User login
Toenail thickening
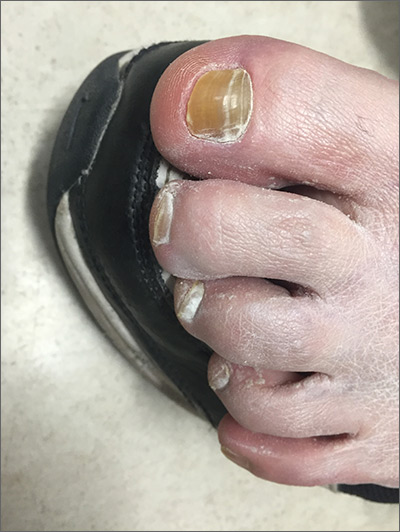
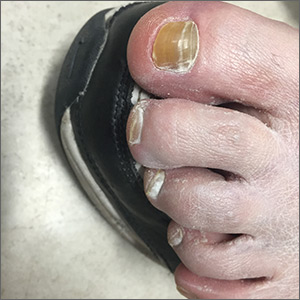
The FP suspected onychauxis, more commonly called hypertrophic nail. The patient’s toenail had the characteristic features of onychauxis, which include discoloration (usually yellow or brown) and a dull appearance. Often, there is an increased curvature or deviation of the nail and a “clam shell” appearance with transverse lines or a lamellar pattern like a ram’s horn. This is in contrast to the longitudinal lines and furrows that one would see with brittle nails associated with old age or the longitudinal melanonychia (hyperpigmented lines) seen in melanoma. Trauma to the nailbed, including trauma from ill-fitting shoes, is the most common cause of onychauxis.
Although nail discoloration and thickening raise the concern for onychomycosis, not all thickened and discolored nails are due to fungal infection. In this case, the thickening of the nail itself (as opposed to the subungal hyperkeratosis typical of onychomycosis) and a lack of improvement with antifungal treatment prompted the FP to consider other causes of nail dystrophy besides onychomycosis.
Nail trimming and filing can minimize discomfort and limit nail margin trauma caused by the nail’s abnormal shape. If this does not provide relief, the curative treatment for onychauxis is toenail removal and matrix ablation. In this case, the patient chose to defer nail removal and resection of the matrix. She said she would consider this treatment option if her nail became more bothersome.
Photos and text courtesy of Sabrina Gill, MD, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Abdullah L, Abba O. Common nail changes and disorders in older people: Diagnosis and management. Can Fam Physician. 2011;57:173–181.
The FP suspected onychauxis, more commonly called hypertrophic nail. The patient’s toenail had the characteristic features of onychauxis, which include discoloration (usually yellow or brown) and a dull appearance. Often, there is an increased curvature or deviation of the nail and a “clam shell” appearance with transverse lines or a lamellar pattern like a ram’s horn. This is in contrast to the longitudinal lines and furrows that one would see with brittle nails associated with old age or the longitudinal melanonychia (hyperpigmented lines) seen in melanoma. Trauma to the nailbed, including trauma from ill-fitting shoes, is the most common cause of onychauxis.
Although nail discoloration and thickening raise the concern for onychomycosis, not all thickened and discolored nails are due to fungal infection. In this case, the thickening of the nail itself (as opposed to the subungal hyperkeratosis typical of onychomycosis) and a lack of improvement with antifungal treatment prompted the FP to consider other causes of nail dystrophy besides onychomycosis.
Nail trimming and filing can minimize discomfort and limit nail margin trauma caused by the nail’s abnormal shape. If this does not provide relief, the curative treatment for onychauxis is toenail removal and matrix ablation. In this case, the patient chose to defer nail removal and resection of the matrix. She said she would consider this treatment option if her nail became more bothersome.
Photos and text courtesy of Sabrina Gill, MD, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
The FP suspected onychauxis, more commonly called hypertrophic nail. The patient’s toenail had the characteristic features of onychauxis, which include discoloration (usually yellow or brown) and a dull appearance. Often, there is an increased curvature or deviation of the nail and a “clam shell” appearance with transverse lines or a lamellar pattern like a ram’s horn. This is in contrast to the longitudinal lines and furrows that one would see with brittle nails associated with old age or the longitudinal melanonychia (hyperpigmented lines) seen in melanoma. Trauma to the nailbed, including trauma from ill-fitting shoes, is the most common cause of onychauxis.
Although nail discoloration and thickening raise the concern for onychomycosis, not all thickened and discolored nails are due to fungal infection. In this case, the thickening of the nail itself (as opposed to the subungal hyperkeratosis typical of onychomycosis) and a lack of improvement with antifungal treatment prompted the FP to consider other causes of nail dystrophy besides onychomycosis.
Nail trimming and filing can minimize discomfort and limit nail margin trauma caused by the nail’s abnormal shape. If this does not provide relief, the curative treatment for onychauxis is toenail removal and matrix ablation. In this case, the patient chose to defer nail removal and resection of the matrix. She said she would consider this treatment option if her nail became more bothersome.
Photos and text courtesy of Sabrina Gill, MD, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Abdullah L, Abba O. Common nail changes and disorders in older people: Diagnosis and management. Can Fam Physician. 2011;57:173–181.
Abdullah L, Abba O. Common nail changes and disorders in older people: Diagnosis and management. Can Fam Physician. 2011;57:173–181.
2019-nCoV outbreak: A few lessons learned for pediatric practices
In late January, signs were posted in all of the offices in our faculty medical practice building.
Combined with current worldwide health concerns and flu season, we are now asking all patients two questions:
1. Do you have a fever, cough or shortness of breath?
2. Have you traveled to China in the last 2 weeks, or have you had contact with someone who has and who now is sick?
Similar signs appeared in medical offices and EDs across the city. Truth be told, when the signs first went up, some thought it was an overreaction. I practice in a city in the Southeast that is not a port of entry and has no scheduled international passenger flights. Wuhan City, China and the threat of 2019 novel coronavirus (2019-nCoV) seemed very far away.
As the international tally of cases has grown, so have local concerns.
Hopefully, proactive public health measures to care for the few individuals currently infected in the United States and appropriately assessing individuals arriving from mainland China will prevent widespread circulation of 2019-nCoV here. If this is the case, most of us likely will never see a case of the virus. Still, there are important lessons to be learned from current preparedness efforts.
A travel history is important. Several years ago, during the height of concern over the spread of Ebola, the health care systems in which I practice asked everyone about travel to West Africa as soon as they approached the registration desk. In the intervening years, asking about a travel history largely was delegated to providers, and I suspect it largely was driven by patient presentation. Child presenting with 10 days of fever? The clinician likely took a travel history. Child presenting for runny nose, ear ache, or rash? Maybe not. With more consistent screening, we are learning how frequently our patients and their families do travel, and that is helping us expand our differential diagnosis.
We need to practice cough etiquette. Patients who endorse respiratory symptoms as part of 2019 n-CoV screening are handed a mask. Those who have traveled to China in the last 14 days are promptly escorted to an exam room. In truth, we should be following cough etiquette and offering all patients with respiratory symptoms a mask. Heightened awareness of this practice may help prevent the spread of much more common viruses such as influenza. Reliable processes to recognize and rapidly triage patients with an infectious illness are critically important in ambulatory settings, and now we have an opportunity to trial and improve these processes. No one wants a child with measles or chicken pox to sit in the waiting room!
Offices must stock personal protective equipment to comply with standard precautions. The recommended PPE when caring for a patient with 2019 n-CoV includes a gown, gloves, mask (n95 or PAPR if available), and eye protection, such as a face shield or goggles. An initial survey of PPE supplies locally revealed of shortage of PPE for eye protection in some offices. Eye protection should be readily available in pediatric and other primary care offices because it must be used as part of standard precautions during procedures likely to generate droplets of blood or body fluids. Examples of common procedures that require eye protection include swabbing the nasopharynx to obtain a specimen for respiratory virus testing or swabbing the throat to test for group A streptococcus.
We should use diagnostic testing judiciously. Over the last couple of weeks, we’ve had a couple of patients who wanted to be tested for 2019 n-CoV but did not meet person under investigation (PUI) criteria. Public health authorities, who must approve all 2019 n-CoV testing, said no. This is enforced diagnostic stewardship, but it is a reminder that, when a diagnostic test is performed in a person with a low likelihood of disease, there is a risk of a false-positive result. What if we applied this principle to tests we send routinely? We would send fewer urine cultures in patients with normal urinalyses and stop testing infants for Clostridioides difficile.
Frontline providers must partner with public health colleagues during outbreaks. Providers have been instructed to immediately notify local or state health departments when a patient is suspected of having 2019 n-CoV specifically because the PUI criteria are met. This notification was crucial in diagnosing the first cases of 2019 n-CoV in the United States. Nine of the first 11 U.S. cases were in travelers from Wuhan, and according to the Centers for Disease Control and Prevention, eight of these “were identified as a result of patients seeking clinical care for symptoms and clinicians connecting with the appropriate public health systems.” Locally, daytime and after hours phone numbers for the health department have been posted in offices across our health care system. The state health department is hosting well-attended webinars to provide updates and answer questions from clinicians. We may never have a case of 2019 n-CoV in Kentucky, but activities like these build relationships between providers and our colleagues in public health, strengthening infrastructure and the capacity to respond to future outbreaks. I suspect the same is true in many other communities.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
In late January, signs were posted in all of the offices in our faculty medical practice building.
Combined with current worldwide health concerns and flu season, we are now asking all patients two questions:
1. Do you have a fever, cough or shortness of breath?
2. Have you traveled to China in the last 2 weeks, or have you had contact with someone who has and who now is sick?
Similar signs appeared in medical offices and EDs across the city. Truth be told, when the signs first went up, some thought it was an overreaction. I practice in a city in the Southeast that is not a port of entry and has no scheduled international passenger flights. Wuhan City, China and the threat of 2019 novel coronavirus (2019-nCoV) seemed very far away.
As the international tally of cases has grown, so have local concerns.
Hopefully, proactive public health measures to care for the few individuals currently infected in the United States and appropriately assessing individuals arriving from mainland China will prevent widespread circulation of 2019-nCoV here. If this is the case, most of us likely will never see a case of the virus. Still, there are important lessons to be learned from current preparedness efforts.
A travel history is important. Several years ago, during the height of concern over the spread of Ebola, the health care systems in which I practice asked everyone about travel to West Africa as soon as they approached the registration desk. In the intervening years, asking about a travel history largely was delegated to providers, and I suspect it largely was driven by patient presentation. Child presenting with 10 days of fever? The clinician likely took a travel history. Child presenting for runny nose, ear ache, or rash? Maybe not. With more consistent screening, we are learning how frequently our patients and their families do travel, and that is helping us expand our differential diagnosis.
We need to practice cough etiquette. Patients who endorse respiratory symptoms as part of 2019 n-CoV screening are handed a mask. Those who have traveled to China in the last 14 days are promptly escorted to an exam room. In truth, we should be following cough etiquette and offering all patients with respiratory symptoms a mask. Heightened awareness of this practice may help prevent the spread of much more common viruses such as influenza. Reliable processes to recognize and rapidly triage patients with an infectious illness are critically important in ambulatory settings, and now we have an opportunity to trial and improve these processes. No one wants a child with measles or chicken pox to sit in the waiting room!
Offices must stock personal protective equipment to comply with standard precautions. The recommended PPE when caring for a patient with 2019 n-CoV includes a gown, gloves, mask (n95 or PAPR if available), and eye protection, such as a face shield or goggles. An initial survey of PPE supplies locally revealed of shortage of PPE for eye protection in some offices. Eye protection should be readily available in pediatric and other primary care offices because it must be used as part of standard precautions during procedures likely to generate droplets of blood or body fluids. Examples of common procedures that require eye protection include swabbing the nasopharynx to obtain a specimen for respiratory virus testing or swabbing the throat to test for group A streptococcus.
We should use diagnostic testing judiciously. Over the last couple of weeks, we’ve had a couple of patients who wanted to be tested for 2019 n-CoV but did not meet person under investigation (PUI) criteria. Public health authorities, who must approve all 2019 n-CoV testing, said no. This is enforced diagnostic stewardship, but it is a reminder that, when a diagnostic test is performed in a person with a low likelihood of disease, there is a risk of a false-positive result. What if we applied this principle to tests we send routinely? We would send fewer urine cultures in patients with normal urinalyses and stop testing infants for Clostridioides difficile.
Frontline providers must partner with public health colleagues during outbreaks. Providers have been instructed to immediately notify local or state health departments when a patient is suspected of having 2019 n-CoV specifically because the PUI criteria are met. This notification was crucial in diagnosing the first cases of 2019 n-CoV in the United States. Nine of the first 11 U.S. cases were in travelers from Wuhan, and according to the Centers for Disease Control and Prevention, eight of these “were identified as a result of patients seeking clinical care for symptoms and clinicians connecting with the appropriate public health systems.” Locally, daytime and after hours phone numbers for the health department have been posted in offices across our health care system. The state health department is hosting well-attended webinars to provide updates and answer questions from clinicians. We may never have a case of 2019 n-CoV in Kentucky, but activities like these build relationships between providers and our colleagues in public health, strengthening infrastructure and the capacity to respond to future outbreaks. I suspect the same is true in many other communities.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
In late January, signs were posted in all of the offices in our faculty medical practice building.
Combined with current worldwide health concerns and flu season, we are now asking all patients two questions:
1. Do you have a fever, cough or shortness of breath?
2. Have you traveled to China in the last 2 weeks, or have you had contact with someone who has and who now is sick?
Similar signs appeared in medical offices and EDs across the city. Truth be told, when the signs first went up, some thought it was an overreaction. I practice in a city in the Southeast that is not a port of entry and has no scheduled international passenger flights. Wuhan City, China and the threat of 2019 novel coronavirus (2019-nCoV) seemed very far away.
As the international tally of cases has grown, so have local concerns.
Hopefully, proactive public health measures to care for the few individuals currently infected in the United States and appropriately assessing individuals arriving from mainland China will prevent widespread circulation of 2019-nCoV here. If this is the case, most of us likely will never see a case of the virus. Still, there are important lessons to be learned from current preparedness efforts.
A travel history is important. Several years ago, during the height of concern over the spread of Ebola, the health care systems in which I practice asked everyone about travel to West Africa as soon as they approached the registration desk. In the intervening years, asking about a travel history largely was delegated to providers, and I suspect it largely was driven by patient presentation. Child presenting with 10 days of fever? The clinician likely took a travel history. Child presenting for runny nose, ear ache, or rash? Maybe not. With more consistent screening, we are learning how frequently our patients and their families do travel, and that is helping us expand our differential diagnosis.
We need to practice cough etiquette. Patients who endorse respiratory symptoms as part of 2019 n-CoV screening are handed a mask. Those who have traveled to China in the last 14 days are promptly escorted to an exam room. In truth, we should be following cough etiquette and offering all patients with respiratory symptoms a mask. Heightened awareness of this practice may help prevent the spread of much more common viruses such as influenza. Reliable processes to recognize and rapidly triage patients with an infectious illness are critically important in ambulatory settings, and now we have an opportunity to trial and improve these processes. No one wants a child with measles or chicken pox to sit in the waiting room!
Offices must stock personal protective equipment to comply with standard precautions. The recommended PPE when caring for a patient with 2019 n-CoV includes a gown, gloves, mask (n95 or PAPR if available), and eye protection, such as a face shield or goggles. An initial survey of PPE supplies locally revealed of shortage of PPE for eye protection in some offices. Eye protection should be readily available in pediatric and other primary care offices because it must be used as part of standard precautions during procedures likely to generate droplets of blood or body fluids. Examples of common procedures that require eye protection include swabbing the nasopharynx to obtain a specimen for respiratory virus testing or swabbing the throat to test for group A streptococcus.
We should use diagnostic testing judiciously. Over the last couple of weeks, we’ve had a couple of patients who wanted to be tested for 2019 n-CoV but did not meet person under investigation (PUI) criteria. Public health authorities, who must approve all 2019 n-CoV testing, said no. This is enforced diagnostic stewardship, but it is a reminder that, when a diagnostic test is performed in a person with a low likelihood of disease, there is a risk of a false-positive result. What if we applied this principle to tests we send routinely? We would send fewer urine cultures in patients with normal urinalyses and stop testing infants for Clostridioides difficile.
Frontline providers must partner with public health colleagues during outbreaks. Providers have been instructed to immediately notify local or state health departments when a patient is suspected of having 2019 n-CoV specifically because the PUI criteria are met. This notification was crucial in diagnosing the first cases of 2019 n-CoV in the United States. Nine of the first 11 U.S. cases were in travelers from Wuhan, and according to the Centers for Disease Control and Prevention, eight of these “were identified as a result of patients seeking clinical care for symptoms and clinicians connecting with the appropriate public health systems.” Locally, daytime and after hours phone numbers for the health department have been posted in offices across our health care system. The state health department is hosting well-attended webinars to provide updates and answer questions from clinicians. We may never have a case of 2019 n-CoV in Kentucky, but activities like these build relationships between providers and our colleagues in public health, strengthening infrastructure and the capacity to respond to future outbreaks. I suspect the same is true in many other communities.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
FDA issues MiniMed600 insulin pump recall
according to a Food and Drug Administration MedWatch release.
