User login
Psychiatric patients and pandemics
What can psychiatric clinicians do to keep their patients healthy in this coronavirus time?
In the 3 days between starting this column and finishing it, the world has gone into a tailspin. Perhaps what I write is no longer relevant. But hopefully it is.
I have no right or wrong answers here but thoughts about factors to consider.
- On inpatient psychiatry wards, the emphasis is on communal living. On our ward, bedrooms and bathrooms are shared. Patients eat together. There are numerous group therapies.
- We have decided to restrict visitors out of the concern that one may infect a ward of patients and staff. We are hoping to do video visitation, but that may take a while to implement.
- An open question is how we are going to provide our involuntary patients with access to the public defense attorneys. Public defenders still have the ability to come onto the inpatient ward, but we will start screening them first.
- In terms of sanitation, wall sanitizers are forbidden, since sanitizers may be drank or made into a firebomb. So we are incessantly wiping down the shared phones and game board pieces.
- Looking at the outpatient arena, we have moved our chairs around, so that there are 3 feet between chairs. We have opened up another waiting room to provide more distance.
- We are trying to decide whether to cancel groups. We did cancel our senior group, and I think I will cancel the rest of them shortly.
- We are seriously looking at telepsychiatry.
- Schools are closed. Many of my clinicians have young children, so they may be out. We are expecting many patients to cancel and will see how that plays out. Others of us have elderly parents. My mother’s assisted-living facility is on lockdown. So, having been locked out after a visit, she is with me tonight.
- Psychiatrists are expected to keep up their relative value unit count. Can they meet their targets? Probably not. Will it matter?
- And what about all our homeless patients, who cannot disinfect their tents or shelters?
- Conferences no longer seem so important. I am less worried about coverage for the American Psychiatric Association meeting, since the 2020 conference has been canceled.
On the rosy side, maybe this will be a wake-up call about climate change. So we live in interesting times.
Take care of your patients and each other.
Dr. Ritchie is chair of psychiatry at Medstar Washington Hospital Center and professor of psychiatry at Georgetown University, Washington. She has no disclosures.
What can psychiatric clinicians do to keep their patients healthy in this coronavirus time?
In the 3 days between starting this column and finishing it, the world has gone into a tailspin. Perhaps what I write is no longer relevant. But hopefully it is.
I have no right or wrong answers here but thoughts about factors to consider.
- On inpatient psychiatry wards, the emphasis is on communal living. On our ward, bedrooms and bathrooms are shared. Patients eat together. There are numerous group therapies.
- We have decided to restrict visitors out of the concern that one may infect a ward of patients and staff. We are hoping to do video visitation, but that may take a while to implement.
- An open question is how we are going to provide our involuntary patients with access to the public defense attorneys. Public defenders still have the ability to come onto the inpatient ward, but we will start screening them first.
- In terms of sanitation, wall sanitizers are forbidden, since sanitizers may be drank or made into a firebomb. So we are incessantly wiping down the shared phones and game board pieces.
- Looking at the outpatient arena, we have moved our chairs around, so that there are 3 feet between chairs. We have opened up another waiting room to provide more distance.
- We are trying to decide whether to cancel groups. We did cancel our senior group, and I think I will cancel the rest of them shortly.
- We are seriously looking at telepsychiatry.
- Schools are closed. Many of my clinicians have young children, so they may be out. We are expecting many patients to cancel and will see how that plays out. Others of us have elderly parents. My mother’s assisted-living facility is on lockdown. So, having been locked out after a visit, she is with me tonight.
- Psychiatrists are expected to keep up their relative value unit count. Can they meet their targets? Probably not. Will it matter?
- And what about all our homeless patients, who cannot disinfect their tents or shelters?
- Conferences no longer seem so important. I am less worried about coverage for the American Psychiatric Association meeting, since the 2020 conference has been canceled.
On the rosy side, maybe this will be a wake-up call about climate change. So we live in interesting times.
Take care of your patients and each other.
Dr. Ritchie is chair of psychiatry at Medstar Washington Hospital Center and professor of psychiatry at Georgetown University, Washington. She has no disclosures.
What can psychiatric clinicians do to keep their patients healthy in this coronavirus time?
In the 3 days between starting this column and finishing it, the world has gone into a tailspin. Perhaps what I write is no longer relevant. But hopefully it is.
I have no right or wrong answers here but thoughts about factors to consider.
- On inpatient psychiatry wards, the emphasis is on communal living. On our ward, bedrooms and bathrooms are shared. Patients eat together. There are numerous group therapies.
- We have decided to restrict visitors out of the concern that one may infect a ward of patients and staff. We are hoping to do video visitation, but that may take a while to implement.
- An open question is how we are going to provide our involuntary patients with access to the public defense attorneys. Public defenders still have the ability to come onto the inpatient ward, but we will start screening them first.
- In terms of sanitation, wall sanitizers are forbidden, since sanitizers may be drank or made into a firebomb. So we are incessantly wiping down the shared phones and game board pieces.
- Looking at the outpatient arena, we have moved our chairs around, so that there are 3 feet between chairs. We have opened up another waiting room to provide more distance.
- We are trying to decide whether to cancel groups. We did cancel our senior group, and I think I will cancel the rest of them shortly.
- We are seriously looking at telepsychiatry.
- Schools are closed. Many of my clinicians have young children, so they may be out. We are expecting many patients to cancel and will see how that plays out. Others of us have elderly parents. My mother’s assisted-living facility is on lockdown. So, having been locked out after a visit, she is with me tonight.
- Psychiatrists are expected to keep up their relative value unit count. Can they meet their targets? Probably not. Will it matter?
- And what about all our homeless patients, who cannot disinfect their tents or shelters?
- Conferences no longer seem so important. I am less worried about coverage for the American Psychiatric Association meeting, since the 2020 conference has been canceled.
On the rosy side, maybe this will be a wake-up call about climate change. So we live in interesting times.
Take care of your patients and each other.
Dr. Ritchie is chair of psychiatry at Medstar Washington Hospital Center and professor of psychiatry at Georgetown University, Washington. She has no disclosures.
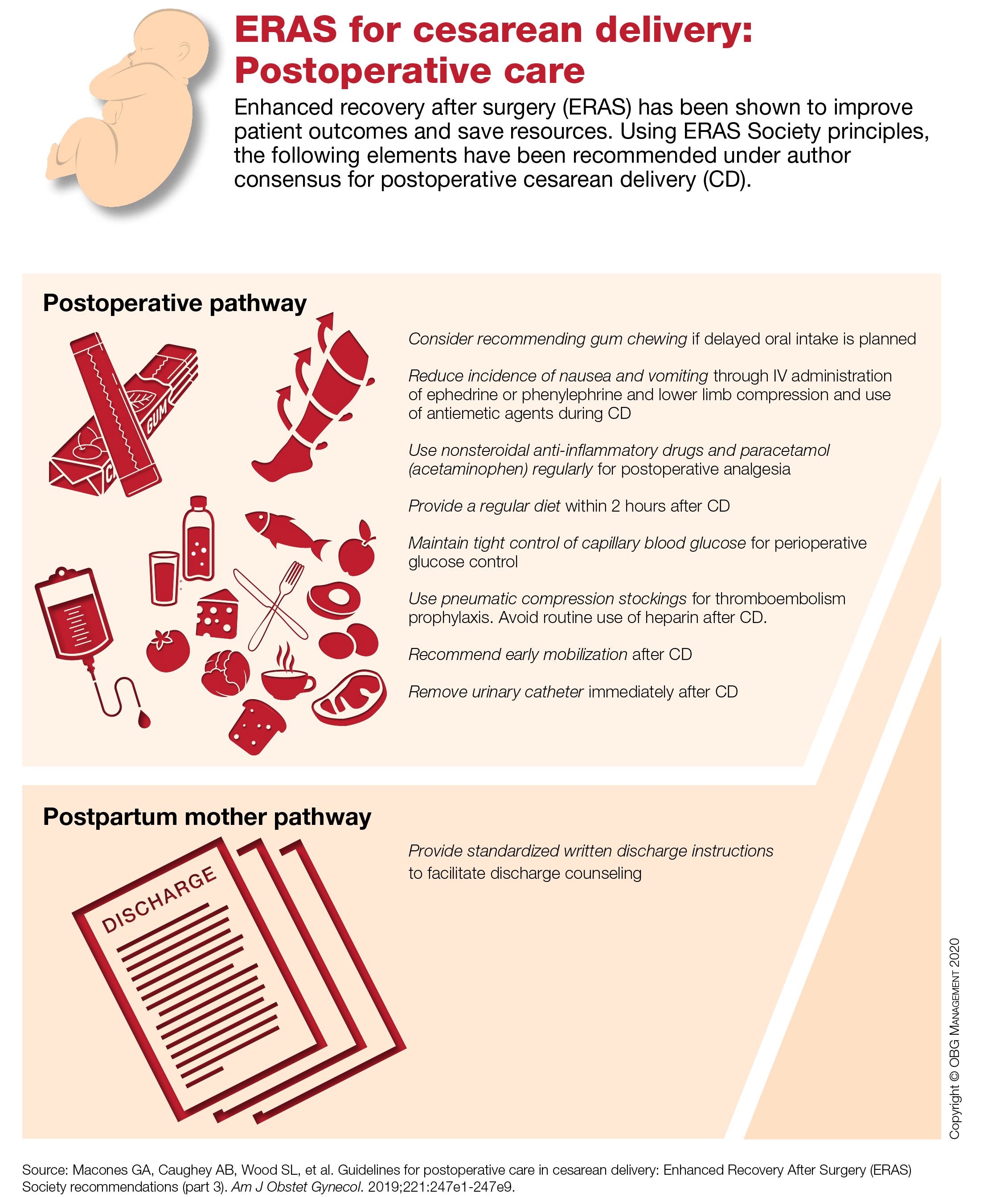
ERAS for cesarean delivery: Postoperative care


COVID-19: ASTCT provides interim guidelines for transplantation
The American Society for Transplantation and Cellular Therapy (ASTCT) has released interim guidelines for the care of hematopoietic cell transplantation (HCT) and cellular therapy patients in the light of the global SARS-CoV-2 pandemic.
The guidelines, summarized briefly below, focus on diagnostic and treatment considerations, evaluation of patients prior to initializing HCT and cellular therapy, and cell donor evaluation. Much of the guideline relies upon recommendations developed by the European Society for Blood and Marrow Transplantation (ESBMT). These guidelines were updated on March 16.
The ASTCT document focuses on patient-treatment specifics and does not cover specific infection-prevention policies and procedures, instead suggesting that local and institutional guidelines, such as those from the Centers for Disease Control and Prevention, should be followed. They did recommend that, in the local presence of COVID-19, “clinic visits that are not critical should be either deferred or substituted with telemedicine visits if deemed appropriate and feasible.”
Diagnostic considerations
In any patient with upper or lower respiratory symptoms, obtain polymerase chain reaction (PCR) testing for SARS-CoV-2, where possible, in addition to other respiratory virus PCR testing from any respiratory sample obtained, following CDC recommendations for sample collection and processing, which are continuously being updated on the CDC website.
These recommendations include nasal sampling, rather than oral sampling, and the discouraging of nasal washes where avoidable. If nasal washing is performed, it should be done with appropriate personal protective equipment as described by the CDC. The CDC has also provided additional infection prevention and control information for known and suspected COVID-19 patients in health care settings.
In patients positive for SARS-CoV-2 in an upper respiratory tract sample, chest imaging should be considered.
Preliminary reports suggest that there may be a discrepancy between upper- and lower-tract specimen positivity. Therefore, even when SARS-CoV-2 is not detected in an upper respiratory sample, the ASTCT recommends that chest imaging should be considered for lower respiratory tract infection when clinical symptoms of lower respiratory tract infection are present, including shortness of breath, hypoxia, and tachypnea.
With regard to routine bronchoalveolar lavage, the ASTCT recommends against it if a patient tests positive for SARS-CoV-2 given the risk of transmission among health care workers. The exception is in the case of suspected coinfection based on abnormal chest imaging and in patients for whom it is clinically indicated (for example, those receiving invasive mechanical ventilation). In addition to testing bronchoalveolar lavage samples for SARS-CoV-2, “copathogens should be evaluated and treated.”
Treatment considerations
“At this point no recommendations can be made on specific therapies due to limited data and unknown risk versus benefit; additional recommendations will be forthcoming. Even less data is available for pediatric patients. Treatment for viral, bacterial, and fungal copathogens should be optimized,” according to the ASTCT.
However, the society lists several therapies currently under consideration, which may be available through compassionate-use programs and are being investigated in current clinical trials in several countries, “including lopinavir/ritonavir, ribavirin, hydroxychloroquine, darunavir/cobicistat, and interferons-alpha and -beta.” Remdesivir, in particular, is being evaluated in a National Institutes of Health–sponsored, placebo-controlled clinical trial (NCT04280705).
In case of known or suspected COVID-19 with normal imaging and no or mild symptoms, no therapy is recommended. However, if symptoms progress or imaging is abnormal, an infectious disease specialist or department should be consulted, according to the ASTCT.
Evaluation prior to HCT or cellular therapy
“There is sufficient concern that COVID-19 could have a significant impact on posttransplant or posttherapy outcomes,” according to the guidelines, and the ASTCT provided the following recommendations to be considered in known or suspected COVID-19 patients. In particular, practitioners need to weigh the risk of delaying or altering therapy plans with the risk of progression of underlying disease.
If SARS-CoV-2 is detected in a respiratory specimen, HCT or cellular therapy procedures should be deferred. Therapy should also be deferred in HCT and cellular therapy candidates with close contact with a person infected with SARS-CoV-2 and in those patients who have traveled to a high-risk area or had close contact with a person traveling from an area at high risk for COVID-19.
In the case of a patient in a community with widespread disease, “all HCT and cellular therapy candidates should undergo screening for SARS-CoV-2 infection by PCR in respiratory specimens at the time of initial evaluation and 2 days prior to conditioning/lymphodepletion, regardless of the presence of symptoms, if testing is available.”
Procedures to be deferred include peripheral blood stem cell mobilization, bone marrow harvest, T-cell collections, and conditioning/lymphodepletion. These should not be performed for at least 14 days (preferably 21 days) from the day of last contact, according to the ASTCT. Two consecutive negative PCR tests each approximately 1 week apart (deferral for 14 days minimum), should be obtained, if available.
In areas with high community spread, the guidelines also state that “interim treatment and/or longer deferral of definite therapy should be considered when feasible (for example, multiple myeloma, germ cell tumors, consolidative transplants).”
Similar considerations should be afforded to potential cellular donors. Donors with SARS-CoV-2 detected in a respiratory sample are considered ineligible. Those meeting exposure criteria for patients, as listed above, should be excluded from donation for at least 28 days. “In individual circumstances, a donor may be considered eligible if respiratory samples are negative for SARS-CoV-2 by PCR and donor is asymptomatic. Donor should be closely monitored for COVID-19.”
