User login
Make the Diagnosis - March 2020
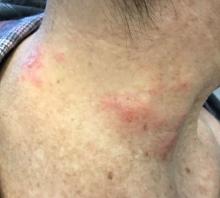
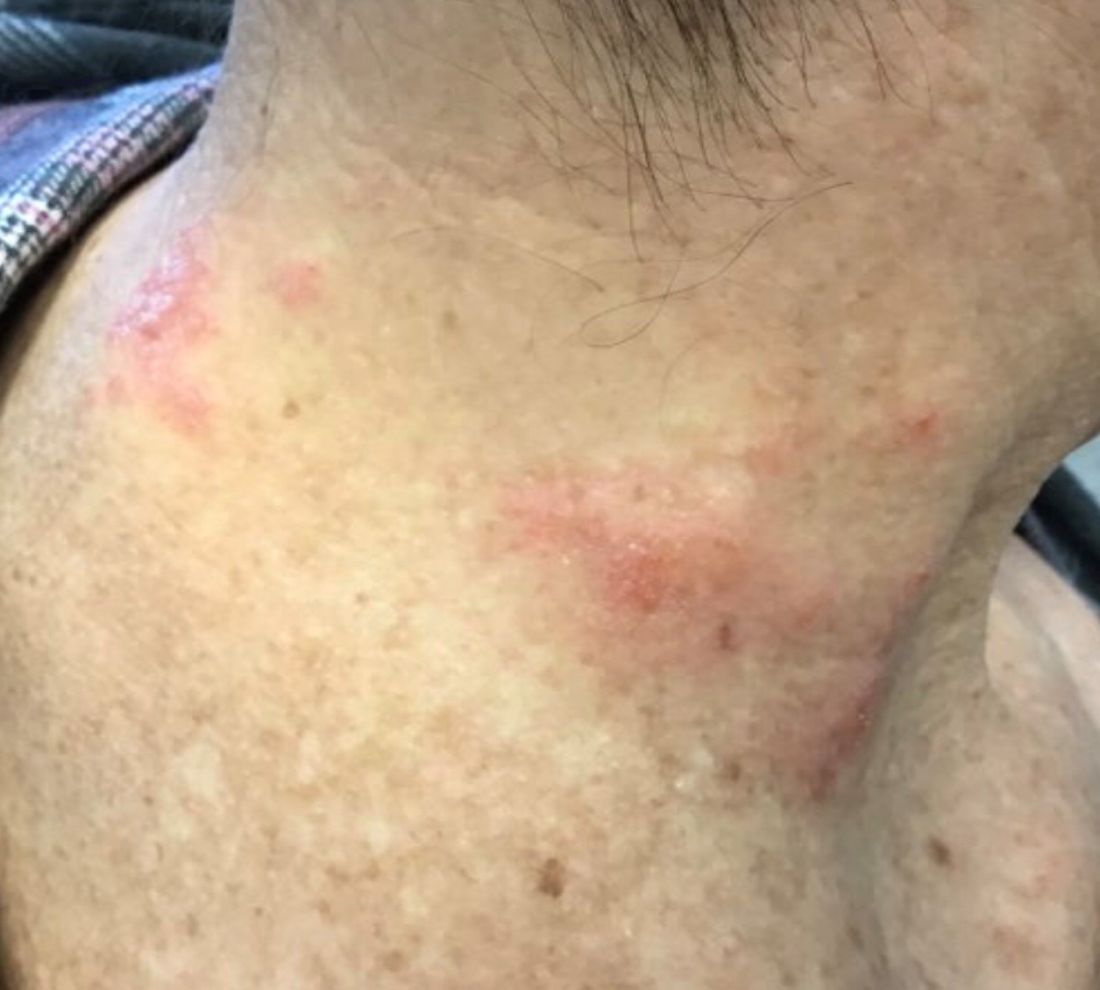
The patient’s biopsy showed sparse and grouped and slightly enlarged atypical stained mononuclear cells in mostly perifollicular areas with focal epidermotropism. CD30 staining was positive. She responded to potent topical steroids.
The etiology of LyP is unknown. It is unclear whether the proliferation of T-cells is a benign and chronic disorder, or an indolent T-cell malignancy.
In addition, 10% of LyP cases are associated with anaplastic large-cell lymphoma, cutaneous T-cell lymphoma (mycosis fungoides), or Hodgkin lymphoma. Borderline cases are those that overlap LyP and lymphoma.
Patients typically present with crops of asymptomatic erythematous to brown papules that may become pustular, vesicular, or necrotic. Lesions tend to resolve within 2-8 weeks with or without scarring. The trunk and extremities are commonly affected. The condition tends to be chronic over months to years. The waxing and waning course is characteristic of LyP. Constitutional symptoms are generally absent in cases not associated with systemic disease.
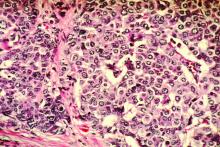
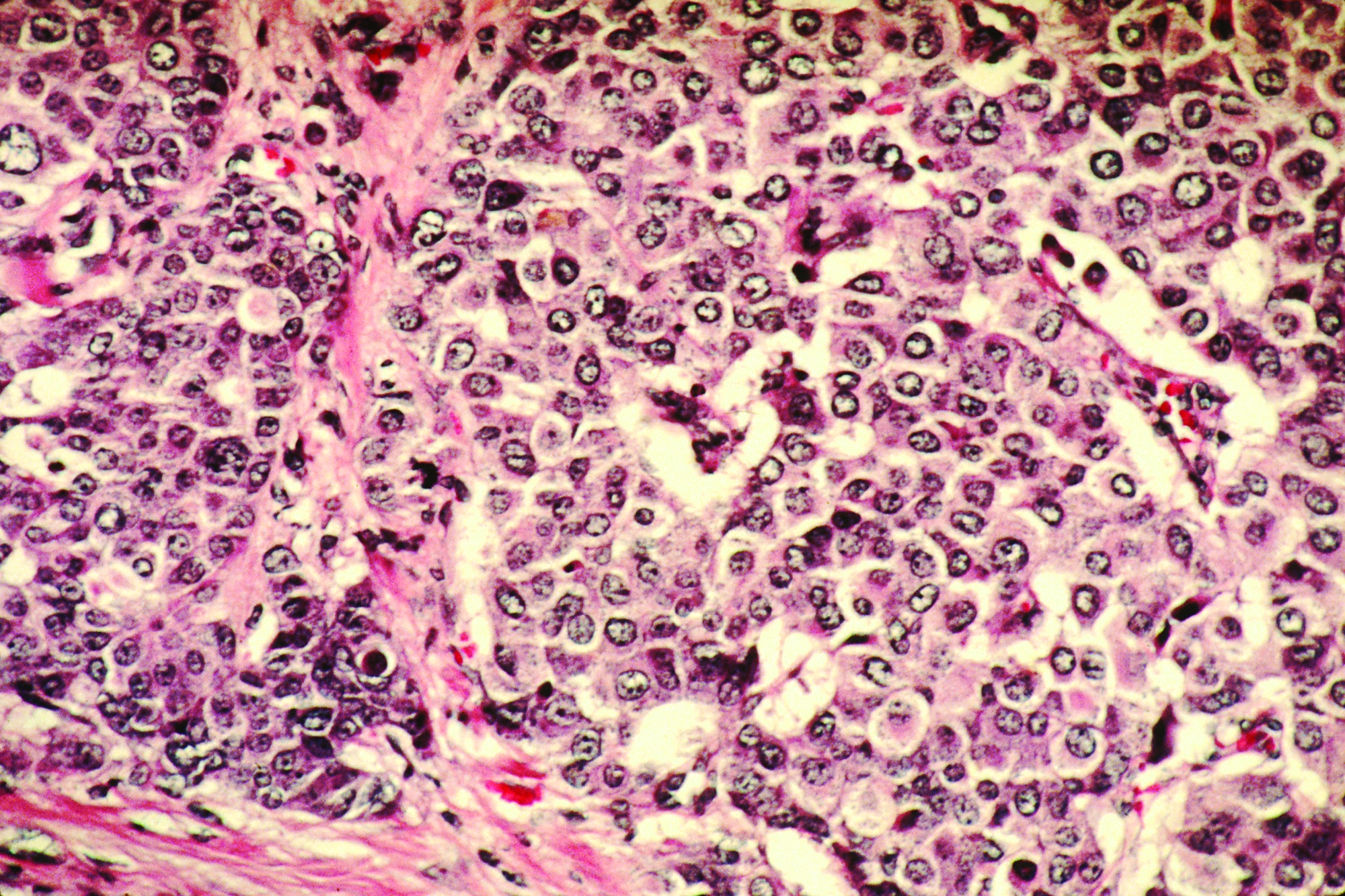
Histopathologic examination reveals a dense wedge-shaped dermal infiltrate of atypical lymphocytes along with numerous eosinophils and neutrophils. Epidermotropism may be present and lymphocytes stain positive for CD30+. Vessels in the dermis may exhibit fibrin deposition and red blood cell extravasation. Histologically, LyP can be classified as Type A to E. These subtypes are determined by the size and type of atypical cells, location and amount of infiltrate, and staining of CD30 and CD8.
The differential diagnosis of LyP includes pityriasis lichenoides, anaplastic large cell lymphoma, cutaneous T-cell lymphoma, folliculitis, arthropod assault, Langerhans cell histiocytosis, and leukemia cutis. Treatment is symptomatic. Mild forms of LyP can many times be managed with superpotent topical corticosteroids. Bexarotene gel has been used for early lesions. For more widespread or persistent disease, intralesional corticosteroids, phototherapy (UVB or PUVA), tetracycline antibiotics, and methotrexate have been reported to be effective. Refractory cases may respond to interferon alpha or oral bexarotene. Routine evaluations are recommended as patients may be at increased risk for the development of lymphoma.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
The patient’s biopsy showed sparse and grouped and slightly enlarged atypical stained mononuclear cells in mostly perifollicular areas with focal epidermotropism. CD30 staining was positive. She responded to potent topical steroids.
The etiology of LyP is unknown. It is unclear whether the proliferation of T-cells is a benign and chronic disorder, or an indolent T-cell malignancy.
In addition, 10% of LyP cases are associated with anaplastic large-cell lymphoma, cutaneous T-cell lymphoma (mycosis fungoides), or Hodgkin lymphoma. Borderline cases are those that overlap LyP and lymphoma.
Patients typically present with crops of asymptomatic erythematous to brown papules that may become pustular, vesicular, or necrotic. Lesions tend to resolve within 2-8 weeks with or without scarring. The trunk and extremities are commonly affected. The condition tends to be chronic over months to years. The waxing and waning course is characteristic of LyP. Constitutional symptoms are generally absent in cases not associated with systemic disease.
Histopathologic examination reveals a dense wedge-shaped dermal infiltrate of atypical lymphocytes along with numerous eosinophils and neutrophils. Epidermotropism may be present and lymphocytes stain positive for CD30+. Vessels in the dermis may exhibit fibrin deposition and red blood cell extravasation. Histologically, LyP can be classified as Type A to E. These subtypes are determined by the size and type of atypical cells, location and amount of infiltrate, and staining of CD30 and CD8.
The differential diagnosis of LyP includes pityriasis lichenoides, anaplastic large cell lymphoma, cutaneous T-cell lymphoma, folliculitis, arthropod assault, Langerhans cell histiocytosis, and leukemia cutis. Treatment is symptomatic. Mild forms of LyP can many times be managed with superpotent topical corticosteroids. Bexarotene gel has been used for early lesions. For more widespread or persistent disease, intralesional corticosteroids, phototherapy (UVB or PUVA), tetracycline antibiotics, and methotrexate have been reported to be effective. Refractory cases may respond to interferon alpha or oral bexarotene. Routine evaluations are recommended as patients may be at increased risk for the development of lymphoma.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
The patient’s biopsy showed sparse and grouped and slightly enlarged atypical stained mononuclear cells in mostly perifollicular areas with focal epidermotropism. CD30 staining was positive. She responded to potent topical steroids.
The etiology of LyP is unknown. It is unclear whether the proliferation of T-cells is a benign and chronic disorder, or an indolent T-cell malignancy.
In addition, 10% of LyP cases are associated with anaplastic large-cell lymphoma, cutaneous T-cell lymphoma (mycosis fungoides), or Hodgkin lymphoma. Borderline cases are those that overlap LyP and lymphoma.
Patients typically present with crops of asymptomatic erythematous to brown papules that may become pustular, vesicular, or necrotic. Lesions tend to resolve within 2-8 weeks with or without scarring. The trunk and extremities are commonly affected. The condition tends to be chronic over months to years. The waxing and waning course is characteristic of LyP. Constitutional symptoms are generally absent in cases not associated with systemic disease.
Histopathologic examination reveals a dense wedge-shaped dermal infiltrate of atypical lymphocytes along with numerous eosinophils and neutrophils. Epidermotropism may be present and lymphocytes stain positive for CD30+. Vessels in the dermis may exhibit fibrin deposition and red blood cell extravasation. Histologically, LyP can be classified as Type A to E. These subtypes are determined by the size and type of atypical cells, location and amount of infiltrate, and staining of CD30 and CD8.
The differential diagnosis of LyP includes pityriasis lichenoides, anaplastic large cell lymphoma, cutaneous T-cell lymphoma, folliculitis, arthropod assault, Langerhans cell histiocytosis, and leukemia cutis. Treatment is symptomatic. Mild forms of LyP can many times be managed with superpotent topical corticosteroids. Bexarotene gel has been used for early lesions. For more widespread or persistent disease, intralesional corticosteroids, phototherapy (UVB or PUVA), tetracycline antibiotics, and methotrexate have been reported to be effective. Refractory cases may respond to interferon alpha or oral bexarotene. Routine evaluations are recommended as patients may be at increased risk for the development of lymphoma.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
Pediatricians twice as happy outside work than at work
Pediatricians are twice as likely to be happy outside the office than they are at work, according to Medscape’s 2020 Lifestyle, Happiness, and Burnout Report.
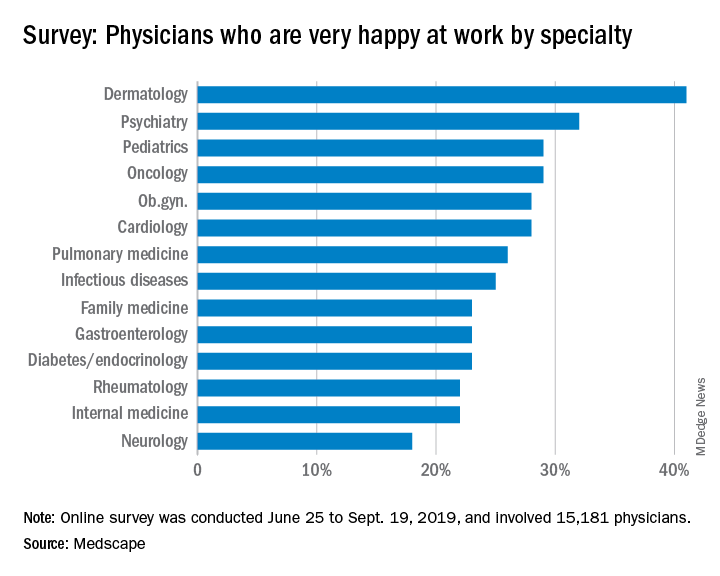
About 29% of pediatricians reported being happy at work, with dermatologists taking the top spot at 41%. Pediatricians did much better when it came to finding happiness outside the office, with 57% reporting that they were very happy when away from work, according to the Medscape report.
The biggest contributing factors to burnout in pediatricians were an overabundance of bureaucratic tasks (59%), insufficient compensation/reimbursement (37%), and spending too many hours at work (34%).
Pediatricians most commonly dealt with burnout by talking with friends/family (54%), exercising (47%), and sleeping (41%). Just over half of pediatricians reported taking 3-4 weeks of vacation, compared with 44% of all physicians; 32% took less than 3 weeks’ vacation.
About 8% of pediatricians reported that they’d contemplated suicide, but 0% reported that they’d attempted it; 85% said that they’d never thought about it. Just under one-quarter of pediatricians said that were currently seeking or planning to seek professional help for depression and/or burnout; 55% said they were not seeking help and had never made use of it in the past.
The Medscape survey was conducted from June 25 to Sept. 19, 2019, and involved 15,181 physicians.
We all feel it. It is not surprising that only 29% of today's pediatricians report that they are "happy" at work and 30% report "burnout"!
This report serves to identify only some of the countless ways in which we are forced to compromise the 24-hour clock, leaving too little time for ourselves and families.
We spend too many hours at work, and other data show we are undercompensated for our efforts.
Today, electronically, most of us are reachable even when out of the office. It is difficult, if not impossible, to completely disconnect. The challenge to achieve the work/life balance we have all imagined is too great!
I try to carve out "forced escapes from reality" through novels, movies, and when possible, distant travel with my spouse. However, the bliss is too short lived. When I return to reality, some bliss fades as I jump back onto the "merry-go-round" for a few more turns.
Lillian M. Beard, MD, is a clinical professor of pediatrics at George Washington University, Washington. She is a Pediatric News Editorial Advisory Board member.
We all feel it. It is not surprising that only 29% of today's pediatricians report that they are "happy" at work and 30% report "burnout"!
This report serves to identify only some of the countless ways in which we are forced to compromise the 24-hour clock, leaving too little time for ourselves and families.
We spend too many hours at work, and other data show we are undercompensated for our efforts.
Today, electronically, most of us are reachable even when out of the office. It is difficult, if not impossible, to completely disconnect. The challenge to achieve the work/life balance we have all imagined is too great!
I try to carve out "forced escapes from reality" through novels, movies, and when possible, distant travel with my spouse. However, the bliss is too short lived. When I return to reality, some bliss fades as I jump back onto the "merry-go-round" for a few more turns.
Lillian M. Beard, MD, is a clinical professor of pediatrics at George Washington University, Washington. She is a Pediatric News Editorial Advisory Board member.
We all feel it. It is not surprising that only 29% of today's pediatricians report that they are "happy" at work and 30% report "burnout"!
This report serves to identify only some of the countless ways in which we are forced to compromise the 24-hour clock, leaving too little time for ourselves and families.
We spend too many hours at work, and other data show we are undercompensated for our efforts.