A class I recall such as this indicates that there is reasonable probability that using a defective pump will cause serious adverse health consequences or death, the agency said in the recall notice. It said the company has received more than 26,000 complaints regarding this problem and is aware of 2,175 injuries and 1 death so far. In all, 322,005 devices have been recalled.
If the pumps in question – Model 630G (distributed September 2016 to October 2019) and 670G (June 2017 to August 2019) – have broken or missing retainer rings, the insulin cartridge can end up loose in the reservoir compartment, which can lead to incorrect dosing and therefore potentially to hyperglycemia or hypoglycemia, according to the statement.
Model 630G was approved by the FDA in August 2016, and the 670G in September that same year.
On Nov. 21, 2019, Medtronic advised patients with type 1 diabetes who use the pumps to:
- Examine the retainer ring to see if it is loose, broken, or missing.
- Stop using the pump if the reservoir does not lock correctly or if the retainer ring is loose, damaged, or missing. Patients should contact Medtronic for a replacement pump and follow their doctor’s recommendations and perform manual insulin injections.
- Continue using the pump if the reservoir locks in place correctly.
- Check pump and retainer ring if the pump is dropped by accident, and stop use if it is damaged.
- Check the pump and retainer ring every set change to verify the reservoir is locked correctly.
More information regarding this recall, including how to contact Medtronic Technical Support, can be found on the FDA website.
according to a Food and Drug Administration MedWatch release.
A class I recall such as this indicates that there is reasonable probability that using a defective pump will cause serious adverse health consequences or death, the agency said in the recall notice. It said the company has received more than 26,000 complaints regarding this problem and is aware of 2,175 injuries and 1 death so far. In all, 322,005 devices have been recalled.
If the pumps in question – Model 630G (distributed September 2016 to October 2019) and 670G (June 2017 to August 2019) – have broken or missing retainer rings, the insulin cartridge can end up loose in the reservoir compartment, which can lead to incorrect dosing and therefore potentially to hyperglycemia or hypoglycemia, according to the statement.
Model 630G was approved by the FDA in August 2016, and the 670G in September that same year.
On Nov. 21, 2019, Medtronic advised patients with type 1 diabetes who use the pumps to:
- Examine the retainer ring to see if it is loose, broken, or missing.
- Stop using the pump if the reservoir does not lock correctly or if the retainer ring is loose, damaged, or missing. Patients should contact Medtronic for a replacement pump and follow their doctor’s recommendations and perform manual insulin injections.
- Continue using the pump if the reservoir locks in place correctly.
- Check pump and retainer ring if the pump is dropped by accident, and stop use if it is damaged.
- Check the pump and retainer ring every set change to verify the reservoir is locked correctly.
More information regarding this recall, including how to contact Medtronic Technical Support, can be found on the FDA website.
according to a Food and Drug Administration MedWatch release.
A class I recall such as this indicates that there is reasonable probability that using a defective pump will cause serious adverse health consequences or death, the agency said in the recall notice. It said the company has received more than 26,000 complaints regarding this problem and is aware of 2,175 injuries and 1 death so far. In all, 322,005 devices have been recalled.
If the pumps in question – Model 630G (distributed September 2016 to October 2019) and 670G (June 2017 to August 2019) – have broken or missing retainer rings, the insulin cartridge can end up loose in the reservoir compartment, which can lead to incorrect dosing and therefore potentially to hyperglycemia or hypoglycemia, according to the statement.
Model 630G was approved by the FDA in August 2016, and the 670G in September that same year.
On Nov. 21, 2019, Medtronic advised patients with type 1 diabetes who use the pumps to:
- Examine the retainer ring to see if it is loose, broken, or missing.
- Stop using the pump if the reservoir does not lock correctly or if the retainer ring is loose, damaged, or missing. Patients should contact Medtronic for a replacement pump and follow their doctor’s recommendations and perform manual insulin injections.
- Continue using the pump if the reservoir locks in place correctly.
- Check pump and retainer ring if the pump is dropped by accident, and stop use if it is damaged.
- Check the pump and retainer ring every set change to verify the reservoir is locked correctly.
More information regarding this recall, including how to contact Medtronic Technical Support, can be found on the FDA website.
Thrombectomy access lags for U.S. stroke patients
In 2017, roughly 3 years after evidence from several studies made endovascular thrombectomy first-line treatment for selected acute ischemic stroke patients, the treatment was available at barely more than one-third of all U.S. stroke centers, available within 30-minute access to just over 30% of Americans, and available within 15-minute access to one-fifth of U.S. residents, based on information in a comprehensive U.S. database.
These numbers showed that “current direct EVT [endovascular thrombectomy] access in the United States is suboptimal under predominate EMS routing protocols,” Amrou Sarraj, MD, and his associates wrote in an article published online in Stroke on Feb. 12. “Only in eight states did the coverage exceed 25% of the population, and nine states had coverage for less than 10% of the population. These results reflect limited access to an effective treatment modality that would improve clinical outcomes in patients with large strokes and prevent potentially devastating disability,” wrote Dr. Sarraj, chief of the general neurology service at Memorial-Hermann Hospital in Houston and coauthors.
Their analysis of data collected in 2017 by the Medicare Provider Analysis and Review (MEDPAR) database, maintained by the Centers for Medicare & Medicaid Services, identified two apparently effective ways to improve EVT access for acute ischemic stroke patients: First, systematically divert patients to a nearby center that offers EVT even when it means bypassing a closer stroke center that does not perform EVT when the added travel time is less than 15 minutes. Second, convert selected stroke centers that currently do not perform EVT into centers that do. Between these two approaches, the strategy of having ambulances bypass stroke centers that do not perform EVT and continuing to ones that do generally has the greater potential to boost access, the authors found. They based their analysis exclusively on their calculations of expected consequences rather than actual experience.
The calculations showed that bypassing non-EVT centers when the added bypass time computed to less than 15 minutes linked with an anticipated overall U.S. gain in access of about 17%, or 52 million people, extending the ability of acute ischemic stroke patients able to quickly reach an EVT center to about 37% of the American public. The second approach to boost access, converting the top 10% of stroke centers based on case volume that currently do not provide EVT to centers that do offer it, would result in expanded access for about 23 million additional Americans, raising the total with access to about 27% of the public, the new report said.
As part of this analysis, the MEDPAR data identified 1,941 U.S. centers providing stroke services during 2017, of which 713 (37%) had performed at least one EVT procedure. By comparison, 2015 MEDPAR data showed 577 U.S. stroke centers performing EVT, indicating that during the 2-3 years following several reports in early 2015 on the net benefits of EVT for acute ischemic stroke patients, the number of U.S. stroke centers offering this treatment had grown by a relative 24%. Based on the locations of the stroke centers that made EVT available in 2017, Dr. Sarraj and coauthors calculated that the 713 EVT-capable stroke centers provided emergency access within a 15-minute ground-ambulance trip for 61 million Americans (20% of the U.S. population), and within a 30-minute ground-transport trip to 95 million residents (31%).
Boosting these numbers by implementing a systematic bypass of stroke patients past non-EVT stroke centers to nearby centers that are EVT capable “has the benefit of ease of implementation and requires less time and resources,” the authors said. However, they also noted the heterogeneity of circumstances based on variables like population density and stroke center distribution, which means that in some locations the most effective way to boost access would be by increasing the number of stroke centers that provide EVT.
In 2018, Dr. Sarraj and associates reported results from a similar analysis of MEDPAR data that used 30-minute and 60-minute ground-transport times as the criteria for their calculations.
The study received no commercial funding. Dr. Sarraj reported receiving research funding from Stryker Neurovascular outside of this work. One coauthor reported serving in roles for the University of Texas Health System for which the institution has been funded via various industry and government grants, and another coauthor reported receiving research funding from the Patient-Centered Outcomes Research Institute, the National Institutes of Health, Genentech, and CSL Behring, as well as consulting fees from Frazer Ltd.
SOURCE: Sarraj A et al. Stroke. 2020 Feb 12. doi: 10.1161/STROKEAHA.120.028850.
In 2017, roughly 3 years after evidence from several studies made endovascular thrombectomy first-line treatment for selected acute ischemic stroke patients, the treatment was available at barely more than one-third of all U.S. stroke centers, available within 30-minute access to just over 30% of Americans, and available within 15-minute access to one-fifth of U.S. residents, based on information in a comprehensive U.S. database.
These numbers showed that “current direct EVT [endovascular thrombectomy] access in the United States is suboptimal under predominate EMS routing protocols,” Amrou Sarraj, MD, and his associates wrote in an article published online in Stroke on Feb. 12. “Only in eight states did the coverage exceed 25% of the population, and nine states had coverage for less than 10% of the population. These results reflect limited access to an effective treatment modality that would improve clinical outcomes in patients with large strokes and prevent potentially devastating disability,” wrote Dr. Sarraj, chief of the general neurology service at Memorial-Hermann Hospital in Houston and coauthors.
Their analysis of data collected in 2017 by the Medicare Provider Analysis and Review (MEDPAR) database, maintained by the Centers for Medicare & Medicaid Services, identified two apparently effective ways to improve EVT access for acute ischemic stroke patients: First, systematically divert patients to a nearby center that offers EVT even when it means bypassing a closer stroke center that does not perform EVT when the added travel time is less than 15 minutes. Second, convert selected stroke centers that currently do not perform EVT into centers that do. Between these two approaches, the strategy of having ambulances bypass stroke centers that do not perform EVT and continuing to ones that do generally has the greater potential to boost access, the authors found. They based their analysis exclusively on their calculations of expected consequences rather than actual experience.
The calculations showed that bypassing non-EVT centers when the added bypass time computed to less than 15 minutes linked with an anticipated overall U.S. gain in access of about 17%, or 52 million people, extending the ability of acute ischemic stroke patients able to quickly reach an EVT center to about 37% of the American public. The second approach to boost access, converting the top 10% of stroke centers based on case volume that currently do not provide EVT to centers that do offer it, would result in expanded access for about 23 million additional Americans, raising the total with access to about 27% of the public, the new report said.
As part of this analysis, the MEDPAR data identified 1,941 U.S. centers providing stroke services during 2017, of which 713 (37%) had performed at least one EVT procedure. By comparison, 2015 MEDPAR data showed 577 U.S. stroke centers performing EVT, indicating that during the 2-3 years following several reports in early 2015 on the net benefits of EVT for acute ischemic stroke patients, the number of U.S. stroke centers offering this treatment had grown by a relative 24%. Based on the locations of the stroke centers that made EVT available in 2017, Dr. Sarraj and coauthors calculated that the 713 EVT-capable stroke centers provided emergency access within a 15-minute ground-ambulance trip for 61 million Americans (20% of the U.S. population), and within a 30-minute ground-transport trip to 95 million residents (31%).
Boosting these numbers by implementing a systematic bypass of stroke patients past non-EVT stroke centers to nearby centers that are EVT capable “has the benefit of ease of implementation and requires less time and resources,” the authors said. However, they also noted the heterogeneity of circumstances based on variables like population density and stroke center distribution, which means that in some locations the most effective way to boost access would be by increasing the number of stroke centers that provide EVT.
In 2018, Dr. Sarraj and associates reported results from a similar analysis of MEDPAR data that used 30-minute and 60-minute ground-transport times as the criteria for their calculations.
The study received no commercial funding. Dr. Sarraj reported receiving research funding from Stryker Neurovascular outside of this work. One coauthor reported serving in roles for the University of Texas Health System for which the institution has been funded via various industry and government grants, and another coauthor reported receiving research funding from the Patient-Centered Outcomes Research Institute, the National Institutes of Health, Genentech, and CSL Behring, as well as consulting fees from Frazer Ltd.
SOURCE: Sarraj A et al. Stroke. 2020 Feb 12. doi: 10.1161/STROKEAHA.120.028850.
In 2017, roughly 3 years after evidence from several studies made endovascular thrombectomy first-line treatment for selected acute ischemic stroke patients, the treatment was available at barely more than one-third of all U.S. stroke centers, available within 30-minute access to just over 30% of Americans, and available within 15-minute access to one-fifth of U.S. residents, based on information in a comprehensive U.S. database.
These numbers showed that “current direct EVT [endovascular thrombectomy] access in the United States is suboptimal under predominate EMS routing protocols,” Amrou Sarraj, MD, and his associates wrote in an article published online in Stroke on Feb. 12. “Only in eight states did the coverage exceed 25% of the population, and nine states had coverage for less than 10% of the population. These results reflect limited access to an effective treatment modality that would improve clinical outcomes in patients with large strokes and prevent potentially devastating disability,” wrote Dr. Sarraj, chief of the general neurology service at Memorial-Hermann Hospital in Houston and coauthors.
Their analysis of data collected in 2017 by the Medicare Provider Analysis and Review (MEDPAR) database, maintained by the Centers for Medicare & Medicaid Services, identified two apparently effective ways to improve EVT access for acute ischemic stroke patients: First, systematically divert patients to a nearby center that offers EVT even when it means bypassing a closer stroke center that does not perform EVT when the added travel time is less than 15 minutes. Second, convert selected stroke centers that currently do not perform EVT into centers that do. Between these two approaches, the strategy of having ambulances bypass stroke centers that do not perform EVT and continuing to ones that do generally has the greater potential to boost access, the authors found. They based their analysis exclusively on their calculations of expected consequences rather than actual experience.
The calculations showed that bypassing non-EVT centers when the added bypass time computed to less than 15 minutes linked with an anticipated overall U.S. gain in access of about 17%, or 52 million people, extending the ability of acute ischemic stroke patients able to quickly reach an EVT center to about 37% of the American public. The second approach to boost access, converting the top 10% of stroke centers based on case volume that currently do not provide EVT to centers that do offer it, would result in expanded access for about 23 million additional Americans, raising the total with access to about 27% of the public, the new report said.
As part of this analysis, the MEDPAR data identified 1,941 U.S. centers providing stroke services during 2017, of which 713 (37%) had performed at least one EVT procedure. By comparison, 2015 MEDPAR data showed 577 U.S. stroke centers performing EVT, indicating that during the 2-3 years following several reports in early 2015 on the net benefits of EVT for acute ischemic stroke patients, the number of U.S. stroke centers offering this treatment had grown by a relative 24%. Based on the locations of the stroke centers that made EVT available in 2017, Dr. Sarraj and coauthors calculated that the 713 EVT-capable stroke centers provided emergency access within a 15-minute ground-ambulance trip for 61 million Americans (20% of the U.S. population), and within a 30-minute ground-transport trip to 95 million residents (31%).
Boosting these numbers by implementing a systematic bypass of stroke patients past non-EVT stroke centers to nearby centers that are EVT capable “has the benefit of ease of implementation and requires less time and resources,” the authors said. However, they also noted the heterogeneity of circumstances based on variables like population density and stroke center distribution, which means that in some locations the most effective way to boost access would be by increasing the number of stroke centers that provide EVT.
In 2018, Dr. Sarraj and associates reported results from a similar analysis of MEDPAR data that used 30-minute and 60-minute ground-transport times as the criteria for their calculations.
The study received no commercial funding. Dr. Sarraj reported receiving research funding from Stryker Neurovascular outside of this work. One coauthor reported serving in roles for the University of Texas Health System for which the institution has been funded via various industry and government grants, and another coauthor reported receiving research funding from the Patient-Centered Outcomes Research Institute, the National Institutes of Health, Genentech, and CSL Behring, as well as consulting fees from Frazer Ltd.
SOURCE: Sarraj A et al. Stroke. 2020 Feb 12. doi: 10.1161/STROKEAHA.120.028850.
FROM STROKE
AAN publishes guideline on the treatment of sleep problems in children with autism
The guideline was published online ahead of print Feb. 12 in Neurology.
“While up to 40% of children and teens in the general population will have sleep problems at some point during their childhood, such problems usually lessen with age,” lead author Ashura Williams Buckley, MD, director of the Sleep and Neurodevelopment Service at the National Institute of Mental Health in Bethesda, Md., said in a press release. “For children and teens with autism, sleep problems are more common and more likely to persist, resulting in poor health and poor quality of life. Some sleep problems may be directly related to autism, but others are not. Regardless, autism symptoms may make sleep problems worse.”
Few evidence-based treatments are available
Dr. Williams Buckley and colleagues developed the current guideline to evaluate which pharmacologic, behavioral, and complementary and alternative medicine (CAM) interventions improve bedtime resistance, sleep onset latency, sleep continuity, total sleep time, and daytime behavior in children and adolescents with ASD. The panel evaluated 900 abstracts of articles that had been included in systematic reviews, as well as 1,087 additional abstracts. One hundred thirty-nine articles were potentially relevant, 12 met criteria for data extraction, and eight were rated class III or higher and were included in the panel’s review.
