User login
Beta-blockers in COPD
Airway disorders
Beta-blockers in COPD: A settled debate?
Beta-blockers are the cornerstone in the management of patients with heart failure and myocardial infarction where they have shown to improve morbidity and mortality. Cardiovascular disease is common in patients with COPD. A 2014 meta-analysis of retrospective studies involving patients with COPD using a beta-blocker has shown lower death and lower exacerbation rate (Du Q, et al. PLoS One. 2014;9[11]:e113048). More recent studies continue to note underutilization of beta-blockers in patients with COPD due to concerns for adverse effects on pulmonary function (Lipworth B, et al. Heart. 2016;102[23]:1909).
To further study these concerns, Dransfield and colleagues conducted a randomized controlled trial (BLOCK COPD) of 532 randomly assigned patients to receive either metoprolol or placebo (Dransfield, et al. N Engl J Med. 2019;381[24]:2304). Primary outcome was time to first COPD exacerbation whereas secondary outcomes included rate of exacerbation, mortality, hospitalization, symptoms, and spirometry data. Median time to exacerbation was similar between the two groups; however, metoprolol was associated with higher incidence of severe exacerbation requiring hospitalization (HR 1.91, 95% CI 1.29-2.83). There was nonstatistical increase in deaths in metoprolol group, mainly contributed by fatal COPD events (seven in metoprolol vs one in placebo). The study results validated some of the concerns of worsening pulmonary function with beta-blocker use; however, in order to better understand the study results, we must pay attention to the study cohort.
In summary, patients did not have significant cardiac disease and, therefore, did not have an overt indication for beta-blocker use. Patients with COPD in this study were sicker than average patients. Lastly, there were more patients in the metoprolol group who had COPD exacerbations requiring ED visit or hospitalization in 12 months prior to study enrollment. For the above-mentioned reasons, the conclusion of this study should not discourage the use of beta-blockers in patients with COPD when underlying cardiac disease warrants their use, after careful consideration of benefits and risks.
Muhammad Adrish, MD, FCCP, Steering Committee Member
Navitha Ramesh, MD, FCCP, Steering Committee Member
Clinical research
Nintedanib in progressive fibrosing interstitial lung diseases: Does one size really fit all?
Interstitial lung diseases (ILDs) include a variety of lung disorders, such as idiopathic interstitial pneumonias (IIPs), autoimmune diseases, granulomatous lung disease, and environmental diseases. They all have one thing in common—a progressive fibrosing phenotype that is almost universally fatal. It has been suggested that such diseases have a shared pathophysiologic mechanism irrespective of the cause and, hence, could respond to similar therapy. Nintedanib acts intracellularly by inhibiting multiple tyrosine kinases. Previous clinical trials have suggested that nintedanib inhibits the progression of lung fibrosis in patients with idiopathic pulmonary fibrosis (Richeldi, et al. N Engl J Med. 2014;370[22]:2071) and systemic sclerosis-associated ILD (Distler, et al. N Engl J Med. 2019;380[26]:2518). The INBUILD trial was conducted to study the efficacy and safety of nintedanib in patients with fibrosing interstitial lung diseases (Flaherty, et al. N Engl J Med. 2019;381[18]:1718).
Patients with a wide spectrum of progressive fibrosing ILD were enrolled in the INBUILD trial. This gave the phenotypic approach needed to study the effects of nintedanib in fibrosing ILDs. The authors reported an absolute difference of 107 mL in the annual rate of decline in forced vital capacity in the overall population, 128.2 mL (95% CI 65.4 to 148.5; P less than .001) in patients with UIP-like fibrotic pattern and 75.3 mL in patients with other fibrotic patterns, between patients who received nintedanib and those who received placebo. Earlier studies have shown similar results in patients with IPF. The most frequent adverse event was diarrhea (66.9% in the nintedanib group and 23.9% in placebo group). Liver enzymes derangement was more common in the nintedanib group. Nausea, vomiting, abdominal pain, decreased appetite, and weight decrease were also more frequent in the nintedanib group than in those in the placebo group. In conclusion, this study not only explored the effects of nintedanib on progressive fibrosing ILDs but also helped to enhance the understanding of their natural history, suggesting a final common pathway toward lung fibrosis.
Mohsin Ijaz, MD, FCCP, Steering Committee Member
Critical care
Vaping-related acute lung injury: Where there’s smoke, there’s fire
E-cigarette or vaping product use–associated lung injury (EVALI) is a burgeoning public health problem in the United States. There have been more than 2,506 hospitalizations and 54 deaths from EVALI (cdc.gov). Unfortunately, the diagnosis is one of exclusion at present. The CDC defines EVALI as lung disease associated with e-cigarette or vaping exposure within 90 days, infiltrates, and absence of other causes (Layden, et al. N Engl J Med. 2019 Sep 6. doi: 10.1056/NEJMoa1911614). As critical care providers, we are uniquely poised to detect and treat this illness, given that roughly 1 in 3 patients with EVALI require mechanical ventilation. Moreover, one-quarter of rehospitalizations and deaths occur 2 days after discharge from initial hospitalization (Mikosz, et al. MMWR 2020;68[5152]:1183). .
To better identify EVALI, the Centers for Disease Control and Prevention (CDC) recommends that health-care providers ask e-cigarette or vaping product users about respiratory, gastrointestinal, and constitutional symptoms, obtain chest imaging in those suspected of EVALI, consider outpatient management of stable patients, test for influenza, and use caution when prescribing steroids in the outpatient setting. Emphasizing cessation and advocating for annual influenza vaccination is also recommended (Update: Interim Guidance for Health Care Providers for Managing Patients with Suspected E-cigarette, or Vaping, Product Use–Associated Lung Injury. (MMWR. 2019;68[46]:1081).
So how can critical care providers assist in the understanding and treatment of EVALI? Critical care physicians treating patients with EVALI face unique challenges moving forward. We need to develop a better understanding of the triggers and pathophysiology of EVALI and learn to improve our recognition of the disease. We should study interventions that may improve outcomes such as corticosteroids. We know little about the long-term outcomes and sequalae of EVALI.
The best treatment for EVALI is prevention. Critical care physicians are experts at identifying and treating life-threatening conditions but as a community have less experience in the public health arena. If as physicians we are called upon to advocate for our patients, then perhaps there is a role for critical care physicians to advocate for a ban on vaping.
Matthew K. Hensley, MD, MPH, Fellow-in-Training
Daniel R. Ouellette, MD, MS, FCCP, NetWork Vice-Chair
Home-based mechanical ventilation and neuromuscular disease
Keeping up with the times: incorporating home mechanical ventilation education into pulmonary and critical care fellowship and clinical practice
Home mechanical ventilation (HMV) utilization for patients with chronic respiratory conditions is rapidly increasing in both pediatric and adult populations. By 2016, the estimated prevalence of HMV was 2.9-12.9/100,000 (3.1-18% via tracheotomy) (Rose, et al. Respir Care. 2015;60[5]:695; Valko, et al. BMC Pulm Med. 2018;18[1]:190). In 2012 limited regional U.S. data were extrapolated to approximate a prevalence of 4.7-6.4/100,000 children utilizing HMV (King, A. Respir Care. 2012;57[6]921), but there is currently no comprehensive registry of HMV use in the United States. A U.S. Department of Health and Human Services report in 2016 described an 85-fold increase in Medicare claims for home ventilators in 2015 compared with 2009 (OEI-12-15-00370; 9/22/2016).
With increasing demand, educating clinicians responsible for providing and managing HMV is paramount. Education specific to longitudinal management of the HMV is noticeably overlooked. The ACGME core competencies for PCCM fellowships include principles inherent to HMV, including modes/principles of ventilation, modalities/principles of oxygen supplementation, tracheostomy tube management, as well as the use of “masks for delivery of supplemental oxygen, humidifiers, nebulizers, and incentive spirometry” (ACGME Common Program Requirements 7/1/2019). However, training programs are not required to provide skills essential in HMV management, including: (1) appropriate patient selection for long-term HMV, (2) selection of well-matched home ventilators suited to patients’ chronic conditions, (3) assessment/timing of transition to invasive ventilation, or (4) adjustments necessary to maintain optimal ventilator support. Life-sustaining ventilators used in ICUs differ from life-supporting HMV systems in modes, interface, cost, algorithms, circuitry, and available adjuncts.
There is an opportunity (and responsibility) to improve current training guidelines to meet growing needs of the population and anticipate needs of trainees as they enter unsupervised practice. Although simulation initiatives at national CHEST meetings attempt to bridge education gaps, it is incumbent upon fellowship training programs to prepare pulmonologists with skills to manage HMV in order to maintain high standards of care in a safe, financially responsible and evidence-based manner.
Bethany L. Lussier, MD, FCCP, NetWork Member
Won Y. Lee, MD, FCCP. Steering Committee Member
Interstitial and diffuse lung disease
Granulomatous lymphocytic interstitial lung disease (GL-ILD)
Among the granulomatous lung diseases, Gl-ILD is hardly a new discovery, but for many reasons, it often goes undiagnosed for years. The relative rareness of the disease itself and, hence, the lack of awareness makes it an uncommon differential for granulomatous ILD. Patients with GL-ILD are often misdiagnosed with sarcoidosis, unspecified ILD, or lymphoid interstitial pneumonia, etc, before receiving a diagnosis of GL-ILD.
GL-ILD is seen in 5% to 22% of patients with common variable immunoglobulin deficiency (CVID). There are instances where patients are diagnosed with CVID based on a radiologic or histologic diagnosis of GL-ILD. Although GL-ILD suggests a pulmonary process, it actually encompasses a multisystemic granulomatous inflammatory disease that may affect the liver, spleen, bowels, lymphoid tissue, and conceivably any other organ system (Hartono, et al. Ann Allergy Asthma Immunol. 2017;118[5]:614. Pathogenesis of GL-ILD in CVID includes dysfunctional antigen handling (due to impaired T cell function) and aberrant immune response to viruses (Hurst, et al. J Allergy Clin Immunol Pract. 2017;5[4]:938).
Patients with GL-ILD often present with progressive shortness of breath, restrictive lung functions with a background of CVID. Imaging findings are 5-30 mm lower lobe-predominant, nodules, ground glass opacities, and splenomegaly. Histopathology varies with predominant granulomas vs lymphocytic infiltrates. The process can be treated and often reversed with use of high dose immunoglobulin replacement, immunomodulatory therapy with agents like azathioprine, and rituximab. However, steroids are not helpful. Due to the lymphocytic dysregulation in GL-ILD, patients are at high risk of death from lymphoma. Part of the management is surveillance for malignancy and involvement of other organ systems.
A. Thanushi Wynn, MD, Fellow-in-Training
Airway disorders
Beta-blockers in COPD: A settled debate?
Beta-blockers are the cornerstone in the management of patients with heart failure and myocardial infarction where they have shown to improve morbidity and mortality. Cardiovascular disease is common in patients with COPD. A 2014 meta-analysis of retrospective studies involving patients with COPD using a beta-blocker has shown lower death and lower exacerbation rate (Du Q, et al. PLoS One. 2014;9[11]:e113048). More recent studies continue to note underutilization of beta-blockers in patients with COPD due to concerns for adverse effects on pulmonary function (Lipworth B, et al. Heart. 2016;102[23]:1909).
To further study these concerns, Dransfield and colleagues conducted a randomized controlled trial (BLOCK COPD) of 532 randomly assigned patients to receive either metoprolol or placebo (Dransfield, et al. N Engl J Med. 2019;381[24]:2304). Primary outcome was time to first COPD exacerbation whereas secondary outcomes included rate of exacerbation, mortality, hospitalization, symptoms, and spirometry data. Median time to exacerbation was similar between the two groups; however, metoprolol was associated with higher incidence of severe exacerbation requiring hospitalization (HR 1.91, 95% CI 1.29-2.83). There was nonstatistical increase in deaths in metoprolol group, mainly contributed by fatal COPD events (seven in metoprolol vs one in placebo). The study results validated some of the concerns of worsening pulmonary function with beta-blocker use; however, in order to better understand the study results, we must pay attention to the study cohort.
In summary, patients did not have significant cardiac disease and, therefore, did not have an overt indication for beta-blocker use. Patients with COPD in this study were sicker than average patients. Lastly, there were more patients in the metoprolol group who had COPD exacerbations requiring ED visit or hospitalization in 12 months prior to study enrollment. For the above-mentioned reasons, the conclusion of this study should not discourage the use of beta-blockers in patients with COPD when underlying cardiac disease warrants their use, after careful consideration of benefits and risks.
Muhammad Adrish, MD, FCCP, Steering Committee Member
Navitha Ramesh, MD, FCCP, Steering Committee Member
Clinical research
Nintedanib in progressive fibrosing interstitial lung diseases: Does one size really fit all?
Interstitial lung diseases (ILDs) include a variety of lung disorders, such as idiopathic interstitial pneumonias (IIPs), autoimmune diseases, granulomatous lung disease, and environmental diseases. They all have one thing in common—a progressive fibrosing phenotype that is almost universally fatal. It has been suggested that such diseases have a shared pathophysiologic mechanism irrespective of the cause and, hence, could respond to similar therapy. Nintedanib acts intracellularly by inhibiting multiple tyrosine kinases. Previous clinical trials have suggested that nintedanib inhibits the progression of lung fibrosis in patients with idiopathic pulmonary fibrosis (Richeldi, et al. N Engl J Med. 2014;370[22]:2071) and systemic sclerosis-associated ILD (Distler, et al. N Engl J Med. 2019;380[26]:2518). The INBUILD trial was conducted to study the efficacy and safety of nintedanib in patients with fibrosing interstitial lung diseases (Flaherty, et al. N Engl J Med. 2019;381[18]:1718).
Patients with a wide spectrum of progressive fibrosing ILD were enrolled in the INBUILD trial. This gave the phenotypic approach needed to study the effects of nintedanib in fibrosing ILDs. The authors reported an absolute difference of 107 mL in the annual rate of decline in forced vital capacity in the overall population, 128.2 mL (95% CI 65.4 to 148.5; P less than .001) in patients with UIP-like fibrotic pattern and 75.3 mL in patients with other fibrotic patterns, between patients who received nintedanib and those who received placebo. Earlier studies have shown similar results in patients with IPF. The most frequent adverse event was diarrhea (66.9% in the nintedanib group and 23.9% in placebo group). Liver enzymes derangement was more common in the nintedanib group. Nausea, vomiting, abdominal pain, decreased appetite, and weight decrease were also more frequent in the nintedanib group than in those in the placebo group. In conclusion, this study not only explored the effects of nintedanib on progressive fibrosing ILDs but also helped to enhance the understanding of their natural history, suggesting a final common pathway toward lung fibrosis.
Mohsin Ijaz, MD, FCCP, Steering Committee Member
Critical care
Vaping-related acute lung injury: Where there’s smoke, there’s fire
E-cigarette or vaping product use–associated lung injury (EVALI) is a burgeoning public health problem in the United States. There have been more than 2,506 hospitalizations and 54 deaths from EVALI (cdc.gov). Unfortunately, the diagnosis is one of exclusion at present. The CDC defines EVALI as lung disease associated with e-cigarette or vaping exposure within 90 days, infiltrates, and absence of other causes (Layden, et al. N Engl J Med. 2019 Sep 6. doi: 10.1056/NEJMoa1911614). As critical care providers, we are uniquely poised to detect and treat this illness, given that roughly 1 in 3 patients with EVALI require mechanical ventilation. Moreover, one-quarter of rehospitalizations and deaths occur 2 days after discharge from initial hospitalization (Mikosz, et al. MMWR 2020;68[5152]:1183). .
To better identify EVALI, the Centers for Disease Control and Prevention (CDC) recommends that health-care providers ask e-cigarette or vaping product users about respiratory, gastrointestinal, and constitutional symptoms, obtain chest imaging in those suspected of EVALI, consider outpatient management of stable patients, test for influenza, and use caution when prescribing steroids in the outpatient setting. Emphasizing cessation and advocating for annual influenza vaccination is also recommended (Update: Interim Guidance for Health Care Providers for Managing Patients with Suspected E-cigarette, or Vaping, Product Use–Associated Lung Injury. (MMWR. 2019;68[46]:1081).
So how can critical care providers assist in the understanding and treatment of EVALI? Critical care physicians treating patients with EVALI face unique challenges moving forward. We need to develop a better understanding of the triggers and pathophysiology of EVALI and learn to improve our recognition of the disease. We should study interventions that may improve outcomes such as corticosteroids. We know little about the long-term outcomes and sequalae of EVALI.
The best treatment for EVALI is prevention. Critical care physicians are experts at identifying and treating life-threatening conditions but as a community have less experience in the public health arena. If as physicians we are called upon to advocate for our patients, then perhaps there is a role for critical care physicians to advocate for a ban on vaping.
Matthew K. Hensley, MD, MPH, Fellow-in-Training
Daniel R. Ouellette, MD, MS, FCCP, NetWork Vice-Chair
Home-based mechanical ventilation and neuromuscular disease
Keeping up with the times: incorporating home mechanical ventilation education into pulmonary and critical care fellowship and clinical practice
Home mechanical ventilation (HMV) utilization for patients with chronic respiratory conditions is rapidly increasing in both pediatric and adult populations. By 2016, the estimated prevalence of HMV was 2.9-12.9/100,000 (3.1-18% via tracheotomy) (Rose, et al. Respir Care. 2015;60[5]:695; Valko, et al. BMC Pulm Med. 2018;18[1]:190). In 2012 limited regional U.S. data were extrapolated to approximate a prevalence of 4.7-6.4/100,000 children utilizing HMV (King, A. Respir Care. 2012;57[6]921), but there is currently no comprehensive registry of HMV use in the United States. A U.S. Department of Health and Human Services report in 2016 described an 85-fold increase in Medicare claims for home ventilators in 2015 compared with 2009 (OEI-12-15-00370; 9/22/2016).
With increasing demand, educating clinicians responsible for providing and managing HMV is paramount. Education specific to longitudinal management of the HMV is noticeably overlooked. The ACGME core competencies for PCCM fellowships include principles inherent to HMV, including modes/principles of ventilation, modalities/principles of oxygen supplementation, tracheostomy tube management, as well as the use of “masks for delivery of supplemental oxygen, humidifiers, nebulizers, and incentive spirometry” (ACGME Common Program Requirements 7/1/2019). However, training programs are not required to provide skills essential in HMV management, including: (1) appropriate patient selection for long-term HMV, (2) selection of well-matched home ventilators suited to patients’ chronic conditions, (3) assessment/timing of transition to invasive ventilation, or (4) adjustments necessary to maintain optimal ventilator support. Life-sustaining ventilators used in ICUs differ from life-supporting HMV systems in modes, interface, cost, algorithms, circuitry, and available adjuncts.
There is an opportunity (and responsibility) to improve current training guidelines to meet growing needs of the population and anticipate needs of trainees as they enter unsupervised practice. Although simulation initiatives at national CHEST meetings attempt to bridge education gaps, it is incumbent upon fellowship training programs to prepare pulmonologists with skills to manage HMV in order to maintain high standards of care in a safe, financially responsible and evidence-based manner.
Bethany L. Lussier, MD, FCCP, NetWork Member
Won Y. Lee, MD, FCCP. Steering Committee Member
Interstitial and diffuse lung disease
Granulomatous lymphocytic interstitial lung disease (GL-ILD)
Among the granulomatous lung diseases, Gl-ILD is hardly a new discovery, but for many reasons, it often goes undiagnosed for years. The relative rareness of the disease itself and, hence, the lack of awareness makes it an uncommon differential for granulomatous ILD. Patients with GL-ILD are often misdiagnosed with sarcoidosis, unspecified ILD, or lymphoid interstitial pneumonia, etc, before receiving a diagnosis of GL-ILD.
GL-ILD is seen in 5% to 22% of patients with common variable immunoglobulin deficiency (CVID). There are instances where patients are diagnosed with CVID based on a radiologic or histologic diagnosis of GL-ILD. Although GL-ILD suggests a pulmonary process, it actually encompasses a multisystemic granulomatous inflammatory disease that may affect the liver, spleen, bowels, lymphoid tissue, and conceivably any other organ system (Hartono, et al. Ann Allergy Asthma Immunol. 2017;118[5]:614. Pathogenesis of GL-ILD in CVID includes dysfunctional antigen handling (due to impaired T cell function) and aberrant immune response to viruses (Hurst, et al. J Allergy Clin Immunol Pract. 2017;5[4]:938).
Patients with GL-ILD often present with progressive shortness of breath, restrictive lung functions with a background of CVID. Imaging findings are 5-30 mm lower lobe-predominant, nodules, ground glass opacities, and splenomegaly. Histopathology varies with predominant granulomas vs lymphocytic infiltrates. The process can be treated and often reversed with use of high dose immunoglobulin replacement, immunomodulatory therapy with agents like azathioprine, and rituximab. However, steroids are not helpful. Due to the lymphocytic dysregulation in GL-ILD, patients are at high risk of death from lymphoma. Part of the management is surveillance for malignancy and involvement of other organ systems.
A. Thanushi Wynn, MD, Fellow-in-Training
Airway disorders
Beta-blockers in COPD: A settled debate?
Beta-blockers are the cornerstone in the management of patients with heart failure and myocardial infarction where they have shown to improve morbidity and mortality. Cardiovascular disease is common in patients with COPD. A 2014 meta-analysis of retrospective studies involving patients with COPD using a beta-blocker has shown lower death and lower exacerbation rate (Du Q, et al. PLoS One. 2014;9[11]:e113048). More recent studies continue to note underutilization of beta-blockers in patients with COPD due to concerns for adverse effects on pulmonary function (Lipworth B, et al. Heart. 2016;102[23]:1909).
To further study these concerns, Dransfield and colleagues conducted a randomized controlled trial (BLOCK COPD) of 532 randomly assigned patients to receive either metoprolol or placebo (Dransfield, et al. N Engl J Med. 2019;381[24]:2304). Primary outcome was time to first COPD exacerbation whereas secondary outcomes included rate of exacerbation, mortality, hospitalization, symptoms, and spirometry data. Median time to exacerbation was similar between the two groups; however, metoprolol was associated with higher incidence of severe exacerbation requiring hospitalization (HR 1.91, 95% CI 1.29-2.83). There was nonstatistical increase in deaths in metoprolol group, mainly contributed by fatal COPD events (seven in metoprolol vs one in placebo). The study results validated some of the concerns of worsening pulmonary function with beta-blocker use; however, in order to better understand the study results, we must pay attention to the study cohort.
In summary, patients did not have significant cardiac disease and, therefore, did not have an overt indication for beta-blocker use. Patients with COPD in this study were sicker than average patients. Lastly, there were more patients in the metoprolol group who had COPD exacerbations requiring ED visit or hospitalization in 12 months prior to study enrollment. For the above-mentioned reasons, the conclusion of this study should not discourage the use of beta-blockers in patients with COPD when underlying cardiac disease warrants their use, after careful consideration of benefits and risks.
Muhammad Adrish, MD, FCCP, Steering Committee Member
Navitha Ramesh, MD, FCCP, Steering Committee Member
Clinical research
Nintedanib in progressive fibrosing interstitial lung diseases: Does one size really fit all?
Interstitial lung diseases (ILDs) include a variety of lung disorders, such as idiopathic interstitial pneumonias (IIPs), autoimmune diseases, granulomatous lung disease, and environmental diseases. They all have one thing in common—a progressive fibrosing phenotype that is almost universally fatal. It has been suggested that such diseases have a shared pathophysiologic mechanism irrespective of the cause and, hence, could respond to similar therapy. Nintedanib acts intracellularly by inhibiting multiple tyrosine kinases. Previous clinical trials have suggested that nintedanib inhibits the progression of lung fibrosis in patients with idiopathic pulmonary fibrosis (Richeldi, et al. N Engl J Med. 2014;370[22]:2071) and systemic sclerosis-associated ILD (Distler, et al. N Engl J Med. 2019;380[26]:2518). The INBUILD trial was conducted to study the efficacy and safety of nintedanib in patients with fibrosing interstitial lung diseases (Flaherty, et al. N Engl J Med. 2019;381[18]:1718).
Patients with a wide spectrum of progressive fibrosing ILD were enrolled in the INBUILD trial. This gave the phenotypic approach needed to study the effects of nintedanib in fibrosing ILDs. The authors reported an absolute difference of 107 mL in the annual rate of decline in forced vital capacity in the overall population, 128.2 mL (95% CI 65.4 to 148.5; P less than .001) in patients with UIP-like fibrotic pattern and 75.3 mL in patients with other fibrotic patterns, between patients who received nintedanib and those who received placebo. Earlier studies have shown similar results in patients with IPF. The most frequent adverse event was diarrhea (66.9% in the nintedanib group and 23.9% in placebo group). Liver enzymes derangement was more common in the nintedanib group. Nausea, vomiting, abdominal pain, decreased appetite, and weight decrease were also more frequent in the nintedanib group than in those in the placebo group. In conclusion, this study not only explored the effects of nintedanib on progressive fibrosing ILDs but also helped to enhance the understanding of their natural history, suggesting a final common pathway toward lung fibrosis.
Mohsin Ijaz, MD, FCCP, Steering Committee Member
Critical care
Vaping-related acute lung injury: Where there’s smoke, there’s fire
E-cigarette or vaping product use–associated lung injury (EVALI) is a burgeoning public health problem in the United States. There have been more than 2,506 hospitalizations and 54 deaths from EVALI (cdc.gov). Unfortunately, the diagnosis is one of exclusion at present. The CDC defines EVALI as lung disease associated with e-cigarette or vaping exposure within 90 days, infiltrates, and absence of other causes (Layden, et al. N Engl J Med. 2019 Sep 6. doi: 10.1056/NEJMoa1911614). As critical care providers, we are uniquely poised to detect and treat this illness, given that roughly 1 in 3 patients with EVALI require mechanical ventilation. Moreover, one-quarter of rehospitalizations and deaths occur 2 days after discharge from initial hospitalization (Mikosz, et al. MMWR 2020;68[5152]:1183). .
To better identify EVALI, the Centers for Disease Control and Prevention (CDC) recommends that health-care providers ask e-cigarette or vaping product users about respiratory, gastrointestinal, and constitutional symptoms, obtain chest imaging in those suspected of EVALI, consider outpatient management of stable patients, test for influenza, and use caution when prescribing steroids in the outpatient setting. Emphasizing cessation and advocating for annual influenza vaccination is also recommended (Update: Interim Guidance for Health Care Providers for Managing Patients with Suspected E-cigarette, or Vaping, Product Use–Associated Lung Injury. (MMWR. 2019;68[46]:1081).
So how can critical care providers assist in the understanding and treatment of EVALI? Critical care physicians treating patients with EVALI face unique challenges moving forward. We need to develop a better understanding of the triggers and pathophysiology of EVALI and learn to improve our recognition of the disease. We should study interventions that may improve outcomes such as corticosteroids. We know little about the long-term outcomes and sequalae of EVALI.
The best treatment for EVALI is prevention. Critical care physicians are experts at identifying and treating life-threatening conditions but as a community have less experience in the public health arena. If as physicians we are called upon to advocate for our patients, then perhaps there is a role for critical care physicians to advocate for a ban on vaping.
Matthew K. Hensley, MD, MPH, Fellow-in-Training
Daniel R. Ouellette, MD, MS, FCCP, NetWork Vice-Chair
Home-based mechanical ventilation and neuromuscular disease
Keeping up with the times: incorporating home mechanical ventilation education into pulmonary and critical care fellowship and clinical practice
Home mechanical ventilation (HMV) utilization for patients with chronic respiratory conditions is rapidly increasing in both pediatric and adult populations. By 2016, the estimated prevalence of HMV was 2.9-12.9/100,000 (3.1-18% via tracheotomy) (Rose, et al. Respir Care. 2015;60[5]:695; Valko, et al. BMC Pulm Med. 2018;18[1]:190). In 2012 limited regional U.S. data were extrapolated to approximate a prevalence of 4.7-6.4/100,000 children utilizing HMV (King, A. Respir Care. 2012;57[6]921), but there is currently no comprehensive registry of HMV use in the United States. A U.S. Department of Health and Human Services report in 2016 described an 85-fold increase in Medicare claims for home ventilators in 2015 compared with 2009 (OEI-12-15-00370; 9/22/2016).
With increasing demand, educating clinicians responsible for providing and managing HMV is paramount. Education specific to longitudinal management of the HMV is noticeably overlooked. The ACGME core competencies for PCCM fellowships include principles inherent to HMV, including modes/principles of ventilation, modalities/principles of oxygen supplementation, tracheostomy tube management, as well as the use of “masks for delivery of supplemental oxygen, humidifiers, nebulizers, and incentive spirometry” (ACGME Common Program Requirements 7/1/2019). However, training programs are not required to provide skills essential in HMV management, including: (1) appropriate patient selection for long-term HMV, (2) selection of well-matched home ventilators suited to patients’ chronic conditions, (3) assessment/timing of transition to invasive ventilation, or (4) adjustments necessary to maintain optimal ventilator support. Life-sustaining ventilators used in ICUs differ from life-supporting HMV systems in modes, interface, cost, algorithms, circuitry, and available adjuncts.
There is an opportunity (and responsibility) to improve current training guidelines to meet growing needs of the population and anticipate needs of trainees as they enter unsupervised practice. Although simulation initiatives at national CHEST meetings attempt to bridge education gaps, it is incumbent upon fellowship training programs to prepare pulmonologists with skills to manage HMV in order to maintain high standards of care in a safe, financially responsible and evidence-based manner.
Bethany L. Lussier, MD, FCCP, NetWork Member
Won Y. Lee, MD, FCCP. Steering Committee Member
Interstitial and diffuse lung disease
Granulomatous lymphocytic interstitial lung disease (GL-ILD)
Among the granulomatous lung diseases, Gl-ILD is hardly a new discovery, but for many reasons, it often goes undiagnosed for years. The relative rareness of the disease itself and, hence, the lack of awareness makes it an uncommon differential for granulomatous ILD. Patients with GL-ILD are often misdiagnosed with sarcoidosis, unspecified ILD, or lymphoid interstitial pneumonia, etc, before receiving a diagnosis of GL-ILD.
GL-ILD is seen in 5% to 22% of patients with common variable immunoglobulin deficiency (CVID). There are instances where patients are diagnosed with CVID based on a radiologic or histologic diagnosis of GL-ILD. Although GL-ILD suggests a pulmonary process, it actually encompasses a multisystemic granulomatous inflammatory disease that may affect the liver, spleen, bowels, lymphoid tissue, and conceivably any other organ system (Hartono, et al. Ann Allergy Asthma Immunol. 2017;118[5]:614. Pathogenesis of GL-ILD in CVID includes dysfunctional antigen handling (due to impaired T cell function) and aberrant immune response to viruses (Hurst, et al. J Allergy Clin Immunol Pract. 2017;5[4]:938).
Patients with GL-ILD often present with progressive shortness of breath, restrictive lung functions with a background of CVID. Imaging findings are 5-30 mm lower lobe-predominant, nodules, ground glass opacities, and splenomegaly. Histopathology varies with predominant granulomas vs lymphocytic infiltrates. The process can be treated and often reversed with use of high dose immunoglobulin replacement, immunomodulatory therapy with agents like azathioprine, and rituximab. However, steroids are not helpful. Due to the lymphocytic dysregulation in GL-ILD, patients are at high risk of death from lymphoma. Part of the management is surveillance for malignancy and involvement of other organ systems.
A. Thanushi Wynn, MD, Fellow-in-Training
Psoriasis ointment helped with itch, healing in phase 2 EB study
LONDON – , in a small, placebo-controlled, phase 2 study.
More importantly, use of the ointment promoted wound healing in those with the severe skin-blistering condition. Indeed, compared with placebo, a greater reduction in wound size was observed after 2 weeks when the ointment was applied (a mean reduction of 65.5% vs. 88.4%; P less than .006). However, at 1 month, no significant differences were seen in the size of the wounds between the two treatment arms.
“Calcipotriol is a vitamin D analog and it is well known that vitamin D is a very critical factor for skin homeostasis and proper wound healing,” Christina Guttmann-Gruber, PhD, said at the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association (DEBRA). Dr. Guttmann-Gruber, a group lead researcher for EB House Austria, which is based at the Salzburg (Austria) University Clinic for Dermatology, noted that vitamin D also helps with tissue repair and immune modulation, and enhances local antimicrobial activity.
During an oral poster presentation at the meeting, Dr. Guttmann-Gruber explained that in previous in vitro studies, it was found that low concentrations (100 nmol) of calcipotriol inhibited proliferation of RDEB tumor cells (Sci Rep. 2018 Sep 7;8:13430). Calcipotriol (also known as calcipotriene) also was found to improve the expression of antimicrobial peptides and promote wound closure. “Therefore, we thought that applying calcipotriol at the site of injury, on chronic wounds prone to superinfection where it is needed, might be beneficial for our patients.”
She and her associates designed a two-arm, randomized, double-blind crossover study to assess the effects of an existing calcipotriol-containing ointment on wound healing in patients with RDEB. The ointment used in the study is approved for treating psoriasis but was adapted by the in-house pharmacy team to reduce the concentration of calcipotriol to about 0.05 mcg/g, or around 121 nmol. The reason for the reduction was that, at higher doses, keratinocyte proliferation was reduced, which would be detrimental in RDEB patients.
Nine patients were included in the study and were randomized to either apply 1 g of the active or placebo ointment to each of two designated wounds, of at least 6 cm2 in size, every day for 4 weeks. A 2-month washout period then followed before the groups switched to use the other ointment for 1 month. Six out of the nine patients completed both treatment phases. The reasons for the patients not completing both intervention phases were not related to the drug.
Calcipotriol treatment resulted in a significant and steady reduction in itch over the entire course of treatment, which was not seen among those on placebo, Dr. Guttmann-Gruber observed. The reduction in itch was “striking,” but only while the treatment was being used, she said. Results for pain were less clear, with a significant reduction in pain after 2 weeks seen only in the placebo group, while both treatments reduced pain to the same degree by 1 month.
No serious adverse events were observed at any time point and topical use of the low-dose calcipotriol did not significantly change serum levels of calcium or vitamin D in the two patients in which this was studied, Dr. Guttmann-Gruber said.
“This is an approved drug; it’s used in psoriasis, but at a very high concentration. We were able to use it off label and make a diluted version,” she observed. “Any pharmacy can do it.” Although it was applied topically, it could be done by applying it to the dressing rather directly onto the wounded skin, she said.
Data on the skin microbiome response to treatment were also collected but were not available to analyze in time for presentation, but it appeared that there was improvement with the low-dose calcipotriol treatment, Dr. Guttmann-Gruber said. “When the wounds are healing, the microbial flora is improving.”
The next step will probably be to plan a multicenter trial of this treatment, Dr. Guttmann-Gruber said in an interview. The questions is whether such a trial would get the financial backing it needed, but if an orphan drug designation could be obtained for calcipotriol for EB, then it would be possible to conduct such a trial.
The study was funded by DEBRA Austria. The presenting author, Dr. Guttmann-Gruber, had no conflicts of interest to disclose.
SOURCE: Guttmann-Gruber C et al. EB World Congress 2020. Poster 34.
LONDON – , in a small, placebo-controlled, phase 2 study.
More importantly, use of the ointment promoted wound healing in those with the severe skin-blistering condition. Indeed, compared with placebo, a greater reduction in wound size was observed after 2 weeks when the ointment was applied (a mean reduction of 65.5% vs. 88.4%; P less than .006). However, at 1 month, no significant differences were seen in the size of the wounds between the two treatment arms.
“Calcipotriol is a vitamin D analog and it is well known that vitamin D is a very critical factor for skin homeostasis and proper wound healing,” Christina Guttmann-Gruber, PhD, said at the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association (DEBRA). Dr. Guttmann-Gruber, a group lead researcher for EB House Austria, which is based at the Salzburg (Austria) University Clinic for Dermatology, noted that vitamin D also helps with tissue repair and immune modulation, and enhances local antimicrobial activity.
During an oral poster presentation at the meeting, Dr. Guttmann-Gruber explained that in previous in vitro studies, it was found that low concentrations (100 nmol) of calcipotriol inhibited proliferation of RDEB tumor cells (Sci Rep. 2018 Sep 7;8:13430). Calcipotriol (also known as calcipotriene) also was found to improve the expression of antimicrobial peptides and promote wound closure. “Therefore, we thought that applying calcipotriol at the site of injury, on chronic wounds prone to superinfection where it is needed, might be beneficial for our patients.”
She and her associates designed a two-arm, randomized, double-blind crossover study to assess the effects of an existing calcipotriol-containing ointment on wound healing in patients with RDEB. The ointment used in the study is approved for treating psoriasis but was adapted by the in-house pharmacy team to reduce the concentration of calcipotriol to about 0.05 mcg/g, or around 121 nmol. The reason for the reduction was that, at higher doses, keratinocyte proliferation was reduced, which would be detrimental in RDEB patients.
Nine patients were included in the study and were randomized to either apply 1 g of the active or placebo ointment to each of two designated wounds, of at least 6 cm2 in size, every day for 4 weeks. A 2-month washout period then followed before the groups switched to use the other ointment for 1 month. Six out of the nine patients completed both treatment phases. The reasons for the patients not completing both intervention phases were not related to the drug.
Calcipotriol treatment resulted in a significant and steady reduction in itch over the entire course of treatment, which was not seen among those on placebo, Dr. Guttmann-Gruber observed. The reduction in itch was “striking,” but only while the treatment was being used, she said. Results for pain were less clear, with a significant reduction in pain after 2 weeks seen only in the placebo group, while both treatments reduced pain to the same degree by 1 month.
No serious adverse events were observed at any time point and topical use of the low-dose calcipotriol did not significantly change serum levels of calcium or vitamin D in the two patients in which this was studied, Dr. Guttmann-Gruber said.
“This is an approved drug; it’s used in psoriasis, but at a very high concentration. We were able to use it off label and make a diluted version,” she observed. “Any pharmacy can do it.” Although it was applied topically, it could be done by applying it to the dressing rather directly onto the wounded skin, she said.
Data on the skin microbiome response to treatment were also collected but were not available to analyze in time for presentation, but it appeared that there was improvement with the low-dose calcipotriol treatment, Dr. Guttmann-Gruber said. “When the wounds are healing, the microbial flora is improving.”
The next step will probably be to plan a multicenter trial of this treatment, Dr. Guttmann-Gruber said in an interview. The questions is whether such a trial would get the financial backing it needed, but if an orphan drug designation could be obtained for calcipotriol for EB, then it would be possible to conduct such a trial.
The study was funded by DEBRA Austria. The presenting author, Dr. Guttmann-Gruber, had no conflicts of interest to disclose.
SOURCE: Guttmann-Gruber C et al. EB World Congress 2020. Poster 34.
LONDON – , in a small, placebo-controlled, phase 2 study.
More importantly, use of the ointment promoted wound healing in those with the severe skin-blistering condition. Indeed, compared with placebo, a greater reduction in wound size was observed after 2 weeks when the ointment was applied (a mean reduction of 65.5% vs. 88.4%; P less than .006). However, at 1 month, no significant differences were seen in the size of the wounds between the two treatment arms.
“Calcipotriol is a vitamin D analog and it is well known that vitamin D is a very critical factor for skin homeostasis and proper wound healing,” Christina Guttmann-Gruber, PhD, said at the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association (DEBRA). Dr. Guttmann-Gruber, a group lead researcher for EB House Austria, which is based at the Salzburg (Austria) University Clinic for Dermatology, noted that vitamin D also helps with tissue repair and immune modulation, and enhances local antimicrobial activity.
During an oral poster presentation at the meeting, Dr. Guttmann-Gruber explained that in previous in vitro studies, it was found that low concentrations (100 nmol) of calcipotriol inhibited proliferation of RDEB tumor cells (Sci Rep. 2018 Sep 7;8:13430). Calcipotriol (also known as calcipotriene) also was found to improve the expression of antimicrobial peptides and promote wound closure. “Therefore, we thought that applying calcipotriol at the site of injury, on chronic wounds prone to superinfection where it is needed, might be beneficial for our patients.”
She and her associates designed a two-arm, randomized, double-blind crossover study to assess the effects of an existing calcipotriol-containing ointment on wound healing in patients with RDEB. The ointment used in the study is approved for treating psoriasis but was adapted by the in-house pharmacy team to reduce the concentration of calcipotriol to about 0.05 mcg/g, or around 121 nmol. The reason for the reduction was that, at higher doses, keratinocyte proliferation was reduced, which would be detrimental in RDEB patients.
Nine patients were included in the study and were randomized to either apply 1 g of the active or placebo ointment to each of two designated wounds, of at least 6 cm2 in size, every day for 4 weeks. A 2-month washout period then followed before the groups switched to use the other ointment for 1 month. Six out of the nine patients completed both treatment phases. The reasons for the patients not completing both intervention phases were not related to the drug.
Calcipotriol treatment resulted in a significant and steady reduction in itch over the entire course of treatment, which was not seen among those on placebo, Dr. Guttmann-Gruber observed. The reduction in itch was “striking,” but only while the treatment was being used, she said. Results for pain were less clear, with a significant reduction in pain after 2 weeks seen only in the placebo group, while both treatments reduced pain to the same degree by 1 month.
No serious adverse events were observed at any time point and topical use of the low-dose calcipotriol did not significantly change serum levels of calcium or vitamin D in the two patients in which this was studied, Dr. Guttmann-Gruber said.
“This is an approved drug; it’s used in psoriasis, but at a very high concentration. We were able to use it off label and make a diluted version,” she observed. “Any pharmacy can do it.” Although it was applied topically, it could be done by applying it to the dressing rather directly onto the wounded skin, she said.
Data on the skin microbiome response to treatment were also collected but were not available to analyze in time for presentation, but it appeared that there was improvement with the low-dose calcipotriol treatment, Dr. Guttmann-Gruber said. “When the wounds are healing, the microbial flora is improving.”
The next step will probably be to plan a multicenter trial of this treatment, Dr. Guttmann-Gruber said in an interview. The questions is whether such a trial would get the financial backing it needed, but if an orphan drug designation could be obtained for calcipotriol for EB, then it would be possible to conduct such a trial.
The study was funded by DEBRA Austria. The presenting author, Dr. Guttmann-Gruber, had no conflicts of interest to disclose.
SOURCE: Guttmann-Gruber C et al. EB World Congress 2020. Poster 34.
REPORTING FROM EB 2020
Pharmacologic prophylaxis fails in pediatric migraine
Clinicians hoped that medications used in adults – such as antidepressants, antiepileptics, antihypertensive agents, calcium channel blockers, and food supplements – would find similar success in children. Unfortunately, researchers found only short-term signs of efficacy over placebo, with no benefit lasting more than 6 months.
The study, conducted by a team led by Cosima Locher, PhD, of Boston Children’s Hospital, included 23 double-blind, randomized, controlled trials with a total of 2,217 patients; the mean age was 11 years. They compared 12 pharmacologic agents with each other or with placebo in the study, published online in JAMA Pediatrics.
In a main efficacy analysis that included 19 studies, only two treatments outperformed placebo: propranolol (standardized mean difference, 0.60; 95% confidence interval, 0.03-1.17) and topiramate (SMD, 0.59; 95% CI, 0.03-1.15). There were no statistically significant between-treatment differences.
The results had an overall low to moderate certainty.
When propranolol was compared to placebo, the 95% prediction interval (–0.62 to 1.82) was wider than the significant confidence interval (0.03-1.17), and comprised both beneficial and detrimental effects. A similar result was found with topiramate, with a prediction interval of –0.62 to 1.80 extending into nonsignificant effects (95% CI, 0.03-1.15). In both cases, significant effects were found only when the prediction interval was 70%.
In a long-term analysis (greater than 6 months), no treatment outperformed placebo.
The treatments generally were acceptable. The researchers found no significant difference in tolerability between any of the treatments and each other or placebo. Safety data analyzed from 13 trials revealed no significant differences between treatments and placebo.
“Because specific effects of drugs are associated with the size of the placebo effect, the lack of drug efficacy in our NMA [network meta-analysis] could be owing to a comparatively high placebo effect in children. In fact, there is indirect evidence [from other studies] that the placebo effect is more pronounced in children and adolescents than in adults,” Dr. Locher and associates said. They suggested that studies were needed to quantify the placebo effect in pediatric migraine, and if it was large, to develop innovative therapies making use of this.
The findings should lead to some changes in practice, Boris Zernikow, MD, PhD, of Children’s and Adolescents’ Hospital Datteln (Germany) wrote in an accompanying editorial.
Pharmacological prophylactic treatment of childhood migraine should be an exception rather than the rule, and nonpharmacologic approaches should be emphasized, particularly because the placebo effect is magnified in children, he said.
Many who suffer migraines in childhood will continue to be affected in adulthood, so pediatric intervention is a good opportunity to instill effective strategies. These include: using abortive medication early in an attack and using antimigraine medications for only that specific type of headache; engaging in physical activity to reduce migraine attacks; getting sufficient sleep; and learning relaxation and other psychological approaches to counter migraines.
Dr. Zernikow had no relevant financial disclosures. One study author received grants from Amgen and other support from Grunenthal and Akelos. The study received funding from the Sara Page Mayo Endowment for Pediatric Pain Research, Education, and Treatment; the Swiss National Science Foundation; the Schweizer-Arau-Foundation; and the Theophrastus Foundation.
SOURCES: Locher C et al. JAMA Pediatrics. 2020 Feb 10. doi: 10.1001/jamapediatrics.2019.5856; Zernikow B. JAMA Pediatrics. 2020 Feb 10. doi: 10.1001/jamapediatrics.2019.5907.
Clinicians hoped that medications used in adults – such as antidepressants, antiepileptics, antihypertensive agents, calcium channel blockers, and food supplements – would find similar success in children. Unfortunately, researchers found only short-term signs of efficacy over placebo, with no benefit lasting more than 6 months.
The study, conducted by a team led by Cosima Locher, PhD, of Boston Children’s Hospital, included 23 double-blind, randomized, controlled trials with a total of 2,217 patients; the mean age was 11 years. They compared 12 pharmacologic agents with each other or with placebo in the study, published online in JAMA Pediatrics.
In a main efficacy analysis that included 19 studies, only two treatments outperformed placebo: propranolol (standardized mean difference, 0.60; 95% confidence interval, 0.03-1.17) and topiramate (SMD, 0.59; 95% CI, 0.03-1.15). There were no statistically significant between-treatment differences.
The results had an overall low to moderate certainty.
When propranolol was compared to placebo, the 95% prediction interval (–0.62 to 1.82) was wider than the significant confidence interval (0.03-1.17), and comprised both beneficial and detrimental effects. A similar result was found with topiramate, with a prediction interval of –0.62 to 1.80 extending into nonsignificant effects (95% CI, 0.03-1.15). In both cases, significant effects were found only when the prediction interval was 70%.
In a long-term analysis (greater than 6 months), no treatment outperformed placebo.
The treatments generally were acceptable. The researchers found no significant difference in tolerability between any of the treatments and each other or placebo. Safety data analyzed from 13 trials revealed no significant differences between treatments and placebo.
“Because specific effects of drugs are associated with the size of the placebo effect, the lack of drug efficacy in our NMA [network meta-analysis] could be owing to a comparatively high placebo effect in children. In fact, there is indirect evidence [from other studies] that the placebo effect is more pronounced in children and adolescents than in adults,” Dr. Locher and associates said. They suggested that studies were needed to quantify the placebo effect in pediatric migraine, and if it was large, to develop innovative therapies making use of this.
The findings should lead to some changes in practice, Boris Zernikow, MD, PhD, of Children’s and Adolescents’ Hospital Datteln (Germany) wrote in an accompanying editorial.
Pharmacological prophylactic treatment of childhood migraine should be an exception rather than the rule, and nonpharmacologic approaches should be emphasized, particularly because the placebo effect is magnified in children, he said.
Many who suffer migraines in childhood will continue to be affected in adulthood, so pediatric intervention is a good opportunity to instill effective strategies. These include: using abortive medication early in an attack and using antimigraine medications for only that specific type of headache; engaging in physical activity to reduce migraine attacks; getting sufficient sleep; and learning relaxation and other psychological approaches to counter migraines.
Dr. Zernikow had no relevant financial disclosures. One study author received grants from Amgen and other support from Grunenthal and Akelos. The study received funding from the Sara Page Mayo Endowment for Pediatric Pain Research, Education, and Treatment; the Swiss National Science Foundation; the Schweizer-Arau-Foundation; and the Theophrastus Foundation.
SOURCES: Locher C et al. JAMA Pediatrics. 2020 Feb 10. doi: 10.1001/jamapediatrics.2019.5856; Zernikow B. JAMA Pediatrics. 2020 Feb 10. doi: 10.1001/jamapediatrics.2019.5907.
Clinicians hoped that medications used in adults – such as antidepressants, antiepileptics, antihypertensive agents, calcium channel blockers, and food supplements – would find similar success in children. Unfortunately, researchers found only short-term signs of efficacy over placebo, with no benefit lasting more than 6 months.
The study, conducted by a team led by Cosima Locher, PhD, of Boston Children’s Hospital, included 23 double-blind, randomized, controlled trials with a total of 2,217 patients; the mean age was 11 years. They compared 12 pharmacologic agents with each other or with placebo in the study, published online in JAMA Pediatrics.
In a main efficacy analysis that included 19 studies, only two treatments outperformed placebo: propranolol (standardized mean difference, 0.60; 95% confidence interval, 0.03-1.17) and topiramate (SMD, 0.59; 95% CI, 0.03-1.15). There were no statistically significant between-treatment differences.
The results had an overall low to moderate certainty.
When propranolol was compared to placebo, the 95% prediction interval (–0.62 to 1.82) was wider than the significant confidence interval (0.03-1.17), and comprised both beneficial and detrimental effects. A similar result was found with topiramate, with a prediction interval of –0.62 to 1.80 extending into nonsignificant effects (95% CI, 0.03-1.15). In both cases, significant effects were found only when the prediction interval was 70%.
In a long-term analysis (greater than 6 months), no treatment outperformed placebo.
The treatments generally were acceptable. The researchers found no significant difference in tolerability between any of the treatments and each other or placebo. Safety data analyzed from 13 trials revealed no significant differences between treatments and placebo.
“Because specific effects of drugs are associated with the size of the placebo effect, the lack of drug efficacy in our NMA [network meta-analysis] could be owing to a comparatively high placebo effect in children. In fact, there is indirect evidence [from other studies] that the placebo effect is more pronounced in children and adolescents than in adults,” Dr. Locher and associates said. They suggested that studies were needed to quantify the placebo effect in pediatric migraine, and if it was large, to develop innovative therapies making use of this.
The findings should lead to some changes in practice, Boris Zernikow, MD, PhD, of Children’s and Adolescents’ Hospital Datteln (Germany) wrote in an accompanying editorial.
Pharmacological prophylactic treatment of childhood migraine should be an exception rather than the rule, and nonpharmacologic approaches should be emphasized, particularly because the placebo effect is magnified in children, he said.
Many who suffer migraines in childhood will continue to be affected in adulthood, so pediatric intervention is a good opportunity to instill effective strategies. These include: using abortive medication early in an attack and using antimigraine medications for only that specific type of headache; engaging in physical activity to reduce migraine attacks; getting sufficient sleep; and learning relaxation and other psychological approaches to counter migraines.
Dr. Zernikow had no relevant financial disclosures. One study author received grants from Amgen and other support from Grunenthal and Akelos. The study received funding from the Sara Page Mayo Endowment for Pediatric Pain Research, Education, and Treatment; the Swiss National Science Foundation; the Schweizer-Arau-Foundation; and the Theophrastus Foundation.
SOURCES: Locher C et al. JAMA Pediatrics. 2020 Feb 10. doi: 10.1001/jamapediatrics.2019.5856; Zernikow B. JAMA Pediatrics. 2020 Feb 10. doi: 10.1001/jamapediatrics.2019.5907.
FROM JAMA PEDIATRICS
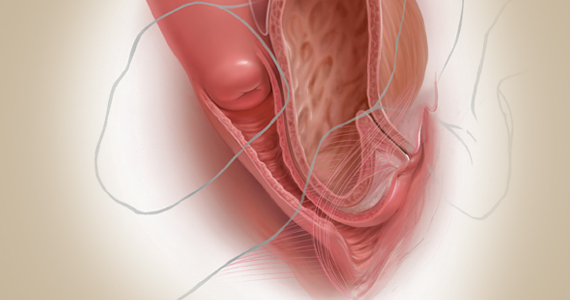
Exploring options for POP treatment: Patient selection, surgical approaches, and ways to manage risks
A number of presentations at the 2019 Pelvic Anatomy and Gynecologic Surgery (PAGS) Symposium (Las Vegas, Nevada, December 12-14, 2019) focused on pelvic organ prolapse (POP) repair, including anatomic considerations, the evolution of surgical procedures, and transvaginal repair. OBG M
Nonsurgical approaches for POP: A good option for the right patient
John B. Gebhart, MD, MS: What are the nonsurgical options for POP?
Mark D. Walters, MD: Women who have prolapse could, of course, choose to continue to live with the prolapse. If they desire treatment, however, the main nonsurgical option is a combination of pessary use, possibly with some estrogen, and possibly with pelvic muscle exercises. Women who have a well-fitting pessary can be managed satisfactorily for years. If possible, women should be taught to take the pessary in and out on a regular basis to minimize their long-term complications.
Dr. Gebhart: How can nonsurgical treatment options be maximized?
Beri M. Ridgeway, MD: It depends on patient commitment. This is important to assess at the first visit when you are making management decisions, because if someone is not going to attend physical therapy or not going to continue to do the exercises, the expectation for the outcome is not going to be great.
Also, if a patient feels very uncomfortable using a pessary and really does not want it, I am fine proceeding with surgery as a first-line treatment. If the patient is committed, the ideal is to educate her and connect her with the right people, either a pelvic floor physical therapist or someone in your office who will encourage her and manage pessary use.
Dr. Gebhart: It goes back to assessing patient goals and expectations.
Mickey M. Karram, MD: If you have a patient who is a good candidate for a pessary—say she has a well-supported distal vagina and maybe a cervical prolapse or an apical prolapse—and you can fit a small pessary that will sit in the upper vagina in a comfortable fashion, it is worthwhile to explain to the patient that she is a really good candidate for this option. By contrast, someone who has a wide genital hiatus and a large rectocele will not have good success with a pessary.
Dr. Gebhart: That is important: Choose your nonsurgical patients well, those who will respond to therapy and maybe not get frustrated with it.
Dr. Walters: A problem I see is that some people are good at fitting a pessary, but they do not teach how to use it very well. When I see the patient back, she says, “What’s my long term on the pessary?” I say, “If we teach you to take it in and out, you are less likely to have any problems with it, and then you can manage it for years that way. Otherwise, you have to keep visiting a practitioner to change it and that is not necessarily a good long-term option.” At the very first visit, I teach them what a pessary is, its purpose, and how to maintain it themselves. I think that gives patients the best chance for long-term satisfaction.
Dr. Gebhart: Surgery is always an option if pessary management is not satisfactory.
Dr. Ridgeway: I also tell patients, especially those uncertain about using a pessary, “Worst case, you spend a little time to figure this out, but if it works, you can avoid surgery. If it doesn’t—the risks are very low and you perhaps wasted some time—but at least you’ll know you tried the conservative management.”
Dr. Gebhart: Mickey made an excellent point earlier that it can be a diagnostic treatment strategy as well.
Dr. Karram: If you are concerned about the prolapse worsening or negatively impacting a functional problem related to the bladder or bowel, it is good to place a pessary for a short period of time. This can potentially give you an idea of how your surgery will impact a patient’s bladder or bowel function.
Continue to: Decisions to make before choosing a surgical approach...
Decisions to make before choosing a surgical approach
Dr. Gebhart: Would you elaborate on the surgical options for managing POP?
Dr. Walters: For women with prolapse who decide they want to have surgery, the woman and the surgeon need to make a number of decisions. Some of these include whether the uterus, if present, needs to be removed; whether the woman would like to maintain sexual function or not; whether the repair would best be done vaginally only with native tissue suturing, vaginally with some augmentation (although that is not likely in the United States at this time), or through the abdomen, usually laparoscopically or robotically with a mesh-augmented sacrocolpopexy repair.
Also, we must decide whether to do additional cystocele and rectocele repairs and whether to add slings for stress incontinence, which can coexist or could develop after the prolapse repair. A lot of different decisions need to be made when choosing a prolapse repair for different women.
Dr. Ridgeway: It is shared decision-making with the patient. You need to understand her goals, the degree of prolapse, whether she has contraindications to uterine preservation, and how much risk she is willing to take.
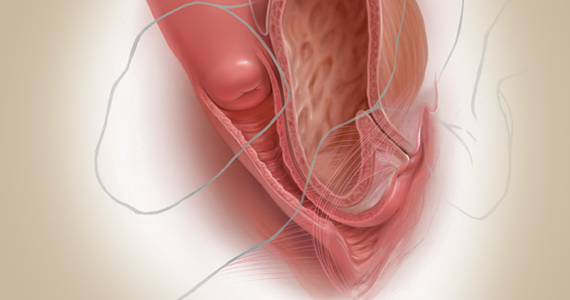
Fundamentals of the clinical evaluation
Dr. Gebhart: For a woman who wants to manage her prolapse surgically, let us consider some fundamentals of clinical diagnosis. Take me through your office evaluation of the patient reporting prolapse symptoms—her history, yes, but from a physical exam standpoint, what is important?
Dr. Karram: You want to know if this is a primary prolapse or recurrent prolapse. You want to distinguish the various segments of the pelvic floor that are prolapsing and try to quantitate that in whatever way you would like. A standardized quantification system is useful, but you should have a system within your practice that you can standardize. Then, determine if there are coexisting functional derangements and how those are being impacted by the prolapse, because that is very important.
Take a good history, and identify how badly the prolapse bothers the patient and affects her quality of life. Understand how much she is willing to do about it. Does she just want to know what it is and has no interest in a surgical intervention, versus something she definitely wants to get corrected? Then do whatever potential testing around the bladder, and bowel, based on any functional derangements and finally determine interest in maintaining sexual function. Once all this information is obtained, a detailed discussion of surgical options can be undertaken.
Dr. Gebhart: What are your clinical pearls for a patient who has prolapse and does not describe any incontinence, voiding dysfunction, or defecatory symptoms? Do we need imaging testing of any sort or is the physical exam adequate for assessing prolapse?
Dr. Walters: When you do the standardized examination of the prolapse, it is important to measure how much prolapse affects the anterior wall of the apex and/or cervix and the posterior wall. Then note that in your notes and plan your surgery accordingly.
It is useful to have the patient fully bear down and then make your measurements; then, especially if she has a full bladder, have her cough while you hold up the prolapse with a speculum or your hand to see if she has stress urinary incontinence.
Continue to: I agree that to diagnose prolapse...
Dr. Ridgeway: I agree that to diagnose prolapse, it is physical exam alone. I would not recommend any significant testing other than testing for the potential for stress incontinence.
Dr. Gebhart: Is it necessary to use the POP-Q (Pelvic Organ Prolapse Quantification system) in a nonacademic private practice setting? Or are other systems, like a Baden-Walker scoring system, adequate in the everyday practice of the experienced generalist?
Dr. Walters: The Baden-Walker system actually is adequate for use in everyday practice. However, Baden-Walker is an outdated measurement system that really is not taught anymore. I think that as older physicians finish and newer doctors come in, no one will even know what Baden-Walker is.
It is better to go ahead and start learning the POP-Q system. Everyone has electronic charts now and if you learn to use the POP-Q, you can do it very quickly and get a grading system for your chart that is reproducible for everyone.
Dr. Ridgeway: The most important thing is to assess all 3 compartments and document the amount of prolapse of each compartment. A modified POP-Q is often adequate. To do this, perform a split speculum exam and use the hymen as the reference. Zero is at the hymen, +1 is 1 cm beyond the hyman. Covering the rectum, how much does the anterior compartment prolapse in reference to the hymen? Covering the anterior compartment, get an idea of what is happening posteriorly. And the crux of any decision in my mind is what is happening at the apex or to the uterus/cervix if it is still present. It is really important to document at least those 3 compartments.
Dr. Karram: I agree. The POP-Q is the ideal, but I don’t think generalists are motivated to use it. It is very important, though, to have some anatomic landmarks, as already mentioned by Dr. Ridgeway.
Choose a surgical approach based on the clinical situation
Dr. Gebhart: How do you choose the surgical approach for someone with prolapse?
Dr. Karram: Most surgeons do what they think they do best. I have spent the majority of my career operating through the vagina, and most of that involves native tissue repairs. I almost always will do a primary prolapse through the vagina and not consider augmentation except in rare circumstances. A recurrent prolapse, a prolapsed shortened vagina, scarring, or a situation that is not straightforward has to be individualized. My basic intervention initially is almost always vaginally with native tissue.
Dr. Ridgeway: For a primary prolapse repair, I also will almost always use native tissue repair as firstline. Whether that is with hysterectomy or without, most people in the long term do very well with that. At least 70% of my repairs are done with a native tissue approach.
For a woman who has a significant prolapse posthysterectomy, especially of the anterior wall or with recurrent prolapse, I offer a laparoscopic sacrocolpopexy. The only other time I offer that as a primary approach would be for a younger woman with very significant prolapse. In that case, I will review risks and benefits with the patient and, using shared decision-making, offer either a native tissue repair or a sacrocolpopexy. For that patient, no matter what you do, given that she has many years to live, the chances are that she will likely need a second intervention.
Dr. Gebhart: Mark, how do you choose an approach for prolapse?
Dr. Walters: I do things pretty much the way Dr. Karram and Dr. Ridgeway do. For women who have a primary prolapse, I usually take a vaginal approach, and for recurrences I frequently do sacrocolpopexy with mesh or I refer to one of my partners who does more laparoscopic or robotic sacrocolpopexy.
Whether the patient needs a hysterectomy or not is evolving. Traditionally, hysterectomy is almost always done at the first prolapse repair. That is being reassessed in the United States to match what is happening in some other countries. It is possible to do nice primary prolapse repair vaginally or laparoscopically and leave the uterus in, in selected women who desire that.
Continue to: Transvaginal prolapse repair: Mesh is no longer an option...
Transvaginal prolapse repair: Mesh is no longer an option
Dr. Gebhart: What led up to the US Food and Drug Administration’s (FDA) market removal of mesh for transvaginal repair of POP?
Dr. Ridgeway: To clarify, it was not a recall—a word that many people use—it was an order to stop producing and distributing surgical mesh intended for transvaginal repair of POP.1 There is a very long history. Transvaginal mesh was introduced with the goal of improving prolapse anatomic and subjective outcomes. Over the last 13 years or so, there were adverse events that led to FDA public health notifications. Consequently, these devices were reclassified, and now require additional testing prior to approval. The newest transvaginal mesh kits were studied.
These 522 studies were completed recently and needed to show superior outcomes because, historically, the risks associated with transvaginal mesh compared to those associated with native tissue repairs are higher: higher reoperation rates, higher rates of other complications, and very minimal improvements in subjective and objective outcomes. Data were presented to the FDA, and it was deemed that these mesh kits did not improve outcomes significantly compared with native tissue repairs.
Dr. Karram: Beri, you stated that very accurately. The pro-mesh advocates were taken back by the idea that the FDA made this recommendation without allowing the outcomes to be followed longer.
Dr. Gebhart: My understanding is that the FDA had a timeline where they had to do a report and the studies had not matured to that end point; thus, they had to go with the data they had even though the studies were not completed. I think they are requesting that they be completed.
Dr. Ridgeway: Additional data will be available, some through the 522 studies, others through randomized controlled trials in which patients were already enrolled and had surgery. As far as I know, I do not think that the decision will be reversed.
Continue to: Native tissue repair and failure risk...
Native tissue repair and failure risk
Dr. Gebhart: I hear a lot that native tissue repairs fail. Mickey, as you do a lot of vaginal surgery, what are your thoughts? Should you use augmentation of some sort because native tissue fails?
Dr. Karram: There is going to be a failure rate with whatever surgery you do. I think that the failure rate with native tissue is somewhat overstated. I think a lot of that dates back to some of the things that were being promoted by mesh advocates. Initially, there was a lot of cherry-picking of native tissue data in some of those studies to promote the idea that the recurrent prolapse rates were 40% to 80%. We certainly do not see that in our patient population.
Based on our 5-year data, we have a recurrence rate of about 15% and a reoperation rate of less than 10%. That is the best I can quote based on our data. We have not followed patients longer than 5 years.
I can’t do much better than that with an augmentation; even if I get another 5% or 10% better anatomic outcome, that will be at the expense of some erosions and other complications specific to the mesh. I do think that the native tissue failure rate being promoted by a lot of individuals is a higher failure rate than what we are seeing.
Dr. Gebhart: What do you think, Mark?
Dr. Walters: Large cohort studies both at your institution, Mayo Clinic, and ours at the Cleveland Clinic mirror what Dr. Karram said, in that we have a reoperation rate somewhere between 8% and 15%. Of course, we have some failures that are stage 2 failures where patients choose not to have another operation. In general, a 10% or 12% reoperation rate at 5 to 7 years is acceptable.
Native tissue repairs probably fail at the apex a little more than mesh sacrocolpopexy. Mesh sacrocolpopexy, depending on what else you do with that operation, may have more distal vaginal failures, rates like distal rectoceles and more de novo stress urinary incontinence than we probably get with native tissue. I get some failures of the apex with native tissue repairs, but I am okay with using sacrocolpopexy as the second-line therapy in those patients.
Hysteropexy technique and pros and cons
Dr. Gebhart: Is hysteropexy a fad, or is there something to this?
Dr. Ridgeway: I do not think it is a fad. Women do feel strongly about this, and we now have data supporting this choice: randomized controlled trials of hysterectomy and prolapse repair versus hysteropexy with comparable outcomes at the short and medium term.2
The outcomes are similar, but as we said, outcomes for all prolapse repair types are not perfect. We have recurrences with sacrocolpopexy, native tissue repair, and hysteropexy. We need more data on types of hysteropexy and long-term outcomes for uterine preservation.
Dr. Walters: We have been discussing what patients think of their uterus, and some patients have very strong opinions. Some prefer to have a hysterectomy because then they don’t need to worry about cancer or do screening for cancer, and they are very happy with that. Other women with the same kind of prolapse prefer not to have a hysterectomy because philosophically they think they are better off keeping their organs. Since satisfaction is an outcome, it is useful to know what the patient wants and what she thinks about the surgical procedure.
Dr. Gebhart: For hysteropexy, do the data show that suture or a mesh augment provide an advantage one way or the other? Do we know that yet?
Dr. Walters: No, there are not enough studies with suture. There are only a few very good studies with suture hysteropexy, and they are mostly sacrospinous suture hysteropexies. Only a few studies look at mesh hysteropexy (with the Uphold device that was put on hold), or with variations of uterosacral support using strips of mesh, mostly done in other countries.
A point I want to add, if native tissue repairs fail at the apex more, why don’t you just always do sacrocolpopexy? One reason is because it might have a little higher complication rate due to the abdominal access and the fact that you are putting mesh in. If you have, for example, a 4% complication rate with the mesh but you get a better cure rate, those things balance out, and the woman may not be that much better off because of the extra complications. You have to assess the pro and con with each patient to pick what is best for her—either a more durable repair with a mesh or a little safer repair with native tissue.
Continue to: Women feel very strongly about risk...
Dr. Ridgeway: Women feel very strongly about risk. Within the same clinic I will have similar patients, and I say, “Probably in the long term this one may last a little longer but the surgery takes longer and it has a little higher complication rate.” One patient will say, “I’m not worried about the risk, I want what’s going to last the longest,” whereas a very similar patient will say, “Why would anyone pick the higher-risk operation? I want the lower risk that probably will last a long time.”
Dr. Gebhart: Beri, who should not have a hysteropexy?
Dr. Ridgeway: The biggest factor would be someone who has ever had postmenopausal bleeding. From our data, we know that if they have even had a work-up with benign results, the risk of unanticipated pathology is high. I do not recommend hysteropexy for anyone who has had postmenopausal bleeding.
For a premenopausal woman who has irregular bleeding, I also do not recommend it, because you just do not know what that future will hold. If a patient has anatomic abnormalities like large fibroids, I would not recommend it either. I would like patients to have had standard cervical cancer screening without any abnormalities for about 10 years or so.
Dr. Gebhart: What about prior cervical dysplasia?
Dr. Ridgeway: If a patient had ASCUS or low-grade dysplasia decades ago, has been normal for at least 10 years, and is currently negative for human papillomavirus, I have no problem.
Dr. Gebhart: How about women at high genetic risk for cancer?
Dr. Ridgeway: If they are at high risk for endometrial cancer, I would not recommend hysteropexy. If they are going to need an oophorectomy and/or salpingectomy for risk reduction during prolapse treatment, I usually perform a hysterectomy.
Plan surgical steps and prepare for “what if’s”
Dr. Gebhart: What tips can you provide, either regarding the evaluation or something you do surgically, that are important in a transvaginal native tissue repair?
Dr. Karram: If you have a case of posthysterectomy apical prolapse, that you think is an indication for sacrocolpopexy, in reality these are very good candidates for either sacrospinous or uterosacral suspensions. I prefer a uterosacral suspension as I feel there is less distortion of the vaginal apex compared to a sacrospinous suspension.
Dr. Ridgeway: The most critical step is setting up the OR and positioning the patient. That sets up the case for success, preventing struggles during the case. I use a high lithotomy, with careful positioning of course, but I use candy cane stirrups so that I can have an instrument stand in front of me and not struggle during the case.
Dr. Walters: My tip for everyone who is doing native tissue surgery, whether it is high McCall colpopexy or uterosacral ligament suspension or sacrocolpopexy, would be to really learn well the anatomy of each operation, including how close the ureter is, where the risk for bleeding is, and where the risk for nerve damage is.
The complications for each of these surgeries are slightly different, but there is a small risk of kinking the ureter with both uterosacral ligament suspension and the McCall, so you should do a cystoscopy as part of that operation. If you do a sacrospinous ligament suspension, use an instrument that can get a stitch into a ligament—not too close to the ischial spine and not too close to the sacrum—to avoid the risk of damage to major nerves and blood vessels and to minimize buttock and leg pain.
Continue to: Another tip is to understand...
Dr. Karram: Another tip is to understand that you are going to have potential complications intraoperatively. Think through those presurgically. You do not want to start thinking about these things and making decisions as they are happening. For example, what if I do a uterosacral suspension and I don’t see efflux of urine from the ureter? What am I going to do, and how long am I going to wait before I intervene? If I do a sacrospinous and I start to see a lot of bleeding from that area, what am I going to do? My plan would be, “I will pack the area, get extra suction, etc.” Thinking these ideas through before they occur is very helpful.
Dr. Gebhart: That is critical, to have an algorithm or a scheme in your mind. You want to think through it before it occurs because you are not always thinking as clearly when things are not going well.
I would say get good at physical examination skills in the office, then have a plan for the OR based on what you see in the office. If what is going on with the prolapse is not completely investigated and other issues are not addressed, then failure results because you did not make the diagnosis. Certainly, modify the procedure according to what you find intraoperatively, but follow through.
Indications and tips for sacrocolpopexy
Dr. Gebhart: What are the indications for sacrocolpopexy?
Dr. Ridgeway: Indications include recurrent apical prolapse, posthysterectomy prolapse, or severe prolapse in someone quite young. It is a fantastic operation with overall low risks, but this needs to be discussed with the patient.
Dr. Walters: There are some unusual circumstances—for example, the woman has a short prolapsed vagina, usually after a prior surgery—in which the best repair is a bridging piece of mesh, usually done laparoscopically, because those operations cannot be done very well vaginally to obtain a durable result.
Dr. Karram: I agree. I do not think that all recurrent prolapses mandate a sacrocolpopexy. You need to individualize, but in general the short prolapsed vagina and patients who are very young are at high risk for a recurrence.
Dr. Gebhart: An older patient might be a very good candidate, even if she had recurrence from another vaginal repair.
Beri, does the patient with a high body mass index need augmentation?
Dr. Ridgeway: That is a great question, and this has to be individualized because, while heavier patients can benefit from augmentation, in a very heavy patient, getting into that abdomen has its own set of challenges. Anatomically they get a better repair with a mesh-augmented repair like a sacrocolpopexy, but they do have increased risks. That is important to acknowledge and clarify with the patient.
Dr. Gebhart: Any surgical tip you might offer on sacrocolpopexy?
Dr. Ridgeway: Perform the operation in the same way you would an open procedure. Meaning, use the same materials, the same sutures, the same placement, and the same type of dissection in order to obtain results similar to those with an open operation. Using your assistants to manipulate the vagina and rectum is important, as well as exposure and typical careful surgical technique.
Dr. Gebhart: What is important about the placement of sutures on the anterior longitudinal ligament, and what do you need to be cognizant of?
Dr. Ridgeway: Be careful of that left common iliac vein that is a little more medial than you would expect and of the middle sacral artery, and try to differentiate between L5 and S1. In an ideal circumstance, place the suture at S1 or L5 but not the inner disc space, which is the area to avoid placement.
Historically, the recommendation is S1. Some people do L5 because of some pull out strength studies, but also because it is easier, and sometimes in that area of the anterior longitudinal ligament is much better. The key is to do enough dissection and use haptic feedback, especially with conventional laparoscopy or an open approach, to avoid placing sutures through the disc space, as there is some concern that it increases the risk for discitis or osteomyelitis in that area.
Continue to: We also have found...
Dr. Gebhart: We also have found that if you have a combined surgery with colorectal colleagues, like a rectal prolapse repair, there is a little higher risk of discitis.
Dr. Ridgeway: In my own practice I saw a combined case with a rectopexy in someone who had a biologic mesh erosion. When we reviewed the literature, a number of reported cases of discitis had either an early post-op or concurrent urinary tract infection or vaginal infection that likely predisposed them to an infection that traveled up the material.
Dr. Karram: My final comment is that a sacrocolpopexy is not a few stitches or a little mesh right at the apex. If the patient has an isolated enterocele, okay, but it is a wide mesh for a reason and it should connect to the endopelvic fascia anteriorly, posteriorly. It is a mistake to suture just a little bit of the cuff and grab it and think, “I’ve done a colpopexy” when the procedure has not been executed as it should be.
Dr. Gebhart: I want to thank our expert panel and OBG M
Continue to: Some procedures call for cystoscopy...
Dr. Gebhart: Is cystoscopy necessary in patients undergoing native tissue repair or abdominal approaches to prolapse, and should the experienced generalist have this skill?
Dr. Walters: If you are going to do prolapse surgery or surgery for stress urinary incontinence, you need to learn to do cystoscopy. Almost all specialists in urogynecology and urology would do a cystoscopy at the time of a native tissue prolapse repair, a mesh-augmented prolapse repair, or a sling procedure. Whether a generalist doing simple hysterectomies needs to do cystoscopy is controversial, and it is probably based on risk assessment of the kind of hysterectomy being done. Definitely, if you are doing prolapse repair, you probably should be doing cystoscopy at the same time.
Dr. Karram: I would take it further. For certain procedures, cystoscopy is standard of care. For example, if you are doing anything around the uterosacral ligaments, whether a McCall culdoplasty or uterosacral suspension, it is standard of care. It would be a difficult medical-legal defense issue if it was not done in those cases.
To Mark’s point, it is controversial whether universal cystoscopy should be performed on every hysterectomy or every anterior to posterior repair. We are not there yet, but certainly it is in your best interest to have a very low threshold, so if you think about doing cystoscopy, you should probably do it.
Dr. Gebhart: Is cystoscopy needed in sacrocolpopexy?
Dr. Ridgeway: We know from our own data that the risk of lower urinary tract injury is very low with sacrocolpopexy. Having said that, I agree with the position statement of the American Urogynecologic Society that says, “Universal cystoscopy should be performed at the time of all pelvic reconstruction surgeries, with the exception of operations solely for posterior compartment defects.”1
Dr. Gebhart: The reality is that we just want to identify if there is a problem or not at the time of the surgery. It does not mean you have to manage it. You could get your partner, your urologist, or another person with expertise to come in to help you.
Dr. Ridgeway: Absolutely, because intraoperative identification and treatment will prevent many unfavorable outcomes in the postoperative period.
Reference
1. Cohen SA, Carberry CL, Smilen SW. American Urogynecologic Society Consensus Statement: cystoscopy at the time of prolapse repair. Female Pelvic Med Reconstr Surg. 2018;24:258-259.
Dr. Gebhart: If a patient is a smoker and/or utilizes tobacco and you think she is a candidate for a sacrocolpopexy, are there any special considerations? How would you counsel that patient?
Dr. Walters: The risk of mesh erosion is high enough that I would try to not do any mesh prolapse repair in a woman who was a smoker, especially a heavy smoker. A more common situation is, would I put a polypropylene midurethral sling in that patient? I usually am willing to do that because it is still the best option compared with the no-mesh options. In a patient who would be a good candidate for sacrocolpopexy, I can usually do a no-mesh surgery and keep the risk low. I could always give the woman an option to quit smoking, but that tends not to be successful.
Dr. Gebhart: What is the risk of using mesh in a smoker?
Dr. Walters: An increased risk of erosion through the vaginal walls. I am not sure of the magnitude of risk, maybe 2 or 3 times higher. That is high enough that I probably would not take the risk except in unusual circumstances.
Dr. Ridgeway: A good amount of data show increased risk of mesh exposure for smokers. Those patients also tend to have a higher risk of prolapse recurrence because of coughing. Sacrocolpopexy is not my favorite operation to do in a smoker. I will work with the patient to quit, but often if it is the right operation, I will do it, with preoperative estrogen and appropriate conseling.
Dr. Gebhart: Is there still a role for vaginal mesh? While it is no longer being sold in the United States, could you fashion your own mesh for a prolapse procedure?
Dr. Walters: I can do pretty much everything I need to do without adding transvaginal mesh, and if I need a meshaugmented repair, then I would go with the sacrocolpopexy route. Having said that, data for hysteropexy do show that a mesh-augmented hysteropexy could have some advantages, whether you do it with a kit or some fashioned pieces of mesh. Most of the experiences with this are outside of the United States, so we need much more standardization of technique and tracking to answer that question.
Dr. Gebhart: Mickey, what are your thoughts regarding someone who thinks, “Mesh has been good for me, I want to stay with that. I’m going to cut my own mesh”? Are they assuming some liability now that companies are no longer marketing mesh for vaginal repair?
Dr. Karram: Unfortunately, I really think they are. It would be easy to be put in a legal corner and asked, the FDA felt that this should be pulled off the market, why are you still utilizing it? At the end of the day, what the FDA said was not inaccurate.
The studies have not shown a significant better outcome with mesh, and it is an extra intervention that, again, in the best of hands is going to have some issues. That is a dilemma many surgeons faced because they felt that that was their main way of treating prolapse—”they took away my way of successfully treating patients for years.” I do think it increases their medical-legal liability.
Dr. Ridgeway: I agree that it does increase medical-legal liability, and I can’t imagine a situation in which I would offer that. Dr. Gebhart: There are risks with all procedures, including slings for stress incontinence, but sling use is appropriate in appropriately counseled patients.
Dr. Ridgeway: Correct. I feel very strongly that the risk profile for the midurethral sling is very different from that for transvaginal mesh. Very large data sets in large groups of people support that the outcomes are favorable and the risk profile is low. Having said that, slings are not risk free, but living with severe incontinence is not risk free either.
- US Food and Drug Administration. FDA takes action to protect women's health, orders manufacturers of surgical mesh intended for transvaginal repair of pelvic organ prolapse to stop selling all devices. https://www.fda.gov/news-events/press-announcements/fda-takes-action-protect-womens-health-orders-manufacturers-surgical-mesh-intended-transvaginal. April 16, 2019. Accessed January 14, 2020.
- Detollenaere RJ, den Boon J, Stekelenburg J, et al. Sacrospinous hysteropexy versus vaginal hysterectomy with suspension of the uterosacral ligaments in women with uterine prolapse stage 2 or higher: multicentre randomised non-inferiority trial. BMJ. 2015;351:h3717.
A number of presentations at the 2019 Pelvic Anatomy and Gynecologic Surgery (PAGS) Symposium (Las Vegas, Nevada, December 12-14, 2019) focused on pelvic organ prolapse (POP) repair, including anatomic considerations, the evolution of surgical procedures, and transvaginal repair. OBG M
Nonsurgical approaches for POP: A good option for the right patient
John B. Gebhart, MD, MS: What are the nonsurgical options for POP?
Mark D. Walters, MD: Women who have prolapse could, of course, choose to continue to live with the prolapse. If they desire treatment, however, the main nonsurgical option is a combination of pessary use, possibly with some estrogen, and possibly with pelvic muscle exercises. Women who have a well-fitting pessary can be managed satisfactorily for years. If possible, women should be taught to take the pessary in and out on a regular basis to minimize their long-term complications.
Dr. Gebhart: How can nonsurgical treatment options be maximized?
Beri M. Ridgeway, MD: It depends on patient commitment. This is important to assess at the first visit when you are making management decisions, because if someone is not going to attend physical therapy or not going to continue to do the exercises, the expectation for the outcome is not going to be great.
Also, if a patient feels very uncomfortable using a pessary and really does not want it, I am fine proceeding with surgery as a first-line treatment. If the patient is committed, the ideal is to educate her and connect her with the right people, either a pelvic floor physical therapist or someone in your office who will encourage her and manage pessary use.
Dr. Gebhart: It goes back to assessing patient goals and expectations.
Mickey M. Karram, MD: If you have a patient who is a good candidate for a pessary—say she has a well-supported distal vagina and maybe a cervical prolapse or an apical prolapse—and you can fit a small pessary that will sit in the upper vagina in a comfortable fashion, it is worthwhile to explain to the patient that she is a really good candidate for this option. By contrast, someone who has a wide genital hiatus and a large rectocele will not have good success with a pessary.
Dr. Gebhart: That is important: Choose your nonsurgical patients well, those who will respond to therapy and maybe not get frustrated with it.
Dr. Walters: A problem I see is that some people are good at fitting a pessary, but they do not teach how to use it very well. When I see the patient back, she says, “What’s my long term on the pessary?” I say, “If we teach you to take it in and out, you are less likely to have any problems with it, and then you can manage it for years that way. Otherwise, you have to keep visiting a practitioner to change it and that is not necessarily a good long-term option.” At the very first visit, I teach them what a pessary is, its purpose, and how to maintain it themselves. I think that gives patients the best chance for long-term satisfaction.
Dr. Gebhart: Surgery is always an option if pessary management is not satisfactory.
Dr. Ridgeway: I also tell patients, especially those uncertain about using a pessary, “Worst case, you spend a little time to figure this out, but if it works, you can avoid surgery. If it doesn’t—the risks are very low and you perhaps wasted some time—but at least you’ll know you tried the conservative management.”
Dr. Gebhart: Mickey made an excellent point earlier that it can be a diagnostic treatment strategy as well.
Dr. Karram: If you are concerned about the prolapse worsening or negatively impacting a functional problem related to the bladder or bowel, it is good to place a pessary for a short period of time. This can potentially give you an idea of how your surgery will impact a patient’s bladder or bowel function.
Continue to: Decisions to make before choosing a surgical approach...
Decisions to make before choosing a surgical approach
Dr. Gebhart: Would you elaborate on the surgical options for managing POP?
Dr. Walters: For women with prolapse who decide they want to have surgery, the woman and the surgeon need to make a number of decisions. Some of these include whether the uterus, if present, needs to be removed; whether the woman would like to maintain sexual function or not; whether the repair would best be done vaginally only with native tissue suturing, vaginally with some augmentation (although that is not likely in the United States at this time), or through the abdomen, usually laparoscopically or robotically with a mesh-augmented sacrocolpopexy repair.
Also, we must decide whether to do additional cystocele and rectocele repairs and whether to add slings for stress incontinence, which can coexist or could develop after the prolapse repair. A lot of different decisions need to be made when choosing a prolapse repair for different women.
Dr. Ridgeway: It is shared decision-making with the patient. You need to understand her goals, the degree of prolapse, whether she has contraindications to uterine preservation, and how much risk she is willing to take.

