User login
Relapsing MS: Ozanimod Tops Fingolimod in Benefit-Risk Profile
Key clinical point: Ozanimod has a superior benefit-risk profile compared with fingolimod for the treatment of relapsing multiple sclerosis (RMS).
Major finding: Compared with fingolimod, ozanimod was associated with lower rates of conduction abnormalities (risk difference, −3.5%) and first-degree atrioventricular block (risk difference, −3.0%), lower risk of extended first-dose cardiac monitoring, and lower risk of adverse events over 1-2 years of follow-up. Efficacy outcomes were comparable.
Study details: Using a matching-adjusted indirect comparison, the first-dose cardiac monitoring outcomes and 1- and 2-year safety and efficacy outcomes were compared for ozanimod and fingolimod in patients with RMS.
Disclosures: The study received research support from Celgene, a wholly-owned subsidiary of Bristol-Myers Squibb. Five authors are employees of Bristol-Myers Squibb. Four authors are employees of Analysis Group, Inc., who have received consulting fees from Bristol-Myers Squibb.
Citation: Swallow E et al. J Comp Eff Res. 2020 Jan 17. doi: 10.2217/cer-2019-0169.
Key clinical point: Ozanimod has a superior benefit-risk profile compared with fingolimod for the treatment of relapsing multiple sclerosis (RMS).
Major finding: Compared with fingolimod, ozanimod was associated with lower rates of conduction abnormalities (risk difference, −3.5%) and first-degree atrioventricular block (risk difference, −3.0%), lower risk of extended first-dose cardiac monitoring, and lower risk of adverse events over 1-2 years of follow-up. Efficacy outcomes were comparable.
Study details: Using a matching-adjusted indirect comparison, the first-dose cardiac monitoring outcomes and 1- and 2-year safety and efficacy outcomes were compared for ozanimod and fingolimod in patients with RMS.
Disclosures: The study received research support from Celgene, a wholly-owned subsidiary of Bristol-Myers Squibb. Five authors are employees of Bristol-Myers Squibb. Four authors are employees of Analysis Group, Inc., who have received consulting fees from Bristol-Myers Squibb.
Citation: Swallow E et al. J Comp Eff Res. 2020 Jan 17. doi: 10.2217/cer-2019-0169.
Key clinical point: Ozanimod has a superior benefit-risk profile compared with fingolimod for the treatment of relapsing multiple sclerosis (RMS).
Major finding: Compared with fingolimod, ozanimod was associated with lower rates of conduction abnormalities (risk difference, −3.5%) and first-degree atrioventricular block (risk difference, −3.0%), lower risk of extended first-dose cardiac monitoring, and lower risk of adverse events over 1-2 years of follow-up. Efficacy outcomes were comparable.
Study details: Using a matching-adjusted indirect comparison, the first-dose cardiac monitoring outcomes and 1- and 2-year safety and efficacy outcomes were compared for ozanimod and fingolimod in patients with RMS.
Disclosures: The study received research support from Celgene, a wholly-owned subsidiary of Bristol-Myers Squibb. Five authors are employees of Bristol-Myers Squibb. Four authors are employees of Analysis Group, Inc., who have received consulting fees from Bristol-Myers Squibb.
Citation: Swallow E et al. J Comp Eff Res. 2020 Jan 17. doi: 10.2217/cer-2019-0169.
Polypharmacy is Associated With Adverse Health Outcomes in Patients With MS
Key clinical point: Polypharmacy is common in patients with multiple sclerosis (MS) and is negatively associated with health outcomes.
Major finding: The rates of polypharmacy among patients with MS ranged from 14.9% to 59% across the studies that were reviewed. Polypharmacy was significantly associated with increased hospitalization rates, cognitive deficits, fatigue, higher relapse rates, and a lower quality of life, particularly among elderly patients.
Study details: The data come from a qualitative systematic review of 7 studies.
Disclosures: Niklas Frahm has received travel funds for research meetings from Novartis. Michael Hecker received speaking fees and travel funds from Bayer HealthCare, Biogen, Merck Serono, Novartis, and Teva. Uwe Klaus Zettl received research support and lecture fees or travel funds from Almirall, Alexion, Bayer HealthCare, Biogen, Merck Serono, Novartis, Roche, Sanofi, and Teva.
Citation: Frahm N et al. Expert Opin Drug Saf. 2020 Jan 27. doi: 10.1080/14740338.2020.1720646.
Key clinical point: Polypharmacy is common in patients with multiple sclerosis (MS) and is negatively associated with health outcomes.
Major finding: The rates of polypharmacy among patients with MS ranged from 14.9% to 59% across the studies that were reviewed. Polypharmacy was significantly associated with increased hospitalization rates, cognitive deficits, fatigue, higher relapse rates, and a lower quality of life, particularly among elderly patients.
Study details: The data come from a qualitative systematic review of 7 studies.
Disclosures: Niklas Frahm has received travel funds for research meetings from Novartis. Michael Hecker received speaking fees and travel funds from Bayer HealthCare, Biogen, Merck Serono, Novartis, and Teva. Uwe Klaus Zettl received research support and lecture fees or travel funds from Almirall, Alexion, Bayer HealthCare, Biogen, Merck Serono, Novartis, Roche, Sanofi, and Teva.
Citation: Frahm N et al. Expert Opin Drug Saf. 2020 Jan 27. doi: 10.1080/14740338.2020.1720646.
Key clinical point: Polypharmacy is common in patients with multiple sclerosis (MS) and is negatively associated with health outcomes.
Major finding: The rates of polypharmacy among patients with MS ranged from 14.9% to 59% across the studies that were reviewed. Polypharmacy was significantly associated with increased hospitalization rates, cognitive deficits, fatigue, higher relapse rates, and a lower quality of life, particularly among elderly patients.
Study details: The data come from a qualitative systematic review of 7 studies.
Disclosures: Niklas Frahm has received travel funds for research meetings from Novartis. Michael Hecker received speaking fees and travel funds from Bayer HealthCare, Biogen, Merck Serono, Novartis, and Teva. Uwe Klaus Zettl received research support and lecture fees or travel funds from Almirall, Alexion, Bayer HealthCare, Biogen, Merck Serono, Novartis, Roche, Sanofi, and Teva.
Citation: Frahm N et al. Expert Opin Drug Saf. 2020 Jan 27. doi: 10.1080/14740338.2020.1720646.
Air Pollution is a Risk Factor for MS
Key clinical point: Air pollution may be a potential etiological factor in the development of multiple sclerosis (MS).
Major finding: The age-standardized prevalence of MS was found to be 97.4 per 100,000 persons in the polluted city and 47.2 per 100,000 persons in the rural and clean city.
Study details: A cross-sectional, population-based, epidemiologic study compared the prevalence of MS among 2 cities in Turkey: Eregli city, which has an iron-and-steel factory with known air pollution, and Devrek city, which is a rural and clean city.
Disclosures: The authors declared no conflicts of interest.
Citation: Börü UT et al. Acta Neurol Scand. 2020 Jan 18. doi: 10.1111/ane.13223.
Key clinical point: Air pollution may be a potential etiological factor in the development of multiple sclerosis (MS).
Major finding: The age-standardized prevalence of MS was found to be 97.4 per 100,000 persons in the polluted city and 47.2 per 100,000 persons in the rural and clean city.
Study details: A cross-sectional, population-based, epidemiologic study compared the prevalence of MS among 2 cities in Turkey: Eregli city, which has an iron-and-steel factory with known air pollution, and Devrek city, which is a rural and clean city.
Disclosures: The authors declared no conflicts of interest.
Citation: Börü UT et al. Acta Neurol Scand. 2020 Jan 18. doi: 10.1111/ane.13223.
Key clinical point: Air pollution may be a potential etiological factor in the development of multiple sclerosis (MS).
Major finding: The age-standardized prevalence of MS was found to be 97.4 per 100,000 persons in the polluted city and 47.2 per 100,000 persons in the rural and clean city.
Study details: A cross-sectional, population-based, epidemiologic study compared the prevalence of MS among 2 cities in Turkey: Eregli city, which has an iron-and-steel factory with known air pollution, and Devrek city, which is a rural and clean city.
Disclosures: The authors declared no conflicts of interest.
Citation: Börü UT et al. Acta Neurol Scand. 2020 Jan 18. doi: 10.1111/ane.13223.
High Prevalence of Alexithymia in Patients With Relapse-Remitting MS
Key clinical point: Patients with relapse-remitting multiple sclerosis (RRMS) have a high prevalence of alexithymia.
Major finding: Alexithymia was observed in about 29.55% of patients and borderline alexithymia was observed in 31.15% of patients; alexithymia positively correlated with anxiety and depression in patients with RRMS (P less than .01).
Study details: The data come from a cross-sectional study that included 106 consecutively assessed adult patients with RRMS (74 female and 32 male patients).
Disclosures: The authors declared no conflicts of interest.
Citation: Stojanov J et al. J Postgrad Med. 2020 Jan 13. doi: 10.4103/jpgm.JPGM_499_19.
Key clinical point: Patients with relapse-remitting multiple sclerosis (RRMS) have a high prevalence of alexithymia.
Major finding: Alexithymia was observed in about 29.55% of patients and borderline alexithymia was observed in 31.15% of patients; alexithymia positively correlated with anxiety and depression in patients with RRMS (P less than .01).
Study details: The data come from a cross-sectional study that included 106 consecutively assessed adult patients with RRMS (74 female and 32 male patients).
Disclosures: The authors declared no conflicts of interest.
Citation: Stojanov J et al. J Postgrad Med. 2020 Jan 13. doi: 10.4103/jpgm.JPGM_499_19.
