User login
Fear drives activity changes in hemophilia patients
Fear of negative events can drive changes in activity levels among patients with hemophilia A, results of the HemACTIVE study suggest.
Patients were more likely to adjust their level of physical activity due to fear of bleeding and joint damage rather than previously experienced bleeding or joint damage.
However, past experience was more likely than fear to make patients stop physical activities altogether.
Mark Skinner, of the Institute for Policy Advancement in Washington, D.C., and colleagues presented these findings in a poster from the annual congress of the European Association for Haemophilia and Allied Disorders.
Mr. Skinner, who is a hemophilia patient himself, said the goal of the HemACTIVE study is to better understand how hemophilia affects patients’ lives.
“We wanted to understand the limitations, challenges, and compromises of individuals living with hemophilia,” Mr. Skinner said. “What has motivated them or prevented them from living more full, active lives doing the kind of work, leisure, and social activities that those without hemophilia do? Is it treatment choice, is it satisfaction with treatment, is it fear?
“We wanted to do a comprehensive study that really looked at the intersection of treatment adherence and satisfaction, the emotional components that relate to those decisions, and the challenges and compromises so that we could better identify what we need to consider as patients think about either changing their therapy or changing their treatment regimen on existing therapy.”
Previous results from the HemACTIVE study showed that, although activity levels differed among hemophilia patients, all patients surveyed wanted greater activity levels, better protection from bleeding, better pain relief, and less-frequent infusions (EAHAD 2019, Abstract P084). In addition, patients who used factor VIII products with an extended half-life were more active and more likely to adhere to their prescribed treatment (ISTH 2019, Abstract PB0210).
The results reported at EAHAD 2020 focus on patients’ reasons for modifying physical activity. Patients and caregivers completed a screening phone interview, followed by a 25-minute, web-based questionnaire on patient activity.
There were 275 respondents – 194 patients with hemophilia A and 81 caregivers – from Canada, France, Germany, Italy, and the United States. Patients had severe (61%) or moderate (39%) hemophilia A, and most (67%) were receiving prophylaxis.
Most patients (70%) were “active” or “extremely/very active,” 77% of patients adjusted their activities because of their hemophilia, and nearly half of patients stopped activities because of their disease.
Fear drives adjustments in activity
Patients were sometimes more likely to adjust their activities based on fear of experiencing an event, as opposed to previously experiencing that event.
Specifically, 44% of patients adjusted their activities due to fear of joint damage, compared with 36% of patients who made adjustments because of past significant joint damage.
Similarly, 41% of patients adjusted activities due to fear of breakthrough bleeds, compared with 36% of patients who made adjustments because of past experience with bleeds and 25% who made adjustments because of significant past bleeds.
On the other hand, a similar percentage of patients adjusted activities because of past experience with pain (43%) and fear of pain (41%). And a similar percentage of patients adjusted activities because of existing joint damage restrictions (35%) and fear of joint deterioration (32%).
Past experience prompts discontinuation of activity
Overall, 47% of patients said anxiety was the most common emotional reason for stopping physical activities. However, patients were consistently more likely to stop activities because of past experience rather than fear or anxiety.
Specifically, 50% of patients stopped activities because of significant past joint damage, 46% stopped because of developing joint problems, and 38% stopped due to fear of joint damage.
More patients stopped activities because of significant past bleeds (41%) rather than fear of breakthrough bleeds (26%). More patients stopped activities because they developed chronic pain (38%) rather than fear of pain (less than 15%). And more patients stopped activities because of existing joint damage restrictions (62%) rather than fear of joint damage (34%).
Applying results to practice: Changing the conversation
Ideally, these findings would be used to promote individualized treatment of hemophilia driven by patients’ goals, Mr. Skinner said. By better understanding patients’ feelings and motivations, clinicians may devise more personalized treatment regimens that align with patients’ goals and improve their quality of life.
Rather than adjusting treatment based only on “hard metrics” such as bleeding events, “we need to take a more holistic approach to looking at outcomes that are more important to patients,” Mr. Skinner said. This type of approach is particularly important to Mr. Skinner as someone who has severe hemophilia A.
“Because hemophilia is a life-long disease, and you’re born with it, you make conscious or unconscious adaptations throughout your life,” he explained. “Your expectations or aspirations adjust to what you’ve been told you can or cannot do because of your hemophilia. The choices I made for my career, where I live, the type of vacations I go on, the type of sports I participate in have all been limited over the course of time, which has meant that I’ve made compromises. There are a lot of individuals with hemophilia who are making decisions that are not what their life goals are.
“What this research helps me understand is that we can change the conversation and build it around an individual patient and understand what their aspirations are. If a clinician understands what I’m wanting to achieve in life … we can build a treatment regime around helping me achieve those goals. That is known to improve adherence.
“The goal, really, is to have hemophilia as a secondary consideration. Instead of saying: ‘You have hemophilia, so these are the options available to you,’ you can say, ‘what is it that you would like to achieve, and then we’ll figure out how your treatment for hemophilia can be adjusted to help you achieve those goals.’ It may sound like a nuance, but it really is reversing the conversation. The goal setting first versus your disease comes first.”
The HemACTIVE study was supported by Bayer. Mr. Skinner disclosed relationships with Bayer and other pharmaceutical companies.
SOURCE: Skinner M et al. EAHAD 2020, Abstract P304.
Fear of negative events can drive changes in activity levels among patients with hemophilia A, results of the HemACTIVE study suggest.
Patients were more likely to adjust their level of physical activity due to fear of bleeding and joint damage rather than previously experienced bleeding or joint damage.
However, past experience was more likely than fear to make patients stop physical activities altogether.
Mark Skinner, of the Institute for Policy Advancement in Washington, D.C., and colleagues presented these findings in a poster from the annual congress of the European Association for Haemophilia and Allied Disorders.
Mr. Skinner, who is a hemophilia patient himself, said the goal of the HemACTIVE study is to better understand how hemophilia affects patients’ lives.
“We wanted to understand the limitations, challenges, and compromises of individuals living with hemophilia,” Mr. Skinner said. “What has motivated them or prevented them from living more full, active lives doing the kind of work, leisure, and social activities that those without hemophilia do? Is it treatment choice, is it satisfaction with treatment, is it fear?
“We wanted to do a comprehensive study that really looked at the intersection of treatment adherence and satisfaction, the emotional components that relate to those decisions, and the challenges and compromises so that we could better identify what we need to consider as patients think about either changing their therapy or changing their treatment regimen on existing therapy.”
Previous results from the HemACTIVE study showed that, although activity levels differed among hemophilia patients, all patients surveyed wanted greater activity levels, better protection from bleeding, better pain relief, and less-frequent infusions (EAHAD 2019, Abstract P084). In addition, patients who used factor VIII products with an extended half-life were more active and more likely to adhere to their prescribed treatment (ISTH 2019, Abstract PB0210).
The results reported at EAHAD 2020 focus on patients’ reasons for modifying physical activity. Patients and caregivers completed a screening phone interview, followed by a 25-minute, web-based questionnaire on patient activity.
There were 275 respondents – 194 patients with hemophilia A and 81 caregivers – from Canada, France, Germany, Italy, and the United States. Patients had severe (61%) or moderate (39%) hemophilia A, and most (67%) were receiving prophylaxis.
Most patients (70%) were “active” or “extremely/very active,” 77% of patients adjusted their activities because of their hemophilia, and nearly half of patients stopped activities because of their disease.
Fear drives adjustments in activity
Patients were sometimes more likely to adjust their activities based on fear of experiencing an event, as opposed to previously experiencing that event.
Specifically, 44% of patients adjusted their activities due to fear of joint damage, compared with 36% of patients who made adjustments because of past significant joint damage.
Similarly, 41% of patients adjusted activities due to fear of breakthrough bleeds, compared with 36% of patients who made adjustments because of past experience with bleeds and 25% who made adjustments because of significant past bleeds.
On the other hand, a similar percentage of patients adjusted activities because of past experience with pain (43%) and fear of pain (41%). And a similar percentage of patients adjusted activities because of existing joint damage restrictions (35%) and fear of joint deterioration (32%).
Past experience prompts discontinuation of activity
Overall, 47% of patients said anxiety was the most common emotional reason for stopping physical activities. However, patients were consistently more likely to stop activities because of past experience rather than fear or anxiety.
Specifically, 50% of patients stopped activities because of significant past joint damage, 46% stopped because of developing joint problems, and 38% stopped due to fear of joint damage.
More patients stopped activities because of significant past bleeds (41%) rather than fear of breakthrough bleeds (26%). More patients stopped activities because they developed chronic pain (38%) rather than fear of pain (less than 15%). And more patients stopped activities because of existing joint damage restrictions (62%) rather than fear of joint damage (34%).
Applying results to practice: Changing the conversation
Ideally, these findings would be used to promote individualized treatment of hemophilia driven by patients’ goals, Mr. Skinner said. By better understanding patients’ feelings and motivations, clinicians may devise more personalized treatment regimens that align with patients’ goals and improve their quality of life.
Rather than adjusting treatment based only on “hard metrics” such as bleeding events, “we need to take a more holistic approach to looking at outcomes that are more important to patients,” Mr. Skinner said. This type of approach is particularly important to Mr. Skinner as someone who has severe hemophilia A.
“Because hemophilia is a life-long disease, and you’re born with it, you make conscious or unconscious adaptations throughout your life,” he explained. “Your expectations or aspirations adjust to what you’ve been told you can or cannot do because of your hemophilia. The choices I made for my career, where I live, the type of vacations I go on, the type of sports I participate in have all been limited over the course of time, which has meant that I’ve made compromises. There are a lot of individuals with hemophilia who are making decisions that are not what their life goals are.
“What this research helps me understand is that we can change the conversation and build it around an individual patient and understand what their aspirations are. If a clinician understands what I’m wanting to achieve in life … we can build a treatment regime around helping me achieve those goals. That is known to improve adherence.
“The goal, really, is to have hemophilia as a secondary consideration. Instead of saying: ‘You have hemophilia, so these are the options available to you,’ you can say, ‘what is it that you would like to achieve, and then we’ll figure out how your treatment for hemophilia can be adjusted to help you achieve those goals.’ It may sound like a nuance, but it really is reversing the conversation. The goal setting first versus your disease comes first.”
The HemACTIVE study was supported by Bayer. Mr. Skinner disclosed relationships with Bayer and other pharmaceutical companies.
SOURCE: Skinner M et al. EAHAD 2020, Abstract P304.
Fear of negative events can drive changes in activity levels among patients with hemophilia A, results of the HemACTIVE study suggest.
Patients were more likely to adjust their level of physical activity due to fear of bleeding and joint damage rather than previously experienced bleeding or joint damage.
However, past experience was more likely than fear to make patients stop physical activities altogether.
Mark Skinner, of the Institute for Policy Advancement in Washington, D.C., and colleagues presented these findings in a poster from the annual congress of the European Association for Haemophilia and Allied Disorders.
Mr. Skinner, who is a hemophilia patient himself, said the goal of the HemACTIVE study is to better understand how hemophilia affects patients’ lives.
“We wanted to understand the limitations, challenges, and compromises of individuals living with hemophilia,” Mr. Skinner said. “What has motivated them or prevented them from living more full, active lives doing the kind of work, leisure, and social activities that those without hemophilia do? Is it treatment choice, is it satisfaction with treatment, is it fear?
“We wanted to do a comprehensive study that really looked at the intersection of treatment adherence and satisfaction, the emotional components that relate to those decisions, and the challenges and compromises so that we could better identify what we need to consider as patients think about either changing their therapy or changing their treatment regimen on existing therapy.”
Previous results from the HemACTIVE study showed that, although activity levels differed among hemophilia patients, all patients surveyed wanted greater activity levels, better protection from bleeding, better pain relief, and less-frequent infusions (EAHAD 2019, Abstract P084). In addition, patients who used factor VIII products with an extended half-life were more active and more likely to adhere to their prescribed treatment (ISTH 2019, Abstract PB0210).
The results reported at EAHAD 2020 focus on patients’ reasons for modifying physical activity. Patients and caregivers completed a screening phone interview, followed by a 25-minute, web-based questionnaire on patient activity.
There were 275 respondents – 194 patients with hemophilia A and 81 caregivers – from Canada, France, Germany, Italy, and the United States. Patients had severe (61%) or moderate (39%) hemophilia A, and most (67%) were receiving prophylaxis.
Most patients (70%) were “active” or “extremely/very active,” 77% of patients adjusted their activities because of their hemophilia, and nearly half of patients stopped activities because of their disease.
Fear drives adjustments in activity
Patients were sometimes more likely to adjust their activities based on fear of experiencing an event, as opposed to previously experiencing that event.
Specifically, 44% of patients adjusted their activities due to fear of joint damage, compared with 36% of patients who made adjustments because of past significant joint damage.
Similarly, 41% of patients adjusted activities due to fear of breakthrough bleeds, compared with 36% of patients who made adjustments because of past experience with bleeds and 25% who made adjustments because of significant past bleeds.
On the other hand, a similar percentage of patients adjusted activities because of past experience with pain (43%) and fear of pain (41%). And a similar percentage of patients adjusted activities because of existing joint damage restrictions (35%) and fear of joint deterioration (32%).
Past experience prompts discontinuation of activity
Overall, 47% of patients said anxiety was the most common emotional reason for stopping physical activities. However, patients were consistently more likely to stop activities because of past experience rather than fear or anxiety.
Specifically, 50% of patients stopped activities because of significant past joint damage, 46% stopped because of developing joint problems, and 38% stopped due to fear of joint damage.
More patients stopped activities because of significant past bleeds (41%) rather than fear of breakthrough bleeds (26%). More patients stopped activities because they developed chronic pain (38%) rather than fear of pain (less than 15%). And more patients stopped activities because of existing joint damage restrictions (62%) rather than fear of joint damage (34%).
Applying results to practice: Changing the conversation
Ideally, these findings would be used to promote individualized treatment of hemophilia driven by patients’ goals, Mr. Skinner said. By better understanding patients’ feelings and motivations, clinicians may devise more personalized treatment regimens that align with patients’ goals and improve their quality of life.
Rather than adjusting treatment based only on “hard metrics” such as bleeding events, “we need to take a more holistic approach to looking at outcomes that are more important to patients,” Mr. Skinner said. This type of approach is particularly important to Mr. Skinner as someone who has severe hemophilia A.
