic resonance imaging revealed semi-lobar holoprosencephaly. A holoprosencephaly-specific gene panel revealed a deletion in the ZIC2 gene, which is known to cause holoprosencephaly.
Careful re-examination of the ES data revealed some abnormality in the ZIC2 signal, which might have been studied in greater detail and thereby detected if the prenatal diagnosis of holoprosencephaly had been made.
Case 4. An ultrasound evaluation at 12 weeks’ gestation revealed a cystic hygroma, short long bones, and possible absent hand and fibula. A postnatal fetal autopsy at 14 weeks showed split-hand and split-foot malformations, which were not appreciated on ultrasonography.
In filtering the ES data with this information, a pathogenic variant in the PRCN gene was identified as causal, and the diagnosis of Goltz syndrome was made.
Challenges facing prenatal diagnosis
A case series is inherently limited by its small sample size. Nevertheless, the authors suggest 2 major challenges in our ability to make the above diagnoses in the prenatal setting:
1) the prenatal assessment being limited to major structural abnormalities, and 2) commercial laboratories not having enough experience or volume to interpret the limited information provided by prenatal imaging.
Prenatal genetic diagnosis often is limited by incomplete information about the features seen on ultrasonography. Although not all features are visible prenatally, more diagnoses can be made if laboratories are provided with detailed information about the structural abnormalities that are seen. Furthermore, if ES does not provide a prenatal diagnosis, the data should be reviewed postnatally if more detailed phenotypic information becomes available.
Can AI technology be incorporated to make a genetic diagnosis?
Hsieh TC, Mensah MA, Pantel JT, et al. PEDIA: prioritization of exome data by image analysis. Genet Med. 2019;21:2807-2814.
Increasingly, ES is used in all types of undiagnosed, rare genetic diseases. Although there is a high diagnostic yield in many populations, ES’s clinical utility is limited by the labor-intensive process of interpreting each variant in the context of detailed phenotypic information. The widespread use of HPO would be one step toward standardizing the information that is entered into the analysis of ES data, but even HPO cannot capture certain visual clues.
Hsieh and colleagues attempted to use artificial intelligence (AI) for “next-generation phenotyping” to assess facial dysmorphology and integrate the information into variant classification.5 The authors described their approach of incorporating AI as “prioritization of exome data by image analysis” (PEDIA).
Continue to: Designing dysmorphology machine learning...
Designing dysmorphology machine learning
The cohort included 679 individuals with 105 different genetic disorders. All individuals had a previously confirmed molecular diagnosis that would be detected by ES. Each individual had a frontal facial photograph analyzed and detailed clinical features documented in HPO terms extracted by 2 clinicians.
A facial analysis software called DeepGestalt, trained on 17,000 patient images, was used to create a Gestalt score. Each individual had 4 different predicted gene scoring approaches:
- a molecular deleteriousness score
- facial analysis with the Gestalt score
- a combination of molecular deleteriousness score and HPO-based gene-prioritization tool (termed semantic similarity score)
- the PEDIA score, which included all 3 prior approaches.
A type of machine learning algorithm (support vector machine, or SVM) was applied, validated, and used to prioritize genes based on the combined scores.
AI seemed to improve diagnostic accuracy
Utilizing the combination of machine learning, HPO terms, and facial analysis software greatly improved the accuracy of variant classification predictions over any approach alone.
Using only the sequence variant and molecular deleteriousness score, the causative variant was ranked in the top 10 of all identified variants in less than 45% of cases. Adding the HPO-based gene prioritization tools increased the accuracy to 63% to 94%. Use of the PEDIA score, which incorporated all 3, increased the accuracy to 99% for the top 10 ranking.
Even more impressive improvements were made in the top 1 ranking accuracy rate, which went from 36% to 74% without facial image information to 86% to 89% with inclusion of DeepGestalt scores.
Study strengths and limitations
This study’s innovative application of facial analysis and machine learning, combined with HPO-driven variant classification, showed added benefit. To achieve this with available patient photographs and thorough phenotyping, previously diagnosed patients were used. Because complete ES information was not available for those patients, their known pathogenic variant was inserted into randomly selected exomes from the 1000 Genomes Project (healthy individuals). The authors additionally noted that the PEDIA score performance was diminished for rare disorders in which limited data were available.
The accuracy of gene prediction in pediatric and adult populations is enhanced by the use of computer-assisted image analysis and machine-learning algorithms. These computational methods may be employed to automate variant classification, making it more accurate, efficient, and less laborious. Detailed descriptions or characteristic images of prenatal findings also may allow this technology to be introduced in the prenatal setting.
- Lord J, McMullan DJ, Eberhardt RY, et al; for the Prenatal Assessment of Genomes and Exomes Consortium. Prenatal exome sequencing analysis in fetal structural anomalies detected by ultrasonography (PAGE): a cohort study. Lancet. 2019;393:747-757.
- Petrovski S, Aggarwal V, Giordano JL, et al. Whole-exome sequencing in the evaluation of fetal structural anomalies: a prospective cohort study. Lancet. 2019;393:758-767.
- Tomar S, Sethi R, Lai PS. Specific phenotype semantics facilitate gene prioritization in clinical exome sequencing. Eur J Hum Genet. 2019;27:1389-1397.
- Aarabi M, Sniezek O, Jiang H, et al. Importance of complete phenotyping in prenatal whole exome sequencing. Hum Genet. 2018;137:175-181.
- Hsieh TC, Mensah MA, Pantel JT, et al. PEDIA: prioritization of exome data by image analysis. Genet Med. 2019;21:2807-2814.
As prenatal genetic testing and imaging have advanced, the diagnosis of genetic disorders has moved from the postnatal to the prenatal time frame. This has largely been facilitated by the increasing use of exome sequencing (ES) in the prenatal setting. Two landmark trials published in January 2019 highlighted the overall diagnostic yields of prenatal ES as 8.5% and 10% in fetuses with normal karyotype and microarray.1,2
Although this is a huge step forward in prenatal diagnosis, ES is currently a manually curated, labor-intensive task. The process involves reviewing thousands of sequence variants for any given sample and prioritizing each variant based on bioinformatic data, prediction models, literature review, and specific patient characteristics. The patient characteristics, or phenotypic information, are critically important in prioritizing candidate variants.
To date, prenatal ES has been limited by the use of inconsistent terminology and the lack of well-understood prenatal phenotypes. In this Update, we highlight how recently published work draws attention to these critical gaps in prenatal diagnosis.
Standardizing phenotyping language in the prenatal setting
Tomar S, Sethi R, Lai PS. Specific phenotype semantics facilitate gene prioritization in clinical exome sequencing. Eur J Hum Genet. 2019;27:1389-1397.
Clinical ES in pediatric and adult populations is enhanced by the use of standardized vocabulary to describe disorders. Standardized language ensures that identified variants are filtered correctly and in a systematic fashion based on the patient characteristics that are provided. One commonly used platform is the Human Phenotype Ontology (HPO).
Tomar and colleagues assessed the impact of HPO-based clinical information on the performance of a gene prioritization tool.3 Gene prioritization (or simulation) tools are used for interpretation of ES data to help analysts efficiently sort through the thousands of variants in an individual’s genetic sequence. The performance, or accuracy, of a prioritization tool can be assessed by looking at the location of the disease-causing gene in the suggested gene list.
Continue to: Cohort of diagnosed patients and gene prioritization...
Cohort of diagnosed patients and gene prioritization
In this experimental model, Tomar and colleagues included 50 cases with neuromuscular disorders; all had available sequencing data, fully described phenotypes, and known causal genes. The authors varied the level of available clinical information in the HPO terms used for simulated variant analysis. Using 3 web-based gene prioritization tools on the 50 cases, they varied the HPO input to include a random selection of 10%, 30%, and 50% of HPO terms derived from deep phenotyping.
The 3 prioritization tools ranked input genes based on gene-phenotype associations that were derived from gene-phenotype databases. The authors then assessed the quality of the candidate gene lists by the location of the known causative gene on the generated rank lists. They repeated this analysis 4 times with different randomly selected HPO terms.
Inclusion of more HPO terms allowed for more accurate diagnoses in rare disorders
The authors found that the phenotype input for ES matters. When only 10% and 30% of the HPO terms were used to create a candidate gene list, the causative gene was less likely to be in the top portions of gene lists than when 50% or 100% of the available HPO terms were used.
For well-characterized disorders, use of the top 10% HPO terms performed as well as when all available HPO terms were used. For previously undescribed disease-gene associations, identification of the disease gene suffered with more limited HPO term availability.
What this study contributes
This study was a simulation of previously sequenced patients with neuromuscular disorders. It examined a small sample size for a narrow spectrum of disease. However, it clearly illustrated the principle that completeness of phenotypic information for ES pipelines is relevant for interpretation.
The quantity and quality of phenotype input into ES matters for assessing genetic variants. HPO terms have been developed to represent prenatal sonographic findings, and these have been extended to include gestational age of onset in some cases. Providing as much data as possible about the prenatal phenotype through accepted uniform vocabulary (such as HPO) will increase the likelihood that a prenatal diagnosis can be made.
Detailed description of prenatal findings is essential to diagnosis
Aarabi M, Sniezek O, Jiang H, et al. Importance of complete phenotyping in prenatal whole exome sequencing. Hum Genet. 2018;137:175-181.
In a retrospective cohort study, Aarabi and colleagues evaluated the diagnostic utility and limitations of ES in prenatal cases with structural birth defects.4
A case series study
The investigators included 20 pregnancies with structural birth defects that were referred to their center for prenatal diagnosis between 12 and 20 weeks’ gestation. All pregnancies had normal karyotype and microarray analyses prior to enrollment.
ES was performed on trio samples, which included fetal and parental DNA samples (extracted from peripheral blood). Reports provided by the commercial laboratories were normal for all cases and included no pathogenic or likely pathogenic variants. The laboratory provided the investigators with the FASTQ (genetic sequence) files for reanalysis, which was performed using both prenatal and postnatal detailed phenotypic information.
Continue to: Use of postnatal information facilitated diagnoses...
Use of postnatal information facilitated diagnoses
Reanalysis of ES data using detailed postnatal findings revealed a possible diagnosis in 20% of cases. Each case in which a diagnosis was made, detailed below, highlights an important limitation in our current ability to make prenatal diagnoses.
