User login
FDA: Cell phones still look safe
according to a review by the Food and Drug Administration.
The FDA reviewed the published literature from 2008 to 2018 and concluded that the data don’t support any quantifiable adverse health risks from RFR. However, the evidence is not without limitations.
The FDA’s evaluation included evidence from in vivo animal studies from Jan. 1, 2008, to Aug. 1, 2018, and epidemiologic studies in humans from Jan. 1, 2008, to May 8, 2018. Both kinds of evidence had limitations, but neither produced strong indications of any causal risks from cell phone use.
The FDA noted that in vivo animal studies are limited by variability of methods and RFR exposure, which make comparisons of results difficult. These studies are also impacted by the indirect effects of temperature increases (the only currently established biological effect of RFR) and stress experienced by the animals, which make teasing out the direct effects of RFR difficult.
The FDA noted that strong epidemiologic studies can provide more relevant and accurate information than in vivo studies, but epidemiologic studies are not without limitations. For example, most have participants track and self-report their cell phone use. There’s also no way to directly track certain factors of RFR exposure, such as frequency, duration, or intensity.
Even with those caveats in mind, the FDA wrote that, “based on the studies that are described in detail in this report, there is insufficient evidence to support a causal association between RFR exposure and tumorigenesis. There is a lack of clear dose-response relationship, a lack of consistent findings or specificity, and a lack of biological mechanistic plausibility.”
The full review is available on the FDA website.
according to a review by the Food and Drug Administration.
The FDA reviewed the published literature from 2008 to 2018 and concluded that the data don’t support any quantifiable adverse health risks from RFR. However, the evidence is not without limitations.
The FDA’s evaluation included evidence from in vivo animal studies from Jan. 1, 2008, to Aug. 1, 2018, and epidemiologic studies in humans from Jan. 1, 2008, to May 8, 2018. Both kinds of evidence had limitations, but neither produced strong indications of any causal risks from cell phone use.
The FDA noted that in vivo animal studies are limited by variability of methods and RFR exposure, which make comparisons of results difficult. These studies are also impacted by the indirect effects of temperature increases (the only currently established biological effect of RFR) and stress experienced by the animals, which make teasing out the direct effects of RFR difficult.
The FDA noted that strong epidemiologic studies can provide more relevant and accurate information than in vivo studies, but epidemiologic studies are not without limitations. For example, most have participants track and self-report their cell phone use. There’s also no way to directly track certain factors of RFR exposure, such as frequency, duration, or intensity.
Even with those caveats in mind, the FDA wrote that, “based on the studies that are described in detail in this report, there is insufficient evidence to support a causal association between RFR exposure and tumorigenesis. There is a lack of clear dose-response relationship, a lack of consistent findings or specificity, and a lack of biological mechanistic plausibility.”
The full review is available on the FDA website.
according to a review by the Food and Drug Administration.
The FDA reviewed the published literature from 2008 to 2018 and concluded that the data don’t support any quantifiable adverse health risks from RFR. However, the evidence is not without limitations.
The FDA’s evaluation included evidence from in vivo animal studies from Jan. 1, 2008, to Aug. 1, 2018, and epidemiologic studies in humans from Jan. 1, 2008, to May 8, 2018. Both kinds of evidence had limitations, but neither produced strong indications of any causal risks from cell phone use.
The FDA noted that in vivo animal studies are limited by variability of methods and RFR exposure, which make comparisons of results difficult. These studies are also impacted by the indirect effects of temperature increases (the only currently established biological effect of RFR) and stress experienced by the animals, which make teasing out the direct effects of RFR difficult.
The FDA noted that strong epidemiologic studies can provide more relevant and accurate information than in vivo studies, but epidemiologic studies are not without limitations. For example, most have participants track and self-report their cell phone use. There’s also no way to directly track certain factors of RFR exposure, such as frequency, duration, or intensity.
Even with those caveats in mind, the FDA wrote that, “based on the studies that are described in detail in this report, there is insufficient evidence to support a causal association between RFR exposure and tumorigenesis. There is a lack of clear dose-response relationship, a lack of consistent findings or specificity, and a lack of biological mechanistic plausibility.”
The full review is available on the FDA website.
U.S. Multi-Society Task Force publishes polypectomy guidance
The U.S. Multi-Society Task Force (USMSTF) on Colorectal Cancer recently published recommendations for endoscopic removal of precancerous colorectal lesions.
According to lead author Tonya Kaltenbach, MD, of the University of California, San Francisco, and fellow panelists, the publication aims to improve complete resection rates, which can vary widely between endoscopists; almost one out of four lesions (22.7%) may be incompletely removed by some practitioners, leading to higher rates of colorectal cancer.
“[A]lthough the majority (50%) of postcolonoscopy colon cancers [are] likely due to missed lesions, close to one-fifth of incident cancers [are] related to incomplete resection,” the panelists wrote in Gastroenterology, referring to a pooled analysis of eight surveillance studies.
The panelists’ recommendations, which were based on both evidence and clinical experience, range from specific polyp removal techniques to guidance for institution-wide quality assurance of polypectomies. Each statement is described by both strength of recommendation and level of evidence, the latter of which was determined by Grading of Recommendations, Assessment, Development, and Evaluation Ratings of Evidence (GRADE) criteria. Recommendations were written by a panel of nine experts and approved by the governing boards of the three societies they represented – the American College of Gastroenterology, the American Gastroenterological Association, and the American Society for Gastrointestinal Endoscopy. The recommendations were copublished in the March issues of the American Journal of Gastroenterology, Gastroenterology, and Gastrointestinal Endoscopy.
Central to the publication are recommended polypectomy techniques for specific types of lesions.
“Polypectomy techniques vary widely in clinical practice,” the panelists wrote. “They are often driven by physician preference based on how they were taught and on trial and error, due to the lack of standardized training and the paucity of published evidence. In the past decade, evidence has evolved on the superiority of specific methods.”
“Optimal techniques encompass effectiveness, safety, and efficiency,” they wrote. “Colorectal lesion characteristics, including location, size, morphology, and histology, influence the optimal removal method.”
For lesions up to 9 mm, the panelists recommended cold snare polypectomy “due to high complete resection rates and safety profile.” In contrast, they recommended against both cold and hot biopsy forceps, which have been associated with higher rates of incomplete resection. Furthermore, they cautioned that hot biopsy forceps may increase risks of complications and produce inadequate tissue samples for histopathology.
For nonpedunculated lesions between 10 and 19 mm, guidance is minimal. The panelists recommended cold or hot snare polypectomy, although this statement was conditional and based on low-quality evidence.
Recommendations were more extensive for large nonpedunculated lesions (at least 20 mm). For such lesions, the panelists strongly recommended endoscopic mucosal resection (EMR). They emphasized that large lesions should be removed in the fewest possible pieces by an appropriately experienced endoscopist during a single colonoscopy session. The panelists recommended the use of a viscous injection solution with a contrast agent and adjuvant thermal ablation of the post-EMR margin. They recommended against the use of tattoo as a submucosal injection solution, and ablation of residual lesion tissue that is endoscopically visible. Additional recommendations for large lesions, including prophylactic closure of resection defects and coagulation techniques, were based on low-quality evidence.
For pedunculated lesions greater than 10 mm, the panelists recommended hot snare polypectomy. For pedunculated lesions with a head greater than 20 mm or a stalk thickness greater than 5 mm, they recommended prophylactic mechanical ligation.
Beyond lesion assessment and removal, recommendations addressed lesion marking, equipment, surveillance, and quality of polypectomy.
Concerning quality, the panelists recommended that endoscopists participate in a quality assurance program that documents adverse events, and that institutions use standardized polypectomy competency assessments, such as Cold Snare Polypectomy Competency Assessment Tool and/or Direct Observation of Polypectomy Skills.
“Focused teaching is needed to ensure the optimal endoscopic management of colorectal lesions,” the panelists wrote. They went on to suggest that “development and implementation of polypectomy quality metrics may be necessary to optimize practice and outcomes.”
“For example, the type of resection method used for the colorectal lesion removal in the procedure report should be documented, and the inclusion of adequate resection technique as a quality indicator in colorectal cancer screening programs should be considered,” they wrote. “Adverse events, including bleeding, perforation, hospital admissions, and the number of benign colorectal lesions referred for surgical management, should be measured and reported. Finally, standards for pathology preparation and reporting of lesions suspicious for submucosal invasion should be in place to provide accurate staging and management.”
The investigators reported relationships with Covidien, Ironwood, Medtronic, and others.
SOURCE: Kaltenbach T et al. Gastroenterology. 2020 Jan 18. doi: 10.1053/j.gastro.2019.12.018.
The U.S. Multi-Society Task Force (USMSTF) on Colorectal Cancer recently published recommendations for endoscopic removal of precancerous colorectal lesions.
According to lead author Tonya Kaltenbach, MD, of the University of California, San Francisco, and fellow panelists, the publication aims to improve complete resection rates, which can vary widely between endoscopists; almost one out of four lesions (22.7%) may be incompletely removed by some practitioners, leading to higher rates of colorectal cancer.
“[A]lthough the majority (50%) of postcolonoscopy colon cancers [are] likely due to missed lesions, close to one-fifth of incident cancers [are] related to incomplete resection,” the panelists wrote in Gastroenterology, referring to a pooled analysis of eight surveillance studies.
The panelists’ recommendations, which were based on both evidence and clinical experience, range from specific polyp removal techniques to guidance for institution-wide quality assurance of polypectomies. Each statement is described by both strength of recommendation and level of evidence, the latter of which was determined by Grading of Recommendations, Assessment, Development, and Evaluation Ratings of Evidence (GRADE) criteria. Recommendations were written by a panel of nine experts and approved by the governing boards of the three societies they represented – the American College of Gastroenterology, the American Gastroenterological Association, and the American Society for Gastrointestinal Endoscopy. The recommendations were copublished in the March issues of the American Journal of Gastroenterology, Gastroenterology, and Gastrointestinal Endoscopy.
Central to the publication are recommended polypectomy techniques for specific types of lesions.
“Polypectomy techniques vary widely in clinical practice,” the panelists wrote. “They are often driven by physician preference based on how they were taught and on trial and error, due to the lack of standardized training and the paucity of published evidence. In the past decade, evidence has evolved on the superiority of specific methods.”
“Optimal techniques encompass effectiveness, safety, and efficiency,” they wrote. “Colorectal lesion characteristics, including location, size, morphology, and histology, influence the optimal removal method.”
For lesions up to 9 mm, the panelists recommended cold snare polypectomy “due to high complete resection rates and safety profile.” In contrast, they recommended against both cold and hot biopsy forceps, which have been associated with higher rates of incomplete resection. Furthermore, they cautioned that hot biopsy forceps may increase risks of complications and produce inadequate tissue samples for histopathology.
For nonpedunculated lesions between 10 and 19 mm, guidance is minimal. The panelists recommended cold or hot snare polypectomy, although this statement was conditional and based on low-quality evidence.
Recommendations were more extensive for large nonpedunculated lesions (at least 20 mm). For such lesions, the panelists strongly recommended endoscopic mucosal resection (EMR). They emphasized that large lesions should be removed in the fewest possible pieces by an appropriately experienced endoscopist during a single colonoscopy session. The panelists recommended the use of a viscous injection solution with a contrast agent and adjuvant thermal ablation of the post-EMR margin. They recommended against the use of tattoo as a submucosal injection solution, and ablation of residual lesion tissue that is endoscopically visible. Additional recommendations for large lesions, including prophylactic closure of resection defects and coagulation techniques, were based on low-quality evidence.
For pedunculated lesions greater than 10 mm, the panelists recommended hot snare polypectomy. For pedunculated lesions with a head greater than 20 mm or a stalk thickness greater than 5 mm, they recommended prophylactic mechanical ligation.
Beyond lesion assessment and removal, recommendations addressed lesion marking, equipment, surveillance, and quality of polypectomy.
Concerning quality, the panelists recommended that endoscopists participate in a quality assurance program that documents adverse events, and that institutions use standardized polypectomy competency assessments, such as Cold Snare Polypectomy Competency Assessment Tool and/or Direct Observation of Polypectomy Skills.
“Focused teaching is needed to ensure the optimal endoscopic management of colorectal lesions,” the panelists wrote. They went on to suggest that “development and implementation of polypectomy quality metrics may be necessary to optimize practice and outcomes.”
“For example, the type of resection method used for the colorectal lesion removal in the procedure report should be documented, and the inclusion of adequate resection technique as a quality indicator in colorectal cancer screening programs should be considered,” they wrote. “Adverse events, including bleeding, perforation, hospital admissions, and the number of benign colorectal lesions referred for surgical management, should be measured and reported. Finally, standards for pathology preparation and reporting of lesions suspicious for submucosal invasion should be in place to provide accurate staging and management.”
The investigators reported relationships with Covidien, Ironwood, Medtronic, and others.
SOURCE: Kaltenbach T et al. Gastroenterology. 2020 Jan 18. doi: 10.1053/j.gastro.2019.12.018.
The U.S. Multi-Society Task Force (USMSTF) on Colorectal Cancer recently published recommendations for endoscopic removal of precancerous colorectal lesions.
According to lead author Tonya Kaltenbach, MD, of the University of California, San Francisco, and fellow panelists, the publication aims to improve complete resection rates, which can vary widely between endoscopists; almost one out of four lesions (22.7%) may be incompletely removed by some practitioners, leading to higher rates of colorectal cancer.
“[A]lthough the majority (50%) of postcolonoscopy colon cancers [are] likely due to missed lesions, close to one-fifth of incident cancers [are] related to incomplete resection,” the panelists wrote in Gastroenterology, referring to a pooled analysis of eight surveillance studies.
The panelists’ recommendations, which were based on both evidence and clinical experience, range from specific polyp removal techniques to guidance for institution-wide quality assurance of polypectomies. Each statement is described by both strength of recommendation and level of evidence, the latter of which was determined by Grading of Recommendations, Assessment, Development, and Evaluation Ratings of Evidence (GRADE) criteria. Recommendations were written by a panel of nine experts and approved by the governing boards of the three societies they represented – the American College of Gastroenterology, the American Gastroenterological Association, and the American Society for Gastrointestinal Endoscopy. The recommendations were copublished in the March issues of the American Journal of Gastroenterology, Gastroenterology, and Gastrointestinal Endoscopy.
Central to the publication are recommended polypectomy techniques for specific types of lesions.
“Polypectomy techniques vary widely in clinical practice,” the panelists wrote. “They are often driven by physician preference based on how they were taught and on trial and error, due to the lack of standardized training and the paucity of published evidence. In the past decade, evidence has evolved on the superiority of specific methods.”
“Optimal techniques encompass effectiveness, safety, and efficiency,” they wrote. “Colorectal lesion characteristics, including location, size, morphology, and histology, influence the optimal removal method.”
For lesions up to 9 mm, the panelists recommended cold snare polypectomy “due to high complete resection rates and safety profile.” In contrast, they recommended against both cold and hot biopsy forceps, which have been associated with higher rates of incomplete resection. Furthermore, they cautioned that hot biopsy forceps may increase risks of complications and produce inadequate tissue samples for histopathology.
For nonpedunculated lesions between 10 and 19 mm, guidance is minimal. The panelists recommended cold or hot snare polypectomy, although this statement was conditional and based on low-quality evidence.
Recommendations were more extensive for large nonpedunculated lesions (at least 20 mm). For such lesions, the panelists strongly recommended endoscopic mucosal resection (EMR). They emphasized that large lesions should be removed in the fewest possible pieces by an appropriately experienced endoscopist during a single colonoscopy session. The panelists recommended the use of a viscous injection solution with a contrast agent and adjuvant thermal ablation of the post-EMR margin. They recommended against the use of tattoo as a submucosal injection solution, and ablation of residual lesion tissue that is endoscopically visible. Additional recommendations for large lesions, including prophylactic closure of resection defects and coagulation techniques, were based on low-quality evidence.
For pedunculated lesions greater than 10 mm, the panelists recommended hot snare polypectomy. For pedunculated lesions with a head greater than 20 mm or a stalk thickness greater than 5 mm, they recommended prophylactic mechanical ligation.
Beyond lesion assessment and removal, recommendations addressed lesion marking, equipment, surveillance, and quality of polypectomy.
Concerning quality, the panelists recommended that endoscopists participate in a quality assurance program that documents adverse events, and that institutions use standardized polypectomy competency assessments, such as Cold Snare Polypectomy Competency Assessment Tool and/or Direct Observation of Polypectomy Skills.
“Focused teaching is needed to ensure the optimal endoscopic management of colorectal lesions,” the panelists wrote. They went on to suggest that “development and implementation of polypectomy quality metrics may be necessary to optimize practice and outcomes.”
“For example, the type of resection method used for the colorectal lesion removal in the procedure report should be documented, and the inclusion of adequate resection technique as a quality indicator in colorectal cancer screening programs should be considered,” they wrote. “Adverse events, including bleeding, perforation, hospital admissions, and the number of benign colorectal lesions referred for surgical management, should be measured and reported. Finally, standards for pathology preparation and reporting of lesions suspicious for submucosal invasion should be in place to provide accurate staging and management.”
The investigators reported relationships with Covidien, Ironwood, Medtronic, and others.
SOURCE: Kaltenbach T et al. Gastroenterology. 2020 Jan 18. doi: 10.1053/j.gastro.2019.12.018.