“Because hemophilia is a life-long disease, and you’re born with it, you make conscious or unconscious adaptations throughout your life,” he explained. “Your expectations or aspirations adjust to what you’ve been told you can or cannot do because of your hemophilia. The choices I made for my career, where I live, the type of vacations I go on, the type of sports I participate in have all been limited over the course of time, which has meant that I’ve made compromises. There are a lot of individuals with hemophilia who are making decisions that are not what their life goals are.
“What this research helps me understand is that we can change the conversation and build it around an individual patient and understand what their aspirations are. If a clinician understands what I’m wanting to achieve in life … we can build a treatment regime around helping me achieve those goals. That is known to improve adherence.
“The goal, really, is to have hemophilia as a secondary consideration. Instead of saying: ‘You have hemophilia, so these are the options available to you,’ you can say, ‘what is it that you would like to achieve, and then we’ll figure out how your treatment for hemophilia can be adjusted to help you achieve those goals.’ It may sound like a nuance, but it really is reversing the conversation. The goal setting first versus your disease comes first.”
The HemACTIVE study was supported by Bayer. Mr. Skinner disclosed relationships with Bayer and other pharmaceutical companies.
SOURCE: Skinner M et al. EAHAD 2020, Abstract P304.
REPORTING FROM EAHAD 2020
Coronavirus outbreak: Putting it into perspective
References
- Centers for Disease Control and Prevention. 2019 novel coronavirus. https://www.cdc.gov/coronavirus/2019-nCoV/hcp/index.html. Last reviewed February 8, 2020. Accessed February 9, 2020.
- World Health Organization. Novel coronavirus (2019-nCoV) situation as of 10 February 2020. http://who.maps.arcgis.com/apps/opsdashboard/index.html#/c88e37cfc43b4ed3baf977d77e4a0667. Accessed February 9, 2020.
- Centers for Disease Control and Prevention. 2019 novel coronavirus: Evaluating and reporting persons under investigation (PUI). https://www.cdc.gov/coronavirus/2019-nCoV/hcp/clinical-criteria.html Last reviewed February 3, 2020. Accessed February 9, 2020.
- Centers for Disease Control and Prevention. Weekly US influenza surveillance report. https://www.cdc.gov/flu/weekly/index.htm. Last reviewed February 7, 2020. Accessed February 12, 2020.
References
- Centers for Disease Control and Prevention. 2019 novel coronavirus. https://www.cdc.gov/coronavirus/2019-nCoV/hcp/index.html. Last reviewed February 8, 2020. Accessed February 9, 2020.
- World Health Organization. Novel coronavirus (2019-nCoV) situation as of 10 February 2020. http://who.maps.arcgis.com/apps/opsdashboard/index.html#/c88e37cfc43b4ed3baf977d77e4a0667. Accessed February 9, 2020.
- Centers for Disease Control and Prevention. 2019 novel coronavirus: Evaluating and reporting persons under investigation (PUI). https://www.cdc.gov/coronavirus/2019-nCoV/hcp/clinical-criteria.html Last reviewed February 3, 2020. Accessed February 9, 2020.
- Centers for Disease Control and Prevention. Weekly US influenza surveillance report. https://www.cdc.gov/flu/weekly/index.htm. Last reviewed February 7, 2020. Accessed February 12, 2020.
References
- Centers for Disease Control and Prevention. 2019 novel coronavirus. https://www.cdc.gov/coronavirus/2019-nCoV/hcp/index.html. Last reviewed February 8, 2020. Accessed February 9, 2020.
- World Health Organization. Novel coronavirus (2019-nCoV) situation as of 10 February 2020. http://who.maps.arcgis.com/apps/opsdashboard/index.html#/c88e37cfc43b4ed3baf977d77e4a0667. Accessed February 9, 2020.
- Centers for Disease Control and Prevention. 2019 novel coronavirus: Evaluating and reporting persons under investigation (PUI). https://www.cdc.gov/coronavirus/2019-nCoV/hcp/clinical-criteria.html Last reviewed February 3, 2020. Accessed February 9, 2020.
- Centers for Disease Control and Prevention. Weekly US influenza surveillance report. https://www.cdc.gov/flu/weekly/index.htm. Last reviewed February 7, 2020. Accessed February 12, 2020.
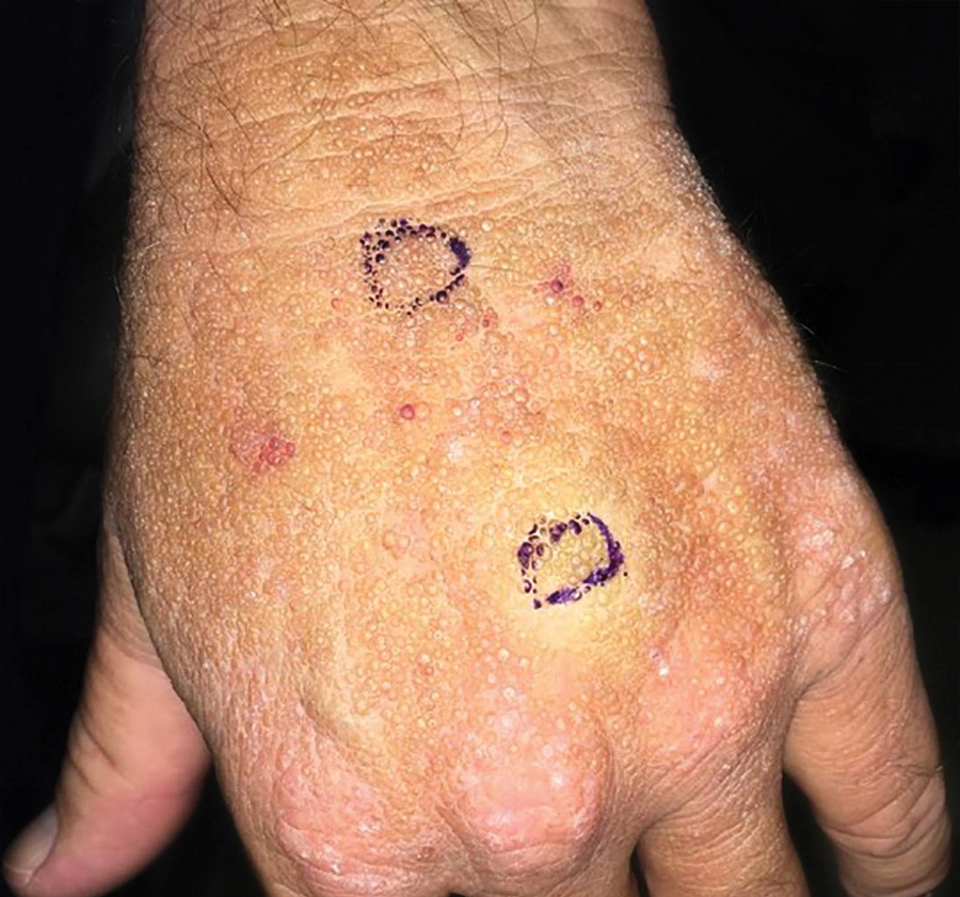
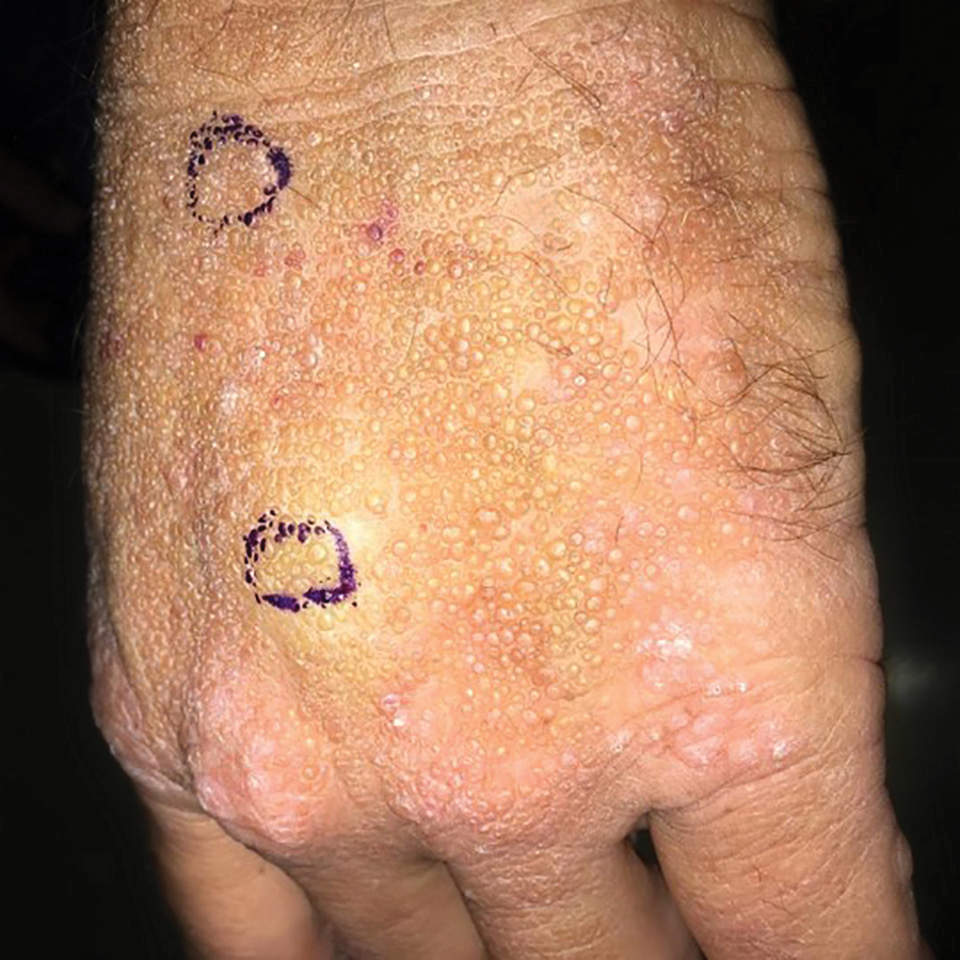
Smooth Papules on the Left Hand
The Diagnosis: Adult Colloid Milium
A 4-mm punch biopsy was performed and histopathologic evaluation revealed collections of amorphic eosinophilic material and fissures in the papillary dermis with sparing of the dermoepidermal junction, indicating adult colloid milium (Figure 1).
Adult colloid milium is an uncommon condition with grouped translucent to whitish papules that present on sun-exposed skin on the hands, face, neck, or ears in middle-aged adults.1 It has been associated with petrochemical exposure, tanning bed use, and excessive sun exposure. Our patient had a history of sun exposure, specifically to the left hand while driving. This condition is widely thought to be a result of photoinduced damage to elastic fibers and may potentially be a popular variant of severe solar elastosis.2 Due to vascular fragility, trauma to these locations often will result in hemorrhage into individual lesions, as observed in our patient (Figure 2).
Adult colloid milium is diagnosed clinically and may mimic lichen or systemic amyloidosis, syringomas, lipoid proteinosis, molluscum contagiosum, steatocystoma multiplex, and sarcoidosis.2
Biopsy often is helpful in determining the diagnosis. Histopathology reveals amorphous eosinophilic deposits with fissures in the papillary dermis. These deposits are thought to be remnants of degenerated elastic fibers. Stains often are helpful, as the deposits are weakly apple-green birefringent on Congo red stain and are periodic acid-Schiff and thioflavin T positive. Laminin and type IV collagen stains are negative with adult colloid milium but are positive with amyloidosis and lipoid proteinosis.3 Electron microscopy also may help distinguish between amyloidosis and adult colloid milium, as these conditions may have a similar histologic appearance.
Treatment has not proven to be consistently helpful, as cryotherapy and dermabrasion have been the mainstay of treatment, often with disappointing results.4 Laser treatment has been shown to be of some benefit in treating these lesions.2
- Touart DM, Sau P. Cutaneous deposition diseases. part I. J Am Acad Dermatol. 1998;39(2, pt 1):149-171.
- Pourrabbani S, Marra DE, Iwasaki J, et al. Colloid milium: a review and update. J Drugs Dermatol. 2007;6:293-296.
- Calonje JE, Brenn T, Lazar A, et al. McKee's Pathology of the Skin. 4th ed. Philadelphia, PA: Saunders; 2012.
- Netscher DT, Sharma S, Kinner BM, et al. Adult-type colloid milium of hands and face successfully treated with dermabrasion. South Med J. 1996;89:1004-1007.
The Diagnosis: Adult Colloid Milium
A 4-mm punch biopsy was performed and histopathologic evaluation revealed collections of amorphic eosinophilic material and fissures in the papillary dermis with sparing of the dermoepidermal junction, indicating adult colloid milium (Figure 1).
Adult colloid milium is an uncommon condition with grouped translucent to whitish papules that present on sun-exposed skin on the hands, face, neck, or ears in middle-aged adults.1 It has been associated with petrochemical exposure, tanning bed use, and excessive sun exposure. Our patient had a history of sun exposure, specifically to the left hand while driving. This condition is widely thought to be a result of photoinduced damage to elastic fibers and may potentially be a popular variant of severe solar elastosis.2 Due to vascular fragility, trauma to these locations often will result in hemorrhage into individual lesions, as observed in our patient (Figure 2).
Adult colloid milium is diagnosed clinically and may mimic lichen or systemic amyloidosis, syringomas, lipoid proteinosis, molluscum contagiosum, steatocystoma multiplex, and sarcoidosis.2
Biopsy often is helpful in determining the diagnosis. Histopathology reveals amorphous eosinophilic deposits with fissures in the papillary dermis. These deposits are thought to be remnants of degenerated elastic fibers. Stains often are helpful, as the deposits are weakly apple-green birefringent on Congo red stain and are periodic acid-Schiff and thioflavin T positive. Laminin and type IV collagen stains are negative with adult colloid milium but are positive with amyloidosis and lipoid proteinosis.3 Electron microscopy also may help distinguish between amyloidosis and adult colloid milium, as these conditions may have a similar histologic appearance.
Treatment has not proven to be consistently helpful, as cryotherapy and dermabrasion have been the mainstay of treatment, often with disappointing results.4 Laser treatment has been shown to be of some benefit in treating these lesions.2
The Diagnosis: Adult Colloid Milium
A 4-mm punch biopsy was performed and histopathologic evaluation revealed collections of amorphic eosinophilic material and fissures in the papillary dermis with sparing of the dermoepidermal junction, indicating adult colloid milium (Figure 1).
Adult colloid milium is an uncommon condition with grouped translucent to whitish papules that present on sun-exposed skin on the hands, face, neck, or ears in middle-aged adults.1 It has been associated with petrochemical exposure, tanning bed use, and excessive sun exposure. Our patient had a history of sun exposure, specifically to the left hand while driving. This condition is widely thought to be a result of photoinduced damage to elastic fibers and may potentially be a popular variant of severe solar elastosis.2 Due to vascular fragility, trauma to these locations often will result in hemorrhage into individual lesions, as observed in our patient (Figure 2).
