User login
Teriflunomide increases the likelihood of achieving NEDA in relapsing-remitting MS
WEST PALM BEACH, FLA. – , according to an analysis presented at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. Teriflunomide reduces the risks of relapse, relapse resulting in hospital admission, and relapse resulting in prolonged hospitalization, compared with placebo.
Teriflunomide modulates the immune system and is an approved treatment for relapsing-remitting MS and clinically isolated syndrome. The phase 3 TEMSO study provided evidence that established the treatment’s safety and efficacy. In that study, significantly more patients who received a 14-mg dose of teriflunomide achieved NEDA, compared with patients who received placebo. Researchers generally weight all components of NEDA (i.e., confirmed disability worsening [CDW], relapse, and unique active MRI lesions) equally, but this approach could limit the interpretation of how each endpoint contributes to the effectiveness of a disease-modifying therapy.
A new analysis of TEMSO data
Keith R. Edwards, MD, director of the MS Center of Northeastern New York in Latham and colleagues conducted a win ratio matched-pairs analysis of TEMSO data to evaluate the efficacy of teriflunomide in enabling patients to achieve NEDA. In this analysis, the components of NEDA were assessed in order of priority, rather than as factors of equal weight.
In TEMSO, patients with relapsing-remitting MS received placebo or 14 mg of teriflunomide for 108 weeks. Dr. Edwards and colleagues matched active and control patients according to baseline characteristics. They compared the occurrence of disease activity events between the members of each pair. If a patient receiving teriflunomide had an event later than a control did, or did not have the event at all, teriflunomide was considered to “win.” If neither patient in a pair had a given event, the researchers omitted the pair from their analysis. Dr. Edwards and colleagues counted wins and summarized them as ratios. They conducted a second win ratio analysis of all relapses and relapses resulting in deaths, life-threatening events, prolonged hospitalizations, and hospital admissions.
NEDA components were ranked and assessed in the following order of decreasing priority: CDW, relapse, unique active MRI lesions. In a sensitivity analysis, the investigators ranked and assessed these components in the reverse order.
Sensitivity analysis supported primary analysis
Dr. Edwards and colleagues included 363 participants who received placebo and 358 who received teriflunomide in their analysis. Baseline characteristics did not differ significantly between the two groups. The population’s mean age was approximately 38 years, and about 73% of participants were female. The population’s mean baseline Expanded Disability Status Scale score was 2.7. Overall, about 72% of participants completed the study.
The researchers created 321 risk-matched pairs of participants. The win ratio analysis indicated that patients who received teriflunomide were significantly more likely to achieve NEDA, compared with controls (win ratio, 1.33). When the investigators analyzed the data by prioritizing the NEDA components in the reverse order, they found similar results (win ratio, 1.41).
When Dr. Edwards and colleagues analyzed relapse severity, they found that no relapses resulting in death or life-threatening events occurred in the active or control groups. Compared with placebo, teriflunomide significantly reduced the risk of relapse, relapses resulting in hospital admissions, and relapses resulting in prolonged hospitalizations (win ratio, 1.37).
The TEMSO study was funded by Sanofi. Dr. Edwards received grant or research support from Biogen, Genentech, Genzyme, and Novartis. Several authors received funding from Sanofi.
SOURCE: Edwards KR et al. ACTRIMS 2020, Abstract P036.
WEST PALM BEACH, FLA. – , according to an analysis presented at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. Teriflunomide reduces the risks of relapse, relapse resulting in hospital admission, and relapse resulting in prolonged hospitalization, compared with placebo.
Teriflunomide modulates the immune system and is an approved treatment for relapsing-remitting MS and clinically isolated syndrome. The phase 3 TEMSO study provided evidence that established the treatment’s safety and efficacy. In that study, significantly more patients who received a 14-mg dose of teriflunomide achieved NEDA, compared with patients who received placebo. Researchers generally weight all components of NEDA (i.e., confirmed disability worsening [CDW], relapse, and unique active MRI lesions) equally, but this approach could limit the interpretation of how each endpoint contributes to the effectiveness of a disease-modifying therapy.
A new analysis of TEMSO data
Keith R. Edwards, MD, director of the MS Center of Northeastern New York in Latham and colleagues conducted a win ratio matched-pairs analysis of TEMSO data to evaluate the efficacy of teriflunomide in enabling patients to achieve NEDA. In this analysis, the components of NEDA were assessed in order of priority, rather than as factors of equal weight.
In TEMSO, patients with relapsing-remitting MS received placebo or 14 mg of teriflunomide for 108 weeks. Dr. Edwards and colleagues matched active and control patients according to baseline characteristics. They compared the occurrence of disease activity events between the members of each pair. If a patient receiving teriflunomide had an event later than a control did, or did not have the event at all, teriflunomide was considered to “win.” If neither patient in a pair had a given event, the researchers omitted the pair from their analysis. Dr. Edwards and colleagues counted wins and summarized them as ratios. They conducted a second win ratio analysis of all relapses and relapses resulting in deaths, life-threatening events, prolonged hospitalizations, and hospital admissions.
NEDA components were ranked and assessed in the following order of decreasing priority: CDW, relapse, unique active MRI lesions. In a sensitivity analysis, the investigators ranked and assessed these components in the reverse order.
Sensitivity analysis supported primary analysis
Dr. Edwards and colleagues included 363 participants who received placebo and 358 who received teriflunomide in their analysis. Baseline characteristics did not differ significantly between the two groups. The population’s mean age was approximately 38 years, and about 73% of participants were female. The population’s mean baseline Expanded Disability Status Scale score was 2.7. Overall, about 72% of participants completed the study.
The researchers created 321 risk-matched pairs of participants. The win ratio analysis indicated that patients who received teriflunomide were significantly more likely to achieve NEDA, compared with controls (win ratio, 1.33). When the investigators analyzed the data by prioritizing the NEDA components in the reverse order, they found similar results (win ratio, 1.41).
When Dr. Edwards and colleagues analyzed relapse severity, they found that no relapses resulting in death or life-threatening events occurred in the active or control groups. Compared with placebo, teriflunomide significantly reduced the risk of relapse, relapses resulting in hospital admissions, and relapses resulting in prolonged hospitalizations (win ratio, 1.37).
The TEMSO study was funded by Sanofi. Dr. Edwards received grant or research support from Biogen, Genentech, Genzyme, and Novartis. Several authors received funding from Sanofi.
SOURCE: Edwards KR et al. ACTRIMS 2020, Abstract P036.
WEST PALM BEACH, FLA. – , according to an analysis presented at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. Teriflunomide reduces the risks of relapse, relapse resulting in hospital admission, and relapse resulting in prolonged hospitalization, compared with placebo.
Teriflunomide modulates the immune system and is an approved treatment for relapsing-remitting MS and clinically isolated syndrome. The phase 3 TEMSO study provided evidence that established the treatment’s safety and efficacy. In that study, significantly more patients who received a 14-mg dose of teriflunomide achieved NEDA, compared with patients who received placebo. Researchers generally weight all components of NEDA (i.e., confirmed disability worsening [CDW], relapse, and unique active MRI lesions) equally, but this approach could limit the interpretation of how each endpoint contributes to the effectiveness of a disease-modifying therapy.
A new analysis of TEMSO data
Keith R. Edwards, MD, director of the MS Center of Northeastern New York in Latham and colleagues conducted a win ratio matched-pairs analysis of TEMSO data to evaluate the efficacy of teriflunomide in enabling patients to achieve NEDA. In this analysis, the components of NEDA were assessed in order of priority, rather than as factors of equal weight.
In TEMSO, patients with relapsing-remitting MS received placebo or 14 mg of teriflunomide for 108 weeks. Dr. Edwards and colleagues matched active and control patients according to baseline characteristics. They compared the occurrence of disease activity events between the members of each pair. If a patient receiving teriflunomide had an event later than a control did, or did not have the event at all, teriflunomide was considered to “win.” If neither patient in a pair had a given event, the researchers omitted the pair from their analysis. Dr. Edwards and colleagues counted wins and summarized them as ratios. They conducted a second win ratio analysis of all relapses and relapses resulting in deaths, life-threatening events, prolonged hospitalizations, and hospital admissions.
NEDA components were ranked and assessed in the following order of decreasing priority: CDW, relapse, unique active MRI lesions. In a sensitivity analysis, the investigators ranked and assessed these components in the reverse order.
Sensitivity analysis supported primary analysis
Dr. Edwards and colleagues included 363 participants who received placebo and 358 who received teriflunomide in their analysis. Baseline characteristics did not differ significantly between the two groups. The population’s mean age was approximately 38 years, and about 73% of participants were female. The population’s mean baseline Expanded Disability Status Scale score was 2.7. Overall, about 72% of participants completed the study.
The researchers created 321 risk-matched pairs of participants. The win ratio analysis indicated that patients who received teriflunomide were significantly more likely to achieve NEDA, compared with controls (win ratio, 1.33). When the investigators analyzed the data by prioritizing the NEDA components in the reverse order, they found similar results (win ratio, 1.41).
When Dr. Edwards and colleagues analyzed relapse severity, they found that no relapses resulting in death or life-threatening events occurred in the active or control groups. Compared with placebo, teriflunomide significantly reduced the risk of relapse, relapses resulting in hospital admissions, and relapses resulting in prolonged hospitalizations (win ratio, 1.37).
The TEMSO study was funded by Sanofi. Dr. Edwards received grant or research support from Biogen, Genentech, Genzyme, and Novartis. Several authors received funding from Sanofi.
SOURCE: Edwards KR et al. ACTRIMS 2020, Abstract P036.
REPORTING FROM ACTRIMS Forum 2020
FDA, FTC uniting to promote biosimilars
The Food and Drug Administration is collaborating with the Federal Trade Commission (FTC) to expand the biosimilars market.
The two agencies signed a joint statement on Feb. 3, 2020, outlining four sets of goals aimed at creating meaningful competition from biosimilars against their reference biologic products.
“Competition is key for helping American patients have access to affordable medicines,” FDA Commissioner Stephen Hahn, MD, said in a statement. “Strengthening efforts to curtail and discourage anticompetitive behavior is key for facilitating robust competition for patients in the biologics marketplace, including through biosimilars, bringing down the costs of these crucial products for patients.”
The statement highlighted four goals. First is that the agencies will coordinate to promote greater competition in the biologic market, including the development of materials to educate the market about biosimilars. The FDA and FTC also sponsored a public workshop on March 9 to discuss competition for biologics.
The second goal has the FDA and FTC working together “to deter behavior that impedes access to samples needed for the development of biologics, including biosimilars,” the joint statement notes.
Third, the agencies will crack down on “false or misleading communications about biologics, including biosimilars, within their respective authorities,” according to the joint statement.
“FDA and FTC, as authorized by their respective statutes, will work together to address false or misleading communications about biologics, including biosimilars,” the statement continues. “In particular, if a communication makes a false or misleading comparison between a reference product and a biosimilar in a manner that misrepresents the safety or efficacy of biosimilars, deceives consumers, or deters competition, FDA and FTC intend to take appropriate action within their respective authorities. FDA intends to take appropriate action to address such communications where those communications have the potential to impact public health.”
Finally, the FTC committed to review patent settlement agreements involving biologics, including biosimilars, for antitrust violations.
Separately, the FDA issued a draft guidance document for comment on manufacturers seeking licensure of biosimilar products that do not cover all the approved uses of the reference product, as well as how to add uses over time that were not part of the initial license of the biosimilar product. The draft guidance covers licensure of products, labeling of biosimilars with fewer indications than the reference product, supplemental applications for indications not on the initial biosimilar application but covered by the reference product, and the timing of applications.
The FDA notes in the draft guidance that this is needed to cover situations such as when some indications on the reference product are covered by exclusivity, although it does encourage a biosimilar manufacturer to seek licensure for all indications that the reference product does have.
The Food and Drug Administration is collaborating with the Federal Trade Commission (FTC) to expand the biosimilars market.
The two agencies signed a joint statement on Feb. 3, 2020, outlining four sets of goals aimed at creating meaningful competition from biosimilars against their reference biologic products.
“Competition is key for helping American patients have access to affordable medicines,” FDA Commissioner Stephen Hahn, MD, said in a statement. “Strengthening efforts to curtail and discourage anticompetitive behavior is key for facilitating robust competition for patients in the biologics marketplace, including through biosimilars, bringing down the costs of these crucial products for patients.”
The statement highlighted four goals. First is that the agencies will coordinate to promote greater competition in the biologic market, including the development of materials to educate the market about biosimilars. The FDA and FTC also sponsored a public workshop on March 9 to discuss competition for biologics.
The second goal has the FDA and FTC working together “to deter behavior that impedes access to samples needed for the development of biologics, including biosimilars,” the joint statement notes.
Third, the agencies will crack down on “false or misleading communications about biologics, including biosimilars, within their respective authorities,” according to the joint statement.
“FDA and FTC, as authorized by their respective statutes, will work together to address false or misleading communications about biologics, including biosimilars,” the statement continues. “In particular, if a communication makes a false or misleading comparison between a reference product and a biosimilar in a manner that misrepresents the safety or efficacy of biosimilars, deceives consumers, or deters competition, FDA and FTC intend to take appropriate action within their respective authorities. FDA intends to take appropriate action to address such communications where those communications have the potential to impact public health.”
Finally, the FTC committed to review patent settlement agreements involving biologics, including biosimilars, for antitrust violations.
Separately, the FDA issued a draft guidance document for comment on manufacturers seeking licensure of biosimilar products that do not cover all the approved uses of the reference product, as well as how to add uses over time that were not part of the initial license of the biosimilar product. The draft guidance covers licensure of products, labeling of biosimilars with fewer indications than the reference product, supplemental applications for indications not on the initial biosimilar application but covered by the reference product, and the timing of applications.
The FDA notes in the draft guidance that this is needed to cover situations such as when some indications on the reference product are covered by exclusivity, although it does encourage a biosimilar manufacturer to seek licensure for all indications that the reference product does have.
The Food and Drug Administration is collaborating with the Federal Trade Commission (FTC) to expand the biosimilars market.
The two agencies signed a joint statement on Feb. 3, 2020, outlining four sets of goals aimed at creating meaningful competition from biosimilars against their reference biologic products.