Key clinical point: Patients with relapse-remitting multiple sclerosis (RRMS) have a high prevalence of alexithymia.
Major finding: Alexithymia was observed in about 29.55% of patients and borderline alexithymia was observed in 31.15% of patients; alexithymia positively correlated with anxiety and depression in patients with RRMS (P less than .01).
Study details: The data come from a cross-sectional study that included 106 consecutively assessed adult patients with RRMS (74 female and 32 male patients).
Disclosures: The authors declared no conflicts of interest.
Citation: Stojanov J et al. J Postgrad Med. 2020 Jan 13. doi: 10.4103/jpgm.JPGM_499_19.
Lower Urinary Tract Symptoms Are Common in Patients With MS
Key clinical point: Patients with multiple sclerosis (MS) have a high prevalence of lower urinary tract symptoms (LUTS).
Major finding: The prevalence of LUTS among patients with MS was 87.7%. The likelihood of urinary problems was higher in patients with a high Expanded Disability Status Scale score (adjusted odds ratio, 0.677; 95% CI, 0.507-0.903; P = .008).
Study details: The data come from a cross-sectional study that included 602 patients with MS.
Disclosures: The authors declared no conflicts of interest.
Citation: Nazari F et al. BMC Neurol. 2020 Jan 17. doi: 10.1186/s12883-019-1582-1.
Key clinical point: Patients with multiple sclerosis (MS) have a high prevalence of lower urinary tract symptoms (LUTS).
Major finding: The prevalence of LUTS among patients with MS was 87.7%. The likelihood of urinary problems was higher in patients with a high Expanded Disability Status Scale score (adjusted odds ratio, 0.677; 95% CI, 0.507-0.903; P = .008).
Study details: The data come from a cross-sectional study that included 602 patients with MS.
Disclosures: The authors declared no conflicts of interest.
Citation: Nazari F et al. BMC Neurol. 2020 Jan 17. doi: 10.1186/s12883-019-1582-1.
Key clinical point: Patients with multiple sclerosis (MS) have a high prevalence of lower urinary tract symptoms (LUTS).
Major finding: The prevalence of LUTS among patients with MS was 87.7%. The likelihood of urinary problems was higher in patients with a high Expanded Disability Status Scale score (adjusted odds ratio, 0.677; 95% CI, 0.507-0.903; P = .008).
Study details: The data come from a cross-sectional study that included 602 patients with MS.
Disclosures: The authors declared no conflicts of interest.
Citation: Nazari F et al. BMC Neurol. 2020 Jan 17. doi: 10.1186/s12883-019-1582-1.
More than 12 weeks needed for x-ray resolution of pneumonia in the elderly
Stress incontinence surgery found to improve sexual dysfunction
An analysis of four commonly performed surgical procedures for stress urinary incontinence found that they all improved sexual dysfunction to a similar degree over the course of 24 months.
“There is a growing body of literature concerning female sexual function after treatment for urinary incontinence,” Stephanie M. Glass Clark, MD, of the University of Pittsburgh, and colleagues wrote in a study published in Obstetrics & Gynecology. “Pelvic floor muscle therapy has been shown to improve sexual function as well as urinary incontinence symptoms. Surgical treatment, on the other hand, has had unclear effects on sexual function.”
Dr. Glass Clark and colleagues conducted a combined secondary analysis of the SISTEr (Stress Incontinence Surgical Treatment Efficacy Trial) and TOMUS (Trial of Mid-Urethral Slings) studies. Women in the original trials were randomized to receive surgical treatment for stress urinary incontinence with an autologous fascial sling or Burch colposuspension (SISTEr), or a retropubic or transobturator midurethral sling (TOMUS). Sexual function as assessed by the short version of the Pelvic Organ Prolapse/ Urinary Incontinence Sexual Questionnaire (PISQ-12) was compared between groups at baseline, 12 months, and 24 months.
Of the 924 women included, 249 (27%) had an autologous fascial sling, 239 (26%) underwent Burch colposuspension, 216 (23%) had a retropubic midurethral sling placed, and 220 (24%) had a transobturator midurethral sling placed. The researchers observed no significant differences in mean PISQ-12 scores between the four treatment groups at the time of baseline (P = .07) or at the 12- and 24-month visits (P = .42 and P = .50, respectively). Patients in the two studies showed an overall improvement in sexual function over the 24-month study period.
Specifically, PISQ-12 scores at baseline were 32.6 in the transobturator sling group, 33.1 in the retropubic sling group, 31.9 in the Burch procedure group, and 31.4 in the fascial sling group. At 12 months, the PISQ-12 scores rose to 37.7 in the transobturator sling group, 37.8 in the retropubic sling group, 36.9 in the Burch procedure group, and 37.1 in the fascial sling group. These scores were generally maintained at 24 months (37.7 in the transobturator sling group, 37.1 in the retropubic sling group, 36.7 in the Burch procedure group, and 37.4 in the fascial sling group), and were not statistically different than the scores tabulated at the 12-month follow-up visit (P = .97).
“This study and others demonstrate that sexual function improves with surgical improvement of stress incontinence which may suggest a possible association of urinary incontinence and sexual dysfunction,” Dr. Glass Clark and colleagues concluded. “As we continue to explore the complex and multifaceted problem of sexual dysfunction, further evaluation of the effect of pelvic floor disorders – and their treatments – will be important and necessary research.”
The researchers acknowledged certain limitations of the study, including the fact that there was a low degree of diversity among women in the studied trials, which limits the generalizability of the findings. They also pointed out that the PISQ-12 does not address sexual stimulation or nonpenetrative vaginal intercourse. “Additionally, it limits partner-related problems to erectile dysfunction and premature ejaculation; some eligible participants may be excluded secondary to sexual preferences given the assumptions inherent to the questionnaire that the partner is male,” they wrote.
This secondary analysis had no outside sources of funding. Dr. Glass Clark reported that she received a travel stipend from the Society of Gynecologic Surgeons, sponsored by OB-STATS. Her coauthors reported having no financial conflicts.
SOURCE: Glass Clark SM et al. Obstet Gynecol 2020;135(2):352-60.
At face value, this is a retrospective analysis of sexual function after surgical correction for urinary incontinence. However, the researchers looked at two well-known and well-respected randomized, controlled trials comparing two types of incontinence procedures head to head, each. So the reader gets an opportunity to examine the influence of four different surgical procedures on sexual function.
Although I expected to see there would be an initial improvement with surgical correction, I did not expect that improvement would be so well maintained over time. There was sustained – and even continued – improvement in many cases, and this suggests a closer link to urinary incontinence that just embarrassment or worry about leakage during sex. I think the “take-home message” is that women who undergo anti-incontinence procedures can expect an improvement in sexual function from baseline, with the majority happening within the first year, and maintain this improvement between years 1 and 2.
I think this is the type of study that we all envisioned being able to do 25 years ago when female pelvic medicine and reconstructive surgery was in its infancy as an “official” subspecialty, and the National Institutes of Health had developed the Urinary Incontinence Network and the Pelvic Floor Disorders Network. It is gratifying that enough good research has been done to finally enjoy the fruits of their/our labor! The study had large numbers, used a widely known, validated questionnaire, and used data generated from randomized, controlled trials. Although the subjects may not represent all demographics, the study findings can be an aid to most practicing gynecologists to help counsel their patients.
The major limitations of any retrospective study are the inability to go back and ask questions not addressed in the Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire Short Form. For instance, the authors discussed that it might be nice to have an “open-ended” question about why the nonresponders were not having sex.
Patrick Woodman, DO, MS , is a urogynecologist with the Michigan State University, East Lansing. He is also the program director for the obstetrics and gynecology residency for Ascension Macomb-Oakland Hospital, Warren (Michigan) Campus. Dr. Woodman is a member of the Ob.Gyn. News editorial advisory board.
At face value, this is a retrospective analysis of sexual function after surgical correction for urinary incontinence. However, the researchers looked at two well-known and well-respected randomized, controlled trials comparing two types of incontinence procedures head to head, each. So the reader gets an opportunity to examine the influence of four different surgical procedures on sexual function.
Although I expected to see there would be an initial improvement with surgical correction, I did not expect that improvement would be so well maintained over time. There was sustained – and even continued – improvement in many cases, and this suggests a closer link to urinary incontinence that just embarrassment or worry about leakage during sex. I think the “take-home message” is that women who undergo anti-incontinence procedures can expect an improvement in sexual function from baseline, with the majority happening within the first year, and maintain this improvement between years 1 and 2.
I think this is the type of study that we all envisioned being able to do 25 years ago when female pelvic medicine and reconstructive surgery was in its infancy as an “official” subspecialty, and the National Institutes of Health had developed the Urinary Incontinence Network and the Pelvic Floor Disorders Network. It is gratifying that enough good research has been done to finally enjoy the fruits of their/our labor! The study had large numbers, used a widely known, validated questionnaire, and used data generated from randomized, controlled trials. Although the subjects may not represent all demographics, the study findings can be an aid to most practicing gynecologists to help counsel their patients.
The major limitations of any retrospective study are the inability to go back and ask questions not addressed in the Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire Short Form. For instance, the authors discussed that it might be nice to have an “open-ended” question about why the nonresponders were not having sex.
Patrick Woodman, DO, MS , is a urogynecologist with the Michigan State University, East Lansing. He is also the program director for the obstetrics and gynecology residency for Ascension Macomb-Oakland Hospital, Warren (Michigan) Campus. Dr. Woodman is a member of the Ob.Gyn. News editorial advisory board.