Adult colloid milium is diagnosed clinically and may mimic lichen or systemic amyloidosis, syringomas, lipoid proteinosis, molluscum contagiosum, steatocystoma multiplex, and sarcoidosis.2
Biopsy often is helpful in determining the diagnosis. Histopathology reveals amorphous eosinophilic deposits with fissures in the papillary dermis. These deposits are thought to be remnants of degenerated elastic fibers. Stains often are helpful, as the deposits are weakly apple-green birefringent on Congo red stain and are periodic acid-Schiff and thioflavin T positive. Laminin and type IV collagen stains are negative with adult colloid milium but are positive with amyloidosis and lipoid proteinosis.3 Electron microscopy also may help distinguish between amyloidosis and adult colloid milium, as these conditions may have a similar histologic appearance.
Treatment has not proven to be consistently helpful, as cryotherapy and dermabrasion have been the mainstay of treatment, often with disappointing results.4 Laser treatment has been shown to be of some benefit in treating these lesions.2
- Touart DM, Sau P. Cutaneous deposition diseases. part I. J Am Acad Dermatol. 1998;39(2, pt 1):149-171.
- Pourrabbani S, Marra DE, Iwasaki J, et al. Colloid milium: a review and update. J Drugs Dermatol. 2007;6:293-296.
- Calonje JE, Brenn T, Lazar A, et al. McKee's Pathology of the Skin. 4th ed. Philadelphia, PA: Saunders; 2012.
- Netscher DT, Sharma S, Kinner BM, et al. Adult-type colloid milium of hands and face successfully treated with dermabrasion. South Med J. 1996;89:1004-1007.
- Touart DM, Sau P. Cutaneous deposition diseases. part I. J Am Acad Dermatol. 1998;39(2, pt 1):149-171.
- Pourrabbani S, Marra DE, Iwasaki J, et al. Colloid milium: a review and update. J Drugs Dermatol. 2007;6:293-296.
- Calonje JE, Brenn T, Lazar A, et al. McKee's Pathology of the Skin. 4th ed. Philadelphia, PA: Saunders; 2012.
- Netscher DT, Sharma S, Kinner BM, et al. Adult-type colloid milium of hands and face successfully treated with dermabrasion. South Med J. 1996;89:1004-1007.
A 41-year-old man presented to the outpatient dermatology clinic with multiple smooth papules on the left hand of 7 years' duration. The papules had been steadily increasing in number, and the patient reported that they were frequently symptomatic with a burning itching sensation. Physical examination revealed multiple 1- to 3-mm, dome-shaped, translucent to flesh-colored papules on the left hand with a few scattered bright red papules. No similar lesions were present on the right hand or elsewhere on the body. He had a history of hypertension but was otherwise healthy with no other chronic medical conditions.
When Should I Refer My CKD Patient to Nephrology?
Q) When should I refer patients with chronic kidney disease (CKD) to a nephrology specialist?
Nephrology specialists can be of particular assistance to primary care providers in treating patients who are at different stages of CKD.1 Nephrologists can help determine the etiology of CKD, recommend specific disease-related therapy, suggest treatments to delay disease progression in patients who have not responded to conventional therapies, recognize and treat for disease-related complications, and plan for renal replacement therapy.1
Indications for referral vary across guidelines but there is one commonality: Patients with a severely decreased estimated glomerular filtration rate (eGFR) of < 30 mL/min per 1.73 m2 require prompt referral to a nephrologist for comanaged care.1-4 Patients with CKD who have an eGFR at or below this threshold are likely at an advanced stage of disease and are therefore at greater risk for progression to end-stage renal disease (ESRD), which requires dialysis.1 Research shows that late referral to nephrology is associated with significantly higher rates of mortality within the first 90 days of dialysis.5 Furthermore, the Renal Physicians Association Clinical Practice Guideline states that patients with advanced CKD (stages 4 and 5) have a greater predisposition for quick progression to ESRD with multiple comorbid conditions and poor outcomes.6
Clinical outcomes can improve when referrals are made before patients with CKD register a low eGFR—but the appropriate threshold (or when to refer patients with a higher eGFR) is less clear.1 Based in part on practice guidelines,2,3,6,7 referral to a nephrologist or clinician with expertise in CKD should be considered for patients with CKD who meet 1 or more of the following criteria:
- Urine albumin-to-creatinine ratio > 300 mg/g (34 mg/mmoL), including nephrotic syndrome
- Hematuria that is not secondary to urologic conditions
- Inability to identify a presumed cause of CKD
- eGFR decline of > 30% in less than 4 months without an obvious explanation
- Difficult-to-manage complications, such as anemia requiring erythropoietin therapy or abnormalities of bone and mineral metabolism requiring phosphorus binders or vitamin D preparations
- Serum potassium > 5.5 mEq/L
- Difficult-to-manage drug complications
- Age < 18 y
- Resistant hypertension
- Recurrent or extensive nephrolithiasis
- Confirmed or presumed hereditary kidney disease (eg, polycystic kidney disease, Alport syndrome, or autosomal dominant interstitial kidney disease).1,2,4,7
These criteria can aid clinicians in deciding when a preemptive referral is needed to prevent advanced CKD stages and ESRD in their patients. Also, because patients with CKD can be at high risk for adverse cardiovascular outcomes, referral to cardiology (eg, for patients with complicated cardiovascular disease) should be considered.1–YTM
Yolanda Thompson-Martin, DNP, RN, ANP-C, FNKF
University Health Physicians/Truman Medical Center, Kansas City, Missouri
1. Levey AS, Inker LA. Definition and staging of chronic kidney disease in adults. UpToDate. www.uptodate.com/contents/definition-and-staging-of-chronic-kidney-disease-in-adults. Accessed January 29, 2020.
2. National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification and stratification. Am J of Kidney Dis. 2002;39(suppl 1):S1-S266.
3. Kidney Disease Outcomes Quality Initiative (K/DOQI). K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis. 2004;43(suppl 1):11-13.
4. Luxton G; Caring for Australasians with Renal Impairment. The CARI Guidelines. Timing of referral of chronic kidney disease patients to nephrology services (adult). Nephrology (Carlton). 2010;15(suppl 1):S2-S11.
5. Jungers P, Massy Z, Nguyen-Khoa T, et al. Longer duration of predialysis nephrological care is associated with improved long-term survival of dialysis patients. Nephrol Dial Transplant. 2001;16(12):2357-2364.
6. WK Bolton. Renal Physicians Association Clinical Practice Guidelines: appropriate patient preparation for renal replacement therapy: guide line number 3. J Am Soc Nephrol. 2003;14(5):1406-1410.
7. Kidney Disease Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guidelines for the evaluation and management of chronic kidney disease. Kidney Int. 2013;3(suppl):1-150.
Q) When should I refer patients with chronic kidney disease (CKD) to a nephrology specialist?
Nephrology specialists can be of particular assistance to primary care providers in treating patients who are at different stages of CKD.1 Nephrologists can help determine the etiology of CKD, recommend specific disease-related therapy, suggest treatments to delay disease progression in patients who have not responded to conventional therapies, recognize and treat for disease-related complications, and plan for renal replacement therapy.1
Indications for referral vary across guidelines but there is one commonality: Patients with a severely decreased estimated glomerular filtration rate (eGFR) of < 30 mL/min per 1.73 m2 require prompt referral to a nephrologist for comanaged care.1-4 Patients with CKD who have an eGFR at or below this threshold are likely at an advanced stage of disease and are therefore at greater risk for progression to end-stage renal disease (ESRD), which requires dialysis.1 Research shows that late referral to nephrology is associated with significantly higher rates of mortality within the first 90 days of dialysis.5 Furthermore, the Renal Physicians Association Clinical Practice Guideline states that patients with advanced CKD (stages 4 and 5) have a greater predisposition for quick progression to ESRD with multiple comorbid conditions and poor outcomes.6
Clinical outcomes can improve when referrals are made before patients with CKD register a low eGFR—but the appropriate threshold (or when to refer patients with a higher eGFR) is less clear.1 Based in part on practice guidelines,2,3,6,7 referral to a nephrologist or clinician with expertise in CKD should be considered for patients with CKD who meet 1 or more of the following criteria:
- Urine albumin-to-creatinine ratio > 300 mg/g (34 mg/mmoL), including nephrotic syndrome
- Hematuria that is not secondary to urologic conditions
- Inability to identify a presumed cause of CKD
- eGFR decline of > 30% in less than 4 months without an obvious explanation
- Difficult-to-manage complications, such as anemia requiring erythropoietin therapy or abnormalities of bone and mineral metabolism requiring phosphorus binders or vitamin D preparations
- Serum potassium > 5.5 mEq/L
- Difficult-to-manage drug complications
- Age < 18 y
- Resistant hypertension
- Recurrent or extensive nephrolithiasis
- Confirmed or presumed hereditary kidney disease (eg, polycystic kidney disease, Alport syndrome, or autosomal dominant interstitial kidney disease).1,2,4,7
These criteria can aid clinicians in deciding when a preemptive referral is needed to prevent advanced CKD stages and ESRD in their patients. Also, because patients with CKD can be at high risk for adverse cardiovascular outcomes, referral to cardiology (eg, for patients with complicated cardiovascular disease) should be considered.1–YTM
Yolanda Thompson-Martin, DNP, RN, ANP-C, FNKF
University Health Physicians/Truman Medical Center, Kansas City, Missouri
Q) When should I refer patients with chronic kidney disease (CKD) to a nephrology specialist?
Nephrology specialists can be of particular assistance to primary care providers in treating patients who are at different stages of CKD.1 Nephrologists can help determine the etiology of CKD, recommend specific disease-related therapy, suggest treatments to delay disease progression in patients who have not responded to conventional therapies, recognize and treat for disease-related complications, and plan for renal replacement therapy.1
Indications for referral vary across guidelines but there is one commonality: Patients with a severely decreased estimated glomerular filtration rate (eGFR) of < 30 mL/min per 1.73 m2 require prompt referral to a nephrologist for comanaged care.1-4 Patients with CKD who have an eGFR at or below this threshold are likely at an advanced stage of disease and are therefore at greater risk for progression to end-stage renal disease (ESRD), which requires dialysis.1 Research shows that late referral to nephrology is associated with significantly higher rates of mortality within the first 90 days of dialysis.5 Furthermore, the Renal Physicians Association Clinical Practice Guideline states that patients with advanced CKD (stages 4 and 5) have a greater predisposition for quick progression to ESRD with multiple comorbid conditions and poor outcomes.6
Clinical outcomes can improve when referrals are made before patients with CKD register a low eGFR—but the appropriate threshold (or when to refer patients with a higher eGFR) is less clear.1 Based in part on practice guidelines,2,3,6,7 referral to a nephrologist or clinician with expertise in CKD should be considered for patients with CKD who meet 1 or more of the following criteria:
- Urine albumin-to-creatinine ratio > 300 mg/g (34 mg/mmoL), including nephrotic syndrome
- Hematuria that is not secondary to urologic conditions
- Inability to identify a presumed cause of CKD
- eGFR decline of > 30% in less than 4 months without an obvious explanation
- Difficult-to-manage complications, such as anemia requiring erythropoietin therapy or abnormalities of bone and mineral metabolism requiring phosphorus binders or vitamin D preparations
- Serum potassium > 5.5 mEq/L
- Difficult-to-manage drug complications
- Age < 18 y
- Resistant hypertension
- Recurrent or extensive nephrolithiasis
- Confirmed or presumed hereditary kidney disease (eg, polycystic kidney disease, Alport syndrome, or autosomal dominant interstitial kidney disease).1,2,4,7
These criteria can aid clinicians in deciding when a preemptive referral is needed to prevent advanced CKD stages and ESRD in their patients. Also, because patients with CKD can be at high risk for adverse cardiovascular outcomes, referral to cardiology (eg, for patients with complicated cardiovascular disease) should be considered.1–YTM
Yolanda Thompson-Martin, DNP, RN, ANP-C, FNKF
University Health Physicians/Truman Medical Center, Kansas City, Missouri
1. Levey AS, Inker LA. Definition and staging of chronic kidney disease in adults. UpToDate. www.uptodate.com/contents/definition-and-staging-of-chronic-kidney-disease-in-adults. Accessed January 29, 2020.
2. National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification and stratification. Am J of Kidney Dis. 2002;39(suppl 1):S1-S266.
3. Kidney Disease Outcomes Quality Initiative (K/DOQI). K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis. 2004;43(suppl 1):11-13.
4. Luxton G; Caring for Australasians with Renal Impairment. The CARI Guidelines. Timing of referral of chronic kidney disease patients to nephrology services (adult). Nephrology (Carlton). 2010;15(suppl 1):S2-S11.
5. Jungers P, Massy Z, Nguyen-Khoa T, et al. Longer duration of predialysis nephrological care is associated with improved long-term survival of dialysis patients. Nephrol Dial Transplant. 2001;16(12):2357-2364.
6. WK Bolton. Renal Physicians Association Clinical Practice Guidelines: appropriate patient preparation for renal replacement therapy: guide line number 3. J Am Soc Nephrol. 2003;14(5):1406-1410.
7. Kidney Disease Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guidelines for the evaluation and management of chronic kidney disease. Kidney Int. 2013;3(suppl):1-150.
1. Levey AS, Inker LA. Definition and staging of chronic kidney disease in adults. UpToDate. www.uptodate.com/contents/definition-and-staging-of-chronic-kidney-disease-in-adults. Accessed January 29, 2020.
2. National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification and stratification. Am J of Kidney Dis. 2002;39(suppl 1):S1-S266.
3. Kidney Disease Outcomes Quality Initiative (K/DOQI). K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis. 2004;43(suppl 1):11-13.
4. Luxton G; Caring for Australasians with Renal Impairment. The CARI Guidelines. Timing of referral of chronic kidney disease patients to nephrology services (adult). Nephrology (Carlton). 2010;15(suppl 1):S2-S11.
5. Jungers P, Massy Z, Nguyen-Khoa T, et al. Longer duration of predialysis nephrological care is associated with improved long-term survival of dialysis patients. Nephrol Dial Transplant. 2001;16(12):2357-2364.
6. WK Bolton. Renal Physicians Association Clinical Practice Guidelines: appropriate patient preparation for renal replacement therapy: guide line number 3. J Am Soc Nephrol. 2003;14(5):1406-1410.