Case 1. A fetus was diagnosed prenatally with arthrogryposis, plagiocephaly, and club feet. After birth, the infant also was found to have generalized muscle weakness, elevated creatine phosphokinase, and congenital hip dislocation.
Reanalysis of the ES data revealed compound heterozygous missense variants in the nebulin gene (NEB). Although classified as variants of uncertain significance (VUS), these are consistent with the phenotype, the authors argued, and with the diagnosis of autosomal recessive nemaline myopathy 2.
Case 2. Prenatal diagnosis was made of a right limb anomaly, tetralogy of Fallot, intrauterine growth restriction, ambiguous genitalia, and dextrocardia. Postnatal evaluation revealed absent pulmonary valve syndrome, right arm dysplasia, pectus carinatum deformity, and failure to thrive.
In this case, ES with the postnatal information revealed a VUS in the NOTCH1 gene, which has been associated with Adams-Oliver syndrome. Although by strict criteria this variant is also of uncertain significance, Adams-Oliver syndrome is characterized, in part, by transverse limb defects and congenital heart disease, as was found in the proband.
Case 3. Prenatal ultrasonography revealed microcephaly and absence of the septum pellucidum. Postnatal magnetic resonance imaging revealed semi-lobar holoprosencephaly. A holoprosencephaly-specific gene panel revealed a deletion in the ZIC2 gene, which is known to cause holoprosencephaly.
Careful re-examination of the ES data revealed some abnormality in the ZIC2 signal, which might have been studied in greater detail and thereby detected if the prenatal diagnosis of holoprosencephaly had been made.
Case 4. An ultrasound evaluation at 12 weeks’ gestation revealed a cystic hygroma, short long bones, and possible absent hand and fibula. A postnatal fetal autopsy at 14 weeks showed split-hand and split-foot malformations, which were not appreciated on ultrasonography.
In filtering the ES data with this information, a pathogenic variant in the PRCN gene was identified as causal, and the diagnosis of Goltz syndrome was made.
Challenges facing prenatal diagnosis
A case series is inherently limited by its small sample size. Nevertheless, the authors suggest 2 major challenges in our ability to make the above diagnoses in the prenatal setting:
1) the prenatal assessment being limited to major structural abnormalities, and 2) commercial laboratories not having enough experience or volume to interpret the limited information provided by prenatal imaging.
Prenatal genetic diagnosis often is limited by incomplete information about the features seen on ultrasonography. Although not all features are visible prenatally, more diagnoses can be made if laboratories are provided with detailed information about the structural abnormalities that are seen. Furthermore, if ES does not provide a prenatal diagnosis, the data should be reviewed postnatally if more detailed phenotypic information becomes available.
Can AI technology be incorporated to make a genetic diagnosis?
Hsieh TC, Mensah MA, Pantel JT, et al. PEDIA: prioritization of exome data by image analysis. Genet Med. 2019;21:2807-2814.
Increasingly, ES is used in all types of undiagnosed, rare genetic diseases. Although there is a high diagnostic yield in many populations, ES’s clinical utility is limited by the labor-intensive process of interpreting each variant in the context of detailed phenotypic information. The widespread use of HPO would be one step toward standardizing the information that is entered into the analysis of ES data, but even HPO cannot capture certain visual clues.
Hsieh and colleagues attempted to use artificial intelligence (AI) for “next-generation phenotyping” to assess facial dysmorphology and integrate the information into variant classification.5 The authors described their approach of incorporating AI as “prioritization of exome data by image analysis” (PEDIA).
Continue to: Designing dysmorphology machine learning...
Designing dysmorphology machine learning
The cohort included 679 individuals with 105 different genetic disorders. All individuals had a previously confirmed molecular diagnosis that would be detected by ES. Each individual had a frontal facial photograph analyzed and detailed clinical features documented in HPO terms extracted by 2 clinicians.
A facial analysis software called DeepGestalt, trained on 17,000 patient images, was used to create a Gestalt score. Each individual had 4 different predicted gene scoring approaches:
- a molecular deleteriousness score
- facial analysis with the Gestalt score
- a combination of molecular deleteriousness score and HPO-based gene-prioritization tool (termed semantic similarity score)
- the PEDIA score, which included all 3 prior approaches.
A type of machine learning algorithm (support vector machine, or SVM) was applied, validated, and used to prioritize genes based on the combined scores.
AI seemed to improve diagnostic accuracy
Utilizing the combination of machine learning, HPO terms, and facial analysis software greatly improved the accuracy of variant classification predictions over any approach alone.
Using only the sequence variant and molecular deleteriousness score, the causative variant was ranked in the top 10 of all identified variants in less than 45% of cases. Adding the HPO-based gene prioritization tools increased the accuracy to 63% to 94%. Use of the PEDIA score, which incorporated all 3, increased the accuracy to 99% for the top 10 ranking.
Even more impressive improvements were made in the top 1 ranking accuracy rate, which went from 36% to 74% without facial image information to 86% to 89% with inclusion of DeepGestalt scores.
Study strengths and limitations
This study’s innovative application of facial analysis and machine learning, combined with HPO-driven variant classification, showed added benefit. To achieve this with available patient photographs and thorough phenotyping, previously diagnosed patients were used. Because complete ES information was not available for those patients, their known pathogenic variant was inserted into randomly selected exomes from the 1000 Genomes Project (healthy individuals). The authors additionally noted that the PEDIA score performance was diminished for rare disorders in which limited data were available.
The accuracy of gene prediction in pediatric and adult populations is enhanced by the use of computer-assisted image analysis and machine-learning algorithms. These computational methods may be employed to automate variant classification, making it more accurate, efficient, and less laborious. Detailed descriptions or characteristic images of prenatal findings also may allow this technology to be introduced in the prenatal setting.
As prenatal genetic testing and imaging have advanced, the diagnosis of genetic disorders has moved from the postnatal to the prenatal time frame. This has largely been facilitated by the increasing use of exome sequencing (ES) in the prenatal setting. Two landmark trials published in January 2019 highlighted the overall diagnostic yields of prenatal ES as 8.5% and 10% in fetuses with normal karyotype and microarray.1,2
Although this is a huge step forward in prenatal diagnosis, ES is currently a manually curated, labor-intensive task. The process involves reviewing thousands of sequence variants for any given sample and prioritizing each variant based on bioinformatic data, prediction models, literature review, and specific patient characteristics. The patient characteristics, or phenotypic information, are critically important in prioritizing candidate variants.
To date, prenatal ES has been limited by the use of inconsistent terminology and the lack of well-understood prenatal phenotypes. In this Update, we highlight how recently published work draws attention to these critical gaps in prenatal diagnosis.
Standardizing phenotyping language in the prenatal setting
Tomar S, Sethi R, Lai PS. Specific phenotype semantics facilitate gene prioritization in clinical exome sequencing. Eur J Hum Genet. 2019;27:1389-1397.
Clinical ES in pediatric and adult populations is enhanced by the use of standardized vocabulary to describe disorders. Standardized language ensures that identified variants are filtered correctly and in a systematic fashion based on the patient characteristics that are provided. One commonly used platform is the Human Phenotype Ontology (HPO).
Tomar and colleagues assessed the impact of HPO-based clinical information on the performance of a gene prioritization tool.3 Gene prioritization (or simulation) tools are used for interpretation of ES data to help analysts efficiently sort through the thousands of variants in an individual’s genetic sequence. The performance, or accuracy, of a prioritization tool can be assessed by looking at the location of the disease-causing gene in the suggested gene list.
Continue to: Cohort of diagnosed patients and gene prioritization...
Cohort of diagnosed patients and gene prioritization
In this experimental model, Tomar and colleagues included 50 cases with neuromuscular disorders; all had available sequencing data, fully described phenotypes, and known causal genes. The authors varied the level of available clinical information in the HPO terms used for simulated variant analysis. Using 3 web-based gene prioritization tools on the 50 cases, they varied the HPO input to include a random selection of 10%, 30%, and 50% of HPO terms derived from deep phenotyping.
The 3 prioritization tools ranked input genes based on gene-phenotype associations that were derived from gene-phenotype databases. The authors then assessed the quality of the candidate gene lists by the location of the known causative gene on the generated rank lists. They repeated this analysis 4 times with different randomly selected HPO terms.
Inclusion of more HPO terms allowed for more accurate diagnoses in rare disorders
The authors found that the phenotype input for ES matters. When only 10% and 30% of the HPO terms were used to create a candidate gene list, the causative gene was less likely to be in the top portions of gene lists than when 50% or 100% of the available HPO terms were used.
For well-characterized disorders, use of the top 10% HPO terms performed as well as when all available HPO terms were used. For previously undescribed disease-gene associations, identification of the disease gene suffered with more limited HPO term availability.
What this study contributes
This study was a simulation of previously sequenced patients with neuromuscular disorders. It examined a small sample size for a narrow spectrum of disease. However, it clearly illustrated the principle that completeness of phenotypic information for ES pipelines is relevant for interpretation.
The quantity and quality of phenotype input into ES matters for assessing genetic variants. HPO terms have been developed to represent prenatal sonographic findings, and these have been extended to include gestational age of onset in some cases. Providing as much data as possible about the prenatal phenotype through accepted uniform vocabulary (such as HPO) will increase the likelihood that a prenatal diagnosis can be made.
Detailed description of prenatal findings is essential to diagnosis
Aarabi M, Sniezek O, Jiang H, et al. Importance of complete phenotyping in prenatal whole exome sequencing. Hum Genet. 2018;137:175-181.
In a retrospective cohort study, Aarabi and colleagues evaluated the diagnostic utility and limitations of ES in prenatal cases with structural birth defects.4
A case series study
The investigators included 20 pregnancies with structural birth defects that were referred to their center for prenatal diagnosis between 12 and 20 weeks’ gestation. All pregnancies had normal karyotype and microarray analyses prior to enrollment.
ES was performed on trio samples, which included fetal and parental DNA samples (extracted from peripheral blood). Reports provided by the commercial laboratories were normal for all cases and included no pathogenic or likely pathogenic variants. The laboratory provided the investigators with the FASTQ (genetic sequence) files for reanalysis, which was performed using both prenatal and postnatal detailed phenotypic information.