Fundamentals of the clinical evaluation
Dr. Gebhart: For a woman who wants to manage her prolapse surgically, let us consider some fundamentals of clinical diagnosis. Take me through your office evaluation of the patient reporting prolapse symptoms—her history, yes, but from a physical exam standpoint, what is important?
Dr. Karram: You want to know if this is a primary prolapse or recurrent prolapse. You want to distinguish the various segments of the pelvic floor that are prolapsing and try to quantitate that in whatever way you would like. A standardized quantification system is useful, but you should have a system within your practice that you can standardize. Then, determine if there are coexisting functional derangements and how those are being impacted by the prolapse, because that is very important.
Take a good history, and identify how badly the prolapse bothers the patient and affects her quality of life. Understand how much she is willing to do about it. Does she just want to know what it is and has no interest in a surgical intervention, versus something she definitely wants to get corrected? Then do whatever potential testing around the bladder, and bowel, based on any functional derangements and finally determine interest in maintaining sexual function. Once all this information is obtained, a detailed discussion of surgical options can be undertaken.
Dr. Gebhart: What are your clinical pearls for a patient who has prolapse and does not describe any incontinence, voiding dysfunction, or defecatory symptoms? Do we need imaging testing of any sort or is the physical exam adequate for assessing prolapse?
Dr. Walters: When you do the standardized examination of the prolapse, it is important to measure how much prolapse affects the anterior wall of the apex and/or cervix and the posterior wall. Then note that in your notes and plan your surgery accordingly.
It is useful to have the patient fully bear down and then make your measurements; then, especially if she has a full bladder, have her cough while you hold up the prolapse with a speculum or your hand to see if she has stress urinary incontinence.
Continue to: I agree that to diagnose prolapse...
Dr. Ridgeway: I agree that to diagnose prolapse, it is physical exam alone. I would not recommend any significant testing other than testing for the potential for stress incontinence.
Dr. Gebhart: Is it necessary to use the POP-Q (Pelvic Organ Prolapse Quantification system) in a nonacademic private practice setting? Or are other systems, like a Baden-Walker scoring system, adequate in the everyday practice of the experienced generalist?
Dr. Walters: The Baden-Walker system actually is adequate for use in everyday practice. However, Baden-Walker is an outdated measurement system that really is not taught anymore. I think that as older physicians finish and newer doctors come in, no one will even know what Baden-Walker is.
It is better to go ahead and start learning the POP-Q system. Everyone has electronic charts now and if you learn to use the POP-Q, you can do it very quickly and get a grading system for your chart that is reproducible for everyone.
Dr. Ridgeway: The most important thing is to assess all 3 compartments and document the amount of prolapse of each compartment. A modified POP-Q is often adequate. To do this, perform a split speculum exam and use the hymen as the reference. Zero is at the hymen, +1 is 1 cm beyond the hyman. Covering the rectum, how much does the anterior compartment prolapse in reference to the hymen? Covering the anterior compartment, get an idea of what is happening posteriorly. And the crux of any decision in my mind is what is happening at the apex or to the uterus/cervix if it is still present. It is really important to document at least those 3 compartments.
Dr. Karram: I agree. The POP-Q is the ideal, but I don’t think generalists are motivated to use it. It is very important, though, to have some anatomic landmarks, as already mentioned by Dr. Ridgeway.
Choose a surgical approach based on the clinical situation
Dr. Gebhart: How do you choose the surgical approach for someone with prolapse?
Dr. Karram: Most surgeons do what they think they do best. I have spent the majority of my career operating through the vagina, and most of that involves native tissue repairs. I almost always will do a primary prolapse through the vagina and not consider augmentation except in rare circumstances. A recurrent prolapse, a prolapsed shortened vagina, scarring, or a situation that is not straightforward has to be individualized. My basic intervention initially is almost always vaginally with native tissue.
Dr. Ridgeway: For a primary prolapse repair, I also will almost always use native tissue repair as firstline. Whether that is with hysterectomy or without, most people in the long term do very well with that. At least 70% of my repairs are done with a native tissue approach.
For a woman who has a significant prolapse posthysterectomy, especially of the anterior wall or with recurrent prolapse, I offer a laparoscopic sacrocolpopexy. The only other time I offer that as a primary approach would be for a younger woman with very significant prolapse. In that case, I will review risks and benefits with the patient and, using shared decision-making, offer either a native tissue repair or a sacrocolpopexy. For that patient, no matter what you do, given that she has many years to live, the chances are that she will likely need a second intervention.
Dr. Gebhart: Mark, how do you choose an approach for prolapse?
Dr. Walters: I do things pretty much the way Dr. Karram and Dr. Ridgeway do. For women who have a primary prolapse, I usually take a vaginal approach, and for recurrences I frequently do sacrocolpopexy with mesh or I refer to one of my partners who does more laparoscopic or robotic sacrocolpopexy.
Whether the patient needs a hysterectomy or not is evolving. Traditionally, hysterectomy is almost always done at the first prolapse repair. That is being reassessed in the United States to match what is happening in some other countries. It is possible to do nice primary prolapse repair vaginally or laparoscopically and leave the uterus in, in selected women who desire that.
Continue to: Transvaginal prolapse repair: Mesh is no longer an option...
Transvaginal prolapse repair: Mesh is no longer an option
Dr. Gebhart: What led up to the US Food and Drug Administration’s (FDA) market removal of mesh for transvaginal repair of POP?
Dr. Ridgeway: To clarify, it was not a recall—a word that many people use—it was an order to stop producing and distributing surgical mesh intended for transvaginal repair of POP.1 There is a very long history. Transvaginal mesh was introduced with the goal of improving prolapse anatomic and subjective outcomes. Over the last 13 years or so, there were adverse events that led to FDA public health notifications. Consequently, these devices were reclassified, and now require additional testing prior to approval. The newest transvaginal mesh kits were studied.
These 522 studies were completed recently and needed to show superior outcomes because, historically, the risks associated with transvaginal mesh compared to those associated with native tissue repairs are higher: higher reoperation rates, higher rates of other complications, and very minimal improvements in subjective and objective outcomes. Data were presented to the FDA, and it was deemed that these mesh kits did not improve outcomes significantly compared with native tissue repairs.
Dr. Karram: Beri, you stated that very accurately. The pro-mesh advocates were taken back by the idea that the FDA made this recommendation without allowing the outcomes to be followed longer.
Dr. Gebhart: My understanding is that the FDA had a timeline where they had to do a report and the studies had not matured to that end point; thus, they had to go with the data they had even though the studies were not completed. I think they are requesting that they be completed.
Dr. Ridgeway: Additional data will be available, some through the 522 studies, others through randomized controlled trials in which patients were already enrolled and had surgery. As far as I know, I do not think that the decision will be reversed.
Continue to: Native tissue repair and failure risk...
Native tissue repair and failure risk
Dr. Gebhart: I hear a lot that native tissue repairs fail. Mickey, as you do a lot of vaginal surgery, what are your thoughts? Should you use augmentation of some sort because native tissue fails?
Dr. Karram: There is going to be a failure rate with whatever surgery you do. I think that the failure rate with native tissue is somewhat overstated. I think a lot of that dates back to some of the things that were being promoted by mesh advocates. Initially, there was a lot of cherry-picking of native tissue data in some of those studies to promote the idea that the recurrent prolapse rates were 40% to 80%. We certainly do not see that in our patient population.
Based on our 5-year data, we have a recurrence rate of about 15% and a reoperation rate of less than 10%. That is the best I can quote based on our data. We have not followed patients longer than 5 years.
I can’t do much better than that with an augmentation; even if I get another 5% or 10% better anatomic outcome, that will be at the expense of some erosions and other complications specific to the mesh. I do think that the native tissue failure rate being promoted by a lot of individuals is a higher failure rate than what we are seeing.
Dr. Gebhart: What do you think, Mark?
Dr. Walters: Large cohort studies both at your institution, Mayo Clinic, and ours at the Cleveland Clinic mirror what Dr. Karram said, in that we have a reoperation rate somewhere between 8% and 15%. Of course, we have some failures that are stage 2 failures where patients choose not to have another operation. In general, a 10% or 12% reoperation rate at 5 to 7 years is acceptable.
Native tissue repairs probably fail at the apex a little more than mesh sacrocolpopexy. Mesh sacrocolpopexy, depending on what else you do with that operation, may have more distal vaginal failures, rates like distal rectoceles and more de novo stress urinary incontinence than we probably get with native tissue. I get some failures of the apex with native tissue repairs, but I am okay with using sacrocolpopexy as the second-line therapy in those patients.
Hysteropexy technique and pros and cons
Dr. Gebhart: Is hysteropexy a fad, or is there something to this?
Dr. Ridgeway: I do not think it is a fad. Women do feel strongly about this, and we now have data supporting this choice: randomized controlled trials of hysterectomy and prolapse repair versus hysteropexy with comparable outcomes at the short and medium term.2
The outcomes are similar, but as we said, outcomes for all prolapse repair types are not perfect. We have recurrences with sacrocolpopexy, native tissue repair, and hysteropexy. We need more data on types of hysteropexy and long-term outcomes for uterine preservation.
Dr. Walters: We have been discussing what patients think of their uterus, and some patients have very strong opinions. Some prefer to have a hysterectomy because then they don’t need to worry about cancer or do screening for cancer, and they are very happy with that. Other women with the same kind of prolapse prefer not to have a hysterectomy because philosophically they think they are better off keeping their organs. Since satisfaction is an outcome, it is useful to know what the patient wants and what she thinks about the surgical procedure.
Dr. Gebhart: For hysteropexy, do the data show that suture or a mesh augment provide an advantage one way or the other? Do we know that yet?
Dr. Walters: No, there are not enough studies with suture. There are only a few very good studies with suture hysteropexy, and they are mostly sacrospinous suture hysteropexies. Only a few studies look at mesh hysteropexy (with the Uphold device that was put on hold), or with variations of uterosacral support using strips of mesh, mostly done in other countries.
A point I want to add, if native tissue repairs fail at the apex more, why don’t you just always do sacrocolpopexy? One reason is because it might have a little higher complication rate due to the abdominal access and the fact that you are putting mesh in. If you have, for example, a 4% complication rate with the mesh but you get a better cure rate, those things balance out, and the woman may not be that much better off because of the extra complications. You have to assess the pro and con with each patient to pick what is best for her—either a more durable repair with a mesh or a little safer repair with native tissue.
Continue to: Women feel very strongly about risk...
Dr. Ridgeway: Women feel very strongly about risk. Within the same clinic I will have similar patients, and I say, “Probably in the long term this one may last a little longer but the surgery takes longer and it has a little higher complication rate.” One patient will say, “I’m not worried about the risk, I want what’s going to last the longest,” whereas a very similar patient will say, “Why would anyone pick the higher-risk operation? I want the lower risk that probably will last a long time.”
Dr. Gebhart: Beri, who should not have a hysteropexy?
Dr. Ridgeway: The biggest factor would be someone who has ever had postmenopausal bleeding. From our data, we know that if they have even had a work-up with benign results, the risk of unanticipated pathology is high. I do not recommend hysteropexy for anyone who has had postmenopausal bleeding.
For a premenopausal woman who has irregular bleeding, I also do not recommend it, because you just do not know what that future will hold. If a patient has anatomic abnormalities like large fibroids, I would not recommend it either. I would like patients to have had standard cervical cancer screening without any abnormalities for about 10 years or so.
Dr. Gebhart: What about prior cervical dysplasia?
Dr. Ridgeway: If a patient had ASCUS or low-grade dysplasia decades ago, has been normal for at least 10 years, and is currently negative for human papillomavirus, I have no problem.
Dr. Gebhart: How about women at high genetic risk for cancer?
Dr. Ridgeway: If they are at high risk for endometrial cancer, I would not recommend hysteropexy. If they are going to need an oophorectomy and/or salpingectomy for risk reduction during prolapse treatment, I usually perform a hysterectomy.
Plan surgical steps and prepare for “what if’s”
Dr. Gebhart: What tips can you provide, either regarding the evaluation or something you do surgically, that are important in a transvaginal native tissue repair?
Dr. Karram: If you have a case of posthysterectomy apical prolapse, that you think is an indication for sacrocolpopexy, in reality these are very good candidates for either sacrospinous or uterosacral suspensions. I prefer a uterosacral suspension as I feel there is less distortion of the vaginal apex compared to a sacrospinous suspension.
Dr. Ridgeway: The most critical step is setting up the OR and positioning the patient. That sets up the case for success, preventing struggles during the case. I use a high lithotomy, with careful positioning of course, but I use candy cane stirrups so that I can have an instrument stand in front of me and not struggle during the case.
Dr. Walters: My tip for everyone who is doing native tissue surgery, whether it is high McCall colpopexy or uterosacral ligament suspension or sacrocolpopexy, would be to really learn well the anatomy of each operation, including how close the ureter is, where the risk for bleeding is, and where the risk for nerve damage is.
The complications for each of these surgeries are slightly different, but there is a small risk of kinking the ureter with both uterosacral ligament suspension and the McCall, so you should do a cystoscopy as part of that operation. If you do a sacrospinous ligament suspension, use an instrument that can get a stitch into a ligament—not too close to the ischial spine and not too close to the sacrum—to avoid the risk of damage to major nerves and blood vessels and to minimize buttock and leg pain.
Continue to: Another tip is to understand...
Dr. Karram: Another tip is to understand that you are going to have potential complications intraoperatively. Think through those presurgically. You do not want to start thinking about these things and making decisions as they are happening. For example, what if I do a uterosacral suspension and I don’t see efflux of urine from the ureter? What am I going to do, and how long am I going to wait before I intervene? If I do a sacrospinous and I start to see a lot of bleeding from that area, what am I going to do? My plan would be, “I will pack the area, get extra suction, etc.” Thinking these ideas through before they occur is very helpful.
Dr. Gebhart: That is critical, to have an algorithm or a scheme in your mind. You want to think through it before it occurs because you are not always thinking as clearly when things are not going well.
I would say get good at physical examination skills in the office, then have a plan for the OR based on what you see in the office. If what is going on with the prolapse is not completely investigated and other issues are not addressed, then failure results because you did not make the diagnosis. Certainly, modify the procedure according to what you find intraoperatively, but follow through.
Indications and tips for sacrocolpopexy
Dr. Gebhart: What are the indications for sacrocolpopexy?
Dr. Ridgeway: Indications include recurrent apical prolapse, posthysterectomy prolapse, or severe prolapse in someone quite young. It is a fantastic operation with overall low risks, but this needs to be discussed with the patient.
Dr. Walters: There are some unusual circumstances—for example, the woman has a short prolapsed vagina, usually after a prior surgery—in which the best repair is a bridging piece of mesh, usually done laparoscopically, because those operations cannot be done very well vaginally to obtain a durable result.
Dr. Karram: I agree. I do not think that all recurrent prolapses mandate a sacrocolpopexy. You need to individualize, but in general the short prolapsed vagina and patients who are very young are at high risk for a recurrence.
Dr. Gebhart: An older patient might be a very good candidate, even if she had recurrence from another vaginal repair.
Beri, does the patient with a high body mass index need augmentation?
Dr. Ridgeway: That is a great question, and this has to be individualized because, while heavier patients can benefit from augmentation, in a very heavy patient, getting into that abdomen has its own set of challenges. Anatomically they get a better repair with a mesh-augmented repair like a sacrocolpopexy, but they do have increased risks. That is important to acknowledge and clarify with the patient.
Dr. Gebhart: Any surgical tip you might offer on sacrocolpopexy?
Dr. Ridgeway: Perform the operation in the same way you would an open procedure. Meaning, use the same materials, the same sutures, the same placement, and the same type of dissection in order to obtain results similar to those with an open operation. Using your assistants to manipulate the vagina and rectum is important, as well as exposure and typical careful surgical technique.
Dr. Gebhart: What is important about the placement of sutures on the anterior longitudinal ligament, and what do you need to be cognizant of?
Dr. Ridgeway: Be careful of that left common iliac vein that is a little more medial than you would expect and of the middle sacral artery, and try to differentiate between L5 and S1. In an ideal circumstance, place the suture at S1 or L5 but not the inner disc space, which is the area to avoid placement.
Historically, the recommendation is S1. Some people do L5 because of some pull out strength studies, but also because it is easier, and sometimes in that area of the anterior longitudinal ligament is much better. The key is to do enough dissection and use haptic feedback, especially with conventional laparoscopy or an open approach, to avoid placing sutures through the disc space, as there is some concern that it increases the risk for discitis or osteomyelitis in that area.
Continue to: We also have found...
Dr. Gebhart: We also have found that if you have a combined surgery with colorectal colleagues, like a rectal prolapse repair, there is a little higher risk of discitis.
Dr. Ridgeway: In my own practice I saw a combined case with a rectopexy in someone who had a biologic mesh erosion. When we reviewed the literature, a number of reported cases of discitis had either an early post-op or concurrent urinary tract infection or vaginal infection that likely predisposed them to an infection that traveled up the material.
Dr. Karram: My final comment is that a sacrocolpopexy is not a few stitches or a little mesh right at the apex. If the patient has an isolated enterocele, okay, but it is a wide mesh for a reason and it should connect to the endopelvic fascia anteriorly, posteriorly. It is a mistake to suture just a little bit of the cuff and grab it and think, “I’ve done a colpopexy” when the procedure has not been executed as it should be.
Dr. Gebhart: I want to thank our expert panel and OBG M
Continue to: Some procedures call for cystoscopy...
Dr. Gebhart: Is cystoscopy necessary in patients undergoing native tissue repair or abdominal approaches to prolapse, and should the experienced generalist have this skill?
Dr. Walters: If you are going to do prolapse surgery or surgery for stress urinary incontinence, you need to learn to do cystoscopy. Almost all specialists in urogynecology and urology would do a cystoscopy at the time of a native tissue prolapse repair, a mesh-augmented prolapse repair, or a sling procedure. Whether a generalist doing simple hysterectomies needs to do cystoscopy is controversial, and it is probably based on risk assessment of the kind of hysterectomy being done. Definitely, if you are doing prolapse repair, you probably should be doing cystoscopy at the same time.
Dr. Karram: I would take it further. For certain procedures, cystoscopy is standard of care. For example, if you are doing anything around the uterosacral ligaments, whether a McCall culdoplasty or uterosacral suspension, it is standard of care. It would be a difficult medical-legal defense issue if it was not done in those cases.
To Mark’s point, it is controversial whether universal cystoscopy should be performed on every hysterectomy or every anterior to posterior repair. We are not there yet, but certainly it is in your best interest to have a very low threshold, so if you think about doing cystoscopy, you should probably do it.
Dr. Gebhart: Is cystoscopy needed in sacrocolpopexy?
Dr. Ridgeway: We know from our own data that the risk of lower urinary tract injury is very low with sacrocolpopexy. Having said that, I agree with the position statement of the American Urogynecologic Society that says, “Universal cystoscopy should be performed at the time of all pelvic reconstruction surgeries, with the exception of operations solely for posterior compartment defects.”1
Dr. Gebhart: The reality is that we just want to identify if there is a problem or not at the time of the surgery. It does not mean you have to manage it. You could get your partner, your urologist, or another person with expertise to come in to help you.
Dr. Ridgeway: Absolutely, because intraoperative identification and treatment will prevent many unfavorable outcomes in the postoperative period.
Reference
1. Cohen SA, Carberry CL, Smilen SW. American Urogynecologic Society Consensus Statement: cystoscopy at the time of prolapse repair. Female Pelvic Med Reconstr Surg. 2018;24:258-259.
Dr. Gebhart: If a patient is a smoker and/or utilizes tobacco and you think she is a candidate for a sacrocolpopexy, are there any special considerations? How would you counsel that patient?
Dr. Walters: The risk of mesh erosion is high enough that I would try to not do any mesh prolapse repair in a woman who was a smoker, especially a heavy smoker. A more common situation is, would I put a polypropylene midurethral sling in that patient? I usually am willing to do that because it is still the best option compared with the no-mesh options. In a patient who would be a good candidate for sacrocolpopexy, I can usually do a no-mesh surgery and keep the risk low. I could always give the woman an option to quit smoking, but that tends not to be successful.
Dr. Gebhart: What is the risk of using mesh in a smoker?
Dr. Walters: An increased risk of erosion through the vaginal walls. I am not sure of the magnitude of risk, maybe 2 or 3 times higher. That is high enough that I probably would not take the risk except in unusual circumstances.
Dr. Ridgeway: A good amount of data show increased risk of mesh exposure for smokers. Those patients also tend to have a higher risk of prolapse recurrence because of coughing. Sacrocolpopexy is not my favorite operation to do in a smoker. I will work with the patient to quit, but often if it is the right operation, I will do it, with preoperative estrogen and appropriate conseling.
Dr. Gebhart: Is there still a role for vaginal mesh? While it is no longer being sold in the United States, could you fashion your own mesh for a prolapse procedure?
Dr. Walters: I can do pretty much everything I need to do without adding transvaginal mesh, and if I need a meshaugmented repair, then I would go with the sacrocolpopexy route. Having said that, data for hysteropexy do show that a mesh-augmented hysteropexy could have some advantages, whether you do it with a kit or some fashioned pieces of mesh. Most of the experiences with this are outside of the United States, so we need much more standardization of technique and tracking to answer that question.
Dr. Gebhart: Mickey, what are your thoughts regarding someone who thinks, “Mesh has been good for me, I want to stay with that. I’m going to cut my own mesh”? Are they assuming some liability now that companies are no longer marketing mesh for vaginal repair?
Dr. Karram: Unfortunately, I really think they are. It would be easy to be put in a legal corner and asked, the FDA felt that this should be pulled off the market, why are you still utilizing it? At the end of the day, what the FDA said was not inaccurate.
The studies have not shown a significant better outcome with mesh, and it is an extra intervention that, again, in the best of hands is going to have some issues. That is a dilemma many surgeons faced because they felt that that was their main way of treating prolapse—”they took away my way of successfully treating patients for years.” I do think it increases their medical-legal liability.
Dr. Ridgeway: I agree that it does increase medical-legal liability, and I can’t imagine a situation in which I would offer that. Dr. Gebhart: There are risks with all procedures, including slings for stress incontinence, but sling use is appropriate in appropriately counseled patients.
Dr. Ridgeway: Correct. I feel very strongly that the risk profile for the midurethral sling is very different from that for transvaginal mesh. Very large data sets in large groups of people support that the outcomes are favorable and the risk profile is low. Having said that, slings are not risk free, but living with severe incontinence is not risk free either.
A number of presentations at the 2019 Pelvic Anatomy and Gynecologic Surgery (PAGS) Symposium (Las Vegas, Nevada, December 12-14, 2019) focused on pelvic organ prolapse (POP) repair, including anatomic considerations, the evolution of surgical procedures, and transvaginal repair. OBG M
Nonsurgical approaches for POP: A good option for the right patient
John B. Gebhart, MD, MS: What are the nonsurgical options for POP?
Mark D. Walters, MD: Women who have prolapse could, of course, choose to continue to live with the prolapse. If they desire treatment, however, the main nonsurgical option is a combination of pessary use, possibly with some estrogen, and possibly with pelvic muscle exercises. Women who have a well-fitting pessary can be managed satisfactorily for years. If possible, women should be taught to take the pessary in and out on a regular basis to minimize their long-term complications.
Dr. Gebhart: How can nonsurgical treatment options be maximized?
Beri M. Ridgeway, MD: It depends on patient commitment. This is important to assess at the first visit when you are making management decisions, because if someone is not going to attend physical therapy or not going to continue to do the exercises, the expectation for the outcome is not going to be great.
Also, if a patient feels very uncomfortable using a pessary and really does not want it, I am fine proceeding with surgery as a first-line treatment. If the patient is committed, the ideal is to educate her and connect her with the right people, either a pelvic floor physical therapist or someone in your office who will encourage her and manage pessary use.
Dr. Gebhart: It goes back to assessing patient goals and expectations.
Mickey M. Karram, MD: If you have a patient who is a good candidate for a pessary—say she has a well-supported distal vagina and maybe a cervical prolapse or an apical prolapse—and you can fit a small pessary that will sit in the upper vagina in a comfortable fashion, it is worthwhile to explain to the patient that she is a really good candidate for this option. By contrast, someone who has a wide genital hiatus and a large rectocele will not have good success with a pessary.
Dr. Gebhart: That is important: Choose your nonsurgical patients well, those who will respond to therapy and maybe not get frustrated with it.
Dr. Walters: A problem I see is that some people are good at fitting a pessary, but they do not teach how to use it very well. When I see the patient back, she says, “What’s my long term on the pessary?” I say, “If we teach you to take it in and out, you are less likely to have any problems with it, and then you can manage it for years that way. Otherwise, you have to keep visiting a practitioner to change it and that is not necessarily a good long-term option.” At the very first visit, I teach them what a pessary is, its purpose, and how to maintain it themselves. I think that gives patients the best chance for long-term satisfaction.
Dr. Gebhart: Surgery is always an option if pessary management is not satisfactory.
Dr. Ridgeway: I also tell patients, especially those uncertain about using a pessary, “Worst case, you spend a little time to figure this out, but if it works, you can avoid surgery. If it doesn’t—the risks are very low and you perhaps wasted some time—but at least you’ll know you tried the conservative management.”
Dr. Gebhart: Mickey made an excellent point earlier that it can be a diagnostic treatment strategy as well.
Dr. Karram: If you are concerned about the prolapse worsening or negatively impacting a functional problem related to the bladder or bowel, it is good to place a pessary for a short period of time. This can potentially give you an idea of how your surgery will impact a patient’s bladder or bowel function.
Continue to: Decisions to make before choosing a surgical approach...
Decisions to make before choosing a surgical approach
Dr. Gebhart: Would you elaborate on the surgical options for managing POP?
Dr. Walters: For women with prolapse who decide they want to have surgery, the woman and the surgeon need to make a number of decisions. Some of these include whether the uterus, if present, needs to be removed; whether the woman would like to maintain sexual function or not; whether the repair would best be done vaginally only with native tissue suturing, vaginally with some augmentation (although that is not likely in the United States at this time), or through the abdomen, usually laparoscopically or robotically with a mesh-augmented sacrocolpopexy repair.
Also, we must decide whether to do additional cystocele and rectocele repairs and whether to add slings for stress incontinence, which can coexist or could develop after the prolapse repair. A lot of different decisions need to be made when choosing a prolapse repair for different women.
Dr. Ridgeway: It is shared decision-making with the patient. You need to understand her goals, the degree of prolapse, whether she has contraindications to uterine preservation, and how much risk she is willing to take.