“Competition is key for helping American patients have access to affordable medicines,” FDA Commissioner Stephen Hahn, MD, said in a statement. “Strengthening efforts to curtail and discourage anticompetitive behavior is key for facilitating robust competition for patients in the biologics marketplace, including through biosimilars, bringing down the costs of these crucial products for patients.”
The statement highlighted four goals. First is that the agencies will coordinate to promote greater competition in the biologic market, including the development of materials to educate the market about biosimilars. The FDA and FTC also sponsored a public workshop on March 9 to discuss competition for biologics.
The second goal has the FDA and FTC working together “to deter behavior that impedes access to samples needed for the development of biologics, including biosimilars,” the joint statement notes.
Third, the agencies will crack down on “false or misleading communications about biologics, including biosimilars, within their respective authorities,” according to the joint statement.
“FDA and FTC, as authorized by their respective statutes, will work together to address false or misleading communications about biologics, including biosimilars,” the statement continues. “In particular, if a communication makes a false or misleading comparison between a reference product and a biosimilar in a manner that misrepresents the safety or efficacy of biosimilars, deceives consumers, or deters competition, FDA and FTC intend to take appropriate action within their respective authorities. FDA intends to take appropriate action to address such communications where those communications have the potential to impact public health.”
Finally, the FTC committed to review patent settlement agreements involving biologics, including biosimilars, for antitrust violations.
Separately, the FDA issued a draft guidance document for comment on manufacturers seeking licensure of biosimilar products that do not cover all the approved uses of the reference product, as well as how to add uses over time that were not part of the initial license of the biosimilar product. The draft guidance covers licensure of products, labeling of biosimilars with fewer indications than the reference product, supplemental applications for indications not on the initial biosimilar application but covered by the reference product, and the timing of applications.
The FDA notes in the draft guidance that this is needed to cover situations such as when some indications on the reference product are covered by exclusivity, although it does encourage a biosimilar manufacturer to seek licensure for all indications that the reference product does have.
COVID-19: Older patients with cancer especially vulnerable
For oncologists and other clinicians caring for patients with cancer, the COVID-19 pandemic represents a dynamic clinical challenge that is changing daily and that can feel overwhelming at times, say experts.
“Oncology clinicians are well versed in caring for immunosuppressed patients with cancer, of all ages,” Merry-Jennifer Markham, MD, interim chief of the Division of Hematology and Oncology at the University of Florida Health, Gainesville, told Medscape Medical News.
However, she emphasized that, during this COVID-19 outbreak, “we must be especially diligent about screening for symptoms and exposure, and we must recognize that our older patients with cancer may be especially vulnerable.”
Patients with cancer who are in active treatment are immunosuppressed and are more susceptible to infection and to complications from infection, Markham pointed out. “While we don’t yet have much data on how COVID-19 impacts patients with cancer, I have to suspect that patients undergoing active cancer treatment may be especially vulnerable to the more severe illness associated with COVID-19,” she said.
Indeed, a recent report from China that was published in the Lancet Oncology supports this. The authors suggest that patients with cancer are at higher risk for COVID-19 and have a worse prognosis if they become infected than do those without cancer.
Commonsense rules
Commonsense rules apply for all patients with cancer, regardless of age, said Markham. Measures include thorough handwashing, staying home when sick, and avoiding sick contacts.
Markham, who acts as an expert spokesperson for the American Society of Clinical Oncology, provides information on what patients with cancer need to know about COVID-19 at Cancer.net, the society’s website for patients with cancer.
“Unfortunately, this outbreak of COVID-19 is happening rapidly and in real time,” Markham noted. “The entire medical community is learning as we go, rather than having the luxury of years of evidence-based literature to guide us.”
Another expert agrees. “Unfortunately, there are not a lot of data on how COVID-19 affects cancer patients,” Cardinale Smith, MD, PhD, director of Quality for Cancer Services in the Mount Sinai Health System, New York City, said in an interview.
“We need to minimize the risk for patients and minimize our own exposure by treating this situation like we would a really bad flu season,” Smith told Medscape Medical News. “Some patients have had a bad outcome, but the vast majority do not. The best we can do is stay calm and focused.”
At Mount Sinai, for patients with cancer, routine, nonurgent appointments are being rescheduled for May, Smith said. Those in active treatment are screened by telephone 24 to 48 hours before arrival, after which they undergo a full risk assessment in an isolation room. Those with a respiratory infection are given a mask.
“Patients are very anxious and worried about COVID-19,” said Smith, who has young children and an elderly parent at home. “We don’t have all the answers, and this can heighten anxiety.”
To help allay fears, social workers are asking patients with cancer who express anxiety to discuss their concerns and provide information. A one-page handout on both flu and COVID-10 is available in the waiting room.
The Web portal MyChart gives patients access to updated information on COVID-19 precautions and provides links to the hospital website and to the US Centers for Disease Control and Prevention. Patients who are not feeling well can speak to someone or get answers if they have additional questions.
When counseling patients, Smith advises them to use “an abundance of caution” and to be creative in efforts to minimize risk. “My suggestion is to use FaceTime and Skype to connect and communicate with your community,” she said.
Some churches are conducting services via teleconferencing to minimize risk, and seniors’ centers that offer yoga and other classes are also beginning to provide services virtually, she pointed out.
Data from China
A report published February 14 in the Lancet Oncology appears to be the first analysis in the literature to focus on COVID-19 in patients with cancer.
“Patients with cancer are more susceptible to infection than individuals without cancer because of their systemic immunosuppressive state caused by the malignancy and anticancer treatments, such as chemotherapy or surgery,” write the authors, led by Wenhua Liang, MD, of Guangzhou Medical University. However, in correspondence published in the Lancet Oncology, other experts in China question some of Liang’s and colleagues’ findings.
The report by Liang and colleagues concerns a prospective cohort of 1590 patients with COVID-19.
There were 2007 laboratory-confirmed cases of COVID-19 among patients admitted to 575 hospitals throughout China as of January 31. Of those cases, 417 were excluded from the analysis because of insufficient information regarding disease history.
The team reports that of 18 patients with cancer and COVID-19, 39% were at significantly higher risk for “severe events.” By comparison, of 1572 patients with COVID-19 who did not have cancer, 8% were at significantly higher risk (P = .0003). These events included rapid clinical deterioration that required admission to intensive care; invasive ventilation; or death.
Patients with cancer experienced a much more rapid deterioration in clinical status than did those without cancer. The median time to severe events was 13 days, vs 43 days (hazard ratio [HR] adjusted for age, 3.56; P < .0001).
The analysis also shows that patients who underwent chemotherapy or surgery in the past month had a 75% risk of experiencing clinically severe events, compared with a 43% risk for those who had not received recent treatment.
After adjusting for other risk factors, including age and smoking history, older age was the only risk factor for severe events (odds ratio [OR], 1.43; 95% confidence interval [CI], 0.97 – 2.12; P = .072), the study authors say.
Patients with lung cancer did not have a higher probability of severe events compared with patients with other cancer types (20% vs 62%, respectively; P = .294).
Liang and colleagues conclude that these findings provide “a timely reminder to physicians that more intensive attention should be paid to patients with cancer, in case of rapid deterioration.”
The team also proposes three strategies for managing patients with cancer who are at risk for COVID-19 or any other severe infectious disease. They recommend that intentional postponement of adjuvant chemotherapy or elective surgery be considered for patients with stable cancer who live in areas where disease is endemic. Stronger “personal protection provisions” could also be made for patients with cancer or for cancer survivors. Lastly, for patients with cancer who have COVID-19, especially those who are older or who have comorbidities, more intensive surveillance or treatment should be considered.
However, in comments in the Lancet Oncology, other authors in China say these findings should be interpreted with caution.
One group suggests that the increased susceptibility to COVID-19 in patients with cancer could be the result of higher rates of smoking compared with patients who did not have cancer. “Overall, current evidence remains insufficient to explain a conclusive association between cancer and COVID-19,” say Huahao Shen, PhD, of Zhejiang University School of Medicine, Hangzhou, Zhejiang, and colleagues.
Another group suggests that the significantly higher median age of patients with cancer compared with noncancer patients (63 years vs 49 years) may have contributed to poor prognosis.
These authors, led by Li Zhang, MD, PhD, and Hanping Wang, MD, of Peking Union Medical College and the Chinese Academy of Medical Sciences, Beijing, emphasize that patients with cancer need online medical counseling and that critical cases need to be identified and treated.
“In endemic areas outside Wuhan, decisions on whether or not to postpone cancer treatment need to made on a patient-by-patient basis and according to the risk to the patient and the prevailing situation because delays could lead to tumor progression and ultimately poorer outcomes,” they write.
The study was funded by the China National Science Foundation and the Key Project of Guangzhou Scientific Research Project. Liang and coauthors, Shen and coauthors, Zhang, Wang, and Smith have disclosed no relevant financial relationships. Markham has relationships with Aduro Biotech, Lilly, Tesaro, Novartis, and VBL Therapeutics.
This article first appeared on Medscape.com.
For oncologists and other clinicians caring for patients with cancer, the COVID-19 pandemic represents a dynamic clinical challenge that is changing daily and that can feel overwhelming at times, say experts.
“Oncology clinicians are well versed in caring for immunosuppressed patients with cancer, of all ages,” Merry-Jennifer Markham, MD, interim chief of the Division of Hematology and Oncology at the University of Florida Health, Gainesville, told Medscape Medical News.
However, she emphasized that, during this COVID-19 outbreak, “we must be especially diligent about screening for symptoms and exposure, and we must recognize that our older patients with cancer may be especially vulnerable.”
Patients with cancer who are in active treatment are immunosuppressed and are more susceptible to infection and to complications from infection, Markham pointed out. “While we don’t yet have much data on how COVID-19 impacts patients with cancer, I have to suspect that patients undergoing active cancer treatment may be especially vulnerable to the more severe illness associated with COVID-19,” she said.
Indeed, a recent report from China that was published in the Lancet Oncology supports this. The authors suggest that patients with cancer are at higher risk for COVID-19 and have a worse prognosis if they become infected than do those without cancer.
Commonsense rules
Commonsense rules apply for all patients with cancer, regardless of age, said Markham. Measures include thorough handwashing, staying home when sick, and avoiding sick contacts.
Markham, who acts as an expert spokesperson for the American Society of Clinical Oncology, provides information on what patients with cancer need to know about COVID-19 at Cancer.net, the society’s website for patients with cancer.
“Unfortunately, this outbreak of COVID-19 is happening rapidly and in real time,” Markham noted. “The entire medical community is learning as we go, rather than having the luxury of years of evidence-based literature to guide us.”
Another expert agrees. “Unfortunately, there are not a lot of data on how COVID-19 affects cancer patients,” Cardinale Smith, MD, PhD, director of Quality for Cancer Services in the Mount Sinai Health System, New York City, said in an interview.
“We need to minimize the risk for patients and minimize our own exposure by treating this situation like we would a really bad flu season,” Smith told Medscape Medical News. “Some patients have had a bad outcome, but the vast majority do not. The best we can do is stay calm and focused.”
At Mount Sinai, for patients with cancer, routine, nonurgent appointments are being rescheduled for May, Smith said. Those in active treatment are screened by telephone 24 to 48 hours before arrival, after which they undergo a full risk assessment in an isolation room. Those with a respiratory infection are given a mask.
“Patients are very anxious and worried about COVID-19,” said Smith, who has young children and an elderly parent at home. “We don’t have all the answers, and this can heighten anxiety.”
To help allay fears, social workers are asking patients with cancer who express anxiety to discuss their concerns and provide information. A one-page handout on both flu and COVID-10 is available in the waiting room.
The Web portal MyChart gives patients access to updated information on COVID-19 precautions and provides links to the hospital website and to the US Centers for Disease Control and Prevention. Patients who are not feeling well can speak to someone or get answers if they have additional questions.
When counseling patients, Smith advises them to use “an abundance of caution” and to be creative in efforts to minimize risk. “My suggestion is to use FaceTime and Skype to connect and communicate with your community,” she said.
Some churches are conducting services via teleconferencing to minimize risk, and seniors’ centers that offer yoga and other classes are also beginning to provide services virtually, she pointed out.
Data from China
A report published February 14 in the Lancet Oncology appears to be the first analysis in the literature to focus on COVID-19 in patients with cancer.
“Patients with cancer are more susceptible to infection than individuals without cancer because of their systemic immunosuppressive state caused by the malignancy and anticancer treatments, such as chemotherapy or surgery,” write the authors, led by Wenhua Liang, MD, of Guangzhou Medical University. However, in correspondence published in the Lancet Oncology, other experts in China question some of Liang’s and colleagues’ findings.
The report by Liang and colleagues concerns a prospective cohort of 1590 patients with COVID-19.
There were 2007 laboratory-confirmed cases of COVID-19 among patients admitted to 575 hospitals throughout China as of January 31. Of those cases, 417 were excluded from the analysis because of insufficient information regarding disease history.
The team reports that of 18 patients with cancer and COVID-19, 39% were at significantly higher risk for “severe events.” By comparison, of 1572 patients with COVID-19 who did not have cancer, 8% were at significantly higher risk (P = .0003). These events included rapid clinical deterioration that required admission to intensive care; invasive ventilation; or death.
Patients with cancer experienced a much more rapid deterioration in clinical status than did those without cancer. The median time to severe events was 13 days, vs 43 days (hazard ratio [HR] adjusted for age, 3.56; P < .0001).
The analysis also shows that patients who underwent chemotherapy or surgery in the past month had a 75% risk of experiencing clinically severe events, compared with a 43% risk for those who had not received recent treatment.
After adjusting for other risk factors, including age and smoking history, older age was the only risk factor for severe events (odds ratio [OR], 1.43; 95% confidence interval [CI], 0.97 – 2.12; P = .072), the study authors say.
Patients with lung cancer did not have a higher probability of severe events compared with patients with other cancer types (20% vs 62%, respectively; P = .294).
Liang and colleagues conclude that these findings provide “a timely reminder to physicians that more intensive attention should be paid to patients with cancer, in case of rapid deterioration.”
