User login
American Academy of Neurology cancels annual meeting amid COVID-19 pandemic
“Protecting the health, safety, and well-being of our members, attendees, and ultimately our neurology patients is paramount, and serves as the reason for our decision to cancel the AAN annual meeting for the first time in our 72-year history,” AAN President James Stevens, MD, said in a statement. “Put simply, canceling the AAN annual meeting is the right thing to do during this historic time.”
Dr. Stevens added that it is “important to keep our members in their communities – where you stand by to help patients during this time of uncertainty. We also have a professional responsibility to model social distancing and not contribute to the spread of the virus through a large public gathering.”
AAN said it is currently processing full registration fee refunds for those who had registered to attend. Information for exhibitors and sponsors will be forthcoming.
As for missed CME opportunities related to attending the annual meeting, AAN will provide different educational opportunities throughout the remainder of 2020.
Further questions should be directed via email to memberservices@aan.com. Additional information related to the cancellation will be posted to the AAN website and via social media.
“Protecting the health, safety, and well-being of our members, attendees, and ultimately our neurology patients is paramount, and serves as the reason for our decision to cancel the AAN annual meeting for the first time in our 72-year history,” AAN President James Stevens, MD, said in a statement. “Put simply, canceling the AAN annual meeting is the right thing to do during this historic time.”
Dr. Stevens added that it is “important to keep our members in their communities – where you stand by to help patients during this time of uncertainty. We also have a professional responsibility to model social distancing and not contribute to the spread of the virus through a large public gathering.”
AAN said it is currently processing full registration fee refunds for those who had registered to attend. Information for exhibitors and sponsors will be forthcoming.
As for missed CME opportunities related to attending the annual meeting, AAN will provide different educational opportunities throughout the remainder of 2020.
Further questions should be directed via email to memberservices@aan.com. Additional information related to the cancellation will be posted to the AAN website and via social media.
“Protecting the health, safety, and well-being of our members, attendees, and ultimately our neurology patients is paramount, and serves as the reason for our decision to cancel the AAN annual meeting for the first time in our 72-year history,” AAN President James Stevens, MD, said in a statement. “Put simply, canceling the AAN annual meeting is the right thing to do during this historic time.”
Dr. Stevens added that it is “important to keep our members in their communities – where you stand by to help patients during this time of uncertainty. We also have a professional responsibility to model social distancing and not contribute to the spread of the virus through a large public gathering.”
AAN said it is currently processing full registration fee refunds for those who had registered to attend. Information for exhibitors and sponsors will be forthcoming.
As for missed CME opportunities related to attending the annual meeting, AAN will provide different educational opportunities throughout the remainder of 2020.
Further questions should be directed via email to memberservices@aan.com. Additional information related to the cancellation will be posted to the AAN website and via social media.
Expert says progress in gut-brain research requires an open mind
A growing body of research links the gut with the brain and behavior, but compartmentalization within the medical community may be slowing investigation of the gut-brain axis, according to a leading expert.
Studies have shown that the microbiome may influence a diverse range of behavioral and neurological processes, from acute and chronic stress responses to development of Parkinson’s and Alzheimer’s disease, reported John F. Cryan, PhD, of University College Cork, Ireland.
Dr. Cryan began his presentation at the annual Gut Microbiota for Health World Summit by citing Hippocrates, who is thought to have stated that all diseases begin in the gut.
“That can be quite strange when I talk to my neurology or psychiatry colleagues,” Dr. Cryan said. “They sometimes look at me like I have two heads. Because in medicine we compartmentalize, and if you are studying neurology or psychiatry or [you are] in clinical practice, you are focusing on everything from the neck upwards.”
For more than a decade, Dr. Cryan and colleagues have been investigating the gut-brain axis, predominantly in mouse models, but also across animal species and in humans.
At the meeting, sponsored by the American Gastroenterological Association and the European Society for Neurogastroenterology and Motility, Dr. Cryan reviewed a variety of representative studies.
For instance, in both mice and humans, research has shown that C-section, which is associated with poorer microbiome diversity than vaginal delivery, has also been linked with social deficits and elevated stress responses. And in the case of mice, coprophagia, in which cesarean-delivered mice eat the feces of vaginally born mice, has been shown to ameliorate these psychiatric effects.
Dr. Cryan likened this process to an “artificial fecal transplant.”
“You know, co-housing and eating each other’s poo is not the translational approach that we were advocating by any means,” Dr. Cryan said. “But at least it tells us – in a proof-of-concept way – that if we change the microbiome, then we can reverse what’s going on.”
While the mechanisms behind the gut-brain axis remain incompletely understood, Dr. Cryan noted that the vagus nerve, which travels from the gut to the brain, plays a central role, and that transecting this nerve in mice stops the microbiome from affecting the brain.
“What happens in vagus doesn’t just stay in vagus, but will actually affect our emotions in different ways,” Dr. Cryan said.
He emphasized that communication travels both ways along the gut-brain axis, and went on to describe how this phenomenon has been demonstrated across a wide array of animals.
“From insects all the way through to primates, if you start to interfere with social behavior, you change the microbiome,” Dr. Cryan said. “But the opposite is also true; if you start to change the microbiome you can start to have widespread effects on social behavior.”
In humans, manipulating the microbiome could open up new psychiatric frontiers, Dr. Cryan said.
“[In the past 30 years], there really have been no real advances in how we manage mental health,” he said. “That’s very sobering when we are having such a mental health problem across all ages right now. And so perhaps it’s time for what we’ve coined the ‘psychobiotic revolution’ – time for a new way of thinking about mental health.”
According to Dr. Cryan, psychobiotics are interventions that target the microbiome for mental health purposes, including fermented foods, probiotics, prebiotics, synbiotics, parabiotics, and postbiotics.
Among these, probiotics have been a focal point of interventional research. Although results have been mixed, Dr. Cryan suggested that negative probiotic studies are more likely due to bacterial strain than a failure of the concept as a whole.
“Most strains of bacteria will do absolutely nothing,” Dr. Cryan said. “Strain is really important.”
In demonstration of this concept, he recounted a 2017 study conducted at University College Cork in which 22 healthy volunteers were given Bifidobacterium longum 1714, and then subjected to a social stress test. The results, published in Translational Psychiatry, showed that the probiotic, compared with placebo, was associated with attenuated stress responses, reduced daily stress, and enhanced visuospatial memory.
In contrast, a similar study by Dr. Cryan and colleagues, which tested Lactobacillus rhamnosus (JB-1), fell short.
“You [could not have gotten] more negative data into one paper if you tried,” Dr. Cryan said, referring to the study. “It did absolutely nothing.”
To find out which psychobiotics may have an impact, and how, Dr. Cryan called for more research.
“It’s still early days,” he said. “We probably have more meta-analyses and systematic reviews of the field than we have primary research papers.
Dr. Cryan concluded his presentation on an optimistic note.
“Neurology is waking up ... to understand that the microbiome could be playing a key role in many, many other disorders. ... Overall, what we’re beginning to see is that our state of gut markedly affects our state of mind.”
Dr. Cryan disclosed relationships with Abbott Nutrition, Roche Pharma, Nutricia, and others.
A growing body of research links the gut with the brain and behavior, but compartmentalization within the medical community may be slowing investigation of the gut-brain axis, according to a leading expert.
Studies have shown that the microbiome may influence a diverse range of behavioral and neurological processes, from acute and chronic stress responses to development of Parkinson’s and Alzheimer’s disease, reported John F. Cryan, PhD, of University College Cork, Ireland.
Dr. Cryan began his presentation at the annual Gut Microbiota for Health World Summit by citing Hippocrates, who is thought to have stated that all diseases begin in the gut.
“That can be quite strange when I talk to my neurology or psychiatry colleagues,” Dr. Cryan said. “They sometimes look at me like I have two heads. Because in medicine we compartmentalize, and if you are studying neurology or psychiatry or [you are] in clinical practice, you are focusing on everything from the neck upwards.”
For more than a decade, Dr. Cryan and colleagues have been investigating the gut-brain axis, predominantly in mouse models, but also across animal species and in humans.
At the meeting, sponsored by the American Gastroenterological Association and the European Society for Neurogastroenterology and Motility, Dr. Cryan reviewed a variety of representative studies.
For instance, in both mice and humans, research has shown that C-section, which is associated with poorer microbiome diversity than vaginal delivery, has also been linked with social deficits and elevated stress responses. And in the case of mice, coprophagia, in which cesarean-delivered mice eat the feces of vaginally born mice, has been shown to ameliorate these psychiatric effects.
Dr. Cryan likened this process to an “artificial fecal transplant.”
“You know, co-housing and eating each other’s poo is not the translational approach that we were advocating by any means,” Dr. Cryan said. “But at least it tells us – in a proof-of-concept way – that if we change the microbiome, then we can reverse what’s going on.”
While the mechanisms behind the gut-brain axis remain incompletely understood, Dr. Cryan noted that the vagus nerve, which travels from the gut to the brain, plays a central role, and that transecting this nerve in mice stops the microbiome from affecting the brain.
“What happens in vagus doesn’t just stay in vagus, but will actually affect our emotions in different ways,” Dr. Cryan said.
He emphasized that communication travels both ways along the gut-brain axis, and went on to describe how this phenomenon has been demonstrated across a wide array of animals.
“From insects all the way through to primates, if you start to interfere with social behavior, you change the microbiome,” Dr. Cryan said. “But the opposite is also true; if you start to change the microbiome you can start to have widespread effects on social behavior.”
In humans, manipulating the microbiome could open up new psychiatric frontiers, Dr. Cryan said.
“[In the past 30 years], there really have been no real advances in how we manage mental health,” he said. “That’s very sobering when we are having such a mental health problem across all ages right now. And so perhaps it’s time for what we’ve coined the ‘psychobiotic revolution’ – time for a new way of thinking about mental health.”
According to Dr. Cryan, psychobiotics are interventions that target the microbiome for mental health purposes, including fermented foods, probiotics, prebiotics, synbiotics, parabiotics, and postbiotics.
Among these, probiotics have been a focal point of interventional research. Although results have been mixed, Dr. Cryan suggested that negative probiotic studies are more likely due to bacterial strain than a failure of the concept as a whole.
“Most strains of bacteria will do absolutely nothing,” Dr. Cryan said. “Strain is really important.”
In demonstration of this concept, he recounted a 2017 study conducted at University College Cork in which 22 healthy volunteers were given Bifidobacterium longum 1714, and then subjected to a social stress test. The results, published in Translational Psychiatry, showed that the probiotic, compared with placebo, was associated with attenuated stress responses, reduced daily stress, and enhanced visuospatial memory.
In contrast, a similar study by Dr. Cryan and colleagues, which tested Lactobacillus rhamnosus (JB-1), fell short.
“You [could not have gotten] more negative data into one paper if you tried,” Dr. Cryan said, referring to the study. “It did absolutely nothing.”
To find out which psychobiotics may have an impact, and how, Dr. Cryan called for more research.
“It’s still early days,” he said. “We probably have more meta-analyses and systematic reviews of the field than we have primary research papers.
Dr. Cryan concluded his presentation on an optimistic note.
“Neurology is waking up ... to understand that the microbiome could be playing a key role in many, many other disorders. ... Overall, what we’re beginning to see is that our state of gut markedly affects our state of mind.”
Dr. Cryan disclosed relationships with Abbott Nutrition, Roche Pharma, Nutricia, and others.
A growing body of research links the gut with the brain and behavior, but compartmentalization within the medical community may be slowing investigation of the gut-brain axis, according to a leading expert.
Studies have shown that the microbiome may influence a diverse range of behavioral and neurological processes, from acute and chronic stress responses to development of Parkinson’s and Alzheimer’s disease, reported John F. Cryan, PhD, of University College Cork, Ireland.
Dr. Cryan began his presentation at the annual Gut Microbiota for Health World Summit by citing Hippocrates, who is thought to have stated that all diseases begin in the gut.
“That can be quite strange when I talk to my neurology or psychiatry colleagues,” Dr. Cryan said. “They sometimes look at me like I have two heads. Because in medicine we compartmentalize, and if you are studying neurology or psychiatry or [you are] in clinical practice, you are focusing on everything from the neck upwards.”
For more than a decade, Dr. Cryan and colleagues have been investigating the gut-brain axis, predominantly in mouse models, but also across animal species and in humans.
At the meeting, sponsored by the American Gastroenterological Association and the European Society for Neurogastroenterology and Motility, Dr. Cryan reviewed a variety of representative studies.
For instance, in both mice and humans, research has shown that C-section, which is associated with poorer microbiome diversity than vaginal delivery, has also been linked with social deficits and elevated stress responses. And in the case of mice, coprophagia, in which cesarean-delivered mice eat the feces of vaginally born mice, has been shown to ameliorate these psychiatric effects.
Dr. Cryan likened this process to an “artificial fecal transplant.”
“You know, co-housing and eating each other’s poo is not the translational approach that we were advocating by any means,” Dr. Cryan said. “But at least it tells us – in a proof-of-concept way – that if we change the microbiome, then we can reverse what’s going on.”
While the mechanisms behind the gut-brain axis remain incompletely understood, Dr. Cryan noted that the vagus nerve, which travels from the gut to the brain, plays a central role, and that transecting this nerve in mice stops the microbiome from affecting the brain.
“What happens in vagus doesn’t just stay in vagus, but will actually affect our emotions in different ways,” Dr. Cryan said.
He emphasized that communication travels both ways along the gut-brain axis, and went on to describe how this phenomenon has been demonstrated across a wide array of animals.
“From insects all the way through to primates, if you start to interfere with social behavior, you change the microbiome,” Dr. Cryan said. “But the opposite is also true; if you start to change the microbiome you can start to have widespread effects on social behavior.”