In the case of unrelated donors, the ASTCT recommends referral to the National Marrow Donor Program (NMDP) guidelines for updated guidance, but points out that, according to the NMDP, the Food and Drug Administration reports that there have been no reported or suspected cases of transfusion-transmitted COVID-19 to date and that “no cases of transfusion-transmission were ever reported for the other two coronaviruses that emerged during the past 2 decades [SARS, the severe acute respiratory syndrome coronavirus, and MERS-CoV, which causes Mideast respiratory syndrome].”
In the updated ESBMT guidelines, this recommendation was made in reference to the greater spread of COVID-19: “It is therefore strongly recommended to have secured stem cell product access by freezing the product before start of conditioning and, in situations when this is not possible, to have an alternative donor as a backup. For low-risk patients, it is recommended to postpone the start of the transplant procedure if deemed to be safe to do so. This includes both allogeneic and autologous transplant procedures.”
In a recent webinar, Pavan Reddy, MD, of the University of Michigan, Ann Arbor, and ASTCT President; Alpana Waghmare, MD, of the Fred Hutchinson Cancer Research Center, Seattle; and Roy Chemaly, MD, of the MD Anderson Cancer Center, Houston, and chair of the ASTCT Transplant Infectious Disease Special Interest Group, discussed the guidelines and provided some updated information.
Dr. Reddy stated that, at the University of Michigan, they were delaying all nonurgent transplants, largely for myeloma, and are postponing even allotransplants. “The transplants we are not delaying are the high-risk AMLs … and in cases where we truly cannot delay transplants because of patient condition or, in some cases, the donor situation.”
Dr. Chemaly and Dr. Waghmare both agreed that their centers were following a similar approach.
With regard to patient testing, all three institution have recently moved to testing everyone a few days before transplant regardless of symptoms.
They also pointed out that essentially all clinical trials were being put on hold during the crisis, except for those few where patients would be put in danger if the trial were interrupted.
The guidelines discuss in depth the rationale, toxicity, and dosages for use of select agents, including remdesivir, chloroquine/hydroxychloroquine, ribavirin, and tocilizumab. There was some concern expressed about shortages developing in these drugs, which serve a number of other patient communities, in particular the possibility of a tocilizumab shortage was of concern.
Steroids and intravenous immunoglobulins are not are not recommended, according to the guidelines, which also stated that adjunctive therapies such as antibiotics should be considered.
Dr. Chemaly, Dr. Reddy, and Dr. Waghmare did not provide disclosure in the webinar.
The ASTCT recommends following the World Health Organization and CDC COVID-19 pages for continued updates and information on other aspects of the pandemic.
This article was updated 3/26/20.
SOURCE: ASTCT Response to COVID-19. 2020. www.astct.org/connect/astct-response-to-covid-19.
There is emerging data regarding coinfection of SARS-CoV-2 with other viruses including infleunza. Immunocompromised hosts, especially transplantation and cellular therapy (TCT) recipients, are known to frequently have more than one pathogen present, especially in pulmonary infections. As the community spread increases, it would be reasonable to obtain concomitant testing for respiratory viruses along with SARS-CoV-2 as recommended. In addition, viral infection can cause secondary bacterial and fungal infections (especially Aspergillus). In the presence of SARS-CoV-2, where it is recommended to avoid bronchoalveolar lavage, we have to keep a high clinical suspicion based on patients’ risk factors.
Acute Respiratory Distress Syndrome (ARDS) caused by an intense inflammatory response is the main cause of death in COVID-19. Early reports on the use of tocilizumab (an IL-6 receptor blocker) for ARDS to block cytokine mediated injury to the lung should be a consideration early in the course of COVID-19 pneumonitis, especially in setting of high risk for ARDS mortality.
We are considering other IL-6–blocking agents like siltuximab in case of a shortage of tocilizumab while centers scramble to get these agents. It is important to note that any such usages for COVID-19 would be considered off-label.
TCT candidates should of course be practicing social distancing in days leading to transplant to reduce their risk of exposure regardless of state or federal recommendations. Household members of TCT candidates should practice similar caution because transmission has been reported by asymptomatic individuals.
Zainab Shahid, MD, is the medical director of Bone Marrow Transplant Infectious Diseases at the Levine Cancer Institute/Atrium Health and a clinical associate professor of medicine at University of North Carolina at Chapel Hill. She reported that she had no relevant disclosures.
There is emerging data regarding coinfection of SARS-CoV-2 with other viruses including infleunza. Immunocompromised hosts, especially transplantation and cellular therapy (TCT) recipients, are known to frequently have more than one pathogen present, especially in pulmonary infections. As the community spread increases, it would be reasonable to obtain concomitant testing for respiratory viruses along with SARS-CoV-2 as recommended. In addition, viral infection can cause secondary bacterial and fungal infections (especially Aspergillus). In the presence of SARS-CoV-2, where it is recommended to avoid bronchoalveolar lavage, we have to keep a high clinical suspicion based on patients’ risk factors.
Acute Respiratory Distress Syndrome (ARDS) caused by an intense inflammatory response is the main cause of death in COVID-19. Early reports on the use of tocilizumab (an IL-6 receptor blocker) for ARDS to block cytokine mediated injury to the lung should be a consideration early in the course of COVID-19 pneumonitis, especially in setting of high risk for ARDS mortality.
We are considering other IL-6–blocking agents like siltuximab in case of a shortage of tocilizumab while centers scramble to get these agents. It is important to note that any such usages for COVID-19 would be considered off-label.
TCT candidates should of course be practicing social distancing in days leading to transplant to reduce their risk of exposure regardless of state or federal recommendations. Household members of TCT candidates should practice similar caution because transmission has been reported by asymptomatic individuals.
Zainab Shahid, MD, is the medical director of Bone Marrow Transplant Infectious Diseases at the Levine Cancer Institute/Atrium Health and a clinical associate professor of medicine at University of North Carolina at Chapel Hill. She reported that she had no relevant disclosures.
There is emerging data regarding coinfection of SARS-CoV-2 with other viruses including infleunza. Immunocompromised hosts, especially transplantation and cellular therapy (TCT) recipients, are known to frequently have more than one pathogen present, especially in pulmonary infections. As the community spread increases, it would be reasonable to obtain concomitant testing for respiratory viruses along with SARS-CoV-2 as recommended. In addition, viral infection can cause secondary bacterial and fungal infections (especially Aspergillus). In the presence of SARS-CoV-2, where it is recommended to avoid bronchoalveolar lavage, we have to keep a high clinical suspicion based on patients’ risk factors.
Acute Respiratory Distress Syndrome (ARDS) caused by an intense inflammatory response is the main cause of death in COVID-19. Early reports on the use of tocilizumab (an IL-6 receptor blocker) for ARDS to block cytokine mediated injury to the lung should be a consideration early in the course of COVID-19 pneumonitis, especially in setting of high risk for ARDS mortality.
We are considering other IL-6–blocking agents like siltuximab in case of a shortage of tocilizumab while centers scramble to get these agents. It is important to note that any such usages for COVID-19 would be considered off-label.
TCT candidates should of course be practicing social distancing in days leading to transplant to reduce their risk of exposure regardless of state or federal recommendations. Household members of TCT candidates should practice similar caution because transmission has been reported by asymptomatic individuals.
Zainab Shahid, MD, is the medical director of Bone Marrow Transplant Infectious Diseases at the Levine Cancer Institute/Atrium Health and a clinical associate professor of medicine at University of North Carolina at Chapel Hill. She reported that she had no relevant disclosures.
The American Society for Transplantation and Cellular Therapy (ASTCT) has released interim guidelines for the care of hematopoietic cell transplantation (HCT) and cellular therapy patients in the light of the global SARS-CoV-2 pandemic.
The guidelines, summarized briefly below, focus on diagnostic and treatment considerations, evaluation of patients prior to initializing HCT and cellular therapy, and cell donor evaluation. Much of the guideline relies upon recommendations developed by the European Society for Blood and Marrow Transplantation (ESBMT). These guidelines were updated on March 16.
The ASTCT document focuses on patient-treatment specifics and does not cover specific infection-prevention policies and procedures, instead suggesting that local and institutional guidelines, such as those from the Centers for Disease Control and Prevention, should be followed. They did recommend that, in the local presence of COVID-19, “clinic visits that are not critical should be either deferred or substituted with telemedicine visits if deemed appropriate and feasible.”
Diagnostic considerations
In any patient with upper or lower respiratory symptoms, obtain polymerase chain reaction (PCR) testing for SARS-CoV-2, where possible, in addition to other respiratory virus PCR testing from any respiratory sample obtained, following CDC recommendations for sample collection and processing, which are continuously being updated on the CDC website.
These recommendations include nasal sampling, rather than oral sampling, and the discouraging of nasal washes where avoidable. If nasal washing is performed, it should be done with appropriate personal protective equipment as described by the CDC. The CDC has also provided additional infection prevention and control information for known and suspected COVID-19 patients in health care settings.
In patients positive for SARS-CoV-2 in an upper respiratory tract sample, chest imaging should be considered.
Preliminary reports suggest that there may be a discrepancy between upper- and lower-tract specimen positivity. Therefore, even when SARS-CoV-2 is not detected in an upper respiratory sample, the ASTCT recommends that chest imaging should be considered for lower respiratory tract infection when clinical symptoms of lower respiratory tract infection are present, including shortness of breath, hypoxia, and tachypnea.
With regard to routine bronchoalveolar lavage, the ASTCT recommends against it if a patient tests positive for SARS-CoV-2 given the risk of transmission among health care workers. The exception is in the case of suspected coinfection based on abnormal chest imaging and in patients for whom it is clinically indicated (for example, those receiving invasive mechanical ventilation). In addition to testing bronchoalveolar lavage samples for SARS-CoV-2, “copathogens should be evaluated and treated.”
Treatment considerations
“At this point no recommendations can be made on specific therapies due to limited data and unknown risk versus benefit; additional recommendations will be forthcoming. Even less data is available for pediatric patients. Treatment for viral, bacterial, and fungal copathogens should be optimized,” according to the ASTCT.
However, the society lists several therapies currently under consideration, which may be available through compassionate-use programs and are being investigated in current clinical trials in several countries, “including lopinavir/ritonavir, ribavirin, hydroxychloroquine, darunavir/cobicistat, and interferons-alpha and -beta.” Remdesivir, in particular, is being evaluated in a National Institutes of Health–sponsored, placebo-controlled clinical trial (NCT04280705).
In case of known or suspected COVID-19 with normal imaging and no or mild symptoms, no therapy is recommended. However, if symptoms progress or imaging is abnormal, an infectious disease specialist or department should be consulted, according to the ASTCT.
Evaluation prior to HCT or cellular therapy
“There is sufficient concern that COVID-19 could have a significant impact on posttransplant or posttherapy outcomes,” according to the guidelines, and the ASTCT provided the following recommendations to be considered in known or suspected COVID-19 patients. In particular, practitioners need to weigh the risk of delaying or altering therapy plans with the risk of progression of underlying disease.
If SARS-CoV-2 is detected in a respiratory specimen, HCT or cellular therapy procedures should be deferred. Therapy should also be deferred in HCT and cellular therapy candidates with close contact with a person infected with SARS-CoV-2 and in those patients who have traveled to a high-risk area or had close contact with a person traveling from an area at high risk for COVID-19.
In the case of a patient in a community with widespread disease, “all HCT and cellular therapy candidates should undergo screening for SARS-CoV-2 infection by PCR in respiratory specimens at the time of initial evaluation and 2 days prior to conditioning/lymphodepletion, regardless of the presence of symptoms, if testing is available.”
Procedures to be deferred include peripheral blood stem cell mobilization, bone marrow harvest, T-cell collections, and conditioning/lymphodepletion. These should not be performed for at least 14 days (preferably 21 days) from the day of last contact, according to the ASTCT. Two consecutive negative PCR tests each approximately 1 week apart (deferral for 14 days minimum), should be obtained, if available.
In areas with high community spread, the guidelines also state that “interim treatment and/or longer deferral of definite therapy should be considered when feasible (for example, multiple myeloma, germ cell tumors, consolidative transplants).”
Similar considerations should be afforded to potential cellular donors. Donors with SARS-CoV-2 detected in a respiratory sample are considered ineligible. Those meeting exposure criteria for patients, as listed above, should be excluded from donation for at least 28 days. “In individual circumstances, a donor may be considered eligible if respiratory samples are negative for SARS-CoV-2 by PCR and donor is asymptomatic. Donor should be closely monitored for COVID-19.”
In the case of unrelated donors, the ASTCT recommends referral to the National Marrow Donor Program (NMDP) guidelines for updated guidance, but points out that, according to the NMDP, the Food and Drug Administration reports that there have been no reported or suspected cases of transfusion-transmitted COVID-19 to date and that “no cases of transfusion-transmission were ever reported for the other two coronaviruses that emerged during the past 2 decades [SARS, the severe acute respiratory syndrome coronavirus, and MERS-CoV, which causes Mideast respiratory syndrome].”
In the updated ESBMT guidelines, this recommendation was made in reference to the greater spread of COVID-19: “It is therefore strongly recommended to have secured stem cell product access by freezing the product before start of conditioning and, in situations when this is not possible, to have an alternative donor as a backup. For low-risk patients, it is recommended to postpone the start of the transplant procedure if deemed to be safe to do so. This includes both allogeneic and autologous transplant procedures.”
In a recent webinar, Pavan Reddy, MD, of the University of Michigan, Ann Arbor, and ASTCT President; Alpana Waghmare, MD, of the Fred Hutchinson Cancer Research Center, Seattle; and Roy Chemaly, MD, of the MD Anderson Cancer Center, Houston, and chair of the ASTCT Transplant Infectious Disease Special Interest Group, discussed the guidelines and provided some updated information.
Dr. Reddy stated that, at the University of Michigan, they were delaying all nonurgent transplants, largely for myeloma, and are postponing even allotransplants. “The transplants we are not delaying are the high-risk AMLs … and in cases where we truly cannot delay transplants because of patient condition or, in some cases, the donor situation.”
Dr. Chemaly and Dr. Waghmare both agreed that their centers were following a similar approach.
With regard to patient testing, all three institution have recently moved to testing everyone a few days before transplant regardless of symptoms.
They also pointed out that essentially all clinical trials were being put on hold during the crisis, except for those few where patients would be put in danger if the trial were interrupted.
The guidelines discuss in depth the rationale, toxicity, and dosages for use of select agents, including remdesivir, chloroquine/hydroxychloroquine, ribavirin, and tocilizumab. There was some concern expressed about shortages developing in these drugs, which serve a number of other patient communities, in particular the possibility of a tocilizumab shortage was of concern.
Steroids and intravenous immunoglobulins are not are not recommended, according to the guidelines, which also stated that adjunctive therapies such as antibiotics should be considered.
Dr. Chemaly, Dr. Reddy, and Dr. Waghmare did not provide disclosure in the webinar.
The ASTCT recommends following the World Health Organization and CDC COVID-19 pages for continued updates and information on other aspects of the pandemic.
This article was updated 3/26/20.
SOURCE: ASTCT Response to COVID-19. 2020. www.astct.org/connect/astct-response-to-covid-19.
The American Society for Transplantation and Cellular Therapy (ASTCT) has released interim guidelines for the care of hematopoietic cell transplantation (HCT) and cellular therapy patients in the light of the global SARS-CoV-2 pandemic.
