User login
As novel coronavirus outbreak evolves, critical care providers need to be prepared
ORLANDO – While the impact of the 2019 novel coronavirus outbreak on hospitals outside of China remains to be determined, there are several practical points critical care professionals need to know to be prepared in the face of this dynamic and rapidly evolving outbreak, speakers said at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
“Priorities for us in our hospitals are early detection, infection prevention, staff safety, and obviously, taking care of sick people,” said Ryan C. Maves, MD, of the Naval Medical Center San Diego in a special session on the 2019 Novel Coronavirus outbreak.*
Approximately 72,000 cases of coronavirus disease 2019 (COVID-19) had been reported as of Feb. 17, 2020, the day of Dr. Maves’ talk, according to statistics from Johns Hopkins Center for Science and Engineering in Baltimore. A total of 1,775 deaths had been recorded, nearly all of which were in Hubei Province, the central point of the outbreak. In the United States, the number of cases stood at 15, with no deaths reported.
While the dynamics of the 2019 novel coronavirus are still being learned, the estimated range of spread for droplet transmission is 2 meters, according to Dr. Maves. The duration of environmental persistence is not yet known, but he said that other coronaviruses persist in low-humidity conditions for up to 4 days.
The number of secondary cases that arise from a primary infection, or R0, is estimated to be between 1.5 and 3, though it can change as exposure evolves; by comparison, the R0 for H1N1 influenza has been reported as 1.5, while measles is 12-18, indicating that it is “very contagious,” said Dr. Maves. Severe acute respiratory syndrome had an initial R0 of about 3.5, which he said declined rapidly to 0.7 as environmental and policy controls were put into place.
Critical care professionals need to know how to identify patients at risk of having COVID-19 and determine whether they need further work-up, according to Dr. Maves, who highlighted recent criteria released by the Centers for Disease Control and Prevention.
The highest-risk category, he said, are individuals exposed to a laboratory-confirmed coronavirus case, which along with fever or signs and symptoms of a lower respiratory illness would be sufficient to classify them as a “person of interest” requiring further evaluation for disease. A history of travel from Hubei Province plus fever and signs/symptoms of lower respiratory illness would also meet criteria for evaluation, according to the CDC, while travel to mainland China would also meet the threshold, if those symptoms required hospitalization.
The CDC also published a step-wise flowchart to evaluate patients who may have been exposed to the 2019 novel coronavirus. According to that flowchart, if an individual has traveled to China or had close contact with someone infected with the 2019 Novel Coronavirus within 14 days of symptoms, and that individual has fever or symptoms of lower respiratory illness such as cough or shortness of breath, then providers should isolate that individual and assess clinical status, in addition to contacting the local health department.
Laura E. Evans, MD, MS, FCCM, of New York University, said she might recommend providers “flip the script” on that CDC algorithm when it comes to identifying patients who may have been exposed.
“I think perhaps what we should be doing at sites of entry is not talking about travel as the first question, but rather fever or symptoms of lower respiratory illnesses as the first question, and use that as the opportunity to implement risk mitigation at that stage,” Dr. Evans said in a presentation on preparing for COVID-19.
Even with “substantial uncertainty” about the potential impact of the 2019 Novel Coronavirus, a significant influx of seriously ill patients would put strain the U.S. health care delivery system, she added.
“None of us have tons of extra capacity in our emergency departments, inpatient units, or ICUs, and I think we need to be prepared for that,” she added. “We need to know what our process is for ‘identify, isolate, and inform,’ and we need to be testing that now.”
Dr. Maves and Dr. Evans both reported that they had no financial conflicts of interest to report. Dr. Maves indicated that the views expressed in his presentation did not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, or the United States government.
*Correction, 2/19/20: An earlier version of this article misstated the location of the naval center.
ORLANDO – While the impact of the 2019 novel coronavirus outbreak on hospitals outside of China remains to be determined, there are several practical points critical care professionals need to know to be prepared in the face of this dynamic and rapidly evolving outbreak, speakers said at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
“Priorities for us in our hospitals are early detection, infection prevention, staff safety, and obviously, taking care of sick people,” said Ryan C. Maves, MD, of the Naval Medical Center San Diego in a special session on the 2019 Novel Coronavirus outbreak.*
Approximately 72,000 cases of coronavirus disease 2019 (COVID-19) had been reported as of Feb. 17, 2020, the day of Dr. Maves’ talk, according to statistics from Johns Hopkins Center for Science and Engineering in Baltimore. A total of 1,775 deaths had been recorded, nearly all of which were in Hubei Province, the central point of the outbreak. In the United States, the number of cases stood at 15, with no deaths reported.
While the dynamics of the 2019 novel coronavirus are still being learned, the estimated range of spread for droplet transmission is 2 meters, according to Dr. Maves. The duration of environmental persistence is not yet known, but he said that other coronaviruses persist in low-humidity conditions for up to 4 days.
The number of secondary cases that arise from a primary infection, or R0, is estimated to be between 1.5 and 3, though it can change as exposure evolves; by comparison, the R0 for H1N1 influenza has been reported as 1.5, while measles is 12-18, indicating that it is “very contagious,” said Dr. Maves. Severe acute respiratory syndrome had an initial R0 of about 3.5, which he said declined rapidly to 0.7 as environmental and policy controls were put into place.
Critical care professionals need to know how to identify patients at risk of having COVID-19 and determine whether they need further work-up, according to Dr. Maves, who highlighted recent criteria released by the Centers for Disease Control and Prevention.
The highest-risk category, he said, are individuals exposed to a laboratory-confirmed coronavirus case, which along with fever or signs and symptoms of a lower respiratory illness would be sufficient to classify them as a “person of interest” requiring further evaluation for disease. A history of travel from Hubei Province plus fever and signs/symptoms of lower respiratory illness would also meet criteria for evaluation, according to the CDC, while travel to mainland China would also meet the threshold, if those symptoms required hospitalization.
The CDC also published a step-wise flowchart to evaluate patients who may have been exposed to the 2019 novel coronavirus. According to that flowchart, if an individual has traveled to China or had close contact with someone infected with the 2019 Novel Coronavirus within 14 days of symptoms, and that individual has fever or symptoms of lower respiratory illness such as cough or shortness of breath, then providers should isolate that individual and assess clinical status, in addition to contacting the local health department.
Laura E. Evans, MD, MS, FCCM, of New York University, said she might recommend providers “flip the script” on that CDC algorithm when it comes to identifying patients who may have been exposed.
“I think perhaps what we should be doing at sites of entry is not talking about travel as the first question, but rather fever or symptoms of lower respiratory illnesses as the first question, and use that as the opportunity to implement risk mitigation at that stage,” Dr. Evans said in a presentation on preparing for COVID-19.
Even with “substantial uncertainty” about the potential impact of the 2019 Novel Coronavirus, a significant influx of seriously ill patients would put strain the U.S. health care delivery system, she added.
“None of us have tons of extra capacity in our emergency departments, inpatient units, or ICUs, and I think we need to be prepared for that,” she added. “We need to know what our process is for ‘identify, isolate, and inform,’ and we need to be testing that now.”
Dr. Maves and Dr. Evans both reported that they had no financial conflicts of interest to report. Dr. Maves indicated that the views expressed in his presentation did not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, or the United States government.
*Correction, 2/19/20: An earlier version of this article misstated the location of the naval center.
ORLANDO – While the impact of the 2019 novel coronavirus outbreak on hospitals outside of China remains to be determined, there are several practical points critical care professionals need to know to be prepared in the face of this dynamic and rapidly evolving outbreak, speakers said at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
“Priorities for us in our hospitals are early detection, infection prevention, staff safety, and obviously, taking care of sick people,” said Ryan C. Maves, MD, of the Naval Medical Center San Diego in a special session on the 2019 Novel Coronavirus outbreak.*
Approximately 72,000 cases of coronavirus disease 2019 (COVID-19) had been reported as of Feb. 17, 2020, the day of Dr. Maves’ talk, according to statistics from Johns Hopkins Center for Science and Engineering in Baltimore. A total of 1,775 deaths had been recorded, nearly all of which were in Hubei Province, the central point of the outbreak. In the United States, the number of cases stood at 15, with no deaths reported.
While the dynamics of the 2019 novel coronavirus are still being learned, the estimated range of spread for droplet transmission is 2 meters, according to Dr. Maves. The duration of environmental persistence is not yet known, but he said that other coronaviruses persist in low-humidity conditions for up to 4 days.
The number of secondary cases that arise from a primary infection, or R0, is estimated to be between 1.5 and 3, though it can change as exposure evolves; by comparison, the R0 for H1N1 influenza has been reported as 1.5, while measles is 12-18, indicating that it is “very contagious,” said Dr. Maves. Severe acute respiratory syndrome had an initial R0 of about 3.5, which he said declined rapidly to 0.7 as environmental and policy controls were put into place.
Critical care professionals need to know how to identify patients at risk of having COVID-19 and determine whether they need further work-up, according to Dr. Maves, who highlighted recent criteria released by the Centers for Disease Control and Prevention.
The highest-risk category, he said, are individuals exposed to a laboratory-confirmed coronavirus case, which along with fever or signs and symptoms of a lower respiratory illness would be sufficient to classify them as a “person of interest” requiring further evaluation for disease. A history of travel from Hubei Province plus fever and signs/symptoms of lower respiratory illness would also meet criteria for evaluation, according to the CDC, while travel to mainland China would also meet the threshold, if those symptoms required hospitalization.
The CDC also published a step-wise flowchart to evaluate patients who may have been exposed to the 2019 novel coronavirus. According to that flowchart, if an individual has traveled to China or had close contact with someone infected with the 2019 Novel Coronavirus within 14 days of symptoms, and that individual has fever or symptoms of lower respiratory illness such as cough or shortness of breath, then providers should isolate that individual and assess clinical status, in addition to contacting the local health department.
Laura E. Evans, MD, MS, FCCM, of New York University, said she might recommend providers “flip the script” on that CDC algorithm when it comes to identifying patients who may have been exposed.
“I think perhaps what we should be doing at sites of entry is not talking about travel as the first question, but rather fever or symptoms of lower respiratory illnesses as the first question, and use that as the opportunity to implement risk mitigation at that stage,” Dr. Evans said in a presentation on preparing for COVID-19.
Even with “substantial uncertainty” about the potential impact of the 2019 Novel Coronavirus, a significant influx of seriously ill patients would put strain the U.S. health care delivery system, she added.
“None of us have tons of extra capacity in our emergency departments, inpatient units, or ICUs, and I think we need to be prepared for that,” she added. “We need to know what our process is for ‘identify, isolate, and inform,’ and we need to be testing that now.”
Dr. Maves and Dr. Evans both reported that they had no financial conflicts of interest to report. Dr. Maves indicated that the views expressed in his presentation did not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, or the United States government.
*Correction, 2/19/20: An earlier version of this article misstated the location of the naval center.
EXPERT ANALYSIS FROM CCC49
Low-dose methotrexate trial pins down adverse event rates
A new study has found an elevated risk of some adverse events in patients treated with low-dose methotrexate, compared with patients treated with placebo.
“The data presented here provide an important source of new evidence to improve the monitoring guidelines and safe prescribing of LD-MTX [low-dose methotrexate],” wrote Daniel H. Solomon, MD, of Brigham and Women’s Hospital and Harvard Medical School in Boston, and coauthors. The study was published in Annals of Internal Medicine.
To determine the rates of adverse events (AEs) among LD-MTX users, along with assessing the risks of certain predefined AEs, the researchers enrolled 6,158 patients in the Cardiovascular Inflammation Reduction Trial (CIRT) and randomized 4,786 of those patients to two groups: those receiving LD-MTX (n = 2,391) and those receiving placebo (n = 2,395). The median dose was 15 mg per week, and median follow-up was 23 months. All participants in CIRT had a history of cardiovascular disease, along with diabetes or metabolic syndrome. Just over 81% of the participants were male, and nearly 85% were white. Their median age was nearly 66 years.
Of the participants in the LD-MTX group, 2,156 (90.2%) had an AE and 2,080 (87.0%) had an AE of interest, which included infectious, hematologic, pulmonary, hepatic, cancerous, and gastrointestinal AEs. Of the participants in the placebo group, 2,076 (86.7%) had an AE and 1,951 (81.5%) had an AE of interest. As such, the relative rate of an AE of interest was 17% higher in the LD-MTX group (hazard ratio, 1.17; 95% confidence interval, 1.10-1.25).
In regard to specific types of AEs, the rates of gastrointestinal (HR, 1.23; 95% CI, 1.03-1.47), pulmonary (HR, 1.42; 95% CI, 1.14-1.77), infectious (HR, 1.15; 95% CI, 1.01-1.30) and hematologic (HR, 1.22; 95% CI, 1.11-1.34) were higher for participants in the LD-MTX group. Five cases of cirrhosis were found in the LD-MTX group, compared with none in the placebo group; none of the patients with cirrhosis had severe liver test abnormalities before their diagnosis. While the risk of cancer overall was not elevated in the LD-MTX group, 53 participants in that group developed skin cancer, compared with 26 in the placebo group (HR, 2.04; 95% CI, 1.28-3.26). Renal AEs were among the few that decreased in LD-MTX users (HR, 0.85; 95% CI, 0.78-0.93).
“Methotrexate has become the standard of care for RA patients,” Dr. Solomon said in an interview, “and because it worked so well, we accepted it without large placebo-controlled trials and without a precise understanding of the risk factors for AEs. Until this study, our evidence basis for the side-effect profile was relatively weak.