Today, electronically, most of us are reachable even when out of the office. It is difficult, if not impossible, to completely disconnect. The challenge to achieve the work/life balance we have all imagined is too great!
I try to carve out "forced escapes from reality" through novels, movies, and when possible, distant travel with my spouse. However, the bliss is too short lived. When I return to reality, some bliss fades as I jump back onto the "merry-go-round" for a few more turns.
Lillian M. Beard, MD, is a clinical professor of pediatrics at George Washington University, Washington. She is a Pediatric News Editorial Advisory Board member.
Pediatricians are twice as likely to be happy outside the office than they are at work, according to Medscape’s 2020 Lifestyle, Happiness, and Burnout Report.

About 29% of pediatricians reported being happy at work, with dermatologists taking the top spot at 41%. Pediatricians did much better when it came to finding happiness outside the office, with 57% reporting that they were very happy when away from work, according to the Medscape report.
The biggest contributing factors to burnout in pediatricians were an overabundance of bureaucratic tasks (59%), insufficient compensation/reimbursement (37%), and spending too many hours at work (34%).
Pediatricians most commonly dealt with burnout by talking with friends/family (54%), exercising (47%), and sleeping (41%). Just over half of pediatricians reported taking 3-4 weeks of vacation, compared with 44% of all physicians; 32% took less than 3 weeks’ vacation.
About 8% of pediatricians reported that they’d contemplated suicide, but 0% reported that they’d attempted it; 85% said that they’d never thought about it. Just under one-quarter of pediatricians said that were currently seeking or planning to seek professional help for depression and/or burnout; 55% said they were not seeking help and had never made use of it in the past.
The Medscape survey was conducted from June 25 to Sept. 19, 2019, and involved 15,181 physicians.
Pediatricians are twice as likely to be happy outside the office than they are at work, according to Medscape’s 2020 Lifestyle, Happiness, and Burnout Report.

About 29% of pediatricians reported being happy at work, with dermatologists taking the top spot at 41%. Pediatricians did much better when it came to finding happiness outside the office, with 57% reporting that they were very happy when away from work, according to the Medscape report.
The biggest contributing factors to burnout in pediatricians were an overabundance of bureaucratic tasks (59%), insufficient compensation/reimbursement (37%), and spending too many hours at work (34%).
Pediatricians most commonly dealt with burnout by talking with friends/family (54%), exercising (47%), and sleeping (41%). Just over half of pediatricians reported taking 3-4 weeks of vacation, compared with 44% of all physicians; 32% took less than 3 weeks’ vacation.
About 8% of pediatricians reported that they’d contemplated suicide, but 0% reported that they’d attempted it; 85% said that they’d never thought about it. Just under one-quarter of pediatricians said that were currently seeking or planning to seek professional help for depression and/or burnout; 55% said they were not seeking help and had never made use of it in the past.
The Medscape survey was conducted from June 25 to Sept. 19, 2019, and involved 15,181 physicians.
Newborn transfer may not reflect true rate of complications
Neonatal transfer was the factor most often associated with unexpected, severe complications at birth, particularly at hospitals that had the highest rates of complications, according to a cross-sectional study published online in JAMA Network Open (2020;3[2]:e1919498).
Mark A. Clapp, MD, MPH, of Massachusetts General Hospital in Boston, and colleagues wrote. “Thus, if this metric is to be used in its current form, it would appear that accreditors, regulatory bodies, and payers should consider adjusting for or stratifying by a hospital’s level of neonatal care to avoid disincentivizing against appropriate transfers.”
The Joint Commission recently included unexpected complications in term newborns as a marker of quality of obstetric care, but it does not currently recommend any risk adjustment for the metric. The authors aimed to learn which factors regarding patients and hospitals were associated with such complications. Severe, unexpected newborn complications include death, seizure, use of assisted ventilation for at least 6 hours, transfer to another facility, or a 5-minute Apgar score of 3 or less.
“This measure has been proposed to serve as a balancing measure to maternal metrics, such as the rate of nulliparous, term, singleton, vertex-presenting cesarean deliveries,” the authors explained.
This study was supported by a Health Policy Award from the Society for Maternal-Fetal Medicine. The authors reported no relevant financial disclosures.
This story first appeared on Medscape.
Neonatal transfer was the factor most often associated with unexpected, severe complications at birth, particularly at hospitals that had the highest rates of complications, according to a cross-sectional study published online in JAMA Network Open (2020;3[2]:e1919498).
Mark A. Clapp, MD, MPH, of Massachusetts General Hospital in Boston, and colleagues wrote. “Thus, if this metric is to be used in its current form, it would appear that accreditors, regulatory bodies, and payers should consider adjusting for or stratifying by a hospital’s level of neonatal care to avoid disincentivizing against appropriate transfers.”
The Joint Commission recently included unexpected complications in term newborns as a marker of quality of obstetric care, but it does not currently recommend any risk adjustment for the metric. The authors aimed to learn which factors regarding patients and hospitals were associated with such complications. Severe, unexpected newborn complications include death, seizure, use of assisted ventilation for at least 6 hours, transfer to another facility, or a 5-minute Apgar score of 3 or less.
“This measure has been proposed to serve as a balancing measure to maternal metrics, such as the rate of nulliparous, term, singleton, vertex-presenting cesarean deliveries,” the authors explained.
This study was supported by a Health Policy Award from the Society for Maternal-Fetal Medicine. The authors reported no relevant financial disclosures.
This story first appeared on Medscape.
Neonatal transfer was the factor most often associated with unexpected, severe complications at birth, particularly at hospitals that had the highest rates of complications, according to a cross-sectional study published online in JAMA Network Open (2020;3[2]:e1919498).
Mark A. Clapp, MD, MPH, of Massachusetts General Hospital in Boston, and colleagues wrote. “Thus, if this metric is to be used in its current form, it would appear that accreditors, regulatory bodies, and payers should consider adjusting for or stratifying by a hospital’s level of neonatal care to avoid disincentivizing against appropriate transfers.”
The Joint Commission recently included unexpected complications in term newborns as a marker of quality of obstetric care, but it does not currently recommend any risk adjustment for the metric. The authors aimed to learn which factors regarding patients and hospitals were associated with such complications. Severe, unexpected newborn complications include death, seizure, use of assisted ventilation for at least 6 hours, transfer to another facility, or a 5-minute Apgar score of 3 or less.
“This measure has been proposed to serve as a balancing measure to maternal metrics, such as the rate of nulliparous, term, singleton, vertex-presenting cesarean deliveries,” the authors explained.
This study was supported by a Health Policy Award from the Society for Maternal-Fetal Medicine. The authors reported no relevant financial disclosures.
This story first appeared on Medscape.
FROM JAMA NETWORK OPEN
Adrenal-permissive genotype linked to poor prognosis in prostate cancer
Men with low-volume, advanced prostate cancer who carry one or more adrenal-permissive alleles of the HSD3B1 gene have worse prognosis than do men with adrenal-restrictive alleles of HSD3B1, a retrospective analysis suggests.
Among 475 white men in the CHAARTED trial, those with the adrenal-permissive allele, HSD3B1(1245C), had a nearly twofold greater risk for developing early castration-resistant prostate cancer (CRPC) and for lower overall survival, compared with men who had the adrenal-restrictive allele, HSD3B1(1245A).
“Our findings suggest that the HSD3B1 genotype can be used to risk stratify white men with low-volume, metastatic prostate cancer,” Jason W.D. Hearn, MD, of the University of Michigan in Ann Arbor and colleagues wrote in JAMA Oncology.
HSD3B1 encodes for an enzyme involved in regulating the rate of metabolic conversion of the precursor hormone dehydroepiandrosterone into testosterone and dihydrotestosterone in prostate tissues. A common missense-encoding germline mutation in the gene leads to variant metabolic phenotypes, including the adrenal-permissive type that allows for ramped-up conversion of the precursor hormone into dihydrotestosterone and the adrenal-restrictive type that results in more rapid enzyme degradation, limiting the conversion from the precursor into the active hormone.
“The population frequency of the adrenal-permissive HSD3B1(1245C) allele varies tremendously by race and is disproportionally high in white men (e.g., carried in approximately 70% of Italian and Spanish men and only about 9% of Yoruba Nigerian men),” Dr. Hearn and colleagues wrote.
Previous studies have suggested that men with both metastatic and nonmetastatic prostate cancer who inherit the adrenal permissive HSD3B1(1245C) allele have worse clinical outcomes, compared with men without that allele.
To validate these findings, Dr. Hearn and colleagues retrospectively determined the germline genotype of HSD3B1 for 475 white men enrolled in CHAARTED. In that trial, men with metastatic, hormone-sensitive prostate cancer were randomized to either castration alone or castration plus docetaxel. The current analysis showed that 270 of those patients carried the adrenal-permissive allele.
Among patients with low-volume disease, the adrenal-permissive genotype was associated with significantly lower freedom from CRPC at 2 years, compared with the adrenal-restrictive genotype: 51% and 70.5%, respectively (hazard ratio, 1.89; P = .02). The 5-year overall survival rate was significantly lower for adrenal-permissive allele carriers than adrenal-restrictive allele carriers: 57.5% and 70.8%, respectively (HR, 1.74; P = .045).
Among men with high-volume disease, there was no association between HSD3B1 genotypes and outcomes, and there was no interaction between genotype and benefit from docetaxel. High-volume disease was defined as the presence of visceral metastases or four or more bone metastases with one or more lesions beyond the pelvis and vertebral bodies.
“Regardless of genotype, men with high-volume disease achieved better outcomes when docetaxel was added to ADT [androgen deprivation therapy], whereas outcomes in men with low-volume disease varied only by genotype, with no clear benefit from docetaxel,” the investigators wrote.
The trial was funded by the National Cancer Institute. The authors disclosed relationships with several pharmaceutical companies. One author is a coinventor on a patent for a treatment method based on HSD3B1.
SOURCE: Hearn JWD et al. JAMA Oncol. 2020 Feb 13. doi: 10.1001/jamaoncol.2019.6496.
Men with low-volume, advanced prostate cancer who carry one or more adrenal-permissive alleles of the HSD3B1 gene have worse prognosis than do men with adrenal-restrictive alleles of HSD3B1, a retrospective analysis suggests.
Among 475 white men in the CHAARTED trial, those with the adrenal-permissive allele, HSD3B1(1245C), had a nearly twofold greater risk for developing early castration-resistant prostate cancer (CRPC) and for lower overall survival, compared with men who had the adrenal-restrictive allele, HSD3B1(1245A).
“Our findings suggest that the HSD3B1 genotype can be used to risk stratify white men with low-volume, metastatic prostate cancer,” Jason W.D. Hearn, MD, of the University of Michigan in Ann Arbor and colleagues wrote in JAMA Oncology.
HSD3B1 encodes for an enzyme involved in regulating the rate of metabolic conversion of the precursor hormone dehydroepiandrosterone into testosterone and dihydrotestosterone in prostate tissues. A common missense-encoding germline mutation in the gene leads to variant metabolic phenotypes, including the adrenal-permissive type that allows for ramped-up conversion of the precursor hormone into dihydrotestosterone and the adrenal-restrictive type that results in more rapid enzyme degradation, limiting the conversion from the precursor into the active hormone.
“The population frequency of the adrenal-permissive HSD3B1(1245C) allele varies tremendously by race and is disproportionally high in white men (e.g., carried in approximately 70% of Italian and Spanish men and only about 9% of Yoruba Nigerian men),” Dr. Hearn and colleagues wrote.
Previous studies have suggested that men with both metastatic and nonmetastatic prostate cancer who inherit the adrenal permissive HSD3B1(1245C) allele have worse clinical outcomes, compared with men without that allele.
To validate these findings, Dr. Hearn and colleagues retrospectively determined the germline genotype of HSD3B1 for 475 white men enrolled in CHAARTED. In that trial, men with metastatic, hormone-sensitive prostate cancer were randomized to either castration alone or castration plus docetaxel. The current analysis showed that 270 of those patients carried the adrenal-permissive allele.
Among patients with low-volume disease, the adrenal-permissive genotype was associated with significantly lower freedom from CRPC at 2 years, compared with the adrenal-restrictive genotype: 51% and 70.5%, respectively (hazard ratio, 1.89; P = .02). The 5-year overall survival rate was significantly lower for adrenal-permissive allele carriers than adrenal-restrictive allele carriers: 57.5% and 70.8%, respectively (HR, 1.74; P = .045).
Among men with high-volume disease, there was no association between HSD3B1 genotypes and outcomes, and there was no interaction between genotype and benefit from docetaxel. High-volume disease was defined as the presence of visceral metastases or four or more bone metastases with one or more lesions beyond the pelvis and vertebral bodies.
“Regardless of genotype, men with high-volume disease achieved better outcomes when docetaxel was added to ADT [androgen deprivation therapy], whereas outcomes in men with low-volume disease varied only by genotype, with no clear benefit from docetaxel,” the investigators wrote.
The trial was funded by the National Cancer Institute. The authors disclosed relationships with several pharmaceutical companies. One author is a coinventor on a patent for a treatment method based on HSD3B1.
SOURCE: Hearn JWD et al. JAMA Oncol. 2020 Feb 13. doi: 10.1001/jamaoncol.2019.6496.
Men with low-volume, advanced prostate cancer who carry one or more adrenal-permissive alleles of the HSD3B1 gene have worse prognosis than do men with adrenal-restrictive alleles of HSD3B1, a retrospective analysis suggests.
Among 475 white men in the CHAARTED trial, those with the adrenal-permissive allele, HSD3B1(1245C), had a nearly twofold greater risk for developing early castration-resistant prostate cancer (CRPC) and for lower overall survival, compared with men who had the adrenal-restrictive allele, HSD3B1(1245A).
“Our findings suggest that the HSD3B1 genotype can be used to risk stratify white men with low-volume, metastatic prostate cancer,” Jason W.D. Hearn, MD, of the University of Michigan in Ann Arbor and colleagues wrote in JAMA Oncology.
HSD3B1 encodes for an enzyme involved in regulating the rate of metabolic conversion of the precursor hormone dehydroepiandrosterone into testosterone and dihydrotestosterone in prostate tissues. A common missense-encoding germline mutation in the gene leads to variant metabolic phenotypes, including the adrenal-permissive type that allows for ramped-up conversion of the precursor hormone into dihydrotestosterone and the adrenal-restrictive type that results in more rapid enzyme degradation, limiting the conversion from the precursor into the active hormone.
“The population frequency of the adrenal-permissive HSD3B1(1245C) allele varies tremendously by race and is disproportionally high in white men (e.g., carried in approximately 70% of Italian and Spanish men and only about 9% of Yoruba Nigerian men),” Dr. Hearn and colleagues wrote.
Previous studies have suggested that men with both metastatic and nonmetastatic prostate cancer who inherit the adrenal permissive HSD3B1(1245C) allele have worse clinical outcomes, compared with men without that allele.
To validate these findings, Dr. Hearn and colleagues retrospectively determined the germline genotype of HSD3B1 for 475 white men enrolled in CHAARTED. In that trial, men with metastatic, hormone-sensitive prostate cancer were randomized to either castration alone or castration plus docetaxel. The current analysis showed that 270 of those patients carried the adrenal-permissive allele.
Among patients with low-volume disease, the adrenal-permissive genotype was associated with significantly lower freedom from CRPC at 2 years, compared with the adrenal-restrictive genotype: 51% and 70.5%, respectively (hazard ratio, 1.89; P = .02). The 5-year overall survival rate was significantly lower for adrenal-permissive allele carriers than adrenal-restrictive allele carriers: 57.5% and 70.8%, respectively (HR, 1.74; P = .045).
Among men with high-volume disease, there was no association between HSD3B1 genotypes and outcomes, and there was no interaction between genotype and benefit from docetaxel. High-volume disease was defined as the presence of visceral metastases or four or more bone metastases with one or more lesions beyond the pelvis and vertebral bodies.
“Regardless of genotype, men with high-volume disease achieved better outcomes when docetaxel was added to ADT [androgen deprivation therapy], whereas outcomes in men with low-volume disease varied only by genotype, with no clear benefit from docetaxel,” the investigators wrote.
The trial was funded by the National Cancer Institute. The authors disclosed relationships with several pharmaceutical companies. One author is a coinventor on a patent for a treatment method based on HSD3B1.
SOURCE: Hearn JWD et al. JAMA Oncol. 2020 Feb 13. doi: 10.1001/jamaoncol.2019.6496.
FROM JAMA ONCOLOGY
Adding pembrolizumab to chemo doubled pCR rates in early-stage breast cancer
Adding pembrolizumab to neoadjuvant chemotherapy more than doubled the rate of pathologic complete response, compared with chemotherapy alone, in women with early-stage breast cancer enrolled in the phase 2 I-SPY2 trial.
Pathologic complete response (pCR) rates up to 60% were reported for patients with high-risk, stage II/III breast cancer who received pembrolizumab plus chemotherapy in I-SPY2, an ongoing platform trial designed to rapidly screen multiple agents and pinpoint those with a high probability of success.
The doubling of pCR rates was seen in all three biomarker signatures studied, including ERBB2(HER2)-negative, hormone receptor (HR)-positive/ERBB2-negative, or triple-negative breast cancer.
These results mean that pembrolizumab can “graduate” from I-SPY2 and suggest a greater than 99% predictive probability that the pembrolizumab-plus-chemotherapy approach will be superior to chemotherapy alone in a phase 3 trial, according to Rita Nanda, MD, of the University of Chicago, and colleagues.
“Notably, pembrolizumab was the first agent of 10 studied to graduate in the HR-positive/ERBB2-negative signature since I-SPY2 opened in 2010,” Dr. Nanda and colleagues wrote in JAMA Oncology.
The I-SPY2 study has enrolled adult women with stage II/III breast cancer at high risk of recurrence. The control arm included 181 patients randomized to receive standard neoadjuvant paclitaxel followed by doxorubicin plus cyclophosphamide. The pembrolizumab arm included 69 patients who received the same chemotherapy regimen plus pembrolizumab given concurrently with paclitaxel.
In ERBB2-negative patients, the estimated pCR rates were 44% in the pembrolizumab arm and 17% in the control arm. In HR-positive/ERBB2-negative patients, the estimated pCR rates were 30% and 13%, respectively. In triple-negative patients, the estimated pCR rates were 60% and 22%, respectively.