The guidelines, summarized briefly below, focus on diagnostic and treatment considerations, evaluation of patients prior to initializing HCT and cellular therapy, and cell donor evaluation. Much of the guideline relies upon recommendations developed by the European Society for Blood and Marrow Transplantation (ESBMT). These guidelines were updated on March 16.
The ASTCT document focuses on patient-treatment specifics and does not cover specific infection-prevention policies and procedures, instead suggesting that local and institutional guidelines, such as those from the Centers for Disease Control and Prevention, should be followed. They did recommend that, in the local presence of COVID-19, “clinic visits that are not critical should be either deferred or substituted with telemedicine visits if deemed appropriate and feasible.”
Diagnostic considerations
In any patient with upper or lower respiratory symptoms, obtain polymerase chain reaction (PCR) testing for SARS-CoV-2, where possible, in addition to other respiratory virus PCR testing from any respiratory sample obtained, following CDC recommendations for sample collection and processing, which are continuously being updated on the CDC website.
These recommendations include nasal sampling, rather than oral sampling, and the discouraging of nasal washes where avoidable. If nasal washing is performed, it should be done with appropriate personal protective equipment as described by the CDC. The CDC has also provided additional infection prevention and control information for known and suspected COVID-19 patients in health care settings.
In patients positive for SARS-CoV-2 in an upper respiratory tract sample, chest imaging should be considered.
Preliminary reports suggest that there may be a discrepancy between upper- and lower-tract specimen positivity. Therefore, even when SARS-CoV-2 is not detected in an upper respiratory sample, the ASTCT recommends that chest imaging should be considered for lower respiratory tract infection when clinical symptoms of lower respiratory tract infection are present, including shortness of breath, hypoxia, and tachypnea.
With regard to routine bronchoalveolar lavage, the ASTCT recommends against it if a patient tests positive for SARS-CoV-2 given the risk of transmission among health care workers. The exception is in the case of suspected coinfection based on abnormal chest imaging and in patients for whom it is clinically indicated (for example, those receiving invasive mechanical ventilation). In addition to testing bronchoalveolar lavage samples for SARS-CoV-2, “copathogens should be evaluated and treated.”
Treatment considerations
“At this point no recommendations can be made on specific therapies due to limited data and unknown risk versus benefit; additional recommendations will be forthcoming. Even less data is available for pediatric patients. Treatment for viral, bacterial, and fungal copathogens should be optimized,” according to the ASTCT.
However, the society lists several therapies currently under consideration, which may be available through compassionate-use programs and are being investigated in current clinical trials in several countries, “including lopinavir/ritonavir, ribavirin, hydroxychloroquine, darunavir/cobicistat, and interferons-alpha and -beta.” Remdesivir, in particular, is being evaluated in a National Institutes of Health–sponsored, placebo-controlled clinical trial (NCT04280705).
In case of known or suspected COVID-19 with normal imaging and no or mild symptoms, no therapy is recommended. However, if symptoms progress or imaging is abnormal, an infectious disease specialist or department should be consulted, according to the ASTCT.
Evaluation prior to HCT or cellular therapy
“There is sufficient concern that COVID-19 could have a significant impact on posttransplant or posttherapy outcomes,” according to the guidelines, and the ASTCT provided the following recommendations to be considered in known or suspected COVID-19 patients. In particular, practitioners need to weigh the risk of delaying or altering therapy plans with the risk of progression of underlying disease.
If SARS-CoV-2 is detected in a respiratory specimen, HCT or cellular therapy procedures should be deferred. Therapy should also be deferred in HCT and cellular therapy candidates with close contact with a person infected with SARS-CoV-2 and in those patients who have traveled to a high-risk area or had close contact with a person traveling from an area at high risk for COVID-19.
In the case of a patient in a community with widespread disease, “all HCT and cellular therapy candidates should undergo screening for SARS-CoV-2 infection by PCR in respiratory specimens at the time of initial evaluation and 2 days prior to conditioning/lymphodepletion, regardless of the presence of symptoms, if testing is available.”
Procedures to be deferred include peripheral blood stem cell mobilization, bone marrow harvest, T-cell collections, and conditioning/lymphodepletion. These should not be performed for at least 14 days (preferably 21 days) from the day of last contact, according to the ASTCT. Two consecutive negative PCR tests each approximately 1 week apart (deferral for 14 days minimum), should be obtained, if available.
In areas with high community spread, the guidelines also state that “interim treatment and/or longer deferral of definite therapy should be considered when feasible (for example, multiple myeloma, germ cell tumors, consolidative transplants).”
Similar considerations should be afforded to potential cellular donors. Donors with SARS-CoV-2 detected in a respiratory sample are considered ineligible. Those meeting exposure criteria for patients, as listed above, should be excluded from donation for at least 28 days. “In individual circumstances, a donor may be considered eligible if respiratory samples are negative for SARS-CoV-2 by PCR and donor is asymptomatic. Donor should be closely monitored for COVID-19.”
In the case of unrelated donors, the ASTCT recommends referral to the National Marrow Donor Program (NMDP) guidelines for updated guidance, but points out that, according to the NMDP, the Food and Drug Administration reports that there have been no reported or suspected cases of transfusion-transmitted COVID-19 to date and that “no cases of transfusion-transmission were ever reported for the other two coronaviruses that emerged during the past 2 decades [SARS, the severe acute respiratory syndrome coronavirus, and MERS-CoV, which causes Mideast respiratory syndrome].”
In the updated ESBMT guidelines, this recommendation was made in reference to the greater spread of COVID-19: “It is therefore strongly recommended to have secured stem cell product access by freezing the product before start of conditioning and, in situations when this is not possible, to have an alternative donor as a backup. For low-risk patients, it is recommended to postpone the start of the transplant procedure if deemed to be safe to do so. This includes both allogeneic and autologous transplant procedures.”
In a recent webinar, Pavan Reddy, MD, of the University of Michigan, Ann Arbor, and ASTCT President; Alpana Waghmare, MD, of the Fred Hutchinson Cancer Research Center, Seattle; and Roy Chemaly, MD, of the MD Anderson Cancer Center, Houston, and chair of the ASTCT Transplant Infectious Disease Special Interest Group, discussed the guidelines and provided some updated information.
Dr. Reddy stated that, at the University of Michigan, they were delaying all nonurgent transplants, largely for myeloma, and are postponing even allotransplants. “The transplants we are not delaying are the high-risk AMLs … and in cases where we truly cannot delay transplants because of patient condition or, in some cases, the donor situation.”
Dr. Chemaly and Dr. Waghmare both agreed that their centers were following a similar approach.
With regard to patient testing, all three institution have recently moved to testing everyone a few days before transplant regardless of symptoms.
They also pointed out that essentially all clinical trials were being put on hold during the crisis, except for those few where patients would be put in danger if the trial were interrupted.
The guidelines discuss in depth the rationale, toxicity, and dosages for use of select agents, including remdesivir, chloroquine/hydroxychloroquine, ribavirin, and tocilizumab. There was some concern expressed about shortages developing in these drugs, which serve a number of other patient communities, in particular the possibility of a tocilizumab shortage was of concern.
Steroids and intravenous immunoglobulins are not are not recommended, according to the guidelines, which also stated that adjunctive therapies such as antibiotics should be considered.
Dr. Chemaly, Dr. Reddy, and Dr. Waghmare did not provide disclosure in the webinar.
The ASTCT recommends following the World Health Organization and CDC COVID-19 pages for continued updates and information on other aspects of the pandemic.
This article was updated 3/26/20.
SOURCE: ASTCT Response to COVID-19. 2020. www.astct.org/connect/astct-response-to-covid-19.
Here’s what ICUs are putting up against COVID-19
As COVID-19 spreads across the United States, it is important to understand the extent of the nation’s ICU resources, according to the Society of Critical Care Medicine. The SCCM has updated its statistics on the resources available to care for what could become “an overwhelming number of critically ill patients, many of whom may require mechanical ventilation,” the society said in a blog post on March 13.
That overwhelming number was considered at an American Hospital Association webinar in February: Investigators projected that 4.8 million patients could be hospitalized with COVID-19, of whom 1.9 million would be admitted to ICUs and 960,000 would require ventilator support, Neil A. Halpern, MD, director of the critical care center at Memorial Sloan Kettering Cancer Center, New York, and Kay See Tan, PhD, of the hospital’s department of epidemiology and biostatistics, reported in that post.
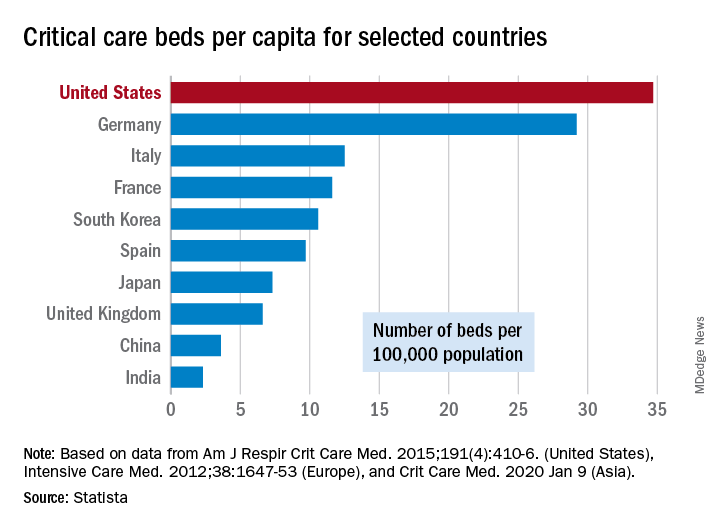
As far as critical care beds are concerned, the United States is in better shape than are other countries dealing with the coronavirus. The United States’ 34.7 critical care beds per 100,000 population put it a good bit ahead of Germany, which has 29.2 beds per 100,000, while other countries in both Europe and Asia are well behind, Dr. Halpern and Dr. Tan noted.
More recent data from the AHA show that just over half of its registered community hospitals deliver ICU services and have at least 10 acute care beds and one ICU bed, they reported.
Those 2,704 hospitals have nearly 535,000 acute care beds, of which almost 97,000 are ICU beds. Almost 71% of those ICU beds are for adults, with the rest located in neonatal and pediatric units, data from an AHA 2018 survey show.
Since patients with COVID-19 are most often admitted to ICUs with severe hypoxic respiratory failure, the nation’s supply of ventilators also may be tested. U.S. acute care hospitals own about 62,000 full-featured mechanical ventilators and almost 99,000 older ventilators that “may not be capable of adequately supporting patients with severe acute respiratory failure,” Dr. Halpern and Dr. Tan said.
As U.S. hospitals reach the crisis levels anticipated in the COVID-19 pandemic, staffing shortages can be expected as well. Almost half (48%) of acute care hospitals have no intensivists, so “other physicians (e.g., pulmonologists, surgeons, anesthesiologists, etc) may be pressed into service as outpatient clinics and elective surgery are suspended,” they wrote.
The blog post includes a tiered staffing strategy that the SCCM “encourages hospitals to adopt in pandemic situations such as COVID-19.”
As COVID-19 spreads across the United States, it is important to understand the extent of the nation’s ICU resources, according to the Society of Critical Care Medicine. The SCCM has updated its statistics on the resources available to care for what could become “an overwhelming number of critically ill patients, many of whom may require mechanical ventilation,” the society said in a blog post on March 13.

That overwhelming number was considered at an American Hospital Association webinar in February: Investigators projected that 4.8 million patients could be hospitalized with COVID-19, of whom 1.9 million would be admitted to ICUs and 960,000 would require ventilator support, Neil A. Halpern, MD, director of the critical care center at Memorial Sloan Kettering Cancer Center, New York, and Kay See Tan, PhD, of the hospital’s department of epidemiology and biostatistics, reported in that post.
As far as critical care beds are concerned, the United States is in better shape than are other countries dealing with the coronavirus. The United States’ 34.7 critical care beds per 100,000 population put it a good bit ahead of Germany, which has 29.2 beds per 100,000, while other countries in both Europe and Asia are well behind, Dr. Halpern and Dr. Tan noted.
More recent data from the AHA show that just over half of its registered community hospitals deliver ICU services and have at least 10 acute care beds and one ICU bed, they reported.
Those 2,704 hospitals have nearly 535,000 acute care beds, of which almost 97,000 are ICU beds. Almost 71% of those ICU beds are for adults, with the rest located in neonatal and pediatric units, data from an AHA 2018 survey show.
Since patients with COVID-19 are most often admitted to ICUs with severe hypoxic respiratory failure, the nation’s supply of ventilators also may be tested. U.S. acute care hospitals own about 62,000 full-featured mechanical ventilators and almost 99,000 older ventilators that “may not be capable of adequately supporting patients with severe acute respiratory failure,” Dr. Halpern and Dr. Tan said.
As U.S. hospitals reach the crisis levels anticipated in the COVID-19 pandemic, staffing shortages can be expected as well. Almost half (48%) of acute care hospitals have no intensivists, so “other physicians (e.g., pulmonologists, surgeons, anesthesiologists, etc) may be pressed into service as outpatient clinics and elective surgery are suspended,” they wrote.
The blog post includes a tiered staffing strategy that the SCCM “encourages hospitals to adopt in pandemic situations such as COVID-19.”
As COVID-19 spreads across the United States, it is important to understand the extent of the nation’s ICU resources, according to the Society of Critical Care Medicine. The SCCM has updated its statistics on the resources available to care for what could become “an overwhelming number of critically ill patients, many of whom may require mechanical ventilation,” the society said in a blog post on March 13.

That overwhelming number was considered at an American Hospital Association webinar in February: Investigators projected that 4.8 million patients could be hospitalized with COVID-19, of whom 1.9 million would be admitted to ICUs and 960,000 would require ventilator support, Neil A. Halpern, MD, director of the critical care center at Memorial Sloan Kettering Cancer Center, New York, and Kay See Tan, PhD, of the hospital’s department of epidemiology and biostatistics, reported in that post.
As far as critical care beds are concerned, the United States is in better shape than are other countries dealing with the coronavirus. The United States’ 34.7 critical care beds per 100,000 population put it a good bit ahead of Germany, which has 29.2 beds per 100,000, while other countries in both Europe and Asia are well behind, Dr. Halpern and Dr. Tan noted.
More recent data from the AHA show that just over half of its registered community hospitals deliver ICU services and have at least 10 acute care beds and one ICU bed, they reported.
Those 2,704 hospitals have nearly 535,000 acute care beds, of which almost 97,000 are ICU beds. Almost 71% of those ICU beds are for adults, with the rest located in neonatal and pediatric units, data from an AHA 2018 survey show.
Since patients with COVID-19 are most often admitted to ICUs with severe hypoxic respiratory failure, the nation’s supply of ventilators also may be tested. U.S. acute care hospitals own about 62,000 full-featured mechanical ventilators and almost 99,000 older ventilators that “may not be capable of adequately supporting patients with severe acute respiratory failure,” Dr. Halpern and Dr. Tan said.