The authors observed what they called a dearth of evidence-based treatments for sleep dysregulation in ASD. Evidence indicates that melatonin, with or without cognitive–behavioral therapy (CBT), improves several sleep outcomes, compared with placebo. “Evidence for other interventions is largely lacking,” wrote Dr. Williams Buckley and colleagues. They observed a lack of long-term safety data for melatonin in children, which they considered concerning, because melatonin affects the hypothalamic–gonadal axis and can potentially influence pubertal development.
Screening for comorbid conditions and concomitant medications
The guideline recommends that clinicians assess children with ASD and sleep disturbances for coexisting conditions and concomitant medications that could be contributing to these sleep disturbances. They should ensure that children receive appropriate treatment for coexisting conditions and adjust or discontinue potentially problematic medications appropriately, according to the guideline.
Furthermore, clinicians should counsel parents or guardians about behavioral strategies as a first-line treatment for improving sleep function. These strategies could be administered alone or with pharmacologic or neutraceutical approaches as needed, according to the authors. Suggested behavioral approaches include unmodified extinction (i.e., imposing a bedtime and ignoring a child’s protests), graduated extinction (i.e., ignoring protests for a specified period before responding), positive routines (i.e., establishing pre-bedtime calming rituals), and bedtime fading (i.e., putting a child to bed close to the time he or she begins to fall asleep).
If a child’s contributing coexisting conditions and medications have been addressed and behavioral strategies have not been helpful, clinicians should offer melatonin, according to the guideline. Because over-the-counter formulations contain variable concentrations of melatonin, clinicians should write a prescription for it or recommend high-purity pharmaceutical grade melatonin. The initial dose should be 1-3 mg/day at 60-30 minutes before bedtime. The dose can be titrated to 10 mg/day. Clinicians also should counsel children and their parents about potential adverse events of melatonin and the lack of long-term safety data, according to the guideline.
In addition, clinicians should advise children and parents that no evidence supports the routine use of weighted blankets or specialized mattress technology for improving sleep. Parents who ask about weighted blankets should be told that the reviewed trial reported no serious adverse events with this intervention, and that blankets could be a reasonable nonpharmacologic approach for some patients, according to the guideline.
Optimal outcome measures are undefined
Dr. Williams Buckley and colleagues also suggested areas for future research. Investigators have not yet defined optimal outcome measures (e.g., questionnaires, polysomnography, and actigraphy) that balance tolerability and accuracy, they wrote. Clinically important differences for most measures also have yet to be determined. Researchers should investigate whether long-term adverse events are associated with chronic melatonin use and study patients with ASD and comorbid mood disorders, wrote the authors. “Research tying the underlying neurobiology in early-life sleep disruption to behavior might help clinicians and researchers understand which treatments might work for which people with ASD,” they concluded.
The AAN supported the development of the guideline. Dr. Williams Buckley had no conflicts of interest. Six authors had conflicts of interest that the AAN deemed not significant enough to prevent their participation in the development of the guideline.
SOURCE: Williams Buckley A et al. Neurology. 2020;94:393-405. doi: 10.1212/WNL0000000000009033.
The guideline was published online ahead of print Feb. 12 in Neurology.
“While up to 40% of children and teens in the general population will have sleep problems at some point during their childhood, such problems usually lessen with age,” lead author Ashura Williams Buckley, MD, director of the Sleep and Neurodevelopment Service at the National Institute of Mental Health in Bethesda, Md., said in a press release. “For children and teens with autism, sleep problems are more common and more likely to persist, resulting in poor health and poor quality of life. Some sleep problems may be directly related to autism, but others are not. Regardless, autism symptoms may make sleep problems worse.”
Few evidence-based treatments are available
Dr. Williams Buckley and colleagues developed the current guideline to evaluate which pharmacologic, behavioral, and complementary and alternative medicine (CAM) interventions improve bedtime resistance, sleep onset latency, sleep continuity, total sleep time, and daytime behavior in children and adolescents with ASD. The panel evaluated 900 abstracts of articles that had been included in systematic reviews, as well as 1,087 additional abstracts. One hundred thirty-nine articles were potentially relevant, 12 met criteria for data extraction, and eight were rated class III or higher and were included in the panel’s review.
The authors observed what they called a dearth of evidence-based treatments for sleep dysregulation in ASD. Evidence indicates that melatonin, with or without cognitive–behavioral therapy (CBT), improves several sleep outcomes, compared with placebo. “Evidence for other interventions is largely lacking,” wrote Dr. Williams Buckley and colleagues. They observed a lack of long-term safety data for melatonin in children, which they considered concerning, because melatonin affects the hypothalamic–gonadal axis and can potentially influence pubertal development.
Screening for comorbid conditions and concomitant medications
The guideline recommends that clinicians assess children with ASD and sleep disturbances for coexisting conditions and concomitant medications that could be contributing to these sleep disturbances. They should ensure that children receive appropriate treatment for coexisting conditions and adjust or discontinue potentially problematic medications appropriately, according to the guideline.
Furthermore, clinicians should counsel parents or guardians about behavioral strategies as a first-line treatment for improving sleep function. These strategies could be administered alone or with pharmacologic or neutraceutical approaches as needed, according to the authors. Suggested behavioral approaches include unmodified extinction (i.e., imposing a bedtime and ignoring a child’s protests), graduated extinction (i.e., ignoring protests for a specified period before responding), positive routines (i.e., establishing pre-bedtime calming rituals), and bedtime fading (i.e., putting a child to bed close to the time he or she begins to fall asleep).
If a child’s contributing coexisting conditions and medications have been addressed and behavioral strategies have not been helpful, clinicians should offer melatonin, according to the guideline. Because over-the-counter formulations contain variable concentrations of melatonin, clinicians should write a prescription for it or recommend high-purity pharmaceutical grade melatonin. The initial dose should be 1-3 mg/day at 60-30 minutes before bedtime. The dose can be titrated to 10 mg/day. Clinicians also should counsel children and their parents about potential adverse events of melatonin and the lack of long-term safety data, according to the guideline.
In addition, clinicians should advise children and parents that no evidence supports the routine use of weighted blankets or specialized mattress technology for improving sleep. Parents who ask about weighted blankets should be told that the reviewed trial reported no serious adverse events with this intervention, and that blankets could be a reasonable nonpharmacologic approach for some patients, according to the guideline.
Optimal outcome measures are undefined
Dr. Williams Buckley and colleagues also suggested areas for future research. Investigators have not yet defined optimal outcome measures (e.g., questionnaires, polysomnography, and actigraphy) that balance tolerability and accuracy, they wrote. Clinically important differences for most measures also have yet to be determined. Researchers should investigate whether long-term adverse events are associated with chronic melatonin use and study patients with ASD and comorbid mood disorders, wrote the authors. “Research tying the underlying neurobiology in early-life sleep disruption to behavior might help clinicians and researchers understand which treatments might work for which people with ASD,” they concluded.
The AAN supported the development of the guideline. Dr. Williams Buckley had no conflicts of interest. Six authors had conflicts of interest that the AAN deemed not significant enough to prevent their participation in the development of the guideline.
SOURCE: Williams Buckley A et al. Neurology. 2020;94:393-405. doi: 10.1212/WNL0000000000009033.
The guideline was published online ahead of print Feb. 12 in Neurology.
“While up to 40% of children and teens in the general population will have sleep problems at some point during their childhood, such problems usually lessen with age,” lead author Ashura Williams Buckley, MD, director of the Sleep and Neurodevelopment Service at the National Institute of Mental Health in Bethesda, Md., said in a press release. “For children and teens with autism, sleep problems are more common and more likely to persist, resulting in poor health and poor quality of life. Some sleep problems may be directly related to autism, but others are not. Regardless, autism symptoms may make sleep problems worse.”
Few evidence-based treatments are available
Dr. Williams Buckley and colleagues developed the current guideline to evaluate which pharmacologic, behavioral, and complementary and alternative medicine (CAM) interventions improve bedtime resistance, sleep onset latency, sleep continuity, total sleep time, and daytime behavior in children and adolescents with ASD. The panel evaluated 900 abstracts of articles that had been included in systematic reviews, as well as 1,087 additional abstracts. One hundred thirty-nine articles were potentially relevant, 12 met criteria for data extraction, and eight were rated class III or higher and were included in the panel’s review.
The authors observed what they called a dearth of evidence-based treatments for sleep dysregulation in ASD. Evidence indicates that melatonin, with or without cognitive–behavioral therapy (CBT), improves several sleep outcomes, compared with placebo. “Evidence for other interventions is largely lacking,” wrote Dr. Williams Buckley and colleagues. They observed a lack of long-term safety data for melatonin in children, which they considered concerning, because melatonin affects the hypothalamic–gonadal axis and can potentially influence pubertal development.
Screening for comorbid conditions and concomitant medications
The guideline recommends that clinicians assess children with ASD and sleep disturbances for coexisting conditions and concomitant medications that could be contributing to these sleep disturbances. They should ensure that children receive appropriate treatment for coexisting conditions and adjust or discontinue potentially problematic medications appropriately, according to the guideline.
Furthermore, clinicians should counsel parents or guardians about behavioral strategies as a first-line treatment for improving sleep function. These strategies could be administered alone or with pharmacologic or neutraceutical approaches as needed, according to the authors. Suggested behavioral approaches include unmodified extinction (i.e., imposing a bedtime and ignoring a child’s protests), graduated extinction (i.e., ignoring protests for a specified period before responding), positive routines (i.e., establishing pre-bedtime calming rituals), and bedtime fading (i.e., putting a child to bed close to the time he or she begins to fall asleep).
If a child’s contributing coexisting conditions and medications have been addressed and behavioral strategies have not been helpful, clinicians should offer melatonin, according to the guideline. Because over-the-counter formulations contain variable concentrations of melatonin, clinicians should write a prescription for it or recommend high-purity pharmaceutical grade melatonin. The initial dose should be 1-3 mg/day at 60-30 minutes before bedtime. The dose can be titrated to 10 mg/day. Clinicians also should counsel children and their parents about potential adverse events of melatonin and the lack of long-term safety data, according to the guideline.
In addition, clinicians should advise children and parents that no evidence supports the routine use of weighted blankets or specialized mattress technology for improving sleep. Parents who ask about weighted blankets should be told that the reviewed trial reported no serious adverse events with this intervention, and that blankets could be a reasonable nonpharmacologic approach for some patients, according to the guideline.
Optimal outcome measures are undefined
Dr. Williams Buckley and colleagues also suggested areas for future research. Investigators have not yet defined optimal outcome measures (e.g., questionnaires, polysomnography, and actigraphy) that balance tolerability and accuracy, they wrote. Clinically important differences for most measures also have yet to be determined. Researchers should investigate whether long-term adverse events are associated with chronic melatonin use and study patients with ASD and comorbid mood disorders, wrote the authors. “Research tying the underlying neurobiology in early-life sleep disruption to behavior might help clinicians and researchers understand which treatments might work for which people with ASD,” they concluded.
The AAN supported the development of the guideline. Dr. Williams Buckley had no conflicts of interest. Six authors had conflicts of interest that the AAN deemed not significant enough to prevent their participation in the development of the guideline.
SOURCE: Williams Buckley A et al. Neurology. 2020;94:393-405. doi: 10.1212/WNL0000000000009033.
FROM NEUROLOGY
Key clinical point: The AAN has published a guideline on the treatment of sleep problems in children with autism.
Major finding: The guideline recommends behavioral strategies as a first-line treatment.
Study details: A review of 1,987 peer-reviewed studies.
Disclosures: The AAN funded the development of the guideline. The first author had no conflicts of interest, and the other authors had no significant conflicts.
Source: Williams Buckley A et al. Neurology. 2020;94:393-405. doi: 10.1212/WNL0000000000009033.
More conflicting evidence on paclitaxel devices in PAD
The controversy regarding the safety of treating peripheral artery disease (PAD) with paclitaxel-coated devices has only deepened in the new year, with two recent studies suggesting opposite safety findings.
The debate began with a 2018 meta-analysis showing a late mortality signal associated with paclitaxel drug-coated balloons (DCBs) that sent reverberations through the interventional cardiology community (J Am Heart Assoc. 2018 Dec 18;7[24]:e011245).
Now, in a new meta-analysis involving eight randomized controlled trials (RCTs) and more than 1,400 patients with critical limb ischemia (CLI), the same researchers found significantly more early amputations and deaths in those treated with DCB below the knee, compared with conventional balloon angioplasty.
“The findings of our latest report add to previous evidence underpinning major safety concerns around use of paclitaxel in lower limb angioplasties – increased long-term patient mortality in cases of intermittent claudication,” lead author Konstantinos Katsanos MD, MSc, PhD, Patras University Hospital, Greece, said in an interview.
By contrast, a retrospective study of insurance claims in Germany showed no heightened mortality with paclitaxel-coated balloons and stents, compared with uncoated devices, in close to 38,000 patients with PAD.
On the contrary, use of paclitaxel-coated devices was associated with higher long-term survival, better amputation-free survival (AFS), and lower rates of major cardiovascular events in the treatment of chronic limb-threatening ischemia (CLTI).
These findings “emphasize the difference between population-based evidence and randomized trials,” lead author Christian-Alexander Behrendt, MD, University Medical Center Hamburg-Eppendorf, Germany, said in an interview.
Downstream “showers”
In the new meta-analysis led by Dr. Katsanos, published online Jan. 15, the 1,420 patients were treated with five different DCBs and 97% had CLI (J Vasc Intervent Radiol 2020 Feb;31[2]:202-12).
In up to 1-year follow-up, the paclitaxel DCB group had fewer target lesion revascularizations (TLR) than those of the uncoated device group (11.8% vs. 25.6%; risk ratio, 0.53; 95% confidence interval, 0.35-0.81) but worse AFS (13.7% vs. 9.4%; hazard ratio [HR], 1.52; 95% CI, 1.12-2.07).
The latter finding was driven by nonsignificant increased risks for all-cause death (odds ratio [OR], 1.39; 95% CI, 0.94-2.07) and major amputations (OR, 1.63; 95% CI, 0.92-2.90).
In dose-subgroup analyses, AFS was significantly worse in cases with high-dose (3.0-3.5 mcg/mm2) devices, but not in the single trial with a low-dose DCB (2.0 mcg/mm2).
“Considering the well-described downstream ‘showers’ of paclitaxel particles with current drug-coated balloons, we hypothesize that nontarget paclitaxel embolization is a plausible mechanism for distal foot and systemic toxicity,” Dr. Katsanos said.
Short time frame
Eric Secemsky, MD, of Harvard Medical School, and director of vascular intervention at Beth Israel Deaconess Medical Center, Boston, suggested in an interview that this theorized mechanism of harm in below-the-knee procedures could potentially shed light on a similar mechanism at play in above-the-knee procedures.
“We didn’t understand why people could potentially be dying in above-the-knee [procedures], and the suggestion here is that these devices might perhaps be causing particular embolization or maybe delayed wound healing,” Dr. Secemsky speculated.
However, “I don’t know that this is true, so I am cautious to say this is true,” he emphasized.
Dr. Secemsky said a strength of the Katsanos analysis is that the RCTs included more than 1,000 patients, but noted that it is hard to vet the quality and rigor of the data, as some of the studies have not yet been published. He also noted that paclitaxel-coated devices are not approved by the Food and Drug Administration in the United States for below-the-knee procedures.
Moreover, he continued, “two studies were driving the signal of harm: the IN.PACT DEEP, which included an iteration of their DCB that is no longer being tested; and the unpublished SINGA-PACLI trial. Those studies contributed most of the adverse events seen in this meta-analysis.”
In addition, the trials had different lengths of follow-up (6-12 months), he said. “Thus, the five trials with data available to 12 months are driving the 1-year findings, whereas three RCTs, including the primary RCT showing safety [Lutonix-BTK trial], only contribute data to 6 months.”
For this reason, “we are not too excited about this meta-analysis as of now, [because] all it tells us is that we need more data to support the safety of drug-coated devices in this population,” Dr. Secemsky said.
Dr. Katsanos explained that, “to address the differences in follow-up period and number of cases lost to follow-up, the primary endpoint was calculated on the log-hazard scale and expressed as a hazard ratio, as recommended for time-to-event outcomes.”
He highlighted that a short-term time frame of 6 months to 1 year was chosen “because it is clinically relevant to limb-threatening CLI.”
Sensitivity tests also “showed consistent direction and magnitude of the summary treatment effects in case of both AFS and freedom from TLR,” Dr. Katsanos emphasized.
Lower mortality, fewer amputations
The second study, published online Jan. 8, drew on health insurance claims in the German BARMER database to analyze 37,914 patients (mean age, 73.3 years, 49% female) and 21,546 propensity-score-matched patients with symptomatic CLTI or intermittent claudication (IC) with an index revascularization during 2010-2018 (Eur J Vasc Endovasc Surg. 2020 Jan 8. doi: 10.1016/j.ejvs.2019.12.034).
Patients were first stratified by CLTI or IC, and then by balloon vs. stent use. Paclitaxel-coated devices were then compared with uncoated devices within each stratum. The primary outcome was all-cause mortality at the end of follow-up.
From 2010 to 2018, the annual use of paclitaxel-coated devices increased dramatically from 3% to 39% in the CLTI group and from 4% to 48% in the IC group (P less than .001 for both).