FROM GASTROENTEROLOGY
Thank you to the CHEST 2020 Scientific Program Committee
The CHEST 2020 Scientific Program Committee has been working tirelessly to select the best and most clinically relevant sessions for the upcoming meeting. CHEST would like to extend a heartfelt thank you to all that actively participated in grading, curriculum group calls, the live meeting in February, and all the homework in between. We’re not done, but your work has been instrumental in making the CHEST Annual Meeting 2020 a success.
The CHEST 2020 Scientific Program Committee has been working tirelessly to select the best and most clinically relevant sessions for the upcoming meeting. CHEST would like to extend a heartfelt thank you to all that actively participated in grading, curriculum group calls, the live meeting in February, and all the homework in between. We’re not done, but your work has been instrumental in making the CHEST Annual Meeting 2020 a success.
The CHEST 2020 Scientific Program Committee has been working tirelessly to select the best and most clinically relevant sessions for the upcoming meeting. CHEST would like to extend a heartfelt thank you to all that actively participated in grading, curriculum group calls, the live meeting in February, and all the homework in between. We’re not done, but your work has been instrumental in making the CHEST Annual Meeting 2020 a success.
Antineutrophil Cytoplasmic Antibody Vasculitis Induced by Hydralazine
To the Editor:
Hydralazine-induced antineutrophil cytoplasmic antibody vasculitis (HIAV) is a rare side effect that may develop in patients treated with hydralazine. Without early recognition and hydralazine cessation, patients often develop acute renal failure and pulmonary hemorrhage that may result in death. We present a case of HIAV.
A 67-year-old woman presented with progressive, tense, hemorrhagic, and necrotic bullae on both sides of the face and neck as well as the extremities of 2 weeks’ duration. She had a history of hypertension and a thyroid nodule after unilateral thyroid lobectomy. A review of symptoms was positive for worsening dyspnea and progressive generalized weakness. Noteworthy medications included amlodipine, metoprolol, levothyroxine, and oral hydralazine 75 mg 3 times daily for 13 months.
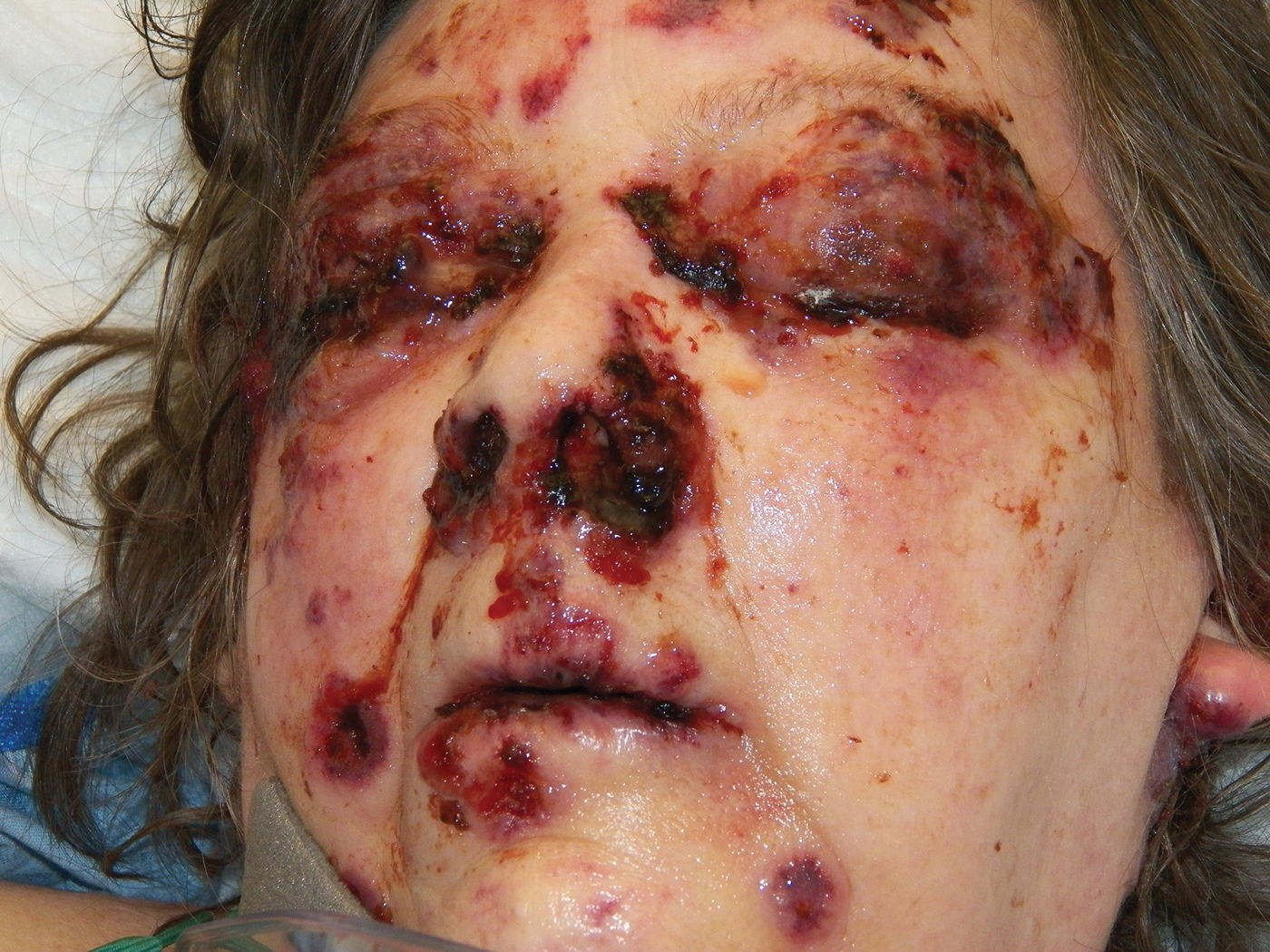
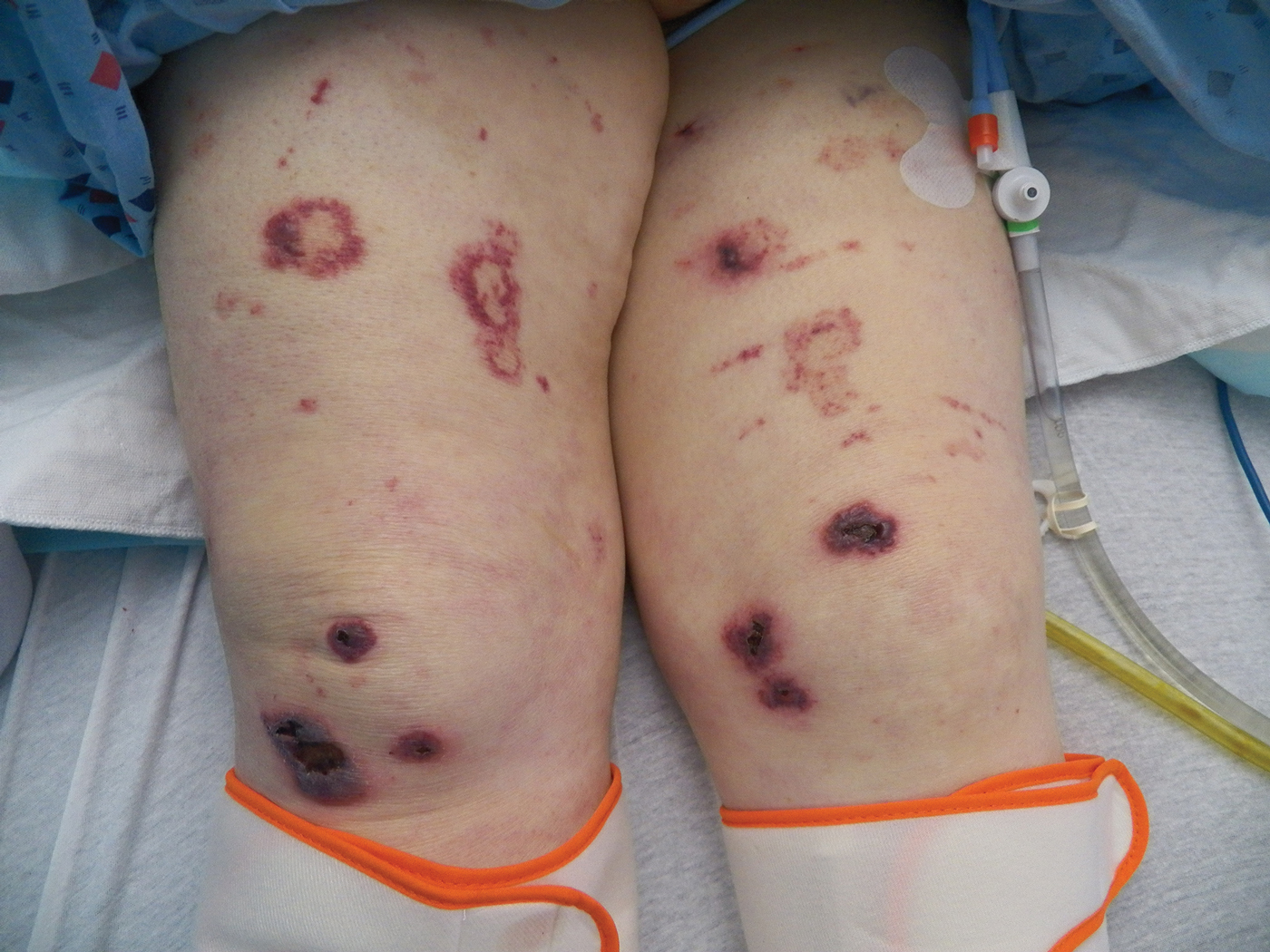
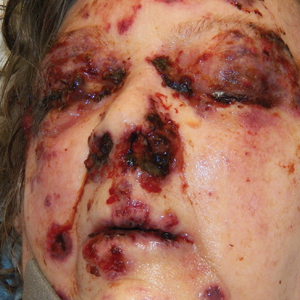
Bullae first appeared on the patient’s scalp and quickly progressed with a cephalocaudal pattern with a propensity for the eyes, nostrils, and labial mucosa (Figure 1). The tongue was covered by an eschar, and she had diffuse periorbital edema. Additionally, concentric purpuric patches were noted on the thighs and lower legs (Figure 2).
Pertinent laboratory findings included a positive antinuclear antibody titer of 1:320 and perinuclear antineutrophil cytoplasmic antibody (ANCA) titer of 1:160, along with an elevated serum creatinine level (2.31 mg/dL [reference range, 0.6–1.2 mg/dL]). Bilateral perihilar infiltrates with bilateral pleural effusions were noted on a chest radiograph.
While hospitalized, she developed pulmonary hemorrhages and a progressive decline in respiratory status. She subsequently was admitted to the medical intensive care unit. Aggressive support was administered, and several skin biopsy specimens were obtained along with an endobronchial biopsy of the right middle lobe.
Skin histopathology revealed a necrotic vasculitis (Figure 3). Direct immunofluorescence was not performed. Lung histopathology showed fragments of bronchial tissue with acute and chronic inflammation, focal necrosis, granulation tissue formation, edema, and squamous metaplasia. Together with the clinical history, these findings were consistent with HIAV.
Hydralazine was immediately discontinued, and the patient was started on 65 mg daily of intravenous methylprednisolone; methylprednisolone was later changed to oral prednisone 30 mg daily. Due to multiple organ involvement—lung and kidney—intravenous rituximab 375 mg/m2 every week for 4 weeks, per lymphoma protocol, was started. Within 2 weeks of beginning therapy, her renal function and respiratory status improved, and by week 4, the skin lesions had completely resolved. Although initially she did well on immunosuppressive therapy with resolution of all symptoms, the patient contracted Clostridium difficile–induced systemic inflammatory response syndrome after 5 weeks of therapy and died.
Hydralazine was first introduced in 1951 for adjunctive hypertension therapy due to its vasodilation effects.1-3 Since its introduction, it has been implicated in 2 important disease processes: HIAV and hydralazine-induced lupus.
Hydralazine-induced ANCA vasculitis was first documented in 1980; by 2011, multiple cases had been reported.1-7 Hydralazine-induced ANCA vasculitis has occurred in patients aged 11 to 79 years taking 50 to 300 mg daily. Symptom onset varies from 6 months to 14 years, with a mean exposure duration of 4.7 years and mean daily dose of 142 mg.1-7
Clinical manifestations range from less specific, such as fever, malaise, arthralgia, myalgia, and weight loss, to single tissue or organ involvement that may be fatal. The most frequent clinical features include kidney involvement (81%), cutaneous vasculitis (25%), arthralgia (24%), and pleuropulmonary involvement (19%). Cutaneous manifestations include but are not limited to palpable lower extremity purpura; morbilliform eruptions; and hemorrhagic blisters on the lower legs, arms, trunk, nasal septum, and uvula.1-4,8
The most commonly affected organ is the kidney, which commonly presents as hematuria, proteinuria, and elevated serum creatinine level. Histopathologically, patients most likely will have necrotizing and crescentic glomerulonephritis that is pauci-immune by immunofluorescence.7,9 The lungs are the next most commonly affected organ, with a classic presentation of cough, dyspnea, and hemoptysis in the setting of intra-alveolar hemorrhage.6,8 When both the kidneys and lungs are involved, the patient is said to have pulmonary-renal syndrome that is characterized by lung infiltrates or nodules with or without hemorrhage, hemoptysis, and pleuritis in the setting of glomerulonephritis.1,6
Clear data on incidence and prevalence of HIAV does not exist due to the rarity of the disease and the lack of prospective studies. To identify a clear incidence and prevalence, prospective longitudinal studies with larger cohorts along with better recognition and diagnosis are needed.2,8,10 A few predisposing risk factors have been identified, including older age, a cumulative dose of 100 g at the time of presentation, female sex, a history of thyroid disease, HLA-DR4 genotypes, slow hepatic acetylation, and the null gene for C4.1,3,5,9-11 Our patient was an older woman with a history of thyroid disease who had been taking oral hydralazine 75 mg 3 times daily for 13 months. During this 13-month duration, she had no dose adjustments.
Currently, the pathomechanism for HIAV is unclear and may be multifactorial. There are 4 main theories2,8-10,12,13:
1. Hydralazine and its metabolites accumulate inside neutrophils, then subsequently bind and alter the configuration of myeloperoxidase (MPO). This alteration leads to spreading of the autoimmune response to other autoantigens, making neutrophil proteins (eg, elastase, lactoferrin, nuclear antigens) immunogenic.
2. Hydralazine binds MPO in neutrophils, creating cytotoxic products that induce neutrophil apoptosis. Neutrophil apoptosis without priming then results in ANCA antigen presence on the neutrophil cell membrane and the formation of MPO-ANCA. Myeloperoxidase-ANCA then binds to these membrane-bound antigens that cause self-perpetuating, constitutive activation through cross-linking with proteinase 3 or MPO and Fcγ receptors.
3. Activated neutrophils in the presence of hydrogen peroxidase release MPO that converts hydralazine into a cytotoxic product that is immunogenic for T cells that activate ANCA-producing B cells.
4. Histone H3 trimethyl Lys27 (H3K27me3) levels are perturbed in HIAV, which leads to aberrant gene silencing of proteinase 3 and MPO.In contrast, the demethylase Jumonji domain-containing protein 3 for the H3K27me3 histone is increased in patients without HIAV. Based on this data and the data showing a role for hydralazine in reversing epigenetic silencing of tumor suppressor genes in cancer cells,13 it has been proposed that hydralazine may reverse epigenetic silencing of proteinase 3 and MPO.
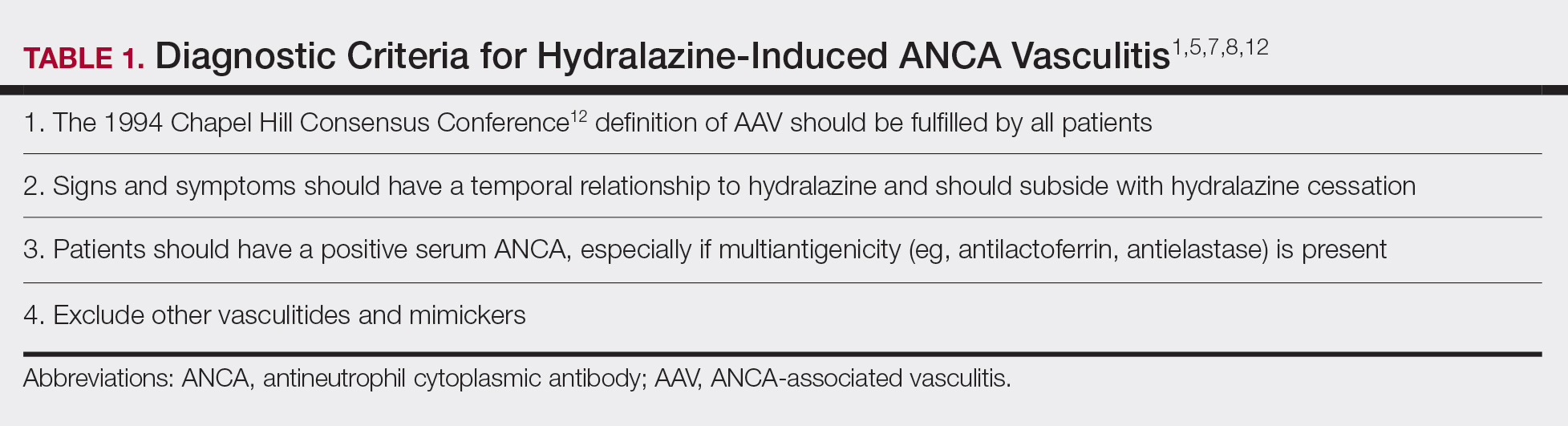
Diagnosing HIAV is still difficult because physicians do not recognize the drug as the etiologic agent, there is extensive variability in duration between starting the drug and onset of symptoms, and there often is a failure to order the appropriate laboratory and invasive tests needed for evaluation and diagnosis.3,5,8,10,12 Despite these difficulties, a set of criteria and practices for diagnosis are delineated in Table 1, with the key diagnostic feature being resolution with hydralazine cessation.1,5,7,8,12
A comprehensive drug history from at least 6 months prior to presentation is essential. Biopsies also are strongly encouraged to confirm the presence of vasculitis and to determine its severity.8,12 If renal biopsies are performed, they typically show scant IgG, IgM, and C3 deposition that is characteristic of ANCA-positive pauci-immune glomerulonephritis. Compared to hydralazine-induced lupus, renal involvement in the setting of HIAV has a relative lack of immunoglobulin and complement deposition with histopathology and immunostaining.14
Laboratory test results including serum MPO-ANCA (perinuclear ANCA) with coexisting elastase and/or lactoferrin autoantibodies is characteristic of HIAV. Antinuclear antibody, antihistone, anti–double-stranded DNA, and antiphospholipid antibodies along with low complement levels also may be present.2,4,9,10,13,15 It is recommended that ANCA assays combine indirect immunofluorescence with antigen-specific enzyme-linked immunosorbent assay.8 With respect to its idiopathic counterpart, patients may only present with MPO-ANCA, while other aforementioned antibodies (eg, antihistone, anti–double-stranded DNA) are rarely found or are entirely absent.2,9 Patients with HIAV often have higher titers of MPO-ANCA.9,15 In hydralazine-induced lupus, patients rarely have MPO-ANCA.