Fundamentals of the clinical evaluation
Dr. Gebhart: For a woman who wants to manage her prolapse surgically, let us consider some fundamentals of clinical diagnosis. Take me through your office evaluation of the patient reporting prolapse symptoms—her history, yes, but from a physical exam standpoint, what is important?
Dr. Karram: You want to know if this is a primary prolapse or recurrent prolapse. You want to distinguish the various segments of the pelvic floor that are prolapsing and try to quantitate that in whatever way you would like. A standardized quantification system is useful, but you should have a system within your practice that you can standardize. Then, determine if there are coexisting functional derangements and how those are being impacted by the prolapse, because that is very important.
Take a good history, and identify how badly the prolapse bothers the patient and affects her quality of life. Understand how much she is willing to do about it. Does she just want to know what it is and has no interest in a surgical intervention, versus something she definitely wants to get corrected? Then do whatever potential testing around the bladder, and bowel, based on any functional derangements and finally determine interest in maintaining sexual function. Once all this information is obtained, a detailed discussion of surgical options can be undertaken.
Dr. Gebhart: What are your clinical pearls for a patient who has prolapse and does not describe any incontinence, voiding dysfunction, or defecatory symptoms? Do we need imaging testing of any sort or is the physical exam adequate for assessing prolapse?
Dr. Walters: When you do the standardized examination of the prolapse, it is important to measure how much prolapse affects the anterior wall of the apex and/or cervix and the posterior wall. Then note that in your notes and plan your surgery accordingly.
It is useful to have the patient fully bear down and then make your measurements; then, especially if she has a full bladder, have her cough while you hold up the prolapse with a speculum or your hand to see if she has stress urinary incontinence.
Continue to: I agree that to diagnose prolapse...
Dr. Ridgeway: I agree that to diagnose prolapse, it is physical exam alone. I would not recommend any significant testing other than testing for the potential for stress incontinence.
Dr. Gebhart: Is it necessary to use the POP-Q (Pelvic Organ Prolapse Quantification system) in a nonacademic private practice setting? Or are other systems, like a Baden-Walker scoring system, adequate in the everyday practice of the experienced generalist?
Dr. Walters: The Baden-Walker system actually is adequate for use in everyday practice. However, Baden-Walker is an outdated measurement system that really is not taught anymore. I think that as older physicians finish and newer doctors come in, no one will even know what Baden-Walker is.
It is better to go ahead and start learning the POP-Q system. Everyone has electronic charts now and if you learn to use the POP-Q, you can do it very quickly and get a grading system for your chart that is reproducible for everyone.
Dr. Ridgeway: The most important thing is to assess all 3 compartments and document the amount of prolapse of each compartment. A modified POP-Q is often adequate. To do this, perform a split speculum exam and use the hymen as the reference. Zero is at the hymen, +1 is 1 cm beyond the hyman. Covering the rectum, how much does the anterior compartment prolapse in reference to the hymen? Covering the anterior compartment, get an idea of what is happening posteriorly. And the crux of any decision in my mind is what is happening at the apex or to the uterus/cervix if it is still present. It is really important to document at least those 3 compartments.
Dr. Karram: I agree. The POP-Q is the ideal, but I don’t think generalists are motivated to use it. It is very important, though, to have some anatomic landmarks, as already mentioned by Dr. Ridgeway.
Choose a surgical approach based on the clinical situation
Dr. Gebhart: How do you choose the surgical approach for someone with prolapse?
Dr. Karram: Most surgeons do what they think they do best. I have spent the majority of my career operating through the vagina, and most of that involves native tissue repairs. I almost always will do a primary prolapse through the vagina and not consider augmentation except in rare circumstances. A recurrent prolapse, a prolapsed shortened vagina, scarring, or a situation that is not straightforward has to be individualized. My basic intervention initially is almost always vaginally with native tissue.
Dr. Ridgeway: For a primary prolapse repair, I also will almost always use native tissue repair as firstline. Whether that is with hysterectomy or without, most people in the long term do very well with that. At least 70% of my repairs are done with a native tissue approach.
For a woman who has a significant prolapse posthysterectomy, especially of the anterior wall or with recurrent prolapse, I offer a laparoscopic sacrocolpopexy. The only other time I offer that as a primary approach would be for a younger woman with very significant prolapse. In that case, I will review risks and benefits with the patient and, using shared decision-making, offer either a native tissue repair or a sacrocolpopexy. For that patient, no matter what you do, given that she has many years to live, the chances are that she will likely need a second intervention.
Dr. Gebhart: Mark, how do you choose an approach for prolapse?
Dr. Walters: I do things pretty much the way Dr. Karram and Dr. Ridgeway do. For women who have a primary prolapse, I usually take a vaginal approach, and for recurrences I frequently do sacrocolpopexy with mesh or I refer to one of my partners who does more laparoscopic or robotic sacrocolpopexy.
Whether the patient needs a hysterectomy or not is evolving. Traditionally, hysterectomy is almost always done at the first prolapse repair. That is being reassessed in the United States to match what is happening in some other countries. It is possible to do nice primary prolapse repair vaginally or laparoscopically and leave the uterus in, in selected women who desire that.
Continue to: Transvaginal prolapse repair: Mesh is no longer an option...
Transvaginal prolapse repair: Mesh is no longer an option
Dr. Gebhart: What led up to the US Food and Drug Administration’s (FDA) market removal of mesh for transvaginal repair of POP?
Dr. Ridgeway: To clarify, it was not a recall—a word that many people use—it was an order to stop producing and distributing surgical mesh intended for transvaginal repair of POP.1 There is a very long history. Transvaginal mesh was introduced with the goal of improving prolapse anatomic and subjective outcomes. Over the last 13 years or so, there were adverse events that led to FDA public health notifications. Consequently, these devices were reclassified, and now require additional testing prior to approval. The newest transvaginal mesh kits were studied.
These 522 studies were completed recently and needed to show superior outcomes because, historically, the risks associated with transvaginal mesh compared to those associated with native tissue repairs are higher: higher reoperation rates, higher rates of other complications, and very minimal improvements in subjective and objective outcomes. Data were presented to the FDA, and it was deemed that these mesh kits did not improve outcomes significantly compared with native tissue repairs.
Dr. Karram: Beri, you stated that very accurately. The pro-mesh advocates were taken back by the idea that the FDA made this recommendation without allowing the outcomes to be followed longer.
Dr. Gebhart: My understanding is that the FDA had a timeline where they had to do a report and the studies had not matured to that end point; thus, they had to go with the data they had even though the studies were not completed. I think they are requesting that they be completed.
Dr. Ridgeway: Additional data will be available, some through the 522 studies, others through randomized controlled trials in which patients were already enrolled and had surgery. As far as I know, I do not think that the decision will be reversed.
Continue to: Native tissue repair and failure risk...
Native tissue repair and failure risk
Dr. Gebhart: I hear a lot that native tissue repairs fail. Mickey, as you do a lot of vaginal surgery, what are your thoughts? Should you use augmentation of some sort because native tissue fails?
Dr. Karram: There is going to be a failure rate with whatever surgery you do. I think that the failure rate with native tissue is somewhat overstated. I think a lot of that dates back to some of the things that were being promoted by mesh advocates. Initially, there was a lot of cherry-picking of native tissue data in some of those studies to promote the idea that the recurrent prolapse rates were 40% to 80%. We certainly do not see that in our patient population.
Based on our 5-year data, we have a recurrence rate of about 15% and a reoperation rate of less than 10%. That is the best I can quote based on our data. We have not followed patients longer than 5 years.
I can’t do much better than that with an augmentation; even if I get another 5% or 10% better anatomic outcome, that will be at the expense of some erosions and other complications specific to the mesh. I do think that the native tissue failure rate being promoted by a lot of individuals is a higher failure rate than what we are seeing.
Dr. Gebhart: What do you think, Mark?
Dr. Walters: Large cohort studies both at your institution, Mayo Clinic, and ours at the Cleveland Clinic mirror what Dr. Karram said, in that we have a reoperation rate somewhere between 8% and 15%. Of course, we have some failures that are stage 2 failures where patients choose not to have another operation. In general, a 10% or 12% reoperation rate at 5 to 7 years is acceptable.
Native tissue repairs probably fail at the apex a little more than mesh sacrocolpopexy. Mesh sacrocolpopexy, depending on what else you do with that operation, may have more distal vaginal failures, rates like distal rectoceles and more de novo stress urinary incontinence than we probably get with native tissue. I get some failures of the apex with native tissue repairs, but I am okay with using sacrocolpopexy as the second-line therapy in those patients.
Hysteropexy technique and pros and cons
Dr. Gebhart: Is hysteropexy a fad, or is there something to this?
Dr. Ridgeway: I do not think it is a fad. Women do feel strongly about this, and we now have data supporting this choice: randomized controlled trials of hysterectomy and prolapse repair versus hysteropexy with comparable outcomes at the short and medium term.2
The outcomes are similar, but as we said, outcomes for all prolapse repair types are not perfect. We have recurrences with sacrocolpopexy, native tissue repair, and hysteropexy. We need more data on types of hysteropexy and long-term outcomes for uterine preservation.
Dr. Walters: We have been discussing what patients think of their uterus, and some patients have very strong opinions. Some prefer to have a hysterectomy because then they don’t need to worry about cancer or do screening for cancer, and they are very happy with that. Other women with the same kind of prolapse prefer not to have a hysterectomy because philosophically they think they are better off keeping their organs. Since satisfaction is an outcome, it is useful to know what the patient wants and what she thinks about the surgical procedure.
Dr. Gebhart: For hysteropexy, do the data show that suture or a mesh augment provide an advantage one way or the other? Do we know that yet?
Dr. Walters: No, there are not enough studies with suture. There are only a few very good studies with suture hysteropexy, and they are mostly sacrospinous suture hysteropexies. Only a few studies look at mesh hysteropexy (with the Uphold device that was put on hold), or with variations of uterosacral support using strips of mesh, mostly done in other countries.
A point I want to add, if native tissue repairs fail at the apex more, why don’t you just always do sacrocolpopexy? One reason is because it might have a little higher complication rate due to the abdominal access and the fact that you are putting mesh in. If you have, for example, a 4% complication rate with the mesh but you get a better cure rate, those things balance out, and the woman may not be that much better off because of the extra complications. You have to assess the pro and con with each patient to pick what is best for her—either a more durable repair with a mesh or a little safer repair with native tissue.
Continue to: Women feel very strongly about risk...
Dr. Ridgeway: Women feel very strongly about risk. Within the same clinic I will have similar patients, and I say, “Probably in the long term this one may last a little longer but the surgery takes longer and it has a little higher complication rate.” One patient will say, “I’m not worried about the risk, I want what’s going to last the longest,” whereas a very similar patient will say, “Why would anyone pick the higher-risk operation? I want the lower risk that probably will last a long time.”
Dr. Gebhart: Beri, who should not have a hysteropexy?
Dr. Ridgeway: The biggest factor would be someone who has ever had postmenopausal bleeding. From our data, we know that if they have even had a work-up with benign results, the risk of unanticipated pathology is high. I do not recommend hysteropexy for anyone who has had postmenopausal bleeding.
For a premenopausal woman who has irregular bleeding, I also do not recommend it, because you just do not know what that future will hold. If a patient has anatomic abnormalities like large fibroids, I would not recommend it either. I would like patients to have had standard cervical cancer screening without any abnormalities for about 10 years or so.
Dr. Gebhart: What about prior cervical dysplasia?
Dr. Ridgeway: If a patient had ASCUS or low-grade dysplasia decades ago, has been normal for at least 10 years, and is currently negative for human papillomavirus, I have no problem.
Dr. Gebhart: How about women at high genetic risk for cancer?
Dr. Ridgeway: If they are at high risk for endometrial cancer, I would not recommend hysteropexy. If they are going to need an oophorectomy and/or salpingectomy for risk reduction during prolapse treatment, I usually perform a hysterectomy.
Plan surgical steps and prepare for “what if’s”
Dr. Gebhart: What tips can you provide, either regarding the evaluation or something you do surgically, that are important in a transvaginal native tissue repair?
Dr. Karram: If you have a case of posthysterectomy apical prolapse, that you think is an indication for sacrocolpopexy, in reality these are very good candidates for either sacrospinous or uterosacral suspensions. I prefer a uterosacral suspension as I feel there is less distortion of the vaginal apex compared to a sacrospinous suspension.
Dr. Ridgeway: The most critical step is setting up the OR and positioning the patient. That sets up the case for success, preventing struggles during the case. I use a high lithotomy, with careful positioning of course, but I use candy cane stirrups so that I can have an instrument stand in front of me and not struggle during the case.
Dr. Walters: My tip for everyone who is doing native tissue surgery, whether it is high McCall colpopexy or uterosacral ligament suspension or sacrocolpopexy, would be to really learn well the anatomy of each operation, including how close the ureter is, where the risk for bleeding is, and where the risk for nerve damage is.
The complications for each of these surgeries are slightly different, but there is a small risk of kinking the ureter with both uterosacral ligament suspension and the McCall, so you should do a cystoscopy as part of that operation. If you do a sacrospinous ligament suspension, use an instrument that can get a stitch into a ligament—not too close to the ischial spine and not too close to the sacrum—to avoid the risk of damage to major nerves and blood vessels and to minimize buttock and leg pain.
Continue to: Another tip is to understand...
Dr. Karram: Another tip is to understand that you are going to have potential complications intraoperatively. Think through those presurgically. You do not want to start thinking about these things and making decisions as they are happening. For example, what if I do a uterosacral suspension and I don’t see efflux of urine from the ureter? What am I going to do, and how long am I going to wait before I intervene? If I do a sacrospinous and I start to see a lot of bleeding from that area, what am I going to do? My plan would be, “I will pack the area, get extra suction, etc.” Thinking these ideas through before they occur is very helpful.
Dr. Gebhart: That is critical, to have an algorithm or a scheme in your mind. You want to think through it before it occurs because you are not always thinking as clearly when things are not going well.
I would say get good at physical examination skills in the office, then have a plan for the OR based on what you see in the office. If what is going on with the prolapse is not completely investigated and other issues are not addressed, then failure results because you did not make the diagnosis. Certainly, modify the procedure according to what you find intraoperatively, but follow through.
Indications and tips for sacrocolpopexy
Dr. Gebhart: What are the indications for sacrocolpopexy?
Dr. Ridgeway: Indications include recurrent apical prolapse, posthysterectomy prolapse, or severe prolapse in someone quite young. It is a fantastic operation with overall low risks, but this needs to be discussed with the patient.
Dr. Walters: There are some unusual circumstances—for example, the woman has a short prolapsed vagina, usually after a prior surgery—in which the best repair is a bridging piece of mesh, usually done laparoscopically, because those operations cannot be done very well vaginally to obtain a durable result.
Dr. Karram: I agree. I do not think that all recurrent prolapses mandate a sacrocolpopexy. You need to individualize, but in general the short prolapsed vagina and patients who are very young are at high risk for a recurrence.
Dr. Gebhart: An older patient might be a very good candidate, even if she had recurrence from another vaginal repair.
Beri, does the patient with a high body mass index need augmentation?
Dr. Ridgeway: That is a great question, and this has to be individualized because, while heavier patients can benefit from augmentation, in a very heavy patient, getting into that abdomen has its own set of challenges. Anatomically they get a better repair with a mesh-augmented repair like a sacrocolpopexy, but they do have increased risks. That is important to acknowledge and clarify with the patient.
Dr. Gebhart: Any surgical tip you might offer on sacrocolpopexy?
Dr. Ridgeway: Perform the operation in the same way you would an open procedure. Meaning, use the same materials, the same sutures, the same placement, and the same type of dissection in order to obtain results similar to those with an open operation. Using your assistants to manipulate the vagina and rectum is important, as well as exposure and typical careful surgical technique.
Dr. Gebhart: What is important about the placement of sutures on the anterior longitudinal ligament, and what do you need to be cognizant of?
Dr. Ridgeway: Be careful of that left common iliac vein that is a little more medial than you would expect and of the middle sacral artery, and try to differentiate between L5 and S1. In an ideal circumstance, place the suture at S1 or L5 but not the inner disc space, which is the area to avoid placement.
Historically, the recommendation is S1. Some people do L5 because of some pull out strength studies, but also because it is easier, and sometimes in that area of the anterior longitudinal ligament is much better. The key is to do enough dissection and use haptic feedback, especially with conventional laparoscopy or an open approach, to avoid placing sutures through the disc space, as there is some concern that it increases the risk for discitis or osteomyelitis in that area.
Continue to: We also have found...
Dr. Gebhart: We also have found that if you have a combined surgery with colorectal colleagues, like a rectal prolapse repair, there is a little higher risk of discitis.
Dr. Ridgeway: In my own practice I saw a combined case with a rectopexy in someone who had a biologic mesh erosion. When we reviewed the literature, a number of reported cases of discitis had either an early post-op or concurrent urinary tract infection or vaginal infection that likely predisposed them to an infection that traveled up the material.
Dr. Karram: My final comment is that a sacrocolpopexy is not a few stitches or a little mesh right at the apex. If the patient has an isolated enterocele, okay, but it is a wide mesh for a reason and it should connect to the endopelvic fascia anteriorly, posteriorly. It is a mistake to suture just a little bit of the cuff and grab it and think, “I’ve done a colpopexy” when the procedure has not been executed as it should be.
Dr. Gebhart: I want to thank our expert panel and OBG M
Continue to: Some procedures call for cystoscopy...
Dr. Gebhart: Is cystoscopy necessary in patients undergoing native tissue repair or abdominal approaches to prolapse, and should the experienced generalist have this skill?
Dr. Walters: If you are going to do prolapse surgery or surgery for stress urinary incontinence, you need to learn to do cystoscopy. Almost all specialists in urogynecology and urology would do a cystoscopy at the time of a native tissue prolapse repair, a mesh-augmented prolapse repair, or a sling procedure. Whether a generalist doing simple hysterectomies needs to do cystoscopy is controversial, and it is probably based on risk assessment of the kind of hysterectomy being done. Definitely, if you are doing prolapse repair, you probably should be doing cystoscopy at the same time.
Dr. Karram: I would take it further. For certain procedures, cystoscopy is standard of care. For example, if you are doing anything around the uterosacral ligaments, whether a McCall culdoplasty or uterosacral suspension, it is standard of care. It would be a difficult medical-legal defense issue if it was not done in those cases.
To Mark’s point, it is controversial whether universal cystoscopy should be performed on every hysterectomy or every anterior to posterior repair. We are not there yet, but certainly it is in your best interest to have a very low threshold, so if you think about doing cystoscopy, you should probably do it.
Dr. Gebhart: Is cystoscopy needed in sacrocolpopexy?
Dr. Ridgeway: We know from our own data that the risk of lower urinary tract injury is very low with sacrocolpopexy. Having said that, I agree with the position statement of the American Urogynecologic Society that says, “Universal cystoscopy should be performed at the time of all pelvic reconstruction surgeries, with the exception of operations solely for posterior compartment defects.”1
Dr. Gebhart: The reality is that we just want to identify if there is a problem or not at the time of the surgery. It does not mean you have to manage it. You could get your partner, your urologist, or another person with expertise to come in to help you.
Dr. Ridgeway: Absolutely, because intraoperative identification and treatment will prevent many unfavorable outcomes in the postoperative period.
Reference
1. Cohen SA, Carberry CL, Smilen SW. American Urogynecologic Society Consensus Statement: cystoscopy at the time of prolapse repair. Female Pelvic Med Reconstr Surg. 2018;24:258-259.
Dr. Gebhart: If a patient is a smoker and/or utilizes tobacco and you think she is a candidate for a sacrocolpopexy, are there any special considerations? How would you counsel that patient?
Dr. Walters: The risk of mesh erosion is high enough that I would try to not do any mesh prolapse repair in a woman who was a smoker, especially a heavy smoker. A more common situation is, would I put a polypropylene midurethral sling in that patient? I usually am willing to do that because it is still the best option compared with the no-mesh options. In a patient who would be a good candidate for sacrocolpopexy, I can usually do a no-mesh surgery and keep the risk low. I could always give the woman an option to quit smoking, but that tends not to be successful.
Dr. Gebhart: What is the risk of using mesh in a smoker?
Dr. Walters: An increased risk of erosion through the vaginal walls. I am not sure of the magnitude of risk, maybe 2 or 3 times higher. That is high enough that I probably would not take the risk except in unusual circumstances.
Dr. Ridgeway: A good amount of data show increased risk of mesh exposure for smokers. Those patients also tend to have a higher risk of prolapse recurrence because of coughing. Sacrocolpopexy is not my favorite operation to do in a smoker. I will work with the patient to quit, but often if it is the right operation, I will do it, with preoperative estrogen and appropriate conseling.
Dr. Gebhart: Is there still a role for vaginal mesh? While it is no longer being sold in the United States, could you fashion your own mesh for a prolapse procedure?
Dr. Walters: I can do pretty much everything I need to do without adding transvaginal mesh, and if I need a meshaugmented repair, then I would go with the sacrocolpopexy route. Having said that, data for hysteropexy do show that a mesh-augmented hysteropexy could have some advantages, whether you do it with a kit or some fashioned pieces of mesh. Most of the experiences with this are outside of the United States, so we need much more standardization of technique and tracking to answer that question.
Dr. Gebhart: Mickey, what are your thoughts regarding someone who thinks, “Mesh has been good for me, I want to stay with that. I’m going to cut my own mesh”? Are they assuming some liability now that companies are no longer marketing mesh for vaginal repair?
Dr. Karram: Unfortunately, I really think they are. It would be easy to be put in a legal corner and asked, the FDA felt that this should be pulled off the market, why are you still utilizing it? At the end of the day, what the FDA said was not inaccurate.
The studies have not shown a significant better outcome with mesh, and it is an extra intervention that, again, in the best of hands is going to have some issues. That is a dilemma many surgeons faced because they felt that that was their main way of treating prolapse—”they took away my way of successfully treating patients for years.” I do think it increases their medical-legal liability.
Dr. Ridgeway: I agree that it does increase medical-legal liability, and I can’t imagine a situation in which I would offer that. Dr. Gebhart: There are risks with all procedures, including slings for stress incontinence, but sling use is appropriate in appropriately counseled patients.
Dr. Ridgeway: Correct. I feel very strongly that the risk profile for the midurethral sling is very different from that for transvaginal mesh. Very large data sets in large groups of people support that the outcomes are favorable and the risk profile is low. Having said that, slings are not risk free, but living with severe incontinence is not risk free either.
- US Food and Drug Administration. FDA takes action to protect women's health, orders manufacturers of surgical mesh intended for transvaginal repair of pelvic organ prolapse to stop selling all devices. https://www.fda.gov/news-events/press-announcements/fda-takes-action-protect-womens-health-orders-manufacturers-surgical-mesh-intended-transvaginal. April 16, 2019. Accessed January 14, 2020.
- Detollenaere RJ, den Boon J, Stekelenburg J, et al. Sacrospinous hysteropexy versus vaginal hysterectomy with suspension of the uterosacral ligaments in women with uterine prolapse stage 2 or higher: multicentre randomised non-inferiority trial. BMJ. 2015;351:h3717.
- US Food and Drug Administration. FDA takes action to protect women's health, orders manufacturers of surgical mesh intended for transvaginal repair of pelvic organ prolapse to stop selling all devices. https://www.fda.gov/news-events/press-announcements/fda-takes-action-protect-womens-health-orders-manufacturers-surgical-mesh-intended-transvaginal. April 16, 2019. Accessed January 14, 2020.
- Detollenaere RJ, den Boon J, Stekelenburg J, et al. Sacrospinous hysteropexy versus vaginal hysterectomy with suspension of the uterosacral ligaments in women with uterine prolapse stage 2 or higher: multicentre randomised non-inferiority trial. BMJ. 2015;351:h3717.
2020 Update on fertility
Although we are not able to cover all of the important developments in fertility medicine over the past year, there were 3 important articles published in the past 12 months that we highlight here. First, we discuss an American College of Obstetricians and Gynecologists (ACOG) committee opinion on genetic carrier screening that was reaffirmed in 2019. Second, we explore an interesting retrospective analysis of time-lapse videos and clinical outcomes of more than 10,000 embryos from 8 IVF clinics, across 4 countries. The authors assessed whether a deep learning model could predict the probability of pregnancy with fetal heart from time-lapse videos in the hopes that their research can improve prioritization of the most viable embryo for single embryo transfer. Last, we consider a review of the data on obstetric and reproductive health effects of preconception and prenatal exposure to several environmental toxicants, including heavy metals, endocrine-disrupting chemicals, pesticides, and air pollution.
Preconception genetic carrier screening: Standardize your counseling approach
American College of Obstetricians and Gynecologists Committee on Genetics. Committee Opinion No. 690: carrier screening in the age of genomic medicine. Obstet Gynecol. 2017;129:e35-e40.
With the rapid development of advanced and high throughput platforms for DNA sequencing in the past several years, the cost of genetic testing has decreased dramatically. Women's health care providers in general, and fertility specialists in particular, are uniquely positioned to take advantage of these novel and yet affordable technologies by counseling prospective parents during the preconception counseling, or early prenatal period, about the availability of genetic carrier screening and its potential to provide actionable information in a timely manner. The ultimate objective of genetic carrier screening is to enable individuals to make an informed decision regarding their reproductive choices based on their personal values. In a study by Larsen and colleagues, the uptake of genetic carrier screening was significantly higher when offered in the preconception period (68.7%), compared with during pregnancy (35.1%), which highlights the significance of early counseling.1
Based on the Centers for Disease Control and Prevention's Birth/Infant Death Data set, birth defects affect 1 in every 33 (about 3%) of all babies born in the United States each year and account for 20% of infant mortality.2 About 20% of birth defects are caused by single-gene (monogenic) disorders, and although some of these are due to dominant conditions or de novo mutations, a significant proportion are due to autosomal recessive, or X-chromosome linked conditions that are commonly assessed by genetic carrier screening.
ACOG published a committee opinion on "Carrier Screening in the Age of Genomic Medicine" in March 2017, which was reaffirmed in 2019.3
Residual risk. Several points discussed in this document are of paramount importance, including the need for pretest and posttest counseling and consent, as well as a discussion of "residual risk." Newer platforms employ sequencing techniques that potentially can detect most, if not all, of the disease-causing variants in the tested genes, such as the gene for cystic fibrosis and, therefore, have a higher detection rate compared with the older PCR-based techniques for a limited number of specific mutations included in the panel. Due to a variety of technical and biological limitations, however, such as allelic dropouts and the occurrence of de novo mutations, the detection rate is not 100%; there is always a residual risk that needs to be estimated and provided to individuals based on the existing knowledge on frequency of gene, penetrance of phenotype, and prevalence of condition in the general and specific ethnic populations.
Continue to: Expanded vs panethnic screening...
Expanded vs panethnic screening. Furthermore, although sequencing technology has made "expanded carrier screening" for several hundred conditions, simultaneous to and independent of ethnicity and family history, more easily available and affordable, ethnic-specific and panethnic screening for a more limited number of conditions are still acceptable approaches. Having said this, when the first partner screened is identified to be a carrier, his/her reproductive partners must be offered next-generation sequencing to identify less common disease-causing variants.4
A cautionary point to consider when expanded carrier screening panels are requested is the significant variability among commercial laboratories with regard to the conditions included in their panels. In addition, consider the absence of a well-defined or predictable phenotype for some of the included conditions.
Perhaps the most important matter when it comes to genetic carrier screening is to have a standard counseling approach that is persistently followed and offers the opportunity for individuals to know about their genetic testing options and available reproductive choices, including the use of donor gametes, preimplantation genetic testing for monogenic disease (PGT-M, formerly known as preimplantation genetic diagnosis, or PGD), prenatal testing, and pregnancy management options. For couples and/or individuals who decide to proceed with an affected pregnancy, earlier diagnosis can assist with postnatal management.
Medicolegal responsibility. Genetic carrier screening also is of specific relevance to the field of fertility medicine and assisted reproductive technology (ART) as a potential liability issue. Couples and individuals who are undergoing fertility treatment with in vitro fertilization (IVF) for a variety of medical or personal reasons are a specific group that certainly should be offered genetic carrier screening, as they have the option of "adding on" PGT-M (PGD) to their existing treatment plan at a fraction of the cost and treatment burden that would have otherwise been needed if they were not undergoing IVF. After counseling, some individuals and couples may ultimately opt out of genetic carrier screening. The counseling discussion needs to be clearly documented in the medical chart.
The preconception period is the perfect time to have a discussion about genetic carrier screening; it offers the opportunity for timely interventions if desired by the couples or individuals.
Continue to: Artificial intelligence and embryo selection...
Artificial intelligence and embryo selection
With continued improvements in embryo culture conditions and cryopreservation technology, there has been a tremendous amount of interest in developing better methods for embryo selection. These efforts are aimed at encouraging elective single embryo transfer (eSET) for women of all ages, thereby lowering the risk of multiple pregnancy and its associated adverse neonatal and obstetric outcomes—without compromising the pregnancy rates per transfer or lengthening the time to pregnancy.
One of the most extensively studied methods for this purpose is preimplantation genetic testing for aneuploidy (PGT-A, formerly known as PGS), but emerging data from large multicenter randomized clinical trials (RCTs) have again cast significant doubt on PGT-A's efficacy and utility.5 Meanwhile, alternative methods for embryo selection are currently under investigation, including noninvasive PGT-A and morphokinetic assessment of embryo development via analysis of images obtained by time-lapse imaging.
The potential of time-lapse imaging
Despite the initial promising results from time-lapse imaging, subsequent RCTs have not shown a significant clinical benefit.6 However, these early methods of morphokinetic assessment are mainly dependent on the embryologists' subjective assessment of individual static frames and "annotation" of observed spatial and temporal features of embryo development. In addition to being a very time-consuming task, this process is subject to significant interobserver and intraobserver variability.
Considering these limitations, even machine-based algorithms that incorporate these annotations along with such other clinical variables as parental age and prior obstetric history, have a low predictive power for the outcome of embryo transfer, with an area under the curve (AUC) of the ROC curve of 0.65 to 0.74. (An AUC of 0.5 represents completely random prediction and an AUC of 1.0 suggests perfect prediction.)7
A recent study by Tran and colleagues has employed a deep learning (neural network) model to analyze the entire raw time-lapse videos in an automated manner without prior annotation by embryologists. After analysis of 10,638 embryos from 8 different IVF clinics in 4 different countries, they have reported an AUC of 0.93 (95% confidence interval, 0.92-0.94) for prediction of fetal heart rate activity detected at 7 weeks of gestation or beyond. Although these data are very preliminary and have not yet been validated prospectively in larger datasets for live birth, it may herald the beginning of a new era for the automation and standardization of embryo assessment with artificial intelligence—similar to the rapidly increasing role of facial recognition technology for various applications.
Improved standardization of noninvasive embryo selection with growing use of artificial intelligence is a promising new tool to improve the safety and efficacy of ART.
Continue to: Environmental toxicants: The hidden danger...
Environmental toxicants: The hidden danger
Segal TR, Giudice LC. Before the beginning: environmental exposures and reproductive and obstetrical outcomes. Fertil Steril. 2019;112:613-621.
We receive news daily about the existential risk to humans of climate change. However, a risk that is likely as serious goes almost unseen by the public and most health care providers. That risk is environmental toxicants.8
More than 80,000 chemicals are registered in the United States, most in the last 75 years. These chemicals are ubiquitous. All of us are continuously exposed to and suffused with these toxicants and their metabolites. Air pollution adds insult to injury. Since this exposure has especially significant implications for fertility, infertility, pregnancy, perinatal health, childhood development, adult diseases, and later generational reproduction, it is imperative that reproductive health professionals take responsibility for helping mitigate this environmental crisis.
The problem is exceptionally complicated
The risks posed by environmental toxicants are much less visible than those for climate change, so the public, policymakers, and providers are largely unaware or may even seem uncaring. Few health professionals have sufficient knowledge to deliver care in this area, know which questions to ask, or have adequate information/medical record tools to assist them in care—and what are the possible interventions?
Addressing risk posed by individual toxicants
Addressing the problem clinically requires asking patients questions about exposure and recommending interventions. Toxicant chemicals include the neurotoxin mercury, which can be addressed by limiting intake of fish, especially certain types.
Lead was used before 1978 in paint, it also was used in gas and in water pipes. People living in older homes may be exposed, as well as those in occupations exposed to lead. Others with lead exposure risk include immigrants from areas without lead regulations and people using pica- or lead-glazed pottery. Lead exposure has been associated with multiple pregnancy complications and permanently impaired intellectual development in children. If lead testing reveals high levels, chelation therapy can help.
Cadmium is a heavy metal used in rechargeable batteries, paint pigment, and plastic production. Exposure results from food intake, smoking, and second-hand smoke. Cadmium accumulates in the liver, kidneys, testes, ovaries, and placenta. Exposure causes itai-itai disease, which is characterized by osteomalacia and renal tubular dysfunction as well as epigenetic changes in placental DNA and damage to the reproductive system. Eating organic food and reducing industrial exposure to cadmium are preventive strategies.
Pesticides are ubiquitous, with 90% of the US population having detectable levels. Exposure during the preconception period can lead to intrauterine growth restriction, low birth weight, subsequent cancers, and other problems. Eating organic food can reduce risk, as can frequent hand washing when exposed to pesticides, using protective gear, and removing shoes in the home.
Endocrine-disrupting chemicals (EDCs) are chemicals that can mimic or block endogenous hormones, which leads to adverse health outcomes. In addition to heavy metals, 3 important EDCs are bisphenol A (BPA), phthalates, and polybrominated diethyl ethers (PBDEs). Exposure is ubiquitous from industrial food processing, personal care products, cosmetics, and dust. Phthalates and BPA have short half-lives of hours to days, while PBDEs can persist in adipose tissue for months. Abnormal urogenital and neurologic development and thyroid disruption can result. Eating organic food, eating at home, and decreasing processed food intake can reduce exposure.
BPA is used in plastics, canned food liners, cash register receipts, and epoxy resins. Exposure is through inhalation, ingestion, and dermal absorption and affects semen quality, fertilization, placentation, and early reproduction. Limiting the use of plastic containers, not microwaving food in plastic, and avoiding thermal paper cash register receipts can reduce exposure.
Phthalates are synthetically derived and used as plasticizers in personal and medical products. The major source of phthalate exposure is food; exposure causes sperm, egg, and DNA damage. Phthalate avoidance involves replacing plastic bottles with glass or stainless steel, avoiding reheating food in plastic containers, and choosing "fragrance free" products.
PBDEs are used in flame retardants on upholstery, textiles, carpeting, and some electronics. Most PBDEs have been replaced by alternatives; however, their half-life is up to 12 years. Complications caused by PBDEs include thyroid disruption, resulting in abnormal fetal brain development. Avoiding dust and furniture that contain PBDEs, as well as hand washing, reduces exposure risk.
Air pollutants are associated with adverse obstetric outcomes and lower cognitive function in children. Avoiding areas with heavy traffic, staying indoors when air is heavily polluted, and using a HEPA filter in the home can reduce chemicals from air pollution.
Recommendations
The magnitude of the problem that environmental toxicant exposure creates requires health care providers to take action. The table in the publication by Segal and Giudice can be used as a tool that patients can answer first themselves before review by their provider.2 It can be added to your electronic health record and/or patient portal. Even making general comments to raise awareness, asking questions regarding exposure, and making recommendations can be helpful (TABLES 1 and 2). When possible, we also should advocate for public awareness and policy changes that address this significant health issue.