Continue to: Use of postnatal information facilitated diagnoses...
Use of postnatal information facilitated diagnoses
Reanalysis of ES data using detailed postnatal findings revealed a possible diagnosis in 20% of cases. Each case in which a diagnosis was made, detailed below, highlights an important limitation in our current ability to make prenatal diagnoses.
Case 1. A fetus was diagnosed prenatally with arthrogryposis, plagiocephaly, and club feet. After birth, the infant also was found to have generalized muscle weakness, elevated creatine phosphokinase, and congenital hip dislocation.
Reanalysis of the ES data revealed compound heterozygous missense variants in the nebulin gene (NEB). Although classified as variants of uncertain significance (VUS), these are consistent with the phenotype, the authors argued, and with the diagnosis of autosomal recessive nemaline myopathy 2.
Case 2. Prenatal diagnosis was made of a right limb anomaly, tetralogy of Fallot, intrauterine growth restriction, ambiguous genitalia, and dextrocardia. Postnatal evaluation revealed absent pulmonary valve syndrome, right arm dysplasia, pectus carinatum deformity, and failure to thrive.
In this case, ES with the postnatal information revealed a VUS in the NOTCH1 gene, which has been associated with Adams-Oliver syndrome. Although by strict criteria this variant is also of uncertain significance, Adams-Oliver syndrome is characterized, in part, by transverse limb defects and congenital heart disease, as was found in the proband.
Case 3. Prenatal ultrasonography revealed microcephaly and absence of the septum pellucidum. Postnatal magnetic resonance imaging revealed semi-lobar holoprosencephaly. A holoprosencephaly-specific gene panel revealed a deletion in the ZIC2 gene, which is known to cause holoprosencephaly.
Careful re-examination of the ES data revealed some abnormality in the ZIC2 signal, which might have been studied in greater detail and thereby detected if the prenatal diagnosis of holoprosencephaly had been made.
Case 4. An ultrasound evaluation at 12 weeks’ gestation revealed a cystic hygroma, short long bones, and possible absent hand and fibula. A postnatal fetal autopsy at 14 weeks showed split-hand and split-foot malformations, which were not appreciated on ultrasonography.
In filtering the ES data with this information, a pathogenic variant in the PRCN gene was identified as causal, and the diagnosis of Goltz syndrome was made.
Challenges facing prenatal diagnosis
A case series is inherently limited by its small sample size. Nevertheless, the authors suggest 2 major challenges in our ability to make the above diagnoses in the prenatal setting:
1) the prenatal assessment being limited to major structural abnormalities, and 2) commercial laboratories not having enough experience or volume to interpret the limited information provided by prenatal imaging.
Prenatal genetic diagnosis often is limited by incomplete information about the features seen on ultrasonography. Although not all features are visible prenatally, more diagnoses can be made if laboratories are provided with detailed information about the structural abnormalities that are seen. Furthermore, if ES does not provide a prenatal diagnosis, the data should be reviewed postnatally if more detailed phenotypic information becomes available.
Can AI technology be incorporated to make a genetic diagnosis?
Hsieh TC, Mensah MA, Pantel JT, et al. PEDIA: prioritization of exome data by image analysis. Genet Med. 2019;21:2807-2814.
Increasingly, ES is used in all types of undiagnosed, rare genetic diseases. Although there is a high diagnostic yield in many populations, ES’s clinical utility is limited by the labor-intensive process of interpreting each variant in the context of detailed phenotypic information. The widespread use of HPO would be one step toward standardizing the information that is entered into the analysis of ES data, but even HPO cannot capture certain visual clues.
Hsieh and colleagues attempted to use artificial intelligence (AI) for “next-generation phenotyping” to assess facial dysmorphology and integrate the information into variant classification.5 The authors described their approach of incorporating AI as “prioritization of exome data by image analysis” (PEDIA).
Continue to: Designing dysmorphology machine learning...
Designing dysmorphology machine learning
The cohort included 679 individuals with 105 different genetic disorders. All individuals had a previously confirmed molecular diagnosis that would be detected by ES. Each individual had a frontal facial photograph analyzed and detailed clinical features documented in HPO terms extracted by 2 clinicians.
A facial analysis software called DeepGestalt, trained on 17,000 patient images, was used to create a Gestalt score. Each individual had 4 different predicted gene scoring approaches:
- a molecular deleteriousness score
- facial analysis with the Gestalt score
- a combination of molecular deleteriousness score and HPO-based gene-prioritization tool (termed semantic similarity score)
- the PEDIA score, which included all 3 prior approaches.
A type of machine learning algorithm (support vector machine, or SVM) was applied, validated, and used to prioritize genes based on the combined scores.
AI seemed to improve diagnostic accuracy
Utilizing the combination of machine learning, HPO terms, and facial analysis software greatly improved the accuracy of variant classification predictions over any approach alone.
Using only the sequence variant and molecular deleteriousness score, the causative variant was ranked in the top 10 of all identified variants in less than 45% of cases. Adding the HPO-based gene prioritization tools increased the accuracy to 63% to 94%. Use of the PEDIA score, which incorporated all 3, increased the accuracy to 99% for the top 10 ranking.
Even more impressive improvements were made in the top 1 ranking accuracy rate, which went from 36% to 74% without facial image information to 86% to 89% with inclusion of DeepGestalt scores.
Study strengths and limitations
This study’s innovative application of facial analysis and machine learning, combined with HPO-driven variant classification, showed added benefit. To achieve this with available patient photographs and thorough phenotyping, previously diagnosed patients were used. Because complete ES information was not available for those patients, their known pathogenic variant was inserted into randomly selected exomes from the 1000 Genomes Project (healthy individuals). The authors additionally noted that the PEDIA score performance was diminished for rare disorders in which limited data were available.
The accuracy of gene prediction in pediatric and adult populations is enhanced by the use of computer-assisted image analysis and machine-learning algorithms. These computational methods may be employed to automate variant classification, making it more accurate, efficient, and less laborious. Detailed descriptions or characteristic images of prenatal findings also may allow this technology to be introduced in the prenatal setting.
- Lord J, McMullan DJ, Eberhardt RY, et al; for the Prenatal Assessment of Genomes and Exomes Consortium. Prenatal exome sequencing analysis in fetal structural anomalies detected by ultrasonography (PAGE): a cohort study. Lancet. 2019;393:747-757.
- Petrovski S, Aggarwal V, Giordano JL, et al. Whole-exome sequencing in the evaluation of fetal structural anomalies: a prospective cohort study. Lancet. 2019;393:758-767.
- Tomar S, Sethi R, Lai PS. Specific phenotype semantics facilitate gene prioritization in clinical exome sequencing. Eur J Hum Genet. 2019;27:1389-1397.
- Aarabi M, Sniezek O, Jiang H, et al. Importance of complete phenotyping in prenatal whole exome sequencing. Hum Genet. 2018;137:175-181.
- Hsieh TC, Mensah MA, Pantel JT, et al. PEDIA: prioritization of exome data by image analysis. Genet Med. 2019;21:2807-2814.
- Lord J, McMullan DJ, Eberhardt RY, et al; for the Prenatal Assessment of Genomes and Exomes Consortium. Prenatal exome sequencing analysis in fetal structural anomalies detected by ultrasonography (PAGE): a cohort study. Lancet. 2019;393:747-757.
- Petrovski S, Aggarwal V, Giordano JL, et al. Whole-exome sequencing in the evaluation of fetal structural anomalies: a prospective cohort study. Lancet. 2019;393:758-767.
- Tomar S, Sethi R, Lai PS. Specific phenotype semantics facilitate gene prioritization in clinical exome sequencing. Eur J Hum Genet. 2019;27:1389-1397.
- Aarabi M, Sniezek O, Jiang H, et al. Importance of complete phenotyping in prenatal whole exome sequencing. Hum Genet. 2018;137:175-181.
- Hsieh TC, Mensah MA, Pantel JT, et al. PEDIA: prioritization of exome data by image analysis. Genet Med. 2019;21:2807-2814.
Progesterone for preterm delivery prevention
Researchers have been studying the use of exogenous progestins for prevention of preterm delivery (PTD) for almost 60 years, but conflicting results contribute to an ongoing debate. Interpretation of the available data is particularly difficult because different forms and doses of progestins have been used in disparate study populations.
Based on available data, progesterone supplementation is not effective as a primary prevention strategy for PTD in the general low-risk obstetric population. PTD is a complex problem with varied and incompletely elucidated pathogenic pathways, making it unlikely that one interventional approach would be effective for all pregnant women. As a result, emerging indications for the use of progesterone are based on risk factors for PTD (ie, prior PTD and/or short cervix). However, this secondary prevention approach is a limiting factor in itself because 50% of women destined to have a PTD have no identifiable risk factors.1 In addition, researchers have found that progestins are ineffective at delaying delivery for women with multiple gestation, suggesting that a distinct underlying mechanism of early parturition is present in these women, and that this mechanism is unresponsive to progestins.2
The formulations used in the study of progestin supplementation for PTD prevention have been almost exclusively either the synthetic 17 alpha-hydroxyprogesterone caproate (17-OHPC) or natural progesterone administered orally or vaginally. In 2003, the American College of Obstetricians and Gynecologists (ACOG) supported the use of progesterone to reduce the rate of PTD,3 and in 2011, the US Food and Drug Administration (FDA) approved 17-OHPC for use as prophylaxis against recurrent PTD. As a result, in recent years, the perceived standard of care for a majority of practitioners in the United States had been that all women with a previous preterm birth should be offered 17-OHPC. It may be interesting to note that in other parts of the world, the same enthusiastic adoption did not occur. For example, in Australia and New Zealand in 2007, only 5% of practitioners were using progesterone for this indication.4 Further, 17-OHPC is not recommended by professional guidelines in the United Kingdom and has remained unavailable in Germany.
The publication in 2019 of the PROLONG trial called into question the use of 17-OHPC for the prevention of PTD.5 In the December 2019 issue of OBG Management (“Managing preterm birth in those at risk: Expert strategies”), I expressed the opinion that with only rare exceptions, 17-OHPC is no longer a viable option for recurrent PTD prevention.6 In light of these developments, what scientific evidence is relevant and applicable to the care of women at risk for PTD?