“We had a limited data set but decades of experience,” he added. “Now we have better evidence, for example, that methotrexate is associated with elevations in liver function tests. We even found five cases of cirrhosis. And the people who developed cirrhosis didn’t have severe test abnormalities; just minor ones over many months. So now we have a better understanding of the potential impact of minor, yet chronic abnormalities.”
Dr. Solomon and coauthors acknowledged their study’s limitations, including CIRT not including patients with systemic rheumatic disease and the possibility that participants did not report AEs that occurred in between routine study visits. In addition, although the median follow-up of nearly 2 years was longer than in other LD-MTX trials, they noted that “it may still be too short to observe some AEs that require long-term exposure.”
Dr. Solomon and colleagues should be commended for undertaking a long-awaited randomized, placebo-controlled trial that adds much-needed insight into how and when to monitor patients being treated with MTX, Vivian P. Bykerk, MD, of the Hospital for Special Surgery and Weill Cornell Medical College in New York, wrote in an editorial (Ann Intern Med. 2020 Feb 17. doi: 10.7326/M20-0435).
Dr. Bykerk noted that although the results may not be applicable to patients with RA and other inflammatory arthritides who are treated with MTX – RA patients in particular are younger, more often female, have lower rates of diabetes, and usually receive higher doses than those used in CIRT — the risk estimates from the CIRT study are “largely congruent with those expected in MTX-treated patients with rheumatic diseases.”
Regardless, she emphasized that this is a step in a much-needed direction, reminding physicians that “MTX use has inherent risks” and that its AEs, although infrequent, are clinically serious.
The National Institutes of Health funded the study. Various authors reported receiving grants from the National Heart, Lung, and Blood Institute, along with grants, research support, and personal fees from numerous pharmaceutical companies before and during the study. Dr. Bykerk reported receiving personal fees, grants, and nonfinancial support from pharmaceutical companies, foundations, and the NIH.
SOURCE: Solomon DH et al. Ann Intern Med. 2020 Feb 17. doi: 10.7326/M19-3369.
A new study has found an elevated risk of some adverse events in patients treated with low-dose methotrexate, compared with patients treated with placebo.
“The data presented here provide an important source of new evidence to improve the monitoring guidelines and safe prescribing of LD-MTX [low-dose methotrexate],” wrote Daniel H. Solomon, MD, of Brigham and Women’s Hospital and Harvard Medical School in Boston, and coauthors. The study was published in Annals of Internal Medicine.
To determine the rates of adverse events (AEs) among LD-MTX users, along with assessing the risks of certain predefined AEs, the researchers enrolled 6,158 patients in the Cardiovascular Inflammation Reduction Trial (CIRT) and randomized 4,786 of those patients to two groups: those receiving LD-MTX (n = 2,391) and those receiving placebo (n = 2,395). The median dose was 15 mg per week, and median follow-up was 23 months. All participants in CIRT had a history of cardiovascular disease, along with diabetes or metabolic syndrome. Just over 81% of the participants were male, and nearly 85% were white. Their median age was nearly 66 years.
Of the participants in the LD-MTX group, 2,156 (90.2%) had an AE and 2,080 (87.0%) had an AE of interest, which included infectious, hematologic, pulmonary, hepatic, cancerous, and gastrointestinal AEs. Of the participants in the placebo group, 2,076 (86.7%) had an AE and 1,951 (81.5%) had an AE of interest. As such, the relative rate of an AE of interest was 17% higher in the LD-MTX group (hazard ratio, 1.17; 95% confidence interval, 1.10-1.25).
In regard to specific types of AEs, the rates of gastrointestinal (HR, 1.23; 95% CI, 1.03-1.47), pulmonary (HR, 1.42; 95% CI, 1.14-1.77), infectious (HR, 1.15; 95% CI, 1.01-1.30) and hematologic (HR, 1.22; 95% CI, 1.11-1.34) were higher for participants in the LD-MTX group. Five cases of cirrhosis were found in the LD-MTX group, compared with none in the placebo group; none of the patients with cirrhosis had severe liver test abnormalities before their diagnosis. While the risk of cancer overall was not elevated in the LD-MTX group, 53 participants in that group developed skin cancer, compared with 26 in the placebo group (HR, 2.04; 95% CI, 1.28-3.26). Renal AEs were among the few that decreased in LD-MTX users (HR, 0.85; 95% CI, 0.78-0.93).
“Methotrexate has become the standard of care for RA patients,” Dr. Solomon said in an interview, “and because it worked so well, we accepted it without large placebo-controlled trials and without a precise understanding of the risk factors for AEs. Until this study, our evidence basis for the side-effect profile was relatively weak.
“We had a limited data set but decades of experience,” he added. “Now we have better evidence, for example, that methotrexate is associated with elevations in liver function tests. We even found five cases of cirrhosis. And the people who developed cirrhosis didn’t have severe test abnormalities; just minor ones over many months. So now we have a better understanding of the potential impact of minor, yet chronic abnormalities.”
Dr. Solomon and coauthors acknowledged their study’s limitations, including CIRT not including patients with systemic rheumatic disease and the possibility that participants did not report AEs that occurred in between routine study visits. In addition, although the median follow-up of nearly 2 years was longer than in other LD-MTX trials, they noted that “it may still be too short to observe some AEs that require long-term exposure.”
Dr. Solomon and colleagues should be commended for undertaking a long-awaited randomized, placebo-controlled trial that adds much-needed insight into how and when to monitor patients being treated with MTX, Vivian P. Bykerk, MD, of the Hospital for Special Surgery and Weill Cornell Medical College in New York, wrote in an editorial (Ann Intern Med. 2020 Feb 17. doi: 10.7326/M20-0435).
Dr. Bykerk noted that although the results may not be applicable to patients with RA and other inflammatory arthritides who are treated with MTX – RA patients in particular are younger, more often female, have lower rates of diabetes, and usually receive higher doses than those used in CIRT — the risk estimates from the CIRT study are “largely congruent with those expected in MTX-treated patients with rheumatic diseases.”
Regardless, she emphasized that this is a step in a much-needed direction, reminding physicians that “MTX use has inherent risks” and that its AEs, although infrequent, are clinically serious.
The National Institutes of Health funded the study. Various authors reported receiving grants from the National Heart, Lung, and Blood Institute, along with grants, research support, and personal fees from numerous pharmaceutical companies before and during the study. Dr. Bykerk reported receiving personal fees, grants, and nonfinancial support from pharmaceutical companies, foundations, and the NIH.
SOURCE: Solomon DH et al. Ann Intern Med. 2020 Feb 17. doi: 10.7326/M19-3369.
A new study has found an elevated risk of some adverse events in patients treated with low-dose methotrexate, compared with patients treated with placebo.
“The data presented here provide an important source of new evidence to improve the monitoring guidelines and safe prescribing of LD-MTX [low-dose methotrexate],” wrote Daniel H. Solomon, MD, of Brigham and Women’s Hospital and Harvard Medical School in Boston, and coauthors. The study was published in Annals of Internal Medicine.
To determine the rates of adverse events (AEs) among LD-MTX users, along with assessing the risks of certain predefined AEs, the researchers enrolled 6,158 patients in the Cardiovascular Inflammation Reduction Trial (CIRT) and randomized 4,786 of those patients to two groups: those receiving LD-MTX (n = 2,391) and those receiving placebo (n = 2,395). The median dose was 15 mg per week, and median follow-up was 23 months. All participants in CIRT had a history of cardiovascular disease, along with diabetes or metabolic syndrome. Just over 81% of the participants were male, and nearly 85% were white. Their median age was nearly 66 years.
Of the participants in the LD-MTX group, 2,156 (90.2%) had an AE and 2,080 (87.0%) had an AE of interest, which included infectious, hematologic, pulmonary, hepatic, cancerous, and gastrointestinal AEs. Of the participants in the placebo group, 2,076 (86.7%) had an AE and 1,951 (81.5%) had an AE of interest. As such, the relative rate of an AE of interest was 17% higher in the LD-MTX group (hazard ratio, 1.17; 95% confidence interval, 1.10-1.25).
In regard to specific types of AEs, the rates of gastrointestinal (HR, 1.23; 95% CI, 1.03-1.47), pulmonary (HR, 1.42; 95% CI, 1.14-1.77), infectious (HR, 1.15; 95% CI, 1.01-1.30) and hematologic (HR, 1.22; 95% CI, 1.11-1.34) were higher for participants in the LD-MTX group. Five cases of cirrhosis were found in the LD-MTX group, compared with none in the placebo group; none of the patients with cirrhosis had severe liver test abnormalities before their diagnosis. While the risk of cancer overall was not elevated in the LD-MTX group, 53 participants in that group developed skin cancer, compared with 26 in the placebo group (HR, 2.04; 95% CI, 1.28-3.26). Renal AEs were among the few that decreased in LD-MTX users (HR, 0.85; 95% CI, 0.78-0.93).
“Methotrexate has become the standard of care for RA patients,” Dr. Solomon said in an interview, “and because it worked so well, we accepted it without large placebo-controlled trials and without a precise understanding of the risk factors for AEs. Until this study, our evidence basis for the side-effect profile was relatively weak.
“We had a limited data set but decades of experience,” he added. “Now we have better evidence, for example, that methotrexate is associated with elevations in liver function tests. We even found five cases of cirrhosis. And the people who developed cirrhosis didn’t have severe test abnormalities; just minor ones over many months. So now we have a better understanding of the potential impact of minor, yet chronic abnormalities.”
Dr. Solomon and coauthors acknowledged their study’s limitations, including CIRT not including patients with systemic rheumatic disease and the possibility that participants did not report AEs that occurred in between routine study visits. In addition, although the median follow-up of nearly 2 years was longer than in other LD-MTX trials, they noted that “it may still be too short to observe some AEs that require long-term exposure.”
Dr. Solomon and colleagues should be commended for undertaking a long-awaited randomized, placebo-controlled trial that adds much-needed insight into how and when to monitor patients being treated with MTX, Vivian P. Bykerk, MD, of the Hospital for Special Surgery and Weill Cornell Medical College in New York, wrote in an editorial (Ann Intern Med. 2020 Feb 17. doi: 10.7326/M20-0435).
Dr. Bykerk noted that although the results may not be applicable to patients with RA and other inflammatory arthritides who are treated with MTX – RA patients in particular are younger, more often female, have lower rates of diabetes, and usually receive higher doses than those used in CIRT — the risk estimates from the CIRT study are “largely congruent with those expected in MTX-treated patients with rheumatic diseases.”
Regardless, she emphasized that this is a step in a much-needed direction, reminding physicians that “MTX use has inherent risks” and that its AEs, although infrequent, are clinically serious.
The National Institutes of Health funded the study. Various authors reported receiving grants from the National Heart, Lung, and Blood Institute, along with grants, research support, and personal fees from numerous pharmaceutical companies before and during the study. Dr. Bykerk reported receiving personal fees, grants, and nonfinancial support from pharmaceutical companies, foundations, and the NIH.
SOURCE: Solomon DH et al. Ann Intern Med. 2020 Feb 17. doi: 10.7326/M19-3369.
FROM ANNALS OF INTERNAL MEDICINE
Dupilumab for severe AD: Expert advocates continuous treatment
LAHAINA, HAWAII – rather than treatment on an as-needed basis, Andrew Blauvelt, MD, advised at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“I view atopic dermatitis as a chronic disease requiring chronic treatment. So be very careful about stopping. We know that if you start and stop biologics you’re going to be far more prone to develop antidrug antibodies resulting in drug resistance than with continual dosing,” said Dr. Blauvelt, a dermatologist and clinical trialist who is president of the Oregon Medical Research Center, Portland.
He said dupilumab (Dupixent) seldom induces disease remission as defined by clear skin while off all drugs for at least 1 year, although he has a few patients who seem to be exceptions. Yet clearly dupilumab doesn’t change an individual’s predisposing genetics or environmental allergen exposure pattern, so it’s best to think of it as a treatment for the long haul.
Dr. Blauvelt considers dupilumab far and away the best medication for treatment of adults and teenagers whose atopic dermatitis (AD) is uncontrolled with topical therapy. Payers often balk at authorizing dupilumab unless a patient has first undergone an unsuccessful trial of cyclosporine or methotrexate, which are far less expensive. But that’s not what the expert consensus guidelines recommend (Ann Allergy Asthma Immunol. 2018 Jan;120[1]:10-22.e2).
“The guidelines don’t suggest that failure on methotrexate or cyclosporine should be a prerequisite for dupilumab. So if you’re having problems with an insurance company and you really want to use dupilumab, you can point to this paper and say, ‘Look, the experts do not recommend step therapy, we can go directly to dupilumab.’ And the dupilumab label says simply that failure of topical therapy is required before being allowed to use dupilumab. So both the label and the experts say you don’t have to go through a bunch of steps in order to get to what I consider the very best drug for our patients,” he explained.
Both cyclosporine and methotrexate are far more broadly immunosuppressive and hence less safe than dupilumab. Both require laboratory monitoring. In contrast, blood work isn’t required in patients on dupilumab; in fact, Dr. Blauvelt considers it an unwise use of resources. Nor is tuberculosis testing advised prior to starting dupilumab.
When he can’t get authorization for dupilumab, Dr. Blauvelt’s go-to drug is methotrexate at 15-25 mg/week. It’s not as effective as cyclosporine for rapid clearing, but it’s safer for long-term use.