7. Kidney Disease Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guidelines for the evaluation and management of chronic kidney disease. Kidney Int. 2013;3(suppl):1-150.
Resurgence of black lung among U.S. coal miners
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention.
Advances in technology over the last century, as well as the exportation of many high exposure jobs, nearly eliminated lung diseases caused by occupational exposure to respirable dust (the pneumoconioses) in the United States. One such example of this near elimination is black lung, or coal workers' pneumoconiosis (CWP), following the 1969 Federal Coal Mine Health and Safety Act. The Act established permissible exposure limits to respirable dust, designed to prevent the most severe forms of CWP from occurring, and a national respiratory health screening program for underground coal miners. Between 1970 and the mid-1990s, disease prevalence plummeted from nearly 35% to less than 5% prevalence among longer tenured miners, and from 3% to less than 1% in miners with less than 10 years of mining tenure (Hall NB, et al. Curr Environ Health Rep. 2019;6[3]:137).
Many assumed that this was the last we'd hear of black lung - that the cases of disease existing in the 1990s were likely caused by exposures that occurred prior to the 1969 Act, and within a few years, no further cases would be detected. This appeared to be an entirely reasonable assumption in the 1990s given the 30 years of declining prevalence and the continuous technological advances designed to continue reductions in dust exposures. In fact, the precipitous decline in black lung was briefly viewed as a public health triumph, as the most severe forms appeared to be near eradication in the United States just 2 decades ago (Attfield MD, et al. Am J Public Health. 1992;82[7]:971; Attfield MD, et al. Am J Public Health. 1992;82[7]:964). However, what has since been observed is a strong and ongoing resurgence of the potentially deadly fibrotic interstitial disease starting in the early 2000s (Figure 1), with the most striking increase observed in the Central Appalachian states of Kentucky, Virginia, and West Virginia (Blackley DJ, et al. Am J Respir Crit Care Med. 2014;190[6]:708; Blackley DJ, et al. Am J Public Health. 2018;108[9]:1220).
Of great concern is the resurgence of complicated Black Lung (progressive massive fibrosis [PMF]), which is completely disabling and leads to premature mortality. The prevalence of PMF is higher today than when NIOSH started formally tracking the disease in the 1970s, especially among specific populations.
Since the mid-2000s, NIOSH and others have described the following(Hall NB, et al. Curr Environ Health Rep. 2019;6[3]:137):
· Increasing prevalence and severity of CWP both nationwide and specifically in Central Appalachia.
· Rapid progression of CWP.
· Increases in the frequency of lung transplantation for CWP.
· Severe disease among surface coal miners with no underground mining tenure.
· Increased severity of disease among former and retired miners.
· Hundreds of cases of PMF among coal miners seeking care at clinics in eastern Kentucky and southwestern Virginia.
· Increasing numbers of miners with PMF filing for federal black lung compensation.
· Radiologic and pathologic indications of increased respirable silica exposure among coal miners.
· Premature mortality in miners diagnosed with CWP.
· Underutilization of a secondary prevention worker removal program designed to reduce the exposure of miners with disease.
· Former miners with severe disease describing extreme pressure to operate. outside of applicable protective federal standards in order to increase productivity
In our surveillance work, we have talked to many miners who, after having months or years' worth of extensive workups for pneumonia, sarcoidosis, lung cancer, and/or diseases other than the pneumoconioses, have eventually learned that they actually had dust-induced lung disease attributable to their work. Additionally, through our evaluation of the transplantation data, it has become clear that dust-related lung disease is likely underreported or underrecognized among those receiving lung transplants. Finally, through analysis of mortality data, it is apparent that CWP is also underreported as a cause of death among miners with black lung. We mention these points to emphasize how important it is to document a full occupational history for proper diagnoses, early intervention, and improved public health information to inform primary and secondary disease prevention efforts.
Resources for clinicians
CWP is most commonly identified using plain posterior-anterior chest radiography and presence/severity of fibrotic change is described using an international standard established by the International Labour Office (International Labour Office. Guidelines for the use of the ILO international classification of radiographs of pneumoconioses. Geneva: International Labour Office; 2011). In the United States, NIOSH operates the B Reader Training and Certification Program, which offers a free self-study syllabus, https://www.cdc.gov/niosh/topics/chestradiography/breader.html, and in-person training courses on occasion, to assist physicians in learning and demonstrating continuous competency in classifying chest radiographs of dust-exposed workers according to the ILO Standards (Halldin CN, et al. J Occup Environ Med. 2019;61[12]:1045). The B Reader Program and ILO Standards are currently undergoing a decade-long revision process where both will feature digitally acquired chest radiograph images. This process should be fully complete in the following months.
To educate miners, mine operators, and others about the risks of respirable dust, NIOSH produced an educational video, Faces of Black Lung, in 2008 that featured two miners in their 50s and 60s who had complicated Black Lung. Because of the resurgence of disease and particularly severe cases being identified among much younger miners, NIOSH recently released an updated version of the video, Faces of Black Lung II, where three Kentucky underground miners, ages 39, 42, and 48, describe the incredible disability and quality of life lost due to a disease caused by gross overexposure of respirable coal mine dust.
Unfortunately, the 42-year-old miner died from complications stemming from Black Lung less than a year after filming his part in the video, and the other two miners have been advised to be evaluated for lung transplantation. We hope that these men's stories will help younger miners relate to the risks of respirable coal mine dust and help others understand the severity of disease as all three of these men struggled to breathe just describing their day to day tasks.
Parting message
No one should ever have to consider a lung transplant at the age of 40 because they went to work attempting to provide for their family. No one should ever be faced with end-of-life planning while their kids are in grade school because of a disease they acquired at work. Respirable coal mine dust is the only cause of black lung, and the coal mining industry has the necessary technology and tools to prevent harmful exposures to respirable dust, and, together with miners, must successfully and consistently implement dust suppression controls. There is no cure for black lung; it's irreversible and can be first recognized and continue to progress even after a miner has left exposure. However, early identification and appropriate intervention can prevent progression to the most disabling manifestations. The role of the clinician is to be part of the early identification of black lung through including CWP in the differential diagnosis for unusual or unexpected respiratory illness in otherwise healthy primarily working aged miners. The public health community must continue to monitor disease prevalence in working populations and implement policies and recommendations to support the efforts of those on the frontline - the miners, industry, and health-care workers.
The Energy Information Agency projects that coal will continue to be a substantial source of U.S. energy production and consumption well into the mid- to late-century. Unfortunately, Black Lung has made a resurgence and is killing miners, and each of us has a role to play in eliminating it once and for all. We will continue to carry out our mandate to screen working coal miners for respiratory disease; however, given the continued contraction of the coal mining industry, it's much more likely for cases of disease to be recognized in the clinic setting. Therefore, we reiterate our previous plea to clinicians: when identifying an individual with interstitial fibrosis consider their full occupational history.
Dr. Halldin and Dr. Laney are from the Surveillance Branch, Respiratory Health Division, National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention, Morgantown, WV.
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention.
Advances in technology over the last century, as well as the exportation of many high exposure jobs, nearly eliminated lung diseases caused by occupational exposure to respirable dust (the pneumoconioses) in the United States. One such example of this near elimination is black lung, or coal workers' pneumoconiosis (CWP), following the 1969 Federal Coal Mine Health and Safety Act. The Act established permissible exposure limits to respirable dust, designed to prevent the most severe forms of CWP from occurring, and a national respiratory health screening program for underground coal miners. Between 1970 and the mid-1990s, disease prevalence plummeted from nearly 35% to less than 5% prevalence among longer tenured miners, and from 3% to less than 1% in miners with less than 10 years of mining tenure (Hall NB, et al. Curr Environ Health Rep. 2019;6[3]:137).
Many assumed that this was the last we'd hear of black lung - that the cases of disease existing in the 1990s were likely caused by exposures that occurred prior to the 1969 Act, and within a few years, no further cases would be detected. This appeared to be an entirely reasonable assumption in the 1990s given the 30 years of declining prevalence and the continuous technological advances designed to continue reductions in dust exposures. In fact, the precipitous decline in black lung was briefly viewed as a public health triumph, as the most severe forms appeared to be near eradication in the United States just 2 decades ago (Attfield MD, et al. Am J Public Health. 1992;82[7]:971; Attfield MD, et al. Am J Public Health. 1992;82[7]:964). However, what has since been observed is a strong and ongoing resurgence of the potentially deadly fibrotic interstitial disease starting in the early 2000s (Figure 1), with the most striking increase observed in the Central Appalachian states of Kentucky, Virginia, and West Virginia (Blackley DJ, et al. Am J Respir Crit Care Med. 2014;190[6]:708; Blackley DJ, et al. Am J Public Health. 2018;108[9]:1220).
Of great concern is the resurgence of complicated Black Lung (progressive massive fibrosis [PMF]), which is completely disabling and leads to premature mortality. The prevalence of PMF is higher today than when NIOSH started formally tracking the disease in the 1970s, especially among specific populations.
Since the mid-2000s, NIOSH and others have described the following(Hall NB, et al. Curr Environ Health Rep. 2019;6[3]:137):
· Increasing prevalence and severity of CWP both nationwide and specifically in Central Appalachia.
· Rapid progression of CWP.
· Increases in the frequency of lung transplantation for CWP.
· Severe disease among surface coal miners with no underground mining tenure.
· Increased severity of disease among former and retired miners.
· Hundreds of cases of PMF among coal miners seeking care at clinics in eastern Kentucky and southwestern Virginia.
· Increasing numbers of miners with PMF filing for federal black lung compensation.
· Radiologic and pathologic indications of increased respirable silica exposure among coal miners.
· Premature mortality in miners diagnosed with CWP.
· Underutilization of a secondary prevention worker removal program designed to reduce the exposure of miners with disease.
· Former miners with severe disease describing extreme pressure to operate. outside of applicable protective federal standards in order to increase productivity
In our surveillance work, we have talked to many miners who, after having months or years' worth of extensive workups for pneumonia, sarcoidosis, lung cancer, and/or diseases other than the pneumoconioses, have eventually learned that they actually had dust-induced lung disease attributable to their work. Additionally, through our evaluation of the transplantation data, it has become clear that dust-related lung disease is likely underreported or underrecognized among those receiving lung transplants. Finally, through analysis of mortality data, it is apparent that CWP is also underreported as a cause of death among miners with black lung. We mention these points to emphasize how important it is to document a full occupational history for proper diagnoses, early intervention, and improved public health information to inform primary and secondary disease prevention efforts.
Resources for clinicians
CWP is most commonly identified using plain posterior-anterior chest radiography and presence/severity of fibrotic change is described using an international standard established by the International Labour Office (International Labour Office. Guidelines for the use of the ILO international classification of radiographs of pneumoconioses. Geneva: International Labour Office; 2011). In the United States, NIOSH operates the B Reader Training and Certification Program, which offers a free self-study syllabus, https://www.cdc.gov/niosh/topics/chestradiography/breader.html, and in-person training courses on occasion, to assist physicians in learning and demonstrating continuous competency in classifying chest radiographs of dust-exposed workers according to the ILO Standards (Halldin CN, et al. J Occup Environ Med. 2019;61[12]:1045). The B Reader Program and ILO Standards are currently undergoing a decade-long revision process where both will feature digitally acquired chest radiograph images. This process should be fully complete in the following months.
To educate miners, mine operators, and others about the risks of respirable dust, NIOSH produced an educational video, Faces of Black Lung, in 2008 that featured two miners in their 50s and 60s who had complicated Black Lung. Because of the resurgence of disease and particularly severe cases being identified among much younger miners, NIOSH recently released an updated version of the video, Faces of Black Lung II, where three Kentucky underground miners, ages 39, 42, and 48, describe the incredible disability and quality of life lost due to a disease caused by gross overexposure of respirable coal mine dust.
Unfortunately, the 42-year-old miner died from complications stemming from Black Lung less than a year after filming his part in the video, and the other two miners have been advised to be evaluated for lung transplantation. We hope that these men's stories will help younger miners relate to the risks of respirable coal mine dust and help others understand the severity of disease as all three of these men struggled to breathe just describing their day to day tasks.
Parting message
No one should ever have to consider a lung transplant at the age of 40 because they went to work attempting to provide for their family. No one should ever be faced with end-of-life planning while their kids are in grade school because of a disease they acquired at work. Respirable coal mine dust is the only cause of black lung, and the coal mining industry has the necessary technology and tools to prevent harmful exposures to respirable dust, and, together with miners, must successfully and consistently implement dust suppression controls. There is no cure for black lung; it's irreversible and can be first recognized and continue to progress even after a miner has left exposure. However, early identification and appropriate intervention can prevent progression to the most disabling manifestations. The role of the clinician is to be part of the early identification of black lung through including CWP in the differential diagnosis for unusual or unexpected respiratory illness in otherwise healthy primarily working aged miners. The public health community must continue to monitor disease prevalence in working populations and implement policies and recommendations to support the efforts of those on the frontline - the miners, industry, and health-care workers.
The Energy Information Agency projects that coal will continue to be a substantial source of U.S. energy production and consumption well into the mid- to late-century. Unfortunately, Black Lung has made a resurgence and is killing miners, and each of us has a role to play in eliminating it once and for all. We will continue to carry out our mandate to screen working coal miners for respiratory disease; however, given the continued contraction of the coal mining industry, it's much more likely for cases of disease to be recognized in the clinic setting. Therefore, we reiterate our previous plea to clinicians: when identifying an individual with interstitial fibrosis consider their full occupational history.
Dr. Halldin and Dr. Laney are from the Surveillance Branch, Respiratory Health Division, National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention, Morgantown, WV.
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention.
Advances in technology over the last century, as well as the exportation of many high exposure jobs, nearly eliminated lung diseases caused by occupational exposure to respirable dust (the pneumoconioses) in the United States. One such example of this near elimination is black lung, or coal workers' pneumoconiosis (CWP), following the 1969 Federal Coal Mine Health and Safety Act. The Act established permissible exposure limits to respirable dust, designed to prevent the most severe forms of CWP from occurring, and a national respiratory health screening program for underground coal miners. Between 1970 and the mid-1990s, disease prevalence plummeted from nearly 35% to less than 5% prevalence among longer tenured miners, and from 3% to less than 1% in miners with less than 10 years of mining tenure (Hall NB, et al. Curr Environ Health Rep. 2019;6[3]:137).