Environmental toxicants are a significant health problem that can be effectively mitigated through patient questions and recommended interventions.
- Larsen D, Ma J, Strassberg M, et al. The uptake of pan-ethnic expanded carrier screening is higher when offered during preconception or early prenatal genetic counseling. Prenat Diagn. 2019;39:319-323.
- Matthews TJ, MacDorman MF, Thoma ME. Infant Mortality Statistics From the 2013 Period Linked Birth/Infant Death Data Set. Natl Vital Stat Rep. 2015;64:1-30.
- American College of Obstetricians and Gynecologists Committee on Genetics. Committee Opinion No. 690: carrier screening in the age of genomic medicine. Obstet Gynecol. 2017;129:e35-e40.
- Gregg AR, Edwards JG. Prenatal genetic carrier screening in the genomic age. Semin Perinatol. 2018;42:303-306.
- Munné S, Kaplan B, Frattarelli JL, et al; STAR Study Group. Preimplantation genetic testing for aneuploidy versus morphology as selection criteria for single frozen-thawed embryo transfer in good-prognosis patients: a multicenter randomized clinical trial. Fertil Steril. 2019;112:1071-1079. e7.
- Goodman LR, Goldberg J, Falcone T, et al. Does the addition of time-lapse morphokinetics in the selection of embryos for transfer improve pregnancy rates? A randomized controlled trial. Fertil Steril. 2016;105:275-285.e10.
- Blank C, Wildeboer RR, DeCroo I, et al. Prediction of implantation after blastocyst transfer in in vitro fertilization: a machine-learning perspective. Fertil Steril. 2019;111:318- 326.
- The American College of Obstetricians and Gynecologists Committee on Health Care for Underserved Women; American Society for Reproductive Medicine Practice Committee; The University of California, San Francisco Program on Reproductive Health and the Environment. ACOG Committee Opinion No. 575. Exposure to environmental toxic agents. Fertil Steril. 2013;100:931-934.
Although we are not able to cover all of the important developments in fertility medicine over the past year, there were 3 important articles published in the past 12 months that we highlight here. First, we discuss an American College of Obstetricians and Gynecologists (ACOG) committee opinion on genetic carrier screening that was reaffirmed in 2019. Second, we explore an interesting retrospective analysis of time-lapse videos and clinical outcomes of more than 10,000 embryos from 8 IVF clinics, across 4 countries. The authors assessed whether a deep learning model could predict the probability of pregnancy with fetal heart from time-lapse videos in the hopes that their research can improve prioritization of the most viable embryo for single embryo transfer. Last, we consider a review of the data on obstetric and reproductive health effects of preconception and prenatal exposure to several environmental toxicants, including heavy metals, endocrine-disrupting chemicals, pesticides, and air pollution.
Preconception genetic carrier screening: Standardize your counseling approach
American College of Obstetricians and Gynecologists Committee on Genetics. Committee Opinion No. 690: carrier screening in the age of genomic medicine. Obstet Gynecol. 2017;129:e35-e40.
With the rapid development of advanced and high throughput platforms for DNA sequencing in the past several years, the cost of genetic testing has decreased dramatically. Women's health care providers in general, and fertility specialists in particular, are uniquely positioned to take advantage of these novel and yet affordable technologies by counseling prospective parents during the preconception counseling, or early prenatal period, about the availability of genetic carrier screening and its potential to provide actionable information in a timely manner. The ultimate objective of genetic carrier screening is to enable individuals to make an informed decision regarding their reproductive choices based on their personal values. In a study by Larsen and colleagues, the uptake of genetic carrier screening was significantly higher when offered in the preconception period (68.7%), compared with during pregnancy (35.1%), which highlights the significance of early counseling.1
Based on the Centers for Disease Control and Prevention's Birth/Infant Death Data set, birth defects affect 1 in every 33 (about 3%) of all babies born in the United States each year and account for 20% of infant mortality.2 About 20% of birth defects are caused by single-gene (monogenic) disorders, and although some of these are due to dominant conditions or de novo mutations, a significant proportion are due to autosomal recessive, or X-chromosome linked conditions that are commonly assessed by genetic carrier screening.
ACOG published a committee opinion on "Carrier Screening in the Age of Genomic Medicine" in March 2017, which was reaffirmed in 2019.3
Residual risk. Several points discussed in this document are of paramount importance, including the need for pretest and posttest counseling and consent, as well as a discussion of "residual risk." Newer platforms employ sequencing techniques that potentially can detect most, if not all, of the disease-causing variants in the tested genes, such as the gene for cystic fibrosis and, therefore, have a higher detection rate compared with the older PCR-based techniques for a limited number of specific mutations included in the panel. Due to a variety of technical and biological limitations, however, such as allelic dropouts and the occurrence of de novo mutations, the detection rate is not 100%; there is always a residual risk that needs to be estimated and provided to individuals based on the existing knowledge on frequency of gene, penetrance of phenotype, and prevalence of condition in the general and specific ethnic populations.
Continue to: Expanded vs panethnic screening...
Expanded vs panethnic screening. Furthermore, although sequencing technology has made "expanded carrier screening" for several hundred conditions, simultaneous to and independent of ethnicity and family history, more easily available and affordable, ethnic-specific and panethnic screening for a more limited number of conditions are still acceptable approaches. Having said this, when the first partner screened is identified to be a carrier, his/her reproductive partners must be offered next-generation sequencing to identify less common disease-causing variants.4
A cautionary point to consider when expanded carrier screening panels are requested is the significant variability among commercial laboratories with regard to the conditions included in their panels. In addition, consider the absence of a well-defined or predictable phenotype for some of the included conditions.
Perhaps the most important matter when it comes to genetic carrier screening is to have a standard counseling approach that is persistently followed and offers the opportunity for individuals to know about their genetic testing options and available reproductive choices, including the use of donor gametes, preimplantation genetic testing for monogenic disease (PGT-M, formerly known as preimplantation genetic diagnosis, or PGD), prenatal testing, and pregnancy management options. For couples and/or individuals who decide to proceed with an affected pregnancy, earlier diagnosis can assist with postnatal management.
Medicolegal responsibility. Genetic carrier screening also is of specific relevance to the field of fertility medicine and assisted reproductive technology (ART) as a potential liability issue. Couples and individuals who are undergoing fertility treatment with in vitro fertilization (IVF) for a variety of medical or personal reasons are a specific group that certainly should be offered genetic carrier screening, as they have the option of "adding on" PGT-M (PGD) to their existing treatment plan at a fraction of the cost and treatment burden that would have otherwise been needed if they were not undergoing IVF. After counseling, some individuals and couples may ultimately opt out of genetic carrier screening. The counseling discussion needs to be clearly documented in the medical chart.
The preconception period is the perfect time to have a discussion about genetic carrier screening; it offers the opportunity for timely interventions if desired by the couples or individuals.
Continue to: Artificial intelligence and embryo selection...
Artificial intelligence and embryo selection
With continued improvements in embryo culture conditions and cryopreservation technology, there has been a tremendous amount of interest in developing better methods for embryo selection. These efforts are aimed at encouraging elective single embryo transfer (eSET) for women of all ages, thereby lowering the risk of multiple pregnancy and its associated adverse neonatal and obstetric outcomes—without compromising the pregnancy rates per transfer or lengthening the time to pregnancy.
One of the most extensively studied methods for this purpose is preimplantation genetic testing for aneuploidy (PGT-A, formerly known as PGS), but emerging data from large multicenter randomized clinical trials (RCTs) have again cast significant doubt on PGT-A's efficacy and utility.5 Meanwhile, alternative methods for embryo selection are currently under investigation, including noninvasive PGT-A and morphokinetic assessment of embryo development via analysis of images obtained by time-lapse imaging.
The potential of time-lapse imaging
Despite the initial promising results from time-lapse imaging, subsequent RCTs have not shown a significant clinical benefit.6 However, these early methods of morphokinetic assessment are mainly dependent on the embryologists' subjective assessment of individual static frames and "annotation" of observed spatial and temporal features of embryo development. In addition to being a very time-consuming task, this process is subject to significant interobserver and intraobserver variability.
Considering these limitations, even machine-based algorithms that incorporate these annotations along with such other clinical variables as parental age and prior obstetric history, have a low predictive power for the outcome of embryo transfer, with an area under the curve (AUC) of the ROC curve of 0.65 to 0.74. (An AUC of 0.5 represents completely random prediction and an AUC of 1.0 suggests perfect prediction.)7
A recent study by Tran and colleagues has employed a deep learning (neural network) model to analyze the entire raw time-lapse videos in an automated manner without prior annotation by embryologists. After analysis of 10,638 embryos from 8 different IVF clinics in 4 different countries, they have reported an AUC of 0.93 (95% confidence interval, 0.92-0.94) for prediction of fetal heart rate activity detected at 7 weeks of gestation or beyond. Although these data are very preliminary and have not yet been validated prospectively in larger datasets for live birth, it may herald the beginning of a new era for the automation and standardization of embryo assessment with artificial intelligence—similar to the rapidly increasing role of facial recognition technology for various applications.
Improved standardization of noninvasive embryo selection with growing use of artificial intelligence is a promising new tool to improve the safety and efficacy of ART.
Continue to: Environmental toxicants: The hidden danger...
Environmental toxicants: The hidden danger
Segal TR, Giudice LC. Before the beginning: environmental exposures and reproductive and obstetrical outcomes. Fertil Steril. 2019;112:613-621.
We receive news daily about the existential risk to humans of climate change. However, a risk that is likely as serious goes almost unseen by the public and most health care providers. That risk is environmental toxicants.8
More than 80,000 chemicals are registered in the United States, most in the last 75 years. These chemicals are ubiquitous. All of us are continuously exposed to and suffused with these toxicants and their metabolites. Air pollution adds insult to injury. Since this exposure has especially significant implications for fertility, infertility, pregnancy, perinatal health, childhood development, adult diseases, and later generational reproduction, it is imperative that reproductive health professionals take responsibility for helping mitigate this environmental crisis.
The problem is exceptionally complicated
The risks posed by environmental toxicants are much less visible than those for climate change, so the public, policymakers, and providers are largely unaware or may even seem uncaring. Few health professionals have sufficient knowledge to deliver care in this area, know which questions to ask, or have adequate information/medical record tools to assist them in care—and what are the possible interventions?
Addressing risk posed by individual toxicants
Addressing the problem clinically requires asking patients questions about exposure and recommending interventions. Toxicant chemicals include the neurotoxin mercury, which can be addressed by limiting intake of fish, especially certain types.
Lead was used before 1978 in paint, it also was used in gas and in water pipes. People living in older homes may be exposed, as well as those in occupations exposed to lead. Others with lead exposure risk include immigrants from areas without lead regulations and people using pica- or lead-glazed pottery. Lead exposure has been associated with multiple pregnancy complications and permanently impaired intellectual development in children. If lead testing reveals high levels, chelation therapy can help.
Cadmium is a heavy metal used in rechargeable batteries, paint pigment, and plastic production. Exposure results from food intake, smoking, and second-hand smoke. Cadmium accumulates in the liver, kidneys, testes, ovaries, and placenta. Exposure causes itai-itai disease, which is characterized by osteomalacia and renal tubular dysfunction as well as epigenetic changes in placental DNA and damage to the reproductive system. Eating organic food and reducing industrial exposure to cadmium are preventive strategies.
Pesticides are ubiquitous, with 90% of the US population having detectable levels. Exposure during the preconception period can lead to intrauterine growth restriction, low birth weight, subsequent cancers, and other problems. Eating organic food can reduce risk, as can frequent hand washing when exposed to pesticides, using protective gear, and removing shoes in the home.
Endocrine-disrupting chemicals (EDCs) are chemicals that can mimic or block endogenous hormones, which leads to adverse health outcomes. In addition to heavy metals, 3 important EDCs are bisphenol A (BPA), phthalates, and polybrominated diethyl ethers (PBDEs). Exposure is ubiquitous from industrial food processing, personal care products, cosmetics, and dust. Phthalates and BPA have short half-lives of hours to days, while PBDEs can persist in adipose tissue for months. Abnormal urogenital and neurologic development and thyroid disruption can result. Eating organic food, eating at home, and decreasing processed food intake can reduce exposure.
BPA is used in plastics, canned food liners, cash register receipts, and epoxy resins. Exposure is through inhalation, ingestion, and dermal absorption and affects semen quality, fertilization, placentation, and early reproduction. Limiting the use of plastic containers, not microwaving food in plastic, and avoiding thermal paper cash register receipts can reduce exposure.
Phthalates are synthetically derived and used as plasticizers in personal and medical products. The major source of phthalate exposure is food; exposure causes sperm, egg, and DNA damage. Phthalate avoidance involves replacing plastic bottles with glass or stainless steel, avoiding reheating food in plastic containers, and choosing "fragrance free" products.
PBDEs are used in flame retardants on upholstery, textiles, carpeting, and some electronics. Most PBDEs have been replaced by alternatives; however, their half-life is up to 12 years. Complications caused by PBDEs include thyroid disruption, resulting in abnormal fetal brain development. Avoiding dust and furniture that contain PBDEs, as well as hand washing, reduces exposure risk.
Air pollutants are associated with adverse obstetric outcomes and lower cognitive function in children. Avoiding areas with heavy traffic, staying indoors when air is heavily polluted, and using a HEPA filter in the home can reduce chemicals from air pollution.
Recommendations
The magnitude of the problem that environmental toxicant exposure creates requires health care providers to take action. The table in the publication by Segal and Giudice can be used as a tool that patients can answer first themselves before review by their provider.2 It can be added to your electronic health record and/or patient portal. Even making general comments to raise awareness, asking questions regarding exposure, and making recommendations can be helpful (TABLES 1 and 2). When possible, we also should advocate for public awareness and policy changes that address this significant health issue.