The team also proposes three strategies for managing patients with cancer who are at risk for COVID-19 or any other severe infectious disease. They recommend that intentional postponement of adjuvant chemotherapy or elective surgery be considered for patients with stable cancer who live in areas where disease is endemic. Stronger “personal protection provisions” could also be made for patients with cancer or for cancer survivors. Lastly, for patients with cancer who have COVID-19, especially those who are older or who have comorbidities, more intensive surveillance or treatment should be considered.
However, in comments in the Lancet Oncology, other authors in China say these findings should be interpreted with caution.
One group suggests that the increased susceptibility to COVID-19 in patients with cancer could be the result of higher rates of smoking compared with patients who did not have cancer. “Overall, current evidence remains insufficient to explain a conclusive association between cancer and COVID-19,” say Huahao Shen, PhD, of Zhejiang University School of Medicine, Hangzhou, Zhejiang, and colleagues.
Another group suggests that the significantly higher median age of patients with cancer compared with noncancer patients (63 years vs 49 years) may have contributed to poor prognosis.
These authors, led by Li Zhang, MD, PhD, and Hanping Wang, MD, of Peking Union Medical College and the Chinese Academy of Medical Sciences, Beijing, emphasize that patients with cancer need online medical counseling and that critical cases need to be identified and treated.
“In endemic areas outside Wuhan, decisions on whether or not to postpone cancer treatment need to made on a patient-by-patient basis and according to the risk to the patient and the prevailing situation because delays could lead to tumor progression and ultimately poorer outcomes,” they write.
The study was funded by the China National Science Foundation and the Key Project of Guangzhou Scientific Research Project. Liang and coauthors, Shen and coauthors, Zhang, Wang, and Smith have disclosed no relevant financial relationships. Markham has relationships with Aduro Biotech, Lilly, Tesaro, Novartis, and VBL Therapeutics.
This article first appeared on Medscape.com.
For oncologists and other clinicians caring for patients with cancer, the COVID-19 pandemic represents a dynamic clinical challenge that is changing daily and that can feel overwhelming at times, say experts.
“Oncology clinicians are well versed in caring for immunosuppressed patients with cancer, of all ages,” Merry-Jennifer Markham, MD, interim chief of the Division of Hematology and Oncology at the University of Florida Health, Gainesville, told Medscape Medical News.
However, she emphasized that, during this COVID-19 outbreak, “we must be especially diligent about screening for symptoms and exposure, and we must recognize that our older patients with cancer may be especially vulnerable.”
Patients with cancer who are in active treatment are immunosuppressed and are more susceptible to infection and to complications from infection, Markham pointed out. “While we don’t yet have much data on how COVID-19 impacts patients with cancer, I have to suspect that patients undergoing active cancer treatment may be especially vulnerable to the more severe illness associated with COVID-19,” she said.
Indeed, a recent report from China that was published in the Lancet Oncology supports this. The authors suggest that patients with cancer are at higher risk for COVID-19 and have a worse prognosis if they become infected than do those without cancer.
Commonsense rules
Commonsense rules apply for all patients with cancer, regardless of age, said Markham. Measures include thorough handwashing, staying home when sick, and avoiding sick contacts.
Markham, who acts as an expert spokesperson for the American Society of Clinical Oncology, provides information on what patients with cancer need to know about COVID-19 at Cancer.net, the society’s website for patients with cancer.
“Unfortunately, this outbreak of COVID-19 is happening rapidly and in real time,” Markham noted. “The entire medical community is learning as we go, rather than having the luxury of years of evidence-based literature to guide us.”
Another expert agrees. “Unfortunately, there are not a lot of data on how COVID-19 affects cancer patients,” Cardinale Smith, MD, PhD, director of Quality for Cancer Services in the Mount Sinai Health System, New York City, said in an interview.
“We need to minimize the risk for patients and minimize our own exposure by treating this situation like we would a really bad flu season,” Smith told Medscape Medical News. “Some patients have had a bad outcome, but the vast majority do not. The best we can do is stay calm and focused.”
At Mount Sinai, for patients with cancer, routine, nonurgent appointments are being rescheduled for May, Smith said. Those in active treatment are screened by telephone 24 to 48 hours before arrival, after which they undergo a full risk assessment in an isolation room. Those with a respiratory infection are given a mask.
“Patients are very anxious and worried about COVID-19,” said Smith, who has young children and an elderly parent at home. “We don’t have all the answers, and this can heighten anxiety.”
To help allay fears, social workers are asking patients with cancer who express anxiety to discuss their concerns and provide information. A one-page handout on both flu and COVID-10 is available in the waiting room.
The Web portal MyChart gives patients access to updated information on COVID-19 precautions and provides links to the hospital website and to the US Centers for Disease Control and Prevention. Patients who are not feeling well can speak to someone or get answers if they have additional questions.
When counseling patients, Smith advises them to use “an abundance of caution” and to be creative in efforts to minimize risk. “My suggestion is to use FaceTime and Skype to connect and communicate with your community,” she said.
Some churches are conducting services via teleconferencing to minimize risk, and seniors’ centers that offer yoga and other classes are also beginning to provide services virtually, she pointed out.
Data from China
A report published February 14 in the Lancet Oncology appears to be the first analysis in the literature to focus on COVID-19 in patients with cancer.
“Patients with cancer are more susceptible to infection than individuals without cancer because of their systemic immunosuppressive state caused by the malignancy and anticancer treatments, such as chemotherapy or surgery,” write the authors, led by Wenhua Liang, MD, of Guangzhou Medical University. However, in correspondence published in the Lancet Oncology, other experts in China question some of Liang’s and colleagues’ findings.
The report by Liang and colleagues concerns a prospective cohort of 1590 patients with COVID-19.
There were 2007 laboratory-confirmed cases of COVID-19 among patients admitted to 575 hospitals throughout China as of January 31. Of those cases, 417 were excluded from the analysis because of insufficient information regarding disease history.
The team reports that of 18 patients with cancer and COVID-19, 39% were at significantly higher risk for “severe events.” By comparison, of 1572 patients with COVID-19 who did not have cancer, 8% were at significantly higher risk (P = .0003). These events included rapid clinical deterioration that required admission to intensive care; invasive ventilation; or death.
Patients with cancer experienced a much more rapid deterioration in clinical status than did those without cancer. The median time to severe events was 13 days, vs 43 days (hazard ratio [HR] adjusted for age, 3.56; P < .0001).
The analysis also shows that patients who underwent chemotherapy or surgery in the past month had a 75% risk of experiencing clinically severe events, compared with a 43% risk for those who had not received recent treatment.
After adjusting for other risk factors, including age and smoking history, older age was the only risk factor for severe events (odds ratio [OR], 1.43; 95% confidence interval [CI], 0.97 – 2.12; P = .072), the study authors say.
Patients with lung cancer did not have a higher probability of severe events compared with patients with other cancer types (20% vs 62%, respectively; P = .294).
Liang and colleagues conclude that these findings provide “a timely reminder to physicians that more intensive attention should be paid to patients with cancer, in case of rapid deterioration.”
The team also proposes three strategies for managing patients with cancer who are at risk for COVID-19 or any other severe infectious disease. They recommend that intentional postponement of adjuvant chemotherapy or elective surgery be considered for patients with stable cancer who live in areas where disease is endemic. Stronger “personal protection provisions” could also be made for patients with cancer or for cancer survivors. Lastly, for patients with cancer who have COVID-19, especially those who are older or who have comorbidities, more intensive surveillance or treatment should be considered.
However, in comments in the Lancet Oncology, other authors in China say these findings should be interpreted with caution.
One group suggests that the increased susceptibility to COVID-19 in patients with cancer could be the result of higher rates of smoking compared with patients who did not have cancer. “Overall, current evidence remains insufficient to explain a conclusive association between cancer and COVID-19,” say Huahao Shen, PhD, of Zhejiang University School of Medicine, Hangzhou, Zhejiang, and colleagues.
Another group suggests that the significantly higher median age of patients with cancer compared with noncancer patients (63 years vs 49 years) may have contributed to poor prognosis.
These authors, led by Li Zhang, MD, PhD, and Hanping Wang, MD, of Peking Union Medical College and the Chinese Academy of Medical Sciences, Beijing, emphasize that patients with cancer need online medical counseling and that critical cases need to be identified and treated.
“In endemic areas outside Wuhan, decisions on whether or not to postpone cancer treatment need to made on a patient-by-patient basis and according to the risk to the patient and the prevailing situation because delays could lead to tumor progression and ultimately poorer outcomes,” they write.
The study was funded by the China National Science Foundation and the Key Project of Guangzhou Scientific Research Project. Liang and coauthors, Shen and coauthors, Zhang, Wang, and Smith have disclosed no relevant financial relationships. Markham has relationships with Aduro Biotech, Lilly, Tesaro, Novartis, and VBL Therapeutics.
This article first appeared on Medscape.com.
A match made in medicine: Match Day 2020
Match Day is the celebration of the National Resident Matching Program® (NRMP®) results, which seals the fate not only of future medical professionals, but of the program placements dedicated to supporting the acceleration of their careers.
Daniel Ricotta, MD, FHM, an academic hospitalist at Beth Israel Deaconess Medical Center (BIDMC) in Boston, and an active SHM member since 2013, offers unique insight into the value of understanding both sides of this interview table.
As the associate program director of BIDMC’s Internal Medicine Residency Program and the director of Simulation Education at the Carl J. Shapiro Center for Education & Research, Dr. Ricotta is able to act on his passions for medical education and clinical care.
“I was attracted to the breadth of medicine and enjoyed learning everything,” Dr. Ricotta said. “I knew I wanted to do academic medicine and education, and I was able to get involved by working with students and residents early on in my career.”
A natural fit for his current roles, Dr. Ricotta has gained a unique perspective on the match process and how it has evolved since he began his residency nine years ago.
Preparing for Match Day includes an extensive checklist of life-altering to-dos that shape your career trajectory. Medical students must have noteworthy CV points, scholarly recommendations, stand-out interviews, and a thoughtful rank list – among many other things to consider throughout the course of the match. Dr. Ricotta said that while this application process has generally remained the same since his participation, he has noticed that the students themselves have changed.
“Students going into residency are more mature and further along professionally,” he explained. “I’ve seen more students go on to do something else for a while and have gained more experience. They’re taking time off for research or getting dual degrees.”
Additionally, according to Dr. Ricotta, students are applying to double the number of programs than in years past, and are even using technology to their benefit. Because interview slots are limited, some students set up “bot automation” to help lock in interviews.
Amidst what can feel like a free-for-all, Dr. Ricotta reminds his students that the match process is a two-sided relationship.
“I certainly didn’t realize how much work goes into recruitment when I was a student,” Dr. Ricotta admitted. “What students don’t think about is the amount of care that goes into trying to match students who share similar values, the mission, or are a good cultural fit.”
He went on to emphasize the importance of environmental compatibility.
“Go somewhere that you feel you will fit in. Where you will thrive,” he said. “Go somewhere that has a mission that resonates with your mission and think about your fellow applicants and potential mentorship. Could you see yourself being their classmate? Does this program have people there who can help you to achieve your goals?”
Keeping in mind questions like these, it is no surprise that because of hospital medicine’s scheduling flexibility and hands-on learning opportunities that more and more students are interested in exploring this specialty.
“What is amazing about hospital medicine is the ample opportunity for you to get involved earlier in your career and build from that,” he said. “There is more face time with patients, more training for medical students available, countless academic opportunities in research and scholarships, and even conferences.”
Because of the multiple career pathways available in hospital medicine, SHM aims to provide students and residents with professional tools and opportunities as early as possible to allow them to get a preview of what they can expect as a hospitalist – no matter which path they choose.
“SHM is about getting involved,” said Dr. Ricotta. “SHM encourages residents to become actively incorporated into the community through chapters, conferences, and other networking opportunities on both local and national levels. That’s really difficult to do as a resident.”
Whether you’re waiting on the NRMP® results this year or you are in the beginning stages of gathering your application materials, one thing is clear according to Dr. Ricotta, you’re not just an applicant number.
Are you a student interested in exploring a career in hospital medicine? SHM supports educational and professional needs at all stages of your career. When you join SHM during your residency training, you receive access to programs, resources, and opportunities that will enhance your skills and raise your professional profile. For more information about our Residents & Fellows membership opportunity, please visit: hospitalmedicine.org/residents.
Ms. Cowan is a marketing communications specialist at the Society of Hospital Medicine.
Match Day is the celebration of the National Resident Matching Program® (NRMP®) results, which seals the fate not only of future medical professionals, but of the program placements dedicated to supporting the acceleration of their careers.
Daniel Ricotta, MD, FHM, an academic hospitalist at Beth Israel Deaconess Medical Center (BIDMC) in Boston, and an active SHM member since 2013, offers unique insight into the value of understanding both sides of this interview table.
As the associate program director of BIDMC’s Internal Medicine Residency Program and the director of Simulation Education at the Carl J. Shapiro Center for Education & Research, Dr. Ricotta is able to act on his passions for medical education and clinical care.
“I was attracted to the breadth of medicine and enjoyed learning everything,” Dr. Ricotta said. “I knew I wanted to do academic medicine and education, and I was able to get involved by working with students and residents early on in my career.”
A natural fit for his current roles, Dr. Ricotta has gained a unique perspective on the match process and how it has evolved since he began his residency nine years ago.
Preparing for Match Day includes an extensive checklist of life-altering to-dos that shape your career trajectory. Medical students must have noteworthy CV points, scholarly recommendations, stand-out interviews, and a thoughtful rank list – among many other things to consider throughout the course of the match. Dr. Ricotta said that while this application process has generally remained the same since his participation, he has noticed that the students themselves have changed.
“Students going into residency are more mature and further along professionally,” he explained. “I’ve seen more students go on to do something else for a while and have gained more experience. They’re taking time off for research or getting dual degrees.”
Additionally, according to Dr. Ricotta, students are applying to double the number of programs than in years past, and are even using technology to their benefit. Because interview slots are limited, some students set up “bot automation” to help lock in interviews.
Amidst what can feel like a free-for-all, Dr. Ricotta reminds his students that the match process is a two-sided relationship.
“I certainly didn’t realize how much work goes into recruitment when I was a student,” Dr. Ricotta admitted. “What students don’t think about is the amount of care that goes into trying to match students who share similar values, the mission, or are a good cultural fit.”
He went on to emphasize the importance of environmental compatibility.