When a diagnosis of HIAV is made, it cannot be confirmed until hydralazine is discontinued and the patient’s symptoms resolve. Therefore, it is both diagnostic and therapeutic to discontinue hydralazine when HIAV is suspected. If recognized when the patient is only presenting with nonspecific symptoms, simple hydralazine cessation may be all that is needed; however, because recognition and diagnosis of HIAV is difficult, most patients present when the disease is severe and has progressed to organ involvement.8-10
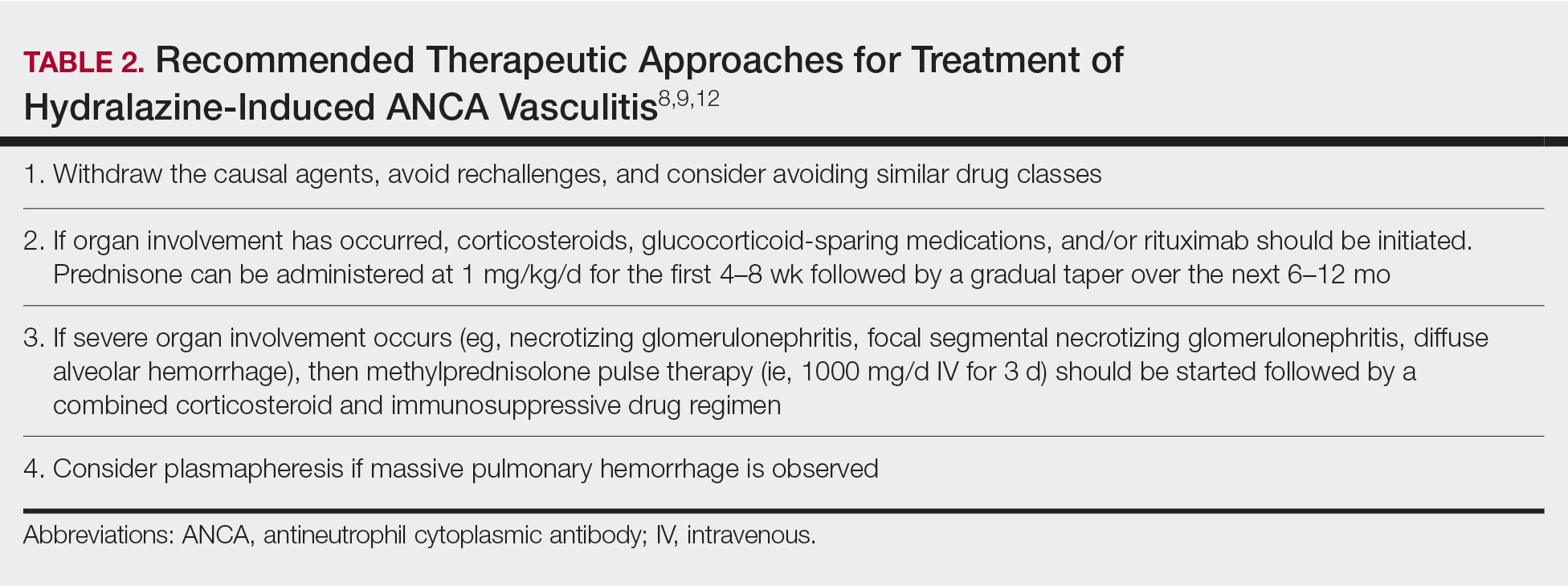
Treatment recommendations are highlighted in Table 2.8,9,12 Glucocorticoid therapy is believed to work by preventing T-cell and B-cell maturation needed to produce MPO-ANCA. Rituximab, on the other hand, is suspected to act by clearing the peripheral blood of MPO-ANCA B cells.12,16 Of note, patients with HIAV are different from their idiopathic counterparts because they usually need shorter courses of immunosuppressive therapy, long-term maintenance usually is unnecessary, and their prognosis generally is good if the offending agent is withdrawn.7-9,12 Once the appropriate therapy is instituted, vasculitic manifestations are expected to resolve 10 days to 8 months after hydralazine cessation; however, a response often is seen within 1 to 4 weeks after initiation of systemic treatment.4,8 Serum ANCA should be monitored, and there should be surveillance for the emergence of a chronic underlying vasculitis.8,12
Our patient highlights the importance of identifying individuals at risk for HIAV. We seek to increase recognition of this entity, as it is not commonly seen in a dermatologic setting and is associated with high morbidity and mortality, as seen in our patient.
- Yokogawa N, Vivino FB. Hydralazine-induced autoimmune disease: comparison to idiopathic lupus and ANCA-positive vasculitis. Mod Rheumatol. 2009;19:338-347.
- Agarwal G, Sultan G, Werner SL, et al. Hydralazine induces myeloperoxidase and proteinase 3 anti-neutrophil cytoplasmic antibody vasculitis and leads to pulmonary renal syndrome. Case Rep Nephrol. 2014;2014:868590.
- Keasberry J, Frazier J, Isbel NM, et al. Hydralazine-induced anti-neutrophilic cytoplasmic antibody-positive renal vasculitis presenting with a vasculitic syndrome, acute nephritis and a puzzling skin rash: a case report. J Med Case Rep. 2013;7:20.
- ten Holder SM, Joy MS, Falk RJ. Cutaneous and systemic manifestations of drug-induced vasculitis. Ann Pharmacother. 2002;36:130-147.
- Namas R, Rubin B, Adwar W, et al. A challenging twist in pulmonary renal syndrome. Case Rep Rheumatol. 2014;2014:516362.
- Dobre M, Wish J, Negrea L. Hydralazine-induced ANCA-positive pauci-immune glomerulonephritis. Ren Fail. 2009;31:745-748.
- Hogan JJ, Markowitz GS, Radhakrishnan J. Drug-induced glomerular disease: immune-mediated injury. Clin J Am Soc Nephrol. 2015;10:1300-1310.
- Radic M, Martinovic Kaliterna D, Radic J. Drug-induced vasculitis: a clinical and pathological review. Neth J Med. 2012;70:12-17.
- Babar F, Posner JN, Obah EA. Hydralazine-induced pauci-immune glomerulonephritis: intriguing case series misleading diagnoses. J Community Hosp Intern Med Perspect. 2016;6:30632.
- Marina VP, Malhotra D, Kaw D. Hydralazine-induced ANCA vasculitis with pulmonary renal syndrome: a rare clinical presentation. Int Urol Nephrol. 2012;44:1907-1909.
- Magro CM. Associated ANCA positive vasculitis. The Dermatologist. 2015;23(7). http://www.the-dermatologist.com/content/associated-anca-positive-vasculitis. Accessed January 30, 2020.
- Gao Y, Zhao MH. Review article: Drug-induced anti-neutrophil cytoplasmic antibody-associated vasculitis. Nephrology (Carlton). 2009;14:33-41.
- Grau RG. Drug-induced vasculitis: new insights and a changing lineup of suspects. Curr Rheumatol Rep. 2015;17:71.
- Sangala N, Lee RW, Horsfield C, et al. Combined ANCA-associated vasculitis and lupus syndrome following prolonged use of hydralazine: a timely reminder of an old foe. Int Urol Nephrol. 2010;42:503-506.
- Choi HK, Merkel PA, Walker AM, et al. Drug-associated antineutrophil cytoplasmic antibody-positive vasculitis: prevalence among patients with high titers of antimyeloperoxidase antibodies. Arthritis Rheum. 2000;43:405-413.
- Coutinho AE, Chapman KE. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol. 2011;335:2-13.
To the Editor:
Hydralazine-induced antineutrophil cytoplasmic antibody vasculitis (HIAV) is a rare side effect that may develop in patients treated with hydralazine. Without early recognition and hydralazine cessation, patients often develop acute renal failure and pulmonary hemorrhage that may result in death. We present a case of HIAV.
A 67-year-old woman presented with progressive, tense, hemorrhagic, and necrotic bullae on both sides of the face and neck as well as the extremities of 2 weeks’ duration. She had a history of hypertension and a thyroid nodule after unilateral thyroid lobectomy. A review of symptoms was positive for worsening dyspnea and progressive generalized weakness. Noteworthy medications included amlodipine, metoprolol, levothyroxine, and oral hydralazine 75 mg 3 times daily for 13 months.
Bullae first appeared on the patient’s scalp and quickly progressed with a cephalocaudal pattern with a propensity for the eyes, nostrils, and labial mucosa (Figure 1). The tongue was covered by an eschar, and she had diffuse periorbital edema. Additionally, concentric purpuric patches were noted on the thighs and lower legs (Figure 2).
Pertinent laboratory findings included a positive antinuclear antibody titer of 1:320 and perinuclear antineutrophil cytoplasmic antibody (ANCA) titer of 1:160, along with an elevated serum creatinine level (2.31 mg/dL [reference range, 0.6–1.2 mg/dL]). Bilateral perihilar infiltrates with bilateral pleural effusions were noted on a chest radiograph.
While hospitalized, she developed pulmonary hemorrhages and a progressive decline in respiratory status. She subsequently was admitted to the medical intensive care unit. Aggressive support was administered, and several skin biopsy specimens were obtained along with an endobronchial biopsy of the right middle lobe.
Skin histopathology revealed a necrotic vasculitis (Figure 3). Direct immunofluorescence was not performed. Lung histopathology showed fragments of bronchial tissue with acute and chronic inflammation, focal necrosis, granulation tissue formation, edema, and squamous metaplasia. Together with the clinical history, these findings were consistent with HIAV.
Hydralazine was immediately discontinued, and the patient was started on 65 mg daily of intravenous methylprednisolone; methylprednisolone was later changed to oral prednisone 30 mg daily. Due to multiple organ involvement—lung and kidney—intravenous rituximab 375 mg/m2 every week for 4 weeks, per lymphoma protocol, was started. Within 2 weeks of beginning therapy, her renal function and respiratory status improved, and by week 4, the skin lesions had completely resolved. Although initially she did well on immunosuppressive therapy with resolution of all symptoms, the patient contracted Clostridium difficile–induced systemic inflammatory response syndrome after 5 weeks of therapy and died.
Hydralazine was first introduced in 1951 for adjunctive hypertension therapy due to its vasodilation effects.1-3 Since its introduction, it has been implicated in 2 important disease processes: HIAV and hydralazine-induced lupus.
Hydralazine-induced ANCA vasculitis was first documented in 1980; by 2011, multiple cases had been reported.1-7 Hydralazine-induced ANCA vasculitis has occurred in patients aged 11 to 79 years taking 50 to 300 mg daily. Symptom onset varies from 6 months to 14 years, with a mean exposure duration of 4.7 years and mean daily dose of 142 mg.1-7
Clinical manifestations range from less specific, such as fever, malaise, arthralgia, myalgia, and weight loss, to single tissue or organ involvement that may be fatal. The most frequent clinical features include kidney involvement (81%), cutaneous vasculitis (25%), arthralgia (24%), and pleuropulmonary involvement (19%). Cutaneous manifestations include but are not limited to palpable lower extremity purpura; morbilliform eruptions; and hemorrhagic blisters on the lower legs, arms, trunk, nasal septum, and uvula.1-4,8
The most commonly affected organ is the kidney, which commonly presents as hematuria, proteinuria, and elevated serum creatinine level. Histopathologically, patients most likely will have necrotizing and crescentic glomerulonephritis that is pauci-immune by immunofluorescence.7,9 The lungs are the next most commonly affected organ, with a classic presentation of cough, dyspnea, and hemoptysis in the setting of intra-alveolar hemorrhage.6,8 When both the kidneys and lungs are involved, the patient is said to have pulmonary-renal syndrome that is characterized by lung infiltrates or nodules with or without hemorrhage, hemoptysis, and pleuritis in the setting of glomerulonephritis.1,6
Clear data on incidence and prevalence of HIAV does not exist due to the rarity of the disease and the lack of prospective studies. To identify a clear incidence and prevalence, prospective longitudinal studies with larger cohorts along with better recognition and diagnosis are needed.2,8,10 A few predisposing risk factors have been identified, including older age, a cumulative dose of 100 g at the time of presentation, female sex, a history of thyroid disease, HLA-DR4 genotypes, slow hepatic acetylation, and the null gene for C4.1,3,5,9-11 Our patient was an older woman with a history of thyroid disease who had been taking oral hydralazine 75 mg 3 times daily for 13 months. During this 13-month duration, she had no dose adjustments.
Currently, the pathomechanism for HIAV is unclear and may be multifactorial. There are 4 main theories2,8-10,12,13:
1. Hydralazine and its metabolites accumulate inside neutrophils, then subsequently bind and alter the configuration of myeloperoxidase (MPO). This alteration leads to spreading of the autoimmune response to other autoantigens, making neutrophil proteins (eg, elastase, lactoferrin, nuclear antigens) immunogenic.
2. Hydralazine binds MPO in neutrophils, creating cytotoxic products that induce neutrophil apoptosis. Neutrophil apoptosis without priming then results in ANCA antigen presence on the neutrophil cell membrane and the formation of MPO-ANCA. Myeloperoxidase-ANCA then binds to these membrane-bound antigens that cause self-perpetuating, constitutive activation through cross-linking with proteinase 3 or MPO and Fcγ receptors.
3. Activated neutrophils in the presence of hydrogen peroxidase release MPO that converts hydralazine into a cytotoxic product that is immunogenic for T cells that activate ANCA-producing B cells.
4. Histone H3 trimethyl Lys27 (H3K27me3) levels are perturbed in HIAV, which leads to aberrant gene silencing of proteinase 3 and MPO.In contrast, the demethylase Jumonji domain-containing protein 3 for the H3K27me3 histone is increased in patients without HIAV. Based on this data and the data showing a role for hydralazine in reversing epigenetic silencing of tumor suppressor genes in cancer cells,13 it has been proposed that hydralazine may reverse epigenetic silencing of proteinase 3 and MPO.
Diagnosing HIAV is still difficult because physicians do not recognize the drug as the etiologic agent, there is extensive variability in duration between starting the drug and onset of symptoms, and there often is a failure to order the appropriate laboratory and invasive tests needed for evaluation and diagnosis.3,5,8,10,12 Despite these difficulties, a set of criteria and practices for diagnosis are delineated in Table 1, with the key diagnostic feature being resolution with hydralazine cessation.1,5,7,8,12
A comprehensive drug history from at least 6 months prior to presentation is essential. Biopsies also are strongly encouraged to confirm the presence of vasculitis and to determine its severity.8,12 If renal biopsies are performed, they typically show scant IgG, IgM, and C3 deposition that is characteristic of ANCA-positive pauci-immune glomerulonephritis. Compared to hydralazine-induced lupus, renal involvement in the setting of HIAV has a relative lack of immunoglobulin and complement deposition with histopathology and immunostaining.14
Laboratory test results including serum MPO-ANCA (perinuclear ANCA) with coexisting elastase and/or lactoferrin autoantibodies is characteristic of HIAV. Antinuclear antibody, antihistone, anti–double-stranded DNA, and antiphospholipid antibodies along with low complement levels also may be present.2,4,9,10,13,15 It is recommended that ANCA assays combine indirect immunofluorescence with antigen-specific enzyme-linked immunosorbent assay.8 With respect to its idiopathic counterpart, patients may only present with MPO-ANCA, while other aforementioned antibodies (eg, antihistone, anti–double-stranded DNA) are rarely found or are entirely absent.2,9 Patients with HIAV often have higher titers of MPO-ANCA.9,15 In hydralazine-induced lupus, patients rarely have MPO-ANCA.