Environmental toxicants are a significant health problem that can be effectively mitigated through patient questions and recommended interventions.
Although we are not able to cover all of the important developments in fertility medicine over the past year, there were 3 important articles published in the past 12 months that we highlight here. First, we discuss an American College of Obstetricians and Gynecologists (ACOG) committee opinion on genetic carrier screening that was reaffirmed in 2019. Second, we explore an interesting retrospective analysis of time-lapse videos and clinical outcomes of more than 10,000 embryos from 8 IVF clinics, across 4 countries. The authors assessed whether a deep learning model could predict the probability of pregnancy with fetal heart from time-lapse videos in the hopes that their research can improve prioritization of the most viable embryo for single embryo transfer. Last, we consider a review of the data on obstetric and reproductive health effects of preconception and prenatal exposure to several environmental toxicants, including heavy metals, endocrine-disrupting chemicals, pesticides, and air pollution.
Preconception genetic carrier screening: Standardize your counseling approach
American College of Obstetricians and Gynecologists Committee on Genetics. Committee Opinion No. 690: carrier screening in the age of genomic medicine. Obstet Gynecol. 2017;129:e35-e40.
With the rapid development of advanced and high throughput platforms for DNA sequencing in the past several years, the cost of genetic testing has decreased dramatically. Women's health care providers in general, and fertility specialists in particular, are uniquely positioned to take advantage of these novel and yet affordable technologies by counseling prospective parents during the preconception counseling, or early prenatal period, about the availability of genetic carrier screening and its potential to provide actionable information in a timely manner. The ultimate objective of genetic carrier screening is to enable individuals to make an informed decision regarding their reproductive choices based on their personal values. In a study by Larsen and colleagues, the uptake of genetic carrier screening was significantly higher when offered in the preconception period (68.7%), compared with during pregnancy (35.1%), which highlights the significance of early counseling.1
Based on the Centers for Disease Control and Prevention's Birth/Infant Death Data set, birth defects affect 1 in every 33 (about 3%) of all babies born in the United States each year and account for 20% of infant mortality.2 About 20% of birth defects are caused by single-gene (monogenic) disorders, and although some of these are due to dominant conditions or de novo mutations, a significant proportion are due to autosomal recessive, or X-chromosome linked conditions that are commonly assessed by genetic carrier screening.
ACOG published a committee opinion on "Carrier Screening in the Age of Genomic Medicine" in March 2017, which was reaffirmed in 2019.3
Residual risk. Several points discussed in this document are of paramount importance, including the need for pretest and posttest counseling and consent, as well as a discussion of "residual risk." Newer platforms employ sequencing techniques that potentially can detect most, if not all, of the disease-causing variants in the tested genes, such as the gene for cystic fibrosis and, therefore, have a higher detection rate compared with the older PCR-based techniques for a limited number of specific mutations included in the panel. Due to a variety of technical and biological limitations, however, such as allelic dropouts and the occurrence of de novo mutations, the detection rate is not 100%; there is always a residual risk that needs to be estimated and provided to individuals based on the existing knowledge on frequency of gene, penetrance of phenotype, and prevalence of condition in the general and specific ethnic populations.
Continue to: Expanded vs panethnic screening...
Expanded vs panethnic screening. Furthermore, although sequencing technology has made "expanded carrier screening" for several hundred conditions, simultaneous to and independent of ethnicity and family history, more easily available and affordable, ethnic-specific and panethnic screening for a more limited number of conditions are still acceptable approaches. Having said this, when the first partner screened is identified to be a carrier, his/her reproductive partners must be offered next-generation sequencing to identify less common disease-causing variants.4
A cautionary point to consider when expanded carrier screening panels are requested is the significant variability among commercial laboratories with regard to the conditions included in their panels. In addition, consider the absence of a well-defined or predictable phenotype for some of the included conditions.
Perhaps the most important matter when it comes to genetic carrier screening is to have a standard counseling approach that is persistently followed and offers the opportunity for individuals to know about their genetic testing options and available reproductive choices, including the use of donor gametes, preimplantation genetic testing for monogenic disease (PGT-M, formerly known as preimplantation genetic diagnosis, or PGD), prenatal testing, and pregnancy management options. For couples and/or individuals who decide to proceed with an affected pregnancy, earlier diagnosis can assist with postnatal management.
Medicolegal responsibility. Genetic carrier screening also is of specific relevance to the field of fertility medicine and assisted reproductive technology (ART) as a potential liability issue. Couples and individuals who are undergoing fertility treatment with in vitro fertilization (IVF) for a variety of medical or personal reasons are a specific group that certainly should be offered genetic carrier screening, as they have the option of "adding on" PGT-M (PGD) to their existing treatment plan at a fraction of the cost and treatment burden that would have otherwise been needed if they were not undergoing IVF. After counseling, some individuals and couples may ultimately opt out of genetic carrier screening. The counseling discussion needs to be clearly documented in the medical chart.
The preconception period is the perfect time to have a discussion about genetic carrier screening; it offers the opportunity for timely interventions if desired by the couples or individuals.
Continue to: Artificial intelligence and embryo selection...
Artificial intelligence and embryo selection
With continued improvements in embryo culture conditions and cryopreservation technology, there has been a tremendous amount of interest in developing better methods for embryo selection. These efforts are aimed at encouraging elective single embryo transfer (eSET) for women of all ages, thereby lowering the risk of multiple pregnancy and its associated adverse neonatal and obstetric outcomes—without compromising the pregnancy rates per transfer or lengthening the time to pregnancy.
One of the most extensively studied methods for this purpose is preimplantation genetic testing for aneuploidy (PGT-A, formerly known as PGS), but emerging data from large multicenter randomized clinical trials (RCTs) have again cast significant doubt on PGT-A's efficacy and utility.5 Meanwhile, alternative methods for embryo selection are currently under investigation, including noninvasive PGT-A and morphokinetic assessment of embryo development via analysis of images obtained by time-lapse imaging.
The potential of time-lapse imaging
Despite the initial promising results from time-lapse imaging, subsequent RCTs have not shown a significant clinical benefit.6 However, these early methods of morphokinetic assessment are mainly dependent on the embryologists' subjective assessment of individual static frames and "annotation" of observed spatial and temporal features of embryo development. In addition to being a very time-consuming task, this process is subject to significant interobserver and intraobserver variability.
Considering these limitations, even machine-based algorithms that incorporate these annotations along with such other clinical variables as parental age and prior obstetric history, have a low predictive power for the outcome of embryo transfer, with an area under the curve (AUC) of the ROC curve of 0.65 to 0.74. (An AUC of 0.5 represents completely random prediction and an AUC of 1.0 suggests perfect prediction.)7
A recent study by Tran and colleagues has employed a deep learning (neural network) model to analyze the entire raw time-lapse videos in an automated manner without prior annotation by embryologists. After analysis of 10,638 embryos from 8 different IVF clinics in 4 different countries, they have reported an AUC of 0.93 (95% confidence interval, 0.92-0.94) for prediction of fetal heart rate activity detected at 7 weeks of gestation or beyond. Although these data are very preliminary and have not yet been validated prospectively in larger datasets for live birth, it may herald the beginning of a new era for the automation and standardization of embryo assessment with artificial intelligence—similar to the rapidly increasing role of facial recognition technology for various applications.
Improved standardization of noninvasive embryo selection with growing use of artificial intelligence is a promising new tool to improve the safety and efficacy of ART.
Continue to: Environmental toxicants: The hidden danger...
Environmental toxicants: The hidden danger
Segal TR, Giudice LC. Before the beginning: environmental exposures and reproductive and obstetrical outcomes. Fertil Steril. 2019;112:613-621.
We receive news daily about the existential risk to humans of climate change. However, a risk that is likely as serious goes almost unseen by the public and most health care providers. That risk is environmental toxicants.8
More than 80,000 chemicals are registered in the United States, most in the last 75 years. These chemicals are ubiquitous. All of us are continuously exposed to and suffused with these toxicants and their metabolites. Air pollution adds insult to injury. Since this exposure has especially significant implications for fertility, infertility, pregnancy, perinatal health, childhood development, adult diseases, and later generational reproduction, it is imperative that reproductive health professionals take responsibility for helping mitigate this environmental crisis.
The problem is exceptionally complicated
The risks posed by environmental toxicants are much less visible than those for climate change, so the public, policymakers, and providers are largely unaware or may even seem uncaring. Few health professionals have sufficient knowledge to deliver care in this area, know which questions to ask, or have adequate information/medical record tools to assist them in care—and what are the possible interventions?
Addressing risk posed by individual toxicants
Addressing the problem clinically requires asking patients questions about exposure and recommending interventions. Toxicant chemicals include the neurotoxin mercury, which can be addressed by limiting intake of fish, especially certain types.
Lead was used before 1978 in paint, it also was used in gas and in water pipes. People living in older homes may be exposed, as well as those in occupations exposed to lead. Others with lead exposure risk include immigrants from areas without lead regulations and people using pica- or lead-glazed pottery. Lead exposure has been associated with multiple pregnancy complications and permanently impaired intellectual development in children. If lead testing reveals high levels, chelation therapy can help.
Cadmium is a heavy metal used in rechargeable batteries, paint pigment, and plastic production. Exposure results from food intake, smoking, and second-hand smoke. Cadmium accumulates in the liver, kidneys, testes, ovaries, and placenta. Exposure causes itai-itai disease, which is characterized by osteomalacia and renal tubular dysfunction as well as epigenetic changes in placental DNA and damage to the reproductive system. Eating organic food and reducing industrial exposure to cadmium are preventive strategies.
Pesticides are ubiquitous, with 90% of the US population having detectable levels. Exposure during the preconception period can lead to intrauterine growth restriction, low birth weight, subsequent cancers, and other problems. Eating organic food can reduce risk, as can frequent hand washing when exposed to pesticides, using protective gear, and removing shoes in the home.
Endocrine-disrupting chemicals (EDCs) are chemicals that can mimic or block endogenous hormones, which leads to adverse health outcomes. In addition to heavy metals, 3 important EDCs are bisphenol A (BPA), phthalates, and polybrominated diethyl ethers (PBDEs). Exposure is ubiquitous from industrial food processing, personal care products, cosmetics, and dust. Phthalates and BPA have short half-lives of hours to days, while PBDEs can persist in adipose tissue for months. Abnormal urogenital and neurologic development and thyroid disruption can result. Eating organic food, eating at home, and decreasing processed food intake can reduce exposure.
BPA is used in plastics, canned food liners, cash register receipts, and epoxy resins. Exposure is through inhalation, ingestion, and dermal absorption and affects semen quality, fertilization, placentation, and early reproduction. Limiting the use of plastic containers, not microwaving food in plastic, and avoiding thermal paper cash register receipts can reduce exposure.
Phthalates are synthetically derived and used as plasticizers in personal and medical products. The major source of phthalate exposure is food; exposure causes sperm, egg, and DNA damage. Phthalate avoidance involves replacing plastic bottles with glass or stainless steel, avoiding reheating food in plastic containers, and choosing "fragrance free" products.
PBDEs are used in flame retardants on upholstery, textiles, carpeting, and some electronics. Most PBDEs have been replaced by alternatives; however, their half-life is up to 12 years. Complications caused by PBDEs include thyroid disruption, resulting in abnormal fetal brain development. Avoiding dust and furniture that contain PBDEs, as well as hand washing, reduces exposure risk.
Air pollutants are associated with adverse obstetric outcomes and lower cognitive function in children. Avoiding areas with heavy traffic, staying indoors when air is heavily polluted, and using a HEPA filter in the home can reduce chemicals from air pollution.
Recommendations
The magnitude of the problem that environmental toxicant exposure creates requires health care providers to take action. The table in the publication by Segal and Giudice can be used as a tool that patients can answer first themselves before review by their provider.2 It can be added to your electronic health record and/or patient portal. Even making general comments to raise awareness, asking questions regarding exposure, and making recommendations can be helpful (TABLES 1 and 2). When possible, we also should advocate for public awareness and policy changes that address this significant health issue.


