User login
Dermatologists best at finding work satisfaction in the office
, according to Medscape’s 2020 Lifestyle, Happiness, and Burnout Report.

About 41% of dermatologists reported being very happy at work, making their specialty the only one to break the 40% barrier. While dermatologists weren’t the happiest outside of work – that honor went to rheumatologists – dermatology was only 4 percentage points behind (60% vs. 56%).
Perhaps unsurprisingly, the percentage of dermatologists who were burned out was lower than that of physicians overall (36% vs. 41%). The biggest factors leading to burnout in dermatologists were an overabundance of bureaucratic tasks (58%), increased time devoted to EHRs (38%), and compliance with government regulations (35%).
Dermatologists dealt with burnout through a variety of ways, with the most common being exercise (44%), talk with family/friends (44%), and isolation from others (40%). In addition, dermatologists took slightly more vacation time than did physicians overall, with 51% of dermatologists taking 3-4 weeks of vacation, compared with 44% for physicians overall.
About 16% of dermatologists have contemplated suicide; however, none reported attempting suicide, and 72% of dermatologists have never felt suicidal. Most dermatologists also plan to deal with burnout or depression on their own, with only 31% reporting that they are currently seeking professional help, planning to seek help, or are not currently looking but have been treated in the past.
The Medscape survey was conducted from June 25 to Sept. 19, 2019, and involved 15,181 physicians.
, according to Medscape’s 2020 Lifestyle, Happiness, and Burnout Report.

About 41% of dermatologists reported being very happy at work, making their specialty the only one to break the 40% barrier. While dermatologists weren’t the happiest outside of work – that honor went to rheumatologists – dermatology was only 4 percentage points behind (60% vs. 56%).
Perhaps unsurprisingly, the percentage of dermatologists who were burned out was lower than that of physicians overall (36% vs. 41%). The biggest factors leading to burnout in dermatologists were an overabundance of bureaucratic tasks (58%), increased time devoted to EHRs (38%), and compliance with government regulations (35%).
Dermatologists dealt with burnout through a variety of ways, with the most common being exercise (44%), talk with family/friends (44%), and isolation from others (40%). In addition, dermatologists took slightly more vacation time than did physicians overall, with 51% of dermatologists taking 3-4 weeks of vacation, compared with 44% for physicians overall.
About 16% of dermatologists have contemplated suicide; however, none reported attempting suicide, and 72% of dermatologists have never felt suicidal. Most dermatologists also plan to deal with burnout or depression on their own, with only 31% reporting that they are currently seeking professional help, planning to seek help, or are not currently looking but have been treated in the past.
The Medscape survey was conducted from June 25 to Sept. 19, 2019, and involved 15,181 physicians.
, according to Medscape’s 2020 Lifestyle, Happiness, and Burnout Report.

About 41% of dermatologists reported being very happy at work, making their specialty the only one to break the 40% barrier. While dermatologists weren’t the happiest outside of work – that honor went to rheumatologists – dermatology was only 4 percentage points behind (60% vs. 56%).
Perhaps unsurprisingly, the percentage of dermatologists who were burned out was lower than that of physicians overall (36% vs. 41%). The biggest factors leading to burnout in dermatologists were an overabundance of bureaucratic tasks (58%), increased time devoted to EHRs (38%), and compliance with government regulations (35%).
Dermatologists dealt with burnout through a variety of ways, with the most common being exercise (44%), talk with family/friends (44%), and isolation from others (40%). In addition, dermatologists took slightly more vacation time than did physicians overall, with 51% of dermatologists taking 3-4 weeks of vacation, compared with 44% for physicians overall.
About 16% of dermatologists have contemplated suicide; however, none reported attempting suicide, and 72% of dermatologists have never felt suicidal. Most dermatologists also plan to deal with burnout or depression on their own, with only 31% reporting that they are currently seeking professional help, planning to seek help, or are not currently looking but have been treated in the past.
The Medscape survey was conducted from June 25 to Sept. 19, 2019, and involved 15,181 physicians.
Testing times for epidermolysis bullosa topical therapies
LONDON –
Results from trials such as ESSENCE, with allantoin, and DELIVERS, with diacerein, were “disappointing,” Dédée Murrell, BMBCh, MD, pointed out at the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association (DEBRA).
Those two topical agents were most likely let down by the trials’ design, said Dr. Murrell, of St. George Hospital, University of New South Wales, Sydney, but she noted that there were still some promising trials that were either ongoing, such as EASE, with Oleogel-S10, or that were about to be unblinded, such as SISTERS, with sirolimus.
Epidermolysis bullosa (EB) is a group of rare genetic diseases that can cause the skin to blister and peel away to varying degrees, causing itchy and painful skin, as well as recurrent wounds, some of which may seem never to heal and that increase the risk for squamous cell carcinoma. Although finding a cure for the disease is high on the research agenda, finding a reliable therapy that can soothe and protect the skin is of equal importance.
Trials and tribulations
Conducting trials in rare diseases can be difficult because the studies are often small and poorly controlled, Dr. Murrell said during an oral presentation at the meeting. To gain regulatory approval, trials need to have an active and a placebo arm, because “even though we’re dealing with a rare disease, we still have to show statistical significance between the two arms.”
However, it is not just about finding enough participants who meet the inclusion criteria and adequately controlling the study, as finding funding can also be a significant hurdle. That is the case particularly when an existing drug with no patent protection is proposed to be repurposed. As an example, Dr. Murrell said that many patients with EB may use gentian violet to treat their condition, but it has been around for so long and is so widely used, that funding a trial to formally prove its merit is unlikely. In addition, “there are special caveats that occur in dermatology clinical trials with topical drugs that don’t exist [in trials] with systemic treatments, one of which is that it is very important to keep other variables the same,” Dr. Murrell said. “So, for example, the dressings need to stay the same throughout a trial with a topical therapy, because if you improve the dressings [during the course of the trial], you could mask the effect of the treatment.” Similarly, the bathing and cleansing routines of the participants need to remain the same throughout the trial.
“We also need to have validated instruments to prove whether these treatments are working, and the instruments need to be objective as well as subjective,” Dr. Murrell advised. For example, inflammation and blistering need to be scored separately from scarring and skin damage. “You have to conduct a clinical trial to be able to verify that there is diminished scarring or damage, because those are the longer-term complications.” Inflammation and blistering are valid endpoints to use in shorter-term studies.
Dr. Murrell also cautioned on getting too enthused about the results of case reports. “We do get excited when we see a patient using something new and they seem to be getting much better,” but such reports do not have a placebo arm, or, if there is one, then there is no vehicle control, she said. It’s important to include a run-in period in a trial to establish a new baseline and to ensure that any effects seen with a topical agent are independent of the carrier substance or any altered bathing behavior or dressing habits, which could skew the results.
ESSENCE and allantoin
So what went wrong in the phase 3 ESSENCE trial with allantoin, which was halted early in September 2017? The trial had included 169 patients with any type of EB – simplex, recessive dystrophic, and junctional non-Herlitz – who were randomized to treatment with the allantoin-containing cream SD-101 or a placebo cream containing only the vehicle. The creams were applied daily to the entire body for 3 months, with the primary endpoint being total wound closure at the end of the treatment period. Total wound closure was a requirement of the Food and Drug Administration, Dr. Murrell said, but it is now known that 100% closure is not always likely, which the agency itself now concedes.
“Most disappointingly, no significant difference was found [between the study drug and placebo], therefore it didn’t meet the primary endpoint, and you’re not even allowed to consider secondary endpoints – those are the rules of the game,” she said. As a result, the trial was stopped in 2017.
For inclusion in the study, patients had to have at least one target wound that had been present for at least 3 weeks, but there was no stratification on the duration of wounds in the randomization process. That meant that some individuals with wounds of shorter duration had unintentionally ended up in the placebo arm – favoring healing – and those with more chronic wounds had been in the allantoin arm. So, because the study arms might not have been equally balanced at baseline, it would have been harder for the actual treatment to demonstrate a benefit, Dr. Murrell suggested.
Another problem with the trial was that the vehicle cream contained elements, such as lanolin, already associated with wound healing. That would have given patients in the placebo arm an advantage because anyone applying the cream every day would probably get better or improve to some degree.
The patients were also required to have daily dressing changes and baths and, “if you give any patient that advice and they comply with it for a period of time, they are going to improve,” whether or not they are applying the study drug. Dr. Murrell said that the researchers likely should have done a run-in period first and then established a new baseline to randomize the patients.
“Lastly, no one had ever done a study of what we essentially tell eczema patients to do every day … to moisturize, because that will provide extra protection and barrier to their skin. So, if anything, the ESSENCE study shows that moisturizing has a protective effect of the vehicle for patients with EB,” she said.
DELIVERS and diacerein
Another trial that was stopped prematurely was the phase 2 DELIVERS study, which was set up to assess the benefits of topical diacerein in people with EB simplex. Diacerein, an extract of rhubarb root, was tested in 54 patients, who were randomized to apply either diacerein or vehicle ointment for 8 weeks.
Initially, the results “looked very promising,” Dr. Murrell said, because there was a trend toward improved EB simplex lesions, with the primary endpoint of at least a 60% reduction in lesions met by 57.1% of diacerein-treated and 53.8% of vehicle-treated patients.
However, the trial included use of the Investigator’s Global Assessment Scale at the FDA’s behest, but the tool had not been validated in previous EB trials, and which didn’t seem to show any benefit of the active over the placebo ointment. (The Investigator’s Global Assessment is a 5-point scale used for overall clinical assessment of severity of disease, ranging from 0 to 4, where a higher score denotes worse outcome.)In a poster presented separately at the meeting, the DELIVERS researchers noted that “the lack of statistical significance in the primary endpoint could be explained in part by milder disease in the diacerein group.” The mean body surface area of EB simplex lesions within the assessment area at baseline was 5.76% in the diacerein group and 7.13% in the vehicle group. The researchers proposed that perhaps a higher concentration of diacerein than the 1% used in the trial might have been needed.
Sirolimus and EB simplex
Dr. Murrell noted that a pilot study, known as the SISTERS trial, had been conducted with a 2% sirolimus topical ointment at her institution and at Stanford (Calif.) University. This prospective, double-blind study had involved 16 patients with EB simplex, in which blisters tend to be confined to the palms of the hands and soles of the feet. The patients were assigned to treat both feet with either topical sirolimus or a placebo cream for 12 weeks. After a 4-week wash-out period, the patients switched to using the opposite cream for an additional 12 weeks.
Sirolimus is an inhibitor of the mTOR pathway, and, according to a description of the study on ClinicalTrials.gov, the researchers’ aim was to inhibit “the mTOR pathway to down-regulate the translation of defective keratin proteins.” That would allow a transition from supportive care, which is the current practice for EB simplex, to using a targeted molecular therapy to improve patient mobility and quality of life, they note on the site.
“We look forward to having that study unblinded,” Dr. Murrell said, adding that “data should be ready in a few months.”
EASE and Oleogel-S10
Oleogel-S10 is a gel that contains a birch bark extract dissolved in sunflower oil. It is already approved in Europe (Episalvan) for the treatment of partial-thickness skin wounds, but its use in EB remains investigational.
In a poster presentation at the meeting, Stella Gewert, MD, of the University of Freiburg (Germany) and colleagues discussed their experience using Oleogel-S10 in the treatment of four patients – each with a different type of EB – who applied the gel for between 6 days and 3 months.
Promising effects were seen, including reduced pruritus and pain, wounds healing more quickly, and reductions in lesion size. “During treatment, dressing requirements were reduced, and patient quality of life improved,” the researchers observed.
Mark Sumeray, MD, the chief medical officer of Amryt Pharmaceuticals, which is developing Oleogel-S10, said it was important to emphasize that Oleogel-S10 is a gel and not a cream. Gels are mixed with oil and are easier to apply – an important consideration for those with EB, he explained, whereas creams tend to be mixed with water and are stickier.
The phase 3 EASE trial is looking at the efficacy and safety of the gel in patients with junctional and dystrophic EB, and recruitment is ongoing, Dr. Murrell said. The primary endpoint is the proportion of patients with the first complete closure of a target wound within 45 days of treatment initiation. The estimated primary completion date for the trial is June 2020, and it is projected to end by 2022.
Scioderm, in collaboration with Amicus, funded the ESSENCE trial; Castle Creek financed the DELIVERS study; Amryt is supporting the EASE study; and Stanford University is sponsor of the SISTERS study. Dr. Murrell has been the principal investigator for trials run by Amicus, Amryt, Castle Creek, and Shire, and she acknowledged receipt of honoraria or consultation fees from those companies and others. Dr. Gewert did not report any financial disclosures. Dr. Sumeray is an employee and shareholder of Amryt.