When a diagnosis of HIAV is made, it cannot be confirmed until hydralazine is discontinued and the patient’s symptoms resolve. Therefore, it is both diagnostic and therapeutic to discontinue hydralazine when HIAV is suspected. If recognized when the patient is only presenting with nonspecific symptoms, simple hydralazine cessation may be all that is needed; however, because recognition and diagnosis of HIAV is difficult, most patients present when the disease is severe and has progressed to organ involvement.8-10
Treatment recommendations are highlighted in Table 2.8,9,12 Glucocorticoid therapy is believed to work by preventing T-cell and B-cell maturation needed to produce MPO-ANCA. Rituximab, on the other hand, is suspected to act by clearing the peripheral blood of MPO-ANCA B cells.12,16 Of note, patients with HIAV are different from their idiopathic counterparts because they usually need shorter courses of immunosuppressive therapy, long-term maintenance usually is unnecessary, and their prognosis generally is good if the offending agent is withdrawn.7-9,12 Once the appropriate therapy is instituted, vasculitic manifestations are expected to resolve 10 days to 8 months after hydralazine cessation; however, a response often is seen within 1 to 4 weeks after initiation of systemic treatment.4,8 Serum ANCA should be monitored, and there should be surveillance for the emergence of a chronic underlying vasculitis.8,12
Our patient highlights the importance of identifying individuals at risk for HIAV. We seek to increase recognition of this entity, as it is not commonly seen in a dermatologic setting and is associated with high morbidity and mortality, as seen in our patient.
To the Editor:
Hydralazine-induced antineutrophil cytoplasmic antibody vasculitis (HIAV) is a rare side effect that may develop in patients treated with hydralazine. Without early recognition and hydralazine cessation, patients often develop acute renal failure and pulmonary hemorrhage that may result in death. We present a case of HIAV.
A 67-year-old woman presented with progressive, tense, hemorrhagic, and necrotic bullae on both sides of the face and neck as well as the extremities of 2 weeks’ duration. She had a history of hypertension and a thyroid nodule after unilateral thyroid lobectomy. A review of symptoms was positive for worsening dyspnea and progressive generalized weakness. Noteworthy medications included amlodipine, metoprolol, levothyroxine, and oral hydralazine 75 mg 3 times daily for 13 months.
Bullae first appeared on the patient’s scalp and quickly progressed with a cephalocaudal pattern with a propensity for the eyes, nostrils, and labial mucosa (Figure 1). The tongue was covered by an eschar, and she had diffuse periorbital edema. Additionally, concentric purpuric patches were noted on the thighs and lower legs (Figure 2).
Pertinent laboratory findings included a positive antinuclear antibody titer of 1:320 and perinuclear antineutrophil cytoplasmic antibody (ANCA) titer of 1:160, along with an elevated serum creatinine level (2.31 mg/dL [reference range, 0.6–1.2 mg/dL]). Bilateral perihilar infiltrates with bilateral pleural effusions were noted on a chest radiograph.
While hospitalized, she developed pulmonary hemorrhages and a progressive decline in respiratory status. She subsequently was admitted to the medical intensive care unit. Aggressive support was administered, and several skin biopsy specimens were obtained along with an endobronchial biopsy of the right middle lobe.
Skin histopathology revealed a necrotic vasculitis (Figure 3). Direct immunofluorescence was not performed. Lung histopathology showed fragments of bronchial tissue with acute and chronic inflammation, focal necrosis, granulation tissue formation, edema, and squamous metaplasia. Together with the clinical history, these findings were consistent with HIAV.
Hydralazine was immediately discontinued, and the patient was started on 65 mg daily of intravenous methylprednisolone; methylprednisolone was later changed to oral prednisone 30 mg daily. Due to multiple organ involvement—lung and kidney—intravenous rituximab 375 mg/m2 every week for 4 weeks, per lymphoma protocol, was started. Within 2 weeks of beginning therapy, her renal function and respiratory status improved, and by week 4, the skin lesions had completely resolved. Although initially she did well on immunosuppressive therapy with resolution of all symptoms, the patient contracted Clostridium difficile–induced systemic inflammatory response syndrome after 5 weeks of therapy and died.
Hydralazine was first introduced in 1951 for adjunctive hypertension therapy due to its vasodilation effects.1-3 Since its introduction, it has been implicated in 2 important disease processes: HIAV and hydralazine-induced lupus.
Hydralazine-induced ANCA vasculitis was first documented in 1980; by 2011, multiple cases had been reported.1-7 Hydralazine-induced ANCA vasculitis has occurred in patients aged 11 to 79 years taking 50 to 300 mg daily. Symptom onset varies from 6 months to 14 years, with a mean exposure duration of 4.7 years and mean daily dose of 142 mg.1-7
Clinical manifestations range from less specific, such as fever, malaise, arthralgia, myalgia, and weight loss, to single tissue or organ involvement that may be fatal. The most frequent clinical features include kidney involvement (81%), cutaneous vasculitis (25%), arthralgia (24%), and pleuropulmonary involvement (19%). Cutaneous manifestations include but are not limited to palpable lower extremity purpura; morbilliform eruptions; and hemorrhagic blisters on the lower legs, arms, trunk, nasal septum, and uvula.1-4,8
The most commonly affected organ is the kidney, which commonly presents as hematuria, proteinuria, and elevated serum creatinine level. Histopathologically, patients most likely will have necrotizing and crescentic glomerulonephritis that is pauci-immune by immunofluorescence.7,9 The lungs are the next most commonly affected organ, with a classic presentation of cough, dyspnea, and hemoptysis in the setting of intra-alveolar hemorrhage.6,8 When both the kidneys and lungs are involved, the patient is said to have pulmonary-renal syndrome that is characterized by lung infiltrates or nodules with or without hemorrhage, hemoptysis, and pleuritis in the setting of glomerulonephritis.1,6
Clear data on incidence and prevalence of HIAV does not exist due to the rarity of the disease and the lack of prospective studies. To identify a clear incidence and prevalence, prospective longitudinal studies with larger cohorts along with better recognition and diagnosis are needed.2,8,10 A few predisposing risk factors have been identified, including older age, a cumulative dose of 100 g at the time of presentation, female sex, a history of thyroid disease, HLA-DR4 genotypes, slow hepatic acetylation, and the null gene for C4.1,3,5,9-11 Our patient was an older woman with a history of thyroid disease who had been taking oral hydralazine 75 mg 3 times daily for 13 months. During this 13-month duration, she had no dose adjustments.
Currently, the pathomechanism for HIAV is unclear and may be multifactorial. There are 4 main theories2,8-10,12,13:
1. Hydralazine and its metabolites accumulate inside neutrophils, then subsequently bind and alter the configuration of myeloperoxidase (MPO). This alteration leads to spreading of the autoimmune response to other autoantigens, making neutrophil proteins (eg, elastase, lactoferrin, nuclear antigens) immunogenic.
2. Hydralazine binds MPO in neutrophils, creating cytotoxic products that induce neutrophil apoptosis. Neutrophil apoptosis without priming then results in ANCA antigen presence on the neutrophil cell membrane and the formation of MPO-ANCA. Myeloperoxidase-ANCA then binds to these membrane-bound antigens that cause self-perpetuating, constitutive activation through cross-linking with proteinase 3 or MPO and Fcγ receptors.
3. Activated neutrophils in the presence of hydrogen peroxidase release MPO that converts hydralazine into a cytotoxic product that is immunogenic for T cells that activate ANCA-producing B cells.
4. Histone H3 trimethyl Lys27 (H3K27me3) levels are perturbed in HIAV, which leads to aberrant gene silencing of proteinase 3 and MPO.In contrast, the demethylase Jumonji domain-containing protein 3 for the H3K27me3 histone is increased in patients without HIAV. Based on this data and the data showing a role for hydralazine in reversing epigenetic silencing of tumor suppressor genes in cancer cells,13 it has been proposed that hydralazine may reverse epigenetic silencing of proteinase 3 and MPO.
Diagnosing HIAV is still difficult because physicians do not recognize the drug as the etiologic agent, there is extensive variability in duration between starting the drug and onset of symptoms, and there often is a failure to order the appropriate laboratory and invasive tests needed for evaluation and diagnosis.3,5,8,10,12 Despite these difficulties, a set of criteria and practices for diagnosis are delineated in Table 1, with the key diagnostic feature being resolution with hydralazine cessation.1,5,7,8,12
A comprehensive drug history from at least 6 months prior to presentation is essential. Biopsies also are strongly encouraged to confirm the presence of vasculitis and to determine its severity.8,12 If renal biopsies are performed, they typically show scant IgG, IgM, and C3 deposition that is characteristic of ANCA-positive pauci-immune glomerulonephritis. Compared to hydralazine-induced lupus, renal involvement in the setting of HIAV has a relative lack of immunoglobulin and complement deposition with histopathology and immunostaining.14
Laboratory test results including serum MPO-ANCA (perinuclear ANCA) with coexisting elastase and/or lactoferrin autoantibodies is characteristic of HIAV. Antinuclear antibody, antihistone, anti–double-stranded DNA, and antiphospholipid antibodies along with low complement levels also may be present.2,4,9,10,13,15 It is recommended that ANCA assays combine indirect immunofluorescence with antigen-specific enzyme-linked immunosorbent assay.8 With respect to its idiopathic counterpart, patients may only present with MPO-ANCA, while other aforementioned antibodies (eg, antihistone, anti–double-stranded DNA) are rarely found or are entirely absent.2,9 Patients with HIAV often have higher titers of MPO-ANCA.9,15 In hydralazine-induced lupus, patients rarely have MPO-ANCA.
When a diagnosis of HIAV is made, it cannot be confirmed until hydralazine is discontinued and the patient’s symptoms resolve. Therefore, it is both diagnostic and therapeutic to discontinue hydralazine when HIAV is suspected. If recognized when the patient is only presenting with nonspecific symptoms, simple hydralazine cessation may be all that is needed; however, because recognition and diagnosis of HIAV is difficult, most patients present when the disease is severe and has progressed to organ involvement.8-10
Treatment recommendations are highlighted in Table 2.8,9,12 Glucocorticoid therapy is believed to work by preventing T-cell and B-cell maturation needed to produce MPO-ANCA. Rituximab, on the other hand, is suspected to act by clearing the peripheral blood of MPO-ANCA B cells.12,16 Of note, patients with HIAV are different from their idiopathic counterparts because they usually need shorter courses of immunosuppressive therapy, long-term maintenance usually is unnecessary, and their prognosis generally is good if the offending agent is withdrawn.7-9,12 Once the appropriate therapy is instituted, vasculitic manifestations are expected to resolve 10 days to 8 months after hydralazine cessation; however, a response often is seen within 1 to 4 weeks after initiation of systemic treatment.4,8 Serum ANCA should be monitored, and there should be surveillance for the emergence of a chronic underlying vasculitis.8,12
Our patient highlights the importance of identifying individuals at risk for HIAV. We seek to increase recognition of this entity, as it is not commonly seen in a dermatologic setting and is associated with high morbidity and mortality, as seen in our patient.
- Yokogawa N, Vivino FB. Hydralazine-induced autoimmune disease: comparison to idiopathic lupus and ANCA-positive vasculitis. Mod Rheumatol. 2009;19:338-347.
- Agarwal G, Sultan G, Werner SL, et al. Hydralazine induces myeloperoxidase and proteinase 3 anti-neutrophil cytoplasmic antibody vasculitis and leads to pulmonary renal syndrome. Case Rep Nephrol. 2014;2014:868590.
- Keasberry J, Frazier J, Isbel NM, et al. Hydralazine-induced anti-neutrophilic cytoplasmic antibody-positive renal vasculitis presenting with a vasculitic syndrome, acute nephritis and a puzzling skin rash: a case report. J Med Case Rep. 2013;7:20.
- ten Holder SM, Joy MS, Falk RJ. Cutaneous and systemic manifestations of drug-induced vasculitis. Ann Pharmacother. 2002;36:130-147.
- Namas R, Rubin B, Adwar W, et al. A challenging twist in pulmonary renal syndrome. Case Rep Rheumatol. 2014;2014:516362.
- Dobre M, Wish J, Negrea L. Hydralazine-induced ANCA-positive pauci-immune glomerulonephritis. Ren Fail. 2009;31:745-748.
- Hogan JJ, Markowitz GS, Radhakrishnan J. Drug-induced glomerular disease: immune-mediated injury. Clin J Am Soc Nephrol. 2015;10:1300-1310.
- Radic M, Martinovic Kaliterna D, Radic J. Drug-induced vasculitis: a clinical and pathological review. Neth J Med. 2012;70:12-17.
- Babar F, Posner JN, Obah EA. Hydralazine-induced pauci-immune glomerulonephritis: intriguing case series misleading diagnoses. J Community Hosp Intern Med Perspect. 2016;6:30632.
- Marina VP, Malhotra D, Kaw D. Hydralazine-induced ANCA vasculitis with pulmonary renal syndrome: a rare clinical presentation. Int Urol Nephrol. 2012;44:1907-1909.
- Magro CM. Associated ANCA positive vasculitis. The Dermatologist. 2015;23(7). http://www.the-dermatologist.com/content/associated-anca-positive-vasculitis. Accessed January 30, 2020.
- Gao Y, Zhao MH. Review article: Drug-induced anti-neutrophil cytoplasmic antibody-associated vasculitis. Nephrology (Carlton). 2009;14:33-41.
- Grau RG. Drug-induced vasculitis: new insights and a changing lineup of suspects. Curr Rheumatol Rep. 2015;17:71.
- Sangala N, Lee RW, Horsfield C, et al. Combined ANCA-associated vasculitis and lupus syndrome following prolonged use of hydralazine: a timely reminder of an old foe. Int Urol Nephrol. 2010;42:503-506.
- Choi HK, Merkel PA, Walker AM, et al. Drug-associated antineutrophil cytoplasmic antibody-positive vasculitis: prevalence among patients with high titers of antimyeloperoxidase antibodies. Arthritis Rheum. 2000;43:405-413.
- Coutinho AE, Chapman KE. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol. 2011;335:2-13.
- Yokogawa N, Vivino FB. Hydralazine-induced autoimmune disease: comparison to idiopathic lupus and ANCA-positive vasculitis. Mod Rheumatol. 2009;19:338-347.
- Agarwal G, Sultan G, Werner SL, et al. Hydralazine induces myeloperoxidase and proteinase 3 anti-neutrophil cytoplasmic antibody vasculitis and leads to pulmonary renal syndrome. Case Rep Nephrol. 2014;2014:868590.
- Keasberry J, Frazier J, Isbel NM, et al. Hydralazine-induced anti-neutrophilic cytoplasmic antibody-positive renal vasculitis presenting with a vasculitic syndrome, acute nephritis and a puzzling skin rash: a case report. J Med Case Rep. 2013;7:20.
- ten Holder SM, Joy MS, Falk RJ. Cutaneous and systemic manifestations of drug-induced vasculitis. Ann Pharmacother. 2002;36:130-147.
- Namas R, Rubin B, Adwar W, et al. A challenging twist in pulmonary renal syndrome. Case Rep Rheumatol. 2014;2014:516362.
- Dobre M, Wish J, Negrea L. Hydralazine-induced ANCA-positive pauci-immune glomerulonephritis. Ren Fail. 2009;31:745-748.
- Hogan JJ, Markowitz GS, Radhakrishnan J. Drug-induced glomerular disease: immune-mediated injury. Clin J Am Soc Nephrol. 2015;10:1300-1310.
- Radic M, Martinovic Kaliterna D, Radic J. Drug-induced vasculitis: a clinical and pathological review. Neth J Med. 2012;70:12-17.
- Babar F, Posner JN, Obah EA. Hydralazine-induced pauci-immune glomerulonephritis: intriguing case series misleading diagnoses. J Community Hosp Intern Med Perspect. 2016;6:30632.
- Marina VP, Malhotra D, Kaw D. Hydralazine-induced ANCA vasculitis with pulmonary renal syndrome: a rare clinical presentation. Int Urol Nephrol. 2012;44:1907-1909.
- Magro CM. Associated ANCA positive vasculitis. The Dermatologist. 2015;23(7). http://www.the-dermatologist.com/content/associated-anca-positive-vasculitis. Accessed January 30, 2020.
- Gao Y, Zhao MH. Review article: Drug-induced anti-neutrophil cytoplasmic antibody-associated vasculitis. Nephrology (Carlton). 2009;14:33-41.
- Grau RG. Drug-induced vasculitis: new insights and a changing lineup of suspects. Curr Rheumatol Rep. 2015;17:71.
- Sangala N, Lee RW, Horsfield C, et al. Combined ANCA-associated vasculitis and lupus syndrome following prolonged use of hydralazine: a timely reminder of an old foe. Int Urol Nephrol. 2010;42:503-506.
- Choi HK, Merkel PA, Walker AM, et al. Drug-associated antineutrophil cytoplasmic antibody-positive vasculitis: prevalence among patients with high titers of antimyeloperoxidase antibodies. Arthritis Rheum. 2000;43:405-413.
- Coutinho AE, Chapman KE. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol. 2011;335:2-13.
Practice Points
- Hydralazine-induced antineutrophil cytoplasmic antibody vasculitis (HIAV) is a rare side effect of hydralazine treatment and can have notable morbidity and mortality.
- Incidence and prevalence of HIAV is unclear due to its rarity, but risk factors that have been identified are older age, a cumulative dose of 100 g of hydralazine at the time of presentation, female sex, thyroid disease, HLA-DR4 genotypes, slow hepatic acetylation, and the null gene for C4.
- Symptoms of HIAV can include fever, malaise, arthralgia, weight loss, or even involvement of organs such as the kidneys and lungs.
- If recognized early, cessation of hydralazine and supportive therapy generally are sufficient; however, severe cases may need management with high-dose corticosteroids, rituximab, and even plasmapheresis.
Baby’s Got Back Rash
ANSWER
The correct answer is psoriasis vulgaris (choice “a”).