In humans, manipulating the microbiome could open up new psychiatric frontiers, Dr. Cryan said.
“[In the past 30 years], there really have been no real advances in how we manage mental health,” he said. “That’s very sobering when we are having such a mental health problem across all ages right now. And so perhaps it’s time for what we’ve coined the ‘psychobiotic revolution’ – time for a new way of thinking about mental health.”
According to Dr. Cryan, psychobiotics are interventions that target the microbiome for mental health purposes, including fermented foods, probiotics, prebiotics, synbiotics, parabiotics, and postbiotics.
Among these, probiotics have been a focal point of interventional research. Although results have been mixed, Dr. Cryan suggested that negative probiotic studies are more likely due to bacterial strain than a failure of the concept as a whole.
“Most strains of bacteria will do absolutely nothing,” Dr. Cryan said. “Strain is really important.”
In demonstration of this concept, he recounted a 2017 study conducted at University College Cork in which 22 healthy volunteers were given Bifidobacterium longum 1714, and then subjected to a social stress test. The results, published in Translational Psychiatry, showed that the probiotic, compared with placebo, was associated with attenuated stress responses, reduced daily stress, and enhanced visuospatial memory.
In contrast, a similar study by Dr. Cryan and colleagues, which tested Lactobacillus rhamnosus (JB-1), fell short.
“You [could not have gotten] more negative data into one paper if you tried,” Dr. Cryan said, referring to the study. “It did absolutely nothing.”
To find out which psychobiotics may have an impact, and how, Dr. Cryan called for more research.
“It’s still early days,” he said. “We probably have more meta-analyses and systematic reviews of the field than we have primary research papers.
Dr. Cryan concluded his presentation on an optimistic note.
“Neurology is waking up ... to understand that the microbiome could be playing a key role in many, many other disorders. ... Overall, what we’re beginning to see is that our state of gut markedly affects our state of mind.”
Dr. Cryan disclosed relationships with Abbott Nutrition, Roche Pharma, Nutricia, and others.
FROM GMFH 2020
Real-world data are a wake-up call
In this edition of “Applying research to practice,” I highlight a study revealing real-world information about the clinical care of breast cancer patients with deleterious germline mutations.
While germline testing among breast cancer patients is becoming more commonplace, it isn’t clear how test results influence patient care. To gain some insight, Allison W. Kurian, MD, of Stanford (Calif.) University, and colleagues analyzed data on 20,568 women with stage 0-III breast cancer from the Surveillance, Epidemiology, and End Results (SEER) registries of Georgia and California (JAMA Oncol. 2020 Feb 6. doi: 10.1001/jamaoncol.2019.6400).
The researchers aimed to determine whether women with mutations in breast cancer–associated genes (BRCA1/2 or others) received guideline-concordant care to the same degree as women who lacked deleterious mutations. The authors evaluated guideline concordance with respect to three treatment modalities: surgery (bilateral vs. unilateral mastectomy in women who were eligible for unilateral surgery), radiotherapy after lumpectomy (for women aged less than 70 years with hormonally responsive, ErbB2-negative, stage I cancers), and chemotherapy (among women eligible for consideration of chemotherapy omission)
In alignment with guidelines, many clinicians correctly used genetic test results to guide surgical decisions. For example, 61.7% of women with BRCA mutations underwent bilateral mastectomy, compared with 24.3% who were mutation negative (odds ratio, 5.52). For other pathogenic variants (ATM, CDH1, CHEK2, NBN, NF1, PALB2, PTEN, and TP53), the rate of bilateral mastectomy was still elevated, albeit to a lesser degree (OR, 2.41).
In discord with guidelines, women with BRCA mutations were 78% less likely to receive radiotherapy after lumpectomy (OR, 0.22) and 76% more likely to receive chemotherapy for early-stage, hormone-positive disease (OR, 1.76), suggesting possible trends in under- and overtreatment, respectively. Chemotherapy utilization rates among mutation carriers and noncarriers became more similar after adjustment for clinical and demographic factors.
There are limits on the granularity of the SEER database, such that, if a patient had a mastectomy a year or more after lumpectomy in an effort to avoid radiotherapy, the database would not have reflected that. Clinical factors could have appropriately influenced chemotherapy receipt among patients with mutations, but those additional factors (including patient preference) would not be included in the SEER data.
The authors concluded that research should be conducted to confirm the results of this retrospective, population-based cohort analysis, in an effort to understand the decision-making process and consequences for long-term outcome.
How these findings should influence practice
With every new development, there are challenges – some expected, some unanticipated.
It is now feasible to obtain multigene panel testing reasonably inexpensively. There are concerns about undertesting of patients on the basis of family history alone. And some major professional organizations have endorsed routine gene panel testing for all breast cancer patients.
As a consequence of these factors, genetic test results are routinely available to clinicians who may lack formal training in clinical genetics. Whether these results influence the receipt of evidence-based clinical care is uncertain.
The information published by Dr. Kurian and colleagues is inherently limited by the methodology of a SEER database review. Among other limitations, as the authors comment:
- The genetic test results could have arrived after treatment decisions were made.
- Treatment delivered more than a year after diagnosis would not have been captured.
- There was selection of patients for genetic testing.
- There were few patients with particular germline mutations other than BRCA1/2 on whom to judge whether treatment was guideline concordant.
- The rationale for the treatment choices made by physicians and patients was not available.
- Impact of treatment choices on survival for carriers of deleterious mutations is uncertain.
Nonetheless, these data suggest a need to redouble efforts to educate patients, their family members, and health care professionals about evidence-based guidelines for care and the rationale for those recommendations.
Careful, prospective monitoring of any resultant differences in treatment outcome in patients treated with guideline-concordant and nonconcordant care is needed. When treatment choices appear to systematically deviate from published guidelines with no obvious rationale, it is a wake-up call for all of us.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations.
In this edition of “Applying research to practice,” I highlight a study revealing real-world information about the clinical care of breast cancer patients with deleterious germline mutations.
While germline testing among breast cancer patients is becoming more commonplace, it isn’t clear how test results influence patient care. To gain some insight, Allison W. Kurian, MD, of Stanford (Calif.) University, and colleagues analyzed data on 20,568 women with stage 0-III breast cancer from the Surveillance, Epidemiology, and End Results (SEER) registries of Georgia and California (JAMA Oncol. 2020 Feb 6. doi: 10.1001/jamaoncol.2019.6400).
The researchers aimed to determine whether women with mutations in breast cancer–associated genes (BRCA1/2 or others) received guideline-concordant care to the same degree as women who lacked deleterious mutations. The authors evaluated guideline concordance with respect to three treatment modalities: surgery (bilateral vs. unilateral mastectomy in women who were eligible for unilateral surgery), radiotherapy after lumpectomy (for women aged less than 70 years with hormonally responsive, ErbB2-negative, stage I cancers), and chemotherapy (among women eligible for consideration of chemotherapy omission)
In alignment with guidelines, many clinicians correctly used genetic test results to guide surgical decisions. For example, 61.7% of women with BRCA mutations underwent bilateral mastectomy, compared with 24.3% who were mutation negative (odds ratio, 5.52). For other pathogenic variants (ATM, CDH1, CHEK2, NBN, NF1, PALB2, PTEN, and TP53), the rate of bilateral mastectomy was still elevated, albeit to a lesser degree (OR, 2.41).
In discord with guidelines, women with BRCA mutations were 78% less likely to receive radiotherapy after lumpectomy (OR, 0.22) and 76% more likely to receive chemotherapy for early-stage, hormone-positive disease (OR, 1.76), suggesting possible trends in under- and overtreatment, respectively. Chemotherapy utilization rates among mutation carriers and noncarriers became more similar after adjustment for clinical and demographic factors.
There are limits on the granularity of the SEER database, such that, if a patient had a mastectomy a year or more after lumpectomy in an effort to avoid radiotherapy, the database would not have reflected that. Clinical factors could have appropriately influenced chemotherapy receipt among patients with mutations, but those additional factors (including patient preference) would not be included in the SEER data.
The authors concluded that research should be conducted to confirm the results of this retrospective, population-based cohort analysis, in an effort to understand the decision-making process and consequences for long-term outcome.
How these findings should influence practice
With every new development, there are challenges – some expected, some unanticipated.
It is now feasible to obtain multigene panel testing reasonably inexpensively. There are concerns about undertesting of patients on the basis of family history alone. And some major professional organizations have endorsed routine gene panel testing for all breast cancer patients.
As a consequence of these factors, genetic test results are routinely available to clinicians who may lack formal training in clinical genetics. Whether these results influence the receipt of evidence-based clinical care is uncertain.
The information published by Dr. Kurian and colleagues is inherently limited by the methodology of a SEER database review. Among other limitations, as the authors comment:
- The genetic test results could have arrived after treatment decisions were made.
- Treatment delivered more than a year after diagnosis would not have been captured.
- There was selection of patients for genetic testing.
- There were few patients with particular germline mutations other than BRCA1/2 on whom to judge whether treatment was guideline concordant.
- The rationale for the treatment choices made by physicians and patients was not available.
- Impact of treatment choices on survival for carriers of deleterious mutations is uncertain.
Nonetheless, these data suggest a need to redouble efforts to educate patients, their family members, and health care professionals about evidence-based guidelines for care and the rationale for those recommendations.
Careful, prospective monitoring of any resultant differences in treatment outcome in patients treated with guideline-concordant and nonconcordant care is needed. When treatment choices appear to systematically deviate from published guidelines with no obvious rationale, it is a wake-up call for all of us.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations.
In this edition of “Applying research to practice,” I highlight a study revealing real-world information about the clinical care of breast cancer patients with deleterious germline mutations.
While germline testing among breast cancer patients is becoming more commonplace, it isn’t clear how test results influence patient care. To gain some insight, Allison W. Kurian, MD, of Stanford (Calif.) University, and colleagues analyzed data on 20,568 women with stage 0-III breast cancer from the Surveillance, Epidemiology, and End Results (SEER) registries of Georgia and California (JAMA Oncol. 2020 Feb 6. doi: 10.1001/jamaoncol.2019.6400).
The researchers aimed to determine whether women with mutations in breast cancer–associated genes (BRCA1/2 or others) received guideline-concordant care to the same degree as women who lacked deleterious mutations. The authors evaluated guideline concordance with respect to three treatment modalities: surgery (bilateral vs. unilateral mastectomy in women who were eligible for unilateral surgery), radiotherapy after lumpectomy (for women aged less than 70 years with hormonally responsive, ErbB2-negative, stage I cancers), and chemotherapy (among women eligible for consideration of chemotherapy omission)
In alignment with guidelines, many clinicians correctly used genetic test results to guide surgical decisions. For example, 61.7% of women with BRCA mutations underwent bilateral mastectomy, compared with 24.3% who were mutation negative (odds ratio, 5.52). For other pathogenic variants (ATM, CDH1, CHEK2, NBN, NF1, PALB2, PTEN, and TP53), the rate of bilateral mastectomy was still elevated, albeit to a lesser degree (OR, 2.41).
In discord with guidelines, women with BRCA mutations were 78% less likely to receive radiotherapy after lumpectomy (OR, 0.22) and 76% more likely to receive chemotherapy for early-stage, hormone-positive disease (OR, 1.76), suggesting possible trends in under- and overtreatment, respectively. Chemotherapy utilization rates among mutation carriers and noncarriers became more similar after adjustment for clinical and demographic factors.
There are limits on the granularity of the SEER database, such that, if a patient had a mastectomy a year or more after lumpectomy in an effort to avoid radiotherapy, the database would not have reflected that. Clinical factors could have appropriately influenced chemotherapy receipt among patients with mutations, but those additional factors (including patient preference) would not be included in the SEER data.
The authors concluded that research should be conducted to confirm the results of this retrospective, population-based cohort analysis, in an effort to understand the decision-making process and consequences for long-term outcome.
How these findings should influence practice
With every new development, there are challenges – some expected, some unanticipated.
It is now feasible to obtain multigene panel testing reasonably inexpensively. There are concerns about undertesting of patients on the basis of family history alone. And some major professional organizations have endorsed routine gene panel testing for all breast cancer patients.
As a consequence of these factors, genetic test results are routinely available to clinicians who may lack formal training in clinical genetics. Whether these results influence the receipt of evidence-based clinical care is uncertain.
The information published by Dr. Kurian and colleagues is inherently limited by the methodology of a SEER database review. Among other limitations, as the authors comment:
- The genetic test results could have arrived after treatment decisions were made.
- Treatment delivered more than a year after diagnosis would not have been captured.
- There was selection of patients for genetic testing.
- There were few patients with particular germline mutations other than BRCA1/2 on whom to judge whether treatment was guideline concordant.
- The rationale for the treatment choices made by physicians and patients was not available.
- Impact of treatment choices on survival for carriers of deleterious mutations is uncertain.
Nonetheless, these data suggest a need to redouble efforts to educate patients, their family members, and health care professionals about evidence-based guidelines for care and the rationale for those recommendations.
Careful, prospective monitoring of any resultant differences in treatment outcome in patients treated with guideline-concordant and nonconcordant care is needed. When treatment choices appear to systematically deviate from published guidelines with no obvious rationale, it is a wake-up call for all of us.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations.
Hospital medicine physician leaders
The right skills and time to develop them
“When you get someone who knows what quality looks like and pair that with curiosity about new ways to think about leading, you end up with the people who are able to produce dramatic innovations in the field.”1
In medicine, a physician is trained to take charge in emergent situations and make potentially lifesaving efforts. However, when it comes to leading teams of individuals, not only must successful leaders have the right skills, they also need time to dedicate to the work of leadership.