“Methotrexate is the devil we know – we know how to use it, and we know how to monitor for it,” he commented, adding that he reserves cyclosporine for a maximum of a month or 2 of acute crisis management, or as a bridge in getting patients off of systemic corticosteroids.
Set realistic efficacy expectations
Dermatologists who prescribe the newest biologics for psoriasis are accustomed to routinely seeing PASI 90 responses and even complete disease clearing. However, AD is a more challenging disease. In the landmark dupilumab phase 3 randomized trials, roughly two-thirds of patients achieved an Eczema Area and Severity Index (EASI) 75 response, with a mean 80% improvement in EASI symptom scores over baseline. Roughly 20% of dupilumab-treated adults with AD achieve disease clearance, and a similar percentage become almost clear. The improvements are durable in long-term follow-up studies.
“Dupilumab doesn’t get a lot of people to zero. They’re not going to be completely clearing their eczema. So they shouldn’t be freaking out if they still have eczema. What they can expect is diminution of the disease to much lower levels,” Dr. Blauvelt said.
The marked improvement in quality of life that occurs with dupilumab therapy isn’t adequately captured by EASI scores. “In my experience, more than 80%-90% of patients are happy on this drug,” Dr. Blauvelt said.
Conference codirector Linda Stein Gold, MD, agreed, commenting that she has found dupilumab to be “absolutely life altering” for her patients with severe AD.
“They know they still have AD, but now they can go whole days without thinking about it,” said Dr. Stein Gold, director of dermatology research and head of the division of dermatology at the Henry Ford Health System in Detroit.
Dr. Blauvelt noted that most of his patients on dupilumab remain on topical therapy, typically with triamcinolone on the body and hydrocortisone on the face. What he terms “miniflares” in patients on dupilumab are not at all unusual, but they’re readily manageable.
“Flares that used to last for weeks now last for a day or 2, maybe 3, and then it’s back to normal in patients on dupilumab,” Dr. Blauvelt said.
Safety
Dupilumab is a targeted inhibitor of interleukins-4 and -13, cytokines involved in allergy-mediated inflammation and the control of parasitic infections, but which have no bearing on control of bacterial or viral infections or malignancies. Indeed, the randomized trials have demonstrated that the incidence of skin infections is actually lower with dupilumab than with placebo.
“You’re improving the skin barrier so much that they’re not going to be getting staph or herpes simplex,” he explained.
The main side effect consists of dupilumab-associated eye issues. These occur in up to 20% of treated patients and encompass a spectrum ranging from dry eye to nonallergic conjunctivitis, inflammation of the eyelid, and keratitis. The mechanism is unknown. The condition is not infectious and doesn’t affect vision. Intriguingly, it doesn’t occur in patients with asthma, a disease for which dupilumab is also approved.
“Ask about eye issues at every office visit,” the dermatologist urged.
He sends all of his AD patients with dupilumab-associated eye issues to a single trusted local ophthalmologist and lets him manage the condition, which is generally mild to moderate. Eye issues have resulted in discontinuation of dupilumab in only 2 of the roughly 150 AD patients Dr. Blauvelt has placed on the biologic. The ophthalmologist generally relies upon lubricating eye drops and a couple of weeks of steroid eye drops or, in some cases, topical cyclosporine 0.05% ophthalmic emulsion, followed by episodic use of the steroid eye drops on an as-needed basis.
Residual facial disease in AD patients on dupilumab can be caused by a variety of causes, including breakthrough AD, rosacea, allergic contact dermatitis, steroid withdrawal, or photosensitivity, with Demodex thought to play a role in some cases.
Dr. Blauvelt reported serving as a scientific adviser to and paid clinical trial investigator for several dozen pharmaceutical companies. SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
LAHAINA, HAWAII – rather than treatment on an as-needed basis, Andrew Blauvelt, MD, advised at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“I view atopic dermatitis as a chronic disease requiring chronic treatment. So be very careful about stopping. We know that if you start and stop biologics you’re going to be far more prone to develop antidrug antibodies resulting in drug resistance than with continual dosing,” said Dr. Blauvelt, a dermatologist and clinical trialist who is president of the Oregon Medical Research Center, Portland.
He said dupilumab (Dupixent) seldom induces disease remission as defined by clear skin while off all drugs for at least 1 year, although he has a few patients who seem to be exceptions. Yet clearly dupilumab doesn’t change an individual’s predisposing genetics or environmental allergen exposure pattern, so it’s best to think of it as a treatment for the long haul.
Dr. Blauvelt considers dupilumab far and away the best medication for treatment of adults and teenagers whose atopic dermatitis (AD) is uncontrolled with topical therapy. Payers often balk at authorizing dupilumab unless a patient has first undergone an unsuccessful trial of cyclosporine or methotrexate, which are far less expensive. But that’s not what the expert consensus guidelines recommend (Ann Allergy Asthma Immunol. 2018 Jan;120[1]:10-22.e2).
“The guidelines don’t suggest that failure on methotrexate or cyclosporine should be a prerequisite for dupilumab. So if you’re having problems with an insurance company and you really want to use dupilumab, you can point to this paper and say, ‘Look, the experts do not recommend step therapy, we can go directly to dupilumab.’ And the dupilumab label says simply that failure of topical therapy is required before being allowed to use dupilumab. So both the label and the experts say you don’t have to go through a bunch of steps in order to get to what I consider the very best drug for our patients,” he explained.
Both cyclosporine and methotrexate are far more broadly immunosuppressive and hence less safe than dupilumab. Both require laboratory monitoring. In contrast, blood work isn’t required in patients on dupilumab; in fact, Dr. Blauvelt considers it an unwise use of resources. Nor is tuberculosis testing advised prior to starting dupilumab.
When he can’t get authorization for dupilumab, Dr. Blauvelt’s go-to drug is methotrexate at 15-25 mg/week. It’s not as effective as cyclosporine for rapid clearing, but it’s safer for long-term use.
“Methotrexate is the devil we know – we know how to use it, and we know how to monitor for it,” he commented, adding that he reserves cyclosporine for a maximum of a month or 2 of acute crisis management, or as a bridge in getting patients off of systemic corticosteroids.
Set realistic efficacy expectations
Dermatologists who prescribe the newest biologics for psoriasis are accustomed to routinely seeing PASI 90 responses and even complete disease clearing. However, AD is a more challenging disease. In the landmark dupilumab phase 3 randomized trials, roughly two-thirds of patients achieved an Eczema Area and Severity Index (EASI) 75 response, with a mean 80% improvement in EASI symptom scores over baseline. Roughly 20% of dupilumab-treated adults with AD achieve disease clearance, and a similar percentage become almost clear. The improvements are durable in long-term follow-up studies.
“Dupilumab doesn’t get a lot of people to zero. They’re not going to be completely clearing their eczema. So they shouldn’t be freaking out if they still have eczema. What they can expect is diminution of the disease to much lower levels,” Dr. Blauvelt said.
The marked improvement in quality of life that occurs with dupilumab therapy isn’t adequately captured by EASI scores. “In my experience, more than 80%-90% of patients are happy on this drug,” Dr. Blauvelt said.
Conference codirector Linda Stein Gold, MD, agreed, commenting that she has found dupilumab to be “absolutely life altering” for her patients with severe AD.
“They know they still have AD, but now they can go whole days without thinking about it,” said Dr. Stein Gold, director of dermatology research and head of the division of dermatology at the Henry Ford Health System in Detroit.
Dr. Blauvelt noted that most of his patients on dupilumab remain on topical therapy, typically with triamcinolone on the body and hydrocortisone on the face. What he terms “miniflares” in patients on dupilumab are not at all unusual, but they’re readily manageable.
“Flares that used to last for weeks now last for a day or 2, maybe 3, and then it’s back to normal in patients on dupilumab,” Dr. Blauvelt said.
Safety
Dupilumab is a targeted inhibitor of interleukins-4 and -13, cytokines involved in allergy-mediated inflammation and the control of parasitic infections, but which have no bearing on control of bacterial or viral infections or malignancies. Indeed, the randomized trials have demonstrated that the incidence of skin infections is actually lower with dupilumab than with placebo.
“You’re improving the skin barrier so much that they’re not going to be getting staph or herpes simplex,” he explained.
The main side effect consists of dupilumab-associated eye issues. These occur in up to 20% of treated patients and encompass a spectrum ranging from dry eye to nonallergic conjunctivitis, inflammation of the eyelid, and keratitis. The mechanism is unknown. The condition is not infectious and doesn’t affect vision. Intriguingly, it doesn’t occur in patients with asthma, a disease for which dupilumab is also approved.
“Ask about eye issues at every office visit,” the dermatologist urged.
He sends all of his AD patients with dupilumab-associated eye issues to a single trusted local ophthalmologist and lets him manage the condition, which is generally mild to moderate. Eye issues have resulted in discontinuation of dupilumab in only 2 of the roughly 150 AD patients Dr. Blauvelt has placed on the biologic. The ophthalmologist generally relies upon lubricating eye drops and a couple of weeks of steroid eye drops or, in some cases, topical cyclosporine 0.05% ophthalmic emulsion, followed by episodic use of the steroid eye drops on an as-needed basis.
Residual facial disease in AD patients on dupilumab can be caused by a variety of causes, including breakthrough AD, rosacea, allergic contact dermatitis, steroid withdrawal, or photosensitivity, with Demodex thought to play a role in some cases.
Dr. Blauvelt reported serving as a scientific adviser to and paid clinical trial investigator for several dozen pharmaceutical companies. SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
LAHAINA, HAWAII – rather than treatment on an as-needed basis, Andrew Blauvelt, MD, advised at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“I view atopic dermatitis as a chronic disease requiring chronic treatment. So be very careful about stopping. We know that if you start and stop biologics you’re going to be far more prone to develop antidrug antibodies resulting in drug resistance than with continual dosing,” said Dr. Blauvelt, a dermatologist and clinical trialist who is president of the Oregon Medical Research Center, Portland.
He said dupilumab (Dupixent) seldom induces disease remission as defined by clear skin while off all drugs for at least 1 year, although he has a few patients who seem to be exceptions. Yet clearly dupilumab doesn’t change an individual’s predisposing genetics or environmental allergen exposure pattern, so it’s best to think of it as a treatment for the long haul.
Dr. Blauvelt considers dupilumab far and away the best medication for treatment of adults and teenagers whose atopic dermatitis (AD) is uncontrolled with topical therapy. Payers often balk at authorizing dupilumab unless a patient has first undergone an unsuccessful trial of cyclosporine or methotrexate, which are far less expensive. But that’s not what the expert consensus guidelines recommend (Ann Allergy Asthma Immunol. 2018 Jan;120[1]:10-22.e2).
“The guidelines don’t suggest that failure on methotrexate or cyclosporine should be a prerequisite for dupilumab. So if you’re having problems with an insurance company and you really want to use dupilumab, you can point to this paper and say, ‘Look, the experts do not recommend step therapy, we can go directly to dupilumab.’ And the dupilumab label says simply that failure of topical therapy is required before being allowed to use dupilumab. So both the label and the experts say you don’t have to go through a bunch of steps in order to get to what I consider the very best drug for our patients,” he explained.
Both cyclosporine and methotrexate are far more broadly immunosuppressive and hence less safe than dupilumab. Both require laboratory monitoring. In contrast, blood work isn’t required in patients on dupilumab; in fact, Dr. Blauvelt considers it an unwise use of resources. Nor is tuberculosis testing advised prior to starting dupilumab.
When he can’t get authorization for dupilumab, Dr. Blauvelt’s go-to drug is methotrexate at 15-25 mg/week. It’s not as effective as cyclosporine for rapid clearing, but it’s safer for long-term use.
“Methotrexate is the devil we know – we know how to use it, and we know how to monitor for it,” he commented, adding that he reserves cyclosporine for a maximum of a month or 2 of acute crisis management, or as a bridge in getting patients off of systemic corticosteroids.
Set realistic efficacy expectations
Dermatologists who prescribe the newest biologics for psoriasis are accustomed to routinely seeing PASI 90 responses and even complete disease clearing. However, AD is a more challenging disease. In the landmark dupilumab phase 3 randomized trials, roughly two-thirds of patients achieved an Eczema Area and Severity Index (EASI) 75 response, with a mean 80% improvement in EASI symptom scores over baseline. Roughly 20% of dupilumab-treated adults with AD achieve disease clearance, and a similar percentage become almost clear. The improvements are durable in long-term follow-up studies.
“Dupilumab doesn’t get a lot of people to zero. They’re not going to be completely clearing their eczema. So they shouldn’t be freaking out if they still have eczema. What they can expect is diminution of the disease to much lower levels,” Dr. Blauvelt said.
The marked improvement in quality of life that occurs with dupilumab therapy isn’t adequately captured by EASI scores. “In my experience, more than 80%-90% of patients are happy on this drug,” Dr. Blauvelt said.
Conference codirector Linda Stein Gold, MD, agreed, commenting that she has found dupilumab to be “absolutely life altering” for her patients with severe AD.
“They know they still have AD, but now they can go whole days without thinking about it,” said Dr. Stein Gold, director of dermatology research and head of the division of dermatology at the Henry Ford Health System in Detroit.
Dr. Blauvelt noted that most of his patients on dupilumab remain on topical therapy, typically with triamcinolone on the body and hydrocortisone on the face. What he terms “miniflares” in patients on dupilumab are not at all unusual, but they’re readily manageable.
“Flares that used to last for weeks now last for a day or 2, maybe 3, and then it’s back to normal in patients on dupilumab,” Dr. Blauvelt said.