Continue to: Case 1 Previous spontaneous PTD at 31 weeks...
Case 1 Previous spontaneous PTD at 31 weeks
MC is an asymptomatic 32-year-old woman with a singleton pregnancy at 13 weeks’ gestation. You see her for a maternal-fetal medicine consultation because 2 years ago she had a spontaneous PTD at 31 weeks’ gestation. What management recommendations can you make to decrease her risk of recurrent PTD?
Cervical length measurement narrows in on risk
The indication “previous preterm birth” is largely meaningless because of the heterogeneity in preterm birth pathways (preterm birth as a syndrome7) and inadequate risk characterization. Among women who experience a spontaneous PTD, 70% to 80% do not deliver prematurely in subsequent pregnancies.8 To better characterize the risk of PTD recurrence, ultrasound assessment of cervical length should be used. Research has shown that among women with a prior spontaneous PTD who maintain a normal cervical length until 24 weeks’ gestation, more than 90% will deliver at 35 weeks or after without intervention.9 Such an approach not only identifies the subgroup of women at significantly increased risk of recurrence but also eliminates unnecessary interventions.
Cervical ultrasound surveillance should be initiated at 16 weeks’ gestation. A short cervix before 16 weeks is not associated with a statistically significant increase in risk for PTD.10 Shortening of the cervix begins approximately 10 weeks before delivery in any gestational age group.11 Therefore, ultrasound assessment of the cervix at 28 weeks and after is irrelevant. In addition, after 28 weeks, cervical length varies greatly leading to loss in the predictive power of the cervical measurement.12 Based on these considerations, cervical surveillance may be extended up to 26 weeks. Although cervical cerclage is not an option in the United States in cases in which a short cervix is detected between 24 and 26 weeks, vaginal progesterone supplementation may still be considered.
Case 1 Continued
MC was started on ultrasound cervical surveillance at 16 weeks’ gestation. Her cervical length was initially normal (> 2.5 cm), but at 18 weeks the measurement was 2.2 cm. What is your recommendation?
The value of vaginal progesterone
There appears to be increasing consensus on the value of vaginal progesterone for women with a midtrimester short cervix on sonography, with or without a history of PTD. An individual patient data meta-analysis demonstrated the benefits of vaginal progesterone.13 Although there was no evidence of an effect on PTD at less than 37 weeks, the rates of PTD at less than 36 weeks and spontaneous PTD at less than 34 weeks were significantly reduced (by 20% and 28%, respectively). Also, there was a significant reduction in the risk of respiratory distress syndrome (53%) and composite neonatal morbidity and mortality (41%), with no significant impact on infant development up to the second year of life.13
The lack of generalizable evidence of benefit on childhood outcomes, combined with considerable uncertainty about the exact role and mechanism of action of exogenous progestins, contribute to the ongoing debate. Vaginal progesterone dosage regimens have been based on extrapolations from experience with progesterone in nonpregnant women, and recent pharmacokinetic studies have revealed how precarious such extrapolations may be. As an example, in nonpregnant women, the bioavailability of oral and vaginal progesterone is similar.14 In pregnancy, however, while daily oral progesterone doubles a pregnant woman’s serum progesterone level,15 daily vaginal administration of progesterone results in only a modest rise in serum progesterone, with a coefficient of variation among individuals that is double that outside of pregnancy.16 It is, therefore, considered that vaginal progesterone in pregnancy may have a local action secondary to the uterine first-pass effect. The uterine first-pass effect for vaginal progesterone was described in nonpregnant women and is only assumed to occur in pregnancy as well. 17
After evaluating the data from the largest available study of vaginal progesterone,18 the FDA concluded in 2012 that the study did not meet the statistical significance generally expected to support the approval of a new product. However, according to a more comprehensive evidence review developed in 2019 by the National Guideline Alliance in the United Kingdom, women with a history of PTD and women with a short cervix derive an important benefit from the use of vaginal progesterone; thus, this intervention should be offered to them.19 At this time, a short cervix and PTD prevention are not considered FDA-approved indications for progesterone supplementation in pregnancy. However, vaginal progesterone is FDA approved for use in pregnant women with a history of infertility.
Continue to: Case 1 Continued...
Case 1 Continued
MC initiated treatment with daily vaginal progesterone at 18 weeks’ gestation and returned for ultrasound cervical length examination weekly instead of every other week. At 20 weeks’ gestation, cervical length was 2.0 cm; the following week it was 1.4 cm. What would you recommend at this point?
When to consider cerclage
If cervical shortening progresses to about 1.5 cm while a woman is being treated with vaginal progesterone, cerclage may be considered. The benefit of cerclage in patients with prior PTD and a short cervix was highlighted in a 2018 Cochrane Review.20 In this stepwise management approach to a short cervix, waiting for a cervix to be less than 1.5 cm may be unadvisable. Under conditions of a very short cervix that is frequently dilated with exposure of fetal membranes, ascending subclinical intra-amniotic infection may already be present, reducing the efficacy of any preventive measures. Preferential consideration for cerclage from the start over initial vaginal progesterone also may be appropriate when there is a history of 2 spontaneous PTDs or mid-trimester losses, a history of a successful cerclage, or with a very short cervix (< 1.0 cm) at the initial evaluation. As for the latter, a 2018 individual patient data meta-analysis of vaginal progesterone found no benefit when the cervix was less than 1.0 cm.13
Progesterone plus cerclage likely to add benefit
The results of an adjusted indirect comparison meta-analysis suggest that both interventions—vaginal progesterone and cerclage—are equally effective.21 Assuming that there is no clinically meaningful difference in benefit associated with these 2 treatments, the next logical question is whether combining the 2 therapies provides any added benefit; limited observational data seem to suggest that it does. In a retrospective cohort of 86 consecutive singleton pregnancies among women who underwent ultrasound-indicated cerclage, those who used vaginal progesterone after cerclage (n = 45) had a lower rate of PTD.22 Also, a small (66 cases) case-control study demonstrated the benefit of administration of vaginal progesterone as a rescue intervention in women with cerclage and progressive cervical shortening despite cerclage.23
Case 2 Woman experiences adverse effects from vaginal progesterone
MS is a 25-year-old G2P0101 who was started on vaginal progesterone as prophylaxis for recurrent PTD. She is now at 20 weeks’ gestation, with a stable remnant cervical length of 2.0 cm. She is reporting an increasing vaginal burning sensation and vaginal discharge caused by the nightly vaginal progesterone applications, to the point that she is unwilling to continue the treatment. She asks if any alternatives to vaginal progesterone are available to decrease her risk of PTD.
Continue to: Is oral progesterone an option?...
Is oral progesterone an option?
In the 1980s and 1990s, oral micronized progesterone was widely used in France at doses of 900 to 1,200 mg/d for women at risk for PTD. The practice was stopped when secondary hepatic effects, including cholestasis of pregnancy, were reported at a higher rate in treated women.24 A rise in the serum concentration of progesterone metabolites has been associated with impaired biliary excretion and subsequent accumulation of bile acids.25 In other reports, elevated serum transaminase activity was found in pregnant women treated with oral micronized progesterone, and withdrawal of treatment frequently has led to improvement in transaminase levels.26 The synthesis of endogenous progesterone during normal pregnancy is between 250 and 500 mg/d,26 and experts have expressed concern that exogenous progesterone supplementation may impose an additional load on the hepatic transport of sulfated metabolites. Unlike orally administered progesterone, progestins given by the vaginal route avoid the hepatic first-pass effect. For this reason, they may be associated with less hepatic dysfunction.
Although not recommended by professional guidelines, oral progesterone administration for the prevention of PTD has been used in the United States. A 2015 survey of Wisconsin prenatal care providers found that of those who prescribed any progesterone for PTD prevention, oral progesterone was prescribed by 13.1% of obstetricians, 24.4% of midwives, and 40.7% of family medicine practitioners.27
Some limited recent evidence from a meta-analysis of 3 trials investigating oral progesterone versus placebo suggests effectiveness in the prevention of recurrent PTD and reduction in perinatal morbidity and mortality.15 However, the number of cases included in the meta-analysis (386) was too small to support definitive clinical recommendations. Furthermore, questions have been raised in the literature about the reliability of the largest trial included in that meta-analysis.28
Case 3 Two previous spontaneous PTDs
A 29-year-old G3P0201 presents for her first prenatal appointment at 10 weeks’ gestation. With her first pregnancy she had a spontaneous PTD at 23 weeks, and the neonate did not survive. In her second pregnancy, she was treated with 17-OHPC from 16 weeks’ gestation. She had a spontaneous PTD at 29 weeks, and that child is developing normally by her report. She believes that 17-OHPC helped her in her last pregnancy and is anxious about the risk for still another PTD. Consistent with the concept of shared decision-making, you inform her of the results of the recent PROLONG trial and statements on the subject released by professional organizations such as ACOG and the Society for Maternal-Fetal Medicine (SMFM). What options does she have?
17-OHPC may be a possibility in very high-risk women
According to a SMFM statement released in the wake of the PROLONG trial publication, “. . . SMFM believes that it is reasonable for providers to use 17-OHPC in women with a profile more representative of the very high-risk population reported in the Meis trial”.29 Only a few women will have a recurrence risk of PTD over 50%, as was the background event rate in the Meis trial.30 Such a risk level may be suspected, as an example, in women with 2 or more prior early (before 34 weeks) PTDs without intervening term deliveries. Even in those cases, if treatment with 17-OHPC is decided upon, ultrasound cervical surveillance should be added as an additional safety measure. ●
- Iams JD, Goldenberg RL, Mercer BM, et al. The preterm prediction study: can low-risk women destined for spontaneous preterm birth be identified? Am J Obstet Gynecol. 2001;184:652-655.
- Murray SR, Stock SJ, Cowan S, et al. Spontaneous preterm birth prevention in multiple pregnancy. Obstet Gynecol. 2018;20:57-63.
- American College of Obstetricians and Gynecologists. ACOG committee opinion. Use of progesterone to reduce preterm birth. Obstet Gynecol. 2003;102:1115-1116.