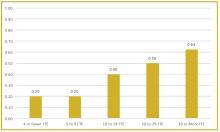
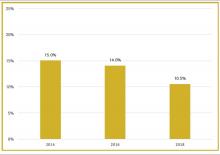
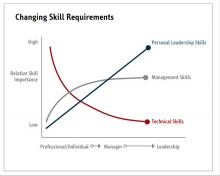
To better understand current approaches to dedicated hospital medicine group (HMG) leadership time, let’s examine the 2018 State of Hospital Medicine (SoHM) Report. The survey, upon which the Report was based, examined two aspects of leadership: 1) how much dedicated time a leader receives to manage the group; and 2) how the leader’s time is compensated. Looking closely at the data displayed in graphs from the SoHM Report (Figures 1, 2, and 3), we can see that dedicated administrative time is directly proportional to the size of the group.
In my current role as a regional medical director in the Dallas-Fort Worth market, I oversee some programs where the size is greater than 30 full-time equivalents (FTEs), and requires a full-time administrative physician leader to manage the group. Their daily administrative duties include, but are not limited to, addressing physician performance and behaviors, managing team performance metrics, dealing with consultants’ expectations, attending and leading various committee meetings at the hospital or the system level, attending and presenting performance reviews, leading and preparing for team meetings, as well as addressing and being innovative in leading new initiatives from the hospital partner system.
Although physician leaders are paid more for their work, the 2018 SoHM Report reveals a decline in the premium year over year. One of the reasons for the payment decline that I have encountered in various groups is that their incentives for leading the group are based on performance, as opposed to receiving a fixed stipend. Another reason is the presence of dedicated administrative support or the inclusion of a performance improvement staffer, such as an additional nurse or advanced practice provider, in the group.
Evidence suggests that organizations and patients benefit when physicians take on leadership roles. Physician leaders play critical roles in providing high-quality patient care. How can the Society of Hospital Medicine help? Management degrees and leadership workshops have become a common pathway for many physicians, including myself. SHM provides one of the most thorough and relevant experiences through the SHM Leadership Academy. The focus of the Leadership Academy is on developing a broad set of additional leadership competencies across a spectrum of experience.5 As hospitalist physicians are often expected to fulfill a broader leadership void, we must pay attention to developing the leadership skills depicted in Figure 3. Hospital medicine is an ideal “proving ground” for future physician executives and leaders, as they often share the same characteristics required for success.
The leadership paths available in my organization, Sound Physicians, were recently highlighted in a New York Times article.3 Sound Physicians employs more than 3,000 physicians across the country, and has a pipeline for doctors to advance through structured rungs of leadership – emphasizing a different mix of clinical, strategic, and business skills at each stage, from individual practitioner to the C-suite. The training includes in-person and online courses, as well as an annual conference, to help doctors develop management and leadership competencies, and learn how to apply these skills within their organizations. Since introducing its leadership development program, the company reports less turnover, higher morale, and better growth. I personally have gone through the leadership training provided by Sound Physicians, and reflecting back, it has been a transformational experience for me. Leadership is a journey, not a destination, and as physicians we should strive to learn more from the health care leaders around us.
The administrative workload for hospital-based physician leaders will increase with the arrival of value-based programs and alternative payment models promoted by the Centers for Medicare and Medicaid Services. Lead hospitalist duties are not limited to daily operations, but can extend to leading the strategic vision of the hospital or health system. The 2020 SoHM Report will reflect these changes, as well as provide further information about how to manage and set expectations for physician leaders, based on group size and employment model.
Dr. Patel is a regional medical director with Sound Physicians. He manages more than 100 FTE hospitalists and advanced-practice providers (APPs) within multiple health systems and hospitals in the Texas market. He also serves as a member of the SHM Practice Analysis Committee and as a vice president of SHM North Texas Chapter.
References
1. Angood P and Birk S. The Value of Physician Leadership. Physician Exec. 2014 May-Jun;40(3):6-20.
2. Rice JA. Expanding the Need for Physician Leaders. Executive Insight, Advance Healthcare Network, Nov 16, 2011. Available at: http://healthcare-executive-insight.advanceweb.com/Features/Articles/Expanding-the-Need-for-Physician-Leaders.aspx.
3. Khullar D. Good leaders make good doctors. New York Times. 2019 Nov 21.
4. Beresford L. The State of Hospital Medicine in 2018. Hospitalist. 2019;23(1):1-11.
5. Harte B. Hospitalists can meet the demand for physician executives. Hospitalist. 2018 Nov 29.
The right skills and time to develop them
The right skills and time to develop them
“When you get someone who knows what quality looks like and pair that with curiosity about new ways to think about leading, you end up with the people who are able to produce dramatic innovations in the field.”1
In medicine, a physician is trained to take charge in emergent situations and make potentially lifesaving efforts. However, when it comes to leading teams of individuals, not only must successful leaders have the right skills, they also need time to dedicate to the work of leadership.
To better understand current approaches to dedicated hospital medicine group (HMG) leadership time, let’s examine the 2018 State of Hospital Medicine (SoHM) Report. The survey, upon which the Report was based, examined two aspects of leadership: 1) how much dedicated time a leader receives to manage the group; and 2) how the leader’s time is compensated. Looking closely at the data displayed in graphs from the SoHM Report (Figures 1, 2, and 3), we can see that dedicated administrative time is directly proportional to the size of the group.
In my current role as a regional medical director in the Dallas-Fort Worth market, I oversee some programs where the size is greater than 30 full-time equivalents (FTEs), and requires a full-time administrative physician leader to manage the group. Their daily administrative duties include, but are not limited to, addressing physician performance and behaviors, managing team performance metrics, dealing with consultants’ expectations, attending and leading various committee meetings at the hospital or the system level, attending and presenting performance reviews, leading and preparing for team meetings, as well as addressing and being innovative in leading new initiatives from the hospital partner system.
Although physician leaders are paid more for their work, the 2018 SoHM Report reveals a decline in the premium year over year. One of the reasons for the payment decline that I have encountered in various groups is that their incentives for leading the group are based on performance, as opposed to receiving a fixed stipend. Another reason is the presence of dedicated administrative support or the inclusion of a performance improvement staffer, such as an additional nurse or advanced practice provider, in the group.
Evidence suggests that organizations and patients benefit when physicians take on leadership roles. Physician leaders play critical roles in providing high-quality patient care. How can the Society of Hospital Medicine help? Management degrees and leadership workshops have become a common pathway for many physicians, including myself. SHM provides one of the most thorough and relevant experiences through the SHM Leadership Academy. The focus of the Leadership Academy is on developing a broad set of additional leadership competencies across a spectrum of experience.5 As hospitalist physicians are often expected to fulfill a broader leadership void, we must pay attention to developing the leadership skills depicted in Figure 3. Hospital medicine is an ideal “proving ground” for future physician executives and leaders, as they often share the same characteristics required for success.
The leadership paths available in my organization, Sound Physicians, were recently highlighted in a New York Times article.3 Sound Physicians employs more than 3,000 physicians across the country, and has a pipeline for doctors to advance through structured rungs of leadership – emphasizing a different mix of clinical, strategic, and business skills at each stage, from individual practitioner to the C-suite. The training includes in-person and online courses, as well as an annual conference, to help doctors develop management and leadership competencies, and learn how to apply these skills within their organizations. Since introducing its leadership development program, the company reports less turnover, higher morale, and better growth. I personally have gone through the leadership training provided by Sound Physicians, and reflecting back, it has been a transformational experience for me. Leadership is a journey, not a destination, and as physicians we should strive to learn more from the health care leaders around us.
The administrative workload for hospital-based physician leaders will increase with the arrival of value-based programs and alternative payment models promoted by the Centers for Medicare and Medicaid Services. Lead hospitalist duties are not limited to daily operations, but can extend to leading the strategic vision of the hospital or health system. The 2020 SoHM Report will reflect these changes, as well as provide further information about how to manage and set expectations for physician leaders, based on group size and employment model.
Dr. Patel is a regional medical director with Sound Physicians. He manages more than 100 FTE hospitalists and advanced-practice providers (APPs) within multiple health systems and hospitals in the Texas market. He also serves as a member of the SHM Practice Analysis Committee and as a vice president of SHM North Texas Chapter.
References
1. Angood P and Birk S. The Value of Physician Leadership. Physician Exec. 2014 May-Jun;40(3):6-20.
2. Rice JA. Expanding the Need for Physician Leaders. Executive Insight, Advance Healthcare Network, Nov 16, 2011. Available at: http://healthcare-executive-insight.advanceweb.com/Features/Articles/Expanding-the-Need-for-Physician-Leaders.aspx.
3. Khullar D. Good leaders make good doctors. New York Times. 2019 Nov 21.
4. Beresford L. The State of Hospital Medicine in 2018. Hospitalist. 2019;23(1):1-11.
5. Harte B. Hospitalists can meet the demand for physician executives. Hospitalist. 2018 Nov 29.
“When you get someone who knows what quality looks like and pair that with curiosity about new ways to think about leading, you end up with the people who are able to produce dramatic innovations in the field.”1
In medicine, a physician is trained to take charge in emergent situations and make potentially lifesaving efforts. However, when it comes to leading teams of individuals, not only must successful leaders have the right skills, they also need time to dedicate to the work of leadership.
To better understand current approaches to dedicated hospital medicine group (HMG) leadership time, let’s examine the 2018 State of Hospital Medicine (SoHM) Report. The survey, upon which the Report was based, examined two aspects of leadership: 1) how much dedicated time a leader receives to manage the group; and 2) how the leader’s time is compensated. Looking closely at the data displayed in graphs from the SoHM Report (Figures 1, 2, and 3), we can see that dedicated administrative time is directly proportional to the size of the group.
In my current role as a regional medical director in the Dallas-Fort Worth market, I oversee some programs where the size is greater than 30 full-time equivalents (FTEs), and requires a full-time administrative physician leader to manage the group. Their daily administrative duties include, but are not limited to, addressing physician performance and behaviors, managing team performance metrics, dealing with consultants’ expectations, attending and leading various committee meetings at the hospital or the system level, attending and presenting performance reviews, leading and preparing for team meetings, as well as addressing and being innovative in leading new initiatives from the hospital partner system.
Although physician leaders are paid more for their work, the 2018 SoHM Report reveals a decline in the premium year over year. One of the reasons for the payment decline that I have encountered in various groups is that their incentives for leading the group are based on performance, as opposed to receiving a fixed stipend. Another reason is the presence of dedicated administrative support or the inclusion of a performance improvement staffer, such as an additional nurse or advanced practice provider, in the group.
Evidence suggests that organizations and patients benefit when physicians take on leadership roles. Physician leaders play critical roles in providing high-quality patient care. How can the Society of Hospital Medicine help? Management degrees and leadership workshops have become a common pathway for many physicians, including myself. SHM provides one of the most thorough and relevant experiences through the SHM Leadership Academy. The focus of the Leadership Academy is on developing a broad set of additional leadership competencies across a spectrum of experience.5 As hospitalist physicians are often expected to fulfill a broader leadership void, we must pay attention to developing the leadership skills depicted in Figure 3. Hospital medicine is an ideal “proving ground” for future physician executives and leaders, as they often share the same characteristics required for success.
The leadership paths available in my organization, Sound Physicians, were recently highlighted in a New York Times article.3 Sound Physicians employs more than 3,000 physicians across the country, and has a pipeline for doctors to advance through structured rungs of leadership – emphasizing a different mix of clinical, strategic, and business skills at each stage, from individual practitioner to the C-suite. The training includes in-person and online courses, as well as an annual conference, to help doctors develop management and leadership competencies, and learn how to apply these skills within their organizations. Since introducing its leadership development program, the company reports less turnover, higher morale, and better growth. I personally have gone through the leadership training provided by Sound Physicians, and reflecting back, it has been a transformational experience for me. Leadership is a journey, not a destination, and as physicians we should strive to learn more from the health care leaders around us.
The administrative workload for hospital-based physician leaders will increase with the arrival of value-based programs and alternative payment models promoted by the Centers for Medicare and Medicaid Services. Lead hospitalist duties are not limited to daily operations, but can extend to leading the strategic vision of the hospital or health system. The 2020 SoHM Report will reflect these changes, as well as provide further information about how to manage and set expectations for physician leaders, based on group size and employment model.
Dr. Patel is a regional medical director with Sound Physicians. He manages more than 100 FTE hospitalists and advanced-practice providers (APPs) within multiple health systems and hospitals in the Texas market. He also serves as a member of the SHM Practice Analysis Committee and as a vice president of SHM North Texas Chapter.
References
1. Angood P and Birk S. The Value of Physician Leadership. Physician Exec. 2014 May-Jun;40(3):6-20.
2. Rice JA. Expanding the Need for Physician Leaders. Executive Insight, Advance Healthcare Network, Nov 16, 2011. Available at: http://healthcare-executive-insight.advanceweb.com/Features/Articles/Expanding-the-Need-for-Physician-Leaders.aspx.
3. Khullar D. Good leaders make good doctors. New York Times. 2019 Nov 21.
4. Beresford L. The State of Hospital Medicine in 2018. Hospitalist. 2019;23(1):1-11.
5. Harte B. Hospitalists can meet the demand for physician executives. Hospitalist. 2018 Nov 29.
Lombardy ICU capacity stressed to breaking point by COVID-19 outbreak
The outbreak of COVID-19 in the Lombardy region of Italy has severely stressed the medical system and the current level of activity may not be sustainable for long, according to Maurizio Cecconi, MD, of the department of anesthesia and intensive care, Humanitas Research Hospital, Milan. Dr. Cecconi spoke via JAMA Live Stream interview with Howard Bauchner, MD, the Editor in Chief of JAMA.