Residual cancer burden classified as extensive was less often seen in the pembrolizumab-treated patients, the investigators noted.
Event-free survival was qualitatively similar between the pembrolizumab and control arms, although the investigators cautioned against drawing conclusions based on this exploratory analysis in a small number of patients.
“Patients who achieved pCR had excellent outcomes regardless of arm,” the investigators wrote.
Immune-related adverse events (irAEs) were seen in the pembrolizumab-treated patients, although most were grade 1 or 2 and managed with dose interruption or corticosteroid therapy.
The most common irAE was thyroid dysfunction in 13% of patients, which was on par with what was seen in previously published reports. By contrast, adrenal insufficiency was observed in about 9% of patients, which is higher than in published reports for reasons that are unclear.
“Future work to characterize the risk factors for developing irAEs is warranted to improve the therapeutic index of these agents,” Dr. Nanda and colleagues wrote.
Pembrolizumab plus standard neoadjuvant chemotherapy is being evaluated in two ongoing, randomized phase 3 trials – KEYNOTE 522, which is evaluating patients with triple-negative breast cancer, and KEYNOTE 756, which is focused on high-risk, HR-positive/ERBB2-negative breast cancer.
The ongoing I-SPY2 study is supported by a grant from the National Cancer Institute as well as funding from charitable organizations, pharmaceutical companies, and private individuals. The investigators disclosed relationships with a range of pharmaceutical companies.
SOURCE: Nanda R et al. JAMA Oncol. 2020 Feb 13. doi: 10.1001/jamaoncol.2019.6650.
Adding pembrolizumab to neoadjuvant chemotherapy more than doubled the rate of pathologic complete response, compared with chemotherapy alone, in women with early-stage breast cancer enrolled in the phase 2 I-SPY2 trial.
Pathologic complete response (pCR) rates up to 60% were reported for patients with high-risk, stage II/III breast cancer who received pembrolizumab plus chemotherapy in I-SPY2, an ongoing platform trial designed to rapidly screen multiple agents and pinpoint those with a high probability of success.
The doubling of pCR rates was seen in all three biomarker signatures studied, including ERBB2(HER2)-negative, hormone receptor (HR)-positive/ERBB2-negative, or triple-negative breast cancer.
These results mean that pembrolizumab can “graduate” from I-SPY2 and suggest a greater than 99% predictive probability that the pembrolizumab-plus-chemotherapy approach will be superior to chemotherapy alone in a phase 3 trial, according to Rita Nanda, MD, of the University of Chicago, and colleagues.
“Notably, pembrolizumab was the first agent of 10 studied to graduate in the HR-positive/ERBB2-negative signature since I-SPY2 opened in 2010,” Dr. Nanda and colleagues wrote in JAMA Oncology.
The I-SPY2 study has enrolled adult women with stage II/III breast cancer at high risk of recurrence. The control arm included 181 patients randomized to receive standard neoadjuvant paclitaxel followed by doxorubicin plus cyclophosphamide. The pembrolizumab arm included 69 patients who received the same chemotherapy regimen plus pembrolizumab given concurrently with paclitaxel.
In ERBB2-negative patients, the estimated pCR rates were 44% in the pembrolizumab arm and 17% in the control arm. In HR-positive/ERBB2-negative patients, the estimated pCR rates were 30% and 13%, respectively. In triple-negative patients, the estimated pCR rates were 60% and 22%, respectively.
Residual cancer burden classified as extensive was less often seen in the pembrolizumab-treated patients, the investigators noted.
Event-free survival was qualitatively similar between the pembrolizumab and control arms, although the investigators cautioned against drawing conclusions based on this exploratory analysis in a small number of patients.
“Patients who achieved pCR had excellent outcomes regardless of arm,” the investigators wrote.
Immune-related adverse events (irAEs) were seen in the pembrolizumab-treated patients, although most were grade 1 or 2 and managed with dose interruption or corticosteroid therapy.
The most common irAE was thyroid dysfunction in 13% of patients, which was on par with what was seen in previously published reports. By contrast, adrenal insufficiency was observed in about 9% of patients, which is higher than in published reports for reasons that are unclear.
“Future work to characterize the risk factors for developing irAEs is warranted to improve the therapeutic index of these agents,” Dr. Nanda and colleagues wrote.
Pembrolizumab plus standard neoadjuvant chemotherapy is being evaluated in two ongoing, randomized phase 3 trials – KEYNOTE 522, which is evaluating patients with triple-negative breast cancer, and KEYNOTE 756, which is focused on high-risk, HR-positive/ERBB2-negative breast cancer.
The ongoing I-SPY2 study is supported by a grant from the National Cancer Institute as well as funding from charitable organizations, pharmaceutical companies, and private individuals. The investigators disclosed relationships with a range of pharmaceutical companies.
SOURCE: Nanda R et al. JAMA Oncol. 2020 Feb 13. doi: 10.1001/jamaoncol.2019.6650.
Adding pembrolizumab to neoadjuvant chemotherapy more than doubled the rate of pathologic complete response, compared with chemotherapy alone, in women with early-stage breast cancer enrolled in the phase 2 I-SPY2 trial.
Pathologic complete response (pCR) rates up to 60% were reported for patients with high-risk, stage II/III breast cancer who received pembrolizumab plus chemotherapy in I-SPY2, an ongoing platform trial designed to rapidly screen multiple agents and pinpoint those with a high probability of success.
The doubling of pCR rates was seen in all three biomarker signatures studied, including ERBB2(HER2)-negative, hormone receptor (HR)-positive/ERBB2-negative, or triple-negative breast cancer.
These results mean that pembrolizumab can “graduate” from I-SPY2 and suggest a greater than 99% predictive probability that the pembrolizumab-plus-chemotherapy approach will be superior to chemotherapy alone in a phase 3 trial, according to Rita Nanda, MD, of the University of Chicago, and colleagues.
“Notably, pembrolizumab was the first agent of 10 studied to graduate in the HR-positive/ERBB2-negative signature since I-SPY2 opened in 2010,” Dr. Nanda and colleagues wrote in JAMA Oncology.
The I-SPY2 study has enrolled adult women with stage II/III breast cancer at high risk of recurrence. The control arm included 181 patients randomized to receive standard neoadjuvant paclitaxel followed by doxorubicin plus cyclophosphamide. The pembrolizumab arm included 69 patients who received the same chemotherapy regimen plus pembrolizumab given concurrently with paclitaxel.
In ERBB2-negative patients, the estimated pCR rates were 44% in the pembrolizumab arm and 17% in the control arm. In HR-positive/ERBB2-negative patients, the estimated pCR rates were 30% and 13%, respectively. In triple-negative patients, the estimated pCR rates were 60% and 22%, respectively.
Residual cancer burden classified as extensive was less often seen in the pembrolizumab-treated patients, the investigators noted.
Event-free survival was qualitatively similar between the pembrolizumab and control arms, although the investigators cautioned against drawing conclusions based on this exploratory analysis in a small number of patients.
“Patients who achieved pCR had excellent outcomes regardless of arm,” the investigators wrote.
Immune-related adverse events (irAEs) were seen in the pembrolizumab-treated patients, although most were grade 1 or 2 and managed with dose interruption or corticosteroid therapy.
The most common irAE was thyroid dysfunction in 13% of patients, which was on par with what was seen in previously published reports. By contrast, adrenal insufficiency was observed in about 9% of patients, which is higher than in published reports for reasons that are unclear.
“Future work to characterize the risk factors for developing irAEs is warranted to improve the therapeutic index of these agents,” Dr. Nanda and colleagues wrote.
Pembrolizumab plus standard neoadjuvant chemotherapy is being evaluated in two ongoing, randomized phase 3 trials – KEYNOTE 522, which is evaluating patients with triple-negative breast cancer, and KEYNOTE 756, which is focused on high-risk, HR-positive/ERBB2-negative breast cancer.
The ongoing I-SPY2 study is supported by a grant from the National Cancer Institute as well as funding from charitable organizations, pharmaceutical companies, and private individuals. The investigators disclosed relationships with a range of pharmaceutical companies.
SOURCE: Nanda R et al. JAMA Oncol. 2020 Feb 13. doi: 10.1001/jamaoncol.2019.6650.
FROM JAMA ONCOLOGY
Key clinical point: Adding pembrolizumab to neoadjuvant chemotherapy more than doubled the rate of pathologic complete response (pCR) in women with early-stage breast cancer.
Major finding: In ERBB2-negative patients, the estimated pCR rates were 44% in the pembrolizumab arm and 17% in the control arm. In HR-positive/ERBB2-negative patients, the estimated pCR rates were 30% and 13%, respectively. In triple-negative patients, the estimated pCR rates were 60% and 22%, respectively.
Study details: Phase 2 trial of 69 patients treated with pembrolizumab and chemotherapy, compared with 181 chemotherapy-treated control subjects.
Disclosures: The trial is supported by a grant from the National Cancer Institute as well as funding from charitable organizations, pharmaceutical companies, and private individuals. The investigators disclosed relationships with a range of pharmaceutical companies.
Source: Nanda R et al. JAMA Oncol. 2020 Feb 13.
Broadly Distributed Vascular Macules in a Pediatric Patient
The Diagnosis: Capillary Malformation-Arteriovenous Malformation Syndrome
Capillary malformation-arteriovenous malformation (CM-AVM) was suspected, and a sample of the patient's blood was sent for a diagnostic genetic workup. DNA sequencing evaluated the following 5 genes that have been implicated in telangiectasia or AVM disorders: ACVRL1 (activin A receptorlike type 1), ENG (endoglin), GDF2 (growth differentiation factor 2), RASA1 (RAS p21 protein activator 1), and SMAD4 (SMAD family member 4). The patient was found to be heterozygous for a known pathogenic splice-site mutation in the RASA1 gene, consistent with a diagnosis of CM-AVM.
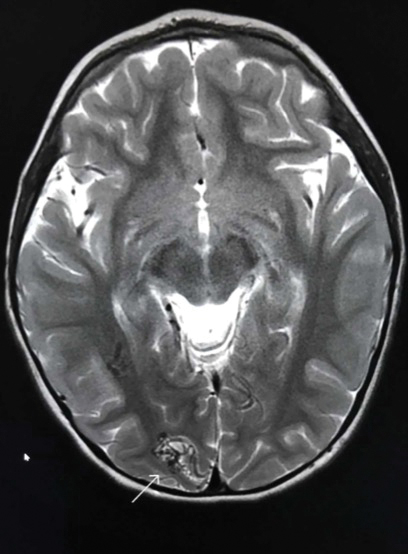
Capillary malformation-arteriovenous malformation presents with multiple small cutaneous CMs and associated arteriovenous fistulas as well as high-flow AVMs located in the soft tissues, bones, or central nervous system (CNS). Occasionally, the cutaneous CMs are surrounded by a blanched halo.1 Because of the potential for CNS involvement in CM-AVM, our patient was further evaluated with spine and brain magnetic resonance imaging (MRI). The brain MRI revealed 2 right occipital pole and fusiform gyral AVMs (Figure). No vascular abnormalities were found in the spine. The patient was referred to interventional neuroradiology to assess the feasibility of ablation to reduce the risk for complications, including intracranial hemorrhage.
Compared to other well-established congenital vascular disorders, CM-AVM has only recently been described in the literature. It was first reported by Eerola and colleagues2 in 2003. They studied several families with CMs and identified heterozygous inactivating RASA1 mutations in 6 families manifesting atypical CMs that were multiple small, round to oval, and pinkish red.2
It has been estimated that RASA1 mutations contribute to 68% of CM-AVM cases. Another gene--EPHB4 (EPH receptor B4)--has been implicated in patients with RASA1-negative disease. Two separate subtypes for patients with CM-AVM have been described: (1) CM-AVM type 1 for patients with RASA1 mutations, and (2) CM-AVM type 2 for those with EPHB4 mutations.3
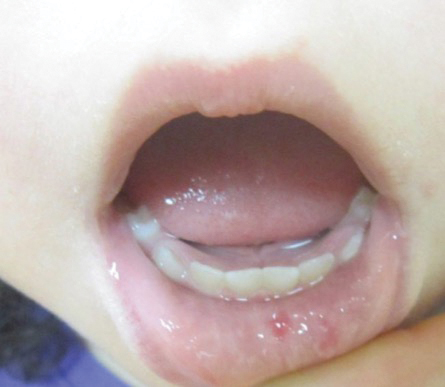
Both CM-AVM types are characterized by small multifocal CMs and an increased risk for CNS fast-flow vascular malformations.4 It has been suggested that there are morphologic differences between the cutaneous manifestations of the 2 types. For example, one group stated Bier spots are more frequently observed in CM-AVM type 2. This same group suggested telangiectases seen primarily on the lips but also in the perioral region and on the upper thorax were seen in CM-AVM type 2 but not in CM-AVM type 1.4 In our patient, it is plausible that the pinpoint red macules on the lips and oral mucosa could be confused for telangiectases (quiz image [bottom]). At this time, we do not feel that there is sufficient evidence to clinically distinguish between CM-AVM types 1 and 2.
Central nervous system involvement seems to be more common in patients with CM-AVM type 1 (10%) than those with CM-AVM type 2 (3%).1,4 Of the 2 CM-AVM type 2 patients found to have intracranial AVMs in one study, both were found to have vein of Galen aneurysmal malformations (VGAMs).4 The study examining CNS involvement in CM-AVM type 1 did not comment on the percentage of VGAMs seen in all patients.1 However, in the retrospective component of the study, the authors reported that in 161 patients with CM-AVM type 1, 24 AVMs were observed, 6 of which were intracranial. Half of these intracranial AVMs were at the vein of Galen, demonstrating that VGAMs are seen in both types of CM-AVM.1 Further study is necessary to better characterize potential phenotypic differences between the 2 forms of CM-AVM.
Overall, the annual risk for hemorrhage associated with brain AVMs is approximately 2% per year.5 Because the morbidity and mortality of undiagnosed CNS malformations is high, it is recommended that patients with both types of CM-AVM undergo spine and brain MRI evaluation. If CNS malformations are identified, patients should be referred to interventional neuroradiology to assess the feasibility of ablation.
It is unclear if patients who initially screen negative for AVMs will go on to develop these fast-flow lesions later. We have noted that new CMs develop over time in our patients. Therefore, it does not seem far-fetched to hypothesize that AVMs of CNS are similarly dynamic. Ultimately, we recommend ongoing screening for brain and spinal AVMs at regular intervals, determined by discussions of risks and benefits between the treating team and patient/family.
It is important to distinguish CM-AVM from hereditary hemorrhagic telangiectasia (HHT), as the distinction affects patient management. Unlike the AVMs found in HHT, AVMs in CM-AVM seldom are found in the lungs or liver.1 Thus, asymptomatic patients with HHT, but not CM-AVM, often are screened for pulmonary AVMs.
The diagnosis of HHT is based on the following 4 findings: spontaneous and recurrent epistaxis; multiple mucocutaneous telangiectasia at characteristic sites, including the lips, oral cavity, fingers, and nose; visceral involvement, such as gastrointestinal, pulmonary, cerebral, or hepatic AVMs; and a first-degree relative with the disorder. Three of the criteria are required for diagnosis.
Notably, the lesions seen in HHT and CM-AVM are morphologically different. Our patient did have 1-mm red macules on the lower lip that had clinical features overlapping with telangiectases, but other cutaneous findings including the presence of red macules and small patches, some with blanched halos, were clearly characteristic of CMs, not telangiectases.6 Furthermore, our patient did not have a personal history of epistaxis or a family history of any affected first-degree relatives. Finally, individuals with HHT tend to develop symptoms later in life compared to patients with CM-AVM, starting with epistaxis at 12 years of age.6
Patients with Henoch-Schönlein purpura also present in childhood but typically demonstrate palpable purpura and acute abdominal pain. Patients with Klippel-Trenaunay syndrome present with CM and venous malformation but also typically display limb overgrowth. Most patients with Klippel-Trenaunay syndrome are born with a port-wine stain.
Diffuse neonatal hemangiomatosis is characterized by multiple progressive, rapidly growing cutaneous hemangiomas associated with widespread visceral hemangiomas in the liver, lungs, gastrointestinal tract, brain, and meninges. Our patient's macules were much more slowly progressive.
- Revencu N, Boon LM, Mendola A, et al. RASA1 mutations and associated phenotypes in 68 families with capillary malformation-arteriovenous malformation. Hum Mutat. 2013;34:1632-1641.
- Eerola I, Boon LM, Mulliken JB, et al. Capillary malformation-arteriovenous malformation, a new clinical and genetic disorder caused by RASA1 mutations. Am J Hum Genet. 2003;73:1240-1249.
- Yu J, Streicher JL, Medne L, et al. EPHB4 mutation implicated in capillary malformation-arteriovenous malformation syndrome: a case report. Pediatr Dermatol. 2017;34:227-230.
- Amyere M, Revencu N, Helaers R, et al. Germline loss-of-function mutations in EPHB4 cause a second form of capillary malformation-arteriovenous malformation (CM-AVM2) deregulating RAS-MAPK signaling. Circulation. 2017;136:1037-1048.
- Mohr JP, Parides MK, Stapf C, et al. Medical management with or without interventional therapy for unruptured brain arteriovenous malformations (ARUBA): a multicentre, non-blinded, randomised trial. Lancet. 2014;383:614-621.
- Edwards LR, Blechman AB, Zlotoff BJ. RASA1 mutation in a family with capillary malformation-arteriovenous malformation syndrome: a discussion of the differential diagnosis. Pediatr Dermatol. 2017;35:e9-e12.
The Diagnosis: Capillary Malformation-Arteriovenous Malformation Syndrome
Capillary malformation-arteriovenous malformation (CM-AVM) was suspected, and a sample of the patient's blood was sent for a diagnostic genetic workup. DNA sequencing evaluated the following 5 genes that have been implicated in telangiectasia or AVM disorders: ACVRL1 (activin A receptorlike type 1), ENG (endoglin), GDF2 (growth differentiation factor 2), RASA1 (RAS p21 protein activator 1), and SMAD4 (SMAD family member 4). The patient was found to be heterozygous for a known pathogenic splice-site mutation in the RASA1 gene, consistent with a diagnosis of CM-AVM.