DISCUSSION
At least 30% of patients with psoriasis have a family history of the disease—a meaningful clue in developing a differential. Besides asking about the history, always look for corroborating signs in areas where the disease is commonly seen (eg, the fingernails). In this case, further corroboration was provided by the history of illness at the time of the rash’s onset; what was initially strep-driven guttate psoriasis morphed into full-blown psoriasis vulgaris.
The heavy scales, with their salmon-pink base, tipped the scales in favor of psoriasis as the diagnosis. The pinpoint bleeding (known as the Auspitz sign), although not pathognomic for psoriasis, is certainly suggestive of it.
In adults, these findings would probably have been sufficient to settle on psoriasis. But before labeling a young child with a serious, lifelong diagnosis, it was necessary to be sure. For one thing, advanced psoriasis is very unusual in children as young as this patient, and for another, treatment would likely be problematic. Fortunately for clarity’s sake, the biopsy was consistent with psoriasis and inconsistent with the other items in the differential.
TREATMENT
The patient was prescribed a topical steroid cream to apply every other day, alternating with vitamin D–derived ointment. In addition, he was advised to increase his exposure to natural sunlight. Phototherapy with narrow-band ultraviolet light B would be a superior option, but his family lives too far from the clinic to make 3 roundtrips per week for such treatment.
If these measures fail, a biologic agent may be appropriate. Unfortunately, the patient’s insurance carrier requires the failure of several other modalities before it will approve use of such therapy.
ANSWER
The correct answer is psoriasis vulgaris (choice “a”).
DISCUSSION
At least 30% of patients with psoriasis have a family history of the disease—a meaningful clue in developing a differential. Besides asking about the history, always look for corroborating signs in areas where the disease is commonly seen (eg, the fingernails). In this case, further corroboration was provided by the history of illness at the time of the rash’s onset; what was initially strep-driven guttate psoriasis morphed into full-blown psoriasis vulgaris.
The heavy scales, with their salmon-pink base, tipped the scales in favor of psoriasis as the diagnosis. The pinpoint bleeding (known as the Auspitz sign), although not pathognomic for psoriasis, is certainly suggestive of it.
In adults, these findings would probably have been sufficient to settle on psoriasis. But before labeling a young child with a serious, lifelong diagnosis, it was necessary to be sure. For one thing, advanced psoriasis is very unusual in children as young as this patient, and for another, treatment would likely be problematic. Fortunately for clarity’s sake, the biopsy was consistent with psoriasis and inconsistent with the other items in the differential.
TREATMENT
The patient was prescribed a topical steroid cream to apply every other day, alternating with vitamin D–derived ointment. In addition, he was advised to increase his exposure to natural sunlight. Phototherapy with narrow-band ultraviolet light B would be a superior option, but his family lives too far from the clinic to make 3 roundtrips per week for such treatment.
If these measures fail, a biologic agent may be appropriate. Unfortunately, the patient’s insurance carrier requires the failure of several other modalities before it will approve use of such therapy.
ANSWER
The correct answer is psoriasis vulgaris (choice “a”).
DISCUSSION
At least 30% of patients with psoriasis have a family history of the disease—a meaningful clue in developing a differential. Besides asking about the history, always look for corroborating signs in areas where the disease is commonly seen (eg, the fingernails). In this case, further corroboration was provided by the history of illness at the time of the rash’s onset; what was initially strep-driven guttate psoriasis morphed into full-blown psoriasis vulgaris.
The heavy scales, with their salmon-pink base, tipped the scales in favor of psoriasis as the diagnosis. The pinpoint bleeding (known as the Auspitz sign), although not pathognomic for psoriasis, is certainly suggestive of it.
In adults, these findings would probably have been sufficient to settle on psoriasis. But before labeling a young child with a serious, lifelong diagnosis, it was necessary to be sure. For one thing, advanced psoriasis is very unusual in children as young as this patient, and for another, treatment would likely be problematic. Fortunately for clarity’s sake, the biopsy was consistent with psoriasis and inconsistent with the other items in the differential.
TREATMENT
The patient was prescribed a topical steroid cream to apply every other day, alternating with vitamin D–derived ointment. In addition, he was advised to increase his exposure to natural sunlight. Phototherapy with narrow-band ultraviolet light B would be a superior option, but his family lives too far from the clinic to make 3 roundtrips per week for such treatment.
If these measures fail, a biologic agent may be appropriate. Unfortunately, the patient’s insurance carrier requires the failure of several other modalities before it will approve use of such therapy.
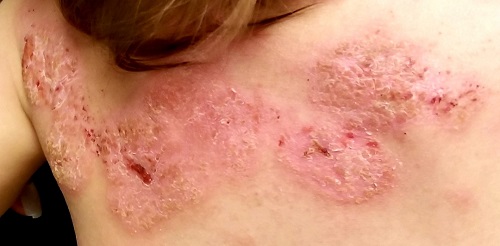
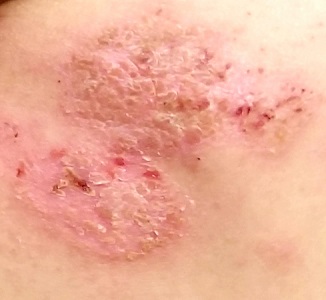
Several months ago, a rash of numerous small, red, scaly papules and patches manifested on this 3-year-old boy’s back and shoulders. At the time, he had been ill for about a week, and his primary care provider diagnosed chickenpox—even though the child had been immunized.
Although the patient’s health soon improved, the appearance of the rash worsened. Treatment with various products—including calamine lotion, OTC tolnaftate and miconazole, and a 2-week course of oral antibiotics—was of no help. Finally, the patient was referred to dermatology.
Family history is positive for psoriasis. However, the parents are quick to note that the boy’s rash appears far different from that of affected family members, and previous providers have dismissed this diagnosis from the differential. There is no family or personal history of atopy.
Examination reveals a dense papulosquamous rash mainly confined to the child’s back and posterior shoulders (the area over the scapula). No other areas are similarly affected, but 1 fingernail is mildly pitted.
A #10 blade lifts the edge of one of the scales gently (and painlessly) until there is pinpoint bleeding from 2 tiny foci. A 5-mm full-thickness punch biopsy with primary closure shows marked parakeratosis, collections of neutrophils on the crests of dermal papillae, and fusing of rete ridges, which effectively obscure the normal wave-like pattern of the dermoepidermal junction.
Weight bias against teens: Understand it and combat it
NEW ORLEANS – and support your patients regardless of weight.
Rebecca Puhl, PhD, a deputy director of the Rudd Center for Food Policy and Obesity at the University of Connecticut, Hartford, said that weight-based discrimination can occur whatever a person’s size or body shape, but it’s most often targeted at youth who are overweight or obese.
These children and teens commonly face teasing, harassment, cyberbullying, physical aggression, and social bullying from peers, coaches, teachers, and even parents, Dr. Puhl told attendees at the annual meeting of the American Academy of Pediatrics.
Common beliefs about overweight people – that they have little self discipline or poor eating and activity habits – only perpetuate stereotypes, she said. Common stereotypes are that people with obesity are noncompliant, lazy, sloppy, unsuccessful, unintelligent, dishonest, and awkward.
And health professionals of every type have been found to harbor these biases. In one study of more than 4,000 first-year medical students, well over half the respondents revealed explicit (74%) and implicit (67%) weight bias (Obesity. 2014 Apr;22[4]:1201-8). The study also found that explicit weight bias was stronger than explicit bias against blacks, Hispanics, LGBTQ people, and poor people.
Know the effects of weight stigma
Far from a minor issue, the discrimination that begins in childhood against those who are overweight can have a long-lasting impact on their future prospects and mental health. Being overweight is overwhelmingly cited as the most common reason for bullying (Pediatr Obes. 2016 Aug;11[4]:241-50). Dr. Puhl described to attendees how weight bias shifts throughout a lifetime, beginning as early as preschool. In childhood, the stereotypes about being overweight worsen, and the teasing and bullying increase. By adolescence, this treatment affects teens’ psychological, social, and physical well-being. It then translates in adulthood into reduced opportunities in employment and education, and poorer access and treatment in health care.
The mental distress caused by weight bullying often takes the form of depression, anxiety, and substance use, Dr. Puhl said, and children’s academic success can be hampered by bullying about their weight. One study found a higher risk of poor grades and school avoidance with each additional teasing incident (J Youth Adolesc. 2012 Jan;41[1]:27-40).
Weight stigma also can contribute to more weight gain, obesity, and lower physical activity levels. Maladaptive eating behaviors can result from weight stigmatization as well: binge eating, emotional eating, increased consumption in general, and other eating disorders. Severe binge eating is 80% more likely among teens who are bullied about their weight, Dr. Puhl said, and the risk increases with increased frequency and types of bullying.
Children who are teased about their weight often become less willing to engage in physical activity, she noted. They may skip gym class, feel less competent about physical activity, and end up enjoying sports participation less.
Further, sexual- and gender-minority youth report high rates of weight-related teasing from friends and family regardless of their body mass index (BMI) percentile, Dr. Puhl emphasized. Researchers have found bullying about weight in this population linked to dieting, difficulty sleeping, high stress levels, binge drinking, smoking, and marijuana use.
Know how to combat weight bias
Dr. Puhl described strategies for reducing weight bias based on clinical practice recommendations in the American Academy of Pediatrics’ policy statement entitled “Stigma Experienced by Children and Adolescents With Obesity” (Pediatrics. 2017 Dec;140[6]:e20173034).
Be aware. Consider how personal assumptions and attitudes about weight can affect your body language, tone of voice, facial expression, gestures, eye contact (or lack thereof), and spatial distance from the patient.
Recognizing the biological, genetic, and environmental causes of obesity can reduce stigma and improve understanding of the complexity of obesity etiology. It’s also important that you help parents understand this complexity and the negative impact of weight stigma.
Consider language and word choice. “Carefully consider language that might unintentionally communicate bias, blame, or negative judgment,” Dr. Puhl told attendees. “Use language that is supportive and empowering.”
Terms such as “unhealthy weight” and “high BMI” are less stigmatizing than “fat” and “morbidly obese” to parents, she said, and research has found nearly a quarter of parents would avoid future doctor appointments if their child’s doctor used stigmatizing terms to discuss weight (Pediatrics. 2011 Oct;128[4]:e786-93).
Teens themselves may have diverse preferences for the language used. Start by asking: “Could we talk about your weight today?” and then follow up by directly asking, “what words would you feel most comfortable with as we talk about your weight?”
Person-first language – such as “person with obesity” instead of “obese person” also is important to reducing stigma, she said.
It’s normal for you to feel uneasy about bringing up weight with patients, so Dr. Puhl recommended you practice dialogue out loud.
“Acknowledge your strengths,” she said. “You already have the skills and experience of engaging in difficult conversations with patients and families on a range of other health issues,” so apply that in this context as well.
Screen for negative experiences that could indicate weight-based bullying. These could include teasing and bullying, low self-esteem, poor school performance, depression, and anxiety.
“Remember that weight-based victimization can occur at diverse body sizes, not just in youth with obesity,” Dr. Puhl said. If you discover your patient is experiencing weight-related bullying, determine whether they have a support system in place and whether a mental health referral is appropriate. Provide or refer for behavior change counseling with motivational interviewing and patient-centered, empathic approaches. Parents should be aware of the issue and should contact the child’s teachers and school administration to help address it.
But before you do that, keep in mind that it’s not just peers doing the bullying. According to a study of teens with obesity enrolled in a national weight-loss camp, 37% of teen participants in 2012 said that their parents bully them (Pediatrics. 2013 Jan;131[1]:e1-9).
You should assess whether family interactions or the parents’ own history with weight is involved. If parents make disparaging comments about their child’s weight, “use this as an opportunity to model appropriate language and educate parents about weight bias,” Dr. Puhl said.
It’s also important to realize that parents themselves often are frustrated, so critical comments about their language or approach can backfire, she warned. Instead, help parents understand how to create a home setting that encourages healthy food choices, praises children for healthy decision making, and models positive health behaviors. It is key for them to focus on improving their children’s health behaviors rather than focusing on weight.
And before you contact the school, remember teachers also are common perpetrators of weight stigma, Dr. Puhl noted. She gave as an example a study in which investigators assessed 133 teachers’ perceptions of middle or high school students’ abilities based on photos that had been digitally altered to show each girl both as average weight or as overweight. Each photo was associated with an essay specifically chosen because it was neither particularly good nor bad (Brit J Educ Psychol. 2019 Oct 26. doi: 10.1111/bjep.12322). The teachers judged the essays believed to be submitted by overweight girls to be “similar in structural quality,” but they gave the overweight girls lower grades than the average-weight girls. They also indicated that they considered the overweight girls “put forth more effort, needed more remedial assistance, and had lower overall grades in school.” The teachers also rated other teachers’ weight bias to be at a low level, and their own weight bias to be “significantly lower” than the others.
Assess your clinical environment. Be aware of your clinical environment and whether it meets the needs of youth with diverse body sizes. That includes having a range of sturdy armless seating options and adequately sized doorways, hallways, and restrooms. You also should have beds, wheelchairs, and exam tables with adequate weight capacity. Also check that you have supplies, such as robes or blood pressure cuffs, on hand for a variety of body sizes.
NEW ORLEANS – and support your patients regardless of weight.
Rebecca Puhl, PhD, a deputy director of the Rudd Center for Food Policy and Obesity at the University of Connecticut, Hartford, said that weight-based discrimination can occur whatever a person’s size or body shape, but it’s most often targeted at youth who are overweight or obese.
These children and teens commonly face teasing, harassment, cyberbullying, physical aggression, and social bullying from peers, coaches, teachers, and even parents, Dr. Puhl told attendees at the annual meeting of the American Academy of Pediatrics.
Common beliefs about overweight people – that they have little self discipline or poor eating and activity habits – only perpetuate stereotypes, she said. Common stereotypes are that people with obesity are noncompliant, lazy, sloppy, unsuccessful, unintelligent, dishonest, and awkward.
And health professionals of every type have been found to harbor these biases. In one study of more than 4,000 first-year medical students, well over half the respondents revealed explicit (74%) and implicit (67%) weight bias (Obesity. 2014 Apr;22[4]:1201-8). The study also found that explicit weight bias was stronger than explicit bias against blacks, Hispanics, LGBTQ people, and poor people.
Know the effects of weight stigma
Far from a minor issue, the discrimination that begins in childhood against those who are overweight can have a long-lasting impact on their future prospects and mental health. Being overweight is overwhelmingly cited as the most common reason for bullying (Pediatr Obes. 2016 Aug;11[4]:241-50). Dr. Puhl described to attendees how weight bias shifts throughout a lifetime, beginning as early as preschool. In childhood, the stereotypes about being overweight worsen, and the teasing and bullying increase. By adolescence, this treatment affects teens’ psychological, social, and physical well-being. It then translates in adulthood into reduced opportunities in employment and education, and poorer access and treatment in health care.
The mental distress caused by weight bullying often takes the form of depression, anxiety, and substance use, Dr. Puhl said, and children’s academic success can be hampered by bullying about their weight. One study found a higher risk of poor grades and school avoidance with each additional teasing incident (J Youth Adolesc. 2012 Jan;41[1]:27-40).
Weight stigma also can contribute to more weight gain, obesity, and lower physical activity levels. Maladaptive eating behaviors can result from weight stigmatization as well: binge eating, emotional eating, increased consumption in general, and other eating disorders. Severe binge eating is 80% more likely among teens who are bullied about their weight, Dr. Puhl said, and the risk increases with increased frequency and types of bullying.
Children who are teased about their weight often become less willing to engage in physical activity, she noted. They may skip gym class, feel less competent about physical activity, and end up enjoying sports participation less.
Further, sexual- and gender-minority youth report high rates of weight-related teasing from friends and family regardless of their body mass index (BMI) percentile, Dr. Puhl emphasized. Researchers have found bullying about weight in this population linked to dieting, difficulty sleeping, high stress levels, binge drinking, smoking, and marijuana use.
Know how to combat weight bias
Dr. Puhl described strategies for reducing weight bias based on clinical practice recommendations in the American Academy of Pediatrics’ policy statement entitled “Stigma Experienced by Children and Adolescents With Obesity” (Pediatrics. 2017 Dec;140[6]:e20173034).
Be aware. Consider how personal assumptions and attitudes about weight can affect your body language, tone of voice, facial expression, gestures, eye contact (or lack thereof), and spatial distance from the patient.
Recognizing the biological, genetic, and environmental causes of obesity can reduce stigma and improve understanding of the complexity of obesity etiology. It’s also important that you help parents understand this complexity and the negative impact of weight stigma.
Consider language and word choice. “Carefully consider language that might unintentionally communicate bias, blame, or negative judgment,” Dr. Puhl told attendees. “Use language that is supportive and empowering.”
Terms such as “unhealthy weight” and “high BMI” are less stigmatizing than “fat” and “morbidly obese” to parents, she said, and research has found nearly a quarter of parents would avoid future doctor appointments if their child’s doctor used stigmatizing terms to discuss weight (Pediatrics. 2011 Oct;128[4]:e786-93).
Teens themselves may have diverse preferences for the language used. Start by asking: “Could we talk about your weight today?” and then follow up by directly asking, “what words would you feel most comfortable with as we talk about your weight?”
Person-first language – such as “person with obesity” instead of “obese person” also is important to reducing stigma, she said.
It’s normal for you to feel uneasy about bringing up weight with patients, so Dr. Puhl recommended you practice dialogue out loud.
“Acknowledge your strengths,” she said. “You already have the skills and experience of engaging in difficult conversations with patients and families on a range of other health issues,” so apply that in this context as well.