- Dodd JM, Ashwood P, Flenady V, et al. A survey of clinician and patient attitudes towards the use of progesterone for women at risk of preterm birth. Aust N Z J Obstet Gynaecol. 2007;47:106-109.
- Blackwell SC, Gyamfi -Bannerman C, Biggio JR, et al. 17-OHPC to prevent recurrent preterm birth in singleton gestations (PROLONG study): a multicenter, international, randomized double-blind trial. Am J Perinatol. 2020;37:127-136.
- Duff P, Vidaeff AC, Ross MG, Norwitz ER. Managing preterm birth in those at risk: expert strategies. OBG Manag. 2019;31:39-42.
- Romero R, Mazor M, Munoz H, et al. The preterm labor syndrome. Ann N Y Acad Sci. 1994;734:414-429.
- Phillips C, Velji Z, Hanly C, et al. Risk of recurrent spontaneous preterm birth: a systematic review and meta-analysis. BMJ Open. 2017;7:e015402.
- Berghella V, Seibel-Seamon J. Contemporary use of cervical cerclage. Clin Obstet Gynecol. 2007;50:468-477.
- Naim A, Haberman S, Burgess T, et al. Changes in cervical length and the risk of preterm labor. Am J Obstet Gynecol. 2002;186:887-889.
- Zilianti M, Azuaga A, Calderon F, et al. Monitoring the effacement of the uterine cervix by transperineal sonography: a new perspective. J Ultrasound Med. 1995;14:719-724.
- Goldenberg RL, Iams JD, Miodovnik M, et al. The preterm prediction study: risk factors in twin gestation. Am J Obstet Gynecol. 1996;175:1047-1053.
- Romero R, Conde-Agudelo A, Da Fonseca E, et al. Vaginal progesterone for preventing preterm birth and adverse perinatal outcomes in singleton gestations with a short cervix: a meta-analysis of individual patient data. Am J Obstet Gynecol. 2018;218:161-180.
- Norman T, Morse C, Dennerstein L. Comparative bioavailability of orally and vaginally administered progesterone. Fertil Steril. 1991;56:1034-1039.
- Boelig RC, Della Corte L, Ashoush S, et al. Oral progesterone for the prevention of recurrent preterm birth: systematic review and metaanalysis. Am J Obstet Gynecol MFM. 2019;1:50-62.
- Boelig RC, Zuppa AF, Kraft WK, et al. Pharmacokinetics of vaginal progesterone in pregnancy. Am J Obstet Gynecol. 2019;221:263.e1-7.
- Bulletti C, de Ziegler D, Flamigni C, et al. Targeted drug delivery in gynaecology: the first uterine pass effect. Hum Reprod. 1997;12:1073-1079.
- Hassan SS, Romero R, Vidyadhari D, et al. Vaginal progesterone reduces the rate of preterm birth in women with a sonographic short cervix: a multicenter, randomized, double-blind, placebocontrolled trial. Ultrasound Obstet Gynecol. 2011;38:18-31.
- Preterm labour and birth. Evidence review for clinical effectiveness of prophylactic progesterone in preventing preterm labour. London: National Institute for Health and Care Excellence (UK); August 2019.
- Alfirevic Z, Stampalija T, Medley N. Cervical stitch (cerclage) for preventing preterm birth in singleton pregnancy. Cochrane Database Syst Rev. 2017;6:CD008991.
- Conde-Agudelo A, Romero R, Da Fonseca E, et al. Vaginal progesterone is as effective as cervical cerclage to prevent preterm birth in women with a singleton gestation, previous spontaneous preterm birth, and a short cervix: updated indirect comparison meta-analysis. Am J Obstet Gynecol. 2018;219:10-25.
- Park JY, Jung YM, Kook S-Y, et al. The effect of postoperative vaginal progesterone in ultrasound-indicated cerclage to prevent preterm birth. J Matern Fetal Neonatal Med. 2019:1-8.
- Roman AR, Da Silva Costa F, et al. Rescue adjuvant vaginal progesterone may improve outcomes in cervical cerclage failure. Geburt Frauen. 2018;78:785-790.
- Benifle JL, Dumont M, Levardon M, et al. Effects of natural micronized progesterone on the liver in the third trimester of pregnancy. Contracept Fertil Sex. 1997;25:165-169.
- Vallejo M, Briz O, Serrano MA, et al. Potential role of transinhibition of the bile salt export pump by progesterone metabolites in the etiopathogenesis of intrahepatic cholestasis of pregnancy. J Hepatol. 2006;44:1150-1157.
- Bacq Y, Sapey T, Bréchot MC, et al. Intrahepatic cholestasis of pregnancy: a French prospective study. Hepatology. 1997;26:358-364.
- Hoppe K, Kramer RD, Ha B, et al. Progesterone supplementation for the prevention of preterm birth: provider practice in Wisconsin. WMJ. 2019;118:126-131.
- Katsanevakis E, Mol BW, Thornton J. A question about the reliability of a recent trial of progesterone for preterm birth prevention, published in Acta. Acta Obstet Gynecol Scand. 2020;99:426.
- Society for Maternal-Fetal Medicine (SMFM) Publications Committee. SMFM Statement: use of 17-alpha hydroxyprogesterone caproate for prevention of recurrent preterm birth. https://www.smfm.org/publications/280smfm-statement-use-of-17-alpha-hydroxyprogesteronecaproate-for-prevention-of-recurrent-preterm-birth. Accessed March 23, 2020.
- Meis PJ, Klebanoff M, Thom E, et al. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;384:2379-2385.
Researchers have been studying the use of exogenous progestins for prevention of preterm delivery (PTD) for almost 60 years, but conflicting results contribute to an ongoing debate. Interpretation of the available data is particularly difficult because different forms and doses of progestins have been used in disparate study populations.
Based on available data, progesterone supplementation is not effective as a primary prevention strategy for PTD in the general low-risk obstetric population. PTD is a complex problem with varied and incompletely elucidated pathogenic pathways, making it unlikely that one interventional approach would be effective for all pregnant women. As a result, emerging indications for the use of progesterone are based on risk factors for PTD (ie, prior PTD and/or short cervix). However, this secondary prevention approach is a limiting factor in itself because 50% of women destined to have a PTD have no identifiable risk factors.1 In addition, researchers have found that progestins are ineffective at delaying delivery for women with multiple gestation, suggesting that a distinct underlying mechanism of early parturition is present in these women, and that this mechanism is unresponsive to progestins.2
The formulations used in the study of progestin supplementation for PTD prevention have been almost exclusively either the synthetic 17 alpha-hydroxyprogesterone caproate (17-OHPC) or natural progesterone administered orally or vaginally. In 2003, the American College of Obstetricians and Gynecologists (ACOG) supported the use of progesterone to reduce the rate of PTD,3 and in 2011, the US Food and Drug Administration (FDA) approved 17-OHPC for use as prophylaxis against recurrent PTD. As a result, in recent years, the perceived standard of care for a majority of practitioners in the United States had been that all women with a previous preterm birth should be offered 17-OHPC. It may be interesting to note that in other parts of the world, the same enthusiastic adoption did not occur. For example, in Australia and New Zealand in 2007, only 5% of practitioners were using progesterone for this indication.4 Further, 17-OHPC is not recommended by professional guidelines in the United Kingdom and has remained unavailable in Germany.
The publication in 2019 of the PROLONG trial called into question the use of 17-OHPC for the prevention of PTD.5 In the December 2019 issue of OBG Management (“Managing preterm birth in those at risk: Expert strategies”), I expressed the opinion that with only rare exceptions, 17-OHPC is no longer a viable option for recurrent PTD prevention.6 In light of these developments, what scientific evidence is relevant and applicable to the care of women at risk for PTD?
Continue to: Case 1 Previous spontaneous PTD at 31 weeks...
Case 1 Previous spontaneous PTD at 31 weeks
MC is an asymptomatic 32-year-old woman with a singleton pregnancy at 13 weeks’ gestation. You see her for a maternal-fetal medicine consultation because 2 years ago she had a spontaneous PTD at 31 weeks’ gestation. What management recommendations can you make to decrease her risk of recurrent PTD?
Cervical length measurement narrows in on risk
The indication “previous preterm birth” is largely meaningless because of the heterogeneity in preterm birth pathways (preterm birth as a syndrome7) and inadequate risk characterization. Among women who experience a spontaneous PTD, 70% to 80% do not deliver prematurely in subsequent pregnancies.8 To better characterize the risk of PTD recurrence, ultrasound assessment of cervical length should be used. Research has shown that among women with a prior spontaneous PTD who maintain a normal cervical length until 24 weeks’ gestation, more than 90% will deliver at 35 weeks or after without intervention.9 Such an approach not only identifies the subgroup of women at significantly increased risk of recurrence but also eliminates unnecessary interventions.
Cervical ultrasound surveillance should be initiated at 16 weeks’ gestation. A short cervix before 16 weeks is not associated with a statistically significant increase in risk for PTD.10 Shortening of the cervix begins approximately 10 weeks before delivery in any gestational age group.11 Therefore, ultrasound assessment of the cervix at 28 weeks and after is irrelevant. In addition, after 28 weeks, cervical length varies greatly leading to loss in the predictive power of the cervical measurement.12 Based on these considerations, cervical surveillance may be extended up to 26 weeks. Although cervical cerclage is not an option in the United States in cases in which a short cervix is detected between 24 and 26 weeks, vaginal progesterone supplementation may still be considered.
Case 1 Continued
MC was started on ultrasound cervical surveillance at 16 weeks’ gestation. Her cervical length was initially normal (> 2.5 cm), but at 18 weeks the measurement was 2.2 cm. What is your recommendation?