A summary of comments by Dr. Cecconi and two colleagues was simultaneously published in JAMA (2020 Mar 13. doi: 10.1001/jama.2020.4031).
Dr. Cecconi discussed the progress and medical response to the swiftly expanding outbreak that began on Feb. 20. A man in his 30s was admitted to the Codogno Hospital, Lodi, Lombardy, Italy, in respiratory distress. He tested positive for a new coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19). In less than 24 hours, the hospital had 36 cases of COVID-19.
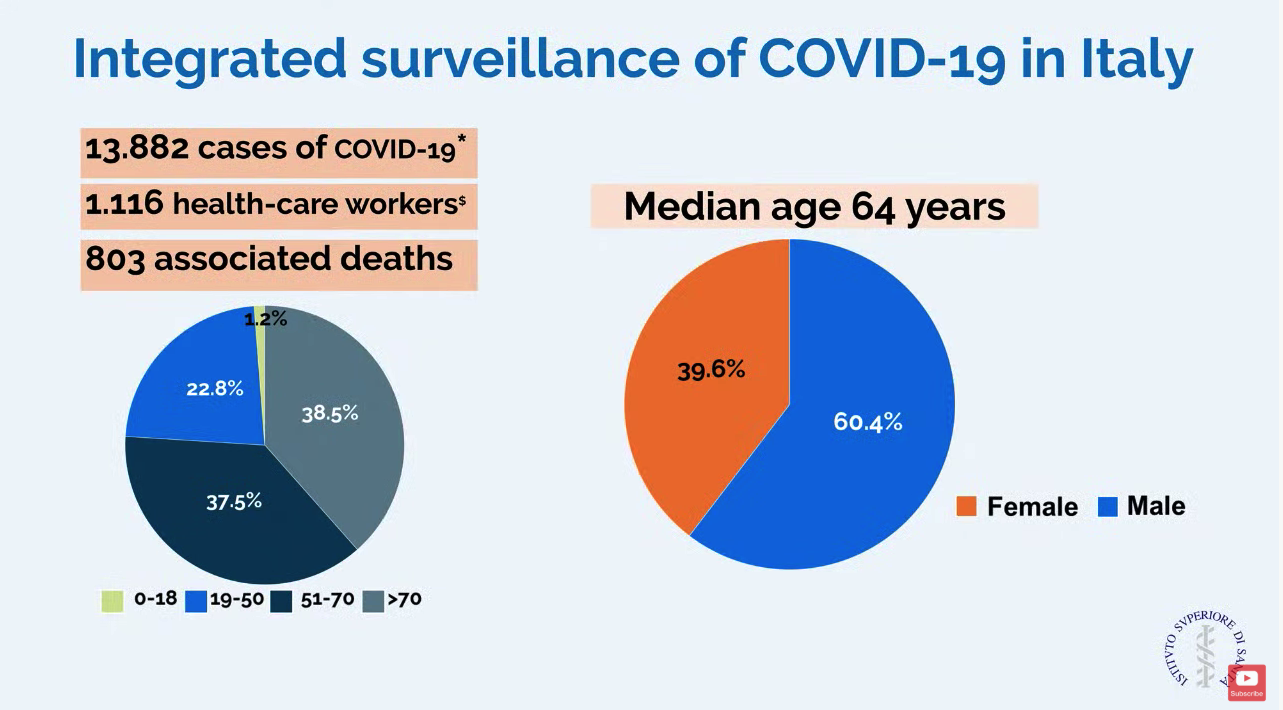
In a slide provided by the Italian National Health Service, the number of cases in Italy stands at 13,882 with 803 associated deaths.
ICU resources have been severely stressed. Before the outbreak, Lombardy had 720 ICU beds (about 5% of total beds). Within 48 hours of the first case, ICU cohorts were formed in 15 hub hospitals totaling 130 COVID-19 ICU beds. By March 7, the total number of dedicated cohorted COVID-19 ICU beds was 482.
“The proportion of ICU admissions represents 12% of the total positive cases, and 16% of all hospitalized patients,” compared with about 5% of ICU admissions reported from China. The difference may be attributable to different criteria for ICU admissions in Italy, compared with China, according to Dr. Cecconi and colleagues.
Dr. Cecconi mentioned that there were relatively few cases in children, and they had relatively mild disease. The death rate among patients remained under 1% up to age 59. For patients aged 60-69 years, the rate was 2.7%; for patients aged 70-79 years, the rate was 9.6%; for those aged 80-89, the rate was much higher at 16.6%.
Modeled forecasts of the potential number of cases in Lombardy are daunting. “The linear model forecasts that approximately 869 ICU admissions could occur by March 20, 2020, whereas the exponential model growth projects that approximately 14,542 ICU admissions could occur by then. Even though these projections are hypothetical and involve various assumptions, any substantial increase in the number of critically ill patients would rapidly exceed total ICU capacity, without even considering other critical admissions, such as for trauma, stroke, and other emergencies,” wrote Dr. Cecconi and his colleagues in JAMA. He said, “We could be on our knees very soon,” referring to the potential dramatic increase in cases.
Dr. Cecconi had some recommendations for other countries in which a major outbreak has not yet occurred. He recommended going beyond expanding ICU and isolation capacity and focus on training staff with simulation for treating these highly contagious patients. His medical center has worked hard to protect staff but 1,116 health care workers have tested positive for the virus. Conditions for staff are very difficult in full protective gear, and Dr. Cecconi commended the heroic work by these doctors and nurses.
In addition, Dr. Cecconi is focused on supportive care for patients and does not recommend using untried approaches on these patients that could cause harm. “Everyone wants to find a specific drug for these patients, but I say there is not particular drug at the moment.” He stressed that, despite the crisis, doctors should focus on evidence-based treatment and tried-and-true supportive care.
Disclosures by Dr. Cecconi are available on the JAMA website.
CORRECTION 3/13/2020 2.18 P.M. The death rate for patients aged 70-79 was corrected.
The outbreak of COVID-19 in the Lombardy region of Italy has severely stressed the medical system and the current level of activity may not be sustainable for long, according to Maurizio Cecconi, MD, of the department of anesthesia and intensive care, Humanitas Research Hospital, Milan. Dr. Cecconi spoke via JAMA Live Stream interview with Howard Bauchner, MD, the Editor in Chief of JAMA.

A summary of comments by Dr. Cecconi and two colleagues was simultaneously published in JAMA (2020 Mar 13. doi: 10.1001/jama.2020.4031).
Dr. Cecconi discussed the progress and medical response to the swiftly expanding outbreak that began on Feb. 20. A man in his 30s was admitted to the Codogno Hospital, Lodi, Lombardy, Italy, in respiratory distress. He tested positive for a new coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19). In less than 24 hours, the hospital had 36 cases of COVID-19.
In a slide provided by the Italian National Health Service, the number of cases in Italy stands at 13,882 with 803 associated deaths.
ICU resources have been severely stressed. Before the outbreak, Lombardy had 720 ICU beds (about 5% of total beds). Within 48 hours of the first case, ICU cohorts were formed in 15 hub hospitals totaling 130 COVID-19 ICU beds. By March 7, the total number of dedicated cohorted COVID-19 ICU beds was 482.
“The proportion of ICU admissions represents 12% of the total positive cases, and 16% of all hospitalized patients,” compared with about 5% of ICU admissions reported from China. The difference may be attributable to different criteria for ICU admissions in Italy, compared with China, according to Dr. Cecconi and colleagues.
Dr. Cecconi mentioned that there were relatively few cases in children, and they had relatively mild disease. The death rate among patients remained under 1% up to age 59. For patients aged 60-69 years, the rate was 2.7%; for patients aged 70-79 years, the rate was 9.6%; for those aged 80-89, the rate was much higher at 16.6%.

Modeled forecasts of the potential number of cases in Lombardy are daunting. “The linear model forecasts that approximately 869 ICU admissions could occur by March 20, 2020, whereas the exponential model growth projects that approximately 14,542 ICU admissions could occur by then. Even though these projections are hypothetical and involve various assumptions, any substantial increase in the number of critically ill patients would rapidly exceed total ICU capacity, without even considering other critical admissions, such as for trauma, stroke, and other emergencies,” wrote Dr. Cecconi and his colleagues in JAMA. He said, “We could be on our knees very soon,” referring to the potential dramatic increase in cases.
Dr. Cecconi had some recommendations for other countries in which a major outbreak has not yet occurred. He recommended going beyond expanding ICU and isolation capacity and focus on training staff with simulation for treating these highly contagious patients. His medical center has worked hard to protect staff but 1,116 health care workers have tested positive for the virus. Conditions for staff are very difficult in full protective gear, and Dr. Cecconi commended the heroic work by these doctors and nurses.
In addition, Dr. Cecconi is focused on supportive care for patients and does not recommend using untried approaches on these patients that could cause harm. “Everyone wants to find a specific drug for these patients, but I say there is not particular drug at the moment.” He stressed that, despite the crisis, doctors should focus on evidence-based treatment and tried-and-true supportive care.
Disclosures by Dr. Cecconi are available on the JAMA website.
CORRECTION 3/13/2020 2.18 P.M. The death rate for patients aged 70-79 was corrected.
The outbreak of COVID-19 in the Lombardy region of Italy has severely stressed the medical system and the current level of activity may not be sustainable for long, according to Maurizio Cecconi, MD, of the department of anesthesia and intensive care, Humanitas Research Hospital, Milan. Dr. Cecconi spoke via JAMA Live Stream interview with Howard Bauchner, MD, the Editor in Chief of JAMA.

A summary of comments by Dr. Cecconi and two colleagues was simultaneously published in JAMA (2020 Mar 13. doi: 10.1001/jama.2020.4031).
Dr. Cecconi discussed the progress and medical response to the swiftly expanding outbreak that began on Feb. 20. A man in his 30s was admitted to the Codogno Hospital, Lodi, Lombardy, Italy, in respiratory distress. He tested positive for a new coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19). In less than 24 hours, the hospital had 36 cases of COVID-19.
In a slide provided by the Italian National Health Service, the number of cases in Italy stands at 13,882 with 803 associated deaths.
ICU resources have been severely stressed. Before the outbreak, Lombardy had 720 ICU beds (about 5% of total beds). Within 48 hours of the first case, ICU cohorts were formed in 15 hub hospitals totaling 130 COVID-19 ICU beds. By March 7, the total number of dedicated cohorted COVID-19 ICU beds was 482.
“The proportion of ICU admissions represents 12% of the total positive cases, and 16% of all hospitalized patients,” compared with about 5% of ICU admissions reported from China. The difference may be attributable to different criteria for ICU admissions in Italy, compared with China, according to Dr. Cecconi and colleagues.
Dr. Cecconi mentioned that there were relatively few cases in children, and they had relatively mild disease. The death rate among patients remained under 1% up to age 59. For patients aged 60-69 years, the rate was 2.7%; for patients aged 70-79 years, the rate was 9.6%; for those aged 80-89, the rate was much higher at 16.6%.

Modeled forecasts of the potential number of cases in Lombardy are daunting. “The linear model forecasts that approximately 869 ICU admissions could occur by March 20, 2020, whereas the exponential model growth projects that approximately 14,542 ICU admissions could occur by then. Even though these projections are hypothetical and involve various assumptions, any substantial increase in the number of critically ill patients would rapidly exceed total ICU capacity, without even considering other critical admissions, such as for trauma, stroke, and other emergencies,” wrote Dr. Cecconi and his colleagues in JAMA. He said, “We could be on our knees very soon,” referring to the potential dramatic increase in cases.
Dr. Cecconi had some recommendations for other countries in which a major outbreak has not yet occurred. He recommended going beyond expanding ICU and isolation capacity and focus on training staff with simulation for treating these highly contagious patients. His medical center has worked hard to protect staff but 1,116 health care workers have tested positive for the virus. Conditions for staff are very difficult in full protective gear, and Dr. Cecconi commended the heroic work by these doctors and nurses.
In addition, Dr. Cecconi is focused on supportive care for patients and does not recommend using untried approaches on these patients that could cause harm. “Everyone wants to find a specific drug for these patients, but I say there is not particular drug at the moment.” He stressed that, despite the crisis, doctors should focus on evidence-based treatment and tried-and-true supportive care.
Disclosures by Dr. Cecconi are available on the JAMA website.
CORRECTION 3/13/2020 2.18 P.M. The death rate for patients aged 70-79 was corrected.
REPORTING FROM JAMA LIVE STREAM
Internist reports from COVID-19 front lines near Seattle
KENT, WASHINGTON – The first thing I learned in this outbreak is that my sense of alarm has been deadened by years of medical practice. As a primary care doctor working south of Seattle, in the University of Washington’s Kent neighborhood clinic, I have dealt with long hours, the sometimes-insurmountable problems of the patients I care for, and the constant, gnawing fear of missing something and doing harm. To get through my day, I’ve done my best to rationalize that fear, to explain it away.
I can’t explain how, when I heard the news of the coronavirus epidemic in China, I didn’t think it would affect me. I can’t explain how news of the first patient presenting to an urgent care north of Seattle didn’t cause me, or all health care providers, to think about how we would respond. I can’t explain why so many doctors were dismissive of the very real threat that was about to explode. I can’t explain why it took 6 weeks for the COVID-19 outbreak to seem real to me.
If you work in a doctor’s office, emergency department, hospital, or urgent care center and have not seen a coronavirus case yet, you may have time to think through what is likely to happen in your community. We did not activate a chain of command or decide how information was going to be communicated to the front line and back to leadership. Few of us ran worst-case scenarios.