“Go somewhere that you feel you will fit in. Where you will thrive,” he said. “Go somewhere that has a mission that resonates with your mission and think about your fellow applicants and potential mentorship. Could you see yourself being their classmate? Does this program have people there who can help you to achieve your goals?”
Keeping in mind questions like these, it is no surprise that because of hospital medicine’s scheduling flexibility and hands-on learning opportunities that more and more students are interested in exploring this specialty.
“What is amazing about hospital medicine is the ample opportunity for you to get involved earlier in your career and build from that,” he said. “There is more face time with patients, more training for medical students available, countless academic opportunities in research and scholarships, and even conferences.”
Because of the multiple career pathways available in hospital medicine, SHM aims to provide students and residents with professional tools and opportunities as early as possible to allow them to get a preview of what they can expect as a hospitalist – no matter which path they choose.
“SHM is about getting involved,” said Dr. Ricotta. “SHM encourages residents to become actively incorporated into the community through chapters, conferences, and other networking opportunities on both local and national levels. That’s really difficult to do as a resident.”
Whether you’re waiting on the NRMP® results this year or you are in the beginning stages of gathering your application materials, one thing is clear according to Dr. Ricotta, you’re not just an applicant number.
Are you a student interested in exploring a career in hospital medicine? SHM supports educational and professional needs at all stages of your career. When you join SHM during your residency training, you receive access to programs, resources, and opportunities that will enhance your skills and raise your professional profile. For more information about our Residents & Fellows membership opportunity, please visit: hospitalmedicine.org/residents.
Ms. Cowan is a marketing communications specialist at the Society of Hospital Medicine.
Match Day is the celebration of the National Resident Matching Program® (NRMP®) results, which seals the fate not only of future medical professionals, but of the program placements dedicated to supporting the acceleration of their careers.
Daniel Ricotta, MD, FHM, an academic hospitalist at Beth Israel Deaconess Medical Center (BIDMC) in Boston, and an active SHM member since 2013, offers unique insight into the value of understanding both sides of this interview table.
As the associate program director of BIDMC’s Internal Medicine Residency Program and the director of Simulation Education at the Carl J. Shapiro Center for Education & Research, Dr. Ricotta is able to act on his passions for medical education and clinical care.
“I was attracted to the breadth of medicine and enjoyed learning everything,” Dr. Ricotta said. “I knew I wanted to do academic medicine and education, and I was able to get involved by working with students and residents early on in my career.”
A natural fit for his current roles, Dr. Ricotta has gained a unique perspective on the match process and how it has evolved since he began his residency nine years ago.
Preparing for Match Day includes an extensive checklist of life-altering to-dos that shape your career trajectory. Medical students must have noteworthy CV points, scholarly recommendations, stand-out interviews, and a thoughtful rank list – among many other things to consider throughout the course of the match. Dr. Ricotta said that while this application process has generally remained the same since his participation, he has noticed that the students themselves have changed.
“Students going into residency are more mature and further along professionally,” he explained. “I’ve seen more students go on to do something else for a while and have gained more experience. They’re taking time off for research or getting dual degrees.”
Additionally, according to Dr. Ricotta, students are applying to double the number of programs than in years past, and are even using technology to their benefit. Because interview slots are limited, some students set up “bot automation” to help lock in interviews.
Amidst what can feel like a free-for-all, Dr. Ricotta reminds his students that the match process is a two-sided relationship.
“I certainly didn’t realize how much work goes into recruitment when I was a student,” Dr. Ricotta admitted. “What students don’t think about is the amount of care that goes into trying to match students who share similar values, the mission, or are a good cultural fit.”
He went on to emphasize the importance of environmental compatibility.
“Go somewhere that you feel you will fit in. Where you will thrive,” he said. “Go somewhere that has a mission that resonates with your mission and think about your fellow applicants and potential mentorship. Could you see yourself being their classmate? Does this program have people there who can help you to achieve your goals?”
Keeping in mind questions like these, it is no surprise that because of hospital medicine’s scheduling flexibility and hands-on learning opportunities that more and more students are interested in exploring this specialty.
“What is amazing about hospital medicine is the ample opportunity for you to get involved earlier in your career and build from that,” he said. “There is more face time with patients, more training for medical students available, countless academic opportunities in research and scholarships, and even conferences.”
Because of the multiple career pathways available in hospital medicine, SHM aims to provide students and residents with professional tools and opportunities as early as possible to allow them to get a preview of what they can expect as a hospitalist – no matter which path they choose.
“SHM is about getting involved,” said Dr. Ricotta. “SHM encourages residents to become actively incorporated into the community through chapters, conferences, and other networking opportunities on both local and national levels. That’s really difficult to do as a resident.”
Whether you’re waiting on the NRMP® results this year or you are in the beginning stages of gathering your application materials, one thing is clear according to Dr. Ricotta, you’re not just an applicant number.
Are you a student interested in exploring a career in hospital medicine? SHM supports educational and professional needs at all stages of your career. When you join SHM during your residency training, you receive access to programs, resources, and opportunities that will enhance your skills and raise your professional profile. For more information about our Residents & Fellows membership opportunity, please visit: hospitalmedicine.org/residents.
Ms. Cowan is a marketing communications specialist at the Society of Hospital Medicine.
Program Helps Native Americans Get Back to the Roots of Good Health
Diabetes used to be rare among Native Americans. Before the 1950s, there were few accounts of lifestyle diseases like type 2 diabetes mellitus (T2DM), says Valarie Blue Bird Jernigan, a member of the Choctaw Nation and University of Oklahoma researcher who studies the impacts of food environments on Native American health: “They couldn’t really be found in Native American communities. The major problem was malnutrition.” In 1940, only 21 cases of T2DM were identified among the Akimel O’odham people living in the Sonoran Desert. By 2006, 38% of adults in that tribe had T2DM.
The rate of diagnosed T2DM among American Indian/Alaska Native (AI/AN) adults is now double that of white adults, and the incidence among children and young adults is > 10 times that of other groups.
“Focusing on biologic factors alone overlooks factors that propel development of chronic diseases,” say researchers from the University of New Mexico and the Centers for Disease Control and Prevention (CDC) Native Diabetes Wellness program. Poverty, historical trauma, and adverse childhood experiences all play a part in AI/AN health issues. But food insecurity—uncertain or limited access to enough food for a healthy life—also correlates with greater risk of T2DM. In 2016, nearly 30% of AI/AN households were food insecure, compared with 16% of non-AI/AN households. Rates of food insecurity among AI/AN children are about double the national rates. Compounding the problem, “food deserts” are still common in Indian Country.
Native Americans used to eat healthier, living off the land, hunting, and fishing. Then federal mandates affected the land and water resources of tribal nations, disrupting indigenous food systems and reducing access to traditional foods, the researchers say. In the 1970s, the federal government began buying up surplus foods to support prices for farmers, then providing them to Native communities. The food was needed—the problem was that it consisted largely of high-salt, high-fat, high-sugar canned foods. One consequence of the calorie-dense commodities-based diet was “commod bod,” a phrase coined in Native communities.
Recently some traditional foods, like hand-harvested wild rice, grass-fed bison, and wild-caught Pacific salmon, have been added to the food assistance programs; the US Department of Agriculture cites high rates of participant satisfaction. About one-third of 103 tribal organizations also now have “grocery-store–like models” where aid recipients can select their own foods, including fresh fruits and vegetables.
However, in February, the Trump administration released a proposal to overhaul the Supplemental Nutrition Assistance Program, replacing half the benefits people receive with boxed, nonperishable foods. According to recent research, Jernigan says, 60% of Native Americans who receive food assistance through the Supplemental Nutrition Assistance Program rely on the program as their primary source of food.
It became clear that one way to help AI/AN communities reclaim their health was to bring back the old ways. The Indian Health Service (IHS) Tribal Leaders Diabetes Committee has supported programs in which AI/AN communities integrate their own cultures and history, to encourage healthier lifestyles. The concept of a “food sovereignty movement” evolved into programs like the Traditional Foods Project (TFP).
The TFP has provided “modest” funding to AI/AN communities to design their own interventions promoting access to traditional foods, physical activity, and social support. The project began in 2008 with 11 tribes and tribal organizations, and expanded to 17 in 2009.
Recently, the CDC researchers reported on how the TFP was doing, evaluating data the tribal partners collected between 2008 and 2014 in 3 domains: traditional foods, physical activity, and social support. Each partner used various strategies aimed at behavior changes, with unique solutions in each group. Some of their initiatives covered > 1 domain: gardening, for instance, involved physical activity, social support, and traditional foods.
From 82% to 94% of the partners (numbers varied as more communities joined the TFP) reported gardening during summer months; 59% to 82% also gardened during the winter. Many started community gardens, but school gardens had the most participants. In 1 year, 6 communities had school gardens involving 3,017 people. Most of the partners also began focusing on sustainability, using heirloom seeds, for instance. One coordinator took a course to become a Master Composter, balancing traditional ecological knowledge and Western science, leading to “large yields of harvested produce.”
Healthy food outlets increased, reported by 11 of 16 communities in T10, up from 2 of 11 in the first test period. Moreover, by T10, nearly two-thirds of the partners reported that healthy food selections were available at 1 or more venues, including worksites, supermarkets, vending machines, and restaurants.
Most partners reported health education activities for each period, involving nearly 11,000 participants. Storytelling was an important teaching activity, the researchers say. Head Start organizations added physical activities, gardening, and a health education curriculum.
The partners measured changes such as weight loss, improved physical activity, and healthy food choices in 69 of 156 data points recorded during the 10 periods. In most periods, almost half of the partners measured participant change in 1 or more domains. As many as 7,500 participants took part in organized physical activities for 1 partner during 1 period. Involvement in activities peaked in the middle years but leveled off at a median of about 65%.
The researchers also gathered observations from the partners. The program’s impact was visible not only community-wide, but among individuals. One young man who had struggled with substance abuse said he “found himself through connection with the earth” in the community garden. Another participant said, “Food is good medicine.”
A thread in every discussion, the researchers say, was: “Traditional foods have become a way to talk about health.” The way to reclaim health, the partners came to believe, was to reconnect with the land, water, traditional foodways, and “all that they mean.”
Diabetes used to be rare among Native Americans. Before the 1950s, there were few accounts of lifestyle diseases like type 2 diabetes mellitus (T2DM), says Valarie Blue Bird Jernigan, a member of the Choctaw Nation and University of Oklahoma researcher who studies the impacts of food environments on Native American health: “They couldn’t really be found in Native American communities. The major problem was malnutrition.” In 1940, only 21 cases of T2DM were identified among the Akimel O’odham people living in the Sonoran Desert. By 2006, 38% of adults in that tribe had T2DM.
The rate of diagnosed T2DM among American Indian/Alaska Native (AI/AN) adults is now double that of white adults, and the incidence among children and young adults is > 10 times that of other groups.
“Focusing on biologic factors alone overlooks factors that propel development of chronic diseases,” say researchers from the University of New Mexico and the Centers for Disease Control and Prevention (CDC) Native Diabetes Wellness program. Poverty, historical trauma, and adverse childhood experiences all play a part in AI/AN health issues. But food insecurity—uncertain or limited access to enough food for a healthy life—also correlates with greater risk of T2DM. In 2016, nearly 30% of AI/AN households were food insecure, compared with 16% of non-AI/AN households. Rates of food insecurity among AI/AN children are about double the national rates. Compounding the problem, “food deserts” are still common in Indian Country.
Native Americans used to eat healthier, living off the land, hunting, and fishing. Then federal mandates affected the land and water resources of tribal nations, disrupting indigenous food systems and reducing access to traditional foods, the researchers say. In the 1970s, the federal government began buying up surplus foods to support prices for farmers, then providing them to Native communities. The food was needed—the problem was that it consisted largely of high-salt, high-fat, high-sugar canned foods. One consequence of the calorie-dense commodities-based diet was “commod bod,” a phrase coined in Native communities.
Recently some traditional foods, like hand-harvested wild rice, grass-fed bison, and wild-caught Pacific salmon, have been added to the food assistance programs; the US Department of Agriculture cites high rates of participant satisfaction. About one-third of 103 tribal organizations also now have “grocery-store–like models” where aid recipients can select their own foods, including fresh fruits and vegetables.
However, in February, the Trump administration released a proposal to overhaul the Supplemental Nutrition Assistance Program, replacing half the benefits people receive with boxed, nonperishable foods. According to recent research, Jernigan says, 60% of Native Americans who receive food assistance through the Supplemental Nutrition Assistance Program rely on the program as their primary source of food.
It became clear that one way to help AI/AN communities reclaim their health was to bring back the old ways. The Indian Health Service (IHS) Tribal Leaders Diabetes Committee has supported programs in which AI/AN communities integrate their own cultures and history, to encourage healthier lifestyles. The concept of a “food sovereignty movement” evolved into programs like the Traditional Foods Project (TFP).
The TFP has provided “modest” funding to AI/AN communities to design their own interventions promoting access to traditional foods, physical activity, and social support. The project began in 2008 with 11 tribes and tribal organizations, and expanded to 17 in 2009.
Recently, the CDC researchers reported on how the TFP was doing, evaluating data the tribal partners collected between 2008 and 2014 in 3 domains: traditional foods, physical activity, and social support. Each partner used various strategies aimed at behavior changes, with unique solutions in each group. Some of their initiatives covered > 1 domain: gardening, for instance, involved physical activity, social support, and traditional foods.
From 82% to 94% of the partners (numbers varied as more communities joined the TFP) reported gardening during summer months; 59% to 82% also gardened during the winter. Many started community gardens, but school gardens had the most participants. In 1 year, 6 communities had school gardens involving 3,017 people. Most of the partners also began focusing on sustainability, using heirloom seeds, for instance. One coordinator took a course to become a Master Composter, balancing traditional ecological knowledge and Western science, leading to “large yields of harvested produce.”
Healthy food outlets increased, reported by 11 of 16 communities in T10, up from 2 of 11 in the first test period. Moreover, by T10, nearly two-thirds of the partners reported that healthy food selections were available at 1 or more venues, including worksites, supermarkets, vending machines, and restaurants.
Most partners reported health education activities for each period, involving nearly 11,000 participants. Storytelling was an important teaching activity, the researchers say. Head Start organizations added physical activities, gardening, and a health education curriculum.