The value of vaginal progesterone
There appears to be increasing consensus on the value of vaginal progesterone for women with a midtrimester short cervix on sonography, with or without a history of PTD. An individual patient data meta-analysis demonstrated the benefits of vaginal progesterone.13 Although there was no evidence of an effect on PTD at less than 37 weeks, the rates of PTD at less than 36 weeks and spontaneous PTD at less than 34 weeks were significantly reduced (by 20% and 28%, respectively). Also, there was a significant reduction in the risk of respiratory distress syndrome (53%) and composite neonatal morbidity and mortality (41%), with no significant impact on infant development up to the second year of life.13
The lack of generalizable evidence of benefit on childhood outcomes, combined with considerable uncertainty about the exact role and mechanism of action of exogenous progestins, contribute to the ongoing debate. Vaginal progesterone dosage regimens have been based on extrapolations from experience with progesterone in nonpregnant women, and recent pharmacokinetic studies have revealed how precarious such extrapolations may be. As an example, in nonpregnant women, the bioavailability of oral and vaginal progesterone is similar.14 In pregnancy, however, while daily oral progesterone doubles a pregnant woman’s serum progesterone level,15 daily vaginal administration of progesterone results in only a modest rise in serum progesterone, with a coefficient of variation among individuals that is double that outside of pregnancy.16 It is, therefore, considered that vaginal progesterone in pregnancy may have a local action secondary to the uterine first-pass effect. The uterine first-pass effect for vaginal progesterone was described in nonpregnant women and is only assumed to occur in pregnancy as well. 17
After evaluating the data from the largest available study of vaginal progesterone,18 the FDA concluded in 2012 that the study did not meet the statistical significance generally expected to support the approval of a new product. However, according to a more comprehensive evidence review developed in 2019 by the National Guideline Alliance in the United Kingdom, women with a history of PTD and women with a short cervix derive an important benefit from the use of vaginal progesterone; thus, this intervention should be offered to them.19 At this time, a short cervix and PTD prevention are not considered FDA-approved indications for progesterone supplementation in pregnancy. However, vaginal progesterone is FDA approved for use in pregnant women with a history of infertility.
Continue to: Case 1 Continued...
Case 1 Continued
MC initiated treatment with daily vaginal progesterone at 18 weeks’ gestation and returned for ultrasound cervical length examination weekly instead of every other week. At 20 weeks’ gestation, cervical length was 2.0 cm; the following week it was 1.4 cm. What would you recommend at this point?
When to consider cerclage
If cervical shortening progresses to about 1.5 cm while a woman is being treated with vaginal progesterone, cerclage may be considered. The benefit of cerclage in patients with prior PTD and a short cervix was highlighted in a 2018 Cochrane Review.20 In this stepwise management approach to a short cervix, waiting for a cervix to be less than 1.5 cm may be unadvisable. Under conditions of a very short cervix that is frequently dilated with exposure of fetal membranes, ascending subclinical intra-amniotic infection may already be present, reducing the efficacy of any preventive measures. Preferential consideration for cerclage from the start over initial vaginal progesterone also may be appropriate when there is a history of 2 spontaneous PTDs or mid-trimester losses, a history of a successful cerclage, or with a very short cervix (< 1.0 cm) at the initial evaluation. As for the latter, a 2018 individual patient data meta-analysis of vaginal progesterone found no benefit when the cervix was less than 1.0 cm.13
Progesterone plus cerclage likely to add benefit
The results of an adjusted indirect comparison meta-analysis suggest that both interventions—vaginal progesterone and cerclage—are equally effective.21 Assuming that there is no clinically meaningful difference in benefit associated with these 2 treatments, the next logical question is whether combining the 2 therapies provides any added benefit; limited observational data seem to suggest that it does. In a retrospective cohort of 86 consecutive singleton pregnancies among women who underwent ultrasound-indicated cerclage, those who used vaginal progesterone after cerclage (n = 45) had a lower rate of PTD.22 Also, a small (66 cases) case-control study demonstrated the benefit of administration of vaginal progesterone as a rescue intervention in women with cerclage and progressive cervical shortening despite cerclage.23
Case 2 Woman experiences adverse effects from vaginal progesterone
MS is a 25-year-old G2P0101 who was started on vaginal progesterone as prophylaxis for recurrent PTD. She is now at 20 weeks’ gestation, with a stable remnant cervical length of 2.0 cm. She is reporting an increasing vaginal burning sensation and vaginal discharge caused by the nightly vaginal progesterone applications, to the point that she is unwilling to continue the treatment. She asks if any alternatives to vaginal progesterone are available to decrease her risk of PTD.
Continue to: Is oral progesterone an option?...
Is oral progesterone an option?
In the 1980s and 1990s, oral micronized progesterone was widely used in France at doses of 900 to 1,200 mg/d for women at risk for PTD. The practice was stopped when secondary hepatic effects, including cholestasis of pregnancy, were reported at a higher rate in treated women.24 A rise in the serum concentration of progesterone metabolites has been associated with impaired biliary excretion and subsequent accumulation of bile acids.25 In other reports, elevated serum transaminase activity was found in pregnant women treated with oral micronized progesterone, and withdrawal of treatment frequently has led to improvement in transaminase levels.26 The synthesis of endogenous progesterone during normal pregnancy is between 250 and 500 mg/d,26 and experts have expressed concern that exogenous progesterone supplementation may impose an additional load on the hepatic transport of sulfated metabolites. Unlike orally administered progesterone, progestins given by the vaginal route avoid the hepatic first-pass effect. For this reason, they may be associated with less hepatic dysfunction.
Although not recommended by professional guidelines, oral progesterone administration for the prevention of PTD has been used in the United States. A 2015 survey of Wisconsin prenatal care providers found that of those who prescribed any progesterone for PTD prevention, oral progesterone was prescribed by 13.1% of obstetricians, 24.4% of midwives, and 40.7% of family medicine practitioners.27
Some limited recent evidence from a meta-analysis of 3 trials investigating oral progesterone versus placebo suggests effectiveness in the prevention of recurrent PTD and reduction in perinatal morbidity and mortality.15 However, the number of cases included in the meta-analysis (386) was too small to support definitive clinical recommendations. Furthermore, questions have been raised in the literature about the reliability of the largest trial included in that meta-analysis.28
Case 3 Two previous spontaneous PTDs
A 29-year-old G3P0201 presents for her first prenatal appointment at 10 weeks’ gestation. With her first pregnancy she had a spontaneous PTD at 23 weeks, and the neonate did not survive. In her second pregnancy, she was treated with 17-OHPC from 16 weeks’ gestation. She had a spontaneous PTD at 29 weeks, and that child is developing normally by her report. She believes that 17-OHPC helped her in her last pregnancy and is anxious about the risk for still another PTD. Consistent with the concept of shared decision-making, you inform her of the results of the recent PROLONG trial and statements on the subject released by professional organizations such as ACOG and the Society for Maternal-Fetal Medicine (SMFM). What options does she have?
17-OHPC may be a possibility in very high-risk women
According to a SMFM statement released in the wake of the PROLONG trial publication, “. . . SMFM believes that it is reasonable for providers to use 17-OHPC in women with a profile more representative of the very high-risk population reported in the Meis trial”.29 Only a few women will have a recurrence risk of PTD over 50%, as was the background event rate in the Meis trial.30 Such a risk level may be suspected, as an example, in women with 2 or more prior early (before 34 weeks) PTDs without intervening term deliveries. Even in those cases, if treatment with 17-OHPC is decided upon, ultrasound cervical surveillance should be added as an additional safety measure. ●
Researchers have been studying the use of exogenous progestins for prevention of preterm delivery (PTD) for almost 60 years, but conflicting results contribute to an ongoing debate. Interpretation of the available data is particularly difficult because different forms and doses of progestins have been used in disparate study populations.
Based on available data, progesterone supplementation is not effective as a primary prevention strategy for PTD in the general low-risk obstetric population. PTD is a complex problem with varied and incompletely elucidated pathogenic pathways, making it unlikely that one interventional approach would be effective for all pregnant women. As a result, emerging indications for the use of progesterone are based on risk factors for PTD (ie, prior PTD and/or short cervix). However, this secondary prevention approach is a limiting factor in itself because 50% of women destined to have a PTD have no identifiable risk factors.1 In addition, researchers have found that progestins are ineffective at delaying delivery for women with multiple gestation, suggesting that a distinct underlying mechanism of early parturition is present in these women, and that this mechanism is unresponsive to progestins.2
The formulations used in the study of progestin supplementation for PTD prevention have been almost exclusively either the synthetic 17 alpha-hydroxyprogesterone caproate (17-OHPC) or natural progesterone administered orally or vaginally. In 2003, the American College of Obstetricians and Gynecologists (ACOG) supported the use of progesterone to reduce the rate of PTD,3 and in 2011, the US Food and Drug Administration (FDA) approved 17-OHPC for use as prophylaxis against recurrent PTD. As a result, in recent years, the perceived standard of care for a majority of practitioners in the United States had been that all women with a previous preterm birth should be offered 17-OHPC. It may be interesting to note that in other parts of the world, the same enthusiastic adoption did not occur. For example, in Australia and New Zealand in 2007, only 5% of practitioners were using progesterone for this indication.4 Further, 17-OHPC is not recommended by professional guidelines in the United Kingdom and has remained unavailable in Germany.
The publication in 2019 of the PROLONG trial called into question the use of 17-OHPC for the prevention of PTD.5 In the December 2019 issue of OBG Management (“Managing preterm birth in those at risk: Expert strategies”), I expressed the opinion that with only rare exceptions, 17-OHPC is no longer a viable option for recurrent PTD prevention.6 In light of these developments, what scientific evidence is relevant and applicable to the care of women at risk for PTD?
Continue to: Case 1 Previous spontaneous PTD at 31 weeks...
Case 1 Previous spontaneous PTD at 31 weeks
MC is an asymptomatic 32-year-old woman with a singleton pregnancy at 13 weeks’ gestation. You see her for a maternal-fetal medicine consultation because 2 years ago she had a spontaneous PTD at 31 weeks’ gestation. What management recommendations can you make to decrease her risk of recurrent PTD?
Cervical length measurement narrows in on risk
The indication “previous preterm birth” is largely meaningless because of the heterogeneity in preterm birth pathways (preterm birth as a syndrome7) and inadequate risk characterization. Among women who experience a spontaneous PTD, 70% to 80% do not deliver prematurely in subsequent pregnancies.8 To better characterize the risk of PTD recurrence, ultrasound assessment of cervical length should be used. Research has shown that among women with a prior spontaneous PTD who maintain a normal cervical length until 24 weeks’ gestation, more than 90% will deliver at 35 weeks or after without intervention.9 Such an approach not only identifies the subgroup of women at significantly increased risk of recurrence but also eliminates unnecessary interventions.