By March 12, we had 376 confirmed cases, and likely more than a thousand are undetected. The moment of realization of the severity of the outbreak didn’t come to me until Saturday, Feb. 29. In the week prior, several patients had come into the clinic with symptoms and potential exposures, but not meeting the narrow Centers for Disease Control and Prevention testing criteria. They were all advised by the Washington Department of Health to go home. At the time, it seemed like decent advice. Frontline providers didn’t know that there had been two cases of community transmission weeks before, or that one was about to become the first death in Washington state. I still advised patients to quarantine themselves. In the absence of testing, we had to assume everyone was positive and should stay home until 72 hours after their symptoms resolved. Studying the state’s FMLA [Family and Medical Leave Act] intently, I wrote insistent letters to inflexible bosses, explaining that their employees needed to stay home.
I worked that Saturday. Half of my patients had coughs. Our team insisted that they wear masks. One woman refused, and I refused to see her until she did. In a customer service–oriented health care system, I had been schooled to accommodate almost any patient request. But I was not about to put my staff and other patients at risk. Reluctantly, she complied.
On my lunch break, my partner called me to tell me he was at the grocery store. “Why?” I asked, since we usually went together. It became clear he was worried about an outbreak. He had been following the news closely and tried to tell me how deadly this could get and how quickly the disease could spread. I brushed his fears aside, as more evidence of his sweet and overly cautious nature. “It’ll be fine,” I said with misplaced confidence.
Later that day, I heard about the first death and the outbreak at Life Care, a nursing home north of Seattle. I learned that firefighters who had responded to distress calls were under quarantine. I learned through an epidemiologist that there were likely hundreds of undetected cases throughout Washington.
On Monday, our clinic decided to convert all cases with symptoms into telemedicine visits. Luckily, we had been building the capacity to see and treat patients virtually for a while. We have ramped up quickly, but there have been bumps along the way. It’s difficult to convince those who are anxious about their symptoms to allow us to use telemedicine for everyone’s safety. It is unclear how much liability we are taking on as individual providers with this approach or who will speak up for us if something goes wrong.
Patients don’t seem to know where to get their information, and they have been turning to increasingly bizarre sources. For the poorest, who have had so much trouble accessing care, I cannot blame them for not knowing whom to trust. I post what I know on Twitter and Facebook, but I know I’m no match for cynical social media algorithms.
Testing was still not available at my clinic the first week of March, and it remains largely unavailable throughout much of the country. We have lost weeks of opportunity to contain this. Luckily, on March 4, the University of Washington was finally allowed to use their homegrown test and bypass the limited supply from the CDC. But our capacity at UW is still limited, and the test remained unavailable to the majority of those potentially showing symptoms until March 9.
I am used to being less worried than my patients. I am used to reassuring them. But over the first week of March, I had an eerie sense that my alarm far outstripped theirs. I got relatively few questions about coronavirus, even as the number of cases continued to rise. It wasn’t until the end of the week that I noticed a few were truly fearful. Patients started stealing the gloves and the hand sanitizer, and we had to zealously guard them. My hands are raw from washing.
Throughout this time, I have been grateful for a centralized drive with clear protocols. I am grateful for clear messages at the beginning and end of the day from our CEO. I hope that other clinics model this and have daily in-person meetings, because too much cannot be conveyed in an email when the situation changes hourly.
But our health system nationally was already stretched thin before, and providers have sacrificed a lot, especially in the most critical settings, to provide decent patient care. Now we are asked to risk our health and safety, and our family’s, and I worry about the erosion of trust and work conditions for those on the front lines. I also worry our patients won’t believe us when we have allowed the costs of care to continue to rise and ruin their lives. I worry about the millions of people without doctors to call because they have no insurance, and because so many primary care physicians have left unsustainable jobs.
I am grateful that few of my colleagues have been sick and that those that were called out. I am grateful for the new nurse practitioners in our clinic who took the lion’s share of possibly affected patients and triaged hundreds of phone calls, creating note and message templates that we all use. I am grateful that my clinic manager insisted on doing a drill with all the staff members.
I am grateful that we were reminded that we are a team and that if the call center and cleaning crews and front desk are excluded, then our protocols are useless. I am grateful that our registered nurses quickly shifted to triage. I am grateful that I have testing available.
This week, for the first time since I started working, multiple patients asked how I am doing and expressed their thanks. I am most grateful for them.
I can’t tell you what to do or what is going to happen, but I can tell you that you need to prepare now. You need to run drills and catch the holes in your plans before the pandemic reaches you. You need to be creative and honest about the flaws in your organization that this pandemic will inevitably expose. You need to meet with your team every day and remember that we are all going to be stretched even thinner than before.
Most of us will get through this, but many of us won’t. And for those who do, we need to be honest about our successes and failures. We need to build a system that can do better next time. Because this is not the last pandemic we will face.
Dr. Elisabeth Poorman is a general internist at a University of Washington neighborhood clinic in Kent. She completed her residency at Cambridge (Mass.) Health Alliance and specializes in addiction medicine. She also serves on the editorial advisory board of Internal Medicine News.
KENT, WASHINGTON – The first thing I learned in this outbreak is that my sense of alarm has been deadened by years of medical practice. As a primary care doctor working south of Seattle, in the University of Washington’s Kent neighborhood clinic, I have dealt with long hours, the sometimes-insurmountable problems of the patients I care for, and the constant, gnawing fear of missing something and doing harm. To get through my day, I’ve done my best to rationalize that fear, to explain it away.
I can’t explain how, when I heard the news of the coronavirus epidemic in China, I didn’t think it would affect me. I can’t explain how news of the first patient presenting to an urgent care north of Seattle didn’t cause me, or all health care providers, to think about how we would respond. I can’t explain why so many doctors were dismissive of the very real threat that was about to explode. I can’t explain why it took 6 weeks for the COVID-19 outbreak to seem real to me.
If you work in a doctor’s office, emergency department, hospital, or urgent care center and have not seen a coronavirus case yet, you may have time to think through what is likely to happen in your community. We did not activate a chain of command or decide how information was going to be communicated to the front line and back to leadership. Few of us ran worst-case scenarios.
By March 12, we had 376 confirmed cases, and likely more than a thousand are undetected. The moment of realization of the severity of the outbreak didn’t come to me until Saturday, Feb. 29. In the week prior, several patients had come into the clinic with symptoms and potential exposures, but not meeting the narrow Centers for Disease Control and Prevention testing criteria. They were all advised by the Washington Department of Health to go home. At the time, it seemed like decent advice. Frontline providers didn’t know that there had been two cases of community transmission weeks before, or that one was about to become the first death in Washington state. I still advised patients to quarantine themselves. In the absence of testing, we had to assume everyone was positive and should stay home until 72 hours after their symptoms resolved. Studying the state’s FMLA [Family and Medical Leave Act] intently, I wrote insistent letters to inflexible bosses, explaining that their employees needed to stay home.
I worked that Saturday. Half of my patients had coughs. Our team insisted that they wear masks. One woman refused, and I refused to see her until she did. In a customer service–oriented health care system, I had been schooled to accommodate almost any patient request. But I was not about to put my staff and other patients at risk. Reluctantly, she complied.
On my lunch break, my partner called me to tell me he was at the grocery store. “Why?” I asked, since we usually went together. It became clear he was worried about an outbreak. He had been following the news closely and tried to tell me how deadly this could get and how quickly the disease could spread. I brushed his fears aside, as more evidence of his sweet and overly cautious nature. “It’ll be fine,” I said with misplaced confidence.
Later that day, I heard about the first death and the outbreak at Life Care, a nursing home north of Seattle. I learned that firefighters who had responded to distress calls were under quarantine. I learned through an epidemiologist that there were likely hundreds of undetected cases throughout Washington.
On Monday, our clinic decided to convert all cases with symptoms into telemedicine visits. Luckily, we had been building the capacity to see and treat patients virtually for a while. We have ramped up quickly, but there have been bumps along the way. It’s difficult to convince those who are anxious about their symptoms to allow us to use telemedicine for everyone’s safety. It is unclear how much liability we are taking on as individual providers with this approach or who will speak up for us if something goes wrong.
Patients don’t seem to know where to get their information, and they have been turning to increasingly bizarre sources. For the poorest, who have had so much trouble accessing care, I cannot blame them for not knowing whom to trust. I post what I know on Twitter and Facebook, but I know I’m no match for cynical social media algorithms.
Testing was still not available at my clinic the first week of March, and it remains largely unavailable throughout much of the country. We have lost weeks of opportunity to contain this. Luckily, on March 4, the University of Washington was finally allowed to use their homegrown test and bypass the limited supply from the CDC. But our capacity at UW is still limited, and the test remained unavailable to the majority of those potentially showing symptoms until March 9.
I am used to being less worried than my patients. I am used to reassuring them. But over the first week of March, I had an eerie sense that my alarm far outstripped theirs. I got relatively few questions about coronavirus, even as the number of cases continued to rise. It wasn’t until the end of the week that I noticed a few were truly fearful. Patients started stealing the gloves and the hand sanitizer, and we had to zealously guard them. My hands are raw from washing.
Throughout this time, I have been grateful for a centralized drive with clear protocols. I am grateful for clear messages at the beginning and end of the day from our CEO. I hope that other clinics model this and have daily in-person meetings, because too much cannot be conveyed in an email when the situation changes hourly.
But our health system nationally was already stretched thin before, and providers have sacrificed a lot, especially in the most critical settings, to provide decent patient care. Now we are asked to risk our health and safety, and our family’s, and I worry about the erosion of trust and work conditions for those on the front lines. I also worry our patients won’t believe us when we have allowed the costs of care to continue to rise and ruin their lives. I worry about the millions of people without doctors to call because they have no insurance, and because so many primary care physicians have left unsustainable jobs.
I am grateful that few of my colleagues have been sick and that those that were called out. I am grateful for the new nurse practitioners in our clinic who took the lion’s share of possibly affected patients and triaged hundreds of phone calls, creating note and message templates that we all use. I am grateful that my clinic manager insisted on doing a drill with all the staff members.
I am grateful that we were reminded that we are a team and that if the call center and cleaning crews and front desk are excluded, then our protocols are useless. I am grateful that our registered nurses quickly shifted to triage. I am grateful that I have testing available.
This week, for the first time since I started working, multiple patients asked how I am doing and expressed their thanks. I am most grateful for them.
I can’t tell you what to do or what is going to happen, but I can tell you that you need to prepare now. You need to run drills and catch the holes in your plans before the pandemic reaches you. You need to be creative and honest about the flaws in your organization that this pandemic will inevitably expose. You need to meet with your team every day and remember that we are all going to be stretched even thinner than before.
Most of us will get through this, but many of us won’t. And for those who do, we need to be honest about our successes and failures. We need to build a system that can do better next time. Because this is not the last pandemic we will face.
Dr. Elisabeth Poorman is a general internist at a University of Washington neighborhood clinic in Kent. She completed her residency at Cambridge (Mass.) Health Alliance and specializes in addiction medicine. She also serves on the editorial advisory board of Internal Medicine News.
KENT, WASHINGTON – The first thing I learned in this outbreak is that my sense of alarm has been deadened by years of medical practice. As a primary care doctor working south of Seattle, in the University of Washington’s Kent neighborhood clinic, I have dealt with long hours, the sometimes-insurmountable problems of the patients I care for, and the constant, gnawing fear of missing something and doing harm. To get through my day, I’ve done my best to rationalize that fear, to explain it away.
I can’t explain how, when I heard the news of the coronavirus epidemic in China, I didn’t think it would affect me. I can’t explain how news of the first patient presenting to an urgent care north of Seattle didn’t cause me, or all health care providers, to think about how we would respond. I can’t explain why so many doctors were dismissive of the very real threat that was about to explode. I can’t explain why it took 6 weeks for the COVID-19 outbreak to seem real to me.
If you work in a doctor’s office, emergency department, hospital, or urgent care center and have not seen a coronavirus case yet, you may have time to think through what is likely to happen in your community. We did not activate a chain of command or decide how information was going to be communicated to the front line and back to leadership. Few of us ran worst-case scenarios.
By March 12, we had 376 confirmed cases, and likely more than a thousand are undetected. The moment of realization of the severity of the outbreak didn’t come to me until Saturday, Feb. 29. In the week prior, several patients had come into the clinic with symptoms and potential exposures, but not meeting the narrow Centers for Disease Control and Prevention testing criteria. They were all advised by the Washington Department of Health to go home. At the time, it seemed like decent advice. Frontline providers didn’t know that there had been two cases of community transmission weeks before, or that one was about to become the first death in Washington state. I still advised patients to quarantine themselves. In the absence of testing, we had to assume everyone was positive and should stay home until 72 hours after their symptoms resolved. Studying the state’s FMLA [Family and Medical Leave Act] intently, I wrote insistent letters to inflexible bosses, explaining that their employees needed to stay home.
I worked that Saturday. Half of my patients had coughs. Our team insisted that they wear masks. One woman refused, and I refused to see her until she did. In a customer service–oriented health care system, I had been schooled to accommodate almost any patient request. But I was not about to put my staff and other patients at risk. Reluctantly, she complied.
On my lunch break, my partner called me to tell me he was at the grocery store. “Why?” I asked, since we usually went together. It became clear he was worried about an outbreak. He had been following the news closely and tried to tell me how deadly this could get and how quickly the disease could spread. I brushed his fears aside, as more evidence of his sweet and overly cautious nature. “It’ll be fine,” I said with misplaced confidence.
Later that day, I heard about the first death and the outbreak at Life Care, a nursing home north of Seattle. I learned that firefighters who had responded to distress calls were under quarantine. I learned through an epidemiologist that there were likely hundreds of undetected cases throughout Washington.