Many assumed that this was the last we'd hear of black lung - that the cases of disease existing in the 1990s were likely caused by exposures that occurred prior to the 1969 Act, and within a few years, no further cases would be detected. This appeared to be an entirely reasonable assumption in the 1990s given the 30 years of declining prevalence and the continuous technological advances designed to continue reductions in dust exposures. In fact, the precipitous decline in black lung was briefly viewed as a public health triumph, as the most severe forms appeared to be near eradication in the United States just 2 decades ago (Attfield MD, et al. Am J Public Health. 1992;82[7]:971; Attfield MD, et al. Am J Public Health. 1992;82[7]:964). However, what has since been observed is a strong and ongoing resurgence of the potentially deadly fibrotic interstitial disease starting in the early 2000s (Figure 1), with the most striking increase observed in the Central Appalachian states of Kentucky, Virginia, and West Virginia (Blackley DJ, et al. Am J Respir Crit Care Med. 2014;190[6]:708; Blackley DJ, et al. Am J Public Health. 2018;108[9]:1220).
Of great concern is the resurgence of complicated Black Lung (progressive massive fibrosis [PMF]), which is completely disabling and leads to premature mortality. The prevalence of PMF is higher today than when NIOSH started formally tracking the disease in the 1970s, especially among specific populations.
Since the mid-2000s, NIOSH and others have described the following(Hall NB, et al. Curr Environ Health Rep. 2019;6[3]:137):
· Increasing prevalence and severity of CWP both nationwide and specifically in Central Appalachia.
· Rapid progression of CWP.
· Increases in the frequency of lung transplantation for CWP.
· Severe disease among surface coal miners with no underground mining tenure.
· Increased severity of disease among former and retired miners.
· Hundreds of cases of PMF among coal miners seeking care at clinics in eastern Kentucky and southwestern Virginia.
· Increasing numbers of miners with PMF filing for federal black lung compensation.
· Radiologic and pathologic indications of increased respirable silica exposure among coal miners.
· Premature mortality in miners diagnosed with CWP.
· Underutilization of a secondary prevention worker removal program designed to reduce the exposure of miners with disease.
· Former miners with severe disease describing extreme pressure to operate. outside of applicable protective federal standards in order to increase productivity
In our surveillance work, we have talked to many miners who, after having months or years' worth of extensive workups for pneumonia, sarcoidosis, lung cancer, and/or diseases other than the pneumoconioses, have eventually learned that they actually had dust-induced lung disease attributable to their work. Additionally, through our evaluation of the transplantation data, it has become clear that dust-related lung disease is likely underreported or underrecognized among those receiving lung transplants. Finally, through analysis of mortality data, it is apparent that CWP is also underreported as a cause of death among miners with black lung. We mention these points to emphasize how important it is to document a full occupational history for proper diagnoses, early intervention, and improved public health information to inform primary and secondary disease prevention efforts.
Resources for clinicians
CWP is most commonly identified using plain posterior-anterior chest radiography and presence/severity of fibrotic change is described using an international standard established by the International Labour Office (International Labour Office. Guidelines for the use of the ILO international classification of radiographs of pneumoconioses. Geneva: International Labour Office; 2011). In the United States, NIOSH operates the B Reader Training and Certification Program, which offers a free self-study syllabus, https://www.cdc.gov/niosh/topics/chestradiography/breader.html, and in-person training courses on occasion, to assist physicians in learning and demonstrating continuous competency in classifying chest radiographs of dust-exposed workers according to the ILO Standards (Halldin CN, et al. J Occup Environ Med. 2019;61[12]:1045). The B Reader Program and ILO Standards are currently undergoing a decade-long revision process where both will feature digitally acquired chest radiograph images. This process should be fully complete in the following months.
To educate miners, mine operators, and others about the risks of respirable dust, NIOSH produced an educational video, Faces of Black Lung, in 2008 that featured two miners in their 50s and 60s who had complicated Black Lung. Because of the resurgence of disease and particularly severe cases being identified among much younger miners, NIOSH recently released an updated version of the video, Faces of Black Lung II, where three Kentucky underground miners, ages 39, 42, and 48, describe the incredible disability and quality of life lost due to a disease caused by gross overexposure of respirable coal mine dust.
Unfortunately, the 42-year-old miner died from complications stemming from Black Lung less than a year after filming his part in the video, and the other two miners have been advised to be evaluated for lung transplantation. We hope that these men's stories will help younger miners relate to the risks of respirable coal mine dust and help others understand the severity of disease as all three of these men struggled to breathe just describing their day to day tasks.
Parting message
No one should ever have to consider a lung transplant at the age of 40 because they went to work attempting to provide for their family. No one should ever be faced with end-of-life planning while their kids are in grade school because of a disease they acquired at work. Respirable coal mine dust is the only cause of black lung, and the coal mining industry has the necessary technology and tools to prevent harmful exposures to respirable dust, and, together with miners, must successfully and consistently implement dust suppression controls. There is no cure for black lung; it's irreversible and can be first recognized and continue to progress even after a miner has left exposure. However, early identification and appropriate intervention can prevent progression to the most disabling manifestations. The role of the clinician is to be part of the early identification of black lung through including CWP in the differential diagnosis for unusual or unexpected respiratory illness in otherwise healthy primarily working aged miners. The public health community must continue to monitor disease prevalence in working populations and implement policies and recommendations to support the efforts of those on the frontline - the miners, industry, and health-care workers.
The Energy Information Agency projects that coal will continue to be a substantial source of U.S. energy production and consumption well into the mid- to late-century. Unfortunately, Black Lung has made a resurgence and is killing miners, and each of us has a role to play in eliminating it once and for all. We will continue to carry out our mandate to screen working coal miners for respiratory disease; however, given the continued contraction of the coal mining industry, it's much more likely for cases of disease to be recognized in the clinic setting. Therefore, we reiterate our previous plea to clinicians: when identifying an individual with interstitial fibrosis consider their full occupational history.
Dr. Halldin and Dr. Laney are from the Surveillance Branch, Respiratory Health Division, National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention, Morgantown, WV.
This month in the journal CHEST®
Editor’s picks
CHEST Reviews
Critically ill patients with the HIV: 30 years later. By Dr. E. Azoulay, et al.
Phenotypic subtypes of obstructive sleep apnea: a challenge and opportunity for precision medicine. By Drs. A. Zinchuk and H. K. Yaggi.
Basic primer for finances in academic adult and pediatric pulmonary divisions. By Dr. L. Schnapp, et al.
Original research
Eligibility for lung volume reduction surgery in chronic obstructive pulmonary disease patients identified in a UK primary care setting. By Dr. H. Whittaker, et al.
Early life exposure to oral antibiotics and lung function into early adulthood. By Dr. K. dos Santos, et al.
Editor’s picks
Editor’s picks
CHEST Reviews
Critically ill patients with the HIV: 30 years later. By Dr. E. Azoulay, et al.
Phenotypic subtypes of obstructive sleep apnea: a challenge and opportunity for precision medicine. By Drs. A. Zinchuk and H. K. Yaggi.
Basic primer for finances in academic adult and pediatric pulmonary divisions. By Dr. L. Schnapp, et al.
Original research
Eligibility for lung volume reduction surgery in chronic obstructive pulmonary disease patients identified in a UK primary care setting. By Dr. H. Whittaker, et al.
Early life exposure to oral antibiotics and lung function into early adulthood. By Dr. K. dos Santos, et al.
CHEST Reviews
Critically ill patients with the HIV: 30 years later. By Dr. E. Azoulay, et al.
Phenotypic subtypes of obstructive sleep apnea: a challenge and opportunity for precision medicine. By Drs. A. Zinchuk and H. K. Yaggi.
Basic primer for finances in academic adult and pediatric pulmonary divisions. By Dr. L. Schnapp, et al.
Original research
Eligibility for lung volume reduction surgery in chronic obstructive pulmonary disease patients identified in a UK primary care setting. By Dr. H. Whittaker, et al.
Early life exposure to oral antibiotics and lung function into early adulthood. By Dr. K. dos Santos, et al.
Meet the FISH Bowl finalists
CHEST 2019 marked the inaugural FISH Bowl competition for attendees. Inspired by Shark Tank, our kinder, gentler, yet still competitive and cutting-edge FISH Bowl (Furthering Innovation and Science for Health) featured CHEST members disrupting our beliefs about how clinical care and education are performed. As health-care providers, they presented innovative ideas pertaining to education and clinical disease for pulmonary, critical care, and sleep medicine. Six finalists were chosen from dozens of submissions, and three emerged winners! In this new Meet the FISH Bowl Finalists series, CHEST introduces you to many of them – including Education Category Finalist Dr. Bhavani.
Name: Siva Bhavani
Institution: University of Chicago
Position: Pulmonary Critical Care Fellow
Title: Quizomics
Brief summary: Quizomics is a cutting-edge mobile app that hosts trivia competitions for medical conferences. Quizomics is unlike any medical trivia competition you have ever seen, because the Quizomics app can host 20,000 medical professionals simultaneously competing in the world’s largest medical trivia competition. Physicians compete among thousands of peers in their respective specialties to prepare for boards, obtain CME, and gain recognition in their fields as they fight their way to the top of the leaderboard!
1. What inspired your innovation? The average person checks their phone every 12 minutes, and this is no different at medical conferences. Whether you are in line for coffee, looking around at posters, or listening to a lecture - very little time passes before you are again checking your phone. The natural engagement we have with our phones can be leveraged for educational purposes by introducing gamified medical education platforms like Quizomics. I was inspired because the future of the medical conference demands digital engagement, gamified education, and large-scale social interaction. There is currently no platform that offers these services to prepare medical conferences for the digital education revolution that is coming.
2. Who do you think can benefit most from it, and why? The highest benefit is going to be to the physicians who are tired of the traditional CME options. Quizomics provides a high quality entertaining and educational platform for physicians to get CME while engaging and interacting with their peers. Further, physicians preparing for boards will find Quizomics an engaging alternative to the traditional textbooks. Finally, medical conferences will find that Quizomics can increase engagement, education, and attendance.
3. What do you see as challenges to your innovation gaining widespread acceptance? How can they be overcome? Content creation (trivia questions and explanations) is the biggest challenge to Quizomics. To overcome this, we plan to partner with tech-forward medical organizations that have high quality question banks in order to provide physicians with top-notch gamified education.
4. Why was it meaningful for you to emerge as a finalist in FISH Bowl 2019? FISH Bowl was an amazing opportunity to present Quizomics to others in the pulmonary/critical care specialty. Further, it was an opportunity to get direct feedback from leading educators in the field, and much of the resulting feedback has been incorporated into Quizomics.
5. What future do you envision for your innovation beyond FISH Bowl 2019? Quizomics is launching at a national neurosurgery board review course this winter. Following this pilot launch, Quizomics is scheduled for roll-out at Chicago area internal medicine residency programs through the summer of 2020. You can expect to see Quizomics at national conferences by 2021!
CHEST 2019 marked the inaugural FISH Bowl competition for attendees. Inspired by Shark Tank, our kinder, gentler, yet still competitive and cutting-edge FISH Bowl (Furthering Innovation and Science for Health) featured CHEST members disrupting our beliefs about how clinical care and education are performed. As health-care providers, they presented innovative ideas pertaining to education and clinical disease for pulmonary, critical care, and sleep medicine. Six finalists were chosen from dozens of submissions, and three emerged winners! In this new Meet the FISH Bowl Finalists series, CHEST introduces you to many of them – including Education Category Finalist Dr. Bhavani.
Name: Siva Bhavani
Institution: University of Chicago
Position: Pulmonary Critical Care Fellow
Title: Quizomics
Brief summary: Quizomics is a cutting-edge mobile app that hosts trivia competitions for medical conferences. Quizomics is unlike any medical trivia competition you have ever seen, because the Quizomics app can host 20,000 medical professionals simultaneously competing in the world’s largest medical trivia competition. Physicians compete among thousands of peers in their respective specialties to prepare for boards, obtain CME, and gain recognition in their fields as they fight their way to the top of the leaderboard!
1. What inspired your innovation? The average person checks their phone every 12 minutes, and this is no different at medical conferences. Whether you are in line for coffee, looking around at posters, or listening to a lecture - very little time passes before you are again checking your phone. The natural engagement we have with our phones can be leveraged for educational purposes by introducing gamified medical education platforms like Quizomics. I was inspired because the future of the medical conference demands digital engagement, gamified education, and large-scale social interaction. There is currently no platform that offers these services to prepare medical conferences for the digital education revolution that is coming.
2. Who do you think can benefit most from it, and why? The highest benefit is going to be to the physicians who are tired of the traditional CME options. Quizomics provides a high quality entertaining and educational platform for physicians to get CME while engaging and interacting with their peers. Further, physicians preparing for boards will find Quizomics an engaging alternative to the traditional textbooks. Finally, medical conferences will find that Quizomics can increase engagement, education, and attendance.
3. What do you see as challenges to your innovation gaining widespread acceptance? How can they be overcome? Content creation (trivia questions and explanations) is the biggest challenge to Quizomics. To overcome this, we plan to partner with tech-forward medical organizations that have high quality question banks in order to provide physicians with top-notch gamified education.
4. Why was it meaningful for you to emerge as a finalist in FISH Bowl 2019? FISH Bowl was an amazing opportunity to present Quizomics to others in the pulmonary/critical care specialty. Further, it was an opportunity to get direct feedback from leading educators in the field, and much of the resulting feedback has been incorporated into Quizomics.
5. What future do you envision for your innovation beyond FISH Bowl 2019? Quizomics is launching at a national neurosurgery board review course this winter. Following this pilot launch, Quizomics is scheduled for roll-out at Chicago area internal medicine residency programs through the summer of 2020. You can expect to see Quizomics at national conferences by 2021!
CHEST 2019 marked the inaugural FISH Bowl competition for attendees. Inspired by Shark Tank, our kinder, gentler, yet still competitive and cutting-edge FISH Bowl (Furthering Innovation and Science for Health) featured CHEST members disrupting our beliefs about how clinical care and education are performed. As health-care providers, they presented innovative ideas pertaining to education and clinical disease for pulmonary, critical care, and sleep medicine. Six finalists were chosen from dozens of submissions, and three emerged winners! In this new Meet the FISH Bowl Finalists series, CHEST introduces you to many of them – including Education Category Finalist Dr. Bhavani.
Name: Siva Bhavani
Institution: University of Chicago
Position: Pulmonary Critical Care Fellow
Title: Quizomics
Brief summary: Quizomics is a cutting-edge mobile app that hosts trivia competitions for medical conferences. Quizomics is unlike any medical trivia competition you have ever seen, because the Quizomics app can host 20,000 medical professionals simultaneously competing in the world’s largest medical trivia competition. Physicians compete among thousands of peers in their respective specialties to prepare for boards, obtain CME, and gain recognition in their fields as they fight their way to the top of the leaderboard!