As U.S. hospitals reach the crisis levels anticipated in the COVID-19 pandemic, staffing shortages can be expected as well. Almost half (48%) of acute care hospitals have no intensivists, so “other physicians (e.g., pulmonologists, surgeons, anesthesiologists, etc) may be pressed into service as outpatient clinics and elective surgery are suspended,” they wrote.
The blog post includes a tiered staffing strategy that the SCCM “encourages hospitals to adopt in pandemic situations such as COVID-19.”
Study supports genetic testing for all breast cancer patients age 65 and younger
Current National Comprehensive Cancer Network (NCCN) criteria may prevent genetic testing in “a substantial proportion” of women who carry germline pathogenic variants in breast cancer predisposition genes, according to investigators.
They found that, by expanding NCCN criteria to include germline genetic testing for all women diagnosed with breast cancer at age 65 or younger, the sensitivity of testing for nine well-established breast cancer predisposition genes would improve from 70% to more than 90%. The sensitivity for detection of BRCA1 and BRCA2 only would improve from 87% to greater than 98%.
Siddhartha Yadav, MD, of the Mayo Clinic, Rochester, Minn., and colleagues reported these findings in the Journal of Clinical Oncology.
“In a large unselected series of women with breast cancer, we demonstrate that expanding the NCCN testing criteria to include all women diagnosed with breast cancer at or before the age of 65 years has the potential to improve the sensitivity of germline genetic testing without the need for evaluation of all women with breast cancer,” Dr. Yadav and colleagues wrote.
Robert Pilarski, who was vice-chair of the panel that drew up the NCCN guidelines, said in an interview that the guideline authors tried to achieve a balance.
“We’ve known that NCCN misses cases and indications, but it comes down to whether the goal is to test all women with mutations or to have criteria that are a cost-effective and reasonable compromise to capture as many patients as possible,” said Mr. Pilarski, a licensed genetic counselor at the Ohio State University Wexner Medical Center in Columbus.
Current NCCN criteria for genetic/familial high-risk assessment for breast, ovarian, and pancreatic cancer recommend testing for individuals with blood relatives who have known or likely pathogenic variants, as well as patients with breast cancer diagnosed at age 45 or younger, patients aged 46-50 years with unknown or limited family history, patients with a second breast cancer diagnosed at any age, patients with triple-negative breast cancer diagnosed at age 60 or younger, and patients with breast cancer diagnosed at any age if they are of Ashkenazi Jewish ancestry.
But as Dr. Yadav and colleagues note, two recent studies (J Clin Oncol. 2019 Feb 20;37[6]:453-60; Ann Surg Oncol. 2018 Oct;25[10]:2925-31) suggested that up to 50% of germline pathogenic variants could be missed if testing were based solely on NCCN criteria.
Based on these findings, the American Society of Breast Surgeons issued a consensus guideline on genetic testing for hereditary breast cancer (Ann Surg Oncol. 2019 Oct;26[10]:3025-31), which states that, “genetic testing should be made available to all patients with a personal history of breast cancer.”
“Without question, if your goal is to identify everyone with a mutation, you’d have to test every cancer patient,” Mr. Pilarski said. “At this point, the ASBrS [American Society of Breast Surgeons] are the only group that have proposed that, and a lot of us feel that’s going too far at this point in time, and so the issue becomes what’s reasonable before that, and I think this paper is a great step forward.”
Cutting through the confusion
To see whether tweaking the existing guidelines could help clarify the issues surrounding genetic testing for breast cancer, Dr. Yadav and colleagues looked at a cohort of patients from the Mayo Clinic Breast Cancer Study. This prospective registry was open to all women evaluated at the Mayo Clinic Rochester for a first diagnosis of invasive breast cancer or ductal carcinoma in situ from May 2000 through May 2016.
The women were evaluated for germline pathogenic variants in nine breast cancer predisposition genes: ATM, BRCA1, BRCA2, CDH1, CHEK2, NF1, PALB2, PTEN, and TP53.
The researchers found that, of the 3,907 women in the sample, 1,872 (47.9%) would have been recommended for testing under the NCCN criteria, but the remaining 2,035 would not.
Women who met NCCN criteria were significantly more likely to carry a pathogenic variant (9% vs. 3.5%, P less than .001). However, 29.9% of women with pathogenic variants in the nine-gene panel and 13.1% of those with pathogenic variants in BRCA1 or BRCA2 did not qualify for testing by NCCN criteria.
The sensitivity of NCCN criteria was 70% for the nine-gene panel and 87% for BRCA 1 and BRCA 2, with a 53% specificity.
But if the criteria were expanded to include all women age 65 years and younger with a breast cancer diagnosis, the sensitivity for the nine-gene panel would increase to 92.1%, and the sensitivity for BRCA1 and BRCA2 only would climb to greater than 98.1%, with a specificity of approximately 22% for each test combination.
The authors acknowledged that they did not assess the cost-effectiveness of the testing criteria.
This study was supported by grants from the National Institutes of Health and the Breast Cancer Research Foundation. Authors disclosed relationships with Grail, bioTheranostics, Myriad Genetics, and other companies. Mr. Pilarski reported no conflicts of interest.
SOURCE: Yadav S et al. J Clin Oncol. 2020 Mar 3. doi: 10.1200/JCO.19.02190
Current National Comprehensive Cancer Network (NCCN) criteria may prevent genetic testing in “a substantial proportion” of women who carry germline pathogenic variants in breast cancer predisposition genes, according to investigators.
They found that, by expanding NCCN criteria to include germline genetic testing for all women diagnosed with breast cancer at age 65 or younger, the sensitivity of testing for nine well-established breast cancer predisposition genes would improve from 70% to more than 90%. The sensitivity for detection of BRCA1 and BRCA2 only would improve from 87% to greater than 98%.
Siddhartha Yadav, MD, of the Mayo Clinic, Rochester, Minn., and colleagues reported these findings in the Journal of Clinical Oncology.
“In a large unselected series of women with breast cancer, we demonstrate that expanding the NCCN testing criteria to include all women diagnosed with breast cancer at or before the age of 65 years has the potential to improve the sensitivity of germline genetic testing without the need for evaluation of all women with breast cancer,” Dr. Yadav and colleagues wrote.
Robert Pilarski, who was vice-chair of the panel that drew up the NCCN guidelines, said in an interview that the guideline authors tried to achieve a balance.
“We’ve known that NCCN misses cases and indications, but it comes down to whether the goal is to test all women with mutations or to have criteria that are a cost-effective and reasonable compromise to capture as many patients as possible,” said Mr. Pilarski, a licensed genetic counselor at the Ohio State University Wexner Medical Center in Columbus.
Current NCCN criteria for genetic/familial high-risk assessment for breast, ovarian, and pancreatic cancer recommend testing for individuals with blood relatives who have known or likely pathogenic variants, as well as patients with breast cancer diagnosed at age 45 or younger, patients aged 46-50 years with unknown or limited family history, patients with a second breast cancer diagnosed at any age, patients with triple-negative breast cancer diagnosed at age 60 or younger, and patients with breast cancer diagnosed at any age if they are of Ashkenazi Jewish ancestry.
But as Dr. Yadav and colleagues note, two recent studies (J Clin Oncol. 2019 Feb 20;37[6]:453-60; Ann Surg Oncol. 2018 Oct;25[10]:2925-31) suggested that up to 50% of germline pathogenic variants could be missed if testing were based solely on NCCN criteria.
Based on these findings, the American Society of Breast Surgeons issued a consensus guideline on genetic testing for hereditary breast cancer (Ann Surg Oncol. 2019 Oct;26[10]:3025-31), which states that, “genetic testing should be made available to all patients with a personal history of breast cancer.”
“Without question, if your goal is to identify everyone with a mutation, you’d have to test every cancer patient,” Mr. Pilarski said. “At this point, the ASBrS [American Society of Breast Surgeons] are the only group that have proposed that, and a lot of us feel that’s going too far at this point in time, and so the issue becomes what’s reasonable before that, and I think this paper is a great step forward.”
Cutting through the confusion
To see whether tweaking the existing guidelines could help clarify the issues surrounding genetic testing for breast cancer, Dr. Yadav and colleagues looked at a cohort of patients from the Mayo Clinic Breast Cancer Study. This prospective registry was open to all women evaluated at the Mayo Clinic Rochester for a first diagnosis of invasive breast cancer or ductal carcinoma in situ from May 2000 through May 2016.
The women were evaluated for germline pathogenic variants in nine breast cancer predisposition genes: ATM, BRCA1, BRCA2, CDH1, CHEK2, NF1, PALB2, PTEN, and TP53.
The researchers found that, of the 3,907 women in the sample, 1,872 (47.9%) would have been recommended for testing under the NCCN criteria, but the remaining 2,035 would not.
Women who met NCCN criteria were significantly more likely to carry a pathogenic variant (9% vs. 3.5%, P less than .001). However, 29.9% of women with pathogenic variants in the nine-gene panel and 13.1% of those with pathogenic variants in BRCA1 or BRCA2 did not qualify for testing by NCCN criteria.
The sensitivity of NCCN criteria was 70% for the nine-gene panel and 87% for BRCA 1 and BRCA 2, with a 53% specificity.
But if the criteria were expanded to include all women age 65 years and younger with a breast cancer diagnosis, the sensitivity for the nine-gene panel would increase to 92.1%, and the sensitivity for BRCA1 and BRCA2 only would climb to greater than 98.1%, with a specificity of approximately 22% for each test combination.
The authors acknowledged that they did not assess the cost-effectiveness of the testing criteria.
This study was supported by grants from the National Institutes of Health and the Breast Cancer Research Foundation. Authors disclosed relationships with Grail, bioTheranostics, Myriad Genetics, and other companies. Mr. Pilarski reported no conflicts of interest.
SOURCE: Yadav S et al. J Clin Oncol. 2020 Mar 3. doi: 10.1200/JCO.19.02190
Current National Comprehensive Cancer Network (NCCN) criteria may prevent genetic testing in “a substantial proportion” of women who carry germline pathogenic variants in breast cancer predisposition genes, according to investigators.
They found that, by expanding NCCN criteria to include germline genetic testing for all women diagnosed with breast cancer at age 65 or younger, the sensitivity of testing for nine well-established breast cancer predisposition genes would improve from 70% to more than 90%. The sensitivity for detection of BRCA1 and BRCA2 only would improve from 87% to greater than 98%.
Siddhartha Yadav, MD, of the Mayo Clinic, Rochester, Minn., and colleagues reported these findings in the Journal of Clinical Oncology.
“In a large unselected series of women with breast cancer, we demonstrate that expanding the NCCN testing criteria to include all women diagnosed with breast cancer at or before the age of 65 years has the potential to improve the sensitivity of germline genetic testing without the need for evaluation of all women with breast cancer,” Dr. Yadav and colleagues wrote.
Robert Pilarski, who was vice-chair of the panel that drew up the NCCN guidelines, said in an interview that the guideline authors tried to achieve a balance.
“We’ve known that NCCN misses cases and indications, but it comes down to whether the goal is to test all women with mutations or to have criteria that are a cost-effective and reasonable compromise to capture as many patients as possible,” said Mr. Pilarski, a licensed genetic counselor at the Ohio State University Wexner Medical Center in Columbus.
Current NCCN criteria for genetic/familial high-risk assessment for breast, ovarian, and pancreatic cancer recommend testing for individuals with blood relatives who have known or likely pathogenic variants, as well as patients with breast cancer diagnosed at age 45 or younger, patients aged 46-50 years with unknown or limited family history, patients with a second breast cancer diagnosed at any age, patients with triple-negative breast cancer diagnosed at age 60 or younger, and patients with breast cancer diagnosed at any age if they are of Ashkenazi Jewish ancestry.
But as Dr. Yadav and colleagues note, two recent studies (J Clin Oncol. 2019 Feb 20;37[6]:453-60; Ann Surg Oncol. 2018 Oct;25[10]:2925-31) suggested that up to 50% of germline pathogenic variants could be missed if testing were based solely on NCCN criteria.
Based on these findings, the American Society of Breast Surgeons issued a consensus guideline on genetic testing for hereditary breast cancer (Ann Surg Oncol. 2019 Oct;26[10]:3025-31), which states that, “genetic testing should be made available to all patients with a personal history of breast cancer.”
“Without question, if your goal is to identify everyone with a mutation, you’d have to test every cancer patient,” Mr. Pilarski said. “At this point, the ASBrS [American Society of Breast Surgeons] are the only group that have proposed that, and a lot of us feel that’s going too far at this point in time, and so the issue becomes what’s reasonable before that, and I think this paper is a great step forward.”
Cutting through the confusion
To see whether tweaking the existing guidelines could help clarify the issues surrounding genetic testing for breast cancer, Dr. Yadav and colleagues looked at a cohort of patients from the Mayo Clinic Breast Cancer Study. This prospective registry was open to all women evaluated at the Mayo Clinic Rochester for a first diagnosis of invasive breast cancer or ductal carcinoma in situ from May 2000 through May 2016.
The women were evaluated for germline pathogenic variants in nine breast cancer predisposition genes: ATM, BRCA1, BRCA2, CDH1, CHEK2, NF1, PALB2, PTEN, and TP53.
The researchers found that, of the 3,907 women in the sample, 1,872 (47.9%) would have been recommended for testing under the NCCN criteria, but the remaining 2,035 would not.
Women who met NCCN criteria were significantly more likely to carry a pathogenic variant (9% vs. 3.5%, P less than .001). However, 29.9% of women with pathogenic variants in the nine-gene panel and 13.1% of those with pathogenic variants in BRCA1 or BRCA2 did not qualify for testing by NCCN criteria.
The sensitivity of NCCN criteria was 70% for the nine-gene panel and 87% for BRCA 1 and BRCA 2, with a 53% specificity.
But if the criteria were expanded to include all women age 65 years and younger with a breast cancer diagnosis, the sensitivity for the nine-gene panel would increase to 92.1%, and the sensitivity for BRCA1 and BRCA2 only would climb to greater than 98.1%, with a specificity of approximately 22% for each test combination.
The authors acknowledged that they did not assess the cost-effectiveness of the testing criteria.
This study was supported by grants from the National Institutes of Health and the Breast Cancer Research Foundation. Authors disclosed relationships with Grail, bioTheranostics, Myriad Genetics, and other companies. Mr. Pilarski reported no conflicts of interest.
SOURCE: Yadav S et al. J Clin Oncol. 2020 Mar 3. doi: 10.1200/JCO.19.02190
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Reduced TNFi dose does not maintain axial spondyloarthritis improvement
Patients with axial spondyloarthritis have a significantly lower likelihood of achieving improvement in disease activity or remission when their dose of tumor necrosis factor inhibitor therapy is reduced, based on a systematic review and meta-analysis of six trials that included 747 adults.
Tumor necrosis factor inhibitors (TNFi) “have shown significant sustained clinical improvement in axSpA and are introduced in patients with axial disease or as the next line of treatment after inadequate response to nonsteroidal anti-inflammatory drugs,” but this improvement comes with a degree of immunosuppression that can increase infection risk, wrote Daeria O. Lawson of Toronto Western Hospital and colleagues. However, the impact of reducing or discontinuing TNFi therapy, compared with standard dosing, has not been well examined, they said.