At face value, this is a retrospective analysis of sexual function after surgical correction for urinary incontinence. However, the researchers looked at two well-known and well-respected randomized, controlled trials comparing two types of incontinence procedures head to head, each. So the reader gets an opportunity to examine the influence of four different surgical procedures on sexual function.
Although I expected to see there would be an initial improvement with surgical correction, I did not expect that improvement would be so well maintained over time. There was sustained – and even continued – improvement in many cases, and this suggests a closer link to urinary incontinence that just embarrassment or worry about leakage during sex. I think the “take-home message” is that women who undergo anti-incontinence procedures can expect an improvement in sexual function from baseline, with the majority happening within the first year, and maintain this improvement between years 1 and 2.
I think this is the type of study that we all envisioned being able to do 25 years ago when female pelvic medicine and reconstructive surgery was in its infancy as an “official” subspecialty, and the National Institutes of Health had developed the Urinary Incontinence Network and the Pelvic Floor Disorders Network. It is gratifying that enough good research has been done to finally enjoy the fruits of their/our labor! The study had large numbers, used a widely known, validated questionnaire, and used data generated from randomized, controlled trials. Although the subjects may not represent all demographics, the study findings can be an aid to most practicing gynecologists to help counsel their patients.
The major limitations of any retrospective study are the inability to go back and ask questions not addressed in the Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire Short Form. For instance, the authors discussed that it might be nice to have an “open-ended” question about why the nonresponders were not having sex.
Patrick Woodman, DO, MS , is a urogynecologist with the Michigan State University, East Lansing. He is also the program director for the obstetrics and gynecology residency for Ascension Macomb-Oakland Hospital, Warren (Michigan) Campus. Dr. Woodman is a member of the Ob.Gyn. News editorial advisory board.
An analysis of four commonly performed surgical procedures for stress urinary incontinence found that they all improved sexual dysfunction to a similar degree over the course of 24 months.
“There is a growing body of literature concerning female sexual function after treatment for urinary incontinence,” Stephanie M. Glass Clark, MD, of the University of Pittsburgh, and colleagues wrote in a study published in Obstetrics & Gynecology. “Pelvic floor muscle therapy has been shown to improve sexual function as well as urinary incontinence symptoms. Surgical treatment, on the other hand, has had unclear effects on sexual function.”
Dr. Glass Clark and colleagues conducted a combined secondary analysis of the SISTEr (Stress Incontinence Surgical Treatment Efficacy Trial) and TOMUS (Trial of Mid-Urethral Slings) studies. Women in the original trials were randomized to receive surgical treatment for stress urinary incontinence with an autologous fascial sling or Burch colposuspension (SISTEr), or a retropubic or transobturator midurethral sling (TOMUS). Sexual function as assessed by the short version of the Pelvic Organ Prolapse/ Urinary Incontinence Sexual Questionnaire (PISQ-12) was compared between groups at baseline, 12 months, and 24 months.
Of the 924 women included, 249 (27%) had an autologous fascial sling, 239 (26%) underwent Burch colposuspension, 216 (23%) had a retropubic midurethral sling placed, and 220 (24%) had a transobturator midurethral sling placed. The researchers observed no significant differences in mean PISQ-12 scores between the four treatment groups at the time of baseline (P = .07) or at the 12- and 24-month visits (P = .42 and P = .50, respectively). Patients in the two studies showed an overall improvement in sexual function over the 24-month study period.
Specifically, PISQ-12 scores at baseline were 32.6 in the transobturator sling group, 33.1 in the retropubic sling group, 31.9 in the Burch procedure group, and 31.4 in the fascial sling group. At 12 months, the PISQ-12 scores rose to 37.7 in the transobturator sling group, 37.8 in the retropubic sling group, 36.9 in the Burch procedure group, and 37.1 in the fascial sling group. These scores were generally maintained at 24 months (37.7 in the transobturator sling group, 37.1 in the retropubic sling group, 36.7 in the Burch procedure group, and 37.4 in the fascial sling group), and were not statistically different than the scores tabulated at the 12-month follow-up visit (P = .97).
“This study and others demonstrate that sexual function improves with surgical improvement of stress incontinence which may suggest a possible association of urinary incontinence and sexual dysfunction,” Dr. Glass Clark and colleagues concluded. “As we continue to explore the complex and multifaceted problem of sexual dysfunction, further evaluation of the effect of pelvic floor disorders – and their treatments – will be important and necessary research.”
The researchers acknowledged certain limitations of the study, including the fact that there was a low degree of diversity among women in the studied trials, which limits the generalizability of the findings. They also pointed out that the PISQ-12 does not address sexual stimulation or nonpenetrative vaginal intercourse. “Additionally, it limits partner-related problems to erectile dysfunction and premature ejaculation; some eligible participants may be excluded secondary to sexual preferences given the assumptions inherent to the questionnaire that the partner is male,” they wrote.
This secondary analysis had no outside sources of funding. Dr. Glass Clark reported that she received a travel stipend from the Society of Gynecologic Surgeons, sponsored by OB-STATS. Her coauthors reported having no financial conflicts.
SOURCE: Glass Clark SM et al. Obstet Gynecol 2020;135(2):352-60.
An analysis of four commonly performed surgical procedures for stress urinary incontinence found that they all improved sexual dysfunction to a similar degree over the course of 24 months.
“There is a growing body of literature concerning female sexual function after treatment for urinary incontinence,” Stephanie M. Glass Clark, MD, of the University of Pittsburgh, and colleagues wrote in a study published in Obstetrics & Gynecology. “Pelvic floor muscle therapy has been shown to improve sexual function as well as urinary incontinence symptoms. Surgical treatment, on the other hand, has had unclear effects on sexual function.”
Dr. Glass Clark and colleagues conducted a combined secondary analysis of the SISTEr (Stress Incontinence Surgical Treatment Efficacy Trial) and TOMUS (Trial of Mid-Urethral Slings) studies. Women in the original trials were randomized to receive surgical treatment for stress urinary incontinence with an autologous fascial sling or Burch colposuspension (SISTEr), or a retropubic or transobturator midurethral sling (TOMUS). Sexual function as assessed by the short version of the Pelvic Organ Prolapse/ Urinary Incontinence Sexual Questionnaire (PISQ-12) was compared between groups at baseline, 12 months, and 24 months.
Of the 924 women included, 249 (27%) had an autologous fascial sling, 239 (26%) underwent Burch colposuspension, 216 (23%) had a retropubic midurethral sling placed, and 220 (24%) had a transobturator midurethral sling placed. The researchers observed no significant differences in mean PISQ-12 scores between the four treatment groups at the time of baseline (P = .07) or at the 12- and 24-month visits (P = .42 and P = .50, respectively). Patients in the two studies showed an overall improvement in sexual function over the 24-month study period.
Specifically, PISQ-12 scores at baseline were 32.6 in the transobturator sling group, 33.1 in the retropubic sling group, 31.9 in the Burch procedure group, and 31.4 in the fascial sling group. At 12 months, the PISQ-12 scores rose to 37.7 in the transobturator sling group, 37.8 in the retropubic sling group, 36.9 in the Burch procedure group, and 37.1 in the fascial sling group. These scores were generally maintained at 24 months (37.7 in the transobturator sling group, 37.1 in the retropubic sling group, 36.7 in the Burch procedure group, and 37.4 in the fascial sling group), and were not statistically different than the scores tabulated at the 12-month follow-up visit (P = .97).
“This study and others demonstrate that sexual function improves with surgical improvement of stress incontinence which may suggest a possible association of urinary incontinence and sexual dysfunction,” Dr. Glass Clark and colleagues concluded. “As we continue to explore the complex and multifaceted problem of sexual dysfunction, further evaluation of the effect of pelvic floor disorders – and their treatments – will be important and necessary research.”
The researchers acknowledged certain limitations of the study, including the fact that there was a low degree of diversity among women in the studied trials, which limits the generalizability of the findings. They also pointed out that the PISQ-12 does not address sexual stimulation or nonpenetrative vaginal intercourse. “Additionally, it limits partner-related problems to erectile dysfunction and premature ejaculation; some eligible participants may be excluded secondary to sexual preferences given the assumptions inherent to the questionnaire that the partner is male,” they wrote.
This secondary analysis had no outside sources of funding. Dr. Glass Clark reported that she received a travel stipend from the Society of Gynecologic Surgeons, sponsored by OB-STATS. Her coauthors reported having no financial conflicts.
SOURCE: Glass Clark SM et al. Obstet Gynecol 2020;135(2):352-60.