The partners measured changes such as weight loss, improved physical activity, and healthy food choices in 69 of 156 data points recorded during the 10 periods. In most periods, almost half of the partners measured participant change in 1 or more domains. As many as 7,500 participants took part in organized physical activities for 1 partner during 1 period. Involvement in activities peaked in the middle years but leveled off at a median of about 65%.
The researchers also gathered observations from the partners. The program’s impact was visible not only community-wide, but among individuals. One young man who had struggled with substance abuse said he “found himself through connection with the earth” in the community garden. Another participant said, “Food is good medicine.”
A thread in every discussion, the researchers say, was: “Traditional foods have become a way to talk about health.” The way to reclaim health, the partners came to believe, was to reconnect with the land, water, traditional foodways, and “all that they mean.”
Diabetes used to be rare among Native Americans. Before the 1950s, there were few accounts of lifestyle diseases like type 2 diabetes mellitus (T2DM), says Valarie Blue Bird Jernigan, a member of the Choctaw Nation and University of Oklahoma researcher who studies the impacts of food environments on Native American health: “They couldn’t really be found in Native American communities. The major problem was malnutrition.” In 1940, only 21 cases of T2DM were identified among the Akimel O’odham people living in the Sonoran Desert. By 2006, 38% of adults in that tribe had T2DM.
The rate of diagnosed T2DM among American Indian/Alaska Native (AI/AN) adults is now double that of white adults, and the incidence among children and young adults is > 10 times that of other groups.
“Focusing on biologic factors alone overlooks factors that propel development of chronic diseases,” say researchers from the University of New Mexico and the Centers for Disease Control and Prevention (CDC) Native Diabetes Wellness program. Poverty, historical trauma, and adverse childhood experiences all play a part in AI/AN health issues. But food insecurity—uncertain or limited access to enough food for a healthy life—also correlates with greater risk of T2DM. In 2016, nearly 30% of AI/AN households were food insecure, compared with 16% of non-AI/AN households. Rates of food insecurity among AI/AN children are about double the national rates. Compounding the problem, “food deserts” are still common in Indian Country.
Native Americans used to eat healthier, living off the land, hunting, and fishing. Then federal mandates affected the land and water resources of tribal nations, disrupting indigenous food systems and reducing access to traditional foods, the researchers say. In the 1970s, the federal government began buying up surplus foods to support prices for farmers, then providing them to Native communities. The food was needed—the problem was that it consisted largely of high-salt, high-fat, high-sugar canned foods. One consequence of the calorie-dense commodities-based diet was “commod bod,” a phrase coined in Native communities.
Recently some traditional foods, like hand-harvested wild rice, grass-fed bison, and wild-caught Pacific salmon, have been added to the food assistance programs; the US Department of Agriculture cites high rates of participant satisfaction. About one-third of 103 tribal organizations also now have “grocery-store–like models” where aid recipients can select their own foods, including fresh fruits and vegetables.
However, in February, the Trump administration released a proposal to overhaul the Supplemental Nutrition Assistance Program, replacing half the benefits people receive with boxed, nonperishable foods. According to recent research, Jernigan says, 60% of Native Americans who receive food assistance through the Supplemental Nutrition Assistance Program rely on the program as their primary source of food.
It became clear that one way to help AI/AN communities reclaim their health was to bring back the old ways. The Indian Health Service (IHS) Tribal Leaders Diabetes Committee has supported programs in which AI/AN communities integrate their own cultures and history, to encourage healthier lifestyles. The concept of a “food sovereignty movement” evolved into programs like the Traditional Foods Project (TFP).
The TFP has provided “modest” funding to AI/AN communities to design their own interventions promoting access to traditional foods, physical activity, and social support. The project began in 2008 with 11 tribes and tribal organizations, and expanded to 17 in 2009.
Recently, the CDC researchers reported on how the TFP was doing, evaluating data the tribal partners collected between 2008 and 2014 in 3 domains: traditional foods, physical activity, and social support. Each partner used various strategies aimed at behavior changes, with unique solutions in each group. Some of their initiatives covered > 1 domain: gardening, for instance, involved physical activity, social support, and traditional foods.
From 82% to 94% of the partners (numbers varied as more communities joined the TFP) reported gardening during summer months; 59% to 82% also gardened during the winter. Many started community gardens, but school gardens had the most participants. In 1 year, 6 communities had school gardens involving 3,017 people. Most of the partners also began focusing on sustainability, using heirloom seeds, for instance. One coordinator took a course to become a Master Composter, balancing traditional ecological knowledge and Western science, leading to “large yields of harvested produce.”
Healthy food outlets increased, reported by 11 of 16 communities in T10, up from 2 of 11 in the first test period. Moreover, by T10, nearly two-thirds of the partners reported that healthy food selections were available at 1 or more venues, including worksites, supermarkets, vending machines, and restaurants.
Most partners reported health education activities for each period, involving nearly 11,000 participants. Storytelling was an important teaching activity, the researchers say. Head Start organizations added physical activities, gardening, and a health education curriculum.
The partners measured changes such as weight loss, improved physical activity, and healthy food choices in 69 of 156 data points recorded during the 10 periods. In most periods, almost half of the partners measured participant change in 1 or more domains. As many as 7,500 participants took part in organized physical activities for 1 partner during 1 period. Involvement in activities peaked in the middle years but leveled off at a median of about 65%.
The researchers also gathered observations from the partners. The program’s impact was visible not only community-wide, but among individuals. One young man who had struggled with substance abuse said he “found himself through connection with the earth” in the community garden. Another participant said, “Food is good medicine.”
A thread in every discussion, the researchers say, was: “Traditional foods have become a way to talk about health.” The way to reclaim health, the partners came to believe, was to reconnect with the land, water, traditional foodways, and “all that they mean.”
Red meat intake linked to increased risk of fatal CHD in men
PHOENIX – Consumption of red meat, particularly the processed form, is linked to a higher risk of developing coronary heart disease in men, results from a large prospective analysis demonstrated.
“The findings of this study are in line with randomized trials showing that the consumption of red meat, as compared with plant-based protein sources, increases LDL cholesterol levels, and with previous studies on red meat and risk of coronary heart disease,” lead study author Laila Al-Shaar, MPH, PhD, said in an interview in advance of the Epidemiology and Prevention/Lifestyle and Cardiometabolic Health meeting.
According to Dr. Al-Shaar, a postdoctoral research fellow in the department of nutrition at the T.H. Chan School of Public Health at Harvard University, Boston, most of the existing studies on red meat and heart disease have examined the impact of increasing consumption of red meat while decreasing consumption of all other foods. For the current study, she and her colleagues used a substitution analysis approach to understand how replacing red meat (total, processed, or unprocessed) with another protein-rich food was associated with the risk of heart disease. “This would potentially provide more specific guidance for healthier alternatives for those planning to cut down their red meat intake,” she said.
She and her colleagues prospectively followed 43,259 men in the Health Professionals Follow-up Study (1986-2012) who had no known history of cancer or cardiovascular disease. Diet was assessed by a standardized and validated food frequency questionnaire that was updated every 4 years. Dr. Al-Shaar and her colleagues used multivariate Cox models to estimate hazard ratios and 95% confidence intervals of CHD risk across categories of red meat consumption. They performed substitution analyses by comparing coefficients in models including alternative foods as continuous variables.
Over roughly 933,000 person-years of follow-up, the researchers documented 4,148 incident CHD cases. Of these, 1,680 were fatal. After multivariate adjustment for dietary and nondietary risk factors, both total and processed red meat intake were associated with a modestly higher risk of CHD (hazard ratio for a one serving/day increment, 1.08; 95% confidence interval, 1.01-1.14 for total red meat; and HR, 1.13; 95% CI, 1.03-1.22 for processed red meat). Substitutions of one serving per day of other foods (including nuts, legumes, soy, whole grains, and low- and high-fat dairy) for one serving per day of total red meat were associated with a 10%-47% lower CHD risk.
Stronger inverse associations were observed between some of these substitutions for red meat and risk of fatal CHD. Substituting nuts lowered the risk of fatal heart disease by 17%, while replacing red meat with whole grains was linked to a 48% reduction in that outcome. Those associations were more pronounced when replacing processed red meat.
“Processed meats and meats in general have been thought to be potentially not favorable in terms of cardiovascular disease and cardiovascular disease risk,” Robert H. Eckel, MD, professor emeritus of medicine at the University of Colorado Anschutz Medical Campus, Aurora, said in an interview. “Now we have increasing data that not only is there a negative cardiovascular disease impact of animal protein, but we see this on all-cause mortality, including cancer.”
Dr. Al-Shaar said that the findings “support current recommendations to limit consumption of red meat and suggest that high-quality plant-based proteins such as nuts, legumes, and soy are good alternatives for individuals planning to have better food choices and healthier eating patterns.”
She acknowledged certain limitations of the study, including its observational design and the fact that it was limited to non-Hispanic white health professionals, “thus limiting the generalizability of its findings to the whole population.”
Dr. Eckel, who is a past president of the American Heart Association, underscored the importance of one’s overall diet in mitigating the risk of developing coronary heart disease. “It’s not simply substituting animal protein with plant protein,” he said. “Fruits and vegetables and whole grains, lean protein from fish – a Mediterranean-style diet – is what the AHA recommends.”
Dr. Al-Shaar reported having no financial disclosures. The study was supported by a T32 training grant from the National Institutes of Health and by other grants from the NIH. The meeting was sponsored by the AHA.
SOURCE: Al-Shaar L et al. Epi/Lifestyle 2020, Abstract P512.
PHOENIX – Consumption of red meat, particularly the processed form, is linked to a higher risk of developing coronary heart disease in men, results from a large prospective analysis demonstrated.
“The findings of this study are in line with randomized trials showing that the consumption of red meat, as compared with plant-based protein sources, increases LDL cholesterol levels, and with previous studies on red meat and risk of coronary heart disease,” lead study author Laila Al-Shaar, MPH, PhD, said in an interview in advance of the Epidemiology and Prevention/Lifestyle and Cardiometabolic Health meeting.
According to Dr. Al-Shaar, a postdoctoral research fellow in the department of nutrition at the T.H. Chan School of Public Health at Harvard University, Boston, most of the existing studies on red meat and heart disease have examined the impact of increasing consumption of red meat while decreasing consumption of all other foods. For the current study, she and her colleagues used a substitution analysis approach to understand how replacing red meat (total, processed, or unprocessed) with another protein-rich food was associated with the risk of heart disease. “This would potentially provide more specific guidance for healthier alternatives for those planning to cut down their red meat intake,” she said.
She and her colleagues prospectively followed 43,259 men in the Health Professionals Follow-up Study (1986-2012) who had no known history of cancer or cardiovascular disease. Diet was assessed by a standardized and validated food frequency questionnaire that was updated every 4 years. Dr. Al-Shaar and her colleagues used multivariate Cox models to estimate hazard ratios and 95% confidence intervals of CHD risk across categories of red meat consumption. They performed substitution analyses by comparing coefficients in models including alternative foods as continuous variables.
Over roughly 933,000 person-years of follow-up, the researchers documented 4,148 incident CHD cases. Of these, 1,680 were fatal. After multivariate adjustment for dietary and nondietary risk factors, both total and processed red meat intake were associated with a modestly higher risk of CHD (hazard ratio for a one serving/day increment, 1.08; 95% confidence interval, 1.01-1.14 for total red meat; and HR, 1.13; 95% CI, 1.03-1.22 for processed red meat). Substitutions of one serving per day of other foods (including nuts, legumes, soy, whole grains, and low- and high-fat dairy) for one serving per day of total red meat were associated with a 10%-47% lower CHD risk.
Stronger inverse associations were observed between some of these substitutions for red meat and risk of fatal CHD. Substituting nuts lowered the risk of fatal heart disease by 17%, while replacing red meat with whole grains was linked to a 48% reduction in that outcome. Those associations were more pronounced when replacing processed red meat.
“Processed meats and meats in general have been thought to be potentially not favorable in terms of cardiovascular disease and cardiovascular disease risk,” Robert H. Eckel, MD, professor emeritus of medicine at the University of Colorado Anschutz Medical Campus, Aurora, said in an interview. “Now we have increasing data that not only is there a negative cardiovascular disease impact of animal protein, but we see this on all-cause mortality, including cancer.”
Dr. Al-Shaar said that the findings “support current recommendations to limit consumption of red meat and suggest that high-quality plant-based proteins such as nuts, legumes, and soy are good alternatives for individuals planning to have better food choices and healthier eating patterns.”
She acknowledged certain limitations of the study, including its observational design and the fact that it was limited to non-Hispanic white health professionals, “thus limiting the generalizability of its findings to the whole population.”
Dr. Eckel, who is a past president of the American Heart Association, underscored the importance of one’s overall diet in mitigating the risk of developing coronary heart disease. “It’s not simply substituting animal protein with plant protein,” he said. “Fruits and vegetables and whole grains, lean protein from fish – a Mediterranean-style diet – is what the AHA recommends.”
Dr. Al-Shaar reported having no financial disclosures. The study was supported by a T32 training grant from the National Institutes of Health and by other grants from the NIH. The meeting was sponsored by the AHA.
SOURCE: Al-Shaar L et al. Epi/Lifestyle 2020, Abstract P512.
PHOENIX – Consumption of red meat, particularly the processed form, is linked to a higher risk of developing coronary heart disease in men, results from a large prospective analysis demonstrated.
“The findings of this study are in line with randomized trials showing that the consumption of red meat, as compared with plant-based protein sources, increases LDL cholesterol levels, and with previous studies on red meat and risk of coronary heart disease,” lead study author Laila Al-Shaar, MPH, PhD, said in an interview in advance of the Epidemiology and Prevention/Lifestyle and Cardiometabolic Health meeting.
According to Dr. Al-Shaar, a postdoctoral research fellow in the department of nutrition at the T.H. Chan School of Public Health at Harvard University, Boston, most of the existing studies on red meat and heart disease have examined the impact of increasing consumption of red meat while decreasing consumption of all other foods. For the current study, she and her colleagues used a substitution analysis approach to understand how replacing red meat (total, processed, or unprocessed) with another protein-rich food was associated with the risk of heart disease. “This would potentially provide more specific guidance for healthier alternatives for those planning to cut down their red meat intake,” she said.