Screen for negative experiences that could indicate weight-based bullying. These could include teasing and bullying, low self-esteem, poor school performance, depression, and anxiety.
“Remember that weight-based victimization can occur at diverse body sizes, not just in youth with obesity,” Dr. Puhl said. If you discover your patient is experiencing weight-related bullying, determine whether they have a support system in place and whether a mental health referral is appropriate. Provide or refer for behavior change counseling with motivational interviewing and patient-centered, empathic approaches. Parents should be aware of the issue and should contact the child’s teachers and school administration to help address it.
But before you do that, keep in mind that it’s not just peers doing the bullying. According to a study of teens with obesity enrolled in a national weight-loss camp, 37% of teen participants in 2012 said that their parents bully them (Pediatrics. 2013 Jan;131[1]:e1-9).
You should assess whether family interactions or the parents’ own history with weight is involved. If parents make disparaging comments about their child’s weight, “use this as an opportunity to model appropriate language and educate parents about weight bias,” Dr. Puhl said.
It’s also important to realize that parents themselves often are frustrated, so critical comments about their language or approach can backfire, she warned. Instead, help parents understand how to create a home setting that encourages healthy food choices, praises children for healthy decision making, and models positive health behaviors. It is key for them to focus on improving their children’s health behaviors rather than focusing on weight.
And before you contact the school, remember teachers also are common perpetrators of weight stigma, Dr. Puhl noted. She gave as an example a study in which investigators assessed 133 teachers’ perceptions of middle or high school students’ abilities based on photos that had been digitally altered to show each girl both as average weight or as overweight. Each photo was associated with an essay specifically chosen because it was neither particularly good nor bad (Brit J Educ Psychol. 2019 Oct 26. doi: 10.1111/bjep.12322). The teachers judged the essays believed to be submitted by overweight girls to be “similar in structural quality,” but they gave the overweight girls lower grades than the average-weight girls. They also indicated that they considered the overweight girls “put forth more effort, needed more remedial assistance, and had lower overall grades in school.” The teachers also rated other teachers’ weight bias to be at a low level, and their own weight bias to be “significantly lower” than the others.
Assess your clinical environment. Be aware of your clinical environment and whether it meets the needs of youth with diverse body sizes. That includes having a range of sturdy armless seating options and adequately sized doorways, hallways, and restrooms. You also should have beds, wheelchairs, and exam tables with adequate weight capacity. Also check that you have supplies, such as robes or blood pressure cuffs, on hand for a variety of body sizes.
NEW ORLEANS – and support your patients regardless of weight.
Rebecca Puhl, PhD, a deputy director of the Rudd Center for Food Policy and Obesity at the University of Connecticut, Hartford, said that weight-based discrimination can occur whatever a person’s size or body shape, but it’s most often targeted at youth who are overweight or obese.
These children and teens commonly face teasing, harassment, cyberbullying, physical aggression, and social bullying from peers, coaches, teachers, and even parents, Dr. Puhl told attendees at the annual meeting of the American Academy of Pediatrics.
Common beliefs about overweight people – that they have little self discipline or poor eating and activity habits – only perpetuate stereotypes, she said. Common stereotypes are that people with obesity are noncompliant, lazy, sloppy, unsuccessful, unintelligent, dishonest, and awkward.
And health professionals of every type have been found to harbor these biases. In one study of more than 4,000 first-year medical students, well over half the respondents revealed explicit (74%) and implicit (67%) weight bias (Obesity. 2014 Apr;22[4]:1201-8). The study also found that explicit weight bias was stronger than explicit bias against blacks, Hispanics, LGBTQ people, and poor people.
Know the effects of weight stigma
Far from a minor issue, the discrimination that begins in childhood against those who are overweight can have a long-lasting impact on their future prospects and mental health. Being overweight is overwhelmingly cited as the most common reason for bullying (Pediatr Obes. 2016 Aug;11[4]:241-50). Dr. Puhl described to attendees how weight bias shifts throughout a lifetime, beginning as early as preschool. In childhood, the stereotypes about being overweight worsen, and the teasing and bullying increase. By adolescence, this treatment affects teens’ psychological, social, and physical well-being. It then translates in adulthood into reduced opportunities in employment and education, and poorer access and treatment in health care.
The mental distress caused by weight bullying often takes the form of depression, anxiety, and substance use, Dr. Puhl said, and children’s academic success can be hampered by bullying about their weight. One study found a higher risk of poor grades and school avoidance with each additional teasing incident (J Youth Adolesc. 2012 Jan;41[1]:27-40).
Weight stigma also can contribute to more weight gain, obesity, and lower physical activity levels. Maladaptive eating behaviors can result from weight stigmatization as well: binge eating, emotional eating, increased consumption in general, and other eating disorders. Severe binge eating is 80% more likely among teens who are bullied about their weight, Dr. Puhl said, and the risk increases with increased frequency and types of bullying.
Children who are teased about their weight often become less willing to engage in physical activity, she noted. They may skip gym class, feel less competent about physical activity, and end up enjoying sports participation less.
Further, sexual- and gender-minority youth report high rates of weight-related teasing from friends and family regardless of their body mass index (BMI) percentile, Dr. Puhl emphasized. Researchers have found bullying about weight in this population linked to dieting, difficulty sleeping, high stress levels, binge drinking, smoking, and marijuana use.
Know how to combat weight bias
Dr. Puhl described strategies for reducing weight bias based on clinical practice recommendations in the American Academy of Pediatrics’ policy statement entitled “Stigma Experienced by Children and Adolescents With Obesity” (Pediatrics. 2017 Dec;140[6]:e20173034).
Be aware. Consider how personal assumptions and attitudes about weight can affect your body language, tone of voice, facial expression, gestures, eye contact (or lack thereof), and spatial distance from the patient.
Recognizing the biological, genetic, and environmental causes of obesity can reduce stigma and improve understanding of the complexity of obesity etiology. It’s also important that you help parents understand this complexity and the negative impact of weight stigma.
Consider language and word choice. “Carefully consider language that might unintentionally communicate bias, blame, or negative judgment,” Dr. Puhl told attendees. “Use language that is supportive and empowering.”
Terms such as “unhealthy weight” and “high BMI” are less stigmatizing than “fat” and “morbidly obese” to parents, she said, and research has found nearly a quarter of parents would avoid future doctor appointments if their child’s doctor used stigmatizing terms to discuss weight (Pediatrics. 2011 Oct;128[4]:e786-93).
Teens themselves may have diverse preferences for the language used. Start by asking: “Could we talk about your weight today?” and then follow up by directly asking, “what words would you feel most comfortable with as we talk about your weight?”
Person-first language – such as “person with obesity” instead of “obese person” also is important to reducing stigma, she said.
It’s normal for you to feel uneasy about bringing up weight with patients, so Dr. Puhl recommended you practice dialogue out loud.
“Acknowledge your strengths,” she said. “You already have the skills and experience of engaging in difficult conversations with patients and families on a range of other health issues,” so apply that in this context as well.
Screen for negative experiences that could indicate weight-based bullying. These could include teasing and bullying, low self-esteem, poor school performance, depression, and anxiety.
“Remember that weight-based victimization can occur at diverse body sizes, not just in youth with obesity,” Dr. Puhl said. If you discover your patient is experiencing weight-related bullying, determine whether they have a support system in place and whether a mental health referral is appropriate. Provide or refer for behavior change counseling with motivational interviewing and patient-centered, empathic approaches. Parents should be aware of the issue and should contact the child’s teachers and school administration to help address it.
But before you do that, keep in mind that it’s not just peers doing the bullying. According to a study of teens with obesity enrolled in a national weight-loss camp, 37% of teen participants in 2012 said that their parents bully them (Pediatrics. 2013 Jan;131[1]:e1-9).
You should assess whether family interactions or the parents’ own history with weight is involved. If parents make disparaging comments about their child’s weight, “use this as an opportunity to model appropriate language and educate parents about weight bias,” Dr. Puhl said.
It’s also important to realize that parents themselves often are frustrated, so critical comments about their language or approach can backfire, she warned. Instead, help parents understand how to create a home setting that encourages healthy food choices, praises children for healthy decision making, and models positive health behaviors. It is key for them to focus on improving their children’s health behaviors rather than focusing on weight.
And before you contact the school, remember teachers also are common perpetrators of weight stigma, Dr. Puhl noted. She gave as an example a study in which investigators assessed 133 teachers’ perceptions of middle or high school students’ abilities based on photos that had been digitally altered to show each girl both as average weight or as overweight. Each photo was associated with an essay specifically chosen because it was neither particularly good nor bad (Brit J Educ Psychol. 2019 Oct 26. doi: 10.1111/bjep.12322). The teachers judged the essays believed to be submitted by overweight girls to be “similar in structural quality,” but they gave the overweight girls lower grades than the average-weight girls. They also indicated that they considered the overweight girls “put forth more effort, needed more remedial assistance, and had lower overall grades in school.” The teachers also rated other teachers’ weight bias to be at a low level, and their own weight bias to be “significantly lower” than the others.
Assess your clinical environment. Be aware of your clinical environment and whether it meets the needs of youth with diverse body sizes. That includes having a range of sturdy armless seating options and adequately sized doorways, hallways, and restrooms. You also should have beds, wheelchairs, and exam tables with adequate weight capacity. Also check that you have supplies, such as robes or blood pressure cuffs, on hand for a variety of body sizes.
EXPERT ANALYSIS FROM AAP 19
Consider PET/CT when infectious source is a puzzler
CHICAGO – Dual positron emission tomography-computed tomography (PET/CT) scans changed the treatment course of nearly half of patients whose scans were positive for infection. In a single-center systematic review of 18fluorodeoxyglucose (FDG)–PET/CT scans, 55 of the 138 scans (40%) changed clinical management.
Presenting the findings at the annual meeting of the Radiological Society of North America, Benjamin Viglianti, MD, PhD, said that PET/CT had particular utility in cases of bacteremia and endocarditis, in which the scans changed treatment in 46% of those cases.
Dr. Viglianti, a radiologist at the University of Michigan, Ann Arbor, explained that medical student and first author Anitha Menon, himself, and their collaborators deliberately used a broad definition of clinical management change. The management course was considered to change not only if an unknown infection site was discovered or if a new intervention was initiated after the scan, but also if antibiotic choice or duration was changed or an additional specialty was consulted.
Scans were included in the study if an infectious etiology was found in the scan and if the patient received an infectious disease consult. Bacteremia and endocarditis were the most frequent indications for scans and also the indications for which management was most frequently changed. When a vascular cause was the indication for the scan, management changed 41% of the time. For fevers of unknown origin, the scan changed management in 30% of the cases, while for osteomyelitis, management was changed for 28% of patients.
The investigators identified several broad themes from their review that pointed toward when clinicians might consider FDG-PET/CT imaging in infectious disease management.
The first, said Dr. Viglianti, was that “for patients with suspected vascular graft infection, PET/CT using FDG may be a good first-choice imaging modality.” He pointed to an illustrative case of a patient who was 1 month out from open repair of a thoracoabdominal aortic aneurysm. The patient had abdominal pain, epigastric tenderness and nausea, as well as an erythematous incision site. A CT scan just revealed an abdominal fluid collection, but the PET/CT scan showed radiotracer uptake at the prior repair site, indicating infection.
For patients with bacteremia, the investigators judged that FDG-PET/CT might be particularly useful in patients who have a graft, prosthetic valve, or cardiac device. Here, Dr. Viglianti and his collaborators highlighted the scan of a woman with DiGeorge syndrome who had received aortic root replacement for truncus arteriosis. She had been found to have persistent enterococcal bacteremia at high levels, but had been symptom free. To take a close look at the suspected infectious nidus, a transesophageal echocardiogram had been obtained, but this study didn’t turn up any clear masses or vegetations. The PET/CT scan, though, revealed avid FDG uptake in the area of the prosthesis.
Management course was not likely to be changed for patients with fever of unknown origin, but the investigators did note that whole-body PET/CT was useful to distinguish infectious etiologies from hematologic and oncologic processes. Their review included a patient who had Crohn’s disease and fever, myalgias, and upper abdominal pain, as well as liver enzyme elevation. The PET/CT showed radiotracer uptake within the spleen, which was enlarged. The scan also showed bone marrow uptake; these findings pointed toward hemophagocytic lymphohistiocytosis rather than an infectious etiology.
For osteomyelitis, said Dr. Viglianti, FDG-PET may have limited utility; it might be most useful when MRI is contraindicated. Within the study population, the investigators identified a patient who had chills and fever along with focal tenderness over the lumbar spine in the context of recent pyelonephritis of a graft kidney. Here, MRI findings were suspicious for osteomyelitis and diskitis, and the FDG uptake at the L4-L5 vertebral levels confirmed the MRI results.
When a patient with a prosthetic valve is suspected of having endocarditis, “cardiac PET/CT may be of high diagnostic value,” said Dr. Viglianti. For patients with endocarditis of native valves, though, a full-body FDG-PET/CT scan may spot septic emboli. A patient identified in the investigators’ review had been admitted for methicillin-resistant Staphylococcus aureus endocarditis. The patient, who had a history of intravenous drug use, received a transesophageal echocardiogram that found severe tricuspid valve regurgitation and vegetations. The whole-body PET/CT scan, though, revealed avid uptake in both buttocks, as well as thigh, ankle and calf muscles – a pattern “suspicious for infectious myositis,” said the researchers.
In discussion during the poster session, Dr. Viglianti said that, although reimbursement for PET/CT scans for infectious etiologies might not be feasible, it can still be a reasonable and even cost-effective choice. At his institution, he said, the requisite radioisotope is made in-house, twice daily, so it’s relatively easy to arrange scans. Since PET/CT scans can be acquired relatively quickly and there’s no delay while waiting for radiotracer uptake, clinical decisions can be made more quickly than when waiting for bone uptake for a technetium-99 scan, he said. This can have the effect of saving a night of hospitalization in many cases.
Dr. Viglianti and Ms. Menon reported that they had no relevant conflicts of interest. No outside sources of funding were reported.
SOURCE: Menon A et al. RSNA 2019, Abstract NM203-SDSUB1.
CHICAGO – Dual positron emission tomography-computed tomography (PET/CT) scans changed the treatment course of nearly half of patients whose scans were positive for infection. In a single-center systematic review of 18fluorodeoxyglucose (FDG)–PET/CT scans, 55 of the 138 scans (40%) changed clinical management.
Presenting the findings at the annual meeting of the Radiological Society of North America, Benjamin Viglianti, MD, PhD, said that PET/CT had particular utility in cases of bacteremia and endocarditis, in which the scans changed treatment in 46% of those cases.
Dr. Viglianti, a radiologist at the University of Michigan, Ann Arbor, explained that medical student and first author Anitha Menon, himself, and their collaborators deliberately used a broad definition of clinical management change. The management course was considered to change not only if an unknown infection site was discovered or if a new intervention was initiated after the scan, but also if antibiotic choice or duration was changed or an additional specialty was consulted.
Scans were included in the study if an infectious etiology was found in the scan and if the patient received an infectious disease consult. Bacteremia and endocarditis were the most frequent indications for scans and also the indications for which management was most frequently changed. When a vascular cause was the indication for the scan, management changed 41% of the time. For fevers of unknown origin, the scan changed management in 30% of the cases, while for osteomyelitis, management was changed for 28% of patients.
The investigators identified several broad themes from their review that pointed toward when clinicians might consider FDG-PET/CT imaging in infectious disease management.
The first, said Dr. Viglianti, was that “for patients with suspected vascular graft infection, PET/CT using FDG may be a good first-choice imaging modality.” He pointed to an illustrative case of a patient who was 1 month out from open repair of a thoracoabdominal aortic aneurysm. The patient had abdominal pain, epigastric tenderness and nausea, as well as an erythematous incision site. A CT scan just revealed an abdominal fluid collection, but the PET/CT scan showed radiotracer uptake at the prior repair site, indicating infection.
For patients with bacteremia, the investigators judged that FDG-PET/CT might be particularly useful in patients who have a graft, prosthetic valve, or cardiac device. Here, Dr. Viglianti and his collaborators highlighted the scan of a woman with DiGeorge syndrome who had received aortic root replacement for truncus arteriosis. She had been found to have persistent enterococcal bacteremia at high levels, but had been symptom free. To take a close look at the suspected infectious nidus, a transesophageal echocardiogram had been obtained, but this study didn’t turn up any clear masses or vegetations. The PET/CT scan, though, revealed avid FDG uptake in the area of the prosthesis.
Management course was not likely to be changed for patients with fever of unknown origin, but the investigators did note that whole-body PET/CT was useful to distinguish infectious etiologies from hematologic and oncologic processes. Their review included a patient who had Crohn’s disease and fever, myalgias, and upper abdominal pain, as well as liver enzyme elevation. The PET/CT showed radiotracer uptake within the spleen, which was enlarged. The scan also showed bone marrow uptake; these findings pointed toward hemophagocytic lymphohistiocytosis rather than an infectious etiology.
For osteomyelitis, said Dr. Viglianti, FDG-PET may have limited utility; it might be most useful when MRI is contraindicated. Within the study population, the investigators identified a patient who had chills and fever along with focal tenderness over the lumbar spine in the context of recent pyelonephritis of a graft kidney. Here, MRI findings were suspicious for osteomyelitis and diskitis, and the FDG uptake at the L4-L5 vertebral levels confirmed the MRI results.