FROM OBSTETRICS & GYNECOLOGY
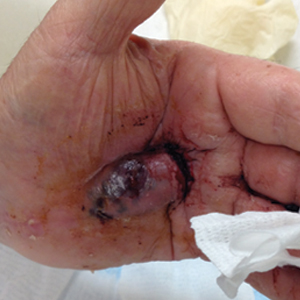
Bleeding Hand Mass in an Older Man
The Diagnosis: Epithelioid Angiosarcoma
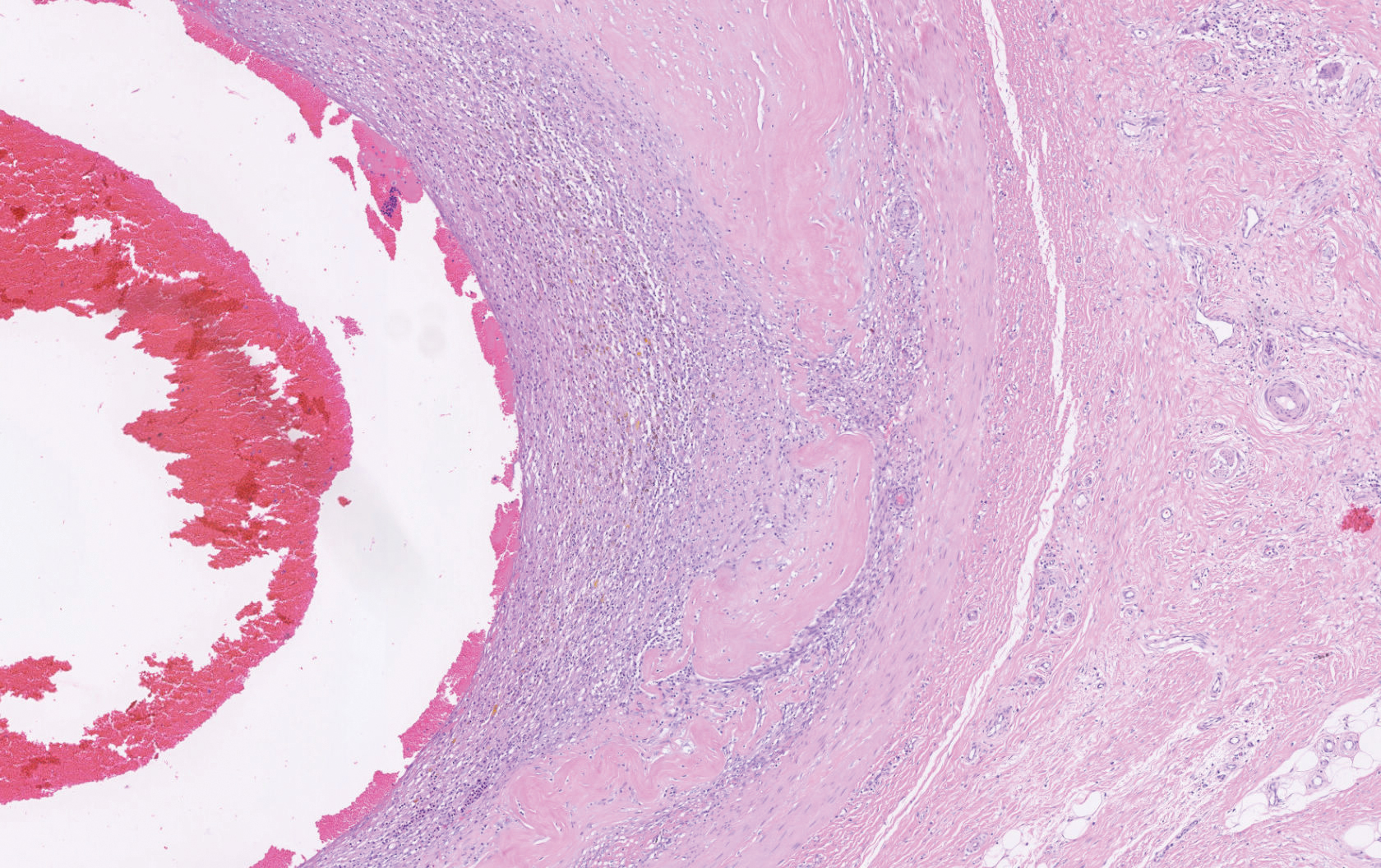
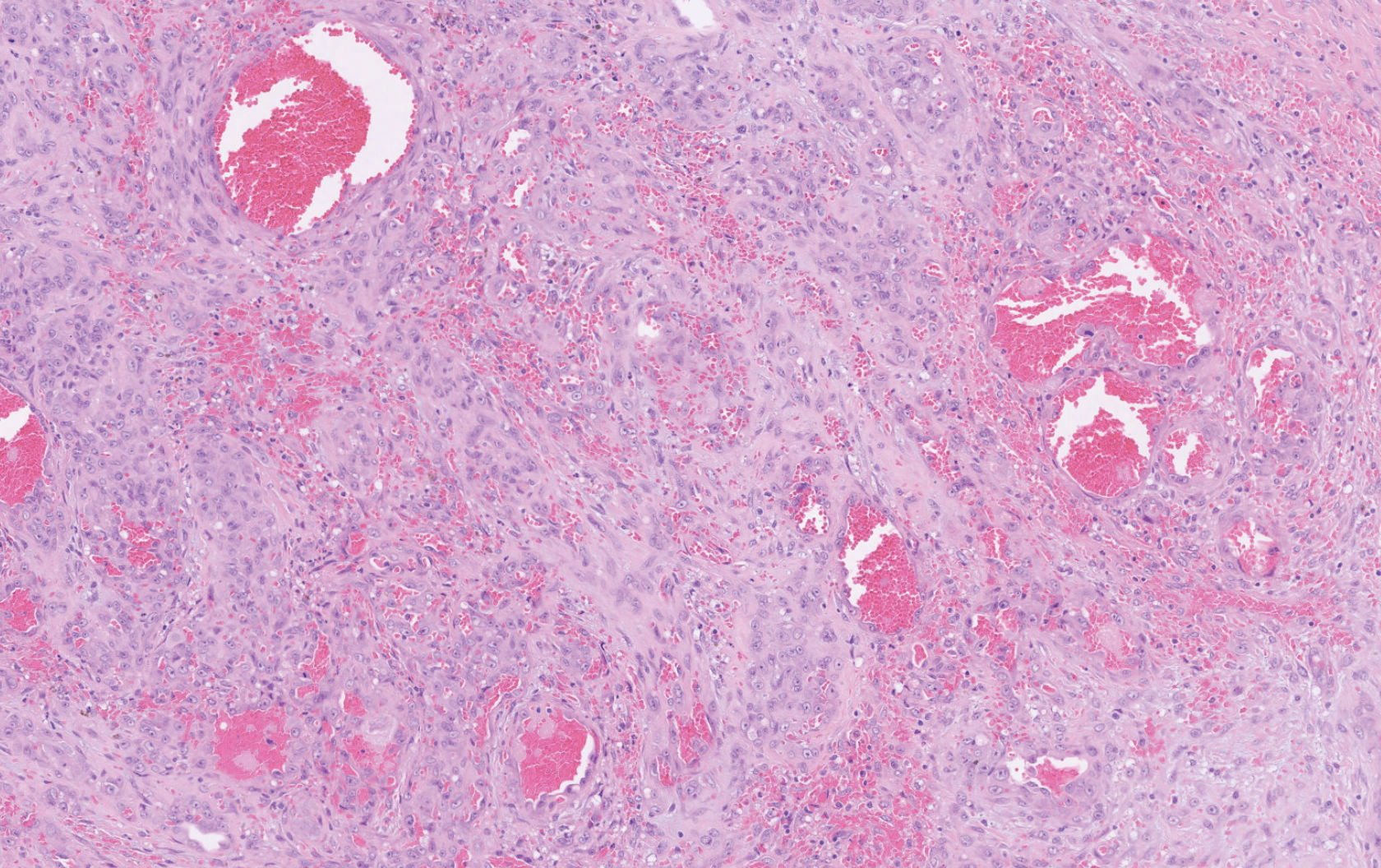
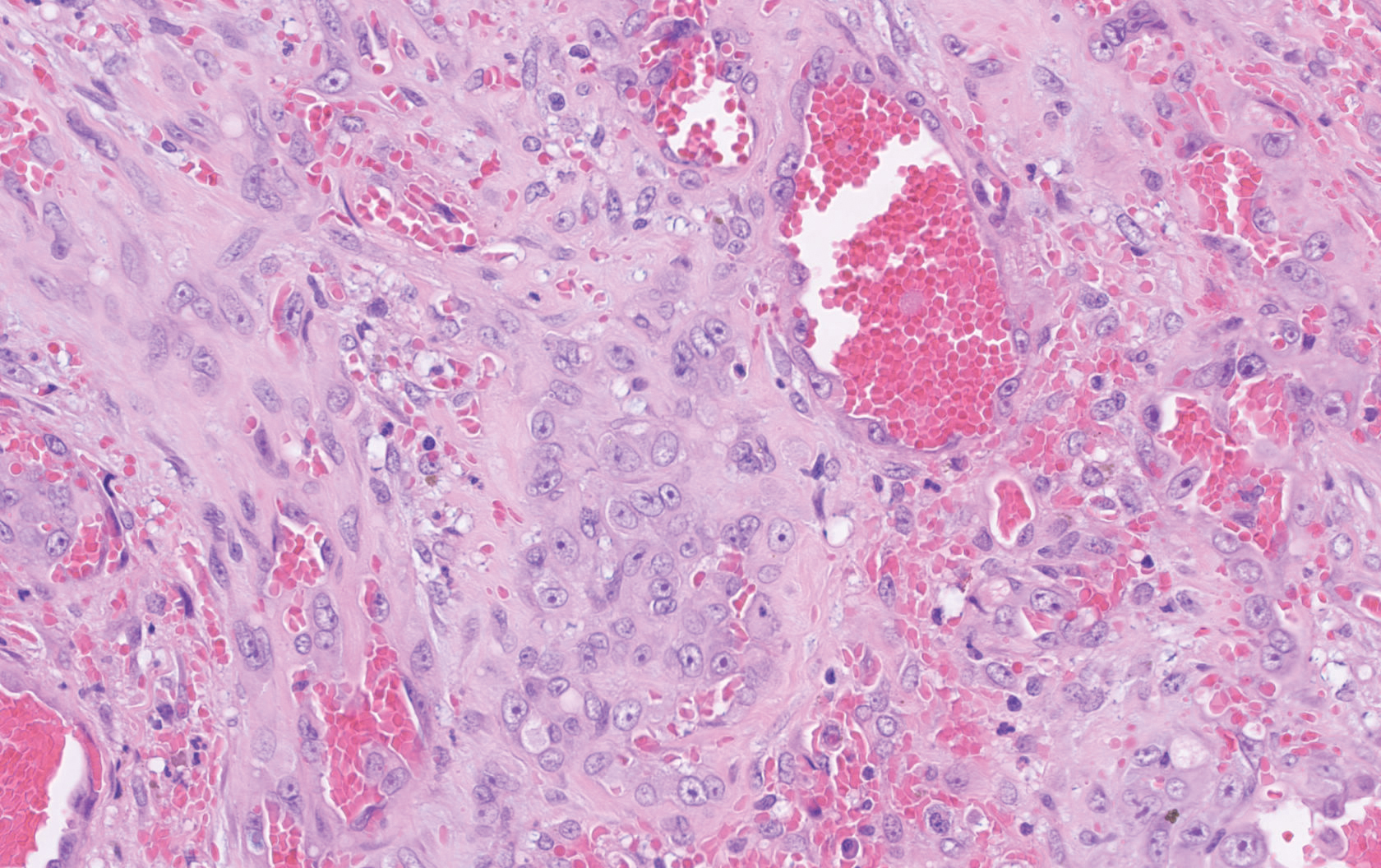
Histopathology showed a large soft-tissue neoplasm with extensive hemorrhage (Figure 1). The epithelioid angiosarcoma (EA) consisted mostly of irregular slit-shaped vessels lined by sheets of atypical endothelial cells (Figure 2). At higher-power magnification, the cellular atypia was prominent and diffuse (Figure 3). Immunostaining of the tumor cells showed positive uptake for CD31, confirming vascular origin (Figure 4). Other vascular markers, including CD34 and factor VIII, as well as nuclear positivity for the erythroblast transformation-specific transcription factor gene, ERG, can be demonstrated by EA. Irregular, smooth muscle actin-positive spindle cells are distributed around some of the vessels. The human herpesvirus 8 stain is negative.