In a study published in Arthritis Care & Research, the investigators identified six randomized, controlled trials with a total of 747 adults. Overall, patients on a reduced dose had a lower likelihood of achieving 40% improvement in Assessment of SpondyloArthritis international Society response criteria (ASAS40) or ASAS partial remission, compared with those on a standard TNFi dose (risk ratios, 0.62 and 0.17, respectively).
In addition, the mean increase in the Bath Ankylosing Spondylitis Disease Activity Index score was 0.35 for patients on reduced TNFi therapy, and no differences were seen in C-reactive protein levels, infection rates, or injection/infusion reactions in patients on a reduced dose, compared with those on the standard dose.
Patients on the reduced TNFi dose also had more disease flares and/or relapses, compared with the standard group (risk ratio, 1.73).
The study findings were limited by several factors including the inability to compare subgroups based on dosing regimens, potential blinding and selection bias, and inadequate data to assess certain patient outcomes, including maintenance of disease remission and quality of life, the researchers noted. The results confirm findings from previous studies and support the benefit of standard dosing for maintaining stable disease, they said.
However, more research is needed to identify patients who may be more responsive to TNFi reduction, they wrote. “Although treatment recommendations for the best dose reduction strategies cannot be made at this time given the heterogeneity in tapering strategies reported in the literature, this decision should be an individualized one between the patient and their physician,” the researchers emphasized.
The study received no outside funding. Dr. Lawson is supported in part by the Ontario Drug Policy Research Network Student Training Program.
SOURCE: Lawson DO et al. Arthritis Care Res. 2020 Mar 12. doi: 10.1002/ACR.24184.
Patients with axial spondyloarthritis have a significantly lower likelihood of achieving improvement in disease activity or remission when their dose of tumor necrosis factor inhibitor therapy is reduced, based on a systematic review and meta-analysis of six trials that included 747 adults.
Tumor necrosis factor inhibitors (TNFi) “have shown significant sustained clinical improvement in axSpA and are introduced in patients with axial disease or as the next line of treatment after inadequate response to nonsteroidal anti-inflammatory drugs,” but this improvement comes with a degree of immunosuppression that can increase infection risk, wrote Daeria O. Lawson of Toronto Western Hospital and colleagues. However, the impact of reducing or discontinuing TNFi therapy, compared with standard dosing, has not been well examined, they said.
In a study published in Arthritis Care & Research, the investigators identified six randomized, controlled trials with a total of 747 adults. Overall, patients on a reduced dose had a lower likelihood of achieving 40% improvement in Assessment of SpondyloArthritis international Society response criteria (ASAS40) or ASAS partial remission, compared with those on a standard TNFi dose (risk ratios, 0.62 and 0.17, respectively).
In addition, the mean increase in the Bath Ankylosing Spondylitis Disease Activity Index score was 0.35 for patients on reduced TNFi therapy, and no differences were seen in C-reactive protein levels, infection rates, or injection/infusion reactions in patients on a reduced dose, compared with those on the standard dose.
Patients on the reduced TNFi dose also had more disease flares and/or relapses, compared with the standard group (risk ratio, 1.73).
The study findings were limited by several factors including the inability to compare subgroups based on dosing regimens, potential blinding and selection bias, and inadequate data to assess certain patient outcomes, including maintenance of disease remission and quality of life, the researchers noted. The results confirm findings from previous studies and support the benefit of standard dosing for maintaining stable disease, they said.
However, more research is needed to identify patients who may be more responsive to TNFi reduction, they wrote. “Although treatment recommendations for the best dose reduction strategies cannot be made at this time given the heterogeneity in tapering strategies reported in the literature, this decision should be an individualized one between the patient and their physician,” the researchers emphasized.
The study received no outside funding. Dr. Lawson is supported in part by the Ontario Drug Policy Research Network Student Training Program.
SOURCE: Lawson DO et al. Arthritis Care Res. 2020 Mar 12. doi: 10.1002/ACR.24184.
Patients with axial spondyloarthritis have a significantly lower likelihood of achieving improvement in disease activity or remission when their dose of tumor necrosis factor inhibitor therapy is reduced, based on a systematic review and meta-analysis of six trials that included 747 adults.
Tumor necrosis factor inhibitors (TNFi) “have shown significant sustained clinical improvement in axSpA and are introduced in patients with axial disease or as the next line of treatment after inadequate response to nonsteroidal anti-inflammatory drugs,” but this improvement comes with a degree of immunosuppression that can increase infection risk, wrote Daeria O. Lawson of Toronto Western Hospital and colleagues. However, the impact of reducing or discontinuing TNFi therapy, compared with standard dosing, has not been well examined, they said.
In a study published in Arthritis Care & Research, the investigators identified six randomized, controlled trials with a total of 747 adults. Overall, patients on a reduced dose had a lower likelihood of achieving 40% improvement in Assessment of SpondyloArthritis international Society response criteria (ASAS40) or ASAS partial remission, compared with those on a standard TNFi dose (risk ratios, 0.62 and 0.17, respectively).
In addition, the mean increase in the Bath Ankylosing Spondylitis Disease Activity Index score was 0.35 for patients on reduced TNFi therapy, and no differences were seen in C-reactive protein levels, infection rates, or injection/infusion reactions in patients on a reduced dose, compared with those on the standard dose.
Patients on the reduced TNFi dose also had more disease flares and/or relapses, compared with the standard group (risk ratio, 1.73).
The study findings were limited by several factors including the inability to compare subgroups based on dosing regimens, potential blinding and selection bias, and inadequate data to assess certain patient outcomes, including maintenance of disease remission and quality of life, the researchers noted. The results confirm findings from previous studies and support the benefit of standard dosing for maintaining stable disease, they said.
However, more research is needed to identify patients who may be more responsive to TNFi reduction, they wrote. “Although treatment recommendations for the best dose reduction strategies cannot be made at this time given the heterogeneity in tapering strategies reported in the literature, this decision should be an individualized one between the patient and their physician,” the researchers emphasized.
The study received no outside funding. Dr. Lawson is supported in part by the Ontario Drug Policy Research Network Student Training Program.
SOURCE: Lawson DO et al. Arthritis Care Res. 2020 Mar 12. doi: 10.1002/ACR.24184.
FROM ARTHRITIS CARE & RESEARCH
COVID-19 in children, pregnant women: What do we know?
A novel coronavirus, the causative agent of the current pandemic of viral respiratory illness and pneumonia, was first identified in Wuhan, Hubei, China. The disease has been given the name, coronavirus disease 2019 (COVID-19). The virus at last report has spread to more than 100 countries. Much of what we suspect about this virus comes from work on other severe coronavirus respiratory disease outbreaks – Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome (SARS). MERS-CoV was a viral respiratory disease, first reported in Saudi Arabia, that was identified in more than 27 additional countries. The disease was characterized by severe acute respiratory illness, including fever, cough, and shortness of breath. Among 2,499 cases, only two patients tested positive for MERS-CoV in the United States. SARS-CoV also caused a severe viral respiratory illness. SARS was first recognized in Asia in 2003 and was subsequently reported in approximately 25 countries. The last case reported was in 2004.
As of March 13, there are 137,066 cases worldwide of COVID-19 and 1,701 in the United States, according to the John Hopkins University Coronavirus COVID-19 resource center.
What about children?
The remarkable observation is how few seriously ill children have been identified in the face of global spread. Unlike the H1N1 influenza epidemic of 2009, where older adults were relatively spared and children were a major target population, COVID-19 appears to be relatively infrequent in children or too mild to come to diagnosis, to date. Specifically, among China’s first approximately 44,000 cases, less than 2% were identified in children less than 20 years of age, and severe disease was uncommon with no deaths in children less than 10 years of age reported. One child, 13 months of age, with acute respiratory distress syndrome and septic shock was reported in China. According to the Centers for Disease Control and Prevention webcast , children present with fever in about 50% of cases, cough, fatigue, and subsequently some (3%-30%) progress to shortness of breath. Some children and adults have presented with gastrointestinal disease initially. Viral RNA has been detected in respiratory secretions, blood, and stool of affected children; however, the samples were not cultured for virus so whether stool is a potential source for transmission is unclear. In adults, the disease appears to be most severe – with development of pneumonia – in the second week of illness. In both children and adults, the chest x-ray findings are an interstitial pneumonitis, ground glass appearance, and/or patchy infiltrates.

Are some children at greater risk? Are children the source of community transmission? Will children become a greater part of the disease pattern as further cases are identified and further testing is available? We cannot answer many of these questions about COVID-19 in children as yet, but as you are aware, data are accumulating daily, and the Centers for Disease Control and Prevention and the National Institutes of Health are providing regular updates.
A report from China gave us some idea about community transmission and infection risk for children. The Shenzhen CDC identified 391 COVID-19 cases and 1,286 close contacts. Household contacts and those persons traveling with a case of the virus were at highest risk of acquisition. The secondary attack rates within households was 15%; children were as likely to become infected as adults (medRxiv preprint. 2020. doi: 10.1101/2020.03.03.20028423).
What about pregnant women?
The data on pregnant women are even more limited. The concern about COVID-19 during pregnancy comes from our knowledge of adverse outcomes from other respiratory viral infections. For example, respiratory viral infections such as influenza have been associated with increased maternal risk of severe disease, and adverse neonatal outcomes, including low birth weight and preterm birth. The experience with SARS also is concerning for excess adverse maternal and neonatal complications such as spontaneous miscarriage, preterm delivery, intrauterine growth restriction, admission to the ICU, renal failure, and disseminated intravascular coagulopathy all were reported as complications of SARS infection during pregnancy.
Two studies on COVID-19 in pregnancy have been reported to date. In nine pregnant women reported by Chen et al., COVID-19 pneumonia was identified in the third trimester. The women presented with fever, cough, myalgia, sore throat, and/or malaise. Fetal distress was reported in two; all nine infants were born alive. Apgar scores were 8-10 at 1 minute. Five were found to have lymphopenia; three had increases in hepatic enzymes. None of the infants developed severe COVID-19 pneumonia. Amniotic fluid, cord blood, neonatal throat swab, and breast milk samples from six of the nine patients were tested for the novel coronavirus 2019, and all results were negative (Lancet. 2020 Feb 12. doi: 10.1016/S0140-6736[20]30360-3)https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)30360-3/fulltext.
In a study by Zhu et al., nine pregnant women with confirmed COVID-19 infection were identified during Jan. 20-Feb. 5, 2020. The onset of clinical symptoms in these women occurred before delivery in four cases, on the day of delivery in two cases, and after delivery in three cases. Of the 10 neonates (one set of twins) many had clinical symptoms, but none were proven to be COVID-19 positive in their pharyngeal swabs. Shortness of breath was observed in six, fever in two, tachycardia in one. GI symptoms such as feeding intolerance, bloating, GI bleed, and vomiting also were observed. Chest radiography showed abnormalities in seven neonates at admission. Thrombocytopenia and/or disseminated intravascular coagulopathy also was reported. Five neonates recovered and were discharged, one died, and four neonates remained in hospital in a stable condition. It is unclear if the illness in these infants was related to COVID-19 (Transl Pediatrics. 2020 Feb. doi: 10.21037/tp.2020.02.06)http://tp.amegroups.com/article/view/35919/28274.
In the limited experience to date, no evidence of virus has been found in the breast milk of women with COVID-19, which is consistent with the SARS experience. Current recommendations are to separate the infant from known COVID-19 infected mothers either in a different room or in the mother’s room using a six foot rule, a barrier curtain of some type, and mask and hand washing prior to any contact between mother and infant. If the mother desires to breastfeed her child, the same precautions – mask and hand washing – should be in place.
What about treatment?
There are no proven effective therapies and supportive care has been the mainstay to date. Clinical trials of remdesivir have been initiated both by Gilead (compassionate use, open label) and by the National Institutes of Health (randomized remdesivirhttps://www.drugs.com/history/remdesivir.html vs. placebo) in adults based on in vitro data suggesting activity again COVID-19. Lopinavir/ritonavir (combination protease inhibitors) also have been administered off label, but no results are available as yet.
Keeping up
I suggest several valuable resources to keep yourself abreast of the rapidly changing COVID-19 story. First the CDC website or your local Department of Health. These are being updated frequently and include advisories on personal protective equipment, clusters of cases in your local community, and current recommendations for mitigation of the epidemic. I have listened to Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, and Robert R. Redfield, MD, the director of the CDC almost daily. I trust their viewpoints and transparency about what is and what is not known, as well as the why and wherefore of their guidance, remembering that each day brings new information and new guidance.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University and public health and senior attending physician at Boston Medical Center. He has no relevant financial disclosures. Email him at pdnews@mdedge.com.
A novel coronavirus, the causative agent of the current pandemic of viral respiratory illness and pneumonia, was first identified in Wuhan, Hubei, China. The disease has been given the name, coronavirus disease 2019 (COVID-19). The virus at last report has spread to more than 100 countries. Much of what we suspect about this virus comes from work on other severe coronavirus respiratory disease outbreaks – Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome (SARS). MERS-CoV was a viral respiratory disease, first reported in Saudi Arabia, that was identified in more than 27 additional countries. The disease was characterized by severe acute respiratory illness, including fever, cough, and shortness of breath. Among 2,499 cases, only two patients tested positive for MERS-CoV in the United States. SARS-CoV also caused a severe viral respiratory illness. SARS was first recognized in Asia in 2003 and was subsequently reported in approximately 25 countries. The last case reported was in 2004.
As of March 13, there are 137,066 cases worldwide of COVID-19 and 1,701 in the United States, according to the John Hopkins University Coronavirus COVID-19 resource center.
What about children?
The remarkable observation is how few seriously ill children have been identified in the face of global spread. Unlike the H1N1 influenza epidemic of 2009, where older adults were relatively spared and children were a major target population, COVID-19 appears to be relatively infrequent in children or too mild to come to diagnosis, to date. Specifically, among China’s first approximately 44,000 cases, less than 2% were identified in children less than 20 years of age, and severe disease was uncommon with no deaths in children less than 10 years of age reported. One child, 13 months of age, with acute respiratory distress syndrome and septic shock was reported in China. According to the Centers for Disease Control and Prevention webcast , children present with fever in about 50% of cases, cough, fatigue, and subsequently some (3%-30%) progress to shortness of breath. Some children and adults have presented with gastrointestinal disease initially. Viral RNA has been detected in respiratory secretions, blood, and stool of affected children; however, the samples were not cultured for virus so whether stool is a potential source for transmission is unclear. In adults, the disease appears to be most severe – with development of pneumonia – in the second week of illness. In both children and adults, the chest x-ray findings are an interstitial pneumonitis, ground glass appearance, and/or patchy infiltrates.

Are some children at greater risk? Are children the source of community transmission? Will children become a greater part of the disease pattern as further cases are identified and further testing is available? We cannot answer many of these questions about COVID-19 in children as yet, but as you are aware, data are accumulating daily, and the Centers for Disease Control and Prevention and the National Institutes of Health are providing regular updates.
A report from China gave us some idea about community transmission and infection risk for children. The Shenzhen CDC identified 391 COVID-19 cases and 1,286 close contacts. Household contacts and those persons traveling with a case of the virus were at highest risk of acquisition. The secondary attack rates within households was 15%; children were as likely to become infected as adults (medRxiv preprint. 2020. doi: 10.1101/2020.03.03.20028423).