When a patient with a prosthetic valve is suspected of having endocarditis, “cardiac PET/CT may be of high diagnostic value,” said Dr. Viglianti. For patients with endocarditis of native valves, though, a full-body FDG-PET/CT scan may spot septic emboli. A patient identified in the investigators’ review had been admitted for methicillin-resistant Staphylococcus aureus endocarditis. The patient, who had a history of intravenous drug use, received a transesophageal echocardiogram that found severe tricuspid valve regurgitation and vegetations. The whole-body PET/CT scan, though, revealed avid uptake in both buttocks, as well as thigh, ankle and calf muscles – a pattern “suspicious for infectious myositis,” said the researchers.
In discussion during the poster session, Dr. Viglianti said that, although reimbursement for PET/CT scans for infectious etiologies might not be feasible, it can still be a reasonable and even cost-effective choice. At his institution, he said, the requisite radioisotope is made in-house, twice daily, so it’s relatively easy to arrange scans. Since PET/CT scans can be acquired relatively quickly and there’s no delay while waiting for radiotracer uptake, clinical decisions can be made more quickly than when waiting for bone uptake for a technetium-99 scan, he said. This can have the effect of saving a night of hospitalization in many cases.
Dr. Viglianti and Ms. Menon reported that they had no relevant conflicts of interest. No outside sources of funding were reported.
SOURCE: Menon A et al. RSNA 2019, Abstract NM203-SDSUB1.
CHICAGO – Dual positron emission tomography-computed tomography (PET/CT) scans changed the treatment course of nearly half of patients whose scans were positive for infection. In a single-center systematic review of 18fluorodeoxyglucose (FDG)–PET/CT scans, 55 of the 138 scans (40%) changed clinical management.
Presenting the findings at the annual meeting of the Radiological Society of North America, Benjamin Viglianti, MD, PhD, said that PET/CT had particular utility in cases of bacteremia and endocarditis, in which the scans changed treatment in 46% of those cases.
Dr. Viglianti, a radiologist at the University of Michigan, Ann Arbor, explained that medical student and first author Anitha Menon, himself, and their collaborators deliberately used a broad definition of clinical management change. The management course was considered to change not only if an unknown infection site was discovered or if a new intervention was initiated after the scan, but also if antibiotic choice or duration was changed or an additional specialty was consulted.
Scans were included in the study if an infectious etiology was found in the scan and if the patient received an infectious disease consult. Bacteremia and endocarditis were the most frequent indications for scans and also the indications for which management was most frequently changed. When a vascular cause was the indication for the scan, management changed 41% of the time. For fevers of unknown origin, the scan changed management in 30% of the cases, while for osteomyelitis, management was changed for 28% of patients.
The investigators identified several broad themes from their review that pointed toward when clinicians might consider FDG-PET/CT imaging in infectious disease management.
The first, said Dr. Viglianti, was that “for patients with suspected vascular graft infection, PET/CT using FDG may be a good first-choice imaging modality.” He pointed to an illustrative case of a patient who was 1 month out from open repair of a thoracoabdominal aortic aneurysm. The patient had abdominal pain, epigastric tenderness and nausea, as well as an erythematous incision site. A CT scan just revealed an abdominal fluid collection, but the PET/CT scan showed radiotracer uptake at the prior repair site, indicating infection.
For patients with bacteremia, the investigators judged that FDG-PET/CT might be particularly useful in patients who have a graft, prosthetic valve, or cardiac device. Here, Dr. Viglianti and his collaborators highlighted the scan of a woman with DiGeorge syndrome who had received aortic root replacement for truncus arteriosis. She had been found to have persistent enterococcal bacteremia at high levels, but had been symptom free. To take a close look at the suspected infectious nidus, a transesophageal echocardiogram had been obtained, but this study didn’t turn up any clear masses or vegetations. The PET/CT scan, though, revealed avid FDG uptake in the area of the prosthesis.
Management course was not likely to be changed for patients with fever of unknown origin, but the investigators did note that whole-body PET/CT was useful to distinguish infectious etiologies from hematologic and oncologic processes. Their review included a patient who had Crohn’s disease and fever, myalgias, and upper abdominal pain, as well as liver enzyme elevation. The PET/CT showed radiotracer uptake within the spleen, which was enlarged. The scan also showed bone marrow uptake; these findings pointed toward hemophagocytic lymphohistiocytosis rather than an infectious etiology.
For osteomyelitis, said Dr. Viglianti, FDG-PET may have limited utility; it might be most useful when MRI is contraindicated. Within the study population, the investigators identified a patient who had chills and fever along with focal tenderness over the lumbar spine in the context of recent pyelonephritis of a graft kidney. Here, MRI findings were suspicious for osteomyelitis and diskitis, and the FDG uptake at the L4-L5 vertebral levels confirmed the MRI results.
When a patient with a prosthetic valve is suspected of having endocarditis, “cardiac PET/CT may be of high diagnostic value,” said Dr. Viglianti. For patients with endocarditis of native valves, though, a full-body FDG-PET/CT scan may spot septic emboli. A patient identified in the investigators’ review had been admitted for methicillin-resistant Staphylococcus aureus endocarditis. The patient, who had a history of intravenous drug use, received a transesophageal echocardiogram that found severe tricuspid valve regurgitation and vegetations. The whole-body PET/CT scan, though, revealed avid uptake in both buttocks, as well as thigh, ankle and calf muscles – a pattern “suspicious for infectious myositis,” said the researchers.
In discussion during the poster session, Dr. Viglianti said that, although reimbursement for PET/CT scans for infectious etiologies might not be feasible, it can still be a reasonable and even cost-effective choice. At his institution, he said, the requisite radioisotope is made in-house, twice daily, so it’s relatively easy to arrange scans. Since PET/CT scans can be acquired relatively quickly and there’s no delay while waiting for radiotracer uptake, clinical decisions can be made more quickly than when waiting for bone uptake for a technetium-99 scan, he said. This can have the effect of saving a night of hospitalization in many cases.
Dr. Viglianti and Ms. Menon reported that they had no relevant conflicts of interest. No outside sources of funding were reported.
SOURCE: Menon A et al. RSNA 2019, Abstract NM203-SDSUB1.
REPORTING FROM RSNA 2019
Older NHL survivors show worse cognitive decline
Older long-term survivors of non-Hodgkin lymphoma (NHL) may have worse cognitive outcomes compared with the noncancer aging population, according to a cross-sectional study.
The findings suggest additional research is needed to better understand cognitive decline in older survivors of NHL.
“The aim of the present study was to examine the difference in cognitive status between a group of long-term older survivors of NHL compared with a group of noncancer controls of the same age,” wrote Domenico La Carpia, MD, of Fondazione ANT Italia Onlus, Florence, Italy, and colleagues.
The researchers conducted a multicenter cross-sectional cohort study involving 63 long-term survivors of NHL and 61 age-matched controls. Their report was published in the Journal of Geriatric Oncology.
Eligible survivors and controls were aged 65 years and older. Among both groups, the mean age of study participants was 74 years, and most survivors were women (58.7%).
While cognitive decline was assessed via standardized neuropsychological testing, the team also evaluated polypharmacy, functional status, and level of multimorbidity in the cohort.
Other clinical data, including the time from complete remission, type of treatment received, and histopathological type of tumor, were collected from patient charts and included in the analysis.
After analysis, the researchers found that NHL survivors had a higher mean number of chronic conditions (3.4 vs. 2.3; P = .003), were receiving more medications (3.4 vs. 2.3; P = .03), and had worse functional status compared with controls.
In addition, survivors had impaired executive functioning compared with control subjects (Trail Making Test B-A, 47.9 vs. 32.1; P = .04), but scores on the Mini Mental State Examination (MMSE) did not differ between the groups.
“A small, statistically significant difference was also observed in verbal memory scores between the two groups,” they reported.
The researchers acknowledged that a key limitation was the cross-sectional nature of the study; hence, causality cannot be inferred from the data.
“Comprehensive geriatric assessment for older cancer survivors is advisable to identify those individuals who are at highest risk of developing disability and to implement tailored early interventions,” they concluded.
No funding sources were reported. The authors reported having no conflicts of interest.
SOURCE: La Carpia D et al. J Geriatr Oncol. 2020 Jan 31. doi: 10.1016/j.jgo.2020.01.007.
Older long-term survivors of non-Hodgkin lymphoma (NHL) may have worse cognitive outcomes compared with the noncancer aging population, according to a cross-sectional study.
The findings suggest additional research is needed to better understand cognitive decline in older survivors of NHL.
“The aim of the present study was to examine the difference in cognitive status between a group of long-term older survivors of NHL compared with a group of noncancer controls of the same age,” wrote Domenico La Carpia, MD, of Fondazione ANT Italia Onlus, Florence, Italy, and colleagues.
The researchers conducted a multicenter cross-sectional cohort study involving 63 long-term survivors of NHL and 61 age-matched controls. Their report was published in the Journal of Geriatric Oncology.
Eligible survivors and controls were aged 65 years and older. Among both groups, the mean age of study participants was 74 years, and most survivors were women (58.7%).
While cognitive decline was assessed via standardized neuropsychological testing, the team also evaluated polypharmacy, functional status, and level of multimorbidity in the cohort.
Other clinical data, including the time from complete remission, type of treatment received, and histopathological type of tumor, were collected from patient charts and included in the analysis.
After analysis, the researchers found that NHL survivors had a higher mean number of chronic conditions (3.4 vs. 2.3; P = .003), were receiving more medications (3.4 vs. 2.3; P = .03), and had worse functional status compared with controls.
In addition, survivors had impaired executive functioning compared with control subjects (Trail Making Test B-A, 47.9 vs. 32.1; P = .04), but scores on the Mini Mental State Examination (MMSE) did not differ between the groups.
“A small, statistically significant difference was also observed in verbal memory scores between the two groups,” they reported.
The researchers acknowledged that a key limitation was the cross-sectional nature of the study; hence, causality cannot be inferred from the data.
“Comprehensive geriatric assessment for older cancer survivors is advisable to identify those individuals who are at highest risk of developing disability and to implement tailored early interventions,” they concluded.
No funding sources were reported. The authors reported having no conflicts of interest.
SOURCE: La Carpia D et al. J Geriatr Oncol. 2020 Jan 31. doi: 10.1016/j.jgo.2020.01.007.
Older long-term survivors of non-Hodgkin lymphoma (NHL) may have worse cognitive outcomes compared with the noncancer aging population, according to a cross-sectional study.
The findings suggest additional research is needed to better understand cognitive decline in older survivors of NHL.
“The aim of the present study was to examine the difference in cognitive status between a group of long-term older survivors of NHL compared with a group of noncancer controls of the same age,” wrote Domenico La Carpia, MD, of Fondazione ANT Italia Onlus, Florence, Italy, and colleagues.
The researchers conducted a multicenter cross-sectional cohort study involving 63 long-term survivors of NHL and 61 age-matched controls. Their report was published in the Journal of Geriatric Oncology.
Eligible survivors and controls were aged 65 years and older. Among both groups, the mean age of study participants was 74 years, and most survivors were women (58.7%).
While cognitive decline was assessed via standardized neuropsychological testing, the team also evaluated polypharmacy, functional status, and level of multimorbidity in the cohort.
Other clinical data, including the time from complete remission, type of treatment received, and histopathological type of tumor, were collected from patient charts and included in the analysis.
After analysis, the researchers found that NHL survivors had a higher mean number of chronic conditions (3.4 vs. 2.3; P = .003), were receiving more medications (3.4 vs. 2.3; P = .03), and had worse functional status compared with controls.
In addition, survivors had impaired executive functioning compared with control subjects (Trail Making Test B-A, 47.9 vs. 32.1; P = .04), but scores on the Mini Mental State Examination (MMSE) did not differ between the groups.
“A small, statistically significant difference was also observed in verbal memory scores between the two groups,” they reported.
The researchers acknowledged that a key limitation was the cross-sectional nature of the study; hence, causality cannot be inferred from the data.
“Comprehensive geriatric assessment for older cancer survivors is advisable to identify those individuals who are at highest risk of developing disability and to implement tailored early interventions,” they concluded.
No funding sources were reported. The authors reported having no conflicts of interest.
SOURCE: La Carpia D et al. J Geriatr Oncol. 2020 Jan 31. doi: 10.1016/j.jgo.2020.01.007.
FROM THE JOURNAL OF GERIATRIC ONCOLOGY
Genetic testing helps avoid false hemoglobinopathy diagnoses in newborns
Confirmatory genetic testing may be useful in the diagnosis of hemoglobinopathies for newborns with an abnormal hemoglobin (Hb) pattern, according to a recent study.
The findings suggest further research is needed to evaluate whether genetic testing programs for newborns could have diagnostic value in the clinical setting.
“We studied a consecutive cohort of newborns with an ‘FSA’ pattern (a suspected diagnosis of HbSbeta+) on the initial newborn screening test,” explained Lisa M. Shook of the University of Cincinnati and colleagues. The results were published in the International Journal of Neonatal Screening.
The retrospective study included a total of 1,151 newborns with an abnormal Hb pattern, 31 of which had an FSA pattern. The newborns were screened for hemoglobinopathies from 2015 to 2018. The findings of the initial newborn screening test (a suspected diagnosis of HbSbeta+) were compared with the diagnosis established using both protein-based and genetic confirmatory testing. Protein-based testing cannot accurately detect several hemoglobinopathies in newborns, especially when beta-thalassemia mutations are involved, according to the authors.
“During this study period, genetic testing was not universally applied in advance; it was used based on clinical suspicion,” the researchers wrote.
Among newborns with an FSA pattern, the mean gestational age was 38.7 weeks. In total, 17 newborns received genetic testing, and 30 had protein-based confirmatory testing.
“In this consecutive cohort of 31 newborns with a suspected diagnosis of HbSbeta+ based on initial newborn screening (an FSA pattern), none actually had HbSbeta+. All had the sickle cell trait (HbAS), instead; that is, we found that an initial FSA pattern was much more likely to indicate a final diagnosis of HbAS than HbSbeta+,” the authors wrote.
This meant that two-thirds of these newborns had a correct diagnosis of HbAS established at 2-4 weeks of age by protein-based confirmatory testing (and confirmed by genetic testing in a subset), but that the remaining one-third still had an incorrect, suspected diagnosis of HbSbeta+. This could lead to unnecessary treatment and testing of infants and incorrect, disease-focused counseling of parents and family members, according to the authors.
Two key limitations of the study were the small sample size and retrospective design.
“Based on this experience in which genetic testing was not universally applied, we now perform simultaneous protein-based and genetic testing as our standard clinical practice,” they concluded.
The study was funded by the National Institutes of Health and the Ohio Department of Health. The authors reported having no conflicts of interest.
SOURCE: Shook LM et al. Int J Neonatal Screen. 2020 Jan 31. doi: 10.3390/ijns6010007
Confirmatory genetic testing may be useful in the diagnosis of hemoglobinopathies for newborns with an abnormal hemoglobin (Hb) pattern, according to a recent study.
The findings suggest further research is needed to evaluate whether genetic testing programs for newborns could have diagnostic value in the clinical setting.
“We studied a consecutive cohort of newborns with an ‘FSA’ pattern (a suspected diagnosis of HbSbeta+) on the initial newborn screening test,” explained Lisa M. Shook of the University of Cincinnati and colleagues. The results were published in the International Journal of Neonatal Screening.
The retrospective study included a total of 1,151 newborns with an abnormal Hb pattern, 31 of which had an FSA pattern. The newborns were screened for hemoglobinopathies from 2015 to 2018. The findings of the initial newborn screening test (a suspected diagnosis of HbSbeta+) were compared with the diagnosis established using both protein-based and genetic confirmatory testing. Protein-based testing cannot accurately detect several hemoglobinopathies in newborns, especially when beta-thalassemia mutations are involved, according to the authors.
“During this study period, genetic testing was not universally applied in advance; it was used based on clinical suspicion,” the researchers wrote.
Among newborns with an FSA pattern, the mean gestational age was 38.7 weeks. In total, 17 newborns received genetic testing, and 30 had protein-based confirmatory testing.
“In this consecutive cohort of 31 newborns with a suspected diagnosis of HbSbeta+ based on initial newborn screening (an FSA pattern), none actually had HbSbeta+. All had the sickle cell trait (HbAS), instead; that is, we found that an initial FSA pattern was much more likely to indicate a final diagnosis of HbAS than HbSbeta+,” the authors wrote.
This meant that two-thirds of these newborns had a correct diagnosis of HbAS established at 2-4 weeks of age by protein-based confirmatory testing (and confirmed by genetic testing in a subset), but that the remaining one-third still had an incorrect, suspected diagnosis of HbSbeta+. This could lead to unnecessary treatment and testing of infants and incorrect, disease-focused counseling of parents and family members, according to the authors.
Two key limitations of the study were the small sample size and retrospective design.
“Based on this experience in which genetic testing was not universally applied, we now perform simultaneous protein-based and genetic testing as our standard clinical practice,” they concluded.
The study was funded by the National Institutes of Health and the Ohio Department of Health. The authors reported having no conflicts of interest.
SOURCE: Shook LM et al. Int J Neonatal Screen. 2020 Jan 31. doi: 10.3390/ijns6010007
Confirmatory genetic testing may be useful in the diagnosis of hemoglobinopathies for newborns with an abnormal hemoglobin (Hb) pattern, according to a recent study.
The findings suggest further research is needed to evaluate whether genetic testing programs for newborns could have diagnostic value in the clinical setting.
“We studied a consecutive cohort of newborns with an ‘FSA’ pattern (a suspected diagnosis of HbSbeta+) on the initial newborn screening test,” explained Lisa M. Shook of the University of Cincinnati and colleagues. The results were published in the International Journal of Neonatal Screening.