Capillary malformation-arteriovenous malformation presents with multiple small cutaneous CMs and associated arteriovenous fistulas as well as high-flow AVMs located in the soft tissues, bones, or central nervous system (CNS). Occasionally, the cutaneous CMs are surrounded by a blanched halo.1 Because of the potential for CNS involvement in CM-AVM, our patient was further evaluated with spine and brain magnetic resonance imaging (MRI). The brain MRI revealed 2 right occipital pole and fusiform gyral AVMs (Figure). No vascular abnormalities were found in the spine. The patient was referred to interventional neuroradiology to assess the feasibility of ablation to reduce the risk for complications, including intracranial hemorrhage.
Compared to other well-established congenital vascular disorders, CM-AVM has only recently been described in the literature. It was first reported by Eerola and colleagues2 in 2003. They studied several families with CMs and identified heterozygous inactivating RASA1 mutations in 6 families manifesting atypical CMs that were multiple small, round to oval, and pinkish red.2
It has been estimated that RASA1 mutations contribute to 68% of CM-AVM cases. Another gene--EPHB4 (EPH receptor B4)--has been implicated in patients with RASA1-negative disease. Two separate subtypes for patients with CM-AVM have been described: (1) CM-AVM type 1 for patients with RASA1 mutations, and (2) CM-AVM type 2 for those with EPHB4 mutations.3
Both CM-AVM types are characterized by small multifocal CMs and an increased risk for CNS fast-flow vascular malformations.4 It has been suggested that there are morphologic differences between the cutaneous manifestations of the 2 types. For example, one group stated Bier spots are more frequently observed in CM-AVM type 2. This same group suggested telangiectases seen primarily on the lips but also in the perioral region and on the upper thorax were seen in CM-AVM type 2 but not in CM-AVM type 1.4 In our patient, it is plausible that the pinpoint red macules on the lips and oral mucosa could be confused for telangiectases (quiz image [bottom]). At this time, we do not feel that there is sufficient evidence to clinically distinguish between CM-AVM types 1 and 2.
Central nervous system involvement seems to be more common in patients with CM-AVM type 1 (10%) than those with CM-AVM type 2 (3%).1,4 Of the 2 CM-AVM type 2 patients found to have intracranial AVMs in one study, both were found to have vein of Galen aneurysmal malformations (VGAMs).4 The study examining CNS involvement in CM-AVM type 1 did not comment on the percentage of VGAMs seen in all patients.1 However, in the retrospective component of the study, the authors reported that in 161 patients with CM-AVM type 1, 24 AVMs were observed, 6 of which were intracranial. Half of these intracranial AVMs were at the vein of Galen, demonstrating that VGAMs are seen in both types of CM-AVM.1 Further study is necessary to better characterize potential phenotypic differences between the 2 forms of CM-AVM.
Overall, the annual risk for hemorrhage associated with brain AVMs is approximately 2% per year.5 Because the morbidity and mortality of undiagnosed CNS malformations is high, it is recommended that patients with both types of CM-AVM undergo spine and brain MRI evaluation. If CNS malformations are identified, patients should be referred to interventional neuroradiology to assess the feasibility of ablation.
It is unclear if patients who initially screen negative for AVMs will go on to develop these fast-flow lesions later. We have noted that new CMs develop over time in our patients. Therefore, it does not seem far-fetched to hypothesize that AVMs of CNS are similarly dynamic. Ultimately, we recommend ongoing screening for brain and spinal AVMs at regular intervals, determined by discussions of risks and benefits between the treating team and patient/family.
It is important to distinguish CM-AVM from hereditary hemorrhagic telangiectasia (HHT), as the distinction affects patient management. Unlike the AVMs found in HHT, AVMs in CM-AVM seldom are found in the lungs or liver.1 Thus, asymptomatic patients with HHT, but not CM-AVM, often are screened for pulmonary AVMs.
The diagnosis of HHT is based on the following 4 findings: spontaneous and recurrent epistaxis; multiple mucocutaneous telangiectasia at characteristic sites, including the lips, oral cavity, fingers, and nose; visceral involvement, such as gastrointestinal, pulmonary, cerebral, or hepatic AVMs; and a first-degree relative with the disorder. Three of the criteria are required for diagnosis.
Notably, the lesions seen in HHT and CM-AVM are morphologically different. Our patient did have 1-mm red macules on the lower lip that had clinical features overlapping with telangiectases, but other cutaneous findings including the presence of red macules and small patches, some with blanched halos, were clearly characteristic of CMs, not telangiectases.6 Furthermore, our patient did not have a personal history of epistaxis or a family history of any affected first-degree relatives. Finally, individuals with HHT tend to develop symptoms later in life compared to patients with CM-AVM, starting with epistaxis at 12 years of age.6
Patients with Henoch-Schönlein purpura also present in childhood but typically demonstrate palpable purpura and acute abdominal pain. Patients with Klippel-Trenaunay syndrome present with CM and venous malformation but also typically display limb overgrowth. Most patients with Klippel-Trenaunay syndrome are born with a port-wine stain.
Diffuse neonatal hemangiomatosis is characterized by multiple progressive, rapidly growing cutaneous hemangiomas associated with widespread visceral hemangiomas in the liver, lungs, gastrointestinal tract, brain, and meninges. Our patient's macules were much more slowly progressive.
The Diagnosis: Capillary Malformation-Arteriovenous Malformation Syndrome
Capillary malformation-arteriovenous malformation (CM-AVM) was suspected, and a sample of the patient's blood was sent for a diagnostic genetic workup. DNA sequencing evaluated the following 5 genes that have been implicated in telangiectasia or AVM disorders: ACVRL1 (activin A receptorlike type 1), ENG (endoglin), GDF2 (growth differentiation factor 2), RASA1 (RAS p21 protein activator 1), and SMAD4 (SMAD family member 4). The patient was found to be heterozygous for a known pathogenic splice-site mutation in the RASA1 gene, consistent with a diagnosis of CM-AVM.
Capillary malformation-arteriovenous malformation presents with multiple small cutaneous CMs and associated arteriovenous fistulas as well as high-flow AVMs located in the soft tissues, bones, or central nervous system (CNS). Occasionally, the cutaneous CMs are surrounded by a blanched halo.1 Because of the potential for CNS involvement in CM-AVM, our patient was further evaluated with spine and brain magnetic resonance imaging (MRI). The brain MRI revealed 2 right occipital pole and fusiform gyral AVMs (Figure). No vascular abnormalities were found in the spine. The patient was referred to interventional neuroradiology to assess the feasibility of ablation to reduce the risk for complications, including intracranial hemorrhage.
Compared to other well-established congenital vascular disorders, CM-AVM has only recently been described in the literature. It was first reported by Eerola and colleagues2 in 2003. They studied several families with CMs and identified heterozygous inactivating RASA1 mutations in 6 families manifesting atypical CMs that were multiple small, round to oval, and pinkish red.2
It has been estimated that RASA1 mutations contribute to 68% of CM-AVM cases. Another gene--EPHB4 (EPH receptor B4)--has been implicated in patients with RASA1-negative disease. Two separate subtypes for patients with CM-AVM have been described: (1) CM-AVM type 1 for patients with RASA1 mutations, and (2) CM-AVM type 2 for those with EPHB4 mutations.3
Both CM-AVM types are characterized by small multifocal CMs and an increased risk for CNS fast-flow vascular malformations.4 It has been suggested that there are morphologic differences between the cutaneous manifestations of the 2 types. For example, one group stated Bier spots are more frequently observed in CM-AVM type 2. This same group suggested telangiectases seen primarily on the lips but also in the perioral region and on the upper thorax were seen in CM-AVM type 2 but not in CM-AVM type 1.4 In our patient, it is plausible that the pinpoint red macules on the lips and oral mucosa could be confused for telangiectases (quiz image [bottom]). At this time, we do not feel that there is sufficient evidence to clinically distinguish between CM-AVM types 1 and 2.
Central nervous system involvement seems to be more common in patients with CM-AVM type 1 (10%) than those with CM-AVM type 2 (3%).1,4 Of the 2 CM-AVM type 2 patients found to have intracranial AVMs in one study, both were found to have vein of Galen aneurysmal malformations (VGAMs).4 The study examining CNS involvement in CM-AVM type 1 did not comment on the percentage of VGAMs seen in all patients.1 However, in the retrospective component of the study, the authors reported that in 161 patients with CM-AVM type 1, 24 AVMs were observed, 6 of which were intracranial. Half of these intracranial AVMs were at the vein of Galen, demonstrating that VGAMs are seen in both types of CM-AVM.1 Further study is necessary to better characterize potential phenotypic differences between the 2 forms of CM-AVM.
Overall, the annual risk for hemorrhage associated with brain AVMs is approximately 2% per year.5 Because the morbidity and mortality of undiagnosed CNS malformations is high, it is recommended that patients with both types of CM-AVM undergo spine and brain MRI evaluation. If CNS malformations are identified, patients should be referred to interventional neuroradiology to assess the feasibility of ablation.
It is unclear if patients who initially screen negative for AVMs will go on to develop these fast-flow lesions later. We have noted that new CMs develop over time in our patients. Therefore, it does not seem far-fetched to hypothesize that AVMs of CNS are similarly dynamic. Ultimately, we recommend ongoing screening for brain and spinal AVMs at regular intervals, determined by discussions of risks and benefits between the treating team and patient/family.
It is important to distinguish CM-AVM from hereditary hemorrhagic telangiectasia (HHT), as the distinction affects patient management. Unlike the AVMs found in HHT, AVMs in CM-AVM seldom are found in the lungs or liver.1 Thus, asymptomatic patients with HHT, but not CM-AVM, often are screened for pulmonary AVMs.
The diagnosis of HHT is based on the following 4 findings: spontaneous and recurrent epistaxis; multiple mucocutaneous telangiectasia at characteristic sites, including the lips, oral cavity, fingers, and nose; visceral involvement, such as gastrointestinal, pulmonary, cerebral, or hepatic AVMs; and a first-degree relative with the disorder. Three of the criteria are required for diagnosis.
Notably, the lesions seen in HHT and CM-AVM are morphologically different. Our patient did have 1-mm red macules on the lower lip that had clinical features overlapping with telangiectases, but other cutaneous findings including the presence of red macules and small patches, some with blanched halos, were clearly characteristic of CMs, not telangiectases.6 Furthermore, our patient did not have a personal history of epistaxis or a family history of any affected first-degree relatives. Finally, individuals with HHT tend to develop symptoms later in life compared to patients with CM-AVM, starting with epistaxis at 12 years of age.6
Patients with Henoch-Schönlein purpura also present in childhood but typically demonstrate palpable purpura and acute abdominal pain. Patients with Klippel-Trenaunay syndrome present with CM and venous malformation but also typically display limb overgrowth. Most patients with Klippel-Trenaunay syndrome are born with a port-wine stain.
Diffuse neonatal hemangiomatosis is characterized by multiple progressive, rapidly growing cutaneous hemangiomas associated with widespread visceral hemangiomas in the liver, lungs, gastrointestinal tract, brain, and meninges. Our patient's macules were much more slowly progressive.
- Revencu N, Boon LM, Mendola A, et al. RASA1 mutations and associated phenotypes in 68 families with capillary malformation-arteriovenous malformation. Hum Mutat. 2013;34:1632-1641.
- Eerola I, Boon LM, Mulliken JB, et al. Capillary malformation-arteriovenous malformation, a new clinical and genetic disorder caused by RASA1 mutations. Am J Hum Genet. 2003;73:1240-1249.
- Yu J, Streicher JL, Medne L, et al. EPHB4 mutation implicated in capillary malformation-arteriovenous malformation syndrome: a case report. Pediatr Dermatol. 2017;34:227-230.
- Amyere M, Revencu N, Helaers R, et al. Germline loss-of-function mutations in EPHB4 cause a second form of capillary malformation-arteriovenous malformation (CM-AVM2) deregulating RAS-MAPK signaling. Circulation. 2017;136:1037-1048.
- Mohr JP, Parides MK, Stapf C, et al. Medical management with or without interventional therapy for unruptured brain arteriovenous malformations (ARUBA): a multicentre, non-blinded, randomised trial. Lancet. 2014;383:614-621.
- Edwards LR, Blechman AB, Zlotoff BJ. RASA1 mutation in a family with capillary malformation-arteriovenous malformation syndrome: a discussion of the differential diagnosis. Pediatr Dermatol. 2017;35:e9-e12.
- Revencu N, Boon LM, Mendola A, et al. RASA1 mutations and associated phenotypes in 68 families with capillary malformation-arteriovenous malformation. Hum Mutat. 2013;34:1632-1641.
- Eerola I, Boon LM, Mulliken JB, et al. Capillary malformation-arteriovenous malformation, a new clinical and genetic disorder caused by RASA1 mutations. Am J Hum Genet. 2003;73:1240-1249.
- Yu J, Streicher JL, Medne L, et al. EPHB4 mutation implicated in capillary malformation-arteriovenous malformation syndrome: a case report. Pediatr Dermatol. 2017;34:227-230.
- Amyere M, Revencu N, Helaers R, et al. Germline loss-of-function mutations in EPHB4 cause a second form of capillary malformation-arteriovenous malformation (CM-AVM2) deregulating RAS-MAPK signaling. Circulation. 2017;136:1037-1048.
- Mohr JP, Parides MK, Stapf C, et al. Medical management with or without interventional therapy for unruptured brain arteriovenous malformations (ARUBA): a multicentre, non-blinded, randomised trial. Lancet. 2014;383:614-621.
- Edwards LR, Blechman AB, Zlotoff BJ. RASA1 mutation in a family with capillary malformation-arteriovenous malformation syndrome: a discussion of the differential diagnosis. Pediatr Dermatol. 2017;35:e9-e12.
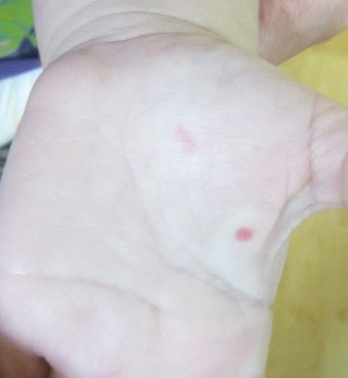
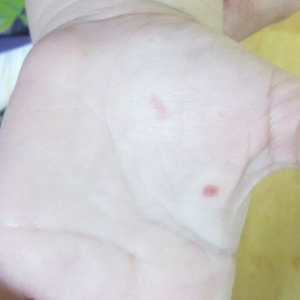
A 2-year-old girl presented with an erythematous macule on the left nasal sidewall that had been present since birth as well as other similar-appearing macules that had slowly evolved over the last 2 years. The patient was born via normal spontaneous vaginal delivery to healthy parents. She had 2 healthy siblings. Her parents reported that she was otherwise growing and developing normally. The patient had no history of epistaxis, and there was no family history of vascular anomalies. Physical examination revealed 2- to 6-mm vascular macules that blanched with pressure and filled quickly thereafter on the left nasal sidewall, upper (top) and lower extremities, and trunk. Some macules were surrounded by blanched halos. Several 1-mm red macules also were noted on the exterior and interior of the mucosal lower lip (bottom).
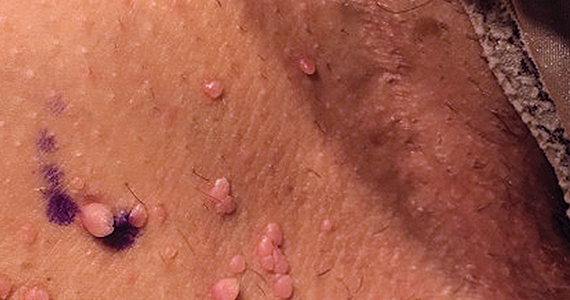
Can you identify these numerous papules in the groin area?