What about pregnant women?
The data on pregnant women are even more limited. The concern about COVID-19 during pregnancy comes from our knowledge of adverse outcomes from other respiratory viral infections. For example, respiratory viral infections such as influenza have been associated with increased maternal risk of severe disease, and adverse neonatal outcomes, including low birth weight and preterm birth. The experience with SARS also is concerning for excess adverse maternal and neonatal complications such as spontaneous miscarriage, preterm delivery, intrauterine growth restriction, admission to the ICU, renal failure, and disseminated intravascular coagulopathy all were reported as complications of SARS infection during pregnancy.
Two studies on COVID-19 in pregnancy have been reported to date. In nine pregnant women reported by Chen et al., COVID-19 pneumonia was identified in the third trimester. The women presented with fever, cough, myalgia, sore throat, and/or malaise. Fetal distress was reported in two; all nine infants were born alive. Apgar scores were 8-10 at 1 minute. Five were found to have lymphopenia; three had increases in hepatic enzymes. None of the infants developed severe COVID-19 pneumonia. Amniotic fluid, cord blood, neonatal throat swab, and breast milk samples from six of the nine patients were tested for the novel coronavirus 2019, and all results were negative (Lancet. 2020 Feb 12. doi: 10.1016/S0140-6736[20]30360-3)https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)30360-3/fulltext.
In a study by Zhu et al., nine pregnant women with confirmed COVID-19 infection were identified during Jan. 20-Feb. 5, 2020. The onset of clinical symptoms in these women occurred before delivery in four cases, on the day of delivery in two cases, and after delivery in three cases. Of the 10 neonates (one set of twins) many had clinical symptoms, but none were proven to be COVID-19 positive in their pharyngeal swabs. Shortness of breath was observed in six, fever in two, tachycardia in one. GI symptoms such as feeding intolerance, bloating, GI bleed, and vomiting also were observed. Chest radiography showed abnormalities in seven neonates at admission. Thrombocytopenia and/or disseminated intravascular coagulopathy also was reported. Five neonates recovered and were discharged, one died, and four neonates remained in hospital in a stable condition. It is unclear if the illness in these infants was related to COVID-19 (Transl Pediatrics. 2020 Feb. doi: 10.21037/tp.2020.02.06)http://tp.amegroups.com/article/view/35919/28274.
In the limited experience to date, no evidence of virus has been found in the breast milk of women with COVID-19, which is consistent with the SARS experience. Current recommendations are to separate the infant from known COVID-19 infected mothers either in a different room or in the mother’s room using a six foot rule, a barrier curtain of some type, and mask and hand washing prior to any contact between mother and infant. If the mother desires to breastfeed her child, the same precautions – mask and hand washing – should be in place.
What about treatment?
There are no proven effective therapies and supportive care has been the mainstay to date. Clinical trials of remdesivir have been initiated both by Gilead (compassionate use, open label) and by the National Institutes of Health (randomized remdesivirhttps://www.drugs.com/history/remdesivir.html vs. placebo) in adults based on in vitro data suggesting activity again COVID-19. Lopinavir/ritonavir (combination protease inhibitors) also have been administered off label, but no results are available as yet.
Keeping up
I suggest several valuable resources to keep yourself abreast of the rapidly changing COVID-19 story. First the CDC website or your local Department of Health. These are being updated frequently and include advisories on personal protective equipment, clusters of cases in your local community, and current recommendations for mitigation of the epidemic. I have listened to Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, and Robert R. Redfield, MD, the director of the CDC almost daily. I trust their viewpoints and transparency about what is and what is not known, as well as the why and wherefore of their guidance, remembering that each day brings new information and new guidance.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University and public health and senior attending physician at Boston Medical Center. He has no relevant financial disclosures. Email him at pdnews@mdedge.com.
A novel coronavirus, the causative agent of the current pandemic of viral respiratory illness and pneumonia, was first identified in Wuhan, Hubei, China. The disease has been given the name, coronavirus disease 2019 (COVID-19). The virus at last report has spread to more than 100 countries. Much of what we suspect about this virus comes from work on other severe coronavirus respiratory disease outbreaks – Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome (SARS). MERS-CoV was a viral respiratory disease, first reported in Saudi Arabia, that was identified in more than 27 additional countries. The disease was characterized by severe acute respiratory illness, including fever, cough, and shortness of breath. Among 2,499 cases, only two patients tested positive for MERS-CoV in the United States. SARS-CoV also caused a severe viral respiratory illness. SARS was first recognized in Asia in 2003 and was subsequently reported in approximately 25 countries. The last case reported was in 2004.
As of March 13, there are 137,066 cases worldwide of COVID-19 and 1,701 in the United States, according to the John Hopkins University Coronavirus COVID-19 resource center.
What about children?
The remarkable observation is how few seriously ill children have been identified in the face of global spread. Unlike the H1N1 influenza epidemic of 2009, where older adults were relatively spared and children were a major target population, COVID-19 appears to be relatively infrequent in children or too mild to come to diagnosis, to date. Specifically, among China’s first approximately 44,000 cases, less than 2% were identified in children less than 20 years of age, and severe disease was uncommon with no deaths in children less than 10 years of age reported. One child, 13 months of age, with acute respiratory distress syndrome and septic shock was reported in China. According to the Centers for Disease Control and Prevention webcast , children present with fever in about 50% of cases, cough, fatigue, and subsequently some (3%-30%) progress to shortness of breath. Some children and adults have presented with gastrointestinal disease initially. Viral RNA has been detected in respiratory secretions, blood, and stool of affected children; however, the samples were not cultured for virus so whether stool is a potential source for transmission is unclear. In adults, the disease appears to be most severe – with development of pneumonia – in the second week of illness. In both children and adults, the chest x-ray findings are an interstitial pneumonitis, ground glass appearance, and/or patchy infiltrates.

Are some children at greater risk? Are children the source of community transmission? Will children become a greater part of the disease pattern as further cases are identified and further testing is available? We cannot answer many of these questions about COVID-19 in children as yet, but as you are aware, data are accumulating daily, and the Centers for Disease Control and Prevention and the National Institutes of Health are providing regular updates.
A report from China gave us some idea about community transmission and infection risk for children. The Shenzhen CDC identified 391 COVID-19 cases and 1,286 close contacts. Household contacts and those persons traveling with a case of the virus were at highest risk of acquisition. The secondary attack rates within households was 15%; children were as likely to become infected as adults (medRxiv preprint. 2020. doi: 10.1101/2020.03.03.20028423).
What about pregnant women?
The data on pregnant women are even more limited. The concern about COVID-19 during pregnancy comes from our knowledge of adverse outcomes from other respiratory viral infections. For example, respiratory viral infections such as influenza have been associated with increased maternal risk of severe disease, and adverse neonatal outcomes, including low birth weight and preterm birth. The experience with SARS also is concerning for excess adverse maternal and neonatal complications such as spontaneous miscarriage, preterm delivery, intrauterine growth restriction, admission to the ICU, renal failure, and disseminated intravascular coagulopathy all were reported as complications of SARS infection during pregnancy.
Two studies on COVID-19 in pregnancy have been reported to date. In nine pregnant women reported by Chen et al., COVID-19 pneumonia was identified in the third trimester. The women presented with fever, cough, myalgia, sore throat, and/or malaise. Fetal distress was reported in two; all nine infants were born alive. Apgar scores were 8-10 at 1 minute. Five were found to have lymphopenia; three had increases in hepatic enzymes. None of the infants developed severe COVID-19 pneumonia. Amniotic fluid, cord blood, neonatal throat swab, and breast milk samples from six of the nine patients were tested for the novel coronavirus 2019, and all results were negative (Lancet. 2020 Feb 12. doi: 10.1016/S0140-6736[20]30360-3)https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)30360-3/fulltext.
In a study by Zhu et al., nine pregnant women with confirmed COVID-19 infection were identified during Jan. 20-Feb. 5, 2020. The onset of clinical symptoms in these women occurred before delivery in four cases, on the day of delivery in two cases, and after delivery in three cases. Of the 10 neonates (one set of twins) many had clinical symptoms, but none were proven to be COVID-19 positive in their pharyngeal swabs. Shortness of breath was observed in six, fever in two, tachycardia in one. GI symptoms such as feeding intolerance, bloating, GI bleed, and vomiting also were observed. Chest radiography showed abnormalities in seven neonates at admission. Thrombocytopenia and/or disseminated intravascular coagulopathy also was reported. Five neonates recovered and were discharged, one died, and four neonates remained in hospital in a stable condition. It is unclear if the illness in these infants was related to COVID-19 (Transl Pediatrics. 2020 Feb. doi: 10.21037/tp.2020.02.06)http://tp.amegroups.com/article/view/35919/28274.
In the limited experience to date, no evidence of virus has been found in the breast milk of women with COVID-19, which is consistent with the SARS experience. Current recommendations are to separate the infant from known COVID-19 infected mothers either in a different room or in the mother’s room using a six foot rule, a barrier curtain of some type, and mask and hand washing prior to any contact between mother and infant. If the mother desires to breastfeed her child, the same precautions – mask and hand washing – should be in place.
What about treatment?
There are no proven effective therapies and supportive care has been the mainstay to date. Clinical trials of remdesivir have been initiated both by Gilead (compassionate use, open label) and by the National Institutes of Health (randomized remdesivirhttps://www.drugs.com/history/remdesivir.html vs. placebo) in adults based on in vitro data suggesting activity again COVID-19. Lopinavir/ritonavir (combination protease inhibitors) also have been administered off label, but no results are available as yet.
Keeping up
I suggest several valuable resources to keep yourself abreast of the rapidly changing COVID-19 story. First the CDC website or your local Department of Health. These are being updated frequently and include advisories on personal protective equipment, clusters of cases in your local community, and current recommendations for mitigation of the epidemic. I have listened to Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, and Robert R. Redfield, MD, the director of the CDC almost daily. I trust their viewpoints and transparency about what is and what is not known, as well as the why and wherefore of their guidance, remembering that each day brings new information and new guidance.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University and public health and senior attending physician at Boston Medical Center. He has no relevant financial disclosures. Email him at pdnews@mdedge.com.
Detection of COVID-19 in children in early January 2020 in Wuhan, China
Clinical question: What were the clinical characteristics of children in Wuhan, China hospitalized with SARS-CoV-2?
Background: The coronavirus disease 2019 (COVID-19) was recently described by researchers in Wuhan, China.1 However, there has been limited discussion on how the disease has affected children. Based on the Chinese Center for Disease Control and Prevention report, Wu et al. found that 1% of the affected population was less than 10 years, and another 1% of the affected population was 10-19 years.2 However, little information regarding hospitalizations of children with viral infections was previously reported.
Study design: A retrospective analysis of hospitalized children.
Setting: Three sites of a multisite urban teaching hospital in central Wuhan, China.
Synopsis: Over an 8-day period, hospitalized pediatric patients were retrospectively enrolled into this study. The authors defined pediatric patients as those aged 16 years or younger. The patients had one throat swab specimen collected on admission. Throat swab specimens were tested for viral etiologies. In response to the COVID-19 outbreak, the throat samples were retrospectively tested for SARS-CoV-2. If two independent experiments and a clinically verified diagnostic test confirmed the SARS-CoV-2, the cases were confirmed as COVID-19 cases. During the 8-day period, 366 hospitalized pediatric patients were included in the study. Of the 366 patients, 6 tested positive for SARS-CoV-2, while 23 tested positive for influenza A and 20 tested positive for influenza B. The median age of the six patients was 3 years (range, 1-7 years), and all were previously healthy. All six pediatric patients with COVID-19 had high fevers (greater than 39°C), cough, and lymphopenia. Four of the six affected patients had vomiting and leukopenia, while three of the six patients had neutropenia. Four of the six affected patients had pneumonia, as diagnosed on CT scans. Of the six patients, one patient was admitted to the ICU and received intravenous immunoglobulin. The patient admitted to ICU underwent a CT scan which showed “patchy ground-glass opacities in both lungs,” while three of the five children requiring non-ICU hospitalization had chest radiographs showing “patchy shadows in both lungs.” The median length of stay in the hospital was 7.5 days (range, 5-13 days).
Bottom line: COVID-19 causes moderate to severe respiratory illness in pediatric patients with SARS-CoV-2, possibly leading to critical illness. During this time period of the Wuhan COVID-19 outbreak, pediatric patients were more likely to be hospitalized with influenza A or B, than they were with SARS-CoV-2.
Citation: Liu W et al. Detection of Covid-19 in Children in Early January 2020 in Wuhan, China. N Engl J Med. 2020 Mar 12. doi: 10.1056/NEJMc2003717.
Dr. Kumar is clinical assistant professor of pediatrics at Case Western Reserve University, Cleveland, and a pediatric hospitalist at Cleveland Clinic Children’s. She is the pediatric editor of the Hospitalist.
References
1. Zhu N et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727-33.
2. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72,314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020 Feb 24 (Epub ahead of print).
From the Hospitalist editors: The pediatrics “In the Literature” series generally focuses on original articles. However, given the urgency to learn more about SARS-CoV-2/COVID-19 pandemic and the limited literature about hospitalized pediatric patients with the disease, the editors of the Hospitalist thought it was appropriate to share an article reviewing this letter that was recently published in the New England Journal of Medicine.
Clinical question: What were the clinical characteristics of children in Wuhan, China hospitalized with SARS-CoV-2?
Background: The coronavirus disease 2019 (COVID-19) was recently described by researchers in Wuhan, China.1 However, there has been limited discussion on how the disease has affected children. Based on the Chinese Center for Disease Control and Prevention report, Wu et al. found that 1% of the affected population was less than 10 years, and another 1% of the affected population was 10-19 years.2 However, little information regarding hospitalizations of children with viral infections was previously reported.
Study design: A retrospective analysis of hospitalized children.
Setting: Three sites of a multisite urban teaching hospital in central Wuhan, China.
Synopsis: Over an 8-day period, hospitalized pediatric patients were retrospectively enrolled into this study. The authors defined pediatric patients as those aged 16 years or younger. The patients had one throat swab specimen collected on admission. Throat swab specimens were tested for viral etiologies. In response to the COVID-19 outbreak, the throat samples were retrospectively tested for SARS-CoV-2. If two independent experiments and a clinically verified diagnostic test confirmed the SARS-CoV-2, the cases were confirmed as COVID-19 cases. During the 8-day period, 366 hospitalized pediatric patients were included in the study. Of the 366 patients, 6 tested positive for SARS-CoV-2, while 23 tested positive for influenza A and 20 tested positive for influenza B. The median age of the six patients was 3 years (range, 1-7 years), and all were previously healthy. All six pediatric patients with COVID-19 had high fevers (greater than 39°C), cough, and lymphopenia. Four of the six affected patients had vomiting and leukopenia, while three of the six patients had neutropenia. Four of the six affected patients had pneumonia, as diagnosed on CT scans. Of the six patients, one patient was admitted to the ICU and received intravenous immunoglobulin. The patient admitted to ICU underwent a CT scan which showed “patchy ground-glass opacities in both lungs,” while three of the five children requiring non-ICU hospitalization had chest radiographs showing “patchy shadows in both lungs.” The median length of stay in the hospital was 7.5 days (range, 5-13 days).