A total of 2,454 deaths occurred within 5 years of follow-up (median, 2.7 years; longest, 8 years).
A Cox proportional hazards model (based on propensity-score-matched cohorts at 5 years) showed that, compared with uncoated devices, use of paclitaxel-coated devices in the CLTI group was associated with several improvements:
- Overall survival: HR, 0.83; 95% CI, 0.77-0.90.
- Amputation-free survival: HR, 0.85; 95% CI, 0.78-0.91.
- Major cardiovascular events: HR, 0.82; 95% CI, 0.77-0.88.
In the IC group, mortality was significantly better with DCB (HR, 0.87; 95% CI, 0.76-0.99) or a combination of DCB and drug-eluting stents (HR, 0.88; 95% CI, 0.80-0.98) than with uncoated devices, but similar for DES alone (HR, 0.91; 95% CI, 0.77-1.08).
No benefit was found for paclitaxel-coated devices in the IC group for AFS (HR, 0.91; 95% CI, 0.82-1.00) or major cardiovascular events (HR, 0.93; 95% CI, 0.87-1.00).
The authors acknowledge that “unmeasured confounding” may partly explain the results. It may be that patients revascularized with DCB or DES “are more likely to be treated in highly specialized trial centers with clear follow-up protocol.”
Moreover, these patients may have received “the best treatment,” including statin therapy, added Dr. Behrendt.
More evidence needed
Dr. Secemsky, who was not involved with either study, said the German investigators “did a wonderful job with this analysis in a large population of several thousand patients, showing nicely that after accounting for differences in comorbidities, the patients had no evidence of harm with [paclitaxel-coated] devices through 5 years.”
However, he cautioned, median follow-up time was just over 2 years. “Although the investigators had data all the way out to 5 years, over time, the number of patients contributing data became smaller, which results in more uncertainty with these longer-term findings,” he said. “As such, we still need to look at additional long-term data in this patient population to confirm the safety of these devices.”
At present, the “major consideration we want to address is whether it’s safe to use these devices, and we’re undertaking these analyses to examine safety, not to see if they improve mortality,” although the present study “has a suggestion of mortality benefit,” Dr. Secemsky said.
Dr. Katsanos added that paclitaxel-coated balloons “remain under investigation for below-knee arteries and critical limb ischemia,” with “a few randomized controlled trials on the way.”
“We need definitive evidence from high-quality multicenter controlled trials that these devices may improve wound healing and limb salvage without any systemic mortality risk,” he said.
Dr. Katsanos receives personal fees from Boston Scientific and Philips Healthcare. The study by Dr. Behrendt was part of the IDOMENEO project funded by the German Joint Federal Committee. Dr. Behrendt reports no relevant financial relationships. Dr. Secemsky reports institutional grants from Cook Medical, BD Bard, Medtronic, Beth Israel Deaconess Medical Center, and Boston Scientific, and reports consultancy for Cook Medical, BD Bard, and Medtronic.
This article first appeared on Medscape.com.
The controversy regarding the safety of treating peripheral artery disease (PAD) with paclitaxel-coated devices has only deepened in the new year, with two recent studies suggesting opposite safety findings.
The debate began with a 2018 meta-analysis showing a late mortality signal associated with paclitaxel drug-coated balloons (DCBs) that sent reverberations through the interventional cardiology community (J Am Heart Assoc. 2018 Dec 18;7[24]:e011245).
Now, in a new meta-analysis involving eight randomized controlled trials (RCTs) and more than 1,400 patients with critical limb ischemia (CLI), the same researchers found significantly more early amputations and deaths in those treated with DCB below the knee, compared with conventional balloon angioplasty.
“The findings of our latest report add to previous evidence underpinning major safety concerns around use of paclitaxel in lower limb angioplasties – increased long-term patient mortality in cases of intermittent claudication,” lead author Konstantinos Katsanos MD, MSc, PhD, Patras University Hospital, Greece, said in an interview.
By contrast, a retrospective study of insurance claims in Germany showed no heightened mortality with paclitaxel-coated balloons and stents, compared with uncoated devices, in close to 38,000 patients with PAD.
On the contrary, use of paclitaxel-coated devices was associated with higher long-term survival, better amputation-free survival (AFS), and lower rates of major cardiovascular events in the treatment of chronic limb-threatening ischemia (CLTI).
These findings “emphasize the difference between population-based evidence and randomized trials,” lead author Christian-Alexander Behrendt, MD, University Medical Center Hamburg-Eppendorf, Germany, said in an interview.
Downstream “showers”
In the new meta-analysis led by Dr. Katsanos, published online Jan. 15, the 1,420 patients were treated with five different DCBs and 97% had CLI (J Vasc Intervent Radiol 2020 Feb;31[2]:202-12).
In up to 1-year follow-up, the paclitaxel DCB group had fewer target lesion revascularizations (TLR) than those of the uncoated device group (11.8% vs. 25.6%; risk ratio, 0.53; 95% confidence interval, 0.35-0.81) but worse AFS (13.7% vs. 9.4%; hazard ratio [HR], 1.52; 95% CI, 1.12-2.07).
The latter finding was driven by nonsignificant increased risks for all-cause death (odds ratio [OR], 1.39; 95% CI, 0.94-2.07) and major amputations (OR, 1.63; 95% CI, 0.92-2.90).
In dose-subgroup analyses, AFS was significantly worse in cases with high-dose (3.0-3.5 mcg/mm2) devices, but not in the single trial with a low-dose DCB (2.0 mcg/mm2).
“Considering the well-described downstream ‘showers’ of paclitaxel particles with current drug-coated balloons, we hypothesize that nontarget paclitaxel embolization is a plausible mechanism for distal foot and systemic toxicity,” Dr. Katsanos said.
Short time frame
Eric Secemsky, MD, of Harvard Medical School, and director of vascular intervention at Beth Israel Deaconess Medical Center, Boston, suggested in an interview that this theorized mechanism of harm in below-the-knee procedures could potentially shed light on a similar mechanism at play in above-the-knee procedures.
“We didn’t understand why people could potentially be dying in above-the-knee [procedures], and the suggestion here is that these devices might perhaps be causing particular embolization or maybe delayed wound healing,” Dr. Secemsky speculated.
However, “I don’t know that this is true, so I am cautious to say this is true,” he emphasized.
Dr. Secemsky said a strength of the Katsanos analysis is that the RCTs included more than 1,000 patients, but noted that it is hard to vet the quality and rigor of the data, as some of the studies have not yet been published. He also noted that paclitaxel-coated devices are not approved by the Food and Drug Administration in the United States for below-the-knee procedures.
Moreover, he continued, “two studies were driving the signal of harm: the IN.PACT DEEP, which included an iteration of their DCB that is no longer being tested; and the unpublished SINGA-PACLI trial. Those studies contributed most of the adverse events seen in this meta-analysis.”
In addition, the trials had different lengths of follow-up (6-12 months), he said. “Thus, the five trials with data available to 12 months are driving the 1-year findings, whereas three RCTs, including the primary RCT showing safety [Lutonix-BTK trial], only contribute data to 6 months.”
For this reason, “we are not too excited about this meta-analysis as of now, [because] all it tells us is that we need more data to support the safety of drug-coated devices in this population,” Dr. Secemsky said.
Dr. Katsanos explained that, “to address the differences in follow-up period and number of cases lost to follow-up, the primary endpoint was calculated on the log-hazard scale and expressed as a hazard ratio, as recommended for time-to-event outcomes.”
He highlighted that a short-term time frame of 6 months to 1 year was chosen “because it is clinically relevant to limb-threatening CLI.”
Sensitivity tests also “showed consistent direction and magnitude of the summary treatment effects in case of both AFS and freedom from TLR,” Dr. Katsanos emphasized.
Lower mortality, fewer amputations
The second study, published online Jan. 8, drew on health insurance claims in the German BARMER database to analyze 37,914 patients (mean age, 73.3 years, 49% female) and 21,546 propensity-score-matched patients with symptomatic CLTI or intermittent claudication (IC) with an index revascularization during 2010-2018 (Eur J Vasc Endovasc Surg. 2020 Jan 8. doi: 10.1016/j.ejvs.2019.12.034).
Patients were first stratified by CLTI or IC, and then by balloon vs. stent use. Paclitaxel-coated devices were then compared with uncoated devices within each stratum. The primary outcome was all-cause mortality at the end of follow-up.
From 2010 to 2018, the annual use of paclitaxel-coated devices increased dramatically from 3% to 39% in the CLTI group and from 4% to 48% in the IC group (P less than .001 for both).
A total of 2,454 deaths occurred within 5 years of follow-up (median, 2.7 years; longest, 8 years).
A Cox proportional hazards model (based on propensity-score-matched cohorts at 5 years) showed that, compared with uncoated devices, use of paclitaxel-coated devices in the CLTI group was associated with several improvements:
- Overall survival: HR, 0.83; 95% CI, 0.77-0.90.
- Amputation-free survival: HR, 0.85; 95% CI, 0.78-0.91.
- Major cardiovascular events: HR, 0.82; 95% CI, 0.77-0.88.
In the IC group, mortality was significantly better with DCB (HR, 0.87; 95% CI, 0.76-0.99) or a combination of DCB and drug-eluting stents (HR, 0.88; 95% CI, 0.80-0.98) than with uncoated devices, but similar for DES alone (HR, 0.91; 95% CI, 0.77-1.08).
No benefit was found for paclitaxel-coated devices in the IC group for AFS (HR, 0.91; 95% CI, 0.82-1.00) or major cardiovascular events (HR, 0.93; 95% CI, 0.87-1.00).
The authors acknowledge that “unmeasured confounding” may partly explain the results. It may be that patients revascularized with DCB or DES “are more likely to be treated in highly specialized trial centers with clear follow-up protocol.”
Moreover, these patients may have received “the best treatment,” including statin therapy, added Dr. Behrendt.
More evidence needed
Dr. Secemsky, who was not involved with either study, said the German investigators “did a wonderful job with this analysis in a large population of several thousand patients, showing nicely that after accounting for differences in comorbidities, the patients had no evidence of harm with [paclitaxel-coated] devices through 5 years.”
However, he cautioned, median follow-up time was just over 2 years. “Although the investigators had data all the way out to 5 years, over time, the number of patients contributing data became smaller, which results in more uncertainty with these longer-term findings,” he said. “As such, we still need to look at additional long-term data in this patient population to confirm the safety of these devices.”
At present, the “major consideration we want to address is whether it’s safe to use these devices, and we’re undertaking these analyses to examine safety, not to see if they improve mortality,” although the present study “has a suggestion of mortality benefit,” Dr. Secemsky said.
Dr. Katsanos added that paclitaxel-coated balloons “remain under investigation for below-knee arteries and critical limb ischemia,” with “a few randomized controlled trials on the way.”
“We need definitive evidence from high-quality multicenter controlled trials that these devices may improve wound healing and limb salvage without any systemic mortality risk,” he said.
Dr. Katsanos receives personal fees from Boston Scientific and Philips Healthcare. The study by Dr. Behrendt was part of the IDOMENEO project funded by the German Joint Federal Committee. Dr. Behrendt reports no relevant financial relationships. Dr. Secemsky reports institutional grants from Cook Medical, BD Bard, Medtronic, Beth Israel Deaconess Medical Center, and Boston Scientific, and reports consultancy for Cook Medical, BD Bard, and Medtronic.
This article first appeared on Medscape.com.
The controversy regarding the safety of treating peripheral artery disease (PAD) with paclitaxel-coated devices has only deepened in the new year, with two recent studies suggesting opposite safety findings.
The debate began with a 2018 meta-analysis showing a late mortality signal associated with paclitaxel drug-coated balloons (DCBs) that sent reverberations through the interventional cardiology community (J Am Heart Assoc. 2018 Dec 18;7[24]:e011245).
Now, in a new meta-analysis involving eight randomized controlled trials (RCTs) and more than 1,400 patients with critical limb ischemia (CLI), the same researchers found significantly more early amputations and deaths in those treated with DCB below the knee, compared with conventional balloon angioplasty.
“The findings of our latest report add to previous evidence underpinning major safety concerns around use of paclitaxel in lower limb angioplasties – increased long-term patient mortality in cases of intermittent claudication,” lead author Konstantinos Katsanos MD, MSc, PhD, Patras University Hospital, Greece, said in an interview.
By contrast, a retrospective study of insurance claims in Germany showed no heightened mortality with paclitaxel-coated balloons and stents, compared with uncoated devices, in close to 38,000 patients with PAD.
On the contrary, use of paclitaxel-coated devices was associated with higher long-term survival, better amputation-free survival (AFS), and lower rates of major cardiovascular events in the treatment of chronic limb-threatening ischemia (CLTI).
These findings “emphasize the difference between population-based evidence and randomized trials,” lead author Christian-Alexander Behrendt, MD, University Medical Center Hamburg-Eppendorf, Germany, said in an interview.
Downstream “showers”
In the new meta-analysis led by Dr. Katsanos, published online Jan. 15, the 1,420 patients were treated with five different DCBs and 97% had CLI (J Vasc Intervent Radiol 2020 Feb;31[2]:202-12).
In up to 1-year follow-up, the paclitaxel DCB group had fewer target lesion revascularizations (TLR) than those of the uncoated device group (11.8% vs. 25.6%; risk ratio, 0.53; 95% confidence interval, 0.35-0.81) but worse AFS (13.7% vs. 9.4%; hazard ratio [HR], 1.52; 95% CI, 1.12-2.07).
The latter finding was driven by nonsignificant increased risks for all-cause death (odds ratio [OR], 1.39; 95% CI, 0.94-2.07) and major amputations (OR, 1.63; 95% CI, 0.92-2.90).
In dose-subgroup analyses, AFS was significantly worse in cases with high-dose (3.0-3.5 mcg/mm2) devices, but not in the single trial with a low-dose DCB (2.0 mcg/mm2).
“Considering the well-described downstream ‘showers’ of paclitaxel particles with current drug-coated balloons, we hypothesize that nontarget paclitaxel embolization is a plausible mechanism for distal foot and systemic toxicity,” Dr. Katsanos said.
Short time frame
Eric Secemsky, MD, of Harvard Medical School, and director of vascular intervention at Beth Israel Deaconess Medical Center, Boston, suggested in an interview that this theorized mechanism of harm in below-the-knee procedures could potentially shed light on a similar mechanism at play in above-the-knee procedures.
“We didn’t understand why people could potentially be dying in above-the-knee [procedures], and the suggestion here is that these devices might perhaps be causing particular embolization or maybe delayed wound healing,” Dr. Secemsky speculated.
However, “I don’t know that this is true, so I am cautious to say this is true,” he emphasized.
Dr. Secemsky said a strength of the Katsanos analysis is that the RCTs included more than 1,000 patients, but noted that it is hard to vet the quality and rigor of the data, as some of the studies have not yet been published. He also noted that paclitaxel-coated devices are not approved by the Food and Drug Administration in the United States for below-the-knee procedures.
Moreover, he continued, “two studies were driving the signal of harm: the IN.PACT DEEP, which included an iteration of their DCB that is no longer being tested; and the unpublished SINGA-PACLI trial. Those studies contributed most of the adverse events seen in this meta-analysis.”
In addition, the trials had different lengths of follow-up (6-12 months), he said. “Thus, the five trials with data available to 12 months are driving the 1-year findings, whereas three RCTs, including the primary RCT showing safety [Lutonix-BTK trial], only contribute data to 6 months.”
For this reason, “we are not too excited about this meta-analysis as of now, [because] all it tells us is that we need more data to support the safety of drug-coated devices in this population,” Dr. Secemsky said.
Dr. Katsanos explained that, “to address the differences in follow-up period and number of cases lost to follow-up, the primary endpoint was calculated on the log-hazard scale and expressed as a hazard ratio, as recommended for time-to-event outcomes.”
He highlighted that a short-term time frame of 6 months to 1 year was chosen “because it is clinically relevant to limb-threatening CLI.”
Sensitivity tests also “showed consistent direction and magnitude of the summary treatment effects in case of both AFS and freedom from TLR,” Dr. Katsanos emphasized.
Lower mortality, fewer amputations
The second study, published online Jan. 8, drew on health insurance claims in the German BARMER database to analyze 37,914 patients (mean age, 73.3 years, 49% female) and 21,546 propensity-score-matched patients with symptomatic CLTI or intermittent claudication (IC) with an index revascularization during 2010-2018 (Eur J Vasc Endovasc Surg. 2020 Jan 8. doi: 10.1016/j.ejvs.2019.12.034).
Patients were first stratified by CLTI or IC, and then by balloon vs. stent use. Paclitaxel-coated devices were then compared with uncoated devices within each stratum. The primary outcome was all-cause mortality at the end of follow-up.
From 2010 to 2018, the annual use of paclitaxel-coated devices increased dramatically from 3% to 39% in the CLTI group and from 4% to 48% in the IC group (P less than .001 for both).
A total of 2,454 deaths occurred within 5 years of follow-up (median, 2.7 years; longest, 8 years).