Condylomata acuminata
Condylomata acuminata (CA), or anogenital warts, are the cutaneous manifestation of infection by human papillomavirus (HPV). The virus is transmitted primarily via sexual contact with infected skin or mucosa, although it also may result from nonsexual contact or vertical transmission during vaginal delivery.1 More than 200 types of HPV have been identified; however, genotypes 6 and 11 are most commonly implicated in the development of CA and are associated with a low risk for oncogenesis. Nevertheless, CA pose a tremendous economic and psychological burden on the health care system and those affected, respectively, representing the most common sexually transmitted viral disease in the United States.2
Clinical presentation
CA present as discrete or clustered smooth, papillomatous, sessile, exophytic papules or plaques, often lacking the thick, horny scale seen in common warts, and they may be broad based or pedunculated.2 The anogenital region is affected, including the external genitalia, perineum, perianal area, and adjacent skin such as the mons pubis and inguinal folds. Extension into the urethra or vaginal, cervical, and anal canals is possible, although rarely beyond the dentate line.2,3 Lesions typically are asymptomatic but may be extensive or disfiguring, often noticed by patients upon self-inspection and leading to significant distress. Symptoms such as pruritus, pain, bleeding, or discharge may develop in traumatized or secondarily infected lesions.1,3
Diagnosis
Although CA can be diagnosed clinically, biopsy facilitates definitive diagnosis in less clear-cut cases.1,3 Histologically, CA are characterized by hyperkeratosis, parakeratosis, acanthosis, and papillomatosis, with the presence of koilocytes in the epidermis.2
Treatment
Treatment of CA is challenging, as there are currently no antiviral therapies available to cure the condition. Treatment options include destructive, immunomodulatory, and antiproliferative therapies, either alone or in combination. There is no first-line therapy indicated for CA, and treatment selection is dependent on multiple patient-specific factors, including the size, number, and anatomic location of the lesions, as well as ease of treatment and adverse effects.2
Topical therapies. For external CA, there are several treatments that may be applied by patients themselves, including topical podophyllotoxin, imiquimod, and sinecatechins (TABLE).1 Podophyllotoxin (brand name Condylox) is an antiproliferative agent available as a 0.15% cream or 0.5% solution.1,2 It should be applied twice daily for 3 consecutive days per week for up to 4 weeks. Podophyllotoxin is contraindicated in pregnancy and may cause local irritation.2
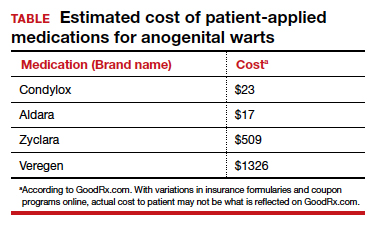
Imiquimod (brand names Aldara and Zyclara) is an immunomodulatory, available as a 5% and 3.75% cream. For external genital warts, the cream should be applied 3 times per week for up to 16 weeks; for perianal warts it should be applied daily for up to 8 weeks. Adverse effects of imiquimod include local irritation and systemic flu-like symptoms and are prominent with the 3.75% formulation, reducing adherence.1,2,4
In-office treatment options include cryotherapy, trichloroacetic acid (TCA), intralesional immunotherapy, laser therapy, phototherapy, and surgical options.2 Liquid nitrogen is cost-effective, efficacious, and safe for use in pregnancy; it is used in 2 to 3 freeze/thaw cycles per cryotherapy session to induce cellular damage.1,2 Its disadvantages include adverse effects, such as blistering, ulceration, dyspigmentation, and scarring. In addition, subclinical lesions in adjacent skin are not addressed during treatment.2
TCA is a caustic agent applied in the office once weekly or every 2 to 3 weeks for a maximum of 3 to 4 months, with similar benefits to cryotherapy in terms of ease of application and safety in pregnancy. There is the risk of blistering and ulceration in treated lesions as well as in inadvertently treated adjacent skin.1
Intralesional immunotherapy with Candida antigen (brand name Candin) is used in 3 sessions 4 to 6 weeks apart and is safe, with minimal adverse effects.2
Laser therapy treatment options include carbon dioxide laser therapy and ND:YAG laser. Their use is limited, however, by availability and cost.1,2
CA may be removed surgically via shave excision, scissor excision, curettage, and electrosurgery. These procedures can be painful, however, requiring local anesthesia and having a prolonged healing course.1,2
CA recurrence
CA unfortunately has a high rate of recurrence despite treatment, and patients require extensive counseling. Patients should be screened for other sexually transmitted infections and advised to notify their sexual partners. If followed properly, safe sexual practices, including condom use and limiting sexual partners, may prevent further transmission.1 The quadrivalent HPV vaccine (effective for the prevention of infection with HPV genotypes 6, 11, 16, and 18 in unexposed individuals) is ineffective in treating patients with pre-existing CA but can protect against the acquisition of other HPV genotypes included in the vaccine.1,5
Arriving at the diagnosis
Acrochordons are a common skin finding in the groin, but the onset is more gradual and the individual lesions tend to be more pedunculated. Molluscum is also on the differential and can affect the genitalia. Molluscum lesions have a characteristic central dimple or dell, which is absent in CA.
CASE Treatment course
The patient was treated with successive sessions of cryotherapy in combination with a course of topical imiquimod followed by several injections with Candida antigen, with persistence of some lesions as well as recurrence.
- Steben M, Garland SM. Genital warts. Best Prac Res Clin Obstet Gynaecol. 2014;28:1063-1073.
- Fathi R, Tsoukas MM. Genital warts and other HPV infections: established and novel therapies. Clin Dermatol. 2014;32:299-306.
- Lynde C, Vender R, Bourcier M, et al. Clinical features of external genital warts. J Cutan Med Surg. 2013;17 (suppl 2):S55-60.
- Scheinfeld N. Update on the treatment of genital warts. Dermatol Online J. 2013;19:18559.
- Markowitz LE, Dunne EF, Saraiya M, et al; Centers for Disease Control and Prevention (CDC); Advisory Committee on Immunization Practices (ACIP). Quadrivalent Human Papillomavirus Vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2007;56:1-24.
Condylomata acuminata
Condylomata acuminata (CA), or anogenital warts, are the cutaneous manifestation of infection by human papillomavirus (HPV). The virus is transmitted primarily via sexual contact with infected skin or mucosa, although it also may result from nonsexual contact or vertical transmission during vaginal delivery.1 More than 200 types of HPV have been identified; however, genotypes 6 and 11 are most commonly implicated in the development of CA and are associated with a low risk for oncogenesis. Nevertheless, CA pose a tremendous economic and psychological burden on the health care system and those affected, respectively, representing the most common sexually transmitted viral disease in the United States.2
Clinical presentation
CA present as discrete or clustered smooth, papillomatous, sessile, exophytic papules or plaques, often lacking the thick, horny scale seen in common warts, and they may be broad based or pedunculated.2 The anogenital region is affected, including the external genitalia, perineum, perianal area, and adjacent skin such as the mons pubis and inguinal folds. Extension into the urethra or vaginal, cervical, and anal canals is possible, although rarely beyond the dentate line.2,3 Lesions typically are asymptomatic but may be extensive or disfiguring, often noticed by patients upon self-inspection and leading to significant distress. Symptoms such as pruritus, pain, bleeding, or discharge may develop in traumatized or secondarily infected lesions.1,3
Diagnosis
Although CA can be diagnosed clinically, biopsy facilitates definitive diagnosis in less clear-cut cases.1,3 Histologically, CA are characterized by hyperkeratosis, parakeratosis, acanthosis, and papillomatosis, with the presence of koilocytes in the epidermis.2
Treatment
Treatment of CA is challenging, as there are currently no antiviral therapies available to cure the condition. Treatment options include destructive, immunomodulatory, and antiproliferative therapies, either alone or in combination. There is no first-line therapy indicated for CA, and treatment selection is dependent on multiple patient-specific factors, including the size, number, and anatomic location of the lesions, as well as ease of treatment and adverse effects.2
Topical therapies. For external CA, there are several treatments that may be applied by patients themselves, including topical podophyllotoxin, imiquimod, and sinecatechins (TABLE).1 Podophyllotoxin (brand name Condylox) is an antiproliferative agent available as a 0.15% cream or 0.5% solution.1,2 It should be applied twice daily for 3 consecutive days per week for up to 4 weeks. Podophyllotoxin is contraindicated in pregnancy and may cause local irritation.2

Imiquimod (brand names Aldara and Zyclara) is an immunomodulatory, available as a 5% and 3.75% cream. For external genital warts, the cream should be applied 3 times per week for up to 16 weeks; for perianal warts it should be applied daily for up to 8 weeks. Adverse effects of imiquimod include local irritation and systemic flu-like symptoms and are prominent with the 3.75% formulation, reducing adherence.1,2,4
In-office treatment options include cryotherapy, trichloroacetic acid (TCA), intralesional immunotherapy, laser therapy, phototherapy, and surgical options.2 Liquid nitrogen is cost-effective, efficacious, and safe for use in pregnancy; it is used in 2 to 3 freeze/thaw cycles per cryotherapy session to induce cellular damage.1,2 Its disadvantages include adverse effects, such as blistering, ulceration, dyspigmentation, and scarring. In addition, subclinical lesions in adjacent skin are not addressed during treatment.2
TCA is a caustic agent applied in the office once weekly or every 2 to 3 weeks for a maximum of 3 to 4 months, with similar benefits to cryotherapy in terms of ease of application and safety in pregnancy. There is the risk of blistering and ulceration in treated lesions as well as in inadvertently treated adjacent skin.1
Intralesional immunotherapy with Candida antigen (brand name Candin) is used in 3 sessions 4 to 6 weeks apart and is safe, with minimal adverse effects.2
Laser therapy treatment options include carbon dioxide laser therapy and ND:YAG laser. Their use is limited, however, by availability and cost.1,2
CA may be removed surgically via shave excision, scissor excision, curettage, and electrosurgery. These procedures can be painful, however, requiring local anesthesia and having a prolonged healing course.1,2
CA recurrence
CA unfortunately has a high rate of recurrence despite treatment, and patients require extensive counseling. Patients should be screened for other sexually transmitted infections and advised to notify their sexual partners. If followed properly, safe sexual practices, including condom use and limiting sexual partners, may prevent further transmission.1 The quadrivalent HPV vaccine (effective for the prevention of infection with HPV genotypes 6, 11, 16, and 18 in unexposed individuals) is ineffective in treating patients with pre-existing CA but can protect against the acquisition of other HPV genotypes included in the vaccine.1,5
Arriving at the diagnosis
Acrochordons are a common skin finding in the groin, but the onset is more gradual and the individual lesions tend to be more pedunculated. Molluscum is also on the differential and can affect the genitalia. Molluscum lesions have a characteristic central dimple or dell, which is absent in CA.
CASE Treatment course
The patient was treated with successive sessions of cryotherapy in combination with a course of topical imiquimod followed by several injections with Candida antigen, with persistence of some lesions as well as recurrence.
Condylomata acuminata
Condylomata acuminata (CA), or anogenital warts, are the cutaneous manifestation of infection by human papillomavirus (HPV). The virus is transmitted primarily via sexual contact with infected skin or mucosa, although it also may result from nonsexual contact or vertical transmission during vaginal delivery.1 More than 200 types of HPV have been identified; however, genotypes 6 and 11 are most commonly implicated in the development of CA and are associated with a low risk for oncogenesis. Nevertheless, CA pose a tremendous economic and psychological burden on the health care system and those affected, respectively, representing the most common sexually transmitted viral disease in the United States.2
Clinical presentation
CA present as discrete or clustered smooth, papillomatous, sessile, exophytic papules or plaques, often lacking the thick, horny scale seen in common warts, and they may be broad based or pedunculated.2 The anogenital region is affected, including the external genitalia, perineum, perianal area, and adjacent skin such as the mons pubis and inguinal folds. Extension into the urethra or vaginal, cervical, and anal canals is possible, although rarely beyond the dentate line.2,3 Lesions typically are asymptomatic but may be extensive or disfiguring, often noticed by patients upon self-inspection and leading to significant distress. Symptoms such as pruritus, pain, bleeding, or discharge may develop in traumatized or secondarily infected lesions.1,3
Diagnosis
Although CA can be diagnosed clinically, biopsy facilitates definitive diagnosis in less clear-cut cases.1,3 Histologically, CA are characterized by hyperkeratosis, parakeratosis, acanthosis, and papillomatosis, with the presence of koilocytes in the epidermis.2
Treatment
Treatment of CA is challenging, as there are currently no antiviral therapies available to cure the condition. Treatment options include destructive, immunomodulatory, and antiproliferative therapies, either alone or in combination. There is no first-line therapy indicated for CA, and treatment selection is dependent on multiple patient-specific factors, including the size, number, and anatomic location of the lesions, as well as ease of treatment and adverse effects.2
Topical therapies. For external CA, there are several treatments that may be applied by patients themselves, including topical podophyllotoxin, imiquimod, and sinecatechins (TABLE).1 Podophyllotoxin (brand name Condylox) is an antiproliferative agent available as a 0.15% cream or 0.5% solution.1,2 It should be applied twice daily for 3 consecutive days per week for up to 4 weeks. Podophyllotoxin is contraindicated in pregnancy and may cause local irritation.2

Imiquimod (brand names Aldara and Zyclara) is an immunomodulatory, available as a 5% and 3.75% cream. For external genital warts, the cream should be applied 3 times per week for up to 16 weeks; for perianal warts it should be applied daily for up to 8 weeks. Adverse effects of imiquimod include local irritation and systemic flu-like symptoms and are prominent with the 3.75% formulation, reducing adherence.1,2,4
In-office treatment options include cryotherapy, trichloroacetic acid (TCA), intralesional immunotherapy, laser therapy, phototherapy, and surgical options.2 Liquid nitrogen is cost-effective, efficacious, and safe for use in pregnancy; it is used in 2 to 3 freeze/thaw cycles per cryotherapy session to induce cellular damage.1,2 Its disadvantages include adverse effects, such as blistering, ulceration, dyspigmentation, and scarring. In addition, subclinical lesions in adjacent skin are not addressed during treatment.2
TCA is a caustic agent applied in the office once weekly or every 2 to 3 weeks for a maximum of 3 to 4 months, with similar benefits to cryotherapy in terms of ease of application and safety in pregnancy. There is the risk of blistering and ulceration in treated lesions as well as in inadvertently treated adjacent skin.1
Intralesional immunotherapy with Candida antigen (brand name Candin) is used in 3 sessions 4 to 6 weeks apart and is safe, with minimal adverse effects.2
Laser therapy treatment options include carbon dioxide laser therapy and ND:YAG laser. Their use is limited, however, by availability and cost.1,2
CA may be removed surgically via shave excision, scissor excision, curettage, and electrosurgery. These procedures can be painful, however, requiring local anesthesia and having a prolonged healing course.1,2
CA recurrence
CA unfortunately has a high rate of recurrence despite treatment, and patients require extensive counseling. Patients should be screened for other sexually transmitted infections and advised to notify their sexual partners. If followed properly, safe sexual practices, including condom use and limiting sexual partners, may prevent further transmission.1 The quadrivalent HPV vaccine (effective for the prevention of infection with HPV genotypes 6, 11, 16, and 18 in unexposed individuals) is ineffective in treating patients with pre-existing CA but can protect against the acquisition of other HPV genotypes included in the vaccine.1,5
Arriving at the diagnosis
Acrochordons are a common skin finding in the groin, but the onset is more gradual and the individual lesions tend to be more pedunculated. Molluscum is also on the differential and can affect the genitalia. Molluscum lesions have a characteristic central dimple or dell, which is absent in CA.
CASE Treatment course
The patient was treated with successive sessions of cryotherapy in combination with a course of topical imiquimod followed by several injections with Candida antigen, with persistence of some lesions as well as recurrence.
- Steben M, Garland SM. Genital warts. Best Prac Res Clin Obstet Gynaecol. 2014;28:1063-1073.
- Fathi R, Tsoukas MM. Genital warts and other HPV infections: established and novel therapies. Clin Dermatol. 2014;32:299-306.
- Lynde C, Vender R, Bourcier M, et al. Clinical features of external genital warts. J Cutan Med Surg. 2013;17 (suppl 2):S55-60.
- Scheinfeld N. Update on the treatment of genital warts. Dermatol Online J. 2013;19:18559.
- Markowitz LE, Dunne EF, Saraiya M, et al; Centers for Disease Control and Prevention (CDC); Advisory Committee on Immunization Practices (ACIP). Quadrivalent Human Papillomavirus Vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2007;56:1-24.
- Steben M, Garland SM. Genital warts. Best Prac Res Clin Obstet Gynaecol. 2014;28:1063-1073.
- Fathi R, Tsoukas MM. Genital warts and other HPV infections: established and novel therapies. Clin Dermatol. 2014;32:299-306.
- Lynde C, Vender R, Bourcier M, et al. Clinical features of external genital warts. J Cutan Med Surg. 2013;17 (suppl 2):S55-60.
- Scheinfeld N. Update on the treatment of genital warts. Dermatol Online J. 2013;19:18559.
- Markowitz LE, Dunne EF, Saraiya M, et al; Centers for Disease Control and Prevention (CDC); Advisory Committee on Immunization Practices (ACIP). Quadrivalent Human Papillomavirus Vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2007;56:1-24.
CASE Skin tags on the groin
A 47-year-old woman with no personal history of skin cancer presents to a dermatologist for annual skin surveillance examination. She notes multiple “pink skin tags” on the groin, present for 4 months. She says they are asymptomatic and have not been treated previously. She states that she is in a long-term monogamous relationship. Physical examination reveals multiple smooth, flat-topped, pedunculated pink papules on the bilateral upper inner thighs. Shave biopsy of a lesion on the right upper medial thigh is performed to aid in diagnosis (FIGURE 1).
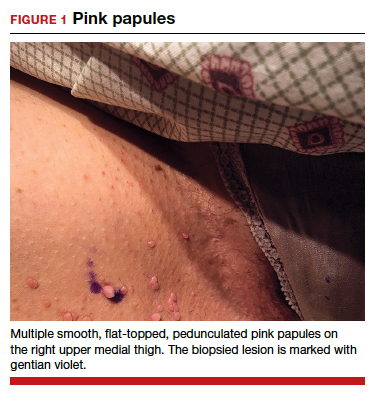
ERAS for cesarean delivery: Intraoperative care



Tools for preventing heart failure
SNOWMASS, COLO. – If ever there was a major chronic disease that’s teed up and ready to be stamped into submission through diligent application of preventive medicine, it’s the epidemic of heart failure.
“The best way to treat heart failure is to prevent it in the first place. There will be more than 1 million new cases of heart failure this year, and the vast majority of them could have been prevented,” Gregg C. Fonarow, MD, asserted at the annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
Using firmly evidence-based, guideline-directed therapies, it’s often possible to prevent patients at high risk for developing heart failure (HF) from actually doing so. Or, in the terminology of the ACC/American Heart Association heart failure guidelines coauthored by Dr. Fonarow, the goal is to keep patients who are stage A – that is, pre-HF but at high risk because of hypertension, coronary artery disease, diabetes, family history of cardiomyopathy, or other reasons – from progressing to stage B, marked by asymptomatic left ventricular dysfunction, a prior MI, or asymptomatic valvular disease; and blocking those who are stage B from then moving on to stage C, the classic symptomatic form of HF; and thence to end-stage stage D disease.
Heart failure is an enormous public health problem, and one of the most expensive of all diseases. The prognostic impact of newly diagnosed HF is profound, with 10-15 years of life lost, compared with the general population. Even today, roughly one in five newly diagnosed patients won’t survive for a year, and the 5-year mortality is about 50%, said Dr. Fonarow, who is professor of cardiovascular medicine and chief of the division of cardiology at the University of California, Los Angeles, and director of the Ahmanson-UCLA Cardiomyopathy Center, also in Los Angeles.
Symptomatic stage C is “the tip of the iceberg,” the cardiologist stressed. Vastly more patients are in stages A and B. In order to keep them from progressing to stage C, it’s first necessary to identify them. That’s why the 2013 guidelines give a class IC recommendation for periodic evaluation for signs and symptoms of HF in patients who are at high risk, and for a noninvasive assessment of left ventricular ejection fraction in those with a strong family history of cardiomyopathy or who are on cardiotoxic drugs (J Am Coll Cardiol. 2013 Oct 15;62[16]:e147-239).
The two biggest risk factors for the development of symptomatic stage C HF are hypertension and atherosclerotic cardiovascular disease. Close to 80% of patients presenting with heart failure have prevalent hypertension, and a history of ischemic heart disease is nearly as common.