Safety
Dupilumab is a targeted inhibitor of interleukins-4 and -13, cytokines involved in allergy-mediated inflammation and the control of parasitic infections, but which have no bearing on control of bacterial or viral infections or malignancies. Indeed, the randomized trials have demonstrated that the incidence of skin infections is actually lower with dupilumab than with placebo.
“You’re improving the skin barrier so much that they’re not going to be getting staph or herpes simplex,” he explained.
The main side effect consists of dupilumab-associated eye issues. These occur in up to 20% of treated patients and encompass a spectrum ranging from dry eye to nonallergic conjunctivitis, inflammation of the eyelid, and keratitis. The mechanism is unknown. The condition is not infectious and doesn’t affect vision. Intriguingly, it doesn’t occur in patients with asthma, a disease for which dupilumab is also approved.
“Ask about eye issues at every office visit,” the dermatologist urged.
He sends all of his AD patients with dupilumab-associated eye issues to a single trusted local ophthalmologist and lets him manage the condition, which is generally mild to moderate. Eye issues have resulted in discontinuation of dupilumab in only 2 of the roughly 150 AD patients Dr. Blauvelt has placed on the biologic. The ophthalmologist generally relies upon lubricating eye drops and a couple of weeks of steroid eye drops or, in some cases, topical cyclosporine 0.05% ophthalmic emulsion, followed by episodic use of the steroid eye drops on an as-needed basis.
Residual facial disease in AD patients on dupilumab can be caused by a variety of causes, including breakthrough AD, rosacea, allergic contact dermatitis, steroid withdrawal, or photosensitivity, with Demodex thought to play a role in some cases.
Dr. Blauvelt reported serving as a scientific adviser to and paid clinical trial investigator for several dozen pharmaceutical companies. SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT OPINION FROM SDEF HAWAII DERMATOLOGY SEMINAR
Heart disease risk rises with gut metabolite linked to red meat
Changes in gut microbiota linked to red meat intake over time were significantly associated with increased risk of coronary heart disease, regardless of baseline microbiota measures, based on data from 760 participants in the Nurses’ Health Study.
“A gut microbiota–related metabolite, trimethylamine N-oxide (TMAO), has been related to risks of major adverse cardiovascular events including myocardial infarction and coronary heart disease (CHD) in epidemiological studies,” but previous studies have not examined the impact of long-term changes in TMAO on CHD risk, wrote Yoriko Heianza, RD, PhD, of Tulane University, New Orleans, and colleagues.
Red meat has been shown to increase TMAO levels, whereas discontinuation of red meat intake reduced plasma TMAO levels (Eur Heart J 2019;40:583-94), the investigators wrote.
In their study, published in the Journal of the American College of Cardiology, the researchers evaluated blood samples from 760 women who were participants in the Nurses’ Health Study. The samples were collected at two time points: 1989-1990 and 2000-2002. The researchers identified 360 incident cases of CHD over the study period and compared them with matched controls.
Over roughly 10 years, increases in TMAO over time were significantly associated with increased CHD risk, with a relative risk of 1.58 for the top tertile and a relative risk of 1.33 per each standard deviation.
Women with elevated levels of TMAO both at baseline and at the 10-year point had the highest CHD risk (relative risk 1.79), compared with women with low TMAO levels at baseline and 10 years later.
The researchers also found an impact of diet on the TMAO-CHD relationship. Individuals with unhealthy eating patterns based on the Alternate Healthy Eating Index showed greater increases in TMAO and greater CHD risk. By contrast, greater adherence to healthy eating habits attenuated the impact of TMAO and CHD.
The study findings were limited by several factors, including the inability to assess the timing of the changes in the metabolites that contributed to CHD, the reliance on self-reports for dietary patterns and other variables, and the inclusion only of women health professionals in the study population, the researchers noted. However, the results were strengthened by the availability of long-term blood samples and a patient population free of disease at baseline.
In addition, “adherence to healthy dietary patterns may modulate the adverse relationship between TMAO changes and CHD, suggesting that TMAO as a potential intermediate endpoint of interventions focusing on dietary modifications for CHD prevention,” the researchers wrote.
“The findings of the study provide further evidence for the role of TMAO as a predictive biomarker for atherosclerotic heart disease and strengthen the case for TMAO as a potential intervention target in CV [cardiovascular] disease prevention,” wrote Paul A. Heidenreich, MD, and Petra Mamic, MD, of Stanford (Calif.) University, in an accompanying editorial.
In addition, “It is increasingly clear that GMB [gut microbiota] metabolites have biological activity, and that dietary changes alter the GMB and its metabolic output, with subsequent modulation of downstream host effects,” they wrote.
“While acknowledging the limitations of self-reported dietary pattern assessment, this is an important finding because it suggests that healthy dietary patterns may in some ways neutralize TMAO’s harmful effects on the CV system, potentially through other identified and unidentified GMB-mediated pathways,” they added.
The study was sponsored in part by the National Institutes of Health, the Boston Obesity Nutrition Research Center, and the United States–Israel Binational Science Foundation. Neither the researchers nor the editorialists had any financial conflicts to disclose.
SOURCES: Heianza Y et al. J Am Coll Cardiol. 2020 Feb 17. doi: 0.1016/j.jacc.2019.11.060; Heidenreich PA, Mamic P. J Am Coll Cardiol. 2020 Feb 17. doi: 10.1016/j.jacc.2019.12.023.
Changes in gut microbiota linked to red meat intake over time were significantly associated with increased risk of coronary heart disease, regardless of baseline microbiota measures, based on data from 760 participants in the Nurses’ Health Study.
“A gut microbiota–related metabolite, trimethylamine N-oxide (TMAO), has been related to risks of major adverse cardiovascular events including myocardial infarction and coronary heart disease (CHD) in epidemiological studies,” but previous studies have not examined the impact of long-term changes in TMAO on CHD risk, wrote Yoriko Heianza, RD, PhD, of Tulane University, New Orleans, and colleagues.
Red meat has been shown to increase TMAO levels, whereas discontinuation of red meat intake reduced plasma TMAO levels (Eur Heart J 2019;40:583-94), the investigators wrote.
In their study, published in the Journal of the American College of Cardiology, the researchers evaluated blood samples from 760 women who were participants in the Nurses’ Health Study. The samples were collected at two time points: 1989-1990 and 2000-2002. The researchers identified 360 incident cases of CHD over the study period and compared them with matched controls.
Over roughly 10 years, increases in TMAO over time were significantly associated with increased CHD risk, with a relative risk of 1.58 for the top tertile and a relative risk of 1.33 per each standard deviation.
Women with elevated levels of TMAO both at baseline and at the 10-year point had the highest CHD risk (relative risk 1.79), compared with women with low TMAO levels at baseline and 10 years later.
The researchers also found an impact of diet on the TMAO-CHD relationship. Individuals with unhealthy eating patterns based on the Alternate Healthy Eating Index showed greater increases in TMAO and greater CHD risk. By contrast, greater adherence to healthy eating habits attenuated the impact of TMAO and CHD.
The study findings were limited by several factors, including the inability to assess the timing of the changes in the metabolites that contributed to CHD, the reliance on self-reports for dietary patterns and other variables, and the inclusion only of women health professionals in the study population, the researchers noted. However, the results were strengthened by the availability of long-term blood samples and a patient population free of disease at baseline.
In addition, “adherence to healthy dietary patterns may modulate the adverse relationship between TMAO changes and CHD, suggesting that TMAO as a potential intermediate endpoint of interventions focusing on dietary modifications for CHD prevention,” the researchers wrote.
“The findings of the study provide further evidence for the role of TMAO as a predictive biomarker for atherosclerotic heart disease and strengthen the case for TMAO as a potential intervention target in CV [cardiovascular] disease prevention,” wrote Paul A. Heidenreich, MD, and Petra Mamic, MD, of Stanford (Calif.) University, in an accompanying editorial.
In addition, “It is increasingly clear that GMB [gut microbiota] metabolites have biological activity, and that dietary changes alter the GMB and its metabolic output, with subsequent modulation of downstream host effects,” they wrote.
“While acknowledging the limitations of self-reported dietary pattern assessment, this is an important finding because it suggests that healthy dietary patterns may in some ways neutralize TMAO’s harmful effects on the CV system, potentially through other identified and unidentified GMB-mediated pathways,” they added.
The study was sponsored in part by the National Institutes of Health, the Boston Obesity Nutrition Research Center, and the United States–Israel Binational Science Foundation. Neither the researchers nor the editorialists had any financial conflicts to disclose.
SOURCES: Heianza Y et al. J Am Coll Cardiol. 2020 Feb 17. doi: 0.1016/j.jacc.2019.11.060; Heidenreich PA, Mamic P. J Am Coll Cardiol. 2020 Feb 17. doi: 10.1016/j.jacc.2019.12.023.
Changes in gut microbiota linked to red meat intake over time were significantly associated with increased risk of coronary heart disease, regardless of baseline microbiota measures, based on data from 760 participants in the Nurses’ Health Study.
“A gut microbiota–related metabolite, trimethylamine N-oxide (TMAO), has been related to risks of major adverse cardiovascular events including myocardial infarction and coronary heart disease (CHD) in epidemiological studies,” but previous studies have not examined the impact of long-term changes in TMAO on CHD risk, wrote Yoriko Heianza, RD, PhD, of Tulane University, New Orleans, and colleagues.
Red meat has been shown to increase TMAO levels, whereas discontinuation of red meat intake reduced plasma TMAO levels (Eur Heart J 2019;40:583-94), the investigators wrote.
In their study, published in the Journal of the American College of Cardiology, the researchers evaluated blood samples from 760 women who were participants in the Nurses’ Health Study. The samples were collected at two time points: 1989-1990 and 2000-2002. The researchers identified 360 incident cases of CHD over the study period and compared them with matched controls.
Over roughly 10 years, increases in TMAO over time were significantly associated with increased CHD risk, with a relative risk of 1.58 for the top tertile and a relative risk of 1.33 per each standard deviation.
Women with elevated levels of TMAO both at baseline and at the 10-year point had the highest CHD risk (relative risk 1.79), compared with women with low TMAO levels at baseline and 10 years later.
The researchers also found an impact of diet on the TMAO-CHD relationship. Individuals with unhealthy eating patterns based on the Alternate Healthy Eating Index showed greater increases in TMAO and greater CHD risk. By contrast, greater adherence to healthy eating habits attenuated the impact of TMAO and CHD.
The study findings were limited by several factors, including the inability to assess the timing of the changes in the metabolites that contributed to CHD, the reliance on self-reports for dietary patterns and other variables, and the inclusion only of women health professionals in the study population, the researchers noted. However, the results were strengthened by the availability of long-term blood samples and a patient population free of disease at baseline.
In addition, “adherence to healthy dietary patterns may modulate the adverse relationship between TMAO changes and CHD, suggesting that TMAO as a potential intermediate endpoint of interventions focusing on dietary modifications for CHD prevention,” the researchers wrote.
“The findings of the study provide further evidence for the role of TMAO as a predictive biomarker for atherosclerotic heart disease and strengthen the case for TMAO as a potential intervention target in CV [cardiovascular] disease prevention,” wrote Paul A. Heidenreich, MD, and Petra Mamic, MD, of Stanford (Calif.) University, in an accompanying editorial.
In addition, “It is increasingly clear that GMB [gut microbiota] metabolites have biological activity, and that dietary changes alter the GMB and its metabolic output, with subsequent modulation of downstream host effects,” they wrote.
“While acknowledging the limitations of self-reported dietary pattern assessment, this is an important finding because it suggests that healthy dietary patterns may in some ways neutralize TMAO’s harmful effects on the CV system, potentially through other identified and unidentified GMB-mediated pathways,” they added.
The study was sponsored in part by the National Institutes of Health, the Boston Obesity Nutrition Research Center, and the United States–Israel Binational Science Foundation. Neither the researchers nor the editorialists had any financial conflicts to disclose.
SOURCES: Heianza Y et al. J Am Coll Cardiol. 2020 Feb 17. doi: 0.1016/j.jacc.2019.11.060; Heidenreich PA, Mamic P. J Am Coll Cardiol. 2020 Feb 17. doi: 10.1016/j.jacc.2019.12.023.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Some relevant financial conflicts go undisclosed in ACR guidelines
Over one-third of undisclosed industry payments made to physician-authors of American College of Rheumatology clinical practice guidelines were relevant to guideline recommendations, according to a recent review in Arthritis & Rheumatology.
Since 2014, 56 of 89 total physician-authors across five ACR clinical practice guidelines have been paid a total of $9,728,751 from industry sources. Nineteen of 89 authors received $1,961,362 in industry payments that were directly relevant to a guideline’s recommendations, and $699,561 of these payments (35.7%) were undisclosed, according to Cole Wayant, of the Oklahoma State University Center for Health Sciences, Tulsa, and colleagues.
The ACR’s Policy and Procedure Manual for Clinical Practice Guidelines, last updated in January 2015, allows up to 49% of authors in a clinical practice guideline to have financial conflicts of interest, including intellectual conflicts of interest, and requires them to report those relationships. When the ACR creates a call for letters of interest for a guideline, it includes a list of companies and organizations that could be affected by the guideline topic. To be considered conflict free, an author must not have ties to these companies and organizations for 1 year before the deadline on the letter of interest and 1 year after a guideline is published. This policy extends to members of an ACR guideline development group, literature review team, and voting panel. Under these guidelines, an author who has any relationship with a company is considered conflicted, which counts toward this total.