A Cox proportional hazards model (based on propensity-score-matched cohorts at 5 years) showed that, compared with uncoated devices, use of paclitaxel-coated devices in the CLTI group was associated with several improvements:
- Overall survival: HR, 0.83; 95% CI, 0.77-0.90.
- Amputation-free survival: HR, 0.85; 95% CI, 0.78-0.91.
- Major cardiovascular events: HR, 0.82; 95% CI, 0.77-0.88.
In the IC group, mortality was significantly better with DCB (HR, 0.87; 95% CI, 0.76-0.99) or a combination of DCB and drug-eluting stents (HR, 0.88; 95% CI, 0.80-0.98) than with uncoated devices, but similar for DES alone (HR, 0.91; 95% CI, 0.77-1.08).
No benefit was found for paclitaxel-coated devices in the IC group for AFS (HR, 0.91; 95% CI, 0.82-1.00) or major cardiovascular events (HR, 0.93; 95% CI, 0.87-1.00).
The authors acknowledge that “unmeasured confounding” may partly explain the results. It may be that patients revascularized with DCB or DES “are more likely to be treated in highly specialized trial centers with clear follow-up protocol.”
Moreover, these patients may have received “the best treatment,” including statin therapy, added Dr. Behrendt.
More evidence needed
Dr. Secemsky, who was not involved with either study, said the German investigators “did a wonderful job with this analysis in a large population of several thousand patients, showing nicely that after accounting for differences in comorbidities, the patients had no evidence of harm with [paclitaxel-coated] devices through 5 years.”
However, he cautioned, median follow-up time was just over 2 years. “Although the investigators had data all the way out to 5 years, over time, the number of patients contributing data became smaller, which results in more uncertainty with these longer-term findings,” he said. “As such, we still need to look at additional long-term data in this patient population to confirm the safety of these devices.”
At present, the “major consideration we want to address is whether it’s safe to use these devices, and we’re undertaking these analyses to examine safety, not to see if they improve mortality,” although the present study “has a suggestion of mortality benefit,” Dr. Secemsky said.
Dr. Katsanos added that paclitaxel-coated balloons “remain under investigation for below-knee arteries and critical limb ischemia,” with “a few randomized controlled trials on the way.”
“We need definitive evidence from high-quality multicenter controlled trials that these devices may improve wound healing and limb salvage without any systemic mortality risk,” he said.
Dr. Katsanos receives personal fees from Boston Scientific and Philips Healthcare. The study by Dr. Behrendt was part of the IDOMENEO project funded by the German Joint Federal Committee. Dr. Behrendt reports no relevant financial relationships. Dr. Secemsky reports institutional grants from Cook Medical, BD Bard, Medtronic, Beth Israel Deaconess Medical Center, and Boston Scientific, and reports consultancy for Cook Medical, BD Bard, and Medtronic.
This article first appeared on Medscape.com.
Trump seeks to cut NIH, CDC budgets, some Medicare spending
The Trump administration on Feb. 10 argued for cutting spending for a federal agency at the forefront of the efforts to combat the coronavirus, while also seeking to slow spending in certain parts of the Medicare and Medicaid programs.
President Donald Trump presented his fiscal 2021 request to Congress for refilling the coffers of federal agencies. In any administration, an annual budget serves only as a political blueprint, as the White House document itself makes no changes in federal spending.
In Mr. Trump’s case, several of his requests for agencies within the Department of Health & Human Services run counter to recent budget trends. Republicans and Democrats in Congress have worked together in recent years to increase budgets for major federal health agencies.
But Mr. Trump asked Congress to cut annual budget authority for the National Institute of Allergy and Infectious Diseases by $430 million to $5.446 billion for fiscal 2021. In contrast, Congress has raised the annual budget for NIAID, a key agency in combating the coronavirus, from $5.545 billion in fiscal 2019 to $5.876 billion in fiscal 2020, which began in October, according to an HHS summary of Mr. Trump’s request.
For the Centers for Disease Control and Prevention, which is central to the battle against the coronavirus, Mr. Trump proposed a drop in discretionary funding to $5.627 billion. In contrast, Congress raised the CDC budget from $6.544 billion in fiscal 2019 to $6.917 in fiscal 2020.
Mr. Trump also wants to cut $559 million from the budget of the National Cancer Institute, dropping it to $5.881 billion in fiscal 2021. In contrast, Congress raised NCI’s budget from $6.121 billion in fiscal 2019 to $6.440 billion in fiscal 2020.
Mr. Trump requested a $2.6 billion reduction in the National Institutes of Health’s total discretionary budget, seeking to drop it to $37.70 billion. In contrast, Congress raised NIH’s budget from $37.887 in fiscal 2019 to $40.304 billion in fiscal 2020.
Mr. Trump’s budget proposal also includes an estimate of $152 billion in savings over a decade for Medicaid through the implementation of what the administration calls “community engagement” requirements.
The Trump administration has been at odds with Democrats for years about whether work requirements should be attached to Medicaid. “Well-designed community engagement incentives have great potential to improve health and well-being while empowering beneficiaries to rise out of poverty,” HHS said in a budget document.
Yet researchers last year reported that Arkansas’ attempt to attach work requirements to Medicaid caused almost 17,000 adults to lose this health care coverage within the first 6 months, and there was no significant difference in employment.
The researchers say this loss of coverage was partly a result of bureaucratic obstacles and confusion about the new rules. In June 2018, Arkansas became the first state to implement work requirements for Medicaid, Benjamin D. Sommers, MD, PhD, of the Harvard T.H. Chan School of Public Health, Boston, and colleagues wrote in the New England Journal of Medicine (2019 Sep 12;381[11]:1073-82).
Budget ‘would thwart’ progress
A few medical groups on Monday quickly criticized Mr. Trump’s proposals.
“In a time where our nation continues to face significant public health challenges — including 2019 novel coronavirus, climate change, gun violence, and costly chronic diseases such as heart disease and cancer – the administration should be investing more resources in better health, not cutting federal health budgets,” said Georges C. Benjamin, MD, executive director of the American Public Health Association, in a statement.
David J. Skorton, MD, chief executive and president of the Association of American Medical Colleges (AAMC) also urged increased investment in fighting disease.
“We must continue the bipartisan budget trajectory set forth by Congress over the last several years, not reverse course,” Dr. Skorton said in a statement.
Mr. Trump’s proposed cuts in medical research “would thwart scientific progress on strategies to prevent, diagnose, treat, and cure medical conditions that affect countless patients nationwide,” he said.
In total, the new 2021 appropriations for HHS would fall by $9.46 billion to $85.667 billion under Mr. Trump’s proposal. Appropriations, also called discretionary budget authority, represents the operating budgets for federal agencies. These are decided through annual spending bills.
Congress has separate sets of laws for handling payments the federal government makes through Medicare and Medicaid. These are known as mandatory spending.
‘Untenable cuts’
AAMC’s Dr. Skorton also objected to what he termed Mr. Trump’s bid “to reduce and consolidate Medicare, Medicaid, and children’s hospital graduate medical education into a single grant program.”
This would force teaching hospitals to absorb $52 billion in “untenable cuts,” he said.
“The proposal ignores the intent of the Medicare GME program, which is to ensure an adequate physician workforce to care for Medicare beneficiaries and support the critical patient care missions of America’s teaching hospitals,” Dr. Skorton said.
The budget also seeks cuts to Medicaid, which come in addition to the administration’s “recent proposals to scale back Medicaid coverage,” Dr. Skorton said.
“More than 26% of all Medicaid hospitalizations occur at AAMC-member teaching hospitals, even though these institutions represent only 5% of all hospitals,” Dr. Skorton said. “Each of the administration’s proposals on their own would be devastating for patients – and combined, they would be disastrous.”
Rick Pollack, the chief executive and president of the American Hospital Association, described Mr. Trump’s fiscal 2021 proposal as another bid to undermine medical care in the United States.
“Every year, we adapt to a constantly changing environment, but every year, the administration aims to gut our nation’s health care infrastructure,” Mr. Pollack said in a statement.
In it, he noted that about one in five people in America depend on Medicaid, with children accounting for a large proportion of those covered by the state-federal program.
“The budget’s proposal on Medicaid financing and service delivery would cut hundreds of billions of dollars from the Medicaid program annually,” Mr. Pollack said.
He also objected to “hundreds of billions of proposed reductions to Medicare” endorsed by Mr. Trump.
Medical malpractice overhaul
The Trump administration also offered many suggestions for changing federal laws to reduce health care spending. Among these was a proposed overhaul of the approach to medical malpractice cases.
The president’s budget proposal estimates $40 billion in savings over a decade from steps to limit medical liability, according to a report from the Office of Management and Budget (OMB).
“The current medical liability system does not work for patients or providers, nor does it promote high-quality, evidence-based care,” OMB said. “Providers practice with a threat of potentially frivolous lawsuits, and injured patients often do not receive just compensation for their injuries.”
Mr. Trump’s fiscal 2021 budget calls for a cap on noneconomic damage awards of $250,000, which would increase with inflation over time, and a 3-year statute of limitations. Under this plan, courts could also modify attorney’s fee arrangements. HHS could provide guidance to states on how to create expert panels and administrative health care tribunals to review medical liability.
These steps would lead to lower health care spending, with clinicians dropping “defensive medicine practices,” OMB said. That would benefit the Medicare and Medicaid programs as well as lowering costs of health insurance in general.
Mr. Trump’s fiscal 2021 budget also includes a series of proposals for Medicare that it estimates would, in aggregate, save $755.5 billion over a decade.
Site-neutral policy
A large chunk of the estimated Medicare savings in Mr. Trump’s fiscal 2021 health budget would come from lowering payments to hospitals for services provided in their outpatient and physician offices.
In the fiscal 2021 proposal, HHS noted that “Medicare generally pays on-campus hospital outpatient departments substantially more than physician offices for the same services.”
Mr. Trump’s budget proposal seeks a more expansive shift to what’s called a “site-neutral” payment for services delivered in hospital outpatient programs or physician offices. This would bring these payments more in line with those made to independent physician practices.
“This proposal would eliminate the often significant disparity between what Medicare pays in these different settings for the same services,” HHS said in the budget summary.
HHS estimated this change in policy would generate $117.2 billion in savings over a decade. Combined with saving from medical malpractice reforms, the Trump administration estimates these two moves combined could save about $164 billion over a decade.
The site-neutral policy has been a legal battleground, with hospital and physician groups winning a round last year.
Another Medicare proposal included in Mr. Trump’s fiscal 2021 budget homes in on this issue for cases where a hospital owns a physician office. Medicare now pays most off-campus hospital outpatient departments higher rates than the program’s physician fee schedule dictates for the same services.
Switching to a site-neutral policy for these hospital-owned physician offices would result in $47.2 billion in savings over a decade, HHS said in the budget document.
This article first appeared on Medscape.com.
The Trump administration on Feb. 10 argued for cutting spending for a federal agency at the forefront of the efforts to combat the coronavirus, while also seeking to slow spending in certain parts of the Medicare and Medicaid programs.
President Donald Trump presented his fiscal 2021 request to Congress for refilling the coffers of federal agencies. In any administration, an annual budget serves only as a political blueprint, as the White House document itself makes no changes in federal spending.
In Mr. Trump’s case, several of his requests for agencies within the Department of Health & Human Services run counter to recent budget trends. Republicans and Democrats in Congress have worked together in recent years to increase budgets for major federal health agencies.
But Mr. Trump asked Congress to cut annual budget authority for the National Institute of Allergy and Infectious Diseases by $430 million to $5.446 billion for fiscal 2021. In contrast, Congress has raised the annual budget for NIAID, a key agency in combating the coronavirus, from $5.545 billion in fiscal 2019 to $5.876 billion in fiscal 2020, which began in October, according to an HHS summary of Mr. Trump’s request.
For the Centers for Disease Control and Prevention, which is central to the battle against the coronavirus, Mr. Trump proposed a drop in discretionary funding to $5.627 billion. In contrast, Congress raised the CDC budget from $6.544 billion in fiscal 2019 to $6.917 in fiscal 2020.
Mr. Trump also wants to cut $559 million from the budget of the National Cancer Institute, dropping it to $5.881 billion in fiscal 2021. In contrast, Congress raised NCI’s budget from $6.121 billion in fiscal 2019 to $6.440 billion in fiscal 2020.
Mr. Trump requested a $2.6 billion reduction in the National Institutes of Health’s total discretionary budget, seeking to drop it to $37.70 billion. In contrast, Congress raised NIH’s budget from $37.887 in fiscal 2019 to $40.304 billion in fiscal 2020.
Mr. Trump’s budget proposal also includes an estimate of $152 billion in savings over a decade for Medicaid through the implementation of what the administration calls “community engagement” requirements.
The Trump administration has been at odds with Democrats for years about whether work requirements should be attached to Medicaid. “Well-designed community engagement incentives have great potential to improve health and well-being while empowering beneficiaries to rise out of poverty,” HHS said in a budget document.
Yet researchers last year reported that Arkansas’ attempt to attach work requirements to Medicaid caused almost 17,000 adults to lose this health care coverage within the first 6 months, and there was no significant difference in employment.
The researchers say this loss of coverage was partly a result of bureaucratic obstacles and confusion about the new rules. In June 2018, Arkansas became the first state to implement work requirements for Medicaid, Benjamin D. Sommers, MD, PhD, of the Harvard T.H. Chan School of Public Health, Boston, and colleagues wrote in the New England Journal of Medicine (2019 Sep 12;381[11]:1073-82).
Budget ‘would thwart’ progress
A few medical groups on Monday quickly criticized Mr. Trump’s proposals.
“In a time where our nation continues to face significant public health challenges — including 2019 novel coronavirus, climate change, gun violence, and costly chronic diseases such as heart disease and cancer – the administration should be investing more resources in better health, not cutting federal health budgets,” said Georges C. Benjamin, MD, executive director of the American Public Health Association, in a statement.
David J. Skorton, MD, chief executive and president of the Association of American Medical Colleges (AAMC) also urged increased investment in fighting disease.
“We must continue the bipartisan budget trajectory set forth by Congress over the last several years, not reverse course,” Dr. Skorton said in a statement.
Mr. Trump’s proposed cuts in medical research “would thwart scientific progress on strategies to prevent, diagnose, treat, and cure medical conditions that affect countless patients nationwide,” he said.
In total, the new 2021 appropriations for HHS would fall by $9.46 billion to $85.667 billion under Mr. Trump’s proposal. Appropriations, also called discretionary budget authority, represents the operating budgets for federal agencies. These are decided through annual spending bills.
Congress has separate sets of laws for handling payments the federal government makes through Medicare and Medicaid. These are known as mandatory spending.
‘Untenable cuts’
AAMC’s Dr. Skorton also objected to what he termed Mr. Trump’s bid “to reduce and consolidate Medicare, Medicaid, and children’s hospital graduate medical education into a single grant program.”
This would force teaching hospitals to absorb $52 billion in “untenable cuts,” he said.
“The proposal ignores the intent of the Medicare GME program, which is to ensure an adequate physician workforce to care for Medicare beneficiaries and support the critical patient care missions of America’s teaching hospitals,” Dr. Skorton said.
The budget also seeks cuts to Medicaid, which come in addition to the administration’s “recent proposals to scale back Medicaid coverage,” Dr. Skorton said.
“More than 26% of all Medicaid hospitalizations occur at AAMC-member teaching hospitals, even though these institutions represent only 5% of all hospitals,” Dr. Skorton said. “Each of the administration’s proposals on their own would be devastating for patients – and combined, they would be disastrous.”
Rick Pollack, the chief executive and president of the American Hospital Association, described Mr. Trump’s fiscal 2021 proposal as another bid to undermine medical care in the United States.
“Every year, we adapt to a constantly changing environment, but every year, the administration aims to gut our nation’s health care infrastructure,” Mr. Pollack said in a statement.
In it, he noted that about one in five people in America depend on Medicaid, with children accounting for a large proportion of those covered by the state-federal program.
“The budget’s proposal on Medicaid financing and service delivery would cut hundreds of billions of dollars from the Medicaid program annually,” Mr. Pollack said.
He also objected to “hundreds of billions of proposed reductions to Medicare” endorsed by Mr. Trump.
Medical malpractice overhaul
The Trump administration also offered many suggestions for changing federal laws to reduce health care spending. Among these was a proposed overhaul of the approach to medical malpractice cases.
The president’s budget proposal estimates $40 billion in savings over a decade from steps to limit medical liability, according to a report from the Office of Management and Budget (OMB).
“The current medical liability system does not work for patients or providers, nor does it promote high-quality, evidence-based care,” OMB said. “Providers practice with a threat of potentially frivolous lawsuits, and injured patients often do not receive just compensation for their injuries.”
Mr. Trump’s fiscal 2021 budget calls for a cap on noneconomic damage awards of $250,000, which would increase with inflation over time, and a 3-year statute of limitations. Under this plan, courts could also modify attorney’s fee arrangements. HHS could provide guidance to states on how to create expert panels and administrative health care tribunals to review medical liability.