LONDON –
Results from trials such as ESSENCE, with allantoin, and DELIVERS, with diacerein, were “disappointing,” Dédée Murrell, BMBCh, MD, pointed out at the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association (DEBRA).
Those two topical agents were most likely let down by the trials’ design, said Dr. Murrell, of St. George Hospital, University of New South Wales, Sydney, but she noted that there were still some promising trials that were either ongoing, such as EASE, with Oleogel-S10, or that were about to be unblinded, such as SISTERS, with sirolimus.
Epidermolysis bullosa (EB) is a group of rare genetic diseases that can cause the skin to blister and peel away to varying degrees, causing itchy and painful skin, as well as recurrent wounds, some of which may seem never to heal and that increase the risk for squamous cell carcinoma. Although finding a cure for the disease is high on the research agenda, finding a reliable therapy that can soothe and protect the skin is of equal importance.
Trials and tribulations
Conducting trials in rare diseases can be difficult because the studies are often small and poorly controlled, Dr. Murrell said during an oral presentation at the meeting. To gain regulatory approval, trials need to have an active and a placebo arm, because “even though we’re dealing with a rare disease, we still have to show statistical significance between the two arms.”
However, it is not just about finding enough participants who meet the inclusion criteria and adequately controlling the study, as finding funding can also be a significant hurdle. That is the case particularly when an existing drug with no patent protection is proposed to be repurposed. As an example, Dr. Murrell said that many patients with EB may use gentian violet to treat their condition, but it has been around for so long and is so widely used, that funding a trial to formally prove its merit is unlikely. In addition, “there are special caveats that occur in dermatology clinical trials with topical drugs that don’t exist [in trials] with systemic treatments, one of which is that it is very important to keep other variables the same,” Dr. Murrell said. “So, for example, the dressings need to stay the same throughout a trial with a topical therapy, because if you improve the dressings [during the course of the trial], you could mask the effect of the treatment.” Similarly, the bathing and cleansing routines of the participants need to remain the same throughout the trial.
“We also need to have validated instruments to prove whether these treatments are working, and the instruments need to be objective as well as subjective,” Dr. Murrell advised. For example, inflammation and blistering need to be scored separately from scarring and skin damage. “You have to conduct a clinical trial to be able to verify that there is diminished scarring or damage, because those are the longer-term complications.” Inflammation and blistering are valid endpoints to use in shorter-term studies.
Dr. Murrell also cautioned on getting too enthused about the results of case reports. “We do get excited when we see a patient using something new and they seem to be getting much better,” but such reports do not have a placebo arm, or, if there is one, then there is no vehicle control, she said. It’s important to include a run-in period in a trial to establish a new baseline and to ensure that any effects seen with a topical agent are independent of the carrier substance or any altered bathing behavior or dressing habits, which could skew the results.
ESSENCE and allantoin
So what went wrong in the phase 3 ESSENCE trial with allantoin, which was halted early in September 2017? The trial had included 169 patients with any type of EB – simplex, recessive dystrophic, and junctional non-Herlitz – who were randomized to treatment with the allantoin-containing cream SD-101 or a placebo cream containing only the vehicle. The creams were applied daily to the entire body for 3 months, with the primary endpoint being total wound closure at the end of the treatment period. Total wound closure was a requirement of the Food and Drug Administration, Dr. Murrell said, but it is now known that 100% closure is not always likely, which the agency itself now concedes.
“Most disappointingly, no significant difference was found [between the study drug and placebo], therefore it didn’t meet the primary endpoint, and you’re not even allowed to consider secondary endpoints – those are the rules of the game,” she said. As a result, the trial was stopped in 2017.
For inclusion in the study, patients had to have at least one target wound that had been present for at least 3 weeks, but there was no stratification on the duration of wounds in the randomization process. That meant that some individuals with wounds of shorter duration had unintentionally ended up in the placebo arm – favoring healing – and those with more chronic wounds had been in the allantoin arm. So, because the study arms might not have been equally balanced at baseline, it would have been harder for the actual treatment to demonstrate a benefit, Dr. Murrell suggested.
Another problem with the trial was that the vehicle cream contained elements, such as lanolin, already associated with wound healing. That would have given patients in the placebo arm an advantage because anyone applying the cream every day would probably get better or improve to some degree.
The patients were also required to have daily dressing changes and baths and, “if you give any patient that advice and they comply with it for a period of time, they are going to improve,” whether or not they are applying the study drug. Dr. Murrell said that the researchers likely should have done a run-in period first and then established a new baseline to randomize the patients.
“Lastly, no one had ever done a study of what we essentially tell eczema patients to do every day … to moisturize, because that will provide extra protection and barrier to their skin. So, if anything, the ESSENCE study shows that moisturizing has a protective effect of the vehicle for patients with EB,” she said.
DELIVERS and diacerein
Another trial that was stopped prematurely was the phase 2 DELIVERS study, which was set up to assess the benefits of topical diacerein in people with EB simplex. Diacerein, an extract of rhubarb root, was tested in 54 patients, who were randomized to apply either diacerein or vehicle ointment for 8 weeks.
Initially, the results “looked very promising,” Dr. Murrell said, because there was a trend toward improved EB simplex lesions, with the primary endpoint of at least a 60% reduction in lesions met by 57.1% of diacerein-treated and 53.8% of vehicle-treated patients.
However, the trial included use of the Investigator’s Global Assessment Scale at the FDA’s behest, but the tool had not been validated in previous EB trials, and which didn’t seem to show any benefit of the active over the placebo ointment. (The Investigator’s Global Assessment is a 5-point scale used for overall clinical assessment of severity of disease, ranging from 0 to 4, where a higher score denotes worse outcome.)In a poster presented separately at the meeting, the DELIVERS researchers noted that “the lack of statistical significance in the primary endpoint could be explained in part by milder disease in the diacerein group.” The mean body surface area of EB simplex lesions within the assessment area at baseline was 5.76% in the diacerein group and 7.13% in the vehicle group. The researchers proposed that perhaps a higher concentration of diacerein than the 1% used in the trial might have been needed.
Sirolimus and EB simplex
Dr. Murrell noted that a pilot study, known as the SISTERS trial, had been conducted with a 2% sirolimus topical ointment at her institution and at Stanford (Calif.) University. This prospective, double-blind study had involved 16 patients with EB simplex, in which blisters tend to be confined to the palms of the hands and soles of the feet. The patients were assigned to treat both feet with either topical sirolimus or a placebo cream for 12 weeks. After a 4-week wash-out period, the patients switched to using the opposite cream for an additional 12 weeks.
Sirolimus is an inhibitor of the mTOR pathway, and, according to a description of the study on ClinicalTrials.gov, the researchers’ aim was to inhibit “the mTOR pathway to down-regulate the translation of defective keratin proteins.” That would allow a transition from supportive care, which is the current practice for EB simplex, to using a targeted molecular therapy to improve patient mobility and quality of life, they note on the site.
“We look forward to having that study unblinded,” Dr. Murrell said, adding that “data should be ready in a few months.”
EASE and Oleogel-S10
Oleogel-S10 is a gel that contains a birch bark extract dissolved in sunflower oil. It is already approved in Europe (Episalvan) for the treatment of partial-thickness skin wounds, but its use in EB remains investigational.
In a poster presentation at the meeting, Stella Gewert, MD, of the University of Freiburg (Germany) and colleagues discussed their experience using Oleogel-S10 in the treatment of four patients – each with a different type of EB – who applied the gel for between 6 days and 3 months.
Promising effects were seen, including reduced pruritus and pain, wounds healing more quickly, and reductions in lesion size. “During treatment, dressing requirements were reduced, and patient quality of life improved,” the researchers observed.
Mark Sumeray, MD, the chief medical officer of Amryt Pharmaceuticals, which is developing Oleogel-S10, said it was important to emphasize that Oleogel-S10 is a gel and not a cream. Gels are mixed with oil and are easier to apply – an important consideration for those with EB, he explained, whereas creams tend to be mixed with water and are stickier.
The phase 3 EASE trial is looking at the efficacy and safety of the gel in patients with junctional and dystrophic EB, and recruitment is ongoing, Dr. Murrell said. The primary endpoint is the proportion of patients with the first complete closure of a target wound within 45 days of treatment initiation. The estimated primary completion date for the trial is June 2020, and it is projected to end by 2022.
Scioderm, in collaboration with Amicus, funded the ESSENCE trial; Castle Creek financed the DELIVERS study; Amryt is supporting the EASE study; and Stanford University is sponsor of the SISTERS study. Dr. Murrell has been the principal investigator for trials run by Amicus, Amryt, Castle Creek, and Shire, and she acknowledged receipt of honoraria or consultation fees from those companies and others. Dr. Gewert did not report any financial disclosures. Dr. Sumeray is an employee and shareholder of Amryt.
LONDON –
Results from trials such as ESSENCE, with allantoin, and DELIVERS, with diacerein, were “disappointing,” Dédée Murrell, BMBCh, MD, pointed out at the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association (DEBRA).
Those two topical agents were most likely let down by the trials’ design, said Dr. Murrell, of St. George Hospital, University of New South Wales, Sydney, but she noted that there were still some promising trials that were either ongoing, such as EASE, with Oleogel-S10, or that were about to be unblinded, such as SISTERS, with sirolimus.
Epidermolysis bullosa (EB) is a group of rare genetic diseases that can cause the skin to blister and peel away to varying degrees, causing itchy and painful skin, as well as recurrent wounds, some of which may seem never to heal and that increase the risk for squamous cell carcinoma. Although finding a cure for the disease is high on the research agenda, finding a reliable therapy that can soothe and protect the skin is of equal importance.
Trials and tribulations
Conducting trials in rare diseases can be difficult because the studies are often small and poorly controlled, Dr. Murrell said during an oral presentation at the meeting. To gain regulatory approval, trials need to have an active and a placebo arm, because “even though we’re dealing with a rare disease, we still have to show statistical significance between the two arms.”
However, it is not just about finding enough participants who meet the inclusion criteria and adequately controlling the study, as finding funding can also be a significant hurdle. That is the case particularly when an existing drug with no patent protection is proposed to be repurposed. As an example, Dr. Murrell said that many patients with EB may use gentian violet to treat their condition, but it has been around for so long and is so widely used, that funding a trial to formally prove its merit is unlikely. In addition, “there are special caveats that occur in dermatology clinical trials with topical drugs that don’t exist [in trials] with systemic treatments, one of which is that it is very important to keep other variables the same,” Dr. Murrell said. “So, for example, the dressings need to stay the same throughout a trial with a topical therapy, because if you improve the dressings [during the course of the trial], you could mask the effect of the treatment.” Similarly, the bathing and cleansing routines of the participants need to remain the same throughout the trial.
“We also need to have validated instruments to prove whether these treatments are working, and the instruments need to be objective as well as subjective,” Dr. Murrell advised. For example, inflammation and blistering need to be scored separately from scarring and skin damage. “You have to conduct a clinical trial to be able to verify that there is diminished scarring or damage, because those are the longer-term complications.” Inflammation and blistering are valid endpoints to use in shorter-term studies.
Dr. Murrell also cautioned on getting too enthused about the results of case reports. “We do get excited when we see a patient using something new and they seem to be getting much better,” but such reports do not have a placebo arm, or, if there is one, then there is no vehicle control, she said. It’s important to include a run-in period in a trial to establish a new baseline and to ensure that any effects seen with a topical agent are independent of the carrier substance or any altered bathing behavior or dressing habits, which could skew the results.
ESSENCE and allantoin
So what went wrong in the phase 3 ESSENCE trial with allantoin, which was halted early in September 2017? The trial had included 169 patients with any type of EB – simplex, recessive dystrophic, and junctional non-Herlitz – who were randomized to treatment with the allantoin-containing cream SD-101 or a placebo cream containing only the vehicle. The creams were applied daily to the entire body for 3 months, with the primary endpoint being total wound closure at the end of the treatment period. Total wound closure was a requirement of the Food and Drug Administration, Dr. Murrell said, but it is now known that 100% closure is not always likely, which the agency itself now concedes.
“Most disappointingly, no significant difference was found [between the study drug and placebo], therefore it didn’t meet the primary endpoint, and you’re not even allowed to consider secondary endpoints – those are the rules of the game,” she said. As a result, the trial was stopped in 2017.
For inclusion in the study, patients had to have at least one target wound that had been present for at least 3 weeks, but there was no stratification on the duration of wounds in the randomization process. That meant that some individuals with wounds of shorter duration had unintentionally ended up in the placebo arm – favoring healing – and those with more chronic wounds had been in the allantoin arm. So, because the study arms might not have been equally balanced at baseline, it would have been harder for the actual treatment to demonstrate a benefit, Dr. Murrell suggested.
Another problem with the trial was that the vehicle cream contained elements, such as lanolin, already associated with wound healing. That would have given patients in the placebo arm an advantage because anyone applying the cream every day would probably get better or improve to some degree.