She and her colleagues prospectively followed 43,259 men in the Health Professionals Follow-up Study (1986-2012) who had no known history of cancer or cardiovascular disease. Diet was assessed by a standardized and validated food frequency questionnaire that was updated every 4 years. Dr. Al-Shaar and her colleagues used multivariate Cox models to estimate hazard ratios and 95% confidence intervals of CHD risk across categories of red meat consumption. They performed substitution analyses by comparing coefficients in models including alternative foods as continuous variables.
Over roughly 933,000 person-years of follow-up, the researchers documented 4,148 incident CHD cases. Of these, 1,680 were fatal. After multivariate adjustment for dietary and nondietary risk factors, both total and processed red meat intake were associated with a modestly higher risk of CHD (hazard ratio for a one serving/day increment, 1.08; 95% confidence interval, 1.01-1.14 for total red meat; and HR, 1.13; 95% CI, 1.03-1.22 for processed red meat). Substitutions of one serving per day of other foods (including nuts, legumes, soy, whole grains, and low- and high-fat dairy) for one serving per day of total red meat were associated with a 10%-47% lower CHD risk.
Stronger inverse associations were observed between some of these substitutions for red meat and risk of fatal CHD. Substituting nuts lowered the risk of fatal heart disease by 17%, while replacing red meat with whole grains was linked to a 48% reduction in that outcome. Those associations were more pronounced when replacing processed red meat.
“Processed meats and meats in general have been thought to be potentially not favorable in terms of cardiovascular disease and cardiovascular disease risk,” Robert H. Eckel, MD, professor emeritus of medicine at the University of Colorado Anschutz Medical Campus, Aurora, said in an interview. “Now we have increasing data that not only is there a negative cardiovascular disease impact of animal protein, but we see this on all-cause mortality, including cancer.”
Dr. Al-Shaar said that the findings “support current recommendations to limit consumption of red meat and suggest that high-quality plant-based proteins such as nuts, legumes, and soy are good alternatives for individuals planning to have better food choices and healthier eating patterns.”
She acknowledged certain limitations of the study, including its observational design and the fact that it was limited to non-Hispanic white health professionals, “thus limiting the generalizability of its findings to the whole population.”
Dr. Eckel, who is a past president of the American Heart Association, underscored the importance of one’s overall diet in mitigating the risk of developing coronary heart disease. “It’s not simply substituting animal protein with plant protein,” he said. “Fruits and vegetables and whole grains, lean protein from fish – a Mediterranean-style diet – is what the AHA recommends.”
Dr. Al-Shaar reported having no financial disclosures. The study was supported by a T32 training grant from the National Institutes of Health and by other grants from the NIH. The meeting was sponsored by the AHA.
SOURCE: Al-Shaar L et al. Epi/Lifestyle 2020, Abstract P512.
REPORTING FROM EPI/LIFESTYLE 2020
Fracture risk for MM patients remains higher despite improvements in treatments, survival
Bone lesions are one of the primary symptoms in multiple myeloma (MM), and approximately 80% of patients experience a pathological fracture at initial presentation or during the course of the disease, according to the authors of a study published online in Bone.
The authors performed a study to determine if improved treatment strategies and supportive care over time, including the use of bisphosphonates, reduced the overall fracture risk in MM patients.
Their retrospective case-control study included 1,334 patients with MM in Denmark from the Danish National Health Service, of which 881 sustained a fracture between 1996 and 2011. MM patients were matched to patients from the database without MM.
The researchers found that the risk of any fracture was significantly higher in MM patients, compared with patients without MM at all three time periods examined, and remained high over time: adjusted odds ratio (95% confidence interval) 1996-2000: 1.7 (1.3–2.3); 2001-2006: 1.3 (1.1-1.6); 2007-2011: 1.7 (1.4-2.2)). The risk of vertebral fractures was particularly higher in MM patients and also remained high over time: ORadj (95% CI) 1996-2000: 3.5 (1.4–8.6); 2001-2006: 4.0 (1.9-8.2); 2007-2011: 3.0 (1.6-5.7).
In addition, they found that the risk of any fracture was equally high in men and women MM patients, and in patients aged ≤ 65 or > 65 years.
“New treatment strategies, even if they have a positive impact on overall survival, offer no guarantee for a corresponding reduction in bone lesions,” the authors concluded.
The majority of authors reported having no disclosures. One author reported grants and personal fees from three pharmaceutical companies, outside the submitted work.
SOURCE: Oortgiesen BE et al. Bone. 2020 doi.org/10.1016/j.bone.2020.115299.
Bone lesions are one of the primary symptoms in multiple myeloma (MM), and approximately 80% of patients experience a pathological fracture at initial presentation or during the course of the disease, according to the authors of a study published online in Bone.
The authors performed a study to determine if improved treatment strategies and supportive care over time, including the use of bisphosphonates, reduced the overall fracture risk in MM patients.
Their retrospective case-control study included 1,334 patients with MM in Denmark from the Danish National Health Service, of which 881 sustained a fracture between 1996 and 2011. MM patients were matched to patients from the database without MM.
The researchers found that the risk of any fracture was significantly higher in MM patients, compared with patients without MM at all three time periods examined, and remained high over time: adjusted odds ratio (95% confidence interval) 1996-2000: 1.7 (1.3–2.3); 2001-2006: 1.3 (1.1-1.6); 2007-2011: 1.7 (1.4-2.2)). The risk of vertebral fractures was particularly higher in MM patients and also remained high over time: ORadj (95% CI) 1996-2000: 3.5 (1.4–8.6); 2001-2006: 4.0 (1.9-8.2); 2007-2011: 3.0 (1.6-5.7).
In addition, they found that the risk of any fracture was equally high in men and women MM patients, and in patients aged ≤ 65 or > 65 years.
“New treatment strategies, even if they have a positive impact on overall survival, offer no guarantee for a corresponding reduction in bone lesions,” the authors concluded.
The majority of authors reported having no disclosures. One author reported grants and personal fees from three pharmaceutical companies, outside the submitted work.
SOURCE: Oortgiesen BE et al. Bone. 2020 doi.org/10.1016/j.bone.2020.115299.
Bone lesions are one of the primary symptoms in multiple myeloma (MM), and approximately 80% of patients experience a pathological fracture at initial presentation or during the course of the disease, according to the authors of a study published online in Bone.
The authors performed a study to determine if improved treatment strategies and supportive care over time, including the use of bisphosphonates, reduced the overall fracture risk in MM patients.
Their retrospective case-control study included 1,334 patients with MM in Denmark from the Danish National Health Service, of which 881 sustained a fracture between 1996 and 2011. MM patients were matched to patients from the database without MM.
The researchers found that the risk of any fracture was significantly higher in MM patients, compared with patients without MM at all three time periods examined, and remained high over time: adjusted odds ratio (95% confidence interval) 1996-2000: 1.7 (1.3–2.3); 2001-2006: 1.3 (1.1-1.6); 2007-2011: 1.7 (1.4-2.2)). The risk of vertebral fractures was particularly higher in MM patients and also remained high over time: ORadj (95% CI) 1996-2000: 3.5 (1.4–8.6); 2001-2006: 4.0 (1.9-8.2); 2007-2011: 3.0 (1.6-5.7).
In addition, they found that the risk of any fracture was equally high in men and women MM patients, and in patients aged ≤ 65 or > 65 years.
“New treatment strategies, even if they have a positive impact on overall survival, offer no guarantee for a corresponding reduction in bone lesions,” the authors concluded.
The majority of authors reported having no disclosures. One author reported grants and personal fees from three pharmaceutical companies, outside the submitted work.
SOURCE: Oortgiesen BE et al. Bone. 2020 doi.org/10.1016/j.bone.2020.115299.
FROM BONE
Health professionals fight against COVID-19 myths and misinformation
Misinformation about the COVID-19 travels faster than the virus and complicates the job of doctors who are treating those infected and responding to concerns of their other patients.
An array of myths springing up around this disease can be found on the Internet. The main themes appear to be false narratives about the origin of the virus, the size of the outbreak in the United States and in other countries, the availability of cures and treatments, and ways to prevent infection. Widespread misinformation hampers public health efforts to control the disease outbreak, confuses the public, and requires medical professionals to spend time refuting myths and re-educating patients.
A group of infectious disease experts became so alarmed by the misinformation trend they published a statement in The Lancet decrying the spread of false statements being circulated by some media outlets. “The rapid, open, and transparent sharing of data on this outbreak is now being threatened by rumours and misinformation ... Conspiracy theories do nothing but create fear, rumours, and prejudice that jeopardise our global collaboration in the fight against this virus,” wrote Charles H. Calisher, PhD, of Colorado State University, Fort Collins, and colleagues.
What can physicians do to counter misinformation?
Pulmonologist and critical care physician Cedric “Jamie” Rutland, MD, who practices in Riverside, Calif., sees misinformation about the novel coronavirus every day at home and on the job. His patients worry that everyone who gets infected will die or end up in the ICU. His neighbors ask him to pilfer surgical masks to protect them from the false notion that Chinese people in their community posed some kind of COVID-19 risk.
As he pondered how to counter myths with facts, Dr. Rutland turned to an unusual resource: His 7-year-old daughter Amelia. He explained to her how COVID-19 works and found that she could easily understand the basics. Now, Dr. Rutland draws upon the lessons from chats with his daughter as he explains COVID-19 to his patient audience on his YouTube channel “Medicine Deconstructed.” Simplicity, but not too much simplicity, is key, he said. Dr. Rutland uses a visual aid – a rough drawing of a virus – and shows how inflammation and antibodies enter the picture after infection. “I just teach them that if you’re a healthy person, this is how the body works, and this is what the immune system will do,” he said. “For the most part, you can calm people down when you make time for education.”
What are best practices? In a series of interviews, specialists emphasized the importance of fact-finding, wide-ranging communication, and – perhaps most difficult of all – humility.
Dr. Rutland emphasizes thoughtful communication based on facts and humility when communicating to patients about this potential health risk. “A lot of people finish medical school and think, ‘Everyone should trust me because I’m the pulmonologist or the GI doc.’ That’s not how it works. You still have to earn people’s trust,” he said.
Make sure all staff get reliable information
Hospitals are scrambling to keep staff safe with up-to-date directives and debunk false narratives about the virus. Keeping all hospital staff informed with verified and authoritative facts about the coronavirus is a key objective of the Massachusetts General Hospital’s Center for Disaster Medicine. The Center’s coronavirus educational materials are distributed to all staffers from physicians to janitors. “These provide information that they need to understand the risks and keep themselves safe,” said Eileen Searle, PhD, the Biothreats Clinical Operations program manager in the CDM.
According to Dr. Searle, the hospital keeps a continually updated COVID-19 Frequently Asked Questions document in its internal computer system. All employees can access it, she said, and it’s updated to include questions as they come up.
Even valets and front-desk volunteers are encouraged to read the FAQ, she said, since “they’re the first people that family and patients are interacting with.” The document “gives them reassurance about delivering messages,” she said.
Use patience with your patients
Dr. Rutland urges colleagues to take the time to listen to patients and educate them. “Reduce the gap between you and them,” said Dr. Rutland, who treats patients in Orange and Riverside counties. “Take off your white coat, sit down, and talk to the person about their concerns.”
Boston cardiologist Haider Warraich, MD, of Brigham and Women’s Hospital, Boston, said it’s important to “put medical information into a greater human context.” For example, he has told patients that he’s still taking his daughter to school despite COVID-19 risks. “I take the information I provide and apply it to my own life,” he said.
The Washington State Department of Health offers this advice to physicians to counter false information and stigma: “Stay updated and informed on COVID-19 to avoid miscommunication or inaccurate information. Talk openly about the harm of stigma. View people directly impacted by stigma as people first. Be conscious of your language. Acknowledge access and language barriers.”
Speak out on social media – but don’t fight
Should medical professionals speak out about COVID-19 misinformation via social media? It’s an individual decision, Dr. Warraich said, “but my sense is that it’s never been more important for physicians to be part of the fray and help quell the epidemic of misinformation that almost always follows any type of medial calamity.”
Dr. Rutland, vice president and founding member of the Association for Healthcare Social Media, cautioned that effective communication via social media requires care. Avoid confrontation, he advised. “Don’t call people stupid or say things like, ‘I went to medical school and I’m smarter than you.’ ”
Instead, he said, “it’s important to just state the facts: These are the people who are dying, these are the people who are getting infected.”
And, he added, remember to push the most important message of all: Wash your hands!
Public health organizations fight the ‘infodemic’
In a trend that hearkens back to the days of snake oil cures for all maladies, advertisements for fake treatments are popping up on the Internet and on other media.
Facebook and Amazon have acted to remove these ads but these messages continue to flood social media such as Twitter, WhatsApp, and other sites. Discussion groups on platforms such as Reddit continue to pump out misinformation about COVID-19. Conspiracy theories that link the virus to espionage and bioweapons are making the rounds on the Internet and talk radio. Wrong information about the effectiveness of non-N95 face masks to protect wearers against infection is widespread, leading to shortages for medical personnel and price gouging. Pernicious rumors about the effectiveness of substances such a vinegar, silver, garlic, lemon juice, and even vodka to disinfect hands and surfaces abound on the Internet. An especially dangerous stream of misinformation stigmatizes ethnic groups and individuals as sources of the infection.
The World Health Organization identified early in the COVID-19 outbreak the global wave of misinformation about the virus and dubbed the problem the “infodemic.” The WHO “Q & A” page on COVID-19 is updated frequently and addresses myths and rumors currently circulating.
According to the WHO website, the agency has reached out to social media players such as Facebook, Twitter, Instagram, LinkedIn, Pinterest, TikTok, and Weibo, the microblogging site in China. WHO has worked with these sites to curb the “infodemic” of misinformation and has used these sites for public education outreach on COVID-19. “Myth busting” infographics posted on a WHO web page are also reposted on major social media sites.