These steps would lead to lower health care spending, with clinicians dropping “defensive medicine practices,” OMB said. That would benefit the Medicare and Medicaid programs as well as lowering costs of health insurance in general.
Mr. Trump’s fiscal 2021 budget also includes a series of proposals for Medicare that it estimates would, in aggregate, save $755.5 billion over a decade.
Site-neutral policy
A large chunk of the estimated Medicare savings in Mr. Trump’s fiscal 2021 health budget would come from lowering payments to hospitals for services provided in their outpatient and physician offices.
In the fiscal 2021 proposal, HHS noted that “Medicare generally pays on-campus hospital outpatient departments substantially more than physician offices for the same services.”
Mr. Trump’s budget proposal seeks a more expansive shift to what’s called a “site-neutral” payment for services delivered in hospital outpatient programs or physician offices. This would bring these payments more in line with those made to independent physician practices.
“This proposal would eliminate the often significant disparity between what Medicare pays in these different settings for the same services,” HHS said in the budget summary.
HHS estimated this change in policy would generate $117.2 billion in savings over a decade. Combined with saving from medical malpractice reforms, the Trump administration estimates these two moves combined could save about $164 billion over a decade.
The site-neutral policy has been a legal battleground, with hospital and physician groups winning a round last year.
Another Medicare proposal included in Mr. Trump’s fiscal 2021 budget homes in on this issue for cases where a hospital owns a physician office. Medicare now pays most off-campus hospital outpatient departments higher rates than the program’s physician fee schedule dictates for the same services.
Switching to a site-neutral policy for these hospital-owned physician offices would result in $47.2 billion in savings over a decade, HHS said in the budget document.
This article first appeared on Medscape.com.
The Trump administration on Feb. 10 argued for cutting spending for a federal agency at the forefront of the efforts to combat the coronavirus, while also seeking to slow spending in certain parts of the Medicare and Medicaid programs.
President Donald Trump presented his fiscal 2021 request to Congress for refilling the coffers of federal agencies. In any administration, an annual budget serves only as a political blueprint, as the White House document itself makes no changes in federal spending.
In Mr. Trump’s case, several of his requests for agencies within the Department of Health & Human Services run counter to recent budget trends. Republicans and Democrats in Congress have worked together in recent years to increase budgets for major federal health agencies.
But Mr. Trump asked Congress to cut annual budget authority for the National Institute of Allergy and Infectious Diseases by $430 million to $5.446 billion for fiscal 2021. In contrast, Congress has raised the annual budget for NIAID, a key agency in combating the coronavirus, from $5.545 billion in fiscal 2019 to $5.876 billion in fiscal 2020, which began in October, according to an HHS summary of Mr. Trump’s request.
For the Centers for Disease Control and Prevention, which is central to the battle against the coronavirus, Mr. Trump proposed a drop in discretionary funding to $5.627 billion. In contrast, Congress raised the CDC budget from $6.544 billion in fiscal 2019 to $6.917 in fiscal 2020.
Mr. Trump also wants to cut $559 million from the budget of the National Cancer Institute, dropping it to $5.881 billion in fiscal 2021. In contrast, Congress raised NCI’s budget from $6.121 billion in fiscal 2019 to $6.440 billion in fiscal 2020.
Mr. Trump requested a $2.6 billion reduction in the National Institutes of Health’s total discretionary budget, seeking to drop it to $37.70 billion. In contrast, Congress raised NIH’s budget from $37.887 in fiscal 2019 to $40.304 billion in fiscal 2020.
Mr. Trump’s budget proposal also includes an estimate of $152 billion in savings over a decade for Medicaid through the implementation of what the administration calls “community engagement” requirements.
The Trump administration has been at odds with Democrats for years about whether work requirements should be attached to Medicaid. “Well-designed community engagement incentives have great potential to improve health and well-being while empowering beneficiaries to rise out of poverty,” HHS said in a budget document.
Yet researchers last year reported that Arkansas’ attempt to attach work requirements to Medicaid caused almost 17,000 adults to lose this health care coverage within the first 6 months, and there was no significant difference in employment.
The researchers say this loss of coverage was partly a result of bureaucratic obstacles and confusion about the new rules. In June 2018, Arkansas became the first state to implement work requirements for Medicaid, Benjamin D. Sommers, MD, PhD, of the Harvard T.H. Chan School of Public Health, Boston, and colleagues wrote in the New England Journal of Medicine (2019 Sep 12;381[11]:1073-82).
Budget ‘would thwart’ progress
A few medical groups on Monday quickly criticized Mr. Trump’s proposals.
“In a time where our nation continues to face significant public health challenges — including 2019 novel coronavirus, climate change, gun violence, and costly chronic diseases such as heart disease and cancer – the administration should be investing more resources in better health, not cutting federal health budgets,” said Georges C. Benjamin, MD, executive director of the American Public Health Association, in a statement.
David J. Skorton, MD, chief executive and president of the Association of American Medical Colleges (AAMC) also urged increased investment in fighting disease.
“We must continue the bipartisan budget trajectory set forth by Congress over the last several years, not reverse course,” Dr. Skorton said in a statement.
Mr. Trump’s proposed cuts in medical research “would thwart scientific progress on strategies to prevent, diagnose, treat, and cure medical conditions that affect countless patients nationwide,” he said.
In total, the new 2021 appropriations for HHS would fall by $9.46 billion to $85.667 billion under Mr. Trump’s proposal. Appropriations, also called discretionary budget authority, represents the operating budgets for federal agencies. These are decided through annual spending bills.
Congress has separate sets of laws for handling payments the federal government makes through Medicare and Medicaid. These are known as mandatory spending.
‘Untenable cuts’
AAMC’s Dr. Skorton also objected to what he termed Mr. Trump’s bid “to reduce and consolidate Medicare, Medicaid, and children’s hospital graduate medical education into a single grant program.”
This would force teaching hospitals to absorb $52 billion in “untenable cuts,” he said.
“The proposal ignores the intent of the Medicare GME program, which is to ensure an adequate physician workforce to care for Medicare beneficiaries and support the critical patient care missions of America’s teaching hospitals,” Dr. Skorton said.
The budget also seeks cuts to Medicaid, which come in addition to the administration’s “recent proposals to scale back Medicaid coverage,” Dr. Skorton said.
“More than 26% of all Medicaid hospitalizations occur at AAMC-member teaching hospitals, even though these institutions represent only 5% of all hospitals,” Dr. Skorton said. “Each of the administration’s proposals on their own would be devastating for patients – and combined, they would be disastrous.”
Rick Pollack, the chief executive and president of the American Hospital Association, described Mr. Trump’s fiscal 2021 proposal as another bid to undermine medical care in the United States.
“Every year, we adapt to a constantly changing environment, but every year, the administration aims to gut our nation’s health care infrastructure,” Mr. Pollack said in a statement.
In it, he noted that about one in five people in America depend on Medicaid, with children accounting for a large proportion of those covered by the state-federal program.
“The budget’s proposal on Medicaid financing and service delivery would cut hundreds of billions of dollars from the Medicaid program annually,” Mr. Pollack said.
He also objected to “hundreds of billions of proposed reductions to Medicare” endorsed by Mr. Trump.
Medical malpractice overhaul
The Trump administration also offered many suggestions for changing federal laws to reduce health care spending. Among these was a proposed overhaul of the approach to medical malpractice cases.
The president’s budget proposal estimates $40 billion in savings over a decade from steps to limit medical liability, according to a report from the Office of Management and Budget (OMB).
“The current medical liability system does not work for patients or providers, nor does it promote high-quality, evidence-based care,” OMB said. “Providers practice with a threat of potentially frivolous lawsuits, and injured patients often do not receive just compensation for their injuries.”
Mr. Trump’s fiscal 2021 budget calls for a cap on noneconomic damage awards of $250,000, which would increase with inflation over time, and a 3-year statute of limitations. Under this plan, courts could also modify attorney’s fee arrangements. HHS could provide guidance to states on how to create expert panels and administrative health care tribunals to review medical liability.
These steps would lead to lower health care spending, with clinicians dropping “defensive medicine practices,” OMB said. That would benefit the Medicare and Medicaid programs as well as lowering costs of health insurance in general.
Mr. Trump’s fiscal 2021 budget also includes a series of proposals for Medicare that it estimates would, in aggregate, save $755.5 billion over a decade.
Site-neutral policy
A large chunk of the estimated Medicare savings in Mr. Trump’s fiscal 2021 health budget would come from lowering payments to hospitals for services provided in their outpatient and physician offices.
In the fiscal 2021 proposal, HHS noted that “Medicare generally pays on-campus hospital outpatient departments substantially more than physician offices for the same services.”
Mr. Trump’s budget proposal seeks a more expansive shift to what’s called a “site-neutral” payment for services delivered in hospital outpatient programs or physician offices. This would bring these payments more in line with those made to independent physician practices.
“This proposal would eliminate the often significant disparity between what Medicare pays in these different settings for the same services,” HHS said in the budget summary.
HHS estimated this change in policy would generate $117.2 billion in savings over a decade. Combined with saving from medical malpractice reforms, the Trump administration estimates these two moves combined could save about $164 billion over a decade.
The site-neutral policy has been a legal battleground, with hospital and physician groups winning a round last year.
Another Medicare proposal included in Mr. Trump’s fiscal 2021 budget homes in on this issue for cases where a hospital owns a physician office. Medicare now pays most off-campus hospital outpatient departments higher rates than the program’s physician fee schedule dictates for the same services.
Switching to a site-neutral policy for these hospital-owned physician offices would result in $47.2 billion in savings over a decade, HHS said in the budget document.
This article first appeared on Medscape.com.
The dementia height advantage, and ‘human textile’
Soylent stitches are people!
Ask anyone in advertising and they’ll tell you that branding is everything. Now, there may be a bit of self-promotion involved there. But you can’t deny that naming your product appropriately is important, and we’re going to say with some degree of confidence that “human textile” is not the greatest name in the world.
Now, we don’t want to question the team of French researchers too much. After all, according to their research published in Acta Biomaterialia, they’ve come up with quite the nifty and potentially lifesaving innovation: stitches made from human skin.
By taking sheets of human skin cells (eww) and cutting them into strips, the researchers were able to weave the strips into a sort of yarn, the advantages of which should be obvious. Patients can say goodbye to pesky issues of compatibility and adverse immune response when doctors and surgeons can stitch wounds, sew pouches, and create tubes and valves with yarn crafted from themselves.
We just can’t get past the name they chose. Human textile. The process of making them is gruesome enough already. No need to call to mind some horrific dystopian future in which cotton can no longer grow and we have to recycle humans (alive or dead, depending on how grim you’re feeling) in big industrial textile mills to craft clothing for ourselves.
It’s just too bad Charlton Heston is dead; he’d have made a great spokesperson.
Towering over dementia
Let us take a moment to pity the plight of the shorter brother. Always losing the battle of the boards in fraternal driveway basketball games. Never reaching the Pop-Tarts cruelly stashed high in the pantry by one’s taller, greedier sibling. Always being addressed during family dinners as “Frodo.”
And new research findings add to the burden borne by altitude-impaired brothers everywhere: Being the short one may boost your risk of dementia.
Danish researchers at the University of Copenhagen examined the potential role that height in young adulthood plays later in dementia risk. The planet’s taller-brother Danes (the world’s third-tallest nation) analyzed data from more than 666,000 Danish men, including more than 70,000 brothers.
They found that, for every 6 cm of height in those above average height, the risk of dementia dropped 10%. And that inverse relationship between height and dementia risk held even in the shared environments of families: Being the taller brother delivered relatively more dementia protection. Even being smarter, better educated, and savvier at playing point guard didn’t erase shorter brothers’ dementia/height disadvantage.
Before you take solace in a Coors stubby, littler brothers, let’s remember the advantages shorter siblings still enjoy: Never being called “Ichabod.” Walking tall in low-ceilinged parking garages. Fitting comfortably into 911s and F-18s alike. Draining threes from anywhere in the driveway.
Oh, and clearly being Mom’s favorite.
Swinging for longevity
They say that laughter is the best medicine, but we always assumed that it applied to the people doing the laughing.
That may not be the case, according to a report presented at the American Stroke Association International Stroke Conference in Dallas.
It may be even better to get laughed at, and Adnan Qureshi, MD, of the University of Missouri, Columbia, and associates have data from the Cardiovascular Health Study of adults aged 65 years and older to prove it.
It’s all about the golf. The 384 golfers among the almost 5,900 participants had a death rate of 15.1% over the 10-year follow-up.
As for the nongolfers – the ones who make fun of golfers’ clothes and say that golf is boring, who joke about riding around in carts and hanging around with old people, who laugh because the lowest score wins, who say it’s easy to hit a little white ball that’s not even moving, who think an albatross is just a bird ... um, we seem to have gotten a bit off topic here.
Anyway, the death rate for the nongolfers in the study was a significantly higher 24.6%. So, suck on that, nongolfers, because it looks like the golfers will be having the last laugh. In plaid pants.

Soylent stitches are people!
Ask anyone in advertising and they’ll tell you that branding is everything. Now, there may be a bit of self-promotion involved there. But you can’t deny that naming your product appropriately is important, and we’re going to say with some degree of confidence that “human textile” is not the greatest name in the world.
Now, we don’t want to question the team of French researchers too much. After all, according to their research published in Acta Biomaterialia, they’ve come up with quite the nifty and potentially lifesaving innovation: stitches made from human skin.
By taking sheets of human skin cells (eww) and cutting them into strips, the researchers were able to weave the strips into a sort of yarn, the advantages of which should be obvious. Patients can say goodbye to pesky issues of compatibility and adverse immune response when doctors and surgeons can stitch wounds, sew pouches, and create tubes and valves with yarn crafted from themselves.
We just can’t get past the name they chose. Human textile. The process of making them is gruesome enough already. No need to call to mind some horrific dystopian future in which cotton can no longer grow and we have to recycle humans (alive or dead, depending on how grim you’re feeling) in big industrial textile mills to craft clothing for ourselves.
It’s just too bad Charlton Heston is dead; he’d have made a great spokesperson.
Towering over dementia
Let us take a moment to pity the plight of the shorter brother. Always losing the battle of the boards in fraternal driveway basketball games. Never reaching the Pop-Tarts cruelly stashed high in the pantry by one’s taller, greedier sibling. Always being addressed during family dinners as “Frodo.”
And new research findings add to the burden borne by altitude-impaired brothers everywhere: Being the short one may boost your risk of dementia.
Danish researchers at the University of Copenhagen examined the potential role that height in young adulthood plays later in dementia risk. The planet’s taller-brother Danes (the world’s third-tallest nation) analyzed data from more than 666,000 Danish men, including more than 70,000 brothers.
They found that, for every 6 cm of height in those above average height, the risk of dementia dropped 10%. And that inverse relationship between height and dementia risk held even in the shared environments of families: Being the taller brother delivered relatively more dementia protection. Even being smarter, better educated, and savvier at playing point guard didn’t erase shorter brothers’ dementia/height disadvantage.
Before you take solace in a Coors stubby, littler brothers, let’s remember the advantages shorter siblings still enjoy: Never being called “Ichabod.” Walking tall in low-ceilinged parking garages. Fitting comfortably into 911s and F-18s alike. Draining threes from anywhere in the driveway.
Oh, and clearly being Mom’s favorite.
Swinging for longevity
They say that laughter is the best medicine, but we always assumed that it applied to the people doing the laughing.
That may not be the case, according to a report presented at the American Stroke Association International Stroke Conference in Dallas.
It may be even better to get laughed at, and Adnan Qureshi, MD, of the University of Missouri, Columbia, and associates have data from the Cardiovascular Health Study of adults aged 65 years and older to prove it.
It’s all about the golf. The 384 golfers among the almost 5,900 participants had a death rate of 15.1% over the 10-year follow-up.
As for the nongolfers – the ones who make fun of golfers’ clothes and say that golf is boring, who joke about riding around in carts and hanging around with old people, who laugh because the lowest score wins, who say it’s easy to hit a little white ball that’s not even moving, who think an albatross is just a bird ... um, we seem to have gotten a bit off topic here.
Anyway, the death rate for the nongolfers in the study was a significantly higher 24.6%. So, suck on that, nongolfers, because it looks like the golfers will be having the last laugh. In plaid pants.

Soylent stitches are people!
Ask anyone in advertising and they’ll tell you that branding is everything. Now, there may be a bit of self-promotion involved there. But you can’t deny that naming your product appropriately is important, and we’re going to say with some degree of confidence that “human textile” is not the greatest name in the world.
Now, we don’t want to question the team of French researchers too much. After all, according to their research published in Acta Biomaterialia, they’ve come up with quite the nifty and potentially lifesaving innovation: stitches made from human skin.
By taking sheets of human skin cells (eww) and cutting them into strips, the researchers were able to weave the strips into a sort of yarn, the advantages of which should be obvious. Patients can say goodbye to pesky issues of compatibility and adverse immune response when doctors and surgeons can stitch wounds, sew pouches, and create tubes and valves with yarn crafted from themselves.