The patients were also required to have daily dressing changes and baths and, “if you give any patient that advice and they comply with it for a period of time, they are going to improve,” whether or not they are applying the study drug. Dr. Murrell said that the researchers likely should have done a run-in period first and then established a new baseline to randomize the patients.
“Lastly, no one had ever done a study of what we essentially tell eczema patients to do every day … to moisturize, because that will provide extra protection and barrier to their skin. So, if anything, the ESSENCE study shows that moisturizing has a protective effect of the vehicle for patients with EB,” she said.
DELIVERS and diacerein
Another trial that was stopped prematurely was the phase 2 DELIVERS study, which was set up to assess the benefits of topical diacerein in people with EB simplex. Diacerein, an extract of rhubarb root, was tested in 54 patients, who were randomized to apply either diacerein or vehicle ointment for 8 weeks.
Initially, the results “looked very promising,” Dr. Murrell said, because there was a trend toward improved EB simplex lesions, with the primary endpoint of at least a 60% reduction in lesions met by 57.1% of diacerein-treated and 53.8% of vehicle-treated patients.
However, the trial included use of the Investigator’s Global Assessment Scale at the FDA’s behest, but the tool had not been validated in previous EB trials, and which didn’t seem to show any benefit of the active over the placebo ointment. (The Investigator’s Global Assessment is a 5-point scale used for overall clinical assessment of severity of disease, ranging from 0 to 4, where a higher score denotes worse outcome.)In a poster presented separately at the meeting, the DELIVERS researchers noted that “the lack of statistical significance in the primary endpoint could be explained in part by milder disease in the diacerein group.” The mean body surface area of EB simplex lesions within the assessment area at baseline was 5.76% in the diacerein group and 7.13% in the vehicle group. The researchers proposed that perhaps a higher concentration of diacerein than the 1% used in the trial might have been needed.
Sirolimus and EB simplex
Dr. Murrell noted that a pilot study, known as the SISTERS trial, had been conducted with a 2% sirolimus topical ointment at her institution and at Stanford (Calif.) University. This prospective, double-blind study had involved 16 patients with EB simplex, in which blisters tend to be confined to the palms of the hands and soles of the feet. The patients were assigned to treat both feet with either topical sirolimus or a placebo cream for 12 weeks. After a 4-week wash-out period, the patients switched to using the opposite cream for an additional 12 weeks.
Sirolimus is an inhibitor of the mTOR pathway, and, according to a description of the study on ClinicalTrials.gov, the researchers’ aim was to inhibit “the mTOR pathway to down-regulate the translation of defective keratin proteins.” That would allow a transition from supportive care, which is the current practice for EB simplex, to using a targeted molecular therapy to improve patient mobility and quality of life, they note on the site.
“We look forward to having that study unblinded,” Dr. Murrell said, adding that “data should be ready in a few months.”
EASE and Oleogel-S10
Oleogel-S10 is a gel that contains a birch bark extract dissolved in sunflower oil. It is already approved in Europe (Episalvan) for the treatment of partial-thickness skin wounds, but its use in EB remains investigational.
In a poster presentation at the meeting, Stella Gewert, MD, of the University of Freiburg (Germany) and colleagues discussed their experience using Oleogel-S10 in the treatment of four patients – each with a different type of EB – who applied the gel for between 6 days and 3 months.
Promising effects were seen, including reduced pruritus and pain, wounds healing more quickly, and reductions in lesion size. “During treatment, dressing requirements were reduced, and patient quality of life improved,” the researchers observed.
Mark Sumeray, MD, the chief medical officer of Amryt Pharmaceuticals, which is developing Oleogel-S10, said it was important to emphasize that Oleogel-S10 is a gel and not a cream. Gels are mixed with oil and are easier to apply – an important consideration for those with EB, he explained, whereas creams tend to be mixed with water and are stickier.
The phase 3 EASE trial is looking at the efficacy and safety of the gel in patients with junctional and dystrophic EB, and recruitment is ongoing, Dr. Murrell said. The primary endpoint is the proportion of patients with the first complete closure of a target wound within 45 days of treatment initiation. The estimated primary completion date for the trial is June 2020, and it is projected to end by 2022.
Scioderm, in collaboration with Amicus, funded the ESSENCE trial; Castle Creek financed the DELIVERS study; Amryt is supporting the EASE study; and Stanford University is sponsor of the SISTERS study. Dr. Murrell has been the principal investigator for trials run by Amicus, Amryt, Castle Creek, and Shire, and she acknowledged receipt of honoraria or consultation fees from those companies and others. Dr. Gewert did not report any financial disclosures. Dr. Sumeray is an employee and shareholder of Amryt.
EXPERT ANALYSIS FROM EB 2020
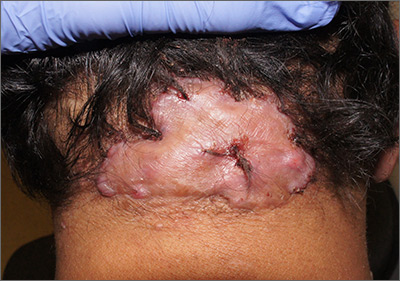
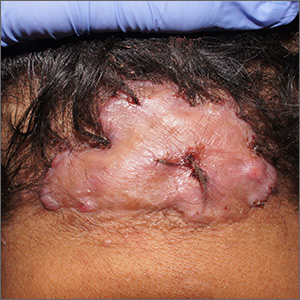
Enlarging scalp plaque
The findings of follicular-based papules, pustules, and scars led to the diagnosis of folliculitis keloidalis nuchae.
Follicular keloidalis, also called acne keloidalis nuchae, is more common in patients with darker skin types (Fitzpatrick skin types IV-VI). The pathogenesis is unclear, but the condition may arise from mechanical occlusion with a retained short hair that leads to follicular destruction. It also may be a primary disorder arising from bacterial infection and subsequent vigorous inflammation.
In the earliest stages, when only small inflammatory papules are present, patients should be instructed not to cut their hair shorter than 0.25-in long to avoid hair retention. Also, topical clindamycin lotion or topical chlorhexidine solution may serve as sufficient treatment. As the disease progresses, oral doxycycline 100 mg bid, combination rifampin 300 mg bid and clindamycin 300 mg bid for 3 months, or isotretinoin 1 mg/kg daily for 6 to 8 months are medical therapeutic options. Procedural options include radiation and laser hair removal.
In this patient, trials of intralesional triamcinolone acetonide 10 mg/mL helped to flatten the plaque, but hair loss persisted. Ultimately, he was referred to Plastic Surgery for excision and was treated for several months with doxycycline 100 mg bid and monitored for recurrence.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
Chouk C, Litaiem N, Jones M, et al. Acne keloidalis nuchae: clinical and dermoscopic features. BMJ Case Rep. 2017. pii: bcr-2017-222222. doi: 10.1136/bcr-2017-222222.
The findings of follicular-based papules, pustules, and scars led to the diagnosis of folliculitis keloidalis nuchae.
Follicular keloidalis, also called acne keloidalis nuchae, is more common in patients with darker skin types (Fitzpatrick skin types IV-VI). The pathogenesis is unclear, but the condition may arise from mechanical occlusion with a retained short hair that leads to follicular destruction. It also may be a primary disorder arising from bacterial infection and subsequent vigorous inflammation.
In the earliest stages, when only small inflammatory papules are present, patients should be instructed not to cut their hair shorter than 0.25-in long to avoid hair retention. Also, topical clindamycin lotion or topical chlorhexidine solution may serve as sufficient treatment. As the disease progresses, oral doxycycline 100 mg bid, combination rifampin 300 mg bid and clindamycin 300 mg bid for 3 months, or isotretinoin 1 mg/kg daily for 6 to 8 months are medical therapeutic options. Procedural options include radiation and laser hair removal.
In this patient, trials of intralesional triamcinolone acetonide 10 mg/mL helped to flatten the plaque, but hair loss persisted. Ultimately, he was referred to Plastic Surgery for excision and was treated for several months with doxycycline 100 mg bid and monitored for recurrence.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
The findings of follicular-based papules, pustules, and scars led to the diagnosis of folliculitis keloidalis nuchae.
Follicular keloidalis, also called acne keloidalis nuchae, is more common in patients with darker skin types (Fitzpatrick skin types IV-VI). The pathogenesis is unclear, but the condition may arise from mechanical occlusion with a retained short hair that leads to follicular destruction. It also may be a primary disorder arising from bacterial infection and subsequent vigorous inflammation.
In the earliest stages, when only small inflammatory papules are present, patients should be instructed not to cut their hair shorter than 0.25-in long to avoid hair retention. Also, topical clindamycin lotion or topical chlorhexidine solution may serve as sufficient treatment. As the disease progresses, oral doxycycline 100 mg bid, combination rifampin 300 mg bid and clindamycin 300 mg bid for 3 months, or isotretinoin 1 mg/kg daily for 6 to 8 months are medical therapeutic options. Procedural options include radiation and laser hair removal.
In this patient, trials of intralesional triamcinolone acetonide 10 mg/mL helped to flatten the plaque, but hair loss persisted. Ultimately, he was referred to Plastic Surgery for excision and was treated for several months with doxycycline 100 mg bid and monitored for recurrence.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
Chouk C, Litaiem N, Jones M, et al. Acne keloidalis nuchae: clinical and dermoscopic features. BMJ Case Rep. 2017. pii: bcr-2017-222222. doi: 10.1136/bcr-2017-222222.
Chouk C, Litaiem N, Jones M, et al. Acne keloidalis nuchae: clinical and dermoscopic features. BMJ Case Rep. 2017. pii: bcr-2017-222222. doi: 10.1136/bcr-2017-222222.
Merkel cell carcinoma management undergoes revolution
LAHAINA, HAWAII – The , Paul Nghiem, MD, PhD, declared at the SDEF Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
That’s because dermatologists are typically the physicians who make the diagnosis of Merkel cell carcinoma (MCC), so they’re on the scene from the outset and well positioned to help direct early management of this particularly aggressive malignancy, explained Dr. Nghiem, professor and head of dermatology at the University of Washington, Seattle.
“The management of Merkel is pretty high stakes, and if you get it right at the beginning it makes a huge difference in the side effects, as well as the chances that the patient will have the disease under control,” said Dr. Nghiem, who is sometimes called “the Merkel man” because of his many pioneering contributions to the field.
Better early management
Getting early management right, he added, hinges upon ordering a baseline PET-CT scan to search for metastases before performing definitive surgical excision of the primary tumor.
“There are really important prognostic and therapeutic implications for a baseline scan in almost any patient with early Merkel – and that’s a very different situation than with melanoma,” the dermatologist said. “There’s at least a threefold higher likelihood that the cancer has spread asymptomatically at baseline with Merkel cell carcinoma than with melanoma.”
In a soon-to-be-published study by Dr. Nghiem and coworkers, baseline imaging resulted in prognostically important upstaging that led to an altered management strategy in 12% of 584 patients with MCC, or 1 in 8.
“You don’t want to overtreat locally a lesion that has already spread distantly; you want to start focusing on the distant disease. The local disease is secondary,” he said.
The surgical excision of the primary lesion should be thoughtfully wide without being aggressive or mutilating, and it should involve primary closure. “Definitely avoid flaps and grafts, which delay your further management with radiotherapy by months and months,” Dr. Nghiem advised.
Adjuvant radiotherapy of the primary tumor site is extremely effective at preventing recurrent MCC. In Dr. Nghiem’s view, almost everyone is a candidate: In a series of 803 patients in the Seattle MCC cohort, 92% received local adjuvant radiotherapy. The national rate, in contrast, is only about 50%, highlighting the need for additional physician education.
“A little bit of radiation – one dose – appears to be just as effective as 6 weeks in controlling microscopic disease. That’s probably something we’re going to be moving towards as a field,” he predicted.
Indeed, local adjuvant radiotherapy is so effective in MCC that the surgical margins make no difference. This was demonstrated in a study by Dr. Nghiem and his coinvestigators involving 70 patients with margins greater than 1 cm who received radiotherapy, 70 others with smaller or even positive margins who received radiotherapy, and 35 patients with margins of 1 cm or less who did not receive radiotherapy. There were no MCC recurrences in any of the radiotherapy recipients, regardless of their margin status. In contrast, 7 of the 35 patients who didn’t receive radiation therapy developed a cancer recurrence. Of note, the recurrence rate of MCC is historically about 40% – far greater than for any other skin cancer. Most recurrences happen within the first 2-3 years, Dr. Nghiem observed.