Mr. Wayant and colleagues performed a cross-sectional study of five ACR guidelines published since August 2014 on axial spondyloarthritis (27 authors), glucocorticoid-induced osteoporosis (21 authors), RA (26 authors), perioperative management of antirheumatic medication (31 authors), and polymyalgia rheumatica (46 authors). Using the Open Payments Database, the researchers searched for any general (speaking fees, consulting fees, education, honoraria, travel, food, or beverage payments) research, associated research, and ownership (stocks or dividends) relationships reported by guideline authors in the 12 months before a guideline was published. The guidelines on axial spondyloarthritis, glucocorticoid-induced osteoporosis, and RA contained specific recommendations for classes of medications or branded drugs, and conflicts from authors in those guidelines were assessed to determine relevancy of those payments.
Of the 56 physician-authors who received at least one payment (62.9%), the median payment was $522. However, 51 authors reported receiving more than $1,000, 42 authors reported more than $10,000, 20 authors reported more than $100,000, and 2 authors reported more than $1 million. Overall, 14 of 56 authors (25.0%) reported having no financial conflicts of interest, but did in fact receive some payment, and $4,189,090 of the $9,728,751 (43.1%) was not reported. The researchers said that the 19 authors with directly relevant payments were members of the voting panel (11 authors), literature review team (6 authors), and core leadership team (3 authors).
Physician-authors of clinical practice guidelines receiving payments from industry is not an issue specific to rheumatology. In an interview, Mr. Wayant said that authors of clinical guidelines across many different medical specialties often work closely with industry and hold “numerous conflicts of interest.”
“If professional societies are meant to be the public face of specialty providers, one would expect the guideline authors to resemble all society members,” Mr. Wayant said. “However, we routinely find that authors of professional society guidelines have large financial conflicts of interest that exceed the national average, indicating that the views and opinions of guideline authors may not reflect the opinion of most providers.”
These financial relationships between industry and physician authors have been shown to affect research results. A Cochrane Review published in 2017 evaluating industry sponsorship and research outcomes found that studies sponsored by industry were more likely to have favorable efficacy results and conclusions, compared with studies not sponsored by industry sources (Cochrane Database Syst Rev. 2017 Feb 16;2:MR000033). As medical societies continue to become more involved with clinical practice guidelines, recommendations from physician-authors with financial ties to industry can present a conflict of interest. Recommendations in clinical practice guidelines often affect reimbursement of a drug from insurance, and an author can vote for a drug recommendation in a guideline that may not match patient values and preferences, noted Mr. Wayant.
“These authors are fundamentally different from the average rheumatologist that stays up to date with the medical literature, in terms of financial ties to industry,” he said. “Removing the influence of for-profit companies from guideline development cannot harm the rigor of the guideline recommendations, since many medical professionals without conflicts are experts in evidence-based medicine and study appraisal.”
Being financially linked to industry does not automatically make one the most qualified candidate for deciding which therapies are best for patients, Mr. Wayant explained, and guidelines should reflect the values of patients and the medical profession, rather than industry.
“Given the importance of guidelines, [we] encourage the ACR and all professional societies to do everything possible to be above reproach and seek out authors who do not have financial conflicts to write the guidelines,” he said.
The authors reported having no funding source for the study. One author reported serving on an advisory board for Janssen involving infliximab and golimumab, for Sanofi Genzyme involving sarilumab, and receiving payment for a survey from Comsort. The other authors reported having no conflicts of interest.
SOURCE: Wayant C et al. Arthritis Rheumatol. 2020 Feb 10. doi: 10.1002/art.41224.
Over one-third of undisclosed industry payments made to physician-authors of American College of Rheumatology clinical practice guidelines were relevant to guideline recommendations, according to a recent review in Arthritis & Rheumatology.
Since 2014, 56 of 89 total physician-authors across five ACR clinical practice guidelines have been paid a total of $9,728,751 from industry sources. Nineteen of 89 authors received $1,961,362 in industry payments that were directly relevant to a guideline’s recommendations, and $699,561 of these payments (35.7%) were undisclosed, according to Cole Wayant, of the Oklahoma State University Center for Health Sciences, Tulsa, and colleagues.
The ACR’s Policy and Procedure Manual for Clinical Practice Guidelines, last updated in January 2015, allows up to 49% of authors in a clinical practice guideline to have financial conflicts of interest, including intellectual conflicts of interest, and requires them to report those relationships. When the ACR creates a call for letters of interest for a guideline, it includes a list of companies and organizations that could be affected by the guideline topic. To be considered conflict free, an author must not have ties to these companies and organizations for 1 year before the deadline on the letter of interest and 1 year after a guideline is published. This policy extends to members of an ACR guideline development group, literature review team, and voting panel. Under these guidelines, an author who has any relationship with a company is considered conflicted, which counts toward this total.
Mr. Wayant and colleagues performed a cross-sectional study of five ACR guidelines published since August 2014 on axial spondyloarthritis (27 authors), glucocorticoid-induced osteoporosis (21 authors), RA (26 authors), perioperative management of antirheumatic medication (31 authors), and polymyalgia rheumatica (46 authors). Using the Open Payments Database, the researchers searched for any general (speaking fees, consulting fees, education, honoraria, travel, food, or beverage payments) research, associated research, and ownership (stocks or dividends) relationships reported by guideline authors in the 12 months before a guideline was published. The guidelines on axial spondyloarthritis, glucocorticoid-induced osteoporosis, and RA contained specific recommendations for classes of medications or branded drugs, and conflicts from authors in those guidelines were assessed to determine relevancy of those payments.
Of the 56 physician-authors who received at least one payment (62.9%), the median payment was $522. However, 51 authors reported receiving more than $1,000, 42 authors reported more than $10,000, 20 authors reported more than $100,000, and 2 authors reported more than $1 million. Overall, 14 of 56 authors (25.0%) reported having no financial conflicts of interest, but did in fact receive some payment, and $4,189,090 of the $9,728,751 (43.1%) was not reported. The researchers said that the 19 authors with directly relevant payments were members of the voting panel (11 authors), literature review team (6 authors), and core leadership team (3 authors).
Physician-authors of clinical practice guidelines receiving payments from industry is not an issue specific to rheumatology. In an interview, Mr. Wayant said that authors of clinical guidelines across many different medical specialties often work closely with industry and hold “numerous conflicts of interest.”
“If professional societies are meant to be the public face of specialty providers, one would expect the guideline authors to resemble all society members,” Mr. Wayant said. “However, we routinely find that authors of professional society guidelines have large financial conflicts of interest that exceed the national average, indicating that the views and opinions of guideline authors may not reflect the opinion of most providers.”
These financial relationships between industry and physician authors have been shown to affect research results. A Cochrane Review published in 2017 evaluating industry sponsorship and research outcomes found that studies sponsored by industry were more likely to have favorable efficacy results and conclusions, compared with studies not sponsored by industry sources (Cochrane Database Syst Rev. 2017 Feb 16;2:MR000033). As medical societies continue to become more involved with clinical practice guidelines, recommendations from physician-authors with financial ties to industry can present a conflict of interest. Recommendations in clinical practice guidelines often affect reimbursement of a drug from insurance, and an author can vote for a drug recommendation in a guideline that may not match patient values and preferences, noted Mr. Wayant.
“These authors are fundamentally different from the average rheumatologist that stays up to date with the medical literature, in terms of financial ties to industry,” he said. “Removing the influence of for-profit companies from guideline development cannot harm the rigor of the guideline recommendations, since many medical professionals without conflicts are experts in evidence-based medicine and study appraisal.”
Being financially linked to industry does not automatically make one the most qualified candidate for deciding which therapies are best for patients, Mr. Wayant explained, and guidelines should reflect the values of patients and the medical profession, rather than industry.
“Given the importance of guidelines, [we] encourage the ACR and all professional societies to do everything possible to be above reproach and seek out authors who do not have financial conflicts to write the guidelines,” he said.
The authors reported having no funding source for the study. One author reported serving on an advisory board for Janssen involving infliximab and golimumab, for Sanofi Genzyme involving sarilumab, and receiving payment for a survey from Comsort. The other authors reported having no conflicts of interest.
SOURCE: Wayant C et al. Arthritis Rheumatol. 2020 Feb 10. doi: 10.1002/art.41224.
Over one-third of undisclosed industry payments made to physician-authors of American College of Rheumatology clinical practice guidelines were relevant to guideline recommendations, according to a recent review in Arthritis & Rheumatology.
Since 2014, 56 of 89 total physician-authors across five ACR clinical practice guidelines have been paid a total of $9,728,751 from industry sources. Nineteen of 89 authors received $1,961,362 in industry payments that were directly relevant to a guideline’s recommendations, and $699,561 of these payments (35.7%) were undisclosed, according to Cole Wayant, of the Oklahoma State University Center for Health Sciences, Tulsa, and colleagues.
The ACR’s Policy and Procedure Manual for Clinical Practice Guidelines, last updated in January 2015, allows up to 49% of authors in a clinical practice guideline to have financial conflicts of interest, including intellectual conflicts of interest, and requires them to report those relationships. When the ACR creates a call for letters of interest for a guideline, it includes a list of companies and organizations that could be affected by the guideline topic. To be considered conflict free, an author must not have ties to these companies and organizations for 1 year before the deadline on the letter of interest and 1 year after a guideline is published. This policy extends to members of an ACR guideline development group, literature review team, and voting panel. Under these guidelines, an author who has any relationship with a company is considered conflicted, which counts toward this total.
Mr. Wayant and colleagues performed a cross-sectional study of five ACR guidelines published since August 2014 on axial spondyloarthritis (27 authors), glucocorticoid-induced osteoporosis (21 authors), RA (26 authors), perioperative management of antirheumatic medication (31 authors), and polymyalgia rheumatica (46 authors). Using the Open Payments Database, the researchers searched for any general (speaking fees, consulting fees, education, honoraria, travel, food, or beverage payments) research, associated research, and ownership (stocks or dividends) relationships reported by guideline authors in the 12 months before a guideline was published. The guidelines on axial spondyloarthritis, glucocorticoid-induced osteoporosis, and RA contained specific recommendations for classes of medications or branded drugs, and conflicts from authors in those guidelines were assessed to determine relevancy of those payments.
Of the 56 physician-authors who received at least one payment (62.9%), the median payment was $522. However, 51 authors reported receiving more than $1,000, 42 authors reported more than $10,000, 20 authors reported more than $100,000, and 2 authors reported more than $1 million. Overall, 14 of 56 authors (25.0%) reported having no financial conflicts of interest, but did in fact receive some payment, and $4,189,090 of the $9,728,751 (43.1%) was not reported. The researchers said that the 19 authors with directly relevant payments were members of the voting panel (11 authors), literature review team (6 authors), and core leadership team (3 authors).
Physician-authors of clinical practice guidelines receiving payments from industry is not an issue specific to rheumatology. In an interview, Mr. Wayant said that authors of clinical guidelines across many different medical specialties often work closely with industry and hold “numerous conflicts of interest.”
“If professional societies are meant to be the public face of specialty providers, one would expect the guideline authors to resemble all society members,” Mr. Wayant said. “However, we routinely find that authors of professional society guidelines have large financial conflicts of interest that exceed the national average, indicating that the views and opinions of guideline authors may not reflect the opinion of most providers.”
These financial relationships between industry and physician authors have been shown to affect research results. A Cochrane Review published in 2017 evaluating industry sponsorship and research outcomes found that studies sponsored by industry were more likely to have favorable efficacy results and conclusions, compared with studies not sponsored by industry sources (Cochrane Database Syst Rev. 2017 Feb 16;2:MR000033). As medical societies continue to become more involved with clinical practice guidelines, recommendations from physician-authors with financial ties to industry can present a conflict of interest. Recommendations in clinical practice guidelines often affect reimbursement of a drug from insurance, and an author can vote for a drug recommendation in a guideline that may not match patient values and preferences, noted Mr. Wayant.
“These authors are fundamentally different from the average rheumatologist that stays up to date with the medical literature, in terms of financial ties to industry,” he said. “Removing the influence of for-profit companies from guideline development cannot harm the rigor of the guideline recommendations, since many medical professionals without conflicts are experts in evidence-based medicine and study appraisal.”
Being financially linked to industry does not automatically make one the most qualified candidate for deciding which therapies are best for patients, Mr. Wayant explained, and guidelines should reflect the values of patients and the medical profession, rather than industry.
“Given the importance of guidelines, [we] encourage the ACR and all professional societies to do everything possible to be above reproach and seek out authors who do not have financial conflicts to write the guidelines,” he said.
The authors reported having no funding source for the study. One author reported serving on an advisory board for Janssen involving infliximab and golimumab, for Sanofi Genzyme involving sarilumab, and receiving payment for a survey from Comsort. The other authors reported having no conflicts of interest.
SOURCE: Wayant C et al. Arthritis Rheumatol. 2020 Feb 10. doi: 10.1002/art.41224.
FROM ARTHRITIS & RHEUMATOLOGY
Less than a quarter of endocrinologists happy at work
according to Medscape’s Endocrinologist Lifestyle, Happiness, and Burnout Report 2020.
The report, which surveyed more than 15,000 physicians from various specialties, found that 31% of endocrinologists are burned out, 2% are depressed, and 16% are both depressed and burned out. Among them, 71% report having too many bureaucratic tasks as the biggest contributor, followed by insufficient reimbursement/compensation at 46% and increasing computerization of tasks (including EHR) at 34%.