On Monday, our clinic decided to convert all cases with symptoms into telemedicine visits. Luckily, we had been building the capacity to see and treat patients virtually for a while. We have ramped up quickly, but there have been bumps along the way. It’s difficult to convince those who are anxious about their symptoms to allow us to use telemedicine for everyone’s safety. It is unclear how much liability we are taking on as individual providers with this approach or who will speak up for us if something goes wrong.
Patients don’t seem to know where to get their information, and they have been turning to increasingly bizarre sources. For the poorest, who have had so much trouble accessing care, I cannot blame them for not knowing whom to trust. I post what I know on Twitter and Facebook, but I know I’m no match for cynical social media algorithms.
Testing was still not available at my clinic the first week of March, and it remains largely unavailable throughout much of the country. We have lost weeks of opportunity to contain this. Luckily, on March 4, the University of Washington was finally allowed to use their homegrown test and bypass the limited supply from the CDC. But our capacity at UW is still limited, and the test remained unavailable to the majority of those potentially showing symptoms until March 9.
I am used to being less worried than my patients. I am used to reassuring them. But over the first week of March, I had an eerie sense that my alarm far outstripped theirs. I got relatively few questions about coronavirus, even as the number of cases continued to rise. It wasn’t until the end of the week that I noticed a few were truly fearful. Patients started stealing the gloves and the hand sanitizer, and we had to zealously guard them. My hands are raw from washing.
Throughout this time, I have been grateful for a centralized drive with clear protocols. I am grateful for clear messages at the beginning and end of the day from our CEO. I hope that other clinics model this and have daily in-person meetings, because too much cannot be conveyed in an email when the situation changes hourly.
But our health system nationally was already stretched thin before, and providers have sacrificed a lot, especially in the most critical settings, to provide decent patient care. Now we are asked to risk our health and safety, and our family’s, and I worry about the erosion of trust and work conditions for those on the front lines. I also worry our patients won’t believe us when we have allowed the costs of care to continue to rise and ruin their lives. I worry about the millions of people without doctors to call because they have no insurance, and because so many primary care physicians have left unsustainable jobs.
I am grateful that few of my colleagues have been sick and that those that were called out. I am grateful for the new nurse practitioners in our clinic who took the lion’s share of possibly affected patients and triaged hundreds of phone calls, creating note and message templates that we all use. I am grateful that my clinic manager insisted on doing a drill with all the staff members.
I am grateful that we were reminded that we are a team and that if the call center and cleaning crews and front desk are excluded, then our protocols are useless. I am grateful that our registered nurses quickly shifted to triage. I am grateful that I have testing available.
This week, for the first time since I started working, multiple patients asked how I am doing and expressed their thanks. I am most grateful for them.
I can’t tell you what to do or what is going to happen, but I can tell you that you need to prepare now. You need to run drills and catch the holes in your plans before the pandemic reaches you. You need to be creative and honest about the flaws in your organization that this pandemic will inevitably expose. You need to meet with your team every day and remember that we are all going to be stretched even thinner than before.
Most of us will get through this, but many of us won’t. And for those who do, we need to be honest about our successes and failures. We need to build a system that can do better next time. Because this is not the last pandemic we will face.
Dr. Elisabeth Poorman is a general internist at a University of Washington neighborhood clinic in Kent. She completed her residency at Cambridge (Mass.) Health Alliance and specializes in addiction medicine. She also serves on the editorial advisory board of Internal Medicine News.
Emeritus
“So what do you do all day?”
.
Asking what an emeritus does all day sounds fair, but the question is harder to answer than it sounds. Last year, I asked Dave, who was already retired. He took a day to think about it.
“Sometimes I sit on the back porch and watch the birds,” he said.
Dave has long been an avid bird-watcher. Along with golf and Facebook, watching birds is a pursuit that really engages many people, but I never understood it. I still don’t.
Over the years, I’ve met people whose experience of retirement has ranged from, “I’m so busy, I don’t know how I had time to work!” to, “I miss the gang and I’m bored,” to everything in between. Before I (semi-) retired, I made a plan to not make plans, at least at first: No new hobbies, cooking lessons, or anthropology courses. I figured I would figure it out.
So I am figuring it out. No rush. After a lifetime of rushing, not rushing is part of the point.
One hobby I cultivate is napping. I always get up early, no alarm needed. By late morning I am sometimes inclined to lie down for a bit. Taking a midmorning nap has always struck me as one of life’s great pleasures, though one I could rarely enjoy, unless you count dozing off standing up while a patient described an itch that started 17 years before, on a Thursday.
Now I can shut my eyes for half an hour and wake up refreshed, ready for the rest of the day.
During which I will do ...
An older friend of mine, now long gone, wrote a witty essay on being embarrassed to work at home. He refused to answer the phone during the day and hid from the postman. Contemplating retirement, I was afraid I would also feel that way, picturing myself a pitiful pensioner shuffling abroad at mid-day, looking for a park to poison pigeons in. That of course was before “working remotely” became a goal for cool young strivers. You see them around at all hours, with things sticking out of their ears, talking urgently to no one you can see.
Now I also walk the streets proudly at 11 a.m. or 2:45 p.m. I may get one of those earbuds that stick out at 45 degrees, so people can think my ear fungus has grown branches. Maybe they’ll imagine me a mastermind of an international CBD cartel. What they think doesn’t really matter.
One thing that I actually do all day is wonder why I spent so much of my career worrying about what other people think. Dr. Smith used to refer patients. No longer. Did I fail to meet her expectations? Mr. Trelawney came in weekly with itches and pains. No more. Did I roll my eyes too obviously?
Questions like these used to trouble me. Now I can’t recall why. Instead I worry about more important things, like who will play right field for the Red Sox this year.
Though I never signed up, I am an enrolled Baby Boomer, that navel-gazing cohort now passing from the scene while pretending it won’t. I never understood my generation when it was claiming to overturn the universe in the 1960s. Now its members write and read books with chirpy titles like “Amazing Aging!” as though – because we are so wonderfully special – age, infirmity, and decline will repeal themselves just for us.
Well, anyone can dream.
I go into the office a couple of half-days a week, when I’m in town. I like bantering with the gang and chatting with old patients. They wish me well and hope I’ll refer them to someone worthy when I hang them up for good, as many of their (and my) doctors already have.
Here is one thing I don’t do all day – manage human resource issues in the office. What’s to miss?
Now and then, with lessening frequency, I muse, “Well, if I do get bored, I can always spend more time in the office.”
Time for another nap.
Dr. Rockoff, who wrote the Dermatology News column “Under My Skin,” is now semi-retired, after 40 years of practice in Brookline, Mass. He served on the clinical faculty at Tufts University, Boston, and taught senior medical students and other trainees for 30 years. His second book, “Act Like a Doctor, Think Like a Patient,” is available at amazon.com and barnesandnoble.com. Write to him at dermnews@mdedge.com.
“So what do you do all day?”
.
Asking what an emeritus does all day sounds fair, but the question is harder to answer than it sounds. Last year, I asked Dave, who was already retired. He took a day to think about it.
“Sometimes I sit on the back porch and watch the birds,” he said.
Dave has long been an avid bird-watcher. Along with golf and Facebook, watching birds is a pursuit that really engages many people, but I never understood it. I still don’t.
Over the years, I’ve met people whose experience of retirement has ranged from, “I’m so busy, I don’t know how I had time to work!” to, “I miss the gang and I’m bored,” to everything in between. Before I (semi-) retired, I made a plan to not make plans, at least at first: No new hobbies, cooking lessons, or anthropology courses. I figured I would figure it out.
So I am figuring it out. No rush. After a lifetime of rushing, not rushing is part of the point.
One hobby I cultivate is napping. I always get up early, no alarm needed. By late morning I am sometimes inclined to lie down for a bit. Taking a midmorning nap has always struck me as one of life’s great pleasures, though one I could rarely enjoy, unless you count dozing off standing up while a patient described an itch that started 17 years before, on a Thursday.
Now I can shut my eyes for half an hour and wake up refreshed, ready for the rest of the day.
During which I will do ...
An older friend of mine, now long gone, wrote a witty essay on being embarrassed to work at home. He refused to answer the phone during the day and hid from the postman. Contemplating retirement, I was afraid I would also feel that way, picturing myself a pitiful pensioner shuffling abroad at mid-day, looking for a park to poison pigeons in. That of course was before “working remotely” became a goal for cool young strivers. You see them around at all hours, with things sticking out of their ears, talking urgently to no one you can see.
Now I also walk the streets proudly at 11 a.m. or 2:45 p.m. I may get one of those earbuds that stick out at 45 degrees, so people can think my ear fungus has grown branches. Maybe they’ll imagine me a mastermind of an international CBD cartel. What they think doesn’t really matter.
One thing that I actually do all day is wonder why I spent so much of my career worrying about what other people think. Dr. Smith used to refer patients. No longer. Did I fail to meet her expectations? Mr. Trelawney came in weekly with itches and pains. No more. Did I roll my eyes too obviously?
Questions like these used to trouble me. Now I can’t recall why. Instead I worry about more important things, like who will play right field for the Red Sox this year.
Though I never signed up, I am an enrolled Baby Boomer, that navel-gazing cohort now passing from the scene while pretending it won’t. I never understood my generation when it was claiming to overturn the universe in the 1960s. Now its members write and read books with chirpy titles like “Amazing Aging!” as though – because we are so wonderfully special – age, infirmity, and decline will repeal themselves just for us.
Well, anyone can dream.
I go into the office a couple of half-days a week, when I’m in town. I like bantering with the gang and chatting with old patients. They wish me well and hope I’ll refer them to someone worthy when I hang them up for good, as many of their (and my) doctors already have.
Here is one thing I don’t do all day – manage human resource issues in the office. What’s to miss?
Now and then, with lessening frequency, I muse, “Well, if I do get bored, I can always spend more time in the office.”
Time for another nap.
Dr. Rockoff, who wrote the Dermatology News column “Under My Skin,” is now semi-retired, after 40 years of practice in Brookline, Mass. He served on the clinical faculty at Tufts University, Boston, and taught senior medical students and other trainees for 30 years. His second book, “Act Like a Doctor, Think Like a Patient,” is available at amazon.com and barnesandnoble.com. Write to him at dermnews@mdedge.com.
“So what do you do all day?”
.
Asking what an emeritus does all day sounds fair, but the question is harder to answer than it sounds. Last year, I asked Dave, who was already retired. He took a day to think about it.
“Sometimes I sit on the back porch and watch the birds,” he said.
Dave has long been an avid bird-watcher. Along with golf and Facebook, watching birds is a pursuit that really engages many people, but I never understood it. I still don’t.
Over the years, I’ve met people whose experience of retirement has ranged from, “I’m so busy, I don’t know how I had time to work!” to, “I miss the gang and I’m bored,” to everything in between. Before I (semi-) retired, I made a plan to not make plans, at least at first: No new hobbies, cooking lessons, or anthropology courses. I figured I would figure it out.
So I am figuring it out. No rush. After a lifetime of rushing, not rushing is part of the point.
One hobby I cultivate is napping. I always get up early, no alarm needed. By late morning I am sometimes inclined to lie down for a bit. Taking a midmorning nap has always struck me as one of life’s great pleasures, though one I could rarely enjoy, unless you count dozing off standing up while a patient described an itch that started 17 years before, on a Thursday.
Now I can shut my eyes for half an hour and wake up refreshed, ready for the rest of the day.
During which I will do ...
An older friend of mine, now long gone, wrote a witty essay on being embarrassed to work at home. He refused to answer the phone during the day and hid from the postman. Contemplating retirement, I was afraid I would also feel that way, picturing myself a pitiful pensioner shuffling abroad at mid-day, looking for a park to poison pigeons in. That of course was before “working remotely” became a goal for cool young strivers. You see them around at all hours, with things sticking out of their ears, talking urgently to no one you can see.
Now I also walk the streets proudly at 11 a.m. or 2:45 p.m. I may get one of those earbuds that stick out at 45 degrees, so people can think my ear fungus has grown branches. Maybe they’ll imagine me a mastermind of an international CBD cartel. What they think doesn’t really matter.
One thing that I actually do all day is wonder why I spent so much of my career worrying about what other people think. Dr. Smith used to refer patients. No longer. Did I fail to meet her expectations? Mr. Trelawney came in weekly with itches and pains. No more. Did I roll my eyes too obviously?
Questions like these used to trouble me. Now I can’t recall why. Instead I worry about more important things, like who will play right field for the Red Sox this year.
Though I never signed up, I am an enrolled Baby Boomer, that navel-gazing cohort now passing from the scene while pretending it won’t. I never understood my generation when it was claiming to overturn the universe in the 1960s. Now its members write and read books with chirpy titles like “Amazing Aging!” as though – because we are so wonderfully special – age, infirmity, and decline will repeal themselves just for us.
Well, anyone can dream.
I go into the office a couple of half-days a week, when I’m in town. I like bantering with the gang and chatting with old patients. They wish me well and hope I’ll refer them to someone worthy when I hang them up for good, as many of their (and my) doctors already have.
Here is one thing I don’t do all day – manage human resource issues in the office. What’s to miss?
Now and then, with lessening frequency, I muse, “Well, if I do get bored, I can always spend more time in the office.”
Time for another nap.
Dr. Rockoff, who wrote the Dermatology News column “Under My Skin,” is now semi-retired, after 40 years of practice in Brookline, Mass. He served on the clinical faculty at Tufts University, Boston, and taught senior medical students and other trainees for 30 years. His second book, “Act Like a Doctor, Think Like a Patient,” is available at amazon.com and barnesandnoble.com. Write to him at dermnews@mdedge.com.