Immune therapy takes center stage
Another major transformation in MCC management has been the emergence of immune therapy as first-line systemic therapy. It has replaced chemotherapy, which is more toxic and has a much shorter average duration of response. Avelumab (Bavencio) and pembrolizumab (Keytruda), the two monoclonal antibodies directed against the protein programmed death–ligand 1 (PD-L1) receptor which are approved for MCC and have been incorporated into the National Comprehensive Cancer Network (NCCN) guidelines, provide a sixfold improvement in survival, compared with chemotherapy. For example, Dr. Nghiem was first author of a multicenter phase 2 study of pembrolizumab in which the 12- and 24-month overall survival rates in pembrolizumab responders were 85% and 79%, compared with just 12% and 6%, respectively, in historical controls on first-line chemotherapy (J Clin Oncol. 2019 Mar 20;37[9]:693-702).
“Merkel cell carcinoma is the most responsive solid tumor to immune therapy,” Dr. Nghiem commented.
Why MCC matters
Although rare, MCC is important because it’s five times more lethal than melanoma. Moreover, its incidence has been rising at a rate roughly twice that of the increase in melanoma since the turn of the century. There are now more than 3,000 new cases of MCC annually, about the same as for cutaneous T-cell lymphoma (CTCL).
“It’s just that you live a long time with CTCL and you don’t with Merkel cell carcinoma. You either get rid of Merkel fast or it gets rid of you,” the dermatologist observed.
It’s a fascinating malignancy, he continued. Eight of 10 cases are caused by Merkel cell polyomavirus, discovered in 2008. The virus is ubiquitously acquired in childhood and then lies dormant on the skin for the next 6 or 7 decades, at which point MCC rates shoot up dramatically, probably due to immunosenescence. Immunosuppressed patients are at 10-fold increased risk for MCC.
Given the rarity of MCC, it doesn’t make sense to actively hunt for it. But Dr. Nghiem and coworkers have developed a handy vowel-based mnemonic that serves to raise the index of suspicion: the “AEIOU” features.
- A = asymptomatic.
- E = expanding rapidly within past 3 months.
- I = immune-mediated.
- O = older than age 50.
- U = UV-exposed skin.
The investigators found in a series of 195 MCC patients that 89% of them possessed three or more of these features (J Am Acad Dermatol. 2008 Mar;58[3]:375-81). But while the AEIOU guide is quite sensitive, it’s not specific.
“If you have any three or more of these features, that lesion probably deserves a biopsy if it’s not readily explained. Even if it’s not a Merkel, it may turn out to be a different nonmelanoma skin cancer, something you want to know about,” Dr. Nghiem said.
A shift in surveillance strategy
Dr. Nghiem was senior author of a major study that validated the clinical utility of a Merkel polyomavirus serology test for monitoring the disease status of patients treated for MCC (Cancer. 2017 Apr 15;123[8]:1464-74). The test, which measures antibodies to Merkel cell polyomavirus oncoproteins, has been incorporated in NCCN guidelines. The blood test is used initially in newly diagnosed MCC to stratify patients into two subgroups: the half who are seropositive at baseline, and the other half who are seronegative. The seropositive group undergoes surveillance via repeat blood testing every 3 months. If antibody levels are low, there is a high degree of certainty that immune therapy is working and remission is present. Thus, the blood test spares patients in this group the expense and radiation exposure entailed in repeated surveillance scans. However, rising antibody levels indicate the cancer has already recurred or will do so within the next several months.
Unfortunately, the blood test cannot be used serially to track disease status in patients who are seronegative at baseline. That group is at 42% increased risk of MCC recurrence.
Immune therapy works in only about two-thirds of MCC patients with distant disease. Leaving the visible primary tumor in place to serve as a real-time window into immune treatment effectiveness is a useful contemporary surveillance strategy.
“By leaving the visible primary there, you will rapidly know if that patient is in the favorable two-thirds group or not,” he explained.
Historically, surgery and surveillance of MCC were based upon the melanoma model, and medical oncologists were trained to treat the malignancy as they would small cell lung cancer. These are now outmoded approaches, Dr. Nghiem said. That’s why a multidisciplinary approach is highly desirable for management of MCC, including dermatologists, pathologists, surgeons, radiation oncologists, medical oncologists, and imaging experts.
Dr. Nghiem and his colleagues have created a comprehensive source of information about Merkel cell carcinoma for physicians and patients at merkelcell.org.
He reported receiving research grants from Bristol-Myers Squibb and serving as a consultant to EMD Serono, Merck, Sanofi/Regeneron, and 4SC.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
LAHAINA, HAWAII – The , Paul Nghiem, MD, PhD, declared at the SDEF Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
That’s because dermatologists are typically the physicians who make the diagnosis of Merkel cell carcinoma (MCC), so they’re on the scene from the outset and well positioned to help direct early management of this particularly aggressive malignancy, explained Dr. Nghiem, professor and head of dermatology at the University of Washington, Seattle.
“The management of Merkel is pretty high stakes, and if you get it right at the beginning it makes a huge difference in the side effects, as well as the chances that the patient will have the disease under control,” said Dr. Nghiem, who is sometimes called “the Merkel man” because of his many pioneering contributions to the field.
Better early management
Getting early management right, he added, hinges upon ordering a baseline PET-CT scan to search for metastases before performing definitive surgical excision of the primary tumor.
“There are really important prognostic and therapeutic implications for a baseline scan in almost any patient with early Merkel – and that’s a very different situation than with melanoma,” the dermatologist said. “There’s at least a threefold higher likelihood that the cancer has spread asymptomatically at baseline with Merkel cell carcinoma than with melanoma.”
In a soon-to-be-published study by Dr. Nghiem and coworkers, baseline imaging resulted in prognostically important upstaging that led to an altered management strategy in 12% of 584 patients with MCC, or 1 in 8.
“You don’t want to overtreat locally a lesion that has already spread distantly; you want to start focusing on the distant disease. The local disease is secondary,” he said.
The surgical excision of the primary lesion should be thoughtfully wide without being aggressive or mutilating, and it should involve primary closure. “Definitely avoid flaps and grafts, which delay your further management with radiotherapy by months and months,” Dr. Nghiem advised.
Adjuvant radiotherapy of the primary tumor site is extremely effective at preventing recurrent MCC. In Dr. Nghiem’s view, almost everyone is a candidate: In a series of 803 patients in the Seattle MCC cohort, 92% received local adjuvant radiotherapy. The national rate, in contrast, is only about 50%, highlighting the need for additional physician education.
“A little bit of radiation – one dose – appears to be just as effective as 6 weeks in controlling microscopic disease. That’s probably something we’re going to be moving towards as a field,” he predicted.
Indeed, local adjuvant radiotherapy is so effective in MCC that the surgical margins make no difference. This was demonstrated in a study by Dr. Nghiem and his coinvestigators involving 70 patients with margins greater than 1 cm who received radiotherapy, 70 others with smaller or even positive margins who received radiotherapy, and 35 patients with margins of 1 cm or less who did not receive radiotherapy. There were no MCC recurrences in any of the radiotherapy recipients, regardless of their margin status. In contrast, 7 of the 35 patients who didn’t receive radiation therapy developed a cancer recurrence. Of note, the recurrence rate of MCC is historically about 40% – far greater than for any other skin cancer. Most recurrences happen within the first 2-3 years, Dr. Nghiem observed.
Immune therapy takes center stage
Another major transformation in MCC management has been the emergence of immune therapy as first-line systemic therapy. It has replaced chemotherapy, which is more toxic and has a much shorter average duration of response. Avelumab (Bavencio) and pembrolizumab (Keytruda), the two monoclonal antibodies directed against the protein programmed death–ligand 1 (PD-L1) receptor which are approved for MCC and have been incorporated into the National Comprehensive Cancer Network (NCCN) guidelines, provide a sixfold improvement in survival, compared with chemotherapy. For example, Dr. Nghiem was first author of a multicenter phase 2 study of pembrolizumab in which the 12- and 24-month overall survival rates in pembrolizumab responders were 85% and 79%, compared with just 12% and 6%, respectively, in historical controls on first-line chemotherapy (J Clin Oncol. 2019 Mar 20;37[9]:693-702).
“Merkel cell carcinoma is the most responsive solid tumor to immune therapy,” Dr. Nghiem commented.
Why MCC matters
Although rare, MCC is important because it’s five times more lethal than melanoma. Moreover, its incidence has been rising at a rate roughly twice that of the increase in melanoma since the turn of the century. There are now more than 3,000 new cases of MCC annually, about the same as for cutaneous T-cell lymphoma (CTCL).
“It’s just that you live a long time with CTCL and you don’t with Merkel cell carcinoma. You either get rid of Merkel fast or it gets rid of you,” the dermatologist observed.
It’s a fascinating malignancy, he continued. Eight of 10 cases are caused by Merkel cell polyomavirus, discovered in 2008. The virus is ubiquitously acquired in childhood and then lies dormant on the skin for the next 6 or 7 decades, at which point MCC rates shoot up dramatically, probably due to immunosenescence. Immunosuppressed patients are at 10-fold increased risk for MCC.
Given the rarity of MCC, it doesn’t make sense to actively hunt for it. But Dr. Nghiem and coworkers have developed a handy vowel-based mnemonic that serves to raise the index of suspicion: the “AEIOU” features.
- A = asymptomatic.
- E = expanding rapidly within past 3 months.
- I = immune-mediated.
- O = older than age 50.
- U = UV-exposed skin.
The investigators found in a series of 195 MCC patients that 89% of them possessed three or more of these features (J Am Acad Dermatol. 2008 Mar;58[3]:375-81). But while the AEIOU guide is quite sensitive, it’s not specific.
“If you have any three or more of these features, that lesion probably deserves a biopsy if it’s not readily explained. Even if it’s not a Merkel, it may turn out to be a different nonmelanoma skin cancer, something you want to know about,” Dr. Nghiem said.
A shift in surveillance strategy
Dr. Nghiem was senior author of a major study that validated the clinical utility of a Merkel polyomavirus serology test for monitoring the disease status of patients treated for MCC (Cancer. 2017 Apr 15;123[8]:1464-74). The test, which measures antibodies to Merkel cell polyomavirus oncoproteins, has been incorporated in NCCN guidelines. The blood test is used initially in newly diagnosed MCC to stratify patients into two subgroups: the half who are seropositive at baseline, and the other half who are seronegative. The seropositive group undergoes surveillance via repeat blood testing every 3 months. If antibody levels are low, there is a high degree of certainty that immune therapy is working and remission is present. Thus, the blood test spares patients in this group the expense and radiation exposure entailed in repeated surveillance scans. However, rising antibody levels indicate the cancer has already recurred or will do so within the next several months.
Unfortunately, the blood test cannot be used serially to track disease status in patients who are seronegative at baseline. That group is at 42% increased risk of MCC recurrence.
Immune therapy works in only about two-thirds of MCC patients with distant disease. Leaving the visible primary tumor in place to serve as a real-time window into immune treatment effectiveness is a useful contemporary surveillance strategy.
“By leaving the visible primary there, you will rapidly know if that patient is in the favorable two-thirds group or not,” he explained.
Historically, surgery and surveillance of MCC were based upon the melanoma model, and medical oncologists were trained to treat the malignancy as they would small cell lung cancer. These are now outmoded approaches, Dr. Nghiem said. That’s why a multidisciplinary approach is highly desirable for management of MCC, including dermatologists, pathologists, surgeons, radiation oncologists, medical oncologists, and imaging experts.
Dr. Nghiem and his colleagues have created a comprehensive source of information about Merkel cell carcinoma for physicians and patients at merkelcell.org.
He reported receiving research grants from Bristol-Myers Squibb and serving as a consultant to EMD Serono, Merck, Sanofi/Regeneron, and 4SC.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
LAHAINA, HAWAII – The , Paul Nghiem, MD, PhD, declared at the SDEF Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
That’s because dermatologists are typically the physicians who make the diagnosis of Merkel cell carcinoma (MCC), so they’re on the scene from the outset and well positioned to help direct early management of this particularly aggressive malignancy, explained Dr. Nghiem, professor and head of dermatology at the University of Washington, Seattle.
“The management of Merkel is pretty high stakes, and if you get it right at the beginning it makes a huge difference in the side effects, as well as the chances that the patient will have the disease under control,” said Dr. Nghiem, who is sometimes called “the Merkel man” because of his many pioneering contributions to the field.
Better early management
Getting early management right, he added, hinges upon ordering a baseline PET-CT scan to search for metastases before performing definitive surgical excision of the primary tumor.
“There are really important prognostic and therapeutic implications for a baseline scan in almost any patient with early Merkel – and that’s a very different situation than with melanoma,” the dermatologist said. “There’s at least a threefold higher likelihood that the cancer has spread asymptomatically at baseline with Merkel cell carcinoma than with melanoma.”
In a soon-to-be-published study by Dr. Nghiem and coworkers, baseline imaging resulted in prognostically important upstaging that led to an altered management strategy in 12% of 584 patients with MCC, or 1 in 8.