The top way endocrinologists cope is by talking with family and close friends (42%), with almost equal amounts coping by either eating junk food or exercising (39% and 37%, respectively). Regarding professional help, 60% said they would not and have not previously sought professional help, while 12% said they were currently seeking professional help. Although 40% said they would engage a workplace program, 31% said they would not.
Among the reasons they gave for not seeking professional help, 45% felt they were too busy, 40% didn’t think their symptoms were severe enough, and 36% said they could handle it without help from professionals.
A slideshow of the full report can be found on Medscape.com.
according to Medscape’s Endocrinologist Lifestyle, Happiness, and Burnout Report 2020.
The report, which surveyed more than 15,000 physicians from various specialties, found that 31% of endocrinologists are burned out, 2% are depressed, and 16% are both depressed and burned out. Among them, 71% report having too many bureaucratic tasks as the biggest contributor, followed by insufficient reimbursement/compensation at 46% and increasing computerization of tasks (including EHR) at 34%.
The top way endocrinologists cope is by talking with family and close friends (42%), with almost equal amounts coping by either eating junk food or exercising (39% and 37%, respectively). Regarding professional help, 60% said they would not and have not previously sought professional help, while 12% said they were currently seeking professional help. Although 40% said they would engage a workplace program, 31% said they would not.
Among the reasons they gave for not seeking professional help, 45% felt they were too busy, 40% didn’t think their symptoms were severe enough, and 36% said they could handle it without help from professionals.
A slideshow of the full report can be found on Medscape.com.
according to Medscape’s Endocrinologist Lifestyle, Happiness, and Burnout Report 2020.
The report, which surveyed more than 15,000 physicians from various specialties, found that 31% of endocrinologists are burned out, 2% are depressed, and 16% are both depressed and burned out. Among them, 71% report having too many bureaucratic tasks as the biggest contributor, followed by insufficient reimbursement/compensation at 46% and increasing computerization of tasks (including EHR) at 34%.
The top way endocrinologists cope is by talking with family and close friends (42%), with almost equal amounts coping by either eating junk food or exercising (39% and 37%, respectively). Regarding professional help, 60% said they would not and have not previously sought professional help, while 12% said they were currently seeking professional help. Although 40% said they would engage a workplace program, 31% said they would not.
Among the reasons they gave for not seeking professional help, 45% felt they were too busy, 40% didn’t think their symptoms were severe enough, and 36% said they could handle it without help from professionals.
A slideshow of the full report can be found on Medscape.com.
Sharpest spikes in pediatric diabetes seen in Asian, Pacific Islander youth
according to a review of almost 70,000 children in the SEARCH for Diabetes in Youth Study, an ongoing, population-based surveillance project of individuals younger than 20 years.
“For both type 1 and type 2 diabetes, the rates of increase were generally higher among racial/ethnic minority populations than those among whites,” wrote the investigators, led by Jasmin Divers, PhD, of the division of health services research, department of foundations of medicine, at New York University. “These findings highlight the need for continued surveillance for diabetes among youths to monitor overall and group-specific trends, identify factors driving these trends, and inform health care planning.”
SEARCH identified 14,638 cases of pediatric type 1 diabetes and 3,916 cases of type 2 diabetes from 2002 to 2015. The study draws participants from all 64 counties in Colorado, plus selected Indian reservations in Arizona and New Mexico under the direction of Colorado; all 46 counties in South Carolina; 8 in Ohio; 5 in Washington; and Kaiser Permanente Southern California health plan enrollees in 7 counties.
The investigators found steeper increases in age- and sex-adjusted incidence of type 1 diabetes from 2002 to 2015 among black youth (2.7% per year), Hispanic youth (4%), and Asian and Pacific Islander youth (4.4%), than among their white counterparts (0.7%). Incidence among Asians and Pacific Islanders did not change significantly during 2002-2010, but increased steeply during 2011-2015 (8.5% per year) for unknown reasons.
“In parallel with increased obesity prevalence in U.S. youths, the incidence of type 2 diabetes among adolescents has increased at a higher rate than that of type 1 diabetes, especially among racial-/ethnic-minority youths,” the authors noted.
The number of new cases of type 2 diagnosed in children younger than 10 years were too few to report on (181 total cases during 2002-2015), so the incidence analysis was limited to children who were aged 10-19 years at diagnosis. The steepest annual percentage changes were among Asians and Pacific Islander youth (7.7% per year), followed by Hispanic (6.5%), black (6.0%), and American Indian (3.7%) youth.
“Although the SEARCH population is similar demographically to the U.S. youth population, it is not designed to be nationally representative,” which is one of the limitations of the study, the investigators wrote.
The authors reported having no conflicts of interest.
SOURCE: Divers J et al. MMWR Morb Mortal Wkly Rep. 2020;69:161-5.
according to a review of almost 70,000 children in the SEARCH for Diabetes in Youth Study, an ongoing, population-based surveillance project of individuals younger than 20 years.
“For both type 1 and type 2 diabetes, the rates of increase were generally higher among racial/ethnic minority populations than those among whites,” wrote the investigators, led by Jasmin Divers, PhD, of the division of health services research, department of foundations of medicine, at New York University. “These findings highlight the need for continued surveillance for diabetes among youths to monitor overall and group-specific trends, identify factors driving these trends, and inform health care planning.”
SEARCH identified 14,638 cases of pediatric type 1 diabetes and 3,916 cases of type 2 diabetes from 2002 to 2015. The study draws participants from all 64 counties in Colorado, plus selected Indian reservations in Arizona and New Mexico under the direction of Colorado; all 46 counties in South Carolina; 8 in Ohio; 5 in Washington; and Kaiser Permanente Southern California health plan enrollees in 7 counties.
The investigators found steeper increases in age- and sex-adjusted incidence of type 1 diabetes from 2002 to 2015 among black youth (2.7% per year), Hispanic youth (4%), and Asian and Pacific Islander youth (4.4%), than among their white counterparts (0.7%). Incidence among Asians and Pacific Islanders did not change significantly during 2002-2010, but increased steeply during 2011-2015 (8.5% per year) for unknown reasons.
“In parallel with increased obesity prevalence in U.S. youths, the incidence of type 2 diabetes among adolescents has increased at a higher rate than that of type 1 diabetes, especially among racial-/ethnic-minority youths,” the authors noted.
The number of new cases of type 2 diagnosed in children younger than 10 years were too few to report on (181 total cases during 2002-2015), so the incidence analysis was limited to children who were aged 10-19 years at diagnosis. The steepest annual percentage changes were among Asians and Pacific Islander youth (7.7% per year), followed by Hispanic (6.5%), black (6.0%), and American Indian (3.7%) youth.
“Although the SEARCH population is similar demographically to the U.S. youth population, it is not designed to be nationally representative,” which is one of the limitations of the study, the investigators wrote.
The authors reported having no conflicts of interest.
SOURCE: Divers J et al. MMWR Morb Mortal Wkly Rep. 2020;69:161-5.
according to a review of almost 70,000 children in the SEARCH for Diabetes in Youth Study, an ongoing, population-based surveillance project of individuals younger than 20 years.
“For both type 1 and type 2 diabetes, the rates of increase were generally higher among racial/ethnic minority populations than those among whites,” wrote the investigators, led by Jasmin Divers, PhD, of the division of health services research, department of foundations of medicine, at New York University. “These findings highlight the need for continued surveillance for diabetes among youths to monitor overall and group-specific trends, identify factors driving these trends, and inform health care planning.”
SEARCH identified 14,638 cases of pediatric type 1 diabetes and 3,916 cases of type 2 diabetes from 2002 to 2015. The study draws participants from all 64 counties in Colorado, plus selected Indian reservations in Arizona and New Mexico under the direction of Colorado; all 46 counties in South Carolina; 8 in Ohio; 5 in Washington; and Kaiser Permanente Southern California health plan enrollees in 7 counties.
The investigators found steeper increases in age- and sex-adjusted incidence of type 1 diabetes from 2002 to 2015 among black youth (2.7% per year), Hispanic youth (4%), and Asian and Pacific Islander youth (4.4%), than among their white counterparts (0.7%). Incidence among Asians and Pacific Islanders did not change significantly during 2002-2010, but increased steeply during 2011-2015 (8.5% per year) for unknown reasons.
“In parallel with increased obesity prevalence in U.S. youths, the incidence of type 2 diabetes among adolescents has increased at a higher rate than that of type 1 diabetes, especially among racial-/ethnic-minority youths,” the authors noted.
The number of new cases of type 2 diagnosed in children younger than 10 years were too few to report on (181 total cases during 2002-2015), so the incidence analysis was limited to children who were aged 10-19 years at diagnosis. The steepest annual percentage changes were among Asians and Pacific Islander youth (7.7% per year), followed by Hispanic (6.5%), black (6.0%), and American Indian (3.7%) youth.
“Although the SEARCH population is similar demographically to the U.S. youth population, it is not designed to be nationally representative,” which is one of the limitations of the study, the investigators wrote.
The authors reported having no conflicts of interest.
SOURCE: Divers J et al. MMWR Morb Mortal Wkly Rep. 2020;69:161-5.
FROM THE MORBIDITY AND MORTALITY WEEKLY REPORT
Subtype appears to predict docetaxel benefit in prostate cancer
SAN FRANCISCO – (ADT), according to a correlative analysis of the CHAARTED trial.
Researchers found that adding docetaxel to ADT reduced the risk of death by 55% among men with luminal B subtype tumors, which corresponded to about 22 more months of life on average. In contrast, there was no significant reduction in risk of death among men with the basal subtype.
Anis Hamid, MD, of the Dana-Farber Cancer Institute in Boston, presented these results at the 2020 Genitourinary Cancers Symposium, sponsored by the American Society for Clinical Oncology, ASTRO, and the Society of Urologic Oncology.
The phase 3, randomized CHAARTED trial included 790 men with newly diagnosed, metastatic, hormone-sensitive prostate cancer. Previously reported results showed a survival benefit of adding six cycles of docetaxel to ADT (N Engl J Med. 2015;373:737-46), with long-term durability driven by benefit in men with high-volume disease (J Clin Oncol. 2018;36:1080-7).
These findings, along with those from similar chemotherapy trials, changed the treatment paradigm for metastatic hormone-sensitive disease, Dr. Hamid noted. Androgen receptor signaling inhibitors and prostate radiation have since entered this treatment space as well.
“There is a critical need to identify and better understand patients who do and do not benefit from upfront chemotherapy or potent hormonal therapy in order to guide optimal therapy selection,” Dr. Hamid said. “It is hypothesized that understanding the underlying biology will provide critical insights.”
Study details
Dr. Hamid and colleagues analyzed primary biopsies obtained at the time of metastatic diagnosis, before ADT exposure, in 160 patients treated on the CHAARTED trial. The researchers performed whole-transcriptome profiling and classification using the PAM50 gene set.
Tumors were classified as basal subtype in 52.1% of patients, as luminal B subtype in 46.1%, and as luminal A subtype in 1.8%.
Among patients given ADT alone, those with luminal B subtype tended to have poorer overall survival than those with basal subtype (hazard ratio, 1.75; P = .052). Among patients who received ADT plus docetaxel, there was no significant difference in overall survival according to subtype (HR, 0.92; P = .14).
The addition of docetaxel to ADT significantly prolonged median overall survival among men with luminal B subtype (52.1 months vs. 29.8 months; HR, 0.45; P = .007) but not among men with basal subtype (49.2 months vs. 47.1 months; HR, 0.85; P = .60). Findings were similar in an analysis restricted to patients with high-volume disease, according to Dr. Hamid.
“Luminal B tumors are associated with a poorer prognosis on ADT alone but significantly benefit from the addition of docetaxel chemotherapy upfront,” he said. “Conversely, basal subtype predicts for a lack of survival benefit with docetaxel.”
Dr. Hamid and colleagues also found that adding docetaxel significantly prolonged median time to castration-resistant prostate cancer, regardless of whether the subtype was luminal B (16.9 months vs. 8.0 months; HR, 0.43; P = .001) or basal (17.7 months vs. 6.4 months; HR, 0.51; P = .013).
Dr. Hamid acknowledged that prediction of treatment response is likely to be more complex than this research suggests and additionally influenced by as-yet-unidentified biomarkers.
“I await more biological information from CHAARTED but also independent metastatic cohorts,” he said. “I don’t think it’s likely there will be just one particular very discrete story like we have presented today. The effect size that we saw in the luminal B tumors, for example, with respect to the benefit of docetaxel, wasn’t subtle. It was big, and I am ever keen to know what is driving that.”
Clinical implications
“The landscape of treatments for metastatic hormone-sensitive prostate cancer is very complicated, and there are many, many good options and probably more than one best answer for each patient,” said invited discussant Dana E. Rathkopf, MD, of the Memorial Sloan Kettering Cancer Center in New York.
Historically, the luminal B subtype has been associated with a good response to ADT but also with a high proliferation rate and fairly poor prognosis. “So maybe the luminal B response to ADT was good, but it wasn’t good enough, given the underlying biology of this poor prognostic subtype,” Dr. Rathkopf said.
She added that the efficacy of ADT alone may have been worse than expected in the luminal B group, making the addition of docetaxel appear more favorable, as overall survival with ADT plus docetaxel was similar between these subtypes, at about 50 months.
“But still, it looks like the addition of docetaxel added benefit to these luminal B cells regardless of whether they did better or worse than was expected on the ADT,” Dr. Rathkopf said. Taken together, the findings identify luminal B subtype as a predictive biomarker of response to docetaxel in men with metastatic hormone-sensitive prostate cancer, she added.