AGA guideline favors biologics for moderate to severe ulcerative colitis
For moderate to severe ulcerative colitis, treatment with any one of the leading biologic agents is superior to no treatment at all. And in treatment-naive patients, infliximab or vedolizumab should be used rather than adalimumab for inducing remission. These are key recommendations from the American Gastroenterological Association guideline for patients with moderate to severe ulcerative colitis (UC), published in Gastroenterology (doi: 10.1053/j.gastro.2020.01.006).
In all, the guideline comprises 11 recommendations for using immunomodulators, biologics, and small-molecule agents to induce and maintain remission in outpatients with moderate to severe UC and to decrease the need for colectomy in hospitalized patients with acute severe UC. The latest guideline follows a guideline for mild to moderate UC published last year (Gastroenterology. 2019;156[3]:748-64). A technical review accompanied the most recent publication (Gastroenterology. 2020. doi: 10.1053/j.gastro.2020.01.007).
An updated guideline was long overdue, lead author Joseph D. Feuerstein, MD, of Beth Israel Deaconess Medical Center, Boston, said in an interview. “The care of patients with inflammatory bowel disease – both ulcerative colitis and Crohn’s – has become increasingly complicated with many newer drugs becoming available,” he said. “The paradigm of how we are treating the disease is evolving, but we haven’t had updated, evidence-based guidelines.” Dr. Feuerstein is the lead author of this guideline.
The guideline can also aid in influencing payers’ policies that now require step-up therapy – that is, failing with the least costly drug before moving onto newer and more effective but costlier agents – Dr. Feuerstein said. “These guidelines show now that we should be treating people based on the evidence and not based on just an insurance company’s preferred policy,” he said.
The strongest recommendation is to use the tumor necrosis factor–alpha (TNF-alpha) antagonists infliximab, adalimumab, and golimumab, the anti-integrin agent vedolizumab, or the anti-interleukin 12/23 agent ustekinumab — all biologics — or the synthetic JAK inhibitor tofacitinib rather than not treating the UC. This is the only recommendation labeled as “strong,” based on “moderate quality evidence.” The relative risk profiles the committee analyzed all favored the biologics over the JAK inhibitor.
Also based on moderate evidence is the recommendation to use infliximab or vedolizumab rather than adalimumab to induce remission in patients who had taken biologic agents before. The other recommendations are based on evidence listed at “low” or “very low” quality, or citing a “knowledge gap.”
“The quality of the evidence available is variable, and we can only make our recommendations based on the quality of the evidence there, but it doesn’t negate the effects of the guideline itself,” Dr. Feuerstein said. The strong recommendation is based on randomized clinical trials that led to the Food and Drug Administration approvals, said Aline Charabaty-Pishvaian, MD, AGAF, associate professor at Johns Hopkins University, Baltimore, and director of the Inflammatory Bowel Disease Center at Sibley Memorial Hospital, Washington. “For everything else, we do not have randomized controlled trials to help make a decision, and the recommendations are made based on the interpretation of different RCTs [randomized controlled trials], knowing that these trials have different designs, patient populations, and endpoints, as well as on experts’ opinions,” she said in an interview.
The only other recommendation based on moderate quality evidence is to use infliximab or vedolizumab rather than adalimumab to induce remission in patients who haven’t previously used biologic agents. The guideline also recommends using tofacitinib in these patients in the confines of a clinical trial; and using ustekinumab or tofacitinib rather than vedolizumab or adalimumab in patients who’ve already been on infliximab, particularly if they haven’t responded to treatment.
The guideline also recommends against thiopurine monotherapy to induce remission, but, for maintenance of remission, recommends such treatment vs. none. However, the guideline suggests against methotrexate monotherapy to induce or maintain remission. And biologic monotherapy is preferred to thiopurine to induce remission, but the guideline makes no recommendation for biologic vs. thiopurine monotherapy to maintain remission. Likewise, combining vedolizumab or ustekinumab with thiopurines or methotrexate is preferred to monotherapy with either a biologic or thiopurine.
The guideline also addresses step-up therapy. It suggests biologics as a first treatment, either as monotherapy or in combination with an immunomodulator, rather than a step-up after failure with 5-aminosalicylates. Also, it recommends against continuing 5-aminosalicylates to induce or maintain remission after a patient has achieved remission with biologics as monotherapy or in combination with immunomodulators or tofacitinib.
The guideline also offers four recommendations for hospitalized patients with acute severe ulcerative colitis: use of intravenous 40-60 mg/d methylprednisolone rather than higher dose IV corticosteroids, no adjunctive antibiotics in the absence of infection; use of infliximab or cyclosporine when IV corticosteroids fail, and no recommendation on the use of intensive vs. standard infliximab dosing when IV corticosteroids fail and the patient is already on infliximab.
The guideline will be meaningful in closing the evidence gap going forward because it can help direct the design of clinical trials, Dr. Charabaty-Pishvaian said. “The guideline highlights areas of need in terms of randomized clinical trials,” she said. “We need these trials to answer the questions we ask ourselves in our daily practice when managing patients with UC: Which drug to choose as the first-line agent? Which drug is the second-line therapy when the disease doesn’t respond or loses response to the first-line agent? Do we need to use combination therapy with all biologics, or only with anti-TNF-alpha agents? For how long? Can we use vedolizumab or ustekinumab as monotherapy when used as a first-line agent? And is there any advantage in adding an immunomodulator when these agents are used as third- or fourth-line therapy?”
Dr. Feuerstein has no relevant financial relationships to disclose. Guideline author Kim Isaacs, MD, disclosed relationships with AbbVie, Takeda, UCB, Janssen and Hoffmann-Laroche. All other committee members have no relevant disclosures.
*This story was updated on 5/7/2020.
SOURCE: Feuerstein JD et al. on behalf of the AGA Institute Clinical Guidelines Committee. Gastroenterology. 2020. doi: 10.1053/j.gastro.2020.01.006.
For moderate to severe ulcerative colitis, treatment with any one of the leading biologic agents is superior to no treatment at all. And in treatment-naive patients, infliximab or vedolizumab should be used rather than adalimumab for inducing remission. These are key recommendations from the American Gastroenterological Association guideline for patients with moderate to severe ulcerative colitis (UC), published in Gastroenterology (doi: 10.1053/j.gastro.2020.01.006).
In all, the guideline comprises 11 recommendations for using immunomodulators, biologics, and small-molecule agents to induce and maintain remission in outpatients with moderate to severe UC and to decrease the need for colectomy in hospitalized patients with acute severe UC. The latest guideline follows a guideline for mild to moderate UC published last year (Gastroenterology. 2019;156[3]:748-64). A technical review accompanied the most recent publication (Gastroenterology. 2020. doi: 10.1053/j.gastro.2020.01.007).
An updated guideline was long overdue, lead author Joseph D. Feuerstein, MD, of Beth Israel Deaconess Medical Center, Boston, said in an interview. “The care of patients with inflammatory bowel disease – both ulcerative colitis and Crohn’s – has become increasingly complicated with many newer drugs becoming available,” he said. “The paradigm of how we are treating the disease is evolving, but we haven’t had updated, evidence-based guidelines.” Dr. Feuerstein is the lead author of this guideline.
The guideline can also aid in influencing payers’ policies that now require step-up therapy – that is, failing with the least costly drug before moving onto newer and more effective but costlier agents – Dr. Feuerstein said. “These guidelines show now that we should be treating people based on the evidence and not based on just an insurance company’s preferred policy,” he said.
The strongest recommendation is to use the tumor necrosis factor–alpha (TNF-alpha) antagonists infliximab, adalimumab, and golimumab, the anti-integrin agent vedolizumab, or the anti-interleukin 12/23 agent ustekinumab — all biologics — or the synthetic JAK inhibitor tofacitinib rather than not treating the UC. This is the only recommendation labeled as “strong,” based on “moderate quality evidence.” The relative risk profiles the committee analyzed all favored the biologics over the JAK inhibitor.
Also based on moderate evidence is the recommendation to use infliximab or vedolizumab rather than adalimumab to induce remission in patients who had taken biologic agents before. The other recommendations are based on evidence listed at “low” or “very low” quality, or citing a “knowledge gap.”
“The quality of the evidence available is variable, and we can only make our recommendations based on the quality of the evidence there, but it doesn’t negate the effects of the guideline itself,” Dr. Feuerstein said. The strong recommendation is based on randomized clinical trials that led to the Food and Drug Administration approvals, said Aline Charabaty-Pishvaian, MD, AGAF, associate professor at Johns Hopkins University, Baltimore, and director of the Inflammatory Bowel Disease Center at Sibley Memorial Hospital, Washington. “For everything else, we do not have randomized controlled trials to help make a decision, and the recommendations are made based on the interpretation of different RCTs [randomized controlled trials], knowing that these trials have different designs, patient populations, and endpoints, as well as on experts’ opinions,” she said in an interview.
The only other recommendation based on moderate quality evidence is to use infliximab or vedolizumab rather than adalimumab to induce remission in patients who haven’t previously used biologic agents. The guideline also recommends using tofacitinib in these patients in the confines of a clinical trial; and using ustekinumab or tofacitinib rather than vedolizumab or adalimumab in patients who’ve already been on infliximab, particularly if they haven’t responded to treatment.
The guideline also recommends against thiopurine monotherapy to induce remission, but, for maintenance of remission, recommends such treatment vs. none. However, the guideline suggests against methotrexate monotherapy to induce or maintain remission. And biologic monotherapy is preferred to thiopurine to induce remission, but the guideline makes no recommendation for biologic vs. thiopurine monotherapy to maintain remission. Likewise, combining vedolizumab or ustekinumab with thiopurines or methotrexate is preferred to monotherapy with either a biologic or thiopurine.
The guideline also addresses step-up therapy. It suggests biologics as a first treatment, either as monotherapy or in combination with an immunomodulator, rather than a step-up after failure with 5-aminosalicylates. Also, it recommends against continuing 5-aminosalicylates to induce or maintain remission after a patient has achieved remission with biologics as monotherapy or in combination with immunomodulators or tofacitinib.
The guideline also offers four recommendations for hospitalized patients with acute severe ulcerative colitis: use of intravenous 40-60 mg/d methylprednisolone rather than higher dose IV corticosteroids, no adjunctive antibiotics in the absence of infection; use of infliximab or cyclosporine when IV corticosteroids fail, and no recommendation on the use of intensive vs. standard infliximab dosing when IV corticosteroids fail and the patient is already on infliximab.
The guideline will be meaningful in closing the evidence gap going forward because it can help direct the design of clinical trials, Dr. Charabaty-Pishvaian said. “The guideline highlights areas of need in terms of randomized clinical trials,” she said. “We need these trials to answer the questions we ask ourselves in our daily practice when managing patients with UC: Which drug to choose as the first-line agent? Which drug is the second-line therapy when the disease doesn’t respond or loses response to the first-line agent? Do we need to use combination therapy with all biologics, or only with anti-TNF-alpha agents? For how long? Can we use vedolizumab or ustekinumab as monotherapy when used as a first-line agent? And is there any advantage in adding an immunomodulator when these agents are used as third- or fourth-line therapy?”
Dr. Feuerstein has no relevant financial relationships to disclose. Guideline author Kim Isaacs, MD, disclosed relationships with AbbVie, Takeda, UCB, Janssen and Hoffmann-Laroche. All other committee members have no relevant disclosures.
*This story was updated on 5/7/2020.
SOURCE: Feuerstein JD et al. on behalf of the AGA Institute Clinical Guidelines Committee. Gastroenterology. 2020. doi: 10.1053/j.gastro.2020.01.006.
For moderate to severe ulcerative colitis, treatment with any one of the leading biologic agents is superior to no treatment at all. And in treatment-naive patients, infliximab or vedolizumab should be used rather than adalimumab for inducing remission. These are key recommendations from the American Gastroenterological Association guideline for patients with moderate to severe ulcerative colitis (UC), published in Gastroenterology (doi: 10.1053/j.gastro.2020.01.006).
In all, the guideline comprises 11 recommendations for using immunomodulators, biologics, and small-molecule agents to induce and maintain remission in outpatients with moderate to severe UC and to decrease the need for colectomy in hospitalized patients with acute severe UC. The latest guideline follows a guideline for mild to moderate UC published last year (Gastroenterology. 2019;156[3]:748-64). A technical review accompanied the most recent publication (Gastroenterology. 2020. doi: 10.1053/j.gastro.2020.01.007).
An updated guideline was long overdue, lead author Joseph D. Feuerstein, MD, of Beth Israel Deaconess Medical Center, Boston, said in an interview. “The care of patients with inflammatory bowel disease – both ulcerative colitis and Crohn’s – has become increasingly complicated with many newer drugs becoming available,” he said. “The paradigm of how we are treating the disease is evolving, but we haven’t had updated, evidence-based guidelines.” Dr. Feuerstein is the lead author of this guideline.
The guideline can also aid in influencing payers’ policies that now require step-up therapy – that is, failing with the least costly drug before moving onto newer and more effective but costlier agents – Dr. Feuerstein said. “These guidelines show now that we should be treating people based on the evidence and not based on just an insurance company’s preferred policy,” he said.
The strongest recommendation is to use the tumor necrosis factor–alpha (TNF-alpha) antagonists infliximab, adalimumab, and golimumab, the anti-integrin agent vedolizumab, or the anti-interleukin 12/23 agent ustekinumab — all biologics — or the synthetic JAK inhibitor tofacitinib rather than not treating the UC. This is the only recommendation labeled as “strong,” based on “moderate quality evidence.” The relative risk profiles the committee analyzed all favored the biologics over the JAK inhibitor.