“You don’t want to overtreat locally a lesion that has already spread distantly; you want to start focusing on the distant disease. The local disease is secondary,” he said.
The surgical excision of the primary lesion should be thoughtfully wide without being aggressive or mutilating, and it should involve primary closure. “Definitely avoid flaps and grafts, which delay your further management with radiotherapy by months and months,” Dr. Nghiem advised.
Adjuvant radiotherapy of the primary tumor site is extremely effective at preventing recurrent MCC. In Dr. Nghiem’s view, almost everyone is a candidate: In a series of 803 patients in the Seattle MCC cohort, 92% received local adjuvant radiotherapy. The national rate, in contrast, is only about 50%, highlighting the need for additional physician education.
“A little bit of radiation – one dose – appears to be just as effective as 6 weeks in controlling microscopic disease. That’s probably something we’re going to be moving towards as a field,” he predicted.
Indeed, local adjuvant radiotherapy is so effective in MCC that the surgical margins make no difference. This was demonstrated in a study by Dr. Nghiem and his coinvestigators involving 70 patients with margins greater than 1 cm who received radiotherapy, 70 others with smaller or even positive margins who received radiotherapy, and 35 patients with margins of 1 cm or less who did not receive radiotherapy. There were no MCC recurrences in any of the radiotherapy recipients, regardless of their margin status. In contrast, 7 of the 35 patients who didn’t receive radiation therapy developed a cancer recurrence. Of note, the recurrence rate of MCC is historically about 40% – far greater than for any other skin cancer. Most recurrences happen within the first 2-3 years, Dr. Nghiem observed.
Immune therapy takes center stage
Another major transformation in MCC management has been the emergence of immune therapy as first-line systemic therapy. It has replaced chemotherapy, which is more toxic and has a much shorter average duration of response. Avelumab (Bavencio) and pembrolizumab (Keytruda), the two monoclonal antibodies directed against the protein programmed death–ligand 1 (PD-L1) receptor which are approved for MCC and have been incorporated into the National Comprehensive Cancer Network (NCCN) guidelines, provide a sixfold improvement in survival, compared with chemotherapy. For example, Dr. Nghiem was first author of a multicenter phase 2 study of pembrolizumab in which the 12- and 24-month overall survival rates in pembrolizumab responders were 85% and 79%, compared with just 12% and 6%, respectively, in historical controls on first-line chemotherapy (J Clin Oncol. 2019 Mar 20;37[9]:693-702).
“Merkel cell carcinoma is the most responsive solid tumor to immune therapy,” Dr. Nghiem commented.
Why MCC matters
Although rare, MCC is important because it’s five times more lethal than melanoma. Moreover, its incidence has been rising at a rate roughly twice that of the increase in melanoma since the turn of the century. There are now more than 3,000 new cases of MCC annually, about the same as for cutaneous T-cell lymphoma (CTCL).
“It’s just that you live a long time with CTCL and you don’t with Merkel cell carcinoma. You either get rid of Merkel fast or it gets rid of you,” the dermatologist observed.
It’s a fascinating malignancy, he continued. Eight of 10 cases are caused by Merkel cell polyomavirus, discovered in 2008. The virus is ubiquitously acquired in childhood and then lies dormant on the skin for the next 6 or 7 decades, at which point MCC rates shoot up dramatically, probably due to immunosenescence. Immunosuppressed patients are at 10-fold increased risk for MCC.
Given the rarity of MCC, it doesn’t make sense to actively hunt for it. But Dr. Nghiem and coworkers have developed a handy vowel-based mnemonic that serves to raise the index of suspicion: the “AEIOU” features.
- A = asymptomatic.
- E = expanding rapidly within past 3 months.
- I = immune-mediated.
- O = older than age 50.
- U = UV-exposed skin.
The investigators found in a series of 195 MCC patients that 89% of them possessed three or more of these features (J Am Acad Dermatol. 2008 Mar;58[3]:375-81). But while the AEIOU guide is quite sensitive, it’s not specific.
“If you have any three or more of these features, that lesion probably deserves a biopsy if it’s not readily explained. Even if it’s not a Merkel, it may turn out to be a different nonmelanoma skin cancer, something you want to know about,” Dr. Nghiem said.
A shift in surveillance strategy
Dr. Nghiem was senior author of a major study that validated the clinical utility of a Merkel polyomavirus serology test for monitoring the disease status of patients treated for MCC (Cancer. 2017 Apr 15;123[8]:1464-74). The test, which measures antibodies to Merkel cell polyomavirus oncoproteins, has been incorporated in NCCN guidelines. The blood test is used initially in newly diagnosed MCC to stratify patients into two subgroups: the half who are seropositive at baseline, and the other half who are seronegative. The seropositive group undergoes surveillance via repeat blood testing every 3 months. If antibody levels are low, there is a high degree of certainty that immune therapy is working and remission is present. Thus, the blood test spares patients in this group the expense and radiation exposure entailed in repeated surveillance scans. However, rising antibody levels indicate the cancer has already recurred or will do so within the next several months.
Unfortunately, the blood test cannot be used serially to track disease status in patients who are seronegative at baseline. That group is at 42% increased risk of MCC recurrence.
Immune therapy works in only about two-thirds of MCC patients with distant disease. Leaving the visible primary tumor in place to serve as a real-time window into immune treatment effectiveness is a useful contemporary surveillance strategy.
“By leaving the visible primary there, you will rapidly know if that patient is in the favorable two-thirds group or not,” he explained.
Historically, surgery and surveillance of MCC were based upon the melanoma model, and medical oncologists were trained to treat the malignancy as they would small cell lung cancer. These are now outmoded approaches, Dr. Nghiem said. That’s why a multidisciplinary approach is highly desirable for management of MCC, including dermatologists, pathologists, surgeons, radiation oncologists, medical oncologists, and imaging experts.
Dr. Nghiem and his colleagues have created a comprehensive source of information about Merkel cell carcinoma for physicians and patients at merkelcell.org.
He reported receiving research grants from Bristol-Myers Squibb and serving as a consultant to EMD Serono, Merck, Sanofi/Regeneron, and 4SC.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
REPORTING FROM SDEF HAWAII DERMATOLOGY SEMINAR
Risk factors for death from COVID-19 identified in Wuhan patients
Patients who did not survive hospitalization for COVID-19 in Wuhan were more likely to be older, have comorbidities, and elevated D-dimer, according to the first study to examine risk factors associated with death among adults hospitalized with COVID-19. “Older age, showing signs of sepsis on admission, underlying diseases like high blood pressure and diabetes, and the prolonged use of noninvasive ventilation were important factors in the deaths of these patients,” coauthor Zhibo Liu said in a news release. Abnormal blood clotting was part of the clinical picture too.
Fei Zhou, MD, from the Chinese Academy of Medical Sciences, and colleagues conducted a retrospective, observational, multicenter cohort study of 191 patients, 137 of whom were discharged and 54 of whom died in the hospital.
The study, published online today in The Lancet, included all adult inpatients with laboratory-confirmed COVID-19 from Jinyintan Hospital and Wuhan Pulmonary Hospital who had been discharged or died by January 31 of this year. Severely ill patients in the province were transferred to these hospitals until February 1.
The researchers compared demographic, clinical, treatment, and laboratory data from electronic medical records between survivors and those who succumbed to the disease. The analysis also tested serial samples for viral RNA. Overall, 91 (48%) of the 191 patients had comorbidity. Most common was hypertension (30%), followed by diabetes (19%) and coronary heart disease (8%).
The odds of dying in the hospital increased with age (odds ratio 1.10; 95% confidence interval, 1.03-1.17; per year increase in age), higher Sequential Organ Failure Assessment (SOFA) score (5.65, 2.61-12.23; P < .0001), and D-dimer level exceeding 1 mcg/L on admission. The SOFA was previously called the “sepsis-related organ failure assessment score” and assesses rate of organ failure in intensive care units. Elevated D-dimer indicates increased risk of abnormal blood clotting, such as deep vein thrombosis.
Nonsurvivors compared with survivors had higher frequencies of respiratory failure (98% vs 36%), sepsis (100%, vs 42%), and secondary infections (50% vs 1%).
The average age of survivors was 52 years compared to 69 for those who died. Liu cited weakening of the immune system and increased inflammation, which damages organs and also promotes viral replication, as explanations for the age effect.
From the time of initial symptoms, median time to discharge from the hospital was 22 days. Average time to death was 18.5 days.
Fever persisted for a median of 12 days among all patients, and cough persisted for a median 19 days; 45% of the survivors were still coughing on discharge. In survivors, shortness of breath improved after 13 days, but persisted until death in the others.
Viral shedding persisted for a median duration of 20 days in survivors, ranging from 8 to 37. The virus (SARS-CoV-2) was detectable in nonsurvivors until death. Antiviral treatment did not curtail viral shedding.
But the viral shedding data come with a caveat. “The extended viral shedding noted in our study has important implications for guiding decisions around isolation precautions and antiviral treatment in patients with confirmed COVID-19 infection. However, we need to be clear that viral shedding time should not be confused with other self-isolation guidance for people who may have been exposed to COVID-19 but do not have symptoms, as this guidance is based on the incubation time of the virus,” explained colead author Bin Cao.
“Older age, elevated D-dimer levels, and high SOFA score could help clinicians to identify at an early stage those patients with COVID-19 who have poor prognosis. Prolonged viral shedding provides the rationale for a strategy of isolation of infected patients and optimal antiviral interventions in the future,” the researchers conclude.
A limitation in interpreting the findings of the study is that hospitalized patients do not represent the entire infected population. The researchers caution that “the number of deaths does not reflect the true mortality of COVID-19.” They also note that they did not have enough genetic material to accurately assess duration of viral shedding.
This article first appeared on Medscape.com.
Patients who did not survive hospitalization for COVID-19 in Wuhan were more likely to be older, have comorbidities, and elevated D-dimer, according to the first study to examine risk factors associated with death among adults hospitalized with COVID-19. “Older age, showing signs of sepsis on admission, underlying diseases like high blood pressure and diabetes, and the prolonged use of noninvasive ventilation were important factors in the deaths of these patients,” coauthor Zhibo Liu said in a news release. Abnormal blood clotting was part of the clinical picture too.
Fei Zhou, MD, from the Chinese Academy of Medical Sciences, and colleagues conducted a retrospective, observational, multicenter cohort study of 191 patients, 137 of whom were discharged and 54 of whom died in the hospital.
The study, published online today in The Lancet, included all adult inpatients with laboratory-confirmed COVID-19 from Jinyintan Hospital and Wuhan Pulmonary Hospital who had been discharged or died by January 31 of this year. Severely ill patients in the province were transferred to these hospitals until February 1.
The researchers compared demographic, clinical, treatment, and laboratory data from electronic medical records between survivors and those who succumbed to the disease. The analysis also tested serial samples for viral RNA. Overall, 91 (48%) of the 191 patients had comorbidity. Most common was hypertension (30%), followed by diabetes (19%) and coronary heart disease (8%).
The odds of dying in the hospital increased with age (odds ratio 1.10; 95% confidence interval, 1.03-1.17; per year increase in age), higher Sequential Organ Failure Assessment (SOFA) score (5.65, 2.61-12.23; P < .0001), and D-dimer level exceeding 1 mcg/L on admission. The SOFA was previously called the “sepsis-related organ failure assessment score” and assesses rate of organ failure in intensive care units. Elevated D-dimer indicates increased risk of abnormal blood clotting, such as deep vein thrombosis.
Nonsurvivors compared with survivors had higher frequencies of respiratory failure (98% vs 36%), sepsis (100%, vs 42%), and secondary infections (50% vs 1%).
The average age of survivors was 52 years compared to 69 for those who died. Liu cited weakening of the immune system and increased inflammation, which damages organs and also promotes viral replication, as explanations for the age effect.
From the time of initial symptoms, median time to discharge from the hospital was 22 days. Average time to death was 18.5 days.
Fever persisted for a median of 12 days among all patients, and cough persisted for a median 19 days; 45% of the survivors were still coughing on discharge. In survivors, shortness of breath improved after 13 days, but persisted until death in the others.
Viral shedding persisted for a median duration of 20 days in survivors, ranging from 8 to 37. The virus (SARS-CoV-2) was detectable in nonsurvivors until death. Antiviral treatment did not curtail viral shedding.
But the viral shedding data come with a caveat. “The extended viral shedding noted in our study has important implications for guiding decisions around isolation precautions and antiviral treatment in patients with confirmed COVID-19 infection. However, we need to be clear that viral shedding time should not be confused with other self-isolation guidance for people who may have been exposed to COVID-19 but do not have symptoms, as this guidance is based on the incubation time of the virus,” explained colead author Bin Cao.