1. What inspired your innovation? The average person checks their phone every 12 minutes, and this is no different at medical conferences. Whether you are in line for coffee, looking around at posters, or listening to a lecture - very little time passes before you are again checking your phone. The natural engagement we have with our phones can be leveraged for educational purposes by introducing gamified medical education platforms like Quizomics. I was inspired because the future of the medical conference demands digital engagement, gamified education, and large-scale social interaction. There is currently no platform that offers these services to prepare medical conferences for the digital education revolution that is coming.
2. Who do you think can benefit most from it, and why? The highest benefit is going to be to the physicians who are tired of the traditional CME options. Quizomics provides a high quality entertaining and educational platform for physicians to get CME while engaging and interacting with their peers. Further, physicians preparing for boards will find Quizomics an engaging alternative to the traditional textbooks. Finally, medical conferences will find that Quizomics can increase engagement, education, and attendance.
3. What do you see as challenges to your innovation gaining widespread acceptance? How can they be overcome? Content creation (trivia questions and explanations) is the biggest challenge to Quizomics. To overcome this, we plan to partner with tech-forward medical organizations that have high quality question banks in order to provide physicians with top-notch gamified education.
4. Why was it meaningful for you to emerge as a finalist in FISH Bowl 2019? FISH Bowl was an amazing opportunity to present Quizomics to others in the pulmonary/critical care specialty. Further, it was an opportunity to get direct feedback from leading educators in the field, and much of the resulting feedback has been incorporated into Quizomics.
5. What future do you envision for your innovation beyond FISH Bowl 2019? Quizomics is launching at a national neurosurgery board review course this winter. Following this pilot launch, Quizomics is scheduled for roll-out at Chicago area internal medicine residency programs through the summer of 2020. You can expect to see Quizomics at national conferences by 2021!
CHEST Foundation Casino Night promises fun for a good cause
Keeping the momentum from our first-ever CHEST Foundation Reception and Casino Night at CHEST 2019, where champions in attendance raised more than $35,000 for pulmonary fibrosis research, the CHEST Foundation continues our long-standing partnership with the Feldman Family Foundation and invites you to the 7th Annual Irv Feldman Texas Hold ‘Em Annual Tournament & Casino Night!
Funds raised at the event support the CHEST Foundation’s mission-based programming and directly impact patients living with pulmonary fibrosis by providing them with access to chest medicine experts; assistance in securing medication and portable oxygen; and empowering the patients and their clinicians to better manage their disease.
Join us at 6:00 PM on Saturday, March 7, at Chevy Chase Country Club in Wheeling, Illinois, for an exciting evening of play. The grand prize winner of the poker tournament receives a coveted seat at the World Series of Poker Main Event – allowing them to test their mettle against the world’s best players. We will also be hosting a plethora of other casino games like blackjack, craps, and roulette and an ever-expanding silent auction giving everyone a chance to join in on the fun and contribute to the fight against pulmonary fibrosis.
Interested in sponsoring the event, purchasing tickets, or receiving more information about the tournament? Contact Angela Perillo, Director of Development and Foundation Operations, at aperillo@chestnet.org.
Hope to see you March 7th!
Keeping the momentum from our first-ever CHEST Foundation Reception and Casino Night at CHEST 2019, where champions in attendance raised more than $35,000 for pulmonary fibrosis research, the CHEST Foundation continues our long-standing partnership with the Feldman Family Foundation and invites you to the 7th Annual Irv Feldman Texas Hold ‘Em Annual Tournament & Casino Night!
Funds raised at the event support the CHEST Foundation’s mission-based programming and directly impact patients living with pulmonary fibrosis by providing them with access to chest medicine experts; assistance in securing medication and portable oxygen; and empowering the patients and their clinicians to better manage their disease.
Join us at 6:00 PM on Saturday, March 7, at Chevy Chase Country Club in Wheeling, Illinois, for an exciting evening of play. The grand prize winner of the poker tournament receives a coveted seat at the World Series of Poker Main Event – allowing them to test their mettle against the world’s best players. We will also be hosting a plethora of other casino games like blackjack, craps, and roulette and an ever-expanding silent auction giving everyone a chance to join in on the fun and contribute to the fight against pulmonary fibrosis.
Interested in sponsoring the event, purchasing tickets, or receiving more information about the tournament? Contact Angela Perillo, Director of Development and Foundation Operations, at aperillo@chestnet.org.
Hope to see you March 7th!
Keeping the momentum from our first-ever CHEST Foundation Reception and Casino Night at CHEST 2019, where champions in attendance raised more than $35,000 for pulmonary fibrosis research, the CHEST Foundation continues our long-standing partnership with the Feldman Family Foundation and invites you to the 7th Annual Irv Feldman Texas Hold ‘Em Annual Tournament & Casino Night!
Funds raised at the event support the CHEST Foundation’s mission-based programming and directly impact patients living with pulmonary fibrosis by providing them with access to chest medicine experts; assistance in securing medication and portable oxygen; and empowering the patients and their clinicians to better manage their disease.
Join us at 6:00 PM on Saturday, March 7, at Chevy Chase Country Club in Wheeling, Illinois, for an exciting evening of play. The grand prize winner of the poker tournament receives a coveted seat at the World Series of Poker Main Event – allowing them to test their mettle against the world’s best players. We will also be hosting a plethora of other casino games like blackjack, craps, and roulette and an ever-expanding silent auction giving everyone a chance to join in on the fun and contribute to the fight against pulmonary fibrosis.
Interested in sponsoring the event, purchasing tickets, or receiving more information about the tournament? Contact Angela Perillo, Director of Development and Foundation Operations, at aperillo@chestnet.org.
Hope to see you March 7th!
President’s report
After an outstanding annual meeting in New Orleans, with the greatest number of attendees and a number of other firsts, and with the holidays rapidly approaching, you might think there would be a lull in activity, but your CHEST leadership and staff have been busy. Let’s start with a CHEST 2019 recap.
This year’s meeting had a total of 5,960 medical professionals and 8,593 total attendees. All were the highest in CHEST history! In addition, there were more international attendees, and CHEST 2019 saw the largest number of fellows-in-training and the largest number of advanced practice providers attending.
Since CHEST 2019, we have held five live learning sessions at headquarters in Glenview, with a total of 281 attendees, including: Extracorporeal Support for Respiratory and Cardiac Failure in Adults; Critical Care Ultrasound: Integration Into Clinical Practice; Comprehensive Pleural Procedures; Ultrasonography: Essentials in Critical Care; and the Advanced Critical Care Echocardiography Board Review Exam Course. In case you missed those opportunities, in the near future, CHEST will be holding the following 2020 courses: Comprehensive Bronchoscopy With Endobronchial Ultrasound February 20 – 22, Mechanical Ventilation: Advanced Critical Care Management February 27 - 29, Ultrasonography: Essentials in Critical Care March 5 - 7, Bronchoscopy and Chest Tubes in the ICU March 20 - 21, Advanced Clinical Training in Pulmonary Function Testing March 27 - 28, Critical Skills for Critical Care: A State-of-the-Art Update, and Procedures for ICU Providers April 30 - May 2. For additional information, check out the events at chestnet.org.
Internationally, the program for the Italian CHEST Congress, to be held with the Italian CHEST Chapter in Bologna in June (June 25-27), is finished. This meeting will be designed on a smaller scale of that of the annual CHEST meeting, with plenty of educational opportunities in the areas of pulmonary, critical care, and sleep medicine, and will also feature faculty from around the world. Come experience all the education, as well as the beauty of Italy in June! CHEST has continued other international activities with leadership attendance and lectures at the Asian Pacific Society of Respirology (APSR), where we engaged with multiple societies as CHEST continues to grow our international strategy to educate those who request further education in our fields. CHEST also sent selected young investigators to the APSR meeting.
Plans are well under way to hold another successful annual meeting in Chicago - CHEST 2020. The call for topics has ended, and proposal grading is ongoing. The call for abstracts has gone out and will close March 31. We encourage all, including our learners in training, to submit high quality abstracts and case reports, and we will offer suggestions for those needing editorial assistance. This is one of the many ways to get CHEST-involved. In addition to the innovations and experiences we offered last year, there will be continued social media presence and new exciting offerings at this year’s annual meeting. Save the dates - October 17-21, in our home town of Chicago!
One of my goals for this year is to evaluate ways to increase engagement and leadership opportunities within the organization, with our CHEST NetWorks being one example. The work of the NetWorks task force is ongoing. Expect to see pilots of twitter handles, infographics, and e-bytes coming from some NetWorks in the near future.
The editorial board for the next volume of SEEK Critical Care has been selected, and work is under way for delivery of the next print edition and library update at the summer Board Review Courses in August in Washington DC. Your CHEST Journal Editorial Board has also been busy. The redesigned issue with the new content structure has hit mailboxes, and you can expect to see updated guidelines for “Managing Chronic Cough as a Symptom in Children and Management Algorithms: CHEST Guideline and Expert Panel Report” and “Chronic Cough Due to Stable Chronic Bronchitis: CHEST Expert Panel Report” out soon. Also, look for publications that CHEST has endorsed to include the College of American Pathologists’ supplement “Collection and Handling of Thoracic Small Biopsy and Cytology Specimens for Ancillary Studies” and the Society of Critical Care Medicine’s algorithm and bundle for the “Surviving Sepsis Campaign International Guidelines for the Management of Septic Shock and Sepsis-Associated Organ Dysfunction in Children.” CHEST had representatives to both of these writing groups. In addition, more podcasts will soon be on the horizon.
The CHEST Foundation gala, The Golden Era of EP (Erin Popovich) was held in early December at the AT & T center in San Antonio, with over 500 people in attendance, including many from the San Antonio community, current and former Spurs players and coaches, in addition to our leadership and staff. The Erin Popovich (EP) endowment is dedicated to empowerment and access for patients with interstitial lung disease, as well as research in this area. Over 3 million dollars have been raised to date to directly support this endowment. One of the early products from this endowment is the soon to be available Oxygen Access Toolkit, developed for use by provider offices, clinicians, DME suppliers, patients, and caregivers to answer some of the basic facts about access to oxygen that so many of our patients with ILD and other lung diseases need. Other resources will include the ILD Tree, Get a Second Opinion, You’re Not Alone Patient Journey, Mnemonic for ILD Patients, the Patients’ Bill of Rights, and a co-morbidities one–page information sheet.
After the next quarterly Board Meeting in January, I will update you on decisions regarding future strategy that emerge from that meeting. The agenda will include many of the topics mentioned above, in addition to a strategic discussion regarding CHEST’s increased role in advocacy, which has been requested by many members.
Of course, all these events and activities could not be accomplished without the incredible effort by your CHEST staff and volunteer leadership. I look forward to many updates in my next report. As always, please reach out to me with any comments, questions, or suggestions, and if I am unable to respond, I will address it with the appropriate staff person. Thank you all for being the most important reason that CHEST exists. Have a great 2020!
After an outstanding annual meeting in New Orleans, with the greatest number of attendees and a number of other firsts, and with the holidays rapidly approaching, you might think there would be a lull in activity, but your CHEST leadership and staff have been busy. Let’s start with a CHEST 2019 recap.
This year’s meeting had a total of 5,960 medical professionals and 8,593 total attendees. All were the highest in CHEST history! In addition, there were more international attendees, and CHEST 2019 saw the largest number of fellows-in-training and the largest number of advanced practice providers attending.
Since CHEST 2019, we have held five live learning sessions at headquarters in Glenview, with a total of 281 attendees, including: Extracorporeal Support for Respiratory and Cardiac Failure in Adults; Critical Care Ultrasound: Integration Into Clinical Practice; Comprehensive Pleural Procedures; Ultrasonography: Essentials in Critical Care; and the Advanced Critical Care Echocardiography Board Review Exam Course. In case you missed those opportunities, in the near future, CHEST will be holding the following 2020 courses: Comprehensive Bronchoscopy With Endobronchial Ultrasound February 20 – 22, Mechanical Ventilation: Advanced Critical Care Management February 27 - 29, Ultrasonography: Essentials in Critical Care March 5 - 7, Bronchoscopy and Chest Tubes in the ICU March 20 - 21, Advanced Clinical Training in Pulmonary Function Testing March 27 - 28, Critical Skills for Critical Care: A State-of-the-Art Update, and Procedures for ICU Providers April 30 - May 2. For additional information, check out the events at chestnet.org.
Internationally, the program for the Italian CHEST Congress, to be held with the Italian CHEST Chapter in Bologna in June (June 25-27), is finished. This meeting will be designed on a smaller scale of that of the annual CHEST meeting, with plenty of educational opportunities in the areas of pulmonary, critical care, and sleep medicine, and will also feature faculty from around the world. Come experience all the education, as well as the beauty of Italy in June! CHEST has continued other international activities with leadership attendance and lectures at the Asian Pacific Society of Respirology (APSR), where we engaged with multiple societies as CHEST continues to grow our international strategy to educate those who request further education in our fields. CHEST also sent selected young investigators to the APSR meeting.
Plans are well under way to hold another successful annual meeting in Chicago - CHEST 2020. The call for topics has ended, and proposal grading is ongoing. The call for abstracts has gone out and will close March 31. We encourage all, including our learners in training, to submit high quality abstracts and case reports, and we will offer suggestions for those needing editorial assistance. This is one of the many ways to get CHEST-involved. In addition to the innovations and experiences we offered last year, there will be continued social media presence and new exciting offerings at this year’s annual meeting. Save the dates - October 17-21, in our home town of Chicago!
One of my goals for this year is to evaluate ways to increase engagement and leadership opportunities within the organization, with our CHEST NetWorks being one example. The work of the NetWorks task force is ongoing. Expect to see pilots of twitter handles, infographics, and e-bytes coming from some NetWorks in the near future.
The editorial board for the next volume of SEEK Critical Care has been selected, and work is under way for delivery of the next print edition and library update at the summer Board Review Courses in August in Washington DC. Your CHEST Journal Editorial Board has also been busy. The redesigned issue with the new content structure has hit mailboxes, and you can expect to see updated guidelines for “Managing Chronic Cough as a Symptom in Children and Management Algorithms: CHEST Guideline and Expert Panel Report” and “Chronic Cough Due to Stable Chronic Bronchitis: CHEST Expert Panel Report” out soon. Also, look for publications that CHEST has endorsed to include the College of American Pathologists’ supplement “Collection and Handling of Thoracic Small Biopsy and Cytology Specimens for Ancillary Studies” and the Society of Critical Care Medicine’s algorithm and bundle for the “Surviving Sepsis Campaign International Guidelines for the Management of Septic Shock and Sepsis-Associated Organ Dysfunction in Children.” CHEST had representatives to both of these writing groups. In addition, more podcasts will soon be on the horizon.