Cervical ultrasound surveillance should be initiated at 16 weeks’ gestation. A short cervix before 16 weeks is not associated with a statistically significant increase in risk for PTD.10 Shortening of the cervix begins approximately 10 weeks before delivery in any gestational age group.11 Therefore, ultrasound assessment of the cervix at 28 weeks and after is irrelevant. In addition, after 28 weeks, cervical length varies greatly leading to loss in the predictive power of the cervical measurement.12 Based on these considerations, cervical surveillance may be extended up to 26 weeks. Although cervical cerclage is not an option in the United States in cases in which a short cervix is detected between 24 and 26 weeks, vaginal progesterone supplementation may still be considered.
Case 1 Continued
MC was started on ultrasound cervical surveillance at 16 weeks’ gestation. Her cervical length was initially normal (> 2.5 cm), but at 18 weeks the measurement was 2.2 cm. What is your recommendation?
The value of vaginal progesterone
There appears to be increasing consensus on the value of vaginal progesterone for women with a midtrimester short cervix on sonography, with or without a history of PTD. An individual patient data meta-analysis demonstrated the benefits of vaginal progesterone.13 Although there was no evidence of an effect on PTD at less than 37 weeks, the rates of PTD at less than 36 weeks and spontaneous PTD at less than 34 weeks were significantly reduced (by 20% and 28%, respectively). Also, there was a significant reduction in the risk of respiratory distress syndrome (53%) and composite neonatal morbidity and mortality (41%), with no significant impact on infant development up to the second year of life.13
The lack of generalizable evidence of benefit on childhood outcomes, combined with considerable uncertainty about the exact role and mechanism of action of exogenous progestins, contribute to the ongoing debate. Vaginal progesterone dosage regimens have been based on extrapolations from experience with progesterone in nonpregnant women, and recent pharmacokinetic studies have revealed how precarious such extrapolations may be. As an example, in nonpregnant women, the bioavailability of oral and vaginal progesterone is similar.14 In pregnancy, however, while daily oral progesterone doubles a pregnant woman’s serum progesterone level,15 daily vaginal administration of progesterone results in only a modest rise in serum progesterone, with a coefficient of variation among individuals that is double that outside of pregnancy.16 It is, therefore, considered that vaginal progesterone in pregnancy may have a local action secondary to the uterine first-pass effect. The uterine first-pass effect for vaginal progesterone was described in nonpregnant women and is only assumed to occur in pregnancy as well. 17
After evaluating the data from the largest available study of vaginal progesterone,18 the FDA concluded in 2012 that the study did not meet the statistical significance generally expected to support the approval of a new product. However, according to a more comprehensive evidence review developed in 2019 by the National Guideline Alliance in the United Kingdom, women with a history of PTD and women with a short cervix derive an important benefit from the use of vaginal progesterone; thus, this intervention should be offered to them.19 At this time, a short cervix and PTD prevention are not considered FDA-approved indications for progesterone supplementation in pregnancy. However, vaginal progesterone is FDA approved for use in pregnant women with a history of infertility.
Continue to: Case 1 Continued...
Case 1 Continued
MC initiated treatment with daily vaginal progesterone at 18 weeks’ gestation and returned for ultrasound cervical length examination weekly instead of every other week. At 20 weeks’ gestation, cervical length was 2.0 cm; the following week it was 1.4 cm. What would you recommend at this point?
When to consider cerclage
If cervical shortening progresses to about 1.5 cm while a woman is being treated with vaginal progesterone, cerclage may be considered. The benefit of cerclage in patients with prior PTD and a short cervix was highlighted in a 2018 Cochrane Review.20 In this stepwise management approach to a short cervix, waiting for a cervix to be less than 1.5 cm may be unadvisable. Under conditions of a very short cervix that is frequently dilated with exposure of fetal membranes, ascending subclinical intra-amniotic infection may already be present, reducing the efficacy of any preventive measures. Preferential consideration for cerclage from the start over initial vaginal progesterone also may be appropriate when there is a history of 2 spontaneous PTDs or mid-trimester losses, a history of a successful cerclage, or with a very short cervix (< 1.0 cm) at the initial evaluation. As for the latter, a 2018 individual patient data meta-analysis of vaginal progesterone found no benefit when the cervix was less than 1.0 cm.13
Progesterone plus cerclage likely to add benefit
The results of an adjusted indirect comparison meta-analysis suggest that both interventions—vaginal progesterone and cerclage—are equally effective.21 Assuming that there is no clinically meaningful difference in benefit associated with these 2 treatments, the next logical question is whether combining the 2 therapies provides any added benefit; limited observational data seem to suggest that it does. In a retrospective cohort of 86 consecutive singleton pregnancies among women who underwent ultrasound-indicated cerclage, those who used vaginal progesterone after cerclage (n = 45) had a lower rate of PTD.22 Also, a small (66 cases) case-control study demonstrated the benefit of administration of vaginal progesterone as a rescue intervention in women with cerclage and progressive cervical shortening despite cerclage.23
Case 2 Woman experiences adverse effects from vaginal progesterone
MS is a 25-year-old G2P0101 who was started on vaginal progesterone as prophylaxis for recurrent PTD. She is now at 20 weeks’ gestation, with a stable remnant cervical length of 2.0 cm. She is reporting an increasing vaginal burning sensation and vaginal discharge caused by the nightly vaginal progesterone applications, to the point that she is unwilling to continue the treatment. She asks if any alternatives to vaginal progesterone are available to decrease her risk of PTD.
Continue to: Is oral progesterone an option?...
Is oral progesterone an option?
In the 1980s and 1990s, oral micronized progesterone was widely used in France at doses of 900 to 1,200 mg/d for women at risk for PTD. The practice was stopped when secondary hepatic effects, including cholestasis of pregnancy, were reported at a higher rate in treated women.24 A rise in the serum concentration of progesterone metabolites has been associated with impaired biliary excretion and subsequent accumulation of bile acids.25 In other reports, elevated serum transaminase activity was found in pregnant women treated with oral micronized progesterone, and withdrawal of treatment frequently has led to improvement in transaminase levels.26 The synthesis of endogenous progesterone during normal pregnancy is between 250 and 500 mg/d,26 and experts have expressed concern that exogenous progesterone supplementation may impose an additional load on the hepatic transport of sulfated metabolites. Unlike orally administered progesterone, progestins given by the vaginal route avoid the hepatic first-pass effect. For this reason, they may be associated with less hepatic dysfunction.
Although not recommended by professional guidelines, oral progesterone administration for the prevention of PTD has been used in the United States. A 2015 survey of Wisconsin prenatal care providers found that of those who prescribed any progesterone for PTD prevention, oral progesterone was prescribed by 13.1% of obstetricians, 24.4% of midwives, and 40.7% of family medicine practitioners.27
Some limited recent evidence from a meta-analysis of 3 trials investigating oral progesterone versus placebo suggests effectiveness in the prevention of recurrent PTD and reduction in perinatal morbidity and mortality.15 However, the number of cases included in the meta-analysis (386) was too small to support definitive clinical recommendations. Furthermore, questions have been raised in the literature about the reliability of the largest trial included in that meta-analysis.28
Case 3 Two previous spontaneous PTDs
A 29-year-old G3P0201 presents for her first prenatal appointment at 10 weeks’ gestation. With her first pregnancy she had a spontaneous PTD at 23 weeks, and the neonate did not survive. In her second pregnancy, she was treated with 17-OHPC from 16 weeks’ gestation. She had a spontaneous PTD at 29 weeks, and that child is developing normally by her report. She believes that 17-OHPC helped her in her last pregnancy and is anxious about the risk for still another PTD. Consistent with the concept of shared decision-making, you inform her of the results of the recent PROLONG trial and statements on the subject released by professional organizations such as ACOG and the Society for Maternal-Fetal Medicine (SMFM). What options does she have?
17-OHPC may be a possibility in very high-risk women
According to a SMFM statement released in the wake of the PROLONG trial publication, “. . . SMFM believes that it is reasonable for providers to use 17-OHPC in women with a profile more representative of the very high-risk population reported in the Meis trial”.29 Only a few women will have a recurrence risk of PTD over 50%, as was the background event rate in the Meis trial.30 Such a risk level may be suspected, as an example, in women with 2 or more prior early (before 34 weeks) PTDs without intervening term deliveries. Even in those cases, if treatment with 17-OHPC is decided upon, ultrasound cervical surveillance should be added as an additional safety measure. ●
- Iams JD, Goldenberg RL, Mercer BM, et al. The preterm prediction study: can low-risk women destined for spontaneous preterm birth be identified? Am J Obstet Gynecol. 2001;184:652-655.
- Murray SR, Stock SJ, Cowan S, et al. Spontaneous preterm birth prevention in multiple pregnancy. Obstet Gynecol. 2018;20:57-63.
- American College of Obstetricians and Gynecologists. ACOG committee opinion. Use of progesterone to reduce preterm birth. Obstet Gynecol. 2003;102:1115-1116.
- Dodd JM, Ashwood P, Flenady V, et al. A survey of clinician and patient attitudes towards the use of progesterone for women at risk of preterm birth. Aust N Z J Obstet Gynaecol. 2007;47:106-109.
- Blackwell SC, Gyamfi -Bannerman C, Biggio JR, et al. 17-OHPC to prevent recurrent preterm birth in singleton gestations (PROLONG study): a multicenter, international, randomized double-blind trial. Am J Perinatol. 2020;37:127-136.
- Duff P, Vidaeff AC, Ross MG, Norwitz ER. Managing preterm birth in those at risk: expert strategies. OBG Manag. 2019;31:39-42.
- Romero R, Mazor M, Munoz H, et al. The preterm labor syndrome. Ann N Y Acad Sci. 1994;734:414-429.
- Phillips C, Velji Z, Hanly C, et al. Risk of recurrent spontaneous preterm birth: a systematic review and meta-analysis. BMJ Open. 2017;7:e015402.
- Berghella V, Seibel-Seamon J. Contemporary use of cervical cerclage. Clin Obstet Gynecol. 2007;50:468-477.
- Naim A, Haberman S, Burgess T, et al. Changes in cervical length and the risk of preterm labor. Am J Obstet Gynecol. 2002;186:887-889.