“Older age, elevated D-dimer levels, and high SOFA score could help clinicians to identify at an early stage those patients with COVID-19 who have poor prognosis. Prolonged viral shedding provides the rationale for a strategy of isolation of infected patients and optimal antiviral interventions in the future,” the researchers conclude.
A limitation in interpreting the findings of the study is that hospitalized patients do not represent the entire infected population. The researchers caution that “the number of deaths does not reflect the true mortality of COVID-19.” They also note that they did not have enough genetic material to accurately assess duration of viral shedding.
This article first appeared on Medscape.com.
Patients who did not survive hospitalization for COVID-19 in Wuhan were more likely to be older, have comorbidities, and elevated D-dimer, according to the first study to examine risk factors associated with death among adults hospitalized with COVID-19. “Older age, showing signs of sepsis on admission, underlying diseases like high blood pressure and diabetes, and the prolonged use of noninvasive ventilation were important factors in the deaths of these patients,” coauthor Zhibo Liu said in a news release. Abnormal blood clotting was part of the clinical picture too.
Fei Zhou, MD, from the Chinese Academy of Medical Sciences, and colleagues conducted a retrospective, observational, multicenter cohort study of 191 patients, 137 of whom were discharged and 54 of whom died in the hospital.
The study, published online today in The Lancet, included all adult inpatients with laboratory-confirmed COVID-19 from Jinyintan Hospital and Wuhan Pulmonary Hospital who had been discharged or died by January 31 of this year. Severely ill patients in the province were transferred to these hospitals until February 1.
The researchers compared demographic, clinical, treatment, and laboratory data from electronic medical records between survivors and those who succumbed to the disease. The analysis also tested serial samples for viral RNA. Overall, 91 (48%) of the 191 patients had comorbidity. Most common was hypertension (30%), followed by diabetes (19%) and coronary heart disease (8%).
The odds of dying in the hospital increased with age (odds ratio 1.10; 95% confidence interval, 1.03-1.17; per year increase in age), higher Sequential Organ Failure Assessment (SOFA) score (5.65, 2.61-12.23; P < .0001), and D-dimer level exceeding 1 mcg/L on admission. The SOFA was previously called the “sepsis-related organ failure assessment score” and assesses rate of organ failure in intensive care units. Elevated D-dimer indicates increased risk of abnormal blood clotting, such as deep vein thrombosis.
Nonsurvivors compared with survivors had higher frequencies of respiratory failure (98% vs 36%), sepsis (100%, vs 42%), and secondary infections (50% vs 1%).
The average age of survivors was 52 years compared to 69 for those who died. Liu cited weakening of the immune system and increased inflammation, which damages organs and also promotes viral replication, as explanations for the age effect.
From the time of initial symptoms, median time to discharge from the hospital was 22 days. Average time to death was 18.5 days.
Fever persisted for a median of 12 days among all patients, and cough persisted for a median 19 days; 45% of the survivors were still coughing on discharge. In survivors, shortness of breath improved after 13 days, but persisted until death in the others.
Viral shedding persisted for a median duration of 20 days in survivors, ranging from 8 to 37. The virus (SARS-CoV-2) was detectable in nonsurvivors until death. Antiviral treatment did not curtail viral shedding.
But the viral shedding data come with a caveat. “The extended viral shedding noted in our study has important implications for guiding decisions around isolation precautions and antiviral treatment in patients with confirmed COVID-19 infection. However, we need to be clear that viral shedding time should not be confused with other self-isolation guidance for people who may have been exposed to COVID-19 but do not have symptoms, as this guidance is based on the incubation time of the virus,” explained colead author Bin Cao.
“Older age, elevated D-dimer levels, and high SOFA score could help clinicians to identify at an early stage those patients with COVID-19 who have poor prognosis. Prolonged viral shedding provides the rationale for a strategy of isolation of infected patients and optimal antiviral interventions in the future,” the researchers conclude.
A limitation in interpreting the findings of the study is that hospitalized patients do not represent the entire infected population. The researchers caution that “the number of deaths does not reflect the true mortality of COVID-19.” They also note that they did not have enough genetic material to accurately assess duration of viral shedding.
This article first appeared on Medscape.com.
Does misplaced faith in modern medicine run at odds against healthier lifestyles?
Recently, a study in the Journal of the American Heart Association found that taking statins and blood pressure medications doesn’t lead to healthier lifestyles.
This should surprise no one practicing medicine today. With absolutely no scientific data to back up the next statement, I’m willing to bet a study on oral antiglycemics for type 2 diabetes would yield similar results.
The problem here is that these drugs don’t change human nature, and I’m not belittling their ability to reduce morbidity and mortality.
Developed nations nowadays live in a world of plenty. For most of us, there’s not only no shortage of food options, but the majority of what’s out there is the worst stuff for your health. Salty, dense calories, high-fat, sweetened – for most of us that’s a normal day of eating. It tastes good. Two million years of evolution have programmed us to eat similar stuff because in the wild it sustains survival.
In the city and suburbs, however, that’s not the case.
Food manufacturers make it and stores sell it because, quite bluntly, it makes money. The profit margin for unhealthy stuff is higher than that for fruits and vegetables. If you’re trying to run a successful business, which one would you choose to sell?
As long as people are going to eat unhealthy stuff, others will sell it to them. All the medical breakthroughs in the world won’t change that.
Same with exercise. Some people love to exercise. Some people catch the bug to do it consistently. But most try for a few weeks, usually in January-February, then give up because they don’t have time, or the will, or both.
Medical breakthroughs won’t fix that, either.
There’s also, I suspect, a component of misplaced faith in modern medicine. Like the mysterious “anti-calories” in a can of diet soda. You really do encounter people who think that drinking a diet soda and having a slice of chocolate cake will cancel each other out. Any doctor or nutritionist will scoff at this, but it’s amazing how many people think that doing one good thing (health wise) means you can equally do one bad thing at no penalty. Humans love magical thinking like that.
Unintentionally, the medications contribute to this belief. People figure if they’re lowering blood sugar or lipids, maybe they can eat more steak and ice cream. That’s an unintended consequence of modern medicine. It’s not even limited to nonmedical people. When Lipitor came to market in the late 1990s, one of my attendings called it “a license to eat.” Sadly, as the new study proves, he wasn’t that far from the truth.
People want an easy cure. A pill that makes it all better. That’s human nature. But the real problem, for all the great work we’ve done in medications, is that many patients don’t want to be an active participant in their own care. Exercising and maintaining a healthy diet are hard work, in spite of all the evidence showing their benefits (especially when combined with modern medicine, which is the whole idea in the first place). So it’s much easier for them to place all the responsibility on doctors and medications, and just take a simple pill to fix everything.
As this study shows, it doesn’t work that way.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Recently, a study in the Journal of the American Heart Association found that taking statins and blood pressure medications doesn’t lead to healthier lifestyles.
This should surprise no one practicing medicine today. With absolutely no scientific data to back up the next statement, I’m willing to bet a study on oral antiglycemics for type 2 diabetes would yield similar results.
The problem here is that these drugs don’t change human nature, and I’m not belittling their ability to reduce morbidity and mortality.
Developed nations nowadays live in a world of plenty. For most of us, there’s not only no shortage of food options, but the majority of what’s out there is the worst stuff for your health. Salty, dense calories, high-fat, sweetened – for most of us that’s a normal day of eating. It tastes good. Two million years of evolution have programmed us to eat similar stuff because in the wild it sustains survival.
In the city and suburbs, however, that’s not the case.
Food manufacturers make it and stores sell it because, quite bluntly, it makes money. The profit margin for unhealthy stuff is higher than that for fruits and vegetables. If you’re trying to run a successful business, which one would you choose to sell?
As long as people are going to eat unhealthy stuff, others will sell it to them. All the medical breakthroughs in the world won’t change that.
Same with exercise. Some people love to exercise. Some people catch the bug to do it consistently. But most try for a few weeks, usually in January-February, then give up because they don’t have time, or the will, or both.
Medical breakthroughs won’t fix that, either.
There’s also, I suspect, a component of misplaced faith in modern medicine. Like the mysterious “anti-calories” in a can of diet soda. You really do encounter people who think that drinking a diet soda and having a slice of chocolate cake will cancel each other out. Any doctor or nutritionist will scoff at this, but it’s amazing how many people think that doing one good thing (health wise) means you can equally do one bad thing at no penalty. Humans love magical thinking like that.
Unintentionally, the medications contribute to this belief. People figure if they’re lowering blood sugar or lipids, maybe they can eat more steak and ice cream. That’s an unintended consequence of modern medicine. It’s not even limited to nonmedical people. When Lipitor came to market in the late 1990s, one of my attendings called it “a license to eat.” Sadly, as the new study proves, he wasn’t that far from the truth.
People want an easy cure. A pill that makes it all better. That’s human nature. But the real problem, for all the great work we’ve done in medications, is that many patients don’t want to be an active participant in their own care. Exercising and maintaining a healthy diet are hard work, in spite of all the evidence showing their benefits (especially when combined with modern medicine, which is the whole idea in the first place). So it’s much easier for them to place all the responsibility on doctors and medications, and just take a simple pill to fix everything.
As this study shows, it doesn’t work that way.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Recently, a study in the Journal of the American Heart Association found that taking statins and blood pressure medications doesn’t lead to healthier lifestyles.
This should surprise no one practicing medicine today. With absolutely no scientific data to back up the next statement, I’m willing to bet a study on oral antiglycemics for type 2 diabetes would yield similar results.
The problem here is that these drugs don’t change human nature, and I’m not belittling their ability to reduce morbidity and mortality.
Developed nations nowadays live in a world of plenty. For most of us, there’s not only no shortage of food options, but the majority of what’s out there is the worst stuff for your health. Salty, dense calories, high-fat, sweetened – for most of us that’s a normal day of eating. It tastes good. Two million years of evolution have programmed us to eat similar stuff because in the wild it sustains survival.
In the city and suburbs, however, that’s not the case.
Food manufacturers make it and stores sell it because, quite bluntly, it makes money. The profit margin for unhealthy stuff is higher than that for fruits and vegetables. If you’re trying to run a successful business, which one would you choose to sell?
As long as people are going to eat unhealthy stuff, others will sell it to them. All the medical breakthroughs in the world won’t change that.
Same with exercise. Some people love to exercise. Some people catch the bug to do it consistently. But most try for a few weeks, usually in January-February, then give up because they don’t have time, or the will, or both.
Medical breakthroughs won’t fix that, either.
There’s also, I suspect, a component of misplaced faith in modern medicine. Like the mysterious “anti-calories” in a can of diet soda. You really do encounter people who think that drinking a diet soda and having a slice of chocolate cake will cancel each other out. Any doctor or nutritionist will scoff at this, but it’s amazing how many people think that doing one good thing (health wise) means you can equally do one bad thing at no penalty. Humans love magical thinking like that.
Unintentionally, the medications contribute to this belief. People figure if they’re lowering blood sugar or lipids, maybe they can eat more steak and ice cream. That’s an unintended consequence of modern medicine. It’s not even limited to nonmedical people. When Lipitor came to market in the late 1990s, one of my attendings called it “a license to eat.” Sadly, as the new study proves, he wasn’t that far from the truth.
People want an easy cure. A pill that makes it all better. That’s human nature. But the real problem, for all the great work we’ve done in medications, is that many patients don’t want to be an active participant in their own care. Exercising and maintaining a healthy diet are hard work, in spite of all the evidence showing their benefits (especially when combined with modern medicine, which is the whole idea in the first place). So it’s much easier for them to place all the responsibility on doctors and medications, and just take a simple pill to fix everything.
As this study shows, it doesn’t work that way.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Belapectin misses endpoints in NASH trial
For patients with nonalcoholic steatohepatitis (NASH) with cirrhosis and portal hypertension, belapectin therapy was safe but did not significantly improve fibrosis or hepatic venous pressure gradient, compared with placebo, according to the results of a multicenter phase 2b study.
After 52 weeks of infusions, the change in hepatic venous pressure gradient did not significantly differ between the 2-mg/kg group (–0.28 mm Hg) and the placebo group (0.10 mm Hg) or between the 8-mg/kg group (–0.25 mm Hg) and the placebo group (P = .1 for both comparisons). Belapectin also did not significantly improve fibrosis, nonalcoholic fatty liver disease activity score, or the frequency of various complications of cirrhosis. “However, in a subgroup analysis of patients without esophageal varices, 2 mg/kg belapectin did reduce hepatic venous pressure gradient and development of varices,” wrote Naga Chalasani, MD, of Indiana University in Indianapolis and his associates. The findings were published in Gastroenterology.