We just can’t get past the name they chose. Human textile. The process of making them is gruesome enough already. No need to call to mind some horrific dystopian future in which cotton can no longer grow and we have to recycle humans (alive or dead, depending on how grim you’re feeling) in big industrial textile mills to craft clothing for ourselves.
It’s just too bad Charlton Heston is dead; he’d have made a great spokesperson.
Towering over dementia
Let us take a moment to pity the plight of the shorter brother. Always losing the battle of the boards in fraternal driveway basketball games. Never reaching the Pop-Tarts cruelly stashed high in the pantry by one’s taller, greedier sibling. Always being addressed during family dinners as “Frodo.”
And new research findings add to the burden borne by altitude-impaired brothers everywhere: Being the short one may boost your risk of dementia.
Danish researchers at the University of Copenhagen examined the potential role that height in young adulthood plays later in dementia risk. The planet’s taller-brother Danes (the world’s third-tallest nation) analyzed data from more than 666,000 Danish men, including more than 70,000 brothers.
They found that, for every 6 cm of height in those above average height, the risk of dementia dropped 10%. And that inverse relationship between height and dementia risk held even in the shared environments of families: Being the taller brother delivered relatively more dementia protection. Even being smarter, better educated, and savvier at playing point guard didn’t erase shorter brothers’ dementia/height disadvantage.
Before you take solace in a Coors stubby, littler brothers, let’s remember the advantages shorter siblings still enjoy: Never being called “Ichabod.” Walking tall in low-ceilinged parking garages. Fitting comfortably into 911s and F-18s alike. Draining threes from anywhere in the driveway.
Oh, and clearly being Mom’s favorite.
Swinging for longevity
They say that laughter is the best medicine, but we always assumed that it applied to the people doing the laughing.
That may not be the case, according to a report presented at the American Stroke Association International Stroke Conference in Dallas.
It may be even better to get laughed at, and Adnan Qureshi, MD, of the University of Missouri, Columbia, and associates have data from the Cardiovascular Health Study of adults aged 65 years and older to prove it.
It’s all about the golf. The 384 golfers among the almost 5,900 participants had a death rate of 15.1% over the 10-year follow-up.
As for the nongolfers – the ones who make fun of golfers’ clothes and say that golf is boring, who joke about riding around in carts and hanging around with old people, who laugh because the lowest score wins, who say it’s easy to hit a little white ball that’s not even moving, who think an albatross is just a bird ... um, we seem to have gotten a bit off topic here.
Anyway, the death rate for the nongolfers in the study was a significantly higher 24.6%. So, suck on that, nongolfers, because it looks like the golfers will be having the last laugh. In plaid pants.

Seminal, highly anticipated Alzheimer’s trial falters
DIAN-TU top-line results negative
Top-line results from the seminal phase 2/3 Dominantly Inherited Alzheimer’s Network–Trials Unit (DIAN-TU) study show that the novel drugs gantenerumab (Roche) and solanezumab (Lilly) did not meet the primary endpoint in patients with early-stage, dominantly inherited Alzheimer’s disease (AD), investigators have announced.
In the international trial, which included almost 200 participants, the two experimental agents were evaluated separately. However, initial analyses showed that neither significantly slowed cognitive decline, the primary outcome measure, nor memory loss.
Still, the researchers noted that they will continue exploring data from DIAN-TU’s cognitive and clinical outcomes and are awaiting analyses of various biomarkers.
“Although the drugs we evaluated were not successful, the trial will move us forward in understanding Alzheimer’s,” principal investigator Randall J. Bateman, MD, of Washington University, St. Louis, said in a news release.
Funders for the trial included the National Institute on Aging and the Alzheimer’s Association.
“While the top-line data fell short, the Alzheimer’s Association looks forward to a more complete report at upcoming scientific conferences. We learn from every trial,” Maria Carrillo, PhD, chief scientific officer at the Alzheimer’s Association, noted in the organization’s own release.
Rebecca M. Edelmayer, PhD, director of scientific engagement for the Alzheimer’s Association, agreed with Dr. Carrillo that, although the results were disappointing, the data will be beneficial for the field.
“It’s always a difficult day when we get news like this,” Dr. Edelmayer said in an interview. However, “this research is going to absolutely provide valuable information once we can really pick through all of the data.”
Rare condition
Dominantly inherited AD, also known as familial AD or autosomal dominant AD, is rare but can affect memory and cognitive skills in individuals as young as age 30. It is caused by mutations on chromosomes 21, 14, and/or 1 that play a part in the breakdown of amyloid proteins and formation of amyloid plaques.
Both gantenerumab and solanezumab were created to target and neutralize amyloid-beta, albeit through different mechanisms. Both are also being assessed in other trials as treatment for more common forms of AD.
As reported by Medscape Medical News, results of the phase 3 EXPEDITION3 trial of solanezumab in patients with mild AD were negative, as were two other phase 3 trials. The drug is now being evaluated in the ongoing solanezumab Anti-Amyloid Treatment in Asymptomatic Alzheimer’s (A4) study.
Although the phase 3 SCARLET ROAD trial of gantenerumab for mild AD was stopped early for futility in 2014, it was continued as an open-label extension at the high dose for 2 years. During that period, follow-up analyses showed a dramatic decline in amyloid-beta deposition in the participants – leading to the launch of the phase 3 GRADUATE 1 and GRADUATE 2 trials.
Starting in 2012, DIAN-TU was conducted at 24 sites in the United States, the United Kingdom, Canada, Europe, and Australia. It followed 194 adult patients for up to 7 years (average duration, 5 years). Its original estimated completion date was December 2020, as stated on clinicaltrials.gov.
All participants had family members with a genetic mutation that causes early-onset Alzheimer’s disease. They already had very mild symptoms of cognitive decline and memory loss at the start of the trial or were expected to develop symptoms within 15 years of enrollment.
“People who inherit the mutation are all but guaranteed to develop symptoms at about the same age their parents did,” the release noted.
“While devastating for families, such mutations allow researchers to identify people in the early stages of the disease before their behavior and memory begin to change,” it added.
The Alzheimer’s Association noted in its release that a child of a parent with the mutation has a 50/50 chance of inheriting the disease. “This form affects less than 1% of the individuals living with Alzheimer’s disease today,” Dr. Edelmayer noted.
Detailed data coming soon
Trial participants were randomly assigned to receive either solanezumab, gantenerumab, or matching placebo. To act as a comparator group, family members without the AD mutation were also included.
The primary measure was change from baseline in the DIAN-TU cognitive composite score. Secondary measures included changes on the Mini-Mental State Examination, the Functional Assessment Scale, the Neuropsychiatric Inventory Questionnaire, the 12-item International Shopping List Test, the Memory Complaint Questionnaire, and the Wechsler Memory Scale Logical Memory/Paragraph Memory test.
The researchers also conducted imaging scans and collected samples of blood and cerebrospinal fluid.
Along with announcing the negative top-line results for the trial, the investigators noted that “a more detailed analysis of the trial’s data” will be presented at the Advances in Alzheimer’s and Parkinson’s Therapies in Vienna on April 2, 2020, and at the Alzheimer’s Association International Conference in Amsterdam in July.
The researchers will continue to explore all data gathered – but already new insights have been discovered into the development and progression of AD, Dr. Bateman noted.
Included among these discoveries is that brain changes that occur as the disease progresses are similar among those with the inherited, early-onset form of AD and the late-onset form.
“The trial’s innovative design ... will make advances for future Alzheimer’s trials. Ongoing and continued research and trials will bring us closer to our goal to stop Alzheimer’s,” Dr. Bateman said. “We will continue until we are successful.”
“These results reflect the difficult nature of treating [AD] and the great need for continued research,” said Daniel Skovronsky, MD, PhD, chief scientific officer and president of Lilly Research Labs.
“If we have learned one thing after more than 30 years of Alzheimer’s research, it is that even negative results propel the science forward,” he added.
Lilly noted in a statement that the DIAN-TU top-line results will not affect its ongoing A4 study of solanezumab. Roche noted in its own statement that the findings also will not affect the company’s ongoing GRADUATE studies of gantenerumab.
“The work doesn’t stop here”
Richard J. Hodes, MD, director of the National Institute on Aging, said that DIAN-TU will advance the field’s knowledge about a complex disease.
“We look forward to learning more through the published, peer-reviewed data, which will provide a broad range of scientists with crucial information and guidance for future research,” he said.
Howard Fillit, MD, founding executive director and chief science officer at the Alzheimer’s Drug Discovery Foundation (ADDF), agreed.
“While we are disappointed that patients in this study did not see a benefit, we need to keep in mind that Alzheimer’s is a complicated disease due to complex, multifactorial causes,” he said in a statement.
“ADDF has long supported a broader approach that moves past targeting beta-amyloid and advances a diverse pipeline of drugs addressing multiple targets” in AD, Dr. Fillit added. “We need multiple ‘shots on goals’ to discover effective drugs.”
Dr. Edelmayer said the results emphasize that “this story isn’t yet completely told” and that there is still a lot to learn from the data, especially regarding the biomarkers that were tested.
“With that information, we will gain valuable insight into the outcomes that have been released but will also probably better understand where we should be putting our energies and focus moving forward,” she said.
Going forward, “we will continue this fight until we have an effective treatment for all individuals living with Alzheimer’s, whether it’s dominantly inherited [AD] or the more common version, which is the late-onset or sporadic form of the disease,” said Dr. Edelmayer.
“We have to stay optimistic. The work doesn’t stop here.”
The trial was funded by Eli Lilly, Roche, the Alzheimer’s Association, the National Institute on Aging, the GHR Foundation, and FBRI.
This article first appeared on Medscape.com.
DIAN-TU top-line results negative
DIAN-TU top-line results negative
Top-line results from the seminal phase 2/3 Dominantly Inherited Alzheimer’s Network–Trials Unit (DIAN-TU) study show that the novel drugs gantenerumab (Roche) and solanezumab (Lilly) did not meet the primary endpoint in patients with early-stage, dominantly inherited Alzheimer’s disease (AD), investigators have announced.
In the international trial, which included almost 200 participants, the two experimental agents were evaluated separately. However, initial analyses showed that neither significantly slowed cognitive decline, the primary outcome measure, nor memory loss.
Still, the researchers noted that they will continue exploring data from DIAN-TU’s cognitive and clinical outcomes and are awaiting analyses of various biomarkers.
“Although the drugs we evaluated were not successful, the trial will move us forward in understanding Alzheimer’s,” principal investigator Randall J. Bateman, MD, of Washington University, St. Louis, said in a news release.
Funders for the trial included the National Institute on Aging and the Alzheimer’s Association.
“While the top-line data fell short, the Alzheimer’s Association looks forward to a more complete report at upcoming scientific conferences. We learn from every trial,” Maria Carrillo, PhD, chief scientific officer at the Alzheimer’s Association, noted in the organization’s own release.
Rebecca M. Edelmayer, PhD, director of scientific engagement for the Alzheimer’s Association, agreed with Dr. Carrillo that, although the results were disappointing, the data will be beneficial for the field.
“It’s always a difficult day when we get news like this,” Dr. Edelmayer said in an interview. However, “this research is going to absolutely provide valuable information once we can really pick through all of the data.”
Rare condition
Dominantly inherited AD, also known as familial AD or autosomal dominant AD, is rare but can affect memory and cognitive skills in individuals as young as age 30. It is caused by mutations on chromosomes 21, 14, and/or 1 that play a part in the breakdown of amyloid proteins and formation of amyloid plaques.
Both gantenerumab and solanezumab were created to target and neutralize amyloid-beta, albeit through different mechanisms. Both are also being assessed in other trials as treatment for more common forms of AD.
As reported by Medscape Medical News, results of the phase 3 EXPEDITION3 trial of solanezumab in patients with mild AD were negative, as were two other phase 3 trials. The drug is now being evaluated in the ongoing solanezumab Anti-Amyloid Treatment in Asymptomatic Alzheimer’s (A4) study.
Although the phase 3 SCARLET ROAD trial of gantenerumab for mild AD was stopped early for futility in 2014, it was continued as an open-label extension at the high dose for 2 years. During that period, follow-up analyses showed a dramatic decline in amyloid-beta deposition in the participants – leading to the launch of the phase 3 GRADUATE 1 and GRADUATE 2 trials.
Starting in 2012, DIAN-TU was conducted at 24 sites in the United States, the United Kingdom, Canada, Europe, and Australia. It followed 194 adult patients for up to 7 years (average duration, 5 years). Its original estimated completion date was December 2020, as stated on clinicaltrials.gov.
All participants had family members with a genetic mutation that causes early-onset Alzheimer’s disease. They already had very mild symptoms of cognitive decline and memory loss at the start of the trial or were expected to develop symptoms within 15 years of enrollment.
“People who inherit the mutation are all but guaranteed to develop symptoms at about the same age their parents did,” the release noted.
“While devastating for families, such mutations allow researchers to identify people in the early stages of the disease before their behavior and memory begin to change,” it added.
The Alzheimer’s Association noted in its release that a child of a parent with the mutation has a 50/50 chance of inheriting the disease. “This form affects less than 1% of the individuals living with Alzheimer’s disease today,” Dr. Edelmayer noted.
Detailed data coming soon
Trial participants were randomly assigned to receive either solanezumab, gantenerumab, or matching placebo. To act as a comparator group, family members without the AD mutation were also included.
The primary measure was change from baseline in the DIAN-TU cognitive composite score. Secondary measures included changes on the Mini-Mental State Examination, the Functional Assessment Scale, the Neuropsychiatric Inventory Questionnaire, the 12-item International Shopping List Test, the Memory Complaint Questionnaire, and the Wechsler Memory Scale Logical Memory/Paragraph Memory test.
The researchers also conducted imaging scans and collected samples of blood and cerebrospinal fluid.
Along with announcing the negative top-line results for the trial, the investigators noted that “a more detailed analysis of the trial’s data” will be presented at the Advances in Alzheimer’s and Parkinson’s Therapies in Vienna on April 2, 2020, and at the Alzheimer’s Association International Conference in Amsterdam in July.
The researchers will continue to explore all data gathered – but already new insights have been discovered into the development and progression of AD, Dr. Bateman noted.
Included among these discoveries is that brain changes that occur as the disease progresses are similar among those with the inherited, early-onset form of AD and the late-onset form.
“The trial’s innovative design ... will make advances for future Alzheimer’s trials. Ongoing and continued research and trials will bring us closer to our goal to stop Alzheimer’s,” Dr. Bateman said. “We will continue until we are successful.”
“These results reflect the difficult nature of treating [AD] and the great need for continued research,” said Daniel Skovronsky, MD, PhD, chief scientific officer and president of Lilly Research Labs.
“If we have learned one thing after more than 30 years of Alzheimer’s research, it is that even negative results propel the science forward,” he added.
Lilly noted in a statement that the DIAN-TU top-line results will not affect its ongoing A4 study of solanezumab. Roche noted in its own statement that the findings also will not affect the company’s ongoing GRADUATE studies of gantenerumab.
“The work doesn’t stop here”
Richard J. Hodes, MD, director of the National Institute on Aging, said that DIAN-TU will advance the field’s knowledge about a complex disease.
“We look forward to learning more through the published, peer-reviewed data, which will provide a broad range of scientists with crucial information and guidance for future research,” he said.
Howard Fillit, MD, founding executive director and chief science officer at the Alzheimer’s Drug Discovery Foundation (ADDF), agreed.
“While we are disappointed that patients in this study did not see a benefit, we need to keep in mind that Alzheimer’s is a complicated disease due to complex, multifactorial causes,” he said in a statement.
“ADDF has long supported a broader approach that moves past targeting beta-amyloid and advances a diverse pipeline of drugs addressing multiple targets” in AD, Dr. Fillit added. “We need multiple ‘shots on goals’ to discover effective drugs.”
Dr. Edelmayer said the results emphasize that “this story isn’t yet completely told” and that there is still a lot to learn from the data, especially regarding the biomarkers that were tested.
“With that information, we will gain valuable insight into the outcomes that have been released but will also probably better understand where we should be putting our energies and focus moving forward,” she said.
Going forward, “we will continue this fight until we have an effective treatment for all individuals living with Alzheimer’s, whether it’s dominantly inherited [AD] or the more common version, which is the late-onset or sporadic form of the disease,” said Dr. Edelmayer.
“We have to stay optimistic. The work doesn’t stop here.”
The trial was funded by Eli Lilly, Roche, the Alzheimer’s Association, the National Institute on Aging, the GHR Foundation, and FBRI.
This article first appeared on Medscape.com.
Top-line results from the seminal phase 2/3 Dominantly Inherited Alzheimer’s Network–Trials Unit (DIAN-TU) study show that the novel drugs gantenerumab (Roche) and solanezumab (Lilly) did not meet the primary endpoint in patients with early-stage, dominantly inherited Alzheimer’s disease (AD), investigators have announced.
In the international trial, which included almost 200 participants, the two experimental agents were evaluated separately. However, initial analyses showed that neither significantly slowed cognitive decline, the primary outcome measure, nor memory loss.
Still, the researchers noted that they will continue exploring data from DIAN-TU’s cognitive and clinical outcomes and are awaiting analyses of various biomarkers.