The CHEST Foundation gala, The Golden Era of EP (Erin Popovich) was held in early December at the AT & T center in San Antonio, with over 500 people in attendance, including many from the San Antonio community, current and former Spurs players and coaches, in addition to our leadership and staff. The Erin Popovich (EP) endowment is dedicated to empowerment and access for patients with interstitial lung disease, as well as research in this area. Over 3 million dollars have been raised to date to directly support this endowment. One of the early products from this endowment is the soon to be available Oxygen Access Toolkit, developed for use by provider offices, clinicians, DME suppliers, patients, and caregivers to answer some of the basic facts about access to oxygen that so many of our patients with ILD and other lung diseases need. Other resources will include the ILD Tree, Get a Second Opinion, You’re Not Alone Patient Journey, Mnemonic for ILD Patients, the Patients’ Bill of Rights, and a co-morbidities one–page information sheet.
After the next quarterly Board Meeting in January, I will update you on decisions regarding future strategy that emerge from that meeting. The agenda will include many of the topics mentioned above, in addition to a strategic discussion regarding CHEST’s increased role in advocacy, which has been requested by many members.
Of course, all these events and activities could not be accomplished without the incredible effort by your CHEST staff and volunteer leadership. I look forward to many updates in my next report. As always, please reach out to me with any comments, questions, or suggestions, and if I am unable to respond, I will address it with the appropriate staff person. Thank you all for being the most important reason that CHEST exists. Have a great 2020!
After an outstanding annual meeting in New Orleans, with the greatest number of attendees and a number of other firsts, and with the holidays rapidly approaching, you might think there would be a lull in activity, but your CHEST leadership and staff have been busy. Let’s start with a CHEST 2019 recap.
This year’s meeting had a total of 5,960 medical professionals and 8,593 total attendees. All were the highest in CHEST history! In addition, there were more international attendees, and CHEST 2019 saw the largest number of fellows-in-training and the largest number of advanced practice providers attending.
Since CHEST 2019, we have held five live learning sessions at headquarters in Glenview, with a total of 281 attendees, including: Extracorporeal Support for Respiratory and Cardiac Failure in Adults; Critical Care Ultrasound: Integration Into Clinical Practice; Comprehensive Pleural Procedures; Ultrasonography: Essentials in Critical Care; and the Advanced Critical Care Echocardiography Board Review Exam Course. In case you missed those opportunities, in the near future, CHEST will be holding the following 2020 courses: Comprehensive Bronchoscopy With Endobronchial Ultrasound February 20 – 22, Mechanical Ventilation: Advanced Critical Care Management February 27 - 29, Ultrasonography: Essentials in Critical Care March 5 - 7, Bronchoscopy and Chest Tubes in the ICU March 20 - 21, Advanced Clinical Training in Pulmonary Function Testing March 27 - 28, Critical Skills for Critical Care: A State-of-the-Art Update, and Procedures for ICU Providers April 30 - May 2. For additional information, check out the events at chestnet.org.
Internationally, the program for the Italian CHEST Congress, to be held with the Italian CHEST Chapter in Bologna in June (June 25-27), is finished. This meeting will be designed on a smaller scale of that of the annual CHEST meeting, with plenty of educational opportunities in the areas of pulmonary, critical care, and sleep medicine, and will also feature faculty from around the world. Come experience all the education, as well as the beauty of Italy in June! CHEST has continued other international activities with leadership attendance and lectures at the Asian Pacific Society of Respirology (APSR), where we engaged with multiple societies as CHEST continues to grow our international strategy to educate those who request further education in our fields. CHEST also sent selected young investigators to the APSR meeting.
Plans are well under way to hold another successful annual meeting in Chicago - CHEST 2020. The call for topics has ended, and proposal grading is ongoing. The call for abstracts has gone out and will close March 31. We encourage all, including our learners in training, to submit high quality abstracts and case reports, and we will offer suggestions for those needing editorial assistance. This is one of the many ways to get CHEST-involved. In addition to the innovations and experiences we offered last year, there will be continued social media presence and new exciting offerings at this year’s annual meeting. Save the dates - October 17-21, in our home town of Chicago!
One of my goals for this year is to evaluate ways to increase engagement and leadership opportunities within the organization, with our CHEST NetWorks being one example. The work of the NetWorks task force is ongoing. Expect to see pilots of twitter handles, infographics, and e-bytes coming from some NetWorks in the near future.
The editorial board for the next volume of SEEK Critical Care has been selected, and work is under way for delivery of the next print edition and library update at the summer Board Review Courses in August in Washington DC. Your CHEST Journal Editorial Board has also been busy. The redesigned issue with the new content structure has hit mailboxes, and you can expect to see updated guidelines for “Managing Chronic Cough as a Symptom in Children and Management Algorithms: CHEST Guideline and Expert Panel Report” and “Chronic Cough Due to Stable Chronic Bronchitis: CHEST Expert Panel Report” out soon. Also, look for publications that CHEST has endorsed to include the College of American Pathologists’ supplement “Collection and Handling of Thoracic Small Biopsy and Cytology Specimens for Ancillary Studies” and the Society of Critical Care Medicine’s algorithm and bundle for the “Surviving Sepsis Campaign International Guidelines for the Management of Septic Shock and Sepsis-Associated Organ Dysfunction in Children.” CHEST had representatives to both of these writing groups. In addition, more podcasts will soon be on the horizon.
The CHEST Foundation gala, The Golden Era of EP (Erin Popovich) was held in early December at the AT & T center in San Antonio, with over 500 people in attendance, including many from the San Antonio community, current and former Spurs players and coaches, in addition to our leadership and staff. The Erin Popovich (EP) endowment is dedicated to empowerment and access for patients with interstitial lung disease, as well as research in this area. Over 3 million dollars have been raised to date to directly support this endowment. One of the early products from this endowment is the soon to be available Oxygen Access Toolkit, developed for use by provider offices, clinicians, DME suppliers, patients, and caregivers to answer some of the basic facts about access to oxygen that so many of our patients with ILD and other lung diseases need. Other resources will include the ILD Tree, Get a Second Opinion, You’re Not Alone Patient Journey, Mnemonic for ILD Patients, the Patients’ Bill of Rights, and a co-morbidities one–page information sheet.
After the next quarterly Board Meeting in January, I will update you on decisions regarding future strategy that emerge from that meeting. The agenda will include many of the topics mentioned above, in addition to a strategic discussion regarding CHEST’s increased role in advocacy, which has been requested by many members.
Of course, all these events and activities could not be accomplished without the incredible effort by your CHEST staff and volunteer leadership. I look forward to many updates in my next report. As always, please reach out to me with any comments, questions, or suggestions, and if I am unable to respond, I will address it with the appropriate staff person. Thank you all for being the most important reason that CHEST exists. Have a great 2020!
Neuromuscular blockade for ARDS in the ICU
The ability to control the delivery of ventilation to patients having the acute respiratory distress syndrome (ARDS) without encountering patient respiratory effort via the administration of neuromuscular blocking drugs has been a potentially appealing therapeutic option for decades (Light RW, et al. Anesth Analg. 1975;54[2]:219). This practice had been common in the late 20th century in order to avoid excessive tachypnea and appearance of patient discomfort with the collateral benefit of improving oxygenation and decreasing the fraction of inspired oxygen (FiO2) (Hansen-Flaschen JH, et al. JAMA. 1991;26:2870). Following the publication by the NIH-sponsored ARDS Network of the landmark low tidal volume lung protective ventilation trial, whereupon study subjects had been allowed to breathe up to 35 times per minute (ARDS Network, N Engl J Med. 2000;342[18]:1301) and additional concerns that neuromuscular blockade could potentially be associated with neuromuscular weakness, this practice fell out of favor.
Although the validity of using lung protective ventilation in ARDS, with a plateau pressure of less than 30 cm/H2O via delivery of a low tidal volume, has withstood the test of time, subsequent attempts to utilize methods that would further protect the lung with additional “rescue” approaches to mechanical ventilation led to a partial renaissance of the neuromuscular blockade (NMB) approach. For example, high frequency oscillatory ventilation, with its idiosyncratic delivery of minute volumes of ventilator gas, requires NMB in order to be used. However, the publication of two negative trials, including one demonstrating an increased mortality, sidelined this approach (Ferguson ND, et al. N Engl J Med. 2013;368[9]:795).
More notably, the use of NMB in patients with ARDS has been advocated during conventional mechanical ventilation to avoid the generation of large tidal volumes via ventilator asynchrony occurring during patient-triggered breaths. Ostensibly, wiping out any patient effort via NMB eliminates manifestations of asynchrony, such as double triggering, which can generate areas of regional tidal hyperinflation in the injured lung and thereby worsen ventilator-induced lung injury. The utilization of NMB early in the course of ARDS (less than 48 hours) resulted in less lung inflammation (Forel JM, et al. Crit Care Med. 2006;34[11]:2749). Subsequently, the ACURASYS trial found that patients with moderately severe or severe ARDS treated with NMB had a mortality benefit comparable to that seen in the original ARDS low tidal volume trial (Papazian L, et al. N Engl J Med. 2010;369:980).
Several criticisms of ACURASYS led to the desire for a larger confirmatory trial be undertaken. The NIH-sponsored successor to the ARDS Network, the Prevention and Early Treatment of Acute Lung Injury (PETAL) Network, took this on straight away with its formation in 2014 (disclosure: the author is a Principal Investigator of one of the 13 PETAL Network Clinical Centers). This trial, called the Re-Evaluation of Systemic Early Neuromuscular Blockade, the ROSE trial, was published last year in the New England Journal of Medicine and failed to confirm a mortality benefit to NMB when used early in the course of ARDS, such as had been done earlier (Moss M, et al. N Engl J Med. 2019;380[21]:1997).
What then, should clinicians consider the proper use of NMB in ARDS to be?
There has been a recent spate of large negative trials of once-promising interventions in critical care medicine (Laffey. Lancet Respir Med. 2018;6[9]659). Among these were trials related to early mobility, vitamin D administration, transpulmonary pressure titrated positive end-expiratory pressure (PEEP), and of course, high frequency oscillatory ventilation, just to name a few disappointments. Recognition of heterogeneity of treatment effect (HTE), with some subgroups being more likely to respond to an intervention than others (Iwashyna. Am J Respir Crit Care Med. 2015;192[9]:1045), is cold comfort to the bedside clinician and all but the most dedicated health services researcher. At least to date, personalized medicine has fallen short of prospective validation in ARDS (Constantin et al. Lancet Respir Med. 2019;7[10]:870).
The failure of the ROSE trial to demonstrate a mortality benefit to ARDS patients with a P/F ratio of less than 150 on at least 8 cm H2O treated with early NMB means the routine use of this approach in all such patients isn’t warranted. In a prescient nod to HTE, “a foolish consistency,” as Emerson said, “is the hobgoblin of little minds.” Importantly, there were several subtle but not necessarily irrelevant differences between ACURASYS and ROSE. ROSE used a high PEEP algorithm to titrate PEEP to FiO2, rather than the conventional low PEEP approach used in the original ARDS Network and ACURASYS trials. Potentially, the benefits of NMB on the injured lung in ARDS may have been mitigated by using higher PEEP levels. ROSE also failed to demonstrate a decrease in barotrauma as had been reported earlier. That said, it is difficult to ascribe the lack of benefit of NMB mechanistically to less asynchrony induced regional tidal hyperinflation in the NMB group at high PEEP, especially given the lighter sedation targets employed in both the NMB and the placebo group. Meanwhile, ROSE did confirm patients were not harmed by NMB by resulting in more neuromuscular weakness upon recovery.
Among patients with Berlin severe ARDS (ie. P/F less than 100 on at least 5 cm H2O PEEP) evaluated between publication of ACURASYS and ROSE, clinicians were far more inclined to use NMB than other rescue modalities, including prone ventilation (Duan, Ann Am Thorac Soc. 2017;12:1818). It seems unlikely the publication of ROSE will alter this. As rescue modalities go, NMB is relatively inexpensive, widely available and easily performed (Co, I and Hyzy RC, Crit Care Med. 2019 Dec 18. doi: 10.1097/CCM.0000000000004198). Ultimately, though the question isn’t whether NMB will be used in ARDS patients with refractory hypoxemia early or even later, but whether prone ventilation should be simultaneously initiated at the time of, or even before the institution of NMB.
As in ACURASYS, patients in the landmark PROSEVA prone ventilation trial were treated with a low PEEP algorithm (Guérin C et al. N Engl J Med. 2013;368[23]:2159). Prone ventilation has many salutary physiologic benefits, not the least of which is recruitment of areas of collapsed lung. Patients who are recruitable with PEEP, i.e. whose PaO2 increases with increasing PEEP in the face of an unchanged or minimally changed plateau pressure, may also demonstrate a mortality benefit (Goligher, EC et al. Am J Respir Crit Care Med. 2014;190[1]:70). It remains unknown whether prone ventilation would remain of significant benefit should a high PEEP approach be employed.
Prone ventilation clearly has its adherents (Albert, RK, Ann Am Thorac Soc. 2020;17[1]:24), although underutilization remains prevalent perhaps due to its somewhat cumbersome nature. While it might have been interesting had ROSE performed a simultaneous assessment of prone ventilation along with NMB via a factorial trial design, clinicians remain at the crossroads of how to escalate ventilator support in the ARDS patient with worsening, if not refractory hypoxemia. The use of NMB with a high PEEP approach often allows for recruitment and a concomitant lowering of FiO2 to acceptable levels in advance of the utilization of prone ventilation. Although some clinicians are able to successfully utilize prone ventilation without NMB, many are not, and NMB use was widespread in PROSEVA.
With no evidence of harm, the employment of NMB in the setting of Berlin severe ARDS is entirely justifiable, whether occurring early or late in the clinical course, regardless of, or potentially with the concomitant employment of prone ventilation. These two rescue modalities remain first line and, despite evidence to the contrary (Li, et al. Am J Respir Crit Care Med. 2018;197[8]:991) should be employed in advance of others, most notably extracorporeal support.
Dr. Hyzy is with the Division of Pulmonary and Critical Care Medicine, University of Michigan, Ann Arbor.