Also based on moderate evidence is the recommendation to use infliximab or vedolizumab rather than adalimumab to induce remission in patients who had taken biologic agents before. The other recommendations are based on evidence listed at “low” or “very low” quality, or citing a “knowledge gap.”
“The quality of the evidence available is variable, and we can only make our recommendations based on the quality of the evidence there, but it doesn’t negate the effects of the guideline itself,” Dr. Feuerstein said. The strong recommendation is based on randomized clinical trials that led to the Food and Drug Administration approvals, said Aline Charabaty-Pishvaian, MD, AGAF, associate professor at Johns Hopkins University, Baltimore, and director of the Inflammatory Bowel Disease Center at Sibley Memorial Hospital, Washington. “For everything else, we do not have randomized controlled trials to help make a decision, and the recommendations are made based on the interpretation of different RCTs [randomized controlled trials], knowing that these trials have different designs, patient populations, and endpoints, as well as on experts’ opinions,” she said in an interview.
The only other recommendation based on moderate quality evidence is to use infliximab or vedolizumab rather than adalimumab to induce remission in patients who haven’t previously used biologic agents. The guideline also recommends using tofacitinib in these patients in the confines of a clinical trial; and using ustekinumab or tofacitinib rather than vedolizumab or adalimumab in patients who’ve already been on infliximab, particularly if they haven’t responded to treatment.
The guideline also recommends against thiopurine monotherapy to induce remission, but, for maintenance of remission, recommends such treatment vs. none. However, the guideline suggests against methotrexate monotherapy to induce or maintain remission. And biologic monotherapy is preferred to thiopurine to induce remission, but the guideline makes no recommendation for biologic vs. thiopurine monotherapy to maintain remission. Likewise, combining vedolizumab or ustekinumab with thiopurines or methotrexate is preferred to monotherapy with either a biologic or thiopurine.
The guideline also addresses step-up therapy. It suggests biologics as a first treatment, either as monotherapy or in combination with an immunomodulator, rather than a step-up after failure with 5-aminosalicylates. Also, it recommends against continuing 5-aminosalicylates to induce or maintain remission after a patient has achieved remission with biologics as monotherapy or in combination with immunomodulators or tofacitinib.
The guideline also offers four recommendations for hospitalized patients with acute severe ulcerative colitis: use of intravenous 40-60 mg/d methylprednisolone rather than higher dose IV corticosteroids, no adjunctive antibiotics in the absence of infection; use of infliximab or cyclosporine when IV corticosteroids fail, and no recommendation on the use of intensive vs. standard infliximab dosing when IV corticosteroids fail and the patient is already on infliximab.
The guideline will be meaningful in closing the evidence gap going forward because it can help direct the design of clinical trials, Dr. Charabaty-Pishvaian said. “The guideline highlights areas of need in terms of randomized clinical trials,” she said. “We need these trials to answer the questions we ask ourselves in our daily practice when managing patients with UC: Which drug to choose as the first-line agent? Which drug is the second-line therapy when the disease doesn’t respond or loses response to the first-line agent? Do we need to use combination therapy with all biologics, or only with anti-TNF-alpha agents? For how long? Can we use vedolizumab or ustekinumab as monotherapy when used as a first-line agent? And is there any advantage in adding an immunomodulator when these agents are used as third- or fourth-line therapy?”
Dr. Feuerstein has no relevant financial relationships to disclose. Guideline author Kim Isaacs, MD, disclosed relationships with AbbVie, Takeda, UCB, Janssen and Hoffmann-Laroche. All other committee members have no relevant disclosures.
*This story was updated on 5/7/2020.
SOURCE: Feuerstein JD et al. on behalf of the AGA Institute Clinical Guidelines Committee. Gastroenterology. 2020. doi: 10.1053/j.gastro.2020.01.006.
FROM GASTROENTEROLOGY
Age divide seen in colorectal cancer screening use
Roughly 79% of adults aged 65-75 years were up to date with their colorectal cancer (CRC) screening in 2018, compared with a significantly lower 63% of those aged 50-64, according to the Centers for Disease Control and Prevention.
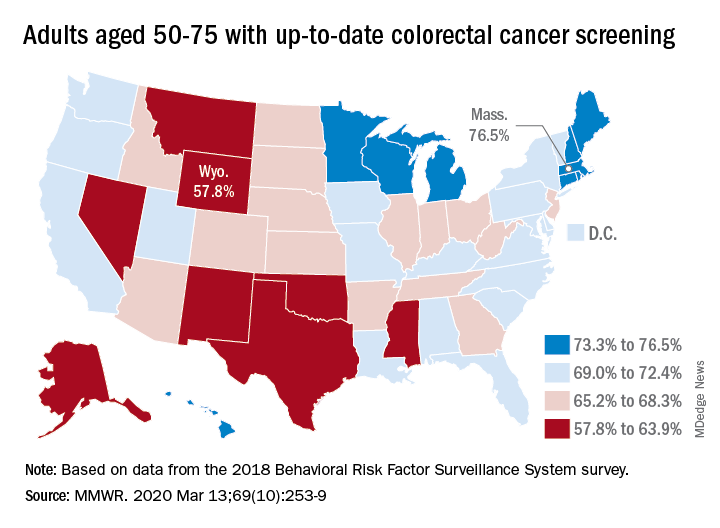
That works out to almost 69% of Americans aged 50-75 years with up-to-date CRC screening, which was defined as a blood stool test in the past year, sigmoidoscopy in the past 5 years, and/or colonoscopy in the past 10 years, Djenaba A. Joseph, MD, and associates at the CDC’s National Center for Chronic Disease Prevention and Health Promotion wrote in the Morbidity and Mortality Weekly Report.
“CRC screening has increased steadily among adults over the past 20 years,” the authors noted. However, they observed a lower rate of screening in the 50-64 age group (vs. the 65-75 age group) that was consistent and significant across all demographic groups studied: sex, race/ethnicity, education, annual income, residence location, health insurance status, and regular health care provider status.
The range of screening rates in those groups went from a low of 32.6% (in 50- to 64-year-olds who had no health insurance) to a high of 87.1% (in 65- to 75-year-olds who had annual household incomes of $75,000 and over).
Up-to-date CRC screening rates were higher for the 65-75 age group in every state. The highest screening rate was observed in Rhode Island, at 84.9% in the 65-75 age group. Massachusetts had the highest rate for 50- to 64-year-olds, at 72.1%, and the highest rate for all ages studied (50-75 years), at 76.5%. Wyoming was lowest in all three categories: 51.5% in the 50-64 age group, 68.5% in the 65-75 age group, and 57.8% in the 50-75 age group.
“To achieve further increases in CRC screening to maximize benefit, specific efforts to increase screening in persons aged 50-64 years are needed,” Dr. Joseph and colleagues wrote. They added that efforts might include “providing education about insurance coverage for preventive services, providing clear communication about test options, and conducting research to identify and understand barriers and facilitators to CRC screening specific to this younger age group to inform effective interventions to increase screening.”
SOURCE: Joseph DA et al. MMWR. 2020 Mar 13;69(10):253-9.
Roughly 79% of adults aged 65-75 years were up to date with their colorectal cancer (CRC) screening in 2018, compared with a significantly lower 63% of those aged 50-64, according to the Centers for Disease Control and Prevention.

That works out to almost 69% of Americans aged 50-75 years with up-to-date CRC screening, which was defined as a blood stool test in the past year, sigmoidoscopy in the past 5 years, and/or colonoscopy in the past 10 years, Djenaba A. Joseph, MD, and associates at the CDC’s National Center for Chronic Disease Prevention and Health Promotion wrote in the Morbidity and Mortality Weekly Report.
“CRC screening has increased steadily among adults over the past 20 years,” the authors noted. However, they observed a lower rate of screening in the 50-64 age group (vs. the 65-75 age group) that was consistent and significant across all demographic groups studied: sex, race/ethnicity, education, annual income, residence location, health insurance status, and regular health care provider status.
The range of screening rates in those groups went from a low of 32.6% (in 50- to 64-year-olds who had no health insurance) to a high of 87.1% (in 65- to 75-year-olds who had annual household incomes of $75,000 and over).
Up-to-date CRC screening rates were higher for the 65-75 age group in every state. The highest screening rate was observed in Rhode Island, at 84.9% in the 65-75 age group. Massachusetts had the highest rate for 50- to 64-year-olds, at 72.1%, and the highest rate for all ages studied (50-75 years), at 76.5%. Wyoming was lowest in all three categories: 51.5% in the 50-64 age group, 68.5% in the 65-75 age group, and 57.8% in the 50-75 age group.
“To achieve further increases in CRC screening to maximize benefit, specific efforts to increase screening in persons aged 50-64 years are needed,” Dr. Joseph and colleagues wrote. They added that efforts might include “providing education about insurance coverage for preventive services, providing clear communication about test options, and conducting research to identify and understand barriers and facilitators to CRC screening specific to this younger age group to inform effective interventions to increase screening.”
SOURCE: Joseph DA et al. MMWR. 2020 Mar 13;69(10):253-9.
Roughly 79% of adults aged 65-75 years were up to date with their colorectal cancer (CRC) screening in 2018, compared with a significantly lower 63% of those aged 50-64, according to the Centers for Disease Control and Prevention.

That works out to almost 69% of Americans aged 50-75 years with up-to-date CRC screening, which was defined as a blood stool test in the past year, sigmoidoscopy in the past 5 years, and/or colonoscopy in the past 10 years, Djenaba A. Joseph, MD, and associates at the CDC’s National Center for Chronic Disease Prevention and Health Promotion wrote in the Morbidity and Mortality Weekly Report.
“CRC screening has increased steadily among adults over the past 20 years,” the authors noted. However, they observed a lower rate of screening in the 50-64 age group (vs. the 65-75 age group) that was consistent and significant across all demographic groups studied: sex, race/ethnicity, education, annual income, residence location, health insurance status, and regular health care provider status.
The range of screening rates in those groups went from a low of 32.6% (in 50- to 64-year-olds who had no health insurance) to a high of 87.1% (in 65- to 75-year-olds who had annual household incomes of $75,000 and over).
Up-to-date CRC screening rates were higher for the 65-75 age group in every state. The highest screening rate was observed in Rhode Island, at 84.9% in the 65-75 age group. Massachusetts had the highest rate for 50- to 64-year-olds, at 72.1%, and the highest rate for all ages studied (50-75 years), at 76.5%. Wyoming was lowest in all three categories: 51.5% in the 50-64 age group, 68.5% in the 65-75 age group, and 57.8% in the 50-75 age group.
“To achieve further increases in CRC screening to maximize benefit, specific efforts to increase screening in persons aged 50-64 years are needed,” Dr. Joseph and colleagues wrote. They added that efforts might include “providing education about insurance coverage for preventive services, providing clear communication about test options, and conducting research to identify and understand barriers and facilitators to CRC screening specific to this younger age group to inform effective interventions to increase screening.”
SOURCE: Joseph DA et al. MMWR. 2020 Mar 13;69(10):253-9.
FROM MMWR
FDA warns of serious infection risk after FMT
The Food and Drug Administration has issued a Safety Alert warning of the potential risk of serious, life-threatening infection in patients who receive fecal microbiota transplant for Clostridioides difficile infection.
The FDA has received six reports of infection associated with fecal microbiota transplant from a stool bank company based in the United States: Two patients had enteropathogenic Escherichia coli (EPEC) infection, and four had shiga toxin–producing E. coli (STEC). The two EPEC infections came from two separate donors, but the four STEC infections came from a single donor, according to the FDA.
In addition, two patients died after receiving fecal microbiota transplant from the donor associated with the STEC infections. These patients died before any of the STEC infections were reported to the FDA; as their stool was not tested for STEC, it is unclear whether it contributed to their deaths.
The use of fecal microbiota transplant is still investigational, and as such, patients should be made aware by health care providers of the risks, which include the potential for transmission of pathogenic bacteria and the resultant adverse events, the FDA said in the press release.
The Food and Drug Administration has issued a Safety Alert warning of the potential risk of serious, life-threatening infection in patients who receive fecal microbiota transplant for Clostridioides difficile infection.
The FDA has received six reports of infection associated with fecal microbiota transplant from a stool bank company based in the United States: Two patients had enteropathogenic Escherichia coli (EPEC) infection, and four had shiga toxin–producing E. coli (STEC). The two EPEC infections came from two separate donors, but the four STEC infections came from a single donor, according to the FDA.
In addition, two patients died after receiving fecal microbiota transplant from the donor associated with the STEC infections. These patients died before any of the STEC infections were reported to the FDA; as their stool was not tested for STEC, it is unclear whether it contributed to their deaths.
The use of fecal microbiota transplant is still investigational, and as such, patients should be made aware by health care providers of the risks, which include the potential for transmission of pathogenic bacteria and the resultant adverse events, the FDA said in the press release.
The Food and Drug Administration has issued a Safety Alert warning of the potential risk of serious, life-threatening infection in patients who receive fecal microbiota transplant for Clostridioides difficile infection.
The FDA has received six reports of infection associated with fecal microbiota transplant from a stool bank company based in the United States: Two patients had enteropathogenic Escherichia coli (EPEC) infection, and four had shiga toxin–producing E. coli (STEC). The two EPEC infections came from two separate donors, but the four STEC infections came from a single donor, according to the FDA.
In addition, two patients died after receiving fecal microbiota transplant from the donor associated with the STEC infections. These patients died before any of the STEC infections were reported to the FDA; as their stool was not tested for STEC, it is unclear whether it contributed to their deaths.
The use of fecal microbiota transplant is still investigational, and as such, patients should be made aware by health care providers of the risks, which include the potential for transmission of pathogenic bacteria and the resultant adverse events, the FDA said in the press release.