Environmental toxicants are a significant health problem that can be effectively mitigated through patient questions and recommended interventions.
- Larsen D, Ma J, Strassberg M, et al. The uptake of pan-ethnic expanded carrier screening is higher when offered during preconception or early prenatal genetic counseling. Prenat Diagn. 2019;39:319-323.
- Matthews TJ, MacDorman MF, Thoma ME. Infant Mortality Statistics From the 2013 Period Linked Birth/Infant Death Data Set. Natl Vital Stat Rep. 2015;64:1-30.
- American College of Obstetricians and Gynecologists Committee on Genetics. Committee Opinion No. 690: carrier screening in the age of genomic medicine. Obstet Gynecol. 2017;129:e35-e40.
- Gregg AR, Edwards JG. Prenatal genetic carrier screening in the genomic age. Semin Perinatol. 2018;42:303-306.
- Munné S, Kaplan B, Frattarelli JL, et al; STAR Study Group. Preimplantation genetic testing for aneuploidy versus morphology as selection criteria for single frozen-thawed embryo transfer in good-prognosis patients: a multicenter randomized clinical trial. Fertil Steril. 2019;112:1071-1079. e7.
- Goodman LR, Goldberg J, Falcone T, et al. Does the addition of time-lapse morphokinetics in the selection of embryos for transfer improve pregnancy rates? A randomized controlled trial. Fertil Steril. 2016;105:275-285.e10.
- Blank C, Wildeboer RR, DeCroo I, et al. Prediction of implantation after blastocyst transfer in in vitro fertilization: a machine-learning perspective. Fertil Steril. 2019;111:318- 326.
- The American College of Obstetricians and Gynecologists Committee on Health Care for Underserved Women; American Society for Reproductive Medicine Practice Committee; The University of California, San Francisco Program on Reproductive Health and the Environment. ACOG Committee Opinion No. 575. Exposure to environmental toxic agents. Fertil Steril. 2013;100:931-934.
- Larsen D, Ma J, Strassberg M, et al. The uptake of pan-ethnic expanded carrier screening is higher when offered during preconception or early prenatal genetic counseling. Prenat Diagn. 2019;39:319-323.
- Matthews TJ, MacDorman MF, Thoma ME. Infant Mortality Statistics From the 2013 Period Linked Birth/Infant Death Data Set. Natl Vital Stat Rep. 2015;64:1-30.
- American College of Obstetricians and Gynecologists Committee on Genetics. Committee Opinion No. 690: carrier screening in the age of genomic medicine. Obstet Gynecol. 2017;129:e35-e40.
- Gregg AR, Edwards JG. Prenatal genetic carrier screening in the genomic age. Semin Perinatol. 2018;42:303-306.
- Munné S, Kaplan B, Frattarelli JL, et al; STAR Study Group. Preimplantation genetic testing for aneuploidy versus morphology as selection criteria for single frozen-thawed embryo transfer in good-prognosis patients: a multicenter randomized clinical trial. Fertil Steril. 2019;112:1071-1079. e7.
- Goodman LR, Goldberg J, Falcone T, et al. Does the addition of time-lapse morphokinetics in the selection of embryos for transfer improve pregnancy rates? A randomized controlled trial. Fertil Steril. 2016;105:275-285.e10.
- Blank C, Wildeboer RR, DeCroo I, et al. Prediction of implantation after blastocyst transfer in in vitro fertilization: a machine-learning perspective. Fertil Steril. 2019;111:318- 326.
- The American College of Obstetricians and Gynecologists Committee on Health Care for Underserved Women; American Society for Reproductive Medicine Practice Committee; The University of California, San Francisco Program on Reproductive Health and the Environment. ACOG Committee Opinion No. 575. Exposure to environmental toxic agents. Fertil Steril. 2013;100:931-934.
Breast cancer treatments veer from guidelines
Women with breast cancer may be receiving treatments that are discordant with guideline recommendations for genetic subtypes of disease, based on a retrospective analysis of more than 20,000 patients.
Radiotherapy and chemotherapy practices were particularly out of alignment with guidelines, reported lead author Allison W. Kurian, MD, of Stanford (Calif.) University, and colleagues.
“Integrating genetic testing into breast cancer care has been complex and challenging,” the investigators wrote in JAMA Oncology. “There is wide variability in which clinicians order testing and disclose results, in the clinical significance of results, and in how clinicians interpret results to patients.”
According to the investigators, while germline testing is on the rise, little is known about how these test results are translating to clinical care.
To learn more, the investigators evaluated data from 20,568 women with stage 0-III breast cancer who entered the Surveillance, Epidemiology, and End Results registries of Georgia and California between 2014 and 2016.
Three treatment types were evaluated: surgery (bilateral vs. unilateral mastectomy), radiotherapy after lumpectomy, and chemotherapy. Treatment selection was compared with test results for breast cancer–associated genes, such as BRCA1/2, TP53, PTEN, and others. Associations were then compared with guideline recommendations.
Data analysis suggested that many clinicians were correctly using genetic test results to guide surgical decisions. For example, almost two-thirds (61.7%) of women with a BRCA mutation underwent bilateral mastectomy, compared with one-quarter (24.3%) who were BRCA negative (odds ratio, 5.52). For other pathogenic variants, the rate of bilateral mastectomy was still elevated, albeit to a lesser degree (OR, 2.41).
Generally, these practices align with recommendations, the investigators wrote, noting that research supports bilateral mastectomy with BRCA1/2, TP53, and PTEN variants, while data are lacking for other genetic subtypes.
Radiotherapy and chemotherapy practices were more discordant with guidelines. For example, women with a BRCA mutation were 78% less likely to receive radiotherapy after lumpectomy (OR, 0.22) and 76% more likely to receive chemotherapy for early-stage, hormone-positive disease (OR, 1.76). According to investigators, these findings suggest possible trends in undertreatment and overtreatment, respectively.
“We believe more research is needed to confirm our results and to evaluate long-term outcomes of pathogenic variant carriers to understand treatment decision making and consequences,” the investigators concluded.
The study was funded by the National Institutes of Health and the California Department of Public Health. The investigators reported relationships with Myriad Genetics, Genomic Health, Roche, and other companies.
SOURCE: Kurian AW et al. JAMA Oncol. 2020 Feb 6. doi: 10.1001/jamaoncol.2019.6400.
Women with breast cancer may be receiving treatments that are discordant with guideline recommendations for genetic subtypes of disease, based on a retrospective analysis of more than 20,000 patients.
Radiotherapy and chemotherapy practices were particularly out of alignment with guidelines, reported lead author Allison W. Kurian, MD, of Stanford (Calif.) University, and colleagues.
“Integrating genetic testing into breast cancer care has been complex and challenging,” the investigators wrote in JAMA Oncology. “There is wide variability in which clinicians order testing and disclose results, in the clinical significance of results, and in how clinicians interpret results to patients.”
According to the investigators, while germline testing is on the rise, little is known about how these test results are translating to clinical care.
To learn more, the investigators evaluated data from 20,568 women with stage 0-III breast cancer who entered the Surveillance, Epidemiology, and End Results registries of Georgia and California between 2014 and 2016.
Three treatment types were evaluated: surgery (bilateral vs. unilateral mastectomy), radiotherapy after lumpectomy, and chemotherapy. Treatment selection was compared with test results for breast cancer–associated genes, such as BRCA1/2, TP53, PTEN, and others. Associations were then compared with guideline recommendations.
Data analysis suggested that many clinicians were correctly using genetic test results to guide surgical decisions. For example, almost two-thirds (61.7%) of women with a BRCA mutation underwent bilateral mastectomy, compared with one-quarter (24.3%) who were BRCA negative (odds ratio, 5.52). For other pathogenic variants, the rate of bilateral mastectomy was still elevated, albeit to a lesser degree (OR, 2.41).
Generally, these practices align with recommendations, the investigators wrote, noting that research supports bilateral mastectomy with BRCA1/2, TP53, and PTEN variants, while data are lacking for other genetic subtypes.
Radiotherapy and chemotherapy practices were more discordant with guidelines. For example, women with a BRCA mutation were 78% less likely to receive radiotherapy after lumpectomy (OR, 0.22) and 76% more likely to receive chemotherapy for early-stage, hormone-positive disease (OR, 1.76). According to investigators, these findings suggest possible trends in undertreatment and overtreatment, respectively.
“We believe more research is needed to confirm our results and to evaluate long-term outcomes of pathogenic variant carriers to understand treatment decision making and consequences,” the investigators concluded.
The study was funded by the National Institutes of Health and the California Department of Public Health. The investigators reported relationships with Myriad Genetics, Genomic Health, Roche, and other companies.
SOURCE: Kurian AW et al. JAMA Oncol. 2020 Feb 6. doi: 10.1001/jamaoncol.2019.6400.
Women with breast cancer may be receiving treatments that are discordant with guideline recommendations for genetic subtypes of disease, based on a retrospective analysis of more than 20,000 patients.
Radiotherapy and chemotherapy practices were particularly out of alignment with guidelines, reported lead author Allison W. Kurian, MD, of Stanford (Calif.) University, and colleagues.
“Integrating genetic testing into breast cancer care has been complex and challenging,” the investigators wrote in JAMA Oncology. “There is wide variability in which clinicians order testing and disclose results, in the clinical significance of results, and in how clinicians interpret results to patients.”
According to the investigators, while germline testing is on the rise, little is known about how these test results are translating to clinical care.
To learn more, the investigators evaluated data from 20,568 women with stage 0-III breast cancer who entered the Surveillance, Epidemiology, and End Results registries of Georgia and California between 2014 and 2016.
Three treatment types were evaluated: surgery (bilateral vs. unilateral mastectomy), radiotherapy after lumpectomy, and chemotherapy. Treatment selection was compared with test results for breast cancer–associated genes, such as BRCA1/2, TP53, PTEN, and others. Associations were then compared with guideline recommendations.
Data analysis suggested that many clinicians were correctly using genetic test results to guide surgical decisions. For example, almost two-thirds (61.7%) of women with a BRCA mutation underwent bilateral mastectomy, compared with one-quarter (24.3%) who were BRCA negative (odds ratio, 5.52). For other pathogenic variants, the rate of bilateral mastectomy was still elevated, albeit to a lesser degree (OR, 2.41).
Generally, these practices align with recommendations, the investigators wrote, noting that research supports bilateral mastectomy with BRCA1/2, TP53, and PTEN variants, while data are lacking for other genetic subtypes.
Radiotherapy and chemotherapy practices were more discordant with guidelines. For example, women with a BRCA mutation were 78% less likely to receive radiotherapy after lumpectomy (OR, 0.22) and 76% more likely to receive chemotherapy for early-stage, hormone-positive disease (OR, 1.76). According to investigators, these findings suggest possible trends in undertreatment and overtreatment, respectively.
“We believe more research is needed to confirm our results and to evaluate long-term outcomes of pathogenic variant carriers to understand treatment decision making and consequences,” the investigators concluded.
The study was funded by the National Institutes of Health and the California Department of Public Health. The investigators reported relationships with Myriad Genetics, Genomic Health, Roche, and other companies.
SOURCE: Kurian AW et al. JAMA Oncol. 2020 Feb 6. doi: 10.1001/jamaoncol.2019.6400.
FROM JAMA ONCOLOGY
Screen pregnant women with suspected 2019-nCoV infection
It is too early yet to explicitly determine the effects of the Novel Coronavirus (2019-nCoV) on pregnant women and their fetuses. This is a critical concern, because members of the coronavirus family, which have been responsible for previous outbreaks of severe acute respiratory syndrome (SARS-CoV) and Middle East respiratory syndrome (MERS-CoV), have demonstrated their ability to cause severe complications during pregnancy, according to researchers.
The SARS virus outbreak and the more recent MERS virus outbreak provide the best available models with which to examine the potential impact of 2019-nCoV on pregnancy, according to a letter published online in the Lancet.
Twelve pregnant women were infected with SARS-CoV during the 2002-2003 pandemic. Three (25%) of these women died during pregnancy. Overall, four of seven women had a miscarriage in the first trimester. In the second or third trimester, two out of five women had fetal growth restriction, and four of the five had preterm birth (one case was spontaneous and three were induced because of the maternal condition), according to corresponding author David Baud, MD, PhD, of the maternal-fetal and obstetrics research unit at Lausanne (Switzerland) University Hospital, and colleagues.
A review of 11 pregnant women infected with the virus showed that 10 women (91%) presented with adverse outcomes. Six (55%) neonates were admitted to the ICU; three (27%) died. Two neonates were delivered prematurely because their mothers developed severe respiratory failure.
Because 2019-nCov has a potential for similar behavior, “we recommend systematic screening of any suspected 2019-nCoV infection during pregnancy. If 2019-nCoV infection during pregnancy is confirmed, extended follow-up should be recommended for mothers and their fetuses,” concluded Dr. Baud and colleagues.
Dr. Baud and associates are known for their previous research on the impacts of the Zika virus on pregnancy. They reported having no competing interests.
SOURCE: Baud D et al. Lancet. 2020 Feb 6. doi: 10.1016/S0140-6736(20)30311-1.
The coronavirus has been spreading rapidly in China, and recently, international cases have been identified, including within the United States. As the article by Locher et al. suggests, mechanical, physiological, and immune adaptations in pregnancy leave pregnant women at risk of severe complications from respiratory illnesses.
Obstetricians need to be prepared to screen, test, and promptly treat pregnant women with any severe respiratory illness to reduce maternal and perinatal morbidity. At this time, the Centers for Disease Control and Prevention advises that any patient with fever and signs of a lower respiratory infection, as well as an epidemiologic risk factor (such as recent travel to China), should be considered at risk for the coronavirus. Samples are collected and sent to the CDC as testing can be done only at the CDC at this time. Please refer to the CDC website for up-to-date guidance for health care professionals.
Unfortunately, there is no specific treatment for coronavirus. Clinical management includes prompt implementation of recommended infection prevention and control measures. Supportive management of complications, including fever reduction and advanced organ support, should be provided as necessary.
While coronavirus is a terrifying potential threat, it’s worth mentioning that, for most pregnant women, a much more likely threat is influenza. Pregnant women with influenza virus infection are at increased risk for progression to pneumonia, ICU admission, preterm delivery, and maternal death. The influenza vaccine can help reduce these risks, and we should continue to encourage vaccination for all pregnant women. Prompt treatment is important! Treatment within 48 hours of the onset of symptoms is ideal, but treatment should not be withheld if the ideal window is missed.
Finally, don’t forget to remind your pregnant patients to avoid close contact with sick family members and friends, wash hands frequently, and call the doctor’s office with any sign of a flu-like illness!
Angela Martin, MD, is an assistant professor of gynecology and obstetrics in the division of maternal-fetal medicine at the University of Kansas Medical Center in Kansas City. She is a member of the Ob.Gyn. News editorial advisory board.
The coronavirus has been spreading rapidly in China, and recently, international cases have been identified, including within the United States. As the article by Locher et al. suggests, mechanical, physiological, and immune adaptations in pregnancy leave pregnant women at risk of severe complications from respiratory illnesses.
Obstetricians need to be prepared to screen, test, and promptly treat pregnant women with any severe respiratory illness to reduce maternal and perinatal morbidity. At this time, the Centers for Disease Control and Prevention advises that any patient with fever and signs of a lower respiratory infection, as well as an epidemiologic risk factor (such as recent travel to China), should be considered at risk for the coronavirus. Samples are collected and sent to the CDC as testing can be done only at the CDC at this time. Please refer to the CDC website for up-to-date guidance for health care professionals.
Unfortunately, there is no specific treatment for coronavirus. Clinical management includes prompt implementation of recommended infection prevention and control measures. Supportive management of complications, including fever reduction and advanced organ support, should be provided as necessary.
While coronavirus is a terrifying potential threat, it’s worth mentioning that, for most pregnant women, a much more likely threat is influenza. Pregnant women with influenza virus infection are at increased risk for progression to pneumonia, ICU admission, preterm delivery, and maternal death. The influenza vaccine can help reduce these risks, and we should continue to encourage vaccination for all pregnant women. Prompt treatment is important! Treatment within 48 hours of the onset of symptoms is ideal, but treatment should not be withheld if the ideal window is missed.
Finally, don’t forget to remind your pregnant patients to avoid close contact with sick family members and friends, wash hands frequently, and call the doctor’s office with any sign of a flu-like illness!
Angela Martin, MD, is an assistant professor of gynecology and obstetrics in the division of maternal-fetal medicine at the University of Kansas Medical Center in Kansas City. She is a member of the Ob.Gyn. News editorial advisory board.
The coronavirus has been spreading rapidly in China, and recently, international cases have been identified, including within the United States. As the article by Locher et al. suggests, mechanical, physiological, and immune adaptations in pregnancy leave pregnant women at risk of severe complications from respiratory illnesses.
Obstetricians need to be prepared to screen, test, and promptly treat pregnant women with any severe respiratory illness to reduce maternal and perinatal morbidity. At this time, the Centers for Disease Control and Prevention advises that any patient with fever and signs of a lower respiratory infection, as well as an epidemiologic risk factor (such as recent travel to China), should be considered at risk for the coronavirus. Samples are collected and sent to the CDC as testing can be done only at the CDC at this time. Please refer to the CDC website for up-to-date guidance for health care professionals.
Unfortunately, there is no specific treatment for coronavirus. Clinical management includes prompt implementation of recommended infection prevention and control measures. Supportive management of complications, including fever reduction and advanced organ support, should be provided as necessary.
While coronavirus is a terrifying potential threat, it’s worth mentioning that, for most pregnant women, a much more likely threat is influenza. Pregnant women with influenza virus infection are at increased risk for progression to pneumonia, ICU admission, preterm delivery, and maternal death. The influenza vaccine can help reduce these risks, and we should continue to encourage vaccination for all pregnant women. Prompt treatment is important! Treatment within 48 hours of the onset of symptoms is ideal, but treatment should not be withheld if the ideal window is missed.
Finally, don’t forget to remind your pregnant patients to avoid close contact with sick family members and friends, wash hands frequently, and call the doctor’s office with any sign of a flu-like illness!
Angela Martin, MD, is an assistant professor of gynecology and obstetrics in the division of maternal-fetal medicine at the University of Kansas Medical Center in Kansas City. She is a member of the Ob.Gyn. News editorial advisory board.
It is too early yet to explicitly determine the effects of the Novel Coronavirus (2019-nCoV) on pregnant women and their fetuses. This is a critical concern, because members of the coronavirus family, which have been responsible for previous outbreaks of severe acute respiratory syndrome (SARS-CoV) and Middle East respiratory syndrome (MERS-CoV), have demonstrated their ability to cause severe complications during pregnancy, according to researchers.
The SARS virus outbreak and the more recent MERS virus outbreak provide the best available models with which to examine the potential impact of 2019-nCoV on pregnancy, according to a letter published online in the Lancet.
Twelve pregnant women were infected with SARS-CoV during the 2002-2003 pandemic. Three (25%) of these women died during pregnancy. Overall, four of seven women had a miscarriage in the first trimester. In the second or third trimester, two out of five women had fetal growth restriction, and four of the five had preterm birth (one case was spontaneous and three were induced because of the maternal condition), according to corresponding author David Baud, MD, PhD, of the maternal-fetal and obstetrics research unit at Lausanne (Switzerland) University Hospital, and colleagues.
A review of 11 pregnant women infected with the virus showed that 10 women (91%) presented with adverse outcomes. Six (55%) neonates were admitted to the ICU; three (27%) died. Two neonates were delivered prematurely because their mothers developed severe respiratory failure.
Because 2019-nCov has a potential for similar behavior, “we recommend systematic screening of any suspected 2019-nCoV infection during pregnancy. If 2019-nCoV infection during pregnancy is confirmed, extended follow-up should be recommended for mothers and their fetuses,” concluded Dr. Baud and colleagues.
Dr. Baud and associates are known for their previous research on the impacts of the Zika virus on pregnancy. They reported having no competing interests.
SOURCE: Baud D et al. Lancet. 2020 Feb 6. doi: 10.1016/S0140-6736(20)30311-1.
It is too early yet to explicitly determine the effects of the Novel Coronavirus (2019-nCoV) on pregnant women and their fetuses. This is a critical concern, because members of the coronavirus family, which have been responsible for previous outbreaks of severe acute respiratory syndrome (SARS-CoV) and Middle East respiratory syndrome (MERS-CoV), have demonstrated their ability to cause severe complications during pregnancy, according to researchers.
The SARS virus outbreak and the more recent MERS virus outbreak provide the best available models with which to examine the potential impact of 2019-nCoV on pregnancy, according to a letter published online in the Lancet.
Twelve pregnant women were infected with SARS-CoV during the 2002-2003 pandemic. Three (25%) of these women died during pregnancy. Overall, four of seven women had a miscarriage in the first trimester. In the second or third trimester, two out of five women had fetal growth restriction, and four of the five had preterm birth (one case was spontaneous and three were induced because of the maternal condition), according to corresponding author David Baud, MD, PhD, of the maternal-fetal and obstetrics research unit at Lausanne (Switzerland) University Hospital, and colleagues.
A review of 11 pregnant women infected with the virus showed that 10 women (91%) presented with adverse outcomes. Six (55%) neonates were admitted to the ICU; three (27%) died. Two neonates were delivered prematurely because their mothers developed severe respiratory failure.
Because 2019-nCov has a potential for similar behavior, “we recommend systematic screening of any suspected 2019-nCoV infection during pregnancy. If 2019-nCoV infection during pregnancy is confirmed, extended follow-up should be recommended for mothers and their fetuses,” concluded Dr. Baud and colleagues.
Dr. Baud and associates are known for their previous research on the impacts of the Zika virus on pregnancy. They reported having no competing interests.
SOURCE: Baud D et al. Lancet. 2020 Feb 6. doi: 10.1016/S0140-6736(20)30311-1.
FROM THE LANCET
What you absolutely need to know about tail coverage
A 28-year-old pediatrician working in a large group practice in California found a new job in Pennsylvania. The job would allow her to live with her husband, who was a nonphysician.
On her last day of work at the California job, the practice’s office manager asked her, “Do you know about the tail coverage?”
He explained that it is malpractice insurance for any cases filed against her after leaving the job. Without it, he said, she would not be covered for those claims.
The physician (who asked not to be identified) had very little savings and suddenly had to pay a five-figure bill for tail coverage. To provide the extra malpractice coverage, she and her husband had to use savings they’d set aside to buy a house.
Getting tail coverage, known formally as an extended reporting endorsement, often comes as a complete and costly surprise for new doctors, says Dennis Hursh, Esq, a health care attorney based in Middletown, Penn., who deals with physicians’ employment contracts.
“Having to pay for a tail can disrupt lives,” Hursh said. “A tail can cost about one third of a young doctor’s salary. If you don’t feel you can afford to pay that, you may be forced to stay with a job you don’t like.”
Most medical residents don’t think about tail coverage until they apply for their first job, but last year, residents at Hahnemann University Hospital in Philadelphia got a painful early lesson.
In the summer, the hospital went out of business because of financial problems. Hundreds of medical residents and fellows not only were forced to find new programs but also had to prepare to buy tail coverage for their training years at Hahnemann.
“All the guarantees have been yanked out from under us,” said Tom Sibert, MD, a former internal medicine resident at the hospital, who is now finishing his training in California. “Residents don’t have that kind of money.”
Hahnemann trainees have asked the judge in the bankruptcy proceedings to put them ahead of other creditors and to ensure their tail coverage is paid. As of early February, the issue had not been resolved.
Meanwhile, Sibert and many other former trainees were trying to get quotes for purchasing tail coverage. They have been shocked by the amounts they would have to pay.
How tail coverage works
Medical malpractice tail coverage protects from incidents that took place when doctors were at their previous jobs but that later resulted in malpractice claims after they had left that employer.
One type of malpractice insurance, an occurrence policy, does not need tail coverage. Occurrence policies cover any incident that occurred when the policy was in force, no matter when a claim was filed – even if it is filed many years after the claims-filing period of the policy ends.
However, most malpractice policies – as many as 85%, according to one estimate – are claims-made policies. Claims-made policies are more much common because they’re significantly less expensive than occurrence policies.
Under a claims-made policy, coverage for malpractice claims completely stops when the policy ends. It does not cover incidents that occurred when the policy was in force but for which the patients later filed claims, as the occurrence policy does. So a tail is needed to cover these claims.
Physicians in all stages of their career may need tail coverage when they leave a job, change malpractice carriers, or retire.
But young physicians often have greater problems with tail coverage, for several reasons. They tend to be employed, and as such, they cannot choose the coverage they want. As a result, they most likely get claims-made coverage. In addition, the job turnover tends to be higher for these doctors. When leaving a job, the tail comes into play. More than half of new physicians leave their first job within 5 years, and of those, more than half leave after only 1 or 2 years.
Young physicians have no experience with tails and may not even know what they are. “In training, malpractice coverage is not a problem because the program handles it,” Mr. Hursh said. Accreditation standards require that teaching hospitals buy coverage, including a tail when residents leave.
So when young physicians are offered their first job and are handed an employment contract to sign, they may not even look for tail coverage, says Mr. Hursh, who wrote The Final Hurdle, a Physician’s Guide to Negotiating a Fair Employment Agreement. Instead, “young physicians tend to focus on issues like salary, benefits, and signing bonuses,” he said.
Mr. Hursh says the tail is usually the most expensive potential cost in the contract.
There’s no easy way to get out of paying the tail coverage once it is enshrined in the contract. The full tail can cost five or even six figures, depending on the physicians’ specialty, the local malpractice premium, and the physician’s own claims history.
Can you negotiate your tail coverage?
Negotiating tail coverage in the employment contract involves some familiarity with medical malpractice insurance and a close reading of the contract. First, you have to determine that the employer is providing claims-made coverage, which would require a tail if you leave. Then you have to determine whether the employer will pay for the tail coverage.
Often, the contract does not even mention tail coverage. “It could merely state that the practice will be responsible for malpractice coverage while you are working there,” Mr. Hursh said. Although it never specifies the tail, this language indicates that you will be paying for it, he says.
Therefore, it’s wise to have a conversation with your prospective employer about the tail. “Some new doctors never ask the question ‘What happens if I leave? Do I get tail coverage?’ ” said Israel Teitelbaum, an attorney who is chairman of Contemporary Insurance Services, an insurance broker in Silver Spring, Md.
Talking about the tail, however, can be a touchy subject for many young doctors applying for their first job. The tail matters only if you leave the job, and you may not want to imply that you would ever want to leave. Too much money, however, is on the line for you not to ask, Mr. Teitelbaum said.
Even if the employer verbally agrees to pay for the tail coverage, experts advise that you try to get the employer’s commitment in writing and have it put it into the contract.
Getting the employer to cover the tail in the initial contract is crucial because once you have agreed to work there, “it’s much more difficult to get it changed,” Mr. Teitelbaum said. However, even if tail coverage is not in the first contract, you shouldn’t give up, he says. You should try again in the next contract a few years later.
“It’s never too late to bring it up,” Mr. Teitelbaum said. After a few years of employment, you have a track record at the job. “A doctor who is very desirable to the employer may be able to get tail coverage on contract renewal.”
Coverage: Large employers vs. small employers
Willingness to pay for an employee’s tail coverage varies depending on the size of the employer. Large employers – systems, hospitals, and large practices – are much more likely to cover the tail than small and medium-sized practices.
Large employers tend to pay for at least part of the tail because they realize that it is in their interest to do so. Since they have the deepest pockets, they’re often the first to be named in a lawsuit. They might have to pay the whole claim if the physician did not have tail coverage.
However, many large employers want to use tail coverage as a bargaining chip to make sure doctors stay for a while at least. One typical arrangement, Mr. Hursh says, is to pay only one-fifth of the tail if the physician leaves in the first year of employment and then to pay one fifth more in each succeeding year until year five, when the employer assumes the entire cost of the tail.
Smaller practices, on the other hand, are usually close-fisted about tail coverage. “They tend to view the tail as an unnecessary expense,” Mr. Hursh said. “They don’t want to pay for a doctor who is not generating revenue for them any more.”
Traditionally, when physicians become partners, practices are more generous and agree to pay their tails if they leave, Mr. Hursh says. But he thinks this is changing, too – recent partnership contracts he has reviewed did not provide for tail coverage.
Times you don’t need to pay for tail coverage
Even if you’re responsible for the tail coverage, your insurance arrangement may be such that you don’t have to pay for it, says Michelle Perron, a malpractice insurance broker in North Hampton, N.H.
For example, if the carrier at your new job is the same as the one at your old job, your coverage would continue with no break, and you would not need a tail, she says. Even if you move to another state, your old carrier might also sell policies there, and you would then likely have seamless coverage, Ms. Perron says. This would be handy if you could choose your new carrier.
Even when you change carriers, Ms. Perron says, the new one might agree to pick up the old carrier’s coverage in return for getting your business, assuming you are an independent physician buying your own coverage. The new carrier would issue prior acts coverage, also known as nose coverage.
Older doctors going into retirement also have a potential tail coverage problem, but their tail coverage premium is often waived, Ms. Perron says. The need for a tail has to do with claims arising post retirement, after your coverage has ended. Typically, if you have been with the carrier for at least 5 years and you are age 55 years or older, your carrier will waive the tail coverage premium, she says.
However, if the retired doctor starts practicing again, even part time, the carrier may want to take back the free tail, she says. Some retired doctors get around this by buying a lower-priced tail from another company, but the former carrier may still want its money back, Ms. Perron says.
Can you just go without tail coverage?
What happens if physicians with a tail commitment choose to wing it and not pay for the tail? If a claim was never made against them, they may believe that the expense is unnecessary. The situation, however, is not so simple.
Some states require having tail coverage. Malpractice coverage is required in seven states, and at least some of those states explicitly extend this requirement to tails. They are Colorado, Connecticut, Kansas, Massachusetts, New Jersey, Rhode Island, and Wisconsin. Eleven more states tie malpractice coverage, perhaps including tails, to some benefit for the doctor, such as tort reform. These states include Indiana, Nebraska, New Mexico, New York, and Pennsylvania.
Many hospitals require tail coverage for privileges, and some insurers do as well. In addition, Ms. Perron says a missing tail reduces your prospects when looking for a job. “For the employer, having to pay coverage for a new hire will cost more than starting fresh with someone else,” she said.
Still, it’s important to remember the risk of being sued. “If you don’t buy the tail coverage, you are at risk for a lawsuit for many years to come,” Mr. Teitelbaum said.
Doctors should consider their potential lifetime risk, not just their current risk. Although only 8% of doctors younger than age 40 have been sued for malpractice, that figure climbs to almost half by the time doctors reach age 55.
The risks are higher in some specialties. About 63% of general surgeons and ob.gyns. have been sued.
Many of these claims are without merit, and doctors pay only the legal expenses of defending the case. Some doctors may think they could risk frivolous suits and cover legal expenses out of pocket. An American Medical Association survey showed that 68% of closed claims against doctors were dropped, dismissed, or withdrawn. It said these claims cost an average of more than $30,000 to defend.
However, Mr. Teitelbaum puts the defense costs for so-called frivolous suits much higher than the AMA, at $250,000 or more. “Even if you’re sure you won’t have to pay a claim, you still have to defend yourself against frivolous suits,” he said. “You won’t recover those expenses.”
How to lower your tail coverage cost
Physicians typically have 60 days to buy tail coverage after their regular coverage has ended. Specialized brokers such as Mr. Teitelbaum and Ms. Perron help physicians look for the best tails to buy.
The cost of the tail depends on how long you’ve been at your job when you leave it, Ms. Perron says. If you leave in the first 1 or 2 years of the policy, she says, the tail price will be lower because the coverage period is shorter.
Usually the most expensive tail available is from the carrier that issued the original policy. Why is this? “Carriers rarely sell a tail that undercuts their retail price,” Mr. Teitelbaum said. “They don’t want to compete with themselves, and in fact doing so could pose regulatory problems for them.”
Instead of buying from their own carrier, doctors can purchase stand-alone tails from competitors, which Mr. Teitelbaum says are 10%-30% less expensive than the policy the original carrier issues. However, stand-alone tails are not always easy to find, especially for high-cost specialties such as neurosurgery and ob.gyn., he says.
Some physicians try to bring down the cost of the tail by limiting the duration of the tail. You can buy tails that only cover claims filed 1-5 years after the incident took place, rather than indefinitely. These limits mirror the typical statute of limitations – the time limit to file a claim in each state. This limit is as little as 2 years in some states, though it can be as long as 6 years in others.
However, some states make exceptions to the statute of limitations. The 2- to 6-year clock doesn’t start ticking until the mistake is discovered or, in the case of children, when they reach adulthood. “This means that with a limited tail, you always have risk,” Perron said.
And yet some doctors insist on these time-limited tails. “If a doctor opts for 3 years’ coverage, that’s better than no years,” Mr. Teitelbaum said. “But I would advise them to take at least 5 years because that gives you coverage for the basic statute of limitations in most states. Three-year tails do yield savings, but often they’re not enough to warrant the risk.”
Another way to reduce costs is to lower the coverage limits of the tail. The standard coverage limit is $1 million per case and $3 million per year, so doctors might be able to save money on the premium by buying limits of $200,000/$600,000. But Mr. Teitelbaum says most companies would refuse to sell a policy with a limit lower than that of the expiring policy.
Further ways to reduce the cost of the tail include buying tail coverage that doesn’t give the physician the right to approve a settlement or that doesn’t include legal fees in the coverage limits. But these options, too, raise the physician’s risks. Whichever option you choose, the important thing is to protect yourself against costly lawsuits.
This article first appeared on Medscape.com.
A 28-year-old pediatrician working in a large group practice in California found a new job in Pennsylvania. The job would allow her to live with her husband, who was a nonphysician.
On her last day of work at the California job, the practice’s office manager asked her, “Do you know about the tail coverage?”
He explained that it is malpractice insurance for any cases filed against her after leaving the job. Without it, he said, she would not be covered for those claims.
The physician (who asked not to be identified) had very little savings and suddenly had to pay a five-figure bill for tail coverage. To provide the extra malpractice coverage, she and her husband had to use savings they’d set aside to buy a house.
Getting tail coverage, known formally as an extended reporting endorsement, often comes as a complete and costly surprise for new doctors, says Dennis Hursh, Esq, a health care attorney based in Middletown, Penn., who deals with physicians’ employment contracts.
“Having to pay for a tail can disrupt lives,” Hursh said. “A tail can cost about one third of a young doctor’s salary. If you don’t feel you can afford to pay that, you may be forced to stay with a job you don’t like.”
Most medical residents don’t think about tail coverage until they apply for their first job, but last year, residents at Hahnemann University Hospital in Philadelphia got a painful early lesson.
In the summer, the hospital went out of business because of financial problems. Hundreds of medical residents and fellows not only were forced to find new programs but also had to prepare to buy tail coverage for their training years at Hahnemann.
“All the guarantees have been yanked out from under us,” said Tom Sibert, MD, a former internal medicine resident at the hospital, who is now finishing his training in California. “Residents don’t have that kind of money.”
Hahnemann trainees have asked the judge in the bankruptcy proceedings to put them ahead of other creditors and to ensure their tail coverage is paid. As of early February, the issue had not been resolved.
Meanwhile, Sibert and many other former trainees were trying to get quotes for purchasing tail coverage. They have been shocked by the amounts they would have to pay.
How tail coverage works
Medical malpractice tail coverage protects from incidents that took place when doctors were at their previous jobs but that later resulted in malpractice claims after they had left that employer.
One type of malpractice insurance, an occurrence policy, does not need tail coverage. Occurrence policies cover any incident that occurred when the policy was in force, no matter when a claim was filed – even if it is filed many years after the claims-filing period of the policy ends.
However, most malpractice policies – as many as 85%, according to one estimate – are claims-made policies. Claims-made policies are more much common because they’re significantly less expensive than occurrence policies.
Under a claims-made policy, coverage for malpractice claims completely stops when the policy ends. It does not cover incidents that occurred when the policy was in force but for which the patients later filed claims, as the occurrence policy does. So a tail is needed to cover these claims.
Physicians in all stages of their career may need tail coverage when they leave a job, change malpractice carriers, or retire.
But young physicians often have greater problems with tail coverage, for several reasons. They tend to be employed, and as such, they cannot choose the coverage they want. As a result, they most likely get claims-made coverage. In addition, the job turnover tends to be higher for these doctors. When leaving a job, the tail comes into play. More than half of new physicians leave their first job within 5 years, and of those, more than half leave after only 1 or 2 years.
Young physicians have no experience with tails and may not even know what they are. “In training, malpractice coverage is not a problem because the program handles it,” Mr. Hursh said. Accreditation standards require that teaching hospitals buy coverage, including a tail when residents leave.
So when young physicians are offered their first job and are handed an employment contract to sign, they may not even look for tail coverage, says Mr. Hursh, who wrote The Final Hurdle, a Physician’s Guide to Negotiating a Fair Employment Agreement. Instead, “young physicians tend to focus on issues like salary, benefits, and signing bonuses,” he said.
Mr. Hursh says the tail is usually the most expensive potential cost in the contract.
There’s no easy way to get out of paying the tail coverage once it is enshrined in the contract. The full tail can cost five or even six figures, depending on the physicians’ specialty, the local malpractice premium, and the physician’s own claims history.
Can you negotiate your tail coverage?
Negotiating tail coverage in the employment contract involves some familiarity with medical malpractice insurance and a close reading of the contract. First, you have to determine that the employer is providing claims-made coverage, which would require a tail if you leave. Then you have to determine whether the employer will pay for the tail coverage.
Often, the contract does not even mention tail coverage. “It could merely state that the practice will be responsible for malpractice coverage while you are working there,” Mr. Hursh said. Although it never specifies the tail, this language indicates that you will be paying for it, he says.
Therefore, it’s wise to have a conversation with your prospective employer about the tail. “Some new doctors never ask the question ‘What happens if I leave? Do I get tail coverage?’ ” said Israel Teitelbaum, an attorney who is chairman of Contemporary Insurance Services, an insurance broker in Silver Spring, Md.
Talking about the tail, however, can be a touchy subject for many young doctors applying for their first job. The tail matters only if you leave the job, and you may not want to imply that you would ever want to leave. Too much money, however, is on the line for you not to ask, Mr. Teitelbaum said.
Even if the employer verbally agrees to pay for the tail coverage, experts advise that you try to get the employer’s commitment in writing and have it put it into the contract.
Getting the employer to cover the tail in the initial contract is crucial because once you have agreed to work there, “it’s much more difficult to get it changed,” Mr. Teitelbaum said. However, even if tail coverage is not in the first contract, you shouldn’t give up, he says. You should try again in the next contract a few years later.
“It’s never too late to bring it up,” Mr. Teitelbaum said. After a few years of employment, you have a track record at the job. “A doctor who is very desirable to the employer may be able to get tail coverage on contract renewal.”
Coverage: Large employers vs. small employers
Willingness to pay for an employee’s tail coverage varies depending on the size of the employer. Large employers – systems, hospitals, and large practices – are much more likely to cover the tail than small and medium-sized practices.
Large employers tend to pay for at least part of the tail because they realize that it is in their interest to do so. Since they have the deepest pockets, they’re often the first to be named in a lawsuit. They might have to pay the whole claim if the physician did not have tail coverage.
However, many large employers want to use tail coverage as a bargaining chip to make sure doctors stay for a while at least. One typical arrangement, Mr. Hursh says, is to pay only one-fifth of the tail if the physician leaves in the first year of employment and then to pay one fifth more in each succeeding year until year five, when the employer assumes the entire cost of the tail.
Smaller practices, on the other hand, are usually close-fisted about tail coverage. “They tend to view the tail as an unnecessary expense,” Mr. Hursh said. “They don’t want to pay for a doctor who is not generating revenue for them any more.”
Traditionally, when physicians become partners, practices are more generous and agree to pay their tails if they leave, Mr. Hursh says. But he thinks this is changing, too – recent partnership contracts he has reviewed did not provide for tail coverage.
Times you don’t need to pay for tail coverage
Even if you’re responsible for the tail coverage, your insurance arrangement may be such that you don’t have to pay for it, says Michelle Perron, a malpractice insurance broker in North Hampton, N.H.
For example, if the carrier at your new job is the same as the one at your old job, your coverage would continue with no break, and you would not need a tail, she says. Even if you move to another state, your old carrier might also sell policies there, and you would then likely have seamless coverage, Ms. Perron says. This would be handy if you could choose your new carrier.
Even when you change carriers, Ms. Perron says, the new one might agree to pick up the old carrier’s coverage in return for getting your business, assuming you are an independent physician buying your own coverage. The new carrier would issue prior acts coverage, also known as nose coverage.
Older doctors going into retirement also have a potential tail coverage problem, but their tail coverage premium is often waived, Ms. Perron says. The need for a tail has to do with claims arising post retirement, after your coverage has ended. Typically, if you have been with the carrier for at least 5 years and you are age 55 years or older, your carrier will waive the tail coverage premium, she says.
However, if the retired doctor starts practicing again, even part time, the carrier may want to take back the free tail, she says. Some retired doctors get around this by buying a lower-priced tail from another company, but the former carrier may still want its money back, Ms. Perron says.
Can you just go without tail coverage?
What happens if physicians with a tail commitment choose to wing it and not pay for the tail? If a claim was never made against them, they may believe that the expense is unnecessary. The situation, however, is not so simple.
Some states require having tail coverage. Malpractice coverage is required in seven states, and at least some of those states explicitly extend this requirement to tails. They are Colorado, Connecticut, Kansas, Massachusetts, New Jersey, Rhode Island, and Wisconsin. Eleven more states tie malpractice coverage, perhaps including tails, to some benefit for the doctor, such as tort reform. These states include Indiana, Nebraska, New Mexico, New York, and Pennsylvania.
Many hospitals require tail coverage for privileges, and some insurers do as well. In addition, Ms. Perron says a missing tail reduces your prospects when looking for a job. “For the employer, having to pay coverage for a new hire will cost more than starting fresh with someone else,” she said.
Still, it’s important to remember the risk of being sued. “If you don’t buy the tail coverage, you are at risk for a lawsuit for many years to come,” Mr. Teitelbaum said.
Doctors should consider their potential lifetime risk, not just their current risk. Although only 8% of doctors younger than age 40 have been sued for malpractice, that figure climbs to almost half by the time doctors reach age 55.
The risks are higher in some specialties. About 63% of general surgeons and ob.gyns. have been sued.
Many of these claims are without merit, and doctors pay only the legal expenses of defending the case. Some doctors may think they could risk frivolous suits and cover legal expenses out of pocket. An American Medical Association survey showed that 68% of closed claims against doctors were dropped, dismissed, or withdrawn. It said these claims cost an average of more than $30,000 to defend.
However, Mr. Teitelbaum puts the defense costs for so-called frivolous suits much higher than the AMA, at $250,000 or more. “Even if you’re sure you won’t have to pay a claim, you still have to defend yourself against frivolous suits,” he said. “You won’t recover those expenses.”
How to lower your tail coverage cost
Physicians typically have 60 days to buy tail coverage after their regular coverage has ended. Specialized brokers such as Mr. Teitelbaum and Ms. Perron help physicians look for the best tails to buy.
The cost of the tail depends on how long you’ve been at your job when you leave it, Ms. Perron says. If you leave in the first 1 or 2 years of the policy, she says, the tail price will be lower because the coverage period is shorter.
Usually the most expensive tail available is from the carrier that issued the original policy. Why is this? “Carriers rarely sell a tail that undercuts their retail price,” Mr. Teitelbaum said. “They don’t want to compete with themselves, and in fact doing so could pose regulatory problems for them.”
Instead of buying from their own carrier, doctors can purchase stand-alone tails from competitors, which Mr. Teitelbaum says are 10%-30% less expensive than the policy the original carrier issues. However, stand-alone tails are not always easy to find, especially for high-cost specialties such as neurosurgery and ob.gyn., he says.
Some physicians try to bring down the cost of the tail by limiting the duration of the tail. You can buy tails that only cover claims filed 1-5 years after the incident took place, rather than indefinitely. These limits mirror the typical statute of limitations – the time limit to file a claim in each state. This limit is as little as 2 years in some states, though it can be as long as 6 years in others.
However, some states make exceptions to the statute of limitations. The 2- to 6-year clock doesn’t start ticking until the mistake is discovered or, in the case of children, when they reach adulthood. “This means that with a limited tail, you always have risk,” Perron said.
And yet some doctors insist on these time-limited tails. “If a doctor opts for 3 years’ coverage, that’s better than no years,” Mr. Teitelbaum said. “But I would advise them to take at least 5 years because that gives you coverage for the basic statute of limitations in most states. Three-year tails do yield savings, but often they’re not enough to warrant the risk.”
Another way to reduce costs is to lower the coverage limits of the tail. The standard coverage limit is $1 million per case and $3 million per year, so doctors might be able to save money on the premium by buying limits of $200,000/$600,000. But Mr. Teitelbaum says most companies would refuse to sell a policy with a limit lower than that of the expiring policy.
Further ways to reduce the cost of the tail include buying tail coverage that doesn’t give the physician the right to approve a settlement or that doesn’t include legal fees in the coverage limits. But these options, too, raise the physician’s risks. Whichever option you choose, the important thing is to protect yourself against costly lawsuits.
This article first appeared on Medscape.com.
A 28-year-old pediatrician working in a large group practice in California found a new job in Pennsylvania. The job would allow her to live with her husband, who was a nonphysician.
On her last day of work at the California job, the practice’s office manager asked her, “Do you know about the tail coverage?”
He explained that it is malpractice insurance for any cases filed against her after leaving the job. Without it, he said, she would not be covered for those claims.
The physician (who asked not to be identified) had very little savings and suddenly had to pay a five-figure bill for tail coverage. To provide the extra malpractice coverage, she and her husband had to use savings they’d set aside to buy a house.
Getting tail coverage, known formally as an extended reporting endorsement, often comes as a complete and costly surprise for new doctors, says Dennis Hursh, Esq, a health care attorney based in Middletown, Penn., who deals with physicians’ employment contracts.
“Having to pay for a tail can disrupt lives,” Hursh said. “A tail can cost about one third of a young doctor’s salary. If you don’t feel you can afford to pay that, you may be forced to stay with a job you don’t like.”
Most medical residents don’t think about tail coverage until they apply for their first job, but last year, residents at Hahnemann University Hospital in Philadelphia got a painful early lesson.
In the summer, the hospital went out of business because of financial problems. Hundreds of medical residents and fellows not only were forced to find new programs but also had to prepare to buy tail coverage for their training years at Hahnemann.
“All the guarantees have been yanked out from under us,” said Tom Sibert, MD, a former internal medicine resident at the hospital, who is now finishing his training in California. “Residents don’t have that kind of money.”
Hahnemann trainees have asked the judge in the bankruptcy proceedings to put them ahead of other creditors and to ensure their tail coverage is paid. As of early February, the issue had not been resolved.
Meanwhile, Sibert and many other former trainees were trying to get quotes for purchasing tail coverage. They have been shocked by the amounts they would have to pay.
How tail coverage works
Medical malpractice tail coverage protects from incidents that took place when doctors were at their previous jobs but that later resulted in malpractice claims after they had left that employer.
One type of malpractice insurance, an occurrence policy, does not need tail coverage. Occurrence policies cover any incident that occurred when the policy was in force, no matter when a claim was filed – even if it is filed many years after the claims-filing period of the policy ends.
However, most malpractice policies – as many as 85%, according to one estimate – are claims-made policies. Claims-made policies are more much common because they’re significantly less expensive than occurrence policies.
Under a claims-made policy, coverage for malpractice claims completely stops when the policy ends. It does not cover incidents that occurred when the policy was in force but for which the patients later filed claims, as the occurrence policy does. So a tail is needed to cover these claims.
Physicians in all stages of their career may need tail coverage when they leave a job, change malpractice carriers, or retire.
But young physicians often have greater problems with tail coverage, for several reasons. They tend to be employed, and as such, they cannot choose the coverage they want. As a result, they most likely get claims-made coverage. In addition, the job turnover tends to be higher for these doctors. When leaving a job, the tail comes into play. More than half of new physicians leave their first job within 5 years, and of those, more than half leave after only 1 or 2 years.
Young physicians have no experience with tails and may not even know what they are. “In training, malpractice coverage is not a problem because the program handles it,” Mr. Hursh said. Accreditation standards require that teaching hospitals buy coverage, including a tail when residents leave.
So when young physicians are offered their first job and are handed an employment contract to sign, they may not even look for tail coverage, says Mr. Hursh, who wrote The Final Hurdle, a Physician’s Guide to Negotiating a Fair Employment Agreement. Instead, “young physicians tend to focus on issues like salary, benefits, and signing bonuses,” he said.
Mr. Hursh says the tail is usually the most expensive potential cost in the contract.
There’s no easy way to get out of paying the tail coverage once it is enshrined in the contract. The full tail can cost five or even six figures, depending on the physicians’ specialty, the local malpractice premium, and the physician’s own claims history.
Can you negotiate your tail coverage?
Negotiating tail coverage in the employment contract involves some familiarity with medical malpractice insurance and a close reading of the contract. First, you have to determine that the employer is providing claims-made coverage, which would require a tail if you leave. Then you have to determine whether the employer will pay for the tail coverage.
Often, the contract does not even mention tail coverage. “It could merely state that the practice will be responsible for malpractice coverage while you are working there,” Mr. Hursh said. Although it never specifies the tail, this language indicates that you will be paying for it, he says.
Therefore, it’s wise to have a conversation with your prospective employer about the tail. “Some new doctors never ask the question ‘What happens if I leave? Do I get tail coverage?’ ” said Israel Teitelbaum, an attorney who is chairman of Contemporary Insurance Services, an insurance broker in Silver Spring, Md.
Talking about the tail, however, can be a touchy subject for many young doctors applying for their first job. The tail matters only if you leave the job, and you may not want to imply that you would ever want to leave. Too much money, however, is on the line for you not to ask, Mr. Teitelbaum said.
Even if the employer verbally agrees to pay for the tail coverage, experts advise that you try to get the employer’s commitment in writing and have it put it into the contract.
Getting the employer to cover the tail in the initial contract is crucial because once you have agreed to work there, “it’s much more difficult to get it changed,” Mr. Teitelbaum said. However, even if tail coverage is not in the first contract, you shouldn’t give up, he says. You should try again in the next contract a few years later.
“It’s never too late to bring it up,” Mr. Teitelbaum said. After a few years of employment, you have a track record at the job. “A doctor who is very desirable to the employer may be able to get tail coverage on contract renewal.”
Coverage: Large employers vs. small employers
Willingness to pay for an employee’s tail coverage varies depending on the size of the employer. Large employers – systems, hospitals, and large practices – are much more likely to cover the tail than small and medium-sized practices.
Large employers tend to pay for at least part of the tail because they realize that it is in their interest to do so. Since they have the deepest pockets, they’re often the first to be named in a lawsuit. They might have to pay the whole claim if the physician did not have tail coverage.
However, many large employers want to use tail coverage as a bargaining chip to make sure doctors stay for a while at least. One typical arrangement, Mr. Hursh says, is to pay only one-fifth of the tail if the physician leaves in the first year of employment and then to pay one fifth more in each succeeding year until year five, when the employer assumes the entire cost of the tail.
Smaller practices, on the other hand, are usually close-fisted about tail coverage. “They tend to view the tail as an unnecessary expense,” Mr. Hursh said. “They don’t want to pay for a doctor who is not generating revenue for them any more.”
Traditionally, when physicians become partners, practices are more generous and agree to pay their tails if they leave, Mr. Hursh says. But he thinks this is changing, too – recent partnership contracts he has reviewed did not provide for tail coverage.
Times you don’t need to pay for tail coverage
Even if you’re responsible for the tail coverage, your insurance arrangement may be such that you don’t have to pay for it, says Michelle Perron, a malpractice insurance broker in North Hampton, N.H.
For example, if the carrier at your new job is the same as the one at your old job, your coverage would continue with no break, and you would not need a tail, she says. Even if you move to another state, your old carrier might also sell policies there, and you would then likely have seamless coverage, Ms. Perron says. This would be handy if you could choose your new carrier.
Even when you change carriers, Ms. Perron says, the new one might agree to pick up the old carrier’s coverage in return for getting your business, assuming you are an independent physician buying your own coverage. The new carrier would issue prior acts coverage, also known as nose coverage.
Older doctors going into retirement also have a potential tail coverage problem, but their tail coverage premium is often waived, Ms. Perron says. The need for a tail has to do with claims arising post retirement, after your coverage has ended. Typically, if you have been with the carrier for at least 5 years and you are age 55 years or older, your carrier will waive the tail coverage premium, she says.
However, if the retired doctor starts practicing again, even part time, the carrier may want to take back the free tail, she says. Some retired doctors get around this by buying a lower-priced tail from another company, but the former carrier may still want its money back, Ms. Perron says.
Can you just go without tail coverage?
What happens if physicians with a tail commitment choose to wing it and not pay for the tail? If a claim was never made against them, they may believe that the expense is unnecessary. The situation, however, is not so simple.
Some states require having tail coverage. Malpractice coverage is required in seven states, and at least some of those states explicitly extend this requirement to tails. They are Colorado, Connecticut, Kansas, Massachusetts, New Jersey, Rhode Island, and Wisconsin. Eleven more states tie malpractice coverage, perhaps including tails, to some benefit for the doctor, such as tort reform. These states include Indiana, Nebraska, New Mexico, New York, and Pennsylvania.
Many hospitals require tail coverage for privileges, and some insurers do as well. In addition, Ms. Perron says a missing tail reduces your prospects when looking for a job. “For the employer, having to pay coverage for a new hire will cost more than starting fresh with someone else,” she said.
Still, it’s important to remember the risk of being sued. “If you don’t buy the tail coverage, you are at risk for a lawsuit for many years to come,” Mr. Teitelbaum said.
Doctors should consider their potential lifetime risk, not just their current risk. Although only 8% of doctors younger than age 40 have been sued for malpractice, that figure climbs to almost half by the time doctors reach age 55.
The risks are higher in some specialties. About 63% of general surgeons and ob.gyns. have been sued.
Many of these claims are without merit, and doctors pay only the legal expenses of defending the case. Some doctors may think they could risk frivolous suits and cover legal expenses out of pocket. An American Medical Association survey showed that 68% of closed claims against doctors were dropped, dismissed, or withdrawn. It said these claims cost an average of more than $30,000 to defend.
However, Mr. Teitelbaum puts the defense costs for so-called frivolous suits much higher than the AMA, at $250,000 or more. “Even if you’re sure you won’t have to pay a claim, you still have to defend yourself against frivolous suits,” he said. “You won’t recover those expenses.”
How to lower your tail coverage cost
Physicians typically have 60 days to buy tail coverage after their regular coverage has ended. Specialized brokers such as Mr. Teitelbaum and Ms. Perron help physicians look for the best tails to buy.
The cost of the tail depends on how long you’ve been at your job when you leave it, Ms. Perron says. If you leave in the first 1 or 2 years of the policy, she says, the tail price will be lower because the coverage period is shorter.
Usually the most expensive tail available is from the carrier that issued the original policy. Why is this? “Carriers rarely sell a tail that undercuts their retail price,” Mr. Teitelbaum said. “They don’t want to compete with themselves, and in fact doing so could pose regulatory problems for them.”
Instead of buying from their own carrier, doctors can purchase stand-alone tails from competitors, which Mr. Teitelbaum says are 10%-30% less expensive than the policy the original carrier issues. However, stand-alone tails are not always easy to find, especially for high-cost specialties such as neurosurgery and ob.gyn., he says.
Some physicians try to bring down the cost of the tail by limiting the duration of the tail. You can buy tails that only cover claims filed 1-5 years after the incident took place, rather than indefinitely. These limits mirror the typical statute of limitations – the time limit to file a claim in each state. This limit is as little as 2 years in some states, though it can be as long as 6 years in others.
However, some states make exceptions to the statute of limitations. The 2- to 6-year clock doesn’t start ticking until the mistake is discovered or, in the case of children, when they reach adulthood. “This means that with a limited tail, you always have risk,” Perron said.
And yet some doctors insist on these time-limited tails. “If a doctor opts for 3 years’ coverage, that’s better than no years,” Mr. Teitelbaum said. “But I would advise them to take at least 5 years because that gives you coverage for the basic statute of limitations in most states. Three-year tails do yield savings, but often they’re not enough to warrant the risk.”
Another way to reduce costs is to lower the coverage limits of the tail. The standard coverage limit is $1 million per case and $3 million per year, so doctors might be able to save money on the premium by buying limits of $200,000/$600,000. But Mr. Teitelbaum says most companies would refuse to sell a policy with a limit lower than that of the expiring policy.
Further ways to reduce the cost of the tail include buying tail coverage that doesn’t give the physician the right to approve a settlement or that doesn’t include legal fees in the coverage limits. But these options, too, raise the physician’s risks. Whichever option you choose, the important thing is to protect yourself against costly lawsuits.
This article first appeared on Medscape.com.
Cancer research foundation awards ‘breakthrough scientists’
The Damon Runyon Cancer Research Foundation has awarded $100,000 each to six “breakthrough scientists.”
The Damon Runyon–Dale F. Frey Award for Breakthrough Scientists provides additional funding to scientists completing a Damon Runyon Fellowship Award who “are most likely to make paradigm-shifting breakthroughs that transform the way we prevent, diagnose, and treat cancer,” according to the foundation announcement.
Lindsay B. Case, PhD, of the University of Texas Southwestern Medical Center, Dallas, received the award for her research investigating the molecular interactions that contribute to integrin signaling and focal adhesion function. This work could reveal new strategies for disrupting integrin signaling in cancer.
Ivana Gasic, DrSc, of Harvard Medical School, Boston, was awarded for her research on tubulin autoregulation and the microtubule integrity response. Her research could provide insight into the workings of microtubule-targeting chemotherapeutic drugs and reveal new pathways to target cancer cells.
Natasha M. O’Brown, PhD, of Harvard Medical School, Boston, was awarded for her research using CRISPR technology and zebrafish to investigate the molecular mechanisms that regulate the blood-brain barrier. This work could reveal new approaches to drug delivery for patients with brain tumors.
Benjamin M. Stinson, PhD, of Harvard Medical School, Boston, was awarded for his work investigating the mechanisms of two main DNA double-strand break repair pathways. This research could shed light on the causes of cancers and have applications for cancer treatment.
Iva A. Tchasovnikarova, PhD, of Massachusetts General Hospital, Boston, was awarded for epigenetics research that may reveal targets for cancer therapy. She developed a method to identify cell-based reporters of any epigenetic process inside the nucleus and aims to use this method to better understand the biology underlying epigenetic mechanisms.
Yi Yin, PhD, of the University of California, Los Angeles, was awarded for work that may help inform the treatment of many cancers. Dr. Yin developed single-cell assays that she will combine with statistical modeling to better understand homologous recombination.
The Damon Runyon Cancer Research Foundation has awarded $100,000 each to six “breakthrough scientists.”
The Damon Runyon–Dale F. Frey Award for Breakthrough Scientists provides additional funding to scientists completing a Damon Runyon Fellowship Award who “are most likely to make paradigm-shifting breakthroughs that transform the way we prevent, diagnose, and treat cancer,” according to the foundation announcement.
Lindsay B. Case, PhD, of the University of Texas Southwestern Medical Center, Dallas, received the award for her research investigating the molecular interactions that contribute to integrin signaling and focal adhesion function. This work could reveal new strategies for disrupting integrin signaling in cancer.
Ivana Gasic, DrSc, of Harvard Medical School, Boston, was awarded for her research on tubulin autoregulation and the microtubule integrity response. Her research could provide insight into the workings of microtubule-targeting chemotherapeutic drugs and reveal new pathways to target cancer cells.
Natasha M. O’Brown, PhD, of Harvard Medical School, Boston, was awarded for her research using CRISPR technology and zebrafish to investigate the molecular mechanisms that regulate the blood-brain barrier. This work could reveal new approaches to drug delivery for patients with brain tumors.
Benjamin M. Stinson, PhD, of Harvard Medical School, Boston, was awarded for his work investigating the mechanisms of two main DNA double-strand break repair pathways. This research could shed light on the causes of cancers and have applications for cancer treatment.
Iva A. Tchasovnikarova, PhD, of Massachusetts General Hospital, Boston, was awarded for epigenetics research that may reveal targets for cancer therapy. She developed a method to identify cell-based reporters of any epigenetic process inside the nucleus and aims to use this method to better understand the biology underlying epigenetic mechanisms.
Yi Yin, PhD, of the University of California, Los Angeles, was awarded for work that may help inform the treatment of many cancers. Dr. Yin developed single-cell assays that she will combine with statistical modeling to better understand homologous recombination.
The Damon Runyon Cancer Research Foundation has awarded $100,000 each to six “breakthrough scientists.”
The Damon Runyon–Dale F. Frey Award for Breakthrough Scientists provides additional funding to scientists completing a Damon Runyon Fellowship Award who “are most likely to make paradigm-shifting breakthroughs that transform the way we prevent, diagnose, and treat cancer,” according to the foundation announcement.
Lindsay B. Case, PhD, of the University of Texas Southwestern Medical Center, Dallas, received the award for her research investigating the molecular interactions that contribute to integrin signaling and focal adhesion function. This work could reveal new strategies for disrupting integrin signaling in cancer.
Ivana Gasic, DrSc, of Harvard Medical School, Boston, was awarded for her research on tubulin autoregulation and the microtubule integrity response. Her research could provide insight into the workings of microtubule-targeting chemotherapeutic drugs and reveal new pathways to target cancer cells.
Natasha M. O’Brown, PhD, of Harvard Medical School, Boston, was awarded for her research using CRISPR technology and zebrafish to investigate the molecular mechanisms that regulate the blood-brain barrier. This work could reveal new approaches to drug delivery for patients with brain tumors.
Benjamin M. Stinson, PhD, of Harvard Medical School, Boston, was awarded for his work investigating the mechanisms of two main DNA double-strand break repair pathways. This research could shed light on the causes of cancers and have applications for cancer treatment.
Iva A. Tchasovnikarova, PhD, of Massachusetts General Hospital, Boston, was awarded for epigenetics research that may reveal targets for cancer therapy. She developed a method to identify cell-based reporters of any epigenetic process inside the nucleus and aims to use this method to better understand the biology underlying epigenetic mechanisms.
Yi Yin, PhD, of the University of California, Los Angeles, was awarded for work that may help inform the treatment of many cancers. Dr. Yin developed single-cell assays that she will combine with statistical modeling to better understand homologous recombination.
ObGyn malpractice liability risk: 2020 developments and probabilities
In this second in a series of 3 articles discussing medical malpractice and the ObGyn we look at the reasons for malpractice claims and liability, what happens to malpractice claims, and the direction and future of medical malpractice. The first article dealt with 2 sources of major malpractice damages: the “big verdict” and physicians with multiple malpractice paid claims. Next month we look at the place of apology in medicine, in cases in which error, including negligence, may have caused a patient injury.
CASE 1 Long-term brachial plexus injury
Right upper extremity injury occurs in the neonate at delivery with sequela of long-term brachial plexus injury (which is diagnosed around 6 months of age). Physical therapy and orthopedic assessment are rendered. Despite continued treatment, discrepancy in arm lengths (ie, affected side arm is noticeably shorter than opposite side) remains. The child cannot play basketball with his older brother and is the victim of ridicule, the plaintiff’s attorney emphasizes. He is unable to properly pronate or supinate the affected arm.
The defendant ObGyn maintains that there was “no shoulder dystocia [at delivery] and the shoulder did not get obstructed in the pelvis; shoulder was delivered 15 seconds after delivery of the head.” The nursing staff testifies that if shoulder dystocia had been the problem they would have launched upon a series of procedures to address such, in accord with the delivering obstetrician. The defense expert witness testifies that a brachial plexus injury can happen without shoulder dystocia.
A defense verdict is rendered by the Florida jury.1
CASE 2 Shoulder dystocia
During delivery, the obstetrician notes a shoulder dystocia (“turtle sign”). After initial attempts to release the shoulder were unsuccessful, the physician applies traction several times to the head of the child, and the baby is delivered. There is permanent injury to the right brachial plexus. The defendant ObGyn says that traction was necessary to dislodge the shoulder, and that the injury was the result of the forces of labor (not the traction). The expert witness for the plaintiff testifies that the medical standard of care did not permit traction under these circumstances, and that the traction was the likely cause of the injury.
The Virginia jury awards $2.32 million in damages.2
Note: The above vignettes are drawn from actual cases but are only outlines of those cases and are not complete descriptions of the claims in the cases. Because the information comes from informal sources, not formal court records, the facts may be inaccurate and incomplete. They should be viewed as illustrations only.