Compared to classic angiosarcomas, EAs have a predilection for the extremities rather than the head and scalp. Histopathologically, the cells are epithelioid and are strongly positive for vimentin and CD31, in addition to factor VIII, friend leukemia integration 1 transcription factor, and CD34.1,2 In contrast, epithelioid sarcomas more typically are seen in younger adults and less likely to be CD31 positive.3 An epithelioid hemangioendothelioma is more focal in cellular atypia and forms small nests and trabeculae rather than sheets of atypical cells. Melanoma cells stain positive for human melanoma black 45, Melan-A, and S-100 but not for CD31.3 Glomangiosarcomas typically stain positive for smooth muscle actin and muscle-specific actin.4
Epithelioid angiosarcomas are rare and aggressive malignancies of endothelial origin.3 They are more prevalent in men and have a peak incidence in the seventh decade of life. They most commonly occur in the deep soft tissues of the extremities but have been reported to form in a variety of primary sites, including the skin, bones, thyroid, and adrenal glands.3
Tumors tend to be highly aggressive and demonstrate early nodal and solid organ metastases.3 Our case demonstrated the aggressive nature of this high-grade malignancy by showing neoplastic invasion through a vascular wall. Within 2 to 3 years of diagnosis, 50% of patients die of the disease, and the 5-year survival rate is estimated to be 12% to 20%.3,5 The etiology remains unknown, but EA has been linked to prior exposure to toxic chemicals, irradiation, or Thorotrast contrast media, and it may arise in the setting of arteriovenous fistulae and chronic lymphedema.6
Although radiation therapy often is utilized, surgery is the primary treatment modality.5 Even with wide excision, local recurrence is common. Tumor size is one of the most important prognostic features, with a worse prognosis for tumors larger than 5 cm. Evidence suggests that paclitaxel-based chemotherapeutic regimens may improve survival, and a combination of paclitaxel and sorafenib has been reported to induce remission in metastatic angiosarcoma of parietal EA.5 Currently, no standardized treatment regimen for this condition exists.
Acknowledgment
The authors thank Amanda Marsch, MD (Chicago, Illinois), for obtaining outside pathology consultation.
- Suchak R, Thway K, Zelger B, et al. Primary cutaneous epithelioid angiosarcoma: a clinicopathologic study of 13 cases of a rare neoplasm occurring outside the setting of conventional angiosarcomas and with predilection for the limbs. Am J Surg Pathol. 2011;35:60-69.
- Prescott RJ, Banerjee SS, Eyden BP, et al. Cutaneous epithelioid angiosarcoma: a clinicopathological study of four cases. Histopathology. 1994;25:421-429.
- Hart J, Mandavilli S. Epithelioid angiosarcoma: a brief diagnostic review and differential diagnosis. Arch Pathol Lab Med. 2011;135:268-272.
- Maselli AM, Jambhekar AV, Hunter JG. Glomangiosarcoma arising from a prior biopsy site. Plast Reconstr Surg Glob Open. 2017;5:e1219.
- Donghi D, Dummer R, Cozzio A. Complete remission in a patient with multifocal metastatic cutaneous angiosarcoma with a combination of paclitaxel and sorafenib. Br J Dermatol. 2010;162:697-699.
- Wu J, Li X, Liu X. Epithelioid angiosarcoma: a clinicopathological study of 16 Chinese cases. Int J Clin Exp Pathol. 2015;8:3901-3909.
The Diagnosis: Epithelioid Angiosarcoma
Histopathology showed a large soft-tissue neoplasm with extensive hemorrhage (Figure 1). The epithelioid angiosarcoma (EA) consisted mostly of irregular slit-shaped vessels lined by sheets of atypical endothelial cells (Figure 2). At higher-power magnification, the cellular atypia was prominent and diffuse (Figure 3). Immunostaining of the tumor cells showed positive uptake for CD31, confirming vascular origin (Figure 4). Other vascular markers, including CD34 and factor VIII, as well as nuclear positivity for the erythroblast transformation-specific transcription factor gene, ERG, can be demonstrated by EA. Irregular, smooth muscle actin-positive spindle cells are distributed around some of the vessels. The human herpesvirus 8 stain is negative.
Compared to classic angiosarcomas, EAs have a predilection for the extremities rather than the head and scalp. Histopathologically, the cells are epithelioid and are strongly positive for vimentin and CD31, in addition to factor VIII, friend leukemia integration 1 transcription factor, and CD34.1,2 In contrast, epithelioid sarcomas more typically are seen in younger adults and less likely to be CD31 positive.3 An epithelioid hemangioendothelioma is more focal in cellular atypia and forms small nests and trabeculae rather than sheets of atypical cells. Melanoma cells stain positive for human melanoma black 45, Melan-A, and S-100 but not for CD31.3 Glomangiosarcomas typically stain positive for smooth muscle actin and muscle-specific actin.4
Epithelioid angiosarcomas are rare and aggressive malignancies of endothelial origin.3 They are more prevalent in men and have a peak incidence in the seventh decade of life. They most commonly occur in the deep soft tissues of the extremities but have been reported to form in a variety of primary sites, including the skin, bones, thyroid, and adrenal glands.3
Tumors tend to be highly aggressive and demonstrate early nodal and solid organ metastases.3 Our case demonstrated the aggressive nature of this high-grade malignancy by showing neoplastic invasion through a vascular wall. Within 2 to 3 years of diagnosis, 50% of patients die of the disease, and the 5-year survival rate is estimated to be 12% to 20%.3,5 The etiology remains unknown, but EA has been linked to prior exposure to toxic chemicals, irradiation, or Thorotrast contrast media, and it may arise in the setting of arteriovenous fistulae and chronic lymphedema.6
Although radiation therapy often is utilized, surgery is the primary treatment modality.5 Even with wide excision, local recurrence is common. Tumor size is one of the most important prognostic features, with a worse prognosis for tumors larger than 5 cm. Evidence suggests that paclitaxel-based chemotherapeutic regimens may improve survival, and a combination of paclitaxel and sorafenib has been reported to induce remission in metastatic angiosarcoma of parietal EA.5 Currently, no standardized treatment regimen for this condition exists.
Acknowledgment
The authors thank Amanda Marsch, MD (Chicago, Illinois), for obtaining outside pathology consultation.
The Diagnosis: Epithelioid Angiosarcoma
Histopathology showed a large soft-tissue neoplasm with extensive hemorrhage (Figure 1). The epithelioid angiosarcoma (EA) consisted mostly of irregular slit-shaped vessels lined by sheets of atypical endothelial cells (Figure 2). At higher-power magnification, the cellular atypia was prominent and diffuse (Figure 3). Immunostaining of the tumor cells showed positive uptake for CD31, confirming vascular origin (Figure 4). Other vascular markers, including CD34 and factor VIII, as well as nuclear positivity for the erythroblast transformation-specific transcription factor gene, ERG, can be demonstrated by EA. Irregular, smooth muscle actin-positive spindle cells are distributed around some of the vessels. The human herpesvirus 8 stain is negative.