The retrospective study included a total of 1,151 newborns with an abnormal Hb pattern, 31 of which had an FSA pattern. The newborns were screened for hemoglobinopathies from 2015 to 2018. The findings of the initial newborn screening test (a suspected diagnosis of HbSbeta+) were compared with the diagnosis established using both protein-based and genetic confirmatory testing. Protein-based testing cannot accurately detect several hemoglobinopathies in newborns, especially when beta-thalassemia mutations are involved, according to the authors.
“During this study period, genetic testing was not universally applied in advance; it was used based on clinical suspicion,” the researchers wrote.
Among newborns with an FSA pattern, the mean gestational age was 38.7 weeks. In total, 17 newborns received genetic testing, and 30 had protein-based confirmatory testing.
“In this consecutive cohort of 31 newborns with a suspected diagnosis of HbSbeta+ based on initial newborn screening (an FSA pattern), none actually had HbSbeta+. All had the sickle cell trait (HbAS), instead; that is, we found that an initial FSA pattern was much more likely to indicate a final diagnosis of HbAS than HbSbeta+,” the authors wrote.
This meant that two-thirds of these newborns had a correct diagnosis of HbAS established at 2-4 weeks of age by protein-based confirmatory testing (and confirmed by genetic testing in a subset), but that the remaining one-third still had an incorrect, suspected diagnosis of HbSbeta+. This could lead to unnecessary treatment and testing of infants and incorrect, disease-focused counseling of parents and family members, according to the authors.
Two key limitations of the study were the small sample size and retrospective design.
“Based on this experience in which genetic testing was not universally applied, we now perform simultaneous protein-based and genetic testing as our standard clinical practice,” they concluded.
The study was funded by the National Institutes of Health and the Ohio Department of Health. The authors reported having no conflicts of interest.
SOURCE: Shook LM et al. Int J Neonatal Screen. 2020 Jan 31. doi: 10.3390/ijns6010007
FROM THE INTERNATIONAL JOURNAL OF NEONATAL SCREENING
Fear drives activity changes in hemophilia patients
Fear of negative events can drive changes in activity levels among patients with hemophilia A, results of the HemACTIVE study suggest.
Patients were more likely to adjust their level of physical activity due to fear of bleeding and joint damage rather than previously experienced bleeding or joint damage.
However, past experience was more likely than fear to make patients stop physical activities altogether.
Mark Skinner, of the Institute for Policy Advancement in Washington, D.C., and colleagues presented these findings in a poster from the annual congress of the European Association for Haemophilia and Allied Disorders.
Mr. Skinner, who is a hemophilia patient himself, said the goal of the HemACTIVE study is to better understand how hemophilia affects patients’ lives.
“We wanted to understand the limitations, challenges, and compromises of individuals living with hemophilia,” Mr. Skinner said. “What has motivated them or prevented them from living more full, active lives doing the kind of work, leisure, and social activities that those without hemophilia do? Is it treatment choice, is it satisfaction with treatment, is it fear?
“We wanted to do a comprehensive study that really looked at the intersection of treatment adherence and satisfaction, the emotional components that relate to those decisions, and the challenges and compromises so that we could better identify what we need to consider as patients think about either changing their therapy or changing their treatment regimen on existing therapy.”
Previous results from the HemACTIVE study showed that, although activity levels differed among hemophilia patients, all patients surveyed wanted greater activity levels, better protection from bleeding, better pain relief, and less-frequent infusions (EAHAD 2019, Abstract P084). In addition, patients who used factor VIII products with an extended half-life were more active and more likely to adhere to their prescribed treatment (ISTH 2019, Abstract PB0210).
The results reported at EAHAD 2020 focus on patients’ reasons for modifying physical activity. Patients and caregivers completed a screening phone interview, followed by a 25-minute, web-based questionnaire on patient activity.
There were 275 respondents – 194 patients with hemophilia A and 81 caregivers – from Canada, France, Germany, Italy, and the United States. Patients had severe (61%) or moderate (39%) hemophilia A, and most (67%) were receiving prophylaxis.
Most patients (70%) were “active” or “extremely/very active,” 77% of patients adjusted their activities because of their hemophilia, and nearly half of patients stopped activities because of their disease.
Fear drives adjustments in activity
Patients were sometimes more likely to adjust their activities based on fear of experiencing an event, as opposed to previously experiencing that event.
Specifically, 44% of patients adjusted their activities due to fear of joint damage, compared with 36% of patients who made adjustments because of past significant joint damage.
Similarly, 41% of patients adjusted activities due to fear of breakthrough bleeds, compared with 36% of patients who made adjustments because of past experience with bleeds and 25% who made adjustments because of significant past bleeds.
On the other hand, a similar percentage of patients adjusted activities because of past experience with pain (43%) and fear of pain (41%). And a similar percentage of patients adjusted activities because of existing joint damage restrictions (35%) and fear of joint deterioration (32%).
Past experience prompts discontinuation of activity
Overall, 47% of patients said anxiety was the most common emotional reason for stopping physical activities. However, patients were consistently more likely to stop activities because of past experience rather than fear or anxiety.
Specifically, 50% of patients stopped activities because of significant past joint damage, 46% stopped because of developing joint problems, and 38% stopped due to fear of joint damage.
More patients stopped activities because of significant past bleeds (41%) rather than fear of breakthrough bleeds (26%). More patients stopped activities because they developed chronic pain (38%) rather than fear of pain (less than 15%). And more patients stopped activities because of existing joint damage restrictions (62%) rather than fear of joint damage (34%).
Applying results to practice: Changing the conversation
Ideally, these findings would be used to promote individualized treatment of hemophilia driven by patients’ goals, Mr. Skinner said. By better understanding patients’ feelings and motivations, clinicians may devise more personalized treatment regimens that align with patients’ goals and improve their quality of life.
Rather than adjusting treatment based only on “hard metrics” such as bleeding events, “we need to take a more holistic approach to looking at outcomes that are more important to patients,” Mr. Skinner said. This type of approach is particularly important to Mr. Skinner as someone who has severe hemophilia A.
“Because hemophilia is a life-long disease, and you’re born with it, you make conscious or unconscious adaptations throughout your life,” he explained. “Your expectations or aspirations adjust to what you’ve been told you can or cannot do because of your hemophilia. The choices I made for my career, where I live, the type of vacations I go on, the type of sports I participate in have all been limited over the course of time, which has meant that I’ve made compromises. There are a lot of individuals with hemophilia who are making decisions that are not what their life goals are.
“What this research helps me understand is that we can change the conversation and build it around an individual patient and understand what their aspirations are. If a clinician understands what I’m wanting to achieve in life … we can build a treatment regime around helping me achieve those goals. That is known to improve adherence.
“The goal, really, is to have hemophilia as a secondary consideration. Instead of saying: ‘You have hemophilia, so these are the options available to you,’ you can say, ‘what is it that you would like to achieve, and then we’ll figure out how your treatment for hemophilia can be adjusted to help you achieve those goals.’ It may sound like a nuance, but it really is reversing the conversation. The goal setting first versus your disease comes first.”
The HemACTIVE study was supported by Bayer. Mr. Skinner disclosed relationships with Bayer and other pharmaceutical companies.
SOURCE: Skinner M et al. EAHAD 2020, Abstract P304.
Fear of negative events can drive changes in activity levels among patients with hemophilia A, results of the HemACTIVE study suggest.
Patients were more likely to adjust their level of physical activity due to fear of bleeding and joint damage rather than previously experienced bleeding or joint damage.
However, past experience was more likely than fear to make patients stop physical activities altogether.
Mark Skinner, of the Institute for Policy Advancement in Washington, D.C., and colleagues presented these findings in a poster from the annual congress of the European Association for Haemophilia and Allied Disorders.
Mr. Skinner, who is a hemophilia patient himself, said the goal of the HemACTIVE study is to better understand how hemophilia affects patients’ lives.
“We wanted to understand the limitations, challenges, and compromises of individuals living with hemophilia,” Mr. Skinner said. “What has motivated them or prevented them from living more full, active lives doing the kind of work, leisure, and social activities that those without hemophilia do? Is it treatment choice, is it satisfaction with treatment, is it fear?
“We wanted to do a comprehensive study that really looked at the intersection of treatment adherence and satisfaction, the emotional components that relate to those decisions, and the challenges and compromises so that we could better identify what we need to consider as patients think about either changing their therapy or changing their treatment regimen on existing therapy.”
Previous results from the HemACTIVE study showed that, although activity levels differed among hemophilia patients, all patients surveyed wanted greater activity levels, better protection from bleeding, better pain relief, and less-frequent infusions (EAHAD 2019, Abstract P084). In addition, patients who used factor VIII products with an extended half-life were more active and more likely to adhere to their prescribed treatment (ISTH 2019, Abstract PB0210).
The results reported at EAHAD 2020 focus on patients’ reasons for modifying physical activity. Patients and caregivers completed a screening phone interview, followed by a 25-minute, web-based questionnaire on patient activity.
There were 275 respondents – 194 patients with hemophilia A and 81 caregivers – from Canada, France, Germany, Italy, and the United States. Patients had severe (61%) or moderate (39%) hemophilia A, and most (67%) were receiving prophylaxis.
Most patients (70%) were “active” or “extremely/very active,” 77% of patients adjusted their activities because of their hemophilia, and nearly half of patients stopped activities because of their disease.
Fear drives adjustments in activity
Patients were sometimes more likely to adjust their activities based on fear of experiencing an event, as opposed to previously experiencing that event.
Specifically, 44% of patients adjusted their activities due to fear of joint damage, compared with 36% of patients who made adjustments because of past significant joint damage.
Similarly, 41% of patients adjusted activities due to fear of breakthrough bleeds, compared with 36% of patients who made adjustments because of past experience with bleeds and 25% who made adjustments because of significant past bleeds.
On the other hand, a similar percentage of patients adjusted activities because of past experience with pain (43%) and fear of pain (41%). And a similar percentage of patients adjusted activities because of existing joint damage restrictions (35%) and fear of joint deterioration (32%).
Past experience prompts discontinuation of activity
Overall, 47% of patients said anxiety was the most common emotional reason for stopping physical activities. However, patients were consistently more likely to stop activities because of past experience rather than fear or anxiety.
Specifically, 50% of patients stopped activities because of significant past joint damage, 46% stopped because of developing joint problems, and 38% stopped due to fear of joint damage.
More patients stopped activities because of significant past bleeds (41%) rather than fear of breakthrough bleeds (26%). More patients stopped activities because they developed chronic pain (38%) rather than fear of pain (less than 15%). And more patients stopped activities because of existing joint damage restrictions (62%) rather than fear of joint damage (34%).
Applying results to practice: Changing the conversation
Ideally, these findings would be used to promote individualized treatment of hemophilia driven by patients’ goals, Mr. Skinner said. By better understanding patients’ feelings and motivations, clinicians may devise more personalized treatment regimens that align with patients’ goals and improve their quality of life.
Rather than adjusting treatment based only on “hard metrics” such as bleeding events, “we need to take a more holistic approach to looking at outcomes that are more important to patients,” Mr. Skinner said. This type of approach is particularly important to Mr. Skinner as someone who has severe hemophilia A.
“Because hemophilia is a life-long disease, and you’re born with it, you make conscious or unconscious adaptations throughout your life,” he explained. “Your expectations or aspirations adjust to what you’ve been told you can or cannot do because of your hemophilia. The choices I made for my career, where I live, the type of vacations I go on, the type of sports I participate in have all been limited over the course of time, which has meant that I’ve made compromises. There are a lot of individuals with hemophilia who are making decisions that are not what their life goals are.
“What this research helps me understand is that we can change the conversation and build it around an individual patient and understand what their aspirations are. If a clinician understands what I’m wanting to achieve in life … we can build a treatment regime around helping me achieve those goals. That is known to improve adherence.
“The goal, really, is to have hemophilia as a secondary consideration. Instead of saying: ‘You have hemophilia, so these are the options available to you,’ you can say, ‘what is it that you would like to achieve, and then we’ll figure out how your treatment for hemophilia can be adjusted to help you achieve those goals.’ It may sound like a nuance, but it really is reversing the conversation. The goal setting first versus your disease comes first.”
The HemACTIVE study was supported by Bayer. Mr. Skinner disclosed relationships with Bayer and other pharmaceutical companies.
SOURCE: Skinner M et al. EAHAD 2020, Abstract P304.
Fear of negative events can drive changes in activity levels among patients with hemophilia A, results of the HemACTIVE study suggest.
Patients were more likely to adjust their level of physical activity due to fear of bleeding and joint damage rather than previously experienced bleeding or joint damage.
However, past experience was more likely than fear to make patients stop physical activities altogether.
Mark Skinner, of the Institute for Policy Advancement in Washington, D.C., and colleagues presented these findings in a poster from the annual congress of the European Association for Haemophilia and Allied Disorders.
Mr. Skinner, who is a hemophilia patient himself, said the goal of the HemACTIVE study is to better understand how hemophilia affects patients’ lives.
“We wanted to understand the limitations, challenges, and compromises of individuals living with hemophilia,” Mr. Skinner said. “What has motivated them or prevented them from living more full, active lives doing the kind of work, leisure, and social activities that those without hemophilia do? Is it treatment choice, is it satisfaction with treatment, is it fear?
“We wanted to do a comprehensive study that really looked at the intersection of treatment adherence and satisfaction, the emotional components that relate to those decisions, and the challenges and compromises so that we could better identify what we need to consider as patients think about either changing their therapy or changing their treatment regimen on existing therapy.”
Previous results from the HemACTIVE study showed that, although activity levels differed among hemophilia patients, all patients surveyed wanted greater activity levels, better protection from bleeding, better pain relief, and less-frequent infusions (EAHAD 2019, Abstract P084). In addition, patients who used factor VIII products with an extended half-life were more active and more likely to adhere to their prescribed treatment (ISTH 2019, Abstract PB0210).
The results reported at EAHAD 2020 focus on patients’ reasons for modifying physical activity. Patients and caregivers completed a screening phone interview, followed by a 25-minute, web-based questionnaire on patient activity.
There were 275 respondents – 194 patients with hemophilia A and 81 caregivers – from Canada, France, Germany, Italy, and the United States. Patients had severe (61%) or moderate (39%) hemophilia A, and most (67%) were receiving prophylaxis.
Most patients (70%) were “active” or “extremely/very active,” 77% of patients adjusted their activities because of their hemophilia, and nearly half of patients stopped activities because of their disease.
Fear drives adjustments in activity
Patients were sometimes more likely to adjust their activities based on fear of experiencing an event, as opposed to previously experiencing that event.
Specifically, 44% of patients adjusted their activities due to fear of joint damage, compared with 36% of patients who made adjustments because of past significant joint damage.
Similarly, 41% of patients adjusted activities due to fear of breakthrough bleeds, compared with 36% of patients who made adjustments because of past experience with bleeds and 25% who made adjustments because of significant past bleeds.
On the other hand, a similar percentage of patients adjusted activities because of past experience with pain (43%) and fear of pain (41%). And a similar percentage of patients adjusted activities because of existing joint damage restrictions (35%) and fear of joint deterioration (32%).
Past experience prompts discontinuation of activity
Overall, 47% of patients said anxiety was the most common emotional reason for stopping physical activities. However, patients were consistently more likely to stop activities because of past experience rather than fear or anxiety.
Specifically, 50% of patients stopped activities because of significant past joint damage, 46% stopped because of developing joint problems, and 38% stopped due to fear of joint damage.
More patients stopped activities because of significant past bleeds (41%) rather than fear of breakthrough bleeds (26%). More patients stopped activities because they developed chronic pain (38%) rather than fear of pain (less than 15%). And more patients stopped activities because of existing joint damage restrictions (62%) rather than fear of joint damage (34%).
Applying results to practice: Changing the conversation
Ideally, these findings would be used to promote individualized treatment of hemophilia driven by patients’ goals, Mr. Skinner said. By better understanding patients’ feelings and motivations, clinicians may devise more personalized treatment regimens that align with patients’ goals and improve their quality of life.
Rather than adjusting treatment based only on “hard metrics” such as bleeding events, “we need to take a more holistic approach to looking at outcomes that are more important to patients,” Mr. Skinner said. This type of approach is particularly important to Mr. Skinner as someone who has severe hemophilia A.
“Because hemophilia is a life-long disease, and you’re born with it, you make conscious or unconscious adaptations throughout your life,” he explained. “Your expectations or aspirations adjust to what you’ve been told you can or cannot do because of your hemophilia. The choices I made for my career, where I live, the type of vacations I go on, the type of sports I participate in have all been limited over the course of time, which has meant that I’ve made compromises. There are a lot of individuals with hemophilia who are making decisions that are not what their life goals are.
“What this research helps me understand is that we can change the conversation and build it around an individual patient and understand what their aspirations are. If a clinician understands what I’m wanting to achieve in life … we can build a treatment regime around helping me achieve those goals. That is known to improve adherence.
“The goal, really, is to have hemophilia as a secondary consideration. Instead of saying: ‘You have hemophilia, so these are the options available to you,’ you can say, ‘what is it that you would like to achieve, and then we’ll figure out how your treatment for hemophilia can be adjusted to help you achieve those goals.’ It may sound like a nuance, but it really is reversing the conversation. The goal setting first versus your disease comes first.”
The HemACTIVE study was supported by Bayer. Mr. Skinner disclosed relationships with Bayer and other pharmaceutical companies.
SOURCE: Skinner M et al. EAHAD 2020, Abstract P304.
REPORTING FROM EAHAD 2020