Other major modifiable risk factors are diabetes, overweight and obesity, metabolic syndrome, dyslipidemia, smoking, valvular heart disease, and chronic kidney disease.
Hypertension
Most patients with high blood pressure believe they’re on antihypertensive medication to prevent MI and stroke, but in reality the largest benefit is what Dr. Fonarow termed the “phenomenal” reduction in the risk of developing HF, which amounted to a 52% relative risk reduction in one meta-analysis of older randomized trials. In the contemporary era, the landmark SPRINT trial of close to 10,000 randomized hypertensive patients showed that more-intensive blood pressure lowering to a target systolic BP of less than 120 mm Hg resulted in a 38% reduction in the risk of new-onset HF, compared with standard treatment to a target of less than 140 mm Hg. That’s why the 2017 focused update of the HF guidelines gives a strong class IB recommendation for a target blood pressure of less than 130/80 mm Hg in hypertensive patients with stage A HF (J Am Coll Cardiol. 2017 Aug 8;70[6]:776-803).
Atherosclerotic cardiovascular disease
Within 6 years after diagnosis of an MI, 22% of men and 46% of women will develop symptomatic heart failure. Intensive statin therapy gets a strong recommendation post MI in the guidelines, not only because in a meta-analysis of four major randomized trials it resulted in a further 64% reduction in the risk of coronary death or recurrent MI, compared with moderate statin therapy, but also because of the 27% relative risk reduction in new-onset HF. ACE inhibitors get a class IA recommendation for prevention of symptomatic HF in patients who are stage A with a history of atherosclerotic disease, diabetes, or hypertension. Angiotensin receptor blockers get a class IC recommendation.
Diabetes
Diabetes markedly increases the risk of developing HF: by two to four times overall and by four to eight times in younger diabetes patients. The two chronic diseases are highly comorbid, with roughly 45% of patients with HF also having diabetes. Moreover, diabetes in HF patients is associated with a substantially worse prognosis, even when standard HF therapies are applied.
Choices regarding glycemic management can markedly affect HF risk and outcomes. Randomized trials show that the peroxisome proliferator-activated receptor agonists double the risk of HF. The glucagonlike peptide–1 receptor agonists are absolutely neutral with regard to HF outcomes. Similarly, the dipeptidyl peptidase–4 inhibitors have no impact on the risks of major adverse cardiovascular events or HF. Intensive glycemic control has no impact on the risk of new-onset HF. Insulin therapy, too, is neutral on this score.
“Depressingly, even lifestyle modification with weight loss, once you have type 2 diabetes, does not lower the risk,” Dr. Fonarow continued.
In contrast, the sodium-glucose transporter 2 (SGLT2) inhibitors have impressive cardiovascular and renal protective benefits in patients with type 2 diabetes, as demonstrated in a meta-analysis of more than 34,000 participants in the randomized trials of empagliflozin (Jardiance) in EMPA-REG OUTCOME, canagliflozin (Invokana) in CANVAS/CANVAS-R, and dapagliflozin (Farxiga) in DECLARE-TIMI 58. The SGLT2 inhibitors collectively reduced the risk of HF hospitalization by 21% in participants with no baseline history of the disease and by 29% in those with a history of HF. Moreover, the risk of progression of renal disease was reduced by 45% (Lancet. 2019 Jan 5;393[10166]:31-9).
More recently, the landmark DAPA-HF trial established SGLT2 inhibitor therapy as part of standard-of-care, guideline-directed medical therapy for patients with HF with reduced ejection fraction regardless of whether they have comorbid type 2 diabetes (N Engl J Med. 2019 Nov 21;381[21]:1995-2008).
These are remarkable medications, generally very well tolerated, and it’s critical that cardiologists get on board in prescribing them, Dr. Fonarow emphasized. He alerted his colleagues to what he called an “incredibly helpful” review article that provides practical guidance for cardiologists in how to start using the SGLT2 inhibitors (JACC Heart Fail. 2019 Feb;7[2]:169-72).
“It’s pretty straightforward,” according to Dr. Fonarow. “If you’re comfortable enough in using ACE inhibitors, angiotensin receptor blockers, and beta-blockers, I think you’ll find these medications fit similarly when you actually get experience in utilizing them.”
He reported serving as a consultant to 10 pharmaceutical or medical device companies.
SNOWMASS, COLO. – If ever there was a major chronic disease that’s teed up and ready to be stamped into submission through diligent application of preventive medicine, it’s the epidemic of heart failure.
“The best way to treat heart failure is to prevent it in the first place. There will be more than 1 million new cases of heart failure this year, and the vast majority of them could have been prevented,” Gregg C. Fonarow, MD, asserted at the annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
Using firmly evidence-based, guideline-directed therapies, it’s often possible to prevent patients at high risk for developing heart failure (HF) from actually doing so. Or, in the terminology of the ACC/American Heart Association heart failure guidelines coauthored by Dr. Fonarow, the goal is to keep patients who are stage A – that is, pre-HF but at high risk because of hypertension, coronary artery disease, diabetes, family history of cardiomyopathy, or other reasons – from progressing to stage B, marked by asymptomatic left ventricular dysfunction, a prior MI, or asymptomatic valvular disease; and blocking those who are stage B from then moving on to stage C, the classic symptomatic form of HF; and thence to end-stage stage D disease.
Heart failure is an enormous public health problem, and one of the most expensive of all diseases. The prognostic impact of newly diagnosed HF is profound, with 10-15 years of life lost, compared with the general population. Even today, roughly one in five newly diagnosed patients won’t survive for a year, and the 5-year mortality is about 50%, said Dr. Fonarow, who is professor of cardiovascular medicine and chief of the division of cardiology at the University of California, Los Angeles, and director of the Ahmanson-UCLA Cardiomyopathy Center, also in Los Angeles.
Symptomatic stage C is “the tip of the iceberg,” the cardiologist stressed. Vastly more patients are in stages A and B. In order to keep them from progressing to stage C, it’s first necessary to identify them. That’s why the 2013 guidelines give a class IC recommendation for periodic evaluation for signs and symptoms of HF in patients who are at high risk, and for a noninvasive assessment of left ventricular ejection fraction in those with a strong family history of cardiomyopathy or who are on cardiotoxic drugs (J Am Coll Cardiol. 2013 Oct 15;62[16]:e147-239).
The two biggest risk factors for the development of symptomatic stage C HF are hypertension and atherosclerotic cardiovascular disease. Close to 80% of patients presenting with heart failure have prevalent hypertension, and a history of ischemic heart disease is nearly as common.
Other major modifiable risk factors are diabetes, overweight and obesity, metabolic syndrome, dyslipidemia, smoking, valvular heart disease, and chronic kidney disease.
Hypertension
Most patients with high blood pressure believe they’re on antihypertensive medication to prevent MI and stroke, but in reality the largest benefit is what Dr. Fonarow termed the “phenomenal” reduction in the risk of developing HF, which amounted to a 52% relative risk reduction in one meta-analysis of older randomized trials. In the contemporary era, the landmark SPRINT trial of close to 10,000 randomized hypertensive patients showed that more-intensive blood pressure lowering to a target systolic BP of less than 120 mm Hg resulted in a 38% reduction in the risk of new-onset HF, compared with standard treatment to a target of less than 140 mm Hg. That’s why the 2017 focused update of the HF guidelines gives a strong class IB recommendation for a target blood pressure of less than 130/80 mm Hg in hypertensive patients with stage A HF (J Am Coll Cardiol. 2017 Aug 8;70[6]:776-803).
Atherosclerotic cardiovascular disease
Within 6 years after diagnosis of an MI, 22% of men and 46% of women will develop symptomatic heart failure. Intensive statin therapy gets a strong recommendation post MI in the guidelines, not only because in a meta-analysis of four major randomized trials it resulted in a further 64% reduction in the risk of coronary death or recurrent MI, compared with moderate statin therapy, but also because of the 27% relative risk reduction in new-onset HF. ACE inhibitors get a class IA recommendation for prevention of symptomatic HF in patients who are stage A with a history of atherosclerotic disease, diabetes, or hypertension. Angiotensin receptor blockers get a class IC recommendation.
Diabetes
Diabetes markedly increases the risk of developing HF: by two to four times overall and by four to eight times in younger diabetes patients. The two chronic diseases are highly comorbid, with roughly 45% of patients with HF also having diabetes. Moreover, diabetes in HF patients is associated with a substantially worse prognosis, even when standard HF therapies are applied.
Choices regarding glycemic management can markedly affect HF risk and outcomes. Randomized trials show that the peroxisome proliferator-activated receptor agonists double the risk of HF. The glucagonlike peptide–1 receptor agonists are absolutely neutral with regard to HF outcomes. Similarly, the dipeptidyl peptidase–4 inhibitors have no impact on the risks of major adverse cardiovascular events or HF. Intensive glycemic control has no impact on the risk of new-onset HF. Insulin therapy, too, is neutral on this score.
“Depressingly, even lifestyle modification with weight loss, once you have type 2 diabetes, does not lower the risk,” Dr. Fonarow continued.
In contrast, the sodium-glucose transporter 2 (SGLT2) inhibitors have impressive cardiovascular and renal protective benefits in patients with type 2 diabetes, as demonstrated in a meta-analysis of more than 34,000 participants in the randomized trials of empagliflozin (Jardiance) in EMPA-REG OUTCOME, canagliflozin (Invokana) in CANVAS/CANVAS-R, and dapagliflozin (Farxiga) in DECLARE-TIMI 58. The SGLT2 inhibitors collectively reduced the risk of HF hospitalization by 21% in participants with no baseline history of the disease and by 29% in those with a history of HF. Moreover, the risk of progression of renal disease was reduced by 45% (Lancet. 2019 Jan 5;393[10166]:31-9).
More recently, the landmark DAPA-HF trial established SGLT2 inhibitor therapy as part of standard-of-care, guideline-directed medical therapy for patients with HF with reduced ejection fraction regardless of whether they have comorbid type 2 diabetes (N Engl J Med. 2019 Nov 21;381[21]:1995-2008).
These are remarkable medications, generally very well tolerated, and it’s critical that cardiologists get on board in prescribing them, Dr. Fonarow emphasized. He alerted his colleagues to what he called an “incredibly helpful” review article that provides practical guidance for cardiologists in how to start using the SGLT2 inhibitors (JACC Heart Fail. 2019 Feb;7[2]:169-72).
“It’s pretty straightforward,” according to Dr. Fonarow. “If you’re comfortable enough in using ACE inhibitors, angiotensin receptor blockers, and beta-blockers, I think you’ll find these medications fit similarly when you actually get experience in utilizing them.”
He reported serving as a consultant to 10 pharmaceutical or medical device companies.
SNOWMASS, COLO. – If ever there was a major chronic disease that’s teed up and ready to be stamped into submission through diligent application of preventive medicine, it’s the epidemic of heart failure.
“The best way to treat heart failure is to prevent it in the first place. There will be more than 1 million new cases of heart failure this year, and the vast majority of them could have been prevented,” Gregg C. Fonarow, MD, asserted at the annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
Using firmly evidence-based, guideline-directed therapies, it’s often possible to prevent patients at high risk for developing heart failure (HF) from actually doing so. Or, in the terminology of the ACC/American Heart Association heart failure guidelines coauthored by Dr. Fonarow, the goal is to keep patients who are stage A – that is, pre-HF but at high risk because of hypertension, coronary artery disease, diabetes, family history of cardiomyopathy, or other reasons – from progressing to stage B, marked by asymptomatic left ventricular dysfunction, a prior MI, or asymptomatic valvular disease; and blocking those who are stage B from then moving on to stage C, the classic symptomatic form of HF; and thence to end-stage stage D disease.
Heart failure is an enormous public health problem, and one of the most expensive of all diseases. The prognostic impact of newly diagnosed HF is profound, with 10-15 years of life lost, compared with the general population. Even today, roughly one in five newly diagnosed patients won’t survive for a year, and the 5-year mortality is about 50%, said Dr. Fonarow, who is professor of cardiovascular medicine and chief of the division of cardiology at the University of California, Los Angeles, and director of the Ahmanson-UCLA Cardiomyopathy Center, also in Los Angeles.
Symptomatic stage C is “the tip of the iceberg,” the cardiologist stressed. Vastly more patients are in stages A and B. In order to keep them from progressing to stage C, it’s first necessary to identify them. That’s why the 2013 guidelines give a class IC recommendation for periodic evaluation for signs and symptoms of HF in patients who are at high risk, and for a noninvasive assessment of left ventricular ejection fraction in those with a strong family history of cardiomyopathy or who are on cardiotoxic drugs (J Am Coll Cardiol. 2013 Oct 15;62[16]:e147-239).
The two biggest risk factors for the development of symptomatic stage C HF are hypertension and atherosclerotic cardiovascular disease. Close to 80% of patients presenting with heart failure have prevalent hypertension, and a history of ischemic heart disease is nearly as common.
Other major modifiable risk factors are diabetes, overweight and obesity, metabolic syndrome, dyslipidemia, smoking, valvular heart disease, and chronic kidney disease.
Hypertension
Most patients with high blood pressure believe they’re on antihypertensive medication to prevent MI and stroke, but in reality the largest benefit is what Dr. Fonarow termed the “phenomenal” reduction in the risk of developing HF, which amounted to a 52% relative risk reduction in one meta-analysis of older randomized trials. In the contemporary era, the landmark SPRINT trial of close to 10,000 randomized hypertensive patients showed that more-intensive blood pressure lowering to a target systolic BP of less than 120 mm Hg resulted in a 38% reduction in the risk of new-onset HF, compared with standard treatment to a target of less than 140 mm Hg. That’s why the 2017 focused update of the HF guidelines gives a strong class IB recommendation for a target blood pressure of less than 130/80 mm Hg in hypertensive patients with stage A HF (J Am Coll Cardiol. 2017 Aug 8;70[6]:776-803).
Atherosclerotic cardiovascular disease
Within 6 years after diagnosis of an MI, 22% of men and 46% of women will develop symptomatic heart failure. Intensive statin therapy gets a strong recommendation post MI in the guidelines, not only because in a meta-analysis of four major randomized trials it resulted in a further 64% reduction in the risk of coronary death or recurrent MI, compared with moderate statin therapy, but also because of the 27% relative risk reduction in new-onset HF. ACE inhibitors get a class IA recommendation for prevention of symptomatic HF in patients who are stage A with a history of atherosclerotic disease, diabetes, or hypertension. Angiotensin receptor blockers get a class IC recommendation.
Diabetes
Diabetes markedly increases the risk of developing HF: by two to four times overall and by four to eight times in younger diabetes patients. The two chronic diseases are highly comorbid, with roughly 45% of patients with HF also having diabetes. Moreover, diabetes in HF patients is associated with a substantially worse prognosis, even when standard HF therapies are applied.
Choices regarding glycemic management can markedly affect HF risk and outcomes. Randomized trials show that the peroxisome proliferator-activated receptor agonists double the risk of HF. The glucagonlike peptide–1 receptor agonists are absolutely neutral with regard to HF outcomes. Similarly, the dipeptidyl peptidase–4 inhibitors have no impact on the risks of major adverse cardiovascular events or HF. Intensive glycemic control has no impact on the risk of new-onset HF. Insulin therapy, too, is neutral on this score.
“Depressingly, even lifestyle modification with weight loss, once you have type 2 diabetes, does not lower the risk,” Dr. Fonarow continued.
In contrast, the sodium-glucose transporter 2 (SGLT2) inhibitors have impressive cardiovascular and renal protective benefits in patients with type 2 diabetes, as demonstrated in a meta-analysis of more than 34,000 participants in the randomized trials of empagliflozin (Jardiance) in EMPA-REG OUTCOME, canagliflozin (Invokana) in CANVAS/CANVAS-R, and dapagliflozin (Farxiga) in DECLARE-TIMI 58. The SGLT2 inhibitors collectively reduced the risk of HF hospitalization by 21% in participants with no baseline history of the disease and by 29% in those with a history of HF. Moreover, the risk of progression of renal disease was reduced by 45% (Lancet. 2019 Jan 5;393[10166]:31-9).
More recently, the landmark DAPA-HF trial established SGLT2 inhibitor therapy as part of standard-of-care, guideline-directed medical therapy for patients with HF with reduced ejection fraction regardless of whether they have comorbid type 2 diabetes (N Engl J Med. 2019 Nov 21;381[21]:1995-2008).
These are remarkable medications, generally very well tolerated, and it’s critical that cardiologists get on board in prescribing them, Dr. Fonarow emphasized. He alerted his colleagues to what he called an “incredibly helpful” review article that provides practical guidance for cardiologists in how to start using the SGLT2 inhibitors (JACC Heart Fail. 2019 Feb;7[2]:169-72).
“It’s pretty straightforward,” according to Dr. Fonarow. “If you’re comfortable enough in using ACE inhibitors, angiotensin receptor blockers, and beta-blockers, I think you’ll find these medications fit similarly when you actually get experience in utilizing them.”
He reported serving as a consultant to 10 pharmaceutical or medical device companies.
EXPERT ANALYSIS FROM ACC SNOWMASS 2020
Cosmeceutical ingredients to use before and after antiaging procedures
Outcomes are improved when proper skincare is practiced before and after any type of dermatologic procedure. This column reviews These are ingredients commonly used before, during, and after procedures.
I will use the first person when I am expressing my personal opinion or experience versus data reported in published studies that I reference.
Ascorbic acid
Ascorbic acid (vitamin C) is an essential cofactor necessary for lysyl hydroxylase and prolyl hydroxylase to produce collagen. Many studies have demonstrated that the use of oral and topical ascorbic acid increases collagen production by fibroblasts.1-3 Several different ascorbic acid products, varying greatly in quality, are available on the market.
Ascorbic acid is very sensitive to light and air exposure and does not penetrate well if not at a pH of 2 or 2.5. There are aqueous and lipophilic formulations. Some are produced from L-ascorbic acid, while others are made from ascorbyl palmitate, or salts such as calcium ascorbate, magnesium ascorbate, magnesium ascorbyl phosphate, sodium ascorbate, and sodium ascorbyl phosphate. Consequently, one must closely evaluate any chosen ascorbic acid preparation and pay close attention to the form used in any studies. I am discussing ascorbic acid in general, but my statements only apply to properly formulated products. Most of the studies I quote used L-ascorbic acid, which is the form studied by the late Sheldon Pinnell, MD, who was an expert on ascorbic acid.