- Zilianti M, Azuaga A, Calderon F, et al. Monitoring the effacement of the uterine cervix by transperineal sonography: a new perspective. J Ultrasound Med. 1995;14:719-724.
- Goldenberg RL, Iams JD, Miodovnik M, et al. The preterm prediction study: risk factors in twin gestation. Am J Obstet Gynecol. 1996;175:1047-1053.
- Romero R, Conde-Agudelo A, Da Fonseca E, et al. Vaginal progesterone for preventing preterm birth and adverse perinatal outcomes in singleton gestations with a short cervix: a meta-analysis of individual patient data. Am J Obstet Gynecol. 2018;218:161-180.
- Norman T, Morse C, Dennerstein L. Comparative bioavailability of orally and vaginally administered progesterone. Fertil Steril. 1991;56:1034-1039.
- Boelig RC, Della Corte L, Ashoush S, et al. Oral progesterone for the prevention of recurrent preterm birth: systematic review and metaanalysis. Am J Obstet Gynecol MFM. 2019;1:50-62.
- Boelig RC, Zuppa AF, Kraft WK, et al. Pharmacokinetics of vaginal progesterone in pregnancy. Am J Obstet Gynecol. 2019;221:263.e1-7.
- Bulletti C, de Ziegler D, Flamigni C, et al. Targeted drug delivery in gynaecology: the first uterine pass effect. Hum Reprod. 1997;12:1073-1079.
- Hassan SS, Romero R, Vidyadhari D, et al. Vaginal progesterone reduces the rate of preterm birth in women with a sonographic short cervix: a multicenter, randomized, double-blind, placebocontrolled trial. Ultrasound Obstet Gynecol. 2011;38:18-31.
- Preterm labour and birth. Evidence review for clinical effectiveness of prophylactic progesterone in preventing preterm labour. London: National Institute for Health and Care Excellence (UK); August 2019.
- Alfirevic Z, Stampalija T, Medley N. Cervical stitch (cerclage) for preventing preterm birth in singleton pregnancy. Cochrane Database Syst Rev. 2017;6:CD008991.
- Conde-Agudelo A, Romero R, Da Fonseca E, et al. Vaginal progesterone is as effective as cervical cerclage to prevent preterm birth in women with a singleton gestation, previous spontaneous preterm birth, and a short cervix: updated indirect comparison meta-analysis. Am J Obstet Gynecol. 2018;219:10-25.
- Park JY, Jung YM, Kook S-Y, et al. The effect of postoperative vaginal progesterone in ultrasound-indicated cerclage to prevent preterm birth. J Matern Fetal Neonatal Med. 2019:1-8.
- Roman AR, Da Silva Costa F, et al. Rescue adjuvant vaginal progesterone may improve outcomes in cervical cerclage failure. Geburt Frauen. 2018;78:785-790.
- Benifle JL, Dumont M, Levardon M, et al. Effects of natural micronized progesterone on the liver in the third trimester of pregnancy. Contracept Fertil Sex. 1997;25:165-169.
- Vallejo M, Briz O, Serrano MA, et al. Potential role of transinhibition of the bile salt export pump by progesterone metabolites in the etiopathogenesis of intrahepatic cholestasis of pregnancy. J Hepatol. 2006;44:1150-1157.
- Bacq Y, Sapey T, Bréchot MC, et al. Intrahepatic cholestasis of pregnancy: a French prospective study. Hepatology. 1997;26:358-364.
- Hoppe K, Kramer RD, Ha B, et al. Progesterone supplementation for the prevention of preterm birth: provider practice in Wisconsin. WMJ. 2019;118:126-131.
- Katsanevakis E, Mol BW, Thornton J. A question about the reliability of a recent trial of progesterone for preterm birth prevention, published in Acta. Acta Obstet Gynecol Scand. 2020;99:426.
- Society for Maternal-Fetal Medicine (SMFM) Publications Committee. SMFM Statement: use of 17-alpha hydroxyprogesterone caproate for prevention of recurrent preterm birth. https://www.smfm.org/publications/280smfm-statement-use-of-17-alpha-hydroxyprogesteronecaproate-for-prevention-of-recurrent-preterm-birth. Accessed March 23, 2020.
- Meis PJ, Klebanoff M, Thom E, et al. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;384:2379-2385.
- Iams JD, Goldenberg RL, Mercer BM, et al. The preterm prediction study: can low-risk women destined for spontaneous preterm birth be identified? Am J Obstet Gynecol. 2001;184:652-655.
- Murray SR, Stock SJ, Cowan S, et al. Spontaneous preterm birth prevention in multiple pregnancy. Obstet Gynecol. 2018;20:57-63.
- American College of Obstetricians and Gynecologists. ACOG committee opinion. Use of progesterone to reduce preterm birth. Obstet Gynecol. 2003;102:1115-1116.
- Dodd JM, Ashwood P, Flenady V, et al. A survey of clinician and patient attitudes towards the use of progesterone for women at risk of preterm birth. Aust N Z J Obstet Gynaecol. 2007;47:106-109.
- Blackwell SC, Gyamfi -Bannerman C, Biggio JR, et al. 17-OHPC to prevent recurrent preterm birth in singleton gestations (PROLONG study): a multicenter, international, randomized double-blind trial. Am J Perinatol. 2020;37:127-136.
- Duff P, Vidaeff AC, Ross MG, Norwitz ER. Managing preterm birth in those at risk: expert strategies. OBG Manag. 2019;31:39-42.
- Romero R, Mazor M, Munoz H, et al. The preterm labor syndrome. Ann N Y Acad Sci. 1994;734:414-429.
- Phillips C, Velji Z, Hanly C, et al. Risk of recurrent spontaneous preterm birth: a systematic review and meta-analysis. BMJ Open. 2017;7:e015402.
- Berghella V, Seibel-Seamon J. Contemporary use of cervical cerclage. Clin Obstet Gynecol. 2007;50:468-477.
- Naim A, Haberman S, Burgess T, et al. Changes in cervical length and the risk of preterm labor. Am J Obstet Gynecol. 2002;186:887-889.
- Zilianti M, Azuaga A, Calderon F, et al. Monitoring the effacement of the uterine cervix by transperineal sonography: a new perspective. J Ultrasound Med. 1995;14:719-724.
- Goldenberg RL, Iams JD, Miodovnik M, et al. The preterm prediction study: risk factors in twin gestation. Am J Obstet Gynecol. 1996;175:1047-1053.
- Romero R, Conde-Agudelo A, Da Fonseca E, et al. Vaginal progesterone for preventing preterm birth and adverse perinatal outcomes in singleton gestations with a short cervix: a meta-analysis of individual patient data. Am J Obstet Gynecol. 2018;218:161-180.
- Norman T, Morse C, Dennerstein L. Comparative bioavailability of orally and vaginally administered progesterone. Fertil Steril. 1991;56:1034-1039.
- Boelig RC, Della Corte L, Ashoush S, et al. Oral progesterone for the prevention of recurrent preterm birth: systematic review and metaanalysis. Am J Obstet Gynecol MFM. 2019;1:50-62.
- Boelig RC, Zuppa AF, Kraft WK, et al. Pharmacokinetics of vaginal progesterone in pregnancy. Am J Obstet Gynecol. 2019;221:263.e1-7.
- Bulletti C, de Ziegler D, Flamigni C, et al. Targeted drug delivery in gynaecology: the first uterine pass effect. Hum Reprod. 1997;12:1073-1079.
- Hassan SS, Romero R, Vidyadhari D, et al. Vaginal progesterone reduces the rate of preterm birth in women with a sonographic short cervix: a multicenter, randomized, double-blind, placebocontrolled trial. Ultrasound Obstet Gynecol. 2011;38:18-31.
- Preterm labour and birth. Evidence review for clinical effectiveness of prophylactic progesterone in preventing preterm labour. London: National Institute for Health and Care Excellence (UK); August 2019.
- Alfirevic Z, Stampalija T, Medley N. Cervical stitch (cerclage) for preventing preterm birth in singleton pregnancy. Cochrane Database Syst Rev. 2017;6:CD008991.
- Conde-Agudelo A, Romero R, Da Fonseca E, et al. Vaginal progesterone is as effective as cervical cerclage to prevent preterm birth in women with a singleton gestation, previous spontaneous preterm birth, and a short cervix: updated indirect comparison meta-analysis. Am J Obstet Gynecol. 2018;219:10-25.
- Park JY, Jung YM, Kook S-Y, et al. The effect of postoperative vaginal progesterone in ultrasound-indicated cerclage to prevent preterm birth. J Matern Fetal Neonatal Med. 2019:1-8.
- Roman AR, Da Silva Costa F, et al. Rescue adjuvant vaginal progesterone may improve outcomes in cervical cerclage failure. Geburt Frauen. 2018;78:785-790.
- Benifle JL, Dumont M, Levardon M, et al. Effects of natural micronized progesterone on the liver in the third trimester of pregnancy. Contracept Fertil Sex. 1997;25:165-169.
- Vallejo M, Briz O, Serrano MA, et al. Potential role of transinhibition of the bile salt export pump by progesterone metabolites in the etiopathogenesis of intrahepatic cholestasis of pregnancy. J Hepatol. 2006;44:1150-1157.
- Bacq Y, Sapey T, Bréchot MC, et al. Intrahepatic cholestasis of pregnancy: a French prospective study. Hepatology. 1997;26:358-364.
- Hoppe K, Kramer RD, Ha B, et al. Progesterone supplementation for the prevention of preterm birth: provider practice in Wisconsin. WMJ. 2019;118:126-131.
- Katsanevakis E, Mol BW, Thornton J. A question about the reliability of a recent trial of progesterone for preterm birth prevention, published in Acta. Acta Obstet Gynecol Scand. 2020;99:426.
- Society for Maternal-Fetal Medicine (SMFM) Publications Committee. SMFM Statement: use of 17-alpha hydroxyprogesterone caproate for prevention of recurrent preterm birth. https://www.smfm.org/publications/280smfm-statement-use-of-17-alpha-hydroxyprogesteronecaproate-for-prevention-of-recurrent-preterm-birth. Accessed March 23, 2020.
- Meis PJ, Klebanoff M, Thom E, et al. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;384:2379-2385.