The CDC has followed with its own “frequently asked questions” page to address questions and rumors. State health agencies have put up COVID-19 pages to address public concerns and offer advice on prevention. The Maryland Department of Health web page directly addresses dangerous misinformation: “Do not stigmatize people of any specific ethnicities or racial background. Viruses do not target people from specific populations, ethnicities or racial backgrounds. Stay informed and seek information from reliable, official sources. Be wary of myths, rumors and misinformation circulating online and elsewhere. Health information shared through social media is frequently inaccurate, unless coming from an official, reliable source such as the CDC, MDH or local health departments.”
The Washington State Department of Health has taken a more assertive stance on stigma. The COVID-19 web page recommends to the public: “Show compassion and support for individuals and communities more closely impacted. Avoid stigmatizing people who are in quarantine. They are making the right choice for their communities. Do not make assumptions about someone’s health status based on their ethnicity, race or national origin.”
Misinformation about the COVID-19 travels faster than the virus and complicates the job of doctors who are treating those infected and responding to concerns of their other patients.
An array of myths springing up around this disease can be found on the Internet. The main themes appear to be false narratives about the origin of the virus, the size of the outbreak in the United States and in other countries, the availability of cures and treatments, and ways to prevent infection. Widespread misinformation hampers public health efforts to control the disease outbreak, confuses the public, and requires medical professionals to spend time refuting myths and re-educating patients.
A group of infectious disease experts became so alarmed by the misinformation trend they published a statement in The Lancet decrying the spread of false statements being circulated by some media outlets. “The rapid, open, and transparent sharing of data on this outbreak is now being threatened by rumours and misinformation ... Conspiracy theories do nothing but create fear, rumours, and prejudice that jeopardise our global collaboration in the fight against this virus,” wrote Charles H. Calisher, PhD, of Colorado State University, Fort Collins, and colleagues.
What can physicians do to counter misinformation?
Pulmonologist and critical care physician Cedric “Jamie” Rutland, MD, who practices in Riverside, Calif., sees misinformation about the novel coronavirus every day at home and on the job. His patients worry that everyone who gets infected will die or end up in the ICU. His neighbors ask him to pilfer surgical masks to protect them from the false notion that Chinese people in their community posed some kind of COVID-19 risk.
As he pondered how to counter myths with facts, Dr. Rutland turned to an unusual resource: His 7-year-old daughter Amelia. He explained to her how COVID-19 works and found that she could easily understand the basics. Now, Dr. Rutland draws upon the lessons from chats with his daughter as he explains COVID-19 to his patient audience on his YouTube channel “Medicine Deconstructed.” Simplicity, but not too much simplicity, is key, he said. Dr. Rutland uses a visual aid – a rough drawing of a virus – and shows how inflammation and antibodies enter the picture after infection. “I just teach them that if you’re a healthy person, this is how the body works, and this is what the immune system will do,” he said. “For the most part, you can calm people down when you make time for education.”
What are best practices? In a series of interviews, specialists emphasized the importance of fact-finding, wide-ranging communication, and – perhaps most difficult of all – humility.
Dr. Rutland emphasizes thoughtful communication based on facts and humility when communicating to patients about this potential health risk. “A lot of people finish medical school and think, ‘Everyone should trust me because I’m the pulmonologist or the GI doc.’ That’s not how it works. You still have to earn people’s trust,” he said.
Make sure all staff get reliable information
Hospitals are scrambling to keep staff safe with up-to-date directives and debunk false narratives about the virus. Keeping all hospital staff informed with verified and authoritative facts about the coronavirus is a key objective of the Massachusetts General Hospital’s Center for Disaster Medicine. The Center’s coronavirus educational materials are distributed to all staffers from physicians to janitors. “These provide information that they need to understand the risks and keep themselves safe,” said Eileen Searle, PhD, the Biothreats Clinical Operations program manager in the CDM.
According to Dr. Searle, the hospital keeps a continually updated COVID-19 Frequently Asked Questions document in its internal computer system. All employees can access it, she said, and it’s updated to include questions as they come up.
Even valets and front-desk volunteers are encouraged to read the FAQ, she said, since “they’re the first people that family and patients are interacting with.” The document “gives them reassurance about delivering messages,” she said.
Use patience with your patients
Dr. Rutland urges colleagues to take the time to listen to patients and educate them. “Reduce the gap between you and them,” said Dr. Rutland, who treats patients in Orange and Riverside counties. “Take off your white coat, sit down, and talk to the person about their concerns.”
Boston cardiologist Haider Warraich, MD, of Brigham and Women’s Hospital, Boston, said it’s important to “put medical information into a greater human context.” For example, he has told patients that he’s still taking his daughter to school despite COVID-19 risks. “I take the information I provide and apply it to my own life,” he said.
The Washington State Department of Health offers this advice to physicians to counter false information and stigma: “Stay updated and informed on COVID-19 to avoid miscommunication or inaccurate information. Talk openly about the harm of stigma. View people directly impacted by stigma as people first. Be conscious of your language. Acknowledge access and language barriers.”
Speak out on social media – but don’t fight
Should medical professionals speak out about COVID-19 misinformation via social media? It’s an individual decision, Dr. Warraich said, “but my sense is that it’s never been more important for physicians to be part of the fray and help quell the epidemic of misinformation that almost always follows any type of medial calamity.”
Dr. Rutland, vice president and founding member of the Association for Healthcare Social Media, cautioned that effective communication via social media requires care. Avoid confrontation, he advised. “Don’t call people stupid or say things like, ‘I went to medical school and I’m smarter than you.’ ”
Instead, he said, “it’s important to just state the facts: These are the people who are dying, these are the people who are getting infected.”
And, he added, remember to push the most important message of all: Wash your hands!
Public health organizations fight the ‘infodemic’
In a trend that hearkens back to the days of snake oil cures for all maladies, advertisements for fake treatments are popping up on the Internet and on other media.
Facebook and Amazon have acted to remove these ads but these messages continue to flood social media such as Twitter, WhatsApp, and other sites. Discussion groups on platforms such as Reddit continue to pump out misinformation about COVID-19. Conspiracy theories that link the virus to espionage and bioweapons are making the rounds on the Internet and talk radio. Wrong information about the effectiveness of non-N95 face masks to protect wearers against infection is widespread, leading to shortages for medical personnel and price gouging. Pernicious rumors about the effectiveness of substances such a vinegar, silver, garlic, lemon juice, and even vodka to disinfect hands and surfaces abound on the Internet. An especially dangerous stream of misinformation stigmatizes ethnic groups and individuals as sources of the infection.
The World Health Organization identified early in the COVID-19 outbreak the global wave of misinformation about the virus and dubbed the problem the “infodemic.” The WHO “Q & A” page on COVID-19 is updated frequently and addresses myths and rumors currently circulating.
According to the WHO website, the agency has reached out to social media players such as Facebook, Twitter, Instagram, LinkedIn, Pinterest, TikTok, and Weibo, the microblogging site in China. WHO has worked with these sites to curb the “infodemic” of misinformation and has used these sites for public education outreach on COVID-19. “Myth busting” infographics posted on a WHO web page are also reposted on major social media sites.
The CDC has followed with its own “frequently asked questions” page to address questions and rumors. State health agencies have put up COVID-19 pages to address public concerns and offer advice on prevention. The Maryland Department of Health web page directly addresses dangerous misinformation: “Do not stigmatize people of any specific ethnicities or racial background. Viruses do not target people from specific populations, ethnicities or racial backgrounds. Stay informed and seek information from reliable, official sources. Be wary of myths, rumors and misinformation circulating online and elsewhere. Health information shared through social media is frequently inaccurate, unless coming from an official, reliable source such as the CDC, MDH or local health departments.”
The Washington State Department of Health has taken a more assertive stance on stigma. The COVID-19 web page recommends to the public: “Show compassion and support for individuals and communities more closely impacted. Avoid stigmatizing people who are in quarantine. They are making the right choice for their communities. Do not make assumptions about someone’s health status based on their ethnicity, race or national origin.”
Misinformation about the COVID-19 travels faster than the virus and complicates the job of doctors who are treating those infected and responding to concerns of their other patients.
An array of myths springing up around this disease can be found on the Internet. The main themes appear to be false narratives about the origin of the virus, the size of the outbreak in the United States and in other countries, the availability of cures and treatments, and ways to prevent infection. Widespread misinformation hampers public health efforts to control the disease outbreak, confuses the public, and requires medical professionals to spend time refuting myths and re-educating patients.
A group of infectious disease experts became so alarmed by the misinformation trend they published a statement in The Lancet decrying the spread of false statements being circulated by some media outlets. “The rapid, open, and transparent sharing of data on this outbreak is now being threatened by rumours and misinformation ... Conspiracy theories do nothing but create fear, rumours, and prejudice that jeopardise our global collaboration in the fight against this virus,” wrote Charles H. Calisher, PhD, of Colorado State University, Fort Collins, and colleagues.
What can physicians do to counter misinformation?
Pulmonologist and critical care physician Cedric “Jamie” Rutland, MD, who practices in Riverside, Calif., sees misinformation about the novel coronavirus every day at home and on the job. His patients worry that everyone who gets infected will die or end up in the ICU. His neighbors ask him to pilfer surgical masks to protect them from the false notion that Chinese people in their community posed some kind of COVID-19 risk.
As he pondered how to counter myths with facts, Dr. Rutland turned to an unusual resource: His 7-year-old daughter Amelia. He explained to her how COVID-19 works and found that she could easily understand the basics. Now, Dr. Rutland draws upon the lessons from chats with his daughter as he explains COVID-19 to his patient audience on his YouTube channel “Medicine Deconstructed.” Simplicity, but not too much simplicity, is key, he said. Dr. Rutland uses a visual aid – a rough drawing of a virus – and shows how inflammation and antibodies enter the picture after infection. “I just teach them that if you’re a healthy person, this is how the body works, and this is what the immune system will do,” he said. “For the most part, you can calm people down when you make time for education.”
What are best practices? In a series of interviews, specialists emphasized the importance of fact-finding, wide-ranging communication, and – perhaps most difficult of all – humility.
Dr. Rutland emphasizes thoughtful communication based on facts and humility when communicating to patients about this potential health risk. “A lot of people finish medical school and think, ‘Everyone should trust me because I’m the pulmonologist or the GI doc.’ That’s not how it works. You still have to earn people’s trust,” he said.
Make sure all staff get reliable information
Hospitals are scrambling to keep staff safe with up-to-date directives and debunk false narratives about the virus. Keeping all hospital staff informed with verified and authoritative facts about the coronavirus is a key objective of the Massachusetts General Hospital’s Center for Disaster Medicine. The Center’s coronavirus educational materials are distributed to all staffers from physicians to janitors. “These provide information that they need to understand the risks and keep themselves safe,” said Eileen Searle, PhD, the Biothreats Clinical Operations program manager in the CDM.
According to Dr. Searle, the hospital keeps a continually updated COVID-19 Frequently Asked Questions document in its internal computer system. All employees can access it, she said, and it’s updated to include questions as they come up.
Even valets and front-desk volunteers are encouraged to read the FAQ, she said, since “they’re the first people that family and patients are interacting with.” The document “gives them reassurance about delivering messages,” she said.
Use patience with your patients
Dr. Rutland urges colleagues to take the time to listen to patients and educate them. “Reduce the gap between you and them,” said Dr. Rutland, who treats patients in Orange and Riverside counties. “Take off your white coat, sit down, and talk to the person about their concerns.”
Boston cardiologist Haider Warraich, MD, of Brigham and Women’s Hospital, Boston, said it’s important to “put medical information into a greater human context.” For example, he has told patients that he’s still taking his daughter to school despite COVID-19 risks. “I take the information I provide and apply it to my own life,” he said.
The Washington State Department of Health offers this advice to physicians to counter false information and stigma: “Stay updated and informed on COVID-19 to avoid miscommunication or inaccurate information. Talk openly about the harm of stigma. View people directly impacted by stigma as people first. Be conscious of your language. Acknowledge access and language barriers.”
Speak out on social media – but don’t fight
Should medical professionals speak out about COVID-19 misinformation via social media? It’s an individual decision, Dr. Warraich said, “but my sense is that it’s never been more important for physicians to be part of the fray and help quell the epidemic of misinformation that almost always follows any type of medial calamity.”
Dr. Rutland, vice president and founding member of the Association for Healthcare Social Media, cautioned that effective communication via social media requires care. Avoid confrontation, he advised. “Don’t call people stupid or say things like, ‘I went to medical school and I’m smarter than you.’ ”
Instead, he said, “it’s important to just state the facts: These are the people who are dying, these are the people who are getting infected.”
And, he added, remember to push the most important message of all: Wash your hands!
Public health organizations fight the ‘infodemic’
In a trend that hearkens back to the days of snake oil cures for all maladies, advertisements for fake treatments are popping up on the Internet and on other media.
Facebook and Amazon have acted to remove these ads but these messages continue to flood social media such as Twitter, WhatsApp, and other sites. Discussion groups on platforms such as Reddit continue to pump out misinformation about COVID-19. Conspiracy theories that link the virus to espionage and bioweapons are making the rounds on the Internet and talk radio. Wrong information about the effectiveness of non-N95 face masks to protect wearers against infection is widespread, leading to shortages for medical personnel and price gouging. Pernicious rumors about the effectiveness of substances such a vinegar, silver, garlic, lemon juice, and even vodka to disinfect hands and surfaces abound on the Internet. An especially dangerous stream of misinformation stigmatizes ethnic groups and individuals as sources of the infection.
The World Health Organization identified early in the COVID-19 outbreak the global wave of misinformation about the virus and dubbed the problem the “infodemic.” The WHO “Q & A” page on COVID-19 is updated frequently and addresses myths and rumors currently circulating.
According to the WHO website, the agency has reached out to social media players such as Facebook, Twitter, Instagram, LinkedIn, Pinterest, TikTok, and Weibo, the microblogging site in China. WHO has worked with these sites to curb the “infodemic” of misinformation and has used these sites for public education outreach on COVID-19. “Myth busting” infographics posted on a WHO web page are also reposted on major social media sites.
The CDC has followed with its own “frequently asked questions” page to address questions and rumors. State health agencies have put up COVID-19 pages to address public concerns and offer advice on prevention. The Maryland Department of Health web page directly addresses dangerous misinformation: “Do not stigmatize people of any specific ethnicities or racial background. Viruses do not target people from specific populations, ethnicities or racial backgrounds. Stay informed and seek information from reliable, official sources. Be wary of myths, rumors and misinformation circulating online and elsewhere. Health information shared through social media is frequently inaccurate, unless coming from an official, reliable source such as the CDC, MDH or local health departments.”