Properly formulated L-ascorbic acid products have a low pH. Unless formulated specifically to deter stinging, these low-pH preparations will sting wounded skin. For this reason, most ascorbic acid preparations should be avoided until the skin has completely re-epithelialized. I prefer using it preprocedure and after the procedure once the skin has re-epithelialized. Alster and West showed that use of ascorbic acid – in an aqueous solution formulated not to sting – after laser resurfacing resulted in a significant decrease in post‐CO2 laser resurfacing erythema by the eighth postoperative week when compared with laser‐irradiated skin that had not received topical vitamin C.4
I prefer using ascorbic acid in patients before and after procedures involving fillers, toxins, skin tightening, and nonablative lasers. In my experience, this improves collagen production. Also, I use ascorbic acid before microneedling, but not during or after. Several case reports have cited allergic granulomatous reactions when ascorbic acid is used during microneedling procedures,5 although these reports did not involve aqueous formulations.
Defensin
Defensins are peptides that play an important role in wound repair. Defensin has exhibited the capacity to activate the leucine-rich repeat-containing G-protein–coupled receptors 5 and 6 (also known as LGR5+ and LGR6+) stem cells.6 This accelerates wound healing by stimulating LGR stem cells to form new keratinocytes that populate the epidermis.7 Using defensins prior to procedures would theoretically speed wound healing, but no studies have been published in this area. Anecdotally, it has been used after microneedling without complication. I have not used defensin in this situation, but when I have asked the audience during lectures, many practitioners have reported using it and found that it accelerates healing.
Growth factors
Growth factors are essential in the skin because they are responsible for immunomodulation, regulation of cell division, wound healing, and tissue generation.1 There are several important growth factor families, including: transforming growth factor-beta (TGF-beta), epidermal growth factor (EGF), insulin-like growth factor (IGF), platelet-derived growth factor (PDGF), and fibroblast growth factor (FGF).2 Because of the numerous different variables that play a role with growth factor function, it is difficult to know exactly which combinations are the most helpful to improve outcomes after procedures. There is some evidence to support the use of FGF, TGF-beta, and EGF, IGF, and PDGF to hasten skin healing.8,9 It is certain that growth factors play an important role in pre- and postprocedure skincare, but we do not yet know which growth factor combinations are the most effective.
Heparan sulfate
Heparan sulfate is a glycosaminoglycan found in the skin. Older cells are less responsive to growth factors than are younger cells; therefore, it is desirable to amplify the growth factor signal in older patients. Heparan sulfate has been shown to contribute to growth factors reaching the receptors on the cell surface and enhancing the cell’s ability to “hear” growth factor signals. Combining growth factors with enhancers such as heparan sulfate, defensins, ascorbic acid, and matrikines can improve outcomes of cosmetic procedures. There are not enough studies yet to substantiate which combinations are the most effective. However, I believe that if you are using a growth factor–containing product after a procedure, you should combine it with heparan sulfate to improve efficacy.
Heparan sulfate is not the same as the blood thinner heparin; however, it may affect clotting factors. It is prudent to stop heparan sulfate the day before a dermal filler procedure because of this theoretical risk. (I have not seen an increase in bruising in patients who use heparan sulfate prior to getting fillers.) I suggest using heparan sulfate–containing products with growth factors 24 hours after injecting fillers to try and enhance collagen synthesis that occurs after hyaluronic acid (HA) filler injections.10
Hyaluronic acid
Hyaluronic acid (HA) is known to increase penetration of drugs, as well as cosmeceutical ingredients.11 For this reason, it is often used before a procedure to increase efficacy of growth factors. Many practitioners report using it during microneedling to help the device glide across the skin. I have not observed or heard of any reports of adverse events from using it during microneedling.
HA has been shown to accelerate wound healing in rats12 and dental procedures.13 For this reason, it is often used after laser resurfacing and microneedling procedures and on sutured and open wounds. HA can vary in chain link and molecular weight and whether or not it is cross linked. These differences affect efficacy and should be taken into consideration when choosing an HA product. Some formulations combine various forms of HA. Because HA may increase bruising because of its effects on fibrin formation,14 I prefer not to use it 2 days prior to or the day of filler injections.15
Hydroxy acids
Pretreating skin with hydroxy acids increases dermal matrix formation,16 promotes collagen synthesis,17 and hastens stratum corneum turnover.18 Although postprocedure healing times after pretreatment with hydroxy acids has not been studied, it is very likely that pretreatment with hydroxy acids speeds healing time by increasing collagen production and cell turnover. West and Alster showed that pretreating skin with hydroxy acids prior to CO2 resurfacing did not affect the incidence of postprocedure hyperpigmentation.19
Matrikines
Matrikines are peptides that occur when extracellular matrix (ECM) macromolecules are partially degraded. These peptides interact with cell surface receptors and activate intracellular signalling pathways to modulate ECM remodeling.20 Matrikines, such as tripeptides and hexapeptides, have been shown to remove damaged collagen and elastin from the ECM.21 It is thought that these matrikines help to prepare the skin for procedures by freeing up space to allow room for newly formed collagen. Using matrikines at least 2 weeks before procedures may precondition the skin to heal faster.22
The tripeptide glycyl-histidyl-lysine (GHK) is a good example of a matrikine. When it forms a complex with copper (II) ions (GHK–Cu) it can stimulate collagen and glycosaminoglycan synthesis23 and increase tissue inhibitors of metalloproteinases, TIMP-1 and TIMP-2, which play a role in wound remodeling.24
A serum that contains tripeptide-1, hexapeptide-12, lactoferrin, and phosphatidyl serine has been shown to speed resolution of bruises and inflammation when applied after procedures. It is believed that these ingredients activate macrophages to clear hemosiderin from the skin.
Retinoids
Derived from vitamin A, the retinoid family includes compounds such as adapalene, retinol, tazarotene, trifarotene, and tretinoin. Retinoids should be used for at least 2-4 weeks prior to procedures to improve outcomes. Multiple studies have cogently revealed that pretreatment with tretinoin accelerates wound healing.25-27 Kligman assessed healing after punch biopsy in the mid-1990s and found that the wounds on arms pretreated with tretinoin cream 0.05%-0.1% were significantly diminished by 35%-37% on days 1 and 4 and 47%-50% reduced on days 6, 8, and 11 as compared with the wounds on untreated arms.28 A tretinoin pretreatment regimen of 2-4 weeks is supported by the preponderance of studies29 because peak epidermal hypertrophy emerges after 7 days of tretinoin application and normalizes after 14 days of continued treatment.30 Such an approach gives the skin time to recover from any retinoid dermatitis before the procedure is performed. Pretreatment with adapalene requires an earlier initiation period and should be introduced 5-6 weeks before procedures because it exhibits a longer half-life.31
Topical retinoids should not be used after a procedure until re-epithelialization is complete. Hung et al. applied 0.05% tretinoin cream daily for 10 days prior to partial-thickness skin wounding in a porcine model, with results revealing that re-epithelialization was accelerated with preprocedure treatment while use after the procedure slowed wound healing.32
Skin care regimen design by procedure type
Procedures can be divided into six main types: nonablative, such as peels, intense pulsed light (IPL), and vascular or pigmented lasers; microneedling or other procedures that cause open channels into the dermis; injectables such as toxins and fillers; ablative, such as CO2, erbium, and fractionated lasers; sutured wounds; and unsutured wounds. Skincare regimens that are prescribed before and after each of these procedures should take into account the Baumann Skin Type, the procedure type, whether it is pre- or postprocedure, and lifestyle issues such as sun exposure. Once the pre- and postprocedure regimen has been designed, patients should be given specific instructions as to which brands, the exact products, and the order in which to apply them.
Conclusion
To ensure the best outcomes from surgical treatments, patient education is a key step. The more that patients know and understand about the ways in which they can prepare for their procedure and treat their skin after the procedure, the better the results. Providers should give this type of information in an easy-to-follow printed instruction sheet because studies show that patients cannot remember most of the oral instructions offered by practitioners. Patients should be encouraged to ask questions during their consultation and procedure and to express any concerns with the practitioner’s office should any arise after they have returned home. These steps help improve patient compliance, satisfaction, and outcomes. Please discuss your opinions and experience with me on LinkedIn. You can also see a lecture on this topic on my website, SkinGuru.com.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), as well as a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems. Write to her at dermnews@mdedge.com.
References
1. Murad S et al. Proc Natl Acad Sci U S A. 1981 May;78(5):2879-82.
2. Tajima S, Pinnell SR. J Dermatol Sci. 1996 Mar;11(3):250-3.
3. Geesin JC et al. J Invest Dermatol. 1988 Apr;90(4):420-4.
4. Alster TS, West TB. Dermatol Surg. 1998 Mar;24(3):331-4.
5. Soltani-Arabshahi R et al. JAMA Dermatol. 2014 Jan;150(1):68-72.
6. Lough D et al. Plast Reconstr Surg. 2013 Nov;132(5):1159-71.
7. Hirsch T et al. J Gene Med. 2009 Mar;11(3):220-8.
8. Van Brunt J, Klausner A. Nat Biotechnol. 1988 Jan 1;6:25-30.
9. Lynch SE et al. J Clin Invest. 1989 Aug;84(2):640-6.
10. Wang F et al. Arch Dermatol. 2007 Feb;143(2):155-63.
11. Huang G, Huang H. Drug Deliv. 2018 Nov;25(1):766-72.
12. Celani LM. J Surg Clin Res. 2019 Oct. doi: 10.20398/jscr.v10i2.18825.
13. Yildirim S et al. J Periodontol. 2018 Jan;89(1):36-45.
14. Weigel PH et al. Ciba Found Symp. 1989;143:248-61; discussion 261-4, 281-5.
15. Basora JF et al. Am J Case Rep. 2014 May 9;15:199-202.
16. Okano Yet al. Exp Dermatol. 2003;12 Suppl 2:57-63.
17. Bernstein EF et al. Dermatol Surg. 2001 May;27(5):429-33.
18. Hood HL et al. Food Chem Toxicol. 1999 Nov;37(11):1105-11.
19. West TB, Alster TS. Dermatol Surg. 1999 Jan;25(1):15-7.
20. Maquart FX et al. M. Biochimie. 2005 Mar-Apr;87(3-4):353-60.
21. Pickart L et al. Biomed Res Int. 2015;2015:648108.
22. Widgerow AD et al. Aesthet Surg J. 2019 Apr 8;39 (Supplement 3):S103-11.
23. Maquart FX et al. FEBS Lett. 1988 Oct 10;238(2):343-6.
24. Siméon A et al. J Invest Dermatol. 1999 Jun;112(6):957-64.
25. Vagotis FL, Brundage SR. Aesthetic Plast Surg. 1995 May-Jun;19(3):243-6.
26. Stuzin JM. Plast Reconstr Surg. 2011 Mar;127(3):1343-5.
27. Elson ML. J Am Acad Dermatol. 1998 Aug;39:S79-81.
28. Popp C et al. Br J Dermatol. 1995 Jan;132(1):46-53.
29. Orringer JS et al. J Am Acad Dermatol. 2004 Dec;51(6):940-6.
30. Kim IH et al. J Korean Med Sci. 1996 Aug;11(4):335-41.
31. Basak PY et al. Eur J Dermatol. 2002 Mar-Apr;12(2):145-8.
32. Hung VC et al. Arch Dermatol. 1989 Jan;125(1):65-9.
Outcomes are improved when proper skincare is practiced before and after any type of dermatologic procedure. This column reviews These are ingredients commonly used before, during, and after procedures.
I will use the first person when I am expressing my personal opinion or experience versus data reported in published studies that I reference.
Ascorbic acid
Ascorbic acid (vitamin C) is an essential cofactor necessary for lysyl hydroxylase and prolyl hydroxylase to produce collagen. Many studies have demonstrated that the use of oral and topical ascorbic acid increases collagen production by fibroblasts.1-3 Several different ascorbic acid products, varying greatly in quality, are available on the market.
Ascorbic acid is very sensitive to light and air exposure and does not penetrate well if not at a pH of 2 or 2.5. There are aqueous and lipophilic formulations. Some are produced from L-ascorbic acid, while others are made from ascorbyl palmitate, or salts such as calcium ascorbate, magnesium ascorbate, magnesium ascorbyl phosphate, sodium ascorbate, and sodium ascorbyl phosphate. Consequently, one must closely evaluate any chosen ascorbic acid preparation and pay close attention to the form used in any studies. I am discussing ascorbic acid in general, but my statements only apply to properly formulated products. Most of the studies I quote used L-ascorbic acid, which is the form studied by the late Sheldon Pinnell, MD, who was an expert on ascorbic acid.
Properly formulated L-ascorbic acid products have a low pH. Unless formulated specifically to deter stinging, these low-pH preparations will sting wounded skin. For this reason, most ascorbic acid preparations should be avoided until the skin has completely re-epithelialized. I prefer using it preprocedure and after the procedure once the skin has re-epithelialized. Alster and West showed that use of ascorbic acid – in an aqueous solution formulated not to sting – after laser resurfacing resulted in a significant decrease in post‐CO2 laser resurfacing erythema by the eighth postoperative week when compared with laser‐irradiated skin that had not received topical vitamin C.4
I prefer using ascorbic acid in patients before and after procedures involving fillers, toxins, skin tightening, and nonablative lasers. In my experience, this improves collagen production. Also, I use ascorbic acid before microneedling, but not during or after. Several case reports have cited allergic granulomatous reactions when ascorbic acid is used during microneedling procedures,5 although these reports did not involve aqueous formulations.
Defensin
Defensins are peptides that play an important role in wound repair. Defensin has exhibited the capacity to activate the leucine-rich repeat-containing G-protein–coupled receptors 5 and 6 (also known as LGR5+ and LGR6+) stem cells.6 This accelerates wound healing by stimulating LGR stem cells to form new keratinocytes that populate the epidermis.7 Using defensins prior to procedures would theoretically speed wound healing, but no studies have been published in this area. Anecdotally, it has been used after microneedling without complication. I have not used defensin in this situation, but when I have asked the audience during lectures, many practitioners have reported using it and found that it accelerates healing.
Growth factors
Growth factors are essential in the skin because they are responsible for immunomodulation, regulation of cell division, wound healing, and tissue generation.1 There are several important growth factor families, including: transforming growth factor-beta (TGF-beta), epidermal growth factor (EGF), insulin-like growth factor (IGF), platelet-derived growth factor (PDGF), and fibroblast growth factor (FGF).2 Because of the numerous different variables that play a role with growth factor function, it is difficult to know exactly which combinations are the most helpful to improve outcomes after procedures. There is some evidence to support the use of FGF, TGF-beta, and EGF, IGF, and PDGF to hasten skin healing.8,9 It is certain that growth factors play an important role in pre- and postprocedure skincare, but we do not yet know which growth factor combinations are the most effective.
Heparan sulfate
Heparan sulfate is a glycosaminoglycan found in the skin. Older cells are less responsive to growth factors than are younger cells; therefore, it is desirable to amplify the growth factor signal in older patients. Heparan sulfate has been shown to contribute to growth factors reaching the receptors on the cell surface and enhancing the cell’s ability to “hear” growth factor signals. Combining growth factors with enhancers such as heparan sulfate, defensins, ascorbic acid, and matrikines can improve outcomes of cosmetic procedures. There are not enough studies yet to substantiate which combinations are the most effective. However, I believe that if you are using a growth factor–containing product after a procedure, you should combine it with heparan sulfate to improve efficacy.
Heparan sulfate is not the same as the blood thinner heparin; however, it may affect clotting factors. It is prudent to stop heparan sulfate the day before a dermal filler procedure because of this theoretical risk. (I have not seen an increase in bruising in patients who use heparan sulfate prior to getting fillers.) I suggest using heparan sulfate–containing products with growth factors 24 hours after injecting fillers to try and enhance collagen synthesis that occurs after hyaluronic acid (HA) filler injections.10
Hyaluronic acid
Hyaluronic acid (HA) is known to increase penetration of drugs, as well as cosmeceutical ingredients.11 For this reason, it is often used before a procedure to increase efficacy of growth factors. Many practitioners report using it during microneedling to help the device glide across the skin. I have not observed or heard of any reports of adverse events from using it during microneedling.
HA has been shown to accelerate wound healing in rats12 and dental procedures.13 For this reason, it is often used after laser resurfacing and microneedling procedures and on sutured and open wounds. HA can vary in chain link and molecular weight and whether or not it is cross linked. These differences affect efficacy and should be taken into consideration when choosing an HA product. Some formulations combine various forms of HA. Because HA may increase bruising because of its effects on fibrin formation,14 I prefer not to use it 2 days prior to or the day of filler injections.15
Hydroxy acids
Pretreating skin with hydroxy acids increases dermal matrix formation,16 promotes collagen synthesis,17 and hastens stratum corneum turnover.18 Although postprocedure healing times after pretreatment with hydroxy acids has not been studied, it is very likely that pretreatment with hydroxy acids speeds healing time by increasing collagen production and cell turnover. West and Alster showed that pretreating skin with hydroxy acids prior to CO2 resurfacing did not affect the incidence of postprocedure hyperpigmentation.19
Matrikines
Matrikines are peptides that occur when extracellular matrix (ECM) macromolecules are partially degraded. These peptides interact with cell surface receptors and activate intracellular signalling pathways to modulate ECM remodeling.20 Matrikines, such as tripeptides and hexapeptides, have been shown to remove damaged collagen and elastin from the ECM.21 It is thought that these matrikines help to prepare the skin for procedures by freeing up space to allow room for newly formed collagen. Using matrikines at least 2 weeks before procedures may precondition the skin to heal faster.22
The tripeptide glycyl-histidyl-lysine (GHK) is a good example of a matrikine. When it forms a complex with copper (II) ions (GHK–Cu) it can stimulate collagen and glycosaminoglycan synthesis23 and increase tissue inhibitors of metalloproteinases, TIMP-1 and TIMP-2, which play a role in wound remodeling.24
A serum that contains tripeptide-1, hexapeptide-12, lactoferrin, and phosphatidyl serine has been shown to speed resolution of bruises and inflammation when applied after procedures. It is believed that these ingredients activate macrophages to clear hemosiderin from the skin.