The ability to control the delivery of ventilation to patients having the acute respiratory distress syndrome (ARDS) without encountering patient respiratory effort via the administration of neuromuscular blocking drugs has been a potentially appealing therapeutic option for decades (Light RW, et al. Anesth Analg. 1975;54[2]:219). This practice had been common in the late 20th century in order to avoid excessive tachypnea and appearance of patient discomfort with the collateral benefit of improving oxygenation and decreasing the fraction of inspired oxygen (FiO2) (Hansen-Flaschen JH, et al. JAMA. 1991;26:2870). Following the publication by the NIH-sponsored ARDS Network of the landmark low tidal volume lung protective ventilation trial, whereupon study subjects had been allowed to breathe up to 35 times per minute (ARDS Network, N Engl J Med. 2000;342[18]:1301) and additional concerns that neuromuscular blockade could potentially be associated with neuromuscular weakness, this practice fell out of favor.
Although the validity of using lung protective ventilation in ARDS, with a plateau pressure of less than 30 cm/H2O via delivery of a low tidal volume, has withstood the test of time, subsequent attempts to utilize methods that would further protect the lung with additional “rescue” approaches to mechanical ventilation led to a partial renaissance of the neuromuscular blockade (NMB) approach. For example, high frequency oscillatory ventilation, with its idiosyncratic delivery of minute volumes of ventilator gas, requires NMB in order to be used. However, the publication of two negative trials, including one demonstrating an increased mortality, sidelined this approach (Ferguson ND, et al. N Engl J Med. 2013;368[9]:795).
More notably, the use of NMB in patients with ARDS has been advocated during conventional mechanical ventilation to avoid the generation of large tidal volumes via ventilator asynchrony occurring during patient-triggered breaths. Ostensibly, wiping out any patient effort via NMB eliminates manifestations of asynchrony, such as double triggering, which can generate areas of regional tidal hyperinflation in the injured lung and thereby worsen ventilator-induced lung injury. The utilization of NMB early in the course of ARDS (less than 48 hours) resulted in less lung inflammation (Forel JM, et al. Crit Care Med. 2006;34[11]:2749). Subsequently, the ACURASYS trial found that patients with moderately severe or severe ARDS treated with NMB had a mortality benefit comparable to that seen in the original ARDS low tidal volume trial (Papazian L, et al. N Engl J Med. 2010;369:980).
Several criticisms of ACURASYS led to the desire for a larger confirmatory trial be undertaken. The NIH-sponsored successor to the ARDS Network, the Prevention and Early Treatment of Acute Lung Injury (PETAL) Network, took this on straight away with its formation in 2014 (disclosure: the author is a Principal Investigator of one of the 13 PETAL Network Clinical Centers). This trial, called the Re-Evaluation of Systemic Early Neuromuscular Blockade, the ROSE trial, was published last year in the New England Journal of Medicine and failed to confirm a mortality benefit to NMB when used early in the course of ARDS, such as had been done earlier (Moss M, et al. N Engl J Med. 2019;380[21]:1997).
What then, should clinicians consider the proper use of NMB in ARDS to be?
There has been a recent spate of large negative trials of once-promising interventions in critical care medicine (Laffey. Lancet Respir Med. 2018;6[9]659). Among these were trials related to early mobility, vitamin D administration, transpulmonary pressure titrated positive end-expiratory pressure (PEEP), and of course, high frequency oscillatory ventilation, just to name a few disappointments. Recognition of heterogeneity of treatment effect (HTE), with some subgroups being more likely to respond to an intervention than others (Iwashyna. Am J Respir Crit Care Med. 2015;192[9]:1045), is cold comfort to the bedside clinician and all but the most dedicated health services researcher. At least to date, personalized medicine has fallen short of prospective validation in ARDS (Constantin et al. Lancet Respir Med. 2019;7[10]:870).
The failure of the ROSE trial to demonstrate a mortality benefit to ARDS patients with a P/F ratio of less than 150 on at least 8 cm H2O treated with early NMB means the routine use of this approach in all such patients isn’t warranted. In a prescient nod to HTE, “a foolish consistency,” as Emerson said, “is the hobgoblin of little minds.” Importantly, there were several subtle but not necessarily irrelevant differences between ACURASYS and ROSE. ROSE used a high PEEP algorithm to titrate PEEP to FiO2, rather than the conventional low PEEP approach used in the original ARDS Network and ACURASYS trials. Potentially, the benefits of NMB on the injured lung in ARDS may have been mitigated by using higher PEEP levels. ROSE also failed to demonstrate a decrease in barotrauma as had been reported earlier. That said, it is difficult to ascribe the lack of benefit of NMB mechanistically to less asynchrony induced regional tidal hyperinflation in the NMB group at high PEEP, especially given the lighter sedation targets employed in both the NMB and the placebo group. Meanwhile, ROSE did confirm patients were not harmed by NMB by resulting in more neuromuscular weakness upon recovery.
Among patients with Berlin severe ARDS (ie. P/F less than 100 on at least 5 cm H2O PEEP) evaluated between publication of ACURASYS and ROSE, clinicians were far more inclined to use NMB than other rescue modalities, including prone ventilation (Duan, Ann Am Thorac Soc. 2017;12:1818). It seems unlikely the publication of ROSE will alter this. As rescue modalities go, NMB is relatively inexpensive, widely available and easily performed (Co, I and Hyzy RC, Crit Care Med. 2019 Dec 18. doi: 10.1097/CCM.0000000000004198). Ultimately, though the question isn’t whether NMB will be used in ARDS patients with refractory hypoxemia early or even later, but whether prone ventilation should be simultaneously initiated at the time of, or even before the institution of NMB.
As in ACURASYS, patients in the landmark PROSEVA prone ventilation trial were treated with a low PEEP algorithm (Guérin C et al. N Engl J Med. 2013;368[23]:2159). Prone ventilation has many salutary physiologic benefits, not the least of which is recruitment of areas of collapsed lung. Patients who are recruitable with PEEP, i.e. whose PaO2 increases with increasing PEEP in the face of an unchanged or minimally changed plateau pressure, may also demonstrate a mortality benefit (Goligher, EC et al. Am J Respir Crit Care Med. 2014;190[1]:70). It remains unknown whether prone ventilation would remain of significant benefit should a high PEEP approach be employed.
Prone ventilation clearly has its adherents (Albert, RK, Ann Am Thorac Soc. 2020;17[1]:24), although underutilization remains prevalent perhaps due to its somewhat cumbersome nature. While it might have been interesting had ROSE performed a simultaneous assessment of prone ventilation along with NMB via a factorial trial design, clinicians remain at the crossroads of how to escalate ventilator support in the ARDS patient with worsening, if not refractory hypoxemia. The use of NMB with a high PEEP approach often allows for recruitment and a concomitant lowering of FiO2 to acceptable levels in advance of the utilization of prone ventilation. Although some clinicians are able to successfully utilize prone ventilation without NMB, many are not, and NMB use was widespread in PROSEVA.
With no evidence of harm, the employment of NMB in the setting of Berlin severe ARDS is entirely justifiable, whether occurring early or late in the clinical course, regardless of, or potentially with the concomitant employment of prone ventilation. These two rescue modalities remain first line and, despite evidence to the contrary (Li, et al. Am J Respir Crit Care Med. 2018;197[8]:991) should be employed in advance of others, most notably extracorporeal support.
Dr. Hyzy is with the Division of Pulmonary and Critical Care Medicine, University of Michigan, Ann Arbor.
The ability to control the delivery of ventilation to patients having the acute respiratory distress syndrome (ARDS) without encountering patient respiratory effort via the administration of neuromuscular blocking drugs has been a potentially appealing therapeutic option for decades (Light RW, et al. Anesth Analg. 1975;54[2]:219). This practice had been common in the late 20th century in order to avoid excessive tachypnea and appearance of patient discomfort with the collateral benefit of improving oxygenation and decreasing the fraction of inspired oxygen (FiO2) (Hansen-Flaschen JH, et al. JAMA. 1991;26:2870). Following the publication by the NIH-sponsored ARDS Network of the landmark low tidal volume lung protective ventilation trial, whereupon study subjects had been allowed to breathe up to 35 times per minute (ARDS Network, N Engl J Med. 2000;342[18]:1301) and additional concerns that neuromuscular blockade could potentially be associated with neuromuscular weakness, this practice fell out of favor.
Although the validity of using lung protective ventilation in ARDS, with a plateau pressure of less than 30 cm/H2O via delivery of a low tidal volume, has withstood the test of time, subsequent attempts to utilize methods that would further protect the lung with additional “rescue” approaches to mechanical ventilation led to a partial renaissance of the neuromuscular blockade (NMB) approach. For example, high frequency oscillatory ventilation, with its idiosyncratic delivery of minute volumes of ventilator gas, requires NMB in order to be used. However, the publication of two negative trials, including one demonstrating an increased mortality, sidelined this approach (Ferguson ND, et al. N Engl J Med. 2013;368[9]:795).
More notably, the use of NMB in patients with ARDS has been advocated during conventional mechanical ventilation to avoid the generation of large tidal volumes via ventilator asynchrony occurring during patient-triggered breaths. Ostensibly, wiping out any patient effort via NMB eliminates manifestations of asynchrony, such as double triggering, which can generate areas of regional tidal hyperinflation in the injured lung and thereby worsen ventilator-induced lung injury. The utilization of NMB early in the course of ARDS (less than 48 hours) resulted in less lung inflammation (Forel JM, et al. Crit Care Med. 2006;34[11]:2749). Subsequently, the ACURASYS trial found that patients with moderately severe or severe ARDS treated with NMB had a mortality benefit comparable to that seen in the original ARDS low tidal volume trial (Papazian L, et al. N Engl J Med. 2010;369:980).
Several criticisms of ACURASYS led to the desire for a larger confirmatory trial be undertaken. The NIH-sponsored successor to the ARDS Network, the Prevention and Early Treatment of Acute Lung Injury (PETAL) Network, took this on straight away with its formation in 2014 (disclosure: the author is a Principal Investigator of one of the 13 PETAL Network Clinical Centers). This trial, called the Re-Evaluation of Systemic Early Neuromuscular Blockade, the ROSE trial, was published last year in the New England Journal of Medicine and failed to confirm a mortality benefit to NMB when used early in the course of ARDS, such as had been done earlier (Moss M, et al. N Engl J Med. 2019;380[21]:1997).
What then, should clinicians consider the proper use of NMB in ARDS to be?
There has been a recent spate of large negative trials of once-promising interventions in critical care medicine (Laffey. Lancet Respir Med. 2018;6[9]659). Among these were trials related to early mobility, vitamin D administration, transpulmonary pressure titrated positive end-expiratory pressure (PEEP), and of course, high frequency oscillatory ventilation, just to name a few disappointments. Recognition of heterogeneity of treatment effect (HTE), with some subgroups being more likely to respond to an intervention than others (Iwashyna. Am J Respir Crit Care Med. 2015;192[9]:1045), is cold comfort to the bedside clinician and all but the most dedicated health services researcher. At least to date, personalized medicine has fallen short of prospective validation in ARDS (Constantin et al. Lancet Respir Med. 2019;7[10]:870).
The failure of the ROSE trial to demonstrate a mortality benefit to ARDS patients with a P/F ratio of less than 150 on at least 8 cm H2O treated with early NMB means the routine use of this approach in all such patients isn’t warranted. In a prescient nod to HTE, “a foolish consistency,” as Emerson said, “is the hobgoblin of little minds.” Importantly, there were several subtle but not necessarily irrelevant differences between ACURASYS and ROSE. ROSE used a high PEEP algorithm to titrate PEEP to FiO2, rather than the conventional low PEEP approach used in the original ARDS Network and ACURASYS trials. Potentially, the benefits of NMB on the injured lung in ARDS may have been mitigated by using higher PEEP levels. ROSE also failed to demonstrate a decrease in barotrauma as had been reported earlier. That said, it is difficult to ascribe the lack of benefit of NMB mechanistically to less asynchrony induced regional tidal hyperinflation in the NMB group at high PEEP, especially given the lighter sedation targets employed in both the NMB and the placebo group. Meanwhile, ROSE did confirm patients were not harmed by NMB by resulting in more neuromuscular weakness upon recovery.
Among patients with Berlin severe ARDS (ie. P/F less than 100 on at least 5 cm H2O PEEP) evaluated between publication of ACURASYS and ROSE, clinicians were far more inclined to use NMB than other rescue modalities, including prone ventilation (Duan, Ann Am Thorac Soc. 2017;12:1818). It seems unlikely the publication of ROSE will alter this. As rescue modalities go, NMB is relatively inexpensive, widely available and easily performed (Co, I and Hyzy RC, Crit Care Med. 2019 Dec 18. doi: 10.1097/CCM.0000000000004198). Ultimately, though the question isn’t whether NMB will be used in ARDS patients with refractory hypoxemia early or even later, but whether prone ventilation should be simultaneously initiated at the time of, or even before the institution of NMB.
As in ACURASYS, patients in the landmark PROSEVA prone ventilation trial were treated with a low PEEP algorithm (Guérin C et al. N Engl J Med. 2013;368[23]:2159). Prone ventilation has many salutary physiologic benefits, not the least of which is recruitment of areas of collapsed lung. Patients who are recruitable with PEEP, i.e. whose PaO2 increases with increasing PEEP in the face of an unchanged or minimally changed plateau pressure, may also demonstrate a mortality benefit (Goligher, EC et al. Am J Respir Crit Care Med. 2014;190[1]:70). It remains unknown whether prone ventilation would remain of significant benefit should a high PEEP approach be employed.
Prone ventilation clearly has its adherents (Albert, RK, Ann Am Thorac Soc. 2020;17[1]:24), although underutilization remains prevalent perhaps due to its somewhat cumbersome nature. While it might have been interesting had ROSE performed a simultaneous assessment of prone ventilation along with NMB via a factorial trial design, clinicians remain at the crossroads of how to escalate ventilator support in the ARDS patient with worsening, if not refractory hypoxemia. The use of NMB with a high PEEP approach often allows for recruitment and a concomitant lowering of FiO2 to acceptable levels in advance of the utilization of prone ventilation. Although some clinicians are able to successfully utilize prone ventilation without NMB, many are not, and NMB use was widespread in PROSEVA.
With no evidence of harm, the employment of NMB in the setting of Berlin severe ARDS is entirely justifiable, whether occurring early or late in the clinical course, regardless of, or potentially with the concomitant employment of prone ventilation. These two rescue modalities remain first line and, despite evidence to the contrary (Li, et al. Am J Respir Crit Care Med. 2018;197[8]:991) should be employed in advance of others, most notably extracorporeal support.
Dr. Hyzy is with the Division of Pulmonary and Critical Care Medicine, University of Michigan, Ann Arbor.