NASH leads to portal hypertension, variceal bleeding, ascites with bacterial peritonitis, hepatic encephalopathy, and liver-related death and is a leading reason for liver transplantation among women and men. Galectin-3, which is primarily secreted by macrophages, is elevated in patients with NASH and has been linked to the pathophysiology of liver fibrosis in mice. Belapectin (GR-MD-02), a complex carbohydrate that targets and disrupts galectin-3, has been found to reduce liver fibrosis and portal hypertension in rats and was safe and well tolerated in phase 1 studies.
For this double-blind trial, the researchers randomly assigned 162 patients with NASH, cirrhosis, and portal hypertension (hepatic venous pressure gradient at least 6 mm Hg) to receive biweekly infusions of belapectin 2 mg/kg (54 patients), belapectin 8 mg/kg (54 patients), or placebo (54 patients). Patients were treated for 52 weeks. The primary endpoint was change from baseline in hepatic venous pressure gradient.
In a post-hoc analysis of the 81 patients who had no esophageal varices at baseline, 2 mg/kg belapectin was associated with an average 1.61-mm Hg reduction in hepatic venous pressure gradient from baseline (P = .02) and with a reduction in the development of new varices (P = .03).These effects did not extend to subgroups of patients with varices at baseline, clinically significant portal hypertension, or mild portal hypertension. Moreover, 2 mg/kg belapectin did not improve fibrosis, and the higher dose of belapectin (8 mg/kg) met neither the primary endpoint nor the secondary endpoints in the overall cohort or in subgroup analyses. In the subgroup with no varices at baseline, Galectin Technologies is proceeding to initiating a phase 3 clinical trial.
“Interestingly and somewhat unexpectedly, belapectin was associated with an improvement in hepatocyte ballooning,” which “is considered fundamental to the pathogenesis of disease progression in nonalcoholic steatohepatitis,” the researchers wrote. “The significance of such improvement in hepatocyte ballooning in the absence of improvement of other histological components, especially inflammation, is unknown.”
Galectin Therapeutics provided funding. Dr. Chalasani disclosed grant support from Galectin Therapeutics and relevant consulting relationships with NuSirt, AbbVie, Afimmune (DS Biopharma), and several other pharmaceutical companies. Sixteen coinvestigators also disclosed relationships with pharmaceutical companies, of whom eight disclosed consulting relationships, received research funding, or were employed by Galectin.
SOURCE: Chalasani N et al. Gastroenterology. 2019 Dec 5. doi: 10.1053/j.gastro.2019.11.296.
*This story was updated on 3/18/2020.
For patients with nonalcoholic steatohepatitis (NASH) with cirrhosis and portal hypertension, belapectin therapy was safe but did not significantly improve fibrosis or hepatic venous pressure gradient, compared with placebo, according to the results of a multicenter phase 2b study.
After 52 weeks of infusions, the change in hepatic venous pressure gradient did not significantly differ between the 2-mg/kg group (–0.28 mm Hg) and the placebo group (0.10 mm Hg) or between the 8-mg/kg group (–0.25 mm Hg) and the placebo group (P = .1 for both comparisons). Belapectin also did not significantly improve fibrosis, nonalcoholic fatty liver disease activity score, or the frequency of various complications of cirrhosis. “However, in a subgroup analysis of patients without esophageal varices, 2 mg/kg belapectin did reduce hepatic venous pressure gradient and development of varices,” wrote Naga Chalasani, MD, of Indiana University in Indianapolis and his associates. The findings were published in Gastroenterology.
NASH leads to portal hypertension, variceal bleeding, ascites with bacterial peritonitis, hepatic encephalopathy, and liver-related death and is a leading reason for liver transplantation among women and men. Galectin-3, which is primarily secreted by macrophages, is elevated in patients with NASH and has been linked to the pathophysiology of liver fibrosis in mice. Belapectin (GR-MD-02), a complex carbohydrate that targets and disrupts galectin-3, has been found to reduce liver fibrosis and portal hypertension in rats and was safe and well tolerated in phase 1 studies.
For this double-blind trial, the researchers randomly assigned 162 patients with NASH, cirrhosis, and portal hypertension (hepatic venous pressure gradient at least 6 mm Hg) to receive biweekly infusions of belapectin 2 mg/kg (54 patients), belapectin 8 mg/kg (54 patients), or placebo (54 patients). Patients were treated for 52 weeks. The primary endpoint was change from baseline in hepatic venous pressure gradient.
In a post-hoc analysis of the 81 patients who had no esophageal varices at baseline, 2 mg/kg belapectin was associated with an average 1.61-mm Hg reduction in hepatic venous pressure gradient from baseline (P = .02) and with a reduction in the development of new varices (P = .03).These effects did not extend to subgroups of patients with varices at baseline, clinically significant portal hypertension, or mild portal hypertension. Moreover, 2 mg/kg belapectin did not improve fibrosis, and the higher dose of belapectin (8 mg/kg) met neither the primary endpoint nor the secondary endpoints in the overall cohort or in subgroup analyses. In the subgroup with no varices at baseline, Galectin Technologies is proceeding to initiating a phase 3 clinical trial.
“Interestingly and somewhat unexpectedly, belapectin was associated with an improvement in hepatocyte ballooning,” which “is considered fundamental to the pathogenesis of disease progression in nonalcoholic steatohepatitis,” the researchers wrote. “The significance of such improvement in hepatocyte ballooning in the absence of improvement of other histological components, especially inflammation, is unknown.”
Galectin Therapeutics provided funding. Dr. Chalasani disclosed grant support from Galectin Therapeutics and relevant consulting relationships with NuSirt, AbbVie, Afimmune (DS Biopharma), and several other pharmaceutical companies. Sixteen coinvestigators also disclosed relationships with pharmaceutical companies, of whom eight disclosed consulting relationships, received research funding, or were employed by Galectin.
SOURCE: Chalasani N et al. Gastroenterology. 2019 Dec 5. doi: 10.1053/j.gastro.2019.11.296.
*This story was updated on 3/18/2020.
For patients with nonalcoholic steatohepatitis (NASH) with cirrhosis and portal hypertension, belapectin therapy was safe but did not significantly improve fibrosis or hepatic venous pressure gradient, compared with placebo, according to the results of a multicenter phase 2b study.
After 52 weeks of infusions, the change in hepatic venous pressure gradient did not significantly differ between the 2-mg/kg group (–0.28 mm Hg) and the placebo group (0.10 mm Hg) or between the 8-mg/kg group (–0.25 mm Hg) and the placebo group (P = .1 for both comparisons). Belapectin also did not significantly improve fibrosis, nonalcoholic fatty liver disease activity score, or the frequency of various complications of cirrhosis. “However, in a subgroup analysis of patients without esophageal varices, 2 mg/kg belapectin did reduce hepatic venous pressure gradient and development of varices,” wrote Naga Chalasani, MD, of Indiana University in Indianapolis and his associates. The findings were published in Gastroenterology.
NASH leads to portal hypertension, variceal bleeding, ascites with bacterial peritonitis, hepatic encephalopathy, and liver-related death and is a leading reason for liver transplantation among women and men. Galectin-3, which is primarily secreted by macrophages, is elevated in patients with NASH and has been linked to the pathophysiology of liver fibrosis in mice. Belapectin (GR-MD-02), a complex carbohydrate that targets and disrupts galectin-3, has been found to reduce liver fibrosis and portal hypertension in rats and was safe and well tolerated in phase 1 studies.
For this double-blind trial, the researchers randomly assigned 162 patients with NASH, cirrhosis, and portal hypertension (hepatic venous pressure gradient at least 6 mm Hg) to receive biweekly infusions of belapectin 2 mg/kg (54 patients), belapectin 8 mg/kg (54 patients), or placebo (54 patients). Patients were treated for 52 weeks. The primary endpoint was change from baseline in hepatic venous pressure gradient.
In a post-hoc analysis of the 81 patients who had no esophageal varices at baseline, 2 mg/kg belapectin was associated with an average 1.61-mm Hg reduction in hepatic venous pressure gradient from baseline (P = .02) and with a reduction in the development of new varices (P = .03).These effects did not extend to subgroups of patients with varices at baseline, clinically significant portal hypertension, or mild portal hypertension. Moreover, 2 mg/kg belapectin did not improve fibrosis, and the higher dose of belapectin (8 mg/kg) met neither the primary endpoint nor the secondary endpoints in the overall cohort or in subgroup analyses. In the subgroup with no varices at baseline, Galectin Technologies is proceeding to initiating a phase 3 clinical trial.
“Interestingly and somewhat unexpectedly, belapectin was associated with an improvement in hepatocyte ballooning,” which “is considered fundamental to the pathogenesis of disease progression in nonalcoholic steatohepatitis,” the researchers wrote. “The significance of such improvement in hepatocyte ballooning in the absence of improvement of other histological components, especially inflammation, is unknown.”
Galectin Therapeutics provided funding. Dr. Chalasani disclosed grant support from Galectin Therapeutics and relevant consulting relationships with NuSirt, AbbVie, Afimmune (DS Biopharma), and several other pharmaceutical companies. Sixteen coinvestigators also disclosed relationships with pharmaceutical companies, of whom eight disclosed consulting relationships, received research funding, or were employed by Galectin.
SOURCE: Chalasani N et al. Gastroenterology. 2019 Dec 5. doi: 10.1053/j.gastro.2019.11.296.
*This story was updated on 3/18/2020.
FROM GASTROENTEROLOGY
CMS to test model limiting out-of-pocket costs for insulin
The plan, dubbed the Part D Senior Savings Model, would lower out-of-pocket costs to a maximum of a $35 copay for a 30-day supply throughout the plan year. The agency estimates that beneficiaries enrolling in a plan that is participating in the model would save an average of $446 annually in out-of-pocket costs for insulin.
The maximum copay will apply to all phases of the Part D benefit, including the deductible, initial coverage, and coverage gap phases, according to a fact sheet issued March 11 by the agency. CMS will be proving additional risk corridor protections to Part D plan sponsors to encourage participation in the voluntary model.
The agency has released two requests for application to participate in the model, one for Part D sponsors and one for insulin manufacturers, both of which can be found on a CMS web page providing more details on the program.
The agency said in the fact sheet that, on or around March 20, 2020, it will announce the manufacturers that are participating in the model for the 2021 plan year.
The plan, dubbed the Part D Senior Savings Model, would lower out-of-pocket costs to a maximum of a $35 copay for a 30-day supply throughout the plan year. The agency estimates that beneficiaries enrolling in a plan that is participating in the model would save an average of $446 annually in out-of-pocket costs for insulin.
The maximum copay will apply to all phases of the Part D benefit, including the deductible, initial coverage, and coverage gap phases, according to a fact sheet issued March 11 by the agency. CMS will be proving additional risk corridor protections to Part D plan sponsors to encourage participation in the voluntary model.
The agency has released two requests for application to participate in the model, one for Part D sponsors and one for insulin manufacturers, both of which can be found on a CMS web page providing more details on the program.
The agency said in the fact sheet that, on or around March 20, 2020, it will announce the manufacturers that are participating in the model for the 2021 plan year.
The plan, dubbed the Part D Senior Savings Model, would lower out-of-pocket costs to a maximum of a $35 copay for a 30-day supply throughout the plan year. The agency estimates that beneficiaries enrolling in a plan that is participating in the model would save an average of $446 annually in out-of-pocket costs for insulin.
The maximum copay will apply to all phases of the Part D benefit, including the deductible, initial coverage, and coverage gap phases, according to a fact sheet issued March 11 by the agency. CMS will be proving additional risk corridor protections to Part D plan sponsors to encourage participation in the voluntary model.
The agency has released two requests for application to participate in the model, one for Part D sponsors and one for insulin manufacturers, both of which can be found on a CMS web page providing more details on the program.
The agency said in the fact sheet that, on or around March 20, 2020, it will announce the manufacturers that are participating in the model for the 2021 plan year.
FDA approves immunotherapy combo for liver cancer
Patients with advanced liver cancer have a new treatment option – the immunotherapy combination of nivolumab (Opdivo, Bristol-Myers Squibb) and ipilimumab (Yervoy, Bristol-Myers Squibb).
The US Food and Drug Administration (FDA) granted an accelerated approval of the combination for use in patients with advanced hepatocellular carcinoma (HCC) who have previously been treated with sorafenib (Nexavar, Bayer). Nivolumab is already approved as monotherapy for use in advanced HCC in patients who have previously been treated with sorafenib.
The immunotherapy combination has shown a response rate that is more than twice that seen with nivolumab alone. The combination was tested at three different dosage schedules in the single-arm phase 1/2 trial known as CheckMate-040, which was conducted in 148 patients with advanced HCC who had previously been treated with sorafenib.
The approval was based on one arm of this trial, a cohort of 49 patients who were treated with nivolumab 1 mg/kg IV and ipilimumab 3 mg/kg IV every 3 weeks for four doses, followed by nivolumab 240 mg every 2 weeks until disease progression or unacceptable toxicity.