The trend in malpractice
It has been clear for many years that medical malpractice claims are not randomly or evenly distributed among physicians. Notably, the variation among specialties has, and continues to be, substantial (FIGURE 1).3 Recent data suggest that, although paid claims per “1,000 physician-years” averages 14 paid claims per 1,000 physician years, it ranges from 4 or 5 in 1,000 (psychiatry and pediatrics) to 53 and 49 claims per 1,000 (neurology and plastic surgery, respectively). Obstetrics and gynecology has the fourth highest rate at 42.5 paid claims per 1,000 physician years.4 (These data are for the years 1992–2014.)
Continue to: The number of ObGyn paid malpractice claims has decreased over time...
The number of ObGyn paid malpractice claims has decreased over time. Although large verdicts and physicians with multiple paid malpractice claims receive a good deal of attention (as we noted in part 1 of our series), in fact, paid medical malpractice claims have trended downward in recent decades.5 When the data above are disaggregated by 5-year periods, for example, in obstetrics and gynecology, there has been a consistent reduction in paid malpractice claims from 1992 to 2014. Paid claims went from 58 per 1,000 physician-years in 1992–1996 to 25 per 1,000 in 2009–2014 (FIGURE 2).4,6 In short, the rate dropped by half over approximately 20 years.4
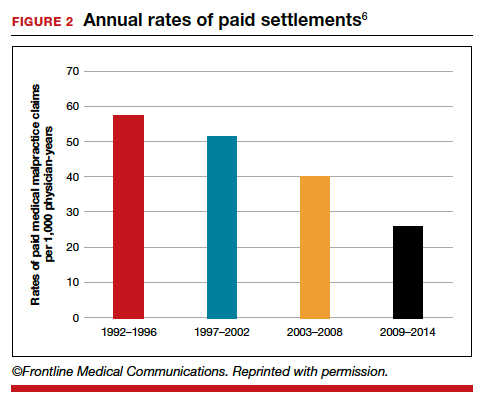
It is reasonable to expect that such a decline in the cost of malpractice insurance premiums would follow. Robert L. Barbieri, MD, who practices in Boston, Massachusetts, in his excellent recent editorial in OBG M
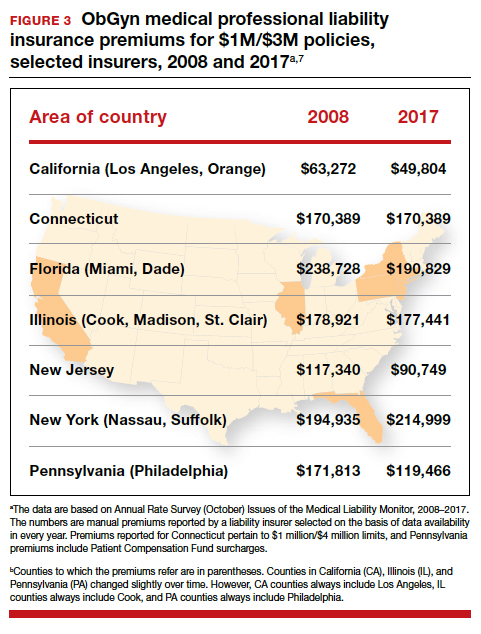
Why have malpractice payouts declined overall?
Have medical errors declined?
It would be wonderful if the reduction in malpractice claims represented a significant decrease in medical errors. Attention to medical errors was driven by the first widely noticed study of medical error deaths. The Institute of Medicine (IOM) study in 2000, put the number of deaths annually at 44,000 to 98,000.8 There have been many efforts to reduce such errors, and it is possible that those efforts have indeed reduced errors somewhat.4 Barbieri provided a helpful digest of many of the error-reduction suggestions for ObGyn practice (TABLE 1).6 But the number of medical errors remains high. More recent studies have suggested that the IOM’s reported number of injuries may have been low.9 In 2013, one study suggested that 210,000 deaths annually were “associated with preventable harm” in hospitals. Because of how the data were gathered the authors estimated that the actual number of preventable deaths was closer to 400,000 annually. Serious harm to patients was estimated at 10 to 20 times the IOM rate.9
Therefore, a dramatic reduction in preventable medical errors does not appear to explain the reduction in malpractice claims. Some portion of it may be explained by malpractice reforms—see "The medical reform factor" section below.
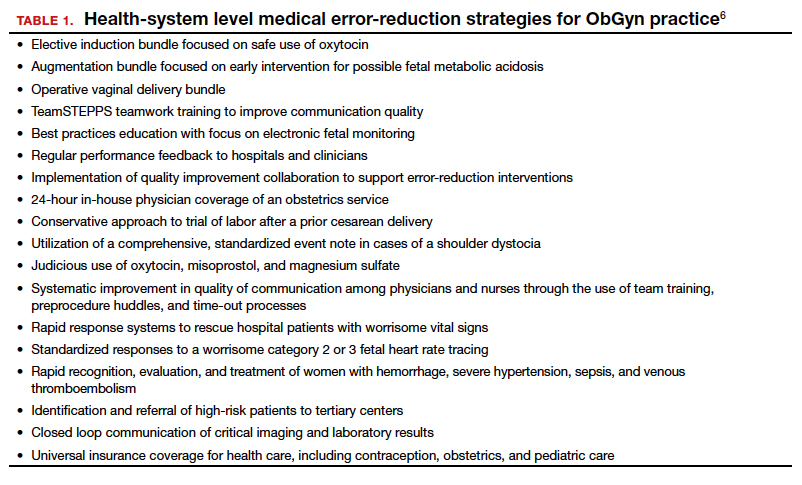
The collective accountability factor
The way malpractice claims are paid (FIGURE 4),10 reported, and handled may explain some of the apparent reduction in overall paid claims. Perhaps the advent of “collective accountability,” in which patient care is rendered by teams and responsibility accepted at a team level, can alleviate a significant amount of individual physician medical malpractice claims.11 This “enterprise liability” may shift the burden of medical error from physicians to health care organizations.12 Collective accountability may, therefore, focus on institutional responsibility rather than individual physician negligence.11,13 Institutions frequently hire multiple specialists and cover their medical malpractice costs as well as stand to be named in suits.
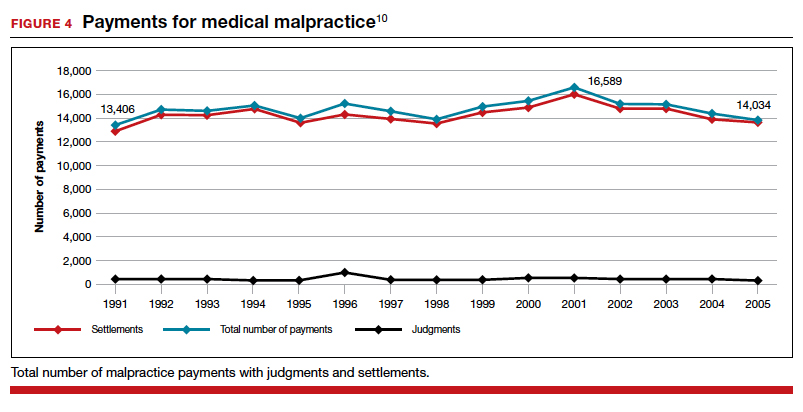
Continue to: The institutional involvement in malpractice cases also may affect...
The institutional involvement in malpractice cases also may affect apparent malpractice rates in another way. The National Practitioner Data Bank, which is the source of information for many malpractice studies, only requires reporting about individual physicians, not institutions.14 If, therefore, claims are settled on behalf of an institution, without implicating the physician, the number of physician malpractice cases may appear to decline without any real change in malpractice rates.14 In addition, institutions have taken the lead in informal resolution of injuries that occur in the institution, and these programs may reduce the direct malpractice claims against physicians. (These “disclosure, apology, and offer,” and similar programs, are discussed in the upcoming third part of this series.)
The medical reform factor
As noted, annual rates paid for medical malpractice in our specialty are trending downward. Many commentators look to malpractice reforms as the reason for the drop in malpractice rates.15-17 Because medical malpractice is essentially a matter of state law, the medical malpractice reform has occurred primarily at the state level.18 There have been many different reforms tried—limits on expert witnesses, review panels, and a variety of procedural limitations.19 Perhaps the most effective reform has been caps being placed on noneconomic damages (generally pain and suffering).20 These caps vary by state (FIGURE 5)21,22 and, of course, affect the “big verdict” cases. (As we saw in the second case scenario above, Virginia is an example of a state with a cap on malpractice awards.) They also have the secondary effect of reducing the number of malpractice cases. They make malpractice cases less attractive to some attorneys because they reduce the opportunity of large contingency fees from large verdicts. (Virtually all medical malpractice cases in the United States are tried on a contingency-fee basis, meaning that the plaintiff does not pay the attorney handling the case but rather the attorney takes a percentage of any recovery—typically in the neighborhood of 35%.) The reform process continues, although, presently, there is less pressure to act on the malpractice crisis.
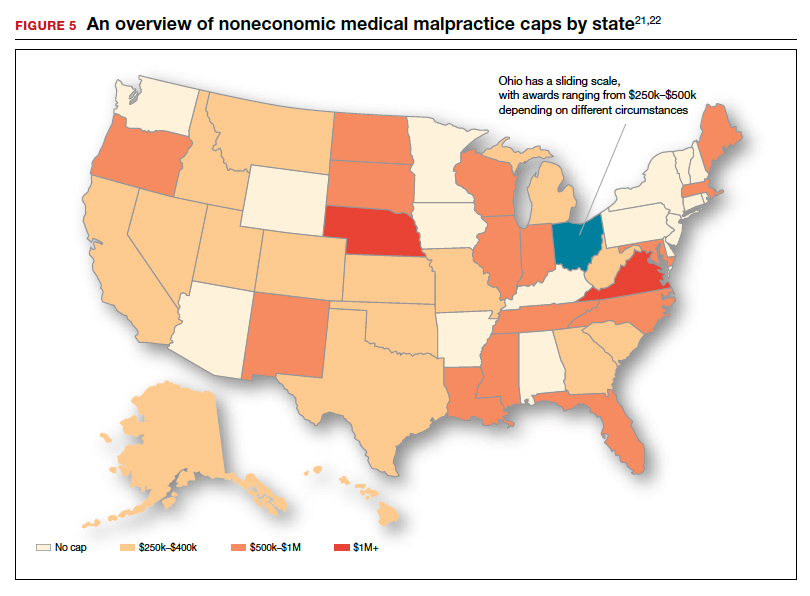
Medical malpractice cases are emotional and costly
Another reason for the relatively low rate of paid claims is that medical malpractice cases are difficult, emotionally challenging, time consuming, and expensive to pursue.23 They typically drag on for years, require extensive and expensive expert consultants as well as witnesses, and face stiff defense (compared with many other torts). The settlement of medical malpractice cases, for example, is less likely than other kinds of personal injury cases.
The contingency-fee basis does mean that injured patients do not have to pay attorney fees up front; however, plaintiffs may have to pay substantial costs along the way. The other side of this coin is that lawyers can be reluctant to take malpractice cases in which the damages are likely to be small, or where the legal uncertainty reduces the odds of achieving any damages. Thus, many potential malpractice cases are never filed.
A word of caution
The news of a reduction in malpractice paid claims may not be permanent. The numbers can conceivably be cyclical, and political reforms achieved can be changed. In addition, new technology will likely bring new kinds of malpractice claims. That appears to be the case, for example, with electronic health records (EHRs). One insurer reports that EHR malpractice claims have increased over the last 8 years.24 The most common injury in these claims was death (25%), as well as a magnitude of less serious injuries. EHR-related claims result from system failures, copy-paste inaccuracies, faulty drop-down menu use, and uncorrected “auto-populated” fields. Obstetrics is tied for fifth on the list of 14 specialties with claims related to EHRs, and gynecology is tied for eighth place.24
Continue to: A federal court ruled that a hospital that changed from...
A federal court ruled that a hospital that changed from paper records to EHRs for test results had a duty to “‘implement a reasonable procedure during the transition phase’ to ensure the timely delivery of test results” to health care providers.25 We will address this in a future “What’s the Verdict?”.
Rates of harm, malpractice cases, and the disposition of cases
There are many surprises when looking at medical malpractice claims data generally. The first surprise is how few claims are filed relative to the number of error-related injuries. Given the estimate of 210,000 to 400,000 deaths “associated with preventable harm” in hospitals, plus 10 to 20 times that number of serious injuries, it would be reasonable to expect claims of many hundreds of thousands per year. Compare the probability of a malpractice claim from an error-related injury, for example, with the probability of other personal injuries—eg, of traffic deaths associated with preventable harm.
The second key observation is how many of the claims filed are not successful—even when there was evidence in the record of errors associated with the injury. Studies slice the data in different ways but collectively suggest that only a small proportion of malpractice claims filed (a claim is generally regarded as some written demand for compensation for injuries) result in payments, either through settlement or by trial. A 2006 study by Studdert and colleagues determined that 63% of formal malpractice claims filed did involve injuries resulting from errors.26 The study found that in 16% of the claims (not injuries) there was no payment even though there was error. In 10% of the claims there was payment, even in the absence of error.
Overall, in this study, 56% of the claims received some compensation.26 That is higher than a more recent study by Jena and others, which found only 22% of claims resulted in compensation.3
How malpractice claims are decided is also interesting. Jena and colleagues found that only 55% of claims resulted in litigation.27 Presumably, the other 45% may have resulted in the plaintiff dropping the case, or in some form of settlement. Of the claims that were litigated, 54% were dismissed by the court, and another 35% were settled before a trial verdict. The cases that went to trial (about 10%), overwhelmingly (80%) resulted in verdicts for the defense.3,27 A different study found that only 9% of cases went to trial, and 87% were a defense verdict.28 The high level of defense verdicts may suggest that malpractice defense lawyers, and their client physicians, do a good job of assessing cases they are likely to lose, and settling them before trial.
ObGyns generally have larger numbers of claims and among the largest payment amounts when there is payment. Fewer of their cases are dismissed by the courts, so more go to trial. At trial, however, ObGyns prevail at a remarkably high rate.27 As for the probability of payment of a malpractice claim for ObGyns, one study suggested that there is approximately a 16% annual probability of a claim being filed, but only a 3% annual probability of a payment being made (suggesting about a 20% probability of payment per claim).3
Continue to: The purposes and effects of the medical malpractice system...
The purposes and effects of the medical malpractice system
The essential goals of tort law (including medical malpractice) include compensation for those who are injured and deterrence of future injuries (TABLE 2). What are the overall effects to the medical malpractice system? Unfortunately, the answer is that the law delivers disappointing results at best. It has a fairly high error rate. Many people who deserve some compensation for their injuries never seek compensation, and many deserving injured patients fail in efforts to receive compensation. At the same time, a few of the injured receive huge recoveries (even windfalls), and at least a small fraction receive compensation when there was no medical error. In addition to the high error rate, the system is inefficient and very expensive. Both defendants (through their insurance carriers) and plaintiffs spend a lot of money, years of time, and untold emotional pain dealing with these cases. The system also exacts high emotional and personal costs on plaintiffs and defendants.

Malpractice reform has not really addressed these issues—it has generally been focused on ways to reduce the cost of malpractice insurance. The most effective reform in reducing rates—caps—has had the effect of compensating the most seriously injured as though they were more modestly injured, and dissuading attorneys from taking the cases of those less seriously injured.
The medical and legal professions exist to help patients (the public). It does not seem that we have arrived at a system that does that very fairly or efficiently when a patient is injured because of preventable medical error.
The two vignettes described at the beginning, with similar injuries (shoulder dystocia), had disparate outcomes. In one there was a defense verdict and in the other a verdict for the plaintiffs of more than $2 million. The differences explain a number of important elements related to malpractice claims. (We have only very abbreviated and incomplete descriptions of the cases, so this discussion necessarily assumes facts and jumps to conclusions that may not be entirely consistent with the actual cases.)
These vignettes are unusual in that they went to trial. As we have noted, only a small percentage of malpractice cases are tried. And the verdict for the plaintiff-patient (in the second case) is unusual among those cases that go to trial, where plaintiffs seldom prevail.
From the facts we have, one significant difference in the 2 cases is that the plaintiff’s expert witness specifically testified in the second case that the “medical standard of care did not permit traction under these circumstances.” That is an essential element of a successful plaintiff’s malpractice case. In this case, the expert could also draw a connection between that breach of standard of care and harm to the child. In the case without liability, the nursing staff was able to testify that there was no shoulder dystocia because if there had been such an injury, they would have immediately launched into special action, which did not happen. By contrast, in the liability case, there seemed to be critical gaps in the medical record.
It is also important to remember that these cases were tried in different states, with different laws. The juries and judges in the 2 cases were different. Finally, the quality of the attorneys representing the plaintiffs and defendants were different. We mention these factors to point out that medical malpractice is not an exact science. It depends on many human elements that make the outcome of cases somewhat unpredictable. This unpredictability is one reason why parties and attorneys like to settle cases.
Watch for the third and final article in this series next month, as we are going to look at “apology in medicine and a proactive response” to communication regarding a complication.
- Shoulder dystocia—Florida defense verdict. Medical Malpractice: Verdicts, Settlements & Experts. 2019;35(1):18.
- Shoulder dystocia improperly managed--$2.320 million Virginia verdict. Medical Malpractice: Verdicts, Settlements & Experts. 2019;35(2):13.
- Jena AB, Seabury S, Lakdawalla D, et al. Malpractice risk according to physician specialty. N Engl J Med. 2011;365:629-636.
- Schaffer AC, Jena AB, Seabury SA, et al. Rates and characteristics of paid malpractice claims among US physicians by specialty, 1992-2014. JAMA Intern Med. 2017;177:710-718.
- Lowes R. Malpractice premiums trail inflation for some physicians. Medscape. December 16, 2016. https://www.medscape.com/viewarticle/873422. Accessed January 10, 2020.
- Barbieri RL. Good news for ObGyns: medical liability claims resulting in payment are decreasing! OBG Manag. 2019;31:10-13.
- Guardado JR. Medical professional liability insurance premiums: an overview of the market from 2008 to 2017. AMA Policy Research Perspectives, 2018. https://www.ama-assn.org/sites/ama-assn.org/files/corp/media-browser/public/government/advocacy/policy-research-perspective-liability-insurance-premiums.pdf. Accessed January 10, 2020.
- Institute of Medicine Committee on Quality Health Care in America; Kohn LT, Corrigan JM, Donaldson MS, eds. To Err is Human: Building a Safer Health System. Washington, DC: National Academies Press; 2000.
- James JT. A new, evidence-based estimate of patient harms associated with hospital care. J Patient Saf. 2013;9:122-128. https://journals.lww.com/journalpatientsafety/Fulltext/
2013/09000/A_New,_Evidence_based_Estimate_of_Patient_
Harms.2.aspx. Accessed January 10, 2020. - Public Citizen Congress Watch. The great medical malpractice hoax: NPDB data continue to show medical liability system produces rational outcomes. January 2007. https://www.citizen.org/wp-content/uploads/npdb_report_
final.pdf. Accessed January 23, 2020. - Bell SK, Delbanco T, Anderson-Shaw L, et al. Accountability for medical error: moving beyond blame to advocacy. Chest. 2011;140:519-526.
- Ramanathan T. Legal mechanisms supporting accountable care principles. Am J Public Health. 2014;104:2048-2051.
- Kachalia A, Kaufman SR, Boothman R, et al. Liability claims and costs before and after implementation of a medical error disclosure program. Ann Intern Med. 2010;153:213-221.
- National Practitioner Data Bank web site. What you must report to the NPDB. https://www.npdb.hrsa.gov/hcorg/whatYouMustReport
ToTheDataBank.jsp. Accessed January 10, 2020. - Bovbjerg RR. Malpractice crisis and reform. Clin Perinatol. 2005;32:203-233, viii-ix.
- Viscusi WK. Medical malpractice reform: what works and what doesn't. Denver Law Rev. 2019;96:775-791. https://static1.squarespace.com/static/5cb79f7efd6793296c0eb738 /t/5d5f4ffabd6c5400011a12f6/1566527483118/Vol96_Issue4_Viscusi_
FINAL.pdf. Accessed January 10, 2020. - National Conference of State Legislatures. Medical malpractice reform. Health Cost Containment and Efficiencies: NCSL Briefs for State Legislators. 2011;(16). http://www.ncsl.org/research/health/medical-malpractice-reform-health-cost-brief.aspx. Accessed January 10, 2020.
- Kass JS, Rose RV. Medical malpractice reform: historical approaches, alternative models, and communication and resolution programs. AMA J Ethics. 2016;18:299-310.
- Boehm G. Debunking medical malpractice myths: unraveling the false premises behind "tort reform". Yale J Health Policy Law Ethics. 2005;5:357-369.
- Hellinger FJ, Encinosa WE. The impact of state laws limiting malpractice damage awards on health care expenditures. Am J Public Health. 2006;96:1375-1381.
- Perry G. Medical malpractice caps by state [infographic]. January 3, 2013. https://www.business2community.com/infographics/medical-malpractice-caps-by-state-infographic-0368345. Accessed January 23, 2020.
- Goguen D. State-by-state medical malpractice damages caps. An in-depth look at state laws limiting compensation for medical malpractice plaintiffs. https://www.nolo.com/legal-encyclopedia/state-state-medical-malpractice-damages-caps.html. Accessed January 23, 2020.
- Berlin L. Medical errors, malpractice, and defensive medicine: an ill-fated triad. Diagnosis (Berl). 2017;4:133-139.
- Ranum D. Electronic health records continue to lead to medical malpractice suits. The Doctors Company. August 2019. https://www.thedoctors.com/articles/electronic-health-records-continue-to-lead-to-medical-malpractice-suits/. Accessed January 10, 2020.
- Mangalmurti SS, Murtagh L, Mello MM. Medical malpractice liability in the age of electronic health records. N Engl J Med. 2010;363:2060-2067.
- Studdert DM, Mello MM, Gawande AA, et al. Claims, errors, and compensation payments in medical malpractice litigation. N Engl J Med. 2006;354(19):2024-2033.
- Jena AB, Chandra A, Lakdawalla D, et al. Outcomes of medical malpractice litigation against US physicians. Arch Intern Med. 2012;172:892-894.
- Glaser LM, Alvi FA, Milad MP. Trends in malpractice claims for obstetric and gynecologic procedures, 2005 through 2014. Am J Obstet Gynecol. 2017;217:340.e1-340.e6.
In this second in a series of 3 articles discussing medical malpractice and the ObGyn we look at the reasons for malpractice claims and liability, what happens to malpractice claims, and the direction and future of medical malpractice. The first article dealt with 2 sources of major malpractice damages: the “big verdict” and physicians with multiple malpractice paid claims. Next month we look at the place of apology in medicine, in cases in which error, including negligence, may have caused a patient injury.
CASE 1 Long-term brachial plexus injury
Right upper extremity injury occurs in the neonate at delivery with sequela of long-term brachial plexus injury (which is diagnosed around 6 months of age). Physical therapy and orthopedic assessment are rendered. Despite continued treatment, discrepancy in arm lengths (ie, affected side arm is noticeably shorter than opposite side) remains. The child cannot play basketball with his older brother and is the victim of ridicule, the plaintiff’s attorney emphasizes. He is unable to properly pronate or supinate the affected arm.
The defendant ObGyn maintains that there was “no shoulder dystocia [at delivery] and the shoulder did not get obstructed in the pelvis; shoulder was delivered 15 seconds after delivery of the head.” The nursing staff testifies that if shoulder dystocia had been the problem they would have launched upon a series of procedures to address such, in accord with the delivering obstetrician. The defense expert witness testifies that a brachial plexus injury can happen without shoulder dystocia.
A defense verdict is rendered by the Florida jury.1
CASE 2 Shoulder dystocia
During delivery, the obstetrician notes a shoulder dystocia (“turtle sign”). After initial attempts to release the shoulder were unsuccessful, the physician applies traction several times to the head of the child, and the baby is delivered. There is permanent injury to the right brachial plexus. The defendant ObGyn says that traction was necessary to dislodge the shoulder, and that the injury was the result of the forces of labor (not the traction). The expert witness for the plaintiff testifies that the medical standard of care did not permit traction under these circumstances, and that the traction was the likely cause of the injury.
The Virginia jury awards $2.32 million in damages.2
Note: The above vignettes are drawn from actual cases but are only outlines of those cases and are not complete descriptions of the claims in the cases. Because the information comes from informal sources, not formal court records, the facts may be inaccurate and incomplete. They should be viewed as illustrations only.