Compared to classic angiosarcomas, EAs have a predilection for the extremities rather than the head and scalp. Histopathologically, the cells are epithelioid and are strongly positive for vimentin and CD31, in addition to factor VIII, friend leukemia integration 1 transcription factor, and CD34.1,2 In contrast, epithelioid sarcomas more typically are seen in younger adults and less likely to be CD31 positive.3 An epithelioid hemangioendothelioma is more focal in cellular atypia and forms small nests and trabeculae rather than sheets of atypical cells. Melanoma cells stain positive for human melanoma black 45, Melan-A, and S-100 but not for CD31.3 Glomangiosarcomas typically stain positive for smooth muscle actin and muscle-specific actin.4
Epithelioid angiosarcomas are rare and aggressive malignancies of endothelial origin.3 They are more prevalent in men and have a peak incidence in the seventh decade of life. They most commonly occur in the deep soft tissues of the extremities but have been reported to form in a variety of primary sites, including the skin, bones, thyroid, and adrenal glands.3
Tumors tend to be highly aggressive and demonstrate early nodal and solid organ metastases.3 Our case demonstrated the aggressive nature of this high-grade malignancy by showing neoplastic invasion through a vascular wall. Within 2 to 3 years of diagnosis, 50% of patients die of the disease, and the 5-year survival rate is estimated to be 12% to 20%.3,5 The etiology remains unknown, but EA has been linked to prior exposure to toxic chemicals, irradiation, or Thorotrast contrast media, and it may arise in the setting of arteriovenous fistulae and chronic lymphedema.6
Although radiation therapy often is utilized, surgery is the primary treatment modality.5 Even with wide excision, local recurrence is common. Tumor size is one of the most important prognostic features, with a worse prognosis for tumors larger than 5 cm. Evidence suggests that paclitaxel-based chemotherapeutic regimens may improve survival, and a combination of paclitaxel and sorafenib has been reported to induce remission in metastatic angiosarcoma of parietal EA.5 Currently, no standardized treatment regimen for this condition exists.
Acknowledgment
The authors thank Amanda Marsch, MD (Chicago, Illinois), for obtaining outside pathology consultation.
- Suchak R, Thway K, Zelger B, et al. Primary cutaneous epithelioid angiosarcoma: a clinicopathologic study of 13 cases of a rare neoplasm occurring outside the setting of conventional angiosarcomas and with predilection for the limbs. Am J Surg Pathol. 2011;35:60-69.
- Prescott RJ, Banerjee SS, Eyden BP, et al. Cutaneous epithelioid angiosarcoma: a clinicopathological study of four cases. Histopathology. 1994;25:421-429.
- Hart J, Mandavilli S. Epithelioid angiosarcoma: a brief diagnostic review and differential diagnosis. Arch Pathol Lab Med. 2011;135:268-272.
- Maselli AM, Jambhekar AV, Hunter JG. Glomangiosarcoma arising from a prior biopsy site. Plast Reconstr Surg Glob Open. 2017;5:e1219.
- Donghi D, Dummer R, Cozzio A. Complete remission in a patient with multifocal metastatic cutaneous angiosarcoma with a combination of paclitaxel and sorafenib. Br J Dermatol. 2010;162:697-699.
- Wu J, Li X, Liu X. Epithelioid angiosarcoma: a clinicopathological study of 16 Chinese cases. Int J Clin Exp Pathol. 2015;8:3901-3909.
- Suchak R, Thway K, Zelger B, et al. Primary cutaneous epithelioid angiosarcoma: a clinicopathologic study of 13 cases of a rare neoplasm occurring outside the setting of conventional angiosarcomas and with predilection for the limbs. Am J Surg Pathol. 2011;35:60-69.
- Prescott RJ, Banerjee SS, Eyden BP, et al. Cutaneous epithelioid angiosarcoma: a clinicopathological study of four cases. Histopathology. 1994;25:421-429.
- Hart J, Mandavilli S. Epithelioid angiosarcoma: a brief diagnostic review and differential diagnosis. Arch Pathol Lab Med. 2011;135:268-272.
- Maselli AM, Jambhekar AV, Hunter JG. Glomangiosarcoma arising from a prior biopsy site. Plast Reconstr Surg Glob Open. 2017;5:e1219.
- Donghi D, Dummer R, Cozzio A. Complete remission in a patient with multifocal metastatic cutaneous angiosarcoma with a combination of paclitaxel and sorafenib. Br J Dermatol. 2010;162:697-699.
- Wu J, Li X, Liu X. Epithelioid angiosarcoma: a clinicopathological study of 16 Chinese cases. Int J Clin Exp Pathol. 2015;8:3901-3909.
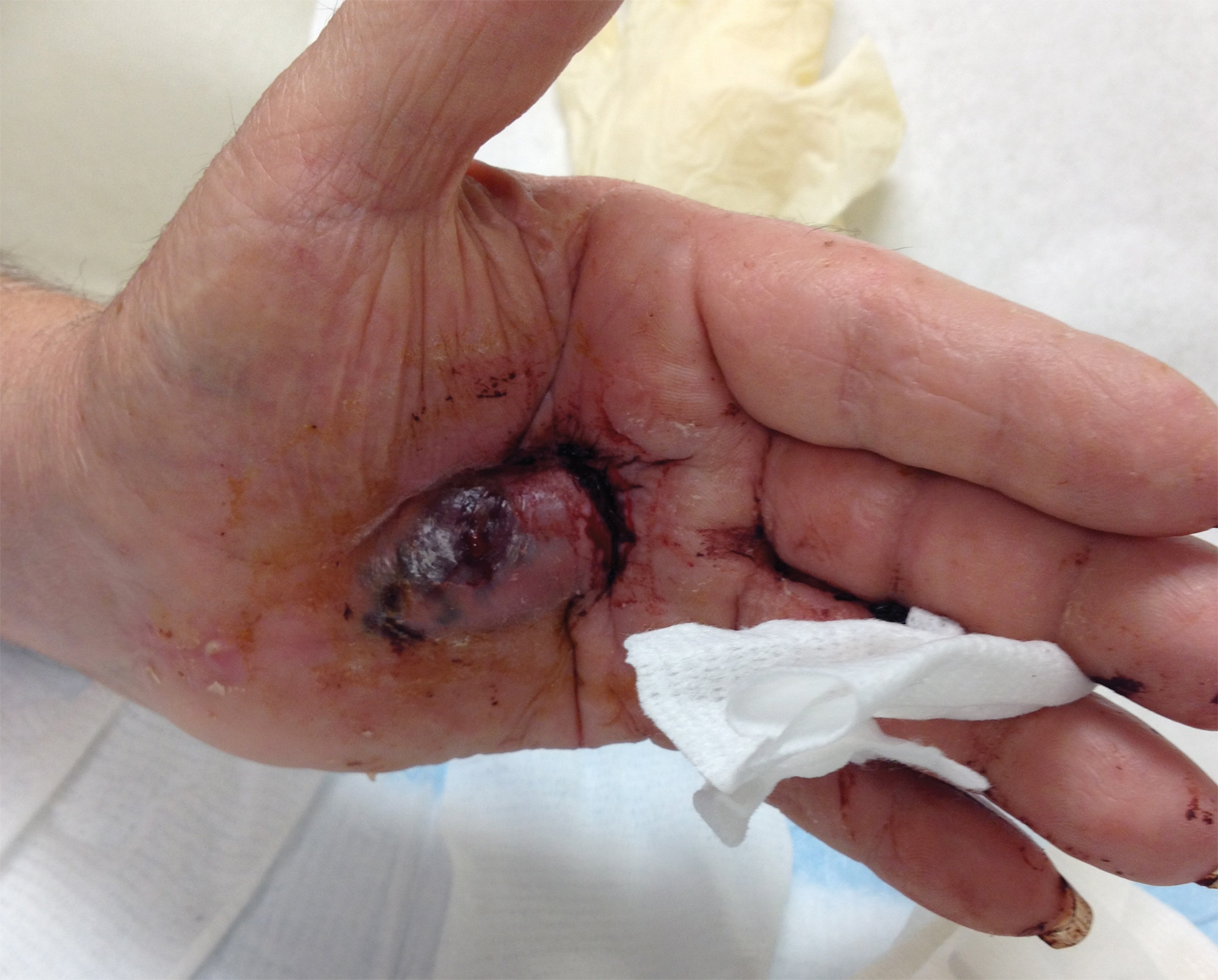
A 72-year-old man presented for evaluation of a mass on the left hand that continued to grow over the last few months and eventually bled. The patient first noticed a small firm lump on the palm approximately 1 year prior to presentation, and it was originally diagnosed as a Dupuytren contracture by his primary care physician. Months later, the lesion grew and began to bleed. Magnetic resonance imaging showed large hematomas of the hand with areas of nodular enhancement. The mass was located between the third and fourth proximal phalanges and abutted the extensor tendon. Complete excision yielded a definitive diagnosis.
ACC issues guidance on cardiac implications of coronavirus
The American College of Cardiology on Feb. 13, 2020, released a clinical bulletin that aims to address cardiac implications of the current epidemic of the novel coronavirus, now known as COVID-19.
The bulletin, reviewed and approved by the college’s Science and Quality Oversight Committee, “provides background on the epidemic, which was first reported in late December 2019, and looks at early cardiac implications from case reports,” the ACC noted in a press release. “It also provides information on the potential cardiac implications from analog viral respiratory pandemics and offers early clinical guidance given current COVID-19 uncertainty.”
The document looks at some early cardiac implications of the infection. For example, early case reports suggest patients with underlying conditions are at higher risk of complications or mortality from the virus, with up to 50% of hospitalized patients having a chronic medical illness, the authors wrote.
About 40% of hospitalized patients confirmed to have the virus have cardiovascular or cerebrovascular disease, they noted.
In a recent case report on 138 hospitalized COVID-19 patients, they noted, 19.6% developed acute respiratory distress syndrome, 16.7% developed arrhythmia, 8.7% developed shock, 7.2% developed acute cardiac injury, and 3.6% developed acute kidney injury. “Rates of complication were universally higher for ICU patients,” they wrote.