“Can this help select patients who should and should not receive docetaxel? I think we would all agree that further validation is needed before we move this into clinical practice,” Dr. Rathkopf said. “It’s not that easy, and there are probably additional tests and additional molecular factors that we need to incorporate into these types of models.”
“As a clinician, if I see a patient tomorrow with newly diagnosed metastatic hormone-sensitive prostate cancer who is basal subtype on PAM50, I certainly wouldn’t withhold docetaxel,” Dr. Hamid said. “Withholding a proven life-prolonging therapy needs to meet a very high bar of biomarker discovery and validation independently for us to confidently translate that to the clinics.”
This research was funded by Decipher Biosciences and the National Institutes of Health. The CHAARTED trial was funded by the ECOG-ACRIN Cancer Research Group in collaboration with the National Cancer Institute.
Dr. Hamid disclosed relationships with Bayer and Merck Sharp and Dohme. Dr. Rathkopf disclosed relationships with AstraZeneca, Genentech/Roche, Janssen, Celgene, Ferring, Medivation, Millennium, Novartis, Taiho Pharmaceutical, Takeda, and TRACON Pharma.
SOURCE: Hamid A et al. GUCS 2020. Abstract 162.
SAN FRANCISCO – (ADT), according to a correlative analysis of the CHAARTED trial.
Researchers found that adding docetaxel to ADT reduced the risk of death by 55% among men with luminal B subtype tumors, which corresponded to about 22 more months of life on average. In contrast, there was no significant reduction in risk of death among men with the basal subtype.
Anis Hamid, MD, of the Dana-Farber Cancer Institute in Boston, presented these results at the 2020 Genitourinary Cancers Symposium, sponsored by the American Society for Clinical Oncology, ASTRO, and the Society of Urologic Oncology.
The phase 3, randomized CHAARTED trial included 790 men with newly diagnosed, metastatic, hormone-sensitive prostate cancer. Previously reported results showed a survival benefit of adding six cycles of docetaxel to ADT (N Engl J Med. 2015;373:737-46), with long-term durability driven by benefit in men with high-volume disease (J Clin Oncol. 2018;36:1080-7).
These findings, along with those from similar chemotherapy trials, changed the treatment paradigm for metastatic hormone-sensitive disease, Dr. Hamid noted. Androgen receptor signaling inhibitors and prostate radiation have since entered this treatment space as well.
“There is a critical need to identify and better understand patients who do and do not benefit from upfront chemotherapy or potent hormonal therapy in order to guide optimal therapy selection,” Dr. Hamid said. “It is hypothesized that understanding the underlying biology will provide critical insights.”
Study details
Dr. Hamid and colleagues analyzed primary biopsies obtained at the time of metastatic diagnosis, before ADT exposure, in 160 patients treated on the CHAARTED trial. The researchers performed whole-transcriptome profiling and classification using the PAM50 gene set.
Tumors were classified as basal subtype in 52.1% of patients, as luminal B subtype in 46.1%, and as luminal A subtype in 1.8%.
Among patients given ADT alone, those with luminal B subtype tended to have poorer overall survival than those with basal subtype (hazard ratio, 1.75; P = .052). Among patients who received ADT plus docetaxel, there was no significant difference in overall survival according to subtype (HR, 0.92; P = .14).
The addition of docetaxel to ADT significantly prolonged median overall survival among men with luminal B subtype (52.1 months vs. 29.8 months; HR, 0.45; P = .007) but not among men with basal subtype (49.2 months vs. 47.1 months; HR, 0.85; P = .60). Findings were similar in an analysis restricted to patients with high-volume disease, according to Dr. Hamid.
“Luminal B tumors are associated with a poorer prognosis on ADT alone but significantly benefit from the addition of docetaxel chemotherapy upfront,” he said. “Conversely, basal subtype predicts for a lack of survival benefit with docetaxel.”
Dr. Hamid and colleagues also found that adding docetaxel significantly prolonged median time to castration-resistant prostate cancer, regardless of whether the subtype was luminal B (16.9 months vs. 8.0 months; HR, 0.43; P = .001) or basal (17.7 months vs. 6.4 months; HR, 0.51; P = .013).
Dr. Hamid acknowledged that prediction of treatment response is likely to be more complex than this research suggests and additionally influenced by as-yet-unidentified biomarkers.
“I await more biological information from CHAARTED but also independent metastatic cohorts,” he said. “I don’t think it’s likely there will be just one particular very discrete story like we have presented today. The effect size that we saw in the luminal B tumors, for example, with respect to the benefit of docetaxel, wasn’t subtle. It was big, and I am ever keen to know what is driving that.”
Clinical implications
“The landscape of treatments for metastatic hormone-sensitive prostate cancer is very complicated, and there are many, many good options and probably more than one best answer for each patient,” said invited discussant Dana E. Rathkopf, MD, of the Memorial Sloan Kettering Cancer Center in New York.
Historically, the luminal B subtype has been associated with a good response to ADT but also with a high proliferation rate and fairly poor prognosis. “So maybe the luminal B response to ADT was good, but it wasn’t good enough, given the underlying biology of this poor prognostic subtype,” Dr. Rathkopf said.
She added that the efficacy of ADT alone may have been worse than expected in the luminal B group, making the addition of docetaxel appear more favorable, as overall survival with ADT plus docetaxel was similar between these subtypes, at about 50 months.
“But still, it looks like the addition of docetaxel added benefit to these luminal B cells regardless of whether they did better or worse than was expected on the ADT,” Dr. Rathkopf said. Taken together, the findings identify luminal B subtype as a predictive biomarker of response to docetaxel in men with metastatic hormone-sensitive prostate cancer, she added.
“Can this help select patients who should and should not receive docetaxel? I think we would all agree that further validation is needed before we move this into clinical practice,” Dr. Rathkopf said. “It’s not that easy, and there are probably additional tests and additional molecular factors that we need to incorporate into these types of models.”
“As a clinician, if I see a patient tomorrow with newly diagnosed metastatic hormone-sensitive prostate cancer who is basal subtype on PAM50, I certainly wouldn’t withhold docetaxel,” Dr. Hamid said. “Withholding a proven life-prolonging therapy needs to meet a very high bar of biomarker discovery and validation independently for us to confidently translate that to the clinics.”
This research was funded by Decipher Biosciences and the National Institutes of Health. The CHAARTED trial was funded by the ECOG-ACRIN Cancer Research Group in collaboration with the National Cancer Institute.
Dr. Hamid disclosed relationships with Bayer and Merck Sharp and Dohme. Dr. Rathkopf disclosed relationships with AstraZeneca, Genentech/Roche, Janssen, Celgene, Ferring, Medivation, Millennium, Novartis, Taiho Pharmaceutical, Takeda, and TRACON Pharma.
SOURCE: Hamid A et al. GUCS 2020. Abstract 162.
SAN FRANCISCO – (ADT), according to a correlative analysis of the CHAARTED trial.
Researchers found that adding docetaxel to ADT reduced the risk of death by 55% among men with luminal B subtype tumors, which corresponded to about 22 more months of life on average. In contrast, there was no significant reduction in risk of death among men with the basal subtype.
Anis Hamid, MD, of the Dana-Farber Cancer Institute in Boston, presented these results at the 2020 Genitourinary Cancers Symposium, sponsored by the American Society for Clinical Oncology, ASTRO, and the Society of Urologic Oncology.
The phase 3, randomized CHAARTED trial included 790 men with newly diagnosed, metastatic, hormone-sensitive prostate cancer. Previously reported results showed a survival benefit of adding six cycles of docetaxel to ADT (N Engl J Med. 2015;373:737-46), with long-term durability driven by benefit in men with high-volume disease (J Clin Oncol. 2018;36:1080-7).
These findings, along with those from similar chemotherapy trials, changed the treatment paradigm for metastatic hormone-sensitive disease, Dr. Hamid noted. Androgen receptor signaling inhibitors and prostate radiation have since entered this treatment space as well.
“There is a critical need to identify and better understand patients who do and do not benefit from upfront chemotherapy or potent hormonal therapy in order to guide optimal therapy selection,” Dr. Hamid said. “It is hypothesized that understanding the underlying biology will provide critical insights.”
Study details
Dr. Hamid and colleagues analyzed primary biopsies obtained at the time of metastatic diagnosis, before ADT exposure, in 160 patients treated on the CHAARTED trial. The researchers performed whole-transcriptome profiling and classification using the PAM50 gene set.
Tumors were classified as basal subtype in 52.1% of patients, as luminal B subtype in 46.1%, and as luminal A subtype in 1.8%.
Among patients given ADT alone, those with luminal B subtype tended to have poorer overall survival than those with basal subtype (hazard ratio, 1.75; P = .052). Among patients who received ADT plus docetaxel, there was no significant difference in overall survival according to subtype (HR, 0.92; P = .14).
The addition of docetaxel to ADT significantly prolonged median overall survival among men with luminal B subtype (52.1 months vs. 29.8 months; HR, 0.45; P = .007) but not among men with basal subtype (49.2 months vs. 47.1 months; HR, 0.85; P = .60). Findings were similar in an analysis restricted to patients with high-volume disease, according to Dr. Hamid.
“Luminal B tumors are associated with a poorer prognosis on ADT alone but significantly benefit from the addition of docetaxel chemotherapy upfront,” he said. “Conversely, basal subtype predicts for a lack of survival benefit with docetaxel.”
Dr. Hamid and colleagues also found that adding docetaxel significantly prolonged median time to castration-resistant prostate cancer, regardless of whether the subtype was luminal B (16.9 months vs. 8.0 months; HR, 0.43; P = .001) or basal (17.7 months vs. 6.4 months; HR, 0.51; P = .013).
Dr. Hamid acknowledged that prediction of treatment response is likely to be more complex than this research suggests and additionally influenced by as-yet-unidentified biomarkers.
“I await more biological information from CHAARTED but also independent metastatic cohorts,” he said. “I don’t think it’s likely there will be just one particular very discrete story like we have presented today. The effect size that we saw in the luminal B tumors, for example, with respect to the benefit of docetaxel, wasn’t subtle. It was big, and I am ever keen to know what is driving that.”
Clinical implications
“The landscape of treatments for metastatic hormone-sensitive prostate cancer is very complicated, and there are many, many good options and probably more than one best answer for each patient,” said invited discussant Dana E. Rathkopf, MD, of the Memorial Sloan Kettering Cancer Center in New York.
Historically, the luminal B subtype has been associated with a good response to ADT but also with a high proliferation rate and fairly poor prognosis. “So maybe the luminal B response to ADT was good, but it wasn’t good enough, given the underlying biology of this poor prognostic subtype,” Dr. Rathkopf said.
She added that the efficacy of ADT alone may have been worse than expected in the luminal B group, making the addition of docetaxel appear more favorable, as overall survival with ADT plus docetaxel was similar between these subtypes, at about 50 months.
“But still, it looks like the addition of docetaxel added benefit to these luminal B cells regardless of whether they did better or worse than was expected on the ADT,” Dr. Rathkopf said. Taken together, the findings identify luminal B subtype as a predictive biomarker of response to docetaxel in men with metastatic hormone-sensitive prostate cancer, she added.
“Can this help select patients who should and should not receive docetaxel? I think we would all agree that further validation is needed before we move this into clinical practice,” Dr. Rathkopf said. “It’s not that easy, and there are probably additional tests and additional molecular factors that we need to incorporate into these types of models.”
“As a clinician, if I see a patient tomorrow with newly diagnosed metastatic hormone-sensitive prostate cancer who is basal subtype on PAM50, I certainly wouldn’t withhold docetaxel,” Dr. Hamid said. “Withholding a proven life-prolonging therapy needs to meet a very high bar of biomarker discovery and validation independently for us to confidently translate that to the clinics.”
This research was funded by Decipher Biosciences and the National Institutes of Health. The CHAARTED trial was funded by the ECOG-ACRIN Cancer Research Group in collaboration with the National Cancer Institute.
Dr. Hamid disclosed relationships with Bayer and Merck Sharp and Dohme. Dr. Rathkopf disclosed relationships with AstraZeneca, Genentech/Roche, Janssen, Celgene, Ferring, Medivation, Millennium, Novartis, Taiho Pharmaceutical, Takeda, and TRACON Pharma.
SOURCE: Hamid A et al. GUCS 2020. Abstract 162.
REPORTING FROM GUCS 2020
50 years of growth: More dermatologists, more demand
A dive into the dermatology data pool reveals a great deal of change in the 50 years that Dermatology News and Skin & Allergy News have been covering the specialty.