Retinoids
Derived from vitamin A, the retinoid family includes compounds such as adapalene, retinol, tazarotene, trifarotene, and tretinoin. Retinoids should be used for at least 2-4 weeks prior to procedures to improve outcomes. Multiple studies have cogently revealed that pretreatment with tretinoin accelerates wound healing.25-27 Kligman assessed healing after punch biopsy in the mid-1990s and found that the wounds on arms pretreated with tretinoin cream 0.05%-0.1% were significantly diminished by 35%-37% on days 1 and 4 and 47%-50% reduced on days 6, 8, and 11 as compared with the wounds on untreated arms.28 A tretinoin pretreatment regimen of 2-4 weeks is supported by the preponderance of studies29 because peak epidermal hypertrophy emerges after 7 days of tretinoin application and normalizes after 14 days of continued treatment.30 Such an approach gives the skin time to recover from any retinoid dermatitis before the procedure is performed. Pretreatment with adapalene requires an earlier initiation period and should be introduced 5-6 weeks before procedures because it exhibits a longer half-life.31
Topical retinoids should not be used after a procedure until re-epithelialization is complete. Hung et al. applied 0.05% tretinoin cream daily for 10 days prior to partial-thickness skin wounding in a porcine model, with results revealing that re-epithelialization was accelerated with preprocedure treatment while use after the procedure slowed wound healing.32
Skin care regimen design by procedure type
Procedures can be divided into six main types: nonablative, such as peels, intense pulsed light (IPL), and vascular or pigmented lasers; microneedling or other procedures that cause open channels into the dermis; injectables such as toxins and fillers; ablative, such as CO2, erbium, and fractionated lasers; sutured wounds; and unsutured wounds. Skincare regimens that are prescribed before and after each of these procedures should take into account the Baumann Skin Type, the procedure type, whether it is pre- or postprocedure, and lifestyle issues such as sun exposure. Once the pre- and postprocedure regimen has been designed, patients should be given specific instructions as to which brands, the exact products, and the order in which to apply them.
Conclusion
To ensure the best outcomes from surgical treatments, patient education is a key step. The more that patients know and understand about the ways in which they can prepare for their procedure and treat their skin after the procedure, the better the results. Providers should give this type of information in an easy-to-follow printed instruction sheet because studies show that patients cannot remember most of the oral instructions offered by practitioners. Patients should be encouraged to ask questions during their consultation and procedure and to express any concerns with the practitioner’s office should any arise after they have returned home. These steps help improve patient compliance, satisfaction, and outcomes. Please discuss your opinions and experience with me on LinkedIn. You can also see a lecture on this topic on my website, SkinGuru.com.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), as well as a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems. Write to her at dermnews@mdedge.com.
References
1. Murad S et al. Proc Natl Acad Sci U S A. 1981 May;78(5):2879-82.
2. Tajima S, Pinnell SR. J Dermatol Sci. 1996 Mar;11(3):250-3.
3. Geesin JC et al. J Invest Dermatol. 1988 Apr;90(4):420-4.
4. Alster TS, West TB. Dermatol Surg. 1998 Mar;24(3):331-4.
5. Soltani-Arabshahi R et al. JAMA Dermatol. 2014 Jan;150(1):68-72.
6. Lough D et al. Plast Reconstr Surg. 2013 Nov;132(5):1159-71.
7. Hirsch T et al. J Gene Med. 2009 Mar;11(3):220-8.
8. Van Brunt J, Klausner A. Nat Biotechnol. 1988 Jan 1;6:25-30.
9. Lynch SE et al. J Clin Invest. 1989 Aug;84(2):640-6.
10. Wang F et al. Arch Dermatol. 2007 Feb;143(2):155-63.
11. Huang G, Huang H. Drug Deliv. 2018 Nov;25(1):766-72.
12. Celani LM. J Surg Clin Res. 2019 Oct. doi: 10.20398/jscr.v10i2.18825.
13. Yildirim S et al. J Periodontol. 2018 Jan;89(1):36-45.
14. Weigel PH et al. Ciba Found Symp. 1989;143:248-61; discussion 261-4, 281-5.
15. Basora JF et al. Am J Case Rep. 2014 May 9;15:199-202.
16. Okano Yet al. Exp Dermatol. 2003;12 Suppl 2:57-63.
17. Bernstein EF et al. Dermatol Surg. 2001 May;27(5):429-33.
18. Hood HL et al. Food Chem Toxicol. 1999 Nov;37(11):1105-11.
19. West TB, Alster TS. Dermatol Surg. 1999 Jan;25(1):15-7.
20. Maquart FX et al. M. Biochimie. 2005 Mar-Apr;87(3-4):353-60.
21. Pickart L et al. Biomed Res Int. 2015;2015:648108.
22. Widgerow AD et al. Aesthet Surg J. 2019 Apr 8;39 (Supplement 3):S103-11.
23. Maquart FX et al. FEBS Lett. 1988 Oct 10;238(2):343-6.
24. Siméon A et al. J Invest Dermatol. 1999 Jun;112(6):957-64.
25. Vagotis FL, Brundage SR. Aesthetic Plast Surg. 1995 May-Jun;19(3):243-6.
26. Stuzin JM. Plast Reconstr Surg. 2011 Mar;127(3):1343-5.
27. Elson ML. J Am Acad Dermatol. 1998 Aug;39:S79-81.
28. Popp C et al. Br J Dermatol. 1995 Jan;132(1):46-53.
29. Orringer JS et al. J Am Acad Dermatol. 2004 Dec;51(6):940-6.
30. Kim IH et al. J Korean Med Sci. 1996 Aug;11(4):335-41.
31. Basak PY et al. Eur J Dermatol. 2002 Mar-Apr;12(2):145-8.
32. Hung VC et al. Arch Dermatol. 1989 Jan;125(1):65-9.
Outcomes are improved when proper skincare is practiced before and after any type of dermatologic procedure. This column reviews These are ingredients commonly used before, during, and after procedures.
I will use the first person when I am expressing my personal opinion or experience versus data reported in published studies that I reference.
Ascorbic acid
Ascorbic acid (vitamin C) is an essential cofactor necessary for lysyl hydroxylase and prolyl hydroxylase to produce collagen. Many studies have demonstrated that the use of oral and topical ascorbic acid increases collagen production by fibroblasts.1-3 Several different ascorbic acid products, varying greatly in quality, are available on the market.
Ascorbic acid is very sensitive to light and air exposure and does not penetrate well if not at a pH of 2 or 2.5. There are aqueous and lipophilic formulations. Some are produced from L-ascorbic acid, while others are made from ascorbyl palmitate, or salts such as calcium ascorbate, magnesium ascorbate, magnesium ascorbyl phosphate, sodium ascorbate, and sodium ascorbyl phosphate. Consequently, one must closely evaluate any chosen ascorbic acid preparation and pay close attention to the form used in any studies. I am discussing ascorbic acid in general, but my statements only apply to properly formulated products. Most of the studies I quote used L-ascorbic acid, which is the form studied by the late Sheldon Pinnell, MD, who was an expert on ascorbic acid.
Properly formulated L-ascorbic acid products have a low pH. Unless formulated specifically to deter stinging, these low-pH preparations will sting wounded skin. For this reason, most ascorbic acid preparations should be avoided until the skin has completely re-epithelialized. I prefer using it preprocedure and after the procedure once the skin has re-epithelialized. Alster and West showed that use of ascorbic acid – in an aqueous solution formulated not to sting – after laser resurfacing resulted in a significant decrease in post‐CO2 laser resurfacing erythema by the eighth postoperative week when compared with laser‐irradiated skin that had not received topical vitamin C.4
I prefer using ascorbic acid in patients before and after procedures involving fillers, toxins, skin tightening, and nonablative lasers. In my experience, this improves collagen production. Also, I use ascorbic acid before microneedling, but not during or after. Several case reports have cited allergic granulomatous reactions when ascorbic acid is used during microneedling procedures,5 although these reports did not involve aqueous formulations.
Defensin
Defensins are peptides that play an important role in wound repair. Defensin has exhibited the capacity to activate the leucine-rich repeat-containing G-protein–coupled receptors 5 and 6 (also known as LGR5+ and LGR6+) stem cells.6 This accelerates wound healing by stimulating LGR stem cells to form new keratinocytes that populate the epidermis.7 Using defensins prior to procedures would theoretically speed wound healing, but no studies have been published in this area. Anecdotally, it has been used after microneedling without complication. I have not used defensin in this situation, but when I have asked the audience during lectures, many practitioners have reported using it and found that it accelerates healing.
Growth factors
Growth factors are essential in the skin because they are responsible for immunomodulation, regulation of cell division, wound healing, and tissue generation.1 There are several important growth factor families, including: transforming growth factor-beta (TGF-beta), epidermal growth factor (EGF), insulin-like growth factor (IGF), platelet-derived growth factor (PDGF), and fibroblast growth factor (FGF).2 Because of the numerous different variables that play a role with growth factor function, it is difficult to know exactly which combinations are the most helpful to improve outcomes after procedures. There is some evidence to support the use of FGF, TGF-beta, and EGF, IGF, and PDGF to hasten skin healing.8,9 It is certain that growth factors play an important role in pre- and postprocedure skincare, but we do not yet know which growth factor combinations are the most effective.
Heparan sulfate
Heparan sulfate is a glycosaminoglycan found in the skin. Older cells are less responsive to growth factors than are younger cells; therefore, it is desirable to amplify the growth factor signal in older patients. Heparan sulfate has been shown to contribute to growth factors reaching the receptors on the cell surface and enhancing the cell’s ability to “hear” growth factor signals. Combining growth factors with enhancers such as heparan sulfate, defensins, ascorbic acid, and matrikines can improve outcomes of cosmetic procedures. There are not enough studies yet to substantiate which combinations are the most effective. However, I believe that if you are using a growth factor–containing product after a procedure, you should combine it with heparan sulfate to improve efficacy.
Heparan sulfate is not the same as the blood thinner heparin; however, it may affect clotting factors. It is prudent to stop heparan sulfate the day before a dermal filler procedure because of this theoretical risk. (I have not seen an increase in bruising in patients who use heparan sulfate prior to getting fillers.) I suggest using heparan sulfate–containing products with growth factors 24 hours after injecting fillers to try and enhance collagen synthesis that occurs after hyaluronic acid (HA) filler injections.10
Hyaluronic acid
Hyaluronic acid (HA) is known to increase penetration of drugs, as well as cosmeceutical ingredients.11 For this reason, it is often used before a procedure to increase efficacy of growth factors. Many practitioners report using it during microneedling to help the device glide across the skin. I have not observed or heard of any reports of adverse events from using it during microneedling.
HA has been shown to accelerate wound healing in rats12 and dental procedures.13 For this reason, it is often used after laser resurfacing and microneedling procedures and on sutured and open wounds. HA can vary in chain link and molecular weight and whether or not it is cross linked. These differences affect efficacy and should be taken into consideration when choosing an HA product. Some formulations combine various forms of HA. Because HA may increase bruising because of its effects on fibrin formation,14 I prefer not to use it 2 days prior to or the day of filler injections.15
Hydroxy acids
Pretreating skin with hydroxy acids increases dermal matrix formation,16 promotes collagen synthesis,17 and hastens stratum corneum turnover.18 Although postprocedure healing times after pretreatment with hydroxy acids has not been studied, it is very likely that pretreatment with hydroxy acids speeds healing time by increasing collagen production and cell turnover. West and Alster showed that pretreating skin with hydroxy acids prior to CO2 resurfacing did not affect the incidence of postprocedure hyperpigmentation.19
Matrikines
Matrikines are peptides that occur when extracellular matrix (ECM) macromolecules are partially degraded. These peptides interact with cell surface receptors and activate intracellular signalling pathways to modulate ECM remodeling.20 Matrikines, such as tripeptides and hexapeptides, have been shown to remove damaged collagen and elastin from the ECM.21 It is thought that these matrikines help to prepare the skin for procedures by freeing up space to allow room for newly formed collagen. Using matrikines at least 2 weeks before procedures may precondition the skin to heal faster.22
The tripeptide glycyl-histidyl-lysine (GHK) is a good example of a matrikine. When it forms a complex with copper (II) ions (GHK–Cu) it can stimulate collagen and glycosaminoglycan synthesis23 and increase tissue inhibitors of metalloproteinases, TIMP-1 and TIMP-2, which play a role in wound remodeling.24
A serum that contains tripeptide-1, hexapeptide-12, lactoferrin, and phosphatidyl serine has been shown to speed resolution of bruises and inflammation when applied after procedures. It is believed that these ingredients activate macrophages to clear hemosiderin from the skin.
Retinoids
Derived from vitamin A, the retinoid family includes compounds such as adapalene, retinol, tazarotene, trifarotene, and tretinoin. Retinoids should be used for at least 2-4 weeks prior to procedures to improve outcomes. Multiple studies have cogently revealed that pretreatment with tretinoin accelerates wound healing.25-27 Kligman assessed healing after punch biopsy in the mid-1990s and found that the wounds on arms pretreated with tretinoin cream 0.05%-0.1% were significantly diminished by 35%-37% on days 1 and 4 and 47%-50% reduced on days 6, 8, and 11 as compared with the wounds on untreated arms.28 A tretinoin pretreatment regimen of 2-4 weeks is supported by the preponderance of studies29 because peak epidermal hypertrophy emerges after 7 days of tretinoin application and normalizes after 14 days of continued treatment.30 Such an approach gives the skin time to recover from any retinoid dermatitis before the procedure is performed. Pretreatment with adapalene requires an earlier initiation period and should be introduced 5-6 weeks before procedures because it exhibits a longer half-life.31
Topical retinoids should not be used after a procedure until re-epithelialization is complete. Hung et al. applied 0.05% tretinoin cream daily for 10 days prior to partial-thickness skin wounding in a porcine model, with results revealing that re-epithelialization was accelerated with preprocedure treatment while use after the procedure slowed wound healing.32
Skin care regimen design by procedure type
Procedures can be divided into six main types: nonablative, such as peels, intense pulsed light (IPL), and vascular or pigmented lasers; microneedling or other procedures that cause open channels into the dermis; injectables such as toxins and fillers; ablative, such as CO2, erbium, and fractionated lasers; sutured wounds; and unsutured wounds. Skincare regimens that are prescribed before and after each of these procedures should take into account the Baumann Skin Type, the procedure type, whether it is pre- or postprocedure, and lifestyle issues such as sun exposure. Once the pre- and postprocedure regimen has been designed, patients should be given specific instructions as to which brands, the exact products, and the order in which to apply them.
Conclusion
To ensure the best outcomes from surgical treatments, patient education is a key step. The more that patients know and understand about the ways in which they can prepare for their procedure and treat their skin after the procedure, the better the results. Providers should give this type of information in an easy-to-follow printed instruction sheet because studies show that patients cannot remember most of the oral instructions offered by practitioners. Patients should be encouraged to ask questions during their consultation and procedure and to express any concerns with the practitioner’s office should any arise after they have returned home. These steps help improve patient compliance, satisfaction, and outcomes. Please discuss your opinions and experience with me on LinkedIn. You can also see a lecture on this topic on my website, SkinGuru.com.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), as well as a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems. Write to her at dermnews@mdedge.com.
References
1. Murad S et al. Proc Natl Acad Sci U S A. 1981 May;78(5):2879-82.
2. Tajima S, Pinnell SR. J Dermatol Sci. 1996 Mar;11(3):250-3.
3. Geesin JC et al. J Invest Dermatol. 1988 Apr;90(4):420-4.
4. Alster TS, West TB. Dermatol Surg. 1998 Mar;24(3):331-4.
5. Soltani-Arabshahi R et al. JAMA Dermatol. 2014 Jan;150(1):68-72.
6. Lough D et al. Plast Reconstr Surg. 2013 Nov;132(5):1159-71.
7. Hirsch T et al. J Gene Med. 2009 Mar;11(3):220-8.
8. Van Brunt J, Klausner A. Nat Biotechnol. 1988 Jan 1;6:25-30.
9. Lynch SE et al. J Clin Invest. 1989 Aug;84(2):640-6.
10. Wang F et al. Arch Dermatol. 2007 Feb;143(2):155-63.
11. Huang G, Huang H. Drug Deliv. 2018 Nov;25(1):766-72.
12. Celani LM. J Surg Clin Res. 2019 Oct. doi: 10.20398/jscr.v10i2.18825.
13. Yildirim S et al. J Periodontol. 2018 Jan;89(1):36-45.
14. Weigel PH et al. Ciba Found Symp. 1989;143:248-61; discussion 261-4, 281-5.
15. Basora JF et al. Am J Case Rep. 2014 May 9;15:199-202.
16. Okano Yet al. Exp Dermatol. 2003;12 Suppl 2:57-63.
17. Bernstein EF et al. Dermatol Surg. 2001 May;27(5):429-33.
18. Hood HL et al. Food Chem Toxicol. 1999 Nov;37(11):1105-11.
19. West TB, Alster TS. Dermatol Surg. 1999 Jan;25(1):15-7.
20. Maquart FX et al. M. Biochimie. 2005 Mar-Apr;87(3-4):353-60.
21. Pickart L et al. Biomed Res Int. 2015;2015:648108.
22. Widgerow AD et al. Aesthet Surg J. 2019 Apr 8;39 (Supplement 3):S103-11.
23. Maquart FX et al. FEBS Lett. 1988 Oct 10;238(2):343-6.
24. Siméon A et al. J Invest Dermatol. 1999 Jun;112(6):957-64.
25. Vagotis FL, Brundage SR. Aesthetic Plast Surg. 1995 May-Jun;19(3):243-6.
26. Stuzin JM. Plast Reconstr Surg. 2011 Mar;127(3):1343-5.
27. Elson ML. J Am Acad Dermatol. 1998 Aug;39:S79-81.
28. Popp C et al. Br J Dermatol. 1995 Jan;132(1):46-53.
29. Orringer JS et al. J Am Acad Dermatol. 2004 Dec;51(6):940-6.
30. Kim IH et al. J Korean Med Sci. 1996 Aug;11(4):335-41.
31. Basak PY et al. Eur J Dermatol. 2002 Mar-Apr;12(2):145-8.
32. Hung VC et al. Arch Dermatol. 1989 Jan;125(1):65-9.