The Washington State Department of Health has taken a more assertive stance on stigma. The COVID-19 web page recommends to the public: “Show compassion and support for individuals and communities more closely impacted. Avoid stigmatizing people who are in quarantine. They are making the right choice for their communities. Do not make assumptions about someone’s health status based on their ethnicity, race or national origin.”
Cancer mortality continues to decline while cancer incidence rises in women
according to the Annual Report to the Nation on the Status of Cancer.
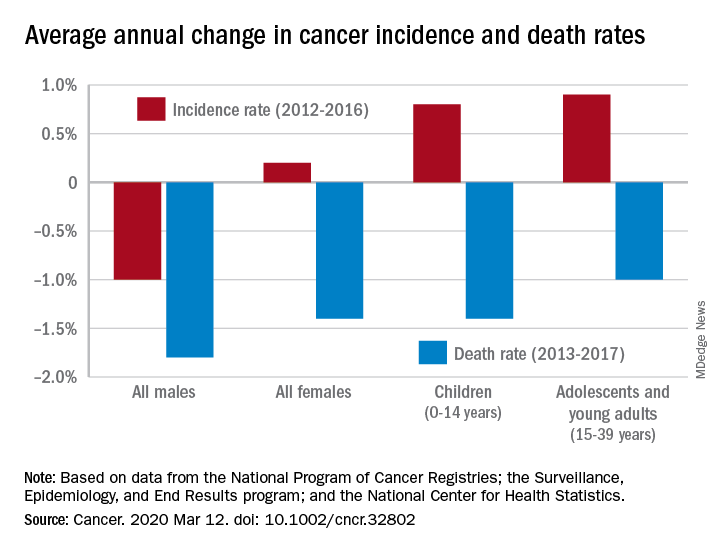
During 2013-2017, the overall age-standardized death rate for all cancers was 158.2 per 100,000 population, and the average decline over that period was 1.5% per year. The average annual change was greater for men (–1.8%) than women (–1.4%) for 2013-2017, but the death rate was higher for men (189.3 per 100,000 vs. 135.5 per 100,000) for those years, S. Jane Henley of the Centers for Disease Control and Prevention and associates reported in Cancer.
“The drops in mortality we’re seeing are real, sustained, and a strong indication of what we can do when we work to prevent and treat cancer,” William G. Cance, MD, chief medical and scientific officer of the America Cancer Society, said in a written statement accompanying the report.
Overall cancer incidence for the most recent 5-year period (2012-2016) was 447.9 per 100,000, with rates of 487.9 for men and 421.4 for women, the investigators said.
Incidence dropped by 0.6% per year overall, but that hides a major difference between men, who saw a decrease of 1.0% a year, and women, who experienced an annual increase of 0.2%.
Over those 5 years, cancer incidence also increased by 0.8% annually among children aged 0-14 years and by 0.9% in adolescents and young adults aged 15-39 years, Ms. Henley and associates said in the report, which is a collaborative effort between the CDC, the National Cancer Institute, the American Cancer Society, and the North American Association of Central Cancer Registries.
“[W]e must not be complacent. The cancer incidence data – especially the increase in cancer among women – is a clear reminder that there is more work ahead,” Norman E. Sharpless, MD, director of the National Cancer Institute, said in the accompanying statement.
SOURCE: Henley SJ et al. Cancer. 2020 Mar 12. doi: 10.1002/cncr.32802.
according to the Annual Report to the Nation on the Status of Cancer.

During 2013-2017, the overall age-standardized death rate for all cancers was 158.2 per 100,000 population, and the average decline over that period was 1.5% per year. The average annual change was greater for men (–1.8%) than women (–1.4%) for 2013-2017, but the death rate was higher for men (189.3 per 100,000 vs. 135.5 per 100,000) for those years, S. Jane Henley of the Centers for Disease Control and Prevention and associates reported in Cancer.
“The drops in mortality we’re seeing are real, sustained, and a strong indication of what we can do when we work to prevent and treat cancer,” William G. Cance, MD, chief medical and scientific officer of the America Cancer Society, said in a written statement accompanying the report.
Overall cancer incidence for the most recent 5-year period (2012-2016) was 447.9 per 100,000, with rates of 487.9 for men and 421.4 for women, the investigators said.
Incidence dropped by 0.6% per year overall, but that hides a major difference between men, who saw a decrease of 1.0% a year, and women, who experienced an annual increase of 0.2%.
Over those 5 years, cancer incidence also increased by 0.8% annually among children aged 0-14 years and by 0.9% in adolescents and young adults aged 15-39 years, Ms. Henley and associates said in the report, which is a collaborative effort between the CDC, the National Cancer Institute, the American Cancer Society, and the North American Association of Central Cancer Registries.
“[W]e must not be complacent. The cancer incidence data – especially the increase in cancer among women – is a clear reminder that there is more work ahead,” Norman E. Sharpless, MD, director of the National Cancer Institute, said in the accompanying statement.
SOURCE: Henley SJ et al. Cancer. 2020 Mar 12. doi: 10.1002/cncr.32802.
according to the Annual Report to the Nation on the Status of Cancer.

During 2013-2017, the overall age-standardized death rate for all cancers was 158.2 per 100,000 population, and the average decline over that period was 1.5% per year. The average annual change was greater for men (–1.8%) than women (–1.4%) for 2013-2017, but the death rate was higher for men (189.3 per 100,000 vs. 135.5 per 100,000) for those years, S. Jane Henley of the Centers for Disease Control and Prevention and associates reported in Cancer.
“The drops in mortality we’re seeing are real, sustained, and a strong indication of what we can do when we work to prevent and treat cancer,” William G. Cance, MD, chief medical and scientific officer of the America Cancer Society, said in a written statement accompanying the report.
Overall cancer incidence for the most recent 5-year period (2012-2016) was 447.9 per 100,000, with rates of 487.9 for men and 421.4 for women, the investigators said.
Incidence dropped by 0.6% per year overall, but that hides a major difference between men, who saw a decrease of 1.0% a year, and women, who experienced an annual increase of 0.2%.
Over those 5 years, cancer incidence also increased by 0.8% annually among children aged 0-14 years and by 0.9% in adolescents and young adults aged 15-39 years, Ms. Henley and associates said in the report, which is a collaborative effort between the CDC, the National Cancer Institute, the American Cancer Society, and the North American Association of Central Cancer Registries.
“[W]e must not be complacent. The cancer incidence data – especially the increase in cancer among women – is a clear reminder that there is more work ahead,” Norman E. Sharpless, MD, director of the National Cancer Institute, said in the accompanying statement.
SOURCE: Henley SJ et al. Cancer. 2020 Mar 12. doi: 10.1002/cncr.32802.
FROM CANCER
FDA to revise safety evaluation of type 2 diabetes drugs
The US Food and Drug Administration (FDA) has issued new draft guidance for industry on evaluating the safety of new drugs for type 2 diabetes and removed the “outdated” 12-year-old requirement for standardized cardiovascular outcomes trials (CVOTs).
The new draft guidance, “Type 2 Diabetes Mellitus: Evaluating the Safety of New Drugs for Improving Glycemic Control,” will replace the December 2008 requirement that manufacturers conduct CVOTs to rule out unacceptable cardiovascular safety risk. That move followed concerns raised at the time about the thiazolidinedione class of glucose-lowering drugs.
Since then, “FDA has reviewed the results of several [CVOTs] conducted to meet the December 2008 guidance recommendations. None of the CVOTs to date have identified an increased risk of ischemic cardiovascular events; some of the CVOTs have instead demonstrated a reduced risk for cardiovascular events,” according to the federal register announcement.
In October 2018, the FDA’s Endocrinologic and Metabolic Drugs Advisory Committee narrowly voted (10 to 9) to continue requiring CVOTs, but most panel members also recommended some changes to them, including requirements for safety data beyond cardiovascular events.
Based on the CVOT results over the years and the panel’s recommendations, “FDA is revisiting the recommendations of the December 2008 guidance and is now proposing an updated approach to evaluating the safety of new drugs and biologics to improve glycemic control.”
“The new draft guidance does not contain the recommendation that sponsors of all new therapies for type 2 diabetes uniformly rule out a specific degree of risk for ischemic cardiovascular adverse outcomes,” the FDA said.
Instead, the draft calls for at least 4000 patient-years of exposure to the new drug in phase 3 trials and inclusion of study participants with comorbid conditions and/or diabetes complications, including at least 500 with stage 3-4 chronic kidney disease, 600 with established cardiovascular disease, and at least 600 over the age of 65 years.
The FDA is soliciting stakeholder input on these and other issues, including study duration, subject demographics, specific safety concerns, and event adjudication.
In a statement, Lisa Yanoff, MD, acting director of the Division for Metabolism and Endocrinology Products in the FDA’s Center for Drug Evaluation and Research, said: “By following previous FDA recommendations, sponsors have shown that new type 2 diabetes drugs do not have excess ischemic cardiovascular risk, which has provided reassuring cardiovascular safety information for millions of diabetes patients. Now, with this proposed approach, we will have broader, valuable safety information for these medications.”
The draft is open for comments for 90 days after March 9, 2020. It is available online, along with a link for submitting comments.
This article first appeared on Medscape.com.
The US Food and Drug Administration (FDA) has issued new draft guidance for industry on evaluating the safety of new drugs for type 2 diabetes and removed the “outdated” 12-year-old requirement for standardized cardiovascular outcomes trials (CVOTs).
The new draft guidance, “Type 2 Diabetes Mellitus: Evaluating the Safety of New Drugs for Improving Glycemic Control,” will replace the December 2008 requirement that manufacturers conduct CVOTs to rule out unacceptable cardiovascular safety risk. That move followed concerns raised at the time about the thiazolidinedione class of glucose-lowering drugs.
Since then, “FDA has reviewed the results of several [CVOTs] conducted to meet the December 2008 guidance recommendations. None of the CVOTs to date have identified an increased risk of ischemic cardiovascular events; some of the CVOTs have instead demonstrated a reduced risk for cardiovascular events,” according to the federal register announcement.
In October 2018, the FDA’s Endocrinologic and Metabolic Drugs Advisory Committee narrowly voted (10 to 9) to continue requiring CVOTs, but most panel members also recommended some changes to them, including requirements for safety data beyond cardiovascular events.
Based on the CVOT results over the years and the panel’s recommendations, “FDA is revisiting the recommendations of the December 2008 guidance and is now proposing an updated approach to evaluating the safety of new drugs and biologics to improve glycemic control.”
“The new draft guidance does not contain the recommendation that sponsors of all new therapies for type 2 diabetes uniformly rule out a specific degree of risk for ischemic cardiovascular adverse outcomes,” the FDA said.
Instead, the draft calls for at least 4000 patient-years of exposure to the new drug in phase 3 trials and inclusion of study participants with comorbid conditions and/or diabetes complications, including at least 500 with stage 3-4 chronic kidney disease, 600 with established cardiovascular disease, and at least 600 over the age of 65 years.
The FDA is soliciting stakeholder input on these and other issues, including study duration, subject demographics, specific safety concerns, and event adjudication.
In a statement, Lisa Yanoff, MD, acting director of the Division for Metabolism and Endocrinology Products in the FDA’s Center for Drug Evaluation and Research, said: “By following previous FDA recommendations, sponsors have shown that new type 2 diabetes drugs do not have excess ischemic cardiovascular risk, which has provided reassuring cardiovascular safety information for millions of diabetes patients. Now, with this proposed approach, we will have broader, valuable safety information for these medications.”
The draft is open for comments for 90 days after March 9, 2020. It is available online, along with a link for submitting comments.
This article first appeared on Medscape.com.
The US Food and Drug Administration (FDA) has issued new draft guidance for industry on evaluating the safety of new drugs for type 2 diabetes and removed the “outdated” 12-year-old requirement for standardized cardiovascular outcomes trials (CVOTs).
The new draft guidance, “Type 2 Diabetes Mellitus: Evaluating the Safety of New Drugs for Improving Glycemic Control,” will replace the December 2008 requirement that manufacturers conduct CVOTs to rule out unacceptable cardiovascular safety risk. That move followed concerns raised at the time about the thiazolidinedione class of glucose-lowering drugs.
Since then, “FDA has reviewed the results of several [CVOTs] conducted to meet the December 2008 guidance recommendations. None of the CVOTs to date have identified an increased risk of ischemic cardiovascular events; some of the CVOTs have instead demonstrated a reduced risk for cardiovascular events,” according to the federal register announcement.
In October 2018, the FDA’s Endocrinologic and Metabolic Drugs Advisory Committee narrowly voted (10 to 9) to continue requiring CVOTs, but most panel members also recommended some changes to them, including requirements for safety data beyond cardiovascular events.
Based on the CVOT results over the years and the panel’s recommendations, “FDA is revisiting the recommendations of the December 2008 guidance and is now proposing an updated approach to evaluating the safety of new drugs and biologics to improve glycemic control.”
“The new draft guidance does not contain the recommendation that sponsors of all new therapies for type 2 diabetes uniformly rule out a specific degree of risk for ischemic cardiovascular adverse outcomes,” the FDA said.
Instead, the draft calls for at least 4000 patient-years of exposure to the new drug in phase 3 trials and inclusion of study participants with comorbid conditions and/or diabetes complications, including at least 500 with stage 3-4 chronic kidney disease, 600 with established cardiovascular disease, and at least 600 over the age of 65 years.
The FDA is soliciting stakeholder input on these and other issues, including study duration, subject demographics, specific safety concerns, and event adjudication.
In a statement, Lisa Yanoff, MD, acting director of the Division for Metabolism and Endocrinology Products in the FDA’s Center for Drug Evaluation and Research, said: “By following previous FDA recommendations, sponsors have shown that new type 2 diabetes drugs do not have excess ischemic cardiovascular risk, which has provided reassuring cardiovascular safety information for millions of diabetes patients. Now, with this proposed approach, we will have broader, valuable safety information for these medications.”
The draft is open for comments for 90 days after March 9, 2020. It is available online, along with a link for submitting comments.
This article first appeared on Medscape.com.