“The first reported death was a 61-year-old male, with a long history of smoking, who succumbed to acute respiratory distress, heart failure, and cardiac arrest,” the document noted. “Early, unpublished first-hand reports suggest at least some patients develop myocarditis.”
Stressing the current uncertainty about the virus, the bulletin provides the following clinical guidance:
- COVID-19 is spread through droplets and can live for substantial periods outside the body; containment and prevention using standard public health and personal strategies for preventing the spread of communicable disease remains the priority.
- In geographies with active COVID-19 transmission (mainly China), it is reasonable to advise patients with underlying cardiovascular disease of the potential increased risk and to encourage additional, reasonable precautions.
- Older adults are less likely to present with fever, thus close assessment for other symptoms such as cough or shortness of breath is warranted.
- Some experts have suggested that the rigorous use of guideline-directed, plaque-stabilizing agents could offer additional protection to cardiovascular disease (CVD) patients during a widespread outbreak (statins, beta-blockers, ACE inhibitors, acetylsalicylic acid); however, such therapies should be tailored to individual patients.
- It is important for patients with CVD to remain current with vaccinations, including the pneumococcal vaccine, given the increased risk of secondary bacterial infection; it would also be prudent to receive vaccination to prevent another source of fever which could be initially confused with coronavirus infection.
- It may be reasonable to triage COVID-19 patients according to the presence of underlying cardiovascular, respiratory, renal, and other chronic diseases for prioritized treatment.
- Providers are cautioned that classic symptoms and presentation of acute MI may be overshadowed in the context of coronavirus, resulting in underdiagnosis.
- For CVD patients in geographies without widespread COVID-19, emphasis should remain on the threat from influenza, the importance of vaccination and frequent handwashing, and continued adherence to all guideline-directed therapy for underlying chronic conditions.
- COVID-19 is a fast-moving epidemic with an uncertain clinical profile; providers should be prepared for guidance to shift as more information becomes available.
The full clinical update is available here.
This article first appeared on Medscape.com.
The American College of Cardiology on Feb. 13, 2020, released a clinical bulletin that aims to address cardiac implications of the current epidemic of the novel coronavirus, now known as COVID-19.
The bulletin, reviewed and approved by the college’s Science and Quality Oversight Committee, “provides background on the epidemic, which was first reported in late December 2019, and looks at early cardiac implications from case reports,” the ACC noted in a press release. “It also provides information on the potential cardiac implications from analog viral respiratory pandemics and offers early clinical guidance given current COVID-19 uncertainty.”
The document looks at some early cardiac implications of the infection. For example, early case reports suggest patients with underlying conditions are at higher risk of complications or mortality from the virus, with up to 50% of hospitalized patients having a chronic medical illness, the authors wrote.
About 40% of hospitalized patients confirmed to have the virus have cardiovascular or cerebrovascular disease, they noted.
In a recent case report on 138 hospitalized COVID-19 patients, they noted, 19.6% developed acute respiratory distress syndrome, 16.7% developed arrhythmia, 8.7% developed shock, 7.2% developed acute cardiac injury, and 3.6% developed acute kidney injury. “Rates of complication were universally higher for ICU patients,” they wrote.
“The first reported death was a 61-year-old male, with a long history of smoking, who succumbed to acute respiratory distress, heart failure, and cardiac arrest,” the document noted. “Early, unpublished first-hand reports suggest at least some patients develop myocarditis.”
Stressing the current uncertainty about the virus, the bulletin provides the following clinical guidance:
- COVID-19 is spread through droplets and can live for substantial periods outside the body; containment and prevention using standard public health and personal strategies for preventing the spread of communicable disease remains the priority.
- In geographies with active COVID-19 transmission (mainly China), it is reasonable to advise patients with underlying cardiovascular disease of the potential increased risk and to encourage additional, reasonable precautions.
- Older adults are less likely to present with fever, thus close assessment for other symptoms such as cough or shortness of breath is warranted.
- Some experts have suggested that the rigorous use of guideline-directed, plaque-stabilizing agents could offer additional protection to cardiovascular disease (CVD) patients during a widespread outbreak (statins, beta-blockers, ACE inhibitors, acetylsalicylic acid); however, such therapies should be tailored to individual patients.
- It is important for patients with CVD to remain current with vaccinations, including the pneumococcal vaccine, given the increased risk of secondary bacterial infection; it would also be prudent to receive vaccination to prevent another source of fever which could be initially confused with coronavirus infection.
- It may be reasonable to triage COVID-19 patients according to the presence of underlying cardiovascular, respiratory, renal, and other chronic diseases for prioritized treatment.
- Providers are cautioned that classic symptoms and presentation of acute MI may be overshadowed in the context of coronavirus, resulting in underdiagnosis.
- For CVD patients in geographies without widespread COVID-19, emphasis should remain on the threat from influenza, the importance of vaccination and frequent handwashing, and continued adherence to all guideline-directed therapy for underlying chronic conditions.
- COVID-19 is a fast-moving epidemic with an uncertain clinical profile; providers should be prepared for guidance to shift as more information becomes available.
The full clinical update is available here.
This article first appeared on Medscape.com.
The American College of Cardiology on Feb. 13, 2020, released a clinical bulletin that aims to address cardiac implications of the current epidemic of the novel coronavirus, now known as COVID-19.
The bulletin, reviewed and approved by the college’s Science and Quality Oversight Committee, “provides background on the epidemic, which was first reported in late December 2019, and looks at early cardiac implications from case reports,” the ACC noted in a press release. “It also provides information on the potential cardiac implications from analog viral respiratory pandemics and offers early clinical guidance given current COVID-19 uncertainty.”
The document looks at some early cardiac implications of the infection. For example, early case reports suggest patients with underlying conditions are at higher risk of complications or mortality from the virus, with up to 50% of hospitalized patients having a chronic medical illness, the authors wrote.
About 40% of hospitalized patients confirmed to have the virus have cardiovascular or cerebrovascular disease, they noted.
In a recent case report on 138 hospitalized COVID-19 patients, they noted, 19.6% developed acute respiratory distress syndrome, 16.7% developed arrhythmia, 8.7% developed shock, 7.2% developed acute cardiac injury, and 3.6% developed acute kidney injury. “Rates of complication were universally higher for ICU patients,” they wrote.
“The first reported death was a 61-year-old male, with a long history of smoking, who succumbed to acute respiratory distress, heart failure, and cardiac arrest,” the document noted. “Early, unpublished first-hand reports suggest at least some patients develop myocarditis.”
Stressing the current uncertainty about the virus, the bulletin provides the following clinical guidance:
- COVID-19 is spread through droplets and can live for substantial periods outside the body; containment and prevention using standard public health and personal strategies for preventing the spread of communicable disease remains the priority.
- In geographies with active COVID-19 transmission (mainly China), it is reasonable to advise patients with underlying cardiovascular disease of the potential increased risk and to encourage additional, reasonable precautions.
- Older adults are less likely to present with fever, thus close assessment for other symptoms such as cough or shortness of breath is warranted.
- Some experts have suggested that the rigorous use of guideline-directed, plaque-stabilizing agents could offer additional protection to cardiovascular disease (CVD) patients during a widespread outbreak (statins, beta-blockers, ACE inhibitors, acetylsalicylic acid); however, such therapies should be tailored to individual patients.
- It is important for patients with CVD to remain current with vaccinations, including the pneumococcal vaccine, given the increased risk of secondary bacterial infection; it would also be prudent to receive vaccination to prevent another source of fever which could be initially confused with coronavirus infection.
- It may be reasonable to triage COVID-19 patients according to the presence of underlying cardiovascular, respiratory, renal, and other chronic diseases for prioritized treatment.
- Providers are cautioned that classic symptoms and presentation of acute MI may be overshadowed in the context of coronavirus, resulting in underdiagnosis.
- For CVD patients in geographies without widespread COVID-19, emphasis should remain on the threat from influenza, the importance of vaccination and frequent handwashing, and continued adherence to all guideline-directed therapy for underlying chronic conditions.
- COVID-19 is a fast-moving epidemic with an uncertain clinical profile; providers should be prepared for guidance to shift as more information becomes available.
The full clinical update is available here.
This article first appeared on Medscape.com.
Resetting your compensation
Using the State of Hospital Medicine Report to bolster your proposal
In the ever-changing world of health care, one thing is for sure: If you’re not paying attention, you’re falling behind. In this column, I will discuss how you may utilize the Society of Hospital Medicine (SHM) State of Hospital Medicine Report (SoHM) to evaluate your current compensation structure and strengthen your business plan for change. For purposes of this exercise, I will focus on data referenced as Internal Medicine only, hospital-owned Hospital Medicine Groups (HMG).
Issues with retention, recruitment, or burnout may be among the first factors that lead you to reevaluate your compensation plan. The SoHM Report can help you to take a dive into feedback for these areas. Look for any indications that compensation may be affecting your turnover, inability to hire, or leaving your current team frustrated with their current pay structure. Feedback surrounding each of these factors may drive you to evaluate your comp plan but remain mindful that money is not always the answer.
You may complete your evaluation and find the data could suggest you are in fact well paid for the work you do. Even though this may be the case, the evaluation and transparency to your provider team may help flush out the real reason you are struggling with recruitment, retention, or burnout. However, if you do find you have an opportunity to improve your compensation structure, remember that you will need a compelling, data-driven case, to present to your C-suite.