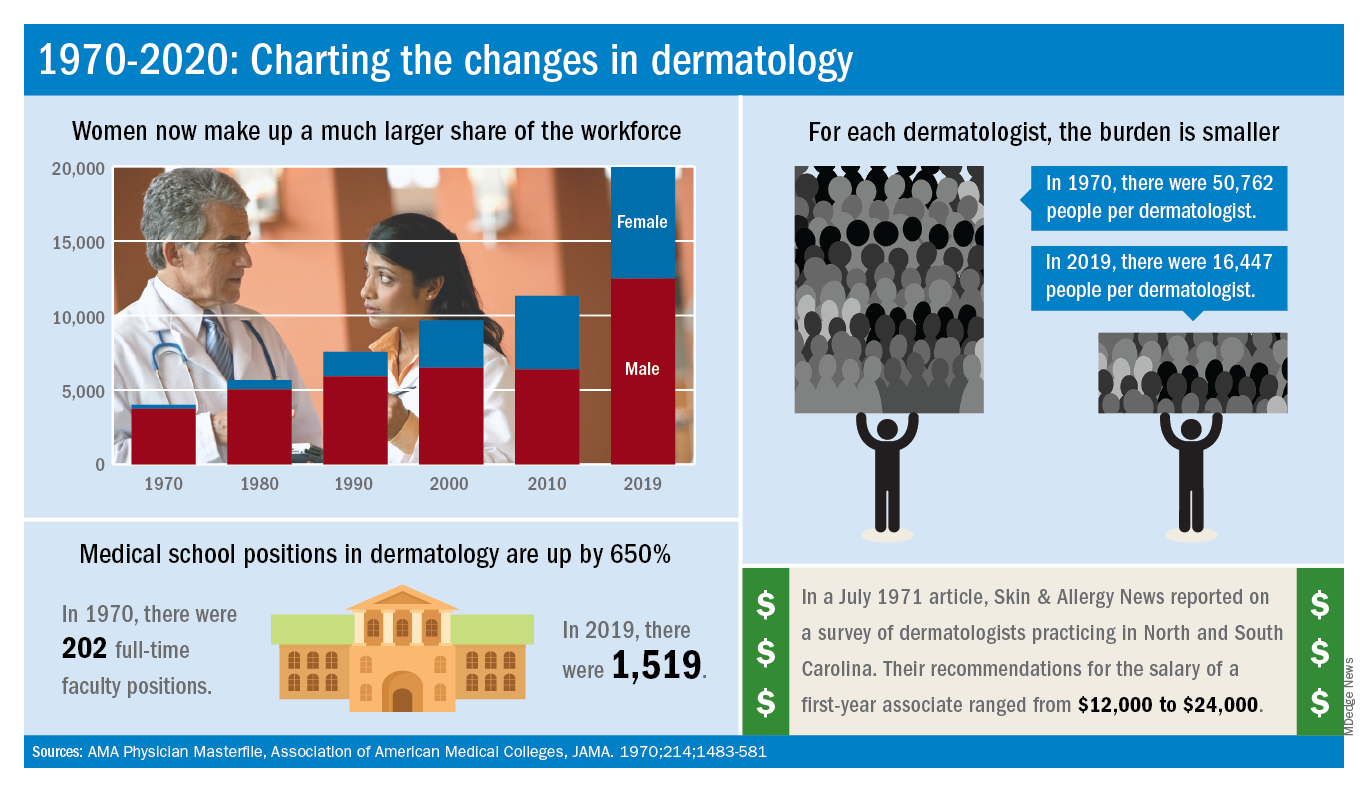
For one thing, there are a lot more dermatologists now. The American Academy of Dermatology puts its 2020 membership at a somewhat lower 18,898, noting that not all dermatologists are AAD members.
Much of that 50-year increase comes from the larger proportion of women entering the specialty. In 1970, only 7% of dermatologists were women, but by 2019 they represented over 37% of the dermatology workforce, according to the AMA numbers. The AAD, however, reports more women than the AMA (8,940 vs. 7,482), so the proportion of female academy members in 2020 is about 47%.
The population of dermatologists has increased faster than the general population since 1970, leading to a rising density of providers. A report from 1973 put the national figure at 1.7 dermatologists per 100,000 population in 1970, and two more recent studies in JAMA Dermatology reported density levels of 3.65 per 100,000 in 2013 and 3.36 per 100,000 in 2016.
In that 1973 report, the authors said that there was “no evidence to suggest that there will not be sufficient dermatologists in the future, if training centers are maintained and adequately financed,” noting that “the current rate of filling of dermatologic residency positions should not diminish and may continue to increase.”
In 2017, the conclusion was quite different: “Dermatologists alone have been unable to meet increasing patient demand for dermatologic services. The number of dermatology residency training positions has been relatively stagnant, suggesting that the current supply of dermatologists in training will be insufficient to fully meet growing future demand.”
The current state of strong demand for dermatologists does come with some benefits. In a 2019 survey by physician recruitment firm Merritt Hawkins, dermatologists had the 6th-highest starting salary at $420,000 a year. An article in the July 1971 issue of Skin & Allergy News offered a somewhat different perspective on compensation for first-year dermatologists: Recommendations offered by those already in practice ranged from $12,000 to $24,000.
Those first-year physicians are coming from residencies that, not too surprisingly, are now producing more new dermatologists than they did in 1970, although perhaps not as many more as might be expected.
There were an estimated 250 individuals completing dermatology residencies annually in 1976 (J Am Acad Dermatol. 1981;4[3]:344-5), which suggests a total of approximately 750 active residents at a time when there were about 5,000 practicing dermatologists in the country.
For the 2018-2019 academic year, there were 1,439 active residents in U.S. training programs, according to the Association of American Medical Colleges, so there were twice the number of residents but four times as many dermatologists in practice, compared with 1976.
The next link in the dermatology supply chain would be the medical schools, and there the numbers of full-time faculty have more than kept up. For the 1969-1970 academic year, there were 202 full-time positions in dermatology (JAMA. 1970;214:1483-581). By 2019, the number had risen to 1,519, according to the Association of American Medical Colleges.
A dive into the dermatology data pool reveals a great deal of change in the 50 years that Dermatology News and Skin & Allergy News have been covering the specialty.

For one thing, there are a lot more dermatologists now. The American Academy of Dermatology puts its 2020 membership at a somewhat lower 18,898, noting that not all dermatologists are AAD members.
Much of that 50-year increase comes from the larger proportion of women entering the specialty. In 1970, only 7% of dermatologists were women, but by 2019 they represented over 37% of the dermatology workforce, according to the AMA numbers. The AAD, however, reports more women than the AMA (8,940 vs. 7,482), so the proportion of female academy members in 2020 is about 47%.
The population of dermatologists has increased faster than the general population since 1970, leading to a rising density of providers. A report from 1973 put the national figure at 1.7 dermatologists per 100,000 population in 1970, and two more recent studies in JAMA Dermatology reported density levels of 3.65 per 100,000 in 2013 and 3.36 per 100,000 in 2016.
In that 1973 report, the authors said that there was “no evidence to suggest that there will not be sufficient dermatologists in the future, if training centers are maintained and adequately financed,” noting that “the current rate of filling of dermatologic residency positions should not diminish and may continue to increase.”
In 2017, the conclusion was quite different: “Dermatologists alone have been unable to meet increasing patient demand for dermatologic services. The number of dermatology residency training positions has been relatively stagnant, suggesting that the current supply of dermatologists in training will be insufficient to fully meet growing future demand.”
The current state of strong demand for dermatologists does come with some benefits. In a 2019 survey by physician recruitment firm Merritt Hawkins, dermatologists had the 6th-highest starting salary at $420,000 a year. An article in the July 1971 issue of Skin & Allergy News offered a somewhat different perspective on compensation for first-year dermatologists: Recommendations offered by those already in practice ranged from $12,000 to $24,000.
Those first-year physicians are coming from residencies that, not too surprisingly, are now producing more new dermatologists than they did in 1970, although perhaps not as many more as might be expected.
There were an estimated 250 individuals completing dermatology residencies annually in 1976 (J Am Acad Dermatol. 1981;4[3]:344-5), which suggests a total of approximately 750 active residents at a time when there were about 5,000 practicing dermatologists in the country.
For the 2018-2019 academic year, there were 1,439 active residents in U.S. training programs, according to the Association of American Medical Colleges, so there were twice the number of residents but four times as many dermatologists in practice, compared with 1976.
The next link in the dermatology supply chain would be the medical schools, and there the numbers of full-time faculty have more than kept up. For the 1969-1970 academic year, there were 202 full-time positions in dermatology (JAMA. 1970;214:1483-581). By 2019, the number had risen to 1,519, according to the Association of American Medical Colleges.
A dive into the dermatology data pool reveals a great deal of change in the 50 years that Dermatology News and Skin & Allergy News have been covering the specialty.

For one thing, there are a lot more dermatologists now. The American Academy of Dermatology puts its 2020 membership at a somewhat lower 18,898, noting that not all dermatologists are AAD members.
Much of that 50-year increase comes from the larger proportion of women entering the specialty. In 1970, only 7% of dermatologists were women, but by 2019 they represented over 37% of the dermatology workforce, according to the AMA numbers. The AAD, however, reports more women than the AMA (8,940 vs. 7,482), so the proportion of female academy members in 2020 is about 47%.
The population of dermatologists has increased faster than the general population since 1970, leading to a rising density of providers. A report from 1973 put the national figure at 1.7 dermatologists per 100,000 population in 1970, and two more recent studies in JAMA Dermatology reported density levels of 3.65 per 100,000 in 2013 and 3.36 per 100,000 in 2016.
In that 1973 report, the authors said that there was “no evidence to suggest that there will not be sufficient dermatologists in the future, if training centers are maintained and adequately financed,” noting that “the current rate of filling of dermatologic residency positions should not diminish and may continue to increase.”
In 2017, the conclusion was quite different: “Dermatologists alone have been unable to meet increasing patient demand for dermatologic services. The number of dermatology residency training positions has been relatively stagnant, suggesting that the current supply of dermatologists in training will be insufficient to fully meet growing future demand.”
The current state of strong demand for dermatologists does come with some benefits. In a 2019 survey by physician recruitment firm Merritt Hawkins, dermatologists had the 6th-highest starting salary at $420,000 a year. An article in the July 1971 issue of Skin & Allergy News offered a somewhat different perspective on compensation for first-year dermatologists: Recommendations offered by those already in practice ranged from $12,000 to $24,000.
Those first-year physicians are coming from residencies that, not too surprisingly, are now producing more new dermatologists than they did in 1970, although perhaps not as many more as might be expected.
There were an estimated 250 individuals completing dermatology residencies annually in 1976 (J Am Acad Dermatol. 1981;4[3]:344-5), which suggests a total of approximately 750 active residents at a time when there were about 5,000 practicing dermatologists in the country.
For the 2018-2019 academic year, there were 1,439 active residents in U.S. training programs, according to the Association of American Medical Colleges, so there were twice the number of residents but four times as many dermatologists in practice, compared with 1976.
The next link in the dermatology supply chain would be the medical schools, and there the numbers of full-time faculty have more than kept up. For the 1969-1970 academic year, there were 202 full-time positions in dermatology (JAMA. 1970;214:1483-581). By 2019, the number had risen to 1,519, according to the Association of American Medical Colleges.
Flu increases activity but not its severity

The CDC’s latest report shows that 6.8% of outpatients visiting health care providers had influenza-like illness during the week ending Feb. 8. That’s up from the previous week’s 6.6%, but that rise of 0.2 percentage points is smaller than the 0.6-point rises that occurred each of the 2 weeks before, and that could mean that activity is slowing.
That slowing, however, is not noticeable from this week’s map, which puts 41 states (there were 35 last week) and Puerto Rico in the red at the highest level of activity on the CDC’s 1-10 scale and another three states in the “high” range with levels of 8 or 9, the CDC’s influenza division reported.
That leaves Nevada and Oregon at level 7; Alaska, Florida, and the District of Columbia at level 5; Idaho at level 3, and Delaware with insufficient data (it was at level 5 last week), the CDC said.
The 2019-2020 season’s high activity, fortunately, has not translated into high severity, as overall hospitalization and mortality rates continue to remain at fairly typical levels. Hospitalization rates are elevated among children and young adults, however, and pediatric deaths are now up to 92, the CDC said, which is high for this point in the season.

The CDC’s latest report shows that 6.8% of outpatients visiting health care providers had influenza-like illness during the week ending Feb. 8. That’s up from the previous week’s 6.6%, but that rise of 0.2 percentage points is smaller than the 0.6-point rises that occurred each of the 2 weeks before, and that could mean that activity is slowing.
That slowing, however, is not noticeable from this week’s map, which puts 41 states (there were 35 last week) and Puerto Rico in the red at the highest level of activity on the CDC’s 1-10 scale and another three states in the “high” range with levels of 8 or 9, the CDC’s influenza division reported.
That leaves Nevada and Oregon at level 7; Alaska, Florida, and the District of Columbia at level 5; Idaho at level 3, and Delaware with insufficient data (it was at level 5 last week), the CDC said.
The 2019-2020 season’s high activity, fortunately, has not translated into high severity, as overall hospitalization and mortality rates continue to remain at fairly typical levels. Hospitalization rates are elevated among children and young adults, however, and pediatric deaths are now up to 92, the CDC said, which is high for this point in the season.

The CDC’s latest report shows that 6.8% of outpatients visiting health care providers had influenza-like illness during the week ending Feb. 8. That’s up from the previous week’s 6.6%, but that rise of 0.2 percentage points is smaller than the 0.6-point rises that occurred each of the 2 weeks before, and that could mean that activity is slowing.
That slowing, however, is not noticeable from this week’s map, which puts 41 states (there were 35 last week) and Puerto Rico in the red at the highest level of activity on the CDC’s 1-10 scale and another three states in the “high” range with levels of 8 or 9, the CDC’s influenza division reported.
That leaves Nevada and Oregon at level 7; Alaska, Florida, and the District of Columbia at level 5; Idaho at level 3, and Delaware with insufficient data (it was at level 5 last week), the CDC said.
The 2019-2020 season’s high activity, fortunately, has not translated into high severity, as overall hospitalization and mortality rates continue to remain at fairly typical levels. Hospitalization rates are elevated among children and young adults, however, and pediatric deaths are now up to 92, the CDC said, which is high for this point in the season.