After a minimum follow-up of 28 months, 33% (16/49) of these patients showed a response, with 8% (4/49) showing a complete response and 24% (12/49) a partial response.
In terms of duration of responses, 88% of the responses lasted at least 6 months, 56% at least 12 months, and 31% at least 24 months, according to the company.
The results that led to the 2017 approval of nivolumab monotherapy for advanced HCC, as previously reported by Medscape Medical News, come from a cohort of 154 patients who received nivolumab 3 mg/kg administered intravenously every 2 weeks.
The overall response rate was 14.3% (22 of 154 patients), with three patients (1.9%) showing a complete response and 19 patients (12.3%) a partial response. The duration of the responses ranged from 3.2 to 38.2+ months; 91% of those patients had responses of 6 months or longer, and 55% had responses of 12 months or longer.
Notably, patient responses in all arms were achieved regardless of baseline tumor PD-L1 status.
Aggressive disease, incidence is rising
“HCC is an aggressive disease in need of different treatment approaches,” said Anthony B. El-Khoueiry, MD, of the Norris Comprehensive Cancer Center, University of Southern California, Los Angeles, in a company press statement.“The overall response rate observed in the Opdivo + Yervoy cohort of the CheckMate-040 trial underscores the potential of this dual immunotherapy as a possible treatment option for patients,” he commented. El-Khoueiry was lead investigator of the study and has received honoraria and consulting fees from Bristol-Myers Squibb.
“The incidence of liver cancer is rising in the United States, and HCC is the most common and aggressive form of the disease,” said Andrea Wilson, president and founder, Blue Faery: The Adrienne Wilson Liver Cancer Association.
“Today’s approval provides a new option for patients with HCC previously treated with sorafenib, giving the community more hope,” she said in the company press statement.
Safety profile
The nivolumab-ipilimumab combination had an “acceptable” safety profile overall in the CheckMate-040 trial, wrote lead study author Thomas Yao, MD, of the University at Hong Kong, China, and colleagues in their study abstract, which was presented at the 2019 annual meeting of the American Society of Clinical Oncology. Yao has received honoraria from Bristol-Myers Squibb and has served as a consultant to the company.
According to those data, 37% of patients had a grade 3-4 treatment-related adverse event (TRAE), the most common of which were pruritus and rash; 5% had grade 3–4 TRAEs that led to discontinuation.
Nivolumab is associated with pneumonitis, colitis, hepatitis, endocrinopathies, nephritis, renal dysfunction, skin adverse reactions, encephalitis, other adverse reactions, and infusion-related reactions, as well as embryo-fetal toxicity. Ipilimumab has a boxed warning for immune-mediated adverse reactions.
Nivolumab alone is approved for use in the treatment of unresectable or metastatic melanoma, non–small cell lung cancer, small cell lung cancer, and classical Hodgkin lymphoma.
The combination of nivolumab with ipilimumab is also approved for use in the treatment of melanoma and renal cell carcinoma.
This article first appeared on Medscape.com.
Patients with advanced liver cancer have a new treatment option – the immunotherapy combination of nivolumab (Opdivo, Bristol-Myers Squibb) and ipilimumab (Yervoy, Bristol-Myers Squibb).
The US Food and Drug Administration (FDA) granted an accelerated approval of the combination for use in patients with advanced hepatocellular carcinoma (HCC) who have previously been treated with sorafenib (Nexavar, Bayer). Nivolumab is already approved as monotherapy for use in advanced HCC in patients who have previously been treated with sorafenib.
The immunotherapy combination has shown a response rate that is more than twice that seen with nivolumab alone. The combination was tested at three different dosage schedules in the single-arm phase 1/2 trial known as CheckMate-040, which was conducted in 148 patients with advanced HCC who had previously been treated with sorafenib.
The approval was based on one arm of this trial, a cohort of 49 patients who were treated with nivolumab 1 mg/kg IV and ipilimumab 3 mg/kg IV every 3 weeks for four doses, followed by nivolumab 240 mg every 2 weeks until disease progression or unacceptable toxicity.
After a minimum follow-up of 28 months, 33% (16/49) of these patients showed a response, with 8% (4/49) showing a complete response and 24% (12/49) a partial response.
In terms of duration of responses, 88% of the responses lasted at least 6 months, 56% at least 12 months, and 31% at least 24 months, according to the company.
The results that led to the 2017 approval of nivolumab monotherapy for advanced HCC, as previously reported by Medscape Medical News, come from a cohort of 154 patients who received nivolumab 3 mg/kg administered intravenously every 2 weeks.
The overall response rate was 14.3% (22 of 154 patients), with three patients (1.9%) showing a complete response and 19 patients (12.3%) a partial response. The duration of the responses ranged from 3.2 to 38.2+ months; 91% of those patients had responses of 6 months or longer, and 55% had responses of 12 months or longer.
Notably, patient responses in all arms were achieved regardless of baseline tumor PD-L1 status.
Aggressive disease, incidence is rising
“HCC is an aggressive disease in need of different treatment approaches,” said Anthony B. El-Khoueiry, MD, of the Norris Comprehensive Cancer Center, University of Southern California, Los Angeles, in a company press statement.“The overall response rate observed in the Opdivo + Yervoy cohort of the CheckMate-040 trial underscores the potential of this dual immunotherapy as a possible treatment option for patients,” he commented. El-Khoueiry was lead investigator of the study and has received honoraria and consulting fees from Bristol-Myers Squibb.
“The incidence of liver cancer is rising in the United States, and HCC is the most common and aggressive form of the disease,” said Andrea Wilson, president and founder, Blue Faery: The Adrienne Wilson Liver Cancer Association.
“Today’s approval provides a new option for patients with HCC previously treated with sorafenib, giving the community more hope,” she said in the company press statement.
Safety profile
The nivolumab-ipilimumab combination had an “acceptable” safety profile overall in the CheckMate-040 trial, wrote lead study author Thomas Yao, MD, of the University at Hong Kong, China, and colleagues in their study abstract, which was presented at the 2019 annual meeting of the American Society of Clinical Oncology. Yao has received honoraria from Bristol-Myers Squibb and has served as a consultant to the company.
According to those data, 37% of patients had a grade 3-4 treatment-related adverse event (TRAE), the most common of which were pruritus and rash; 5% had grade 3–4 TRAEs that led to discontinuation.
Nivolumab is associated with pneumonitis, colitis, hepatitis, endocrinopathies, nephritis, renal dysfunction, skin adverse reactions, encephalitis, other adverse reactions, and infusion-related reactions, as well as embryo-fetal toxicity. Ipilimumab has a boxed warning for immune-mediated adverse reactions.
Nivolumab alone is approved for use in the treatment of unresectable or metastatic melanoma, non–small cell lung cancer, small cell lung cancer, and classical Hodgkin lymphoma.
The combination of nivolumab with ipilimumab is also approved for use in the treatment of melanoma and renal cell carcinoma.
This article first appeared on Medscape.com.
Patients with advanced liver cancer have a new treatment option – the immunotherapy combination of nivolumab (Opdivo, Bristol-Myers Squibb) and ipilimumab (Yervoy, Bristol-Myers Squibb).
The US Food and Drug Administration (FDA) granted an accelerated approval of the combination for use in patients with advanced hepatocellular carcinoma (HCC) who have previously been treated with sorafenib (Nexavar, Bayer). Nivolumab is already approved as monotherapy for use in advanced HCC in patients who have previously been treated with sorafenib.
The immunotherapy combination has shown a response rate that is more than twice that seen with nivolumab alone. The combination was tested at three different dosage schedules in the single-arm phase 1/2 trial known as CheckMate-040, which was conducted in 148 patients with advanced HCC who had previously been treated with sorafenib.
The approval was based on one arm of this trial, a cohort of 49 patients who were treated with nivolumab 1 mg/kg IV and ipilimumab 3 mg/kg IV every 3 weeks for four doses, followed by nivolumab 240 mg every 2 weeks until disease progression or unacceptable toxicity.
After a minimum follow-up of 28 months, 33% (16/49) of these patients showed a response, with 8% (4/49) showing a complete response and 24% (12/49) a partial response.
In terms of duration of responses, 88% of the responses lasted at least 6 months, 56% at least 12 months, and 31% at least 24 months, according to the company.
The results that led to the 2017 approval of nivolumab monotherapy for advanced HCC, as previously reported by Medscape Medical News, come from a cohort of 154 patients who received nivolumab 3 mg/kg administered intravenously every 2 weeks.
The overall response rate was 14.3% (22 of 154 patients), with three patients (1.9%) showing a complete response and 19 patients (12.3%) a partial response. The duration of the responses ranged from 3.2 to 38.2+ months; 91% of those patients had responses of 6 months or longer, and 55% had responses of 12 months or longer.
Notably, patient responses in all arms were achieved regardless of baseline tumor PD-L1 status.
Aggressive disease, incidence is rising
“HCC is an aggressive disease in need of different treatment approaches,” said Anthony B. El-Khoueiry, MD, of the Norris Comprehensive Cancer Center, University of Southern California, Los Angeles, in a company press statement.“The overall response rate observed in the Opdivo + Yervoy cohort of the CheckMate-040 trial underscores the potential of this dual immunotherapy as a possible treatment option for patients,” he commented. El-Khoueiry was lead investigator of the study and has received honoraria and consulting fees from Bristol-Myers Squibb.
“The incidence of liver cancer is rising in the United States, and HCC is the most common and aggressive form of the disease,” said Andrea Wilson, president and founder, Blue Faery: The Adrienne Wilson Liver Cancer Association.
“Today’s approval provides a new option for patients with HCC previously treated with sorafenib, giving the community more hope,” she said in the company press statement.
Safety profile
The nivolumab-ipilimumab combination had an “acceptable” safety profile overall in the CheckMate-040 trial, wrote lead study author Thomas Yao, MD, of the University at Hong Kong, China, and colleagues in their study abstract, which was presented at the 2019 annual meeting of the American Society of Clinical Oncology. Yao has received honoraria from Bristol-Myers Squibb and has served as a consultant to the company.
According to those data, 37% of patients had a grade 3-4 treatment-related adverse event (TRAE), the most common of which were pruritus and rash; 5% had grade 3–4 TRAEs that led to discontinuation.
Nivolumab is associated with pneumonitis, colitis, hepatitis, endocrinopathies, nephritis, renal dysfunction, skin adverse reactions, encephalitis, other adverse reactions, and infusion-related reactions, as well as embryo-fetal toxicity. Ipilimumab has a boxed warning for immune-mediated adverse reactions.
Nivolumab alone is approved for use in the treatment of unresectable or metastatic melanoma, non–small cell lung cancer, small cell lung cancer, and classical Hodgkin lymphoma.
The combination of nivolumab with ipilimumab is also approved for use in the treatment of melanoma and renal cell carcinoma.
This article first appeared on Medscape.com.
ACR cancels March conference, symposium
The American College of Rheumatology decided to cancel its upcoming Division & Program Directors Conference and State-of-the-Art Clinical Symposium because of “the escalation in the number of people affected [by the COVID-19 situation], and the likelihood of potentially increasing the exposure to COVID-19.”
The Division & Program Directors Conference was slated to take place March 13-14 in Chicago, while the State-of-the-Art Clinical Symposium was scheduled to happen March 27-29 in New Orleans. In both cases, the organizers are exploring alternative ways to deliver or present the content.
The ACR has not made a decision on the status of its Pediatric Rheumatology Symposium, April 29-May 2, New Orleans, but plans to do so by March 30.
The American College of Rheumatology decided to cancel its upcoming Division & Program Directors Conference and State-of-the-Art Clinical Symposium because of “the escalation in the number of people affected [by the COVID-19 situation], and the likelihood of potentially increasing the exposure to COVID-19.”
The Division & Program Directors Conference was slated to take place March 13-14 in Chicago, while the State-of-the-Art Clinical Symposium was scheduled to happen March 27-29 in New Orleans. In both cases, the organizers are exploring alternative ways to deliver or present the content.
The ACR has not made a decision on the status of its Pediatric Rheumatology Symposium, April 29-May 2, New Orleans, but plans to do so by March 30.
The American College of Rheumatology decided to cancel its upcoming Division & Program Directors Conference and State-of-the-Art Clinical Symposium because of “the escalation in the number of people affected [by the COVID-19 situation], and the likelihood of potentially increasing the exposure to COVID-19.”
The Division & Program Directors Conference was slated to take place March 13-14 in Chicago, while the State-of-the-Art Clinical Symposium was scheduled to happen March 27-29 in New Orleans. In both cases, the organizers are exploring alternative ways to deliver or present the content.
The ACR has not made a decision on the status of its Pediatric Rheumatology Symposium, April 29-May 2, New Orleans, but plans to do so by March 30.