Bottom line: COVID-19 causes moderate to severe respiratory illness in pediatric patients with SARS-CoV-2, possibly leading to critical illness. During this time period of the Wuhan COVID-19 outbreak, pediatric patients were more likely to be hospitalized with influenza A or B, than they were with SARS-CoV-2.
Citation: Liu W et al. Detection of Covid-19 in Children in Early January 2020 in Wuhan, China. N Engl J Med. 2020 Mar 12. doi: 10.1056/NEJMc2003717.
Dr. Kumar is clinical assistant professor of pediatrics at Case Western Reserve University, Cleveland, and a pediatric hospitalist at Cleveland Clinic Children’s. She is the pediatric editor of the Hospitalist.
References
1. Zhu N et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727-33.
2. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72,314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020 Feb 24 (Epub ahead of print).
From the Hospitalist editors: The pediatrics “In the Literature” series generally focuses on original articles. However, given the urgency to learn more about SARS-CoV-2/COVID-19 pandemic and the limited literature about hospitalized pediatric patients with the disease, the editors of the Hospitalist thought it was appropriate to share an article reviewing this letter that was recently published in the New England Journal of Medicine.
Clinical question: What were the clinical characteristics of children in Wuhan, China hospitalized with SARS-CoV-2?
Background: The coronavirus disease 2019 (COVID-19) was recently described by researchers in Wuhan, China.1 However, there has been limited discussion on how the disease has affected children. Based on the Chinese Center for Disease Control and Prevention report, Wu et al. found that 1% of the affected population was less than 10 years, and another 1% of the affected population was 10-19 years.2 However, little information regarding hospitalizations of children with viral infections was previously reported.
Study design: A retrospective analysis of hospitalized children.
Setting: Three sites of a multisite urban teaching hospital in central Wuhan, China.
Synopsis: Over an 8-day period, hospitalized pediatric patients were retrospectively enrolled into this study. The authors defined pediatric patients as those aged 16 years or younger. The patients had one throat swab specimen collected on admission. Throat swab specimens were tested for viral etiologies. In response to the COVID-19 outbreak, the throat samples were retrospectively tested for SARS-CoV-2. If two independent experiments and a clinically verified diagnostic test confirmed the SARS-CoV-2, the cases were confirmed as COVID-19 cases. During the 8-day period, 366 hospitalized pediatric patients were included in the study. Of the 366 patients, 6 tested positive for SARS-CoV-2, while 23 tested positive for influenza A and 20 tested positive for influenza B. The median age of the six patients was 3 years (range, 1-7 years), and all were previously healthy. All six pediatric patients with COVID-19 had high fevers (greater than 39°C), cough, and lymphopenia. Four of the six affected patients had vomiting and leukopenia, while three of the six patients had neutropenia. Four of the six affected patients had pneumonia, as diagnosed on CT scans. Of the six patients, one patient was admitted to the ICU and received intravenous immunoglobulin. The patient admitted to ICU underwent a CT scan which showed “patchy ground-glass opacities in both lungs,” while three of the five children requiring non-ICU hospitalization had chest radiographs showing “patchy shadows in both lungs.” The median length of stay in the hospital was 7.5 days (range, 5-13 days).
Bottom line: COVID-19 causes moderate to severe respiratory illness in pediatric patients with SARS-CoV-2, possibly leading to critical illness. During this time period of the Wuhan COVID-19 outbreak, pediatric patients were more likely to be hospitalized with influenza A or B, than they were with SARS-CoV-2.
Citation: Liu W et al. Detection of Covid-19 in Children in Early January 2020 in Wuhan, China. N Engl J Med. 2020 Mar 12. doi: 10.1056/NEJMc2003717.
Dr. Kumar is clinical assistant professor of pediatrics at Case Western Reserve University, Cleveland, and a pediatric hospitalist at Cleveland Clinic Children’s. She is the pediatric editor of the Hospitalist.
References
1. Zhu N et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727-33.
2. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72,314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020 Feb 24 (Epub ahead of print).
From the Hospitalist editors: The pediatrics “In the Literature” series generally focuses on original articles. However, given the urgency to learn more about SARS-CoV-2/COVID-19 pandemic and the limited literature about hospitalized pediatric patients with the disease, the editors of the Hospitalist thought it was appropriate to share an article reviewing this letter that was recently published in the New England Journal of Medicine.
Second transplant a good salvage option for children with ALL, AML, or MDS
ORLANDO – A second hematopoietic stem cell transplant can be a successful salvage therapy for a child who has experienced a relapse following a first allogeneic transplant, investigators report.
A retrospective study of 221 children who experienced a relapse after a first hematopoietic stem cell transplant (HSCT) showed that 3-year overall survival (OS) was six times higher among those who had second HSCT, compared with those who did not, reported Akshay Sharma, MBBS, from St. Jude’s Children’s Research Hospital in Memphis.
“We found that factors that are typically associated with poor outcomes after transplant such as disease status at the time of first transplantation – being in remission or not, type of transplant – myeloablative or reduced intensity, and choice of donor were generally not significantly predictive of outcomes following posttransplant relapse in our multivariable model,” he said at the Transplantation and Cellular Therapy Meetings.
Relapse is the most common cause of death after HSCT, and 20%-30% of children who undergo allogeneic HSCT will experience a relapse.
To study this issue, Dr. Sharma and colleagues took a retrospective look at 703 patients 21 and younger who received a first alloHSCT at St. Jude’s for acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML) or myelodysplastic syndrome from 1990 through 2018. Of this cohort, 211 patients (31%) experienced a relapse after transplant.
There were no significant differences between patients who had a relapse and those who did not in sex, race, conditioning-regimen intensity, performance status, donor type, or graft type (peripheral blood stem cell, umbilical cord blood, bone marrow), or in the incidence of acute graft-versus-host disease GVHD.
The investigators found that, as expected, outcomes were poor for patients who experienced a posttransplant relapse, with 3-year overall survival from relapse for the 221 patients of just 10%.
In multivariable analysis controlling for sex, disease status at the time of first transplant, interval from first transplant to relapse, management after relapse, chronic GVHD and year of relapse, factors significantly associated with worse overall survival were relapse within 6 months of transplant vs. later than 6 months (hazard ratio, 4.6; P < .001) and decade of transplant (HR, 2.6 for 1990-2000 and 1.6 for 2001-2010 vs. 2011-2018; P < .001).
In contrast, both second HSCT and donor lymphocyte infusion were associated with better overall survival, compared with postrelapse chemotherapy or supportive care (HR, 0.04 and 0.6, respectively; P < .001 for both comparisons).
A longer interval from first transplant to relapse was the strongest predictor of long-term survival, Dr. Sharma said at the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
Among the 221 patients who had a relapse, 61 (28%) had a second HSCT, 28 (13%) received only donor lymphocyte infusions, without second transplant, and 132 (62%) received either chemotherapy or supportive care.
The 3-year overall survival rate for patients who received a second transplant was 28%, compared with 4% for those who did not have a repeat HSCT. The most important independent predictors for getting a second transplant were longer time to relapse after first transplant, first transplantation from a matched sibling donor instead of from a haploidentical donor, some degree of acute GVHD, and decade of first transplant (current decade vs. earlier decades).
The investigators also looked at guideline recommendations from both the American Society of Hematology and UK National Health Service regarding second allogenenic transplant after relapse.
ASH guidelines say that “patients with chemo-sensitive disease in remission who had a long initial remission (> 6-12 months) after first transplant and who never developed any GVHD” are most likely to benefit from a second transplant.
NHS guidelines say that a second transplant can be considered for patients who experience relapse more than 12 months after first alloHSCT. But as Dr. Sharma and colleagues discovered, patients who had a second transplant had better overall survival regardless of time from first to second transplant, compared with patients who had later relapses but no second transplant.
“With these data in mind, we submit that these ASH and NHS guidelines, which are based on older data from the 1900s and 2000 and are mostly based on adult data and do not include much pediatric data should be reconsidered, at least in the context of pediatric patients as we approach them,” he said.
Jaap-Jan Boelens, MD, PhD, a pediatric transplant specialist at Memorial Sloan Kettering Cancer Center in New York City, who was not involved in the study, said that the use of second transplant as salvage therapy is becoming more common at his center.
“But there is a little nuance,” he said in an interview. “If someone relapses a month after transplant, let’s say, it doesn’t make sense to go for another allo transplant. But if the interval between transplant is longer, more than half a year, we usually consider going for a second allo transplant.”
The decision to attempt a second transplant may also hinge on the disease the patient is being treated for, and on the depth of remission prior to relapse, he said.
Reggie E. Duerst, MD, director of the Stem Cell Transplant Program at Ann & Robert H. Lurie Children’s Hospital of Chicago, said that trying to replicate the conditions of the first transplant may not work.
“It’s also a function of who was the donor for the first transplant – was it a matched sibling? And then for the second one do you go to an unrelated donor, or a half-matched relative, banking on the fact that the donor’s immune system for the second transplant is going to be able to mediate some kind of graft-versus-leukemia effect that wasn’t there the first time around?” he said.
Dr. Duerst, who was not involved in the study, noted that, for some patients with lymphoid malignancies, chimeric antigen receptor (CAR) T-cell therapy may be a more effective salvage strategy than second transplant, but added that it’s still too soon to know which strategy will be more effective.
The study was supported by St. Jude’s, the American Society of Hematology, and the American Society for Transplantation and Cellular Therapy. Dr. Sharma, Dr. Boelens, and Dr. Duerst reported having no relevant disclosures.
SOURCE: Sharma A et al. TCT 2020, Abstract 116.
ORLANDO – A second hematopoietic stem cell transplant can be a successful salvage therapy for a child who has experienced a relapse following a first allogeneic transplant, investigators report.
A retrospective study of 221 children who experienced a relapse after a first hematopoietic stem cell transplant (HSCT) showed that 3-year overall survival (OS) was six times higher among those who had second HSCT, compared with those who did not, reported Akshay Sharma, MBBS, from St. Jude’s Children’s Research Hospital in Memphis.
“We found that factors that are typically associated with poor outcomes after transplant such as disease status at the time of first transplantation – being in remission or not, type of transplant – myeloablative or reduced intensity, and choice of donor were generally not significantly predictive of outcomes following posttransplant relapse in our multivariable model,” he said at the Transplantation and Cellular Therapy Meetings.
Relapse is the most common cause of death after HSCT, and 20%-30% of children who undergo allogeneic HSCT will experience a relapse.
To study this issue, Dr. Sharma and colleagues took a retrospective look at 703 patients 21 and younger who received a first alloHSCT at St. Jude’s for acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML) or myelodysplastic syndrome from 1990 through 2018. Of this cohort, 211 patients (31%) experienced a relapse after transplant.
There were no significant differences between patients who had a relapse and those who did not in sex, race, conditioning-regimen intensity, performance status, donor type, or graft type (peripheral blood stem cell, umbilical cord blood, bone marrow), or in the incidence of acute graft-versus-host disease GVHD.
The investigators found that, as expected, outcomes were poor for patients who experienced a posttransplant relapse, with 3-year overall survival from relapse for the 221 patients of just 10%.
In multivariable analysis controlling for sex, disease status at the time of first transplant, interval from first transplant to relapse, management after relapse, chronic GVHD and year of relapse, factors significantly associated with worse overall survival were relapse within 6 months of transplant vs. later than 6 months (hazard ratio, 4.6; P < .001) and decade of transplant (HR, 2.6 for 1990-2000 and 1.6 for 2001-2010 vs. 2011-2018; P < .001).
In contrast, both second HSCT and donor lymphocyte infusion were associated with better overall survival, compared with postrelapse chemotherapy or supportive care (HR, 0.04 and 0.6, respectively; P < .001 for both comparisons).
A longer interval from first transplant to relapse was the strongest predictor of long-term survival, Dr. Sharma said at the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
Among the 221 patients who had a relapse, 61 (28%) had a second HSCT, 28 (13%) received only donor lymphocyte infusions, without second transplant, and 132 (62%) received either chemotherapy or supportive care.
The 3-year overall survival rate for patients who received a second transplant was 28%, compared with 4% for those who did not have a repeat HSCT. The most important independent predictors for getting a second transplant were longer time to relapse after first transplant, first transplantation from a matched sibling donor instead of from a haploidentical donor, some degree of acute GVHD, and decade of first transplant (current decade vs. earlier decades).
The investigators also looked at guideline recommendations from both the American Society of Hematology and UK National Health Service regarding second allogenenic transplant after relapse.
ASH guidelines say that “patients with chemo-sensitive disease in remission who had a long initial remission (> 6-12 months) after first transplant and who never developed any GVHD” are most likely to benefit from a second transplant.
NHS guidelines say that a second transplant can be considered for patients who experience relapse more than 12 months after first alloHSCT. But as Dr. Sharma and colleagues discovered, patients who had a second transplant had better overall survival regardless of time from first to second transplant, compared with patients who had later relapses but no second transplant.
“With these data in mind, we submit that these ASH and NHS guidelines, which are based on older data from the 1900s and 2000 and are mostly based on adult data and do not include much pediatric data should be reconsidered, at least in the context of pediatric patients as we approach them,” he said.
Jaap-Jan Boelens, MD, PhD, a pediatric transplant specialist at Memorial Sloan Kettering Cancer Center in New York City, who was not involved in the study, said that the use of second transplant as salvage therapy is becoming more common at his center.
“But there is a little nuance,” he said in an interview. “If someone relapses a month after transplant, let’s say, it doesn’t make sense to go for another allo transplant. But if the interval between transplant is longer, more than half a year, we usually consider going for a second allo transplant.”
The decision to attempt a second transplant may also hinge on the disease the patient is being treated for, and on the depth of remission prior to relapse, he said.
Reggie E. Duerst, MD, director of the Stem Cell Transplant Program at Ann & Robert H. Lurie Children’s Hospital of Chicago, said that trying to replicate the conditions of the first transplant may not work.
“It’s also a function of who was the donor for the first transplant – was it a matched sibling? And then for the second one do you go to an unrelated donor, or a half-matched relative, banking on the fact that the donor’s immune system for the second transplant is going to be able to mediate some kind of graft-versus-leukemia effect that wasn’t there the first time around?” he said.
Dr. Duerst, who was not involved in the study, noted that, for some patients with lymphoid malignancies, chimeric antigen receptor (CAR) T-cell therapy may be a more effective salvage strategy than second transplant, but added that it’s still too soon to know which strategy will be more effective.
The study was supported by St. Jude’s, the American Society of Hematology, and the American Society for Transplantation and Cellular Therapy. Dr. Sharma, Dr. Boelens, and Dr. Duerst reported having no relevant disclosures.
SOURCE: Sharma A et al. TCT 2020, Abstract 116.
ORLANDO – A second hematopoietic stem cell transplant can be a successful salvage therapy for a child who has experienced a relapse following a first allogeneic transplant, investigators report.