Let us start by understanding how the market has changed over time using data from the 2014, 2016, and 2018 SoHM Reports. Of note, each report is based on data from the prior year. Since 2013, hospital owned HMGs have seen a 16% increase in total compensation while experiencing only a 9% increase in collections. Meanwhile RVU productivity has remained relatively stable over time. From this, we see hospitalists are earning more for similar productivity. The hospital reimbursement for professional fees has not grown at the same rate as compensation. Also, the collection per RVU has remained relatively flat over time.
It’s simple: Hospitalists are earning more and professional revenues are not making up the difference. This market change is driving hospitals to invest more money to maintain their HMGs. If your hospital hasn’t been responding to these data, you will need a strong business plan to get buy in from your hospital administration.
Now that you have evaluated the market change, it is time to put some optics on where your compensation falls in the current market. When you combine your total compensation with your total RVU productivity, you can use the SoHM Report to evaluate the current reported benchmarks for Compensation per RVU. Plotting these benchmarks against your own compensation and any proposed changes can help your administration really begin to see whether a change should be considered. Providing that clear picture in relevance to the SoHM benchmark is important, as a chart or graph can simplify your C-suite’s understanding of your proposal.
By simplifying your example using Compensation per RVU, you are making the conversation easier to follow. Your hospital leaders can clearly see the cost for every RVU generated and understand the impact. This is not to say that you should base your compensation around productivity. It is merely a way to roll in all compensation factors, whether quality related, performance based, or productivity driven, and tie them to a metric that is clear and easy for administration to understand.
Remember, when designing your new compensation plan, you can reference the SoHM Report to see how HMGs around the country are providing incentive and what percentage of compensation is based on incentive. There are sections within the report directly outlining these data points.
Now that we have reviewed market change and how to visualize change between your current and proposed future state, I will leave you with some final thoughts regarding other considerations when building your business plan:
- Focus on only physician-generated RVUs.
- Consider Length of Stay impact on productivity.
- Decide if Case Mix Index changes have impacted your staffing needs.
- Understand your E and M coding practices in reference to industry benchmarks. The SoHM Report provides benchmarks for billing practices across the country.
- Lastly, clearly identify the issues you want to address and set goals with measurable outcomes.
There is still time for your group to be part of the 2020 State of Hospital Medicine Report data by participating in the 2020 Survey. Data are being accepted through Feb. 28, 2020. Submit your data at www.hospitalmedicine.org/2020survey.
Mr. Sandroni is director of operations, hospitalists, at Rochester (N.Y.) Regional Health.
Using the State of Hospital Medicine Report to bolster your proposal
Using the State of Hospital Medicine Report to bolster your proposal
In the ever-changing world of health care, one thing is for sure: If you’re not paying attention, you’re falling behind. In this column, I will discuss how you may utilize the Society of Hospital Medicine (SHM) State of Hospital Medicine Report (SoHM) to evaluate your current compensation structure and strengthen your business plan for change. For purposes of this exercise, I will focus on data referenced as Internal Medicine only, hospital-owned Hospital Medicine Groups (HMG).
Issues with retention, recruitment, or burnout may be among the first factors that lead you to reevaluate your compensation plan. The SoHM Report can help you to take a dive into feedback for these areas. Look for any indications that compensation may be affecting your turnover, inability to hire, or leaving your current team frustrated with their current pay structure. Feedback surrounding each of these factors may drive you to evaluate your comp plan but remain mindful that money is not always the answer.
You may complete your evaluation and find the data could suggest you are in fact well paid for the work you do. Even though this may be the case, the evaluation and transparency to your provider team may help flush out the real reason you are struggling with recruitment, retention, or burnout. However, if you do find you have an opportunity to improve your compensation structure, remember that you will need a compelling, data-driven case, to present to your C-suite.
Let us start by understanding how the market has changed over time using data from the 2014, 2016, and 2018 SoHM Reports. Of note, each report is based on data from the prior year. Since 2013, hospital owned HMGs have seen a 16% increase in total compensation while experiencing only a 9% increase in collections. Meanwhile RVU productivity has remained relatively stable over time. From this, we see hospitalists are earning more for similar productivity. The hospital reimbursement for professional fees has not grown at the same rate as compensation. Also, the collection per RVU has remained relatively flat over time.
It’s simple: Hospitalists are earning more and professional revenues are not making up the difference. This market change is driving hospitals to invest more money to maintain their HMGs. If your hospital hasn’t been responding to these data, you will need a strong business plan to get buy in from your hospital administration.
Now that you have evaluated the market change, it is time to put some optics on where your compensation falls in the current market. When you combine your total compensation with your total RVU productivity, you can use the SoHM Report to evaluate the current reported benchmarks for Compensation per RVU. Plotting these benchmarks against your own compensation and any proposed changes can help your administration really begin to see whether a change should be considered. Providing that clear picture in relevance to the SoHM benchmark is important, as a chart or graph can simplify your C-suite’s understanding of your proposal.
By simplifying your example using Compensation per RVU, you are making the conversation easier to follow. Your hospital leaders can clearly see the cost for every RVU generated and understand the impact. This is not to say that you should base your compensation around productivity. It is merely a way to roll in all compensation factors, whether quality related, performance based, or productivity driven, and tie them to a metric that is clear and easy for administration to understand.
Remember, when designing your new compensation plan, you can reference the SoHM Report to see how HMGs around the country are providing incentive and what percentage of compensation is based on incentive. There are sections within the report directly outlining these data points.
Now that we have reviewed market change and how to visualize change between your current and proposed future state, I will leave you with some final thoughts regarding other considerations when building your business plan:
- Focus on only physician-generated RVUs.
- Consider Length of Stay impact on productivity.
- Decide if Case Mix Index changes have impacted your staffing needs.
- Understand your E and M coding practices in reference to industry benchmarks. The SoHM Report provides benchmarks for billing practices across the country.
- Lastly, clearly identify the issues you want to address and set goals with measurable outcomes.
There is still time for your group to be part of the 2020 State of Hospital Medicine Report data by participating in the 2020 Survey. Data are being accepted through Feb. 28, 2020. Submit your data at www.hospitalmedicine.org/2020survey.
Mr. Sandroni is director of operations, hospitalists, at Rochester (N.Y.) Regional Health.
In the ever-changing world of health care, one thing is for sure: If you’re not paying attention, you’re falling behind. In this column, I will discuss how you may utilize the Society of Hospital Medicine (SHM) State of Hospital Medicine Report (SoHM) to evaluate your current compensation structure and strengthen your business plan for change. For purposes of this exercise, I will focus on data referenced as Internal Medicine only, hospital-owned Hospital Medicine Groups (HMG).
Issues with retention, recruitment, or burnout may be among the first factors that lead you to reevaluate your compensation plan. The SoHM Report can help you to take a dive into feedback for these areas. Look for any indications that compensation may be affecting your turnover, inability to hire, or leaving your current team frustrated with their current pay structure. Feedback surrounding each of these factors may drive you to evaluate your comp plan but remain mindful that money is not always the answer.
You may complete your evaluation and find the data could suggest you are in fact well paid for the work you do. Even though this may be the case, the evaluation and transparency to your provider team may help flush out the real reason you are struggling with recruitment, retention, or burnout. However, if you do find you have an opportunity to improve your compensation structure, remember that you will need a compelling, data-driven case, to present to your C-suite.
Let us start by understanding how the market has changed over time using data from the 2014, 2016, and 2018 SoHM Reports. Of note, each report is based on data from the prior year. Since 2013, hospital owned HMGs have seen a 16% increase in total compensation while experiencing only a 9% increase in collections. Meanwhile RVU productivity has remained relatively stable over time. From this, we see hospitalists are earning more for similar productivity. The hospital reimbursement for professional fees has not grown at the same rate as compensation. Also, the collection per RVU has remained relatively flat over time.
It’s simple: Hospitalists are earning more and professional revenues are not making up the difference. This market change is driving hospitals to invest more money to maintain their HMGs. If your hospital hasn’t been responding to these data, you will need a strong business plan to get buy in from your hospital administration.
Now that you have evaluated the market change, it is time to put some optics on where your compensation falls in the current market. When you combine your total compensation with your total RVU productivity, you can use the SoHM Report to evaluate the current reported benchmarks for Compensation per RVU. Plotting these benchmarks against your own compensation and any proposed changes can help your administration really begin to see whether a change should be considered. Providing that clear picture in relevance to the SoHM benchmark is important, as a chart or graph can simplify your C-suite’s understanding of your proposal.
By simplifying your example using Compensation per RVU, you are making the conversation easier to follow. Your hospital leaders can clearly see the cost for every RVU generated and understand the impact. This is not to say that you should base your compensation around productivity. It is merely a way to roll in all compensation factors, whether quality related, performance based, or productivity driven, and tie them to a metric that is clear and easy for administration to understand.
Remember, when designing your new compensation plan, you can reference the SoHM Report to see how HMGs around the country are providing incentive and what percentage of compensation is based on incentive. There are sections within the report directly outlining these data points.
Now that we have reviewed market change and how to visualize change between your current and proposed future state, I will leave you with some final thoughts regarding other considerations when building your business plan:
- Focus on only physician-generated RVUs.
- Consider Length of Stay impact on productivity.
- Decide if Case Mix Index changes have impacted your staffing needs.
- Understand your E and M coding practices in reference to industry benchmarks. The SoHM Report provides benchmarks for billing practices across the country.
- Lastly, clearly identify the issues you want to address and set goals with measurable outcomes.
There is still time for your group to be part of the 2020 State of Hospital Medicine Report data by participating in the 2020 Survey. Data are being accepted through Feb. 28, 2020. Submit your data at www.hospitalmedicine.org/2020survey.
Mr. Sandroni is director of operations, hospitalists, at Rochester (N.Y.) Regional Health.