The trend in malpractice
It has been clear for many years that medical malpractice claims are not randomly or evenly distributed among physicians. Notably, the variation among specialties has, and continues to be, substantial (FIGURE 1).3 Recent data suggest that, although paid claims per “1,000 physician-years” averages 14 paid claims per 1,000 physician years, it ranges from 4 or 5 in 1,000 (psychiatry and pediatrics) to 53 and 49 claims per 1,000 (neurology and plastic surgery, respectively). Obstetrics and gynecology has the fourth highest rate at 42.5 paid claims per 1,000 physician years.4 (These data are for the years 1992–2014.)

Continue to: The number of ObGyn paid malpractice claims has decreased over time...
The number of ObGyn paid malpractice claims has decreased over time. Although large verdicts and physicians with multiple paid malpractice claims receive a good deal of attention (as we noted in part 1 of our series), in fact, paid medical malpractice claims have trended downward in recent decades.5 When the data above are disaggregated by 5-year periods, for example, in obstetrics and gynecology, there has been a consistent reduction in paid malpractice claims from 1992 to 2014. Paid claims went from 58 per 1,000 physician-years in 1992–1996 to 25 per 1,000 in 2009–2014 (FIGURE 2).4,6 In short, the rate dropped by half over approximately 20 years.4

It is reasonable to expect that such a decline in the cost of malpractice insurance premiums would follow. Robert L. Barbieri, MD, who practices in Boston, Massachusetts, in his excellent recent editorial in OBG M

Why have malpractice payouts declined overall?
Have medical errors declined?
It would be wonderful if the reduction in malpractice claims represented a significant decrease in medical errors. Attention to medical errors was driven by the first widely noticed study of medical error deaths. The Institute of Medicine (IOM) study in 2000, put the number of deaths annually at 44,000 to 98,000.8 There have been many efforts to reduce such errors, and it is possible that those efforts have indeed reduced errors somewhat.4 Barbieri provided a helpful digest of many of the error-reduction suggestions for ObGyn practice (TABLE 1).6 But the number of medical errors remains high. More recent studies have suggested that the IOM’s reported number of injuries may have been low.9 In 2013, one study suggested that 210,000 deaths annually were “associated with preventable harm” in hospitals. Because of how the data were gathered the authors estimated that the actual number of preventable deaths was closer to 400,000 annually. Serious harm to patients was estimated at 10 to 20 times the IOM rate.9
Therefore, a dramatic reduction in preventable medical errors does not appear to explain the reduction in malpractice claims. Some portion of it may be explained by malpractice reforms—see "The medical reform factor" section below.

The collective accountability factor
The way malpractice claims are paid (FIGURE 4),10 reported, and handled may explain some of the apparent reduction in overall paid claims. Perhaps the advent of “collective accountability,” in which patient care is rendered by teams and responsibility accepted at a team level, can alleviate a significant amount of individual physician medical malpractice claims.11 This “enterprise liability” may shift the burden of medical error from physicians to health care organizations.12 Collective accountability may, therefore, focus on institutional responsibility rather than individual physician negligence.11,13 Institutions frequently hire multiple specialists and cover their medical malpractice costs as well as stand to be named in suits.

Continue to: The institutional involvement in malpractice cases also may affect...
The institutional involvement in malpractice cases also may affect apparent malpractice rates in another way. The National Practitioner Data Bank, which is the source of information for many malpractice studies, only requires reporting about individual physicians, not institutions.14 If, therefore, claims are settled on behalf of an institution, without implicating the physician, the number of physician malpractice cases may appear to decline without any real change in malpractice rates.14 In addition, institutions have taken the lead in informal resolution of injuries that occur in the institution, and these programs may reduce the direct malpractice claims against physicians. (These “disclosure, apology, and offer,” and similar programs, are discussed in the upcoming third part of this series.)
The medical reform factor
As noted, annual rates paid for medical malpractice in our specialty are trending downward. Many commentators look to malpractice reforms as the reason for the drop in malpractice rates.15-17 Because medical malpractice is essentially a matter of state law, the medical malpractice reform has occurred primarily at the state level.18 There have been many different reforms tried—limits on expert witnesses, review panels, and a variety of procedural limitations.19 Perhaps the most effective reform has been caps being placed on noneconomic damages (generally pain and suffering).20 These caps vary by state (FIGURE 5)21,22 and, of course, affect the “big verdict” cases. (As we saw in the second case scenario above, Virginia is an example of a state with a cap on malpractice awards.) They also have the secondary effect of reducing the number of malpractice cases. They make malpractice cases less attractive to some attorneys because they reduce the opportunity of large contingency fees from large verdicts. (Virtually all medical malpractice cases in the United States are tried on a contingency-fee basis, meaning that the plaintiff does not pay the attorney handling the case but rather the attorney takes a percentage of any recovery—typically in the neighborhood of 35%.) The reform process continues, although, presently, there is less pressure to act on the malpractice crisis.

Medical malpractice cases are emotional and costly
Another reason for the relatively low rate of paid claims is that medical malpractice cases are difficult, emotionally challenging, time consuming, and expensive to pursue.23 They typically drag on for years, require extensive and expensive expert consultants as well as witnesses, and face stiff defense (compared with many other torts). The settlement of medical malpractice cases, for example, is less likely than other kinds of personal injury cases.
The contingency-fee basis does mean that injured patients do not have to pay attorney fees up front; however, plaintiffs may have to pay substantial costs along the way. The other side of this coin is that lawyers can be reluctant to take malpractice cases in which the damages are likely to be small, or where the legal uncertainty reduces the odds of achieving any damages. Thus, many potential malpractice cases are never filed.
A word of caution
The news of a reduction in malpractice paid claims may not be permanent. The numbers can conceivably be cyclical, and political reforms achieved can be changed. In addition, new technology will likely bring new kinds of malpractice claims. That appears to be the case, for example, with electronic health records (EHRs). One insurer reports that EHR malpractice claims have increased over the last 8 years.24 The most common injury in these claims was death (25%), as well as a magnitude of less serious injuries. EHR-related claims result from system failures, copy-paste inaccuracies, faulty drop-down menu use, and uncorrected “auto-populated” fields. Obstetrics is tied for fifth on the list of 14 specialties with claims related to EHRs, and gynecology is tied for eighth place.24
Continue to: A federal court ruled that a hospital that changed from...
A federal court ruled that a hospital that changed from paper records to EHRs for test results had a duty to “‘implement a reasonable procedure during the transition phase’ to ensure the timely delivery of test results” to health care providers.25 We will address this in a future “What’s the Verdict?”.
Rates of harm, malpractice cases, and the disposition of cases
There are many surprises when looking at medical malpractice claims data generally. The first surprise is how few claims are filed relative to the number of error-related injuries. Given the estimate of 210,000 to 400,000 deaths “associated with preventable harm” in hospitals, plus 10 to 20 times that number of serious injuries, it would be reasonable to expect claims of many hundreds of thousands per year. Compare the probability of a malpractice claim from an error-related injury, for example, with the probability of other personal injuries—eg, of traffic deaths associated with preventable harm.
The second key observation is how many of the claims filed are not successful—even when there was evidence in the record of errors associated with the injury. Studies slice the data in different ways but collectively suggest that only a small proportion of malpractice claims filed (a claim is generally regarded as some written demand for compensation for injuries) result in payments, either through settlement or by trial. A 2006 study by Studdert and colleagues determined that 63% of formal malpractice claims filed did involve injuries resulting from errors.26 The study found that in 16% of the claims (not injuries) there was no payment even though there was error. In 10% of the claims there was payment, even in the absence of error.
Overall, in this study, 56% of the claims received some compensation.26 That is higher than a more recent study by Jena and others, which found only 22% of claims resulted in compensation.3
How malpractice claims are decided is also interesting. Jena and colleagues found that only 55% of claims resulted in litigation.27 Presumably, the other 45% may have resulted in the plaintiff dropping the case, or in some form of settlement. Of the claims that were litigated, 54% were dismissed by the court, and another 35% were settled before a trial verdict. The cases that went to trial (about 10%), overwhelmingly (80%) resulted in verdicts for the defense.3,27 A different study found that only 9% of cases went to trial, and 87% were a defense verdict.28 The high level of defense verdicts may suggest that malpractice defense lawyers, and their client physicians, do a good job of assessing cases they are likely to lose, and settling them before trial.
ObGyns generally have larger numbers of claims and among the largest payment amounts when there is payment. Fewer of their cases are dismissed by the courts, so more go to trial. At trial, however, ObGyns prevail at a remarkably high rate.27 As for the probability of payment of a malpractice claim for ObGyns, one study suggested that there is approximately a 16% annual probability of a claim being filed, but only a 3% annual probability of a payment being made (suggesting about a 20% probability of payment per claim).3
Continue to: The purposes and effects of the medical malpractice system...
The purposes and effects of the medical malpractice system
The essential goals of tort law (including medical malpractice) include compensation for those who are injured and deterrence of future injuries (TABLE 2). What are the overall effects to the medical malpractice system? Unfortunately, the answer is that the law delivers disappointing results at best. It has a fairly high error rate. Many people who deserve some compensation for their injuries never seek compensation, and many deserving injured patients fail in efforts to receive compensation. At the same time, a few of the injured receive huge recoveries (even windfalls), and at least a small fraction receive compensation when there was no medical error. In addition to the high error rate, the system is inefficient and very expensive. Both defendants (through their insurance carriers) and plaintiffs spend a lot of money, years of time, and untold emotional pain dealing with these cases. The system also exacts high emotional and personal costs on plaintiffs and defendants.

Malpractice reform has not really addressed these issues—it has generally been focused on ways to reduce the cost of malpractice insurance. The most effective reform in reducing rates—caps—has had the effect of compensating the most seriously injured as though they were more modestly injured, and dissuading attorneys from taking the cases of those less seriously injured.
The medical and legal professions exist to help patients (the public). It does not seem that we have arrived at a system that does that very fairly or efficiently when a patient is injured because of preventable medical error.
The two vignettes described at the beginning, with similar injuries (shoulder dystocia), had disparate outcomes. In one there was a defense verdict and in the other a verdict for the plaintiffs of more than $2 million. The differences explain a number of important elements related to malpractice claims. (We have only very abbreviated and incomplete descriptions of the cases, so this discussion necessarily assumes facts and jumps to conclusions that may not be entirely consistent with the actual cases.)
These vignettes are unusual in that they went to trial. As we have noted, only a small percentage of malpractice cases are tried. And the verdict for the plaintiff-patient (in the second case) is unusual among those cases that go to trial, where plaintiffs seldom prevail.
From the facts we have, one significant difference in the 2 cases is that the plaintiff’s expert witness specifically testified in the second case that the “medical standard of care did not permit traction under these circumstances.” That is an essential element of a successful plaintiff’s malpractice case. In this case, the expert could also draw a connection between that breach of standard of care and harm to the child. In the case without liability, the nursing staff was able to testify that there was no shoulder dystocia because if there had been such an injury, they would have immediately launched into special action, which did not happen. By contrast, in the liability case, there seemed to be critical gaps in the medical record.
It is also important to remember that these cases were tried in different states, with different laws. The juries and judges in the 2 cases were different. Finally, the quality of the attorneys representing the plaintiffs and defendants were different. We mention these factors to point out that medical malpractice is not an exact science. It depends on many human elements that make the outcome of cases somewhat unpredictable. This unpredictability is one reason why parties and attorneys like to settle cases.
Watch for the third and final article in this series next month, as we are going to look at “apology in medicine and a proactive response” to communication regarding a complication.
In this second in a series of 3 articles discussing medical malpractice and the ObGyn we look at the reasons for malpractice claims and liability, what happens to malpractice claims, and the direction and future of medical malpractice. The first article dealt with 2 sources of major malpractice damages: the “big verdict” and physicians with multiple malpractice paid claims. Next month we look at the place of apology in medicine, in cases in which error, including negligence, may have caused a patient injury.
CASE 1 Long-term brachial plexus injury
Right upper extremity injury occurs in the neonate at delivery with sequela of long-term brachial plexus injury (which is diagnosed around 6 months of age). Physical therapy and orthopedic assessment are rendered. Despite continued treatment, discrepancy in arm lengths (ie, affected side arm is noticeably shorter than opposite side) remains. The child cannot play basketball with his older brother and is the victim of ridicule, the plaintiff’s attorney emphasizes. He is unable to properly pronate or supinate the affected arm.
The defendant ObGyn maintains that there was “no shoulder dystocia [at delivery] and the shoulder did not get obstructed in the pelvis; shoulder was delivered 15 seconds after delivery of the head.” The nursing staff testifies that if shoulder dystocia had been the problem they would have launched upon a series of procedures to address such, in accord with the delivering obstetrician. The defense expert witness testifies that a brachial plexus injury can happen without shoulder dystocia.
A defense verdict is rendered by the Florida jury.1
CASE 2 Shoulder dystocia
During delivery, the obstetrician notes a shoulder dystocia (“turtle sign”). After initial attempts to release the shoulder were unsuccessful, the physician applies traction several times to the head of the child, and the baby is delivered. There is permanent injury to the right brachial plexus. The defendant ObGyn says that traction was necessary to dislodge the shoulder, and that the injury was the result of the forces of labor (not the traction). The expert witness for the plaintiff testifies that the medical standard of care did not permit traction under these circumstances, and that the traction was the likely cause of the injury.
The Virginia jury awards $2.32 million in damages.2
Note: The above vignettes are drawn from actual cases but are only outlines of those cases and are not complete descriptions of the claims in the cases. Because the information comes from informal sources, not formal court records, the facts may be inaccurate and incomplete. They should be viewed as illustrations only.

The trend in malpractice
It has been clear for many years that medical malpractice claims are not randomly or evenly distributed among physicians. Notably, the variation among specialties has, and continues to be, substantial (FIGURE 1).3 Recent data suggest that, although paid claims per “1,000 physician-years” averages 14 paid claims per 1,000 physician years, it ranges from 4 or 5 in 1,000 (psychiatry and pediatrics) to 53 and 49 claims per 1,000 (neurology and plastic surgery, respectively). Obstetrics and gynecology has the fourth highest rate at 42.5 paid claims per 1,000 physician years.4 (These data are for the years 1992–2014.)

Continue to: The number of ObGyn paid malpractice claims has decreased over time...
The number of ObGyn paid malpractice claims has decreased over time. Although large verdicts and physicians with multiple paid malpractice claims receive a good deal of attention (as we noted in part 1 of our series), in fact, paid medical malpractice claims have trended downward in recent decades.5 When the data above are disaggregated by 5-year periods, for example, in obstetrics and gynecology, there has been a consistent reduction in paid malpractice claims from 1992 to 2014. Paid claims went from 58 per 1,000 physician-years in 1992–1996 to 25 per 1,000 in 2009–2014 (FIGURE 2).4,6 In short, the rate dropped by half over approximately 20 years.4

It is reasonable to expect that such a decline in the cost of malpractice insurance premiums would follow. Robert L. Barbieri, MD, who practices in Boston, Massachusetts, in his excellent recent editorial in OBG M

Why have malpractice payouts declined overall?
Have medical errors declined?
It would be wonderful if the reduction in malpractice claims represented a significant decrease in medical errors. Attention to medical errors was driven by the first widely noticed study of medical error deaths. The Institute of Medicine (IOM) study in 2000, put the number of deaths annually at 44,000 to 98,000.8 There have been many efforts to reduce such errors, and it is possible that those efforts have indeed reduced errors somewhat.4 Barbieri provided a helpful digest of many of the error-reduction suggestions for ObGyn practice (TABLE 1).6 But the number of medical errors remains high. More recent studies have suggested that the IOM’s reported number of injuries may have been low.9 In 2013, one study suggested that 210,000 deaths annually were “associated with preventable harm” in hospitals. Because of how the data were gathered the authors estimated that the actual number of preventable deaths was closer to 400,000 annually. Serious harm to patients was estimated at 10 to 20 times the IOM rate.9
Therefore, a dramatic reduction in preventable medical errors does not appear to explain the reduction in malpractice claims. Some portion of it may be explained by malpractice reforms—see "The medical reform factor" section below.

The collective accountability factor
The way malpractice claims are paid (FIGURE 4),10 reported, and handled may explain some of the apparent reduction in overall paid claims. Perhaps the advent of “collective accountability,” in which patient care is rendered by teams and responsibility accepted at a team level, can alleviate a significant amount of individual physician medical malpractice claims.11 This “enterprise liability” may shift the burden of medical error from physicians to health care organizations.12 Collective accountability may, therefore, focus on institutional responsibility rather than individual physician negligence.11,13 Institutions frequently hire multiple specialists and cover their medical malpractice costs as well as stand to be named in suits.

Continue to: The institutional involvement in malpractice cases also may affect...
The institutional involvement in malpractice cases also may affect apparent malpractice rates in another way. The National Practitioner Data Bank, which is the source of information for many malpractice studies, only requires reporting about individual physicians, not institutions.14 If, therefore, claims are settled on behalf of an institution, without implicating the physician, the number of physician malpractice cases may appear to decline without any real change in malpractice rates.14 In addition, institutions have taken the lead in informal resolution of injuries that occur in the institution, and these programs may reduce the direct malpractice claims against physicians. (These “disclosure, apology, and offer,” and similar programs, are discussed in the upcoming third part of this series.)
The medical reform factor
As noted, annual rates paid for medical malpractice in our specialty are trending downward. Many commentators look to malpractice reforms as the reason for the drop in malpractice rates.15-17 Because medical malpractice is essentially a matter of state law, the medical malpractice reform has occurred primarily at the state level.18 There have been many different reforms tried—limits on expert witnesses, review panels, and a variety of procedural limitations.19 Perhaps the most effective reform has been caps being placed on noneconomic damages (generally pain and suffering).20 These caps vary by state (FIGURE 5)21,22 and, of course, affect the “big verdict” cases. (As we saw in the second case scenario above, Virginia is an example of a state with a cap on malpractice awards.) They also have the secondary effect of reducing the number of malpractice cases. They make malpractice cases less attractive to some attorneys because they reduce the opportunity of large contingency fees from large verdicts. (Virtually all medical malpractice cases in the United States are tried on a contingency-fee basis, meaning that the plaintiff does not pay the attorney handling the case but rather the attorney takes a percentage of any recovery—typically in the neighborhood of 35%.) The reform process continues, although, presently, there is less pressure to act on the malpractice crisis.

Medical malpractice cases are emotional and costly
Another reason for the relatively low rate of paid claims is that medical malpractice cases are difficult, emotionally challenging, time consuming, and expensive to pursue.23 They typically drag on for years, require extensive and expensive expert consultants as well as witnesses, and face stiff defense (compared with many other torts). The settlement of medical malpractice cases, for example, is less likely than other kinds of personal injury cases.
The contingency-fee basis does mean that injured patients do not have to pay attorney fees up front; however, plaintiffs may have to pay substantial costs along the way. The other side of this coin is that lawyers can be reluctant to take malpractice cases in which the damages are likely to be small, or where the legal uncertainty reduces the odds of achieving any damages. Thus, many potential malpractice cases are never filed.
A word of caution
The news of a reduction in malpractice paid claims may not be permanent. The numbers can conceivably be cyclical, and political reforms achieved can be changed. In addition, new technology will likely bring new kinds of malpractice claims. That appears to be the case, for example, with electronic health records (EHRs). One insurer reports that EHR malpractice claims have increased over the last 8 years.24 The most common injury in these claims was death (25%), as well as a magnitude of less serious injuries. EHR-related claims result from system failures, copy-paste inaccuracies, faulty drop-down menu use, and uncorrected “auto-populated” fields. Obstetrics is tied for fifth on the list of 14 specialties with claims related to EHRs, and gynecology is tied for eighth place.24
Continue to: A federal court ruled that a hospital that changed from...
A federal court ruled that a hospital that changed from paper records to EHRs for test results had a duty to “‘implement a reasonable procedure during the transition phase’ to ensure the timely delivery of test results” to health care providers.25 We will address this in a future “What’s the Verdict?”.
Rates of harm, malpractice cases, and the disposition of cases
There are many surprises when looking at medical malpractice claims data generally. The first surprise is how few claims are filed relative to the number of error-related injuries. Given the estimate of 210,000 to 400,000 deaths “associated with preventable harm” in hospitals, plus 10 to 20 times that number of serious injuries, it would be reasonable to expect claims of many hundreds of thousands per year. Compare the probability of a malpractice claim from an error-related injury, for example, with the probability of other personal injuries—eg, of traffic deaths associated with preventable harm.
The second key observation is how many of the claims filed are not successful—even when there was evidence in the record of errors associated with the injury. Studies slice the data in different ways but collectively suggest that only a small proportion of malpractice claims filed (a claim is generally regarded as some written demand for compensation for injuries) result in payments, either through settlement or by trial. A 2006 study by Studdert and colleagues determined that 63% of formal malpractice claims filed did involve injuries resulting from errors.26 The study found that in 16% of the claims (not injuries) there was no payment even though there was error. In 10% of the claims there was payment, even in the absence of error.
Overall, in this study, 56% of the claims received some compensation.26 That is higher than a more recent study by Jena and others, which found only 22% of claims resulted in compensation.3
How malpractice claims are decided is also interesting. Jena and colleagues found that only 55% of claims resulted in litigation.27 Presumably, the other 45% may have resulted in the plaintiff dropping the case, or in some form of settlement. Of the claims that were litigated, 54% were dismissed by the court, and another 35% were settled before a trial verdict. The cases that went to trial (about 10%), overwhelmingly (80%) resulted in verdicts for the defense.3,27 A different study found that only 9% of cases went to trial, and 87% were a defense verdict.28 The high level of defense verdicts may suggest that malpractice defense lawyers, and their client physicians, do a good job of assessing cases they are likely to lose, and settling them before trial.
ObGyns generally have larger numbers of claims and among the largest payment amounts when there is payment. Fewer of their cases are dismissed by the courts, so more go to trial. At trial, however, ObGyns prevail at a remarkably high rate.27 As for the probability of payment of a malpractice claim for ObGyns, one study suggested that there is approximately a 16% annual probability of a claim being filed, but only a 3% annual probability of a payment being made (suggesting about a 20% probability of payment per claim).3
Continue to: The purposes and effects of the medical malpractice system...
The purposes and effects of the medical malpractice system
The essential goals of tort law (including medical malpractice) include compensation for those who are injured and deterrence of future injuries (TABLE 2). What are the overall effects to the medical malpractice system? Unfortunately, the answer is that the law delivers disappointing results at best. It has a fairly high error rate. Many people who deserve some compensation for their injuries never seek compensation, and many deserving injured patients fail in efforts to receive compensation. At the same time, a few of the injured receive huge recoveries (even windfalls), and at least a small fraction receive compensation when there was no medical error. In addition to the high error rate, the system is inefficient and very expensive. Both defendants (through their insurance carriers) and plaintiffs spend a lot of money, years of time, and untold emotional pain dealing with these cases. The system also exacts high emotional and personal costs on plaintiffs and defendants.

Malpractice reform has not really addressed these issues—it has generally been focused on ways to reduce the cost of malpractice insurance. The most effective reform in reducing rates—caps—has had the effect of compensating the most seriously injured as though they were more modestly injured, and dissuading attorneys from taking the cases of those less seriously injured.
The medical and legal professions exist to help patients (the public). It does not seem that we have arrived at a system that does that very fairly or efficiently when a patient is injured because of preventable medical error.
The two vignettes described at the beginning, with similar injuries (shoulder dystocia), had disparate outcomes. In one there was a defense verdict and in the other a verdict for the plaintiffs of more than $2 million. The differences explain a number of important elements related to malpractice claims. (We have only very abbreviated and incomplete descriptions of the cases, so this discussion necessarily assumes facts and jumps to conclusions that may not be entirely consistent with the actual cases.)
These vignettes are unusual in that they went to trial. As we have noted, only a small percentage of malpractice cases are tried. And the verdict for the plaintiff-patient (in the second case) is unusual among those cases that go to trial, where plaintiffs seldom prevail.
From the facts we have, one significant difference in the 2 cases is that the plaintiff’s expert witness specifically testified in the second case that the “medical standard of care did not permit traction under these circumstances.” That is an essential element of a successful plaintiff’s malpractice case. In this case, the expert could also draw a connection between that breach of standard of care and harm to the child. In the case without liability, the nursing staff was able to testify that there was no shoulder dystocia because if there had been such an injury, they would have immediately launched into special action, which did not happen. By contrast, in the liability case, there seemed to be critical gaps in the medical record.
It is also important to remember that these cases were tried in different states, with different laws. The juries and judges in the 2 cases were different. Finally, the quality of the attorneys representing the plaintiffs and defendants were different. We mention these factors to point out that medical malpractice is not an exact science. It depends on many human elements that make the outcome of cases somewhat unpredictable. This unpredictability is one reason why parties and attorneys like to settle cases.
Watch for the third and final article in this series next month, as we are going to look at “apology in medicine and a proactive response” to communication regarding a complication.
- Shoulder dystocia—Florida defense verdict. Medical Malpractice: Verdicts, Settlements & Experts. 2019;35(1):18.
- Shoulder dystocia improperly managed--$2.320 million Virginia verdict. Medical Malpractice: Verdicts, Settlements & Experts. 2019;35(2):13.
- Jena AB, Seabury S, Lakdawalla D, et al. Malpractice risk according to physician specialty. N Engl J Med. 2011;365:629-636.
- Schaffer AC, Jena AB, Seabury SA, et al. Rates and characteristics of paid malpractice claims among US physicians by specialty, 1992-2014. JAMA Intern Med. 2017;177:710-718.
- Lowes R. Malpractice premiums trail inflation for some physicians. Medscape. December 16, 2016. https://www.medscape.com/viewarticle/873422. Accessed January 10, 2020.
- Barbieri RL. Good news for ObGyns: medical liability claims resulting in payment are decreasing! OBG Manag. 2019;31:10-13.
- Guardado JR. Medical professional liability insurance premiums: an overview of the market from 2008 to 2017. AMA Policy Research Perspectives, 2018. https://www.ama-assn.org/sites/ama-assn.org/files/corp/media-browser/public/government/advocacy/policy-research-perspective-liability-insurance-premiums.pdf. Accessed January 10, 2020.
- Institute of Medicine Committee on Quality Health Care in America; Kohn LT, Corrigan JM, Donaldson MS, eds. To Err is Human: Building a Safer Health System. Washington, DC: National Academies Press; 2000.
- James JT. A new, evidence-based estimate of patient harms associated with hospital care. J Patient Saf. 2013;9:122-128. https://journals.lww.com/journalpatientsafety/Fulltext/
2013/09000/A_New,_Evidence_based_Estimate_of_Patient_
Harms.2.aspx. Accessed January 10, 2020. - Public Citizen Congress Watch. The great medical malpractice hoax: NPDB data continue to show medical liability system produces rational outcomes. January 2007. https://www.citizen.org/wp-content/uploads/npdb_report_
final.pdf. Accessed January 23, 2020. - Bell SK, Delbanco T, Anderson-Shaw L, et al. Accountability for medical error: moving beyond blame to advocacy. Chest. 2011;140:519-526.
- Ramanathan T. Legal mechanisms supporting accountable care principles. Am J Public Health. 2014;104:2048-2051.
- Kachalia A, Kaufman SR, Boothman R, et al. Liability claims and costs before and after implementation of a medical error disclosure program. Ann Intern Med. 2010;153:213-221.
- National Practitioner Data Bank web site. What you must report to the NPDB. https://www.npdb.hrsa.gov/hcorg/whatYouMustReport
ToTheDataBank.jsp. Accessed January 10, 2020. - Bovbjerg RR. Malpractice crisis and reform. Clin Perinatol. 2005;32:203-233, viii-ix.
- Viscusi WK. Medical malpractice reform: what works and what doesn't. Denver Law Rev. 2019;96:775-791. https://static1.squarespace.com/static/5cb79f7efd6793296c0eb738 /t/5d5f4ffabd6c5400011a12f6/1566527483118/Vol96_Issue4_Viscusi_
FINAL.pdf. Accessed January 10, 2020. - National Conference of State Legislatures. Medical malpractice reform. Health Cost Containment and Efficiencies: NCSL Briefs for State Legislators. 2011;(16). http://www.ncsl.org/research/health/medical-malpractice-reform-health-cost-brief.aspx. Accessed January 10, 2020.
- Kass JS, Rose RV. Medical malpractice reform: historical approaches, alternative models, and communication and resolution programs. AMA J Ethics. 2016;18:299-310.
- Boehm G. Debunking medical malpractice myths: unraveling the false premises behind "tort reform". Yale J Health Policy Law Ethics. 2005;5:357-369.
- Hellinger FJ, Encinosa WE. The impact of state laws limiting malpractice damage awards on health care expenditures. Am J Public Health. 2006;96:1375-1381.
- Perry G. Medical malpractice caps by state [infographic]. January 3, 2013. https://www.business2community.com/infographics/medical-malpractice-caps-by-state-infographic-0368345. Accessed January 23, 2020.
- Goguen D. State-by-state medical malpractice damages caps. An in-depth look at state laws limiting compensation for medical malpractice plaintiffs. https://www.nolo.com/legal-encyclopedia/state-state-medical-malpractice-damages-caps.html. Accessed January 23, 2020.
- Berlin L. Medical errors, malpractice, and defensive medicine: an ill-fated triad. Diagnosis (Berl). 2017;4:133-139.
- Ranum D. Electronic health records continue to lead to medical malpractice suits. The Doctors Company. August 2019. https://www.thedoctors.com/articles/electronic-health-records-continue-to-lead-to-medical-malpractice-suits/. Accessed January 10, 2020.
- Mangalmurti SS, Murtagh L, Mello MM. Medical malpractice liability in the age of electronic health records. N Engl J Med. 2010;363:2060-2067.
- Studdert DM, Mello MM, Gawande AA, et al. Claims, errors, and compensation payments in medical malpractice litigation. N Engl J Med. 2006;354(19):2024-2033.
- Jena AB, Chandra A, Lakdawalla D, et al. Outcomes of medical malpractice litigation against US physicians. Arch Intern Med. 2012;172:892-894.
- Glaser LM, Alvi FA, Milad MP. Trends in malpractice claims for obstetric and gynecologic procedures, 2005 through 2014. Am J Obstet Gynecol. 2017;217:340.e1-340.e6.
- Shoulder dystocia—Florida defense verdict. Medical Malpractice: Verdicts, Settlements & Experts. 2019;35(1):18.
- Shoulder dystocia improperly managed--$2.320 million Virginia verdict. Medical Malpractice: Verdicts, Settlements & Experts. 2019;35(2):13.
- Jena AB, Seabury S, Lakdawalla D, et al. Malpractice risk according to physician specialty. N Engl J Med. 2011;365:629-636.
- Schaffer AC, Jena AB, Seabury SA, et al. Rates and characteristics of paid malpractice claims among US physicians by specialty, 1992-2014. JAMA Intern Med. 2017;177:710-718.
- Lowes R. Malpractice premiums trail inflation for some physicians. Medscape. December 16, 2016. https://www.medscape.com/viewarticle/873422. Accessed January 10, 2020.
- Barbieri RL. Good news for ObGyns: medical liability claims resulting in payment are decreasing! OBG Manag. 2019;31:10-13.
- Guardado JR. Medical professional liability insurance premiums: an overview of the market from 2008 to 2017. AMA Policy Research Perspectives, 2018. https://www.ama-assn.org/sites/ama-assn.org/files/corp/media-browser/public/government/advocacy/policy-research-perspective-liability-insurance-premiums.pdf. Accessed January 10, 2020.
- Institute of Medicine Committee on Quality Health Care in America; Kohn LT, Corrigan JM, Donaldson MS, eds. To Err is Human: Building a Safer Health System. Washington, DC: National Academies Press; 2000.
- James JT. A new, evidence-based estimate of patient harms associated with hospital care. J Patient Saf. 2013;9:122-128. https://journals.lww.com/journalpatientsafety/Fulltext/
2013/09000/A_New,_Evidence_based_Estimate_of_Patient_
Harms.2.aspx. Accessed January 10, 2020. - Public Citizen Congress Watch. The great medical malpractice hoax: NPDB data continue to show medical liability system produces rational outcomes. January 2007. https://www.citizen.org/wp-content/uploads/npdb_report_
final.pdf. Accessed January 23, 2020. - Bell SK, Delbanco T, Anderson-Shaw L, et al. Accountability for medical error: moving beyond blame to advocacy. Chest. 2011;140:519-526.
- Ramanathan T. Legal mechanisms supporting accountable care principles. Am J Public Health. 2014;104:2048-2051.
- Kachalia A, Kaufman SR, Boothman R, et al. Liability claims and costs before and after implementation of a medical error disclosure program. Ann Intern Med. 2010;153:213-221.
- National Practitioner Data Bank web site. What you must report to the NPDB. https://www.npdb.hrsa.gov/hcorg/whatYouMustReport
ToTheDataBank.jsp. Accessed January 10, 2020. - Bovbjerg RR. Malpractice crisis and reform. Clin Perinatol. 2005;32:203-233, viii-ix.
- Viscusi WK. Medical malpractice reform: what works and what doesn't. Denver Law Rev. 2019;96:775-791. https://static1.squarespace.com/static/5cb79f7efd6793296c0eb738 /t/5d5f4ffabd6c5400011a12f6/1566527483118/Vol96_Issue4_Viscusi_
FINAL.pdf. Accessed January 10, 2020. - National Conference of State Legislatures. Medical malpractice reform. Health Cost Containment and Efficiencies: NCSL Briefs for State Legislators. 2011;(16). http://www.ncsl.org/research/health/medical-malpractice-reform-health-cost-brief.aspx. Accessed January 10, 2020.
- Kass JS, Rose RV. Medical malpractice reform: historical approaches, alternative models, and communication and resolution programs. AMA J Ethics. 2016;18:299-310.
- Boehm G. Debunking medical malpractice myths: unraveling the false premises behind "tort reform". Yale J Health Policy Law Ethics. 2005;5:357-369.
- Hellinger FJ, Encinosa WE. The impact of state laws limiting malpractice damage awards on health care expenditures. Am J Public Health. 2006;96:1375-1381.
- Perry G. Medical malpractice caps by state [infographic]. January 3, 2013. https://www.business2community.com/infographics/medical-malpractice-caps-by-state-infographic-0368345. Accessed January 23, 2020.
- Goguen D. State-by-state medical malpractice damages caps. An in-depth look at state laws limiting compensation for medical malpractice plaintiffs. https://www.nolo.com/legal-encyclopedia/state-state-medical-malpractice-damages-caps.html. Accessed January 23, 2020.
- Berlin L. Medical errors, malpractice, and defensive medicine: an ill-fated triad. Diagnosis (Berl). 2017;4:133-139.
- Ranum D. Electronic health records continue to lead to medical malpractice suits. The Doctors Company. August 2019. https://www.thedoctors.com/articles/electronic-health-records-continue-to-lead-to-medical-malpractice-suits/. Accessed January 10, 2020.
- Mangalmurti SS, Murtagh L, Mello MM. Medical malpractice liability in the age of electronic health records. N Engl J Med. 2010;363:2060-2067.
- Studdert DM, Mello MM, Gawande AA, et al. Claims, errors, and compensation payments in medical malpractice litigation. N Engl J Med. 2006;354(19):2024-2033.
- Jena AB, Chandra A, Lakdawalla D, et al. Outcomes of medical malpractice litigation against US physicians. Arch Intern Med. 2012;172:892-894.
- Glaser LM, Alvi FA, Milad MP. Trends in malpractice claims for obstetric and gynecologic procedures, 2005 through 2014. Am J Obstet Gynecol. 2017;217:340.e1-340.e6.