A retrospective study of 221 children who experienced a relapse after a first hematopoietic stem cell transplant (HSCT) showed that 3-year overall survival (OS) was six times higher among those who had second HSCT, compared with those who did not, reported Akshay Sharma, MBBS, from St. Jude’s Children’s Research Hospital in Memphis.
“We found that factors that are typically associated with poor outcomes after transplant such as disease status at the time of first transplantation – being in remission or not, type of transplant – myeloablative or reduced intensity, and choice of donor were generally not significantly predictive of outcomes following posttransplant relapse in our multivariable model,” he said at the Transplantation and Cellular Therapy Meetings.
Relapse is the most common cause of death after HSCT, and 20%-30% of children who undergo allogeneic HSCT will experience a relapse.
To study this issue, Dr. Sharma and colleagues took a retrospective look at 703 patients 21 and younger who received a first alloHSCT at St. Jude’s for acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML) or myelodysplastic syndrome from 1990 through 2018. Of this cohort, 211 patients (31%) experienced a relapse after transplant.
There were no significant differences between patients who had a relapse and those who did not in sex, race, conditioning-regimen intensity, performance status, donor type, or graft type (peripheral blood stem cell, umbilical cord blood, bone marrow), or in the incidence of acute graft-versus-host disease GVHD.
The investigators found that, as expected, outcomes were poor for patients who experienced a posttransplant relapse, with 3-year overall survival from relapse for the 221 patients of just 10%.
In multivariable analysis controlling for sex, disease status at the time of first transplant, interval from first transplant to relapse, management after relapse, chronic GVHD and year of relapse, factors significantly associated with worse overall survival were relapse within 6 months of transplant vs. later than 6 months (hazard ratio, 4.6; P < .001) and decade of transplant (HR, 2.6 for 1990-2000 and 1.6 for 2001-2010 vs. 2011-2018; P < .001).
In contrast, both second HSCT and donor lymphocyte infusion were associated with better overall survival, compared with postrelapse chemotherapy or supportive care (HR, 0.04 and 0.6, respectively; P < .001 for both comparisons).
A longer interval from first transplant to relapse was the strongest predictor of long-term survival, Dr. Sharma said at the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
Among the 221 patients who had a relapse, 61 (28%) had a second HSCT, 28 (13%) received only donor lymphocyte infusions, without second transplant, and 132 (62%) received either chemotherapy or supportive care.
The 3-year overall survival rate for patients who received a second transplant was 28%, compared with 4% for those who did not have a repeat HSCT. The most important independent predictors for getting a second transplant were longer time to relapse after first transplant, first transplantation from a matched sibling donor instead of from a haploidentical donor, some degree of acute GVHD, and decade of first transplant (current decade vs. earlier decades).
The investigators also looked at guideline recommendations from both the American Society of Hematology and UK National Health Service regarding second allogenenic transplant after relapse.
ASH guidelines say that “patients with chemo-sensitive disease in remission who had a long initial remission (> 6-12 months) after first transplant and who never developed any GVHD” are most likely to benefit from a second transplant.
NHS guidelines say that a second transplant can be considered for patients who experience relapse more than 12 months after first alloHSCT. But as Dr. Sharma and colleagues discovered, patients who had a second transplant had better overall survival regardless of time from first to second transplant, compared with patients who had later relapses but no second transplant.
“With these data in mind, we submit that these ASH and NHS guidelines, which are based on older data from the 1900s and 2000 and are mostly based on adult data and do not include much pediatric data should be reconsidered, at least in the context of pediatric patients as we approach them,” he said.
Jaap-Jan Boelens, MD, PhD, a pediatric transplant specialist at Memorial Sloan Kettering Cancer Center in New York City, who was not involved in the study, said that the use of second transplant as salvage therapy is becoming more common at his center.
“But there is a little nuance,” he said in an interview. “If someone relapses a month after transplant, let’s say, it doesn’t make sense to go for another allo transplant. But if the interval between transplant is longer, more than half a year, we usually consider going for a second allo transplant.”
The decision to attempt a second transplant may also hinge on the disease the patient is being treated for, and on the depth of remission prior to relapse, he said.
Reggie E. Duerst, MD, director of the Stem Cell Transplant Program at Ann & Robert H. Lurie Children’s Hospital of Chicago, said that trying to replicate the conditions of the first transplant may not work.
“It’s also a function of who was the donor for the first transplant – was it a matched sibling? And then for the second one do you go to an unrelated donor, or a half-matched relative, banking on the fact that the donor’s immune system for the second transplant is going to be able to mediate some kind of graft-versus-leukemia effect that wasn’t there the first time around?” he said.
Dr. Duerst, who was not involved in the study, noted that, for some patients with lymphoid malignancies, chimeric antigen receptor (CAR) T-cell therapy may be a more effective salvage strategy than second transplant, but added that it’s still too soon to know which strategy will be more effective.
The study was supported by St. Jude’s, the American Society of Hematology, and the American Society for Transplantation and Cellular Therapy. Dr. Sharma, Dr. Boelens, and Dr. Duerst reported having no relevant disclosures.
SOURCE: Sharma A et al. TCT 2020, Abstract 116.
REPORTING FROM TCT 2020
Flattening the curve: Viral graphic shows COVID-19 containment needs
Editor’s note: Find the latest COVID-19 news and guidance in Medscape’s Coronavirus Resource Center.
The “Flattening the Curve” graphic, which has, to not use the term lightly, gone viral on social media, visually explains the best currently available strategy to stop the COVID-19 spread, experts told Medscape Medical News.
The height of the curve is the number of potential cases in the United States; along the horizontal X axis, or the breadth, is the amount of time. The line across the middle represents the point at which too many cases in too short a time overwhelm the healthcare system.
Jeanne Marrazzo, MD, MPH, director of the Division of Infectious Diseases at the University of Alabama at Birmingham’s School of Medicine explained.
“Not only are you spreading out the new cases but the rate at which people recover,” she told Medscape Medical News. “You have time to get people out of the hospital so you can get new people in and clear out those beds.”
The strategy, with its own Twitter hashtag, #Flattenthecurve, “is about all we have,” without a vaccine, Marrazzo said.
Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases, said avoiding spikes in cases could mean fewer deaths.
“If you look at the curves of outbreaks, you know, they go big peaks, and then they come down. What we need to do is flatten that down,” Fauci said March 10 in a White House briefing. “You do that by trying to interfere with the natural flow of the outbreak.”
Wuhan, China, at the epicenter of the pandemic, “had an explosive curve” and quickly got overwhelmed without early containment measures, Marrazzo noted. “If you look at Italy right now, it’s clearly in the same situation.”
The Race Is On to Interrupt the Spread
The race is on in the US to interrupt the transmission of the virus and slow the spread, meaning containment measures have increasingly higher and wider stakes.
Closing down Broadway shows and some theme parks and massive sporting events; the escalating numbers of people working from home; and businesses cutting hours or closing all demonstrate the level of US confidence that “social distancing” will work, Marrazzo said.
“We’re clearly ready to disrupt the economy and social infrastructure,” she said.
That appears to have made a difference in Wuhan, Marrazzo said, as the new infections are coming down.
The question, she said, is “we’re not China – so are Americans really going to take to this? Americans greatly value their liberty and there’s some skepticism about public health and its directives. People have never seen a pandemic like this before.”
Dena Grayson, MD, PhD, a Florida-based expert in Ebola and other pandemic threats, told Medscape Medical News that EvergreenHealth in Kirkland, Washington, is a good example of what it means when a virus overwhelms healthcare operations.
The New York Times reported that supplies were so strained at the facility that staff were using sanitary napkins to pad protective helmets.
As of March 11, 65 people who had come into the hospital have tested positive for the virus, and 15 of them had died.
Grayson points out that the COVID-19 cases come on top of a severe flu season and the usual cases hospitals see, so the bar on the graphic is even lower than it usually would be.
“We have a relatively limited capacity with ICU beds to begin with,” she said.
So far, closures, postponements, and cancellations are woefully inadequate, Grayson said.
“We can’t stop this virus. We can hope to contain it and slow down the rate of infection,” she said.
“We need to right now shut down all the schools, preschools, and universities,” Grayson said. “We need to look at shutting down public transportation. We need people to stay home – and not for a day but for a couple of weeks.”
The graphic was developed by visual-data journalist Rosamund Pearce, based on a graphic that had appeared in a Centers for Disease Control and Prevention (CDC) article titled “Community Mitigation Guidelines to Prevent Pandemic Influenza,” the Times reports.
Marrazzo and Grayson have disclosed no relevant financial relationships.
This story first appeared on Medscape.com .
Editor’s note: Find the latest COVID-19 news and guidance in Medscape’s Coronavirus Resource Center.
The “Flattening the Curve” graphic, which has, to not use the term lightly, gone viral on social media, visually explains the best currently available strategy to stop the COVID-19 spread, experts told Medscape Medical News.
The height of the curve is the number of potential cases in the United States; along the horizontal X axis, or the breadth, is the amount of time. The line across the middle represents the point at which too many cases in too short a time overwhelm the healthcare system.

Jeanne Marrazzo, MD, MPH, director of the Division of Infectious Diseases at the University of Alabama at Birmingham’s School of Medicine explained.
“Not only are you spreading out the new cases but the rate at which people recover,” she told Medscape Medical News. “You have time to get people out of the hospital so you can get new people in and clear out those beds.”
The strategy, with its own Twitter hashtag, #Flattenthecurve, “is about all we have,” without a vaccine, Marrazzo said.
Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases, said avoiding spikes in cases could mean fewer deaths.
“If you look at the curves of outbreaks, you know, they go big peaks, and then they come down. What we need to do is flatten that down,” Fauci said March 10 in a White House briefing. “You do that by trying to interfere with the natural flow of the outbreak.”
Wuhan, China, at the epicenter of the pandemic, “had an explosive curve” and quickly got overwhelmed without early containment measures, Marrazzo noted. “If you look at Italy right now, it’s clearly in the same situation.”
The Race Is On to Interrupt the Spread
The race is on in the US to interrupt the transmission of the virus and slow the spread, meaning containment measures have increasingly higher and wider stakes.
Closing down Broadway shows and some theme parks and massive sporting events; the escalating numbers of people working from home; and businesses cutting hours or closing all demonstrate the level of US confidence that “social distancing” will work, Marrazzo said.
“We’re clearly ready to disrupt the economy and social infrastructure,” she said.
That appears to have made a difference in Wuhan, Marrazzo said, as the new infections are coming down.
The question, she said, is “we’re not China – so are Americans really going to take to this? Americans greatly value their liberty and there’s some skepticism about public health and its directives. People have never seen a pandemic like this before.”
Dena Grayson, MD, PhD, a Florida-based expert in Ebola and other pandemic threats, told Medscape Medical News that EvergreenHealth in Kirkland, Washington, is a good example of what it means when a virus overwhelms healthcare operations.
The New York Times reported that supplies were so strained at the facility that staff were using sanitary napkins to pad protective helmets.
As of March 11, 65 people who had come into the hospital have tested positive for the virus, and 15 of them had died.
Grayson points out that the COVID-19 cases come on top of a severe flu season and the usual cases hospitals see, so the bar on the graphic is even lower than it usually would be.
“We have a relatively limited capacity with ICU beds to begin with,” she said.
So far, closures, postponements, and cancellations are woefully inadequate, Grayson said.
“We can’t stop this virus. We can hope to contain it and slow down the rate of infection,” she said.
“We need to right now shut down all the schools, preschools, and universities,” Grayson said. “We need to look at shutting down public transportation. We need people to stay home – and not for a day but for a couple of weeks.”
The graphic was developed by visual-data journalist Rosamund Pearce, based on a graphic that had appeared in a Centers for Disease Control and Prevention (CDC) article titled “Community Mitigation Guidelines to Prevent Pandemic Influenza,” the Times reports.
Marrazzo and Grayson have disclosed no relevant financial relationships.
This story first appeared on Medscape.com .
Editor’s note: Find the latest COVID-19 news and guidance in Medscape’s Coronavirus Resource Center.
The “Flattening the Curve” graphic, which has, to not use the term lightly, gone viral on social media, visually explains the best currently available strategy to stop the COVID-19 spread, experts told Medscape Medical News.
The height of the curve is the number of potential cases in the United States; along the horizontal X axis, or the breadth, is the amount of time. The line across the middle represents the point at which too many cases in too short a time overwhelm the healthcare system.

Jeanne Marrazzo, MD, MPH, director of the Division of Infectious Diseases at the University of Alabama at Birmingham’s School of Medicine explained.
“Not only are you spreading out the new cases but the rate at which people recover,” she told Medscape Medical News. “You have time to get people out of the hospital so you can get new people in and clear out those beds.”
The strategy, with its own Twitter hashtag, #Flattenthecurve, “is about all we have,” without a vaccine, Marrazzo said.
Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases, said avoiding spikes in cases could mean fewer deaths.
“If you look at the curves of outbreaks, you know, they go big peaks, and then they come down. What we need to do is flatten that down,” Fauci said March 10 in a White House briefing. “You do that by trying to interfere with the natural flow of the outbreak.”
Wuhan, China, at the epicenter of the pandemic, “had an explosive curve” and quickly got overwhelmed without early containment measures, Marrazzo noted. “If you look at Italy right now, it’s clearly in the same situation.”
The Race Is On to Interrupt the Spread
The race is on in the US to interrupt the transmission of the virus and slow the spread, meaning containment measures have increasingly higher and wider stakes.
Closing down Broadway shows and some theme parks and massive sporting events; the escalating numbers of people working from home; and businesses cutting hours or closing all demonstrate the level of US confidence that “social distancing” will work, Marrazzo said.
“We’re clearly ready to disrupt the economy and social infrastructure,” she said.
That appears to have made a difference in Wuhan, Marrazzo said, as the new infections are coming down.
The question, she said, is “we’re not China – so are Americans really going to take to this? Americans greatly value their liberty and there’s some skepticism about public health and its directives. People have never seen a pandemic like this before.”
Dena Grayson, MD, PhD, a Florida-based expert in Ebola and other pandemic threats, told Medscape Medical News that EvergreenHealth in Kirkland, Washington, is a good example of what it means when a virus overwhelms healthcare operations.
The New York Times reported that supplies were so strained at the facility that staff were using sanitary napkins to pad protective helmets.
As of March 11, 65 people who had come into the hospital have tested positive for the virus, and 15 of them had died.
Grayson points out that the COVID-19 cases come on top of a severe flu season and the usual cases hospitals see, so the bar on the graphic is even lower than it usually would be.
“We have a relatively limited capacity with ICU beds to begin with,” she said.
So far, closures, postponements, and cancellations are woefully inadequate, Grayson said.
“We can’t stop this virus. We can hope to contain it and slow down the rate of infection,” she said.
“We need to right now shut down all the schools, preschools, and universities,” Grayson said. “We need to look at shutting down public transportation. We need people to stay home – and not for a day but for a couple of weeks.”
The graphic was developed by visual-data journalist Rosamund Pearce, based on a graphic that had appeared in a Centers for Disease Control and Prevention (CDC) article titled “Community Mitigation Guidelines to Prevent Pandemic Influenza,” the Times reports.
Marrazzo and Grayson have disclosed no relevant financial relationships.
This story first appeared on Medscape.com .