“Although the drugs we evaluated were not successful, the trial will move us forward in understanding Alzheimer’s,” principal investigator Randall J. Bateman, MD, of Washington University, St. Louis, said in a news release.
Funders for the trial included the National Institute on Aging and the Alzheimer’s Association.
“While the top-line data fell short, the Alzheimer’s Association looks forward to a more complete report at upcoming scientific conferences. We learn from every trial,” Maria Carrillo, PhD, chief scientific officer at the Alzheimer’s Association, noted in the organization’s own release.
Rebecca M. Edelmayer, PhD, director of scientific engagement for the Alzheimer’s Association, agreed with Dr. Carrillo that, although the results were disappointing, the data will be beneficial for the field.
“It’s always a difficult day when we get news like this,” Dr. Edelmayer said in an interview. However, “this research is going to absolutely provide valuable information once we can really pick through all of the data.”
Rare condition
Dominantly inherited AD, also known as familial AD or autosomal dominant AD, is rare but can affect memory and cognitive skills in individuals as young as age 30. It is caused by mutations on chromosomes 21, 14, and/or 1 that play a part in the breakdown of amyloid proteins and formation of amyloid plaques.
Both gantenerumab and solanezumab were created to target and neutralize amyloid-beta, albeit through different mechanisms. Both are also being assessed in other trials as treatment for more common forms of AD.
As reported by Medscape Medical News, results of the phase 3 EXPEDITION3 trial of solanezumab in patients with mild AD were negative, as were two other phase 3 trials. The drug is now being evaluated in the ongoing solanezumab Anti-Amyloid Treatment in Asymptomatic Alzheimer’s (A4) study.
Although the phase 3 SCARLET ROAD trial of gantenerumab for mild AD was stopped early for futility in 2014, it was continued as an open-label extension at the high dose for 2 years. During that period, follow-up analyses showed a dramatic decline in amyloid-beta deposition in the participants – leading to the launch of the phase 3 GRADUATE 1 and GRADUATE 2 trials.
Starting in 2012, DIAN-TU was conducted at 24 sites in the United States, the United Kingdom, Canada, Europe, and Australia. It followed 194 adult patients for up to 7 years (average duration, 5 years). Its original estimated completion date was December 2020, as stated on clinicaltrials.gov.
All participants had family members with a genetic mutation that causes early-onset Alzheimer’s disease. They already had very mild symptoms of cognitive decline and memory loss at the start of the trial or were expected to develop symptoms within 15 years of enrollment.
“People who inherit the mutation are all but guaranteed to develop symptoms at about the same age their parents did,” the release noted.
“While devastating for families, such mutations allow researchers to identify people in the early stages of the disease before their behavior and memory begin to change,” it added.
The Alzheimer’s Association noted in its release that a child of a parent with the mutation has a 50/50 chance of inheriting the disease. “This form affects less than 1% of the individuals living with Alzheimer’s disease today,” Dr. Edelmayer noted.
Detailed data coming soon
Trial participants were randomly assigned to receive either solanezumab, gantenerumab, or matching placebo. To act as a comparator group, family members without the AD mutation were also included.
The primary measure was change from baseline in the DIAN-TU cognitive composite score. Secondary measures included changes on the Mini-Mental State Examination, the Functional Assessment Scale, the Neuropsychiatric Inventory Questionnaire, the 12-item International Shopping List Test, the Memory Complaint Questionnaire, and the Wechsler Memory Scale Logical Memory/Paragraph Memory test.
The researchers also conducted imaging scans and collected samples of blood and cerebrospinal fluid.
Along with announcing the negative top-line results for the trial, the investigators noted that “a more detailed analysis of the trial’s data” will be presented at the Advances in Alzheimer’s and Parkinson’s Therapies in Vienna on April 2, 2020, and at the Alzheimer’s Association International Conference in Amsterdam in July.
The researchers will continue to explore all data gathered – but already new insights have been discovered into the development and progression of AD, Dr. Bateman noted.
Included among these discoveries is that brain changes that occur as the disease progresses are similar among those with the inherited, early-onset form of AD and the late-onset form.
“The trial’s innovative design ... will make advances for future Alzheimer’s trials. Ongoing and continued research and trials will bring us closer to our goal to stop Alzheimer’s,” Dr. Bateman said. “We will continue until we are successful.”
“These results reflect the difficult nature of treating [AD] and the great need for continued research,” said Daniel Skovronsky, MD, PhD, chief scientific officer and president of Lilly Research Labs.
“If we have learned one thing after more than 30 years of Alzheimer’s research, it is that even negative results propel the science forward,” he added.
Lilly noted in a statement that the DIAN-TU top-line results will not affect its ongoing A4 study of solanezumab. Roche noted in its own statement that the findings also will not affect the company’s ongoing GRADUATE studies of gantenerumab.
“The work doesn’t stop here”
Richard J. Hodes, MD, director of the National Institute on Aging, said that DIAN-TU will advance the field’s knowledge about a complex disease.
“We look forward to learning more through the published, peer-reviewed data, which will provide a broad range of scientists with crucial information and guidance for future research,” he said.
Howard Fillit, MD, founding executive director and chief science officer at the Alzheimer’s Drug Discovery Foundation (ADDF), agreed.
“While we are disappointed that patients in this study did not see a benefit, we need to keep in mind that Alzheimer’s is a complicated disease due to complex, multifactorial causes,” he said in a statement.
“ADDF has long supported a broader approach that moves past targeting beta-amyloid and advances a diverse pipeline of drugs addressing multiple targets” in AD, Dr. Fillit added. “We need multiple ‘shots on goals’ to discover effective drugs.”
Dr. Edelmayer said the results emphasize that “this story isn’t yet completely told” and that there is still a lot to learn from the data, especially regarding the biomarkers that were tested.
“With that information, we will gain valuable insight into the outcomes that have been released but will also probably better understand where we should be putting our energies and focus moving forward,” she said.
Going forward, “we will continue this fight until we have an effective treatment for all individuals living with Alzheimer’s, whether it’s dominantly inherited [AD] or the more common version, which is the late-onset or sporadic form of the disease,” said Dr. Edelmayer.
“We have to stay optimistic. The work doesn’t stop here.”
The trial was funded by Eli Lilly, Roche, the Alzheimer’s Association, the National Institute on Aging, the GHR Foundation, and FBRI.
This article first appeared on Medscape.com.
IBD fertility has improved
AUSTIN, TEX. – Patients with inflammatory bowel disease (IBD) who want to have children can benefit from better education about recent findings that disease control, laparoscopic surgery, and in vitro fertilization (IVF) have improved their chances of conceiving, according to a review of published reports presented here at the Crohn’s & Colitis Congress, a partnership of the Crohn’s & Colitis Congress Foundation and the American Gastroenterological Association.
“Decreased fertility in IBD is due to voluntary childlessness, which we can change with education; surgery for IBD, which we can improve with laparoscopic surgery; and increased disease activity, which we can also make a difference in,” Sonia Friedman, MD, of Harvard Medical School, Boston, said in an interview.
Dr. Friedman and coauthors last year published an analysis of the Danish National Birth Cohort, which showed women with IBD had an 28% greater relative risk of taking a year or more to get pregnant than controls without IBD, and that the relative risk was even higher in women with Crohn’s disease — 54% (Clin Gastroenterol Hepatol. 2019. doi: 10.1016/j.cgh.2019.08.031). “We found that women with Crohn’s surgery had decreased fertility by 2.54 times greater relative risk,” she said.
“Fertility, pregnancy is the most important thing to patients,” Dr. Friedman said in an interview. “That’s what people ask me about the most. In the population of IBD patients, the onset is age 15-35, and these people are in the prime of their reproductive years.” Sexual function, known to be decreased in men and women with IBD, is also an overriding concern in these patients, she said. “There needs to be a lot more information out there about it.”
She said gastroenterologists should keep in mind that much of the evidence documenting reduced fertility after ileo-pouch anal anastomosis is dated and focused on open surgery, which caused profound scarring of the pelvis and fallopian tubes, thus hindering conception. Laparoscopic ileoanal J-pouch surgery (IPAA) has yielded much improved outcomes in women of child-bearing age, she said, citing a study late last year that reported women who had laparoscopic IPAA had a median time to pregnancy of 3.5 months versus 9 months for women who had open IPAA (Surgery. 2019;166:670-7).
“It’s really important to discuss the issues of fertility, especially for patients contemplating surgery,” Dr. Friedman said. “Emphasize that there are good outcomes with laparoscopic surgery, and they can have assisted reproductive technology [ART], or in vitro fertilization, if needed. Never withhold surgery based on fear of infertility.”
Her practice is to refer women with IBD in remission for IVF if they’ve tried to get pregnant every month for a year or more and to refer women with IBD surgery for IVF after trying to get pregnant for 6 months. Dr. Friedman coauthored two studies of the Danish National Birth Cohort of ART in women with Crohn’s disease and ulcerative colitis (UC) along with controls (Gut. 2016;65:767-76; Gut. 2017;66:556-58). “We found that women with Crohn’s and UC had a decreased chance of having a clinical pregnancy, but they had no problem carrying the pregnancy to term,” she said.
Those findings raised questions about the etiology of decreased fertility in IBD patients, which could include factors such as IVF technique, reproductive hormone and microbiome changes, or IBD medications. “How can we carry that forward to all women with IBD?” she said. Women with IBD have less chance of conceiving with each IVF treatment cycle than do women without IBD, she said. “The most interesting thing is that the reduced chance of live birth after IVF treatment in Crohn’s and UC is related to the stages of implantation and not to the ability to maintain the fetus throughout pregnancy,” she said.
Dr. Friedman has no financial relationships to disclose.
The AGA IBD Parenthood Project can help guide your patients with IBD throughout their pregnancy, from trying to conceive through postpartum care. Learn more at IBDParenthoodProject.org.
SOURCE: Friedman S. Crohn’s & Colitis Congress, Session Sp86.
AUSTIN, TEX. – Patients with inflammatory bowel disease (IBD) who want to have children can benefit from better education about recent findings that disease control, laparoscopic surgery, and in vitro fertilization (IVF) have improved their chances of conceiving, according to a review of published reports presented here at the Crohn’s & Colitis Congress, a partnership of the Crohn’s & Colitis Congress Foundation and the American Gastroenterological Association.
“Decreased fertility in IBD is due to voluntary childlessness, which we can change with education; surgery for IBD, which we can improve with laparoscopic surgery; and increased disease activity, which we can also make a difference in,” Sonia Friedman, MD, of Harvard Medical School, Boston, said in an interview.
Dr. Friedman and coauthors last year published an analysis of the Danish National Birth Cohort, which showed women with IBD had an 28% greater relative risk of taking a year or more to get pregnant than controls without IBD, and that the relative risk was even higher in women with Crohn’s disease — 54% (Clin Gastroenterol Hepatol. 2019. doi: 10.1016/j.cgh.2019.08.031). “We found that women with Crohn’s surgery had decreased fertility by 2.54 times greater relative risk,” she said.
“Fertility, pregnancy is the most important thing to patients,” Dr. Friedman said in an interview. “That’s what people ask me about the most. In the population of IBD patients, the onset is age 15-35, and these people are in the prime of their reproductive years.” Sexual function, known to be decreased in men and women with IBD, is also an overriding concern in these patients, she said. “There needs to be a lot more information out there about it.”
She said gastroenterologists should keep in mind that much of the evidence documenting reduced fertility after ileo-pouch anal anastomosis is dated and focused on open surgery, which caused profound scarring of the pelvis and fallopian tubes, thus hindering conception. Laparoscopic ileoanal J-pouch surgery (IPAA) has yielded much improved outcomes in women of child-bearing age, she said, citing a study late last year that reported women who had laparoscopic IPAA had a median time to pregnancy of 3.5 months versus 9 months for women who had open IPAA (Surgery. 2019;166:670-7).
“It’s really important to discuss the issues of fertility, especially for patients contemplating surgery,” Dr. Friedman said. “Emphasize that there are good outcomes with laparoscopic surgery, and they can have assisted reproductive technology [ART], or in vitro fertilization, if needed. Never withhold surgery based on fear of infertility.”
Her practice is to refer women with IBD in remission for IVF if they’ve tried to get pregnant every month for a year or more and to refer women with IBD surgery for IVF after trying to get pregnant for 6 months. Dr. Friedman coauthored two studies of the Danish National Birth Cohort of ART in women with Crohn’s disease and ulcerative colitis (UC) along with controls (Gut. 2016;65:767-76; Gut. 2017;66:556-58). “We found that women with Crohn’s and UC had a decreased chance of having a clinical pregnancy, but they had no problem carrying the pregnancy to term,” she said.
Those findings raised questions about the etiology of decreased fertility in IBD patients, which could include factors such as IVF technique, reproductive hormone and microbiome changes, or IBD medications. “How can we carry that forward to all women with IBD?” she said. Women with IBD have less chance of conceiving with each IVF treatment cycle than do women without IBD, she said. “The most interesting thing is that the reduced chance of live birth after IVF treatment in Crohn’s and UC is related to the stages of implantation and not to the ability to maintain the fetus throughout pregnancy,” she said.
Dr. Friedman has no financial relationships to disclose.
The AGA IBD Parenthood Project can help guide your patients with IBD throughout their pregnancy, from trying to conceive through postpartum care. Learn more at IBDParenthoodProject.org.
SOURCE: Friedman S. Crohn’s & Colitis Congress, Session Sp86.
AUSTIN, TEX. – Patients with inflammatory bowel disease (IBD) who want to have children can benefit from better education about recent findings that disease control, laparoscopic surgery, and in vitro fertilization (IVF) have improved their chances of conceiving, according to a review of published reports presented here at the Crohn’s & Colitis Congress, a partnership of the Crohn’s & Colitis Congress Foundation and the American Gastroenterological Association.
“Decreased fertility in IBD is due to voluntary childlessness, which we can change with education; surgery for IBD, which we can improve with laparoscopic surgery; and increased disease activity, which we can also make a difference in,” Sonia Friedman, MD, of Harvard Medical School, Boston, said in an interview.
Dr. Friedman and coauthors last year published an analysis of the Danish National Birth Cohort, which showed women with IBD had an 28% greater relative risk of taking a year or more to get pregnant than controls without IBD, and that the relative risk was even higher in women with Crohn’s disease — 54% (Clin Gastroenterol Hepatol. 2019. doi: 10.1016/j.cgh.2019.08.031). “We found that women with Crohn’s surgery had decreased fertility by 2.54 times greater relative risk,” she said.
“Fertility, pregnancy is the most important thing to patients,” Dr. Friedman said in an interview. “That’s what people ask me about the most. In the population of IBD patients, the onset is age 15-35, and these people are in the prime of their reproductive years.” Sexual function, known to be decreased in men and women with IBD, is also an overriding concern in these patients, she said. “There needs to be a lot more information out there about it.”
She said gastroenterologists should keep in mind that much of the evidence documenting reduced fertility after ileo-pouch anal anastomosis is dated and focused on open surgery, which caused profound scarring of the pelvis and fallopian tubes, thus hindering conception. Laparoscopic ileoanal J-pouch surgery (IPAA) has yielded much improved outcomes in women of child-bearing age, she said, citing a study late last year that reported women who had laparoscopic IPAA had a median time to pregnancy of 3.5 months versus 9 months for women who had open IPAA (Surgery. 2019;166:670-7).
“It’s really important to discuss the issues of fertility, especially for patients contemplating surgery,” Dr. Friedman said. “Emphasize that there are good outcomes with laparoscopic surgery, and they can have assisted reproductive technology [ART], or in vitro fertilization, if needed. Never withhold surgery based on fear of infertility.”
Her practice is to refer women with IBD in remission for IVF if they’ve tried to get pregnant every month for a year or more and to refer women with IBD surgery for IVF after trying to get pregnant for 6 months. Dr. Friedman coauthored two studies of the Danish National Birth Cohort of ART in women with Crohn’s disease and ulcerative colitis (UC) along with controls (Gut. 2016;65:767-76; Gut. 2017;66:556-58). “We found that women with Crohn’s and UC had a decreased chance of having a clinical pregnancy, but they had no problem carrying the pregnancy to term,” she said.
Those findings raised questions about the etiology of decreased fertility in IBD patients, which could include factors such as IVF technique, reproductive hormone and microbiome changes, or IBD medications. “How can we carry that forward to all women with IBD?” she said. Women with IBD have less chance of conceiving with each IVF treatment cycle than do women without IBD, she said. “The most interesting thing is that the reduced chance of live birth after IVF treatment in Crohn’s and UC is related to the stages of implantation and not to the ability to maintain the fetus throughout pregnancy,” she said.
Dr. Friedman has no financial relationships to disclose.
The AGA IBD Parenthood Project can help guide your patients with IBD throughout their pregnancy, from trying to conceive through postpartum care. Learn more at IBDParenthoodProject.org.
SOURCE: Friedman S. Crohn’s & Colitis Congress, Session Sp86.
REPORTING FROM CROHN’S & COLITIS CONGRESS